User login
Cutis Tricolor
Circumscribed Acral Hypokeratosis
Paranoid, agitated, and manipulative
CASE: Agitation
Mrs. M, age 39, presents to the emergency department (ED) with altered mental status. She is escorted by her husband and the police. She has a history of severe alcohol dependence, bipolar disorder (BD), anxiety, borderline personality disorder (BPD), hypothyroidism, and bulimia, and had gastric bypass surgery 4 years ago. Her husband called 911 when he could no longer manage Mrs. M’s agitated state. The police found her to be extremely paranoid, restless, and disoriented. Her husband reports that she shouted “the world is going to end” before she escaped naked into her neighborhood streets.
On several occasions Mrs. M had been admitted to the same hospital for alcohol withdrawal and dependence with subsequent liver failure, leading to jaundice, coagulopathy, and ascites. During these hospitalizations, she exhibited poor behavioral tendencies, unhealthy psychological defenses, and chronic maladaptive coping and defense mechanisms congruent with her BPD diagnosis. Specifically, she engaged in splitting of hospital staff, ranging from extreme flattery to overt devaluation and hostility. Other defense mechanisms included denial, distortion, acting out, and passive-aggressive behavior. During these admissions, Mrs. M often displayed deficits in recall and attention on Mini-Mental State Examination (MMSE), but these deficits were associated with concurrent alcohol use and improved rapidly during her stay.
In her current presentation, Mrs. M’s mental status change is more pronounced and atypical compared with earlier admissions. Her outpatient medication regimen includes lamotrigine, 100 mg/d, levothyroxine, 88 mcg/d, venlafaxine extended release (XR), 75 mg/d, clonazepam, 3 mg/d, docusate as needed for constipation, and a daily multivitamin.
The authors’ observations
Delirium is a disturbance of consciousness manifested by a reduced clarity of awareness (impairment in attention) and change in cognition (impairment in orientation, memory, and language).1,2 The disturbance develops over a short time and tends to fluctuate during the day. Delirium is a direct physiological consequence of a general medical condition, substance use (intoxication or withdrawal), or both (Table).3
Delirium generally is a reversible mental disorder but can progress to irreversible brain damage. Prompt and accurate diagnosis of delirium is essential,4 although the condition often is underdiagnosed or misdiagnosed because of lack of recognition.
Table
DSM-IV-TR diagnostic criteria for delirium
|
| Source: Reference 3 |
Patients who have convoluted histories, such as Mrs. M, are common and difficult to manage and treat. These patients become substantially more complex when they are admitted to inpatient medical or surgical services. The need to clarify between delirium (primarily medical) and depression (primarily psychiatric) becomes paramount when administering treatment and evaluating decision-making capacity.5 In Mrs. M’s case, internal medicine, neurology, and psychiatry teams each had a different approach to altered mental status. Each team’s different terminology, assessment, and objectives further complicated an already challenging case.6
EVALUATION: Confounding results
The ED physicians offer a working diagnosis of acute mental status change, administer IV lorazepam, 4 mg, and order restraints for Mrs. M’s severe agitation. Her initial vital signs reveal slightly elevated blood pressure (140/90 mm Hg) and tachycardia (115 beats per minute). Internal medicine clinicians note that Mrs. M is not in acute distress, although she refuses to speak and has a small amount of dried blood on her lips, presumably from a struggle with the police before coming to the hospital, but this is not certain. Her abdomen is not tender; she has normal bowel sounds, and no asterixis is noted on neurologic exam. Physical exam is otherwise normal. A noncontrast head CT scan shows no acute process. Initial lab values show elevations in ammonia (277 μg/dL) and γ-glutamyl transpeptidase (68 U/L). Thyroid-stimulating hormone is 1.45 mlU/L, prothrombin time is 19.5 s, partial thromboplastin time is 40.3 s, and international normalized ratio is 1.67. The internal medicine team admits Mrs. M to the intensive care unit (ICU) for further management of her mental status change with alcohol withdrawal or hepatic encephalopathy as the most likely etiologies.
Mrs. M’s husband says that his wife has not consumed alcohol in the last 4 months in preparation for a possible liver transplant; however, past interactions with Mrs. M’s family suggest they are unreliable. The Clinical Institute Withdrawal Assessment (CIWA) protocol is implemented in case her symptoms are caused by alcohol withdrawal. Her vital signs are stable and IV lorazepam, 4 mg, is administered once for agitation. Mrs. M’s husband also reports that 1 month ago his wife underwent a transjugular intrahepatic portosystemic shunt (TIPS) procedure for portal hypertension. Outpatient psychotropics (lamotrigine, 100 mg/d, and venlafaxine XR, 75 mg/d) are restarted because withdrawal from these drugs may exacerbate her symptoms. In the ICU Mrs. M experiences a tonic-clonic seizure with fecal incontinence and bitten tongue, which results in a consultation from neurology and the psychiatry consultation-liaison service.
Psychiatry recommends withholding psychotropics, stopping CIWA, and using vital sign parameters along with objective signs of diaphoresis and tremors as indicators of alcohol withdrawal for lorazepam administration. Mrs. M receives IV haloperidol, 1 mg, once during her second day in the hospital for severe agitation, but this medication is discontinued because of concern about lowering her seizure threshold.7 After treatment with lactulose, her ammonia levels trend down to 33 μg/dL, but her altered mental state persists with significant deficits in attention and orientation.
The neurology service performs an EEG that shows no slow-wave, triphasic waves, or epileptiform activity, which likely would be present in delirium or seizures. See Figure 1 for an example of triphasic waves on an EEG and Figure 2 for Mrs. M's EEG results. Subsequent lumbar puncture, MRI, and a second EEG are unremarkable. By the fifth hospital day, Mrs. M is calm and her paranoia has subsided, but she still is confused and disoriented. Psychiatry orders a third EEG while she is in this confused state; it shows no pathologic process. Based on these examinations, neurology posits that Mrs. M is not encephalopathic.
Figure 1: Representative sample of triphasic waves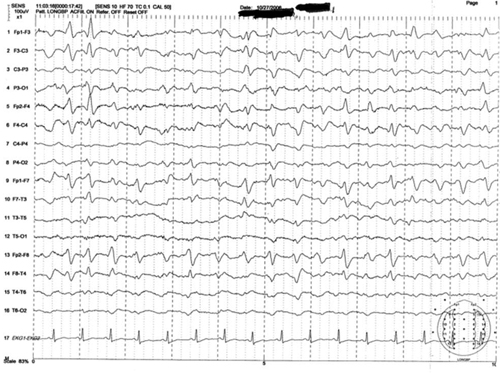
This EEG tracing is from a 54-year-old woman who underwent prolonged abdominal surgery for lysis of adhesions during which she suffered an intraoperative left subinsular stroke followed by nonconvulsive status epilepticus. The tracing demonstrates typical morphology with the positive sharp transient preceded and followed by smaller amplitude negative deflections. Symmetric, frontal predominance of findings seen is this tracing is common
Figure 2: Mrs. M’s EEG results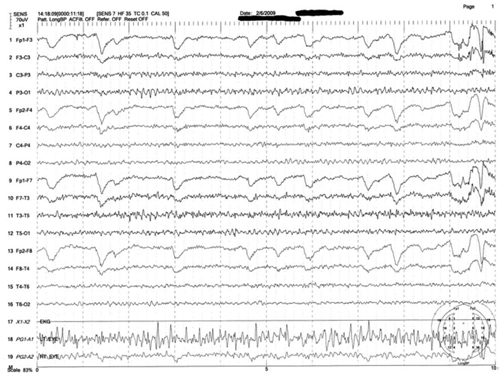
This is a representative tracing of Mrs. M’s 3 EEGs revealing an 8.5 to 9 Hz dominant alpha rhythm. There is superimposed frontally dominant beta fast activity, which is consistent with known administration of benzodiazepines
The authors’ observations
Mrs. M had repeated admissions for alcohol dependence and subsequent liver failure. Her recent hospitalization was complicated by a TIPS procedure done 1 month ago. The incidence of hepatic encephalopathy in patients undergoing TIPS is >30%, especially in the first month post-procedure, which raised suspicion that hepatic encephalopathy played a significant role in Mrs. M’s delirium.8
Because of frequent hospitalization, Mrs. M was well known to the internal medicine, neurology, and psychiatry teams, and each used different terms to describe her mental state. Internal medicine used the phrase “acute mental status change,” which covers a broad differential. Neurology used “encephalopathy,” which also is a general term. Psychiatry used “delirium,” which has narrower and more specific diagnostic criteria. Engel et al9 described the delirious patient as having “cerebral insufficiency” with universally abnormal EEG. Regardless of terminology, based on Mrs. M’s acute confusion, one would expect an abnormal EEG, but repeat EEGs were unremarkable.
Interpreting EEG
EEG is one of the few tools available for measuring acute changes in cerebral function, and an EEG slowing remains a hallmark in encephalopathic processes.10,11 Initially, the 3 specialties agreed that Mrs. M’s presentation likely was caused by underlying medical issues or substances (alcohol or others). EEG can help recognize delirium, and, in some cases, elucidate the underlying cause.10,12 It was surprising that Mrs. M’s EEGs were normal despite a clinical presentation of delirium. Because of the normal EEG findings, neurology leaned toward a primary psychiatric (“functional”) etiology as the cause of her delirium vs a general medical condition or alcohol withdrawal (“organic”).
A literature search in regards to sensitivity of EEG in delirium revealed conflicting statements and data. A standard textbook in neurology and psychiatry states that “a normal EEG virtually excludes a toxic-metabolic encephalopathy.”13 The American Psychiatric Association’s (APA) practice guidelines for delirium states: “The presence of EEG abnormalities has fairly good sensitivities for delirium (in one study, the sensitivity was found to be 75%), but the absence does not rule out the diagnosis; thus the EEG is no substitute for careful clinical observation.”6
At the beginning of Mrs. M’s care, in discussion with the neurology and internal medicine teams, we argued that Mrs. M was experiencing delirium despite her initial normal EEG. We did not expect that 2 subsequent EEGs would be normal, especially because the teams witnessed the final EEG being performed while Mrs. M was clinically evaluated and observed to be in a state of delirium.
OUTCOME: Cause still unknown
By the 6th day of hospitalization, Mrs. M’s vitals are normal and she remains hemodynamically stable. Differential diagnosis remains wide and unclear. The psychiatry team feels she could have atypical catatonia due to an underlying mood disorder. One hour after a trial of IV lorazepam, 1 mg, Mrs. M is more lucid and fully oriented, with MMSE of 28/30 (recall was 1/3), indicating normal cognition. During the exam, a psychiatry resident notes Mrs. M winks and feigns a yawn at the medical students and nurses in the room, displaying her boredom with the interview and simplicity of the mental status exam questions. Later that evening, Mrs. M exhibits bizarre sexual gestures toward male hospital staff, including licking a male nursing staff member’s hand.
Although Mrs. M’s initial confusion resolved, the severity of her comorbid psychiatric history warrants inpatient psychiatric hospitalization. She agrees to transfer to the psychiatric ward after she confesses anxiety regarding death, intense demoralization, and guilt related to her condition and her relationship with her 12-year-old daughter. She tearfully reports that she discontinued her psychotropic medications shortly after stopping alcohol 4 months ago. Shortly before her transfer, psychiatry is called back to the medicine floor because of Mrs. M’s disruptive behavior.
The team finds Mrs. M in her hospital gown, pursuing her husband in the hallway as he is leaving, yelling profanities and blaming him for her horrible experience in the hospital. Based on her demeanor, the team determines that she is back to her baseline mental state despite her mood disorder, and that her upcoming inpatient psychiatric stay likely would be too short to address her comorbid personality disorder. The next day she signs out of the hospital against medical advice.
The authors’ observations
We never clearly identified the specific etiology responsible for Mrs. M’s delirium. We assume at the initial presentation she had toxic-metabolic encephalopathy that rapidly resolved with lactulose treatment and lowering her ammonia. She then had a single tonic-clonic seizure, perhaps related to stopping and then restarting her psychotropics. Her subsequent confusion, bizarre sexual behavior, and demeanor on her final hospital days were more indicative of her psychiatric diagnoses. We now suspect that Mrs. M’s delirium was briefer than presumed and she returned to her baseline borderline personality, resulting in some factitious staging of delirium to confuse her 3 treating teams (a psychoanalyst may say this was a form of projective identification).
We felt that if Mrs. M truly was delirious due to metabolic or hepatic dysfunction or alcohol withdrawal, she would have had abnormal EEG findings. We discovered that the notion of “75% sensitivity” of EEG abnormalities cited in the APA guidelines comes from studies that include patients with “psychogenic” and “organic” delirium. Acute manias and agitated psychoses were termed “psychogenic delirium” and acute confusion due to medical conditions or substance issues was termed “organic delirium.”9,12,14-16
This poses a circular reasoning in the diagnostic criteria and clinical approach to delirium. The fallacy is that, according to DSM-IV-TR, delirium is supposed to be the result of a direct physiological consequence of a general medical condition or substance use (criterion D), and cannot be due to psychosis (eg, schizophrenia) or mania (eg, BD). We question the presumptive 75% sensitivity of EEG abnormalities in patients with delirium because it is possible that when some of these studies were conducted the definition of delirium was not solidified or fully understood. We suspect the sensitivity would be much higher if the correct definition of delirium according to DSM-IV-TR is used in future studies. To improve interdisciplinary communication and future research, it would be constructive if all disciplines could agree on a single term, with the same diagnostic criteria, when evaluating a patient with acute confusion.
Related Resources
- Meagher D. Delirium: the role of psychiatry. Advances in Psychiatric Treatment. 2001;7:433-442.
- Casey DA, DeFazio JV Jr, Vansickle K, et al. Delirium. Quick recognition, careful evaluation, and appropriate treatment. Postgrad Med. 1996;100(1):121-4, 128, 133-134.
Drug Brand Names
- Clonazepam • Klonopin
- Docusate • Surfak
- Haloperidol • Haldol
- Lamotrigine • Lamictal
- Lorazepam • Ativan
- Levothyroxine • Levoxyl, Synthtoid
- Venlafaxine XR • Effexor XR
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Acknowledgment
The views expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Department of the Navy, Department of Defense, or the U.S. Government. The authors are employees of the U.S. Government. This work was prepared as part of their official duties. Title 17 U.S.C. 105 provides that “Copyright protection under this title is not available for any work of the U.S. Government.” Title 17 U.S.C. 101 defines a U.S. Government work as a work prepared by a military service member or employee of the U.S. Government as part of that person’s official duties.
1. Katz IR, Mossey J, Sussman N, et al. Bedside clinical and electrophysiological assessment: assessment of change in vulnerable patients. Int Psychogeriatr. 1991;3(2):289-300.
2. Inouye SK. Delirium in older persons. N Engl J Med. 2006;354(11):1157-1165.
3. Diagnostic and statistical manual of mental disorders, 4th ed, text rev. Washington DC: American Psychiatric Association; 2000.
4. McPhee SJ, Papadakis M, Rabow MW. CURRENT medical diagnosis and treatment. New York NY: McGraw Hill Medical; 2012.
5. Brody B. Who has capacity? N Engl J Med. 2009;361(3):232-233.
6. Practice guideline for the treatment of patients with delirium. American Psychiatric Association. Am J Psychiatry. 1999;156(5 suppl):1-20.
7. Fricchione GL, Nejad SH, Esses JA, et al. Postoperative delirium. Am J Psychiatry. 2008;165(7):803-812.
8. Sanyal AJ, Freedman AM, Shiffman ML, et al. Portosystemic encephalopathy after transjugular intrahepatic portosystemic shunt: results of a prospective controlled study. Hepatology. 1994;20(1 pt 1):46-55.
9. Engel GL, Romano J. Delirium a syndrome of cerebral insufficiency. 1959. J Neuropsychiatry Clin Neurosci. 2004;16(4):526-538.
10. Pro JD, Wells CE. The use of the electroencephalogram in the diagnosis of delirium. Dis Nerv Syst. 1977;38(10):804-808.
11. Sidhu KS, Balon R, Ajluni V, et al. Standard EEG and the difficult-to-assess mental status. Ann Clin Psychiatry. 2009;21(2):103-108.
12. Brenner RP. Utility of EEG in delirium: past views and current practice. Int Psychogeriatr. 1991;3(2):211-229.
13. Kaufman DM. Clinical neurology for psychiatrists. 5th ed. Philadelphia PA: Saunders; 2001: 230-232.
14. Bond TC. Recognition of acute delirious mania. Arch Gen Psychiatry. 1980;37(5):553-554.
15. Krauthammer C, Klerman GL. Secondary mania: manic syndromes associated with antecedent physical illness or drugs. Arch Gen Psychiatry. 1978;35(11):1333-1339.
16. Larson EW, Richelson E. Organic causes of mania. Mayo Clin Proc. 1988;63(9):906-912.
CASE: Agitation
Mrs. M, age 39, presents to the emergency department (ED) with altered mental status. She is escorted by her husband and the police. She has a history of severe alcohol dependence, bipolar disorder (BD), anxiety, borderline personality disorder (BPD), hypothyroidism, and bulimia, and had gastric bypass surgery 4 years ago. Her husband called 911 when he could no longer manage Mrs. M’s agitated state. The police found her to be extremely paranoid, restless, and disoriented. Her husband reports that she shouted “the world is going to end” before she escaped naked into her neighborhood streets.
On several occasions Mrs. M had been admitted to the same hospital for alcohol withdrawal and dependence with subsequent liver failure, leading to jaundice, coagulopathy, and ascites. During these hospitalizations, she exhibited poor behavioral tendencies, unhealthy psychological defenses, and chronic maladaptive coping and defense mechanisms congruent with her BPD diagnosis. Specifically, she engaged in splitting of hospital staff, ranging from extreme flattery to overt devaluation and hostility. Other defense mechanisms included denial, distortion, acting out, and passive-aggressive behavior. During these admissions, Mrs. M often displayed deficits in recall and attention on Mini-Mental State Examination (MMSE), but these deficits were associated with concurrent alcohol use and improved rapidly during her stay.
In her current presentation, Mrs. M’s mental status change is more pronounced and atypical compared with earlier admissions. Her outpatient medication regimen includes lamotrigine, 100 mg/d, levothyroxine, 88 mcg/d, venlafaxine extended release (XR), 75 mg/d, clonazepam, 3 mg/d, docusate as needed for constipation, and a daily multivitamin.
The authors’ observations
Delirium is a disturbance of consciousness manifested by a reduced clarity of awareness (impairment in attention) and change in cognition (impairment in orientation, memory, and language).1,2 The disturbance develops over a short time and tends to fluctuate during the day. Delirium is a direct physiological consequence of a general medical condition, substance use (intoxication or withdrawal), or both (Table).3
Delirium generally is a reversible mental disorder but can progress to irreversible brain damage. Prompt and accurate diagnosis of delirium is essential,4 although the condition often is underdiagnosed or misdiagnosed because of lack of recognition.
Table
DSM-IV-TR diagnostic criteria for delirium
|
| Source: Reference 3 |
Patients who have convoluted histories, such as Mrs. M, are common and difficult to manage and treat. These patients become substantially more complex when they are admitted to inpatient medical or surgical services. The need to clarify between delirium (primarily medical) and depression (primarily psychiatric) becomes paramount when administering treatment and evaluating decision-making capacity.5 In Mrs. M’s case, internal medicine, neurology, and psychiatry teams each had a different approach to altered mental status. Each team’s different terminology, assessment, and objectives further complicated an already challenging case.6
EVALUATION: Confounding results
The ED physicians offer a working diagnosis of acute mental status change, administer IV lorazepam, 4 mg, and order restraints for Mrs. M’s severe agitation. Her initial vital signs reveal slightly elevated blood pressure (140/90 mm Hg) and tachycardia (115 beats per minute). Internal medicine clinicians note that Mrs. M is not in acute distress, although she refuses to speak and has a small amount of dried blood on her lips, presumably from a struggle with the police before coming to the hospital, but this is not certain. Her abdomen is not tender; she has normal bowel sounds, and no asterixis is noted on neurologic exam. Physical exam is otherwise normal. A noncontrast head CT scan shows no acute process. Initial lab values show elevations in ammonia (277 μg/dL) and γ-glutamyl transpeptidase (68 U/L). Thyroid-stimulating hormone is 1.45 mlU/L, prothrombin time is 19.5 s, partial thromboplastin time is 40.3 s, and international normalized ratio is 1.67. The internal medicine team admits Mrs. M to the intensive care unit (ICU) for further management of her mental status change with alcohol withdrawal or hepatic encephalopathy as the most likely etiologies.
Mrs. M’s husband says that his wife has not consumed alcohol in the last 4 months in preparation for a possible liver transplant; however, past interactions with Mrs. M’s family suggest they are unreliable. The Clinical Institute Withdrawal Assessment (CIWA) protocol is implemented in case her symptoms are caused by alcohol withdrawal. Her vital signs are stable and IV lorazepam, 4 mg, is administered once for agitation. Mrs. M’s husband also reports that 1 month ago his wife underwent a transjugular intrahepatic portosystemic shunt (TIPS) procedure for portal hypertension. Outpatient psychotropics (lamotrigine, 100 mg/d, and venlafaxine XR, 75 mg/d) are restarted because withdrawal from these drugs may exacerbate her symptoms. In the ICU Mrs. M experiences a tonic-clonic seizure with fecal incontinence and bitten tongue, which results in a consultation from neurology and the psychiatry consultation-liaison service.
Psychiatry recommends withholding psychotropics, stopping CIWA, and using vital sign parameters along with objective signs of diaphoresis and tremors as indicators of alcohol withdrawal for lorazepam administration. Mrs. M receives IV haloperidol, 1 mg, once during her second day in the hospital for severe agitation, but this medication is discontinued because of concern about lowering her seizure threshold.7 After treatment with lactulose, her ammonia levels trend down to 33 μg/dL, but her altered mental state persists with significant deficits in attention and orientation.
The neurology service performs an EEG that shows no slow-wave, triphasic waves, or epileptiform activity, which likely would be present in delirium or seizures. See Figure 1 for an example of triphasic waves on an EEG and Figure 2 for Mrs. M's EEG results. Subsequent lumbar puncture, MRI, and a second EEG are unremarkable. By the fifth hospital day, Mrs. M is calm and her paranoia has subsided, but she still is confused and disoriented. Psychiatry orders a third EEG while she is in this confused state; it shows no pathologic process. Based on these examinations, neurology posits that Mrs. M is not encephalopathic.
Figure 1: Representative sample of triphasic waves
This EEG tracing is from a 54-year-old woman who underwent prolonged abdominal surgery for lysis of adhesions during which she suffered an intraoperative left subinsular stroke followed by nonconvulsive status epilepticus. The tracing demonstrates typical morphology with the positive sharp transient preceded and followed by smaller amplitude negative deflections. Symmetric, frontal predominance of findings seen is this tracing is common
Figure 2: Mrs. M’s EEG results
This is a representative tracing of Mrs. M’s 3 EEGs revealing an 8.5 to 9 Hz dominant alpha rhythm. There is superimposed frontally dominant beta fast activity, which is consistent with known administration of benzodiazepines
The authors’ observations
Mrs. M had repeated admissions for alcohol dependence and subsequent liver failure. Her recent hospitalization was complicated by a TIPS procedure done 1 month ago. The incidence of hepatic encephalopathy in patients undergoing TIPS is >30%, especially in the first month post-procedure, which raised suspicion that hepatic encephalopathy played a significant role in Mrs. M’s delirium.8
Because of frequent hospitalization, Mrs. M was well known to the internal medicine, neurology, and psychiatry teams, and each used different terms to describe her mental state. Internal medicine used the phrase “acute mental status change,” which covers a broad differential. Neurology used “encephalopathy,” which also is a general term. Psychiatry used “delirium,” which has narrower and more specific diagnostic criteria. Engel et al9 described the delirious patient as having “cerebral insufficiency” with universally abnormal EEG. Regardless of terminology, based on Mrs. M’s acute confusion, one would expect an abnormal EEG, but repeat EEGs were unremarkable.
Interpreting EEG
EEG is one of the few tools available for measuring acute changes in cerebral function, and an EEG slowing remains a hallmark in encephalopathic processes.10,11 Initially, the 3 specialties agreed that Mrs. M’s presentation likely was caused by underlying medical issues or substances (alcohol or others). EEG can help recognize delirium, and, in some cases, elucidate the underlying cause.10,12 It was surprising that Mrs. M’s EEGs were normal despite a clinical presentation of delirium. Because of the normal EEG findings, neurology leaned toward a primary psychiatric (“functional”) etiology as the cause of her delirium vs a general medical condition or alcohol withdrawal (“organic”).
A literature search in regards to sensitivity of EEG in delirium revealed conflicting statements and data. A standard textbook in neurology and psychiatry states that “a normal EEG virtually excludes a toxic-metabolic encephalopathy.”13 The American Psychiatric Association’s (APA) practice guidelines for delirium states: “The presence of EEG abnormalities has fairly good sensitivities for delirium (in one study, the sensitivity was found to be 75%), but the absence does not rule out the diagnosis; thus the EEG is no substitute for careful clinical observation.”6
At the beginning of Mrs. M’s care, in discussion with the neurology and internal medicine teams, we argued that Mrs. M was experiencing delirium despite her initial normal EEG. We did not expect that 2 subsequent EEGs would be normal, especially because the teams witnessed the final EEG being performed while Mrs. M was clinically evaluated and observed to be in a state of delirium.
OUTCOME: Cause still unknown
By the 6th day of hospitalization, Mrs. M’s vitals are normal and she remains hemodynamically stable. Differential diagnosis remains wide and unclear. The psychiatry team feels she could have atypical catatonia due to an underlying mood disorder. One hour after a trial of IV lorazepam, 1 mg, Mrs. M is more lucid and fully oriented, with MMSE of 28/30 (recall was 1/3), indicating normal cognition. During the exam, a psychiatry resident notes Mrs. M winks and feigns a yawn at the medical students and nurses in the room, displaying her boredom with the interview and simplicity of the mental status exam questions. Later that evening, Mrs. M exhibits bizarre sexual gestures toward male hospital staff, including licking a male nursing staff member’s hand.
Although Mrs. M’s initial confusion resolved, the severity of her comorbid psychiatric history warrants inpatient psychiatric hospitalization. She agrees to transfer to the psychiatric ward after she confesses anxiety regarding death, intense demoralization, and guilt related to her condition and her relationship with her 12-year-old daughter. She tearfully reports that she discontinued her psychotropic medications shortly after stopping alcohol 4 months ago. Shortly before her transfer, psychiatry is called back to the medicine floor because of Mrs. M’s disruptive behavior.
The team finds Mrs. M in her hospital gown, pursuing her husband in the hallway as he is leaving, yelling profanities and blaming him for her horrible experience in the hospital. Based on her demeanor, the team determines that she is back to her baseline mental state despite her mood disorder, and that her upcoming inpatient psychiatric stay likely would be too short to address her comorbid personality disorder. The next day she signs out of the hospital against medical advice.
The authors’ observations
We never clearly identified the specific etiology responsible for Mrs. M’s delirium. We assume at the initial presentation she had toxic-metabolic encephalopathy that rapidly resolved with lactulose treatment and lowering her ammonia. She then had a single tonic-clonic seizure, perhaps related to stopping and then restarting her psychotropics. Her subsequent confusion, bizarre sexual behavior, and demeanor on her final hospital days were more indicative of her psychiatric diagnoses. We now suspect that Mrs. M’s delirium was briefer than presumed and she returned to her baseline borderline personality, resulting in some factitious staging of delirium to confuse her 3 treating teams (a psychoanalyst may say this was a form of projective identification).
We felt that if Mrs. M truly was delirious due to metabolic or hepatic dysfunction or alcohol withdrawal, she would have had abnormal EEG findings. We discovered that the notion of “75% sensitivity” of EEG abnormalities cited in the APA guidelines comes from studies that include patients with “psychogenic” and “organic” delirium. Acute manias and agitated psychoses were termed “psychogenic delirium” and acute confusion due to medical conditions or substance issues was termed “organic delirium.”9,12,14-16
This poses a circular reasoning in the diagnostic criteria and clinical approach to delirium. The fallacy is that, according to DSM-IV-TR, delirium is supposed to be the result of a direct physiological consequence of a general medical condition or substance use (criterion D), and cannot be due to psychosis (eg, schizophrenia) or mania (eg, BD). We question the presumptive 75% sensitivity of EEG abnormalities in patients with delirium because it is possible that when some of these studies were conducted the definition of delirium was not solidified or fully understood. We suspect the sensitivity would be much higher if the correct definition of delirium according to DSM-IV-TR is used in future studies. To improve interdisciplinary communication and future research, it would be constructive if all disciplines could agree on a single term, with the same diagnostic criteria, when evaluating a patient with acute confusion.
Related Resources
- Meagher D. Delirium: the role of psychiatry. Advances in Psychiatric Treatment. 2001;7:433-442.
- Casey DA, DeFazio JV Jr, Vansickle K, et al. Delirium. Quick recognition, careful evaluation, and appropriate treatment. Postgrad Med. 1996;100(1):121-4, 128, 133-134.
Drug Brand Names
- Clonazepam • Klonopin
- Docusate • Surfak
- Haloperidol • Haldol
- Lamotrigine • Lamictal
- Lorazepam • Ativan
- Levothyroxine • Levoxyl, Synthtoid
- Venlafaxine XR • Effexor XR
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Acknowledgment
The views expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Department of the Navy, Department of Defense, or the U.S. Government. The authors are employees of the U.S. Government. This work was prepared as part of their official duties. Title 17 U.S.C. 105 provides that “Copyright protection under this title is not available for any work of the U.S. Government.” Title 17 U.S.C. 101 defines a U.S. Government work as a work prepared by a military service member or employee of the U.S. Government as part of that person’s official duties.
CASE: Agitation
Mrs. M, age 39, presents to the emergency department (ED) with altered mental status. She is escorted by her husband and the police. She has a history of severe alcohol dependence, bipolar disorder (BD), anxiety, borderline personality disorder (BPD), hypothyroidism, and bulimia, and had gastric bypass surgery 4 years ago. Her husband called 911 when he could no longer manage Mrs. M’s agitated state. The police found her to be extremely paranoid, restless, and disoriented. Her husband reports that she shouted “the world is going to end” before she escaped naked into her neighborhood streets.
On several occasions Mrs. M had been admitted to the same hospital for alcohol withdrawal and dependence with subsequent liver failure, leading to jaundice, coagulopathy, and ascites. During these hospitalizations, she exhibited poor behavioral tendencies, unhealthy psychological defenses, and chronic maladaptive coping and defense mechanisms congruent with her BPD diagnosis. Specifically, she engaged in splitting of hospital staff, ranging from extreme flattery to overt devaluation and hostility. Other defense mechanisms included denial, distortion, acting out, and passive-aggressive behavior. During these admissions, Mrs. M often displayed deficits in recall and attention on Mini-Mental State Examination (MMSE), but these deficits were associated with concurrent alcohol use and improved rapidly during her stay.
In her current presentation, Mrs. M’s mental status change is more pronounced and atypical compared with earlier admissions. Her outpatient medication regimen includes lamotrigine, 100 mg/d, levothyroxine, 88 mcg/d, venlafaxine extended release (XR), 75 mg/d, clonazepam, 3 mg/d, docusate as needed for constipation, and a daily multivitamin.
The authors’ observations
Delirium is a disturbance of consciousness manifested by a reduced clarity of awareness (impairment in attention) and change in cognition (impairment in orientation, memory, and language).1,2 The disturbance develops over a short time and tends to fluctuate during the day. Delirium is a direct physiological consequence of a general medical condition, substance use (intoxication or withdrawal), or both (Table).3
Delirium generally is a reversible mental disorder but can progress to irreversible brain damage. Prompt and accurate diagnosis of delirium is essential,4 although the condition often is underdiagnosed or misdiagnosed because of lack of recognition.
Table
DSM-IV-TR diagnostic criteria for delirium
|
| Source: Reference 3 |
Patients who have convoluted histories, such as Mrs. M, are common and difficult to manage and treat. These patients become substantially more complex when they are admitted to inpatient medical or surgical services. The need to clarify between delirium (primarily medical) and depression (primarily psychiatric) becomes paramount when administering treatment and evaluating decision-making capacity.5 In Mrs. M’s case, internal medicine, neurology, and psychiatry teams each had a different approach to altered mental status. Each team’s different terminology, assessment, and objectives further complicated an already challenging case.6
EVALUATION: Confounding results
The ED physicians offer a working diagnosis of acute mental status change, administer IV lorazepam, 4 mg, and order restraints for Mrs. M’s severe agitation. Her initial vital signs reveal slightly elevated blood pressure (140/90 mm Hg) and tachycardia (115 beats per minute). Internal medicine clinicians note that Mrs. M is not in acute distress, although she refuses to speak and has a small amount of dried blood on her lips, presumably from a struggle with the police before coming to the hospital, but this is not certain. Her abdomen is not tender; she has normal bowel sounds, and no asterixis is noted on neurologic exam. Physical exam is otherwise normal. A noncontrast head CT scan shows no acute process. Initial lab values show elevations in ammonia (277 μg/dL) and γ-glutamyl transpeptidase (68 U/L). Thyroid-stimulating hormone is 1.45 mlU/L, prothrombin time is 19.5 s, partial thromboplastin time is 40.3 s, and international normalized ratio is 1.67. The internal medicine team admits Mrs. M to the intensive care unit (ICU) for further management of her mental status change with alcohol withdrawal or hepatic encephalopathy as the most likely etiologies.
Mrs. M’s husband says that his wife has not consumed alcohol in the last 4 months in preparation for a possible liver transplant; however, past interactions with Mrs. M’s family suggest they are unreliable. The Clinical Institute Withdrawal Assessment (CIWA) protocol is implemented in case her symptoms are caused by alcohol withdrawal. Her vital signs are stable and IV lorazepam, 4 mg, is administered once for agitation. Mrs. M’s husband also reports that 1 month ago his wife underwent a transjugular intrahepatic portosystemic shunt (TIPS) procedure for portal hypertension. Outpatient psychotropics (lamotrigine, 100 mg/d, and venlafaxine XR, 75 mg/d) are restarted because withdrawal from these drugs may exacerbate her symptoms. In the ICU Mrs. M experiences a tonic-clonic seizure with fecal incontinence and bitten tongue, which results in a consultation from neurology and the psychiatry consultation-liaison service.
Psychiatry recommends withholding psychotropics, stopping CIWA, and using vital sign parameters along with objective signs of diaphoresis and tremors as indicators of alcohol withdrawal for lorazepam administration. Mrs. M receives IV haloperidol, 1 mg, once during her second day in the hospital for severe agitation, but this medication is discontinued because of concern about lowering her seizure threshold.7 After treatment with lactulose, her ammonia levels trend down to 33 μg/dL, but her altered mental state persists with significant deficits in attention and orientation.
The neurology service performs an EEG that shows no slow-wave, triphasic waves, or epileptiform activity, which likely would be present in delirium or seizures. See Figure 1 for an example of triphasic waves on an EEG and Figure 2 for Mrs. M's EEG results. Subsequent lumbar puncture, MRI, and a second EEG are unremarkable. By the fifth hospital day, Mrs. M is calm and her paranoia has subsided, but she still is confused and disoriented. Psychiatry orders a third EEG while she is in this confused state; it shows no pathologic process. Based on these examinations, neurology posits that Mrs. M is not encephalopathic.
Figure 1: Representative sample of triphasic waves
This EEG tracing is from a 54-year-old woman who underwent prolonged abdominal surgery for lysis of adhesions during which she suffered an intraoperative left subinsular stroke followed by nonconvulsive status epilepticus. The tracing demonstrates typical morphology with the positive sharp transient preceded and followed by smaller amplitude negative deflections. Symmetric, frontal predominance of findings seen is this tracing is common
Figure 2: Mrs. M’s EEG results
This is a representative tracing of Mrs. M’s 3 EEGs revealing an 8.5 to 9 Hz dominant alpha rhythm. There is superimposed frontally dominant beta fast activity, which is consistent with known administration of benzodiazepines
The authors’ observations
Mrs. M had repeated admissions for alcohol dependence and subsequent liver failure. Her recent hospitalization was complicated by a TIPS procedure done 1 month ago. The incidence of hepatic encephalopathy in patients undergoing TIPS is >30%, especially in the first month post-procedure, which raised suspicion that hepatic encephalopathy played a significant role in Mrs. M’s delirium.8
Because of frequent hospitalization, Mrs. M was well known to the internal medicine, neurology, and psychiatry teams, and each used different terms to describe her mental state. Internal medicine used the phrase “acute mental status change,” which covers a broad differential. Neurology used “encephalopathy,” which also is a general term. Psychiatry used “delirium,” which has narrower and more specific diagnostic criteria. Engel et al9 described the delirious patient as having “cerebral insufficiency” with universally abnormal EEG. Regardless of terminology, based on Mrs. M’s acute confusion, one would expect an abnormal EEG, but repeat EEGs were unremarkable.
Interpreting EEG
EEG is one of the few tools available for measuring acute changes in cerebral function, and an EEG slowing remains a hallmark in encephalopathic processes.10,11 Initially, the 3 specialties agreed that Mrs. M’s presentation likely was caused by underlying medical issues or substances (alcohol or others). EEG can help recognize delirium, and, in some cases, elucidate the underlying cause.10,12 It was surprising that Mrs. M’s EEGs were normal despite a clinical presentation of delirium. Because of the normal EEG findings, neurology leaned toward a primary psychiatric (“functional”) etiology as the cause of her delirium vs a general medical condition or alcohol withdrawal (“organic”).
A literature search in regards to sensitivity of EEG in delirium revealed conflicting statements and data. A standard textbook in neurology and psychiatry states that “a normal EEG virtually excludes a toxic-metabolic encephalopathy.”13 The American Psychiatric Association’s (APA) practice guidelines for delirium states: “The presence of EEG abnormalities has fairly good sensitivities for delirium (in one study, the sensitivity was found to be 75%), but the absence does not rule out the diagnosis; thus the EEG is no substitute for careful clinical observation.”6
At the beginning of Mrs. M’s care, in discussion with the neurology and internal medicine teams, we argued that Mrs. M was experiencing delirium despite her initial normal EEG. We did not expect that 2 subsequent EEGs would be normal, especially because the teams witnessed the final EEG being performed while Mrs. M was clinically evaluated and observed to be in a state of delirium.
OUTCOME: Cause still unknown
By the 6th day of hospitalization, Mrs. M’s vitals are normal and she remains hemodynamically stable. Differential diagnosis remains wide and unclear. The psychiatry team feels she could have atypical catatonia due to an underlying mood disorder. One hour after a trial of IV lorazepam, 1 mg, Mrs. M is more lucid and fully oriented, with MMSE of 28/30 (recall was 1/3), indicating normal cognition. During the exam, a psychiatry resident notes Mrs. M winks and feigns a yawn at the medical students and nurses in the room, displaying her boredom with the interview and simplicity of the mental status exam questions. Later that evening, Mrs. M exhibits bizarre sexual gestures toward male hospital staff, including licking a male nursing staff member’s hand.
Although Mrs. M’s initial confusion resolved, the severity of her comorbid psychiatric history warrants inpatient psychiatric hospitalization. She agrees to transfer to the psychiatric ward after she confesses anxiety regarding death, intense demoralization, and guilt related to her condition and her relationship with her 12-year-old daughter. She tearfully reports that she discontinued her psychotropic medications shortly after stopping alcohol 4 months ago. Shortly before her transfer, psychiatry is called back to the medicine floor because of Mrs. M’s disruptive behavior.
The team finds Mrs. M in her hospital gown, pursuing her husband in the hallway as he is leaving, yelling profanities and blaming him for her horrible experience in the hospital. Based on her demeanor, the team determines that she is back to her baseline mental state despite her mood disorder, and that her upcoming inpatient psychiatric stay likely would be too short to address her comorbid personality disorder. The next day she signs out of the hospital against medical advice.
The authors’ observations
We never clearly identified the specific etiology responsible for Mrs. M’s delirium. We assume at the initial presentation she had toxic-metabolic encephalopathy that rapidly resolved with lactulose treatment and lowering her ammonia. She then had a single tonic-clonic seizure, perhaps related to stopping and then restarting her psychotropics. Her subsequent confusion, bizarre sexual behavior, and demeanor on her final hospital days were more indicative of her psychiatric diagnoses. We now suspect that Mrs. M’s delirium was briefer than presumed and she returned to her baseline borderline personality, resulting in some factitious staging of delirium to confuse her 3 treating teams (a psychoanalyst may say this was a form of projective identification).
We felt that if Mrs. M truly was delirious due to metabolic or hepatic dysfunction or alcohol withdrawal, she would have had abnormal EEG findings. We discovered that the notion of “75% sensitivity” of EEG abnormalities cited in the APA guidelines comes from studies that include patients with “psychogenic” and “organic” delirium. Acute manias and agitated psychoses were termed “psychogenic delirium” and acute confusion due to medical conditions or substance issues was termed “organic delirium.”9,12,14-16
This poses a circular reasoning in the diagnostic criteria and clinical approach to delirium. The fallacy is that, according to DSM-IV-TR, delirium is supposed to be the result of a direct physiological consequence of a general medical condition or substance use (criterion D), and cannot be due to psychosis (eg, schizophrenia) or mania (eg, BD). We question the presumptive 75% sensitivity of EEG abnormalities in patients with delirium because it is possible that when some of these studies were conducted the definition of delirium was not solidified or fully understood. We suspect the sensitivity would be much higher if the correct definition of delirium according to DSM-IV-TR is used in future studies. To improve interdisciplinary communication and future research, it would be constructive if all disciplines could agree on a single term, with the same diagnostic criteria, when evaluating a patient with acute confusion.
Related Resources
- Meagher D. Delirium: the role of psychiatry. Advances in Psychiatric Treatment. 2001;7:433-442.
- Casey DA, DeFazio JV Jr, Vansickle K, et al. Delirium. Quick recognition, careful evaluation, and appropriate treatment. Postgrad Med. 1996;100(1):121-4, 128, 133-134.
Drug Brand Names
- Clonazepam • Klonopin
- Docusate • Surfak
- Haloperidol • Haldol
- Lamotrigine • Lamictal
- Lorazepam • Ativan
- Levothyroxine • Levoxyl, Synthtoid
- Venlafaxine XR • Effexor XR
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Acknowledgment
The views expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Department of the Navy, Department of Defense, or the U.S. Government. The authors are employees of the U.S. Government. This work was prepared as part of their official duties. Title 17 U.S.C. 105 provides that “Copyright protection under this title is not available for any work of the U.S. Government.” Title 17 U.S.C. 101 defines a U.S. Government work as a work prepared by a military service member or employee of the U.S. Government as part of that person’s official duties.
1. Katz IR, Mossey J, Sussman N, et al. Bedside clinical and electrophysiological assessment: assessment of change in vulnerable patients. Int Psychogeriatr. 1991;3(2):289-300.
2. Inouye SK. Delirium in older persons. N Engl J Med. 2006;354(11):1157-1165.
3. Diagnostic and statistical manual of mental disorders, 4th ed, text rev. Washington DC: American Psychiatric Association; 2000.
4. McPhee SJ, Papadakis M, Rabow MW. CURRENT medical diagnosis and treatment. New York NY: McGraw Hill Medical; 2012.
5. Brody B. Who has capacity? N Engl J Med. 2009;361(3):232-233.
6. Practice guideline for the treatment of patients with delirium. American Psychiatric Association. Am J Psychiatry. 1999;156(5 suppl):1-20.
7. Fricchione GL, Nejad SH, Esses JA, et al. Postoperative delirium. Am J Psychiatry. 2008;165(7):803-812.
8. Sanyal AJ, Freedman AM, Shiffman ML, et al. Portosystemic encephalopathy after transjugular intrahepatic portosystemic shunt: results of a prospective controlled study. Hepatology. 1994;20(1 pt 1):46-55.
9. Engel GL, Romano J. Delirium a syndrome of cerebral insufficiency. 1959. J Neuropsychiatry Clin Neurosci. 2004;16(4):526-538.
10. Pro JD, Wells CE. The use of the electroencephalogram in the diagnosis of delirium. Dis Nerv Syst. 1977;38(10):804-808.
11. Sidhu KS, Balon R, Ajluni V, et al. Standard EEG and the difficult-to-assess mental status. Ann Clin Psychiatry. 2009;21(2):103-108.
12. Brenner RP. Utility of EEG in delirium: past views and current practice. Int Psychogeriatr. 1991;3(2):211-229.
13. Kaufman DM. Clinical neurology for psychiatrists. 5th ed. Philadelphia PA: Saunders; 2001: 230-232.
14. Bond TC. Recognition of acute delirious mania. Arch Gen Psychiatry. 1980;37(5):553-554.
15. Krauthammer C, Klerman GL. Secondary mania: manic syndromes associated with antecedent physical illness or drugs. Arch Gen Psychiatry. 1978;35(11):1333-1339.
16. Larson EW, Richelson E. Organic causes of mania. Mayo Clin Proc. 1988;63(9):906-912.
1. Katz IR, Mossey J, Sussman N, et al. Bedside clinical and electrophysiological assessment: assessment of change in vulnerable patients. Int Psychogeriatr. 1991;3(2):289-300.
2. Inouye SK. Delirium in older persons. N Engl J Med. 2006;354(11):1157-1165.
3. Diagnostic and statistical manual of mental disorders, 4th ed, text rev. Washington DC: American Psychiatric Association; 2000.
4. McPhee SJ, Papadakis M, Rabow MW. CURRENT medical diagnosis and treatment. New York NY: McGraw Hill Medical; 2012.
5. Brody B. Who has capacity? N Engl J Med. 2009;361(3):232-233.
6. Practice guideline for the treatment of patients with delirium. American Psychiatric Association. Am J Psychiatry. 1999;156(5 suppl):1-20.
7. Fricchione GL, Nejad SH, Esses JA, et al. Postoperative delirium. Am J Psychiatry. 2008;165(7):803-812.
8. Sanyal AJ, Freedman AM, Shiffman ML, et al. Portosystemic encephalopathy after transjugular intrahepatic portosystemic shunt: results of a prospective controlled study. Hepatology. 1994;20(1 pt 1):46-55.
9. Engel GL, Romano J. Delirium a syndrome of cerebral insufficiency. 1959. J Neuropsychiatry Clin Neurosci. 2004;16(4):526-538.
10. Pro JD, Wells CE. The use of the electroencephalogram in the diagnosis of delirium. Dis Nerv Syst. 1977;38(10):804-808.
11. Sidhu KS, Balon R, Ajluni V, et al. Standard EEG and the difficult-to-assess mental status. Ann Clin Psychiatry. 2009;21(2):103-108.
12. Brenner RP. Utility of EEG in delirium: past views and current practice. Int Psychogeriatr. 1991;3(2):211-229.
13. Kaufman DM. Clinical neurology for psychiatrists. 5th ed. Philadelphia PA: Saunders; 2001: 230-232.
14. Bond TC. Recognition of acute delirious mania. Arch Gen Psychiatry. 1980;37(5):553-554.
15. Krauthammer C, Klerman GL. Secondary mania: manic syndromes associated with antecedent physical illness or drugs. Arch Gen Psychiatry. 1978;35(11):1333-1339.
16. Larson EW, Richelson E. Organic causes of mania. Mayo Clin Proc. 1988;63(9):906-912.
One twin has cerebral palsy; $103M verdict … and more
AFTER PREMATURE RUPTURE OF MEMBRANES at 25 weeks’ gestation, a woman went to the emergency department (ED) and was later released. Eight days later, she returned to the ED with abdominal pain; a soporific drug was administered. After several hours, it was determined that she was in labor. Twins were delivered vaginally. One child has cerebral palsy and requires assistance in daily activities, although her cognitive function is intact.
PARENTS’ CLAIM The mother should not have been released after premature rupture of her membranes. The nurses and ObGyns failed to timely recognize that the mother was in labor, and failed to prevent premature delivery. Proper recognition of contractions would have allowed for administration of a tocolytic to delay delivery. That drug had been effectively administered during the first two trimesters of the pregnancy. A cesarean delivery should have been performed.
DEFENDANTS’ DEFENSE There was no negligence. The hospital argued that fetal heart-rate monitors did not suggest contractions.
VERDICT A $103 million New York verdict was returned against the hospital; a defense verdict was returned for the physicians.
Perforated uterus and severed iliac artery after D&C
A GYNECOLOGIC SURGEON performed a dilation and curettage (D&C) on a 47-year-old woman. During surgery, the patient suffered a perforated uterus and a severed iliac artery, resulting in a myocardial infarction.
PATIENT’S CLAIM The surgeon failed to dilate the cervix appropriately to assess the cervical and endometrial cavity length, and then failed to use proper instrumentation in the uterus. He did not assess uterine shape before the D&C. The patient suffered cognitive and emotional injuries, and will require additional surgery.
PHYSICIAN’S DEFENSE The patient’s anatomy is abnormal. A perforation is a known complication of a D&C.
VERDICT A $350,000 Wisconsin settlement was reached.
Failure to monitor a high-risk patient
A WOMAN WITH A HEART CONDITION who routinely took a beta-blocker plus migraine medication also had lupus. Her pregnancy was therefore at high risk for developing intrauterine growth restriction. Her US Navy ObGyn was advised by a maternal-fetal medicine (MFM) specialist to monitor the pregnancy closely with frequent ultrasonography and other tests that were never performed.
The baby was born by emergency cesarean delivery at 36 weeks’ gestation. The child suffered severe hypoxia and a brain hemorrhage just before delivery, which caused serious, permanent physical and neurologic injuries. He needs 24-hour care, is confined to a wheelchair, and requires a feeding tube.
PATIENT’S CLAIM The ObGyn failed to monitor the mother for fetal growth restriction as recommended by the MFM specialist.
DEFENDANTS’ DEFENSE There was no negligence; the mother was treated properly.
VERDICT After a $28 million Virginia verdict was awarded, the parties continued to dispute whether the judgment would be paid under California law (where the child was born) or Virginia law (where the case was filed). Prior to a rehearing, a $25 million settlement was reached.
Uterine cancer went undiagnosed
A WOMAN IN HER 50s saw her gynecologist in March 2004 to report vaginal staining. She did not return to the physician’s office until January 2005, when she reported daily vaginal bleeding. Ultrasonography showed a 4-cm mass in the endometrial cavity, consistent with a large polyp. A hysteroscopy and biopsy revealed that the woman had uterine cancer. She underwent a hysterectomy and radiation therapy, but the cancer metastasized to her lungs and she died in October 2006.
ESTATE’S CLAIM The gynecologist failed to diagnose uterine cancer in a timely manner.
PHYSICIAN’S DEFENSE The patient’s cancer was aggressive; an earlier diagnosis would not have changed the outcome.
VERDICT A $820,000 Massachusetts settlement was reached.
WHEN A 51-YEAR-OLD WOMAN NOTICED A BULGE in her vagina, she consulted her gynecologist. He determined the cause to be a cystocele and rectocele, and recommended a tension-free vaginal tape–obturator (TVT-O) procedure with anterior and posterior colporrhaphy.
The patient awoke from surgery in severe pain and was told that she had lost a lot of blood. Two weeks later, the physician explained that the stitches, not yet absorbed, were causing an abrasion, and that more vaginal tissue had been removed than planned.
Two more weeks passed, and the patient used a mirror to look at her vagina but could not see the opening. The TVT-O tape had created a ridge of tissue in the anterior vagina, causing severe stenosis. Vaginal dilators were required to expand the vagina. Entrapment of the dorsal clitoral nerve by the TVT-O tape was also discovered. The patient continues to experience dyspareunia and groin pain.
PATIENT’S CLAIM The gynecologist failed to tell her that, 2 months before surgery, the FDA had issued a public health warning about complications associated with transvaginal placement of surgical mesh during prolapse and urinary incontinence repair. Nor was she informed that the defendant had just completed training in TVT-O surgery, was not fully credentialed, and was proctored during the procedure.
PHYSICIAN’S DEFENSE The case was settled before the trial concluded.
VERDICT A $390,000 Virginia settlement was reached.
Lumpectomy, though no mass palpated
A 52-YEAR-OLD WOMAN FOUND A LUMP in her left breast. Her internist ordered mammography, which identified a 2-cm oval, asymmetrical density in the upper inner quadrant of the left breast. The radiologist recommended ultrasonography (US).
The patient consulted a surgical oncologist, who performed fine-needle aspiration. Pathology identified “clusters of malignant cells consistent with carcinoma,” and suggested a confirmatory biopsy. The oncologist recommended lumpectomy and sentinel node biopsy.
On the day of surgery, the patient could not locate the mass. The oncologist testified that he had palpated it. During surgery, gross examination did not show a mass or tumor. Frozen sections of sentinel nodes did not reveal evidence of cancer.
The patient suffered postsurgical seromas and lymphedema. The lymphedema has partially resolved, but causes pain in her left arm and breast.
PATIENT’S CLAIM The surgical oncologist should have performed US before surgery. It was negligent to continue with surgery when there were negative intraoperative findings for cancer or a mass.
PHYSICIAN’S DEFENSE Proper care was provided.
VERDICT A $950,000 Illinois verdict was returned.
Genetic testing fails to identify cystic fibrosis in one twin
AFTER HAVING ONE CHILD with cystic fibrosis (CF), parents underwent genetic testing. Embryos were prepared for in vitro fertilization (IVF) and sent to a genetic-testing laboratory. The lab reported that the embryos were negative for CF. Two embryos were implanted, and the mother gave birth to twins, one of which has CF.
PARENTS’ CLAIM Multiple errors by the genetic-testing laboratory led to an incorrect report on the embryos. The parents claimed wrongful birth.
DEFENDANTS’ DEFENSE The testing laboratory and physician owner argued that amniocentesis should have been performed during the pregnancy to rule out CF.
VERDICT The trial judge denied the use of the amniocentesis defense because an abortion would have been the only option available, and abortion is against the public policy of Tennessee. The court entered summary judgment on liability for the parents.
A $13 million verdict was returned, including $7 million to the parents for emotional distress.
These cases were selected by the editors of OBG Management from Medical Malpractice Verdicts, Settlements & Experts, with permission of the editor, Lewis Laska (www.verdictslaska.com). The information available to the editors about the cases presented here is sometimes incomplete. Moreover, the cases may or may not have merit. Nevertheless, these cases represent the types of clinical situations that typically result in litigation and are meant to illustrate nationwide variation in jury verdicts and awards.
We want to hear from you! Tell us what you think.
AFTER PREMATURE RUPTURE OF MEMBRANES at 25 weeks’ gestation, a woman went to the emergency department (ED) and was later released. Eight days later, she returned to the ED with abdominal pain; a soporific drug was administered. After several hours, it was determined that she was in labor. Twins were delivered vaginally. One child has cerebral palsy and requires assistance in daily activities, although her cognitive function is intact.
PARENTS’ CLAIM The mother should not have been released after premature rupture of her membranes. The nurses and ObGyns failed to timely recognize that the mother was in labor, and failed to prevent premature delivery. Proper recognition of contractions would have allowed for administration of a tocolytic to delay delivery. That drug had been effectively administered during the first two trimesters of the pregnancy. A cesarean delivery should have been performed.
DEFENDANTS’ DEFENSE There was no negligence. The hospital argued that fetal heart-rate monitors did not suggest contractions.
VERDICT A $103 million New York verdict was returned against the hospital; a defense verdict was returned for the physicians.
Perforated uterus and severed iliac artery after D&C
A GYNECOLOGIC SURGEON performed a dilation and curettage (D&C) on a 47-year-old woman. During surgery, the patient suffered a perforated uterus and a severed iliac artery, resulting in a myocardial infarction.
PATIENT’S CLAIM The surgeon failed to dilate the cervix appropriately to assess the cervical and endometrial cavity length, and then failed to use proper instrumentation in the uterus. He did not assess uterine shape before the D&C. The patient suffered cognitive and emotional injuries, and will require additional surgery.
PHYSICIAN’S DEFENSE The patient’s anatomy is abnormal. A perforation is a known complication of a D&C.
VERDICT A $350,000 Wisconsin settlement was reached.
Failure to monitor a high-risk patient
A WOMAN WITH A HEART CONDITION who routinely took a beta-blocker plus migraine medication also had lupus. Her pregnancy was therefore at high risk for developing intrauterine growth restriction. Her US Navy ObGyn was advised by a maternal-fetal medicine (MFM) specialist to monitor the pregnancy closely with frequent ultrasonography and other tests that were never performed.
The baby was born by emergency cesarean delivery at 36 weeks’ gestation. The child suffered severe hypoxia and a brain hemorrhage just before delivery, which caused serious, permanent physical and neurologic injuries. He needs 24-hour care, is confined to a wheelchair, and requires a feeding tube.
PATIENT’S CLAIM The ObGyn failed to monitor the mother for fetal growth restriction as recommended by the MFM specialist.
DEFENDANTS’ DEFENSE There was no negligence; the mother was treated properly.
VERDICT After a $28 million Virginia verdict was awarded, the parties continued to dispute whether the judgment would be paid under California law (where the child was born) or Virginia law (where the case was filed). Prior to a rehearing, a $25 million settlement was reached.
Uterine cancer went undiagnosed
A WOMAN IN HER 50s saw her gynecologist in March 2004 to report vaginal staining. She did not return to the physician’s office until January 2005, when she reported daily vaginal bleeding. Ultrasonography showed a 4-cm mass in the endometrial cavity, consistent with a large polyp. A hysteroscopy and biopsy revealed that the woman had uterine cancer. She underwent a hysterectomy and radiation therapy, but the cancer metastasized to her lungs and she died in October 2006.
ESTATE’S CLAIM The gynecologist failed to diagnose uterine cancer in a timely manner.
PHYSICIAN’S DEFENSE The patient’s cancer was aggressive; an earlier diagnosis would not have changed the outcome.
VERDICT A $820,000 Massachusetts settlement was reached.
WHEN A 51-YEAR-OLD WOMAN NOTICED A BULGE in her vagina, she consulted her gynecologist. He determined the cause to be a cystocele and rectocele, and recommended a tension-free vaginal tape–obturator (TVT-O) procedure with anterior and posterior colporrhaphy.
The patient awoke from surgery in severe pain and was told that she had lost a lot of blood. Two weeks later, the physician explained that the stitches, not yet absorbed, were causing an abrasion, and that more vaginal tissue had been removed than planned.
Two more weeks passed, and the patient used a mirror to look at her vagina but could not see the opening. The TVT-O tape had created a ridge of tissue in the anterior vagina, causing severe stenosis. Vaginal dilators were required to expand the vagina. Entrapment of the dorsal clitoral nerve by the TVT-O tape was also discovered. The patient continues to experience dyspareunia and groin pain.
PATIENT’S CLAIM The gynecologist failed to tell her that, 2 months before surgery, the FDA had issued a public health warning about complications associated with transvaginal placement of surgical mesh during prolapse and urinary incontinence repair. Nor was she informed that the defendant had just completed training in TVT-O surgery, was not fully credentialed, and was proctored during the procedure.
PHYSICIAN’S DEFENSE The case was settled before the trial concluded.
VERDICT A $390,000 Virginia settlement was reached.
Lumpectomy, though no mass palpated
A 52-YEAR-OLD WOMAN FOUND A LUMP in her left breast. Her internist ordered mammography, which identified a 2-cm oval, asymmetrical density in the upper inner quadrant of the left breast. The radiologist recommended ultrasonography (US).
The patient consulted a surgical oncologist, who performed fine-needle aspiration. Pathology identified “clusters of malignant cells consistent with carcinoma,” and suggested a confirmatory biopsy. The oncologist recommended lumpectomy and sentinel node biopsy.
On the day of surgery, the patient could not locate the mass. The oncologist testified that he had palpated it. During surgery, gross examination did not show a mass or tumor. Frozen sections of sentinel nodes did not reveal evidence of cancer.
The patient suffered postsurgical seromas and lymphedema. The lymphedema has partially resolved, but causes pain in her left arm and breast.
PATIENT’S CLAIM The surgical oncologist should have performed US before surgery. It was negligent to continue with surgery when there were negative intraoperative findings for cancer or a mass.
PHYSICIAN’S DEFENSE Proper care was provided.
VERDICT A $950,000 Illinois verdict was returned.
Genetic testing fails to identify cystic fibrosis in one twin
AFTER HAVING ONE CHILD with cystic fibrosis (CF), parents underwent genetic testing. Embryos were prepared for in vitro fertilization (IVF) and sent to a genetic-testing laboratory. The lab reported that the embryos were negative for CF. Two embryos were implanted, and the mother gave birth to twins, one of which has CF.
PARENTS’ CLAIM Multiple errors by the genetic-testing laboratory led to an incorrect report on the embryos. The parents claimed wrongful birth.
DEFENDANTS’ DEFENSE The testing laboratory and physician owner argued that amniocentesis should have been performed during the pregnancy to rule out CF.
VERDICT The trial judge denied the use of the amniocentesis defense because an abortion would have been the only option available, and abortion is against the public policy of Tennessee. The court entered summary judgment on liability for the parents.
A $13 million verdict was returned, including $7 million to the parents for emotional distress.
AFTER PREMATURE RUPTURE OF MEMBRANES at 25 weeks’ gestation, a woman went to the emergency department (ED) and was later released. Eight days later, she returned to the ED with abdominal pain; a soporific drug was administered. After several hours, it was determined that she was in labor. Twins were delivered vaginally. One child has cerebral palsy and requires assistance in daily activities, although her cognitive function is intact.
PARENTS’ CLAIM The mother should not have been released after premature rupture of her membranes. The nurses and ObGyns failed to timely recognize that the mother was in labor, and failed to prevent premature delivery. Proper recognition of contractions would have allowed for administration of a tocolytic to delay delivery. That drug had been effectively administered during the first two trimesters of the pregnancy. A cesarean delivery should have been performed.
DEFENDANTS’ DEFENSE There was no negligence. The hospital argued that fetal heart-rate monitors did not suggest contractions.
VERDICT A $103 million New York verdict was returned against the hospital; a defense verdict was returned for the physicians.
Perforated uterus and severed iliac artery after D&C
A GYNECOLOGIC SURGEON performed a dilation and curettage (D&C) on a 47-year-old woman. During surgery, the patient suffered a perforated uterus and a severed iliac artery, resulting in a myocardial infarction.
PATIENT’S CLAIM The surgeon failed to dilate the cervix appropriately to assess the cervical and endometrial cavity length, and then failed to use proper instrumentation in the uterus. He did not assess uterine shape before the D&C. The patient suffered cognitive and emotional injuries, and will require additional surgery.
PHYSICIAN’S DEFENSE The patient’s anatomy is abnormal. A perforation is a known complication of a D&C.
VERDICT A $350,000 Wisconsin settlement was reached.
Failure to monitor a high-risk patient
A WOMAN WITH A HEART CONDITION who routinely took a beta-blocker plus migraine medication also had lupus. Her pregnancy was therefore at high risk for developing intrauterine growth restriction. Her US Navy ObGyn was advised by a maternal-fetal medicine (MFM) specialist to monitor the pregnancy closely with frequent ultrasonography and other tests that were never performed.
The baby was born by emergency cesarean delivery at 36 weeks’ gestation. The child suffered severe hypoxia and a brain hemorrhage just before delivery, which caused serious, permanent physical and neurologic injuries. He needs 24-hour care, is confined to a wheelchair, and requires a feeding tube.
PATIENT’S CLAIM The ObGyn failed to monitor the mother for fetal growth restriction as recommended by the MFM specialist.
DEFENDANTS’ DEFENSE There was no negligence; the mother was treated properly.
VERDICT After a $28 million Virginia verdict was awarded, the parties continued to dispute whether the judgment would be paid under California law (where the child was born) or Virginia law (where the case was filed). Prior to a rehearing, a $25 million settlement was reached.
Uterine cancer went undiagnosed
A WOMAN IN HER 50s saw her gynecologist in March 2004 to report vaginal staining. She did not return to the physician’s office until January 2005, when she reported daily vaginal bleeding. Ultrasonography showed a 4-cm mass in the endometrial cavity, consistent with a large polyp. A hysteroscopy and biopsy revealed that the woman had uterine cancer. She underwent a hysterectomy and radiation therapy, but the cancer metastasized to her lungs and she died in October 2006.
ESTATE’S CLAIM The gynecologist failed to diagnose uterine cancer in a timely manner.
PHYSICIAN’S DEFENSE The patient’s cancer was aggressive; an earlier diagnosis would not have changed the outcome.
VERDICT A $820,000 Massachusetts settlement was reached.
WHEN A 51-YEAR-OLD WOMAN NOTICED A BULGE in her vagina, she consulted her gynecologist. He determined the cause to be a cystocele and rectocele, and recommended a tension-free vaginal tape–obturator (TVT-O) procedure with anterior and posterior colporrhaphy.
The patient awoke from surgery in severe pain and was told that she had lost a lot of blood. Two weeks later, the physician explained that the stitches, not yet absorbed, were causing an abrasion, and that more vaginal tissue had been removed than planned.
Two more weeks passed, and the patient used a mirror to look at her vagina but could not see the opening. The TVT-O tape had created a ridge of tissue in the anterior vagina, causing severe stenosis. Vaginal dilators were required to expand the vagina. Entrapment of the dorsal clitoral nerve by the TVT-O tape was also discovered. The patient continues to experience dyspareunia and groin pain.
PATIENT’S CLAIM The gynecologist failed to tell her that, 2 months before surgery, the FDA had issued a public health warning about complications associated with transvaginal placement of surgical mesh during prolapse and urinary incontinence repair. Nor was she informed that the defendant had just completed training in TVT-O surgery, was not fully credentialed, and was proctored during the procedure.
PHYSICIAN’S DEFENSE The case was settled before the trial concluded.
VERDICT A $390,000 Virginia settlement was reached.
Lumpectomy, though no mass palpated
A 52-YEAR-OLD WOMAN FOUND A LUMP in her left breast. Her internist ordered mammography, which identified a 2-cm oval, asymmetrical density in the upper inner quadrant of the left breast. The radiologist recommended ultrasonography (US).
The patient consulted a surgical oncologist, who performed fine-needle aspiration. Pathology identified “clusters of malignant cells consistent with carcinoma,” and suggested a confirmatory biopsy. The oncologist recommended lumpectomy and sentinel node biopsy.
On the day of surgery, the patient could not locate the mass. The oncologist testified that he had palpated it. During surgery, gross examination did not show a mass or tumor. Frozen sections of sentinel nodes did not reveal evidence of cancer.
The patient suffered postsurgical seromas and lymphedema. The lymphedema has partially resolved, but causes pain in her left arm and breast.
PATIENT’S CLAIM The surgical oncologist should have performed US before surgery. It was negligent to continue with surgery when there were negative intraoperative findings for cancer or a mass.
PHYSICIAN’S DEFENSE Proper care was provided.
VERDICT A $950,000 Illinois verdict was returned.
Genetic testing fails to identify cystic fibrosis in one twin
AFTER HAVING ONE CHILD with cystic fibrosis (CF), parents underwent genetic testing. Embryos were prepared for in vitro fertilization (IVF) and sent to a genetic-testing laboratory. The lab reported that the embryos were negative for CF. Two embryos were implanted, and the mother gave birth to twins, one of which has CF.
PARENTS’ CLAIM Multiple errors by the genetic-testing laboratory led to an incorrect report on the embryos. The parents claimed wrongful birth.
DEFENDANTS’ DEFENSE The testing laboratory and physician owner argued that amniocentesis should have been performed during the pregnancy to rule out CF.
VERDICT The trial judge denied the use of the amniocentesis defense because an abortion would have been the only option available, and abortion is against the public policy of Tennessee. The court entered summary judgment on liability for the parents.
A $13 million verdict was returned, including $7 million to the parents for emotional distress.
These cases were selected by the editors of OBG Management from Medical Malpractice Verdicts, Settlements & Experts, with permission of the editor, Lewis Laska (www.verdictslaska.com). The information available to the editors about the cases presented here is sometimes incomplete. Moreover, the cases may or may not have merit. Nevertheless, these cases represent the types of clinical situations that typically result in litigation and are meant to illustrate nationwide variation in jury verdicts and awards.
We want to hear from you! Tell us what you think.
These cases were selected by the editors of OBG Management from Medical Malpractice Verdicts, Settlements & Experts, with permission of the editor, Lewis Laska (www.verdictslaska.com). The information available to the editors about the cases presented here is sometimes incomplete. Moreover, the cases may or may not have merit. Nevertheless, these cases represent the types of clinical situations that typically result in litigation and are meant to illustrate nationwide variation in jury verdicts and awards.
We want to hear from you! Tell us what you think.
The new year brings refinements to CPT and Medicare codes
Ms. Witt reports no financial relationships relevant to this article.
Among changes to Current Procedural Terminology (CPT) that took effect on January 1 are several of interest to our specialty:
- the addition of “typical” times to the evaluation and management (E/M) codes for same-day admission and discharge
- a new code for bladder injection
- bundling of imaging guidance associated with percutaneous implantation of a neurostimulator electrode array, if performed, using code 64561, Percutaneous implantation of neurostimulator electrode array; sacral nerve (transforaminal placement).
In addition, CPT made it clear that all E/M codes can be reported by qualified nonphysician health-care providers, as well as physicians. As for Medicare, coding for administration of depot medroxyprogesterone acetate (Depo-Provera) has been modified, as has the billing process for interpretation of ultrasonography performed outside of the office.
Because of requirements in the Health Insurance Portability and Accountability Act (HIPAA), insurers were required to accept the new codes and revisions on January 1.
Providers can now characterize their level of service by how long it took to provide
As I mentioned, typical times have been added to the set of observation and inpatient care codes that involve admission and discharge on the same date of service. Until now, these codes did not have a pre-assigned typical time, and the provider had to select the level of service based solely on three key components: history, examination, and medical decision-making. The addition of times allows the provider to select the level of service based on counseling or coordination of care, if that activity dominated the visit.
The typical times are:
- 99234, 40 minutes
- 99235, 50 minutes
- 99236, 55 minutes.
Chemodenervation of the bladder gets its own code
A new code, 52287, cystourethroscopy, with injection(s) for chemodenervation of the bladder, has been added to CPT. This procedure is performed to treat idiopathic overactive bladder that can’t be managed any other way. It typically involves the injection of botulinum. Before January 1, this procedure was reported using codes 52000 and 64614, but this approach represented an inexact match.
Payers will be looking closely at diagnostic coding for this procedure. The most frequently accepted diagnostic codes are:
- 596.51, hypertonicity of bladder
- 596.54, neurogenic bladder NOS
- 596.55, detrusor sphincter dyssynergia
- 596.59, other functional disorder of bladder
- 788.41, urinary frequency.
Because costs will vary, depending on the chemotoxin used, the agent may be reported separately using the descriptive “J” code or another Medicare-designated alphanumeric code, such as J0585, injection of botulinum toxin type A, 1 unit.
Qualified providers now include nonphysicians as well as physicians
CPT has clarified that all E/M codes can be reported not only by physicians but by qualified nonphysicians as well.
CPT also changed wording in each of the codes so that the use of counseling time applies to all providers when counseling dominates the visit. In other words, if a payer allows someone other than a physician to provide and bill for a service, the CPT E/M codes can be used by all providers who qualify and have documented the service. These changes have no effect on the codes themselves.
Please note, however, that registered nurses and licensed practical nurses are not normally recognized as billing providers and will still be restricted to code 99211, Office or other outpatient visit for the evaluation and management of an established patient, that may not require the presence of a physician. Usually, with this code, presenting problems are minimal. Typically, 5 minutes are spent performing or supervising these services. This code is often referred to as the “nurse-only” code.
As a result of this clarification, references to physicians have been removed from CPT code 59300, Episiotomy or vaginal repair, by other than attending. This change signifies that this code may be reported by any qualified provider who did not perform the delivery or was not covering for a physician group who billed for the delivery.
Three new codes for the flu vaccine
Two of the new codes are CPT codes, and the other is for Medicare:
- 90653, Influenza vaccine, inactivated, subunit, adjuvanted, for intramuscular use
- 90672, Influenza virus vaccine, live, for intranasal use
- Q2034, Agriflu.
Keep in mind that the administration of the flu vaccine is reported differently for Medicare, compared with private payers. Administration code G0008 and diagnosis code V04.81 would be reported in conjunction with the appropriate vaccine code for Medicare. CPT requires that code 90471 be reported for administration.
CPT also revised all flu vaccine codes (90655–90660) to include the term “trivalent” to signify that all flu vaccines are made up of three strains of the virus.
Medicare refines billing for MPA administration
When billing for MPA or MPA in combination with estradiol, be aware that Medicare has eliminated the J codes for these drugs, replacing them with a single new code.
The deleted codes are:
- J1051, medroxyprogesterone acetate, 50 mg
- J1055, medroxyprogesterone acetate, 150 mg, for contraceptive use
- J1056, medroxyprogesterone acetate/ estradiol cypionate, 5 mg/25 mg.
The new code is J1050, medroxyprogesterone acetate, 1 mg. To use it, you must indicate the dosage as a quantity. For example, if you injected 150 mg, you would use code J1050 x 150 on the claim. The diagnosis code will indicate the reason for the injection—that is, medical treatment or contraception. In the event that the combination drug is being administered, separate billing of J1000, Injection, depo-estradiol cypionate, up to 5 mg, would need to be reported in addition to J1050.
Medicare has also issued a national policy on Place of Service (POS) billing because the office of the inspector general has found that physicians and other suppliers frequently report an incorrect POS, and Medicare pays more for some sites. Medicare rules for the billing of POS for the professional component of an imaging service are changing, effective April 1, 2013. This rule was postponed from its original date of October 1, 2012. Under this rule, when the professional and technical components of a service are performed in different locations, the appropriate POS to report for the interpretive aspect is the location where the technical component was performed. This change would apply to an ObGyn practice that contracts out for the technical component of an ultrasound but performs the interpretation in the office. In that case, the POS should not be listed as “office” or POS 11, but should match the POS of the imaging contractor.
We want to hear from you! Tell us what you think.
Ms. Witt reports no financial relationships relevant to this article.
Among changes to Current Procedural Terminology (CPT) that took effect on January 1 are several of interest to our specialty:
- the addition of “typical” times to the evaluation and management (E/M) codes for same-day admission and discharge
- a new code for bladder injection
- bundling of imaging guidance associated with percutaneous implantation of a neurostimulator electrode array, if performed, using code 64561, Percutaneous implantation of neurostimulator electrode array; sacral nerve (transforaminal placement).
In addition, CPT made it clear that all E/M codes can be reported by qualified nonphysician health-care providers, as well as physicians. As for Medicare, coding for administration of depot medroxyprogesterone acetate (Depo-Provera) has been modified, as has the billing process for interpretation of ultrasonography performed outside of the office.
Because of requirements in the Health Insurance Portability and Accountability Act (HIPAA), insurers were required to accept the new codes and revisions on January 1.
Providers can now characterize their level of service by how long it took to provide
As I mentioned, typical times have been added to the set of observation and inpatient care codes that involve admission and discharge on the same date of service. Until now, these codes did not have a pre-assigned typical time, and the provider had to select the level of service based solely on three key components: history, examination, and medical decision-making. The addition of times allows the provider to select the level of service based on counseling or coordination of care, if that activity dominated the visit.
The typical times are:
- 99234, 40 minutes
- 99235, 50 minutes
- 99236, 55 minutes.
Chemodenervation of the bladder gets its own code
A new code, 52287, cystourethroscopy, with injection(s) for chemodenervation of the bladder, has been added to CPT. This procedure is performed to treat idiopathic overactive bladder that can’t be managed any other way. It typically involves the injection of botulinum. Before January 1, this procedure was reported using codes 52000 and 64614, but this approach represented an inexact match.
Payers will be looking closely at diagnostic coding for this procedure. The most frequently accepted diagnostic codes are:
- 596.51, hypertonicity of bladder
- 596.54, neurogenic bladder NOS
- 596.55, detrusor sphincter dyssynergia
- 596.59, other functional disorder of bladder
- 788.41, urinary frequency.
Because costs will vary, depending on the chemotoxin used, the agent may be reported separately using the descriptive “J” code or another Medicare-designated alphanumeric code, such as J0585, injection of botulinum toxin type A, 1 unit.
Qualified providers now include nonphysicians as well as physicians
CPT has clarified that all E/M codes can be reported not only by physicians but by qualified nonphysicians as well.
CPT also changed wording in each of the codes so that the use of counseling time applies to all providers when counseling dominates the visit. In other words, if a payer allows someone other than a physician to provide and bill for a service, the CPT E/M codes can be used by all providers who qualify and have documented the service. These changes have no effect on the codes themselves.
Please note, however, that registered nurses and licensed practical nurses are not normally recognized as billing providers and will still be restricted to code 99211, Office or other outpatient visit for the evaluation and management of an established patient, that may not require the presence of a physician. Usually, with this code, presenting problems are minimal. Typically, 5 minutes are spent performing or supervising these services. This code is often referred to as the “nurse-only” code.
As a result of this clarification, references to physicians have been removed from CPT code 59300, Episiotomy or vaginal repair, by other than attending. This change signifies that this code may be reported by any qualified provider who did not perform the delivery or was not covering for a physician group who billed for the delivery.
Three new codes for the flu vaccine
Two of the new codes are CPT codes, and the other is for Medicare:
- 90653, Influenza vaccine, inactivated, subunit, adjuvanted, for intramuscular use
- 90672, Influenza virus vaccine, live, for intranasal use
- Q2034, Agriflu.
Keep in mind that the administration of the flu vaccine is reported differently for Medicare, compared with private payers. Administration code G0008 and diagnosis code V04.81 would be reported in conjunction with the appropriate vaccine code for Medicare. CPT requires that code 90471 be reported for administration.
CPT also revised all flu vaccine codes (90655–90660) to include the term “trivalent” to signify that all flu vaccines are made up of three strains of the virus.
Medicare refines billing for MPA administration
When billing for MPA or MPA in combination with estradiol, be aware that Medicare has eliminated the J codes for these drugs, replacing them with a single new code.
The deleted codes are:
- J1051, medroxyprogesterone acetate, 50 mg
- J1055, medroxyprogesterone acetate, 150 mg, for contraceptive use
- J1056, medroxyprogesterone acetate/ estradiol cypionate, 5 mg/25 mg.
The new code is J1050, medroxyprogesterone acetate, 1 mg. To use it, you must indicate the dosage as a quantity. For example, if you injected 150 mg, you would use code J1050 x 150 on the claim. The diagnosis code will indicate the reason for the injection—that is, medical treatment or contraception. In the event that the combination drug is being administered, separate billing of J1000, Injection, depo-estradiol cypionate, up to 5 mg, would need to be reported in addition to J1050.
Medicare has also issued a national policy on Place of Service (POS) billing because the office of the inspector general has found that physicians and other suppliers frequently report an incorrect POS, and Medicare pays more for some sites. Medicare rules for the billing of POS for the professional component of an imaging service are changing, effective April 1, 2013. This rule was postponed from its original date of October 1, 2012. Under this rule, when the professional and technical components of a service are performed in different locations, the appropriate POS to report for the interpretive aspect is the location where the technical component was performed. This change would apply to an ObGyn practice that contracts out for the technical component of an ultrasound but performs the interpretation in the office. In that case, the POS should not be listed as “office” or POS 11, but should match the POS of the imaging contractor.
We want to hear from you! Tell us what you think.
Ms. Witt reports no financial relationships relevant to this article.
Among changes to Current Procedural Terminology (CPT) that took effect on January 1 are several of interest to our specialty:
- the addition of “typical” times to the evaluation and management (E/M) codes for same-day admission and discharge
- a new code for bladder injection
- bundling of imaging guidance associated with percutaneous implantation of a neurostimulator electrode array, if performed, using code 64561, Percutaneous implantation of neurostimulator electrode array; sacral nerve (transforaminal placement).
In addition, CPT made it clear that all E/M codes can be reported by qualified nonphysician health-care providers, as well as physicians. As for Medicare, coding for administration of depot medroxyprogesterone acetate (Depo-Provera) has been modified, as has the billing process for interpretation of ultrasonography performed outside of the office.
Because of requirements in the Health Insurance Portability and Accountability Act (HIPAA), insurers were required to accept the new codes and revisions on January 1.
Providers can now characterize their level of service by how long it took to provide
As I mentioned, typical times have been added to the set of observation and inpatient care codes that involve admission and discharge on the same date of service. Until now, these codes did not have a pre-assigned typical time, and the provider had to select the level of service based solely on three key components: history, examination, and medical decision-making. The addition of times allows the provider to select the level of service based on counseling or coordination of care, if that activity dominated the visit.
The typical times are:
- 99234, 40 minutes
- 99235, 50 minutes
- 99236, 55 minutes.
Chemodenervation of the bladder gets its own code
A new code, 52287, cystourethroscopy, with injection(s) for chemodenervation of the bladder, has been added to CPT. This procedure is performed to treat idiopathic overactive bladder that can’t be managed any other way. It typically involves the injection of botulinum. Before January 1, this procedure was reported using codes 52000 and 64614, but this approach represented an inexact match.
Payers will be looking closely at diagnostic coding for this procedure. The most frequently accepted diagnostic codes are:
- 596.51, hypertonicity of bladder
- 596.54, neurogenic bladder NOS
- 596.55, detrusor sphincter dyssynergia
- 596.59, other functional disorder of bladder
- 788.41, urinary frequency.
Because costs will vary, depending on the chemotoxin used, the agent may be reported separately using the descriptive “J” code or another Medicare-designated alphanumeric code, such as J0585, injection of botulinum toxin type A, 1 unit.
Qualified providers now include nonphysicians as well as physicians
CPT has clarified that all E/M codes can be reported not only by physicians but by qualified nonphysicians as well.
CPT also changed wording in each of the codes so that the use of counseling time applies to all providers when counseling dominates the visit. In other words, if a payer allows someone other than a physician to provide and bill for a service, the CPT E/M codes can be used by all providers who qualify and have documented the service. These changes have no effect on the codes themselves.
Please note, however, that registered nurses and licensed practical nurses are not normally recognized as billing providers and will still be restricted to code 99211, Office or other outpatient visit for the evaluation and management of an established patient, that may not require the presence of a physician. Usually, with this code, presenting problems are minimal. Typically, 5 minutes are spent performing or supervising these services. This code is often referred to as the “nurse-only” code.
As a result of this clarification, references to physicians have been removed from CPT code 59300, Episiotomy or vaginal repair, by other than attending. This change signifies that this code may be reported by any qualified provider who did not perform the delivery or was not covering for a physician group who billed for the delivery.
Three new codes for the flu vaccine
Two of the new codes are CPT codes, and the other is for Medicare:
- 90653, Influenza vaccine, inactivated, subunit, adjuvanted, for intramuscular use
- 90672, Influenza virus vaccine, live, for intranasal use
- Q2034, Agriflu.
Keep in mind that the administration of the flu vaccine is reported differently for Medicare, compared with private payers. Administration code G0008 and diagnosis code V04.81 would be reported in conjunction with the appropriate vaccine code for Medicare. CPT requires that code 90471 be reported for administration.
CPT also revised all flu vaccine codes (90655–90660) to include the term “trivalent” to signify that all flu vaccines are made up of three strains of the virus.
Medicare refines billing for MPA administration
When billing for MPA or MPA in combination with estradiol, be aware that Medicare has eliminated the J codes for these drugs, replacing them with a single new code.
The deleted codes are:
- J1051, medroxyprogesterone acetate, 50 mg
- J1055, medroxyprogesterone acetate, 150 mg, for contraceptive use
- J1056, medroxyprogesterone acetate/ estradiol cypionate, 5 mg/25 mg.
The new code is J1050, medroxyprogesterone acetate, 1 mg. To use it, you must indicate the dosage as a quantity. For example, if you injected 150 mg, you would use code J1050 x 150 on the claim. The diagnosis code will indicate the reason for the injection—that is, medical treatment or contraception. In the event that the combination drug is being administered, separate billing of J1000, Injection, depo-estradiol cypionate, up to 5 mg, would need to be reported in addition to J1050.
Medicare has also issued a national policy on Place of Service (POS) billing because the office of the inspector general has found that physicians and other suppliers frequently report an incorrect POS, and Medicare pays more for some sites. Medicare rules for the billing of POS for the professional component of an imaging service are changing, effective April 1, 2013. This rule was postponed from its original date of October 1, 2012. Under this rule, when the professional and technical components of a service are performed in different locations, the appropriate POS to report for the interpretive aspect is the location where the technical component was performed. This change would apply to an ObGyn practice that contracts out for the technical component of an ultrasound but performs the interpretation in the office. In that case, the POS should not be listed as “office” or POS 11, but should match the POS of the imaging contractor.
We want to hear from you! Tell us what you think.
Drug interactions with tobacco smoke: Implications for patient care
- Tobacco smokers often are treated with medications that are metabolized by hepatic cytochrome (CYP) 1A2 enzymes. Starting or stopping tobacco smoking may cause drug interactions because polycyclic aromatic hydrocarbons in cigarette smoke induce CYP1A2 enzymes.
- Drugs that are significantly metabolized by CYP1A2 (major substrates) are more likely to be impacted by changes in tobacco smoking compared with minor substrates.
- Induction of hepatic CYP1A2 enzymes may be greater in heavy or moderate smokers compared with light smokers (eg, <10 cigarettes per day).
- Evidence-based approaches for treating tobacco use in health care settings should address the risk of CYP1A2 drug interactions in tobacco smokers and how this impacts their clinical care.
Mrs. C, age 51, experiences exacerbated asthma and difficulty breathing and is admitted to a non-smoking hospital. She also has chronic obstructive pulmonary disease, type 2 diabetes mellitus, hypertension, hypercholesterolemia, hypothyroidism, gastroesophageal reflux disease, overactive bladder, muscle spasms, fibromyalgia, bipolar disorder, insomnia, and nicotine and caffeine dependence. She takes 19 prescribed and over-the-counter medications, drinks up to 8 cups of coffee per day, and smokes 20 to 30 cigarettes per day. In the emergency room, she receives albuterol/ipratropium inhalation therapy to help her breathing and a 21-mg nicotine replacement patch to avoid nicotine withdrawal.
In the United States, 19% of adults smoke cigarettes.1 Heavy tobacco smoking and nicotine dependence are common among psychiatric patients and contribute to higher rates of tobacco-related morbidity and mortality.2 When smokers stop smoking or are admitted to smoke-free facilities and are forced to abstain, nicotine withdrawal symptoms and changes in drug metabolism can develop over several days.3-5
Smokers such as Mrs. C are at risk for cytochrome (CYP) P450 drug interactions when they are admitted to or discharged from a smoke-free facility. Nine of Mrs. C’s medications are substrates of CYP1A2 (acetaminophen, caffeine, cyclobenzaprine, diazepam, duloxetine, melatonin, olanzapine, ondansetron, and zolpidem). When Mrs. C stops smoking while in the hospital, she could experience higher serum concentrations and adverse effects of these medications. If Mrs. C resumes smoking after bring discharged, metabolism and clearance of any medications started while she was hospitalized that are substrates of CYP1A2 enzymes could be increased, which could lead to reduced efficacy and poor clinical outcomes.
Pharmacokinetic effects
Polycyclic aromatic hydrocarbons in tobacco smoke induce hepatic CYP1A1, 1A2, and possibly 2E1 isoenzymes.6-12 CYP1A2 is a hepatic enzyme responsible for metabolizing and eliminating several classes of substrates (eg, drugs, hormones, endogenous compounds, and procarcinogens).6,13 Genetic, epigenetic, and environmental factors such as smoking impact the expression and activity of CYP1A2 and result in large interpatient variability in pharmacokinetic drug interactions.6,12,13 CYP1A2 enzymes can be induced or inhibited by drugs and substances, which can result in decreased or increased serum concentrations of substrates, respectively. When individuals stop smoking and switch to other nicotine products or devices, CYP1A2 induction of hepatic enzymes will revert to normal metabolism over several weeks to a month.10 Besides tobacco smoke, other CYP1A2 inducers include charbroiled food, carbamazepine, omeprazole, phenobarbital, primidone, and rifampin.4,5 Nicotine replacement products—such as gum, inhalers, lozenges, patches, and nasal spray—and nicotine delivery devices such as electronic cigarettes do not induce hepatic CYP1A2 enzymes or cause the same drug interactions as cigarette smoking.
Table 13-11 and Table 23-11 list commonly prescribed CYP1A2 substrates that could be affected by tobacco smoke. There are no specific guidelines for how to assess, monitor, or manage pharmacokinetic drug interactions with tobacco smoke.6-13 Induction of hepatic CYP1A2 enzymes by cigarette smoke may require increased dosages of some psychotropics—such as tricyclic antidepressants, duloxetine, mirtazapine, and some first- and second-generation antipsychotics (SGAs)—to achieve serum concentrations adequate for clinical efficacy. Serum concentrations may increase to toxic levels and result in adverse effects when a person quits smoking cigarettes or if a CYP1A2 inhibitor, such as amlodipine, cimetidine, ciprofloxacin, diclofenac, fluoxetine, fluvoxamine, or nifedipine, is added.5
Table 1
Common major cytochrome P450 (CYP) 1A2 substrates
| Drug | Class |
|---|---|
| Alosetron3,5,6 | Irritable bowel syndrome: serotonin 3 antagonist |
| Aminophylline3,5 | Bronchodilator: theophylline derivative |
| Betaxolol3,5 | β-1 selective adrenergic receptor blocking agent |
| Caffeine3-9 | Stimulant |
| Clomipramine3-11 | Tricyclic antidepressant |
| Clozapine3-10 | Second-generation antipsychotic |
| Cyclobenzaprine3-7 | Skeletal muscle relaxant |
| Doxepin3,7,10,11 | Tricyclic antidepressant |
| Duloxetine3-6 | Serotonin-norepinephrine reuptake inhibitor |
| Estradiol3,5-8 | Estrogen (active) |
| Estrogens: conjugated and estropipate3,5; estrone3,7 | Estrogen (derivatives) |
| Fluvoxamine3,8,9 | Selective serotonin reuptake inhibitor |
| Guanabenz3,5-7 | α-2 adrenergic agonist |
| Mirtazapine3-7 | Antidepressant: α-2 antagonist/serotonin 2A, 2C antagonist |
| Olanzapine3-11 | Second-generation antipsychotic |
| Pimozide3,5,7 | First-generation antipsychotic |
| Propranolol3-11 | β-adrenergic blocker |
| Ramelteon3,5,10 | Melatonin receptor agonist |
| Rasagiline3,5 | Antiparkinson: type B monoamine oxidase inhibitor |
| Riluzole3-7,10 | Glutamate inhibitor |
| Ropinirole3,5-7 | Antiparkinson: dopamine agonist |
| Theophylline3-6,8-11 | Bronchodilator: methylxanthine |
| Thiothixene3,5 | First-generation antipsychotic |
| Trifluoperazine3,5,9 | First-generation antipsychotic |
| Several classes of CYP1A2 substrates are not included and may cause toxicity with smoking cessation or require dosage increases in tobacco smokers (eg, antiarrhythmic, antifungal, antimalarial, antineoplastic, antiretroviral, and anthelmintic agents and the antibiotic quinolone). Clinicians should be most concerned about drugs with a narrow therapeutic index and those that may be toxic with smoking cessation (eg, bleeding from warfarin and clopidogrel; high serum concentrations of caffeine, clozapine, olanzapine, propranolol, and theophylline) | |
Table 2
Common minor cytochrome P450 (CYP) 1A2 substrates
| Drug | Class |
|---|---|
| Acetaminophen3-9 | Analgesic |
| Almotriptan6 | Antimigraine: serotonin 1B, 1D receptor agonist |
| Amitriptyline3-7,9-11 | Tricyclic antidepressant |
| Asenapine9 | Second-generation antipsychotic |
| Carvedilol5-7 | β and α adrenergic blocking activity |
| Chlorpromazine3,4,7-9,11 | First-generation antipsychotic |
| Chlorzoxazone4,7 | Skeletal muscle relaxant |
| Clopidogrel5 | Antiplatelet |
| Desipramine4,7,10,11 | Tricyclic antidepressant |
| Diazepam4,7,9,10 | Benzodiazepine |
| Diclofenac5,7 | Nonsteroidal anti-inflammatory drug |
| Diphenhydramine6 | Antihistamine |
| Febuxostat5 | Xanthine oxidase inhibitor |
| Fluphenazine3,9 | First-generation antipsychotic |
| Frovatriptan3 | Antimigraine: serotonin 1 agonist |
| Haloperidol3,4,6,8,9 | First-generation antipsychotic |
| Imipramine3,4,6-11 | Tricyclic antidepressant |
| Maprotiline6 | Tetracyclic antidepressant |
| Melatonin3,4,6,7 | Sleep-regulating hormone |
| Metoclopramide3 | Antiemetic: prokinetic gastrointestinal agent |
| Nabumetone6 | Nonsteroidal anti-inflammatory drug |
| Naproxen3,4,6,7 | Nonsteroidal anti-inflammatory drug |
| Naratriptan10 | Antimigraine: serotonin 1B, 1D receptor agonist |
| Nicardipine3,7 | Calcium channel blocker |
| Nortriptyline4,6,7,9-11 | Tricyclic antidepressant |
| Ondansetron3,4,6,7 | Antiemetic: serotonin 3 antagonist |
| Palonosetron5 | Antiemetic: serotonin 3 antagonist |
| Perphenazine3,7 | First-generation antipsychotic |
| Progesterone5,7 | Progestin |
| Propofol4,6,7 | General anesthetic |
| Ranitidine5,7 | H2 antagonist |
| Rivastigmine10 | Acetylcholinesterase inhibitor |
| Selegiline6,7 | Antiparkinson: type B monoamine oxidase inhibitor |
| Thioridazine3,4,6 | First-generation antipsychotic |
| Tizanidine3-6 | Skeletal muscle relaxant: α-2 adrenergic agonist |
| Trazodone6,9 | Serotonin reuptake inhibitor and antagonist |
| Triamterene6 | Diuretic: potassium sparing |
| Verapamil3,4,6,7,10 | Calcium channel blocker |
| Warfarin3,4,6-10 | Anticoagulant: coumarin derivative |
| Zileuton3,4,6,7 | Asthma agent: 5-lipoxygenase inhibitor |
| Ziprasidone3,4 | Second-generation antipsychotic |
| Zolmitriptan3,6,7 | Antimigraine: serotonin 1B, 1D receptor agonist |
| Zolpidem4,6,7 | Nonbenzodiazepine hypnotic |
| Several classes of CYP1A2 substrates are not included and may cause toxicity with smoking cessation or require dosage increases in tobacco smokers (eg, antiarrhythmic, antifungal, antimalarial, antineoplastic, antiretroviral and anthelmintic agents and the antibiotic quinolone). Clinicians should be most concerned about drugs with a narrow therapeutic index and those that may be toxic with smoking cessation (eg, bleeding from warfarin and clopidogrel; high serum concentrations of caffeine, clozapine, olanzapine, propranolol, and theophylline) | |
SGA such as clozapine and olanzapine are major substrates of CYP1A2 and dosages may need to be adjusted when smoking status changes, depending on clinical efficacy and adverse effects.10,14,15 Maximum induction of clozapine and olanzapine metabolism may occur with 7 to 12 cigarettes per day and smokers may have 40% to 50% lower serum concentrations compared with nonsmokers.14 When a patient stops smoking, clozapine and olanzapine dosages may need to be reduced by 30% to 40% (eg, a stepwise 10% reduction in daily dose until day 4) to avoid elevated serum concentrations and risk of toxicity symptoms.15
Tobacco smokers can tolerate high daily intake of caffeinated beverages because of increased metabolism and clearance of caffeine, a major substrate of CYP1A2.11 When patients stop smoking, increased caffeine serum concentrations may cause anxiety, irritability, restlessness, insomnia, tremors, palpitations, and tachycardia. Caffeine toxicity also can mimic symptoms of nicotine withdrawal; therefore, smokers should be advised to reduce their caffeine intake by half to avoid adverse effects when they stop smoking.10,11
Adjusting dosing
Factors such as the amount and frequency of tobacco smoking, how quickly CYP1A2 enzymes change when starting and stopping smoking, exposure to secondhand smoke, and other concomitant drugs contribute to variability in pharmacokinetic drug interactions. Heavy smokers (≥30 cigarettes per day) should be closely monitored because variations in drug serum concentrations may be affected significantly by changes in smoking status.4,9,11 Dosage reductions of potentially toxic drugs should be done immediately when a heavy tobacco user stops smoking.10 For CYP1A2 substrates with a narrow therapeutic range, a conservative approach is to reduce the daily dose by 10% per day for several days after smoking cessation.11,16 The impact on drug metabolism may continue for weeks to a month after the person stops smoking; therefore, there may be a delay until CYP1A2 enzymes return to normal hepatic metabolism.4,8,9,15 In most situations, smoking cessation reverses induction of hepatic CYP1A2 enzymes back to normal metabolism and causes serum drug concentrations to increase.10 Because secondhand smoke induces hepatic CYP1A2 enzymes, those exposed to smoke may have changes in drug metabolism due to environmental smoke exposure.11
Tobacco smokers who take medications and hormones that are metabolized by CYP1A2 enzymes should be closely monitored because dosage adjustments may be necessary when they start or stop smoking cigarettes. An assessment of CYP drug interactions and routine monitoring of efficacy and/or toxicity should be done to avoid potential adverse effects from medications and to determine if changes in dosages and disease state management are required.
Related Resources
- Rx for Change. Drug interactions with smoking. http://smokingcessationleadership.ucsf.edu/interactions.pdf.
- Fiore MC, Baker TB. Treating smokers in the health care setting. N Engl J Med. 2011;365(13):1222-1231.
Drug Brand Names
- Albuterol/ipratropium • Combivent
- Almotriptan • Axert
- Alosetron • Lotronex
- Aminophylline • Phyllocontin, Truphylline
- Amitriptyline • Elavil
- Amlodipine • Norvasc
- Asenapine • Saphris
- Betaxolol • Kerlone
- Carbamazepine • Carbatrol, Tegretol
- Carvedilol • Coreg
- Chlorpromazine • Thorazine
- Chlorzoxazone • Parafon Forte
- Cimetidine • Tagamet
- Ciprofloxacin • Cipro
- Clomipramine • Anafranil
- Clopidogrel • Plavix
- Clozapine • Clozaril
- Cyclobenzaprine • Flexeril
- Desipramine • Norpramin
- Diazepam • Valium
- Diclofenac • Voltaren
- Diphenhydramine • Benadryl
- Doxepin • Silenor, Sinequan
- Duloxetine • Cymbalta
- Estradiol • Estrace
- Estrogens (conjugated) • Cenestin, Premarin
- Estropipate • Ogen
- Febuxostat • Uloric
- Fluoxetine • Prozac
- Fluphenazine • Prolixin
- Fluvoxamine • Luvox
- Frovatriptan • Frova
- Guanabenz • Wytensin
- Haloperidol • Haldol
- Imipramine • Tofranil
- Maprotiline • Ludiomil
- Metoclopramide • Reglan
- Mirtazapine • Remeron
- Nabumetone • Relafen
- Naratriptan • Amerge
- Nicardipine • Cardene
- Nifedipine • Adalat, Procardia
- Nortriptyline • Aventyl, Pamelor
- Olanzapine • Zyprexa
- Omeprazole • Prilosec
- Ondansetron • Zofran
- Palonosetron • Aloxi
- Perphenazine • Trilafon
- Pimozide • Orap
- Primidone • Mysoline
- Progesterone • Prometrium
- Propofol • Diprivan
- Propranolol • Inderal
- Ramelteon • Rozerem
- Ranitidine • Zantac
- Rasagiline • Azilect
- Rifampin • Rifadin, Rimactane
- Riluzole • Rilutek
- Rivastigmine • Exelon
- Ropinirole • Requip
- Selegiline • Eldepryl, EMSAM, others
- Theophylline • Elixophyllin
- Thioridazine • Mellaril
- Thiothixene • Navane
- Tizanidine • Zanaflex
- Trazodone • Desyrel, Oleptro
- Triamterene • Dyrenium
- Trifluoperazine • Stelazine
- Verapamil • Calan, Verelan
- Warfarin • Coumadin, Jantoven
- Zileuton • Zyflo
- Ziprasidone • Geodon
- Zolmitriptan • Zomig
- Zolpidem • Ambien, Edluar
Disclosure
Ms. Fankhauser reports no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Centers for Disease Control and Prevention (CDC). Vital signs: current cigarette smoking among adults aged ≥18 years—United States 2005-2010. MMWR Morb Mortal Wkly Rep. 2011;60(35):1207-1212.
2. Ziedonis D, Hitsman B, Beckham JC, et al. Tobacco use and cessation in psychiatric disorders: National Institute of Mental Health report. Nicotine Tob Res. 2008;10(12):1691-1715.
3. Choe JY. Drug actions and interactions. New York NY: McGraw-Hill Medical; 2011.
4. Tatro DS. Drug interaction facts. St. Louis MO: Wolters Kluwer Health; 2011.
5. Lacy CF, Armstrong LL, Goldman MP, et al. eds. Drug information handbook, 20th ed. Hudson, OH: Lexicomp; 2011.
6. Zhou SF, Yang LP, Zhou ZW, et al. Insights into the substrate specificity, inhibitors, regulation, and polymorphisms and the clinical impact of human cytochrome P450 1A2. AAPS J. 2009;11(3):481-494.
7. Rendic S. Summary of information on human CYP enzymes: human P450 metabolism data. Drug Metab Rev. 2002;34(1-2):83-448.
8. Zevin S, Benowitz NL. Drug interactions with tobacco smoking. An update. Clin Pharmacokinet. 1999;36(6):425-438.
9. Desai HD, Seabolt J, Jann MW. Smoking in patients receiving psychotropic medications: a pharmacokinetic perspective. CNS Drugs. 2001;15(6):469-494.
10. Schaffer SD, Yoon S, Zadezensky I. A review of smoking cessation: potentially risky effects on prescribed medications. J Clin Nurs. 2009;18(11):1533-1540.
11. Kroon LA. Drug interactions with smoking. Am J Health Syst Pharm. 2007;64(18):1917-1921.
12. Plowchalk DR, Yeo KR. Prediction of drug clearance in a smoking population: modeling the impact of variable cigarette consumption on the induction of CYP1A2. Eur J Pharmacol. 2012;68(6):951-960.
13. Faber MS, Jetter A, Fuhr U. Assessment of CYP1A2 activity in clinical practice: why how, and when? Basic Clin Pharmacol Toxicol. 2005;97(3):125-134.
14. Haslemo T, Eikeseth PH, Tanum L, et al. The effect of variable cigarette consumption on the interaction with clozapine and olanzapine. Eur J Clin Pharmacol. 2006;62(12):1049-1053.
15. Lowe EJ, Ackman ML. Impact of tobacco smoking cessation on stable clozapine or olanzapine treatment. Ann Pharmacother. 2010;44(4):727-732.
16. Faber MS, Fuhr U. Time response of cytochrome P4501A2 activity on cessation of heavy smoking. Clin Pharmacol Ther. 2004;76(2):178-184.
- Tobacco smokers often are treated with medications that are metabolized by hepatic cytochrome (CYP) 1A2 enzymes. Starting or stopping tobacco smoking may cause drug interactions because polycyclic aromatic hydrocarbons in cigarette smoke induce CYP1A2 enzymes.
- Drugs that are significantly metabolized by CYP1A2 (major substrates) are more likely to be impacted by changes in tobacco smoking compared with minor substrates.
- Induction of hepatic CYP1A2 enzymes may be greater in heavy or moderate smokers compared with light smokers (eg, <10 cigarettes per day).
- Evidence-based approaches for treating tobacco use in health care settings should address the risk of CYP1A2 drug interactions in tobacco smokers and how this impacts their clinical care.
Mrs. C, age 51, experiences exacerbated asthma and difficulty breathing and is admitted to a non-smoking hospital. She also has chronic obstructive pulmonary disease, type 2 diabetes mellitus, hypertension, hypercholesterolemia, hypothyroidism, gastroesophageal reflux disease, overactive bladder, muscle spasms, fibromyalgia, bipolar disorder, insomnia, and nicotine and caffeine dependence. She takes 19 prescribed and over-the-counter medications, drinks up to 8 cups of coffee per day, and smokes 20 to 30 cigarettes per day. In the emergency room, she receives albuterol/ipratropium inhalation therapy to help her breathing and a 21-mg nicotine replacement patch to avoid nicotine withdrawal.
In the United States, 19% of adults smoke cigarettes.1 Heavy tobacco smoking and nicotine dependence are common among psychiatric patients and contribute to higher rates of tobacco-related morbidity and mortality.2 When smokers stop smoking or are admitted to smoke-free facilities and are forced to abstain, nicotine withdrawal symptoms and changes in drug metabolism can develop over several days.3-5
Smokers such as Mrs. C are at risk for cytochrome (CYP) P450 drug interactions when they are admitted to or discharged from a smoke-free facility. Nine of Mrs. C’s medications are substrates of CYP1A2 (acetaminophen, caffeine, cyclobenzaprine, diazepam, duloxetine, melatonin, olanzapine, ondansetron, and zolpidem). When Mrs. C stops smoking while in the hospital, she could experience higher serum concentrations and adverse effects of these medications. If Mrs. C resumes smoking after bring discharged, metabolism and clearance of any medications started while she was hospitalized that are substrates of CYP1A2 enzymes could be increased, which could lead to reduced efficacy and poor clinical outcomes.
Pharmacokinetic effects
Polycyclic aromatic hydrocarbons in tobacco smoke induce hepatic CYP1A1, 1A2, and possibly 2E1 isoenzymes.6-12 CYP1A2 is a hepatic enzyme responsible for metabolizing and eliminating several classes of substrates (eg, drugs, hormones, endogenous compounds, and procarcinogens).6,13 Genetic, epigenetic, and environmental factors such as smoking impact the expression and activity of CYP1A2 and result in large interpatient variability in pharmacokinetic drug interactions.6,12,13 CYP1A2 enzymes can be induced or inhibited by drugs and substances, which can result in decreased or increased serum concentrations of substrates, respectively. When individuals stop smoking and switch to other nicotine products or devices, CYP1A2 induction of hepatic enzymes will revert to normal metabolism over several weeks to a month.10 Besides tobacco smoke, other CYP1A2 inducers include charbroiled food, carbamazepine, omeprazole, phenobarbital, primidone, and rifampin.4,5 Nicotine replacement products—such as gum, inhalers, lozenges, patches, and nasal spray—and nicotine delivery devices such as electronic cigarettes do not induce hepatic CYP1A2 enzymes or cause the same drug interactions as cigarette smoking.
Table 13-11 and Table 23-11 list commonly prescribed CYP1A2 substrates that could be affected by tobacco smoke. There are no specific guidelines for how to assess, monitor, or manage pharmacokinetic drug interactions with tobacco smoke.6-13 Induction of hepatic CYP1A2 enzymes by cigarette smoke may require increased dosages of some psychotropics—such as tricyclic antidepressants, duloxetine, mirtazapine, and some first- and second-generation antipsychotics (SGAs)—to achieve serum concentrations adequate for clinical efficacy. Serum concentrations may increase to toxic levels and result in adverse effects when a person quits smoking cigarettes or if a CYP1A2 inhibitor, such as amlodipine, cimetidine, ciprofloxacin, diclofenac, fluoxetine, fluvoxamine, or nifedipine, is added.5
Table 1
Common major cytochrome P450 (CYP) 1A2 substrates
| Drug | Class |
|---|---|
| Alosetron3,5,6 | Irritable bowel syndrome: serotonin 3 antagonist |
| Aminophylline3,5 | Bronchodilator: theophylline derivative |
| Betaxolol3,5 | β-1 selective adrenergic receptor blocking agent |
| Caffeine3-9 | Stimulant |
| Clomipramine3-11 | Tricyclic antidepressant |
| Clozapine3-10 | Second-generation antipsychotic |
| Cyclobenzaprine3-7 | Skeletal muscle relaxant |
| Doxepin3,7,10,11 | Tricyclic antidepressant |
| Duloxetine3-6 | Serotonin-norepinephrine reuptake inhibitor |
| Estradiol3,5-8 | Estrogen (active) |
| Estrogens: conjugated and estropipate3,5; estrone3,7 | Estrogen (derivatives) |
| Fluvoxamine3,8,9 | Selective serotonin reuptake inhibitor |
| Guanabenz3,5-7 | α-2 adrenergic agonist |
| Mirtazapine3-7 | Antidepressant: α-2 antagonist/serotonin 2A, 2C antagonist |
| Olanzapine3-11 | Second-generation antipsychotic |
| Pimozide3,5,7 | First-generation antipsychotic |
| Propranolol3-11 | β-adrenergic blocker |
| Ramelteon3,5,10 | Melatonin receptor agonist |
| Rasagiline3,5 | Antiparkinson: type B monoamine oxidase inhibitor |
| Riluzole3-7,10 | Glutamate inhibitor |
| Ropinirole3,5-7 | Antiparkinson: dopamine agonist |
| Theophylline3-6,8-11 | Bronchodilator: methylxanthine |
| Thiothixene3,5 | First-generation antipsychotic |
| Trifluoperazine3,5,9 | First-generation antipsychotic |
| Several classes of CYP1A2 substrates are not included and may cause toxicity with smoking cessation or require dosage increases in tobacco smokers (eg, antiarrhythmic, antifungal, antimalarial, antineoplastic, antiretroviral, and anthelmintic agents and the antibiotic quinolone). Clinicians should be most concerned about drugs with a narrow therapeutic index and those that may be toxic with smoking cessation (eg, bleeding from warfarin and clopidogrel; high serum concentrations of caffeine, clozapine, olanzapine, propranolol, and theophylline) | |
Table 2
Common minor cytochrome P450 (CYP) 1A2 substrates
| Drug | Class |
|---|---|
| Acetaminophen3-9 | Analgesic |
| Almotriptan6 | Antimigraine: serotonin 1B, 1D receptor agonist |
| Amitriptyline3-7,9-11 | Tricyclic antidepressant |
| Asenapine9 | Second-generation antipsychotic |
| Carvedilol5-7 | β and α adrenergic blocking activity |
| Chlorpromazine3,4,7-9,11 | First-generation antipsychotic |
| Chlorzoxazone4,7 | Skeletal muscle relaxant |
| Clopidogrel5 | Antiplatelet |
| Desipramine4,7,10,11 | Tricyclic antidepressant |
| Diazepam4,7,9,10 | Benzodiazepine |
| Diclofenac5,7 | Nonsteroidal anti-inflammatory drug |
| Diphenhydramine6 | Antihistamine |
| Febuxostat5 | Xanthine oxidase inhibitor |
| Fluphenazine3,9 | First-generation antipsychotic |
| Frovatriptan3 | Antimigraine: serotonin 1 agonist |
| Haloperidol3,4,6,8,9 | First-generation antipsychotic |
| Imipramine3,4,6-11 | Tricyclic antidepressant |
| Maprotiline6 | Tetracyclic antidepressant |
| Melatonin3,4,6,7 | Sleep-regulating hormone |
| Metoclopramide3 | Antiemetic: prokinetic gastrointestinal agent |
| Nabumetone6 | Nonsteroidal anti-inflammatory drug |
| Naproxen3,4,6,7 | Nonsteroidal anti-inflammatory drug |
| Naratriptan10 | Antimigraine: serotonin 1B, 1D receptor agonist |
| Nicardipine3,7 | Calcium channel blocker |
| Nortriptyline4,6,7,9-11 | Tricyclic antidepressant |
| Ondansetron3,4,6,7 | Antiemetic: serotonin 3 antagonist |
| Palonosetron5 | Antiemetic: serotonin 3 antagonist |
| Perphenazine3,7 | First-generation antipsychotic |
| Progesterone5,7 | Progestin |
| Propofol4,6,7 | General anesthetic |
| Ranitidine5,7 | H2 antagonist |
| Rivastigmine10 | Acetylcholinesterase inhibitor |
| Selegiline6,7 | Antiparkinson: type B monoamine oxidase inhibitor |
| Thioridazine3,4,6 | First-generation antipsychotic |
| Tizanidine3-6 | Skeletal muscle relaxant: α-2 adrenergic agonist |
| Trazodone6,9 | Serotonin reuptake inhibitor and antagonist |
| Triamterene6 | Diuretic: potassium sparing |
| Verapamil3,4,6,7,10 | Calcium channel blocker |
| Warfarin3,4,6-10 | Anticoagulant: coumarin derivative |
| Zileuton3,4,6,7 | Asthma agent: 5-lipoxygenase inhibitor |
| Ziprasidone3,4 | Second-generation antipsychotic |
| Zolmitriptan3,6,7 | Antimigraine: serotonin 1B, 1D receptor agonist |
| Zolpidem4,6,7 | Nonbenzodiazepine hypnotic |
| Several classes of CYP1A2 substrates are not included and may cause toxicity with smoking cessation or require dosage increases in tobacco smokers (eg, antiarrhythmic, antifungal, antimalarial, antineoplastic, antiretroviral and anthelmintic agents and the antibiotic quinolone). Clinicians should be most concerned about drugs with a narrow therapeutic index and those that may be toxic with smoking cessation (eg, bleeding from warfarin and clopidogrel; high serum concentrations of caffeine, clozapine, olanzapine, propranolol, and theophylline) | |
SGA such as clozapine and olanzapine are major substrates of CYP1A2 and dosages may need to be adjusted when smoking status changes, depending on clinical efficacy and adverse effects.10,14,15 Maximum induction of clozapine and olanzapine metabolism may occur with 7 to 12 cigarettes per day and smokers may have 40% to 50% lower serum concentrations compared with nonsmokers.14 When a patient stops smoking, clozapine and olanzapine dosages may need to be reduced by 30% to 40% (eg, a stepwise 10% reduction in daily dose until day 4) to avoid elevated serum concentrations and risk of toxicity symptoms.15
Tobacco smokers can tolerate high daily intake of caffeinated beverages because of increased metabolism and clearance of caffeine, a major substrate of CYP1A2.11 When patients stop smoking, increased caffeine serum concentrations may cause anxiety, irritability, restlessness, insomnia, tremors, palpitations, and tachycardia. Caffeine toxicity also can mimic symptoms of nicotine withdrawal; therefore, smokers should be advised to reduce their caffeine intake by half to avoid adverse effects when they stop smoking.10,11
Adjusting dosing
Factors such as the amount and frequency of tobacco smoking, how quickly CYP1A2 enzymes change when starting and stopping smoking, exposure to secondhand smoke, and other concomitant drugs contribute to variability in pharmacokinetic drug interactions. Heavy smokers (≥30 cigarettes per day) should be closely monitored because variations in drug serum concentrations may be affected significantly by changes in smoking status.4,9,11 Dosage reductions of potentially toxic drugs should be done immediately when a heavy tobacco user stops smoking.10 For CYP1A2 substrates with a narrow therapeutic range, a conservative approach is to reduce the daily dose by 10% per day for several days after smoking cessation.11,16 The impact on drug metabolism may continue for weeks to a month after the person stops smoking; therefore, there may be a delay until CYP1A2 enzymes return to normal hepatic metabolism.4,8,9,15 In most situations, smoking cessation reverses induction of hepatic CYP1A2 enzymes back to normal metabolism and causes serum drug concentrations to increase.10 Because secondhand smoke induces hepatic CYP1A2 enzymes, those exposed to smoke may have changes in drug metabolism due to environmental smoke exposure.11
Tobacco smokers who take medications and hormones that are metabolized by CYP1A2 enzymes should be closely monitored because dosage adjustments may be necessary when they start or stop smoking cigarettes. An assessment of CYP drug interactions and routine monitoring of efficacy and/or toxicity should be done to avoid potential adverse effects from medications and to determine if changes in dosages and disease state management are required.
Related Resources
- Rx for Change. Drug interactions with smoking. http://smokingcessationleadership.ucsf.edu/interactions.pdf.
- Fiore MC, Baker TB. Treating smokers in the health care setting. N Engl J Med. 2011;365(13):1222-1231.
Drug Brand Names
- Albuterol/ipratropium • Combivent
- Almotriptan • Axert
- Alosetron • Lotronex
- Aminophylline • Phyllocontin, Truphylline
- Amitriptyline • Elavil
- Amlodipine • Norvasc
- Asenapine • Saphris
- Betaxolol • Kerlone
- Carbamazepine • Carbatrol, Tegretol
- Carvedilol • Coreg
- Chlorpromazine • Thorazine
- Chlorzoxazone • Parafon Forte
- Cimetidine • Tagamet
- Ciprofloxacin • Cipro
- Clomipramine • Anafranil
- Clopidogrel • Plavix
- Clozapine • Clozaril
- Cyclobenzaprine • Flexeril
- Desipramine • Norpramin
- Diazepam • Valium
- Diclofenac • Voltaren
- Diphenhydramine • Benadryl
- Doxepin • Silenor, Sinequan
- Duloxetine • Cymbalta
- Estradiol • Estrace
- Estrogens (conjugated) • Cenestin, Premarin
- Estropipate • Ogen
- Febuxostat • Uloric
- Fluoxetine • Prozac
- Fluphenazine • Prolixin
- Fluvoxamine • Luvox
- Frovatriptan • Frova
- Guanabenz • Wytensin
- Haloperidol • Haldol
- Imipramine • Tofranil
- Maprotiline • Ludiomil
- Metoclopramide • Reglan
- Mirtazapine • Remeron
- Nabumetone • Relafen
- Naratriptan • Amerge
- Nicardipine • Cardene
- Nifedipine • Adalat, Procardia
- Nortriptyline • Aventyl, Pamelor
- Olanzapine • Zyprexa
- Omeprazole • Prilosec
- Ondansetron • Zofran
- Palonosetron • Aloxi
- Perphenazine • Trilafon
- Pimozide • Orap
- Primidone • Mysoline
- Progesterone • Prometrium
- Propofol • Diprivan
- Propranolol • Inderal
- Ramelteon • Rozerem
- Ranitidine • Zantac
- Rasagiline • Azilect
- Rifampin • Rifadin, Rimactane
- Riluzole • Rilutek
- Rivastigmine • Exelon
- Ropinirole • Requip
- Selegiline • Eldepryl, EMSAM, others
- Theophylline • Elixophyllin
- Thioridazine • Mellaril
- Thiothixene • Navane
- Tizanidine • Zanaflex
- Trazodone • Desyrel, Oleptro
- Triamterene • Dyrenium
- Trifluoperazine • Stelazine
- Verapamil • Calan, Verelan
- Warfarin • Coumadin, Jantoven
- Zileuton • Zyflo
- Ziprasidone • Geodon
- Zolmitriptan • Zomig
- Zolpidem • Ambien, Edluar
Disclosure
Ms. Fankhauser reports no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
- Tobacco smokers often are treated with medications that are metabolized by hepatic cytochrome (CYP) 1A2 enzymes. Starting or stopping tobacco smoking may cause drug interactions because polycyclic aromatic hydrocarbons in cigarette smoke induce CYP1A2 enzymes.
- Drugs that are significantly metabolized by CYP1A2 (major substrates) are more likely to be impacted by changes in tobacco smoking compared with minor substrates.
- Induction of hepatic CYP1A2 enzymes may be greater in heavy or moderate smokers compared with light smokers (eg, <10 cigarettes per day).
- Evidence-based approaches for treating tobacco use in health care settings should address the risk of CYP1A2 drug interactions in tobacco smokers and how this impacts their clinical care.
Mrs. C, age 51, experiences exacerbated asthma and difficulty breathing and is admitted to a non-smoking hospital. She also has chronic obstructive pulmonary disease, type 2 diabetes mellitus, hypertension, hypercholesterolemia, hypothyroidism, gastroesophageal reflux disease, overactive bladder, muscle spasms, fibromyalgia, bipolar disorder, insomnia, and nicotine and caffeine dependence. She takes 19 prescribed and over-the-counter medications, drinks up to 8 cups of coffee per day, and smokes 20 to 30 cigarettes per day. In the emergency room, she receives albuterol/ipratropium inhalation therapy to help her breathing and a 21-mg nicotine replacement patch to avoid nicotine withdrawal.
In the United States, 19% of adults smoke cigarettes.1 Heavy tobacco smoking and nicotine dependence are common among psychiatric patients and contribute to higher rates of tobacco-related morbidity and mortality.2 When smokers stop smoking or are admitted to smoke-free facilities and are forced to abstain, nicotine withdrawal symptoms and changes in drug metabolism can develop over several days.3-5
Smokers such as Mrs. C are at risk for cytochrome (CYP) P450 drug interactions when they are admitted to or discharged from a smoke-free facility. Nine of Mrs. C’s medications are substrates of CYP1A2 (acetaminophen, caffeine, cyclobenzaprine, diazepam, duloxetine, melatonin, olanzapine, ondansetron, and zolpidem). When Mrs. C stops smoking while in the hospital, she could experience higher serum concentrations and adverse effects of these medications. If Mrs. C resumes smoking after bring discharged, metabolism and clearance of any medications started while she was hospitalized that are substrates of CYP1A2 enzymes could be increased, which could lead to reduced efficacy and poor clinical outcomes.
Pharmacokinetic effects
Polycyclic aromatic hydrocarbons in tobacco smoke induce hepatic CYP1A1, 1A2, and possibly 2E1 isoenzymes.6-12 CYP1A2 is a hepatic enzyme responsible for metabolizing and eliminating several classes of substrates (eg, drugs, hormones, endogenous compounds, and procarcinogens).6,13 Genetic, epigenetic, and environmental factors such as smoking impact the expression and activity of CYP1A2 and result in large interpatient variability in pharmacokinetic drug interactions.6,12,13 CYP1A2 enzymes can be induced or inhibited by drugs and substances, which can result in decreased or increased serum concentrations of substrates, respectively. When individuals stop smoking and switch to other nicotine products or devices, CYP1A2 induction of hepatic enzymes will revert to normal metabolism over several weeks to a month.10 Besides tobacco smoke, other CYP1A2 inducers include charbroiled food, carbamazepine, omeprazole, phenobarbital, primidone, and rifampin.4,5 Nicotine replacement products—such as gum, inhalers, lozenges, patches, and nasal spray—and nicotine delivery devices such as electronic cigarettes do not induce hepatic CYP1A2 enzymes or cause the same drug interactions as cigarette smoking.
Table 13-11 and Table 23-11 list commonly prescribed CYP1A2 substrates that could be affected by tobacco smoke. There are no specific guidelines for how to assess, monitor, or manage pharmacokinetic drug interactions with tobacco smoke.6-13 Induction of hepatic CYP1A2 enzymes by cigarette smoke may require increased dosages of some psychotropics—such as tricyclic antidepressants, duloxetine, mirtazapine, and some first- and second-generation antipsychotics (SGAs)—to achieve serum concentrations adequate for clinical efficacy. Serum concentrations may increase to toxic levels and result in adverse effects when a person quits smoking cigarettes or if a CYP1A2 inhibitor, such as amlodipine, cimetidine, ciprofloxacin, diclofenac, fluoxetine, fluvoxamine, or nifedipine, is added.5
Table 1
Common major cytochrome P450 (CYP) 1A2 substrates
| Drug | Class |
|---|---|
| Alosetron3,5,6 | Irritable bowel syndrome: serotonin 3 antagonist |
| Aminophylline3,5 | Bronchodilator: theophylline derivative |
| Betaxolol3,5 | β-1 selective adrenergic receptor blocking agent |
| Caffeine3-9 | Stimulant |
| Clomipramine3-11 | Tricyclic antidepressant |
| Clozapine3-10 | Second-generation antipsychotic |
| Cyclobenzaprine3-7 | Skeletal muscle relaxant |
| Doxepin3,7,10,11 | Tricyclic antidepressant |
| Duloxetine3-6 | Serotonin-norepinephrine reuptake inhibitor |
| Estradiol3,5-8 | Estrogen (active) |
| Estrogens: conjugated and estropipate3,5; estrone3,7 | Estrogen (derivatives) |
| Fluvoxamine3,8,9 | Selective serotonin reuptake inhibitor |
| Guanabenz3,5-7 | α-2 adrenergic agonist |
| Mirtazapine3-7 | Antidepressant: α-2 antagonist/serotonin 2A, 2C antagonist |
| Olanzapine3-11 | Second-generation antipsychotic |
| Pimozide3,5,7 | First-generation antipsychotic |
| Propranolol3-11 | β-adrenergic blocker |
| Ramelteon3,5,10 | Melatonin receptor agonist |
| Rasagiline3,5 | Antiparkinson: type B monoamine oxidase inhibitor |
| Riluzole3-7,10 | Glutamate inhibitor |
| Ropinirole3,5-7 | Antiparkinson: dopamine agonist |
| Theophylline3-6,8-11 | Bronchodilator: methylxanthine |
| Thiothixene3,5 | First-generation antipsychotic |
| Trifluoperazine3,5,9 | First-generation antipsychotic |
| Several classes of CYP1A2 substrates are not included and may cause toxicity with smoking cessation or require dosage increases in tobacco smokers (eg, antiarrhythmic, antifungal, antimalarial, antineoplastic, antiretroviral, and anthelmintic agents and the antibiotic quinolone). Clinicians should be most concerned about drugs with a narrow therapeutic index and those that may be toxic with smoking cessation (eg, bleeding from warfarin and clopidogrel; high serum concentrations of caffeine, clozapine, olanzapine, propranolol, and theophylline) | |
Table 2
Common minor cytochrome P450 (CYP) 1A2 substrates
| Drug | Class |
|---|---|
| Acetaminophen3-9 | Analgesic |
| Almotriptan6 | Antimigraine: serotonin 1B, 1D receptor agonist |
| Amitriptyline3-7,9-11 | Tricyclic antidepressant |
| Asenapine9 | Second-generation antipsychotic |
| Carvedilol5-7 | β and α adrenergic blocking activity |
| Chlorpromazine3,4,7-9,11 | First-generation antipsychotic |
| Chlorzoxazone4,7 | Skeletal muscle relaxant |
| Clopidogrel5 | Antiplatelet |
| Desipramine4,7,10,11 | Tricyclic antidepressant |
| Diazepam4,7,9,10 | Benzodiazepine |
| Diclofenac5,7 | Nonsteroidal anti-inflammatory drug |
| Diphenhydramine6 | Antihistamine |
| Febuxostat5 | Xanthine oxidase inhibitor |
| Fluphenazine3,9 | First-generation antipsychotic |
| Frovatriptan3 | Antimigraine: serotonin 1 agonist |
| Haloperidol3,4,6,8,9 | First-generation antipsychotic |
| Imipramine3,4,6-11 | Tricyclic antidepressant |
| Maprotiline6 | Tetracyclic antidepressant |
| Melatonin3,4,6,7 | Sleep-regulating hormone |
| Metoclopramide3 | Antiemetic: prokinetic gastrointestinal agent |
| Nabumetone6 | Nonsteroidal anti-inflammatory drug |
| Naproxen3,4,6,7 | Nonsteroidal anti-inflammatory drug |
| Naratriptan10 | Antimigraine: serotonin 1B, 1D receptor agonist |
| Nicardipine3,7 | Calcium channel blocker |
| Nortriptyline4,6,7,9-11 | Tricyclic antidepressant |
| Ondansetron3,4,6,7 | Antiemetic: serotonin 3 antagonist |
| Palonosetron5 | Antiemetic: serotonin 3 antagonist |
| Perphenazine3,7 | First-generation antipsychotic |
| Progesterone5,7 | Progestin |
| Propofol4,6,7 | General anesthetic |
| Ranitidine5,7 | H2 antagonist |
| Rivastigmine10 | Acetylcholinesterase inhibitor |
| Selegiline6,7 | Antiparkinson: type B monoamine oxidase inhibitor |
| Thioridazine3,4,6 | First-generation antipsychotic |
| Tizanidine3-6 | Skeletal muscle relaxant: α-2 adrenergic agonist |
| Trazodone6,9 | Serotonin reuptake inhibitor and antagonist |
| Triamterene6 | Diuretic: potassium sparing |
| Verapamil3,4,6,7,10 | Calcium channel blocker |
| Warfarin3,4,6-10 | Anticoagulant: coumarin derivative |
| Zileuton3,4,6,7 | Asthma agent: 5-lipoxygenase inhibitor |
| Ziprasidone3,4 | Second-generation antipsychotic |
| Zolmitriptan3,6,7 | Antimigraine: serotonin 1B, 1D receptor agonist |
| Zolpidem4,6,7 | Nonbenzodiazepine hypnotic |
| Several classes of CYP1A2 substrates are not included and may cause toxicity with smoking cessation or require dosage increases in tobacco smokers (eg, antiarrhythmic, antifungal, antimalarial, antineoplastic, antiretroviral and anthelmintic agents and the antibiotic quinolone). Clinicians should be most concerned about drugs with a narrow therapeutic index and those that may be toxic with smoking cessation (eg, bleeding from warfarin and clopidogrel; high serum concentrations of caffeine, clozapine, olanzapine, propranolol, and theophylline) | |
SGA such as clozapine and olanzapine are major substrates of CYP1A2 and dosages may need to be adjusted when smoking status changes, depending on clinical efficacy and adverse effects.10,14,15 Maximum induction of clozapine and olanzapine metabolism may occur with 7 to 12 cigarettes per day and smokers may have 40% to 50% lower serum concentrations compared with nonsmokers.14 When a patient stops smoking, clozapine and olanzapine dosages may need to be reduced by 30% to 40% (eg, a stepwise 10% reduction in daily dose until day 4) to avoid elevated serum concentrations and risk of toxicity symptoms.15
Tobacco smokers can tolerate high daily intake of caffeinated beverages because of increased metabolism and clearance of caffeine, a major substrate of CYP1A2.11 When patients stop smoking, increased caffeine serum concentrations may cause anxiety, irritability, restlessness, insomnia, tremors, palpitations, and tachycardia. Caffeine toxicity also can mimic symptoms of nicotine withdrawal; therefore, smokers should be advised to reduce their caffeine intake by half to avoid adverse effects when they stop smoking.10,11
Adjusting dosing
Factors such as the amount and frequency of tobacco smoking, how quickly CYP1A2 enzymes change when starting and stopping smoking, exposure to secondhand smoke, and other concomitant drugs contribute to variability in pharmacokinetic drug interactions. Heavy smokers (≥30 cigarettes per day) should be closely monitored because variations in drug serum concentrations may be affected significantly by changes in smoking status.4,9,11 Dosage reductions of potentially toxic drugs should be done immediately when a heavy tobacco user stops smoking.10 For CYP1A2 substrates with a narrow therapeutic range, a conservative approach is to reduce the daily dose by 10% per day for several days after smoking cessation.11,16 The impact on drug metabolism may continue for weeks to a month after the person stops smoking; therefore, there may be a delay until CYP1A2 enzymes return to normal hepatic metabolism.4,8,9,15 In most situations, smoking cessation reverses induction of hepatic CYP1A2 enzymes back to normal metabolism and causes serum drug concentrations to increase.10 Because secondhand smoke induces hepatic CYP1A2 enzymes, those exposed to smoke may have changes in drug metabolism due to environmental smoke exposure.11
Tobacco smokers who take medications and hormones that are metabolized by CYP1A2 enzymes should be closely monitored because dosage adjustments may be necessary when they start or stop smoking cigarettes. An assessment of CYP drug interactions and routine monitoring of efficacy and/or toxicity should be done to avoid potential adverse effects from medications and to determine if changes in dosages and disease state management are required.
Related Resources
- Rx for Change. Drug interactions with smoking. http://smokingcessationleadership.ucsf.edu/interactions.pdf.
- Fiore MC, Baker TB. Treating smokers in the health care setting. N Engl J Med. 2011;365(13):1222-1231.
Drug Brand Names
- Albuterol/ipratropium • Combivent
- Almotriptan • Axert
- Alosetron • Lotronex
- Aminophylline • Phyllocontin, Truphylline
- Amitriptyline • Elavil
- Amlodipine • Norvasc
- Asenapine • Saphris
- Betaxolol • Kerlone
- Carbamazepine • Carbatrol, Tegretol
- Carvedilol • Coreg
- Chlorpromazine • Thorazine
- Chlorzoxazone • Parafon Forte
- Cimetidine • Tagamet
- Ciprofloxacin • Cipro
- Clomipramine • Anafranil
- Clopidogrel • Plavix
- Clozapine • Clozaril
- Cyclobenzaprine • Flexeril
- Desipramine • Norpramin
- Diazepam • Valium
- Diclofenac • Voltaren
- Diphenhydramine • Benadryl
- Doxepin • Silenor, Sinequan
- Duloxetine • Cymbalta
- Estradiol • Estrace
- Estrogens (conjugated) • Cenestin, Premarin
- Estropipate • Ogen
- Febuxostat • Uloric
- Fluoxetine • Prozac
- Fluphenazine • Prolixin
- Fluvoxamine • Luvox
- Frovatriptan • Frova
- Guanabenz • Wytensin
- Haloperidol • Haldol
- Imipramine • Tofranil
- Maprotiline • Ludiomil
- Metoclopramide • Reglan
- Mirtazapine • Remeron
- Nabumetone • Relafen
- Naratriptan • Amerge
- Nicardipine • Cardene
- Nifedipine • Adalat, Procardia
- Nortriptyline • Aventyl, Pamelor
- Olanzapine • Zyprexa
- Omeprazole • Prilosec
- Ondansetron • Zofran
- Palonosetron • Aloxi
- Perphenazine • Trilafon
- Pimozide • Orap
- Primidone • Mysoline
- Progesterone • Prometrium
- Propofol • Diprivan
- Propranolol • Inderal
- Ramelteon • Rozerem
- Ranitidine • Zantac
- Rasagiline • Azilect
- Rifampin • Rifadin, Rimactane
- Riluzole • Rilutek
- Rivastigmine • Exelon
- Ropinirole • Requip
- Selegiline • Eldepryl, EMSAM, others
- Theophylline • Elixophyllin
- Thioridazine • Mellaril
- Thiothixene • Navane
- Tizanidine • Zanaflex
- Trazodone • Desyrel, Oleptro
- Triamterene • Dyrenium
- Trifluoperazine • Stelazine
- Verapamil • Calan, Verelan
- Warfarin • Coumadin, Jantoven
- Zileuton • Zyflo
- Ziprasidone • Geodon
- Zolmitriptan • Zomig
- Zolpidem • Ambien, Edluar
Disclosure
Ms. Fankhauser reports no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Centers for Disease Control and Prevention (CDC). Vital signs: current cigarette smoking among adults aged ≥18 years—United States 2005-2010. MMWR Morb Mortal Wkly Rep. 2011;60(35):1207-1212.
2. Ziedonis D, Hitsman B, Beckham JC, et al. Tobacco use and cessation in psychiatric disorders: National Institute of Mental Health report. Nicotine Tob Res. 2008;10(12):1691-1715.
3. Choe JY. Drug actions and interactions. New York NY: McGraw-Hill Medical; 2011.
4. Tatro DS. Drug interaction facts. St. Louis MO: Wolters Kluwer Health; 2011.
5. Lacy CF, Armstrong LL, Goldman MP, et al. eds. Drug information handbook, 20th ed. Hudson, OH: Lexicomp; 2011.
6. Zhou SF, Yang LP, Zhou ZW, et al. Insights into the substrate specificity, inhibitors, regulation, and polymorphisms and the clinical impact of human cytochrome P450 1A2. AAPS J. 2009;11(3):481-494.
7. Rendic S. Summary of information on human CYP enzymes: human P450 metabolism data. Drug Metab Rev. 2002;34(1-2):83-448.
8. Zevin S, Benowitz NL. Drug interactions with tobacco smoking. An update. Clin Pharmacokinet. 1999;36(6):425-438.
9. Desai HD, Seabolt J, Jann MW. Smoking in patients receiving psychotropic medications: a pharmacokinetic perspective. CNS Drugs. 2001;15(6):469-494.
10. Schaffer SD, Yoon S, Zadezensky I. A review of smoking cessation: potentially risky effects on prescribed medications. J Clin Nurs. 2009;18(11):1533-1540.
11. Kroon LA. Drug interactions with smoking. Am J Health Syst Pharm. 2007;64(18):1917-1921.
12. Plowchalk DR, Yeo KR. Prediction of drug clearance in a smoking population: modeling the impact of variable cigarette consumption on the induction of CYP1A2. Eur J Pharmacol. 2012;68(6):951-960.
13. Faber MS, Jetter A, Fuhr U. Assessment of CYP1A2 activity in clinical practice: why how, and when? Basic Clin Pharmacol Toxicol. 2005;97(3):125-134.
14. Haslemo T, Eikeseth PH, Tanum L, et al. The effect of variable cigarette consumption on the interaction with clozapine and olanzapine. Eur J Clin Pharmacol. 2006;62(12):1049-1053.
15. Lowe EJ, Ackman ML. Impact of tobacco smoking cessation on stable clozapine or olanzapine treatment. Ann Pharmacother. 2010;44(4):727-732.
16. Faber MS, Fuhr U. Time response of cytochrome P4501A2 activity on cessation of heavy smoking. Clin Pharmacol Ther. 2004;76(2):178-184.
1. Centers for Disease Control and Prevention (CDC). Vital signs: current cigarette smoking among adults aged ≥18 years—United States 2005-2010. MMWR Morb Mortal Wkly Rep. 2011;60(35):1207-1212.
2. Ziedonis D, Hitsman B, Beckham JC, et al. Tobacco use and cessation in psychiatric disorders: National Institute of Mental Health report. Nicotine Tob Res. 2008;10(12):1691-1715.
3. Choe JY. Drug actions and interactions. New York NY: McGraw-Hill Medical; 2011.
4. Tatro DS. Drug interaction facts. St. Louis MO: Wolters Kluwer Health; 2011.
5. Lacy CF, Armstrong LL, Goldman MP, et al. eds. Drug information handbook, 20th ed. Hudson, OH: Lexicomp; 2011.
6. Zhou SF, Yang LP, Zhou ZW, et al. Insights into the substrate specificity, inhibitors, regulation, and polymorphisms and the clinical impact of human cytochrome P450 1A2. AAPS J. 2009;11(3):481-494.
7. Rendic S. Summary of information on human CYP enzymes: human P450 metabolism data. Drug Metab Rev. 2002;34(1-2):83-448.
8. Zevin S, Benowitz NL. Drug interactions with tobacco smoking. An update. Clin Pharmacokinet. 1999;36(6):425-438.
9. Desai HD, Seabolt J, Jann MW. Smoking in patients receiving psychotropic medications: a pharmacokinetic perspective. CNS Drugs. 2001;15(6):469-494.
10. Schaffer SD, Yoon S, Zadezensky I. A review of smoking cessation: potentially risky effects on prescribed medications. J Clin Nurs. 2009;18(11):1533-1540.
11. Kroon LA. Drug interactions with smoking. Am J Health Syst Pharm. 2007;64(18):1917-1921.
12. Plowchalk DR, Yeo KR. Prediction of drug clearance in a smoking population: modeling the impact of variable cigarette consumption on the induction of CYP1A2. Eur J Pharmacol. 2012;68(6):951-960.
13. Faber MS, Jetter A, Fuhr U. Assessment of CYP1A2 activity in clinical practice: why how, and when? Basic Clin Pharmacol Toxicol. 2005;97(3):125-134.
14. Haslemo T, Eikeseth PH, Tanum L, et al. The effect of variable cigarette consumption on the interaction with clozapine and olanzapine. Eur J Clin Pharmacol. 2006;62(12):1049-1053.
15. Lowe EJ, Ackman ML. Impact of tobacco smoking cessation on stable clozapine or olanzapine treatment. Ann Pharmacother. 2010;44(4):727-732.
16. Faber MS, Fuhr U. Time response of cytochrome P4501A2 activity on cessation of heavy smoking. Clin Pharmacol Ther. 2004;76(2):178-184.
Vitamin deficiencies and mental health: How are they linked?
Discuss this article at www.facebook.com/CurrentPsychiatry
Patients today often are overfed but undernourished. A growing body of literature links dietary choices to brain health and the risk of psychiatric illness. Vitamin deficiencies can affect psychiatric patients in several ways:
- deficiencies may play a causative role in mental illness and exacerbate symptoms
- psychiatric symptoms can result in poor nutrition
- vitamin insufficiency—defined as subclinical deficiency—may compromise patient recovery.
Additionally, genetic differences may compromise vitamin and essential nutrient pathways.
Vitamins are dietary components other than carbohydrates, fats, minerals, and proteins that are necessary for life. B vitamins are required for proper functioning of the methylation cycle, monoamine production, DNA synthesis, and maintenance of phospholipids such as myelin (Figure). Fat-soluble vitamins A, D, and E play important roles in genetic transcription, antioxidant recycling, and inflammatory regulation in the brain.
Figure: The methylation cycle
Vitamins B2, B6, B9, and B12 directly impact the functioning of the methylation cycle. Deficiencies pertain to brain function, as neurotransmitters, myelin, and active glutathione are dependent on one-carbon metabolism
Illustration: Mala Nimalasuriya with permission from DrewRamseyMD.com
To help clinicians recognize and treat vitamin deficiencies among psychiatric patients, this article reviews the role of the 6 essential water-soluble vitamins (B1, B2, B6, B9, B12, and C; Table 1,1) and 3 fat-soluble vitamins (A, D, and E; Table 2,1) in brain metabolism and psychiatric pathology. Because numerous sources address using supplements to treat vitamin deficiencies, this article emphasizes food sources, which for many patients are adequate to sustain nutrient status.
Table 1
Water-soluble vitamins: Deficiency, insufficiency, symptoms, and dietary sources
| Deficiency | Insufficiency | Symptoms | At-risk patients | Dietary sources |
|---|---|---|---|---|
| B1 (thiamine): Glycolysis, tricarboxylic acid cycle | ||||
| Rare; 7% in heart failure patients | 5% total, 12% of older women | Wernicke-Korsakoff syndrome, memory impairment, confusion, lack of coordination, paralysis | Older adults, malabsorptive conditions, heavy alcohol use. Those with diabetes are at risk because of increased clearance | Pork, fish, beans, lentils, nuts, rice, and wheat germ. Raw fish, tea, and betel nuts impair absorption |
| B2 (riboflavin): FMN, FAD cofactors in glycolysis and oxidative pathways. B6, folate, and glutathione synthesis | ||||
| 10% to 27% of older adults | <3%; 95% of adolescent girls (measured by EGRAC) | Fatigue, cracked lips, sore throat, bloodshot eyes | Older adults, low intake of animal and dairy products, heavy alcohol use | Dairy, meat and fish, eggs, mushrooms, almonds, leafy greens, and legumes |
| B6 (pyridoxal): Methylation cycle | ||||
| 11% to 24% (<5 ng/mL); 38% of heart failure patients | 14% total, 26% of adults | Dermatitis, glossitis, convulsions, migraine, chronic pain, depression | Older adults, women who use oral contraceptives, alcoholism. 33% to 49% of women age >51 have inadequate intake | Bananas, beans, potatoes, navy beans, salmon, steak, and whole grains |
| B9 (folate): Methylation cycle | ||||
| 0.5% total; up to 50% of depressed patients | 16% of adults, 19% of adolescent girls | Loss of appetite, weight loss, weakness, heart palpitations, behavioral disorders | Depression, pregnancy and lactation, alcoholism, dialysis, liver disease. Deficiency during pregnancy is linked to neural tube defects | Leafy green vegetables, fruits, dried beans, and peas |
| B12 (cobalamin): Methylation cycle (cofactor methionine synthase) | ||||
| 10% to 15% of older adults | <3% to 9% | Depression, irritability, anemia, fatigue, shortness of breath, high blood pressure | Vegetarian or vegan diet, achlorhydria, older adults. Deficiency more often due to poor absorption than low consumption | Meat, seafood, eggs, and dairy |
| C (ascorbic acid): Antioxidant | ||||
| 7.1% | 31% | Scurvy, fatigue, anemia, joint pain, petechia. Symptoms develop after 1 to 3 months of no dietary intake | Smokers, infants fed boiled or evaporated milk, limited dietary variation, patients with malabsorption, chronic illnesses | Citrus fruits, tomatoes and tomato juice, and potatoes |
| EGRAC: erythrocyte glutathione reductase activation coefficient; FAD: flavin adenine dinucleotide; FMN: flavin mononucleotide Source: Reference 1 | ||||
Table 2
Fat-soluble vitamins: Deficiency, insufficiency, symptoms, and dietary sources
| Deficiency | Insufficiency | Symptoms | At-risk patients | Dietary sources |
|---|---|---|---|---|
| A (retinol): Transcription regulation, vision | ||||
| <5% of U.S. population | 44% | Blindness, decreased immunity, corneal and retinal damage | Pregnant women, individuals with strict dietary restrictions, heavy alcohol use, chronic diarrhea, fat malabsorptive conditions | Beef liver, dairy products. Convertible beta-carotene sources: sweet potatoes, carrots, spinach, butternut squash, greens, broccoli, cantaloupe |
| D (cholecalciferol): Hormone, transcriptional regulation | ||||
| ≥50%, 90% of adults age >50 | 69% | Rickets, osteoporosis, muscle twitching | Breast-fed infants, older adults, limited sun exposure, pigmented skin, fat malabsorption, obesity. Older adults have an impaired ability to make vitamin D from the sun. SPF 15 reduces production by 99% | Fatty fish and fish liver oils, sun-dried mushrooms |
| E (tocopherols and tocotrienols): Antioxidant, PUFA protectant, gene regulation | ||||
| Rare | 93% | Anemia, neuropathy, myopathy, abnormal eye movements, weakness, retinal damage | Malabsorptive conditions, HIV, depression | Sunflower, wheat germ, and safflower oils; meats; fish; dairy; green vegetables |
| HIV: human immunodeficiency virus; PUFA: polyunsaturated fatty acids; SPF: sun protection factor Source: Reference 1 | ||||
Water-soluble vitamins
Vitamin B1 (thiamine) is essential for glucose metabolism. Pregnancy, lactation, and fever increase the need for thiamine, and tea, coffee, and shellfish can impair its absorption. Although rare, severe B1 deficiency can lead to beriberi, Wernicke’s encephalopathy (confusion, ataxia, nystagmus), and Korsakoff’s psychosis (confabulation, lack of insight, retrograde and anterograde amnesia, and apathy). Confusion and disorientation stem from the brain’s inability to oxidize glucose for energy because B1 is a critical cofactor in glycolysis and the tricarboxylic acid cycle. Deficiency leads to an increase in reactive oxygen species, proinflammatory cytokines, and blood-brain barrier dysfunction.2 Wernicke’s encephalopathy is most frequently encountered in patients with chronic alcoholism, diabetes, or eating disorders, and after bariatric surgery.3 Iatrogenic Wernicke’s encephalopathy may occur when depleted patients receive IV saline with dextrose without receiving thiamine. Top dietary sources of B1 include pork, fish, beans, lentils, nuts, rice, and wheat germ.
Vitamin B2 (riboflavin) is essential for oxidative pathways, monoamine synthesis, and the methylation cycle. B2 is needed to create the essential flavoprotein coenzymes for synthesis of L-methylfolate—the active form of folate—and for proper utilization of B6. Deficiency can occur after 4 months of inadequate intake.
Although generally B2 deficiency is rare, surveys in the United States have found that 10% to 27% of older adults (age ≥65) are deficient.4 Low intake of dairy products and meat and chronic, excessive alcohol intake are associated with deficiency. Marginal B2 levels are more prevalent in depressed patients, possibly because of B2’s role in the function of glutathione, an endogenous antioxidant.5 Top dietary sources of B2 are dairy products, meat and fish, eggs, mushrooms, almonds, leafy greens, and legumes.
Vitamin B6 refers to 3 distinct compounds: pyridoxine, pyridoxal, and pyridoxamine. B6 is essential to glycolysis, the methylation cycle, and recharging glutathione, an innate antioxidant in the brain. Higher levels of vitamin B6 are associated with a lower prevalence of depression in adolescents,6 and low dietary and plasma B6 increases the risk and severity of depression in geriatric patients7 and predicts depression in prospective trials.8 Deficiency is common (24% to 56%) among patients receiving hemodialysis.9 Women who take oral contraceptives are at increased risk of vitamin B6 deficiency.10 Top dietary sources are fish, beef, poultry, potatoes, legumes, and spinach.
Vitamin B9 (folate) is needed for proper one-carbon metabolism and thus requisite in synthesis of serotonin, norepinephrine, dopamine, and DNA and in phospholipid production. Low maternal folate status increases the risk of neural tube defects in newborns. Folate deficiency and insufficiency are common among patients with mood disorders and correlate with illness severity.11 In a study of 2,682 Finnish men, those in the lowest one-third of folate consumption had a 67% increased relative risk of depression.12 A meta-analysis of 11 studies of 15,315 persons found those who had low folate levels had a significant risk of depression.13 Patients without deficiency but with folate levels near the low end of the normal range also report low mood.14 Compared with controls, patients experiencing a first episode of psychosis have lower levels of folate, B12, and docosahexaenoic acid.15
Dietary folate must be converted to L-methylfolate for use in the brain. Patients with a methylenetetrahydrofolate reductase (MTHFR) C677T polymorphism produce a less active form of the enzyme. The TT genotype is associated with major depression and bipolar disorder.16 Clinical trials have shown that several forms of folate can enhance antidepressant treatment.17 Augmentation with L-methylfolate, which bypasses the MTHFR enzyme, can be an effective strategy for treating depression in these patients.18
Leafy greens and legumes such as lentils are top dietary sources of folate; supplemental folic acid has been linked to an increased risk of cancer and overall mortality.19,20
Vitamin B12 (cobalamin). An essential cofactor in one-carbon metabolism, B12 is needed to produce monoamine neurotransmitters and maintain myelin. Deficiency is found in up to one-third of depressed patients11 and compromises antidepressant response,21 whereas higher vitamin B12 levels are associated with better treatment outcomes.22 B12 deficiency can cause depression, irritability, agitation, psychosis, and obsessive symptoms.23,24 Low B12 levels and elevated homocysteine increase the risk of cognitive decline and Alzheimer’s disease and are linked to a 5-fold increase in the rate of brain atrophy.26
B12 deficiencies may be seen in patients with gastrointestinal illness, older adults with achlorhydria, and vegans and vegetarians, in whom B12 intake can be low. Proton pump inhibitors such as omeprazole interfere with B12 absorption from food.
Psychiatric symptoms of B12 deficiency may present before hematologic findings.23 Folic acid supplementation may mask a B12 deficiency by delaying anemia but will not delay psychiatric symptoms. Ten percent of patients with an insufficiency (low normal levels of 200 to 400 pg/mL) have elevated homocysteine, which increases the risk of psychiatric disorders as well as comorbid illnesses such as cardiovascular disease. Top dietary sources include fish, mollusks (oysters, mussels, and clams), meat, and dairy products.
Vitamin C is vital for the synthesis of monoamines such as serotonin and norepinephrine. Vitamin C’s primary role in the brain is as an antioxidant. As a necessary cofactor, it keeps the copper and iron in metalloenzymes reduced, and also recycles vitamin E. Proper function of the methylation cycle depends on vitamin C, as does collagen synthesis and metabolism of xenobiotics by the liver. It is concentrated in cerebrospinal fluid.
Humans cannot manufacture vitamin C. Although the need for vitamin C (90 mg/d) is thought to be met by diet, studies have found that up to 13.7% of healthy, middle class patients in the United States are depleted.27 Older adults and patients with a poor diet due to drug or alcohol abuse, eating disorders, or affective symptoms are at risk.
Scurvy is caused by vitamin C deficiency and leads to bleeding gums and petechiae. Patients with insufficiency report irritability, loss of appetite, weight loss, and hypochondriasis. Vitamin C intake is significantly lower in older adults (age ≥60) with depression.28 Some research indicates patients with schizophrenia have decreased vitamin C levels and dysfunction of antioxidant defenses.29 Citrus, potatoes, and tomatoes are top dietary sources of vitamin C.
Fat-soluble vitamins
Vitamin A. Although vitamin A activity in the brain is poorly understood, retinol—the active form of vitamin A—is crucial for formation of opsins, which are the basis for vision. Childhood vitamin A deficiency may lead to blindness. Vitamin A also plays an important role in maintaining bone growth, reproduction, cell division, and immune system integrity.30 Animal sources such as beef liver, dairy products, and eggs provide retinol, and plant sources such as carrots, sweet potatoes, and leafy greens provide provitamin A carotenoids that humans convert into retinol.
Deficiency rarely is observed in the United States but remains a common problem for developing nations. In the United States, vitamin A deficiency is most often seen with excessive alcohol use, rigorous dietary restrictions, and gastrointestinal diseases accompanied by poor fat absorption.
Excess vitamin A ingestion may result in bone abnormalities, liver damage, birth defects, and depression. Isotretinoin—a form of vitamin A used to treat severe acne—carries an FDA “black-box” warning for psychiatric adverse effects, including aggression, depression, psychosis, and suicide.
Vitamin D is produced from cholesterol in the epidermis through exposure to sunlight, namely ultraviolet B radiation. After dermal synthesis or ingestion, vitamin D is converted through a series of steps into the active form of vitamin D, calcitriol, which also is known as 25(OH)D3.
Although vitamin D is known for its role in bone growth and mineralization,31 increasing evidence reveals vitamin D’s role in brain function and development.32 Both glial and neuronal cells possess vitamin D receptors in the hippocampus, prefrontal cortex, hypothalamus, thalamus, and substantia nigra—all regions theorized to be linked to depression pathophysiology.33 A review of the association of vitamin D deficiency and psychiatric illnesses will be published in a future issue of Current Psychiatry.
Vitamin D exists in food as either D2 or D3, from plant and animal sources, respectively. Concentrated sources include oily fish, sun-dried or “UVB-irradiated” mushrooms, and milk.
Vitamin E. There are 8 isoforms of vitamin E—4 tocopherols and 4 tocotrienols—that function as fat-soluble antioxidants and also promote innate antioxidant enzymes. Because vitamin E protects neuronal membranes from oxidation, low levels may affect the brain via increased inflammation. Alpha-tocopherol is the most common form of vitamin E in humans, but emerging evidence suggests tocotrienols mediate disease by modifying transcription factors in the brain, such as glutathione reductase, superoxide dismutase, and nuclear factor-kappaB.34 Low plasma vitamin E levels are found in depressed patients, although some data suggest this may be caused by factors other than dietary intake.35 Low vitamin status has been found in up to 70% of older adults.36 Although deficiency is rare, most of the U.S. population (93%) has inadequate dietary intake of vitamin E.1 The reasons for this discrepancy are unclear. Foods rich in vitamin E include almonds, sunflower seeds, leafy greens, and wheat germ.
Recommendations
Patients with depression, alcohol abuse, eating disorders, obsessive-compulsive disorder, or schizophrenia may neglect to care for themselves or adopt particular eating patterns. Deficiencies are more common among geriatric patients and those who are medically ill. Because dietary patterns are linked to the risk of psychiatric disorders, nutritional inquiry often identifies multiple modifiable risk factors, such as folate, vitamin B12, and vitamin D intake.37,38 Nutritional counseling offers clinicians an intervention with minimal side effect risks and the opportunity to modify a behavior that patients engage in 3 times a day.
Psychiatrists should assess patients’ dietary patterns and vitamin status, particularly older adults and those with:
- lower socioeconomic status or food insecurity
- a history of treatment resistance
- restrictive dietary patterns such as veganism
- alcohol abuse.
On initial assessment, test or obtain from other health care providers your patient’s blood levels of folate and vitamins D and B12. In some patients, assessing B2 and B6 levels may provide etiological guidance regarding onset of psychiatric symptoms or failure to respond to pharmacologic treatment. Because treating vitamin deficiencies often includes using supplements, evaluate recent reviews of specific deficiencies and consider consulting with the patient’s primary care provider.
Conduct a simple assessment of dietary patterns by asking patients about a typical breakfast, lunch, and dinner, their favorite snacks and foods, and specific dietary habits or restrictions (eg, not consuming seafood, dairy, meat, etc.). Rudimentary nutritional recommendations can be effective in changing a patient’s eating habits, particularly when provided by a physician. Encourage patients to eat nutrient-dense foods such as leafy greens, beans and legumes, seafood, whole grains, and a variety of vegetables and fruits. For more complex patients, consult with a clinical nutritionist.
Related Resources
- Institute of Medicine. Dietary Reference Intakes: Recommended intakes for individuals. SummaryDRIs/~/media/Files
/Activity%20Files/Nutrition
/DRIs/5_Summary%20Table%20Tables%201-4.pdf" target="_blank">www.iom.edu/Activities/Nutrition/
SummaryDRIs/~/media/Files
/Activity%20Files/Nutrition
/DRIs/5_Summary%20Table%20Tables%201-4.pdf. - The Farmacy: Vitamins. http://drewramseymd.com/index.php/resources/farmacy/category/vitamins.
- Office of Dietary Supplements. National Institutes of Health. Dietary supplements fact sheets. http://ods.od.nih.gov/factsheets/list-all.
- Oregon State University. Linus Pauling Institute. Micronutrient information center. http://lpi.oregonstate.edu/infocenter/vitamins.html.
Drug Brand Names
- Isotretinoin • Accutane
- L-methylfolate • Deplin
- Omeprazole • Prilosec
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Moshfegh A, Goldman J, Cleveland L. United States Department of Agriculture, Agricultural Research Service. What we eat in America NHANES 2001-2002: Usual nutrient intakes from food compared to dietary reference intakes. http://www.ars.usda.gov/SP2UserFiles/Place/12355000/pdf/0102/usualintaketables2001-02.pdf. Published September 2005. Accessed November 27, 2012.
2. Page GL, Laight D, Cummings MH. Thiamine deficiency in diabetes mellitus and the impact of thiamine replacement on glucose metabolism and vascular disease. Int J Clin Pract. 2011;65(6):684-690.
3. McCormick LM, Buchanan JR, Onwuameze OE, et al. Beyond alcoholism: Wernicke-Korsakoff syndrome in patients with psychiatric disorders. Cogn Behav Neurol. 2011;24(4):209-216.
4. Powers HJ. Riboflavin (vitamin B-2) and health. Am J Clin Nutr. 2003;77(6):1352-1360.
5. Naghashpour M, Amani R, Nutr R, et al. Riboflavin status and its association with serum hs-CRP levels among clinical nurses with depression. J Am Coll Nutr. 2011;30(5):340-347.
6. Murakami K, Miyake Y, Sasaki S, et al. Dietary folate, riboflavin, vitamin B-6, and vitamin B-12 and depressive symptoms in early adolescence: the Ryukyus Child Health Study. Psychosom Med. 2010;72(8):763-768.
7. Merete C, Falcon LM, Tucker KL. Vitamin B6 is associated with depressive symptomatology in Massachusetts elders. J Am Coll Nutr. 2008;27(3):421-427.
8. Skarupski KA, Tangney C, Li H, et al. Longitudinal association of vitamin B-6, folate, and vitamin B-12 with depressive symptoms among older adults over time. Am J Clin Nutr. 2010;92(2):330-335.
9. Corken M, Porter J. Is vitamin B(6) deficiency an under-recognized risk in patients receiving haemodialysis? A systematic review: 2000-2010. Nephrology (Carlton). 2011;16(7):619-625.
10. Wilson SM, Bivins BN, Russell KA, et al. Oral contraceptive use: impact on folate, vitamin B6, and vitamin B12 status. Nutr Rev. 2011;69(10):572-583.
11. Coppen A, Bolander-Gouaille C. Treatment of depression: time to consider folic acid and vitamin B12. J Psychopharmacol. 2005;19(1):59-65.
12. Tolmunen T, Voutilainen S, Hintikka J, et al. Dietary folate and depressive symptoms are associated in middle-aged Finnish men. J Nutr. 2003;133(10):3233-3236.
13. Gilbody S, Lightfoot T, Sheldon T. Is low folate a risk factor for depression? A meta-analysis and exploration of heterogeneity. J Epidemiol Community Health. 2007;61(7):631-637.
14. Rösche J, Uhlmann C, Fröscher W. Low serum folate levels as a risk factor for depressive mood in patients with chronic epilepsy. J Neuropsychiatry Clin Neurosci. 2003;15(1):64-66.
15. Kale A, Naphade N, Sapkale S, et al. Reduced folic acid, vitamin B12 and docosahexaenoic acid and increased homocysteine and cortisol in never-medicated schizophrenia patients: implications for altered one-carbon metabolism. Psychiatry Res. 2010;175(1-2):47-53.
16. Gilbody S, Lewis S, Lightfoot T. Methylenetetrahydrofolate reductase (MTHFR) genetic polymorphisms and psychiatric disorders: a HuGE review. Am J Epidemiol. 2007;165(1):1-13.
17. Di Palma C, Urani R, Agricola R, et al. Is methylfolate effective in relieving major depression in chronic alcoholics? A hypothesis of treatment. Curr Ther Res Clin Exp. 1994;55(5):559-568.
18. Papakostas GI, Shelton RC, Zajecka JM, et al. l-Methylfolate as adjunctive therapy for ssri-resistant major depression: results of two randomized, double-blind, parallel-sequential trials. Am J Psychiatry. 2012;169(12):1267-1274.
19. Baggott JE, Oster RA, Tamura T. Meta-analysis of cancer risk in folic acid supplementation trials. Cancer Epidemiol. 2012;36(1):78-81.
20. Figueiredo JC, Grau MV, Haile RW, et al. Folic acid and risk of prostate cancer: results from a randomized clinical trial. J Natl Cancer Inst. 2009;101(6):432-435.
21. Kate N, Grover S, Agarwal M. Does B12 deficiency lead to lack of treatment response to conventional antidepressants? Psychiatry (Edgmont). 2010;7(11):42-44.
22. Hintikka J, Tolmunen T, Tanskanen A, et al. High vitamin B12 level and good treatment outcome may be associated in major depressive disorder. BMC Psychiatry. 2003;3:17.-
23. Lindenbaum J, Healton EB, Savage DG, et al. Neuropsychiatric disorders caused by cobalamin deficiency in the absence of anemia or macrocytosis. N Engl J Med. 1988;318(26):1720-1728.
24. Bar-Shai M, Gott D, Marmor S. Acute psychotic depression as a sole manifestation of vitamin B12 deficiency. Psychosomatics. 2011;52(4):384-386.
25. Sharma V, Biswas D. Cobalamin deficiency presenting as obsessive compulsive disorder: case report. Gen Hosp Psychiatry. 2012;34(5):578.e7-e8.
26. Vogiatzoglou A, Refsum H, Johnston C, et al. Vitamin B12 status and rate of brain volume loss in community-dwelling elderly. Neurology. 2008;71(11):826-832.
27. Smith A, Di Primio G, Humphrey-Murto S. Scurvy in the developed world. CMAJ. 2011;183(11):E752-E725.
28. Payne ME, Steck SE, George RR, et al. Fruit, vegetable, and antioxidant intakes are lower in older adults with depression. J Acad Nutr Diet. 2012;112(12):2022-2027.
29. Dadheech G, Mishra S, Gautam S, et al. Oxidative stress, α-tocopherol, ascorbic acid and reduced glutathione status in schizophrenics. Indian J Clin Biochem. 2006;21(2):34-38.
30. Hinds TS, West WL, Knight EM. Carotenoids and retinoids: a review of research clinical, and public health applications. J Clin Pharmacol. 1997;37(7):551-558.
31. Thacher TD, Clarke BL. Vitamin D insufficiency. Mayo Clin Proc. 2011;86(1):50-60.
32. Berk M, Sanders KM, Pasco JA, et al. Vitamin D deficiency may play a role in depression. Med Hypotheses. 2007;69(6):1316-1319.
33. Eyles DW, Smith S, Kinobe R, et al. Distribution of the vitamin D receptor and 1 alpha-hydroxylase in human brain. J Chem Neuroanat. 2005;29(1):21-30.
34. Sen CK, Khanna S, Roy S. Tocotrienol: the natural vitamin E to defend the nervous system? Ann N Y Acad Sci. 2004;1031:127-142.
35. Owen AJ, Batterham MJ, Probst YC, et al. Low plasma vitamin E levels in major depression: diet or disease? Eur J Clin Nutr. 2005;59(2):304-306.
36. Panemangalore M, Lee CJ. Evaluation of the indices of retinol and alpha-tocopherol status in free-living elderly. J Gerontol. 1992;47(3):B98-B104.
37. Sánchez-Villegas A, Delgado-Rodríguez M, Alonso A, et al. Association of the Mediterranean dietary pattern with the incidence of depression: the Seguimiento Universidad de Navarra/University of Navarra follow-up (SUN) cohort. Arch Gen Psychiatry. 2009;66(10):1090-1098.
38. Jacka FN, Pasco JA, Mykletun A, et al. Association of Western and traditional diets with depression and anxiety in women. Am J Psychiatry. 2010;167(3):305-311.
Discuss this article at www.facebook.com/CurrentPsychiatry
Patients today often are overfed but undernourished. A growing body of literature links dietary choices to brain health and the risk of psychiatric illness. Vitamin deficiencies can affect psychiatric patients in several ways:
- deficiencies may play a causative role in mental illness and exacerbate symptoms
- psychiatric symptoms can result in poor nutrition
- vitamin insufficiency—defined as subclinical deficiency—may compromise patient recovery.
Additionally, genetic differences may compromise vitamin and essential nutrient pathways.
Vitamins are dietary components other than carbohydrates, fats, minerals, and proteins that are necessary for life. B vitamins are required for proper functioning of the methylation cycle, monoamine production, DNA synthesis, and maintenance of phospholipids such as myelin (Figure). Fat-soluble vitamins A, D, and E play important roles in genetic transcription, antioxidant recycling, and inflammatory regulation in the brain.

Figure: The methylation cycle
Vitamins B2, B6, B9, and B12 directly impact the functioning of the methylation cycle. Deficiencies pertain to brain function, as neurotransmitters, myelin, and active glutathione are dependent on one-carbon metabolism
Illustration: Mala Nimalasuriya with permission from DrewRamseyMD.com
To help clinicians recognize and treat vitamin deficiencies among psychiatric patients, this article reviews the role of the 6 essential water-soluble vitamins (B1, B2, B6, B9, B12, and C; Table 1,1) and 3 fat-soluble vitamins (A, D, and E; Table 2,1) in brain metabolism and psychiatric pathology. Because numerous sources address using supplements to treat vitamin deficiencies, this article emphasizes food sources, which for many patients are adequate to sustain nutrient status.
Table 1
Water-soluble vitamins: Deficiency, insufficiency, symptoms, and dietary sources
| Deficiency | Insufficiency | Symptoms | At-risk patients | Dietary sources |
|---|---|---|---|---|
| B1 (thiamine): Glycolysis, tricarboxylic acid cycle | ||||
| Rare; 7% in heart failure patients | 5% total, 12% of older women | Wernicke-Korsakoff syndrome, memory impairment, confusion, lack of coordination, paralysis | Older adults, malabsorptive conditions, heavy alcohol use. Those with diabetes are at risk because of increased clearance | Pork, fish, beans, lentils, nuts, rice, and wheat germ. Raw fish, tea, and betel nuts impair absorption |
| B2 (riboflavin): FMN, FAD cofactors in glycolysis and oxidative pathways. B6, folate, and glutathione synthesis | ||||
| 10% to 27% of older adults | <3%; 95% of adolescent girls (measured by EGRAC) | Fatigue, cracked lips, sore throat, bloodshot eyes | Older adults, low intake of animal and dairy products, heavy alcohol use | Dairy, meat and fish, eggs, mushrooms, almonds, leafy greens, and legumes |
| B6 (pyridoxal): Methylation cycle | ||||
| 11% to 24% (<5 ng/mL); 38% of heart failure patients | 14% total, 26% of adults | Dermatitis, glossitis, convulsions, migraine, chronic pain, depression | Older adults, women who use oral contraceptives, alcoholism. 33% to 49% of women age >51 have inadequate intake | Bananas, beans, potatoes, navy beans, salmon, steak, and whole grains |
| B9 (folate): Methylation cycle | ||||
| 0.5% total; up to 50% of depressed patients | 16% of adults, 19% of adolescent girls | Loss of appetite, weight loss, weakness, heart palpitations, behavioral disorders | Depression, pregnancy and lactation, alcoholism, dialysis, liver disease. Deficiency during pregnancy is linked to neural tube defects | Leafy green vegetables, fruits, dried beans, and peas |
| B12 (cobalamin): Methylation cycle (cofactor methionine synthase) | ||||
| 10% to 15% of older adults | <3% to 9% | Depression, irritability, anemia, fatigue, shortness of breath, high blood pressure | Vegetarian or vegan diet, achlorhydria, older adults. Deficiency more often due to poor absorption than low consumption | Meat, seafood, eggs, and dairy |
| C (ascorbic acid): Antioxidant | ||||
| 7.1% | 31% | Scurvy, fatigue, anemia, joint pain, petechia. Symptoms develop after 1 to 3 months of no dietary intake | Smokers, infants fed boiled or evaporated milk, limited dietary variation, patients with malabsorption, chronic illnesses | Citrus fruits, tomatoes and tomato juice, and potatoes |
| EGRAC: erythrocyte glutathione reductase activation coefficient; FAD: flavin adenine dinucleotide; FMN: flavin mononucleotide Source: Reference 1 | ||||
Table 2
Fat-soluble vitamins: Deficiency, insufficiency, symptoms, and dietary sources
| Deficiency | Insufficiency | Symptoms | At-risk patients | Dietary sources |
|---|---|---|---|---|
| A (retinol): Transcription regulation, vision | ||||
| <5% of U.S. population | 44% | Blindness, decreased immunity, corneal and retinal damage | Pregnant women, individuals with strict dietary restrictions, heavy alcohol use, chronic diarrhea, fat malabsorptive conditions | Beef liver, dairy products. Convertible beta-carotene sources: sweet potatoes, carrots, spinach, butternut squash, greens, broccoli, cantaloupe |
| D (cholecalciferol): Hormone, transcriptional regulation | ||||
| ≥50%, 90% of adults age >50 | 69% | Rickets, osteoporosis, muscle twitching | Breast-fed infants, older adults, limited sun exposure, pigmented skin, fat malabsorption, obesity. Older adults have an impaired ability to make vitamin D from the sun. SPF 15 reduces production by 99% | Fatty fish and fish liver oils, sun-dried mushrooms |
| E (tocopherols and tocotrienols): Antioxidant, PUFA protectant, gene regulation | ||||
| Rare | 93% | Anemia, neuropathy, myopathy, abnormal eye movements, weakness, retinal damage | Malabsorptive conditions, HIV, depression | Sunflower, wheat germ, and safflower oils; meats; fish; dairy; green vegetables |
| HIV: human immunodeficiency virus; PUFA: polyunsaturated fatty acids; SPF: sun protection factor Source: Reference 1 | ||||
Water-soluble vitamins
Vitamin B1 (thiamine) is essential for glucose metabolism. Pregnancy, lactation, and fever increase the need for thiamine, and tea, coffee, and shellfish can impair its absorption. Although rare, severe B1 deficiency can lead to beriberi, Wernicke’s encephalopathy (confusion, ataxia, nystagmus), and Korsakoff’s psychosis (confabulation, lack of insight, retrograde and anterograde amnesia, and apathy). Confusion and disorientation stem from the brain’s inability to oxidize glucose for energy because B1 is a critical cofactor in glycolysis and the tricarboxylic acid cycle. Deficiency leads to an increase in reactive oxygen species, proinflammatory cytokines, and blood-brain barrier dysfunction.2 Wernicke’s encephalopathy is most frequently encountered in patients with chronic alcoholism, diabetes, or eating disorders, and after bariatric surgery.3 Iatrogenic Wernicke’s encephalopathy may occur when depleted patients receive IV saline with dextrose without receiving thiamine. Top dietary sources of B1 include pork, fish, beans, lentils, nuts, rice, and wheat germ.
Vitamin B2 (riboflavin) is essential for oxidative pathways, monoamine synthesis, and the methylation cycle. B2 is needed to create the essential flavoprotein coenzymes for synthesis of L-methylfolate—the active form of folate—and for proper utilization of B6. Deficiency can occur after 4 months of inadequate intake.
Although generally B2 deficiency is rare, surveys in the United States have found that 10% to 27% of older adults (age ≥65) are deficient.4 Low intake of dairy products and meat and chronic, excessive alcohol intake are associated with deficiency. Marginal B2 levels are more prevalent in depressed patients, possibly because of B2’s role in the function of glutathione, an endogenous antioxidant.5 Top dietary sources of B2 are dairy products, meat and fish, eggs, mushrooms, almonds, leafy greens, and legumes.
Vitamin B6 refers to 3 distinct compounds: pyridoxine, pyridoxal, and pyridoxamine. B6 is essential to glycolysis, the methylation cycle, and recharging glutathione, an innate antioxidant in the brain. Higher levels of vitamin B6 are associated with a lower prevalence of depression in adolescents,6 and low dietary and plasma B6 increases the risk and severity of depression in geriatric patients7 and predicts depression in prospective trials.8 Deficiency is common (24% to 56%) among patients receiving hemodialysis.9 Women who take oral contraceptives are at increased risk of vitamin B6 deficiency.10 Top dietary sources are fish, beef, poultry, potatoes, legumes, and spinach.
Vitamin B9 (folate) is needed for proper one-carbon metabolism and thus requisite in synthesis of serotonin, norepinephrine, dopamine, and DNA and in phospholipid production. Low maternal folate status increases the risk of neural tube defects in newborns. Folate deficiency and insufficiency are common among patients with mood disorders and correlate with illness severity.11 In a study of 2,682 Finnish men, those in the lowest one-third of folate consumption had a 67% increased relative risk of depression.12 A meta-analysis of 11 studies of 15,315 persons found those who had low folate levels had a significant risk of depression.13 Patients without deficiency but with folate levels near the low end of the normal range also report low mood.14 Compared with controls, patients experiencing a first episode of psychosis have lower levels of folate, B12, and docosahexaenoic acid.15
Dietary folate must be converted to L-methylfolate for use in the brain. Patients with a methylenetetrahydrofolate reductase (MTHFR) C677T polymorphism produce a less active form of the enzyme. The TT genotype is associated with major depression and bipolar disorder.16 Clinical trials have shown that several forms of folate can enhance antidepressant treatment.17 Augmentation with L-methylfolate, which bypasses the MTHFR enzyme, can be an effective strategy for treating depression in these patients.18
Leafy greens and legumes such as lentils are top dietary sources of folate; supplemental folic acid has been linked to an increased risk of cancer and overall mortality.19,20
Vitamin B12 (cobalamin). An essential cofactor in one-carbon metabolism, B12 is needed to produce monoamine neurotransmitters and maintain myelin. Deficiency is found in up to one-third of depressed patients11 and compromises antidepressant response,21 whereas higher vitamin B12 levels are associated with better treatment outcomes.22 B12 deficiency can cause depression, irritability, agitation, psychosis, and obsessive symptoms.23,24 Low B12 levels and elevated homocysteine increase the risk of cognitive decline and Alzheimer’s disease and are linked to a 5-fold increase in the rate of brain atrophy.26
B12 deficiencies may be seen in patients with gastrointestinal illness, older adults with achlorhydria, and vegans and vegetarians, in whom B12 intake can be low. Proton pump inhibitors such as omeprazole interfere with B12 absorption from food.
Psychiatric symptoms of B12 deficiency may present before hematologic findings.23 Folic acid supplementation may mask a B12 deficiency by delaying anemia but will not delay psychiatric symptoms. Ten percent of patients with an insufficiency (low normal levels of 200 to 400 pg/mL) have elevated homocysteine, which increases the risk of psychiatric disorders as well as comorbid illnesses such as cardiovascular disease. Top dietary sources include fish, mollusks (oysters, mussels, and clams), meat, and dairy products.
Vitamin C is vital for the synthesis of monoamines such as serotonin and norepinephrine. Vitamin C’s primary role in the brain is as an antioxidant. As a necessary cofactor, it keeps the copper and iron in metalloenzymes reduced, and also recycles vitamin E. Proper function of the methylation cycle depends on vitamin C, as does collagen synthesis and metabolism of xenobiotics by the liver. It is concentrated in cerebrospinal fluid.
Humans cannot manufacture vitamin C. Although the need for vitamin C (90 mg/d) is thought to be met by diet, studies have found that up to 13.7% of healthy, middle class patients in the United States are depleted.27 Older adults and patients with a poor diet due to drug or alcohol abuse, eating disorders, or affective symptoms are at risk.
Scurvy is caused by vitamin C deficiency and leads to bleeding gums and petechiae. Patients with insufficiency report irritability, loss of appetite, weight loss, and hypochondriasis. Vitamin C intake is significantly lower in older adults (age ≥60) with depression.28 Some research indicates patients with schizophrenia have decreased vitamin C levels and dysfunction of antioxidant defenses.29 Citrus, potatoes, and tomatoes are top dietary sources of vitamin C.
Fat-soluble vitamins
Vitamin A. Although vitamin A activity in the brain is poorly understood, retinol—the active form of vitamin A—is crucial for formation of opsins, which are the basis for vision. Childhood vitamin A deficiency may lead to blindness. Vitamin A also plays an important role in maintaining bone growth, reproduction, cell division, and immune system integrity.30 Animal sources such as beef liver, dairy products, and eggs provide retinol, and plant sources such as carrots, sweet potatoes, and leafy greens provide provitamin A carotenoids that humans convert into retinol.
Deficiency rarely is observed in the United States but remains a common problem for developing nations. In the United States, vitamin A deficiency is most often seen with excessive alcohol use, rigorous dietary restrictions, and gastrointestinal diseases accompanied by poor fat absorption.
Excess vitamin A ingestion may result in bone abnormalities, liver damage, birth defects, and depression. Isotretinoin—a form of vitamin A used to treat severe acne—carries an FDA “black-box” warning for psychiatric adverse effects, including aggression, depression, psychosis, and suicide.
Vitamin D is produced from cholesterol in the epidermis through exposure to sunlight, namely ultraviolet B radiation. After dermal synthesis or ingestion, vitamin D is converted through a series of steps into the active form of vitamin D, calcitriol, which also is known as 25(OH)D3.
Although vitamin D is known for its role in bone growth and mineralization,31 increasing evidence reveals vitamin D’s role in brain function and development.32 Both glial and neuronal cells possess vitamin D receptors in the hippocampus, prefrontal cortex, hypothalamus, thalamus, and substantia nigra—all regions theorized to be linked to depression pathophysiology.33 A review of the association of vitamin D deficiency and psychiatric illnesses will be published in a future issue of Current Psychiatry.
Vitamin D exists in food as either D2 or D3, from plant and animal sources, respectively. Concentrated sources include oily fish, sun-dried or “UVB-irradiated” mushrooms, and milk.
Vitamin E. There are 8 isoforms of vitamin E—4 tocopherols and 4 tocotrienols—that function as fat-soluble antioxidants and also promote innate antioxidant enzymes. Because vitamin E protects neuronal membranes from oxidation, low levels may affect the brain via increased inflammation. Alpha-tocopherol is the most common form of vitamin E in humans, but emerging evidence suggests tocotrienols mediate disease by modifying transcription factors in the brain, such as glutathione reductase, superoxide dismutase, and nuclear factor-kappaB.34 Low plasma vitamin E levels are found in depressed patients, although some data suggest this may be caused by factors other than dietary intake.35 Low vitamin status has been found in up to 70% of older adults.36 Although deficiency is rare, most of the U.S. population (93%) has inadequate dietary intake of vitamin E.1 The reasons for this discrepancy are unclear. Foods rich in vitamin E include almonds, sunflower seeds, leafy greens, and wheat germ.
Recommendations
Patients with depression, alcohol abuse, eating disorders, obsessive-compulsive disorder, or schizophrenia may neglect to care for themselves or adopt particular eating patterns. Deficiencies are more common among geriatric patients and those who are medically ill. Because dietary patterns are linked to the risk of psychiatric disorders, nutritional inquiry often identifies multiple modifiable risk factors, such as folate, vitamin B12, and vitamin D intake.37,38 Nutritional counseling offers clinicians an intervention with minimal side effect risks and the opportunity to modify a behavior that patients engage in 3 times a day.
Psychiatrists should assess patients’ dietary patterns and vitamin status, particularly older adults and those with:
- lower socioeconomic status or food insecurity
- a history of treatment resistance
- restrictive dietary patterns such as veganism
- alcohol abuse.
On initial assessment, test or obtain from other health care providers your patient’s blood levels of folate and vitamins D and B12. In some patients, assessing B2 and B6 levels may provide etiological guidance regarding onset of psychiatric symptoms or failure to respond to pharmacologic treatment. Because treating vitamin deficiencies often includes using supplements, evaluate recent reviews of specific deficiencies and consider consulting with the patient’s primary care provider.
Conduct a simple assessment of dietary patterns by asking patients about a typical breakfast, lunch, and dinner, their favorite snacks and foods, and specific dietary habits or restrictions (eg, not consuming seafood, dairy, meat, etc.). Rudimentary nutritional recommendations can be effective in changing a patient’s eating habits, particularly when provided by a physician. Encourage patients to eat nutrient-dense foods such as leafy greens, beans and legumes, seafood, whole grains, and a variety of vegetables and fruits. For more complex patients, consult with a clinical nutritionist.
Related Resources
- Institute of Medicine. Dietary Reference Intakes: Recommended intakes for individuals. SummaryDRIs/~/media/Files
/Activity%20Files/Nutrition
/DRIs/5_Summary%20Table%20Tables%201-4.pdf" target="_blank">www.iom.edu/Activities/Nutrition/
SummaryDRIs/~/media/Files
/Activity%20Files/Nutrition
/DRIs/5_Summary%20Table%20Tables%201-4.pdf. - The Farmacy: Vitamins. http://drewramseymd.com/index.php/resources/farmacy/category/vitamins.
- Office of Dietary Supplements. National Institutes of Health. Dietary supplements fact sheets. http://ods.od.nih.gov/factsheets/list-all.
- Oregon State University. Linus Pauling Institute. Micronutrient information center. http://lpi.oregonstate.edu/infocenter/vitamins.html.
Drug Brand Names
- Isotretinoin • Accutane
- L-methylfolate • Deplin
- Omeprazole • Prilosec
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Discuss this article at www.facebook.com/CurrentPsychiatry
Patients today often are overfed but undernourished. A growing body of literature links dietary choices to brain health and the risk of psychiatric illness. Vitamin deficiencies can affect psychiatric patients in several ways:
- deficiencies may play a causative role in mental illness and exacerbate symptoms
- psychiatric symptoms can result in poor nutrition
- vitamin insufficiency—defined as subclinical deficiency—may compromise patient recovery.
Additionally, genetic differences may compromise vitamin and essential nutrient pathways.
Vitamins are dietary components other than carbohydrates, fats, minerals, and proteins that are necessary for life. B vitamins are required for proper functioning of the methylation cycle, monoamine production, DNA synthesis, and maintenance of phospholipids such as myelin (Figure). Fat-soluble vitamins A, D, and E play important roles in genetic transcription, antioxidant recycling, and inflammatory regulation in the brain.

Figure: The methylation cycle
Vitamins B2, B6, B9, and B12 directly impact the functioning of the methylation cycle. Deficiencies pertain to brain function, as neurotransmitters, myelin, and active glutathione are dependent on one-carbon metabolism
Illustration: Mala Nimalasuriya with permission from DrewRamseyMD.com
To help clinicians recognize and treat vitamin deficiencies among psychiatric patients, this article reviews the role of the 6 essential water-soluble vitamins (B1, B2, B6, B9, B12, and C; Table 1,1) and 3 fat-soluble vitamins (A, D, and E; Table 2,1) in brain metabolism and psychiatric pathology. Because numerous sources address using supplements to treat vitamin deficiencies, this article emphasizes food sources, which for many patients are adequate to sustain nutrient status.
Table 1
Water-soluble vitamins: Deficiency, insufficiency, symptoms, and dietary sources
| Deficiency | Insufficiency | Symptoms | At-risk patients | Dietary sources |
|---|---|---|---|---|
| B1 (thiamine): Glycolysis, tricarboxylic acid cycle | ||||
| Rare; 7% in heart failure patients | 5% total, 12% of older women | Wernicke-Korsakoff syndrome, memory impairment, confusion, lack of coordination, paralysis | Older adults, malabsorptive conditions, heavy alcohol use. Those with diabetes are at risk because of increased clearance | Pork, fish, beans, lentils, nuts, rice, and wheat germ. Raw fish, tea, and betel nuts impair absorption |
| B2 (riboflavin): FMN, FAD cofactors in glycolysis and oxidative pathways. B6, folate, and glutathione synthesis | ||||
| 10% to 27% of older adults | <3%; 95% of adolescent girls (measured by EGRAC) | Fatigue, cracked lips, sore throat, bloodshot eyes | Older adults, low intake of animal and dairy products, heavy alcohol use | Dairy, meat and fish, eggs, mushrooms, almonds, leafy greens, and legumes |
| B6 (pyridoxal): Methylation cycle | ||||
| 11% to 24% (<5 ng/mL); 38% of heart failure patients | 14% total, 26% of adults | Dermatitis, glossitis, convulsions, migraine, chronic pain, depression | Older adults, women who use oral contraceptives, alcoholism. 33% to 49% of women age >51 have inadequate intake | Bananas, beans, potatoes, navy beans, salmon, steak, and whole grains |
| B9 (folate): Methylation cycle | ||||
| 0.5% total; up to 50% of depressed patients | 16% of adults, 19% of adolescent girls | Loss of appetite, weight loss, weakness, heart palpitations, behavioral disorders | Depression, pregnancy and lactation, alcoholism, dialysis, liver disease. Deficiency during pregnancy is linked to neural tube defects | Leafy green vegetables, fruits, dried beans, and peas |
| B12 (cobalamin): Methylation cycle (cofactor methionine synthase) | ||||
| 10% to 15% of older adults | <3% to 9% | Depression, irritability, anemia, fatigue, shortness of breath, high blood pressure | Vegetarian or vegan diet, achlorhydria, older adults. Deficiency more often due to poor absorption than low consumption | Meat, seafood, eggs, and dairy |
| C (ascorbic acid): Antioxidant | ||||
| 7.1% | 31% | Scurvy, fatigue, anemia, joint pain, petechia. Symptoms develop after 1 to 3 months of no dietary intake | Smokers, infants fed boiled or evaporated milk, limited dietary variation, patients with malabsorption, chronic illnesses | Citrus fruits, tomatoes and tomato juice, and potatoes |
| EGRAC: erythrocyte glutathione reductase activation coefficient; FAD: flavin adenine dinucleotide; FMN: flavin mononucleotide Source: Reference 1 | ||||
Table 2
Fat-soluble vitamins: Deficiency, insufficiency, symptoms, and dietary sources
| Deficiency | Insufficiency | Symptoms | At-risk patients | Dietary sources |
|---|---|---|---|---|
| A (retinol): Transcription regulation, vision | ||||
| <5% of U.S. population | 44% | Blindness, decreased immunity, corneal and retinal damage | Pregnant women, individuals with strict dietary restrictions, heavy alcohol use, chronic diarrhea, fat malabsorptive conditions | Beef liver, dairy products. Convertible beta-carotene sources: sweet potatoes, carrots, spinach, butternut squash, greens, broccoli, cantaloupe |
| D (cholecalciferol): Hormone, transcriptional regulation | ||||
| ≥50%, 90% of adults age >50 | 69% | Rickets, osteoporosis, muscle twitching | Breast-fed infants, older adults, limited sun exposure, pigmented skin, fat malabsorption, obesity. Older adults have an impaired ability to make vitamin D from the sun. SPF 15 reduces production by 99% | Fatty fish and fish liver oils, sun-dried mushrooms |
| E (tocopherols and tocotrienols): Antioxidant, PUFA protectant, gene regulation | ||||
| Rare | 93% | Anemia, neuropathy, myopathy, abnormal eye movements, weakness, retinal damage | Malabsorptive conditions, HIV, depression | Sunflower, wheat germ, and safflower oils; meats; fish; dairy; green vegetables |
| HIV: human immunodeficiency virus; PUFA: polyunsaturated fatty acids; SPF: sun protection factor Source: Reference 1 | ||||
Water-soluble vitamins
Vitamin B1 (thiamine) is essential for glucose metabolism. Pregnancy, lactation, and fever increase the need for thiamine, and tea, coffee, and shellfish can impair its absorption. Although rare, severe B1 deficiency can lead to beriberi, Wernicke’s encephalopathy (confusion, ataxia, nystagmus), and Korsakoff’s psychosis (confabulation, lack of insight, retrograde and anterograde amnesia, and apathy). Confusion and disorientation stem from the brain’s inability to oxidize glucose for energy because B1 is a critical cofactor in glycolysis and the tricarboxylic acid cycle. Deficiency leads to an increase in reactive oxygen species, proinflammatory cytokines, and blood-brain barrier dysfunction.2 Wernicke’s encephalopathy is most frequently encountered in patients with chronic alcoholism, diabetes, or eating disorders, and after bariatric surgery.3 Iatrogenic Wernicke’s encephalopathy may occur when depleted patients receive IV saline with dextrose without receiving thiamine. Top dietary sources of B1 include pork, fish, beans, lentils, nuts, rice, and wheat germ.
Vitamin B2 (riboflavin) is essential for oxidative pathways, monoamine synthesis, and the methylation cycle. B2 is needed to create the essential flavoprotein coenzymes for synthesis of L-methylfolate—the active form of folate—and for proper utilization of B6. Deficiency can occur after 4 months of inadequate intake.
Although generally B2 deficiency is rare, surveys in the United States have found that 10% to 27% of older adults (age ≥65) are deficient.4 Low intake of dairy products and meat and chronic, excessive alcohol intake are associated with deficiency. Marginal B2 levels are more prevalent in depressed patients, possibly because of B2’s role in the function of glutathione, an endogenous antioxidant.5 Top dietary sources of B2 are dairy products, meat and fish, eggs, mushrooms, almonds, leafy greens, and legumes.
Vitamin B6 refers to 3 distinct compounds: pyridoxine, pyridoxal, and pyridoxamine. B6 is essential to glycolysis, the methylation cycle, and recharging glutathione, an innate antioxidant in the brain. Higher levels of vitamin B6 are associated with a lower prevalence of depression in adolescents,6 and low dietary and plasma B6 increases the risk and severity of depression in geriatric patients7 and predicts depression in prospective trials.8 Deficiency is common (24% to 56%) among patients receiving hemodialysis.9 Women who take oral contraceptives are at increased risk of vitamin B6 deficiency.10 Top dietary sources are fish, beef, poultry, potatoes, legumes, and spinach.
Vitamin B9 (folate) is needed for proper one-carbon metabolism and thus requisite in synthesis of serotonin, norepinephrine, dopamine, and DNA and in phospholipid production. Low maternal folate status increases the risk of neural tube defects in newborns. Folate deficiency and insufficiency are common among patients with mood disorders and correlate with illness severity.11 In a study of 2,682 Finnish men, those in the lowest one-third of folate consumption had a 67% increased relative risk of depression.12 A meta-analysis of 11 studies of 15,315 persons found those who had low folate levels had a significant risk of depression.13 Patients without deficiency but with folate levels near the low end of the normal range also report low mood.14 Compared with controls, patients experiencing a first episode of psychosis have lower levels of folate, B12, and docosahexaenoic acid.15
Dietary folate must be converted to L-methylfolate for use in the brain. Patients with a methylenetetrahydrofolate reductase (MTHFR) C677T polymorphism produce a less active form of the enzyme. The TT genotype is associated with major depression and bipolar disorder.16 Clinical trials have shown that several forms of folate can enhance antidepressant treatment.17 Augmentation with L-methylfolate, which bypasses the MTHFR enzyme, can be an effective strategy for treating depression in these patients.18
Leafy greens and legumes such as lentils are top dietary sources of folate; supplemental folic acid has been linked to an increased risk of cancer and overall mortality.19,20
Vitamin B12 (cobalamin). An essential cofactor in one-carbon metabolism, B12 is needed to produce monoamine neurotransmitters and maintain myelin. Deficiency is found in up to one-third of depressed patients11 and compromises antidepressant response,21 whereas higher vitamin B12 levels are associated with better treatment outcomes.22 B12 deficiency can cause depression, irritability, agitation, psychosis, and obsessive symptoms.23,24 Low B12 levels and elevated homocysteine increase the risk of cognitive decline and Alzheimer’s disease and are linked to a 5-fold increase in the rate of brain atrophy.26
B12 deficiencies may be seen in patients with gastrointestinal illness, older adults with achlorhydria, and vegans and vegetarians, in whom B12 intake can be low. Proton pump inhibitors such as omeprazole interfere with B12 absorption from food.
Psychiatric symptoms of B12 deficiency may present before hematologic findings.23 Folic acid supplementation may mask a B12 deficiency by delaying anemia but will not delay psychiatric symptoms. Ten percent of patients with an insufficiency (low normal levels of 200 to 400 pg/mL) have elevated homocysteine, which increases the risk of psychiatric disorders as well as comorbid illnesses such as cardiovascular disease. Top dietary sources include fish, mollusks (oysters, mussels, and clams), meat, and dairy products.
Vitamin C is vital for the synthesis of monoamines such as serotonin and norepinephrine. Vitamin C’s primary role in the brain is as an antioxidant. As a necessary cofactor, it keeps the copper and iron in metalloenzymes reduced, and also recycles vitamin E. Proper function of the methylation cycle depends on vitamin C, as does collagen synthesis and metabolism of xenobiotics by the liver. It is concentrated in cerebrospinal fluid.
Humans cannot manufacture vitamin C. Although the need for vitamin C (90 mg/d) is thought to be met by diet, studies have found that up to 13.7% of healthy, middle class patients in the United States are depleted.27 Older adults and patients with a poor diet due to drug or alcohol abuse, eating disorders, or affective symptoms are at risk.
Scurvy is caused by vitamin C deficiency and leads to bleeding gums and petechiae. Patients with insufficiency report irritability, loss of appetite, weight loss, and hypochondriasis. Vitamin C intake is significantly lower in older adults (age ≥60) with depression.28 Some research indicates patients with schizophrenia have decreased vitamin C levels and dysfunction of antioxidant defenses.29 Citrus, potatoes, and tomatoes are top dietary sources of vitamin C.
Fat-soluble vitamins
Vitamin A. Although vitamin A activity in the brain is poorly understood, retinol—the active form of vitamin A—is crucial for formation of opsins, which are the basis for vision. Childhood vitamin A deficiency may lead to blindness. Vitamin A also plays an important role in maintaining bone growth, reproduction, cell division, and immune system integrity.30 Animal sources such as beef liver, dairy products, and eggs provide retinol, and plant sources such as carrots, sweet potatoes, and leafy greens provide provitamin A carotenoids that humans convert into retinol.
Deficiency rarely is observed in the United States but remains a common problem for developing nations. In the United States, vitamin A deficiency is most often seen with excessive alcohol use, rigorous dietary restrictions, and gastrointestinal diseases accompanied by poor fat absorption.
Excess vitamin A ingestion may result in bone abnormalities, liver damage, birth defects, and depression. Isotretinoin—a form of vitamin A used to treat severe acne—carries an FDA “black-box” warning for psychiatric adverse effects, including aggression, depression, psychosis, and suicide.
Vitamin D is produced from cholesterol in the epidermis through exposure to sunlight, namely ultraviolet B radiation. After dermal synthesis or ingestion, vitamin D is converted through a series of steps into the active form of vitamin D, calcitriol, which also is known as 25(OH)D3.
Although vitamin D is known for its role in bone growth and mineralization,31 increasing evidence reveals vitamin D’s role in brain function and development.32 Both glial and neuronal cells possess vitamin D receptors in the hippocampus, prefrontal cortex, hypothalamus, thalamus, and substantia nigra—all regions theorized to be linked to depression pathophysiology.33 A review of the association of vitamin D deficiency and psychiatric illnesses will be published in a future issue of Current Psychiatry.
Vitamin D exists in food as either D2 or D3, from plant and animal sources, respectively. Concentrated sources include oily fish, sun-dried or “UVB-irradiated” mushrooms, and milk.
Vitamin E. There are 8 isoforms of vitamin E—4 tocopherols and 4 tocotrienols—that function as fat-soluble antioxidants and also promote innate antioxidant enzymes. Because vitamin E protects neuronal membranes from oxidation, low levels may affect the brain via increased inflammation. Alpha-tocopherol is the most common form of vitamin E in humans, but emerging evidence suggests tocotrienols mediate disease by modifying transcription factors in the brain, such as glutathione reductase, superoxide dismutase, and nuclear factor-kappaB.34 Low plasma vitamin E levels are found in depressed patients, although some data suggest this may be caused by factors other than dietary intake.35 Low vitamin status has been found in up to 70% of older adults.36 Although deficiency is rare, most of the U.S. population (93%) has inadequate dietary intake of vitamin E.1 The reasons for this discrepancy are unclear. Foods rich in vitamin E include almonds, sunflower seeds, leafy greens, and wheat germ.
Recommendations
Patients with depression, alcohol abuse, eating disorders, obsessive-compulsive disorder, or schizophrenia may neglect to care for themselves or adopt particular eating patterns. Deficiencies are more common among geriatric patients and those who are medically ill. Because dietary patterns are linked to the risk of psychiatric disorders, nutritional inquiry often identifies multiple modifiable risk factors, such as folate, vitamin B12, and vitamin D intake.37,38 Nutritional counseling offers clinicians an intervention with minimal side effect risks and the opportunity to modify a behavior that patients engage in 3 times a day.
Psychiatrists should assess patients’ dietary patterns and vitamin status, particularly older adults and those with:
- lower socioeconomic status or food insecurity
- a history of treatment resistance
- restrictive dietary patterns such as veganism
- alcohol abuse.
On initial assessment, test or obtain from other health care providers your patient’s blood levels of folate and vitamins D and B12. In some patients, assessing B2 and B6 levels may provide etiological guidance regarding onset of psychiatric symptoms or failure to respond to pharmacologic treatment. Because treating vitamin deficiencies often includes using supplements, evaluate recent reviews of specific deficiencies and consider consulting with the patient’s primary care provider.
Conduct a simple assessment of dietary patterns by asking patients about a typical breakfast, lunch, and dinner, their favorite snacks and foods, and specific dietary habits or restrictions (eg, not consuming seafood, dairy, meat, etc.). Rudimentary nutritional recommendations can be effective in changing a patient’s eating habits, particularly when provided by a physician. Encourage patients to eat nutrient-dense foods such as leafy greens, beans and legumes, seafood, whole grains, and a variety of vegetables and fruits. For more complex patients, consult with a clinical nutritionist.
Related Resources
- Institute of Medicine. Dietary Reference Intakes: Recommended intakes for individuals. SummaryDRIs/~/media/Files
/Activity%20Files/Nutrition
/DRIs/5_Summary%20Table%20Tables%201-4.pdf" target="_blank">www.iom.edu/Activities/Nutrition/
SummaryDRIs/~/media/Files
/Activity%20Files/Nutrition
/DRIs/5_Summary%20Table%20Tables%201-4.pdf. - The Farmacy: Vitamins. http://drewramseymd.com/index.php/resources/farmacy/category/vitamins.
- Office of Dietary Supplements. National Institutes of Health. Dietary supplements fact sheets. http://ods.od.nih.gov/factsheets/list-all.
- Oregon State University. Linus Pauling Institute. Micronutrient information center. http://lpi.oregonstate.edu/infocenter/vitamins.html.
Drug Brand Names
- Isotretinoin • Accutane
- L-methylfolate • Deplin
- Omeprazole • Prilosec
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Moshfegh A, Goldman J, Cleveland L. United States Department of Agriculture, Agricultural Research Service. What we eat in America NHANES 2001-2002: Usual nutrient intakes from food compared to dietary reference intakes. http://www.ars.usda.gov/SP2UserFiles/Place/12355000/pdf/0102/usualintaketables2001-02.pdf. Published September 2005. Accessed November 27, 2012.
2. Page GL, Laight D, Cummings MH. Thiamine deficiency in diabetes mellitus and the impact of thiamine replacement on glucose metabolism and vascular disease. Int J Clin Pract. 2011;65(6):684-690.
3. McCormick LM, Buchanan JR, Onwuameze OE, et al. Beyond alcoholism: Wernicke-Korsakoff syndrome in patients with psychiatric disorders. Cogn Behav Neurol. 2011;24(4):209-216.
4. Powers HJ. Riboflavin (vitamin B-2) and health. Am J Clin Nutr. 2003;77(6):1352-1360.
5. Naghashpour M, Amani R, Nutr R, et al. Riboflavin status and its association with serum hs-CRP levels among clinical nurses with depression. J Am Coll Nutr. 2011;30(5):340-347.
6. Murakami K, Miyake Y, Sasaki S, et al. Dietary folate, riboflavin, vitamin B-6, and vitamin B-12 and depressive symptoms in early adolescence: the Ryukyus Child Health Study. Psychosom Med. 2010;72(8):763-768.
7. Merete C, Falcon LM, Tucker KL. Vitamin B6 is associated with depressive symptomatology in Massachusetts elders. J Am Coll Nutr. 2008;27(3):421-427.
8. Skarupski KA, Tangney C, Li H, et al. Longitudinal association of vitamin B-6, folate, and vitamin B-12 with depressive symptoms among older adults over time. Am J Clin Nutr. 2010;92(2):330-335.
9. Corken M, Porter J. Is vitamin B(6) deficiency an under-recognized risk in patients receiving haemodialysis? A systematic review: 2000-2010. Nephrology (Carlton). 2011;16(7):619-625.
10. Wilson SM, Bivins BN, Russell KA, et al. Oral contraceptive use: impact on folate, vitamin B6, and vitamin B12 status. Nutr Rev. 2011;69(10):572-583.
11. Coppen A, Bolander-Gouaille C. Treatment of depression: time to consider folic acid and vitamin B12. J Psychopharmacol. 2005;19(1):59-65.
12. Tolmunen T, Voutilainen S, Hintikka J, et al. Dietary folate and depressive symptoms are associated in middle-aged Finnish men. J Nutr. 2003;133(10):3233-3236.
13. Gilbody S, Lightfoot T, Sheldon T. Is low folate a risk factor for depression? A meta-analysis and exploration of heterogeneity. J Epidemiol Community Health. 2007;61(7):631-637.
14. Rösche J, Uhlmann C, Fröscher W. Low serum folate levels as a risk factor for depressive mood in patients with chronic epilepsy. J Neuropsychiatry Clin Neurosci. 2003;15(1):64-66.
15. Kale A, Naphade N, Sapkale S, et al. Reduced folic acid, vitamin B12 and docosahexaenoic acid and increased homocysteine and cortisol in never-medicated schizophrenia patients: implications for altered one-carbon metabolism. Psychiatry Res. 2010;175(1-2):47-53.
16. Gilbody S, Lewis S, Lightfoot T. Methylenetetrahydrofolate reductase (MTHFR) genetic polymorphisms and psychiatric disorders: a HuGE review. Am J Epidemiol. 2007;165(1):1-13.
17. Di Palma C, Urani R, Agricola R, et al. Is methylfolate effective in relieving major depression in chronic alcoholics? A hypothesis of treatment. Curr Ther Res Clin Exp. 1994;55(5):559-568.
18. Papakostas GI, Shelton RC, Zajecka JM, et al. l-Methylfolate as adjunctive therapy for ssri-resistant major depression: results of two randomized, double-blind, parallel-sequential trials. Am J Psychiatry. 2012;169(12):1267-1274.
19. Baggott JE, Oster RA, Tamura T. Meta-analysis of cancer risk in folic acid supplementation trials. Cancer Epidemiol. 2012;36(1):78-81.
20. Figueiredo JC, Grau MV, Haile RW, et al. Folic acid and risk of prostate cancer: results from a randomized clinical trial. J Natl Cancer Inst. 2009;101(6):432-435.
21. Kate N, Grover S, Agarwal M. Does B12 deficiency lead to lack of treatment response to conventional antidepressants? Psychiatry (Edgmont). 2010;7(11):42-44.
22. Hintikka J, Tolmunen T, Tanskanen A, et al. High vitamin B12 level and good treatment outcome may be associated in major depressive disorder. BMC Psychiatry. 2003;3:17.-
23. Lindenbaum J, Healton EB, Savage DG, et al. Neuropsychiatric disorders caused by cobalamin deficiency in the absence of anemia or macrocytosis. N Engl J Med. 1988;318(26):1720-1728.
24. Bar-Shai M, Gott D, Marmor S. Acute psychotic depression as a sole manifestation of vitamin B12 deficiency. Psychosomatics. 2011;52(4):384-386.
25. Sharma V, Biswas D. Cobalamin deficiency presenting as obsessive compulsive disorder: case report. Gen Hosp Psychiatry. 2012;34(5):578.e7-e8.
26. Vogiatzoglou A, Refsum H, Johnston C, et al. Vitamin B12 status and rate of brain volume loss in community-dwelling elderly. Neurology. 2008;71(11):826-832.
27. Smith A, Di Primio G, Humphrey-Murto S. Scurvy in the developed world. CMAJ. 2011;183(11):E752-E725.
28. Payne ME, Steck SE, George RR, et al. Fruit, vegetable, and antioxidant intakes are lower in older adults with depression. J Acad Nutr Diet. 2012;112(12):2022-2027.
29. Dadheech G, Mishra S, Gautam S, et al. Oxidative stress, α-tocopherol, ascorbic acid and reduced glutathione status in schizophrenics. Indian J Clin Biochem. 2006;21(2):34-38.
30. Hinds TS, West WL, Knight EM. Carotenoids and retinoids: a review of research clinical, and public health applications. J Clin Pharmacol. 1997;37(7):551-558.
31. Thacher TD, Clarke BL. Vitamin D insufficiency. Mayo Clin Proc. 2011;86(1):50-60.
32. Berk M, Sanders KM, Pasco JA, et al. Vitamin D deficiency may play a role in depression. Med Hypotheses. 2007;69(6):1316-1319.
33. Eyles DW, Smith S, Kinobe R, et al. Distribution of the vitamin D receptor and 1 alpha-hydroxylase in human brain. J Chem Neuroanat. 2005;29(1):21-30.
34. Sen CK, Khanna S, Roy S. Tocotrienol: the natural vitamin E to defend the nervous system? Ann N Y Acad Sci. 2004;1031:127-142.
35. Owen AJ, Batterham MJ, Probst YC, et al. Low plasma vitamin E levels in major depression: diet or disease? Eur J Clin Nutr. 2005;59(2):304-306.
36. Panemangalore M, Lee CJ. Evaluation of the indices of retinol and alpha-tocopherol status in free-living elderly. J Gerontol. 1992;47(3):B98-B104.
37. Sánchez-Villegas A, Delgado-Rodríguez M, Alonso A, et al. Association of the Mediterranean dietary pattern with the incidence of depression: the Seguimiento Universidad de Navarra/University of Navarra follow-up (SUN) cohort. Arch Gen Psychiatry. 2009;66(10):1090-1098.
38. Jacka FN, Pasco JA, Mykletun A, et al. Association of Western and traditional diets with depression and anxiety in women. Am J Psychiatry. 2010;167(3):305-311.
1. Moshfegh A, Goldman J, Cleveland L. United States Department of Agriculture, Agricultural Research Service. What we eat in America NHANES 2001-2002: Usual nutrient intakes from food compared to dietary reference intakes. http://www.ars.usda.gov/SP2UserFiles/Place/12355000/pdf/0102/usualintaketables2001-02.pdf. Published September 2005. Accessed November 27, 2012.
2. Page GL, Laight D, Cummings MH. Thiamine deficiency in diabetes mellitus and the impact of thiamine replacement on glucose metabolism and vascular disease. Int J Clin Pract. 2011;65(6):684-690.
3. McCormick LM, Buchanan JR, Onwuameze OE, et al. Beyond alcoholism: Wernicke-Korsakoff syndrome in patients with psychiatric disorders. Cogn Behav Neurol. 2011;24(4):209-216.
4. Powers HJ. Riboflavin (vitamin B-2) and health. Am J Clin Nutr. 2003;77(6):1352-1360.
5. Naghashpour M, Amani R, Nutr R, et al. Riboflavin status and its association with serum hs-CRP levels among clinical nurses with depression. J Am Coll Nutr. 2011;30(5):340-347.
6. Murakami K, Miyake Y, Sasaki S, et al. Dietary folate, riboflavin, vitamin B-6, and vitamin B-12 and depressive symptoms in early adolescence: the Ryukyus Child Health Study. Psychosom Med. 2010;72(8):763-768.
7. Merete C, Falcon LM, Tucker KL. Vitamin B6 is associated with depressive symptomatology in Massachusetts elders. J Am Coll Nutr. 2008;27(3):421-427.
8. Skarupski KA, Tangney C, Li H, et al. Longitudinal association of vitamin B-6, folate, and vitamin B-12 with depressive symptoms among older adults over time. Am J Clin Nutr. 2010;92(2):330-335.
9. Corken M, Porter J. Is vitamin B(6) deficiency an under-recognized risk in patients receiving haemodialysis? A systematic review: 2000-2010. Nephrology (Carlton). 2011;16(7):619-625.
10. Wilson SM, Bivins BN, Russell KA, et al. Oral contraceptive use: impact on folate, vitamin B6, and vitamin B12 status. Nutr Rev. 2011;69(10):572-583.
11. Coppen A, Bolander-Gouaille C. Treatment of depression: time to consider folic acid and vitamin B12. J Psychopharmacol. 2005;19(1):59-65.
12. Tolmunen T, Voutilainen S, Hintikka J, et al. Dietary folate and depressive symptoms are associated in middle-aged Finnish men. J Nutr. 2003;133(10):3233-3236.
13. Gilbody S, Lightfoot T, Sheldon T. Is low folate a risk factor for depression? A meta-analysis and exploration of heterogeneity. J Epidemiol Community Health. 2007;61(7):631-637.
14. Rösche J, Uhlmann C, Fröscher W. Low serum folate levels as a risk factor for depressive mood in patients with chronic epilepsy. J Neuropsychiatry Clin Neurosci. 2003;15(1):64-66.
15. Kale A, Naphade N, Sapkale S, et al. Reduced folic acid, vitamin B12 and docosahexaenoic acid and increased homocysteine and cortisol in never-medicated schizophrenia patients: implications for altered one-carbon metabolism. Psychiatry Res. 2010;175(1-2):47-53.
16. Gilbody S, Lewis S, Lightfoot T. Methylenetetrahydrofolate reductase (MTHFR) genetic polymorphisms and psychiatric disorders: a HuGE review. Am J Epidemiol. 2007;165(1):1-13.
17. Di Palma C, Urani R, Agricola R, et al. Is methylfolate effective in relieving major depression in chronic alcoholics? A hypothesis of treatment. Curr Ther Res Clin Exp. 1994;55(5):559-568.
18. Papakostas GI, Shelton RC, Zajecka JM, et al. l-Methylfolate as adjunctive therapy for ssri-resistant major depression: results of two randomized, double-blind, parallel-sequential trials. Am J Psychiatry. 2012;169(12):1267-1274.
19. Baggott JE, Oster RA, Tamura T. Meta-analysis of cancer risk in folic acid supplementation trials. Cancer Epidemiol. 2012;36(1):78-81.
20. Figueiredo JC, Grau MV, Haile RW, et al. Folic acid and risk of prostate cancer: results from a randomized clinical trial. J Natl Cancer Inst. 2009;101(6):432-435.
21. Kate N, Grover S, Agarwal M. Does B12 deficiency lead to lack of treatment response to conventional antidepressants? Psychiatry (Edgmont). 2010;7(11):42-44.
22. Hintikka J, Tolmunen T, Tanskanen A, et al. High vitamin B12 level and good treatment outcome may be associated in major depressive disorder. BMC Psychiatry. 2003;3:17.-
23. Lindenbaum J, Healton EB, Savage DG, et al. Neuropsychiatric disorders caused by cobalamin deficiency in the absence of anemia or macrocytosis. N Engl J Med. 1988;318(26):1720-1728.
24. Bar-Shai M, Gott D, Marmor S. Acute psychotic depression as a sole manifestation of vitamin B12 deficiency. Psychosomatics. 2011;52(4):384-386.
25. Sharma V, Biswas D. Cobalamin deficiency presenting as obsessive compulsive disorder: case report. Gen Hosp Psychiatry. 2012;34(5):578.e7-e8.
26. Vogiatzoglou A, Refsum H, Johnston C, et al. Vitamin B12 status and rate of brain volume loss in community-dwelling elderly. Neurology. 2008;71(11):826-832.
27. Smith A, Di Primio G, Humphrey-Murto S. Scurvy in the developed world. CMAJ. 2011;183(11):E752-E725.
28. Payne ME, Steck SE, George RR, et al. Fruit, vegetable, and antioxidant intakes are lower in older adults with depression. J Acad Nutr Diet. 2012;112(12):2022-2027.
29. Dadheech G, Mishra S, Gautam S, et al. Oxidative stress, α-tocopherol, ascorbic acid and reduced glutathione status in schizophrenics. Indian J Clin Biochem. 2006;21(2):34-38.
30. Hinds TS, West WL, Knight EM. Carotenoids and retinoids: a review of research clinical, and public health applications. J Clin Pharmacol. 1997;37(7):551-558.
31. Thacher TD, Clarke BL. Vitamin D insufficiency. Mayo Clin Proc. 2011;86(1):50-60.
32. Berk M, Sanders KM, Pasco JA, et al. Vitamin D deficiency may play a role in depression. Med Hypotheses. 2007;69(6):1316-1319.
33. Eyles DW, Smith S, Kinobe R, et al. Distribution of the vitamin D receptor and 1 alpha-hydroxylase in human brain. J Chem Neuroanat. 2005;29(1):21-30.
34. Sen CK, Khanna S, Roy S. Tocotrienol: the natural vitamin E to defend the nervous system? Ann N Y Acad Sci. 2004;1031:127-142.
35. Owen AJ, Batterham MJ, Probst YC, et al. Low plasma vitamin E levels in major depression: diet or disease? Eur J Clin Nutr. 2005;59(2):304-306.
36. Panemangalore M, Lee CJ. Evaluation of the indices of retinol and alpha-tocopherol status in free-living elderly. J Gerontol. 1992;47(3):B98-B104.
37. Sánchez-Villegas A, Delgado-Rodríguez M, Alonso A, et al. Association of the Mediterranean dietary pattern with the incidence of depression: the Seguimiento Universidad de Navarra/University of Navarra follow-up (SUN) cohort. Arch Gen Psychiatry. 2009;66(10):1090-1098.
38. Jacka FN, Pasco JA, Mykletun A, et al. Association of Western and traditional diets with depression and anxiety in women. Am J Psychiatry. 2010;167(3):305-311.
The delirium/dementia treatment paradox
Delirium is the most common psychiatric disorder on medical/surgical units of general hospitals and is a major cause of consultations for consultation-liaison (C-L) psychiatrists in medical centers.
It primarily afflicts older patients and is characterized by a rapid onset of confusion with hallucinations, delusions, agitation (but sometimes hypoactivity), and fluctuating severity and outcomes. It can be triggered by multiple factors, including dehydration, electrolyte imbalance, upper respiratory infection, urinary tract infections, post-surgical state, and, very commonly, iatrogenic factors. Many medications, especially those with anticholinergic activity, can cause delirium in older patients. Delirium can lead to pronged hospital stays, functional decline, cognitive impairment, and accelerated mortality.1
So what is the evidence-based treatment for this common and serious neuropsychiatric brain disorder? None exists! Delirium is managed by identifying and addressing underlying factors, as well as supportive medical care (hydration, nutrition, sleep, monitoring vital signs, preventing aspiration, quiet rooms, orientation, and ambulation). Physical restraints are undesirable but may be hard to avoid for severely disinhibited and agitated patients who try to rip out their IV or endotracheal tube or assault staff or family members who try to calm them during their psychotic confusional state.
Doesn’t this sound similar to dementia patients in nursing homes who develop psychosis and agitation and pose management problems for their family and staff? These 2 neuropsychiatric conditions are clinically similar, and the use of antipsychotic agents is not FDA-approved for either of them. Antipsychotics have not been found efficacious for delirium2 or dementia with psychosis3 but their use continues. The paradox is that C-L psychiatrists use small doses of antipsychotics routinely for delirium and generally believe that such pharmacologic intervention works in many patients, although some older agents (eg, haloperidol) have been reported to be neurotoxic4 and associated with higher mortality in dementia patients with psychosis.5,6
The relationship of antipsychotic treatment with delirium and dementia is complex, paradoxical, and unsettled. Consider the following:
- Antipsychotics are not approved for the psychosis of dementia despite 17 large, placebo-controlled trials with 4 different second-generation agents.
- The FDA issued a “black-box” warning on all first- and second-generation antipsychotics because of a 1.6-times higher mortality rate among older patients with dementia.
- No placebo-controlled, FDA trials of delirium have been conducted with any antipsychotic agents.
- Clinicians frequently use first- and second-generation antipsychotics for delirium despite the lack of evidence.
- The FDA has not issued a “black-box” warning on antipsychotics for use in delirium as they did for dementia, although typically both populations are older and may be susceptible to the same complications observed in Alzheimer’s disease trials (eg, strokes, transient ischemic attack, and aspiration pneumonia). This may be because of the absence of safety data of antipsychotics in delirium based on industry-sponsored registration clinical trials. No data means no warning, although the risk factors may be present for millions of older patients who have suffered from delirium and have been treated with first-or second-generation antipsychotics.
The current convoluted state of pharmacotherapy for delirium and dementia is likely to continue: dementia patients with psychosis may or may not receive antipsychotics because of the FDA’s “black-box” warning while delirium patients readily receive various antipsychotics based on long-term practice, although not a single antipsychotic has been FDA-approved for delirium. Until a pharmaceutical company decides to conduct a large placebo-controlled trial for delirium, the current widespread, off-label use of antipsychotics in delirium will continue and “black-box” warning will apply only to 1 clinical population of older persons (those with dementia) but not another (those with delirium). Go figure!
1. Martins S, Fernandes L. Delirium in elderly people: a review. Front Neurol. 2012;3:101.-
2. Flaherty JH, Gonzales JP, Dong B. Antipsychotics in the treatment of delirium in older hospitalized adults: a systematic review. J Am Geriatr Soc. 2011;59(suppl 2):S269-S276.
3. Seitz DP, Gill SS, Herrmann N, et al. Pharmacological treatments for neuropsychiatric symptoms of dementia in long-term care: a systematic review. [published online October 19, 2012]. Int Psychogeriatr. 2012:1-19. doi: http://dx.doi.org/10.1017/S1041610212001627.
4. Nasrallah HA. Does the neurotoxicity of haloperidol explain the higher mortality in dementia patients compared with the second generation agents? Am J Psychiatry. 2012;169(6):663-664;author reply 664–665.
5. Nasrallah HA, White T, Nasrallah AT. Lower mortality in geriatric patients receiving risperidone and olanzapine versus haloperidol: preliminary analysis of retrospective data. Am J Geriatr Psychiatry. 2004;12(4):437-439.
6. Kales HC, Kim HM, Zivin K, et al. Risk of mortality among individual antipsychotics in patients with dementia. Am J Psychiatry. 2012;169(1):71-79.
Delirium is the most common psychiatric disorder on medical/surgical units of general hospitals and is a major cause of consultations for consultation-liaison (C-L) psychiatrists in medical centers.
It primarily afflicts older patients and is characterized by a rapid onset of confusion with hallucinations, delusions, agitation (but sometimes hypoactivity), and fluctuating severity and outcomes. It can be triggered by multiple factors, including dehydration, electrolyte imbalance, upper respiratory infection, urinary tract infections, post-surgical state, and, very commonly, iatrogenic factors. Many medications, especially those with anticholinergic activity, can cause delirium in older patients. Delirium can lead to pronged hospital stays, functional decline, cognitive impairment, and accelerated mortality.1
So what is the evidence-based treatment for this common and serious neuropsychiatric brain disorder? None exists! Delirium is managed by identifying and addressing underlying factors, as well as supportive medical care (hydration, nutrition, sleep, monitoring vital signs, preventing aspiration, quiet rooms, orientation, and ambulation). Physical restraints are undesirable but may be hard to avoid for severely disinhibited and agitated patients who try to rip out their IV or endotracheal tube or assault staff or family members who try to calm them during their psychotic confusional state.
Doesn’t this sound similar to dementia patients in nursing homes who develop psychosis and agitation and pose management problems for their family and staff? These 2 neuropsychiatric conditions are clinically similar, and the use of antipsychotic agents is not FDA-approved for either of them. Antipsychotics have not been found efficacious for delirium2 or dementia with psychosis3 but their use continues. The paradox is that C-L psychiatrists use small doses of antipsychotics routinely for delirium and generally believe that such pharmacologic intervention works in many patients, although some older agents (eg, haloperidol) have been reported to be neurotoxic4 and associated with higher mortality in dementia patients with psychosis.5,6
The relationship of antipsychotic treatment with delirium and dementia is complex, paradoxical, and unsettled. Consider the following:
- Antipsychotics are not approved for the psychosis of dementia despite 17 large, placebo-controlled trials with 4 different second-generation agents.
- The FDA issued a “black-box” warning on all first- and second-generation antipsychotics because of a 1.6-times higher mortality rate among older patients with dementia.
- No placebo-controlled, FDA trials of delirium have been conducted with any antipsychotic agents.
- Clinicians frequently use first- and second-generation antipsychotics for delirium despite the lack of evidence.
- The FDA has not issued a “black-box” warning on antipsychotics for use in delirium as they did for dementia, although typically both populations are older and may be susceptible to the same complications observed in Alzheimer’s disease trials (eg, strokes, transient ischemic attack, and aspiration pneumonia). This may be because of the absence of safety data of antipsychotics in delirium based on industry-sponsored registration clinical trials. No data means no warning, although the risk factors may be present for millions of older patients who have suffered from delirium and have been treated with first-or second-generation antipsychotics.
The current convoluted state of pharmacotherapy for delirium and dementia is likely to continue: dementia patients with psychosis may or may not receive antipsychotics because of the FDA’s “black-box” warning while delirium patients readily receive various antipsychotics based on long-term practice, although not a single antipsychotic has been FDA-approved for delirium. Until a pharmaceutical company decides to conduct a large placebo-controlled trial for delirium, the current widespread, off-label use of antipsychotics in delirium will continue and “black-box” warning will apply only to 1 clinical population of older persons (those with dementia) but not another (those with delirium). Go figure!
Delirium is the most common psychiatric disorder on medical/surgical units of general hospitals and is a major cause of consultations for consultation-liaison (C-L) psychiatrists in medical centers.
It primarily afflicts older patients and is characterized by a rapid onset of confusion with hallucinations, delusions, agitation (but sometimes hypoactivity), and fluctuating severity and outcomes. It can be triggered by multiple factors, including dehydration, electrolyte imbalance, upper respiratory infection, urinary tract infections, post-surgical state, and, very commonly, iatrogenic factors. Many medications, especially those with anticholinergic activity, can cause delirium in older patients. Delirium can lead to pronged hospital stays, functional decline, cognitive impairment, and accelerated mortality.1
So what is the evidence-based treatment for this common and serious neuropsychiatric brain disorder? None exists! Delirium is managed by identifying and addressing underlying factors, as well as supportive medical care (hydration, nutrition, sleep, monitoring vital signs, preventing aspiration, quiet rooms, orientation, and ambulation). Physical restraints are undesirable but may be hard to avoid for severely disinhibited and agitated patients who try to rip out their IV or endotracheal tube or assault staff or family members who try to calm them during their psychotic confusional state.
Doesn’t this sound similar to dementia patients in nursing homes who develop psychosis and agitation and pose management problems for their family and staff? These 2 neuropsychiatric conditions are clinically similar, and the use of antipsychotic agents is not FDA-approved for either of them. Antipsychotics have not been found efficacious for delirium2 or dementia with psychosis3 but their use continues. The paradox is that C-L psychiatrists use small doses of antipsychotics routinely for delirium and generally believe that such pharmacologic intervention works in many patients, although some older agents (eg, haloperidol) have been reported to be neurotoxic4 and associated with higher mortality in dementia patients with psychosis.5,6
The relationship of antipsychotic treatment with delirium and dementia is complex, paradoxical, and unsettled. Consider the following:
- Antipsychotics are not approved for the psychosis of dementia despite 17 large, placebo-controlled trials with 4 different second-generation agents.
- The FDA issued a “black-box” warning on all first- and second-generation antipsychotics because of a 1.6-times higher mortality rate among older patients with dementia.
- No placebo-controlled, FDA trials of delirium have been conducted with any antipsychotic agents.
- Clinicians frequently use first- and second-generation antipsychotics for delirium despite the lack of evidence.
- The FDA has not issued a “black-box” warning on antipsychotics for use in delirium as they did for dementia, although typically both populations are older and may be susceptible to the same complications observed in Alzheimer’s disease trials (eg, strokes, transient ischemic attack, and aspiration pneumonia). This may be because of the absence of safety data of antipsychotics in delirium based on industry-sponsored registration clinical trials. No data means no warning, although the risk factors may be present for millions of older patients who have suffered from delirium and have been treated with first-or second-generation antipsychotics.
The current convoluted state of pharmacotherapy for delirium and dementia is likely to continue: dementia patients with psychosis may or may not receive antipsychotics because of the FDA’s “black-box” warning while delirium patients readily receive various antipsychotics based on long-term practice, although not a single antipsychotic has been FDA-approved for delirium. Until a pharmaceutical company decides to conduct a large placebo-controlled trial for delirium, the current widespread, off-label use of antipsychotics in delirium will continue and “black-box” warning will apply only to 1 clinical population of older persons (those with dementia) but not another (those with delirium). Go figure!
1. Martins S, Fernandes L. Delirium in elderly people: a review. Front Neurol. 2012;3:101.-
2. Flaherty JH, Gonzales JP, Dong B. Antipsychotics in the treatment of delirium in older hospitalized adults: a systematic review. J Am Geriatr Soc. 2011;59(suppl 2):S269-S276.
3. Seitz DP, Gill SS, Herrmann N, et al. Pharmacological treatments for neuropsychiatric symptoms of dementia in long-term care: a systematic review. [published online October 19, 2012]. Int Psychogeriatr. 2012:1-19. doi: http://dx.doi.org/10.1017/S1041610212001627.
4. Nasrallah HA. Does the neurotoxicity of haloperidol explain the higher mortality in dementia patients compared with the second generation agents? Am J Psychiatry. 2012;169(6):663-664;author reply 664–665.
5. Nasrallah HA, White T, Nasrallah AT. Lower mortality in geriatric patients receiving risperidone and olanzapine versus haloperidol: preliminary analysis of retrospective data. Am J Geriatr Psychiatry. 2004;12(4):437-439.
6. Kales HC, Kim HM, Zivin K, et al. Risk of mortality among individual antipsychotics in patients with dementia. Am J Psychiatry. 2012;169(1):71-79.
1. Martins S, Fernandes L. Delirium in elderly people: a review. Front Neurol. 2012;3:101.-
2. Flaherty JH, Gonzales JP, Dong B. Antipsychotics in the treatment of delirium in older hospitalized adults: a systematic review. J Am Geriatr Soc. 2011;59(suppl 2):S269-S276.
3. Seitz DP, Gill SS, Herrmann N, et al. Pharmacological treatments for neuropsychiatric symptoms of dementia in long-term care: a systematic review. [published online October 19, 2012]. Int Psychogeriatr. 2012:1-19. doi: http://dx.doi.org/10.1017/S1041610212001627.
4. Nasrallah HA. Does the neurotoxicity of haloperidol explain the higher mortality in dementia patients compared with the second generation agents? Am J Psychiatry. 2012;169(6):663-664;author reply 664–665.
5. Nasrallah HA, White T, Nasrallah AT. Lower mortality in geriatric patients receiving risperidone and olanzapine versus haloperidol: preliminary analysis of retrospective data. Am J Geriatr Psychiatry. 2004;12(4):437-439.
6. Kales HC, Kim HM, Zivin K, et al. Risk of mortality among individual antipsychotics in patients with dementia. Am J Psychiatry. 2012;169(1):71-79.
Does a higher frequency of difficult patient encounters lead to lower quality care?
Background Difficult patient encounters in the primary care office are frequent and are associated with physician burnout. However, their relationship to patient care outcomes is not known.
Objective To determine the effect of difficult encounters on patient health outcomes and the role of physician dissatisfaction and burnout as mediators of this effect.
Design A total of 422 physicians were sorted into 3 clusters based on perceived frequency of difficult patient encounters in their practices. Patient charts were audited to assess the quality of hypertension and diabetes management and preventive care based on national guidelines. Summary measures of quality and errors were compared among the 3 physician clusters.
Results Of the 1384 patients, 359 were cared for by high-cluster physicians (those who had a high frequency of difficult encounters), 871 by medium-cluster physicians, and 154 by low-cluster physicians. Dissatisfaction and burnout were higher among physicians reporting higher frequencies of difficult encounters. However, quality of patient care and management errors were similar across all 3 groups.
Conclusions Physician perception of frequent difficult encounters was not associated with worse patient care quality or more medical errors. Future studies should investigate whether other patient outcomes, including acute care and patient satisfaction, are affected by difficult encounters.
Physicians who have high numbers of difficult patient encounters are more likely to report burnout and related stressor effects than are colleagues with fewer difficult encounters.1 More of them also perceive that they provide suboptimal care than do colleagues who report fewer difficult patients.1 These were some of the findings taken from the Minimizing Error, Maximizing Outcome (MEMO) Study that we conducted from 2001 to 2005.1 But these findings prompted us to wonder: Is that perception accurate?
Whether physicians reporting high numbers of difficult patient encounters actually provide poorer care is unknown. In a recent study of physicians from one large primary care system, patient panels that were more challenging—as determined by higher rates of underinsured, minority, and non-English-speaking patients—were associated with lower quality care.2 Hinchey and Jackson found that 2 weeks after initial presentation, patients involved in difficult encounters at a walk-in clinic experienced worsening physical symptoms.3 However, this study did not address whether difficult patient encounters affected the care rendered by providers to patients in general.
A detailed, rigorous model describing the interplay and relationships among difficult encounters, adverse physician outcomes (eg, burnout, dissatisfaction), and patient health outcomes has yet to be developed. To better understand the effects of these interactions, we revisited data from the MEMO study.
The findings that prompted another look at the data
When we conducted the MEMO study, we surveyed 422 physicians working in 119 primary care clinics in the upper Midwest and New York City.4 Almost half (49%) of the physicians reported moderately or highly stressful jobs; 27% reported burnout; and 30% were at least moderately likely to leave their practices within 2 years. Of these physicians, 113 (27%) reported high numbers of difficult encounters, which corresponds with other reports of 10% to 37% in primary care settings.5-7 These 113 physicians were 12.2 times more likely to report burnout compared with colleagues with fewer difficult encounters.1 They also reported lower job satisfaction, increased stress, more time pressure, and greater intent to leave practice, which are also echoed in other studies.8-10
We found in our study (and at least one other) that physicians experiencing burnout are often younger and female, work long hours, and practice in a medicine subspecialty.1,11 Many physicians who care for difficult patients report that they secretly hope these patients will not return.6
Our hypothesis
We hypothesized that frequent difficult encounters may amplify an adverse work environment, and that physicians facing time pressure and a lack of work control brought on by these encounters might be unable to sustain a high standard of care for their overall patient load.
METHODS
Participants
Physician and patient participants and design of the MEMO study are described in detail elsewhere.12 The following, though, is a recap:
We recruited 422 general internists and family physicians from 119 ambulatory care clinics in New York City and the upper Midwest. These regions offered a diverse patient and payer mix. Physicians were asked via on-site presentations and mailed invitations to complete a survey derived from focus groups and the Physician Worklife Survey.13,14
We also recruited up to 8 patients per participating physician via mailed invitations. Inclusion criteria were a minimum age of 18; a diagnosis of at least one target condition (hypertension, diabetes, congestive heart failure); ability to read in English, Spanish, or Chinese; and at least 2 visits with their primary physician in the previous year.
Here we report on outcomes for those patients with diabetes and hypertension.
Measures
When we initially conducted the study, physicians completed an 8-item Burden of Difficult Encounters measure designed to approximate the frequency of difficult encounters experienced. Latent cluster analyses of this survey measure defined 3 distinct groups of physicians: those who estimated a high, medium, and low frequency of difficult encounters in their practices. Via chart audits, we determined quality of care and errors related to guideline-recommended management and preventive care for hypertension and diabetes. Details of these audits are found elsewhere.4
We defined quality care for hypertension as successful blood pressure control (<140/90), and for diabetes, successful control of hemoglobin A1c (≤7.5) and blood pressure (<135/80). One quality point was awarded for each of these 3 measures if achieved for at least 50% of recorded visits over an 18-month period. We calculated the quality score as the proportion of total possible quality points (with 100%=best).
We defined errors as guideline non-adherence and missed opportunities for prevention or management, tailored to each patient’s age, sex, and diagnoses. We calculated the error score as the proportion of total applicable error points (maximum=15; 0%=best). We assigned an error point for each missing process of care, including missed treatment opportunities, inattention to behavioral factors, guideline nonadherence, lack of tobacco use documentation, and missed prevention activities, such as mammograms, cervical cancer screening, colon cancer screening, and depression assessment.
We normalized scores to a range of 0 to 100 by dividing the number of quality or error points by the number of applicable items and multiplying by 100. We calculated quality and error scores for hypertension or diabetes for each patient and averaged them to determine total scores per physician.
Data analysis
Latent cluster analyses identified 3 distinct clusters of physicians based on their reported frequency of difficult encounters.1 We used a 2-level hierarchical linear model of patients nested under physicians to assess if a higher number of perceived difficult patients was associated with poorer patient care, as measured by quality of care and medical errors, controlling for physician age, sex, and racial/ethnic minority status. To further adjust for negatively biased standard errors (physicians recruited from the same clinics, for example), we applied the Huber-White sandwich estimator.15,16
We analyzed the association between levels of difficult patients and patient outcomes following a conceptual model. Using Cluster 3 (low frequency of difficult encounters) as the reference group, we tested the direct association of Cluster 1 (high frequency of difficult encounters) and Cluster 2 (medium frequency of encounters) with patient outcomes (eg, errors in diabetes and hypertension management, missed prevention activities, quality benchmarks met). We also tested the adjusted influence of Clusters 1 and 2 on patient outcomes, controlling for the mediators of burnout and satisfaction. Finally, we examined the direct influence of Clusters 1 and 2 on the mediators of burnout and satisfaction.
RESULTS
A total of 449 physicians from 119 clinics consented to participate in MEMO (59.8% of those approached), and 94% of these (n=422) completed the survey.4 Compared with participants, nonparticipants did not differ significantly by specialty or sex. Physicians were evenly divided between general internists (51.9%) and family physicians (48.1%). The mean age was 43 (range, 29-89), 44.4% were women, most (83.3%) worked full-time, and 22.0% were from a racial or ethnic minority group. Specific results of the Burden of Encounters measure, depicted in TABLE 1, have been reported previously.1
TABLE 1
Burden of Difficult Encounters measure1
Latent cluster analyses of this survey measure were used to assign physicians to one of 3 clusters: those who estimated a low, medium, or high frequency of difficult encounters in their practice.
| How often do the following interactions occur? (1=never; 4=often) | |
|---|---|
| Patients who: | No. of physicians providing ratings of 3 or 4 (%); n=422 |
| Visit regularly, but ignore medical advice | 155 (37) |
| Have expectations for care that are unrealistic | 68 (16) |
| Insist on being prescribed an unnecessary drug | 58 (14) |
| Insist on an unnecessary test | 54 (13) |
| Persistently complain, although you have done everything possible to help | 50 (12) |
| Do not express appropriate respect | 16 (4) |
| Show dissatisfaction with your care | 4 (1) |
| Are verbally abusive | 1 (0.2) |
Physicians were more likely to sort into the high (n=113) and medium (n=268) frequency of difficult encounter clusters as opposed to the low-frequency cluster (n=41) (TABLE 2). Of the 1384 patients whose records were audited, 359 were cared for by high-cluster physicians, 871 by medium-cluster physicians, and 154 by low-cluster physicians. Patients had a mean age of 59.6, 65.6% were women, and they had an average of 4.5 chronic medical conditions. A greater percentage of patients with physicians in the high-frequency cluster had a diagnosis of hypertension, compared with the medium cluster (92.4% vs 87.7%; P<.05). Patients did not differ across physician clusters by age, sex, prevalence of diabetes, or number of chronic diagnoses.
TABLE 2
Physician characteristics across frequency clusters (n=422)1
| Physician characteristic | Frequency-of-difficult-encounter cluster | ||
|---|---|---|---|
| High, % (n=113) | Medium, % (n=268) | Low, % (n=41) | |
| Family physicians (vs general internists) | 41.6 | 49.6 | 58.5 |
| Age, mean (SD) | 40.8 (9.0)*† | 43.3 (9.0) | 46.1 (13.4) |
| Female sex | 50.4† | 44.6‡ | 26.8 |
| Racial/ethnic minority | |||
| • Black or African American | 8.0 | 4.1‡ | 14.6 |
| • Asian | 13.3 | 11.9 | 9.8 |
| • Hispanic or Latino | 6.6 | 3.1 | 0 |
| • Other | 6.2 | 3.4 | 0 |
| Full-time work status | 83.8 | 83.5 | 80.5 |
| Exact probability tests were used to contrast proportional differences. *P<.05 for high vs medium frequency of difficult encounter clusters. †P<.05 for high vs low frequency of difficult encounter clusters. ‡P<.05 for medium vs low frequency of difficult encounter clusters. | |||
We examined the relationship between perceived frequency of difficult encounters and patient outcomes using a double-mediation model with physician burnout and satisfaction as mediators. We found that the greater the perceived number of difficult encounters, the greater the burnout and job dissatisfaction. For example, on a 5-point Likert scale measuring burnout where 1 = no burnout and 5 = significant and persistent burnout, medium-cluster physicians scored 0.48 points higher than the low-cluster physician cohort. High-cluster physicians scored 0.84 points higher than their low-cluster colleagues (both P<.05). Similarly, high-cluster physicians were less satisfied with their jobs; on a 5-point scale where 1 = low satisfaction and 5 = high satisfaction, high-cluster physicians scored 0.60 points lower than low-cluster physicians (P<.05).
Yet, there was no clear association between perceived frequency of difficult encounters and patient outcomes. High-cluster physicians had a 5.57% lower overall error rate compared with low-cluster physicians (P<.05), although this was not true for specific errors, such as those in hypertension or diabetes management, where rates were similar. High-cluster physicians also had a 7.68% lower overall quality rate (P<.05), although, again, this was not true for management of specific conditions such as hypertension and diabetes, where rates were similar. In sum, in our double-mediation model, there was no consistent influence of a physician’s difficult-encounter cluster on patient outcomes, even when including physician burnout and level of satisfaction as mediators.
DISCUSSION
Our principal finding is that the perception of frequent difficult encounters—while associated with significant physician burnout and dissatisfaction—was not associated with worse quality of patient care or higher rates of error. Physicians with a high volume of difficult encounters and burnout maintained standards of care for their patients comparable to those of their peers who experienced less frequent difficult encounters. We propose several hypotheses to explain this observation.
First, the Conservation of Resources (COR) Theory suggests that when resources are depleted or stressed by work demands (difficult encounters), burnout will result.17 In response, burned-out individuals will reduce their resource expenditure (attention, time) and focus their resources on the most important aspects of their work—in our case, measured quality of care. In the physician-patient communication literature, Williams et al suggest that burned-out physicians use a strictly biomedical style of communication,18 which is less resource intensive than more patient-centered forms of communication.19 Thus, while a physician may be burned out and dissatisfied, she or he will focus communication on key clinical aspects of the encounter (the presenting complaint, necessary preventive care) while de-emphasizing the psychosocial aspects of care. Consequently, a physician may be burned out by difficult encounters, but may continue to provide adequate patient care.
Second, these results may reflect (in part) the professional socialization of physicians. The rigors of medical school and residency training provide physicians with a high level of personal hardiness. The nursing literature defines hardiness as the interrelatedness of 3 factors controlled by the individual through lifestyle: control of the environment, commitment to self-fulfilling goals, and reasonable levels of challenge in daily life. Thomsens et al found that these traits serve as buffers to protect individuals from the psychological repercussions of stress.20
Nikou designed a study to investigate the relationships among hardiness, stress, and health-promoting behaviors in students attending a nursing student conference.21 The results indicated that hardiness was inversely related to stress and positively related to health-promoting behaviors. Thus, while physicians face challenging and difficult encounters and become burned out and dissatisfied, they are able to deliver acceptable patient care due to the buffering effect of their professional socialization.
Third, physicians’ responses to performance measurement pressures—ubiquitous in the culture of primary care medicine today—may also contribute to our findings. Physicians are called on to meet both national and local standards of care, and are expected to keep patients satisfied. Such objectives may be tied to financial incentives.22 In this environment, many doctors are likely to respond so that quality measures are met, even when faced with a challenging patient encounter. Higashi et al found that the quality of care delivered to patients was better as the number of chronic conditions increased.23 Others have argued that current clinical practice guidelines, which have driven quality measurement, have led to unintended consequences—for example, polypharmacy with inadequate consideration of adverse drug-drug interactions.22,24,25
Study limitations. This study is limited by its sample size, which may have restricted our ability to discern small but meaningful differences in quality and errors. In addition, enrollment bias—given that a small number of patients per physician were enrolled—could have muted potential positive findings. If possible, future studies should include outcomes from entire patient panels.
While the objective recording of quality and errors is a strength of this study, data on the frequency of difficult encounters were cross-sectional. As a result, causal relationships between physician-experienced difficulty and patient outcomes were not possible to determine.
Lastly, throughout this study the term “patient outcomes” has been limited to the particular medical outcomes used in our investigation. But it is well recognized that important patient outcomes could also include measures such as satisfaction, trust, medication adherence, and costs.
More to explore. We found that the perception of frequent difficult patient encounters was not associated with poorer patient outcomes, even in the setting of physician dissatisfaction and burnout. Although difficult encounters were associated with physician burnout and job dissatisfaction, it appears that physicians who perceived very frequent difficult patient encounters had comparable standards of care relative to their peers who reported fewer difficult encounters.
Future research should examine additional patient outcomes related to chronic conditions and acute care and their relationship to difficult encounters. Furthermore, other potential consequences of difficult encounters need to be explored, especially those that may result from poor physician-patient communication such as medication adherence, patient satisfaction, and trust.
CORRESPONDENCE
Perry G. An, MD, Newton-Wellesley Hospital, 2014 Washington Street, 2nd Floor, Newton, MA 02462; [email protected]
1. An PG, Rabatin JS, Manwell LB, et al. Burden of difficult patient encounters in primary care: data from the Minimizing Error, Maximizing Outcome Study. Arch Intern Med. 2009;169:410-414.
2. Hong CS, Atlas SJ, Chang Y, et al. Relationship between patient panel characteristics and primary care physician clinical performance rankings. JAMA. 2010;304:1107-1113.
3. Hinchey SA, Jackson JL. A cohort study assessing difficult patient encounters in a walk-in primary care clinic, predictors and outcomes. J Gen Intern Med. 2011;26:588-594.
4. Linzer M, Manwell LB, Williams ES, et al. Working conditions in primary care: physician reactions and care quality. Ann Intern Med. 2009;151:28-36.
5. Jackson JL, Kroenke K. Difficult patient encounters in the ambulatory clinic: clinical predictors and outcomes. Arch Intern Med. 1999;159:1069-1075.
6. Hahn SR, Kroenke K, Spitzer RL, et al. The difficult patient: prevalence, psychopathology, and functional impairment. J Gen Intern Med. 1996;11:1-8.
7. Hahn SR, Thompson KS, Wills TA, et al. The difficult doctor-patient relationship: somatization, personality and psychopathology. J Clin Epidemiol. 1994;47:647-657.
8. Wetterneck TB, Linzer M, McMurray JE, et al. Worklife and satisfaction of general internists. Arch Intern Med. 2002;162:649-656.
9. Calnan M, Wainwright D, Forsythe M, et al. General practice. All stressed up and nowhere to go? Health Serv J. 2000;110:28-29.
10. Mathers N, Jones N, Hannay D. Heartsink patients: a study of their general practitioners. Br J Gen Pract. 1995;45:293-296.
11. Krebs EE, Garrett JM, Konrad TR. The difficult doctor? Characteristics of physicians who report frustration with patients: an analysis of survey data. BMC Health Serv Res. 2006;6:128-135.
12. Linzer M, Manwell LB, Mundt M, et al. Organizational climate, stress, and error in primary care: the MEMO study. In: Advances in Patient Safety: From Research to Implementation. Vol. 1. AHRQ publication no. 050021 (1). Rockville, Md: Agency for Healthcare Research and Quality, 2005:65-77. Available at: http://www.ncbi.nlm.nih.gov/books/NBK20448. Accessed February 16, 2012.
13. Konrad TR, Williams ES, Linzer M, et al. Measuring physician job satisfaction in a changing workplace and a challenging environment. Med Care. 1999;37:1174-1182.
14. Williams ES, Konrad TR, Linzer M, et al. Refining the measurement of physician job satisfaction: results from the Physician Worklife Study. Med Care. 1999;37:1140-1154.
15. Huber PJ. Proceedings of the Fifth Berkeley Symposium on Mathematical Statistics and Probability. Berkeley, Calif: University of California Press; 1967:221–233.
16. White H. Maximum likelihood estimation of misspecified models. Econometrica. 1982;50:1-25.
17. Hobfoll SE. Conservation of resources: a new attempt at conceptualizing stress. Am Psychol. 1989;44:513-524.
18. Williams ES, Lawrence ER, Campbell KS, et al. The effect of emotional exhaustion and depersonalization on physician-patient communication: a theoretical model, implications, and directions for future research. Adv Health Care Manag. 2009;8:3-20.
19. Roter DL, Stewart M, Putnam SM, et al. Communication patterns of primary care physicians. JAMA. 1997;277:350-356.
20. Thomsens SB, Arnetz P, Nolan J, et al. Individual and organizational well-being in psychiatric nursing. J Adv Nursing. 1999;30:749-757.
21. Nikou VR. The relationships of hardiness, stress, and health-promoting behaviors in undergraduate female nursing students. Paper presented at: Promoting Students’ Success, 14th International Nursing Research Congress, Sigma Theta Tau International; July 12, 2003; St. Thomas, US Virgin Islands.
22. Stearns CR, Gonzales R, Camargo CA Jr, et al. Antibiotic prescriptions are associated with increased patient satisfaction with emergency department visits for acute respiratory tract infections. Acad Emerg Med. 2009;16:934-941.
23. Higashi T, Wenger NS, Adams JL, et al. Relationship between number of medical conditions and quality of care. N Engl J Med. 2007;356:2496-2504.
24. Boyd CM, Darer J, Boult C, et al. Clinical practice guidelines and quality of care for older patients with multiple comorbid disease: implications for pay for performance. JAMA. 2005;294:716-724.
25. Linder JA, Singer DE. Desire for antibiotics and antibiotic prescribing for adults with upper respiratory tract infections. J Gen Intern Med. 2003;18:795-801.
Background Difficult patient encounters in the primary care office are frequent and are associated with physician burnout. However, their relationship to patient care outcomes is not known.
Objective To determine the effect of difficult encounters on patient health outcomes and the role of physician dissatisfaction and burnout as mediators of this effect.
Design A total of 422 physicians were sorted into 3 clusters based on perceived frequency of difficult patient encounters in their practices. Patient charts were audited to assess the quality of hypertension and diabetes management and preventive care based on national guidelines. Summary measures of quality and errors were compared among the 3 physician clusters.
Results Of the 1384 patients, 359 were cared for by high-cluster physicians (those who had a high frequency of difficult encounters), 871 by medium-cluster physicians, and 154 by low-cluster physicians. Dissatisfaction and burnout were higher among physicians reporting higher frequencies of difficult encounters. However, quality of patient care and management errors were similar across all 3 groups.
Conclusions Physician perception of frequent difficult encounters was not associated with worse patient care quality or more medical errors. Future studies should investigate whether other patient outcomes, including acute care and patient satisfaction, are affected by difficult encounters.
Physicians who have high numbers of difficult patient encounters are more likely to report burnout and related stressor effects than are colleagues with fewer difficult encounters.1 More of them also perceive that they provide suboptimal care than do colleagues who report fewer difficult patients.1 These were some of the findings taken from the Minimizing Error, Maximizing Outcome (MEMO) Study that we conducted from 2001 to 2005.1 But these findings prompted us to wonder: Is that perception accurate?
Whether physicians reporting high numbers of difficult patient encounters actually provide poorer care is unknown. In a recent study of physicians from one large primary care system, patient panels that were more challenging—as determined by higher rates of underinsured, minority, and non-English-speaking patients—were associated with lower quality care.2 Hinchey and Jackson found that 2 weeks after initial presentation, patients involved in difficult encounters at a walk-in clinic experienced worsening physical symptoms.3 However, this study did not address whether difficult patient encounters affected the care rendered by providers to patients in general.
A detailed, rigorous model describing the interplay and relationships among difficult encounters, adverse physician outcomes (eg, burnout, dissatisfaction), and patient health outcomes has yet to be developed. To better understand the effects of these interactions, we revisited data from the MEMO study.
The findings that prompted another look at the data
When we conducted the MEMO study, we surveyed 422 physicians working in 119 primary care clinics in the upper Midwest and New York City.4 Almost half (49%) of the physicians reported moderately or highly stressful jobs; 27% reported burnout; and 30% were at least moderately likely to leave their practices within 2 years. Of these physicians, 113 (27%) reported high numbers of difficult encounters, which corresponds with other reports of 10% to 37% in primary care settings.5-7 These 113 physicians were 12.2 times more likely to report burnout compared with colleagues with fewer difficult encounters.1 They also reported lower job satisfaction, increased stress, more time pressure, and greater intent to leave practice, which are also echoed in other studies.8-10
We found in our study (and at least one other) that physicians experiencing burnout are often younger and female, work long hours, and practice in a medicine subspecialty.1,11 Many physicians who care for difficult patients report that they secretly hope these patients will not return.6
Our hypothesis
We hypothesized that frequent difficult encounters may amplify an adverse work environment, and that physicians facing time pressure and a lack of work control brought on by these encounters might be unable to sustain a high standard of care for their overall patient load.
METHODS
Participants
Physician and patient participants and design of the MEMO study are described in detail elsewhere.12 The following, though, is a recap:
We recruited 422 general internists and family physicians from 119 ambulatory care clinics in New York City and the upper Midwest. These regions offered a diverse patient and payer mix. Physicians were asked via on-site presentations and mailed invitations to complete a survey derived from focus groups and the Physician Worklife Survey.13,14
We also recruited up to 8 patients per participating physician via mailed invitations. Inclusion criteria were a minimum age of 18; a diagnosis of at least one target condition (hypertension, diabetes, congestive heart failure); ability to read in English, Spanish, or Chinese; and at least 2 visits with their primary physician in the previous year.
Here we report on outcomes for those patients with diabetes and hypertension.
Measures
When we initially conducted the study, physicians completed an 8-item Burden of Difficult Encounters measure designed to approximate the frequency of difficult encounters experienced. Latent cluster analyses of this survey measure defined 3 distinct groups of physicians: those who estimated a high, medium, and low frequency of difficult encounters in their practices. Via chart audits, we determined quality of care and errors related to guideline-recommended management and preventive care for hypertension and diabetes. Details of these audits are found elsewhere.4
We defined quality care for hypertension as successful blood pressure control (<140/90), and for diabetes, successful control of hemoglobin A1c (≤7.5) and blood pressure (<135/80). One quality point was awarded for each of these 3 measures if achieved for at least 50% of recorded visits over an 18-month period. We calculated the quality score as the proportion of total possible quality points (with 100%=best).
We defined errors as guideline non-adherence and missed opportunities for prevention or management, tailored to each patient’s age, sex, and diagnoses. We calculated the error score as the proportion of total applicable error points (maximum=15; 0%=best). We assigned an error point for each missing process of care, including missed treatment opportunities, inattention to behavioral factors, guideline nonadherence, lack of tobacco use documentation, and missed prevention activities, such as mammograms, cervical cancer screening, colon cancer screening, and depression assessment.
We normalized scores to a range of 0 to 100 by dividing the number of quality or error points by the number of applicable items and multiplying by 100. We calculated quality and error scores for hypertension or diabetes for each patient and averaged them to determine total scores per physician.
Data analysis
Latent cluster analyses identified 3 distinct clusters of physicians based on their reported frequency of difficult encounters.1 We used a 2-level hierarchical linear model of patients nested under physicians to assess if a higher number of perceived difficult patients was associated with poorer patient care, as measured by quality of care and medical errors, controlling for physician age, sex, and racial/ethnic minority status. To further adjust for negatively biased standard errors (physicians recruited from the same clinics, for example), we applied the Huber-White sandwich estimator.15,16
We analyzed the association between levels of difficult patients and patient outcomes following a conceptual model. Using Cluster 3 (low frequency of difficult encounters) as the reference group, we tested the direct association of Cluster 1 (high frequency of difficult encounters) and Cluster 2 (medium frequency of encounters) with patient outcomes (eg, errors in diabetes and hypertension management, missed prevention activities, quality benchmarks met). We also tested the adjusted influence of Clusters 1 and 2 on patient outcomes, controlling for the mediators of burnout and satisfaction. Finally, we examined the direct influence of Clusters 1 and 2 on the mediators of burnout and satisfaction.
RESULTS
A total of 449 physicians from 119 clinics consented to participate in MEMO (59.8% of those approached), and 94% of these (n=422) completed the survey.4 Compared with participants, nonparticipants did not differ significantly by specialty or sex. Physicians were evenly divided between general internists (51.9%) and family physicians (48.1%). The mean age was 43 (range, 29-89), 44.4% were women, most (83.3%) worked full-time, and 22.0% were from a racial or ethnic minority group. Specific results of the Burden of Encounters measure, depicted in TABLE 1, have been reported previously.1
TABLE 1
Burden of Difficult Encounters measure1
Latent cluster analyses of this survey measure were used to assign physicians to one of 3 clusters: those who estimated a low, medium, or high frequency of difficult encounters in their practice.
| How often do the following interactions occur? (1=never; 4=often) | |
|---|---|
| Patients who: | No. of physicians providing ratings of 3 or 4 (%); n=422 |
| Visit regularly, but ignore medical advice | 155 (37) |
| Have expectations for care that are unrealistic | 68 (16) |
| Insist on being prescribed an unnecessary drug | 58 (14) |
| Insist on an unnecessary test | 54 (13) |
| Persistently complain, although you have done everything possible to help | 50 (12) |
| Do not express appropriate respect | 16 (4) |
| Show dissatisfaction with your care | 4 (1) |
| Are verbally abusive | 1 (0.2) |
Physicians were more likely to sort into the high (n=113) and medium (n=268) frequency of difficult encounter clusters as opposed to the low-frequency cluster (n=41) (TABLE 2). Of the 1384 patients whose records were audited, 359 were cared for by high-cluster physicians, 871 by medium-cluster physicians, and 154 by low-cluster physicians. Patients had a mean age of 59.6, 65.6% were women, and they had an average of 4.5 chronic medical conditions. A greater percentage of patients with physicians in the high-frequency cluster had a diagnosis of hypertension, compared with the medium cluster (92.4% vs 87.7%; P<.05). Patients did not differ across physician clusters by age, sex, prevalence of diabetes, or number of chronic diagnoses.
TABLE 2
Physician characteristics across frequency clusters (n=422)1
| Physician characteristic | Frequency-of-difficult-encounter cluster | ||
|---|---|---|---|
| High, % (n=113) | Medium, % (n=268) | Low, % (n=41) | |
| Family physicians (vs general internists) | 41.6 | 49.6 | 58.5 |
| Age, mean (SD) | 40.8 (9.0)*† | 43.3 (9.0) | 46.1 (13.4) |
| Female sex | 50.4† | 44.6‡ | 26.8 |
| Racial/ethnic minority | |||
| • Black or African American | 8.0 | 4.1‡ | 14.6 |
| • Asian | 13.3 | 11.9 | 9.8 |
| • Hispanic or Latino | 6.6 | 3.1 | 0 |
| • Other | 6.2 | 3.4 | 0 |
| Full-time work status | 83.8 | 83.5 | 80.5 |
| Exact probability tests were used to contrast proportional differences. *P<.05 for high vs medium frequency of difficult encounter clusters. †P<.05 for high vs low frequency of difficult encounter clusters. ‡P<.05 for medium vs low frequency of difficult encounter clusters. | |||
We examined the relationship between perceived frequency of difficult encounters and patient outcomes using a double-mediation model with physician burnout and satisfaction as mediators. We found that the greater the perceived number of difficult encounters, the greater the burnout and job dissatisfaction. For example, on a 5-point Likert scale measuring burnout where 1 = no burnout and 5 = significant and persistent burnout, medium-cluster physicians scored 0.48 points higher than the low-cluster physician cohort. High-cluster physicians scored 0.84 points higher than their low-cluster colleagues (both P<.05). Similarly, high-cluster physicians were less satisfied with their jobs; on a 5-point scale where 1 = low satisfaction and 5 = high satisfaction, high-cluster physicians scored 0.60 points lower than low-cluster physicians (P<.05).
Yet, there was no clear association between perceived frequency of difficult encounters and patient outcomes. High-cluster physicians had a 5.57% lower overall error rate compared with low-cluster physicians (P<.05), although this was not true for specific errors, such as those in hypertension or diabetes management, where rates were similar. High-cluster physicians also had a 7.68% lower overall quality rate (P<.05), although, again, this was not true for management of specific conditions such as hypertension and diabetes, where rates were similar. In sum, in our double-mediation model, there was no consistent influence of a physician’s difficult-encounter cluster on patient outcomes, even when including physician burnout and level of satisfaction as mediators.
DISCUSSION
Our principal finding is that the perception of frequent difficult encounters—while associated with significant physician burnout and dissatisfaction—was not associated with worse quality of patient care or higher rates of error. Physicians with a high volume of difficult encounters and burnout maintained standards of care for their patients comparable to those of their peers who experienced less frequent difficult encounters. We propose several hypotheses to explain this observation.
First, the Conservation of Resources (COR) Theory suggests that when resources are depleted or stressed by work demands (difficult encounters), burnout will result.17 In response, burned-out individuals will reduce their resource expenditure (attention, time) and focus their resources on the most important aspects of their work—in our case, measured quality of care. In the physician-patient communication literature, Williams et al suggest that burned-out physicians use a strictly biomedical style of communication,18 which is less resource intensive than more patient-centered forms of communication.19 Thus, while a physician may be burned out and dissatisfied, she or he will focus communication on key clinical aspects of the encounter (the presenting complaint, necessary preventive care) while de-emphasizing the psychosocial aspects of care. Consequently, a physician may be burned out by difficult encounters, but may continue to provide adequate patient care.
Second, these results may reflect (in part) the professional socialization of physicians. The rigors of medical school and residency training provide physicians with a high level of personal hardiness. The nursing literature defines hardiness as the interrelatedness of 3 factors controlled by the individual through lifestyle: control of the environment, commitment to self-fulfilling goals, and reasonable levels of challenge in daily life. Thomsens et al found that these traits serve as buffers to protect individuals from the psychological repercussions of stress.20
Nikou designed a study to investigate the relationships among hardiness, stress, and health-promoting behaviors in students attending a nursing student conference.21 The results indicated that hardiness was inversely related to stress and positively related to health-promoting behaviors. Thus, while physicians face challenging and difficult encounters and become burned out and dissatisfied, they are able to deliver acceptable patient care due to the buffering effect of their professional socialization.
Third, physicians’ responses to performance measurement pressures—ubiquitous in the culture of primary care medicine today—may also contribute to our findings. Physicians are called on to meet both national and local standards of care, and are expected to keep patients satisfied. Such objectives may be tied to financial incentives.22 In this environment, many doctors are likely to respond so that quality measures are met, even when faced with a challenging patient encounter. Higashi et al found that the quality of care delivered to patients was better as the number of chronic conditions increased.23 Others have argued that current clinical practice guidelines, which have driven quality measurement, have led to unintended consequences—for example, polypharmacy with inadequate consideration of adverse drug-drug interactions.22,24,25
Study limitations. This study is limited by its sample size, which may have restricted our ability to discern small but meaningful differences in quality and errors. In addition, enrollment bias—given that a small number of patients per physician were enrolled—could have muted potential positive findings. If possible, future studies should include outcomes from entire patient panels.
While the objective recording of quality and errors is a strength of this study, data on the frequency of difficult encounters were cross-sectional. As a result, causal relationships between physician-experienced difficulty and patient outcomes were not possible to determine.
Lastly, throughout this study the term “patient outcomes” has been limited to the particular medical outcomes used in our investigation. But it is well recognized that important patient outcomes could also include measures such as satisfaction, trust, medication adherence, and costs.
More to explore. We found that the perception of frequent difficult patient encounters was not associated with poorer patient outcomes, even in the setting of physician dissatisfaction and burnout. Although difficult encounters were associated with physician burnout and job dissatisfaction, it appears that physicians who perceived very frequent difficult patient encounters had comparable standards of care relative to their peers who reported fewer difficult encounters.
Future research should examine additional patient outcomes related to chronic conditions and acute care and their relationship to difficult encounters. Furthermore, other potential consequences of difficult encounters need to be explored, especially those that may result from poor physician-patient communication such as medication adherence, patient satisfaction, and trust.
CORRESPONDENCE
Perry G. An, MD, Newton-Wellesley Hospital, 2014 Washington Street, 2nd Floor, Newton, MA 02462; [email protected]
Background Difficult patient encounters in the primary care office are frequent and are associated with physician burnout. However, their relationship to patient care outcomes is not known.
Objective To determine the effect of difficult encounters on patient health outcomes and the role of physician dissatisfaction and burnout as mediators of this effect.
Design A total of 422 physicians were sorted into 3 clusters based on perceived frequency of difficult patient encounters in their practices. Patient charts were audited to assess the quality of hypertension and diabetes management and preventive care based on national guidelines. Summary measures of quality and errors were compared among the 3 physician clusters.
Results Of the 1384 patients, 359 were cared for by high-cluster physicians (those who had a high frequency of difficult encounters), 871 by medium-cluster physicians, and 154 by low-cluster physicians. Dissatisfaction and burnout were higher among physicians reporting higher frequencies of difficult encounters. However, quality of patient care and management errors were similar across all 3 groups.
Conclusions Physician perception of frequent difficult encounters was not associated with worse patient care quality or more medical errors. Future studies should investigate whether other patient outcomes, including acute care and patient satisfaction, are affected by difficult encounters.
Physicians who have high numbers of difficult patient encounters are more likely to report burnout and related stressor effects than are colleagues with fewer difficult encounters.1 More of them also perceive that they provide suboptimal care than do colleagues who report fewer difficult patients.1 These were some of the findings taken from the Minimizing Error, Maximizing Outcome (MEMO) Study that we conducted from 2001 to 2005.1 But these findings prompted us to wonder: Is that perception accurate?
Whether physicians reporting high numbers of difficult patient encounters actually provide poorer care is unknown. In a recent study of physicians from one large primary care system, patient panels that were more challenging—as determined by higher rates of underinsured, minority, and non-English-speaking patients—were associated with lower quality care.2 Hinchey and Jackson found that 2 weeks after initial presentation, patients involved in difficult encounters at a walk-in clinic experienced worsening physical symptoms.3 However, this study did not address whether difficult patient encounters affected the care rendered by providers to patients in general.
A detailed, rigorous model describing the interplay and relationships among difficult encounters, adverse physician outcomes (eg, burnout, dissatisfaction), and patient health outcomes has yet to be developed. To better understand the effects of these interactions, we revisited data from the MEMO study.
The findings that prompted another look at the data
When we conducted the MEMO study, we surveyed 422 physicians working in 119 primary care clinics in the upper Midwest and New York City.4 Almost half (49%) of the physicians reported moderately or highly stressful jobs; 27% reported burnout; and 30% were at least moderately likely to leave their practices within 2 years. Of these physicians, 113 (27%) reported high numbers of difficult encounters, which corresponds with other reports of 10% to 37% in primary care settings.5-7 These 113 physicians were 12.2 times more likely to report burnout compared with colleagues with fewer difficult encounters.1 They also reported lower job satisfaction, increased stress, more time pressure, and greater intent to leave practice, which are also echoed in other studies.8-10
We found in our study (and at least one other) that physicians experiencing burnout are often younger and female, work long hours, and practice in a medicine subspecialty.1,11 Many physicians who care for difficult patients report that they secretly hope these patients will not return.6
Our hypothesis
We hypothesized that frequent difficult encounters may amplify an adverse work environment, and that physicians facing time pressure and a lack of work control brought on by these encounters might be unable to sustain a high standard of care for their overall patient load.
METHODS
Participants
Physician and patient participants and design of the MEMO study are described in detail elsewhere.12 The following, though, is a recap:
We recruited 422 general internists and family physicians from 119 ambulatory care clinics in New York City and the upper Midwest. These regions offered a diverse patient and payer mix. Physicians were asked via on-site presentations and mailed invitations to complete a survey derived from focus groups and the Physician Worklife Survey.13,14
We also recruited up to 8 patients per participating physician via mailed invitations. Inclusion criteria were a minimum age of 18; a diagnosis of at least one target condition (hypertension, diabetes, congestive heart failure); ability to read in English, Spanish, or Chinese; and at least 2 visits with their primary physician in the previous year.
Here we report on outcomes for those patients with diabetes and hypertension.
Measures
When we initially conducted the study, physicians completed an 8-item Burden of Difficult Encounters measure designed to approximate the frequency of difficult encounters experienced. Latent cluster analyses of this survey measure defined 3 distinct groups of physicians: those who estimated a high, medium, and low frequency of difficult encounters in their practices. Via chart audits, we determined quality of care and errors related to guideline-recommended management and preventive care for hypertension and diabetes. Details of these audits are found elsewhere.4
We defined quality care for hypertension as successful blood pressure control (<140/90), and for diabetes, successful control of hemoglobin A1c (≤7.5) and blood pressure (<135/80). One quality point was awarded for each of these 3 measures if achieved for at least 50% of recorded visits over an 18-month period. We calculated the quality score as the proportion of total possible quality points (with 100%=best).
We defined errors as guideline non-adherence and missed opportunities for prevention or management, tailored to each patient’s age, sex, and diagnoses. We calculated the error score as the proportion of total applicable error points (maximum=15; 0%=best). We assigned an error point for each missing process of care, including missed treatment opportunities, inattention to behavioral factors, guideline nonadherence, lack of tobacco use documentation, and missed prevention activities, such as mammograms, cervical cancer screening, colon cancer screening, and depression assessment.
We normalized scores to a range of 0 to 100 by dividing the number of quality or error points by the number of applicable items and multiplying by 100. We calculated quality and error scores for hypertension or diabetes for each patient and averaged them to determine total scores per physician.
Data analysis
Latent cluster analyses identified 3 distinct clusters of physicians based on their reported frequency of difficult encounters.1 We used a 2-level hierarchical linear model of patients nested under physicians to assess if a higher number of perceived difficult patients was associated with poorer patient care, as measured by quality of care and medical errors, controlling for physician age, sex, and racial/ethnic minority status. To further adjust for negatively biased standard errors (physicians recruited from the same clinics, for example), we applied the Huber-White sandwich estimator.15,16
We analyzed the association between levels of difficult patients and patient outcomes following a conceptual model. Using Cluster 3 (low frequency of difficult encounters) as the reference group, we tested the direct association of Cluster 1 (high frequency of difficult encounters) and Cluster 2 (medium frequency of encounters) with patient outcomes (eg, errors in diabetes and hypertension management, missed prevention activities, quality benchmarks met). We also tested the adjusted influence of Clusters 1 and 2 on patient outcomes, controlling for the mediators of burnout and satisfaction. Finally, we examined the direct influence of Clusters 1 and 2 on the mediators of burnout and satisfaction.
RESULTS
A total of 449 physicians from 119 clinics consented to participate in MEMO (59.8% of those approached), and 94% of these (n=422) completed the survey.4 Compared with participants, nonparticipants did not differ significantly by specialty or sex. Physicians were evenly divided between general internists (51.9%) and family physicians (48.1%). The mean age was 43 (range, 29-89), 44.4% were women, most (83.3%) worked full-time, and 22.0% were from a racial or ethnic minority group. Specific results of the Burden of Encounters measure, depicted in TABLE 1, have been reported previously.1
TABLE 1
Burden of Difficult Encounters measure1
Latent cluster analyses of this survey measure were used to assign physicians to one of 3 clusters: those who estimated a low, medium, or high frequency of difficult encounters in their practice.
| How often do the following interactions occur? (1=never; 4=often) | |
|---|---|
| Patients who: | No. of physicians providing ratings of 3 or 4 (%); n=422 |
| Visit regularly, but ignore medical advice | 155 (37) |
| Have expectations for care that are unrealistic | 68 (16) |
| Insist on being prescribed an unnecessary drug | 58 (14) |
| Insist on an unnecessary test | 54 (13) |
| Persistently complain, although you have done everything possible to help | 50 (12) |
| Do not express appropriate respect | 16 (4) |
| Show dissatisfaction with your care | 4 (1) |
| Are verbally abusive | 1 (0.2) |
Physicians were more likely to sort into the high (n=113) and medium (n=268) frequency of difficult encounter clusters as opposed to the low-frequency cluster (n=41) (TABLE 2). Of the 1384 patients whose records were audited, 359 were cared for by high-cluster physicians, 871 by medium-cluster physicians, and 154 by low-cluster physicians. Patients had a mean age of 59.6, 65.6% were women, and they had an average of 4.5 chronic medical conditions. A greater percentage of patients with physicians in the high-frequency cluster had a diagnosis of hypertension, compared with the medium cluster (92.4% vs 87.7%; P<.05). Patients did not differ across physician clusters by age, sex, prevalence of diabetes, or number of chronic diagnoses.
TABLE 2
Physician characteristics across frequency clusters (n=422)1
| Physician characteristic | Frequency-of-difficult-encounter cluster | ||
|---|---|---|---|
| High, % (n=113) | Medium, % (n=268) | Low, % (n=41) | |
| Family physicians (vs general internists) | 41.6 | 49.6 | 58.5 |
| Age, mean (SD) | 40.8 (9.0)*† | 43.3 (9.0) | 46.1 (13.4) |
| Female sex | 50.4† | 44.6‡ | 26.8 |
| Racial/ethnic minority | |||
| • Black or African American | 8.0 | 4.1‡ | 14.6 |
| • Asian | 13.3 | 11.9 | 9.8 |
| • Hispanic or Latino | 6.6 | 3.1 | 0 |
| • Other | 6.2 | 3.4 | 0 |
| Full-time work status | 83.8 | 83.5 | 80.5 |
| Exact probability tests were used to contrast proportional differences. *P<.05 for high vs medium frequency of difficult encounter clusters. †P<.05 for high vs low frequency of difficult encounter clusters. ‡P<.05 for medium vs low frequency of difficult encounter clusters. | |||
We examined the relationship between perceived frequency of difficult encounters and patient outcomes using a double-mediation model with physician burnout and satisfaction as mediators. We found that the greater the perceived number of difficult encounters, the greater the burnout and job dissatisfaction. For example, on a 5-point Likert scale measuring burnout where 1 = no burnout and 5 = significant and persistent burnout, medium-cluster physicians scored 0.48 points higher than the low-cluster physician cohort. High-cluster physicians scored 0.84 points higher than their low-cluster colleagues (both P<.05). Similarly, high-cluster physicians were less satisfied with their jobs; on a 5-point scale where 1 = low satisfaction and 5 = high satisfaction, high-cluster physicians scored 0.60 points lower than low-cluster physicians (P<.05).
Yet, there was no clear association between perceived frequency of difficult encounters and patient outcomes. High-cluster physicians had a 5.57% lower overall error rate compared with low-cluster physicians (P<.05), although this was not true for specific errors, such as those in hypertension or diabetes management, where rates were similar. High-cluster physicians also had a 7.68% lower overall quality rate (P<.05), although, again, this was not true for management of specific conditions such as hypertension and diabetes, where rates were similar. In sum, in our double-mediation model, there was no consistent influence of a physician’s difficult-encounter cluster on patient outcomes, even when including physician burnout and level of satisfaction as mediators.
DISCUSSION
Our principal finding is that the perception of frequent difficult encounters—while associated with significant physician burnout and dissatisfaction—was not associated with worse quality of patient care or higher rates of error. Physicians with a high volume of difficult encounters and burnout maintained standards of care for their patients comparable to those of their peers who experienced less frequent difficult encounters. We propose several hypotheses to explain this observation.
First, the Conservation of Resources (COR) Theory suggests that when resources are depleted or stressed by work demands (difficult encounters), burnout will result.17 In response, burned-out individuals will reduce their resource expenditure (attention, time) and focus their resources on the most important aspects of their work—in our case, measured quality of care. In the physician-patient communication literature, Williams et al suggest that burned-out physicians use a strictly biomedical style of communication,18 which is less resource intensive than more patient-centered forms of communication.19 Thus, while a physician may be burned out and dissatisfied, she or he will focus communication on key clinical aspects of the encounter (the presenting complaint, necessary preventive care) while de-emphasizing the psychosocial aspects of care. Consequently, a physician may be burned out by difficult encounters, but may continue to provide adequate patient care.
Second, these results may reflect (in part) the professional socialization of physicians. The rigors of medical school and residency training provide physicians with a high level of personal hardiness. The nursing literature defines hardiness as the interrelatedness of 3 factors controlled by the individual through lifestyle: control of the environment, commitment to self-fulfilling goals, and reasonable levels of challenge in daily life. Thomsens et al found that these traits serve as buffers to protect individuals from the psychological repercussions of stress.20
Nikou designed a study to investigate the relationships among hardiness, stress, and health-promoting behaviors in students attending a nursing student conference.21 The results indicated that hardiness was inversely related to stress and positively related to health-promoting behaviors. Thus, while physicians face challenging and difficult encounters and become burned out and dissatisfied, they are able to deliver acceptable patient care due to the buffering effect of their professional socialization.
Third, physicians’ responses to performance measurement pressures—ubiquitous in the culture of primary care medicine today—may also contribute to our findings. Physicians are called on to meet both national and local standards of care, and are expected to keep patients satisfied. Such objectives may be tied to financial incentives.22 In this environment, many doctors are likely to respond so that quality measures are met, even when faced with a challenging patient encounter. Higashi et al found that the quality of care delivered to patients was better as the number of chronic conditions increased.23 Others have argued that current clinical practice guidelines, which have driven quality measurement, have led to unintended consequences—for example, polypharmacy with inadequate consideration of adverse drug-drug interactions.22,24,25
Study limitations. This study is limited by its sample size, which may have restricted our ability to discern small but meaningful differences in quality and errors. In addition, enrollment bias—given that a small number of patients per physician were enrolled—could have muted potential positive findings. If possible, future studies should include outcomes from entire patient panels.
While the objective recording of quality and errors is a strength of this study, data on the frequency of difficult encounters were cross-sectional. As a result, causal relationships between physician-experienced difficulty and patient outcomes were not possible to determine.
Lastly, throughout this study the term “patient outcomes” has been limited to the particular medical outcomes used in our investigation. But it is well recognized that important patient outcomes could also include measures such as satisfaction, trust, medication adherence, and costs.
More to explore. We found that the perception of frequent difficult patient encounters was not associated with poorer patient outcomes, even in the setting of physician dissatisfaction and burnout. Although difficult encounters were associated with physician burnout and job dissatisfaction, it appears that physicians who perceived very frequent difficult patient encounters had comparable standards of care relative to their peers who reported fewer difficult encounters.
Future research should examine additional patient outcomes related to chronic conditions and acute care and their relationship to difficult encounters. Furthermore, other potential consequences of difficult encounters need to be explored, especially those that may result from poor physician-patient communication such as medication adherence, patient satisfaction, and trust.
CORRESPONDENCE
Perry G. An, MD, Newton-Wellesley Hospital, 2014 Washington Street, 2nd Floor, Newton, MA 02462; [email protected]
1. An PG, Rabatin JS, Manwell LB, et al. Burden of difficult patient encounters in primary care: data from the Minimizing Error, Maximizing Outcome Study. Arch Intern Med. 2009;169:410-414.
2. Hong CS, Atlas SJ, Chang Y, et al. Relationship between patient panel characteristics and primary care physician clinical performance rankings. JAMA. 2010;304:1107-1113.
3. Hinchey SA, Jackson JL. A cohort study assessing difficult patient encounters in a walk-in primary care clinic, predictors and outcomes. J Gen Intern Med. 2011;26:588-594.
4. Linzer M, Manwell LB, Williams ES, et al. Working conditions in primary care: physician reactions and care quality. Ann Intern Med. 2009;151:28-36.
5. Jackson JL, Kroenke K. Difficult patient encounters in the ambulatory clinic: clinical predictors and outcomes. Arch Intern Med. 1999;159:1069-1075.
6. Hahn SR, Kroenke K, Spitzer RL, et al. The difficult patient: prevalence, psychopathology, and functional impairment. J Gen Intern Med. 1996;11:1-8.
7. Hahn SR, Thompson KS, Wills TA, et al. The difficult doctor-patient relationship: somatization, personality and psychopathology. J Clin Epidemiol. 1994;47:647-657.
8. Wetterneck TB, Linzer M, McMurray JE, et al. Worklife and satisfaction of general internists. Arch Intern Med. 2002;162:649-656.
9. Calnan M, Wainwright D, Forsythe M, et al. General practice. All stressed up and nowhere to go? Health Serv J. 2000;110:28-29.
10. Mathers N, Jones N, Hannay D. Heartsink patients: a study of their general practitioners. Br J Gen Pract. 1995;45:293-296.
11. Krebs EE, Garrett JM, Konrad TR. The difficult doctor? Characteristics of physicians who report frustration with patients: an analysis of survey data. BMC Health Serv Res. 2006;6:128-135.
12. Linzer M, Manwell LB, Mundt M, et al. Organizational climate, stress, and error in primary care: the MEMO study. In: Advances in Patient Safety: From Research to Implementation. Vol. 1. AHRQ publication no. 050021 (1). Rockville, Md: Agency for Healthcare Research and Quality, 2005:65-77. Available at: http://www.ncbi.nlm.nih.gov/books/NBK20448. Accessed February 16, 2012.
13. Konrad TR, Williams ES, Linzer M, et al. Measuring physician job satisfaction in a changing workplace and a challenging environment. Med Care. 1999;37:1174-1182.
14. Williams ES, Konrad TR, Linzer M, et al. Refining the measurement of physician job satisfaction: results from the Physician Worklife Study. Med Care. 1999;37:1140-1154.
15. Huber PJ. Proceedings of the Fifth Berkeley Symposium on Mathematical Statistics and Probability. Berkeley, Calif: University of California Press; 1967:221–233.
16. White H. Maximum likelihood estimation of misspecified models. Econometrica. 1982;50:1-25.
17. Hobfoll SE. Conservation of resources: a new attempt at conceptualizing stress. Am Psychol. 1989;44:513-524.
18. Williams ES, Lawrence ER, Campbell KS, et al. The effect of emotional exhaustion and depersonalization on physician-patient communication: a theoretical model, implications, and directions for future research. Adv Health Care Manag. 2009;8:3-20.
19. Roter DL, Stewart M, Putnam SM, et al. Communication patterns of primary care physicians. JAMA. 1997;277:350-356.
20. Thomsens SB, Arnetz P, Nolan J, et al. Individual and organizational well-being in psychiatric nursing. J Adv Nursing. 1999;30:749-757.
21. Nikou VR. The relationships of hardiness, stress, and health-promoting behaviors in undergraduate female nursing students. Paper presented at: Promoting Students’ Success, 14th International Nursing Research Congress, Sigma Theta Tau International; July 12, 2003; St. Thomas, US Virgin Islands.
22. Stearns CR, Gonzales R, Camargo CA Jr, et al. Antibiotic prescriptions are associated with increased patient satisfaction with emergency department visits for acute respiratory tract infections. Acad Emerg Med. 2009;16:934-941.
23. Higashi T, Wenger NS, Adams JL, et al. Relationship between number of medical conditions and quality of care. N Engl J Med. 2007;356:2496-2504.
24. Boyd CM, Darer J, Boult C, et al. Clinical practice guidelines and quality of care for older patients with multiple comorbid disease: implications for pay for performance. JAMA. 2005;294:716-724.
25. Linder JA, Singer DE. Desire for antibiotics and antibiotic prescribing for adults with upper respiratory tract infections. J Gen Intern Med. 2003;18:795-801.
1. An PG, Rabatin JS, Manwell LB, et al. Burden of difficult patient encounters in primary care: data from the Minimizing Error, Maximizing Outcome Study. Arch Intern Med. 2009;169:410-414.
2. Hong CS, Atlas SJ, Chang Y, et al. Relationship between patient panel characteristics and primary care physician clinical performance rankings. JAMA. 2010;304:1107-1113.
3. Hinchey SA, Jackson JL. A cohort study assessing difficult patient encounters in a walk-in primary care clinic, predictors and outcomes. J Gen Intern Med. 2011;26:588-594.
4. Linzer M, Manwell LB, Williams ES, et al. Working conditions in primary care: physician reactions and care quality. Ann Intern Med. 2009;151:28-36.
5. Jackson JL, Kroenke K. Difficult patient encounters in the ambulatory clinic: clinical predictors and outcomes. Arch Intern Med. 1999;159:1069-1075.
6. Hahn SR, Kroenke K, Spitzer RL, et al. The difficult patient: prevalence, psychopathology, and functional impairment. J Gen Intern Med. 1996;11:1-8.
7. Hahn SR, Thompson KS, Wills TA, et al. The difficult doctor-patient relationship: somatization, personality and psychopathology. J Clin Epidemiol. 1994;47:647-657.
8. Wetterneck TB, Linzer M, McMurray JE, et al. Worklife and satisfaction of general internists. Arch Intern Med. 2002;162:649-656.
9. Calnan M, Wainwright D, Forsythe M, et al. General practice. All stressed up and nowhere to go? Health Serv J. 2000;110:28-29.
10. Mathers N, Jones N, Hannay D. Heartsink patients: a study of their general practitioners. Br J Gen Pract. 1995;45:293-296.
11. Krebs EE, Garrett JM, Konrad TR. The difficult doctor? Characteristics of physicians who report frustration with patients: an analysis of survey data. BMC Health Serv Res. 2006;6:128-135.
12. Linzer M, Manwell LB, Mundt M, et al. Organizational climate, stress, and error in primary care: the MEMO study. In: Advances in Patient Safety: From Research to Implementation. Vol. 1. AHRQ publication no. 050021 (1). Rockville, Md: Agency for Healthcare Research and Quality, 2005:65-77. Available at: http://www.ncbi.nlm.nih.gov/books/NBK20448. Accessed February 16, 2012.
13. Konrad TR, Williams ES, Linzer M, et al. Measuring physician job satisfaction in a changing workplace and a challenging environment. Med Care. 1999;37:1174-1182.
14. Williams ES, Konrad TR, Linzer M, et al. Refining the measurement of physician job satisfaction: results from the Physician Worklife Study. Med Care. 1999;37:1140-1154.
15. Huber PJ. Proceedings of the Fifth Berkeley Symposium on Mathematical Statistics and Probability. Berkeley, Calif: University of California Press; 1967:221–233.
16. White H. Maximum likelihood estimation of misspecified models. Econometrica. 1982;50:1-25.
17. Hobfoll SE. Conservation of resources: a new attempt at conceptualizing stress. Am Psychol. 1989;44:513-524.
18. Williams ES, Lawrence ER, Campbell KS, et al. The effect of emotional exhaustion and depersonalization on physician-patient communication: a theoretical model, implications, and directions for future research. Adv Health Care Manag. 2009;8:3-20.
19. Roter DL, Stewart M, Putnam SM, et al. Communication patterns of primary care physicians. JAMA. 1997;277:350-356.
20. Thomsens SB, Arnetz P, Nolan J, et al. Individual and organizational well-being in psychiatric nursing. J Adv Nursing. 1999;30:749-757.
21. Nikou VR. The relationships of hardiness, stress, and health-promoting behaviors in undergraduate female nursing students. Paper presented at: Promoting Students’ Success, 14th International Nursing Research Congress, Sigma Theta Tau International; July 12, 2003; St. Thomas, US Virgin Islands.
22. Stearns CR, Gonzales R, Camargo CA Jr, et al. Antibiotic prescriptions are associated with increased patient satisfaction with emergency department visits for acute respiratory tract infections. Acad Emerg Med. 2009;16:934-941.
23. Higashi T, Wenger NS, Adams JL, et al. Relationship between number of medical conditions and quality of care. N Engl J Med. 2007;356:2496-2504.
24. Boyd CM, Darer J, Boult C, et al. Clinical practice guidelines and quality of care for older patients with multiple comorbid disease: implications for pay for performance. JAMA. 2005;294:716-724.
25. Linder JA, Singer DE. Desire for antibiotics and antibiotic prescribing for adults with upper respiratory tract infections. J Gen Intern Med. 2003;18:795-801.
Keeping older patients healthy and safe as they travel
• Advise older adults to prepare a health travel kit containing all their medications and medical supplies, a list of chronic conditions, and emergency contact information, and to pack it in their carry-on luggage. C
• Instruct patients who will be airborne for ≥4 hours to stay hydrated, avoid alcohol and sedating drugs, and either do seated calf exercises or get up and move about the cabin periodically. B
• Remind patients who will spend time in developing countries to drink only bottled beverages, eat only hot food and fruit that can be peeled, and avoid ice cubes and food from street vendors. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE Larry R, a 77-year-old retired college professor, comes in for a checkup because he is planning a trip to Kenya—on a safari he describes excitedly as “the trip of a lifetime.” He’ll be going with a group, but before he signs on he wants to be sure you think he can manage the tour’s “moderate pace.” He also thinks that he’ll “need to get some shots.”
The patient is overweight (BMI 29) and smokes a pipe daily. He has a history of hypertension, hyperlipidemia, and mild osteoarthritis in both knees and hips, all of which are well controlled.
What would you advise Professor R about the health care preparations needed for his big trip?
Chances are you have patients like Professor R—retired and relatively healthy, and endowed with a sense of adventure and the financial resources that make it possible to visit distant lands. With the nation’s 78 million baby boomers starting to reach retirement age—the oldest cohort turned 65 in 2011—you’re likely to see increasing numbers of older patients with plans for international travel in the years ahead.1
Like their younger counterparts, older people travel for a variety of reasons: Some have planned for decades to take the “trip of a lifetime” when they retire. Others plan longer excursions, sometimes referred to as an adult “gap year,” to relive a long-ago experience, volunteer in an underdeveloped country, or hike and bird watch in a rainforest. Many more are immigrants who travel to visit relatives or friends in their country of origin, usually a lower-income, environmentally depressed locale with a higher incidence of infectious diseases like malaria, typhoid, and hepatitis A. 2
And while the older traveler will have to take many of the same steps to stay healthy as his or her younger counterpart, it is the older traveler who is more likely to have chronic conditions and special needs that require additional preparation. With careful planning, however, even those with decreased faculties, ranging from impaired vision or hearing to mild cognitive impairment, can safely travel abroad.3
A pretravel visit is your opportunity to assess the patient’s fitness to make the trip being planned, ensure optimal management of chronic conditions while traveling, and identify (and recommend steps to mitigate) travel-related risks.
Morbidity and mortality abroad: A review of the risks
Although much pretravel advice centers on the prevention of tropical infectious diseases, such infections account for a very small percentage of deaths of Americans outside of the United States.1 In fact, the major health risks facing older adults traveling abroad are similar to those they face at home: Cardiovascular events are responsible for the preponderance of deaths and for half of all travel-related illnesses.1
International travel can be physically demanding for older individuals and injuries are common, accounting for a large proportion of deaths of Americans overseas4 and an estimated 25% to 38% of travel-related incidents.1,5 A third of injury-related deaths of US citizens traveling abroad involve traffic accidents, followed by homicide (17% of cases) and drowning (13%).1,5 Thus, injury prevention and management of chronic conditions are key issues to address in a pretravel consult.
Even small steps help safeguard older travelers
Older patients planning to travel abroad should schedule an appointment at least 4 to 6 weeks before their departure.2 Ask about the locale, political and environmental climate, length of stay, location and type of accommodations, accessibility to health care, and activities planned,6 which will enable you to offer both general and destination-specific health and safety tips. When advising older adults with complex comorbidities and/or particularly high-risk itineraries, referral to a travel medicine specialist should be strongly considered.
Exercise. Encourage older patients to initiate a graduated exercise program, starting several months before the trip.3 Even a modest improvement in endurance, strength, and flexibility can reduce the likelihood of injury.
Luggage. The right luggage can benefit your patients. Recommend that older patients purchase lightweight suitcases with wheels, which are easier to maneuver in and out of airports6 and less likely to cause muscle strain or musculoskeletal injury.
Insurance. If an individual becomes ill or sustains an injury overseas, the right insurance can be crucial. Advise older adults to review their health insurance policy to see whether it provides overseas coverage. If not, suggest they consider a short-term supplemental policy to cover medical care and evacuation, if needed. Recommend trip cancellation insurance, as well.
Patients should pack pills, medical supplies in a carry-on kit
Encourage all older travelers to compile a personalized travel health kit equipped with common over-the-counter (OTC) medications, prescription drugs, and any personal medical supplies they’ll need, such as a continuous positive airway pressure (CPAP) machine.1,3 Remind patients to take an ample supply of both prescription and OTC drugs, each in its original labeled container.3,6 Buying medications outside the United States is not advisable, given the variation in international regulatory standards. Stress the importance of keeping the kit in a carry-on bag.
The health kit should also include descriptions of the patient’s preexisting medical conditions, which you or a nurse or medical assistant in the practice can help to prepare; a list of prescription drugs he or she takes (using both the generic and brand names); and a copy of a recent electrocardiogram, if available, along with contact information in case of an emergency.1,3,6 A patient who uses injectable medication, such as insulin, should obtain a letter (on the practice’s letterhead) from the prescribing physician and be prepared to show the letter to airport security personnel.3,6
Staying safe in the air
The lower barometric and oxygen partial pressures found in aircraft cabins, which are pressurized at 5000 to 8000 feet, can affect both the respiratory and cardiovascular systems of older adults—particularly those with pulmonary or cardiac disorders.6,7 Individuals who do not routinely require oxygen and are able to walk the equivalent of one city block or climb one flight of stairs without shortness of breath should have little trouble compensating for the reduced oxygen in the cabin.3 Patients with stable heart failure, including New York Heart Association grades III and IV, can tolerate flights of up to one hour without additional oxygen.7
Advise older adults who will require oxygen that they are not permitted to bring their own oxygen canisters onboard an airplane.1 In-flight oxygen needs to be ordered at least 7 days before departure, and there may be a charge.7 Most airlines have medical consultants available to help patients who will need oxygen or other medical provisions.7 In addition, tour companies or travel consultants can help older patients with special needs ensure that they have access to oxygen or other medical supplies at their destination.
Thrombosis—the other in-flight risk
Sometimes referred to as “economy class syndrome” or “traveler’s thrombosis,” the venous stasis of air travel is responsible for a 3-fold increase in the risk of venous thromboembolism (VTE).8 While fatal pulmonary embolism is rare, duration of travel and risk of VTE follow a dose-response relationship, with each 2-hour increase in flight time conferring an additional 18% risk.8 Other risk factors for VTE include varicose veins, metastatic cancer, major surgery within the past 2 weeks, prior VTE, and BMI >40. Advanced age increases travelers’ risk of VTE, as well.7,8 The absolute risk, however, is low.
Among travelers older than 50 years, symptomatic VTE occurs at an estimated rate of one in 600 for flights >4 hours and one in 500 for flights >12 hours.9,10 While there is no evidence that first-class seating lowers the risk, there are preventive measures that patients can take.11
Tell patients to stay hydrated, drinking plenty of fluids but avoiding alcohol during flights of ≥4 hours’ duration. Sedating drugs should be avoided, as well. Advise anyone planning a long flight to either do seated exercises (intermittent calf contractions) or to periodically get up and walk about the cabin.9 You may also want to recommend that patients purchase below-the-knee elastic compression stockings to help decrease venous stasis.12
There is no evidence to recommend the use of aspirin to prevent VTE.12,13 But you may consider prescribing a single 40-mg dose of enoxaparin for a patient who has multiple risk factors and will be airborne for >6 hours.13
Promote safety and comfort on the ground
It is crucial to remind all travelers about the risks associated with traveling in motor vehicles in other countries. Remind patients to wear seat belts whenever they’re available; exercise caution regarding public transportation, which may be overcrowded and have an increased risk of pickpockets and robbery; and avoid riding on motorcycles and scooters. If they do opt to ride on a scooter, tell them that it’s imperative that they wear a helmet.
Minimize the effects of jet lag
Travelers of any age may experience jet lag, which occurs when the individual’s circadian clock cannot keep pace with travel across time zones.14 Notably, however, older people appear to suffer less than their younger counterparts.3 Patients traveling great distances are not likely to avoid jet lag completely, of course. Recommend the following strategies:
Start adjusting your schedule in the week before you depart, gradually shifting 2 hours toward congruence with the time zone at your destination.14
Help reset your circadian rhythm through exposure to bright light, in the morning after eastward travel and in the evening after westward travel.14
Take it easy at first. An itinerary that accounts for initial fatigue is an important nonpharmacologic management strategy.14
Avoid sedating medications, including antihistamines, tranquilizers, anti-motion sickness agents, and benzodiazepines, as these can increase falls and confusion in older adults and make jet lag worse.3
Take melatonin. A dose of 0.5 to 5 mg, taken at bedtime, may promote sleep and decrease jet lag symptoms in travelers crossing multiple time zones.14
Prepare patients to cope with heat …
Unusually hot, humid weather increases morbidity and mortality in the elderly,3,15 and older patients traveling to such climates will need to take extra precautions. Strenuous exercise in the heat should be avoided, because both thirst and the capacity to conserve salt and water decrease with age.16 Acclimatization is helped by rest, air-conditioning, loose cotton clothing, brimmed hats, and cool baths or showers.3 Diuretics may have to be adjusted for fluids lost by increased perspiration, and a discussion about a dose reduction should be included in the pretravel consult for patients who take diuretics and will be traveling to a hot, humid climate.
… and increases in altitude
For older adults, exposure to a moderate altitude (<2500 meters) is initially associated with hypoxemia and a reduced exercise capacity, until acclimatization occurs by Day 5.17,18 Although older adults generally acclimatize well, advise them to limit their activities for the first few days at a higher altitude. This is especially important for patients with coronary artery disease (CAD).
To further ease the effects of a higher altitude, advise patients to drink plenty of fluids, but little or no alcohol.19 Review the medications of an older patient who will be spending time at very high altitude. Rarely, antihypertensive medication may need to be adjusted. The body compensates for lower oxygen with a faster heart rate, and some antihypertensives may interfere with this compensatory mechanism.3
Precautions (and prophylaxis) may prevent travelers’ diarrhea
Diarrhea—among the most common travel-related conditions20—affects an estimated 30% to 70% of international travelers.2 The incidence is highest among visitors to developing countries. Most (80%-90%) of travelers’ diarrhea is due to bacterial infection,21 10% of cases are caused by parasites, and 5% to 8% by viral infection.2,22
Although increasing age lowers the risk of travelers’ diarrhea,1 older patients traveling to developing areas should be cautioned to only eat food that is served hot or fruit they can peel themselves; drink only bottled water and sealed liquids; and avoid salad, ice, and food from street vendors.1 Studies have shown, however, that tourists often get diarrhea despite these safety measures.2
Treatment and prophylaxis. Prophylactic antibiotics can prevent travelers’ diarrhea. But the increased sun sensitivity, drug-drug interactions, and gastrointestinal (GI) adverse effects associated with antibiotics limit their usefulness. Prophylaxis is indicated, however, for older adults for whom the complications of dehydration would likely be so severe that the benefits of using antibiotics to prevent diarrhea clearly outweigh the risks.23
Fluoroquinolones are a first-line treatment for travelers’ diarrhea. But increasing microbial resistance to this class of drugs, especially among Campylobacter isolates,24 may limit their usefulness in some destinations.25 Azithromycin is recommended in such cases, and has been shown to be equally effective.26,27 Single-dose therapy is well established with fluoroquinolones, but the best regimen for azithromycin (1 vs 3 days) is still under evaluation.28,29 Along with instructions on when to take an antibiotic, travelers should be given prescriptions for treatment of travelers’ diarrhea before the start of their trip. Suggest that patients purchase oral rehydration packets to take on their trip, and stress the importance of using them and staying hydrated if diarrhea develops.
Decreased upper GI acidity due to acid-blocking medications such as proton-pump inhibitors can increase the risk for many infections, including salmonella and cholera. Patients taking such medications should be made aware of the risk, and the risks vs benefits of temporarily stopping them should be discussed. Vaccination against cholera should not routinely be recommended.29,30
Vaccines and pills protect against preventable diseases
An impending trip abroad also presents an opportunity to review the patient’s immunization status, catch up on recommended vaccines, and determine whether any additional vaccinations are needed.
Herpes zoster (HZ). Patients older than 60 years should receive a single dose of the HZ vaccine, whether or not they have a history of this condition. Because this is a live virus-containing vaccine, however, it should not be given to anyone who is immunocompromised.31
MMR booster. Adults born before 1957 can be considered immune from both measles and mumps, but not rubella. There is no data on immunization to rubella, but guidelines do not recommend MMR vaccination in the elderly.1
Pneumococcal polyvalent-23 (PPV-23). One dose of the PPV-23 vaccine is indicated for all adults at age 65. This is especially important for travelers, as the prevalence of pneumococcal disease is likely higher in crowded, urban environments within less developed countries.31
Tetanus. Although tetanus is mainly a disease of the elderly, only 45% of men ages 70 years or older and 21% of women in this age group were found to have protective antibodies.1,32 In 2011, the Advisory Committee on Immunization Practices (ACIP) recommended one dose of tetanus and diphtheria toxoid (Td) every 10 years, with a single dose of tetanus toxoid, diphtheria toxoid, and acellular pertussis (Tdap) vaccine given in place of Td for adults older than 65.33 Despite ACIP’s recommendation, the vaccine’s use in adults 65 years and older is an off-label indication, as Tdap is only approved for use in those 11 to 64 years of age.33
Additional vaccines are recommended for travelers, with some indicated for all travelers and others that are destination-specific (TABLE).
TABLE
Which travel-related vaccines does your patient need?
| Disease | Type of vaccine | Primary course | Booster/ follow-up | Route | For which destinations? |
|---|---|---|---|---|---|
| Vaccines for all travelers | |||||
| Hepatitis A* | Killed virus | 2 doses (6-18 mo apart)† | None | IM | All |
| Hepatitis B* | Recombinant viral antigen | 3 doses (0, 1, 6 mo) | None | IM | All |
| Influenza | Inactivated viral | Single dose | Annually | IM | All |
| Typhoid | Capsular polysaccharide Live attenuated bacteria | Single dose 4 doses (0, 2, 4, 6 mo) | 2-3 y 5 y | IM Oral | All |
| Vaccines for travelers to select destinations | |||||
| Japanese encephalitis | Inactivated viral | 2 doses (28 d apart) | Unknown | IM | Rural Asia‡ |
| Meningococcus | Quadrivalent conjugated polysaccharide | Single dose | >10 y | IM | Sub-Saharan Africa; Saudi Arabia |
| Polio | Inactivated viral | Single dose if patient had childhood series | None | SC; IM | Anyplace where polio still occurs |
| Rabies | Inactivated cell culture viral | 3 doses (0, 7, 21-28 d) | None unless exposure occurs | IM | |
| Yellow fever | Live attenuated virus | Single dose | 10 y | SC | Sub-Saharan Africa; tropical South America |
| IM, intramuscular; SC, subcutaneous. *A combined hepatitis A/B vaccine is approved for use in older adults. †Second dose may be delayed up to 8 years without diminished efficacy. ‡Required only for prolonged stays in rural areas of Asia. Adapted from: Mandell GL, Bennett JE, Dolin R, eds. Principles and Practice of Infectious Diseases. 7th ed. Philadelphia, Pa: Elsevier; 2010. | |||||
Meds and safety measures can minimize malaria risk
The risk of acquiring malaria differs significantly among travelers, based on destination, duration and type of travel, and season. Choice of antimalarial agents (eg, atovaquone/proguanil, chloroquine, doxycycline, mefloquine, and primaquine) should be made on an individual basis after considering these factors, as well as the resistance patterns of the countries on the patient’s itinerary, his or her medical history, and the adverse effects profile of potential agents. Because many older adults take multiple medications, the possibility of drug-drug interactions must be considered.1 You’ll find destination-specific recommendations on malaria prevention on the Centers for Disease Control and Prevention’s Travelers’ Health Web site, listed in “Travel and health: Resources for patients and physicians”. For guidance on the best drug to prescribe, you can also consult a travel medicine specialist.2
Access-able Travel Source Web site provides information for older adult travelers with special needs who need help traveling with oxygen or getting around despite decreased mobility (www.access-able.com/tips/).
American Diabetes Association publishes detailed information about traveling with diabetes (http://www.diabetes.org/living-with-diabetes/know-your-rights/discrimination/public-accommodations/air-travel-and-diabetes/).
Bureau of Consular Affairs publishes information regarding VISA and security requirements at various destinations and travel warnings (http://travel.state.gov/about/about_304.html).
Centers for Disease Control and Prevention publishes “The Yellow Book”—a reference for clinicians who advise international travelers about health risks. There is also a range of other travel-related information on its Travelers’ Health Web site (www.cdc.gov/travel/).
International Travel Medicine Society provides a global travel clinic directory (http://www.istm.org/Webforms/Searchclinics/Default.aspx?SearchType=advanced).
Transportation Security Administration Web site provides information on what can be brought on-board a plane. See “Can I bring my … through the checkpoint?” (http://apps.tsa.dhs.gov/mytsa/cib_home.aspx).
Travel Health Online offers a list of medical providers around the world (www.tripprep.com/scripts).
World Health Organization’s Travel and Health Web site provides free access to selected chapters of its book, “International Travel and Health 2012,” as well as interactive maps, information about infectious diseases and food safety, and more (www.who.int/ith/en/).
Patients should be mindful of mosquitos. Stress the importance of preventing mosquito bites (as much as possible). Advise patients traveling to mosquito-infested areas to use insect repellents containing 30% N-diethyl-meta-toluamide (DEET) and permethrin-treated clothing.34 Tell them, too, to wear long sleeves, pants, and footwear that provides full coverage.35 Ensuring that sleeping areas are properly screened or air-conditioned will further reduce the likelihood of mosquito bites.36
CASE After seeing the chief complaint listed as “Traveling to Kenya” on Professor R’s chart, you quickly review the CDC’s Travelers’ Health Web site. You encourage him to stay with his tour group and to wear a seatbelt whenever possible. You also review how to make a personalized travel health kit, and encourage him to register with the Smart Traveler Enrollment Program (STEP) (detailed at https://step.state.gov/rep) before leaving for the safari. You strongly suggest that he consider purchasing additional medical evacuation insurance, as well.
Given the prevalence of travelers’ diarrhea, along with dengue and malaria, in Kenya, you review food and water safety and avoidance of insect-transmitted diseases with the patient, and write a prescription for ciprofloxacin to be taken if he develops diarrhea. Professor R is not at high risk for VTE, but you encourage him to stay hydrated, avoid sedating medications, and be diligent about mobilization during lengthy flights. You recommend melatonin for jet lag.
To adjust to the heat, you recommend that he avoid strenuous exercise in the first few days and drink sufficient fluids throughout the trip. You administer the Tdap vaccine, an adult polio booster, and the hepatitis A vaccine, verify that he has received his pneumococcal and influenza vaccines, and prescribe an antimalarial medication.
And as you walk him toward the door, you offer him one final piece of advice: Take plenty of pictures.
CORRESPONDENCE
Jeffrey D. Schlaudecker, MD, The Christ Hospital/University of Cincinnati Family Medicine Residency Program, 2123 Auburn Avenue #340, Cincinnati, OH 45219; [email protected]
1. Reed CM. Travel recommendations for older adults. Clin Geriatr Med. 2007;23:687-713, ix.
2. Centers for Disease Control and Prevention. Health Information for International Travel 2012. New York, NY:Oxford University Press; 2012. Available at http://wwwnc.cdc.gov/travel/page/yellowboth-2012-home.htm. Accessed December 18, 2012.
3. Cooper MC. The elderly travellers. Travel Med Infect Dis. 2006;4:218-222.
4. Guse CE, Cortes LM, Hargarten SW, et al. Fatal injuries of US citizens abroad. J Travel Med. 2007;14:279-287
5. Tonellato DJ, Guse CE, Hargarten SW. Injury deaths of US citizens abroad: new data source, old travel problem. J Travel Med. 2009;16:304-310.
6. Fenner P. Fitness to travel - assessment in the elderly and medically impaired. Aust Fam Physician. 2007;36:312-315.
7. Smith D, Toff W, Joy M, et al. Fitness to fly for passengers with cardiovascular disease. Heart. 2010;96(suppl 2):Sii1-S16.
8. Chandra D, Parisini E, Mozaffarian D. Meta-analysis: travel and risk for venous thromboembolism. Ann Intern Med. 2009;151:180-190.
9. Gavish I, Brenner B. Air travel and the risk of thromboembolism. Intern Emerg Med. 2011;6:113-116.
10. Ansari MT, Cheung BM, Qing Huang J, et al. Traveler’s thrombosis: a systematic review. J Travel Med. 2005;12:142-154.
11. Schwarz T, Siegert G, Oettler W, et al. Venous thrombosis after long-haul flights. Arch Intern Med. 2003;163:2759-2764.
12. Cesarone MR, Belcaro G, Errichi BM, et al. The LONFLIT4—Concorde Deep Venous Thrombosis and Edema Study: prevention with travel stockings. Angiology. 2003;54:143-154.
13. Cesarone MR, Belcaro G, Nicolaides AN, et al. Venous thrombosis from air travel: the LONFLIT3 study—prevention with aspirin vs low-molecular-weight heparin (LMWH) in high-risk subjects: a randomized trial. Angiology. 2002;53:1-6.
14. Sack RL. Clinical practice. Jet lag. N Engl J Med. 2010: 440-447.
15. Davies I, O’Neill PA, McLean KA, et al. Age-associated alterations in thirst and arginine vasopressin in response to a water or sodium load. Age Ageing. 1995;24:151-159.
16. Rikkert MG, Melis RJ, Claassen JA. Heat waves and dehydration in the elderly. BMJ. 2009;339:b2663.-
17. Levine BD, Zuckerman JH, deFilippi CR. Effect of high-altitude exposure in the elderly: the Tenth Mountain Division study. Circulation. 1997;96:1224-1232.
18. Agostoni P, Cattadori G, Guazzi M, et al. Effects of simulated altitude-induced hypoxia on exercise capacity in patients with chronic heart failure. Am J Med. 2000;109:450-455.
19. Higgins JP, Tuttle T, Higgins JA. Altitude and the heart: is going high safe for your cardiac patient? Am Heart J. 2010;159:25-32.
20. Gautret P, Schlagenhauf P, Gaudart J, et al. Multicenter EuroTravNet/GeoSentinel study of travel-related infectious diseases in Europe. Emerg Infect Dis. 2009;15:1783-1790.
21. Adachi JA, Jiang ZD, Mathewson JJ, et al. Enteroaggregative Escherichia coli as a major etiologic agent in traveler’s diarrhea in 3 regions of the world. Clin Infect Dis. 2001;32:1706-1709.
22. Black RE. Epidemiology of travelers’ diarrhea and relative importance of various pathogens. Rev Infect Dis. 1990;12 (suppl 1):S73-S79.
23. Rendi-Wagner P, Kollaritsch H. Drug prophylaxis for travelers’ diarrhea. Clin Infect Dis. 2002;34:628-633.
24. Butzler JP. Campylobacter, from obscurity to celebrity. Clin Microbiol Infect. 2004;10:868-876.
25. Hoge CW, Gambel JM, Srijan A, et al. Trends in antibiotic resistance among diarrheal pathogens isolated in Thailand over 15 years. Clin Infect Dis. 1998;26:341-345.
26. Adachi JA, Ericsson CD, Jiang ZD, et al. Azithromycin found to be comparable to levofloxacin for the treatment of US travelers with acute diarrhea acquired in Mexico. Clin Infect Dis. 2003;37:1165-1171.
27. Tribble DR, Sanders JW, Pang LW, et al. Traveler’s diarrhea in Thailand: randomized, double-blind trial comparing single-dose and 3-day azithromycin-based regimens with a 3-day levofloxacin regimen. Clin Infect Dis. 2007;44:338-346.
28. Shanks GD, Smoak BL, Aleman GM, et al. Single dose of azithromycin or three-day course of ciprofloxacin as therapy for epidemic dysentery in Kenya. Acute Dysentery Study Group. Clin Infect Dis. 1999;29:942-943.
29. Heatley RV, Sobala GM. Acid suppression and the gastric flora. Baillieres Clin Gastroenterol. 1993;7:167-181.
30. Hill DR, Ford L, Lalloo DG. Oral cholera vaccines: use in clinical practice. Lancet Infect Dis. 2006;6:361-373.
31. Vaughn JA, Miller RA. Update on immunizations in adults. Am Fam Physician. 2011;84:1015-1020.
32. Gergen PJ, McQuillan GM, Kiely M, et al. A population-based serologic survey of immunity to tetanus in the United States. N Engl J Med. 1995;332:761-766.
33. World Health Organization. Typhoid vaccines: WHO position paper. Wkly Epidemiol Rec. 2008;83:49-59.
34. Fradin MS, Day JF. Comparative efficacy of insect repellents against mosquito bites. N Engl J Med. 2002;347:13-18.
35. Soto J, Medina F, Dember N, et al. Efficacy of permethrin-impregnated uniforms in the prevention of malaria and leishmaniasis in Colombian soldiers. Clin Infect Dis. 1995;21:599-602.
36. Freedman DO. Clinical practice. Malaria prevention in short-term travelers. N Engl J Med. 2008;359:603-612.
• Advise older adults to prepare a health travel kit containing all their medications and medical supplies, a list of chronic conditions, and emergency contact information, and to pack it in their carry-on luggage. C
• Instruct patients who will be airborne for ≥4 hours to stay hydrated, avoid alcohol and sedating drugs, and either do seated calf exercises or get up and move about the cabin periodically. B
• Remind patients who will spend time in developing countries to drink only bottled beverages, eat only hot food and fruit that can be peeled, and avoid ice cubes and food from street vendors. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE Larry R, a 77-year-old retired college professor, comes in for a checkup because he is planning a trip to Kenya—on a safari he describes excitedly as “the trip of a lifetime.” He’ll be going with a group, but before he signs on he wants to be sure you think he can manage the tour’s “moderate pace.” He also thinks that he’ll “need to get some shots.”
The patient is overweight (BMI 29) and smokes a pipe daily. He has a history of hypertension, hyperlipidemia, and mild osteoarthritis in both knees and hips, all of which are well controlled.
What would you advise Professor R about the health care preparations needed for his big trip?
Chances are you have patients like Professor R—retired and relatively healthy, and endowed with a sense of adventure and the financial resources that make it possible to visit distant lands. With the nation’s 78 million baby boomers starting to reach retirement age—the oldest cohort turned 65 in 2011—you’re likely to see increasing numbers of older patients with plans for international travel in the years ahead.1
Like their younger counterparts, older people travel for a variety of reasons: Some have planned for decades to take the “trip of a lifetime” when they retire. Others plan longer excursions, sometimes referred to as an adult “gap year,” to relive a long-ago experience, volunteer in an underdeveloped country, or hike and bird watch in a rainforest. Many more are immigrants who travel to visit relatives or friends in their country of origin, usually a lower-income, environmentally depressed locale with a higher incidence of infectious diseases like malaria, typhoid, and hepatitis A. 2
And while the older traveler will have to take many of the same steps to stay healthy as his or her younger counterpart, it is the older traveler who is more likely to have chronic conditions and special needs that require additional preparation. With careful planning, however, even those with decreased faculties, ranging from impaired vision or hearing to mild cognitive impairment, can safely travel abroad.3
A pretravel visit is your opportunity to assess the patient’s fitness to make the trip being planned, ensure optimal management of chronic conditions while traveling, and identify (and recommend steps to mitigate) travel-related risks.
Morbidity and mortality abroad: A review of the risks
Although much pretravel advice centers on the prevention of tropical infectious diseases, such infections account for a very small percentage of deaths of Americans outside of the United States.1 In fact, the major health risks facing older adults traveling abroad are similar to those they face at home: Cardiovascular events are responsible for the preponderance of deaths and for half of all travel-related illnesses.1
International travel can be physically demanding for older individuals and injuries are common, accounting for a large proportion of deaths of Americans overseas4 and an estimated 25% to 38% of travel-related incidents.1,5 A third of injury-related deaths of US citizens traveling abroad involve traffic accidents, followed by homicide (17% of cases) and drowning (13%).1,5 Thus, injury prevention and management of chronic conditions are key issues to address in a pretravel consult.
Even small steps help safeguard older travelers
Older patients planning to travel abroad should schedule an appointment at least 4 to 6 weeks before their departure.2 Ask about the locale, political and environmental climate, length of stay, location and type of accommodations, accessibility to health care, and activities planned,6 which will enable you to offer both general and destination-specific health and safety tips. When advising older adults with complex comorbidities and/or particularly high-risk itineraries, referral to a travel medicine specialist should be strongly considered.
Exercise. Encourage older patients to initiate a graduated exercise program, starting several months before the trip.3 Even a modest improvement in endurance, strength, and flexibility can reduce the likelihood of injury.
Luggage. The right luggage can benefit your patients. Recommend that older patients purchase lightweight suitcases with wheels, which are easier to maneuver in and out of airports6 and less likely to cause muscle strain or musculoskeletal injury.
Insurance. If an individual becomes ill or sustains an injury overseas, the right insurance can be crucial. Advise older adults to review their health insurance policy to see whether it provides overseas coverage. If not, suggest they consider a short-term supplemental policy to cover medical care and evacuation, if needed. Recommend trip cancellation insurance, as well.
Patients should pack pills, medical supplies in a carry-on kit
Encourage all older travelers to compile a personalized travel health kit equipped with common over-the-counter (OTC) medications, prescription drugs, and any personal medical supplies they’ll need, such as a continuous positive airway pressure (CPAP) machine.1,3 Remind patients to take an ample supply of both prescription and OTC drugs, each in its original labeled container.3,6 Buying medications outside the United States is not advisable, given the variation in international regulatory standards. Stress the importance of keeping the kit in a carry-on bag.
The health kit should also include descriptions of the patient’s preexisting medical conditions, which you or a nurse or medical assistant in the practice can help to prepare; a list of prescription drugs he or she takes (using both the generic and brand names); and a copy of a recent electrocardiogram, if available, along with contact information in case of an emergency.1,3,6 A patient who uses injectable medication, such as insulin, should obtain a letter (on the practice’s letterhead) from the prescribing physician and be prepared to show the letter to airport security personnel.3,6
Staying safe in the air
The lower barometric and oxygen partial pressures found in aircraft cabins, which are pressurized at 5000 to 8000 feet, can affect both the respiratory and cardiovascular systems of older adults—particularly those with pulmonary or cardiac disorders.6,7 Individuals who do not routinely require oxygen and are able to walk the equivalent of one city block or climb one flight of stairs without shortness of breath should have little trouble compensating for the reduced oxygen in the cabin.3 Patients with stable heart failure, including New York Heart Association grades III and IV, can tolerate flights of up to one hour without additional oxygen.7
Advise older adults who will require oxygen that they are not permitted to bring their own oxygen canisters onboard an airplane.1 In-flight oxygen needs to be ordered at least 7 days before departure, and there may be a charge.7 Most airlines have medical consultants available to help patients who will need oxygen or other medical provisions.7 In addition, tour companies or travel consultants can help older patients with special needs ensure that they have access to oxygen or other medical supplies at their destination.
Thrombosis—the other in-flight risk
Sometimes referred to as “economy class syndrome” or “traveler’s thrombosis,” the venous stasis of air travel is responsible for a 3-fold increase in the risk of venous thromboembolism (VTE).8 While fatal pulmonary embolism is rare, duration of travel and risk of VTE follow a dose-response relationship, with each 2-hour increase in flight time conferring an additional 18% risk.8 Other risk factors for VTE include varicose veins, metastatic cancer, major surgery within the past 2 weeks, prior VTE, and BMI >40. Advanced age increases travelers’ risk of VTE, as well.7,8 The absolute risk, however, is low.
Among travelers older than 50 years, symptomatic VTE occurs at an estimated rate of one in 600 for flights >4 hours and one in 500 for flights >12 hours.9,10 While there is no evidence that first-class seating lowers the risk, there are preventive measures that patients can take.11
Tell patients to stay hydrated, drinking plenty of fluids but avoiding alcohol during flights of ≥4 hours’ duration. Sedating drugs should be avoided, as well. Advise anyone planning a long flight to either do seated exercises (intermittent calf contractions) or to periodically get up and walk about the cabin.9 You may also want to recommend that patients purchase below-the-knee elastic compression stockings to help decrease venous stasis.12
There is no evidence to recommend the use of aspirin to prevent VTE.12,13 But you may consider prescribing a single 40-mg dose of enoxaparin for a patient who has multiple risk factors and will be airborne for >6 hours.13
Promote safety and comfort on the ground
It is crucial to remind all travelers about the risks associated with traveling in motor vehicles in other countries. Remind patients to wear seat belts whenever they’re available; exercise caution regarding public transportation, which may be overcrowded and have an increased risk of pickpockets and robbery; and avoid riding on motorcycles and scooters. If they do opt to ride on a scooter, tell them that it’s imperative that they wear a helmet.
Minimize the effects of jet lag
Travelers of any age may experience jet lag, which occurs when the individual’s circadian clock cannot keep pace with travel across time zones.14 Notably, however, older people appear to suffer less than their younger counterparts.3 Patients traveling great distances are not likely to avoid jet lag completely, of course. Recommend the following strategies:
Start adjusting your schedule in the week before you depart, gradually shifting 2 hours toward congruence with the time zone at your destination.14
Help reset your circadian rhythm through exposure to bright light, in the morning after eastward travel and in the evening after westward travel.14
Take it easy at first. An itinerary that accounts for initial fatigue is an important nonpharmacologic management strategy.14
Avoid sedating medications, including antihistamines, tranquilizers, anti-motion sickness agents, and benzodiazepines, as these can increase falls and confusion in older adults and make jet lag worse.3
Take melatonin. A dose of 0.5 to 5 mg, taken at bedtime, may promote sleep and decrease jet lag symptoms in travelers crossing multiple time zones.14
Prepare patients to cope with heat …
Unusually hot, humid weather increases morbidity and mortality in the elderly,3,15 and older patients traveling to such climates will need to take extra precautions. Strenuous exercise in the heat should be avoided, because both thirst and the capacity to conserve salt and water decrease with age.16 Acclimatization is helped by rest, air-conditioning, loose cotton clothing, brimmed hats, and cool baths or showers.3 Diuretics may have to be adjusted for fluids lost by increased perspiration, and a discussion about a dose reduction should be included in the pretravel consult for patients who take diuretics and will be traveling to a hot, humid climate.
… and increases in altitude
For older adults, exposure to a moderate altitude (<2500 meters) is initially associated with hypoxemia and a reduced exercise capacity, until acclimatization occurs by Day 5.17,18 Although older adults generally acclimatize well, advise them to limit their activities for the first few days at a higher altitude. This is especially important for patients with coronary artery disease (CAD).
To further ease the effects of a higher altitude, advise patients to drink plenty of fluids, but little or no alcohol.19 Review the medications of an older patient who will be spending time at very high altitude. Rarely, antihypertensive medication may need to be adjusted. The body compensates for lower oxygen with a faster heart rate, and some antihypertensives may interfere with this compensatory mechanism.3
Precautions (and prophylaxis) may prevent travelers’ diarrhea
Diarrhea—among the most common travel-related conditions20—affects an estimated 30% to 70% of international travelers.2 The incidence is highest among visitors to developing countries. Most (80%-90%) of travelers’ diarrhea is due to bacterial infection,21 10% of cases are caused by parasites, and 5% to 8% by viral infection.2,22
Although increasing age lowers the risk of travelers’ diarrhea,1 older patients traveling to developing areas should be cautioned to only eat food that is served hot or fruit they can peel themselves; drink only bottled water and sealed liquids; and avoid salad, ice, and food from street vendors.1 Studies have shown, however, that tourists often get diarrhea despite these safety measures.2
Treatment and prophylaxis. Prophylactic antibiotics can prevent travelers’ diarrhea. But the increased sun sensitivity, drug-drug interactions, and gastrointestinal (GI) adverse effects associated with antibiotics limit their usefulness. Prophylaxis is indicated, however, for older adults for whom the complications of dehydration would likely be so severe that the benefits of using antibiotics to prevent diarrhea clearly outweigh the risks.23
Fluoroquinolones are a first-line treatment for travelers’ diarrhea. But increasing microbial resistance to this class of drugs, especially among Campylobacter isolates,24 may limit their usefulness in some destinations.25 Azithromycin is recommended in such cases, and has been shown to be equally effective.26,27 Single-dose therapy is well established with fluoroquinolones, but the best regimen for azithromycin (1 vs 3 days) is still under evaluation.28,29 Along with instructions on when to take an antibiotic, travelers should be given prescriptions for treatment of travelers’ diarrhea before the start of their trip. Suggest that patients purchase oral rehydration packets to take on their trip, and stress the importance of using them and staying hydrated if diarrhea develops.
Decreased upper GI acidity due to acid-blocking medications such as proton-pump inhibitors can increase the risk for many infections, including salmonella and cholera. Patients taking such medications should be made aware of the risk, and the risks vs benefits of temporarily stopping them should be discussed. Vaccination against cholera should not routinely be recommended.29,30
Vaccines and pills protect against preventable diseases
An impending trip abroad also presents an opportunity to review the patient’s immunization status, catch up on recommended vaccines, and determine whether any additional vaccinations are needed.
Herpes zoster (HZ). Patients older than 60 years should receive a single dose of the HZ vaccine, whether or not they have a history of this condition. Because this is a live virus-containing vaccine, however, it should not be given to anyone who is immunocompromised.31
MMR booster. Adults born before 1957 can be considered immune from both measles and mumps, but not rubella. There is no data on immunization to rubella, but guidelines do not recommend MMR vaccination in the elderly.1
Pneumococcal polyvalent-23 (PPV-23). One dose of the PPV-23 vaccine is indicated for all adults at age 65. This is especially important for travelers, as the prevalence of pneumococcal disease is likely higher in crowded, urban environments within less developed countries.31
Tetanus. Although tetanus is mainly a disease of the elderly, only 45% of men ages 70 years or older and 21% of women in this age group were found to have protective antibodies.1,32 In 2011, the Advisory Committee on Immunization Practices (ACIP) recommended one dose of tetanus and diphtheria toxoid (Td) every 10 years, with a single dose of tetanus toxoid, diphtheria toxoid, and acellular pertussis (Tdap) vaccine given in place of Td for adults older than 65.33 Despite ACIP’s recommendation, the vaccine’s use in adults 65 years and older is an off-label indication, as Tdap is only approved for use in those 11 to 64 years of age.33
Additional vaccines are recommended for travelers, with some indicated for all travelers and others that are destination-specific (TABLE).
TABLE
Which travel-related vaccines does your patient need?
| Disease | Type of vaccine | Primary course | Booster/ follow-up | Route | For which destinations? |
|---|---|---|---|---|---|
| Vaccines for all travelers | |||||
| Hepatitis A* | Killed virus | 2 doses (6-18 mo apart)† | None | IM | All |
| Hepatitis B* | Recombinant viral antigen | 3 doses (0, 1, 6 mo) | None | IM | All |
| Influenza | Inactivated viral | Single dose | Annually | IM | All |
| Typhoid | Capsular polysaccharide Live attenuated bacteria | Single dose 4 doses (0, 2, 4, 6 mo) | 2-3 y 5 y | IM Oral | All |
| Vaccines for travelers to select destinations | |||||
| Japanese encephalitis | Inactivated viral | 2 doses (28 d apart) | Unknown | IM | Rural Asia‡ |
| Meningococcus | Quadrivalent conjugated polysaccharide | Single dose | >10 y | IM | Sub-Saharan Africa; Saudi Arabia |
| Polio | Inactivated viral | Single dose if patient had childhood series | None | SC; IM | Anyplace where polio still occurs |
| Rabies | Inactivated cell culture viral | 3 doses (0, 7, 21-28 d) | None unless exposure occurs | IM | |
| Yellow fever | Live attenuated virus | Single dose | 10 y | SC | Sub-Saharan Africa; tropical South America |
| IM, intramuscular; SC, subcutaneous. *A combined hepatitis A/B vaccine is approved for use in older adults. †Second dose may be delayed up to 8 years without diminished efficacy. ‡Required only for prolonged stays in rural areas of Asia. Adapted from: Mandell GL, Bennett JE, Dolin R, eds. Principles and Practice of Infectious Diseases. 7th ed. Philadelphia, Pa: Elsevier; 2010. | |||||
Meds and safety measures can minimize malaria risk
The risk of acquiring malaria differs significantly among travelers, based on destination, duration and type of travel, and season. Choice of antimalarial agents (eg, atovaquone/proguanil, chloroquine, doxycycline, mefloquine, and primaquine) should be made on an individual basis after considering these factors, as well as the resistance patterns of the countries on the patient’s itinerary, his or her medical history, and the adverse effects profile of potential agents. Because many older adults take multiple medications, the possibility of drug-drug interactions must be considered.1 You’ll find destination-specific recommendations on malaria prevention on the Centers for Disease Control and Prevention’s Travelers’ Health Web site, listed in “Travel and health: Resources for patients and physicians”. For guidance on the best drug to prescribe, you can also consult a travel medicine specialist.2
Access-able Travel Source Web site provides information for older adult travelers with special needs who need help traveling with oxygen or getting around despite decreased mobility (www.access-able.com/tips/).
American Diabetes Association publishes detailed information about traveling with diabetes (http://www.diabetes.org/living-with-diabetes/know-your-rights/discrimination/public-accommodations/air-travel-and-diabetes/).
Bureau of Consular Affairs publishes information regarding VISA and security requirements at various destinations and travel warnings (http://travel.state.gov/about/about_304.html).
Centers for Disease Control and Prevention publishes “The Yellow Book”—a reference for clinicians who advise international travelers about health risks. There is also a range of other travel-related information on its Travelers’ Health Web site (www.cdc.gov/travel/).
International Travel Medicine Society provides a global travel clinic directory (http://www.istm.org/Webforms/Searchclinics/Default.aspx?SearchType=advanced).
Transportation Security Administration Web site provides information on what can be brought on-board a plane. See “Can I bring my … through the checkpoint?” (http://apps.tsa.dhs.gov/mytsa/cib_home.aspx).
Travel Health Online offers a list of medical providers around the world (www.tripprep.com/scripts).
World Health Organization’s Travel and Health Web site provides free access to selected chapters of its book, “International Travel and Health 2012,” as well as interactive maps, information about infectious diseases and food safety, and more (www.who.int/ith/en/).
Patients should be mindful of mosquitos. Stress the importance of preventing mosquito bites (as much as possible). Advise patients traveling to mosquito-infested areas to use insect repellents containing 30% N-diethyl-meta-toluamide (DEET) and permethrin-treated clothing.34 Tell them, too, to wear long sleeves, pants, and footwear that provides full coverage.35 Ensuring that sleeping areas are properly screened or air-conditioned will further reduce the likelihood of mosquito bites.36
CASE After seeing the chief complaint listed as “Traveling to Kenya” on Professor R’s chart, you quickly review the CDC’s Travelers’ Health Web site. You encourage him to stay with his tour group and to wear a seatbelt whenever possible. You also review how to make a personalized travel health kit, and encourage him to register with the Smart Traveler Enrollment Program (STEP) (detailed at https://step.state.gov/rep) before leaving for the safari. You strongly suggest that he consider purchasing additional medical evacuation insurance, as well.
Given the prevalence of travelers’ diarrhea, along with dengue and malaria, in Kenya, you review food and water safety and avoidance of insect-transmitted diseases with the patient, and write a prescription for ciprofloxacin to be taken if he develops diarrhea. Professor R is not at high risk for VTE, but you encourage him to stay hydrated, avoid sedating medications, and be diligent about mobilization during lengthy flights. You recommend melatonin for jet lag.
To adjust to the heat, you recommend that he avoid strenuous exercise in the first few days and drink sufficient fluids throughout the trip. You administer the Tdap vaccine, an adult polio booster, and the hepatitis A vaccine, verify that he has received his pneumococcal and influenza vaccines, and prescribe an antimalarial medication.
And as you walk him toward the door, you offer him one final piece of advice: Take plenty of pictures.
CORRESPONDENCE
Jeffrey D. Schlaudecker, MD, The Christ Hospital/University of Cincinnati Family Medicine Residency Program, 2123 Auburn Avenue #340, Cincinnati, OH 45219; [email protected]
• Advise older adults to prepare a health travel kit containing all their medications and medical supplies, a list of chronic conditions, and emergency contact information, and to pack it in their carry-on luggage. C
• Instruct patients who will be airborne for ≥4 hours to stay hydrated, avoid alcohol and sedating drugs, and either do seated calf exercises or get up and move about the cabin periodically. B
• Remind patients who will spend time in developing countries to drink only bottled beverages, eat only hot food and fruit that can be peeled, and avoid ice cubes and food from street vendors. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE Larry R, a 77-year-old retired college professor, comes in for a checkup because he is planning a trip to Kenya—on a safari he describes excitedly as “the trip of a lifetime.” He’ll be going with a group, but before he signs on he wants to be sure you think he can manage the tour’s “moderate pace.” He also thinks that he’ll “need to get some shots.”
The patient is overweight (BMI 29) and smokes a pipe daily. He has a history of hypertension, hyperlipidemia, and mild osteoarthritis in both knees and hips, all of which are well controlled.
What would you advise Professor R about the health care preparations needed for his big trip?
Chances are you have patients like Professor R—retired and relatively healthy, and endowed with a sense of adventure and the financial resources that make it possible to visit distant lands. With the nation’s 78 million baby boomers starting to reach retirement age—the oldest cohort turned 65 in 2011—you’re likely to see increasing numbers of older patients with plans for international travel in the years ahead.1
Like their younger counterparts, older people travel for a variety of reasons: Some have planned for decades to take the “trip of a lifetime” when they retire. Others plan longer excursions, sometimes referred to as an adult “gap year,” to relive a long-ago experience, volunteer in an underdeveloped country, or hike and bird watch in a rainforest. Many more are immigrants who travel to visit relatives or friends in their country of origin, usually a lower-income, environmentally depressed locale with a higher incidence of infectious diseases like malaria, typhoid, and hepatitis A. 2
And while the older traveler will have to take many of the same steps to stay healthy as his or her younger counterpart, it is the older traveler who is more likely to have chronic conditions and special needs that require additional preparation. With careful planning, however, even those with decreased faculties, ranging from impaired vision or hearing to mild cognitive impairment, can safely travel abroad.3
A pretravel visit is your opportunity to assess the patient’s fitness to make the trip being planned, ensure optimal management of chronic conditions while traveling, and identify (and recommend steps to mitigate) travel-related risks.
Morbidity and mortality abroad: A review of the risks
Although much pretravel advice centers on the prevention of tropical infectious diseases, such infections account for a very small percentage of deaths of Americans outside of the United States.1 In fact, the major health risks facing older adults traveling abroad are similar to those they face at home: Cardiovascular events are responsible for the preponderance of deaths and for half of all travel-related illnesses.1
International travel can be physically demanding for older individuals and injuries are common, accounting for a large proportion of deaths of Americans overseas4 and an estimated 25% to 38% of travel-related incidents.1,5 A third of injury-related deaths of US citizens traveling abroad involve traffic accidents, followed by homicide (17% of cases) and drowning (13%).1,5 Thus, injury prevention and management of chronic conditions are key issues to address in a pretravel consult.
Even small steps help safeguard older travelers
Older patients planning to travel abroad should schedule an appointment at least 4 to 6 weeks before their departure.2 Ask about the locale, political and environmental climate, length of stay, location and type of accommodations, accessibility to health care, and activities planned,6 which will enable you to offer both general and destination-specific health and safety tips. When advising older adults with complex comorbidities and/or particularly high-risk itineraries, referral to a travel medicine specialist should be strongly considered.
Exercise. Encourage older patients to initiate a graduated exercise program, starting several months before the trip.3 Even a modest improvement in endurance, strength, and flexibility can reduce the likelihood of injury.
Luggage. The right luggage can benefit your patients. Recommend that older patients purchase lightweight suitcases with wheels, which are easier to maneuver in and out of airports6 and less likely to cause muscle strain or musculoskeletal injury.
Insurance. If an individual becomes ill or sustains an injury overseas, the right insurance can be crucial. Advise older adults to review their health insurance policy to see whether it provides overseas coverage. If not, suggest they consider a short-term supplemental policy to cover medical care and evacuation, if needed. Recommend trip cancellation insurance, as well.
Patients should pack pills, medical supplies in a carry-on kit
Encourage all older travelers to compile a personalized travel health kit equipped with common over-the-counter (OTC) medications, prescription drugs, and any personal medical supplies they’ll need, such as a continuous positive airway pressure (CPAP) machine.1,3 Remind patients to take an ample supply of both prescription and OTC drugs, each in its original labeled container.3,6 Buying medications outside the United States is not advisable, given the variation in international regulatory standards. Stress the importance of keeping the kit in a carry-on bag.
The health kit should also include descriptions of the patient’s preexisting medical conditions, which you or a nurse or medical assistant in the practice can help to prepare; a list of prescription drugs he or she takes (using both the generic and brand names); and a copy of a recent electrocardiogram, if available, along with contact information in case of an emergency.1,3,6 A patient who uses injectable medication, such as insulin, should obtain a letter (on the practice’s letterhead) from the prescribing physician and be prepared to show the letter to airport security personnel.3,6
Staying safe in the air
The lower barometric and oxygen partial pressures found in aircraft cabins, which are pressurized at 5000 to 8000 feet, can affect both the respiratory and cardiovascular systems of older adults—particularly those with pulmonary or cardiac disorders.6,7 Individuals who do not routinely require oxygen and are able to walk the equivalent of one city block or climb one flight of stairs without shortness of breath should have little trouble compensating for the reduced oxygen in the cabin.3 Patients with stable heart failure, including New York Heart Association grades III and IV, can tolerate flights of up to one hour without additional oxygen.7
Advise older adults who will require oxygen that they are not permitted to bring their own oxygen canisters onboard an airplane.1 In-flight oxygen needs to be ordered at least 7 days before departure, and there may be a charge.7 Most airlines have medical consultants available to help patients who will need oxygen or other medical provisions.7 In addition, tour companies or travel consultants can help older patients with special needs ensure that they have access to oxygen or other medical supplies at their destination.
Thrombosis—the other in-flight risk
Sometimes referred to as “economy class syndrome” or “traveler’s thrombosis,” the venous stasis of air travel is responsible for a 3-fold increase in the risk of venous thromboembolism (VTE).8 While fatal pulmonary embolism is rare, duration of travel and risk of VTE follow a dose-response relationship, with each 2-hour increase in flight time conferring an additional 18% risk.8 Other risk factors for VTE include varicose veins, metastatic cancer, major surgery within the past 2 weeks, prior VTE, and BMI >40. Advanced age increases travelers’ risk of VTE, as well.7,8 The absolute risk, however, is low.
Among travelers older than 50 years, symptomatic VTE occurs at an estimated rate of one in 600 for flights >4 hours and one in 500 for flights >12 hours.9,10 While there is no evidence that first-class seating lowers the risk, there are preventive measures that patients can take.11
Tell patients to stay hydrated, drinking plenty of fluids but avoiding alcohol during flights of ≥4 hours’ duration. Sedating drugs should be avoided, as well. Advise anyone planning a long flight to either do seated exercises (intermittent calf contractions) or to periodically get up and walk about the cabin.9 You may also want to recommend that patients purchase below-the-knee elastic compression stockings to help decrease venous stasis.12
There is no evidence to recommend the use of aspirin to prevent VTE.12,13 But you may consider prescribing a single 40-mg dose of enoxaparin for a patient who has multiple risk factors and will be airborne for >6 hours.13
Promote safety and comfort on the ground
It is crucial to remind all travelers about the risks associated with traveling in motor vehicles in other countries. Remind patients to wear seat belts whenever they’re available; exercise caution regarding public transportation, which may be overcrowded and have an increased risk of pickpockets and robbery; and avoid riding on motorcycles and scooters. If they do opt to ride on a scooter, tell them that it’s imperative that they wear a helmet.
Minimize the effects of jet lag
Travelers of any age may experience jet lag, which occurs when the individual’s circadian clock cannot keep pace with travel across time zones.14 Notably, however, older people appear to suffer less than their younger counterparts.3 Patients traveling great distances are not likely to avoid jet lag completely, of course. Recommend the following strategies:
Start adjusting your schedule in the week before you depart, gradually shifting 2 hours toward congruence with the time zone at your destination.14
Help reset your circadian rhythm through exposure to bright light, in the morning after eastward travel and in the evening after westward travel.14
Take it easy at first. An itinerary that accounts for initial fatigue is an important nonpharmacologic management strategy.14
Avoid sedating medications, including antihistamines, tranquilizers, anti-motion sickness agents, and benzodiazepines, as these can increase falls and confusion in older adults and make jet lag worse.3
Take melatonin. A dose of 0.5 to 5 mg, taken at bedtime, may promote sleep and decrease jet lag symptoms in travelers crossing multiple time zones.14
Prepare patients to cope with heat …
Unusually hot, humid weather increases morbidity and mortality in the elderly,3,15 and older patients traveling to such climates will need to take extra precautions. Strenuous exercise in the heat should be avoided, because both thirst and the capacity to conserve salt and water decrease with age.16 Acclimatization is helped by rest, air-conditioning, loose cotton clothing, brimmed hats, and cool baths or showers.3 Diuretics may have to be adjusted for fluids lost by increased perspiration, and a discussion about a dose reduction should be included in the pretravel consult for patients who take diuretics and will be traveling to a hot, humid climate.
… and increases in altitude
For older adults, exposure to a moderate altitude (<2500 meters) is initially associated with hypoxemia and a reduced exercise capacity, until acclimatization occurs by Day 5.17,18 Although older adults generally acclimatize well, advise them to limit their activities for the first few days at a higher altitude. This is especially important for patients with coronary artery disease (CAD).
To further ease the effects of a higher altitude, advise patients to drink plenty of fluids, but little or no alcohol.19 Review the medications of an older patient who will be spending time at very high altitude. Rarely, antihypertensive medication may need to be adjusted. The body compensates for lower oxygen with a faster heart rate, and some antihypertensives may interfere with this compensatory mechanism.3
Precautions (and prophylaxis) may prevent travelers’ diarrhea
Diarrhea—among the most common travel-related conditions20—affects an estimated 30% to 70% of international travelers.2 The incidence is highest among visitors to developing countries. Most (80%-90%) of travelers’ diarrhea is due to bacterial infection,21 10% of cases are caused by parasites, and 5% to 8% by viral infection.2,22
Although increasing age lowers the risk of travelers’ diarrhea,1 older patients traveling to developing areas should be cautioned to only eat food that is served hot or fruit they can peel themselves; drink only bottled water and sealed liquids; and avoid salad, ice, and food from street vendors.1 Studies have shown, however, that tourists often get diarrhea despite these safety measures.2
Treatment and prophylaxis. Prophylactic antibiotics can prevent travelers’ diarrhea. But the increased sun sensitivity, drug-drug interactions, and gastrointestinal (GI) adverse effects associated with antibiotics limit their usefulness. Prophylaxis is indicated, however, for older adults for whom the complications of dehydration would likely be so severe that the benefits of using antibiotics to prevent diarrhea clearly outweigh the risks.23
Fluoroquinolones are a first-line treatment for travelers’ diarrhea. But increasing microbial resistance to this class of drugs, especially among Campylobacter isolates,24 may limit their usefulness in some destinations.25 Azithromycin is recommended in such cases, and has been shown to be equally effective.26,27 Single-dose therapy is well established with fluoroquinolones, but the best regimen for azithromycin (1 vs 3 days) is still under evaluation.28,29 Along with instructions on when to take an antibiotic, travelers should be given prescriptions for treatment of travelers’ diarrhea before the start of their trip. Suggest that patients purchase oral rehydration packets to take on their trip, and stress the importance of using them and staying hydrated if diarrhea develops.
Decreased upper GI acidity due to acid-blocking medications such as proton-pump inhibitors can increase the risk for many infections, including salmonella and cholera. Patients taking such medications should be made aware of the risk, and the risks vs benefits of temporarily stopping them should be discussed. Vaccination against cholera should not routinely be recommended.29,30
Vaccines and pills protect against preventable diseases
An impending trip abroad also presents an opportunity to review the patient’s immunization status, catch up on recommended vaccines, and determine whether any additional vaccinations are needed.
Herpes zoster (HZ). Patients older than 60 years should receive a single dose of the HZ vaccine, whether or not they have a history of this condition. Because this is a live virus-containing vaccine, however, it should not be given to anyone who is immunocompromised.31
MMR booster. Adults born before 1957 can be considered immune from both measles and mumps, but not rubella. There is no data on immunization to rubella, but guidelines do not recommend MMR vaccination in the elderly.1
Pneumococcal polyvalent-23 (PPV-23). One dose of the PPV-23 vaccine is indicated for all adults at age 65. This is especially important for travelers, as the prevalence of pneumococcal disease is likely higher in crowded, urban environments within less developed countries.31
Tetanus. Although tetanus is mainly a disease of the elderly, only 45% of men ages 70 years or older and 21% of women in this age group were found to have protective antibodies.1,32 In 2011, the Advisory Committee on Immunization Practices (ACIP) recommended one dose of tetanus and diphtheria toxoid (Td) every 10 years, with a single dose of tetanus toxoid, diphtheria toxoid, and acellular pertussis (Tdap) vaccine given in place of Td for adults older than 65.33 Despite ACIP’s recommendation, the vaccine’s use in adults 65 years and older is an off-label indication, as Tdap is only approved for use in those 11 to 64 years of age.33
Additional vaccines are recommended for travelers, with some indicated for all travelers and others that are destination-specific (TABLE).
TABLE
Which travel-related vaccines does your patient need?
| Disease | Type of vaccine | Primary course | Booster/ follow-up | Route | For which destinations? |
|---|---|---|---|---|---|
| Vaccines for all travelers | |||||
| Hepatitis A* | Killed virus | 2 doses (6-18 mo apart)† | None | IM | All |
| Hepatitis B* | Recombinant viral antigen | 3 doses (0, 1, 6 mo) | None | IM | All |
| Influenza | Inactivated viral | Single dose | Annually | IM | All |
| Typhoid | Capsular polysaccharide Live attenuated bacteria | Single dose 4 doses (0, 2, 4, 6 mo) | 2-3 y 5 y | IM Oral | All |
| Vaccines for travelers to select destinations | |||||
| Japanese encephalitis | Inactivated viral | 2 doses (28 d apart) | Unknown | IM | Rural Asia‡ |
| Meningococcus | Quadrivalent conjugated polysaccharide | Single dose | >10 y | IM | Sub-Saharan Africa; Saudi Arabia |
| Polio | Inactivated viral | Single dose if patient had childhood series | None | SC; IM | Anyplace where polio still occurs |
| Rabies | Inactivated cell culture viral | 3 doses (0, 7, 21-28 d) | None unless exposure occurs | IM | |
| Yellow fever | Live attenuated virus | Single dose | 10 y | SC | Sub-Saharan Africa; tropical South America |
| IM, intramuscular; SC, subcutaneous. *A combined hepatitis A/B vaccine is approved for use in older adults. †Second dose may be delayed up to 8 years without diminished efficacy. ‡Required only for prolonged stays in rural areas of Asia. Adapted from: Mandell GL, Bennett JE, Dolin R, eds. Principles and Practice of Infectious Diseases. 7th ed. Philadelphia, Pa: Elsevier; 2010. | |||||
Meds and safety measures can minimize malaria risk
The risk of acquiring malaria differs significantly among travelers, based on destination, duration and type of travel, and season. Choice of antimalarial agents (eg, atovaquone/proguanil, chloroquine, doxycycline, mefloquine, and primaquine) should be made on an individual basis after considering these factors, as well as the resistance patterns of the countries on the patient’s itinerary, his or her medical history, and the adverse effects profile of potential agents. Because many older adults take multiple medications, the possibility of drug-drug interactions must be considered.1 You’ll find destination-specific recommendations on malaria prevention on the Centers for Disease Control and Prevention’s Travelers’ Health Web site, listed in “Travel and health: Resources for patients and physicians”. For guidance on the best drug to prescribe, you can also consult a travel medicine specialist.2
Access-able Travel Source Web site provides information for older adult travelers with special needs who need help traveling with oxygen or getting around despite decreased mobility (www.access-able.com/tips/).
American Diabetes Association publishes detailed information about traveling with diabetes (http://www.diabetes.org/living-with-diabetes/know-your-rights/discrimination/public-accommodations/air-travel-and-diabetes/).
Bureau of Consular Affairs publishes information regarding VISA and security requirements at various destinations and travel warnings (http://travel.state.gov/about/about_304.html).
Centers for Disease Control and Prevention publishes “The Yellow Book”—a reference for clinicians who advise international travelers about health risks. There is also a range of other travel-related information on its Travelers’ Health Web site (www.cdc.gov/travel/).
International Travel Medicine Society provides a global travel clinic directory (http://www.istm.org/Webforms/Searchclinics/Default.aspx?SearchType=advanced).
Transportation Security Administration Web site provides information on what can be brought on-board a plane. See “Can I bring my … through the checkpoint?” (http://apps.tsa.dhs.gov/mytsa/cib_home.aspx).
Travel Health Online offers a list of medical providers around the world (www.tripprep.com/scripts).
World Health Organization’s Travel and Health Web site provides free access to selected chapters of its book, “International Travel and Health 2012,” as well as interactive maps, information about infectious diseases and food safety, and more (www.who.int/ith/en/).
Patients should be mindful of mosquitos. Stress the importance of preventing mosquito bites (as much as possible). Advise patients traveling to mosquito-infested areas to use insect repellents containing 30% N-diethyl-meta-toluamide (DEET) and permethrin-treated clothing.34 Tell them, too, to wear long sleeves, pants, and footwear that provides full coverage.35 Ensuring that sleeping areas are properly screened or air-conditioned will further reduce the likelihood of mosquito bites.36
CASE After seeing the chief complaint listed as “Traveling to Kenya” on Professor R’s chart, you quickly review the CDC’s Travelers’ Health Web site. You encourage him to stay with his tour group and to wear a seatbelt whenever possible. You also review how to make a personalized travel health kit, and encourage him to register with the Smart Traveler Enrollment Program (STEP) (detailed at https://step.state.gov/rep) before leaving for the safari. You strongly suggest that he consider purchasing additional medical evacuation insurance, as well.
Given the prevalence of travelers’ diarrhea, along with dengue and malaria, in Kenya, you review food and water safety and avoidance of insect-transmitted diseases with the patient, and write a prescription for ciprofloxacin to be taken if he develops diarrhea. Professor R is not at high risk for VTE, but you encourage him to stay hydrated, avoid sedating medications, and be diligent about mobilization during lengthy flights. You recommend melatonin for jet lag.
To adjust to the heat, you recommend that he avoid strenuous exercise in the first few days and drink sufficient fluids throughout the trip. You administer the Tdap vaccine, an adult polio booster, and the hepatitis A vaccine, verify that he has received his pneumococcal and influenza vaccines, and prescribe an antimalarial medication.
And as you walk him toward the door, you offer him one final piece of advice: Take plenty of pictures.
CORRESPONDENCE
Jeffrey D. Schlaudecker, MD, The Christ Hospital/University of Cincinnati Family Medicine Residency Program, 2123 Auburn Avenue #340, Cincinnati, OH 45219; [email protected]
1. Reed CM. Travel recommendations for older adults. Clin Geriatr Med. 2007;23:687-713, ix.
2. Centers for Disease Control and Prevention. Health Information for International Travel 2012. New York, NY:Oxford University Press; 2012. Available at http://wwwnc.cdc.gov/travel/page/yellowboth-2012-home.htm. Accessed December 18, 2012.
3. Cooper MC. The elderly travellers. Travel Med Infect Dis. 2006;4:218-222.
4. Guse CE, Cortes LM, Hargarten SW, et al. Fatal injuries of US citizens abroad. J Travel Med. 2007;14:279-287
5. Tonellato DJ, Guse CE, Hargarten SW. Injury deaths of US citizens abroad: new data source, old travel problem. J Travel Med. 2009;16:304-310.
6. Fenner P. Fitness to travel - assessment in the elderly and medically impaired. Aust Fam Physician. 2007;36:312-315.
7. Smith D, Toff W, Joy M, et al. Fitness to fly for passengers with cardiovascular disease. Heart. 2010;96(suppl 2):Sii1-S16.
8. Chandra D, Parisini E, Mozaffarian D. Meta-analysis: travel and risk for venous thromboembolism. Ann Intern Med. 2009;151:180-190.
9. Gavish I, Brenner B. Air travel and the risk of thromboembolism. Intern Emerg Med. 2011;6:113-116.
10. Ansari MT, Cheung BM, Qing Huang J, et al. Traveler’s thrombosis: a systematic review. J Travel Med. 2005;12:142-154.
11. Schwarz T, Siegert G, Oettler W, et al. Venous thrombosis after long-haul flights. Arch Intern Med. 2003;163:2759-2764.
12. Cesarone MR, Belcaro G, Errichi BM, et al. The LONFLIT4—Concorde Deep Venous Thrombosis and Edema Study: prevention with travel stockings. Angiology. 2003;54:143-154.
13. Cesarone MR, Belcaro G, Nicolaides AN, et al. Venous thrombosis from air travel: the LONFLIT3 study—prevention with aspirin vs low-molecular-weight heparin (LMWH) in high-risk subjects: a randomized trial. Angiology. 2002;53:1-6.
14. Sack RL. Clinical practice. Jet lag. N Engl J Med. 2010: 440-447.
15. Davies I, O’Neill PA, McLean KA, et al. Age-associated alterations in thirst and arginine vasopressin in response to a water or sodium load. Age Ageing. 1995;24:151-159.
16. Rikkert MG, Melis RJ, Claassen JA. Heat waves and dehydration in the elderly. BMJ. 2009;339:b2663.-
17. Levine BD, Zuckerman JH, deFilippi CR. Effect of high-altitude exposure in the elderly: the Tenth Mountain Division study. Circulation. 1997;96:1224-1232.
18. Agostoni P, Cattadori G, Guazzi M, et al. Effects of simulated altitude-induced hypoxia on exercise capacity in patients with chronic heart failure. Am J Med. 2000;109:450-455.
19. Higgins JP, Tuttle T, Higgins JA. Altitude and the heart: is going high safe for your cardiac patient? Am Heart J. 2010;159:25-32.
20. Gautret P, Schlagenhauf P, Gaudart J, et al. Multicenter EuroTravNet/GeoSentinel study of travel-related infectious diseases in Europe. Emerg Infect Dis. 2009;15:1783-1790.
21. Adachi JA, Jiang ZD, Mathewson JJ, et al. Enteroaggregative Escherichia coli as a major etiologic agent in traveler’s diarrhea in 3 regions of the world. Clin Infect Dis. 2001;32:1706-1709.
22. Black RE. Epidemiology of travelers’ diarrhea and relative importance of various pathogens. Rev Infect Dis. 1990;12 (suppl 1):S73-S79.
23. Rendi-Wagner P, Kollaritsch H. Drug prophylaxis for travelers’ diarrhea. Clin Infect Dis. 2002;34:628-633.
24. Butzler JP. Campylobacter, from obscurity to celebrity. Clin Microbiol Infect. 2004;10:868-876.
25. Hoge CW, Gambel JM, Srijan A, et al. Trends in antibiotic resistance among diarrheal pathogens isolated in Thailand over 15 years. Clin Infect Dis. 1998;26:341-345.
26. Adachi JA, Ericsson CD, Jiang ZD, et al. Azithromycin found to be comparable to levofloxacin for the treatment of US travelers with acute diarrhea acquired in Mexico. Clin Infect Dis. 2003;37:1165-1171.
27. Tribble DR, Sanders JW, Pang LW, et al. Traveler’s diarrhea in Thailand: randomized, double-blind trial comparing single-dose and 3-day azithromycin-based regimens with a 3-day levofloxacin regimen. Clin Infect Dis. 2007;44:338-346.
28. Shanks GD, Smoak BL, Aleman GM, et al. Single dose of azithromycin or three-day course of ciprofloxacin as therapy for epidemic dysentery in Kenya. Acute Dysentery Study Group. Clin Infect Dis. 1999;29:942-943.
29. Heatley RV, Sobala GM. Acid suppression and the gastric flora. Baillieres Clin Gastroenterol. 1993;7:167-181.
30. Hill DR, Ford L, Lalloo DG. Oral cholera vaccines: use in clinical practice. Lancet Infect Dis. 2006;6:361-373.
31. Vaughn JA, Miller RA. Update on immunizations in adults. Am Fam Physician. 2011;84:1015-1020.
32. Gergen PJ, McQuillan GM, Kiely M, et al. A population-based serologic survey of immunity to tetanus in the United States. N Engl J Med. 1995;332:761-766.
33. World Health Organization. Typhoid vaccines: WHO position paper. Wkly Epidemiol Rec. 2008;83:49-59.
34. Fradin MS, Day JF. Comparative efficacy of insect repellents against mosquito bites. N Engl J Med. 2002;347:13-18.
35. Soto J, Medina F, Dember N, et al. Efficacy of permethrin-impregnated uniforms in the prevention of malaria and leishmaniasis in Colombian soldiers. Clin Infect Dis. 1995;21:599-602.
36. Freedman DO. Clinical practice. Malaria prevention in short-term travelers. N Engl J Med. 2008;359:603-612.
1. Reed CM. Travel recommendations for older adults. Clin Geriatr Med. 2007;23:687-713, ix.
2. Centers for Disease Control and Prevention. Health Information for International Travel 2012. New York, NY:Oxford University Press; 2012. Available at http://wwwnc.cdc.gov/travel/page/yellowboth-2012-home.htm. Accessed December 18, 2012.
3. Cooper MC. The elderly travellers. Travel Med Infect Dis. 2006;4:218-222.
4. Guse CE, Cortes LM, Hargarten SW, et al. Fatal injuries of US citizens abroad. J Travel Med. 2007;14:279-287
5. Tonellato DJ, Guse CE, Hargarten SW. Injury deaths of US citizens abroad: new data source, old travel problem. J Travel Med. 2009;16:304-310.
6. Fenner P. Fitness to travel - assessment in the elderly and medically impaired. Aust Fam Physician. 2007;36:312-315.
7. Smith D, Toff W, Joy M, et al. Fitness to fly for passengers with cardiovascular disease. Heart. 2010;96(suppl 2):Sii1-S16.
8. Chandra D, Parisini E, Mozaffarian D. Meta-analysis: travel and risk for venous thromboembolism. Ann Intern Med. 2009;151:180-190.
9. Gavish I, Brenner B. Air travel and the risk of thromboembolism. Intern Emerg Med. 2011;6:113-116.
10. Ansari MT, Cheung BM, Qing Huang J, et al. Traveler’s thrombosis: a systematic review. J Travel Med. 2005;12:142-154.
11. Schwarz T, Siegert G, Oettler W, et al. Venous thrombosis after long-haul flights. Arch Intern Med. 2003;163:2759-2764.
12. Cesarone MR, Belcaro G, Errichi BM, et al. The LONFLIT4—Concorde Deep Venous Thrombosis and Edema Study: prevention with travel stockings. Angiology. 2003;54:143-154.
13. Cesarone MR, Belcaro G, Nicolaides AN, et al. Venous thrombosis from air travel: the LONFLIT3 study—prevention with aspirin vs low-molecular-weight heparin (LMWH) in high-risk subjects: a randomized trial. Angiology. 2002;53:1-6.
14. Sack RL. Clinical practice. Jet lag. N Engl J Med. 2010: 440-447.
15. Davies I, O’Neill PA, McLean KA, et al. Age-associated alterations in thirst and arginine vasopressin in response to a water or sodium load. Age Ageing. 1995;24:151-159.
16. Rikkert MG, Melis RJ, Claassen JA. Heat waves and dehydration in the elderly. BMJ. 2009;339:b2663.-
17. Levine BD, Zuckerman JH, deFilippi CR. Effect of high-altitude exposure in the elderly: the Tenth Mountain Division study. Circulation. 1997;96:1224-1232.
18. Agostoni P, Cattadori G, Guazzi M, et al. Effects of simulated altitude-induced hypoxia on exercise capacity in patients with chronic heart failure. Am J Med. 2000;109:450-455.
19. Higgins JP, Tuttle T, Higgins JA. Altitude and the heart: is going high safe for your cardiac patient? Am Heart J. 2010;159:25-32.
20. Gautret P, Schlagenhauf P, Gaudart J, et al. Multicenter EuroTravNet/GeoSentinel study of travel-related infectious diseases in Europe. Emerg Infect Dis. 2009;15:1783-1790.
21. Adachi JA, Jiang ZD, Mathewson JJ, et al. Enteroaggregative Escherichia coli as a major etiologic agent in traveler’s diarrhea in 3 regions of the world. Clin Infect Dis. 2001;32:1706-1709.
22. Black RE. Epidemiology of travelers’ diarrhea and relative importance of various pathogens. Rev Infect Dis. 1990;12 (suppl 1):S73-S79.
23. Rendi-Wagner P, Kollaritsch H. Drug prophylaxis for travelers’ diarrhea. Clin Infect Dis. 2002;34:628-633.
24. Butzler JP. Campylobacter, from obscurity to celebrity. Clin Microbiol Infect. 2004;10:868-876.
25. Hoge CW, Gambel JM, Srijan A, et al. Trends in antibiotic resistance among diarrheal pathogens isolated in Thailand over 15 years. Clin Infect Dis. 1998;26:341-345.
26. Adachi JA, Ericsson CD, Jiang ZD, et al. Azithromycin found to be comparable to levofloxacin for the treatment of US travelers with acute diarrhea acquired in Mexico. Clin Infect Dis. 2003;37:1165-1171.
27. Tribble DR, Sanders JW, Pang LW, et al. Traveler’s diarrhea in Thailand: randomized, double-blind trial comparing single-dose and 3-day azithromycin-based regimens with a 3-day levofloxacin regimen. Clin Infect Dis. 2007;44:338-346.
28. Shanks GD, Smoak BL, Aleman GM, et al. Single dose of azithromycin or three-day course of ciprofloxacin as therapy for epidemic dysentery in Kenya. Acute Dysentery Study Group. Clin Infect Dis. 1999;29:942-943.
29. Heatley RV, Sobala GM. Acid suppression and the gastric flora. Baillieres Clin Gastroenterol. 1993;7:167-181.
30. Hill DR, Ford L, Lalloo DG. Oral cholera vaccines: use in clinical practice. Lancet Infect Dis. 2006;6:361-373.
31. Vaughn JA, Miller RA. Update on immunizations in adults. Am Fam Physician. 2011;84:1015-1020.
32. Gergen PJ, McQuillan GM, Kiely M, et al. A population-based serologic survey of immunity to tetanus in the United States. N Engl J Med. 1995;332:761-766.
33. World Health Organization. Typhoid vaccines: WHO position paper. Wkly Epidemiol Rec. 2008;83:49-59.
34. Fradin MS, Day JF. Comparative efficacy of insect repellents against mosquito bites. N Engl J Med. 2002;347:13-18.
35. Soto J, Medina F, Dember N, et al. Efficacy of permethrin-impregnated uniforms in the prevention of malaria and leishmaniasis in Colombian soldiers. Clin Infect Dis. 1995;21:599-602.
36. Freedman DO. Clinical practice. Malaria prevention in short-term travelers. N Engl J Med. 2008;359:603-612.