User login
Implantable Direct Current Spinal Fusion Stimulators Do Not Decrease Implant-Related Infections in a Rabbit Model
USPSTF: What’s recommended, what’s not
The United States Preventive Services Task Force (USPSTF) was busy in 2013, issuing 26 recommendations on 16 topics (TABLES 1-3). We have covered some of these topics previously in Practice Alerts or audiocasts—vitamin D for bone health and fall prevention,1 screening for lung cancer,2 human immunodeficiency virus infection,3 and the use of multivitamins to prevent cancer and cardiovascular disease (CVD).4 Another Practice Alert on chronic hepatitis C virus infection reviewed recommendations of the Centers for Disease Control and Prevention,5 which agree with those of the USPSTF. This Practice Alert discusses the remaining USPSTF recommendations.
Alcohol and tobacco
The Task Force (TF) reports that 30% of adults are affected by alcohol-related problems and that alcohol causes 85,000 deaths per year, making it the third leading cause of preventable death.6 The TF reviewed evidence on screening and counseling and now recommends screening adults ≥18 years for alcohol misuse and providing brief counseling to reduce alcohol use for those who engage in risky or hazardous drinking.6 The TF recommends any of 3 screening tools: using either the Alcohol Use Disorders Identification Test (AUDIT) or the abbreviated AUDIT-Consumption (AUDIT-C), or asking a single-question, such as “How many times in the past year have you had 5 (for men) or 4 (for women and all adults >65 years) or more drinks in a day?”6
Counseling for 5 to 15 minutes during the initial clinical encounter and then at subsequent visits is more effective than very brief (<5 minutes) or single-episode counseling. Counseling can include action plans, drinking diaries, stress management, or problem solving, and it can be done face-to-face or with written self-help materials, computer- or Web-based programs, or telephone support. Despite the importance of alcohol misuse as a health problem, the TF could find no evidence that screening and behavioral counseling is effective for adolescents.
For tobacco use, however, the TF now recommends providing prevention advice to school-age children and adolescents,7 presented in person or through written materials, videos, or other media. Over 8% of middle school children and close to 24% of high school students use tobacco.7 Tobacco is the leading cause of preventable deaths in the United States, and most smokers start before they are adults.7
Cancer screening and prevention
In addition to the recommendation for lung cancer screening, 2 other cancer screening/prevention recommendations were made in 2013. One is a modification of the previous recommendation on the use of BRCA gene testing to detect increased risk of breast and ovarian cancer. The recommendation now states that if a woman has a family member with breast, ovarian, tubal, or peritoneal cancer, her physician should use a screening tool to determine if her family history suggests high risk for having either BRCA1 or BRCA2. With a positive screening result, referral for genetic counseling is warranted. After counseling, the patient may choose to undergo BRCA testing. Screening tools reviewed by the TF are the Ontario Family History Assessment Tool, the Manchester Scoring System, the Referral Screening Tool, the Pedigree Assessment Tool, and the Family History Screening-7 instrument.8
The second recommendation is complex and concerns whether to prescribe tamoxifen or raloxifene to prevent breast cancer in women at high risk—ie, a 5-year risk ≥3%.9 One tool for estimating risk can be found at http://www.cancer.gov/bcrisktool/. It calculates risk based on age, race, genetic profile, age at menopause and menarche, family history of breast cancer, and personal history of breast cancer and biopsies. The TF recommends that physicians share decision making with women who are at high risk of breast cancer and offer medication to those at low risk of complications (those who have had a hysterectomy). Use of tamoxifen or raloxifene can reduce risk of the invasive cancer by 7 to 9 cases per 1000 women over 5 years. However, the risk of venous thromboembolism increases by 4 to 7 cases per 1000 over 5 years, and tamoxifen increases the risk of endometrial cancer by 4 in 1000. Both medications can cause hot flashes.9
Gestational diabetes
For a number of years the TF has assigned an “I” statement (insufficient evidence to assess benefits and harms) to screening for gestational diabetes. It recently changed that to a “B” recommendation for all pregnant women after the 24th week of pregnancy. Screening before 24 weeks is still listed as an I. Possible screening tools include a fasting blood glucose test, a 50-g oral glucose challenge test, or an assessment of risk factors. The TF did not find evidence of superiority with any of these methods. The TF found that diet modifications, glucose monitoring, and use of insulin can, in some cases, moderately reduce the incidence of preeclampsia, macrosomia, and shoulder dystocia.10
Intimate partner violence
Another change from a previous “I” statement pertains to intimate partner violence (IPV). The TF now recommends screening women of childbearing age for IPV and either providing intervention services for those who screen positive for IPV or referring for services. Reproductive age is defined as 14 to 46 years, although the TF admits that most studies have looked at women ≥18 years.11 Most of the benefits from screening and counseling have been demonstrated in pregnant women.
IPV can include physical, sexual, or psychological harm by a current or former partner or spouse, and it is not limited to opposite sex couples.11 Screening tools with the highest sensitivity and specificity include the Hurt, Insult, Threaten, and Scream (HITS) scale. Potential interventions include counseling, home visits, information cards, referrals to community services, and mentoring support.
While the TF acknowledges that both child abuse and elder abuse are prominent problems, there is not enough evidence to assess and recommend interventions.11,12
D recommendations
There were 4 “D” recommendations (recommend against) in 2013: testing for BRCA or using tamoxifen or raloxifene in women at low risk of breast cancer; using β-carotene or vitamin E to prevent CVD and cancer; and using low doses of vitamin D and calcium to prevent fractures in noninstitutionalized postmenopausal women (TABLE 2). In each instance the harms of the intervention were deemed to exceed potential benefits.
I statements
The TF still finds little evidence to support some common practices (TABLE 3). Physicians who use these interventions should realize that the TF, after thorough systematic reviews of the available evidence, does not find enough evidence to assess their relative benefits and harms. A description of the evidence on each condition can be found in the recommendations section of the USPSTF Web site (http://www.uspreventiveservicestaskforce.org/uspstopics.htm).
1. Campos-Outcalt D. Vitamin D: when it helps, when it harms. J Fam Pract. 2013;62:368-370.
2. Campos-Outcalt D. Lung cancer screening: USPSTF revises its recommendation. J Fam Pract. 2013;62:733-740.
3. Campos-Outcalt D. HIV screening: what the USPSTF says now. [Audiocast]. Parsippany, NJ; The Journal of Family Practice: 2013. Available at: http://www.jfponline.com/multimedia/audio/article/hiv-screening-what-the-uspstf-says-now/a1c4bc0fc9405f18820bb19fe971f743.html. Accessed March 14, 2014.
4. Campos-Outcalt D. Does your patient really need a supplement? [Audiocast]. Parsippany, NJ: The Journal of Family Practice; 2013. Available at: http://www.jfponline.com/index.php?id=21643&cHash=071010&tx_ttnews[tt_news]=226385. Accessed March 14, 2014.
5. Campos-Outcalt D. Hepatitis C: new CDC screening recommendations. J Fam Pract. 2012;61:744-746.
6. US Preventive Services Task Force Web site. Screening and behavioral counseling interventions in primary care to reduce alcohol misuse. Available at: http://www.uspreventiveservicestaskforce.org/uspstf/uspsdrin.htm. Accessed March 14, 2014.
7. US Preventive Services Task Force Web site. Primary care interventions to prevent tobacco use in children and adolescents. Available at: http://www.uspreventiveservicestaskforce.org/uspstf/uspstbac.htm. Accessed March 14, 2014.
8. US Preventive Services Task Force Web site. Risk assessment, genetic counseling, and genetic testing for BRCA-related cancer in women. Available at: http://www.uspreventiveservicestaskforce.org/uspstf/uspsbrgen.htm. Accessed March 14, 2014.
9. US Preventive Services Task Force Web site. Medication for risk reduction of primary breast cancer in women. Available at: http://www.uspreventiveservicestaskforce.org/uspstf/uspsbrpv.htm. Accessed March 14, 2014.
10. US Preventive Services Task Force Web site. Screening for gestational diabetes mellitius. Available at: http://www.uspreventiveservicestaskforce.org/uspstf/uspsgdm.htm. Accessed March 14, 2014.
11. US Preventive Services Task Force Web site. Screening for intimate partner violence and abuse of elderly and vulnerable adults. Available at: http://www.uspreventiveservicestaskforce.org/uspstf/uspsipv.htm. Accessed March 14, 2014.
12. US Preventive Services Task Force Web site. Primary interventions to prevent child maltreatment. Available at: http://www.uspreventiveservicestaskforce.org/uspstf/uspsfamv.htm. Accessed March 14, 2014.
The United States Preventive Services Task Force (USPSTF) was busy in 2013, issuing 26 recommendations on 16 topics (TABLES 1-3). We have covered some of these topics previously in Practice Alerts or audiocasts—vitamin D for bone health and fall prevention,1 screening for lung cancer,2 human immunodeficiency virus infection,3 and the use of multivitamins to prevent cancer and cardiovascular disease (CVD).4 Another Practice Alert on chronic hepatitis C virus infection reviewed recommendations of the Centers for Disease Control and Prevention,5 which agree with those of the USPSTF. This Practice Alert discusses the remaining USPSTF recommendations.
Alcohol and tobacco
The Task Force (TF) reports that 30% of adults are affected by alcohol-related problems and that alcohol causes 85,000 deaths per year, making it the third leading cause of preventable death.6 The TF reviewed evidence on screening and counseling and now recommends screening adults ≥18 years for alcohol misuse and providing brief counseling to reduce alcohol use for those who engage in risky or hazardous drinking.6 The TF recommends any of 3 screening tools: using either the Alcohol Use Disorders Identification Test (AUDIT) or the abbreviated AUDIT-Consumption (AUDIT-C), or asking a single-question, such as “How many times in the past year have you had 5 (for men) or 4 (for women and all adults >65 years) or more drinks in a day?”6
Counseling for 5 to 15 minutes during the initial clinical encounter and then at subsequent visits is more effective than very brief (<5 minutes) or single-episode counseling. Counseling can include action plans, drinking diaries, stress management, or problem solving, and it can be done face-to-face or with written self-help materials, computer- or Web-based programs, or telephone support. Despite the importance of alcohol misuse as a health problem, the TF could find no evidence that screening and behavioral counseling is effective for adolescents.
For tobacco use, however, the TF now recommends providing prevention advice to school-age children and adolescents,7 presented in person or through written materials, videos, or other media. Over 8% of middle school children and close to 24% of high school students use tobacco.7 Tobacco is the leading cause of preventable deaths in the United States, and most smokers start before they are adults.7
Cancer screening and prevention
In addition to the recommendation for lung cancer screening, 2 other cancer screening/prevention recommendations were made in 2013. One is a modification of the previous recommendation on the use of BRCA gene testing to detect increased risk of breast and ovarian cancer. The recommendation now states that if a woman has a family member with breast, ovarian, tubal, or peritoneal cancer, her physician should use a screening tool to determine if her family history suggests high risk for having either BRCA1 or BRCA2. With a positive screening result, referral for genetic counseling is warranted. After counseling, the patient may choose to undergo BRCA testing. Screening tools reviewed by the TF are the Ontario Family History Assessment Tool, the Manchester Scoring System, the Referral Screening Tool, the Pedigree Assessment Tool, and the Family History Screening-7 instrument.8
The second recommendation is complex and concerns whether to prescribe tamoxifen or raloxifene to prevent breast cancer in women at high risk—ie, a 5-year risk ≥3%.9 One tool for estimating risk can be found at http://www.cancer.gov/bcrisktool/. It calculates risk based on age, race, genetic profile, age at menopause and menarche, family history of breast cancer, and personal history of breast cancer and biopsies. The TF recommends that physicians share decision making with women who are at high risk of breast cancer and offer medication to those at low risk of complications (those who have had a hysterectomy). Use of tamoxifen or raloxifene can reduce risk of the invasive cancer by 7 to 9 cases per 1000 women over 5 years. However, the risk of venous thromboembolism increases by 4 to 7 cases per 1000 over 5 years, and tamoxifen increases the risk of endometrial cancer by 4 in 1000. Both medications can cause hot flashes.9
Gestational diabetes
For a number of years the TF has assigned an “I” statement (insufficient evidence to assess benefits and harms) to screening for gestational diabetes. It recently changed that to a “B” recommendation for all pregnant women after the 24th week of pregnancy. Screening before 24 weeks is still listed as an I. Possible screening tools include a fasting blood glucose test, a 50-g oral glucose challenge test, or an assessment of risk factors. The TF did not find evidence of superiority with any of these methods. The TF found that diet modifications, glucose monitoring, and use of insulin can, in some cases, moderately reduce the incidence of preeclampsia, macrosomia, and shoulder dystocia.10
Intimate partner violence
Another change from a previous “I” statement pertains to intimate partner violence (IPV). The TF now recommends screening women of childbearing age for IPV and either providing intervention services for those who screen positive for IPV or referring for services. Reproductive age is defined as 14 to 46 years, although the TF admits that most studies have looked at women ≥18 years.11 Most of the benefits from screening and counseling have been demonstrated in pregnant women.
IPV can include physical, sexual, or psychological harm by a current or former partner or spouse, and it is not limited to opposite sex couples.11 Screening tools with the highest sensitivity and specificity include the Hurt, Insult, Threaten, and Scream (HITS) scale. Potential interventions include counseling, home visits, information cards, referrals to community services, and mentoring support.
While the TF acknowledges that both child abuse and elder abuse are prominent problems, there is not enough evidence to assess and recommend interventions.11,12
D recommendations
There were 4 “D” recommendations (recommend against) in 2013: testing for BRCA or using tamoxifen or raloxifene in women at low risk of breast cancer; using β-carotene or vitamin E to prevent CVD and cancer; and using low doses of vitamin D and calcium to prevent fractures in noninstitutionalized postmenopausal women (TABLE 2). In each instance the harms of the intervention were deemed to exceed potential benefits.
I statements
The TF still finds little evidence to support some common practices (TABLE 3). Physicians who use these interventions should realize that the TF, after thorough systematic reviews of the available evidence, does not find enough evidence to assess their relative benefits and harms. A description of the evidence on each condition can be found in the recommendations section of the USPSTF Web site (http://www.uspreventiveservicestaskforce.org/uspstopics.htm).
The United States Preventive Services Task Force (USPSTF) was busy in 2013, issuing 26 recommendations on 16 topics (TABLES 1-3). We have covered some of these topics previously in Practice Alerts or audiocasts—vitamin D for bone health and fall prevention,1 screening for lung cancer,2 human immunodeficiency virus infection,3 and the use of multivitamins to prevent cancer and cardiovascular disease (CVD).4 Another Practice Alert on chronic hepatitis C virus infection reviewed recommendations of the Centers for Disease Control and Prevention,5 which agree with those of the USPSTF. This Practice Alert discusses the remaining USPSTF recommendations.
Alcohol and tobacco
The Task Force (TF) reports that 30% of adults are affected by alcohol-related problems and that alcohol causes 85,000 deaths per year, making it the third leading cause of preventable death.6 The TF reviewed evidence on screening and counseling and now recommends screening adults ≥18 years for alcohol misuse and providing brief counseling to reduce alcohol use for those who engage in risky or hazardous drinking.6 The TF recommends any of 3 screening tools: using either the Alcohol Use Disorders Identification Test (AUDIT) or the abbreviated AUDIT-Consumption (AUDIT-C), or asking a single-question, such as “How many times in the past year have you had 5 (for men) or 4 (for women and all adults >65 years) or more drinks in a day?”6
Counseling for 5 to 15 minutes during the initial clinical encounter and then at subsequent visits is more effective than very brief (<5 minutes) or single-episode counseling. Counseling can include action plans, drinking diaries, stress management, or problem solving, and it can be done face-to-face or with written self-help materials, computer- or Web-based programs, or telephone support. Despite the importance of alcohol misuse as a health problem, the TF could find no evidence that screening and behavioral counseling is effective for adolescents.
For tobacco use, however, the TF now recommends providing prevention advice to school-age children and adolescents,7 presented in person or through written materials, videos, or other media. Over 8% of middle school children and close to 24% of high school students use tobacco.7 Tobacco is the leading cause of preventable deaths in the United States, and most smokers start before they are adults.7
Cancer screening and prevention
In addition to the recommendation for lung cancer screening, 2 other cancer screening/prevention recommendations were made in 2013. One is a modification of the previous recommendation on the use of BRCA gene testing to detect increased risk of breast and ovarian cancer. The recommendation now states that if a woman has a family member with breast, ovarian, tubal, or peritoneal cancer, her physician should use a screening tool to determine if her family history suggests high risk for having either BRCA1 or BRCA2. With a positive screening result, referral for genetic counseling is warranted. After counseling, the patient may choose to undergo BRCA testing. Screening tools reviewed by the TF are the Ontario Family History Assessment Tool, the Manchester Scoring System, the Referral Screening Tool, the Pedigree Assessment Tool, and the Family History Screening-7 instrument.8
The second recommendation is complex and concerns whether to prescribe tamoxifen or raloxifene to prevent breast cancer in women at high risk—ie, a 5-year risk ≥3%.9 One tool for estimating risk can be found at http://www.cancer.gov/bcrisktool/. It calculates risk based on age, race, genetic profile, age at menopause and menarche, family history of breast cancer, and personal history of breast cancer and biopsies. The TF recommends that physicians share decision making with women who are at high risk of breast cancer and offer medication to those at low risk of complications (those who have had a hysterectomy). Use of tamoxifen or raloxifene can reduce risk of the invasive cancer by 7 to 9 cases per 1000 women over 5 years. However, the risk of venous thromboembolism increases by 4 to 7 cases per 1000 over 5 years, and tamoxifen increases the risk of endometrial cancer by 4 in 1000. Both medications can cause hot flashes.9
Gestational diabetes
For a number of years the TF has assigned an “I” statement (insufficient evidence to assess benefits and harms) to screening for gestational diabetes. It recently changed that to a “B” recommendation for all pregnant women after the 24th week of pregnancy. Screening before 24 weeks is still listed as an I. Possible screening tools include a fasting blood glucose test, a 50-g oral glucose challenge test, or an assessment of risk factors. The TF did not find evidence of superiority with any of these methods. The TF found that diet modifications, glucose monitoring, and use of insulin can, in some cases, moderately reduce the incidence of preeclampsia, macrosomia, and shoulder dystocia.10
Intimate partner violence
Another change from a previous “I” statement pertains to intimate partner violence (IPV). The TF now recommends screening women of childbearing age for IPV and either providing intervention services for those who screen positive for IPV or referring for services. Reproductive age is defined as 14 to 46 years, although the TF admits that most studies have looked at women ≥18 years.11 Most of the benefits from screening and counseling have been demonstrated in pregnant women.
IPV can include physical, sexual, or psychological harm by a current or former partner or spouse, and it is not limited to opposite sex couples.11 Screening tools with the highest sensitivity and specificity include the Hurt, Insult, Threaten, and Scream (HITS) scale. Potential interventions include counseling, home visits, information cards, referrals to community services, and mentoring support.
While the TF acknowledges that both child abuse and elder abuse are prominent problems, there is not enough evidence to assess and recommend interventions.11,12
D recommendations
There were 4 “D” recommendations (recommend against) in 2013: testing for BRCA or using tamoxifen or raloxifene in women at low risk of breast cancer; using β-carotene or vitamin E to prevent CVD and cancer; and using low doses of vitamin D and calcium to prevent fractures in noninstitutionalized postmenopausal women (TABLE 2). In each instance the harms of the intervention were deemed to exceed potential benefits.
I statements
The TF still finds little evidence to support some common practices (TABLE 3). Physicians who use these interventions should realize that the TF, after thorough systematic reviews of the available evidence, does not find enough evidence to assess their relative benefits and harms. A description of the evidence on each condition can be found in the recommendations section of the USPSTF Web site (http://www.uspreventiveservicestaskforce.org/uspstopics.htm).
1. Campos-Outcalt D. Vitamin D: when it helps, when it harms. J Fam Pract. 2013;62:368-370.
2. Campos-Outcalt D. Lung cancer screening: USPSTF revises its recommendation. J Fam Pract. 2013;62:733-740.
3. Campos-Outcalt D. HIV screening: what the USPSTF says now. [Audiocast]. Parsippany, NJ; The Journal of Family Practice: 2013. Available at: http://www.jfponline.com/multimedia/audio/article/hiv-screening-what-the-uspstf-says-now/a1c4bc0fc9405f18820bb19fe971f743.html. Accessed March 14, 2014.
4. Campos-Outcalt D. Does your patient really need a supplement? [Audiocast]. Parsippany, NJ: The Journal of Family Practice; 2013. Available at: http://www.jfponline.com/index.php?id=21643&cHash=071010&tx_ttnews[tt_news]=226385. Accessed March 14, 2014.
5. Campos-Outcalt D. Hepatitis C: new CDC screening recommendations. J Fam Pract. 2012;61:744-746.
6. US Preventive Services Task Force Web site. Screening and behavioral counseling interventions in primary care to reduce alcohol misuse. Available at: http://www.uspreventiveservicestaskforce.org/uspstf/uspsdrin.htm. Accessed March 14, 2014.
7. US Preventive Services Task Force Web site. Primary care interventions to prevent tobacco use in children and adolescents. Available at: http://www.uspreventiveservicestaskforce.org/uspstf/uspstbac.htm. Accessed March 14, 2014.
8. US Preventive Services Task Force Web site. Risk assessment, genetic counseling, and genetic testing for BRCA-related cancer in women. Available at: http://www.uspreventiveservicestaskforce.org/uspstf/uspsbrgen.htm. Accessed March 14, 2014.
9. US Preventive Services Task Force Web site. Medication for risk reduction of primary breast cancer in women. Available at: http://www.uspreventiveservicestaskforce.org/uspstf/uspsbrpv.htm. Accessed March 14, 2014.
10. US Preventive Services Task Force Web site. Screening for gestational diabetes mellitius. Available at: http://www.uspreventiveservicestaskforce.org/uspstf/uspsgdm.htm. Accessed March 14, 2014.
11. US Preventive Services Task Force Web site. Screening for intimate partner violence and abuse of elderly and vulnerable adults. Available at: http://www.uspreventiveservicestaskforce.org/uspstf/uspsipv.htm. Accessed March 14, 2014.
12. US Preventive Services Task Force Web site. Primary interventions to prevent child maltreatment. Available at: http://www.uspreventiveservicestaskforce.org/uspstf/uspsfamv.htm. Accessed March 14, 2014.
1. Campos-Outcalt D. Vitamin D: when it helps, when it harms. J Fam Pract. 2013;62:368-370.
2. Campos-Outcalt D. Lung cancer screening: USPSTF revises its recommendation. J Fam Pract. 2013;62:733-740.
3. Campos-Outcalt D. HIV screening: what the USPSTF says now. [Audiocast]. Parsippany, NJ; The Journal of Family Practice: 2013. Available at: http://www.jfponline.com/multimedia/audio/article/hiv-screening-what-the-uspstf-says-now/a1c4bc0fc9405f18820bb19fe971f743.html. Accessed March 14, 2014.
4. Campos-Outcalt D. Does your patient really need a supplement? [Audiocast]. Parsippany, NJ: The Journal of Family Practice; 2013. Available at: http://www.jfponline.com/index.php?id=21643&cHash=071010&tx_ttnews[tt_news]=226385. Accessed March 14, 2014.
5. Campos-Outcalt D. Hepatitis C: new CDC screening recommendations. J Fam Pract. 2012;61:744-746.
6. US Preventive Services Task Force Web site. Screening and behavioral counseling interventions in primary care to reduce alcohol misuse. Available at: http://www.uspreventiveservicestaskforce.org/uspstf/uspsdrin.htm. Accessed March 14, 2014.
7. US Preventive Services Task Force Web site. Primary care interventions to prevent tobacco use in children and adolescents. Available at: http://www.uspreventiveservicestaskforce.org/uspstf/uspstbac.htm. Accessed March 14, 2014.
8. US Preventive Services Task Force Web site. Risk assessment, genetic counseling, and genetic testing for BRCA-related cancer in women. Available at: http://www.uspreventiveservicestaskforce.org/uspstf/uspsbrgen.htm. Accessed March 14, 2014.
9. US Preventive Services Task Force Web site. Medication for risk reduction of primary breast cancer in women. Available at: http://www.uspreventiveservicestaskforce.org/uspstf/uspsbrpv.htm. Accessed March 14, 2014.
10. US Preventive Services Task Force Web site. Screening for gestational diabetes mellitius. Available at: http://www.uspreventiveservicestaskforce.org/uspstf/uspsgdm.htm. Accessed March 14, 2014.
11. US Preventive Services Task Force Web site. Screening for intimate partner violence and abuse of elderly and vulnerable adults. Available at: http://www.uspreventiveservicestaskforce.org/uspstf/uspsipv.htm. Accessed March 14, 2014.
12. US Preventive Services Task Force Web site. Primary interventions to prevent child maltreatment. Available at: http://www.uspreventiveservicestaskforce.org/uspstf/uspsfamv.htm. Accessed March 14, 2014.
Studies hint at safety, efficacy of spinal muscular atrophy drug
PHILADELPHIA – The latest interim results from open-label studies of the investigational antisense oligonucleotide therapy ISIS-SMNRx for the treatment of patients with type 1, 2, or 3 spinal muscular atrophy support its safety and are starting to show its potential efficacy in treating the range of severity seen in the disease.
In two ongoing studies with up to 9 months of follow-up data, no safety or tolerability concerns arose with total doses of up to 18 mg in patients with type 2 or 3 spinal muscular atrophy (SMA) and in total doses of up to 48 mg in infants with type 1 SMA. Children aged 2-15 years with type 2 or 3 SMA had a dose- and time-dependent improvement in scores on the Hammersmith Functional Motor Scale-Expanded (HFMSE) that also correlated well with levels of SMN protein in cerebrospinal fluid. Infants with type 1 SMA achieved motor milestones on the Hammersmith Infant Neurological Exam that were consistent with increases in motor function test scores, according to investigators who presented the results at the annual meeting of the American Academy of Neurology.
"It’s very encouraging that we can do this safely and that the children tolerate the lumbar punctures, and there’s hope that the measures [used in the studies] are sensitive to change," said primary investigator Dr. Claudia Chiriboga, who presented the interim results of a study in patients with SMA types 2 or 3.
In that study, ISIS-SMNRx, an antisense oligonucleotide that promotes transcription of the full-length SMN protein from the SMN2 gene, was administered in an intrathecal bolus via lumbar puncture at points during a 3-month period; patients were then followed for 6 months. A total of eight patients received 3 mg at each dose (total dose, 9 mg); eight received 6 mg at each dose (total dose, 18 mg); and nine received 9 mg at each dose (18 mg total). Later, investigators added a 12-mg dose cohort that currently has eight patients enrolled, but results in that cohort are not yet available, said Dr. Chiriboga of the division of child neurology at Columbia University, New York.
The SMA type 2 and 3 patients included 10 patients with type 2 and 15 with type 3. They were medically stable and 2-15 years old, with a mean age of 7.5 years. Most (20) had three copies of the SMN2 gene; 4 had four copies and 1 had two copies. A majority of the patients (16) were nonambulatory.
None of the adverse events reported were considered related to the study drug, and most of the 143 adverse events were mild or moderate, the investigators found. Two severe adverse events were back pain and myalgia. Most of the adverse events were related to the lumbar punctures.
Scores on the HFMSE improved from baseline by a mean of 1.5 points in the 3-mg group, 2.3 points in the 6-mg group, and a statistically significant 3.7 points in the 9-mg group. SMN levels in cerebrospinal fluid at day 85 increased from baseline in all groups but were significantly increased in the 9-mg group only.
Additional secondary endpoints showed nonsignificant improvement of 22.7 m at 9 months on the 6-minute walk test in those who could walk, and an improvement of 2.3 points on an 18-point scale measuring upper limb function in weaker nonambulatory patients, but the open-label nature of the study and small numbers of patients make it difficult to interpret such findings, Dr. Chiriboga said.
"The feeling is that when there’s chronicity, like end-stage type of changes – severe scoliosis, for example – that those individuals don’t do as well. ... It’s not so much the age," Dr. Chiriboga said in an interview. Patients with type 3 disease also do better because they have more SMN2 to begin with, she said.
Similarly, in the ongoing open-label study of infants with type 1 SMA, ISIS-SMNRx was administered to 4 patients in 6-mg doses at days 1, 15, 85, and 253, and in 12-mg doses to 11 patients at the same time points. These infants were all aged 7 months or younger. Their mean age at symptom onset was 7 weeks, and they were enrolled in the study at a mean age of 18-21 weeks. All but one patient had two copies of the SMN2 gene, reported primary investigator Dr. Richard S. Finkel.
None of the adverse events in the infants were deemed to be related to ISIS-SMNRx. Of 14 severe adverse events, 11 were respiratory infections, and all were considered to be consistent with severe infant SMA, said Dr. Finkel, chief of the division of neurology at Nemours Children’s Hospital and professor of neurology at the University of Central Florida, both in Orlando.
One patient in the 6-mg group died accidentally, and another underwent permanent ventilation. Two of 11 patients in the 12-mg group died of pulmonary infection, and 1 required permanent ventilation (16 or more hours per day continuously for more than 2 weeks in the absence of an acute reversible illness), although 4 of the patients in this group have not yet received all their doses. At the last follow-up, or at the time of death or permanent ventilation, the median age was 14 months in the 6-mg group and 9.6 months in the 12-mg group (which has not been followed as long).
Scores on the Children’s Hospital of Philadelphia Infant Test of Neuromuscular Disorders (CHOP-INTEND) showed increases in 8 of 11 infants who had completed treatment and evaluation. The scores increased by a mean of 5.4 points overall and by 8.3 points in those in the 12-mg group. Incremental milestones on the Hammersmith Infant Neurological Exam were achieved by 9 of 11 infants, including 6 of 7 in the 12-mg group.
The median age at death or need for permanent ventilation is 10.5 months in infants with two SMN2 gene copies, and 85% reach this endpoint at 18 months. Scores on the CHOP-INTEND also declined by 1.27 points per year, according to a study of the natural history of type 1 SMA in 34 patients by Dr. Finkel and his colleagues that is under review for publication.
Compound muscle action potentials measured in the ulnar nerve–innervated abductor digiti minimi and peroneal nerve–innervated anterior tibialis were stable or increased in most infants, he said.
These encouraging results with ISIS-SMNRx have led Isis to begin plans for phase III trials in patients with SMA types 1-3, the investigators said.
The studies are funded by Isis Pharmaceuticals, the Department of Defense, and the National Institute of Neurological Disorders and Stroke. Neither Dr. Finkel nor Dr. Chiriboga had conflicts of interest. Some of the coauthors in each study were employees of Isis.
PHILADELPHIA – The latest interim results from open-label studies of the investigational antisense oligonucleotide therapy ISIS-SMNRx for the treatment of patients with type 1, 2, or 3 spinal muscular atrophy support its safety and are starting to show its potential efficacy in treating the range of severity seen in the disease.
In two ongoing studies with up to 9 months of follow-up data, no safety or tolerability concerns arose with total doses of up to 18 mg in patients with type 2 or 3 spinal muscular atrophy (SMA) and in total doses of up to 48 mg in infants with type 1 SMA. Children aged 2-15 years with type 2 or 3 SMA had a dose- and time-dependent improvement in scores on the Hammersmith Functional Motor Scale-Expanded (HFMSE) that also correlated well with levels of SMN protein in cerebrospinal fluid. Infants with type 1 SMA achieved motor milestones on the Hammersmith Infant Neurological Exam that were consistent with increases in motor function test scores, according to investigators who presented the results at the annual meeting of the American Academy of Neurology.
"It’s very encouraging that we can do this safely and that the children tolerate the lumbar punctures, and there’s hope that the measures [used in the studies] are sensitive to change," said primary investigator Dr. Claudia Chiriboga, who presented the interim results of a study in patients with SMA types 2 or 3.
In that study, ISIS-SMNRx, an antisense oligonucleotide that promotes transcription of the full-length SMN protein from the SMN2 gene, was administered in an intrathecal bolus via lumbar puncture at points during a 3-month period; patients were then followed for 6 months. A total of eight patients received 3 mg at each dose (total dose, 9 mg); eight received 6 mg at each dose (total dose, 18 mg); and nine received 9 mg at each dose (18 mg total). Later, investigators added a 12-mg dose cohort that currently has eight patients enrolled, but results in that cohort are not yet available, said Dr. Chiriboga of the division of child neurology at Columbia University, New York.
The SMA type 2 and 3 patients included 10 patients with type 2 and 15 with type 3. They were medically stable and 2-15 years old, with a mean age of 7.5 years. Most (20) had three copies of the SMN2 gene; 4 had four copies and 1 had two copies. A majority of the patients (16) were nonambulatory.
None of the adverse events reported were considered related to the study drug, and most of the 143 adverse events were mild or moderate, the investigators found. Two severe adverse events were back pain and myalgia. Most of the adverse events were related to the lumbar punctures.
Scores on the HFMSE improved from baseline by a mean of 1.5 points in the 3-mg group, 2.3 points in the 6-mg group, and a statistically significant 3.7 points in the 9-mg group. SMN levels in cerebrospinal fluid at day 85 increased from baseline in all groups but were significantly increased in the 9-mg group only.
Additional secondary endpoints showed nonsignificant improvement of 22.7 m at 9 months on the 6-minute walk test in those who could walk, and an improvement of 2.3 points on an 18-point scale measuring upper limb function in weaker nonambulatory patients, but the open-label nature of the study and small numbers of patients make it difficult to interpret such findings, Dr. Chiriboga said.
"The feeling is that when there’s chronicity, like end-stage type of changes – severe scoliosis, for example – that those individuals don’t do as well. ... It’s not so much the age," Dr. Chiriboga said in an interview. Patients with type 3 disease also do better because they have more SMN2 to begin with, she said.
Similarly, in the ongoing open-label study of infants with type 1 SMA, ISIS-SMNRx was administered to 4 patients in 6-mg doses at days 1, 15, 85, and 253, and in 12-mg doses to 11 patients at the same time points. These infants were all aged 7 months or younger. Their mean age at symptom onset was 7 weeks, and they were enrolled in the study at a mean age of 18-21 weeks. All but one patient had two copies of the SMN2 gene, reported primary investigator Dr. Richard S. Finkel.
None of the adverse events in the infants were deemed to be related to ISIS-SMNRx. Of 14 severe adverse events, 11 were respiratory infections, and all were considered to be consistent with severe infant SMA, said Dr. Finkel, chief of the division of neurology at Nemours Children’s Hospital and professor of neurology at the University of Central Florida, both in Orlando.
One patient in the 6-mg group died accidentally, and another underwent permanent ventilation. Two of 11 patients in the 12-mg group died of pulmonary infection, and 1 required permanent ventilation (16 or more hours per day continuously for more than 2 weeks in the absence of an acute reversible illness), although 4 of the patients in this group have not yet received all their doses. At the last follow-up, or at the time of death or permanent ventilation, the median age was 14 months in the 6-mg group and 9.6 months in the 12-mg group (which has not been followed as long).
Scores on the Children’s Hospital of Philadelphia Infant Test of Neuromuscular Disorders (CHOP-INTEND) showed increases in 8 of 11 infants who had completed treatment and evaluation. The scores increased by a mean of 5.4 points overall and by 8.3 points in those in the 12-mg group. Incremental milestones on the Hammersmith Infant Neurological Exam were achieved by 9 of 11 infants, including 6 of 7 in the 12-mg group.
The median age at death or need for permanent ventilation is 10.5 months in infants with two SMN2 gene copies, and 85% reach this endpoint at 18 months. Scores on the CHOP-INTEND also declined by 1.27 points per year, according to a study of the natural history of type 1 SMA in 34 patients by Dr. Finkel and his colleagues that is under review for publication.
Compound muscle action potentials measured in the ulnar nerve–innervated abductor digiti minimi and peroneal nerve–innervated anterior tibialis were stable or increased in most infants, he said.
These encouraging results with ISIS-SMNRx have led Isis to begin plans for phase III trials in patients with SMA types 1-3, the investigators said.
The studies are funded by Isis Pharmaceuticals, the Department of Defense, and the National Institute of Neurological Disorders and Stroke. Neither Dr. Finkel nor Dr. Chiriboga had conflicts of interest. Some of the coauthors in each study were employees of Isis.
PHILADELPHIA – The latest interim results from open-label studies of the investigational antisense oligonucleotide therapy ISIS-SMNRx for the treatment of patients with type 1, 2, or 3 spinal muscular atrophy support its safety and are starting to show its potential efficacy in treating the range of severity seen in the disease.
In two ongoing studies with up to 9 months of follow-up data, no safety or tolerability concerns arose with total doses of up to 18 mg in patients with type 2 or 3 spinal muscular atrophy (SMA) and in total doses of up to 48 mg in infants with type 1 SMA. Children aged 2-15 years with type 2 or 3 SMA had a dose- and time-dependent improvement in scores on the Hammersmith Functional Motor Scale-Expanded (HFMSE) that also correlated well with levels of SMN protein in cerebrospinal fluid. Infants with type 1 SMA achieved motor milestones on the Hammersmith Infant Neurological Exam that were consistent with increases in motor function test scores, according to investigators who presented the results at the annual meeting of the American Academy of Neurology.
"It’s very encouraging that we can do this safely and that the children tolerate the lumbar punctures, and there’s hope that the measures [used in the studies] are sensitive to change," said primary investigator Dr. Claudia Chiriboga, who presented the interim results of a study in patients with SMA types 2 or 3.
In that study, ISIS-SMNRx, an antisense oligonucleotide that promotes transcription of the full-length SMN protein from the SMN2 gene, was administered in an intrathecal bolus via lumbar puncture at points during a 3-month period; patients were then followed for 6 months. A total of eight patients received 3 mg at each dose (total dose, 9 mg); eight received 6 mg at each dose (total dose, 18 mg); and nine received 9 mg at each dose (18 mg total). Later, investigators added a 12-mg dose cohort that currently has eight patients enrolled, but results in that cohort are not yet available, said Dr. Chiriboga of the division of child neurology at Columbia University, New York.
The SMA type 2 and 3 patients included 10 patients with type 2 and 15 with type 3. They were medically stable and 2-15 years old, with a mean age of 7.5 years. Most (20) had three copies of the SMN2 gene; 4 had four copies and 1 had two copies. A majority of the patients (16) were nonambulatory.
None of the adverse events reported were considered related to the study drug, and most of the 143 adverse events were mild or moderate, the investigators found. Two severe adverse events were back pain and myalgia. Most of the adverse events were related to the lumbar punctures.
Scores on the HFMSE improved from baseline by a mean of 1.5 points in the 3-mg group, 2.3 points in the 6-mg group, and a statistically significant 3.7 points in the 9-mg group. SMN levels in cerebrospinal fluid at day 85 increased from baseline in all groups but were significantly increased in the 9-mg group only.
Additional secondary endpoints showed nonsignificant improvement of 22.7 m at 9 months on the 6-minute walk test in those who could walk, and an improvement of 2.3 points on an 18-point scale measuring upper limb function in weaker nonambulatory patients, but the open-label nature of the study and small numbers of patients make it difficult to interpret such findings, Dr. Chiriboga said.
"The feeling is that when there’s chronicity, like end-stage type of changes – severe scoliosis, for example – that those individuals don’t do as well. ... It’s not so much the age," Dr. Chiriboga said in an interview. Patients with type 3 disease also do better because they have more SMN2 to begin with, she said.
Similarly, in the ongoing open-label study of infants with type 1 SMA, ISIS-SMNRx was administered to 4 patients in 6-mg doses at days 1, 15, 85, and 253, and in 12-mg doses to 11 patients at the same time points. These infants were all aged 7 months or younger. Their mean age at symptom onset was 7 weeks, and they were enrolled in the study at a mean age of 18-21 weeks. All but one patient had two copies of the SMN2 gene, reported primary investigator Dr. Richard S. Finkel.
None of the adverse events in the infants were deemed to be related to ISIS-SMNRx. Of 14 severe adverse events, 11 were respiratory infections, and all were considered to be consistent with severe infant SMA, said Dr. Finkel, chief of the division of neurology at Nemours Children’s Hospital and professor of neurology at the University of Central Florida, both in Orlando.
One patient in the 6-mg group died accidentally, and another underwent permanent ventilation. Two of 11 patients in the 12-mg group died of pulmonary infection, and 1 required permanent ventilation (16 or more hours per day continuously for more than 2 weeks in the absence of an acute reversible illness), although 4 of the patients in this group have not yet received all their doses. At the last follow-up, or at the time of death or permanent ventilation, the median age was 14 months in the 6-mg group and 9.6 months in the 12-mg group (which has not been followed as long).
Scores on the Children’s Hospital of Philadelphia Infant Test of Neuromuscular Disorders (CHOP-INTEND) showed increases in 8 of 11 infants who had completed treatment and evaluation. The scores increased by a mean of 5.4 points overall and by 8.3 points in those in the 12-mg group. Incremental milestones on the Hammersmith Infant Neurological Exam were achieved by 9 of 11 infants, including 6 of 7 in the 12-mg group.
The median age at death or need for permanent ventilation is 10.5 months in infants with two SMN2 gene copies, and 85% reach this endpoint at 18 months. Scores on the CHOP-INTEND also declined by 1.27 points per year, according to a study of the natural history of type 1 SMA in 34 patients by Dr. Finkel and his colleagues that is under review for publication.
Compound muscle action potentials measured in the ulnar nerve–innervated abductor digiti minimi and peroneal nerve–innervated anterior tibialis were stable or increased in most infants, he said.
These encouraging results with ISIS-SMNRx have led Isis to begin plans for phase III trials in patients with SMA types 1-3, the investigators said.
The studies are funded by Isis Pharmaceuticals, the Department of Defense, and the National Institute of Neurological Disorders and Stroke. Neither Dr. Finkel nor Dr. Chiriboga had conflicts of interest. Some of the coauthors in each study were employees of Isis.
AT THE AAN 2014 ANNUAL MEETING
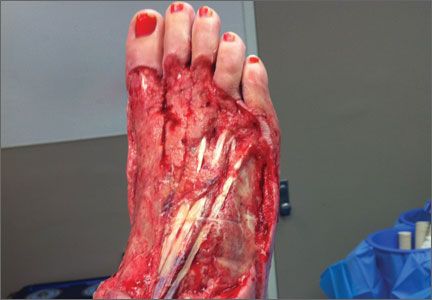
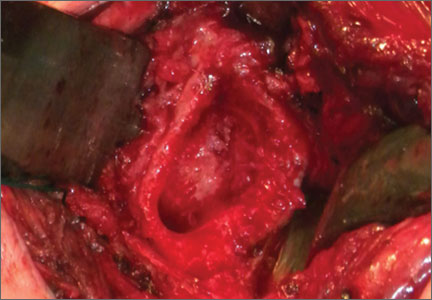
Necrotizing Fasciitis of Lower Extremity Caused by Haemophilus influenzae in a Healthy Adult With a Closed Lisfranc Injury
Hospitalists Share Strategies to Overcome Career-Related Struggles
LAS VEGAS—If she said it once, Patience Reich, MD, SFHM, said it a half-dozen times during SHM’s annual meeting: “Let it go.”
“You can’t be Martha Stewart and a perfect doctor. Just let it go,” said Dr. Reich, associate professor of internal medicine and associate faculty for the Office of Women in Medicine and Science at the Wake Forest School of Medicine in Winston-Salem, N.C., told about 75 female hospitalists during a two-hour workshop focused on women’s issues at the Mandalay Bay Resort and Casino. “Even in 2014, there are trade-offs to be made.
Dr. Reich and Rachel George, MD, MBA, CPE, SFHM, of Cogent Healthcare, have been moderating the workshop at SHM meetings for several years. They said the issues they encounter among hospitalists around the country, which are no different today than they were in years past, include gender bias, career advancement challenges, and the guilt some feel spending time away from their children or communicating with their stay-at-home husbands.
At HM14, workshop attendees searched for solutions to common struggles.
“Don’t pretend you can have it all,” Dr. George said. “It’s a myth ruining womankind. There’s nothing that says you have to be June Cleaver and Marcus Welby all rolled into one. We have to stop thinking that we have to do it.”
Dr. George told the workshop attendees that cooking and cleaning are so far down on her priority list that “they practically don’t exist.”
“It’s OK. My kids are happy and healthy,” she said. “It doesn’t matter if they come home to a dirty house or if they eat pizza. They’re going to survive. I think women put all that guilt on themselves. Some of it society does, but a lot of women put the guilt on themselves just because they don’t cook a three-course meal every night.”
Open Forum
The issues were much the same during a Special Interest Group attended by nearly 35 hospitalists and moderated by Melissa Mattison, MD, FACP, SFHM, of Beth Israel Deaconess Medical Center in Boston. Topics ranged from personal experiences with workplace discrimination to apprehension in pursuing leadership roles to “partner envy” and dealing with the “guilt” of being a working parent.
One hospitalist wondered how others dealt with harassment from patients. “I’m young, petite, and a minority,” she said. “I get ‘sweetie’ and ‘honey’ all the time from my patients.”
Another explained the difficulty of working full time while taking care of an elderly parent. Yet another admitted her desire for a role model, “as there are none in my area.”
“Men seem to have an innate drive to be the breadwinner,” one attendee said. “No matter how much help you have at home, it doesn’t take away the guilt I feel.”
Another said, “I think about all of these issues constantly.”
Dr. Mattison, a member of the annual meeting committee, left the 45-minute open forum with four action items:
- Increase the exposure of programming for issues related to work-life balance at annual meeting;
- Suggest keynote speakers who are not men;
- Create a toolkit for HM leaders and department of medicine leaders to help them understand work-life issues; and
- Create a community on the HMX portal to discuss work-life issues, “whether they are related to being a mother or father, juggling work and home, or whatever issues come up.”
The Key: Flex Schedules
Many physicians who choose a career in HM do it because of the work-life balance the specialty affords, and many of the challenges women hospitalists face at the local level revolve around the schedule. That’s how Zenobia JonesFoster, MD, MPH, a hospitalist at Wellstar Health in Atlanta, views it.
“I think it’s very facility-dependent. I think when we look for a job and decide where we want to go, we really need to understand the culture and how people advance within that culture,” said Dr. JonesFoster, who attended the women’s issues workshop. “The academic environment has a lot more deferred policy and bureaucracy versus a private institution, but you’re going to find that anywhere.”
A hospitalist for a little more than two years, Dr. JonesFoster has two young children, ages one and three, and works in a group with 30 full-time hospitalists and 10 nurse practitioners and physician assistants. Her husband is a businessman, so schedules and work-life balance are a major concern.
“If I was given a job opportunity Monday through Friday, regular work hours, there’s no way I would take it because of the flexibility of hospital medicine hours, with the seven-on seven-off schedule,” she said. “The time I have off, I get to just be a mom and not think about work. But when I’m at work, I love it.”
Dr. JonesFoster’s group has seen an increase in patient census recently and just went live with a new hospital-wide electronic health records system, which has opened up more shifts and moonlighting opportunities. Attending her first annual meeting, she was most interested in learning the pros and cons of leadership positions, because her health system “offers a lot of opportunity for advancement” and is “talking about adding a residency program.”
“Another thing I wanted to learn about was mentorship,” she said. “I wanted to meet women who have done this before, who have had children, who are working full-time trying to do a little bit of everything. I wanted to see how they did it and try and learn from their experiences.”
From all accounts, mission accomplished.
Richard Quinn is a freelance writer in New Jersey.
LAS VEGAS—If she said it once, Patience Reich, MD, SFHM, said it a half-dozen times during SHM’s annual meeting: “Let it go.”
“You can’t be Martha Stewart and a perfect doctor. Just let it go,” said Dr. Reich, associate professor of internal medicine and associate faculty for the Office of Women in Medicine and Science at the Wake Forest School of Medicine in Winston-Salem, N.C., told about 75 female hospitalists during a two-hour workshop focused on women’s issues at the Mandalay Bay Resort and Casino. “Even in 2014, there are trade-offs to be made.
Dr. Reich and Rachel George, MD, MBA, CPE, SFHM, of Cogent Healthcare, have been moderating the workshop at SHM meetings for several years. They said the issues they encounter among hospitalists around the country, which are no different today than they were in years past, include gender bias, career advancement challenges, and the guilt some feel spending time away from their children or communicating with their stay-at-home husbands.
At HM14, workshop attendees searched for solutions to common struggles.
“Don’t pretend you can have it all,” Dr. George said. “It’s a myth ruining womankind. There’s nothing that says you have to be June Cleaver and Marcus Welby all rolled into one. We have to stop thinking that we have to do it.”
Dr. George told the workshop attendees that cooking and cleaning are so far down on her priority list that “they practically don’t exist.”
“It’s OK. My kids are happy and healthy,” she said. “It doesn’t matter if they come home to a dirty house or if they eat pizza. They’re going to survive. I think women put all that guilt on themselves. Some of it society does, but a lot of women put the guilt on themselves just because they don’t cook a three-course meal every night.”
Open Forum
The issues were much the same during a Special Interest Group attended by nearly 35 hospitalists and moderated by Melissa Mattison, MD, FACP, SFHM, of Beth Israel Deaconess Medical Center in Boston. Topics ranged from personal experiences with workplace discrimination to apprehension in pursuing leadership roles to “partner envy” and dealing with the “guilt” of being a working parent.
One hospitalist wondered how others dealt with harassment from patients. “I’m young, petite, and a minority,” she said. “I get ‘sweetie’ and ‘honey’ all the time from my patients.”
Another explained the difficulty of working full time while taking care of an elderly parent. Yet another admitted her desire for a role model, “as there are none in my area.”
“Men seem to have an innate drive to be the breadwinner,” one attendee said. “No matter how much help you have at home, it doesn’t take away the guilt I feel.”
Another said, “I think about all of these issues constantly.”
Dr. Mattison, a member of the annual meeting committee, left the 45-minute open forum with four action items:
- Increase the exposure of programming for issues related to work-life balance at annual meeting;
- Suggest keynote speakers who are not men;
- Create a toolkit for HM leaders and department of medicine leaders to help them understand work-life issues; and
- Create a community on the HMX portal to discuss work-life issues, “whether they are related to being a mother or father, juggling work and home, or whatever issues come up.”
The Key: Flex Schedules
Many physicians who choose a career in HM do it because of the work-life balance the specialty affords, and many of the challenges women hospitalists face at the local level revolve around the schedule. That’s how Zenobia JonesFoster, MD, MPH, a hospitalist at Wellstar Health in Atlanta, views it.
“I think it’s very facility-dependent. I think when we look for a job and decide where we want to go, we really need to understand the culture and how people advance within that culture,” said Dr. JonesFoster, who attended the women’s issues workshop. “The academic environment has a lot more deferred policy and bureaucracy versus a private institution, but you’re going to find that anywhere.”
A hospitalist for a little more than two years, Dr. JonesFoster has two young children, ages one and three, and works in a group with 30 full-time hospitalists and 10 nurse practitioners and physician assistants. Her husband is a businessman, so schedules and work-life balance are a major concern.
“If I was given a job opportunity Monday through Friday, regular work hours, there’s no way I would take it because of the flexibility of hospital medicine hours, with the seven-on seven-off schedule,” she said. “The time I have off, I get to just be a mom and not think about work. But when I’m at work, I love it.”
Dr. JonesFoster’s group has seen an increase in patient census recently and just went live with a new hospital-wide electronic health records system, which has opened up more shifts and moonlighting opportunities. Attending her first annual meeting, she was most interested in learning the pros and cons of leadership positions, because her health system “offers a lot of opportunity for advancement” and is “talking about adding a residency program.”
“Another thing I wanted to learn about was mentorship,” she said. “I wanted to meet women who have done this before, who have had children, who are working full-time trying to do a little bit of everything. I wanted to see how they did it and try and learn from their experiences.”
From all accounts, mission accomplished.
Richard Quinn is a freelance writer in New Jersey.
LAS VEGAS—If she said it once, Patience Reich, MD, SFHM, said it a half-dozen times during SHM’s annual meeting: “Let it go.”
“You can’t be Martha Stewart and a perfect doctor. Just let it go,” said Dr. Reich, associate professor of internal medicine and associate faculty for the Office of Women in Medicine and Science at the Wake Forest School of Medicine in Winston-Salem, N.C., told about 75 female hospitalists during a two-hour workshop focused on women’s issues at the Mandalay Bay Resort and Casino. “Even in 2014, there are trade-offs to be made.
Dr. Reich and Rachel George, MD, MBA, CPE, SFHM, of Cogent Healthcare, have been moderating the workshop at SHM meetings for several years. They said the issues they encounter among hospitalists around the country, which are no different today than they were in years past, include gender bias, career advancement challenges, and the guilt some feel spending time away from their children or communicating with their stay-at-home husbands.
At HM14, workshop attendees searched for solutions to common struggles.
“Don’t pretend you can have it all,” Dr. George said. “It’s a myth ruining womankind. There’s nothing that says you have to be June Cleaver and Marcus Welby all rolled into one. We have to stop thinking that we have to do it.”
Dr. George told the workshop attendees that cooking and cleaning are so far down on her priority list that “they practically don’t exist.”
“It’s OK. My kids are happy and healthy,” she said. “It doesn’t matter if they come home to a dirty house or if they eat pizza. They’re going to survive. I think women put all that guilt on themselves. Some of it society does, but a lot of women put the guilt on themselves just because they don’t cook a three-course meal every night.”
Open Forum
The issues were much the same during a Special Interest Group attended by nearly 35 hospitalists and moderated by Melissa Mattison, MD, FACP, SFHM, of Beth Israel Deaconess Medical Center in Boston. Topics ranged from personal experiences with workplace discrimination to apprehension in pursuing leadership roles to “partner envy” and dealing with the “guilt” of being a working parent.
One hospitalist wondered how others dealt with harassment from patients. “I’m young, petite, and a minority,” she said. “I get ‘sweetie’ and ‘honey’ all the time from my patients.”
Another explained the difficulty of working full time while taking care of an elderly parent. Yet another admitted her desire for a role model, “as there are none in my area.”
“Men seem to have an innate drive to be the breadwinner,” one attendee said. “No matter how much help you have at home, it doesn’t take away the guilt I feel.”
Another said, “I think about all of these issues constantly.”
Dr. Mattison, a member of the annual meeting committee, left the 45-minute open forum with four action items:
- Increase the exposure of programming for issues related to work-life balance at annual meeting;
- Suggest keynote speakers who are not men;
- Create a toolkit for HM leaders and department of medicine leaders to help them understand work-life issues; and
- Create a community on the HMX portal to discuss work-life issues, “whether they are related to being a mother or father, juggling work and home, or whatever issues come up.”
The Key: Flex Schedules
Many physicians who choose a career in HM do it because of the work-life balance the specialty affords, and many of the challenges women hospitalists face at the local level revolve around the schedule. That’s how Zenobia JonesFoster, MD, MPH, a hospitalist at Wellstar Health in Atlanta, views it.
“I think it’s very facility-dependent. I think when we look for a job and decide where we want to go, we really need to understand the culture and how people advance within that culture,” said Dr. JonesFoster, who attended the women’s issues workshop. “The academic environment has a lot more deferred policy and bureaucracy versus a private institution, but you’re going to find that anywhere.”
A hospitalist for a little more than two years, Dr. JonesFoster has two young children, ages one and three, and works in a group with 30 full-time hospitalists and 10 nurse practitioners and physician assistants. Her husband is a businessman, so schedules and work-life balance are a major concern.
“If I was given a job opportunity Monday through Friday, regular work hours, there’s no way I would take it because of the flexibility of hospital medicine hours, with the seven-on seven-off schedule,” she said. “The time I have off, I get to just be a mom and not think about work. But when I’m at work, I love it.”
Dr. JonesFoster’s group has seen an increase in patient census recently and just went live with a new hospital-wide electronic health records system, which has opened up more shifts and moonlighting opportunities. Attending her first annual meeting, she was most interested in learning the pros and cons of leadership positions, because her health system “offers a lot of opportunity for advancement” and is “talking about adding a residency program.”
“Another thing I wanted to learn about was mentorship,” she said. “I wanted to meet women who have done this before, who have had children, who are working full-time trying to do a little bit of everything. I wanted to see how they did it and try and learn from their experiences.”
From all accounts, mission accomplished.
Richard Quinn is a freelance writer in New Jersey.
Open Surgical Dislocation Versus Arthroscopic Treatment of Femoroacetabular Impingement
Analysis of Intermediate Outcomes of Glenoid Bone Grafting in Revision Shoulder Arthroplasty
The Applications of Biologics in Orthopedic Surgery
As orthopedic surgeons, we have done a great job continually trying to improve the outcomes of our patients. During the first decade of the 21st century, many of these advances centered on strengthening the biomechanics of constructs used to repair patients’ pathologies. Trauma surgeons incorporated minimally invasive osteosynthesis with locked plates; shoulder surgeons began using double-row and transosseous-equivalent rotator cuff repairs. As a result of these shifts in treatment methods, healing rates and outcomes have improved. Unfortunately, to take rotator cuff repair as an example, healing rates have still not achieved 100%. To reach this goal in the future, biologic manipulation of the healing milieu will play a critical role.
This issue of The American Journal of Orthopedics features an article on the “Analysis of Intermediate Outcomes of Glenoid Bone Grafting in Revision Shoulder Arthroplasty” by Dr. Schubkegel and colleagues. While not as cutting edge or in vogue as growth factors or stem cells, bone graft is one of the original biologics used by orthopedic surgeons. The authors review the midterm results of glenoid bone grafting secondary to failed total shoulder arthroplasty and find that bone grafting resulted in good functional outcomes. Studies such as this one highlight the important role that biologics play, particularly in challenging or revision cases.
Platelet-rich plasma (PRP) is another biologic that is presently available for use. Reviewing its use as it pertains to orthopedics highlights both the potential benefits
as well as the difficulties associated with incorporating biologics into everyday practice. In 2006, Mishra and colleagues1 published one of the first studies that looked at the potential benefits of using PRP to treat lateral epicondylitis. While, from a purist’s standpoint, it wasn’t the best-designed study, it did provide cause for optimism with regard to a novel treatment option for an age-old problem. Since that time, hundreds of studies have been done on PRP looking at its potential treatment uses in everything from tennis elbow to rotator cuff repairs.
Study designs have improved, and with that, so have our indications for using PRP. Interestingly though, the more we study PRP (and other exogenous growth factors), it almost seems as if more questions are raised than answered. For instance, preparing PRP from a given patient will result in different concentrations of the PRP depending on what time of the day the patient’s blood is drawn. What is the ideal time to prepare the PRP? Additionally, PRP prepared using different companies’ systems results in different concentrations of growth factors. So, not only is a given patient’s PRP different at different times of day, but these differences get magnified by using different preparation systems.
One of the main issues with tendon healing is that the tissue heals via reactive scar formation instead of truly regenerating new tendon. In this scenario, it is possible that adding PRP or other growth factors to the repair construct may only increase scar formation. Along these lines, newer work is focusing on cellular solutions to healing problems. Stem cells, which are undifferentiated, unspecialized cells, have shown potential to improve healing when added to injury/repair sites. Thus far, unfortunately, there is very little clinical data pertaining to their use in orthopedic surgery. Compounding this problem are the US Food and Drug Administration’s regulations on manipulating stem cells.
In the future, it is likely that growth factors, cytokines, PRP, and cellular approaches will be used to enhance healing. For now, a significant amount of preclinical work is being done to figure out the most advantageous ways to use such adjuvants. This is an extremely exciting field with ample opportunities to
answer well-designed research questions. Future issues of this journal will likely highlight such studies. ◾
Reference
1. Mishra A, Pavelko T. Treatment of chronic elbow tendinosis with buffered
platelet-rich plasma. Am J Sports Med. 2006;34(11):1774-1778.
As orthopedic surgeons, we have done a great job continually trying to improve the outcomes of our patients. During the first decade of the 21st century, many of these advances centered on strengthening the biomechanics of constructs used to repair patients’ pathologies. Trauma surgeons incorporated minimally invasive osteosynthesis with locked plates; shoulder surgeons began using double-row and transosseous-equivalent rotator cuff repairs. As a result of these shifts in treatment methods, healing rates and outcomes have improved. Unfortunately, to take rotator cuff repair as an example, healing rates have still not achieved 100%. To reach this goal in the future, biologic manipulation of the healing milieu will play a critical role.
This issue of The American Journal of Orthopedics features an article on the “Analysis of Intermediate Outcomes of Glenoid Bone Grafting in Revision Shoulder Arthroplasty” by Dr. Schubkegel and colleagues. While not as cutting edge or in vogue as growth factors or stem cells, bone graft is one of the original biologics used by orthopedic surgeons. The authors review the midterm results of glenoid bone grafting secondary to failed total shoulder arthroplasty and find that bone grafting resulted in good functional outcomes. Studies such as this one highlight the important role that biologics play, particularly in challenging or revision cases.
Platelet-rich plasma (PRP) is another biologic that is presently available for use. Reviewing its use as it pertains to orthopedics highlights both the potential benefits
as well as the difficulties associated with incorporating biologics into everyday practice. In 2006, Mishra and colleagues1 published one of the first studies that looked at the potential benefits of using PRP to treat lateral epicondylitis. While, from a purist’s standpoint, it wasn’t the best-designed study, it did provide cause for optimism with regard to a novel treatment option for an age-old problem. Since that time, hundreds of studies have been done on PRP looking at its potential treatment uses in everything from tennis elbow to rotator cuff repairs.
Study designs have improved, and with that, so have our indications for using PRP. Interestingly though, the more we study PRP (and other exogenous growth factors), it almost seems as if more questions are raised than answered. For instance, preparing PRP from a given patient will result in different concentrations of the PRP depending on what time of the day the patient’s blood is drawn. What is the ideal time to prepare the PRP? Additionally, PRP prepared using different companies’ systems results in different concentrations of growth factors. So, not only is a given patient’s PRP different at different times of day, but these differences get magnified by using different preparation systems.
One of the main issues with tendon healing is that the tissue heals via reactive scar formation instead of truly regenerating new tendon. In this scenario, it is possible that adding PRP or other growth factors to the repair construct may only increase scar formation. Along these lines, newer work is focusing on cellular solutions to healing problems. Stem cells, which are undifferentiated, unspecialized cells, have shown potential to improve healing when added to injury/repair sites. Thus far, unfortunately, there is very little clinical data pertaining to their use in orthopedic surgery. Compounding this problem are the US Food and Drug Administration’s regulations on manipulating stem cells.
In the future, it is likely that growth factors, cytokines, PRP, and cellular approaches will be used to enhance healing. For now, a significant amount of preclinical work is being done to figure out the most advantageous ways to use such adjuvants. This is an extremely exciting field with ample opportunities to
answer well-designed research questions. Future issues of this journal will likely highlight such studies. ◾
Reference
1. Mishra A, Pavelko T. Treatment of chronic elbow tendinosis with buffered
platelet-rich plasma. Am J Sports Med. 2006;34(11):1774-1778.
As orthopedic surgeons, we have done a great job continually trying to improve the outcomes of our patients. During the first decade of the 21st century, many of these advances centered on strengthening the biomechanics of constructs used to repair patients’ pathologies. Trauma surgeons incorporated minimally invasive osteosynthesis with locked plates; shoulder surgeons began using double-row and transosseous-equivalent rotator cuff repairs. As a result of these shifts in treatment methods, healing rates and outcomes have improved. Unfortunately, to take rotator cuff repair as an example, healing rates have still not achieved 100%. To reach this goal in the future, biologic manipulation of the healing milieu will play a critical role.
This issue of The American Journal of Orthopedics features an article on the “Analysis of Intermediate Outcomes of Glenoid Bone Grafting in Revision Shoulder Arthroplasty” by Dr. Schubkegel and colleagues. While not as cutting edge or in vogue as growth factors or stem cells, bone graft is one of the original biologics used by orthopedic surgeons. The authors review the midterm results of glenoid bone grafting secondary to failed total shoulder arthroplasty and find that bone grafting resulted in good functional outcomes. Studies such as this one highlight the important role that biologics play, particularly in challenging or revision cases.
Platelet-rich plasma (PRP) is another biologic that is presently available for use. Reviewing its use as it pertains to orthopedics highlights both the potential benefits
as well as the difficulties associated with incorporating biologics into everyday practice. In 2006, Mishra and colleagues1 published one of the first studies that looked at the potential benefits of using PRP to treat lateral epicondylitis. While, from a purist’s standpoint, it wasn’t the best-designed study, it did provide cause for optimism with regard to a novel treatment option for an age-old problem. Since that time, hundreds of studies have been done on PRP looking at its potential treatment uses in everything from tennis elbow to rotator cuff repairs.
Study designs have improved, and with that, so have our indications for using PRP. Interestingly though, the more we study PRP (and other exogenous growth factors), it almost seems as if more questions are raised than answered. For instance, preparing PRP from a given patient will result in different concentrations of the PRP depending on what time of the day the patient’s blood is drawn. What is the ideal time to prepare the PRP? Additionally, PRP prepared using different companies’ systems results in different concentrations of growth factors. So, not only is a given patient’s PRP different at different times of day, but these differences get magnified by using different preparation systems.
One of the main issues with tendon healing is that the tissue heals via reactive scar formation instead of truly regenerating new tendon. In this scenario, it is possible that adding PRP or other growth factors to the repair construct may only increase scar formation. Along these lines, newer work is focusing on cellular solutions to healing problems. Stem cells, which are undifferentiated, unspecialized cells, have shown potential to improve healing when added to injury/repair sites. Thus far, unfortunately, there is very little clinical data pertaining to their use in orthopedic surgery. Compounding this problem are the US Food and Drug Administration’s regulations on manipulating stem cells.
In the future, it is likely that growth factors, cytokines, PRP, and cellular approaches will be used to enhance healing. For now, a significant amount of preclinical work is being done to figure out the most advantageous ways to use such adjuvants. This is an extremely exciting field with ample opportunities to
answer well-designed research questions. Future issues of this journal will likely highlight such studies. ◾
Reference
1. Mishra A, Pavelko T. Treatment of chronic elbow tendinosis with buffered
platelet-rich plasma. Am J Sports Med. 2006;34(11):1774-1778.
Vomiting, abdominal pain, compulsive bathing—Dx?
THE CASE
A 33-year-old multiparous pregnant woman at 7 weeks gestation came to our clinic after 3 days of vomiting. She had been vomiting up to 7 times a day and had right lower quadrant pain radiating into her flank. She described the pain as continuous, severe, and “crampy” in nature. The patient also complained of a loss of appetite, nonbloody diarrhea, fever, chills, night sweats, and urinary urgency. She’d tried acetaminophen without relief and repeatedly took hot showers—for up to 6 hours each day—which she said temporarily improved her symptoms.
At presentation, the patient’s vital signs were normal, with no orthostatic changes in blood pressure or heart rate. A physical and pelvic examination revealed tenderness in her right lower quadrant and flank and a mildly tender uterus. Chlamydia culture was positive. Pelvic ultrasound showed a normal intrauterine pregnancy, a surgically absent right ovary and tube, and a normal left ovary and tube. Her appendix was not visualized. Laboratory results, including a basic metabolic panel, complete blood count, liver function tests, amylase test, lipase test, and urinalysis were normal.
On admission, the patient received intravenous (IV) fluids, oral ondansetron 4 mg every 6 hours and IV hydromorphone 2 mg every 3 to 6 hours. Because her symptoms did not respond to initial therapy, we administered IV metoclopramide 10 mg every 6 to 8 hours and promethazine 12.5 mg rectally 3 times daily. After a discussion of the risks associated with benzodiazepine use during pregnancy, the patient agreed to treatment with IV lorazepam 2 mg. She was also informed of the risks of radiation during pregnancy,1 and opted to undergo an abdominal computed tomography (CT) scan and ultrasound. No abnormalities were found.
The patient said that in prior pregnancies, she had experienced nausea and vomiting during the first trimester, but that her current symptoms were much worse. She also said she’d been smoking cannabis twice a day for a year.
THE DIAGNOSIS
Based on our patient’s symptoms, her history of daily cannabis use, and the lack of improvement from antiemetics and analgesics, we concluded that she was suffering from cannabinoid hyperemesis. By Day 3, her symptoms improved and she could tolerate oral fluids. We advised her to stop using cannabis and discharged her.
One week later, the patient reported that she had not smoked cannabis since she’d been admitted to the hospital and that her symptoms, including her compulsive bathing, continued to improve. The patient subsequently delivered a healthy newborn at term.
DISCUSSION
A drug sometimes used to relieve nausea may make it much worse
First described in 2004, cannabinoid hyperemesis is a triad of vomiting, abdominal pain, and compulsive bathing in patients with chronic cannabis use.2 Other symptoms include subjective fevers, chills, and diaphoresis. Symptoms of cannabinoid hyperemesis may persist for weeks and spontaneously remit for weeks to months. The syndrome can lead to serious complications, including volume depletion, weight loss of 5 to 10 kg per episode, burns caused by frequent hot showers, and esophageal rupture.2-4 The frequent hot baths or showers associated with cannabinoid hyperemesis are potentially harmful to the fetus—hyperthermia during early gestation can cause fetal mental deficiency, seizures, and neural tube defects.5-8
The association between cannabinoid hyperemesis and pregnancy is unknown. However, because 11% of pregnant women use cannabis,9 many women may be at risk for the condition. Pregnant women may use cannabis recreationally or to combat morning sickness. The high rate of cannabis use in this population may be due in part to a lack of perceived risk to the fetus, although prenatal cannabis exposure is associated with reduced fetal growth.10
Active component of cannabis may trigger vomiting, pain
While chronic cannabis use causes cannabinoid hyperemesis, the pathogenesis and pathophysiology of the disease remain unknown. Delta-9-tetrahydrocannabinol (THC), the active ingredient in cannabis, appears to be to blame.11-15
THC is lipophilic and accumulates in the body with chronic cannabis use. At higher accumulated levels, THC binds to cannabinoid receptors in the intestinal nerve plexus, causing lower esophageal sphincter relaxation and gastrointestinal motility inhibition.11,12 At high concentrations, THC also binds to CB1 receptors in the hypothalamus, which is responsible for integrating satiety, thirst, digestive function, and thermoregulation.13-15 This causes hypothalamic dysregulation that may be temporarily relieved by hot showers.2
Possible causes of our patient’s signs and symptoms included appendicitis, pyelonephritis, nephrolithiasis, ectopic pregnancy, and pelvic inflammatory disease. Our patient’s normal laboratory and imaging studies made these diagnoses less likely. Her lack of a fallopian tube or ovary on the right side prompted us to quickly rule out a right-sided ectopic implantation.
We considered our patient’s chlamydial infection as the source of her symptoms. However, she had experienced no vaginal discharge or bleeding, and her nausea and abdominal pain did not improve with a therapeutic dose of azithromycin. Imaging showed no evidence of chlamydia-associated complications, such as pelvic inflammatory disease or tubo-ovarian abscess, both of which are uncommon in pregnancy.
THE TAKEAWAY
Consider cannabinoid hyperemesis in patients who are experiencing vomiting and abdominal pain that are temporarily relieved by bathing in hot water. Ask such patients about their cannabis use, and strongly encourage them to discontinue the drug. Counsel pregnant women about the maternal and fetal risks of cannabis use and the potential teratogenicity of hot water bathing. Provide IV fluids to ensure adequate hydration and a benzodiazepine as appropriate for anxiety and cannabis withdrawal symptoms.16 Advise patients that their symptoms may return within weeks to months if they resume using cannabis.2,17
1. ACOG Committee on Obstetric Practice. ACOG Committee Opinion. Number 299, September 2004 (replaces No. 158, September 1995). Guidelines for diagnostic imaging during pregnancy. Obstet Gynecol. 2004;104:647-651.
2. Allen JH, de Moore GM, Heddle R, et al. Cannabinoid hyperemesis: cyclical hyperemesis in association with chronic cannabis abuse. Gut. 2004;53:1566-1570.
3. Sontineni SP, Chaudhary S, Sontineni V, et al. Cannabinoid hyperemesis syndrome: clinical diagnosis of an underrecognised manifestation of chronic cannabis abuse. World J Gastroenterol. 2009;15:1264-1266.
4. de Moore GM, Baker J, Bui T. Psychogenic vomiting complicated by marijuana abuse and spontaneous pneumomediastinum. Aust N Z J Psychiatry. 1996;30:290-294.
5. Smith DW, Clarren SK, Harvey MA. Hyperthermia as a possible teratogenic agent. J Pediatr. 1978;92:878-883.
6. Shiota K. Neural tube defects and maternal hyperthermia in early pregnancy: epidemiology in a human embryo population. Am J Med Genet. 1982;12:281-288.
7. Milunsky A, Ulcickas M, Rothman K, et al. Maternal heat exposure and neural tube defects. JAMA. 1992;268:882-885.
8. Miller P, Smith DW, Shepard TH. Maternal hyperthermia as a possible cause of anencephaly. Lancet. 1978;11:1519-1521.
9. Substance Abuse and Mental Health Services Administration Web site. Results from the 2008 National Survey on Drug Use and Health: national findings. Available at: http://www.samhsa.gov/data/nsduh/2k8nsduh/2k8Results.htm. Accessed December 15, 2011.
10. Gray TR, Eiden RD, Leonard KD, et al. Identifying prenatal cannabis exposure and effects of concurrent tobacco exposure on neonatal growth. Clin Chem. 2010;56:1442-1450.
11. Izzo AA, Coutts AA. Cannabinoids and the digestive tract. Handb Exp Pharmacol. 2005;(168):573-598.
12. McCallum RW, Soykan I, Sridhar KR, et al. Delta-9-tetrahydrocannabinol delays the gastric emptying of solid food in humans: a double-blind, randomized study. Aliment Pharmacol Ther. 1999;13:77-80.
13. Pertwee RG. Pharmacology of cannabinoid CB1 and CB2 receptors. Pharmacol Ther. 1997;74:129-180.
14. Childers SR, Breivogel CS. Cannabis and endogenous cannabinoid systems. Drug Alcohol Dependence. 1998;51:173-187.
15. Herkenham M, Lynn AB, Little MD, et al. Cannabinoid receptor localization in brain. Proc Natl Acad Sci. 1990;87:1932-1936.
16. Bergman U, Rosa FW, Baum C, et al. Effects of exposure to benzodiazepine during fetal life. Lancet. 1992;340:694-696.
17. Wallace D, Martin AL, Park B. Cannabinoid hyperemesis: marijuana puts patients in hot water. Australas Psychiatry. 2007;15:156-158.
THE CASE
A 33-year-old multiparous pregnant woman at 7 weeks gestation came to our clinic after 3 days of vomiting. She had been vomiting up to 7 times a day and had right lower quadrant pain radiating into her flank. She described the pain as continuous, severe, and “crampy” in nature. The patient also complained of a loss of appetite, nonbloody diarrhea, fever, chills, night sweats, and urinary urgency. She’d tried acetaminophen without relief and repeatedly took hot showers—for up to 6 hours each day—which she said temporarily improved her symptoms.
At presentation, the patient’s vital signs were normal, with no orthostatic changes in blood pressure or heart rate. A physical and pelvic examination revealed tenderness in her right lower quadrant and flank and a mildly tender uterus. Chlamydia culture was positive. Pelvic ultrasound showed a normal intrauterine pregnancy, a surgically absent right ovary and tube, and a normal left ovary and tube. Her appendix was not visualized. Laboratory results, including a basic metabolic panel, complete blood count, liver function tests, amylase test, lipase test, and urinalysis were normal.
On admission, the patient received intravenous (IV) fluids, oral ondansetron 4 mg every 6 hours and IV hydromorphone 2 mg every 3 to 6 hours. Because her symptoms did not respond to initial therapy, we administered IV metoclopramide 10 mg every 6 to 8 hours and promethazine 12.5 mg rectally 3 times daily. After a discussion of the risks associated with benzodiazepine use during pregnancy, the patient agreed to treatment with IV lorazepam 2 mg. She was also informed of the risks of radiation during pregnancy,1 and opted to undergo an abdominal computed tomography (CT) scan and ultrasound. No abnormalities were found.
The patient said that in prior pregnancies, she had experienced nausea and vomiting during the first trimester, but that her current symptoms were much worse. She also said she’d been smoking cannabis twice a day for a year.
THE DIAGNOSIS
Based on our patient’s symptoms, her history of daily cannabis use, and the lack of improvement from antiemetics and analgesics, we concluded that she was suffering from cannabinoid hyperemesis. By Day 3, her symptoms improved and she could tolerate oral fluids. We advised her to stop using cannabis and discharged her.
One week later, the patient reported that she had not smoked cannabis since she’d been admitted to the hospital and that her symptoms, including her compulsive bathing, continued to improve. The patient subsequently delivered a healthy newborn at term.
DISCUSSION
A drug sometimes used to relieve nausea may make it much worse
First described in 2004, cannabinoid hyperemesis is a triad of vomiting, abdominal pain, and compulsive bathing in patients with chronic cannabis use.2 Other symptoms include subjective fevers, chills, and diaphoresis. Symptoms of cannabinoid hyperemesis may persist for weeks and spontaneously remit for weeks to months. The syndrome can lead to serious complications, including volume depletion, weight loss of 5 to 10 kg per episode, burns caused by frequent hot showers, and esophageal rupture.2-4 The frequent hot baths or showers associated with cannabinoid hyperemesis are potentially harmful to the fetus—hyperthermia during early gestation can cause fetal mental deficiency, seizures, and neural tube defects.5-8
The association between cannabinoid hyperemesis and pregnancy is unknown. However, because 11% of pregnant women use cannabis,9 many women may be at risk for the condition. Pregnant women may use cannabis recreationally or to combat morning sickness. The high rate of cannabis use in this population may be due in part to a lack of perceived risk to the fetus, although prenatal cannabis exposure is associated with reduced fetal growth.10
Active component of cannabis may trigger vomiting, pain
While chronic cannabis use causes cannabinoid hyperemesis, the pathogenesis and pathophysiology of the disease remain unknown. Delta-9-tetrahydrocannabinol (THC), the active ingredient in cannabis, appears to be to blame.11-15
THC is lipophilic and accumulates in the body with chronic cannabis use. At higher accumulated levels, THC binds to cannabinoid receptors in the intestinal nerve plexus, causing lower esophageal sphincter relaxation and gastrointestinal motility inhibition.11,12 At high concentrations, THC also binds to CB1 receptors in the hypothalamus, which is responsible for integrating satiety, thirst, digestive function, and thermoregulation.13-15 This causes hypothalamic dysregulation that may be temporarily relieved by hot showers.2
Possible causes of our patient’s signs and symptoms included appendicitis, pyelonephritis, nephrolithiasis, ectopic pregnancy, and pelvic inflammatory disease. Our patient’s normal laboratory and imaging studies made these diagnoses less likely. Her lack of a fallopian tube or ovary on the right side prompted us to quickly rule out a right-sided ectopic implantation.
We considered our patient’s chlamydial infection as the source of her symptoms. However, she had experienced no vaginal discharge or bleeding, and her nausea and abdominal pain did not improve with a therapeutic dose of azithromycin. Imaging showed no evidence of chlamydia-associated complications, such as pelvic inflammatory disease or tubo-ovarian abscess, both of which are uncommon in pregnancy.
THE TAKEAWAY
Consider cannabinoid hyperemesis in patients who are experiencing vomiting and abdominal pain that are temporarily relieved by bathing in hot water. Ask such patients about their cannabis use, and strongly encourage them to discontinue the drug. Counsel pregnant women about the maternal and fetal risks of cannabis use and the potential teratogenicity of hot water bathing. Provide IV fluids to ensure adequate hydration and a benzodiazepine as appropriate for anxiety and cannabis withdrawal symptoms.16 Advise patients that their symptoms may return within weeks to months if they resume using cannabis.2,17
THE CASE
A 33-year-old multiparous pregnant woman at 7 weeks gestation came to our clinic after 3 days of vomiting. She had been vomiting up to 7 times a day and had right lower quadrant pain radiating into her flank. She described the pain as continuous, severe, and “crampy” in nature. The patient also complained of a loss of appetite, nonbloody diarrhea, fever, chills, night sweats, and urinary urgency. She’d tried acetaminophen without relief and repeatedly took hot showers—for up to 6 hours each day—which she said temporarily improved her symptoms.
At presentation, the patient’s vital signs were normal, with no orthostatic changes in blood pressure or heart rate. A physical and pelvic examination revealed tenderness in her right lower quadrant and flank and a mildly tender uterus. Chlamydia culture was positive. Pelvic ultrasound showed a normal intrauterine pregnancy, a surgically absent right ovary and tube, and a normal left ovary and tube. Her appendix was not visualized. Laboratory results, including a basic metabolic panel, complete blood count, liver function tests, amylase test, lipase test, and urinalysis were normal.
On admission, the patient received intravenous (IV) fluids, oral ondansetron 4 mg every 6 hours and IV hydromorphone 2 mg every 3 to 6 hours. Because her symptoms did not respond to initial therapy, we administered IV metoclopramide 10 mg every 6 to 8 hours and promethazine 12.5 mg rectally 3 times daily. After a discussion of the risks associated with benzodiazepine use during pregnancy, the patient agreed to treatment with IV lorazepam 2 mg. She was also informed of the risks of radiation during pregnancy,1 and opted to undergo an abdominal computed tomography (CT) scan and ultrasound. No abnormalities were found.
The patient said that in prior pregnancies, she had experienced nausea and vomiting during the first trimester, but that her current symptoms were much worse. She also said she’d been smoking cannabis twice a day for a year.
THE DIAGNOSIS
Based on our patient’s symptoms, her history of daily cannabis use, and the lack of improvement from antiemetics and analgesics, we concluded that she was suffering from cannabinoid hyperemesis. By Day 3, her symptoms improved and she could tolerate oral fluids. We advised her to stop using cannabis and discharged her.
One week later, the patient reported that she had not smoked cannabis since she’d been admitted to the hospital and that her symptoms, including her compulsive bathing, continued to improve. The patient subsequently delivered a healthy newborn at term.
DISCUSSION
A drug sometimes used to relieve nausea may make it much worse
First described in 2004, cannabinoid hyperemesis is a triad of vomiting, abdominal pain, and compulsive bathing in patients with chronic cannabis use.2 Other symptoms include subjective fevers, chills, and diaphoresis. Symptoms of cannabinoid hyperemesis may persist for weeks and spontaneously remit for weeks to months. The syndrome can lead to serious complications, including volume depletion, weight loss of 5 to 10 kg per episode, burns caused by frequent hot showers, and esophageal rupture.2-4 The frequent hot baths or showers associated with cannabinoid hyperemesis are potentially harmful to the fetus—hyperthermia during early gestation can cause fetal mental deficiency, seizures, and neural tube defects.5-8
The association between cannabinoid hyperemesis and pregnancy is unknown. However, because 11% of pregnant women use cannabis,9 many women may be at risk for the condition. Pregnant women may use cannabis recreationally or to combat morning sickness. The high rate of cannabis use in this population may be due in part to a lack of perceived risk to the fetus, although prenatal cannabis exposure is associated with reduced fetal growth.10
Active component of cannabis may trigger vomiting, pain
While chronic cannabis use causes cannabinoid hyperemesis, the pathogenesis and pathophysiology of the disease remain unknown. Delta-9-tetrahydrocannabinol (THC), the active ingredient in cannabis, appears to be to blame.11-15
THC is lipophilic and accumulates in the body with chronic cannabis use. At higher accumulated levels, THC binds to cannabinoid receptors in the intestinal nerve plexus, causing lower esophageal sphincter relaxation and gastrointestinal motility inhibition.11,12 At high concentrations, THC also binds to CB1 receptors in the hypothalamus, which is responsible for integrating satiety, thirst, digestive function, and thermoregulation.13-15 This causes hypothalamic dysregulation that may be temporarily relieved by hot showers.2
Possible causes of our patient’s signs and symptoms included appendicitis, pyelonephritis, nephrolithiasis, ectopic pregnancy, and pelvic inflammatory disease. Our patient’s normal laboratory and imaging studies made these diagnoses less likely. Her lack of a fallopian tube or ovary on the right side prompted us to quickly rule out a right-sided ectopic implantation.
We considered our patient’s chlamydial infection as the source of her symptoms. However, she had experienced no vaginal discharge or bleeding, and her nausea and abdominal pain did not improve with a therapeutic dose of azithromycin. Imaging showed no evidence of chlamydia-associated complications, such as pelvic inflammatory disease or tubo-ovarian abscess, both of which are uncommon in pregnancy.
THE TAKEAWAY
Consider cannabinoid hyperemesis in patients who are experiencing vomiting and abdominal pain that are temporarily relieved by bathing in hot water. Ask such patients about their cannabis use, and strongly encourage them to discontinue the drug. Counsel pregnant women about the maternal and fetal risks of cannabis use and the potential teratogenicity of hot water bathing. Provide IV fluids to ensure adequate hydration and a benzodiazepine as appropriate for anxiety and cannabis withdrawal symptoms.16 Advise patients that their symptoms may return within weeks to months if they resume using cannabis.2,17
1. ACOG Committee on Obstetric Practice. ACOG Committee Opinion. Number 299, September 2004 (replaces No. 158, September 1995). Guidelines for diagnostic imaging during pregnancy. Obstet Gynecol. 2004;104:647-651.
2. Allen JH, de Moore GM, Heddle R, et al. Cannabinoid hyperemesis: cyclical hyperemesis in association with chronic cannabis abuse. Gut. 2004;53:1566-1570.
3. Sontineni SP, Chaudhary S, Sontineni V, et al. Cannabinoid hyperemesis syndrome: clinical diagnosis of an underrecognised manifestation of chronic cannabis abuse. World J Gastroenterol. 2009;15:1264-1266.
4. de Moore GM, Baker J, Bui T. Psychogenic vomiting complicated by marijuana abuse and spontaneous pneumomediastinum. Aust N Z J Psychiatry. 1996;30:290-294.
5. Smith DW, Clarren SK, Harvey MA. Hyperthermia as a possible teratogenic agent. J Pediatr. 1978;92:878-883.
6. Shiota K. Neural tube defects and maternal hyperthermia in early pregnancy: epidemiology in a human embryo population. Am J Med Genet. 1982;12:281-288.
7. Milunsky A, Ulcickas M, Rothman K, et al. Maternal heat exposure and neural tube defects. JAMA. 1992;268:882-885.
8. Miller P, Smith DW, Shepard TH. Maternal hyperthermia as a possible cause of anencephaly. Lancet. 1978;11:1519-1521.
9. Substance Abuse and Mental Health Services Administration Web site. Results from the 2008 National Survey on Drug Use and Health: national findings. Available at: http://www.samhsa.gov/data/nsduh/2k8nsduh/2k8Results.htm. Accessed December 15, 2011.
10. Gray TR, Eiden RD, Leonard KD, et al. Identifying prenatal cannabis exposure and effects of concurrent tobacco exposure on neonatal growth. Clin Chem. 2010;56:1442-1450.
11. Izzo AA, Coutts AA. Cannabinoids and the digestive tract. Handb Exp Pharmacol. 2005;(168):573-598.
12. McCallum RW, Soykan I, Sridhar KR, et al. Delta-9-tetrahydrocannabinol delays the gastric emptying of solid food in humans: a double-blind, randomized study. Aliment Pharmacol Ther. 1999;13:77-80.
13. Pertwee RG. Pharmacology of cannabinoid CB1 and CB2 receptors. Pharmacol Ther. 1997;74:129-180.
14. Childers SR, Breivogel CS. Cannabis and endogenous cannabinoid systems. Drug Alcohol Dependence. 1998;51:173-187.
15. Herkenham M, Lynn AB, Little MD, et al. Cannabinoid receptor localization in brain. Proc Natl Acad Sci. 1990;87:1932-1936.
16. Bergman U, Rosa FW, Baum C, et al. Effects of exposure to benzodiazepine during fetal life. Lancet. 1992;340:694-696.
17. Wallace D, Martin AL, Park B. Cannabinoid hyperemesis: marijuana puts patients in hot water. Australas Psychiatry. 2007;15:156-158.
1. ACOG Committee on Obstetric Practice. ACOG Committee Opinion. Number 299, September 2004 (replaces No. 158, September 1995). Guidelines for diagnostic imaging during pregnancy. Obstet Gynecol. 2004;104:647-651.
2. Allen JH, de Moore GM, Heddle R, et al. Cannabinoid hyperemesis: cyclical hyperemesis in association with chronic cannabis abuse. Gut. 2004;53:1566-1570.
3. Sontineni SP, Chaudhary S, Sontineni V, et al. Cannabinoid hyperemesis syndrome: clinical diagnosis of an underrecognised manifestation of chronic cannabis abuse. World J Gastroenterol. 2009;15:1264-1266.
4. de Moore GM, Baker J, Bui T. Psychogenic vomiting complicated by marijuana abuse and spontaneous pneumomediastinum. Aust N Z J Psychiatry. 1996;30:290-294.
5. Smith DW, Clarren SK, Harvey MA. Hyperthermia as a possible teratogenic agent. J Pediatr. 1978;92:878-883.
6. Shiota K. Neural tube defects and maternal hyperthermia in early pregnancy: epidemiology in a human embryo population. Am J Med Genet. 1982;12:281-288.
7. Milunsky A, Ulcickas M, Rothman K, et al. Maternal heat exposure and neural tube defects. JAMA. 1992;268:882-885.
8. Miller P, Smith DW, Shepard TH. Maternal hyperthermia as a possible cause of anencephaly. Lancet. 1978;11:1519-1521.
9. Substance Abuse and Mental Health Services Administration Web site. Results from the 2008 National Survey on Drug Use and Health: national findings. Available at: http://www.samhsa.gov/data/nsduh/2k8nsduh/2k8Results.htm. Accessed December 15, 2011.
10. Gray TR, Eiden RD, Leonard KD, et al. Identifying prenatal cannabis exposure and effects of concurrent tobacco exposure on neonatal growth. Clin Chem. 2010;56:1442-1450.
11. Izzo AA, Coutts AA. Cannabinoids and the digestive tract. Handb Exp Pharmacol. 2005;(168):573-598.
12. McCallum RW, Soykan I, Sridhar KR, et al. Delta-9-tetrahydrocannabinol delays the gastric emptying of solid food in humans: a double-blind, randomized study. Aliment Pharmacol Ther. 1999;13:77-80.
13. Pertwee RG. Pharmacology of cannabinoid CB1 and CB2 receptors. Pharmacol Ther. 1997;74:129-180.
14. Childers SR, Breivogel CS. Cannabis and endogenous cannabinoid systems. Drug Alcohol Dependence. 1998;51:173-187.
15. Herkenham M, Lynn AB, Little MD, et al. Cannabinoid receptor localization in brain. Proc Natl Acad Sci. 1990;87:1932-1936.
16. Bergman U, Rosa FW, Baum C, et al. Effects of exposure to benzodiazepine during fetal life. Lancet. 1992;340:694-696.
17. Wallace D, Martin AL, Park B. Cannabinoid hyperemesis: marijuana puts patients in hot water. Australas Psychiatry. 2007;15:156-158.
A simple way to reduce catheter-associated UTIs
Ensure that antibiotics are administered to surgical patients when their urinary catheter is removed to reduce the risk of urinary tract infections (UTIs).1
Strength of recommendation
B: Based on a meta-analysis.
Marschall J, Carpenter CR, Fowler S, et al; CDC Prevention Epicenters Program. Antibiotic prophylaxis for urinary tract infections after removal of urinary catheter: meta-analysis. BMJ. 2013;346:f3147.
Illustrative case
A 49-year-old man was admitted to the hospital for resection of a vertebral mass. He is almost ready for discharge, and his urinary catheter soon will be removed. Should he be given an antibiotic when his catheter is removed to prevent a UTI?
Approximately 15% to 25% of hospitalized patients receive a urinary catheter, typically during the perioperative period.2 UTIs are the most common hospital-acquired infections, and virtually all of these UTIs are caused by instrumentation of the urinary tract, primarily by catheters.2 Although the mortality rate among patients with catheter-associated UTIs (CAUTIs) is just 2.3%, CAUTIs are the leading cause of hospital-acquired bacteremia, which increases morbidity and length of stay.2 The most common pathogens for CAUTIs are Escherichia coli (21.4%), Candida species (21%), and Enterococcus species (14.9%).2Pseudomonas aeruginosa, Klebsiella, and Enterobacter species make up the bulk of the remainder.2
Support for antibiotic prophylaxis has historically been equivocal
Until now, no data clearly supported routine use of prophylactic antibiotics after urinary catheterization. Centers for Disease Control and Prevention (CDC) guidelines published in 2009 outline which patients are appropriate for catheterization, but do not recommend routine use of antibiotics to prevent CAUTIs.2 The 2014 Infectious Diseases Society of America guidelines, which came out before the study reported on here was published, state the benefit of antibiotics at the time of catheter removal is an unresolved issue.3
STUDY SUMMARY: Meta-analysis shows prophylactic antibiotics reduce UTI ris
Marschall et al1 searched multiple databases for studies published between 1947 and 2012 that evaluated prophylactic use of antibiotics at the time of urinary catheter removal. The endpoint for their analysis was symptomatic UTI, which they defined as bacteriuria plus at least one clinical symptom. Trials were excluded if patients had suprapubic catheters or if antibiotics were started shortly after the catheter was inserted.
The authors analyzed 7 studies. Six were randomized controlled trials, of which one was unpublished. The seventh trial was a nonrandomized study that compared outcomes of patients of 2 surgeons, one of whom used prophylactic antibiotics and one who did not. Five studies enrolled surgical patients exclusively, including 2 that focused on urology patients. In all of the studies, patients had a urinary catheter in place for fewer than 15 days. The duration of antibiotic treatment varied from a single dose to 3 days. The antibiotics used included trimethoprim/sulfamethoxazole, nitrofurantoin, ciprofloxacin, and a cephalosporin.
Antibiotic prophylaxis significantly reduced the rate of CAUTIs. The absolute risk reduction was 5.8%; the rate of CAUTIs was 4.7% in the group treated with antibiotics vs 10.5% in the control group. The number needed to treat to prevent one CAUTI was 17 (95% confidence interval [CI], 12-30), with a risk ratio (RR) of .45 (95% CI, .28-.72). The RR varied only slightly (.36) when the researchers repeated their analysis but excluded the unpublished trial, and remained at .45 when they analyzed only studies of surgical patients.
The reduction in CAUTIs remained consistent despite varying lengths of antibiotic administration and choice of antimicrobial agents. However, when the authors looked at pooled results just from the 2 studies that included both surgical and medical patients, they found no decrease in CAUTIs.
WHAT'S NEW: We now have an effective way to reduce CAUTIs
Prophylactic use of antibiotics when a urinary catheter is removed appears to reduce the rate of CAUTIs by more than 50% in surgical patients. The 2009 CDC guidelines on CAUTI prevention emphasize the use of appropriate infection control measures and limiting the duration of urinary catheter use.2 Now there are data showing a reduction in the incidence of CAUTIs when prophylactic antibiotics are given during catheter removal.
CAVEATS: Results may not apply to nonsurgical patients
This meta-analysis does not provide enough information to identify which patients are most likely to benefit from antibiotic prophylaxis. Most patients (92%) in this analysis had undergone surgery, but urinary catheterization is common among medically hospitalized patients. Studies of antibiotic prophylaxis at the time of catheter removal in nonsurgical patients are needed to strengthen the recommendation of this practice for all patients.
Some of the studies analyzed may have been biased. The authors determined that most of the studies in their meta-analysis were at high risk of attrition bias because there was potential for systematic differences in withdrawals between the treatment and control groups. In addition, in most studies, the randomization and allocation appeared to be inadequate, which increased the risk for selection bias.
CHALLENGES TO IMPLEMENTATION: Which antibiotics to use, and for how long, remains unclear
Antibiotic choice depends upon institutional policies and local resistance patterns, which complicates making universal recommendations. The optimal duration of treatment also is unknown, although this meta-analysis suggests that prophylaxis for 3 days or less can reduce CAUTI risk.
Catheters impregnated with antimicrobials or with microbial resistance barriers may be an alternative to administering antibiotics at catheter removal, but in preliminary studies, these devices have not been shown to reduce the incidence of CAUTIs.4,5 Increasing antimicrobial resistance also complicates the widespread use of prophylaxis.
Acknowledgement
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
1. Marschall J, Carpenter CR, Fowler S, et al; CDC Prevention Epicenters Program. Antibiotic prophylaxis for urinary tract infections after removal of urinary catheter: meta-analysis. BMJ. 2013;346:f3147.
2. Gould CV, Umscheid CA, Agarwal RK, et al. Guideline for prevention of catheter-associated urinary tract infections 2009. Available at: http://www.cdc.gov/hicpac/pdf/cauti/cautiguideline2009final.pdf. Accessed April 15, 2014.
3. Lo E, Nicolle LE, Coffin SE, et al. Strategies to prevent catheter-associated urinary tract infections in acute care hospitals: 2014 update. Infect Control Hosp Epidemiol. 2014;35:464-479.
4. Pickard R, Lam T, Maclennan G, et al. Types of urethral catheter for reducing symptomatic urinary tract infections in hospitalised adults requiring short-term catheterisation: multicentre randomised controlled trial and economic evaluation of antimicrobial- and antiseptic-impregnated urethral catheters (the CATHETER trial). Health Technol Assess. 2012;16:1-197.
5. Pickard R, Lam T, MacLennan G, et al. Antimicrobial catheters for reduction of symptomatic urinary tract infection in adults requiring short-term catheterisation in hospital: a multicentre randomised controlled trial. Lancet. 2012;380:1927-1935.
Ensure that antibiotics are administered to surgical patients when their urinary catheter is removed to reduce the risk of urinary tract infections (UTIs).1
Strength of recommendation
B: Based on a meta-analysis.
Marschall J, Carpenter CR, Fowler S, et al; CDC Prevention Epicenters Program. Antibiotic prophylaxis for urinary tract infections after removal of urinary catheter: meta-analysis. BMJ. 2013;346:f3147.
Illustrative case
A 49-year-old man was admitted to the hospital for resection of a vertebral mass. He is almost ready for discharge, and his urinary catheter soon will be removed. Should he be given an antibiotic when his catheter is removed to prevent a UTI?
Approximately 15% to 25% of hospitalized patients receive a urinary catheter, typically during the perioperative period.2 UTIs are the most common hospital-acquired infections, and virtually all of these UTIs are caused by instrumentation of the urinary tract, primarily by catheters.2 Although the mortality rate among patients with catheter-associated UTIs (CAUTIs) is just 2.3%, CAUTIs are the leading cause of hospital-acquired bacteremia, which increases morbidity and length of stay.2 The most common pathogens for CAUTIs are Escherichia coli (21.4%), Candida species (21%), and Enterococcus species (14.9%).2Pseudomonas aeruginosa, Klebsiella, and Enterobacter species make up the bulk of the remainder.2
Support for antibiotic prophylaxis has historically been equivocal
Until now, no data clearly supported routine use of prophylactic antibiotics after urinary catheterization. Centers for Disease Control and Prevention (CDC) guidelines published in 2009 outline which patients are appropriate for catheterization, but do not recommend routine use of antibiotics to prevent CAUTIs.2 The 2014 Infectious Diseases Society of America guidelines, which came out before the study reported on here was published, state the benefit of antibiotics at the time of catheter removal is an unresolved issue.3
STUDY SUMMARY: Meta-analysis shows prophylactic antibiotics reduce UTI ris
Marschall et al1 searched multiple databases for studies published between 1947 and 2012 that evaluated prophylactic use of antibiotics at the time of urinary catheter removal. The endpoint for their analysis was symptomatic UTI, which they defined as bacteriuria plus at least one clinical symptom. Trials were excluded if patients had suprapubic catheters or if antibiotics were started shortly after the catheter was inserted.
The authors analyzed 7 studies. Six were randomized controlled trials, of which one was unpublished. The seventh trial was a nonrandomized study that compared outcomes of patients of 2 surgeons, one of whom used prophylactic antibiotics and one who did not. Five studies enrolled surgical patients exclusively, including 2 that focused on urology patients. In all of the studies, patients had a urinary catheter in place for fewer than 15 days. The duration of antibiotic treatment varied from a single dose to 3 days. The antibiotics used included trimethoprim/sulfamethoxazole, nitrofurantoin, ciprofloxacin, and a cephalosporin.
Antibiotic prophylaxis significantly reduced the rate of CAUTIs. The absolute risk reduction was 5.8%; the rate of CAUTIs was 4.7% in the group treated with antibiotics vs 10.5% in the control group. The number needed to treat to prevent one CAUTI was 17 (95% confidence interval [CI], 12-30), with a risk ratio (RR) of .45 (95% CI, .28-.72). The RR varied only slightly (.36) when the researchers repeated their analysis but excluded the unpublished trial, and remained at .45 when they analyzed only studies of surgical patients.
The reduction in CAUTIs remained consistent despite varying lengths of antibiotic administration and choice of antimicrobial agents. However, when the authors looked at pooled results just from the 2 studies that included both surgical and medical patients, they found no decrease in CAUTIs.
WHAT'S NEW: We now have an effective way to reduce CAUTIs
Prophylactic use of antibiotics when a urinary catheter is removed appears to reduce the rate of CAUTIs by more than 50% in surgical patients. The 2009 CDC guidelines on CAUTI prevention emphasize the use of appropriate infection control measures and limiting the duration of urinary catheter use.2 Now there are data showing a reduction in the incidence of CAUTIs when prophylactic antibiotics are given during catheter removal.
CAVEATS: Results may not apply to nonsurgical patients
This meta-analysis does not provide enough information to identify which patients are most likely to benefit from antibiotic prophylaxis. Most patients (92%) in this analysis had undergone surgery, but urinary catheterization is common among medically hospitalized patients. Studies of antibiotic prophylaxis at the time of catheter removal in nonsurgical patients are needed to strengthen the recommendation of this practice for all patients.
Some of the studies analyzed may have been biased. The authors determined that most of the studies in their meta-analysis were at high risk of attrition bias because there was potential for systematic differences in withdrawals between the treatment and control groups. In addition, in most studies, the randomization and allocation appeared to be inadequate, which increased the risk for selection bias.
CHALLENGES TO IMPLEMENTATION: Which antibiotics to use, and for how long, remains unclear
Antibiotic choice depends upon institutional policies and local resistance patterns, which complicates making universal recommendations. The optimal duration of treatment also is unknown, although this meta-analysis suggests that prophylaxis for 3 days or less can reduce CAUTI risk.
Catheters impregnated with antimicrobials or with microbial resistance barriers may be an alternative to administering antibiotics at catheter removal, but in preliminary studies, these devices have not been shown to reduce the incidence of CAUTIs.4,5 Increasing antimicrobial resistance also complicates the widespread use of prophylaxis.
Acknowledgement
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
Ensure that antibiotics are administered to surgical patients when their urinary catheter is removed to reduce the risk of urinary tract infections (UTIs).1
Strength of recommendation
B: Based on a meta-analysis.
Marschall J, Carpenter CR, Fowler S, et al; CDC Prevention Epicenters Program. Antibiotic prophylaxis for urinary tract infections after removal of urinary catheter: meta-analysis. BMJ. 2013;346:f3147.
Illustrative case
A 49-year-old man was admitted to the hospital for resection of a vertebral mass. He is almost ready for discharge, and his urinary catheter soon will be removed. Should he be given an antibiotic when his catheter is removed to prevent a UTI?
Approximately 15% to 25% of hospitalized patients receive a urinary catheter, typically during the perioperative period.2 UTIs are the most common hospital-acquired infections, and virtually all of these UTIs are caused by instrumentation of the urinary tract, primarily by catheters.2 Although the mortality rate among patients with catheter-associated UTIs (CAUTIs) is just 2.3%, CAUTIs are the leading cause of hospital-acquired bacteremia, which increases morbidity and length of stay.2 The most common pathogens for CAUTIs are Escherichia coli (21.4%), Candida species (21%), and Enterococcus species (14.9%).2Pseudomonas aeruginosa, Klebsiella, and Enterobacter species make up the bulk of the remainder.2
Support for antibiotic prophylaxis has historically been equivocal
Until now, no data clearly supported routine use of prophylactic antibiotics after urinary catheterization. Centers for Disease Control and Prevention (CDC) guidelines published in 2009 outline which patients are appropriate for catheterization, but do not recommend routine use of antibiotics to prevent CAUTIs.2 The 2014 Infectious Diseases Society of America guidelines, which came out before the study reported on here was published, state the benefit of antibiotics at the time of catheter removal is an unresolved issue.3
STUDY SUMMARY: Meta-analysis shows prophylactic antibiotics reduce UTI ris
Marschall et al1 searched multiple databases for studies published between 1947 and 2012 that evaluated prophylactic use of antibiotics at the time of urinary catheter removal. The endpoint for their analysis was symptomatic UTI, which they defined as bacteriuria plus at least one clinical symptom. Trials were excluded if patients had suprapubic catheters or if antibiotics were started shortly after the catheter was inserted.
The authors analyzed 7 studies. Six were randomized controlled trials, of which one was unpublished. The seventh trial was a nonrandomized study that compared outcomes of patients of 2 surgeons, one of whom used prophylactic antibiotics and one who did not. Five studies enrolled surgical patients exclusively, including 2 that focused on urology patients. In all of the studies, patients had a urinary catheter in place for fewer than 15 days. The duration of antibiotic treatment varied from a single dose to 3 days. The antibiotics used included trimethoprim/sulfamethoxazole, nitrofurantoin, ciprofloxacin, and a cephalosporin.
Antibiotic prophylaxis significantly reduced the rate of CAUTIs. The absolute risk reduction was 5.8%; the rate of CAUTIs was 4.7% in the group treated with antibiotics vs 10.5% in the control group. The number needed to treat to prevent one CAUTI was 17 (95% confidence interval [CI], 12-30), with a risk ratio (RR) of .45 (95% CI, .28-.72). The RR varied only slightly (.36) when the researchers repeated their analysis but excluded the unpublished trial, and remained at .45 when they analyzed only studies of surgical patients.
The reduction in CAUTIs remained consistent despite varying lengths of antibiotic administration and choice of antimicrobial agents. However, when the authors looked at pooled results just from the 2 studies that included both surgical and medical patients, they found no decrease in CAUTIs.
WHAT'S NEW: We now have an effective way to reduce CAUTIs
Prophylactic use of antibiotics when a urinary catheter is removed appears to reduce the rate of CAUTIs by more than 50% in surgical patients. The 2009 CDC guidelines on CAUTI prevention emphasize the use of appropriate infection control measures and limiting the duration of urinary catheter use.2 Now there are data showing a reduction in the incidence of CAUTIs when prophylactic antibiotics are given during catheter removal.
CAVEATS: Results may not apply to nonsurgical patients
This meta-analysis does not provide enough information to identify which patients are most likely to benefit from antibiotic prophylaxis. Most patients (92%) in this analysis had undergone surgery, but urinary catheterization is common among medically hospitalized patients. Studies of antibiotic prophylaxis at the time of catheter removal in nonsurgical patients are needed to strengthen the recommendation of this practice for all patients.
Some of the studies analyzed may have been biased. The authors determined that most of the studies in their meta-analysis were at high risk of attrition bias because there was potential for systematic differences in withdrawals between the treatment and control groups. In addition, in most studies, the randomization and allocation appeared to be inadequate, which increased the risk for selection bias.
CHALLENGES TO IMPLEMENTATION: Which antibiotics to use, and for how long, remains unclear
Antibiotic choice depends upon institutional policies and local resistance patterns, which complicates making universal recommendations. The optimal duration of treatment also is unknown, although this meta-analysis suggests that prophylaxis for 3 days or less can reduce CAUTI risk.
Catheters impregnated with antimicrobials or with microbial resistance barriers may be an alternative to administering antibiotics at catheter removal, but in preliminary studies, these devices have not been shown to reduce the incidence of CAUTIs.4,5 Increasing antimicrobial resistance also complicates the widespread use of prophylaxis.
Acknowledgement
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
1. Marschall J, Carpenter CR, Fowler S, et al; CDC Prevention Epicenters Program. Antibiotic prophylaxis for urinary tract infections after removal of urinary catheter: meta-analysis. BMJ. 2013;346:f3147.
2. Gould CV, Umscheid CA, Agarwal RK, et al. Guideline for prevention of catheter-associated urinary tract infections 2009. Available at: http://www.cdc.gov/hicpac/pdf/cauti/cautiguideline2009final.pdf. Accessed April 15, 2014.
3. Lo E, Nicolle LE, Coffin SE, et al. Strategies to prevent catheter-associated urinary tract infections in acute care hospitals: 2014 update. Infect Control Hosp Epidemiol. 2014;35:464-479.
4. Pickard R, Lam T, Maclennan G, et al. Types of urethral catheter for reducing symptomatic urinary tract infections in hospitalised adults requiring short-term catheterisation: multicentre randomised controlled trial and economic evaluation of antimicrobial- and antiseptic-impregnated urethral catheters (the CATHETER trial). Health Technol Assess. 2012;16:1-197.
5. Pickard R, Lam T, MacLennan G, et al. Antimicrobial catheters for reduction of symptomatic urinary tract infection in adults requiring short-term catheterisation in hospital: a multicentre randomised controlled trial. Lancet. 2012;380:1927-1935.
1. Marschall J, Carpenter CR, Fowler S, et al; CDC Prevention Epicenters Program. Antibiotic prophylaxis for urinary tract infections after removal of urinary catheter: meta-analysis. BMJ. 2013;346:f3147.
2. Gould CV, Umscheid CA, Agarwal RK, et al. Guideline for prevention of catheter-associated urinary tract infections 2009. Available at: http://www.cdc.gov/hicpac/pdf/cauti/cautiguideline2009final.pdf. Accessed April 15, 2014.
3. Lo E, Nicolle LE, Coffin SE, et al. Strategies to prevent catheter-associated urinary tract infections in acute care hospitals: 2014 update. Infect Control Hosp Epidemiol. 2014;35:464-479.
4. Pickard R, Lam T, Maclennan G, et al. Types of urethral catheter for reducing symptomatic urinary tract infections in hospitalised adults requiring short-term catheterisation: multicentre randomised controlled trial and economic evaluation of antimicrobial- and antiseptic-impregnated urethral catheters (the CATHETER trial). Health Technol Assess. 2012;16:1-197.
5. Pickard R, Lam T, MacLennan G, et al. Antimicrobial catheters for reduction of symptomatic urinary tract infection in adults requiring short-term catheterisation in hospital: a multicentre randomised controlled trial. Lancet. 2012;380:1927-1935.
Copyright © 2014 Family Physicians Inquiries Network. All rights reserved.