User login
Discharge Summary Quality
Hospitalized patients are often cared for by physicians who do not follow them in the community, creating a discontinuity of care that must be bridged through communication. This communication between inpatient and outpatient physicians occurs, in part via a discharge summary, which is intended to summarize events during hospitalization and prepare the outpatient physician to resume care of the patient. Yet, this form of communication has long been problematic.[1, 2, 3] In a 1960 study, only 30% of discharge letters were received by the primary care physician within 48 hours of discharge.[1]
More recent studies have shown little improvement. Direct communication between hospital and outpatient physicians is rare, and discharge summaries are still largely unavailable at the time of follow‐up.[4] In 1 study, primary care physicians were unaware of 62% of laboratory tests or study results that were pending on discharge,[5] in part because this information is missing from most discharge summaries.[6] Deficits such as these persist despite the fact that the rate of postdischarge completion of recommended tests, referrals, or procedures is significantly increased when the recommendation is included in the discharge summary.[7]
Regulatory mandates for discharge summaries from the Centers for Medicare and Medicaid Services[8] and from The Joint Commission[9] appear to be generally met[10, 11]; however, these mandates have no requirements for timeliness stricter than 30 days, do not require that summaries be transmitted to outpatient physicians, and do not require several content elements that might be useful to outside physicians such as condition of the patient at discharge, cognitive and functional status, goals of care, or pending studies. Expert opinion guidelines have more comprehensive recommendations,[12, 13] but it is uncertain how widely they are followed.
The existence of a discharge summary does not necessarily mean it serves a patient well in the transitional period.[11, 14, 15] Discharge summaries are a complex intervention, and we do not yet understand the best ways discharge summaries may fulfill needs specific to transitional care. Furthermore, it is uncertain what factors improve aspects of discharge summary quality as defined by timeliness, transmission, and content.[6, 16]
The goal of the DIagnosing Systemic failures, Complexities and HARm in GEriatric discharges study (DISCHARGE) was to comprehensively assess the discharge process for older patients discharged to the community. In this article we examine discharge summaries of patients enrolled in the study to determine the timeliness, transmission to outside physicians, and content of the summaries. We further examine the effect of provider training level and timeliness of dictation on discharge summary quality.
METHODS
Study Cohort
The DISCHARGE study was a prospective, observational cohort study of patients 65 years or older discharged to home from YaleNew Haven Hospital (YNHH) who were admitted with acute coronary syndrome (ACS), community‐acquired pneumonia, or heart failure (HF). Patients were screened by physicians for eligibility within 24 hours of admission using specialty society guidelines[17, 18, 19, 20] and were enrolled by telephone within 1 week of discharge. Additional inclusion criteria included speaking English or Spanish, and ability of the patient or caregiver to participate in a telephone interview. Patients enrolled in hospice were excluded, as were patients who failed the Mini‐Cog mental status screen (3‐item recall and a clock draw)[21] while in the hospital or appeared confused or delirious during the telephone interview. Caregivers of cognitively impaired patients were eligible for enrollment instead if the patient provided permission.
Study Setting
YNHH is a 966‐bed urban tertiary care hospital with statistically lower than the national average mortality for acute myocardial infarction, HF, and pneumonia but statistically higher than the national average for 30‐day readmission rates for HF and pneumonia at the time this study was conducted. Advanced practice registered nurses (APRNs) working under the supervision of private or university cardiologists provided care for cardiology service patients. Housestaff under the supervision of university or hospitalist attending physicians, or physician assistants or APRNs under the supervision of hospitalist attending physicians provided care for patients on medical services. Discharge summaries were typically dictated by APRNs for cardiology patients, by 2nd‐ or 3rd‐year residents for housestaff patients, and by hospitalists for hospitalist patients. A dictation guideline was provided to housestaff and hospitalists (see Supporting Information, Appendix 1, in the online version of this article); this guideline suggested including basic demographic information, disposition and diagnoses, the admission history and physical, hospital course, discharge medications, and follow‐up appointments. Additionally, housestaff received a lecture about discharge summaries at the start of their 2nd year. Discharge instructions including medications and follow‐up appointment information were automatically appended to the discharge summaries. Summaries were sent by the medical records department only to physicians in the system who were listed by the dictating physician as needing to receive a copy of the summary; no summary was automatically sent (ie, to the primary care physician) if not requested by the dictating physician.
Data Collection
Experienced registered nurses trained in chart abstraction conducted explicit reviews of medical charts using a standardized review tool. The tool included 24 questions about the discharge summary applicable to all 3 conditions, with 7 additional questions for patients with HF and 1 additional question for patients with ACS. These questions included the 6 elements required by The Joint Commission for all discharge summaries (reason for hospitalization, significant findings, procedures and treatment provided, patient's discharge condition, patient and family instructions, and attending physician's signature)[9] as well as the 7 elements (principal diagnosis and problem list, medication list, transferring physician name and contact information, cognitive status of the patient, test results, and pending test results) recommended by the Transitions of Care Consensus Conference (TOCCC), a recent consensus statement produced by 6 major medical societies.[13] Each content element is shown in (see Supporting Information, Appendix 2, in the online version of this article), which also indicates the elements included in the 2 guidelines.
Main Measures
We assessed quality in 3 main domains: timeliness, transmission, and content. We defined timeliness as days between discharge date and dictation date (not final signature date, which may occur later), and measured both median timeliness and proportion of discharge summaries completed on the day of discharge. We defined transmission as successful fax or mail of the discharge summary to an outside physician as reported by the medical records department, and measured the proportion of discharge summaries sent to any outside physician as well as the median number of physicians per discharge summary who were scheduled to follow‐up with the patient postdischarge but who did not receive a copy of the summary. We defined 21 individual content items and assessed the frequency of each individual content item. We also measured compliance with The Joint Commission mandates and TOCCC recommendations, which included several of the individual content items.
To measure compliance with The Joint Commission requirements, we created a composite score in which 1 point was provided for the presence of each of the 6 required elements (maximum score=6). Every discharge summary received 1 point for attending physician signature, because all discharge summaries were electronically signed. Discharge instructions to family/patients were automatically appended to every discharge summary; however, we gave credit for patient and family instructions only to those that included any information about signs and symptoms to monitor for at home. We defined discharge condition as any information about functional status, cognitive status, physical exam, or laboratory findings at discharge.
To measure compliance with specialty society recommendations for discharge summaries, we created a composite score in which 1 point was provided for the presence of each of the 7 recommended elements (maximum score=7). Every discharge summary received 1 point for discharge medications, because these are automatically appended.
We obtained data on age, race, gender, and length of stay from hospital administrative databases. The study was approved by the Yale Human Investigation Committee, and verbal informed consent was obtained from all study participants.
Statistical Analysis
Characteristics of the sample are described with counts and percentages or means and standard deviations. Medians and interquartile ranges (IQRs) or counts and percentages were calculated for summary measures of timeliness, transmission, and content. We assessed differences in quality measures between APRNs, housestaff, and hospitalists using 2 tests. We conducted multivariable logistic regression analyses for timeliness and for transmission to any outside physician. All discharge summaries included at least 4 of The Joint Commission elements; consequently, we coded this content outcome as an ordinal variable with 3 levels indicating inclusion of 4, 5, or 6 of The Joint Commission elements. We coded the TOCCC content outcome as a 3‐level variable indicating <4, 4, or >4 elements satisfied. Accordingly, proportional odds models were used, in which the reported odds ratios (ORs) can be interpreted as the average effect of the explanatory variable on the odds of having more recommendations, for any dichotomization of the outcome. Residual analysis and goodness‐of‐fit statistics were used to assess model fit; the proportional odds assumption was tested. Statistical analyses were conducted with SAS 9.2 (SAS Institute, Cary, NC). P values <0.05 were interpreted as statistically significant for 2‐sided tests.
RESULTS
Enrollment and Study Sample
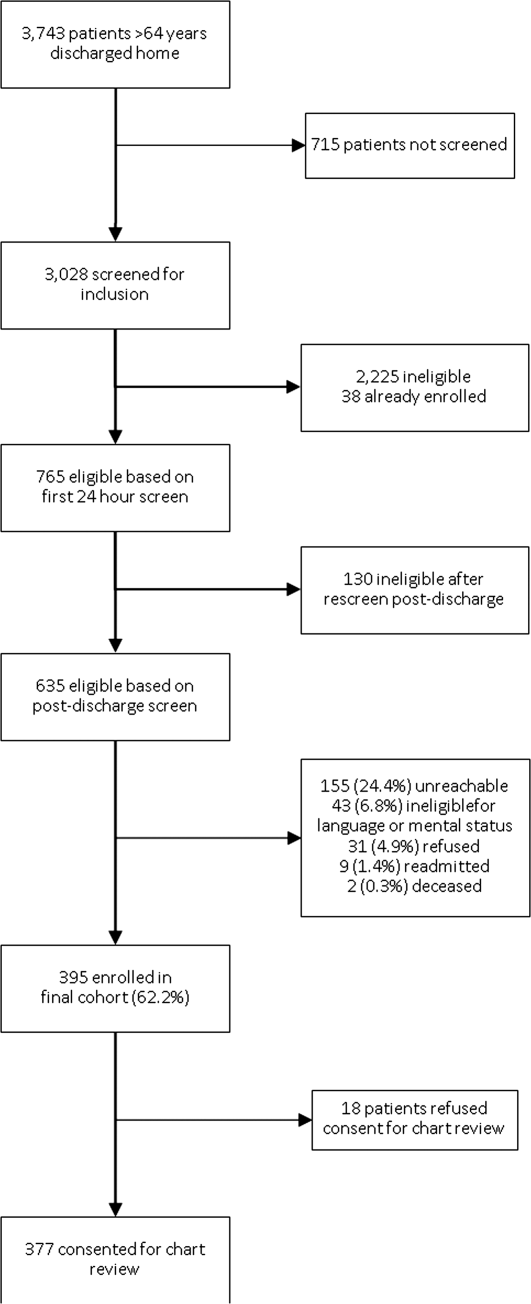
A total of 3743 patients over 64 years old were discharged home from the medical service at YNHH during the study period; 3028 patients were screened for eligibility within 24 hours of admission. We identified 635 eligible admissions and enrolled 395 patients (62.2%) in the study. Of these, 377 granted permission for chart review and were included in this analysis (Figure 1).
The study sample had a mean age of 77.1 years (standard deviation: 7.8); 205 (54.4%) were male and 310 (82.5%) were non‐Hispanic white. A total of 195 (51.7%) had ACS, 91 (24.1%) had pneumonia, and 146 (38.7%) had HF; 54 (14.3%) patients had more than 1 qualifying condition. There were similar numbers of patients on the cardiology, medicine housestaff, and medicine hospitalist teams (Table 1).
| Characteristic | N (%) or Mean (SD) |
|---|---|
| |
| Condition | |
| Acute coronary syndrome | 195 (51.7) |
| Community‐acquired pneumonia | 91 (24.1) |
| Heart failure | 146 (38.7) |
| Training level of summary dictator | |
| APRN | 140 (37.1) |
| House staff | 123 (32.6) |
| Hospitalist | 114 (30.2) |
| Length of stay, mean, d | 3.5 (2.5) |
| Total number of medications | 8.9 (3.3) |
| Identify a usual source of care | 360 (96.0) |
| Age, mean, y | 77.1 (7.8) |
| Male | 205 (54.4) |
| English‐speaking | 366 (98.1) |
| Race/ethnicity | |
| Non‐Hispanic white | 310 (82.5) |
| Non‐Hispanic black | 44 (11.7) |
| Hispanic | 15 (4.0) |
| Other | 7 (1.9) |
| High school graduate or GED Admission source | 268 (73.4) |
| Emergency department | 248 (66.0) |
| Direct transfer from hospital or nursing facility | 94 (25.0) |
| Direct admission from office | 34 (9.0) |
Timeliness
Discharge summaries were completed for 376/377 patients, of which 174 (46.3%) were dictated on the day of discharge. However, 122 (32.4%) summaries were dictated more than 48 hours after discharge, including 93 (24.7%) that were dictated more than 1 week after discharge (see Supporting Information, Appendix 3, in the online version of this article).
Summaries dictated by hospitalists were most likely to be done on the day of discharge (35.3% APRNs, 38.2% housestaff, 68.4% hospitalists, P<0.001). After adjustment for diagnosis and length of stay, hospitalists were still significantly more likely to produce a timely discharge summary than APRNs (OR: 2.82; 95% confidence interval [CI]: 1.56‐5.09), whereas housestaff were no different than APRNs (OR: 0.84; 95% CI: 0.48‐1.46).
Transmission
A total of 144 (38.3%) discharge summaries were not sent to any physician besides the inpatient attending, and 209/374 (55.9%) were not sent to at least 1 physician listed as having a follow‐up appointment planned with the patient. Each discharge summary was sent to a median of 1 physician besides the dictating physician (IQR: 01). However, for each summary, a median of 1 physician (IQR: 01) who had a scheduled follow‐up with the patient did not receive the summary. Summaries dictated by hospitalists were most likely to be sent to at least 1 outside physician (54.7% APRNs, 58.5% housestaff, 73.7% hospitalists, P=0.006). Summaries dictated on the day of discharge were more likely than delayed summaries to be sent to at least 1 outside physician (75.9% vs 49.5%, P<0.001). After adjustment for diagnosis and length of stay, there was no longer a difference in likelihood of transmitting a discharge summary to any outpatient physician according to training level; however, dictations completed on the day of discharge remained significantly more likely to be transmitted to an outside physician (OR: 3.05; 95% CI: 1.88‐4.93) (Table 2).
| Explanatory Variable | Proportion Transmitted to at Least 1 Outside Physician | OR for Transmission to Any Outside Physician (95% CI) | Adjusted P Value |
|---|---|---|---|
| |||
| Training level | 0.52 | ||
| APRN | 54.7% | REF | |
| Housestaff | 58.5% | 1.17 (0.66‐2.06) | |
| Hospitalist | 73.7% | 1.46 (0.76‐2.79) | |
| Timeliness | |||
| Dictated after discharge | 49.5% | REF | <0.001 |
| Dictated day of discharge | 75.9% | 3.05 (1.88‐4.93) | |
| Acute coronary syndrome vs nota | 52.1 % | 1.05 (0.49‐2.26) | 0.89 |
| Pneumonia vs nota | 69.2 % | 1.59 (0.66‐3.79) | 0.30 |
| Heart failure vs nota | 74.7 % | 3.32 (1.61‐6.84) | 0.001 |
| Length of stay, d | 0.91 (0.83‐1.00) | 0.06 | |
Content
Rate of inclusion of each content element is shown in Table 3, overall and by training level. Nearly every discharge summary included information about admitting diagnosis, hospital course, and procedures or tests performed during the hospitalization. However, few summaries included information about the patient's condition at discharge. Less than half included discharge laboratory results; less than one‐third included functional capacity, cognitive capacity, or discharge physical exam. Only 4.1% overall of discharge summaries for patients with HF included the patient's weight at discharge; best were hospitalists who still included this information in only 7.7% of summaries. Information about postdischarge care, including home social support, pending tests, or recommended follow‐up tests/procedures was also rarely specified. Last, only 6.2% of discharge summaries included the name and contact number of the inpatient physician; this information was least likely to be provided by housestaff (1.6%) and most likely to be provided by hospitalists (15.2%) (P<0.001).
| Discharge Summary Component | Overall, n=377, n (%) | APRN, n=140, n (%) | Housestaff, n=123, n (%) | Hospitalist, n=114, n (%) | P Value |
|---|---|---|---|---|---|
| |||||
| Diagnosisab | 368 (97.9) | 136 (97.8) | 120 (97.6) | 112 (98.3) | 1.00 |
| Discharge second diagnosisb | 289 (76.9) | 100 (71.9) | 89 (72.4) | 100 (87.7) | <0.001 |
| Hospital coursea | 375 (100.0) | 138 (100) | 123 (100) | 114 (100) | N/A |
| Procedures/tests performed during admissionab | 374 (99.7) | 138 (99.3) | 123 (100) | 113 (100) | N/A |
| Patient and family instructionsa | 371 (98.4) | 136 (97.1) | 122 (99.2) | 113 (99.1) | .43 |
| Social support or living situation of patient | 148 (39.5) | 18 (12.9) | 62 (50.4) | 68 (60.2) | <0.001 |
| Functional capacity at dischargea | 99 (26.4) | 37 (26.6) | 32 (26.0) | 30 (26.6) | 0.99 |
| Cognitive capacity at dischargeab | 30 (8.0) | 6 (4.4) | 11 (8.9) | 13 (11.5) | 0.10 |
| Physical exam at dischargea | 62 (16.7) | 19 (13.8) | 16 (13.1) | 27 (24.1) | 0.04 |
| Laboratory results at time of dischargea | 164 (43.9) | 63 (45.3) | 50 (40.7) | 51 (45.5) | 0.68 |
| Back to baseline or other nonspecific remark about discharge statusa | 71 (19.0) | 30 (21.6) | 18 (14.8) | 23 (20.4) | 0.34 |
| Any test or result still pending or specific comment that nothing is pendingb | 46 (12.2) | 9 (6.4) | 20 (16.3) | 17 (14.9) | 0.03 |
| Recommendation for follow‐up tests/procedures | 157 (41.9) | 43 (30.9) | 54 (43.9) | 60 (53.1) | 0.002 |
| Call‐back number of responsible in‐house physicianb | 23 (6.2) | 4 (2.9) | 2 (1.6) | 17 (15.2) | <0.001 |
| Resuscitation status | 27 (7.7) | 2 (1.5) | 18 (15.4) | 7 (6.7) | <0.001 |
| Etiology of heart failurec | 120 (82.8) | 44 (81.5) | 34 (87.2) | 42 (80.8) | 0.69 |
| Reason/trigger for exacerbationc | 86 (58.9) | 30 (55.6) | 27 (67.5) | 29 (55.8) | 0.43 |
| Ejection fractionc | 107 (73.3) | 40 (74.1) | 32 (80.0) | 35 (67.3) | 0.39 |
| Discharge weightc | 6 (4.1) | 1 (1.9) | 1 (2.5) | 4 (7.7) | 0.33 |
| Target weight rangec | 5 (3.4) | 0 (0) | 2 (5.0) | 3 (5.8) | 0.22 |
| Discharge creatinine or GFRc | 34 (23.3) | 14 (25.9) | 10 (25.0) | 10 (19.2) | 0.69 |
| If stent placed, whether drug‐eluting or notd | 89 (81.7) | 58 (87.9) | 27 (81.8) | 4 (40.0) | 0.001 |
On average, summaries included 5.6 of the 6 Joint Commission elements and 4.0 of the 7 TOCCC elements. A total of 63.0% of discharge summaries included all 6 elements required by The Joint Commission, whereas no discharge summary included all 7 TOCCC elements.
APRNs, housestaff and hospitalists included the same average number of The Joint Commission elements (5.6 each), but hospitalists on average included slightly more TOCCC elements (4.3) than did housestaff (4.0) or APRNs (3.8) (P<0.001). Summaries dictated on the day of discharge included an average of 4.2 TOCCC elements, compared to 3.9 TOCCC elements in delayed discharge. In multivariable analyses adjusted for diagnosis and length of stay, there was still no difference by training level in presence of The Joint Commission elements, but hospitalists were significantly more likely to include more TOCCC elements than APRNs (OR: 2.70; 95% CI: 1.49‐4.90) (Table 4). Summaries dictated on the day of discharge were significantly more likely to include more TOCCC elements (OR: 1.92; 95% CI: 1.23‐2.99).
| Explanatory Variable | Average Number of TOCCC Elements Included | OR (95% CI) | Adjusted P Value |
|---|---|---|---|
| |||
| Training level | 0.004 | ||
| APRN | 3.8 | REF | |
| Housestaff | 4.0 | 1.54 (0.90‐2.62) | |
| Hospitalist | 4.3 | 2.70 (1.49‐4.90) | |
| Timeliness | |||
| Dictated after discharge | 3.9 | REF | |
| Dictated day of discharge | 4.2 | 1.92 (1.23‐2.99) | 0.004 |
| Acute coronary syndrome vs nota | 3.9 | 0.72 (0.37‐1.39) | 0.33 |
| Pneumonia vs nota | 4.2 | 1.02 (0.49‐2.14) | 0.95 |
| Heart failure vs nota | 4.1 | 1.49 (0.80‐2.78) | 0.21 |
| Length of stay, d | 0.99 (0.90‐1.07) | 0.73 | |
No discharge summary included all 7 TOCCC‐endorsed content elements, was dictated on the day of discharge, and was sent to an outside physician.
DISCUSSION
In this prospective single‐site study of medical patients with 3 common conditions, we found that discharge summaries were completed relatively promptly, but were often not sent to the appropriate outpatient physicians. We also found that summaries were uniformly excellent at providing details of the hospitalization, but less reliable at providing details relevant to transitional care such as the patient's condition on discharge or existence of pending tests. On average, summaries included 57% of the elements included in consensus guidelines by 6 major medical societies. The content of discharge summaries dictated by hospitalists was slightly more comprehensive than that of APRNs and trainees, but no group exhibited high performance. In fact, not one discharge summary fully met all 3 quality criteria of timeliness, transmission, and content.
Our study, unlike most in the field, focused on multiple dimensions of discharge summary quality simultaneously. For instance, previous studies have found that timely receipt of a discharge summary does not reduce readmission rates.[11, 14, 15] Yet, if the content of the discharge summary is inadequate for postdischarge care, the summary may not be useful even if it is received by the follow‐up visit. Conversely, high‐quality content is ineffective if the summary is not sent to the outpatient physician.
This study suggests several avenues for improving summary quality. Timely discharge summaries in this study were more likely to include key content and to be transmitted to the appropriate physician. Strategies to improve discharge summary quality should therefore prioritize timely summaries, which can be expected to have downstream benefits for other aspects of quality. Some studies have found that templates improve discharge summary content.[22] In our institution, a template exists, but it favors a hospitalization‐focused rather than transition‐focused approach to the discharge summary. For instance, it includes instructions to dictate the admission exam, but not the discharge exam. Thus, designing templates specifically for transitional care is key. Maximizing capabilities of electronic records may help; many content elements that were commonly missing (e.g., pending results, discharge vitals, discharge weight) could be automatically inserted from electronic records. Likewise, automatic transmission of the summary to care providers listed in the electronic record might ameliorate many transmission failures. Some efforts have been made to convert existing electronic data into discharge summaries.[23, 24, 25] However, these activities are very preliminary, and some studies have found the quality of electronic summaries to be lower than dictated or handwritten summaries.[26] As with all automated or electronic applications, it will be essential to consider workflow, readability, and ability to synthesize information prior to adoption.
Hospitalists consistently produced highest‐quality summaries, even though they did not receive explicit training, suggesting experience may be beneficial,[27, 28, 29] or that the hospitalist community focus on transitional care has been effective. In addition, hospitalists at our institution explicitly prioritize timely and comprehensive discharge dictations, because their business relies on maintaining good relationships with outpatient physicians who contract for their services. Housestaff and APRNs have no such incentives or policies; rather, they typically consider discharge summaries to be a useful source of patient history at the time of an admission or readmission. Other academic centers have found similar results.[6, 16] Nonetheless, even though hospitalists had slightly better performance in our study, large gaps in the quality of summaries remained for all groups including hospitalists.
This study has several limitations. First, as a single‐site study at an academic hospital, it may not be generalizable to other hospitals or other settings. It is noteworthy, however, that the average time to dictation in this study was much lower than that of other studies,[4, 14, 30, 31, 32] suggesting that practices at this institution are at least no worse and possibly better than elsewhere. Second, although there are some mandates and expert opinion‐based guidelines for discharge summary content, there is no validated evidence base to confirm what content ought to be present in discharge summaries to improve patient outcomes. Third, we had too few readmissions in the dataset to have enough power to determine whether discharge summary content, timeliness, or transmission predicts readmission. Fourth, we did not determine whether the information in discharge summaries was accurate or complete; we merely assessed whether it was present. For example, we gave every discharge summary full credit for including discharge medications because they are automatically appended. Yet medication reconciliation errors at discharge are common.[33, 34] In fact, in the DISCHARGE study cohort, more than a quarter of discharge medication lists contained a suspected error.[35]
In summary, this study demonstrated the inadequacy of the contemporary discharge summary for conveying information that is critical to the transition from hospital to home. It may be that hospital culture treats hospitalizations as discrete and self‐contained events rather than as components of a larger episode of care. As interest in reducing readmissions rises, reframing the discharge summary to serve as a transitional tool and targeting it for quality assessment will likely be necessary.
Acknowledgments
The authors would like to acknowledge Amy Browning and the staff of the Center for Outcomes Research and Evaluation Follow‐Up Center for conducting patient interviews, Mark Abroms and Katherine Herman for patient recruitment and screening, and Peter Charpentier for Web site development.
Disclosures
At the time this study was conducted, Dr. Horwitz was supported by the CTSA Grant UL1 RR024139 and KL2 RR024138 from the National Center for Advancing Translational Sciences (NCATS), a component of the National Institutes of Health (NIH), and NIH roadmap for Medical Research, and was a Centers of Excellence Scholar in Geriatric Medicine by the John A. Hartford Foundation and the American Federation for Aging Research. Dr. Horwitz is now supported by the National Institute on Aging (K08 AG038336) and by the American Federation for Aging Research through the Paul B. Beeson Career Development Award Program. This work was also supported by a grant from the Claude D. Pepper Older Americans Independence Center at Yale University School of Medicine (P30AG021342 NIH/NIA). Dr. Krumholz is supported by grant U01 HL105270‐01 (Center for Cardiovascular Outcomes Research at Yale University) from the National Heart, Lung, and Blood Institute. No funding source had any role in the study design; in the collection, analysis, and interpretation of data; in the writing of the report; or in the decision to submit the article for publication. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute on Aging, the National Center for Advancing Translational Sciences, the National Institutes of Health, The John A. Hartford Foundation, the National Heart, Lung, and Blood Institute, or the American Federation for Aging Research. Dr. Horwitz had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. An earlier version of this work was presented as an oral presentation at the Society of General Internal Medicine Annual Meeting in Orlando, Florida on May 12, 2012. Dr. Krumholz chairs a cardiac scientific advisory board for UnitedHealth. Dr. Krumholz receives support from the Centers of Medicare and Medicaid Services (CMS) to develop and maintain performance measures that are used for public reporting, including readmission measures.
APPENDIX
A
Dictation guidelines provided to house staff and hospitalists
DICTATION GUIDELINES
FORMAT OF DISCHARGE SUMMARY
- Your name(spell it out), andPatient name(spell it out as well)
- Medical record number, date of admission, date of discharge
- Attending physician
- Disposition
- Principal and other diagnoses, Principal and other operations/procedures
- Copies to be sent to other physicians
- Begin narrative: CC, HPI, PMHx, Medications on admit, Social, Family Hx, Physical exam on admission, Data (labs on admission, plus labs relevant to workup, significant changes at discharge, admission EKG, radiologic and other data),Hospital course by problem, discharge meds, follow‐up appointments
APPENDIX
B
| Diagnosis |
| Discharge Second Diagnosis |
| Hospital course |
| Procedures/tests performed during admission |
| Patient and Family Instructions |
| Social support or living situation of patient |
| Functional capacity at discharge |
| Cognitive capacity at discharge |
| Physical exam at discharge |
| Laboratory results at time of discharge |
| Back to baseline or other nonspecific remark about discharge status |
| Any test or result still pending |
| Specific comment that nothing is pending |
| Recommendation for follow up tests/procedures |
| Call back number of responsible in‐house physician |
| Resuscitation status |
| Etiology of heart failure |
| Reason/trigger for exacerbation |
| Ejection fraction |
| Discharge weight |
| Target weight range |
| Discharge creatinine or GFR |
| If stent placed, whether drug‐eluting or not |
| Composite element | Data elements abstracted that qualify as meeting measure |
|---|---|
| Reason for hospitalization | Diagnosis |
| Significant findings | Hospital course |
| Procedures and treatment provided | Procedures/tests performed during admission |
| Patient's discharge condition | Functional capacity at discharge, Cognitive capacity at discharge, Physical exam at discharge, Laboratory results at time of discharge, Back to baseline or other nonspecific remark about discharge status |
| Patient and family instructions | Signs and symptoms to monitor at home |
| Attending physician's signature | Attending signature |
| Composite element | Data elements abstracted that qualify as meeting measure |
|---|---|
| Principal diagnosis | Diagnosis |
| Problem list | Discharge second diagnosis |
| Medication list | [Automatically appended; full credit to every summary] |
| Transferring physician name and contact information | Call back number of responsible in‐house physician |
| Cognitive status of the patient | Cognitive capacity at discharge |
| Test results | Procedures/tests performed during admission |
| Pending test results | Any test or result still pending or specific comment that nothing is pending |
APPENDIX
C
Histogram of days between discharge and dictation
- , , . Value of the specialist's report. Br Med J. 1960;2(5213):1663–1664.
- , . Communications between general practitioners and consultants. Br Med J. 1974;4(5942):456–459.
- , , . A functional hospital discharge summary. J Pediatr. 1975;86(1):97–98.
- , , , , , . Deficits in communication and information transfer between hospital‐based and primary care physicians: implications for patient safety and continuity of care. JAMA. 2007;297(8):831–841.
- , , , et al. Patient safety concerns arising from test results that return after hospital discharge. Ann Intern Med. 2005;143(2):121–128.
- , , , et al. Adequacy of hospital discharge summaries in documenting tests with pending results and outpatient follow‐up providers. J Gen Intern Med. 2009;24(9):1002–1006.
- , , . Tying up loose ends: discharging patients with unresolved medical issues. Arch Intern Med. 2007;167(12):1305–1311.
- Centers for Medicare and Medicaid Services. Condition of participation: medical record services. 42. Vol 482.C.F.R. § 482.24 (2012).
- Joint Commission on Accreditation of Healthcare Organizations. Hospital Accreditation Standards. Standard IM 6.10 EP 7–9. Oakbrook Terrace, IL: The Joint Commission; 2008.
- , . Documentation of mandated discharge summary components in transitions from acute to subacute care. In: Agency for Healthcare Research and Quality, ed. Advances in Patient Safety: New Directions and Alternative Approaches. Vol 2: Culture and Redesign. AHRQ Publication No. 08-0034‐2. Rockville, MD: Agency for Healthcare Research and Quality; 2008:179–188.
- , , , et al. Hospital discharge documentation and risk of rehospitalisation. BMJ Qual Saf. 2011;20(9):773–778.
- , , , et al. Transition of care for hospitalized elderly patients‐development of a discharge checklist for hospitalists. J Hosp Med. 2006;1(6):354–360.
- , , , et al. Transitions of Care Consensus Policy Statement American College of Physicians‐Society of General Internal Medicine‐Society of Hospital Medicine‐American Geriatrics Society‐American College of Emergency Physicians‐Society of Academic Emergency Medicine. J Gen Intern Med. 2009;24(8):971–976.
- , , , et al. Association of communication between hospital‐based physicians and primary care providers with patient outcomes. J Gen Intern Med. 2009;24(3):381–386.
- , , , . Effect of discharge summary availability during post‐discharge visits on hospital readmission. J Gen Intern Med. 2002;17(3):186–192.
- , , , , . Provider characteristics, clinical‐work processes and their relationship to discharge summary quality for sub‐acute care patients. J Gen Intern Med. 2012;27(1):78–84.
- , , , et al. ACC/AHA 2007 guidelines for the management of patients with unstable angina/non‐ST‐elevation myocardial infarction: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the 2002 Guidelines for the Management of Patients With Unstable Angina/Non‐ST‐Elevation Myocardial Infarction) developed in collaboration with the American College of Emergency Physicians, the Society for Cardiovascular Angiography and Interventions, and the Society of Thoracic Surgeons endorsed by the American Association of Cardiovascular and Pulmonary Rehabilitation and the Society for Academic Emergency Medicine. J Am Coll Cardiol. 2007;50(7):e1–e157.
- , , . Universal definition of myocardial infarction. Eur Heart J. 2007;28(20):2525–2538.
- , , , et al. ESC guidelines for the diagnosis and treatment of acute and chronic heart failure 2008: the Task Force for the diagnosis and treatment of acute and chronic heart failure 2008 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association of the ESC (HFA) and endorsed by the European Society of Intensive Care Medicine (ESICM). Eur J Heart Fail. 2008;10(10):933–989.
- , , , et al. Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community‐acquired pneumonia in adults. Clin Infect Dis. 2007;44(suppl 2):S27–S72.
- , , , et al. Clock drawing in Alzheimer's disease. A novel measure of dementia severity. J Am Geriatr Soc. 1989;37(8):725–729.
- , , , , . Assessing quality and efficiency of discharge summaries. Am J Med Qual. 2005;20(6):337–343.
- , , , et al. Electronic versus dictated hospital discharge summaries: a randomized controlled trial. J Gen Intern Med. 2009;24(9):995–1001.
- , , , . Dictated versus database‐generated discharge summaries: a randomized clinical trial. CMAJ. 1999;160(3):319–326.
- , , , , . Computerised updating of clinical summaries: new opportunities for clinical practice and research? BMJ. 1988;297(6662):1504–1506.
- , , . Evaluation of electronic discharge summaries: a comparison of documentation in electronic and handwritten discharge summaries. Int J Med Inform. 2008;77(9):613–620.
- , , , , . Did I do as best as the system would let me? Healthcare professional views on hospital to home care transitions. J Gen Intern Med. 2012;27(12):1649–1656.
- , , , , . Learning by doing—resident perspectives on developing competency in high‐quality discharge care. J Gen Intern Med. 2012;27(9):1188–1194.
- , , , , . Out of sight, out of mind: housestaff perceptions of quality‐limiting factors in discharge care at teaching hospitals. J Hosp Med. 2012;7(5):376–381.
- , , . Dissemination of discharge summaries. Not reaching follow‐up physicians. Can Fam Physician. 2002;48:737–742.
- , , , . Primary care physician attitudes regarding communication with hospitalists. Am J Med. 2001;111(9B):15S–20S.
- , , , . General practitioner‐hospital communications: a review of discharge summaries. J Qual Clin Pract. 2001;21(4):104–108.
- , , . Accuracy of information on medicines in hospital discharge summaries. Intern Med J. 2006;36(4):221–225.
- , , . Accuracy of medication documentation in hospital discharge summaries: A retrospective analysis of medication transcription errors in manual and electronic discharge summaries. Int J Med Inform. 2010;79(1):58–64.
- , , , . Medication reconciliation accuracy and patient understanding of intended medication changes on hospital discharge. J Gen Intern Med. 2012;27(11):1513–1520.
Hospitalized patients are often cared for by physicians who do not follow them in the community, creating a discontinuity of care that must be bridged through communication. This communication between inpatient and outpatient physicians occurs, in part via a discharge summary, which is intended to summarize events during hospitalization and prepare the outpatient physician to resume care of the patient. Yet, this form of communication has long been problematic.[1, 2, 3] In a 1960 study, only 30% of discharge letters were received by the primary care physician within 48 hours of discharge.[1]
More recent studies have shown little improvement. Direct communication between hospital and outpatient physicians is rare, and discharge summaries are still largely unavailable at the time of follow‐up.[4] In 1 study, primary care physicians were unaware of 62% of laboratory tests or study results that were pending on discharge,[5] in part because this information is missing from most discharge summaries.[6] Deficits such as these persist despite the fact that the rate of postdischarge completion of recommended tests, referrals, or procedures is significantly increased when the recommendation is included in the discharge summary.[7]
Regulatory mandates for discharge summaries from the Centers for Medicare and Medicaid Services[8] and from The Joint Commission[9] appear to be generally met[10, 11]; however, these mandates have no requirements for timeliness stricter than 30 days, do not require that summaries be transmitted to outpatient physicians, and do not require several content elements that might be useful to outside physicians such as condition of the patient at discharge, cognitive and functional status, goals of care, or pending studies. Expert opinion guidelines have more comprehensive recommendations,[12, 13] but it is uncertain how widely they are followed.
The existence of a discharge summary does not necessarily mean it serves a patient well in the transitional period.[11, 14, 15] Discharge summaries are a complex intervention, and we do not yet understand the best ways discharge summaries may fulfill needs specific to transitional care. Furthermore, it is uncertain what factors improve aspects of discharge summary quality as defined by timeliness, transmission, and content.[6, 16]
The goal of the DIagnosing Systemic failures, Complexities and HARm in GEriatric discharges study (DISCHARGE) was to comprehensively assess the discharge process for older patients discharged to the community. In this article we examine discharge summaries of patients enrolled in the study to determine the timeliness, transmission to outside physicians, and content of the summaries. We further examine the effect of provider training level and timeliness of dictation on discharge summary quality.
METHODS
Study Cohort
The DISCHARGE study was a prospective, observational cohort study of patients 65 years or older discharged to home from YaleNew Haven Hospital (YNHH) who were admitted with acute coronary syndrome (ACS), community‐acquired pneumonia, or heart failure (HF). Patients were screened by physicians for eligibility within 24 hours of admission using specialty society guidelines[17, 18, 19, 20] and were enrolled by telephone within 1 week of discharge. Additional inclusion criteria included speaking English or Spanish, and ability of the patient or caregiver to participate in a telephone interview. Patients enrolled in hospice were excluded, as were patients who failed the Mini‐Cog mental status screen (3‐item recall and a clock draw)[21] while in the hospital or appeared confused or delirious during the telephone interview. Caregivers of cognitively impaired patients were eligible for enrollment instead if the patient provided permission.
Study Setting
YNHH is a 966‐bed urban tertiary care hospital with statistically lower than the national average mortality for acute myocardial infarction, HF, and pneumonia but statistically higher than the national average for 30‐day readmission rates for HF and pneumonia at the time this study was conducted. Advanced practice registered nurses (APRNs) working under the supervision of private or university cardiologists provided care for cardiology service patients. Housestaff under the supervision of university or hospitalist attending physicians, or physician assistants or APRNs under the supervision of hospitalist attending physicians provided care for patients on medical services. Discharge summaries were typically dictated by APRNs for cardiology patients, by 2nd‐ or 3rd‐year residents for housestaff patients, and by hospitalists for hospitalist patients. A dictation guideline was provided to housestaff and hospitalists (see Supporting Information, Appendix 1, in the online version of this article); this guideline suggested including basic demographic information, disposition and diagnoses, the admission history and physical, hospital course, discharge medications, and follow‐up appointments. Additionally, housestaff received a lecture about discharge summaries at the start of their 2nd year. Discharge instructions including medications and follow‐up appointment information were automatically appended to the discharge summaries. Summaries were sent by the medical records department only to physicians in the system who were listed by the dictating physician as needing to receive a copy of the summary; no summary was automatically sent (ie, to the primary care physician) if not requested by the dictating physician.
Data Collection
Experienced registered nurses trained in chart abstraction conducted explicit reviews of medical charts using a standardized review tool. The tool included 24 questions about the discharge summary applicable to all 3 conditions, with 7 additional questions for patients with HF and 1 additional question for patients with ACS. These questions included the 6 elements required by The Joint Commission for all discharge summaries (reason for hospitalization, significant findings, procedures and treatment provided, patient's discharge condition, patient and family instructions, and attending physician's signature)[9] as well as the 7 elements (principal diagnosis and problem list, medication list, transferring physician name and contact information, cognitive status of the patient, test results, and pending test results) recommended by the Transitions of Care Consensus Conference (TOCCC), a recent consensus statement produced by 6 major medical societies.[13] Each content element is shown in (see Supporting Information, Appendix 2, in the online version of this article), which also indicates the elements included in the 2 guidelines.
Main Measures
We assessed quality in 3 main domains: timeliness, transmission, and content. We defined timeliness as days between discharge date and dictation date (not final signature date, which may occur later), and measured both median timeliness and proportion of discharge summaries completed on the day of discharge. We defined transmission as successful fax or mail of the discharge summary to an outside physician as reported by the medical records department, and measured the proportion of discharge summaries sent to any outside physician as well as the median number of physicians per discharge summary who were scheduled to follow‐up with the patient postdischarge but who did not receive a copy of the summary. We defined 21 individual content items and assessed the frequency of each individual content item. We also measured compliance with The Joint Commission mandates and TOCCC recommendations, which included several of the individual content items.
To measure compliance with The Joint Commission requirements, we created a composite score in which 1 point was provided for the presence of each of the 6 required elements (maximum score=6). Every discharge summary received 1 point for attending physician signature, because all discharge summaries were electronically signed. Discharge instructions to family/patients were automatically appended to every discharge summary; however, we gave credit for patient and family instructions only to those that included any information about signs and symptoms to monitor for at home. We defined discharge condition as any information about functional status, cognitive status, physical exam, or laboratory findings at discharge.
To measure compliance with specialty society recommendations for discharge summaries, we created a composite score in which 1 point was provided for the presence of each of the 7 recommended elements (maximum score=7). Every discharge summary received 1 point for discharge medications, because these are automatically appended.
We obtained data on age, race, gender, and length of stay from hospital administrative databases. The study was approved by the Yale Human Investigation Committee, and verbal informed consent was obtained from all study participants.
Statistical Analysis
Characteristics of the sample are described with counts and percentages or means and standard deviations. Medians and interquartile ranges (IQRs) or counts and percentages were calculated for summary measures of timeliness, transmission, and content. We assessed differences in quality measures between APRNs, housestaff, and hospitalists using 2 tests. We conducted multivariable logistic regression analyses for timeliness and for transmission to any outside physician. All discharge summaries included at least 4 of The Joint Commission elements; consequently, we coded this content outcome as an ordinal variable with 3 levels indicating inclusion of 4, 5, or 6 of The Joint Commission elements. We coded the TOCCC content outcome as a 3‐level variable indicating <4, 4, or >4 elements satisfied. Accordingly, proportional odds models were used, in which the reported odds ratios (ORs) can be interpreted as the average effect of the explanatory variable on the odds of having more recommendations, for any dichotomization of the outcome. Residual analysis and goodness‐of‐fit statistics were used to assess model fit; the proportional odds assumption was tested. Statistical analyses were conducted with SAS 9.2 (SAS Institute, Cary, NC). P values <0.05 were interpreted as statistically significant for 2‐sided tests.
RESULTS
Enrollment and Study Sample
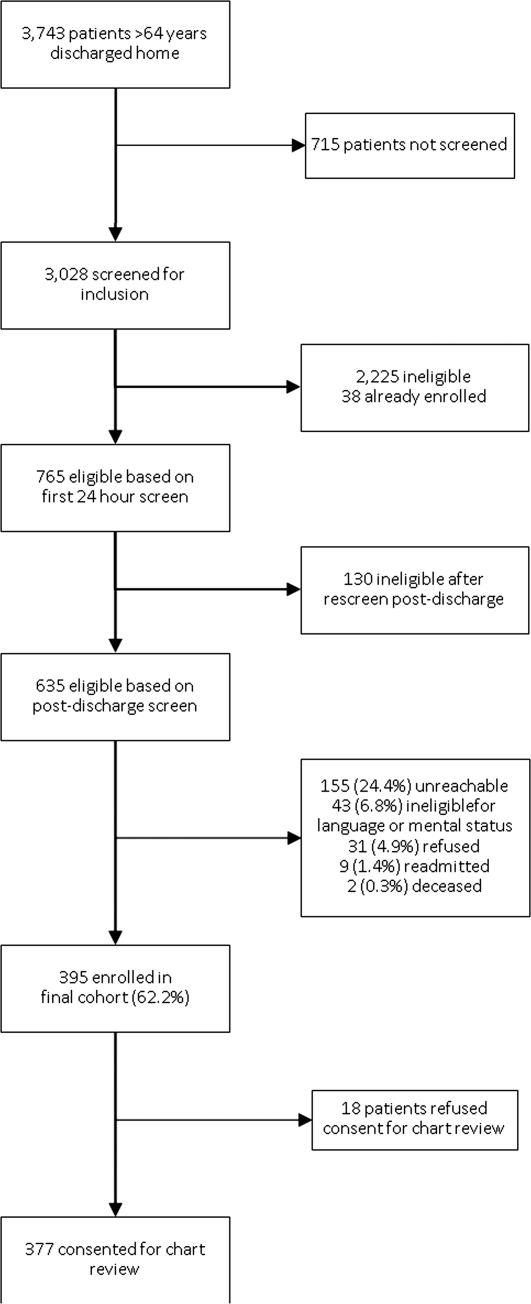
A total of 3743 patients over 64 years old were discharged home from the medical service at YNHH during the study period; 3028 patients were screened for eligibility within 24 hours of admission. We identified 635 eligible admissions and enrolled 395 patients (62.2%) in the study. Of these, 377 granted permission for chart review and were included in this analysis (Figure 1).

The study sample had a mean age of 77.1 years (standard deviation: 7.8); 205 (54.4%) were male and 310 (82.5%) were non‐Hispanic white. A total of 195 (51.7%) had ACS, 91 (24.1%) had pneumonia, and 146 (38.7%) had HF; 54 (14.3%) patients had more than 1 qualifying condition. There were similar numbers of patients on the cardiology, medicine housestaff, and medicine hospitalist teams (Table 1).
| Characteristic | N (%) or Mean (SD) |
|---|---|
| |
| Condition | |
| Acute coronary syndrome | 195 (51.7) |
| Community‐acquired pneumonia | 91 (24.1) |
| Heart failure | 146 (38.7) |
| Training level of summary dictator | |
| APRN | 140 (37.1) |
| House staff | 123 (32.6) |
| Hospitalist | 114 (30.2) |
| Length of stay, mean, d | 3.5 (2.5) |
| Total number of medications | 8.9 (3.3) |
| Identify a usual source of care | 360 (96.0) |
| Age, mean, y | 77.1 (7.8) |
| Male | 205 (54.4) |
| English‐speaking | 366 (98.1) |
| Race/ethnicity | |
| Non‐Hispanic white | 310 (82.5) |
| Non‐Hispanic black | 44 (11.7) |
| Hispanic | 15 (4.0) |
| Other | 7 (1.9) |
| High school graduate or GED Admission source | 268 (73.4) |
| Emergency department | 248 (66.0) |
| Direct transfer from hospital or nursing facility | 94 (25.0) |
| Direct admission from office | 34 (9.0) |
Timeliness
Discharge summaries were completed for 376/377 patients, of which 174 (46.3%) were dictated on the day of discharge. However, 122 (32.4%) summaries were dictated more than 48 hours after discharge, including 93 (24.7%) that were dictated more than 1 week after discharge (see Supporting Information, Appendix 3, in the online version of this article).
Summaries dictated by hospitalists were most likely to be done on the day of discharge (35.3% APRNs, 38.2% housestaff, 68.4% hospitalists, P<0.001). After adjustment for diagnosis and length of stay, hospitalists were still significantly more likely to produce a timely discharge summary than APRNs (OR: 2.82; 95% confidence interval [CI]: 1.56‐5.09), whereas housestaff were no different than APRNs (OR: 0.84; 95% CI: 0.48‐1.46).
Transmission
A total of 144 (38.3%) discharge summaries were not sent to any physician besides the inpatient attending, and 209/374 (55.9%) were not sent to at least 1 physician listed as having a follow‐up appointment planned with the patient. Each discharge summary was sent to a median of 1 physician besides the dictating physician (IQR: 01). However, for each summary, a median of 1 physician (IQR: 01) who had a scheduled follow‐up with the patient did not receive the summary. Summaries dictated by hospitalists were most likely to be sent to at least 1 outside physician (54.7% APRNs, 58.5% housestaff, 73.7% hospitalists, P=0.006). Summaries dictated on the day of discharge were more likely than delayed summaries to be sent to at least 1 outside physician (75.9% vs 49.5%, P<0.001). After adjustment for diagnosis and length of stay, there was no longer a difference in likelihood of transmitting a discharge summary to any outpatient physician according to training level; however, dictations completed on the day of discharge remained significantly more likely to be transmitted to an outside physician (OR: 3.05; 95% CI: 1.88‐4.93) (Table 2).
| Explanatory Variable | Proportion Transmitted to at Least 1 Outside Physician | OR for Transmission to Any Outside Physician (95% CI) | Adjusted P Value |
|---|---|---|---|
| |||
| Training level | 0.52 | ||
| APRN | 54.7% | REF | |
| Housestaff | 58.5% | 1.17 (0.66‐2.06) | |
| Hospitalist | 73.7% | 1.46 (0.76‐2.79) | |
| Timeliness | |||
| Dictated after discharge | 49.5% | REF | <0.001 |
| Dictated day of discharge | 75.9% | 3.05 (1.88‐4.93) | |
| Acute coronary syndrome vs nota | 52.1 % | 1.05 (0.49‐2.26) | 0.89 |
| Pneumonia vs nota | 69.2 % | 1.59 (0.66‐3.79) | 0.30 |
| Heart failure vs nota | 74.7 % | 3.32 (1.61‐6.84) | 0.001 |
| Length of stay, d | 0.91 (0.83‐1.00) | 0.06 | |
Content
Rate of inclusion of each content element is shown in Table 3, overall and by training level. Nearly every discharge summary included information about admitting diagnosis, hospital course, and procedures or tests performed during the hospitalization. However, few summaries included information about the patient's condition at discharge. Less than half included discharge laboratory results; less than one‐third included functional capacity, cognitive capacity, or discharge physical exam. Only 4.1% overall of discharge summaries for patients with HF included the patient's weight at discharge; best were hospitalists who still included this information in only 7.7% of summaries. Information about postdischarge care, including home social support, pending tests, or recommended follow‐up tests/procedures was also rarely specified. Last, only 6.2% of discharge summaries included the name and contact number of the inpatient physician; this information was least likely to be provided by housestaff (1.6%) and most likely to be provided by hospitalists (15.2%) (P<0.001).
| Discharge Summary Component | Overall, n=377, n (%) | APRN, n=140, n (%) | Housestaff, n=123, n (%) | Hospitalist, n=114, n (%) | P Value |
|---|---|---|---|---|---|
| |||||
| Diagnosisab | 368 (97.9) | 136 (97.8) | 120 (97.6) | 112 (98.3) | 1.00 |
| Discharge second diagnosisb | 289 (76.9) | 100 (71.9) | 89 (72.4) | 100 (87.7) | <0.001 |
| Hospital coursea | 375 (100.0) | 138 (100) | 123 (100) | 114 (100) | N/A |
| Procedures/tests performed during admissionab | 374 (99.7) | 138 (99.3) | 123 (100) | 113 (100) | N/A |
| Patient and family instructionsa | 371 (98.4) | 136 (97.1) | 122 (99.2) | 113 (99.1) | .43 |
| Social support or living situation of patient | 148 (39.5) | 18 (12.9) | 62 (50.4) | 68 (60.2) | <0.001 |
| Functional capacity at dischargea | 99 (26.4) | 37 (26.6) | 32 (26.0) | 30 (26.6) | 0.99 |
| Cognitive capacity at dischargeab | 30 (8.0) | 6 (4.4) | 11 (8.9) | 13 (11.5) | 0.10 |
| Physical exam at dischargea | 62 (16.7) | 19 (13.8) | 16 (13.1) | 27 (24.1) | 0.04 |
| Laboratory results at time of dischargea | 164 (43.9) | 63 (45.3) | 50 (40.7) | 51 (45.5) | 0.68 |
| Back to baseline or other nonspecific remark about discharge statusa | 71 (19.0) | 30 (21.6) | 18 (14.8) | 23 (20.4) | 0.34 |
| Any test or result still pending or specific comment that nothing is pendingb | 46 (12.2) | 9 (6.4) | 20 (16.3) | 17 (14.9) | 0.03 |
| Recommendation for follow‐up tests/procedures | 157 (41.9) | 43 (30.9) | 54 (43.9) | 60 (53.1) | 0.002 |
| Call‐back number of responsible in‐house physicianb | 23 (6.2) | 4 (2.9) | 2 (1.6) | 17 (15.2) | <0.001 |
| Resuscitation status | 27 (7.7) | 2 (1.5) | 18 (15.4) | 7 (6.7) | <0.001 |
| Etiology of heart failurec | 120 (82.8) | 44 (81.5) | 34 (87.2) | 42 (80.8) | 0.69 |
| Reason/trigger for exacerbationc | 86 (58.9) | 30 (55.6) | 27 (67.5) | 29 (55.8) | 0.43 |
| Ejection fractionc | 107 (73.3) | 40 (74.1) | 32 (80.0) | 35 (67.3) | 0.39 |
| Discharge weightc | 6 (4.1) | 1 (1.9) | 1 (2.5) | 4 (7.7) | 0.33 |
| Target weight rangec | 5 (3.4) | 0 (0) | 2 (5.0) | 3 (5.8) | 0.22 |
| Discharge creatinine or GFRc | 34 (23.3) | 14 (25.9) | 10 (25.0) | 10 (19.2) | 0.69 |
| If stent placed, whether drug‐eluting or notd | 89 (81.7) | 58 (87.9) | 27 (81.8) | 4 (40.0) | 0.001 |
On average, summaries included 5.6 of the 6 Joint Commission elements and 4.0 of the 7 TOCCC elements. A total of 63.0% of discharge summaries included all 6 elements required by The Joint Commission, whereas no discharge summary included all 7 TOCCC elements.
APRNs, housestaff and hospitalists included the same average number of The Joint Commission elements (5.6 each), but hospitalists on average included slightly more TOCCC elements (4.3) than did housestaff (4.0) or APRNs (3.8) (P<0.001). Summaries dictated on the day of discharge included an average of 4.2 TOCCC elements, compared to 3.9 TOCCC elements in delayed discharge. In multivariable analyses adjusted for diagnosis and length of stay, there was still no difference by training level in presence of The Joint Commission elements, but hospitalists were significantly more likely to include more TOCCC elements than APRNs (OR: 2.70; 95% CI: 1.49‐4.90) (Table 4). Summaries dictated on the day of discharge were significantly more likely to include more TOCCC elements (OR: 1.92; 95% CI: 1.23‐2.99).
| Explanatory Variable | Average Number of TOCCC Elements Included | OR (95% CI) | Adjusted P Value |
|---|---|---|---|
| |||
| Training level | 0.004 | ||
| APRN | 3.8 | REF | |
| Housestaff | 4.0 | 1.54 (0.90‐2.62) | |
| Hospitalist | 4.3 | 2.70 (1.49‐4.90) | |
| Timeliness | |||
| Dictated after discharge | 3.9 | REF | |
| Dictated day of discharge | 4.2 | 1.92 (1.23‐2.99) | 0.004 |
| Acute coronary syndrome vs nota | 3.9 | 0.72 (0.37‐1.39) | 0.33 |
| Pneumonia vs nota | 4.2 | 1.02 (0.49‐2.14) | 0.95 |
| Heart failure vs nota | 4.1 | 1.49 (0.80‐2.78) | 0.21 |
| Length of stay, d | 0.99 (0.90‐1.07) | 0.73 | |
No discharge summary included all 7 TOCCC‐endorsed content elements, was dictated on the day of discharge, and was sent to an outside physician.
DISCUSSION
In this prospective single‐site study of medical patients with 3 common conditions, we found that discharge summaries were completed relatively promptly, but were often not sent to the appropriate outpatient physicians. We also found that summaries were uniformly excellent at providing details of the hospitalization, but less reliable at providing details relevant to transitional care such as the patient's condition on discharge or existence of pending tests. On average, summaries included 57% of the elements included in consensus guidelines by 6 major medical societies. The content of discharge summaries dictated by hospitalists was slightly more comprehensive than that of APRNs and trainees, but no group exhibited high performance. In fact, not one discharge summary fully met all 3 quality criteria of timeliness, transmission, and content.
Our study, unlike most in the field, focused on multiple dimensions of discharge summary quality simultaneously. For instance, previous studies have found that timely receipt of a discharge summary does not reduce readmission rates.[11, 14, 15] Yet, if the content of the discharge summary is inadequate for postdischarge care, the summary may not be useful even if it is received by the follow‐up visit. Conversely, high‐quality content is ineffective if the summary is not sent to the outpatient physician.
This study suggests several avenues for improving summary quality. Timely discharge summaries in this study were more likely to include key content and to be transmitted to the appropriate physician. Strategies to improve discharge summary quality should therefore prioritize timely summaries, which can be expected to have downstream benefits for other aspects of quality. Some studies have found that templates improve discharge summary content.[22] In our institution, a template exists, but it favors a hospitalization‐focused rather than transition‐focused approach to the discharge summary. For instance, it includes instructions to dictate the admission exam, but not the discharge exam. Thus, designing templates specifically for transitional care is key. Maximizing capabilities of electronic records may help; many content elements that were commonly missing (e.g., pending results, discharge vitals, discharge weight) could be automatically inserted from electronic records. Likewise, automatic transmission of the summary to care providers listed in the electronic record might ameliorate many transmission failures. Some efforts have been made to convert existing electronic data into discharge summaries.[23, 24, 25] However, these activities are very preliminary, and some studies have found the quality of electronic summaries to be lower than dictated or handwritten summaries.[26] As with all automated or electronic applications, it will be essential to consider workflow, readability, and ability to synthesize information prior to adoption.
Hospitalists consistently produced highest‐quality summaries, even though they did not receive explicit training, suggesting experience may be beneficial,[27, 28, 29] or that the hospitalist community focus on transitional care has been effective. In addition, hospitalists at our institution explicitly prioritize timely and comprehensive discharge dictations, because their business relies on maintaining good relationships with outpatient physicians who contract for their services. Housestaff and APRNs have no such incentives or policies; rather, they typically consider discharge summaries to be a useful source of patient history at the time of an admission or readmission. Other academic centers have found similar results.[6, 16] Nonetheless, even though hospitalists had slightly better performance in our study, large gaps in the quality of summaries remained for all groups including hospitalists.
This study has several limitations. First, as a single‐site study at an academic hospital, it may not be generalizable to other hospitals or other settings. It is noteworthy, however, that the average time to dictation in this study was much lower than that of other studies,[4, 14, 30, 31, 32] suggesting that practices at this institution are at least no worse and possibly better than elsewhere. Second, although there are some mandates and expert opinion‐based guidelines for discharge summary content, there is no validated evidence base to confirm what content ought to be present in discharge summaries to improve patient outcomes. Third, we had too few readmissions in the dataset to have enough power to determine whether discharge summary content, timeliness, or transmission predicts readmission. Fourth, we did not determine whether the information in discharge summaries was accurate or complete; we merely assessed whether it was present. For example, we gave every discharge summary full credit for including discharge medications because they are automatically appended. Yet medication reconciliation errors at discharge are common.[33, 34] In fact, in the DISCHARGE study cohort, more than a quarter of discharge medication lists contained a suspected error.[35]
In summary, this study demonstrated the inadequacy of the contemporary discharge summary for conveying information that is critical to the transition from hospital to home. It may be that hospital culture treats hospitalizations as discrete and self‐contained events rather than as components of a larger episode of care. As interest in reducing readmissions rises, reframing the discharge summary to serve as a transitional tool and targeting it for quality assessment will likely be necessary.
Acknowledgments
The authors would like to acknowledge Amy Browning and the staff of the Center for Outcomes Research and Evaluation Follow‐Up Center for conducting patient interviews, Mark Abroms and Katherine Herman for patient recruitment and screening, and Peter Charpentier for Web site development.
Disclosures
At the time this study was conducted, Dr. Horwitz was supported by the CTSA Grant UL1 RR024139 and KL2 RR024138 from the National Center for Advancing Translational Sciences (NCATS), a component of the National Institutes of Health (NIH), and NIH roadmap for Medical Research, and was a Centers of Excellence Scholar in Geriatric Medicine by the John A. Hartford Foundation and the American Federation for Aging Research. Dr. Horwitz is now supported by the National Institute on Aging (K08 AG038336) and by the American Federation for Aging Research through the Paul B. Beeson Career Development Award Program. This work was also supported by a grant from the Claude D. Pepper Older Americans Independence Center at Yale University School of Medicine (P30AG021342 NIH/NIA). Dr. Krumholz is supported by grant U01 HL105270‐01 (Center for Cardiovascular Outcomes Research at Yale University) from the National Heart, Lung, and Blood Institute. No funding source had any role in the study design; in the collection, analysis, and interpretation of data; in the writing of the report; or in the decision to submit the article for publication. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute on Aging, the National Center for Advancing Translational Sciences, the National Institutes of Health, The John A. Hartford Foundation, the National Heart, Lung, and Blood Institute, or the American Federation for Aging Research. Dr. Horwitz had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. An earlier version of this work was presented as an oral presentation at the Society of General Internal Medicine Annual Meeting in Orlando, Florida on May 12, 2012. Dr. Krumholz chairs a cardiac scientific advisory board for UnitedHealth. Dr. Krumholz receives support from the Centers of Medicare and Medicaid Services (CMS) to develop and maintain performance measures that are used for public reporting, including readmission measures.
APPENDIX
A
Dictation guidelines provided to house staff and hospitalists
DICTATION GUIDELINES
FORMAT OF DISCHARGE SUMMARY
- Your name(spell it out), andPatient name(spell it out as well)
- Medical record number, date of admission, date of discharge
- Attending physician
- Disposition
- Principal and other diagnoses, Principal and other operations/procedures
- Copies to be sent to other physicians
- Begin narrative: CC, HPI, PMHx, Medications on admit, Social, Family Hx, Physical exam on admission, Data (labs on admission, plus labs relevant to workup, significant changes at discharge, admission EKG, radiologic and other data),Hospital course by problem, discharge meds, follow‐up appointments
APPENDIX
B
| Diagnosis |
| Discharge Second Diagnosis |
| Hospital course |
| Procedures/tests performed during admission |
| Patient and Family Instructions |
| Social support or living situation of patient |
| Functional capacity at discharge |
| Cognitive capacity at discharge |
| Physical exam at discharge |
| Laboratory results at time of discharge |
| Back to baseline or other nonspecific remark about discharge status |
| Any test or result still pending |
| Specific comment that nothing is pending |
| Recommendation for follow up tests/procedures |
| Call back number of responsible in‐house physician |
| Resuscitation status |
| Etiology of heart failure |
| Reason/trigger for exacerbation |
| Ejection fraction |
| Discharge weight |
| Target weight range |
| Discharge creatinine or GFR |
| If stent placed, whether drug‐eluting or not |
| Composite element | Data elements abstracted that qualify as meeting measure |
|---|---|
| Reason for hospitalization | Diagnosis |
| Significant findings | Hospital course |
| Procedures and treatment provided | Procedures/tests performed during admission |
| Patient's discharge condition | Functional capacity at discharge, Cognitive capacity at discharge, Physical exam at discharge, Laboratory results at time of discharge, Back to baseline or other nonspecific remark about discharge status |
| Patient and family instructions | Signs and symptoms to monitor at home |
| Attending physician's signature | Attending signature |
| Composite element | Data elements abstracted that qualify as meeting measure |
|---|---|
| Principal diagnosis | Diagnosis |
| Problem list | Discharge second diagnosis |
| Medication list | [Automatically appended; full credit to every summary] |
| Transferring physician name and contact information | Call back number of responsible in‐house physician |
| Cognitive status of the patient | Cognitive capacity at discharge |
| Test results | Procedures/tests performed during admission |
| Pending test results | Any test or result still pending or specific comment that nothing is pending |
APPENDIX
C
Histogram of days between discharge and dictation
Hospitalized patients are often cared for by physicians who do not follow them in the community, creating a discontinuity of care that must be bridged through communication. This communication between inpatient and outpatient physicians occurs, in part via a discharge summary, which is intended to summarize events during hospitalization and prepare the outpatient physician to resume care of the patient. Yet, this form of communication has long been problematic.[1, 2, 3] In a 1960 study, only 30% of discharge letters were received by the primary care physician within 48 hours of discharge.[1]
More recent studies have shown little improvement. Direct communication between hospital and outpatient physicians is rare, and discharge summaries are still largely unavailable at the time of follow‐up.[4] In 1 study, primary care physicians were unaware of 62% of laboratory tests or study results that were pending on discharge,[5] in part because this information is missing from most discharge summaries.[6] Deficits such as these persist despite the fact that the rate of postdischarge completion of recommended tests, referrals, or procedures is significantly increased when the recommendation is included in the discharge summary.[7]
Regulatory mandates for discharge summaries from the Centers for Medicare and Medicaid Services[8] and from The Joint Commission[9] appear to be generally met[10, 11]; however, these mandates have no requirements for timeliness stricter than 30 days, do not require that summaries be transmitted to outpatient physicians, and do not require several content elements that might be useful to outside physicians such as condition of the patient at discharge, cognitive and functional status, goals of care, or pending studies. Expert opinion guidelines have more comprehensive recommendations,[12, 13] but it is uncertain how widely they are followed.
The existence of a discharge summary does not necessarily mean it serves a patient well in the transitional period.[11, 14, 15] Discharge summaries are a complex intervention, and we do not yet understand the best ways discharge summaries may fulfill needs specific to transitional care. Furthermore, it is uncertain what factors improve aspects of discharge summary quality as defined by timeliness, transmission, and content.[6, 16]
The goal of the DIagnosing Systemic failures, Complexities and HARm in GEriatric discharges study (DISCHARGE) was to comprehensively assess the discharge process for older patients discharged to the community. In this article we examine discharge summaries of patients enrolled in the study to determine the timeliness, transmission to outside physicians, and content of the summaries. We further examine the effect of provider training level and timeliness of dictation on discharge summary quality.
METHODS
Study Cohort
The DISCHARGE study was a prospective, observational cohort study of patients 65 years or older discharged to home from YaleNew Haven Hospital (YNHH) who were admitted with acute coronary syndrome (ACS), community‐acquired pneumonia, or heart failure (HF). Patients were screened by physicians for eligibility within 24 hours of admission using specialty society guidelines[17, 18, 19, 20] and were enrolled by telephone within 1 week of discharge. Additional inclusion criteria included speaking English or Spanish, and ability of the patient or caregiver to participate in a telephone interview. Patients enrolled in hospice were excluded, as were patients who failed the Mini‐Cog mental status screen (3‐item recall and a clock draw)[21] while in the hospital or appeared confused or delirious during the telephone interview. Caregivers of cognitively impaired patients were eligible for enrollment instead if the patient provided permission.
Study Setting
YNHH is a 966‐bed urban tertiary care hospital with statistically lower than the national average mortality for acute myocardial infarction, HF, and pneumonia but statistically higher than the national average for 30‐day readmission rates for HF and pneumonia at the time this study was conducted. Advanced practice registered nurses (APRNs) working under the supervision of private or university cardiologists provided care for cardiology service patients. Housestaff under the supervision of university or hospitalist attending physicians, or physician assistants or APRNs under the supervision of hospitalist attending physicians provided care for patients on medical services. Discharge summaries were typically dictated by APRNs for cardiology patients, by 2nd‐ or 3rd‐year residents for housestaff patients, and by hospitalists for hospitalist patients. A dictation guideline was provided to housestaff and hospitalists (see Supporting Information, Appendix 1, in the online version of this article); this guideline suggested including basic demographic information, disposition and diagnoses, the admission history and physical, hospital course, discharge medications, and follow‐up appointments. Additionally, housestaff received a lecture about discharge summaries at the start of their 2nd year. Discharge instructions including medications and follow‐up appointment information were automatically appended to the discharge summaries. Summaries were sent by the medical records department only to physicians in the system who were listed by the dictating physician as needing to receive a copy of the summary; no summary was automatically sent (ie, to the primary care physician) if not requested by the dictating physician.
Data Collection
Experienced registered nurses trained in chart abstraction conducted explicit reviews of medical charts using a standardized review tool. The tool included 24 questions about the discharge summary applicable to all 3 conditions, with 7 additional questions for patients with HF and 1 additional question for patients with ACS. These questions included the 6 elements required by The Joint Commission for all discharge summaries (reason for hospitalization, significant findings, procedures and treatment provided, patient's discharge condition, patient and family instructions, and attending physician's signature)[9] as well as the 7 elements (principal diagnosis and problem list, medication list, transferring physician name and contact information, cognitive status of the patient, test results, and pending test results) recommended by the Transitions of Care Consensus Conference (TOCCC), a recent consensus statement produced by 6 major medical societies.[13] Each content element is shown in (see Supporting Information, Appendix 2, in the online version of this article), which also indicates the elements included in the 2 guidelines.
Main Measures
We assessed quality in 3 main domains: timeliness, transmission, and content. We defined timeliness as days between discharge date and dictation date (not final signature date, which may occur later), and measured both median timeliness and proportion of discharge summaries completed on the day of discharge. We defined transmission as successful fax or mail of the discharge summary to an outside physician as reported by the medical records department, and measured the proportion of discharge summaries sent to any outside physician as well as the median number of physicians per discharge summary who were scheduled to follow‐up with the patient postdischarge but who did not receive a copy of the summary. We defined 21 individual content items and assessed the frequency of each individual content item. We also measured compliance with The Joint Commission mandates and TOCCC recommendations, which included several of the individual content items.
To measure compliance with The Joint Commission requirements, we created a composite score in which 1 point was provided for the presence of each of the 6 required elements (maximum score=6). Every discharge summary received 1 point for attending physician signature, because all discharge summaries were electronically signed. Discharge instructions to family/patients were automatically appended to every discharge summary; however, we gave credit for patient and family instructions only to those that included any information about signs and symptoms to monitor for at home. We defined discharge condition as any information about functional status, cognitive status, physical exam, or laboratory findings at discharge.
To measure compliance with specialty society recommendations for discharge summaries, we created a composite score in which 1 point was provided for the presence of each of the 7 recommended elements (maximum score=7). Every discharge summary received 1 point for discharge medications, because these are automatically appended.
We obtained data on age, race, gender, and length of stay from hospital administrative databases. The study was approved by the Yale Human Investigation Committee, and verbal informed consent was obtained from all study participants.
Statistical Analysis
Characteristics of the sample are described with counts and percentages or means and standard deviations. Medians and interquartile ranges (IQRs) or counts and percentages were calculated for summary measures of timeliness, transmission, and content. We assessed differences in quality measures between APRNs, housestaff, and hospitalists using 2 tests. We conducted multivariable logistic regression analyses for timeliness and for transmission to any outside physician. All discharge summaries included at least 4 of The Joint Commission elements; consequently, we coded this content outcome as an ordinal variable with 3 levels indicating inclusion of 4, 5, or 6 of The Joint Commission elements. We coded the TOCCC content outcome as a 3‐level variable indicating <4, 4, or >4 elements satisfied. Accordingly, proportional odds models were used, in which the reported odds ratios (ORs) can be interpreted as the average effect of the explanatory variable on the odds of having more recommendations, for any dichotomization of the outcome. Residual analysis and goodness‐of‐fit statistics were used to assess model fit; the proportional odds assumption was tested. Statistical analyses were conducted with SAS 9.2 (SAS Institute, Cary, NC). P values <0.05 were interpreted as statistically significant for 2‐sided tests.
RESULTS
Enrollment and Study Sample
A total of 3743 patients over 64 years old were discharged home from the medical service at YNHH during the study period; 3028 patients were screened for eligibility within 24 hours of admission. We identified 635 eligible admissions and enrolled 395 patients (62.2%) in the study. Of these, 377 granted permission for chart review and were included in this analysis (Figure 1).

The study sample had a mean age of 77.1 years (standard deviation: 7.8); 205 (54.4%) were male and 310 (82.5%) were non‐Hispanic white. A total of 195 (51.7%) had ACS, 91 (24.1%) had pneumonia, and 146 (38.7%) had HF; 54 (14.3%) patients had more than 1 qualifying condition. There were similar numbers of patients on the cardiology, medicine housestaff, and medicine hospitalist teams (Table 1).
| Characteristic | N (%) or Mean (SD) |
|---|---|
| |
| Condition | |
| Acute coronary syndrome | 195 (51.7) |
| Community‐acquired pneumonia | 91 (24.1) |
| Heart failure | 146 (38.7) |
| Training level of summary dictator | |
| APRN | 140 (37.1) |
| House staff | 123 (32.6) |
| Hospitalist | 114 (30.2) |
| Length of stay, mean, d | 3.5 (2.5) |
| Total number of medications | 8.9 (3.3) |
| Identify a usual source of care | 360 (96.0) |
| Age, mean, y | 77.1 (7.8) |
| Male | 205 (54.4) |
| English‐speaking | 366 (98.1) |
| Race/ethnicity | |
| Non‐Hispanic white | 310 (82.5) |
| Non‐Hispanic black | 44 (11.7) |
| Hispanic | 15 (4.0) |
| Other | 7 (1.9) |
| High school graduate or GED Admission source | 268 (73.4) |
| Emergency department | 248 (66.0) |
| Direct transfer from hospital or nursing facility | 94 (25.0) |
| Direct admission from office | 34 (9.0) |
Timeliness
Discharge summaries were completed for 376/377 patients, of which 174 (46.3%) were dictated on the day of discharge. However, 122 (32.4%) summaries were dictated more than 48 hours after discharge, including 93 (24.7%) that were dictated more than 1 week after discharge (see Supporting Information, Appendix 3, in the online version of this article).
Summaries dictated by hospitalists were most likely to be done on the day of discharge (35.3% APRNs, 38.2% housestaff, 68.4% hospitalists, P<0.001). After adjustment for diagnosis and length of stay, hospitalists were still significantly more likely to produce a timely discharge summary than APRNs (OR: 2.82; 95% confidence interval [CI]: 1.56‐5.09), whereas housestaff were no different than APRNs (OR: 0.84; 95% CI: 0.48‐1.46).
Transmission
A total of 144 (38.3%) discharge summaries were not sent to any physician besides the inpatient attending, and 209/374 (55.9%) were not sent to at least 1 physician listed as having a follow‐up appointment planned with the patient. Each discharge summary was sent to a median of 1 physician besides the dictating physician (IQR: 01). However, for each summary, a median of 1 physician (IQR: 01) who had a scheduled follow‐up with the patient did not receive the summary. Summaries dictated by hospitalists were most likely to be sent to at least 1 outside physician (54.7% APRNs, 58.5% housestaff, 73.7% hospitalists, P=0.006). Summaries dictated on the day of discharge were more likely than delayed summaries to be sent to at least 1 outside physician (75.9% vs 49.5%, P<0.001). After adjustment for diagnosis and length of stay, there was no longer a difference in likelihood of transmitting a discharge summary to any outpatient physician according to training level; however, dictations completed on the day of discharge remained significantly more likely to be transmitted to an outside physician (OR: 3.05; 95% CI: 1.88‐4.93) (Table 2).
| Explanatory Variable | Proportion Transmitted to at Least 1 Outside Physician | OR for Transmission to Any Outside Physician (95% CI) | Adjusted P Value |
|---|---|---|---|
| |||
| Training level | 0.52 | ||
| APRN | 54.7% | REF | |
| Housestaff | 58.5% | 1.17 (0.66‐2.06) | |
| Hospitalist | 73.7% | 1.46 (0.76‐2.79) | |
| Timeliness | |||
| Dictated after discharge | 49.5% | REF | <0.001 |
| Dictated day of discharge | 75.9% | 3.05 (1.88‐4.93) | |
| Acute coronary syndrome vs nota | 52.1 % | 1.05 (0.49‐2.26) | 0.89 |
| Pneumonia vs nota | 69.2 % | 1.59 (0.66‐3.79) | 0.30 |
| Heart failure vs nota | 74.7 % | 3.32 (1.61‐6.84) | 0.001 |
| Length of stay, d | 0.91 (0.83‐1.00) | 0.06 | |
Content
Rate of inclusion of each content element is shown in Table 3, overall and by training level. Nearly every discharge summary included information about admitting diagnosis, hospital course, and procedures or tests performed during the hospitalization. However, few summaries included information about the patient's condition at discharge. Less than half included discharge laboratory results; less than one‐third included functional capacity, cognitive capacity, or discharge physical exam. Only 4.1% overall of discharge summaries for patients with HF included the patient's weight at discharge; best were hospitalists who still included this information in only 7.7% of summaries. Information about postdischarge care, including home social support, pending tests, or recommended follow‐up tests/procedures was also rarely specified. Last, only 6.2% of discharge summaries included the name and contact number of the inpatient physician; this information was least likely to be provided by housestaff (1.6%) and most likely to be provided by hospitalists (15.2%) (P<0.001).
| Discharge Summary Component | Overall, n=377, n (%) | APRN, n=140, n (%) | Housestaff, n=123, n (%) | Hospitalist, n=114, n (%) | P Value |
|---|---|---|---|---|---|
| |||||
| Diagnosisab | 368 (97.9) | 136 (97.8) | 120 (97.6) | 112 (98.3) | 1.00 |
| Discharge second diagnosisb | 289 (76.9) | 100 (71.9) | 89 (72.4) | 100 (87.7) | <0.001 |
| Hospital coursea | 375 (100.0) | 138 (100) | 123 (100) | 114 (100) | N/A |
| Procedures/tests performed during admissionab | 374 (99.7) | 138 (99.3) | 123 (100) | 113 (100) | N/A |
| Patient and family instructionsa | 371 (98.4) | 136 (97.1) | 122 (99.2) | 113 (99.1) | .43 |
| Social support or living situation of patient | 148 (39.5) | 18 (12.9) | 62 (50.4) | 68 (60.2) | <0.001 |
| Functional capacity at dischargea | 99 (26.4) | 37 (26.6) | 32 (26.0) | 30 (26.6) | 0.99 |
| Cognitive capacity at dischargeab | 30 (8.0) | 6 (4.4) | 11 (8.9) | 13 (11.5) | 0.10 |
| Physical exam at dischargea | 62 (16.7) | 19 (13.8) | 16 (13.1) | 27 (24.1) | 0.04 |
| Laboratory results at time of dischargea | 164 (43.9) | 63 (45.3) | 50 (40.7) | 51 (45.5) | 0.68 |
| Back to baseline or other nonspecific remark about discharge statusa | 71 (19.0) | 30 (21.6) | 18 (14.8) | 23 (20.4) | 0.34 |
| Any test or result still pending or specific comment that nothing is pendingb | 46 (12.2) | 9 (6.4) | 20 (16.3) | 17 (14.9) | 0.03 |
| Recommendation for follow‐up tests/procedures | 157 (41.9) | 43 (30.9) | 54 (43.9) | 60 (53.1) | 0.002 |
| Call‐back number of responsible in‐house physicianb | 23 (6.2) | 4 (2.9) | 2 (1.6) | 17 (15.2) | <0.001 |
| Resuscitation status | 27 (7.7) | 2 (1.5) | 18 (15.4) | 7 (6.7) | <0.001 |
| Etiology of heart failurec | 120 (82.8) | 44 (81.5) | 34 (87.2) | 42 (80.8) | 0.69 |
| Reason/trigger for exacerbationc | 86 (58.9) | 30 (55.6) | 27 (67.5) | 29 (55.8) | 0.43 |
| Ejection fractionc | 107 (73.3) | 40 (74.1) | 32 (80.0) | 35 (67.3) | 0.39 |
| Discharge weightc | 6 (4.1) | 1 (1.9) | 1 (2.5) | 4 (7.7) | 0.33 |
| Target weight rangec | 5 (3.4) | 0 (0) | 2 (5.0) | 3 (5.8) | 0.22 |
| Discharge creatinine or GFRc | 34 (23.3) | 14 (25.9) | 10 (25.0) | 10 (19.2) | 0.69 |
| If stent placed, whether drug‐eluting or notd | 89 (81.7) | 58 (87.9) | 27 (81.8) | 4 (40.0) | 0.001 |
On average, summaries included 5.6 of the 6 Joint Commission elements and 4.0 of the 7 TOCCC elements. A total of 63.0% of discharge summaries included all 6 elements required by The Joint Commission, whereas no discharge summary included all 7 TOCCC elements.
APRNs, housestaff and hospitalists included the same average number of The Joint Commission elements (5.6 each), but hospitalists on average included slightly more TOCCC elements (4.3) than did housestaff (4.0) or APRNs (3.8) (P<0.001). Summaries dictated on the day of discharge included an average of 4.2 TOCCC elements, compared to 3.9 TOCCC elements in delayed discharge. In multivariable analyses adjusted for diagnosis and length of stay, there was still no difference by training level in presence of The Joint Commission elements, but hospitalists were significantly more likely to include more TOCCC elements than APRNs (OR: 2.70; 95% CI: 1.49‐4.90) (Table 4). Summaries dictated on the day of discharge were significantly more likely to include more TOCCC elements (OR: 1.92; 95% CI: 1.23‐2.99).
| Explanatory Variable | Average Number of TOCCC Elements Included | OR (95% CI) | Adjusted P Value |
|---|---|---|---|
| |||
| Training level | 0.004 | ||
| APRN | 3.8 | REF | |
| Housestaff | 4.0 | 1.54 (0.90‐2.62) | |
| Hospitalist | 4.3 | 2.70 (1.49‐4.90) | |
| Timeliness | |||
| Dictated after discharge | 3.9 | REF | |
| Dictated day of discharge | 4.2 | 1.92 (1.23‐2.99) | 0.004 |
| Acute coronary syndrome vs nota | 3.9 | 0.72 (0.37‐1.39) | 0.33 |
| Pneumonia vs nota | 4.2 | 1.02 (0.49‐2.14) | 0.95 |
| Heart failure vs nota | 4.1 | 1.49 (0.80‐2.78) | 0.21 |
| Length of stay, d | 0.99 (0.90‐1.07) | 0.73 | |
No discharge summary included all 7 TOCCC‐endorsed content elements, was dictated on the day of discharge, and was sent to an outside physician.
DISCUSSION
In this prospective single‐site study of medical patients with 3 common conditions, we found that discharge summaries were completed relatively promptly, but were often not sent to the appropriate outpatient physicians. We also found that summaries were uniformly excellent at providing details of the hospitalization, but less reliable at providing details relevant to transitional care such as the patient's condition on discharge or existence of pending tests. On average, summaries included 57% of the elements included in consensus guidelines by 6 major medical societies. The content of discharge summaries dictated by hospitalists was slightly more comprehensive than that of APRNs and trainees, but no group exhibited high performance. In fact, not one discharge summary fully met all 3 quality criteria of timeliness, transmission, and content.
Our study, unlike most in the field, focused on multiple dimensions of discharge summary quality simultaneously. For instance, previous studies have found that timely receipt of a discharge summary does not reduce readmission rates.[11, 14, 15] Yet, if the content of the discharge summary is inadequate for postdischarge care, the summary may not be useful even if it is received by the follow‐up visit. Conversely, high‐quality content is ineffective if the summary is not sent to the outpatient physician.
This study suggests several avenues for improving summary quality. Timely discharge summaries in this study were more likely to include key content and to be transmitted to the appropriate physician. Strategies to improve discharge summary quality should therefore prioritize timely summaries, which can be expected to have downstream benefits for other aspects of quality. Some studies have found that templates improve discharge summary content.[22] In our institution, a template exists, but it favors a hospitalization‐focused rather than transition‐focused approach to the discharge summary. For instance, it includes instructions to dictate the admission exam, but not the discharge exam. Thus, designing templates specifically for transitional care is key. Maximizing capabilities of electronic records may help; many content elements that were commonly missing (e.g., pending results, discharge vitals, discharge weight) could be automatically inserted from electronic records. Likewise, automatic transmission of the summary to care providers listed in the electronic record might ameliorate many transmission failures. Some efforts have been made to convert existing electronic data into discharge summaries.[23, 24, 25] However, these activities are very preliminary, and some studies have found the quality of electronic summaries to be lower than dictated or handwritten summaries.[26] As with all automated or electronic applications, it will be essential to consider workflow, readability, and ability to synthesize information prior to adoption.
Hospitalists consistently produced highest‐quality summaries, even though they did not receive explicit training, suggesting experience may be beneficial,[27, 28, 29] or that the hospitalist community focus on transitional care has been effective. In addition, hospitalists at our institution explicitly prioritize timely and comprehensive discharge dictations, because their business relies on maintaining good relationships with outpatient physicians who contract for their services. Housestaff and APRNs have no such incentives or policies; rather, they typically consider discharge summaries to be a useful source of patient history at the time of an admission or readmission. Other academic centers have found similar results.[6, 16] Nonetheless, even though hospitalists had slightly better performance in our study, large gaps in the quality of summaries remained for all groups including hospitalists.
This study has several limitations. First, as a single‐site study at an academic hospital, it may not be generalizable to other hospitals or other settings. It is noteworthy, however, that the average time to dictation in this study was much lower than that of other studies,[4, 14, 30, 31, 32] suggesting that practices at this institution are at least no worse and possibly better than elsewhere. Second, although there are some mandates and expert opinion‐based guidelines for discharge summary content, there is no validated evidence base to confirm what content ought to be present in discharge summaries to improve patient outcomes. Third, we had too few readmissions in the dataset to have enough power to determine whether discharge summary content, timeliness, or transmission predicts readmission. Fourth, we did not determine whether the information in discharge summaries was accurate or complete; we merely assessed whether it was present. For example, we gave every discharge summary full credit for including discharge medications because they are automatically appended. Yet medication reconciliation errors at discharge are common.[33, 34] In fact, in the DISCHARGE study cohort, more than a quarter of discharge medication lists contained a suspected error.[35]
In summary, this study demonstrated the inadequacy of the contemporary discharge summary for conveying information that is critical to the transition from hospital to home. It may be that hospital culture treats hospitalizations as discrete and self‐contained events rather than as components of a larger episode of care. As interest in reducing readmissions rises, reframing the discharge summary to serve as a transitional tool and targeting it for quality assessment will likely be necessary.
Acknowledgments
The authors would like to acknowledge Amy Browning and the staff of the Center for Outcomes Research and Evaluation Follow‐Up Center for conducting patient interviews, Mark Abroms and Katherine Herman for patient recruitment and screening, and Peter Charpentier for Web site development.
Disclosures
At the time this study was conducted, Dr. Horwitz was supported by the CTSA Grant UL1 RR024139 and KL2 RR024138 from the National Center for Advancing Translational Sciences (NCATS), a component of the National Institutes of Health (NIH), and NIH roadmap for Medical Research, and was a Centers of Excellence Scholar in Geriatric Medicine by the John A. Hartford Foundation and the American Federation for Aging Research. Dr. Horwitz is now supported by the National Institute on Aging (K08 AG038336) and by the American Federation for Aging Research through the Paul B. Beeson Career Development Award Program. This work was also supported by a grant from the Claude D. Pepper Older Americans Independence Center at Yale University School of Medicine (P30AG021342 NIH/NIA). Dr. Krumholz is supported by grant U01 HL105270‐01 (Center for Cardiovascular Outcomes Research at Yale University) from the National Heart, Lung, and Blood Institute. No funding source had any role in the study design; in the collection, analysis, and interpretation of data; in the writing of the report; or in the decision to submit the article for publication. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute on Aging, the National Center for Advancing Translational Sciences, the National Institutes of Health, The John A. Hartford Foundation, the National Heart, Lung, and Blood Institute, or the American Federation for Aging Research. Dr. Horwitz had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. An earlier version of this work was presented as an oral presentation at the Society of General Internal Medicine Annual Meeting in Orlando, Florida on May 12, 2012. Dr. Krumholz chairs a cardiac scientific advisory board for UnitedHealth. Dr. Krumholz receives support from the Centers of Medicare and Medicaid Services (CMS) to develop and maintain performance measures that are used for public reporting, including readmission measures.
APPENDIX
A
Dictation guidelines provided to house staff and hospitalists
DICTATION GUIDELINES
FORMAT OF DISCHARGE SUMMARY
- Your name(spell it out), andPatient name(spell it out as well)
- Medical record number, date of admission, date of discharge
- Attending physician
- Disposition
- Principal and other diagnoses, Principal and other operations/procedures
- Copies to be sent to other physicians
- Begin narrative: CC, HPI, PMHx, Medications on admit, Social, Family Hx, Physical exam on admission, Data (labs on admission, plus labs relevant to workup, significant changes at discharge, admission EKG, radiologic and other data),Hospital course by problem, discharge meds, follow‐up appointments
APPENDIX
B
| Diagnosis |
| Discharge Second Diagnosis |
| Hospital course |
| Procedures/tests performed during admission |
| Patient and Family Instructions |
| Social support or living situation of patient |
| Functional capacity at discharge |
| Cognitive capacity at discharge |
| Physical exam at discharge |
| Laboratory results at time of discharge |
| Back to baseline or other nonspecific remark about discharge status |
| Any test or result still pending |
| Specific comment that nothing is pending |
| Recommendation for follow up tests/procedures |
| Call back number of responsible in‐house physician |
| Resuscitation status |
| Etiology of heart failure |
| Reason/trigger for exacerbation |
| Ejection fraction |
| Discharge weight |
| Target weight range |
| Discharge creatinine or GFR |
| If stent placed, whether drug‐eluting or not |
| Composite element | Data elements abstracted that qualify as meeting measure |
|---|---|
| Reason for hospitalization | Diagnosis |
| Significant findings | Hospital course |
| Procedures and treatment provided | Procedures/tests performed during admission |
| Patient's discharge condition | Functional capacity at discharge, Cognitive capacity at discharge, Physical exam at discharge, Laboratory results at time of discharge, Back to baseline or other nonspecific remark about discharge status |
| Patient and family instructions | Signs and symptoms to monitor at home |
| Attending physician's signature | Attending signature |
| Composite element | Data elements abstracted that qualify as meeting measure |
|---|---|
| Principal diagnosis | Diagnosis |
| Problem list | Discharge second diagnosis |
| Medication list | [Automatically appended; full credit to every summary] |
| Transferring physician name and contact information | Call back number of responsible in‐house physician |
| Cognitive status of the patient | Cognitive capacity at discharge |
| Test results | Procedures/tests performed during admission |
| Pending test results | Any test or result still pending or specific comment that nothing is pending |
APPENDIX
C
Histogram of days between discharge and dictation
- , , . Value of the specialist's report. Br Med J. 1960;2(5213):1663–1664.
- , . Communications between general practitioners and consultants. Br Med J. 1974;4(5942):456–459.
- , , . A functional hospital discharge summary. J Pediatr. 1975;86(1):97–98.
- , , , , , . Deficits in communication and information transfer between hospital‐based and primary care physicians: implications for patient safety and continuity of care. JAMA. 2007;297(8):831–841.
- , , , et al. Patient safety concerns arising from test results that return after hospital discharge. Ann Intern Med. 2005;143(2):121–128.
- , , , et al. Adequacy of hospital discharge summaries in documenting tests with pending results and outpatient follow‐up providers. J Gen Intern Med. 2009;24(9):1002–1006.
- , , . Tying up loose ends: discharging patients with unresolved medical issues. Arch Intern Med. 2007;167(12):1305–1311.
- Centers for Medicare and Medicaid Services. Condition of participation: medical record services. 42. Vol 482.C.F.R. § 482.24 (2012).
- Joint Commission on Accreditation of Healthcare Organizations. Hospital Accreditation Standards. Standard IM 6.10 EP 7–9. Oakbrook Terrace, IL: The Joint Commission; 2008.
- , . Documentation of mandated discharge summary components in transitions from acute to subacute care. In: Agency for Healthcare Research and Quality, ed. Advances in Patient Safety: New Directions and Alternative Approaches. Vol 2: Culture and Redesign. AHRQ Publication No. 08-0034‐2. Rockville, MD: Agency for Healthcare Research and Quality; 2008:179–188.
- , , , et al. Hospital discharge documentation and risk of rehospitalisation. BMJ Qual Saf. 2011;20(9):773–778.
- , , , et al. Transition of care for hospitalized elderly patients‐development of a discharge checklist for hospitalists. J Hosp Med. 2006;1(6):354–360.
- , , , et al. Transitions of Care Consensus Policy Statement American College of Physicians‐Society of General Internal Medicine‐Society of Hospital Medicine‐American Geriatrics Society‐American College of Emergency Physicians‐Society of Academic Emergency Medicine. J Gen Intern Med. 2009;24(8):971–976.
- , , , et al. Association of communication between hospital‐based physicians and primary care providers with patient outcomes. J Gen Intern Med. 2009;24(3):381–386.
- , , , . Effect of discharge summary availability during post‐discharge visits on hospital readmission. J Gen Intern Med. 2002;17(3):186–192.
- , , , , . Provider characteristics, clinical‐work processes and their relationship to discharge summary quality for sub‐acute care patients. J Gen Intern Med. 2012;27(1):78–84.
- , , , et al. ACC/AHA 2007 guidelines for the management of patients with unstable angina/non‐ST‐elevation myocardial infarction: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the 2002 Guidelines for the Management of Patients With Unstable Angina/Non‐ST‐Elevation Myocardial Infarction) developed in collaboration with the American College of Emergency Physicians, the Society for Cardiovascular Angiography and Interventions, and the Society of Thoracic Surgeons endorsed by the American Association of Cardiovascular and Pulmonary Rehabilitation and the Society for Academic Emergency Medicine. J Am Coll Cardiol. 2007;50(7):e1–e157.
- , , . Universal definition of myocardial infarction. Eur Heart J. 2007;28(20):2525–2538.
- , , , et al. ESC guidelines for the diagnosis and treatment of acute and chronic heart failure 2008: the Task Force for the diagnosis and treatment of acute and chronic heart failure 2008 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association of the ESC (HFA) and endorsed by the European Society of Intensive Care Medicine (ESICM). Eur J Heart Fail. 2008;10(10):933–989.
- , , , et al. Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community‐acquired pneumonia in adults. Clin Infect Dis. 2007;44(suppl 2):S27–S72.
- , , , et al. Clock drawing in Alzheimer's disease. A novel measure of dementia severity. J Am Geriatr Soc. 1989;37(8):725–729.
- , , , , . Assessing quality and efficiency of discharge summaries. Am J Med Qual. 2005;20(6):337–343.
- , , , et al. Electronic versus dictated hospital discharge summaries: a randomized controlled trial. J Gen Intern Med. 2009;24(9):995–1001.
- , , , . Dictated versus database‐generated discharge summaries: a randomized clinical trial. CMAJ. 1999;160(3):319–326.
- , , , , . Computerised updating of clinical summaries: new opportunities for clinical practice and research? BMJ. 1988;297(6662):1504–1506.
- , , . Evaluation of electronic discharge summaries: a comparison of documentation in electronic and handwritten discharge summaries. Int J Med Inform. 2008;77(9):613–620.
- , , , , . Did I do as best as the system would let me? Healthcare professional views on hospital to home care transitions. J Gen Intern Med. 2012;27(12):1649–1656.
- , , , , . Learning by doing—resident perspectives on developing competency in high‐quality discharge care. J Gen Intern Med. 2012;27(9):1188–1194.
- , , , , . Out of sight, out of mind: housestaff perceptions of quality‐limiting factors in discharge care at teaching hospitals. J Hosp Med. 2012;7(5):376–381.
- , , . Dissemination of discharge summaries. Not reaching follow‐up physicians. Can Fam Physician. 2002;48:737–742.
- , , , . Primary care physician attitudes regarding communication with hospitalists. Am J Med. 2001;111(9B):15S–20S.
- , , , . General practitioner‐hospital communications: a review of discharge summaries. J Qual Clin Pract. 2001;21(4):104–108.
- , , . Accuracy of information on medicines in hospital discharge summaries. Intern Med J. 2006;36(4):221–225.
- , , . Accuracy of medication documentation in hospital discharge summaries: A retrospective analysis of medication transcription errors in manual and electronic discharge summaries. Int J Med Inform. 2010;79(1):58–64.
- , , , . Medication reconciliation accuracy and patient understanding of intended medication changes on hospital discharge. J Gen Intern Med. 2012;27(11):1513–1520.
- , , . Value of the specialist's report. Br Med J. 1960;2(5213):1663–1664.
- , . Communications between general practitioners and consultants. Br Med J. 1974;4(5942):456–459.
- , , . A functional hospital discharge summary. J Pediatr. 1975;86(1):97–98.
- , , , , , . Deficits in communication and information transfer between hospital‐based and primary care physicians: implications for patient safety and continuity of care. JAMA. 2007;297(8):831–841.
- , , , et al. Patient safety concerns arising from test results that return after hospital discharge. Ann Intern Med. 2005;143(2):121–128.
- , , , et al. Adequacy of hospital discharge summaries in documenting tests with pending results and outpatient follow‐up providers. J Gen Intern Med. 2009;24(9):1002–1006.
- , , . Tying up loose ends: discharging patients with unresolved medical issues. Arch Intern Med. 2007;167(12):1305–1311.
- Centers for Medicare and Medicaid Services. Condition of participation: medical record services. 42. Vol 482.C.F.R. § 482.24 (2012).
- Joint Commission on Accreditation of Healthcare Organizations. Hospital Accreditation Standards. Standard IM 6.10 EP 7–9. Oakbrook Terrace, IL: The Joint Commission; 2008.
- , . Documentation of mandated discharge summary components in transitions from acute to subacute care. In: Agency for Healthcare Research and Quality, ed. Advances in Patient Safety: New Directions and Alternative Approaches. Vol 2: Culture and Redesign. AHRQ Publication No. 08-0034‐2. Rockville, MD: Agency for Healthcare Research and Quality; 2008:179–188.
- , , , et al. Hospital discharge documentation and risk of rehospitalisation. BMJ Qual Saf. 2011;20(9):773–778.
- , , , et al. Transition of care for hospitalized elderly patients‐development of a discharge checklist for hospitalists. J Hosp Med. 2006;1(6):354–360.
- , , , et al. Transitions of Care Consensus Policy Statement American College of Physicians‐Society of General Internal Medicine‐Society of Hospital Medicine‐American Geriatrics Society‐American College of Emergency Physicians‐Society of Academic Emergency Medicine. J Gen Intern Med. 2009;24(8):971–976.
- , , , et al. Association of communication between hospital‐based physicians and primary care providers with patient outcomes. J Gen Intern Med. 2009;24(3):381–386.
- , , , . Effect of discharge summary availability during post‐discharge visits on hospital readmission. J Gen Intern Med. 2002;17(3):186–192.
- , , , , . Provider characteristics, clinical‐work processes and their relationship to discharge summary quality for sub‐acute care patients. J Gen Intern Med. 2012;27(1):78–84.
- , , , et al. ACC/AHA 2007 guidelines for the management of patients with unstable angina/non‐ST‐elevation myocardial infarction: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the 2002 Guidelines for the Management of Patients With Unstable Angina/Non‐ST‐Elevation Myocardial Infarction) developed in collaboration with the American College of Emergency Physicians, the Society for Cardiovascular Angiography and Interventions, and the Society of Thoracic Surgeons endorsed by the American Association of Cardiovascular and Pulmonary Rehabilitation and the Society for Academic Emergency Medicine. J Am Coll Cardiol. 2007;50(7):e1–e157.
- , , . Universal definition of myocardial infarction. Eur Heart J. 2007;28(20):2525–2538.
- , , , et al. ESC guidelines for the diagnosis and treatment of acute and chronic heart failure 2008: the Task Force for the diagnosis and treatment of acute and chronic heart failure 2008 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association of the ESC (HFA) and endorsed by the European Society of Intensive Care Medicine (ESICM). Eur J Heart Fail. 2008;10(10):933–989.
- , , , et al. Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community‐acquired pneumonia in adults. Clin Infect Dis. 2007;44(suppl 2):S27–S72.
- , , , et al. Clock drawing in Alzheimer's disease. A novel measure of dementia severity. J Am Geriatr Soc. 1989;37(8):725–729.
- , , , , . Assessing quality and efficiency of discharge summaries. Am J Med Qual. 2005;20(6):337–343.
- , , , et al. Electronic versus dictated hospital discharge summaries: a randomized controlled trial. J Gen Intern Med. 2009;24(9):995–1001.
- , , , . Dictated versus database‐generated discharge summaries: a randomized clinical trial. CMAJ. 1999;160(3):319–326.
- , , , , . Computerised updating of clinical summaries: new opportunities for clinical practice and research? BMJ. 1988;297(6662):1504–1506.
- , , . Evaluation of electronic discharge summaries: a comparison of documentation in electronic and handwritten discharge summaries. Int J Med Inform. 2008;77(9):613–620.
- , , , , . Did I do as best as the system would let me? Healthcare professional views on hospital to home care transitions. J Gen Intern Med. 2012;27(12):1649–1656.
- , , , , . Learning by doing—resident perspectives on developing competency in high‐quality discharge care. J Gen Intern Med. 2012;27(9):1188–1194.
- , , , , . Out of sight, out of mind: housestaff perceptions of quality‐limiting factors in discharge care at teaching hospitals. J Hosp Med. 2012;7(5):376–381.
- , , . Dissemination of discharge summaries. Not reaching follow‐up physicians. Can Fam Physician. 2002;48:737–742.
- , , , . Primary care physician attitudes regarding communication with hospitalists. Am J Med. 2001;111(9B):15S–20S.
- , , , . General practitioner‐hospital communications: a review of discharge summaries. J Qual Clin Pract. 2001;21(4):104–108.
- , , . Accuracy of information on medicines in hospital discharge summaries. Intern Med J. 2006;36(4):221–225.
- , , . Accuracy of medication documentation in hospital discharge summaries: A retrospective analysis of medication transcription errors in manual and electronic discharge summaries. Int J Med Inform. 2010;79(1):58–64.
- , , , . Medication reconciliation accuracy and patient understanding of intended medication changes on hospital discharge. J Gen Intern Med. 2012;27(11):1513–1520.
Copyright © 2013 Society of Hospital Medicine
Decreased hospital LOS not associated with increase in 30-day readmission rates
Clinical question
Does decreased length of stay result in increased risk of 30-day readmission for hospitalized patients with acute medical illness?
Bottom line
Reduction in length of stay (LOS) is not associated with increased risk of 30-day readmission for patients with acute medical illness. Although this may suggest that decreased LOS does not affect quality of care, this finding may also be due to improved efficiencies in a previously inefficient Veteran Affairs (VA) system leading to earlier discharges and increased efforts at bettering transitions of care. LOE = 2b
Reference
Study design
Cohort (retrospective)
Funding source
Government
Allocation
Uncertain
Setting
Inpatient (any location)
Synopsis
To determine whether reductions in LOS adversely affect 30-day readmission rates, these investigators used a national VA administrative database to identify all acute medical admissions to VA hospitals from 1997 to 2010. Patients who died, were transferred to another acute care facility, or whose LOS was longer than 30 days were excluded from consideration. Readmissions were defined as those that were linked to the index admission and occurred within 30 days of discharge. The cohort consisted of more than 4 million admissions and was further subdivided into 5 high-volume diagnoses: heart failure, chronic obstructive pulmonary disease (COPD), heart failure, acute myocardial infarction (AMI), community-acquired pneumonia, and gastrointestinal bleed. After adjusting for hospital and patient characteristics, LOS decreased during the 14-year period from 5.44 days to 3.98 days, and 30-day readmission rates decreased from 16.5% to 13.8%. Among the 5 high-volume conditions, LOS decreased the most for AMI (by almost 3 days) while readmission rates decreased the most for COPD (3.3%). Further analysis of all medical conditions showed that each additional day of stay resulted in a 3% increased rate of readmission. This was likely due to unmeasured severity of illness that affected both LOS and readmission. Of note, however, hospitals that had a mean LOS lower than the average LOS across all hospitals had higher readmissions rates (6% increase for each day lower than the average). Despite this, the overall readmission rate decreased over time as LOS decreased. All-cause mortality at 30 days and 90 days also improved over time.
Clinical question
Does decreased length of stay result in increased risk of 30-day readmission for hospitalized patients with acute medical illness?
Bottom line
Reduction in length of stay (LOS) is not associated with increased risk of 30-day readmission for patients with acute medical illness. Although this may suggest that decreased LOS does not affect quality of care, this finding may also be due to improved efficiencies in a previously inefficient Veteran Affairs (VA) system leading to earlier discharges and increased efforts at bettering transitions of care. LOE = 2b
Reference
Study design
Cohort (retrospective)
Funding source
Government
Allocation
Uncertain
Setting
Inpatient (any location)
Synopsis
To determine whether reductions in LOS adversely affect 30-day readmission rates, these investigators used a national VA administrative database to identify all acute medical admissions to VA hospitals from 1997 to 2010. Patients who died, were transferred to another acute care facility, or whose LOS was longer than 30 days were excluded from consideration. Readmissions were defined as those that were linked to the index admission and occurred within 30 days of discharge. The cohort consisted of more than 4 million admissions and was further subdivided into 5 high-volume diagnoses: heart failure, chronic obstructive pulmonary disease (COPD), heart failure, acute myocardial infarction (AMI), community-acquired pneumonia, and gastrointestinal bleed. After adjusting for hospital and patient characteristics, LOS decreased during the 14-year period from 5.44 days to 3.98 days, and 30-day readmission rates decreased from 16.5% to 13.8%. Among the 5 high-volume conditions, LOS decreased the most for AMI (by almost 3 days) while readmission rates decreased the most for COPD (3.3%). Further analysis of all medical conditions showed that each additional day of stay resulted in a 3% increased rate of readmission. This was likely due to unmeasured severity of illness that affected both LOS and readmission. Of note, however, hospitals that had a mean LOS lower than the average LOS across all hospitals had higher readmissions rates (6% increase for each day lower than the average). Despite this, the overall readmission rate decreased over time as LOS decreased. All-cause mortality at 30 days and 90 days also improved over time.
Clinical question
Does decreased length of stay result in increased risk of 30-day readmission for hospitalized patients with acute medical illness?
Bottom line
Reduction in length of stay (LOS) is not associated with increased risk of 30-day readmission for patients with acute medical illness. Although this may suggest that decreased LOS does not affect quality of care, this finding may also be due to improved efficiencies in a previously inefficient Veteran Affairs (VA) system leading to earlier discharges and increased efforts at bettering transitions of care. LOE = 2b
Reference
Study design
Cohort (retrospective)
Funding source
Government
Allocation
Uncertain
Setting
Inpatient (any location)
Synopsis
To determine whether reductions in LOS adversely affect 30-day readmission rates, these investigators used a national VA administrative database to identify all acute medical admissions to VA hospitals from 1997 to 2010. Patients who died, were transferred to another acute care facility, or whose LOS was longer than 30 days were excluded from consideration. Readmissions were defined as those that were linked to the index admission and occurred within 30 days of discharge. The cohort consisted of more than 4 million admissions and was further subdivided into 5 high-volume diagnoses: heart failure, chronic obstructive pulmonary disease (COPD), heart failure, acute myocardial infarction (AMI), community-acquired pneumonia, and gastrointestinal bleed. After adjusting for hospital and patient characteristics, LOS decreased during the 14-year period from 5.44 days to 3.98 days, and 30-day readmission rates decreased from 16.5% to 13.8%. Among the 5 high-volume conditions, LOS decreased the most for AMI (by almost 3 days) while readmission rates decreased the most for COPD (3.3%). Further analysis of all medical conditions showed that each additional day of stay resulted in a 3% increased rate of readmission. This was likely due to unmeasured severity of illness that affected both LOS and readmission. Of note, however, hospitals that had a mean LOS lower than the average LOS across all hospitals had higher readmissions rates (6% increase for each day lower than the average). Despite this, the overall readmission rate decreased over time as LOS decreased. All-cause mortality at 30 days and 90 days also improved over time.
Femoral lines not associated with increased risk of bloodstream infections
Clinical question
Do central venous catheters in the femoral vein increase the risk of catheter-related bloodstream infections as compared with those placed in the subclavian or internal jugular veins?
Bottom line
The risk of catheter-related bloodstream infections (CRBIs) from nontunneled central venous catheters has decreased in the last decade.This review suggests that there is no difference in risk of CRBIs when comparing catheters placed in femoral sites with those placed in subclavian or internal jugular (IJ) sites, especially when looking at data from more recent studies. LOE = 1a
Reference
Study design
Meta-analysis (other)
Funding source
Unknown/not stated
Allocation
Uncertain
Setting
Inpatient (any location)
Synopsis
Current guidelines from the Centers for Disease Control recommend avoiding the femoral vein for central access in adult patients because of a potentially higher risk of CRBI. Two independent investigators searched MEDLINE, EMBASE, the Cochrane Database of Systematic Reviews, and bibliographies of relevant articles, as well as performed an Internet search, to find randomized controlled trials (RCTs) and cohort studies that examined the risk of CRBIs due to nontunneled central venous catheters placed in the femoral site as compared with those placed in the subclavian or IJ sites. Two RCTs, 8 cohort studies, and data from a Welsh infection control surveillance Web site were selected. Two authors independently extracted data from the selected studies. No formal quality assessment of the studies was performed. Data from the RCTs alone showed no difference in CRBIs between femoral sites and subclavian or IJ sites. Data from all the studies that compared femoral sites to subclavian sites showed no significant difference in the risk of CRBIs. For comparisons of femoral and IJ sites, the overall data favored the IJ site (relative risk of infection with femoral site placement = 1.90; 95% CI, 1.21-2.97; P = .005). However, 2 of the 9 included studies in this analysis were "statistical outliers," possibly due to unique circumstances in the hospitals in which they were performed, thus limiting their generalizability. When these 2 studies were removed from the analysis, there was no significant difference between femoral and IJ sites. For both comparisons (femoral vs subclavian and femoral vs IJ), there was an interaction between risk of infection and year of study publication, with earlier studies noting a greater risk of infection with femoral sites. Overall, this data confirms a decrease in incidence of CRBIs by more than 50% in the last 10 years. Additionally, study meta-analysis found no difference in the risk of deep venous thrombosis with femoral versus subclavian and IJ sites.
Clinical question
Do central venous catheters in the femoral vein increase the risk of catheter-related bloodstream infections as compared with those placed in the subclavian or internal jugular veins?
Bottom line
The risk of catheter-related bloodstream infections (CRBIs) from nontunneled central venous catheters has decreased in the last decade.This review suggests that there is no difference in risk of CRBIs when comparing catheters placed in femoral sites with those placed in subclavian or internal jugular (IJ) sites, especially when looking at data from more recent studies. LOE = 1a
Reference
Study design
Meta-analysis (other)
Funding source
Unknown/not stated
Allocation
Uncertain
Setting
Inpatient (any location)
Synopsis
Current guidelines from the Centers for Disease Control recommend avoiding the femoral vein for central access in adult patients because of a potentially higher risk of CRBI. Two independent investigators searched MEDLINE, EMBASE, the Cochrane Database of Systematic Reviews, and bibliographies of relevant articles, as well as performed an Internet search, to find randomized controlled trials (RCTs) and cohort studies that examined the risk of CRBIs due to nontunneled central venous catheters placed in the femoral site as compared with those placed in the subclavian or IJ sites. Two RCTs, 8 cohort studies, and data from a Welsh infection control surveillance Web site were selected. Two authors independently extracted data from the selected studies. No formal quality assessment of the studies was performed. Data from the RCTs alone showed no difference in CRBIs between femoral sites and subclavian or IJ sites. Data from all the studies that compared femoral sites to subclavian sites showed no significant difference in the risk of CRBIs. For comparisons of femoral and IJ sites, the overall data favored the IJ site (relative risk of infection with femoral site placement = 1.90; 95% CI, 1.21-2.97; P = .005). However, 2 of the 9 included studies in this analysis were "statistical outliers," possibly due to unique circumstances in the hospitals in which they were performed, thus limiting their generalizability. When these 2 studies were removed from the analysis, there was no significant difference between femoral and IJ sites. For both comparisons (femoral vs subclavian and femoral vs IJ), there was an interaction between risk of infection and year of study publication, with earlier studies noting a greater risk of infection with femoral sites. Overall, this data confirms a decrease in incidence of CRBIs by more than 50% in the last 10 years. Additionally, study meta-analysis found no difference in the risk of deep venous thrombosis with femoral versus subclavian and IJ sites.
Clinical question
Do central venous catheters in the femoral vein increase the risk of catheter-related bloodstream infections as compared with those placed in the subclavian or internal jugular veins?
Bottom line
The risk of catheter-related bloodstream infections (CRBIs) from nontunneled central venous catheters has decreased in the last decade.This review suggests that there is no difference in risk of CRBIs when comparing catheters placed in femoral sites with those placed in subclavian or internal jugular (IJ) sites, especially when looking at data from more recent studies. LOE = 1a
Reference
Study design
Meta-analysis (other)
Funding source
Unknown/not stated
Allocation
Uncertain
Setting
Inpatient (any location)
Synopsis
Current guidelines from the Centers for Disease Control recommend avoiding the femoral vein for central access in adult patients because of a potentially higher risk of CRBI. Two independent investigators searched MEDLINE, EMBASE, the Cochrane Database of Systematic Reviews, and bibliographies of relevant articles, as well as performed an Internet search, to find randomized controlled trials (RCTs) and cohort studies that examined the risk of CRBIs due to nontunneled central venous catheters placed in the femoral site as compared with those placed in the subclavian or IJ sites. Two RCTs, 8 cohort studies, and data from a Welsh infection control surveillance Web site were selected. Two authors independently extracted data from the selected studies. No formal quality assessment of the studies was performed. Data from the RCTs alone showed no difference in CRBIs between femoral sites and subclavian or IJ sites. Data from all the studies that compared femoral sites to subclavian sites showed no significant difference in the risk of CRBIs. For comparisons of femoral and IJ sites, the overall data favored the IJ site (relative risk of infection with femoral site placement = 1.90; 95% CI, 1.21-2.97; P = .005). However, 2 of the 9 included studies in this analysis were "statistical outliers," possibly due to unique circumstances in the hospitals in which they were performed, thus limiting their generalizability. When these 2 studies were removed from the analysis, there was no significant difference between femoral and IJ sites. For both comparisons (femoral vs subclavian and femoral vs IJ), there was an interaction between risk of infection and year of study publication, with earlier studies noting a greater risk of infection with femoral sites. Overall, this data confirms a decrease in incidence of CRBIs by more than 50% in the last 10 years. Additionally, study meta-analysis found no difference in the risk of deep venous thrombosis with femoral versus subclavian and IJ sites.
Norovirus now top cause of acute gastroenteritis in young U.S. children
Norovirus is now the leading cause of acute gastroenteritis requiring medical care among U.S. children younger than 5 years of age, according to a report published online March 20 in the New England Journal of Medicine.
Now that rotavirus vaccines have dramatically reduced the number of acute gastroenteritis cases attributable to that organism, norovirus infections have taken over the lead in causing the disorder in the young U.S. pediatric population. Norovirus is responsible for an estimated 1 million health care visits each year for this age group, at an estimated cost approaching $300 million, said Daniel C. Payne, Ph.D., of the National Center for Immunization and Respiratory Diseases, Centers for Disease Control and Prevention, and his associates.
"According to our estimation, by their fifth birthday, 1 in 278 U.S. children are hospitalized for norovirus infection, 1 in 14 are seen in the emergency department, and 1 in 6 are seen by outpatient care providers," the investigators noted.
They studied the epidemiology of the infection because now that candidate norovirus vaccines are in development, "there is a need to directly measure the pediatric health care burden of norovirus-associated gastroenteritis."
Dr. Payne and his colleagues analyzed data from the New Vaccine Surveillance Network, which collects information on the medical care of children residing near Rochester, N.Y.; Nashville, Tenn.; and Cincinnati – a catchment population exceeding 141,000 children under age 5.
The researchers prospectively assessed cases of acute gastroenteritis treated at hospitals, emergency departments, and outpatient clinics during two successive 12-month surveillance periods between October 2008 and September 2010. There were 1,077 cases the first year and 820 the second year; the data from these were compared with data from 806 age-matched children attending well-child visits, who served as a control group.
The disease burden of norovirus infection was "consistently high" during both years, accounting for 20%-22% of cases of acute gastroenteritis. Norovirus was detected in 4% of healthy controls in 2009. The overall rate of medical attention for the infection was highest – 47% – among children aged 6-18 months, Dr. Payne and his associates reported (N. Engl. J. Med. 2013;368:1121-30).
This study was supported by the CDC. Dr. Payne reported that he did not have any conflicts of interest relevant to this study. His coauthors reported ties to GlaxoSmithKline, Merck, and Luminex Molecular Diagnostics.
Norovirus is now the leading cause of acute gastroenteritis requiring medical care among U.S. children younger than 5 years of age, according to a report published online March 20 in the New England Journal of Medicine.
Now that rotavirus vaccines have dramatically reduced the number of acute gastroenteritis cases attributable to that organism, norovirus infections have taken over the lead in causing the disorder in the young U.S. pediatric population. Norovirus is responsible for an estimated 1 million health care visits each year for this age group, at an estimated cost approaching $300 million, said Daniel C. Payne, Ph.D., of the National Center for Immunization and Respiratory Diseases, Centers for Disease Control and Prevention, and his associates.
"According to our estimation, by their fifth birthday, 1 in 278 U.S. children are hospitalized for norovirus infection, 1 in 14 are seen in the emergency department, and 1 in 6 are seen by outpatient care providers," the investigators noted.
They studied the epidemiology of the infection because now that candidate norovirus vaccines are in development, "there is a need to directly measure the pediatric health care burden of norovirus-associated gastroenteritis."
Dr. Payne and his colleagues analyzed data from the New Vaccine Surveillance Network, which collects information on the medical care of children residing near Rochester, N.Y.; Nashville, Tenn.; and Cincinnati – a catchment population exceeding 141,000 children under age 5.
The researchers prospectively assessed cases of acute gastroenteritis treated at hospitals, emergency departments, and outpatient clinics during two successive 12-month surveillance periods between October 2008 and September 2010. There were 1,077 cases the first year and 820 the second year; the data from these were compared with data from 806 age-matched children attending well-child visits, who served as a control group.
The disease burden of norovirus infection was "consistently high" during both years, accounting for 20%-22% of cases of acute gastroenteritis. Norovirus was detected in 4% of healthy controls in 2009. The overall rate of medical attention for the infection was highest – 47% – among children aged 6-18 months, Dr. Payne and his associates reported (N. Engl. J. Med. 2013;368:1121-30).
This study was supported by the CDC. Dr. Payne reported that he did not have any conflicts of interest relevant to this study. His coauthors reported ties to GlaxoSmithKline, Merck, and Luminex Molecular Diagnostics.
Norovirus is now the leading cause of acute gastroenteritis requiring medical care among U.S. children younger than 5 years of age, according to a report published online March 20 in the New England Journal of Medicine.
Now that rotavirus vaccines have dramatically reduced the number of acute gastroenteritis cases attributable to that organism, norovirus infections have taken over the lead in causing the disorder in the young U.S. pediatric population. Norovirus is responsible for an estimated 1 million health care visits each year for this age group, at an estimated cost approaching $300 million, said Daniel C. Payne, Ph.D., of the National Center for Immunization and Respiratory Diseases, Centers for Disease Control and Prevention, and his associates.
"According to our estimation, by their fifth birthday, 1 in 278 U.S. children are hospitalized for norovirus infection, 1 in 14 are seen in the emergency department, and 1 in 6 are seen by outpatient care providers," the investigators noted.
They studied the epidemiology of the infection because now that candidate norovirus vaccines are in development, "there is a need to directly measure the pediatric health care burden of norovirus-associated gastroenteritis."
Dr. Payne and his colleagues analyzed data from the New Vaccine Surveillance Network, which collects information on the medical care of children residing near Rochester, N.Y.; Nashville, Tenn.; and Cincinnati – a catchment population exceeding 141,000 children under age 5.
The researchers prospectively assessed cases of acute gastroenteritis treated at hospitals, emergency departments, and outpatient clinics during two successive 12-month surveillance periods between October 2008 and September 2010. There were 1,077 cases the first year and 820 the second year; the data from these were compared with data from 806 age-matched children attending well-child visits, who served as a control group.
The disease burden of norovirus infection was "consistently high" during both years, accounting for 20%-22% of cases of acute gastroenteritis. Norovirus was detected in 4% of healthy controls in 2009. The overall rate of medical attention for the infection was highest – 47% – among children aged 6-18 months, Dr. Payne and his associates reported (N. Engl. J. Med. 2013;368:1121-30).
This study was supported by the CDC. Dr. Payne reported that he did not have any conflicts of interest relevant to this study. His coauthors reported ties to GlaxoSmithKline, Merck, and Luminex Molecular Diagnostics.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Major Finding: By the time U.S. children turn 5, 1 in 278 is admitted to the hospital for a norovirus infection, 1 in 14 is seen in an emergency department, and 1 in 6 is seen by an outpatient health care provider, at a cost of $273 million annually.
Data Source: A prospective, population-based surveillance study of norovirus infections in children under age 5.
Disclosures: This study was supported by the CDC. Dr. Payne said that he did not have any conflicts of interest relevant to this study. His coauthors reported ties to GlaxoSmithKline, Merck, and Luminex Molecular Diagnostics.
FDA Recommends New Opioids Research Prove Abuse-Deterrent Properties
Inappropriate use of prescription opioids is a major public health challenge, prompting the U.S. Food and Drug Administration (FDA) to issue a draft guidance document aimed at helping industry create new formulations of opioids with abuse-deterrent properties.
Released in January, “Guidance for Industry: Abuse-Deterrent Opioids—Evaluation and Labeling” provides recommendations for conducting studies to prove that a particular formulation contains abuse-deterrent properties. It also explains how the FDA will review the results and determine which labeling claims to approve.
This announcement is “one component of our larger effort to prevent prescription drug abuse and misuse, while ensuring that patients in pain continue to have access to these important medicines,” Douglas Throckmorton, MD, deputy director for regulatory programs in the FDA’s Center for Drug Evaluation and Research, said during a teleconference.
According to the FDA guidance, opioid analgesics can be abused in a variety of ways:
- Swallowed whole;
- Crushed and swallowed;
- Crushed and snorted;
- Crushed and smoked; or
- Crushed, dissolved, and injected.
With the science of abuse deterrence being relatively new, the FDA plans to take a flexible and adaptive approach. That’s because the analytical, clinical, and statistical methods for evaluating formulation technologies are still evolving.
“Physicians should care about this because the government is regulating prescribing practices more directly than in the past, especially with pain drugs,” says Daniel Carpenter, PhD, a Harvard University government professor and author on FDA pharmaceutical regulation. “The FDA and federal agencies are going to be leaning more heavily upon physicians.”
To date, the majority of current abuse-deterrent technologies have not been effective in preventing the most widespread type of abuse—ingesting a number of pills or tablets to reach a state of euphoria.
—Daniel Carpenter, PhD, Harvard University government professor and author on FDA pharmaceutical regulation
Science points toward ways that formulations can help thwart abuse. For instance, adding an opioid antagonist can hinder, limit, or defeat euphoria. An antagonist can be sequestered and released only upon the product’s manipulation. In one such scenario, the substance acting as an antagonist could be clinically inactive when swallowed, but then would become active if the product is crushed and injected or snorted.
“The guidance describes advice for the development of abuse-deterrent opioids and does not describe practice guidelines,” says Christopher Kelly, an FDA spokesman. However, he adds, “[FDA] urges all prescribers of extended-release and long-acting opioids to participate in the training under the Risk Evaluation and Mitigation Strategy (REMS).” The first REMS-compliant training is expected to become available by March 1.
Such a strategy is intended to manage known or potential serious risks associated with a drug product. The FDA requires it to ensure that the benefits of a drug outweigh its risks.
Manufacturers of opioid analgesics have worked with the FDA to produce materials for the REMS program that would inform healthcare professionals about safe prescribing. Continuing-education providers also are designing accredited training. (For more information, listen to this NIH podcast about training to help providers prescribe painkillers properly.)
Prescribers are advised to complete a REMS-compliant program through an accredited continuing-education provider for their discipline. They should discuss the safe use, serious risks, storage, and disposal of opioids with patients and caregivers each time they prescribe these medicines. It’s also essential to stress the importance of reading the medication guide they will receive from the pharmacist at drug-dispensing time.
Whether the FDA’s industry guidance for the development of abuse-deterrent opioids will make a difference remains to be seen, according to Carpenter. The addictive potential of opioids has created “a kind of public health epidemic,” he says. “It’s not an infectious epidemic in the sense of the flu, but it’s socially and behaviorally infectious and very destructive.”
Creating better tamper-resistant drugs could impede someone from “taking a longer-acting version and breaking it down into a much more toxic soup for other purposes,” Carpenter says. However, he concedes it won’t be impossible to swallow one or more pills too many, leading to this very common form of pharmaceutical abuse.
The FDA is accepting public comment on the draft guidance, while encouraging further scientific and clinical research to advance the development and assessment of abuse-deterrent technologies.
Susan Kreimer is a freelance writer based in New York.
Inappropriate use of prescription opioids is a major public health challenge, prompting the U.S. Food and Drug Administration (FDA) to issue a draft guidance document aimed at helping industry create new formulations of opioids with abuse-deterrent properties.
Released in January, “Guidance for Industry: Abuse-Deterrent Opioids—Evaluation and Labeling” provides recommendations for conducting studies to prove that a particular formulation contains abuse-deterrent properties. It also explains how the FDA will review the results and determine which labeling claims to approve.
This announcement is “one component of our larger effort to prevent prescription drug abuse and misuse, while ensuring that patients in pain continue to have access to these important medicines,” Douglas Throckmorton, MD, deputy director for regulatory programs in the FDA’s Center for Drug Evaluation and Research, said during a teleconference.
According to the FDA guidance, opioid analgesics can be abused in a variety of ways:
- Swallowed whole;
- Crushed and swallowed;
- Crushed and snorted;
- Crushed and smoked; or
- Crushed, dissolved, and injected.
With the science of abuse deterrence being relatively new, the FDA plans to take a flexible and adaptive approach. That’s because the analytical, clinical, and statistical methods for evaluating formulation technologies are still evolving.
“Physicians should care about this because the government is regulating prescribing practices more directly than in the past, especially with pain drugs,” says Daniel Carpenter, PhD, a Harvard University government professor and author on FDA pharmaceutical regulation. “The FDA and federal agencies are going to be leaning more heavily upon physicians.”
To date, the majority of current abuse-deterrent technologies have not been effective in preventing the most widespread type of abuse—ingesting a number of pills or tablets to reach a state of euphoria.
—Daniel Carpenter, PhD, Harvard University government professor and author on FDA pharmaceutical regulation
Science points toward ways that formulations can help thwart abuse. For instance, adding an opioid antagonist can hinder, limit, or defeat euphoria. An antagonist can be sequestered and released only upon the product’s manipulation. In one such scenario, the substance acting as an antagonist could be clinically inactive when swallowed, but then would become active if the product is crushed and injected or snorted.
“The guidance describes advice for the development of abuse-deterrent opioids and does not describe practice guidelines,” says Christopher Kelly, an FDA spokesman. However, he adds, “[FDA] urges all prescribers of extended-release and long-acting opioids to participate in the training under the Risk Evaluation and Mitigation Strategy (REMS).” The first REMS-compliant training is expected to become available by March 1.
Such a strategy is intended to manage known or potential serious risks associated with a drug product. The FDA requires it to ensure that the benefits of a drug outweigh its risks.
Manufacturers of opioid analgesics have worked with the FDA to produce materials for the REMS program that would inform healthcare professionals about safe prescribing. Continuing-education providers also are designing accredited training. (For more information, listen to this NIH podcast about training to help providers prescribe painkillers properly.)
Prescribers are advised to complete a REMS-compliant program through an accredited continuing-education provider for their discipline. They should discuss the safe use, serious risks, storage, and disposal of opioids with patients and caregivers each time they prescribe these medicines. It’s also essential to stress the importance of reading the medication guide they will receive from the pharmacist at drug-dispensing time.
Whether the FDA’s industry guidance for the development of abuse-deterrent opioids will make a difference remains to be seen, according to Carpenter. The addictive potential of opioids has created “a kind of public health epidemic,” he says. “It’s not an infectious epidemic in the sense of the flu, but it’s socially and behaviorally infectious and very destructive.”
Creating better tamper-resistant drugs could impede someone from “taking a longer-acting version and breaking it down into a much more toxic soup for other purposes,” Carpenter says. However, he concedes it won’t be impossible to swallow one or more pills too many, leading to this very common form of pharmaceutical abuse.
The FDA is accepting public comment on the draft guidance, while encouraging further scientific and clinical research to advance the development and assessment of abuse-deterrent technologies.
Susan Kreimer is a freelance writer based in New York.
Inappropriate use of prescription opioids is a major public health challenge, prompting the U.S. Food and Drug Administration (FDA) to issue a draft guidance document aimed at helping industry create new formulations of opioids with abuse-deterrent properties.
Released in January, “Guidance for Industry: Abuse-Deterrent Opioids—Evaluation and Labeling” provides recommendations for conducting studies to prove that a particular formulation contains abuse-deterrent properties. It also explains how the FDA will review the results and determine which labeling claims to approve.
This announcement is “one component of our larger effort to prevent prescription drug abuse and misuse, while ensuring that patients in pain continue to have access to these important medicines,” Douglas Throckmorton, MD, deputy director for regulatory programs in the FDA’s Center for Drug Evaluation and Research, said during a teleconference.
According to the FDA guidance, opioid analgesics can be abused in a variety of ways:
- Swallowed whole;
- Crushed and swallowed;
- Crushed and snorted;
- Crushed and smoked; or
- Crushed, dissolved, and injected.
With the science of abuse deterrence being relatively new, the FDA plans to take a flexible and adaptive approach. That’s because the analytical, clinical, and statistical methods for evaluating formulation technologies are still evolving.
“Physicians should care about this because the government is regulating prescribing practices more directly than in the past, especially with pain drugs,” says Daniel Carpenter, PhD, a Harvard University government professor and author on FDA pharmaceutical regulation. “The FDA and federal agencies are going to be leaning more heavily upon physicians.”
To date, the majority of current abuse-deterrent technologies have not been effective in preventing the most widespread type of abuse—ingesting a number of pills or tablets to reach a state of euphoria.
—Daniel Carpenter, PhD, Harvard University government professor and author on FDA pharmaceutical regulation
Science points toward ways that formulations can help thwart abuse. For instance, adding an opioid antagonist can hinder, limit, or defeat euphoria. An antagonist can be sequestered and released only upon the product’s manipulation. In one such scenario, the substance acting as an antagonist could be clinically inactive when swallowed, but then would become active if the product is crushed and injected or snorted.
“The guidance describes advice for the development of abuse-deterrent opioids and does not describe practice guidelines,” says Christopher Kelly, an FDA spokesman. However, he adds, “[FDA] urges all prescribers of extended-release and long-acting opioids to participate in the training under the Risk Evaluation and Mitigation Strategy (REMS).” The first REMS-compliant training is expected to become available by March 1.
Such a strategy is intended to manage known or potential serious risks associated with a drug product. The FDA requires it to ensure that the benefits of a drug outweigh its risks.
Manufacturers of opioid analgesics have worked with the FDA to produce materials for the REMS program that would inform healthcare professionals about safe prescribing. Continuing-education providers also are designing accredited training. (For more information, listen to this NIH podcast about training to help providers prescribe painkillers properly.)
Prescribers are advised to complete a REMS-compliant program through an accredited continuing-education provider for their discipline. They should discuss the safe use, serious risks, storage, and disposal of opioids with patients and caregivers each time they prescribe these medicines. It’s also essential to stress the importance of reading the medication guide they will receive from the pharmacist at drug-dispensing time.
Whether the FDA’s industry guidance for the development of abuse-deterrent opioids will make a difference remains to be seen, according to Carpenter. The addictive potential of opioids has created “a kind of public health epidemic,” he says. “It’s not an infectious epidemic in the sense of the flu, but it’s socially and behaviorally infectious and very destructive.”
Creating better tamper-resistant drugs could impede someone from “taking a longer-acting version and breaking it down into a much more toxic soup for other purposes,” Carpenter says. However, he concedes it won’t be impossible to swallow one or more pills too many, leading to this very common form of pharmaceutical abuse.
The FDA is accepting public comment on the draft guidance, while encouraging further scientific and clinical research to advance the development and assessment of abuse-deterrent technologies.
Susan Kreimer is a freelance writer based in New York.
Old gout drug learns new cardiac tricks
SAN FRANCISCO – The venerable antihyperuricemic agent allopurinol has shown early promise for two novel cardiovascular applications: prevention of atrial fibrillation in the setting of heart failure and reduction of left ventricular hypertrophy in patients with type 2 diabetes.
Allopurinol is a xanthine oxidase inhibitor and antigout drug. The rationale for the drug’s use in reducing the incidence of atrial fibrillation in patients with heart failure lies in the observation that serum uric acid has emerged as an independent marker of mortality and a predictor of new-onset atrial fibrillation in heart failure. Xanthine oxidase is not only a source of reactive oxygen species that adversely affect myocardial function, but it also catalyzes the conversion of xanthine to uric acid, Dr. Fernando E. Hernandez explained at the annual meeting of the American College of Cardiology.
He presented a retrospective cohort study involving 603 patients enrolled in the Miami Veterans Affairs heart failure clinic. The 103 on allopurinol, and the 500 who were not, matched up well in terms of baseline characteristics including age, prevalence of coronary artery disease, median left ventricular ejection, left atrial size, and use of guideline-recommended ACE inhibitors and beta-blockers.
During up to 5 years of follow-up, the incidence of new-onset atrial fibrillation was 184 cases/1,000 person-years in the allopurinol users compared with 252/1,000 person-years in controls. In a Cox proportional hazards analysis adjusted for small differences in potential confounders, the use of allopurinol was independently associated with a 47% reduction in the risk of atrial fibrillation (P = .04), reported Dr. Hernandez of the University of Miami.
This intriguing finding needs to be confirmed in randomized prospective trials, he noted.
In a separate presentation, Dr. Benjamin R. Szwejkowski noted that left ventricular hypertrophy (LVH) is common in patients with type 2 diabetes and contributes to their elevated risk of cardiovascular morbidity and mortality.
Based on their hypothesis that LVH is related in part to oxidative stress and reducing that stress via xanthine oxidase inhibition using allopurinol can cause LVH regression, the investigators conducted a randomized, double-blind placebo-controlled clinical trial. Sixty-six patients with type 2 diabetes and echocardiographic evidence of LVH were randomized to allopurinol at 600 mg/day or placebo for 9 months.
The primary study endpoint was change in left ventricular mass between baseline and 9 months, as measured by cardiac MRI. Allopurinol resulted in a significant mean 2.65-g reduction in LV mass, while in the control group LV mass increased by 1.21 g. Similarly, LV mass indexed to body surface area fell significantly by 1.32 g/m2 in the allopurinol group while increasing by 0.65 g/m2 in the placebo arm, reported Dr. Szwejkowski of the University of Dundee(Scotland).
"Allopurinol may be a useful therapy to reduce cardiovascular risk in type 2 diabetic patients with LVH," according to the cardiologist.
Flow-mediated dilatation didn’t change significantly over time in either study group.
Dr. Szwejkowski and Dr. Hernandez reported having no relevant financial conflicts.
SAN FRANCISCO – The venerable antihyperuricemic agent allopurinol has shown early promise for two novel cardiovascular applications: prevention of atrial fibrillation in the setting of heart failure and reduction of left ventricular hypertrophy in patients with type 2 diabetes.
Allopurinol is a xanthine oxidase inhibitor and antigout drug. The rationale for the drug’s use in reducing the incidence of atrial fibrillation in patients with heart failure lies in the observation that serum uric acid has emerged as an independent marker of mortality and a predictor of new-onset atrial fibrillation in heart failure. Xanthine oxidase is not only a source of reactive oxygen species that adversely affect myocardial function, but it also catalyzes the conversion of xanthine to uric acid, Dr. Fernando E. Hernandez explained at the annual meeting of the American College of Cardiology.
He presented a retrospective cohort study involving 603 patients enrolled in the Miami Veterans Affairs heart failure clinic. The 103 on allopurinol, and the 500 who were not, matched up well in terms of baseline characteristics including age, prevalence of coronary artery disease, median left ventricular ejection, left atrial size, and use of guideline-recommended ACE inhibitors and beta-blockers.
During up to 5 years of follow-up, the incidence of new-onset atrial fibrillation was 184 cases/1,000 person-years in the allopurinol users compared with 252/1,000 person-years in controls. In a Cox proportional hazards analysis adjusted for small differences in potential confounders, the use of allopurinol was independently associated with a 47% reduction in the risk of atrial fibrillation (P = .04), reported Dr. Hernandez of the University of Miami.
This intriguing finding needs to be confirmed in randomized prospective trials, he noted.
In a separate presentation, Dr. Benjamin R. Szwejkowski noted that left ventricular hypertrophy (LVH) is common in patients with type 2 diabetes and contributes to their elevated risk of cardiovascular morbidity and mortality.
Based on their hypothesis that LVH is related in part to oxidative stress and reducing that stress via xanthine oxidase inhibition using allopurinol can cause LVH regression, the investigators conducted a randomized, double-blind placebo-controlled clinical trial. Sixty-six patients with type 2 diabetes and echocardiographic evidence of LVH were randomized to allopurinol at 600 mg/day or placebo for 9 months.
The primary study endpoint was change in left ventricular mass between baseline and 9 months, as measured by cardiac MRI. Allopurinol resulted in a significant mean 2.65-g reduction in LV mass, while in the control group LV mass increased by 1.21 g. Similarly, LV mass indexed to body surface area fell significantly by 1.32 g/m2 in the allopurinol group while increasing by 0.65 g/m2 in the placebo arm, reported Dr. Szwejkowski of the University of Dundee(Scotland).
"Allopurinol may be a useful therapy to reduce cardiovascular risk in type 2 diabetic patients with LVH," according to the cardiologist.
Flow-mediated dilatation didn’t change significantly over time in either study group.
Dr. Szwejkowski and Dr. Hernandez reported having no relevant financial conflicts.
SAN FRANCISCO – The venerable antihyperuricemic agent allopurinol has shown early promise for two novel cardiovascular applications: prevention of atrial fibrillation in the setting of heart failure and reduction of left ventricular hypertrophy in patients with type 2 diabetes.
Allopurinol is a xanthine oxidase inhibitor and antigout drug. The rationale for the drug’s use in reducing the incidence of atrial fibrillation in patients with heart failure lies in the observation that serum uric acid has emerged as an independent marker of mortality and a predictor of new-onset atrial fibrillation in heart failure. Xanthine oxidase is not only a source of reactive oxygen species that adversely affect myocardial function, but it also catalyzes the conversion of xanthine to uric acid, Dr. Fernando E. Hernandez explained at the annual meeting of the American College of Cardiology.
He presented a retrospective cohort study involving 603 patients enrolled in the Miami Veterans Affairs heart failure clinic. The 103 on allopurinol, and the 500 who were not, matched up well in terms of baseline characteristics including age, prevalence of coronary artery disease, median left ventricular ejection, left atrial size, and use of guideline-recommended ACE inhibitors and beta-blockers.
During up to 5 years of follow-up, the incidence of new-onset atrial fibrillation was 184 cases/1,000 person-years in the allopurinol users compared with 252/1,000 person-years in controls. In a Cox proportional hazards analysis adjusted for small differences in potential confounders, the use of allopurinol was independently associated with a 47% reduction in the risk of atrial fibrillation (P = .04), reported Dr. Hernandez of the University of Miami.
This intriguing finding needs to be confirmed in randomized prospective trials, he noted.
In a separate presentation, Dr. Benjamin R. Szwejkowski noted that left ventricular hypertrophy (LVH) is common in patients with type 2 diabetes and contributes to their elevated risk of cardiovascular morbidity and mortality.
Based on their hypothesis that LVH is related in part to oxidative stress and reducing that stress via xanthine oxidase inhibition using allopurinol can cause LVH regression, the investigators conducted a randomized, double-blind placebo-controlled clinical trial. Sixty-six patients with type 2 diabetes and echocardiographic evidence of LVH were randomized to allopurinol at 600 mg/day or placebo for 9 months.
The primary study endpoint was change in left ventricular mass between baseline and 9 months, as measured by cardiac MRI. Allopurinol resulted in a significant mean 2.65-g reduction in LV mass, while in the control group LV mass increased by 1.21 g. Similarly, LV mass indexed to body surface area fell significantly by 1.32 g/m2 in the allopurinol group while increasing by 0.65 g/m2 in the placebo arm, reported Dr. Szwejkowski of the University of Dundee(Scotland).
"Allopurinol may be a useful therapy to reduce cardiovascular risk in type 2 diabetic patients with LVH," according to the cardiologist.
Flow-mediated dilatation didn’t change significantly over time in either study group.
Dr. Szwejkowski and Dr. Hernandez reported having no relevant financial conflicts.
AT ACC 13
Major finding: At the end of 5 years of allopurinol use, the incidence of new-onset atrial fibrillation was 184 cases/1,000 person-years in the allopurinol users compared with 252/1,000 person-years in controls.
Data source: A retrospective cohort study involving 603 patients with heart failure.
Disclosures: The study presenters reported having no relevant financial conflicts.
New concussion guidelines stress individualized approach
Any athlete with a possible concussion should be immediately removed from play pending an evaluation by a licensed health care provider trained in assessing concussions and traumatic brain injury, according to a new guideline from the American Academy of Neurology.
The guideline for evaluating and managing athletes with concussion was published online in the journal Neurology on March 18 (doi:10.1212/WNL.0b013e31828d57dd) in conjunction with the annual meeting of the AAN. The guideline replaces the Academy’s 1997 recommendations, which stressed using a grading system to try to predict concussion outcomes.
The new guideline takes a more individualized and conservative approach, especially for younger athletes. The new approach comes as many states have enacted legislation regulating when young athletes can return to play following a concussion.
"If in doubt, sit it out," Dr. Jeffrey S. Kutcher, coauthor of the guideline and a neurologist at the University of Michigan in Ann Arbor, said in a statement. "Being seen by a trained professional is extremely important after a concussion. If headaches or other symptoms return with the start of exercise, stop the activity and consult a doctor. You only get one brain; treat it well."
The new guideline calls for athletes to stay off the field until they are asymptomatic off medication. High school athletes and younger players with a concussion should be managed more conservatively since they take longer to recover than older athletes, according to the AAN.
But there is not enough evidence to support complete rest after a concussion. Activities that do not worsen symptoms and don’t pose a risk of another concussion can be part of the management of the injury, according to the guideline.
"We’re moved away from the concussion grading systems we first established in 1997 and are now recommending concussion and return to play be assessed in each athlete individually," Dr. Christopher C. Giza, the co–lead guideline author and a neurologist at Mattel Children’s Hospital at the University of California, Los Angeles, said in a statement. "There is no set timeline for safe return to play."
The AAN expert panel recommends that sideline providers use symptom checklists such as the Standardized Assessment of Concussion to help identify suspected concussion and that the scores be shared with the physicians involved in the athletes’ care off the field. But these checklists should not be the only tool used in making a diagnosis, according to the guidelines. Also, the checklist scores may be more useful if they are compared against preinjury individual scores, especially in younger athletes and those with prior concussions.
CT imaging should not be used to diagnose a suspected sport-related concussion, according to the guideline. But imaging might be used to rule out more serious traumatic brain injuries, such as intracranial hemorrhage in athletes with a suspected concussion who also have a loss of consciousness, posttraumatic amnesia, persistently altered mental status, focal neurologic deficit, evidence of skull fracture, or signs of clinical deterioration.
Athletes are at greater risk of concussion if they have a history of concussion. The first 10 days after a concussion pose the greatest risk for a repeat injury.
The AAN advises physicians to be on the lookout for ongoing symptoms that are linked to a longer recovery, such as continued headache or fogginess. Athletes with a history of concussions and younger players also tend to have a longer recovery.
The guideline also include level C recommendations stating that health care providers "might" develop individualized graded plans for returning to physical and cognitive activity. They might also provide cognitive restructuring counseling in an effort to shorten the duration of symptoms and the likelihood of developing chronic post-concussion syndrome, according to the guideline.
The guideline also included a number of recommendations on areas for future research, including studies of pre–high school age athletes to determine the natural history of concussion and recovery time for this age group, as well as the best assessment tools. The expert panel also called for clinical trials of different postconcussion management strategies and return-to-play protocols.
The guidelines were developed by a multidisciplinary expert committee that included representatives from neurology, athletic training, neuropsychology, epidemiology and biostatistics, neurosurgery, physical medicine and rehabilitation, and sports medicine. Many of the authors reported serving as consultants for professional sports associations, receiving honoraria and funding for travel for lectures on sports concussion, receiving research support from various foundations and organizations, and providing expert testimony in legal cases involving traumatic brain injury or concussion.
One of the most important statements in the new guideline
is that providers should not rely on a single diagnostic test when evaluating
an athlete, said Dr. Barry Jordan, the assistant medical director and attending
neurologist at the Burke Rehabilitation Hospital in White Plains, N.Y. Dr.
Jordan, who is an expert on sports concussions, said he’s seen too many
providers using a single computerized screening tool to assess whether an
athlete is well enough to return to play.
The new
guideline calls on providers to combine screening checklists with clinical
findings when making the determination about whether an athlete is well enough
to return to the field. Dr. Jordan
said this comprehensive approach is the way to go. And physicians who are
knowledgeable about concussions must be involved with that evaluation, he said.

|
| Dr. Barry Jordan |
The new guideline is an important update reflecting
the movement away from grading concussions to a more individualized approach. "You can't grade the severity until the concussion is over," he said.
Dr. Jordan
said the AAN guideline is "clear and easy to follow" and will results in better
care if followed.
Dr.
Barry Jordan is the director of the Brain Injury Program at Burke
Rehabilitation Hospital in White Plains, N.Y. He works with several sports
organizations including the New York State Athletic Commission, U.S.A. Boxing, and the National
Football League Players Association. He also writes a bimonthly column for
Clinical Neurology News called “On the Sidelines.”
One of the most important statements in the new guideline
is that providers should not rely on a single diagnostic test when evaluating
an athlete, said Dr. Barry Jordan, the assistant medical director and attending
neurologist at the Burke Rehabilitation Hospital in White Plains, N.Y. Dr.
Jordan, who is an expert on sports concussions, said he’s seen too many
providers using a single computerized screening tool to assess whether an
athlete is well enough to return to play.
The new
guideline calls on providers to combine screening checklists with clinical
findings when making the determination about whether an athlete is well enough
to return to the field. Dr. Jordan
said this comprehensive approach is the way to go. And physicians who are
knowledgeable about concussions must be involved with that evaluation, he said.

|
| Dr. Barry Jordan |
The new guideline is an important update reflecting
the movement away from grading concussions to a more individualized approach. "You can't grade the severity until the concussion is over," he said.
Dr. Jordan
said the AAN guideline is "clear and easy to follow" and will results in better
care if followed.
Dr.
Barry Jordan is the director of the Brain Injury Program at Burke
Rehabilitation Hospital in White Plains, N.Y. He works with several sports
organizations including the New York State Athletic Commission, U.S.A. Boxing, and the National
Football League Players Association. He also writes a bimonthly column for
Clinical Neurology News called “On the Sidelines.”
One of the most important statements in the new guideline
is that providers should not rely on a single diagnostic test when evaluating
an athlete, said Dr. Barry Jordan, the assistant medical director and attending
neurologist at the Burke Rehabilitation Hospital in White Plains, N.Y. Dr.
Jordan, who is an expert on sports concussions, said he’s seen too many
providers using a single computerized screening tool to assess whether an
athlete is well enough to return to play.
The new
guideline calls on providers to combine screening checklists with clinical
findings when making the determination about whether an athlete is well enough
to return to the field. Dr. Jordan
said this comprehensive approach is the way to go. And physicians who are
knowledgeable about concussions must be involved with that evaluation, he said.

|
| Dr. Barry Jordan |
The new guideline is an important update reflecting
the movement away from grading concussions to a more individualized approach. "You can't grade the severity until the concussion is over," he said.
Dr. Jordan
said the AAN guideline is "clear and easy to follow" and will results in better
care if followed.
Dr.
Barry Jordan is the director of the Brain Injury Program at Burke
Rehabilitation Hospital in White Plains, N.Y. He works with several sports
organizations including the New York State Athletic Commission, U.S.A. Boxing, and the National
Football League Players Association. He also writes a bimonthly column for
Clinical Neurology News called “On the Sidelines.”
Any athlete with a possible concussion should be immediately removed from play pending an evaluation by a licensed health care provider trained in assessing concussions and traumatic brain injury, according to a new guideline from the American Academy of Neurology.
The guideline for evaluating and managing athletes with concussion was published online in the journal Neurology on March 18 (doi:10.1212/WNL.0b013e31828d57dd) in conjunction with the annual meeting of the AAN. The guideline replaces the Academy’s 1997 recommendations, which stressed using a grading system to try to predict concussion outcomes.
The new guideline takes a more individualized and conservative approach, especially for younger athletes. The new approach comes as many states have enacted legislation regulating when young athletes can return to play following a concussion.
"If in doubt, sit it out," Dr. Jeffrey S. Kutcher, coauthor of the guideline and a neurologist at the University of Michigan in Ann Arbor, said in a statement. "Being seen by a trained professional is extremely important after a concussion. If headaches or other symptoms return with the start of exercise, stop the activity and consult a doctor. You only get one brain; treat it well."
The new guideline calls for athletes to stay off the field until they are asymptomatic off medication. High school athletes and younger players with a concussion should be managed more conservatively since they take longer to recover than older athletes, according to the AAN.
But there is not enough evidence to support complete rest after a concussion. Activities that do not worsen symptoms and don’t pose a risk of another concussion can be part of the management of the injury, according to the guideline.
"We’re moved away from the concussion grading systems we first established in 1997 and are now recommending concussion and return to play be assessed in each athlete individually," Dr. Christopher C. Giza, the co–lead guideline author and a neurologist at Mattel Children’s Hospital at the University of California, Los Angeles, said in a statement. "There is no set timeline for safe return to play."
The AAN expert panel recommends that sideline providers use symptom checklists such as the Standardized Assessment of Concussion to help identify suspected concussion and that the scores be shared with the physicians involved in the athletes’ care off the field. But these checklists should not be the only tool used in making a diagnosis, according to the guidelines. Also, the checklist scores may be more useful if they are compared against preinjury individual scores, especially in younger athletes and those with prior concussions.
CT imaging should not be used to diagnose a suspected sport-related concussion, according to the guideline. But imaging might be used to rule out more serious traumatic brain injuries, such as intracranial hemorrhage in athletes with a suspected concussion who also have a loss of consciousness, posttraumatic amnesia, persistently altered mental status, focal neurologic deficit, evidence of skull fracture, or signs of clinical deterioration.
Athletes are at greater risk of concussion if they have a history of concussion. The first 10 days after a concussion pose the greatest risk for a repeat injury.
The AAN advises physicians to be on the lookout for ongoing symptoms that are linked to a longer recovery, such as continued headache or fogginess. Athletes with a history of concussions and younger players also tend to have a longer recovery.
The guideline also include level C recommendations stating that health care providers "might" develop individualized graded plans for returning to physical and cognitive activity. They might also provide cognitive restructuring counseling in an effort to shorten the duration of symptoms and the likelihood of developing chronic post-concussion syndrome, according to the guideline.
The guideline also included a number of recommendations on areas for future research, including studies of pre–high school age athletes to determine the natural history of concussion and recovery time for this age group, as well as the best assessment tools. The expert panel also called for clinical trials of different postconcussion management strategies and return-to-play protocols.
The guidelines were developed by a multidisciplinary expert committee that included representatives from neurology, athletic training, neuropsychology, epidemiology and biostatistics, neurosurgery, physical medicine and rehabilitation, and sports medicine. Many of the authors reported serving as consultants for professional sports associations, receiving honoraria and funding for travel for lectures on sports concussion, receiving research support from various foundations and organizations, and providing expert testimony in legal cases involving traumatic brain injury or concussion.
Any athlete with a possible concussion should be immediately removed from play pending an evaluation by a licensed health care provider trained in assessing concussions and traumatic brain injury, according to a new guideline from the American Academy of Neurology.
The guideline for evaluating and managing athletes with concussion was published online in the journal Neurology on March 18 (doi:10.1212/WNL.0b013e31828d57dd) in conjunction with the annual meeting of the AAN. The guideline replaces the Academy’s 1997 recommendations, which stressed using a grading system to try to predict concussion outcomes.
The new guideline takes a more individualized and conservative approach, especially for younger athletes. The new approach comes as many states have enacted legislation regulating when young athletes can return to play following a concussion.
"If in doubt, sit it out," Dr. Jeffrey S. Kutcher, coauthor of the guideline and a neurologist at the University of Michigan in Ann Arbor, said in a statement. "Being seen by a trained professional is extremely important after a concussion. If headaches or other symptoms return with the start of exercise, stop the activity and consult a doctor. You only get one brain; treat it well."
The new guideline calls for athletes to stay off the field until they are asymptomatic off medication. High school athletes and younger players with a concussion should be managed more conservatively since they take longer to recover than older athletes, according to the AAN.
But there is not enough evidence to support complete rest after a concussion. Activities that do not worsen symptoms and don’t pose a risk of another concussion can be part of the management of the injury, according to the guideline.
"We’re moved away from the concussion grading systems we first established in 1997 and are now recommending concussion and return to play be assessed in each athlete individually," Dr. Christopher C. Giza, the co–lead guideline author and a neurologist at Mattel Children’s Hospital at the University of California, Los Angeles, said in a statement. "There is no set timeline for safe return to play."
The AAN expert panel recommends that sideline providers use symptom checklists such as the Standardized Assessment of Concussion to help identify suspected concussion and that the scores be shared with the physicians involved in the athletes’ care off the field. But these checklists should not be the only tool used in making a diagnosis, according to the guidelines. Also, the checklist scores may be more useful if they are compared against preinjury individual scores, especially in younger athletes and those with prior concussions.
CT imaging should not be used to diagnose a suspected sport-related concussion, according to the guideline. But imaging might be used to rule out more serious traumatic brain injuries, such as intracranial hemorrhage in athletes with a suspected concussion who also have a loss of consciousness, posttraumatic amnesia, persistently altered mental status, focal neurologic deficit, evidence of skull fracture, or signs of clinical deterioration.
Athletes are at greater risk of concussion if they have a history of concussion. The first 10 days after a concussion pose the greatest risk for a repeat injury.
The AAN advises physicians to be on the lookout for ongoing symptoms that are linked to a longer recovery, such as continued headache or fogginess. Athletes with a history of concussions and younger players also tend to have a longer recovery.
The guideline also include level C recommendations stating that health care providers "might" develop individualized graded plans for returning to physical and cognitive activity. They might also provide cognitive restructuring counseling in an effort to shorten the duration of symptoms and the likelihood of developing chronic post-concussion syndrome, according to the guideline.
The guideline also included a number of recommendations on areas for future research, including studies of pre–high school age athletes to determine the natural history of concussion and recovery time for this age group, as well as the best assessment tools. The expert panel also called for clinical trials of different postconcussion management strategies and return-to-play protocols.
The guidelines were developed by a multidisciplinary expert committee that included representatives from neurology, athletic training, neuropsychology, epidemiology and biostatistics, neurosurgery, physical medicine and rehabilitation, and sports medicine. Many of the authors reported serving as consultants for professional sports associations, receiving honoraria and funding for travel for lectures on sports concussion, receiving research support from various foundations and organizations, and providing expert testimony in legal cases involving traumatic brain injury or concussion.
FROM NEUROLOGY
Sleep in Hospitalized Adults
Lack of sleep is a common problem in hospitalized patients and is associated with poorer health outcomes, especially in older patients.[1, 2, 3] Prior studies highlight a multitude of factors that can result in sleep loss in the hospital[3, 4, 5, 6] with 1 of the most common causes of sleep disruption in the hospital being noise.[7, 8, 9]
In addition to external factors, such as hospital noise, there may be inherent characteristics that predispose certain patients to greater sleep loss when hospitalized. One such measure is the construct of perceived control or the psychological measure of how much individuals expect themselves to be capable of bringing about desired outcomes.[10] Among older patients, low perceived control is associated with increased rates of physician visits, hospitalizations, and death.[11, 12] In contrast, patients who feel more in control of their environment may experience positive health benefits.[13]
Yet, when patients are placed in a hospital setting, they experience a significant reduction in control over their environment along with an increase in dependency on medical staff and therapies.[14, 15] For example, hospitalized patients are restricted in their personal decisions, such as what clothes they can wear and what they can eat and are not in charge of their own schedules, including their sleep time.
Although prior studies suggest that perceived control over sleep is related to actual sleep among community‐dwelling adults,[16, 17] no study has examined this relationship in hospitalized adults. Therefore, the aim of our study was to examine the possible association between perceived control, noise levels, and sleep in hospitalized middle‐aged and older patients.
METHODS
Study Design
We conducted a prospective cohort study of subjects recruited from a large ongoing study of admitted patients at the University of Chicago inpatient general medicine service.[18] Because we were interested in middle‐aged and older adults who are most sensitive to sleep disruptions, patients who were age 50 years and over, ambulatory, and living in the community were eligible for the study.[19] Exclusion criteria were cognitive impairment (telephone version of the Mini‐Mental State Exam <17 out of 22), preexisting sleeping disorders identified via patient charts, such as obstructive sleep apnea and narcolepsy, transfer from the intensive care unit (ICU), and admission to the hospital more than 72 hours prior to enrollment.[20] These inclusion and exclusion criteria were selected to identify a patient population with minimal sleep disturbances at baseline. Patients under isolation were excluded because they are not visited as frequently by the healthcare team.[21, 22] Most general medicine rooms were double occupancy but efforts were made to make patient rooms single when possible or required (ie, isolation for infection control). The study was approved by the University of Chicago Institutional Review Board.
Subjective Data Collection
Baseline levels of perceived control over sleep, or the amount of control patients believe they have over their sleep, were assessed using 2 different scales. The first tool was the 8‐item Sleep Locus of Control (SLOC) scale,[17] which ranges from 8 to 48, with higher values corresponding to a greater internal locus of control over sleep. An internal sleep locus of control indicates beliefs that patients feel that they are primarily responsible for their own sleep as opposed to an external locus of control which indicates beliefs that good sleep is due to luck or chance. For example, patients were asked how strongly they agree or disagree with statements, such as, If I take care of myself, I can avoid insomnia and People who never get insomnia are just plain lucky (see Supporting Information, Appendix 2, in the online version of this article). The second tool was the 9‐item Sleep Self‐Efficacy (SSE) scale,[23] which ranges from 9 to 45, with higher values corresponding to greater confidence patients have in their ability to sleep. One of the items asks, How confident are you that you can lie in bed feeling physically relaxed (see Supporting Information, Appendix 1, in the online version of this article)? Both instruments have been validated in an outpatient setting.[23] These surveys were given immediately on enrollment in the study to measure baseline perceived control.
Baseline sleep habits were also collected on enrollment using the Epworth Sleepiness Scale,[24, 25] a standard validated survey that assesses excess daytime sleepiness in various common situations. For each day in the hospital, patients were asked to report in‐hospital sleep quality using the Karolinska Sleep Log.[26] The Karolinska Sleep Quality Index (KSQI) is calculated from 4 items on the Karolinska Sleep Log (sleep quality, sleep restlessness, slept throughout the night, ease of falling asleep). The questions are on a 5‐point scale and the 4 items are averaged for a final score out of 5 with a higher number indicating better subjective sleep quality. The item How much was your sleep disturbed by noise? on the Karolinska Sleep Log was used to assess the degree to which noise was a disruptor of sleep. This question was also on a 5‐point scale with higher scores indicating greater disruptiveness of noise. Patients were also asked how disruptive noise from roommates was on a nightly basis using this same scale.
Objective Data Collection
Wrist activity monitors (Actiwatch 2; Respironics, Inc., Murrysville, PA)[27, 28, 29, 30] were used to measure patient sleep. Actiware 5 software (Respironics, Inc.)[31] was used to estimate quantitative measures of sleep time and efficiency. Sleep time is defined as the total duration of time spent sleeping at night and sleep efficiency is defined as the fraction of time, reported as a percentage, spent sleeping by actigraphy out of the total time patients reported they were sleeping.
Sound levels in patient rooms were recorded using Larson Davis 720 Sound Level Monitors (Larson Davis, Inc., Provo, UT). These monitors store functional average sound pressure levels in A‐weighted decibels called the Leq over 1‐hour intervals. The Leq is the average sound level over the given time interval. Minimum (Lmin) and maximum (Lmax) sound levels are also stored. The LD SLM Utility Program (Larson Davis, Inc.) was used to extract the sound level measurements recorded by the monitors.
Demographic information (age, gender, race, ethnicity, highest level of education, length of stay in the hospital, and comorbidities) was obtained from hospital charts via an ongoing study of admitted patients at the University of Chicago Medical Center inpatient general medicine service.[18] Chart audits were performed to determine whether patients received pharmacologic sleep aids in the hospital.
Data Analysis
Descriptive statistics were used to summarize mean sleep duration and sleep efficiency in the hospital as well as SLOC and SSE. Because the SSE scores were not normally distributed, the scores were dichotomized at the median to create a variable denoting high and low SSE. Additionally, because the distribution of responses to the noise disruption question was skewed to the right, reports of noise disruptions were grouped into not disruptive (score=1) and disruptive (score>1).
Two‐sample t tests with equal variances were used to assess the relationship between perceived control measures (high/low SLOC, SSE) and objective sleep measures (sleep time, sleep efficiency). Multivariate linear regression was used to test the association between high SSE (independent variable) and sleep time (dependent variable), clustering for multiple nights of data within the subject. Multivariate logistic regression, also adjusting for subject, was used to test the association between high SSE and noise disruptiveness and the association between high SSE and Karolinska scores. Leq, Lmax, and Lmin were all tested using stepwise forward regression. Because our prior work[9] demonstrated that noise levels separated into tertiles were significantly associated with sleep time, our analysis also used noise levels separated into tertiles. Stepwise forward regression was used to add basic patient demographics (gender, race, age) to the models. Statistical significance was defined as P<0.05, and all statistical analysis was done using Stata 11.0 (StataCorp, College Station, TX).
RESULTS
From April 2010 to May 2012, 1134 patients were screened by study personnel for this study via an ongoing study of hospitalized patients on the inpatient general medicine ward. Of the 361 (31.8%) eligible patients, 206 (57.1%) consented to participate. Of the subjects enrolled in the study, 118 were able to complete at least 1 night of actigraphy, sound monitoring, and subjective assessment for a total of 185 patient nights (Figure 1).
The majority of patients were female (57%), African American (67%), and non‐Hispanic (97%). The mean age was 65 years (standard deviation [SD], 11.6 years), and the median length of stay was 4 days (interquartile range [IQR], 36). The majority of patients also had hypertension (67%), with chronic obstructive pulmonary disease [COPD] (31%) and congestive heart failure (31%) being the next most common comorbidities. About two‐thirds of subjects (64%) were characterized as average or above average sleepers with Epworth Sleepiness Scale scores 9[20] (Table 1). Only 5% of patients received pharmacological sleep aids.
| Value, n (%)a | |
|---|---|
| |
| Patient characteristics | |
| Age, mean (SD), y | 63 (12) |
| Length of stay, median (IQR), db | 4 (36) |
| Female | 67 (57) |
| African American | 79 (67) |
| Hispanic | 3 (3) |
| High school graduate | 92 (78) |
| Comorbidities | |
| Hypertension | 79 (66) |
| Chronic obstructive pulmonary disease | 37 (31) |
| Congestive heart failure | 37 (31) |
| Diabetes | 36 (30) |
| End stage renal disease | 23 (19) |
| Baseline sleep characteristics | |
| Sleep duration, mean (SD), minc | 333 (128) |
| Epworth Sleepiness Scale, score 9d | 73 (64) |
The mean baseline SLOC score was 30.4 (SD, 6.7), with a median of 31 (IQR, 2735). The mean baseline SSE score was 32.1 (SD, 9.4), with a median of 34 (IQR, 2441). Fifty‐four patients were categorized as having high sleep self‐efficacy (high SSE), which we defined as scoring above the median of 34.
Average in‐hospital sleep was 5.5 hours (333 minutes; SD, 128 minutes) which was significantly shorter than the self‐reported sleep duration of 6.5 hours prior to admission (387 minutes, SD, 125 minutes; P=0.0001). The mean sleep efficiency was 73% (SD, 19%) with 55% of actigraphy nights below the normal range of 80% efficiency for adults.[19] Median KSQI was 3.5 (IQR, 2.254.75), with 41% of the patients with a KSQI 3, putting them in the insomniac range.[32] The median score on the noise disruptiveness question was 1 (IQR, 14) with 42% of reports coded as disruptive defined as a score >1 on the 5‐point scale. The median score on the roommate disruptiveness question was 1 (IQR, 11) with 77% of responses coded as not disruptive defined as a score of 1 on the 5‐point scale.
A 2‐sample t test with equal variances showed that those patients reporting high SSE were more likely to sleep longer in the hospital than those reporting low SSE (364 minutes 95% confidence interval [CI]: 340, 388 vs 309 minutes 95% CI: 283, 336; P=0.003) (Figure 2). Patients with high SSE were also more likely to have a normal sleep efficiency (above 80%) compared to those with low SSE (54% 95% CI: 43, 65 vs 38% 95% CI: 28,47; P=0.028). Last, there was a trend toward patients reporting higher SSE to also report less noise disruption compared to those patients with low SSE ([42%] 95% CI: 31, 53 vs [56%] 95% CI: 46, 65; P=0.063) (Figure 3).
Linear regression clustered by subject showed that high SSE was associated with longer sleep duration (55 minutes 95% CI: 14, 97; P=0.010). Furthermore, high SSE was significantly associated with longer sleep duration after controlling for both objective noise level and patient demographics in the model using stepwise forward regression (50 minutes 95% CI: 11, 90; P=0.014) (Table 2).
| Sleep Duration (min) | Model 1 Beta [95% CI]a | Model 2 Beta [95% CI]a |
|---|---|---|
| ||
| High SSE | 55 [14, 97]b | 50 [11, 90]b |
| Lmin tert 3 | 14 [59, 29] | |
| Lmin tert 2 | 21 [65, 23] | |
| Female | 49 [10, 89]b | |
| African American | 16 [59, 27] | |
| Age | 1 [0.9, 3] | |
| Karolinska Sleep Quality | Model 1 OR [95% CI]c | Model 2 OR [95% CI]c |
| High SSE | 2.04 [1.12, 3.71]b | 2.01 [1.06, 3.79]b |
| Lmin tert 3 | 0.90 [0.37, 2.2] | |
| Lmin tert 2 | 0.86 [0.38, 1.94] | |
| Female | 1.78 [0.90, 3.52] | |
| African American | 1.19 [0.60, 2.38] | |
| Age | 1.02 [0.99, 1.05] | |
| Noise Complaints | Model 1 OR [95% CI]d | Model 2 OR [95% CI]d |
| High SSE | 0.57 [0.30, 1.12] | 0.49 [0.25, 0.96]b |
| Lmin tert 3 | 0.85 [0.39, 1.84] | |
| Lmin tert 2 | 0.91 [0.43, 1.93] | |
| Female | 1.40 [0.71, 2.78] | |
| African American | 0.35 [0.17, 0.70] | |
| Age | 1.00 [0.96, 1.03] | |
| Age2e | 1.00 [1.00, 1.00] | |
Logistic regression clustered by subject demonstrated that patients with high SSE had 2 times higher odds of having a KSQI score above 3 (95% CI: 1.12, 3.71; P=0.020). This association was still significant after controlling for noise and patient demographics (OR: 2.01; 95% CI: 1.06, 3.79; P=0.032). After controlling for noise levels and patient demographics, there was a statistically significant association between high SSE and lower odds of noise complaints (OR: 0.49; 95% CI: 0.25, 0.96; P=0.039) (Table 2). Although demographic characteristics were not associated with high SSE, those patients with high SSE had lower odds of being in the loudest tertile rooms (OR: 0.34; 95% CI: 0.15, 0.74; P=0.007).
In multivariate linear regression analyses, there were no significant relationships between SLOC scores and KSQI, reported noise disruptiveness, and markers of sleep (sleep duration or sleep efficiency).
DISCUSSION
This study is the first to examine the relationship between perceived control, noise levels, and objective measurements of sleep in a hospital setting. One measure of perceived control, namely SSE, was associated with objective sleep duration, subjective and objective sleep quality, noise levels in patient rooms, and perhaps also patient complaints of noise. These associations remained significant after controlling for objective noise levels and patient demographics, suggesting that SSE is independently related to sleep.
In contrast to SSE, SLOC was not found to be significantly associated with either subjective or objective measures of sleep quality. The lack of association may be due to the fact that the SLOC questionnaire does not translate as well to the inpatient setting as the SSE questionnaire. The SLOC questionnaire focuses on general beliefs about sleep whereas the SSE questionnaire focuses on personal beliefs about one's own ability sleep in the immediate future, which may make it more relevant in the inpatient setting (see Supporting Information, Appendix 1 and 2, in the online version of this article).
Given our findings, it is important to identify why patients with high SSE have better sleep and fewer noise complaints. One possibility is that sleep self‐efficacy is an inherited trait unique to each person that is also predictive of a patient's sleep patterns. However, is it also possible that those patients with high SSE feel more empowered to take control of their environment, allowing them to advocate for better sleep? This hypothesis is further strengthened by the finding that those patients with high SSE on study entry were less likely to be in the noisiest rooms. This raises the possibility that at least 1 of the mechanisms by which high SSE may be protective against sleep loss is through patients taking an active role in noise reduction, such as closing the door or advocating for their sleep with staff. However, we did not directly observe or ask patients whether doors of patient rooms were open or closed or whether the patients took other measures to advocate for their own sleep. Thus, further work is necessary to understand the mechanisms by which sleep self‐efficacy may influence sleep.
One potential avenue for future research is to explore possible interventions for boosting sleep self‐efficacy in the hospital. Although most interventions have focused on environmental noise and staff‐based education, empowering patients through boosting SSE may be a helpful adjunct to improving hospital sleep.[33, 34] Currently, the SSE scale is not commonly used in the inpatient setting. Motivational interviewing and patient coaching could be explored as potential tools for boosting SSE. Furthermore, even if SSE is not easily changed, measuring SSE in patients newly admitted to the hospital may be useful in identifying patients most susceptible to sleep disruptions. Efforts to identify patients with low SSE should go hand‐in‐hand with measures to reduce noise. Addressing both patient‐level and environmental factors simultaneously may be the best strategy for improving sleep in an inpatient hospital setting.
In contrast to our prior study, it is worth noting that we did not find any significant relationships between overall noise levels and sleep.[9] In this dataset, nighttime noise is still a predictor of sleep loss in the hospital. However, when we restrict our sample to those who answered the SSE questionnaire and had nighttime noise recorded, we lose a significant number of observations. Because of our interest in testing the relationship between SSE and sleep, we chose to control for overall noise (which enabled us to retain more observations). We also did not find any interactions between SSE and noise in our regression models. Further work is warranted with larger sample sizes to better understand the role of SSE in the context of sleep and noise levels. In addition, females also received more sleep than males in our study.
There are several limitations to this study. This study was carried out at a single service at a single institution, limiting the ability to generalize the findings to other hospital settings. This study had a relatively high rate of patients who were unable to complete at least 1 night of data collection (42%), often due to watch removal for imaging or procedures, which may also affect the representativeness of our sample. Moreover, we can only examine associations and not causal relationships. The SSE scale has never been used in hospitalized patients, making comparisons between scores from hospitalized patients and population controls difficult. In addition, the SSE scale also has not been dichotomized in previous studies into high and low SSE. However, a sensitivity analysis with raw SSE scores did not change the results of our study. It can be difficult to perform actigraphy measurements in the hospital because many patients spend most of their time in bed. Because we chose a relatively healthy cohort of patients without significant limitations in mobility, actigraphy could still be used to differentiate time spent awake from time spent sleeping. Because we did not perform polysomnography, we cannot explore the role of sleep architecture which is an important component of sleep quality. Although the use of pharmacologic sleep aids is a potential confounding factor, the rate of use was very low in our cohort and unlikely to significantly affect our results. Continued study of this patient population is warranted to further develop the findings.
In conclusion, patients with high SSE sleep better in the hospital, tend to be in quieter rooms, and may report fewer noise complaints. Our findings suggest that a greater confidence in the ability to sleep may be beneficial in hospitalized adults. In addition to noise control, hospitals should also consider targeting patients with low SSE when designing novel interventions to improve in‐hospital sleep.
Disclosures
This work was supported by funding from the National Institute on Aging through a Short‐Term Aging‐Related Research Program (1 T35 AG029795), National Institute on Aging career development award (K23AG033763), a midcareer career development award (1K24AG031326), a program project (P01AG‐11412), an Agency for Healthcare Research and Quality Centers for Education and Research on Therapeutics grant (1U18HS016967), and a National Institute on Aging Clinical Translational Sciences award (UL1 RR024999). Dr. Arora had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the statistical analysis. The funding agencies had no role in the design of the study; the collection, analysis, and interpretation of the data; or the decision to approve publication of the finished manuscript. The authors report no conflicts of interest.
- , , , . The metabolic consequences of sleep deprivation. Sleep Med Rev. 2007;11(3):163–178.
- , , , , , . Poor self‐reported sleep quality predicts mortality within one year of inpatient post‐acute rehabilitation among older adults. Sleep. 2011;34(12):1715–1721.
- , , , et al. The sleep of older people in hospital and nursing homes. J Clin Nurs. 1999;8:360–368.
- , , et al. Sleep in hospitalized medical patients, part 1: factors affecting sleep. J Hosp Med. 2008; 3:473–482.
- , , , et al. Nocturnal care interactions with patients in critical care units. Am J Crit Care. 2004;13:102–112; quiz 114–115.
- , , . Patient perception of sleep quality and etiology of sleep disruption in the intensive care unit. Am J Respir Crit Care Med. 1999;159:1155–1162.
- . Sleep in acute care settings: an integrative review. J Nurs Scholarsh. 2000;32(1):31–38.
- , , , et al. Sleep disruption due to hospital noises: a prospective evaluation. Ann Int Med. 2012;157(3): 170–179.
- , , , et al. Noise and sleep among adult medical inpatients: far from a quiet night. Arch Intern Med. 2012;172:68–70.
- . Generalized expectancies for internal versus external control of reinforcement. Psychol Monogr. 1966;80:1–28.
- , . Psychosocial risk factors and mortality: a prospective study with special focus on social support, social participation, and locus of control in Norway. J Epidemiol Community Health. 1998;52:476–481.
- , . The interactive effect of perceived control and functional status on health and mortality among young‐old and old‐old adults. J Gerontol B Psychol Sci Soc Sci. 1997;52:P118–P126.
- , . Role‐specific feelings of control and mortality. Psychol Aging. 2000;15:617–626.
- , , . Patient empowerment in intensive care—an interview study. Intensive Crit Care Nurs. 2006;22:370–377.
- , , . Exploring the relationship between personal control and the hospital environment. J Clin Nurs. 2008;17:1601–1609.
- , , , et al. Effects of volitional lifestyle on sleep‐life habits in the aged. Psychiatry Clin Neurosci. 1998;52:183–184.
- , , , et al. Sleep locus of control: report on a new scale. Behav Sleep Med. 2004;2:79–93.
- , , , et al. Effects of physician experience on costs and outcomes on an academic general medicine service: results of a trial of hospitalists. Ann Intern Med. 2002;137:866–874.
- , , , et al. The effects of age, sex, ethnicity, and sleep‐disordered breathing on sleep architecture. Arch Intern Med. 2004;164:406–418.
- , , , et al. Validation of a telephone version of the mini‐mental state examination. J Am Geriatr Soc. 1992;40:697–702.
- , , , et al. Contact isolation in surgical patients: a barrier to care? Surgery. 2003;134:180–188.
- , . Adverse effects of contact isolation. Lancet. 1999;354:1177–1178.
- . Behavioral Treatment for Persistent Insomnia. Elmsford, NY: Pergamon Press; 1987.
- . A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep. 1991;14:540–545.
- . Reliability and factor analysis of the Epworth Sleepiness Scale. Sleep. 1992;15:376–381.
- , . Objective components of individual differences in subjective sleep quality. J Sleep Res. 1997;6:217–220.
- , , , et al. The role of actigraphy in the study of sleep and circadian rhythms. Sleep. 2003;26:342–392.
- , , , et al. Practice parameters for the use of actigraphy in the assessment of sleep and sleep disorders: an update for 2007. Sleep. 2007;30:519–529.
- , , , et al. The role of actigraphy in the evaluation of sleep disorders. Sleep. 1995;18:288–302.
- , , , et al. Clinical review: sleep measurement in critical care patients: research and clinical implications. Crit Care. 2007;11:226.
- , , , et al. Evaluation of immobility time for sleep latency in actigraphy. Sleep Med. 2009;10:621–625.
- , , , et al. The subjective meaning of sleep quality: a comparison of individuals with and without insomnia. Sleep. 2008;31:383–393.
- , , , et al. Sleep in hospitalized medical patients, part 2: behavioral and pharmacological management of sleep disturbances. J Hosp Med. 2009;4:50–59.
- , , , . A nonpharmacologic sleep protocol for hospitalized older patients. J Am Geriatr Soc. 1998;46(6):700–705.
Lack of sleep is a common problem in hospitalized patients and is associated with poorer health outcomes, especially in older patients.[1, 2, 3] Prior studies highlight a multitude of factors that can result in sleep loss in the hospital[3, 4, 5, 6] with 1 of the most common causes of sleep disruption in the hospital being noise.[7, 8, 9]
In addition to external factors, such as hospital noise, there may be inherent characteristics that predispose certain patients to greater sleep loss when hospitalized. One such measure is the construct of perceived control or the psychological measure of how much individuals expect themselves to be capable of bringing about desired outcomes.[10] Among older patients, low perceived control is associated with increased rates of physician visits, hospitalizations, and death.[11, 12] In contrast, patients who feel more in control of their environment may experience positive health benefits.[13]
Yet, when patients are placed in a hospital setting, they experience a significant reduction in control over their environment along with an increase in dependency on medical staff and therapies.[14, 15] For example, hospitalized patients are restricted in their personal decisions, such as what clothes they can wear and what they can eat and are not in charge of their own schedules, including their sleep time.
Although prior studies suggest that perceived control over sleep is related to actual sleep among community‐dwelling adults,[16, 17] no study has examined this relationship in hospitalized adults. Therefore, the aim of our study was to examine the possible association between perceived control, noise levels, and sleep in hospitalized middle‐aged and older patients.
METHODS
Study Design
We conducted a prospective cohort study of subjects recruited from a large ongoing study of admitted patients at the University of Chicago inpatient general medicine service.[18] Because we were interested in middle‐aged and older adults who are most sensitive to sleep disruptions, patients who were age 50 years and over, ambulatory, and living in the community were eligible for the study.[19] Exclusion criteria were cognitive impairment (telephone version of the Mini‐Mental State Exam <17 out of 22), preexisting sleeping disorders identified via patient charts, such as obstructive sleep apnea and narcolepsy, transfer from the intensive care unit (ICU), and admission to the hospital more than 72 hours prior to enrollment.[20] These inclusion and exclusion criteria were selected to identify a patient population with minimal sleep disturbances at baseline. Patients under isolation were excluded because they are not visited as frequently by the healthcare team.[21, 22] Most general medicine rooms were double occupancy but efforts were made to make patient rooms single when possible or required (ie, isolation for infection control). The study was approved by the University of Chicago Institutional Review Board.
Subjective Data Collection
Baseline levels of perceived control over sleep, or the amount of control patients believe they have over their sleep, were assessed using 2 different scales. The first tool was the 8‐item Sleep Locus of Control (SLOC) scale,[17] which ranges from 8 to 48, with higher values corresponding to a greater internal locus of control over sleep. An internal sleep locus of control indicates beliefs that patients feel that they are primarily responsible for their own sleep as opposed to an external locus of control which indicates beliefs that good sleep is due to luck or chance. For example, patients were asked how strongly they agree or disagree with statements, such as, If I take care of myself, I can avoid insomnia and People who never get insomnia are just plain lucky (see Supporting Information, Appendix 2, in the online version of this article). The second tool was the 9‐item Sleep Self‐Efficacy (SSE) scale,[23] which ranges from 9 to 45, with higher values corresponding to greater confidence patients have in their ability to sleep. One of the items asks, How confident are you that you can lie in bed feeling physically relaxed (see Supporting Information, Appendix 1, in the online version of this article)? Both instruments have been validated in an outpatient setting.[23] These surveys were given immediately on enrollment in the study to measure baseline perceived control.
Baseline sleep habits were also collected on enrollment using the Epworth Sleepiness Scale,[24, 25] a standard validated survey that assesses excess daytime sleepiness in various common situations. For each day in the hospital, patients were asked to report in‐hospital sleep quality using the Karolinska Sleep Log.[26] The Karolinska Sleep Quality Index (KSQI) is calculated from 4 items on the Karolinska Sleep Log (sleep quality, sleep restlessness, slept throughout the night, ease of falling asleep). The questions are on a 5‐point scale and the 4 items are averaged for a final score out of 5 with a higher number indicating better subjective sleep quality. The item How much was your sleep disturbed by noise? on the Karolinska Sleep Log was used to assess the degree to which noise was a disruptor of sleep. This question was also on a 5‐point scale with higher scores indicating greater disruptiveness of noise. Patients were also asked how disruptive noise from roommates was on a nightly basis using this same scale.
Objective Data Collection
Wrist activity monitors (Actiwatch 2; Respironics, Inc., Murrysville, PA)[27, 28, 29, 30] were used to measure patient sleep. Actiware 5 software (Respironics, Inc.)[31] was used to estimate quantitative measures of sleep time and efficiency. Sleep time is defined as the total duration of time spent sleeping at night and sleep efficiency is defined as the fraction of time, reported as a percentage, spent sleeping by actigraphy out of the total time patients reported they were sleeping.
Sound levels in patient rooms were recorded using Larson Davis 720 Sound Level Monitors (Larson Davis, Inc., Provo, UT). These monitors store functional average sound pressure levels in A‐weighted decibels called the Leq over 1‐hour intervals. The Leq is the average sound level over the given time interval. Minimum (Lmin) and maximum (Lmax) sound levels are also stored. The LD SLM Utility Program (Larson Davis, Inc.) was used to extract the sound level measurements recorded by the monitors.
Demographic information (age, gender, race, ethnicity, highest level of education, length of stay in the hospital, and comorbidities) was obtained from hospital charts via an ongoing study of admitted patients at the University of Chicago Medical Center inpatient general medicine service.[18] Chart audits were performed to determine whether patients received pharmacologic sleep aids in the hospital.
Data Analysis
Descriptive statistics were used to summarize mean sleep duration and sleep efficiency in the hospital as well as SLOC and SSE. Because the SSE scores were not normally distributed, the scores were dichotomized at the median to create a variable denoting high and low SSE. Additionally, because the distribution of responses to the noise disruption question was skewed to the right, reports of noise disruptions were grouped into not disruptive (score=1) and disruptive (score>1).
Two‐sample t tests with equal variances were used to assess the relationship between perceived control measures (high/low SLOC, SSE) and objective sleep measures (sleep time, sleep efficiency). Multivariate linear regression was used to test the association between high SSE (independent variable) and sleep time (dependent variable), clustering for multiple nights of data within the subject. Multivariate logistic regression, also adjusting for subject, was used to test the association between high SSE and noise disruptiveness and the association between high SSE and Karolinska scores. Leq, Lmax, and Lmin were all tested using stepwise forward regression. Because our prior work[9] demonstrated that noise levels separated into tertiles were significantly associated with sleep time, our analysis also used noise levels separated into tertiles. Stepwise forward regression was used to add basic patient demographics (gender, race, age) to the models. Statistical significance was defined as P<0.05, and all statistical analysis was done using Stata 11.0 (StataCorp, College Station, TX).
RESULTS
From April 2010 to May 2012, 1134 patients were screened by study personnel for this study via an ongoing study of hospitalized patients on the inpatient general medicine ward. Of the 361 (31.8%) eligible patients, 206 (57.1%) consented to participate. Of the subjects enrolled in the study, 118 were able to complete at least 1 night of actigraphy, sound monitoring, and subjective assessment for a total of 185 patient nights (Figure 1).

The majority of patients were female (57%), African American (67%), and non‐Hispanic (97%). The mean age was 65 years (standard deviation [SD], 11.6 years), and the median length of stay was 4 days (interquartile range [IQR], 36). The majority of patients also had hypertension (67%), with chronic obstructive pulmonary disease [COPD] (31%) and congestive heart failure (31%) being the next most common comorbidities. About two‐thirds of subjects (64%) were characterized as average or above average sleepers with Epworth Sleepiness Scale scores 9[20] (Table 1). Only 5% of patients received pharmacological sleep aids.
| Value, n (%)a | |
|---|---|
| |
| Patient characteristics | |
| Age, mean (SD), y | 63 (12) |
| Length of stay, median (IQR), db | 4 (36) |
| Female | 67 (57) |
| African American | 79 (67) |
| Hispanic | 3 (3) |
| High school graduate | 92 (78) |
| Comorbidities | |
| Hypertension | 79 (66) |
| Chronic obstructive pulmonary disease | 37 (31) |
| Congestive heart failure | 37 (31) |
| Diabetes | 36 (30) |
| End stage renal disease | 23 (19) |
| Baseline sleep characteristics | |
| Sleep duration, mean (SD), minc | 333 (128) |
| Epworth Sleepiness Scale, score 9d | 73 (64) |
The mean baseline SLOC score was 30.4 (SD, 6.7), with a median of 31 (IQR, 2735). The mean baseline SSE score was 32.1 (SD, 9.4), with a median of 34 (IQR, 2441). Fifty‐four patients were categorized as having high sleep self‐efficacy (high SSE), which we defined as scoring above the median of 34.
Average in‐hospital sleep was 5.5 hours (333 minutes; SD, 128 minutes) which was significantly shorter than the self‐reported sleep duration of 6.5 hours prior to admission (387 minutes, SD, 125 minutes; P=0.0001). The mean sleep efficiency was 73% (SD, 19%) with 55% of actigraphy nights below the normal range of 80% efficiency for adults.[19] Median KSQI was 3.5 (IQR, 2.254.75), with 41% of the patients with a KSQI 3, putting them in the insomniac range.[32] The median score on the noise disruptiveness question was 1 (IQR, 14) with 42% of reports coded as disruptive defined as a score >1 on the 5‐point scale. The median score on the roommate disruptiveness question was 1 (IQR, 11) with 77% of responses coded as not disruptive defined as a score of 1 on the 5‐point scale.
A 2‐sample t test with equal variances showed that those patients reporting high SSE were more likely to sleep longer in the hospital than those reporting low SSE (364 minutes 95% confidence interval [CI]: 340, 388 vs 309 minutes 95% CI: 283, 336; P=0.003) (Figure 2). Patients with high SSE were also more likely to have a normal sleep efficiency (above 80%) compared to those with low SSE (54% 95% CI: 43, 65 vs 38% 95% CI: 28,47; P=0.028). Last, there was a trend toward patients reporting higher SSE to also report less noise disruption compared to those patients with low SSE ([42%] 95% CI: 31, 53 vs [56%] 95% CI: 46, 65; P=0.063) (Figure 3).


Linear regression clustered by subject showed that high SSE was associated with longer sleep duration (55 minutes 95% CI: 14, 97; P=0.010). Furthermore, high SSE was significantly associated with longer sleep duration after controlling for both objective noise level and patient demographics in the model using stepwise forward regression (50 minutes 95% CI: 11, 90; P=0.014) (Table 2).
| Sleep Duration (min) | Model 1 Beta [95% CI]a | Model 2 Beta [95% CI]a |
|---|---|---|
| ||
| High SSE | 55 [14, 97]b | 50 [11, 90]b |
| Lmin tert 3 | 14 [59, 29] | |
| Lmin tert 2 | 21 [65, 23] | |
| Female | 49 [10, 89]b | |
| African American | 16 [59, 27] | |
| Age | 1 [0.9, 3] | |
| Karolinska Sleep Quality | Model 1 OR [95% CI]c | Model 2 OR [95% CI]c |
| High SSE | 2.04 [1.12, 3.71]b | 2.01 [1.06, 3.79]b |
| Lmin tert 3 | 0.90 [0.37, 2.2] | |
| Lmin tert 2 | 0.86 [0.38, 1.94] | |
| Female | 1.78 [0.90, 3.52] | |
| African American | 1.19 [0.60, 2.38] | |
| Age | 1.02 [0.99, 1.05] | |
| Noise Complaints | Model 1 OR [95% CI]d | Model 2 OR [95% CI]d |
| High SSE | 0.57 [0.30, 1.12] | 0.49 [0.25, 0.96]b |
| Lmin tert 3 | 0.85 [0.39, 1.84] | |
| Lmin tert 2 | 0.91 [0.43, 1.93] | |
| Female | 1.40 [0.71, 2.78] | |
| African American | 0.35 [0.17, 0.70] | |
| Age | 1.00 [0.96, 1.03] | |
| Age2e | 1.00 [1.00, 1.00] | |
Logistic regression clustered by subject demonstrated that patients with high SSE had 2 times higher odds of having a KSQI score above 3 (95% CI: 1.12, 3.71; P=0.020). This association was still significant after controlling for noise and patient demographics (OR: 2.01; 95% CI: 1.06, 3.79; P=0.032). After controlling for noise levels and patient demographics, there was a statistically significant association between high SSE and lower odds of noise complaints (OR: 0.49; 95% CI: 0.25, 0.96; P=0.039) (Table 2). Although demographic characteristics were not associated with high SSE, those patients with high SSE had lower odds of being in the loudest tertile rooms (OR: 0.34; 95% CI: 0.15, 0.74; P=0.007).
In multivariate linear regression analyses, there were no significant relationships between SLOC scores and KSQI, reported noise disruptiveness, and markers of sleep (sleep duration or sleep efficiency).
DISCUSSION
This study is the first to examine the relationship between perceived control, noise levels, and objective measurements of sleep in a hospital setting. One measure of perceived control, namely SSE, was associated with objective sleep duration, subjective and objective sleep quality, noise levels in patient rooms, and perhaps also patient complaints of noise. These associations remained significant after controlling for objective noise levels and patient demographics, suggesting that SSE is independently related to sleep.
In contrast to SSE, SLOC was not found to be significantly associated with either subjective or objective measures of sleep quality. The lack of association may be due to the fact that the SLOC questionnaire does not translate as well to the inpatient setting as the SSE questionnaire. The SLOC questionnaire focuses on general beliefs about sleep whereas the SSE questionnaire focuses on personal beliefs about one's own ability sleep in the immediate future, which may make it more relevant in the inpatient setting (see Supporting Information, Appendix 1 and 2, in the online version of this article).
Given our findings, it is important to identify why patients with high SSE have better sleep and fewer noise complaints. One possibility is that sleep self‐efficacy is an inherited trait unique to each person that is also predictive of a patient's sleep patterns. However, is it also possible that those patients with high SSE feel more empowered to take control of their environment, allowing them to advocate for better sleep? This hypothesis is further strengthened by the finding that those patients with high SSE on study entry were less likely to be in the noisiest rooms. This raises the possibility that at least 1 of the mechanisms by which high SSE may be protective against sleep loss is through patients taking an active role in noise reduction, such as closing the door or advocating for their sleep with staff. However, we did not directly observe or ask patients whether doors of patient rooms were open or closed or whether the patients took other measures to advocate for their own sleep. Thus, further work is necessary to understand the mechanisms by which sleep self‐efficacy may influence sleep.
One potential avenue for future research is to explore possible interventions for boosting sleep self‐efficacy in the hospital. Although most interventions have focused on environmental noise and staff‐based education, empowering patients through boosting SSE may be a helpful adjunct to improving hospital sleep.[33, 34] Currently, the SSE scale is not commonly used in the inpatient setting. Motivational interviewing and patient coaching could be explored as potential tools for boosting SSE. Furthermore, even if SSE is not easily changed, measuring SSE in patients newly admitted to the hospital may be useful in identifying patients most susceptible to sleep disruptions. Efforts to identify patients with low SSE should go hand‐in‐hand with measures to reduce noise. Addressing both patient‐level and environmental factors simultaneously may be the best strategy for improving sleep in an inpatient hospital setting.
In contrast to our prior study, it is worth noting that we did not find any significant relationships between overall noise levels and sleep.[9] In this dataset, nighttime noise is still a predictor of sleep loss in the hospital. However, when we restrict our sample to those who answered the SSE questionnaire and had nighttime noise recorded, we lose a significant number of observations. Because of our interest in testing the relationship between SSE and sleep, we chose to control for overall noise (which enabled us to retain more observations). We also did not find any interactions between SSE and noise in our regression models. Further work is warranted with larger sample sizes to better understand the role of SSE in the context of sleep and noise levels. In addition, females also received more sleep than males in our study.
There are several limitations to this study. This study was carried out at a single service at a single institution, limiting the ability to generalize the findings to other hospital settings. This study had a relatively high rate of patients who were unable to complete at least 1 night of data collection (42%), often due to watch removal for imaging or procedures, which may also affect the representativeness of our sample. Moreover, we can only examine associations and not causal relationships. The SSE scale has never been used in hospitalized patients, making comparisons between scores from hospitalized patients and population controls difficult. In addition, the SSE scale also has not been dichotomized in previous studies into high and low SSE. However, a sensitivity analysis with raw SSE scores did not change the results of our study. It can be difficult to perform actigraphy measurements in the hospital because many patients spend most of their time in bed. Because we chose a relatively healthy cohort of patients without significant limitations in mobility, actigraphy could still be used to differentiate time spent awake from time spent sleeping. Because we did not perform polysomnography, we cannot explore the role of sleep architecture which is an important component of sleep quality. Although the use of pharmacologic sleep aids is a potential confounding factor, the rate of use was very low in our cohort and unlikely to significantly affect our results. Continued study of this patient population is warranted to further develop the findings.
In conclusion, patients with high SSE sleep better in the hospital, tend to be in quieter rooms, and may report fewer noise complaints. Our findings suggest that a greater confidence in the ability to sleep may be beneficial in hospitalized adults. In addition to noise control, hospitals should also consider targeting patients with low SSE when designing novel interventions to improve in‐hospital sleep.
Disclosures
This work was supported by funding from the National Institute on Aging through a Short‐Term Aging‐Related Research Program (1 T35 AG029795), National Institute on Aging career development award (K23AG033763), a midcareer career development award (1K24AG031326), a program project (P01AG‐11412), an Agency for Healthcare Research and Quality Centers for Education and Research on Therapeutics grant (1U18HS016967), and a National Institute on Aging Clinical Translational Sciences award (UL1 RR024999). Dr. Arora had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the statistical analysis. The funding agencies had no role in the design of the study; the collection, analysis, and interpretation of the data; or the decision to approve publication of the finished manuscript. The authors report no conflicts of interest.
Lack of sleep is a common problem in hospitalized patients and is associated with poorer health outcomes, especially in older patients.[1, 2, 3] Prior studies highlight a multitude of factors that can result in sleep loss in the hospital[3, 4, 5, 6] with 1 of the most common causes of sleep disruption in the hospital being noise.[7, 8, 9]
In addition to external factors, such as hospital noise, there may be inherent characteristics that predispose certain patients to greater sleep loss when hospitalized. One such measure is the construct of perceived control or the psychological measure of how much individuals expect themselves to be capable of bringing about desired outcomes.[10] Among older patients, low perceived control is associated with increased rates of physician visits, hospitalizations, and death.[11, 12] In contrast, patients who feel more in control of their environment may experience positive health benefits.[13]
Yet, when patients are placed in a hospital setting, they experience a significant reduction in control over their environment along with an increase in dependency on medical staff and therapies.[14, 15] For example, hospitalized patients are restricted in their personal decisions, such as what clothes they can wear and what they can eat and are not in charge of their own schedules, including their sleep time.
Although prior studies suggest that perceived control over sleep is related to actual sleep among community‐dwelling adults,[16, 17] no study has examined this relationship in hospitalized adults. Therefore, the aim of our study was to examine the possible association between perceived control, noise levels, and sleep in hospitalized middle‐aged and older patients.
METHODS
Study Design
We conducted a prospective cohort study of subjects recruited from a large ongoing study of admitted patients at the University of Chicago inpatient general medicine service.[18] Because we were interested in middle‐aged and older adults who are most sensitive to sleep disruptions, patients who were age 50 years and over, ambulatory, and living in the community were eligible for the study.[19] Exclusion criteria were cognitive impairment (telephone version of the Mini‐Mental State Exam <17 out of 22), preexisting sleeping disorders identified via patient charts, such as obstructive sleep apnea and narcolepsy, transfer from the intensive care unit (ICU), and admission to the hospital more than 72 hours prior to enrollment.[20] These inclusion and exclusion criteria were selected to identify a patient population with minimal sleep disturbances at baseline. Patients under isolation were excluded because they are not visited as frequently by the healthcare team.[21, 22] Most general medicine rooms were double occupancy but efforts were made to make patient rooms single when possible or required (ie, isolation for infection control). The study was approved by the University of Chicago Institutional Review Board.
Subjective Data Collection
Baseline levels of perceived control over sleep, or the amount of control patients believe they have over their sleep, were assessed using 2 different scales. The first tool was the 8‐item Sleep Locus of Control (SLOC) scale,[17] which ranges from 8 to 48, with higher values corresponding to a greater internal locus of control over sleep. An internal sleep locus of control indicates beliefs that patients feel that they are primarily responsible for their own sleep as opposed to an external locus of control which indicates beliefs that good sleep is due to luck or chance. For example, patients were asked how strongly they agree or disagree with statements, such as, If I take care of myself, I can avoid insomnia and People who never get insomnia are just plain lucky (see Supporting Information, Appendix 2, in the online version of this article). The second tool was the 9‐item Sleep Self‐Efficacy (SSE) scale,[23] which ranges from 9 to 45, with higher values corresponding to greater confidence patients have in their ability to sleep. One of the items asks, How confident are you that you can lie in bed feeling physically relaxed (see Supporting Information, Appendix 1, in the online version of this article)? Both instruments have been validated in an outpatient setting.[23] These surveys were given immediately on enrollment in the study to measure baseline perceived control.
Baseline sleep habits were also collected on enrollment using the Epworth Sleepiness Scale,[24, 25] a standard validated survey that assesses excess daytime sleepiness in various common situations. For each day in the hospital, patients were asked to report in‐hospital sleep quality using the Karolinska Sleep Log.[26] The Karolinska Sleep Quality Index (KSQI) is calculated from 4 items on the Karolinska Sleep Log (sleep quality, sleep restlessness, slept throughout the night, ease of falling asleep). The questions are on a 5‐point scale and the 4 items are averaged for a final score out of 5 with a higher number indicating better subjective sleep quality. The item How much was your sleep disturbed by noise? on the Karolinska Sleep Log was used to assess the degree to which noise was a disruptor of sleep. This question was also on a 5‐point scale with higher scores indicating greater disruptiveness of noise. Patients were also asked how disruptive noise from roommates was on a nightly basis using this same scale.
Objective Data Collection
Wrist activity monitors (Actiwatch 2; Respironics, Inc., Murrysville, PA)[27, 28, 29, 30] were used to measure patient sleep. Actiware 5 software (Respironics, Inc.)[31] was used to estimate quantitative measures of sleep time and efficiency. Sleep time is defined as the total duration of time spent sleeping at night and sleep efficiency is defined as the fraction of time, reported as a percentage, spent sleeping by actigraphy out of the total time patients reported they were sleeping.
Sound levels in patient rooms were recorded using Larson Davis 720 Sound Level Monitors (Larson Davis, Inc., Provo, UT). These monitors store functional average sound pressure levels in A‐weighted decibels called the Leq over 1‐hour intervals. The Leq is the average sound level over the given time interval. Minimum (Lmin) and maximum (Lmax) sound levels are also stored. The LD SLM Utility Program (Larson Davis, Inc.) was used to extract the sound level measurements recorded by the monitors.
Demographic information (age, gender, race, ethnicity, highest level of education, length of stay in the hospital, and comorbidities) was obtained from hospital charts via an ongoing study of admitted patients at the University of Chicago Medical Center inpatient general medicine service.[18] Chart audits were performed to determine whether patients received pharmacologic sleep aids in the hospital.
Data Analysis
Descriptive statistics were used to summarize mean sleep duration and sleep efficiency in the hospital as well as SLOC and SSE. Because the SSE scores were not normally distributed, the scores were dichotomized at the median to create a variable denoting high and low SSE. Additionally, because the distribution of responses to the noise disruption question was skewed to the right, reports of noise disruptions were grouped into not disruptive (score=1) and disruptive (score>1).
Two‐sample t tests with equal variances were used to assess the relationship between perceived control measures (high/low SLOC, SSE) and objective sleep measures (sleep time, sleep efficiency). Multivariate linear regression was used to test the association between high SSE (independent variable) and sleep time (dependent variable), clustering for multiple nights of data within the subject. Multivariate logistic regression, also adjusting for subject, was used to test the association between high SSE and noise disruptiveness and the association between high SSE and Karolinska scores. Leq, Lmax, and Lmin were all tested using stepwise forward regression. Because our prior work[9] demonstrated that noise levels separated into tertiles were significantly associated with sleep time, our analysis also used noise levels separated into tertiles. Stepwise forward regression was used to add basic patient demographics (gender, race, age) to the models. Statistical significance was defined as P<0.05, and all statistical analysis was done using Stata 11.0 (StataCorp, College Station, TX).
RESULTS
From April 2010 to May 2012, 1134 patients were screened by study personnel for this study via an ongoing study of hospitalized patients on the inpatient general medicine ward. Of the 361 (31.8%) eligible patients, 206 (57.1%) consented to participate. Of the subjects enrolled in the study, 118 were able to complete at least 1 night of actigraphy, sound monitoring, and subjective assessment for a total of 185 patient nights (Figure 1).

The majority of patients were female (57%), African American (67%), and non‐Hispanic (97%). The mean age was 65 years (standard deviation [SD], 11.6 years), and the median length of stay was 4 days (interquartile range [IQR], 36). The majority of patients also had hypertension (67%), with chronic obstructive pulmonary disease [COPD] (31%) and congestive heart failure (31%) being the next most common comorbidities. About two‐thirds of subjects (64%) were characterized as average or above average sleepers with Epworth Sleepiness Scale scores 9[20] (Table 1). Only 5% of patients received pharmacological sleep aids.
| Value, n (%)a | |
|---|---|
| |
| Patient characteristics | |
| Age, mean (SD), y | 63 (12) |
| Length of stay, median (IQR), db | 4 (36) |
| Female | 67 (57) |
| African American | 79 (67) |
| Hispanic | 3 (3) |
| High school graduate | 92 (78) |
| Comorbidities | |
| Hypertension | 79 (66) |
| Chronic obstructive pulmonary disease | 37 (31) |
| Congestive heart failure | 37 (31) |
| Diabetes | 36 (30) |
| End stage renal disease | 23 (19) |
| Baseline sleep characteristics | |
| Sleep duration, mean (SD), minc | 333 (128) |
| Epworth Sleepiness Scale, score 9d | 73 (64) |
The mean baseline SLOC score was 30.4 (SD, 6.7), with a median of 31 (IQR, 2735). The mean baseline SSE score was 32.1 (SD, 9.4), with a median of 34 (IQR, 2441). Fifty‐four patients were categorized as having high sleep self‐efficacy (high SSE), which we defined as scoring above the median of 34.
Average in‐hospital sleep was 5.5 hours (333 minutes; SD, 128 minutes) which was significantly shorter than the self‐reported sleep duration of 6.5 hours prior to admission (387 minutes, SD, 125 minutes; P=0.0001). The mean sleep efficiency was 73% (SD, 19%) with 55% of actigraphy nights below the normal range of 80% efficiency for adults.[19] Median KSQI was 3.5 (IQR, 2.254.75), with 41% of the patients with a KSQI 3, putting them in the insomniac range.[32] The median score on the noise disruptiveness question was 1 (IQR, 14) with 42% of reports coded as disruptive defined as a score >1 on the 5‐point scale. The median score on the roommate disruptiveness question was 1 (IQR, 11) with 77% of responses coded as not disruptive defined as a score of 1 on the 5‐point scale.
A 2‐sample t test with equal variances showed that those patients reporting high SSE were more likely to sleep longer in the hospital than those reporting low SSE (364 minutes 95% confidence interval [CI]: 340, 388 vs 309 minutes 95% CI: 283, 336; P=0.003) (Figure 2). Patients with high SSE were also more likely to have a normal sleep efficiency (above 80%) compared to those with low SSE (54% 95% CI: 43, 65 vs 38% 95% CI: 28,47; P=0.028). Last, there was a trend toward patients reporting higher SSE to also report less noise disruption compared to those patients with low SSE ([42%] 95% CI: 31, 53 vs [56%] 95% CI: 46, 65; P=0.063) (Figure 3).


Linear regression clustered by subject showed that high SSE was associated with longer sleep duration (55 minutes 95% CI: 14, 97; P=0.010). Furthermore, high SSE was significantly associated with longer sleep duration after controlling for both objective noise level and patient demographics in the model using stepwise forward regression (50 minutes 95% CI: 11, 90; P=0.014) (Table 2).
| Sleep Duration (min) | Model 1 Beta [95% CI]a | Model 2 Beta [95% CI]a |
|---|---|---|
| ||
| High SSE | 55 [14, 97]b | 50 [11, 90]b |
| Lmin tert 3 | 14 [59, 29] | |
| Lmin tert 2 | 21 [65, 23] | |
| Female | 49 [10, 89]b | |
| African American | 16 [59, 27] | |
| Age | 1 [0.9, 3] | |
| Karolinska Sleep Quality | Model 1 OR [95% CI]c | Model 2 OR [95% CI]c |
| High SSE | 2.04 [1.12, 3.71]b | 2.01 [1.06, 3.79]b |
| Lmin tert 3 | 0.90 [0.37, 2.2] | |
| Lmin tert 2 | 0.86 [0.38, 1.94] | |
| Female | 1.78 [0.90, 3.52] | |
| African American | 1.19 [0.60, 2.38] | |
| Age | 1.02 [0.99, 1.05] | |
| Noise Complaints | Model 1 OR [95% CI]d | Model 2 OR [95% CI]d |
| High SSE | 0.57 [0.30, 1.12] | 0.49 [0.25, 0.96]b |
| Lmin tert 3 | 0.85 [0.39, 1.84] | |
| Lmin tert 2 | 0.91 [0.43, 1.93] | |
| Female | 1.40 [0.71, 2.78] | |
| African American | 0.35 [0.17, 0.70] | |
| Age | 1.00 [0.96, 1.03] | |
| Age2e | 1.00 [1.00, 1.00] | |
Logistic regression clustered by subject demonstrated that patients with high SSE had 2 times higher odds of having a KSQI score above 3 (95% CI: 1.12, 3.71; P=0.020). This association was still significant after controlling for noise and patient demographics (OR: 2.01; 95% CI: 1.06, 3.79; P=0.032). After controlling for noise levels and patient demographics, there was a statistically significant association between high SSE and lower odds of noise complaints (OR: 0.49; 95% CI: 0.25, 0.96; P=0.039) (Table 2). Although demographic characteristics were not associated with high SSE, those patients with high SSE had lower odds of being in the loudest tertile rooms (OR: 0.34; 95% CI: 0.15, 0.74; P=0.007).
In multivariate linear regression analyses, there were no significant relationships between SLOC scores and KSQI, reported noise disruptiveness, and markers of sleep (sleep duration or sleep efficiency).
DISCUSSION
This study is the first to examine the relationship between perceived control, noise levels, and objective measurements of sleep in a hospital setting. One measure of perceived control, namely SSE, was associated with objective sleep duration, subjective and objective sleep quality, noise levels in patient rooms, and perhaps also patient complaints of noise. These associations remained significant after controlling for objective noise levels and patient demographics, suggesting that SSE is independently related to sleep.
In contrast to SSE, SLOC was not found to be significantly associated with either subjective or objective measures of sleep quality. The lack of association may be due to the fact that the SLOC questionnaire does not translate as well to the inpatient setting as the SSE questionnaire. The SLOC questionnaire focuses on general beliefs about sleep whereas the SSE questionnaire focuses on personal beliefs about one's own ability sleep in the immediate future, which may make it more relevant in the inpatient setting (see Supporting Information, Appendix 1 and 2, in the online version of this article).
Given our findings, it is important to identify why patients with high SSE have better sleep and fewer noise complaints. One possibility is that sleep self‐efficacy is an inherited trait unique to each person that is also predictive of a patient's sleep patterns. However, is it also possible that those patients with high SSE feel more empowered to take control of their environment, allowing them to advocate for better sleep? This hypothesis is further strengthened by the finding that those patients with high SSE on study entry were less likely to be in the noisiest rooms. This raises the possibility that at least 1 of the mechanisms by which high SSE may be protective against sleep loss is through patients taking an active role in noise reduction, such as closing the door or advocating for their sleep with staff. However, we did not directly observe or ask patients whether doors of patient rooms were open or closed or whether the patients took other measures to advocate for their own sleep. Thus, further work is necessary to understand the mechanisms by which sleep self‐efficacy may influence sleep.
One potential avenue for future research is to explore possible interventions for boosting sleep self‐efficacy in the hospital. Although most interventions have focused on environmental noise and staff‐based education, empowering patients through boosting SSE may be a helpful adjunct to improving hospital sleep.[33, 34] Currently, the SSE scale is not commonly used in the inpatient setting. Motivational interviewing and patient coaching could be explored as potential tools for boosting SSE. Furthermore, even if SSE is not easily changed, measuring SSE in patients newly admitted to the hospital may be useful in identifying patients most susceptible to sleep disruptions. Efforts to identify patients with low SSE should go hand‐in‐hand with measures to reduce noise. Addressing both patient‐level and environmental factors simultaneously may be the best strategy for improving sleep in an inpatient hospital setting.
In contrast to our prior study, it is worth noting that we did not find any significant relationships between overall noise levels and sleep.[9] In this dataset, nighttime noise is still a predictor of sleep loss in the hospital. However, when we restrict our sample to those who answered the SSE questionnaire and had nighttime noise recorded, we lose a significant number of observations. Because of our interest in testing the relationship between SSE and sleep, we chose to control for overall noise (which enabled us to retain more observations). We also did not find any interactions between SSE and noise in our regression models. Further work is warranted with larger sample sizes to better understand the role of SSE in the context of sleep and noise levels. In addition, females also received more sleep than males in our study.
There are several limitations to this study. This study was carried out at a single service at a single institution, limiting the ability to generalize the findings to other hospital settings. This study had a relatively high rate of patients who were unable to complete at least 1 night of data collection (42%), often due to watch removal for imaging or procedures, which may also affect the representativeness of our sample. Moreover, we can only examine associations and not causal relationships. The SSE scale has never been used in hospitalized patients, making comparisons between scores from hospitalized patients and population controls difficult. In addition, the SSE scale also has not been dichotomized in previous studies into high and low SSE. However, a sensitivity analysis with raw SSE scores did not change the results of our study. It can be difficult to perform actigraphy measurements in the hospital because many patients spend most of their time in bed. Because we chose a relatively healthy cohort of patients without significant limitations in mobility, actigraphy could still be used to differentiate time spent awake from time spent sleeping. Because we did not perform polysomnography, we cannot explore the role of sleep architecture which is an important component of sleep quality. Although the use of pharmacologic sleep aids is a potential confounding factor, the rate of use was very low in our cohort and unlikely to significantly affect our results. Continued study of this patient population is warranted to further develop the findings.
In conclusion, patients with high SSE sleep better in the hospital, tend to be in quieter rooms, and may report fewer noise complaints. Our findings suggest that a greater confidence in the ability to sleep may be beneficial in hospitalized adults. In addition to noise control, hospitals should also consider targeting patients with low SSE when designing novel interventions to improve in‐hospital sleep.
Disclosures
This work was supported by funding from the National Institute on Aging through a Short‐Term Aging‐Related Research Program (1 T35 AG029795), National Institute on Aging career development award (K23AG033763), a midcareer career development award (1K24AG031326), a program project (P01AG‐11412), an Agency for Healthcare Research and Quality Centers for Education and Research on Therapeutics grant (1U18HS016967), and a National Institute on Aging Clinical Translational Sciences award (UL1 RR024999). Dr. Arora had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the statistical analysis. The funding agencies had no role in the design of the study; the collection, analysis, and interpretation of the data; or the decision to approve publication of the finished manuscript. The authors report no conflicts of interest.
- , , , . The metabolic consequences of sleep deprivation. Sleep Med Rev. 2007;11(3):163–178.
- , , , , , . Poor self‐reported sleep quality predicts mortality within one year of inpatient post‐acute rehabilitation among older adults. Sleep. 2011;34(12):1715–1721.
- , , , et al. The sleep of older people in hospital and nursing homes. J Clin Nurs. 1999;8:360–368.
- , , et al. Sleep in hospitalized medical patients, part 1: factors affecting sleep. J Hosp Med. 2008; 3:473–482.
- , , , et al. Nocturnal care interactions with patients in critical care units. Am J Crit Care. 2004;13:102–112; quiz 114–115.
- , , . Patient perception of sleep quality and etiology of sleep disruption in the intensive care unit. Am J Respir Crit Care Med. 1999;159:1155–1162.
- . Sleep in acute care settings: an integrative review. J Nurs Scholarsh. 2000;32(1):31–38.
- , , , et al. Sleep disruption due to hospital noises: a prospective evaluation. Ann Int Med. 2012;157(3): 170–179.
- , , , et al. Noise and sleep among adult medical inpatients: far from a quiet night. Arch Intern Med. 2012;172:68–70.
- . Generalized expectancies for internal versus external control of reinforcement. Psychol Monogr. 1966;80:1–28.
- , . Psychosocial risk factors and mortality: a prospective study with special focus on social support, social participation, and locus of control in Norway. J Epidemiol Community Health. 1998;52:476–481.
- , . The interactive effect of perceived control and functional status on health and mortality among young‐old and old‐old adults. J Gerontol B Psychol Sci Soc Sci. 1997;52:P118–P126.
- , . Role‐specific feelings of control and mortality. Psychol Aging. 2000;15:617–626.
- , , . Patient empowerment in intensive care—an interview study. Intensive Crit Care Nurs. 2006;22:370–377.
- , , . Exploring the relationship between personal control and the hospital environment. J Clin Nurs. 2008;17:1601–1609.
- , , , et al. Effects of volitional lifestyle on sleep‐life habits in the aged. Psychiatry Clin Neurosci. 1998;52:183–184.
- , , , et al. Sleep locus of control: report on a new scale. Behav Sleep Med. 2004;2:79–93.
- , , , et al. Effects of physician experience on costs and outcomes on an academic general medicine service: results of a trial of hospitalists. Ann Intern Med. 2002;137:866–874.
- , , , et al. The effects of age, sex, ethnicity, and sleep‐disordered breathing on sleep architecture. Arch Intern Med. 2004;164:406–418.
- , , , et al. Validation of a telephone version of the mini‐mental state examination. J Am Geriatr Soc. 1992;40:697–702.
- , , , et al. Contact isolation in surgical patients: a barrier to care? Surgery. 2003;134:180–188.
- , . Adverse effects of contact isolation. Lancet. 1999;354:1177–1178.
- . Behavioral Treatment for Persistent Insomnia. Elmsford, NY: Pergamon Press; 1987.
- . A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep. 1991;14:540–545.
- . Reliability and factor analysis of the Epworth Sleepiness Scale. Sleep. 1992;15:376–381.
- , . Objective components of individual differences in subjective sleep quality. J Sleep Res. 1997;6:217–220.
- , , , et al. The role of actigraphy in the study of sleep and circadian rhythms. Sleep. 2003;26:342–392.
- , , , et al. Practice parameters for the use of actigraphy in the assessment of sleep and sleep disorders: an update for 2007. Sleep. 2007;30:519–529.
- , , , et al. The role of actigraphy in the evaluation of sleep disorders. Sleep. 1995;18:288–302.
- , , , et al. Clinical review: sleep measurement in critical care patients: research and clinical implications. Crit Care. 2007;11:226.
- , , , et al. Evaluation of immobility time for sleep latency in actigraphy. Sleep Med. 2009;10:621–625.
- , , , et al. The subjective meaning of sleep quality: a comparison of individuals with and without insomnia. Sleep. 2008;31:383–393.
- , , , et al. Sleep in hospitalized medical patients, part 2: behavioral and pharmacological management of sleep disturbances. J Hosp Med. 2009;4:50–59.
- , , , . A nonpharmacologic sleep protocol for hospitalized older patients. J Am Geriatr Soc. 1998;46(6):700–705.
- , , , . The metabolic consequences of sleep deprivation. Sleep Med Rev. 2007;11(3):163–178.
- , , , , , . Poor self‐reported sleep quality predicts mortality within one year of inpatient post‐acute rehabilitation among older adults. Sleep. 2011;34(12):1715–1721.
- , , , et al. The sleep of older people in hospital and nursing homes. J Clin Nurs. 1999;8:360–368.
- , , et al. Sleep in hospitalized medical patients, part 1: factors affecting sleep. J Hosp Med. 2008; 3:473–482.
- , , , et al. Nocturnal care interactions with patients in critical care units. Am J Crit Care. 2004;13:102–112; quiz 114–115.
- , , . Patient perception of sleep quality and etiology of sleep disruption in the intensive care unit. Am J Respir Crit Care Med. 1999;159:1155–1162.
- . Sleep in acute care settings: an integrative review. J Nurs Scholarsh. 2000;32(1):31–38.
- , , , et al. Sleep disruption due to hospital noises: a prospective evaluation. Ann Int Med. 2012;157(3): 170–179.
- , , , et al. Noise and sleep among adult medical inpatients: far from a quiet night. Arch Intern Med. 2012;172:68–70.
- . Generalized expectancies for internal versus external control of reinforcement. Psychol Monogr. 1966;80:1–28.
- , . Psychosocial risk factors and mortality: a prospective study with special focus on social support, social participation, and locus of control in Norway. J Epidemiol Community Health. 1998;52:476–481.
- , . The interactive effect of perceived control and functional status on health and mortality among young‐old and old‐old adults. J Gerontol B Psychol Sci Soc Sci. 1997;52:P118–P126.
- , . Role‐specific feelings of control and mortality. Psychol Aging. 2000;15:617–626.
- , , . Patient empowerment in intensive care—an interview study. Intensive Crit Care Nurs. 2006;22:370–377.
- , , . Exploring the relationship between personal control and the hospital environment. J Clin Nurs. 2008;17:1601–1609.
- , , , et al. Effects of volitional lifestyle on sleep‐life habits in the aged. Psychiatry Clin Neurosci. 1998;52:183–184.
- , , , et al. Sleep locus of control: report on a new scale. Behav Sleep Med. 2004;2:79–93.
- , , , et al. Effects of physician experience on costs and outcomes on an academic general medicine service: results of a trial of hospitalists. Ann Intern Med. 2002;137:866–874.
- , , , et al. The effects of age, sex, ethnicity, and sleep‐disordered breathing on sleep architecture. Arch Intern Med. 2004;164:406–418.
- , , , et al. Validation of a telephone version of the mini‐mental state examination. J Am Geriatr Soc. 1992;40:697–702.
- , , , et al. Contact isolation in surgical patients: a barrier to care? Surgery. 2003;134:180–188.
- , . Adverse effects of contact isolation. Lancet. 1999;354:1177–1178.
- . Behavioral Treatment for Persistent Insomnia. Elmsford, NY: Pergamon Press; 1987.
- . A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep. 1991;14:540–545.
- . Reliability and factor analysis of the Epworth Sleepiness Scale. Sleep. 1992;15:376–381.
- , . Objective components of individual differences in subjective sleep quality. J Sleep Res. 1997;6:217–220.
- , , , et al. The role of actigraphy in the study of sleep and circadian rhythms. Sleep. 2003;26:342–392.
- , , , et al. Practice parameters for the use of actigraphy in the assessment of sleep and sleep disorders: an update for 2007. Sleep. 2007;30:519–529.
- , , , et al. The role of actigraphy in the evaluation of sleep disorders. Sleep. 1995;18:288–302.
- , , , et al. Clinical review: sleep measurement in critical care patients: research and clinical implications. Crit Care. 2007;11:226.
- , , , et al. Evaluation of immobility time for sleep latency in actigraphy. Sleep Med. 2009;10:621–625.
- , , , et al. The subjective meaning of sleep quality: a comparison of individuals with and without insomnia. Sleep. 2008;31:383–393.
- , , , et al. Sleep in hospitalized medical patients, part 2: behavioral and pharmacological management of sleep disturbances. J Hosp Med. 2009;4:50–59.
- , , , . A nonpharmacologic sleep protocol for hospitalized older patients. J Am Geriatr Soc. 1998;46(6):700–705.
Copyright © 2013 Society of Hospital Medicine
Hospitalists on Alert as CRE Infections Spike
Hospitalists should be on the lookout for carbapenem-resistant Enterobacteriaceae (CRE) infections, says one author of a CDC report that noted a three-fold increase in the proportion of Enterobacteriaceae bugs that proved resistant to carbapenem within the past decade.
Earlier this month, the CDC's Morbidity and Mortality Weekly Report revealed that the percentage of CRE infections jumped to 4.2% in 2011 from 1.2% in 2001, according to data from the National Nosocomial Infection Surveillance system.
"It is a very serious public health threat," says co-author Alex Kallen, MD, MPH, a medical epidemiologist and outbreak response coordinator in the CDC's Division of Healthcare Quality Promotion. "Maybe it's not that common now, but with no action, it has the potential to become much more common, like a lot of the other MDROs [multidrug-resistant organisms] that hospitalists see regularly. [Hospitalists] have a lot of control over some of the things that could potentially lead to increased transmission."
Dr. Kallen says HM groups can help reduce the spread of CRE through antibiotic stewardship, the review of detailed patient histories to ferret out risk factors, and dedication to contact precautions and hand hygiene. Hospitalists also play a leadership role in coordinating efforts for patients transferring between hospitals and other institutions, such as skilled-nursing or assisted-living facilities, he says.
Dr. Kallen added that hospitalists should not dismiss CRE, even if they rarely encounter it.
"If you're a place that doesn't see this very often, and you see one, that's a big deal," he adds. "It needs to be acted on aggressively. Being proactive is much more effective than waiting until it's common and then trying to intervene."
Visit our website for more information on hospital-acquired infections.
Hospitalists should be on the lookout for carbapenem-resistant Enterobacteriaceae (CRE) infections, says one author of a CDC report that noted a three-fold increase in the proportion of Enterobacteriaceae bugs that proved resistant to carbapenem within the past decade.
Earlier this month, the CDC's Morbidity and Mortality Weekly Report revealed that the percentage of CRE infections jumped to 4.2% in 2011 from 1.2% in 2001, according to data from the National Nosocomial Infection Surveillance system.
"It is a very serious public health threat," says co-author Alex Kallen, MD, MPH, a medical epidemiologist and outbreak response coordinator in the CDC's Division of Healthcare Quality Promotion. "Maybe it's not that common now, but with no action, it has the potential to become much more common, like a lot of the other MDROs [multidrug-resistant organisms] that hospitalists see regularly. [Hospitalists] have a lot of control over some of the things that could potentially lead to increased transmission."
Dr. Kallen says HM groups can help reduce the spread of CRE through antibiotic stewardship, the review of detailed patient histories to ferret out risk factors, and dedication to contact precautions and hand hygiene. Hospitalists also play a leadership role in coordinating efforts for patients transferring between hospitals and other institutions, such as skilled-nursing or assisted-living facilities, he says.
Dr. Kallen added that hospitalists should not dismiss CRE, even if they rarely encounter it.
"If you're a place that doesn't see this very often, and you see one, that's a big deal," he adds. "It needs to be acted on aggressively. Being proactive is much more effective than waiting until it's common and then trying to intervene."
Visit our website for more information on hospital-acquired infections.
Hospitalists should be on the lookout for carbapenem-resistant Enterobacteriaceae (CRE) infections, says one author of a CDC report that noted a three-fold increase in the proportion of Enterobacteriaceae bugs that proved resistant to carbapenem within the past decade.
Earlier this month, the CDC's Morbidity and Mortality Weekly Report revealed that the percentage of CRE infections jumped to 4.2% in 2011 from 1.2% in 2001, according to data from the National Nosocomial Infection Surveillance system.
"It is a very serious public health threat," says co-author Alex Kallen, MD, MPH, a medical epidemiologist and outbreak response coordinator in the CDC's Division of Healthcare Quality Promotion. "Maybe it's not that common now, but with no action, it has the potential to become much more common, like a lot of the other MDROs [multidrug-resistant organisms] that hospitalists see regularly. [Hospitalists] have a lot of control over some of the things that could potentially lead to increased transmission."
Dr. Kallen says HM groups can help reduce the spread of CRE through antibiotic stewardship, the review of detailed patient histories to ferret out risk factors, and dedication to contact precautions and hand hygiene. Hospitalists also play a leadership role in coordinating efforts for patients transferring between hospitals and other institutions, such as skilled-nursing or assisted-living facilities, he says.
Dr. Kallen added that hospitalists should not dismiss CRE, even if they rarely encounter it.
"If you're a place that doesn't see this very often, and you see one, that's a big deal," he adds. "It needs to be acted on aggressively. Being proactive is much more effective than waiting until it's common and then trying to intervene."
Visit our website for more information on hospital-acquired infections.
Foundation Chips in to Reduce 30-Day Readmissions
The Robert Wood Johnson Foundation of Princeton, N.J., the country’s largest healthcare-focused philanthropy, has undertaken a number of initiatives to improve care transitions and reduce preventable hospital readmissions.
One of the key conclusions from these initiatives, says Anne Weiss, MPP, director of the foundation's Quality/Equality Health Care Team, is that hospitals and hospitalists can't do it alone. "Hospitals are now being held financially accountable for something they can't possibly control," Weiss says, referring to whether or not the discharged patient returns to the hospital within 30 days.
The foundation has mobilized broad community coalitions through its Aligning Forces for Quality campaign, bringing together healthcare providers, purchasers, consumers, and other stakeholders to improve care transitions. One such coalition, Better Health Greater Cleveland of Ohio, announced a 10.7% reduction in avoidable hospitalizations for common cardiac conditions in 2011.
Successful care transitions also require healthcare providers to appreciate the need for patients and their families to engage in their plans for post-discharge care, Weiss says. "I have been stunned to learn the kinds of medical tasks patients and families are now expected to conduct when they go home," she adds. "I hear them say, 'Nobody told us we would have to flush IVs.'"
Through another initiative, the foundation produced an interactive map that displays the percentage of patients readmitted to hospitals within 30 days of discharge; it has supported research that found improvements in nurses' work environments helped to reduce avoidable hospital readmissions. It also has produced a "Transitions to Better Care" video contest for hospitals, as well as a national publicity campaign about these issues called "Care About Your Care."
Visit our website for more information about patient care transitions.
The Robert Wood Johnson Foundation of Princeton, N.J., the country’s largest healthcare-focused philanthropy, has undertaken a number of initiatives to improve care transitions and reduce preventable hospital readmissions.
One of the key conclusions from these initiatives, says Anne Weiss, MPP, director of the foundation's Quality/Equality Health Care Team, is that hospitals and hospitalists can't do it alone. "Hospitals are now being held financially accountable for something they can't possibly control," Weiss says, referring to whether or not the discharged patient returns to the hospital within 30 days.
The foundation has mobilized broad community coalitions through its Aligning Forces for Quality campaign, bringing together healthcare providers, purchasers, consumers, and other stakeholders to improve care transitions. One such coalition, Better Health Greater Cleveland of Ohio, announced a 10.7% reduction in avoidable hospitalizations for common cardiac conditions in 2011.
Successful care transitions also require healthcare providers to appreciate the need for patients and their families to engage in their plans for post-discharge care, Weiss says. "I have been stunned to learn the kinds of medical tasks patients and families are now expected to conduct when they go home," she adds. "I hear them say, 'Nobody told us we would have to flush IVs.'"
Through another initiative, the foundation produced an interactive map that displays the percentage of patients readmitted to hospitals within 30 days of discharge; it has supported research that found improvements in nurses' work environments helped to reduce avoidable hospital readmissions. It also has produced a "Transitions to Better Care" video contest for hospitals, as well as a national publicity campaign about these issues called "Care About Your Care."
Visit our website for more information about patient care transitions.
The Robert Wood Johnson Foundation of Princeton, N.J., the country’s largest healthcare-focused philanthropy, has undertaken a number of initiatives to improve care transitions and reduce preventable hospital readmissions.
One of the key conclusions from these initiatives, says Anne Weiss, MPP, director of the foundation's Quality/Equality Health Care Team, is that hospitals and hospitalists can't do it alone. "Hospitals are now being held financially accountable for something they can't possibly control," Weiss says, referring to whether or not the discharged patient returns to the hospital within 30 days.
The foundation has mobilized broad community coalitions through its Aligning Forces for Quality campaign, bringing together healthcare providers, purchasers, consumers, and other stakeholders to improve care transitions. One such coalition, Better Health Greater Cleveland of Ohio, announced a 10.7% reduction in avoidable hospitalizations for common cardiac conditions in 2011.
Successful care transitions also require healthcare providers to appreciate the need for patients and their families to engage in their plans for post-discharge care, Weiss says. "I have been stunned to learn the kinds of medical tasks patients and families are now expected to conduct when they go home," she adds. "I hear them say, 'Nobody told us we would have to flush IVs.'"
Through another initiative, the foundation produced an interactive map that displays the percentage of patients readmitted to hospitals within 30 days of discharge; it has supported research that found improvements in nurses' work environments helped to reduce avoidable hospital readmissions. It also has produced a "Transitions to Better Care" video contest for hospitals, as well as a national publicity campaign about these issues called "Care About Your Care."
Visit our website for more information about patient care transitions.