User login
Smooth Moves
A recent study in the Journal of Hospital Medicine concluded that by rescheduling fewer than 10 elective admissions per week from a weekday to a weekend, hospitals can reduce overcrowding. The report should encourage hospitalists to reconsider their own scheduling strategies, the lead author says.
"If they notice that on certain days their unit or their hospital is very crowded and on other days it's less so, it may be worth working with their organization's quality and safety or operational leadership to learn more about those patterns and see if they can improve on them," says Evan S. Fieldston, MD, MBA, MSHP, pediatric hospitalist at the Children's Hospital of Philadelphia.
The study examined 2007 daily inpatient census data from 39 tertiary-care children's hospitals. The average weekday occupancy ranged from 70.9% to 108.1%, while the average weekend occupancy ranged from 65.7% to 94.9%. After rescheduling, or "smoothing," elective admissions from days with "thresholds of high occupancy," defined as >85% occupancy, to less busy days, 39,607 patients were removed from exposure to occupancy levels greater than 95%.
Eugene Litvak, MD, president and CEO of the nonprofit Institute for Healthcare Optimization and adjunct professor of operations management at the Harvard School of Public Health in Boston, says the issue goes beyond U.S. hospitals. Dr. Litvak says he's discussed overcrowding with more than 100 hospitals in Europe, Japan, Australia, and the U.S. "In talking with their leadership in healthcare, I saw the same problem," he says.
The solution, Dr. Litvak suggests, lies with queueing theory, a mathematical formula that addresses random demand for a fixed capacity. Based on average census data, hospitals can apply queueing theory to determine how many beds and staff they need for ED admissions throughout a typical week.
A recent study in the Journal of Hospital Medicine concluded that by rescheduling fewer than 10 elective admissions per week from a weekday to a weekend, hospitals can reduce overcrowding. The report should encourage hospitalists to reconsider their own scheduling strategies, the lead author says.
"If they notice that on certain days their unit or their hospital is very crowded and on other days it's less so, it may be worth working with their organization's quality and safety or operational leadership to learn more about those patterns and see if they can improve on them," says Evan S. Fieldston, MD, MBA, MSHP, pediatric hospitalist at the Children's Hospital of Philadelphia.
The study examined 2007 daily inpatient census data from 39 tertiary-care children's hospitals. The average weekday occupancy ranged from 70.9% to 108.1%, while the average weekend occupancy ranged from 65.7% to 94.9%. After rescheduling, or "smoothing," elective admissions from days with "thresholds of high occupancy," defined as >85% occupancy, to less busy days, 39,607 patients were removed from exposure to occupancy levels greater than 95%.
Eugene Litvak, MD, president and CEO of the nonprofit Institute for Healthcare Optimization and adjunct professor of operations management at the Harvard School of Public Health in Boston, says the issue goes beyond U.S. hospitals. Dr. Litvak says he's discussed overcrowding with more than 100 hospitals in Europe, Japan, Australia, and the U.S. "In talking with their leadership in healthcare, I saw the same problem," he says.
The solution, Dr. Litvak suggests, lies with queueing theory, a mathematical formula that addresses random demand for a fixed capacity. Based on average census data, hospitals can apply queueing theory to determine how many beds and staff they need for ED admissions throughout a typical week.
A recent study in the Journal of Hospital Medicine concluded that by rescheduling fewer than 10 elective admissions per week from a weekday to a weekend, hospitals can reduce overcrowding. The report should encourage hospitalists to reconsider their own scheduling strategies, the lead author says.
"If they notice that on certain days their unit or their hospital is very crowded and on other days it's less so, it may be worth working with their organization's quality and safety or operational leadership to learn more about those patterns and see if they can improve on them," says Evan S. Fieldston, MD, MBA, MSHP, pediatric hospitalist at the Children's Hospital of Philadelphia.
The study examined 2007 daily inpatient census data from 39 tertiary-care children's hospitals. The average weekday occupancy ranged from 70.9% to 108.1%, while the average weekend occupancy ranged from 65.7% to 94.9%. After rescheduling, or "smoothing," elective admissions from days with "thresholds of high occupancy," defined as >85% occupancy, to less busy days, 39,607 patients were removed from exposure to occupancy levels greater than 95%.
Eugene Litvak, MD, president and CEO of the nonprofit Institute for Healthcare Optimization and adjunct professor of operations management at the Harvard School of Public Health in Boston, says the issue goes beyond U.S. hospitals. Dr. Litvak says he's discussed overcrowding with more than 100 hospitals in Europe, Japan, Australia, and the U.S. "In talking with their leadership in healthcare, I saw the same problem," he says.
The solution, Dr. Litvak suggests, lies with queueing theory, a mathematical formula that addresses random demand for a fixed capacity. Based on average census data, hospitals can apply queueing theory to determine how many beds and staff they need for ED admissions throughout a typical week.
CD19-redirected T cells induce remission in CLL patients
Gene therapy with a lentiviral vector expressing a chimeric antigen receptor with specificity for CD19 (CART19) has induced complete remission in 3 patients with chronic lymphocytic leukemia (CLL), according to research published simultaneously in the August 10 issues of The New England Journal of Medicine and Science Translational Medicine.
The research team, from the University of Pennsylvania, reported that the reinfused, modified T cells expanded to more than 1000 times the initial engraftment level. The patients’ remission was ongoing at 10 months after treatment.
The investigators believe the big difference between this genetically modified T cell and previous ones that had disappointing clinical activity is the addition of the CD137 (4-1BB) costimulatory signaling domain that significantly increases antitumor activity.
The team, led by Carl June, MD, described in the NEJM article the T-cell treatment of one of the patients with advanced, p53-deficient CLL.
A half year prior to enrolling in the trial, the 64-year-old patient’s T cells were collected and frozen. Before reinfusing the T cells into the patient, the investigators thawed the cells and transduced them with lentivirus expressing CD19-specific chimeric antigen receptor.
Four days prior to reinfusion, the patient received chemotherapy with pentostatin and cyclophosphamide to deplete his lymphocytes. After 3 days of chemotherapy, his bone marrow was hypercellular with approximately 40% involvement by CLL.
After 4 days of chemotherapy, the patient received an infusion of T cells, of which 5% were transduced, totaling 1.42 x 107 transduced cells, split into 3 consecutive daily infusions.
Two weeks after the infusion, the patient experienced chills, fever, and fatigue, which intensified over the subsequent days. He was diagnosed with tumor lysis syndrome on day 22 after infusion. On day 23 after the CART19-cell infusion, the patient had no evidence of CLL in the bone marrow, and by day 28, his adenopathy was not palpable.
In addition to tumor lysis syndrome, the only other grade 3/4 toxicity observed was lymphopenia.
The investigators did not expect that such a low dose of chimeric antigen receptor T cells would result in a clinically evident antitumor response. The dose was several orders of magnitude lower than that used in previous studies of modified T cells.
They speculated that the course of chemotherapy administered to the patient prior to the CART19-cell infusion may have been responsible for the increased engraftment and for “potentiating the ability of chimeric antigen receptor T cells to kill stressed tumor cells that would otherwise survive the chemotherapy.”
The researchers conclude that continued study of CD19-redirected T cells is warranted and plan to test the approach in other CD19-positive tumors, including non-Hodgkin lymphoma and acute lymphocytic leukemia. ![]()
Gene therapy with a lentiviral vector expressing a chimeric antigen receptor with specificity for CD19 (CART19) has induced complete remission in 3 patients with chronic lymphocytic leukemia (CLL), according to research published simultaneously in the August 10 issues of The New England Journal of Medicine and Science Translational Medicine.
The research team, from the University of Pennsylvania, reported that the reinfused, modified T cells expanded to more than 1000 times the initial engraftment level. The patients’ remission was ongoing at 10 months after treatment.
The investigators believe the big difference between this genetically modified T cell and previous ones that had disappointing clinical activity is the addition of the CD137 (4-1BB) costimulatory signaling domain that significantly increases antitumor activity.
The team, led by Carl June, MD, described in the NEJM article the T-cell treatment of one of the patients with advanced, p53-deficient CLL.
A half year prior to enrolling in the trial, the 64-year-old patient’s T cells were collected and frozen. Before reinfusing the T cells into the patient, the investigators thawed the cells and transduced them with lentivirus expressing CD19-specific chimeric antigen receptor.
Four days prior to reinfusion, the patient received chemotherapy with pentostatin and cyclophosphamide to deplete his lymphocytes. After 3 days of chemotherapy, his bone marrow was hypercellular with approximately 40% involvement by CLL.
After 4 days of chemotherapy, the patient received an infusion of T cells, of which 5% were transduced, totaling 1.42 x 107 transduced cells, split into 3 consecutive daily infusions.
Two weeks after the infusion, the patient experienced chills, fever, and fatigue, which intensified over the subsequent days. He was diagnosed with tumor lysis syndrome on day 22 after infusion. On day 23 after the CART19-cell infusion, the patient had no evidence of CLL in the bone marrow, and by day 28, his adenopathy was not palpable.
In addition to tumor lysis syndrome, the only other grade 3/4 toxicity observed was lymphopenia.
The investigators did not expect that such a low dose of chimeric antigen receptor T cells would result in a clinically evident antitumor response. The dose was several orders of magnitude lower than that used in previous studies of modified T cells.
They speculated that the course of chemotherapy administered to the patient prior to the CART19-cell infusion may have been responsible for the increased engraftment and for “potentiating the ability of chimeric antigen receptor T cells to kill stressed tumor cells that would otherwise survive the chemotherapy.”
The researchers conclude that continued study of CD19-redirected T cells is warranted and plan to test the approach in other CD19-positive tumors, including non-Hodgkin lymphoma and acute lymphocytic leukemia. ![]()
Gene therapy with a lentiviral vector expressing a chimeric antigen receptor with specificity for CD19 (CART19) has induced complete remission in 3 patients with chronic lymphocytic leukemia (CLL), according to research published simultaneously in the August 10 issues of The New England Journal of Medicine and Science Translational Medicine.
The research team, from the University of Pennsylvania, reported that the reinfused, modified T cells expanded to more than 1000 times the initial engraftment level. The patients’ remission was ongoing at 10 months after treatment.
The investigators believe the big difference between this genetically modified T cell and previous ones that had disappointing clinical activity is the addition of the CD137 (4-1BB) costimulatory signaling domain that significantly increases antitumor activity.
The team, led by Carl June, MD, described in the NEJM article the T-cell treatment of one of the patients with advanced, p53-deficient CLL.
A half year prior to enrolling in the trial, the 64-year-old patient’s T cells were collected and frozen. Before reinfusing the T cells into the patient, the investigators thawed the cells and transduced them with lentivirus expressing CD19-specific chimeric antigen receptor.
Four days prior to reinfusion, the patient received chemotherapy with pentostatin and cyclophosphamide to deplete his lymphocytes. After 3 days of chemotherapy, his bone marrow was hypercellular with approximately 40% involvement by CLL.
After 4 days of chemotherapy, the patient received an infusion of T cells, of which 5% were transduced, totaling 1.42 x 107 transduced cells, split into 3 consecutive daily infusions.
Two weeks after the infusion, the patient experienced chills, fever, and fatigue, which intensified over the subsequent days. He was diagnosed with tumor lysis syndrome on day 22 after infusion. On day 23 after the CART19-cell infusion, the patient had no evidence of CLL in the bone marrow, and by day 28, his adenopathy was not palpable.
In addition to tumor lysis syndrome, the only other grade 3/4 toxicity observed was lymphopenia.
The investigators did not expect that such a low dose of chimeric antigen receptor T cells would result in a clinically evident antitumor response. The dose was several orders of magnitude lower than that used in previous studies of modified T cells.
They speculated that the course of chemotherapy administered to the patient prior to the CART19-cell infusion may have been responsible for the increased engraftment and for “potentiating the ability of chimeric antigen receptor T cells to kill stressed tumor cells that would otherwise survive the chemotherapy.”
The researchers conclude that continued study of CD19-redirected T cells is warranted and plan to test the approach in other CD19-positive tumors, including non-Hodgkin lymphoma and acute lymphocytic leukemia. ![]()
Neuro-HM Gains Numbers, Momentum
“Enter The Neurohospitalist” might sound like a medical spoof of a Bruce Lee movie, but it’s really a subspecialty’s announcement that it’s here to stay.
The clever moniker was the name of a plenary session at the 8th New York Symposium on Neurological Emergencies & Neurological Care, sponsored by Columbia University’s Center for Continuing Medical Education. The two-hour presentation on neurology’s take on HM was a new feature for the annual meeting, and to presenter David Likosky, MD, SFHM, hospitalist and stroke program director at Evergreen Hospital Medical Center in Kirkland, Wash., it was the latest sign that the field of HM is cementing its future.
“The neurohospitalist world right now is where the hospital medicine world was, say, ten, fifteen years ago,” says Dr. Likosky, who is board-certified in both neurology and internal medicine.
Multiple fields have adopted the HM model, to the point that SHM is holding its first national specialty hospitalist meeting, Focused Practice in Hospital Medicine, on Nov. 4 in Las Vegas. The meeting is designed to help promote networking of people interested in the hospitalist model in various specialties, as well as to help identify issues related to those specialties. Click here for more information and registration.
But even within the growth of speciality hospitalist models, neurology might be the cohort embracing it the fastest. Dr. Likosky estimates there are 500 neurohospitalists practicing nationwide. The Neurohospitalist Society held its first meeting earlier this year, and the field’s first textbook, which he is contributing to, is set for release in November. The Academy of Neurology has a dedicated neurohospitalist section. And the subspecialty even has its own quarterly journal, The Neurohospitalist.

—David Likosky, MD, SFHM, hospitalist, stroke program director, Evergreen Hospital Medical Center, Kirkland, Wash.
“There is now a critical mass of neurohospitalists,” Dr. Likosky says. “There’s also an increasing recognition by the neurointensivists that someone has to help them take care of these patients, either before they get to the unit or when they come out of the unit. … Most hospitals don’t have neurointensivists, but they have very ill neurology patients. That’s another niche for neurohospitalists. All specialties of intensivists are looking for help with these patients.”
Another panelist at the four-day Manhattan conference, William D. Freeman, MD, assistant professor of neurology at the Mayo Clinic in Jacksonville, Fla., says the continued success of the field will be judged on data. He says three areas of potential “low-hanging fruit” to focus on are:
- Increased use of intravenous tissue plasminogen activators (tPA). The FDA-approved “clot-busting therapy” has been shown to reverse the effects of ischemic stroke if given within a time-sensitive window of therapeutic opportunity.
- Reduced length of stay for stroke patients. Adherence to best practices, Dr. Freeman says, will most effectively reduce patient stays and will be the ones that also demonstrate quality and patient-safety attributes.
- Focus on stroke patient metrics. Administrators often focus on quality measures that are easily identifiable; Dr. Likosky says new programs have to be able to show they can meet those thresholds.
“Hospital administrators are new to the concept of a neurohospitalist,” Dr. Likosky adds. “It’s easier in that they get the hospitalist model because that’s been around for so long, but figuring out the expense of a neurohospitalist program, how that functionally works, are there enough volumes, are all questions that are being asked.”
Still, Drs. Freeman and Likosky agree that the advantages of the subspecialty—everything from physicians’ quality of life to newly satisfied specialists in other departments (who will have a quicker neuro consult available)—mean the nascent specialty can continue to grow in numbers and influence.
“The future is bright for neurohospitalists,” Dr. Freeman says.
Richard Quinn is a freelance writer based in New Jersey.
“Enter The Neurohospitalist” might sound like a medical spoof of a Bruce Lee movie, but it’s really a subspecialty’s announcement that it’s here to stay.
The clever moniker was the name of a plenary session at the 8th New York Symposium on Neurological Emergencies & Neurological Care, sponsored by Columbia University’s Center for Continuing Medical Education. The two-hour presentation on neurology’s take on HM was a new feature for the annual meeting, and to presenter David Likosky, MD, SFHM, hospitalist and stroke program director at Evergreen Hospital Medical Center in Kirkland, Wash., it was the latest sign that the field of HM is cementing its future.
“The neurohospitalist world right now is where the hospital medicine world was, say, ten, fifteen years ago,” says Dr. Likosky, who is board-certified in both neurology and internal medicine.
Multiple fields have adopted the HM model, to the point that SHM is holding its first national specialty hospitalist meeting, Focused Practice in Hospital Medicine, on Nov. 4 in Las Vegas. The meeting is designed to help promote networking of people interested in the hospitalist model in various specialties, as well as to help identify issues related to those specialties. Click here for more information and registration.
But even within the growth of speciality hospitalist models, neurology might be the cohort embracing it the fastest. Dr. Likosky estimates there are 500 neurohospitalists practicing nationwide. The Neurohospitalist Society held its first meeting earlier this year, and the field’s first textbook, which he is contributing to, is set for release in November. The Academy of Neurology has a dedicated neurohospitalist section. And the subspecialty even has its own quarterly journal, The Neurohospitalist.

—David Likosky, MD, SFHM, hospitalist, stroke program director, Evergreen Hospital Medical Center, Kirkland, Wash.
“There is now a critical mass of neurohospitalists,” Dr. Likosky says. “There’s also an increasing recognition by the neurointensivists that someone has to help them take care of these patients, either before they get to the unit or when they come out of the unit. … Most hospitals don’t have neurointensivists, but they have very ill neurology patients. That’s another niche for neurohospitalists. All specialties of intensivists are looking for help with these patients.”
Another panelist at the four-day Manhattan conference, William D. Freeman, MD, assistant professor of neurology at the Mayo Clinic in Jacksonville, Fla., says the continued success of the field will be judged on data. He says three areas of potential “low-hanging fruit” to focus on are:
- Increased use of intravenous tissue plasminogen activators (tPA). The FDA-approved “clot-busting therapy” has been shown to reverse the effects of ischemic stroke if given within a time-sensitive window of therapeutic opportunity.
- Reduced length of stay for stroke patients. Adherence to best practices, Dr. Freeman says, will most effectively reduce patient stays and will be the ones that also demonstrate quality and patient-safety attributes.
- Focus on stroke patient metrics. Administrators often focus on quality measures that are easily identifiable; Dr. Likosky says new programs have to be able to show they can meet those thresholds.
“Hospital administrators are new to the concept of a neurohospitalist,” Dr. Likosky adds. “It’s easier in that they get the hospitalist model because that’s been around for so long, but figuring out the expense of a neurohospitalist program, how that functionally works, are there enough volumes, are all questions that are being asked.”
Still, Drs. Freeman and Likosky agree that the advantages of the subspecialty—everything from physicians’ quality of life to newly satisfied specialists in other departments (who will have a quicker neuro consult available)—mean the nascent specialty can continue to grow in numbers and influence.
“The future is bright for neurohospitalists,” Dr. Freeman says.
Richard Quinn is a freelance writer based in New Jersey.
“Enter The Neurohospitalist” might sound like a medical spoof of a Bruce Lee movie, but it’s really a subspecialty’s announcement that it’s here to stay.
The clever moniker was the name of a plenary session at the 8th New York Symposium on Neurological Emergencies & Neurological Care, sponsored by Columbia University’s Center for Continuing Medical Education. The two-hour presentation on neurology’s take on HM was a new feature for the annual meeting, and to presenter David Likosky, MD, SFHM, hospitalist and stroke program director at Evergreen Hospital Medical Center in Kirkland, Wash., it was the latest sign that the field of HM is cementing its future.
“The neurohospitalist world right now is where the hospital medicine world was, say, ten, fifteen years ago,” says Dr. Likosky, who is board-certified in both neurology and internal medicine.
Multiple fields have adopted the HM model, to the point that SHM is holding its first national specialty hospitalist meeting, Focused Practice in Hospital Medicine, on Nov. 4 in Las Vegas. The meeting is designed to help promote networking of people interested in the hospitalist model in various specialties, as well as to help identify issues related to those specialties. Click here for more information and registration.
But even within the growth of speciality hospitalist models, neurology might be the cohort embracing it the fastest. Dr. Likosky estimates there are 500 neurohospitalists practicing nationwide. The Neurohospitalist Society held its first meeting earlier this year, and the field’s first textbook, which he is contributing to, is set for release in November. The Academy of Neurology has a dedicated neurohospitalist section. And the subspecialty even has its own quarterly journal, The Neurohospitalist.

—David Likosky, MD, SFHM, hospitalist, stroke program director, Evergreen Hospital Medical Center, Kirkland, Wash.
“There is now a critical mass of neurohospitalists,” Dr. Likosky says. “There’s also an increasing recognition by the neurointensivists that someone has to help them take care of these patients, either before they get to the unit or when they come out of the unit. … Most hospitals don’t have neurointensivists, but they have very ill neurology patients. That’s another niche for neurohospitalists. All specialties of intensivists are looking for help with these patients.”
Another panelist at the four-day Manhattan conference, William D. Freeman, MD, assistant professor of neurology at the Mayo Clinic in Jacksonville, Fla., says the continued success of the field will be judged on data. He says three areas of potential “low-hanging fruit” to focus on are:
- Increased use of intravenous tissue plasminogen activators (tPA). The FDA-approved “clot-busting therapy” has been shown to reverse the effects of ischemic stroke if given within a time-sensitive window of therapeutic opportunity.
- Reduced length of stay for stroke patients. Adherence to best practices, Dr. Freeman says, will most effectively reduce patient stays and will be the ones that also demonstrate quality and patient-safety attributes.
- Focus on stroke patient metrics. Administrators often focus on quality measures that are easily identifiable; Dr. Likosky says new programs have to be able to show they can meet those thresholds.
“Hospital administrators are new to the concept of a neurohospitalist,” Dr. Likosky adds. “It’s easier in that they get the hospitalist model because that’s been around for so long, but figuring out the expense of a neurohospitalist program, how that functionally works, are there enough volumes, are all questions that are being asked.”
Still, Drs. Freeman and Likosky agree that the advantages of the subspecialty—everything from physicians’ quality of life to newly satisfied specialists in other departments (who will have a quicker neuro consult available)—mean the nascent specialty can continue to grow in numbers and influence.
“The future is bright for neurohospitalists,” Dr. Freeman says.
Richard Quinn is a freelance writer based in New Jersey.
Roth Spots—More than Meets the Eye
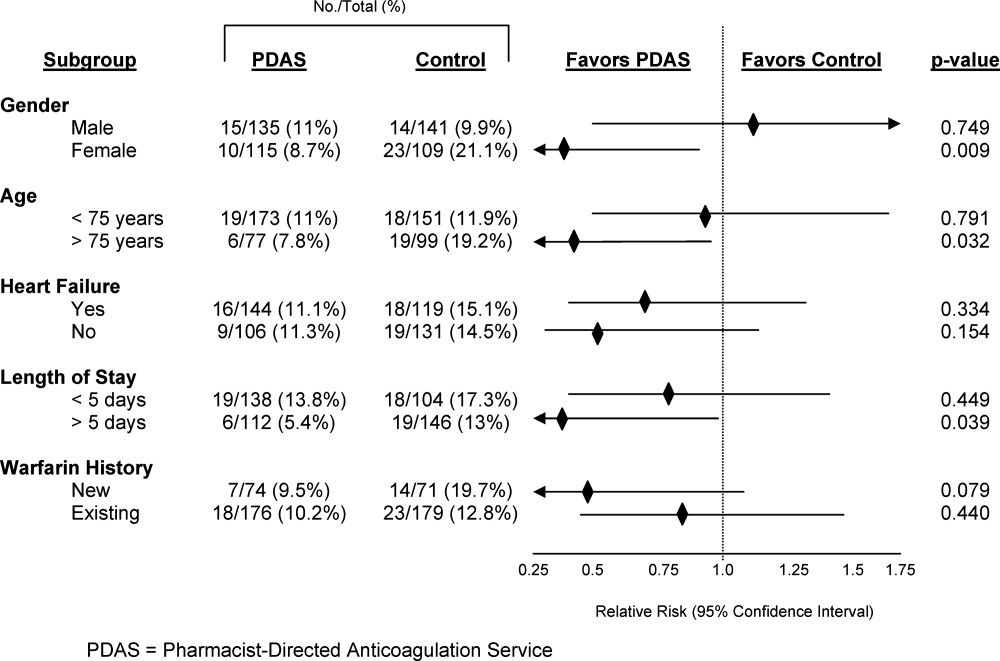
A 50‐year‐old female patient with a past medical history of Sjogren's syndrome and polymyositis presented with fever, rash, swelling, and pain in her extremities. Skin biopsy confirmed vasculitis. She was treated with steroids and azathioprine. However, she developed sudden‐onset central visual blurring in her right eye on the fifth day of hospitalization. Fundoscopic exam showed multiple central white‐centered retinal hemorrhages (Roth spots, Figures 1, 2) and vascular sheathing, consistent with retinal vasculitis. Blood cultures were negative. Transthoracic and transesophageal echocardiograms were normal. She was treated with high‐dose intravenous steroids and cyclophosphamide, with visual improvement and a marked reduction in the number of Roth spots.
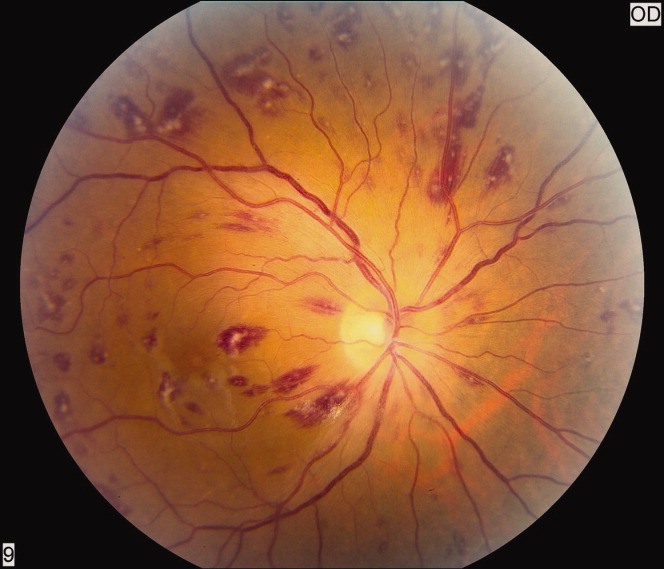
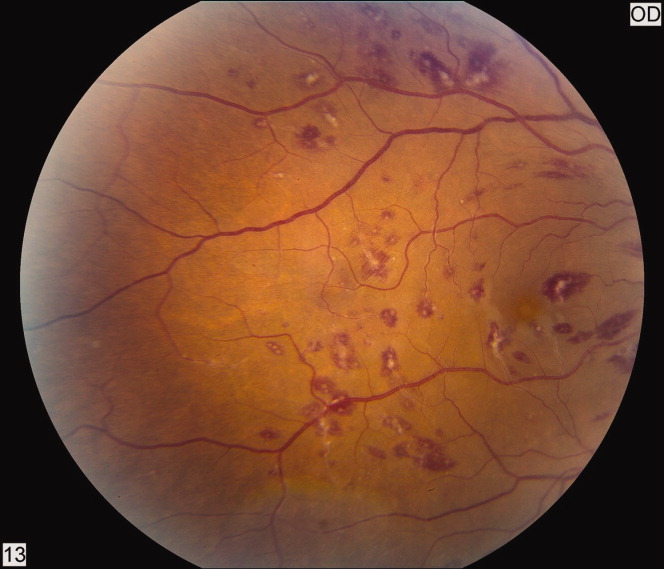
Roth spots 1 are nonspecific intraretinal hemorrhagic lesions with a white center due to fibrin deposition. Although historically associated with infective endocarditis, they can also occur in other systemic diseases such as connective tissue disorders, vasculitis, leukemia, diabetes, hypertension, anemia, trauma, as well as disseminated bacterial and fungal infections.
- , , .White centered hemorrhages: their significance.Ophthalmology.1980;87:66–69.
A 50‐year‐old female patient with a past medical history of Sjogren's syndrome and polymyositis presented with fever, rash, swelling, and pain in her extremities. Skin biopsy confirmed vasculitis. She was treated with steroids and azathioprine. However, she developed sudden‐onset central visual blurring in her right eye on the fifth day of hospitalization. Fundoscopic exam showed multiple central white‐centered retinal hemorrhages (Roth spots, Figures 1, 2) and vascular sheathing, consistent with retinal vasculitis. Blood cultures were negative. Transthoracic and transesophageal echocardiograms were normal. She was treated with high‐dose intravenous steroids and cyclophosphamide, with visual improvement and a marked reduction in the number of Roth spots.


Roth spots 1 are nonspecific intraretinal hemorrhagic lesions with a white center due to fibrin deposition. Although historically associated with infective endocarditis, they can also occur in other systemic diseases such as connective tissue disorders, vasculitis, leukemia, diabetes, hypertension, anemia, trauma, as well as disseminated bacterial and fungal infections.
A 50‐year‐old female patient with a past medical history of Sjogren's syndrome and polymyositis presented with fever, rash, swelling, and pain in her extremities. Skin biopsy confirmed vasculitis. She was treated with steroids and azathioprine. However, she developed sudden‐onset central visual blurring in her right eye on the fifth day of hospitalization. Fundoscopic exam showed multiple central white‐centered retinal hemorrhages (Roth spots, Figures 1, 2) and vascular sheathing, consistent with retinal vasculitis. Blood cultures were negative. Transthoracic and transesophageal echocardiograms were normal. She was treated with high‐dose intravenous steroids and cyclophosphamide, with visual improvement and a marked reduction in the number of Roth spots.


Roth spots 1 are nonspecific intraretinal hemorrhagic lesions with a white center due to fibrin deposition. Although historically associated with infective endocarditis, they can also occur in other systemic diseases such as connective tissue disorders, vasculitis, leukemia, diabetes, hypertension, anemia, trauma, as well as disseminated bacterial and fungal infections.
- , , .White centered hemorrhages: their significance.Ophthalmology.1980;87:66–69.
- , , .White centered hemorrhages: their significance.Ophthalmology.1980;87:66–69.
Pharmacist‐Directed Anticoagulation
Anticoagulants are one of the most common drug classes involved in medication errors and adverse events. Warfarin, an anticoagulant that plays a key role in the management of many disease states, is implicated in approximately 30% of reported anticoagulant‐related errors.1 Anticoagulation with warfarin is complicated by inter‐individual variability in response to therapy, clinically significant drug interactions, a narrow therapeutic window, and the need for frequent and lifelong monitoring.2
In the hospital setting, warfarin use is complicated due to patient handoff among health care providers, and acute illnesses that impact sensitivity and response to warfarin. Common causes of errors with anticoagulants are knowledge deficits, failure to follow policy/procedure/protocol, and communication issues.1 An added opportunity for warfarin‐related medication errors is the risk associated with the transition from the inpatient‐to‐outpatient setting. Due to the risk and complexity associated with anticoagulant medications, the Joint Commission instituted National Patient Safety Goal (NPSG) 03.05.01 (formerly NPSG 3E): a series of requirements intended to Reduce the likelihood of patient harm with the use of anticoagulation therapy.3 In order to optimally address this National Patient Safety Goal, a systematic intervention would be required to impact each step of the medication use process for anticoagulants.
Several studies have suggested that dedicated anticoagulation management services or clinics improve anticoagulation management in the outpatient setting.2 Non‐physician providers, primarily pharmacists and nurses, frequently manage outpatient anticoagulation management services or clinics. However, very few studies have evaluated the impact of a warfarin management service in the inpatient hospital setting.48 While the few available studies suggest some benefit associated with an inpatient anticoagulation management service, a minority of these studies have assessed the role of these services in facilitating the transition of the anticoagulated patient to the outpatient setting.7
In order to improve anticoagulation management and safety, our institution implemented an inpatient Pharmacist‐Directed Anticoagulation Service (PDAS). The purpose of this study was to evaluate the impact of this service on both transition of care and safety of patients receiving warfarin anticoagulation.
METHODS
This study was completed at Henry Ford Hospital, an 802‐bed, tertiary care, level 1 trauma and academic medical center in Detroit, MI. The study was carried out between November 2007 and June 2009. The study was approved by the Henry Ford Hospital Institutional Review Board with waiver of consent.
Patients
This was a prospective cluster randomized study. All patients admitted to two internal medicine units (IM1 or IM2) or two cardiology units (Card1 or Card2), who received at least one inpatient dose of warfarin, were eligible for inclusion. Patients were included regardless of whether warfarin was newly initiated during the index admission (newly initiated patients) or was continuation of existing anticoagulation (existing warfarin patients). In order to ensure that patient data following discharge would be available for analysis, patients were excluded from this analysis if they were not scheduled to follow‐up in the Henry Ford Medical Group outpatient anticoagulation clinics after discharge, however, these patients were cared for by the PDAS service in the usual manner.
Study Design
Prior to implementation of the PDAS, one internal medicine and one cardiology unit was randomly selected to receive the PDAS intervention (IM1 and Card1), while the other two units (IM2 and Card2) served as control units. These hospital units were selected because anticoagulants are frequently used on these units and the patient population is generally similar between the two internal medicine and two cardiology unitswith exception that Card1 unit also contains a specialized service for advanced heart failure and left ventricular assist device (LVAD) patients. Of note, there was significant expansion of the heart failure service and LVAD program during the time frame of the study, accounting for a greater number of more complicated patients on the Card1 (PDAS) unit.
Specific responsibilities of the PDAS related to warfarin are detailed in Table 1. The PDAS was implemented in September 2007 as a system‐based change to improve anticoagulant safety at our institution. The goals of this service were to improve communication regarding anticoagulation; to improve safety as patients transition from the inpatient‐to‐outpatient settings; and to standardize anticoagulant dosing, monitoring, and patient education. For patients taking warfarin, who are cared for by a health system‐affiliated physician, the PDAS collaborates with our outpatient anticoagulation clinics in order to facilitate transition from the inpatient‐to‐outpatient setting. The Henry Ford Health System has an established, multisite outpatient Anticoagulation Clinic with >5000 patients actively receiving warfarin dosing and monitoring. The anticoagulation clinics are staffed by nurses and pharmacists who provide standardized management of warfarin for patients of all physicians within our health system and provide consistent high‐quality care (average time in international normalized ratio [INR] goal range = 68.2%). The anticoagulation clinics have been in existence since 1992. The PDAS is comprised of three full‐time and two part‐time pharmacists whose responsibilities are limited to the management of anticoagulation throughout the hospital.
| Inpatient Care | Patient Education | Transition of Care |
|---|---|---|
| ||
| Initial dose selection and daily dose adjustments after warfarin is initiated by primary team | Comprehensive education provided verbally and via written communication utilizing the Krames database. | Contact anticoagulation‐responsible physician and anticoagulation clinic via phone. |
| Provide written dosing regimen to patient and provide date for first INR postdischarge. | ||
| Daily laboratory monitoring | Education provided is standardized between inpatient and outpatient settings. | Create electronic Anticoagulation Discharge Summary. Document communication with the outpatient clinicians, reason for admission, steps taken to manage warfarin drug interactions, and warfarin doses administered during stay, discharge warfarin dose and follow‐up date. |
The PDAS was staffed by repurposing pharmacist staff. All pharmacists had either several years of general medicine‐based clinical practice experience or residency training, or both. Pharmacists were oriented to service responsibilities by spending approximately one week in the outpatient anticoagulation clinic and completing focused review of internal and external anticoagulation guidelines.
In the control group, management of anticoagulation and transition of care occurred at the discretion of the primary care team. The primary team had access to a clinical pharmacist, who was not part of the PDAS, seven days per week. However, the primary team was not able to consult the PDAS.
This study was primarily designed to assess the impact of the PDAS on both transition of care and patient safety. For study endpoint purposes, transition of care was assessed by satisfactory completion and documentation of four important metrics: 1) appropriate enrollment in the anticoagulation clinic; 2) documented communication between the inpatient service responsible for anticoagulation and the outpatient anticoagulation clinic prior to patient discharge; 3) documented communication between the inpatient service responsible for anticoagulation and the physician responsible for outpatient management of the patient; 4) INR drawn within five days of hospital discharge. Documentation of communication for metric #2 and #3 was obtained by reviewing the electronic medical record system, particularly electronic discharge summaries and telephone encounter notes.
The primary safety endpoint was defined as a composite of any INR >5, any episode of major bleeding, or development of new thrombosis. This endpoint was met if any of these events occurred either during the index hospitalization or within 30 days of hospital discharge. Major bleeding was identified by review of outpatient anticoagulation clinic encounters and the patient's electronic medical record (includes all inpatient and outpatient encounters within Henry Ford Health System) by using the International Society of Thrombosis and Haemostasis standard and was defined as fatal bleeding or symptomatic bleeding in a critical area or organ (intracranial, intraspinal, intraocular, retroperitoneal, intraarticular, pericardial, or intramuscular with compartment syndrome), or bleeding causing a reduction in hemoglobin levels of 2 g/dL or more, or leading to transfusion of two or more units of blood or red cells.9 New thrombosis was defined as documentation of any of the following: deep vein thrombosis, pulmonary embolism, or cardioembolic stroke. Need for dose adjustment at the first anticoagulation clinic visit after discharge was evaluated as a secondary endpoint.
All analyses compared the PDAS to the control group. In addition, a planned comparison of patients in the PDAS and control groups who were newly initiated to warfarin during the study hospitalization (newly initiated subgroup) and those who were taking warfarin on admission (existing warfarin subgroup) was also undertaken. It was expected that these subgroup analyses would likely be underpowered, however, the potential implications of a service such as this could differ based on history of warfarin use. Therefore, these analyses were planned for exploratory purposes. In order to determine the impact of risk factors for altered warfarin pharmacodynamic response on the safety endpoint, post hoc subgroup analyses were performed based on demographics and clinical characteristics.
Data Analysis
Data are presented as mean standard deviation or proportion, as appropriate. A P‐value of less than 0.05 was considered significant for all comparisons and all tests were two‐tailed.
Intervention and control groups were compared with Student's t test, MannWhitney U test, chi‐square or Fishers exact test, as appropriate. Relative risk (RR) and 95% confidence intervals (CI) were calculated for all primary analyses. All statistical analyses were performed with SPSS v.12.0 (SPSS Inc, Chicago, IL).
It was estimated that a sample size of 250 patients per group would provide greater than 80% power to detect at least a 50% improvement in both the transition of care and primary safety endpoints, with implementation of the PDAS. This calculation is based on the following assumptions: alpha = 0.05; expected control group achievement of the four transition of care metrics = 50%; rate of safety endpoint for the control group = 20%.4
RESULTS
Baseline Characteristics
During the study period, 1360 patients were admitted to the study units. A total of 377 and 483 patients were found to be ineligible for inclusion on the PDAS and control units, respectively. These patients were ineligible because they did not follow up in the Henry Ford Medical Group outpatient anticoagulation clinic. In total, 500 patients were included in the analysis. Patients (n = 145) who were newly initiated on warfarin made up 29% of the total population. Table 2 presents baseline clinical characteristics for patients in the PDAS and control groups, showing increased age, and a greater proportion of patients with heart failure and LVADs in the PDAS group. Patients in the PDAS group had significantly longer hospital stays, however, these increases were driven by a longer length of stay among the advanced heart failure service patients that were managed by the PDAS.
| PDAS (n = 250) | Control (n = 250) | P Value | |
|---|---|---|---|
| |||
| Demographic data | |||
| Age (mean SD) | 64.1 15.6 | 68.0 14.9 | 0.004 |
| Male gender | 54.0% | 56.4% | 0.589 |
| Caucasian race | 44.4% | 50.4% | 0.179 |
| Admitted to a cardiology unit | 78.8% | 74.8% | 0.289 |
| Length of stay (mean SD) | 8.13 7.04 | 6.29 5.63 | 0.001 |
| No heart failure history: length of stay (mean SD) | 6.83 4.53 | 6.15 5.14 | 0.288 |
| Heart failure history: length of stay (mean SD) | 9.09 8.31 | 6.45 6.15 | 0.004 |
| History of heart failure* | 57.6% | 47.6% | 0.025 |
| Heart failure with an LVAD | 14.0% | 0.4% | <0.001 |
| Indication for anticoagulation | |||
| Venous thromboembolism | 21.6% | 18.4% | 0.371 |
| Atrial fibrillation | 54.4% | 66.4% | 0.006 |
| Other | 24.0% | 15.2% | 0.013 |
| Primary admission diagnosis* | |||
| Heart failure | 25.6%* | 21.6% | 0.292 |
| Atrial fibrillation | 16.4% | 20.8% | 0.206 |
| Acute coronary syndrome | 13.6% | 17.6% | 0.218 |
| Venous thromboembolism | 4.8% | 4.8% | 1.00 |
| Infection | 12.4% | 10.0% | 0.395 |
| Bleeding | 1.6% | 1.2% | 0.703 |
Early Warfarin Management
Warfarin management metrics are presented in Table 3. The number of inpatient days prescribed warfarin was increased in the PDAS group by greater than one day while PDAS patients required significantly less dosage adjustment at first outpatient follow‐up visit. Similar to increases noted with length of stay, increases in inpatient warfarin days were likely driven by patients with severe heart failure managed by the PDAS.
| Warfarin Dosing | PDAS (n = 250) | Control (n = 250) | P Value |
|---|---|---|---|
| |||
| Initial dose (mean SD) | 5.23 2.37 | 4.99 2.07 | 0.245 |
| Discharge dose (mean SD) | 5.15 2.52 | 4.91 2.14 | 0.258 |
| INR at discharge (mean SD) | 2.07 0.73 | 2.04 0.73 | 0.660 |
| Therapeutic INR at discharge | 40.8% | 38.0% | 0.522 |
| Inpatient warfarin days (mean SD) | 4.97 4.30 | 3.68 2.69 | <0.001 |
| No heart failure: inpatient warfarin days (mean SD) | 4.09 2.49 | 3.60 2.67 | 0.148 |
| Heart failure: inpatient warfarin days (mean SD) | 5.62 5.16 | 3.76 2.71 | <0.001 |
| Dose change required at first follow‐up visit | 44.8% | 72.6% | <0.001 |
Transition of Care
Transition of care results are presented in Table 4. Full compliance and achievement of the transition of care metrics occurred significantly more often in the PDAS versus control patients with markedly increased rates of documented communication between inpatient providers and both outpatient anticoagulation clinic staff and outpatient physicians. Early follow‐up INR monitoring also occurred more frequently in the PDAS patients. The PDAS patients experienced greater compliance with the transition of care metrics regardless of whether they were in the newly initiated or existing warfarin subgroups (data not shown).
| Transition of Care | PDAS (n = 250) | Control (n = 250) | Relative Risk (95% CI) | P Value |
|---|---|---|---|---|
| ||||
| 100% Communication bundle* compliance, % (n) | 75.6% (189) | 2.8% (7) | 27.0 (13.056.2) | <0.001 |
| Appropriately enrolled in the AC clinic, % (n) | 97.2% (243) | 95.2% (238) | 1.02 (0.991.06) | 0.242 |
| Communication: inpatient service and outpatient physician, % (n) | 99.6% (249) | 12.4% (31) | 8.03 (5.7811.2) | <0.001 |
| Communication: inpatient clinicians and AC clinic staff, % (n) | 98.8% (247) | 14.8% (37) | 6.68 (4.969.00) | <0.001 |
| INR drawn within five days of hospital discharge, % (n) | 78.4% (196) | 66.4% (166) | 1.18 (1.061.32) | 0.003 |
| 30‐Day Composite safety endpoint, % (n) | 10.0% (25) | 14.8% (37) | 0.68 (0.421.09) | 0.103 |
| Inpatient + 30‐day INR >5, % (n) | 9.6% (24) | 14.8% (37) | 0.65 (0.401.05) | 0.076 |
| Inpatient + 30‐day major bleeding, % (n) | 0.8% (2) | 0.4% (1) | 2.00 (0.1821.9) | 0.563 |
| Inpatient + 30‐day thrombosis, % (n) | 0% (0) | 0% (0) | N/A | N/A |
Anticoagulant Safety
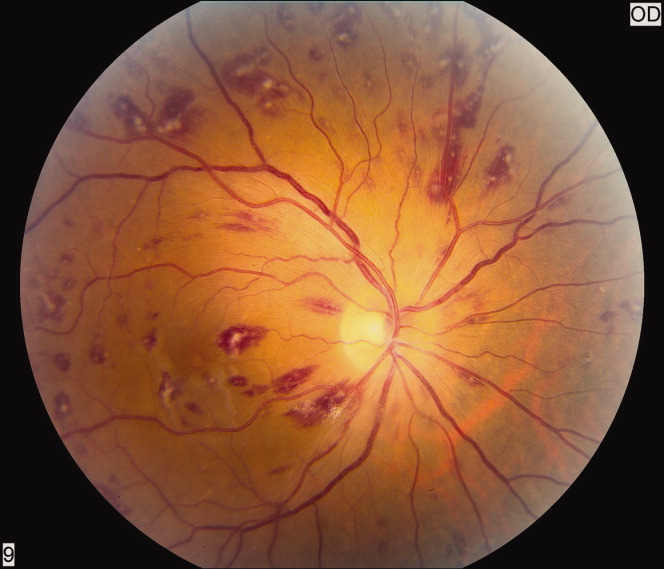
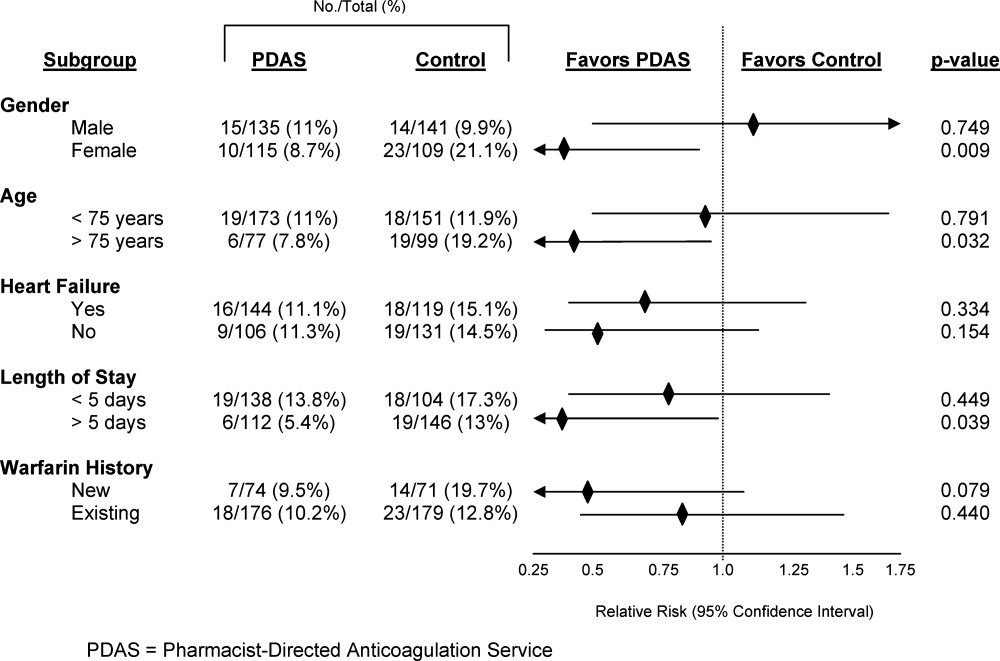
Safety endpoint data is presented in Table 4. The composite safety outcome of INR >5, major bleeding event, or thrombosis occurred in 12.4% of all patients with no early thrombotic events and only three major bleeding events recorded. Excessive INR values >5 occurred less frequently in the PDAS patients, however, differences in this metric and the composite safety outcome were not significantly different. Safety endpoint results in the overall population were driven by a reduction in INR values >5 among newly initiated warfarin patients in the PDAS group (PDAS: 9.5% vs control: 19.7%; P = 0.079; Figure 1). Other subgroup analyses relating to the safety endpoint are presented in Figure 1.
DISCUSSION
This article describes a systematic intervention designed to improve anticoagulation safety and efficacy in the hospital and during the transition to the postdischarge setting. Implementation of a PDAS did not impact patient bleeding and thrombotic outcomes, but did result in improved coordination and documentation of warfarin management and subsequent enhancement in the transition of the anticoagulated patient from the inpatient‐to‐outpatient setting with the Pharmacist‐Directed Anticoagulation Service.
Limited previous work has investigated the role of an anticoagulation service in inpatient management of anticoagulation.48 Only one published study has investigated the impact of this type of service on transition of care issues with warfarin, as was done in our study.7 In that study, management by an inpatient anticoagulation service resulted in a greater proportion of patients referred to an anticoagulation clinic for management (P = 0.001), more patients presenting to the anticoagulation clinic with a therapeutic INR (P = 0.001), and fewer patients presenting to the clinic with supratherapeutic levels of anticoagulation (P = 0.002). These results are somewhat analogous to our findings, in that patients in our study were less likely to require a dose change at the first clinic follow‐up visit after discharge or to have INR values 5.
We completed several subgroup analyses to thoroughly explore the impact of the PDAS on the safety endpoint. While firm conclusions cannot be drawn from these subgroup analyses, some hypothesis‐generating observations can be made. First, there was a greater impact of the PDAS on the safety endpoint in patients who are usually more sensitive to the effects of warfarin and therefore more challenging to manage.2 The impact of the PDAS was also greater among patients whose length of stay was more than five days (population median). This is significant because it suggests that when the opportunity for adverse events and miscommunication is greatest (ie, during hospitalizations of longer duration), there appears to be improvement in the safety endpoint with the PDAS.
To our knowledge, this study was the first to explore the impact of an inpatient anticoagulation service on the care of both newly initiated and existing warfarin patients, rather than only patients newly initiated on warfarin. As expected, the greatest influence of the PDAS on the safety endpoint was observed among the newly initiated patients. While the safety impact of the PDAS was noted most significantly among the newly initiated patients, the PDAS had a positive effect on the transition of care metrics regardless of previous warfarin use.
A limitation of our study should be mentioned. While we employed a design in which randomly selected units were exposed to the PDAS, individual patients were not randomized to the service. This cluster randomized design was chosen because it mimics quality improvement processes that would be rolled out to a hospital nursing unit. While lack of randomization at the patient level is a limitation, our cluster randomized study design is pragmatic and represents an improvement over the existing published before and after quasi‐experimental studies in this area.48
An improved system for documentation of communication was built into the PDAS processes on implementation of the service. However, it should also be noted that inadequate communication between inpatient and outpatient providers was an identified root cause for adverse events with warfarin in our institution prior to implementation of the PDAS. Therefore, improvement in the transition of care metrics with the PDAS were likely due to a combination of both improved documentation and true improvement in communication.
The approach of the PDAS was refined in several ways early after implementation of the service. Some major notable improvements included the development of a systematic approach to ensuring appropriate follow‐up and transition of care for patients being discharged to a skilled nursing facility, and creation of a system that required a mandatory conversation between the PDAS and a surgical service if anticoagulation is ordered within 48 hours of a major procedure. An upcoming improvement to the service will be to transition from a home grown electronic database, which was built for the purposes of streamlining clinical workflow and data collection, to a commercially available software program that has recently become available for management of inpatient anticoagulation. The major advantage of the new program will be the clinical decision support capabilities that will help to further streamline the service and allow for greater efficiency.
To understand implications of our PDAS intervention, it is important to remember that the majority of study patients were prescribed warfarin prior to hospital admission, that patients in both study groups were enrolled in an established, multisite anticoagulation clinic, and that patients in both groups were managed with a comprehensive inpatientoutpatient electronic medical record. Therefore, all providers caring for these patients had real‐time access to all warfarin dosing and dose adjustments, INR results, and anticoagulation clinic encounters, even though formal communication between providers was infrequently documented for control group patients in our electronic medical record. Study patients had low rates of bleeding and no adverse thrombotic outcomes across both treatment groups. Therefore, this type of model is likely to produce larger gains in communication and safety outcomes in health care systems without established anticoagulation clinics or comprehensive electronic medical records.
The PDAS was enthusiastically accepted by providers at our institution and expanded hospital‐wide after completion of this pilot. The PDAS model is a viable approach to standardize anticoagulant management with a goal of improving anticoagulant safety in the inpatient setting. Assessment of the effectiveness of models such as the PDAS for improving anticoagulant safety in the inpatient setting is particularly relevant with the current expectations for hospitals set by The Joint Commission's NPSG.03.05.01.3 More importantly the PDAS model can be an option for improving the transition of the anticoagulated patient from the inpatient‐to‐outpatient setting. Follow‐up with the anticoagulation clinic occurred earlier with the PDAS and, while this study was not designed to evaluate the impact of this new service on rehospitalization, recent literature suggests that earlier follow‐up after discharge leads to less rehospitalization.10 Finally, it may be possible to adapt this model to provide more intensive medication therapy management and monitoring for hospitalized patients with other complicated medication regimens or chronic disease.
CONCLUSION
The clinical pharmacist is uniquely prepared to manage inter‐individual variability in pharmacodynamic response to drug therapy, as well as to provide high‐quality patient education. This study evaluated a new model of inpatient warfarin management, in which warfarin dosing, monitoring, patient education, and transition of care was coordinated by a specialized team of clinical pharmacists that worked in collaboration with physicians and outpatient anticoagulation clinic staff. Safety and efficiency of the care provided by this new service was improved in certain subsets of more complex patients. The major advantage of this service was improvement in patient handoff, improved communication, and earlier patient follow‐up after discharge. Therefore, implementation of a Pharmacist‐Directed Anticoagulation Service provides a net improvement in quality of care for the patient taking warfarin in the inpatient setting.
Acknowledgements
The authors acknowledge the efforts of the PDAS staff: Nassif Abi‐Samra, Pam Holland, Sara Lanfear, and Gail Washington. This work would not have been possible without the dedication of these pharmacists. All authors had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
- U.S. Pharmacopeia. USP Patient Safety CapsLink: January 2008. Available at: http://www.usp.org/pdf/EN/patientSafety/capsLink2008–01‐01.pdf. Accessed March 19,2010.
- ,,,,,.Pharmacology and management of the vitamin K antagonists: American College of Chest Physicians evidence‐based clinical practice guidelines (8th ed.).Chest.2008;133:160S–198S.
- The Joint Commission. 2009 National Patient Safety Goals. Available at: http://www.jointcommission.org/NR/rdonlyres/31666E86‐E7F4–423E‐9BE8‐F05BD1CB0AA8/0/HAP_NPSG.pdf. Accessed May 6,2010.
- ,,, et al.Optimization of inpatient warfarin therapy: impact of a daily consultation by a pharmacist‐managed anticoagulation service.Ann Pharmacother.2000;34:567–572.
- ,,, et al.Pharmacy‐managed protocol for warfarin use in orthopedic surgery patients.Am J Health‐Syst Pharm.1995;52:1310–1316.
- .Pharmacist involvement with warfarin dosing for inpatients.Pharm World Sci.2001;23:31–35.
- ,,.Evaluation of a pharmacy‐managed warfarin‐monitoring service to coordinate inpatient and outpatient therapy.Am J Hosp Pharm.1992;49:387–394.
- ,.Implementation and evaluation of a pharmacist‐assisted warfarin dosing program.Can J Hosp Pharm.1997;50:169–175.
- ,.Definition of major bleeding in clinical investigations of antihemostatic medicinal products in non‐surgical patients.J Thromb Haemostasis.2005;3:692–694.
- ,,.Rehospitalizations among patients in the Medicare fee‐for‐service program.N Engl J Med.2009;360:1418–1428.
Anticoagulants are one of the most common drug classes involved in medication errors and adverse events. Warfarin, an anticoagulant that plays a key role in the management of many disease states, is implicated in approximately 30% of reported anticoagulant‐related errors.1 Anticoagulation with warfarin is complicated by inter‐individual variability in response to therapy, clinically significant drug interactions, a narrow therapeutic window, and the need for frequent and lifelong monitoring.2
In the hospital setting, warfarin use is complicated due to patient handoff among health care providers, and acute illnesses that impact sensitivity and response to warfarin. Common causes of errors with anticoagulants are knowledge deficits, failure to follow policy/procedure/protocol, and communication issues.1 An added opportunity for warfarin‐related medication errors is the risk associated with the transition from the inpatient‐to‐outpatient setting. Due to the risk and complexity associated with anticoagulant medications, the Joint Commission instituted National Patient Safety Goal (NPSG) 03.05.01 (formerly NPSG 3E): a series of requirements intended to Reduce the likelihood of patient harm with the use of anticoagulation therapy.3 In order to optimally address this National Patient Safety Goal, a systematic intervention would be required to impact each step of the medication use process for anticoagulants.
Several studies have suggested that dedicated anticoagulation management services or clinics improve anticoagulation management in the outpatient setting.2 Non‐physician providers, primarily pharmacists and nurses, frequently manage outpatient anticoagulation management services or clinics. However, very few studies have evaluated the impact of a warfarin management service in the inpatient hospital setting.48 While the few available studies suggest some benefit associated with an inpatient anticoagulation management service, a minority of these studies have assessed the role of these services in facilitating the transition of the anticoagulated patient to the outpatient setting.7
In order to improve anticoagulation management and safety, our institution implemented an inpatient Pharmacist‐Directed Anticoagulation Service (PDAS). The purpose of this study was to evaluate the impact of this service on both transition of care and safety of patients receiving warfarin anticoagulation.
METHODS
This study was completed at Henry Ford Hospital, an 802‐bed, tertiary care, level 1 trauma and academic medical center in Detroit, MI. The study was carried out between November 2007 and June 2009. The study was approved by the Henry Ford Hospital Institutional Review Board with waiver of consent.
Patients
This was a prospective cluster randomized study. All patients admitted to two internal medicine units (IM1 or IM2) or two cardiology units (Card1 or Card2), who received at least one inpatient dose of warfarin, were eligible for inclusion. Patients were included regardless of whether warfarin was newly initiated during the index admission (newly initiated patients) or was continuation of existing anticoagulation (existing warfarin patients). In order to ensure that patient data following discharge would be available for analysis, patients were excluded from this analysis if they were not scheduled to follow‐up in the Henry Ford Medical Group outpatient anticoagulation clinics after discharge, however, these patients were cared for by the PDAS service in the usual manner.
Study Design
Prior to implementation of the PDAS, one internal medicine and one cardiology unit was randomly selected to receive the PDAS intervention (IM1 and Card1), while the other two units (IM2 and Card2) served as control units. These hospital units were selected because anticoagulants are frequently used on these units and the patient population is generally similar between the two internal medicine and two cardiology unitswith exception that Card1 unit also contains a specialized service for advanced heart failure and left ventricular assist device (LVAD) patients. Of note, there was significant expansion of the heart failure service and LVAD program during the time frame of the study, accounting for a greater number of more complicated patients on the Card1 (PDAS) unit.
Specific responsibilities of the PDAS related to warfarin are detailed in Table 1. The PDAS was implemented in September 2007 as a system‐based change to improve anticoagulant safety at our institution. The goals of this service were to improve communication regarding anticoagulation; to improve safety as patients transition from the inpatient‐to‐outpatient settings; and to standardize anticoagulant dosing, monitoring, and patient education. For patients taking warfarin, who are cared for by a health system‐affiliated physician, the PDAS collaborates with our outpatient anticoagulation clinics in order to facilitate transition from the inpatient‐to‐outpatient setting. The Henry Ford Health System has an established, multisite outpatient Anticoagulation Clinic with >5000 patients actively receiving warfarin dosing and monitoring. The anticoagulation clinics are staffed by nurses and pharmacists who provide standardized management of warfarin for patients of all physicians within our health system and provide consistent high‐quality care (average time in international normalized ratio [INR] goal range = 68.2%). The anticoagulation clinics have been in existence since 1992. The PDAS is comprised of three full‐time and two part‐time pharmacists whose responsibilities are limited to the management of anticoagulation throughout the hospital.
| Inpatient Care | Patient Education | Transition of Care |
|---|---|---|
| ||
| Initial dose selection and daily dose adjustments after warfarin is initiated by primary team | Comprehensive education provided verbally and via written communication utilizing the Krames database. | Contact anticoagulation‐responsible physician and anticoagulation clinic via phone. |
| Provide written dosing regimen to patient and provide date for first INR postdischarge. | ||
| Daily laboratory monitoring | Education provided is standardized between inpatient and outpatient settings. | Create electronic Anticoagulation Discharge Summary. Document communication with the outpatient clinicians, reason for admission, steps taken to manage warfarin drug interactions, and warfarin doses administered during stay, discharge warfarin dose and follow‐up date. |
The PDAS was staffed by repurposing pharmacist staff. All pharmacists had either several years of general medicine‐based clinical practice experience or residency training, or both. Pharmacists were oriented to service responsibilities by spending approximately one week in the outpatient anticoagulation clinic and completing focused review of internal and external anticoagulation guidelines.
In the control group, management of anticoagulation and transition of care occurred at the discretion of the primary care team. The primary team had access to a clinical pharmacist, who was not part of the PDAS, seven days per week. However, the primary team was not able to consult the PDAS.
This study was primarily designed to assess the impact of the PDAS on both transition of care and patient safety. For study endpoint purposes, transition of care was assessed by satisfactory completion and documentation of four important metrics: 1) appropriate enrollment in the anticoagulation clinic; 2) documented communication between the inpatient service responsible for anticoagulation and the outpatient anticoagulation clinic prior to patient discharge; 3) documented communication between the inpatient service responsible for anticoagulation and the physician responsible for outpatient management of the patient; 4) INR drawn within five days of hospital discharge. Documentation of communication for metric #2 and #3 was obtained by reviewing the electronic medical record system, particularly electronic discharge summaries and telephone encounter notes.
The primary safety endpoint was defined as a composite of any INR >5, any episode of major bleeding, or development of new thrombosis. This endpoint was met if any of these events occurred either during the index hospitalization or within 30 days of hospital discharge. Major bleeding was identified by review of outpatient anticoagulation clinic encounters and the patient's electronic medical record (includes all inpatient and outpatient encounters within Henry Ford Health System) by using the International Society of Thrombosis and Haemostasis standard and was defined as fatal bleeding or symptomatic bleeding in a critical area or organ (intracranial, intraspinal, intraocular, retroperitoneal, intraarticular, pericardial, or intramuscular with compartment syndrome), or bleeding causing a reduction in hemoglobin levels of 2 g/dL or more, or leading to transfusion of two or more units of blood or red cells.9 New thrombosis was defined as documentation of any of the following: deep vein thrombosis, pulmonary embolism, or cardioembolic stroke. Need for dose adjustment at the first anticoagulation clinic visit after discharge was evaluated as a secondary endpoint.
All analyses compared the PDAS to the control group. In addition, a planned comparison of patients in the PDAS and control groups who were newly initiated to warfarin during the study hospitalization (newly initiated subgroup) and those who were taking warfarin on admission (existing warfarin subgroup) was also undertaken. It was expected that these subgroup analyses would likely be underpowered, however, the potential implications of a service such as this could differ based on history of warfarin use. Therefore, these analyses were planned for exploratory purposes. In order to determine the impact of risk factors for altered warfarin pharmacodynamic response on the safety endpoint, post hoc subgroup analyses were performed based on demographics and clinical characteristics.
Data Analysis
Data are presented as mean standard deviation or proportion, as appropriate. A P‐value of less than 0.05 was considered significant for all comparisons and all tests were two‐tailed.
Intervention and control groups were compared with Student's t test, MannWhitney U test, chi‐square or Fishers exact test, as appropriate. Relative risk (RR) and 95% confidence intervals (CI) were calculated for all primary analyses. All statistical analyses were performed with SPSS v.12.0 (SPSS Inc, Chicago, IL).
It was estimated that a sample size of 250 patients per group would provide greater than 80% power to detect at least a 50% improvement in both the transition of care and primary safety endpoints, with implementation of the PDAS. This calculation is based on the following assumptions: alpha = 0.05; expected control group achievement of the four transition of care metrics = 50%; rate of safety endpoint for the control group = 20%.4
RESULTS
Baseline Characteristics
During the study period, 1360 patients were admitted to the study units. A total of 377 and 483 patients were found to be ineligible for inclusion on the PDAS and control units, respectively. These patients were ineligible because they did not follow up in the Henry Ford Medical Group outpatient anticoagulation clinic. In total, 500 patients were included in the analysis. Patients (n = 145) who were newly initiated on warfarin made up 29% of the total population. Table 2 presents baseline clinical characteristics for patients in the PDAS and control groups, showing increased age, and a greater proportion of patients with heart failure and LVADs in the PDAS group. Patients in the PDAS group had significantly longer hospital stays, however, these increases were driven by a longer length of stay among the advanced heart failure service patients that were managed by the PDAS.
| PDAS (n = 250) | Control (n = 250) | P Value | |
|---|---|---|---|
| |||
| Demographic data | |||
| Age (mean SD) | 64.1 15.6 | 68.0 14.9 | 0.004 |
| Male gender | 54.0% | 56.4% | 0.589 |
| Caucasian race | 44.4% | 50.4% | 0.179 |
| Admitted to a cardiology unit | 78.8% | 74.8% | 0.289 |
| Length of stay (mean SD) | 8.13 7.04 | 6.29 5.63 | 0.001 |
| No heart failure history: length of stay (mean SD) | 6.83 4.53 | 6.15 5.14 | 0.288 |
| Heart failure history: length of stay (mean SD) | 9.09 8.31 | 6.45 6.15 | 0.004 |
| History of heart failure* | 57.6% | 47.6% | 0.025 |
| Heart failure with an LVAD | 14.0% | 0.4% | <0.001 |
| Indication for anticoagulation | |||
| Venous thromboembolism | 21.6% | 18.4% | 0.371 |
| Atrial fibrillation | 54.4% | 66.4% | 0.006 |
| Other | 24.0% | 15.2% | 0.013 |
| Primary admission diagnosis* | |||
| Heart failure | 25.6%* | 21.6% | 0.292 |
| Atrial fibrillation | 16.4% | 20.8% | 0.206 |
| Acute coronary syndrome | 13.6% | 17.6% | 0.218 |
| Venous thromboembolism | 4.8% | 4.8% | 1.00 |
| Infection | 12.4% | 10.0% | 0.395 |
| Bleeding | 1.6% | 1.2% | 0.703 |
Early Warfarin Management
Warfarin management metrics are presented in Table 3. The number of inpatient days prescribed warfarin was increased in the PDAS group by greater than one day while PDAS patients required significantly less dosage adjustment at first outpatient follow‐up visit. Similar to increases noted with length of stay, increases in inpatient warfarin days were likely driven by patients with severe heart failure managed by the PDAS.
| Warfarin Dosing | PDAS (n = 250) | Control (n = 250) | P Value |
|---|---|---|---|
| |||
| Initial dose (mean SD) | 5.23 2.37 | 4.99 2.07 | 0.245 |
| Discharge dose (mean SD) | 5.15 2.52 | 4.91 2.14 | 0.258 |
| INR at discharge (mean SD) | 2.07 0.73 | 2.04 0.73 | 0.660 |
| Therapeutic INR at discharge | 40.8% | 38.0% | 0.522 |
| Inpatient warfarin days (mean SD) | 4.97 4.30 | 3.68 2.69 | <0.001 |
| No heart failure: inpatient warfarin days (mean SD) | 4.09 2.49 | 3.60 2.67 | 0.148 |
| Heart failure: inpatient warfarin days (mean SD) | 5.62 5.16 | 3.76 2.71 | <0.001 |
| Dose change required at first follow‐up visit | 44.8% | 72.6% | <0.001 |
Transition of Care
Transition of care results are presented in Table 4. Full compliance and achievement of the transition of care metrics occurred significantly more often in the PDAS versus control patients with markedly increased rates of documented communication between inpatient providers and both outpatient anticoagulation clinic staff and outpatient physicians. Early follow‐up INR monitoring also occurred more frequently in the PDAS patients. The PDAS patients experienced greater compliance with the transition of care metrics regardless of whether they were in the newly initiated or existing warfarin subgroups (data not shown).
| Transition of Care | PDAS (n = 250) | Control (n = 250) | Relative Risk (95% CI) | P Value |
|---|---|---|---|---|
| ||||
| 100% Communication bundle* compliance, % (n) | 75.6% (189) | 2.8% (7) | 27.0 (13.056.2) | <0.001 |
| Appropriately enrolled in the AC clinic, % (n) | 97.2% (243) | 95.2% (238) | 1.02 (0.991.06) | 0.242 |
| Communication: inpatient service and outpatient physician, % (n) | 99.6% (249) | 12.4% (31) | 8.03 (5.7811.2) | <0.001 |
| Communication: inpatient clinicians and AC clinic staff, % (n) | 98.8% (247) | 14.8% (37) | 6.68 (4.969.00) | <0.001 |
| INR drawn within five days of hospital discharge, % (n) | 78.4% (196) | 66.4% (166) | 1.18 (1.061.32) | 0.003 |
| 30‐Day Composite safety endpoint, % (n) | 10.0% (25) | 14.8% (37) | 0.68 (0.421.09) | 0.103 |
| Inpatient + 30‐day INR >5, % (n) | 9.6% (24) | 14.8% (37) | 0.65 (0.401.05) | 0.076 |
| Inpatient + 30‐day major bleeding, % (n) | 0.8% (2) | 0.4% (1) | 2.00 (0.1821.9) | 0.563 |
| Inpatient + 30‐day thrombosis, % (n) | 0% (0) | 0% (0) | N/A | N/A |
Anticoagulant Safety
Safety endpoint data is presented in Table 4. The composite safety outcome of INR >5, major bleeding event, or thrombosis occurred in 12.4% of all patients with no early thrombotic events and only three major bleeding events recorded. Excessive INR values >5 occurred less frequently in the PDAS patients, however, differences in this metric and the composite safety outcome were not significantly different. Safety endpoint results in the overall population were driven by a reduction in INR values >5 among newly initiated warfarin patients in the PDAS group (PDAS: 9.5% vs control: 19.7%; P = 0.079; Figure 1). Other subgroup analyses relating to the safety endpoint are presented in Figure 1.

DISCUSSION
This article describes a systematic intervention designed to improve anticoagulation safety and efficacy in the hospital and during the transition to the postdischarge setting. Implementation of a PDAS did not impact patient bleeding and thrombotic outcomes, but did result in improved coordination and documentation of warfarin management and subsequent enhancement in the transition of the anticoagulated patient from the inpatient‐to‐outpatient setting with the Pharmacist‐Directed Anticoagulation Service.
Limited previous work has investigated the role of an anticoagulation service in inpatient management of anticoagulation.48 Only one published study has investigated the impact of this type of service on transition of care issues with warfarin, as was done in our study.7 In that study, management by an inpatient anticoagulation service resulted in a greater proportion of patients referred to an anticoagulation clinic for management (P = 0.001), more patients presenting to the anticoagulation clinic with a therapeutic INR (P = 0.001), and fewer patients presenting to the clinic with supratherapeutic levels of anticoagulation (P = 0.002). These results are somewhat analogous to our findings, in that patients in our study were less likely to require a dose change at the first clinic follow‐up visit after discharge or to have INR values 5.
We completed several subgroup analyses to thoroughly explore the impact of the PDAS on the safety endpoint. While firm conclusions cannot be drawn from these subgroup analyses, some hypothesis‐generating observations can be made. First, there was a greater impact of the PDAS on the safety endpoint in patients who are usually more sensitive to the effects of warfarin and therefore more challenging to manage.2 The impact of the PDAS was also greater among patients whose length of stay was more than five days (population median). This is significant because it suggests that when the opportunity for adverse events and miscommunication is greatest (ie, during hospitalizations of longer duration), there appears to be improvement in the safety endpoint with the PDAS.
To our knowledge, this study was the first to explore the impact of an inpatient anticoagulation service on the care of both newly initiated and existing warfarin patients, rather than only patients newly initiated on warfarin. As expected, the greatest influence of the PDAS on the safety endpoint was observed among the newly initiated patients. While the safety impact of the PDAS was noted most significantly among the newly initiated patients, the PDAS had a positive effect on the transition of care metrics regardless of previous warfarin use.
A limitation of our study should be mentioned. While we employed a design in which randomly selected units were exposed to the PDAS, individual patients were not randomized to the service. This cluster randomized design was chosen because it mimics quality improvement processes that would be rolled out to a hospital nursing unit. While lack of randomization at the patient level is a limitation, our cluster randomized study design is pragmatic and represents an improvement over the existing published before and after quasi‐experimental studies in this area.48
An improved system for documentation of communication was built into the PDAS processes on implementation of the service. However, it should also be noted that inadequate communication between inpatient and outpatient providers was an identified root cause for adverse events with warfarin in our institution prior to implementation of the PDAS. Therefore, improvement in the transition of care metrics with the PDAS were likely due to a combination of both improved documentation and true improvement in communication.
The approach of the PDAS was refined in several ways early after implementation of the service. Some major notable improvements included the development of a systematic approach to ensuring appropriate follow‐up and transition of care for patients being discharged to a skilled nursing facility, and creation of a system that required a mandatory conversation between the PDAS and a surgical service if anticoagulation is ordered within 48 hours of a major procedure. An upcoming improvement to the service will be to transition from a home grown electronic database, which was built for the purposes of streamlining clinical workflow and data collection, to a commercially available software program that has recently become available for management of inpatient anticoagulation. The major advantage of the new program will be the clinical decision support capabilities that will help to further streamline the service and allow for greater efficiency.
To understand implications of our PDAS intervention, it is important to remember that the majority of study patients were prescribed warfarin prior to hospital admission, that patients in both study groups were enrolled in an established, multisite anticoagulation clinic, and that patients in both groups were managed with a comprehensive inpatientoutpatient electronic medical record. Therefore, all providers caring for these patients had real‐time access to all warfarin dosing and dose adjustments, INR results, and anticoagulation clinic encounters, even though formal communication between providers was infrequently documented for control group patients in our electronic medical record. Study patients had low rates of bleeding and no adverse thrombotic outcomes across both treatment groups. Therefore, this type of model is likely to produce larger gains in communication and safety outcomes in health care systems without established anticoagulation clinics or comprehensive electronic medical records.
The PDAS was enthusiastically accepted by providers at our institution and expanded hospital‐wide after completion of this pilot. The PDAS model is a viable approach to standardize anticoagulant management with a goal of improving anticoagulant safety in the inpatient setting. Assessment of the effectiveness of models such as the PDAS for improving anticoagulant safety in the inpatient setting is particularly relevant with the current expectations for hospitals set by The Joint Commission's NPSG.03.05.01.3 More importantly the PDAS model can be an option for improving the transition of the anticoagulated patient from the inpatient‐to‐outpatient setting. Follow‐up with the anticoagulation clinic occurred earlier with the PDAS and, while this study was not designed to evaluate the impact of this new service on rehospitalization, recent literature suggests that earlier follow‐up after discharge leads to less rehospitalization.10 Finally, it may be possible to adapt this model to provide more intensive medication therapy management and monitoring for hospitalized patients with other complicated medication regimens or chronic disease.
CONCLUSION
The clinical pharmacist is uniquely prepared to manage inter‐individual variability in pharmacodynamic response to drug therapy, as well as to provide high‐quality patient education. This study evaluated a new model of inpatient warfarin management, in which warfarin dosing, monitoring, patient education, and transition of care was coordinated by a specialized team of clinical pharmacists that worked in collaboration with physicians and outpatient anticoagulation clinic staff. Safety and efficiency of the care provided by this new service was improved in certain subsets of more complex patients. The major advantage of this service was improvement in patient handoff, improved communication, and earlier patient follow‐up after discharge. Therefore, implementation of a Pharmacist‐Directed Anticoagulation Service provides a net improvement in quality of care for the patient taking warfarin in the inpatient setting.
Acknowledgements
The authors acknowledge the efforts of the PDAS staff: Nassif Abi‐Samra, Pam Holland, Sara Lanfear, and Gail Washington. This work would not have been possible without the dedication of these pharmacists. All authors had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Anticoagulants are one of the most common drug classes involved in medication errors and adverse events. Warfarin, an anticoagulant that plays a key role in the management of many disease states, is implicated in approximately 30% of reported anticoagulant‐related errors.1 Anticoagulation with warfarin is complicated by inter‐individual variability in response to therapy, clinically significant drug interactions, a narrow therapeutic window, and the need for frequent and lifelong monitoring.2
In the hospital setting, warfarin use is complicated due to patient handoff among health care providers, and acute illnesses that impact sensitivity and response to warfarin. Common causes of errors with anticoagulants are knowledge deficits, failure to follow policy/procedure/protocol, and communication issues.1 An added opportunity for warfarin‐related medication errors is the risk associated with the transition from the inpatient‐to‐outpatient setting. Due to the risk and complexity associated with anticoagulant medications, the Joint Commission instituted National Patient Safety Goal (NPSG) 03.05.01 (formerly NPSG 3E): a series of requirements intended to Reduce the likelihood of patient harm with the use of anticoagulation therapy.3 In order to optimally address this National Patient Safety Goal, a systematic intervention would be required to impact each step of the medication use process for anticoagulants.
Several studies have suggested that dedicated anticoagulation management services or clinics improve anticoagulation management in the outpatient setting.2 Non‐physician providers, primarily pharmacists and nurses, frequently manage outpatient anticoagulation management services or clinics. However, very few studies have evaluated the impact of a warfarin management service in the inpatient hospital setting.48 While the few available studies suggest some benefit associated with an inpatient anticoagulation management service, a minority of these studies have assessed the role of these services in facilitating the transition of the anticoagulated patient to the outpatient setting.7
In order to improve anticoagulation management and safety, our institution implemented an inpatient Pharmacist‐Directed Anticoagulation Service (PDAS). The purpose of this study was to evaluate the impact of this service on both transition of care and safety of patients receiving warfarin anticoagulation.
METHODS
This study was completed at Henry Ford Hospital, an 802‐bed, tertiary care, level 1 trauma and academic medical center in Detroit, MI. The study was carried out between November 2007 and June 2009. The study was approved by the Henry Ford Hospital Institutional Review Board with waiver of consent.
Patients
This was a prospective cluster randomized study. All patients admitted to two internal medicine units (IM1 or IM2) or two cardiology units (Card1 or Card2), who received at least one inpatient dose of warfarin, were eligible for inclusion. Patients were included regardless of whether warfarin was newly initiated during the index admission (newly initiated patients) or was continuation of existing anticoagulation (existing warfarin patients). In order to ensure that patient data following discharge would be available for analysis, patients were excluded from this analysis if they were not scheduled to follow‐up in the Henry Ford Medical Group outpatient anticoagulation clinics after discharge, however, these patients were cared for by the PDAS service in the usual manner.
Study Design
Prior to implementation of the PDAS, one internal medicine and one cardiology unit was randomly selected to receive the PDAS intervention (IM1 and Card1), while the other two units (IM2 and Card2) served as control units. These hospital units were selected because anticoagulants are frequently used on these units and the patient population is generally similar between the two internal medicine and two cardiology unitswith exception that Card1 unit also contains a specialized service for advanced heart failure and left ventricular assist device (LVAD) patients. Of note, there was significant expansion of the heart failure service and LVAD program during the time frame of the study, accounting for a greater number of more complicated patients on the Card1 (PDAS) unit.
Specific responsibilities of the PDAS related to warfarin are detailed in Table 1. The PDAS was implemented in September 2007 as a system‐based change to improve anticoagulant safety at our institution. The goals of this service were to improve communication regarding anticoagulation; to improve safety as patients transition from the inpatient‐to‐outpatient settings; and to standardize anticoagulant dosing, monitoring, and patient education. For patients taking warfarin, who are cared for by a health system‐affiliated physician, the PDAS collaborates with our outpatient anticoagulation clinics in order to facilitate transition from the inpatient‐to‐outpatient setting. The Henry Ford Health System has an established, multisite outpatient Anticoagulation Clinic with >5000 patients actively receiving warfarin dosing and monitoring. The anticoagulation clinics are staffed by nurses and pharmacists who provide standardized management of warfarin for patients of all physicians within our health system and provide consistent high‐quality care (average time in international normalized ratio [INR] goal range = 68.2%). The anticoagulation clinics have been in existence since 1992. The PDAS is comprised of three full‐time and two part‐time pharmacists whose responsibilities are limited to the management of anticoagulation throughout the hospital.
| Inpatient Care | Patient Education | Transition of Care |
|---|---|---|
| ||
| Initial dose selection and daily dose adjustments after warfarin is initiated by primary team | Comprehensive education provided verbally and via written communication utilizing the Krames database. | Contact anticoagulation‐responsible physician and anticoagulation clinic via phone. |
| Provide written dosing regimen to patient and provide date for first INR postdischarge. | ||
| Daily laboratory monitoring | Education provided is standardized between inpatient and outpatient settings. | Create electronic Anticoagulation Discharge Summary. Document communication with the outpatient clinicians, reason for admission, steps taken to manage warfarin drug interactions, and warfarin doses administered during stay, discharge warfarin dose and follow‐up date. |
The PDAS was staffed by repurposing pharmacist staff. All pharmacists had either several years of general medicine‐based clinical practice experience or residency training, or both. Pharmacists were oriented to service responsibilities by spending approximately one week in the outpatient anticoagulation clinic and completing focused review of internal and external anticoagulation guidelines.
In the control group, management of anticoagulation and transition of care occurred at the discretion of the primary care team. The primary team had access to a clinical pharmacist, who was not part of the PDAS, seven days per week. However, the primary team was not able to consult the PDAS.
This study was primarily designed to assess the impact of the PDAS on both transition of care and patient safety. For study endpoint purposes, transition of care was assessed by satisfactory completion and documentation of four important metrics: 1) appropriate enrollment in the anticoagulation clinic; 2) documented communication between the inpatient service responsible for anticoagulation and the outpatient anticoagulation clinic prior to patient discharge; 3) documented communication between the inpatient service responsible for anticoagulation and the physician responsible for outpatient management of the patient; 4) INR drawn within five days of hospital discharge. Documentation of communication for metric #2 and #3 was obtained by reviewing the electronic medical record system, particularly electronic discharge summaries and telephone encounter notes.
The primary safety endpoint was defined as a composite of any INR >5, any episode of major bleeding, or development of new thrombosis. This endpoint was met if any of these events occurred either during the index hospitalization or within 30 days of hospital discharge. Major bleeding was identified by review of outpatient anticoagulation clinic encounters and the patient's electronic medical record (includes all inpatient and outpatient encounters within Henry Ford Health System) by using the International Society of Thrombosis and Haemostasis standard and was defined as fatal bleeding or symptomatic bleeding in a critical area or organ (intracranial, intraspinal, intraocular, retroperitoneal, intraarticular, pericardial, or intramuscular with compartment syndrome), or bleeding causing a reduction in hemoglobin levels of 2 g/dL or more, or leading to transfusion of two or more units of blood or red cells.9 New thrombosis was defined as documentation of any of the following: deep vein thrombosis, pulmonary embolism, or cardioembolic stroke. Need for dose adjustment at the first anticoagulation clinic visit after discharge was evaluated as a secondary endpoint.
All analyses compared the PDAS to the control group. In addition, a planned comparison of patients in the PDAS and control groups who were newly initiated to warfarin during the study hospitalization (newly initiated subgroup) and those who were taking warfarin on admission (existing warfarin subgroup) was also undertaken. It was expected that these subgroup analyses would likely be underpowered, however, the potential implications of a service such as this could differ based on history of warfarin use. Therefore, these analyses were planned for exploratory purposes. In order to determine the impact of risk factors for altered warfarin pharmacodynamic response on the safety endpoint, post hoc subgroup analyses were performed based on demographics and clinical characteristics.
Data Analysis
Data are presented as mean standard deviation or proportion, as appropriate. A P‐value of less than 0.05 was considered significant for all comparisons and all tests were two‐tailed.
Intervention and control groups were compared with Student's t test, MannWhitney U test, chi‐square or Fishers exact test, as appropriate. Relative risk (RR) and 95% confidence intervals (CI) were calculated for all primary analyses. All statistical analyses were performed with SPSS v.12.0 (SPSS Inc, Chicago, IL).
It was estimated that a sample size of 250 patients per group would provide greater than 80% power to detect at least a 50% improvement in both the transition of care and primary safety endpoints, with implementation of the PDAS. This calculation is based on the following assumptions: alpha = 0.05; expected control group achievement of the four transition of care metrics = 50%; rate of safety endpoint for the control group = 20%.4
RESULTS
Baseline Characteristics
During the study period, 1360 patients were admitted to the study units. A total of 377 and 483 patients were found to be ineligible for inclusion on the PDAS and control units, respectively. These patients were ineligible because they did not follow up in the Henry Ford Medical Group outpatient anticoagulation clinic. In total, 500 patients were included in the analysis. Patients (n = 145) who were newly initiated on warfarin made up 29% of the total population. Table 2 presents baseline clinical characteristics for patients in the PDAS and control groups, showing increased age, and a greater proportion of patients with heart failure and LVADs in the PDAS group. Patients in the PDAS group had significantly longer hospital stays, however, these increases were driven by a longer length of stay among the advanced heart failure service patients that were managed by the PDAS.
| PDAS (n = 250) | Control (n = 250) | P Value | |
|---|---|---|---|
| |||
| Demographic data | |||
| Age (mean SD) | 64.1 15.6 | 68.0 14.9 | 0.004 |
| Male gender | 54.0% | 56.4% | 0.589 |
| Caucasian race | 44.4% | 50.4% | 0.179 |
| Admitted to a cardiology unit | 78.8% | 74.8% | 0.289 |
| Length of stay (mean SD) | 8.13 7.04 | 6.29 5.63 | 0.001 |
| No heart failure history: length of stay (mean SD) | 6.83 4.53 | 6.15 5.14 | 0.288 |
| Heart failure history: length of stay (mean SD) | 9.09 8.31 | 6.45 6.15 | 0.004 |
| History of heart failure* | 57.6% | 47.6% | 0.025 |
| Heart failure with an LVAD | 14.0% | 0.4% | <0.001 |
| Indication for anticoagulation | |||
| Venous thromboembolism | 21.6% | 18.4% | 0.371 |
| Atrial fibrillation | 54.4% | 66.4% | 0.006 |
| Other | 24.0% | 15.2% | 0.013 |
| Primary admission diagnosis* | |||
| Heart failure | 25.6%* | 21.6% | 0.292 |
| Atrial fibrillation | 16.4% | 20.8% | 0.206 |
| Acute coronary syndrome | 13.6% | 17.6% | 0.218 |
| Venous thromboembolism | 4.8% | 4.8% | 1.00 |
| Infection | 12.4% | 10.0% | 0.395 |
| Bleeding | 1.6% | 1.2% | 0.703 |
Early Warfarin Management
Warfarin management metrics are presented in Table 3. The number of inpatient days prescribed warfarin was increased in the PDAS group by greater than one day while PDAS patients required significantly less dosage adjustment at first outpatient follow‐up visit. Similar to increases noted with length of stay, increases in inpatient warfarin days were likely driven by patients with severe heart failure managed by the PDAS.
| Warfarin Dosing | PDAS (n = 250) | Control (n = 250) | P Value |
|---|---|---|---|
| |||
| Initial dose (mean SD) | 5.23 2.37 | 4.99 2.07 | 0.245 |
| Discharge dose (mean SD) | 5.15 2.52 | 4.91 2.14 | 0.258 |
| INR at discharge (mean SD) | 2.07 0.73 | 2.04 0.73 | 0.660 |
| Therapeutic INR at discharge | 40.8% | 38.0% | 0.522 |
| Inpatient warfarin days (mean SD) | 4.97 4.30 | 3.68 2.69 | <0.001 |
| No heart failure: inpatient warfarin days (mean SD) | 4.09 2.49 | 3.60 2.67 | 0.148 |
| Heart failure: inpatient warfarin days (mean SD) | 5.62 5.16 | 3.76 2.71 | <0.001 |
| Dose change required at first follow‐up visit | 44.8% | 72.6% | <0.001 |
Transition of Care
Transition of care results are presented in Table 4. Full compliance and achievement of the transition of care metrics occurred significantly more often in the PDAS versus control patients with markedly increased rates of documented communication between inpatient providers and both outpatient anticoagulation clinic staff and outpatient physicians. Early follow‐up INR monitoring also occurred more frequently in the PDAS patients. The PDAS patients experienced greater compliance with the transition of care metrics regardless of whether they were in the newly initiated or existing warfarin subgroups (data not shown).
| Transition of Care | PDAS (n = 250) | Control (n = 250) | Relative Risk (95% CI) | P Value |
|---|---|---|---|---|
| ||||
| 100% Communication bundle* compliance, % (n) | 75.6% (189) | 2.8% (7) | 27.0 (13.056.2) | <0.001 |
| Appropriately enrolled in the AC clinic, % (n) | 97.2% (243) | 95.2% (238) | 1.02 (0.991.06) | 0.242 |
| Communication: inpatient service and outpatient physician, % (n) | 99.6% (249) | 12.4% (31) | 8.03 (5.7811.2) | <0.001 |
| Communication: inpatient clinicians and AC clinic staff, % (n) | 98.8% (247) | 14.8% (37) | 6.68 (4.969.00) | <0.001 |
| INR drawn within five days of hospital discharge, % (n) | 78.4% (196) | 66.4% (166) | 1.18 (1.061.32) | 0.003 |
| 30‐Day Composite safety endpoint, % (n) | 10.0% (25) | 14.8% (37) | 0.68 (0.421.09) | 0.103 |
| Inpatient + 30‐day INR >5, % (n) | 9.6% (24) | 14.8% (37) | 0.65 (0.401.05) | 0.076 |
| Inpatient + 30‐day major bleeding, % (n) | 0.8% (2) | 0.4% (1) | 2.00 (0.1821.9) | 0.563 |
| Inpatient + 30‐day thrombosis, % (n) | 0% (0) | 0% (0) | N/A | N/A |
Anticoagulant Safety
Safety endpoint data is presented in Table 4. The composite safety outcome of INR >5, major bleeding event, or thrombosis occurred in 12.4% of all patients with no early thrombotic events and only three major bleeding events recorded. Excessive INR values >5 occurred less frequently in the PDAS patients, however, differences in this metric and the composite safety outcome were not significantly different. Safety endpoint results in the overall population were driven by a reduction in INR values >5 among newly initiated warfarin patients in the PDAS group (PDAS: 9.5% vs control: 19.7%; P = 0.079; Figure 1). Other subgroup analyses relating to the safety endpoint are presented in Figure 1.

DISCUSSION
This article describes a systematic intervention designed to improve anticoagulation safety and efficacy in the hospital and during the transition to the postdischarge setting. Implementation of a PDAS did not impact patient bleeding and thrombotic outcomes, but did result in improved coordination and documentation of warfarin management and subsequent enhancement in the transition of the anticoagulated patient from the inpatient‐to‐outpatient setting with the Pharmacist‐Directed Anticoagulation Service.
Limited previous work has investigated the role of an anticoagulation service in inpatient management of anticoagulation.48 Only one published study has investigated the impact of this type of service on transition of care issues with warfarin, as was done in our study.7 In that study, management by an inpatient anticoagulation service resulted in a greater proportion of patients referred to an anticoagulation clinic for management (P = 0.001), more patients presenting to the anticoagulation clinic with a therapeutic INR (P = 0.001), and fewer patients presenting to the clinic with supratherapeutic levels of anticoagulation (P = 0.002). These results are somewhat analogous to our findings, in that patients in our study were less likely to require a dose change at the first clinic follow‐up visit after discharge or to have INR values 5.
We completed several subgroup analyses to thoroughly explore the impact of the PDAS on the safety endpoint. While firm conclusions cannot be drawn from these subgroup analyses, some hypothesis‐generating observations can be made. First, there was a greater impact of the PDAS on the safety endpoint in patients who are usually more sensitive to the effects of warfarin and therefore more challenging to manage.2 The impact of the PDAS was also greater among patients whose length of stay was more than five days (population median). This is significant because it suggests that when the opportunity for adverse events and miscommunication is greatest (ie, during hospitalizations of longer duration), there appears to be improvement in the safety endpoint with the PDAS.
To our knowledge, this study was the first to explore the impact of an inpatient anticoagulation service on the care of both newly initiated and existing warfarin patients, rather than only patients newly initiated on warfarin. As expected, the greatest influence of the PDAS on the safety endpoint was observed among the newly initiated patients. While the safety impact of the PDAS was noted most significantly among the newly initiated patients, the PDAS had a positive effect on the transition of care metrics regardless of previous warfarin use.
A limitation of our study should be mentioned. While we employed a design in which randomly selected units were exposed to the PDAS, individual patients were not randomized to the service. This cluster randomized design was chosen because it mimics quality improvement processes that would be rolled out to a hospital nursing unit. While lack of randomization at the patient level is a limitation, our cluster randomized study design is pragmatic and represents an improvement over the existing published before and after quasi‐experimental studies in this area.48
An improved system for documentation of communication was built into the PDAS processes on implementation of the service. However, it should also be noted that inadequate communication between inpatient and outpatient providers was an identified root cause for adverse events with warfarin in our institution prior to implementation of the PDAS. Therefore, improvement in the transition of care metrics with the PDAS were likely due to a combination of both improved documentation and true improvement in communication.
The approach of the PDAS was refined in several ways early after implementation of the service. Some major notable improvements included the development of a systematic approach to ensuring appropriate follow‐up and transition of care for patients being discharged to a skilled nursing facility, and creation of a system that required a mandatory conversation between the PDAS and a surgical service if anticoagulation is ordered within 48 hours of a major procedure. An upcoming improvement to the service will be to transition from a home grown electronic database, which was built for the purposes of streamlining clinical workflow and data collection, to a commercially available software program that has recently become available for management of inpatient anticoagulation. The major advantage of the new program will be the clinical decision support capabilities that will help to further streamline the service and allow for greater efficiency.
To understand implications of our PDAS intervention, it is important to remember that the majority of study patients were prescribed warfarin prior to hospital admission, that patients in both study groups were enrolled in an established, multisite anticoagulation clinic, and that patients in both groups were managed with a comprehensive inpatientoutpatient electronic medical record. Therefore, all providers caring for these patients had real‐time access to all warfarin dosing and dose adjustments, INR results, and anticoagulation clinic encounters, even though formal communication between providers was infrequently documented for control group patients in our electronic medical record. Study patients had low rates of bleeding and no adverse thrombotic outcomes across both treatment groups. Therefore, this type of model is likely to produce larger gains in communication and safety outcomes in health care systems without established anticoagulation clinics or comprehensive electronic medical records.
The PDAS was enthusiastically accepted by providers at our institution and expanded hospital‐wide after completion of this pilot. The PDAS model is a viable approach to standardize anticoagulant management with a goal of improving anticoagulant safety in the inpatient setting. Assessment of the effectiveness of models such as the PDAS for improving anticoagulant safety in the inpatient setting is particularly relevant with the current expectations for hospitals set by The Joint Commission's NPSG.03.05.01.3 More importantly the PDAS model can be an option for improving the transition of the anticoagulated patient from the inpatient‐to‐outpatient setting. Follow‐up with the anticoagulation clinic occurred earlier with the PDAS and, while this study was not designed to evaluate the impact of this new service on rehospitalization, recent literature suggests that earlier follow‐up after discharge leads to less rehospitalization.10 Finally, it may be possible to adapt this model to provide more intensive medication therapy management and monitoring for hospitalized patients with other complicated medication regimens or chronic disease.
CONCLUSION
The clinical pharmacist is uniquely prepared to manage inter‐individual variability in pharmacodynamic response to drug therapy, as well as to provide high‐quality patient education. This study evaluated a new model of inpatient warfarin management, in which warfarin dosing, monitoring, patient education, and transition of care was coordinated by a specialized team of clinical pharmacists that worked in collaboration with physicians and outpatient anticoagulation clinic staff. Safety and efficiency of the care provided by this new service was improved in certain subsets of more complex patients. The major advantage of this service was improvement in patient handoff, improved communication, and earlier patient follow‐up after discharge. Therefore, implementation of a Pharmacist‐Directed Anticoagulation Service provides a net improvement in quality of care for the patient taking warfarin in the inpatient setting.
Acknowledgements
The authors acknowledge the efforts of the PDAS staff: Nassif Abi‐Samra, Pam Holland, Sara Lanfear, and Gail Washington. This work would not have been possible without the dedication of these pharmacists. All authors had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
- U.S. Pharmacopeia. USP Patient Safety CapsLink: January 2008. Available at: http://www.usp.org/pdf/EN/patientSafety/capsLink2008–01‐01.pdf. Accessed March 19,2010.
- ,,,,,.Pharmacology and management of the vitamin K antagonists: American College of Chest Physicians evidence‐based clinical practice guidelines (8th ed.).Chest.2008;133:160S–198S.
- The Joint Commission. 2009 National Patient Safety Goals. Available at: http://www.jointcommission.org/NR/rdonlyres/31666E86‐E7F4–423E‐9BE8‐F05BD1CB0AA8/0/HAP_NPSG.pdf. Accessed May 6,2010.
- ,,, et al.Optimization of inpatient warfarin therapy: impact of a daily consultation by a pharmacist‐managed anticoagulation service.Ann Pharmacother.2000;34:567–572.
- ,,, et al.Pharmacy‐managed protocol for warfarin use in orthopedic surgery patients.Am J Health‐Syst Pharm.1995;52:1310–1316.
- .Pharmacist involvement with warfarin dosing for inpatients.Pharm World Sci.2001;23:31–35.
- ,,.Evaluation of a pharmacy‐managed warfarin‐monitoring service to coordinate inpatient and outpatient therapy.Am J Hosp Pharm.1992;49:387–394.
- ,.Implementation and evaluation of a pharmacist‐assisted warfarin dosing program.Can J Hosp Pharm.1997;50:169–175.
- ,.Definition of major bleeding in clinical investigations of antihemostatic medicinal products in non‐surgical patients.J Thromb Haemostasis.2005;3:692–694.
- ,,.Rehospitalizations among patients in the Medicare fee‐for‐service program.N Engl J Med.2009;360:1418–1428.
- U.S. Pharmacopeia. USP Patient Safety CapsLink: January 2008. Available at: http://www.usp.org/pdf/EN/patientSafety/capsLink2008–01‐01.pdf. Accessed March 19,2010.
- ,,,,,.Pharmacology and management of the vitamin K antagonists: American College of Chest Physicians evidence‐based clinical practice guidelines (8th ed.).Chest.2008;133:160S–198S.
- The Joint Commission. 2009 National Patient Safety Goals. Available at: http://www.jointcommission.org/NR/rdonlyres/31666E86‐E7F4–423E‐9BE8‐F05BD1CB0AA8/0/HAP_NPSG.pdf. Accessed May 6,2010.
- ,,, et al.Optimization of inpatient warfarin therapy: impact of a daily consultation by a pharmacist‐managed anticoagulation service.Ann Pharmacother.2000;34:567–572.
- ,,, et al.Pharmacy‐managed protocol for warfarin use in orthopedic surgery patients.Am J Health‐Syst Pharm.1995;52:1310–1316.
- .Pharmacist involvement with warfarin dosing for inpatients.Pharm World Sci.2001;23:31–35.
- ,,.Evaluation of a pharmacy‐managed warfarin‐monitoring service to coordinate inpatient and outpatient therapy.Am J Hosp Pharm.1992;49:387–394.
- ,.Implementation and evaluation of a pharmacist‐assisted warfarin dosing program.Can J Hosp Pharm.1997;50:169–175.
- ,.Definition of major bleeding in clinical investigations of antihemostatic medicinal products in non‐surgical patients.J Thromb Haemostasis.2005;3:692–694.
- ,,.Rehospitalizations among patients in the Medicare fee‐for‐service program.N Engl J Med.2009;360:1418–1428.
Copyright © 2011 Society of Hospital Medicine
Characteristics of High Cost/LOS Patients
Patients with advanced illness frequently do not receive care that meets their physical and emotional needs at the end of life,1 despite significant expenditures. Palliative care has been recommended as an approach to improve the quality of care for patients with advanced illness,26 while achieving hospital cost savings.7 Studies show that palliative care consults are associated with decreased hospitalization cost712 and length of stay13, 14 in the acute care setting.
Identifying which hospitalized patients are likely to benefit most from palliative care has not been well defined. The Hamilton Chart Audit tool was developed to estimate the number of patients that would benefit from a palliative care consult, in order to determine hospital palliative care staffing and financial needs.15 The CARING criteria identifies patients on admission to the hospital who are at high risk of death within one year and may, therefore, benefit from palliative care.16 The literature from the medical intensive care unit (MICU) identifies palliative care core competencies and quality measures, but does not describe patient factors that should trigger a palliative care consult.1719 Norton et al. studied proactive palliative care consultation in the MICU, finding that palliative care consultation in the high‐risk group (serious illness and high risk of dying) was associated with a shorter MICU length of stay without a significant difference in mortality rates.14
The most specific triggers for a palliative care consult comes from the surgical intensive care guidelines. The American College of Surgeons Surgical Palliative Care Task Force published a consensus guideline based on expert opinion identifying the top ten triggers for a palliative care consultation in the surgical intensive care unit (SICU).20 The top 10 criteria to identify SICU patients for palliative care consultation listed in order of priority were: 1) family request; 2) futility considered or declared by the medical team; 3) family disagreement with the team, advance directive, or each other lasting greater than seven days; 4) death expected during the same SICU stay; 5) SICU stay of greater than one month; 6) diagnosis with a median survival of less than six months; 7) greater than three SICU admissions during the same hospitalization; 8) Glasgow Coma Score of less than eight for greater than one week in a patient greater than 75 years old; 9) Glasgow Outcome Score of less than three (i.e., persistent vegetative state); and 10) multisystem organ failure of greater than three systems.
Studies are lacking that identify hospitalized patients who are more likely to have higher cost per day or length of stay, as these are patients who may benefit from palliative care. We sought to identify patient characteristics that are associated with higher cost per day or longer length of stay in hospitalized patients who died during the hospitalization or were discharged to hospicepatients likely to benefit from targeted palliative care services. We hypothesized that hospitalized patients with the following characteristics who died during the hospitalization or were discharged to hospice would have a higher cost per day or longer length of stay: older patients, lack of insurance, and patients receiving care from a critical care specialty.
METHODS
Study Design
We analyzed administrative data from a single academic hospital, the University of Colorado Hospital, a tertiary care, academic hospital with approximately 400 beds. The study population consisted of hospitalized adult patients (age 18 years) who died during hospitalization or were discharged to hospice in 2006 and 2007. We included both patients discharged to hospice and those who died during hospitalization, as we were seeking to identify a hospitalized patient population who might be expected to benefit from palliative care: those at high risk of death in the near future. Predictors were selected on the basis of clinical experience and the literature. Cost per day and length of stay were the outcome variables. Institutional Review Board (IRB) approval was not necessary because all of the study patients were deceased at the time of analysis.
Due to resource limitations, we were only able to gather clinical information (presence of organ failure [cardiac, respiratory, renal, hepatic, neurologic] or sepsis on admission, and presence or absence of palliative care consultation during hospitalization) from chart review in a subset of the sample population: those that had the highest 10% total hospitalization costs (n = 115). Organ failure was defined as chart documentation of any of the following: 1) cardiac: ST segment elevation myocardial infarction, non‐ST segment elevation myocardial infarction, congestive heart failure, heart failure (n = 28); 2) respiratory: respiratory failure (n = 36); 3) renal: acute kidney injury, acute renal failure, chronic renal failure, dialysis, end‐stage renal disease (n = 42); 4) hepatic: hepatic failure, end‐stage liver disease (n = 10); and 5) neurologic: altered mental status, delirium (n = 4). Sepsis was defined as chart documentation of any of the following: sepsis, severe sepsis, or septic shock.
Outcomes
We found total cost and length of stay to be correlated. Therefore, we used cost per day in lieu of total cost as the primary outcome. Length of stay was the secondary outcome. Using cost per day as the primary outcome reduced the correlation between our primary and secondary outcomes.
Predictors
Potential predictors (age, insurance status, and attending physician specialty) were selected on the basis of clinical experience, the literature, and patient variables available from the administrative data. We also considered diagnosis‐related group (DRG), however, the wide range of unique DRGs for this population did not allow for sensible groupings, so DRG was excluded from further analyses. For descriptive purposes, mean (standard deviation, SD) age was reported. For modeling, age was centered at 65 years, because this is the age of Medicare eligibility and thus a likely point at which insurance status would change. Sixty‐five was also close to the mean age of the full population, 62 years, therefore ensuring that interactions were assessed over the bulk of the data, rather than at outlying points. We also divided age into ten‐year increments for easier interpretation of model estimates. The relationship between age and primary and secondary outcomes differed among younger vs older patients. Therefore, age was included as a piecewise term in the final multivariate linear model which allowed a separate slope to be fit for patients age <65 years vs those 65 years.
Insurance status was dichotomized as insured vs uninsured. Attending physician specialty categories (internal medicine, pulmonary critical care, neurosurgery, surgical oncology, and cardiothoracic surgery) were selected because they were the five most common specialties. The remaining specialties were grouped together as other, which was used as a reference group in the multivariate analyses as it constituted a nontrivial proportion of the study population.
Statistical Analyses
Univariate analyses were performed separately for the primary and secondary outcomes. Univariate associations between the outcomes and categorical predictors were tested using analysis of variance (ANOVA) models with adjustment for multiple comparisons. Associations between the outcomes and the binary predictors were assessed with t‐tests. Predictors that were significant at the 0.10 level and considered clinically relevant were included in the multivariate model. Interaction terms between predictors were examined and included in the final multivariate piecewise linear models, when inclusion of the interaction terms altered the magnitude of the model estimates.
RESULTS
The study population comprised 1155 hospitalizations. Nine hospitalizations were excluded from analysis (five for organ donation, three were erroneousthe patients were not discharged to hospice or did not die during the hospitalization, and one was a pediatric patient), resulting in a study population of n = 1146 hospitalizations.
Table 1 depicts study population characteristics. The average patient age was 62 years (SD = 16), and 96% of patients were insured. The average length of stay was 10.7 days (SD = 14.1), with an average total cost per admission of $44,410 (SD = 76,355), as compared to an overall hospital admission (excluding obstetrics/neonatology) average length of stay of 5.7 days (SD = 8.5) and average total cost per admission of $17,410 (SD = 36,633) during the same time period. The average cost per day was $5095 (SD = $8546). About one‐third of patients were admitted to internal medicine, 20% to pulmonary critical care, and 18% to surgical specialties. The remaining 29% belonged to other specialties.
| Number of patients, n (%) | 1146 |
| Death in hospital | 730 (63.7) |
| Discharged with hospice | 416 (36.3) |
| Age (years), mean (SD) | 61.7 (15.9) |
| Insurance, n (%) | |
| Uninsured | 52 (4.5) |
| Insured | 1,094 (95.5) |
| Length of stay (days), mean (SD) | 10.7 (14.1) |
| Total cost, mean (SD) | $44,410 (76,355) |
| Cost per day, mean (SD) | $5,095 (8,546) |
| Attending MD specialty, n (%) | |
| Cardiothoracic Surgery | 56 (4.9) |
| Pulmonary Critical Care | 230 (20.1) |
| Surgical Oncology | 70 (6.1) |
| Internal Medicine | 383 (33.4) |
| Neurosurgery | 77 (6.7) |
| Other | 330 (28.8) |
Univariate Analyses
Overall, younger patients had a higher cost per day (Pearson 0.09; P = 0.02) and longer length of stay (Pearson 0.15; P < 0.0001) than older patients (data not shown). According to age groups defined by quartiles, patients who were age <51 and between 61‐72 years had significantly higher cost per day than patients age 73 years ($5787 and $5826 vs $3649, respectively; ANOVA P = 0.005; pairwise P < 0.05). The length of stay for the age groups under 73 years of age were significantly longer than for the patients who were 73 years of age and older (11.9, 11.9, and 11.2 vs 8.0 days, respectively; ANOVA P = 0.001; pairwise P < 0.05; Table 2). Uninsured patients had a higher cost per day ($6618 vs $5023; P = 0.02) than insured patients. In pairwise comparisons, patients on the cardiothoracic surgery service had a higher cost per day ($17,942) than any other specialty (ANOVA P < 0.0001; pairwise P < 0.05). Neurosurgery patients had a higher cost per day ($7089) than the internal medicine patients ($3173; pairwise P < 0.05). Cardiothoracic surgery patients also had a significantly higher LOS (18.3 days) than internal medicine (8.0 days), critical care (11.6 days), neurosurgery (10.0 days), and the other (10.9 days) specialties (ANOVA P < 0.0001; pairwise P < 0.05). The LOS for internal medicine (8.0 days) was significantly lower than critical care (11.6 days), surgical oncology (15.9 days), and cardiothoracic surgery (18.3 days; pairwise P < 0.05).
| Variable | N | Cost per day [$] (mean [SD]) | P Value | Length of stay [days] (mean [SD]) | P Value |
|---|---|---|---|---|---|
| |||||
| 1146 | |||||
| Age group, quartiles | |||||
| <51 years | 281 | 5,787 (8,008) | 0.005 | 11.9 (16.4) | 0.001 |
| 51‐60 | 264 | 5,202 (7,643) | 11.9 (15.4) | ||
| 61‐72 | 297 | 5,826 (12,272) | 11.2 (14.1) | ||
| 73 | 304 | 3,649 (3,978) | 8.0 (9.7) | ||
| Insurance | |||||
| Insured | 1094 | 5,023 (8,691) | 0.02 | 10.8 (14.2) | 0.23 |
| Uninsured | 52 | 6,618 (4,297) | 8.4 (13.5) | ||
| Attending MD specialty | |||||
| Internal Medicine | 383 | 3,173 (2,647) | <0.0001* | 8.0 (11.0) | <0.0001 |
| Pulmonary Critical Care | 230 | 4,671 (2,734) | 11.6 (14.3) | ||
| Neurosurgery | 77 | 7,089 (6,103) | 10.0 (13.5) | ||
| Surgical Oncology | 70 | 5,768 (3,521) | 15.9 (17.9) | ||
| Cardiothoracic Surgery | 56 | 17,942 (26,943) | 18.3 (23.6) | ||
| Other | 330 | 4,833 (8,641) | 10.9 (13.6) | ||
Multivariate Analyses
Cost per Day
The final multivariate linear model included age and attending physician specialty. Insurance status was excluded because it lost significant association with cost per day when it was added to the model (Table 3). Compared to the other specialty, internal medicine decreased cost per day by $1531 (P = 0.01), neurosurgery increased cost per day by $2255 (P = 0.03), and cardiothoracic surgery increased cost per day by $12,937 (P < 0.0001). Cost per day decreased by $811 (SE = 349; P = 0.02) for each age decade 65 years, however, no effect was observed on cost per day for those younger than 65 years.
| Predictors | Estimated Effect ($) | 95% Confidence Interval | P Value to Test if = 0 |
|---|---|---|---|
| Other specialty (reference group at 65 yr) | 5209 | (4,133, 6,284) | |
| Internal Medicine | 1,531 | (2,709, 353) | 0.01 |
| Pulmonary Critical Care | 217 | (1,562, 1,128) | 0.75 |
| Neurosurgery | 2255 | (278, 4,232) | 0.03 |
| Surgical Oncology | 1064 | (994, 3,122) | 0.31 |
| Cardiothoracic Surgery | 12937 | (10,676, 15,198) | <0.0001 |
| Age per 10 yr/age <65 | 7 | (506, 519) | 0.98 |
| Age per 10 yr/age 65 | 811 | (1,497, 125) | 0.02 |
Length of Stay
Because age and attending physician specialty had a significant effect on length of stay, multivariate analyses were performed with these two predictor variables (Table 4). Compared to the other specialty, internal medicine decreased length of stay by 2.4 days (P = 0.02), surgical oncology increased LOS by 5.3 days (P = 0.003), and cardiothoracic surgery increased length of stay by 6.9 days (P = 0.001). Length of stay was significantly decreased by 1.8 days (SE = 0.61; P = 0.003) for each age decade 65 years.
| Predictors | Estimated Effect (days) | 95% Confidence Interval | P Value to Test if = 0 |
|---|---|---|---|
| Other specialty (reference group at 65 yr) | 11 | (9.1, 12.9) | |
| Internal Medicine | 2.4 | (4.4, 0.3) | 0.02 |
| Pulmonary Critical Care | 0.5 | (1.9, 2.8) | 0.7 |
| Neurosurgery | 0.9 | (4.3, 2.6) | 0.62 |
| Surgical Oncology | 5.3 | (1.8, 8.9) | 0.003 |
| Cardiothoracic Surgery | 6.9 | (3.0, 10.9) | 0.001 |
| Age per 10 yr/age <65 | 0.8 | (1.7, 0.1) | 0.08 |
| Age per 10 yr/age 65 | 1.8 | (3.0, 0.6) | 0.003 |
DISCUSSION
We found several characteristics that were significantly associated with higher cost per day or longer length of stay in patients who died during hospitalization or were discharged to hospice. Among this patient population, the surgical specialty services had overall higher cost per day and length of stay than other services. Patients cared for on the cardiothoracic surgery service had higher cost per day and length of stay; in contrast, internal medicine patients had lower cost per day and length of stay. Neurosurgery patients had higher cost per day, while surgical oncology patients had higher length of stay. Patients age 65 years and older had a significantly lower cost per day and shorter length of stay than those less than 65 years of age.
Higher cost per day for cardiothoracic surgery and neurosurgery patients may partially be explained by cardiothoracic surgery patients' usage of clinical services, including operating room services, which are higher in costs compared with those of nonsurgical specialties. Some patients may require repeat surgeries in the same hospitalization which further increases the cost per day. Longer length of stay in surgical oncology patients may be related to complex surgeries and possible postoperative complications that may take longer to recover from than standard surgeries.
Our findings that older patients have lower cost per day and shorter length of stay are corroborated by other studies. Lubitz and Riley21 found that in 1976 and 1988, Medicare payments per person year decreased with age. Levinsky et al.22 had similar findings in a review of Medicare data in 2001, but noted smaller reductions in total costabout $400 decrease for each year above 65. Their explanation of the lower cost is that older patients receive less aggressive care. Physicians, as well as patients and families, may continue to pursue expensive, invasive therapies for terminally ill patients who are younger for a longer period of time than with older patients, which would increase cost per day as well as length of stay.
The finding that patients on the surgical specialty services may be a focus for active palliative care intervention has many implications. The American College of Surgeons Surgical Palliative Care Task Force consensus guideline triggers for a palliative care consultation in SICU applied clinically did not result in a change in palliative care consultation rate.23 The use of triggers for palliative care consultation may be an ineffective approach because knowledge and application of the triggers did not change behavior. Focusing on integrating palliative care interventions or consultation for all high‐risk surgical patients, as opposed to relying upon triggers, may be a more effective approach to meeting these patients' palliative care needs while lowering cost per day and length of stay and warrants further study. For instance, palliative care consult teams may consider routine or daily rounds with the surgical specialty services in order to effectively integrate palliative care for these patients. Such an integrative approach may foster familiarity and comfort with palliative care approaches, facilitating access to palliative care services for those patients with palliative needs.
Our study is limited in that it is a retrospective, single‐center study. Our results may not be applicable to the general population. The experience of additional centers analyzed prospectively would provide additional context. The available administrative data limited the analyses to only a small number of predictors. In the subset population with the highest 10% total hospitalization costs, from which clinical information was gathered, the presence of respiratory failure was associated with shorter LOS (33 days vs 42 days; P = 0.03), but not associated with cost per day. Having sepsis at admission was associated with lower cost per day ($5783 vs $10,071; P = 0.04); however, this finding was based on only four patients with sepsis at admission. Patients who were evaluated by the palliative care service (n = 35) had a significantly lower cost per day ($4896 vs $12,210; P = 0.01) but longer LOS (46.5 vs 35.7 days; P = 0.03) than those who were not. These, and other, clinical characteristics need further testing in larger samples. An additional limitation is that we combined hospital decedents with patients discharged to hospice as our study population. These groups were combined since they are both at high risk of death in the near future; the median hospice length of stay in Colorado is 20 days.24 There may exist important differences in these populations that are not accounted for in our findings. Despite these unidentified differences, both populations are at high risk of death in the near future, making it likely that they would benefit from palliative care. Those who died during hospitalization did have a longer LOS (11.5 vs 9.2 days; P = 0.003) and higher cost per day ($6734 vs $2221; P < 0.0001) than those who were discharged to hospice.
Palliative care consultations can lead to improved quality of care for patients and families by addressing suffering and addressing quality of life measures (2, 4, 5, 6). We sought to identify characteristics associated with high cost and prolonged hospitalizations in patients who died during hospitalization, or were discharged to hospice, in order to inform targeting of palliative care services. Our data suggest that younger patients and those cared for by surgical specialty services may have the most palliative needs. Palliative care teams may consider focusing efforts at integrating palliative care with surgical specialty services to address these needs. These findings need to be corroborated in other centers, and include clinical outcomes.
- ,,, et al.Family perspectives on end‐of‐life care at the last place of care.JAMA.2004;291:88–93.
- ,.Do specialist palliative care teams improve outcomes for cancer patients? A systematic literature review.Palliat Med.1998;12:317–332.
- ,,, et al.Evidence‐based interventions to improve the palliative care of pain, dyspnea, and depression at the end of life: a clinical practice guideline from the American College of Physicians.Ann Intern Med.2008;148:141–146.
- ,,, et al.Do palliative consultations improve patient outcomes?JAGS.2008;56:593–599.
- ,,, et al.Effects of a palliative cafe intervention on clinical outcomes in patients with advanced cancer.JAMA.2009;302:741–749.
- ,,, et al.Early palliative care for patients with metastatic non‐small‐cell lung cancer.N Engl J Med.2010;363:733–742.
- ,,, et al.Cost savings associated with US hospital palliative care consultation programs.Arch Intern Med.2008;168(16):1783–1790.
- ,,.Impact of palliative care case management on resource use by patients dying of cancer at a Veterans Affairs Medical Center.J Palliat Med.2005;8(1):26–35.
- ,,, et al.Cost and utilization outcomes of patients receiving hospital‐based palliative care consultation.J Palliat Med.2006;9(4):855–860.
- ,,, et al.A high‐volume specialist palliative care unit and team may reduce in‐hospital end‐of‐life care costs.J Palliat Med.2003;6(5):699–705.
- ,,, et al.The economic and clinical impact of an inpatient palliative care consultation service: a multifaceted approach.J Palliat Med.2007;10(6):1347–1355.
- ,,, et al.Hospital‐based palliative care consultation: effects on hospital cost.J Palliat Med.2010;13(8):1–7.
- ,.Impact of a proactive approach to improve end‐of‐life care in a medical ICU.Chest.2003;123:266–271.
- ,,, et al.Proactive palliative care in the medical intensive care unit: effects on length of stay for selected high‐risk patients.Crit Care Med.2007;35:1530–1535.
- ,,,,.Who needs a palliative care consult? The Hamilton Chart Audit tool.J Palliat Med.2007;10(2):304–307.
- ,,,,,.A practical tool to identify patients who may benefit from a palliative care approach: the CARING criteria.J Pain Symptom Manage.2006;31:285–292.
- ,,, et al.An Official American Thoracic Society Clinical Policy Statement: palliative care for patients with respiratory diseases and critical illnesses.Am J Respir Crit Care Med.2008;177:912–927.
- ,,, et al.Proposed quality measures for palliative care in the critically ill: a consensus from the Robert Wood Johnson Foundation Critical Care Workgroup.Crit Care Med.2006;34:S404–S411.
- ,,, et al.Recommendations for end‐of‐life care in the intensive care unit: a consensus statement by the American Academy of Critical Care Medicine.Crit Care Med.2008;36:953–963.
- ,.Developing guidelines that identify patients who would benefit from palliative care services in the surgical intensive care unit.Crit Care Med.2009;37:946–950.
- ,.Trends in Medicare payments in the last year of life.N Engl J Med.1993;328:1092–1096.
- ,,, et al.Influence of age on Medicare expenditures and medical care in the last year of life.JAMA.2001;286:1349–1355.
- ,,.Addressing access to palliative care services in the surgical intensive care unit.Surgery.2010;147:871–877.
- http://www.coloradocancercoalition.org/…/CCCConferenceKassner Slides11.13.07.ppt. Accessed August 16,2010.
- ,,.The impact of implementing referral criteria on the patterns of referrals and admissions to a palliative care program in Saudi Arabia.J Support Oncol.2010;8:78–81.
Patients with advanced illness frequently do not receive care that meets their physical and emotional needs at the end of life,1 despite significant expenditures. Palliative care has been recommended as an approach to improve the quality of care for patients with advanced illness,26 while achieving hospital cost savings.7 Studies show that palliative care consults are associated with decreased hospitalization cost712 and length of stay13, 14 in the acute care setting.
Identifying which hospitalized patients are likely to benefit most from palliative care has not been well defined. The Hamilton Chart Audit tool was developed to estimate the number of patients that would benefit from a palliative care consult, in order to determine hospital palliative care staffing and financial needs.15 The CARING criteria identifies patients on admission to the hospital who are at high risk of death within one year and may, therefore, benefit from palliative care.16 The literature from the medical intensive care unit (MICU) identifies palliative care core competencies and quality measures, but does not describe patient factors that should trigger a palliative care consult.1719 Norton et al. studied proactive palliative care consultation in the MICU, finding that palliative care consultation in the high‐risk group (serious illness and high risk of dying) was associated with a shorter MICU length of stay without a significant difference in mortality rates.14
The most specific triggers for a palliative care consult comes from the surgical intensive care guidelines. The American College of Surgeons Surgical Palliative Care Task Force published a consensus guideline based on expert opinion identifying the top ten triggers for a palliative care consultation in the surgical intensive care unit (SICU).20 The top 10 criteria to identify SICU patients for palliative care consultation listed in order of priority were: 1) family request; 2) futility considered or declared by the medical team; 3) family disagreement with the team, advance directive, or each other lasting greater than seven days; 4) death expected during the same SICU stay; 5) SICU stay of greater than one month; 6) diagnosis with a median survival of less than six months; 7) greater than three SICU admissions during the same hospitalization; 8) Glasgow Coma Score of less than eight for greater than one week in a patient greater than 75 years old; 9) Glasgow Outcome Score of less than three (i.e., persistent vegetative state); and 10) multisystem organ failure of greater than three systems.
Studies are lacking that identify hospitalized patients who are more likely to have higher cost per day or length of stay, as these are patients who may benefit from palliative care. We sought to identify patient characteristics that are associated with higher cost per day or longer length of stay in hospitalized patients who died during the hospitalization or were discharged to hospicepatients likely to benefit from targeted palliative care services. We hypothesized that hospitalized patients with the following characteristics who died during the hospitalization or were discharged to hospice would have a higher cost per day or longer length of stay: older patients, lack of insurance, and patients receiving care from a critical care specialty.
METHODS
Study Design
We analyzed administrative data from a single academic hospital, the University of Colorado Hospital, a tertiary care, academic hospital with approximately 400 beds. The study population consisted of hospitalized adult patients (age 18 years) who died during hospitalization or were discharged to hospice in 2006 and 2007. We included both patients discharged to hospice and those who died during hospitalization, as we were seeking to identify a hospitalized patient population who might be expected to benefit from palliative care: those at high risk of death in the near future. Predictors were selected on the basis of clinical experience and the literature. Cost per day and length of stay were the outcome variables. Institutional Review Board (IRB) approval was not necessary because all of the study patients were deceased at the time of analysis.
Due to resource limitations, we were only able to gather clinical information (presence of organ failure [cardiac, respiratory, renal, hepatic, neurologic] or sepsis on admission, and presence or absence of palliative care consultation during hospitalization) from chart review in a subset of the sample population: those that had the highest 10% total hospitalization costs (n = 115). Organ failure was defined as chart documentation of any of the following: 1) cardiac: ST segment elevation myocardial infarction, non‐ST segment elevation myocardial infarction, congestive heart failure, heart failure (n = 28); 2) respiratory: respiratory failure (n = 36); 3) renal: acute kidney injury, acute renal failure, chronic renal failure, dialysis, end‐stage renal disease (n = 42); 4) hepatic: hepatic failure, end‐stage liver disease (n = 10); and 5) neurologic: altered mental status, delirium (n = 4). Sepsis was defined as chart documentation of any of the following: sepsis, severe sepsis, or septic shock.
Outcomes
We found total cost and length of stay to be correlated. Therefore, we used cost per day in lieu of total cost as the primary outcome. Length of stay was the secondary outcome. Using cost per day as the primary outcome reduced the correlation between our primary and secondary outcomes.
Predictors
Potential predictors (age, insurance status, and attending physician specialty) were selected on the basis of clinical experience, the literature, and patient variables available from the administrative data. We also considered diagnosis‐related group (DRG), however, the wide range of unique DRGs for this population did not allow for sensible groupings, so DRG was excluded from further analyses. For descriptive purposes, mean (standard deviation, SD) age was reported. For modeling, age was centered at 65 years, because this is the age of Medicare eligibility and thus a likely point at which insurance status would change. Sixty‐five was also close to the mean age of the full population, 62 years, therefore ensuring that interactions were assessed over the bulk of the data, rather than at outlying points. We also divided age into ten‐year increments for easier interpretation of model estimates. The relationship between age and primary and secondary outcomes differed among younger vs older patients. Therefore, age was included as a piecewise term in the final multivariate linear model which allowed a separate slope to be fit for patients age <65 years vs those 65 years.
Insurance status was dichotomized as insured vs uninsured. Attending physician specialty categories (internal medicine, pulmonary critical care, neurosurgery, surgical oncology, and cardiothoracic surgery) were selected because they were the five most common specialties. The remaining specialties were grouped together as other, which was used as a reference group in the multivariate analyses as it constituted a nontrivial proportion of the study population.
Statistical Analyses
Univariate analyses were performed separately for the primary and secondary outcomes. Univariate associations between the outcomes and categorical predictors were tested using analysis of variance (ANOVA) models with adjustment for multiple comparisons. Associations between the outcomes and the binary predictors were assessed with t‐tests. Predictors that were significant at the 0.10 level and considered clinically relevant were included in the multivariate model. Interaction terms between predictors were examined and included in the final multivariate piecewise linear models, when inclusion of the interaction terms altered the magnitude of the model estimates.
RESULTS
The study population comprised 1155 hospitalizations. Nine hospitalizations were excluded from analysis (five for organ donation, three were erroneousthe patients were not discharged to hospice or did not die during the hospitalization, and one was a pediatric patient), resulting in a study population of n = 1146 hospitalizations.
Table 1 depicts study population characteristics. The average patient age was 62 years (SD = 16), and 96% of patients were insured. The average length of stay was 10.7 days (SD = 14.1), with an average total cost per admission of $44,410 (SD = 76,355), as compared to an overall hospital admission (excluding obstetrics/neonatology) average length of stay of 5.7 days (SD = 8.5) and average total cost per admission of $17,410 (SD = 36,633) during the same time period. The average cost per day was $5095 (SD = $8546). About one‐third of patients were admitted to internal medicine, 20% to pulmonary critical care, and 18% to surgical specialties. The remaining 29% belonged to other specialties.
| Number of patients, n (%) | 1146 |
| Death in hospital | 730 (63.7) |
| Discharged with hospice | 416 (36.3) |
| Age (years), mean (SD) | 61.7 (15.9) |
| Insurance, n (%) | |
| Uninsured | 52 (4.5) |
| Insured | 1,094 (95.5) |
| Length of stay (days), mean (SD) | 10.7 (14.1) |
| Total cost, mean (SD) | $44,410 (76,355) |
| Cost per day, mean (SD) | $5,095 (8,546) |
| Attending MD specialty, n (%) | |
| Cardiothoracic Surgery | 56 (4.9) |
| Pulmonary Critical Care | 230 (20.1) |
| Surgical Oncology | 70 (6.1) |
| Internal Medicine | 383 (33.4) |
| Neurosurgery | 77 (6.7) |
| Other | 330 (28.8) |
Univariate Analyses
Overall, younger patients had a higher cost per day (Pearson 0.09; P = 0.02) and longer length of stay (Pearson 0.15; P < 0.0001) than older patients (data not shown). According to age groups defined by quartiles, patients who were age <51 and between 61‐72 years had significantly higher cost per day than patients age 73 years ($5787 and $5826 vs $3649, respectively; ANOVA P = 0.005; pairwise P < 0.05). The length of stay for the age groups under 73 years of age were significantly longer than for the patients who were 73 years of age and older (11.9, 11.9, and 11.2 vs 8.0 days, respectively; ANOVA P = 0.001; pairwise P < 0.05; Table 2). Uninsured patients had a higher cost per day ($6618 vs $5023; P = 0.02) than insured patients. In pairwise comparisons, patients on the cardiothoracic surgery service had a higher cost per day ($17,942) than any other specialty (ANOVA P < 0.0001; pairwise P < 0.05). Neurosurgery patients had a higher cost per day ($7089) than the internal medicine patients ($3173; pairwise P < 0.05). Cardiothoracic surgery patients also had a significantly higher LOS (18.3 days) than internal medicine (8.0 days), critical care (11.6 days), neurosurgery (10.0 days), and the other (10.9 days) specialties (ANOVA P < 0.0001; pairwise P < 0.05). The LOS for internal medicine (8.0 days) was significantly lower than critical care (11.6 days), surgical oncology (15.9 days), and cardiothoracic surgery (18.3 days; pairwise P < 0.05).
| Variable | N | Cost per day [$] (mean [SD]) | P Value | Length of stay [days] (mean [SD]) | P Value |
|---|---|---|---|---|---|
| |||||
| 1146 | |||||
| Age group, quartiles | |||||
| <51 years | 281 | 5,787 (8,008) | 0.005 | 11.9 (16.4) | 0.001 |
| 51‐60 | 264 | 5,202 (7,643) | 11.9 (15.4) | ||
| 61‐72 | 297 | 5,826 (12,272) | 11.2 (14.1) | ||
| 73 | 304 | 3,649 (3,978) | 8.0 (9.7) | ||
| Insurance | |||||
| Insured | 1094 | 5,023 (8,691) | 0.02 | 10.8 (14.2) | 0.23 |
| Uninsured | 52 | 6,618 (4,297) | 8.4 (13.5) | ||
| Attending MD specialty | |||||
| Internal Medicine | 383 | 3,173 (2,647) | <0.0001* | 8.0 (11.0) | <0.0001 |
| Pulmonary Critical Care | 230 | 4,671 (2,734) | 11.6 (14.3) | ||
| Neurosurgery | 77 | 7,089 (6,103) | 10.0 (13.5) | ||
| Surgical Oncology | 70 | 5,768 (3,521) | 15.9 (17.9) | ||
| Cardiothoracic Surgery | 56 | 17,942 (26,943) | 18.3 (23.6) | ||
| Other | 330 | 4,833 (8,641) | 10.9 (13.6) | ||
Multivariate Analyses
Cost per Day
The final multivariate linear model included age and attending physician specialty. Insurance status was excluded because it lost significant association with cost per day when it was added to the model (Table 3). Compared to the other specialty, internal medicine decreased cost per day by $1531 (P = 0.01), neurosurgery increased cost per day by $2255 (P = 0.03), and cardiothoracic surgery increased cost per day by $12,937 (P < 0.0001). Cost per day decreased by $811 (SE = 349; P = 0.02) for each age decade 65 years, however, no effect was observed on cost per day for those younger than 65 years.
| Predictors | Estimated Effect ($) | 95% Confidence Interval | P Value to Test if = 0 |
|---|---|---|---|
| Other specialty (reference group at 65 yr) | 5209 | (4,133, 6,284) | |
| Internal Medicine | 1,531 | (2,709, 353) | 0.01 |
| Pulmonary Critical Care | 217 | (1,562, 1,128) | 0.75 |
| Neurosurgery | 2255 | (278, 4,232) | 0.03 |
| Surgical Oncology | 1064 | (994, 3,122) | 0.31 |
| Cardiothoracic Surgery | 12937 | (10,676, 15,198) | <0.0001 |
| Age per 10 yr/age <65 | 7 | (506, 519) | 0.98 |
| Age per 10 yr/age 65 | 811 | (1,497, 125) | 0.02 |
Length of Stay
Because age and attending physician specialty had a significant effect on length of stay, multivariate analyses were performed with these two predictor variables (Table 4). Compared to the other specialty, internal medicine decreased length of stay by 2.4 days (P = 0.02), surgical oncology increased LOS by 5.3 days (P = 0.003), and cardiothoracic surgery increased length of stay by 6.9 days (P = 0.001). Length of stay was significantly decreased by 1.8 days (SE = 0.61; P = 0.003) for each age decade 65 years.
| Predictors | Estimated Effect (days) | 95% Confidence Interval | P Value to Test if = 0 |
|---|---|---|---|
| Other specialty (reference group at 65 yr) | 11 | (9.1, 12.9) | |
| Internal Medicine | 2.4 | (4.4, 0.3) | 0.02 |
| Pulmonary Critical Care | 0.5 | (1.9, 2.8) | 0.7 |
| Neurosurgery | 0.9 | (4.3, 2.6) | 0.62 |
| Surgical Oncology | 5.3 | (1.8, 8.9) | 0.003 |
| Cardiothoracic Surgery | 6.9 | (3.0, 10.9) | 0.001 |
| Age per 10 yr/age <65 | 0.8 | (1.7, 0.1) | 0.08 |
| Age per 10 yr/age 65 | 1.8 | (3.0, 0.6) | 0.003 |
DISCUSSION
We found several characteristics that were significantly associated with higher cost per day or longer length of stay in patients who died during hospitalization or were discharged to hospice. Among this patient population, the surgical specialty services had overall higher cost per day and length of stay than other services. Patients cared for on the cardiothoracic surgery service had higher cost per day and length of stay; in contrast, internal medicine patients had lower cost per day and length of stay. Neurosurgery patients had higher cost per day, while surgical oncology patients had higher length of stay. Patients age 65 years and older had a significantly lower cost per day and shorter length of stay than those less than 65 years of age.
Higher cost per day for cardiothoracic surgery and neurosurgery patients may partially be explained by cardiothoracic surgery patients' usage of clinical services, including operating room services, which are higher in costs compared with those of nonsurgical specialties. Some patients may require repeat surgeries in the same hospitalization which further increases the cost per day. Longer length of stay in surgical oncology patients may be related to complex surgeries and possible postoperative complications that may take longer to recover from than standard surgeries.
Our findings that older patients have lower cost per day and shorter length of stay are corroborated by other studies. Lubitz and Riley21 found that in 1976 and 1988, Medicare payments per person year decreased with age. Levinsky et al.22 had similar findings in a review of Medicare data in 2001, but noted smaller reductions in total costabout $400 decrease for each year above 65. Their explanation of the lower cost is that older patients receive less aggressive care. Physicians, as well as patients and families, may continue to pursue expensive, invasive therapies for terminally ill patients who are younger for a longer period of time than with older patients, which would increase cost per day as well as length of stay.
The finding that patients on the surgical specialty services may be a focus for active palliative care intervention has many implications. The American College of Surgeons Surgical Palliative Care Task Force consensus guideline triggers for a palliative care consultation in SICU applied clinically did not result in a change in palliative care consultation rate.23 The use of triggers for palliative care consultation may be an ineffective approach because knowledge and application of the triggers did not change behavior. Focusing on integrating palliative care interventions or consultation for all high‐risk surgical patients, as opposed to relying upon triggers, may be a more effective approach to meeting these patients' palliative care needs while lowering cost per day and length of stay and warrants further study. For instance, palliative care consult teams may consider routine or daily rounds with the surgical specialty services in order to effectively integrate palliative care for these patients. Such an integrative approach may foster familiarity and comfort with palliative care approaches, facilitating access to palliative care services for those patients with palliative needs.
Our study is limited in that it is a retrospective, single‐center study. Our results may not be applicable to the general population. The experience of additional centers analyzed prospectively would provide additional context. The available administrative data limited the analyses to only a small number of predictors. In the subset population with the highest 10% total hospitalization costs, from which clinical information was gathered, the presence of respiratory failure was associated with shorter LOS (33 days vs 42 days; P = 0.03), but not associated with cost per day. Having sepsis at admission was associated with lower cost per day ($5783 vs $10,071; P = 0.04); however, this finding was based on only four patients with sepsis at admission. Patients who were evaluated by the palliative care service (n = 35) had a significantly lower cost per day ($4896 vs $12,210; P = 0.01) but longer LOS (46.5 vs 35.7 days; P = 0.03) than those who were not. These, and other, clinical characteristics need further testing in larger samples. An additional limitation is that we combined hospital decedents with patients discharged to hospice as our study population. These groups were combined since they are both at high risk of death in the near future; the median hospice length of stay in Colorado is 20 days.24 There may exist important differences in these populations that are not accounted for in our findings. Despite these unidentified differences, both populations are at high risk of death in the near future, making it likely that they would benefit from palliative care. Those who died during hospitalization did have a longer LOS (11.5 vs 9.2 days; P = 0.003) and higher cost per day ($6734 vs $2221; P < 0.0001) than those who were discharged to hospice.
Palliative care consultations can lead to improved quality of care for patients and families by addressing suffering and addressing quality of life measures (2, 4, 5, 6). We sought to identify characteristics associated with high cost and prolonged hospitalizations in patients who died during hospitalization, or were discharged to hospice, in order to inform targeting of palliative care services. Our data suggest that younger patients and those cared for by surgical specialty services may have the most palliative needs. Palliative care teams may consider focusing efforts at integrating palliative care with surgical specialty services to address these needs. These findings need to be corroborated in other centers, and include clinical outcomes.
Patients with advanced illness frequently do not receive care that meets their physical and emotional needs at the end of life,1 despite significant expenditures. Palliative care has been recommended as an approach to improve the quality of care for patients with advanced illness,26 while achieving hospital cost savings.7 Studies show that palliative care consults are associated with decreased hospitalization cost712 and length of stay13, 14 in the acute care setting.
Identifying which hospitalized patients are likely to benefit most from palliative care has not been well defined. The Hamilton Chart Audit tool was developed to estimate the number of patients that would benefit from a palliative care consult, in order to determine hospital palliative care staffing and financial needs.15 The CARING criteria identifies patients on admission to the hospital who are at high risk of death within one year and may, therefore, benefit from palliative care.16 The literature from the medical intensive care unit (MICU) identifies palliative care core competencies and quality measures, but does not describe patient factors that should trigger a palliative care consult.1719 Norton et al. studied proactive palliative care consultation in the MICU, finding that palliative care consultation in the high‐risk group (serious illness and high risk of dying) was associated with a shorter MICU length of stay without a significant difference in mortality rates.14
The most specific triggers for a palliative care consult comes from the surgical intensive care guidelines. The American College of Surgeons Surgical Palliative Care Task Force published a consensus guideline based on expert opinion identifying the top ten triggers for a palliative care consultation in the surgical intensive care unit (SICU).20 The top 10 criteria to identify SICU patients for palliative care consultation listed in order of priority were: 1) family request; 2) futility considered or declared by the medical team; 3) family disagreement with the team, advance directive, or each other lasting greater than seven days; 4) death expected during the same SICU stay; 5) SICU stay of greater than one month; 6) diagnosis with a median survival of less than six months; 7) greater than three SICU admissions during the same hospitalization; 8) Glasgow Coma Score of less than eight for greater than one week in a patient greater than 75 years old; 9) Glasgow Outcome Score of less than three (i.e., persistent vegetative state); and 10) multisystem organ failure of greater than three systems.
Studies are lacking that identify hospitalized patients who are more likely to have higher cost per day or length of stay, as these are patients who may benefit from palliative care. We sought to identify patient characteristics that are associated with higher cost per day or longer length of stay in hospitalized patients who died during the hospitalization or were discharged to hospicepatients likely to benefit from targeted palliative care services. We hypothesized that hospitalized patients with the following characteristics who died during the hospitalization or were discharged to hospice would have a higher cost per day or longer length of stay: older patients, lack of insurance, and patients receiving care from a critical care specialty.
METHODS
Study Design
We analyzed administrative data from a single academic hospital, the University of Colorado Hospital, a tertiary care, academic hospital with approximately 400 beds. The study population consisted of hospitalized adult patients (age 18 years) who died during hospitalization or were discharged to hospice in 2006 and 2007. We included both patients discharged to hospice and those who died during hospitalization, as we were seeking to identify a hospitalized patient population who might be expected to benefit from palliative care: those at high risk of death in the near future. Predictors were selected on the basis of clinical experience and the literature. Cost per day and length of stay were the outcome variables. Institutional Review Board (IRB) approval was not necessary because all of the study patients were deceased at the time of analysis.
Due to resource limitations, we were only able to gather clinical information (presence of organ failure [cardiac, respiratory, renal, hepatic, neurologic] or sepsis on admission, and presence or absence of palliative care consultation during hospitalization) from chart review in a subset of the sample population: those that had the highest 10% total hospitalization costs (n = 115). Organ failure was defined as chart documentation of any of the following: 1) cardiac: ST segment elevation myocardial infarction, non‐ST segment elevation myocardial infarction, congestive heart failure, heart failure (n = 28); 2) respiratory: respiratory failure (n = 36); 3) renal: acute kidney injury, acute renal failure, chronic renal failure, dialysis, end‐stage renal disease (n = 42); 4) hepatic: hepatic failure, end‐stage liver disease (n = 10); and 5) neurologic: altered mental status, delirium (n = 4). Sepsis was defined as chart documentation of any of the following: sepsis, severe sepsis, or septic shock.
Outcomes
We found total cost and length of stay to be correlated. Therefore, we used cost per day in lieu of total cost as the primary outcome. Length of stay was the secondary outcome. Using cost per day as the primary outcome reduced the correlation between our primary and secondary outcomes.
Predictors
Potential predictors (age, insurance status, and attending physician specialty) were selected on the basis of clinical experience, the literature, and patient variables available from the administrative data. We also considered diagnosis‐related group (DRG), however, the wide range of unique DRGs for this population did not allow for sensible groupings, so DRG was excluded from further analyses. For descriptive purposes, mean (standard deviation, SD) age was reported. For modeling, age was centered at 65 years, because this is the age of Medicare eligibility and thus a likely point at which insurance status would change. Sixty‐five was also close to the mean age of the full population, 62 years, therefore ensuring that interactions were assessed over the bulk of the data, rather than at outlying points. We also divided age into ten‐year increments for easier interpretation of model estimates. The relationship between age and primary and secondary outcomes differed among younger vs older patients. Therefore, age was included as a piecewise term in the final multivariate linear model which allowed a separate slope to be fit for patients age <65 years vs those 65 years.
Insurance status was dichotomized as insured vs uninsured. Attending physician specialty categories (internal medicine, pulmonary critical care, neurosurgery, surgical oncology, and cardiothoracic surgery) were selected because they were the five most common specialties. The remaining specialties were grouped together as other, which was used as a reference group in the multivariate analyses as it constituted a nontrivial proportion of the study population.
Statistical Analyses
Univariate analyses were performed separately for the primary and secondary outcomes. Univariate associations between the outcomes and categorical predictors were tested using analysis of variance (ANOVA) models with adjustment for multiple comparisons. Associations between the outcomes and the binary predictors were assessed with t‐tests. Predictors that were significant at the 0.10 level and considered clinically relevant were included in the multivariate model. Interaction terms between predictors were examined and included in the final multivariate piecewise linear models, when inclusion of the interaction terms altered the magnitude of the model estimates.
RESULTS
The study population comprised 1155 hospitalizations. Nine hospitalizations were excluded from analysis (five for organ donation, three were erroneousthe patients were not discharged to hospice or did not die during the hospitalization, and one was a pediatric patient), resulting in a study population of n = 1146 hospitalizations.
Table 1 depicts study population characteristics. The average patient age was 62 years (SD = 16), and 96% of patients were insured. The average length of stay was 10.7 days (SD = 14.1), with an average total cost per admission of $44,410 (SD = 76,355), as compared to an overall hospital admission (excluding obstetrics/neonatology) average length of stay of 5.7 days (SD = 8.5) and average total cost per admission of $17,410 (SD = 36,633) during the same time period. The average cost per day was $5095 (SD = $8546). About one‐third of patients were admitted to internal medicine, 20% to pulmonary critical care, and 18% to surgical specialties. The remaining 29% belonged to other specialties.
| Number of patients, n (%) | 1146 |
| Death in hospital | 730 (63.7) |
| Discharged with hospice | 416 (36.3) |
| Age (years), mean (SD) | 61.7 (15.9) |
| Insurance, n (%) | |
| Uninsured | 52 (4.5) |
| Insured | 1,094 (95.5) |
| Length of stay (days), mean (SD) | 10.7 (14.1) |
| Total cost, mean (SD) | $44,410 (76,355) |
| Cost per day, mean (SD) | $5,095 (8,546) |
| Attending MD specialty, n (%) | |
| Cardiothoracic Surgery | 56 (4.9) |
| Pulmonary Critical Care | 230 (20.1) |
| Surgical Oncology | 70 (6.1) |
| Internal Medicine | 383 (33.4) |
| Neurosurgery | 77 (6.7) |
| Other | 330 (28.8) |
Univariate Analyses
Overall, younger patients had a higher cost per day (Pearson 0.09; P = 0.02) and longer length of stay (Pearson 0.15; P < 0.0001) than older patients (data not shown). According to age groups defined by quartiles, patients who were age <51 and between 61‐72 years had significantly higher cost per day than patients age 73 years ($5787 and $5826 vs $3649, respectively; ANOVA P = 0.005; pairwise P < 0.05). The length of stay for the age groups under 73 years of age were significantly longer than for the patients who were 73 years of age and older (11.9, 11.9, and 11.2 vs 8.0 days, respectively; ANOVA P = 0.001; pairwise P < 0.05; Table 2). Uninsured patients had a higher cost per day ($6618 vs $5023; P = 0.02) than insured patients. In pairwise comparisons, patients on the cardiothoracic surgery service had a higher cost per day ($17,942) than any other specialty (ANOVA P < 0.0001; pairwise P < 0.05). Neurosurgery patients had a higher cost per day ($7089) than the internal medicine patients ($3173; pairwise P < 0.05). Cardiothoracic surgery patients also had a significantly higher LOS (18.3 days) than internal medicine (8.0 days), critical care (11.6 days), neurosurgery (10.0 days), and the other (10.9 days) specialties (ANOVA P < 0.0001; pairwise P < 0.05). The LOS for internal medicine (8.0 days) was significantly lower than critical care (11.6 days), surgical oncology (15.9 days), and cardiothoracic surgery (18.3 days; pairwise P < 0.05).
| Variable | N | Cost per day [$] (mean [SD]) | P Value | Length of stay [days] (mean [SD]) | P Value |
|---|---|---|---|---|---|
| |||||
| 1146 | |||||
| Age group, quartiles | |||||
| <51 years | 281 | 5,787 (8,008) | 0.005 | 11.9 (16.4) | 0.001 |
| 51‐60 | 264 | 5,202 (7,643) | 11.9 (15.4) | ||
| 61‐72 | 297 | 5,826 (12,272) | 11.2 (14.1) | ||
| 73 | 304 | 3,649 (3,978) | 8.0 (9.7) | ||
| Insurance | |||||
| Insured | 1094 | 5,023 (8,691) | 0.02 | 10.8 (14.2) | 0.23 |
| Uninsured | 52 | 6,618 (4,297) | 8.4 (13.5) | ||
| Attending MD specialty | |||||
| Internal Medicine | 383 | 3,173 (2,647) | <0.0001* | 8.0 (11.0) | <0.0001 |
| Pulmonary Critical Care | 230 | 4,671 (2,734) | 11.6 (14.3) | ||
| Neurosurgery | 77 | 7,089 (6,103) | 10.0 (13.5) | ||
| Surgical Oncology | 70 | 5,768 (3,521) | 15.9 (17.9) | ||
| Cardiothoracic Surgery | 56 | 17,942 (26,943) | 18.3 (23.6) | ||
| Other | 330 | 4,833 (8,641) | 10.9 (13.6) | ||
Multivariate Analyses
Cost per Day
The final multivariate linear model included age and attending physician specialty. Insurance status was excluded because it lost significant association with cost per day when it was added to the model (Table 3). Compared to the other specialty, internal medicine decreased cost per day by $1531 (P = 0.01), neurosurgery increased cost per day by $2255 (P = 0.03), and cardiothoracic surgery increased cost per day by $12,937 (P < 0.0001). Cost per day decreased by $811 (SE = 349; P = 0.02) for each age decade 65 years, however, no effect was observed on cost per day for those younger than 65 years.
| Predictors | Estimated Effect ($) | 95% Confidence Interval | P Value to Test if = 0 |
|---|---|---|---|
| Other specialty (reference group at 65 yr) | 5209 | (4,133, 6,284) | |
| Internal Medicine | 1,531 | (2,709, 353) | 0.01 |
| Pulmonary Critical Care | 217 | (1,562, 1,128) | 0.75 |
| Neurosurgery | 2255 | (278, 4,232) | 0.03 |
| Surgical Oncology | 1064 | (994, 3,122) | 0.31 |
| Cardiothoracic Surgery | 12937 | (10,676, 15,198) | <0.0001 |
| Age per 10 yr/age <65 | 7 | (506, 519) | 0.98 |
| Age per 10 yr/age 65 | 811 | (1,497, 125) | 0.02 |
Length of Stay
Because age and attending physician specialty had a significant effect on length of stay, multivariate analyses were performed with these two predictor variables (Table 4). Compared to the other specialty, internal medicine decreased length of stay by 2.4 days (P = 0.02), surgical oncology increased LOS by 5.3 days (P = 0.003), and cardiothoracic surgery increased length of stay by 6.9 days (P = 0.001). Length of stay was significantly decreased by 1.8 days (SE = 0.61; P = 0.003) for each age decade 65 years.
| Predictors | Estimated Effect (days) | 95% Confidence Interval | P Value to Test if = 0 |
|---|---|---|---|
| Other specialty (reference group at 65 yr) | 11 | (9.1, 12.9) | |
| Internal Medicine | 2.4 | (4.4, 0.3) | 0.02 |
| Pulmonary Critical Care | 0.5 | (1.9, 2.8) | 0.7 |
| Neurosurgery | 0.9 | (4.3, 2.6) | 0.62 |
| Surgical Oncology | 5.3 | (1.8, 8.9) | 0.003 |
| Cardiothoracic Surgery | 6.9 | (3.0, 10.9) | 0.001 |
| Age per 10 yr/age <65 | 0.8 | (1.7, 0.1) | 0.08 |
| Age per 10 yr/age 65 | 1.8 | (3.0, 0.6) | 0.003 |
DISCUSSION
We found several characteristics that were significantly associated with higher cost per day or longer length of stay in patients who died during hospitalization or were discharged to hospice. Among this patient population, the surgical specialty services had overall higher cost per day and length of stay than other services. Patients cared for on the cardiothoracic surgery service had higher cost per day and length of stay; in contrast, internal medicine patients had lower cost per day and length of stay. Neurosurgery patients had higher cost per day, while surgical oncology patients had higher length of stay. Patients age 65 years and older had a significantly lower cost per day and shorter length of stay than those less than 65 years of age.
Higher cost per day for cardiothoracic surgery and neurosurgery patients may partially be explained by cardiothoracic surgery patients' usage of clinical services, including operating room services, which are higher in costs compared with those of nonsurgical specialties. Some patients may require repeat surgeries in the same hospitalization which further increases the cost per day. Longer length of stay in surgical oncology patients may be related to complex surgeries and possible postoperative complications that may take longer to recover from than standard surgeries.
Our findings that older patients have lower cost per day and shorter length of stay are corroborated by other studies. Lubitz and Riley21 found that in 1976 and 1988, Medicare payments per person year decreased with age. Levinsky et al.22 had similar findings in a review of Medicare data in 2001, but noted smaller reductions in total costabout $400 decrease for each year above 65. Their explanation of the lower cost is that older patients receive less aggressive care. Physicians, as well as patients and families, may continue to pursue expensive, invasive therapies for terminally ill patients who are younger for a longer period of time than with older patients, which would increase cost per day as well as length of stay.
The finding that patients on the surgical specialty services may be a focus for active palliative care intervention has many implications. The American College of Surgeons Surgical Palliative Care Task Force consensus guideline triggers for a palliative care consultation in SICU applied clinically did not result in a change in palliative care consultation rate.23 The use of triggers for palliative care consultation may be an ineffective approach because knowledge and application of the triggers did not change behavior. Focusing on integrating palliative care interventions or consultation for all high‐risk surgical patients, as opposed to relying upon triggers, may be a more effective approach to meeting these patients' palliative care needs while lowering cost per day and length of stay and warrants further study. For instance, palliative care consult teams may consider routine or daily rounds with the surgical specialty services in order to effectively integrate palliative care for these patients. Such an integrative approach may foster familiarity and comfort with palliative care approaches, facilitating access to palliative care services for those patients with palliative needs.
Our study is limited in that it is a retrospective, single‐center study. Our results may not be applicable to the general population. The experience of additional centers analyzed prospectively would provide additional context. The available administrative data limited the analyses to only a small number of predictors. In the subset population with the highest 10% total hospitalization costs, from which clinical information was gathered, the presence of respiratory failure was associated with shorter LOS (33 days vs 42 days; P = 0.03), but not associated with cost per day. Having sepsis at admission was associated with lower cost per day ($5783 vs $10,071; P = 0.04); however, this finding was based on only four patients with sepsis at admission. Patients who were evaluated by the palliative care service (n = 35) had a significantly lower cost per day ($4896 vs $12,210; P = 0.01) but longer LOS (46.5 vs 35.7 days; P = 0.03) than those who were not. These, and other, clinical characteristics need further testing in larger samples. An additional limitation is that we combined hospital decedents with patients discharged to hospice as our study population. These groups were combined since they are both at high risk of death in the near future; the median hospice length of stay in Colorado is 20 days.24 There may exist important differences in these populations that are not accounted for in our findings. Despite these unidentified differences, both populations are at high risk of death in the near future, making it likely that they would benefit from palliative care. Those who died during hospitalization did have a longer LOS (11.5 vs 9.2 days; P = 0.003) and higher cost per day ($6734 vs $2221; P < 0.0001) than those who were discharged to hospice.
Palliative care consultations can lead to improved quality of care for patients and families by addressing suffering and addressing quality of life measures (2, 4, 5, 6). We sought to identify characteristics associated with high cost and prolonged hospitalizations in patients who died during hospitalization, or were discharged to hospice, in order to inform targeting of palliative care services. Our data suggest that younger patients and those cared for by surgical specialty services may have the most palliative needs. Palliative care teams may consider focusing efforts at integrating palliative care with surgical specialty services to address these needs. These findings need to be corroborated in other centers, and include clinical outcomes.
- ,,, et al.Family perspectives on end‐of‐life care at the last place of care.JAMA.2004;291:88–93.
- ,.Do specialist palliative care teams improve outcomes for cancer patients? A systematic literature review.Palliat Med.1998;12:317–332.
- ,,, et al.Evidence‐based interventions to improve the palliative care of pain, dyspnea, and depression at the end of life: a clinical practice guideline from the American College of Physicians.Ann Intern Med.2008;148:141–146.
- ,,, et al.Do palliative consultations improve patient outcomes?JAGS.2008;56:593–599.
- ,,, et al.Effects of a palliative cafe intervention on clinical outcomes in patients with advanced cancer.JAMA.2009;302:741–749.
- ,,, et al.Early palliative care for patients with metastatic non‐small‐cell lung cancer.N Engl J Med.2010;363:733–742.
- ,,, et al.Cost savings associated with US hospital palliative care consultation programs.Arch Intern Med.2008;168(16):1783–1790.
- ,,.Impact of palliative care case management on resource use by patients dying of cancer at a Veterans Affairs Medical Center.J Palliat Med.2005;8(1):26–35.
- ,,, et al.Cost and utilization outcomes of patients receiving hospital‐based palliative care consultation.J Palliat Med.2006;9(4):855–860.
- ,,, et al.A high‐volume specialist palliative care unit and team may reduce in‐hospital end‐of‐life care costs.J Palliat Med.2003;6(5):699–705.
- ,,, et al.The economic and clinical impact of an inpatient palliative care consultation service: a multifaceted approach.J Palliat Med.2007;10(6):1347–1355.
- ,,, et al.Hospital‐based palliative care consultation: effects on hospital cost.J Palliat Med.2010;13(8):1–7.
- ,.Impact of a proactive approach to improve end‐of‐life care in a medical ICU.Chest.2003;123:266–271.
- ,,, et al.Proactive palliative care in the medical intensive care unit: effects on length of stay for selected high‐risk patients.Crit Care Med.2007;35:1530–1535.
- ,,,,.Who needs a palliative care consult? The Hamilton Chart Audit tool.J Palliat Med.2007;10(2):304–307.
- ,,,,,.A practical tool to identify patients who may benefit from a palliative care approach: the CARING criteria.J Pain Symptom Manage.2006;31:285–292.
- ,,, et al.An Official American Thoracic Society Clinical Policy Statement: palliative care for patients with respiratory diseases and critical illnesses.Am J Respir Crit Care Med.2008;177:912–927.
- ,,, et al.Proposed quality measures for palliative care in the critically ill: a consensus from the Robert Wood Johnson Foundation Critical Care Workgroup.Crit Care Med.2006;34:S404–S411.
- ,,, et al.Recommendations for end‐of‐life care in the intensive care unit: a consensus statement by the American Academy of Critical Care Medicine.Crit Care Med.2008;36:953–963.
- ,.Developing guidelines that identify patients who would benefit from palliative care services in the surgical intensive care unit.Crit Care Med.2009;37:946–950.
- ,.Trends in Medicare payments in the last year of life.N Engl J Med.1993;328:1092–1096.
- ,,, et al.Influence of age on Medicare expenditures and medical care in the last year of life.JAMA.2001;286:1349–1355.
- ,,.Addressing access to palliative care services in the surgical intensive care unit.Surgery.2010;147:871–877.
- http://www.coloradocancercoalition.org/…/CCCConferenceKassner Slides11.13.07.ppt. Accessed August 16,2010.
- ,,.The impact of implementing referral criteria on the patterns of referrals and admissions to a palliative care program in Saudi Arabia.J Support Oncol.2010;8:78–81.
- ,,, et al.Family perspectives on end‐of‐life care at the last place of care.JAMA.2004;291:88–93.
- ,.Do specialist palliative care teams improve outcomes for cancer patients? A systematic literature review.Palliat Med.1998;12:317–332.
- ,,, et al.Evidence‐based interventions to improve the palliative care of pain, dyspnea, and depression at the end of life: a clinical practice guideline from the American College of Physicians.Ann Intern Med.2008;148:141–146.
- ,,, et al.Do palliative consultations improve patient outcomes?JAGS.2008;56:593–599.
- ,,, et al.Effects of a palliative cafe intervention on clinical outcomes in patients with advanced cancer.JAMA.2009;302:741–749.
- ,,, et al.Early palliative care for patients with metastatic non‐small‐cell lung cancer.N Engl J Med.2010;363:733–742.
- ,,, et al.Cost savings associated with US hospital palliative care consultation programs.Arch Intern Med.2008;168(16):1783–1790.
- ,,.Impact of palliative care case management on resource use by patients dying of cancer at a Veterans Affairs Medical Center.J Palliat Med.2005;8(1):26–35.
- ,,, et al.Cost and utilization outcomes of patients receiving hospital‐based palliative care consultation.J Palliat Med.2006;9(4):855–860.
- ,,, et al.A high‐volume specialist palliative care unit and team may reduce in‐hospital end‐of‐life care costs.J Palliat Med.2003;6(5):699–705.
- ,,, et al.The economic and clinical impact of an inpatient palliative care consultation service: a multifaceted approach.J Palliat Med.2007;10(6):1347–1355.
- ,,, et al.Hospital‐based palliative care consultation: effects on hospital cost.J Palliat Med.2010;13(8):1–7.
- ,.Impact of a proactive approach to improve end‐of‐life care in a medical ICU.Chest.2003;123:266–271.
- ,,, et al.Proactive palliative care in the medical intensive care unit: effects on length of stay for selected high‐risk patients.Crit Care Med.2007;35:1530–1535.
- ,,,,.Who needs a palliative care consult? The Hamilton Chart Audit tool.J Palliat Med.2007;10(2):304–307.
- ,,,,,.A practical tool to identify patients who may benefit from a palliative care approach: the CARING criteria.J Pain Symptom Manage.2006;31:285–292.
- ,,, et al.An Official American Thoracic Society Clinical Policy Statement: palliative care for patients with respiratory diseases and critical illnesses.Am J Respir Crit Care Med.2008;177:912–927.
- ,,, et al.Proposed quality measures for palliative care in the critically ill: a consensus from the Robert Wood Johnson Foundation Critical Care Workgroup.Crit Care Med.2006;34:S404–S411.
- ,,, et al.Recommendations for end‐of‐life care in the intensive care unit: a consensus statement by the American Academy of Critical Care Medicine.Crit Care Med.2008;36:953–963.
- ,.Developing guidelines that identify patients who would benefit from palliative care services in the surgical intensive care unit.Crit Care Med.2009;37:946–950.
- ,.Trends in Medicare payments in the last year of life.N Engl J Med.1993;328:1092–1096.
- ,,, et al.Influence of age on Medicare expenditures and medical care in the last year of life.JAMA.2001;286:1349–1355.
- ,,.Addressing access to palliative care services in the surgical intensive care unit.Surgery.2010;147:871–877.
- http://www.coloradocancercoalition.org/…/CCCConferenceKassner Slides11.13.07.ppt. Accessed August 16,2010.
- ,,.The impact of implementing referral criteria on the patterns of referrals and admissions to a palliative care program in Saudi Arabia.J Support Oncol.2010;8:78–81.
Copyright © 2011 Society of Hospital Medicine
Continuing Medical Education Program in
If you wish to receive credit for this activity, please refer to the website: www.wileyblackwellcme.com.
Accreditation and Designation Statement
Blackwell Futura Media Services designates this journal‐based CME activity for a maximum of 1 AMA PRA Category 1 Credit.. Physicians should only claim credit commensurate with the extent of their participation in the activity.
Blackwell Futura Media Services is accredited by the Accreditation Council for Continuing Medical Education to provide continuing medical education for physicians.
Educational Objectives
The objectives need to be changed. Please remove the existing ones, and include these two:
-
Identify complications elderly patients are at risk for during hospitalization.
-
Suggest evidence‐based strategies to prevent and treat common causes of hospitalization‐related complications in geriatric patients.
This manuscript underwent peer review in line with the standards of editorial integrity and publication ethics maintained by Journal of Hospital Medicine. The peer reviewers have no relevant financial relationships. The peer review process for Journal of Hospital Medicine is single‐blinded. As such, the identities of the reviewers are not disclosed in line with the standard accepted practices of medical journal peer review.
Conflicts of interest have been identified and resolved in accordance with Blackwell Futura Media Services's Policy on Activity Disclosure and Conflict of Interest. The primary resolution method used was peer review and review by a non‐conflicted expert.
Instructions on Receiving Credit
For information on applicability and acceptance of CME credit for this activity, please consult your professional licensing board.
This activity is designed to be completed within an hour; physicians should claim only those credits that reflect the time actually spent in the activity. To successfully earn credit, participants must complete the activity during the valid credit period, which is up to two years from initial publication.
Follow these steps to earn credit:
-
Log on to www.wileyblackwellcme.com
-
Read the target audience, learning objectives, and author disclosures.
-
Read the article in print or online format.
-
Reflect on the article.
-
Access the CME Exam, and choose the best answer to each question.
-
Complete the required evaluation component of the activity.
This activity will be available for CME credit for twelve months following its publication date. At that time, it will be reviewed and potentially updated and extended for an additional twelve months.
If you wish to receive credit for this activity, please refer to the website: www.wileyblackwellcme.com.
Accreditation and Designation Statement
Blackwell Futura Media Services designates this journal‐based CME activity for a maximum of 1 AMA PRA Category 1 Credit.. Physicians should only claim credit commensurate with the extent of their participation in the activity.
Blackwell Futura Media Services is accredited by the Accreditation Council for Continuing Medical Education to provide continuing medical education for physicians.
Educational Objectives
The objectives need to be changed. Please remove the existing ones, and include these two:
-
Identify complications elderly patients are at risk for during hospitalization.
-
Suggest evidence‐based strategies to prevent and treat common causes of hospitalization‐related complications in geriatric patients.
This manuscript underwent peer review in line with the standards of editorial integrity and publication ethics maintained by Journal of Hospital Medicine. The peer reviewers have no relevant financial relationships. The peer review process for Journal of Hospital Medicine is single‐blinded. As such, the identities of the reviewers are not disclosed in line with the standard accepted practices of medical journal peer review.
Conflicts of interest have been identified and resolved in accordance with Blackwell Futura Media Services's Policy on Activity Disclosure and Conflict of Interest. The primary resolution method used was peer review and review by a non‐conflicted expert.
Instructions on Receiving Credit
For information on applicability and acceptance of CME credit for this activity, please consult your professional licensing board.
This activity is designed to be completed within an hour; physicians should claim only those credits that reflect the time actually spent in the activity. To successfully earn credit, participants must complete the activity during the valid credit period, which is up to two years from initial publication.
Follow these steps to earn credit:
-
Log on to www.wileyblackwellcme.com
-
Read the target audience, learning objectives, and author disclosures.
-
Read the article in print or online format.
-
Reflect on the article.
-
Access the CME Exam, and choose the best answer to each question.
-
Complete the required evaluation component of the activity.
This activity will be available for CME credit for twelve months following its publication date. At that time, it will be reviewed and potentially updated and extended for an additional twelve months.
If you wish to receive credit for this activity, please refer to the website: www.wileyblackwellcme.com.
Accreditation and Designation Statement
Blackwell Futura Media Services designates this journal‐based CME activity for a maximum of 1 AMA PRA Category 1 Credit.. Physicians should only claim credit commensurate with the extent of their participation in the activity.
Blackwell Futura Media Services is accredited by the Accreditation Council for Continuing Medical Education to provide continuing medical education for physicians.
Educational Objectives
The objectives need to be changed. Please remove the existing ones, and include these two:
-
Identify complications elderly patients are at risk for during hospitalization.
-
Suggest evidence‐based strategies to prevent and treat common causes of hospitalization‐related complications in geriatric patients.
This manuscript underwent peer review in line with the standards of editorial integrity and publication ethics maintained by Journal of Hospital Medicine. The peer reviewers have no relevant financial relationships. The peer review process for Journal of Hospital Medicine is single‐blinded. As such, the identities of the reviewers are not disclosed in line with the standard accepted practices of medical journal peer review.
Conflicts of interest have been identified and resolved in accordance with Blackwell Futura Media Services's Policy on Activity Disclosure and Conflict of Interest. The primary resolution method used was peer review and review by a non‐conflicted expert.
Instructions on Receiving Credit
For information on applicability and acceptance of CME credit for this activity, please consult your professional licensing board.
This activity is designed to be completed within an hour; physicians should claim only those credits that reflect the time actually spent in the activity. To successfully earn credit, participants must complete the activity during the valid credit period, which is up to two years from initial publication.
Follow these steps to earn credit:
-
Log on to www.wileyblackwellcme.com
-
Read the target audience, learning objectives, and author disclosures.
-
Read the article in print or online format.
-
Reflect on the article.
-
Access the CME Exam, and choose the best answer to each question.
-
Complete the required evaluation component of the activity.
This activity will be available for CME credit for twelve months following its publication date. At that time, it will be reviewed and potentially updated and extended for an additional twelve months.
Medication Reconciliation Barriers
Adverse drug events (ADEs) occur when patients transition between the hospital and other care settings. Medication reconciliation, a process by which a provider obtains and documents a thorough medication history with specific attention to comparing current and previous medication use, can prevent transition‐related errors and harm in a variety of care locations.13 Nevertheless, poor intersite communication,4 flawed reconciliation of drug regimens,2 unreliable patient history‐taking, and poor provider decision‐making5 continue to contribute to transition‐related ADEs.
The Joint Commission introduced medication reconciliation as a hospital National Patient Safety Goal in 2006. However, because organizations have had difficulty implementing the process, it stopped citing medication reconciliation deficiencies in its accreditation surveys.6 Although regional and national initiatives have attempted to improve implementation of medication reconciliationusing provider education, workflow, and process reorganization, and organizational change7a recent field review by the Joint Commission suggests that healthcare organizations remain unable to ensure effective medication reconciliation, citing factors beyond the organizations' control, especially unreliable patient histories.8 Still, the process is slated to return as an accreditation requirement of the Joint Commission on July 1, 2011.8
The objective of this study was to determine factors that influenced physicians' and pharmacists' performance of medication reconciliation in a hospital setting with a computerized medical record and medication reconciliation tool, with the goal of informing an organization's approach to implementation. We conducted individual cognitive task analysis (CTA) interviews and focus group interviews to ascertain physicians' and pharmacists' opinions on the purpose and effectiveness of medication reconciliation, their approach to completing the task, and task facilitators and barriers.
METHODS
Setting and Medication Reconciliation Process
The study setting was an urban, academic, tertiary‐care Veterans Affairs (VA) medical center. A computerized medication reconciliation tool and process were developed in 2005 to comply with the Joint Commission's National Patient Safety Goal.6 The tool was embedded in the VA's Computerized Patient Record System (CPRS) and consisted of a dialogue with which a provider (physician, pharmacist, or other provider) could: 1) view the patient's outpatient medication use, for the last 90 days, from VA computerized pharmacy data; 2) view current VA inpatient orders; 3) record discrepancies between patient‐reported medications, and outpatient and inpatient medications in the VA computerized database; 4) record diagnostic indications for, and responses to, these discrepancies; and 5) produce a medication reconciliation document, which was a separate progress note (Figure 1). The tool did not directly facilitate ordering; however, in CPRS, outpatient orders could easily be copied to inpatient orders and vice versa.

Two versions of the medication reconciliation process were implemented upon hospital admission: one in which the physician initiated and completed a reconciliation that was then reviewed by a pharmacist (Figure 2A)the process primarily used on the medical and surgical services; and one in which, after the physician wrote admission orders, the pharmacist initiated and completed the reconciliation and communicated his or her findings with the physician (Figure 2B)the process primarily used on the psychiatric service. At discharge, after the physician wrote discharge orders, the pharmacist completed a reconciliation of preadmission, inpatient, and discharge orders using a tool similar to the admission one (Figure 1). The pharmacist then communicated the reconciliation findings to the physician, similar to the admission medication reconciliation process shown in Figure 2B. These processes and tools were in place for 18 months at the time of the cognitive task analysis, which took place in June 2007, and 32 months at the time of the focus groups, which took place in August 2008.
Participants
Participants consisted of internal medicine house staff physicians rotating on the inpatient service (n = 23) and inpatient staff pharmacists (n = 12). Overall, 14 (40%) were female. The 23 house staff physicians represented approximately 64% of the total house staff inpatient staffing. Thirteen (57%) were in postgraduate year 1 (PGY1), and 10 (43%) were in PGY2 or higher. The 12 pharmacists represented approximately 50% of the total pharmacist inpatient staffing. Individual CTA interviews took place at the end of the academic year (June) with participants who were highly experienced with the process of medication reconciliation in the VA setting. Focus groups took place at the beginning of the academic year (August) with participants who had to endorse the statement that they were experienced completing medication reconciliation. Subjects participated in either the individual or focus group interviews, but not both. Physicians and pharmacists were interviewed separately. All participants provided written informed consent, and the Institutional Review Board of the James J. Peters VA Medical Center approved the study procedures.
Data Collection
Theoretical Model
The Integrated Change Model9 guided our approach to data collection and analysis. It indicates that a person's motivation, intention, and ability determine whether a behavior will be carried out. A person's motivation is influenced by attitudes (eg, perceived pros and cons of the behavior), social influences, and self‐efficacy (eg, perceived capability). The behavior is also influenced by environmental and physical factorsin this case circumstances of the patient encounter, information systems, and the medication reconciliation tool.
Individual Interviews
We conducted individual CTA interviews with 7 physicians and 5 pharmacists. During CTA, participants verbalized their thoughts while they completed medication reconciliation for at least 1 actual case, and at least 1 standardized (fictitious) case, using the computerized medical record and tool. The purpose of this think aloud exercise was to provide information on medication reconciliation tool functionality and usability, problems in humancomputer interaction, and the impact of the tool on decision‐making, clinical practice, and workflow. As the participants interacted with the medication reconciliation tool, computer screens, mouse clicks, and menu selections were recorded using screen recording software (Hypercam), and the participants' thinking aloud was audio‐recorded. This provided the experimenters with context for analyzing and coding subjects' verbalizations (ie, playback of the screens provided detail about the subjects' interaction with the tool, including what functions of the tool they accessed, and problems they may have encountered). Immediately after completing the tasks, subjects were briefly interviewed about their impressions of the tool and their interaction with it. CTA sessions took 30‐50 minutes per participant. Audio‐ and screen‐recordings were synchronized and transcribed.
Focus Group Interviews
We conducted 3 focus groups, 2 with house staff physicians (n = 9 and n = 7), and 1 with pharmacists (n = 7), for a total of 23 focus group participants. The focus group discussion guide was informed by the results of the CTA and began with broad, open‐ended questions,10 followed by a series of more specific probes. Participants were asked to describe medication reconciliation and its purpose, whether they thought that the process influenced their decision‐making and was effective, and how they go about completing the process. Probes included how the task fit into daily workflow, time needed to complete the task, and its priority relative to other tasks. A set of questions asked participants to report barriers and facilitators to completing the task, training needs and experience, and their suggestions on how to improve task implementation and completion. The discussion also included participants' views of the optimal roles of physicians and pharmacists in performing medication reconciliation. Throughout, participants were encouraged to report both positive and negative perceptions. Group interviews lasted 60‐90 minutes, and were audiotaped and transcribed. The same moderator facilitated all 3 focus groups and participated in the individual CTA interviews.
Data Analysis
CTA and focus group transcripts were analyzed using standard social science methods for analyzing qualitative data.1114 Using multiple close readings, investigators performed initial independent coding of each of the transcripts and generated a list of concepts and domains, and a coding scheme. The coding was reviewed from the perspective of the theoretical model to determine whether additional codes were necessary. A research staff member, blinded to the study hypotheses, applied the codes to each transcript by labeling each word, phrase, or line. To test the coding scheme's reliability, a random 5% of transcript lines were coded by 2 independent coders and interrater reliability was assessed. Disagreement was reconciled by discussion and transcripts recoded as appropriate. The investigators then compared codes within and across interviews to elucidate the larger themes that emerged. Frequencies of mention of each domain were calculated. Although the original intent was to analyze the CTA and focus groups separately, themes arising from each of these 2 techniques overlapped sufficiently to allow for a combined analysis. Data were compiled to provide a description of the factors that affect medication reconciliation completion, a summary of barriers and facilitators to use of the medication reconciliation tool, and user suggestions.
RESULTS
Purpose of Medication Reconciliation
Both physicians and pharmacists agreed that a central goal of medication reconciliation is to prevent prescribing errors and adverse drug events that arise from medication utilization over time, and across place and provider. Respondents also agreed that the medication reconciliation document provides a record of patients' history of medication use and of the provider's rationale for medication changes.
Both physicians and pharmacists also indicated awareness of external necessities for completing medication reconciliation, including Joint Commission accreditation standards and, to a lesser extent, medico‐legal liability concerns. On the other hand, 1 physician expressed concern that documented medication discrepancies could be interpreted as mistakes and a liability problem.
Effectiveness of Medication Reconciliation
There was overall disagreement about whether or not medication reconciliation actually achieves its goal of improving medication safety. Many physicians and pharmacists said that medication reconciliation prompts them to perform additional checking of medication lists, dosing, conditions, and interactions, and to better document medication histories and provider decision‐making. On the other hand, some physicians and pharmacists saw it as mainly an administrative task and doubted whether it had any impact on patient care, as this physician indicated: It just seems like another form to fill out .You could be writing a bunch of whatever and no one would notice and no one would say anything and it would never matter. Likewise, a majority of physicians indicated that other parts of the medical record are better sources of prescribing information than the medication reconciliation document.
Medication Reconciliation Process
Physicians and pharmacists agreed that the key components of medication reconciliation are obtaining a medication history from patients and other available sources, identifying differences among medication histories and medications being given, documenting these discrepancies and their reasons, recording a prescribing plan, and counseling the patient. Respondents indicated that the process varies depending on patient complexity, number of medications, encounter type, and provider. For example, physicians were more likely than pharmacists to complete medication reconciliation at once on admission, rather than begin the task and complete it later, as this physician indicated:
It might seem a little tough, but if you don't do it right at that moment, chances [are high] of you forgetting to do it . So it's best done at the moment when you speak to the patient unless you're waiting for some more information.
On the other hand, this physician routinely put off completing medication reconciliation until the day after admission in order to incorporate a more developed prescribing plan:
You can't do it till the end. Until you have your whole plan. when I do an admission note, I write down every single problem and I account for every single medication that I'm going to put someone on in the hospital or not put someone on. And I actually do the medication reconciliation the next day very easily, because I know exactly what I discontinued or why, or added and why. I don't think it's necessary to do it that night [and] you think about it a little bit more.
These quotes suggests that there is an adherence benefit to completing medication reconciliation on admission as a routine, but that this may not be possible, or even desirable, when more time is needed to obtain and verify medication information and make informed prescribing decisions.
Impact of the Computer on the Medication Reconciliation Process
A majority of respondents cautioned about the impact of computerized information and an electronic tool on medication reconciliation. As indicated in these quotes, the first by a physician and the second by a pharmacist, providers' reliance on the computer can lead to less thorough patient interviews, and computerized medication information may be incomplete, both unintended consequences of the electronic health record:
At the VA, you can just easily import [all the information] and you don't even have to ask the patient. So I would imagine more errors get made because people just import whatever meds are in the computer.
If I didn't have [the computerized record], I would be doing patient interviews much more and finding out what they're taking. On the computer, you do all these beautiful notesso I rely a lot on the computer and the whole patient contact thing kind of slides away. When you go to other hospitals, medication reconciliation is extensively patient interview, family interview, calling neighborhood pharmacies, you know, more like detective work and talking to people versus just sitting here typing, so.But the computer is great.
Who Should Perform Medication Reconciliation
Pharmacists and physicians had mixed responses when asked who should perform medication reconciliation. Several physicians indicated that medication reconciliation duplicates what they already do on admission and in progress notes, and therefore is not a good use of their time.
Respondents from both professions questioned the quality of physicians' medication reconciliation. As 1 pharmacist stated about physicians:
They're busy. Whether you like it or not, they're busy. To compare everything, to go across all the sources whether it's what they get here, asking the patient [viewing data from other facilities].There's nothing wrong with the computer [being] able to copy everything that's active and dropping it in. Butyou have to look it over. That's the problem. They don't look it over.
Thus, there was a tension among a majority of respondents between a belief that pharmacists do a better job at medication history‐taking and reconciliation, and a belief that physicians make the prescribing decisions and should be responsible for them. The quote also suggests that a barrier to self‐efficacy among pharmacists is their dependence on physicians to write the orders needed to address the findings of medication reconciliation. Although many respondents recognized that the task needs to be a component of both profession's jobs, suggestions for collaboration between physicians and pharmacists were limited in vision. Suggestions included pharmacists' checking physicians' work, physicians cosigning pharmacists' work, or some other sequenced completion of the task.
Barriers to Medication Reconciliation
Barriers to efficient and effective completion of medication reconciliation according to respondents are shown in Table 1. Physicians and pharmacists both indicated that patients can be unreliable sources of medication information. A few respondents indicated that whereas patients' health conditions change, medication reconciliation occurs at 1 point in time; this can limit its usefulness or make it immediately obsolete.
| Barrier | Barrier Type/Level | Primary Endorser(s) | No. of Endorsers (MDs/PharmDs) |
|---|---|---|---|
| |||
| Competing clinical tasks have higher priority | Provider | Physician | 9/2 |
| Patients provide unreliable information | Patient | Physician | 6/1 |
| Status (active/expired/discontinued) of medications is unclear | System | Physician/pharmacist | 2/5 |
| Need to complete many medication reconciliations | Provider | Pharmacist | 0/6 |
| Preadmission medication list generated by the tool may show medications in duplicate and may require extensive scrolling | Tool | Physician/pharmacist | 4/2 |
| Medication reconciliation tool only picks up information on medications supplied by the VA | Tool | Pharmacist | 0/5 |
| Process to import non‐local VA medications is slow or does not work | System | Physician/pharmacist | 3/2 |
| Patient's health status changes over time | Patient | Physician/pharmacist | 3/1 |
| It is difficult to determine physicians' rationale for prescribing changes, which is needed for the reconciliation document | Provider | Pharmacist | 0/4 |
| Tool is unclear on where to insert revisions to the medication history, changes to the outpatient or inpatient orders, and unresolved medication discrepancies | Tool | Physician | 3/0 |
Both physicians and pharmacists said that medication reconciliation competed for their time with other responsibilities, and physicians placed acute care responsibilities as a higher priority: One sick patient takes all your timeso your mind is on the patient, not on the reconciliation; that's the last thing you worry about.
Pharmacists emphasized that the volume of patients is a barrier: Give me time and I can do a perfect med rec.Honestly, it's work load.I'm sorry, that day where I had 17 people being discharged, I don't think I did such a great job on their med recs.
Respondents indicated several ways in which the computer system itself was a barrier to effective medication reconciliation. First, as previously noted, the computer only picks up information on medications supplied by the VA, and the medication reconciliation tool may only pick up medications supplied by the local facility. Second, sometimes the computer is unclear on the status of medications, as when outpatient medications are automatically discontinued after hospital admission, or when the system automatically imports a medication that is shown to be active but was only meant to be given for a short period of time, such as an antibiotic. Several barriers specific to tool usability are also shown in Table 1.
Suggestions for Improving Medication Reconciliation
Physicians' and pharmacists' suggestions for improving medication reconciliation are shown in Table 2. First, there was recognition of the need for someone in addition to the author to check the medication reconciliation document to find mistakes. Second, respondents indicated that better provider training might improve medication reconciliation's effectiveness. Both physicians and pharmacists indicated that their education consisted of a limited amount of on‐the‐job training, such as a walk through with a supervisor the first time. When asked for suggestions for improving education, both physicians and pharmacists suggested that physicians should receive case‐based education, during which the purpose of the task is emphasized. These respondents, the first a pharmacist and the second a physician, called for provider feedback to improve and maintain reliability:
| Suggestion | Targeted Level | Primary Endorser(s) | No. of Endorsers (MDs/PharmDs) |
|---|---|---|---|
| |||
| Place checkbox next to each medication | Tool | Physician/pharmacist | 6/2 |
| Order or label medication by condition or diagnosis | Tool | Physician/pharmacist | 2/3 |
| Someone in addition to the author should check the medication reconciliation note and provide feedback and corrections | Provider | Physician/pharmacist | 2/2 |
| Enable searchable medication history | System | Physician | 2/2 |
| Enable automatic importing of medication information from other VAs | System/tool | Physician/pharmacist | 1/2 |
| Reconciliation document should be signed by both physician and pharmacist | Provider | Pharmacist | 0/2 |
| Task should have dedicated staffing | Provider | Pharmacist | 0/2 |
| Facilitate viewing of preadmission medication list and inpatient orders side‐by‐side, instead of topbottom | Tool | Pharmacist | 0/2 |
| Make template more concise | Tool | Pharmacist | 0/2 |
| Automatically convert medication reconciliation planned actions into orders | System/tool | Pharmacist | 0/2 |
| Automatically insert medication reconciliation documentation into admission note | System/tool | Physician | 2/0 |
Have somebody really look at the quality of the reconciliation and speak to whoever did it, whether it's done correctly or not correctly. Because I've seen too many people just use the template, click, click, and then sign. You can finish the note [in] two minutes, but it's not going to be accurate and it's not going to do the patient any good.
We just keep on doing the same thing without ever learning [whether it is] the right way. That's where [we get] this [idea that] we are just doing it for the sake of doing it.
These quotes suggest that a lack of review and feedback about the reconciliation process appears to impact both perceived importance and quality.
DISCUSSION
In this study of hospital physicians' and pharmacists' perspectives on medication reconciliation, we found that although respondents agreed about its main purposeto improve prescribing safetya near majority believed that it was of uncertain benefit to patients and limited use to providers. This might, in part, be because of a tool that was not adequately integrated into workflow, making it extraneous to patient care. As a consequence, many respondents' indicated that when they had competing tasks, especially other acute care responsibilities, medication reconciliation was displaced in priority. Respondents indicated that unreliability of patient medication histories was also a major barrier, which is consistent with a recent Joint Commission field review in which organizations cited this as a task hindrance beyond their control.8 Lastly, respondents revealed limited perception of it being a team‐oriented task.
Our study also probed providers' perceptions of the effect of computerization on completing medication reconciliation. Although study respondents indicated that the computerized tool reduced the time required to complete the task, they also expressed concern that because medication reconciliation was automated in part at the VA, they spent less time with patients on the process. This finding is important because, according to 1 study, many medications are not captured by the VA's Computerized Patient Record System,15 and a patient's lack of medication adherence may not be evident in the CPRS. This finding is also consistent with our prior work that has not demonstrated an inherent advantage to electronic communication of medication information over paper.16
Our study suggests that, for medication reconciliation to be improved, provider self‐efficacy and engagement with the process must be increased. This might involve addressing negative provider attitudes, changing workflow, and improving provider confidence by improving information reliability. With regard to changing attitudes, team members should be briefed on research evidence that shows that medication reconciliation is effective in preventing ADEs13 and is cost‐effective17 to help to increase the value that providers place on medication reconciliation. With regard to workflow, the tool has to be optimized to facilitate information gathering, processing, and medication ordering. Our findings also suggest that medication reconciliation would benefit from widening the time window in which it should be completed (eg, to the first or second business day after admission), since this increases the time available to access multiple data sources, and for providers to update the preadmission medication history and to act on new medication information. Finally, regional electronic health information exchange would improve information reliability and provider confidence in the information.
Our findings also suggest that assignment of productive teamsconsisting of physicians, physician‐extenders, nurses, pharmacists, and/or administrative support staffrather than individuals to the task might improve task completion. Efficacy and perceived capability might be improved by dividing the task into parts more easily manageable by individual team members. One example would be to assign 1 team member to record all of the sources of medication information available for each patient (the patient's home, pharmacies, doctors, hospitals, etc), and assign 1 or more other team members to access these sources as needed. Another example would be to assign the pharmacist to take and document the preadmission medication history (if not for all patients, then perhaps for the highest‐risk patients), and assign the physician to verify the history, specify the planned action on admission for each medication, and complete the admission orders. These suggestions are consistent with a study that suggested that physician engagement and an effective team are strongly correlated with successful implementation of medication reconciliation.7
A strength of our study was its use of multiple methods (focus groups and cognitive task analysis) to collect data from key users individually while they interfaced with the system and in groups. Nevertheless, a limitation of the study is that it took place in a single hospital. Though it had a limited number of physician and pharmacist participants, the study sample represented a large fraction of the inpatient staff in those disciplines. We also did not include nurses, hospitalist attending physicians, or other disciplines that might be involved in medication reconciliation in other facilities or settings. However, our study explored the relationship between two disciplines (physicians and pharmacists), yielding findings that could apply to optimizing the function of other interdisciplinary teams.
Our findings can be used to inform improvement efforts in hospitals that have struggled to implement medication reconciliation. Given that the process is slated to return as an accreditation requirement of the Joint Commission,8 hospitals will need to find ways to strengthen the process. Our findings suggest that increasing providers' perceived capability, and confidence in the process and its outcomes, would improve their engagement in the process. This could be accomplished by improved information gathering, including better computer information systems and regional electronic health information exchange, a flexible timeframe for the process, provider training and feedback, and teamwork. In addition, hospitals can make sure their process is working by ascertaining a gold standard medication history on a subset of patients, and comparing the gold standard to the team's history, and admission and discharge orders. Because it is a central component of safe medication prescribing, medication reconciliation will continue to be a focus of state,18 national,19 and international safety efforts20 in the near future.
Acknowledgements
The authors are grateful for the technical assistance of Daniel Signor in the conduct of this work.
- ,,, et al.Effect of an electronic medication reconciliation application and process redesign on potential adverse drug events: a cluster‐randomized trial.Arch Intern Med.2009;169:771–780.
- ,,, et al.Medication reconciliation: a practical tool to reduce the risk of medication errors.J Crit Care.2003;18:201–205.
- ,,,,.Medication reconciliation for reducing drug‐discrepancy adverse events.Am J Geriatr Pharmacother.2006;4:236–243.
- ,,,.Patient transfer from nursing home to emergency department: outcomes and policy implications.Acad Emerg Med.1997;4:908–915.
- ,,, et al.Systems analysis of adverse drug events. ADE Prevention Study Group.JAMA.1995;274:35–43.
- Joint Commission Hospital National Patient Safety Goal #8. Available at: http://www.jointcommission.org/AccreditationPrograms/ Hospitals/NPSG/. Accessed November 10,2010.
- ,,, et al.Reconciling medications at admission: safe practice recommendations and implementation strategies.Jt Comm J Qual Patient Saf.2006;32:37–50.
- Update: medication reconciliation. National Patient Safety Goal field review results. Joint Commission online June 2, 2010. Available at: http://www.jointcommission.org/NR/rdonlyres/17295FE3–6643‐48E6–89A5‐C629 609E3F36/0/jconlineJune210.pdf. Accessed November 10,2010.
- ,,,.The general public's information needs and perceptions regarding hereditary cancer: an application of the integrated change model.Patient Educ Couns.2005;56:154–165.
- ,.Involving Community Members in Focus Groups.Thousand Oaks, CA:Sage;1998.
- ,.Qualitative Data Analysis.Thousand Oaks, CA:Sage;1994.
- .How to Use Qualitative Methods in Evaluation.Newbury Park, CA:Sage;1987.
- .Handbook of Research Design and Social Measurement,5th ed.Newbury Park, CA:Sage;1991.
- .An Introduction to Qualitative Research.London:Sage;1998.
- ,,,.Assessing the accuracy of computerized medication histories.Am J Manag Care.2004;10:872–877.
- ,,, et al.Electronic health records and adverse drug events after patient transfer.Qual Saf Health Care.2010;19:1–5.
- ,,.Model‐based cost‐effectiveness analysis of interventions aimed at preventing medication error at hospital admission (medicines reconciliation).J Eval Clin Pract.2009;15:299–306.
- Massachusetts Hospital Association. Reconciling medications. A Medication Safety Collaborative sponsored by the MA Coalition for the Prevention of Medical Errors. Available at: http://www.macoalition.org/Initiatives/RecMeds/ProjectBackground.pdf. Accessed November 10,2010.
- Patient safety medication systems tools. Available at: http://www. ihi.org/IHI/Topics/PatientSafety/MedicationSystems/Tools/. Accessed November 10,2010.
- Danish Society for Patient Safety: Operation Life Denmark. Available at: http://patientsikkerhed.dk/en/about_the_danish_society_for_patient_ safety/activities/. Accessed November 10,2010.
Adverse drug events (ADEs) occur when patients transition between the hospital and other care settings. Medication reconciliation, a process by which a provider obtains and documents a thorough medication history with specific attention to comparing current and previous medication use, can prevent transition‐related errors and harm in a variety of care locations.13 Nevertheless, poor intersite communication,4 flawed reconciliation of drug regimens,2 unreliable patient history‐taking, and poor provider decision‐making5 continue to contribute to transition‐related ADEs.
The Joint Commission introduced medication reconciliation as a hospital National Patient Safety Goal in 2006. However, because organizations have had difficulty implementing the process, it stopped citing medication reconciliation deficiencies in its accreditation surveys.6 Although regional and national initiatives have attempted to improve implementation of medication reconciliationusing provider education, workflow, and process reorganization, and organizational change7a recent field review by the Joint Commission suggests that healthcare organizations remain unable to ensure effective medication reconciliation, citing factors beyond the organizations' control, especially unreliable patient histories.8 Still, the process is slated to return as an accreditation requirement of the Joint Commission on July 1, 2011.8
The objective of this study was to determine factors that influenced physicians' and pharmacists' performance of medication reconciliation in a hospital setting with a computerized medical record and medication reconciliation tool, with the goal of informing an organization's approach to implementation. We conducted individual cognitive task analysis (CTA) interviews and focus group interviews to ascertain physicians' and pharmacists' opinions on the purpose and effectiveness of medication reconciliation, their approach to completing the task, and task facilitators and barriers.
METHODS
Setting and Medication Reconciliation Process
The study setting was an urban, academic, tertiary‐care Veterans Affairs (VA) medical center. A computerized medication reconciliation tool and process were developed in 2005 to comply with the Joint Commission's National Patient Safety Goal.6 The tool was embedded in the VA's Computerized Patient Record System (CPRS) and consisted of a dialogue with which a provider (physician, pharmacist, or other provider) could: 1) view the patient's outpatient medication use, for the last 90 days, from VA computerized pharmacy data; 2) view current VA inpatient orders; 3) record discrepancies between patient‐reported medications, and outpatient and inpatient medications in the VA computerized database; 4) record diagnostic indications for, and responses to, these discrepancies; and 5) produce a medication reconciliation document, which was a separate progress note (Figure 1). The tool did not directly facilitate ordering; however, in CPRS, outpatient orders could easily be copied to inpatient orders and vice versa.

Two versions of the medication reconciliation process were implemented upon hospital admission: one in which the physician initiated and completed a reconciliation that was then reviewed by a pharmacist (Figure 2A)the process primarily used on the medical and surgical services; and one in which, after the physician wrote admission orders, the pharmacist initiated and completed the reconciliation and communicated his or her findings with the physician (Figure 2B)the process primarily used on the psychiatric service. At discharge, after the physician wrote discharge orders, the pharmacist completed a reconciliation of preadmission, inpatient, and discharge orders using a tool similar to the admission one (Figure 1). The pharmacist then communicated the reconciliation findings to the physician, similar to the admission medication reconciliation process shown in Figure 2B. These processes and tools were in place for 18 months at the time of the cognitive task analysis, which took place in June 2007, and 32 months at the time of the focus groups, which took place in August 2008.

Participants
Participants consisted of internal medicine house staff physicians rotating on the inpatient service (n = 23) and inpatient staff pharmacists (n = 12). Overall, 14 (40%) were female. The 23 house staff physicians represented approximately 64% of the total house staff inpatient staffing. Thirteen (57%) were in postgraduate year 1 (PGY1), and 10 (43%) were in PGY2 or higher. The 12 pharmacists represented approximately 50% of the total pharmacist inpatient staffing. Individual CTA interviews took place at the end of the academic year (June) with participants who were highly experienced with the process of medication reconciliation in the VA setting. Focus groups took place at the beginning of the academic year (August) with participants who had to endorse the statement that they were experienced completing medication reconciliation. Subjects participated in either the individual or focus group interviews, but not both. Physicians and pharmacists were interviewed separately. All participants provided written informed consent, and the Institutional Review Board of the James J. Peters VA Medical Center approved the study procedures.
Data Collection
Theoretical Model
The Integrated Change Model9 guided our approach to data collection and analysis. It indicates that a person's motivation, intention, and ability determine whether a behavior will be carried out. A person's motivation is influenced by attitudes (eg, perceived pros and cons of the behavior), social influences, and self‐efficacy (eg, perceived capability). The behavior is also influenced by environmental and physical factorsin this case circumstances of the patient encounter, information systems, and the medication reconciliation tool.
Individual Interviews
We conducted individual CTA interviews with 7 physicians and 5 pharmacists. During CTA, participants verbalized their thoughts while they completed medication reconciliation for at least 1 actual case, and at least 1 standardized (fictitious) case, using the computerized medical record and tool. The purpose of this think aloud exercise was to provide information on medication reconciliation tool functionality and usability, problems in humancomputer interaction, and the impact of the tool on decision‐making, clinical practice, and workflow. As the participants interacted with the medication reconciliation tool, computer screens, mouse clicks, and menu selections were recorded using screen recording software (Hypercam), and the participants' thinking aloud was audio‐recorded. This provided the experimenters with context for analyzing and coding subjects' verbalizations (ie, playback of the screens provided detail about the subjects' interaction with the tool, including what functions of the tool they accessed, and problems they may have encountered). Immediately after completing the tasks, subjects were briefly interviewed about their impressions of the tool and their interaction with it. CTA sessions took 30‐50 minutes per participant. Audio‐ and screen‐recordings were synchronized and transcribed.
Focus Group Interviews
We conducted 3 focus groups, 2 with house staff physicians (n = 9 and n = 7), and 1 with pharmacists (n = 7), for a total of 23 focus group participants. The focus group discussion guide was informed by the results of the CTA and began with broad, open‐ended questions,10 followed by a series of more specific probes. Participants were asked to describe medication reconciliation and its purpose, whether they thought that the process influenced their decision‐making and was effective, and how they go about completing the process. Probes included how the task fit into daily workflow, time needed to complete the task, and its priority relative to other tasks. A set of questions asked participants to report barriers and facilitators to completing the task, training needs and experience, and their suggestions on how to improve task implementation and completion. The discussion also included participants' views of the optimal roles of physicians and pharmacists in performing medication reconciliation. Throughout, participants were encouraged to report both positive and negative perceptions. Group interviews lasted 60‐90 minutes, and were audiotaped and transcribed. The same moderator facilitated all 3 focus groups and participated in the individual CTA interviews.
Data Analysis
CTA and focus group transcripts were analyzed using standard social science methods for analyzing qualitative data.1114 Using multiple close readings, investigators performed initial independent coding of each of the transcripts and generated a list of concepts and domains, and a coding scheme. The coding was reviewed from the perspective of the theoretical model to determine whether additional codes were necessary. A research staff member, blinded to the study hypotheses, applied the codes to each transcript by labeling each word, phrase, or line. To test the coding scheme's reliability, a random 5% of transcript lines were coded by 2 independent coders and interrater reliability was assessed. Disagreement was reconciled by discussion and transcripts recoded as appropriate. The investigators then compared codes within and across interviews to elucidate the larger themes that emerged. Frequencies of mention of each domain were calculated. Although the original intent was to analyze the CTA and focus groups separately, themes arising from each of these 2 techniques overlapped sufficiently to allow for a combined analysis. Data were compiled to provide a description of the factors that affect medication reconciliation completion, a summary of barriers and facilitators to use of the medication reconciliation tool, and user suggestions.
RESULTS
Purpose of Medication Reconciliation
Both physicians and pharmacists agreed that a central goal of medication reconciliation is to prevent prescribing errors and adverse drug events that arise from medication utilization over time, and across place and provider. Respondents also agreed that the medication reconciliation document provides a record of patients' history of medication use and of the provider's rationale for medication changes.
Both physicians and pharmacists also indicated awareness of external necessities for completing medication reconciliation, including Joint Commission accreditation standards and, to a lesser extent, medico‐legal liability concerns. On the other hand, 1 physician expressed concern that documented medication discrepancies could be interpreted as mistakes and a liability problem.
Effectiveness of Medication Reconciliation
There was overall disagreement about whether or not medication reconciliation actually achieves its goal of improving medication safety. Many physicians and pharmacists said that medication reconciliation prompts them to perform additional checking of medication lists, dosing, conditions, and interactions, and to better document medication histories and provider decision‐making. On the other hand, some physicians and pharmacists saw it as mainly an administrative task and doubted whether it had any impact on patient care, as this physician indicated: It just seems like another form to fill out .You could be writing a bunch of whatever and no one would notice and no one would say anything and it would never matter. Likewise, a majority of physicians indicated that other parts of the medical record are better sources of prescribing information than the medication reconciliation document.
Medication Reconciliation Process
Physicians and pharmacists agreed that the key components of medication reconciliation are obtaining a medication history from patients and other available sources, identifying differences among medication histories and medications being given, documenting these discrepancies and their reasons, recording a prescribing plan, and counseling the patient. Respondents indicated that the process varies depending on patient complexity, number of medications, encounter type, and provider. For example, physicians were more likely than pharmacists to complete medication reconciliation at once on admission, rather than begin the task and complete it later, as this physician indicated:
It might seem a little tough, but if you don't do it right at that moment, chances [are high] of you forgetting to do it . So it's best done at the moment when you speak to the patient unless you're waiting for some more information.
On the other hand, this physician routinely put off completing medication reconciliation until the day after admission in order to incorporate a more developed prescribing plan:
You can't do it till the end. Until you have your whole plan. when I do an admission note, I write down every single problem and I account for every single medication that I'm going to put someone on in the hospital or not put someone on. And I actually do the medication reconciliation the next day very easily, because I know exactly what I discontinued or why, or added and why. I don't think it's necessary to do it that night [and] you think about it a little bit more.
These quotes suggests that there is an adherence benefit to completing medication reconciliation on admission as a routine, but that this may not be possible, or even desirable, when more time is needed to obtain and verify medication information and make informed prescribing decisions.
Impact of the Computer on the Medication Reconciliation Process
A majority of respondents cautioned about the impact of computerized information and an electronic tool on medication reconciliation. As indicated in these quotes, the first by a physician and the second by a pharmacist, providers' reliance on the computer can lead to less thorough patient interviews, and computerized medication information may be incomplete, both unintended consequences of the electronic health record:
At the VA, you can just easily import [all the information] and you don't even have to ask the patient. So I would imagine more errors get made because people just import whatever meds are in the computer.
If I didn't have [the computerized record], I would be doing patient interviews much more and finding out what they're taking. On the computer, you do all these beautiful notesso I rely a lot on the computer and the whole patient contact thing kind of slides away. When you go to other hospitals, medication reconciliation is extensively patient interview, family interview, calling neighborhood pharmacies, you know, more like detective work and talking to people versus just sitting here typing, so.But the computer is great.
Who Should Perform Medication Reconciliation
Pharmacists and physicians had mixed responses when asked who should perform medication reconciliation. Several physicians indicated that medication reconciliation duplicates what they already do on admission and in progress notes, and therefore is not a good use of their time.
Respondents from both professions questioned the quality of physicians' medication reconciliation. As 1 pharmacist stated about physicians:
They're busy. Whether you like it or not, they're busy. To compare everything, to go across all the sources whether it's what they get here, asking the patient [viewing data from other facilities].There's nothing wrong with the computer [being] able to copy everything that's active and dropping it in. Butyou have to look it over. That's the problem. They don't look it over.
Thus, there was a tension among a majority of respondents between a belief that pharmacists do a better job at medication history‐taking and reconciliation, and a belief that physicians make the prescribing decisions and should be responsible for them. The quote also suggests that a barrier to self‐efficacy among pharmacists is their dependence on physicians to write the orders needed to address the findings of medication reconciliation. Although many respondents recognized that the task needs to be a component of both profession's jobs, suggestions for collaboration between physicians and pharmacists were limited in vision. Suggestions included pharmacists' checking physicians' work, physicians cosigning pharmacists' work, or some other sequenced completion of the task.
Barriers to Medication Reconciliation
Barriers to efficient and effective completion of medication reconciliation according to respondents are shown in Table 1. Physicians and pharmacists both indicated that patients can be unreliable sources of medication information. A few respondents indicated that whereas patients' health conditions change, medication reconciliation occurs at 1 point in time; this can limit its usefulness or make it immediately obsolete.
| Barrier | Barrier Type/Level | Primary Endorser(s) | No. of Endorsers (MDs/PharmDs) |
|---|---|---|---|
| |||
| Competing clinical tasks have higher priority | Provider | Physician | 9/2 |
| Patients provide unreliable information | Patient | Physician | 6/1 |
| Status (active/expired/discontinued) of medications is unclear | System | Physician/pharmacist | 2/5 |
| Need to complete many medication reconciliations | Provider | Pharmacist | 0/6 |
| Preadmission medication list generated by the tool may show medications in duplicate and may require extensive scrolling | Tool | Physician/pharmacist | 4/2 |
| Medication reconciliation tool only picks up information on medications supplied by the VA | Tool | Pharmacist | 0/5 |
| Process to import non‐local VA medications is slow or does not work | System | Physician/pharmacist | 3/2 |
| Patient's health status changes over time | Patient | Physician/pharmacist | 3/1 |
| It is difficult to determine physicians' rationale for prescribing changes, which is needed for the reconciliation document | Provider | Pharmacist | 0/4 |
| Tool is unclear on where to insert revisions to the medication history, changes to the outpatient or inpatient orders, and unresolved medication discrepancies | Tool | Physician | 3/0 |
Both physicians and pharmacists said that medication reconciliation competed for their time with other responsibilities, and physicians placed acute care responsibilities as a higher priority: One sick patient takes all your timeso your mind is on the patient, not on the reconciliation; that's the last thing you worry about.
Pharmacists emphasized that the volume of patients is a barrier: Give me time and I can do a perfect med rec.Honestly, it's work load.I'm sorry, that day where I had 17 people being discharged, I don't think I did such a great job on their med recs.
Respondents indicated several ways in which the computer system itself was a barrier to effective medication reconciliation. First, as previously noted, the computer only picks up information on medications supplied by the VA, and the medication reconciliation tool may only pick up medications supplied by the local facility. Second, sometimes the computer is unclear on the status of medications, as when outpatient medications are automatically discontinued after hospital admission, or when the system automatically imports a medication that is shown to be active but was only meant to be given for a short period of time, such as an antibiotic. Several barriers specific to tool usability are also shown in Table 1.
Suggestions for Improving Medication Reconciliation
Physicians' and pharmacists' suggestions for improving medication reconciliation are shown in Table 2. First, there was recognition of the need for someone in addition to the author to check the medication reconciliation document to find mistakes. Second, respondents indicated that better provider training might improve medication reconciliation's effectiveness. Both physicians and pharmacists indicated that their education consisted of a limited amount of on‐the‐job training, such as a walk through with a supervisor the first time. When asked for suggestions for improving education, both physicians and pharmacists suggested that physicians should receive case‐based education, during which the purpose of the task is emphasized. These respondents, the first a pharmacist and the second a physician, called for provider feedback to improve and maintain reliability:
| Suggestion | Targeted Level | Primary Endorser(s) | No. of Endorsers (MDs/PharmDs) |
|---|---|---|---|
| |||
| Place checkbox next to each medication | Tool | Physician/pharmacist | 6/2 |
| Order or label medication by condition or diagnosis | Tool | Physician/pharmacist | 2/3 |
| Someone in addition to the author should check the medication reconciliation note and provide feedback and corrections | Provider | Physician/pharmacist | 2/2 |
| Enable searchable medication history | System | Physician | 2/2 |
| Enable automatic importing of medication information from other VAs | System/tool | Physician/pharmacist | 1/2 |
| Reconciliation document should be signed by both physician and pharmacist | Provider | Pharmacist | 0/2 |
| Task should have dedicated staffing | Provider | Pharmacist | 0/2 |
| Facilitate viewing of preadmission medication list and inpatient orders side‐by‐side, instead of topbottom | Tool | Pharmacist | 0/2 |
| Make template more concise | Tool | Pharmacist | 0/2 |
| Automatically convert medication reconciliation planned actions into orders | System/tool | Pharmacist | 0/2 |
| Automatically insert medication reconciliation documentation into admission note | System/tool | Physician | 2/0 |
Have somebody really look at the quality of the reconciliation and speak to whoever did it, whether it's done correctly or not correctly. Because I've seen too many people just use the template, click, click, and then sign. You can finish the note [in] two minutes, but it's not going to be accurate and it's not going to do the patient any good.
We just keep on doing the same thing without ever learning [whether it is] the right way. That's where [we get] this [idea that] we are just doing it for the sake of doing it.
These quotes suggest that a lack of review and feedback about the reconciliation process appears to impact both perceived importance and quality.
DISCUSSION
In this study of hospital physicians' and pharmacists' perspectives on medication reconciliation, we found that although respondents agreed about its main purposeto improve prescribing safetya near majority believed that it was of uncertain benefit to patients and limited use to providers. This might, in part, be because of a tool that was not adequately integrated into workflow, making it extraneous to patient care. As a consequence, many respondents' indicated that when they had competing tasks, especially other acute care responsibilities, medication reconciliation was displaced in priority. Respondents indicated that unreliability of patient medication histories was also a major barrier, which is consistent with a recent Joint Commission field review in which organizations cited this as a task hindrance beyond their control.8 Lastly, respondents revealed limited perception of it being a team‐oriented task.
Our study also probed providers' perceptions of the effect of computerization on completing medication reconciliation. Although study respondents indicated that the computerized tool reduced the time required to complete the task, they also expressed concern that because medication reconciliation was automated in part at the VA, they spent less time with patients on the process. This finding is important because, according to 1 study, many medications are not captured by the VA's Computerized Patient Record System,15 and a patient's lack of medication adherence may not be evident in the CPRS. This finding is also consistent with our prior work that has not demonstrated an inherent advantage to electronic communication of medication information over paper.16
Our study suggests that, for medication reconciliation to be improved, provider self‐efficacy and engagement with the process must be increased. This might involve addressing negative provider attitudes, changing workflow, and improving provider confidence by improving information reliability. With regard to changing attitudes, team members should be briefed on research evidence that shows that medication reconciliation is effective in preventing ADEs13 and is cost‐effective17 to help to increase the value that providers place on medication reconciliation. With regard to workflow, the tool has to be optimized to facilitate information gathering, processing, and medication ordering. Our findings also suggest that medication reconciliation would benefit from widening the time window in which it should be completed (eg, to the first or second business day after admission), since this increases the time available to access multiple data sources, and for providers to update the preadmission medication history and to act on new medication information. Finally, regional electronic health information exchange would improve information reliability and provider confidence in the information.
Our findings also suggest that assignment of productive teamsconsisting of physicians, physician‐extenders, nurses, pharmacists, and/or administrative support staffrather than individuals to the task might improve task completion. Efficacy and perceived capability might be improved by dividing the task into parts more easily manageable by individual team members. One example would be to assign 1 team member to record all of the sources of medication information available for each patient (the patient's home, pharmacies, doctors, hospitals, etc), and assign 1 or more other team members to access these sources as needed. Another example would be to assign the pharmacist to take and document the preadmission medication history (if not for all patients, then perhaps for the highest‐risk patients), and assign the physician to verify the history, specify the planned action on admission for each medication, and complete the admission orders. These suggestions are consistent with a study that suggested that physician engagement and an effective team are strongly correlated with successful implementation of medication reconciliation.7
A strength of our study was its use of multiple methods (focus groups and cognitive task analysis) to collect data from key users individually while they interfaced with the system and in groups. Nevertheless, a limitation of the study is that it took place in a single hospital. Though it had a limited number of physician and pharmacist participants, the study sample represented a large fraction of the inpatient staff in those disciplines. We also did not include nurses, hospitalist attending physicians, or other disciplines that might be involved in medication reconciliation in other facilities or settings. However, our study explored the relationship between two disciplines (physicians and pharmacists), yielding findings that could apply to optimizing the function of other interdisciplinary teams.
Our findings can be used to inform improvement efforts in hospitals that have struggled to implement medication reconciliation. Given that the process is slated to return as an accreditation requirement of the Joint Commission,8 hospitals will need to find ways to strengthen the process. Our findings suggest that increasing providers' perceived capability, and confidence in the process and its outcomes, would improve their engagement in the process. This could be accomplished by improved information gathering, including better computer information systems and regional electronic health information exchange, a flexible timeframe for the process, provider training and feedback, and teamwork. In addition, hospitals can make sure their process is working by ascertaining a gold standard medication history on a subset of patients, and comparing the gold standard to the team's history, and admission and discharge orders. Because it is a central component of safe medication prescribing, medication reconciliation will continue to be a focus of state,18 national,19 and international safety efforts20 in the near future.
Acknowledgements
The authors are grateful for the technical assistance of Daniel Signor in the conduct of this work.
Adverse drug events (ADEs) occur when patients transition between the hospital and other care settings. Medication reconciliation, a process by which a provider obtains and documents a thorough medication history with specific attention to comparing current and previous medication use, can prevent transition‐related errors and harm in a variety of care locations.13 Nevertheless, poor intersite communication,4 flawed reconciliation of drug regimens,2 unreliable patient history‐taking, and poor provider decision‐making5 continue to contribute to transition‐related ADEs.
The Joint Commission introduced medication reconciliation as a hospital National Patient Safety Goal in 2006. However, because organizations have had difficulty implementing the process, it stopped citing medication reconciliation deficiencies in its accreditation surveys.6 Although regional and national initiatives have attempted to improve implementation of medication reconciliationusing provider education, workflow, and process reorganization, and organizational change7a recent field review by the Joint Commission suggests that healthcare organizations remain unable to ensure effective medication reconciliation, citing factors beyond the organizations' control, especially unreliable patient histories.8 Still, the process is slated to return as an accreditation requirement of the Joint Commission on July 1, 2011.8
The objective of this study was to determine factors that influenced physicians' and pharmacists' performance of medication reconciliation in a hospital setting with a computerized medical record and medication reconciliation tool, with the goal of informing an organization's approach to implementation. We conducted individual cognitive task analysis (CTA) interviews and focus group interviews to ascertain physicians' and pharmacists' opinions on the purpose and effectiveness of medication reconciliation, their approach to completing the task, and task facilitators and barriers.
METHODS
Setting and Medication Reconciliation Process
The study setting was an urban, academic, tertiary‐care Veterans Affairs (VA) medical center. A computerized medication reconciliation tool and process were developed in 2005 to comply with the Joint Commission's National Patient Safety Goal.6 The tool was embedded in the VA's Computerized Patient Record System (CPRS) and consisted of a dialogue with which a provider (physician, pharmacist, or other provider) could: 1) view the patient's outpatient medication use, for the last 90 days, from VA computerized pharmacy data; 2) view current VA inpatient orders; 3) record discrepancies between patient‐reported medications, and outpatient and inpatient medications in the VA computerized database; 4) record diagnostic indications for, and responses to, these discrepancies; and 5) produce a medication reconciliation document, which was a separate progress note (Figure 1). The tool did not directly facilitate ordering; however, in CPRS, outpatient orders could easily be copied to inpatient orders and vice versa.

Two versions of the medication reconciliation process were implemented upon hospital admission: one in which the physician initiated and completed a reconciliation that was then reviewed by a pharmacist (Figure 2A)the process primarily used on the medical and surgical services; and one in which, after the physician wrote admission orders, the pharmacist initiated and completed the reconciliation and communicated his or her findings with the physician (Figure 2B)the process primarily used on the psychiatric service. At discharge, after the physician wrote discharge orders, the pharmacist completed a reconciliation of preadmission, inpatient, and discharge orders using a tool similar to the admission one (Figure 1). The pharmacist then communicated the reconciliation findings to the physician, similar to the admission medication reconciliation process shown in Figure 2B. These processes and tools were in place for 18 months at the time of the cognitive task analysis, which took place in June 2007, and 32 months at the time of the focus groups, which took place in August 2008.

Participants
Participants consisted of internal medicine house staff physicians rotating on the inpatient service (n = 23) and inpatient staff pharmacists (n = 12). Overall, 14 (40%) were female. The 23 house staff physicians represented approximately 64% of the total house staff inpatient staffing. Thirteen (57%) were in postgraduate year 1 (PGY1), and 10 (43%) were in PGY2 or higher. The 12 pharmacists represented approximately 50% of the total pharmacist inpatient staffing. Individual CTA interviews took place at the end of the academic year (June) with participants who were highly experienced with the process of medication reconciliation in the VA setting. Focus groups took place at the beginning of the academic year (August) with participants who had to endorse the statement that they were experienced completing medication reconciliation. Subjects participated in either the individual or focus group interviews, but not both. Physicians and pharmacists were interviewed separately. All participants provided written informed consent, and the Institutional Review Board of the James J. Peters VA Medical Center approved the study procedures.
Data Collection
Theoretical Model
The Integrated Change Model9 guided our approach to data collection and analysis. It indicates that a person's motivation, intention, and ability determine whether a behavior will be carried out. A person's motivation is influenced by attitudes (eg, perceived pros and cons of the behavior), social influences, and self‐efficacy (eg, perceived capability). The behavior is also influenced by environmental and physical factorsin this case circumstances of the patient encounter, information systems, and the medication reconciliation tool.
Individual Interviews
We conducted individual CTA interviews with 7 physicians and 5 pharmacists. During CTA, participants verbalized their thoughts while they completed medication reconciliation for at least 1 actual case, and at least 1 standardized (fictitious) case, using the computerized medical record and tool. The purpose of this think aloud exercise was to provide information on medication reconciliation tool functionality and usability, problems in humancomputer interaction, and the impact of the tool on decision‐making, clinical practice, and workflow. As the participants interacted with the medication reconciliation tool, computer screens, mouse clicks, and menu selections were recorded using screen recording software (Hypercam), and the participants' thinking aloud was audio‐recorded. This provided the experimenters with context for analyzing and coding subjects' verbalizations (ie, playback of the screens provided detail about the subjects' interaction with the tool, including what functions of the tool they accessed, and problems they may have encountered). Immediately after completing the tasks, subjects were briefly interviewed about their impressions of the tool and their interaction with it. CTA sessions took 30‐50 minutes per participant. Audio‐ and screen‐recordings were synchronized and transcribed.
Focus Group Interviews
We conducted 3 focus groups, 2 with house staff physicians (n = 9 and n = 7), and 1 with pharmacists (n = 7), for a total of 23 focus group participants. The focus group discussion guide was informed by the results of the CTA and began with broad, open‐ended questions,10 followed by a series of more specific probes. Participants were asked to describe medication reconciliation and its purpose, whether they thought that the process influenced their decision‐making and was effective, and how they go about completing the process. Probes included how the task fit into daily workflow, time needed to complete the task, and its priority relative to other tasks. A set of questions asked participants to report barriers and facilitators to completing the task, training needs and experience, and their suggestions on how to improve task implementation and completion. The discussion also included participants' views of the optimal roles of physicians and pharmacists in performing medication reconciliation. Throughout, participants were encouraged to report both positive and negative perceptions. Group interviews lasted 60‐90 minutes, and were audiotaped and transcribed. The same moderator facilitated all 3 focus groups and participated in the individual CTA interviews.
Data Analysis
CTA and focus group transcripts were analyzed using standard social science methods for analyzing qualitative data.1114 Using multiple close readings, investigators performed initial independent coding of each of the transcripts and generated a list of concepts and domains, and a coding scheme. The coding was reviewed from the perspective of the theoretical model to determine whether additional codes were necessary. A research staff member, blinded to the study hypotheses, applied the codes to each transcript by labeling each word, phrase, or line. To test the coding scheme's reliability, a random 5% of transcript lines were coded by 2 independent coders and interrater reliability was assessed. Disagreement was reconciled by discussion and transcripts recoded as appropriate. The investigators then compared codes within and across interviews to elucidate the larger themes that emerged. Frequencies of mention of each domain were calculated. Although the original intent was to analyze the CTA and focus groups separately, themes arising from each of these 2 techniques overlapped sufficiently to allow for a combined analysis. Data were compiled to provide a description of the factors that affect medication reconciliation completion, a summary of barriers and facilitators to use of the medication reconciliation tool, and user suggestions.
RESULTS
Purpose of Medication Reconciliation
Both physicians and pharmacists agreed that a central goal of medication reconciliation is to prevent prescribing errors and adverse drug events that arise from medication utilization over time, and across place and provider. Respondents also agreed that the medication reconciliation document provides a record of patients' history of medication use and of the provider's rationale for medication changes.
Both physicians and pharmacists also indicated awareness of external necessities for completing medication reconciliation, including Joint Commission accreditation standards and, to a lesser extent, medico‐legal liability concerns. On the other hand, 1 physician expressed concern that documented medication discrepancies could be interpreted as mistakes and a liability problem.
Effectiveness of Medication Reconciliation
There was overall disagreement about whether or not medication reconciliation actually achieves its goal of improving medication safety. Many physicians and pharmacists said that medication reconciliation prompts them to perform additional checking of medication lists, dosing, conditions, and interactions, and to better document medication histories and provider decision‐making. On the other hand, some physicians and pharmacists saw it as mainly an administrative task and doubted whether it had any impact on patient care, as this physician indicated: It just seems like another form to fill out .You could be writing a bunch of whatever and no one would notice and no one would say anything and it would never matter. Likewise, a majority of physicians indicated that other parts of the medical record are better sources of prescribing information than the medication reconciliation document.
Medication Reconciliation Process
Physicians and pharmacists agreed that the key components of medication reconciliation are obtaining a medication history from patients and other available sources, identifying differences among medication histories and medications being given, documenting these discrepancies and their reasons, recording a prescribing plan, and counseling the patient. Respondents indicated that the process varies depending on patient complexity, number of medications, encounter type, and provider. For example, physicians were more likely than pharmacists to complete medication reconciliation at once on admission, rather than begin the task and complete it later, as this physician indicated:
It might seem a little tough, but if you don't do it right at that moment, chances [are high] of you forgetting to do it . So it's best done at the moment when you speak to the patient unless you're waiting for some more information.
On the other hand, this physician routinely put off completing medication reconciliation until the day after admission in order to incorporate a more developed prescribing plan:
You can't do it till the end. Until you have your whole plan. when I do an admission note, I write down every single problem and I account for every single medication that I'm going to put someone on in the hospital or not put someone on. And I actually do the medication reconciliation the next day very easily, because I know exactly what I discontinued or why, or added and why. I don't think it's necessary to do it that night [and] you think about it a little bit more.
These quotes suggests that there is an adherence benefit to completing medication reconciliation on admission as a routine, but that this may not be possible, or even desirable, when more time is needed to obtain and verify medication information and make informed prescribing decisions.
Impact of the Computer on the Medication Reconciliation Process
A majority of respondents cautioned about the impact of computerized information and an electronic tool on medication reconciliation. As indicated in these quotes, the first by a physician and the second by a pharmacist, providers' reliance on the computer can lead to less thorough patient interviews, and computerized medication information may be incomplete, both unintended consequences of the electronic health record:
At the VA, you can just easily import [all the information] and you don't even have to ask the patient. So I would imagine more errors get made because people just import whatever meds are in the computer.
If I didn't have [the computerized record], I would be doing patient interviews much more and finding out what they're taking. On the computer, you do all these beautiful notesso I rely a lot on the computer and the whole patient contact thing kind of slides away. When you go to other hospitals, medication reconciliation is extensively patient interview, family interview, calling neighborhood pharmacies, you know, more like detective work and talking to people versus just sitting here typing, so.But the computer is great.
Who Should Perform Medication Reconciliation
Pharmacists and physicians had mixed responses when asked who should perform medication reconciliation. Several physicians indicated that medication reconciliation duplicates what they already do on admission and in progress notes, and therefore is not a good use of their time.
Respondents from both professions questioned the quality of physicians' medication reconciliation. As 1 pharmacist stated about physicians:
They're busy. Whether you like it or not, they're busy. To compare everything, to go across all the sources whether it's what they get here, asking the patient [viewing data from other facilities].There's nothing wrong with the computer [being] able to copy everything that's active and dropping it in. Butyou have to look it over. That's the problem. They don't look it over.
Thus, there was a tension among a majority of respondents between a belief that pharmacists do a better job at medication history‐taking and reconciliation, and a belief that physicians make the prescribing decisions and should be responsible for them. The quote also suggests that a barrier to self‐efficacy among pharmacists is their dependence on physicians to write the orders needed to address the findings of medication reconciliation. Although many respondents recognized that the task needs to be a component of both profession's jobs, suggestions for collaboration between physicians and pharmacists were limited in vision. Suggestions included pharmacists' checking physicians' work, physicians cosigning pharmacists' work, or some other sequenced completion of the task.
Barriers to Medication Reconciliation
Barriers to efficient and effective completion of medication reconciliation according to respondents are shown in Table 1. Physicians and pharmacists both indicated that patients can be unreliable sources of medication information. A few respondents indicated that whereas patients' health conditions change, medication reconciliation occurs at 1 point in time; this can limit its usefulness or make it immediately obsolete.
| Barrier | Barrier Type/Level | Primary Endorser(s) | No. of Endorsers (MDs/PharmDs) |
|---|---|---|---|
| |||
| Competing clinical tasks have higher priority | Provider | Physician | 9/2 |
| Patients provide unreliable information | Patient | Physician | 6/1 |
| Status (active/expired/discontinued) of medications is unclear | System | Physician/pharmacist | 2/5 |
| Need to complete many medication reconciliations | Provider | Pharmacist | 0/6 |
| Preadmission medication list generated by the tool may show medications in duplicate and may require extensive scrolling | Tool | Physician/pharmacist | 4/2 |
| Medication reconciliation tool only picks up information on medications supplied by the VA | Tool | Pharmacist | 0/5 |
| Process to import non‐local VA medications is slow or does not work | System | Physician/pharmacist | 3/2 |
| Patient's health status changes over time | Patient | Physician/pharmacist | 3/1 |
| It is difficult to determine physicians' rationale for prescribing changes, which is needed for the reconciliation document | Provider | Pharmacist | 0/4 |
| Tool is unclear on where to insert revisions to the medication history, changes to the outpatient or inpatient orders, and unresolved medication discrepancies | Tool | Physician | 3/0 |
Both physicians and pharmacists said that medication reconciliation competed for their time with other responsibilities, and physicians placed acute care responsibilities as a higher priority: One sick patient takes all your timeso your mind is on the patient, not on the reconciliation; that's the last thing you worry about.
Pharmacists emphasized that the volume of patients is a barrier: Give me time and I can do a perfect med rec.Honestly, it's work load.I'm sorry, that day where I had 17 people being discharged, I don't think I did such a great job on their med recs.
Respondents indicated several ways in which the computer system itself was a barrier to effective medication reconciliation. First, as previously noted, the computer only picks up information on medications supplied by the VA, and the medication reconciliation tool may only pick up medications supplied by the local facility. Second, sometimes the computer is unclear on the status of medications, as when outpatient medications are automatically discontinued after hospital admission, or when the system automatically imports a medication that is shown to be active but was only meant to be given for a short period of time, such as an antibiotic. Several barriers specific to tool usability are also shown in Table 1.
Suggestions for Improving Medication Reconciliation
Physicians' and pharmacists' suggestions for improving medication reconciliation are shown in Table 2. First, there was recognition of the need for someone in addition to the author to check the medication reconciliation document to find mistakes. Second, respondents indicated that better provider training might improve medication reconciliation's effectiveness. Both physicians and pharmacists indicated that their education consisted of a limited amount of on‐the‐job training, such as a walk through with a supervisor the first time. When asked for suggestions for improving education, both physicians and pharmacists suggested that physicians should receive case‐based education, during which the purpose of the task is emphasized. These respondents, the first a pharmacist and the second a physician, called for provider feedback to improve and maintain reliability:
| Suggestion | Targeted Level | Primary Endorser(s) | No. of Endorsers (MDs/PharmDs) |
|---|---|---|---|
| |||
| Place checkbox next to each medication | Tool | Physician/pharmacist | 6/2 |
| Order or label medication by condition or diagnosis | Tool | Physician/pharmacist | 2/3 |
| Someone in addition to the author should check the medication reconciliation note and provide feedback and corrections | Provider | Physician/pharmacist | 2/2 |
| Enable searchable medication history | System | Physician | 2/2 |
| Enable automatic importing of medication information from other VAs | System/tool | Physician/pharmacist | 1/2 |
| Reconciliation document should be signed by both physician and pharmacist | Provider | Pharmacist | 0/2 |
| Task should have dedicated staffing | Provider | Pharmacist | 0/2 |
| Facilitate viewing of preadmission medication list and inpatient orders side‐by‐side, instead of topbottom | Tool | Pharmacist | 0/2 |
| Make template more concise | Tool | Pharmacist | 0/2 |
| Automatically convert medication reconciliation planned actions into orders | System/tool | Pharmacist | 0/2 |
| Automatically insert medication reconciliation documentation into admission note | System/tool | Physician | 2/0 |
Have somebody really look at the quality of the reconciliation and speak to whoever did it, whether it's done correctly or not correctly. Because I've seen too many people just use the template, click, click, and then sign. You can finish the note [in] two minutes, but it's not going to be accurate and it's not going to do the patient any good.
We just keep on doing the same thing without ever learning [whether it is] the right way. That's where [we get] this [idea that] we are just doing it for the sake of doing it.
These quotes suggest that a lack of review and feedback about the reconciliation process appears to impact both perceived importance and quality.
DISCUSSION
In this study of hospital physicians' and pharmacists' perspectives on medication reconciliation, we found that although respondents agreed about its main purposeto improve prescribing safetya near majority believed that it was of uncertain benefit to patients and limited use to providers. This might, in part, be because of a tool that was not adequately integrated into workflow, making it extraneous to patient care. As a consequence, many respondents' indicated that when they had competing tasks, especially other acute care responsibilities, medication reconciliation was displaced in priority. Respondents indicated that unreliability of patient medication histories was also a major barrier, which is consistent with a recent Joint Commission field review in which organizations cited this as a task hindrance beyond their control.8 Lastly, respondents revealed limited perception of it being a team‐oriented task.
Our study also probed providers' perceptions of the effect of computerization on completing medication reconciliation. Although study respondents indicated that the computerized tool reduced the time required to complete the task, they also expressed concern that because medication reconciliation was automated in part at the VA, they spent less time with patients on the process. This finding is important because, according to 1 study, many medications are not captured by the VA's Computerized Patient Record System,15 and a patient's lack of medication adherence may not be evident in the CPRS. This finding is also consistent with our prior work that has not demonstrated an inherent advantage to electronic communication of medication information over paper.16
Our study suggests that, for medication reconciliation to be improved, provider self‐efficacy and engagement with the process must be increased. This might involve addressing negative provider attitudes, changing workflow, and improving provider confidence by improving information reliability. With regard to changing attitudes, team members should be briefed on research evidence that shows that medication reconciliation is effective in preventing ADEs13 and is cost‐effective17 to help to increase the value that providers place on medication reconciliation. With regard to workflow, the tool has to be optimized to facilitate information gathering, processing, and medication ordering. Our findings also suggest that medication reconciliation would benefit from widening the time window in which it should be completed (eg, to the first or second business day after admission), since this increases the time available to access multiple data sources, and for providers to update the preadmission medication history and to act on new medication information. Finally, regional electronic health information exchange would improve information reliability and provider confidence in the information.
Our findings also suggest that assignment of productive teamsconsisting of physicians, physician‐extenders, nurses, pharmacists, and/or administrative support staffrather than individuals to the task might improve task completion. Efficacy and perceived capability might be improved by dividing the task into parts more easily manageable by individual team members. One example would be to assign 1 team member to record all of the sources of medication information available for each patient (the patient's home, pharmacies, doctors, hospitals, etc), and assign 1 or more other team members to access these sources as needed. Another example would be to assign the pharmacist to take and document the preadmission medication history (if not for all patients, then perhaps for the highest‐risk patients), and assign the physician to verify the history, specify the planned action on admission for each medication, and complete the admission orders. These suggestions are consistent with a study that suggested that physician engagement and an effective team are strongly correlated with successful implementation of medication reconciliation.7
A strength of our study was its use of multiple methods (focus groups and cognitive task analysis) to collect data from key users individually while they interfaced with the system and in groups. Nevertheless, a limitation of the study is that it took place in a single hospital. Though it had a limited number of physician and pharmacist participants, the study sample represented a large fraction of the inpatient staff in those disciplines. We also did not include nurses, hospitalist attending physicians, or other disciplines that might be involved in medication reconciliation in other facilities or settings. However, our study explored the relationship between two disciplines (physicians and pharmacists), yielding findings that could apply to optimizing the function of other interdisciplinary teams.
Our findings can be used to inform improvement efforts in hospitals that have struggled to implement medication reconciliation. Given that the process is slated to return as an accreditation requirement of the Joint Commission,8 hospitals will need to find ways to strengthen the process. Our findings suggest that increasing providers' perceived capability, and confidence in the process and its outcomes, would improve their engagement in the process. This could be accomplished by improved information gathering, including better computer information systems and regional electronic health information exchange, a flexible timeframe for the process, provider training and feedback, and teamwork. In addition, hospitals can make sure their process is working by ascertaining a gold standard medication history on a subset of patients, and comparing the gold standard to the team's history, and admission and discharge orders. Because it is a central component of safe medication prescribing, medication reconciliation will continue to be a focus of state,18 national,19 and international safety efforts20 in the near future.
Acknowledgements
The authors are grateful for the technical assistance of Daniel Signor in the conduct of this work.
- ,,, et al.Effect of an electronic medication reconciliation application and process redesign on potential adverse drug events: a cluster‐randomized trial.Arch Intern Med.2009;169:771–780.
- ,,, et al.Medication reconciliation: a practical tool to reduce the risk of medication errors.J Crit Care.2003;18:201–205.
- ,,,,.Medication reconciliation for reducing drug‐discrepancy adverse events.Am J Geriatr Pharmacother.2006;4:236–243.
- ,,,.Patient transfer from nursing home to emergency department: outcomes and policy implications.Acad Emerg Med.1997;4:908–915.
- ,,, et al.Systems analysis of adverse drug events. ADE Prevention Study Group.JAMA.1995;274:35–43.
- Joint Commission Hospital National Patient Safety Goal #8. Available at: http://www.jointcommission.org/AccreditationPrograms/ Hospitals/NPSG/. Accessed November 10,2010.
- ,,, et al.Reconciling medications at admission: safe practice recommendations and implementation strategies.Jt Comm J Qual Patient Saf.2006;32:37–50.
- Update: medication reconciliation. National Patient Safety Goal field review results. Joint Commission online June 2, 2010. Available at: http://www.jointcommission.org/NR/rdonlyres/17295FE3–6643‐48E6–89A5‐C629 609E3F36/0/jconlineJune210.pdf. Accessed November 10,2010.
- ,,,.The general public's information needs and perceptions regarding hereditary cancer: an application of the integrated change model.Patient Educ Couns.2005;56:154–165.
- ,.Involving Community Members in Focus Groups.Thousand Oaks, CA:Sage;1998.
- ,.Qualitative Data Analysis.Thousand Oaks, CA:Sage;1994.
- .How to Use Qualitative Methods in Evaluation.Newbury Park, CA:Sage;1987.
- .Handbook of Research Design and Social Measurement,5th ed.Newbury Park, CA:Sage;1991.
- .An Introduction to Qualitative Research.London:Sage;1998.
- ,,,.Assessing the accuracy of computerized medication histories.Am J Manag Care.2004;10:872–877.
- ,,, et al.Electronic health records and adverse drug events after patient transfer.Qual Saf Health Care.2010;19:1–5.
- ,,.Model‐based cost‐effectiveness analysis of interventions aimed at preventing medication error at hospital admission (medicines reconciliation).J Eval Clin Pract.2009;15:299–306.
- Massachusetts Hospital Association. Reconciling medications. A Medication Safety Collaborative sponsored by the MA Coalition for the Prevention of Medical Errors. Available at: http://www.macoalition.org/Initiatives/RecMeds/ProjectBackground.pdf. Accessed November 10,2010.
- Patient safety medication systems tools. Available at: http://www. ihi.org/IHI/Topics/PatientSafety/MedicationSystems/Tools/. Accessed November 10,2010.
- Danish Society for Patient Safety: Operation Life Denmark. Available at: http://patientsikkerhed.dk/en/about_the_danish_society_for_patient_ safety/activities/. Accessed November 10,2010.
- ,,, et al.Effect of an electronic medication reconciliation application and process redesign on potential adverse drug events: a cluster‐randomized trial.Arch Intern Med.2009;169:771–780.
- ,,, et al.Medication reconciliation: a practical tool to reduce the risk of medication errors.J Crit Care.2003;18:201–205.
- ,,,,.Medication reconciliation for reducing drug‐discrepancy adverse events.Am J Geriatr Pharmacother.2006;4:236–243.
- ,,,.Patient transfer from nursing home to emergency department: outcomes and policy implications.Acad Emerg Med.1997;4:908–915.
- ,,, et al.Systems analysis of adverse drug events. ADE Prevention Study Group.JAMA.1995;274:35–43.
- Joint Commission Hospital National Patient Safety Goal #8. Available at: http://www.jointcommission.org/AccreditationPrograms/ Hospitals/NPSG/. Accessed November 10,2010.
- ,,, et al.Reconciling medications at admission: safe practice recommendations and implementation strategies.Jt Comm J Qual Patient Saf.2006;32:37–50.
- Update: medication reconciliation. National Patient Safety Goal field review results. Joint Commission online June 2, 2010. Available at: http://www.jointcommission.org/NR/rdonlyres/17295FE3–6643‐48E6–89A5‐C629 609E3F36/0/jconlineJune210.pdf. Accessed November 10,2010.
- ,,,.The general public's information needs and perceptions regarding hereditary cancer: an application of the integrated change model.Patient Educ Couns.2005;56:154–165.
- ,.Involving Community Members in Focus Groups.Thousand Oaks, CA:Sage;1998.
- ,.Qualitative Data Analysis.Thousand Oaks, CA:Sage;1994.
- .How to Use Qualitative Methods in Evaluation.Newbury Park, CA:Sage;1987.
- .Handbook of Research Design and Social Measurement,5th ed.Newbury Park, CA:Sage;1991.
- .An Introduction to Qualitative Research.London:Sage;1998.
- ,,,.Assessing the accuracy of computerized medication histories.Am J Manag Care.2004;10:872–877.
- ,,, et al.Electronic health records and adverse drug events after patient transfer.Qual Saf Health Care.2010;19:1–5.
- ,,.Model‐based cost‐effectiveness analysis of interventions aimed at preventing medication error at hospital admission (medicines reconciliation).J Eval Clin Pract.2009;15:299–306.
- Massachusetts Hospital Association. Reconciling medications. A Medication Safety Collaborative sponsored by the MA Coalition for the Prevention of Medical Errors. Available at: http://www.macoalition.org/Initiatives/RecMeds/ProjectBackground.pdf. Accessed November 10,2010.
- Patient safety medication systems tools. Available at: http://www. ihi.org/IHI/Topics/PatientSafety/MedicationSystems/Tools/. Accessed November 10,2010.
- Danish Society for Patient Safety: Operation Life Denmark. Available at: http://patientsikkerhed.dk/en/about_the_danish_society_for_patient_ safety/activities/. Accessed November 10,2010.
Copyright © 2011 Society of Hospital Medicine
Ten Things About Elderly Patients
Recent studies of the growth of the hospitalist movement have confirmed that hospitalists are taking care of an increasing percentage of hospitalized older adults.1, 2 Unfortunately, few hospitalist groups have specific programs or protocols to address the special needs of older patients.3, 4 We were inspired by a top 10 article written by nephrologists for primary care physicians to compile 10 evidence‐based pearls that may be helpful to hospitalists caring for older persons.5
1. Many Health Conditions in Older Patients are Multifactorial and Require a Multifactorial Approach to Care
A geriatric syndrome is a multifactorial condition occurring primarily in frail elderly which is usually due to multiple contributing factors and results from an interaction between patient‐specific impairments and situation‐specific stressors.68 Geriatric syndromes cannot be treated easily with a single intervention; the approach must target each modifiable risk factor. Table 1 illustrates for an example of how this approach differs from the traditional medical approach in the case of falls, a common geriatric syndrome. Hospitalists commonly encounter the geriatric syndromes that either lead to hospitalization, such as falls or dementia, or that can complicate a hospitalization for another condition, such as delirium, falls, or urinary incontinence. Two geriatric syndromes in particular, delirium and falls, are associated with increased length of stay, increased healthcare costs, and substantial morbidity.9, 10 Multifactorial interventions such as the Hospital Elder Life Project (HELP) have been shown to prevent delirium and falls.1114 Studies of the HELP intervention demonstrated benefits on outcomes that are important to hospitalists, including shortened length of stay.10, 15 While implementing these interventions is not possible at every institution, it may be possible to implement some aspects in a targeted fashion (for example, early physical therapy and mobilization to decrease risk of delirium and falls) or to work with hospital administration to implement the larger interventions.
| Traditional Medical Approach (Diagnosis and Treatment) | Geriatric Approach (Risk Factor Assessment and Reduction) | |
|---|---|---|
| ||
| Search for cause of falls | Search for cause of falls | |
| (eg, syncope workup including echocardiogram, neurologic workup including imaging) | Assess for risk factors and target interventions reduce or eliminate risk factors identified, eg: | |
| Risk factor | Intervention | |
| Leg weakness | Strength training | |
| Falls when transferring | Training in use of assistive device for transfers | |
| Inadequate lighting at home | Home safety evaluation by physical therapist and installation of lights at home | |
2. Screening Elderly Patients for the Presence of Common Geriatric Syndromes can Improve Care
Comprehensive geriatric assessment, a process of multifactorial health evaluation of the older patient, is an important part of geriatric medicine and has been shown to reduce functional decline.16 While comprehensive geriatric assessment may not be practical in the acute care setting, some basic principles and tools of geriatric assessment, namely screening for geriatric syndromes, can be helpful. Here is one efficient, evidence‐based approach to screening hospitalized elderly patients for cognitive impairment and falls, and then using the results of screening to improve care:
Brief cognitive assessment using the Mini‐Cog screening tool
Even with cognitive screening, it can sometimes be difficult to distinguish between dementia and delirium in an acutely ill person. However, knowing who is cognitively impaired on admission tells us who is likely to develop delirium and may need to be discharged to a supervised location, such as a nursing facility.17, 18 The Mini‐Cog (shown in Table 2) is our preferred tool for cognitive screening, because it takes only 24 minutes to administer, has a sensitivity of 76% and specificity of 89% [similar to the Mini‐Mental State Examination (MMSE)], and has been validated in a multiethnic, multilingual sample with low literacy.19, 20 The Mini‐Cog has not been studied extensively in the inpatient setting, but one study did find that abnormal Mini‐Cog scores correlated with the development of delirium among hospitalized elderly patients.21 Other tools appropriate for brief cognitive screening of hospitalized patients include the MMSE, which is widely known but limited by copyright for clinical use and longer time needed for administration, and the Short Portable Mental Status Questionnaire (SPMSQ), which is entirely verbal and does not require that the patient write or draw.22, 23 A new tool, the Sweet 16, which is also entirely verbal and takes about 2 minutes to administer, shows promise but has not been validated in the inpatient setting.24
|
| The Mini‐Cog combines an uncued three‐item recall test with a clock‐drawing test (CDT) that serves as the recall distractor. The Mini‐Cog can be administered in about three minutes, requires no special equipment, and is less influenced by level of education or language differences. |
| Administration |
| 1. Make sure you have the patient's attention. Then instruct the patient to listen carefully to, repeat back to you, and remember (now and later) three unrelated words. You may present the same words up to 3 times if necessary. |
| 2. Instruct the patient to draw the face of a clock, either on a blank sheet of paper, or on a sheet with the clock circle already drawn on the page. After the patient puts the numbers on the clock face, ask him or her to draw the hands to read a specific time (11:10 or 8:20 are most commonly used; however, other times that require use of both halves of the clock face may be effective). These instructions can be repeated, but no additional instructions should be given. If the patient cannot complete the CDT in three minutes or less, move on to the next step. |
| 3. Ask the patient to repeat the three previously presented words. |
| Scoring |
| Give 1 point for each recalled word after the CDT distractor. Score 03 for recall. |
| Give 2 points for a normal CDT, and 0 points for an abnormal CDT. The CDT is considered normal if all numbers are depicted, once each, in the correct sequence and position around the circle, and the hands readably display the requested time. Do not count equal hand length as an error. Add the recall and CDT scores together to get the Mini‐Cog score: |
| 02 positive screen for dementia. |
| 35 negative screen for dementia. |
Fall screen: Have you fallen in the past year? with more detailed inquiry for frequent fallers
An elderly patient with a history of falls is at high risk for falling while in the hospital. Interventions that target multiple risk factors appear to be effective in reducing risk and rate of falling.25 Some aspects of these multifactorial interventions include environmental modifications such as low beds, high‐impact floor mats, restraint removal, bedside posters and patient education materials, and exercise.13, 26 Medication review and reductions in medications that contribute to falls (psychoactive medications, diuretics, blood pressure agents) can also reduce fall risk.13, 14 Many experts recommend checking a 25‐hydroxy vitamin D level for patients who come into the hospital for treatment of a fall‐related injury, starting or continuing vitamin D and calcium supplementation for those with normal vitamin D levels, and starting aggressive vitamin D replacement with 50,000 units of oral ergocalciferol weekly for those with a level below 20 ng/mL. Vitamin D appears to play an important role in neuromuscular function as well as bone density.27 Meta‐analyses have demonstrated that vitamin D supplementation of 7001,000 IU daily may reduce the risk of falls in the elderly by about 20%, that the effect is achieved in the short term (on the order of 25 months), and that the benefit persists for up to 36 months.28, 29
3. Elderly Patients are at Risk for Functional Decline During Hospitalization and Measures Can Be Taken to Prevent This
A cascade to dependency has been described in which the effects of usual aging (eg, fragile skin) and hospitalization (eg, immobilization) interact with each other, often producing complications (eg, pressure ulcers) that can lead to the loss of the ability to live independently.30 Functional decline during hospitalization is common and is associated with poor prognosis. In one study of elderly patients with new Activities of Daily Living (ADL) disability at discharge, over 40% were dead by 12 months after discharge, and only 30% had returned to baseline function.31, 32 Functional status may affect discharge planning, especially if the patient is not able to return safely to his or her previous living environment. Elderly patients are very vulnerable to new ADL dependency and loss of ambulatory ability as a result of a hospitalization.33, 34 The initial evaluation of an elderly patient should always include an inquiry into the patient's functional abilities, including whether the patient requires assistance to perform self‐care (ADL) activities or instrumental activities of daily living (IADLs), and who is available to help at home. We remember ADLs as those activities we learn as a young child (bathing, dressing, feeding, toileting, transfers) and IADLs as activities we learn when we leave our parents' home to live independently (shopping, cooking, laundry, housekeeping, managing money, using public transportation). Patients in assisted living facilities and adult family homes receive varying degrees of help with ADLs and IADLs, and no particular level of assistance can be assumed; assisted living residents may be at higher risk of functional decline during hospitalization than community‐dwelling persons.35
Acute Care of Elderly (ACE) units have been shown to reduce functional disability and discharge to long‐term care settings, with length of stay and hospital charges similar to that of usual care.36 Most hospitals do not have ACE units, but implementing some components of the intervention, such as early physical and occupational therapy, early discharge planning, early mobility, and adequate nutrition may reduce functional disability. A Cochrane review found a small decrease in length of stay of about a day among elderly patients who participated in a structured exercise program, which could be extrapolated to support the usefulness of increased mobility and early physical therapy.37
4. Delirium is a Common but Serious Condition Among Hospitalized Elderly Patients and in Some Cases Can Be Prevented
The incidence of delirium appears to be as high as 60% in hospitalized elderly patients with multiple risk factors for this condition.38, 39 Delirium predicts longer hospital stay, increases likelihood of discharge to a skilled nursing facility, and is a marker for significant morbidity and mortality.4042 Cognitive screening, as discussed above, can help determine who is at risk for delirium, and the Confusion Assessment Method (CAM) is becoming more widely used as a bedside screening tool for detecting delirium.43 Other important risk factors for delirium in addition to preexisting cognitive impairment include age over 65, prior delirium, polypharmacy, functional dependence, and sensory impairment.44 A landmark randomized controlled trial by Sharon Inouye et al. showed that delirium can often be prevented in high‐risk patients by ensuring frequent reorientation, maintenance of the patient's usual sleep‐wake cycle, early mobilization, adequate nutrition and hydration, and regular use of sensory aids such as glasses and hearing aids.11
When delirium does occur despite preventive efforts, it should trigger a thorough investigation for an underlying cause. Common contributing factors to delirium in hospitalized patients include infections such as urinary tract infections or hospital‐acquired pneumonia, pain, electrolyte abnormalities, cardiac ischemia, medication side effects, and urinary retention or constipation. Any psychoactive or anticholinergic medication can contribute to, or exacerbate, delirium; common culprits include diphenhydramine, benzodiazepines, and muscle relaxants.44 Although one or more treatable underlying causes should always be sought, many cases of delirium in the hospital are multifactorial.
If pharmacologic treatment for delirium is necessary, low‐dose haloperidol is the first‐line agent, starting with 0.25 mg intravenously or 0.5 mg by mouth, nightly to twice a day. A recent systematic review supported the use of haloperidol as a first‐line agent in the acute setting; second‐generation antipsychotics are an alternative, but studies have shown no benefits of these agents over haloperidol in terms of safety or efficacy.45 Physical restraints should be avoided in delirious patients, as they may actually worsen delirium and have not been proven to reduce falls or secondary injury.46
5. Treating Nondementia Illnesses in Hospitalized Elders With Dementia Requires Consideration of the Patient's Limited Life Expectancy and Cognitive Deficits
Elderly patients with dementia are frequently hospitalized for medical illness and pose special challenges to the hospitalist. Patients with dementia may not be able to accurately report symptoms and side effects of therapy, and may lack decisional capacity. The clinician should also carefully consider the benefits and burdens of a proposed therapy or diagnostic test, taking into account the patient's cognitive deficits and limited life expectancy.47 The median postdiagnosis life expectancy of a patient with Alzheimer disease is about 4 years for men and about 6 years for women, with a range from about 2 to 9 years, according to population‐based studies of incident Alzheimer dementia.48, 49 In a patient with early or mild dementia, the hospitalist may first need to establish whether the patient does indeed have dementia. It is also important to question family about how the patient is managing at home so that appropriate discharge plans can be made.
Any intervention such as surgery or intubation in a patient with dementia should be considered carefully in the context of the patient's dementia, keeping in mind the remaining life expectancy, increased risk for delirium after surgery, and potential difficulty with adherence to postoperative precautions or participation in rehabilitation. Invasive procedures such as mechanical ventilation or central line placement may not be indicated in patients with advanced dementia. In one prospective cohort study, patients with advanced dementia admitted to the hospital for hip fractures or pneumonia had much higher mortality than those without dementia, despite having equal rates of procedures such as mechanical ventilation and central line placement.50
A common scenario in a patient with advanced dementia is the patient presenting with decreased intake and dysphagia; family and/or caregivers inquire about enteral tube feeding and/or long‐term intravenous hydration. This situation requires a thoughtfully presented, informative discussion with the family about artificial feeding in advanced dementia. There is no evidence that artificial feeding improves outcomes, and a recent Cochrane systematic review found insufficient evidence to suggest that enteral tube feeding is beneficial in patients with dementia.5153 Instead, dementia patients at the end of their lives benefit from a palliative approach, which would include hand‐feeding for comfort if the patient indicates hunger.54
6. Frail Elderly Patients are at High Risk for Iatrogenic Complications During Hospitalization
Frail elders are at increased risk for iatrogenic complications, including surgical‐ and procedure‐related events, adverse drug events, nosocomial infections, pressure ulcers, and falls.5557 In the Harvard Medical Practice Study, a retrospective study of adverse events in 30,000 patients, those age 65 and over accounted for 27% of the hospitalized population but 43% of the adverse events. In this study, four types of adverse events occurred at least twice as often in older patients: fractures, falls, nontechnical postoperative complications, and adverse events related to noninvasive treatments.58 The risk of hospital‐acquired infection also rises dramatically with age; risk is ten times higher in patients over 70 than those aged 49 and under, with urinary tract infections and surgical site infections the most common.59 The oldest, most seriously ill and most functionally impaired elderly patients are at particularly high risk of cascade iatrogenesis, where one medical intervention triggers a series of adverse events.60 Frail elderly patients are at greater risk for adverse events during hospitalization, and careful consideration of the need for all diagnostic tests and procedures may reduce risks. Table 3 lists the iatrogenic complications that disproportionately affect the elderly.
7. Pain Should Be Aggressively Identified and Appropriately Treated
Adequate treatment of pain in the elderly can improve functional status, mobility, and mood. Clinicians are understandably wary of prescribing opioids in the elderly, but undertreatment of pain is also common and in hospitalized patients can lead to delirium, delays in rehabilitation, and higher healthcare costs.44, 61 Acetaminophen is generally safe and is recommended as first‐line therapy in most cases of pain. Nonsteroidal antiinflammatory drugs (NSAIDS) should be used with caution, as the risk of gastrointestinal toxicity increases with age; long‐acting agents such as toradol should be avoided.62 A study of adverse drug reactions causing hospitalization in elderly individuals implicated NSAIDs in 23.5% of cases.63 If a patient has severe pain that limits functional capacity but does not respond to acetaminophen, it is reasonable to consider low‐dose opioids. The 2009 American Geriatrics Society guidelines recommend that opioids be used for severe pain, especially when it limits functional capacity. Opioids should be given orally when possible and started at low doses (for example, 2.5 to 5 milligrams of oxycodone offered every 4 hours as needed). Either oxycodone or morphine can be used as a first‐line, short‐acting opioid, but morphine should be used cautiously in patients with renal insufficiency, as toxic metabolites are renally cleared. Methadone should be used very cautiously and ideally by clinicians experienced in its use because of its long and variable half‐life. Serotonin‐norepinephrine reuptake inhibitors such as duloxetine, and anticonvulsants such as gabapentin, can be helpful adjuncts for neuropathic pain; tricyclics should generally be avoided, because the elderly are particularly sensitive to the anticholinergic side effects (ie, sedation, orthostatic hypotension, urinary retention, constipation) of these agents.61 Nortriptyline, which has the least anticholinergic properties of this medication class, may be the best option if a tricyclic is desired.
8. Hospitalization Can Be an Opportunity for a Thorough Review and Reduction of Outpatient Medications in Collaboration With the Patient's Primary Care Provider
Adverse drug reactions (ADRs) are potentially life‐threatening for all patients, but elderly individuals are disproportionately affected. It is estimated that 30% of hospital admissions of older patients are due to drug‐related toxicity.64 The Beers criteria provide expert consensus on drugs that should either be avoided or used cautiously in the elderly; we have highlighted some of the drugs on the Beers list that are relevant to the hospitalist in Table 4. Notable inclusions on the most recent Beers list include nitrofurantoin, ferrous sulfate at dosages greater than 325 mg per day, doxazosin, and fluoxetine.65 Anticholinergics (such as oxybutynin) and cholinesterase inhibitors (such as donepezil) should not be used together, because the interaction makes them ineffective.
| Falls and fractures58 |
| Nosocomial infections59 |
| Delirium44 |
| Surgical and postoperative complications58 |
| Adverse drug events56 |
| Pressure sores56 |
| Medication | Rationale |
|---|---|
| |
| Benzodiazepines | Can contribute to delirium and falls. |
| Muscle relaxants | Anticholinergic side effects; sedating; can contribute to delirium. |
| Diphenhydramine | Very sedating; can contribute to delirium; do not use as a sleep aid, and use lowest possible dose when treating allergic reactions. |
| Nitrofurantoin | Can cause renal impairment. |
| Ketorolac | Can lead to gastrointestinal bleeds and renal impairment. |
| Ferrous sulfate in doses >325 mg/day | Can cause severe constipation; higher doses not well absorbed. |
| Digoxin in doses of >0.125 mg/day | Decreased renal clearance can increase risk of toxicity (higher doses acceptable for atrial arrythmias). |
| Amiodarone | Multiple toxicities; lack of proven efficacy in older adults. |
| Fluoxetine | Long half‐life; can cause agitation and sleep disturbance. |
| Meperidine | Can cause seizures and contribute to delirium. |
| Doxazosin | Can cause hypotension and contribute to falls. |
Many ADRs are caused by medications that are not on the Beers list but have narrow therapeutic windows, especially warfarin and insulin; digoxin, which is on the Beers list, is another common culprit in ADRs leading to emergency room visits.66 Medications that are clearly causing ADRs should be discontinued, with the knowledge and input of the primary care provider, and with written documentation on the discharge summary and clear instructions to the patient. Even when a hospital admission is not a result of an ADR, it may provide an opportunity to review the patient's medication list, clarify that the patient is taking medications as directed by their primary care provider, and determine if the list contains medications where risks now outweigh benefits, in which case those medications should be discontinued. Studies have shown that elimination of medications that are associated with falls reduces the risk of future falls.67 Adjustments to patient's medications should be made in close collaboration with the primary care provider and with a clinical pharmacist, if available.
9. Discharge Planning for an Elderly Patient Should Start Early, Be Comprehensive, and Involve the Patient and Caregivers
A successful discharge plan for an elderly patient must start early, take functional status into account, and perhaps most importantly, the planning process should involve the patient and family, if appropriate. One study of patients over 70 discharged from an urban, academic public hospital found that only 10% recalled receiving written discharge instructions, and only 53% who were prescribed a new medication actually had that medication at home.68 Growing evidence suggests that older patients have poorer functional health literacy regarding medications.69 Care transitions are an active area of research, and multiple studies have shown lower rates of rehospitalization with comprehensive transitional care programs involving transition coaches or advance practice nurses providing home visits and follow‐up telephone calls.70, 71 More research is needed in this area to develop simple, cost‐effective care transitions programs. In the meantime, hospitalists can actively involve a multidisciplinary team (pharmacists and social workers, for example) early in the hospitalization, provide more patient‐friendly discharge materials such as those available as part of the Society of Hospital Medicine's BOOST, and place targeted follow‐up phone calls after discharge.52, 7274 Ensuring that a frail elderly patient's primary care provider is aware of the hospitalization may be particularly important in avoiding postdischarge problems.75
10. Elderly Patients are Often Very Clear About Their Preferences for Treatment. Discussing This on Admission Can Help Clarify the Goals of the Hospitalization
The risks and benefits of any intervention being considered should be presented and discussed with the patient in the context of the likelihood of the intervention restoring an acceptable quality of life to the patient. Many common chronic conditions in the elderly such as Parkinson disease, congestive heart failure, and dementia are progressive and life‐limiting. Qualitative studies confirm that elderly patients want to know the likelihood that a proposed intervention will help them return to their previous level of functioning or a level that is acceptable to them.76, 77 In one prospective cohort study, older age was found to correlate with a higher rate of withholding ventilatory support, dialysis, and surgery.78 However, a more recent study has suggested that patient preferences are more dynamic, and that older patients will make decisions based on current health status rather than core values.76 While families should be involved in the process of clarification of goals of care, the patients' own values should be elicited whenever possible, with bedside assessment of decision‐making capacity to ensure that patients are allowed to speak for themselves if they possess decisional capacity for the decision at hand.79
Hospitalists should have a special approach to the elderly patient which takes into account the elder's increased vulnerability to iatrogenic complications and acute functional decline, the presence of geriatric syndromes, and individual preferences. Early assessment of an elderly patient's cognitive and physical functioning, level of pain, fall risk, and preferences for care are necessary for optimal inpatient care and may result in preserved function and a decrease in adverse events.
- , , , .Growth in the care of older patients by hospitalists in the United States.N Engl J Med.2009;360(11):1102–1112.
- , , , , , .Continuity of outpatient and inpatient care by primary care physicians for hospitalized older adults.JAMA.2009;301(16):1671–1680.
- , , .Is there a geriatrician in the house? Geriatric care approaches in hospitalist programs.J Hosp Med.2006;1(1):29–35.
- .Care of hospitalized older patients: opportunities for hospital‐based physicians.J Hosp Med.2006;1(1):42–47.
- , .The top 10 things nephrologists wish every primary care physician knew.Mayo Clin Proc.2009;84(2):180–186.
- .What is a geriatric syndrome anyway?J Am Geriatr Soc.2003;51(4):574–576.
- , , , .Geriatric syndromes: clinical, research, and policy implications of a core geriatric concept.J Am Geriatr Soc.2007;55(5):780–791.
- , , .Some considerations regarding geriatric syndromes.Ann Intern Med.2001;135(12):1095.
- , , , et al.Impact and recognition of cognitive impairment among hospitalized elders.J Hosp Med.2010;5(2):69–75.
- , , , , , .A longitudinal analysis of total 3‐year healthcare costs for older adults who experience a fall requiring medical care.J Am Geriatr Soc.2010;58(5):853–860.
- , , , et al.A multicomponent intervention to prevent delirium in hospitalized older patients.N Engl J Med.1999;340(9):669–676.
- , , , .Shared risk factors for falls, incontinence, and functional dependence. Unifying the approach to geriatric syndromes.JAMA.1995;273(17):1348–1353.
- , , , et al.Strategies to prevent falls and fractures in hospitals and care homes and effect of cognitive impairment: systematic review and meta‐analyses.BMJ.2007;334(7584):82.
- , , , et al.Interventions for preventing falls in acute‐ and chronic‐care hospitals: a systematic review and meta‐analysis.J Am Geriatr Soc.2008;56(1):29–36.
- , , , et al.Postoperative delirium in the elderly: risk factors and outcomes.Ann Surg.2009;249(1):173–178.
- , , , et al.A controlled trial of inpatient and outpatient geriatric evaluation and management.N Engl J Med.2002;346(12):905–912.
- , , , et al.Risk factors for preoperative and postoperative delirium in elderly patients with hip fracture.J Am Geriatr Soc.2009;57(8):1354‐1361.
- , , , , , .Risk factors for delirium in intensive care patients: a prospective cohort study.Crit Care.2009;13(3):R77.
- , , , .The Mini‐Cog as a screen for dementia: validation in a population‐based sample.J Am Geriatr Soc.2003;51(10):1451–1454.
- , , , , .Improving identification of cognitive impairment in primary care.Int J Geriatr Psychiatry.2006;21(4):349–355.
- , , , et al.Simple cognitive testing (Mini‐Cog) predicts inhospital delirium in the elderly.J Am Geriatr Soc.2007;55(2):314–316.
- , , , .Short Portable Mental Status Questionnaire as a screening test for dementia and delirium among the elderly.J Am Geriatr Soc.1987;35(5):412–416.
- , , .“Mini‐mental state.” A practical method for grading the cognitive state of patients for the clinician.J Psychiatr Res.1975;12(3):189–198.
- , , , et al.Development and validation of a brief cognitive assessment tool: the Sweet 16.Arch Intern Med.2010. [Epub ahead of Print].
- , , , et al.Interventions for preventing falls in older people in nursing care facilities and hospitals.Cochrane Database Syst Rev.2010;(1):CD005465.
- , , , et al.Fall prevention in acute care hospitals: a randomized trial.JAMA.2010;304(17):1912–1918.
- , , , et al.Vitamin D supplementation improves neuromuscular function in older people who fall.Age Ageing.2004;33(6):589–595.
- , , , et al.Effect of vitamin D on falls: a meta‐analysis.JAMA.2004;291(16):1999–2006.
- , et al.Fall prevention with supplemental and active forms of vitamin D: a meta‐analysis of randomised controlled trials.BMJ.2009;339:b3692.
- .Hazards of hospitalization of the elderly.Ann Intern Med.1993;118(3):219–223.
- , , , .Hospitalization, restricted activity, and the development of disability among older persons.JAMA.2004;292(17):2115–2124.
- , , , et al.Recovery of activities of daily living in older adults after hospitalization for acute medical illness.J Am Geriatr Soc.2008;56(12):2171–2179.
- , , , et al.Loss of independence in activities of daily living in older adults hospitalized with medical illnesses: increased vulnerability with age.J Am Geriatr Soc.2003;51(4):451–458.
- , , .New walking dependence associated with hospitalization for acute medical illness: incidence and significance.J Gerontol A Biol Sci Med Sci.1998;53(4):M307–M312.
- , , , .Hazards of hospitalization: residence prior to admission predicts outcomes.Gerontologist.2008;48(4):537–541.
- , , , , , et al.A randomized trial of care in a hospital medical unit especially designed to improve the functional outcomes of acutely ill older patients.N Engl J Med.1995;332(20):1338–1344.
- , .Exercise for acutely hospitalised older medical inpatients.Cochrane Database Syst Rev.2007;(1):CD005955.
- , , .Occurrence and outcome of delirium in medical in‐patients: a systematic literature review.Age Ageing.2006;35(4):350–364.
- , , .A prospective study of delirium in hospitalized elderly.JAMA.1990;263(8):1097–1101.
- , , , , .Delirium predicts 12‐month mortality.Arch Intern Med.2002;162(4):457–463.
- , , , , .The course of delirium in older medical inpatients: a prospective study.J Gen Intern Med.2003;18(9):696–704.
- , , , .Does delirium increase hospital stay?J Am Geriatr Soc.2003;51(11):1539–1546.
- , , , .The Confusion Assessment Method: a systematic review of current usage.J Am Geriatr Soc.2008;56(5):823–830.
- .Delirium in older persons.N Engl J Med.2006;354(11):1157–1165.
- , , , et al.Pharmacological management of delirium in hospitalized adults—a systematic evidence review.J Gen Intern Med.2009;24(7):848‐853.
- , , .Safety and efficacy of physical restraints for the elderly. Review of the evidence.Can Fam Physician.1996;42:2402–2409.
- , , .Treating nondementia illnesses in patients with dementia.JAMA.2000;283(24):3230–3235.
- , , , , , .Survival in Alzheimer disease: a multiethnic, population‐based study of incident cases.Neurology.2008;71(19):1489–1495.
- , , , et al.Survival after initial diagnosis of Alzheimer disease.Ann Intern Med.2004;140(7):501–509.
- , .Mortality from pneumonia and hip fractures in patients with advanced dementia.JAMA.2000;284(19):2447–2448.
- .Rethinking the role of tube feeding in patients with advanced dementia.N Engl J Med.2000;342(3):206–210.
- Society of Hospital Medicine. BOOSTing Care Transitions Resource Room. Available at: http://www.hospitalmedicine.org/ResourceRoomRedesign/RR_CareTransitions/CT_Home.cfm. Accessed January 25,2011.
- , , .Enteral tube feeding for older people with advanced dementia.Cochrane Database Syst Rev.2009;(2):CD007209.
- .A 93‐year‐old man with advanced dementia and eating problems.JAMA.2007;298(21):2527–2536.
- , , .Patient safety in geriatrics: a call for action.J Gerontol A Biol Sci Med Sci.2003;58(9):M813–M819.
- , , .Preventable medical injuries in older patients.Arch Intern Med.2000;160(18):2717–2728.
- , , , et al.Iatrogenic complications in high‐risk, elderly patients.Arch Intern Med.1992;152(10):2074–2080.
- , , , et al.The nature of adverse events in hospitalized patients. Results of the Harvard Medical Practice Study II.N Engl J Med.1991.
- , , , , et al.Nosocomial infections: decade‐specific risk.Infect Control.1983;4(3):145–147.
- , , , , , .A quality‐of‐care analysis of cascade iatrogenesis in frail elderly hospital patients.QRB Qual Rev Bull.1993;19(6):199‐2.
- American Geriatrics Society Panel on the Pharmacological Management of Persistent Pain in Older Persons.Pharmacological management of persistent pain in older persons.J Am Geriatr Soc.2009;57(8):1331–1346.
- , , , , .The rate of NSAID‐induced endoscopic ulcers increases linearly but not exponentially with age: a pooled analysis of 12 randomised trials.Ann Rheum Dis.2007;66(3):417–418.
- , , , et al.Prevalence, clinical features and avoidability of adverse drug reactions as cause of admission to a geriatric unit: a prospective study of 1756 patients.Drug Saf.2008;31(6):545–556.
- , , , et al.Adverse drug events in high risk older outpatients.J Am Geriatr Soc.1997;45(8):945–948.
- , , , , , .Updating the Beers criteria for potentially inappropriate medication use in older adults: results of a US consensus panel of experts.Arch Intern Med.2003;163(22):2716–2724.
- , , , .Medication use leading to emergency department visits for adverse drug events in older adults.Ann Intern Med.2007;147(11):755–765.
- , .The patient who falls: “It's always a trade‐off.”JAMA.2010;303(3):258–266.
- , , .Hospital discharge information and older patients: do they get what they need?J Hosp Med.2007;2(5):291–296.
- , , .Functional health literacy and understanding of medications at discharge.Mayo Clin Proc.2008;83(5):554–548.
- , , , .The care transitions intervention: results of a randomized controlled trial.Arch Intern Med.2006;166(17):1822–1828.
- , , , , , .Transitional care of older adults hospitalized with heart failure: a randomized, controlled trial.J Am Geriatr Soc.2004;52(5):675–684.
- , , , .The impact of follow‐up telephone calls to patients after hospitalization.Am J Med.2001;111(9B):26S‐30S.
- , , , et al.A quality improvement intervention to facilitate the transition of older adults from three hospitals back to their homes.J Am GeriatrSoc.2009;57(9):1540–1546.
- , , , et al.A reengineered hospital discharge program to decrease rehospitalization: a randomized trial.Ann Intern Med.2009;150(3):178–187.
- , , , et al.Problems after discharge and understanding of communication with their primary care physicians among hospitalized seniors: a mixed methods study.J Hosp Med.2010;5(7):385–391.
- , .What matters to seriously ill older persons making end‐of‐life treatment decisions? A qualitative study.J Palliat Med.2003;6(2):237–244.
- , , .End‐of‐life decision making: a qualitative study of elderly individuals.J Gen Intern Med.2000;15(9):620–625.
- , , , et al.Patient age and decisions to withhold life‐sustaining treatments from seriously ill, hospitalized adults. SUPPORT Investigators. Study to Understand Prognoses and Preferences for Outcomes and Risks of Treatment.Ann Intern Med.1999;130(2):116–125.
- .Clinical practice. Assessment of patients' competence to consent to treatment.N Engl J Med.2007;357(18):1834–1840.
- , , , , .The Mini‐Cog: a cognitive “vital signs” measure for dementia screening in multi‐lingual elderly.Int J Geriatr Psychiatry.2000;15(11):1021–1027.
Recent studies of the growth of the hospitalist movement have confirmed that hospitalists are taking care of an increasing percentage of hospitalized older adults.1, 2 Unfortunately, few hospitalist groups have specific programs or protocols to address the special needs of older patients.3, 4 We were inspired by a top 10 article written by nephrologists for primary care physicians to compile 10 evidence‐based pearls that may be helpful to hospitalists caring for older persons.5
1. Many Health Conditions in Older Patients are Multifactorial and Require a Multifactorial Approach to Care
A geriatric syndrome is a multifactorial condition occurring primarily in frail elderly which is usually due to multiple contributing factors and results from an interaction between patient‐specific impairments and situation‐specific stressors.68 Geriatric syndromes cannot be treated easily with a single intervention; the approach must target each modifiable risk factor. Table 1 illustrates for an example of how this approach differs from the traditional medical approach in the case of falls, a common geriatric syndrome. Hospitalists commonly encounter the geriatric syndromes that either lead to hospitalization, such as falls or dementia, or that can complicate a hospitalization for another condition, such as delirium, falls, or urinary incontinence. Two geriatric syndromes in particular, delirium and falls, are associated with increased length of stay, increased healthcare costs, and substantial morbidity.9, 10 Multifactorial interventions such as the Hospital Elder Life Project (HELP) have been shown to prevent delirium and falls.1114 Studies of the HELP intervention demonstrated benefits on outcomes that are important to hospitalists, including shortened length of stay.10, 15 While implementing these interventions is not possible at every institution, it may be possible to implement some aspects in a targeted fashion (for example, early physical therapy and mobilization to decrease risk of delirium and falls) or to work with hospital administration to implement the larger interventions.
| Traditional Medical Approach (Diagnosis and Treatment) | Geriatric Approach (Risk Factor Assessment and Reduction) | |
|---|---|---|
| ||
| Search for cause of falls | Search for cause of falls | |
| (eg, syncope workup including echocardiogram, neurologic workup including imaging) | Assess for risk factors and target interventions reduce or eliminate risk factors identified, eg: | |
| Risk factor | Intervention | |
| Leg weakness | Strength training | |
| Falls when transferring | Training in use of assistive device for transfers | |
| Inadequate lighting at home | Home safety evaluation by physical therapist and installation of lights at home | |
2. Screening Elderly Patients for the Presence of Common Geriatric Syndromes can Improve Care
Comprehensive geriatric assessment, a process of multifactorial health evaluation of the older patient, is an important part of geriatric medicine and has been shown to reduce functional decline.16 While comprehensive geriatric assessment may not be practical in the acute care setting, some basic principles and tools of geriatric assessment, namely screening for geriatric syndromes, can be helpful. Here is one efficient, evidence‐based approach to screening hospitalized elderly patients for cognitive impairment and falls, and then using the results of screening to improve care:
Brief cognitive assessment using the Mini‐Cog screening tool
Even with cognitive screening, it can sometimes be difficult to distinguish between dementia and delirium in an acutely ill person. However, knowing who is cognitively impaired on admission tells us who is likely to develop delirium and may need to be discharged to a supervised location, such as a nursing facility.17, 18 The Mini‐Cog (shown in Table 2) is our preferred tool for cognitive screening, because it takes only 24 minutes to administer, has a sensitivity of 76% and specificity of 89% [similar to the Mini‐Mental State Examination (MMSE)], and has been validated in a multiethnic, multilingual sample with low literacy.19, 20 The Mini‐Cog has not been studied extensively in the inpatient setting, but one study did find that abnormal Mini‐Cog scores correlated with the development of delirium among hospitalized elderly patients.21 Other tools appropriate for brief cognitive screening of hospitalized patients include the MMSE, which is widely known but limited by copyright for clinical use and longer time needed for administration, and the Short Portable Mental Status Questionnaire (SPMSQ), which is entirely verbal and does not require that the patient write or draw.22, 23 A new tool, the Sweet 16, which is also entirely verbal and takes about 2 minutes to administer, shows promise but has not been validated in the inpatient setting.24
|
| The Mini‐Cog combines an uncued three‐item recall test with a clock‐drawing test (CDT) that serves as the recall distractor. The Mini‐Cog can be administered in about three minutes, requires no special equipment, and is less influenced by level of education or language differences. |
| Administration |
| 1. Make sure you have the patient's attention. Then instruct the patient to listen carefully to, repeat back to you, and remember (now and later) three unrelated words. You may present the same words up to 3 times if necessary. |
| 2. Instruct the patient to draw the face of a clock, either on a blank sheet of paper, or on a sheet with the clock circle already drawn on the page. After the patient puts the numbers on the clock face, ask him or her to draw the hands to read a specific time (11:10 or 8:20 are most commonly used; however, other times that require use of both halves of the clock face may be effective). These instructions can be repeated, but no additional instructions should be given. If the patient cannot complete the CDT in three minutes or less, move on to the next step. |
| 3. Ask the patient to repeat the three previously presented words. |
| Scoring |
| Give 1 point for each recalled word after the CDT distractor. Score 03 for recall. |
| Give 2 points for a normal CDT, and 0 points for an abnormal CDT. The CDT is considered normal if all numbers are depicted, once each, in the correct sequence and position around the circle, and the hands readably display the requested time. Do not count equal hand length as an error. Add the recall and CDT scores together to get the Mini‐Cog score: |
| 02 positive screen for dementia. |
| 35 negative screen for dementia. |
Fall screen: Have you fallen in the past year? with more detailed inquiry for frequent fallers
An elderly patient with a history of falls is at high risk for falling while in the hospital. Interventions that target multiple risk factors appear to be effective in reducing risk and rate of falling.25 Some aspects of these multifactorial interventions include environmental modifications such as low beds, high‐impact floor mats, restraint removal, bedside posters and patient education materials, and exercise.13, 26 Medication review and reductions in medications that contribute to falls (psychoactive medications, diuretics, blood pressure agents) can also reduce fall risk.13, 14 Many experts recommend checking a 25‐hydroxy vitamin D level for patients who come into the hospital for treatment of a fall‐related injury, starting or continuing vitamin D and calcium supplementation for those with normal vitamin D levels, and starting aggressive vitamin D replacement with 50,000 units of oral ergocalciferol weekly for those with a level below 20 ng/mL. Vitamin D appears to play an important role in neuromuscular function as well as bone density.27 Meta‐analyses have demonstrated that vitamin D supplementation of 7001,000 IU daily may reduce the risk of falls in the elderly by about 20%, that the effect is achieved in the short term (on the order of 25 months), and that the benefit persists for up to 36 months.28, 29
3. Elderly Patients are at Risk for Functional Decline During Hospitalization and Measures Can Be Taken to Prevent This
A cascade to dependency has been described in which the effects of usual aging (eg, fragile skin) and hospitalization (eg, immobilization) interact with each other, often producing complications (eg, pressure ulcers) that can lead to the loss of the ability to live independently.30 Functional decline during hospitalization is common and is associated with poor prognosis. In one study of elderly patients with new Activities of Daily Living (ADL) disability at discharge, over 40% were dead by 12 months after discharge, and only 30% had returned to baseline function.31, 32 Functional status may affect discharge planning, especially if the patient is not able to return safely to his or her previous living environment. Elderly patients are very vulnerable to new ADL dependency and loss of ambulatory ability as a result of a hospitalization.33, 34 The initial evaluation of an elderly patient should always include an inquiry into the patient's functional abilities, including whether the patient requires assistance to perform self‐care (ADL) activities or instrumental activities of daily living (IADLs), and who is available to help at home. We remember ADLs as those activities we learn as a young child (bathing, dressing, feeding, toileting, transfers) and IADLs as activities we learn when we leave our parents' home to live independently (shopping, cooking, laundry, housekeeping, managing money, using public transportation). Patients in assisted living facilities and adult family homes receive varying degrees of help with ADLs and IADLs, and no particular level of assistance can be assumed; assisted living residents may be at higher risk of functional decline during hospitalization than community‐dwelling persons.35
Acute Care of Elderly (ACE) units have been shown to reduce functional disability and discharge to long‐term care settings, with length of stay and hospital charges similar to that of usual care.36 Most hospitals do not have ACE units, but implementing some components of the intervention, such as early physical and occupational therapy, early discharge planning, early mobility, and adequate nutrition may reduce functional disability. A Cochrane review found a small decrease in length of stay of about a day among elderly patients who participated in a structured exercise program, which could be extrapolated to support the usefulness of increased mobility and early physical therapy.37
4. Delirium is a Common but Serious Condition Among Hospitalized Elderly Patients and in Some Cases Can Be Prevented
The incidence of delirium appears to be as high as 60% in hospitalized elderly patients with multiple risk factors for this condition.38, 39 Delirium predicts longer hospital stay, increases likelihood of discharge to a skilled nursing facility, and is a marker for significant morbidity and mortality.4042 Cognitive screening, as discussed above, can help determine who is at risk for delirium, and the Confusion Assessment Method (CAM) is becoming more widely used as a bedside screening tool for detecting delirium.43 Other important risk factors for delirium in addition to preexisting cognitive impairment include age over 65, prior delirium, polypharmacy, functional dependence, and sensory impairment.44 A landmark randomized controlled trial by Sharon Inouye et al. showed that delirium can often be prevented in high‐risk patients by ensuring frequent reorientation, maintenance of the patient's usual sleep‐wake cycle, early mobilization, adequate nutrition and hydration, and regular use of sensory aids such as glasses and hearing aids.11
When delirium does occur despite preventive efforts, it should trigger a thorough investigation for an underlying cause. Common contributing factors to delirium in hospitalized patients include infections such as urinary tract infections or hospital‐acquired pneumonia, pain, electrolyte abnormalities, cardiac ischemia, medication side effects, and urinary retention or constipation. Any psychoactive or anticholinergic medication can contribute to, or exacerbate, delirium; common culprits include diphenhydramine, benzodiazepines, and muscle relaxants.44 Although one or more treatable underlying causes should always be sought, many cases of delirium in the hospital are multifactorial.
If pharmacologic treatment for delirium is necessary, low‐dose haloperidol is the first‐line agent, starting with 0.25 mg intravenously or 0.5 mg by mouth, nightly to twice a day. A recent systematic review supported the use of haloperidol as a first‐line agent in the acute setting; second‐generation antipsychotics are an alternative, but studies have shown no benefits of these agents over haloperidol in terms of safety or efficacy.45 Physical restraints should be avoided in delirious patients, as they may actually worsen delirium and have not been proven to reduce falls or secondary injury.46
5. Treating Nondementia Illnesses in Hospitalized Elders With Dementia Requires Consideration of the Patient's Limited Life Expectancy and Cognitive Deficits
Elderly patients with dementia are frequently hospitalized for medical illness and pose special challenges to the hospitalist. Patients with dementia may not be able to accurately report symptoms and side effects of therapy, and may lack decisional capacity. The clinician should also carefully consider the benefits and burdens of a proposed therapy or diagnostic test, taking into account the patient's cognitive deficits and limited life expectancy.47 The median postdiagnosis life expectancy of a patient with Alzheimer disease is about 4 years for men and about 6 years for women, with a range from about 2 to 9 years, according to population‐based studies of incident Alzheimer dementia.48, 49 In a patient with early or mild dementia, the hospitalist may first need to establish whether the patient does indeed have dementia. It is also important to question family about how the patient is managing at home so that appropriate discharge plans can be made.
Any intervention such as surgery or intubation in a patient with dementia should be considered carefully in the context of the patient's dementia, keeping in mind the remaining life expectancy, increased risk for delirium after surgery, and potential difficulty with adherence to postoperative precautions or participation in rehabilitation. Invasive procedures such as mechanical ventilation or central line placement may not be indicated in patients with advanced dementia. In one prospective cohort study, patients with advanced dementia admitted to the hospital for hip fractures or pneumonia had much higher mortality than those without dementia, despite having equal rates of procedures such as mechanical ventilation and central line placement.50
A common scenario in a patient with advanced dementia is the patient presenting with decreased intake and dysphagia; family and/or caregivers inquire about enteral tube feeding and/or long‐term intravenous hydration. This situation requires a thoughtfully presented, informative discussion with the family about artificial feeding in advanced dementia. There is no evidence that artificial feeding improves outcomes, and a recent Cochrane systematic review found insufficient evidence to suggest that enteral tube feeding is beneficial in patients with dementia.5153 Instead, dementia patients at the end of their lives benefit from a palliative approach, which would include hand‐feeding for comfort if the patient indicates hunger.54
6. Frail Elderly Patients are at High Risk for Iatrogenic Complications During Hospitalization
Frail elders are at increased risk for iatrogenic complications, including surgical‐ and procedure‐related events, adverse drug events, nosocomial infections, pressure ulcers, and falls.5557 In the Harvard Medical Practice Study, a retrospective study of adverse events in 30,000 patients, those age 65 and over accounted for 27% of the hospitalized population but 43% of the adverse events. In this study, four types of adverse events occurred at least twice as often in older patients: fractures, falls, nontechnical postoperative complications, and adverse events related to noninvasive treatments.58 The risk of hospital‐acquired infection also rises dramatically with age; risk is ten times higher in patients over 70 than those aged 49 and under, with urinary tract infections and surgical site infections the most common.59 The oldest, most seriously ill and most functionally impaired elderly patients are at particularly high risk of cascade iatrogenesis, where one medical intervention triggers a series of adverse events.60 Frail elderly patients are at greater risk for adverse events during hospitalization, and careful consideration of the need for all diagnostic tests and procedures may reduce risks. Table 3 lists the iatrogenic complications that disproportionately affect the elderly.
7. Pain Should Be Aggressively Identified and Appropriately Treated
Adequate treatment of pain in the elderly can improve functional status, mobility, and mood. Clinicians are understandably wary of prescribing opioids in the elderly, but undertreatment of pain is also common and in hospitalized patients can lead to delirium, delays in rehabilitation, and higher healthcare costs.44, 61 Acetaminophen is generally safe and is recommended as first‐line therapy in most cases of pain. Nonsteroidal antiinflammatory drugs (NSAIDS) should be used with caution, as the risk of gastrointestinal toxicity increases with age; long‐acting agents such as toradol should be avoided.62 A study of adverse drug reactions causing hospitalization in elderly individuals implicated NSAIDs in 23.5% of cases.63 If a patient has severe pain that limits functional capacity but does not respond to acetaminophen, it is reasonable to consider low‐dose opioids. The 2009 American Geriatrics Society guidelines recommend that opioids be used for severe pain, especially when it limits functional capacity. Opioids should be given orally when possible and started at low doses (for example, 2.5 to 5 milligrams of oxycodone offered every 4 hours as needed). Either oxycodone or morphine can be used as a first‐line, short‐acting opioid, but morphine should be used cautiously in patients with renal insufficiency, as toxic metabolites are renally cleared. Methadone should be used very cautiously and ideally by clinicians experienced in its use because of its long and variable half‐life. Serotonin‐norepinephrine reuptake inhibitors such as duloxetine, and anticonvulsants such as gabapentin, can be helpful adjuncts for neuropathic pain; tricyclics should generally be avoided, because the elderly are particularly sensitive to the anticholinergic side effects (ie, sedation, orthostatic hypotension, urinary retention, constipation) of these agents.61 Nortriptyline, which has the least anticholinergic properties of this medication class, may be the best option if a tricyclic is desired.
8. Hospitalization Can Be an Opportunity for a Thorough Review and Reduction of Outpatient Medications in Collaboration With the Patient's Primary Care Provider
Adverse drug reactions (ADRs) are potentially life‐threatening for all patients, but elderly individuals are disproportionately affected. It is estimated that 30% of hospital admissions of older patients are due to drug‐related toxicity.64 The Beers criteria provide expert consensus on drugs that should either be avoided or used cautiously in the elderly; we have highlighted some of the drugs on the Beers list that are relevant to the hospitalist in Table 4. Notable inclusions on the most recent Beers list include nitrofurantoin, ferrous sulfate at dosages greater than 325 mg per day, doxazosin, and fluoxetine.65 Anticholinergics (such as oxybutynin) and cholinesterase inhibitors (such as donepezil) should not be used together, because the interaction makes them ineffective.
| Falls and fractures58 |
| Nosocomial infections59 |
| Delirium44 |
| Surgical and postoperative complications58 |
| Adverse drug events56 |
| Pressure sores56 |
| Medication | Rationale |
|---|---|
| |
| Benzodiazepines | Can contribute to delirium and falls. |
| Muscle relaxants | Anticholinergic side effects; sedating; can contribute to delirium. |
| Diphenhydramine | Very sedating; can contribute to delirium; do not use as a sleep aid, and use lowest possible dose when treating allergic reactions. |
| Nitrofurantoin | Can cause renal impairment. |
| Ketorolac | Can lead to gastrointestinal bleeds and renal impairment. |
| Ferrous sulfate in doses >325 mg/day | Can cause severe constipation; higher doses not well absorbed. |
| Digoxin in doses of >0.125 mg/day | Decreased renal clearance can increase risk of toxicity (higher doses acceptable for atrial arrythmias). |
| Amiodarone | Multiple toxicities; lack of proven efficacy in older adults. |
| Fluoxetine | Long half‐life; can cause agitation and sleep disturbance. |
| Meperidine | Can cause seizures and contribute to delirium. |
| Doxazosin | Can cause hypotension and contribute to falls. |
Many ADRs are caused by medications that are not on the Beers list but have narrow therapeutic windows, especially warfarin and insulin; digoxin, which is on the Beers list, is another common culprit in ADRs leading to emergency room visits.66 Medications that are clearly causing ADRs should be discontinued, with the knowledge and input of the primary care provider, and with written documentation on the discharge summary and clear instructions to the patient. Even when a hospital admission is not a result of an ADR, it may provide an opportunity to review the patient's medication list, clarify that the patient is taking medications as directed by their primary care provider, and determine if the list contains medications where risks now outweigh benefits, in which case those medications should be discontinued. Studies have shown that elimination of medications that are associated with falls reduces the risk of future falls.67 Adjustments to patient's medications should be made in close collaboration with the primary care provider and with a clinical pharmacist, if available.
9. Discharge Planning for an Elderly Patient Should Start Early, Be Comprehensive, and Involve the Patient and Caregivers
A successful discharge plan for an elderly patient must start early, take functional status into account, and perhaps most importantly, the planning process should involve the patient and family, if appropriate. One study of patients over 70 discharged from an urban, academic public hospital found that only 10% recalled receiving written discharge instructions, and only 53% who were prescribed a new medication actually had that medication at home.68 Growing evidence suggests that older patients have poorer functional health literacy regarding medications.69 Care transitions are an active area of research, and multiple studies have shown lower rates of rehospitalization with comprehensive transitional care programs involving transition coaches or advance practice nurses providing home visits and follow‐up telephone calls.70, 71 More research is needed in this area to develop simple, cost‐effective care transitions programs. In the meantime, hospitalists can actively involve a multidisciplinary team (pharmacists and social workers, for example) early in the hospitalization, provide more patient‐friendly discharge materials such as those available as part of the Society of Hospital Medicine's BOOST, and place targeted follow‐up phone calls after discharge.52, 7274 Ensuring that a frail elderly patient's primary care provider is aware of the hospitalization may be particularly important in avoiding postdischarge problems.75
10. Elderly Patients are Often Very Clear About Their Preferences for Treatment. Discussing This on Admission Can Help Clarify the Goals of the Hospitalization
The risks and benefits of any intervention being considered should be presented and discussed with the patient in the context of the likelihood of the intervention restoring an acceptable quality of life to the patient. Many common chronic conditions in the elderly such as Parkinson disease, congestive heart failure, and dementia are progressive and life‐limiting. Qualitative studies confirm that elderly patients want to know the likelihood that a proposed intervention will help them return to their previous level of functioning or a level that is acceptable to them.76, 77 In one prospective cohort study, older age was found to correlate with a higher rate of withholding ventilatory support, dialysis, and surgery.78 However, a more recent study has suggested that patient preferences are more dynamic, and that older patients will make decisions based on current health status rather than core values.76 While families should be involved in the process of clarification of goals of care, the patients' own values should be elicited whenever possible, with bedside assessment of decision‐making capacity to ensure that patients are allowed to speak for themselves if they possess decisional capacity for the decision at hand.79
Hospitalists should have a special approach to the elderly patient which takes into account the elder's increased vulnerability to iatrogenic complications and acute functional decline, the presence of geriatric syndromes, and individual preferences. Early assessment of an elderly patient's cognitive and physical functioning, level of pain, fall risk, and preferences for care are necessary for optimal inpatient care and may result in preserved function and a decrease in adverse events.
Recent studies of the growth of the hospitalist movement have confirmed that hospitalists are taking care of an increasing percentage of hospitalized older adults.1, 2 Unfortunately, few hospitalist groups have specific programs or protocols to address the special needs of older patients.3, 4 We were inspired by a top 10 article written by nephrologists for primary care physicians to compile 10 evidence‐based pearls that may be helpful to hospitalists caring for older persons.5
1. Many Health Conditions in Older Patients are Multifactorial and Require a Multifactorial Approach to Care
A geriatric syndrome is a multifactorial condition occurring primarily in frail elderly which is usually due to multiple contributing factors and results from an interaction between patient‐specific impairments and situation‐specific stressors.68 Geriatric syndromes cannot be treated easily with a single intervention; the approach must target each modifiable risk factor. Table 1 illustrates for an example of how this approach differs from the traditional medical approach in the case of falls, a common geriatric syndrome. Hospitalists commonly encounter the geriatric syndromes that either lead to hospitalization, such as falls or dementia, or that can complicate a hospitalization for another condition, such as delirium, falls, or urinary incontinence. Two geriatric syndromes in particular, delirium and falls, are associated with increased length of stay, increased healthcare costs, and substantial morbidity.9, 10 Multifactorial interventions such as the Hospital Elder Life Project (HELP) have been shown to prevent delirium and falls.1114 Studies of the HELP intervention demonstrated benefits on outcomes that are important to hospitalists, including shortened length of stay.10, 15 While implementing these interventions is not possible at every institution, it may be possible to implement some aspects in a targeted fashion (for example, early physical therapy and mobilization to decrease risk of delirium and falls) or to work with hospital administration to implement the larger interventions.
| Traditional Medical Approach (Diagnosis and Treatment) | Geriatric Approach (Risk Factor Assessment and Reduction) | |
|---|---|---|
| ||
| Search for cause of falls | Search for cause of falls | |
| (eg, syncope workup including echocardiogram, neurologic workup including imaging) | Assess for risk factors and target interventions reduce or eliminate risk factors identified, eg: | |
| Risk factor | Intervention | |
| Leg weakness | Strength training | |
| Falls when transferring | Training in use of assistive device for transfers | |
| Inadequate lighting at home | Home safety evaluation by physical therapist and installation of lights at home | |
2. Screening Elderly Patients for the Presence of Common Geriatric Syndromes can Improve Care
Comprehensive geriatric assessment, a process of multifactorial health evaluation of the older patient, is an important part of geriatric medicine and has been shown to reduce functional decline.16 While comprehensive geriatric assessment may not be practical in the acute care setting, some basic principles and tools of geriatric assessment, namely screening for geriatric syndromes, can be helpful. Here is one efficient, evidence‐based approach to screening hospitalized elderly patients for cognitive impairment and falls, and then using the results of screening to improve care:
Brief cognitive assessment using the Mini‐Cog screening tool
Even with cognitive screening, it can sometimes be difficult to distinguish between dementia and delirium in an acutely ill person. However, knowing who is cognitively impaired on admission tells us who is likely to develop delirium and may need to be discharged to a supervised location, such as a nursing facility.17, 18 The Mini‐Cog (shown in Table 2) is our preferred tool for cognitive screening, because it takes only 24 minutes to administer, has a sensitivity of 76% and specificity of 89% [similar to the Mini‐Mental State Examination (MMSE)], and has been validated in a multiethnic, multilingual sample with low literacy.19, 20 The Mini‐Cog has not been studied extensively in the inpatient setting, but one study did find that abnormal Mini‐Cog scores correlated with the development of delirium among hospitalized elderly patients.21 Other tools appropriate for brief cognitive screening of hospitalized patients include the MMSE, which is widely known but limited by copyright for clinical use and longer time needed for administration, and the Short Portable Mental Status Questionnaire (SPMSQ), which is entirely verbal and does not require that the patient write or draw.22, 23 A new tool, the Sweet 16, which is also entirely verbal and takes about 2 minutes to administer, shows promise but has not been validated in the inpatient setting.24
|
| The Mini‐Cog combines an uncued three‐item recall test with a clock‐drawing test (CDT) that serves as the recall distractor. The Mini‐Cog can be administered in about three minutes, requires no special equipment, and is less influenced by level of education or language differences. |
| Administration |
| 1. Make sure you have the patient's attention. Then instruct the patient to listen carefully to, repeat back to you, and remember (now and later) three unrelated words. You may present the same words up to 3 times if necessary. |
| 2. Instruct the patient to draw the face of a clock, either on a blank sheet of paper, or on a sheet with the clock circle already drawn on the page. After the patient puts the numbers on the clock face, ask him or her to draw the hands to read a specific time (11:10 or 8:20 are most commonly used; however, other times that require use of both halves of the clock face may be effective). These instructions can be repeated, but no additional instructions should be given. If the patient cannot complete the CDT in three minutes or less, move on to the next step. |
| 3. Ask the patient to repeat the three previously presented words. |
| Scoring |
| Give 1 point for each recalled word after the CDT distractor. Score 03 for recall. |
| Give 2 points for a normal CDT, and 0 points for an abnormal CDT. The CDT is considered normal if all numbers are depicted, once each, in the correct sequence and position around the circle, and the hands readably display the requested time. Do not count equal hand length as an error. Add the recall and CDT scores together to get the Mini‐Cog score: |
| 02 positive screen for dementia. |
| 35 negative screen for dementia. |
Fall screen: Have you fallen in the past year? with more detailed inquiry for frequent fallers
An elderly patient with a history of falls is at high risk for falling while in the hospital. Interventions that target multiple risk factors appear to be effective in reducing risk and rate of falling.25 Some aspects of these multifactorial interventions include environmental modifications such as low beds, high‐impact floor mats, restraint removal, bedside posters and patient education materials, and exercise.13, 26 Medication review and reductions in medications that contribute to falls (psychoactive medications, diuretics, blood pressure agents) can also reduce fall risk.13, 14 Many experts recommend checking a 25‐hydroxy vitamin D level for patients who come into the hospital for treatment of a fall‐related injury, starting or continuing vitamin D and calcium supplementation for those with normal vitamin D levels, and starting aggressive vitamin D replacement with 50,000 units of oral ergocalciferol weekly for those with a level below 20 ng/mL. Vitamin D appears to play an important role in neuromuscular function as well as bone density.27 Meta‐analyses have demonstrated that vitamin D supplementation of 7001,000 IU daily may reduce the risk of falls in the elderly by about 20%, that the effect is achieved in the short term (on the order of 25 months), and that the benefit persists for up to 36 months.28, 29
3. Elderly Patients are at Risk for Functional Decline During Hospitalization and Measures Can Be Taken to Prevent This
A cascade to dependency has been described in which the effects of usual aging (eg, fragile skin) and hospitalization (eg, immobilization) interact with each other, often producing complications (eg, pressure ulcers) that can lead to the loss of the ability to live independently.30 Functional decline during hospitalization is common and is associated with poor prognosis. In one study of elderly patients with new Activities of Daily Living (ADL) disability at discharge, over 40% were dead by 12 months after discharge, and only 30% had returned to baseline function.31, 32 Functional status may affect discharge planning, especially if the patient is not able to return safely to his or her previous living environment. Elderly patients are very vulnerable to new ADL dependency and loss of ambulatory ability as a result of a hospitalization.33, 34 The initial evaluation of an elderly patient should always include an inquiry into the patient's functional abilities, including whether the patient requires assistance to perform self‐care (ADL) activities or instrumental activities of daily living (IADLs), and who is available to help at home. We remember ADLs as those activities we learn as a young child (bathing, dressing, feeding, toileting, transfers) and IADLs as activities we learn when we leave our parents' home to live independently (shopping, cooking, laundry, housekeeping, managing money, using public transportation). Patients in assisted living facilities and adult family homes receive varying degrees of help with ADLs and IADLs, and no particular level of assistance can be assumed; assisted living residents may be at higher risk of functional decline during hospitalization than community‐dwelling persons.35
Acute Care of Elderly (ACE) units have been shown to reduce functional disability and discharge to long‐term care settings, with length of stay and hospital charges similar to that of usual care.36 Most hospitals do not have ACE units, but implementing some components of the intervention, such as early physical and occupational therapy, early discharge planning, early mobility, and adequate nutrition may reduce functional disability. A Cochrane review found a small decrease in length of stay of about a day among elderly patients who participated in a structured exercise program, which could be extrapolated to support the usefulness of increased mobility and early physical therapy.37
4. Delirium is a Common but Serious Condition Among Hospitalized Elderly Patients and in Some Cases Can Be Prevented
The incidence of delirium appears to be as high as 60% in hospitalized elderly patients with multiple risk factors for this condition.38, 39 Delirium predicts longer hospital stay, increases likelihood of discharge to a skilled nursing facility, and is a marker for significant morbidity and mortality.4042 Cognitive screening, as discussed above, can help determine who is at risk for delirium, and the Confusion Assessment Method (CAM) is becoming more widely used as a bedside screening tool for detecting delirium.43 Other important risk factors for delirium in addition to preexisting cognitive impairment include age over 65, prior delirium, polypharmacy, functional dependence, and sensory impairment.44 A landmark randomized controlled trial by Sharon Inouye et al. showed that delirium can often be prevented in high‐risk patients by ensuring frequent reorientation, maintenance of the patient's usual sleep‐wake cycle, early mobilization, adequate nutrition and hydration, and regular use of sensory aids such as glasses and hearing aids.11
When delirium does occur despite preventive efforts, it should trigger a thorough investigation for an underlying cause. Common contributing factors to delirium in hospitalized patients include infections such as urinary tract infections or hospital‐acquired pneumonia, pain, electrolyte abnormalities, cardiac ischemia, medication side effects, and urinary retention or constipation. Any psychoactive or anticholinergic medication can contribute to, or exacerbate, delirium; common culprits include diphenhydramine, benzodiazepines, and muscle relaxants.44 Although one or more treatable underlying causes should always be sought, many cases of delirium in the hospital are multifactorial.
If pharmacologic treatment for delirium is necessary, low‐dose haloperidol is the first‐line agent, starting with 0.25 mg intravenously or 0.5 mg by mouth, nightly to twice a day. A recent systematic review supported the use of haloperidol as a first‐line agent in the acute setting; second‐generation antipsychotics are an alternative, but studies have shown no benefits of these agents over haloperidol in terms of safety or efficacy.45 Physical restraints should be avoided in delirious patients, as they may actually worsen delirium and have not been proven to reduce falls or secondary injury.46
5. Treating Nondementia Illnesses in Hospitalized Elders With Dementia Requires Consideration of the Patient's Limited Life Expectancy and Cognitive Deficits
Elderly patients with dementia are frequently hospitalized for medical illness and pose special challenges to the hospitalist. Patients with dementia may not be able to accurately report symptoms and side effects of therapy, and may lack decisional capacity. The clinician should also carefully consider the benefits and burdens of a proposed therapy or diagnostic test, taking into account the patient's cognitive deficits and limited life expectancy.47 The median postdiagnosis life expectancy of a patient with Alzheimer disease is about 4 years for men and about 6 years for women, with a range from about 2 to 9 years, according to population‐based studies of incident Alzheimer dementia.48, 49 In a patient with early or mild dementia, the hospitalist may first need to establish whether the patient does indeed have dementia. It is also important to question family about how the patient is managing at home so that appropriate discharge plans can be made.
Any intervention such as surgery or intubation in a patient with dementia should be considered carefully in the context of the patient's dementia, keeping in mind the remaining life expectancy, increased risk for delirium after surgery, and potential difficulty with adherence to postoperative precautions or participation in rehabilitation. Invasive procedures such as mechanical ventilation or central line placement may not be indicated in patients with advanced dementia. In one prospective cohort study, patients with advanced dementia admitted to the hospital for hip fractures or pneumonia had much higher mortality than those without dementia, despite having equal rates of procedures such as mechanical ventilation and central line placement.50
A common scenario in a patient with advanced dementia is the patient presenting with decreased intake and dysphagia; family and/or caregivers inquire about enteral tube feeding and/or long‐term intravenous hydration. This situation requires a thoughtfully presented, informative discussion with the family about artificial feeding in advanced dementia. There is no evidence that artificial feeding improves outcomes, and a recent Cochrane systematic review found insufficient evidence to suggest that enteral tube feeding is beneficial in patients with dementia.5153 Instead, dementia patients at the end of their lives benefit from a palliative approach, which would include hand‐feeding for comfort if the patient indicates hunger.54
6. Frail Elderly Patients are at High Risk for Iatrogenic Complications During Hospitalization
Frail elders are at increased risk for iatrogenic complications, including surgical‐ and procedure‐related events, adverse drug events, nosocomial infections, pressure ulcers, and falls.5557 In the Harvard Medical Practice Study, a retrospective study of adverse events in 30,000 patients, those age 65 and over accounted for 27% of the hospitalized population but 43% of the adverse events. In this study, four types of adverse events occurred at least twice as often in older patients: fractures, falls, nontechnical postoperative complications, and adverse events related to noninvasive treatments.58 The risk of hospital‐acquired infection also rises dramatically with age; risk is ten times higher in patients over 70 than those aged 49 and under, with urinary tract infections and surgical site infections the most common.59 The oldest, most seriously ill and most functionally impaired elderly patients are at particularly high risk of cascade iatrogenesis, where one medical intervention triggers a series of adverse events.60 Frail elderly patients are at greater risk for adverse events during hospitalization, and careful consideration of the need for all diagnostic tests and procedures may reduce risks. Table 3 lists the iatrogenic complications that disproportionately affect the elderly.
7. Pain Should Be Aggressively Identified and Appropriately Treated
Adequate treatment of pain in the elderly can improve functional status, mobility, and mood. Clinicians are understandably wary of prescribing opioids in the elderly, but undertreatment of pain is also common and in hospitalized patients can lead to delirium, delays in rehabilitation, and higher healthcare costs.44, 61 Acetaminophen is generally safe and is recommended as first‐line therapy in most cases of pain. Nonsteroidal antiinflammatory drugs (NSAIDS) should be used with caution, as the risk of gastrointestinal toxicity increases with age; long‐acting agents such as toradol should be avoided.62 A study of adverse drug reactions causing hospitalization in elderly individuals implicated NSAIDs in 23.5% of cases.63 If a patient has severe pain that limits functional capacity but does not respond to acetaminophen, it is reasonable to consider low‐dose opioids. The 2009 American Geriatrics Society guidelines recommend that opioids be used for severe pain, especially when it limits functional capacity. Opioids should be given orally when possible and started at low doses (for example, 2.5 to 5 milligrams of oxycodone offered every 4 hours as needed). Either oxycodone or morphine can be used as a first‐line, short‐acting opioid, but morphine should be used cautiously in patients with renal insufficiency, as toxic metabolites are renally cleared. Methadone should be used very cautiously and ideally by clinicians experienced in its use because of its long and variable half‐life. Serotonin‐norepinephrine reuptake inhibitors such as duloxetine, and anticonvulsants such as gabapentin, can be helpful adjuncts for neuropathic pain; tricyclics should generally be avoided, because the elderly are particularly sensitive to the anticholinergic side effects (ie, sedation, orthostatic hypotension, urinary retention, constipation) of these agents.61 Nortriptyline, which has the least anticholinergic properties of this medication class, may be the best option if a tricyclic is desired.
8. Hospitalization Can Be an Opportunity for a Thorough Review and Reduction of Outpatient Medications in Collaboration With the Patient's Primary Care Provider
Adverse drug reactions (ADRs) are potentially life‐threatening for all patients, but elderly individuals are disproportionately affected. It is estimated that 30% of hospital admissions of older patients are due to drug‐related toxicity.64 The Beers criteria provide expert consensus on drugs that should either be avoided or used cautiously in the elderly; we have highlighted some of the drugs on the Beers list that are relevant to the hospitalist in Table 4. Notable inclusions on the most recent Beers list include nitrofurantoin, ferrous sulfate at dosages greater than 325 mg per day, doxazosin, and fluoxetine.65 Anticholinergics (such as oxybutynin) and cholinesterase inhibitors (such as donepezil) should not be used together, because the interaction makes them ineffective.
| Falls and fractures58 |
| Nosocomial infections59 |
| Delirium44 |
| Surgical and postoperative complications58 |
| Adverse drug events56 |
| Pressure sores56 |
| Medication | Rationale |
|---|---|
| |
| Benzodiazepines | Can contribute to delirium and falls. |
| Muscle relaxants | Anticholinergic side effects; sedating; can contribute to delirium. |
| Diphenhydramine | Very sedating; can contribute to delirium; do not use as a sleep aid, and use lowest possible dose when treating allergic reactions. |
| Nitrofurantoin | Can cause renal impairment. |
| Ketorolac | Can lead to gastrointestinal bleeds and renal impairment. |
| Ferrous sulfate in doses >325 mg/day | Can cause severe constipation; higher doses not well absorbed. |
| Digoxin in doses of >0.125 mg/day | Decreased renal clearance can increase risk of toxicity (higher doses acceptable for atrial arrythmias). |
| Amiodarone | Multiple toxicities; lack of proven efficacy in older adults. |
| Fluoxetine | Long half‐life; can cause agitation and sleep disturbance. |
| Meperidine | Can cause seizures and contribute to delirium. |
| Doxazosin | Can cause hypotension and contribute to falls. |
Many ADRs are caused by medications that are not on the Beers list but have narrow therapeutic windows, especially warfarin and insulin; digoxin, which is on the Beers list, is another common culprit in ADRs leading to emergency room visits.66 Medications that are clearly causing ADRs should be discontinued, with the knowledge and input of the primary care provider, and with written documentation on the discharge summary and clear instructions to the patient. Even when a hospital admission is not a result of an ADR, it may provide an opportunity to review the patient's medication list, clarify that the patient is taking medications as directed by their primary care provider, and determine if the list contains medications where risks now outweigh benefits, in which case those medications should be discontinued. Studies have shown that elimination of medications that are associated with falls reduces the risk of future falls.67 Adjustments to patient's medications should be made in close collaboration with the primary care provider and with a clinical pharmacist, if available.
9. Discharge Planning for an Elderly Patient Should Start Early, Be Comprehensive, and Involve the Patient and Caregivers
A successful discharge plan for an elderly patient must start early, take functional status into account, and perhaps most importantly, the planning process should involve the patient and family, if appropriate. One study of patients over 70 discharged from an urban, academic public hospital found that only 10% recalled receiving written discharge instructions, and only 53% who were prescribed a new medication actually had that medication at home.68 Growing evidence suggests that older patients have poorer functional health literacy regarding medications.69 Care transitions are an active area of research, and multiple studies have shown lower rates of rehospitalization with comprehensive transitional care programs involving transition coaches or advance practice nurses providing home visits and follow‐up telephone calls.70, 71 More research is needed in this area to develop simple, cost‐effective care transitions programs. In the meantime, hospitalists can actively involve a multidisciplinary team (pharmacists and social workers, for example) early in the hospitalization, provide more patient‐friendly discharge materials such as those available as part of the Society of Hospital Medicine's BOOST, and place targeted follow‐up phone calls after discharge.52, 7274 Ensuring that a frail elderly patient's primary care provider is aware of the hospitalization may be particularly important in avoiding postdischarge problems.75
10. Elderly Patients are Often Very Clear About Their Preferences for Treatment. Discussing This on Admission Can Help Clarify the Goals of the Hospitalization
The risks and benefits of any intervention being considered should be presented and discussed with the patient in the context of the likelihood of the intervention restoring an acceptable quality of life to the patient. Many common chronic conditions in the elderly such as Parkinson disease, congestive heart failure, and dementia are progressive and life‐limiting. Qualitative studies confirm that elderly patients want to know the likelihood that a proposed intervention will help them return to their previous level of functioning or a level that is acceptable to them.76, 77 In one prospective cohort study, older age was found to correlate with a higher rate of withholding ventilatory support, dialysis, and surgery.78 However, a more recent study has suggested that patient preferences are more dynamic, and that older patients will make decisions based on current health status rather than core values.76 While families should be involved in the process of clarification of goals of care, the patients' own values should be elicited whenever possible, with bedside assessment of decision‐making capacity to ensure that patients are allowed to speak for themselves if they possess decisional capacity for the decision at hand.79
Hospitalists should have a special approach to the elderly patient which takes into account the elder's increased vulnerability to iatrogenic complications and acute functional decline, the presence of geriatric syndromes, and individual preferences. Early assessment of an elderly patient's cognitive and physical functioning, level of pain, fall risk, and preferences for care are necessary for optimal inpatient care and may result in preserved function and a decrease in adverse events.
- , , , .Growth in the care of older patients by hospitalists in the United States.N Engl J Med.2009;360(11):1102–1112.
- , , , , , .Continuity of outpatient and inpatient care by primary care physicians for hospitalized older adults.JAMA.2009;301(16):1671–1680.
- , , .Is there a geriatrician in the house? Geriatric care approaches in hospitalist programs.J Hosp Med.2006;1(1):29–35.
- .Care of hospitalized older patients: opportunities for hospital‐based physicians.J Hosp Med.2006;1(1):42–47.
- , .The top 10 things nephrologists wish every primary care physician knew.Mayo Clin Proc.2009;84(2):180–186.
- .What is a geriatric syndrome anyway?J Am Geriatr Soc.2003;51(4):574–576.
- , , , .Geriatric syndromes: clinical, research, and policy implications of a core geriatric concept.J Am Geriatr Soc.2007;55(5):780–791.
- , , .Some considerations regarding geriatric syndromes.Ann Intern Med.2001;135(12):1095.
- , , , et al.Impact and recognition of cognitive impairment among hospitalized elders.J Hosp Med.2010;5(2):69–75.
- , , , , , .A longitudinal analysis of total 3‐year healthcare costs for older adults who experience a fall requiring medical care.J Am Geriatr Soc.2010;58(5):853–860.
- , , , et al.A multicomponent intervention to prevent delirium in hospitalized older patients.N Engl J Med.1999;340(9):669–676.
- , , , .Shared risk factors for falls, incontinence, and functional dependence. Unifying the approach to geriatric syndromes.JAMA.1995;273(17):1348–1353.
- , , , et al.Strategies to prevent falls and fractures in hospitals and care homes and effect of cognitive impairment: systematic review and meta‐analyses.BMJ.2007;334(7584):82.
- , , , et al.Interventions for preventing falls in acute‐ and chronic‐care hospitals: a systematic review and meta‐analysis.J Am Geriatr Soc.2008;56(1):29–36.
- , , , et al.Postoperative delirium in the elderly: risk factors and outcomes.Ann Surg.2009;249(1):173–178.
- , , , et al.A controlled trial of inpatient and outpatient geriatric evaluation and management.N Engl J Med.2002;346(12):905–912.
- , , , et al.Risk factors for preoperative and postoperative delirium in elderly patients with hip fracture.J Am Geriatr Soc.2009;57(8):1354‐1361.
- , , , , , .Risk factors for delirium in intensive care patients: a prospective cohort study.Crit Care.2009;13(3):R77.
- , , , .The Mini‐Cog as a screen for dementia: validation in a population‐based sample.J Am Geriatr Soc.2003;51(10):1451–1454.
- , , , , .Improving identification of cognitive impairment in primary care.Int J Geriatr Psychiatry.2006;21(4):349–355.
- , , , et al.Simple cognitive testing (Mini‐Cog) predicts inhospital delirium in the elderly.J Am Geriatr Soc.2007;55(2):314–316.
- , , , .Short Portable Mental Status Questionnaire as a screening test for dementia and delirium among the elderly.J Am Geriatr Soc.1987;35(5):412–416.
- , , .“Mini‐mental state.” A practical method for grading the cognitive state of patients for the clinician.J Psychiatr Res.1975;12(3):189–198.
- , , , et al.Development and validation of a brief cognitive assessment tool: the Sweet 16.Arch Intern Med.2010. [Epub ahead of Print].
- , , , et al.Interventions for preventing falls in older people in nursing care facilities and hospitals.Cochrane Database Syst Rev.2010;(1):CD005465.
- , , , et al.Fall prevention in acute care hospitals: a randomized trial.JAMA.2010;304(17):1912–1918.
- , , , et al.Vitamin D supplementation improves neuromuscular function in older people who fall.Age Ageing.2004;33(6):589–595.
- , , , et al.Effect of vitamin D on falls: a meta‐analysis.JAMA.2004;291(16):1999–2006.
- , et al.Fall prevention with supplemental and active forms of vitamin D: a meta‐analysis of randomised controlled trials.BMJ.2009;339:b3692.
- .Hazards of hospitalization of the elderly.Ann Intern Med.1993;118(3):219–223.
- , , , .Hospitalization, restricted activity, and the development of disability among older persons.JAMA.2004;292(17):2115–2124.
- , , , et al.Recovery of activities of daily living in older adults after hospitalization for acute medical illness.J Am Geriatr Soc.2008;56(12):2171–2179.
- , , , et al.Loss of independence in activities of daily living in older adults hospitalized with medical illnesses: increased vulnerability with age.J Am Geriatr Soc.2003;51(4):451–458.
- , , .New walking dependence associated with hospitalization for acute medical illness: incidence and significance.J Gerontol A Biol Sci Med Sci.1998;53(4):M307–M312.
- , , , .Hazards of hospitalization: residence prior to admission predicts outcomes.Gerontologist.2008;48(4):537–541.
- , , , , , et al.A randomized trial of care in a hospital medical unit especially designed to improve the functional outcomes of acutely ill older patients.N Engl J Med.1995;332(20):1338–1344.
- , .Exercise for acutely hospitalised older medical inpatients.Cochrane Database Syst Rev.2007;(1):CD005955.
- , , .Occurrence and outcome of delirium in medical in‐patients: a systematic literature review.Age Ageing.2006;35(4):350–364.
- , , .A prospective study of delirium in hospitalized elderly.JAMA.1990;263(8):1097–1101.
- , , , , .Delirium predicts 12‐month mortality.Arch Intern Med.2002;162(4):457–463.
- , , , , .The course of delirium in older medical inpatients: a prospective study.J Gen Intern Med.2003;18(9):696–704.
- , , , .Does delirium increase hospital stay?J Am Geriatr Soc.2003;51(11):1539–1546.
- , , , .The Confusion Assessment Method: a systematic review of current usage.J Am Geriatr Soc.2008;56(5):823–830.
- .Delirium in older persons.N Engl J Med.2006;354(11):1157–1165.
- , , , et al.Pharmacological management of delirium in hospitalized adults—a systematic evidence review.J Gen Intern Med.2009;24(7):848‐853.
- , , .Safety and efficacy of physical restraints for the elderly. Review of the evidence.Can Fam Physician.1996;42:2402–2409.
- , , .Treating nondementia illnesses in patients with dementia.JAMA.2000;283(24):3230–3235.
- , , , , , .Survival in Alzheimer disease: a multiethnic, population‐based study of incident cases.Neurology.2008;71(19):1489–1495.
- , , , et al.Survival after initial diagnosis of Alzheimer disease.Ann Intern Med.2004;140(7):501–509.
- , .Mortality from pneumonia and hip fractures in patients with advanced dementia.JAMA.2000;284(19):2447–2448.
- .Rethinking the role of tube feeding in patients with advanced dementia.N Engl J Med.2000;342(3):206–210.
- Society of Hospital Medicine. BOOSTing Care Transitions Resource Room. Available at: http://www.hospitalmedicine.org/ResourceRoomRedesign/RR_CareTransitions/CT_Home.cfm. Accessed January 25,2011.
- , , .Enteral tube feeding for older people with advanced dementia.Cochrane Database Syst Rev.2009;(2):CD007209.
- .A 93‐year‐old man with advanced dementia and eating problems.JAMA.2007;298(21):2527–2536.
- , , .Patient safety in geriatrics: a call for action.J Gerontol A Biol Sci Med Sci.2003;58(9):M813–M819.
- , , .Preventable medical injuries in older patients.Arch Intern Med.2000;160(18):2717–2728.
- , , , et al.Iatrogenic complications in high‐risk, elderly patients.Arch Intern Med.1992;152(10):2074–2080.
- , , , et al.The nature of adverse events in hospitalized patients. Results of the Harvard Medical Practice Study II.N Engl J Med.1991.
- , , , , et al.Nosocomial infections: decade‐specific risk.Infect Control.1983;4(3):145–147.
- , , , , , .A quality‐of‐care analysis of cascade iatrogenesis in frail elderly hospital patients.QRB Qual Rev Bull.1993;19(6):199‐2.
- American Geriatrics Society Panel on the Pharmacological Management of Persistent Pain in Older Persons.Pharmacological management of persistent pain in older persons.J Am Geriatr Soc.2009;57(8):1331–1346.
- , , , , .The rate of NSAID‐induced endoscopic ulcers increases linearly but not exponentially with age: a pooled analysis of 12 randomised trials.Ann Rheum Dis.2007;66(3):417–418.
- , , , et al.Prevalence, clinical features and avoidability of adverse drug reactions as cause of admission to a geriatric unit: a prospective study of 1756 patients.Drug Saf.2008;31(6):545–556.
- , , , et al.Adverse drug events in high risk older outpatients.J Am Geriatr Soc.1997;45(8):945–948.
- , , , , , .Updating the Beers criteria for potentially inappropriate medication use in older adults: results of a US consensus panel of experts.Arch Intern Med.2003;163(22):2716–2724.
- , , , .Medication use leading to emergency department visits for adverse drug events in older adults.Ann Intern Med.2007;147(11):755–765.
- , .The patient who falls: “It's always a trade‐off.”JAMA.2010;303(3):258–266.
- , , .Hospital discharge information and older patients: do they get what they need?J Hosp Med.2007;2(5):291–296.
- , , .Functional health literacy and understanding of medications at discharge.Mayo Clin Proc.2008;83(5):554–548.
- , , , .The care transitions intervention: results of a randomized controlled trial.Arch Intern Med.2006;166(17):1822–1828.
- , , , , , .Transitional care of older adults hospitalized with heart failure: a randomized, controlled trial.J Am Geriatr Soc.2004;52(5):675–684.
- , , , .The impact of follow‐up telephone calls to patients after hospitalization.Am J Med.2001;111(9B):26S‐30S.
- , , , et al.A quality improvement intervention to facilitate the transition of older adults from three hospitals back to their homes.J Am GeriatrSoc.2009;57(9):1540–1546.
- , , , et al.A reengineered hospital discharge program to decrease rehospitalization: a randomized trial.Ann Intern Med.2009;150(3):178–187.
- , , , et al.Problems after discharge and understanding of communication with their primary care physicians among hospitalized seniors: a mixed methods study.J Hosp Med.2010;5(7):385–391.
- , .What matters to seriously ill older persons making end‐of‐life treatment decisions? A qualitative study.J Palliat Med.2003;6(2):237–244.
- , , .End‐of‐life decision making: a qualitative study of elderly individuals.J Gen Intern Med.2000;15(9):620–625.
- , , , et al.Patient age and decisions to withhold life‐sustaining treatments from seriously ill, hospitalized adults. SUPPORT Investigators. Study to Understand Prognoses and Preferences for Outcomes and Risks of Treatment.Ann Intern Med.1999;130(2):116–125.
- .Clinical practice. Assessment of patients' competence to consent to treatment.N Engl J Med.2007;357(18):1834–1840.
- , , , , .The Mini‐Cog: a cognitive “vital signs” measure for dementia screening in multi‐lingual elderly.Int J Geriatr Psychiatry.2000;15(11):1021–1027.
- , , , .Growth in the care of older patients by hospitalists in the United States.N Engl J Med.2009;360(11):1102–1112.
- , , , , , .Continuity of outpatient and inpatient care by primary care physicians for hospitalized older adults.JAMA.2009;301(16):1671–1680.
- , , .Is there a geriatrician in the house? Geriatric care approaches in hospitalist programs.J Hosp Med.2006;1(1):29–35.
- .Care of hospitalized older patients: opportunities for hospital‐based physicians.J Hosp Med.2006;1(1):42–47.
- , .The top 10 things nephrologists wish every primary care physician knew.Mayo Clin Proc.2009;84(2):180–186.
- .What is a geriatric syndrome anyway?J Am Geriatr Soc.2003;51(4):574–576.
- , , , .Geriatric syndromes: clinical, research, and policy implications of a core geriatric concept.J Am Geriatr Soc.2007;55(5):780–791.
- , , .Some considerations regarding geriatric syndromes.Ann Intern Med.2001;135(12):1095.
- , , , et al.Impact and recognition of cognitive impairment among hospitalized elders.J Hosp Med.2010;5(2):69–75.
- , , , , , .A longitudinal analysis of total 3‐year healthcare costs for older adults who experience a fall requiring medical care.J Am Geriatr Soc.2010;58(5):853–860.
- , , , et al.A multicomponent intervention to prevent delirium in hospitalized older patients.N Engl J Med.1999;340(9):669–676.
- , , , .Shared risk factors for falls, incontinence, and functional dependence. Unifying the approach to geriatric syndromes.JAMA.1995;273(17):1348–1353.
- , , , et al.Strategies to prevent falls and fractures in hospitals and care homes and effect of cognitive impairment: systematic review and meta‐analyses.BMJ.2007;334(7584):82.
- , , , et al.Interventions for preventing falls in acute‐ and chronic‐care hospitals: a systematic review and meta‐analysis.J Am Geriatr Soc.2008;56(1):29–36.
- , , , et al.Postoperative delirium in the elderly: risk factors and outcomes.Ann Surg.2009;249(1):173–178.
- , , , et al.A controlled trial of inpatient and outpatient geriatric evaluation and management.N Engl J Med.2002;346(12):905–912.
- , , , et al.Risk factors for preoperative and postoperative delirium in elderly patients with hip fracture.J Am Geriatr Soc.2009;57(8):1354‐1361.
- , , , , , .Risk factors for delirium in intensive care patients: a prospective cohort study.Crit Care.2009;13(3):R77.
- , , , .The Mini‐Cog as a screen for dementia: validation in a population‐based sample.J Am Geriatr Soc.2003;51(10):1451–1454.
- , , , , .Improving identification of cognitive impairment in primary care.Int J Geriatr Psychiatry.2006;21(4):349–355.
- , , , et al.Simple cognitive testing (Mini‐Cog) predicts inhospital delirium in the elderly.J Am Geriatr Soc.2007;55(2):314–316.
- , , , .Short Portable Mental Status Questionnaire as a screening test for dementia and delirium among the elderly.J Am Geriatr Soc.1987;35(5):412–416.
- , , .“Mini‐mental state.” A practical method for grading the cognitive state of patients for the clinician.J Psychiatr Res.1975;12(3):189–198.
- , , , et al.Development and validation of a brief cognitive assessment tool: the Sweet 16.Arch Intern Med.2010. [Epub ahead of Print].
- , , , et al.Interventions for preventing falls in older people in nursing care facilities and hospitals.Cochrane Database Syst Rev.2010;(1):CD005465.
- , , , et al.Fall prevention in acute care hospitals: a randomized trial.JAMA.2010;304(17):1912–1918.
- , , , et al.Vitamin D supplementation improves neuromuscular function in older people who fall.Age Ageing.2004;33(6):589–595.
- , , , et al.Effect of vitamin D on falls: a meta‐analysis.JAMA.2004;291(16):1999–2006.
- , et al.Fall prevention with supplemental and active forms of vitamin D: a meta‐analysis of randomised controlled trials.BMJ.2009;339:b3692.
- .Hazards of hospitalization of the elderly.Ann Intern Med.1993;118(3):219–223.
- , , , .Hospitalization, restricted activity, and the development of disability among older persons.JAMA.2004;292(17):2115–2124.
- , , , et al.Recovery of activities of daily living in older adults after hospitalization for acute medical illness.J Am Geriatr Soc.2008;56(12):2171–2179.
- , , , et al.Loss of independence in activities of daily living in older adults hospitalized with medical illnesses: increased vulnerability with age.J Am Geriatr Soc.2003;51(4):451–458.
- , , .New walking dependence associated with hospitalization for acute medical illness: incidence and significance.J Gerontol A Biol Sci Med Sci.1998;53(4):M307–M312.
- , , , .Hazards of hospitalization: residence prior to admission predicts outcomes.Gerontologist.2008;48(4):537–541.
- , , , , , et al.A randomized trial of care in a hospital medical unit especially designed to improve the functional outcomes of acutely ill older patients.N Engl J Med.1995;332(20):1338–1344.
- , .Exercise for acutely hospitalised older medical inpatients.Cochrane Database Syst Rev.2007;(1):CD005955.
- , , .Occurrence and outcome of delirium in medical in‐patients: a systematic literature review.Age Ageing.2006;35(4):350–364.
- , , .A prospective study of delirium in hospitalized elderly.JAMA.1990;263(8):1097–1101.
- , , , , .Delirium predicts 12‐month mortality.Arch Intern Med.2002;162(4):457–463.
- , , , , .The course of delirium in older medical inpatients: a prospective study.J Gen Intern Med.2003;18(9):696–704.
- , , , .Does delirium increase hospital stay?J Am Geriatr Soc.2003;51(11):1539–1546.
- , , , .The Confusion Assessment Method: a systematic review of current usage.J Am Geriatr Soc.2008;56(5):823–830.
- .Delirium in older persons.N Engl J Med.2006;354(11):1157–1165.
- , , , et al.Pharmacological management of delirium in hospitalized adults—a systematic evidence review.J Gen Intern Med.2009;24(7):848‐853.
- , , .Safety and efficacy of physical restraints for the elderly. Review of the evidence.Can Fam Physician.1996;42:2402–2409.
- , , .Treating nondementia illnesses in patients with dementia.JAMA.2000;283(24):3230–3235.
- , , , , , .Survival in Alzheimer disease: a multiethnic, population‐based study of incident cases.Neurology.2008;71(19):1489–1495.
- , , , et al.Survival after initial diagnosis of Alzheimer disease.Ann Intern Med.2004;140(7):501–509.
- , .Mortality from pneumonia and hip fractures in patients with advanced dementia.JAMA.2000;284(19):2447–2448.
- .Rethinking the role of tube feeding in patients with advanced dementia.N Engl J Med.2000;342(3):206–210.
- Society of Hospital Medicine. BOOSTing Care Transitions Resource Room. Available at: http://www.hospitalmedicine.org/ResourceRoomRedesign/RR_CareTransitions/CT_Home.cfm. Accessed January 25,2011.
- , , .Enteral tube feeding for older people with advanced dementia.Cochrane Database Syst Rev.2009;(2):CD007209.
- .A 93‐year‐old man with advanced dementia and eating problems.JAMA.2007;298(21):2527–2536.
- , , .Patient safety in geriatrics: a call for action.J Gerontol A Biol Sci Med Sci.2003;58(9):M813–M819.
- , , .Preventable medical injuries in older patients.Arch Intern Med.2000;160(18):2717–2728.
- , , , et al.Iatrogenic complications in high‐risk, elderly patients.Arch Intern Med.1992;152(10):2074–2080.
- , , , et al.The nature of adverse events in hospitalized patients. Results of the Harvard Medical Practice Study II.N Engl J Med.1991.
- , , , , et al.Nosocomial infections: decade‐specific risk.Infect Control.1983;4(3):145–147.
- , , , , , .A quality‐of‐care analysis of cascade iatrogenesis in frail elderly hospital patients.QRB Qual Rev Bull.1993;19(6):199‐2.
- American Geriatrics Society Panel on the Pharmacological Management of Persistent Pain in Older Persons.Pharmacological management of persistent pain in older persons.J Am Geriatr Soc.2009;57(8):1331–1346.
- , , , , .The rate of NSAID‐induced endoscopic ulcers increases linearly but not exponentially with age: a pooled analysis of 12 randomised trials.Ann Rheum Dis.2007;66(3):417–418.
- , , , et al.Prevalence, clinical features and avoidability of adverse drug reactions as cause of admission to a geriatric unit: a prospective study of 1756 patients.Drug Saf.2008;31(6):545–556.
- , , , et al.Adverse drug events in high risk older outpatients.J Am Geriatr Soc.1997;45(8):945–948.
- , , , , , .Updating the Beers criteria for potentially inappropriate medication use in older adults: results of a US consensus panel of experts.Arch Intern Med.2003;163(22):2716–2724.
- , , , .Medication use leading to emergency department visits for adverse drug events in older adults.Ann Intern Med.2007;147(11):755–765.
- , .The patient who falls: “It's always a trade‐off.”JAMA.2010;303(3):258–266.
- , , .Hospital discharge information and older patients: do they get what they need?J Hosp Med.2007;2(5):291–296.
- , , .Functional health literacy and understanding of medications at discharge.Mayo Clin Proc.2008;83(5):554–548.
- , , , .The care transitions intervention: results of a randomized controlled trial.Arch Intern Med.2006;166(17):1822–1828.
- , , , , , .Transitional care of older adults hospitalized with heart failure: a randomized, controlled trial.J Am Geriatr Soc.2004;52(5):675–684.
- , , , .The impact of follow‐up telephone calls to patients after hospitalization.Am J Med.2001;111(9B):26S‐30S.
- , , , et al.A quality improvement intervention to facilitate the transition of older adults from three hospitals back to their homes.J Am GeriatrSoc.2009;57(9):1540–1546.
- , , , et al.A reengineered hospital discharge program to decrease rehospitalization: a randomized trial.Ann Intern Med.2009;150(3):178–187.
- , , , et al.Problems after discharge and understanding of communication with their primary care physicians among hospitalized seniors: a mixed methods study.J Hosp Med.2010;5(7):385–391.
- , .What matters to seriously ill older persons making end‐of‐life treatment decisions? A qualitative study.J Palliat Med.2003;6(2):237–244.
- , , .End‐of‐life decision making: a qualitative study of elderly individuals.J Gen Intern Med.2000;15(9):620–625.
- , , , et al.Patient age and decisions to withhold life‐sustaining treatments from seriously ill, hospitalized adults. SUPPORT Investigators. Study to Understand Prognoses and Preferences for Outcomes and Risks of Treatment.Ann Intern Med.1999;130(2):116–125.
- .Clinical practice. Assessment of patients' competence to consent to treatment.N Engl J Med.2007;357(18):1834–1840.
- , , , , .The Mini‐Cog: a cognitive “vital signs” measure for dementia screening in multi‐lingual elderly.Int J Geriatr Psychiatry.2000;15(11):1021–1027.
Fluoroquinolone‐Resistant Bacteremia
Among Gram‐negative pathogens, Escherichia coli is one of the most common causes of both community‐acquired and nosocomial bloodstream infections.1, 2 Fluoroquinolone resistance among E coli clinical isolates was first observed in patients with hematologic malignancies3, 4 but is no longer restricted to this population5 and has spread in the community.6 Multiple studies have examined potential risk factors for fluoroquinolone resistance in E coli infections.79 Prior fluoroquinolone use stands out as a repeatedly documented risk factor.10 In E coli bacteremias, data on the impact of fluoroquinolone resistance on mortality are limited.9, 11, 12 Ortega and colleagues performed a landmark analysis of a large dataset stemming from bacteremia surveillance data collected over 17 years.9 They found that mortality was associated with both shock and inappropriate empirical treatment, and that inappropriate empirical treatment in turn was linked to fluoroquinolone resistance. Laupland et al reported results from a population‐based study in Canada, and could elicit age, comorbidities, ciprofloxacin resistance, and a nonurinary focus of infection as risk factors for mortality.11 Lastly, a smaller study by Cheong et al found a high APACHE II score (ie, high severity of illness) but not fluoroquinolone resistance (P = .08) to be associated with poor outcomes.12
The prevalence of fluoroquinolone resistance among E coli isolates in our hospital has surpassed 20%. In this setting, an adjustment of recommendations for empirical treatment may become necessary. This is particularly important since some studies have demonstrated that inappropriate empiric therapy in patients with bloodstream infection results in higher mortality.13 The aim of this case‐control study was to determine the impact of fluoroquinolone resistance on in‐hospital mortality among patients with E coli bacteremia.
METHODS
Study Design, Setting, and Patients
This case‐control study was conducted at Barnes‐Jewish Hospital, a 1250‐bed academic medical center in St. Louis, Missouri. A case was defined as any adult patient with a positive blood culture for fluoroquinolone‐resistant E coli between January 1, 2000, and December 31, 2005. Cases were identified from the Medical Informatics database. Patients who were found to be bacteremic but were not admitted (eg, emergency room visit without admission) were excluded. One control patient with a blood culture positive for fluoroquinolone‐sensitive E coli was randomly matched to each case by year of infection. Demographic data such as age, race, gender, and clinical data such as severity‐of‐illness and comorbidity scores and processes of care such as timing of antibiotic administration and appropriateness of empiric therapy were collected from paper and electronic medical records.
Definitions
Appropriate empiric therapy was defined as receipt of an antimicrobial with in vitro activity against the E coli isolate before or within 48 hours of the blood culture being drawn. No antimicrobial therapy given while the blood cultures were under incubation was considered inappropriate empiric therapy.13Cardiac dysfunction was defined as having a history of atrial fibrillation or congestive heart failure. Central venous catheter (CVC) was defined as the presence of central venous catheter for at least 48 hours at the time of the positive culture was drawn. Clinical cure was achieved if the patient was discharged from the hospital or survived 30 days after the bacteremia without a recurrent E coli infection and no positive blood cultures for E coli were recovered within 14 days after initiation of treatment. History of fluoroquinolone use was defined as receipt of any fluoroquinolone within 90 days before the bacteremia. A history of Clostridium difficile disease was defined as having been diagnosed with C difficile disease in the past 6 months before the bacteremia. History of surgery was defined as having had a surgical procedure in the previous 30 days. A history of urinary tract infection (UTI) was defined as a UTI 90 days before the bacteremia. Hospital‐acquired infections were defined as infections that were not active or present at admission and the positive blood cultures were obtained 48 hours or greater after admission. In‐hospital mortality was defined as death in the hospital within 30 days after the positive blood culture. MRSA colonization was defined as a history of colonization with methicillin‐resistant Staphylococcus aureus any time before the bacteremia. Prior antibiotic use was defined as receipt of any antibiotic within 90 days before the bacteremia. Previous hospital admission was defined as admission to a hospital in the last 90 days. Renal dysfunction was defined as acute renal failure (serum creatinine level at the time blood cultures were drawn was twice that of the last available creatinine level), chronic renal insufficiency (creatinine >1.6 mg/dL), or renal failure requiring dialysis. VRE colonization was defined as a history of infection or stool colonization with vancomycin‐resistant enterococci (VRE) any time before the bacteremia.
Statistical Analysis
Univariate analysis of categorical variables in this case‐control study was performed using Mantel‐Haenszel chi‐square or Fisher exact test as appropriate. Continuous variables were compared using the Student t test or the Mann‐Whitney U test depending on the normality assumptions of the variable. Multivariate analysis was performed using backward stepwise conditional logistic regression. Variables that were found to have a P value of .10 on univariate analysis along with age, gender, and race were included in the conditional logistic regression model. Variables which were associated with fewer than five patients were not included in the multivariate analysis despite having a P value of .10 on univariate analysis. Goodness‐of‐fit of the logistic regression model was determined by the Hosmer‐Lemeshow test and the model with the best fit was retained as the final model. A two‐sided P value of .05 was considered statistically significant. Data analysis was performed using SPSS version 17 (SPSS, Chicago, IL).
The study was approved by the Washington University Human Research Protection Office.
RESULTS
Differences Among Patients With Fluoroquinolone‐Resistant and Fluoroquinolone‐Sensitive E coli Bacteremia
Nine‐hundred thirty patients had E coli bacteremia during the study period. Ninety‐eight patients had fluoroquinolone‐resistant E coli but blood cultures from 5 patients were collected in the outpatient setting and no follow‐up information was available; these patients were excluded from the analysis. Ninety‐three patients met the definition of a case and were matched with 93 patients with fluoroquinolone‐sensitive E coli bacteremias by year of infection for each of the cases. A comparison of the baseline demographic data and comorbid illnesses is shown in Table 1. When compared with control patients, cases were more likely to be admitted from a long‐term care facility (35% vs. 9%; P < .001) and to have a hospital‐acquired bacteremia (54% vs. 33%; P = .008). Cases were also more likely to have been admitted to a hospital in the previous 30 days (P < .001), colonized with vancomycin‐resistant enterococci (P = .006), have a central venous catheter in place (P = .04), and have been treated with antibiotics including fluoroquinolones (P < .001). The clinical cure rate was higher among controls (91% vs. 72%; P = .001). Crude mortality was 26% for cases and 8% for controls (P = .002). Although there was no difference in the mean severity‐of‐illness score between cases and controls, cases had a longer mean length of stay (see Table 1).
| Variable | Cases n (%) n = 93 | Controls n (%) n = 93 | P Value |
|---|---|---|---|
| |||
| Demographic characteristics | |||
| Mean age (SD) | 60.1 17.0 years | 63.2 19.4 years | 0.2 |
| Female gender | 61 (66) | 49 (53) | 0.1 |
| Race: | |||
| African American | 26 (28) | 42 (45) | 0.1 |
| Caucasian | 60 (65) | 50 (54) | |
| Other | 7 (7) | 1 (1) | |
| Residence: Home | 55 (59) | 79 (85) | <0.001 |
| LTCF/SNF | 32 (35) | 8 (9) | |
| Other | 6 (6) | 6 (6) | |
| Hospital‐acquired bacteremia | 50 (54) | 31 (33) | 0.008 |
| Comorbidities/Other risk factors | |||
| Alcohol abuse | 6 (6) | 5 (5) | 1.0 |
| APACHE II score 10 | 50 (54) | 49 (53) | 0.9 |
| Mean APACHE II score | 13.4 8.3 | 11.9 6.1 | 0.6 |
| Cardiac dysfunction | 28 (30) | 22 (24) | 0.3 |
| Charlson Index 4 | 36 (39) | 29 (31) | 0.3 |
| Mean Charlson Index | 3.6 2.8 | 3.4 2.8 | 0.7 |
| Chemotherapy | 18 (19) | 11 (12) | 0.2 |
| Cirrhosis | 7 (8) | 4 (4) | 0.5 |
| Diabetes mellitus | 30 (32) | 28 (30) | 0.9 |
| Hypertension | 48 (52) | 47 (51) | 0.9 |
| Malignancy | 35 (38) | 29 (31) | 0.4 |
| MRSA colonization | 11 (12) | 4 (4) | 0.07 |
| Obesity | 17 (18) | 20 (22) | 0.7 |
| Neutropenia | 19 (20) | 9 (10) | 0.07 |
| Previous hospital admission | 43 (46) | 19 (20) | <0.001 |
| Renal dysfunction | 41 (44) | 39 (42) | 0.9 |
| Tobacco use | 20 (22) | 13 (14) | 0.3 |
| Trauma | 3 (3) | 12 (13) | 0.03 |
| VRE colonization | 23 (25) | 8 (9) | 0.006 |
| Previous antibiotic use | 35 (38) | 12 (13) | <0.001 |
| Fluoroquinolone use | 37 (40) | 9 (10) | <0.001 |
| History of UTI | 32 (34) | 23 (25) | 0.2 |
| Corticosteroids | 30 (32) | 9 (10) | <0.001 |
| CVC | 55 (59) | 40 (43) | 0.04 |
| Source of bacteremia | |||
| Urinary tract | 57 (61) | 55 (59) | 0.8 |
| Intra‐abdominal infection | 5 (5) | 11 (12) | 0.1 |
| Primary/catheter‐related | 17 (18) | 4 (4) | 0.005 |
| Chemotherapy‐related/ mucositis | 6 (6) | 1 (1) | 0.09 |
| Pneumonia | 0 (0) | 7 (8) | |
| Other | 8 (9) | 15 (16) | |
| Management and outcome | |||
| Appropriate empiric therapy | 48 (52) | 51 (55) | 0.8 |
| Clinical cure | 67 (72) | 85 (91) | 0.001 |
| Mean length of stay | 18.2 21.9 days | 10.4 10 days | 0.002 |
| Median length of stay | 9 days | 6 days | 0.002 |
| In‐hospital mortality | 24 (26) | 7 (8) | 0.002 |
Risk Factors for Mortality From E coli Bacteremia
On univariate analysis, predictors for in‐hospital mortality included female gender, admission from a nursing home or other long‐term care facility, APACHE II score of >10, Charlson comorbidity score >4, a previous diagnosis of cardiac dysfunction, cirrhosis, renal dysfunction, and treatment with corticosteroids (see Table 2). Fluoroquinolone resistance was also associated with increased mortality (unadjusted odds ratio [uOR], 4.27; 95% confidence interval [CI], 1.710.5). On multivariate analysis (see Table 3), independent risk factors for in‐hospital mortality were cirrhosis (adjusted OR [aOR], 7.2; 95% CI, 1.729.8; P = .007), a history of cardiac dysfunction (aOR, 3.9; 95% CI, 1.69.4; P = .003), and infection with a fluoroquinolone‐resistant E coli isolate (aOR, 3.9; 95% CI, 1.5, 10.2; P = .005). The Hosmer‐Lemeshow test revealed a P value of .54. Both severity‐of‐illness indices were found not to be independent predictors of in‐hospital mortality.
| Variable | Died, n (%) n = 31 | Survived, n (%) n = 155 | P value | Unadjusted odds ratio (uOR) |
|---|---|---|---|---|
| ||||
| Demographic characteristics | ||||
| Mean age (SD) | 61.2 18.9 years | 63.8 14.4 years | 1.0 | |
| Age 65 years | 11 (36) | 66 (43) | 0.4 | 0.73 (0.33, 1.62) |
| Female gender | 19 (61) | 57 (37) | 0.01 | 2.29 (1.18, 4.44) |
| Race: | ||||
| African American | 9 (29) | 59 (38) | 0.1 | 1.86 (0.81, 4.30) |
| Caucasian | 22 (71) | 88 (57) | ||
| Other | 0 (0) | 8 (5) | ||
| Residence: | ||||
| Home | 13 (42) | 121 (78) | 0.02 | 3.13 (1.24, 7.76) |
| LTCF/SNF | 10 (32) | 30 (19) | ||
| Other | 8 (26) | 4 (3) | ||
| Hospital‐acquired bacteremia | 18 (58) | 63 (41) | 0.08 | 2.02 (0.93, 4.42) |
| Comorbidities/Other risk factors | ||||
| Alcohol abuse | 4 (13) | 7 (5) | 0.2 | 3.13 (0.86, 11.44) |
| APACHE II score 10 | 22 (71) | 77 (50) | 0.03 | 2.48 (1.07, 5.72) |
| Mean APACHE II score | 17.8 + 9.9 | 11.6 + 6.2 | 0.002 | |
| Cardiac dysfunction | 15 (48) | 35 (23) | 0.004 | 3.43 (1.53, 7.70) |
| C difficile colitis | 4 (13) | 7 (5) | 0.08 | 3.13 (0.86, 11.43) |
| Charlson Index 4 | 16 (52) | 49 (32) | 0.04 | 2.31 (1.06, 5.04) |
| Mean Charlson Index | 4.8 + 3.0 | 3.2 + 2.7 | 0.006 | |
| Chemotherapy | 6 (19) | 23 (15) | 0.5 | 1.38 (0.51, 3.73) |
| Cirrhosis | 6 (19) | 5 (3) | 0.002 | 7.2 (2.04, 25.4) |
| Diabetes mellitus | 11 (36) | 47 (30) | 0.6 | 1.26 (0.56, 2.85) |
| Hypertension | 17 (55) | 78 (50) | 0.7 | 1.20 (0.55, 2.60) |
| Malignancy | 14 (45) | 50 (32) | 0.2 | 1.73 (0.79, 3.79) |
| MRSA colonization | 3 (10) | 12 (8) | 0.7 | 1.28 (0.34, 4.82) |
| Obesity | 5 (16) | 32 (21) | 0.6 | 0.74 (0.63, 2.08) |
| Neutropenia | 5 (16) | 23 (15) | 0.9 | 1.10 (0.38, 3.17) |
| Previous hospital admission | 15 (48) | 47 (30) | 0.06 | 2.15 (0.98, 4.72) |
| Renal dysfunction | 20 (65) | 60 (39) | 0.01 | 2.88 (1.29, 6.43) |
| Tobacco use | 7 (23) | 26 (17) | 0.4 | 1.45 (0.56, 3.71) |
| Trauma | 1(3) | 14 (9) | 0.3 | 0.34 (0.04, 2.65) |
| VRE colonization | 7 (23) | 24 (16) | 0.3 | 1.59 (0.62, 4.11) |
| Previous antibiotic use | 9 (29) | 38 (25) | 0.6 | 1.26 (0.53, 2.97) |
| Fluoroquinolone use | 8 (26) | 38 (25) | 0.9 | 1.07 (0.44, 2.59) |
| History of UTI | 8 (26) | 47 (30) | 0.6 | 0.80 (0.33, 1.92) |
| Corticosteroids | 12 (39) | 27 (17) | 0.01 | 2.99 (1.30, 6.89) |
| CVC | 17 (55) | 78 (50) | 0.7 | 1.20 (0.55, 2.6) |
| Source of bacteremia | ||||
| Urinary tract | 15 (48) | 97 (63) | 0.1 | 0.56 (0.26, 1.22) |
| Intra‐abdominal infection | 5 (16) | 11 (7) | 0.1 | 2.52 (0.81, 7.85) |
| Primary/catheter‐related | 3 (10) | 18 (12) | 0.8 | 0.82 (0.23, 2.96) |
| Chemotherapy‐related/mucositis | 3 (10) | 4 (3) | 0.08 | 0.05 (0.86, 19.06) |
| Management and outcome | ||||
| Appropriate empiric therapy | 15 (48) | 69 (45) | 0.38 | 0.70 (0.31, 1.56) |
| Mean length of stay | 19.9 + 24.8 days | 13.2 + 15.4 days | 0.2 | |
| In‐hospital mortality | 24 (26) | 7 (8) | 0.002 | |
| Fluoroquinolone resistance | 24 (77) | 69 (45) | 0.002 | 4.27 (1.74, 10.5) |
| Adjusted odds ratio | 95% Confidence interval | P value | |
|---|---|---|---|
| Cirrhosis | 7.2 | (1.7, 29.8) | .007 |
| Fluoroquinolone resistance | 3.9 | (1.5, 10.2) | .005 |
| Cardiac dysfunction | 3.9 | (1.6, 9.4) | .003 |
| Female gender | 0.5 | (0.2, 1.2) | .11 |
DISCUSSION
This case‐control study represents one of the larger studies on fluoroquinolone‐resistant E coli bacteremia and adds to the growing body of literature on the impact of fluoroquinolone resistance and other factors predictive of mortality. In multivariate analysis, fluoroquinolone resistance was associated with in‐hospital mortality from E coli bacteremia, as were the comorbid illnesses cirrhosis and cardiac dysfunction.
Among the risk factors for fluoroquinolone‐resistant E coli bacteremia described in the literature are previous fluoroquinolone exposure,9, 10, 12 nosocomial acquisition,9 presence of a urinary catheter,9 urinary source of bacteremia, previous surgery, and comorbid illnesses.10 If the scope of infections was not limited to the bloodstream, other factors like structural changes in the urinary tract,7 recurrent urinary tract infections,14 residence in a long‐term care facility, age, and prior exposure to aminoglycosides8 were also reported. In our study, previous fluoroquinolone exposure, residence in a long‐term care facility, recent hospitalization, nosocomial acquisition of infection, were associated with cases with fluoroquinolone‐resistant isolates. We also found that a larger proportion of the cases received corticosteroids before the episode of bacteremia; to our knowledge, this finding has not been reported before.
In contrast to results on fluoroquinolone resistance in both E coli and Klebsiella pneumoniae infections reported by Lautenbach et al,13 those patients in our study who were infected with the fluoroquinolone‐resistant phenotype were not more likely to receive inappropriate empiric therapy than control patients (52% vs. 55%; P = .8). This finding may be explained by the relatively low level of appropriate treatment even in the patients with fluoroquinolone‐susceptible E coli. For comparison, Lautenbach and colleagues saw a much higher percentage, 90%, of the patients with the susceptible phenotype received appropriate therapy.13 The high proportion of inappropriate empiric therapy in our study may have played a role in the relatively high overall mortality rate (17%) that we observed. This is in contrast to a recent retrospective study on appropriateness of therapy for E coli bacteremia which found that only 16% of bacteremia episodes (106 of 663) were inadequately treated,15 and the overall mortality was as low as 5%. A significant number of patients in our study, however, did not receive any antimicrobial therapy until blood cultures results were reported as positive and these situations therefore did not meet the definition for appropriate empiric therapy. These same patients did not have all the signs and symptoms associated with sepsis syndrome and so were not treated with any antimicrobials until blood cultures were reported to be positive. Eventually, Lautenbach et al stated thatafter adjusting for inadequate treatmentthere was no longer an association between fluoroquinolone resistance and mortality in their population.13 On the other hand, a recently published landmark Spanish study on factors influencing the outcome of 4758 E coli bacteremias reported that inappropriate treatment and shock were the two independent predictors of mortality; however, inappropriate treatment was significantly associated with fluoroquinolone resistance.9 Laupland et al, who performed a population‐based study of E coli bacteremias in Canada, elicited ciprofloxacin resistance as an independent predictor of mortality but the authors did not adjust for appropriateness of treatment.11 In that study, a urinary source of the bacteremia and younger age turned out to be protective. We studied both variables in our study but failed to confirm their findings.
Previous studies have reported that fluoroquinolone‐resistant clinical isolates collected from urine samples contain less virulence factors compared with the fluoroquinolone‐susceptible E coli.1618 Although no data are available specifically for bloodstream isolates, our finding of increased mortality in fluoroquinolone‐resistant isolates is not consistent with these conceptual findings among E coli isolates from the urinary tract. A delay in delivering the appropriate therapy cannot account for this, because the proportion of patients who did not receive appropriate therapy within 48 hours of the blood cultures being drawn was similar among the cases and control patients. Nevertheless, it would be interesting to assess virulence factor profiles in E coli bloodstream isolates that are stratified by their susceptibility to fluoroquinolones. The pathogens in our cohort may possess unidentified virulence mechanisms as well as resistance mechanisms toward fluoroquinolones. Because only patients with bacteremia were included in this study, it is possible that we have selected for a more virulent subpopulation of E coli strains capable of more invasive disease than uropathogenic isolates. In the past, several small studies have indeed demonstrated differences in virulence factor profiles when comparing E coli isolates strictly from urinary tract infections with those urinary tract isolates causing bacteremia.19, 20 Another potential explanation for the observed association between fluoroquinolone‐resistance and increased mortality may be unmeasured severity of illness among the cases. The cases were more likely to have a health‐care associated infection, more likely to come from a long‐term care facility or have been previously admitted, or associated with a longer length of stay. We did account for severity of illness and risk of mortality from comorbidities using both the APACHE II score and the Charlson Index of Co‐Morbidity, but it is still possible these indices may not be adequate to account for the differences between the cases and controls.
We found a higher crude mortality among patients with fluoroquinolone‐resistant E coli bacteremia than in patients with fluoroquinolone‐susceptible E coli (26% vs. 8%; P = .002). This is similar to the crude mortality rate for fluoroquinolone‐resistant E coli bacteremia reported by Cheong et al (30% in patients with fluoroquinolone‐resistant E coli bacteremia vs. 16% in patients with fluoroquinolone‐susceptible E coli; P = .08).12 In the Cheong et al article, only a high APACHE II score remained an independent risk factor for mortality. And although both Laupland et al and Ortega et al used regression analyses to describe factors associated with mortality, the respective crude mortality rates stratified by fluoroquinolone susceptibility were not reported.9, 11 In our study, the univariate analysis yielded both APACHE II score and Charlson comorbidity score as predictors for in‐hospital mortality but not in the multivariate analysis.
Our findings have important implications in the treatment of Gram‐negative infections. E coli is one of most common Gram‐negative bacilli causing hospital‐acquired infections and is the most common pathogen associated with community‐acquired urinary tract infections. The latest Infectious Diseases Society of America (IDSA) guideline for treatment of acute pyelonephritis recommends the use of fluoroquinolones for empiric therapy of acute pyelonephritis.21 Unfortunately, these guidelines were published in 1999, before reports of the rise in fluoroquinolone resistance among E coli isolates were available. The majority of the patients in our cohort (60%) developed a bacteremia following a complicated urinary tract infection and they would have received a fluoroquinolone for empiric therapy. The risk of providing inappropriate empiric therapy to patients with E coli bacteremia is evident, especially since inappropriate treatment was delivered in approximately half of our patients.
Another group of patients who are at high risk for mortality and are also at risk for development of fluoroquinolone‐resistant E coli bacteremia are patients with liver cirrhosis. Gram‐negative bacilli like E coli are common pathogens implicated in spontaneous bacterial peritonitis (SBP) in these patients.22 Since some patients with cirrhosis are exposed to fluoroquinolones for primary or secondary prophylaxis against SBP,23 they are likely to be colonized and eventually can develop infections with fluoroquinolone‐resistant E coli isolates.8 It may be prudent to select an antimicrobial class that is different from fluoroquinolones in treating sepsis syndrome in this patient population.
Our study has a few limitations. One is that this is a retrospective case‐control study and the accuracy of the data is dependent on the availability of complete medical records. All the admitted patients' charts or medical records were available for review in this study, so we were able to minimize any potential bias that may arise from missing data. This study was conducted at an academic medical center and results may not be generalizable to other healthcare institutions. The rate of inappropriate therapy was particularly high in this study, but it is unlikely to have influenced the final results since this was observed in both cases and controls.
On the basis of our finding that fluoroquinolone resistance is an independent predictor for mortality, we recommend that an alternative antimicrobial class rather than fluoroquinolones be initiated as empiric therapy in patients who are suspected to have an invasive E coli infection. The reason for this increased mortality in fluoroquinolone‐resistant E coli is, at least in our study, not related to inappropriate therapy or a higher severity of illness and may be related to more virulent organisms.
Acknowledgements
We thank Cherie Hill and Dorothy Sinclair for their invaluable help concerning the data management.
- ,.Overview of nosocomial infections caused by gram‐negative bacilli.Clin Infect Dis.2005;41:848–854.
- ,,, et al.Survey of bloodstream infections due to gram‐negative bacilli: frequency of occurrence and antimicrobial susceptibility of isolates collected in the United States, Canada, and Latin America for the SENTRY Antimicrobial Surveillance Program, 1997.Clin Infect Dis.1999;29:595–607.
- ,,,,,.Emergence of fluoroquinolone‐resistant Escherichia coli at a cancer center.Antimicrob Agents Chemother.1994;38:681–687.
- ,,,,.Emergence of quinolone‐resistant Escherichia coli bacteremia in neutropenic patients with cancer who have received prophylactic norfloxacin.Clin Infect Dis.1995;20:557–560; discussion 561–563.
- ,,,,.Antimicrobial‐resistant invasive Escherichia coli, Spain.Emerg Infect Dis.2005;11:546–553.
- ,,, et al.Emergence and dissemination of quinolone‐resistant Escherichia coli in the community.Antimicrob Agents Chemother.1999;43:2736–2741.
- ,,,.Incidence and risk factors for nosocomial infections caused by fluoroquinolone‐resistant Escherichia coli.Eur J Clin Microbiol Infect Dis.2003;22:492–495.
- ,,, et al.Risk factors for fluoroquinolone resistance in nosocomial Escherichia coli and Klebsiella pneumoniae infections.Arch Intern Med.2002;162:2469–2477.
- ,,, et al.Analysis of 4758 Escherichia coli bacteraemia episodes: predictive factors for isolation of an antibiotic‐resistant strain and their impact on the outcome.J Antimicrob Chemother.2009;63:568–574.
- ,,,,,.Relationship between quinolone use and emergence of ciprofloxacin‐resistant Escherichia coli in bloodstream infections.Antimicrob Agents Chemother.1995;39:520–524.
- ,,,,.Incidence, risk factors and outcomes of Escherichia coli bloodstream infections in a large Canadian region.Clin Microbiol Infect.2008;14:1041–1047.
- ,,,,,.Bacteremia due to quinolone‐resistant Escherichia coli in a teaching hospital in South Korea.Clin Infect Dis.2001;33:48–53.
- ,,,,.Association between fluoroquinolone resistance and mortality in Escherichia coli and Klebsiella pneumoniae infections: the role of inadequate empirical antimicrobial therapy.Clin Infect Dis.2005;41:923–929.
- ,,.Risk factors for community‐acquired ciprofloxacin‐resistant Escherichia coli urinary tract infection.Ann Pharmacother. Jul‐2004;38:1148–1152.
- ,,, et al.Impact of antibiotic resistance and of adequate empirical antibiotic treatment in the prognosis of patients with Escherichia coli bacteraemia.J Antimicrob Chemother.2007;60:855–863.
- ,,, et al.Decreased prevalence of virulence factors among ciprofloxacin‐resistant uropathogenic Escherichia coli isolates.J Clin Microbiol.2005;43:4218–4220.
- ,,, et al.Are quinolone‐resistant uropathogenic Escherichia coli less virulent?J Infect Dis.2002;186:1039–1042.
- ,,, et al.Genetic profiles of fluoroquinolone‐resistant Escherichia coli isolates obtained from patients with cystitis: phylogeny, virulence factors, PAIusp subtypes, and mutation patterns.J Clin Microbiol.2009;47:791–795.
- ,,,,,.Comparative study of Escherichia coli virulence determinants in strains causing urinary tract bacteremia versus strains causing pyelonephritis and other sources of bacteremia.Diagn Microbiol Infect Dis.2005;53:93–99.
- ,,,,.Comparative prevalence of virulence factors in Escherichia coli causing urinary tract infection in male infants with and without bacteremia.J Clin Microbiol.2006;44:1156–1158.
- ,,,,,.Guidelines for antimicrobial treatment of uncomplicated acute bacterial cystitis and acute pyelonephritis in women. Infectious Diseases Society of America (IDSA).Clin Infect Dis.1999;29:745–758.
- ,,.Spontaneous bacterial peritonitis: recent data on incidence and treatment.Cleve Clin J Med.2004;71:569–576.
- ,,, et al.Diagnosis, treatment and prophylaxis of spontaneous bacterial peritonitis: a consensus document. International Ascites Club.J Hepatol.2000;32:142–153.
Among Gram‐negative pathogens, Escherichia coli is one of the most common causes of both community‐acquired and nosocomial bloodstream infections.1, 2 Fluoroquinolone resistance among E coli clinical isolates was first observed in patients with hematologic malignancies3, 4 but is no longer restricted to this population5 and has spread in the community.6 Multiple studies have examined potential risk factors for fluoroquinolone resistance in E coli infections.79 Prior fluoroquinolone use stands out as a repeatedly documented risk factor.10 In E coli bacteremias, data on the impact of fluoroquinolone resistance on mortality are limited.9, 11, 12 Ortega and colleagues performed a landmark analysis of a large dataset stemming from bacteremia surveillance data collected over 17 years.9 They found that mortality was associated with both shock and inappropriate empirical treatment, and that inappropriate empirical treatment in turn was linked to fluoroquinolone resistance. Laupland et al reported results from a population‐based study in Canada, and could elicit age, comorbidities, ciprofloxacin resistance, and a nonurinary focus of infection as risk factors for mortality.11 Lastly, a smaller study by Cheong et al found a high APACHE II score (ie, high severity of illness) but not fluoroquinolone resistance (P = .08) to be associated with poor outcomes.12
The prevalence of fluoroquinolone resistance among E coli isolates in our hospital has surpassed 20%. In this setting, an adjustment of recommendations for empirical treatment may become necessary. This is particularly important since some studies have demonstrated that inappropriate empiric therapy in patients with bloodstream infection results in higher mortality.13 The aim of this case‐control study was to determine the impact of fluoroquinolone resistance on in‐hospital mortality among patients with E coli bacteremia.
METHODS
Study Design, Setting, and Patients
This case‐control study was conducted at Barnes‐Jewish Hospital, a 1250‐bed academic medical center in St. Louis, Missouri. A case was defined as any adult patient with a positive blood culture for fluoroquinolone‐resistant E coli between January 1, 2000, and December 31, 2005. Cases were identified from the Medical Informatics database. Patients who were found to be bacteremic but were not admitted (eg, emergency room visit without admission) were excluded. One control patient with a blood culture positive for fluoroquinolone‐sensitive E coli was randomly matched to each case by year of infection. Demographic data such as age, race, gender, and clinical data such as severity‐of‐illness and comorbidity scores and processes of care such as timing of antibiotic administration and appropriateness of empiric therapy were collected from paper and electronic medical records.
Definitions
Appropriate empiric therapy was defined as receipt of an antimicrobial with in vitro activity against the E coli isolate before or within 48 hours of the blood culture being drawn. No antimicrobial therapy given while the blood cultures were under incubation was considered inappropriate empiric therapy.13Cardiac dysfunction was defined as having a history of atrial fibrillation or congestive heart failure. Central venous catheter (CVC) was defined as the presence of central venous catheter for at least 48 hours at the time of the positive culture was drawn. Clinical cure was achieved if the patient was discharged from the hospital or survived 30 days after the bacteremia without a recurrent E coli infection and no positive blood cultures for E coli were recovered within 14 days after initiation of treatment. History of fluoroquinolone use was defined as receipt of any fluoroquinolone within 90 days before the bacteremia. A history of Clostridium difficile disease was defined as having been diagnosed with C difficile disease in the past 6 months before the bacteremia. History of surgery was defined as having had a surgical procedure in the previous 30 days. A history of urinary tract infection (UTI) was defined as a UTI 90 days before the bacteremia. Hospital‐acquired infections were defined as infections that were not active or present at admission and the positive blood cultures were obtained 48 hours or greater after admission. In‐hospital mortality was defined as death in the hospital within 30 days after the positive blood culture. MRSA colonization was defined as a history of colonization with methicillin‐resistant Staphylococcus aureus any time before the bacteremia. Prior antibiotic use was defined as receipt of any antibiotic within 90 days before the bacteremia. Previous hospital admission was defined as admission to a hospital in the last 90 days. Renal dysfunction was defined as acute renal failure (serum creatinine level at the time blood cultures were drawn was twice that of the last available creatinine level), chronic renal insufficiency (creatinine >1.6 mg/dL), or renal failure requiring dialysis. VRE colonization was defined as a history of infection or stool colonization with vancomycin‐resistant enterococci (VRE) any time before the bacteremia.
Statistical Analysis
Univariate analysis of categorical variables in this case‐control study was performed using Mantel‐Haenszel chi‐square or Fisher exact test as appropriate. Continuous variables were compared using the Student t test or the Mann‐Whitney U test depending on the normality assumptions of the variable. Multivariate analysis was performed using backward stepwise conditional logistic regression. Variables that were found to have a P value of .10 on univariate analysis along with age, gender, and race were included in the conditional logistic regression model. Variables which were associated with fewer than five patients were not included in the multivariate analysis despite having a P value of .10 on univariate analysis. Goodness‐of‐fit of the logistic regression model was determined by the Hosmer‐Lemeshow test and the model with the best fit was retained as the final model. A two‐sided P value of .05 was considered statistically significant. Data analysis was performed using SPSS version 17 (SPSS, Chicago, IL).
The study was approved by the Washington University Human Research Protection Office.
RESULTS
Differences Among Patients With Fluoroquinolone‐Resistant and Fluoroquinolone‐Sensitive E coli Bacteremia
Nine‐hundred thirty patients had E coli bacteremia during the study period. Ninety‐eight patients had fluoroquinolone‐resistant E coli but blood cultures from 5 patients were collected in the outpatient setting and no follow‐up information was available; these patients were excluded from the analysis. Ninety‐three patients met the definition of a case and were matched with 93 patients with fluoroquinolone‐sensitive E coli bacteremias by year of infection for each of the cases. A comparison of the baseline demographic data and comorbid illnesses is shown in Table 1. When compared with control patients, cases were more likely to be admitted from a long‐term care facility (35% vs. 9%; P < .001) and to have a hospital‐acquired bacteremia (54% vs. 33%; P = .008). Cases were also more likely to have been admitted to a hospital in the previous 30 days (P < .001), colonized with vancomycin‐resistant enterococci (P = .006), have a central venous catheter in place (P = .04), and have been treated with antibiotics including fluoroquinolones (P < .001). The clinical cure rate was higher among controls (91% vs. 72%; P = .001). Crude mortality was 26% for cases and 8% for controls (P = .002). Although there was no difference in the mean severity‐of‐illness score between cases and controls, cases had a longer mean length of stay (see Table 1).
| Variable | Cases n (%) n = 93 | Controls n (%) n = 93 | P Value |
|---|---|---|---|
| |||
| Demographic characteristics | |||
| Mean age (SD) | 60.1 17.0 years | 63.2 19.4 years | 0.2 |
| Female gender | 61 (66) | 49 (53) | 0.1 |
| Race: | |||
| African American | 26 (28) | 42 (45) | 0.1 |
| Caucasian | 60 (65) | 50 (54) | |
| Other | 7 (7) | 1 (1) | |
| Residence: Home | 55 (59) | 79 (85) | <0.001 |
| LTCF/SNF | 32 (35) | 8 (9) | |
| Other | 6 (6) | 6 (6) | |
| Hospital‐acquired bacteremia | 50 (54) | 31 (33) | 0.008 |
| Comorbidities/Other risk factors | |||
| Alcohol abuse | 6 (6) | 5 (5) | 1.0 |
| APACHE II score 10 | 50 (54) | 49 (53) | 0.9 |
| Mean APACHE II score | 13.4 8.3 | 11.9 6.1 | 0.6 |
| Cardiac dysfunction | 28 (30) | 22 (24) | 0.3 |
| Charlson Index 4 | 36 (39) | 29 (31) | 0.3 |
| Mean Charlson Index | 3.6 2.8 | 3.4 2.8 | 0.7 |
| Chemotherapy | 18 (19) | 11 (12) | 0.2 |
| Cirrhosis | 7 (8) | 4 (4) | 0.5 |
| Diabetes mellitus | 30 (32) | 28 (30) | 0.9 |
| Hypertension | 48 (52) | 47 (51) | 0.9 |
| Malignancy | 35 (38) | 29 (31) | 0.4 |
| MRSA colonization | 11 (12) | 4 (4) | 0.07 |
| Obesity | 17 (18) | 20 (22) | 0.7 |
| Neutropenia | 19 (20) | 9 (10) | 0.07 |
| Previous hospital admission | 43 (46) | 19 (20) | <0.001 |
| Renal dysfunction | 41 (44) | 39 (42) | 0.9 |
| Tobacco use | 20 (22) | 13 (14) | 0.3 |
| Trauma | 3 (3) | 12 (13) | 0.03 |
| VRE colonization | 23 (25) | 8 (9) | 0.006 |
| Previous antibiotic use | 35 (38) | 12 (13) | <0.001 |
| Fluoroquinolone use | 37 (40) | 9 (10) | <0.001 |
| History of UTI | 32 (34) | 23 (25) | 0.2 |
| Corticosteroids | 30 (32) | 9 (10) | <0.001 |
| CVC | 55 (59) | 40 (43) | 0.04 |
| Source of bacteremia | |||
| Urinary tract | 57 (61) | 55 (59) | 0.8 |
| Intra‐abdominal infection | 5 (5) | 11 (12) | 0.1 |
| Primary/catheter‐related | 17 (18) | 4 (4) | 0.005 |
| Chemotherapy‐related/ mucositis | 6 (6) | 1 (1) | 0.09 |
| Pneumonia | 0 (0) | 7 (8) | |
| Other | 8 (9) | 15 (16) | |
| Management and outcome | |||
| Appropriate empiric therapy | 48 (52) | 51 (55) | 0.8 |
| Clinical cure | 67 (72) | 85 (91) | 0.001 |
| Mean length of stay | 18.2 21.9 days | 10.4 10 days | 0.002 |
| Median length of stay | 9 days | 6 days | 0.002 |
| In‐hospital mortality | 24 (26) | 7 (8) | 0.002 |
Risk Factors for Mortality From E coli Bacteremia
On univariate analysis, predictors for in‐hospital mortality included female gender, admission from a nursing home or other long‐term care facility, APACHE II score of >10, Charlson comorbidity score >4, a previous diagnosis of cardiac dysfunction, cirrhosis, renal dysfunction, and treatment with corticosteroids (see Table 2). Fluoroquinolone resistance was also associated with increased mortality (unadjusted odds ratio [uOR], 4.27; 95% confidence interval [CI], 1.710.5). On multivariate analysis (see Table 3), independent risk factors for in‐hospital mortality were cirrhosis (adjusted OR [aOR], 7.2; 95% CI, 1.729.8; P = .007), a history of cardiac dysfunction (aOR, 3.9; 95% CI, 1.69.4; P = .003), and infection with a fluoroquinolone‐resistant E coli isolate (aOR, 3.9; 95% CI, 1.5, 10.2; P = .005). The Hosmer‐Lemeshow test revealed a P value of .54. Both severity‐of‐illness indices were found not to be independent predictors of in‐hospital mortality.
| Variable | Died, n (%) n = 31 | Survived, n (%) n = 155 | P value | Unadjusted odds ratio (uOR) |
|---|---|---|---|---|
| ||||
| Demographic characteristics | ||||
| Mean age (SD) | 61.2 18.9 years | 63.8 14.4 years | 1.0 | |
| Age 65 years | 11 (36) | 66 (43) | 0.4 | 0.73 (0.33, 1.62) |
| Female gender | 19 (61) | 57 (37) | 0.01 | 2.29 (1.18, 4.44) |
| Race: | ||||
| African American | 9 (29) | 59 (38) | 0.1 | 1.86 (0.81, 4.30) |
| Caucasian | 22 (71) | 88 (57) | ||
| Other | 0 (0) | 8 (5) | ||
| Residence: | ||||
| Home | 13 (42) | 121 (78) | 0.02 | 3.13 (1.24, 7.76) |
| LTCF/SNF | 10 (32) | 30 (19) | ||
| Other | 8 (26) | 4 (3) | ||
| Hospital‐acquired bacteremia | 18 (58) | 63 (41) | 0.08 | 2.02 (0.93, 4.42) |
| Comorbidities/Other risk factors | ||||
| Alcohol abuse | 4 (13) | 7 (5) | 0.2 | 3.13 (0.86, 11.44) |
| APACHE II score 10 | 22 (71) | 77 (50) | 0.03 | 2.48 (1.07, 5.72) |
| Mean APACHE II score | 17.8 + 9.9 | 11.6 + 6.2 | 0.002 | |
| Cardiac dysfunction | 15 (48) | 35 (23) | 0.004 | 3.43 (1.53, 7.70) |
| C difficile colitis | 4 (13) | 7 (5) | 0.08 | 3.13 (0.86, 11.43) |
| Charlson Index 4 | 16 (52) | 49 (32) | 0.04 | 2.31 (1.06, 5.04) |
| Mean Charlson Index | 4.8 + 3.0 | 3.2 + 2.7 | 0.006 | |
| Chemotherapy | 6 (19) | 23 (15) | 0.5 | 1.38 (0.51, 3.73) |
| Cirrhosis | 6 (19) | 5 (3) | 0.002 | 7.2 (2.04, 25.4) |
| Diabetes mellitus | 11 (36) | 47 (30) | 0.6 | 1.26 (0.56, 2.85) |
| Hypertension | 17 (55) | 78 (50) | 0.7 | 1.20 (0.55, 2.60) |
| Malignancy | 14 (45) | 50 (32) | 0.2 | 1.73 (0.79, 3.79) |
| MRSA colonization | 3 (10) | 12 (8) | 0.7 | 1.28 (0.34, 4.82) |
| Obesity | 5 (16) | 32 (21) | 0.6 | 0.74 (0.63, 2.08) |
| Neutropenia | 5 (16) | 23 (15) | 0.9 | 1.10 (0.38, 3.17) |
| Previous hospital admission | 15 (48) | 47 (30) | 0.06 | 2.15 (0.98, 4.72) |
| Renal dysfunction | 20 (65) | 60 (39) | 0.01 | 2.88 (1.29, 6.43) |
| Tobacco use | 7 (23) | 26 (17) | 0.4 | 1.45 (0.56, 3.71) |
| Trauma | 1(3) | 14 (9) | 0.3 | 0.34 (0.04, 2.65) |
| VRE colonization | 7 (23) | 24 (16) | 0.3 | 1.59 (0.62, 4.11) |
| Previous antibiotic use | 9 (29) | 38 (25) | 0.6 | 1.26 (0.53, 2.97) |
| Fluoroquinolone use | 8 (26) | 38 (25) | 0.9 | 1.07 (0.44, 2.59) |
| History of UTI | 8 (26) | 47 (30) | 0.6 | 0.80 (0.33, 1.92) |
| Corticosteroids | 12 (39) | 27 (17) | 0.01 | 2.99 (1.30, 6.89) |
| CVC | 17 (55) | 78 (50) | 0.7 | 1.20 (0.55, 2.6) |
| Source of bacteremia | ||||
| Urinary tract | 15 (48) | 97 (63) | 0.1 | 0.56 (0.26, 1.22) |
| Intra‐abdominal infection | 5 (16) | 11 (7) | 0.1 | 2.52 (0.81, 7.85) |
| Primary/catheter‐related | 3 (10) | 18 (12) | 0.8 | 0.82 (0.23, 2.96) |
| Chemotherapy‐related/mucositis | 3 (10) | 4 (3) | 0.08 | 0.05 (0.86, 19.06) |
| Management and outcome | ||||
| Appropriate empiric therapy | 15 (48) | 69 (45) | 0.38 | 0.70 (0.31, 1.56) |
| Mean length of stay | 19.9 + 24.8 days | 13.2 + 15.4 days | 0.2 | |
| In‐hospital mortality | 24 (26) | 7 (8) | 0.002 | |
| Fluoroquinolone resistance | 24 (77) | 69 (45) | 0.002 | 4.27 (1.74, 10.5) |
| Adjusted odds ratio | 95% Confidence interval | P value | |
|---|---|---|---|
| Cirrhosis | 7.2 | (1.7, 29.8) | .007 |
| Fluoroquinolone resistance | 3.9 | (1.5, 10.2) | .005 |
| Cardiac dysfunction | 3.9 | (1.6, 9.4) | .003 |
| Female gender | 0.5 | (0.2, 1.2) | .11 |
DISCUSSION
This case‐control study represents one of the larger studies on fluoroquinolone‐resistant E coli bacteremia and adds to the growing body of literature on the impact of fluoroquinolone resistance and other factors predictive of mortality. In multivariate analysis, fluoroquinolone resistance was associated with in‐hospital mortality from E coli bacteremia, as were the comorbid illnesses cirrhosis and cardiac dysfunction.
Among the risk factors for fluoroquinolone‐resistant E coli bacteremia described in the literature are previous fluoroquinolone exposure,9, 10, 12 nosocomial acquisition,9 presence of a urinary catheter,9 urinary source of bacteremia, previous surgery, and comorbid illnesses.10 If the scope of infections was not limited to the bloodstream, other factors like structural changes in the urinary tract,7 recurrent urinary tract infections,14 residence in a long‐term care facility, age, and prior exposure to aminoglycosides8 were also reported. In our study, previous fluoroquinolone exposure, residence in a long‐term care facility, recent hospitalization, nosocomial acquisition of infection, were associated with cases with fluoroquinolone‐resistant isolates. We also found that a larger proportion of the cases received corticosteroids before the episode of bacteremia; to our knowledge, this finding has not been reported before.
In contrast to results on fluoroquinolone resistance in both E coli and Klebsiella pneumoniae infections reported by Lautenbach et al,13 those patients in our study who were infected with the fluoroquinolone‐resistant phenotype were not more likely to receive inappropriate empiric therapy than control patients (52% vs. 55%; P = .8). This finding may be explained by the relatively low level of appropriate treatment even in the patients with fluoroquinolone‐susceptible E coli. For comparison, Lautenbach and colleagues saw a much higher percentage, 90%, of the patients with the susceptible phenotype received appropriate therapy.13 The high proportion of inappropriate empiric therapy in our study may have played a role in the relatively high overall mortality rate (17%) that we observed. This is in contrast to a recent retrospective study on appropriateness of therapy for E coli bacteremia which found that only 16% of bacteremia episodes (106 of 663) were inadequately treated,15 and the overall mortality was as low as 5%. A significant number of patients in our study, however, did not receive any antimicrobial therapy until blood cultures results were reported as positive and these situations therefore did not meet the definition for appropriate empiric therapy. These same patients did not have all the signs and symptoms associated with sepsis syndrome and so were not treated with any antimicrobials until blood cultures were reported to be positive. Eventually, Lautenbach et al stated thatafter adjusting for inadequate treatmentthere was no longer an association between fluoroquinolone resistance and mortality in their population.13 On the other hand, a recently published landmark Spanish study on factors influencing the outcome of 4758 E coli bacteremias reported that inappropriate treatment and shock were the two independent predictors of mortality; however, inappropriate treatment was significantly associated with fluoroquinolone resistance.9 Laupland et al, who performed a population‐based study of E coli bacteremias in Canada, elicited ciprofloxacin resistance as an independent predictor of mortality but the authors did not adjust for appropriateness of treatment.11 In that study, a urinary source of the bacteremia and younger age turned out to be protective. We studied both variables in our study but failed to confirm their findings.
Previous studies have reported that fluoroquinolone‐resistant clinical isolates collected from urine samples contain less virulence factors compared with the fluoroquinolone‐susceptible E coli.1618 Although no data are available specifically for bloodstream isolates, our finding of increased mortality in fluoroquinolone‐resistant isolates is not consistent with these conceptual findings among E coli isolates from the urinary tract. A delay in delivering the appropriate therapy cannot account for this, because the proportion of patients who did not receive appropriate therapy within 48 hours of the blood cultures being drawn was similar among the cases and control patients. Nevertheless, it would be interesting to assess virulence factor profiles in E coli bloodstream isolates that are stratified by their susceptibility to fluoroquinolones. The pathogens in our cohort may possess unidentified virulence mechanisms as well as resistance mechanisms toward fluoroquinolones. Because only patients with bacteremia were included in this study, it is possible that we have selected for a more virulent subpopulation of E coli strains capable of more invasive disease than uropathogenic isolates. In the past, several small studies have indeed demonstrated differences in virulence factor profiles when comparing E coli isolates strictly from urinary tract infections with those urinary tract isolates causing bacteremia.19, 20 Another potential explanation for the observed association between fluoroquinolone‐resistance and increased mortality may be unmeasured severity of illness among the cases. The cases were more likely to have a health‐care associated infection, more likely to come from a long‐term care facility or have been previously admitted, or associated with a longer length of stay. We did account for severity of illness and risk of mortality from comorbidities using both the APACHE II score and the Charlson Index of Co‐Morbidity, but it is still possible these indices may not be adequate to account for the differences between the cases and controls.
We found a higher crude mortality among patients with fluoroquinolone‐resistant E coli bacteremia than in patients with fluoroquinolone‐susceptible E coli (26% vs. 8%; P = .002). This is similar to the crude mortality rate for fluoroquinolone‐resistant E coli bacteremia reported by Cheong et al (30% in patients with fluoroquinolone‐resistant E coli bacteremia vs. 16% in patients with fluoroquinolone‐susceptible E coli; P = .08).12 In the Cheong et al article, only a high APACHE II score remained an independent risk factor for mortality. And although both Laupland et al and Ortega et al used regression analyses to describe factors associated with mortality, the respective crude mortality rates stratified by fluoroquinolone susceptibility were not reported.9, 11 In our study, the univariate analysis yielded both APACHE II score and Charlson comorbidity score as predictors for in‐hospital mortality but not in the multivariate analysis.
Our findings have important implications in the treatment of Gram‐negative infections. E coli is one of most common Gram‐negative bacilli causing hospital‐acquired infections and is the most common pathogen associated with community‐acquired urinary tract infections. The latest Infectious Diseases Society of America (IDSA) guideline for treatment of acute pyelonephritis recommends the use of fluoroquinolones for empiric therapy of acute pyelonephritis.21 Unfortunately, these guidelines were published in 1999, before reports of the rise in fluoroquinolone resistance among E coli isolates were available. The majority of the patients in our cohort (60%) developed a bacteremia following a complicated urinary tract infection and they would have received a fluoroquinolone for empiric therapy. The risk of providing inappropriate empiric therapy to patients with E coli bacteremia is evident, especially since inappropriate treatment was delivered in approximately half of our patients.
Another group of patients who are at high risk for mortality and are also at risk for development of fluoroquinolone‐resistant E coli bacteremia are patients with liver cirrhosis. Gram‐negative bacilli like E coli are common pathogens implicated in spontaneous bacterial peritonitis (SBP) in these patients.22 Since some patients with cirrhosis are exposed to fluoroquinolones for primary or secondary prophylaxis against SBP,23 they are likely to be colonized and eventually can develop infections with fluoroquinolone‐resistant E coli isolates.8 It may be prudent to select an antimicrobial class that is different from fluoroquinolones in treating sepsis syndrome in this patient population.
Our study has a few limitations. One is that this is a retrospective case‐control study and the accuracy of the data is dependent on the availability of complete medical records. All the admitted patients' charts or medical records were available for review in this study, so we were able to minimize any potential bias that may arise from missing data. This study was conducted at an academic medical center and results may not be generalizable to other healthcare institutions. The rate of inappropriate therapy was particularly high in this study, but it is unlikely to have influenced the final results since this was observed in both cases and controls.
On the basis of our finding that fluoroquinolone resistance is an independent predictor for mortality, we recommend that an alternative antimicrobial class rather than fluoroquinolones be initiated as empiric therapy in patients who are suspected to have an invasive E coli infection. The reason for this increased mortality in fluoroquinolone‐resistant E coli is, at least in our study, not related to inappropriate therapy or a higher severity of illness and may be related to more virulent organisms.
Acknowledgements
We thank Cherie Hill and Dorothy Sinclair for their invaluable help concerning the data management.
Among Gram‐negative pathogens, Escherichia coli is one of the most common causes of both community‐acquired and nosocomial bloodstream infections.1, 2 Fluoroquinolone resistance among E coli clinical isolates was first observed in patients with hematologic malignancies3, 4 but is no longer restricted to this population5 and has spread in the community.6 Multiple studies have examined potential risk factors for fluoroquinolone resistance in E coli infections.79 Prior fluoroquinolone use stands out as a repeatedly documented risk factor.10 In E coli bacteremias, data on the impact of fluoroquinolone resistance on mortality are limited.9, 11, 12 Ortega and colleagues performed a landmark analysis of a large dataset stemming from bacteremia surveillance data collected over 17 years.9 They found that mortality was associated with both shock and inappropriate empirical treatment, and that inappropriate empirical treatment in turn was linked to fluoroquinolone resistance. Laupland et al reported results from a population‐based study in Canada, and could elicit age, comorbidities, ciprofloxacin resistance, and a nonurinary focus of infection as risk factors for mortality.11 Lastly, a smaller study by Cheong et al found a high APACHE II score (ie, high severity of illness) but not fluoroquinolone resistance (P = .08) to be associated with poor outcomes.12
The prevalence of fluoroquinolone resistance among E coli isolates in our hospital has surpassed 20%. In this setting, an adjustment of recommendations for empirical treatment may become necessary. This is particularly important since some studies have demonstrated that inappropriate empiric therapy in patients with bloodstream infection results in higher mortality.13 The aim of this case‐control study was to determine the impact of fluoroquinolone resistance on in‐hospital mortality among patients with E coli bacteremia.
METHODS
Study Design, Setting, and Patients
This case‐control study was conducted at Barnes‐Jewish Hospital, a 1250‐bed academic medical center in St. Louis, Missouri. A case was defined as any adult patient with a positive blood culture for fluoroquinolone‐resistant E coli between January 1, 2000, and December 31, 2005. Cases were identified from the Medical Informatics database. Patients who were found to be bacteremic but were not admitted (eg, emergency room visit without admission) were excluded. One control patient with a blood culture positive for fluoroquinolone‐sensitive E coli was randomly matched to each case by year of infection. Demographic data such as age, race, gender, and clinical data such as severity‐of‐illness and comorbidity scores and processes of care such as timing of antibiotic administration and appropriateness of empiric therapy were collected from paper and electronic medical records.
Definitions
Appropriate empiric therapy was defined as receipt of an antimicrobial with in vitro activity against the E coli isolate before or within 48 hours of the blood culture being drawn. No antimicrobial therapy given while the blood cultures were under incubation was considered inappropriate empiric therapy.13Cardiac dysfunction was defined as having a history of atrial fibrillation or congestive heart failure. Central venous catheter (CVC) was defined as the presence of central venous catheter for at least 48 hours at the time of the positive culture was drawn. Clinical cure was achieved if the patient was discharged from the hospital or survived 30 days after the bacteremia without a recurrent E coli infection and no positive blood cultures for E coli were recovered within 14 days after initiation of treatment. History of fluoroquinolone use was defined as receipt of any fluoroquinolone within 90 days before the bacteremia. A history of Clostridium difficile disease was defined as having been diagnosed with C difficile disease in the past 6 months before the bacteremia. History of surgery was defined as having had a surgical procedure in the previous 30 days. A history of urinary tract infection (UTI) was defined as a UTI 90 days before the bacteremia. Hospital‐acquired infections were defined as infections that were not active or present at admission and the positive blood cultures were obtained 48 hours or greater after admission. In‐hospital mortality was defined as death in the hospital within 30 days after the positive blood culture. MRSA colonization was defined as a history of colonization with methicillin‐resistant Staphylococcus aureus any time before the bacteremia. Prior antibiotic use was defined as receipt of any antibiotic within 90 days before the bacteremia. Previous hospital admission was defined as admission to a hospital in the last 90 days. Renal dysfunction was defined as acute renal failure (serum creatinine level at the time blood cultures were drawn was twice that of the last available creatinine level), chronic renal insufficiency (creatinine >1.6 mg/dL), or renal failure requiring dialysis. VRE colonization was defined as a history of infection or stool colonization with vancomycin‐resistant enterococci (VRE) any time before the bacteremia.
Statistical Analysis
Univariate analysis of categorical variables in this case‐control study was performed using Mantel‐Haenszel chi‐square or Fisher exact test as appropriate. Continuous variables were compared using the Student t test or the Mann‐Whitney U test depending on the normality assumptions of the variable. Multivariate analysis was performed using backward stepwise conditional logistic regression. Variables that were found to have a P value of .10 on univariate analysis along with age, gender, and race were included in the conditional logistic regression model. Variables which were associated with fewer than five patients were not included in the multivariate analysis despite having a P value of .10 on univariate analysis. Goodness‐of‐fit of the logistic regression model was determined by the Hosmer‐Lemeshow test and the model with the best fit was retained as the final model. A two‐sided P value of .05 was considered statistically significant. Data analysis was performed using SPSS version 17 (SPSS, Chicago, IL).
The study was approved by the Washington University Human Research Protection Office.
RESULTS
Differences Among Patients With Fluoroquinolone‐Resistant and Fluoroquinolone‐Sensitive E coli Bacteremia
Nine‐hundred thirty patients had E coli bacteremia during the study period. Ninety‐eight patients had fluoroquinolone‐resistant E coli but blood cultures from 5 patients were collected in the outpatient setting and no follow‐up information was available; these patients were excluded from the analysis. Ninety‐three patients met the definition of a case and were matched with 93 patients with fluoroquinolone‐sensitive E coli bacteremias by year of infection for each of the cases. A comparison of the baseline demographic data and comorbid illnesses is shown in Table 1. When compared with control patients, cases were more likely to be admitted from a long‐term care facility (35% vs. 9%; P < .001) and to have a hospital‐acquired bacteremia (54% vs. 33%; P = .008). Cases were also more likely to have been admitted to a hospital in the previous 30 days (P < .001), colonized with vancomycin‐resistant enterococci (P = .006), have a central venous catheter in place (P = .04), and have been treated with antibiotics including fluoroquinolones (P < .001). The clinical cure rate was higher among controls (91% vs. 72%; P = .001). Crude mortality was 26% for cases and 8% for controls (P = .002). Although there was no difference in the mean severity‐of‐illness score between cases and controls, cases had a longer mean length of stay (see Table 1).
| Variable | Cases n (%) n = 93 | Controls n (%) n = 93 | P Value |
|---|---|---|---|
| |||
| Demographic characteristics | |||
| Mean age (SD) | 60.1 17.0 years | 63.2 19.4 years | 0.2 |
| Female gender | 61 (66) | 49 (53) | 0.1 |
| Race: | |||
| African American | 26 (28) | 42 (45) | 0.1 |
| Caucasian | 60 (65) | 50 (54) | |
| Other | 7 (7) | 1 (1) | |
| Residence: Home | 55 (59) | 79 (85) | <0.001 |
| LTCF/SNF | 32 (35) | 8 (9) | |
| Other | 6 (6) | 6 (6) | |
| Hospital‐acquired bacteremia | 50 (54) | 31 (33) | 0.008 |
| Comorbidities/Other risk factors | |||
| Alcohol abuse | 6 (6) | 5 (5) | 1.0 |
| APACHE II score 10 | 50 (54) | 49 (53) | 0.9 |
| Mean APACHE II score | 13.4 8.3 | 11.9 6.1 | 0.6 |
| Cardiac dysfunction | 28 (30) | 22 (24) | 0.3 |
| Charlson Index 4 | 36 (39) | 29 (31) | 0.3 |
| Mean Charlson Index | 3.6 2.8 | 3.4 2.8 | 0.7 |
| Chemotherapy | 18 (19) | 11 (12) | 0.2 |
| Cirrhosis | 7 (8) | 4 (4) | 0.5 |
| Diabetes mellitus | 30 (32) | 28 (30) | 0.9 |
| Hypertension | 48 (52) | 47 (51) | 0.9 |
| Malignancy | 35 (38) | 29 (31) | 0.4 |
| MRSA colonization | 11 (12) | 4 (4) | 0.07 |
| Obesity | 17 (18) | 20 (22) | 0.7 |
| Neutropenia | 19 (20) | 9 (10) | 0.07 |
| Previous hospital admission | 43 (46) | 19 (20) | <0.001 |
| Renal dysfunction | 41 (44) | 39 (42) | 0.9 |
| Tobacco use | 20 (22) | 13 (14) | 0.3 |
| Trauma | 3 (3) | 12 (13) | 0.03 |
| VRE colonization | 23 (25) | 8 (9) | 0.006 |
| Previous antibiotic use | 35 (38) | 12 (13) | <0.001 |
| Fluoroquinolone use | 37 (40) | 9 (10) | <0.001 |
| History of UTI | 32 (34) | 23 (25) | 0.2 |
| Corticosteroids | 30 (32) | 9 (10) | <0.001 |
| CVC | 55 (59) | 40 (43) | 0.04 |
| Source of bacteremia | |||
| Urinary tract | 57 (61) | 55 (59) | 0.8 |
| Intra‐abdominal infection | 5 (5) | 11 (12) | 0.1 |
| Primary/catheter‐related | 17 (18) | 4 (4) | 0.005 |
| Chemotherapy‐related/ mucositis | 6 (6) | 1 (1) | 0.09 |
| Pneumonia | 0 (0) | 7 (8) | |
| Other | 8 (9) | 15 (16) | |
| Management and outcome | |||
| Appropriate empiric therapy | 48 (52) | 51 (55) | 0.8 |
| Clinical cure | 67 (72) | 85 (91) | 0.001 |
| Mean length of stay | 18.2 21.9 days | 10.4 10 days | 0.002 |
| Median length of stay | 9 days | 6 days | 0.002 |
| In‐hospital mortality | 24 (26) | 7 (8) | 0.002 |
Risk Factors for Mortality From E coli Bacteremia
On univariate analysis, predictors for in‐hospital mortality included female gender, admission from a nursing home or other long‐term care facility, APACHE II score of >10, Charlson comorbidity score >4, a previous diagnosis of cardiac dysfunction, cirrhosis, renal dysfunction, and treatment with corticosteroids (see Table 2). Fluoroquinolone resistance was also associated with increased mortality (unadjusted odds ratio [uOR], 4.27; 95% confidence interval [CI], 1.710.5). On multivariate analysis (see Table 3), independent risk factors for in‐hospital mortality were cirrhosis (adjusted OR [aOR], 7.2; 95% CI, 1.729.8; P = .007), a history of cardiac dysfunction (aOR, 3.9; 95% CI, 1.69.4; P = .003), and infection with a fluoroquinolone‐resistant E coli isolate (aOR, 3.9; 95% CI, 1.5, 10.2; P = .005). The Hosmer‐Lemeshow test revealed a P value of .54. Both severity‐of‐illness indices were found not to be independent predictors of in‐hospital mortality.
| Variable | Died, n (%) n = 31 | Survived, n (%) n = 155 | P value | Unadjusted odds ratio (uOR) |
|---|---|---|---|---|
| ||||
| Demographic characteristics | ||||
| Mean age (SD) | 61.2 18.9 years | 63.8 14.4 years | 1.0 | |
| Age 65 years | 11 (36) | 66 (43) | 0.4 | 0.73 (0.33, 1.62) |
| Female gender | 19 (61) | 57 (37) | 0.01 | 2.29 (1.18, 4.44) |
| Race: | ||||
| African American | 9 (29) | 59 (38) | 0.1 | 1.86 (0.81, 4.30) |
| Caucasian | 22 (71) | 88 (57) | ||
| Other | 0 (0) | 8 (5) | ||
| Residence: | ||||
| Home | 13 (42) | 121 (78) | 0.02 | 3.13 (1.24, 7.76) |
| LTCF/SNF | 10 (32) | 30 (19) | ||
| Other | 8 (26) | 4 (3) | ||
| Hospital‐acquired bacteremia | 18 (58) | 63 (41) | 0.08 | 2.02 (0.93, 4.42) |
| Comorbidities/Other risk factors | ||||
| Alcohol abuse | 4 (13) | 7 (5) | 0.2 | 3.13 (0.86, 11.44) |
| APACHE II score 10 | 22 (71) | 77 (50) | 0.03 | 2.48 (1.07, 5.72) |
| Mean APACHE II score | 17.8 + 9.9 | 11.6 + 6.2 | 0.002 | |
| Cardiac dysfunction | 15 (48) | 35 (23) | 0.004 | 3.43 (1.53, 7.70) |
| C difficile colitis | 4 (13) | 7 (5) | 0.08 | 3.13 (0.86, 11.43) |
| Charlson Index 4 | 16 (52) | 49 (32) | 0.04 | 2.31 (1.06, 5.04) |
| Mean Charlson Index | 4.8 + 3.0 | 3.2 + 2.7 | 0.006 | |
| Chemotherapy | 6 (19) | 23 (15) | 0.5 | 1.38 (0.51, 3.73) |
| Cirrhosis | 6 (19) | 5 (3) | 0.002 | 7.2 (2.04, 25.4) |
| Diabetes mellitus | 11 (36) | 47 (30) | 0.6 | 1.26 (0.56, 2.85) |
| Hypertension | 17 (55) | 78 (50) | 0.7 | 1.20 (0.55, 2.60) |
| Malignancy | 14 (45) | 50 (32) | 0.2 | 1.73 (0.79, 3.79) |
| MRSA colonization | 3 (10) | 12 (8) | 0.7 | 1.28 (0.34, 4.82) |
| Obesity | 5 (16) | 32 (21) | 0.6 | 0.74 (0.63, 2.08) |
| Neutropenia | 5 (16) | 23 (15) | 0.9 | 1.10 (0.38, 3.17) |
| Previous hospital admission | 15 (48) | 47 (30) | 0.06 | 2.15 (0.98, 4.72) |
| Renal dysfunction | 20 (65) | 60 (39) | 0.01 | 2.88 (1.29, 6.43) |
| Tobacco use | 7 (23) | 26 (17) | 0.4 | 1.45 (0.56, 3.71) |
| Trauma | 1(3) | 14 (9) | 0.3 | 0.34 (0.04, 2.65) |
| VRE colonization | 7 (23) | 24 (16) | 0.3 | 1.59 (0.62, 4.11) |
| Previous antibiotic use | 9 (29) | 38 (25) | 0.6 | 1.26 (0.53, 2.97) |
| Fluoroquinolone use | 8 (26) | 38 (25) | 0.9 | 1.07 (0.44, 2.59) |
| History of UTI | 8 (26) | 47 (30) | 0.6 | 0.80 (0.33, 1.92) |
| Corticosteroids | 12 (39) | 27 (17) | 0.01 | 2.99 (1.30, 6.89) |
| CVC | 17 (55) | 78 (50) | 0.7 | 1.20 (0.55, 2.6) |
| Source of bacteremia | ||||
| Urinary tract | 15 (48) | 97 (63) | 0.1 | 0.56 (0.26, 1.22) |
| Intra‐abdominal infection | 5 (16) | 11 (7) | 0.1 | 2.52 (0.81, 7.85) |
| Primary/catheter‐related | 3 (10) | 18 (12) | 0.8 | 0.82 (0.23, 2.96) |
| Chemotherapy‐related/mucositis | 3 (10) | 4 (3) | 0.08 | 0.05 (0.86, 19.06) |
| Management and outcome | ||||
| Appropriate empiric therapy | 15 (48) | 69 (45) | 0.38 | 0.70 (0.31, 1.56) |
| Mean length of stay | 19.9 + 24.8 days | 13.2 + 15.4 days | 0.2 | |
| In‐hospital mortality | 24 (26) | 7 (8) | 0.002 | |
| Fluoroquinolone resistance | 24 (77) | 69 (45) | 0.002 | 4.27 (1.74, 10.5) |
| Adjusted odds ratio | 95% Confidence interval | P value | |
|---|---|---|---|
| Cirrhosis | 7.2 | (1.7, 29.8) | .007 |
| Fluoroquinolone resistance | 3.9 | (1.5, 10.2) | .005 |
| Cardiac dysfunction | 3.9 | (1.6, 9.4) | .003 |
| Female gender | 0.5 | (0.2, 1.2) | .11 |
DISCUSSION
This case‐control study represents one of the larger studies on fluoroquinolone‐resistant E coli bacteremia and adds to the growing body of literature on the impact of fluoroquinolone resistance and other factors predictive of mortality. In multivariate analysis, fluoroquinolone resistance was associated with in‐hospital mortality from E coli bacteremia, as were the comorbid illnesses cirrhosis and cardiac dysfunction.
Among the risk factors for fluoroquinolone‐resistant E coli bacteremia described in the literature are previous fluoroquinolone exposure,9, 10, 12 nosocomial acquisition,9 presence of a urinary catheter,9 urinary source of bacteremia, previous surgery, and comorbid illnesses.10 If the scope of infections was not limited to the bloodstream, other factors like structural changes in the urinary tract,7 recurrent urinary tract infections,14 residence in a long‐term care facility, age, and prior exposure to aminoglycosides8 were also reported. In our study, previous fluoroquinolone exposure, residence in a long‐term care facility, recent hospitalization, nosocomial acquisition of infection, were associated with cases with fluoroquinolone‐resistant isolates. We also found that a larger proportion of the cases received corticosteroids before the episode of bacteremia; to our knowledge, this finding has not been reported before.
In contrast to results on fluoroquinolone resistance in both E coli and Klebsiella pneumoniae infections reported by Lautenbach et al,13 those patients in our study who were infected with the fluoroquinolone‐resistant phenotype were not more likely to receive inappropriate empiric therapy than control patients (52% vs. 55%; P = .8). This finding may be explained by the relatively low level of appropriate treatment even in the patients with fluoroquinolone‐susceptible E coli. For comparison, Lautenbach and colleagues saw a much higher percentage, 90%, of the patients with the susceptible phenotype received appropriate therapy.13 The high proportion of inappropriate empiric therapy in our study may have played a role in the relatively high overall mortality rate (17%) that we observed. This is in contrast to a recent retrospective study on appropriateness of therapy for E coli bacteremia which found that only 16% of bacteremia episodes (106 of 663) were inadequately treated,15 and the overall mortality was as low as 5%. A significant number of patients in our study, however, did not receive any antimicrobial therapy until blood cultures results were reported as positive and these situations therefore did not meet the definition for appropriate empiric therapy. These same patients did not have all the signs and symptoms associated with sepsis syndrome and so were not treated with any antimicrobials until blood cultures were reported to be positive. Eventually, Lautenbach et al stated thatafter adjusting for inadequate treatmentthere was no longer an association between fluoroquinolone resistance and mortality in their population.13 On the other hand, a recently published landmark Spanish study on factors influencing the outcome of 4758 E coli bacteremias reported that inappropriate treatment and shock were the two independent predictors of mortality; however, inappropriate treatment was significantly associated with fluoroquinolone resistance.9 Laupland et al, who performed a population‐based study of E coli bacteremias in Canada, elicited ciprofloxacin resistance as an independent predictor of mortality but the authors did not adjust for appropriateness of treatment.11 In that study, a urinary source of the bacteremia and younger age turned out to be protective. We studied both variables in our study but failed to confirm their findings.
Previous studies have reported that fluoroquinolone‐resistant clinical isolates collected from urine samples contain less virulence factors compared with the fluoroquinolone‐susceptible E coli.1618 Although no data are available specifically for bloodstream isolates, our finding of increased mortality in fluoroquinolone‐resistant isolates is not consistent with these conceptual findings among E coli isolates from the urinary tract. A delay in delivering the appropriate therapy cannot account for this, because the proportion of patients who did not receive appropriate therapy within 48 hours of the blood cultures being drawn was similar among the cases and control patients. Nevertheless, it would be interesting to assess virulence factor profiles in E coli bloodstream isolates that are stratified by their susceptibility to fluoroquinolones. The pathogens in our cohort may possess unidentified virulence mechanisms as well as resistance mechanisms toward fluoroquinolones. Because only patients with bacteremia were included in this study, it is possible that we have selected for a more virulent subpopulation of E coli strains capable of more invasive disease than uropathogenic isolates. In the past, several small studies have indeed demonstrated differences in virulence factor profiles when comparing E coli isolates strictly from urinary tract infections with those urinary tract isolates causing bacteremia.19, 20 Another potential explanation for the observed association between fluoroquinolone‐resistance and increased mortality may be unmeasured severity of illness among the cases. The cases were more likely to have a health‐care associated infection, more likely to come from a long‐term care facility or have been previously admitted, or associated with a longer length of stay. We did account for severity of illness and risk of mortality from comorbidities using both the APACHE II score and the Charlson Index of Co‐Morbidity, but it is still possible these indices may not be adequate to account for the differences between the cases and controls.
We found a higher crude mortality among patients with fluoroquinolone‐resistant E coli bacteremia than in patients with fluoroquinolone‐susceptible E coli (26% vs. 8%; P = .002). This is similar to the crude mortality rate for fluoroquinolone‐resistant E coli bacteremia reported by Cheong et al (30% in patients with fluoroquinolone‐resistant E coli bacteremia vs. 16% in patients with fluoroquinolone‐susceptible E coli; P = .08).12 In the Cheong et al article, only a high APACHE II score remained an independent risk factor for mortality. And although both Laupland et al and Ortega et al used regression analyses to describe factors associated with mortality, the respective crude mortality rates stratified by fluoroquinolone susceptibility were not reported.9, 11 In our study, the univariate analysis yielded both APACHE II score and Charlson comorbidity score as predictors for in‐hospital mortality but not in the multivariate analysis.
Our findings have important implications in the treatment of Gram‐negative infections. E coli is one of most common Gram‐negative bacilli causing hospital‐acquired infections and is the most common pathogen associated with community‐acquired urinary tract infections. The latest Infectious Diseases Society of America (IDSA) guideline for treatment of acute pyelonephritis recommends the use of fluoroquinolones for empiric therapy of acute pyelonephritis.21 Unfortunately, these guidelines were published in 1999, before reports of the rise in fluoroquinolone resistance among E coli isolates were available. The majority of the patients in our cohort (60%) developed a bacteremia following a complicated urinary tract infection and they would have received a fluoroquinolone for empiric therapy. The risk of providing inappropriate empiric therapy to patients with E coli bacteremia is evident, especially since inappropriate treatment was delivered in approximately half of our patients.
Another group of patients who are at high risk for mortality and are also at risk for development of fluoroquinolone‐resistant E coli bacteremia are patients with liver cirrhosis. Gram‐negative bacilli like E coli are common pathogens implicated in spontaneous bacterial peritonitis (SBP) in these patients.22 Since some patients with cirrhosis are exposed to fluoroquinolones for primary or secondary prophylaxis against SBP,23 they are likely to be colonized and eventually can develop infections with fluoroquinolone‐resistant E coli isolates.8 It may be prudent to select an antimicrobial class that is different from fluoroquinolones in treating sepsis syndrome in this patient population.
Our study has a few limitations. One is that this is a retrospective case‐control study and the accuracy of the data is dependent on the availability of complete medical records. All the admitted patients' charts or medical records were available for review in this study, so we were able to minimize any potential bias that may arise from missing data. This study was conducted at an academic medical center and results may not be generalizable to other healthcare institutions. The rate of inappropriate therapy was particularly high in this study, but it is unlikely to have influenced the final results since this was observed in both cases and controls.
On the basis of our finding that fluoroquinolone resistance is an independent predictor for mortality, we recommend that an alternative antimicrobial class rather than fluoroquinolones be initiated as empiric therapy in patients who are suspected to have an invasive E coli infection. The reason for this increased mortality in fluoroquinolone‐resistant E coli is, at least in our study, not related to inappropriate therapy or a higher severity of illness and may be related to more virulent organisms.
Acknowledgements
We thank Cherie Hill and Dorothy Sinclair for their invaluable help concerning the data management.
- ,.Overview of nosocomial infections caused by gram‐negative bacilli.Clin Infect Dis.2005;41:848–854.
- ,,, et al.Survey of bloodstream infections due to gram‐negative bacilli: frequency of occurrence and antimicrobial susceptibility of isolates collected in the United States, Canada, and Latin America for the SENTRY Antimicrobial Surveillance Program, 1997.Clin Infect Dis.1999;29:595–607.
- ,,,,,.Emergence of fluoroquinolone‐resistant Escherichia coli at a cancer center.Antimicrob Agents Chemother.1994;38:681–687.
- ,,,,.Emergence of quinolone‐resistant Escherichia coli bacteremia in neutropenic patients with cancer who have received prophylactic norfloxacin.Clin Infect Dis.1995;20:557–560; discussion 561–563.
- ,,,,.Antimicrobial‐resistant invasive Escherichia coli, Spain.Emerg Infect Dis.2005;11:546–553.
- ,,, et al.Emergence and dissemination of quinolone‐resistant Escherichia coli in the community.Antimicrob Agents Chemother.1999;43:2736–2741.
- ,,,.Incidence and risk factors for nosocomial infections caused by fluoroquinolone‐resistant Escherichia coli.Eur J Clin Microbiol Infect Dis.2003;22:492–495.
- ,,, et al.Risk factors for fluoroquinolone resistance in nosocomial Escherichia coli and Klebsiella pneumoniae infections.Arch Intern Med.2002;162:2469–2477.
- ,,, et al.Analysis of 4758 Escherichia coli bacteraemia episodes: predictive factors for isolation of an antibiotic‐resistant strain and their impact on the outcome.J Antimicrob Chemother.2009;63:568–574.
- ,,,,,.Relationship between quinolone use and emergence of ciprofloxacin‐resistant Escherichia coli in bloodstream infections.Antimicrob Agents Chemother.1995;39:520–524.
- ,,,,.Incidence, risk factors and outcomes of Escherichia coli bloodstream infections in a large Canadian region.Clin Microbiol Infect.2008;14:1041–1047.
- ,,,,,.Bacteremia due to quinolone‐resistant Escherichia coli in a teaching hospital in South Korea.Clin Infect Dis.2001;33:48–53.
- ,,,,.Association between fluoroquinolone resistance and mortality in Escherichia coli and Klebsiella pneumoniae infections: the role of inadequate empirical antimicrobial therapy.Clin Infect Dis.2005;41:923–929.
- ,,.Risk factors for community‐acquired ciprofloxacin‐resistant Escherichia coli urinary tract infection.Ann Pharmacother. Jul‐2004;38:1148–1152.
- ,,, et al.Impact of antibiotic resistance and of adequate empirical antibiotic treatment in the prognosis of patients with Escherichia coli bacteraemia.J Antimicrob Chemother.2007;60:855–863.
- ,,, et al.Decreased prevalence of virulence factors among ciprofloxacin‐resistant uropathogenic Escherichia coli isolates.J Clin Microbiol.2005;43:4218–4220.
- ,,, et al.Are quinolone‐resistant uropathogenic Escherichia coli less virulent?J Infect Dis.2002;186:1039–1042.
- ,,, et al.Genetic profiles of fluoroquinolone‐resistant Escherichia coli isolates obtained from patients with cystitis: phylogeny, virulence factors, PAIusp subtypes, and mutation patterns.J Clin Microbiol.2009;47:791–795.
- ,,,,,.Comparative study of Escherichia coli virulence determinants in strains causing urinary tract bacteremia versus strains causing pyelonephritis and other sources of bacteremia.Diagn Microbiol Infect Dis.2005;53:93–99.
- ,,,,.Comparative prevalence of virulence factors in Escherichia coli causing urinary tract infection in male infants with and without bacteremia.J Clin Microbiol.2006;44:1156–1158.
- ,,,,,.Guidelines for antimicrobial treatment of uncomplicated acute bacterial cystitis and acute pyelonephritis in women. Infectious Diseases Society of America (IDSA).Clin Infect Dis.1999;29:745–758.
- ,,.Spontaneous bacterial peritonitis: recent data on incidence and treatment.Cleve Clin J Med.2004;71:569–576.
- ,,, et al.Diagnosis, treatment and prophylaxis of spontaneous bacterial peritonitis: a consensus document. International Ascites Club.J Hepatol.2000;32:142–153.
- ,.Overview of nosocomial infections caused by gram‐negative bacilli.Clin Infect Dis.2005;41:848–854.
- ,,, et al.Survey of bloodstream infections due to gram‐negative bacilli: frequency of occurrence and antimicrobial susceptibility of isolates collected in the United States, Canada, and Latin America for the SENTRY Antimicrobial Surveillance Program, 1997.Clin Infect Dis.1999;29:595–607.
- ,,,,,.Emergence of fluoroquinolone‐resistant Escherichia coli at a cancer center.Antimicrob Agents Chemother.1994;38:681–687.
- ,,,,.Emergence of quinolone‐resistant Escherichia coli bacteremia in neutropenic patients with cancer who have received prophylactic norfloxacin.Clin Infect Dis.1995;20:557–560; discussion 561–563.
- ,,,,.Antimicrobial‐resistant invasive Escherichia coli, Spain.Emerg Infect Dis.2005;11:546–553.
- ,,, et al.Emergence and dissemination of quinolone‐resistant Escherichia coli in the community.Antimicrob Agents Chemother.1999;43:2736–2741.
- ,,,.Incidence and risk factors for nosocomial infections caused by fluoroquinolone‐resistant Escherichia coli.Eur J Clin Microbiol Infect Dis.2003;22:492–495.
- ,,, et al.Risk factors for fluoroquinolone resistance in nosocomial Escherichia coli and Klebsiella pneumoniae infections.Arch Intern Med.2002;162:2469–2477.
- ,,, et al.Analysis of 4758 Escherichia coli bacteraemia episodes: predictive factors for isolation of an antibiotic‐resistant strain and their impact on the outcome.J Antimicrob Chemother.2009;63:568–574.
- ,,,,,.Relationship between quinolone use and emergence of ciprofloxacin‐resistant Escherichia coli in bloodstream infections.Antimicrob Agents Chemother.1995;39:520–524.
- ,,,,.Incidence, risk factors and outcomes of Escherichia coli bloodstream infections in a large Canadian region.Clin Microbiol Infect.2008;14:1041–1047.
- ,,,,,.Bacteremia due to quinolone‐resistant Escherichia coli in a teaching hospital in South Korea.Clin Infect Dis.2001;33:48–53.
- ,,,,.Association between fluoroquinolone resistance and mortality in Escherichia coli and Klebsiella pneumoniae infections: the role of inadequate empirical antimicrobial therapy.Clin Infect Dis.2005;41:923–929.
- ,,.Risk factors for community‐acquired ciprofloxacin‐resistant Escherichia coli urinary tract infection.Ann Pharmacother. Jul‐2004;38:1148–1152.
- ,,, et al.Impact of antibiotic resistance and of adequate empirical antibiotic treatment in the prognosis of patients with Escherichia coli bacteraemia.J Antimicrob Chemother.2007;60:855–863.
- ,,, et al.Decreased prevalence of virulence factors among ciprofloxacin‐resistant uropathogenic Escherichia coli isolates.J Clin Microbiol.2005;43:4218–4220.
- ,,, et al.Are quinolone‐resistant uropathogenic Escherichia coli less virulent?J Infect Dis.2002;186:1039–1042.
- ,,, et al.Genetic profiles of fluoroquinolone‐resistant Escherichia coli isolates obtained from patients with cystitis: phylogeny, virulence factors, PAIusp subtypes, and mutation patterns.J Clin Microbiol.2009;47:791–795.
- ,,,,,.Comparative study of Escherichia coli virulence determinants in strains causing urinary tract bacteremia versus strains causing pyelonephritis and other sources of bacteremia.Diagn Microbiol Infect Dis.2005;53:93–99.
- ,,,,.Comparative prevalence of virulence factors in Escherichia coli causing urinary tract infection in male infants with and without bacteremia.J Clin Microbiol.2006;44:1156–1158.
- ,,,,,.Guidelines for antimicrobial treatment of uncomplicated acute bacterial cystitis and acute pyelonephritis in women. Infectious Diseases Society of America (IDSA).Clin Infect Dis.1999;29:745–758.
- ,,.Spontaneous bacterial peritonitis: recent data on incidence and treatment.Cleve Clin J Med.2004;71:569–576.
- ,,, et al.Diagnosis, treatment and prophylaxis of spontaneous bacterial peritonitis: a consensus document. International Ascites Club.J Hepatol.2000;32:142–153.
Copyright © 2011 Society of Hospital Medicine