User login
FDA again rejects rivaroxaban for ACS

Credit: CDC
For a second time, the FDA has decided against approving rivaroxaban (Xarelto) to reduce the risk of cardiovascular events in patients with acute coronary syndrome (ACS).
The agency has issued a complete response letter to the drug’s makers, Janssen Pharmaceuticals.
The contents of the letter are not publicly known, but representatives at Janssen have said they “are evaluating the letter and will respond to the agency’s questions.”
This is not the first time the FDA has raised questions about the use of rivaroxaban in ACS. Last June, the agency issued a complete response letter requesting additional information on the drug.
A month before that, an FDA review committee had expressed concerns about missing follow-up data from the ATLAS ACS 2 TIMI 51 trial (JL Mega et al, NEJM, 2012).
So in September, Janssen supplied the FDA with data on the patients who had withdrawn from trial. The company was able to confirm the vital status information for 843 (63%) of the 1338 trial participants who previously had unknown vital status.
Of those 843 patients, 37 had died. The company said the deaths were equally distributed among the treatment groups—rivaroxaban at 2.5 mg, rivaroxaban at 5 mg, and placebo.
“We remain confident in the robustness and results of the ATLAS ACS 2 TIMI 51 trial, evidenced by a significant reduction in cardiovascular events, including a clinically important decrease in cardiovascular death . . . ,” said Christopher Nessel, Vice President at Janssen.
“While we saw an increase in major bleeding, there was no increase in fatal bleeding. We will continue to work with the FDA to address their questions.”
Rivaroxaban is already approved by the FDA to treat and prevent the recurrence of deep vein thrombosis and pulmonary embolism, as thromboprophylaxis in patients who have undergone knee or hip replacement surgery, as well as to reduce the risk of stroke in patients with non-valvular atrial fibrillation. ![]()

Credit: CDC
For a second time, the FDA has decided against approving rivaroxaban (Xarelto) to reduce the risk of cardiovascular events in patients with acute coronary syndrome (ACS).
The agency has issued a complete response letter to the drug’s makers, Janssen Pharmaceuticals.
The contents of the letter are not publicly known, but representatives at Janssen have said they “are evaluating the letter and will respond to the agency’s questions.”
This is not the first time the FDA has raised questions about the use of rivaroxaban in ACS. Last June, the agency issued a complete response letter requesting additional information on the drug.
A month before that, an FDA review committee had expressed concerns about missing follow-up data from the ATLAS ACS 2 TIMI 51 trial (JL Mega et al, NEJM, 2012).
So in September, Janssen supplied the FDA with data on the patients who had withdrawn from trial. The company was able to confirm the vital status information for 843 (63%) of the 1338 trial participants who previously had unknown vital status.
Of those 843 patients, 37 had died. The company said the deaths were equally distributed among the treatment groups—rivaroxaban at 2.5 mg, rivaroxaban at 5 mg, and placebo.
“We remain confident in the robustness and results of the ATLAS ACS 2 TIMI 51 trial, evidenced by a significant reduction in cardiovascular events, including a clinically important decrease in cardiovascular death . . . ,” said Christopher Nessel, Vice President at Janssen.
“While we saw an increase in major bleeding, there was no increase in fatal bleeding. We will continue to work with the FDA to address their questions.”
Rivaroxaban is already approved by the FDA to treat and prevent the recurrence of deep vein thrombosis and pulmonary embolism, as thromboprophylaxis in patients who have undergone knee or hip replacement surgery, as well as to reduce the risk of stroke in patients with non-valvular atrial fibrillation. ![]()

Credit: CDC
For a second time, the FDA has decided against approving rivaroxaban (Xarelto) to reduce the risk of cardiovascular events in patients with acute coronary syndrome (ACS).
The agency has issued a complete response letter to the drug’s makers, Janssen Pharmaceuticals.
The contents of the letter are not publicly known, but representatives at Janssen have said they “are evaluating the letter and will respond to the agency’s questions.”
This is not the first time the FDA has raised questions about the use of rivaroxaban in ACS. Last June, the agency issued a complete response letter requesting additional information on the drug.
A month before that, an FDA review committee had expressed concerns about missing follow-up data from the ATLAS ACS 2 TIMI 51 trial (JL Mega et al, NEJM, 2012).
So in September, Janssen supplied the FDA with data on the patients who had withdrawn from trial. The company was able to confirm the vital status information for 843 (63%) of the 1338 trial participants who previously had unknown vital status.
Of those 843 patients, 37 had died. The company said the deaths were equally distributed among the treatment groups—rivaroxaban at 2.5 mg, rivaroxaban at 5 mg, and placebo.
“We remain confident in the robustness and results of the ATLAS ACS 2 TIMI 51 trial, evidenced by a significant reduction in cardiovascular events, including a clinically important decrease in cardiovascular death . . . ,” said Christopher Nessel, Vice President at Janssen.
“While we saw an increase in major bleeding, there was no increase in fatal bleeding. We will continue to work with the FDA to address their questions.”
Rivaroxaban is already approved by the FDA to treat and prevent the recurrence of deep vein thrombosis and pulmonary embolism, as thromboprophylaxis in patients who have undergone knee or hip replacement surgery, as well as to reduce the risk of stroke in patients with non-valvular atrial fibrillation. ![]()
The Heart Team
Although to many, the concept of Heart Teams, as examined in Mitchel L. Zoler’s article, "Heart teams inch into routine cardiac practice," seem novel, such collaborations were the norm at the dawn of cardiac surgery.
Beginning with the surgical approach to valvular and, later, coronary vascular surgery, the interaction between cardiac physiologists (as they were called then), coronary angiographers, and cardiac surgeons in deciding where and when to operate was often difficult and contentious. Cardiac surgery was a high-risk procedure, and the outcomes were uncertain. Over the last 50 years we have come a long way and much of what we do is almost commonplace, as frequently performed as a cholecystectomy or appendectomy and with similar risks. Over time, we have become casual with our decision-making process. Both cardiologists and cardiac surgeons have staked out their own therapeutic parameters. Specialty society guidelines have provided important boundaries within which we can and should operate.
At the same time, we continue to push the envelope to identify therapeutic targets and technologies. We have developed complex interventional and surgical procedures and have applied them to older and sicker patient populations. New technology has opened avenues of therapy that we could not have imagined at the inception of interventional cardiology and cardiac surgery.
The advanced interventional surgical approach now requires even greater interaction with more special players in both cardiology and surgery. Although the modern cardiology practice is built on everyday procedures that provide the platform on which we treat a variety of cardiac issues that commonly do not require ongoing group interactions, the new treatment options require a more interactive and collegial environment. It is in this domain that the Heart Team has an important role and has found success. It was re-initiated as a result of the development of the transcatheter aortic valve implantation, which requires close cardiology and surgical interaction. It has expanded as a team approach to the treatment choices in the care of patients with structural heart disease.
Definitions of the boundaries of the new therapies raise important economic and professional challenges. The Heart Team as currently organized provides the framework of that discourse. To some, it will represent an inconvenience and an obstruction to their individual professional performance: The requirement to participate in a structured interaction is just one more barrier to the daily performance of their skills. To others, it will provide an important process that will improve performance: It is an opportunity to coordinate the different skills required for the advance treatments and, more importantly, it represents a forum to educate not only the current participants but also the physician, nurses, and technicians for the future. The discussion and planning for the surgical approach for a particular patient provides a dynamic discussion of the therapeutic options and the important decisions about appropriateness of the procedure. This interactive learning process is critical to the interdisciplinary training of all present and future players.
The growth of cardiovascular therapy has led to the construction of large stand-alone units or sections within hospitals identified as heart centers or institutes. The creation of these facilities provides the professional structure and financial environment to create the Heart Team and answer some of the issues raised in the article in this issue. Initially devised as a combination of marketing and professional associations, they now can provide the educational and scientific structure of the Heart Team.
Dr. Goldstein, medical editor of Cardiology News, is professor of medicine at Wayne State University and division head emeritus of cardiovascular medicine at Henry Ford Hospital, both in Detroit. He is on data safety monitoring committees for the National Institutes of Health and several pharmaceutical companies.
Although to many, the concept of Heart Teams, as examined in Mitchel L. Zoler’s article, "Heart teams inch into routine cardiac practice," seem novel, such collaborations were the norm at the dawn of cardiac surgery.
Beginning with the surgical approach to valvular and, later, coronary vascular surgery, the interaction between cardiac physiologists (as they were called then), coronary angiographers, and cardiac surgeons in deciding where and when to operate was often difficult and contentious. Cardiac surgery was a high-risk procedure, and the outcomes were uncertain. Over the last 50 years we have come a long way and much of what we do is almost commonplace, as frequently performed as a cholecystectomy or appendectomy and with similar risks. Over time, we have become casual with our decision-making process. Both cardiologists and cardiac surgeons have staked out their own therapeutic parameters. Specialty society guidelines have provided important boundaries within which we can and should operate.
At the same time, we continue to push the envelope to identify therapeutic targets and technologies. We have developed complex interventional and surgical procedures and have applied them to older and sicker patient populations. New technology has opened avenues of therapy that we could not have imagined at the inception of interventional cardiology and cardiac surgery.
The advanced interventional surgical approach now requires even greater interaction with more special players in both cardiology and surgery. Although the modern cardiology practice is built on everyday procedures that provide the platform on which we treat a variety of cardiac issues that commonly do not require ongoing group interactions, the new treatment options require a more interactive and collegial environment. It is in this domain that the Heart Team has an important role and has found success. It was re-initiated as a result of the development of the transcatheter aortic valve implantation, which requires close cardiology and surgical interaction. It has expanded as a team approach to the treatment choices in the care of patients with structural heart disease.
Definitions of the boundaries of the new therapies raise important economic and professional challenges. The Heart Team as currently organized provides the framework of that discourse. To some, it will represent an inconvenience and an obstruction to their individual professional performance: The requirement to participate in a structured interaction is just one more barrier to the daily performance of their skills. To others, it will provide an important process that will improve performance: It is an opportunity to coordinate the different skills required for the advance treatments and, more importantly, it represents a forum to educate not only the current participants but also the physician, nurses, and technicians for the future. The discussion and planning for the surgical approach for a particular patient provides a dynamic discussion of the therapeutic options and the important decisions about appropriateness of the procedure. This interactive learning process is critical to the interdisciplinary training of all present and future players.
The growth of cardiovascular therapy has led to the construction of large stand-alone units or sections within hospitals identified as heart centers or institutes. The creation of these facilities provides the professional structure and financial environment to create the Heart Team and answer some of the issues raised in the article in this issue. Initially devised as a combination of marketing and professional associations, they now can provide the educational and scientific structure of the Heart Team.
Dr. Goldstein, medical editor of Cardiology News, is professor of medicine at Wayne State University and division head emeritus of cardiovascular medicine at Henry Ford Hospital, both in Detroit. He is on data safety monitoring committees for the National Institutes of Health and several pharmaceutical companies.
Although to many, the concept of Heart Teams, as examined in Mitchel L. Zoler’s article, "Heart teams inch into routine cardiac practice," seem novel, such collaborations were the norm at the dawn of cardiac surgery.
Beginning with the surgical approach to valvular and, later, coronary vascular surgery, the interaction between cardiac physiologists (as they were called then), coronary angiographers, and cardiac surgeons in deciding where and when to operate was often difficult and contentious. Cardiac surgery was a high-risk procedure, and the outcomes were uncertain. Over the last 50 years we have come a long way and much of what we do is almost commonplace, as frequently performed as a cholecystectomy or appendectomy and with similar risks. Over time, we have become casual with our decision-making process. Both cardiologists and cardiac surgeons have staked out their own therapeutic parameters. Specialty society guidelines have provided important boundaries within which we can and should operate.
At the same time, we continue to push the envelope to identify therapeutic targets and technologies. We have developed complex interventional and surgical procedures and have applied them to older and sicker patient populations. New technology has opened avenues of therapy that we could not have imagined at the inception of interventional cardiology and cardiac surgery.
The advanced interventional surgical approach now requires even greater interaction with more special players in both cardiology and surgery. Although the modern cardiology practice is built on everyday procedures that provide the platform on which we treat a variety of cardiac issues that commonly do not require ongoing group interactions, the new treatment options require a more interactive and collegial environment. It is in this domain that the Heart Team has an important role and has found success. It was re-initiated as a result of the development of the transcatheter aortic valve implantation, which requires close cardiology and surgical interaction. It has expanded as a team approach to the treatment choices in the care of patients with structural heart disease.
Definitions of the boundaries of the new therapies raise important economic and professional challenges. The Heart Team as currently organized provides the framework of that discourse. To some, it will represent an inconvenience and an obstruction to their individual professional performance: The requirement to participate in a structured interaction is just one more barrier to the daily performance of their skills. To others, it will provide an important process that will improve performance: It is an opportunity to coordinate the different skills required for the advance treatments and, more importantly, it represents a forum to educate not only the current participants but also the physician, nurses, and technicians for the future. The discussion and planning for the surgical approach for a particular patient provides a dynamic discussion of the therapeutic options and the important decisions about appropriateness of the procedure. This interactive learning process is critical to the interdisciplinary training of all present and future players.
The growth of cardiovascular therapy has led to the construction of large stand-alone units or sections within hospitals identified as heart centers or institutes. The creation of these facilities provides the professional structure and financial environment to create the Heart Team and answer some of the issues raised in the article in this issue. Initially devised as a combination of marketing and professional associations, they now can provide the educational and scientific structure of the Heart Team.
Dr. Goldstein, medical editor of Cardiology News, is professor of medicine at Wayne State University and division head emeritus of cardiovascular medicine at Henry Ford Hospital, both in Detroit. He is on data safety monitoring committees for the National Institutes of Health and several pharmaceutical companies.
Improving Family Engagement During FCR
A growing body of literature suggests that patient‐ and family‐centered care can improve patient outcomes[1, 2, 3, 4, 5] as well as patient, family, and provider satisfaction.[6, 7, 8, 9, 10] Engaging patients and families as a way to improve the quality and safety of care has been widely endorsed by leading healthcare organizations,[11, 12, 13] including the Institute of Medicine.[14] In the pediatric inpatient setting, family‐centered rounds (FCR), defined as bedside rounds in which the patient and family share in the control of the management plan as well as in the evaluation of the process itself,[15] potentially provide a consistent venue for family engagement and are recommended by the American Academy of Pediatrics as standard practice.[13]
According to a recent study by Mittal et al.,[16] FCR are the most common type of rounds practiced among pediatric hospitalists surveyed in the United States and Canada. Despite this widespread shift from rounding in a conference room to the bedside with patients and families, there exist only a few studies that provide specific recommendations on conducting FCR.[15, 17, 18] This research has been limited, primarily focusing how rounds are conducted, and further investigation is needed to identify the impact of other processes and elements within the hospital work system that may also affect family engagement during rounds.
The objectives of this study were to: 1) identify strategies to enhance family engagement during FCR drawing from the viewpoints of the various stakeholders on rounds, and 2) characterize these strategies into known elements of hospital work systems and rounding processes using a recognized human factors engineering approach, The Systems Engineering Initiative for Patient Safety (SEIPS) model.[19] According to the SEIPS model, barriers and facilitators to family engagement during FCR are likely embedded in the design of the hospital work systems and rounding process; therefore, we hypothesized that strategies that influence engagement will target all work system and process elements. This work is part of a larger study in which, after prioritization of this group of strategies based on feasibility and sustainability, a bundle of best practices for conducting FCR will be developed, implemented, and evaluated.
METHODS
Study Design
Semistructured interviews using the stimulated recall approach[20, 21] were conducted to understand the cognitive processes of families and healthcare team (HCT) members during FCR. This qualitative study design allowed us to capture comprehensive information from the perspectives of a diverse group of stakeholders on strategies for improving family engagement during FCR.
Setting and Participants
This study was conducted at a children's hospital in Wisconsin, where FCR were initiated in 2007 with the transition to a new hospital facility. The expectation is that FCR are conducted daily with the family and the patient's HCT, consisting of at least an attending physician and nurse. Typically, multiple residents, interns, and medical students are present along with a combination of other providers, including consulting subspecialists, a fellow, nurse practitioner, respiratory therapist, or pharmacist. When this study was conducted, attendees received little to no formal training regarding their role on FCR. As part of a larger study, English‐speaking patients and/or families admitted to 1 of 4 inpatient services (2 hospitalist, 1 pulmonary, and 1 hematology/oncology), and their associated HCT members were enrolled and their bedside rounds were video recorded. A purposive sampling technique[22, 23] was employed, recruiting interviewees that represented the various groups of stakeholders of rounds, including parents, children, attending physicians, resident physicians, medical students, and nurses. For child interviews, we restricted selection to children aged 8 to 17 years to ensure the ability to understand the interviewing process and provide feedback. Families were consented and children were assented. The University of Wisconsin‐Madison Health Sciences Institutional Review Board approved this study.
Interviews and analysis occurred concurrently in an iterative process, informing each other. Thus, recruitment continued until we reached theoretical saturation,[24, 25] the point at which additional interviews did not provide new information or further conceptual development.
Study Procedures
All interviews were conducted by trained researchers, who used the same semistructured interview guide. During each interview, the interviewee was instructed to watch his/her own rounding video and pause when noticing something that made it easy (facilitator) or hard (barrier) to engage the family. Every time the interviewee paused the video to describe what was noticed, the interviewer then asked follow‐up, open‐ended questions to solicit specific information that focused on strategies for enhancing family engagement during FCR. For instance, if the issue identified was a barrier, the interviewer asked, What would you have wanted to happen differently? and if the issue identified was a facilitator, the interviewer asked, How could we ensure that would happen for everyone? The interviewee rewound the video as needed. If the interviewee had not stopped the video by the halfway point, the interviewer would pause the video and review the instructions. After the interviewee had viewed and commented on the entire rounding video, an opportunity was offered to reflect on other factors that influence family engagement during rounds, and additional questions were asked as necessary to fully understand the interviewee's views. All interviews were audio recorded and personal identifiers were removed prior to data analysis.
Data Analysis
Two research assistants reviewed the audio recordings and identified all instances related to strategies for improving family engagement during FCR. There was no screening of strategies (ie, if an interviewee suggested a strategy was related to improving family engagement, it was categorized as such). To ensure intercoder reliability, these assistants, under the supervision of a researcher (L.D.), reviewed the coding process together, held consensus meetings, and crosschecked interviews for coding consensus. A researcher (A.X.) transcribed all strategy‐related instances, which were then reviewed by two additional researchers (M.K., P.C.). To organize, sort, and code the data, interview transcripts were imported in the NVivo qualitative data analysis software (QSR International, Doncaster, Victoria, Australia). The research group then performed a qualitative content analysis of the transcripts[26] and categorized the strategies in an iterative process (information provided on request).
To ensure that all strategies remained conceptually similar within categories, the constant comparative method[27, 28] was applied to the coding process. This involved comparing: 1) strategy‐related instances from the same participants, 2) strategy‐related instances from different participants in the same groups, 3) strategy‐related instances from different participants in different groups, 4) a coded strategy with other coded strategies, 5) coded strategies with categories, and 6) a category with other categories. A strategy‐related instance could be coded under more than one strategy or category. For instance, one interviewee said conducting things that can be done without family beforehand, and presenting and reviewing the plan with family. This was coded under both the strategy conducting rounds in another location without family and then at the bedside with family in the location of FCR category and the strategy focusing presentation on assessment and plan in the communication style category.
RESULTS
A total of 37 interviews were conducted with 11 parents, 4 children, and 22 HCT members (8 attending physicians, 6 resident physicians, 5 medical students, and 3 nurses) in 24 videos of rounding sessions. The duration of the interviews ranged from 30 to 60 minutes.
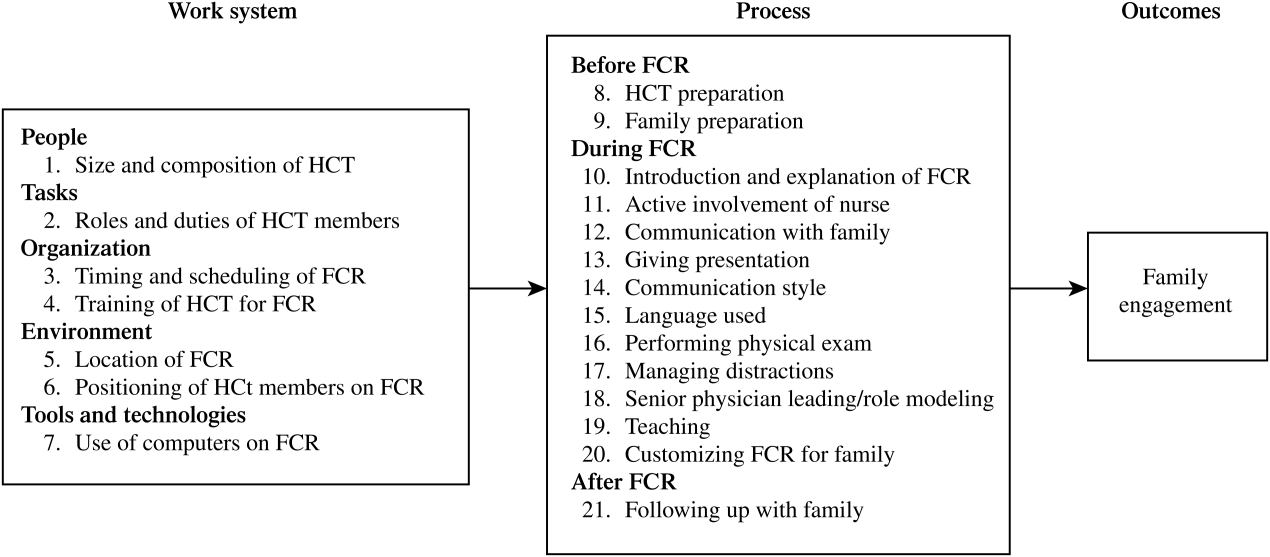
A total of 338 separate instances related to strategies for improving family engagement on FCR were identified and sorted into 21 categories. Using the SEIPS model, these categories were organized into 2 themes: the work system and process of FCR (Figure 1). Of the 21 categories, 12 were mentioned by both families (parents and/or children) and HCT members and 9 were solely mentioned by the HCT.
Work System of FCR
Table 1 shows the categories of strategies related to the 5 elements of the FCR work system.[29, 30] Illustrative quotes from the interviews (Q) are presented in Table 2.
| Work System Elements | Categories | Strategies | P (11) | C (4) | Att. (8) | Res. (6) | MS (5) | RN (3) |
|---|---|---|---|---|---|---|---|---|
| ||||||||
| People | 1. Size and composition of HCT | Have a smaller HCT conduct FCR | X | X | X | X | ||
| Ensure all relevant disciplines present on FCR | X | X | X | X | X | |||
| Task | 2. Roles and duties of HCT members | Define roles/duties of HCT members on FCR | X | X | ||||
| Organization | 3. Timing and scheduling of FCR | Schedule FCR, inform participants beforehand | X | X | X | |||
| 4. Training of HCT for FCR | Train HCT on how to present on FCR | X | X | |||||
| Environment | 5. Location of FCR | At bedside with family and patient | X | X | ||||
| In another location with HCT, then at bedside | X | X | X | X | ||||
| In another location with family but without child | X | |||||||
| 6. Positioning of HCT members on FCR | Sit down with family | X | X | X | X | X | ||
| Stand close to or in a semicircle around family | X | X | X | X | X | |||
| Tools and technologies | 7. Use of computers on FCR | Use computer to support family interaction | X | X | X | X | ||
| Don't use a computer | X | X | ||||||
|
| Q1: I'm intimidated to ask a question. It seems like there are too many peopleI like a smaller group. (P5) |
| Q2: Sometimes rounds are the only time that the parents are there to see the entire teamso in that way, including [the entire team] at the rounds makes more sense. (MS1) |
| Q3: There needs to be much more clear roles about who is supposed to do what, and it should be predictable. (Att.2) |
| Q4: ([T]iming of rounds) is a huge source of frustration for families. If [physicians] know in which order they will go for patients, they can call our charge nurse or unit clerk or page nurses with that information. (RN1) |
| Q5: ([W]ith a notice of the rounding schedule), I can be ahead of time, trying to think of questions. (P10) |
| Q6: [I]t would be really nice to see somebody do a presentation in a medical eye's version and then also in the family‐centered version. (MS5) |
| Q7: [H]aving the medical students practice with the senior residentis a good way of doing it. (Res.4) |
| Q8: [M]aybe some small groups where you practice this among students. (MS5) |
| Q9: It would be better to be in the room for communication. (Att.1) |
| Q10: You could have sort of hallway rounds, which is much more medical oriented, and inside‐the‐room rounds, which is much more talking with the parent. (P1) |
| Q11: [H]ave sit‐down rounds with parents and families. (Res.5) |
| Q12: I've seen some attending physicians who sat down. I think that could be helpful to be on the same level as the patient and family. (Res.2) |
| Q13: [M]aybe formation of semicircle or something like that, where we can see everybody a little more clearly, I think that would be very helpful. (P10) |
| Q14: I find the presence of a computer incredibly offensive and obstructivewhen you are supposed to be able to interact with the patient. (Att.6) |
| Q15: One of the things I started doing is having one of the other resident physicians have the computer, so just relying on them to do the orders, and me just being there mainly for being the presenter of rounds. (Res.4) |
People
Two seemingly contradictory strategies were proposed. Some interviewees suggested a smaller HCT with members most familiar to the family (Q1), whereas other interviewees stressed the need to involve different relevant disciplines (eg, social worker, nutritionist) during rounds (Q2).
Tasks
Both attending and resident physicians emphasized the importance of defining the role of each HCT member before rounding (Q3). Interviewees also suggested these roles should be explained to families, ideally at admission.
Organization
Many interviewees suggested the need to consistently schedule rounds (Q4) and to inform families and nurses of the schedule so all parties could plan ahead (Q5). Some resident physicians and medical students recommended training of learners on how to give a family‐centered presentation using methods such as role modeling (Q6) and practicing with the senior resident physician (Q7) or in small groups (Q8).
Environment
Some interviewees suggested conducting rounds in patient rooms (Q9). Others suggested conducting rounds first in another location (eg, hallway) without the family and then going to the bedside to round with the family (Q10). There were also interviewees who suggested conducting rounds in another location (eg, conference room) with the family (Q11). When conducting rounds in the patient room, some interviewees suggested that some HCT members (eg, attending and senior resident physicians) could sit down with the family (Q12), with the rest of the HCT standing close to the family in a semicircle (Q13).
Tools and Technologies
Some interviewees thought that conversation with families could be negatively affected by the use of computers, and therefore suggested not using them on FCR (Q14). Alternatively, other interviewees considered computers a tool to facilitate the interaction between the HCT and families, such as showing x‐rays or lab values. Several interviewees suggested that computers should not be positioned to block eye contact between HCT members and families; therefore, only HCT members not presenting should use computers (Q15).
Process of FCR
Table 3 shows the categories of strategies related to the process of FCR, which were categorized into 3 phases. Illustrative quotes are presented in Table 4.
| Process Phases | Categories | Strategies | P (11) | C (4) | Att. (8) | Res. (6) | MS (5) | RN (3) |
|---|---|---|---|---|---|---|---|---|
| ||||||||
| Before FCR | 8. HCT preparation | Collect and prepare pertinent information | X | X | X | X | ||
| 9. Family preparation | Orient family to rounding process | X | X | X | X | X | ||
| Build relationship with family | X | X | X | |||||
| Ask family for permission and preferences | X | X | X | |||||
| During FCR | 10. Introduction and explanation of FCR | Introduce HCT and family to each other | X | X | X | X | X | |
| Explain interactive rounding process | X | X | X | |||||
| 11. Active involvement of nurse | Giving nurse opportunity to actively participate | X | X | |||||
| 12. Communication with family | Give family opportunity to actively participate | X | X | X | X | X | X | |
| Address family's questions/concerns | X | X | X | X | X | |||
| Explain tests, findings and results to family | X | X | X | X | X | |||
| Confirm family understanding | X | X | X | |||||
| 13. Giving presentation | Restructure the presentation | X | X | |||||
| Shorten the presentation | X | X | X | |||||
| Focus presentation on assessment and plan | X | X | X | X | X | |||
| Summarize plan for family | X | X | X | |||||
| Avoid discussion of sensitive topics | X | X | X | |||||
| 14. Communication style | Present in a conversational manner | X | X | X | ||||
| Use an engaging communication style | X | X | X | X | X | |||
| 15. Language used | Use qualitative language | X | X | X | ||||
| Use plain language | X | X | X | X | X | |||
| 16. Performing physical exam | Pause and confirm physical exam | X | ||||||
| 17. Managing distractions | Minimize distractions and interruptions | X | X | X | X | X | X | |
| 18. Senior physician leading/role modeling | Attending/senior resident physician should lead, direct and be a role model on FCR rounds | X | X | |||||
| 19. Teaching | Ask family permission and involve in teaching | X | X | |||||
| 20. Customizing FCR for family | Adapt rounds to family's needs | X | X | X | X | |||
| After FCR | 21. Following up with family | HCT members follow up with family | X | X | X | |||
|
| Q1: [Medical students and residents] actually had a chance to do a quick round, an abbreviated presentation to put together an outline of what we're going to talk about before we even do it. (MS2) |
| Q2: I would like to know exactly what's going onbefore I walk in. (Att.6) |
| Q3: [T]he nurse did give me the fore‐warning that rounds would be coming and it was usually like a group of 8 to 10so I was prepared for that. (P7) |
| Q4: What went well is that I had already connected with this mom and the daughter prior to this rounding encounter. (Res.3) |
| Q5: [Y]ou could ask the patient or the parents if they want the child there. (P3) |
| Q6: [Families] really want to know what your role is on the team. (Att.5) |
| Q7: I guess it would be easier to figure out who you need to direct questions to. (P3) |
| Q8: [L]etting the family anticipate what rounding is going to be like and when the opportunity is going to come up to talk. I think that can help. (Res.2) |
| Q9: I think the decision making is probably the most critical partthere is really no substitute for [families] being involved in the decisionwithout a lot of medical conversation and analysis. (P1) |
| Q10: The family was proactive enough to ask questions, but they were never really given entrance to ask questions.No one had said do you have any questions?' (Res.4) |
| Q11: It is really important for the doctors to listen to them, to know that they are the parents and they know their children best. (RN3) |
| Q12: I think sometimes when you are teaching, some of the information could potentially be scary to the family. What I would hope is letting the family feel like they are part of the education process. (Res.2) |
| Q13: Sometimes nurses are asked initially, do you have anything to add,' which I think is a good way to startbecause we have probably the most current and updated information. (RN1) |
| Q14: When I was talking about the physical exam partmaybe at that point, if I could just stop talking, we couldconfirm that exam. (Res.3) |
| Q15: It's distracting if different groups have individual discussions when you are trying to keep the group focused on this particular patient for rounds. (Att.7) |
| Q16: It's just the basic thing that I try to tell residents. I do this hopefully at least once every time I am on services with the team. (Att.1) |
| Q17: ([C]hanging rounds depending on the families) would be the ideal situationthinking about what's helpful for a family. (Att.6) |
| Q18: What I usually see when things work well after we leave is that the nurse can still stick around the family. (Res.4) |
| Q19: [T]he students have to go back in the afternoon to talk with the family about what the treatment point is and answer any questions. (MS2) |
Before FCR
To engage families during FCR, many interviewees suggested that both the HCT and families need preparation. HCT members suggested that medical students should collect up‐to‐date patient information and review it with the senior resident physicians (Q1) to reach a consensus before starting FCR (Q2). To prepare families for rounds, parents and HCT members suggested that the HCT should orient families to the rounding process (Q3), build relationships with families (Q4), and ask for their permission and preference regarding participation in rounds (Q5).
During FCR
A number of strategies focused on the beginning of rounds. Parents, children, and HCT members stressed the need to introduce HCT members by role (Q6) and inform families to whom to direct questions (Q7). It was also suggested that parents introduce themselves to the team. Some interviewees recommended that the HCT explain the rounding process to families at this time (Q8).
Interviewees recommended strategies related to communication between the HCT and families during rounds. Many interviewees suggested restructuring and shortening the presentation by focusing on the assessment and plan (Q9). According to all interviewees, the HCT should present in a conversational manner and use an engaging communication style (eg, smiling, making eye contact, using appropriate humor) and appropriate language (eg, qualitative trend instead of numbers, plain language instead of medical jargon) to communicate with families. To ensure families understanding, HCT members should encourage and address their questions and concerns (Q10). In addition, families should be given the opportunity to provide information (eg, patient history and overnight events) and to express their opinions about the plan (Q11). If teaching is done during rounds, the HCT should involve families and ask for permission (Q12).
Other strategies on rounds were suggested, such as giving nurses the opportunity to actively participate (Q13), pausing and confirming physical exam findings (Q14), minimizing distractions and interruptions (Q15), attending and/or senior resident physicians leading and being role models for FCR (Q16), and adapting rounds to families' needs (Q17).
After FCR
Some HCT members talked about the importance of following up with families after rounds. Specifically, suggestions that nurses could stay with families immediately after rounds (Q18) were made, whereas physicians could return to families later in the day (Q19).
DISCUSSION
Using recognized qualitative systems engineering methods, we identified a broad range of strategies for enhancing family engagement on FCR from the perspectives of a diverse group of stakeholders on rounds and described how these strategies target known fundamental elements in both the hospital work system and rounding process. We highlight recommendations on the content and style of communication during rounds with families, but also introduce more complex system‐wide elements that likely play a role in family engagement, such as the composition of the HCT; organization and environment of rounds; tools and technologies used; and preparation of the HCT, families, and patients beforehand.
Our research both confirms and builds upon practices previously described in the FCR literature.[17, 31, 32] In a case report by Muething et al.,[17] recommendations were developed using a series of plan‐do‐study‐act cycles to determine the components needed to conduct FCR. These components included: 1) determining family preference prior to rounds, 2) defining HCT roles, 3) introducing HCT to family and explaining the purpose of rounds, 4) describing what is shared and how it is said on rounds, 5) describing the contribution of families, nurses, and ancillary staff, and 6) providing teaching recommendations to senior physicians on rounds. All of these components are suggested by one or more of the participants in our study. In addition, our research identifies a variety of new work system‐related strategies, such as scheduling rounds, using computers appropriately on rounds, and providing training of HCT members beforehand.
Of particular interest was the discordance between strategies mentioned by families and the various members of the HCT. Although HCT members mentioned all identified strategies, families were interested in certain ones. Regarding the structure of FCR, families showed particular interest in HCT composition, timing and scheduling of rounds, location of rounds, and positioning of the HCT. In comparison, families did not mention the importance of the roles and duties of HCT members, HCT preparation for rounds, and use of computers during rounds. With respect to the FCR process, families stressed the importance of family preparation beforehand, introduction and explanation of rounds at the beginning, presentation style and communication style, customization, and management of distractions during rounds. None of the families, however, mentioned the rest of the strategies, including HCT preparation before rounds, involvement of the nurse, teaching and performing the physical exam, the role of the attending and senior resident roles during rounds, and following up with the family after rounds. These different perspectives are likely, in part, inherent to the different roles and experiences of parents and HCT members. For example, parents' knowledge of what goes on in the hospital outside of FCR, such as orientation and preparation of HCT members for rounds, is relatively limited. Future research using methods to evaluate and prioritize strategies as well as understanding reasons for contradicting strategies is warranted.
We recognize that, although family engagement is recommended as a critical component of care, strategies to improve engagement may be in direct opposition to other goals of the HCT. For example, some of our participants suggest having a smaller team may be more beneficial for family engagement on rounds. In some settings, it may be feasible to have a small team; however, in institutions that accommodate a large number of learners, excluding students from the teaching opportunity of rounds may actually compromise educational experiences. In patients with chronic and/or complex care, a larger multidisciplinary team may better facilitate information exchange among disciplines and expedite discharge planning. Moreover, one might speculate that it may not be that the size affects family engagement as much as the composition of the team, especially if tailored to the needs of the patient. For example, a large team consisting of primarily physicians and trainees may not be as engaging as the same sized team with one attending physician and a respiratory therapist, case manager, and consulting subspecialist. Finding a balance between engaging families, teaching learners, and maintaining efficiency is paramount and needs to be studied further.
This study has several limitations. Our data are from a single academic children's hospital, which may limit generalizability due to a small sampling of multiple stakeholders on different services, our specific patient population, HCT composition and roles, and teaching needs. However, we face similar barriers to engaging families during rounds as those published from both another single institution[17] and a national sampling of pediatric hospitalists.[16] Furthermore, our recommended strategies to address the FCR process are supported by prior work.[17] Because this study was voluntary, our interviewees were likely more engaged participants in general. Specifically, the viewpoints of engaged families and HCT members may not represent the viewpoints of those who are less engaged or supportive of FCR. We did not enroll nonEnglish‐speaking patients and families, which is a potential direction for future research. In our interviews, we also relied solely on the perceptions of rounding participants, rather than those of outside observers or researchers, which may only provide a partial perspective of potential strategies to improve family engagement. Last, this qualitative research approach does not provide quantitative information regarding whether certain strategies are preferred by a majority of participants, which we hope to address in future research.
This work is part of a larger study that aims to implement a bundle of these strategies after stakeholder prioritization based on impact on family engagement, feasibility, and sustainability. We plan to systematically evaluate the implementation process of these strategies and measure their impact on family engagement and, ultimately, patient safety. One or more of these strategies could be implemented in a similar manner at other hospitals depending on specific institutional needs.
In conclusion, as recently reflected by Barry et al. in The New England Journal of Medicine, Although talk about patient‐centered care is ubiquitous in modern health care, one of the greatest challenges of turning the rhetoric into reality continues to be routinely engaging patients in decision making.[33] FCR provide a crucial opportunity for family involvement in daily care decisions in the pediatric inpatient setting. This study highlights the importance of prior work defining the components of involving families in this process, while emphasizing new systems‐based strategies that further facilitate the expectation of family engagement in the care of hospitalized children.
Disclosures
This work was funded through an Agency for Healthcare Research and Quality Health Services Research Dissemination and Demonstration Grant, R18 HS018680, and also supported by the Arthur Vining Davis Foundation and the National Patient Safety Foundation through the James S. Todd Memorial Research Award. Funding organizations did not contribute to the design and conduct of study; collection, management, analysis, and interpretation of data; or preparation, review, or approval of the manuscript. The authors report no conflicts of interest.
- , , , et al. The impact of patient‐centered care on outcomes. J Fam Pract. 2000;49:796–804.
- , , , et al. Observational study of effect of patient centredness and positive approach on outcomes of general practice consultations. BMJ. 2001;323:908–911.
- , , . Improvement in the family‐centered medical home enhances outcomes for children and youth with special healthcare needs. J Ambul Care Manage. 2009;32:188–196.
- , , . Associations of family‐centered care with health care outcomes for children with special health care needs. Matern Child Health J. 2011;15:794–805.
- , , , , Can a patient‐centered medical home lead to better patient outcomes? The quality implications of Geisinger's ProvenHealth Navigator. Am J Med Qual. 2012;27:210–216.
- , , . Perceptions of health care providers' communication: relationships between patient‐centered communication and satisfaction. Health Commun. 2004;16:363–383.
- , . Satisfaction with care and ease of using health care services among parents of children with special health care needs: the roles of race/ethnicity, insurance, language, and adequacy of family‐centered care. Pediatrics. 2006;117:1184–1196.
- , , , . A randomized, controlled trial of bedside versus conference‐room case presentation in a pediatric intensive care unit. Pediatrics. 2007;120:275–280.
- , , , , . Effect of patient‐centered care on patient satisfaction and quality of care. J Nurs Care Qual. 2008;23:316–321.
- , , . Implementing family‐centered rounds: pediatric residents' perceptions. Clin Pediatr (Phila) 2010;49:228–234.
- ACGME Program Requirements for Graduate Medical Education in Pediatrics. 2007. Available at: http://www.acgme.org/acWebsite/downloads/RRC_progReq320_pediatrics_07012007.pdf. Accessed February 10, 2012.
- Speak up: prevent errors in your child's care. 2011. The Joint Commission Web site. Available at: http://www.jointcommission.org/Speak_Up_Prevent_Errors_in_Your_Childs_Care/. Accessed May 11, 2012.
- Patient‐ and family‐centered care and the pediatrician's role. Pediatrics. 2012;129:394–404.
- Committee on Quality of Health Care in America, Institute of Medicine. Crossing the Quality Chasm: A New Health System for the 21st Century. Washington, DC: The National Academies Press; 2001.
- , , , . Defining family‐centered rounds. Teach Learn Med. 2007;19:319–322.
- , , , et al. Family‐centered rounds on pediatric wards: A PRIS network survey of US and Canadian hospitalists. Pediatrics. 2010;126:37–43.
- , , , , . Family‐centered bedside rounds: a new approach to patient care and teaching. Pediatrics. 2007;119:829–832.
- , , , . Parental responses to involvement in rounds on a pediatric inpatient unit at a teaching hospital: a qualitative study. Acad Med. 2008;83:292–297.
- , , , et al. Work system design for patient safety: the SEIPS model. Qual Saf Health Care. 2006;15:i50–i58.
- , . IPR—a validated model for the 1990s and beyond. Couns Psychol. 1990;18:436–440.
- , . Auto‐ and allo‐confrontation as tools for reflective activities. Appl Ergon. 2004;35:531–540.
- . Qualitative Research and Evaluation Methods. 3rd ed. Thousand Oaks, CA: Sage Publications; 2002.
- , . Doing Qualitative Research. 2nd ed. Thousand Oaks, CA: Sage Publications; 1999.
- . Sample size in qualitative research. Res Nurs Health. 1995;18:179–183.
- , . Basics of Qualitative Research: Techniques and Procedures for Developing Grounded Theory. Newbury Park, CA: Sage Publications; 1998.
- , . Qualitative content analysis in nursing research: concepts, procedures and measures to achieve trustworthiness. Nurse Educ Today. 2004;24:105–112.
- . A purposeful approach to the constant comparative method in the analysis of qualitative interviews. Qual Quant. 2002;36:391–409.
- . Theoretical Sensitivity: Advances in the Methodology of Grounded Theory. Mill Valley, CA: Sociology Press; 1978.
- , . Balance theory of job design. In: Karwowski W, ed. International Encyclopedia of Ergonomics and Human Factors. London: Taylor 2000:1181–1184.
- , . A balance theory of job design for stress reduction. Int J Ind Ergon. 1989;4:67–79.
- Institute for Patient‐ and Family‐Centered Care. Applying patient‐ and family‐centered concepts to bedside rounds. 2010. Available at: http://www.ipfcc.org/advance/topics/PH_RD_Applying_PFCC_Rounds_012009.pdf. Accessed May 11, 2012.
- . A fundamental shift: family‐centered rounds in an academic medical center. Hospitalist. 2006;10:45–46.
- , Shared decision making—pinnacle of patient‐centered care. N Engl J Med. 2012;366:780–781.
A growing body of literature suggests that patient‐ and family‐centered care can improve patient outcomes[1, 2, 3, 4, 5] as well as patient, family, and provider satisfaction.[6, 7, 8, 9, 10] Engaging patients and families as a way to improve the quality and safety of care has been widely endorsed by leading healthcare organizations,[11, 12, 13] including the Institute of Medicine.[14] In the pediatric inpatient setting, family‐centered rounds (FCR), defined as bedside rounds in which the patient and family share in the control of the management plan as well as in the evaluation of the process itself,[15] potentially provide a consistent venue for family engagement and are recommended by the American Academy of Pediatrics as standard practice.[13]
According to a recent study by Mittal et al.,[16] FCR are the most common type of rounds practiced among pediatric hospitalists surveyed in the United States and Canada. Despite this widespread shift from rounding in a conference room to the bedside with patients and families, there exist only a few studies that provide specific recommendations on conducting FCR.[15, 17, 18] This research has been limited, primarily focusing how rounds are conducted, and further investigation is needed to identify the impact of other processes and elements within the hospital work system that may also affect family engagement during rounds.
The objectives of this study were to: 1) identify strategies to enhance family engagement during FCR drawing from the viewpoints of the various stakeholders on rounds, and 2) characterize these strategies into known elements of hospital work systems and rounding processes using a recognized human factors engineering approach, The Systems Engineering Initiative for Patient Safety (SEIPS) model.[19] According to the SEIPS model, barriers and facilitators to family engagement during FCR are likely embedded in the design of the hospital work systems and rounding process; therefore, we hypothesized that strategies that influence engagement will target all work system and process elements. This work is part of a larger study in which, after prioritization of this group of strategies based on feasibility and sustainability, a bundle of best practices for conducting FCR will be developed, implemented, and evaluated.
METHODS
Study Design
Semistructured interviews using the stimulated recall approach[20, 21] were conducted to understand the cognitive processes of families and healthcare team (HCT) members during FCR. This qualitative study design allowed us to capture comprehensive information from the perspectives of a diverse group of stakeholders on strategies for improving family engagement during FCR.
Setting and Participants
This study was conducted at a children's hospital in Wisconsin, where FCR were initiated in 2007 with the transition to a new hospital facility. The expectation is that FCR are conducted daily with the family and the patient's HCT, consisting of at least an attending physician and nurse. Typically, multiple residents, interns, and medical students are present along with a combination of other providers, including consulting subspecialists, a fellow, nurse practitioner, respiratory therapist, or pharmacist. When this study was conducted, attendees received little to no formal training regarding their role on FCR. As part of a larger study, English‐speaking patients and/or families admitted to 1 of 4 inpatient services (2 hospitalist, 1 pulmonary, and 1 hematology/oncology), and their associated HCT members were enrolled and their bedside rounds were video recorded. A purposive sampling technique[22, 23] was employed, recruiting interviewees that represented the various groups of stakeholders of rounds, including parents, children, attending physicians, resident physicians, medical students, and nurses. For child interviews, we restricted selection to children aged 8 to 17 years to ensure the ability to understand the interviewing process and provide feedback. Families were consented and children were assented. The University of Wisconsin‐Madison Health Sciences Institutional Review Board approved this study.
Interviews and analysis occurred concurrently in an iterative process, informing each other. Thus, recruitment continued until we reached theoretical saturation,[24, 25] the point at which additional interviews did not provide new information or further conceptual development.
Study Procedures
All interviews were conducted by trained researchers, who used the same semistructured interview guide. During each interview, the interviewee was instructed to watch his/her own rounding video and pause when noticing something that made it easy (facilitator) or hard (barrier) to engage the family. Every time the interviewee paused the video to describe what was noticed, the interviewer then asked follow‐up, open‐ended questions to solicit specific information that focused on strategies for enhancing family engagement during FCR. For instance, if the issue identified was a barrier, the interviewer asked, What would you have wanted to happen differently? and if the issue identified was a facilitator, the interviewer asked, How could we ensure that would happen for everyone? The interviewee rewound the video as needed. If the interviewee had not stopped the video by the halfway point, the interviewer would pause the video and review the instructions. After the interviewee had viewed and commented on the entire rounding video, an opportunity was offered to reflect on other factors that influence family engagement during rounds, and additional questions were asked as necessary to fully understand the interviewee's views. All interviews were audio recorded and personal identifiers were removed prior to data analysis.
Data Analysis
Two research assistants reviewed the audio recordings and identified all instances related to strategies for improving family engagement during FCR. There was no screening of strategies (ie, if an interviewee suggested a strategy was related to improving family engagement, it was categorized as such). To ensure intercoder reliability, these assistants, under the supervision of a researcher (L.D.), reviewed the coding process together, held consensus meetings, and crosschecked interviews for coding consensus. A researcher (A.X.) transcribed all strategy‐related instances, which were then reviewed by two additional researchers (M.K., P.C.). To organize, sort, and code the data, interview transcripts were imported in the NVivo qualitative data analysis software (QSR International, Doncaster, Victoria, Australia). The research group then performed a qualitative content analysis of the transcripts[26] and categorized the strategies in an iterative process (information provided on request).
To ensure that all strategies remained conceptually similar within categories, the constant comparative method[27, 28] was applied to the coding process. This involved comparing: 1) strategy‐related instances from the same participants, 2) strategy‐related instances from different participants in the same groups, 3) strategy‐related instances from different participants in different groups, 4) a coded strategy with other coded strategies, 5) coded strategies with categories, and 6) a category with other categories. A strategy‐related instance could be coded under more than one strategy or category. For instance, one interviewee said conducting things that can be done without family beforehand, and presenting and reviewing the plan with family. This was coded under both the strategy conducting rounds in another location without family and then at the bedside with family in the location of FCR category and the strategy focusing presentation on assessment and plan in the communication style category.
RESULTS
A total of 37 interviews were conducted with 11 parents, 4 children, and 22 HCT members (8 attending physicians, 6 resident physicians, 5 medical students, and 3 nurses) in 24 videos of rounding sessions. The duration of the interviews ranged from 30 to 60 minutes.
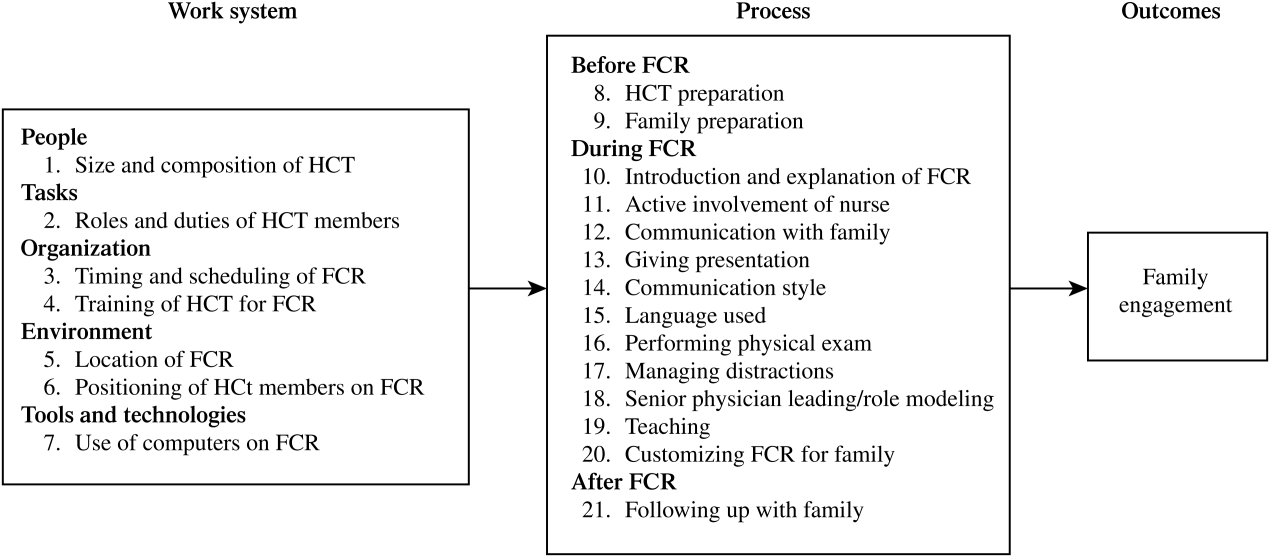
A total of 338 separate instances related to strategies for improving family engagement on FCR were identified and sorted into 21 categories. Using the SEIPS model, these categories were organized into 2 themes: the work system and process of FCR (Figure 1). Of the 21 categories, 12 were mentioned by both families (parents and/or children) and HCT members and 9 were solely mentioned by the HCT.

Work System of FCR
Table 1 shows the categories of strategies related to the 5 elements of the FCR work system.[29, 30] Illustrative quotes from the interviews (Q) are presented in Table 2.
| Work System Elements | Categories | Strategies | P (11) | C (4) | Att. (8) | Res. (6) | MS (5) | RN (3) |
|---|---|---|---|---|---|---|---|---|
| ||||||||
| People | 1. Size and composition of HCT | Have a smaller HCT conduct FCR | X | X | X | X | ||
| Ensure all relevant disciplines present on FCR | X | X | X | X | X | |||
| Task | 2. Roles and duties of HCT members | Define roles/duties of HCT members on FCR | X | X | ||||
| Organization | 3. Timing and scheduling of FCR | Schedule FCR, inform participants beforehand | X | X | X | |||
| 4. Training of HCT for FCR | Train HCT on how to present on FCR | X | X | |||||
| Environment | 5. Location of FCR | At bedside with family and patient | X | X | ||||
| In another location with HCT, then at bedside | X | X | X | X | ||||
| In another location with family but without child | X | |||||||
| 6. Positioning of HCT members on FCR | Sit down with family | X | X | X | X | X | ||
| Stand close to or in a semicircle around family | X | X | X | X | X | |||
| Tools and technologies | 7. Use of computers on FCR | Use computer to support family interaction | X | X | X | X | ||
| Don't use a computer | X | X | ||||||
|
| Q1: I'm intimidated to ask a question. It seems like there are too many peopleI like a smaller group. (P5) |
| Q2: Sometimes rounds are the only time that the parents are there to see the entire teamso in that way, including [the entire team] at the rounds makes more sense. (MS1) |
| Q3: There needs to be much more clear roles about who is supposed to do what, and it should be predictable. (Att.2) |
| Q4: ([T]iming of rounds) is a huge source of frustration for families. If [physicians] know in which order they will go for patients, they can call our charge nurse or unit clerk or page nurses with that information. (RN1) |
| Q5: ([W]ith a notice of the rounding schedule), I can be ahead of time, trying to think of questions. (P10) |
| Q6: [I]t would be really nice to see somebody do a presentation in a medical eye's version and then also in the family‐centered version. (MS5) |
| Q7: [H]aving the medical students practice with the senior residentis a good way of doing it. (Res.4) |
| Q8: [M]aybe some small groups where you practice this among students. (MS5) |
| Q9: It would be better to be in the room for communication. (Att.1) |
| Q10: You could have sort of hallway rounds, which is much more medical oriented, and inside‐the‐room rounds, which is much more talking with the parent. (P1) |
| Q11: [H]ave sit‐down rounds with parents and families. (Res.5) |
| Q12: I've seen some attending physicians who sat down. I think that could be helpful to be on the same level as the patient and family. (Res.2) |
| Q13: [M]aybe formation of semicircle or something like that, where we can see everybody a little more clearly, I think that would be very helpful. (P10) |
| Q14: I find the presence of a computer incredibly offensive and obstructivewhen you are supposed to be able to interact with the patient. (Att.6) |
| Q15: One of the things I started doing is having one of the other resident physicians have the computer, so just relying on them to do the orders, and me just being there mainly for being the presenter of rounds. (Res.4) |
People
Two seemingly contradictory strategies were proposed. Some interviewees suggested a smaller HCT with members most familiar to the family (Q1), whereas other interviewees stressed the need to involve different relevant disciplines (eg, social worker, nutritionist) during rounds (Q2).
Tasks
Both attending and resident physicians emphasized the importance of defining the role of each HCT member before rounding (Q3). Interviewees also suggested these roles should be explained to families, ideally at admission.
Organization
Many interviewees suggested the need to consistently schedule rounds (Q4) and to inform families and nurses of the schedule so all parties could plan ahead (Q5). Some resident physicians and medical students recommended training of learners on how to give a family‐centered presentation using methods such as role modeling (Q6) and practicing with the senior resident physician (Q7) or in small groups (Q8).
Environment
Some interviewees suggested conducting rounds in patient rooms (Q9). Others suggested conducting rounds first in another location (eg, hallway) without the family and then going to the bedside to round with the family (Q10). There were also interviewees who suggested conducting rounds in another location (eg, conference room) with the family (Q11). When conducting rounds in the patient room, some interviewees suggested that some HCT members (eg, attending and senior resident physicians) could sit down with the family (Q12), with the rest of the HCT standing close to the family in a semicircle (Q13).
Tools and Technologies
Some interviewees thought that conversation with families could be negatively affected by the use of computers, and therefore suggested not using them on FCR (Q14). Alternatively, other interviewees considered computers a tool to facilitate the interaction between the HCT and families, such as showing x‐rays or lab values. Several interviewees suggested that computers should not be positioned to block eye contact between HCT members and families; therefore, only HCT members not presenting should use computers (Q15).
Process of FCR
Table 3 shows the categories of strategies related to the process of FCR, which were categorized into 3 phases. Illustrative quotes are presented in Table 4.
| Process Phases | Categories | Strategies | P (11) | C (4) | Att. (8) | Res. (6) | MS (5) | RN (3) |
|---|---|---|---|---|---|---|---|---|
| ||||||||
| Before FCR | 8. HCT preparation | Collect and prepare pertinent information | X | X | X | X | ||
| 9. Family preparation | Orient family to rounding process | X | X | X | X | X | ||
| Build relationship with family | X | X | X | |||||
| Ask family for permission and preferences | X | X | X | |||||
| During FCR | 10. Introduction and explanation of FCR | Introduce HCT and family to each other | X | X | X | X | X | |
| Explain interactive rounding process | X | X | X | |||||
| 11. Active involvement of nurse | Giving nurse opportunity to actively participate | X | X | |||||
| 12. Communication with family | Give family opportunity to actively participate | X | X | X | X | X | X | |
| Address family's questions/concerns | X | X | X | X | X | |||
| Explain tests, findings and results to family | X | X | X | X | X | |||
| Confirm family understanding | X | X | X | |||||
| 13. Giving presentation | Restructure the presentation | X | X | |||||
| Shorten the presentation | X | X | X | |||||
| Focus presentation on assessment and plan | X | X | X | X | X | |||
| Summarize plan for family | X | X | X | |||||
| Avoid discussion of sensitive topics | X | X | X | |||||
| 14. Communication style | Present in a conversational manner | X | X | X | ||||
| Use an engaging communication style | X | X | X | X | X | |||
| 15. Language used | Use qualitative language | X | X | X | ||||
| Use plain language | X | X | X | X | X | |||
| 16. Performing physical exam | Pause and confirm physical exam | X | ||||||
| 17. Managing distractions | Minimize distractions and interruptions | X | X | X | X | X | X | |
| 18. Senior physician leading/role modeling | Attending/senior resident physician should lead, direct and be a role model on FCR rounds | X | X | |||||
| 19. Teaching | Ask family permission and involve in teaching | X | X | |||||
| 20. Customizing FCR for family | Adapt rounds to family's needs | X | X | X | X | |||
| After FCR | 21. Following up with family | HCT members follow up with family | X | X | X | |||
|
| Q1: [Medical students and residents] actually had a chance to do a quick round, an abbreviated presentation to put together an outline of what we're going to talk about before we even do it. (MS2) |
| Q2: I would like to know exactly what's going onbefore I walk in. (Att.6) |
| Q3: [T]he nurse did give me the fore‐warning that rounds would be coming and it was usually like a group of 8 to 10so I was prepared for that. (P7) |
| Q4: What went well is that I had already connected with this mom and the daughter prior to this rounding encounter. (Res.3) |
| Q5: [Y]ou could ask the patient or the parents if they want the child there. (P3) |
| Q6: [Families] really want to know what your role is on the team. (Att.5) |
| Q7: I guess it would be easier to figure out who you need to direct questions to. (P3) |
| Q8: [L]etting the family anticipate what rounding is going to be like and when the opportunity is going to come up to talk. I think that can help. (Res.2) |
| Q9: I think the decision making is probably the most critical partthere is really no substitute for [families] being involved in the decisionwithout a lot of medical conversation and analysis. (P1) |
| Q10: The family was proactive enough to ask questions, but they were never really given entrance to ask questions.No one had said do you have any questions?' (Res.4) |
| Q11: It is really important for the doctors to listen to them, to know that they are the parents and they know their children best. (RN3) |
| Q12: I think sometimes when you are teaching, some of the information could potentially be scary to the family. What I would hope is letting the family feel like they are part of the education process. (Res.2) |
| Q13: Sometimes nurses are asked initially, do you have anything to add,' which I think is a good way to startbecause we have probably the most current and updated information. (RN1) |
| Q14: When I was talking about the physical exam partmaybe at that point, if I could just stop talking, we couldconfirm that exam. (Res.3) |
| Q15: It's distracting if different groups have individual discussions when you are trying to keep the group focused on this particular patient for rounds. (Att.7) |
| Q16: It's just the basic thing that I try to tell residents. I do this hopefully at least once every time I am on services with the team. (Att.1) |
| Q17: ([C]hanging rounds depending on the families) would be the ideal situationthinking about what's helpful for a family. (Att.6) |
| Q18: What I usually see when things work well after we leave is that the nurse can still stick around the family. (Res.4) |
| Q19: [T]he students have to go back in the afternoon to talk with the family about what the treatment point is and answer any questions. (MS2) |
Before FCR
To engage families during FCR, many interviewees suggested that both the HCT and families need preparation. HCT members suggested that medical students should collect up‐to‐date patient information and review it with the senior resident physicians (Q1) to reach a consensus before starting FCR (Q2). To prepare families for rounds, parents and HCT members suggested that the HCT should orient families to the rounding process (Q3), build relationships with families (Q4), and ask for their permission and preference regarding participation in rounds (Q5).
During FCR
A number of strategies focused on the beginning of rounds. Parents, children, and HCT members stressed the need to introduce HCT members by role (Q6) and inform families to whom to direct questions (Q7). It was also suggested that parents introduce themselves to the team. Some interviewees recommended that the HCT explain the rounding process to families at this time (Q8).
Interviewees recommended strategies related to communication between the HCT and families during rounds. Many interviewees suggested restructuring and shortening the presentation by focusing on the assessment and plan (Q9). According to all interviewees, the HCT should present in a conversational manner and use an engaging communication style (eg, smiling, making eye contact, using appropriate humor) and appropriate language (eg, qualitative trend instead of numbers, plain language instead of medical jargon) to communicate with families. To ensure families understanding, HCT members should encourage and address their questions and concerns (Q10). In addition, families should be given the opportunity to provide information (eg, patient history and overnight events) and to express their opinions about the plan (Q11). If teaching is done during rounds, the HCT should involve families and ask for permission (Q12).
Other strategies on rounds were suggested, such as giving nurses the opportunity to actively participate (Q13), pausing and confirming physical exam findings (Q14), minimizing distractions and interruptions (Q15), attending and/or senior resident physicians leading and being role models for FCR (Q16), and adapting rounds to families' needs (Q17).
After FCR
Some HCT members talked about the importance of following up with families after rounds. Specifically, suggestions that nurses could stay with families immediately after rounds (Q18) were made, whereas physicians could return to families later in the day (Q19).
DISCUSSION
Using recognized qualitative systems engineering methods, we identified a broad range of strategies for enhancing family engagement on FCR from the perspectives of a diverse group of stakeholders on rounds and described how these strategies target known fundamental elements in both the hospital work system and rounding process. We highlight recommendations on the content and style of communication during rounds with families, but also introduce more complex system‐wide elements that likely play a role in family engagement, such as the composition of the HCT; organization and environment of rounds; tools and technologies used; and preparation of the HCT, families, and patients beforehand.
Our research both confirms and builds upon practices previously described in the FCR literature.[17, 31, 32] In a case report by Muething et al.,[17] recommendations were developed using a series of plan‐do‐study‐act cycles to determine the components needed to conduct FCR. These components included: 1) determining family preference prior to rounds, 2) defining HCT roles, 3) introducing HCT to family and explaining the purpose of rounds, 4) describing what is shared and how it is said on rounds, 5) describing the contribution of families, nurses, and ancillary staff, and 6) providing teaching recommendations to senior physicians on rounds. All of these components are suggested by one or more of the participants in our study. In addition, our research identifies a variety of new work system‐related strategies, such as scheduling rounds, using computers appropriately on rounds, and providing training of HCT members beforehand.
Of particular interest was the discordance between strategies mentioned by families and the various members of the HCT. Although HCT members mentioned all identified strategies, families were interested in certain ones. Regarding the structure of FCR, families showed particular interest in HCT composition, timing and scheduling of rounds, location of rounds, and positioning of the HCT. In comparison, families did not mention the importance of the roles and duties of HCT members, HCT preparation for rounds, and use of computers during rounds. With respect to the FCR process, families stressed the importance of family preparation beforehand, introduction and explanation of rounds at the beginning, presentation style and communication style, customization, and management of distractions during rounds. None of the families, however, mentioned the rest of the strategies, including HCT preparation before rounds, involvement of the nurse, teaching and performing the physical exam, the role of the attending and senior resident roles during rounds, and following up with the family after rounds. These different perspectives are likely, in part, inherent to the different roles and experiences of parents and HCT members. For example, parents' knowledge of what goes on in the hospital outside of FCR, such as orientation and preparation of HCT members for rounds, is relatively limited. Future research using methods to evaluate and prioritize strategies as well as understanding reasons for contradicting strategies is warranted.
We recognize that, although family engagement is recommended as a critical component of care, strategies to improve engagement may be in direct opposition to other goals of the HCT. For example, some of our participants suggest having a smaller team may be more beneficial for family engagement on rounds. In some settings, it may be feasible to have a small team; however, in institutions that accommodate a large number of learners, excluding students from the teaching opportunity of rounds may actually compromise educational experiences. In patients with chronic and/or complex care, a larger multidisciplinary team may better facilitate information exchange among disciplines and expedite discharge planning. Moreover, one might speculate that it may not be that the size affects family engagement as much as the composition of the team, especially if tailored to the needs of the patient. For example, a large team consisting of primarily physicians and trainees may not be as engaging as the same sized team with one attending physician and a respiratory therapist, case manager, and consulting subspecialist. Finding a balance between engaging families, teaching learners, and maintaining efficiency is paramount and needs to be studied further.
This study has several limitations. Our data are from a single academic children's hospital, which may limit generalizability due to a small sampling of multiple stakeholders on different services, our specific patient population, HCT composition and roles, and teaching needs. However, we face similar barriers to engaging families during rounds as those published from both another single institution[17] and a national sampling of pediatric hospitalists.[16] Furthermore, our recommended strategies to address the FCR process are supported by prior work.[17] Because this study was voluntary, our interviewees were likely more engaged participants in general. Specifically, the viewpoints of engaged families and HCT members may not represent the viewpoints of those who are less engaged or supportive of FCR. We did not enroll nonEnglish‐speaking patients and families, which is a potential direction for future research. In our interviews, we also relied solely on the perceptions of rounding participants, rather than those of outside observers or researchers, which may only provide a partial perspective of potential strategies to improve family engagement. Last, this qualitative research approach does not provide quantitative information regarding whether certain strategies are preferred by a majority of participants, which we hope to address in future research.
This work is part of a larger study that aims to implement a bundle of these strategies after stakeholder prioritization based on impact on family engagement, feasibility, and sustainability. We plan to systematically evaluate the implementation process of these strategies and measure their impact on family engagement and, ultimately, patient safety. One or more of these strategies could be implemented in a similar manner at other hospitals depending on specific institutional needs.
In conclusion, as recently reflected by Barry et al. in The New England Journal of Medicine, Although talk about patient‐centered care is ubiquitous in modern health care, one of the greatest challenges of turning the rhetoric into reality continues to be routinely engaging patients in decision making.[33] FCR provide a crucial opportunity for family involvement in daily care decisions in the pediatric inpatient setting. This study highlights the importance of prior work defining the components of involving families in this process, while emphasizing new systems‐based strategies that further facilitate the expectation of family engagement in the care of hospitalized children.
Disclosures
This work was funded through an Agency for Healthcare Research and Quality Health Services Research Dissemination and Demonstration Grant, R18 HS018680, and also supported by the Arthur Vining Davis Foundation and the National Patient Safety Foundation through the James S. Todd Memorial Research Award. Funding organizations did not contribute to the design and conduct of study; collection, management, analysis, and interpretation of data; or preparation, review, or approval of the manuscript. The authors report no conflicts of interest.
A growing body of literature suggests that patient‐ and family‐centered care can improve patient outcomes[1, 2, 3, 4, 5] as well as patient, family, and provider satisfaction.[6, 7, 8, 9, 10] Engaging patients and families as a way to improve the quality and safety of care has been widely endorsed by leading healthcare organizations,[11, 12, 13] including the Institute of Medicine.[14] In the pediatric inpatient setting, family‐centered rounds (FCR), defined as bedside rounds in which the patient and family share in the control of the management plan as well as in the evaluation of the process itself,[15] potentially provide a consistent venue for family engagement and are recommended by the American Academy of Pediatrics as standard practice.[13]
According to a recent study by Mittal et al.,[16] FCR are the most common type of rounds practiced among pediatric hospitalists surveyed in the United States and Canada. Despite this widespread shift from rounding in a conference room to the bedside with patients and families, there exist only a few studies that provide specific recommendations on conducting FCR.[15, 17, 18] This research has been limited, primarily focusing how rounds are conducted, and further investigation is needed to identify the impact of other processes and elements within the hospital work system that may also affect family engagement during rounds.
The objectives of this study were to: 1) identify strategies to enhance family engagement during FCR drawing from the viewpoints of the various stakeholders on rounds, and 2) characterize these strategies into known elements of hospital work systems and rounding processes using a recognized human factors engineering approach, The Systems Engineering Initiative for Patient Safety (SEIPS) model.[19] According to the SEIPS model, barriers and facilitators to family engagement during FCR are likely embedded in the design of the hospital work systems and rounding process; therefore, we hypothesized that strategies that influence engagement will target all work system and process elements. This work is part of a larger study in which, after prioritization of this group of strategies based on feasibility and sustainability, a bundle of best practices for conducting FCR will be developed, implemented, and evaluated.
METHODS
Study Design
Semistructured interviews using the stimulated recall approach[20, 21] were conducted to understand the cognitive processes of families and healthcare team (HCT) members during FCR. This qualitative study design allowed us to capture comprehensive information from the perspectives of a diverse group of stakeholders on strategies for improving family engagement during FCR.
Setting and Participants
This study was conducted at a children's hospital in Wisconsin, where FCR were initiated in 2007 with the transition to a new hospital facility. The expectation is that FCR are conducted daily with the family and the patient's HCT, consisting of at least an attending physician and nurse. Typically, multiple residents, interns, and medical students are present along with a combination of other providers, including consulting subspecialists, a fellow, nurse practitioner, respiratory therapist, or pharmacist. When this study was conducted, attendees received little to no formal training regarding their role on FCR. As part of a larger study, English‐speaking patients and/or families admitted to 1 of 4 inpatient services (2 hospitalist, 1 pulmonary, and 1 hematology/oncology), and their associated HCT members were enrolled and their bedside rounds were video recorded. A purposive sampling technique[22, 23] was employed, recruiting interviewees that represented the various groups of stakeholders of rounds, including parents, children, attending physicians, resident physicians, medical students, and nurses. For child interviews, we restricted selection to children aged 8 to 17 years to ensure the ability to understand the interviewing process and provide feedback. Families were consented and children were assented. The University of Wisconsin‐Madison Health Sciences Institutional Review Board approved this study.
Interviews and analysis occurred concurrently in an iterative process, informing each other. Thus, recruitment continued until we reached theoretical saturation,[24, 25] the point at which additional interviews did not provide new information or further conceptual development.
Study Procedures
All interviews were conducted by trained researchers, who used the same semistructured interview guide. During each interview, the interviewee was instructed to watch his/her own rounding video and pause when noticing something that made it easy (facilitator) or hard (barrier) to engage the family. Every time the interviewee paused the video to describe what was noticed, the interviewer then asked follow‐up, open‐ended questions to solicit specific information that focused on strategies for enhancing family engagement during FCR. For instance, if the issue identified was a barrier, the interviewer asked, What would you have wanted to happen differently? and if the issue identified was a facilitator, the interviewer asked, How could we ensure that would happen for everyone? The interviewee rewound the video as needed. If the interviewee had not stopped the video by the halfway point, the interviewer would pause the video and review the instructions. After the interviewee had viewed and commented on the entire rounding video, an opportunity was offered to reflect on other factors that influence family engagement during rounds, and additional questions were asked as necessary to fully understand the interviewee's views. All interviews were audio recorded and personal identifiers were removed prior to data analysis.
Data Analysis
Two research assistants reviewed the audio recordings and identified all instances related to strategies for improving family engagement during FCR. There was no screening of strategies (ie, if an interviewee suggested a strategy was related to improving family engagement, it was categorized as such). To ensure intercoder reliability, these assistants, under the supervision of a researcher (L.D.), reviewed the coding process together, held consensus meetings, and crosschecked interviews for coding consensus. A researcher (A.X.) transcribed all strategy‐related instances, which were then reviewed by two additional researchers (M.K., P.C.). To organize, sort, and code the data, interview transcripts were imported in the NVivo qualitative data analysis software (QSR International, Doncaster, Victoria, Australia). The research group then performed a qualitative content analysis of the transcripts[26] and categorized the strategies in an iterative process (information provided on request).
To ensure that all strategies remained conceptually similar within categories, the constant comparative method[27, 28] was applied to the coding process. This involved comparing: 1) strategy‐related instances from the same participants, 2) strategy‐related instances from different participants in the same groups, 3) strategy‐related instances from different participants in different groups, 4) a coded strategy with other coded strategies, 5) coded strategies with categories, and 6) a category with other categories. A strategy‐related instance could be coded under more than one strategy or category. For instance, one interviewee said conducting things that can be done without family beforehand, and presenting and reviewing the plan with family. This was coded under both the strategy conducting rounds in another location without family and then at the bedside with family in the location of FCR category and the strategy focusing presentation on assessment and plan in the communication style category.
RESULTS
A total of 37 interviews were conducted with 11 parents, 4 children, and 22 HCT members (8 attending physicians, 6 resident physicians, 5 medical students, and 3 nurses) in 24 videos of rounding sessions. The duration of the interviews ranged from 30 to 60 minutes.
A total of 338 separate instances related to strategies for improving family engagement on FCR were identified and sorted into 21 categories. Using the SEIPS model, these categories were organized into 2 themes: the work system and process of FCR (Figure 1). Of the 21 categories, 12 were mentioned by both families (parents and/or children) and HCT members and 9 were solely mentioned by the HCT.

Work System of FCR
Table 1 shows the categories of strategies related to the 5 elements of the FCR work system.[29, 30] Illustrative quotes from the interviews (Q) are presented in Table 2.
| Work System Elements | Categories | Strategies | P (11) | C (4) | Att. (8) | Res. (6) | MS (5) | RN (3) |
|---|---|---|---|---|---|---|---|---|
| ||||||||
| People | 1. Size and composition of HCT | Have a smaller HCT conduct FCR | X | X | X | X | ||
| Ensure all relevant disciplines present on FCR | X | X | X | X | X | |||
| Task | 2. Roles and duties of HCT members | Define roles/duties of HCT members on FCR | X | X | ||||
| Organization | 3. Timing and scheduling of FCR | Schedule FCR, inform participants beforehand | X | X | X | |||
| 4. Training of HCT for FCR | Train HCT on how to present on FCR | X | X | |||||
| Environment | 5. Location of FCR | At bedside with family and patient | X | X | ||||
| In another location with HCT, then at bedside | X | X | X | X | ||||
| In another location with family but without child | X | |||||||
| 6. Positioning of HCT members on FCR | Sit down with family | X | X | X | X | X | ||
| Stand close to or in a semicircle around family | X | X | X | X | X | |||
| Tools and technologies | 7. Use of computers on FCR | Use computer to support family interaction | X | X | X | X | ||
| Don't use a computer | X | X | ||||||
|
| Q1: I'm intimidated to ask a question. It seems like there are too many peopleI like a smaller group. (P5) |
| Q2: Sometimes rounds are the only time that the parents are there to see the entire teamso in that way, including [the entire team] at the rounds makes more sense. (MS1) |
| Q3: There needs to be much more clear roles about who is supposed to do what, and it should be predictable. (Att.2) |
| Q4: ([T]iming of rounds) is a huge source of frustration for families. If [physicians] know in which order they will go for patients, they can call our charge nurse or unit clerk or page nurses with that information. (RN1) |
| Q5: ([W]ith a notice of the rounding schedule), I can be ahead of time, trying to think of questions. (P10) |
| Q6: [I]t would be really nice to see somebody do a presentation in a medical eye's version and then also in the family‐centered version. (MS5) |
| Q7: [H]aving the medical students practice with the senior residentis a good way of doing it. (Res.4) |
| Q8: [M]aybe some small groups where you practice this among students. (MS5) |
| Q9: It would be better to be in the room for communication. (Att.1) |
| Q10: You could have sort of hallway rounds, which is much more medical oriented, and inside‐the‐room rounds, which is much more talking with the parent. (P1) |
| Q11: [H]ave sit‐down rounds with parents and families. (Res.5) |
| Q12: I've seen some attending physicians who sat down. I think that could be helpful to be on the same level as the patient and family. (Res.2) |
| Q13: [M]aybe formation of semicircle or something like that, where we can see everybody a little more clearly, I think that would be very helpful. (P10) |
| Q14: I find the presence of a computer incredibly offensive and obstructivewhen you are supposed to be able to interact with the patient. (Att.6) |
| Q15: One of the things I started doing is having one of the other resident physicians have the computer, so just relying on them to do the orders, and me just being there mainly for being the presenter of rounds. (Res.4) |
People
Two seemingly contradictory strategies were proposed. Some interviewees suggested a smaller HCT with members most familiar to the family (Q1), whereas other interviewees stressed the need to involve different relevant disciplines (eg, social worker, nutritionist) during rounds (Q2).
Tasks
Both attending and resident physicians emphasized the importance of defining the role of each HCT member before rounding (Q3). Interviewees also suggested these roles should be explained to families, ideally at admission.
Organization
Many interviewees suggested the need to consistently schedule rounds (Q4) and to inform families and nurses of the schedule so all parties could plan ahead (Q5). Some resident physicians and medical students recommended training of learners on how to give a family‐centered presentation using methods such as role modeling (Q6) and practicing with the senior resident physician (Q7) or in small groups (Q8).
Environment
Some interviewees suggested conducting rounds in patient rooms (Q9). Others suggested conducting rounds first in another location (eg, hallway) without the family and then going to the bedside to round with the family (Q10). There were also interviewees who suggested conducting rounds in another location (eg, conference room) with the family (Q11). When conducting rounds in the patient room, some interviewees suggested that some HCT members (eg, attending and senior resident physicians) could sit down with the family (Q12), with the rest of the HCT standing close to the family in a semicircle (Q13).
Tools and Technologies
Some interviewees thought that conversation with families could be negatively affected by the use of computers, and therefore suggested not using them on FCR (Q14). Alternatively, other interviewees considered computers a tool to facilitate the interaction between the HCT and families, such as showing x‐rays or lab values. Several interviewees suggested that computers should not be positioned to block eye contact between HCT members and families; therefore, only HCT members not presenting should use computers (Q15).
Process of FCR
Table 3 shows the categories of strategies related to the process of FCR, which were categorized into 3 phases. Illustrative quotes are presented in Table 4.
| Process Phases | Categories | Strategies | P (11) | C (4) | Att. (8) | Res. (6) | MS (5) | RN (3) |
|---|---|---|---|---|---|---|---|---|
| ||||||||
| Before FCR | 8. HCT preparation | Collect and prepare pertinent information | X | X | X | X | ||
| 9. Family preparation | Orient family to rounding process | X | X | X | X | X | ||
| Build relationship with family | X | X | X | |||||
| Ask family for permission and preferences | X | X | X | |||||
| During FCR | 10. Introduction and explanation of FCR | Introduce HCT and family to each other | X | X | X | X | X | |
| Explain interactive rounding process | X | X | X | |||||
| 11. Active involvement of nurse | Giving nurse opportunity to actively participate | X | X | |||||
| 12. Communication with family | Give family opportunity to actively participate | X | X | X | X | X | X | |
| Address family's questions/concerns | X | X | X | X | X | |||
| Explain tests, findings and results to family | X | X | X | X | X | |||
| Confirm family understanding | X | X | X | |||||
| 13. Giving presentation | Restructure the presentation | X | X | |||||
| Shorten the presentation | X | X | X | |||||
| Focus presentation on assessment and plan | X | X | X | X | X | |||
| Summarize plan for family | X | X | X | |||||
| Avoid discussion of sensitive topics | X | X | X | |||||
| 14. Communication style | Present in a conversational manner | X | X | X | ||||
| Use an engaging communication style | X | X | X | X | X | |||
| 15. Language used | Use qualitative language | X | X | X | ||||
| Use plain language | X | X | X | X | X | |||
| 16. Performing physical exam | Pause and confirm physical exam | X | ||||||
| 17. Managing distractions | Minimize distractions and interruptions | X | X | X | X | X | X | |
| 18. Senior physician leading/role modeling | Attending/senior resident physician should lead, direct and be a role model on FCR rounds | X | X | |||||
| 19. Teaching | Ask family permission and involve in teaching | X | X | |||||
| 20. Customizing FCR for family | Adapt rounds to family's needs | X | X | X | X | |||
| After FCR | 21. Following up with family | HCT members follow up with family | X | X | X | |||
|
| Q1: [Medical students and residents] actually had a chance to do a quick round, an abbreviated presentation to put together an outline of what we're going to talk about before we even do it. (MS2) |
| Q2: I would like to know exactly what's going onbefore I walk in. (Att.6) |
| Q3: [T]he nurse did give me the fore‐warning that rounds would be coming and it was usually like a group of 8 to 10so I was prepared for that. (P7) |
| Q4: What went well is that I had already connected with this mom and the daughter prior to this rounding encounter. (Res.3) |
| Q5: [Y]ou could ask the patient or the parents if they want the child there. (P3) |
| Q6: [Families] really want to know what your role is on the team. (Att.5) |
| Q7: I guess it would be easier to figure out who you need to direct questions to. (P3) |
| Q8: [L]etting the family anticipate what rounding is going to be like and when the opportunity is going to come up to talk. I think that can help. (Res.2) |
| Q9: I think the decision making is probably the most critical partthere is really no substitute for [families] being involved in the decisionwithout a lot of medical conversation and analysis. (P1) |
| Q10: The family was proactive enough to ask questions, but they were never really given entrance to ask questions.No one had said do you have any questions?' (Res.4) |
| Q11: It is really important for the doctors to listen to them, to know that they are the parents and they know their children best. (RN3) |
| Q12: I think sometimes when you are teaching, some of the information could potentially be scary to the family. What I would hope is letting the family feel like they are part of the education process. (Res.2) |
| Q13: Sometimes nurses are asked initially, do you have anything to add,' which I think is a good way to startbecause we have probably the most current and updated information. (RN1) |
| Q14: When I was talking about the physical exam partmaybe at that point, if I could just stop talking, we couldconfirm that exam. (Res.3) |
| Q15: It's distracting if different groups have individual discussions when you are trying to keep the group focused on this particular patient for rounds. (Att.7) |
| Q16: It's just the basic thing that I try to tell residents. I do this hopefully at least once every time I am on services with the team. (Att.1) |
| Q17: ([C]hanging rounds depending on the families) would be the ideal situationthinking about what's helpful for a family. (Att.6) |
| Q18: What I usually see when things work well after we leave is that the nurse can still stick around the family. (Res.4) |
| Q19: [T]he students have to go back in the afternoon to talk with the family about what the treatment point is and answer any questions. (MS2) |
Before FCR
To engage families during FCR, many interviewees suggested that both the HCT and families need preparation. HCT members suggested that medical students should collect up‐to‐date patient information and review it with the senior resident physicians (Q1) to reach a consensus before starting FCR (Q2). To prepare families for rounds, parents and HCT members suggested that the HCT should orient families to the rounding process (Q3), build relationships with families (Q4), and ask for their permission and preference regarding participation in rounds (Q5).
During FCR
A number of strategies focused on the beginning of rounds. Parents, children, and HCT members stressed the need to introduce HCT members by role (Q6) and inform families to whom to direct questions (Q7). It was also suggested that parents introduce themselves to the team. Some interviewees recommended that the HCT explain the rounding process to families at this time (Q8).
Interviewees recommended strategies related to communication between the HCT and families during rounds. Many interviewees suggested restructuring and shortening the presentation by focusing on the assessment and plan (Q9). According to all interviewees, the HCT should present in a conversational manner and use an engaging communication style (eg, smiling, making eye contact, using appropriate humor) and appropriate language (eg, qualitative trend instead of numbers, plain language instead of medical jargon) to communicate with families. To ensure families understanding, HCT members should encourage and address their questions and concerns (Q10). In addition, families should be given the opportunity to provide information (eg, patient history and overnight events) and to express their opinions about the plan (Q11). If teaching is done during rounds, the HCT should involve families and ask for permission (Q12).
Other strategies on rounds were suggested, such as giving nurses the opportunity to actively participate (Q13), pausing and confirming physical exam findings (Q14), minimizing distractions and interruptions (Q15), attending and/or senior resident physicians leading and being role models for FCR (Q16), and adapting rounds to families' needs (Q17).
After FCR
Some HCT members talked about the importance of following up with families after rounds. Specifically, suggestions that nurses could stay with families immediately after rounds (Q18) were made, whereas physicians could return to families later in the day (Q19).
DISCUSSION
Using recognized qualitative systems engineering methods, we identified a broad range of strategies for enhancing family engagement on FCR from the perspectives of a diverse group of stakeholders on rounds and described how these strategies target known fundamental elements in both the hospital work system and rounding process. We highlight recommendations on the content and style of communication during rounds with families, but also introduce more complex system‐wide elements that likely play a role in family engagement, such as the composition of the HCT; organization and environment of rounds; tools and technologies used; and preparation of the HCT, families, and patients beforehand.
Our research both confirms and builds upon practices previously described in the FCR literature.[17, 31, 32] In a case report by Muething et al.,[17] recommendations were developed using a series of plan‐do‐study‐act cycles to determine the components needed to conduct FCR. These components included: 1) determining family preference prior to rounds, 2) defining HCT roles, 3) introducing HCT to family and explaining the purpose of rounds, 4) describing what is shared and how it is said on rounds, 5) describing the contribution of families, nurses, and ancillary staff, and 6) providing teaching recommendations to senior physicians on rounds. All of these components are suggested by one or more of the participants in our study. In addition, our research identifies a variety of new work system‐related strategies, such as scheduling rounds, using computers appropriately on rounds, and providing training of HCT members beforehand.
Of particular interest was the discordance between strategies mentioned by families and the various members of the HCT. Although HCT members mentioned all identified strategies, families were interested in certain ones. Regarding the structure of FCR, families showed particular interest in HCT composition, timing and scheduling of rounds, location of rounds, and positioning of the HCT. In comparison, families did not mention the importance of the roles and duties of HCT members, HCT preparation for rounds, and use of computers during rounds. With respect to the FCR process, families stressed the importance of family preparation beforehand, introduction and explanation of rounds at the beginning, presentation style and communication style, customization, and management of distractions during rounds. None of the families, however, mentioned the rest of the strategies, including HCT preparation before rounds, involvement of the nurse, teaching and performing the physical exam, the role of the attending and senior resident roles during rounds, and following up with the family after rounds. These different perspectives are likely, in part, inherent to the different roles and experiences of parents and HCT members. For example, parents' knowledge of what goes on in the hospital outside of FCR, such as orientation and preparation of HCT members for rounds, is relatively limited. Future research using methods to evaluate and prioritize strategies as well as understanding reasons for contradicting strategies is warranted.
We recognize that, although family engagement is recommended as a critical component of care, strategies to improve engagement may be in direct opposition to other goals of the HCT. For example, some of our participants suggest having a smaller team may be more beneficial for family engagement on rounds. In some settings, it may be feasible to have a small team; however, in institutions that accommodate a large number of learners, excluding students from the teaching opportunity of rounds may actually compromise educational experiences. In patients with chronic and/or complex care, a larger multidisciplinary team may better facilitate information exchange among disciplines and expedite discharge planning. Moreover, one might speculate that it may not be that the size affects family engagement as much as the composition of the team, especially if tailored to the needs of the patient. For example, a large team consisting of primarily physicians and trainees may not be as engaging as the same sized team with one attending physician and a respiratory therapist, case manager, and consulting subspecialist. Finding a balance between engaging families, teaching learners, and maintaining efficiency is paramount and needs to be studied further.
This study has several limitations. Our data are from a single academic children's hospital, which may limit generalizability due to a small sampling of multiple stakeholders on different services, our specific patient population, HCT composition and roles, and teaching needs. However, we face similar barriers to engaging families during rounds as those published from both another single institution[17] and a national sampling of pediatric hospitalists.[16] Furthermore, our recommended strategies to address the FCR process are supported by prior work.[17] Because this study was voluntary, our interviewees were likely more engaged participants in general. Specifically, the viewpoints of engaged families and HCT members may not represent the viewpoints of those who are less engaged or supportive of FCR. We did not enroll nonEnglish‐speaking patients and families, which is a potential direction for future research. In our interviews, we also relied solely on the perceptions of rounding participants, rather than those of outside observers or researchers, which may only provide a partial perspective of potential strategies to improve family engagement. Last, this qualitative research approach does not provide quantitative information regarding whether certain strategies are preferred by a majority of participants, which we hope to address in future research.
This work is part of a larger study that aims to implement a bundle of these strategies after stakeholder prioritization based on impact on family engagement, feasibility, and sustainability. We plan to systematically evaluate the implementation process of these strategies and measure their impact on family engagement and, ultimately, patient safety. One or more of these strategies could be implemented in a similar manner at other hospitals depending on specific institutional needs.
In conclusion, as recently reflected by Barry et al. in The New England Journal of Medicine, Although talk about patient‐centered care is ubiquitous in modern health care, one of the greatest challenges of turning the rhetoric into reality continues to be routinely engaging patients in decision making.[33] FCR provide a crucial opportunity for family involvement in daily care decisions in the pediatric inpatient setting. This study highlights the importance of prior work defining the components of involving families in this process, while emphasizing new systems‐based strategies that further facilitate the expectation of family engagement in the care of hospitalized children.
Disclosures
This work was funded through an Agency for Healthcare Research and Quality Health Services Research Dissemination and Demonstration Grant, R18 HS018680, and also supported by the Arthur Vining Davis Foundation and the National Patient Safety Foundation through the James S. Todd Memorial Research Award. Funding organizations did not contribute to the design and conduct of study; collection, management, analysis, and interpretation of data; or preparation, review, or approval of the manuscript. The authors report no conflicts of interest.
- , , , et al. The impact of patient‐centered care on outcomes. J Fam Pract. 2000;49:796–804.
- , , , et al. Observational study of effect of patient centredness and positive approach on outcomes of general practice consultations. BMJ. 2001;323:908–911.
- , , . Improvement in the family‐centered medical home enhances outcomes for children and youth with special healthcare needs. J Ambul Care Manage. 2009;32:188–196.
- , , . Associations of family‐centered care with health care outcomes for children with special health care needs. Matern Child Health J. 2011;15:794–805.
- , , , , Can a patient‐centered medical home lead to better patient outcomes? The quality implications of Geisinger's ProvenHealth Navigator. Am J Med Qual. 2012;27:210–216.
- , , . Perceptions of health care providers' communication: relationships between patient‐centered communication and satisfaction. Health Commun. 2004;16:363–383.
- , . Satisfaction with care and ease of using health care services among parents of children with special health care needs: the roles of race/ethnicity, insurance, language, and adequacy of family‐centered care. Pediatrics. 2006;117:1184–1196.
- , , , . A randomized, controlled trial of bedside versus conference‐room case presentation in a pediatric intensive care unit. Pediatrics. 2007;120:275–280.
- , , , , . Effect of patient‐centered care on patient satisfaction and quality of care. J Nurs Care Qual. 2008;23:316–321.
- , , . Implementing family‐centered rounds: pediatric residents' perceptions. Clin Pediatr (Phila) 2010;49:228–234.
- ACGME Program Requirements for Graduate Medical Education in Pediatrics. 2007. Available at: http://www.acgme.org/acWebsite/downloads/RRC_progReq320_pediatrics_07012007.pdf. Accessed February 10, 2012.
- Speak up: prevent errors in your child's care. 2011. The Joint Commission Web site. Available at: http://www.jointcommission.org/Speak_Up_Prevent_Errors_in_Your_Childs_Care/. Accessed May 11, 2012.
- Patient‐ and family‐centered care and the pediatrician's role. Pediatrics. 2012;129:394–404.
- Committee on Quality of Health Care in America, Institute of Medicine. Crossing the Quality Chasm: A New Health System for the 21st Century. Washington, DC: The National Academies Press; 2001.
- , , , . Defining family‐centered rounds. Teach Learn Med. 2007;19:319–322.
- , , , et al. Family‐centered rounds on pediatric wards: A PRIS network survey of US and Canadian hospitalists. Pediatrics. 2010;126:37–43.
- , , , , . Family‐centered bedside rounds: a new approach to patient care and teaching. Pediatrics. 2007;119:829–832.
- , , , . Parental responses to involvement in rounds on a pediatric inpatient unit at a teaching hospital: a qualitative study. Acad Med. 2008;83:292–297.
- , , , et al. Work system design for patient safety: the SEIPS model. Qual Saf Health Care. 2006;15:i50–i58.
- , . IPR—a validated model for the 1990s and beyond. Couns Psychol. 1990;18:436–440.
- , . Auto‐ and allo‐confrontation as tools for reflective activities. Appl Ergon. 2004;35:531–540.
- . Qualitative Research and Evaluation Methods. 3rd ed. Thousand Oaks, CA: Sage Publications; 2002.
- , . Doing Qualitative Research. 2nd ed. Thousand Oaks, CA: Sage Publications; 1999.
- . Sample size in qualitative research. Res Nurs Health. 1995;18:179–183.
- , . Basics of Qualitative Research: Techniques and Procedures for Developing Grounded Theory. Newbury Park, CA: Sage Publications; 1998.
- , . Qualitative content analysis in nursing research: concepts, procedures and measures to achieve trustworthiness. Nurse Educ Today. 2004;24:105–112.
- . A purposeful approach to the constant comparative method in the analysis of qualitative interviews. Qual Quant. 2002;36:391–409.
- . Theoretical Sensitivity: Advances in the Methodology of Grounded Theory. Mill Valley, CA: Sociology Press; 1978.
- , . Balance theory of job design. In: Karwowski W, ed. International Encyclopedia of Ergonomics and Human Factors. London: Taylor 2000:1181–1184.
- , . A balance theory of job design for stress reduction. Int J Ind Ergon. 1989;4:67–79.
- Institute for Patient‐ and Family‐Centered Care. Applying patient‐ and family‐centered concepts to bedside rounds. 2010. Available at: http://www.ipfcc.org/advance/topics/PH_RD_Applying_PFCC_Rounds_012009.pdf. Accessed May 11, 2012.
- . A fundamental shift: family‐centered rounds in an academic medical center. Hospitalist. 2006;10:45–46.
- , Shared decision making—pinnacle of patient‐centered care. N Engl J Med. 2012;366:780–781.
- , , , et al. The impact of patient‐centered care on outcomes. J Fam Pract. 2000;49:796–804.
- , , , et al. Observational study of effect of patient centredness and positive approach on outcomes of general practice consultations. BMJ. 2001;323:908–911.
- , , . Improvement in the family‐centered medical home enhances outcomes for children and youth with special healthcare needs. J Ambul Care Manage. 2009;32:188–196.
- , , . Associations of family‐centered care with health care outcomes for children with special health care needs. Matern Child Health J. 2011;15:794–805.
- , , , , Can a patient‐centered medical home lead to better patient outcomes? The quality implications of Geisinger's ProvenHealth Navigator. Am J Med Qual. 2012;27:210–216.
- , , . Perceptions of health care providers' communication: relationships between patient‐centered communication and satisfaction. Health Commun. 2004;16:363–383.
- , . Satisfaction with care and ease of using health care services among parents of children with special health care needs: the roles of race/ethnicity, insurance, language, and adequacy of family‐centered care. Pediatrics. 2006;117:1184–1196.
- , , , . A randomized, controlled trial of bedside versus conference‐room case presentation in a pediatric intensive care unit. Pediatrics. 2007;120:275–280.
- , , , , . Effect of patient‐centered care on patient satisfaction and quality of care. J Nurs Care Qual. 2008;23:316–321.
- , , . Implementing family‐centered rounds: pediatric residents' perceptions. Clin Pediatr (Phila) 2010;49:228–234.
- ACGME Program Requirements for Graduate Medical Education in Pediatrics. 2007. Available at: http://www.acgme.org/acWebsite/downloads/RRC_progReq320_pediatrics_07012007.pdf. Accessed February 10, 2012.
- Speak up: prevent errors in your child's care. 2011. The Joint Commission Web site. Available at: http://www.jointcommission.org/Speak_Up_Prevent_Errors_in_Your_Childs_Care/. Accessed May 11, 2012.
- Patient‐ and family‐centered care and the pediatrician's role. Pediatrics. 2012;129:394–404.
- Committee on Quality of Health Care in America, Institute of Medicine. Crossing the Quality Chasm: A New Health System for the 21st Century. Washington, DC: The National Academies Press; 2001.
- , , , . Defining family‐centered rounds. Teach Learn Med. 2007;19:319–322.
- , , , et al. Family‐centered rounds on pediatric wards: A PRIS network survey of US and Canadian hospitalists. Pediatrics. 2010;126:37–43.
- , , , , . Family‐centered bedside rounds: a new approach to patient care and teaching. Pediatrics. 2007;119:829–832.
- , , , . Parental responses to involvement in rounds on a pediatric inpatient unit at a teaching hospital: a qualitative study. Acad Med. 2008;83:292–297.
- , , , et al. Work system design for patient safety: the SEIPS model. Qual Saf Health Care. 2006;15:i50–i58.
- , . IPR—a validated model for the 1990s and beyond. Couns Psychol. 1990;18:436–440.
- , . Auto‐ and allo‐confrontation as tools for reflective activities. Appl Ergon. 2004;35:531–540.
- . Qualitative Research and Evaluation Methods. 3rd ed. Thousand Oaks, CA: Sage Publications; 2002.
- , . Doing Qualitative Research. 2nd ed. Thousand Oaks, CA: Sage Publications; 1999.
- . Sample size in qualitative research. Res Nurs Health. 1995;18:179–183.
- , . Basics of Qualitative Research: Techniques and Procedures for Developing Grounded Theory. Newbury Park, CA: Sage Publications; 1998.
- , . Qualitative content analysis in nursing research: concepts, procedures and measures to achieve trustworthiness. Nurse Educ Today. 2004;24:105–112.
- . A purposeful approach to the constant comparative method in the analysis of qualitative interviews. Qual Quant. 2002;36:391–409.
- . Theoretical Sensitivity: Advances in the Methodology of Grounded Theory. Mill Valley, CA: Sociology Press; 1978.
- , . Balance theory of job design. In: Karwowski W, ed. International Encyclopedia of Ergonomics and Human Factors. London: Taylor 2000:1181–1184.
- , . A balance theory of job design for stress reduction. Int J Ind Ergon. 1989;4:67–79.
- Institute for Patient‐ and Family‐Centered Care. Applying patient‐ and family‐centered concepts to bedside rounds. 2010. Available at: http://www.ipfcc.org/advance/topics/PH_RD_Applying_PFCC_Rounds_012009.pdf. Accessed May 11, 2012.
- . A fundamental shift: family‐centered rounds in an academic medical center. Hospitalist. 2006;10:45–46.
- , Shared decision making—pinnacle of patient‐centered care. N Engl J Med. 2012;366:780–781.
Copyright © 2013 Society of Hospital Medicine
Quetiapine for the Treatment of Delirium
Delirium is an acute fluctuation in mental status that includes symptoms of inattention, disorganized thinking, and altered level of consciousness occurring over a short time period.[1] Prevalence of delirium ranges from 10% to 30% in the hospitalized medically ill and ranges from 40% to 60% in intensive care unit (ICU) patients who are not receiving mechanical ventilation.[2, 3] Patients experience delirium more often if they are of older age; have dementia, cancer, or acquired immune deficiency syndrome; have undergone surgery; are terminally ill; or have received multiple psychoactive medications, particularly benzodiazepines or opioids.[3, 4, 5, 6, 7] Delirium is associated with high rates of morbidity and mortality,[8] with patients more likely to develop complications such as pneumonia, decubitus ulcers, and long‐term cognitive deficits.[9, 10, 11, 12] These complications, in turn, lead to longer hospital stays and increased costs of care.[13, 14] Currently, there are no antipsychotics approved by the US Food and Drug Administration for the treatment of delirium.
Both the 2002 American Society of Critical Care Medicine[12] and the 2004 American Psychiatric Association guidelines[15] on delirium recommend haloperidol as the antipsychotic of choice due to its potent tranquilizing effect, lack of active metabolites, and limited anticholinergic and sedating side effects. However, when given intravenously or at high cumulative doses (generally >35 mg/day), haloperidol has been shown to cause QT interval prolongation potentially leading to torsades de pointes and sudden cardiac death.[15] Recent research in delirium treatment has focused on the second‐generation antipsychotics, and these studies have reported positive findings,[16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29] although no significant differences have been found compared to haloperidol.[16, 17, 18, 22, 23, 24] Systematic reviews have also failed to show a significant difference in efficacy or safety between second‐generation and first‐generation antipsychotics and unfortunately report a number of limitations including poor study designs, small sample sizes, lack of a placebo control group, exclusion of ICU patients, and weak primary outcomes.[8, 30, 31, 32] Attempting to correct for a number of these limitations, recent research with quetiapine has reported promising results in 2 controlled studies.[19, 20]
Quetiapine is a second‐generation antipsychotic with a very low affinity for dopamine receptors and a very high affinity for serotonin receptors.[33, 34] Additionally, quetiapine has a high affinity for histamine and 1‐adrenergic receptors, but a very low affinity for M1 muscarinic receptors.[34] This mechanism of action may allow quetiapine to effectively treat delirium and provide sedation without causing significant extrapyramidal side effects associated with potent dopamine receptor antagonism or precipitating delirium through muscarinic receptor antagonism. Quetiapine has a rapid absorption and a short half‐life (36 hours), giving it a quick onset of action and a fast elimination from the body.[35] Unlike haloperidol, it is only available in oral dosage forms, but can be crushed to administer via enteral tube. Common reported adverse effects include somnolence, hypotension, and dizziness. Although quetiapine has been shown to prolong QTc, Harrigan et al. reported a mean increase in QTc from baseline of 5.7 msec; there was no significant effect on this change in the presence of a metabolic inhibitor, ketoconazole.[36] The mean change in QTc from baseline with quetiapine was lower than the change reported with oral haloperidol, 5.7 msec compared to 7.1 msec, respectively. No statistical comparison was performed between these 2 drugs on this measure. Quetiapine does carry a black box warning for increased mortality in elderly patients with dementia‐related psychosis.[35] However, this risk has also been found with first‐generation antipsychotics.[37, 38, 39] A recent study found this risk of sudden cardiac death extended to adult users of both first‐ and second‐generation antipsychotics.[40] In contrast, Elie et al. found no increased risk of mortality in elderly patients with delirium receiving antipsychoticsover 90% were prescribed either haloperidol or risperidonein their nested case‐controlled study.[41] Quetiapine has demonstrated some benefit with limited side effects in studies utilizing antipsychotics for the treatment of delirium. The purpose of this review was to evaluate the role of quetiapine for the treatment of delirium.
LITERATURE REVIEW
We performed an English‐language literature search of MEDLINE and Embase databases to identify journal articles published between January 1960 and December 2012. Keywords included quetiapine, second‐generation antipsychotic, atypical antipsychotic, delirium, and agitation. The search was limited to English‐language articles and adult subjects (>18 years). Based on our review of abstracts, we included both controlled and noncontrolled trials as long as treatment of delirium was the primary focus. We eliminated case reports, foreign language articles, and poster presentations. We identified 8 trials[19, 20, 24, 25, 26, 27, 28, 29] that included 2 double‐blind, randomized, placebo‐controlled trials, which are described in the text below.[19, 20] Six other trials, 5 open‐label[25, 26, 27, 28, 29] and 1 retrospective cohort,[24] are described in Table 1.
| Study | Study Design | No. of Patients Included in Analyses | Patient Type | Treatment | Baseline Delirium Scores | Quetiapine Dose (mg/day) (MeanSD) | Efficacy Measures | Results | Side Effects |
|---|---|---|---|---|---|---|---|---|---|
| |||||||||
| Schwartz et al. (2000)[24] | RC | 22 | General hospital | Quetiapine or haloperidol flexible dose | DRS score 20.9 Q; 18.5 H | 211.4 Q; 3.4 H | >50% improvement in DRS scores | 10/11 in each treatment group had >50% improvement in DRS scores | EPS 2/11 H and 0/11 Q; mild‐to‐moderate sedation 0/11 H and 2/11 Q |
| Pae et al. (2004)[25] | OL | 22 | Neurosurgery and orthopedic surgery and oncology | Flexible dose quetiapine | DRS‐R‐98 21.83.2 and CGI‐s 4.90.8 | 127.172.2 | DRS‐R‐98 and CGI‐s score reduction | DRS‐R‐98 9.33.8 (P0.0001) and CGI‐s 2.11.1 (P0.0001) reduction from baseline; 19/22 (86.3%) and 17/22 (77.3%) showed >50% score reduction for DRS‐R‐98 and CGI‐s, respectively | EPS none; sedation requiring discontinuation 2; mild sedation 3; serious side effects none |
| Maneeton et al. (2007)[26] | OL | 17 | General hospital | Quetiapine 25100 mg/day in 1 or 2 divided doses | DRS 24.53.2 and CGI‐s 4.90.9 | 45.728.7 | 50% reduction in DRS scores | 15/17 (88.2%) had 50% reduction in DRS score; all DRS and CGI‐s scores on days 17 of the 7‐day treatment course were significantly lower than baseline scores | EPS tremor 2; hypotension 2; daytime sleepiness 13; nightmare 3; dry mouth 2; nausea 1 |
| Kim et al. (2003)[27] | OL | 12 (all male and age 64 years) | General hospital | Quetiapine 25 mg BID and increased by 25 mg every 2 days until patient maximally stabilized | DRS 18.256.05; MMSE 14.505.90; CGI‐s 3.000.43; CDT 3.25 2.77 | 93.7523.31 | Reduction in DRS, MMSE, CGI‐s, or CDT scores | All scores were statistically significantly improved from baseline; DRS at end of study 0.631.21 (P=0.03) | No dropouts due to side effects; EPS none; sedation 2; vivid dreams 1 |
| Sasaki et al. (2003)[28] | OL | 12 | General hospital | Quetiapine started at 25 or 50 mg/day and titrated to maximal clinical effect | DRS‐J 18.14.2 | 44.931.0 | Remission was DRS‐J score 12 (cutoff for delirium) and resolution of delirium symptoms | Remission occurred in all patients; mean DRS‐J score was reduced to 9.31.6 after remission | No statistically significant change from baseline in EPS; no excessive daytime sedation, anticholinergic effects, vital sign, or lab parameter changes |
| Lee et al. (2005)[29] | OL | 31 | Neurosurgery and orthopedic surgery, internal medicine, neurology and rehabilitation medicine | Flexible dose quetiapine or amisulpride | DRS‐R‐98 10.14.1 Q and 10.54.1 A | 11385.5 Q; 156.497.5 A | Reduction in DRS‐R‐98 score | DRS‐R‐98 scores significantly reduced from baseline in both groups with 3.52.6 Q (P=0.001) and 3.51.4 A (P=0.000); no difference between groups (P=0.842); 12 (80%) Q and 13 (81.3%) A had >50% reduction in DRS‐R‐98 score | No serious side effects; dropouts 5 Q and 4 A; oversedation 1 Q and 1 A; patient withdrawal 2 Q and 1 A; no statistical differences in total sleep time P=0.767 or quality of sleep P=0.984 |
Randomized Controlled Trials
Tahir et al. published a double‐blind, randomized, placebo‐controlled trial examining the efficacy and tolerability of quetiapine in the treatment of delirium.[19] Inclusion criteria were a Diagnostic and Statistical Manual of Mental Disorders‐IV diagnosis of delirium and a Delirium Rating Scale Revised 98 (DRS‐R‐98) total score of at least 15, indicating the presence of delirium. Subjects were excluded for major preexisting cognitive deficits, alcohol withdrawal, preexisting psychosis, substance dependence, inability to comply with the constraints of the trial, and concurrent medications that interact with quetiapine.
Forty‐two general medicine subjects were enrolled and randomized in the study with no difference in baseline characteristics. Patients received either placebo or quetiapine 25 mg once daily. Doses were titrated by 25 mg daily to a maximum daily dose of 175 mg in divided doses. The primary end point was DRS‐R‐98 total mean score assessed on days 1, 2, 3, 4, 7, and 10, with follow‐up assessment on day 30. Secondary outcome measures were Mini‐Mental State Examination (MMSE), the Brief Psychiatric Rating Scale, and the Clinical Global Improvement Scale. Tolerability was assessed using the Abnormal Involuntary Movements Scale and by clinical examination.
No differences in total mean DRS‐R‐98 score at individual time points reached statistical significance, but these scores improved more quickly in the quetiapine group than placebo. The secondary outcome of rate of delirium improvement on severity score did reach statistical significance; differences on mean severity scores were 0.8270.37 (P=0.026), suggesting that severity scores improved 82% more quickly than placebo. There were no significant differences between groups for any of the other secondary outcomes. Seven patients died within 30 days of entering the study (4 in the quetiapine group and 3 in the placebo group) due to serious medical conditions and not due to study medication as determined through clinical review. One patient withdrew from quetiapine due to sedation. Results of this study are limited by a small sample size, as it was underpowered to detect a statistically significant difference in the primary outcome. Other limitations include subjects who were older, with mean age of 84 years, which may have prevented titration of quetiapine, and subjects with minor cognitive deficits were included, which may have reduced the impact of treatment on the study outcomes. Additionally, strict criteria for exclusion kept those patients most likely to develop delirium from being included, limiting the studies external validity. Finally, the use of DRS‐R‐98 mean total and severity scores is subject to error as outliers in the mean could potentially skew the results.
Devlin et al. investigated the efficacy and safety of quetiapine for ICU delirium in critically ill patients in a double‐blind, randomized, placebo‐controlled trial.[20] Inclusion criteria were an Intensive Care Delirium Screening Checklist Score (ICDSC) >4, which indicates the presence of delirium, an order for as‐needed haloperidol, and tolerating enteral nutrition. Patients were excluded if they had a complicating neurologic condition, current treatment with dexmedetomidine or with medications that interact with quetiapine, baseline QTc interval >500, pregnancy, or poor prognosis. Thirty‐six critically ill subjects were randomized with no significant differences in baseline characteristics including exposure to fentanyl, haloperidol, and benzodiazepines, and in particular, midazolam. If the subject received at least 1 dose of as‐needed haloperidol in the previous 24 hours, then either placebo or quetiapine was given. Quetiapine was initiated at 50 mg every 12 hours and titrated up by 50 mg every 12 hours to a maximum dose of 200 mg every 12 hours. Patients could receive intravenous haloperidol, 1 to 10 mg up to every 2 hours as needed. The primary outcome was time to first resolution of delirium defined as time from administration of the first dose of study drug until an ICDSC 3 was first detected, indicating an absence of delirium. Secondary outcomes were total hours in delirium, total hours spent deeply sedated (Sedation‐Agitation Scale [SAS] 2) or agitated (SAS >5), episodes of subject‐initiated device removal, use of haloperidol therapy including total dose in milligrams, number of doses and number of days of therapy, the use of sedatives (converted to midazolam equivalents) and analgesics, duration of study‐drug administration, average daily and maximum study‐drug dose, length of mechanical ventilation, duration of both ICU and hospital stay, hospital mortality, and disposition of subjects after hospital discharge. Safety measures were total number of adverse and serious adverse events, episodes of somnolence, incidence of extrapyramidal symptoms, and episodes of QTc interval prolongation.
The time to first resolution of delirium was shorter with quetiapine compared to placebo (median [interquartile range]: 1.0 [0.53.0] vs 4.5 days [2.0‐7.0]; P=0.001). Resolution of delirium occurred at least once in all quetiapine patients and in 78% of placebo patients (P=0.05). Statistical significance with quetiapine was reached on the following secondary end points: time spent in delirium (36 [1287] vs 120 hours [60195], P=0.006); shorter duration of study drug (102 [84168] vs 186 hours [108228], P=0.04); lower daily study drug dose (110 [88191] vs 210 mg [116293], P=0.01); less upregulation of the study medication dose (200 [100313] vs 375 mg [25400], P=0.02); fewer hours of agitation (6 [038] vs 36 hours [1166], P=0.02); shorter duration of haloperidol therapy (3 [24] vs 4 days [38], P=0.05); and fewer days of fentanyl (0 [03] vs 4 days [19], P=0.03). There were no significant differences between groups for any other secondary outcomes. As‐needed haloperidol use in the quetiapine versus the placebo group was lower but not statistically significant (1.9 vs 4.3 mg per day; P=0.26). Two main limitations were a small sample size and strict exclusion criteria. Additionally, the primary end point of time to first resolution of delirium may be interpreted as a less rigorous measure, because there is no standard definition for delirium resolution, and delirium is a condition that waxes and wanes on its own.
A post hoc analysis by Devlin et al. was conducted on the above study to compare duration and time to first resolution of 10 delirium symptoms in 29 patients.[21] Symptoms included agitation, decreased level of consciousness, inattention, disorientation, hallucinations/delusions, hyperactivity, hypoactivity, inappropriate speech or mood, sleep/wake cycle disturbance, and symptom fluctuation. Only symptom fluctuation (P=0.009), time to first resolution of symptom fluctuation (P=0.004), time with inattention (47 vs 78 hours, P=0.025), and time with symptom fluctuation (47 vs 89 hours; P =0.04) reached statistical significance with quetiapine compared to placebo. However, quetiapine subjects had a longer time to resolution of agitation (3 vs 1 day, P=0.04) and hyperactivity (5 vs 1 day, P0.04). The authors attribute these findings to the higher use of as‐needed haloperidol in the placebo group (1.9 vs 4.3 mg per day, P=0.26). These results may also be due to a limitation in the study design, which self‐selected for agitation delirium by requiring the use of as‐needed haloperidol as inclusion criteria, as symptoms of agitation would be more likely to receive as‐needed haloperidol doses. In addition, subjects were allowed to receive 1 to 10 mg of haloperidol up to every 2 hours as needed, but there is no discussion of controlling total daily haloperidol dose in each group in the study. Although 1.9 versus 4.3 mg per day is neither statistically nor likely clinically significant, haloperidol is a treatment for delirium, so the study design would be stronger if quetiapine could be directly compared to an equivalent daily dose of haloperidol or if both groups received the same daily dose of haloperidol. Study results are also limited by the nature of a post hoc analysis, missing documentation for individual delirium symptoms, symptoms of delirium not being well characterized and subjective, and delay in enrollment of subjects.
Noncontrolled Trials
Six additional trials are included in the table of this review including 5 open label trials and 1 retrospective cohort study.[24, 25, 26, 27, 28, 29] Schwartz and Masand[24] and Lee et al.[29] compared the efficacy of quetiapine to haloperidol and amisulpride, a second‐generation antipsychotic unavailable in the United States, respectively. In a retrospective cohort of 22 general hospital patients, Schwartz and Masand found quetiapine (average dose, 211.4 mg/day) was as efficacious as haloperidol (average dose, 3.4 mg/day) in improving delirium rating scale (DRS) scores by more than 50% in 10/11 subjects in each group. Lee et al. also found quetiapine (average dose, 113.0 mg/day) to be equally efficacious to amisulpride (156.4 mg/day) in statistically significantly improving DRS‐R‐98 scores in 31 neurosurgery, orthopedic surgery, internal medicine, neurology, and rehabilitation medicine patients. Four open‐label studies tested the efficacy of flexible doses of quetiapine in a total of 63 general hospital, neurosurgery, orthopedic surgery, and oncology patients.[25, 26, 27, 28] Pae et al.,[25] Maneeton et al.,[26] and Kim et al.[27] found that quetiapine statistically significantly reduced DRS‐R‐98, Clinical Global Impression Scale‐Severity, DRS, MMSE, and Clock Drawing Test scores from baseline. Sasaki et al.[28] found all 12 general hospital patients in their study reached remission with an average daily quetiapine dose of 44.9 mg/day. Although these studies have a limited level of evidence due to their small sample sizes, study designs, and heterogeneous subjects, they do still suggest that quetiapine may effectively treat delirium in various patient populations.
DISCUSSION
Although the role of quetiapine for the treatment of delirium continues to develop, the studies evaluated here suggest that quetiapine may be effective and safe for this usage. Resolution of delirium from baseline was shown in all studies. Quetiapine resolved symptoms of delirium more quickly than placebo and had equal efficacy to other antipsychotics, haloperidol and amisulpride. Quetiapine may be most useful for patients with symptom fluctuation as opposed to agitation and hyperactivity or in those patients who may not tolerate haloperidol well. Both randomized control and open‐label trials found a low incidence of adverse effects with quetiapine. There were fewer incidences of QT prolongation and extrapyramidal symptoms, but higher a rate of somnolence with quetiapine compared to other antipsychotics in these trials. However, none of these differences in adverse effects reached statistical significance.
Drawing definitive conclusions about the efficacy of quetiapine in the treatment of delirium is difficult due to multiple study limitations. First, the body of literature is small, with only 2 randomized controlled trials, both of which had limitations. Tahir et al.[19] was underpowered and found no statistically significant difference in the primary end point, DRS‐R‐98 total mean score. Devlin et al.[20] tested the primary end point of time to first resolution of delirium, which may be interpreted as a less rigorous measure because there is no standard definition for delirium resolution. Furthermore, both randomized controlled and observational trials tested the efficacy of quetiapine using different tests, making comparison between these trials difficult. All studies included in this review were carried out in small patient populations, with the largest trial having 42 subjects, and had highly restrictive exclusion criteria limiting the generalizability of the study population to the general hospital population. Although most of the patients included in these studies are general hospital populations, Devlin et al. was performed in critically ill patients, which creates the additional limitation of having heterogeneous study populations. Last, superiority of any 1 antipsychotic is not possible given that none of these studies have done a head‐to‐head comparison of quetiapine with another atypical antipsychotic.
Given the comparable efficacy and safety of antipsychotics, cost is a relevant factor in treatment decisions. Haloperidol is supplied as either a suspension for injection or a tablet. One vial of 5 mg/mL haloperidol is $8.32. Haloperidol 5 mg tablets are approximately $0.29 each, whereas 25 mg tablets of quetiapine are approximately $3.53 each. However, these prices are for consumers and will vary depending on institutional contracts.[42]
CONCLUSION
Quetiapine appears to be an effective and safe agent for the treatment of delirium in both general medicine and ICU patients. Superiority of quetiapine over other antipsychotics for hospital‐associated delirium has not been shown due to limitations in quality and quantity of data. Large, randomized, double‐blind, active control studies with longer study durations are needed to elucidate the efficacy and niche of quetiapine in the treatment of delirium.
Acknowledgment
Disclosure: Nothing to report.
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4th ed. Washington, DC: APA; 1994.
- American Psychiatric Association. Practice guideline for the treatment of patients with delirium. Am J Psychiatry. 1999;156:1–20.
- , . Pharmacological and nonpharmacological management of delirium in critically ill patients. Neurotherapeutics. 2012;9:158–175.
- , . Delirium in cancer patients. Int Psychogeriatr. 1991;3:333–336.
- . Organic mental disorders caused by HIV: update on early‐diagnosis and treatment. Am J Psychiatry. 1990;147:696–710.
- . Post‐operative delirium. Int Psychogeriatr. 1991;3:325–332.
- , , . Delirium in terminally ill cancer patients. Am J Psychiatry. 1983;140:1048–1050.
- , , . Antipsychotics in the treatment of delirium: a systematic review. J Clin Psychiatry. 2007;68:11–21.
- , , , et al. Acute confusional states in the hospitalized elderly: incidence, risk factors and complications [abstract]. Clin Res. 1989;37:524A.
- , . Prognosis of delirium in elderly hospital patients. CMAJ. 1993;149:41–46.
- , , . Duration of delirium shortened by the correction of electrolyte imbalance. Jpn J Psychiatry Neurol. 1988;42:81–88.
- , , , et al. Clinical practice guidelines for the sustained use of sedatives and analgesics in the critically ill adult. Crit Care Med. 2002;30:119–141.
- , , , , , . Intensive care unit delirium is an independent predictor of longer hospital stay: a prospective analysis of 261 non‐ventilated patients. Crit Care. 2005;9:R375–R381.
- , , , et al. Costs associated with delirium in mechanically ventilated patients. Crit Care Med. 2004;32:955–962.
- Cook IA: Guideline Watch: Practice Guideline for the Treatment of Patients With Delirium. Arlington, VA:American Psychiatric Association, 2004. Available at: http://www.psych.org/psych_pract/treatg/pg/prac_guide.cfm. Accessed March 5, 2012.
- , . A double‐blind trial of risperdone and haloperidol for the treatment of delirium. Psychosomatics. 2004;45:297–301.
- , , , . Olanzapine vs haloperidol: treating delirium in a critical care setting. Intensive Care Med. 2004;30:444–449.
- , , , et al. Feasibility, efficacy, and safety of antipsychotics for intensive care unit delirium: the MIND randomized, placebo‐controlled trial. Crit Care Med. 2010;38:428–437.
- , , , et al. A randomized controlled trial of quetiapine versus placebo in the treatment of delirium. Psychosom Res. 2010;69:485–490.
- , , , et al. Efficacy and safety of quetiapine in critically ill patients with delirium: a prospective, multicenter, randomized, double‐blind, placebo‐controlled pilot study. Crit Care Med. 2010;38:419–427.
- , , , et al. Impact of quetiapine on resolution of individual delirium symptoms in critically ill patients with delirium: a post‐hoc analysis of a double‐blind, randomized, placebo‐controlled study. Critical Care. 2011;15:R215.
- , . Olanzapine in the treatment of delirium. Psychosomatics. 1998;39:422–430.
- , , . Comparative efficacy study of haloperidol, olanzapine and risperidone in delirium. J Psychosom Res. 2011;71:277–281.
- , . Treatment of delirium with quetiapine. J Clin Psychiatry. 2000;2:10–12.
- , , , , . A pilot trial of quetiapine for the treatment of patients with delirium. Hum Psychopharmacol Clin Exp. 2004;19:125–127.
- , , . An open‐label study of quetiapine for delirium. J Med Assoc Thai. 2007;10:2158–2163.
- , , , . Treatment of delirium in older adults with quetiapine. J Geriatr Psychiatry Neurol. 2003;16:29–31.
- , , , et al. A prospective, open‐label, flexible‐dose study of quetiapine in the treatment of delirium. J Clin Psychiatry. 2003;64:1316–1321.
- , , , et al. Amisulpride versus quetiapine for the treatment of delirium: a randomized, open prospective study. Int Clin Psychopharmacol. 2005;20:311–314.
- , , , et al. Pharmacological management of delirium in hospitalized adults‐a systematic evidence review. J Gen Intern Med. 2009;24:848–853.
- , , . Systematic review of antipsychotics for the treatment of hospital‐associated delirium in medically or surgically ill patients. Ann Pharmacother. 2006;40:1966–1973.
- , , , . Atypical antipsychotics versus haloperidol for treatment of delirium in acutely ill patients. Pharmacotherapy. 2007;27:588–594.
- , , . Delirium: prevention, treatment, and outcome studies. J Geriatr Psychiatry Neurol. 1998;11:126–137.
- , . Seroquel: biochemical profile of a potential atypical antipsychotic. Psychopharmacology. 1993;112:285–292.
- Seroquel [package insert]. Wilmington, DE: AstraZeneca Pharmaceuticals LP; December 2011.
- , , , et al. A randomized evaluation of the effects of six antipsychotic agents on QTc, in the absence and presence of metabolic inhibition. J Clin Psychopharmacol. 2004;24:62–69.
- , , , et al. Mortality risk in patients with dementia treated with antipsychotics versus other psychiatric medications. Am J Psychiatry. 2007;164:1568–1576.
- , , , et al. Antipsychotic drug use and mortality in older adults with dementia. Ann Intern Med. 2007;146:775–786.
- , , , et al. Risk of death in elderly users of conventional vs. atypical antipsychotic medications. N Engl J Med. 2005;353:2335–2341.
- , , , , . Atypical antipsychotic drugs and the risk of sudden cardiac death. N Engl J Med. 2009;360:225–235.
- , , , , , . A retrospective, exploratory, secondary analysis of the association between antipsychotic use and mortality in elderly patients with delirium. Int Psychogeriatr. 2009;21(3):588–592.
- Walgreens Co. Price your drugs Available at: http://www.walgreens.com/pharmacy/psc/drugpricing/psc_drug_pricing.jsp. Accessed December 17, 2012.
Delirium is an acute fluctuation in mental status that includes symptoms of inattention, disorganized thinking, and altered level of consciousness occurring over a short time period.[1] Prevalence of delirium ranges from 10% to 30% in the hospitalized medically ill and ranges from 40% to 60% in intensive care unit (ICU) patients who are not receiving mechanical ventilation.[2, 3] Patients experience delirium more often if they are of older age; have dementia, cancer, or acquired immune deficiency syndrome; have undergone surgery; are terminally ill; or have received multiple psychoactive medications, particularly benzodiazepines or opioids.[3, 4, 5, 6, 7] Delirium is associated with high rates of morbidity and mortality,[8] with patients more likely to develop complications such as pneumonia, decubitus ulcers, and long‐term cognitive deficits.[9, 10, 11, 12] These complications, in turn, lead to longer hospital stays and increased costs of care.[13, 14] Currently, there are no antipsychotics approved by the US Food and Drug Administration for the treatment of delirium.
Both the 2002 American Society of Critical Care Medicine[12] and the 2004 American Psychiatric Association guidelines[15] on delirium recommend haloperidol as the antipsychotic of choice due to its potent tranquilizing effect, lack of active metabolites, and limited anticholinergic and sedating side effects. However, when given intravenously or at high cumulative doses (generally >35 mg/day), haloperidol has been shown to cause QT interval prolongation potentially leading to torsades de pointes and sudden cardiac death.[15] Recent research in delirium treatment has focused on the second‐generation antipsychotics, and these studies have reported positive findings,[16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29] although no significant differences have been found compared to haloperidol.[16, 17, 18, 22, 23, 24] Systematic reviews have also failed to show a significant difference in efficacy or safety between second‐generation and first‐generation antipsychotics and unfortunately report a number of limitations including poor study designs, small sample sizes, lack of a placebo control group, exclusion of ICU patients, and weak primary outcomes.[8, 30, 31, 32] Attempting to correct for a number of these limitations, recent research with quetiapine has reported promising results in 2 controlled studies.[19, 20]
Quetiapine is a second‐generation antipsychotic with a very low affinity for dopamine receptors and a very high affinity for serotonin receptors.[33, 34] Additionally, quetiapine has a high affinity for histamine and 1‐adrenergic receptors, but a very low affinity for M1 muscarinic receptors.[34] This mechanism of action may allow quetiapine to effectively treat delirium and provide sedation without causing significant extrapyramidal side effects associated with potent dopamine receptor antagonism or precipitating delirium through muscarinic receptor antagonism. Quetiapine has a rapid absorption and a short half‐life (36 hours), giving it a quick onset of action and a fast elimination from the body.[35] Unlike haloperidol, it is only available in oral dosage forms, but can be crushed to administer via enteral tube. Common reported adverse effects include somnolence, hypotension, and dizziness. Although quetiapine has been shown to prolong QTc, Harrigan et al. reported a mean increase in QTc from baseline of 5.7 msec; there was no significant effect on this change in the presence of a metabolic inhibitor, ketoconazole.[36] The mean change in QTc from baseline with quetiapine was lower than the change reported with oral haloperidol, 5.7 msec compared to 7.1 msec, respectively. No statistical comparison was performed between these 2 drugs on this measure. Quetiapine does carry a black box warning for increased mortality in elderly patients with dementia‐related psychosis.[35] However, this risk has also been found with first‐generation antipsychotics.[37, 38, 39] A recent study found this risk of sudden cardiac death extended to adult users of both first‐ and second‐generation antipsychotics.[40] In contrast, Elie et al. found no increased risk of mortality in elderly patients with delirium receiving antipsychoticsover 90% were prescribed either haloperidol or risperidonein their nested case‐controlled study.[41] Quetiapine has demonstrated some benefit with limited side effects in studies utilizing antipsychotics for the treatment of delirium. The purpose of this review was to evaluate the role of quetiapine for the treatment of delirium.
LITERATURE REVIEW
We performed an English‐language literature search of MEDLINE and Embase databases to identify journal articles published between January 1960 and December 2012. Keywords included quetiapine, second‐generation antipsychotic, atypical antipsychotic, delirium, and agitation. The search was limited to English‐language articles and adult subjects (>18 years). Based on our review of abstracts, we included both controlled and noncontrolled trials as long as treatment of delirium was the primary focus. We eliminated case reports, foreign language articles, and poster presentations. We identified 8 trials[19, 20, 24, 25, 26, 27, 28, 29] that included 2 double‐blind, randomized, placebo‐controlled trials, which are described in the text below.[19, 20] Six other trials, 5 open‐label[25, 26, 27, 28, 29] and 1 retrospective cohort,[24] are described in Table 1.
| Study | Study Design | No. of Patients Included in Analyses | Patient Type | Treatment | Baseline Delirium Scores | Quetiapine Dose (mg/day) (MeanSD) | Efficacy Measures | Results | Side Effects |
|---|---|---|---|---|---|---|---|---|---|
| |||||||||
| Schwartz et al. (2000)[24] | RC | 22 | General hospital | Quetiapine or haloperidol flexible dose | DRS score 20.9 Q; 18.5 H | 211.4 Q; 3.4 H | >50% improvement in DRS scores | 10/11 in each treatment group had >50% improvement in DRS scores | EPS 2/11 H and 0/11 Q; mild‐to‐moderate sedation 0/11 H and 2/11 Q |
| Pae et al. (2004)[25] | OL | 22 | Neurosurgery and orthopedic surgery and oncology | Flexible dose quetiapine | DRS‐R‐98 21.83.2 and CGI‐s 4.90.8 | 127.172.2 | DRS‐R‐98 and CGI‐s score reduction | DRS‐R‐98 9.33.8 (P0.0001) and CGI‐s 2.11.1 (P0.0001) reduction from baseline; 19/22 (86.3%) and 17/22 (77.3%) showed >50% score reduction for DRS‐R‐98 and CGI‐s, respectively | EPS none; sedation requiring discontinuation 2; mild sedation 3; serious side effects none |
| Maneeton et al. (2007)[26] | OL | 17 | General hospital | Quetiapine 25100 mg/day in 1 or 2 divided doses | DRS 24.53.2 and CGI‐s 4.90.9 | 45.728.7 | 50% reduction in DRS scores | 15/17 (88.2%) had 50% reduction in DRS score; all DRS and CGI‐s scores on days 17 of the 7‐day treatment course were significantly lower than baseline scores | EPS tremor 2; hypotension 2; daytime sleepiness 13; nightmare 3; dry mouth 2; nausea 1 |
| Kim et al. (2003)[27] | OL | 12 (all male and age 64 years) | General hospital | Quetiapine 25 mg BID and increased by 25 mg every 2 days until patient maximally stabilized | DRS 18.256.05; MMSE 14.505.90; CGI‐s 3.000.43; CDT 3.25 2.77 | 93.7523.31 | Reduction in DRS, MMSE, CGI‐s, or CDT scores | All scores were statistically significantly improved from baseline; DRS at end of study 0.631.21 (P=0.03) | No dropouts due to side effects; EPS none; sedation 2; vivid dreams 1 |
| Sasaki et al. (2003)[28] | OL | 12 | General hospital | Quetiapine started at 25 or 50 mg/day and titrated to maximal clinical effect | DRS‐J 18.14.2 | 44.931.0 | Remission was DRS‐J score 12 (cutoff for delirium) and resolution of delirium symptoms | Remission occurred in all patients; mean DRS‐J score was reduced to 9.31.6 after remission | No statistically significant change from baseline in EPS; no excessive daytime sedation, anticholinergic effects, vital sign, or lab parameter changes |
| Lee et al. (2005)[29] | OL | 31 | Neurosurgery and orthopedic surgery, internal medicine, neurology and rehabilitation medicine | Flexible dose quetiapine or amisulpride | DRS‐R‐98 10.14.1 Q and 10.54.1 A | 11385.5 Q; 156.497.5 A | Reduction in DRS‐R‐98 score | DRS‐R‐98 scores significantly reduced from baseline in both groups with 3.52.6 Q (P=0.001) and 3.51.4 A (P=0.000); no difference between groups (P=0.842); 12 (80%) Q and 13 (81.3%) A had >50% reduction in DRS‐R‐98 score | No serious side effects; dropouts 5 Q and 4 A; oversedation 1 Q and 1 A; patient withdrawal 2 Q and 1 A; no statistical differences in total sleep time P=0.767 or quality of sleep P=0.984 |
Randomized Controlled Trials
Tahir et al. published a double‐blind, randomized, placebo‐controlled trial examining the efficacy and tolerability of quetiapine in the treatment of delirium.[19] Inclusion criteria were a Diagnostic and Statistical Manual of Mental Disorders‐IV diagnosis of delirium and a Delirium Rating Scale Revised 98 (DRS‐R‐98) total score of at least 15, indicating the presence of delirium. Subjects were excluded for major preexisting cognitive deficits, alcohol withdrawal, preexisting psychosis, substance dependence, inability to comply with the constraints of the trial, and concurrent medications that interact with quetiapine.
Forty‐two general medicine subjects were enrolled and randomized in the study with no difference in baseline characteristics. Patients received either placebo or quetiapine 25 mg once daily. Doses were titrated by 25 mg daily to a maximum daily dose of 175 mg in divided doses. The primary end point was DRS‐R‐98 total mean score assessed on days 1, 2, 3, 4, 7, and 10, with follow‐up assessment on day 30. Secondary outcome measures were Mini‐Mental State Examination (MMSE), the Brief Psychiatric Rating Scale, and the Clinical Global Improvement Scale. Tolerability was assessed using the Abnormal Involuntary Movements Scale and by clinical examination.
No differences in total mean DRS‐R‐98 score at individual time points reached statistical significance, but these scores improved more quickly in the quetiapine group than placebo. The secondary outcome of rate of delirium improvement on severity score did reach statistical significance; differences on mean severity scores were 0.8270.37 (P=0.026), suggesting that severity scores improved 82% more quickly than placebo. There were no significant differences between groups for any of the other secondary outcomes. Seven patients died within 30 days of entering the study (4 in the quetiapine group and 3 in the placebo group) due to serious medical conditions and not due to study medication as determined through clinical review. One patient withdrew from quetiapine due to sedation. Results of this study are limited by a small sample size, as it was underpowered to detect a statistically significant difference in the primary outcome. Other limitations include subjects who were older, with mean age of 84 years, which may have prevented titration of quetiapine, and subjects with minor cognitive deficits were included, which may have reduced the impact of treatment on the study outcomes. Additionally, strict criteria for exclusion kept those patients most likely to develop delirium from being included, limiting the studies external validity. Finally, the use of DRS‐R‐98 mean total and severity scores is subject to error as outliers in the mean could potentially skew the results.
Devlin et al. investigated the efficacy and safety of quetiapine for ICU delirium in critically ill patients in a double‐blind, randomized, placebo‐controlled trial.[20] Inclusion criteria were an Intensive Care Delirium Screening Checklist Score (ICDSC) >4, which indicates the presence of delirium, an order for as‐needed haloperidol, and tolerating enteral nutrition. Patients were excluded if they had a complicating neurologic condition, current treatment with dexmedetomidine or with medications that interact with quetiapine, baseline QTc interval >500, pregnancy, or poor prognosis. Thirty‐six critically ill subjects were randomized with no significant differences in baseline characteristics including exposure to fentanyl, haloperidol, and benzodiazepines, and in particular, midazolam. If the subject received at least 1 dose of as‐needed haloperidol in the previous 24 hours, then either placebo or quetiapine was given. Quetiapine was initiated at 50 mg every 12 hours and titrated up by 50 mg every 12 hours to a maximum dose of 200 mg every 12 hours. Patients could receive intravenous haloperidol, 1 to 10 mg up to every 2 hours as needed. The primary outcome was time to first resolution of delirium defined as time from administration of the first dose of study drug until an ICDSC 3 was first detected, indicating an absence of delirium. Secondary outcomes were total hours in delirium, total hours spent deeply sedated (Sedation‐Agitation Scale [SAS] 2) or agitated (SAS >5), episodes of subject‐initiated device removal, use of haloperidol therapy including total dose in milligrams, number of doses and number of days of therapy, the use of sedatives (converted to midazolam equivalents) and analgesics, duration of study‐drug administration, average daily and maximum study‐drug dose, length of mechanical ventilation, duration of both ICU and hospital stay, hospital mortality, and disposition of subjects after hospital discharge. Safety measures were total number of adverse and serious adverse events, episodes of somnolence, incidence of extrapyramidal symptoms, and episodes of QTc interval prolongation.
The time to first resolution of delirium was shorter with quetiapine compared to placebo (median [interquartile range]: 1.0 [0.53.0] vs 4.5 days [2.0‐7.0]; P=0.001). Resolution of delirium occurred at least once in all quetiapine patients and in 78% of placebo patients (P=0.05). Statistical significance with quetiapine was reached on the following secondary end points: time spent in delirium (36 [1287] vs 120 hours [60195], P=0.006); shorter duration of study drug (102 [84168] vs 186 hours [108228], P=0.04); lower daily study drug dose (110 [88191] vs 210 mg [116293], P=0.01); less upregulation of the study medication dose (200 [100313] vs 375 mg [25400], P=0.02); fewer hours of agitation (6 [038] vs 36 hours [1166], P=0.02); shorter duration of haloperidol therapy (3 [24] vs 4 days [38], P=0.05); and fewer days of fentanyl (0 [03] vs 4 days [19], P=0.03). There were no significant differences between groups for any other secondary outcomes. As‐needed haloperidol use in the quetiapine versus the placebo group was lower but not statistically significant (1.9 vs 4.3 mg per day; P=0.26). Two main limitations were a small sample size and strict exclusion criteria. Additionally, the primary end point of time to first resolution of delirium may be interpreted as a less rigorous measure, because there is no standard definition for delirium resolution, and delirium is a condition that waxes and wanes on its own.
A post hoc analysis by Devlin et al. was conducted on the above study to compare duration and time to first resolution of 10 delirium symptoms in 29 patients.[21] Symptoms included agitation, decreased level of consciousness, inattention, disorientation, hallucinations/delusions, hyperactivity, hypoactivity, inappropriate speech or mood, sleep/wake cycle disturbance, and symptom fluctuation. Only symptom fluctuation (P=0.009), time to first resolution of symptom fluctuation (P=0.004), time with inattention (47 vs 78 hours, P=0.025), and time with symptom fluctuation (47 vs 89 hours; P =0.04) reached statistical significance with quetiapine compared to placebo. However, quetiapine subjects had a longer time to resolution of agitation (3 vs 1 day, P=0.04) and hyperactivity (5 vs 1 day, P0.04). The authors attribute these findings to the higher use of as‐needed haloperidol in the placebo group (1.9 vs 4.3 mg per day, P=0.26). These results may also be due to a limitation in the study design, which self‐selected for agitation delirium by requiring the use of as‐needed haloperidol as inclusion criteria, as symptoms of agitation would be more likely to receive as‐needed haloperidol doses. In addition, subjects were allowed to receive 1 to 10 mg of haloperidol up to every 2 hours as needed, but there is no discussion of controlling total daily haloperidol dose in each group in the study. Although 1.9 versus 4.3 mg per day is neither statistically nor likely clinically significant, haloperidol is a treatment for delirium, so the study design would be stronger if quetiapine could be directly compared to an equivalent daily dose of haloperidol or if both groups received the same daily dose of haloperidol. Study results are also limited by the nature of a post hoc analysis, missing documentation for individual delirium symptoms, symptoms of delirium not being well characterized and subjective, and delay in enrollment of subjects.
Noncontrolled Trials
Six additional trials are included in the table of this review including 5 open label trials and 1 retrospective cohort study.[24, 25, 26, 27, 28, 29] Schwartz and Masand[24] and Lee et al.[29] compared the efficacy of quetiapine to haloperidol and amisulpride, a second‐generation antipsychotic unavailable in the United States, respectively. In a retrospective cohort of 22 general hospital patients, Schwartz and Masand found quetiapine (average dose, 211.4 mg/day) was as efficacious as haloperidol (average dose, 3.4 mg/day) in improving delirium rating scale (DRS) scores by more than 50% in 10/11 subjects in each group. Lee et al. also found quetiapine (average dose, 113.0 mg/day) to be equally efficacious to amisulpride (156.4 mg/day) in statistically significantly improving DRS‐R‐98 scores in 31 neurosurgery, orthopedic surgery, internal medicine, neurology, and rehabilitation medicine patients. Four open‐label studies tested the efficacy of flexible doses of quetiapine in a total of 63 general hospital, neurosurgery, orthopedic surgery, and oncology patients.[25, 26, 27, 28] Pae et al.,[25] Maneeton et al.,[26] and Kim et al.[27] found that quetiapine statistically significantly reduced DRS‐R‐98, Clinical Global Impression Scale‐Severity, DRS, MMSE, and Clock Drawing Test scores from baseline. Sasaki et al.[28] found all 12 general hospital patients in their study reached remission with an average daily quetiapine dose of 44.9 mg/day. Although these studies have a limited level of evidence due to their small sample sizes, study designs, and heterogeneous subjects, they do still suggest that quetiapine may effectively treat delirium in various patient populations.
DISCUSSION
Although the role of quetiapine for the treatment of delirium continues to develop, the studies evaluated here suggest that quetiapine may be effective and safe for this usage. Resolution of delirium from baseline was shown in all studies. Quetiapine resolved symptoms of delirium more quickly than placebo and had equal efficacy to other antipsychotics, haloperidol and amisulpride. Quetiapine may be most useful for patients with symptom fluctuation as opposed to agitation and hyperactivity or in those patients who may not tolerate haloperidol well. Both randomized control and open‐label trials found a low incidence of adverse effects with quetiapine. There were fewer incidences of QT prolongation and extrapyramidal symptoms, but higher a rate of somnolence with quetiapine compared to other antipsychotics in these trials. However, none of these differences in adverse effects reached statistical significance.
Drawing definitive conclusions about the efficacy of quetiapine in the treatment of delirium is difficult due to multiple study limitations. First, the body of literature is small, with only 2 randomized controlled trials, both of which had limitations. Tahir et al.[19] was underpowered and found no statistically significant difference in the primary end point, DRS‐R‐98 total mean score. Devlin et al.[20] tested the primary end point of time to first resolution of delirium, which may be interpreted as a less rigorous measure because there is no standard definition for delirium resolution. Furthermore, both randomized controlled and observational trials tested the efficacy of quetiapine using different tests, making comparison between these trials difficult. All studies included in this review were carried out in small patient populations, with the largest trial having 42 subjects, and had highly restrictive exclusion criteria limiting the generalizability of the study population to the general hospital population. Although most of the patients included in these studies are general hospital populations, Devlin et al. was performed in critically ill patients, which creates the additional limitation of having heterogeneous study populations. Last, superiority of any 1 antipsychotic is not possible given that none of these studies have done a head‐to‐head comparison of quetiapine with another atypical antipsychotic.
Given the comparable efficacy and safety of antipsychotics, cost is a relevant factor in treatment decisions. Haloperidol is supplied as either a suspension for injection or a tablet. One vial of 5 mg/mL haloperidol is $8.32. Haloperidol 5 mg tablets are approximately $0.29 each, whereas 25 mg tablets of quetiapine are approximately $3.53 each. However, these prices are for consumers and will vary depending on institutional contracts.[42]
CONCLUSION
Quetiapine appears to be an effective and safe agent for the treatment of delirium in both general medicine and ICU patients. Superiority of quetiapine over other antipsychotics for hospital‐associated delirium has not been shown due to limitations in quality and quantity of data. Large, randomized, double‐blind, active control studies with longer study durations are needed to elucidate the efficacy and niche of quetiapine in the treatment of delirium.
Acknowledgment
Disclosure: Nothing to report.
Delirium is an acute fluctuation in mental status that includes symptoms of inattention, disorganized thinking, and altered level of consciousness occurring over a short time period.[1] Prevalence of delirium ranges from 10% to 30% in the hospitalized medically ill and ranges from 40% to 60% in intensive care unit (ICU) patients who are not receiving mechanical ventilation.[2, 3] Patients experience delirium more often if they are of older age; have dementia, cancer, or acquired immune deficiency syndrome; have undergone surgery; are terminally ill; or have received multiple psychoactive medications, particularly benzodiazepines or opioids.[3, 4, 5, 6, 7] Delirium is associated with high rates of morbidity and mortality,[8] with patients more likely to develop complications such as pneumonia, decubitus ulcers, and long‐term cognitive deficits.[9, 10, 11, 12] These complications, in turn, lead to longer hospital stays and increased costs of care.[13, 14] Currently, there are no antipsychotics approved by the US Food and Drug Administration for the treatment of delirium.
Both the 2002 American Society of Critical Care Medicine[12] and the 2004 American Psychiatric Association guidelines[15] on delirium recommend haloperidol as the antipsychotic of choice due to its potent tranquilizing effect, lack of active metabolites, and limited anticholinergic and sedating side effects. However, when given intravenously or at high cumulative doses (generally >35 mg/day), haloperidol has been shown to cause QT interval prolongation potentially leading to torsades de pointes and sudden cardiac death.[15] Recent research in delirium treatment has focused on the second‐generation antipsychotics, and these studies have reported positive findings,[16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29] although no significant differences have been found compared to haloperidol.[16, 17, 18, 22, 23, 24] Systematic reviews have also failed to show a significant difference in efficacy or safety between second‐generation and first‐generation antipsychotics and unfortunately report a number of limitations including poor study designs, small sample sizes, lack of a placebo control group, exclusion of ICU patients, and weak primary outcomes.[8, 30, 31, 32] Attempting to correct for a number of these limitations, recent research with quetiapine has reported promising results in 2 controlled studies.[19, 20]
Quetiapine is a second‐generation antipsychotic with a very low affinity for dopamine receptors and a very high affinity for serotonin receptors.[33, 34] Additionally, quetiapine has a high affinity for histamine and 1‐adrenergic receptors, but a very low affinity for M1 muscarinic receptors.[34] This mechanism of action may allow quetiapine to effectively treat delirium and provide sedation without causing significant extrapyramidal side effects associated with potent dopamine receptor antagonism or precipitating delirium through muscarinic receptor antagonism. Quetiapine has a rapid absorption and a short half‐life (36 hours), giving it a quick onset of action and a fast elimination from the body.[35] Unlike haloperidol, it is only available in oral dosage forms, but can be crushed to administer via enteral tube. Common reported adverse effects include somnolence, hypotension, and dizziness. Although quetiapine has been shown to prolong QTc, Harrigan et al. reported a mean increase in QTc from baseline of 5.7 msec; there was no significant effect on this change in the presence of a metabolic inhibitor, ketoconazole.[36] The mean change in QTc from baseline with quetiapine was lower than the change reported with oral haloperidol, 5.7 msec compared to 7.1 msec, respectively. No statistical comparison was performed between these 2 drugs on this measure. Quetiapine does carry a black box warning for increased mortality in elderly patients with dementia‐related psychosis.[35] However, this risk has also been found with first‐generation antipsychotics.[37, 38, 39] A recent study found this risk of sudden cardiac death extended to adult users of both first‐ and second‐generation antipsychotics.[40] In contrast, Elie et al. found no increased risk of mortality in elderly patients with delirium receiving antipsychoticsover 90% were prescribed either haloperidol or risperidonein their nested case‐controlled study.[41] Quetiapine has demonstrated some benefit with limited side effects in studies utilizing antipsychotics for the treatment of delirium. The purpose of this review was to evaluate the role of quetiapine for the treatment of delirium.
LITERATURE REVIEW
We performed an English‐language literature search of MEDLINE and Embase databases to identify journal articles published between January 1960 and December 2012. Keywords included quetiapine, second‐generation antipsychotic, atypical antipsychotic, delirium, and agitation. The search was limited to English‐language articles and adult subjects (>18 years). Based on our review of abstracts, we included both controlled and noncontrolled trials as long as treatment of delirium was the primary focus. We eliminated case reports, foreign language articles, and poster presentations. We identified 8 trials[19, 20, 24, 25, 26, 27, 28, 29] that included 2 double‐blind, randomized, placebo‐controlled trials, which are described in the text below.[19, 20] Six other trials, 5 open‐label[25, 26, 27, 28, 29] and 1 retrospective cohort,[24] are described in Table 1.
| Study | Study Design | No. of Patients Included in Analyses | Patient Type | Treatment | Baseline Delirium Scores | Quetiapine Dose (mg/day) (MeanSD) | Efficacy Measures | Results | Side Effects |
|---|---|---|---|---|---|---|---|---|---|
| |||||||||
| Schwartz et al. (2000)[24] | RC | 22 | General hospital | Quetiapine or haloperidol flexible dose | DRS score 20.9 Q; 18.5 H | 211.4 Q; 3.4 H | >50% improvement in DRS scores | 10/11 in each treatment group had >50% improvement in DRS scores | EPS 2/11 H and 0/11 Q; mild‐to‐moderate sedation 0/11 H and 2/11 Q |
| Pae et al. (2004)[25] | OL | 22 | Neurosurgery and orthopedic surgery and oncology | Flexible dose quetiapine | DRS‐R‐98 21.83.2 and CGI‐s 4.90.8 | 127.172.2 | DRS‐R‐98 and CGI‐s score reduction | DRS‐R‐98 9.33.8 (P0.0001) and CGI‐s 2.11.1 (P0.0001) reduction from baseline; 19/22 (86.3%) and 17/22 (77.3%) showed >50% score reduction for DRS‐R‐98 and CGI‐s, respectively | EPS none; sedation requiring discontinuation 2; mild sedation 3; serious side effects none |
| Maneeton et al. (2007)[26] | OL | 17 | General hospital | Quetiapine 25100 mg/day in 1 or 2 divided doses | DRS 24.53.2 and CGI‐s 4.90.9 | 45.728.7 | 50% reduction in DRS scores | 15/17 (88.2%) had 50% reduction in DRS score; all DRS and CGI‐s scores on days 17 of the 7‐day treatment course were significantly lower than baseline scores | EPS tremor 2; hypotension 2; daytime sleepiness 13; nightmare 3; dry mouth 2; nausea 1 |
| Kim et al. (2003)[27] | OL | 12 (all male and age 64 years) | General hospital | Quetiapine 25 mg BID and increased by 25 mg every 2 days until patient maximally stabilized | DRS 18.256.05; MMSE 14.505.90; CGI‐s 3.000.43; CDT 3.25 2.77 | 93.7523.31 | Reduction in DRS, MMSE, CGI‐s, or CDT scores | All scores were statistically significantly improved from baseline; DRS at end of study 0.631.21 (P=0.03) | No dropouts due to side effects; EPS none; sedation 2; vivid dreams 1 |
| Sasaki et al. (2003)[28] | OL | 12 | General hospital | Quetiapine started at 25 or 50 mg/day and titrated to maximal clinical effect | DRS‐J 18.14.2 | 44.931.0 | Remission was DRS‐J score 12 (cutoff for delirium) and resolution of delirium symptoms | Remission occurred in all patients; mean DRS‐J score was reduced to 9.31.6 after remission | No statistically significant change from baseline in EPS; no excessive daytime sedation, anticholinergic effects, vital sign, or lab parameter changes |
| Lee et al. (2005)[29] | OL | 31 | Neurosurgery and orthopedic surgery, internal medicine, neurology and rehabilitation medicine | Flexible dose quetiapine or amisulpride | DRS‐R‐98 10.14.1 Q and 10.54.1 A | 11385.5 Q; 156.497.5 A | Reduction in DRS‐R‐98 score | DRS‐R‐98 scores significantly reduced from baseline in both groups with 3.52.6 Q (P=0.001) and 3.51.4 A (P=0.000); no difference between groups (P=0.842); 12 (80%) Q and 13 (81.3%) A had >50% reduction in DRS‐R‐98 score | No serious side effects; dropouts 5 Q and 4 A; oversedation 1 Q and 1 A; patient withdrawal 2 Q and 1 A; no statistical differences in total sleep time P=0.767 or quality of sleep P=0.984 |
Randomized Controlled Trials
Tahir et al. published a double‐blind, randomized, placebo‐controlled trial examining the efficacy and tolerability of quetiapine in the treatment of delirium.[19] Inclusion criteria were a Diagnostic and Statistical Manual of Mental Disorders‐IV diagnosis of delirium and a Delirium Rating Scale Revised 98 (DRS‐R‐98) total score of at least 15, indicating the presence of delirium. Subjects were excluded for major preexisting cognitive deficits, alcohol withdrawal, preexisting psychosis, substance dependence, inability to comply with the constraints of the trial, and concurrent medications that interact with quetiapine.
Forty‐two general medicine subjects were enrolled and randomized in the study with no difference in baseline characteristics. Patients received either placebo or quetiapine 25 mg once daily. Doses were titrated by 25 mg daily to a maximum daily dose of 175 mg in divided doses. The primary end point was DRS‐R‐98 total mean score assessed on days 1, 2, 3, 4, 7, and 10, with follow‐up assessment on day 30. Secondary outcome measures were Mini‐Mental State Examination (MMSE), the Brief Psychiatric Rating Scale, and the Clinical Global Improvement Scale. Tolerability was assessed using the Abnormal Involuntary Movements Scale and by clinical examination.
No differences in total mean DRS‐R‐98 score at individual time points reached statistical significance, but these scores improved more quickly in the quetiapine group than placebo. The secondary outcome of rate of delirium improvement on severity score did reach statistical significance; differences on mean severity scores were 0.8270.37 (P=0.026), suggesting that severity scores improved 82% more quickly than placebo. There were no significant differences between groups for any of the other secondary outcomes. Seven patients died within 30 days of entering the study (4 in the quetiapine group and 3 in the placebo group) due to serious medical conditions and not due to study medication as determined through clinical review. One patient withdrew from quetiapine due to sedation. Results of this study are limited by a small sample size, as it was underpowered to detect a statistically significant difference in the primary outcome. Other limitations include subjects who were older, with mean age of 84 years, which may have prevented titration of quetiapine, and subjects with minor cognitive deficits were included, which may have reduced the impact of treatment on the study outcomes. Additionally, strict criteria for exclusion kept those patients most likely to develop delirium from being included, limiting the studies external validity. Finally, the use of DRS‐R‐98 mean total and severity scores is subject to error as outliers in the mean could potentially skew the results.
Devlin et al. investigated the efficacy and safety of quetiapine for ICU delirium in critically ill patients in a double‐blind, randomized, placebo‐controlled trial.[20] Inclusion criteria were an Intensive Care Delirium Screening Checklist Score (ICDSC) >4, which indicates the presence of delirium, an order for as‐needed haloperidol, and tolerating enteral nutrition. Patients were excluded if they had a complicating neurologic condition, current treatment with dexmedetomidine or with medications that interact with quetiapine, baseline QTc interval >500, pregnancy, or poor prognosis. Thirty‐six critically ill subjects were randomized with no significant differences in baseline characteristics including exposure to fentanyl, haloperidol, and benzodiazepines, and in particular, midazolam. If the subject received at least 1 dose of as‐needed haloperidol in the previous 24 hours, then either placebo or quetiapine was given. Quetiapine was initiated at 50 mg every 12 hours and titrated up by 50 mg every 12 hours to a maximum dose of 200 mg every 12 hours. Patients could receive intravenous haloperidol, 1 to 10 mg up to every 2 hours as needed. The primary outcome was time to first resolution of delirium defined as time from administration of the first dose of study drug until an ICDSC 3 was first detected, indicating an absence of delirium. Secondary outcomes were total hours in delirium, total hours spent deeply sedated (Sedation‐Agitation Scale [SAS] 2) or agitated (SAS >5), episodes of subject‐initiated device removal, use of haloperidol therapy including total dose in milligrams, number of doses and number of days of therapy, the use of sedatives (converted to midazolam equivalents) and analgesics, duration of study‐drug administration, average daily and maximum study‐drug dose, length of mechanical ventilation, duration of both ICU and hospital stay, hospital mortality, and disposition of subjects after hospital discharge. Safety measures were total number of adverse and serious adverse events, episodes of somnolence, incidence of extrapyramidal symptoms, and episodes of QTc interval prolongation.
The time to first resolution of delirium was shorter with quetiapine compared to placebo (median [interquartile range]: 1.0 [0.53.0] vs 4.5 days [2.0‐7.0]; P=0.001). Resolution of delirium occurred at least once in all quetiapine patients and in 78% of placebo patients (P=0.05). Statistical significance with quetiapine was reached on the following secondary end points: time spent in delirium (36 [1287] vs 120 hours [60195], P=0.006); shorter duration of study drug (102 [84168] vs 186 hours [108228], P=0.04); lower daily study drug dose (110 [88191] vs 210 mg [116293], P=0.01); less upregulation of the study medication dose (200 [100313] vs 375 mg [25400], P=0.02); fewer hours of agitation (6 [038] vs 36 hours [1166], P=0.02); shorter duration of haloperidol therapy (3 [24] vs 4 days [38], P=0.05); and fewer days of fentanyl (0 [03] vs 4 days [19], P=0.03). There were no significant differences between groups for any other secondary outcomes. As‐needed haloperidol use in the quetiapine versus the placebo group was lower but not statistically significant (1.9 vs 4.3 mg per day; P=0.26). Two main limitations were a small sample size and strict exclusion criteria. Additionally, the primary end point of time to first resolution of delirium may be interpreted as a less rigorous measure, because there is no standard definition for delirium resolution, and delirium is a condition that waxes and wanes on its own.
A post hoc analysis by Devlin et al. was conducted on the above study to compare duration and time to first resolution of 10 delirium symptoms in 29 patients.[21] Symptoms included agitation, decreased level of consciousness, inattention, disorientation, hallucinations/delusions, hyperactivity, hypoactivity, inappropriate speech or mood, sleep/wake cycle disturbance, and symptom fluctuation. Only symptom fluctuation (P=0.009), time to first resolution of symptom fluctuation (P=0.004), time with inattention (47 vs 78 hours, P=0.025), and time with symptom fluctuation (47 vs 89 hours; P =0.04) reached statistical significance with quetiapine compared to placebo. However, quetiapine subjects had a longer time to resolution of agitation (3 vs 1 day, P=0.04) and hyperactivity (5 vs 1 day, P0.04). The authors attribute these findings to the higher use of as‐needed haloperidol in the placebo group (1.9 vs 4.3 mg per day, P=0.26). These results may also be due to a limitation in the study design, which self‐selected for agitation delirium by requiring the use of as‐needed haloperidol as inclusion criteria, as symptoms of agitation would be more likely to receive as‐needed haloperidol doses. In addition, subjects were allowed to receive 1 to 10 mg of haloperidol up to every 2 hours as needed, but there is no discussion of controlling total daily haloperidol dose in each group in the study. Although 1.9 versus 4.3 mg per day is neither statistically nor likely clinically significant, haloperidol is a treatment for delirium, so the study design would be stronger if quetiapine could be directly compared to an equivalent daily dose of haloperidol or if both groups received the same daily dose of haloperidol. Study results are also limited by the nature of a post hoc analysis, missing documentation for individual delirium symptoms, symptoms of delirium not being well characterized and subjective, and delay in enrollment of subjects.
Noncontrolled Trials
Six additional trials are included in the table of this review including 5 open label trials and 1 retrospective cohort study.[24, 25, 26, 27, 28, 29] Schwartz and Masand[24] and Lee et al.[29] compared the efficacy of quetiapine to haloperidol and amisulpride, a second‐generation antipsychotic unavailable in the United States, respectively. In a retrospective cohort of 22 general hospital patients, Schwartz and Masand found quetiapine (average dose, 211.4 mg/day) was as efficacious as haloperidol (average dose, 3.4 mg/day) in improving delirium rating scale (DRS) scores by more than 50% in 10/11 subjects in each group. Lee et al. also found quetiapine (average dose, 113.0 mg/day) to be equally efficacious to amisulpride (156.4 mg/day) in statistically significantly improving DRS‐R‐98 scores in 31 neurosurgery, orthopedic surgery, internal medicine, neurology, and rehabilitation medicine patients. Four open‐label studies tested the efficacy of flexible doses of quetiapine in a total of 63 general hospital, neurosurgery, orthopedic surgery, and oncology patients.[25, 26, 27, 28] Pae et al.,[25] Maneeton et al.,[26] and Kim et al.[27] found that quetiapine statistically significantly reduced DRS‐R‐98, Clinical Global Impression Scale‐Severity, DRS, MMSE, and Clock Drawing Test scores from baseline. Sasaki et al.[28] found all 12 general hospital patients in their study reached remission with an average daily quetiapine dose of 44.9 mg/day. Although these studies have a limited level of evidence due to their small sample sizes, study designs, and heterogeneous subjects, they do still suggest that quetiapine may effectively treat delirium in various patient populations.
DISCUSSION
Although the role of quetiapine for the treatment of delirium continues to develop, the studies evaluated here suggest that quetiapine may be effective and safe for this usage. Resolution of delirium from baseline was shown in all studies. Quetiapine resolved symptoms of delirium more quickly than placebo and had equal efficacy to other antipsychotics, haloperidol and amisulpride. Quetiapine may be most useful for patients with symptom fluctuation as opposed to agitation and hyperactivity or in those patients who may not tolerate haloperidol well. Both randomized control and open‐label trials found a low incidence of adverse effects with quetiapine. There were fewer incidences of QT prolongation and extrapyramidal symptoms, but higher a rate of somnolence with quetiapine compared to other antipsychotics in these trials. However, none of these differences in adverse effects reached statistical significance.
Drawing definitive conclusions about the efficacy of quetiapine in the treatment of delirium is difficult due to multiple study limitations. First, the body of literature is small, with only 2 randomized controlled trials, both of which had limitations. Tahir et al.[19] was underpowered and found no statistically significant difference in the primary end point, DRS‐R‐98 total mean score. Devlin et al.[20] tested the primary end point of time to first resolution of delirium, which may be interpreted as a less rigorous measure because there is no standard definition for delirium resolution. Furthermore, both randomized controlled and observational trials tested the efficacy of quetiapine using different tests, making comparison between these trials difficult. All studies included in this review were carried out in small patient populations, with the largest trial having 42 subjects, and had highly restrictive exclusion criteria limiting the generalizability of the study population to the general hospital population. Although most of the patients included in these studies are general hospital populations, Devlin et al. was performed in critically ill patients, which creates the additional limitation of having heterogeneous study populations. Last, superiority of any 1 antipsychotic is not possible given that none of these studies have done a head‐to‐head comparison of quetiapine with another atypical antipsychotic.
Given the comparable efficacy and safety of antipsychotics, cost is a relevant factor in treatment decisions. Haloperidol is supplied as either a suspension for injection or a tablet. One vial of 5 mg/mL haloperidol is $8.32. Haloperidol 5 mg tablets are approximately $0.29 each, whereas 25 mg tablets of quetiapine are approximately $3.53 each. However, these prices are for consumers and will vary depending on institutional contracts.[42]
CONCLUSION
Quetiapine appears to be an effective and safe agent for the treatment of delirium in both general medicine and ICU patients. Superiority of quetiapine over other antipsychotics for hospital‐associated delirium has not been shown due to limitations in quality and quantity of data. Large, randomized, double‐blind, active control studies with longer study durations are needed to elucidate the efficacy and niche of quetiapine in the treatment of delirium.
Acknowledgment
Disclosure: Nothing to report.
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4th ed. Washington, DC: APA; 1994.
- American Psychiatric Association. Practice guideline for the treatment of patients with delirium. Am J Psychiatry. 1999;156:1–20.
- , . Pharmacological and nonpharmacological management of delirium in critically ill patients. Neurotherapeutics. 2012;9:158–175.
- , . Delirium in cancer patients. Int Psychogeriatr. 1991;3:333–336.
- . Organic mental disorders caused by HIV: update on early‐diagnosis and treatment. Am J Psychiatry. 1990;147:696–710.
- . Post‐operative delirium. Int Psychogeriatr. 1991;3:325–332.
- , , . Delirium in terminally ill cancer patients. Am J Psychiatry. 1983;140:1048–1050.
- , , . Antipsychotics in the treatment of delirium: a systematic review. J Clin Psychiatry. 2007;68:11–21.
- , , , et al. Acute confusional states in the hospitalized elderly: incidence, risk factors and complications [abstract]. Clin Res. 1989;37:524A.
- , . Prognosis of delirium in elderly hospital patients. CMAJ. 1993;149:41–46.
- , , . Duration of delirium shortened by the correction of electrolyte imbalance. Jpn J Psychiatry Neurol. 1988;42:81–88.
- , , , et al. Clinical practice guidelines for the sustained use of sedatives and analgesics in the critically ill adult. Crit Care Med. 2002;30:119–141.
- , , , , , . Intensive care unit delirium is an independent predictor of longer hospital stay: a prospective analysis of 261 non‐ventilated patients. Crit Care. 2005;9:R375–R381.
- , , , et al. Costs associated with delirium in mechanically ventilated patients. Crit Care Med. 2004;32:955–962.
- Cook IA: Guideline Watch: Practice Guideline for the Treatment of Patients With Delirium. Arlington, VA:American Psychiatric Association, 2004. Available at: http://www.psych.org/psych_pract/treatg/pg/prac_guide.cfm. Accessed March 5, 2012.
- , . A double‐blind trial of risperdone and haloperidol for the treatment of delirium. Psychosomatics. 2004;45:297–301.
- , , , . Olanzapine vs haloperidol: treating delirium in a critical care setting. Intensive Care Med. 2004;30:444–449.
- , , , et al. Feasibility, efficacy, and safety of antipsychotics for intensive care unit delirium: the MIND randomized, placebo‐controlled trial. Crit Care Med. 2010;38:428–437.
- , , , et al. A randomized controlled trial of quetiapine versus placebo in the treatment of delirium. Psychosom Res. 2010;69:485–490.
- , , , et al. Efficacy and safety of quetiapine in critically ill patients with delirium: a prospective, multicenter, randomized, double‐blind, placebo‐controlled pilot study. Crit Care Med. 2010;38:419–427.
- , , , et al. Impact of quetiapine on resolution of individual delirium symptoms in critically ill patients with delirium: a post‐hoc analysis of a double‐blind, randomized, placebo‐controlled study. Critical Care. 2011;15:R215.
- , . Olanzapine in the treatment of delirium. Psychosomatics. 1998;39:422–430.
- , , . Comparative efficacy study of haloperidol, olanzapine and risperidone in delirium. J Psychosom Res. 2011;71:277–281.
- , . Treatment of delirium with quetiapine. J Clin Psychiatry. 2000;2:10–12.
- , , , , . A pilot trial of quetiapine for the treatment of patients with delirium. Hum Psychopharmacol Clin Exp. 2004;19:125–127.
- , , . An open‐label study of quetiapine for delirium. J Med Assoc Thai. 2007;10:2158–2163.
- , , , . Treatment of delirium in older adults with quetiapine. J Geriatr Psychiatry Neurol. 2003;16:29–31.
- , , , et al. A prospective, open‐label, flexible‐dose study of quetiapine in the treatment of delirium. J Clin Psychiatry. 2003;64:1316–1321.
- , , , et al. Amisulpride versus quetiapine for the treatment of delirium: a randomized, open prospective study. Int Clin Psychopharmacol. 2005;20:311–314.
- , , , et al. Pharmacological management of delirium in hospitalized adults‐a systematic evidence review. J Gen Intern Med. 2009;24:848–853.
- , , . Systematic review of antipsychotics for the treatment of hospital‐associated delirium in medically or surgically ill patients. Ann Pharmacother. 2006;40:1966–1973.
- , , , . Atypical antipsychotics versus haloperidol for treatment of delirium in acutely ill patients. Pharmacotherapy. 2007;27:588–594.
- , , . Delirium: prevention, treatment, and outcome studies. J Geriatr Psychiatry Neurol. 1998;11:126–137.
- , . Seroquel: biochemical profile of a potential atypical antipsychotic. Psychopharmacology. 1993;112:285–292.
- Seroquel [package insert]. Wilmington, DE: AstraZeneca Pharmaceuticals LP; December 2011.
- , , , et al. A randomized evaluation of the effects of six antipsychotic agents on QTc, in the absence and presence of metabolic inhibition. J Clin Psychopharmacol. 2004;24:62–69.
- , , , et al. Mortality risk in patients with dementia treated with antipsychotics versus other psychiatric medications. Am J Psychiatry. 2007;164:1568–1576.
- , , , et al. Antipsychotic drug use and mortality in older adults with dementia. Ann Intern Med. 2007;146:775–786.
- , , , et al. Risk of death in elderly users of conventional vs. atypical antipsychotic medications. N Engl J Med. 2005;353:2335–2341.
- , , , , . Atypical antipsychotic drugs and the risk of sudden cardiac death. N Engl J Med. 2009;360:225–235.
- , , , , , . A retrospective, exploratory, secondary analysis of the association between antipsychotic use and mortality in elderly patients with delirium. Int Psychogeriatr. 2009;21(3):588–592.
- Walgreens Co. Price your drugs Available at: http://www.walgreens.com/pharmacy/psc/drugpricing/psc_drug_pricing.jsp. Accessed December 17, 2012.
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4th ed. Washington, DC: APA; 1994.
- American Psychiatric Association. Practice guideline for the treatment of patients with delirium. Am J Psychiatry. 1999;156:1–20.
- , . Pharmacological and nonpharmacological management of delirium in critically ill patients. Neurotherapeutics. 2012;9:158–175.
- , . Delirium in cancer patients. Int Psychogeriatr. 1991;3:333–336.
- . Organic mental disorders caused by HIV: update on early‐diagnosis and treatment. Am J Psychiatry. 1990;147:696–710.
- . Post‐operative delirium. Int Psychogeriatr. 1991;3:325–332.
- , , . Delirium in terminally ill cancer patients. Am J Psychiatry. 1983;140:1048–1050.
- , , . Antipsychotics in the treatment of delirium: a systematic review. J Clin Psychiatry. 2007;68:11–21.
- , , , et al. Acute confusional states in the hospitalized elderly: incidence, risk factors and complications [abstract]. Clin Res. 1989;37:524A.
- , . Prognosis of delirium in elderly hospital patients. CMAJ. 1993;149:41–46.
- , , . Duration of delirium shortened by the correction of electrolyte imbalance. Jpn J Psychiatry Neurol. 1988;42:81–88.
- , , , et al. Clinical practice guidelines for the sustained use of sedatives and analgesics in the critically ill adult. Crit Care Med. 2002;30:119–141.
- , , , , , . Intensive care unit delirium is an independent predictor of longer hospital stay: a prospective analysis of 261 non‐ventilated patients. Crit Care. 2005;9:R375–R381.
- , , , et al. Costs associated with delirium in mechanically ventilated patients. Crit Care Med. 2004;32:955–962.
- Cook IA: Guideline Watch: Practice Guideline for the Treatment of Patients With Delirium. Arlington, VA:American Psychiatric Association, 2004. Available at: http://www.psych.org/psych_pract/treatg/pg/prac_guide.cfm. Accessed March 5, 2012.
- , . A double‐blind trial of risperdone and haloperidol for the treatment of delirium. Psychosomatics. 2004;45:297–301.
- , , , . Olanzapine vs haloperidol: treating delirium in a critical care setting. Intensive Care Med. 2004;30:444–449.
- , , , et al. Feasibility, efficacy, and safety of antipsychotics for intensive care unit delirium: the MIND randomized, placebo‐controlled trial. Crit Care Med. 2010;38:428–437.
- , , , et al. A randomized controlled trial of quetiapine versus placebo in the treatment of delirium. Psychosom Res. 2010;69:485–490.
- , , , et al. Efficacy and safety of quetiapine in critically ill patients with delirium: a prospective, multicenter, randomized, double‐blind, placebo‐controlled pilot study. Crit Care Med. 2010;38:419–427.
- , , , et al. Impact of quetiapine on resolution of individual delirium symptoms in critically ill patients with delirium: a post‐hoc analysis of a double‐blind, randomized, placebo‐controlled study. Critical Care. 2011;15:R215.
- , . Olanzapine in the treatment of delirium. Psychosomatics. 1998;39:422–430.
- , , . Comparative efficacy study of haloperidol, olanzapine and risperidone in delirium. J Psychosom Res. 2011;71:277–281.
- , . Treatment of delirium with quetiapine. J Clin Psychiatry. 2000;2:10–12.
- , , , , . A pilot trial of quetiapine for the treatment of patients with delirium. Hum Psychopharmacol Clin Exp. 2004;19:125–127.
- , , . An open‐label study of quetiapine for delirium. J Med Assoc Thai. 2007;10:2158–2163.
- , , , . Treatment of delirium in older adults with quetiapine. J Geriatr Psychiatry Neurol. 2003;16:29–31.
- , , , et al. A prospective, open‐label, flexible‐dose study of quetiapine in the treatment of delirium. J Clin Psychiatry. 2003;64:1316–1321.
- , , , et al. Amisulpride versus quetiapine for the treatment of delirium: a randomized, open prospective study. Int Clin Psychopharmacol. 2005;20:311–314.
- , , , et al. Pharmacological management of delirium in hospitalized adults‐a systematic evidence review. J Gen Intern Med. 2009;24:848–853.
- , , . Systematic review of antipsychotics for the treatment of hospital‐associated delirium in medically or surgically ill patients. Ann Pharmacother. 2006;40:1966–1973.
- , , , . Atypical antipsychotics versus haloperidol for treatment of delirium in acutely ill patients. Pharmacotherapy. 2007;27:588–594.
- , , . Delirium: prevention, treatment, and outcome studies. J Geriatr Psychiatry Neurol. 1998;11:126–137.
- , . Seroquel: biochemical profile of a potential atypical antipsychotic. Psychopharmacology. 1993;112:285–292.
- Seroquel [package insert]. Wilmington, DE: AstraZeneca Pharmaceuticals LP; December 2011.
- , , , et al. A randomized evaluation of the effects of six antipsychotic agents on QTc, in the absence and presence of metabolic inhibition. J Clin Psychopharmacol. 2004;24:62–69.
- , , , et al. Mortality risk in patients with dementia treated with antipsychotics versus other psychiatric medications. Am J Psychiatry. 2007;164:1568–1576.
- , , , et al. Antipsychotic drug use and mortality in older adults with dementia. Ann Intern Med. 2007;146:775–786.
- , , , et al. Risk of death in elderly users of conventional vs. atypical antipsychotic medications. N Engl J Med. 2005;353:2335–2341.
- , , , , . Atypical antipsychotic drugs and the risk of sudden cardiac death. N Engl J Med. 2009;360:225–235.
- , , , , , . A retrospective, exploratory, secondary analysis of the association between antipsychotic use and mortality in elderly patients with delirium. Int Psychogeriatr. 2009;21(3):588–592.
- Walgreens Co. Price your drugs Available at: http://www.walgreens.com/pharmacy/psc/drugpricing/psc_drug_pricing.jsp. Accessed December 17, 2012.
Erratum
The author list for the following article, Survey of Overnight Academic Hospitalist Supervision of Trainees, by Jeanne M. Farnan, Alfred Burger, Romsai T. Boonyasai, Luci Leykum, Rebecca Harrison, Julie Machulsky, Vikas Parekh, Bradley A. Sharpe, Anneliese M. Schleyer and Vineet M. Arora, for the SGIM Housestaff Oversight Subcommittee that published in Volume 7, Issue 7, pages 521523 of the Journal of Hospital Medicine contained an error in the spelling of an author name. Romsai T. Boonayasai should be corrected to Romsai T. Boonyasai.
The author list for the following article, Survey of Overnight Academic Hospitalist Supervision of Trainees, by Jeanne M. Farnan, Alfred Burger, Romsai T. Boonyasai, Luci Leykum, Rebecca Harrison, Julie Machulsky, Vikas Parekh, Bradley A. Sharpe, Anneliese M. Schleyer and Vineet M. Arora, for the SGIM Housestaff Oversight Subcommittee that published in Volume 7, Issue 7, pages 521523 of the Journal of Hospital Medicine contained an error in the spelling of an author name. Romsai T. Boonayasai should be corrected to Romsai T. Boonyasai.
The author list for the following article, Survey of Overnight Academic Hospitalist Supervision of Trainees, by Jeanne M. Farnan, Alfred Burger, Romsai T. Boonyasai, Luci Leykum, Rebecca Harrison, Julie Machulsky, Vikas Parekh, Bradley A. Sharpe, Anneliese M. Schleyer and Vineet M. Arora, for the SGIM Housestaff Oversight Subcommittee that published in Volume 7, Issue 7, pages 521523 of the Journal of Hospital Medicine contained an error in the spelling of an author name. Romsai T. Boonayasai should be corrected to Romsai T. Boonyasai.
ONLINE EXCLUSIVE: TKTK
Enter text here
Enter text here
Enter text here
ONLINE EXCLUSIVE: Society of Physician Entrepreneurs Co-Founder Talks about MD Career Changes
Click here to listen to Dr. Hausfeld, managing director of FMS Financial Solutions, Greenbelt, Md., co-founder and treasurer of the Society of Physician Entrepreneurs.
Click here to listen to Dr. Hausfeld, managing director of FMS Financial Solutions, Greenbelt, Md., co-founder and treasurer of the Society of Physician Entrepreneurs.
Click here to listen to Dr. Hausfeld, managing director of FMS Financial Solutions, Greenbelt, Md., co-founder and treasurer of the Society of Physician Entrepreneurs.
ONLINE EXCLUSIVE: TKTK
Enter text here
Enter text here
Enter text here
ONLINE EXCLUSIVE: CMO Discusses 7-on/7-off Schedule at Swedish Hospital Medicine in Seattle
Click here to listen to Dr. Danielsson
Click here to listen to Dr. Danielsson
Click here to listen to Dr. Danielsson
Society of Hospital Medicine Launches Online Training Program for Hospitalists
Hospitalists play an increasingly pivotal role in ensuring the highest quality and safety for patients in hospitals. The implementation of healthcare reform has only heightened the importance of hospital quality and patient safety for hospitalists. To enable education and advancement of quality improvement (QI), SHM has developed the Hospital Quality & Patient Safety (HQPS) Online Academy (http://www.hospitalmedicine.org/hqps).
The HQPS Online Academy consists of Internet-based modules that provide training not included in traditional medical education. These modules bridge the gap between the conceptualization and practice of quality in hospitals, helping hospitalists to prepare and lead quality initiatives to improve patient outcomes. The modules allow healthcare providers to explore and evaluate current quality initiatives and practices, as well as reflect on ways to improve core measures within their hospital.
Each module focuses on a core principle of QI and patient safety, and provides three AMA PRA Category 1 credits.
SHM members who are insured with The Doctors Company can earn a 5% risk-management credit by completing the first five HQPS modules (see below). Eligible members also enjoy premium savings through a 5% program discount and a claims-free credit of up to 25%.
HQPS Online Academy modules
- Quality measurement and stakeholder interests
- Teamwork and communication
- Organizational knowledge and leadership skills
- Patient safety principles
- Quality and safety improvement methods and skills (RCA and FMEA)
Hospitalists play an increasingly pivotal role in ensuring the highest quality and safety for patients in hospitals. The implementation of healthcare reform has only heightened the importance of hospital quality and patient safety for hospitalists. To enable education and advancement of quality improvement (QI), SHM has developed the Hospital Quality & Patient Safety (HQPS) Online Academy (http://www.hospitalmedicine.org/hqps).
The HQPS Online Academy consists of Internet-based modules that provide training not included in traditional medical education. These modules bridge the gap between the conceptualization and practice of quality in hospitals, helping hospitalists to prepare and lead quality initiatives to improve patient outcomes. The modules allow healthcare providers to explore and evaluate current quality initiatives and practices, as well as reflect on ways to improve core measures within their hospital.
Each module focuses on a core principle of QI and patient safety, and provides three AMA PRA Category 1 credits.
SHM members who are insured with The Doctors Company can earn a 5% risk-management credit by completing the first five HQPS modules (see below). Eligible members also enjoy premium savings through a 5% program discount and a claims-free credit of up to 25%.
HQPS Online Academy modules
- Quality measurement and stakeholder interests
- Teamwork and communication
- Organizational knowledge and leadership skills
- Patient safety principles
- Quality and safety improvement methods and skills (RCA and FMEA)
Hospitalists play an increasingly pivotal role in ensuring the highest quality and safety for patients in hospitals. The implementation of healthcare reform has only heightened the importance of hospital quality and patient safety for hospitalists. To enable education and advancement of quality improvement (QI), SHM has developed the Hospital Quality & Patient Safety (HQPS) Online Academy (http://www.hospitalmedicine.org/hqps).
The HQPS Online Academy consists of Internet-based modules that provide training not included in traditional medical education. These modules bridge the gap between the conceptualization and practice of quality in hospitals, helping hospitalists to prepare and lead quality initiatives to improve patient outcomes. The modules allow healthcare providers to explore and evaluate current quality initiatives and practices, as well as reflect on ways to improve core measures within their hospital.
Each module focuses on a core principle of QI and patient safety, and provides three AMA PRA Category 1 credits.
SHM members who are insured with The Doctors Company can earn a 5% risk-management credit by completing the first five HQPS modules (see below). Eligible members also enjoy premium savings through a 5% program discount and a claims-free credit of up to 25%.
HQPS Online Academy modules
- Quality measurement and stakeholder interests
- Teamwork and communication
- Organizational knowledge and leadership skills
- Patient safety principles
- Quality and safety improvement methods and skills (RCA and FMEA)