User login
An 18-year-old woman with hepatic cysts
An 18-year-old woman presents with 3 days of epigastric abdominal pain, with no fever or constitutional symptoms. She was born in the United States and reports yearly trips since age 3 to her family’s farm in a rural area of Mexico, where she is exposed to dogs and horses.
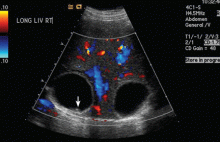
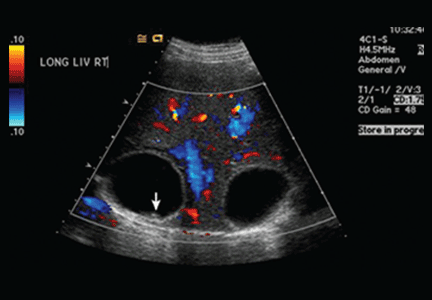
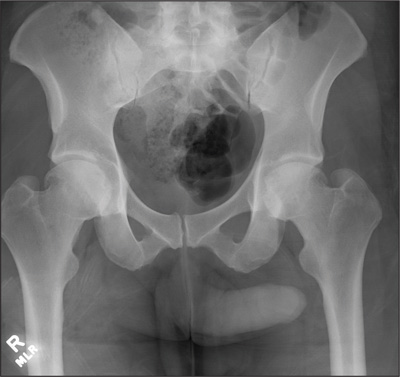
Ultrasonography reveals two large hepatic cysts measuring 5.8 × 6.8 × 5.4 cm and 5.3 × 4.9 × 7 cm, with thickened walls and internal debris (Figure 1). The debris moves to dependent areas when the patient is asked to move onto her side.
Laboratory values at the time of presentation are as follows:
- White blood cell count 11.9 × 109/L (reference range 4.5–11.0), with 20% eosinophils
- Alkaline phosphatase 116 U/L (30–100)
- Total protein 7.3 g/dL (6.0–8.0)
- Albumin 4.3 g/dL (3.5–5.0)
- Aspartate aminotransferase (AST) 19 U/L (10–40)
- Alanine aminotransferase (ALT) 18 U/L (5–40)
- Total bilirubin 0.2 mg/dL (0.3–1.2)
- Direct bilirubin 0.1 mg/dL (0.1–0.3)
- Echinococcus antibody (IgG) testing is positive.
CYSTIC ECHINOCOCCOSIS
The two clinically relevant species of Echinococcus that cause human infection are E granulosus (in cystic echinococcosis) and E multilocularis (in alveolar echinococcosis). Based on clinical and radiographic findings, hepatic hydatid disease from cystic echinococcosis can usually be differentiated from the alveolar form.
E granulosus is a parasitic tapeworm that requires an intermediate host (sheep, goats, cows) and a definite host (dogs, foxes, and related species) for its life cycle. Humans become infected when they ingest food contaminated with feces that contain the eggs of the tapeworm or when they handle carnivorous animals, usually dogs, and accidentally ingest the tapeworm eggs. Once ingested, the egg releases an oncosphere that penetrates the intestinal wall, enters the circulation, and develops into a cyst, most often in the liver and the lungs.1 Human-to-human transmission does not occur.2
Hydatid cysts grow slowly, at a rate of 1 to 50 mm per year,3 so most patients remain asymptomatic for several years. Symptoms occur when a cyst ruptures or impinges on structures.3 Fever and constitutional symptoms usually occur only if there is rupture or bacterial superinfection of the cyst. Tests of liver function tend to be normal unless a cyst obstructs biliary flow. Eosinophilia occurs in 25% of patients.1 Eosinophilia along with the abrupt onset of abdominal pain suggests cyst rupture.
Making the diagnosis
Diagnosis is made by characteristic ultrasonographic findings and by serologic testing. Antibody assays for Echinococcus include indirect hemagglutination, enzyme-linked immunosorbent assay, and latex agglutination. However, these serologic antibody assays for immunoglobulin G cross-react to different echinococcal species as well as to other helminthic infections. Specific serologic studies such as an enzyme-linked immunosorbent assay for E multilocularis are available to confirm the species of Echinococcus but are only used to distinguish cystic echinococcosis from alveolar echinococcosis.
Treatment options
Treatment options include surgery, percutaneous procedures, drug therapy, and observation.
Currently, there is no clear consensus on treatment. To guide treatment decisions, the World Health Organization Informal Working Group on Echinococcosis (WHO-IWGE) recommends management of hepatic hydatid cysts based on classification, size, symptoms, location, and available resources.3
Two percutaneous treatments are aspiration, injection, and re-aspiration to destroy the germinal matrix, and percutaneous therapy to destroy the endocyst. Percutaneous aspiration, injection, and re-aspiration is increasingly used as the first-line treatment for single or easily accessible cysts and for patients who cannot undergo surgery. Surgery is considered for multiple cysts, large cysts, and cysts not easily accessible with a percutaneous technique.3 Complication rates and length of hospital stay with percutaneous aspiration are lower than with surgery.4 Observation is recommended for small, asymptomatic, inactive cysts.
Leakage of cyst contents during surgical or percutaneous intervention or spontaneous rupture can cause a recurrence,5 and anaphylaxis is a potential complication of cyst rupture.1 For this reason, giving oral albendazole (Albenza) is recommended before any intervention. Sterilization of the cyst contents with a protoscolicidal agent (20% NaCl) before evacuation of cyst contents is also standard practice.
The rate of cyst recurrence is 16.2% with open surgery and 3.5% with percutaneous intervention.6 A higher incidence of recurrence in patients who undergo surgical cystectomy likely reflects the more complicated and active nature of the cysts in patients who undergo surgery.6
Albendazole is the drug of choice for hepatic hydatid disease.3 The optimal duration of treatment is unclear but should be guided by a combination of clinical response, medication side effects, serologic titers, and imaging. The most common adverse effects of albendazole are hepatotoxicity, abdominal pain, and nausea.
OUR PATIENT’S DIAGNOSIS AND TREATMENT
In our patient, ultrasonography confirmed the diagnosis of cystic echinococcosis by the finding of active anechoic cysts with echogenic internal debris and with a well-delineated cyst wall. The WHO-IWGE classification was CE1, ie, active anechoic cysts with internal echogenic debris.
Our patient underwent surgical rather than percutaneous cystectomy because of concern about possible cyst leakage, since she had presented with the acute onset of pain and eosinophilia. We were also concerned about the subdiaphragmatic location of the larger cyst, which could have been difficult to reach percutaneously.
Open total pericystectomy involved opening the cyst cavity, sterilizing the contents with 20% NaCl, evacuating the cyst contents, and removing the cyst tissue. Two large cysts were excised and sent for histologic examination, which confirmed E granulosus. Percutaneous aspiration was necessary 4 months later because of a recurrence, and albendazole 400 mg twice daily was continued for another 5 months. Ultrasonography 3 years later showed no evidence of echinococcal cysts, and her antibody titers remain undetectable.
- McManus DP, Gray DJ, Zhang W, Yang Y. Diagnosis, treatment, and management of echinococcosis. BMJ 2012; 344:e3866.
- McManus DP, Zhang W, Li J, Bartley PB. Echinococcosis. Lancet 2003; 362:1295–1304.
- Brunetti E, Kern P, Vuitton DA; Writing Panel for the WHO-IWGE. Expert consensus for the diagnosis and treatment of cystic and alveolar echinococcosis in humans. Acta Trop 2010; 114:1–16.
- Khuroo MS, Wani NA, Javid G, et al. Percutaneous drainage compared with surgery for hepatic hydatid cysts. N Engl J Med 1997; 337:881–887.
- Kayaalp C, Sengul N, Akoglu M. Importance of cyst content in hydatid liver surgery. Arch Surg 2002; 137:159–163.
- Yagci G, Ustunsoz B, Kaymakcioglu N, et al. Results of surgical, laparoscopic, and percutaneous treatment for hydatid disease of the liver: 10 years experience with 355 patients. World J Surg 2005; 29:1670–1679.
An 18-year-old woman presents with 3 days of epigastric abdominal pain, with no fever or constitutional symptoms. She was born in the United States and reports yearly trips since age 3 to her family’s farm in a rural area of Mexico, where she is exposed to dogs and horses.
Ultrasonography reveals two large hepatic cysts measuring 5.8 × 6.8 × 5.4 cm and 5.3 × 4.9 × 7 cm, with thickened walls and internal debris (Figure 1). The debris moves to dependent areas when the patient is asked to move onto her side.
Laboratory values at the time of presentation are as follows:
- White blood cell count 11.9 × 109/L (reference range 4.5–11.0), with 20% eosinophils
- Alkaline phosphatase 116 U/L (30–100)
- Total protein 7.3 g/dL (6.0–8.0)
- Albumin 4.3 g/dL (3.5–5.0)
- Aspartate aminotransferase (AST) 19 U/L (10–40)
- Alanine aminotransferase (ALT) 18 U/L (5–40)
- Total bilirubin 0.2 mg/dL (0.3–1.2)
- Direct bilirubin 0.1 mg/dL (0.1–0.3)
- Echinococcus antibody (IgG) testing is positive.
CYSTIC ECHINOCOCCOSIS
The two clinically relevant species of Echinococcus that cause human infection are E granulosus (in cystic echinococcosis) and E multilocularis (in alveolar echinococcosis). Based on clinical and radiographic findings, hepatic hydatid disease from cystic echinococcosis can usually be differentiated from the alveolar form.
E granulosus is a parasitic tapeworm that requires an intermediate host (sheep, goats, cows) and a definite host (dogs, foxes, and related species) for its life cycle. Humans become infected when they ingest food contaminated with feces that contain the eggs of the tapeworm or when they handle carnivorous animals, usually dogs, and accidentally ingest the tapeworm eggs. Once ingested, the egg releases an oncosphere that penetrates the intestinal wall, enters the circulation, and develops into a cyst, most often in the liver and the lungs.1 Human-to-human transmission does not occur.2
Hydatid cysts grow slowly, at a rate of 1 to 50 mm per year,3 so most patients remain asymptomatic for several years. Symptoms occur when a cyst ruptures or impinges on structures.3 Fever and constitutional symptoms usually occur only if there is rupture or bacterial superinfection of the cyst. Tests of liver function tend to be normal unless a cyst obstructs biliary flow. Eosinophilia occurs in 25% of patients.1 Eosinophilia along with the abrupt onset of abdominal pain suggests cyst rupture.
Making the diagnosis
Diagnosis is made by characteristic ultrasonographic findings and by serologic testing. Antibody assays for Echinococcus include indirect hemagglutination, enzyme-linked immunosorbent assay, and latex agglutination. However, these serologic antibody assays for immunoglobulin G cross-react to different echinococcal species as well as to other helminthic infections. Specific serologic studies such as an enzyme-linked immunosorbent assay for E multilocularis are available to confirm the species of Echinococcus but are only used to distinguish cystic echinococcosis from alveolar echinococcosis.
Treatment options
Treatment options include surgery, percutaneous procedures, drug therapy, and observation.
Currently, there is no clear consensus on treatment. To guide treatment decisions, the World Health Organization Informal Working Group on Echinococcosis (WHO-IWGE) recommends management of hepatic hydatid cysts based on classification, size, symptoms, location, and available resources.3
Two percutaneous treatments are aspiration, injection, and re-aspiration to destroy the germinal matrix, and percutaneous therapy to destroy the endocyst. Percutaneous aspiration, injection, and re-aspiration is increasingly used as the first-line treatment for single or easily accessible cysts and for patients who cannot undergo surgery. Surgery is considered for multiple cysts, large cysts, and cysts not easily accessible with a percutaneous technique.3 Complication rates and length of hospital stay with percutaneous aspiration are lower than with surgery.4 Observation is recommended for small, asymptomatic, inactive cysts.
Leakage of cyst contents during surgical or percutaneous intervention or spontaneous rupture can cause a recurrence,5 and anaphylaxis is a potential complication of cyst rupture.1 For this reason, giving oral albendazole (Albenza) is recommended before any intervention. Sterilization of the cyst contents with a protoscolicidal agent (20% NaCl) before evacuation of cyst contents is also standard practice.
The rate of cyst recurrence is 16.2% with open surgery and 3.5% with percutaneous intervention.6 A higher incidence of recurrence in patients who undergo surgical cystectomy likely reflects the more complicated and active nature of the cysts in patients who undergo surgery.6
Albendazole is the drug of choice for hepatic hydatid disease.3 The optimal duration of treatment is unclear but should be guided by a combination of clinical response, medication side effects, serologic titers, and imaging. The most common adverse effects of albendazole are hepatotoxicity, abdominal pain, and nausea.
OUR PATIENT’S DIAGNOSIS AND TREATMENT
In our patient, ultrasonography confirmed the diagnosis of cystic echinococcosis by the finding of active anechoic cysts with echogenic internal debris and with a well-delineated cyst wall. The WHO-IWGE classification was CE1, ie, active anechoic cysts with internal echogenic debris.
Our patient underwent surgical rather than percutaneous cystectomy because of concern about possible cyst leakage, since she had presented with the acute onset of pain and eosinophilia. We were also concerned about the subdiaphragmatic location of the larger cyst, which could have been difficult to reach percutaneously.
Open total pericystectomy involved opening the cyst cavity, sterilizing the contents with 20% NaCl, evacuating the cyst contents, and removing the cyst tissue. Two large cysts were excised and sent for histologic examination, which confirmed E granulosus. Percutaneous aspiration was necessary 4 months later because of a recurrence, and albendazole 400 mg twice daily was continued for another 5 months. Ultrasonography 3 years later showed no evidence of echinococcal cysts, and her antibody titers remain undetectable.
An 18-year-old woman presents with 3 days of epigastric abdominal pain, with no fever or constitutional symptoms. She was born in the United States and reports yearly trips since age 3 to her family’s farm in a rural area of Mexico, where she is exposed to dogs and horses.
Ultrasonography reveals two large hepatic cysts measuring 5.8 × 6.8 × 5.4 cm and 5.3 × 4.9 × 7 cm, with thickened walls and internal debris (Figure 1). The debris moves to dependent areas when the patient is asked to move onto her side.
Laboratory values at the time of presentation are as follows:
- White blood cell count 11.9 × 109/L (reference range 4.5–11.0), with 20% eosinophils
- Alkaline phosphatase 116 U/L (30–100)
- Total protein 7.3 g/dL (6.0–8.0)
- Albumin 4.3 g/dL (3.5–5.0)
- Aspartate aminotransferase (AST) 19 U/L (10–40)
- Alanine aminotransferase (ALT) 18 U/L (5–40)
- Total bilirubin 0.2 mg/dL (0.3–1.2)
- Direct bilirubin 0.1 mg/dL (0.1–0.3)
- Echinococcus antibody (IgG) testing is positive.
CYSTIC ECHINOCOCCOSIS
The two clinically relevant species of Echinococcus that cause human infection are E granulosus (in cystic echinococcosis) and E multilocularis (in alveolar echinococcosis). Based on clinical and radiographic findings, hepatic hydatid disease from cystic echinococcosis can usually be differentiated from the alveolar form.
E granulosus is a parasitic tapeworm that requires an intermediate host (sheep, goats, cows) and a definite host (dogs, foxes, and related species) for its life cycle. Humans become infected when they ingest food contaminated with feces that contain the eggs of the tapeworm or when they handle carnivorous animals, usually dogs, and accidentally ingest the tapeworm eggs. Once ingested, the egg releases an oncosphere that penetrates the intestinal wall, enters the circulation, and develops into a cyst, most often in the liver and the lungs.1 Human-to-human transmission does not occur.2
Hydatid cysts grow slowly, at a rate of 1 to 50 mm per year,3 so most patients remain asymptomatic for several years. Symptoms occur when a cyst ruptures or impinges on structures.3 Fever and constitutional symptoms usually occur only if there is rupture or bacterial superinfection of the cyst. Tests of liver function tend to be normal unless a cyst obstructs biliary flow. Eosinophilia occurs in 25% of patients.1 Eosinophilia along with the abrupt onset of abdominal pain suggests cyst rupture.
Making the diagnosis
Diagnosis is made by characteristic ultrasonographic findings and by serologic testing. Antibody assays for Echinococcus include indirect hemagglutination, enzyme-linked immunosorbent assay, and latex agglutination. However, these serologic antibody assays for immunoglobulin G cross-react to different echinococcal species as well as to other helminthic infections. Specific serologic studies such as an enzyme-linked immunosorbent assay for E multilocularis are available to confirm the species of Echinococcus but are only used to distinguish cystic echinococcosis from alveolar echinococcosis.
Treatment options
Treatment options include surgery, percutaneous procedures, drug therapy, and observation.
Currently, there is no clear consensus on treatment. To guide treatment decisions, the World Health Organization Informal Working Group on Echinococcosis (WHO-IWGE) recommends management of hepatic hydatid cysts based on classification, size, symptoms, location, and available resources.3
Two percutaneous treatments are aspiration, injection, and re-aspiration to destroy the germinal matrix, and percutaneous therapy to destroy the endocyst. Percutaneous aspiration, injection, and re-aspiration is increasingly used as the first-line treatment for single or easily accessible cysts and for patients who cannot undergo surgery. Surgery is considered for multiple cysts, large cysts, and cysts not easily accessible with a percutaneous technique.3 Complication rates and length of hospital stay with percutaneous aspiration are lower than with surgery.4 Observation is recommended for small, asymptomatic, inactive cysts.
Leakage of cyst contents during surgical or percutaneous intervention or spontaneous rupture can cause a recurrence,5 and anaphylaxis is a potential complication of cyst rupture.1 For this reason, giving oral albendazole (Albenza) is recommended before any intervention. Sterilization of the cyst contents with a protoscolicidal agent (20% NaCl) before evacuation of cyst contents is also standard practice.
The rate of cyst recurrence is 16.2% with open surgery and 3.5% with percutaneous intervention.6 A higher incidence of recurrence in patients who undergo surgical cystectomy likely reflects the more complicated and active nature of the cysts in patients who undergo surgery.6
Albendazole is the drug of choice for hepatic hydatid disease.3 The optimal duration of treatment is unclear but should be guided by a combination of clinical response, medication side effects, serologic titers, and imaging. The most common adverse effects of albendazole are hepatotoxicity, abdominal pain, and nausea.
OUR PATIENT’S DIAGNOSIS AND TREATMENT
In our patient, ultrasonography confirmed the diagnosis of cystic echinococcosis by the finding of active anechoic cysts with echogenic internal debris and with a well-delineated cyst wall. The WHO-IWGE classification was CE1, ie, active anechoic cysts with internal echogenic debris.
Our patient underwent surgical rather than percutaneous cystectomy because of concern about possible cyst leakage, since she had presented with the acute onset of pain and eosinophilia. We were also concerned about the subdiaphragmatic location of the larger cyst, which could have been difficult to reach percutaneously.
Open total pericystectomy involved opening the cyst cavity, sterilizing the contents with 20% NaCl, evacuating the cyst contents, and removing the cyst tissue. Two large cysts were excised and sent for histologic examination, which confirmed E granulosus. Percutaneous aspiration was necessary 4 months later because of a recurrence, and albendazole 400 mg twice daily was continued for another 5 months. Ultrasonography 3 years later showed no evidence of echinococcal cysts, and her antibody titers remain undetectable.
- McManus DP, Gray DJ, Zhang W, Yang Y. Diagnosis, treatment, and management of echinococcosis. BMJ 2012; 344:e3866.
- McManus DP, Zhang W, Li J, Bartley PB. Echinococcosis. Lancet 2003; 362:1295–1304.
- Brunetti E, Kern P, Vuitton DA; Writing Panel for the WHO-IWGE. Expert consensus for the diagnosis and treatment of cystic and alveolar echinococcosis in humans. Acta Trop 2010; 114:1–16.
- Khuroo MS, Wani NA, Javid G, et al. Percutaneous drainage compared with surgery for hepatic hydatid cysts. N Engl J Med 1997; 337:881–887.
- Kayaalp C, Sengul N, Akoglu M. Importance of cyst content in hydatid liver surgery. Arch Surg 2002; 137:159–163.
- Yagci G, Ustunsoz B, Kaymakcioglu N, et al. Results of surgical, laparoscopic, and percutaneous treatment for hydatid disease of the liver: 10 years experience with 355 patients. World J Surg 2005; 29:1670–1679.
- McManus DP, Gray DJ, Zhang W, Yang Y. Diagnosis, treatment, and management of echinococcosis. BMJ 2012; 344:e3866.
- McManus DP, Zhang W, Li J, Bartley PB. Echinococcosis. Lancet 2003; 362:1295–1304.
- Brunetti E, Kern P, Vuitton DA; Writing Panel for the WHO-IWGE. Expert consensus for the diagnosis and treatment of cystic and alveolar echinococcosis in humans. Acta Trop 2010; 114:1–16.
- Khuroo MS, Wani NA, Javid G, et al. Percutaneous drainage compared with surgery for hepatic hydatid cysts. N Engl J Med 1997; 337:881–887.
- Kayaalp C, Sengul N, Akoglu M. Importance of cyst content in hydatid liver surgery. Arch Surg 2002; 137:159–163.
- Yagci G, Ustunsoz B, Kaymakcioglu N, et al. Results of surgical, laparoscopic, and percutaneous treatment for hydatid disease of the liver: 10 years experience with 355 patients. World J Surg 2005; 29:1670–1679.
Syncope during a pharmacologic nuclear stress test
A 60-year-old woman was referred for pharmacologic nuclear stress testing before treatment for breast cancer. She had hypertension, diabetes mellitus, coronary artery disease, and a remote history of stroke, and she was taking clonidine (Catapres), labetalol (Normodyne, Trandate), furosemide (Lasix), hydralazine, valsartan (Diovan), insulin, and the aspirin-dipyridamole combination Aggrenox. Her vital signs and electrocardiogram before the stress test were normal.
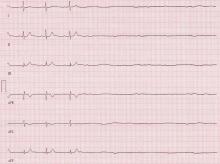
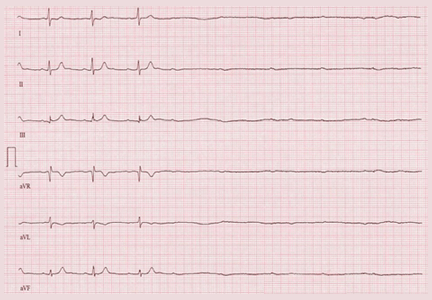
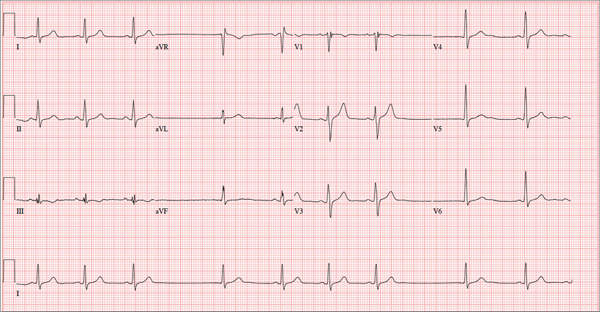
The stress test was started with a standard protocol of adenosine (Adenoscan) infused intravenously over 4 minutes. For the first 2 minutes, she was stable and had no symptoms, but then sinus pauses and second-degree atrioventricular block type 2 developed, after which her heart stopped beating (Figure 1). The infusion was immediately stopped, but she became unresponsive and remained pulseless.
Cardiopulmonary resuscitation was started, aminophylline 100 mg was given intravenously, and she regained a pulse and blood pressure within a few minutes. She was then transferred to the emergency room, where she returned to her baseline clinical and neurologic status without symptoms.
AN UNRECOGNIZED DRUG INTERACTION
Asystole occurred in this patient because of the interaction of intravenous adenosine with the dipyridamole in the medication Aggrenox. Although adenosine, given during pharmacologic stress testing, is known to interact with various medications, the potential for this interaction may be overlooked if the culprit is present in a combination drug. Aggrenox is commonly given for secondary stroke prevention and should be discontinued before pharmacologic nuclear stress testing.
Pharmacologic stress testing involves two commonly used stress agents, adenosine and regadenoson (Lexiscan), which cause coronary vasodilation through their action on A2A receptors in the heart. Coronary vasodilation results in flow heterogeneity in the region of a stenotic artery, which can be detected with nuclear perfusion agents. In addition, adenosine has a short-lived effect on the A1 receptors that block atrioventricular conduction.1
Dipyridamole (Persantine) is contraindicated when either adenosine or regadenoson is used. Dipyridamole enhances the effect of exogenous and endogenous adenosine by inhibiting its uptake by cardiac cells, thus enhancing the action of these coronary vasodilators.2 Atrioventricular block is common during adenosine stress testing but is transient because adenosine has a short half-life (< 10 seconds), and complete heart block or asystole, as seen in this patient, is rare. Giving intravenous adenosine or regadenoson to patients on dipyridamole may have a marked effect on adenosine receptors, so that profound bradycardia and even asystole leading to cardiac collapse may occur. No data are available on the specific interaction of dipyridamole and regadenoson.
Even though the pharmacodynamics of the interaction between dipyridamole and adenosine are known,3 few reports are available detailing serious adverse events. The contraindication to pharmacologic stress testing in patients taking dipyridamole is noted in the American Society of Nuclear Cardiology Guidelines for stress protocols,4 which advise discontinuing dipyridamole-containing drugs at least 48 hours before the use of adenosine or regadenoson. Similarly, the American Heart Association guidelines5 for the management of supraventricular tachycardia recommend an initial dose of 3 mg of adenosine rather than 6 mg in patients who have been taking dipyridamole.
The dose of aminophylline for reversing the adverse effects of adenosine or regadenoson is 50 to 250 mg intravenously over 30 to 60 seconds. But since these adverse effects are short-lived once the infusion is stopped, aminophylline is usually given only if the adverse effects are severe, as in this patient.
Pharmacologic nuclear stress testing with adenosine receptor agonists (eg, adenosine or regadenoson) is contraindicated in patients taking dipyridamole or the combination pill Aggrenox because of the potential for profound bradyarrhythmias or asystole.
- Zoghbi GJ, Iskandrian AE. Selective adenosine agonists and myocardial perfusion imaging. J Nucl Cardiol 2012; 19:126–141.
- Lerman BB, Wesley RC, Belardinelli L. Electrophysiologic effects of dipyridamole on atrioventricular nodal conduction and supraventricular tachycardia. Role of endogenous adenosine. Circulation 1989; 80:1536–1543.
- Biaggioni I, Onrot J, Hollister AS, Robertson D. Cardiovascular effects of adenosine infusion in man and their modulation by dipyridamole. Life Sci 1986; 39:2229–2236.
- Henzlova MJ, Cerqueira MD, Mahmarian JJ, Yao SS; Quality Assurance Committee of the American Society of Nuclear Cardiology. Stress protocols and tracers. J Nucl Cardiol 2006; 13:e80–e90.
- ECC Committee, Subcommittees and Task Forces of the American Heart Association. 2005 American Heart Association guidelines for cardiopulmonary resuscitation and emergency cardiovascular care. Part 7.3: management of symptomatic bradycardia and tachycardia. Circulation 2005; 112(suppl 24):IV67–IV77.
A 60-year-old woman was referred for pharmacologic nuclear stress testing before treatment for breast cancer. She had hypertension, diabetes mellitus, coronary artery disease, and a remote history of stroke, and she was taking clonidine (Catapres), labetalol (Normodyne, Trandate), furosemide (Lasix), hydralazine, valsartan (Diovan), insulin, and the aspirin-dipyridamole combination Aggrenox. Her vital signs and electrocardiogram before the stress test were normal.
The stress test was started with a standard protocol of adenosine (Adenoscan) infused intravenously over 4 minutes. For the first 2 minutes, she was stable and had no symptoms, but then sinus pauses and second-degree atrioventricular block type 2 developed, after which her heart stopped beating (Figure 1). The infusion was immediately stopped, but she became unresponsive and remained pulseless.
Cardiopulmonary resuscitation was started, aminophylline 100 mg was given intravenously, and she regained a pulse and blood pressure within a few minutes. She was then transferred to the emergency room, where she returned to her baseline clinical and neurologic status without symptoms.
AN UNRECOGNIZED DRUG INTERACTION
Asystole occurred in this patient because of the interaction of intravenous adenosine with the dipyridamole in the medication Aggrenox. Although adenosine, given during pharmacologic stress testing, is known to interact with various medications, the potential for this interaction may be overlooked if the culprit is present in a combination drug. Aggrenox is commonly given for secondary stroke prevention and should be discontinued before pharmacologic nuclear stress testing.
Pharmacologic stress testing involves two commonly used stress agents, adenosine and regadenoson (Lexiscan), which cause coronary vasodilation through their action on A2A receptors in the heart. Coronary vasodilation results in flow heterogeneity in the region of a stenotic artery, which can be detected with nuclear perfusion agents. In addition, adenosine has a short-lived effect on the A1 receptors that block atrioventricular conduction.1
Dipyridamole (Persantine) is contraindicated when either adenosine or regadenoson is used. Dipyridamole enhances the effect of exogenous and endogenous adenosine by inhibiting its uptake by cardiac cells, thus enhancing the action of these coronary vasodilators.2 Atrioventricular block is common during adenosine stress testing but is transient because adenosine has a short half-life (< 10 seconds), and complete heart block or asystole, as seen in this patient, is rare. Giving intravenous adenosine or regadenoson to patients on dipyridamole may have a marked effect on adenosine receptors, so that profound bradycardia and even asystole leading to cardiac collapse may occur. No data are available on the specific interaction of dipyridamole and regadenoson.
Even though the pharmacodynamics of the interaction between dipyridamole and adenosine are known,3 few reports are available detailing serious adverse events. The contraindication to pharmacologic stress testing in patients taking dipyridamole is noted in the American Society of Nuclear Cardiology Guidelines for stress protocols,4 which advise discontinuing dipyridamole-containing drugs at least 48 hours before the use of adenosine or regadenoson. Similarly, the American Heart Association guidelines5 for the management of supraventricular tachycardia recommend an initial dose of 3 mg of adenosine rather than 6 mg in patients who have been taking dipyridamole.
The dose of aminophylline for reversing the adverse effects of adenosine or regadenoson is 50 to 250 mg intravenously over 30 to 60 seconds. But since these adverse effects are short-lived once the infusion is stopped, aminophylline is usually given only if the adverse effects are severe, as in this patient.
Pharmacologic nuclear stress testing with adenosine receptor agonists (eg, adenosine or regadenoson) is contraindicated in patients taking dipyridamole or the combination pill Aggrenox because of the potential for profound bradyarrhythmias or asystole.
A 60-year-old woman was referred for pharmacologic nuclear stress testing before treatment for breast cancer. She had hypertension, diabetes mellitus, coronary artery disease, and a remote history of stroke, and she was taking clonidine (Catapres), labetalol (Normodyne, Trandate), furosemide (Lasix), hydralazine, valsartan (Diovan), insulin, and the aspirin-dipyridamole combination Aggrenox. Her vital signs and electrocardiogram before the stress test were normal.
The stress test was started with a standard protocol of adenosine (Adenoscan) infused intravenously over 4 minutes. For the first 2 minutes, she was stable and had no symptoms, but then sinus pauses and second-degree atrioventricular block type 2 developed, after which her heart stopped beating (Figure 1). The infusion was immediately stopped, but she became unresponsive and remained pulseless.
Cardiopulmonary resuscitation was started, aminophylline 100 mg was given intravenously, and she regained a pulse and blood pressure within a few minutes. She was then transferred to the emergency room, where she returned to her baseline clinical and neurologic status without symptoms.
AN UNRECOGNIZED DRUG INTERACTION
Asystole occurred in this patient because of the interaction of intravenous adenosine with the dipyridamole in the medication Aggrenox. Although adenosine, given during pharmacologic stress testing, is known to interact with various medications, the potential for this interaction may be overlooked if the culprit is present in a combination drug. Aggrenox is commonly given for secondary stroke prevention and should be discontinued before pharmacologic nuclear stress testing.
Pharmacologic stress testing involves two commonly used stress agents, adenosine and regadenoson (Lexiscan), which cause coronary vasodilation through their action on A2A receptors in the heart. Coronary vasodilation results in flow heterogeneity in the region of a stenotic artery, which can be detected with nuclear perfusion agents. In addition, adenosine has a short-lived effect on the A1 receptors that block atrioventricular conduction.1
Dipyridamole (Persantine) is contraindicated when either adenosine or regadenoson is used. Dipyridamole enhances the effect of exogenous and endogenous adenosine by inhibiting its uptake by cardiac cells, thus enhancing the action of these coronary vasodilators.2 Atrioventricular block is common during adenosine stress testing but is transient because adenosine has a short half-life (< 10 seconds), and complete heart block or asystole, as seen in this patient, is rare. Giving intravenous adenosine or regadenoson to patients on dipyridamole may have a marked effect on adenosine receptors, so that profound bradycardia and even asystole leading to cardiac collapse may occur. No data are available on the specific interaction of dipyridamole and regadenoson.
Even though the pharmacodynamics of the interaction between dipyridamole and adenosine are known,3 few reports are available detailing serious adverse events. The contraindication to pharmacologic stress testing in patients taking dipyridamole is noted in the American Society of Nuclear Cardiology Guidelines for stress protocols,4 which advise discontinuing dipyridamole-containing drugs at least 48 hours before the use of adenosine or regadenoson. Similarly, the American Heart Association guidelines5 for the management of supraventricular tachycardia recommend an initial dose of 3 mg of adenosine rather than 6 mg in patients who have been taking dipyridamole.
The dose of aminophylline for reversing the adverse effects of adenosine or regadenoson is 50 to 250 mg intravenously over 30 to 60 seconds. But since these adverse effects are short-lived once the infusion is stopped, aminophylline is usually given only if the adverse effects are severe, as in this patient.
Pharmacologic nuclear stress testing with adenosine receptor agonists (eg, adenosine or regadenoson) is contraindicated in patients taking dipyridamole or the combination pill Aggrenox because of the potential for profound bradyarrhythmias or asystole.
- Zoghbi GJ, Iskandrian AE. Selective adenosine agonists and myocardial perfusion imaging. J Nucl Cardiol 2012; 19:126–141.
- Lerman BB, Wesley RC, Belardinelli L. Electrophysiologic effects of dipyridamole on atrioventricular nodal conduction and supraventricular tachycardia. Role of endogenous adenosine. Circulation 1989; 80:1536–1543.
- Biaggioni I, Onrot J, Hollister AS, Robertson D. Cardiovascular effects of adenosine infusion in man and their modulation by dipyridamole. Life Sci 1986; 39:2229–2236.
- Henzlova MJ, Cerqueira MD, Mahmarian JJ, Yao SS; Quality Assurance Committee of the American Society of Nuclear Cardiology. Stress protocols and tracers. J Nucl Cardiol 2006; 13:e80–e90.
- ECC Committee, Subcommittees and Task Forces of the American Heart Association. 2005 American Heart Association guidelines for cardiopulmonary resuscitation and emergency cardiovascular care. Part 7.3: management of symptomatic bradycardia and tachycardia. Circulation 2005; 112(suppl 24):IV67–IV77.
- Zoghbi GJ, Iskandrian AE. Selective adenosine agonists and myocardial perfusion imaging. J Nucl Cardiol 2012; 19:126–141.
- Lerman BB, Wesley RC, Belardinelli L. Electrophysiologic effects of dipyridamole on atrioventricular nodal conduction and supraventricular tachycardia. Role of endogenous adenosine. Circulation 1989; 80:1536–1543.
- Biaggioni I, Onrot J, Hollister AS, Robertson D. Cardiovascular effects of adenosine infusion in man and their modulation by dipyridamole. Life Sci 1986; 39:2229–2236.
- Henzlova MJ, Cerqueira MD, Mahmarian JJ, Yao SS; Quality Assurance Committee of the American Society of Nuclear Cardiology. Stress protocols and tracers. J Nucl Cardiol 2006; 13:e80–e90.
- ECC Committee, Subcommittees and Task Forces of the American Heart Association. 2005 American Heart Association guidelines for cardiopulmonary resuscitation and emergency cardiovascular care. Part 7.3: management of symptomatic bradycardia and tachycardia. Circulation 2005; 112(suppl 24):IV67–IV77.
Niacin's effect on cardiovascular risk: Have we finally learned our lesson?
Randomized controlled trials have unequivocally shown that lowering levels of low-density lipoprotein cholesterol (LDL-C) with statins reduces the rate of cardiovascular events.1–3 Yet many patients still have heart attacks even though they are on statins, so the search continues for other agents to lower cardiovascular risk.4
Niacin has been used for its lipid-modifying effects for more than 50 years. In addition to being the most potent agent for raising the level of high-density lipoprotein cholesterol (HDL-C), niacin decreases the atherogenic lipids triglyceride, LDL-C, and lipoprotein (a)5 and can be very effective in treating mixed dyslipidemias such as hypertriglyceridemia and low HDL-C. This is particularly important for the challenging patients seen in preventive cardiology clinics.
In 1986, before statins were available, the Coronary Drug Project6 showed that immediate-release forms of niacin lowered the rates of nonfatal myocardial infarction and long-term mortality. Later, imaging studies demonstrated that niacin slows progression of carotid intima-medial thickness and coronary atherosclerosis.7–9 Furthermore, meta-analyses of these studies suggest cardiovascular benefit for patients at high vascular risk.10
However, niacin is difficult to use in clinical practice. The near-ubiquitous experience of flushing has limited our ability to give doses high enough to modify plasma lipid levels and rates of clinical events.
To try to mitigate this side effect, investigators developed extended-release formulations and agents such as laropiprant, a chemical antagonist of the interaction between niacin and epidermal prostanoid receptors implicated as the mechanism behind flushing. Although these innovations do not eliminate flushing, they reduce it, and thus have prompted hopes of using niacin more widely in statin-treated patients. However, whether widespread use of niacin on a background of statin therapy would have an impact on cardiovascular events remained to be established.
WHAT WE HAVE LEARNED LATELY ABOUT NIACIN?
More-tolerable formulations of niacin prompted interest in its potential to lower the residual cardiovascular risk observed in statin-treated patients. Two large clinical trials attempted to determine its impact on cardiovascular events in the contemporary era.
The AIM-HIGH study
In the Atherothrombosis Intervention in Metabolic Syndrome With Low HDL/High Triglycerides: Impact on Global Health Outcomes (AIM-HIGH) study,11 3,414 patients at high vascular risk with low HDL-C were treated with niacin or placebo. The trial was stopped early because of no evidence of clinical benefit with niacin and because of concern about an increased risk of stroke, a finding ultimately not observed on a complete review of the data.
I reviewed the limitations of this study earlier in this journal.12 The study was small, use of low-dose niacin was allowed in the placebo group, and physicians could treat high LDL-C as they saw fit during the study, so that more patients in the placebo group received high-dose statin therapy and ezetimibe. All of this likely limited the study’s ability to measure the clinical impact of niacin. As a result, this study was not a pure evaluation of the benefits of niacin vs placebo in addition to standard medical therapy. Hope remained that a much larger study with greater statistical power and a simpler design would provide a definitive answer.
HPS2–THRIVE
The Heart Protection Study 2–Treatment of HDL to Reduce the Incidence of Vascular Events (HPS2-THRIVE), with more than 40,000 patients, was the largest cardiovascular outcomes trial of lipid-modifying therapy to date.13 Its purpose was to determine whether extended-release niacin plus the prostanoid receptor antagonist laropiprant would reduce the rate of cardiovascular events in patients with clinically established vascular disease.
Patients age 50 to 80 with a history of myocardial infarction, ischemic stroke, transient ischemic attack, peripheral arterial disease, or diabetes with other forms of coronary heart disease received a standardized LDL-C-lowering regimen with simvastatin 40 mg daily, with or without ezetimibe 10 mg daily, to achieve a total cholesterol target of 135 mg/dL or below. All were treated with extended-release niacin 2 g daily plus laropiprant 40 mg daily for 1 month to assess compliance. They were then randomized to treatment with extended-release niacin 2 g plus laropiprant 40 mg or placebo daily. At baseline, the mean lipid values were LDL-C 63 mg/dL, HDL-C 44 mg/dL, and triglyceride 125 mg/dL.
Before the end of the trial, the investigators reported a high rate of myopathy-related adverse events in the niacin group, particularly in Chinese patients.13 This contributed to a high dropout rate in the niacin group, in which one quarter of patients stopped taking the study drug.
During the study, niacin lowered the LDL-C level by a mean of 10 mg/dL, lowered triglycerides by 33 mg/dL, and raised HDL-C by 6 mg/dL. On the basis of previous observational studies and randomized clinical trials, the authors calculated that such lipid changes should translate to a 10% to 15% reduction in vascular events. However, no reduction was observed in the primary end point of major vascular events, which included nonfatal myocardial infarction, coronary death, any nonfatal or fatal stroke, and any arterial revascularization, including amputation. The rates were 15% in the placebo group vs 14.5% in the niacin group (P = .96).
A statistically significant 10% reduction in the rate of arterial revascularization was observed in the niacin group, perhaps consistent with earlier observations of an antiatherosclerotic effect.
Subgroup analyses, while always to be interpreted with caution, also provide some interesting findings for consideration. A significant interaction was observed between treatment and baseline LDL-C, with those in the highest LDL-C tertile (> 77 mg/dL) demonstrating a potential reduction in the primary end point with niacin treatment. In addition, a trend toward potential benefit with niacin in patients in Europe, but not in China, was also observed; however, this just failed to meet statistical significance.
HPS2-THRIVE provided important information about the safety of extended-release niacin in combination with laropiprant. The niacin group experienced higher rates not only of myopathy but also of diabetic complications, new diagnosis of diabetes, serious infections, and bleeding. Whether these observations were related to niacin or to laropiprant is unknown. In fact, recent reports suggest laropiprant has adverse effects that may have substantially reduced the potential benefits of niacin.
The overall conclusion of HPS2-THRIVE was that there was no widespread clinical benefit from the combination of niacin and laropiprant in statin-treated patients with vascular disease, and that there was a potential increase in adverse events. Accordingly, the combination treatment will not be integrated into clinical practice.
WHERE DO WE GO FROM HERE?
Despite their limitations, these two large trials suggest that niacin does not reduce cardiovascular risk in patients already receiving a statin.
Might some subgroups be more likely to benefit from niacin? The finding of potential benefit in patients with higher baseline LDL-C suggests this may be true. At baseline, the HPS2-THRIVE patients had very good LDL-C control and had HDL-C levels within the normal range, not necessarily reflecting the patients we see in daily practice, who require more effective reductions in vascular risk. Furthermore, failure of both fibrates and niacin to reduce risk may have reflected the attempt to study these agents in broad patient populations as opposed to focusing on specific cohorts, such as patients with mixed dyslipidemia, for which there is suggestion of benefit.14 It seems unlikely that such a study will be performed in a clinical setting in which niacin may be of greater utility. The experience of adverse events would appear to make that a certainty.
For now, niacin will remain useful in lipid clinics for managing refractory dyslipidemia. Specifically, its ability to lower triglyceride and lipoprotein (a) and to raise HDL-C will continue to be of interest in the clinical management of patients and in the formulation of treatment guidelines. Another reason to use it is to lower LDL-C in patients who cannot tolerate statins. However, there is currently no evidence from randomized controlled trials to support its broader use.
While registry information could provide some sense of real-world effects of niacin’s use, this is a suboptimal way to evaluate the potential efficacy of a therapy—randomized controlled trials are the gold standard. The major flaws of both of the large trials of niacin point out the need for thoughtful study design to avoid incorrectly dismissing potentially useful therapies. But for now, the renaissance of niacin as a means of lowering cardiovascular risk is only wishful thinking.
- Randomised trial of cholesterol lowering in 4444 patients with coronary heart disease: the Scandinavian Simvastatin Survival Study (4S). Lancet 1994; 344:1383–1389.
- Heart Protection Study Collaborative Group. MRC/BHF Heart Protection Study of cholesterol lowering with simvastatin in 20,536 high-risk individuals: a randomised placebo-controlled trial. Lancet 2002; 360:7–22.
- Ridker PM, Danielson E, Fonseca FA, et al; JUPITER Study Group. Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N Engl J Med 2008; 359:2195–2207.
- Libby P. The forgotten majority: unfinished business in cardiovascular risk reduction. J Am Coll Cardiol 2005; 46:1225–1228.
- deLemos AS, Wolfe ML, Long CJ, Sivapackianathan R, Rader DJ. Identification of genetic variants in endothelial lipase in persons with elevated high-density lipoprotein cholesterol. Circulation 2002; 106:1321–1326.
- Canner PL, Berge KG, Wenger NK, et al. Fifteen year mortality in Coronary Drug Project patients: long-term benefit with niacin. J Am Coll Cardiol 1986; 8:1245–1255.
- Taylor AJ, Sullenberger LE, Lee HJ, Lee JK, Grace KA. Arterial Biology for the Investigation of the Treatment Effects of Reducing Cholesterol (ARBITER) 2: a double-blind, placebo-controlled study of extended-release niacin on atherosclerosis progression in secondary prevention patients treated with statins. Circulation 2004; 110:3512–3517.
- Taylor AJ, Lee HJ, Sullenberger LE. The effect of 24 months of combination statin and extended-release niacin on carotid intima-media thickness: ARBITER 3. Curr Med Res Opin 2006; 22:2243–2250.
- Brown BG, Zhao XQ, Chait A, et al. Simvastatin and niacin, antioxidant vitamins, or the combination for the prevention of coronary disease. N Engl J Med 2001; 345:1583–1592.
- Lavigne PM, Karas RH. The current state of niacin in cardiovascular disease prevention: a systematic review and meta-regression. J Am Coll Cardiol 2013; 61:440–446.
- AIM-HIGH Investigators; Boden WE, Probstfield JL, Anderson T, et al. Niacin in patients with low HDL cholesterol levels receiving intensive statin therapy. N Engl J Med 2011; 365:2255–2267.
- Nicholls SJ. Is niacin ineffective? Or did AIM-HIGH miss its target? Cleve Clin J Med 2012; 79:38–43.
- HPS2-THRIVE Collaborative Group. HPS2-THRIVE randomized placebo-controlled trial in 25 673 high-risk patients of ER niacin/laropiprant: trial design, pre-specified muscle and liver outcomes, and reasons for stopping study treatment. Eur Heart J 2013; 34:1279–1291.
- Jun M, Foote C, Lv J, et al. Effects of fibrates on cardiovascular outcomes: a systematic review and meta-analysis. Lancet 2010; 375:1875–1884.
Randomized controlled trials have unequivocally shown that lowering levels of low-density lipoprotein cholesterol (LDL-C) with statins reduces the rate of cardiovascular events.1–3 Yet many patients still have heart attacks even though they are on statins, so the search continues for other agents to lower cardiovascular risk.4
Niacin has been used for its lipid-modifying effects for more than 50 years. In addition to being the most potent agent for raising the level of high-density lipoprotein cholesterol (HDL-C), niacin decreases the atherogenic lipids triglyceride, LDL-C, and lipoprotein (a)5 and can be very effective in treating mixed dyslipidemias such as hypertriglyceridemia and low HDL-C. This is particularly important for the challenging patients seen in preventive cardiology clinics.
In 1986, before statins were available, the Coronary Drug Project6 showed that immediate-release forms of niacin lowered the rates of nonfatal myocardial infarction and long-term mortality. Later, imaging studies demonstrated that niacin slows progression of carotid intima-medial thickness and coronary atherosclerosis.7–9 Furthermore, meta-analyses of these studies suggest cardiovascular benefit for patients at high vascular risk.10
However, niacin is difficult to use in clinical practice. The near-ubiquitous experience of flushing has limited our ability to give doses high enough to modify plasma lipid levels and rates of clinical events.
To try to mitigate this side effect, investigators developed extended-release formulations and agents such as laropiprant, a chemical antagonist of the interaction between niacin and epidermal prostanoid receptors implicated as the mechanism behind flushing. Although these innovations do not eliminate flushing, they reduce it, and thus have prompted hopes of using niacin more widely in statin-treated patients. However, whether widespread use of niacin on a background of statin therapy would have an impact on cardiovascular events remained to be established.
WHAT WE HAVE LEARNED LATELY ABOUT NIACIN?
More-tolerable formulations of niacin prompted interest in its potential to lower the residual cardiovascular risk observed in statin-treated patients. Two large clinical trials attempted to determine its impact on cardiovascular events in the contemporary era.
The AIM-HIGH study
In the Atherothrombosis Intervention in Metabolic Syndrome With Low HDL/High Triglycerides: Impact on Global Health Outcomes (AIM-HIGH) study,11 3,414 patients at high vascular risk with low HDL-C were treated with niacin or placebo. The trial was stopped early because of no evidence of clinical benefit with niacin and because of concern about an increased risk of stroke, a finding ultimately not observed on a complete review of the data.
I reviewed the limitations of this study earlier in this journal.12 The study was small, use of low-dose niacin was allowed in the placebo group, and physicians could treat high LDL-C as they saw fit during the study, so that more patients in the placebo group received high-dose statin therapy and ezetimibe. All of this likely limited the study’s ability to measure the clinical impact of niacin. As a result, this study was not a pure evaluation of the benefits of niacin vs placebo in addition to standard medical therapy. Hope remained that a much larger study with greater statistical power and a simpler design would provide a definitive answer.
HPS2–THRIVE
The Heart Protection Study 2–Treatment of HDL to Reduce the Incidence of Vascular Events (HPS2-THRIVE), with more than 40,000 patients, was the largest cardiovascular outcomes trial of lipid-modifying therapy to date.13 Its purpose was to determine whether extended-release niacin plus the prostanoid receptor antagonist laropiprant would reduce the rate of cardiovascular events in patients with clinically established vascular disease.
Patients age 50 to 80 with a history of myocardial infarction, ischemic stroke, transient ischemic attack, peripheral arterial disease, or diabetes with other forms of coronary heart disease received a standardized LDL-C-lowering regimen with simvastatin 40 mg daily, with or without ezetimibe 10 mg daily, to achieve a total cholesterol target of 135 mg/dL or below. All were treated with extended-release niacin 2 g daily plus laropiprant 40 mg daily for 1 month to assess compliance. They were then randomized to treatment with extended-release niacin 2 g plus laropiprant 40 mg or placebo daily. At baseline, the mean lipid values were LDL-C 63 mg/dL, HDL-C 44 mg/dL, and triglyceride 125 mg/dL.
Before the end of the trial, the investigators reported a high rate of myopathy-related adverse events in the niacin group, particularly in Chinese patients.13 This contributed to a high dropout rate in the niacin group, in which one quarter of patients stopped taking the study drug.
During the study, niacin lowered the LDL-C level by a mean of 10 mg/dL, lowered triglycerides by 33 mg/dL, and raised HDL-C by 6 mg/dL. On the basis of previous observational studies and randomized clinical trials, the authors calculated that such lipid changes should translate to a 10% to 15% reduction in vascular events. However, no reduction was observed in the primary end point of major vascular events, which included nonfatal myocardial infarction, coronary death, any nonfatal or fatal stroke, and any arterial revascularization, including amputation. The rates were 15% in the placebo group vs 14.5% in the niacin group (P = .96).
A statistically significant 10% reduction in the rate of arterial revascularization was observed in the niacin group, perhaps consistent with earlier observations of an antiatherosclerotic effect.
Subgroup analyses, while always to be interpreted with caution, also provide some interesting findings for consideration. A significant interaction was observed between treatment and baseline LDL-C, with those in the highest LDL-C tertile (> 77 mg/dL) demonstrating a potential reduction in the primary end point with niacin treatment. In addition, a trend toward potential benefit with niacin in patients in Europe, but not in China, was also observed; however, this just failed to meet statistical significance.
HPS2-THRIVE provided important information about the safety of extended-release niacin in combination with laropiprant. The niacin group experienced higher rates not only of myopathy but also of diabetic complications, new diagnosis of diabetes, serious infections, and bleeding. Whether these observations were related to niacin or to laropiprant is unknown. In fact, recent reports suggest laropiprant has adverse effects that may have substantially reduced the potential benefits of niacin.
The overall conclusion of HPS2-THRIVE was that there was no widespread clinical benefit from the combination of niacin and laropiprant in statin-treated patients with vascular disease, and that there was a potential increase in adverse events. Accordingly, the combination treatment will not be integrated into clinical practice.
WHERE DO WE GO FROM HERE?
Despite their limitations, these two large trials suggest that niacin does not reduce cardiovascular risk in patients already receiving a statin.
Might some subgroups be more likely to benefit from niacin? The finding of potential benefit in patients with higher baseline LDL-C suggests this may be true. At baseline, the HPS2-THRIVE patients had very good LDL-C control and had HDL-C levels within the normal range, not necessarily reflecting the patients we see in daily practice, who require more effective reductions in vascular risk. Furthermore, failure of both fibrates and niacin to reduce risk may have reflected the attempt to study these agents in broad patient populations as opposed to focusing on specific cohorts, such as patients with mixed dyslipidemia, for which there is suggestion of benefit.14 It seems unlikely that such a study will be performed in a clinical setting in which niacin may be of greater utility. The experience of adverse events would appear to make that a certainty.
For now, niacin will remain useful in lipid clinics for managing refractory dyslipidemia. Specifically, its ability to lower triglyceride and lipoprotein (a) and to raise HDL-C will continue to be of interest in the clinical management of patients and in the formulation of treatment guidelines. Another reason to use it is to lower LDL-C in patients who cannot tolerate statins. However, there is currently no evidence from randomized controlled trials to support its broader use.
While registry information could provide some sense of real-world effects of niacin’s use, this is a suboptimal way to evaluate the potential efficacy of a therapy—randomized controlled trials are the gold standard. The major flaws of both of the large trials of niacin point out the need for thoughtful study design to avoid incorrectly dismissing potentially useful therapies. But for now, the renaissance of niacin as a means of lowering cardiovascular risk is only wishful thinking.
Randomized controlled trials have unequivocally shown that lowering levels of low-density lipoprotein cholesterol (LDL-C) with statins reduces the rate of cardiovascular events.1–3 Yet many patients still have heart attacks even though they are on statins, so the search continues for other agents to lower cardiovascular risk.4
Niacin has been used for its lipid-modifying effects for more than 50 years. In addition to being the most potent agent for raising the level of high-density lipoprotein cholesterol (HDL-C), niacin decreases the atherogenic lipids triglyceride, LDL-C, and lipoprotein (a)5 and can be very effective in treating mixed dyslipidemias such as hypertriglyceridemia and low HDL-C. This is particularly important for the challenging patients seen in preventive cardiology clinics.
In 1986, before statins were available, the Coronary Drug Project6 showed that immediate-release forms of niacin lowered the rates of nonfatal myocardial infarction and long-term mortality. Later, imaging studies demonstrated that niacin slows progression of carotid intima-medial thickness and coronary atherosclerosis.7–9 Furthermore, meta-analyses of these studies suggest cardiovascular benefit for patients at high vascular risk.10
However, niacin is difficult to use in clinical practice. The near-ubiquitous experience of flushing has limited our ability to give doses high enough to modify plasma lipid levels and rates of clinical events.
To try to mitigate this side effect, investigators developed extended-release formulations and agents such as laropiprant, a chemical antagonist of the interaction between niacin and epidermal prostanoid receptors implicated as the mechanism behind flushing. Although these innovations do not eliminate flushing, they reduce it, and thus have prompted hopes of using niacin more widely in statin-treated patients. However, whether widespread use of niacin on a background of statin therapy would have an impact on cardiovascular events remained to be established.
WHAT WE HAVE LEARNED LATELY ABOUT NIACIN?
More-tolerable formulations of niacin prompted interest in its potential to lower the residual cardiovascular risk observed in statin-treated patients. Two large clinical trials attempted to determine its impact on cardiovascular events in the contemporary era.
The AIM-HIGH study
In the Atherothrombosis Intervention in Metabolic Syndrome With Low HDL/High Triglycerides: Impact on Global Health Outcomes (AIM-HIGH) study,11 3,414 patients at high vascular risk with low HDL-C were treated with niacin or placebo. The trial was stopped early because of no evidence of clinical benefit with niacin and because of concern about an increased risk of stroke, a finding ultimately not observed on a complete review of the data.
I reviewed the limitations of this study earlier in this journal.12 The study was small, use of low-dose niacin was allowed in the placebo group, and physicians could treat high LDL-C as they saw fit during the study, so that more patients in the placebo group received high-dose statin therapy and ezetimibe. All of this likely limited the study’s ability to measure the clinical impact of niacin. As a result, this study was not a pure evaluation of the benefits of niacin vs placebo in addition to standard medical therapy. Hope remained that a much larger study with greater statistical power and a simpler design would provide a definitive answer.
HPS2–THRIVE
The Heart Protection Study 2–Treatment of HDL to Reduce the Incidence of Vascular Events (HPS2-THRIVE), with more than 40,000 patients, was the largest cardiovascular outcomes trial of lipid-modifying therapy to date.13 Its purpose was to determine whether extended-release niacin plus the prostanoid receptor antagonist laropiprant would reduce the rate of cardiovascular events in patients with clinically established vascular disease.
Patients age 50 to 80 with a history of myocardial infarction, ischemic stroke, transient ischemic attack, peripheral arterial disease, or diabetes with other forms of coronary heart disease received a standardized LDL-C-lowering regimen with simvastatin 40 mg daily, with or without ezetimibe 10 mg daily, to achieve a total cholesterol target of 135 mg/dL or below. All were treated with extended-release niacin 2 g daily plus laropiprant 40 mg daily for 1 month to assess compliance. They were then randomized to treatment with extended-release niacin 2 g plus laropiprant 40 mg or placebo daily. At baseline, the mean lipid values were LDL-C 63 mg/dL, HDL-C 44 mg/dL, and triglyceride 125 mg/dL.
Before the end of the trial, the investigators reported a high rate of myopathy-related adverse events in the niacin group, particularly in Chinese patients.13 This contributed to a high dropout rate in the niacin group, in which one quarter of patients stopped taking the study drug.
During the study, niacin lowered the LDL-C level by a mean of 10 mg/dL, lowered triglycerides by 33 mg/dL, and raised HDL-C by 6 mg/dL. On the basis of previous observational studies and randomized clinical trials, the authors calculated that such lipid changes should translate to a 10% to 15% reduction in vascular events. However, no reduction was observed in the primary end point of major vascular events, which included nonfatal myocardial infarction, coronary death, any nonfatal or fatal stroke, and any arterial revascularization, including amputation. The rates were 15% in the placebo group vs 14.5% in the niacin group (P = .96).
A statistically significant 10% reduction in the rate of arterial revascularization was observed in the niacin group, perhaps consistent with earlier observations of an antiatherosclerotic effect.
Subgroup analyses, while always to be interpreted with caution, also provide some interesting findings for consideration. A significant interaction was observed between treatment and baseline LDL-C, with those in the highest LDL-C tertile (> 77 mg/dL) demonstrating a potential reduction in the primary end point with niacin treatment. In addition, a trend toward potential benefit with niacin in patients in Europe, but not in China, was also observed; however, this just failed to meet statistical significance.
HPS2-THRIVE provided important information about the safety of extended-release niacin in combination with laropiprant. The niacin group experienced higher rates not only of myopathy but also of diabetic complications, new diagnosis of diabetes, serious infections, and bleeding. Whether these observations were related to niacin or to laropiprant is unknown. In fact, recent reports suggest laropiprant has adverse effects that may have substantially reduced the potential benefits of niacin.
The overall conclusion of HPS2-THRIVE was that there was no widespread clinical benefit from the combination of niacin and laropiprant in statin-treated patients with vascular disease, and that there was a potential increase in adverse events. Accordingly, the combination treatment will not be integrated into clinical practice.
WHERE DO WE GO FROM HERE?
Despite their limitations, these two large trials suggest that niacin does not reduce cardiovascular risk in patients already receiving a statin.
Might some subgroups be more likely to benefit from niacin? The finding of potential benefit in patients with higher baseline LDL-C suggests this may be true. At baseline, the HPS2-THRIVE patients had very good LDL-C control and had HDL-C levels within the normal range, not necessarily reflecting the patients we see in daily practice, who require more effective reductions in vascular risk. Furthermore, failure of both fibrates and niacin to reduce risk may have reflected the attempt to study these agents in broad patient populations as opposed to focusing on specific cohorts, such as patients with mixed dyslipidemia, for which there is suggestion of benefit.14 It seems unlikely that such a study will be performed in a clinical setting in which niacin may be of greater utility. The experience of adverse events would appear to make that a certainty.
For now, niacin will remain useful in lipid clinics for managing refractory dyslipidemia. Specifically, its ability to lower triglyceride and lipoprotein (a) and to raise HDL-C will continue to be of interest in the clinical management of patients and in the formulation of treatment guidelines. Another reason to use it is to lower LDL-C in patients who cannot tolerate statins. However, there is currently no evidence from randomized controlled trials to support its broader use.
While registry information could provide some sense of real-world effects of niacin’s use, this is a suboptimal way to evaluate the potential efficacy of a therapy—randomized controlled trials are the gold standard. The major flaws of both of the large trials of niacin point out the need for thoughtful study design to avoid incorrectly dismissing potentially useful therapies. But for now, the renaissance of niacin as a means of lowering cardiovascular risk is only wishful thinking.
- Randomised trial of cholesterol lowering in 4444 patients with coronary heart disease: the Scandinavian Simvastatin Survival Study (4S). Lancet 1994; 344:1383–1389.
- Heart Protection Study Collaborative Group. MRC/BHF Heart Protection Study of cholesterol lowering with simvastatin in 20,536 high-risk individuals: a randomised placebo-controlled trial. Lancet 2002; 360:7–22.
- Ridker PM, Danielson E, Fonseca FA, et al; JUPITER Study Group. Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N Engl J Med 2008; 359:2195–2207.
- Libby P. The forgotten majority: unfinished business in cardiovascular risk reduction. J Am Coll Cardiol 2005; 46:1225–1228.
- deLemos AS, Wolfe ML, Long CJ, Sivapackianathan R, Rader DJ. Identification of genetic variants in endothelial lipase in persons with elevated high-density lipoprotein cholesterol. Circulation 2002; 106:1321–1326.
- Canner PL, Berge KG, Wenger NK, et al. Fifteen year mortality in Coronary Drug Project patients: long-term benefit with niacin. J Am Coll Cardiol 1986; 8:1245–1255.
- Taylor AJ, Sullenberger LE, Lee HJ, Lee JK, Grace KA. Arterial Biology for the Investigation of the Treatment Effects of Reducing Cholesterol (ARBITER) 2: a double-blind, placebo-controlled study of extended-release niacin on atherosclerosis progression in secondary prevention patients treated with statins. Circulation 2004; 110:3512–3517.
- Taylor AJ, Lee HJ, Sullenberger LE. The effect of 24 months of combination statin and extended-release niacin on carotid intima-media thickness: ARBITER 3. Curr Med Res Opin 2006; 22:2243–2250.
- Brown BG, Zhao XQ, Chait A, et al. Simvastatin and niacin, antioxidant vitamins, or the combination for the prevention of coronary disease. N Engl J Med 2001; 345:1583–1592.
- Lavigne PM, Karas RH. The current state of niacin in cardiovascular disease prevention: a systematic review and meta-regression. J Am Coll Cardiol 2013; 61:440–446.
- AIM-HIGH Investigators; Boden WE, Probstfield JL, Anderson T, et al. Niacin in patients with low HDL cholesterol levels receiving intensive statin therapy. N Engl J Med 2011; 365:2255–2267.
- Nicholls SJ. Is niacin ineffective? Or did AIM-HIGH miss its target? Cleve Clin J Med 2012; 79:38–43.
- HPS2-THRIVE Collaborative Group. HPS2-THRIVE randomized placebo-controlled trial in 25 673 high-risk patients of ER niacin/laropiprant: trial design, pre-specified muscle and liver outcomes, and reasons for stopping study treatment. Eur Heart J 2013; 34:1279–1291.
- Jun M, Foote C, Lv J, et al. Effects of fibrates on cardiovascular outcomes: a systematic review and meta-analysis. Lancet 2010; 375:1875–1884.
- Randomised trial of cholesterol lowering in 4444 patients with coronary heart disease: the Scandinavian Simvastatin Survival Study (4S). Lancet 1994; 344:1383–1389.
- Heart Protection Study Collaborative Group. MRC/BHF Heart Protection Study of cholesterol lowering with simvastatin in 20,536 high-risk individuals: a randomised placebo-controlled trial. Lancet 2002; 360:7–22.
- Ridker PM, Danielson E, Fonseca FA, et al; JUPITER Study Group. Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N Engl J Med 2008; 359:2195–2207.
- Libby P. The forgotten majority: unfinished business in cardiovascular risk reduction. J Am Coll Cardiol 2005; 46:1225–1228.
- deLemos AS, Wolfe ML, Long CJ, Sivapackianathan R, Rader DJ. Identification of genetic variants in endothelial lipase in persons with elevated high-density lipoprotein cholesterol. Circulation 2002; 106:1321–1326.
- Canner PL, Berge KG, Wenger NK, et al. Fifteen year mortality in Coronary Drug Project patients: long-term benefit with niacin. J Am Coll Cardiol 1986; 8:1245–1255.
- Taylor AJ, Sullenberger LE, Lee HJ, Lee JK, Grace KA. Arterial Biology for the Investigation of the Treatment Effects of Reducing Cholesterol (ARBITER) 2: a double-blind, placebo-controlled study of extended-release niacin on atherosclerosis progression in secondary prevention patients treated with statins. Circulation 2004; 110:3512–3517.
- Taylor AJ, Lee HJ, Sullenberger LE. The effect of 24 months of combination statin and extended-release niacin on carotid intima-media thickness: ARBITER 3. Curr Med Res Opin 2006; 22:2243–2250.
- Brown BG, Zhao XQ, Chait A, et al. Simvastatin and niacin, antioxidant vitamins, or the combination for the prevention of coronary disease. N Engl J Med 2001; 345:1583–1592.
- Lavigne PM, Karas RH. The current state of niacin in cardiovascular disease prevention: a systematic review and meta-regression. J Am Coll Cardiol 2013; 61:440–446.
- AIM-HIGH Investigators; Boden WE, Probstfield JL, Anderson T, et al. Niacin in patients with low HDL cholesterol levels receiving intensive statin therapy. N Engl J Med 2011; 365:2255–2267.
- Nicholls SJ. Is niacin ineffective? Or did AIM-HIGH miss its target? Cleve Clin J Med 2012; 79:38–43.
- HPS2-THRIVE Collaborative Group. HPS2-THRIVE randomized placebo-controlled trial in 25 673 high-risk patients of ER niacin/laropiprant: trial design, pre-specified muscle and liver outcomes, and reasons for stopping study treatment. Eur Heart J 2013; 34:1279–1291.
- Jun M, Foote C, Lv J, et al. Effects of fibrates on cardiovascular outcomes: a systematic review and meta-analysis. Lancet 2010; 375:1875–1884.
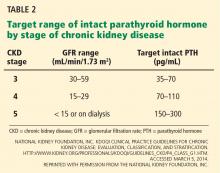
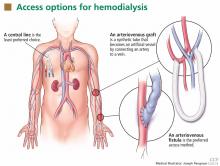
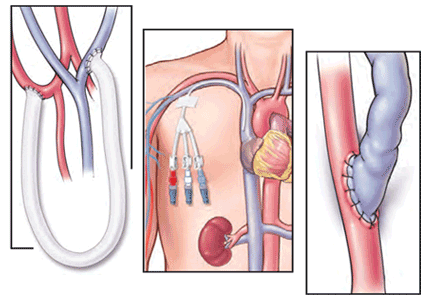
The generalist, the specialist, and the patient with chronic kidney disease
A key part of medical practice is managing professional relationships. This includes effective communication with each other: primary care provider, specialist, and patient in all permutations. I have previously written about how technologic advances both facilitate and hamper interphysician communication. But as payment models morph, as health systems become more complex and insulated, and as the medicine subspecialty workforce changes, the relationship between generalist and nonprocedural specialist will continue to evolve. I can offer personal testimony to the enormous value of sharing our electronic medical record with my nephrology colleagues within the institution; online (nondisruptive) management “conversation” is common in real time while I am with a patient in the office.
Gone is the time when referral was a necessary mechanism to build a practice, when a primary care physician would send everyone with an elevated alkaline phosphatase to the neighboring gastroenterologist, who in turn would send everyone without a primary care doctor to him or her. But there has always been the potential for professional, ego-based tension between primary care and nonprocedural specialist physicians, although this tension is rarely discussed. When does referral to a specialist by a general internist imply a lack of appropriate knowledge or an unwillingness to do an appropriate literature review? When should a specialist be concerned about “interfering” in primary care—by initiating more aggressive blood pressure control, or by giving the patient a needed vaccination? And what should be done if the patient decides to change the captain of the medical team? Maybe in the new medical care arena we will indeed function and be judged as a team, physician communication and transitions will be seamless, and all that matters will be the patient. Time will tell.
For now, the comanagement of patients with a chronic disease is often a challenge. The discussion by Sakhuja et al of patients with chronic kidney disease (CKD) highlights important clinical issues faced by primary care providers and nephrologists. With the increased diagnosis of early CKD, there may not be enough consulting nephrologists to see all these patients. And when CKD is diagnosed at an early stage, not all patients may warrant a specialist consultation. Yet the gaps in clinical care are clear. Too many patients with “a little” proteinuria or microhematuria do not get an adequate microscopic urinalysis to look for a treatable inflammatory renal disorder. Too many patients with a “slightly” elevated creatinine and blood pressure do not have their pressure aggressively treated, despite evidence that a systolic blood pressure in the high 130s is associated with more rapid progression of CKD. Should we establish expectations for ourselves, or should we just take a step back and refer all these patients to a nephrologist and await guidance? This is where I believe that a few clearly written and widely disseminated guidelines would help. Knowledge of appropriate and basic guidelines for diagnosing and managing common disorders (not just CKD) should be the focus of continuing medical education and should be required for maintaining certification for all internists, including specialists. But, as always, guidelines often need to be tailored for the patient in our examining room.
There are nuances in the care of patients with CKD that, as a nonspecialist, I will not automatically know need to be implemented. As an internist, I should know the value of starting inhibition of the angiotensin pathway in patients with proteinuria, but as CKD progresses in a specific patient, should this be decreased? Should I initiate urate-lowering therapy,1 hoping to slow the rate of my patient’s renal demise?
When do we know enough to know that we do not need to ask for a specialist’s input? How well do we self-assess our clinical knowledge and skills? How can we achieve the right balance between referral and self-management? We try to save our patient the cost of the time and the copayment to see a specialist, and with bundled care we try to minimize consultant fees and time. But in the meantime, are we ordering unnecessary tests or delaying appropriate therapy?
As we think about the comanagement of patients with CKD, we need to recognize and utilize the nuanced improvements in care that our nephrology colleagues can provide. As non-nephrologists, we should be able to start a thoughtful diagnostic evaluation. For example, an antinuclear antibody test in the absence of evidence of glomerulonephritis is not likely to be informative in determining the cause of an isolated elevated creatinine; a urinalysis is. We should be able to recognize potential renal injury (proteinuria, decreased glomerular filtration rate, microhematuria, hypertension), and initiate aggressive mitigation of factors that are known to enhance progression of the CKD (proteinuria, hypertension) and contribute to the significant morbidity and mortality of CKD-associated cardiovascular disease.
We should already be managing hypertension, diabetes, and hyperlipidemia, but CKD should be a red flag, driving us to more aggressively control these comorbidities, and driving us to do better than control only the estimated 46.4% of hypertensive patients in 2009 and 2010 whose hypertension was adequately controlled.2 There is no reason for us to step back and wait for direction in addressing these most common issues. And our specialist colleagues will be there to efficiently assist in refining the nuances of care.
- Levy GD, Rashid N, Niu F, Cheetham TC. Effect of urate-lowering therapies on renal disease progression in patients with hyperuricemia. J Rheumatol 2014; Apr 1, doi: 10.3899/jrheum.131159. Epub ahead of print.
- Guo F, He D, Zhang W, Walton RG. Trends in prevalence, awareness, management, and control of hypertension among United States adults, 1999 to 2010. J Am Coll Cardiol 2012; 60:599–606.
A key part of medical practice is managing professional relationships. This includes effective communication with each other: primary care provider, specialist, and patient in all permutations. I have previously written about how technologic advances both facilitate and hamper interphysician communication. But as payment models morph, as health systems become more complex and insulated, and as the medicine subspecialty workforce changes, the relationship between generalist and nonprocedural specialist will continue to evolve. I can offer personal testimony to the enormous value of sharing our electronic medical record with my nephrology colleagues within the institution; online (nondisruptive) management “conversation” is common in real time while I am with a patient in the office.
Gone is the time when referral was a necessary mechanism to build a practice, when a primary care physician would send everyone with an elevated alkaline phosphatase to the neighboring gastroenterologist, who in turn would send everyone without a primary care doctor to him or her. But there has always been the potential for professional, ego-based tension between primary care and nonprocedural specialist physicians, although this tension is rarely discussed. When does referral to a specialist by a general internist imply a lack of appropriate knowledge or an unwillingness to do an appropriate literature review? When should a specialist be concerned about “interfering” in primary care—by initiating more aggressive blood pressure control, or by giving the patient a needed vaccination? And what should be done if the patient decides to change the captain of the medical team? Maybe in the new medical care arena we will indeed function and be judged as a team, physician communication and transitions will be seamless, and all that matters will be the patient. Time will tell.
For now, the comanagement of patients with a chronic disease is often a challenge. The discussion by Sakhuja et al of patients with chronic kidney disease (CKD) highlights important clinical issues faced by primary care providers and nephrologists. With the increased diagnosis of early CKD, there may not be enough consulting nephrologists to see all these patients. And when CKD is diagnosed at an early stage, not all patients may warrant a specialist consultation. Yet the gaps in clinical care are clear. Too many patients with “a little” proteinuria or microhematuria do not get an adequate microscopic urinalysis to look for a treatable inflammatory renal disorder. Too many patients with a “slightly” elevated creatinine and blood pressure do not have their pressure aggressively treated, despite evidence that a systolic blood pressure in the high 130s is associated with more rapid progression of CKD. Should we establish expectations for ourselves, or should we just take a step back and refer all these patients to a nephrologist and await guidance? This is where I believe that a few clearly written and widely disseminated guidelines would help. Knowledge of appropriate and basic guidelines for diagnosing and managing common disorders (not just CKD) should be the focus of continuing medical education and should be required for maintaining certification for all internists, including specialists. But, as always, guidelines often need to be tailored for the patient in our examining room.
There are nuances in the care of patients with CKD that, as a nonspecialist, I will not automatically know need to be implemented. As an internist, I should know the value of starting inhibition of the angiotensin pathway in patients with proteinuria, but as CKD progresses in a specific patient, should this be decreased? Should I initiate urate-lowering therapy,1 hoping to slow the rate of my patient’s renal demise?
When do we know enough to know that we do not need to ask for a specialist’s input? How well do we self-assess our clinical knowledge and skills? How can we achieve the right balance between referral and self-management? We try to save our patient the cost of the time and the copayment to see a specialist, and with bundled care we try to minimize consultant fees and time. But in the meantime, are we ordering unnecessary tests or delaying appropriate therapy?
As we think about the comanagement of patients with CKD, we need to recognize and utilize the nuanced improvements in care that our nephrology colleagues can provide. As non-nephrologists, we should be able to start a thoughtful diagnostic evaluation. For example, an antinuclear antibody test in the absence of evidence of glomerulonephritis is not likely to be informative in determining the cause of an isolated elevated creatinine; a urinalysis is. We should be able to recognize potential renal injury (proteinuria, decreased glomerular filtration rate, microhematuria, hypertension), and initiate aggressive mitigation of factors that are known to enhance progression of the CKD (proteinuria, hypertension) and contribute to the significant morbidity and mortality of CKD-associated cardiovascular disease.
We should already be managing hypertension, diabetes, and hyperlipidemia, but CKD should be a red flag, driving us to more aggressively control these comorbidities, and driving us to do better than control only the estimated 46.4% of hypertensive patients in 2009 and 2010 whose hypertension was adequately controlled.2 There is no reason for us to step back and wait for direction in addressing these most common issues. And our specialist colleagues will be there to efficiently assist in refining the nuances of care.
A key part of medical practice is managing professional relationships. This includes effective communication with each other: primary care provider, specialist, and patient in all permutations. I have previously written about how technologic advances both facilitate and hamper interphysician communication. But as payment models morph, as health systems become more complex and insulated, and as the medicine subspecialty workforce changes, the relationship between generalist and nonprocedural specialist will continue to evolve. I can offer personal testimony to the enormous value of sharing our electronic medical record with my nephrology colleagues within the institution; online (nondisruptive) management “conversation” is common in real time while I am with a patient in the office.
Gone is the time when referral was a necessary mechanism to build a practice, when a primary care physician would send everyone with an elevated alkaline phosphatase to the neighboring gastroenterologist, who in turn would send everyone without a primary care doctor to him or her. But there has always been the potential for professional, ego-based tension between primary care and nonprocedural specialist physicians, although this tension is rarely discussed. When does referral to a specialist by a general internist imply a lack of appropriate knowledge or an unwillingness to do an appropriate literature review? When should a specialist be concerned about “interfering” in primary care—by initiating more aggressive blood pressure control, or by giving the patient a needed vaccination? And what should be done if the patient decides to change the captain of the medical team? Maybe in the new medical care arena we will indeed function and be judged as a team, physician communication and transitions will be seamless, and all that matters will be the patient. Time will tell.
For now, the comanagement of patients with a chronic disease is often a challenge. The discussion by Sakhuja et al of patients with chronic kidney disease (CKD) highlights important clinical issues faced by primary care providers and nephrologists. With the increased diagnosis of early CKD, there may not be enough consulting nephrologists to see all these patients. And when CKD is diagnosed at an early stage, not all patients may warrant a specialist consultation. Yet the gaps in clinical care are clear. Too many patients with “a little” proteinuria or microhematuria do not get an adequate microscopic urinalysis to look for a treatable inflammatory renal disorder. Too many patients with a “slightly” elevated creatinine and blood pressure do not have their pressure aggressively treated, despite evidence that a systolic blood pressure in the high 130s is associated with more rapid progression of CKD. Should we establish expectations for ourselves, or should we just take a step back and refer all these patients to a nephrologist and await guidance? This is where I believe that a few clearly written and widely disseminated guidelines would help. Knowledge of appropriate and basic guidelines for diagnosing and managing common disorders (not just CKD) should be the focus of continuing medical education and should be required for maintaining certification for all internists, including specialists. But, as always, guidelines often need to be tailored for the patient in our examining room.
There are nuances in the care of patients with CKD that, as a nonspecialist, I will not automatically know need to be implemented. As an internist, I should know the value of starting inhibition of the angiotensin pathway in patients with proteinuria, but as CKD progresses in a specific patient, should this be decreased? Should I initiate urate-lowering therapy,1 hoping to slow the rate of my patient’s renal demise?
When do we know enough to know that we do not need to ask for a specialist’s input? How well do we self-assess our clinical knowledge and skills? How can we achieve the right balance between referral and self-management? We try to save our patient the cost of the time and the copayment to see a specialist, and with bundled care we try to minimize consultant fees and time. But in the meantime, are we ordering unnecessary tests or delaying appropriate therapy?
As we think about the comanagement of patients with CKD, we need to recognize and utilize the nuanced improvements in care that our nephrology colleagues can provide. As non-nephrologists, we should be able to start a thoughtful diagnostic evaluation. For example, an antinuclear antibody test in the absence of evidence of glomerulonephritis is not likely to be informative in determining the cause of an isolated elevated creatinine; a urinalysis is. We should be able to recognize potential renal injury (proteinuria, decreased glomerular filtration rate, microhematuria, hypertension), and initiate aggressive mitigation of factors that are known to enhance progression of the CKD (proteinuria, hypertension) and contribute to the significant morbidity and mortality of CKD-associated cardiovascular disease.
We should already be managing hypertension, diabetes, and hyperlipidemia, but CKD should be a red flag, driving us to more aggressively control these comorbidities, and driving us to do better than control only the estimated 46.4% of hypertensive patients in 2009 and 2010 whose hypertension was adequately controlled.2 There is no reason for us to step back and wait for direction in addressing these most common issues. And our specialist colleagues will be there to efficiently assist in refining the nuances of care.
- Levy GD, Rashid N, Niu F, Cheetham TC. Effect of urate-lowering therapies on renal disease progression in patients with hyperuricemia. J Rheumatol 2014; Apr 1, doi: 10.3899/jrheum.131159. Epub ahead of print.
- Guo F, He D, Zhang W, Walton RG. Trends in prevalence, awareness, management, and control of hypertension among United States adults, 1999 to 2010. J Am Coll Cardiol 2012; 60:599–606.
- Levy GD, Rashid N, Niu F, Cheetham TC. Effect of urate-lowering therapies on renal disease progression in patients with hyperuricemia. J Rheumatol 2014; Apr 1, doi: 10.3899/jrheum.131159. Epub ahead of print.
- Guo F, He D, Zhang W, Walton RG. Trends in prevalence, awareness, management, and control of hypertension among United States adults, 1999 to 2010. J Am Coll Cardiol 2012; 60:599–606.
Managing advanced chronic kidney disease: A primary care guide
Accountable-care organizations are becoming more prominent in the United States, and therefore health care systems in the near future will be reimbursed on the basis of their ability to care for patient populations rather than individual patients. As a result, primary care physicians will need to be well versed in the care of patients with common chronic diseases such as chronic kidney disease (CKD). By one estimate, patients with CKD constitute 14% of the US population age 20 and older, or more than 31 million people.1
An earlier article in this journal reviewed how to identify patients with CKD and how to interpret the estimated glomerular filtration rate (GFR).2 This article examines the care of patients with advanced CKD, how to manage their health risks, and how to optimize their care by coordinating with nephrologists.
GOALS OF CKD CARE
CKD is defined either as renal damage (which is most commonly manifested by proteinuria, but which may include pathologic changes on biopsy or other markers of damage on serum, urine, or imaging studies), or as a GFR less than 60 mL/min/1.73 m2 for at least 3 months.3 It is divided into five stages (Table 1).
Since most patients with CKD never reach end-stage renal disease, much of their care is aimed at slowing the progression of renal dysfunction and addressing medical issues that arise as a result of CKD. To these ends, it is important to detect CKD early and refer these patients to a nephrology team in a timely manner. Their care can be separated into several important tasks:
- Identify the cause of CKD, if possible; address potentially reversible causes such as obstruction or medication-related causes. If a primarily glomerular process (marked by heavy proteinuria and dysmorphic red blood cells and red blood cell casts in the urine sediment) or interstitial nephritis (manifested by white blood cells in the urine) is suspected, refer to a nephrologist early.
- Provide treatment to correct the specific cause (if one is present) or slow the deterioration of renal function.
- Address cardiovascular risk factors.
- Address metabolic abnormalities related to CKD.
- If the CKD is advanced, educate the patient about end-stage renal disease and its treatment options, and guide the patient through the transition to end-stage renal disease.
WHEN SHOULD A NEPHROLOGIST BE CONSULTED?
The ideal timing of referral to a nephrologist is not well defined and depends on the comfort level of the primary care provider.
Treatments to slow the progression of CKD and decrease cardiovascular risk should begin early in CKD (ie, in stage 3) and can be managed by the primary care provider with guidance from a nephrologist. Patients referred to a nephrologist while in stage 3 have been shown to go longer without CKD progression than those referred in later stages.4 Early referral to a nephrologist has also been associated with a decreased mortality rate.5 The studies that found these trends, however, were limited by the fact that patients with stage 3 CKD are less likely to progress to end-stage renal disease or to die of cardiovascular disease than patients with stage 4 or 5 CKD.
Once stage 4 CKD develops, the nephrologist should take a more active role in the care plan. In this stage, cardiovascular risk rises, and the risk of developing end-stage renal disease rises dramatically.6 With comprehensive care in a CKD clinic, even patients with advanced CKD are more likely to have a stabilization of renal function.7 Kinchen et al8 found that patients referred to a nephrologist within 4 months of starting dialysis had a lower survival rate than those referred earlier. Therefore, if a nephrologist was not involved in the patient’s care prior to stage 4, then a referral must be made.
Recommendation. Patients with stage 3 CKD can be referred for an initial evaluation and development of a treatment plan, but most of the responsibility for their care can remain with the primary care provider. Once stage 4 CKD develops, the nephrologist should assume an increasing role. However, if glomerular disease is suspected, we recommend referral to a nephrologist regardless of the estimated GFR.
ELEVATED CARDIOVASCULAR RISK
Patients with stage 3 CKD are 20 times more likely to die of a cardiovascular event than to reach end-stage renal disease.6 This increased risk does not quite reach the status of a cardiovascular disease risk equivalent, as does diabetes,9,10 but cardiovascular risk reduction should be a primary focus of care for the CKD patient.
The cardiovascular risk in part is attributed to a high prevalence of traditional cardiovascular risk factors, including diabetes mellitus, hypertension, and hyperlipidemia.11,12 About two-thirds of CKD patients have metabolic syndrome, which is a risk factor for cardiovascular disease and is associated with more rapid progression of CKD.13 In addition, renal dysfunction, proteinuria, and hyperphosphatemia are also risk factors for cardiovascular disease.14–19
The risk of death from a cardiovascular event increases as kidney function declines, with reported 5-year death rates of 19.5% in stage 2, 24.3% in stage 3, and 45.7% in stage 4 CKD. However, imbalance between mortality risk and progression to end-stage renal disease may be age-dependent.20 Younger patients (age 45 and younger) are more likely to progress to end-stage renal disease, whereas in older patients (over age 65), the relative risk of dying of cardiovascular disease is higher.
Aggressive lipid management
Hyperlipidemia is a common risk factor for cardiovascular morbidity and mortality in CKD.21 However, until recently, all studies of outcomes of patients treated for hyperlipidemia excluded patients with CKD. Post hoc analyses of these studies 22–27 showed statins to be beneficial in primary and secondary cardiovascular prevention in patents with “normal” serum creatinine values but estimated GFR levels of 50 to 59 mL/min/1.73 m2.
The SHARP trial28 was the first prospective trial to study lipid-lowering therapy in patients with CKD. In this trial, patients with various stages of CKD, including advanced CKD, had fewer major vascular events if they received the combination of low-dose simvastatin (Zocor) and ezetimibe (Zetia). However, the evidence does not suggest that statin therapy slows the progression of CKD.28–31
Recommendation. Manage hyperlipidemia aggressively using statin therapy with or without ezetimibe, with a target low-density lipoprotein cholesterol level below 100 mg/dL.32
Manage other cardiovascular risk factors
Because hypertension and proteinuria are risk factors not only for cardiovascular disease but also for progression of CKD, they are discussed in the section below.
ATTEMPT TO PREVENT WORSENING OF RENAL FUNCTION
Medications to avoid
It is important to review a CKD patient’s medication list—prescription and over-the-counter drugs—to identify any that may contribute to a worsening of renal function. CKD patients need to be informed about avoiding medications such as nonsteroidal anti-inflammatory drugs, proton pump inhibitors, and herbal supplements because they can cause further renal injury. In addition, other medications (eg, metformin) are contraindicated in CKD because of side effects that may occur in CKD.
Patients should be encouraged to discuss any changes in their medications, including over-the-counter products, with their primary care physicians.
Manage hypertension aggressively
Many patients with CKD also have hypertension,33,34 possibly because they have a higher frequency of underlying essential hypertension or because CKD often worsens preexisting hypertension. Moreover, uncontrolled hypertension is associated with a further decline in renal function.35,36
The ACCORD trial37 found no benefit in lowering systolic blood pressure to less than 120 mm Hg compared with less than 140 mm Hg in patients with diabetes mellitus. (The patients in this study did not necessarily have CKD.)
A meta-analysis38 of trials of antihypertensive treatment in patients with CKD found that the optimal target systolic blood pressure for decreasing the progression of CKD was 110 to 129 mm Hg. The relative risk of progression of renal dysfunction was:
- 1.83 (95% confidence interval [CI] 0.97–3.44) at 130 mm to 139 mm Hg, vs
- 3.14 (95% CI 1.64–5.99) at 160 mm Hg or higher.
There is also evidence that blood pressure control can be relaxed as patients age. While the exact age differs among published guidelines, the evidence supports a goal blood pressure of less than 150/90 mm Hg once a patient reaches the age of 70, regardless of CKD or proteinuria.
Recommendation. Current evidence suggests the following blood pressure goals in CKD patients:
- With diabetes mellitus or proteinuria: < 130/80 mm Hg
- Without proteinuria: < 140/90 mm Hg
- Age 70 and older: <150/90 mm Hg.39
Angiotensin-converting enzyme (ACE) inhibitors and angiotensin receptor blockers (ARBs) are the preferred antihypertensive drugs in patients with diabetes or proteinuria (see below).
Manage proteinuria
Proteinuria is also associated with progression of CKD. AASK,40 a study that included nondiabetic African American patients whose estimated GFRs were between 20 and 60 mL/min/1.73 m2, showed that higher levels of proteinuria were associated with a higher risk of decline in GFR and a higher risk of end-stage renal disease. Findings were similar to those in studies of other CKD populations.41–43 Proteinuria is also an independent risk factor for cardiovascular disease and death. Multiple large studies16,17,44,45 have found associations between higher levels of albumin excretion and risk of major cardiovascular events, cardiovascular death, and death from any cause in people with and without diabetes.
Reducing proteinuria has been shown to both slow progression of renal dysfunction and reduce the cardiovascular risk.44,45 In a substudy of the IDNT46 in patients with diabetic nephropathy, each 50% reduction in urinary protein excretion was associated with a 56% reduction in risk of progression of CKD. Similar effects have been shown in nondiabetic CKD patients.47
ACE inhibitors and ARBs are the preferred treatments for proteinuria in patients with CKD.48–50 Combination therapy with an ACE inhibitor and an ARB has been used,51–53 with a better response in proteinuria reduction. However, combination therapy with these drugs cannot currently be recommended, as the only prospective study of this regimen to date suggested worse renal and overall outcomes in patients at high cardiovascular risk.54 These drugs may also have renoprotective effects independent of their effects on blood pressure and proteinuria.38 Dietary salt restriction and diuretic therapy can further increase the efficacy of proteinuria reduction by ACE inhibitors or ARBs.55,56
On the other hand, stopping ACE inhibitors or ARBs may be beneficial as the patient nears end-stage renal disease. Ahmed et al57 demonstrated that stopping ACE inhibitors or ARBs in advanced stage 4 CKD (mean estimated GFR 16 mL/min/1.73 m2) was associated with improved GFR and delayed onset of renal replacement therapy. This improvement may be due to regaining the slight decrease in GFR that occurred when these medications were started.
Nondihydropyridine calcium channel blockers such as diltiazem (Cardizem) and verapamil (Calan) have also been shown to be useful for reducing proteinuria,58 whereas dihydropyridine calcium channel blockers such as amlodipine (Norvasc) and nifedipine (Procardia), when used without ACE inhibitors or ARBs, can worsen proteinuria.58,59
Correct metabolic acidosis
The kidneys play an important role in maintaining acid-base balance, keeping the blood from becoming too acidic both by reabsorbing bicarbonate filtered into the urine by the glomerulus and by excreting the daily acid load. Metabolic acidosis can develop when these functions break down at more advanced stages of CKD, most often when the estimated GFR declines to less than 20 mL/min/1.73 m2.
Bicarbonate levels of 22 mmol/L or less have been associated with a higher risk of worsening renal function.60 When such patients were treated with sodium bicarbonate to achieve a serum bicarbonate of at least 23 mmol/L, they had an 80% lower rate of progression to end-stage renal disease without any increase in edema, admission for congestive heart failure, or change in blood pressure.61
Susantitaphong et al62 reviewed six randomized trials of bicarbonate supplementation in CKD and found that it was associated with improved kidney function and a 79% lower rate of progression to end-stage renal disease.
The proposed mechanism behind this benefit lies in the increase in ammonia production that each surviving nephron must undertake to handle the daily acid load. The increased ammonia is thought to play a role in activating the alternative complement pathway,63 causing renal inflammation and injury.
Recommendation. Bicarbonate therapy should be used to maintain serum bicarbonate levels above 22 mmol/L in CKD.64
OTHER ASPECTS OF CKD CARE
Bone mineral disorders
Patients with CKD develop secondary hyperparathyroidism, hyperphosphatemia, and (in advanced CKD) hypocalcemia, all leading to disorders of bone mineral metabolism.
Traditionally, it has been thought that decreased production of 1,25-dihydroxyvitamin D by dysfunctional kidneys leads to decreased suppression of the parathyroid gland and to secondary hyperparathyroidism. The major long-term adverse effect of this is a weakened bone matrix resulting from increased calcium and phosphorus efflux from bones (renal osteodystrophy).
The discovery of fibroblast growth factor 23 (FGF-23) has improved our understanding of the physiology behind disordered bone mineral metabolism in CKD. FGF-23, produced by osteoblasts and osteocytes, acts directly on the kidney to increase renal phosphate excretion. It also suppresses 1,25-dihydroxyvitamin D levels by inhibiting 1-alpha-hydroxylase,65 and it stimulates parathyroid hormone secretion. FGF-23 levels rise much earlier in CKD than do parathyroid hormone levels, suggesting that abnormalities in phosphorus balance and FGF-23 may be the earliest pathophysiologic changes.66
The initial treatment of bone mineral disorders is to some extent guided by laboratory values. Phosphate levels higher than 3.5 or 4 mg/dL and elevated FGF-23 levels have been associated with increased mortality rates in CKD patients.18,19,67–69 All patients should also have their 1,25-dihydroxyvitamin D level checked and supplemented if deficient. In many patients with early stage 3 CKD, this may correct secondary hyperparathyroidism.70
Serum phosphorus levels should be kept in the normal range in stage 3 and 4 CKD,71 either by restricting dietary phosphorus intake (< 800 or < 1,000 mg/day) or by using a phosphate binder, which is taken with meals to prevent phosphorus absorption from the gastrointestinal tract. Current US recommendations are to allow graded increases in parathyroid hormone based on the stage of CKD (Table 2).71 However, these targets are still an area of uncertainty, with some guidelines suggesting that wider variations in parathyroid hormone can be allowed, so there may be wider variation in clinical practice in this area.72 If the serum phosphorus level is in the goal range but parathyroid hormone levels are still high, an activated vitamin D analogue such as calcitriol is recommended, although with the emerging role of FGF-23, some experts also call for early use of a phosphate binder in this group.
The treatment of bone mineral disorders in CKD is fairly complex, and we recommend that it be done by or with the close direction of a nephrologist.
Recommendations on bone disorders
- Check levels of calcium, phosphorus, 25-hydroxyvitamin D, and parathyroid hormone in all patients whose estimated GFR is less than 60 mL/min/1.73 m2, with frequency of measurements based on the stage of CKD.71
- Replace vitamin D if deficient.
- Treat elevated phosphorus levels with a protein-restricted diet (nutrition referral) and a phosphate binder.
- Treat elevated hyperparathyroid hormone levels with a vitamin D analogue once phosphorus levels have been controlled.
- Refer patients with an elevated phosphorus or parathyroid hormone level to a nephrology service for consultation before initiating medical therapy.
Anemia is common, treatment controversial
The treatment of anemia attributed to CKD has been a topic of controversy over the past decade, and we recommend that it be done with the guidance of a nephrologist.
Anemia is common in CKD, and declining kidney function is an independent predictor of anemia.73 Anemia is a risk factor for left ventricular hypertrophy, cardiovascular disease,74 and death in CKD.75
The anemia of CKD is attributed to relative erythropoietin deficiency and bone marrow resistance to erythropoietin, but this is a diagnosis of exclusion, and other causes of anemia must be ruled out. Iron deficiency is a common cause of anemia in CKD, and treatment of iron deficiency may correct anemia in more than one-third of these patients.76,77
Erythropoiesis-stimulating agents such as epoetin alfa (Procrit) and darbepoetin (Aranesp) are used to treat renal anemia. However, the target hemoglobin level has been a subject of debate. Three prospective trials78–80 found no benefit in raising the hemoglobin level to normal ranges using these agents, and several found an association with higher rates of stroke and venous thrombosis. The US Food and Drug Administration suggests that the only role for these agents in CKD is to avoid the need for transfusions. They should not be used to normalize the hemoglobin level. The target, although not explicitly specified, is suggested to be around 10 g/dL.81
PREPARE FOR END-STAGE RENAL DISEASE
Discuss the options
Because the risk of developing end-stage renal disease rises dramatically once CKD reaches stage 4, all such patients should have a discussion about renal replacement therapy. They should be educated about their options for treatment (hemodialysis, peritoneal dialysis, and transplantation, as well as not proceeding with renal replacement therapy), often in a formal class. They should then be actively engaged in the decision about how to proceed. Survival and quality of life should be discussed, particularly with patients who are over age 80, who are severely ill, or who are living in a nursing facility, as these groups get limited survival benefit from starting dialysis, and quality of life may actually decrease with dialysis.82,83
The Renal Physicians Association has created clinical practice guidelines for shared decision-making, consisting of 10 practice recommendations that outline a systematic approach to patients needing renal replacement therapy.84
Consider preemptive kidney transplantation
Any patient thought to be a suitable candidate for renal transplantation should be referred to a transplantation center for evaluation. Studies have shown that kidney transplantation offers a survival advantage compared with chronic dialysis and should preferably be done preemptively, ie, before dialysis is required.85–90 Therefore, patients with estimated GFRs in the low 20s should be referred for a transplantation evaluation.
If a living donor is available, the transplantation team usually waits to perform the procedure until the patient is closer to needing dialysis, often when the estimated GFR is around 15 to 16 mL/min/1.73 m2. If no living donor is available, the patient can earn time on the deceased-donor waiting list once his or her estimated GFR falls to below 20 mL/min/1.7 m2.
Plan for dialysis access
Patients starting hemodialysis first need to undergo a procedure to provide access to the blood. The three options are an arteriovenous fistula, an arteriovenous graft, and a central venous catheter (Figure 1).
An arteriovenous fistula is the best option, being the most durable, followed by a graft and then a catheter.91 Arteriovenous fistulas also have the lowest rates of infection,92 thrombosis,93 and intervention to maintain patency.93
The fistula is created by ligating a vein draining an extremity, most often the nondominant arm, and anastomosing the vein to an artery. The higher arterial pressure causes the vein to dilate and thicken (“arterialize”), thus making it able to withstand repeated cannulation necessary for hemodialysis.
An arteriovenous fistula typically takes 1 to 3 months to “mature” to the point where it can be used,94,95 and, depending on the patient and experience of the vascular surgeon, a significant number may never mature. Thus, it is important to discuss hemodialysis access before the patient reaches end-stage renal disease so that he or she can be referred to a vascular surgeon early, when the estimated GFR is about 20 mL/min/1.73 m2.
An arteriovenous graft. Not all patients have suitable vessels for creation of an arteriovenous fistula. In such patients, an arteriovenous graft, typically made of polytetrafluoroethylene, is the next best option. The graft is typically ready to use in 2 weeks and thus does not require as much advance planning. Grafts tend to narrow more often than fistulas and require more procedures to keep them patent.
A central venous catheter is most often inserted into the internal jugular vein and tunneled under the skin to exit in an area covered by the patient’s shirt.
Tunneled dialysis catheters are associated with higher rates of infection, thrombosis, and overall mortality and are therefore the least preferred choice. They are reserved for patients who have not had advance planning for end-stage renal disease, who do not have acceptable vessels for an arteriovenous fistula or graft, or who have refused surgical access.
Protect the fistula arm. It is recommended that venipuncture, intravenous lines, and blood pressure measurements be avoided in the nondominant upper arm of patients with stage 4 and 5 CKD to protect those veins for the potential creation of an arteriovenous fistula.96 For the same reason, peripherally inserted central catheter lines and subclavian catheters should be avoided in these patients. If an arteriovenous fistula has already been placed, this arm must be protected from such procedures at all times.
Studies have shown that late referral to a nephrologist is associated with a lower incidence of starting dialysis with a permanent vascular access.97,98
If the patient wishes to start peritoneal dialysis, the peritoneal dialysis catheter can usually be used 2 weeks after being inserted.
Starting dialysis
The appropriate time for starting dialysis remains controversial, especially in elderly patients with multiple comorbid conditions.
The IDEAL study99 found no benefit in starting dialysis at a GFR of 10 to 14 mL/min compared with 5 to 7 mL/min. Thus, there is no single estimated GFR at which dialysis should be started. Rather, the development of early uremic symptoms and the patient’s quality of life should guide this decision.82,83,99–101
Hemodialysis involves three sessions per week, each taking about 4 hours. Evidence suggests that longer sessions or more sessions per week may offer benefits, especially in terms of blood pressure, volume, and dietary management. This has led to an increase in the popularity of home and in-center nocturnal hemodialysis programs across the United States.
Peritoneal dialysis?
Peritoneal dialysis is an excellent choice for patients who are motivated, can care for themselves at home, and have a support system available to assist them if needed. It allows for daily dialysis, less fluid restriction, and less dietary restriction, and it gives the patient an opportunity to stay independent. It also spares the veins in the arms, which may be needed for vascular access later in life if hemodialysis is needed.
Recommendation. We recommend that peritoneal dialysis be offered to any suitable patient who is approaching end-stage renal disease.
A COMPREHENSIVE, COLLABORATIVE APPROACH
Chronic kidney disease is a multisystem disorder, and its management requires a comprehensive approach (Table 3). Early detection and interventions are key to reducing cardiovascular events and progression to kidney failure.
Early referral to a nephrologist and team collaboration between the primary care provider, the nephrologist, and other health care providers are essential. Early in the course of CKD, it may be appropriate for a nephrologist to evaluate the patient and recommend a set of treatment goals. Follow-up may be infrequent or unnecessary.
As CKD progresses, especially as the patient reaches an estimated GFR of 30 mL/min/1.73 m2, the nephrologist will take a more active role in the patient’s care and medical decision-making. In some circumstances, it may even be appropriate for the nephrologist to be the patient’s source of primary care, with the primary care provider as a consultant.
Caring for patients with CKD includes not only strategies to preserve renal function and prolong survival, but also making critical decisions about starting dialysis and about the need for transplantation. Early involvement of a nephrologist and early preparation for end-stage renal disease with preemptive transplantation and arteriovenous fistula placement are associated with better patient outcomes. Key to this is collaboration between the primary care provider and the nephrologist, with levels of responsibility for patient care that adapt to the patient’s degree of renal dysfunction and other comorbidities. Such strategies to select patients for timely nephrology referral may help improve outcomes in this vulnerable population.
- United States Renal Data System (USRDS). Identification and care of patients with CKD. http://www.usrds.org/2012/pdf/v1_ch2_12.pdf. Accessed March 5, 2014.
- Simon J, Amde M, Poggio ED. Interpreting the estimated glomerular filtration rate in primary care: benefits and pitfalls. Cleve Clin J Med 2011; 78:189–195.
- National Kidney Foundation, Inc. KDOQI Clinical Practice Guidelines for Chronic Kidney Disease: Evaluation, Classification, and Stratification. http://www.kidney.org/professionals/kdoqi/guidelines_ckd/p4_class_g1.htm. Accessed March 5, 2014.
- Orlando LA, Owen WF, Matchar DB. Relationship between nephrologist care and progression of chronic kidney disease. N C Med J 2007; 68:9–16.
- Tseng CL, Kern EF, Miller DR, et al. Survival benefit of nephrologic care in patients with diabetes mellitus and chronic kidney disease. Arch Intern Med 2008; 168:55–62.
- Keith DS, Nichols GA, Gullion CM, Brown JB, Smith DH. Longitudinal follow-up and outcomes among a population with chronic kidney disease in a large managed care organization. Arch Intern Med 2004; 164:659–663.
- Serrano A, Huang J, Ghossein C, et al. Stabilization of glomerular filtration rate in advanced chronic kidney disease: a two-year follow-up of a cohort of chronic kidney disease patients stages 4 and 5. Adv Chronic Kidney Dis 2007; 14:105–112.
- Kinchen KS, Sadler J, Fink N, et al. The timing of specialist evaluation in chronic kidney disease and mortality. Ann Intern Med 2002; 137:479–486.
- Tonelli M, Muntner P, Lloyd A, et al; Alberta Kidney Disease Network. Risk of coronary events in people with chronic kidney disease compared with those with diabetes: a population-level cohort study. Lancet 2012; 380:807–814.
- Wattanakit K, Coresh J, Muntner P, Marsh J, Folsom AR. Cardiovascular risk among adults with chronic kidney disease, with or without prior myocardial infarction. J Am Coll Cardiol 2006; 48:1183–1189.
- Foley RN, Wang C, Collins AJ. Cardiovascular risk factor profiles and kidney function stage in the US general population: the NHANES III study. Mayo Clin Proc 2005; 80:1270–1277.
- Muntner P, He J, Astor BC, Folsom AR, Coresh J. Traditional and nontraditional risk factors predict coronary heart disease in chronic kidney disease: results from the Atherosclerosis Risk in Communities Study. J Am Soc Nephrol 2005; 16:529–538.
- Navaneethan SD, Schold JD, Kirwan JP, et al. Metabolic syndrome, ESRD, and death in CKD. Clin J Am Soc Nephrol 2013; 8:945–952.
- Foley RN, Murray AM, Li S, et al. Chronic kidney disease and the risk for cardiovascular disease, renal replacement, and death in the United States Medicare population, 1998 to 1999. J Am Soc Nephrol 2005; 16:489–495.
- Weiner DE, Tighiouart H, Amin MG, et al. Chronic kidney disease as a risk factor for cardiovascular disease and all-cause mortality: a pooled analysis of community-based studies. J Am Soc Nephrol 2004; 15:1307–1315.
- Gerstein HC, Mann JF, Yi Q, et al; HOPE Study Investigators. Albuminuria and risk of cardiovascular events, death, and heart failure in diabetic and nondiabetic individuals. JAMA 2001; 286:421–426.
- Hillege HL, Fidler V, Diercks GF, et al; Prevention of Renal and Vascular End Stage Disease (PREVEND) Study Group. Urinary albumin excretion predicts cardiovascular and noncardiovascular mortality in general population. Circulation 2002; 106:1777–1782.
- Foley RN, Collins AJ, Ishani A, Kalra PA. Calcium-phosphate levels and cardiovascular disease in community-dwelling adults: the Atherosclerosis Risk in Communities (ARIC) Study. Am Heart J 2008; 156:556–563.
- Tonelli M, Sacks F, Pfeffer M, Gao Z, Curhan G; Cholesterol And Recurrent Events Trial Investigators. Relation between serum phosphate level and cardiovascular event rate in people with coronary disease. Circulation 2005; 112:2627–2633.
- Menon V, Wang X, Sarnak MJ, et al. Long-term outcomes in nondiabetic chronic kidney disease. Kidney Int 2008; 73:1310–1315.
- Kasiske BL. Hyperlipidemia in patients with chronic renal disease. Am J Kidney Dis 1998; 32(suppl 3):S142–S156.
- Kendrick J, Shlipak MG, Targher G, Cook T, Lindenfeld J, Chonchol M. Effect of lovastatin on primary prevention of cardiovascular events in mild CKD and kidney function loss: a post hoc analysis of the Air Force/Texas Coronary Atherosclerosis Prevention Study. Am J Kidney Dis 2010; 55:42–49.
- Colhoun HM, Betteridge DJ, Durrington PN, et al; CARDS Investigators. Effects of atorvastatin on kidney outcomes and cardiovascular disease in patients with diabetes: an analysis from the Collaborative Atorvastatin Diabetes Study (CARDS). Am J Kidney Dis 2009; 54:810–819.
- Koren MJ, Davidson MH, Wilson DJ, Fayyad RS, Zuckerman A, Reed DP; ALLIANCE Investigators. Focused atorvastatin therapy in managed-care patients with coronary heart disease and CKD. Am J Kidney Dis 2009; 53:741–750.
- Fellström BC, Jardine AG, Schmieder RE, et al; AURORA Study Group. Rosuvastatin and cardiovascular events in patients undergoing hemodialysis. N Engl J Med 2009; 360:1395–1407.
- Chonchol M, Cook T, Kjekshus J, Pedersen TR, Lindenfeld J. Simvastatin for secondary prevention of all-cause mortality and major coronary events in patients with mild chronic renal insufficiency. Am J Kidney Dis 2007; 49:373–382.
- Ridker PM, MacFadyen J, Cressman M, Glynn RJ. Efficacy of rosuvastatin among men and women with moderate chronic kidney disease and elevated high-sensitivity C-reactive protein: a secondary analysis from the JUPITER (Justification for the Use of Statins in Prevention-an Intervention Trial Evaluating Rosuvastatin) trial. J Am Coll Cardiol 2010; 55:1266–1273.
- Baigent C, Landray MJ, Reith C, et al; SHARP Investigators. The effects of lowering LDL cholesterol with simvastatin plus ezetimibe in patients with chronic kidney disease (Study of Heart and Renal Protection): a randomised placebo-controlled trial. Lancet 2011; 377:2181–2192.
- Shepherd J, Kastelein JJ, Bittner V, et al; Treating to New Targets Investigators. Effect of intensive lipid lowering with atorvastatin on renal function in patients with coronary heart disease: the Treating to New Targets (TNT) study. Clin J Am Soc Nephrol 2007; 2:1131–1139.
- Tonelli M, Isles C, Craven T, et al. Effect of pravastatin on rate of kidney function loss in people with or at risk for coronary disease. Circulation 2005; 112:171–178.
- Palmer SC, Craig JC, Navaneethan SD, Tonelli M, Pellegrini F, Strippoli GF. Benefits and harms of statin therapy for persons with chronic kidney disease: a systematic review and meta-analysis. Ann Intern Med 2012; 157:263–275.
- National Kidney Foundation, Inc. KDOQI Clinical Practice Guidelines for Managing Dyslipidemias in Chronic Kidney Disease. http://www.kidney.org/professionals/kdoqi/guidelines_lipids/. Accessed March 5, 2014.
- Buckalew VM, Berg RL, Wang SR, Porush JG, Rauch S, Schulman G. Prevalence of hypertension in 1,795 subjects with chronic renal disease: the modification of diet in renal disease study baseline cohort. Modification of Diet in Renal Disease Study Group. Am J Kidney Dis 1996; 28:811–821.
- Coresh J, Wei GL, McQuillan G, et al. Prevalence of high blood pressure and elevated serum creatinine level in the United States: findings from the third National Health and Nutrition Examination Survey (1988–1994). Arch Intern Med 2001; 161:1207–1216.
- Klag MJ, Whelton PK, Randall BL, et al. Blood pressure and end-stage renal disease in men. N Engl J Med 1996; 334:13–18.
- Locatelli F, Marcelli D, Comelli M, et al. Proteinuria and blood pressure as causal components of progression to end-stage renal failure. Northern Italian Cooperative Study Group. Nephrol Dial Transplant 1996; 11:461–467.
- ACCORD Study Group; Cushman WC, Evans GW, Byington RP, et al. Effects of intensive blood-pressure control in type 2 diabetes mellitus. N Engl J Med 2010; 362:1575–1585.
- Jafar TH, Stark PC, Schmid CH, et al. Progression of chronic kidney disease: the role of blood pressure control, proteinuria, and angiotensin-converting enzyme inhibition: a patient-level meta-analysis. Ann Intern Med 2003; 139:244–252.
- Khosla N, Bakris G. Lessons learned from recent hypertension trials about kidney disease. Clin J Am Soc Nephrol 2006; 1:229–235.
- Norris KC, Greene T, Kopple J, et al. Baseline predictors of renal disease progression in the African American Study of Hypertension and Kidney Disease. J Am Soc Nephrol 2006; 17:2928–2936.
- Keane WF, Brenner BM, de Zeeuw D, et al; RENAAL Study Investigators. The risk of developing end-stage renal disease in patients with type 2 diabetes and nephropathy: the RENAAL study. Kidney Int 2003; 63:1499–1507.
- Ruggenenti P, Perna A, Mosconi L, et al. Proteinuria predicts end-stage renal failure in non-diabetic chronic nephropathies. The “Gruppo Italiano di Studi Epidemiologici in Nefrologia” (GISEN). Kidney Int Suppl 1997; 63:S54–S57.
- de Goeij MC, Liem M, de Jager DJ, et al; PREPARE-1 Study Group. Proteinuria as a risk marker for the progression of chronic kidney disease in patients on predialysis care and the role of angiotensin-converting enzyme inhibitor/angiotensin II receptor blocker treatment. Nephron Clin Pract 2012; 121:c73–c82.
- de Zeeuw D, Remuzzi G, Parving HH, et al. Albuminuria, a therapeutic target for cardiovascular protection in type 2 diabetic patients with nephropathy. Circulation 2004; 110:921–927.
- Ibsen H, Olsen MH, Wachtell K, et al. Reduction in albuminuria translates to reduction in cardiovascular events in hypertensive patients: losartan intervention for endpoint reduction in hypertension study. Hypertension 2005; 45:198–202.
- Atkins RC, Briganti EM, Lewis JB, et al. Proteinuria reduction and progression to renal failure in patients with type 2 diabetes mellitus and overt nephropathy. Am J Kidney Dis 2005; 45:281–287.
- Jafar TH, Stark PC, Schmid CH, et al; AIPRD Study Group; Angiotensin-Converting Enzyme Inhibition and Progression of Renal Disease. Proteinuria as a modifiable risk factor for the progression of non-diabetic renal disease. Kidney Int 2001; 60:1131–1140.
- ACE Inhibitors in Diabetic Nephropathy Trialist Group. Should all patients with type 1 diabetes mellitus and microalbuminuria receive angiotensin-converting enzyme inhibitors? A meta-analysis of individual patient data. Ann Intern Med 2001; 134:370–379.
- Casas JP, Chua W, Loukogeorgakis S, et al. Effect of inhibitors of the renin-angiotensin system and other antihypertensive drugs on renal outcomes: systematic review and meta-analysis. Lancet 2005; 366:2026–2033.
- Strippoli GF, Craig M, Deeks JJ, Schena FP, Craig JC. Effects of angiotensin converting enzyme inhibitors and angiotensin II receptor antagonists on mortality and renal outcomes in diabetic nephropathy: systematic review. BMJ 2004; 329:828.
- MacKinnon M, Shurraw S, Akbari A, Knoll GA, Jaffey J, Clark HD. Combination therapy with an angiotensin receptor blocker and an ACE inhibitor in proteinuric renal disease: a systematic review of the efficacy and safety data. Am J Kidney Dis 2006; 48:8–20.
- Kunz R, Friedrich C, Wolbers M, Mann JF. Meta-analysis: effect of mono-therapy and combination therapy with inhibitors of the renin angiotensin system on proteinuria in renal disease. Ann Intern Med 2008; 148:30–48.
- Ruggenenti P, Perticucci E, Cravedi P, et al. Role of remission clinics in the longitudinal treatment of CKD. J Am Soc Nephrol 2008; 19:1213–1224.
- Mann JF, Schmieder RE, McQueen M, et al; ONTARGET investigators. Renal outcomes with telmisartan, ramipril, or both, in people at high vascular risk (the ONTARGET study): a multicentre, randomised, double-blind, controlled trial. Lancet 2008; 372:547–553.
- Esnault VL, Ekhlas A, Delcroix C, Moutel MG, Nguyen JM. Diuretic and enhanced sodium restriction results in improved antiproteinuric response to RAS blocking agents. J Am Soc Nephrol 2005; 16:474–481.
- Vogt L, Waanders F, Boomsma F, de Zeeuw D, Navis G. Effects of dietary sodium and hydrochlorothiazide on the antiproteinuric efficacy of losartan. J Am Soc Nephrol 2008; 19:999–1007.
- Ahmed AK, Kamath NS, El Kossi M, El Nahas AM. The impact of stopping inhibitors of the renin-angiotensin system in patients with advanced chronic kidney disease. Nephrol Dial Transplant 2010; 25:3977–3982.
- Bakris GL, Weir MR, Secic M, Campbell B, Weis-McNulty A. Differential effects of calcium antagonist subclasses on markers of nephropathy progression. Kidney Int 2004; 65:1991–2002.
- Kloke HJ, Wetzels JF, Koene RA, Huysmans FT. Effects of low-dose nifedipine on urinary protein excretion rate in patients with renal disease. Nephrol Dial Transplant 1998; 13:646–650.
- Shah SN, Abramowitz M, Hostetter TH, Melamed ML. Serum bicarbonate levels and the progression of kidney disease: a cohort study. Am J Kidney Dis 2009; 54:270–277.
- de Brito-Ashurst I, Varagunam M, Raftery MJ, Yaqoob MM. Bicarbonate supplementation slows progression of CKD and improves nutritional status. J Am Soc Nephrol 2009; 20:2075–2084.
- Susantitaphong P, Sewaralthahab K, Balk EM, Jaber BL, Madias NE. Short- and long-term effects of alkali therapy in chronic kidney disease: a systematic review. Am J Nephrol 2012; 35:540–547.
- Nath KA, Hostetter MK, Hostetter TH. Ammonia-complement interaction in the pathogenesis of progressive renal injury. Kidney Int Suppl 1989; 27:S52–S54.
- Clinical practice guidelines for nutrition in chronic renal failure. K/DOQI, National Kidney Foundation. Am J Kidney Dis 2000; 35(suppl 2):S1–S140.
- Shimada T, Yamazaki Y, Takahashi M, et al. Vitamin D receptor-independent FGF23 actions in regulating phosphate and vitamin D metabolism. Am J Physiol Renal Physiol 2005; 289:F1088–F1095.
- Hasegawa H, Nagano N, Urakawa I, et al. Direct evidence for a causative role of FGF23 in the abnormal renal phosphate handling and vitamin D metabolism in rats with early-stage chronic kidney disease. Kidney Int 2010; 78:975–980.
- de Boer IH, Rue TC, Kestenbaum B. Serum phosphorus concentrations in the third National Health and Nutrition Examination Survey (NHANES III). Am J Kidney Dis 2009; 53:399–407.
- Kendrick J, Cheung AK, Kaufman JS, et al; HOST Investigators. FGF-23 associates with death, cardiovascular events, and initiation of chronic dialysis. J Am Soc Nephrol 2011; 22:1913–1922.
- Palmer SC, Hayen A, Macaskill P, et al. Serum levels of phosphorus, parathyroid hormone, and calcium and risks of death and cardiovascular disease in individuals with chronic kidney disease: a systematic review and meta-analysis. JAMA. 2011; 305:1119–1127.
- Kooienga L, Fried L, Scragg R, Kendrick J, Smits G, Chonchol M. The effect of combined calcium and vitamin D3 supplementation on serum intact parathyroid hormone in moderate CKD. Am J Kidney Dis 2009; 53:408–416.
- National Kidney Foundation, Inc. KDOQI Clinical Practice Guidelines for Bone Metabolism and Disease in Chronic Kidney Disease. www.kidney.org/professionals/kdoqi/guidelines_bone/guide1.htm#table15. Accessed March 5, 2014.
- Kidney International. KDIGO Clinical Practice Guideline for the Diagnosis, Evaluation, Prevention, and Treatment of Chronic Kidney Disease-Mineral and Bone Disorder (CKD-MBD). http://kdigo.org/home/mineral-bone-disorder. Accessed March 5, 2014.
- Kazmi WH, Kausz AT, Khan S, et al. Anemia: an early complication of chronic renal insufficiency. Am J Kidney Dis 2001; 38:803–812.
- Sarnak MJ, Tighiouart H, Manjunath G, et al. Anemia as a risk factor for cardiovascular disease in the Atherosclerosis Risk in Communities (ARIC) study. J Am Coll Cardiol 2002; 40:27–33.
- Thorp ML, Johnson ES, Yang X, Petrik AF, Platt R, Smith DH. Effect of anaemia on mortality, cardiovascular hospitalizations and end-stage renal disease among patients with chronic kidney disease. Nephrology (Carlton) 2009; 14:240–246.
- Mircescu G, Gârneata L, Capusa C, Ursea N. Intravenous iron supplementation for the treatment of anaemia in pre-dialyzed chronic renal failure patients. Nephrol Dial Transplant 2006; 21:120–124.
- Silverberg DS, Iaina A, Peer G, et al. Intravenous iron supplementation for the treatment of the anemia of moderate to severe chronic renal failure patients not receiving dialysis. Am J Kidney Dis 1996; 27:234–238.
- Singh AK, Szczech L, Tang KL, et al; CHOIR Investigators. Correction of anemia with epoetin alfa in chronic kidney disease. N Engl J Med 2006; 355:2085–2098.
- Drüeke TB, Locatelli F, Clyne N, et al; CREATE Investigators. Normalization of hemoglobin level in patients with chronic kidney disease and anemia. N Engl J Med 2006; 355:2071–2084.
- Pfeffer MA, Burdmann EA, Chen CY, et al; TREAT Investigators. A trial of darbepoetin alfa in type 2 diabetes and chronic kidney disease. N Engl J Med 2009; 361:2019–2032.
- US Food and Drug Administration (FDA). FDA Drug Safety Communication: modified dosing recommendations to improve the safe use of erythropoiesis-stimulating agents (ESAs) in chronic kidney disease. http://www.fda.gov/drugs/drugsafety/ucm259639.htm. Accessed March 5, 2014.
- Kurella M, Covinsky KE, Collins AJ, Chertow GM. Octogenarians and nonagenarians starting dialysis in the United States. Ann Intern Med 2007; 146:177–183.
- Kurella Tamura M, Covinsky KE, Chertow GM, Yaffe K, Landefeld CS, McCulloch CE. Functional status of elderly adults before and after initiation of dialysis. N Engl J Med 2009; 361:1539–1547.
- Renal Physicians Association. Clinical Practice Guideline. Shared Decision-Making in the Appropriate Initiation of and Withdrawal from Dialysis. 2nd ed.
- Vollmer WM, Wahl PW, Blagg CR. Survival with dialysis and transplantation in patients with end-stage renal disease. N Engl J Med 1983; 308:1553–1558.
- Port FK, Wolfe RA, Mauger EA, Berling DP, Jiang K. Comparison of survival probabilities for dialysis patients vs cadaveric renal transplant recipients. JAMA 1993; 270:1339–1343.
- Wolfe RA, Ashby VB, Milford EL, et al. Comparison of mortality in all patients on dialysis, patients on dialysis awaiting transplantation, and recipients of a first cadaveric transplant. N Engl J Med 1999; 341:1725–1730.
- Cosio FG, Alamir A, Yim S, et al. Patient survival after renal transplantation: I. The impact of dialysis pre-transplant. Kidney Int 1998; 53:767–772.
- Meier-Kriesche HU, Port FK, Ojo AO, et al. Effect of waiting time on renal transplant outcome. Kidney Int 2000; 58:1311–1317.
- Mange KC, Joffe MM, Feldman HI. Effect of the use or nonuse of long-term dialysis on the subsequent survival of renal transplants from living donors. N Engl J Med 2001; 344:726–731.
- Dhingra RK, Young EW, Hulbert-Shearon TE, Leavey SF, Port FK. Type of vascular access and mortality in US hemodialysis patients. Kidney Int 2001; 60:1443–1451.
- Nassar GM, Ayus JC. Infectious complications of the hemodialysis access. Kidney Int 2001; 60:1–13.
- Perera GB, Mueller MP, Kubaska SM, Wilson SE, Lawrence PF, Fujitani RM. Superiority of autogenous arteriovenous hemodialysis access: maintenance of function with fewer secondary interventions. Ann Vasc Surg 2004; 18:66–73.
- Basile C, Casucci F, Lomonte C. Timing of first cannulation of arteriovenous fistula: time matters, but there is also something else. Nephrol Dial Transplant 2005; 20:1519–1520.
- Biuckians A, Scott EC, Meier GH, Panneton JM, Glickman MH. The natural history of autologous fistulas as first-time dialysis access in the KDOQI era. J Vasc Surg 2008; 47:415–421.
- National Kidney Foundation, Inc. KDOQI Clinical Practice Guidelines for Vascular Access. http://www.kidney.org/professionals/KDOQI/guideline_upHD_PD_VA/va_guide1.htm. Accessed March 5, 2014.
- Arora P, Obrador GT, Ruthazer R, et al. Prevalence, predictors, and consequences of late nephrology referral at a tertiary care center. J Am Soc Nephrol 1999; 10:1281–1286.
- Gøransson LG, Bergrem H. Consequences of late referral of patients with end-stage renal disease. J Intern Med 2001; 250:154–159.
- Cooper BA, Branley P, Bulfone L, et al. A randomized, controlled trial of early versus late initiation of dialysis. N Engl J Med 2010; 363:609–619.
- Carson RC, Juszczak M, Davenport A, Burns A. Is maximum conservative management an equivalent treatment option to dialysis for elderly patients with significant comorbid disease? Clin J Am Soc Nephrol 2009; 4:1611–1619.
- Murtagh FE, Marsh JE, Donohoe P, Ekbal NJ, Sheerin NS, Harris FE. Dialysis or not? A comparative survival study of patients over 75 years with chronic kidney disease stage 5. Nephrol Dial Transplant 2007; 22:1955–1962.
Accountable-care organizations are becoming more prominent in the United States, and therefore health care systems in the near future will be reimbursed on the basis of their ability to care for patient populations rather than individual patients. As a result, primary care physicians will need to be well versed in the care of patients with common chronic diseases such as chronic kidney disease (CKD). By one estimate, patients with CKD constitute 14% of the US population age 20 and older, or more than 31 million people.1
An earlier article in this journal reviewed how to identify patients with CKD and how to interpret the estimated glomerular filtration rate (GFR).2 This article examines the care of patients with advanced CKD, how to manage their health risks, and how to optimize their care by coordinating with nephrologists.
GOALS OF CKD CARE
CKD is defined either as renal damage (which is most commonly manifested by proteinuria, but which may include pathologic changes on biopsy or other markers of damage on serum, urine, or imaging studies), or as a GFR less than 60 mL/min/1.73 m2 for at least 3 months.3 It is divided into five stages (Table 1).
Since most patients with CKD never reach end-stage renal disease, much of their care is aimed at slowing the progression of renal dysfunction and addressing medical issues that arise as a result of CKD. To these ends, it is important to detect CKD early and refer these patients to a nephrology team in a timely manner. Their care can be separated into several important tasks:
- Identify the cause of CKD, if possible; address potentially reversible causes such as obstruction or medication-related causes. If a primarily glomerular process (marked by heavy proteinuria and dysmorphic red blood cells and red blood cell casts in the urine sediment) or interstitial nephritis (manifested by white blood cells in the urine) is suspected, refer to a nephrologist early.
- Provide treatment to correct the specific cause (if one is present) or slow the deterioration of renal function.
- Address cardiovascular risk factors.
- Address metabolic abnormalities related to CKD.
- If the CKD is advanced, educate the patient about end-stage renal disease and its treatment options, and guide the patient through the transition to end-stage renal disease.
WHEN SHOULD A NEPHROLOGIST BE CONSULTED?
The ideal timing of referral to a nephrologist is not well defined and depends on the comfort level of the primary care provider.
Treatments to slow the progression of CKD and decrease cardiovascular risk should begin early in CKD (ie, in stage 3) and can be managed by the primary care provider with guidance from a nephrologist. Patients referred to a nephrologist while in stage 3 have been shown to go longer without CKD progression than those referred in later stages.4 Early referral to a nephrologist has also been associated with a decreased mortality rate.5 The studies that found these trends, however, were limited by the fact that patients with stage 3 CKD are less likely to progress to end-stage renal disease or to die of cardiovascular disease than patients with stage 4 or 5 CKD.
Once stage 4 CKD develops, the nephrologist should take a more active role in the care plan. In this stage, cardiovascular risk rises, and the risk of developing end-stage renal disease rises dramatically.6 With comprehensive care in a CKD clinic, even patients with advanced CKD are more likely to have a stabilization of renal function.7 Kinchen et al8 found that patients referred to a nephrologist within 4 months of starting dialysis had a lower survival rate than those referred earlier. Therefore, if a nephrologist was not involved in the patient’s care prior to stage 4, then a referral must be made.
Recommendation. Patients with stage 3 CKD can be referred for an initial evaluation and development of a treatment plan, but most of the responsibility for their care can remain with the primary care provider. Once stage 4 CKD develops, the nephrologist should assume an increasing role. However, if glomerular disease is suspected, we recommend referral to a nephrologist regardless of the estimated GFR.
ELEVATED CARDIOVASCULAR RISK
Patients with stage 3 CKD are 20 times more likely to die of a cardiovascular event than to reach end-stage renal disease.6 This increased risk does not quite reach the status of a cardiovascular disease risk equivalent, as does diabetes,9,10 but cardiovascular risk reduction should be a primary focus of care for the CKD patient.
The cardiovascular risk in part is attributed to a high prevalence of traditional cardiovascular risk factors, including diabetes mellitus, hypertension, and hyperlipidemia.11,12 About two-thirds of CKD patients have metabolic syndrome, which is a risk factor for cardiovascular disease and is associated with more rapid progression of CKD.13 In addition, renal dysfunction, proteinuria, and hyperphosphatemia are also risk factors for cardiovascular disease.14–19
The risk of death from a cardiovascular event increases as kidney function declines, with reported 5-year death rates of 19.5% in stage 2, 24.3% in stage 3, and 45.7% in stage 4 CKD. However, imbalance between mortality risk and progression to end-stage renal disease may be age-dependent.20 Younger patients (age 45 and younger) are more likely to progress to end-stage renal disease, whereas in older patients (over age 65), the relative risk of dying of cardiovascular disease is higher.
Aggressive lipid management
Hyperlipidemia is a common risk factor for cardiovascular morbidity and mortality in CKD.21 However, until recently, all studies of outcomes of patients treated for hyperlipidemia excluded patients with CKD. Post hoc analyses of these studies 22–27 showed statins to be beneficial in primary and secondary cardiovascular prevention in patents with “normal” serum creatinine values but estimated GFR levels of 50 to 59 mL/min/1.73 m2.
The SHARP trial28 was the first prospective trial to study lipid-lowering therapy in patients with CKD. In this trial, patients with various stages of CKD, including advanced CKD, had fewer major vascular events if they received the combination of low-dose simvastatin (Zocor) and ezetimibe (Zetia). However, the evidence does not suggest that statin therapy slows the progression of CKD.28–31
Recommendation. Manage hyperlipidemia aggressively using statin therapy with or without ezetimibe, with a target low-density lipoprotein cholesterol level below 100 mg/dL.32
Manage other cardiovascular risk factors
Because hypertension and proteinuria are risk factors not only for cardiovascular disease but also for progression of CKD, they are discussed in the section below.
ATTEMPT TO PREVENT WORSENING OF RENAL FUNCTION
Medications to avoid
It is important to review a CKD patient’s medication list—prescription and over-the-counter drugs—to identify any that may contribute to a worsening of renal function. CKD patients need to be informed about avoiding medications such as nonsteroidal anti-inflammatory drugs, proton pump inhibitors, and herbal supplements because they can cause further renal injury. In addition, other medications (eg, metformin) are contraindicated in CKD because of side effects that may occur in CKD.
Patients should be encouraged to discuss any changes in their medications, including over-the-counter products, with their primary care physicians.
Manage hypertension aggressively
Many patients with CKD also have hypertension,33,34 possibly because they have a higher frequency of underlying essential hypertension or because CKD often worsens preexisting hypertension. Moreover, uncontrolled hypertension is associated with a further decline in renal function.35,36
The ACCORD trial37 found no benefit in lowering systolic blood pressure to less than 120 mm Hg compared with less than 140 mm Hg in patients with diabetes mellitus. (The patients in this study did not necessarily have CKD.)
A meta-analysis38 of trials of antihypertensive treatment in patients with CKD found that the optimal target systolic blood pressure for decreasing the progression of CKD was 110 to 129 mm Hg. The relative risk of progression of renal dysfunction was:
- 1.83 (95% confidence interval [CI] 0.97–3.44) at 130 mm to 139 mm Hg, vs
- 3.14 (95% CI 1.64–5.99) at 160 mm Hg or higher.
There is also evidence that blood pressure control can be relaxed as patients age. While the exact age differs among published guidelines, the evidence supports a goal blood pressure of less than 150/90 mm Hg once a patient reaches the age of 70, regardless of CKD or proteinuria.
Recommendation. Current evidence suggests the following blood pressure goals in CKD patients:
- With diabetes mellitus or proteinuria: < 130/80 mm Hg
- Without proteinuria: < 140/90 mm Hg
- Age 70 and older: <150/90 mm Hg.39
Angiotensin-converting enzyme (ACE) inhibitors and angiotensin receptor blockers (ARBs) are the preferred antihypertensive drugs in patients with diabetes or proteinuria (see below).
Manage proteinuria
Proteinuria is also associated with progression of CKD. AASK,40 a study that included nondiabetic African American patients whose estimated GFRs were between 20 and 60 mL/min/1.73 m2, showed that higher levels of proteinuria were associated with a higher risk of decline in GFR and a higher risk of end-stage renal disease. Findings were similar to those in studies of other CKD populations.41–43 Proteinuria is also an independent risk factor for cardiovascular disease and death. Multiple large studies16,17,44,45 have found associations between higher levels of albumin excretion and risk of major cardiovascular events, cardiovascular death, and death from any cause in people with and without diabetes.
Reducing proteinuria has been shown to both slow progression of renal dysfunction and reduce the cardiovascular risk.44,45 In a substudy of the IDNT46 in patients with diabetic nephropathy, each 50% reduction in urinary protein excretion was associated with a 56% reduction in risk of progression of CKD. Similar effects have been shown in nondiabetic CKD patients.47
ACE inhibitors and ARBs are the preferred treatments for proteinuria in patients with CKD.48–50 Combination therapy with an ACE inhibitor and an ARB has been used,51–53 with a better response in proteinuria reduction. However, combination therapy with these drugs cannot currently be recommended, as the only prospective study of this regimen to date suggested worse renal and overall outcomes in patients at high cardiovascular risk.54 These drugs may also have renoprotective effects independent of their effects on blood pressure and proteinuria.38 Dietary salt restriction and diuretic therapy can further increase the efficacy of proteinuria reduction by ACE inhibitors or ARBs.55,56
On the other hand, stopping ACE inhibitors or ARBs may be beneficial as the patient nears end-stage renal disease. Ahmed et al57 demonstrated that stopping ACE inhibitors or ARBs in advanced stage 4 CKD (mean estimated GFR 16 mL/min/1.73 m2) was associated with improved GFR and delayed onset of renal replacement therapy. This improvement may be due to regaining the slight decrease in GFR that occurred when these medications were started.
Nondihydropyridine calcium channel blockers such as diltiazem (Cardizem) and verapamil (Calan) have also been shown to be useful for reducing proteinuria,58 whereas dihydropyridine calcium channel blockers such as amlodipine (Norvasc) and nifedipine (Procardia), when used without ACE inhibitors or ARBs, can worsen proteinuria.58,59
Correct metabolic acidosis
The kidneys play an important role in maintaining acid-base balance, keeping the blood from becoming too acidic both by reabsorbing bicarbonate filtered into the urine by the glomerulus and by excreting the daily acid load. Metabolic acidosis can develop when these functions break down at more advanced stages of CKD, most often when the estimated GFR declines to less than 20 mL/min/1.73 m2.
Bicarbonate levels of 22 mmol/L or less have been associated with a higher risk of worsening renal function.60 When such patients were treated with sodium bicarbonate to achieve a serum bicarbonate of at least 23 mmol/L, they had an 80% lower rate of progression to end-stage renal disease without any increase in edema, admission for congestive heart failure, or change in blood pressure.61
Susantitaphong et al62 reviewed six randomized trials of bicarbonate supplementation in CKD and found that it was associated with improved kidney function and a 79% lower rate of progression to end-stage renal disease.
The proposed mechanism behind this benefit lies in the increase in ammonia production that each surviving nephron must undertake to handle the daily acid load. The increased ammonia is thought to play a role in activating the alternative complement pathway,63 causing renal inflammation and injury.
Recommendation. Bicarbonate therapy should be used to maintain serum bicarbonate levels above 22 mmol/L in CKD.64
OTHER ASPECTS OF CKD CARE
Bone mineral disorders
Patients with CKD develop secondary hyperparathyroidism, hyperphosphatemia, and (in advanced CKD) hypocalcemia, all leading to disorders of bone mineral metabolism.
Traditionally, it has been thought that decreased production of 1,25-dihydroxyvitamin D by dysfunctional kidneys leads to decreased suppression of the parathyroid gland and to secondary hyperparathyroidism. The major long-term adverse effect of this is a weakened bone matrix resulting from increased calcium and phosphorus efflux from bones (renal osteodystrophy).
The discovery of fibroblast growth factor 23 (FGF-23) has improved our understanding of the physiology behind disordered bone mineral metabolism in CKD. FGF-23, produced by osteoblasts and osteocytes, acts directly on the kidney to increase renal phosphate excretion. It also suppresses 1,25-dihydroxyvitamin D levels by inhibiting 1-alpha-hydroxylase,65 and it stimulates parathyroid hormone secretion. FGF-23 levels rise much earlier in CKD than do parathyroid hormone levels, suggesting that abnormalities in phosphorus balance and FGF-23 may be the earliest pathophysiologic changes.66
The initial treatment of bone mineral disorders is to some extent guided by laboratory values. Phosphate levels higher than 3.5 or 4 mg/dL and elevated FGF-23 levels have been associated with increased mortality rates in CKD patients.18,19,67–69 All patients should also have their 1,25-dihydroxyvitamin D level checked and supplemented if deficient. In many patients with early stage 3 CKD, this may correct secondary hyperparathyroidism.70
Serum phosphorus levels should be kept in the normal range in stage 3 and 4 CKD,71 either by restricting dietary phosphorus intake (< 800 or < 1,000 mg/day) or by using a phosphate binder, which is taken with meals to prevent phosphorus absorption from the gastrointestinal tract. Current US recommendations are to allow graded increases in parathyroid hormone based on the stage of CKD (Table 2).71 However, these targets are still an area of uncertainty, with some guidelines suggesting that wider variations in parathyroid hormone can be allowed, so there may be wider variation in clinical practice in this area.72 If the serum phosphorus level is in the goal range but parathyroid hormone levels are still high, an activated vitamin D analogue such as calcitriol is recommended, although with the emerging role of FGF-23, some experts also call for early use of a phosphate binder in this group.
The treatment of bone mineral disorders in CKD is fairly complex, and we recommend that it be done by or with the close direction of a nephrologist.
Recommendations on bone disorders
- Check levels of calcium, phosphorus, 25-hydroxyvitamin D, and parathyroid hormone in all patients whose estimated GFR is less than 60 mL/min/1.73 m2, with frequency of measurements based on the stage of CKD.71
- Replace vitamin D if deficient.
- Treat elevated phosphorus levels with a protein-restricted diet (nutrition referral) and a phosphate binder.
- Treat elevated hyperparathyroid hormone levels with a vitamin D analogue once phosphorus levels have been controlled.
- Refer patients with an elevated phosphorus or parathyroid hormone level to a nephrology service for consultation before initiating medical therapy.
Anemia is common, treatment controversial
The treatment of anemia attributed to CKD has been a topic of controversy over the past decade, and we recommend that it be done with the guidance of a nephrologist.
Anemia is common in CKD, and declining kidney function is an independent predictor of anemia.73 Anemia is a risk factor for left ventricular hypertrophy, cardiovascular disease,74 and death in CKD.75
The anemia of CKD is attributed to relative erythropoietin deficiency and bone marrow resistance to erythropoietin, but this is a diagnosis of exclusion, and other causes of anemia must be ruled out. Iron deficiency is a common cause of anemia in CKD, and treatment of iron deficiency may correct anemia in more than one-third of these patients.76,77
Erythropoiesis-stimulating agents such as epoetin alfa (Procrit) and darbepoetin (Aranesp) are used to treat renal anemia. However, the target hemoglobin level has been a subject of debate. Three prospective trials78–80 found no benefit in raising the hemoglobin level to normal ranges using these agents, and several found an association with higher rates of stroke and venous thrombosis. The US Food and Drug Administration suggests that the only role for these agents in CKD is to avoid the need for transfusions. They should not be used to normalize the hemoglobin level. The target, although not explicitly specified, is suggested to be around 10 g/dL.81
PREPARE FOR END-STAGE RENAL DISEASE
Discuss the options
Because the risk of developing end-stage renal disease rises dramatically once CKD reaches stage 4, all such patients should have a discussion about renal replacement therapy. They should be educated about their options for treatment (hemodialysis, peritoneal dialysis, and transplantation, as well as not proceeding with renal replacement therapy), often in a formal class. They should then be actively engaged in the decision about how to proceed. Survival and quality of life should be discussed, particularly with patients who are over age 80, who are severely ill, or who are living in a nursing facility, as these groups get limited survival benefit from starting dialysis, and quality of life may actually decrease with dialysis.82,83
The Renal Physicians Association has created clinical practice guidelines for shared decision-making, consisting of 10 practice recommendations that outline a systematic approach to patients needing renal replacement therapy.84
Consider preemptive kidney transplantation
Any patient thought to be a suitable candidate for renal transplantation should be referred to a transplantation center for evaluation. Studies have shown that kidney transplantation offers a survival advantage compared with chronic dialysis and should preferably be done preemptively, ie, before dialysis is required.85–90 Therefore, patients with estimated GFRs in the low 20s should be referred for a transplantation evaluation.
If a living donor is available, the transplantation team usually waits to perform the procedure until the patient is closer to needing dialysis, often when the estimated GFR is around 15 to 16 mL/min/1.73 m2. If no living donor is available, the patient can earn time on the deceased-donor waiting list once his or her estimated GFR falls to below 20 mL/min/1.7 m2.
Plan for dialysis access
Patients starting hemodialysis first need to undergo a procedure to provide access to the blood. The three options are an arteriovenous fistula, an arteriovenous graft, and a central venous catheter (Figure 1).
An arteriovenous fistula is the best option, being the most durable, followed by a graft and then a catheter.91 Arteriovenous fistulas also have the lowest rates of infection,92 thrombosis,93 and intervention to maintain patency.93
The fistula is created by ligating a vein draining an extremity, most often the nondominant arm, and anastomosing the vein to an artery. The higher arterial pressure causes the vein to dilate and thicken (“arterialize”), thus making it able to withstand repeated cannulation necessary for hemodialysis.
An arteriovenous fistula typically takes 1 to 3 months to “mature” to the point where it can be used,94,95 and, depending on the patient and experience of the vascular surgeon, a significant number may never mature. Thus, it is important to discuss hemodialysis access before the patient reaches end-stage renal disease so that he or she can be referred to a vascular surgeon early, when the estimated GFR is about 20 mL/min/1.73 m2.
An arteriovenous graft. Not all patients have suitable vessels for creation of an arteriovenous fistula. In such patients, an arteriovenous graft, typically made of polytetrafluoroethylene, is the next best option. The graft is typically ready to use in 2 weeks and thus does not require as much advance planning. Grafts tend to narrow more often than fistulas and require more procedures to keep them patent.
A central venous catheter is most often inserted into the internal jugular vein and tunneled under the skin to exit in an area covered by the patient’s shirt.
Tunneled dialysis catheters are associated with higher rates of infection, thrombosis, and overall mortality and are therefore the least preferred choice. They are reserved for patients who have not had advance planning for end-stage renal disease, who do not have acceptable vessels for an arteriovenous fistula or graft, or who have refused surgical access.
Protect the fistula arm. It is recommended that venipuncture, intravenous lines, and blood pressure measurements be avoided in the nondominant upper arm of patients with stage 4 and 5 CKD to protect those veins for the potential creation of an arteriovenous fistula.96 For the same reason, peripherally inserted central catheter lines and subclavian catheters should be avoided in these patients. If an arteriovenous fistula has already been placed, this arm must be protected from such procedures at all times.
Studies have shown that late referral to a nephrologist is associated with a lower incidence of starting dialysis with a permanent vascular access.97,98
If the patient wishes to start peritoneal dialysis, the peritoneal dialysis catheter can usually be used 2 weeks after being inserted.
Starting dialysis
The appropriate time for starting dialysis remains controversial, especially in elderly patients with multiple comorbid conditions.
The IDEAL study99 found no benefit in starting dialysis at a GFR of 10 to 14 mL/min compared with 5 to 7 mL/min. Thus, there is no single estimated GFR at which dialysis should be started. Rather, the development of early uremic symptoms and the patient’s quality of life should guide this decision.82,83,99–101
Hemodialysis involves three sessions per week, each taking about 4 hours. Evidence suggests that longer sessions or more sessions per week may offer benefits, especially in terms of blood pressure, volume, and dietary management. This has led to an increase in the popularity of home and in-center nocturnal hemodialysis programs across the United States.
Peritoneal dialysis?
Peritoneal dialysis is an excellent choice for patients who are motivated, can care for themselves at home, and have a support system available to assist them if needed. It allows for daily dialysis, less fluid restriction, and less dietary restriction, and it gives the patient an opportunity to stay independent. It also spares the veins in the arms, which may be needed for vascular access later in life if hemodialysis is needed.
Recommendation. We recommend that peritoneal dialysis be offered to any suitable patient who is approaching end-stage renal disease.
A COMPREHENSIVE, COLLABORATIVE APPROACH
Chronic kidney disease is a multisystem disorder, and its management requires a comprehensive approach (Table 3). Early detection and interventions are key to reducing cardiovascular events and progression to kidney failure.
Early referral to a nephrologist and team collaboration between the primary care provider, the nephrologist, and other health care providers are essential. Early in the course of CKD, it may be appropriate for a nephrologist to evaluate the patient and recommend a set of treatment goals. Follow-up may be infrequent or unnecessary.
As CKD progresses, especially as the patient reaches an estimated GFR of 30 mL/min/1.73 m2, the nephrologist will take a more active role in the patient’s care and medical decision-making. In some circumstances, it may even be appropriate for the nephrologist to be the patient’s source of primary care, with the primary care provider as a consultant.
Caring for patients with CKD includes not only strategies to preserve renal function and prolong survival, but also making critical decisions about starting dialysis and about the need for transplantation. Early involvement of a nephrologist and early preparation for end-stage renal disease with preemptive transplantation and arteriovenous fistula placement are associated with better patient outcomes. Key to this is collaboration between the primary care provider and the nephrologist, with levels of responsibility for patient care that adapt to the patient’s degree of renal dysfunction and other comorbidities. Such strategies to select patients for timely nephrology referral may help improve outcomes in this vulnerable population.
Accountable-care organizations are becoming more prominent in the United States, and therefore health care systems in the near future will be reimbursed on the basis of their ability to care for patient populations rather than individual patients. As a result, primary care physicians will need to be well versed in the care of patients with common chronic diseases such as chronic kidney disease (CKD). By one estimate, patients with CKD constitute 14% of the US population age 20 and older, or more than 31 million people.1
An earlier article in this journal reviewed how to identify patients with CKD and how to interpret the estimated glomerular filtration rate (GFR).2 This article examines the care of patients with advanced CKD, how to manage their health risks, and how to optimize their care by coordinating with nephrologists.
GOALS OF CKD CARE
CKD is defined either as renal damage (which is most commonly manifested by proteinuria, but which may include pathologic changes on biopsy or other markers of damage on serum, urine, or imaging studies), or as a GFR less than 60 mL/min/1.73 m2 for at least 3 months.3 It is divided into five stages (Table 1).
Since most patients with CKD never reach end-stage renal disease, much of their care is aimed at slowing the progression of renal dysfunction and addressing medical issues that arise as a result of CKD. To these ends, it is important to detect CKD early and refer these patients to a nephrology team in a timely manner. Their care can be separated into several important tasks:
- Identify the cause of CKD, if possible; address potentially reversible causes such as obstruction or medication-related causes. If a primarily glomerular process (marked by heavy proteinuria and dysmorphic red blood cells and red blood cell casts in the urine sediment) or interstitial nephritis (manifested by white blood cells in the urine) is suspected, refer to a nephrologist early.
- Provide treatment to correct the specific cause (if one is present) or slow the deterioration of renal function.
- Address cardiovascular risk factors.
- Address metabolic abnormalities related to CKD.
- If the CKD is advanced, educate the patient about end-stage renal disease and its treatment options, and guide the patient through the transition to end-stage renal disease.
WHEN SHOULD A NEPHROLOGIST BE CONSULTED?
The ideal timing of referral to a nephrologist is not well defined and depends on the comfort level of the primary care provider.
Treatments to slow the progression of CKD and decrease cardiovascular risk should begin early in CKD (ie, in stage 3) and can be managed by the primary care provider with guidance from a nephrologist. Patients referred to a nephrologist while in stage 3 have been shown to go longer without CKD progression than those referred in later stages.4 Early referral to a nephrologist has also been associated with a decreased mortality rate.5 The studies that found these trends, however, were limited by the fact that patients with stage 3 CKD are less likely to progress to end-stage renal disease or to die of cardiovascular disease than patients with stage 4 or 5 CKD.
Once stage 4 CKD develops, the nephrologist should take a more active role in the care plan. In this stage, cardiovascular risk rises, and the risk of developing end-stage renal disease rises dramatically.6 With comprehensive care in a CKD clinic, even patients with advanced CKD are more likely to have a stabilization of renal function.7 Kinchen et al8 found that patients referred to a nephrologist within 4 months of starting dialysis had a lower survival rate than those referred earlier. Therefore, if a nephrologist was not involved in the patient’s care prior to stage 4, then a referral must be made.
Recommendation. Patients with stage 3 CKD can be referred for an initial evaluation and development of a treatment plan, but most of the responsibility for their care can remain with the primary care provider. Once stage 4 CKD develops, the nephrologist should assume an increasing role. However, if glomerular disease is suspected, we recommend referral to a nephrologist regardless of the estimated GFR.
ELEVATED CARDIOVASCULAR RISK
Patients with stage 3 CKD are 20 times more likely to die of a cardiovascular event than to reach end-stage renal disease.6 This increased risk does not quite reach the status of a cardiovascular disease risk equivalent, as does diabetes,9,10 but cardiovascular risk reduction should be a primary focus of care for the CKD patient.
The cardiovascular risk in part is attributed to a high prevalence of traditional cardiovascular risk factors, including diabetes mellitus, hypertension, and hyperlipidemia.11,12 About two-thirds of CKD patients have metabolic syndrome, which is a risk factor for cardiovascular disease and is associated with more rapid progression of CKD.13 In addition, renal dysfunction, proteinuria, and hyperphosphatemia are also risk factors for cardiovascular disease.14–19
The risk of death from a cardiovascular event increases as kidney function declines, with reported 5-year death rates of 19.5% in stage 2, 24.3% in stage 3, and 45.7% in stage 4 CKD. However, imbalance between mortality risk and progression to end-stage renal disease may be age-dependent.20 Younger patients (age 45 and younger) are more likely to progress to end-stage renal disease, whereas in older patients (over age 65), the relative risk of dying of cardiovascular disease is higher.
Aggressive lipid management
Hyperlipidemia is a common risk factor for cardiovascular morbidity and mortality in CKD.21 However, until recently, all studies of outcomes of patients treated for hyperlipidemia excluded patients with CKD. Post hoc analyses of these studies 22–27 showed statins to be beneficial in primary and secondary cardiovascular prevention in patents with “normal” serum creatinine values but estimated GFR levels of 50 to 59 mL/min/1.73 m2.
The SHARP trial28 was the first prospective trial to study lipid-lowering therapy in patients with CKD. In this trial, patients with various stages of CKD, including advanced CKD, had fewer major vascular events if they received the combination of low-dose simvastatin (Zocor) and ezetimibe (Zetia). However, the evidence does not suggest that statin therapy slows the progression of CKD.28–31
Recommendation. Manage hyperlipidemia aggressively using statin therapy with or without ezetimibe, with a target low-density lipoprotein cholesterol level below 100 mg/dL.32
Manage other cardiovascular risk factors
Because hypertension and proteinuria are risk factors not only for cardiovascular disease but also for progression of CKD, they are discussed in the section below.
ATTEMPT TO PREVENT WORSENING OF RENAL FUNCTION
Medications to avoid
It is important to review a CKD patient’s medication list—prescription and over-the-counter drugs—to identify any that may contribute to a worsening of renal function. CKD patients need to be informed about avoiding medications such as nonsteroidal anti-inflammatory drugs, proton pump inhibitors, and herbal supplements because they can cause further renal injury. In addition, other medications (eg, metformin) are contraindicated in CKD because of side effects that may occur in CKD.
Patients should be encouraged to discuss any changes in their medications, including over-the-counter products, with their primary care physicians.
Manage hypertension aggressively
Many patients with CKD also have hypertension,33,34 possibly because they have a higher frequency of underlying essential hypertension or because CKD often worsens preexisting hypertension. Moreover, uncontrolled hypertension is associated with a further decline in renal function.35,36
The ACCORD trial37 found no benefit in lowering systolic blood pressure to less than 120 mm Hg compared with less than 140 mm Hg in patients with diabetes mellitus. (The patients in this study did not necessarily have CKD.)
A meta-analysis38 of trials of antihypertensive treatment in patients with CKD found that the optimal target systolic blood pressure for decreasing the progression of CKD was 110 to 129 mm Hg. The relative risk of progression of renal dysfunction was:
- 1.83 (95% confidence interval [CI] 0.97–3.44) at 130 mm to 139 mm Hg, vs
- 3.14 (95% CI 1.64–5.99) at 160 mm Hg or higher.
There is also evidence that blood pressure control can be relaxed as patients age. While the exact age differs among published guidelines, the evidence supports a goal blood pressure of less than 150/90 mm Hg once a patient reaches the age of 70, regardless of CKD or proteinuria.
Recommendation. Current evidence suggests the following blood pressure goals in CKD patients:
- With diabetes mellitus or proteinuria: < 130/80 mm Hg
- Without proteinuria: < 140/90 mm Hg
- Age 70 and older: <150/90 mm Hg.39
Angiotensin-converting enzyme (ACE) inhibitors and angiotensin receptor blockers (ARBs) are the preferred antihypertensive drugs in patients with diabetes or proteinuria (see below).
Manage proteinuria
Proteinuria is also associated with progression of CKD. AASK,40 a study that included nondiabetic African American patients whose estimated GFRs were between 20 and 60 mL/min/1.73 m2, showed that higher levels of proteinuria were associated with a higher risk of decline in GFR and a higher risk of end-stage renal disease. Findings were similar to those in studies of other CKD populations.41–43 Proteinuria is also an independent risk factor for cardiovascular disease and death. Multiple large studies16,17,44,45 have found associations between higher levels of albumin excretion and risk of major cardiovascular events, cardiovascular death, and death from any cause in people with and without diabetes.
Reducing proteinuria has been shown to both slow progression of renal dysfunction and reduce the cardiovascular risk.44,45 In a substudy of the IDNT46 in patients with diabetic nephropathy, each 50% reduction in urinary protein excretion was associated with a 56% reduction in risk of progression of CKD. Similar effects have been shown in nondiabetic CKD patients.47
ACE inhibitors and ARBs are the preferred treatments for proteinuria in patients with CKD.48–50 Combination therapy with an ACE inhibitor and an ARB has been used,51–53 with a better response in proteinuria reduction. However, combination therapy with these drugs cannot currently be recommended, as the only prospective study of this regimen to date suggested worse renal and overall outcomes in patients at high cardiovascular risk.54 These drugs may also have renoprotective effects independent of their effects on blood pressure and proteinuria.38 Dietary salt restriction and diuretic therapy can further increase the efficacy of proteinuria reduction by ACE inhibitors or ARBs.55,56
On the other hand, stopping ACE inhibitors or ARBs may be beneficial as the patient nears end-stage renal disease. Ahmed et al57 demonstrated that stopping ACE inhibitors or ARBs in advanced stage 4 CKD (mean estimated GFR 16 mL/min/1.73 m2) was associated with improved GFR and delayed onset of renal replacement therapy. This improvement may be due to regaining the slight decrease in GFR that occurred when these medications were started.
Nondihydropyridine calcium channel blockers such as diltiazem (Cardizem) and verapamil (Calan) have also been shown to be useful for reducing proteinuria,58 whereas dihydropyridine calcium channel blockers such as amlodipine (Norvasc) and nifedipine (Procardia), when used without ACE inhibitors or ARBs, can worsen proteinuria.58,59
Correct metabolic acidosis
The kidneys play an important role in maintaining acid-base balance, keeping the blood from becoming too acidic both by reabsorbing bicarbonate filtered into the urine by the glomerulus and by excreting the daily acid load. Metabolic acidosis can develop when these functions break down at more advanced stages of CKD, most often when the estimated GFR declines to less than 20 mL/min/1.73 m2.
Bicarbonate levels of 22 mmol/L or less have been associated with a higher risk of worsening renal function.60 When such patients were treated with sodium bicarbonate to achieve a serum bicarbonate of at least 23 mmol/L, they had an 80% lower rate of progression to end-stage renal disease without any increase in edema, admission for congestive heart failure, or change in blood pressure.61
Susantitaphong et al62 reviewed six randomized trials of bicarbonate supplementation in CKD and found that it was associated with improved kidney function and a 79% lower rate of progression to end-stage renal disease.
The proposed mechanism behind this benefit lies in the increase in ammonia production that each surviving nephron must undertake to handle the daily acid load. The increased ammonia is thought to play a role in activating the alternative complement pathway,63 causing renal inflammation and injury.
Recommendation. Bicarbonate therapy should be used to maintain serum bicarbonate levels above 22 mmol/L in CKD.64
OTHER ASPECTS OF CKD CARE
Bone mineral disorders
Patients with CKD develop secondary hyperparathyroidism, hyperphosphatemia, and (in advanced CKD) hypocalcemia, all leading to disorders of bone mineral metabolism.
Traditionally, it has been thought that decreased production of 1,25-dihydroxyvitamin D by dysfunctional kidneys leads to decreased suppression of the parathyroid gland and to secondary hyperparathyroidism. The major long-term adverse effect of this is a weakened bone matrix resulting from increased calcium and phosphorus efflux from bones (renal osteodystrophy).
The discovery of fibroblast growth factor 23 (FGF-23) has improved our understanding of the physiology behind disordered bone mineral metabolism in CKD. FGF-23, produced by osteoblasts and osteocytes, acts directly on the kidney to increase renal phosphate excretion. It also suppresses 1,25-dihydroxyvitamin D levels by inhibiting 1-alpha-hydroxylase,65 and it stimulates parathyroid hormone secretion. FGF-23 levels rise much earlier in CKD than do parathyroid hormone levels, suggesting that abnormalities in phosphorus balance and FGF-23 may be the earliest pathophysiologic changes.66
The initial treatment of bone mineral disorders is to some extent guided by laboratory values. Phosphate levels higher than 3.5 or 4 mg/dL and elevated FGF-23 levels have been associated with increased mortality rates in CKD patients.18,19,67–69 All patients should also have their 1,25-dihydroxyvitamin D level checked and supplemented if deficient. In many patients with early stage 3 CKD, this may correct secondary hyperparathyroidism.70
Serum phosphorus levels should be kept in the normal range in stage 3 and 4 CKD,71 either by restricting dietary phosphorus intake (< 800 or < 1,000 mg/day) or by using a phosphate binder, which is taken with meals to prevent phosphorus absorption from the gastrointestinal tract. Current US recommendations are to allow graded increases in parathyroid hormone based on the stage of CKD (Table 2).71 However, these targets are still an area of uncertainty, with some guidelines suggesting that wider variations in parathyroid hormone can be allowed, so there may be wider variation in clinical practice in this area.72 If the serum phosphorus level is in the goal range but parathyroid hormone levels are still high, an activated vitamin D analogue such as calcitriol is recommended, although with the emerging role of FGF-23, some experts also call for early use of a phosphate binder in this group.
The treatment of bone mineral disorders in CKD is fairly complex, and we recommend that it be done by or with the close direction of a nephrologist.
Recommendations on bone disorders
- Check levels of calcium, phosphorus, 25-hydroxyvitamin D, and parathyroid hormone in all patients whose estimated GFR is less than 60 mL/min/1.73 m2, with frequency of measurements based on the stage of CKD.71
- Replace vitamin D if deficient.
- Treat elevated phosphorus levels with a protein-restricted diet (nutrition referral) and a phosphate binder.
- Treat elevated hyperparathyroid hormone levels with a vitamin D analogue once phosphorus levels have been controlled.
- Refer patients with an elevated phosphorus or parathyroid hormone level to a nephrology service for consultation before initiating medical therapy.
Anemia is common, treatment controversial
The treatment of anemia attributed to CKD has been a topic of controversy over the past decade, and we recommend that it be done with the guidance of a nephrologist.
Anemia is common in CKD, and declining kidney function is an independent predictor of anemia.73 Anemia is a risk factor for left ventricular hypertrophy, cardiovascular disease,74 and death in CKD.75
The anemia of CKD is attributed to relative erythropoietin deficiency and bone marrow resistance to erythropoietin, but this is a diagnosis of exclusion, and other causes of anemia must be ruled out. Iron deficiency is a common cause of anemia in CKD, and treatment of iron deficiency may correct anemia in more than one-third of these patients.76,77
Erythropoiesis-stimulating agents such as epoetin alfa (Procrit) and darbepoetin (Aranesp) are used to treat renal anemia. However, the target hemoglobin level has been a subject of debate. Three prospective trials78–80 found no benefit in raising the hemoglobin level to normal ranges using these agents, and several found an association with higher rates of stroke and venous thrombosis. The US Food and Drug Administration suggests that the only role for these agents in CKD is to avoid the need for transfusions. They should not be used to normalize the hemoglobin level. The target, although not explicitly specified, is suggested to be around 10 g/dL.81
PREPARE FOR END-STAGE RENAL DISEASE
Discuss the options
Because the risk of developing end-stage renal disease rises dramatically once CKD reaches stage 4, all such patients should have a discussion about renal replacement therapy. They should be educated about their options for treatment (hemodialysis, peritoneal dialysis, and transplantation, as well as not proceeding with renal replacement therapy), often in a formal class. They should then be actively engaged in the decision about how to proceed. Survival and quality of life should be discussed, particularly with patients who are over age 80, who are severely ill, or who are living in a nursing facility, as these groups get limited survival benefit from starting dialysis, and quality of life may actually decrease with dialysis.82,83
The Renal Physicians Association has created clinical practice guidelines for shared decision-making, consisting of 10 practice recommendations that outline a systematic approach to patients needing renal replacement therapy.84
Consider preemptive kidney transplantation
Any patient thought to be a suitable candidate for renal transplantation should be referred to a transplantation center for evaluation. Studies have shown that kidney transplantation offers a survival advantage compared with chronic dialysis and should preferably be done preemptively, ie, before dialysis is required.85–90 Therefore, patients with estimated GFRs in the low 20s should be referred for a transplantation evaluation.
If a living donor is available, the transplantation team usually waits to perform the procedure until the patient is closer to needing dialysis, often when the estimated GFR is around 15 to 16 mL/min/1.73 m2. If no living donor is available, the patient can earn time on the deceased-donor waiting list once his or her estimated GFR falls to below 20 mL/min/1.7 m2.
Plan for dialysis access
Patients starting hemodialysis first need to undergo a procedure to provide access to the blood. The three options are an arteriovenous fistula, an arteriovenous graft, and a central venous catheter (Figure 1).
An arteriovenous fistula is the best option, being the most durable, followed by a graft and then a catheter.91 Arteriovenous fistulas also have the lowest rates of infection,92 thrombosis,93 and intervention to maintain patency.93
The fistula is created by ligating a vein draining an extremity, most often the nondominant arm, and anastomosing the vein to an artery. The higher arterial pressure causes the vein to dilate and thicken (“arterialize”), thus making it able to withstand repeated cannulation necessary for hemodialysis.
An arteriovenous fistula typically takes 1 to 3 months to “mature” to the point where it can be used,94,95 and, depending on the patient and experience of the vascular surgeon, a significant number may never mature. Thus, it is important to discuss hemodialysis access before the patient reaches end-stage renal disease so that he or she can be referred to a vascular surgeon early, when the estimated GFR is about 20 mL/min/1.73 m2.
An arteriovenous graft. Not all patients have suitable vessels for creation of an arteriovenous fistula. In such patients, an arteriovenous graft, typically made of polytetrafluoroethylene, is the next best option. The graft is typically ready to use in 2 weeks and thus does not require as much advance planning. Grafts tend to narrow more often than fistulas and require more procedures to keep them patent.
A central venous catheter is most often inserted into the internal jugular vein and tunneled under the skin to exit in an area covered by the patient’s shirt.
Tunneled dialysis catheters are associated with higher rates of infection, thrombosis, and overall mortality and are therefore the least preferred choice. They are reserved for patients who have not had advance planning for end-stage renal disease, who do not have acceptable vessels for an arteriovenous fistula or graft, or who have refused surgical access.
Protect the fistula arm. It is recommended that venipuncture, intravenous lines, and blood pressure measurements be avoided in the nondominant upper arm of patients with stage 4 and 5 CKD to protect those veins for the potential creation of an arteriovenous fistula.96 For the same reason, peripherally inserted central catheter lines and subclavian catheters should be avoided in these patients. If an arteriovenous fistula has already been placed, this arm must be protected from such procedures at all times.
Studies have shown that late referral to a nephrologist is associated with a lower incidence of starting dialysis with a permanent vascular access.97,98
If the patient wishes to start peritoneal dialysis, the peritoneal dialysis catheter can usually be used 2 weeks after being inserted.
Starting dialysis
The appropriate time for starting dialysis remains controversial, especially in elderly patients with multiple comorbid conditions.
The IDEAL study99 found no benefit in starting dialysis at a GFR of 10 to 14 mL/min compared with 5 to 7 mL/min. Thus, there is no single estimated GFR at which dialysis should be started. Rather, the development of early uremic symptoms and the patient’s quality of life should guide this decision.82,83,99–101
Hemodialysis involves three sessions per week, each taking about 4 hours. Evidence suggests that longer sessions or more sessions per week may offer benefits, especially in terms of blood pressure, volume, and dietary management. This has led to an increase in the popularity of home and in-center nocturnal hemodialysis programs across the United States.
Peritoneal dialysis?
Peritoneal dialysis is an excellent choice for patients who are motivated, can care for themselves at home, and have a support system available to assist them if needed. It allows for daily dialysis, less fluid restriction, and less dietary restriction, and it gives the patient an opportunity to stay independent. It also spares the veins in the arms, which may be needed for vascular access later in life if hemodialysis is needed.
Recommendation. We recommend that peritoneal dialysis be offered to any suitable patient who is approaching end-stage renal disease.
A COMPREHENSIVE, COLLABORATIVE APPROACH
Chronic kidney disease is a multisystem disorder, and its management requires a comprehensive approach (Table 3). Early detection and interventions are key to reducing cardiovascular events and progression to kidney failure.
Early referral to a nephrologist and team collaboration between the primary care provider, the nephrologist, and other health care providers are essential. Early in the course of CKD, it may be appropriate for a nephrologist to evaluate the patient and recommend a set of treatment goals. Follow-up may be infrequent or unnecessary.
As CKD progresses, especially as the patient reaches an estimated GFR of 30 mL/min/1.73 m2, the nephrologist will take a more active role in the patient’s care and medical decision-making. In some circumstances, it may even be appropriate for the nephrologist to be the patient’s source of primary care, with the primary care provider as a consultant.
Caring for patients with CKD includes not only strategies to preserve renal function and prolong survival, but also making critical decisions about starting dialysis and about the need for transplantation. Early involvement of a nephrologist and early preparation for end-stage renal disease with preemptive transplantation and arteriovenous fistula placement are associated with better patient outcomes. Key to this is collaboration between the primary care provider and the nephrologist, with levels of responsibility for patient care that adapt to the patient’s degree of renal dysfunction and other comorbidities. Such strategies to select patients for timely nephrology referral may help improve outcomes in this vulnerable population.
- United States Renal Data System (USRDS). Identification and care of patients with CKD. http://www.usrds.org/2012/pdf/v1_ch2_12.pdf. Accessed March 5, 2014.
- Simon J, Amde M, Poggio ED. Interpreting the estimated glomerular filtration rate in primary care: benefits and pitfalls. Cleve Clin J Med 2011; 78:189–195.
- National Kidney Foundation, Inc. KDOQI Clinical Practice Guidelines for Chronic Kidney Disease: Evaluation, Classification, and Stratification. http://www.kidney.org/professionals/kdoqi/guidelines_ckd/p4_class_g1.htm. Accessed March 5, 2014.
- Orlando LA, Owen WF, Matchar DB. Relationship between nephrologist care and progression of chronic kidney disease. N C Med J 2007; 68:9–16.
- Tseng CL, Kern EF, Miller DR, et al. Survival benefit of nephrologic care in patients with diabetes mellitus and chronic kidney disease. Arch Intern Med 2008; 168:55–62.
- Keith DS, Nichols GA, Gullion CM, Brown JB, Smith DH. Longitudinal follow-up and outcomes among a population with chronic kidney disease in a large managed care organization. Arch Intern Med 2004; 164:659–663.
- Serrano A, Huang J, Ghossein C, et al. Stabilization of glomerular filtration rate in advanced chronic kidney disease: a two-year follow-up of a cohort of chronic kidney disease patients stages 4 and 5. Adv Chronic Kidney Dis 2007; 14:105–112.
- Kinchen KS, Sadler J, Fink N, et al. The timing of specialist evaluation in chronic kidney disease and mortality. Ann Intern Med 2002; 137:479–486.
- Tonelli M, Muntner P, Lloyd A, et al; Alberta Kidney Disease Network. Risk of coronary events in people with chronic kidney disease compared with those with diabetes: a population-level cohort study. Lancet 2012; 380:807–814.
- Wattanakit K, Coresh J, Muntner P, Marsh J, Folsom AR. Cardiovascular risk among adults with chronic kidney disease, with or without prior myocardial infarction. J Am Coll Cardiol 2006; 48:1183–1189.
- Foley RN, Wang C, Collins AJ. Cardiovascular risk factor profiles and kidney function stage in the US general population: the NHANES III study. Mayo Clin Proc 2005; 80:1270–1277.
- Muntner P, He J, Astor BC, Folsom AR, Coresh J. Traditional and nontraditional risk factors predict coronary heart disease in chronic kidney disease: results from the Atherosclerosis Risk in Communities Study. J Am Soc Nephrol 2005; 16:529–538.
- Navaneethan SD, Schold JD, Kirwan JP, et al. Metabolic syndrome, ESRD, and death in CKD. Clin J Am Soc Nephrol 2013; 8:945–952.
- Foley RN, Murray AM, Li S, et al. Chronic kidney disease and the risk for cardiovascular disease, renal replacement, and death in the United States Medicare population, 1998 to 1999. J Am Soc Nephrol 2005; 16:489–495.
- Weiner DE, Tighiouart H, Amin MG, et al. Chronic kidney disease as a risk factor for cardiovascular disease and all-cause mortality: a pooled analysis of community-based studies. J Am Soc Nephrol 2004; 15:1307–1315.
- Gerstein HC, Mann JF, Yi Q, et al; HOPE Study Investigators. Albuminuria and risk of cardiovascular events, death, and heart failure in diabetic and nondiabetic individuals. JAMA 2001; 286:421–426.
- Hillege HL, Fidler V, Diercks GF, et al; Prevention of Renal and Vascular End Stage Disease (PREVEND) Study Group. Urinary albumin excretion predicts cardiovascular and noncardiovascular mortality in general population. Circulation 2002; 106:1777–1782.
- Foley RN, Collins AJ, Ishani A, Kalra PA. Calcium-phosphate levels and cardiovascular disease in community-dwelling adults: the Atherosclerosis Risk in Communities (ARIC) Study. Am Heart J 2008; 156:556–563.
- Tonelli M, Sacks F, Pfeffer M, Gao Z, Curhan G; Cholesterol And Recurrent Events Trial Investigators. Relation between serum phosphate level and cardiovascular event rate in people with coronary disease. Circulation 2005; 112:2627–2633.
- Menon V, Wang X, Sarnak MJ, et al. Long-term outcomes in nondiabetic chronic kidney disease. Kidney Int 2008; 73:1310–1315.
- Kasiske BL. Hyperlipidemia in patients with chronic renal disease. Am J Kidney Dis 1998; 32(suppl 3):S142–S156.
- Kendrick J, Shlipak MG, Targher G, Cook T, Lindenfeld J, Chonchol M. Effect of lovastatin on primary prevention of cardiovascular events in mild CKD and kidney function loss: a post hoc analysis of the Air Force/Texas Coronary Atherosclerosis Prevention Study. Am J Kidney Dis 2010; 55:42–49.
- Colhoun HM, Betteridge DJ, Durrington PN, et al; CARDS Investigators. Effects of atorvastatin on kidney outcomes and cardiovascular disease in patients with diabetes: an analysis from the Collaborative Atorvastatin Diabetes Study (CARDS). Am J Kidney Dis 2009; 54:810–819.
- Koren MJ, Davidson MH, Wilson DJ, Fayyad RS, Zuckerman A, Reed DP; ALLIANCE Investigators. Focused atorvastatin therapy in managed-care patients with coronary heart disease and CKD. Am J Kidney Dis 2009; 53:741–750.
- Fellström BC, Jardine AG, Schmieder RE, et al; AURORA Study Group. Rosuvastatin and cardiovascular events in patients undergoing hemodialysis. N Engl J Med 2009; 360:1395–1407.
- Chonchol M, Cook T, Kjekshus J, Pedersen TR, Lindenfeld J. Simvastatin for secondary prevention of all-cause mortality and major coronary events in patients with mild chronic renal insufficiency. Am J Kidney Dis 2007; 49:373–382.
- Ridker PM, MacFadyen J, Cressman M, Glynn RJ. Efficacy of rosuvastatin among men and women with moderate chronic kidney disease and elevated high-sensitivity C-reactive protein: a secondary analysis from the JUPITER (Justification for the Use of Statins in Prevention-an Intervention Trial Evaluating Rosuvastatin) trial. J Am Coll Cardiol 2010; 55:1266–1273.
- Baigent C, Landray MJ, Reith C, et al; SHARP Investigators. The effects of lowering LDL cholesterol with simvastatin plus ezetimibe in patients with chronic kidney disease (Study of Heart and Renal Protection): a randomised placebo-controlled trial. Lancet 2011; 377:2181–2192.
- Shepherd J, Kastelein JJ, Bittner V, et al; Treating to New Targets Investigators. Effect of intensive lipid lowering with atorvastatin on renal function in patients with coronary heart disease: the Treating to New Targets (TNT) study. Clin J Am Soc Nephrol 2007; 2:1131–1139.
- Tonelli M, Isles C, Craven T, et al. Effect of pravastatin on rate of kidney function loss in people with or at risk for coronary disease. Circulation 2005; 112:171–178.
- Palmer SC, Craig JC, Navaneethan SD, Tonelli M, Pellegrini F, Strippoli GF. Benefits and harms of statin therapy for persons with chronic kidney disease: a systematic review and meta-analysis. Ann Intern Med 2012; 157:263–275.
- National Kidney Foundation, Inc. KDOQI Clinical Practice Guidelines for Managing Dyslipidemias in Chronic Kidney Disease. http://www.kidney.org/professionals/kdoqi/guidelines_lipids/. Accessed March 5, 2014.
- Buckalew VM, Berg RL, Wang SR, Porush JG, Rauch S, Schulman G. Prevalence of hypertension in 1,795 subjects with chronic renal disease: the modification of diet in renal disease study baseline cohort. Modification of Diet in Renal Disease Study Group. Am J Kidney Dis 1996; 28:811–821.
- Coresh J, Wei GL, McQuillan G, et al. Prevalence of high blood pressure and elevated serum creatinine level in the United States: findings from the third National Health and Nutrition Examination Survey (1988–1994). Arch Intern Med 2001; 161:1207–1216.
- Klag MJ, Whelton PK, Randall BL, et al. Blood pressure and end-stage renal disease in men. N Engl J Med 1996; 334:13–18.
- Locatelli F, Marcelli D, Comelli M, et al. Proteinuria and blood pressure as causal components of progression to end-stage renal failure. Northern Italian Cooperative Study Group. Nephrol Dial Transplant 1996; 11:461–467.
- ACCORD Study Group; Cushman WC, Evans GW, Byington RP, et al. Effects of intensive blood-pressure control in type 2 diabetes mellitus. N Engl J Med 2010; 362:1575–1585.
- Jafar TH, Stark PC, Schmid CH, et al. Progression of chronic kidney disease: the role of blood pressure control, proteinuria, and angiotensin-converting enzyme inhibition: a patient-level meta-analysis. Ann Intern Med 2003; 139:244–252.
- Khosla N, Bakris G. Lessons learned from recent hypertension trials about kidney disease. Clin J Am Soc Nephrol 2006; 1:229–235.
- Norris KC, Greene T, Kopple J, et al. Baseline predictors of renal disease progression in the African American Study of Hypertension and Kidney Disease. J Am Soc Nephrol 2006; 17:2928–2936.
- Keane WF, Brenner BM, de Zeeuw D, et al; RENAAL Study Investigators. The risk of developing end-stage renal disease in patients with type 2 diabetes and nephropathy: the RENAAL study. Kidney Int 2003; 63:1499–1507.
- Ruggenenti P, Perna A, Mosconi L, et al. Proteinuria predicts end-stage renal failure in non-diabetic chronic nephropathies. The “Gruppo Italiano di Studi Epidemiologici in Nefrologia” (GISEN). Kidney Int Suppl 1997; 63:S54–S57.
- de Goeij MC, Liem M, de Jager DJ, et al; PREPARE-1 Study Group. Proteinuria as a risk marker for the progression of chronic kidney disease in patients on predialysis care and the role of angiotensin-converting enzyme inhibitor/angiotensin II receptor blocker treatment. Nephron Clin Pract 2012; 121:c73–c82.
- de Zeeuw D, Remuzzi G, Parving HH, et al. Albuminuria, a therapeutic target for cardiovascular protection in type 2 diabetic patients with nephropathy. Circulation 2004; 110:921–927.
- Ibsen H, Olsen MH, Wachtell K, et al. Reduction in albuminuria translates to reduction in cardiovascular events in hypertensive patients: losartan intervention for endpoint reduction in hypertension study. Hypertension 2005; 45:198–202.
- Atkins RC, Briganti EM, Lewis JB, et al. Proteinuria reduction and progression to renal failure in patients with type 2 diabetes mellitus and overt nephropathy. Am J Kidney Dis 2005; 45:281–287.
- Jafar TH, Stark PC, Schmid CH, et al; AIPRD Study Group; Angiotensin-Converting Enzyme Inhibition and Progression of Renal Disease. Proteinuria as a modifiable risk factor for the progression of non-diabetic renal disease. Kidney Int 2001; 60:1131–1140.
- ACE Inhibitors in Diabetic Nephropathy Trialist Group. Should all patients with type 1 diabetes mellitus and microalbuminuria receive angiotensin-converting enzyme inhibitors? A meta-analysis of individual patient data. Ann Intern Med 2001; 134:370–379.
- Casas JP, Chua W, Loukogeorgakis S, et al. Effect of inhibitors of the renin-angiotensin system and other antihypertensive drugs on renal outcomes: systematic review and meta-analysis. Lancet 2005; 366:2026–2033.
- Strippoli GF, Craig M, Deeks JJ, Schena FP, Craig JC. Effects of angiotensin converting enzyme inhibitors and angiotensin II receptor antagonists on mortality and renal outcomes in diabetic nephropathy: systematic review. BMJ 2004; 329:828.
- MacKinnon M, Shurraw S, Akbari A, Knoll GA, Jaffey J, Clark HD. Combination therapy with an angiotensin receptor blocker and an ACE inhibitor in proteinuric renal disease: a systematic review of the efficacy and safety data. Am J Kidney Dis 2006; 48:8–20.
- Kunz R, Friedrich C, Wolbers M, Mann JF. Meta-analysis: effect of mono-therapy and combination therapy with inhibitors of the renin angiotensin system on proteinuria in renal disease. Ann Intern Med 2008; 148:30–48.
- Ruggenenti P, Perticucci E, Cravedi P, et al. Role of remission clinics in the longitudinal treatment of CKD. J Am Soc Nephrol 2008; 19:1213–1224.
- Mann JF, Schmieder RE, McQueen M, et al; ONTARGET investigators. Renal outcomes with telmisartan, ramipril, or both, in people at high vascular risk (the ONTARGET study): a multicentre, randomised, double-blind, controlled trial. Lancet 2008; 372:547–553.
- Esnault VL, Ekhlas A, Delcroix C, Moutel MG, Nguyen JM. Diuretic and enhanced sodium restriction results in improved antiproteinuric response to RAS blocking agents. J Am Soc Nephrol 2005; 16:474–481.
- Vogt L, Waanders F, Boomsma F, de Zeeuw D, Navis G. Effects of dietary sodium and hydrochlorothiazide on the antiproteinuric efficacy of losartan. J Am Soc Nephrol 2008; 19:999–1007.
- Ahmed AK, Kamath NS, El Kossi M, El Nahas AM. The impact of stopping inhibitors of the renin-angiotensin system in patients with advanced chronic kidney disease. Nephrol Dial Transplant 2010; 25:3977–3982.
- Bakris GL, Weir MR, Secic M, Campbell B, Weis-McNulty A. Differential effects of calcium antagonist subclasses on markers of nephropathy progression. Kidney Int 2004; 65:1991–2002.
- Kloke HJ, Wetzels JF, Koene RA, Huysmans FT. Effects of low-dose nifedipine on urinary protein excretion rate in patients with renal disease. Nephrol Dial Transplant 1998; 13:646–650.
- Shah SN, Abramowitz M, Hostetter TH, Melamed ML. Serum bicarbonate levels and the progression of kidney disease: a cohort study. Am J Kidney Dis 2009; 54:270–277.
- de Brito-Ashurst I, Varagunam M, Raftery MJ, Yaqoob MM. Bicarbonate supplementation slows progression of CKD and improves nutritional status. J Am Soc Nephrol 2009; 20:2075–2084.
- Susantitaphong P, Sewaralthahab K, Balk EM, Jaber BL, Madias NE. Short- and long-term effects of alkali therapy in chronic kidney disease: a systematic review. Am J Nephrol 2012; 35:540–547.
- Nath KA, Hostetter MK, Hostetter TH. Ammonia-complement interaction in the pathogenesis of progressive renal injury. Kidney Int Suppl 1989; 27:S52–S54.
- Clinical practice guidelines for nutrition in chronic renal failure. K/DOQI, National Kidney Foundation. Am J Kidney Dis 2000; 35(suppl 2):S1–S140.
- Shimada T, Yamazaki Y, Takahashi M, et al. Vitamin D receptor-independent FGF23 actions in regulating phosphate and vitamin D metabolism. Am J Physiol Renal Physiol 2005; 289:F1088–F1095.
- Hasegawa H, Nagano N, Urakawa I, et al. Direct evidence for a causative role of FGF23 in the abnormal renal phosphate handling and vitamin D metabolism in rats with early-stage chronic kidney disease. Kidney Int 2010; 78:975–980.
- de Boer IH, Rue TC, Kestenbaum B. Serum phosphorus concentrations in the third National Health and Nutrition Examination Survey (NHANES III). Am J Kidney Dis 2009; 53:399–407.
- Kendrick J, Cheung AK, Kaufman JS, et al; HOST Investigators. FGF-23 associates with death, cardiovascular events, and initiation of chronic dialysis. J Am Soc Nephrol 2011; 22:1913–1922.
- Palmer SC, Hayen A, Macaskill P, et al. Serum levels of phosphorus, parathyroid hormone, and calcium and risks of death and cardiovascular disease in individuals with chronic kidney disease: a systematic review and meta-analysis. JAMA. 2011; 305:1119–1127.
- Kooienga L, Fried L, Scragg R, Kendrick J, Smits G, Chonchol M. The effect of combined calcium and vitamin D3 supplementation on serum intact parathyroid hormone in moderate CKD. Am J Kidney Dis 2009; 53:408–416.
- National Kidney Foundation, Inc. KDOQI Clinical Practice Guidelines for Bone Metabolism and Disease in Chronic Kidney Disease. www.kidney.org/professionals/kdoqi/guidelines_bone/guide1.htm#table15. Accessed March 5, 2014.
- Kidney International. KDIGO Clinical Practice Guideline for the Diagnosis, Evaluation, Prevention, and Treatment of Chronic Kidney Disease-Mineral and Bone Disorder (CKD-MBD). http://kdigo.org/home/mineral-bone-disorder. Accessed March 5, 2014.
- Kazmi WH, Kausz AT, Khan S, et al. Anemia: an early complication of chronic renal insufficiency. Am J Kidney Dis 2001; 38:803–812.
- Sarnak MJ, Tighiouart H, Manjunath G, et al. Anemia as a risk factor for cardiovascular disease in the Atherosclerosis Risk in Communities (ARIC) study. J Am Coll Cardiol 2002; 40:27–33.
- Thorp ML, Johnson ES, Yang X, Petrik AF, Platt R, Smith DH. Effect of anaemia on mortality, cardiovascular hospitalizations and end-stage renal disease among patients with chronic kidney disease. Nephrology (Carlton) 2009; 14:240–246.
- Mircescu G, Gârneata L, Capusa C, Ursea N. Intravenous iron supplementation for the treatment of anaemia in pre-dialyzed chronic renal failure patients. Nephrol Dial Transplant 2006; 21:120–124.
- Silverberg DS, Iaina A, Peer G, et al. Intravenous iron supplementation for the treatment of the anemia of moderate to severe chronic renal failure patients not receiving dialysis. Am J Kidney Dis 1996; 27:234–238.
- Singh AK, Szczech L, Tang KL, et al; CHOIR Investigators. Correction of anemia with epoetin alfa in chronic kidney disease. N Engl J Med 2006; 355:2085–2098.
- Drüeke TB, Locatelli F, Clyne N, et al; CREATE Investigators. Normalization of hemoglobin level in patients with chronic kidney disease and anemia. N Engl J Med 2006; 355:2071–2084.
- Pfeffer MA, Burdmann EA, Chen CY, et al; TREAT Investigators. A trial of darbepoetin alfa in type 2 diabetes and chronic kidney disease. N Engl J Med 2009; 361:2019–2032.
- US Food and Drug Administration (FDA). FDA Drug Safety Communication: modified dosing recommendations to improve the safe use of erythropoiesis-stimulating agents (ESAs) in chronic kidney disease. http://www.fda.gov/drugs/drugsafety/ucm259639.htm. Accessed March 5, 2014.
- Kurella M, Covinsky KE, Collins AJ, Chertow GM. Octogenarians and nonagenarians starting dialysis in the United States. Ann Intern Med 2007; 146:177–183.
- Kurella Tamura M, Covinsky KE, Chertow GM, Yaffe K, Landefeld CS, McCulloch CE. Functional status of elderly adults before and after initiation of dialysis. N Engl J Med 2009; 361:1539–1547.
- Renal Physicians Association. Clinical Practice Guideline. Shared Decision-Making in the Appropriate Initiation of and Withdrawal from Dialysis. 2nd ed.
- Vollmer WM, Wahl PW, Blagg CR. Survival with dialysis and transplantation in patients with end-stage renal disease. N Engl J Med 1983; 308:1553–1558.
- Port FK, Wolfe RA, Mauger EA, Berling DP, Jiang K. Comparison of survival probabilities for dialysis patients vs cadaveric renal transplant recipients. JAMA 1993; 270:1339–1343.
- Wolfe RA, Ashby VB, Milford EL, et al. Comparison of mortality in all patients on dialysis, patients on dialysis awaiting transplantation, and recipients of a first cadaveric transplant. N Engl J Med 1999; 341:1725–1730.
- Cosio FG, Alamir A, Yim S, et al. Patient survival after renal transplantation: I. The impact of dialysis pre-transplant. Kidney Int 1998; 53:767–772.
- Meier-Kriesche HU, Port FK, Ojo AO, et al. Effect of waiting time on renal transplant outcome. Kidney Int 2000; 58:1311–1317.
- Mange KC, Joffe MM, Feldman HI. Effect of the use or nonuse of long-term dialysis on the subsequent survival of renal transplants from living donors. N Engl J Med 2001; 344:726–731.
- Dhingra RK, Young EW, Hulbert-Shearon TE, Leavey SF, Port FK. Type of vascular access and mortality in US hemodialysis patients. Kidney Int 2001; 60:1443–1451.
- Nassar GM, Ayus JC. Infectious complications of the hemodialysis access. Kidney Int 2001; 60:1–13.
- Perera GB, Mueller MP, Kubaska SM, Wilson SE, Lawrence PF, Fujitani RM. Superiority of autogenous arteriovenous hemodialysis access: maintenance of function with fewer secondary interventions. Ann Vasc Surg 2004; 18:66–73.
- Basile C, Casucci F, Lomonte C. Timing of first cannulation of arteriovenous fistula: time matters, but there is also something else. Nephrol Dial Transplant 2005; 20:1519–1520.
- Biuckians A, Scott EC, Meier GH, Panneton JM, Glickman MH. The natural history of autologous fistulas as first-time dialysis access in the KDOQI era. J Vasc Surg 2008; 47:415–421.
- National Kidney Foundation, Inc. KDOQI Clinical Practice Guidelines for Vascular Access. http://www.kidney.org/professionals/KDOQI/guideline_upHD_PD_VA/va_guide1.htm. Accessed March 5, 2014.
- Arora P, Obrador GT, Ruthazer R, et al. Prevalence, predictors, and consequences of late nephrology referral at a tertiary care center. J Am Soc Nephrol 1999; 10:1281–1286.
- Gøransson LG, Bergrem H. Consequences of late referral of patients with end-stage renal disease. J Intern Med 2001; 250:154–159.
- Cooper BA, Branley P, Bulfone L, et al. A randomized, controlled trial of early versus late initiation of dialysis. N Engl J Med 2010; 363:609–619.
- Carson RC, Juszczak M, Davenport A, Burns A. Is maximum conservative management an equivalent treatment option to dialysis for elderly patients with significant comorbid disease? Clin J Am Soc Nephrol 2009; 4:1611–1619.
- Murtagh FE, Marsh JE, Donohoe P, Ekbal NJ, Sheerin NS, Harris FE. Dialysis or not? A comparative survival study of patients over 75 years with chronic kidney disease stage 5. Nephrol Dial Transplant 2007; 22:1955–1962.
- United States Renal Data System (USRDS). Identification and care of patients with CKD. http://www.usrds.org/2012/pdf/v1_ch2_12.pdf. Accessed March 5, 2014.
- Simon J, Amde M, Poggio ED. Interpreting the estimated glomerular filtration rate in primary care: benefits and pitfalls. Cleve Clin J Med 2011; 78:189–195.
- National Kidney Foundation, Inc. KDOQI Clinical Practice Guidelines for Chronic Kidney Disease: Evaluation, Classification, and Stratification. http://www.kidney.org/professionals/kdoqi/guidelines_ckd/p4_class_g1.htm. Accessed March 5, 2014.
- Orlando LA, Owen WF, Matchar DB. Relationship between nephrologist care and progression of chronic kidney disease. N C Med J 2007; 68:9–16.
- Tseng CL, Kern EF, Miller DR, et al. Survival benefit of nephrologic care in patients with diabetes mellitus and chronic kidney disease. Arch Intern Med 2008; 168:55–62.
- Keith DS, Nichols GA, Gullion CM, Brown JB, Smith DH. Longitudinal follow-up and outcomes among a population with chronic kidney disease in a large managed care organization. Arch Intern Med 2004; 164:659–663.
- Serrano A, Huang J, Ghossein C, et al. Stabilization of glomerular filtration rate in advanced chronic kidney disease: a two-year follow-up of a cohort of chronic kidney disease patients stages 4 and 5. Adv Chronic Kidney Dis 2007; 14:105–112.
- Kinchen KS, Sadler J, Fink N, et al. The timing of specialist evaluation in chronic kidney disease and mortality. Ann Intern Med 2002; 137:479–486.
- Tonelli M, Muntner P, Lloyd A, et al; Alberta Kidney Disease Network. Risk of coronary events in people with chronic kidney disease compared with those with diabetes: a population-level cohort study. Lancet 2012; 380:807–814.
- Wattanakit K, Coresh J, Muntner P, Marsh J, Folsom AR. Cardiovascular risk among adults with chronic kidney disease, with or without prior myocardial infarction. J Am Coll Cardiol 2006; 48:1183–1189.
- Foley RN, Wang C, Collins AJ. Cardiovascular risk factor profiles and kidney function stage in the US general population: the NHANES III study. Mayo Clin Proc 2005; 80:1270–1277.
- Muntner P, He J, Astor BC, Folsom AR, Coresh J. Traditional and nontraditional risk factors predict coronary heart disease in chronic kidney disease: results from the Atherosclerosis Risk in Communities Study. J Am Soc Nephrol 2005; 16:529–538.
- Navaneethan SD, Schold JD, Kirwan JP, et al. Metabolic syndrome, ESRD, and death in CKD. Clin J Am Soc Nephrol 2013; 8:945–952.
- Foley RN, Murray AM, Li S, et al. Chronic kidney disease and the risk for cardiovascular disease, renal replacement, and death in the United States Medicare population, 1998 to 1999. J Am Soc Nephrol 2005; 16:489–495.
- Weiner DE, Tighiouart H, Amin MG, et al. Chronic kidney disease as a risk factor for cardiovascular disease and all-cause mortality: a pooled analysis of community-based studies. J Am Soc Nephrol 2004; 15:1307–1315.
- Gerstein HC, Mann JF, Yi Q, et al; HOPE Study Investigators. Albuminuria and risk of cardiovascular events, death, and heart failure in diabetic and nondiabetic individuals. JAMA 2001; 286:421–426.
- Hillege HL, Fidler V, Diercks GF, et al; Prevention of Renal and Vascular End Stage Disease (PREVEND) Study Group. Urinary albumin excretion predicts cardiovascular and noncardiovascular mortality in general population. Circulation 2002; 106:1777–1782.
- Foley RN, Collins AJ, Ishani A, Kalra PA. Calcium-phosphate levels and cardiovascular disease in community-dwelling adults: the Atherosclerosis Risk in Communities (ARIC) Study. Am Heart J 2008; 156:556–563.
- Tonelli M, Sacks F, Pfeffer M, Gao Z, Curhan G; Cholesterol And Recurrent Events Trial Investigators. Relation between serum phosphate level and cardiovascular event rate in people with coronary disease. Circulation 2005; 112:2627–2633.
- Menon V, Wang X, Sarnak MJ, et al. Long-term outcomes in nondiabetic chronic kidney disease. Kidney Int 2008; 73:1310–1315.
- Kasiske BL. Hyperlipidemia in patients with chronic renal disease. Am J Kidney Dis 1998; 32(suppl 3):S142–S156.
- Kendrick J, Shlipak MG, Targher G, Cook T, Lindenfeld J, Chonchol M. Effect of lovastatin on primary prevention of cardiovascular events in mild CKD and kidney function loss: a post hoc analysis of the Air Force/Texas Coronary Atherosclerosis Prevention Study. Am J Kidney Dis 2010; 55:42–49.
- Colhoun HM, Betteridge DJ, Durrington PN, et al; CARDS Investigators. Effects of atorvastatin on kidney outcomes and cardiovascular disease in patients with diabetes: an analysis from the Collaborative Atorvastatin Diabetes Study (CARDS). Am J Kidney Dis 2009; 54:810–819.
- Koren MJ, Davidson MH, Wilson DJ, Fayyad RS, Zuckerman A, Reed DP; ALLIANCE Investigators. Focused atorvastatin therapy in managed-care patients with coronary heart disease and CKD. Am J Kidney Dis 2009; 53:741–750.
- Fellström BC, Jardine AG, Schmieder RE, et al; AURORA Study Group. Rosuvastatin and cardiovascular events in patients undergoing hemodialysis. N Engl J Med 2009; 360:1395–1407.
- Chonchol M, Cook T, Kjekshus J, Pedersen TR, Lindenfeld J. Simvastatin for secondary prevention of all-cause mortality and major coronary events in patients with mild chronic renal insufficiency. Am J Kidney Dis 2007; 49:373–382.
- Ridker PM, MacFadyen J, Cressman M, Glynn RJ. Efficacy of rosuvastatin among men and women with moderate chronic kidney disease and elevated high-sensitivity C-reactive protein: a secondary analysis from the JUPITER (Justification for the Use of Statins in Prevention-an Intervention Trial Evaluating Rosuvastatin) trial. J Am Coll Cardiol 2010; 55:1266–1273.
- Baigent C, Landray MJ, Reith C, et al; SHARP Investigators. The effects of lowering LDL cholesterol with simvastatin plus ezetimibe in patients with chronic kidney disease (Study of Heart and Renal Protection): a randomised placebo-controlled trial. Lancet 2011; 377:2181–2192.
- Shepherd J, Kastelein JJ, Bittner V, et al; Treating to New Targets Investigators. Effect of intensive lipid lowering with atorvastatin on renal function in patients with coronary heart disease: the Treating to New Targets (TNT) study. Clin J Am Soc Nephrol 2007; 2:1131–1139.
- Tonelli M, Isles C, Craven T, et al. Effect of pravastatin on rate of kidney function loss in people with or at risk for coronary disease. Circulation 2005; 112:171–178.
- Palmer SC, Craig JC, Navaneethan SD, Tonelli M, Pellegrini F, Strippoli GF. Benefits and harms of statin therapy for persons with chronic kidney disease: a systematic review and meta-analysis. Ann Intern Med 2012; 157:263–275.
- National Kidney Foundation, Inc. KDOQI Clinical Practice Guidelines for Managing Dyslipidemias in Chronic Kidney Disease. http://www.kidney.org/professionals/kdoqi/guidelines_lipids/. Accessed March 5, 2014.
- Buckalew VM, Berg RL, Wang SR, Porush JG, Rauch S, Schulman G. Prevalence of hypertension in 1,795 subjects with chronic renal disease: the modification of diet in renal disease study baseline cohort. Modification of Diet in Renal Disease Study Group. Am J Kidney Dis 1996; 28:811–821.
- Coresh J, Wei GL, McQuillan G, et al. Prevalence of high blood pressure and elevated serum creatinine level in the United States: findings from the third National Health and Nutrition Examination Survey (1988–1994). Arch Intern Med 2001; 161:1207–1216.
- Klag MJ, Whelton PK, Randall BL, et al. Blood pressure and end-stage renal disease in men. N Engl J Med 1996; 334:13–18.
- Locatelli F, Marcelli D, Comelli M, et al. Proteinuria and blood pressure as causal components of progression to end-stage renal failure. Northern Italian Cooperative Study Group. Nephrol Dial Transplant 1996; 11:461–467.
- ACCORD Study Group; Cushman WC, Evans GW, Byington RP, et al. Effects of intensive blood-pressure control in type 2 diabetes mellitus. N Engl J Med 2010; 362:1575–1585.
- Jafar TH, Stark PC, Schmid CH, et al. Progression of chronic kidney disease: the role of blood pressure control, proteinuria, and angiotensin-converting enzyme inhibition: a patient-level meta-analysis. Ann Intern Med 2003; 139:244–252.
- Khosla N, Bakris G. Lessons learned from recent hypertension trials about kidney disease. Clin J Am Soc Nephrol 2006; 1:229–235.
- Norris KC, Greene T, Kopple J, et al. Baseline predictors of renal disease progression in the African American Study of Hypertension and Kidney Disease. J Am Soc Nephrol 2006; 17:2928–2936.
- Keane WF, Brenner BM, de Zeeuw D, et al; RENAAL Study Investigators. The risk of developing end-stage renal disease in patients with type 2 diabetes and nephropathy: the RENAAL study. Kidney Int 2003; 63:1499–1507.
- Ruggenenti P, Perna A, Mosconi L, et al. Proteinuria predicts end-stage renal failure in non-diabetic chronic nephropathies. The “Gruppo Italiano di Studi Epidemiologici in Nefrologia” (GISEN). Kidney Int Suppl 1997; 63:S54–S57.
- de Goeij MC, Liem M, de Jager DJ, et al; PREPARE-1 Study Group. Proteinuria as a risk marker for the progression of chronic kidney disease in patients on predialysis care and the role of angiotensin-converting enzyme inhibitor/angiotensin II receptor blocker treatment. Nephron Clin Pract 2012; 121:c73–c82.
- de Zeeuw D, Remuzzi G, Parving HH, et al. Albuminuria, a therapeutic target for cardiovascular protection in type 2 diabetic patients with nephropathy. Circulation 2004; 110:921–927.
- Ibsen H, Olsen MH, Wachtell K, et al. Reduction in albuminuria translates to reduction in cardiovascular events in hypertensive patients: losartan intervention for endpoint reduction in hypertension study. Hypertension 2005; 45:198–202.
- Atkins RC, Briganti EM, Lewis JB, et al. Proteinuria reduction and progression to renal failure in patients with type 2 diabetes mellitus and overt nephropathy. Am J Kidney Dis 2005; 45:281–287.
- Jafar TH, Stark PC, Schmid CH, et al; AIPRD Study Group; Angiotensin-Converting Enzyme Inhibition and Progression of Renal Disease. Proteinuria as a modifiable risk factor for the progression of non-diabetic renal disease. Kidney Int 2001; 60:1131–1140.
- ACE Inhibitors in Diabetic Nephropathy Trialist Group. Should all patients with type 1 diabetes mellitus and microalbuminuria receive angiotensin-converting enzyme inhibitors? A meta-analysis of individual patient data. Ann Intern Med 2001; 134:370–379.
- Casas JP, Chua W, Loukogeorgakis S, et al. Effect of inhibitors of the renin-angiotensin system and other antihypertensive drugs on renal outcomes: systematic review and meta-analysis. Lancet 2005; 366:2026–2033.
- Strippoli GF, Craig M, Deeks JJ, Schena FP, Craig JC. Effects of angiotensin converting enzyme inhibitors and angiotensin II receptor antagonists on mortality and renal outcomes in diabetic nephropathy: systematic review. BMJ 2004; 329:828.
- MacKinnon M, Shurraw S, Akbari A, Knoll GA, Jaffey J, Clark HD. Combination therapy with an angiotensin receptor blocker and an ACE inhibitor in proteinuric renal disease: a systematic review of the efficacy and safety data. Am J Kidney Dis 2006; 48:8–20.
- Kunz R, Friedrich C, Wolbers M, Mann JF. Meta-analysis: effect of mono-therapy and combination therapy with inhibitors of the renin angiotensin system on proteinuria in renal disease. Ann Intern Med 2008; 148:30–48.
- Ruggenenti P, Perticucci E, Cravedi P, et al. Role of remission clinics in the longitudinal treatment of CKD. J Am Soc Nephrol 2008; 19:1213–1224.
- Mann JF, Schmieder RE, McQueen M, et al; ONTARGET investigators. Renal outcomes with telmisartan, ramipril, or both, in people at high vascular risk (the ONTARGET study): a multicentre, randomised, double-blind, controlled trial. Lancet 2008; 372:547–553.
- Esnault VL, Ekhlas A, Delcroix C, Moutel MG, Nguyen JM. Diuretic and enhanced sodium restriction results in improved antiproteinuric response to RAS blocking agents. J Am Soc Nephrol 2005; 16:474–481.
- Vogt L, Waanders F, Boomsma F, de Zeeuw D, Navis G. Effects of dietary sodium and hydrochlorothiazide on the antiproteinuric efficacy of losartan. J Am Soc Nephrol 2008; 19:999–1007.
- Ahmed AK, Kamath NS, El Kossi M, El Nahas AM. The impact of stopping inhibitors of the renin-angiotensin system in patients with advanced chronic kidney disease. Nephrol Dial Transplant 2010; 25:3977–3982.
- Bakris GL, Weir MR, Secic M, Campbell B, Weis-McNulty A. Differential effects of calcium antagonist subclasses on markers of nephropathy progression. Kidney Int 2004; 65:1991–2002.
- Kloke HJ, Wetzels JF, Koene RA, Huysmans FT. Effects of low-dose nifedipine on urinary protein excretion rate in patients with renal disease. Nephrol Dial Transplant 1998; 13:646–650.
- Shah SN, Abramowitz M, Hostetter TH, Melamed ML. Serum bicarbonate levels and the progression of kidney disease: a cohort study. Am J Kidney Dis 2009; 54:270–277.
- de Brito-Ashurst I, Varagunam M, Raftery MJ, Yaqoob MM. Bicarbonate supplementation slows progression of CKD and improves nutritional status. J Am Soc Nephrol 2009; 20:2075–2084.
- Susantitaphong P, Sewaralthahab K, Balk EM, Jaber BL, Madias NE. Short- and long-term effects of alkali therapy in chronic kidney disease: a systematic review. Am J Nephrol 2012; 35:540–547.
- Nath KA, Hostetter MK, Hostetter TH. Ammonia-complement interaction in the pathogenesis of progressive renal injury. Kidney Int Suppl 1989; 27:S52–S54.
- Clinical practice guidelines for nutrition in chronic renal failure. K/DOQI, National Kidney Foundation. Am J Kidney Dis 2000; 35(suppl 2):S1–S140.
- Shimada T, Yamazaki Y, Takahashi M, et al. Vitamin D receptor-independent FGF23 actions in regulating phosphate and vitamin D metabolism. Am J Physiol Renal Physiol 2005; 289:F1088–F1095.
- Hasegawa H, Nagano N, Urakawa I, et al. Direct evidence for a causative role of FGF23 in the abnormal renal phosphate handling and vitamin D metabolism in rats with early-stage chronic kidney disease. Kidney Int 2010; 78:975–980.
- de Boer IH, Rue TC, Kestenbaum B. Serum phosphorus concentrations in the third National Health and Nutrition Examination Survey (NHANES III). Am J Kidney Dis 2009; 53:399–407.
- Kendrick J, Cheung AK, Kaufman JS, et al; HOST Investigators. FGF-23 associates with death, cardiovascular events, and initiation of chronic dialysis. J Am Soc Nephrol 2011; 22:1913–1922.
- Palmer SC, Hayen A, Macaskill P, et al. Serum levels of phosphorus, parathyroid hormone, and calcium and risks of death and cardiovascular disease in individuals with chronic kidney disease: a systematic review and meta-analysis. JAMA. 2011; 305:1119–1127.
- Kooienga L, Fried L, Scragg R, Kendrick J, Smits G, Chonchol M. The effect of combined calcium and vitamin D3 supplementation on serum intact parathyroid hormone in moderate CKD. Am J Kidney Dis 2009; 53:408–416.
- National Kidney Foundation, Inc. KDOQI Clinical Practice Guidelines for Bone Metabolism and Disease in Chronic Kidney Disease. www.kidney.org/professionals/kdoqi/guidelines_bone/guide1.htm#table15. Accessed March 5, 2014.
- Kidney International. KDIGO Clinical Practice Guideline for the Diagnosis, Evaluation, Prevention, and Treatment of Chronic Kidney Disease-Mineral and Bone Disorder (CKD-MBD). http://kdigo.org/home/mineral-bone-disorder. Accessed March 5, 2014.
- Kazmi WH, Kausz AT, Khan S, et al. Anemia: an early complication of chronic renal insufficiency. Am J Kidney Dis 2001; 38:803–812.
- Sarnak MJ, Tighiouart H, Manjunath G, et al. Anemia as a risk factor for cardiovascular disease in the Atherosclerosis Risk in Communities (ARIC) study. J Am Coll Cardiol 2002; 40:27–33.
- Thorp ML, Johnson ES, Yang X, Petrik AF, Platt R, Smith DH. Effect of anaemia on mortality, cardiovascular hospitalizations and end-stage renal disease among patients with chronic kidney disease. Nephrology (Carlton) 2009; 14:240–246.
- Mircescu G, Gârneata L, Capusa C, Ursea N. Intravenous iron supplementation for the treatment of anaemia in pre-dialyzed chronic renal failure patients. Nephrol Dial Transplant 2006; 21:120–124.
- Silverberg DS, Iaina A, Peer G, et al. Intravenous iron supplementation for the treatment of the anemia of moderate to severe chronic renal failure patients not receiving dialysis. Am J Kidney Dis 1996; 27:234–238.
- Singh AK, Szczech L, Tang KL, et al; CHOIR Investigators. Correction of anemia with epoetin alfa in chronic kidney disease. N Engl J Med 2006; 355:2085–2098.
- Drüeke TB, Locatelli F, Clyne N, et al; CREATE Investigators. Normalization of hemoglobin level in patients with chronic kidney disease and anemia. N Engl J Med 2006; 355:2071–2084.
- Pfeffer MA, Burdmann EA, Chen CY, et al; TREAT Investigators. A trial of darbepoetin alfa in type 2 diabetes and chronic kidney disease. N Engl J Med 2009; 361:2019–2032.
- US Food and Drug Administration (FDA). FDA Drug Safety Communication: modified dosing recommendations to improve the safe use of erythropoiesis-stimulating agents (ESAs) in chronic kidney disease. http://www.fda.gov/drugs/drugsafety/ucm259639.htm. Accessed March 5, 2014.
- Kurella M, Covinsky KE, Collins AJ, Chertow GM. Octogenarians and nonagenarians starting dialysis in the United States. Ann Intern Med 2007; 146:177–183.
- Kurella Tamura M, Covinsky KE, Chertow GM, Yaffe K, Landefeld CS, McCulloch CE. Functional status of elderly adults before and after initiation of dialysis. N Engl J Med 2009; 361:1539–1547.
- Renal Physicians Association. Clinical Practice Guideline. Shared Decision-Making in the Appropriate Initiation of and Withdrawal from Dialysis. 2nd ed.
- Vollmer WM, Wahl PW, Blagg CR. Survival with dialysis and transplantation in patients with end-stage renal disease. N Engl J Med 1983; 308:1553–1558.
- Port FK, Wolfe RA, Mauger EA, Berling DP, Jiang K. Comparison of survival probabilities for dialysis patients vs cadaveric renal transplant recipients. JAMA 1993; 270:1339–1343.
- Wolfe RA, Ashby VB, Milford EL, et al. Comparison of mortality in all patients on dialysis, patients on dialysis awaiting transplantation, and recipients of a first cadaveric transplant. N Engl J Med 1999; 341:1725–1730.
- Cosio FG, Alamir A, Yim S, et al. Patient survival after renal transplantation: I. The impact of dialysis pre-transplant. Kidney Int 1998; 53:767–772.
- Meier-Kriesche HU, Port FK, Ojo AO, et al. Effect of waiting time on renal transplant outcome. Kidney Int 2000; 58:1311–1317.
- Mange KC, Joffe MM, Feldman HI. Effect of the use or nonuse of long-term dialysis on the subsequent survival of renal transplants from living donors. N Engl J Med 2001; 344:726–731.
- Dhingra RK, Young EW, Hulbert-Shearon TE, Leavey SF, Port FK. Type of vascular access and mortality in US hemodialysis patients. Kidney Int 2001; 60:1443–1451.
- Nassar GM, Ayus JC. Infectious complications of the hemodialysis access. Kidney Int 2001; 60:1–13.
- Perera GB, Mueller MP, Kubaska SM, Wilson SE, Lawrence PF, Fujitani RM. Superiority of autogenous arteriovenous hemodialysis access: maintenance of function with fewer secondary interventions. Ann Vasc Surg 2004; 18:66–73.
- Basile C, Casucci F, Lomonte C. Timing of first cannulation of arteriovenous fistula: time matters, but there is also something else. Nephrol Dial Transplant 2005; 20:1519–1520.
- Biuckians A, Scott EC, Meier GH, Panneton JM, Glickman MH. The natural history of autologous fistulas as first-time dialysis access in the KDOQI era. J Vasc Surg 2008; 47:415–421.
- National Kidney Foundation, Inc. KDOQI Clinical Practice Guidelines for Vascular Access. http://www.kidney.org/professionals/KDOQI/guideline_upHD_PD_VA/va_guide1.htm. Accessed March 5, 2014.
- Arora P, Obrador GT, Ruthazer R, et al. Prevalence, predictors, and consequences of late nephrology referral at a tertiary care center. J Am Soc Nephrol 1999; 10:1281–1286.
- Gøransson LG, Bergrem H. Consequences of late referral of patients with end-stage renal disease. J Intern Med 2001; 250:154–159.
- Cooper BA, Branley P, Bulfone L, et al. A randomized, controlled trial of early versus late initiation of dialysis. N Engl J Med 2010; 363:609–619.
- Carson RC, Juszczak M, Davenport A, Burns A. Is maximum conservative management an equivalent treatment option to dialysis for elderly patients with significant comorbid disease? Clin J Am Soc Nephrol 2009; 4:1611–1619.
- Murtagh FE, Marsh JE, Donohoe P, Ekbal NJ, Sheerin NS, Harris FE. Dialysis or not? A comparative survival study of patients over 75 years with chronic kidney disease stage 5. Nephrol Dial Transplant 2007; 22:1955–1962.
KEY POINTS
- Steps to stabilize renal function include blood pressure and diabetes control.
- Patients have a very high risk of cardiovascular disease, and one should try to reduce modifiable risk factors such as hypertension (which is also a risk factor for the progression of CKD) and hyperlipidemia.
- In addition to controlling blood pressure, angiotensin-converting enzyme inhibitors and angiotensin receptor blockers reduce proteinuria, a risk factor for progression of CKD.
- Patients with CKD develop secondary hyperparathyroidism, hyperphosphatemia, and, in advanced CKD, hypocalcemia, all leading to disorders of bone mineral metabolism. Low vitamin D levels should be raised with supplements, and high phosphorus levels should be lowered with dietary restriction and phosphate binders.
Hospital-Acquired Clostridium difficile Blamed for Poor Sepsis Outcomes
New research has found that hospital-onset Clostridium difficile infections increase length of stay (LOS), risk of in-hospital mortality, and hospital costs for inpatients with sepsis.
Authors of a new study titled, "The Impact of Hospital-onset Clostridium difficile Infection on Outcomes of Hospitalized Patients with Sepsis," report that after multivariate adjustment, in-hospital mortality rate was 24% for patients with sepsis who develop C. diff infections, versus 15% of inpatient controls, according to the paper that was published online in the Journal of Hospital Medicine earlier this month. Adjusted LOS among cases with C. diff was 5.1 days longer than controls (95% confidence interval: 4.4–5.8), and the median-adjusted cost increase was $4,916 (P<0.001).
"Big numbers, but I'm actually not surprised," says lead author Tara Lagu, MD, MPH, a hospitalist at the Center for Quality of Care Research at Baystate Medical Center in Springfield, Mass. "I know that it happens, because I see it all the time."
Dr. Lagu says that when a patient is on day four of five of a stay for sepsis and develops diarrhea, precautions and treatment will last a minimum of three days, which drives up LOS and cost of care.
In the report, Dr. Lagu did not compare the cost-effectiveness of C. diff prevention programs aimed at sepsis patients, but she's hopeful that is how physicians will use the data.
"I'm just suggesting that if, as a hospital, you're trying to figure out if your program is worth it, think about these numbers in terms of prevention,” she says. "If it looks like the cost is worth it, then you should keep doing what you're doing. If not, then maybe you should do something different if you're not preventing enough cases."
Visit our website for more information on preventing, managing C. diff infections.
New research has found that hospital-onset Clostridium difficile infections increase length of stay (LOS), risk of in-hospital mortality, and hospital costs for inpatients with sepsis.
Authors of a new study titled, "The Impact of Hospital-onset Clostridium difficile Infection on Outcomes of Hospitalized Patients with Sepsis," report that after multivariate adjustment, in-hospital mortality rate was 24% for patients with sepsis who develop C. diff infections, versus 15% of inpatient controls, according to the paper that was published online in the Journal of Hospital Medicine earlier this month. Adjusted LOS among cases with C. diff was 5.1 days longer than controls (95% confidence interval: 4.4–5.8), and the median-adjusted cost increase was $4,916 (P<0.001).
"Big numbers, but I'm actually not surprised," says lead author Tara Lagu, MD, MPH, a hospitalist at the Center for Quality of Care Research at Baystate Medical Center in Springfield, Mass. "I know that it happens, because I see it all the time."
Dr. Lagu says that when a patient is on day four of five of a stay for sepsis and develops diarrhea, precautions and treatment will last a minimum of three days, which drives up LOS and cost of care.
In the report, Dr. Lagu did not compare the cost-effectiveness of C. diff prevention programs aimed at sepsis patients, but she's hopeful that is how physicians will use the data.
"I'm just suggesting that if, as a hospital, you're trying to figure out if your program is worth it, think about these numbers in terms of prevention,” she says. "If it looks like the cost is worth it, then you should keep doing what you're doing. If not, then maybe you should do something different if you're not preventing enough cases."
Visit our website for more information on preventing, managing C. diff infections.
New research has found that hospital-onset Clostridium difficile infections increase length of stay (LOS), risk of in-hospital mortality, and hospital costs for inpatients with sepsis.
Authors of a new study titled, "The Impact of Hospital-onset Clostridium difficile Infection on Outcomes of Hospitalized Patients with Sepsis," report that after multivariate adjustment, in-hospital mortality rate was 24% for patients with sepsis who develop C. diff infections, versus 15% of inpatient controls, according to the paper that was published online in the Journal of Hospital Medicine earlier this month. Adjusted LOS among cases with C. diff was 5.1 days longer than controls (95% confidence interval: 4.4–5.8), and the median-adjusted cost increase was $4,916 (P<0.001).
"Big numbers, but I'm actually not surprised," says lead author Tara Lagu, MD, MPH, a hospitalist at the Center for Quality of Care Research at Baystate Medical Center in Springfield, Mass. "I know that it happens, because I see it all the time."
Dr. Lagu says that when a patient is on day four of five of a stay for sepsis and develops diarrhea, precautions and treatment will last a minimum of three days, which drives up LOS and cost of care.
In the report, Dr. Lagu did not compare the cost-effectiveness of C. diff prevention programs aimed at sepsis patients, but she's hopeful that is how physicians will use the data.
"I'm just suggesting that if, as a hospital, you're trying to figure out if your program is worth it, think about these numbers in terms of prevention,” she says. "If it looks like the cost is worth it, then you should keep doing what you're doing. If not, then maybe you should do something different if you're not preventing enough cases."
Visit our website for more information on preventing, managing C. diff infections.
Pre-Operative Beta Blockers May Benefit Some Cardiac Patients
Clinical question: In patients with ischemic heart disease (IHD) undergoing non-cardiac surgery, do pre-operative beta blockers reduce post-operative major cardiovascular events (MACE) or mortality at 30 days?
Background: Pre-operative beta blocker use has become more restricted, as evidence about which patients derive benefit has become clearer. Opinions and practice vary regarding whether all patients with IHD, or only certain populations within this group, benefit from pre-operative beta blockers.
Study design: Retrospective, national registry-based cohort study.
Setting: Denmark, 2004-2009.
Synopsis: No benefit was found for the overall cohort of 28,263 patients. Patients with IHD and heart failure (n=7990) had lower risk of MACE (HR=0.75, 95% CI, 0.70-0.87) and mortality (HR=0.80, 95% CI, 0.70-0.92). Patients with IHD and myocardial infarction within two years (n=1664) had lower risk of MACE (HR=0.54, 95% CI, 0.37-0.78) but not mortality. Beta blocker dose and compliance were unknown. Whether patients had symptoms or inducible ischemia was not clear. This study supports the concept that higher-risk patients benefit more from pre-operative beta blockers, but it is not high-grade evidence.
Bottom line: Not all patients with IHD benefit from pre-operative beta blockers; those with concomitant heart failure or recent MI have a lower risk of MACE and/or mortality at 30 days with beta blockers.
Citation: Andersson C, Merie C, Jorgensen M, et al. Association of ß-blocker therapy with risks of adverse cardiovascular events and deaths in patients with ischemic heart disease undergoing non-cardiac surgery: A Danish nationwide cohort study JAMA Intern Med. 2014;174(3):336-344.
Clinical question: In patients with ischemic heart disease (IHD) undergoing non-cardiac surgery, do pre-operative beta blockers reduce post-operative major cardiovascular events (MACE) or mortality at 30 days?
Background: Pre-operative beta blocker use has become more restricted, as evidence about which patients derive benefit has become clearer. Opinions and practice vary regarding whether all patients with IHD, or only certain populations within this group, benefit from pre-operative beta blockers.
Study design: Retrospective, national registry-based cohort study.
Setting: Denmark, 2004-2009.
Synopsis: No benefit was found for the overall cohort of 28,263 patients. Patients with IHD and heart failure (n=7990) had lower risk of MACE (HR=0.75, 95% CI, 0.70-0.87) and mortality (HR=0.80, 95% CI, 0.70-0.92). Patients with IHD and myocardial infarction within two years (n=1664) had lower risk of MACE (HR=0.54, 95% CI, 0.37-0.78) but not mortality. Beta blocker dose and compliance were unknown. Whether patients had symptoms or inducible ischemia was not clear. This study supports the concept that higher-risk patients benefit more from pre-operative beta blockers, but it is not high-grade evidence.
Bottom line: Not all patients with IHD benefit from pre-operative beta blockers; those with concomitant heart failure or recent MI have a lower risk of MACE and/or mortality at 30 days with beta blockers.
Citation: Andersson C, Merie C, Jorgensen M, et al. Association of ß-blocker therapy with risks of adverse cardiovascular events and deaths in patients with ischemic heart disease undergoing non-cardiac surgery: A Danish nationwide cohort study JAMA Intern Med. 2014;174(3):336-344.
Clinical question: In patients with ischemic heart disease (IHD) undergoing non-cardiac surgery, do pre-operative beta blockers reduce post-operative major cardiovascular events (MACE) or mortality at 30 days?
Background: Pre-operative beta blocker use has become more restricted, as evidence about which patients derive benefit has become clearer. Opinions and practice vary regarding whether all patients with IHD, or only certain populations within this group, benefit from pre-operative beta blockers.
Study design: Retrospective, national registry-based cohort study.
Setting: Denmark, 2004-2009.
Synopsis: No benefit was found for the overall cohort of 28,263 patients. Patients with IHD and heart failure (n=7990) had lower risk of MACE (HR=0.75, 95% CI, 0.70-0.87) and mortality (HR=0.80, 95% CI, 0.70-0.92). Patients with IHD and myocardial infarction within two years (n=1664) had lower risk of MACE (HR=0.54, 95% CI, 0.37-0.78) but not mortality. Beta blocker dose and compliance were unknown. Whether patients had symptoms or inducible ischemia was not clear. This study supports the concept that higher-risk patients benefit more from pre-operative beta blockers, but it is not high-grade evidence.
Bottom line: Not all patients with IHD benefit from pre-operative beta blockers; those with concomitant heart failure or recent MI have a lower risk of MACE and/or mortality at 30 days with beta blockers.
Citation: Andersson C, Merie C, Jorgensen M, et al. Association of ß-blocker therapy with risks of adverse cardiovascular events and deaths in patients with ischemic heart disease undergoing non-cardiac surgery: A Danish nationwide cohort study JAMA Intern Med. 2014;174(3):336-344.
Eteplirsen showed safety, efficacy over 2 years in Duchenne muscular dystrophy
PHILADELPHIA – Eteplirsen safely maintained its beneficial effect on walking speeds for certain patients with Duchenne muscular dystrophy for more than 2 years and was the first drug to show stabilized diaphragm function over the same period.
The investigational gene therapy drug had a stabilizing effect on patients’ walking speed over the course of 120 weeks regardless of whether they started it earlier or later in the open-label extension of the initial 24-week, randomized trial, but those who started treatment earlier maintained a higher walking speed, Dr. Jerry R. Mendell said at the annual meeting of the American Academy of Neurology.
Coinvestigator Dr. Edward M. Kaye of Sarepta Therapeutics, the study sponsor, presented in-depth safety and pharmacokinetics data that showed no significant treatment-related adverse events with eteplirsen in the small study. No patients have discontinued or disrupted treatment, and no laboratory evidence of toxicity has been seen to date.
The initial 24-week, double-blind, randomized, placebo-controlled study involved four patients who were given the equivalent of 30 mg/kg eteplirsen weekly, four given 50 mg/kg eteplirsen weekly, and four given placebo. At the start of the open-label extension study, two of the placebo-treated patients were transitioned to 30 mg/kg weekly and two to 50 mg/kg weekly.
All patients underwent muscle biopsy at baseline, followed by biopsies at 12 weeks in two of the high-dose patients and in two of the placebo-treated patients, and at 24 weeks in the four patients taking 30 mg/kg weekly and in two placebo-treated patients.
"This design permitted a comparison between high dose over a shorter time interval and low dose over a longer time interval," said Dr. Mendell of the Center for Gene Therapy at Nationwide Children’s Hospital, Columbus, Ohio.
All patients underwent a biopsy at 1 year (48 weeks) in the open-label extension (Ann. Neurol. 2013;74:637-47).
By 48 weeks, the mean percentage of dystrophin-positive muscle fibers was 34% in the placebo/30 mg/kg delayed-treatment group, 43% in the placebo/50 mg/kg delayed-treatment group, 42% in the 50-mg/kg group, and 52% in the 30-mg/kg group.
Distance traveled on the 6-minute walk test began to diverge between active and placebo-treated patients at 12 weeks and stabilized at 24 weeks among those who received active treatment, but continued to decline until 36 weeks for placebo-treated patients who started treatment at 24 weeks.
At 120 weeks, the patients who had received continuous treatment with eteplirsen declined by a mean of 14 m on the 6-minute walk test since baseline, compared with a decline of 79 m in those who underwent delayed treatment with eteplirsen. In comparison, studies of the natural history of Duchenne muscular dystrophy (DMD) have shown steady declines on the 6-minute walk test, ranging from 30 to 115 m at 1 year and 97 to 125 m at 2 years.
There was "remarkable stability" in diaphragm function over the course of treatment with eteplirsen, "which is quite different from the natural history of untreated patients," Dr. Mendell said. In all 12 patients, maximal expiratory pressure remained stable, going from a mean of 90% of predicted at baseline to 95% at 120 weeks. The same was true for maximal inspiratory pressure, moving from 79% of expected at baseline to 80% at 120 weeks. In contrast, the natural history of untreated DMD shows that pulmonary function declines substantially over time: by 3.9%/year for maximal inspiratory pressure after starting at 90% of predicted at 9 years of age, and by 3.6%/year after starting at 45% of predicted at 9 years of age (Pediatr. Pulmonol. 2014;49:473-81).
Eteplirsen was created to help patients with a deletion of exon 51 in dystrophin, which causes a nonfunctional dystrophin protein. The drug is a charge-neutral phosphorodiamidate morpholino oligomer (PMO) that targets the 13% of DMD patients with this mutation by directing alternative splicing of the dystrophin gene through its ability to bind to exon 51 of dystrophin pre-mRNA. This restores transcription and translation of a truncated, yet functional, dystrophin protein, such as those found in Becker muscular dystrophy.
The neutral charge of eteplirsen helps it to avoid binding to serum proteins, which is one of the problems that have occurred with phosphorothioate antisense oligonucleotide-based drugs. Phosphorothioate antisense drugs have also been linked to immune activation, hepatotoxicity, thrombocytopenia, coagulopathy, renal toxicity, and proteinuria. But Dr. Kaye reported that eteplirsen is mainly excreted by the kidneys, has a half-life of about 3 hours, and showed no evidence of those problems. Only 13 of 453 urine protein assessments tested positive, but all were low, transient, and resolved spontaneously.
"The reason that this is important is there has actually been very little human data until recently, despite the fact that [PMOs have] been around for over 30 years. Most of the work has been done in animals and in research laboratories, and it’s only until recently that people have been exposed to the drug," Dr. Kaye said.
The study was funded by Sarepta Therapeutics. Dr. Kaye and two coauthors are employees of the company. Dr. Mendell had no relevant disclosures.
PHILADELPHIA – Eteplirsen safely maintained its beneficial effect on walking speeds for certain patients with Duchenne muscular dystrophy for more than 2 years and was the first drug to show stabilized diaphragm function over the same period.
The investigational gene therapy drug had a stabilizing effect on patients’ walking speed over the course of 120 weeks regardless of whether they started it earlier or later in the open-label extension of the initial 24-week, randomized trial, but those who started treatment earlier maintained a higher walking speed, Dr. Jerry R. Mendell said at the annual meeting of the American Academy of Neurology.
Coinvestigator Dr. Edward M. Kaye of Sarepta Therapeutics, the study sponsor, presented in-depth safety and pharmacokinetics data that showed no significant treatment-related adverse events with eteplirsen in the small study. No patients have discontinued or disrupted treatment, and no laboratory evidence of toxicity has been seen to date.
The initial 24-week, double-blind, randomized, placebo-controlled study involved four patients who were given the equivalent of 30 mg/kg eteplirsen weekly, four given 50 mg/kg eteplirsen weekly, and four given placebo. At the start of the open-label extension study, two of the placebo-treated patients were transitioned to 30 mg/kg weekly and two to 50 mg/kg weekly.
All patients underwent muscle biopsy at baseline, followed by biopsies at 12 weeks in two of the high-dose patients and in two of the placebo-treated patients, and at 24 weeks in the four patients taking 30 mg/kg weekly and in two placebo-treated patients.
"This design permitted a comparison between high dose over a shorter time interval and low dose over a longer time interval," said Dr. Mendell of the Center for Gene Therapy at Nationwide Children’s Hospital, Columbus, Ohio.
All patients underwent a biopsy at 1 year (48 weeks) in the open-label extension (Ann. Neurol. 2013;74:637-47).
By 48 weeks, the mean percentage of dystrophin-positive muscle fibers was 34% in the placebo/30 mg/kg delayed-treatment group, 43% in the placebo/50 mg/kg delayed-treatment group, 42% in the 50-mg/kg group, and 52% in the 30-mg/kg group.
Distance traveled on the 6-minute walk test began to diverge between active and placebo-treated patients at 12 weeks and stabilized at 24 weeks among those who received active treatment, but continued to decline until 36 weeks for placebo-treated patients who started treatment at 24 weeks.
At 120 weeks, the patients who had received continuous treatment with eteplirsen declined by a mean of 14 m on the 6-minute walk test since baseline, compared with a decline of 79 m in those who underwent delayed treatment with eteplirsen. In comparison, studies of the natural history of Duchenne muscular dystrophy (DMD) have shown steady declines on the 6-minute walk test, ranging from 30 to 115 m at 1 year and 97 to 125 m at 2 years.
There was "remarkable stability" in diaphragm function over the course of treatment with eteplirsen, "which is quite different from the natural history of untreated patients," Dr. Mendell said. In all 12 patients, maximal expiratory pressure remained stable, going from a mean of 90% of predicted at baseline to 95% at 120 weeks. The same was true for maximal inspiratory pressure, moving from 79% of expected at baseline to 80% at 120 weeks. In contrast, the natural history of untreated DMD shows that pulmonary function declines substantially over time: by 3.9%/year for maximal inspiratory pressure after starting at 90% of predicted at 9 years of age, and by 3.6%/year after starting at 45% of predicted at 9 years of age (Pediatr. Pulmonol. 2014;49:473-81).
Eteplirsen was created to help patients with a deletion of exon 51 in dystrophin, which causes a nonfunctional dystrophin protein. The drug is a charge-neutral phosphorodiamidate morpholino oligomer (PMO) that targets the 13% of DMD patients with this mutation by directing alternative splicing of the dystrophin gene through its ability to bind to exon 51 of dystrophin pre-mRNA. This restores transcription and translation of a truncated, yet functional, dystrophin protein, such as those found in Becker muscular dystrophy.
The neutral charge of eteplirsen helps it to avoid binding to serum proteins, which is one of the problems that have occurred with phosphorothioate antisense oligonucleotide-based drugs. Phosphorothioate antisense drugs have also been linked to immune activation, hepatotoxicity, thrombocytopenia, coagulopathy, renal toxicity, and proteinuria. But Dr. Kaye reported that eteplirsen is mainly excreted by the kidneys, has a half-life of about 3 hours, and showed no evidence of those problems. Only 13 of 453 urine protein assessments tested positive, but all were low, transient, and resolved spontaneously.
"The reason that this is important is there has actually been very little human data until recently, despite the fact that [PMOs have] been around for over 30 years. Most of the work has been done in animals and in research laboratories, and it’s only until recently that people have been exposed to the drug," Dr. Kaye said.
The study was funded by Sarepta Therapeutics. Dr. Kaye and two coauthors are employees of the company. Dr. Mendell had no relevant disclosures.
PHILADELPHIA – Eteplirsen safely maintained its beneficial effect on walking speeds for certain patients with Duchenne muscular dystrophy for more than 2 years and was the first drug to show stabilized diaphragm function over the same period.
The investigational gene therapy drug had a stabilizing effect on patients’ walking speed over the course of 120 weeks regardless of whether they started it earlier or later in the open-label extension of the initial 24-week, randomized trial, but those who started treatment earlier maintained a higher walking speed, Dr. Jerry R. Mendell said at the annual meeting of the American Academy of Neurology.
Coinvestigator Dr. Edward M. Kaye of Sarepta Therapeutics, the study sponsor, presented in-depth safety and pharmacokinetics data that showed no significant treatment-related adverse events with eteplirsen in the small study. No patients have discontinued or disrupted treatment, and no laboratory evidence of toxicity has been seen to date.
The initial 24-week, double-blind, randomized, placebo-controlled study involved four patients who were given the equivalent of 30 mg/kg eteplirsen weekly, four given 50 mg/kg eteplirsen weekly, and four given placebo. At the start of the open-label extension study, two of the placebo-treated patients were transitioned to 30 mg/kg weekly and two to 50 mg/kg weekly.
All patients underwent muscle biopsy at baseline, followed by biopsies at 12 weeks in two of the high-dose patients and in two of the placebo-treated patients, and at 24 weeks in the four patients taking 30 mg/kg weekly and in two placebo-treated patients.
"This design permitted a comparison between high dose over a shorter time interval and low dose over a longer time interval," said Dr. Mendell of the Center for Gene Therapy at Nationwide Children’s Hospital, Columbus, Ohio.
All patients underwent a biopsy at 1 year (48 weeks) in the open-label extension (Ann. Neurol. 2013;74:637-47).
By 48 weeks, the mean percentage of dystrophin-positive muscle fibers was 34% in the placebo/30 mg/kg delayed-treatment group, 43% in the placebo/50 mg/kg delayed-treatment group, 42% in the 50-mg/kg group, and 52% in the 30-mg/kg group.
Distance traveled on the 6-minute walk test began to diverge between active and placebo-treated patients at 12 weeks and stabilized at 24 weeks among those who received active treatment, but continued to decline until 36 weeks for placebo-treated patients who started treatment at 24 weeks.
At 120 weeks, the patients who had received continuous treatment with eteplirsen declined by a mean of 14 m on the 6-minute walk test since baseline, compared with a decline of 79 m in those who underwent delayed treatment with eteplirsen. In comparison, studies of the natural history of Duchenne muscular dystrophy (DMD) have shown steady declines on the 6-minute walk test, ranging from 30 to 115 m at 1 year and 97 to 125 m at 2 years.
There was "remarkable stability" in diaphragm function over the course of treatment with eteplirsen, "which is quite different from the natural history of untreated patients," Dr. Mendell said. In all 12 patients, maximal expiratory pressure remained stable, going from a mean of 90% of predicted at baseline to 95% at 120 weeks. The same was true for maximal inspiratory pressure, moving from 79% of expected at baseline to 80% at 120 weeks. In contrast, the natural history of untreated DMD shows that pulmonary function declines substantially over time: by 3.9%/year for maximal inspiratory pressure after starting at 90% of predicted at 9 years of age, and by 3.6%/year after starting at 45% of predicted at 9 years of age (Pediatr. Pulmonol. 2014;49:473-81).
Eteplirsen was created to help patients with a deletion of exon 51 in dystrophin, which causes a nonfunctional dystrophin protein. The drug is a charge-neutral phosphorodiamidate morpholino oligomer (PMO) that targets the 13% of DMD patients with this mutation by directing alternative splicing of the dystrophin gene through its ability to bind to exon 51 of dystrophin pre-mRNA. This restores transcription and translation of a truncated, yet functional, dystrophin protein, such as those found in Becker muscular dystrophy.
The neutral charge of eteplirsen helps it to avoid binding to serum proteins, which is one of the problems that have occurred with phosphorothioate antisense oligonucleotide-based drugs. Phosphorothioate antisense drugs have also been linked to immune activation, hepatotoxicity, thrombocytopenia, coagulopathy, renal toxicity, and proteinuria. But Dr. Kaye reported that eteplirsen is mainly excreted by the kidneys, has a half-life of about 3 hours, and showed no evidence of those problems. Only 13 of 453 urine protein assessments tested positive, but all were low, transient, and resolved spontaneously.
"The reason that this is important is there has actually been very little human data until recently, despite the fact that [PMOs have] been around for over 30 years. Most of the work has been done in animals and in research laboratories, and it’s only until recently that people have been exposed to the drug," Dr. Kaye said.
The study was funded by Sarepta Therapeutics. Dr. Kaye and two coauthors are employees of the company. Dr. Mendell had no relevant disclosures.
AT THE AAN 2014 ANNUAL MEETING
Key clinical point: Treatment with eteplirsen stabilized key clinical features of DMD and had no significant adverse events over 2 years.
Major finding: At 120 weeks, patients who received continuous treatment with eteplirsen had a mean decline of 14 m on the 6-minute walk test since baseline, compared with a decline of 79 m in those who underwent delayed treatment with eteplirsen.
Data source: An open-label extension of a 24-week, randomized, double-blind, placebo-controlled trial out to 120 weeks in 12 patients with DMD.
Disclosures: The study is funded by Sarepta Therapeutics. Dr. Kaye and two coauthors are employees of the company. Dr. Mendell had no relevant disclosures.
Inmate Falls From Top Bunk
ANSWER
The radiograph demonstrates no acute osseous injury, such as fracture or dislocation. Of interest and note is increased sclerosis within both femoral heads, more so on the left versus the right side. Given the patient’s young age, such findings could be related to early avascular necrosis. His clinical symptoms certainly correlate. MRI or bone scan, as well as orthopedic evaluation, is warranted in such a case.
Fortunately, subsequent MRI of both hips did not show any avascular necrosis but rather osteoarthritic changes. The MRI of his spinal column was negative as well.
ANSWER
The radiograph demonstrates no acute osseous injury, such as fracture or dislocation. Of interest and note is increased sclerosis within both femoral heads, more so on the left versus the right side. Given the patient’s young age, such findings could be related to early avascular necrosis. His clinical symptoms certainly correlate. MRI or bone scan, as well as orthopedic evaluation, is warranted in such a case.
Fortunately, subsequent MRI of both hips did not show any avascular necrosis but rather osteoarthritic changes. The MRI of his spinal column was negative as well.
ANSWER
The radiograph demonstrates no acute osseous injury, such as fracture or dislocation. Of interest and note is increased sclerosis within both femoral heads, more so on the left versus the right side. Given the patient’s young age, such findings could be related to early avascular necrosis. His clinical symptoms certainly correlate. MRI or bone scan, as well as orthopedic evaluation, is warranted in such a case.
Fortunately, subsequent MRI of both hips did not show any avascular necrosis but rather osteoarthritic changes. The MRI of his spinal column was negative as well.
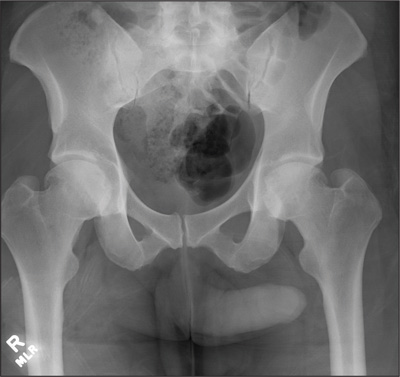
A 30-year-old man is transferred to your facility for evaluation of reported paraplegia after a fall. The patient is an inmate at a local prison. He states he was sleeping on the top bunk when he rolled over and fell off the bed, landing flat on his back on the concrete floor. He immediately started having severe back and hip pain and noticed that he could not move his legs. His primary complaint is severe bilateral hip pain. He was initially evaluated at an outside hospital, where CT of his head, cervical spine, and lumbar spine was negative for any acute pathology. He was sent to your facility for an MRI to rule out contusion or acute herniated disc. The patient denies any significant medical history, including back trauma. Currently, he reports no bowel/bladder issues or saddle anesthesia. On initial exam, he is awake, alert, and oriented, with normal vital signs. Musculoskeletal exam demonstrates a moderate amount of paraspinous tenderness and bilateral hip/pelvis tenderness. There is no instability detected, nor any leg shortening or rotation. He does have bilateral weakness in both lower extremities on the magnitude of 3-/5, although his exam seems limited due to the severity of his hip pain. Sensation is completely intact in both lower extremities. While the patient is awaiting his MRI, you order a portable pelvis radiograph, since none was performed at the outside facility. What is your impression?
Healthy and Active, but Getting Fatigued
ANSWER
The correct interpretation of this ECG includes sinus bradycardia with marked sinus arrhythmia and junctional escape beats with sinus arrest. An intraventricular conduction defect is also present.
Sinus bradycardia is indicated by the normal PQRST complexes at a rate of less than 60 beats/min. A marked sinus arrhythmia is evidenced by more than one pause (between third and fourth beats and seventh and eighth beats on the lead I rhythm strip) on the ECG.
Sinus arrest occurs when the sinus node fails to conduct (absence of P wave during the interval of the pause). A normal QRS complex without a preceding P wave indicates a junctional escape beat. Finally, an intraventricular conduction defect is documented by a QRS duration ≥ 110 ms in the absence of a right or left bundle branch block.
ANSWER
The correct interpretation of this ECG includes sinus bradycardia with marked sinus arrhythmia and junctional escape beats with sinus arrest. An intraventricular conduction defect is also present.
Sinus bradycardia is indicated by the normal PQRST complexes at a rate of less than 60 beats/min. A marked sinus arrhythmia is evidenced by more than one pause (between third and fourth beats and seventh and eighth beats on the lead I rhythm strip) on the ECG.
Sinus arrest occurs when the sinus node fails to conduct (absence of P wave during the interval of the pause). A normal QRS complex without a preceding P wave indicates a junctional escape beat. Finally, an intraventricular conduction defect is documented by a QRS duration ≥ 110 ms in the absence of a right or left bundle branch block.
ANSWER
The correct interpretation of this ECG includes sinus bradycardia with marked sinus arrhythmia and junctional escape beats with sinus arrest. An intraventricular conduction defect is also present.
Sinus bradycardia is indicated by the normal PQRST complexes at a rate of less than 60 beats/min. A marked sinus arrhythmia is evidenced by more than one pause (between third and fourth beats and seventh and eighth beats on the lead I rhythm strip) on the ECG.
Sinus arrest occurs when the sinus node fails to conduct (absence of P wave during the interval of the pause). A normal QRS complex without a preceding P wave indicates a junctional escape beat. Finally, an intraventricular conduction defect is documented by a QRS duration ≥ 110 ms in the absence of a right or left bundle branch block.
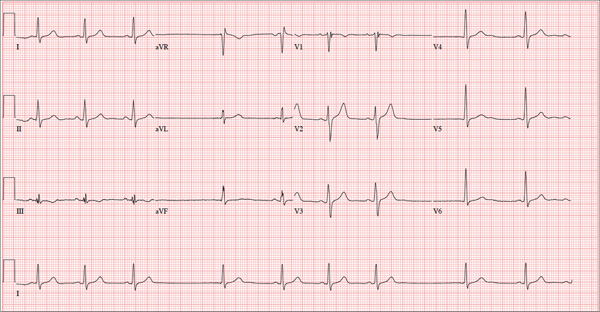
A 68-year-old retired high school teacher became fatigued while doing yardwork. After sitting down to rest, he noticed that his heart seemed to be skipping beats. He asked his daughter, a pediatric nurse, to come over and check his pulse. She confirmed his suspicion and recommended he go to the emergency department. The patient refused but made an appointment to see his primary care provider. Since you are covering for his usual provider (who is on maternity leave), the patient presents to you. Review of his chart indicates that he has been healthy and active his entire life and has never had any cardiac issues. He does not have hypertension, diabetes, hypothyroidism, or pulmonary problems. His history includes GERD, kidney stones, hyperlipidemia, and a fractured left clavicle. All immunizations and tetanus booster are current. The patient denies any history of chest pain, dyspnea, syncope, near-syncope, palpitations, or other heart rhythm issues (eg, tachycardia, bradycardia, or atrial fibrillation). His last ECG, performed three years ago during a routine visit, showed normal sinus rhythm with normal intervals and no evidence of chamber enlargement; hypertrophy; arrhythmia; P, QRS, or QT interval abnormalities; or blocks. His current medications include esomeprazole magnesium, simvastatin, niacin, and aspirin. He denies illicit or homeopathic drug use and has no known drug allergies. He is a widower who does not drink alcohol or smoke cigarettes. Vital signs include a blood pressure of 108/58 mm Hg; pulse, 60 beats/min with occasional pauses; respiratory rate, 14 breaths/min-1; O2 saturation, 98% on room air; and temperature, 98.9°F. His weight is 169 lb and his height, 74 in. Physical exam reveals a tall, thin, healthy-appearing male in no distress. The HEENT exam is remarkable only for corrective lenses. There is no thyromegaly, jugular venous distention, or lymphadenopathy. The lungs are clear in all fields. The cardiac exam reveals a regular rhythm with occasional pauses and no evidence of murmurs, rubs, or extra heart sounds. The abdomen is soft and nontender, without evidence of organomegaly or masses. The peripheral pulses are 2+ bilaterally in all extremities, and the neurologic exam is intact. An ECG is performed, which reveals a ventricular rate of 55 beats/min; PR interval, 146 ms; QRS duration, 122 ms; QT/QTc interval, 424/405 ms; P axis, 60°; R axis, 38°; and T axis, 29°. What is your interpretation of this ECG?