User login
Medications in Dermatology, Part 2: Immunosuppressives
6 ‘M’s to keep in mind when you next see a patient with anorexia nervosa
Anorexia nervosa is associated with comorbid psychiatric disorders, severe physical complications, and high mortality. To help you remember important clinical information when working with patients with anorexia, we propose this “6 M” model for screening, treatment, and prognosis.
Monitor closely. Anorexia can go undiagnosed and untreated for years. During your patients’ office visits, ask about body image, exercise habits, and menstrual irregularities, especially when seeing at-risk youth. During physical examination, reluctance to be weighed, vital sign abnormalities (eg, orthostatic hypotension, variability in pulse), skin abnormalities (lanugo hair, dryness), and marks indicating self-harm can serve as diagnostic indicators. Consider hospitalization for patients at <75% of their ideal body weight, who refuse to eat, or who show vital signs and laboratory abnormalities.
Media. By providing information on healthy eating and nutrition, the Internet can be an excellent resource for people with an eating disorder; however, you should also be aware of the impact of so-called pro-ana Web sites. People with anorexia use these Web sites to discuss their illness, but the sites sometimes glorify eating disorders as a lifestyle choice, and can be a place to share tips and tricks on extreme dieting, and might promote what is known as “thinspiration” in popular culture.
Meals. The American Dietetic Association recommends that anorexic patients begin oral intake at no more than 30 to 40 kcal/kg/day, and then gradually increase it, with a weight gain goal of 0.5 to 1 lb per week.
This graduated weight gain is done to prevent refeeding syndrome. After chronic starvation, intracellular phosphate stores are depleted and once carbohydrate intake resumes, insulin release causes phosphate to enter cells, thereby leading to hypophosphatemia. This electrolyte abnormality can result in cardiac failure. As a result, consider regular monitoring of phosphate levels, especially during the first week of reintroducing food.
Multimodal therapy. Despite being notoriously difficult to treat, patients with anorexia might respond to psychotherapy—especially family therapy—with an increased remission rate and faster return to health, compared with other forms of treatment. With a multimodal regimen involving proper refeeding techniques, family therapy, and medications as appropriate, recovery is possible.
Medications might be a helpful adjunct in patients who do not gain weight despite psychotherapy and proper nutritional measures. For example:
• There is some research on medications such as olanzapine and anxiolytics for treating anorexia.
• A low-dose anxiolytic might benefit patients with preprandial anxiety.
• Comorbid psychiatric disorders might improve during treatment of the eating disorder.
• Selective serotonin reuptake inhibitors and second-generation antipsychotics might help manage severe comorbid psychiatric disorders.
Because of low body weight and altered plasma protein binding, start medications at a low dosage. The risk of adverse effects can increase because more “free” medication is available. Consider avoiding medications such as bupropion and tricyclic antidepressants, because they carry an increased risk of seizures and cardiac effects, respectively.
Morbidity and mortality. Untreated anorexia has the highest mortality among psychiatric disorders: approximately 5.1 deaths for every 1,000 people.1 Recent meta-analyses show that patients with anorexia may have a 5.86 times greater risk of death than the general population.1 Serious sequelae include cardiac complications; osteoporosis; infertility; and comorbid psychiatric conditions such as substance abuse, depression, and obsessive-compulsive disorder.2
1. Arcelus J, Mitchell AJ, Wales J, et al. Mortality rates in patients with anorexia nervosa and other eating disorders. A meta-analysis of 36 studies. Arch Gen Psychiatry. 2011; 68(7):724-731.
2. Yager J, Andersen AE. Clinical practice. Anorexia nervosa. N Engl J Med. 2005;353(14):1481-1488.
Anorexia nervosa is associated with comorbid psychiatric disorders, severe physical complications, and high mortality. To help you remember important clinical information when working with patients with anorexia, we propose this “6 M” model for screening, treatment, and prognosis.
Monitor closely. Anorexia can go undiagnosed and untreated for years. During your patients’ office visits, ask about body image, exercise habits, and menstrual irregularities, especially when seeing at-risk youth. During physical examination, reluctance to be weighed, vital sign abnormalities (eg, orthostatic hypotension, variability in pulse), skin abnormalities (lanugo hair, dryness), and marks indicating self-harm can serve as diagnostic indicators. Consider hospitalization for patients at <75% of their ideal body weight, who refuse to eat, or who show vital signs and laboratory abnormalities.
Media. By providing information on healthy eating and nutrition, the Internet can be an excellent resource for people with an eating disorder; however, you should also be aware of the impact of so-called pro-ana Web sites. People with anorexia use these Web sites to discuss their illness, but the sites sometimes glorify eating disorders as a lifestyle choice, and can be a place to share tips and tricks on extreme dieting, and might promote what is known as “thinspiration” in popular culture.
Meals. The American Dietetic Association recommends that anorexic patients begin oral intake at no more than 30 to 40 kcal/kg/day, and then gradually increase it, with a weight gain goal of 0.5 to 1 lb per week.
This graduated weight gain is done to prevent refeeding syndrome. After chronic starvation, intracellular phosphate stores are depleted and once carbohydrate intake resumes, insulin release causes phosphate to enter cells, thereby leading to hypophosphatemia. This electrolyte abnormality can result in cardiac failure. As a result, consider regular monitoring of phosphate levels, especially during the first week of reintroducing food.
Multimodal therapy. Despite being notoriously difficult to treat, patients with anorexia might respond to psychotherapy—especially family therapy—with an increased remission rate and faster return to health, compared with other forms of treatment. With a multimodal regimen involving proper refeeding techniques, family therapy, and medications as appropriate, recovery is possible.
Medications might be a helpful adjunct in patients who do not gain weight despite psychotherapy and proper nutritional measures. For example:
• There is some research on medications such as olanzapine and anxiolytics for treating anorexia.
• A low-dose anxiolytic might benefit patients with preprandial anxiety.
• Comorbid psychiatric disorders might improve during treatment of the eating disorder.
• Selective serotonin reuptake inhibitors and second-generation antipsychotics might help manage severe comorbid psychiatric disorders.
Because of low body weight and altered plasma protein binding, start medications at a low dosage. The risk of adverse effects can increase because more “free” medication is available. Consider avoiding medications such as bupropion and tricyclic antidepressants, because they carry an increased risk of seizures and cardiac effects, respectively.
Morbidity and mortality. Untreated anorexia has the highest mortality among psychiatric disorders: approximately 5.1 deaths for every 1,000 people.1 Recent meta-analyses show that patients with anorexia may have a 5.86 times greater risk of death than the general population.1 Serious sequelae include cardiac complications; osteoporosis; infertility; and comorbid psychiatric conditions such as substance abuse, depression, and obsessive-compulsive disorder.2
Anorexia nervosa is associated with comorbid psychiatric disorders, severe physical complications, and high mortality. To help you remember important clinical information when working with patients with anorexia, we propose this “6 M” model for screening, treatment, and prognosis.
Monitor closely. Anorexia can go undiagnosed and untreated for years. During your patients’ office visits, ask about body image, exercise habits, and menstrual irregularities, especially when seeing at-risk youth. During physical examination, reluctance to be weighed, vital sign abnormalities (eg, orthostatic hypotension, variability in pulse), skin abnormalities (lanugo hair, dryness), and marks indicating self-harm can serve as diagnostic indicators. Consider hospitalization for patients at <75% of their ideal body weight, who refuse to eat, or who show vital signs and laboratory abnormalities.
Media. By providing information on healthy eating and nutrition, the Internet can be an excellent resource for people with an eating disorder; however, you should also be aware of the impact of so-called pro-ana Web sites. People with anorexia use these Web sites to discuss their illness, but the sites sometimes glorify eating disorders as a lifestyle choice, and can be a place to share tips and tricks on extreme dieting, and might promote what is known as “thinspiration” in popular culture.
Meals. The American Dietetic Association recommends that anorexic patients begin oral intake at no more than 30 to 40 kcal/kg/day, and then gradually increase it, with a weight gain goal of 0.5 to 1 lb per week.
This graduated weight gain is done to prevent refeeding syndrome. After chronic starvation, intracellular phosphate stores are depleted and once carbohydrate intake resumes, insulin release causes phosphate to enter cells, thereby leading to hypophosphatemia. This electrolyte abnormality can result in cardiac failure. As a result, consider regular monitoring of phosphate levels, especially during the first week of reintroducing food.
Multimodal therapy. Despite being notoriously difficult to treat, patients with anorexia might respond to psychotherapy—especially family therapy—with an increased remission rate and faster return to health, compared with other forms of treatment. With a multimodal regimen involving proper refeeding techniques, family therapy, and medications as appropriate, recovery is possible.
Medications might be a helpful adjunct in patients who do not gain weight despite psychotherapy and proper nutritional measures. For example:
• There is some research on medications such as olanzapine and anxiolytics for treating anorexia.
• A low-dose anxiolytic might benefit patients with preprandial anxiety.
• Comorbid psychiatric disorders might improve during treatment of the eating disorder.
• Selective serotonin reuptake inhibitors and second-generation antipsychotics might help manage severe comorbid psychiatric disorders.
Because of low body weight and altered plasma protein binding, start medications at a low dosage. The risk of adverse effects can increase because more “free” medication is available. Consider avoiding medications such as bupropion and tricyclic antidepressants, because they carry an increased risk of seizures and cardiac effects, respectively.
Morbidity and mortality. Untreated anorexia has the highest mortality among psychiatric disorders: approximately 5.1 deaths for every 1,000 people.1 Recent meta-analyses show that patients with anorexia may have a 5.86 times greater risk of death than the general population.1 Serious sequelae include cardiac complications; osteoporosis; infertility; and comorbid psychiatric conditions such as substance abuse, depression, and obsessive-compulsive disorder.2
1. Arcelus J, Mitchell AJ, Wales J, et al. Mortality rates in patients with anorexia nervosa and other eating disorders. A meta-analysis of 36 studies. Arch Gen Psychiatry. 2011; 68(7):724-731.
2. Yager J, Andersen AE. Clinical practice. Anorexia nervosa. N Engl J Med. 2005;353(14):1481-1488.
1. Arcelus J, Mitchell AJ, Wales J, et al. Mortality rates in patients with anorexia nervosa and other eating disorders. A meta-analysis of 36 studies. Arch Gen Psychiatry. 2011; 68(7):724-731.
2. Yager J, Andersen AE. Clinical practice. Anorexia nervosa. N Engl J Med. 2005;353(14):1481-1488.
Rethink clonidine for patients undergoing noncardiac surgery
Close to 1 in 3 Americans has hypertension, and the American Heart Association estimates that number will increase by more than 7% by 2030. The prevalence of obesity, a sedentary lifestyle, cigarette smoking, and a variety of other risk factors create a perfect storm for cardiovascular topsy-turviness.
Hypertension is so common among hospitalized patients, most of us have already chosen our "drugs of choice" to treat it. Unlike the case in primary care, in which physicians may have the luxury of starting with a first-line drug, and perhaps adding a second-line agent a few months later, in the hospital setting, we are often faced with hypertensive emergencies and urgencies that require immediate treatment. The expert opinions outlined in the new JNC-8 guidelines may not be appropriate for our acutely ill patient with a blood pressure of 240/135.
While decreasing the blood pressure is a top priority, there are frequently complicating factors, such as uncontrolled pain, intravenous fluids, or glucocorticoid use that make maintaining a consistently safe blood pressure challenging, to say the least. One reading may be an acceptable 140/85, while a few hours later it may spike to 200/120, and this roller coaster ride may continue for days on end. That\'s when we often reach for a PRN medication to help keep the patient out of danger as we manage a host of other conditions.
Many remember when sublingual nifedipine was the drug of choice for rapid reduction of severe blood pressure elevations, until the rapid drop proved to be devastating to the cerebral perfusion for some very unfortunate patients. Over the years, clonidine has become a highly favored drug if an oral agent is deemed appropriate. Its onset is rapid, and it drops the blood pressure to a moderate degree in most patients. However, a recent article in the New England Journal of Medicine, Clonidine in Patients Undergoing Noncardiac Surgery, may make many rethink their use of clonidine in this subpopulation of patients.
Researchers found clonidine 0.2 mg daily started just before surgery and continued until 72 hours postop was associated with an increase in nonfatal cardiac arrest (0.3% vs. 0.1%) and clinically significant hypotension (47.6% vs. 37.1%). Myocardial infarction occurred in 5.9% in the placebo group, compared to 6.6% in the clonidine group (N. Engl. J. Med. 2014;370:1504-13).
This article is highly significant to me, a frequent prescriber of PRN clonidine. Though the article did not address PRN use of clonidine perioperatively, in my opinion, the results are concerning enough to warrant thoughtful consideration. While it will not likely affect my prescribing practice for most patients, I plan to expand my armamentarium of drugs for those who I think may require surgery in the near future.
Dr. Hester is a hospitalist with Baltimore-Washington Medical Center who has a passion for empowering patients to partner in their health care. She is the creator of the Patient Whiz, a patient-engagement app for iOS. Reach her at [email protected].
Close to 1 in 3 Americans has hypertension, and the American Heart Association estimates that number will increase by more than 7% by 2030. The prevalence of obesity, a sedentary lifestyle, cigarette smoking, and a variety of other risk factors create a perfect storm for cardiovascular topsy-turviness.
Hypertension is so common among hospitalized patients, most of us have already chosen our "drugs of choice" to treat it. Unlike the case in primary care, in which physicians may have the luxury of starting with a first-line drug, and perhaps adding a second-line agent a few months later, in the hospital setting, we are often faced with hypertensive emergencies and urgencies that require immediate treatment. The expert opinions outlined in the new JNC-8 guidelines may not be appropriate for our acutely ill patient with a blood pressure of 240/135.
While decreasing the blood pressure is a top priority, there are frequently complicating factors, such as uncontrolled pain, intravenous fluids, or glucocorticoid use that make maintaining a consistently safe blood pressure challenging, to say the least. One reading may be an acceptable 140/85, while a few hours later it may spike to 200/120, and this roller coaster ride may continue for days on end. That\'s when we often reach for a PRN medication to help keep the patient out of danger as we manage a host of other conditions.
Many remember when sublingual nifedipine was the drug of choice for rapid reduction of severe blood pressure elevations, until the rapid drop proved to be devastating to the cerebral perfusion for some very unfortunate patients. Over the years, clonidine has become a highly favored drug if an oral agent is deemed appropriate. Its onset is rapid, and it drops the blood pressure to a moderate degree in most patients. However, a recent article in the New England Journal of Medicine, Clonidine in Patients Undergoing Noncardiac Surgery, may make many rethink their use of clonidine in this subpopulation of patients.
Researchers found clonidine 0.2 mg daily started just before surgery and continued until 72 hours postop was associated with an increase in nonfatal cardiac arrest (0.3% vs. 0.1%) and clinically significant hypotension (47.6% vs. 37.1%). Myocardial infarction occurred in 5.9% in the placebo group, compared to 6.6% in the clonidine group (N. Engl. J. Med. 2014;370:1504-13).
This article is highly significant to me, a frequent prescriber of PRN clonidine. Though the article did not address PRN use of clonidine perioperatively, in my opinion, the results are concerning enough to warrant thoughtful consideration. While it will not likely affect my prescribing practice for most patients, I plan to expand my armamentarium of drugs for those who I think may require surgery in the near future.
Dr. Hester is a hospitalist with Baltimore-Washington Medical Center who has a passion for empowering patients to partner in their health care. She is the creator of the Patient Whiz, a patient-engagement app for iOS. Reach her at [email protected].
Close to 1 in 3 Americans has hypertension, and the American Heart Association estimates that number will increase by more than 7% by 2030. The prevalence of obesity, a sedentary lifestyle, cigarette smoking, and a variety of other risk factors create a perfect storm for cardiovascular topsy-turviness.
Hypertension is so common among hospitalized patients, most of us have already chosen our "drugs of choice" to treat it. Unlike the case in primary care, in which physicians may have the luxury of starting with a first-line drug, and perhaps adding a second-line agent a few months later, in the hospital setting, we are often faced with hypertensive emergencies and urgencies that require immediate treatment. The expert opinions outlined in the new JNC-8 guidelines may not be appropriate for our acutely ill patient with a blood pressure of 240/135.
While decreasing the blood pressure is a top priority, there are frequently complicating factors, such as uncontrolled pain, intravenous fluids, or glucocorticoid use that make maintaining a consistently safe blood pressure challenging, to say the least. One reading may be an acceptable 140/85, while a few hours later it may spike to 200/120, and this roller coaster ride may continue for days on end. That\'s when we often reach for a PRN medication to help keep the patient out of danger as we manage a host of other conditions.
Many remember when sublingual nifedipine was the drug of choice for rapid reduction of severe blood pressure elevations, until the rapid drop proved to be devastating to the cerebral perfusion for some very unfortunate patients. Over the years, clonidine has become a highly favored drug if an oral agent is deemed appropriate. Its onset is rapid, and it drops the blood pressure to a moderate degree in most patients. However, a recent article in the New England Journal of Medicine, Clonidine in Patients Undergoing Noncardiac Surgery, may make many rethink their use of clonidine in this subpopulation of patients.
Researchers found clonidine 0.2 mg daily started just before surgery and continued until 72 hours postop was associated with an increase in nonfatal cardiac arrest (0.3% vs. 0.1%) and clinically significant hypotension (47.6% vs. 37.1%). Myocardial infarction occurred in 5.9% in the placebo group, compared to 6.6% in the clonidine group (N. Engl. J. Med. 2014;370:1504-13).
This article is highly significant to me, a frequent prescriber of PRN clonidine. Though the article did not address PRN use of clonidine perioperatively, in my opinion, the results are concerning enough to warrant thoughtful consideration. While it will not likely affect my prescribing practice for most patients, I plan to expand my armamentarium of drugs for those who I think may require surgery in the near future.
Dr. Hester is a hospitalist with Baltimore-Washington Medical Center who has a passion for empowering patients to partner in their health care. She is the creator of the Patient Whiz, a patient-engagement app for iOS. Reach her at [email protected].
What are the benefits and risks of inhaled corticosteroids for COPD?
Inhaled corticosteroids (ICS), either alone or with a long-acting β agonist (LABA), reduce the frequency of exacerbations of chronic obstructive pulmonary disease (COPD) and statistically, but not clinically, improve quality of life (QOL) (strength of recommendation [SOR]: B, meta-analyses of heterogeneous studies).
However, ICS have no mortality benefit and don’t consistently improve forced expiratory volume in 1 second (FEV1) (SOR: B, meta-analyses of secondary outcomes). They increase the risk of pneumonia, oropharyngeal candidiasis, and bruising (SOR: B, meta-analyses of secondary outcomes).
Withdrawal of ICS doesn’t significantly increase the risk of COPD exacerbation (SOR: B, a meta-analysis).
EVIDENCE SUMMARY
A Cochrane meta-analysis designed to determine the efficacy of ICS in patients with stable COPD found 55 randomized, controlled trials (RCTs) with a total of 16,154 participants that compared ICS with placebo for 2 weeks to 3 years duration.1 COPD varied from moderate to severe in most studies.
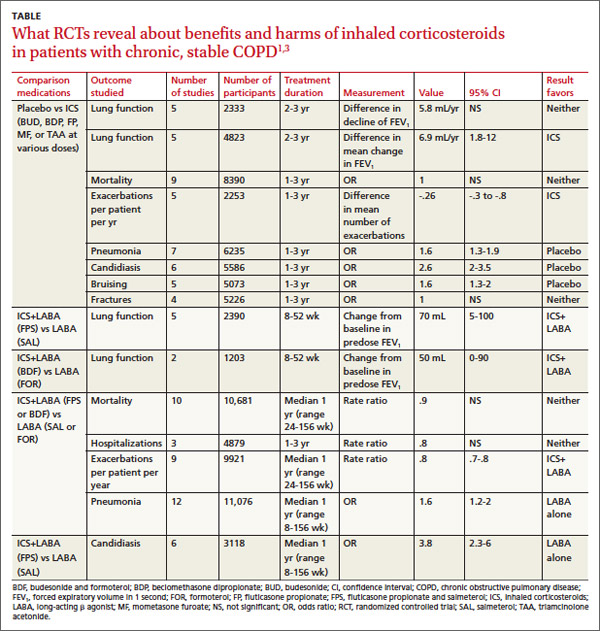
In pooled data, ICS for 2 or more years didn’t consistently improve lung function, the primary outcome (TABLE). However, the largest RCT (N=2617) of 3 years duration showed a small decrease in decline of FEV1 (55 mL compared with 42 mL, P value not provided). Regarding the secondary outcomes of mortality and exacerbations, ICS for a year or longer didn’t reduce mortality but decreased exacerbations by 19%.
Clinically significant adverse effects of ICS use included pneumonia, oropharyngeal candidiasis, and bruising; for ICS treatment longer than one year, the numbers needed to harm (NNH) compared with placebo were 30, 27, and 32, respectively. Bone fractures weren’t more common among ICS users. Investigators observed a statistical, but not clinical, QOL benefit as measured by the St. George’s Respiratory Questionnaire (SGRQ) in 5 RCTs with a total of 2507 patients (mean difference, ‒1.22 units/year; 95% confidence interval, ‒1.83 to ‒.60). The minimum clinically important difference on the 76-item questionnaire was 4 units.2
Adding ICS to LABA increases risk of pneumonia and candidiasis
A Cochrane meta-analysis of 14 double-blind RCTs comprising a total of 11,794 participants with severe COPD compared LABA plus ICS with LABA alone over 8 weeks to 3 years.3 Primary outcomes were exacerbations, mortality, hospitalizations, and pneumonia. Secondary outcomes included oropharyngeal candidiasis and health-related QOL.
The LABA-plus-ICS group had lower rates of exacerbations than the LABA group, but the data were of low quality because of significant heterogeneity among studies and high rates of attrition. No significant difference in mortality or hospitalizations was found between the groups. The risk of pneumonia in the LABA-plus-ICS group was higher than in the LABA-alone group, with a NNH of 48.
Candidiasis occurred more often in patients on combination fluticasone and salmeterol than salmeterol alone, with a NNH of 22. QOL scores (measured by the SGRQ) in patients on combination therapy were statistically better, but clinically insignificant.
Discontinuing ICS doesn’t increase exacerbations
A meta-analysis of 3 RCTs that enrolled a total of 877 patients with COPD compared the number of exacerbations in patients who continued fluticasone 500 mcg inhaled twice daily and patients who were withdrawn from the medication. All patients had been treated with ICS for at least 3 months, and had been on fluticasone for at least 2 weeks. Subjects had a baseline FEV1 between 25% and 80% predicted. No significant increase in exacerbations occurred after discontinuing ICS.4
RECOMMENDATIONS
The American College of Physicians, American College of Chest Physicians, American Thoracic Society, and European Respiratory Society, in a joint guideline, recommend against using ICS as monotherapy for patients with stable COPD. They acknowledge that these drugs are superior to placebo in reducing exacerbations, but note that concerns about their side-effect profile (thrush, potential for bone loss, and moderate to severe easy bruisability) make them less desirable than LABAs or long-acting inhaled anticholinergics.5
The Global Initiative for Chronic Obstructive Lung Disease likewise discourages long-term use of ICS because of the risk of pneumonia and fractures.6 Both groups note that patients with severe COPD may benefit from a combination of ICS and a long-acting medication (usually a LABA).
1. Yang IA, Clarke MS, Sim EH, et al. Inhaled corticosteroids for stable chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2012;(7):CD002991.
2. Jones PW. St. George’s Respiratory Questionnaire: MCID. COPD. 2005;2:75-79.
3. Nannini LJ, Lasserson TJ, Poole P. Combined corticosteroid and long-acting beta(2)-agonist in one inhaler versus long-acting beta(2)-agonists for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2012;9:CD006829.
4. Nadeem NJ, Taylor SJ, Eldridge SM. Withdrawal of inhaled corticosteroids in individuals with COPD—a systemic review and comment on trial methodology. Respir Res. 2011;12:107.
5. Qaseem A, Wilt TJ, Weinberger SE, et al; American College of Physicians; American Thoracic Society; European Respiratory Society. Diagnosis and management of stable chronic obstructive pulmonary disease: a clinical practice guideline update from the American College of Physicians, American College of Chest Physicians, American Thoracic Society, and European Respiratory Society. Ann Intern Med. 2011;155:179-191.
6. Global Initiative for Chronic Obstructive Lung Disease Web site. Global strategy for the diagnosis, management and prevention of COPD. 2014. Available at: www.goldcopd.org/uploads/users/files/GOLD_Report2014_Feb07.pdf. Accessed April 4, 2013.
Inhaled corticosteroids (ICS), either alone or with a long-acting β agonist (LABA), reduce the frequency of exacerbations of chronic obstructive pulmonary disease (COPD) and statistically, but not clinically, improve quality of life (QOL) (strength of recommendation [SOR]: B, meta-analyses of heterogeneous studies).
However, ICS have no mortality benefit and don’t consistently improve forced expiratory volume in 1 second (FEV1) (SOR: B, meta-analyses of secondary outcomes). They increase the risk of pneumonia, oropharyngeal candidiasis, and bruising (SOR: B, meta-analyses of secondary outcomes).
Withdrawal of ICS doesn’t significantly increase the risk of COPD exacerbation (SOR: B, a meta-analysis).
EVIDENCE SUMMARY
A Cochrane meta-analysis designed to determine the efficacy of ICS in patients with stable COPD found 55 randomized, controlled trials (RCTs) with a total of 16,154 participants that compared ICS with placebo for 2 weeks to 3 years duration.1 COPD varied from moderate to severe in most studies.
In pooled data, ICS for 2 or more years didn’t consistently improve lung function, the primary outcome (TABLE). However, the largest RCT (N=2617) of 3 years duration showed a small decrease in decline of FEV1 (55 mL compared with 42 mL, P value not provided). Regarding the secondary outcomes of mortality and exacerbations, ICS for a year or longer didn’t reduce mortality but decreased exacerbations by 19%.
Clinically significant adverse effects of ICS use included pneumonia, oropharyngeal candidiasis, and bruising; for ICS treatment longer than one year, the numbers needed to harm (NNH) compared with placebo were 30, 27, and 32, respectively. Bone fractures weren’t more common among ICS users. Investigators observed a statistical, but not clinical, QOL benefit as measured by the St. George’s Respiratory Questionnaire (SGRQ) in 5 RCTs with a total of 2507 patients (mean difference, ‒1.22 units/year; 95% confidence interval, ‒1.83 to ‒.60). The minimum clinically important difference on the 76-item questionnaire was 4 units.2
Adding ICS to LABA increases risk of pneumonia and candidiasis
A Cochrane meta-analysis of 14 double-blind RCTs comprising a total of 11,794 participants with severe COPD compared LABA plus ICS with LABA alone over 8 weeks to 3 years.3 Primary outcomes were exacerbations, mortality, hospitalizations, and pneumonia. Secondary outcomes included oropharyngeal candidiasis and health-related QOL.
The LABA-plus-ICS group had lower rates of exacerbations than the LABA group, but the data were of low quality because of significant heterogeneity among studies and high rates of attrition. No significant difference in mortality or hospitalizations was found between the groups. The risk of pneumonia in the LABA-plus-ICS group was higher than in the LABA-alone group, with a NNH of 48.

Candidiasis occurred more often in patients on combination fluticasone and salmeterol than salmeterol alone, with a NNH of 22. QOL scores (measured by the SGRQ) in patients on combination therapy were statistically better, but clinically insignificant.
Discontinuing ICS doesn’t increase exacerbations
A meta-analysis of 3 RCTs that enrolled a total of 877 patients with COPD compared the number of exacerbations in patients who continued fluticasone 500 mcg inhaled twice daily and patients who were withdrawn from the medication. All patients had been treated with ICS for at least 3 months, and had been on fluticasone for at least 2 weeks. Subjects had a baseline FEV1 between 25% and 80% predicted. No significant increase in exacerbations occurred after discontinuing ICS.4
RECOMMENDATIONS
The American College of Physicians, American College of Chest Physicians, American Thoracic Society, and European Respiratory Society, in a joint guideline, recommend against using ICS as monotherapy for patients with stable COPD. They acknowledge that these drugs are superior to placebo in reducing exacerbations, but note that concerns about their side-effect profile (thrush, potential for bone loss, and moderate to severe easy bruisability) make them less desirable than LABAs or long-acting inhaled anticholinergics.5
The Global Initiative for Chronic Obstructive Lung Disease likewise discourages long-term use of ICS because of the risk of pneumonia and fractures.6 Both groups note that patients with severe COPD may benefit from a combination of ICS and a long-acting medication (usually a LABA).
Inhaled corticosteroids (ICS), either alone or with a long-acting β agonist (LABA), reduce the frequency of exacerbations of chronic obstructive pulmonary disease (COPD) and statistically, but not clinically, improve quality of life (QOL) (strength of recommendation [SOR]: B, meta-analyses of heterogeneous studies).
However, ICS have no mortality benefit and don’t consistently improve forced expiratory volume in 1 second (FEV1) (SOR: B, meta-analyses of secondary outcomes). They increase the risk of pneumonia, oropharyngeal candidiasis, and bruising (SOR: B, meta-analyses of secondary outcomes).
Withdrawal of ICS doesn’t significantly increase the risk of COPD exacerbation (SOR: B, a meta-analysis).
EVIDENCE SUMMARY
A Cochrane meta-analysis designed to determine the efficacy of ICS in patients with stable COPD found 55 randomized, controlled trials (RCTs) with a total of 16,154 participants that compared ICS with placebo for 2 weeks to 3 years duration.1 COPD varied from moderate to severe in most studies.
In pooled data, ICS for 2 or more years didn’t consistently improve lung function, the primary outcome (TABLE). However, the largest RCT (N=2617) of 3 years duration showed a small decrease in decline of FEV1 (55 mL compared with 42 mL, P value not provided). Regarding the secondary outcomes of mortality and exacerbations, ICS for a year or longer didn’t reduce mortality but decreased exacerbations by 19%.
Clinically significant adverse effects of ICS use included pneumonia, oropharyngeal candidiasis, and bruising; for ICS treatment longer than one year, the numbers needed to harm (NNH) compared with placebo were 30, 27, and 32, respectively. Bone fractures weren’t more common among ICS users. Investigators observed a statistical, but not clinical, QOL benefit as measured by the St. George’s Respiratory Questionnaire (SGRQ) in 5 RCTs with a total of 2507 patients (mean difference, ‒1.22 units/year; 95% confidence interval, ‒1.83 to ‒.60). The minimum clinically important difference on the 76-item questionnaire was 4 units.2
Adding ICS to LABA increases risk of pneumonia and candidiasis
A Cochrane meta-analysis of 14 double-blind RCTs comprising a total of 11,794 participants with severe COPD compared LABA plus ICS with LABA alone over 8 weeks to 3 years.3 Primary outcomes were exacerbations, mortality, hospitalizations, and pneumonia. Secondary outcomes included oropharyngeal candidiasis and health-related QOL.
The LABA-plus-ICS group had lower rates of exacerbations than the LABA group, but the data were of low quality because of significant heterogeneity among studies and high rates of attrition. No significant difference in mortality or hospitalizations was found between the groups. The risk of pneumonia in the LABA-plus-ICS group was higher than in the LABA-alone group, with a NNH of 48.

Candidiasis occurred more often in patients on combination fluticasone and salmeterol than salmeterol alone, with a NNH of 22. QOL scores (measured by the SGRQ) in patients on combination therapy were statistically better, but clinically insignificant.
Discontinuing ICS doesn’t increase exacerbations
A meta-analysis of 3 RCTs that enrolled a total of 877 patients with COPD compared the number of exacerbations in patients who continued fluticasone 500 mcg inhaled twice daily and patients who were withdrawn from the medication. All patients had been treated with ICS for at least 3 months, and had been on fluticasone for at least 2 weeks. Subjects had a baseline FEV1 between 25% and 80% predicted. No significant increase in exacerbations occurred after discontinuing ICS.4
RECOMMENDATIONS
The American College of Physicians, American College of Chest Physicians, American Thoracic Society, and European Respiratory Society, in a joint guideline, recommend against using ICS as monotherapy for patients with stable COPD. They acknowledge that these drugs are superior to placebo in reducing exacerbations, but note that concerns about their side-effect profile (thrush, potential for bone loss, and moderate to severe easy bruisability) make them less desirable than LABAs or long-acting inhaled anticholinergics.5
The Global Initiative for Chronic Obstructive Lung Disease likewise discourages long-term use of ICS because of the risk of pneumonia and fractures.6 Both groups note that patients with severe COPD may benefit from a combination of ICS and a long-acting medication (usually a LABA).
1. Yang IA, Clarke MS, Sim EH, et al. Inhaled corticosteroids for stable chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2012;(7):CD002991.
2. Jones PW. St. George’s Respiratory Questionnaire: MCID. COPD. 2005;2:75-79.
3. Nannini LJ, Lasserson TJ, Poole P. Combined corticosteroid and long-acting beta(2)-agonist in one inhaler versus long-acting beta(2)-agonists for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2012;9:CD006829.
4. Nadeem NJ, Taylor SJ, Eldridge SM. Withdrawal of inhaled corticosteroids in individuals with COPD—a systemic review and comment on trial methodology. Respir Res. 2011;12:107.
5. Qaseem A, Wilt TJ, Weinberger SE, et al; American College of Physicians; American Thoracic Society; European Respiratory Society. Diagnosis and management of stable chronic obstructive pulmonary disease: a clinical practice guideline update from the American College of Physicians, American College of Chest Physicians, American Thoracic Society, and European Respiratory Society. Ann Intern Med. 2011;155:179-191.
6. Global Initiative for Chronic Obstructive Lung Disease Web site. Global strategy for the diagnosis, management and prevention of COPD. 2014. Available at: www.goldcopd.org/uploads/users/files/GOLD_Report2014_Feb07.pdf. Accessed April 4, 2013.
1. Yang IA, Clarke MS, Sim EH, et al. Inhaled corticosteroids for stable chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2012;(7):CD002991.
2. Jones PW. St. George’s Respiratory Questionnaire: MCID. COPD. 2005;2:75-79.
3. Nannini LJ, Lasserson TJ, Poole P. Combined corticosteroid and long-acting beta(2)-agonist in one inhaler versus long-acting beta(2)-agonists for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2012;9:CD006829.
4. Nadeem NJ, Taylor SJ, Eldridge SM. Withdrawal of inhaled corticosteroids in individuals with COPD—a systemic review and comment on trial methodology. Respir Res. 2011;12:107.
5. Qaseem A, Wilt TJ, Weinberger SE, et al; American College of Physicians; American Thoracic Society; European Respiratory Society. Diagnosis and management of stable chronic obstructive pulmonary disease: a clinical practice guideline update from the American College of Physicians, American College of Chest Physicians, American Thoracic Society, and European Respiratory Society. Ann Intern Med. 2011;155:179-191.
6. Global Initiative for Chronic Obstructive Lung Disease Web site. Global strategy for the diagnosis, management and prevention of COPD. 2014. Available at: www.goldcopd.org/uploads/users/files/GOLD_Report2014_Feb07.pdf. Accessed April 4, 2013.
Evidence-based answers from the Family Physicians Inquiries Network
Bruises Be Gone! Treatment of Bruising

An article in Dermatologic Surgery, “Comparative Study on Bruise Reduction Treatments After Bruise Induction Using the Pulsed Dye Laser,” (2013;39:1459-1464) compared the of effectiveness different modalities in reducing time for bruise resolution. The investigators compared cold compresses; hydrogen peroxide; over-the-counter bruise serum containing primrose oil, vitamin E oil, and glycerin; and pulsed dye laser (PDL). Seventeen patients (Fitzpatrick skin types I–IV) were enrolled and had bruise induction with a PDL to produce five 2×2-cm zones of bruising on the lower abdomen. Excluding the control, bruises were randomly treated using a cold compress, bruise serum, 3% hydrogen peroxide–soaked gauze, or PDL. Subjects and 2 blinded physician evaluators graded the bruise severity using a visual scale on days 0, 3, and 7. The investigators found that treatment did not result in statistically significantly shorter bruise resolution time than in controls. They reported that PDL-treated bruises took a longer time to resolve than controls.
What’s the issue?
Although this study found that the PDL-treated bruises took a longer time to resolve, there have been other studies that have shown PDL to hasten time to bruise resolution, which could be due to the fact that the initial bruises in this particular study were actually induced by PDL. In another study, DeFatta et al (Arch Facial Plast Surg. 2009;11:99-103) found maximal efficacy when PDL was performed on or after postoperative day 5. It was reasoned that treatment before then was less effective because of the depth of red blood cell extravasation and overlying inflammation and edema. Karen and Hale (Dermatol Surg. 2010;36:1328-1331) reported that the optimal time for PDL treatment was between 48 and 72 hours, suggesting that hemoglobin predominates during this period instead of the bilirubin predominance seen in the later stages of ecchymosis. Their parameters were 7.5 J/cm2, 10-mm spot, 6-millisecond pulse duration, and cryogen 30 milliseconds with a 20-millisecond delay for a single pass. What do you use for posttreatment bruising?

An article in Dermatologic Surgery, “Comparative Study on Bruise Reduction Treatments After Bruise Induction Using the Pulsed Dye Laser,” (2013;39:1459-1464) compared the of effectiveness different modalities in reducing time for bruise resolution. The investigators compared cold compresses; hydrogen peroxide; over-the-counter bruise serum containing primrose oil, vitamin E oil, and glycerin; and pulsed dye laser (PDL). Seventeen patients (Fitzpatrick skin types I–IV) were enrolled and had bruise induction with a PDL to produce five 2×2-cm zones of bruising on the lower abdomen. Excluding the control, bruises were randomly treated using a cold compress, bruise serum, 3% hydrogen peroxide–soaked gauze, or PDL. Subjects and 2 blinded physician evaluators graded the bruise severity using a visual scale on days 0, 3, and 7. The investigators found that treatment did not result in statistically significantly shorter bruise resolution time than in controls. They reported that PDL-treated bruises took a longer time to resolve than controls.
What’s the issue?
Although this study found that the PDL-treated bruises took a longer time to resolve, there have been other studies that have shown PDL to hasten time to bruise resolution, which could be due to the fact that the initial bruises in this particular study were actually induced by PDL. In another study, DeFatta et al (Arch Facial Plast Surg. 2009;11:99-103) found maximal efficacy when PDL was performed on or after postoperative day 5. It was reasoned that treatment before then was less effective because of the depth of red blood cell extravasation and overlying inflammation and edema. Karen and Hale (Dermatol Surg. 2010;36:1328-1331) reported that the optimal time for PDL treatment was between 48 and 72 hours, suggesting that hemoglobin predominates during this period instead of the bilirubin predominance seen in the later stages of ecchymosis. Their parameters were 7.5 J/cm2, 10-mm spot, 6-millisecond pulse duration, and cryogen 30 milliseconds with a 20-millisecond delay for a single pass. What do you use for posttreatment bruising?

An article in Dermatologic Surgery, “Comparative Study on Bruise Reduction Treatments After Bruise Induction Using the Pulsed Dye Laser,” (2013;39:1459-1464) compared the of effectiveness different modalities in reducing time for bruise resolution. The investigators compared cold compresses; hydrogen peroxide; over-the-counter bruise serum containing primrose oil, vitamin E oil, and glycerin; and pulsed dye laser (PDL). Seventeen patients (Fitzpatrick skin types I–IV) were enrolled and had bruise induction with a PDL to produce five 2×2-cm zones of bruising on the lower abdomen. Excluding the control, bruises were randomly treated using a cold compress, bruise serum, 3% hydrogen peroxide–soaked gauze, or PDL. Subjects and 2 blinded physician evaluators graded the bruise severity using a visual scale on days 0, 3, and 7. The investigators found that treatment did not result in statistically significantly shorter bruise resolution time than in controls. They reported that PDL-treated bruises took a longer time to resolve than controls.
What’s the issue?
Although this study found that the PDL-treated bruises took a longer time to resolve, there have been other studies that have shown PDL to hasten time to bruise resolution, which could be due to the fact that the initial bruises in this particular study were actually induced by PDL. In another study, DeFatta et al (Arch Facial Plast Surg. 2009;11:99-103) found maximal efficacy when PDL was performed on or after postoperative day 5. It was reasoned that treatment before then was less effective because of the depth of red blood cell extravasation and overlying inflammation and edema. Karen and Hale (Dermatol Surg. 2010;36:1328-1331) reported that the optimal time for PDL treatment was between 48 and 72 hours, suggesting that hemoglobin predominates during this period instead of the bilirubin predominance seen in the later stages of ecchymosis. Their parameters were 7.5 J/cm2, 10-mm spot, 6-millisecond pulse duration, and cryogen 30 milliseconds with a 20-millisecond delay for a single pass. What do you use for posttreatment bruising?
Psychosis resolves, but menses stop
CASE Paranoid and hallucinating
Ms. S, age 30, is an unmarried graduate student who has been given a diagnosis of schizophrenia, paranoid type, during inpatient hospitalization that was prompted by impairment in school functioning (difficulty turning in assignments, poor concentration, making careless mistakes on tests), paranoid delusions, and multisensory hallucinations. She says that her roommate and classmates are working together to make her leave school, and recalls seeing them “snare and smirk” as she passes by. Ms. S says that she feels her classmates are calling her names and talking badly about her as soon as she is out of sight.
Ms. S is antipsychotic-naïve and has a baseline body mass index of 17.8 kg/m2, indicating that she is underweight. We believe that olanzapine, 20 mg/d, is a good initial treatment because of its propensity for weight gain; however, she experiences only marginal improvement. Ms. S does not have health insurance, and cannot afford a brand name medication; therefore, she is cross-tapered to perphenazine, 8 mg, and benzatropine, 0.5 mg, both taken twice daily (olanzapine was not available as a generic at the time).
At discharge, Ms. S does not report any hallucinatory experiences, but is guarded, voices suspicions about the treatment team, and asks “What are they doing with all my blood?”—referring to blood draws for laboratory testing during hospitalization.
As an outpatient, Ms. S is continued on the same medications until she has to be switched because she cannot afford the out-of-pocket cost of the antipsychotic, perphenazine ($80 a month). Clozapine is recommended, but Ms. S refuses because of the mandatory weekly blood monitoring. She briefly tries fluphenazine, 2.5 mg/d, but it is discontinued because of malaise and lightheadedness without extrapyramidal symptoms.
Clozapine is again recommended, but Ms. S remains suspicious of the necessary blood draws and refuses. After several trials of antipsychotics, Ms. S starts paliperidone using samples from the clinic, titrated to 6 mg at bedtime. Once tolerance and therapeutic improvement are observed, she is continued on this medication through the manufacturer’s patient assistance program.
Within 3 months, Ms. S and her family find that she has improved significantly. She no longer reports hallucinatory experiences, is less guarded during sessions, and has followed through with paid and volunteer job applications and interviews. She soon finds a job teaching entry-level classes at a community college and is looking forward to a summer trip abroad.
During a follow-up appointment, Ms. S reports that she had missed 2 consecutive menstrual cycles without galactorrhea or fractures. A urine pregnancy test is negative; the prolactin level is 72 μg/L.
Hyperprolactinemia in women is defined as a plasma prolactin level of
a)>2.5 µg/L
b) >5 µg/L
c) >10 µg/L
d) >20 µg/L
e) >25 µg/L
The authors’ observations
A prolactin level >25 μg/L is considered abnormal.1 A level of >250 μg/L may identify a prolactinoma; however, levels >200 μg/L have been observed in patients taking an antipsychotic.1 Given Ms. S’s clinically significant elevation of prolactin, she is referred to her primary care physician. We decide to augment her regimen with aripiprazole, 10 mg/d, because this drug has been noted to help in cases of hyperprolactinemia associated with other antipsychotics.2,3
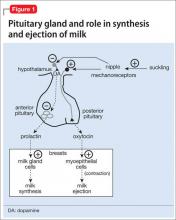
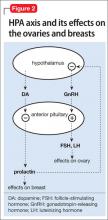
Prolactin serves several roles in the body, including but not limited to lactation, sexual gratification, proliferation of oligodendrocyte precursor cells, surfactant synthesis of fetal lungs at the end of pregnancy, and neurogenesis in maternal and fetal brains (Figure 1 and Figure 2). A 2004 review reported secondary amenorrhea, galactorrhea, and osteopenia as common symptoms of hyperprolactinemia.5 Hyperprolactinemia has been seen with most antipsychotics, both typical and atypical. Although several studies document prolactin elevation with risperidone, fewer have examined the active metabolite (9-hydroxyrisperidone) paliperidone.5-7
In women, a high prolactin level can cause
a) menstrual disturbance
b) galactorrhea
c) breast engorgement
d) sexual dysfunction
e) all of the above
The authors’ observations
Acutely, hyperprolactinemia can cause menstrual abnormalities, decreased libido, breast engorgement, galactorrhea, and sexual dysfunction in women.8 In men, the most common symptoms of hyperprolactinemia are loss of interest in sex, erectile dysfunction, infertility, and gynecomastia. Osteoporosis has been associated with chronic elevation of the prolactin level8 (Table).
TREATMENT Adjunctive aripiprazole
After 8 weeks of adjunctive aripiprazole, Ms. S’s prolactin level decreases to 42 μg/L, but menses do not return. Because her family and primary care providers are eager to have the prolactin level return to normal, reducing her risk of complications, we decide to decrease paliperidone to 3 mg at bedtime.
Eight weeks later, Ms. S shows functional improvement. A repeat test of prolactin is 24 μg/L; she reports a 4-day period of spotting 1 week ago. One month later, the prolactin level is 21 μg/L, and she reports having a normal menstrual period. She continues treatment with paliperidone, 3 mg/d, and aripiprazole, 10 mg/d, experiences regular menses, and continues teaching.
Pharmacotherapy of hyperprolactinemia includes
a) haloperidol
b) perphenazine
c) bromocriptine
d) olanzapine
e) risperidone
The authors' observations
Our goal in treating Ms. S was to address her schizophrenia symptoms and improve her overall functioning. Often, finding an effective treatment can be challenging, and there is little evidence to support the efficacy of one antipsychotic over another.4 In Ms. S’s case, our care was stymied by the cost of medication, challenges related to delusions intrinsic to the illness (she refused clozapine because of required blood draws), and adverse effects. When Ms. S developed amenorrhea while taking paliperidone— the only medication that showed significant improvement in her psychotic symptoms—our goal was to maintain her functional level without significant long-term adverse effects.
Managing hyperprolactinemia
Management of iatrogenic hyperprolactinemia includes decreasing the dosage of the offending agent, using a prolactin-sparing antipsychotic, or initiating a dopamine agonist, such as bromocriptine or cabergoline, in addition to an antipsychotic.1,4 Aripiprazole is considered to be a prolactin-sparing agent because of its propensity to increase the prolactin level to less of a degree than what is seen with other antipsychotics; in fact, it has been shown to reduce an elevated prolactin level.9-11
Most typical and atypical antipsychotics are dopamine—specifically D2—receptor antagonists. These antipsychotics prevent dopamine from binding to the D2 receptor and from inhibiting prolactin release, therefore causing hyperprolactinemia. Aripiprazole differs from other antipsychotics: It is a partial D2 receptor agonist with high affinity, and therefore suppresses prolactin release.8 In a randomized controlled trial, aripiprazole had a lower rate of prolactin elevation compared with placebo.12
Aripiprazole’s ability to reduce an elevated prolactin level caused by other antipsychotics has been demonstrated in several studies with haloperidol,13 olanzapine,14,15 and risperidone.15-17 There has been 1 case report,18 but no controlled studies, of aripiprazole being used to decrease the prolactin level in patients treated with paliperidone.
In Ms. S’s case, adding aripiprazole, 10 mg/d, reduced her prolactin level by approximately 50%. Because several studies have shown that adjunctive aripiprazole with a D2 antagonist normalizes the prolactin level,19 it is reasonable to conclude that adding aripiprazole facilitated reduction of her prolactin level and might have continued to do so if given more time. Regrettably, because of patient and family concerns, paliperidone was reduced before this could be determined. It is unclear whether normalization of Ms. S’s prolactin level and return of her menstrual cycle was caused by adding aripiprazole or by reducing the dosage of paliperidone.
Although additional randomized controlled trials should be conducted on the utility of this approach, it is reasonable to consider augmentation with aripiprazole when treating a patient who is stable on an antipsychotic, including paliperidone, but has developed hyperprolactinemia secondary to treatment.
BOTTOM LINE
Hyperprolactinemia is a relatively common, underreported side effect of both typical and atypical antipsychotics. Paliperidone and risperidone have been shown to have the highest risk among the atypical antipsychotics; aripiprazole has the lowest risk. Treatment of an elevated prolactin level should include reduction or discontinuation of the offending agent and augmentation with aripiprazole.
Related Resources
• Peuskens J, Pani L, Detraux J, et al. The effects of novel and newly approved antipsychotics on serum prolactin levels: a comprehensive review [published online March 28, 2014]. CNS Drugs. doi: 10.1007/s40263-014-0157-3.
• Li X, Tang Y, Wang C. Adjunctive aripiprazole versus placebo for antipsychotic-induced hyperprolactinemia: meta-analysis of randomized controlled trials. PLoS One. 2013;8(8):e70179. doi: 10.1371/journal.pone.0070179.
Drug Brand Names
Aripiprazole • Abilify Haloperidol • Haldol
Benzatropine • Cogentin Olanzapine • Zyprexa
Bromocriptine • Parlodel Paliperidone • Invega
Cabergoline • Dostinex Perphenazine • Trilafon
Clozapine • Clozaril Risperidone • Risperdal
Fluphenazine • Prolixin
DisclosureThe authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Melmed S, Casanueva FF, Hoffman AR, et al. Diagnosis and treatment of hyperprolactinemia: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2011;96(2):273-288.
2. Madhusoodanan S, Parida S, Jimenez C. Hyperprolactinemia associated with psychotropics—a review. Hum Psychopharmacol. 2010;25(4):281-297.
3. Hanssens L, L’Italien G, Loze JY, et al. The effect of antipsychotic medication on sexual function and serum prolactin levels in community-treated schizophrenic patients: results from the Schizophrenia Trial of Aripiprazole (STAR) study (NCT00237913). BMC Psychiatry. 2008;8:95. doi: 10.1186/1471-244X-8-95.
4. Lieberman JA, Stroup TS, McEvoy JP, et al; Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) Investigators. Effectiveness of antipsychotic drugs in patients with chronic schizophrenia. N Engl J Med. 2005;353(12):1209-1223.
5. Haddad PM, Wieck A. Antipsychotic-induced hyperprolactinaemia: mechanisms, clinical features and management. Drugs. 2004;64(20):2291-2314.
6. Knegtering R, Baselmans P, Castelein S, et al. Predominant role of the 9-hydroxy metabolite of risperidone in elevating blood prolactin levels. Am J Psychiatry. 2005;162(5): 1010-1012.
7. Berwaerts J, Cleton A, Rossenu S, et al. A comparison of serum prolactin concentrations after administration of paliperidone extended-release and risperidone tablets in patients with schizophrenia. J Psychopharmacol. 2010; 24(7):1011-1018.
8. Holt RI, Peveler RC. Antipsychotics and hyperprolactinaemia: mechanisms, consequences and management. Clin Endocrinol (Oxf). 2011;74(2):141-147.
9. Friberg LE, Vermeulen AM, Petersson KJ, et al. An agonist-antagonist interaction model for prolactin release following risperidone and paliperidone treatment. Clin Pharmacol Ther. 2009;85(4):409-417.
10. Skopek M, Manoj P. Hyperprolactinaemia during treatment with paliperidone. Australas Psychiatry. 2010; 18(3):261-263.
11. Aihara K, Shimada J, Miwa T, et al. The novel antipsychotic aripiprazole is a partial agonist at short and long isoforms of D2 receptors linked to the regulation of adenylyl cyclase activity and prolactin release. Brain Res. 2004;1003(1-2):9-17.
12. Bushe C, Shaw M, Peveler RC. A review of the association between antipsychotic use and hyperprolactinaemia. J Psychopharmacol. 2008;22(2 suppl):46-55.
13. Yasui-Furukori N, Furukori H, Sugawara N, et al. Dose-dependent effects of adjunctive treatment with aripiprazole on hyperprolactinemia induced by risperidone in female patients with schizophrenia. J Clin Psychopharmacol. 2010;30(5):596-599.
14. Lorenz RA, Weinstein B. Resolution of haloperidol-induced hyperprolactinemia with aripiprazole. J Clin Psychopharmacol. 2007;27(5):524-525.
15. Aggarwal A, Jain M, Garg A, et al. Aripiprazole for olanzapine-induced symptomatic hyper prolactinemia. Indian J Pharmacol. 2010;42(1):58-59.
16. Byerly MJ, Marcus RN, Tran QV, et al. Effects of aripiprazole on prolactin levels in subjects with schizophrenia during cross-titration with risperidone or olanzapine: analysis of a randomized, open-label study. Schizophr Res. 2009; 107(2-3):218-222.
17. Chen CK, Huang YS, Ree SC, et al. Differential add-on effects of aripiprazole in resolving hyperprolactinemia induced by risperidone in comparison to benzamide antipsychotics. Prog Neuropsychopharmacol Biol Psychiatry. 2010;34(8):1495-1499.
18. Chen CY, Lin TY, Wang CC, et al. Improvement of serum prolactin and sexual function after switching to aripiprazole from risperidone in schizophrenia: a case series. Psychiatry Clin Neurosci. 2011;65(1):95-97.
19. Rocha FL, Hara C, Ramos MG. Using aripiprazole to attenuate paliperidone-induced hyperprolactinemia. Prog Neuropsychopharmacol Biol Psychiatry. 2010;34(6):1153-1154.
CASE Paranoid and hallucinating
Ms. S, age 30, is an unmarried graduate student who has been given a diagnosis of schizophrenia, paranoid type, during inpatient hospitalization that was prompted by impairment in school functioning (difficulty turning in assignments, poor concentration, making careless mistakes on tests), paranoid delusions, and multisensory hallucinations. She says that her roommate and classmates are working together to make her leave school, and recalls seeing them “snare and smirk” as she passes by. Ms. S says that she feels her classmates are calling her names and talking badly about her as soon as she is out of sight.
Ms. S is antipsychotic-naïve and has a baseline body mass index of 17.8 kg/m2, indicating that she is underweight. We believe that olanzapine, 20 mg/d, is a good initial treatment because of its propensity for weight gain; however, she experiences only marginal improvement. Ms. S does not have health insurance, and cannot afford a brand name medication; therefore, she is cross-tapered to perphenazine, 8 mg, and benzatropine, 0.5 mg, both taken twice daily (olanzapine was not available as a generic at the time).
At discharge, Ms. S does not report any hallucinatory experiences, but is guarded, voices suspicions about the treatment team, and asks “What are they doing with all my blood?”—referring to blood draws for laboratory testing during hospitalization.
As an outpatient, Ms. S is continued on the same medications until she has to be switched because she cannot afford the out-of-pocket cost of the antipsychotic, perphenazine ($80 a month). Clozapine is recommended, but Ms. S refuses because of the mandatory weekly blood monitoring. She briefly tries fluphenazine, 2.5 mg/d, but it is discontinued because of malaise and lightheadedness without extrapyramidal symptoms.
Clozapine is again recommended, but Ms. S remains suspicious of the necessary blood draws and refuses. After several trials of antipsychotics, Ms. S starts paliperidone using samples from the clinic, titrated to 6 mg at bedtime. Once tolerance and therapeutic improvement are observed, she is continued on this medication through the manufacturer’s patient assistance program.
Within 3 months, Ms. S and her family find that she has improved significantly. She no longer reports hallucinatory experiences, is less guarded during sessions, and has followed through with paid and volunteer job applications and interviews. She soon finds a job teaching entry-level classes at a community college and is looking forward to a summer trip abroad.
During a follow-up appointment, Ms. S reports that she had missed 2 consecutive menstrual cycles without galactorrhea or fractures. A urine pregnancy test is negative; the prolactin level is 72 μg/L.
Hyperprolactinemia in women is defined as a plasma prolactin level of
a)>2.5 µg/L
b) >5 µg/L
c) >10 µg/L
d) >20 µg/L
e) >25 µg/L
The authors’ observations
A prolactin level >25 μg/L is considered abnormal.1 A level of >250 μg/L may identify a prolactinoma; however, levels >200 μg/L have been observed in patients taking an antipsychotic.1 Given Ms. S’s clinically significant elevation of prolactin, she is referred to her primary care physician. We decide to augment her regimen with aripiprazole, 10 mg/d, because this drug has been noted to help in cases of hyperprolactinemia associated with other antipsychotics.2,3
Prolactin serves several roles in the body, including but not limited to lactation, sexual gratification, proliferation of oligodendrocyte precursor cells, surfactant synthesis of fetal lungs at the end of pregnancy, and neurogenesis in maternal and fetal brains (Figure 1 and Figure 2). A 2004 review reported secondary amenorrhea, galactorrhea, and osteopenia as common symptoms of hyperprolactinemia.5 Hyperprolactinemia has been seen with most antipsychotics, both typical and atypical. Although several studies document prolactin elevation with risperidone, fewer have examined the active metabolite (9-hydroxyrisperidone) paliperidone.5-7
In women, a high prolactin level can cause
a) menstrual disturbance
b) galactorrhea
c) breast engorgement
d) sexual dysfunction
e) all of the above
The authors’ observations
Acutely, hyperprolactinemia can cause menstrual abnormalities, decreased libido, breast engorgement, galactorrhea, and sexual dysfunction in women.8 In men, the most common symptoms of hyperprolactinemia are loss of interest in sex, erectile dysfunction, infertility, and gynecomastia. Osteoporosis has been associated with chronic elevation of the prolactin level8 (Table).
TREATMENT Adjunctive aripiprazole
After 8 weeks of adjunctive aripiprazole, Ms. S’s prolactin level decreases to 42 μg/L, but menses do not return. Because her family and primary care providers are eager to have the prolactin level return to normal, reducing her risk of complications, we decide to decrease paliperidone to 3 mg at bedtime.
Eight weeks later, Ms. S shows functional improvement. A repeat test of prolactin is 24 μg/L; she reports a 4-day period of spotting 1 week ago. One month later, the prolactin level is 21 μg/L, and she reports having a normal menstrual period. She continues treatment with paliperidone, 3 mg/d, and aripiprazole, 10 mg/d, experiences regular menses, and continues teaching.
Pharmacotherapy of hyperprolactinemia includes
a) haloperidol
b) perphenazine
c) bromocriptine
d) olanzapine
e) risperidone
The authors' observations
Our goal in treating Ms. S was to address her schizophrenia symptoms and improve her overall functioning. Often, finding an effective treatment can be challenging, and there is little evidence to support the efficacy of one antipsychotic over another.4 In Ms. S’s case, our care was stymied by the cost of medication, challenges related to delusions intrinsic to the illness (she refused clozapine because of required blood draws), and adverse effects. When Ms. S developed amenorrhea while taking paliperidone— the only medication that showed significant improvement in her psychotic symptoms—our goal was to maintain her functional level without significant long-term adverse effects.
Managing hyperprolactinemia
Management of iatrogenic hyperprolactinemia includes decreasing the dosage of the offending agent, using a prolactin-sparing antipsychotic, or initiating a dopamine agonist, such as bromocriptine or cabergoline, in addition to an antipsychotic.1,4 Aripiprazole is considered to be a prolactin-sparing agent because of its propensity to increase the prolactin level to less of a degree than what is seen with other antipsychotics; in fact, it has been shown to reduce an elevated prolactin level.9-11
Most typical and atypical antipsychotics are dopamine—specifically D2—receptor antagonists. These antipsychotics prevent dopamine from binding to the D2 receptor and from inhibiting prolactin release, therefore causing hyperprolactinemia. Aripiprazole differs from other antipsychotics: It is a partial D2 receptor agonist with high affinity, and therefore suppresses prolactin release.8 In a randomized controlled trial, aripiprazole had a lower rate of prolactin elevation compared with placebo.12
Aripiprazole’s ability to reduce an elevated prolactin level caused by other antipsychotics has been demonstrated in several studies with haloperidol,13 olanzapine,14,15 and risperidone.15-17 There has been 1 case report,18 but no controlled studies, of aripiprazole being used to decrease the prolactin level in patients treated with paliperidone.
In Ms. S’s case, adding aripiprazole, 10 mg/d, reduced her prolactin level by approximately 50%. Because several studies have shown that adjunctive aripiprazole with a D2 antagonist normalizes the prolactin level,19 it is reasonable to conclude that adding aripiprazole facilitated reduction of her prolactin level and might have continued to do so if given more time. Regrettably, because of patient and family concerns, paliperidone was reduced before this could be determined. It is unclear whether normalization of Ms. S’s prolactin level and return of her menstrual cycle was caused by adding aripiprazole or by reducing the dosage of paliperidone.
Although additional randomized controlled trials should be conducted on the utility of this approach, it is reasonable to consider augmentation with aripiprazole when treating a patient who is stable on an antipsychotic, including paliperidone, but has developed hyperprolactinemia secondary to treatment.
BOTTOM LINE
Hyperprolactinemia is a relatively common, underreported side effect of both typical and atypical antipsychotics. Paliperidone and risperidone have been shown to have the highest risk among the atypical antipsychotics; aripiprazole has the lowest risk. Treatment of an elevated prolactin level should include reduction or discontinuation of the offending agent and augmentation with aripiprazole.
Related Resources
• Peuskens J, Pani L, Detraux J, et al. The effects of novel and newly approved antipsychotics on serum prolactin levels: a comprehensive review [published online March 28, 2014]. CNS Drugs. doi: 10.1007/s40263-014-0157-3.
• Li X, Tang Y, Wang C. Adjunctive aripiprazole versus placebo for antipsychotic-induced hyperprolactinemia: meta-analysis of randomized controlled trials. PLoS One. 2013;8(8):e70179. doi: 10.1371/journal.pone.0070179.
Drug Brand Names
Aripiprazole • Abilify Haloperidol • Haldol
Benzatropine • Cogentin Olanzapine • Zyprexa
Bromocriptine • Parlodel Paliperidone • Invega
Cabergoline • Dostinex Perphenazine • Trilafon
Clozapine • Clozaril Risperidone • Risperdal
Fluphenazine • Prolixin
DisclosureThe authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
CASE Paranoid and hallucinating
Ms. S, age 30, is an unmarried graduate student who has been given a diagnosis of schizophrenia, paranoid type, during inpatient hospitalization that was prompted by impairment in school functioning (difficulty turning in assignments, poor concentration, making careless mistakes on tests), paranoid delusions, and multisensory hallucinations. She says that her roommate and classmates are working together to make her leave school, and recalls seeing them “snare and smirk” as she passes by. Ms. S says that she feels her classmates are calling her names and talking badly about her as soon as she is out of sight.
Ms. S is antipsychotic-naïve and has a baseline body mass index of 17.8 kg/m2, indicating that she is underweight. We believe that olanzapine, 20 mg/d, is a good initial treatment because of its propensity for weight gain; however, she experiences only marginal improvement. Ms. S does not have health insurance, and cannot afford a brand name medication; therefore, she is cross-tapered to perphenazine, 8 mg, and benzatropine, 0.5 mg, both taken twice daily (olanzapine was not available as a generic at the time).
At discharge, Ms. S does not report any hallucinatory experiences, but is guarded, voices suspicions about the treatment team, and asks “What are they doing with all my blood?”—referring to blood draws for laboratory testing during hospitalization.
As an outpatient, Ms. S is continued on the same medications until she has to be switched because she cannot afford the out-of-pocket cost of the antipsychotic, perphenazine ($80 a month). Clozapine is recommended, but Ms. S refuses because of the mandatory weekly blood monitoring. She briefly tries fluphenazine, 2.5 mg/d, but it is discontinued because of malaise and lightheadedness without extrapyramidal symptoms.
Clozapine is again recommended, but Ms. S remains suspicious of the necessary blood draws and refuses. After several trials of antipsychotics, Ms. S starts paliperidone using samples from the clinic, titrated to 6 mg at bedtime. Once tolerance and therapeutic improvement are observed, she is continued on this medication through the manufacturer’s patient assistance program.
Within 3 months, Ms. S and her family find that she has improved significantly. She no longer reports hallucinatory experiences, is less guarded during sessions, and has followed through with paid and volunteer job applications and interviews. She soon finds a job teaching entry-level classes at a community college and is looking forward to a summer trip abroad.
During a follow-up appointment, Ms. S reports that she had missed 2 consecutive menstrual cycles without galactorrhea or fractures. A urine pregnancy test is negative; the prolactin level is 72 μg/L.
Hyperprolactinemia in women is defined as a plasma prolactin level of
a)>2.5 µg/L
b) >5 µg/L
c) >10 µg/L
d) >20 µg/L
e) >25 µg/L
The authors’ observations
A prolactin level >25 μg/L is considered abnormal.1 A level of >250 μg/L may identify a prolactinoma; however, levels >200 μg/L have been observed in patients taking an antipsychotic.1 Given Ms. S’s clinically significant elevation of prolactin, she is referred to her primary care physician. We decide to augment her regimen with aripiprazole, 10 mg/d, because this drug has been noted to help in cases of hyperprolactinemia associated with other antipsychotics.2,3
Prolactin serves several roles in the body, including but not limited to lactation, sexual gratification, proliferation of oligodendrocyte precursor cells, surfactant synthesis of fetal lungs at the end of pregnancy, and neurogenesis in maternal and fetal brains (Figure 1 and Figure 2). A 2004 review reported secondary amenorrhea, galactorrhea, and osteopenia as common symptoms of hyperprolactinemia.5 Hyperprolactinemia has been seen with most antipsychotics, both typical and atypical. Although several studies document prolactin elevation with risperidone, fewer have examined the active metabolite (9-hydroxyrisperidone) paliperidone.5-7
In women, a high prolactin level can cause
a) menstrual disturbance
b) galactorrhea
c) breast engorgement
d) sexual dysfunction
e) all of the above
The authors’ observations
Acutely, hyperprolactinemia can cause menstrual abnormalities, decreased libido, breast engorgement, galactorrhea, and sexual dysfunction in women.8 In men, the most common symptoms of hyperprolactinemia are loss of interest in sex, erectile dysfunction, infertility, and gynecomastia. Osteoporosis has been associated with chronic elevation of the prolactin level8 (Table).
TREATMENT Adjunctive aripiprazole
After 8 weeks of adjunctive aripiprazole, Ms. S’s prolactin level decreases to 42 μg/L, but menses do not return. Because her family and primary care providers are eager to have the prolactin level return to normal, reducing her risk of complications, we decide to decrease paliperidone to 3 mg at bedtime.
Eight weeks later, Ms. S shows functional improvement. A repeat test of prolactin is 24 μg/L; she reports a 4-day period of spotting 1 week ago. One month later, the prolactin level is 21 μg/L, and she reports having a normal menstrual period. She continues treatment with paliperidone, 3 mg/d, and aripiprazole, 10 mg/d, experiences regular menses, and continues teaching.
Pharmacotherapy of hyperprolactinemia includes
a) haloperidol
b) perphenazine
c) bromocriptine
d) olanzapine
e) risperidone
The authors' observations
Our goal in treating Ms. S was to address her schizophrenia symptoms and improve her overall functioning. Often, finding an effective treatment can be challenging, and there is little evidence to support the efficacy of one antipsychotic over another.4 In Ms. S’s case, our care was stymied by the cost of medication, challenges related to delusions intrinsic to the illness (she refused clozapine because of required blood draws), and adverse effects. When Ms. S developed amenorrhea while taking paliperidone— the only medication that showed significant improvement in her psychotic symptoms—our goal was to maintain her functional level without significant long-term adverse effects.
Managing hyperprolactinemia
Management of iatrogenic hyperprolactinemia includes decreasing the dosage of the offending agent, using a prolactin-sparing antipsychotic, or initiating a dopamine agonist, such as bromocriptine or cabergoline, in addition to an antipsychotic.1,4 Aripiprazole is considered to be a prolactin-sparing agent because of its propensity to increase the prolactin level to less of a degree than what is seen with other antipsychotics; in fact, it has been shown to reduce an elevated prolactin level.9-11
Most typical and atypical antipsychotics are dopamine—specifically D2—receptor antagonists. These antipsychotics prevent dopamine from binding to the D2 receptor and from inhibiting prolactin release, therefore causing hyperprolactinemia. Aripiprazole differs from other antipsychotics: It is a partial D2 receptor agonist with high affinity, and therefore suppresses prolactin release.8 In a randomized controlled trial, aripiprazole had a lower rate of prolactin elevation compared with placebo.12
Aripiprazole’s ability to reduce an elevated prolactin level caused by other antipsychotics has been demonstrated in several studies with haloperidol,13 olanzapine,14,15 and risperidone.15-17 There has been 1 case report,18 but no controlled studies, of aripiprazole being used to decrease the prolactin level in patients treated with paliperidone.
In Ms. S’s case, adding aripiprazole, 10 mg/d, reduced her prolactin level by approximately 50%. Because several studies have shown that adjunctive aripiprazole with a D2 antagonist normalizes the prolactin level,19 it is reasonable to conclude that adding aripiprazole facilitated reduction of her prolactin level and might have continued to do so if given more time. Regrettably, because of patient and family concerns, paliperidone was reduced before this could be determined. It is unclear whether normalization of Ms. S’s prolactin level and return of her menstrual cycle was caused by adding aripiprazole or by reducing the dosage of paliperidone.
Although additional randomized controlled trials should be conducted on the utility of this approach, it is reasonable to consider augmentation with aripiprazole when treating a patient who is stable on an antipsychotic, including paliperidone, but has developed hyperprolactinemia secondary to treatment.
BOTTOM LINE
Hyperprolactinemia is a relatively common, underreported side effect of both typical and atypical antipsychotics. Paliperidone and risperidone have been shown to have the highest risk among the atypical antipsychotics; aripiprazole has the lowest risk. Treatment of an elevated prolactin level should include reduction or discontinuation of the offending agent and augmentation with aripiprazole.
Related Resources
• Peuskens J, Pani L, Detraux J, et al. The effects of novel and newly approved antipsychotics on serum prolactin levels: a comprehensive review [published online March 28, 2014]. CNS Drugs. doi: 10.1007/s40263-014-0157-3.
• Li X, Tang Y, Wang C. Adjunctive aripiprazole versus placebo for antipsychotic-induced hyperprolactinemia: meta-analysis of randomized controlled trials. PLoS One. 2013;8(8):e70179. doi: 10.1371/journal.pone.0070179.
Drug Brand Names
Aripiprazole • Abilify Haloperidol • Haldol
Benzatropine • Cogentin Olanzapine • Zyprexa
Bromocriptine • Parlodel Paliperidone • Invega
Cabergoline • Dostinex Perphenazine • Trilafon
Clozapine • Clozaril Risperidone • Risperdal
Fluphenazine • Prolixin
DisclosureThe authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Melmed S, Casanueva FF, Hoffman AR, et al. Diagnosis and treatment of hyperprolactinemia: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2011;96(2):273-288.
2. Madhusoodanan S, Parida S, Jimenez C. Hyperprolactinemia associated with psychotropics—a review. Hum Psychopharmacol. 2010;25(4):281-297.
3. Hanssens L, L’Italien G, Loze JY, et al. The effect of antipsychotic medication on sexual function and serum prolactin levels in community-treated schizophrenic patients: results from the Schizophrenia Trial of Aripiprazole (STAR) study (NCT00237913). BMC Psychiatry. 2008;8:95. doi: 10.1186/1471-244X-8-95.
4. Lieberman JA, Stroup TS, McEvoy JP, et al; Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) Investigators. Effectiveness of antipsychotic drugs in patients with chronic schizophrenia. N Engl J Med. 2005;353(12):1209-1223.
5. Haddad PM, Wieck A. Antipsychotic-induced hyperprolactinaemia: mechanisms, clinical features and management. Drugs. 2004;64(20):2291-2314.
6. Knegtering R, Baselmans P, Castelein S, et al. Predominant role of the 9-hydroxy metabolite of risperidone in elevating blood prolactin levels. Am J Psychiatry. 2005;162(5): 1010-1012.
7. Berwaerts J, Cleton A, Rossenu S, et al. A comparison of serum prolactin concentrations after administration of paliperidone extended-release and risperidone tablets in patients with schizophrenia. J Psychopharmacol. 2010; 24(7):1011-1018.
8. Holt RI, Peveler RC. Antipsychotics and hyperprolactinaemia: mechanisms, consequences and management. Clin Endocrinol (Oxf). 2011;74(2):141-147.
9. Friberg LE, Vermeulen AM, Petersson KJ, et al. An agonist-antagonist interaction model for prolactin release following risperidone and paliperidone treatment. Clin Pharmacol Ther. 2009;85(4):409-417.
10. Skopek M, Manoj P. Hyperprolactinaemia during treatment with paliperidone. Australas Psychiatry. 2010; 18(3):261-263.
11. Aihara K, Shimada J, Miwa T, et al. The novel antipsychotic aripiprazole is a partial agonist at short and long isoforms of D2 receptors linked to the regulation of adenylyl cyclase activity and prolactin release. Brain Res. 2004;1003(1-2):9-17.
12. Bushe C, Shaw M, Peveler RC. A review of the association between antipsychotic use and hyperprolactinaemia. J Psychopharmacol. 2008;22(2 suppl):46-55.
13. Yasui-Furukori N, Furukori H, Sugawara N, et al. Dose-dependent effects of adjunctive treatment with aripiprazole on hyperprolactinemia induced by risperidone in female patients with schizophrenia. J Clin Psychopharmacol. 2010;30(5):596-599.
14. Lorenz RA, Weinstein B. Resolution of haloperidol-induced hyperprolactinemia with aripiprazole. J Clin Psychopharmacol. 2007;27(5):524-525.
15. Aggarwal A, Jain M, Garg A, et al. Aripiprazole for olanzapine-induced symptomatic hyper prolactinemia. Indian J Pharmacol. 2010;42(1):58-59.
16. Byerly MJ, Marcus RN, Tran QV, et al. Effects of aripiprazole on prolactin levels in subjects with schizophrenia during cross-titration with risperidone or olanzapine: analysis of a randomized, open-label study. Schizophr Res. 2009; 107(2-3):218-222.
17. Chen CK, Huang YS, Ree SC, et al. Differential add-on effects of aripiprazole in resolving hyperprolactinemia induced by risperidone in comparison to benzamide antipsychotics. Prog Neuropsychopharmacol Biol Psychiatry. 2010;34(8):1495-1499.
18. Chen CY, Lin TY, Wang CC, et al. Improvement of serum prolactin and sexual function after switching to aripiprazole from risperidone in schizophrenia: a case series. Psychiatry Clin Neurosci. 2011;65(1):95-97.
19. Rocha FL, Hara C, Ramos MG. Using aripiprazole to attenuate paliperidone-induced hyperprolactinemia. Prog Neuropsychopharmacol Biol Psychiatry. 2010;34(6):1153-1154.
1. Melmed S, Casanueva FF, Hoffman AR, et al. Diagnosis and treatment of hyperprolactinemia: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2011;96(2):273-288.
2. Madhusoodanan S, Parida S, Jimenez C. Hyperprolactinemia associated with psychotropics—a review. Hum Psychopharmacol. 2010;25(4):281-297.
3. Hanssens L, L’Italien G, Loze JY, et al. The effect of antipsychotic medication on sexual function and serum prolactin levels in community-treated schizophrenic patients: results from the Schizophrenia Trial of Aripiprazole (STAR) study (NCT00237913). BMC Psychiatry. 2008;8:95. doi: 10.1186/1471-244X-8-95.
4. Lieberman JA, Stroup TS, McEvoy JP, et al; Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) Investigators. Effectiveness of antipsychotic drugs in patients with chronic schizophrenia. N Engl J Med. 2005;353(12):1209-1223.
5. Haddad PM, Wieck A. Antipsychotic-induced hyperprolactinaemia: mechanisms, clinical features and management. Drugs. 2004;64(20):2291-2314.
6. Knegtering R, Baselmans P, Castelein S, et al. Predominant role of the 9-hydroxy metabolite of risperidone in elevating blood prolactin levels. Am J Psychiatry. 2005;162(5): 1010-1012.
7. Berwaerts J, Cleton A, Rossenu S, et al. A comparison of serum prolactin concentrations after administration of paliperidone extended-release and risperidone tablets in patients with schizophrenia. J Psychopharmacol. 2010; 24(7):1011-1018.
8. Holt RI, Peveler RC. Antipsychotics and hyperprolactinaemia: mechanisms, consequences and management. Clin Endocrinol (Oxf). 2011;74(2):141-147.
9. Friberg LE, Vermeulen AM, Petersson KJ, et al. An agonist-antagonist interaction model for prolactin release following risperidone and paliperidone treatment. Clin Pharmacol Ther. 2009;85(4):409-417.
10. Skopek M, Manoj P. Hyperprolactinaemia during treatment with paliperidone. Australas Psychiatry. 2010; 18(3):261-263.
11. Aihara K, Shimada J, Miwa T, et al. The novel antipsychotic aripiprazole is a partial agonist at short and long isoforms of D2 receptors linked to the regulation of adenylyl cyclase activity and prolactin release. Brain Res. 2004;1003(1-2):9-17.
12. Bushe C, Shaw M, Peveler RC. A review of the association between antipsychotic use and hyperprolactinaemia. J Psychopharmacol. 2008;22(2 suppl):46-55.
13. Yasui-Furukori N, Furukori H, Sugawara N, et al. Dose-dependent effects of adjunctive treatment with aripiprazole on hyperprolactinemia induced by risperidone in female patients with schizophrenia. J Clin Psychopharmacol. 2010;30(5):596-599.
14. Lorenz RA, Weinstein B. Resolution of haloperidol-induced hyperprolactinemia with aripiprazole. J Clin Psychopharmacol. 2007;27(5):524-525.
15. Aggarwal A, Jain M, Garg A, et al. Aripiprazole for olanzapine-induced symptomatic hyper prolactinemia. Indian J Pharmacol. 2010;42(1):58-59.
16. Byerly MJ, Marcus RN, Tran QV, et al. Effects of aripiprazole on prolactin levels in subjects with schizophrenia during cross-titration with risperidone or olanzapine: analysis of a randomized, open-label study. Schizophr Res. 2009; 107(2-3):218-222.
17. Chen CK, Huang YS, Ree SC, et al. Differential add-on effects of aripiprazole in resolving hyperprolactinemia induced by risperidone in comparison to benzamide antipsychotics. Prog Neuropsychopharmacol Biol Psychiatry. 2010;34(8):1495-1499.
18. Chen CY, Lin TY, Wang CC, et al. Improvement of serum prolactin and sexual function after switching to aripiprazole from risperidone in schizophrenia: a case series. Psychiatry Clin Neurosci. 2011;65(1):95-97.
19. Rocha FL, Hara C, Ramos MG. Using aripiprazole to attenuate paliperidone-induced hyperprolactinemia. Prog Neuropsychopharmacol Biol Psychiatry. 2010;34(6):1153-1154.
100 years of solicitude: Do global traumatic events have a transgenerational effect?
Yet, important questions about the impact of these events have not been asked: Can there be a transgenerational neurobiological effect on the children and grandchildren of people who have been subjected to life-threatening, traumatic societal events? Could the psychobiology of widespread anxiety and worry (solicitude) be experienced not only by the generation that witnessed and lived through those devastating events, but also by their progeny, who were not yet born during the traumatic events? And could there be epigenetic consequences on a large scale, producing a generation that shares traits induced by the trauma experienced by the previous generation?
Did the rise of delinquency in the 1950s, followed by the anti-war rebellion, unprecedented sexual promiscuity, and substance abuse of the 1960s, be the result of genetic changes in the previous generation induced by living through World War II—after which the generation that grew up in the 1960s was born?
In the late Gabriel García Márquez’s masterpiece novel, One Hundred Years of Solitude, the 1982 Nobel Laureate’s chronicle of the Buendía family across 7 generations is replete with dark and insalubrious events. The fictional family’s story is considered a metaphor for the tumultuous evolution of Márquez’s native Colombia, but that story is consistent with the concept of transgenerational transmission of the biologic effects of stress, as each generation of the Buendía family manifests unusual, even pathological behaviors.
One hundred years of alarm, panic, and anxiety
Psychiatrists are keenly aware of the impact of stressful events on their patients’ mood and behavior, and of the association of life-threatening events with posttraumatic stress disorder (PTSD). For persons who suffer the generalized anxiety of PTSD, further stressful life events can aggravate their condition and result in additional anxiety and solicitude.
It is not surprising that anxiety has been documented as the most common psychiatric condition in the United States.1 Consider the variety of perturbations that have induced alarm, panic, fear, and simmering anxiety on a global scale over the past 100 years— starting with World War I, exactly a century ago.
War. The ruinous 4-year Great War was followed 20 years later by World War II, which caused tens of millions of casualties and the annihilation of Hiroshima and Nagasaki by the atomic bomb— escalating fear of nuclear warfare and radiation poisoning for decades to come. Add to that the Korean War, the Vietnam conflict, the First Gulf War, and the Iraq and Afghanistan wars. The war fatigue and mental exhaustion of the population are palpable.
Economic upheaval. After the Stock Market Crash of 1929 came the Great Depression, the recessions of the 1970s and early 1980s, another stock market crash in 1987, and, most recently, the financial crisis of 2008. Millions saw their wealth wiped out and their livelihoods disrupted, exerting enormous life-changing stresses on countless families.
Disasters. The sinking of the Titanic in 1912, the crash of the Hindenburg, the Three Mile Island nuclear accident, the meltdown of the Chernobyl and Fukushima Daiichi reactors, the space shuttle disasters, and the 9/11 terrorist attacks—all these trigger and perpetuate fear and worry about the one’s own, and one’s loved ones, abrupt and premature mortality.
Epidemics. Millions died in the 1918 influenza pandemic, prompting widespread societal fears that re-intensified during subsequent epidemics: polio in the 1950s, swine flu in the 1970s, SARS (severe acute respiratory syndrome) in the 1990s, West Nile Virus, and avian influenza.
Assassination. The shooting of Archduke Franz Ferdinand of Austria sparked World War I a century ago, but what baby boomers, such as me, vividly remember is our angst over the assassinations of President John F. Kennedy, his brother Robert, and Rev. Dr. Martin Luther King, Jr; the attempted assassination of President Ronald Reagan; and the murder of John Lennon. Each assassination leaves a communal scar on millions, forever reminding them of the ephemeral nature of life at any rung of the social ladder.
Mass murder. The past 100 years began with the Armenian genocide in 1918, followed by the Holocaust of World War II, the Munich Olympics killings, the Jonestown massacre, the Oklahoma City bombing, and, to name a few, the mass murders at Columbine, Virginia Tech, Newtown, and Fort Hood.
Natural disasters can wreak havoc on peoples’ lives. Consider the annual tally of hurricanes (a long list, some—such as Katrina and Sandy—more infamous than others). Add to those storms the earthquakes, tsunamis, erupting volcanoes, floods, and blizzards, and the result is suffering and anxiety on a massive scale, even among those who are not affected directly.
A surprising facet of these disquieting events is the resiliency of people. Life goes on, despite the agony, despair, and solicitude instigated by deadly events. But of those who buckle under the weight of adversity, many end up in a psychiatric clinic or hospital, and are disabled by their symptoms.
Even ‘good’ change can be disquieting
Juxtaposed against these awful events are 100 years of an array of positive, uplifting discoveries, inventions, and medical advances that have completely transformed our lives. Consider: electricity, clean water, women’s right to vote, automobiles, air and space travel, air conditioning, and highway systems; the momentous discoveries of penicillin, antipsychotics, antidepressants, and mood stabilizers; television, the telephone (evolving from dumb to smart), vaccines, oral contraceptives, genetic discoveries, brain imaging technology, and home appliances (refrigerators, microwave ovens, dishwashers); and not at all least, personal computers and the Internet.
But even these advances can generate anxiety and solicitude: Fear of flying, anyone? Embarrassment about a selfie gone viral on the Web? Worry about being a carrier of a breast cancer gene? Claustrophobia inside an MRI scanner?
Hypothesizing about the transfer of anxiety
Could PTSD and solicitude in one generation be transmitted to the next via epigenetic mechanisms (that is, by over-expression or silencing of genes involved in brain development) and could this transmission result in unusual wide-scale stress reactivity? Might this be an example of the infamous Lamarckian “inheritance of acquired characteristics” at the molecular genetic level, in which the anxiety of traumatized parents is transmitted to their offspring? Or could transmission be mediated by being reared in the emotionally oppressive environment of a family still reeling from the effects of war, disaster, and mass murder?
Such questions might sound rhetorical, but they present a reasonable hypothesis that can be answered by research. Findings from animal studies suggest that such a phenomenon might occur in humans.2 If those findings are validated, opportunities for preventing societal solicitude might emerge.
1. Robins LN, Regier DA, eds. Psychiatric disorders in America: The Epidemiologic Catchment Area Study. New York, New York: The Free Press; 1991.
2. Rechavi O, Minevich G, Hobert O. Transgenerational inheritance of an acquired small RNA-based antiviral response in C. elegans. Cell. 2011;147(6):1248-1256.
Yet, important questions about the impact of these events have not been asked: Can there be a transgenerational neurobiological effect on the children and grandchildren of people who have been subjected to life-threatening, traumatic societal events? Could the psychobiology of widespread anxiety and worry (solicitude) be experienced not only by the generation that witnessed and lived through those devastating events, but also by their progeny, who were not yet born during the traumatic events? And could there be epigenetic consequences on a large scale, producing a generation that shares traits induced by the trauma experienced by the previous generation?
Did the rise of delinquency in the 1950s, followed by the anti-war rebellion, unprecedented sexual promiscuity, and substance abuse of the 1960s, be the result of genetic changes in the previous generation induced by living through World War II—after which the generation that grew up in the 1960s was born?
In the late Gabriel García Márquez’s masterpiece novel, One Hundred Years of Solitude, the 1982 Nobel Laureate’s chronicle of the Buendía family across 7 generations is replete with dark and insalubrious events. The fictional family’s story is considered a metaphor for the tumultuous evolution of Márquez’s native Colombia, but that story is consistent with the concept of transgenerational transmission of the biologic effects of stress, as each generation of the Buendía family manifests unusual, even pathological behaviors.
One hundred years of alarm, panic, and anxiety
Psychiatrists are keenly aware of the impact of stressful events on their patients’ mood and behavior, and of the association of life-threatening events with posttraumatic stress disorder (PTSD). For persons who suffer the generalized anxiety of PTSD, further stressful life events can aggravate their condition and result in additional anxiety and solicitude.
It is not surprising that anxiety has been documented as the most common psychiatric condition in the United States.1 Consider the variety of perturbations that have induced alarm, panic, fear, and simmering anxiety on a global scale over the past 100 years— starting with World War I, exactly a century ago.
War. The ruinous 4-year Great War was followed 20 years later by World War II, which caused tens of millions of casualties and the annihilation of Hiroshima and Nagasaki by the atomic bomb— escalating fear of nuclear warfare and radiation poisoning for decades to come. Add to that the Korean War, the Vietnam conflict, the First Gulf War, and the Iraq and Afghanistan wars. The war fatigue and mental exhaustion of the population are palpable.
Economic upheaval. After the Stock Market Crash of 1929 came the Great Depression, the recessions of the 1970s and early 1980s, another stock market crash in 1987, and, most recently, the financial crisis of 2008. Millions saw their wealth wiped out and their livelihoods disrupted, exerting enormous life-changing stresses on countless families.
Disasters. The sinking of the Titanic in 1912, the crash of the Hindenburg, the Three Mile Island nuclear accident, the meltdown of the Chernobyl and Fukushima Daiichi reactors, the space shuttle disasters, and the 9/11 terrorist attacks—all these trigger and perpetuate fear and worry about the one’s own, and one’s loved ones, abrupt and premature mortality.
Epidemics. Millions died in the 1918 influenza pandemic, prompting widespread societal fears that re-intensified during subsequent epidemics: polio in the 1950s, swine flu in the 1970s, SARS (severe acute respiratory syndrome) in the 1990s, West Nile Virus, and avian influenza.
Assassination. The shooting of Archduke Franz Ferdinand of Austria sparked World War I a century ago, but what baby boomers, such as me, vividly remember is our angst over the assassinations of President John F. Kennedy, his brother Robert, and Rev. Dr. Martin Luther King, Jr; the attempted assassination of President Ronald Reagan; and the murder of John Lennon. Each assassination leaves a communal scar on millions, forever reminding them of the ephemeral nature of life at any rung of the social ladder.
Mass murder. The past 100 years began with the Armenian genocide in 1918, followed by the Holocaust of World War II, the Munich Olympics killings, the Jonestown massacre, the Oklahoma City bombing, and, to name a few, the mass murders at Columbine, Virginia Tech, Newtown, and Fort Hood.
Natural disasters can wreak havoc on peoples’ lives. Consider the annual tally of hurricanes (a long list, some—such as Katrina and Sandy—more infamous than others). Add to those storms the earthquakes, tsunamis, erupting volcanoes, floods, and blizzards, and the result is suffering and anxiety on a massive scale, even among those who are not affected directly.
A surprising facet of these disquieting events is the resiliency of people. Life goes on, despite the agony, despair, and solicitude instigated by deadly events. But of those who buckle under the weight of adversity, many end up in a psychiatric clinic or hospital, and are disabled by their symptoms.
Even ‘good’ change can be disquieting
Juxtaposed against these awful events are 100 years of an array of positive, uplifting discoveries, inventions, and medical advances that have completely transformed our lives. Consider: electricity, clean water, women’s right to vote, automobiles, air and space travel, air conditioning, and highway systems; the momentous discoveries of penicillin, antipsychotics, antidepressants, and mood stabilizers; television, the telephone (evolving from dumb to smart), vaccines, oral contraceptives, genetic discoveries, brain imaging technology, and home appliances (refrigerators, microwave ovens, dishwashers); and not at all least, personal computers and the Internet.
But even these advances can generate anxiety and solicitude: Fear of flying, anyone? Embarrassment about a selfie gone viral on the Web? Worry about being a carrier of a breast cancer gene? Claustrophobia inside an MRI scanner?
Hypothesizing about the transfer of anxiety
Could PTSD and solicitude in one generation be transmitted to the next via epigenetic mechanisms (that is, by over-expression or silencing of genes involved in brain development) and could this transmission result in unusual wide-scale stress reactivity? Might this be an example of the infamous Lamarckian “inheritance of acquired characteristics” at the molecular genetic level, in which the anxiety of traumatized parents is transmitted to their offspring? Or could transmission be mediated by being reared in the emotionally oppressive environment of a family still reeling from the effects of war, disaster, and mass murder?
Such questions might sound rhetorical, but they present a reasonable hypothesis that can be answered by research. Findings from animal studies suggest that such a phenomenon might occur in humans.2 If those findings are validated, opportunities for preventing societal solicitude might emerge.
Yet, important questions about the impact of these events have not been asked: Can there be a transgenerational neurobiological effect on the children and grandchildren of people who have been subjected to life-threatening, traumatic societal events? Could the psychobiology of widespread anxiety and worry (solicitude) be experienced not only by the generation that witnessed and lived through those devastating events, but also by their progeny, who were not yet born during the traumatic events? And could there be epigenetic consequences on a large scale, producing a generation that shares traits induced by the trauma experienced by the previous generation?
Did the rise of delinquency in the 1950s, followed by the anti-war rebellion, unprecedented sexual promiscuity, and substance abuse of the 1960s, be the result of genetic changes in the previous generation induced by living through World War II—after which the generation that grew up in the 1960s was born?
In the late Gabriel García Márquez’s masterpiece novel, One Hundred Years of Solitude, the 1982 Nobel Laureate’s chronicle of the Buendía family across 7 generations is replete with dark and insalubrious events. The fictional family’s story is considered a metaphor for the tumultuous evolution of Márquez’s native Colombia, but that story is consistent with the concept of transgenerational transmission of the biologic effects of stress, as each generation of the Buendía family manifests unusual, even pathological behaviors.
One hundred years of alarm, panic, and anxiety
Psychiatrists are keenly aware of the impact of stressful events on their patients’ mood and behavior, and of the association of life-threatening events with posttraumatic stress disorder (PTSD). For persons who suffer the generalized anxiety of PTSD, further stressful life events can aggravate their condition and result in additional anxiety and solicitude.
It is not surprising that anxiety has been documented as the most common psychiatric condition in the United States.1 Consider the variety of perturbations that have induced alarm, panic, fear, and simmering anxiety on a global scale over the past 100 years— starting with World War I, exactly a century ago.
War. The ruinous 4-year Great War was followed 20 years later by World War II, which caused tens of millions of casualties and the annihilation of Hiroshima and Nagasaki by the atomic bomb— escalating fear of nuclear warfare and radiation poisoning for decades to come. Add to that the Korean War, the Vietnam conflict, the First Gulf War, and the Iraq and Afghanistan wars. The war fatigue and mental exhaustion of the population are palpable.
Economic upheaval. After the Stock Market Crash of 1929 came the Great Depression, the recessions of the 1970s and early 1980s, another stock market crash in 1987, and, most recently, the financial crisis of 2008. Millions saw their wealth wiped out and their livelihoods disrupted, exerting enormous life-changing stresses on countless families.
Disasters. The sinking of the Titanic in 1912, the crash of the Hindenburg, the Three Mile Island nuclear accident, the meltdown of the Chernobyl and Fukushima Daiichi reactors, the space shuttle disasters, and the 9/11 terrorist attacks—all these trigger and perpetuate fear and worry about the one’s own, and one’s loved ones, abrupt and premature mortality.
Epidemics. Millions died in the 1918 influenza pandemic, prompting widespread societal fears that re-intensified during subsequent epidemics: polio in the 1950s, swine flu in the 1970s, SARS (severe acute respiratory syndrome) in the 1990s, West Nile Virus, and avian influenza.
Assassination. The shooting of Archduke Franz Ferdinand of Austria sparked World War I a century ago, but what baby boomers, such as me, vividly remember is our angst over the assassinations of President John F. Kennedy, his brother Robert, and Rev. Dr. Martin Luther King, Jr; the attempted assassination of President Ronald Reagan; and the murder of John Lennon. Each assassination leaves a communal scar on millions, forever reminding them of the ephemeral nature of life at any rung of the social ladder.
Mass murder. The past 100 years began with the Armenian genocide in 1918, followed by the Holocaust of World War II, the Munich Olympics killings, the Jonestown massacre, the Oklahoma City bombing, and, to name a few, the mass murders at Columbine, Virginia Tech, Newtown, and Fort Hood.
Natural disasters can wreak havoc on peoples’ lives. Consider the annual tally of hurricanes (a long list, some—such as Katrina and Sandy—more infamous than others). Add to those storms the earthquakes, tsunamis, erupting volcanoes, floods, and blizzards, and the result is suffering and anxiety on a massive scale, even among those who are not affected directly.
A surprising facet of these disquieting events is the resiliency of people. Life goes on, despite the agony, despair, and solicitude instigated by deadly events. But of those who buckle under the weight of adversity, many end up in a psychiatric clinic or hospital, and are disabled by their symptoms.
Even ‘good’ change can be disquieting
Juxtaposed against these awful events are 100 years of an array of positive, uplifting discoveries, inventions, and medical advances that have completely transformed our lives. Consider: electricity, clean water, women’s right to vote, automobiles, air and space travel, air conditioning, and highway systems; the momentous discoveries of penicillin, antipsychotics, antidepressants, and mood stabilizers; television, the telephone (evolving from dumb to smart), vaccines, oral contraceptives, genetic discoveries, brain imaging technology, and home appliances (refrigerators, microwave ovens, dishwashers); and not at all least, personal computers and the Internet.
But even these advances can generate anxiety and solicitude: Fear of flying, anyone? Embarrassment about a selfie gone viral on the Web? Worry about being a carrier of a breast cancer gene? Claustrophobia inside an MRI scanner?
Hypothesizing about the transfer of anxiety
Could PTSD and solicitude in one generation be transmitted to the next via epigenetic mechanisms (that is, by over-expression or silencing of genes involved in brain development) and could this transmission result in unusual wide-scale stress reactivity? Might this be an example of the infamous Lamarckian “inheritance of acquired characteristics” at the molecular genetic level, in which the anxiety of traumatized parents is transmitted to their offspring? Or could transmission be mediated by being reared in the emotionally oppressive environment of a family still reeling from the effects of war, disaster, and mass murder?
Such questions might sound rhetorical, but they present a reasonable hypothesis that can be answered by research. Findings from animal studies suggest that such a phenomenon might occur in humans.2 If those findings are validated, opportunities for preventing societal solicitude might emerge.
1. Robins LN, Regier DA, eds. Psychiatric disorders in America: The Epidemiologic Catchment Area Study. New York, New York: The Free Press; 1991.
2. Rechavi O, Minevich G, Hobert O. Transgenerational inheritance of an acquired small RNA-based antiviral response in C. elegans. Cell. 2011;147(6):1248-1256.
1. Robins LN, Regier DA, eds. Psychiatric disorders in America: The Epidemiologic Catchment Area Study. New York, New York: The Free Press; 1991.
2. Rechavi O, Minevich G, Hobert O. Transgenerational inheritance of an acquired small RNA-based antiviral response in C. elegans. Cell. 2011;147(6):1248-1256.
HDAC inhibitors aid expansion of HSCs from cord blood

Credit: NHS
New research suggests that histone deacetylase (HDAC) inhibitors can be used to expand hematopoietic stem cells (HSCs) isolated from cord blood.
Investigators found that valproic acid (VPA), in particular, could greatly expand HSCs from cord blood.
These HSCs expressed markers of pluripotency and were more efficient than conventionally expanded HSCs in repopulating the bone marrow and establishing hematopoiesis in immune-deficient mice.
Pratima Chaurasia, PhD, of the Mount Sinai School of Medicine in New York, and colleagues reported these results in The Journal of Clinical Investigation.
For several decades, investigators have used a variety of strategies to expand the numbers of HSCs isolated from cord blood, with limited success. Evidence has suggested the accumulation of epigenetic modifications influences preservation of stem-cell characteristics in HSC daughter cells.
So Dr Chaurasia’s group tested the effects of HDAC inhibitors on CD34+ cells derived from cord blood. The team primed the cells for 16 hours with cytokines, then treated the cells for 7 days, with or without additional cytokines and in the presence or absence of HDAC inhibitors.
The inhibitors included VPA, scriptaid (SCR), trichostatin A, suberoylanilide hydroxamic acid, CAY10433 (C433), CAY10398, and CAY10603 .
VPA, SCR, and C433 were the most active inhibitors. Treatment with these agents led to a similar percentage of CD34+CD90+ cells—75.2% ± 10.7%, 73.4% ± 13.9%, and 70.1% ± 18.4%, respectively—which was significantly higher than control conditions—16.2% ± 9.2% (P<0.0001).
VPA, SCR, and C433 also generated a greater absolute number of CD34+ and CD34+CD90+ cells per cord blood collection, when compared to control conditions (P≤0.0007). And the inhibitors generated a greater absolute number of CD34+CD90+CD184+ cells (P<0.0001)
The investigators conducted subsequent experiments with VPA only. And they found that VPA was more effective under serum-free conditions and in the presence of cytokines.
Additional experiments revealed that VPA influences the expression of pluripotency genes—SOX2, OCT4, and NANOG. And these genes proved essential for the expansion of CD34+ CD90+ cells.
Lastly, the investigators tested VPA-expanded cells by transplanting them into immune-deficient mice. The cells were more efficient than conventionally expanded cord blood HSCs in repopulating the bone marrow and establishing hematopoietic populations.
Specifically, at 13 to 14 weeks after transplant, VPA-treated cord blood CD34+ cells resulted in a greater degree of human CD45+ cell chimerism—32.2% ± 11.3%—when compared to primary cord blood CD34+ cells—19.4% ± 4.9%—and to cells from control cultures—13.2% ± 6.4% (P=0.006 and P=0.0008, respectively).
The investigators evaluated the self-renewal potential of the expanded grafts by transplanting donor-derived cells from primary recipients into secondary recipients.
After 15 to 16 weeks, the secondary recipients transplanted with VPA-treated cord blood CD34+ cells had achieved the greatest degree of human CD45+ cell chimerism, compared to primary cord blood CD34+ cells and cells from control cultures (P<0.0001).
The team also noted that the VPA-treated cells belonged to multiple hematopoietic lineages. And this pattern was distinct from that observed in mice that had received primary cord blood CD34+ cells or cells from control cultures (P<0.0001).
In addition, the VPA-treated HSCs did not cause hematologic malignancies or teratomas in the mice.
The investigators said these results suggest that cord blood cells can be epigenetically reprogrammed by VPA to generate greater numbers of functional HSCs for transplantation.
In a related commentary, Hal Broxmeyer, PhD, of the Indiana University School of Medicine in Indianapolis, discussed how these findings enhance our understanding of HSC function and could potentially provide clinical benefit. ![]()

Credit: NHS
New research suggests that histone deacetylase (HDAC) inhibitors can be used to expand hematopoietic stem cells (HSCs) isolated from cord blood.
Investigators found that valproic acid (VPA), in particular, could greatly expand HSCs from cord blood.
These HSCs expressed markers of pluripotency and were more efficient than conventionally expanded HSCs in repopulating the bone marrow and establishing hematopoiesis in immune-deficient mice.
Pratima Chaurasia, PhD, of the Mount Sinai School of Medicine in New York, and colleagues reported these results in The Journal of Clinical Investigation.
For several decades, investigators have used a variety of strategies to expand the numbers of HSCs isolated from cord blood, with limited success. Evidence has suggested the accumulation of epigenetic modifications influences preservation of stem-cell characteristics in HSC daughter cells.
So Dr Chaurasia’s group tested the effects of HDAC inhibitors on CD34+ cells derived from cord blood. The team primed the cells for 16 hours with cytokines, then treated the cells for 7 days, with or without additional cytokines and in the presence or absence of HDAC inhibitors.
The inhibitors included VPA, scriptaid (SCR), trichostatin A, suberoylanilide hydroxamic acid, CAY10433 (C433), CAY10398, and CAY10603 .
VPA, SCR, and C433 were the most active inhibitors. Treatment with these agents led to a similar percentage of CD34+CD90+ cells—75.2% ± 10.7%, 73.4% ± 13.9%, and 70.1% ± 18.4%, respectively—which was significantly higher than control conditions—16.2% ± 9.2% (P<0.0001).
VPA, SCR, and C433 also generated a greater absolute number of CD34+ and CD34+CD90+ cells per cord blood collection, when compared to control conditions (P≤0.0007). And the inhibitors generated a greater absolute number of CD34+CD90+CD184+ cells (P<0.0001)
The investigators conducted subsequent experiments with VPA only. And they found that VPA was more effective under serum-free conditions and in the presence of cytokines.
Additional experiments revealed that VPA influences the expression of pluripotency genes—SOX2, OCT4, and NANOG. And these genes proved essential for the expansion of CD34+ CD90+ cells.
Lastly, the investigators tested VPA-expanded cells by transplanting them into immune-deficient mice. The cells were more efficient than conventionally expanded cord blood HSCs in repopulating the bone marrow and establishing hematopoietic populations.
Specifically, at 13 to 14 weeks after transplant, VPA-treated cord blood CD34+ cells resulted in a greater degree of human CD45+ cell chimerism—32.2% ± 11.3%—when compared to primary cord blood CD34+ cells—19.4% ± 4.9%—and to cells from control cultures—13.2% ± 6.4% (P=0.006 and P=0.0008, respectively).
The investigators evaluated the self-renewal potential of the expanded grafts by transplanting donor-derived cells from primary recipients into secondary recipients.
After 15 to 16 weeks, the secondary recipients transplanted with VPA-treated cord blood CD34+ cells had achieved the greatest degree of human CD45+ cell chimerism, compared to primary cord blood CD34+ cells and cells from control cultures (P<0.0001).
The team also noted that the VPA-treated cells belonged to multiple hematopoietic lineages. And this pattern was distinct from that observed in mice that had received primary cord blood CD34+ cells or cells from control cultures (P<0.0001).
In addition, the VPA-treated HSCs did not cause hematologic malignancies or teratomas in the mice.
The investigators said these results suggest that cord blood cells can be epigenetically reprogrammed by VPA to generate greater numbers of functional HSCs for transplantation.
In a related commentary, Hal Broxmeyer, PhD, of the Indiana University School of Medicine in Indianapolis, discussed how these findings enhance our understanding of HSC function and could potentially provide clinical benefit. ![]()

Credit: NHS
New research suggests that histone deacetylase (HDAC) inhibitors can be used to expand hematopoietic stem cells (HSCs) isolated from cord blood.
Investigators found that valproic acid (VPA), in particular, could greatly expand HSCs from cord blood.
These HSCs expressed markers of pluripotency and were more efficient than conventionally expanded HSCs in repopulating the bone marrow and establishing hematopoiesis in immune-deficient mice.
Pratima Chaurasia, PhD, of the Mount Sinai School of Medicine in New York, and colleagues reported these results in The Journal of Clinical Investigation.
For several decades, investigators have used a variety of strategies to expand the numbers of HSCs isolated from cord blood, with limited success. Evidence has suggested the accumulation of epigenetic modifications influences preservation of stem-cell characteristics in HSC daughter cells.
So Dr Chaurasia’s group tested the effects of HDAC inhibitors on CD34+ cells derived from cord blood. The team primed the cells for 16 hours with cytokines, then treated the cells for 7 days, with or without additional cytokines and in the presence or absence of HDAC inhibitors.
The inhibitors included VPA, scriptaid (SCR), trichostatin A, suberoylanilide hydroxamic acid, CAY10433 (C433), CAY10398, and CAY10603 .
VPA, SCR, and C433 were the most active inhibitors. Treatment with these agents led to a similar percentage of CD34+CD90+ cells—75.2% ± 10.7%, 73.4% ± 13.9%, and 70.1% ± 18.4%, respectively—which was significantly higher than control conditions—16.2% ± 9.2% (P<0.0001).
VPA, SCR, and C433 also generated a greater absolute number of CD34+ and CD34+CD90+ cells per cord blood collection, when compared to control conditions (P≤0.0007). And the inhibitors generated a greater absolute number of CD34+CD90+CD184+ cells (P<0.0001)
The investigators conducted subsequent experiments with VPA only. And they found that VPA was more effective under serum-free conditions and in the presence of cytokines.
Additional experiments revealed that VPA influences the expression of pluripotency genes—SOX2, OCT4, and NANOG. And these genes proved essential for the expansion of CD34+ CD90+ cells.
Lastly, the investigators tested VPA-expanded cells by transplanting them into immune-deficient mice. The cells were more efficient than conventionally expanded cord blood HSCs in repopulating the bone marrow and establishing hematopoietic populations.
Specifically, at 13 to 14 weeks after transplant, VPA-treated cord blood CD34+ cells resulted in a greater degree of human CD45+ cell chimerism—32.2% ± 11.3%—when compared to primary cord blood CD34+ cells—19.4% ± 4.9%—and to cells from control cultures—13.2% ± 6.4% (P=0.006 and P=0.0008, respectively).
The investigators evaluated the self-renewal potential of the expanded grafts by transplanting donor-derived cells from primary recipients into secondary recipients.
After 15 to 16 weeks, the secondary recipients transplanted with VPA-treated cord blood CD34+ cells had achieved the greatest degree of human CD45+ cell chimerism, compared to primary cord blood CD34+ cells and cells from control cultures (P<0.0001).
The team also noted that the VPA-treated cells belonged to multiple hematopoietic lineages. And this pattern was distinct from that observed in mice that had received primary cord blood CD34+ cells or cells from control cultures (P<0.0001).
In addition, the VPA-treated HSCs did not cause hematologic malignancies or teratomas in the mice.
The investigators said these results suggest that cord blood cells can be epigenetically reprogrammed by VPA to generate greater numbers of functional HSCs for transplantation.
In a related commentary, Hal Broxmeyer, PhD, of the Indiana University School of Medicine in Indianapolis, discussed how these findings enhance our understanding of HSC function and could potentially provide clinical benefit. ![]()
Tracking protein movement to improve patient monitoring, drug development

Credit: Virginia Tech
A novel technique that can detect the subcellular location of a protein may help improve the study of therapies for cancer and other diseases, according to a paper published in Chemical Science.
“Modulation of protein transport inside a cell is practiced as an important therapeutic approach for cancer treatment,” explained Chang Lu, PhD, of Virginia Tech in Blacksburg.
“The subcellular location of a target protein can also serve as a useful read-out for high-content screening of cancer drugs.”
With that in mind, Dr Lu and his colleagues set out to develop a simple and accessible protein detection method that can rapidly screen a large cell population and offers single-cell resolution.
Dr Lu noted that such techniques have been seriously lacking. For instance, fluorescence microscopy can only be used to analyze a limited number of cells.
And data collected by subcellular fractionation only reflects the average properties of the cell populations without revealing the heterogeneity that often exists among cells that seem identical.
Dr Lu and his colleagues had previously made some progress in screening cell populations using an electroporation-based technique, but it did not allow for the examination of native proteins and primary cells isolated from animals and from patients.
Their new work uses a method that “incorporates selective chemical release of cytosolic proteins with a standard procedure for fluorescent labeling of the protein,” Dr Lu said.
This simple tweak to the conventional cell-staining process allowed the researchers to pinpoint the subcellular location of the protein by measuring the amount of the residual protein after release. Using a flow cytometer, the speed of such measurement could reach 10,000 to 100,000 cells per second.
A key ingredient for the team’s process is saponin, an amphipathic glycoside. Saponin dissolves cholesterol and permeates the plasma membrane to allow protein release. And it “shows minimal effects on the state of the cell,” Dr Lu said. ![]()

Credit: Virginia Tech
A novel technique that can detect the subcellular location of a protein may help improve the study of therapies for cancer and other diseases, according to a paper published in Chemical Science.
“Modulation of protein transport inside a cell is practiced as an important therapeutic approach for cancer treatment,” explained Chang Lu, PhD, of Virginia Tech in Blacksburg.
“The subcellular location of a target protein can also serve as a useful read-out for high-content screening of cancer drugs.”
With that in mind, Dr Lu and his colleagues set out to develop a simple and accessible protein detection method that can rapidly screen a large cell population and offers single-cell resolution.
Dr Lu noted that such techniques have been seriously lacking. For instance, fluorescence microscopy can only be used to analyze a limited number of cells.
And data collected by subcellular fractionation only reflects the average properties of the cell populations without revealing the heterogeneity that often exists among cells that seem identical.
Dr Lu and his colleagues had previously made some progress in screening cell populations using an electroporation-based technique, but it did not allow for the examination of native proteins and primary cells isolated from animals and from patients.
Their new work uses a method that “incorporates selective chemical release of cytosolic proteins with a standard procedure for fluorescent labeling of the protein,” Dr Lu said.
This simple tweak to the conventional cell-staining process allowed the researchers to pinpoint the subcellular location of the protein by measuring the amount of the residual protein after release. Using a flow cytometer, the speed of such measurement could reach 10,000 to 100,000 cells per second.
A key ingredient for the team’s process is saponin, an amphipathic glycoside. Saponin dissolves cholesterol and permeates the plasma membrane to allow protein release. And it “shows minimal effects on the state of the cell,” Dr Lu said. ![]()

Credit: Virginia Tech
A novel technique that can detect the subcellular location of a protein may help improve the study of therapies for cancer and other diseases, according to a paper published in Chemical Science.
“Modulation of protein transport inside a cell is practiced as an important therapeutic approach for cancer treatment,” explained Chang Lu, PhD, of Virginia Tech in Blacksburg.
“The subcellular location of a target protein can also serve as a useful read-out for high-content screening of cancer drugs.”
With that in mind, Dr Lu and his colleagues set out to develop a simple and accessible protein detection method that can rapidly screen a large cell population and offers single-cell resolution.
Dr Lu noted that such techniques have been seriously lacking. For instance, fluorescence microscopy can only be used to analyze a limited number of cells.
And data collected by subcellular fractionation only reflects the average properties of the cell populations without revealing the heterogeneity that often exists among cells that seem identical.
Dr Lu and his colleagues had previously made some progress in screening cell populations using an electroporation-based technique, but it did not allow for the examination of native proteins and primary cells isolated from animals and from patients.
Their new work uses a method that “incorporates selective chemical release of cytosolic proteins with a standard procedure for fluorescent labeling of the protein,” Dr Lu said.
This simple tweak to the conventional cell-staining process allowed the researchers to pinpoint the subcellular location of the protein by measuring the amount of the residual protein after release. Using a flow cytometer, the speed of such measurement could reach 10,000 to 100,000 cells per second.
A key ingredient for the team’s process is saponin, an amphipathic glycoside. Saponin dissolves cholesterol and permeates the plasma membrane to allow protein release. And it “shows minimal effects on the state of the cell,” Dr Lu said. ![]()
New insight into PTEN’s role in cancers
Researchers say they’ve uncovered new details that help explain how the PTEN gene exerts its anticancer effects and how PTEN loss or alteration can set cells on a cancerous course.
The team’s study, published in Cell, reveals that PTEN loss and PTEN mutations are not synonymous.
This discovery provides additional insight into basic tumor biology and offers a potential new direction in the pursuit of cancer therapies, according to the researchers.
“By characterizing the ways that 2 specific PTEN mutations regulate the tumor suppressor function of the normal PTEN protein, our findings suggest that different PTEN mutations contribute to tumorigenesis by regulating different aspects of PTEN biology,” said study author Pier Paolo Pandolfi, MD, PhD, of Beth Israel Deaconess Medical Center in Boston.
“It has been suggested that cancer patients harboring mutations in PTEN had poorer outcomes than cancer patients with PTEN loss. Now, using mouse modeling, we are able to demonstrate that this is indeed the case. Because PTEN mutations are extremely frequent in various types of tumors, this discovery could help pave the way for a new level of personalized cancer treatment.”
Several of the proteins that PTEN acts upon, both lipids and proteins, are known to promote cancer when bound to a phosphate. Consequently, when PTEN removes their phosphates, it is acting as a tumor suppressor to prevent cancer. When PTEN is mutated, it loses this suppressive ability, and the cancer-promoting proteins are left intact and uninhibited.
This new study showed that the PTEN mutant protein is not only functionally impaired, it also acquires the ability to affect the function of normal PTEN proteins, thereby gaining a pro-tumorigenic function. But the researchers also wanted to determine the difference between PTEN mutation and PTEN loss.
“We wanted to know, would outcomes differ in cases when PTEN was not expressed, compared with cases when PTEN was expressed but encoded a mutation within its sequence?” said study author Antonella Papa, PhD, an investigator in the Pandolfi lab.
To find out, the team created several genetically modified strains of mice to mimic the PTEN mutations found in human cancer patients.
“All mice [and humans] have 2 copies of the PTEN gene,” Dr Papa explained. “The genetically modified mice in our study had 1 copy of the PTEN gene that contained a cancer-associated mutation [either PTENC124S or PTENG129E] and 1 normal copy of PTEN. Other mice in the study had only 1 copy of the normal PTEN gene, and the second copy was removed.”
The researchers found that the mice with a single mutated copy of PTEN were more tumor-prone than the mice with a deleted copy of PTEN. They also discovered that the mutated protein that was produced by PTENC124S or PTENG129E was binding to and inhibiting the PTEN protein made from the normal copy of the PTEN gene.
“This was very surprising, as we were expecting a reduction in tumorigenesis,” Dr Papa said. “Instead, mechanistically, we found that PTEN exists as a dimer, and, in this new conformation, the mutated protein prevents the normal protein from functioning.”
At the molecular level, this generates an increased activation of a PTEN target—the protein Akt—which is what leads to the augmented tumorigenesis in the mice. Akt is part of a signaling pathway that regulates cell growth, division and metabolism.
When PTEN is prevented from inhibiting Akt, the pathway becomes overactive. As a result, targeting Akt and its pathway may be an effective treatment strategy for patients with PTEN mutations, the researchers said, adding that inhibitors to affect this pathway are currently being tested and developed.
“This defines a new working model for the function and regulation of PTEN and tells us that PTEN mutational status can be used to determine which cancer patients might benefit from earlier and more radical therapeutic interventions and, ultimately, better prognosis,” Dr Pandolfi said.
“Our findings may help to better identify and stratify patients and their response to treatment based on the different genetic alterations found in the PTEN gene. Importantly, our study shows that cancer therapy should be tailored on the basis of the very specific type of mutations that the tumor harbors.”
“This adds a new layer of complexity but also a new opportunity for precision medicine. I would say that, based on these thorough genetic analyses, this story represents the ultimate example of why personalized cancer medicine is so urgently needed.” ![]()

Researchers say they’ve uncovered new details that help explain how the PTEN gene exerts its anticancer effects and how PTEN loss or alteration can set cells on a cancerous course.
The team’s study, published in Cell, reveals that PTEN loss and PTEN mutations are not synonymous.
This discovery provides additional insight into basic tumor biology and offers a potential new direction in the pursuit of cancer therapies, according to the researchers.
“By characterizing the ways that 2 specific PTEN mutations regulate the tumor suppressor function of the normal PTEN protein, our findings suggest that different PTEN mutations contribute to tumorigenesis by regulating different aspects of PTEN biology,” said study author Pier Paolo Pandolfi, MD, PhD, of Beth Israel Deaconess Medical Center in Boston.
“It has been suggested that cancer patients harboring mutations in PTEN had poorer outcomes than cancer patients with PTEN loss. Now, using mouse modeling, we are able to demonstrate that this is indeed the case. Because PTEN mutations are extremely frequent in various types of tumors, this discovery could help pave the way for a new level of personalized cancer treatment.”
Several of the proteins that PTEN acts upon, both lipids and proteins, are known to promote cancer when bound to a phosphate. Consequently, when PTEN removes their phosphates, it is acting as a tumor suppressor to prevent cancer. When PTEN is mutated, it loses this suppressive ability, and the cancer-promoting proteins are left intact and uninhibited.
This new study showed that the PTEN mutant protein is not only functionally impaired, it also acquires the ability to affect the function of normal PTEN proteins, thereby gaining a pro-tumorigenic function. But the researchers also wanted to determine the difference between PTEN mutation and PTEN loss.
“We wanted to know, would outcomes differ in cases when PTEN was not expressed, compared with cases when PTEN was expressed but encoded a mutation within its sequence?” said study author Antonella Papa, PhD, an investigator in the Pandolfi lab.
To find out, the team created several genetically modified strains of mice to mimic the PTEN mutations found in human cancer patients.
“All mice [and humans] have 2 copies of the PTEN gene,” Dr Papa explained. “The genetically modified mice in our study had 1 copy of the PTEN gene that contained a cancer-associated mutation [either PTENC124S or PTENG129E] and 1 normal copy of PTEN. Other mice in the study had only 1 copy of the normal PTEN gene, and the second copy was removed.”
The researchers found that the mice with a single mutated copy of PTEN were more tumor-prone than the mice with a deleted copy of PTEN. They also discovered that the mutated protein that was produced by PTENC124S or PTENG129E was binding to and inhibiting the PTEN protein made from the normal copy of the PTEN gene.
“This was very surprising, as we were expecting a reduction in tumorigenesis,” Dr Papa said. “Instead, mechanistically, we found that PTEN exists as a dimer, and, in this new conformation, the mutated protein prevents the normal protein from functioning.”
At the molecular level, this generates an increased activation of a PTEN target—the protein Akt—which is what leads to the augmented tumorigenesis in the mice. Akt is part of a signaling pathway that regulates cell growth, division and metabolism.
When PTEN is prevented from inhibiting Akt, the pathway becomes overactive. As a result, targeting Akt and its pathway may be an effective treatment strategy for patients with PTEN mutations, the researchers said, adding that inhibitors to affect this pathway are currently being tested and developed.
“This defines a new working model for the function and regulation of PTEN and tells us that PTEN mutational status can be used to determine which cancer patients might benefit from earlier and more radical therapeutic interventions and, ultimately, better prognosis,” Dr Pandolfi said.
“Our findings may help to better identify and stratify patients and their response to treatment based on the different genetic alterations found in the PTEN gene. Importantly, our study shows that cancer therapy should be tailored on the basis of the very specific type of mutations that the tumor harbors.”
“This adds a new layer of complexity but also a new opportunity for precision medicine. I would say that, based on these thorough genetic analyses, this story represents the ultimate example of why personalized cancer medicine is so urgently needed.” ![]()

Researchers say they’ve uncovered new details that help explain how the PTEN gene exerts its anticancer effects and how PTEN loss or alteration can set cells on a cancerous course.
The team’s study, published in Cell, reveals that PTEN loss and PTEN mutations are not synonymous.
This discovery provides additional insight into basic tumor biology and offers a potential new direction in the pursuit of cancer therapies, according to the researchers.
“By characterizing the ways that 2 specific PTEN mutations regulate the tumor suppressor function of the normal PTEN protein, our findings suggest that different PTEN mutations contribute to tumorigenesis by regulating different aspects of PTEN biology,” said study author Pier Paolo Pandolfi, MD, PhD, of Beth Israel Deaconess Medical Center in Boston.
“It has been suggested that cancer patients harboring mutations in PTEN had poorer outcomes than cancer patients with PTEN loss. Now, using mouse modeling, we are able to demonstrate that this is indeed the case. Because PTEN mutations are extremely frequent in various types of tumors, this discovery could help pave the way for a new level of personalized cancer treatment.”
Several of the proteins that PTEN acts upon, both lipids and proteins, are known to promote cancer when bound to a phosphate. Consequently, when PTEN removes their phosphates, it is acting as a tumor suppressor to prevent cancer. When PTEN is mutated, it loses this suppressive ability, and the cancer-promoting proteins are left intact and uninhibited.
This new study showed that the PTEN mutant protein is not only functionally impaired, it also acquires the ability to affect the function of normal PTEN proteins, thereby gaining a pro-tumorigenic function. But the researchers also wanted to determine the difference between PTEN mutation and PTEN loss.
“We wanted to know, would outcomes differ in cases when PTEN was not expressed, compared with cases when PTEN was expressed but encoded a mutation within its sequence?” said study author Antonella Papa, PhD, an investigator in the Pandolfi lab.
To find out, the team created several genetically modified strains of mice to mimic the PTEN mutations found in human cancer patients.
“All mice [and humans] have 2 copies of the PTEN gene,” Dr Papa explained. “The genetically modified mice in our study had 1 copy of the PTEN gene that contained a cancer-associated mutation [either PTENC124S or PTENG129E] and 1 normal copy of PTEN. Other mice in the study had only 1 copy of the normal PTEN gene, and the second copy was removed.”
The researchers found that the mice with a single mutated copy of PTEN were more tumor-prone than the mice with a deleted copy of PTEN. They also discovered that the mutated protein that was produced by PTENC124S or PTENG129E was binding to and inhibiting the PTEN protein made from the normal copy of the PTEN gene.
“This was very surprising, as we were expecting a reduction in tumorigenesis,” Dr Papa said. “Instead, mechanistically, we found that PTEN exists as a dimer, and, in this new conformation, the mutated protein prevents the normal protein from functioning.”
At the molecular level, this generates an increased activation of a PTEN target—the protein Akt—which is what leads to the augmented tumorigenesis in the mice. Akt is part of a signaling pathway that regulates cell growth, division and metabolism.
When PTEN is prevented from inhibiting Akt, the pathway becomes overactive. As a result, targeting Akt and its pathway may be an effective treatment strategy for patients with PTEN mutations, the researchers said, adding that inhibitors to affect this pathway are currently being tested and developed.
“This defines a new working model for the function and regulation of PTEN and tells us that PTEN mutational status can be used to determine which cancer patients might benefit from earlier and more radical therapeutic interventions and, ultimately, better prognosis,” Dr Pandolfi said.
“Our findings may help to better identify and stratify patients and their response to treatment based on the different genetic alterations found in the PTEN gene. Importantly, our study shows that cancer therapy should be tailored on the basis of the very specific type of mutations that the tumor harbors.”
“This adds a new layer of complexity but also a new opportunity for precision medicine. I would say that, based on these thorough genetic analyses, this story represents the ultimate example of why personalized cancer medicine is so urgently needed.” ![]()