User login
Speed your diagnosis of this gallbladder disorder
• Use the Rome III guidelines to diagnose and treat functional gallbladder disorder; when this benchmark is followed, cholecystectomy results in ~90% resolution rate. B
• Keep in mind that classic biliary symptoms, particularly right upper quadrant pain, pain after eating, and reproduction of pain with cholecystokinin injection, are highly predictive of a successful postoperative outcome. C
• Offer cholecystectomy to patients who present with classic biliary symptoms and an abnormal hepatobiliary iminodiacetic acid (HIDA) scan. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE Dionne J, a 38-year-old woman with a BMI of 32, presents with a 2-month history of right upper abdominal pain. The pain is intermittent and often begins after eating, she reports, particularly when a meal includes fatty foods. She has no nausea, vomiting, diarrhea, constipation, or fever, and the pain is not getting progressively worse.
When the pain comes on, Ms. J says, it lasts about an hour, sometimes less. It is colicky in nature, and not relieved with bowel movements or position change. The patient tried ranitidine 150 mg twice a day for 2 weeks, with no relief. You suspect functional gallbladder disorder. But is Ms. J a candidate for a cholecystectomy? What would you do next?
Over the past 2 decades, the incidence of cholecystectomies due to functional gallbladder disorder (FGBD) has multiplied, going from about 5% to 20% to 25%.1 But definitive information about the etiology of FGBD has not kept pace.
Although the Rome III diagnostic guidelines for FGBD, published in 2006,2 remain the standard of care, a number of more recent studies have added to our understanding of this disorder. This review of the diagnosis and treatment of FGBD incorporates both the Rome III guidelines and the latest findings. The text and tables that follow can help you recognize this clinical entity earlier, minimize the number of tests needed to arrive at a definitive diagnosis, and establish a plan of care that is consistent with both the guidelines and the evidence.
As obesity rates rise, so does gallbladder dysfunction
Obesity has been shown to produce a chronic proinflammatory state throughout the body,3-6 which has been linked to fatty infiltration of the gallbladder (among other organs) and impaired contractility.3,6-9
A study by Al-Azzawi et al highlighted the importance of increased fat in the gallbladder wall as a key cause of dysmotility.10 The researchers compared wall thickness, inflammation, and the amount of fat in the walls of gallbladders that had been removed for both acalculous and calculous disease with the characteristics of gallbladders removed for reasons unrelated to organ dysfunction (the controls). Those with dysmotility, they found, had more fat in the wall but the same wall thickness as the controls. The amount of fat in the walls was similar for the acalculous and the calculous groups, but the gallbladders in which stones were found had more inflammation and increased wall thickness.10
Several other studies have found evidence of both inflammation and fatty deposits in the walls of gallbladders removed for acalculous disease.2,4,11-13
In one study, researchers found chronic inflammation in 99% of gallbladders removed from patients who had classic biliary symptoms but no gallstones.11
FGBD appears to be initiated by fatty infiltration of the gallbladder wall, causing increasing levels of inflammation and steatocholecystitis that lead to poor motility.3,4,6-10 This in turn alters bile composition, which can lead to sludge and stone formation.2,6,10 The finding by Al-Azzawi et al of greater thickness and inflammation in the walls of gallbladders with calculi suggests that gallstones result from progressively worsening inflammation and dysmotility.10
Steps to take for a definitive diagnosis
A diagnosis of FGBD requires a history of classic gallbladder symptoms, many but not all of which are specified in the Rome III diagnostic criteria (TABLE). Classic symptoms include nausea, vomiting, right upper quadrant pain, pain after eating, and reproduction of pain with cholecystokinin (CCK) injection. Cramping, bloating, reflux, diarrhea, fullness, and epigastric pain are atypical symptoms.2,11
TABLE
Rome III diagnostic criteria for functional gallbladder disorder
Must include episodes of pain located in the epigastrium and/or right upper quadrant and all of the following findings:
|
| Supportive criteria |
The pain may present with one or more of the following findings:
|
| Source: Behar et al. Gastroenterology. 2006;130:1498-1509.2 Used with permission from Elsevier. |
Rule out structural causes
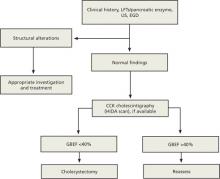
There is no single test for FGBD, and a definitive diagnosis can be made only after structural causes of the symptoms (eg, gallstones, tumor, sclerosis, and cirrhosis) have been ruled out (ALGORITHM).2 Initial tests include liver and pancreatic enzyme laboratory screening and an ultrasound of the upper right quadrant. In patients with FGBD, both the lab tests and the ultrasound will be normal.
ALGORITHM
Diagnostic workup and management of functional gallbladder disorder (Rome III)
CCK, cholecystokinin; EGD, esophagogastroduodenoscopy; GBEF, gallbladder ejection fraction; HIDA, hepatobiliary iminodiacetic acid; LFTs, liver function tests; US, ultrasound.
Source: Behar J et al. Gastroenterology. 2006;130:1498-1509.2 Used with permission from Elsevier.
The Rome III guidelines also call for an esophagogastroduodenoscopy (EGD) to rule out esophagitis, gastritis, and duodenitis, although some researchers have held that if the other tests are normal, this test need not be done.12 If the EGD is also normal—or not done—a hepatobiliary iminodiacetic acid (HIDA) scan is the next step in the diagnostic pathway. The scan tests the gallbladder’s ejection fraction (EF), revealing the percentage of radioactive dye ejected from the organ after CCK is injected (FIGURE).2 The injection of CCK should be done over a minimum of 30 minutes, the guidelines specify. The shorter the time frame used for the injection, the less likely that the pain will be reproduced or that the EF findings will be reliable.14
FIGURE
Abnormal vs normal HIDA scans: What you’ll see
The larger amount of contrast dye retained in the abnormal scan (A) compared with the normal scan (B) is evidence of a poor ejection fraction.
Most researchers define a normal EF as >35%,10,11,13 but the Rome III criteria use a cutoff of 40%. A patient who has an EF <40% and meets the other guideline criteria is diagnosed with FGBD.
CASE On physical examination, Ms. J has pain in the right upper quadrant, with no guarding or rebound, and normal bowel sounds. Her liver and pancreatic enzyme tests are normal, and an ultrasound shows no sludge, no stones, and mild edema of the gallbladder wall. The patient declines an EGD because of the cost but undergoes a HIDA scan—which reveals that she has an EF of 25%.
Will cholecystectomy bring long-term relief?
There are 2 options for a patient diagnosed with FGBD—medical management, consisting of lifestyle modifications such as dietary change and weight loss and medication for symptom relief—or cholecystectomy. Surgery should be offered to any individual who, like Ms. J, meets the Rome III diagnostic criteria and has an abnormal HIDA scan. Recent studies have raised questions about the correlation between HIDA results and postoperative relief,11,12 however, and indicate that patients who have classic biliary symptoms and a normal HIDA scan often have good postoperative outcomes, as well.11,15
A careful workup is key to ensuring maximal benefit from surgery. The resolution of symptoms with a cholecystectomy when the Rome III criteria are followed for patient selection has been found to be close to 90%.11,15-20 Two recent studies have examined the resolution rate for FGBD, with conflicting results.11,12 Both studies were based on long-term postoperative follow-up, ranging from 6 to 24 months. The main difference was the selection bias used in determining eligibility for the study.
The initial selection criteria for the study by Carr et al (N=93) were presenting symptoms (either classic or atypical), followed by a typical workup. The long-term resolution rate for those with classic gallbladder symptoms was 88%11—close to the 90% associated with the Rome III guidelines. The study by Singhal et al (N=141)12 was done retrospectively, using objective data from tests (ie, normal ultrasound and liver biochemistries and abnormal HIDA) rather than patient history as the criteria for inclusion. Among participants in the Singhal study, the long-term resolution rate was just 57%.
Ironically, the patients in the Carr study who had atypical symptoms had mixed postoperative results. The rate of long-term resolution for this cohort was 57%—the same as the overall resolution rate found by Singhal et al.11,12 The fact that a group of patients who presented atypically had the same postoperative resolution rate as those for whom tests (rather than symptoms) were used as the selection criteria illustrates the importance of presenting symptoms as a prognostic indicator.
CASE Ms. J opts for a cholecystectomy and you refer her to a general surgeon. At her annual exam the following year, she reports that she has been symptom free since the surgery.
CORRESPONDENCE
David I. Croteau, MD, FAAFP, LRMC Family Medical Center, 300 Parkview Place, Lakeland, FL 33805; [email protected]
1. Majeski J. Gallbladder ejection fraction: an accurate evaluation of symptomatic acalculous gallbladder disease. Int Surg. 2003;88:95-99.
2. Behar J, Corazziari E, Guelrud M, et al. Functional gallbladder and sphincter of Oddi disorders. Gastroenterology. 2006;130:1498-1509.
3. Goldblatt MI, Swartz-Basile DA, Al-Azzawi HH, et al. Nonalcoholic fatty gallbladder disease: the Influence of diet in lean and obese mice. J Gastrointest Surg. 2006;10:193-201.
4. Chung-Jyi. Steatocholecystitis and fatty gallbladder disease. Dig Dis Sci. 2009;54:1857-1863.
5. Bastard JP, Maachi M, Lagathu C, et al. Recent advances in the relationship between obesity, inflammation, and insulin resistance. Eur Cytokine Netw. 2006;17:4-12.
6. Pitt HA. Hepato-pancreato-biliary fat: the good, the bad and the ugly. HPB (Oxford). 2007;9:92-97.
7. Merg AR, Kalinowski SE, Hinkhouse MM, et al. Mechanisms of impaired gallbladder contractile response in chronic acalculous cholecystitis. J Gastrointest Surg. 2002;6:432-437.
8. Amaral J, Xiao ZL, Chen Q, et al. Gallbladder muscle dysfunction in patients with chronic acalculous disease. Gastroenterology. 2001;120:506-511.
9. Portincasa P, Ciaula AD, Baldassarre G, et al. Gallbladder motor function in gallstone patients: sonographic and in vitro studies on the role of gallstones, smooth muscle function, and gallbladder wall inflammation. J Hepatol. 1994;21:430-440.
10. Al-Azzawi HH, Nakeeb A, Saxena R, et al. Cholecystosteatosis: an explanation for increased cholecystectomy rates. J Gastrointest Surg. 2007;11:835-843.
11. Carr JA, Walls J, Bryan LJ, et al. The treatment of gallbladder dyskinesia based upon symptoms: results of a 2-year, prospective, nonrandomized, concurrent cohort study. Surg Laparosc Endosc Percutan Tech. 2009;19:222-226.
12. Singhal V, Szeto P, Norman H, et al. Biliary dyskinesia: how effective is cholecystectomy? J Gastrointest Surg. 2012;16:135-141.
13. Francis G, Baillie J. Gallbladder dyskinesia: fact or fiction? Curr Gastroenterol Rep. 2011;13:188-192.
14. Ziessman HA. Nuclear medicine hepatobiliary imaging. Clin Gastroenterol Hepatol. 2010;8:111-116.
15. Delgado-Aros S, Cremonini R, Bredenoord AJ, et al. Systemic review and meta-analysis: does gallbladder ejection fraction on cholecystokinin cholescintigraphy predict outcome after cholecystectomy in suspected functional biliary pain? Aliment Pharmacol Ther. 2003;18:167-174.
16. Patel PA, Lamb JJ, Hogle NJ, et al. Therapeutic efficacy of laparoscopic cholecystectomy in the treatment of biliary dyskinesia. Am J Surg. 2004;187:209-212.
17. Mahid SS, Jafri NS, Brangers BC, et al. Meta-analysis of cholecystectomy in symptomatic patients with positive hepatoiminodiacetic acid scan results without gallstones. Arch Surg. 2009;144:180-187.
18. Hansel SL, DiBaise JK. Functional gallbladder disorder: gallbladder dyskinesia. Gastroenterol Clin North Am. 2010;39:369-379.
19. Canfield AJ, Hetz SP, Shriver JP, et al. Biliary dyskinesia: a study of more than 200 patients and review of the literature. J Gastrointest Surg. 1998;2:443-448.
20. Jagannath SB, Singh VK, Cruz-Correa M, et al. A long-term cohort study of outcome after cholecystectomy for chronic acalculous cholecystitis. Am J Surg. 2003;185:91-95.
• Use the Rome III guidelines to diagnose and treat functional gallbladder disorder; when this benchmark is followed, cholecystectomy results in ~90% resolution rate. B
• Keep in mind that classic biliary symptoms, particularly right upper quadrant pain, pain after eating, and reproduction of pain with cholecystokinin injection, are highly predictive of a successful postoperative outcome. C
• Offer cholecystectomy to patients who present with classic biliary symptoms and an abnormal hepatobiliary iminodiacetic acid (HIDA) scan. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE Dionne J, a 38-year-old woman with a BMI of 32, presents with a 2-month history of right upper abdominal pain. The pain is intermittent and often begins after eating, she reports, particularly when a meal includes fatty foods. She has no nausea, vomiting, diarrhea, constipation, or fever, and the pain is not getting progressively worse.
When the pain comes on, Ms. J says, it lasts about an hour, sometimes less. It is colicky in nature, and not relieved with bowel movements or position change. The patient tried ranitidine 150 mg twice a day for 2 weeks, with no relief. You suspect functional gallbladder disorder. But is Ms. J a candidate for a cholecystectomy? What would you do next?
Over the past 2 decades, the incidence of cholecystectomies due to functional gallbladder disorder (FGBD) has multiplied, going from about 5% to 20% to 25%.1 But definitive information about the etiology of FGBD has not kept pace.
Although the Rome III diagnostic guidelines for FGBD, published in 2006,2 remain the standard of care, a number of more recent studies have added to our understanding of this disorder. This review of the diagnosis and treatment of FGBD incorporates both the Rome III guidelines and the latest findings. The text and tables that follow can help you recognize this clinical entity earlier, minimize the number of tests needed to arrive at a definitive diagnosis, and establish a plan of care that is consistent with both the guidelines and the evidence.
As obesity rates rise, so does gallbladder dysfunction
Obesity has been shown to produce a chronic proinflammatory state throughout the body,3-6 which has been linked to fatty infiltration of the gallbladder (among other organs) and impaired contractility.3,6-9
A study by Al-Azzawi et al highlighted the importance of increased fat in the gallbladder wall as a key cause of dysmotility.10 The researchers compared wall thickness, inflammation, and the amount of fat in the walls of gallbladders that had been removed for both acalculous and calculous disease with the characteristics of gallbladders removed for reasons unrelated to organ dysfunction (the controls). Those with dysmotility, they found, had more fat in the wall but the same wall thickness as the controls. The amount of fat in the walls was similar for the acalculous and the calculous groups, but the gallbladders in which stones were found had more inflammation and increased wall thickness.10
Several other studies have found evidence of both inflammation and fatty deposits in the walls of gallbladders removed for acalculous disease.2,4,11-13
In one study, researchers found chronic inflammation in 99% of gallbladders removed from patients who had classic biliary symptoms but no gallstones.11
FGBD appears to be initiated by fatty infiltration of the gallbladder wall, causing increasing levels of inflammation and steatocholecystitis that lead to poor motility.3,4,6-10 This in turn alters bile composition, which can lead to sludge and stone formation.2,6,10 The finding by Al-Azzawi et al of greater thickness and inflammation in the walls of gallbladders with calculi suggests that gallstones result from progressively worsening inflammation and dysmotility.10
Steps to take for a definitive diagnosis
A diagnosis of FGBD requires a history of classic gallbladder symptoms, many but not all of which are specified in the Rome III diagnostic criteria (TABLE). Classic symptoms include nausea, vomiting, right upper quadrant pain, pain after eating, and reproduction of pain with cholecystokinin (CCK) injection. Cramping, bloating, reflux, diarrhea, fullness, and epigastric pain are atypical symptoms.2,11
TABLE
Rome III diagnostic criteria for functional gallbladder disorder
Must include episodes of pain located in the epigastrium and/or right upper quadrant and all of the following findings:
|
| Supportive criteria |
The pain may present with one or more of the following findings:
|
| Source: Behar et al. Gastroenterology. 2006;130:1498-1509.2 Used with permission from Elsevier. |
Rule out structural causes
There is no single test for FGBD, and a definitive diagnosis can be made only after structural causes of the symptoms (eg, gallstones, tumor, sclerosis, and cirrhosis) have been ruled out (ALGORITHM).2 Initial tests include liver and pancreatic enzyme laboratory screening and an ultrasound of the upper right quadrant. In patients with FGBD, both the lab tests and the ultrasound will be normal.
ALGORITHM
Diagnostic workup and management of functional gallbladder disorder (Rome III)
CCK, cholecystokinin; EGD, esophagogastroduodenoscopy; GBEF, gallbladder ejection fraction; HIDA, hepatobiliary iminodiacetic acid; LFTs, liver function tests; US, ultrasound.
Source: Behar J et al. Gastroenterology. 2006;130:1498-1509.2 Used with permission from Elsevier.
The Rome III guidelines also call for an esophagogastroduodenoscopy (EGD) to rule out esophagitis, gastritis, and duodenitis, although some researchers have held that if the other tests are normal, this test need not be done.12 If the EGD is also normal—or not done—a hepatobiliary iminodiacetic acid (HIDA) scan is the next step in the diagnostic pathway. The scan tests the gallbladder’s ejection fraction (EF), revealing the percentage of radioactive dye ejected from the organ after CCK is injected (FIGURE).2 The injection of CCK should be done over a minimum of 30 minutes, the guidelines specify. The shorter the time frame used for the injection, the less likely that the pain will be reproduced or that the EF findings will be reliable.14
FIGURE
Abnormal vs normal HIDA scans: What you’ll see
The larger amount of contrast dye retained in the abnormal scan (A) compared with the normal scan (B) is evidence of a poor ejection fraction.
Most researchers define a normal EF as >35%,10,11,13 but the Rome III criteria use a cutoff of 40%. A patient who has an EF <40% and meets the other guideline criteria is diagnosed with FGBD.
CASE On physical examination, Ms. J has pain in the right upper quadrant, with no guarding or rebound, and normal bowel sounds. Her liver and pancreatic enzyme tests are normal, and an ultrasound shows no sludge, no stones, and mild edema of the gallbladder wall. The patient declines an EGD because of the cost but undergoes a HIDA scan—which reveals that she has an EF of 25%.
Will cholecystectomy bring long-term relief?
There are 2 options for a patient diagnosed with FGBD—medical management, consisting of lifestyle modifications such as dietary change and weight loss and medication for symptom relief—or cholecystectomy. Surgery should be offered to any individual who, like Ms. J, meets the Rome III diagnostic criteria and has an abnormal HIDA scan. Recent studies have raised questions about the correlation between HIDA results and postoperative relief,11,12 however, and indicate that patients who have classic biliary symptoms and a normal HIDA scan often have good postoperative outcomes, as well.11,15
A careful workup is key to ensuring maximal benefit from surgery. The resolution of symptoms with a cholecystectomy when the Rome III criteria are followed for patient selection has been found to be close to 90%.11,15-20 Two recent studies have examined the resolution rate for FGBD, with conflicting results.11,12 Both studies were based on long-term postoperative follow-up, ranging from 6 to 24 months. The main difference was the selection bias used in determining eligibility for the study.
The initial selection criteria for the study by Carr et al (N=93) were presenting symptoms (either classic or atypical), followed by a typical workup. The long-term resolution rate for those with classic gallbladder symptoms was 88%11—close to the 90% associated with the Rome III guidelines. The study by Singhal et al (N=141)12 was done retrospectively, using objective data from tests (ie, normal ultrasound and liver biochemistries and abnormal HIDA) rather than patient history as the criteria for inclusion. Among participants in the Singhal study, the long-term resolution rate was just 57%.
Ironically, the patients in the Carr study who had atypical symptoms had mixed postoperative results. The rate of long-term resolution for this cohort was 57%—the same as the overall resolution rate found by Singhal et al.11,12 The fact that a group of patients who presented atypically had the same postoperative resolution rate as those for whom tests (rather than symptoms) were used as the selection criteria illustrates the importance of presenting symptoms as a prognostic indicator.
CASE Ms. J opts for a cholecystectomy and you refer her to a general surgeon. At her annual exam the following year, she reports that she has been symptom free since the surgery.
CORRESPONDENCE
David I. Croteau, MD, FAAFP, LRMC Family Medical Center, 300 Parkview Place, Lakeland, FL 33805; [email protected]
• Use the Rome III guidelines to diagnose and treat functional gallbladder disorder; when this benchmark is followed, cholecystectomy results in ~90% resolution rate. B
• Keep in mind that classic biliary symptoms, particularly right upper quadrant pain, pain after eating, and reproduction of pain with cholecystokinin injection, are highly predictive of a successful postoperative outcome. C
• Offer cholecystectomy to patients who present with classic biliary symptoms and an abnormal hepatobiliary iminodiacetic acid (HIDA) scan. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE Dionne J, a 38-year-old woman with a BMI of 32, presents with a 2-month history of right upper abdominal pain. The pain is intermittent and often begins after eating, she reports, particularly when a meal includes fatty foods. She has no nausea, vomiting, diarrhea, constipation, or fever, and the pain is not getting progressively worse.
When the pain comes on, Ms. J says, it lasts about an hour, sometimes less. It is colicky in nature, and not relieved with bowel movements or position change. The patient tried ranitidine 150 mg twice a day for 2 weeks, with no relief. You suspect functional gallbladder disorder. But is Ms. J a candidate for a cholecystectomy? What would you do next?
Over the past 2 decades, the incidence of cholecystectomies due to functional gallbladder disorder (FGBD) has multiplied, going from about 5% to 20% to 25%.1 But definitive information about the etiology of FGBD has not kept pace.
Although the Rome III diagnostic guidelines for FGBD, published in 2006,2 remain the standard of care, a number of more recent studies have added to our understanding of this disorder. This review of the diagnosis and treatment of FGBD incorporates both the Rome III guidelines and the latest findings. The text and tables that follow can help you recognize this clinical entity earlier, minimize the number of tests needed to arrive at a definitive diagnosis, and establish a plan of care that is consistent with both the guidelines and the evidence.
As obesity rates rise, so does gallbladder dysfunction
Obesity has been shown to produce a chronic proinflammatory state throughout the body,3-6 which has been linked to fatty infiltration of the gallbladder (among other organs) and impaired contractility.3,6-9
A study by Al-Azzawi et al highlighted the importance of increased fat in the gallbladder wall as a key cause of dysmotility.10 The researchers compared wall thickness, inflammation, and the amount of fat in the walls of gallbladders that had been removed for both acalculous and calculous disease with the characteristics of gallbladders removed for reasons unrelated to organ dysfunction (the controls). Those with dysmotility, they found, had more fat in the wall but the same wall thickness as the controls. The amount of fat in the walls was similar for the acalculous and the calculous groups, but the gallbladders in which stones were found had more inflammation and increased wall thickness.10
Several other studies have found evidence of both inflammation and fatty deposits in the walls of gallbladders removed for acalculous disease.2,4,11-13
In one study, researchers found chronic inflammation in 99% of gallbladders removed from patients who had classic biliary symptoms but no gallstones.11
FGBD appears to be initiated by fatty infiltration of the gallbladder wall, causing increasing levels of inflammation and steatocholecystitis that lead to poor motility.3,4,6-10 This in turn alters bile composition, which can lead to sludge and stone formation.2,6,10 The finding by Al-Azzawi et al of greater thickness and inflammation in the walls of gallbladders with calculi suggests that gallstones result from progressively worsening inflammation and dysmotility.10
Steps to take for a definitive diagnosis
A diagnosis of FGBD requires a history of classic gallbladder symptoms, many but not all of which are specified in the Rome III diagnostic criteria (TABLE). Classic symptoms include nausea, vomiting, right upper quadrant pain, pain after eating, and reproduction of pain with cholecystokinin (CCK) injection. Cramping, bloating, reflux, diarrhea, fullness, and epigastric pain are atypical symptoms.2,11
TABLE
Rome III diagnostic criteria for functional gallbladder disorder
Must include episodes of pain located in the epigastrium and/or right upper quadrant and all of the following findings:
|
| Supportive criteria |
The pain may present with one or more of the following findings:
|
| Source: Behar et al. Gastroenterology. 2006;130:1498-1509.2 Used with permission from Elsevier. |
Rule out structural causes
There is no single test for FGBD, and a definitive diagnosis can be made only after structural causes of the symptoms (eg, gallstones, tumor, sclerosis, and cirrhosis) have been ruled out (ALGORITHM).2 Initial tests include liver and pancreatic enzyme laboratory screening and an ultrasound of the upper right quadrant. In patients with FGBD, both the lab tests and the ultrasound will be normal.
ALGORITHM
Diagnostic workup and management of functional gallbladder disorder (Rome III)
CCK, cholecystokinin; EGD, esophagogastroduodenoscopy; GBEF, gallbladder ejection fraction; HIDA, hepatobiliary iminodiacetic acid; LFTs, liver function tests; US, ultrasound.
Source: Behar J et al. Gastroenterology. 2006;130:1498-1509.2 Used with permission from Elsevier.
The Rome III guidelines also call for an esophagogastroduodenoscopy (EGD) to rule out esophagitis, gastritis, and duodenitis, although some researchers have held that if the other tests are normal, this test need not be done.12 If the EGD is also normal—or not done—a hepatobiliary iminodiacetic acid (HIDA) scan is the next step in the diagnostic pathway. The scan tests the gallbladder’s ejection fraction (EF), revealing the percentage of radioactive dye ejected from the organ after CCK is injected (FIGURE).2 The injection of CCK should be done over a minimum of 30 minutes, the guidelines specify. The shorter the time frame used for the injection, the less likely that the pain will be reproduced or that the EF findings will be reliable.14
FIGURE
Abnormal vs normal HIDA scans: What you’ll see
The larger amount of contrast dye retained in the abnormal scan (A) compared with the normal scan (B) is evidence of a poor ejection fraction.
Most researchers define a normal EF as >35%,10,11,13 but the Rome III criteria use a cutoff of 40%. A patient who has an EF <40% and meets the other guideline criteria is diagnosed with FGBD.
CASE On physical examination, Ms. J has pain in the right upper quadrant, with no guarding or rebound, and normal bowel sounds. Her liver and pancreatic enzyme tests are normal, and an ultrasound shows no sludge, no stones, and mild edema of the gallbladder wall. The patient declines an EGD because of the cost but undergoes a HIDA scan—which reveals that she has an EF of 25%.
Will cholecystectomy bring long-term relief?
There are 2 options for a patient diagnosed with FGBD—medical management, consisting of lifestyle modifications such as dietary change and weight loss and medication for symptom relief—or cholecystectomy. Surgery should be offered to any individual who, like Ms. J, meets the Rome III diagnostic criteria and has an abnormal HIDA scan. Recent studies have raised questions about the correlation between HIDA results and postoperative relief,11,12 however, and indicate that patients who have classic biliary symptoms and a normal HIDA scan often have good postoperative outcomes, as well.11,15
A careful workup is key to ensuring maximal benefit from surgery. The resolution of symptoms with a cholecystectomy when the Rome III criteria are followed for patient selection has been found to be close to 90%.11,15-20 Two recent studies have examined the resolution rate for FGBD, with conflicting results.11,12 Both studies were based on long-term postoperative follow-up, ranging from 6 to 24 months. The main difference was the selection bias used in determining eligibility for the study.
The initial selection criteria for the study by Carr et al (N=93) were presenting symptoms (either classic or atypical), followed by a typical workup. The long-term resolution rate for those with classic gallbladder symptoms was 88%11—close to the 90% associated with the Rome III guidelines. The study by Singhal et al (N=141)12 was done retrospectively, using objective data from tests (ie, normal ultrasound and liver biochemistries and abnormal HIDA) rather than patient history as the criteria for inclusion. Among participants in the Singhal study, the long-term resolution rate was just 57%.
Ironically, the patients in the Carr study who had atypical symptoms had mixed postoperative results. The rate of long-term resolution for this cohort was 57%—the same as the overall resolution rate found by Singhal et al.11,12 The fact that a group of patients who presented atypically had the same postoperative resolution rate as those for whom tests (rather than symptoms) were used as the selection criteria illustrates the importance of presenting symptoms as a prognostic indicator.
CASE Ms. J opts for a cholecystectomy and you refer her to a general surgeon. At her annual exam the following year, she reports that she has been symptom free since the surgery.
CORRESPONDENCE
David I. Croteau, MD, FAAFP, LRMC Family Medical Center, 300 Parkview Place, Lakeland, FL 33805; [email protected]
1. Majeski J. Gallbladder ejection fraction: an accurate evaluation of symptomatic acalculous gallbladder disease. Int Surg. 2003;88:95-99.
2. Behar J, Corazziari E, Guelrud M, et al. Functional gallbladder and sphincter of Oddi disorders. Gastroenterology. 2006;130:1498-1509.
3. Goldblatt MI, Swartz-Basile DA, Al-Azzawi HH, et al. Nonalcoholic fatty gallbladder disease: the Influence of diet in lean and obese mice. J Gastrointest Surg. 2006;10:193-201.
4. Chung-Jyi. Steatocholecystitis and fatty gallbladder disease. Dig Dis Sci. 2009;54:1857-1863.
5. Bastard JP, Maachi M, Lagathu C, et al. Recent advances in the relationship between obesity, inflammation, and insulin resistance. Eur Cytokine Netw. 2006;17:4-12.
6. Pitt HA. Hepato-pancreato-biliary fat: the good, the bad and the ugly. HPB (Oxford). 2007;9:92-97.
7. Merg AR, Kalinowski SE, Hinkhouse MM, et al. Mechanisms of impaired gallbladder contractile response in chronic acalculous cholecystitis. J Gastrointest Surg. 2002;6:432-437.
8. Amaral J, Xiao ZL, Chen Q, et al. Gallbladder muscle dysfunction in patients with chronic acalculous disease. Gastroenterology. 2001;120:506-511.
9. Portincasa P, Ciaula AD, Baldassarre G, et al. Gallbladder motor function in gallstone patients: sonographic and in vitro studies on the role of gallstones, smooth muscle function, and gallbladder wall inflammation. J Hepatol. 1994;21:430-440.
10. Al-Azzawi HH, Nakeeb A, Saxena R, et al. Cholecystosteatosis: an explanation for increased cholecystectomy rates. J Gastrointest Surg. 2007;11:835-843.
11. Carr JA, Walls J, Bryan LJ, et al. The treatment of gallbladder dyskinesia based upon symptoms: results of a 2-year, prospective, nonrandomized, concurrent cohort study. Surg Laparosc Endosc Percutan Tech. 2009;19:222-226.
12. Singhal V, Szeto P, Norman H, et al. Biliary dyskinesia: how effective is cholecystectomy? J Gastrointest Surg. 2012;16:135-141.
13. Francis G, Baillie J. Gallbladder dyskinesia: fact or fiction? Curr Gastroenterol Rep. 2011;13:188-192.
14. Ziessman HA. Nuclear medicine hepatobiliary imaging. Clin Gastroenterol Hepatol. 2010;8:111-116.
15. Delgado-Aros S, Cremonini R, Bredenoord AJ, et al. Systemic review and meta-analysis: does gallbladder ejection fraction on cholecystokinin cholescintigraphy predict outcome after cholecystectomy in suspected functional biliary pain? Aliment Pharmacol Ther. 2003;18:167-174.
16. Patel PA, Lamb JJ, Hogle NJ, et al. Therapeutic efficacy of laparoscopic cholecystectomy in the treatment of biliary dyskinesia. Am J Surg. 2004;187:209-212.
17. Mahid SS, Jafri NS, Brangers BC, et al. Meta-analysis of cholecystectomy in symptomatic patients with positive hepatoiminodiacetic acid scan results without gallstones. Arch Surg. 2009;144:180-187.
18. Hansel SL, DiBaise JK. Functional gallbladder disorder: gallbladder dyskinesia. Gastroenterol Clin North Am. 2010;39:369-379.
19. Canfield AJ, Hetz SP, Shriver JP, et al. Biliary dyskinesia: a study of more than 200 patients and review of the literature. J Gastrointest Surg. 1998;2:443-448.
20. Jagannath SB, Singh VK, Cruz-Correa M, et al. A long-term cohort study of outcome after cholecystectomy for chronic acalculous cholecystitis. Am J Surg. 2003;185:91-95.
1. Majeski J. Gallbladder ejection fraction: an accurate evaluation of symptomatic acalculous gallbladder disease. Int Surg. 2003;88:95-99.
2. Behar J, Corazziari E, Guelrud M, et al. Functional gallbladder and sphincter of Oddi disorders. Gastroenterology. 2006;130:1498-1509.
3. Goldblatt MI, Swartz-Basile DA, Al-Azzawi HH, et al. Nonalcoholic fatty gallbladder disease: the Influence of diet in lean and obese mice. J Gastrointest Surg. 2006;10:193-201.
4. Chung-Jyi. Steatocholecystitis and fatty gallbladder disease. Dig Dis Sci. 2009;54:1857-1863.
5. Bastard JP, Maachi M, Lagathu C, et al. Recent advances in the relationship between obesity, inflammation, and insulin resistance. Eur Cytokine Netw. 2006;17:4-12.
6. Pitt HA. Hepato-pancreato-biliary fat: the good, the bad and the ugly. HPB (Oxford). 2007;9:92-97.
7. Merg AR, Kalinowski SE, Hinkhouse MM, et al. Mechanisms of impaired gallbladder contractile response in chronic acalculous cholecystitis. J Gastrointest Surg. 2002;6:432-437.
8. Amaral J, Xiao ZL, Chen Q, et al. Gallbladder muscle dysfunction in patients with chronic acalculous disease. Gastroenterology. 2001;120:506-511.
9. Portincasa P, Ciaula AD, Baldassarre G, et al. Gallbladder motor function in gallstone patients: sonographic and in vitro studies on the role of gallstones, smooth muscle function, and gallbladder wall inflammation. J Hepatol. 1994;21:430-440.
10. Al-Azzawi HH, Nakeeb A, Saxena R, et al. Cholecystosteatosis: an explanation for increased cholecystectomy rates. J Gastrointest Surg. 2007;11:835-843.
11. Carr JA, Walls J, Bryan LJ, et al. The treatment of gallbladder dyskinesia based upon symptoms: results of a 2-year, prospective, nonrandomized, concurrent cohort study. Surg Laparosc Endosc Percutan Tech. 2009;19:222-226.
12. Singhal V, Szeto P, Norman H, et al. Biliary dyskinesia: how effective is cholecystectomy? J Gastrointest Surg. 2012;16:135-141.
13. Francis G, Baillie J. Gallbladder dyskinesia: fact or fiction? Curr Gastroenterol Rep. 2011;13:188-192.
14. Ziessman HA. Nuclear medicine hepatobiliary imaging. Clin Gastroenterol Hepatol. 2010;8:111-116.
15. Delgado-Aros S, Cremonini R, Bredenoord AJ, et al. Systemic review and meta-analysis: does gallbladder ejection fraction on cholecystokinin cholescintigraphy predict outcome after cholecystectomy in suspected functional biliary pain? Aliment Pharmacol Ther. 2003;18:167-174.
16. Patel PA, Lamb JJ, Hogle NJ, et al. Therapeutic efficacy of laparoscopic cholecystectomy in the treatment of biliary dyskinesia. Am J Surg. 2004;187:209-212.
17. Mahid SS, Jafri NS, Brangers BC, et al. Meta-analysis of cholecystectomy in symptomatic patients with positive hepatoiminodiacetic acid scan results without gallstones. Arch Surg. 2009;144:180-187.
18. Hansel SL, DiBaise JK. Functional gallbladder disorder: gallbladder dyskinesia. Gastroenterol Clin North Am. 2010;39:369-379.
19. Canfield AJ, Hetz SP, Shriver JP, et al. Biliary dyskinesia: a study of more than 200 patients and review of the literature. J Gastrointest Surg. 1998;2:443-448.
20. Jagannath SB, Singh VK, Cruz-Correa M, et al. A long-term cohort study of outcome after cholecystectomy for chronic acalculous cholecystitis. Am J Surg. 2003;185:91-95.
Is Chest Pain Related to Prior Fracture?
ANSWER
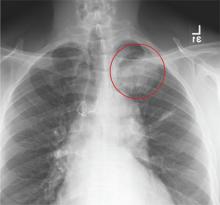
The radiograph demonstrates evidence of previous surgery on the sternum. There also is evidence of scarring or discoid atelectasis along the left mid lung.
Of note, though, is a soft tissue mass (about 5 to 6 cm) within the left pulmonary apex. This lesion could represent a rounded infiltrate, an atypical infection such as a mycetoma, or possibly a pulmonary neoplasm.
Since the patient was stable, he was placed on antibiotics with instructions to follow up with his primary care provider for further work-up on the mass. The patient did follow up; the lesion persisted and subsequent biopsy confirmed carcinoma.
ANSWER
The radiograph demonstrates evidence of previous surgery on the sternum. There also is evidence of scarring or discoid atelectasis along the left mid lung.
Of note, though, is a soft tissue mass (about 5 to 6 cm) within the left pulmonary apex. This lesion could represent a rounded infiltrate, an atypical infection such as a mycetoma, or possibly a pulmonary neoplasm.
Since the patient was stable, he was placed on antibiotics with instructions to follow up with his primary care provider for further work-up on the mass. The patient did follow up; the lesion persisted and subsequent biopsy confirmed carcinoma.
ANSWER
The radiograph demonstrates evidence of previous surgery on the sternum. There also is evidence of scarring or discoid atelectasis along the left mid lung.
Of note, though, is a soft tissue mass (about 5 to 6 cm) within the left pulmonary apex. This lesion could represent a rounded infiltrate, an atypical infection such as a mycetoma, or possibly a pulmonary neoplasm.
Since the patient was stable, he was placed on antibiotics with instructions to follow up with his primary care provider for further work-up on the mass. The patient did follow up; the lesion persisted and subsequent biopsy confirmed carcinoma.
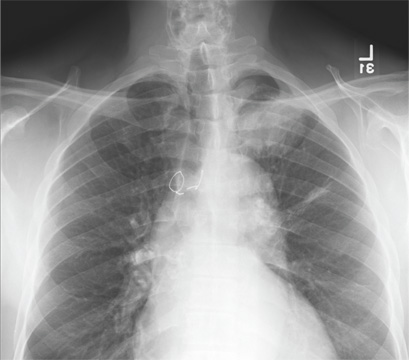
A 61-year-old man presents to your urgent care center for evaluation of “chest pain” he has been experiencing for almost four weeks. He denies any injury or trauma. He describes the pain as “sharp” and “stabbing” and says occasionally it is associated with breathing, localized primarily to the left side. There is no radiation of the pain. He denies fever, nausea, weight loss, night sweats, and hemoptysis. He has smoked a half-pack of cigarettes daily for more than 40 years. His medical history is otherwise unremarkable, except that he was told he had “high blood pressure” and he had his sternum repaired several years ago, following fracture in an accident. Vital signs are as follows: temperature, 36.4°C; blood pressure, 174/100 mm Hg; ventricular rate, 88 beats/min; respiratory rate, 20 breaths/min; and O2 saturation, 100% on room air. He appears to be in no obvious distress. Lung sounds are normal, as is the rest of the physical examination. You obtain a chest radiograph. What is your impression?
Man in Distress With Lower Extremity Pain
ANSWER
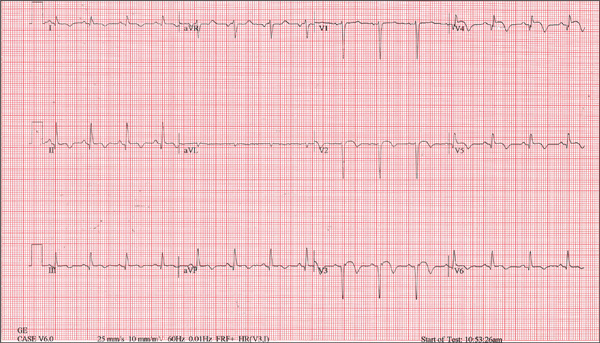
The ECG is diagnostic for a septal and anterolateral ST-elevated myocardial infarction (STEMI), suggestive of a recent MI versus left ventricular aneurysm. The ECG is also diagnostic for a recent inferior wall STEMI.
There are Q waves in leads V1 to V5 and a 2-mm ST elevation in V2 to V6, as well as a T-wave inversion (TWI) in V2 to V6 and lead 1. A 1-mm ST elevation, TWI, and small Q waves are noted in leads II, III, and aVF (inferior leads).
The patient’s abnormal troponin T level assisted with the differential diagnosis. The patient’s normal CK-MB pattern, combined with an elevated troponin T with ECG changes and chest pain reported about one week earlier, supported the diagnosis of a recent acute MI (within the past week).
The stress test was canceled. Cardiac catheterization was performed and showed a 100% mid left anterior descending (LAD) stenosis, with left to left collaterals, and an 80% right coronary artery (RCA) stenosis. No left ventricular aneurysm was noted.
Interventions included an urgent percutaneous intervention (PCI) coronary stent placement to the LAD and a staged PCI to the RCA at a later date.
ANSWER
The ECG is diagnostic for a septal and anterolateral ST-elevated myocardial infarction (STEMI), suggestive of a recent MI versus left ventricular aneurysm. The ECG is also diagnostic for a recent inferior wall STEMI.
There are Q waves in leads V1 to V5 and a 2-mm ST elevation in V2 to V6, as well as a T-wave inversion (TWI) in V2 to V6 and lead 1. A 1-mm ST elevation, TWI, and small Q waves are noted in leads II, III, and aVF (inferior leads).
The patient’s abnormal troponin T level assisted with the differential diagnosis. The patient’s normal CK-MB pattern, combined with an elevated troponin T with ECG changes and chest pain reported about one week earlier, supported the diagnosis of a recent acute MI (within the past week).
The stress test was canceled. Cardiac catheterization was performed and showed a 100% mid left anterior descending (LAD) stenosis, with left to left collaterals, and an 80% right coronary artery (RCA) stenosis. No left ventricular aneurysm was noted.
Interventions included an urgent percutaneous intervention (PCI) coronary stent placement to the LAD and a staged PCI to the RCA at a later date.
ANSWER
The ECG is diagnostic for a septal and anterolateral ST-elevated myocardial infarction (STEMI), suggestive of a recent MI versus left ventricular aneurysm. The ECG is also diagnostic for a recent inferior wall STEMI.
There are Q waves in leads V1 to V5 and a 2-mm ST elevation in V2 to V6, as well as a T-wave inversion (TWI) in V2 to V6 and lead 1. A 1-mm ST elevation, TWI, and small Q waves are noted in leads II, III, and aVF (inferior leads).
The patient’s abnormal troponin T level assisted with the differential diagnosis. The patient’s normal CK-MB pattern, combined with an elevated troponin T with ECG changes and chest pain reported about one week earlier, supported the diagnosis of a recent acute MI (within the past week).
The stress test was canceled. Cardiac catheterization was performed and showed a 100% mid left anterior descending (LAD) stenosis, with left to left collaterals, and an 80% right coronary artery (RCA) stenosis. No left ventricular aneurysm was noted.
Interventions included an urgent percutaneous intervention (PCI) coronary stent placement to the LAD and a staged PCI to the RCA at a later date.
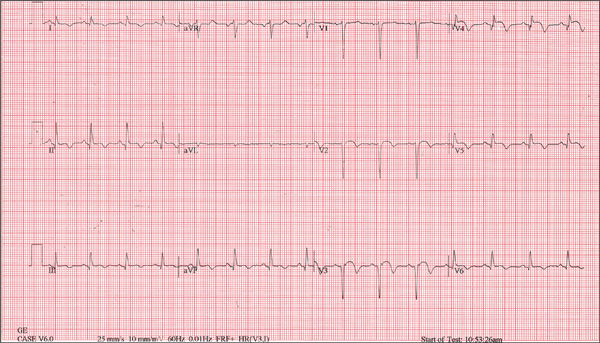
A 53-year-old white man is referred for an adenosine myocardial perfusion scan stress test prior to left femoral popliteal bypass graft surgery. An ECG obtained one month ago was interpreted as normal. A transthoracic echocardiogram at that time revealed a normal left ventricular ejection fraction (> 55%) with normal wall motion and no pathologic valvular heart disease. The patient is in distress due to left lower extremity vascular ischemic rest pain. He admits that earlier this morning, he was slightly dyspneic. However, he denies chest pain. He admits to not taking aspirin (162 mg Q day) as prescribed for the past two days. He also reports an 88–pack-year smoking history. Pulmonary function tests support mild obstructive emphysematous changes. The patient is 68” tall and weighs 128 lb (BMI, 19.5). His vital signs include: a blood pressure of 128/82 mm Hg; ventricular rate, 94 beats/min; respiratory rate, 20 breaths/min; and O2 saturation, 90% on room air. He is afebrile. Pertinent physical findings include no jugular venous distention and no bilateral carotid, femoral, or abdominal bruits. The neurologic exam is unremarkable. The cardiac exam reveals a regular rate and rhythm with an S3 gallop; there is no S4, murmur, or click. There is no peripheral edema. Femoral pulses are +1. Pedal pulses are absent. Stat cardiac enzymes reveal a normal CK-MB (3.3 U/L) and an elevated troponin T level (0.117 μg/L; normal, 0.00 to 0.03). The complete blood count, prothrombin time/partial thromboplastin time/INR tests, and other chemistry panels all yield normal results. This patient’s ECG shows the following: a ventricular rate of 90 beats/min; PR interval, 135 ms; QRS duration, 96 ms; QT/QTc interval, 394/476 ms; P axis, 67°; R axis, 68°; and T axis, 172°. What is your interpretation of this ECG?
Edematous Changes Coincide with New Job
ANSWER
The correct answer is gram-negative bacteria (choice “d”); Pseudomonas is the most likely culprit. Candida albicans (choice “a”), a yeast, is an unlikely cause of this problem and even more unlikely to show up on a bacterial culture. Coagulase-positive staph aureus (choice “b”) is typically associated with infections involving the acute onset of redness, pain, swelling, and pus formation, not the indolent, chronic, low-grade process seen in this case. Trichophyton rubrum (choice “c”) is a dermatophyte, the most common fungal cause of athlete’s feet. The bacterial culture could not have grown a dermatophyte, which needs special media and conditions to grow.
DISCUSSSION
Gram-negative interweb impetigo is a relatively common dermatologic entity, which can be caused by any number of organisms found in fecal material. Pseudomonas, Klebsiella, Proteus, and Acinetobacter are among the more common culprits. These types of infections tend to be much more indolent than the more common staph- and strep-caused cellulitis, which are more likely to create acute redness, swelling, pain, and pus.
Both types of bacterial infections need certain conditions in order to develop. These include excessive heat, sweat, and perhaps most significantly, a break in the skin barrier. Ironically, these fissures are often caused by dermatophytes, in the form of tinea pedis, which is, of course, far better known for causing rashes of the foot.
But tinea pedis is more likely to be found between the third and fourth or the fourth and fifth toes. It creates itching and maceration but rarely causes diffuse redness or edema, and even more rarely leads to pain (unless there is a secondary bacterial infection). As mentioned, given the indolence of this infective process, a culture result showing staph or strep was unlikely.
The culture in this case showed Proteus, for which the minocycline was predictably effective. The rationale for obtaining the acid-fast culture was the possibility of finding Mycobacteria species such as M fortuitum, which is known to cause chronic indolent infections in feet and legs. These, however, more typically manifest with solitary eroded or ulcerated lesions. (Minocycline would have been effective against this organism.)
The use of the topical econazole served two purposes: While this was clearly not classic tinea pedis, it was still possible a dermatophyte or a yeast could have played a role in the creation of the initial fissuring; econazole will help control this, long term. Econazole also has significant antibacterial action and is particularly useful to help prevent future flares.
ANSWER
The correct answer is gram-negative bacteria (choice “d”); Pseudomonas is the most likely culprit. Candida albicans (choice “a”), a yeast, is an unlikely cause of this problem and even more unlikely to show up on a bacterial culture. Coagulase-positive staph aureus (choice “b”) is typically associated with infections involving the acute onset of redness, pain, swelling, and pus formation, not the indolent, chronic, low-grade process seen in this case. Trichophyton rubrum (choice “c”) is a dermatophyte, the most common fungal cause of athlete’s feet. The bacterial culture could not have grown a dermatophyte, which needs special media and conditions to grow.
DISCUSSSION
Gram-negative interweb impetigo is a relatively common dermatologic entity, which can be caused by any number of organisms found in fecal material. Pseudomonas, Klebsiella, Proteus, and Acinetobacter are among the more common culprits. These types of infections tend to be much more indolent than the more common staph- and strep-caused cellulitis, which are more likely to create acute redness, swelling, pain, and pus.
Both types of bacterial infections need certain conditions in order to develop. These include excessive heat, sweat, and perhaps most significantly, a break in the skin barrier. Ironically, these fissures are often caused by dermatophytes, in the form of tinea pedis, which is, of course, far better known for causing rashes of the foot.
But tinea pedis is more likely to be found between the third and fourth or the fourth and fifth toes. It creates itching and maceration but rarely causes diffuse redness or edema, and even more rarely leads to pain (unless there is a secondary bacterial infection). As mentioned, given the indolence of this infective process, a culture result showing staph or strep was unlikely.
The culture in this case showed Proteus, for which the minocycline was predictably effective. The rationale for obtaining the acid-fast culture was the possibility of finding Mycobacteria species such as M fortuitum, which is known to cause chronic indolent infections in feet and legs. These, however, more typically manifest with solitary eroded or ulcerated lesions. (Minocycline would have been effective against this organism.)
The use of the topical econazole served two purposes: While this was clearly not classic tinea pedis, it was still possible a dermatophyte or a yeast could have played a role in the creation of the initial fissuring; econazole will help control this, long term. Econazole also has significant antibacterial action and is particularly useful to help prevent future flares.
ANSWER
The correct answer is gram-negative bacteria (choice “d”); Pseudomonas is the most likely culprit. Candida albicans (choice “a”), a yeast, is an unlikely cause of this problem and even more unlikely to show up on a bacterial culture. Coagulase-positive staph aureus (choice “b”) is typically associated with infections involving the acute onset of redness, pain, swelling, and pus formation, not the indolent, chronic, low-grade process seen in this case. Trichophyton rubrum (choice “c”) is a dermatophyte, the most common fungal cause of athlete’s feet. The bacterial culture could not have grown a dermatophyte, which needs special media and conditions to grow.
DISCUSSSION
Gram-negative interweb impetigo is a relatively common dermatologic entity, which can be caused by any number of organisms found in fecal material. Pseudomonas, Klebsiella, Proteus, and Acinetobacter are among the more common culprits. These types of infections tend to be much more indolent than the more common staph- and strep-caused cellulitis, which are more likely to create acute redness, swelling, pain, and pus.
Both types of bacterial infections need certain conditions in order to develop. These include excessive heat, sweat, and perhaps most significantly, a break in the skin barrier. Ironically, these fissures are often caused by dermatophytes, in the form of tinea pedis, which is, of course, far better known for causing rashes of the foot.
But tinea pedis is more likely to be found between the third and fourth or the fourth and fifth toes. It creates itching and maceration but rarely causes diffuse redness or edema, and even more rarely leads to pain (unless there is a secondary bacterial infection). As mentioned, given the indolence of this infective process, a culture result showing staph or strep was unlikely.
The culture in this case showed Proteus, for which the minocycline was predictably effective. The rationale for obtaining the acid-fast culture was the possibility of finding Mycobacteria species such as M fortuitum, which is known to cause chronic indolent infections in feet and legs. These, however, more typically manifest with solitary eroded or ulcerated lesions. (Minocycline would have been effective against this organism.)
The use of the topical econazole served two purposes: While this was clearly not classic tinea pedis, it was still possible a dermatophyte or a yeast could have played a role in the creation of the initial fissuring; econazole will help control this, long term. Econazole also has significant antibacterial action and is particularly useful to help prevent future flares.
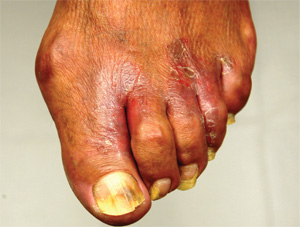
A 50-year-old man presents with a six-month history of worsening redness, swelling, and pain in the interdigital web spaces of both feet. Numerous treatments—most recently, a two-month course of terbinafine 250 mg/d—have not induced any change. The problem manifested as mild cracking between the first and second toes, dorsal aspect, and slowly spread laterally to involve all four web spaces. This pro-cess coincided with the start of a new job in which the patient is on his feet, wearing steel-toed boots, for 12 hours per day in a hot environment. Assuming the problem was athlete’s foot, he tried OTC clotrimazole and terbinafine creams; neither helped at all. In fact, the patient’s pain is now so bad that he has difficult walking. There is no history of smoking, diabetes, or other serious health problems. Examination shows distinct demarcated, dusky-red, edematous changes largely confined to the dorsal aspect. The actual deep interdigital space and the volar aspects of these areas are spared. Slight epidermal fissuring is seen on the dorsal aspect of each web space, from which a small amount of fluid can be coaxed. The fluid is sent for bacterial and acid-fast cultures. In the meantime, the patient is prescribed oral minocycline 100 bid and topical econazole cream and in-structed to return in two weeks. At that time, his condition is almost completely resolved.
Primary care participation ready to rise in 2013
A new report finds that 49% of surveyed primary care physicians expect to participate in an accountable care organization in the next year. Twenty percent already are in ACOs, and most of them are contracting with commercial payers and Medicaid.
Why this dramatic jump? At the end of the day, it is all about the shift from volume-based reimbursement (fee for service) to value-based reimbursement. ACOs are a means to an end, not the end itself. If we will get paid for squeezing waste out of our current system and look at the best way to do it, the elements of an ACO logically fall into place.2
We attribute the big jump in primary care participation to three main factors:
1. Primary care will drive value.
The highest-impact targets for ACOs are:
• Prevention and wellness.
• Chronic disease management.
• Care transitions and navigation.
• Reduced hospitalizations.
• Multispecialty care coordination of complex patients.
While not a monopoly of primary care, all of these opportunities are in your wheelhouse. That is why the Medicare Shared Savings Program ACO regulations correctly require every ACO to include primary care providers. Consequently, primary care providers are being heavily recruited by ACOs.
2. Primary care will derive benefits.
Most ACOs are compensated by receiving some percentage, usually 50%, of savings for a patient population if quality and patient satisfaction metrics are also met. For an ACO to be successful, distribution of savings should be in proportion to their contribution. Primary care physicians should contract only with ACOs that recognize this fundamental connection.
The distribution must be an incentive for every participant to contribute as much value as possible. That will not happen unless the savings distribution is based on merit. As noted, primary care stands to contribute more value, and thus merit more distributions, than any other ACO participant.
The dysfunctions of the fee-for-service system have left primary care underpaid. Now, with the compensation model shifting leverage from costs to savings, primary care physicians are stepping up to close this gap.
3. Primary care is realizing that ACOs are for real.
The move to value-based reimbursement is being driven by unsustainable health care costs, not "Obamacare" or the U.S. Supreme Court. The "fiscal cliff" will have more impact on the growth of ACOs than the Affordable Care Act, as it is forcing us to look at the main drivers of the deficit – entitlements such as Social Security, Medicaid, and Medicare. Notwithstanding, many physicians were waiting until after the election to get serious about ACOs.
3a. This column
(Just kidding!)
So, for these reasons, it is not surprising that primary care physicians are now jumping into ACOs. That said, it is still pretty startling to see a 250% increase in 1 year, which was probably a 250% jump from the year before. Thank you for stepping up to help save American health care.
References
1. Accountable Care Organizations: How Will Payer and Provider Adoption of This Model Impact Prescribing Trends in Cardiometabolic Diseases? Decision Resources, October 2012.
2. As covered in prior articles, besides value-based reimbursement, there are seven other essential elements for a successful ACO: primary care, culture, administration, information technology, patient engagement, scale, and best practices.
Mr. Bobbitt is a senior partner and head of the Health Law Group at the Smith Anderson law firm in Raleigh, N.C. He has many years’ experience assisting physicians to form integrated delivery systems. He has spoken and written nationally to primary care physicians on the strategies and practicalities of forming or joining ACOs. This article is meant to be educational and does not constitute legal advice. For additional information, readers may contact the author at [email protected] or 919-821-6612.
A new report finds that 49% of surveyed primary care physicians expect to participate in an accountable care organization in the next year. Twenty percent already are in ACOs, and most of them are contracting with commercial payers and Medicaid.
Why this dramatic jump? At the end of the day, it is all about the shift from volume-based reimbursement (fee for service) to value-based reimbursement. ACOs are a means to an end, not the end itself. If we will get paid for squeezing waste out of our current system and look at the best way to do it, the elements of an ACO logically fall into place.2
We attribute the big jump in primary care participation to three main factors:
1. Primary care will drive value.
The highest-impact targets for ACOs are:
• Prevention and wellness.
• Chronic disease management.
• Care transitions and navigation.
• Reduced hospitalizations.
• Multispecialty care coordination of complex patients.
While not a monopoly of primary care, all of these opportunities are in your wheelhouse. That is why the Medicare Shared Savings Program ACO regulations correctly require every ACO to include primary care providers. Consequently, primary care providers are being heavily recruited by ACOs.
2. Primary care will derive benefits.
Most ACOs are compensated by receiving some percentage, usually 50%, of savings for a patient population if quality and patient satisfaction metrics are also met. For an ACO to be successful, distribution of savings should be in proportion to their contribution. Primary care physicians should contract only with ACOs that recognize this fundamental connection.
The distribution must be an incentive for every participant to contribute as much value as possible. That will not happen unless the savings distribution is based on merit. As noted, primary care stands to contribute more value, and thus merit more distributions, than any other ACO participant.
The dysfunctions of the fee-for-service system have left primary care underpaid. Now, with the compensation model shifting leverage from costs to savings, primary care physicians are stepping up to close this gap.
3. Primary care is realizing that ACOs are for real.
The move to value-based reimbursement is being driven by unsustainable health care costs, not "Obamacare" or the U.S. Supreme Court. The "fiscal cliff" will have more impact on the growth of ACOs than the Affordable Care Act, as it is forcing us to look at the main drivers of the deficit – entitlements such as Social Security, Medicaid, and Medicare. Notwithstanding, many physicians were waiting until after the election to get serious about ACOs.
3a. This column
(Just kidding!)
So, for these reasons, it is not surprising that primary care physicians are now jumping into ACOs. That said, it is still pretty startling to see a 250% increase in 1 year, which was probably a 250% jump from the year before. Thank you for stepping up to help save American health care.
References
1. Accountable Care Organizations: How Will Payer and Provider Adoption of This Model Impact Prescribing Trends in Cardiometabolic Diseases? Decision Resources, October 2012.
2. As covered in prior articles, besides value-based reimbursement, there are seven other essential elements for a successful ACO: primary care, culture, administration, information technology, patient engagement, scale, and best practices.
Mr. Bobbitt is a senior partner and head of the Health Law Group at the Smith Anderson law firm in Raleigh, N.C. He has many years’ experience assisting physicians to form integrated delivery systems. He has spoken and written nationally to primary care physicians on the strategies and practicalities of forming or joining ACOs. This article is meant to be educational and does not constitute legal advice. For additional information, readers may contact the author at [email protected] or 919-821-6612.
A new report finds that 49% of surveyed primary care physicians expect to participate in an accountable care organization in the next year. Twenty percent already are in ACOs, and most of them are contracting with commercial payers and Medicaid.
Why this dramatic jump? At the end of the day, it is all about the shift from volume-based reimbursement (fee for service) to value-based reimbursement. ACOs are a means to an end, not the end itself. If we will get paid for squeezing waste out of our current system and look at the best way to do it, the elements of an ACO logically fall into place.2
We attribute the big jump in primary care participation to three main factors:
1. Primary care will drive value.
The highest-impact targets for ACOs are:
• Prevention and wellness.
• Chronic disease management.
• Care transitions and navigation.
• Reduced hospitalizations.
• Multispecialty care coordination of complex patients.
While not a monopoly of primary care, all of these opportunities are in your wheelhouse. That is why the Medicare Shared Savings Program ACO regulations correctly require every ACO to include primary care providers. Consequently, primary care providers are being heavily recruited by ACOs.
2. Primary care will derive benefits.
Most ACOs are compensated by receiving some percentage, usually 50%, of savings for a patient population if quality and patient satisfaction metrics are also met. For an ACO to be successful, distribution of savings should be in proportion to their contribution. Primary care physicians should contract only with ACOs that recognize this fundamental connection.
The distribution must be an incentive for every participant to contribute as much value as possible. That will not happen unless the savings distribution is based on merit. As noted, primary care stands to contribute more value, and thus merit more distributions, than any other ACO participant.
The dysfunctions of the fee-for-service system have left primary care underpaid. Now, with the compensation model shifting leverage from costs to savings, primary care physicians are stepping up to close this gap.
3. Primary care is realizing that ACOs are for real.
The move to value-based reimbursement is being driven by unsustainable health care costs, not "Obamacare" or the U.S. Supreme Court. The "fiscal cliff" will have more impact on the growth of ACOs than the Affordable Care Act, as it is forcing us to look at the main drivers of the deficit – entitlements such as Social Security, Medicaid, and Medicare. Notwithstanding, many physicians were waiting until after the election to get serious about ACOs.
3a. This column
(Just kidding!)
So, for these reasons, it is not surprising that primary care physicians are now jumping into ACOs. That said, it is still pretty startling to see a 250% increase in 1 year, which was probably a 250% jump from the year before. Thank you for stepping up to help save American health care.
References
1. Accountable Care Organizations: How Will Payer and Provider Adoption of This Model Impact Prescribing Trends in Cardiometabolic Diseases? Decision Resources, October 2012.
2. As covered in prior articles, besides value-based reimbursement, there are seven other essential elements for a successful ACO: primary care, culture, administration, information technology, patient engagement, scale, and best practices.
Mr. Bobbitt is a senior partner and head of the Health Law Group at the Smith Anderson law firm in Raleigh, N.C. He has many years’ experience assisting physicians to form integrated delivery systems. He has spoken and written nationally to primary care physicians on the strategies and practicalities of forming or joining ACOs. This article is meant to be educational and does not constitute legal advice. For additional information, readers may contact the author at [email protected] or 919-821-6612.
Tailored Health IT Improves VTE Rates
Electronic decision support significantly improves VTE prophylaxis and hospital-acquired VTE rates, according to a new study in the Journal of Hospital Medicine.
The report, "Improving Hospital Venous Thromboembolism Prophylaxis With Electronic Decision Report," saw overall medical service prophylaxis rise to 82.1% from 61.9% (P<0.001) and pharmacologic VTE prophylaxis increase to 74.5% from 59% (P<0.001).
"Healthcare leaders talk about information technology (IT) as a means toward effecting improvements in quality and patient safety and, most of the time, they view that and discuss that in terms of the actual IT system being implemented," says lead author Rohit Bhalla, MD, MPH, associate professor of clinical medicine at Albert Einstein College of Medicine in New York City. "What our intervention really got to was once you've implemented an IT system ... how can it be modified, vis-à-vis decision support, so that it provides an even better result than you get with the product that comes out of the box."
Tailoring a health IT system to improve outcomes requires interdisciplinary work that includes quality officers, physicians, IT staff, and programmers. Hospitalist and fellow author Jason Adelman, MD, MS, patient safety officer at Montefiore Medical Center in the Bronx, N.Y., where the study was conducted, says that the research can help generate future buy-in from physicians who don't value electronic decision support tools.
It can "ease the swallowing of the bitter pill to know that it really makes a difference," Dr. Adelman says. "Don't be up in arms when you're forced to do something a little bit extra, because it really works."
Visit our website for more information about health information technology.
Electronic decision support significantly improves VTE prophylaxis and hospital-acquired VTE rates, according to a new study in the Journal of Hospital Medicine.
The report, "Improving Hospital Venous Thromboembolism Prophylaxis With Electronic Decision Report," saw overall medical service prophylaxis rise to 82.1% from 61.9% (P<0.001) and pharmacologic VTE prophylaxis increase to 74.5% from 59% (P<0.001).
"Healthcare leaders talk about information technology (IT) as a means toward effecting improvements in quality and patient safety and, most of the time, they view that and discuss that in terms of the actual IT system being implemented," says lead author Rohit Bhalla, MD, MPH, associate professor of clinical medicine at Albert Einstein College of Medicine in New York City. "What our intervention really got to was once you've implemented an IT system ... how can it be modified, vis-à-vis decision support, so that it provides an even better result than you get with the product that comes out of the box."
Tailoring a health IT system to improve outcomes requires interdisciplinary work that includes quality officers, physicians, IT staff, and programmers. Hospitalist and fellow author Jason Adelman, MD, MS, patient safety officer at Montefiore Medical Center in the Bronx, N.Y., where the study was conducted, says that the research can help generate future buy-in from physicians who don't value electronic decision support tools.
It can "ease the swallowing of the bitter pill to know that it really makes a difference," Dr. Adelman says. "Don't be up in arms when you're forced to do something a little bit extra, because it really works."
Visit our website for more information about health information technology.
Electronic decision support significantly improves VTE prophylaxis and hospital-acquired VTE rates, according to a new study in the Journal of Hospital Medicine.
The report, "Improving Hospital Venous Thromboembolism Prophylaxis With Electronic Decision Report," saw overall medical service prophylaxis rise to 82.1% from 61.9% (P<0.001) and pharmacologic VTE prophylaxis increase to 74.5% from 59% (P<0.001).
"Healthcare leaders talk about information technology (IT) as a means toward effecting improvements in quality and patient safety and, most of the time, they view that and discuss that in terms of the actual IT system being implemented," says lead author Rohit Bhalla, MD, MPH, associate professor of clinical medicine at Albert Einstein College of Medicine in New York City. "What our intervention really got to was once you've implemented an IT system ... how can it be modified, vis-à-vis decision support, so that it provides an even better result than you get with the product that comes out of the box."
Tailoring a health IT system to improve outcomes requires interdisciplinary work that includes quality officers, physicians, IT staff, and programmers. Hospitalist and fellow author Jason Adelman, MD, MS, patient safety officer at Montefiore Medical Center in the Bronx, N.Y., where the study was conducted, says that the research can help generate future buy-in from physicians who don't value electronic decision support tools.
It can "ease the swallowing of the bitter pill to know that it really makes a difference," Dr. Adelman says. "Don't be up in arms when you're forced to do something a little bit extra, because it really works."
Visit our website for more information about health information technology.
TeamSTEPPS Initiative Teaches Teamwork to Healthcare Providers
University of Minnesota hospitalist Karyn Baum, MD, MSEd, directs one of six regional training centers for Team Strategies and Tools to Enhance Performance and Patient Safety (TeamSTEPPS), an evidence-based, multimedia curriculum, tool set, and system for healthcare organizations to improve their teamwork.
Using the TeamSTEPPS approach, Dr. Baum collaborated with hospitalist Albertine Beard, MD, and the charge nurse on a 28-bed medical unit at the Minneapolis VA Medical Center to present a half-day training session for all VA staff, including four hospitalists. The seminar mixed didactics, discussions, and simulations, similar to traditional role-playing techniques but using a high-fidelity manikin that talks and displays vital signs.
"Teamwork is a set of knowledge, skills, and attitudes that lead to the creation of a culture where it’s about us as a team, not about who is highest in the hierarchy," Dr. Baum says. Hospitalists want to be leaders, "but we have a responsibility to be intentional leaders, learning the skills and modeling them," she adds.
Improved teamwork benefits patients through more effective communication and reduction in medical errors, Dr. Baum says, "but it also helps to create a healthy environment in which to work, where we all have each other’s backs."
TeamSTEPPS, developed jointly by the federal Agency for Healthcare Research and Quality (AHRQ) and the Department of Defense, has reached 25% to 30% of U.S. hospitals by annually training about 700 masters. The masters then go back to their institutions and share the techniques.
Read more about why improving teamwork is good for your patients.
University of Minnesota hospitalist Karyn Baum, MD, MSEd, directs one of six regional training centers for Team Strategies and Tools to Enhance Performance and Patient Safety (TeamSTEPPS), an evidence-based, multimedia curriculum, tool set, and system for healthcare organizations to improve their teamwork.
Using the TeamSTEPPS approach, Dr. Baum collaborated with hospitalist Albertine Beard, MD, and the charge nurse on a 28-bed medical unit at the Minneapolis VA Medical Center to present a half-day training session for all VA staff, including four hospitalists. The seminar mixed didactics, discussions, and simulations, similar to traditional role-playing techniques but using a high-fidelity manikin that talks and displays vital signs.
"Teamwork is a set of knowledge, skills, and attitudes that lead to the creation of a culture where it’s about us as a team, not about who is highest in the hierarchy," Dr. Baum says. Hospitalists want to be leaders, "but we have a responsibility to be intentional leaders, learning the skills and modeling them," she adds.
Improved teamwork benefits patients through more effective communication and reduction in medical errors, Dr. Baum says, "but it also helps to create a healthy environment in which to work, where we all have each other’s backs."
TeamSTEPPS, developed jointly by the federal Agency for Healthcare Research and Quality (AHRQ) and the Department of Defense, has reached 25% to 30% of U.S. hospitals by annually training about 700 masters. The masters then go back to their institutions and share the techniques.
Read more about why improving teamwork is good for your patients.
University of Minnesota hospitalist Karyn Baum, MD, MSEd, directs one of six regional training centers for Team Strategies and Tools to Enhance Performance and Patient Safety (TeamSTEPPS), an evidence-based, multimedia curriculum, tool set, and system for healthcare organizations to improve their teamwork.
Using the TeamSTEPPS approach, Dr. Baum collaborated with hospitalist Albertine Beard, MD, and the charge nurse on a 28-bed medical unit at the Minneapolis VA Medical Center to present a half-day training session for all VA staff, including four hospitalists. The seminar mixed didactics, discussions, and simulations, similar to traditional role-playing techniques but using a high-fidelity manikin that talks and displays vital signs.
"Teamwork is a set of knowledge, skills, and attitudes that lead to the creation of a culture where it’s about us as a team, not about who is highest in the hierarchy," Dr. Baum says. Hospitalists want to be leaders, "but we have a responsibility to be intentional leaders, learning the skills and modeling them," she adds.
Improved teamwork benefits patients through more effective communication and reduction in medical errors, Dr. Baum says, "but it also helps to create a healthy environment in which to work, where we all have each other’s backs."
TeamSTEPPS, developed jointly by the federal Agency for Healthcare Research and Quality (AHRQ) and the Department of Defense, has reached 25% to 30% of U.S. hospitals by annually training about 700 masters. The masters then go back to their institutions and share the techniques.
Read more about why improving teamwork is good for your patients.
FDA approves teduglutide for short bowel syndrome
The Food and Drug Administration on Dec. 21 approved teduglutide, to be marketed under the trade name Gattex, to treat adults with short bowel syndrome (SBS) who need parenteral nutrition.
Teduglutide, a recombinant analogue of human glucagon-like peptide-2 (GLP-2), was unanimously recommended for approval by an FDA advisory panel in October. It is a once-daily subcutaneous injection that helps improve intestinal absorption of fluids and nutrients, reducing the frequency and volume of parenteral nutrition.
"Considering Gattex has been shown to significantly reduce or in some cases even eliminate the requirement for parenteral support, it may become a cornerstone therapy in the management of short bowel syndrome." Dr. Ken Fujioka of the Nutrition and Metabolic Research Center, Scripps Clinic, Del Mar, Calif., said in a statement issued by Gattex’s maker, NPA Pharmaceuticals.
It is the third drug to be approved by the FDA for SBS patients who are dependent on parenteral nutrition. Somatropin (Zorbtive) was approved in 2003 and glutamine (Nutrestore) in 2004.
"Today’s approval expands the available treatment options for patients with this life-threatening condition," Dr. Victoria Kusiak, deputy director of the Office of Drug Evaluation III in the FDA Center for Drug Evaluation and Research, said in a statement. "Because Gattex may cause other serious health conditions, it is critical that patients and health care professionals understand the drug’s potential and known safety risks."
Teduglutide therapy increases the risk of developing cancer and polyps in the intestine, obstructions in the intestine, gallbladder disease, biliary tract disease, and pancreatic disease. The drug will have a Risk Evaluation and Mitigation Strategy, consisting of a communication plan and training for prescribers, according to the FDA.
Pivotal data on the drug were published in September in Gastroenterology (Gastroenterology 2012 Sept. 13 [doi:10.1053/j.gastro.2012.09.007]).
The Food and Drug Administration on Dec. 21 approved teduglutide, to be marketed under the trade name Gattex, to treat adults with short bowel syndrome (SBS) who need parenteral nutrition.
Teduglutide, a recombinant analogue of human glucagon-like peptide-2 (GLP-2), was unanimously recommended for approval by an FDA advisory panel in October. It is a once-daily subcutaneous injection that helps improve intestinal absorption of fluids and nutrients, reducing the frequency and volume of parenteral nutrition.
"Considering Gattex has been shown to significantly reduce or in some cases even eliminate the requirement for parenteral support, it may become a cornerstone therapy in the management of short bowel syndrome." Dr. Ken Fujioka of the Nutrition and Metabolic Research Center, Scripps Clinic, Del Mar, Calif., said in a statement issued by Gattex’s maker, NPA Pharmaceuticals.
It is the third drug to be approved by the FDA for SBS patients who are dependent on parenteral nutrition. Somatropin (Zorbtive) was approved in 2003 and glutamine (Nutrestore) in 2004.
"Today’s approval expands the available treatment options for patients with this life-threatening condition," Dr. Victoria Kusiak, deputy director of the Office of Drug Evaluation III in the FDA Center for Drug Evaluation and Research, said in a statement. "Because Gattex may cause other serious health conditions, it is critical that patients and health care professionals understand the drug’s potential and known safety risks."
Teduglutide therapy increases the risk of developing cancer and polyps in the intestine, obstructions in the intestine, gallbladder disease, biliary tract disease, and pancreatic disease. The drug will have a Risk Evaluation and Mitigation Strategy, consisting of a communication plan and training for prescribers, according to the FDA.
Pivotal data on the drug were published in September in Gastroenterology (Gastroenterology 2012 Sept. 13 [doi:10.1053/j.gastro.2012.09.007]).
The Food and Drug Administration on Dec. 21 approved teduglutide, to be marketed under the trade name Gattex, to treat adults with short bowel syndrome (SBS) who need parenteral nutrition.
Teduglutide, a recombinant analogue of human glucagon-like peptide-2 (GLP-2), was unanimously recommended for approval by an FDA advisory panel in October. It is a once-daily subcutaneous injection that helps improve intestinal absorption of fluids and nutrients, reducing the frequency and volume of parenteral nutrition.
"Considering Gattex has been shown to significantly reduce or in some cases even eliminate the requirement for parenteral support, it may become a cornerstone therapy in the management of short bowel syndrome." Dr. Ken Fujioka of the Nutrition and Metabolic Research Center, Scripps Clinic, Del Mar, Calif., said in a statement issued by Gattex’s maker, NPA Pharmaceuticals.
It is the third drug to be approved by the FDA for SBS patients who are dependent on parenteral nutrition. Somatropin (Zorbtive) was approved in 2003 and glutamine (Nutrestore) in 2004.
"Today’s approval expands the available treatment options for patients with this life-threatening condition," Dr. Victoria Kusiak, deputy director of the Office of Drug Evaluation III in the FDA Center for Drug Evaluation and Research, said in a statement. "Because Gattex may cause other serious health conditions, it is critical that patients and health care professionals understand the drug’s potential and known safety risks."
Teduglutide therapy increases the risk of developing cancer and polyps in the intestine, obstructions in the intestine, gallbladder disease, biliary tract disease, and pancreatic disease. The drug will have a Risk Evaluation and Mitigation Strategy, consisting of a communication plan and training for prescribers, according to the FDA.
Pivotal data on the drug were published in September in Gastroenterology (Gastroenterology 2012 Sept. 13 [doi:10.1053/j.gastro.2012.09.007]).
Flaxseed
Linum usitatissimum, an annual plant native to the eastern Mediterranean and India and better known as flax (or linseed, several decades ago), was cultivated in ancient Egypt and Ethiopia and used for many purposes, including as an ingredient in medicine, soap, and hair products. The oil from the seeds of the plant is thought to possess significant health benefits. Flaxseed oil is one of the richest sources of omega-3 fatty acids, in particular, alpha-linolenic acid (ALA), which represents more than 50% of its total fatty acid content (Br. J. Nutr. 2009;101:440-5; Medical Herbalism: The science and practice of herbal medicine. Healing Arts Press: Rochester, Vt., 2003, p. 57). In addition, flaxseeds are rich in dietary fiber and lignans, which are phytoestrogens with antioxidant properties.
Antioxidant, anti-inflammatory, and antiapoptotic properties have been associated with flaxseed oil and warrant medical consideration. The substantial anti-inflammatory activity of L. usitatissimum has been ascribed to its primary active constituent, ALA (57%), which suppresses arachidonic acid metabolism, thus inhibiting the synthesis of proinflammatory n-6 eicosanoids and reducing vascular permeability (Inflammopharmacology 2010;18:127-36).
In a randomized, double-blind, placebo-controlled application test in 2009, De Spirt et al. studied the cutaneous effects of supplementation with flaxseed or borage oil for 12 weeks in two groups of women (n = 45) aged 18-65 years with sensitive and dry skin. Fifteen women were included in each group, and 15 were randomized to a placebo control group. The placebo group received medium-chain fatty acids. The flaxseed oil included ALA and linoleic acid, and the borage oil contained linoleic and gamma-linolenic acids. ALA contributed to the significant rise in total fatty acids in plasma seen in the flaxseed oil group at weeks 6 and 12. An increase in gamma-linolenic acid was noted in the borage oil group. Erythema, roughness, and scaling were decreased in both treatment groups compared with baseline, while skin hydration was markedly elevated after 12 weeks. In addition, transepidermal water loss was diminished by 10% after 6 weeks in both oil treatment groups, with further reductions after 12 weeks in the flaxseed oil group. The investigators concluded that intervention with dietary lipids can manifest as skin improvements (Br. J. Nutr. 2009;101:440-5).
In 2010, Kaithwas and Majumdar evaluated the anti-inflammatory potential of flaxseed fixed oil against castor oil–induced diarrhea, turpentine oil–induced joint edema, and formaldehyde-induced and complete Freund’s adjuvant (CFA)-induced arthritis in Wistar albino rats. They found that flaxseed oil dose-dependently inhibited the adverse effects of castor oil and turpentine oil as well as CFA, and a significant inhibitory effect was also exerted by flaxseed oil against formaldehyde-induced proliferation of global edematous arthritis. Flaxseed oil also significantly diminished the secondary lesions engendered by CFA by dint of a delayed hypersensitivity reaction. The authors concluded that the significant anti-inflammatory activity imparted by L. usitatissimum fixed oil suggests its therapeutic viability for inflammatory conditions, such as rheumatoid arthritis (Inflammopharmacology 2010;18:127-36).
Recently, de Souza et al. studied the effects on skin wounds in rats of a semisolid formulation of flaxseed oil (1%, 5%, or 10%). The investigators assessed the contraction/re-epithelialization of the wound and resistance to mechanical traction in incisional and excisional models, respectively. They found that the groups treated with flaxseed oil concentrations of 1% or 5% largely started re-epithelialization earlier than the petroleum jelly control group, and achieved 100% re-epithelialization on the 14th day after injury, as compared to 33% of animals in the petroleum jelly group. The investigators concluded that flaxseed oil, at low concentrations, exhibits potential in a solid pharmaceutical preparation, for use in dermal repair (Evid. Based. Complement. Alternat. Med. 2012;2012:270752).
Early in 2012, Tülüce et al. set out to ascertain the antioxidant and antiapoptotic effects of flaxseed oil exerted against ultraviolet C–induced damage in rats. They divided animals into three groups: control, UVC alone, and UVC and flaxseed oil. UVC light exposure lasted for 1 hour twice daily for four weeks in the two exposure groups. In the flaxseed oil group, the oil was administered by gavage prior to each irradiation (4 mL/kg ). The investigators noted that malondialdehyde and protein carbonyl levels were higher in the UVC group than in the controls, but such levels were reduced in the flaxseed oil group compared with the UVC-only group, in skin, lens, and sera. Also, the activities of glutathione peroxidase and superoxide dismutase were found to be higher in the skin, lens, and sera of the flaxseed oil group as compared to the UVC-only group. In addition, retinal apoptosis was lower in the flaxseed group than in the UVC group. The researchers concluded that flaxseed oil may be useful in conferring a photoprotective effect against UVC-induced damage, as manifested in protein carbonylation and reactive oxygen species generation, in rats (Toxicol. Ind. Health. 2012;28:99-107).
Conclusion
Flaxseed oil has gained recent attention for its salutary effects as part of the diet. Rich in omega-3 essential fatty acids and lignans, flaxseed oil has been found to improve fatty acid profiles. Significantly, emerging evidence points to beneficial cutaneous effects derived from dietary use of flaxseed oil. However, more research is necessary to determine whether the beneficial constituents of flaxseed oil can be harnessed in topical products.
Dr. Baumann is in private practice in Miami Beach.
Linum usitatissimum, an annual plant native to the eastern Mediterranean and India and better known as flax (or linseed, several decades ago), was cultivated in ancient Egypt and Ethiopia and used for many purposes, including as an ingredient in medicine, soap, and hair products. The oil from the seeds of the plant is thought to possess significant health benefits. Flaxseed oil is one of the richest sources of omega-3 fatty acids, in particular, alpha-linolenic acid (ALA), which represents more than 50% of its total fatty acid content (Br. J. Nutr. 2009;101:440-5; Medical Herbalism: The science and practice of herbal medicine. Healing Arts Press: Rochester, Vt., 2003, p. 57). In addition, flaxseeds are rich in dietary fiber and lignans, which are phytoestrogens with antioxidant properties.
Antioxidant, anti-inflammatory, and antiapoptotic properties have been associated with flaxseed oil and warrant medical consideration. The substantial anti-inflammatory activity of L. usitatissimum has been ascribed to its primary active constituent, ALA (57%), which suppresses arachidonic acid metabolism, thus inhibiting the synthesis of proinflammatory n-6 eicosanoids and reducing vascular permeability (Inflammopharmacology 2010;18:127-36).
In a randomized, double-blind, placebo-controlled application test in 2009, De Spirt et al. studied the cutaneous effects of supplementation with flaxseed or borage oil for 12 weeks in two groups of women (n = 45) aged 18-65 years with sensitive and dry skin. Fifteen women were included in each group, and 15 were randomized to a placebo control group. The placebo group received medium-chain fatty acids. The flaxseed oil included ALA and linoleic acid, and the borage oil contained linoleic and gamma-linolenic acids. ALA contributed to the significant rise in total fatty acids in plasma seen in the flaxseed oil group at weeks 6 and 12. An increase in gamma-linolenic acid was noted in the borage oil group. Erythema, roughness, and scaling were decreased in both treatment groups compared with baseline, while skin hydration was markedly elevated after 12 weeks. In addition, transepidermal water loss was diminished by 10% after 6 weeks in both oil treatment groups, with further reductions after 12 weeks in the flaxseed oil group. The investigators concluded that intervention with dietary lipids can manifest as skin improvements (Br. J. Nutr. 2009;101:440-5).
In 2010, Kaithwas and Majumdar evaluated the anti-inflammatory potential of flaxseed fixed oil against castor oil–induced diarrhea, turpentine oil–induced joint edema, and formaldehyde-induced and complete Freund’s adjuvant (CFA)-induced arthritis in Wistar albino rats. They found that flaxseed oil dose-dependently inhibited the adverse effects of castor oil and turpentine oil as well as CFA, and a significant inhibitory effect was also exerted by flaxseed oil against formaldehyde-induced proliferation of global edematous arthritis. Flaxseed oil also significantly diminished the secondary lesions engendered by CFA by dint of a delayed hypersensitivity reaction. The authors concluded that the significant anti-inflammatory activity imparted by L. usitatissimum fixed oil suggests its therapeutic viability for inflammatory conditions, such as rheumatoid arthritis (Inflammopharmacology 2010;18:127-36).
Recently, de Souza et al. studied the effects on skin wounds in rats of a semisolid formulation of flaxseed oil (1%, 5%, or 10%). The investigators assessed the contraction/re-epithelialization of the wound and resistance to mechanical traction in incisional and excisional models, respectively. They found that the groups treated with flaxseed oil concentrations of 1% or 5% largely started re-epithelialization earlier than the petroleum jelly control group, and achieved 100% re-epithelialization on the 14th day after injury, as compared to 33% of animals in the petroleum jelly group. The investigators concluded that flaxseed oil, at low concentrations, exhibits potential in a solid pharmaceutical preparation, for use in dermal repair (Evid. Based. Complement. Alternat. Med. 2012;2012:270752).
Early in 2012, Tülüce et al. set out to ascertain the antioxidant and antiapoptotic effects of flaxseed oil exerted against ultraviolet C–induced damage in rats. They divided animals into three groups: control, UVC alone, and UVC and flaxseed oil. UVC light exposure lasted for 1 hour twice daily for four weeks in the two exposure groups. In the flaxseed oil group, the oil was administered by gavage prior to each irradiation (4 mL/kg ). The investigators noted that malondialdehyde and protein carbonyl levels were higher in the UVC group than in the controls, but such levels were reduced in the flaxseed oil group compared with the UVC-only group, in skin, lens, and sera. Also, the activities of glutathione peroxidase and superoxide dismutase were found to be higher in the skin, lens, and sera of the flaxseed oil group as compared to the UVC-only group. In addition, retinal apoptosis was lower in the flaxseed group than in the UVC group. The researchers concluded that flaxseed oil may be useful in conferring a photoprotective effect against UVC-induced damage, as manifested in protein carbonylation and reactive oxygen species generation, in rats (Toxicol. Ind. Health. 2012;28:99-107).
Conclusion
Flaxseed oil has gained recent attention for its salutary effects as part of the diet. Rich in omega-3 essential fatty acids and lignans, flaxseed oil has been found to improve fatty acid profiles. Significantly, emerging evidence points to beneficial cutaneous effects derived from dietary use of flaxseed oil. However, more research is necessary to determine whether the beneficial constituents of flaxseed oil can be harnessed in topical products.
Dr. Baumann is in private practice in Miami Beach.
Linum usitatissimum, an annual plant native to the eastern Mediterranean and India and better known as flax (or linseed, several decades ago), was cultivated in ancient Egypt and Ethiopia and used for many purposes, including as an ingredient in medicine, soap, and hair products. The oil from the seeds of the plant is thought to possess significant health benefits. Flaxseed oil is one of the richest sources of omega-3 fatty acids, in particular, alpha-linolenic acid (ALA), which represents more than 50% of its total fatty acid content (Br. J. Nutr. 2009;101:440-5; Medical Herbalism: The science and practice of herbal medicine. Healing Arts Press: Rochester, Vt., 2003, p. 57). In addition, flaxseeds are rich in dietary fiber and lignans, which are phytoestrogens with antioxidant properties.
Antioxidant, anti-inflammatory, and antiapoptotic properties have been associated with flaxseed oil and warrant medical consideration. The substantial anti-inflammatory activity of L. usitatissimum has been ascribed to its primary active constituent, ALA (57%), which suppresses arachidonic acid metabolism, thus inhibiting the synthesis of proinflammatory n-6 eicosanoids and reducing vascular permeability (Inflammopharmacology 2010;18:127-36).
In a randomized, double-blind, placebo-controlled application test in 2009, De Spirt et al. studied the cutaneous effects of supplementation with flaxseed or borage oil for 12 weeks in two groups of women (n = 45) aged 18-65 years with sensitive and dry skin. Fifteen women were included in each group, and 15 were randomized to a placebo control group. The placebo group received medium-chain fatty acids. The flaxseed oil included ALA and linoleic acid, and the borage oil contained linoleic and gamma-linolenic acids. ALA contributed to the significant rise in total fatty acids in plasma seen in the flaxseed oil group at weeks 6 and 12. An increase in gamma-linolenic acid was noted in the borage oil group. Erythema, roughness, and scaling were decreased in both treatment groups compared with baseline, while skin hydration was markedly elevated after 12 weeks. In addition, transepidermal water loss was diminished by 10% after 6 weeks in both oil treatment groups, with further reductions after 12 weeks in the flaxseed oil group. The investigators concluded that intervention with dietary lipids can manifest as skin improvements (Br. J. Nutr. 2009;101:440-5).
In 2010, Kaithwas and Majumdar evaluated the anti-inflammatory potential of flaxseed fixed oil against castor oil–induced diarrhea, turpentine oil–induced joint edema, and formaldehyde-induced and complete Freund’s adjuvant (CFA)-induced arthritis in Wistar albino rats. They found that flaxseed oil dose-dependently inhibited the adverse effects of castor oil and turpentine oil as well as CFA, and a significant inhibitory effect was also exerted by flaxseed oil against formaldehyde-induced proliferation of global edematous arthritis. Flaxseed oil also significantly diminished the secondary lesions engendered by CFA by dint of a delayed hypersensitivity reaction. The authors concluded that the significant anti-inflammatory activity imparted by L. usitatissimum fixed oil suggests its therapeutic viability for inflammatory conditions, such as rheumatoid arthritis (Inflammopharmacology 2010;18:127-36).
Recently, de Souza et al. studied the effects on skin wounds in rats of a semisolid formulation of flaxseed oil (1%, 5%, or 10%). The investigators assessed the contraction/re-epithelialization of the wound and resistance to mechanical traction in incisional and excisional models, respectively. They found that the groups treated with flaxseed oil concentrations of 1% or 5% largely started re-epithelialization earlier than the petroleum jelly control group, and achieved 100% re-epithelialization on the 14th day after injury, as compared to 33% of animals in the petroleum jelly group. The investigators concluded that flaxseed oil, at low concentrations, exhibits potential in a solid pharmaceutical preparation, for use in dermal repair (Evid. Based. Complement. Alternat. Med. 2012;2012:270752).
Early in 2012, Tülüce et al. set out to ascertain the antioxidant and antiapoptotic effects of flaxseed oil exerted against ultraviolet C–induced damage in rats. They divided animals into three groups: control, UVC alone, and UVC and flaxseed oil. UVC light exposure lasted for 1 hour twice daily for four weeks in the two exposure groups. In the flaxseed oil group, the oil was administered by gavage prior to each irradiation (4 mL/kg ). The investigators noted that malondialdehyde and protein carbonyl levels were higher in the UVC group than in the controls, but such levels were reduced in the flaxseed oil group compared with the UVC-only group, in skin, lens, and sera. Also, the activities of glutathione peroxidase and superoxide dismutase were found to be higher in the skin, lens, and sera of the flaxseed oil group as compared to the UVC-only group. In addition, retinal apoptosis was lower in the flaxseed group than in the UVC group. The researchers concluded that flaxseed oil may be useful in conferring a photoprotective effect against UVC-induced damage, as manifested in protein carbonylation and reactive oxygen species generation, in rats (Toxicol. Ind. Health. 2012;28:99-107).
Conclusion
Flaxseed oil has gained recent attention for its salutary effects as part of the diet. Rich in omega-3 essential fatty acids and lignans, flaxseed oil has been found to improve fatty acid profiles. Significantly, emerging evidence points to beneficial cutaneous effects derived from dietary use of flaxseed oil. However, more research is necessary to determine whether the beneficial constituents of flaxseed oil can be harnessed in topical products.
Dr. Baumann is in private practice in Miami Beach.
You: The next YouTube star
Each month, more than 4 billion hours of video are watched on YouTube. It’s not all for Justin Bieber (though much of it is). People across the globe are flocking to YouTube for medical information and advice. Why not take advantage of this interested audience and free service?
Videos can be made simply, using tools you already have, or they can be done professionally in a studio. Although there are some advantages to professionally produced videos, the beauty of YouTube and the user-generated content movement is that these frills are unnecessary. The most important factor is not the quality of the video, but rather, the quality of the content. Videos that capture your true personality and that deliver useful content to viewers will be successful, regardless of how they are produced.
Videos are powerful on many levels: They’re a platform to educate your patients and prospective patients and market your practice. They showcase you both as a person and a physician. And video content is 50 times more likely to appear on the first page of search engine results than text-only content.
Though you could go to your local camera or electronics store and spend a small fortune on video equipment, I suggest you start off with what you have on hand, such as your smart phone or webcam. Choose a well-lighted, quiet area in your office or at home, such as in front of a bookcase. Outline a script, read through it a few times so that it sounds natural, then videotape yourself and see how it looks.
You won’t be perfect on the first take, but that’s OK. The beauty of short 1- to 2-minute videos is that they’re easy to reshoot.
For your first video, I suggest doing an introduction. Your goal is to appear approachable, friendly, and trustworthy. Introduce yourself and share some personal information, such as where you grew up, where you went to school, your favorite sports teams, your hobbies – anything that provides an opportunity for viewers to connect with you on a personal level. Look straight at the camera, smile often, and speak clearly. Keep it under 90 seconds.
Then do another 90-second video welcoming patients to your practice. Mention your expertise, clinical interests, and anything else that makes your practice stand out.
You’ll find that generating content for videos isn’t difficult. Make videos of procedures that you’re expert in, post-op instructions that you repeat frequently, or cosmetic procedures that patients often inquire about.
Create a channel on a video-sharing site such as YouTube or Vimeo, and upload your videos one at a time. You can then embed those videos on your practice website or blog (see last month’s column on blogging).
Here are some of my best practices for making videos:
• Before you start, ask yourself, "Why would someone want to watch this video?"
• Make a single point in each video and stay focused.
• Choose a well-lighted, quiet area for recording. Place the light source in front of you. Back lighting can create shadows.
• Consider composition. You don’t have to be in the center of the frame. You can be off to one side, especially if you’re including something behind you in the shot, or if you are using props. But always look into the camera.
• Use props when relevant.
• Keep videos under 2 minutes.
• Have a script or an outline, but never read from it.
• Tell stories. Patients will remember them better than statistics.
• Rehearse, rehearse, rehearse.
• Be conversational and smile.
• Watch each take so you can make appropriate changes.
• Don’t waste time trying to make a video "go viral."
• Share your videos on Twitter, Facebook, or other social sharing sites.
When you’re done, have someone from your office view the video critically. Are you looking into the camera or over the heads of the viewers? Are you smiling enough? Do you have too many vocal fillers like "um" and "pretty much?" Are you easily seen and heard? It is interesting and worthy of an audience?
Finally, share your video with me @dermdoc on Twitter. You can count on a retweet.
Dr. Benabio is in private practice in San Diego. Visit his consumer health blog; connect with him on Twitter @Dermdoc, and on Facebook.
Each month, more than 4 billion hours of video are watched on YouTube. It’s not all for Justin Bieber (though much of it is). People across the globe are flocking to YouTube for medical information and advice. Why not take advantage of this interested audience and free service?
Videos can be made simply, using tools you already have, or they can be done professionally in a studio. Although there are some advantages to professionally produced videos, the beauty of YouTube and the user-generated content movement is that these frills are unnecessary. The most important factor is not the quality of the video, but rather, the quality of the content. Videos that capture your true personality and that deliver useful content to viewers will be successful, regardless of how they are produced.
Videos are powerful on many levels: They’re a platform to educate your patients and prospective patients and market your practice. They showcase you both as a person and a physician. And video content is 50 times more likely to appear on the first page of search engine results than text-only content.
Though you could go to your local camera or electronics store and spend a small fortune on video equipment, I suggest you start off with what you have on hand, such as your smart phone or webcam. Choose a well-lighted, quiet area in your office or at home, such as in front of a bookcase. Outline a script, read through it a few times so that it sounds natural, then videotape yourself and see how it looks.
You won’t be perfect on the first take, but that’s OK. The beauty of short 1- to 2-minute videos is that they’re easy to reshoot.
For your first video, I suggest doing an introduction. Your goal is to appear approachable, friendly, and trustworthy. Introduce yourself and share some personal information, such as where you grew up, where you went to school, your favorite sports teams, your hobbies – anything that provides an opportunity for viewers to connect with you on a personal level. Look straight at the camera, smile often, and speak clearly. Keep it under 90 seconds.
Then do another 90-second video welcoming patients to your practice. Mention your expertise, clinical interests, and anything else that makes your practice stand out.
You’ll find that generating content for videos isn’t difficult. Make videos of procedures that you’re expert in, post-op instructions that you repeat frequently, or cosmetic procedures that patients often inquire about.
Create a channel on a video-sharing site such as YouTube or Vimeo, and upload your videos one at a time. You can then embed those videos on your practice website or blog (see last month’s column on blogging).
Here are some of my best practices for making videos:
• Before you start, ask yourself, "Why would someone want to watch this video?"
• Make a single point in each video and stay focused.
• Choose a well-lighted, quiet area for recording. Place the light source in front of you. Back lighting can create shadows.
• Consider composition. You don’t have to be in the center of the frame. You can be off to one side, especially if you’re including something behind you in the shot, or if you are using props. But always look into the camera.
• Use props when relevant.
• Keep videos under 2 minutes.
• Have a script or an outline, but never read from it.
• Tell stories. Patients will remember them better than statistics.
• Rehearse, rehearse, rehearse.
• Be conversational and smile.
• Watch each take so you can make appropriate changes.
• Don’t waste time trying to make a video "go viral."
• Share your videos on Twitter, Facebook, or other social sharing sites.
When you’re done, have someone from your office view the video critically. Are you looking into the camera or over the heads of the viewers? Are you smiling enough? Do you have too many vocal fillers like "um" and "pretty much?" Are you easily seen and heard? It is interesting and worthy of an audience?
Finally, share your video with me @dermdoc on Twitter. You can count on a retweet.
Dr. Benabio is in private practice in San Diego. Visit his consumer health blog; connect with him on Twitter @Dermdoc, and on Facebook.
Each month, more than 4 billion hours of video are watched on YouTube. It’s not all for Justin Bieber (though much of it is). People across the globe are flocking to YouTube for medical information and advice. Why not take advantage of this interested audience and free service?
Videos can be made simply, using tools you already have, or they can be done professionally in a studio. Although there are some advantages to professionally produced videos, the beauty of YouTube and the user-generated content movement is that these frills are unnecessary. The most important factor is not the quality of the video, but rather, the quality of the content. Videos that capture your true personality and that deliver useful content to viewers will be successful, regardless of how they are produced.
Videos are powerful on many levels: They’re a platform to educate your patients and prospective patients and market your practice. They showcase you both as a person and a physician. And video content is 50 times more likely to appear on the first page of search engine results than text-only content.
Though you could go to your local camera or electronics store and spend a small fortune on video equipment, I suggest you start off with what you have on hand, such as your smart phone or webcam. Choose a well-lighted, quiet area in your office or at home, such as in front of a bookcase. Outline a script, read through it a few times so that it sounds natural, then videotape yourself and see how it looks.
You won’t be perfect on the first take, but that’s OK. The beauty of short 1- to 2-minute videos is that they’re easy to reshoot.
For your first video, I suggest doing an introduction. Your goal is to appear approachable, friendly, and trustworthy. Introduce yourself and share some personal information, such as where you grew up, where you went to school, your favorite sports teams, your hobbies – anything that provides an opportunity for viewers to connect with you on a personal level. Look straight at the camera, smile often, and speak clearly. Keep it under 90 seconds.
Then do another 90-second video welcoming patients to your practice. Mention your expertise, clinical interests, and anything else that makes your practice stand out.
You’ll find that generating content for videos isn’t difficult. Make videos of procedures that you’re expert in, post-op instructions that you repeat frequently, or cosmetic procedures that patients often inquire about.
Create a channel on a video-sharing site such as YouTube or Vimeo, and upload your videos one at a time. You can then embed those videos on your practice website or blog (see last month’s column on blogging).
Here are some of my best practices for making videos:
• Before you start, ask yourself, "Why would someone want to watch this video?"
• Make a single point in each video and stay focused.
• Choose a well-lighted, quiet area for recording. Place the light source in front of you. Back lighting can create shadows.
• Consider composition. You don’t have to be in the center of the frame. You can be off to one side, especially if you’re including something behind you in the shot, or if you are using props. But always look into the camera.
• Use props when relevant.
• Keep videos under 2 minutes.
• Have a script or an outline, but never read from it.
• Tell stories. Patients will remember them better than statistics.
• Rehearse, rehearse, rehearse.
• Be conversational and smile.
• Watch each take so you can make appropriate changes.
• Don’t waste time trying to make a video "go viral."
• Share your videos on Twitter, Facebook, or other social sharing sites.
When you’re done, have someone from your office view the video critically. Are you looking into the camera or over the heads of the viewers? Are you smiling enough? Do you have too many vocal fillers like "um" and "pretty much?" Are you easily seen and heard? It is interesting and worthy of an audience?
Finally, share your video with me @dermdoc on Twitter. You can count on a retweet.
Dr. Benabio is in private practice in San Diego. Visit his consumer health blog; connect with him on Twitter @Dermdoc, and on Facebook.