User login
Addressing Inpatient Crowding
High levels of hospital occupancy are associated with compromises to quality of care and access (often referred to as crowding), 18 while low occupancy may be inefficient and also impact quality. 9, 10 Despite this, hospitals typically have uneven occupancy. Although some demand for services is driven by factors beyond the control of a hospital (eg, seasonal variation in viral illness), approximately 15%30% of admissions to children's hospitals are scheduled from days to months in advance, with usual arrivals on weekdays. 1114 For example, of the 3.4 million elective admissions in the 2006 Healthcare Cost and Utilization Project Kids Inpatient Database (HCUP KID), only 13% were admitted on weekends. 14 Combined with short length of stay (LOS) for such patients, this leads to higher midweek and lower weekend occupancy. 12
Hospitals respond to crowding in a number of ways, but often focus on reducing LOS to make room for new patients. 11, 15, 16 For hospitals that are relatively efficient in terms of LOS, efforts to reduce it may not increase functional capacity adequately. In children's hospitals, median lengths of stay are 2 to 3 days, and one‐third of hospitalizations are 1 day or less. 17 Thus, even 10%20% reductions in LOS trims hours, not days, from typical stays. Practical barriers (eg, reluctance to discharge in the middle of the night, or family preferences and work schedules) and undesired outcomes (eg, increased hospital re‐visits) are additional pitfalls encountered by relying on throughput enhancement alone.
Managing scheduled admissions through smoothing is an alternative strategy to reduce variability and high occupancy. 6, 12, 1820 The concept is to proactively control the entry of patients, when possible, to achieve more even levels of occupancy, instead of the peaks and troughs commonly encountered. Nonetheless, it is not a widely used approach. 18, 20, 21 We hypothesized that children's hospitals had substantial unused capacity that could be used to smooth occupancy, which would reduce weekday crowding. While it is obvious that smoothing will reduce peaks to average levels (and also raise troughs), we sought to quantify just how large this difference wasand thereby quantify the potential of smoothing to reduce inpatient crowding (or, conversely, expose more patients to high levels of occupancy). Is there enough variation to justify smoothing, and, if a hospital does smooth, what is the expected result? If the number of patients removed from exposure to high occupancy is not substantial, other means to address inpatient crowding might be of more value. Our aims were to quantify the difference in weekday versus weekend occupancy, report on mathematical feasibility of such an approach, and determine the difference in number of patients exposed to various levels of high occupancy.
Methods
Data Source
This retrospective study was conducted with resource‐utilization data from 39 freestanding, tertiary‐care children's hospitals in the Pediatric Health Information System (PHIS). Participating hospitals are located in noncompeting markets of 23 states, plus the District of Columbia, and affiliated with the Child Health Corporation of America (CHCA, Shawnee Mission, KS). They account for 80% of freestanding, and 20% of all general, tertiary‐care children's hospitals. Data quality and reliability are assured through joint ongoing, systematic monitoring. The Children's Hospital of Philadelphia Committees for the Protection of Human Subjects approved the protocol with a waiver of informed consent.
Patients
Patients admitted January 1December 31, 2007 were eligible for inclusion. Due to variation in the presence of birthing, neonatal intensive care, and behavioral health units across hospitals, these beds and associated patients were excluded. Inpatients enter hospitals either as scheduled (often referred to as elective) or unscheduled (emergent or urgent) admissions. Because PHIS does not include these data, KID was used to standardize the PHIS data for proportion of scheduled admissions. 22 (KID is a healthcare database of 23 million pediatric inpatient discharges developed through federalstateindustry partnership, and sponsored by the Agency for Healthcare Research and Quality [AHRQ].) Each encounter in KID includes a principal International Classification of Diseases, 9th revision (ICD‐9) discharge diagnosis code, and is designated by the hospital as elective (ranging from chemotherapy to tonsillectomy) or not elective. Because admissions, rather than diagnoses, are scheduled, a proportion of patients with each primary diagnosis in KID are scheduled (eg, 28% of patients with a primary diagnosis of esophageal reflux). Proportions in KID were matched to principal diagnoses in PHIS.
Definitions
The census was the number of patients registered as inpatients (including those physically in the emergency department [ED] from time of ED arrival)whether observation or inpatient statusat midnight, the conclusion of the day. Hospital capacity was set using CHCA data (and confirmed by each hospital's administrative personnel) as the number of licensed in‐service beds available for patients in 2007; we assumed beds were staffed and capacity fixed for the year. Occupancy was calculated by dividing census by capacity. Maximum occupancy in a week referred to the highest occupancy level achieved in a seven‐day period (MondaySunday). We analyzed a set of thresholds for high‐occupancy (85%, 90%, 95%, and 100%), because there is no consistent definition for when hospitals are at high occupancy or when crowding occurs, though crowding has been described as starting at 85% occupancy. 2325
Analysis
The hospital was the unit of analysis. We report hospital characteristics, including capacity, number of discharges, and census region, and annual standardized length of stay ratio (SLOSR) as observed‐to‐expected LOS.
Smoothing Technique
A retrospective smoothing algorithm set each hospital's daily occupancy during a week to that hospital's mean occupancy for the week; effectively spreading the week's volume of patients evenly across the days of the week. While inter‐week and inter‐month smoothing were considered, intra‐week smoothing was deemed more practical for the largest number of patients, as it would not mean delaying care by more than one week. In the case of a planned treatment course (eg, chemotherapy), only intra‐week smoothing would maintain the necessary scheduled intervals of treatment.
Mathematical Feasibility
To approximate the number of patient admissions that would require different scheduling during a particular week to achieve smoothed weekly occupancy, we determined the total number of patient‐days in the week that required different scheduling and divided by the average LOS for the week. We then divided the number of admissions‐to‐move by total weekly admissions to compute the percentage at each hospital across 52 weeks of the year.
Measuring the Impact of Smoothing
We focused on the frequency and severity of high occupancy and the number of patients exposed to it. This framework led to 4 measures that assess the opportunity and effect of smoothing:
Difference in hospital weekdayweekend occupancy: Equal to 12‐month median of difference between mean weekday occupancy and mean weekend occupancy for each hospital‐week.
Difference in hospital maximummean occupancy: Equal to median of difference between maximum one‐day occupancy and weekly mean (smoothed) occupancy for each hospital‐week. A regression line was derived from the data for the 39 hospitals to report expected reduction in peak occupancy based on the magnitude of the difference between weekday and weekend occupancy.
Difference in number of hospitals exposed to above‐threshold occupancy: Equal to difference, pre‐ and post‐smoothing, in number of hospitals facing high‐occupancy conditions on an average of at least one weekday midnight per week during the year at different occupancy thresholds.
Difference in number of patients exposed to above‐threshold occupancy: Equal to difference, pre‐ and post‐smoothing, in number of patients exposed to hospital midnight occupancy at the thresholds. We utilized patient‐days for the calculation to avoid double‐counting, and divided this by average LOS, in order to determine the number of patients who would no longer be exposed to over‐threshold occupancy after smoothing, while also adjusting for patients newly exposed to over‐threshold occupancy levels.
All analyses were performed separately for each hospital for the entire year and then for winter (DecemberMarch), the period during which most crowding occurred. Analyses were performed using SAS (version 9.2, SAS Institute, Inc, Cary, NC); P values <0.05 were considered statistically significant.
Results
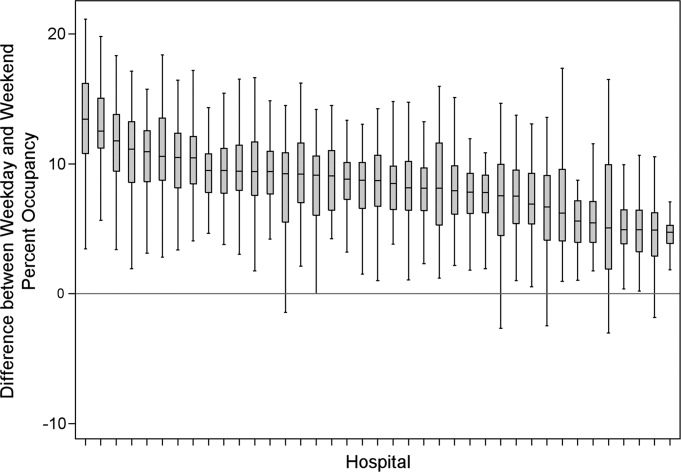
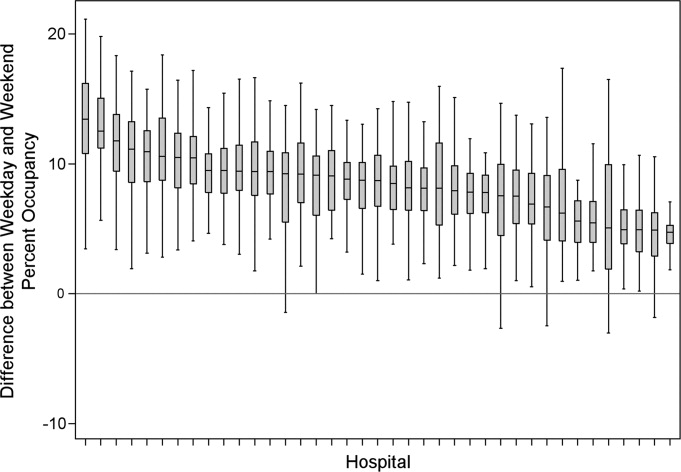
The characteristics of the 39 hospitals are provided in Table 1. Based on standardization with KID, 23.6% of PHIS admissions were scheduled (range: 18.1%35.8%) or a median of 81.5 scheduled admissions per week per hospital; 26.6% of weekday admissions were scheduled versus 16.1% for weekends. Overall, 12.4% of scheduled admissions entered on weekends. For all patients, median LOS was three days (interquartile range [IQR]: twofive days), but median LOS for scheduled admissions was two days (IQR: onefour days). The median LOS and IQR were the same by day of admission for all days of the week. Most hospitals had an overall SLOSR close to one (median: 0.9, IQR: 0.91.1). Overall, hospital mean midnight occupancy ranged from 70.9% to 108.1% on weekdays and 65.7% to 94.9% on weekends. Uniformly, weekday occupancy exceeded weekend occupancy, with a median difference of 8.2% points (IQR: 7.2%9.5% points). There was a wide range of median hospital weekdayweekend occupancy differences across hospitals (Figure 1). The overall difference was less in winter (median difference: 7.7% points; IQR: 6.3%8.8% points) than in summer (median difference: 8.6% points; IQR: 7.4%9.8% points (Wilcoxon Sign Rank test, P < 0.001). Thirty‐five hospitals (89.7%) exceeded the 85% occupancy threshold and 29 (74.4%) exceeded the 95% occupancy threshold on at least 20% of weekdays (Table 2). Across all the hospitals, the median difference in weekly maximum and weekly mean occupancy was 6.6% points (IQR: 6.2%7.4% points) (Figure 2).
| Characteristics | No. (%) |
|---|---|
| |
| Licensed in‐service beds | n = 39 hospitals |
| <200 beds | 6 (15.4) |
| 200249 beds | 10 (25.6) |
| 250300 beds | 14 (35.9) |
| >300 beds | 9 (23.1) |
| No. of discharges | |
| <10,000 | 5 (12.8) |
| 10,00013,999 | 14 (35.9) |
| 14,00017,999 | 11 (28.2) |
| >18,000 | 9 (23.1) |
| Census region | |
| West | 9 (23.1) |
| Midwest | 11 (28.2) |
| Northeast | 6 (15.4) |
| South | 13 (33.3) |
| Admissions | n = 590,352 admissions |
| Medical scheduled admissions* | 79,683 |
| Surgical scheduled admissions* | 59,640 |
| Total scheduled admissions* (% of all admissions) | 139,323 (23.6) |
| Weekend medical scheduled admissions* (% of all medical scheduled admissions) | 13,546 (17.0) |
| Weekend surgical scheduled admissions* (% of all surgical scheduled admissions) | 3,757 (6.3) |
| Weekend total scheduled admissions* (% of total scheduled admissions) | 17,276 (12.4) |
| Entire Year | >85% | Occupancy Threshold | >95% | >100% |
|---|---|---|---|---|
| >90% | ||||
| ||||
| No. of hospitals (n = 39) with mean weekday occupancy above threshold | ||||
| Before smoothing (current state) | 33 | 25 | 14 | 6 |
| After smoothing | 32 | 22 | 10 | 1 |
| No. of hospitals (n = 39) above threshold 20% of weekdays | ||||
| Before smoothing (current state) | 35 | 34 | 29 | 14 |
| After smoothing | 35 | 32 | 21 | 9 |
| Median (IQR) no. of patient‐days per hospital not exposed to occupancy above threshold by smoothing | 3,071 | 281 | 3236 | 3281 |
| (5,552, 919) | (5,288, 3,103) | (0, 7,083) | (962, 8,517) | |
| Median (IQR) no. of patients per hospital not exposed to occupancy above threshold by smoothing | 596 | 50 | 630 | 804 |
| (1,190, 226) | (916, 752) | (0, 1,492) | (231, 2,195) | |
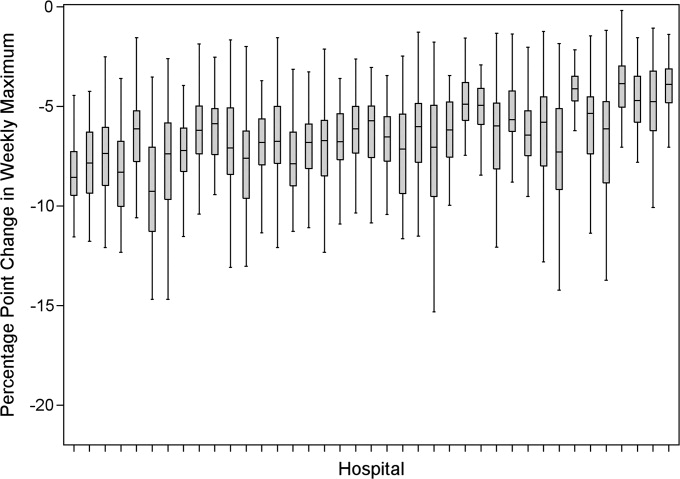
Smoothing reduced the number of hospitals at each occupancy threshold, except 85% (Table 2). As a linear relationship, the reduction in weekday peak occupancy (y) based on a hospital's median difference in weekly maximum and weekly mean occupancy (x) was y = 2.69 + 0.48x. Thus, a hospital with a 10% point difference between weekday and weekend occupancy could reduce weekday peak by 7.5% points.
Smoothing increased the number of patients exposed to the lower thresholds (85% and 90%), but decreased the number of patients exposed to >95% occupancy (Table 2). For example, smoothing at the 95% threshold resulted in 630 fewer patients per hospital exposed to that threshold. If all 39 hospitals had within‐week smoothing, a net of 39,607 patients would have been protected from exposure to >95% occupancy and a net of 50,079 patients from 100% occupancy.
To demonstrate the varied effects of smoothing, Table 3 and Figure 3 present representative categories of response to smoothing depending on pre‐smoothing patterns. While not all hospitals decreased occupancy to below thresholds after smoothing (Types B and D), the overall occupancy was reduced and fewer patients were exposed to extreme levels of high occupancy (eg, >100%).
| Category | Before Smoothing Hospital Description | After Smoothing Hospital Description | No. of Hospitals at 85% Threshold (n = 39) | No. of Hospitals at 95% Threshold (n = 39) |
|---|---|---|---|---|
| ||||
| Type A | Weekdays above threshold | All days below threshold, resulting in net decrease in patients exposed to occupancies above threshold | 3 | 1 |
| Weekends below threshold | ||||
| Type B | Weekdays above threshold | All days above threshold, resulting in net increase in patients exposed to occupancies above threshold | 12 | 18 |
| Weekends below threshold | ||||
| Type C | All days of week below threshold | All days of week below threshold | 6 | 19 |
| Type D | All days of week above threshold | All days of week above threshold, resulting in net decrease in patients exposed to extreme high occupancy | 18 | 1 |
To achieve within‐week smoothing, a median of 7.4 patient‐admissions per week (range: 2.314.4) would have to be scheduled on a different day of the week. This equates to a median of 2.6% (IQR: 2.25%, 2.99%; range: 0.02%9.2%) of all admissionsor 9% of a typical hospital‐week's scheduled admissions.
Discussion
This analysis of 39 children's hospitals found high levels of occupancy and weekend occupancy lower than weekday occupancy (median difference: 8.2% points). Only 12.4% of scheduled admissions entered on weekends. Thus, weekend capacity is available to offset high weekday occupancy. Hospitals at the higher end of the occupancy thresholds (95%, 100%) would reduce the number of days operating at very high occupancy and the number of patients exposed to such levels by smoothing. This change is mathematically feasible, as a median of 7.4 patients would have to be proactively scheduled differently each week, just under one‐tenth of scheduled admissions. Since LOS by day of admission was the same (median: two days), the opportunity to affect occupancy by shifting patients should be relatively similar for all days of the week. In addition, these admissions were short, conferring greater flexibility. Implementing smoothing over the course of the week does not necessarily require admitting patients on weekends. For example, Monday admissions with an anticipated three‐day LOS could enter on Friday with anticipated discharge on Monday to alleviate midweek crowding and take advantage of unoccupied weekend beds. 26
At the highest levels of occupancy, smoothing reduces the frequency of reaching these maximum levels, but can have the effect of actually exposing more patient‐days to a higher occupancy. For example, for nine hospitals in our analysis with >20% of days over 100%, smoothing decreased days over 100%, but exposed weekend patients to higher levels of occupancy (Figure 3). Since most admissions are short and most scheduled admissions currently occur on weekdays, the number of individual patients (not patient‐days) newly exposed to such high occupancy may not increase much after smoothing at these facilities. Regardless, hospitals with such a pattern may not be able to rely solely on smoothing to avoid weekday crowding, and, if they are operating efficiently in terms of SLOSR, might be justified in building more capacity.
Consistent with our findings, the Institute for Healthcare Improvement, the Institute for Healthcare Optimization, and the American Hospital Association Quality Center stress that addressing artificial variability of scheduled admissions is a critical first step to improving patient flow and quality of care while reducing costs. 18, 21, 27 Our study suggests that small numbers of patients need to be proactively scheduled differently to decrease midweek peak occupancy, so only a small proportion of families would need to find this desirable to make it attractive for hospitals and patients. This type of proactive smoothing decreases peak occupancy on weekdays, reducing the safety risks associated with high occupancy, improving acute access for emergent patients, shortening wait‐times and loss of scheduled patients to another facility, and increasing procedure volume (3%74% in one study). 28 Smoothing may also increase quality and safety on weekends, as emergent patients admitted on weekends experience more delays in necessary treatment and have worse outcomes. 2932 In addition, increasing scheduled admissions to span weekends may appeal to some families wishing to avoid absence from work to be with their hospitalized child, to parents concerned about school performanceand may also appeal to staff members seeking flexible schedules. Increasing weekend hospital capacity is safe, feasible, and economical, even when considering the increased wages for weekend work. 33, 34 Finally, smoothing over the whole week allows fixed costs (eg, surgical suites, imaging equipment) to be allocated over 7 days rather than 5, and allows for better matching of revenue to the fixed expenses.
Rather than a prescriptive approach, our work suggests hospitals need to identify only a small number of patients to proactively shift, providing them opportunities to adapt the approach to local circumstances. The particular patients to move around may also depend on the costs and benefits of services (eg, radiologic, laboratory, operative) and the hospital's existing patterns of staffing. A number of hospitals that have engaged in similar work have achieved sustainable results, such as Seattle Children's Hospital, Boston Medical Center, St. John's Regional Health Center, and New York University Langone Medical Center. 19, 26, 3537 In these cases, proactive smoothing took advantage of unused capacity and decreased crowding on days that had been traditionally very full. Hospitals that rarely or never have high‐occupancy days, and that do not expect growth in volume, may not need to employ smoothing, whereas others that have crowding issues primarily in the winter may wish to implement smoothing techniques seasonally.
Aside from attempting to reduce high‐occupancy through modification of admission patterns, other proactive approaches include optimizing staffing and processes around care, improving efficiency of care, and building additional beds. 16, 25, 38, 39 However, the expense of construction and the scarcity of capital often preclude this last option. Among children's hospitals, with SLOSR close to one, implementing strategies to reduce the LOS during periods of high occupancy may not result in meaningful reductions in LOS, as such approaches would only decrease the typical child's hospitalization by hours, not days. In addition to proactive strategies, hospitals also rely on reactive approaches, such as ED boarding, placing patients in hallways on units, diverting ambulances or transfers, or canceling scheduled admissions at the last moment, to decrease crowding. 16, 39, 40
This study has several limitations. First, use of administrative data precluded modeling all responses. For example, some hospitals may be better able to accommodate fluctuations in census or high occupancy without compromising quality or access. Second, we only considered intra‐week smoothing, but hospitals may benefit from smoothing over longer periods of time, especially since children's hospitals are busier in winter months, but incoming scheduled volume is often not reduced. 11 Hospitals with large occupancy variations across months may want to consider broadening the time horizon for smoothing, and weigh the costs and benefits over that period of time, including parental and clinician concerns and preferences for not delaying treatment. At the individual hospital level, discrete‐event simulation would likely be useful to consider the trade‐offs of smoothing to different levels and over different periods of time. Third, we assumed a fixed number of beds for the year, an approach that may not accurately reflect actual available beds on specific days. This limitation was minimized by counting all beds for each hospital as available for all the days of the year, so that hospitals with a high census when all available beds are included would have an even higher percent occupancy if some of those beds were not actually open. In a related way, then, we also do not consider how staffing may need to be altered or augmented to care for additional patients on certain days. Fourth, midnight census, the only universally available measure, was used to determine occupancy rather than peak census. Midnight census provides a standard snapshot, but is lower than mid‐day peak census. 41 In order to account for these limitations, we considered several different thresholds of high occupancy. Fifth, we smoothed at the hospital level, but differential effects may exist at the unit level. Sixth, to determine proportion of scheduled admissions, we used HCUP KID proportions on PHIS admissions. Overall, this approach likely overestimated scheduled medical admissions on weekends, thus biasing our result towards the null hypothesis. Finally, only freestanding children's hospitals were included in this study. While this may limit generalizability, the general concept of smoothing occupancy should apply in any setting with substantial and consistent variation.
In summary, our study revealed that children's hospitals often face high midweek occupancy, but also have substantial unused weekend capacity. Hospitals facing challenges with high weekday occupancy could proactively use a smoothing approach to decrease the frequency and severity of high occupancy. Further qualitative evaluation is also warranted around child, family, and staff preferences concerning scheduled admissions, school, and work.
- , , , . A comparison of in‐hospital mortality risk conferred by high hospital occupancy, differences in nurse staffing levels, weekend admission, and seasonal influenza. Medical Care. 2010;48(3):224–232.
- , , , et al. Hospital workload and adverse events. Med Care. 2007;45(5):448–455.
- , , , , . Impact of admission‐day crowding on the length of stay of pediatric hospitalizations. Pediatrics. 2008;121(4):e718–e730.
- , , , . The effect of hospital bed occupancy on throughput in the pediatric emergency department. Ann Emerg Med. 2009;53(6):767–776.
- . The tipping point: the relationship between volume and patient harm. Am J Med Qual. 2008;23(5):336–341.
- , , , , , . Managing unnecessary variability in patient demand to reduce nursing stress and improve patient safety. Jt Comm J Qual Patient Saf. 2005;31(6):330–338.
- Hospital‐Based Emergency Care: At the Breaking Point. Washington, DC: Institute of Medicine Committee on the Future of Emergency Care in the United States Health System; 2006.
- , , , , . The effect of hospital occupancy on emergency department length of stay and patient disposition. Acad Emerg Med. 2003;10(2):127–133.
- . Interpreting the Volume‐Outcome Relationship in the Context of Health Care Quality: Workshop Summary. Washington, DC: National Academies Press; 2000.
- , , , , . Has recognition of the relationship between mortality rates and hospital volume for major cancer surgery in California made a difference? A follow‐up analysis of another decade. Ann Surg. 2009;250(3):472–483.
- , , , et al. Children's hospitals do not acutely respond to high occupancy. Pediatrics. 2010;125:974–981.
- , , , , , . Scheduled admissions and high occupancy at a children's hospital. J Hosp Med. 2011;6(2):81–87.
- , , . Characteristics of weekday and weekend hospital admissions. HCUP Statistical Brief. 2010;87.
- Agency for Healthcare Research and Quality. HCUP databases, Healthcare Cost and Utilization Project (HCUP); 2008. Available at: http://www.hcup‐us.ahrq.gov/kidoverview.jsp. Accessed July 15, 2009.
- , et al. Managing capacity to reduce emergency department overcrowding and ambulance diversions. J Qual Patient Saf. 2006;32(5):239–245.
- Institute for Healthcare Improvement. Flow initiatives; 2008. Available at: http://www.ihi.org/IHI/Topics/Flow. Accessed February 20, 2008.
- , , , , , . Trends in high‐turnover stays among children hospitalized in the United States, 1993–2003. Pediatrics. 2009;123(3):996–1002.
- Institute for Healthcare Improvement. Smoothing elective surgical admissions. Available at: http://www.ihi.org/IHI/Topics/Flow/PatientFlow/EmergingContent/SmoothingElectiveSurgicalAdmissions.htm. Accessed October 24, 2008.
- Boston hospital sees big impact from smoothing elective schedule. OR Manager. 2004;20:12.
- . Managing Variability in Patient Flow Is the Key to Improving Access to Care, Nursing Staffing, Quality of Care, and Reducing Its Cost. Paper presented at Institute of Medicine, Washington, DC; June 24, 2004.
- American Hospital Association Quality Center. Available at: http://www.ahaqualitycenter.org/ahaqualitycenter/. Accessed October 14, 2008.
- Healthcare Cost and Utilization Project (HCUP). Kids' Inpatient Database (KID); July 2008. Available at: http://www.hcup‐us.ahrq.gov/kidoverview.jsp. Accessed September 10, 2008.
- , , . Using a queuing model to help plan bed allocation in a department of geriatric medicine. Health Care Manag Sci. 2002;5(4):307–313.
- . How many hospital beds? Inquiry. 2002;39(4):400–412.
- . Institute for Healthcare Improvement. Patient flow comments. Available at: http://www.ihi.org/IHI/Topics/Flow. Accessed September 10, 2008.
- . Factory efficiency comes to the hospital. New York Times. July 9, 2010.
- Institute for Healthcare Improvement. Re‐engineering the operating room. Available at: http://www.ihi.org/IHI/Programs/ConferencesAndSeminars/ReengineeringtheOperatingRoomSept08.htm. Accessed November 8, 2008.
- , . Enhanced weekend service: an affordable means to increased hospital procedure volume. CMAJ. 2005;172(4):503–504.
- , . Mortality among patients admitted to hospitals on weekends as compared with weekdays. N Engl J Med. 2001;345:663–668.
- , , , , , . Weekend versus weekday admission and mortality from myocardial infarction. N Engl J Med. 2007;356:1099–1109.
- , . Waiting for urgent procedures on the weekend among emergently hospitalized patients. Am J Med. 2004;117:175–181.
- . Do hospitals provide lower quality care on weekends? Health Serv Res. 2007;42:1589–1612.
- . Hospital saves by working weekends. Mod Healthc. 1996;26:82–99.
- , , , , . Weekend and holiday exercise testing in patients with chest pain. J Gen Intern Med. 1999;14:10–14.
- . Boston Medical Center Case Study: Institute of Healthcare Optimization; 2006. Available at: http://www.ihoptimize.org/8f16e142‐eeaa‐4898–9e62–660218f19ffb/download.htm. Accessed October 3, 2010.
- , , , . The impact of IMPACT on St John's Regional Health Center. Mo Med. 2003;100:590–592.
- NYU Langone Medical Center Extends Access to Non‐Emergent Care as Part of Commitment to Patient‐Centered Care (June 23, 2010). Available at: http://communications.med.nyu.edu/news/2010/nyu‐langone‐medical‐center‐extends‐access‐non‐emergent‐care‐part‐commitment‐patient‐center. Accessed October 3, 2010.
- Carondelet St. Mary's Hospital. A pragmatic approach to improving patient efficiency throughput. Improvement Report 2005. Available at: http://www.ihi.org/IHI/Topics/Flow/PatientFlow/ImprovementStories/APragmaticApproachtoImprovingPatientEfficiencyThroughput.htm. Accessed October 3, 2010.
- AHA Solutions. Patient Flow Challenges Assessment 2009. Chicago, IL; 2009.
- , , , , , . A conceptual model of emergency department crowding. Ann Emerg Med. 2003;42(2):173–180.
- . Annual bed statistics give a misleading picture of hospital surge capacity. Ann Emerg Med. 2006;48(4):384–388.
High levels of hospital occupancy are associated with compromises to quality of care and access (often referred to as crowding), 18 while low occupancy may be inefficient and also impact quality. 9, 10 Despite this, hospitals typically have uneven occupancy. Although some demand for services is driven by factors beyond the control of a hospital (eg, seasonal variation in viral illness), approximately 15%30% of admissions to children's hospitals are scheduled from days to months in advance, with usual arrivals on weekdays. 1114 For example, of the 3.4 million elective admissions in the 2006 Healthcare Cost and Utilization Project Kids Inpatient Database (HCUP KID), only 13% were admitted on weekends. 14 Combined with short length of stay (LOS) for such patients, this leads to higher midweek and lower weekend occupancy. 12
Hospitals respond to crowding in a number of ways, but often focus on reducing LOS to make room for new patients. 11, 15, 16 For hospitals that are relatively efficient in terms of LOS, efforts to reduce it may not increase functional capacity adequately. In children's hospitals, median lengths of stay are 2 to 3 days, and one‐third of hospitalizations are 1 day or less. 17 Thus, even 10%20% reductions in LOS trims hours, not days, from typical stays. Practical barriers (eg, reluctance to discharge in the middle of the night, or family preferences and work schedules) and undesired outcomes (eg, increased hospital re‐visits) are additional pitfalls encountered by relying on throughput enhancement alone.
Managing scheduled admissions through smoothing is an alternative strategy to reduce variability and high occupancy. 6, 12, 1820 The concept is to proactively control the entry of patients, when possible, to achieve more even levels of occupancy, instead of the peaks and troughs commonly encountered. Nonetheless, it is not a widely used approach. 18, 20, 21 We hypothesized that children's hospitals had substantial unused capacity that could be used to smooth occupancy, which would reduce weekday crowding. While it is obvious that smoothing will reduce peaks to average levels (and also raise troughs), we sought to quantify just how large this difference wasand thereby quantify the potential of smoothing to reduce inpatient crowding (or, conversely, expose more patients to high levels of occupancy). Is there enough variation to justify smoothing, and, if a hospital does smooth, what is the expected result? If the number of patients removed from exposure to high occupancy is not substantial, other means to address inpatient crowding might be of more value. Our aims were to quantify the difference in weekday versus weekend occupancy, report on mathematical feasibility of such an approach, and determine the difference in number of patients exposed to various levels of high occupancy.
Methods
Data Source
This retrospective study was conducted with resource‐utilization data from 39 freestanding, tertiary‐care children's hospitals in the Pediatric Health Information System (PHIS). Participating hospitals are located in noncompeting markets of 23 states, plus the District of Columbia, and affiliated with the Child Health Corporation of America (CHCA, Shawnee Mission, KS). They account for 80% of freestanding, and 20% of all general, tertiary‐care children's hospitals. Data quality and reliability are assured through joint ongoing, systematic monitoring. The Children's Hospital of Philadelphia Committees for the Protection of Human Subjects approved the protocol with a waiver of informed consent.
Patients
Patients admitted January 1December 31, 2007 were eligible for inclusion. Due to variation in the presence of birthing, neonatal intensive care, and behavioral health units across hospitals, these beds and associated patients were excluded. Inpatients enter hospitals either as scheduled (often referred to as elective) or unscheduled (emergent or urgent) admissions. Because PHIS does not include these data, KID was used to standardize the PHIS data for proportion of scheduled admissions. 22 (KID is a healthcare database of 23 million pediatric inpatient discharges developed through federalstateindustry partnership, and sponsored by the Agency for Healthcare Research and Quality [AHRQ].) Each encounter in KID includes a principal International Classification of Diseases, 9th revision (ICD‐9) discharge diagnosis code, and is designated by the hospital as elective (ranging from chemotherapy to tonsillectomy) or not elective. Because admissions, rather than diagnoses, are scheduled, a proportion of patients with each primary diagnosis in KID are scheduled (eg, 28% of patients with a primary diagnosis of esophageal reflux). Proportions in KID were matched to principal diagnoses in PHIS.
Definitions
The census was the number of patients registered as inpatients (including those physically in the emergency department [ED] from time of ED arrival)whether observation or inpatient statusat midnight, the conclusion of the day. Hospital capacity was set using CHCA data (and confirmed by each hospital's administrative personnel) as the number of licensed in‐service beds available for patients in 2007; we assumed beds were staffed and capacity fixed for the year. Occupancy was calculated by dividing census by capacity. Maximum occupancy in a week referred to the highest occupancy level achieved in a seven‐day period (MondaySunday). We analyzed a set of thresholds for high‐occupancy (85%, 90%, 95%, and 100%), because there is no consistent definition for when hospitals are at high occupancy or when crowding occurs, though crowding has been described as starting at 85% occupancy. 2325
Analysis
The hospital was the unit of analysis. We report hospital characteristics, including capacity, number of discharges, and census region, and annual standardized length of stay ratio (SLOSR) as observed‐to‐expected LOS.
Smoothing Technique
A retrospective smoothing algorithm set each hospital's daily occupancy during a week to that hospital's mean occupancy for the week; effectively spreading the week's volume of patients evenly across the days of the week. While inter‐week and inter‐month smoothing were considered, intra‐week smoothing was deemed more practical for the largest number of patients, as it would not mean delaying care by more than one week. In the case of a planned treatment course (eg, chemotherapy), only intra‐week smoothing would maintain the necessary scheduled intervals of treatment.
Mathematical Feasibility
To approximate the number of patient admissions that would require different scheduling during a particular week to achieve smoothed weekly occupancy, we determined the total number of patient‐days in the week that required different scheduling and divided by the average LOS for the week. We then divided the number of admissions‐to‐move by total weekly admissions to compute the percentage at each hospital across 52 weeks of the year.
Measuring the Impact of Smoothing
We focused on the frequency and severity of high occupancy and the number of patients exposed to it. This framework led to 4 measures that assess the opportunity and effect of smoothing:
Difference in hospital weekdayweekend occupancy: Equal to 12‐month median of difference between mean weekday occupancy and mean weekend occupancy for each hospital‐week.
Difference in hospital maximummean occupancy: Equal to median of difference between maximum one‐day occupancy and weekly mean (smoothed) occupancy for each hospital‐week. A regression line was derived from the data for the 39 hospitals to report expected reduction in peak occupancy based on the magnitude of the difference between weekday and weekend occupancy.
Difference in number of hospitals exposed to above‐threshold occupancy: Equal to difference, pre‐ and post‐smoothing, in number of hospitals facing high‐occupancy conditions on an average of at least one weekday midnight per week during the year at different occupancy thresholds.
Difference in number of patients exposed to above‐threshold occupancy: Equal to difference, pre‐ and post‐smoothing, in number of patients exposed to hospital midnight occupancy at the thresholds. We utilized patient‐days for the calculation to avoid double‐counting, and divided this by average LOS, in order to determine the number of patients who would no longer be exposed to over‐threshold occupancy after smoothing, while also adjusting for patients newly exposed to over‐threshold occupancy levels.
All analyses were performed separately for each hospital for the entire year and then for winter (DecemberMarch), the period during which most crowding occurred. Analyses were performed using SAS (version 9.2, SAS Institute, Inc, Cary, NC); P values <0.05 were considered statistically significant.
Results
The characteristics of the 39 hospitals are provided in Table 1. Based on standardization with KID, 23.6% of PHIS admissions were scheduled (range: 18.1%35.8%) or a median of 81.5 scheduled admissions per week per hospital; 26.6% of weekday admissions were scheduled versus 16.1% for weekends. Overall, 12.4% of scheduled admissions entered on weekends. For all patients, median LOS was three days (interquartile range [IQR]: twofive days), but median LOS for scheduled admissions was two days (IQR: onefour days). The median LOS and IQR were the same by day of admission for all days of the week. Most hospitals had an overall SLOSR close to one (median: 0.9, IQR: 0.91.1). Overall, hospital mean midnight occupancy ranged from 70.9% to 108.1% on weekdays and 65.7% to 94.9% on weekends. Uniformly, weekday occupancy exceeded weekend occupancy, with a median difference of 8.2% points (IQR: 7.2%9.5% points). There was a wide range of median hospital weekdayweekend occupancy differences across hospitals (Figure 1). The overall difference was less in winter (median difference: 7.7% points; IQR: 6.3%8.8% points) than in summer (median difference: 8.6% points; IQR: 7.4%9.8% points (Wilcoxon Sign Rank test, P < 0.001). Thirty‐five hospitals (89.7%) exceeded the 85% occupancy threshold and 29 (74.4%) exceeded the 95% occupancy threshold on at least 20% of weekdays (Table 2). Across all the hospitals, the median difference in weekly maximum and weekly mean occupancy was 6.6% points (IQR: 6.2%7.4% points) (Figure 2).
| Characteristics | No. (%) |
|---|---|
| |
| Licensed in‐service beds | n = 39 hospitals |
| <200 beds | 6 (15.4) |
| 200249 beds | 10 (25.6) |
| 250300 beds | 14 (35.9) |
| >300 beds | 9 (23.1) |
| No. of discharges | |
| <10,000 | 5 (12.8) |
| 10,00013,999 | 14 (35.9) |
| 14,00017,999 | 11 (28.2) |
| >18,000 | 9 (23.1) |
| Census region | |
| West | 9 (23.1) |
| Midwest | 11 (28.2) |
| Northeast | 6 (15.4) |
| South | 13 (33.3) |
| Admissions | n = 590,352 admissions |
| Medical scheduled admissions* | 79,683 |
| Surgical scheduled admissions* | 59,640 |
| Total scheduled admissions* (% of all admissions) | 139,323 (23.6) |
| Weekend medical scheduled admissions* (% of all medical scheduled admissions) | 13,546 (17.0) |
| Weekend surgical scheduled admissions* (% of all surgical scheduled admissions) | 3,757 (6.3) |
| Weekend total scheduled admissions* (% of total scheduled admissions) | 17,276 (12.4) |

| Entire Year | >85% | Occupancy Threshold | >95% | >100% |
|---|---|---|---|---|
| >90% | ||||
| ||||
| No. of hospitals (n = 39) with mean weekday occupancy above threshold | ||||
| Before smoothing (current state) | 33 | 25 | 14 | 6 |
| After smoothing | 32 | 22 | 10 | 1 |
| No. of hospitals (n = 39) above threshold 20% of weekdays | ||||
| Before smoothing (current state) | 35 | 34 | 29 | 14 |
| After smoothing | 35 | 32 | 21 | 9 |
| Median (IQR) no. of patient‐days per hospital not exposed to occupancy above threshold by smoothing | 3,071 | 281 | 3236 | 3281 |
| (5,552, 919) | (5,288, 3,103) | (0, 7,083) | (962, 8,517) | |
| Median (IQR) no. of patients per hospital not exposed to occupancy above threshold by smoothing | 596 | 50 | 630 | 804 |
| (1,190, 226) | (916, 752) | (0, 1,492) | (231, 2,195) | |

Smoothing reduced the number of hospitals at each occupancy threshold, except 85% (Table 2). As a linear relationship, the reduction in weekday peak occupancy (y) based on a hospital's median difference in weekly maximum and weekly mean occupancy (x) was y = 2.69 + 0.48x. Thus, a hospital with a 10% point difference between weekday and weekend occupancy could reduce weekday peak by 7.5% points.
Smoothing increased the number of patients exposed to the lower thresholds (85% and 90%), but decreased the number of patients exposed to >95% occupancy (Table 2). For example, smoothing at the 95% threshold resulted in 630 fewer patients per hospital exposed to that threshold. If all 39 hospitals had within‐week smoothing, a net of 39,607 patients would have been protected from exposure to >95% occupancy and a net of 50,079 patients from 100% occupancy.
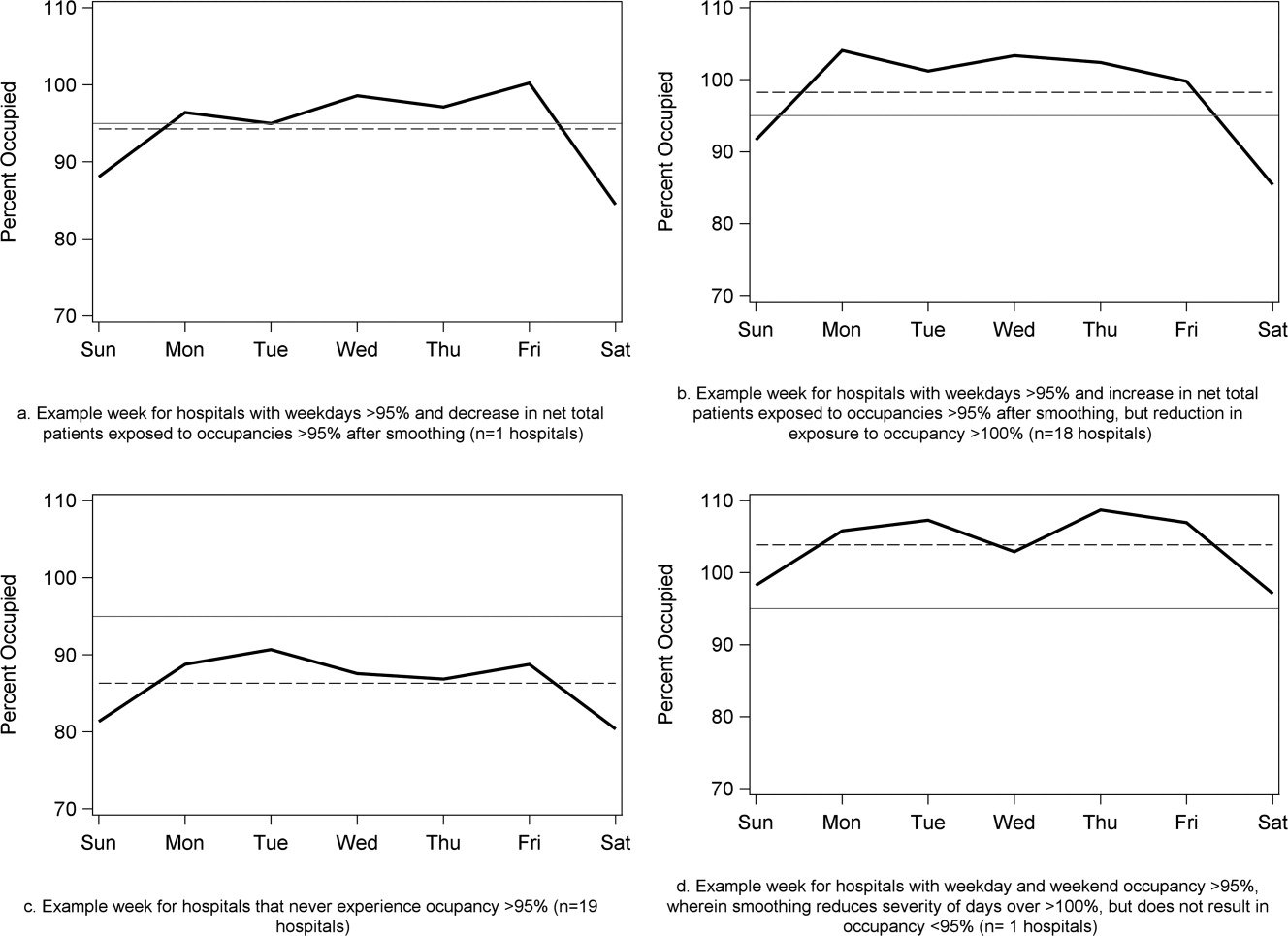
To demonstrate the varied effects of smoothing, Table 3 and Figure 3 present representative categories of response to smoothing depending on pre‐smoothing patterns. While not all hospitals decreased occupancy to below thresholds after smoothing (Types B and D), the overall occupancy was reduced and fewer patients were exposed to extreme levels of high occupancy (eg, >100%).
| Category | Before Smoothing Hospital Description | After Smoothing Hospital Description | No. of Hospitals at 85% Threshold (n = 39) | No. of Hospitals at 95% Threshold (n = 39) |
|---|---|---|---|---|
| ||||
| Type A | Weekdays above threshold | All days below threshold, resulting in net decrease in patients exposed to occupancies above threshold | 3 | 1 |
| Weekends below threshold | ||||
| Type B | Weekdays above threshold | All days above threshold, resulting in net increase in patients exposed to occupancies above threshold | 12 | 18 |
| Weekends below threshold | ||||
| Type C | All days of week below threshold | All days of week below threshold | 6 | 19 |
| Type D | All days of week above threshold | All days of week above threshold, resulting in net decrease in patients exposed to extreme high occupancy | 18 | 1 |

To achieve within‐week smoothing, a median of 7.4 patient‐admissions per week (range: 2.314.4) would have to be scheduled on a different day of the week. This equates to a median of 2.6% (IQR: 2.25%, 2.99%; range: 0.02%9.2%) of all admissionsor 9% of a typical hospital‐week's scheduled admissions.
Discussion
This analysis of 39 children's hospitals found high levels of occupancy and weekend occupancy lower than weekday occupancy (median difference: 8.2% points). Only 12.4% of scheduled admissions entered on weekends. Thus, weekend capacity is available to offset high weekday occupancy. Hospitals at the higher end of the occupancy thresholds (95%, 100%) would reduce the number of days operating at very high occupancy and the number of patients exposed to such levels by smoothing. This change is mathematically feasible, as a median of 7.4 patients would have to be proactively scheduled differently each week, just under one‐tenth of scheduled admissions. Since LOS by day of admission was the same (median: two days), the opportunity to affect occupancy by shifting patients should be relatively similar for all days of the week. In addition, these admissions were short, conferring greater flexibility. Implementing smoothing over the course of the week does not necessarily require admitting patients on weekends. For example, Monday admissions with an anticipated three‐day LOS could enter on Friday with anticipated discharge on Monday to alleviate midweek crowding and take advantage of unoccupied weekend beds. 26
At the highest levels of occupancy, smoothing reduces the frequency of reaching these maximum levels, but can have the effect of actually exposing more patient‐days to a higher occupancy. For example, for nine hospitals in our analysis with >20% of days over 100%, smoothing decreased days over 100%, but exposed weekend patients to higher levels of occupancy (Figure 3). Since most admissions are short and most scheduled admissions currently occur on weekdays, the number of individual patients (not patient‐days) newly exposed to such high occupancy may not increase much after smoothing at these facilities. Regardless, hospitals with such a pattern may not be able to rely solely on smoothing to avoid weekday crowding, and, if they are operating efficiently in terms of SLOSR, might be justified in building more capacity.
Consistent with our findings, the Institute for Healthcare Improvement, the Institute for Healthcare Optimization, and the American Hospital Association Quality Center stress that addressing artificial variability of scheduled admissions is a critical first step to improving patient flow and quality of care while reducing costs. 18, 21, 27 Our study suggests that small numbers of patients need to be proactively scheduled differently to decrease midweek peak occupancy, so only a small proportion of families would need to find this desirable to make it attractive for hospitals and patients. This type of proactive smoothing decreases peak occupancy on weekdays, reducing the safety risks associated with high occupancy, improving acute access for emergent patients, shortening wait‐times and loss of scheduled patients to another facility, and increasing procedure volume (3%74% in one study). 28 Smoothing may also increase quality and safety on weekends, as emergent patients admitted on weekends experience more delays in necessary treatment and have worse outcomes. 2932 In addition, increasing scheduled admissions to span weekends may appeal to some families wishing to avoid absence from work to be with their hospitalized child, to parents concerned about school performanceand may also appeal to staff members seeking flexible schedules. Increasing weekend hospital capacity is safe, feasible, and economical, even when considering the increased wages for weekend work. 33, 34 Finally, smoothing over the whole week allows fixed costs (eg, surgical suites, imaging equipment) to be allocated over 7 days rather than 5, and allows for better matching of revenue to the fixed expenses.
Rather than a prescriptive approach, our work suggests hospitals need to identify only a small number of patients to proactively shift, providing them opportunities to adapt the approach to local circumstances. The particular patients to move around may also depend on the costs and benefits of services (eg, radiologic, laboratory, operative) and the hospital's existing patterns of staffing. A number of hospitals that have engaged in similar work have achieved sustainable results, such as Seattle Children's Hospital, Boston Medical Center, St. John's Regional Health Center, and New York University Langone Medical Center. 19, 26, 3537 In these cases, proactive smoothing took advantage of unused capacity and decreased crowding on days that had been traditionally very full. Hospitals that rarely or never have high‐occupancy days, and that do not expect growth in volume, may not need to employ smoothing, whereas others that have crowding issues primarily in the winter may wish to implement smoothing techniques seasonally.
Aside from attempting to reduce high‐occupancy through modification of admission patterns, other proactive approaches include optimizing staffing and processes around care, improving efficiency of care, and building additional beds. 16, 25, 38, 39 However, the expense of construction and the scarcity of capital often preclude this last option. Among children's hospitals, with SLOSR close to one, implementing strategies to reduce the LOS during periods of high occupancy may not result in meaningful reductions in LOS, as such approaches would only decrease the typical child's hospitalization by hours, not days. In addition to proactive strategies, hospitals also rely on reactive approaches, such as ED boarding, placing patients in hallways on units, diverting ambulances or transfers, or canceling scheduled admissions at the last moment, to decrease crowding. 16, 39, 40
This study has several limitations. First, use of administrative data precluded modeling all responses. For example, some hospitals may be better able to accommodate fluctuations in census or high occupancy without compromising quality or access. Second, we only considered intra‐week smoothing, but hospitals may benefit from smoothing over longer periods of time, especially since children's hospitals are busier in winter months, but incoming scheduled volume is often not reduced. 11 Hospitals with large occupancy variations across months may want to consider broadening the time horizon for smoothing, and weigh the costs and benefits over that period of time, including parental and clinician concerns and preferences for not delaying treatment. At the individual hospital level, discrete‐event simulation would likely be useful to consider the trade‐offs of smoothing to different levels and over different periods of time. Third, we assumed a fixed number of beds for the year, an approach that may not accurately reflect actual available beds on specific days. This limitation was minimized by counting all beds for each hospital as available for all the days of the year, so that hospitals with a high census when all available beds are included would have an even higher percent occupancy if some of those beds were not actually open. In a related way, then, we also do not consider how staffing may need to be altered or augmented to care for additional patients on certain days. Fourth, midnight census, the only universally available measure, was used to determine occupancy rather than peak census. Midnight census provides a standard snapshot, but is lower than mid‐day peak census. 41 In order to account for these limitations, we considered several different thresholds of high occupancy. Fifth, we smoothed at the hospital level, but differential effects may exist at the unit level. Sixth, to determine proportion of scheduled admissions, we used HCUP KID proportions on PHIS admissions. Overall, this approach likely overestimated scheduled medical admissions on weekends, thus biasing our result towards the null hypothesis. Finally, only freestanding children's hospitals were included in this study. While this may limit generalizability, the general concept of smoothing occupancy should apply in any setting with substantial and consistent variation.
In summary, our study revealed that children's hospitals often face high midweek occupancy, but also have substantial unused weekend capacity. Hospitals facing challenges with high weekday occupancy could proactively use a smoothing approach to decrease the frequency and severity of high occupancy. Further qualitative evaluation is also warranted around child, family, and staff preferences concerning scheduled admissions, school, and work.
High levels of hospital occupancy are associated with compromises to quality of care and access (often referred to as crowding), 18 while low occupancy may be inefficient and also impact quality. 9, 10 Despite this, hospitals typically have uneven occupancy. Although some demand for services is driven by factors beyond the control of a hospital (eg, seasonal variation in viral illness), approximately 15%30% of admissions to children's hospitals are scheduled from days to months in advance, with usual arrivals on weekdays. 1114 For example, of the 3.4 million elective admissions in the 2006 Healthcare Cost and Utilization Project Kids Inpatient Database (HCUP KID), only 13% were admitted on weekends. 14 Combined with short length of stay (LOS) for such patients, this leads to higher midweek and lower weekend occupancy. 12
Hospitals respond to crowding in a number of ways, but often focus on reducing LOS to make room for new patients. 11, 15, 16 For hospitals that are relatively efficient in terms of LOS, efforts to reduce it may not increase functional capacity adequately. In children's hospitals, median lengths of stay are 2 to 3 days, and one‐third of hospitalizations are 1 day or less. 17 Thus, even 10%20% reductions in LOS trims hours, not days, from typical stays. Practical barriers (eg, reluctance to discharge in the middle of the night, or family preferences and work schedules) and undesired outcomes (eg, increased hospital re‐visits) are additional pitfalls encountered by relying on throughput enhancement alone.
Managing scheduled admissions through smoothing is an alternative strategy to reduce variability and high occupancy. 6, 12, 1820 The concept is to proactively control the entry of patients, when possible, to achieve more even levels of occupancy, instead of the peaks and troughs commonly encountered. Nonetheless, it is not a widely used approach. 18, 20, 21 We hypothesized that children's hospitals had substantial unused capacity that could be used to smooth occupancy, which would reduce weekday crowding. While it is obvious that smoothing will reduce peaks to average levels (and also raise troughs), we sought to quantify just how large this difference wasand thereby quantify the potential of smoothing to reduce inpatient crowding (or, conversely, expose more patients to high levels of occupancy). Is there enough variation to justify smoothing, and, if a hospital does smooth, what is the expected result? If the number of patients removed from exposure to high occupancy is not substantial, other means to address inpatient crowding might be of more value. Our aims were to quantify the difference in weekday versus weekend occupancy, report on mathematical feasibility of such an approach, and determine the difference in number of patients exposed to various levels of high occupancy.
Methods
Data Source
This retrospective study was conducted with resource‐utilization data from 39 freestanding, tertiary‐care children's hospitals in the Pediatric Health Information System (PHIS). Participating hospitals are located in noncompeting markets of 23 states, plus the District of Columbia, and affiliated with the Child Health Corporation of America (CHCA, Shawnee Mission, KS). They account for 80% of freestanding, and 20% of all general, tertiary‐care children's hospitals. Data quality and reliability are assured through joint ongoing, systematic monitoring. The Children's Hospital of Philadelphia Committees for the Protection of Human Subjects approved the protocol with a waiver of informed consent.
Patients
Patients admitted January 1December 31, 2007 were eligible for inclusion. Due to variation in the presence of birthing, neonatal intensive care, and behavioral health units across hospitals, these beds and associated patients were excluded. Inpatients enter hospitals either as scheduled (often referred to as elective) or unscheduled (emergent or urgent) admissions. Because PHIS does not include these data, KID was used to standardize the PHIS data for proportion of scheduled admissions. 22 (KID is a healthcare database of 23 million pediatric inpatient discharges developed through federalstateindustry partnership, and sponsored by the Agency for Healthcare Research and Quality [AHRQ].) Each encounter in KID includes a principal International Classification of Diseases, 9th revision (ICD‐9) discharge diagnosis code, and is designated by the hospital as elective (ranging from chemotherapy to tonsillectomy) or not elective. Because admissions, rather than diagnoses, are scheduled, a proportion of patients with each primary diagnosis in KID are scheduled (eg, 28% of patients with a primary diagnosis of esophageal reflux). Proportions in KID were matched to principal diagnoses in PHIS.
Definitions
The census was the number of patients registered as inpatients (including those physically in the emergency department [ED] from time of ED arrival)whether observation or inpatient statusat midnight, the conclusion of the day. Hospital capacity was set using CHCA data (and confirmed by each hospital's administrative personnel) as the number of licensed in‐service beds available for patients in 2007; we assumed beds were staffed and capacity fixed for the year. Occupancy was calculated by dividing census by capacity. Maximum occupancy in a week referred to the highest occupancy level achieved in a seven‐day period (MondaySunday). We analyzed a set of thresholds for high‐occupancy (85%, 90%, 95%, and 100%), because there is no consistent definition for when hospitals are at high occupancy or when crowding occurs, though crowding has been described as starting at 85% occupancy. 2325
Analysis
The hospital was the unit of analysis. We report hospital characteristics, including capacity, number of discharges, and census region, and annual standardized length of stay ratio (SLOSR) as observed‐to‐expected LOS.
Smoothing Technique
A retrospective smoothing algorithm set each hospital's daily occupancy during a week to that hospital's mean occupancy for the week; effectively spreading the week's volume of patients evenly across the days of the week. While inter‐week and inter‐month smoothing were considered, intra‐week smoothing was deemed more practical for the largest number of patients, as it would not mean delaying care by more than one week. In the case of a planned treatment course (eg, chemotherapy), only intra‐week smoothing would maintain the necessary scheduled intervals of treatment.
Mathematical Feasibility
To approximate the number of patient admissions that would require different scheduling during a particular week to achieve smoothed weekly occupancy, we determined the total number of patient‐days in the week that required different scheduling and divided by the average LOS for the week. We then divided the number of admissions‐to‐move by total weekly admissions to compute the percentage at each hospital across 52 weeks of the year.
Measuring the Impact of Smoothing
We focused on the frequency and severity of high occupancy and the number of patients exposed to it. This framework led to 4 measures that assess the opportunity and effect of smoothing:
Difference in hospital weekdayweekend occupancy: Equal to 12‐month median of difference between mean weekday occupancy and mean weekend occupancy for each hospital‐week.
Difference in hospital maximummean occupancy: Equal to median of difference between maximum one‐day occupancy and weekly mean (smoothed) occupancy for each hospital‐week. A regression line was derived from the data for the 39 hospitals to report expected reduction in peak occupancy based on the magnitude of the difference between weekday and weekend occupancy.
Difference in number of hospitals exposed to above‐threshold occupancy: Equal to difference, pre‐ and post‐smoothing, in number of hospitals facing high‐occupancy conditions on an average of at least one weekday midnight per week during the year at different occupancy thresholds.
Difference in number of patients exposed to above‐threshold occupancy: Equal to difference, pre‐ and post‐smoothing, in number of patients exposed to hospital midnight occupancy at the thresholds. We utilized patient‐days for the calculation to avoid double‐counting, and divided this by average LOS, in order to determine the number of patients who would no longer be exposed to over‐threshold occupancy after smoothing, while also adjusting for patients newly exposed to over‐threshold occupancy levels.
All analyses were performed separately for each hospital for the entire year and then for winter (DecemberMarch), the period during which most crowding occurred. Analyses were performed using SAS (version 9.2, SAS Institute, Inc, Cary, NC); P values <0.05 were considered statistically significant.
Results
The characteristics of the 39 hospitals are provided in Table 1. Based on standardization with KID, 23.6% of PHIS admissions were scheduled (range: 18.1%35.8%) or a median of 81.5 scheduled admissions per week per hospital; 26.6% of weekday admissions were scheduled versus 16.1% for weekends. Overall, 12.4% of scheduled admissions entered on weekends. For all patients, median LOS was three days (interquartile range [IQR]: twofive days), but median LOS for scheduled admissions was two days (IQR: onefour days). The median LOS and IQR were the same by day of admission for all days of the week. Most hospitals had an overall SLOSR close to one (median: 0.9, IQR: 0.91.1). Overall, hospital mean midnight occupancy ranged from 70.9% to 108.1% on weekdays and 65.7% to 94.9% on weekends. Uniformly, weekday occupancy exceeded weekend occupancy, with a median difference of 8.2% points (IQR: 7.2%9.5% points). There was a wide range of median hospital weekdayweekend occupancy differences across hospitals (Figure 1). The overall difference was less in winter (median difference: 7.7% points; IQR: 6.3%8.8% points) than in summer (median difference: 8.6% points; IQR: 7.4%9.8% points (Wilcoxon Sign Rank test, P < 0.001). Thirty‐five hospitals (89.7%) exceeded the 85% occupancy threshold and 29 (74.4%) exceeded the 95% occupancy threshold on at least 20% of weekdays (Table 2). Across all the hospitals, the median difference in weekly maximum and weekly mean occupancy was 6.6% points (IQR: 6.2%7.4% points) (Figure 2).
| Characteristics | No. (%) |
|---|---|
| |
| Licensed in‐service beds | n = 39 hospitals |
| <200 beds | 6 (15.4) |
| 200249 beds | 10 (25.6) |
| 250300 beds | 14 (35.9) |
| >300 beds | 9 (23.1) |
| No. of discharges | |
| <10,000 | 5 (12.8) |
| 10,00013,999 | 14 (35.9) |
| 14,00017,999 | 11 (28.2) |
| >18,000 | 9 (23.1) |
| Census region | |
| West | 9 (23.1) |
| Midwest | 11 (28.2) |
| Northeast | 6 (15.4) |
| South | 13 (33.3) |
| Admissions | n = 590,352 admissions |
| Medical scheduled admissions* | 79,683 |
| Surgical scheduled admissions* | 59,640 |
| Total scheduled admissions* (% of all admissions) | 139,323 (23.6) |
| Weekend medical scheduled admissions* (% of all medical scheduled admissions) | 13,546 (17.0) |
| Weekend surgical scheduled admissions* (% of all surgical scheduled admissions) | 3,757 (6.3) |
| Weekend total scheduled admissions* (% of total scheduled admissions) | 17,276 (12.4) |

| Entire Year | >85% | Occupancy Threshold | >95% | >100% |
|---|---|---|---|---|
| >90% | ||||
| ||||
| No. of hospitals (n = 39) with mean weekday occupancy above threshold | ||||
| Before smoothing (current state) | 33 | 25 | 14 | 6 |
| After smoothing | 32 | 22 | 10 | 1 |
| No. of hospitals (n = 39) above threshold 20% of weekdays | ||||
| Before smoothing (current state) | 35 | 34 | 29 | 14 |
| After smoothing | 35 | 32 | 21 | 9 |
| Median (IQR) no. of patient‐days per hospital not exposed to occupancy above threshold by smoothing | 3,071 | 281 | 3236 | 3281 |
| (5,552, 919) | (5,288, 3,103) | (0, 7,083) | (962, 8,517) | |
| Median (IQR) no. of patients per hospital not exposed to occupancy above threshold by smoothing | 596 | 50 | 630 | 804 |
| (1,190, 226) | (916, 752) | (0, 1,492) | (231, 2,195) | |

Smoothing reduced the number of hospitals at each occupancy threshold, except 85% (Table 2). As a linear relationship, the reduction in weekday peak occupancy (y) based on a hospital's median difference in weekly maximum and weekly mean occupancy (x) was y = 2.69 + 0.48x. Thus, a hospital with a 10% point difference between weekday and weekend occupancy could reduce weekday peak by 7.5% points.
Smoothing increased the number of patients exposed to the lower thresholds (85% and 90%), but decreased the number of patients exposed to >95% occupancy (Table 2). For example, smoothing at the 95% threshold resulted in 630 fewer patients per hospital exposed to that threshold. If all 39 hospitals had within‐week smoothing, a net of 39,607 patients would have been protected from exposure to >95% occupancy and a net of 50,079 patients from 100% occupancy.
To demonstrate the varied effects of smoothing, Table 3 and Figure 3 present representative categories of response to smoothing depending on pre‐smoothing patterns. While not all hospitals decreased occupancy to below thresholds after smoothing (Types B and D), the overall occupancy was reduced and fewer patients were exposed to extreme levels of high occupancy (eg, >100%).
| Category | Before Smoothing Hospital Description | After Smoothing Hospital Description | No. of Hospitals at 85% Threshold (n = 39) | No. of Hospitals at 95% Threshold (n = 39) |
|---|---|---|---|---|
| ||||
| Type A | Weekdays above threshold | All days below threshold, resulting in net decrease in patients exposed to occupancies above threshold | 3 | 1 |
| Weekends below threshold | ||||
| Type B | Weekdays above threshold | All days above threshold, resulting in net increase in patients exposed to occupancies above threshold | 12 | 18 |
| Weekends below threshold | ||||
| Type C | All days of week below threshold | All days of week below threshold | 6 | 19 |
| Type D | All days of week above threshold | All days of week above threshold, resulting in net decrease in patients exposed to extreme high occupancy | 18 | 1 |

To achieve within‐week smoothing, a median of 7.4 patient‐admissions per week (range: 2.314.4) would have to be scheduled on a different day of the week. This equates to a median of 2.6% (IQR: 2.25%, 2.99%; range: 0.02%9.2%) of all admissionsor 9% of a typical hospital‐week's scheduled admissions.
Discussion
This analysis of 39 children's hospitals found high levels of occupancy and weekend occupancy lower than weekday occupancy (median difference: 8.2% points). Only 12.4% of scheduled admissions entered on weekends. Thus, weekend capacity is available to offset high weekday occupancy. Hospitals at the higher end of the occupancy thresholds (95%, 100%) would reduce the number of days operating at very high occupancy and the number of patients exposed to such levels by smoothing. This change is mathematically feasible, as a median of 7.4 patients would have to be proactively scheduled differently each week, just under one‐tenth of scheduled admissions. Since LOS by day of admission was the same (median: two days), the opportunity to affect occupancy by shifting patients should be relatively similar for all days of the week. In addition, these admissions were short, conferring greater flexibility. Implementing smoothing over the course of the week does not necessarily require admitting patients on weekends. For example, Monday admissions with an anticipated three‐day LOS could enter on Friday with anticipated discharge on Monday to alleviate midweek crowding and take advantage of unoccupied weekend beds. 26
At the highest levels of occupancy, smoothing reduces the frequency of reaching these maximum levels, but can have the effect of actually exposing more patient‐days to a higher occupancy. For example, for nine hospitals in our analysis with >20% of days over 100%, smoothing decreased days over 100%, but exposed weekend patients to higher levels of occupancy (Figure 3). Since most admissions are short and most scheduled admissions currently occur on weekdays, the number of individual patients (not patient‐days) newly exposed to such high occupancy may not increase much after smoothing at these facilities. Regardless, hospitals with such a pattern may not be able to rely solely on smoothing to avoid weekday crowding, and, if they are operating efficiently in terms of SLOSR, might be justified in building more capacity.
Consistent with our findings, the Institute for Healthcare Improvement, the Institute for Healthcare Optimization, and the American Hospital Association Quality Center stress that addressing artificial variability of scheduled admissions is a critical first step to improving patient flow and quality of care while reducing costs. 18, 21, 27 Our study suggests that small numbers of patients need to be proactively scheduled differently to decrease midweek peak occupancy, so only a small proportion of families would need to find this desirable to make it attractive for hospitals and patients. This type of proactive smoothing decreases peak occupancy on weekdays, reducing the safety risks associated with high occupancy, improving acute access for emergent patients, shortening wait‐times and loss of scheduled patients to another facility, and increasing procedure volume (3%74% in one study). 28 Smoothing may also increase quality and safety on weekends, as emergent patients admitted on weekends experience more delays in necessary treatment and have worse outcomes. 2932 In addition, increasing scheduled admissions to span weekends may appeal to some families wishing to avoid absence from work to be with their hospitalized child, to parents concerned about school performanceand may also appeal to staff members seeking flexible schedules. Increasing weekend hospital capacity is safe, feasible, and economical, even when considering the increased wages for weekend work. 33, 34 Finally, smoothing over the whole week allows fixed costs (eg, surgical suites, imaging equipment) to be allocated over 7 days rather than 5, and allows for better matching of revenue to the fixed expenses.
Rather than a prescriptive approach, our work suggests hospitals need to identify only a small number of patients to proactively shift, providing them opportunities to adapt the approach to local circumstances. The particular patients to move around may also depend on the costs and benefits of services (eg, radiologic, laboratory, operative) and the hospital's existing patterns of staffing. A number of hospitals that have engaged in similar work have achieved sustainable results, such as Seattle Children's Hospital, Boston Medical Center, St. John's Regional Health Center, and New York University Langone Medical Center. 19, 26, 3537 In these cases, proactive smoothing took advantage of unused capacity and decreased crowding on days that had been traditionally very full. Hospitals that rarely or never have high‐occupancy days, and that do not expect growth in volume, may not need to employ smoothing, whereas others that have crowding issues primarily in the winter may wish to implement smoothing techniques seasonally.
Aside from attempting to reduce high‐occupancy through modification of admission patterns, other proactive approaches include optimizing staffing and processes around care, improving efficiency of care, and building additional beds. 16, 25, 38, 39 However, the expense of construction and the scarcity of capital often preclude this last option. Among children's hospitals, with SLOSR close to one, implementing strategies to reduce the LOS during periods of high occupancy may not result in meaningful reductions in LOS, as such approaches would only decrease the typical child's hospitalization by hours, not days. In addition to proactive strategies, hospitals also rely on reactive approaches, such as ED boarding, placing patients in hallways on units, diverting ambulances or transfers, or canceling scheduled admissions at the last moment, to decrease crowding. 16, 39, 40
This study has several limitations. First, use of administrative data precluded modeling all responses. For example, some hospitals may be better able to accommodate fluctuations in census or high occupancy without compromising quality or access. Second, we only considered intra‐week smoothing, but hospitals may benefit from smoothing over longer periods of time, especially since children's hospitals are busier in winter months, but incoming scheduled volume is often not reduced. 11 Hospitals with large occupancy variations across months may want to consider broadening the time horizon for smoothing, and weigh the costs and benefits over that period of time, including parental and clinician concerns and preferences for not delaying treatment. At the individual hospital level, discrete‐event simulation would likely be useful to consider the trade‐offs of smoothing to different levels and over different periods of time. Third, we assumed a fixed number of beds for the year, an approach that may not accurately reflect actual available beds on specific days. This limitation was minimized by counting all beds for each hospital as available for all the days of the year, so that hospitals with a high census when all available beds are included would have an even higher percent occupancy if some of those beds were not actually open. In a related way, then, we also do not consider how staffing may need to be altered or augmented to care for additional patients on certain days. Fourth, midnight census, the only universally available measure, was used to determine occupancy rather than peak census. Midnight census provides a standard snapshot, but is lower than mid‐day peak census. 41 In order to account for these limitations, we considered several different thresholds of high occupancy. Fifth, we smoothed at the hospital level, but differential effects may exist at the unit level. Sixth, to determine proportion of scheduled admissions, we used HCUP KID proportions on PHIS admissions. Overall, this approach likely overestimated scheduled medical admissions on weekends, thus biasing our result towards the null hypothesis. Finally, only freestanding children's hospitals were included in this study. While this may limit generalizability, the general concept of smoothing occupancy should apply in any setting with substantial and consistent variation.
In summary, our study revealed that children's hospitals often face high midweek occupancy, but also have substantial unused weekend capacity. Hospitals facing challenges with high weekday occupancy could proactively use a smoothing approach to decrease the frequency and severity of high occupancy. Further qualitative evaluation is also warranted around child, family, and staff preferences concerning scheduled admissions, school, and work.
- , , , . A comparison of in‐hospital mortality risk conferred by high hospital occupancy, differences in nurse staffing levels, weekend admission, and seasonal influenza. Medical Care. 2010;48(3):224–232.
- , , , et al. Hospital workload and adverse events. Med Care. 2007;45(5):448–455.
- , , , , . Impact of admission‐day crowding on the length of stay of pediatric hospitalizations. Pediatrics. 2008;121(4):e718–e730.
- , , , . The effect of hospital bed occupancy on throughput in the pediatric emergency department. Ann Emerg Med. 2009;53(6):767–776.
- . The tipping point: the relationship between volume and patient harm. Am J Med Qual. 2008;23(5):336–341.
- , , , , , . Managing unnecessary variability in patient demand to reduce nursing stress and improve patient safety. Jt Comm J Qual Patient Saf. 2005;31(6):330–338.
- Hospital‐Based Emergency Care: At the Breaking Point. Washington, DC: Institute of Medicine Committee on the Future of Emergency Care in the United States Health System; 2006.
- , , , , . The effect of hospital occupancy on emergency department length of stay and patient disposition. Acad Emerg Med. 2003;10(2):127–133.
- . Interpreting the Volume‐Outcome Relationship in the Context of Health Care Quality: Workshop Summary. Washington, DC: National Academies Press; 2000.
- , , , , . Has recognition of the relationship between mortality rates and hospital volume for major cancer surgery in California made a difference? A follow‐up analysis of another decade. Ann Surg. 2009;250(3):472–483.
- , , , et al. Children's hospitals do not acutely respond to high occupancy. Pediatrics. 2010;125:974–981.
- , , , , , . Scheduled admissions and high occupancy at a children's hospital. J Hosp Med. 2011;6(2):81–87.
- , , . Characteristics of weekday and weekend hospital admissions. HCUP Statistical Brief. 2010;87.
- Agency for Healthcare Research and Quality. HCUP databases, Healthcare Cost and Utilization Project (HCUP); 2008. Available at: http://www.hcup‐us.ahrq.gov/kidoverview.jsp. Accessed July 15, 2009.
- , et al. Managing capacity to reduce emergency department overcrowding and ambulance diversions. J Qual Patient Saf. 2006;32(5):239–245.
- Institute for Healthcare Improvement. Flow initiatives; 2008. Available at: http://www.ihi.org/IHI/Topics/Flow. Accessed February 20, 2008.
- , , , , , . Trends in high‐turnover stays among children hospitalized in the United States, 1993–2003. Pediatrics. 2009;123(3):996–1002.
- Institute for Healthcare Improvement. Smoothing elective surgical admissions. Available at: http://www.ihi.org/IHI/Topics/Flow/PatientFlow/EmergingContent/SmoothingElectiveSurgicalAdmissions.htm. Accessed October 24, 2008.
- Boston hospital sees big impact from smoothing elective schedule. OR Manager. 2004;20:12.
- . Managing Variability in Patient Flow Is the Key to Improving Access to Care, Nursing Staffing, Quality of Care, and Reducing Its Cost. Paper presented at Institute of Medicine, Washington, DC; June 24, 2004.
- American Hospital Association Quality Center. Available at: http://www.ahaqualitycenter.org/ahaqualitycenter/. Accessed October 14, 2008.
- Healthcare Cost and Utilization Project (HCUP). Kids' Inpatient Database (KID); July 2008. Available at: http://www.hcup‐us.ahrq.gov/kidoverview.jsp. Accessed September 10, 2008.
- , , . Using a queuing model to help plan bed allocation in a department of geriatric medicine. Health Care Manag Sci. 2002;5(4):307–313.
- . How many hospital beds? Inquiry. 2002;39(4):400–412.
- . Institute for Healthcare Improvement. Patient flow comments. Available at: http://www.ihi.org/IHI/Topics/Flow. Accessed September 10, 2008.
- . Factory efficiency comes to the hospital. New York Times. July 9, 2010.
- Institute for Healthcare Improvement. Re‐engineering the operating room. Available at: http://www.ihi.org/IHI/Programs/ConferencesAndSeminars/ReengineeringtheOperatingRoomSept08.htm. Accessed November 8, 2008.
- , . Enhanced weekend service: an affordable means to increased hospital procedure volume. CMAJ. 2005;172(4):503–504.
- , . Mortality among patients admitted to hospitals on weekends as compared with weekdays. N Engl J Med. 2001;345:663–668.
- , , , , , . Weekend versus weekday admission and mortality from myocardial infarction. N Engl J Med. 2007;356:1099–1109.
- , . Waiting for urgent procedures on the weekend among emergently hospitalized patients. Am J Med. 2004;117:175–181.
- . Do hospitals provide lower quality care on weekends? Health Serv Res. 2007;42:1589–1612.
- . Hospital saves by working weekends. Mod Healthc. 1996;26:82–99.
- , , , , . Weekend and holiday exercise testing in patients with chest pain. J Gen Intern Med. 1999;14:10–14.
- . Boston Medical Center Case Study: Institute of Healthcare Optimization; 2006. Available at: http://www.ihoptimize.org/8f16e142‐eeaa‐4898–9e62–660218f19ffb/download.htm. Accessed October 3, 2010.
- , , , . The impact of IMPACT on St John's Regional Health Center. Mo Med. 2003;100:590–592.
- NYU Langone Medical Center Extends Access to Non‐Emergent Care as Part of Commitment to Patient‐Centered Care (June 23, 2010). Available at: http://communications.med.nyu.edu/news/2010/nyu‐langone‐medical‐center‐extends‐access‐non‐emergent‐care‐part‐commitment‐patient‐center. Accessed October 3, 2010.
- Carondelet St. Mary's Hospital. A pragmatic approach to improving patient efficiency throughput. Improvement Report 2005. Available at: http://www.ihi.org/IHI/Topics/Flow/PatientFlow/ImprovementStories/APragmaticApproachtoImprovingPatientEfficiencyThroughput.htm. Accessed October 3, 2010.
- AHA Solutions. Patient Flow Challenges Assessment 2009. Chicago, IL; 2009.
- , , , , , . A conceptual model of emergency department crowding. Ann Emerg Med. 2003;42(2):173–180.
- . Annual bed statistics give a misleading picture of hospital surge capacity. Ann Emerg Med. 2006;48(4):384–388.
- , , , . A comparison of in‐hospital mortality risk conferred by high hospital occupancy, differences in nurse staffing levels, weekend admission, and seasonal influenza. Medical Care. 2010;48(3):224–232.
- , , , et al. Hospital workload and adverse events. Med Care. 2007;45(5):448–455.
- , , , , . Impact of admission‐day crowding on the length of stay of pediatric hospitalizations. Pediatrics. 2008;121(4):e718–e730.
- , , , . The effect of hospital bed occupancy on throughput in the pediatric emergency department. Ann Emerg Med. 2009;53(6):767–776.
- . The tipping point: the relationship between volume and patient harm. Am J Med Qual. 2008;23(5):336–341.
- , , , , , . Managing unnecessary variability in patient demand to reduce nursing stress and improve patient safety. Jt Comm J Qual Patient Saf. 2005;31(6):330–338.
- Hospital‐Based Emergency Care: At the Breaking Point. Washington, DC: Institute of Medicine Committee on the Future of Emergency Care in the United States Health System; 2006.
- , , , , . The effect of hospital occupancy on emergency department length of stay and patient disposition. Acad Emerg Med. 2003;10(2):127–133.
- . Interpreting the Volume‐Outcome Relationship in the Context of Health Care Quality: Workshop Summary. Washington, DC: National Academies Press; 2000.
- , , , , . Has recognition of the relationship between mortality rates and hospital volume for major cancer surgery in California made a difference? A follow‐up analysis of another decade. Ann Surg. 2009;250(3):472–483.
- , , , et al. Children's hospitals do not acutely respond to high occupancy. Pediatrics. 2010;125:974–981.
- , , , , , . Scheduled admissions and high occupancy at a children's hospital. J Hosp Med. 2011;6(2):81–87.
- , , . Characteristics of weekday and weekend hospital admissions. HCUP Statistical Brief. 2010;87.
- Agency for Healthcare Research and Quality. HCUP databases, Healthcare Cost and Utilization Project (HCUP); 2008. Available at: http://www.hcup‐us.ahrq.gov/kidoverview.jsp. Accessed July 15, 2009.
- , et al. Managing capacity to reduce emergency department overcrowding and ambulance diversions. J Qual Patient Saf. 2006;32(5):239–245.
- Institute for Healthcare Improvement. Flow initiatives; 2008. Available at: http://www.ihi.org/IHI/Topics/Flow. Accessed February 20, 2008.
- , , , , , . Trends in high‐turnover stays among children hospitalized in the United States, 1993–2003. Pediatrics. 2009;123(3):996–1002.
- Institute for Healthcare Improvement. Smoothing elective surgical admissions. Available at: http://www.ihi.org/IHI/Topics/Flow/PatientFlow/EmergingContent/SmoothingElectiveSurgicalAdmissions.htm. Accessed October 24, 2008.
- Boston hospital sees big impact from smoothing elective schedule. OR Manager. 2004;20:12.
- . Managing Variability in Patient Flow Is the Key to Improving Access to Care, Nursing Staffing, Quality of Care, and Reducing Its Cost. Paper presented at Institute of Medicine, Washington, DC; June 24, 2004.
- American Hospital Association Quality Center. Available at: http://www.ahaqualitycenter.org/ahaqualitycenter/. Accessed October 14, 2008.
- Healthcare Cost and Utilization Project (HCUP). Kids' Inpatient Database (KID); July 2008. Available at: http://www.hcup‐us.ahrq.gov/kidoverview.jsp. Accessed September 10, 2008.
- , , . Using a queuing model to help plan bed allocation in a department of geriatric medicine. Health Care Manag Sci. 2002;5(4):307–313.
- . How many hospital beds? Inquiry. 2002;39(4):400–412.
- . Institute for Healthcare Improvement. Patient flow comments. Available at: http://www.ihi.org/IHI/Topics/Flow. Accessed September 10, 2008.
- . Factory efficiency comes to the hospital. New York Times. July 9, 2010.
- Institute for Healthcare Improvement. Re‐engineering the operating room. Available at: http://www.ihi.org/IHI/Programs/ConferencesAndSeminars/ReengineeringtheOperatingRoomSept08.htm. Accessed November 8, 2008.
- , . Enhanced weekend service: an affordable means to increased hospital procedure volume. CMAJ. 2005;172(4):503–504.
- , . Mortality among patients admitted to hospitals on weekends as compared with weekdays. N Engl J Med. 2001;345:663–668.
- , , , , , . Weekend versus weekday admission and mortality from myocardial infarction. N Engl J Med. 2007;356:1099–1109.
- , . Waiting for urgent procedures on the weekend among emergently hospitalized patients. Am J Med. 2004;117:175–181.
- . Do hospitals provide lower quality care on weekends? Health Serv Res. 2007;42:1589–1612.
- . Hospital saves by working weekends. Mod Healthc. 1996;26:82–99.
- , , , , . Weekend and holiday exercise testing in patients with chest pain. J Gen Intern Med. 1999;14:10–14.
- . Boston Medical Center Case Study: Institute of Healthcare Optimization; 2006. Available at: http://www.ihoptimize.org/8f16e142‐eeaa‐4898–9e62–660218f19ffb/download.htm. Accessed October 3, 2010.
- , , , . The impact of IMPACT on St John's Regional Health Center. Mo Med. 2003;100:590–592.
- NYU Langone Medical Center Extends Access to Non‐Emergent Care as Part of Commitment to Patient‐Centered Care (June 23, 2010). Available at: http://communications.med.nyu.edu/news/2010/nyu‐langone‐medical‐center‐extends‐access‐non‐emergent‐care‐part‐commitment‐patient‐center. Accessed October 3, 2010.
- Carondelet St. Mary's Hospital. A pragmatic approach to improving patient efficiency throughput. Improvement Report 2005. Available at: http://www.ihi.org/IHI/Topics/Flow/PatientFlow/ImprovementStories/APragmaticApproachtoImprovingPatientEfficiencyThroughput.htm. Accessed October 3, 2010.
- AHA Solutions. Patient Flow Challenges Assessment 2009. Chicago, IL; 2009.
- , , , , , . A conceptual model of emergency department crowding. Ann Emerg Med. 2003;42(2):173–180.
- . Annual bed statistics give a misleading picture of hospital surge capacity. Ann Emerg Med. 2006;48(4):384–388.
Copyright © 2011 Society of Hospital Medicine
CCSVI Controversy: A Call for New Research
Multiple sclerosis patients and endovascular interventionalists were elated when Italian researchers reported in 2009 that they had found evidence of chronic cerebrospinal venous insufficiency in nearly every MS patient they had studied and that in many cases, balloon angioplasty and sometimes stent placement of central thoracic veins reduced or eliminated signs of the disease. Neurologists, on the other hand, suggested that hope might be eclipsing reason in the rush to advocate the vascular procedure, given the single-center study's small sample size and nonrandomized, uncontrolled design.
The opposing perspectives incited an apparent turf war within the MS community fueled by accusations on both sides, according to Dr. Jack Burks, chief medical officer of the Multiple Sclerosis Association of America. At issue, he said, is the validity not only of the study results but also of the underlying hypothesis that toxic iron overload in the brain due to chronic cerebrospinal venous insufficiency (CCSVI) might have a primary role in the pathogenesis of MS - a hypothesis that contradicts the compelling body of evidence suggesting that MS is primarily an autoimmune condition.
On one side of the debate are the MS patients and endovascular interventionalists, dubbed the "liberators" by Dr. Burks because of their unflappable advocacy for what has become known as the liberation procedure - the endovascular surgery designed to open the lesions causing the venous insufficiency, he said.
"Neurologists believe the interventionalists are overstating the possible value of CCSVI and that commercial interests are overriding scientific inquiry," according to Dr. Burks, a neurologist and clinical professor of medicine at the University of Nevada, Reno. Patients, armed with anecdotal evidence downloaded from the Internet, are certain that CCSVI surgery is the miracle they've been waiting for and perceive the hesitancy of U.S. and Canadian neurologists to embrace the treatment as evidence of a possible conspiracy with pharmaceutical companies who stand to lose billions of dollars if the surgery becomes a first-line treatment, he said. Further, he noted, advocates of CCSVI claim that neurologists who refuse patients' demands for diagnostic testing and surgical referral for CCSVI are jeopardizing the safety of those patients, who are traveling to foreign countries to get the care that they cannot receive in North America.
To date, the majority of the evidence regarding CCSVI diagnosis and treatment in MS is inconsistent, and can be confusing, Dr. Burks noted. In the initial study, Dr. Paolo Zamboni of the University of Ferrara in Italy, and colleagues, used Doppler ultrasound to examine venous drainage of the brain and spinal cord in 65 patients with different types of MS and 235 controls without MS and observed abnormal venous flow in all of the MS patients and none of the controls. The patterns of venous obstruction differed depending on MS stage and course, although there was no apparent relationship between disease severity and extent of venous obstruction, and MS treatment status did not influence the signs of CCSVI in any of the patients, the authors wrote (J. Neurol. Neurosurg. Psychiatry 2009;80:392-9).
The researchers went on to conduct an open pilot study to determine whether percutaneous transluminal angioplasty could safely and effectively treat the narrowing of the extracranial cerebrospinal veins in the 65 MS patients in which the condition was observed - 35 with relapsing-remitting MS, 20 with secondary progressive MS, and 10 with primary progressive MS. They reported significant improvements in MS clinical outcome measures, significant reductions in new brain lesions on MRI, and significant reductions in the number of relapses experienced by some of the patients.
The findings were limited, however, not only by the study design, but also by the fact that patients remained on their disease-modifying antirheumatic drug therapy during the study period and the timing and type of MRI scans varied among the patients, according to the authors. They also noted that restenosis of the internal jugular veins occurred in nearly half of the patients (J. Vasc. Surg. 2009;50:1348-58).
Since the initial paper, a number of CCSVI studies of various designs have been undertaken, with contradictory results. Following are some of the investigations reported within the past year:
P Researchers at the University of Buffalo found that up to 62% of the 280 patients with MS enrolled in the Combined Transcranial and Extracranial Venous Doppler Evaluation study - the first randomized clinical trial to evaluate MS patients for CCSVI - had the characteristic narrowing of the extracranial veins compared with approximately 22% of 220 healthy controls. While the results, which were reported at the annual meeting of the American Academy of Neurology, did not establish causation, they showed "that narrowing of the extracranial veins, at the very least, is an important association in multiple sclerosis," principal investigator Dr. Robert Zivadinov said in a statement. He acknowledged that the finding of vascular narrowing in nearly a quarter of the healthy controls warranted additional investigation (www.buffalo.edu/news/fast-execute.cgi/article-page.html?article=109370009).
P In an open-label study of extracranial Doppler criteria of CCSVI in 70 MS patients in Poland - 49 with relapsing-remitting MS, 5 with primary progressive MS, and 16 with secondary progressive MS - investigators detected at least two of four extracranial criteria in 90% of the patients. They concluded that, while the extracranial abnormalities could exist in various combinations, "the most common pathology in our patients was the presence of an inverted valve or another pathologic structure [like membranaceous or netlike septum] in the area of junction of the [internal jugular vein] with the brachiocephalic vein (Int. Angiol. 2010;29:109-14).
P A comparison of the internal jugular vein hemodynamics and morphology in 25 patients with MS and 25 controls identified abnormal findings in 92% of the MS patients and 24% of the controls, and evidence of CCSVI in 84% of the MS patients and none of the controls, leading the investigators to conclude that both hemodynamic abnormalities and morphologic changes in the internal jugular vein "are strongly associated with MS" (Int. Angiol. 2010;29:115-20).
P An extended extra- and transcranial color-coded sonography study in 56 MS patients and 20 controls detected no internal jugular vein stenosis and normal blood flow direction in all but 1 patient. There were no between-group differences in intracranial veins and during Valsalva maneuver, and none of the patients fulfilled more than one CCSVI criterion, according to the authors. They concluded that their findings "challenge the hypothesis that cerebral venous congestion plays a significant role in the pathogenesis of MS" (Ann. Neurol. 2010;68:173-83).
P Swedish investigators used phase-contrast MRI to study 21 relapsing-remitting MS patients and 20 healthy controls and found no differences in internal jugular venous outflow, aqueductal cerebrospinal fluid flow, or the presence of internal jugular blood reflux between the two groups. Although contrast-enhanced MR angiography showed internal jugular vein stenosis in 3 of the 21 MS patients, the authors stated they found no evidence "confirming the suggested vascular multiple sclerosis hypothesis" (Ann. Neurol. 2010;68:255-9).
P The authors of an MR venography and flow quantification study in The Netherlands compared the intracranial and extracranial venous anatomy and the intracerebral venous flow profiles of 20 MS patients and 20 age- and gender-matched controls, with image analysis performed by blinded interventional neuroradiologists. They identified venous system anomalies in 50% of the MS patients and 40% of the healthy controls and no venous backflow in either group. "Given the normal intracranial venous flow quantification results, it is likely that these findings reflect anatomical variants of venous drainage rather than clinically relevant venous outflow obstructions," the authors wrote (J. Neurol. Neurosurg. Psychiatry 2010 Oct. 27 [doi: 10.1136/jnnp.2010.223479]).
P Italian researchers investigating the occurrence of CCSVI in 50 consecutive patients with clinically isolated syndromes suggestive of MS reviewed the patients' extracranial and transcranial venous echo-color Doppler sonographs and compared the findings to those of 50 age- and gender-matched healthy controls as well as those of 60 patients with transient global amnesia (TGA) and 60 healthy controls matched to the TGA patients. They found extracranial Doppler sonographic abnormalities in 52% of patients with possible MS, 68.3% of patients with TGA, and 31.8% of the healthy controls. While eight of the patients with possible MS fulfilled the CCSVI criteria, selective phlebography showed no venous anomalies in seven of them. The authors concluded that there was no evidence of CCSVI at MS onset but recommended further studies to "clarify whether CCSVI is associated with later disease stages and characterizes the progressive forms of MS" (Ann. Neurol. 2011;69:90-9).
In a position statement, the Society of Interventional Radiology stated that at present, the published literature is "inconclusive on whether CCSVI is a clinically important factor and on whether balloon angioplasty and/or stent placement are effective in patients with MS" (J. Vasc. Interv. Radiol. 2010;21:1335-7).
Additionally, in a commentary on the treatment of CCSVI, representatives of the Cardiovascular and Interventional Radiological Society of Europe acknowledged that although several centers worldwide are promoting and performing balloon dilatation, with or without stenting for CCSVI, "We believe that until real scientific data are available for CCSVI and balloon dilatation, this treatment should not be offered to MS patients outside of a well designed clinical trial" (Cardiovasc. Intervent. Radiol. 2011;34:1-2).
Toward that end, the National Multiple Sclerosis Society of the United States and the MS Society of Canada have pledged $2.4 million in support of seven CCSVI research studies, including projects designed to evaluate venous abnormalities in children and teens with MS, patients with early and late stage MS, and those at risk for MS.
An international review panel comprising radiologists, vascular surgeons, and neurologists evaluated research applications via an expedited review process, according to the societies.
Dr. Burks disclosed relationships with various pharmaceutical companies. Dr. Baracchini had no relevant conflicts.
To say that the relationship between CCSVI and multiple sclerosis is controversial is an understatement. This article introduces both sides of the story and summarizes the current evidence. While many physicians remain understandably skeptical, it is worth remembering that "conventional wisdom" on the pathophysiology of some diseases has turned out to be way off the mark - consider peptic ulcers. Clearly, more objective data is needed before routine interventions for CCSVI can be recommended, and hopefully, ongoing registries and trials will provide that data. In the meantime, those of us involved with the vascular laboratory should be prepared to evaluate patients for the abnormalities described in CCSVI. Detection of reflux and obstruction in the internal jugular and vertebral veins by duplex ultrasound (in both supine and seated positions) is based on the same general principles as the more familiar upper extremity venous and carotid duplex examinations. Ideally, patients should be screened and entered into a clinical study to establish an evidence base for future decision-making. Until then, we will be stuck between the "liberators" and the "nihilists", and our patients will be caught in the middle.
Robert Eugene Zierler, M.D., is professor of surgery, division of vascular surgery, department of surgery, and medical director, Vascular Diagnostic Service, University of Washington School of Medicine, Seattle, Wash. He is also an associate medical editor for Vascular Specialist.
To say that the relationship between CCSVI and multiple sclerosis is controversial is an understatement. This article introduces both sides of the story and summarizes the current evidence. While many physicians remain understandably skeptical, it is worth remembering that "conventional wisdom" on the pathophysiology of some diseases has turned out to be way off the mark - consider peptic ulcers. Clearly, more objective data is needed before routine interventions for CCSVI can be recommended, and hopefully, ongoing registries and trials will provide that data. In the meantime, those of us involved with the vascular laboratory should be prepared to evaluate patients for the abnormalities described in CCSVI. Detection of reflux and obstruction in the internal jugular and vertebral veins by duplex ultrasound (in both supine and seated positions) is based on the same general principles as the more familiar upper extremity venous and carotid duplex examinations. Ideally, patients should be screened and entered into a clinical study to establish an evidence base for future decision-making. Until then, we will be stuck between the "liberators" and the "nihilists", and our patients will be caught in the middle.
Robert Eugene Zierler, M.D., is professor of surgery, division of vascular surgery, department of surgery, and medical director, Vascular Diagnostic Service, University of Washington School of Medicine, Seattle, Wash. He is also an associate medical editor for Vascular Specialist.
To say that the relationship between CCSVI and multiple sclerosis is controversial is an understatement. This article introduces both sides of the story and summarizes the current evidence. While many physicians remain understandably skeptical, it is worth remembering that "conventional wisdom" on the pathophysiology of some diseases has turned out to be way off the mark - consider peptic ulcers. Clearly, more objective data is needed before routine interventions for CCSVI can be recommended, and hopefully, ongoing registries and trials will provide that data. In the meantime, those of us involved with the vascular laboratory should be prepared to evaluate patients for the abnormalities described in CCSVI. Detection of reflux and obstruction in the internal jugular and vertebral veins by duplex ultrasound (in both supine and seated positions) is based on the same general principles as the more familiar upper extremity venous and carotid duplex examinations. Ideally, patients should be screened and entered into a clinical study to establish an evidence base for future decision-making. Until then, we will be stuck between the "liberators" and the "nihilists", and our patients will be caught in the middle.
Robert Eugene Zierler, M.D., is professor of surgery, division of vascular surgery, department of surgery, and medical director, Vascular Diagnostic Service, University of Washington School of Medicine, Seattle, Wash. He is also an associate medical editor for Vascular Specialist.
Multiple sclerosis patients and endovascular interventionalists were elated when Italian researchers reported in 2009 that they had found evidence of chronic cerebrospinal venous insufficiency in nearly every MS patient they had studied and that in many cases, balloon angioplasty and sometimes stent placement of central thoracic veins reduced or eliminated signs of the disease. Neurologists, on the other hand, suggested that hope might be eclipsing reason in the rush to advocate the vascular procedure, given the single-center study's small sample size and nonrandomized, uncontrolled design.
The opposing perspectives incited an apparent turf war within the MS community fueled by accusations on both sides, according to Dr. Jack Burks, chief medical officer of the Multiple Sclerosis Association of America. At issue, he said, is the validity not only of the study results but also of the underlying hypothesis that toxic iron overload in the brain due to chronic cerebrospinal venous insufficiency (CCSVI) might have a primary role in the pathogenesis of MS - a hypothesis that contradicts the compelling body of evidence suggesting that MS is primarily an autoimmune condition.
On one side of the debate are the MS patients and endovascular interventionalists, dubbed the "liberators" by Dr. Burks because of their unflappable advocacy for what has become known as the liberation procedure - the endovascular surgery designed to open the lesions causing the venous insufficiency, he said.
"Neurologists believe the interventionalists are overstating the possible value of CCSVI and that commercial interests are overriding scientific inquiry," according to Dr. Burks, a neurologist and clinical professor of medicine at the University of Nevada, Reno. Patients, armed with anecdotal evidence downloaded from the Internet, are certain that CCSVI surgery is the miracle they've been waiting for and perceive the hesitancy of U.S. and Canadian neurologists to embrace the treatment as evidence of a possible conspiracy with pharmaceutical companies who stand to lose billions of dollars if the surgery becomes a first-line treatment, he said. Further, he noted, advocates of CCSVI claim that neurologists who refuse patients' demands for diagnostic testing and surgical referral for CCSVI are jeopardizing the safety of those patients, who are traveling to foreign countries to get the care that they cannot receive in North America.
To date, the majority of the evidence regarding CCSVI diagnosis and treatment in MS is inconsistent, and can be confusing, Dr. Burks noted. In the initial study, Dr. Paolo Zamboni of the University of Ferrara in Italy, and colleagues, used Doppler ultrasound to examine venous drainage of the brain and spinal cord in 65 patients with different types of MS and 235 controls without MS and observed abnormal venous flow in all of the MS patients and none of the controls. The patterns of venous obstruction differed depending on MS stage and course, although there was no apparent relationship between disease severity and extent of venous obstruction, and MS treatment status did not influence the signs of CCSVI in any of the patients, the authors wrote (J. Neurol. Neurosurg. Psychiatry 2009;80:392-9).
The researchers went on to conduct an open pilot study to determine whether percutaneous transluminal angioplasty could safely and effectively treat the narrowing of the extracranial cerebrospinal veins in the 65 MS patients in which the condition was observed - 35 with relapsing-remitting MS, 20 with secondary progressive MS, and 10 with primary progressive MS. They reported significant improvements in MS clinical outcome measures, significant reductions in new brain lesions on MRI, and significant reductions in the number of relapses experienced by some of the patients.
The findings were limited, however, not only by the study design, but also by the fact that patients remained on their disease-modifying antirheumatic drug therapy during the study period and the timing and type of MRI scans varied among the patients, according to the authors. They also noted that restenosis of the internal jugular veins occurred in nearly half of the patients (J. Vasc. Surg. 2009;50:1348-58).
Since the initial paper, a number of CCSVI studies of various designs have been undertaken, with contradictory results. Following are some of the investigations reported within the past year:
P Researchers at the University of Buffalo found that up to 62% of the 280 patients with MS enrolled in the Combined Transcranial and Extracranial Venous Doppler Evaluation study - the first randomized clinical trial to evaluate MS patients for CCSVI - had the characteristic narrowing of the extracranial veins compared with approximately 22% of 220 healthy controls. While the results, which were reported at the annual meeting of the American Academy of Neurology, did not establish causation, they showed "that narrowing of the extracranial veins, at the very least, is an important association in multiple sclerosis," principal investigator Dr. Robert Zivadinov said in a statement. He acknowledged that the finding of vascular narrowing in nearly a quarter of the healthy controls warranted additional investigation (www.buffalo.edu/news/fast-execute.cgi/article-page.html?article=109370009).
P In an open-label study of extracranial Doppler criteria of CCSVI in 70 MS patients in Poland - 49 with relapsing-remitting MS, 5 with primary progressive MS, and 16 with secondary progressive MS - investigators detected at least two of four extracranial criteria in 90% of the patients. They concluded that, while the extracranial abnormalities could exist in various combinations, "the most common pathology in our patients was the presence of an inverted valve or another pathologic structure [like membranaceous or netlike septum] in the area of junction of the [internal jugular vein] with the brachiocephalic vein (Int. Angiol. 2010;29:109-14).
P A comparison of the internal jugular vein hemodynamics and morphology in 25 patients with MS and 25 controls identified abnormal findings in 92% of the MS patients and 24% of the controls, and evidence of CCSVI in 84% of the MS patients and none of the controls, leading the investigators to conclude that both hemodynamic abnormalities and morphologic changes in the internal jugular vein "are strongly associated with MS" (Int. Angiol. 2010;29:115-20).
P An extended extra- and transcranial color-coded sonography study in 56 MS patients and 20 controls detected no internal jugular vein stenosis and normal blood flow direction in all but 1 patient. There were no between-group differences in intracranial veins and during Valsalva maneuver, and none of the patients fulfilled more than one CCSVI criterion, according to the authors. They concluded that their findings "challenge the hypothesis that cerebral venous congestion plays a significant role in the pathogenesis of MS" (Ann. Neurol. 2010;68:173-83).
P Swedish investigators used phase-contrast MRI to study 21 relapsing-remitting MS patients and 20 healthy controls and found no differences in internal jugular venous outflow, aqueductal cerebrospinal fluid flow, or the presence of internal jugular blood reflux between the two groups. Although contrast-enhanced MR angiography showed internal jugular vein stenosis in 3 of the 21 MS patients, the authors stated they found no evidence "confirming the suggested vascular multiple sclerosis hypothesis" (Ann. Neurol. 2010;68:255-9).
P The authors of an MR venography and flow quantification study in The Netherlands compared the intracranial and extracranial venous anatomy and the intracerebral venous flow profiles of 20 MS patients and 20 age- and gender-matched controls, with image analysis performed by blinded interventional neuroradiologists. They identified venous system anomalies in 50% of the MS patients and 40% of the healthy controls and no venous backflow in either group. "Given the normal intracranial venous flow quantification results, it is likely that these findings reflect anatomical variants of venous drainage rather than clinically relevant venous outflow obstructions," the authors wrote (J. Neurol. Neurosurg. Psychiatry 2010 Oct. 27 [doi: 10.1136/jnnp.2010.223479]).
P Italian researchers investigating the occurrence of CCSVI in 50 consecutive patients with clinically isolated syndromes suggestive of MS reviewed the patients' extracranial and transcranial venous echo-color Doppler sonographs and compared the findings to those of 50 age- and gender-matched healthy controls as well as those of 60 patients with transient global amnesia (TGA) and 60 healthy controls matched to the TGA patients. They found extracranial Doppler sonographic abnormalities in 52% of patients with possible MS, 68.3% of patients with TGA, and 31.8% of the healthy controls. While eight of the patients with possible MS fulfilled the CCSVI criteria, selective phlebography showed no venous anomalies in seven of them. The authors concluded that there was no evidence of CCSVI at MS onset but recommended further studies to "clarify whether CCSVI is associated with later disease stages and characterizes the progressive forms of MS" (Ann. Neurol. 2011;69:90-9).
In a position statement, the Society of Interventional Radiology stated that at present, the published literature is "inconclusive on whether CCSVI is a clinically important factor and on whether balloon angioplasty and/or stent placement are effective in patients with MS" (J. Vasc. Interv. Radiol. 2010;21:1335-7).
Additionally, in a commentary on the treatment of CCSVI, representatives of the Cardiovascular and Interventional Radiological Society of Europe acknowledged that although several centers worldwide are promoting and performing balloon dilatation, with or without stenting for CCSVI, "We believe that until real scientific data are available for CCSVI and balloon dilatation, this treatment should not be offered to MS patients outside of a well designed clinical trial" (Cardiovasc. Intervent. Radiol. 2011;34:1-2).
Toward that end, the National Multiple Sclerosis Society of the United States and the MS Society of Canada have pledged $2.4 million in support of seven CCSVI research studies, including projects designed to evaluate venous abnormalities in children and teens with MS, patients with early and late stage MS, and those at risk for MS.
An international review panel comprising radiologists, vascular surgeons, and neurologists evaluated research applications via an expedited review process, according to the societies.
Dr. Burks disclosed relationships with various pharmaceutical companies. Dr. Baracchini had no relevant conflicts.
Multiple sclerosis patients and endovascular interventionalists were elated when Italian researchers reported in 2009 that they had found evidence of chronic cerebrospinal venous insufficiency in nearly every MS patient they had studied and that in many cases, balloon angioplasty and sometimes stent placement of central thoracic veins reduced or eliminated signs of the disease. Neurologists, on the other hand, suggested that hope might be eclipsing reason in the rush to advocate the vascular procedure, given the single-center study's small sample size and nonrandomized, uncontrolled design.
The opposing perspectives incited an apparent turf war within the MS community fueled by accusations on both sides, according to Dr. Jack Burks, chief medical officer of the Multiple Sclerosis Association of America. At issue, he said, is the validity not only of the study results but also of the underlying hypothesis that toxic iron overload in the brain due to chronic cerebrospinal venous insufficiency (CCSVI) might have a primary role in the pathogenesis of MS - a hypothesis that contradicts the compelling body of evidence suggesting that MS is primarily an autoimmune condition.
On one side of the debate are the MS patients and endovascular interventionalists, dubbed the "liberators" by Dr. Burks because of their unflappable advocacy for what has become known as the liberation procedure - the endovascular surgery designed to open the lesions causing the venous insufficiency, he said.
"Neurologists believe the interventionalists are overstating the possible value of CCSVI and that commercial interests are overriding scientific inquiry," according to Dr. Burks, a neurologist and clinical professor of medicine at the University of Nevada, Reno. Patients, armed with anecdotal evidence downloaded from the Internet, are certain that CCSVI surgery is the miracle they've been waiting for and perceive the hesitancy of U.S. and Canadian neurologists to embrace the treatment as evidence of a possible conspiracy with pharmaceutical companies who stand to lose billions of dollars if the surgery becomes a first-line treatment, he said. Further, he noted, advocates of CCSVI claim that neurologists who refuse patients' demands for diagnostic testing and surgical referral for CCSVI are jeopardizing the safety of those patients, who are traveling to foreign countries to get the care that they cannot receive in North America.
To date, the majority of the evidence regarding CCSVI diagnosis and treatment in MS is inconsistent, and can be confusing, Dr. Burks noted. In the initial study, Dr. Paolo Zamboni of the University of Ferrara in Italy, and colleagues, used Doppler ultrasound to examine venous drainage of the brain and spinal cord in 65 patients with different types of MS and 235 controls without MS and observed abnormal venous flow in all of the MS patients and none of the controls. The patterns of venous obstruction differed depending on MS stage and course, although there was no apparent relationship between disease severity and extent of venous obstruction, and MS treatment status did not influence the signs of CCSVI in any of the patients, the authors wrote (J. Neurol. Neurosurg. Psychiatry 2009;80:392-9).
The researchers went on to conduct an open pilot study to determine whether percutaneous transluminal angioplasty could safely and effectively treat the narrowing of the extracranial cerebrospinal veins in the 65 MS patients in which the condition was observed - 35 with relapsing-remitting MS, 20 with secondary progressive MS, and 10 with primary progressive MS. They reported significant improvements in MS clinical outcome measures, significant reductions in new brain lesions on MRI, and significant reductions in the number of relapses experienced by some of the patients.
The findings were limited, however, not only by the study design, but also by the fact that patients remained on their disease-modifying antirheumatic drug therapy during the study period and the timing and type of MRI scans varied among the patients, according to the authors. They also noted that restenosis of the internal jugular veins occurred in nearly half of the patients (J. Vasc. Surg. 2009;50:1348-58).
Since the initial paper, a number of CCSVI studies of various designs have been undertaken, with contradictory results. Following are some of the investigations reported within the past year:
P Researchers at the University of Buffalo found that up to 62% of the 280 patients with MS enrolled in the Combined Transcranial and Extracranial Venous Doppler Evaluation study - the first randomized clinical trial to evaluate MS patients for CCSVI - had the characteristic narrowing of the extracranial veins compared with approximately 22% of 220 healthy controls. While the results, which were reported at the annual meeting of the American Academy of Neurology, did not establish causation, they showed "that narrowing of the extracranial veins, at the very least, is an important association in multiple sclerosis," principal investigator Dr. Robert Zivadinov said in a statement. He acknowledged that the finding of vascular narrowing in nearly a quarter of the healthy controls warranted additional investigation (www.buffalo.edu/news/fast-execute.cgi/article-page.html?article=109370009).
P In an open-label study of extracranial Doppler criteria of CCSVI in 70 MS patients in Poland - 49 with relapsing-remitting MS, 5 with primary progressive MS, and 16 with secondary progressive MS - investigators detected at least two of four extracranial criteria in 90% of the patients. They concluded that, while the extracranial abnormalities could exist in various combinations, "the most common pathology in our patients was the presence of an inverted valve or another pathologic structure [like membranaceous or netlike septum] in the area of junction of the [internal jugular vein] with the brachiocephalic vein (Int. Angiol. 2010;29:109-14).
P A comparison of the internal jugular vein hemodynamics and morphology in 25 patients with MS and 25 controls identified abnormal findings in 92% of the MS patients and 24% of the controls, and evidence of CCSVI in 84% of the MS patients and none of the controls, leading the investigators to conclude that both hemodynamic abnormalities and morphologic changes in the internal jugular vein "are strongly associated with MS" (Int. Angiol. 2010;29:115-20).
P An extended extra- and transcranial color-coded sonography study in 56 MS patients and 20 controls detected no internal jugular vein stenosis and normal blood flow direction in all but 1 patient. There were no between-group differences in intracranial veins and during Valsalva maneuver, and none of the patients fulfilled more than one CCSVI criterion, according to the authors. They concluded that their findings "challenge the hypothesis that cerebral venous congestion plays a significant role in the pathogenesis of MS" (Ann. Neurol. 2010;68:173-83).
P Swedish investigators used phase-contrast MRI to study 21 relapsing-remitting MS patients and 20 healthy controls and found no differences in internal jugular venous outflow, aqueductal cerebrospinal fluid flow, or the presence of internal jugular blood reflux between the two groups. Although contrast-enhanced MR angiography showed internal jugular vein stenosis in 3 of the 21 MS patients, the authors stated they found no evidence "confirming the suggested vascular multiple sclerosis hypothesis" (Ann. Neurol. 2010;68:255-9).
P The authors of an MR venography and flow quantification study in The Netherlands compared the intracranial and extracranial venous anatomy and the intracerebral venous flow profiles of 20 MS patients and 20 age- and gender-matched controls, with image analysis performed by blinded interventional neuroradiologists. They identified venous system anomalies in 50% of the MS patients and 40% of the healthy controls and no venous backflow in either group. "Given the normal intracranial venous flow quantification results, it is likely that these findings reflect anatomical variants of venous drainage rather than clinically relevant venous outflow obstructions," the authors wrote (J. Neurol. Neurosurg. Psychiatry 2010 Oct. 27 [doi: 10.1136/jnnp.2010.223479]).
P Italian researchers investigating the occurrence of CCSVI in 50 consecutive patients with clinically isolated syndromes suggestive of MS reviewed the patients' extracranial and transcranial venous echo-color Doppler sonographs and compared the findings to those of 50 age- and gender-matched healthy controls as well as those of 60 patients with transient global amnesia (TGA) and 60 healthy controls matched to the TGA patients. They found extracranial Doppler sonographic abnormalities in 52% of patients with possible MS, 68.3% of patients with TGA, and 31.8% of the healthy controls. While eight of the patients with possible MS fulfilled the CCSVI criteria, selective phlebography showed no venous anomalies in seven of them. The authors concluded that there was no evidence of CCSVI at MS onset but recommended further studies to "clarify whether CCSVI is associated with later disease stages and characterizes the progressive forms of MS" (Ann. Neurol. 2011;69:90-9).
In a position statement, the Society of Interventional Radiology stated that at present, the published literature is "inconclusive on whether CCSVI is a clinically important factor and on whether balloon angioplasty and/or stent placement are effective in patients with MS" (J. Vasc. Interv. Radiol. 2010;21:1335-7).
Additionally, in a commentary on the treatment of CCSVI, representatives of the Cardiovascular and Interventional Radiological Society of Europe acknowledged that although several centers worldwide are promoting and performing balloon dilatation, with or without stenting for CCSVI, "We believe that until real scientific data are available for CCSVI and balloon dilatation, this treatment should not be offered to MS patients outside of a well designed clinical trial" (Cardiovasc. Intervent. Radiol. 2011;34:1-2).
Toward that end, the National Multiple Sclerosis Society of the United States and the MS Society of Canada have pledged $2.4 million in support of seven CCSVI research studies, including projects designed to evaluate venous abnormalities in children and teens with MS, patients with early and late stage MS, and those at risk for MS.
An international review panel comprising radiologists, vascular surgeons, and neurologists evaluated research applications via an expedited review process, according to the societies.
Dr. Burks disclosed relationships with various pharmaceutical companies. Dr. Baracchini had no relevant conflicts.
FDA Panel Divided on Trilipix Labeling Changes
SILVER SPRING, MD. — Members of a Food and Drug Administration advisory panel were divided on whether the approved indication for fenofibric acid, when co-administered with a statin, should be revised based on trial results showing no benefit of the drug in reducing cardiovascular disease risk when added to a statin in patients with type 2 diabetes.
At a meeting May 19, 6 of the 13 members of the FDA’s Endocrinologic and Metabolic Drugs Advisory Committee agreed that the marketing of fenofibric acid should be allowed to continue, with the addition to the label of the main findings of the study – the Action to Control Cardiovascular Risk in Diabetes (ACCORD) Lipid trial. Four panelists, however, voted to recommend that the co-administration indication be withdrawn, citing the lack of solid evidence to support the indication. The remaining three panelists voted to allow continued marketing with no changes to the label.
Fenofibric acid, marketed by Abbott Laboratories as Trilipix, was approved by the FDA in December 2008. The drug’s indication includes its use with a statin as an adjunct to diet to reduce serum triglyceride (TG) levels and increase serum HDL cholesterol levels in patients with mixed dyslipidemia and coronary heart disease (CHD) or a CHD risk equivalent who are on "optimal statin therapy" to achieve their LDL cholesterol goal. Fenofibric acid is the active ingredient of fenofibrate, a fibrate approved in 1993 that is now available in generic formulations.
The FDA convened the panel to review the co-administration indication of fenofibric acid in the context of the ACCORD Lipid study results. That study found no benefit of the combination treatment on major cardiovascular events, compared with treatment with simvastatin alone, over a mean of almost 5 years of follow-up. The study enrolled more than 5,500 patients with type 2 diabetes at high risk of cardiovascular disease, with a range of TG and HDL cholesterol levels. Among women in the study, the rate of major adverse cardiovascular events was higher than that among those on the statin alone. But in another subgroup of patients, those with elevated TG levels (204 mg/dL or higher) and reduced HDL cholesterol levels (34 mg/dL or lower), there was a suggestion of benefit with combination therapy (N. Engl. J. Med. 2010;362:1563-74).
Panelists at the meeting said they were not comfortable drawing any conclusions from analyses of patient subgroups in a negative trial. They voted 13-0 that the FDA should require Abbott to conduct a study to test the hypothesis that add-on therapy with fenofibric acid, compared with placebo, significantly lowers the risk of major adverse cardiovascular events in high-risk patients who have reached their LDL cholesterol goal with a statin but have residual high serum TG and low serum HDL cholesterol levels.
The ACCORD Lipid study was sponsored by the National Heart, Lung and Blood Institute.
The FDA usually follows the recommendations of its advisory panels. Members of FDA advisory panels have been cleared of potential conflicts of interest related to the topic of the meeting; occasionally, they may be given a waiver, but not at this meeting.
SILVER SPRING, MD. — Members of a Food and Drug Administration advisory panel were divided on whether the approved indication for fenofibric acid, when co-administered with a statin, should be revised based on trial results showing no benefit of the drug in reducing cardiovascular disease risk when added to a statin in patients with type 2 diabetes.
At a meeting May 19, 6 of the 13 members of the FDA’s Endocrinologic and Metabolic Drugs Advisory Committee agreed that the marketing of fenofibric acid should be allowed to continue, with the addition to the label of the main findings of the study – the Action to Control Cardiovascular Risk in Diabetes (ACCORD) Lipid trial. Four panelists, however, voted to recommend that the co-administration indication be withdrawn, citing the lack of solid evidence to support the indication. The remaining three panelists voted to allow continued marketing with no changes to the label.
Fenofibric acid, marketed by Abbott Laboratories as Trilipix, was approved by the FDA in December 2008. The drug’s indication includes its use with a statin as an adjunct to diet to reduce serum triglyceride (TG) levels and increase serum HDL cholesterol levels in patients with mixed dyslipidemia and coronary heart disease (CHD) or a CHD risk equivalent who are on "optimal statin therapy" to achieve their LDL cholesterol goal. Fenofibric acid is the active ingredient of fenofibrate, a fibrate approved in 1993 that is now available in generic formulations.
The FDA convened the panel to review the co-administration indication of fenofibric acid in the context of the ACCORD Lipid study results. That study found no benefit of the combination treatment on major cardiovascular events, compared with treatment with simvastatin alone, over a mean of almost 5 years of follow-up. The study enrolled more than 5,500 patients with type 2 diabetes at high risk of cardiovascular disease, with a range of TG and HDL cholesterol levels. Among women in the study, the rate of major adverse cardiovascular events was higher than that among those on the statin alone. But in another subgroup of patients, those with elevated TG levels (204 mg/dL or higher) and reduced HDL cholesterol levels (34 mg/dL or lower), there was a suggestion of benefit with combination therapy (N. Engl. J. Med. 2010;362:1563-74).
Panelists at the meeting said they were not comfortable drawing any conclusions from analyses of patient subgroups in a negative trial. They voted 13-0 that the FDA should require Abbott to conduct a study to test the hypothesis that add-on therapy with fenofibric acid, compared with placebo, significantly lowers the risk of major adverse cardiovascular events in high-risk patients who have reached their LDL cholesterol goal with a statin but have residual high serum TG and low serum HDL cholesterol levels.
The ACCORD Lipid study was sponsored by the National Heart, Lung and Blood Institute.
The FDA usually follows the recommendations of its advisory panels. Members of FDA advisory panels have been cleared of potential conflicts of interest related to the topic of the meeting; occasionally, they may be given a waiver, but not at this meeting.
SILVER SPRING, MD. — Members of a Food and Drug Administration advisory panel were divided on whether the approved indication for fenofibric acid, when co-administered with a statin, should be revised based on trial results showing no benefit of the drug in reducing cardiovascular disease risk when added to a statin in patients with type 2 diabetes.
At a meeting May 19, 6 of the 13 members of the FDA’s Endocrinologic and Metabolic Drugs Advisory Committee agreed that the marketing of fenofibric acid should be allowed to continue, with the addition to the label of the main findings of the study – the Action to Control Cardiovascular Risk in Diabetes (ACCORD) Lipid trial. Four panelists, however, voted to recommend that the co-administration indication be withdrawn, citing the lack of solid evidence to support the indication. The remaining three panelists voted to allow continued marketing with no changes to the label.
Fenofibric acid, marketed by Abbott Laboratories as Trilipix, was approved by the FDA in December 2008. The drug’s indication includes its use with a statin as an adjunct to diet to reduce serum triglyceride (TG) levels and increase serum HDL cholesterol levels in patients with mixed dyslipidemia and coronary heart disease (CHD) or a CHD risk equivalent who are on "optimal statin therapy" to achieve their LDL cholesterol goal. Fenofibric acid is the active ingredient of fenofibrate, a fibrate approved in 1993 that is now available in generic formulations.
The FDA convened the panel to review the co-administration indication of fenofibric acid in the context of the ACCORD Lipid study results. That study found no benefit of the combination treatment on major cardiovascular events, compared with treatment with simvastatin alone, over a mean of almost 5 years of follow-up. The study enrolled more than 5,500 patients with type 2 diabetes at high risk of cardiovascular disease, with a range of TG and HDL cholesterol levels. Among women in the study, the rate of major adverse cardiovascular events was higher than that among those on the statin alone. But in another subgroup of patients, those with elevated TG levels (204 mg/dL or higher) and reduced HDL cholesterol levels (34 mg/dL or lower), there was a suggestion of benefit with combination therapy (N. Engl. J. Med. 2010;362:1563-74).
Panelists at the meeting said they were not comfortable drawing any conclusions from analyses of patient subgroups in a negative trial. They voted 13-0 that the FDA should require Abbott to conduct a study to test the hypothesis that add-on therapy with fenofibric acid, compared with placebo, significantly lowers the risk of major adverse cardiovascular events in high-risk patients who have reached their LDL cholesterol goal with a statin but have residual high serum TG and low serum HDL cholesterol levels.
The ACCORD Lipid study was sponsored by the National Heart, Lung and Blood Institute.
The FDA usually follows the recommendations of its advisory panels. Members of FDA advisory panels have been cleared of potential conflicts of interest related to the topic of the meeting; occasionally, they may be given a waiver, but not at this meeting.
FROM A MEETING OF THE FDA'S ENDOCRINOLOGIC AND METABOLIC DRUGS ADVISORY PANEL
Nonclinical Skills Essential to Successful HM Career
Being a good hospitalist means more than being an adroit clinician. But when it comes to teaching nonclinical aptitude, traditional residency programs often come up short, says Russell Holman, MD, MHM, former SHM president and chief clinical officer of Brentwood, Tenn.-based Cogent Healthcare. He and other HM experts recommend that boots-on-the-ground hospitalists acquire the following nonclinical skills in a purposeful manner as part of their ongoing learning and long-term career goals.
Communication
Hospitalists are the liaison between hospital administrators, managed-care companies, case managers, patients, patients’ families, and primary-care physicians (PCPs), says Isela Sotolongo, executive director of the Southeast region for North Hollywood, Calif.-based IPC: The Hospitalist Company. “A lot of times, more time is spent communicating with all the individuals that are involved in the patient’s admission than is actually spent with the patient,” she says.

—Isela Sotolongo, executive director, Southeast region, IPC: The Hospitalist Company, North Hollywood, Calif.
Hospitalists must quickly establish a relationship with patients and their families; manage specialists and ancillary personnel; brief a PCP on a patient’s needs after discharge; update case managers on a course of hospitalization; and demonstrate effective, efficient patient care to hospital administrators. Being able to express such information in a concise manner that is understood by others will reduce errors and save time and effort, Dr. Holman and Sotolongo say.
Quality Improvement (QI)
An awareness and working knowledge of QI methodologies should be part of a hospitalist’s everyday professional life, Dr. Holman says. QI helps reduce unwanted variation in patient care and enhances the process and outcomes of such care.
“Hospitalists are almost always looked to as being a linchpin in the hospital to improving quality,” he says. Therefore, while QI might not be a hospitalist’s special interest, it is still good to know the basics and how to apply them to actual HM practice, Dr. Holman says.
Leadership
“Even if a hospitalist is not serving as medical director of their hospital medicine group, they are considered to be a leader in their own right,” Dr. Holman says.
A hospitalist takes charge of the admission process and is the person who manages all aspects of a patient’s care throughout the hospital stay to the point of discharge, Sotolongo says. (Click here to listen to Isela Sotolongo discuss hospitalist leadership and communication in detail.)
Also, a hospitalist might be leading and participating in QI activities at the hospital or leading the discussion for a multidisciplinary team, Dr. Holman notes. Having leadership skills helps a hospitalist appreciate the work their medical director must do, or the work that hospital administrators are trying to accomplish, he says.
Teamwork
Teamwork is an essential skill for hospitalists, yet traditional medical training often teaches doctors to be more of a rugged individualist, Dr. Holman says. Hospitalists should make it a point to adopt new behaviors that are teamwork-friendly:
- Value other members of the healthcare team;
- Direct people’s talents to their highest and best use; and
- Delegate tasks to those who are best suited to accomplish those particular needs.
“Teamwork tends to place much more emphasis on the patient being the center of care and others on the healthcare team going to support those patient-centered interests,” Dr. Holman say.
The Patient Perspective
How a patient moves through the healthcare system in theory is often different from reality. A hospitalist should physically follow some of their patients as they move from the hospital back to their caregivers at home or to a rehabilitation center, nursing home, or other facility in order to watch the transition, says Jasen Gundersen, MD, MBA, CPE, SFHM, chief medical officer of the hospital medicine division in Fort Lauderdale, Fla., for Knoxville, Tenn.-based TeamHealth. By doing this, a hospitalist can see where problems arise during handoffs and how to resolve them so that hospital readmissions are reduced.
Teaching
Teaching skills can be applicable in any hospital environment, Dr. Holman adds. Hospitalists who work in nonteaching hospitals often serve as teachers to nursing staff, case management, pharmacists, discharge planners, and other ancillary staff in their daily interaction or over lunchtime educational programs. HM also can provide the bulk of a hospital’s grand rounds or other departmental educational sessions, he says.
“Let's not forget about patients and caregivers,” Dr. Holman adds. “The ability to teach patients and caregivers the necessary information and skills that they need for self-care, for follow-up care, and for compliance to a medical plan is important.”
Professional Evaluation
A hospitalist has to know on which metrics and measurements their success will be based, Dr. Holman says. Will it be patient satisfaction, length of stay, readmission rates, mortality rates, or some other measure?
“We have to be aware of what the hospital’s needs are, what the hospital’s key points are that they look for,” Sotolongo says.
Once the metrics are clear, a hospitalist can set goals to accomplish individually, as a member of an HM group, and partner with the hospital, Dr. Holman says.
Lisa Ryan is a freelance writer based in New Jersey.
Being a good hospitalist means more than being an adroit clinician. But when it comes to teaching nonclinical aptitude, traditional residency programs often come up short, says Russell Holman, MD, MHM, former SHM president and chief clinical officer of Brentwood, Tenn.-based Cogent Healthcare. He and other HM experts recommend that boots-on-the-ground hospitalists acquire the following nonclinical skills in a purposeful manner as part of their ongoing learning and long-term career goals.
Communication
Hospitalists are the liaison between hospital administrators, managed-care companies, case managers, patients, patients’ families, and primary-care physicians (PCPs), says Isela Sotolongo, executive director of the Southeast region for North Hollywood, Calif.-based IPC: The Hospitalist Company. “A lot of times, more time is spent communicating with all the individuals that are involved in the patient’s admission than is actually spent with the patient,” she says.

—Isela Sotolongo, executive director, Southeast region, IPC: The Hospitalist Company, North Hollywood, Calif.
Hospitalists must quickly establish a relationship with patients and their families; manage specialists and ancillary personnel; brief a PCP on a patient’s needs after discharge; update case managers on a course of hospitalization; and demonstrate effective, efficient patient care to hospital administrators. Being able to express such information in a concise manner that is understood by others will reduce errors and save time and effort, Dr. Holman and Sotolongo say.
Quality Improvement (QI)
An awareness and working knowledge of QI methodologies should be part of a hospitalist’s everyday professional life, Dr. Holman says. QI helps reduce unwanted variation in patient care and enhances the process and outcomes of such care.
“Hospitalists are almost always looked to as being a linchpin in the hospital to improving quality,” he says. Therefore, while QI might not be a hospitalist’s special interest, it is still good to know the basics and how to apply them to actual HM practice, Dr. Holman says.
Leadership
“Even if a hospitalist is not serving as medical director of their hospital medicine group, they are considered to be a leader in their own right,” Dr. Holman says.
A hospitalist takes charge of the admission process and is the person who manages all aspects of a patient’s care throughout the hospital stay to the point of discharge, Sotolongo says. (Click here to listen to Isela Sotolongo discuss hospitalist leadership and communication in detail.)
Also, a hospitalist might be leading and participating in QI activities at the hospital or leading the discussion for a multidisciplinary team, Dr. Holman notes. Having leadership skills helps a hospitalist appreciate the work their medical director must do, or the work that hospital administrators are trying to accomplish, he says.
Teamwork
Teamwork is an essential skill for hospitalists, yet traditional medical training often teaches doctors to be more of a rugged individualist, Dr. Holman says. Hospitalists should make it a point to adopt new behaviors that are teamwork-friendly:
- Value other members of the healthcare team;
- Direct people’s talents to their highest and best use; and
- Delegate tasks to those who are best suited to accomplish those particular needs.
“Teamwork tends to place much more emphasis on the patient being the center of care and others on the healthcare team going to support those patient-centered interests,” Dr. Holman say.
The Patient Perspective
How a patient moves through the healthcare system in theory is often different from reality. A hospitalist should physically follow some of their patients as they move from the hospital back to their caregivers at home or to a rehabilitation center, nursing home, or other facility in order to watch the transition, says Jasen Gundersen, MD, MBA, CPE, SFHM, chief medical officer of the hospital medicine division in Fort Lauderdale, Fla., for Knoxville, Tenn.-based TeamHealth. By doing this, a hospitalist can see where problems arise during handoffs and how to resolve them so that hospital readmissions are reduced.
Teaching
Teaching skills can be applicable in any hospital environment, Dr. Holman adds. Hospitalists who work in nonteaching hospitals often serve as teachers to nursing staff, case management, pharmacists, discharge planners, and other ancillary staff in their daily interaction or over lunchtime educational programs. HM also can provide the bulk of a hospital’s grand rounds or other departmental educational sessions, he says.
“Let's not forget about patients and caregivers,” Dr. Holman adds. “The ability to teach patients and caregivers the necessary information and skills that they need for self-care, for follow-up care, and for compliance to a medical plan is important.”
Professional Evaluation
A hospitalist has to know on which metrics and measurements their success will be based, Dr. Holman says. Will it be patient satisfaction, length of stay, readmission rates, mortality rates, or some other measure?
“We have to be aware of what the hospital’s needs are, what the hospital’s key points are that they look for,” Sotolongo says.
Once the metrics are clear, a hospitalist can set goals to accomplish individually, as a member of an HM group, and partner with the hospital, Dr. Holman says.
Lisa Ryan is a freelance writer based in New Jersey.
Being a good hospitalist means more than being an adroit clinician. But when it comes to teaching nonclinical aptitude, traditional residency programs often come up short, says Russell Holman, MD, MHM, former SHM president and chief clinical officer of Brentwood, Tenn.-based Cogent Healthcare. He and other HM experts recommend that boots-on-the-ground hospitalists acquire the following nonclinical skills in a purposeful manner as part of their ongoing learning and long-term career goals.
Communication
Hospitalists are the liaison between hospital administrators, managed-care companies, case managers, patients, patients’ families, and primary-care physicians (PCPs), says Isela Sotolongo, executive director of the Southeast region for North Hollywood, Calif.-based IPC: The Hospitalist Company. “A lot of times, more time is spent communicating with all the individuals that are involved in the patient’s admission than is actually spent with the patient,” she says.

—Isela Sotolongo, executive director, Southeast region, IPC: The Hospitalist Company, North Hollywood, Calif.
Hospitalists must quickly establish a relationship with patients and their families; manage specialists and ancillary personnel; brief a PCP on a patient’s needs after discharge; update case managers on a course of hospitalization; and demonstrate effective, efficient patient care to hospital administrators. Being able to express such information in a concise manner that is understood by others will reduce errors and save time and effort, Dr. Holman and Sotolongo say.
Quality Improvement (QI)
An awareness and working knowledge of QI methodologies should be part of a hospitalist’s everyday professional life, Dr. Holman says. QI helps reduce unwanted variation in patient care and enhances the process and outcomes of such care.
“Hospitalists are almost always looked to as being a linchpin in the hospital to improving quality,” he says. Therefore, while QI might not be a hospitalist’s special interest, it is still good to know the basics and how to apply them to actual HM practice, Dr. Holman says.
Leadership
“Even if a hospitalist is not serving as medical director of their hospital medicine group, they are considered to be a leader in their own right,” Dr. Holman says.
A hospitalist takes charge of the admission process and is the person who manages all aspects of a patient’s care throughout the hospital stay to the point of discharge, Sotolongo says. (Click here to listen to Isela Sotolongo discuss hospitalist leadership and communication in detail.)
Also, a hospitalist might be leading and participating in QI activities at the hospital or leading the discussion for a multidisciplinary team, Dr. Holman notes. Having leadership skills helps a hospitalist appreciate the work their medical director must do, or the work that hospital administrators are trying to accomplish, he says.
Teamwork
Teamwork is an essential skill for hospitalists, yet traditional medical training often teaches doctors to be more of a rugged individualist, Dr. Holman says. Hospitalists should make it a point to adopt new behaviors that are teamwork-friendly:
- Value other members of the healthcare team;
- Direct people’s talents to their highest and best use; and
- Delegate tasks to those who are best suited to accomplish those particular needs.
“Teamwork tends to place much more emphasis on the patient being the center of care and others on the healthcare team going to support those patient-centered interests,” Dr. Holman say.
The Patient Perspective
How a patient moves through the healthcare system in theory is often different from reality. A hospitalist should physically follow some of their patients as they move from the hospital back to their caregivers at home or to a rehabilitation center, nursing home, or other facility in order to watch the transition, says Jasen Gundersen, MD, MBA, CPE, SFHM, chief medical officer of the hospital medicine division in Fort Lauderdale, Fla., for Knoxville, Tenn.-based TeamHealth. By doing this, a hospitalist can see where problems arise during handoffs and how to resolve them so that hospital readmissions are reduced.
Teaching
Teaching skills can be applicable in any hospital environment, Dr. Holman adds. Hospitalists who work in nonteaching hospitals often serve as teachers to nursing staff, case management, pharmacists, discharge planners, and other ancillary staff in their daily interaction or over lunchtime educational programs. HM also can provide the bulk of a hospital’s grand rounds or other departmental educational sessions, he says.
“Let's not forget about patients and caregivers,” Dr. Holman adds. “The ability to teach patients and caregivers the necessary information and skills that they need for self-care, for follow-up care, and for compliance to a medical plan is important.”
Professional Evaluation
A hospitalist has to know on which metrics and measurements their success will be based, Dr. Holman says. Will it be patient satisfaction, length of stay, readmission rates, mortality rates, or some other measure?
“We have to be aware of what the hospital’s needs are, what the hospital’s key points are that they look for,” Sotolongo says.
Once the metrics are clear, a hospitalist can set goals to accomplish individually, as a member of an HM group, and partner with the hospital, Dr. Holman says.
Lisa Ryan is a freelance writer based in New Jersey.
SHM Leaders Field Constituent Questions in Town Hall
The vast majority of the more than 2,500 attendees last week at SHM's annual meeting in Grapevine, Texas, were already on their way to the airport when one last dedicated group discussed the future of the specialty.
The question-and-answer session took place at the annual SHM Town Hall, a chance for rank-and-file hospitalists to query society board members and leaders. Some 75 people took turns asking about internal-medicine residency programs, the challenges of maintaining robust society chapters, and HM's role in critical-care delivery. But the theme of the session kept coming back to whether hospitalists have a seat at the table in how healthcare reform is implemented.
Board member and former SHM Public Policy Committee chairman Eric Siegal, MD, SFHM, says that the society has quickly risen in stature in policy circles despite being a newer specialty. He adds that the field's prominence and credibility is helped by the recent appointment of Patrick Conway, MD, MSc, SFHM, a pediatric hospitalist and director of hospital medicine at Cincinnati Children’s Hospital Medical Center, as chief medical officer of the Centers for Medicare & Medicaid Services (CMS).
"We punch well above our weight class right now," says Dr. Siegal, a critical-care fellow at the University of Wisconsin School of Medicine and Public Health in Madison.
In an interesting twist, James Levy, PA-C, suggested that SHM expand its fellowship programs to nonphysician providers (NPPs) as a way of making them feel more included. Several board members nodded and said they would consider the notion.
"We are actually a big-tent society," says board member Daniel Dressler, MD, MSc, SFHM, associate professor and director of internal-medicine teaching services at Emory University Hospital in Atlanta.
The vast majority of the more than 2,500 attendees last week at SHM's annual meeting in Grapevine, Texas, were already on their way to the airport when one last dedicated group discussed the future of the specialty.
The question-and-answer session took place at the annual SHM Town Hall, a chance for rank-and-file hospitalists to query society board members and leaders. Some 75 people took turns asking about internal-medicine residency programs, the challenges of maintaining robust society chapters, and HM's role in critical-care delivery. But the theme of the session kept coming back to whether hospitalists have a seat at the table in how healthcare reform is implemented.
Board member and former SHM Public Policy Committee chairman Eric Siegal, MD, SFHM, says that the society has quickly risen in stature in policy circles despite being a newer specialty. He adds that the field's prominence and credibility is helped by the recent appointment of Patrick Conway, MD, MSc, SFHM, a pediatric hospitalist and director of hospital medicine at Cincinnati Children’s Hospital Medical Center, as chief medical officer of the Centers for Medicare & Medicaid Services (CMS).
"We punch well above our weight class right now," says Dr. Siegal, a critical-care fellow at the University of Wisconsin School of Medicine and Public Health in Madison.
In an interesting twist, James Levy, PA-C, suggested that SHM expand its fellowship programs to nonphysician providers (NPPs) as a way of making them feel more included. Several board members nodded and said they would consider the notion.
"We are actually a big-tent society," says board member Daniel Dressler, MD, MSc, SFHM, associate professor and director of internal-medicine teaching services at Emory University Hospital in Atlanta.
The vast majority of the more than 2,500 attendees last week at SHM's annual meeting in Grapevine, Texas, were already on their way to the airport when one last dedicated group discussed the future of the specialty.
The question-and-answer session took place at the annual SHM Town Hall, a chance for rank-and-file hospitalists to query society board members and leaders. Some 75 people took turns asking about internal-medicine residency programs, the challenges of maintaining robust society chapters, and HM's role in critical-care delivery. But the theme of the session kept coming back to whether hospitalists have a seat at the table in how healthcare reform is implemented.
Board member and former SHM Public Policy Committee chairman Eric Siegal, MD, SFHM, says that the society has quickly risen in stature in policy circles despite being a newer specialty. He adds that the field's prominence and credibility is helped by the recent appointment of Patrick Conway, MD, MSc, SFHM, a pediatric hospitalist and director of hospital medicine at Cincinnati Children’s Hospital Medical Center, as chief medical officer of the Centers for Medicare & Medicaid Services (CMS).
"We punch well above our weight class right now," says Dr. Siegal, a critical-care fellow at the University of Wisconsin School of Medicine and Public Health in Madison.
In an interesting twist, James Levy, PA-C, suggested that SHM expand its fellowship programs to nonphysician providers (NPPs) as a way of making them feel more included. Several board members nodded and said they would consider the notion.
"We are actually a big-tent society," says board member Daniel Dressler, MD, MSc, SFHM, associate professor and director of internal-medicine teaching services at Emory University Hospital in Atlanta.
In the Literature: Research You Need to Know
Clinical Question: Is chest-compression-only bystander cardiopulmonary resuscitation (CPR) as effective as conventional CPR with rescue breathing for out-of-hospital cardiac arrest?
Background: Out-of-hospital cardiac arrest is a major public health problem, affecting approximately 300,000 people in the U.S. annually. Overall survival is generally less than 10% among those in whom resuscitation is attempted. Bystander CPR significantly improves outcomes but generally is performed in less than 30% of cases.
Study design: Prospective, observational cohort study.
Setting: Communities throughout the state of Arizona.
Synopsis: State officials undertook a multifaceted public service campaign to educate, inform, and encourage the use of compression-only CPR.
Over a period of four years, 5,272 adult out-of-hospital cardiac arrests were documented in Arizona. A total of 4,415 of these incidents met all of the inclusion criteria for analysis; 2,900 received no CPR, 666 received conventional CPR, and 849 received compression-only CPR. Rates of survival to hospital discharge were 5.2% for the no-CPR group, 7.8% for conventional CPR, and 13.3% for compression-only CPR.
Additionally, the successful public service campaign increased the use of lay rescuer conventional CPR by 10% and compression-only CPR by 56%.
Bottom line: Layperson, compression-only CPR was associated with an overall 6.1% increased survival compared with conventional CPR and no bystander CPR.
Citation: Bobrow BJ, Spaite DW, Berg RA, et al. Chest-compression-only CPR by lay rescuers and survival from out-of-hospital cardiac arrest. JAMA. 2010;304(13):1447-1454.
For more physician reviews of HM-related research, visit our website.
Clinical Question: Is chest-compression-only bystander cardiopulmonary resuscitation (CPR) as effective as conventional CPR with rescue breathing for out-of-hospital cardiac arrest?
Background: Out-of-hospital cardiac arrest is a major public health problem, affecting approximately 300,000 people in the U.S. annually. Overall survival is generally less than 10% among those in whom resuscitation is attempted. Bystander CPR significantly improves outcomes but generally is performed in less than 30% of cases.
Study design: Prospective, observational cohort study.
Setting: Communities throughout the state of Arizona.
Synopsis: State officials undertook a multifaceted public service campaign to educate, inform, and encourage the use of compression-only CPR.
Over a period of four years, 5,272 adult out-of-hospital cardiac arrests were documented in Arizona. A total of 4,415 of these incidents met all of the inclusion criteria for analysis; 2,900 received no CPR, 666 received conventional CPR, and 849 received compression-only CPR. Rates of survival to hospital discharge were 5.2% for the no-CPR group, 7.8% for conventional CPR, and 13.3% for compression-only CPR.
Additionally, the successful public service campaign increased the use of lay rescuer conventional CPR by 10% and compression-only CPR by 56%.
Bottom line: Layperson, compression-only CPR was associated with an overall 6.1% increased survival compared with conventional CPR and no bystander CPR.
Citation: Bobrow BJ, Spaite DW, Berg RA, et al. Chest-compression-only CPR by lay rescuers and survival from out-of-hospital cardiac arrest. JAMA. 2010;304(13):1447-1454.
For more physician reviews of HM-related research, visit our website.
Clinical Question: Is chest-compression-only bystander cardiopulmonary resuscitation (CPR) as effective as conventional CPR with rescue breathing for out-of-hospital cardiac arrest?
Background: Out-of-hospital cardiac arrest is a major public health problem, affecting approximately 300,000 people in the U.S. annually. Overall survival is generally less than 10% among those in whom resuscitation is attempted. Bystander CPR significantly improves outcomes but generally is performed in less than 30% of cases.
Study design: Prospective, observational cohort study.
Setting: Communities throughout the state of Arizona.
Synopsis: State officials undertook a multifaceted public service campaign to educate, inform, and encourage the use of compression-only CPR.
Over a period of four years, 5,272 adult out-of-hospital cardiac arrests were documented in Arizona. A total of 4,415 of these incidents met all of the inclusion criteria for analysis; 2,900 received no CPR, 666 received conventional CPR, and 849 received compression-only CPR. Rates of survival to hospital discharge were 5.2% for the no-CPR group, 7.8% for conventional CPR, and 13.3% for compression-only CPR.
Additionally, the successful public service campaign increased the use of lay rescuer conventional CPR by 10% and compression-only CPR by 56%.
Bottom line: Layperson, compression-only CPR was associated with an overall 6.1% increased survival compared with conventional CPR and no bystander CPR.
Citation: Bobrow BJ, Spaite DW, Berg RA, et al. Chest-compression-only CPR by lay rescuers and survival from out-of-hospital cardiac arrest. JAMA. 2010;304(13):1447-1454.
For more physician reviews of HM-related research, visit our website.
Aspirin, Simvastatin Not Effective for Pulmonary Arterial Hypertension
Neither aspirin nor simvastatin improved outcomes for patients with pulmonary arterial hypertension in a phase II safety and efficacy trial published online May 19 in Circulation and simultaneously presented at the annual meeting of the American Thoracic Society.
The primary end point of 6-minute walk distance did not increase after 6 months of aspirin therapy and actually decreased after 6 months of simvastatin therapy, indicating that neither agent should be used as an add-on therapy in PAH, said Dr. Steven M. Kawut of Penn Cardiovascular Institute, University of Pennsylvania, Philadelphia, and his associates.
A recent animal study showed that aspirin decreased pulmonary artery pressure, reduced right ventricular hypertrophy, and improved survival, and several recent studies showed that statins were effective in animal models of pulmonary hypertension. Dr. Kawut and his associates in the ASA-STAT Study Group designed their phase II clinical trial to test the safety and efficacy of both agents against matching placebos, intending to enroll 100 subjects with PAH.
The study, however, was terminated after only 65 subjects had been randomized because an interim analysis showed "a high likelihood of not rejecting the null hypothesis for the simvastatin arm even if fully recruited," they wrote. The investigators reported their findings for those 65 subjects.
The patients’ mean age was 50 years, and 86% were women. Approximately 52% had idiopathic PAH, 19% had PAH associated with systemic sclerosis, 15% had PAH associated with other connective tissue diseases, 9% had congenital systemic-to-pulmonary shunts, and 5% had heritable PAH.
The primary outcome measure was 6-minute walk distance after 6 months of treatment, after adjustment for 6-minute walk distance at baseline.
Patients who received aspirin therapy showed no improvement in this measure, compared with those who received placebo. They also showed no improvement in median Borg dyspnea scores after the walk test, in any scales of the SF-36, or in World Health Organization functional class. And there was no difference between the two groups in time to clinical worsening, Dr. Kawut and his associates said.
Similarly, patients who received simvastatin showed no improvement in 6-minute walk distance after 6 months of treatment, compared with those who received placebo. In fact, the active drug may have reduced this distance, although the number of subjects was not sufficient to detect a statistically significant difference. Moreover, the median Borg dyspnea scores after the walk test tended to be higher in subjects who took simvastatin than in those who took placebo, suggesting greater breathlessness.
As with aspirin, there were no differences on any scales of the SF-36 or in WHO functional class between the subjects who took simvastatin and those who took placebo, the investigators said (Circulation 2011 May 19 [doi:10.1161/CIRCULATIONAHA.110.015693]).
"There was a possible increased risk of major bleeding associated with aspirin use," they noted, but again, the number of subjects was not sufficient to make this determination definitively.
"On the basis of these finding, neither drug can be recommended for the treatment of PAH," Dr. Kawut and his colleagues said.
The study was funded by the National Institutes of Health and the National Center for Research Resources. Aspirin and matching placebo were provided free of charge by Bayer Healthcare. Additional support was provided by Merck. Dr. Kawut and his associates reported ties to numerous industry sources.
Neither aspirin nor simvastatin improved outcomes for patients with pulmonary arterial hypertension in a phase II safety and efficacy trial published online May 19 in Circulation and simultaneously presented at the annual meeting of the American Thoracic Society.
The primary end point of 6-minute walk distance did not increase after 6 months of aspirin therapy and actually decreased after 6 months of simvastatin therapy, indicating that neither agent should be used as an add-on therapy in PAH, said Dr. Steven M. Kawut of Penn Cardiovascular Institute, University of Pennsylvania, Philadelphia, and his associates.
A recent animal study showed that aspirin decreased pulmonary artery pressure, reduced right ventricular hypertrophy, and improved survival, and several recent studies showed that statins were effective in animal models of pulmonary hypertension. Dr. Kawut and his associates in the ASA-STAT Study Group designed their phase II clinical trial to test the safety and efficacy of both agents against matching placebos, intending to enroll 100 subjects with PAH.
The study, however, was terminated after only 65 subjects had been randomized because an interim analysis showed "a high likelihood of not rejecting the null hypothesis for the simvastatin arm even if fully recruited," they wrote. The investigators reported their findings for those 65 subjects.
The patients’ mean age was 50 years, and 86% were women. Approximately 52% had idiopathic PAH, 19% had PAH associated with systemic sclerosis, 15% had PAH associated with other connective tissue diseases, 9% had congenital systemic-to-pulmonary shunts, and 5% had heritable PAH.
The primary outcome measure was 6-minute walk distance after 6 months of treatment, after adjustment for 6-minute walk distance at baseline.
Patients who received aspirin therapy showed no improvement in this measure, compared with those who received placebo. They also showed no improvement in median Borg dyspnea scores after the walk test, in any scales of the SF-36, or in World Health Organization functional class. And there was no difference between the two groups in time to clinical worsening, Dr. Kawut and his associates said.
Similarly, patients who received simvastatin showed no improvement in 6-minute walk distance after 6 months of treatment, compared with those who received placebo. In fact, the active drug may have reduced this distance, although the number of subjects was not sufficient to detect a statistically significant difference. Moreover, the median Borg dyspnea scores after the walk test tended to be higher in subjects who took simvastatin than in those who took placebo, suggesting greater breathlessness.
As with aspirin, there were no differences on any scales of the SF-36 or in WHO functional class between the subjects who took simvastatin and those who took placebo, the investigators said (Circulation 2011 May 19 [doi:10.1161/CIRCULATIONAHA.110.015693]).
"There was a possible increased risk of major bleeding associated with aspirin use," they noted, but again, the number of subjects was not sufficient to make this determination definitively.
"On the basis of these finding, neither drug can be recommended for the treatment of PAH," Dr. Kawut and his colleagues said.
The study was funded by the National Institutes of Health and the National Center for Research Resources. Aspirin and matching placebo were provided free of charge by Bayer Healthcare. Additional support was provided by Merck. Dr. Kawut and his associates reported ties to numerous industry sources.
Neither aspirin nor simvastatin improved outcomes for patients with pulmonary arterial hypertension in a phase II safety and efficacy trial published online May 19 in Circulation and simultaneously presented at the annual meeting of the American Thoracic Society.
The primary end point of 6-minute walk distance did not increase after 6 months of aspirin therapy and actually decreased after 6 months of simvastatin therapy, indicating that neither agent should be used as an add-on therapy in PAH, said Dr. Steven M. Kawut of Penn Cardiovascular Institute, University of Pennsylvania, Philadelphia, and his associates.
A recent animal study showed that aspirin decreased pulmonary artery pressure, reduced right ventricular hypertrophy, and improved survival, and several recent studies showed that statins were effective in animal models of pulmonary hypertension. Dr. Kawut and his associates in the ASA-STAT Study Group designed their phase II clinical trial to test the safety and efficacy of both agents against matching placebos, intending to enroll 100 subjects with PAH.
The study, however, was terminated after only 65 subjects had been randomized because an interim analysis showed "a high likelihood of not rejecting the null hypothesis for the simvastatin arm even if fully recruited," they wrote. The investigators reported their findings for those 65 subjects.
The patients’ mean age was 50 years, and 86% were women. Approximately 52% had idiopathic PAH, 19% had PAH associated with systemic sclerosis, 15% had PAH associated with other connective tissue diseases, 9% had congenital systemic-to-pulmonary shunts, and 5% had heritable PAH.
The primary outcome measure was 6-minute walk distance after 6 months of treatment, after adjustment for 6-minute walk distance at baseline.
Patients who received aspirin therapy showed no improvement in this measure, compared with those who received placebo. They also showed no improvement in median Borg dyspnea scores after the walk test, in any scales of the SF-36, or in World Health Organization functional class. And there was no difference between the two groups in time to clinical worsening, Dr. Kawut and his associates said.
Similarly, patients who received simvastatin showed no improvement in 6-minute walk distance after 6 months of treatment, compared with those who received placebo. In fact, the active drug may have reduced this distance, although the number of subjects was not sufficient to detect a statistically significant difference. Moreover, the median Borg dyspnea scores after the walk test tended to be higher in subjects who took simvastatin than in those who took placebo, suggesting greater breathlessness.
As with aspirin, there were no differences on any scales of the SF-36 or in WHO functional class between the subjects who took simvastatin and those who took placebo, the investigators said (Circulation 2011 May 19 [doi:10.1161/CIRCULATIONAHA.110.015693]).
"There was a possible increased risk of major bleeding associated with aspirin use," they noted, but again, the number of subjects was not sufficient to make this determination definitively.
"On the basis of these finding, neither drug can be recommended for the treatment of PAH," Dr. Kawut and his colleagues said.
The study was funded by the National Institutes of Health and the National Center for Research Resources. Aspirin and matching placebo were provided free of charge by Bayer Healthcare. Additional support was provided by Merck. Dr. Kawut and his associates reported ties to numerous industry sources.
FROM CIRCULATION
Major Finding: Patients with pulmonary arterial hypertension who received 6 months of aspirin or simvastatin therapy showed no improvement in 6-minute walk distance, compared with those who received placebo.
Data Source: A multicenter double-blind randomized phase II clinical trial of the safety and efficacy of aspirin and simvastatin compared with placebo in 65 patients with pulmonary arterial hypertension.
Disclosures: The study was funded by the National Institutes of Health and the National Center for Research Resources. Aspirin and matching placebo were provided free of charge by Bayer Healthcare. Additional support was provided by Merck. Dr. Kawut and his associates reported ties to numerous industry sources.
Low-Dose Irradiation Allays 93% of Gastric MALT Lymphomas at 10 Years
LONDON – Low-dose irradiation of the stomach in patients with gastric MALT lymphoma absent or independent of infection by Helicobacter pylori is associated with a 93% disease control rate after 10 years.
As presented by Dr. Joachim Yahalom, a radiation oncologist at Memorial Sloan-Kettering Cancer Center in New York, these long-term study findings highlight the high disease-control rates that can be obtained using low-dose involved field radiotherapy (IFRT).
Low-dose IFRT has now become a standard of care in the United States, although approaches still vary in Europe, he reported May 9 at the European Society for Therapeutic Radiation Oncology Anniversary Conference.
Indeed, the latest guidelines set by the National Comprehensive Cancer Network on non-Hodgkin’s lymphomas – which includes gastric MALT (mucosa-associated lymphoid tissue) lymphoma of the stomach – recommend the use of radiotherapy as a first-line option when patients have early (stage I/II) H. pylori–negative disease.
"We started using this [low-dose irradiation] treatment approach in the early 90s. Until then, the standard treatment for this lymphoma in the stomach was total gastrectomy, and sometimes it was followed by radiation therapy," said Dr. Yahalom, a professor of radiation oncology at Cornell University in New York. He added that chemotherapy and rituximab (Rituxan) were not generally very effective.
"Obviously the treatment of choice is to give antibiotics if H. pylori is present," Dr. Yahalom added, noting that substantial regression of MALT lymphoma can occur via treatment with antibiotics that target the gastric bacterium in positive cases. However, when H. pylori is not present, or if there is a lack of response to or residual lymphoma after complete response to antibiotic therapy, then radiotherapy is the recommended next step.
Dr. Yahalom presented data representing 16 years of experience in treating 103 patients (60 women and 43 men) with gastric MALT lymphoma in 1992-2009. The majority of patients (76%) had stage I disease, with 19% having stage II, and fewer than 5% having stage IV.
The median age of the patients was 62 years (range, 25-91 years). Symptoms were relatively mild at presentation, Dr. Yahalom observed: In all, 52% had epigastric pain, 36% had nausea, 29% had gastric bleeding, 18% had anorexia, 15% had anemia, and 10% had unknown or no obvious symptoms.
Almost two-thirds of patients were H. pylori negative. This included four patients who had relapsed after a complete response to antibiotic therapy. A further 20% of patients had persistent lymphoma after H. pylori eradication therapy. Another 15% had H. pylori and lymphoma remaining after antibiotic treatment, but had progressive symptoms or disease that could not wait for another antibiotics trial.
Prior to being treated with IFRT, six patients had undergone surgery, and seven had received medications that failed to control their disease.
Approximately 90% of patients were given IFRT at a median dose of 30 Gy in 1.5 Gy fractions for 4 weeks. The lowest and highest total radiation doses administered were 22.5 Gy and 43.5 Gy.
Patients received regular follow up via endoscopic biopsies, and after a median of 5.5 years, the disease control rate was 98%. One-fifth of patients had been followed for 10 years, and the 10-year freedom from local failure was 93%; the 10-year overall survival rate was 74%.
There were no significant acute or late adverse events, Dr. Yahalom noted, and just eight patients relapsed (six with gastric MALT lymphoma and two with diffuse large B-cell lymphoma). Subsequent other cancers included lymphoma in extragastric sites (eight patients), adenocarcinoma of the stomach (two), and second tumors close to the radiotherapy field (one in the colon and two in the pancreas). "Other deaths appeared unrelated to disease or treatment," he said.
Based on this experience, Dr. Yahalom concluded that "low-dose irradiation of the stomach provides excellent long-term disease control [and] is safe and simple.
"Radiation therapy, in our opinion, is the treatment of choice for patients with MALT lymphoma that have exhausted their antibiotic options or are unlikely to respond to it."
Dr. Yahalom said he had no financial conflicts of interest.
LONDON – Low-dose irradiation of the stomach in patients with gastric MALT lymphoma absent or independent of infection by Helicobacter pylori is associated with a 93% disease control rate after 10 years.
As presented by Dr. Joachim Yahalom, a radiation oncologist at Memorial Sloan-Kettering Cancer Center in New York, these long-term study findings highlight the high disease-control rates that can be obtained using low-dose involved field radiotherapy (IFRT).
Low-dose IFRT has now become a standard of care in the United States, although approaches still vary in Europe, he reported May 9 at the European Society for Therapeutic Radiation Oncology Anniversary Conference.
Indeed, the latest guidelines set by the National Comprehensive Cancer Network on non-Hodgkin’s lymphomas – which includes gastric MALT (mucosa-associated lymphoid tissue) lymphoma of the stomach – recommend the use of radiotherapy as a first-line option when patients have early (stage I/II) H. pylori–negative disease.
"We started using this [low-dose irradiation] treatment approach in the early 90s. Until then, the standard treatment for this lymphoma in the stomach was total gastrectomy, and sometimes it was followed by radiation therapy," said Dr. Yahalom, a professor of radiation oncology at Cornell University in New York. He added that chemotherapy and rituximab (Rituxan) were not generally very effective.
"Obviously the treatment of choice is to give antibiotics if H. pylori is present," Dr. Yahalom added, noting that substantial regression of MALT lymphoma can occur via treatment with antibiotics that target the gastric bacterium in positive cases. However, when H. pylori is not present, or if there is a lack of response to or residual lymphoma after complete response to antibiotic therapy, then radiotherapy is the recommended next step.
Dr. Yahalom presented data representing 16 years of experience in treating 103 patients (60 women and 43 men) with gastric MALT lymphoma in 1992-2009. The majority of patients (76%) had stage I disease, with 19% having stage II, and fewer than 5% having stage IV.
The median age of the patients was 62 years (range, 25-91 years). Symptoms were relatively mild at presentation, Dr. Yahalom observed: In all, 52% had epigastric pain, 36% had nausea, 29% had gastric bleeding, 18% had anorexia, 15% had anemia, and 10% had unknown or no obvious symptoms.
Almost two-thirds of patients were H. pylori negative. This included four patients who had relapsed after a complete response to antibiotic therapy. A further 20% of patients had persistent lymphoma after H. pylori eradication therapy. Another 15% had H. pylori and lymphoma remaining after antibiotic treatment, but had progressive symptoms or disease that could not wait for another antibiotics trial.
Prior to being treated with IFRT, six patients had undergone surgery, and seven had received medications that failed to control their disease.
Approximately 90% of patients were given IFRT at a median dose of 30 Gy in 1.5 Gy fractions for 4 weeks. The lowest and highest total radiation doses administered were 22.5 Gy and 43.5 Gy.
Patients received regular follow up via endoscopic biopsies, and after a median of 5.5 years, the disease control rate was 98%. One-fifth of patients had been followed for 10 years, and the 10-year freedom from local failure was 93%; the 10-year overall survival rate was 74%.
There were no significant acute or late adverse events, Dr. Yahalom noted, and just eight patients relapsed (six with gastric MALT lymphoma and two with diffuse large B-cell lymphoma). Subsequent other cancers included lymphoma in extragastric sites (eight patients), adenocarcinoma of the stomach (two), and second tumors close to the radiotherapy field (one in the colon and two in the pancreas). "Other deaths appeared unrelated to disease or treatment," he said.
Based on this experience, Dr. Yahalom concluded that "low-dose irradiation of the stomach provides excellent long-term disease control [and] is safe and simple.
"Radiation therapy, in our opinion, is the treatment of choice for patients with MALT lymphoma that have exhausted their antibiotic options or are unlikely to respond to it."
Dr. Yahalom said he had no financial conflicts of interest.
LONDON – Low-dose irradiation of the stomach in patients with gastric MALT lymphoma absent or independent of infection by Helicobacter pylori is associated with a 93% disease control rate after 10 years.
As presented by Dr. Joachim Yahalom, a radiation oncologist at Memorial Sloan-Kettering Cancer Center in New York, these long-term study findings highlight the high disease-control rates that can be obtained using low-dose involved field radiotherapy (IFRT).
Low-dose IFRT has now become a standard of care in the United States, although approaches still vary in Europe, he reported May 9 at the European Society for Therapeutic Radiation Oncology Anniversary Conference.
Indeed, the latest guidelines set by the National Comprehensive Cancer Network on non-Hodgkin’s lymphomas – which includes gastric MALT (mucosa-associated lymphoid tissue) lymphoma of the stomach – recommend the use of radiotherapy as a first-line option when patients have early (stage I/II) H. pylori–negative disease.
"We started using this [low-dose irradiation] treatment approach in the early 90s. Until then, the standard treatment for this lymphoma in the stomach was total gastrectomy, and sometimes it was followed by radiation therapy," said Dr. Yahalom, a professor of radiation oncology at Cornell University in New York. He added that chemotherapy and rituximab (Rituxan) were not generally very effective.
"Obviously the treatment of choice is to give antibiotics if H. pylori is present," Dr. Yahalom added, noting that substantial regression of MALT lymphoma can occur via treatment with antibiotics that target the gastric bacterium in positive cases. However, when H. pylori is not present, or if there is a lack of response to or residual lymphoma after complete response to antibiotic therapy, then radiotherapy is the recommended next step.
Dr. Yahalom presented data representing 16 years of experience in treating 103 patients (60 women and 43 men) with gastric MALT lymphoma in 1992-2009. The majority of patients (76%) had stage I disease, with 19% having stage II, and fewer than 5% having stage IV.
The median age of the patients was 62 years (range, 25-91 years). Symptoms were relatively mild at presentation, Dr. Yahalom observed: In all, 52% had epigastric pain, 36% had nausea, 29% had gastric bleeding, 18% had anorexia, 15% had anemia, and 10% had unknown or no obvious symptoms.
Almost two-thirds of patients were H. pylori negative. This included four patients who had relapsed after a complete response to antibiotic therapy. A further 20% of patients had persistent lymphoma after H. pylori eradication therapy. Another 15% had H. pylori and lymphoma remaining after antibiotic treatment, but had progressive symptoms or disease that could not wait for another antibiotics trial.
Prior to being treated with IFRT, six patients had undergone surgery, and seven had received medications that failed to control their disease.
Approximately 90% of patients were given IFRT at a median dose of 30 Gy in 1.5 Gy fractions for 4 weeks. The lowest and highest total radiation doses administered were 22.5 Gy and 43.5 Gy.
Patients received regular follow up via endoscopic biopsies, and after a median of 5.5 years, the disease control rate was 98%. One-fifth of patients had been followed for 10 years, and the 10-year freedom from local failure was 93%; the 10-year overall survival rate was 74%.
There were no significant acute or late adverse events, Dr. Yahalom noted, and just eight patients relapsed (six with gastric MALT lymphoma and two with diffuse large B-cell lymphoma). Subsequent other cancers included lymphoma in extragastric sites (eight patients), adenocarcinoma of the stomach (two), and second tumors close to the radiotherapy field (one in the colon and two in the pancreas). "Other deaths appeared unrelated to disease or treatment," he said.
Based on this experience, Dr. Yahalom concluded that "low-dose irradiation of the stomach provides excellent long-term disease control [and] is safe and simple.
"Radiation therapy, in our opinion, is the treatment of choice for patients with MALT lymphoma that have exhausted their antibiotic options or are unlikely to respond to it."
Dr. Yahalom said he had no financial conflicts of interest.
FROM THE EUROPEAN SOCIETY FOR THERAPEUTIC RADIATION ONCOLOGY ANNIVERSARY CONFERENCE
Major Finding: After a median of 5.5 years, the disease control rate was 98%.
Data Source: Data representing 16 years of experience with the use of low-dose irradiation therapy in 103 patients with H. pylori–independent gastric MALT lymphoma.
Disclosures: Dr. Yahalom said he had no financial conflicts of interest.
Fewer Side Effects From Proton Therapy in NSCLC
LONDON – Proton beam therapy for non–small cell lung cancer is associated with fewer radiation-induced side effects than are conventional radiotherapy methods when combined with chemotherapy, according to preliminary data from two retrospective studies conducted at the University of Texas M.D. Anderson Cancer Center in Houston.
Significantly less esophagitis, pneumonitis, and bone marrow toxicity were observed with proton beam therapy (PBT) than with intensity-modulated radiotherapy (IMRT), Dr. Ritsuko Komaki reported May 10, at the European Society for Therapeutic Radiation Oncology (ESTRO) Anniversary Conference.
Proton beam therapy also significantly reduced the incidence of esophagitis when compared with IMRT and three-dimensional conformal radiotherapy (3D-CRT). A mean esophageal dose of 40 Gy or higher was identified as the cut-off point for high-grade esophagitis occurring with any method.
"Radiation dose escalation improves local control but increases toxicity, especially when combined with concurrent chemotherapy for non–small cell lung cancer [NSCLC] or even small cell lung cancer," said Dr. Komaki, a professor of radiation oncology at M.D. Anderson, which opened its 94,000-square-foot Proton Therapy Center in 2006. As such, "radiation chemotherapy is a double-edged sword," she observed. "It will kill cancer cells, but it also kills normal tissues, and more targeted treatment is needed."
"One of the most important benefits of PBT is that there is no exit dose," Dr. Komaki added in an interview with Elsevier Global Medical News. "The protons stop after penetrating the tumor, and there is no dose of radiation beyond it."
This has the potential to spare surrounding cells and organs from damage, she observed. Normal tissues that might be affected by radiation therapy for NSCLC include the lungs, esophagus, heart, and bone marrow, which cannot always be avoided by the use of 3D-CRT or even IMRT.
An expensive new technology that delivers highly targeted radiation with electrically charged particles, proton beam therapy is promising but unproven, according to a 2009 review commissioned by the Agency for Healthcare Research and Quality (AHRQ). The authors found few comparative studies to establish effectiveness or safety for the technology, which is housed in a small but growing number of proton beam centers that can cost $100 million to $225 million to build (Ann. Intern. Med. 2009;151:556-65).
Dr. Komaki and her associates are recruiting patients into the first, prospective randomized trial to directly compare proton beam therapy with IMRT in unresectable stage II /III NSCLC. The phase II trial is supported by a grant from the National Cancer Institute, and involves treatment with 74 Gy proton beam therapy or IMRT with concurrent carboplatin and paclitaxel. To date, 107 of the planned 168 patients have been enrolled in the study at the Texas institution and at the Massachusetts General Hospital in Boston, the other participating center, she said.
A recent report from the M.D. Anderson showed that higher doses of proton radiation could be delivered to lung tumors with a lower risk of esophagitis and pneumonitis than either IMRT or 3D-CRT (Cancer 2011;doi.org/10.1002/cncr.25848).
The new data presented by Dr. Komaki showed significantly reduced rates of grade 2 or higher esophagitis (P less than .0001), pneumonitis (P less than .002), hematologic toxicities (P less than .0001 for neutrophil toxicity and P less than .001 for hemoglobin and white blood cell toxicities), and fatigue (P less than.0001) in 60 patients treated with proton beam therapy compared with 75 patients treated with IMRT (Radiother. Oncol. 2011;99:S89-90, abstract 233).
Other research from the M.D. Anderson team focused on esophagitis, and examined dosimetric and clinical factors that could lead to this side effect following proton beam therapy, IMRT, or 3D-CRT for definitive NSCLC treatment (Radiother. Oncol. 2011;99[Suppl.1]:S210, abstract 518).
Dr. Daniel Gomez, a radiation oncologist at M.D. Anderson, presented data on 678 patients treated at the institution between 1999 and 2008. Dr. Gomez explained that the type of radiation therapy received had altered over the years, with 463 patients treated with 3D-CRT between 1999 and 2005, 122 with IMRT between 2005 and 2007, and 94 patients treated with proton beam therapy between 2006 and 2008.
"Esophagitis is a common toxicity in the treatment of NSCLC with definitive radiation," Dr. Gomez observed. Although studies have looked at what factors might predict this life-limiting side effect, conflicting results have been obtained.
Data show, for example, that the presence of acute toxicity is a predictor of late toxicity (Int. J. Radiat. Oncol. Biol. Phys. 2005,61:335-47), but a variety of dosimetric parameters have been noted and there does not appear to be a single threshold at which a toxic effect is or is not likely to be observed (Int. J. Radiat. Oncol. Biol. Phys. 2010;76:S86-93).
Data presented by Dr. Gomez, however, suggest that a mean delivered esophageal dose of above 40 Gy may be predictive of high-grade inflammation regardless of whether proton beams, IMRT or 3D-CRT is used. This research might eventually help develop dosing guides for clinicians to use in routine practice, he suggested.
"Patients receiving IMRT had a higher rate of esophagitis in all grades, including grade 3," Dr. Gomez said. In contrast, "patients receiving proton therapy had lower rates of esophagitis at all grades." The incidence of grade 3 or higher esophagitis was 14% (n = 65) for 3D-CRT, 27% (n = 33) for IMRT, and 6% (n = 6) for proton beam therapy.
Dr. Gomez also reported that grade 3 or higher esophagitis was more likely in patients who received concurrent chemotherapy than in those who did not (18.4% vs. 7.4%, P less than .001). The mean esophageal dose of radiation delivered to patients given concurrent chemotherapy also was, significantly higher (32.2 Gy vs. 15.8 Gy, P less than .001), however.
The M.D. Anderson investigators said they have just finished (May 12) recruiting patients into a phase III trial (Radiation Therapy Oncology Group [RTOG] 0617) that will compare conventional (60 Gy in 6 weeks) vs. high dose (74 Gy in 7.5 weeks) radiation therapy in combination with paclitaxel and carboplatin, with or without the addition of cetuximab (Erbitux) in 500 patients with NSCLC.
Although the trial is not directly comparing the type of radiation treatment used, it should still be possible to retrospectively analyze the results to determine the individual effects of the radiation modalities used at each participating center, Dr. Komaki noted.
"When we started this trial, it was not acquiring patients because some of the radiation and medical oncologists said that it was obvious that patients given 60 Gy would do worse compared to 74 Gy," she added in the interview. "When we included cetuximab based on the results of the RTOG 0324 trial, however, recruitment started to rocket." The RTOG 0324 trial showed the feasibility of combining cetuximab with chemoradiation in NSCLC (J. Clin. Oncol. 2011 May 9 [Epub ahead of print, doi: 10.1200/JCO.2010.31.7875]).
Discussing the downsides of proton beam therapy vs. IMRT, Dr. Komaki conceded that the newer method involved a lot more sophisticated planning and was more expensive. There is also concern that the sharp drop-off of radiation received with proton beam therapy might mean that important areas of the tumor are missed – although this may explain the lower rate of side effects seen with PBT to date. "There is no give," Dr. Komaki said.
As relatively few proton beam facilities are in operation, large cooperative trials are difficult to perform. The prospective phase II trial comparing proton beam therapy and IMRT now being conducted at M.D. Anderson and the Massachusetts General Hospital will be the proof that such trials are possible, and provide valuable information on the comparative safety and efficacy of the two procedures.
Dr. Komaki and Dr. Gomez said they had no financial conflicts of interest.
LONDON – Proton beam therapy for non–small cell lung cancer is associated with fewer radiation-induced side effects than are conventional radiotherapy methods when combined with chemotherapy, according to preliminary data from two retrospective studies conducted at the University of Texas M.D. Anderson Cancer Center in Houston.
Significantly less esophagitis, pneumonitis, and bone marrow toxicity were observed with proton beam therapy (PBT) than with intensity-modulated radiotherapy (IMRT), Dr. Ritsuko Komaki reported May 10, at the European Society for Therapeutic Radiation Oncology (ESTRO) Anniversary Conference.
Proton beam therapy also significantly reduced the incidence of esophagitis when compared with IMRT and three-dimensional conformal radiotherapy (3D-CRT). A mean esophageal dose of 40 Gy or higher was identified as the cut-off point for high-grade esophagitis occurring with any method.
"Radiation dose escalation improves local control but increases toxicity, especially when combined with concurrent chemotherapy for non–small cell lung cancer [NSCLC] or even small cell lung cancer," said Dr. Komaki, a professor of radiation oncology at M.D. Anderson, which opened its 94,000-square-foot Proton Therapy Center in 2006. As such, "radiation chemotherapy is a double-edged sword," she observed. "It will kill cancer cells, but it also kills normal tissues, and more targeted treatment is needed."
"One of the most important benefits of PBT is that there is no exit dose," Dr. Komaki added in an interview with Elsevier Global Medical News. "The protons stop after penetrating the tumor, and there is no dose of radiation beyond it."
This has the potential to spare surrounding cells and organs from damage, she observed. Normal tissues that might be affected by radiation therapy for NSCLC include the lungs, esophagus, heart, and bone marrow, which cannot always be avoided by the use of 3D-CRT or even IMRT.
An expensive new technology that delivers highly targeted radiation with electrically charged particles, proton beam therapy is promising but unproven, according to a 2009 review commissioned by the Agency for Healthcare Research and Quality (AHRQ). The authors found few comparative studies to establish effectiveness or safety for the technology, which is housed in a small but growing number of proton beam centers that can cost $100 million to $225 million to build (Ann. Intern. Med. 2009;151:556-65).
Dr. Komaki and her associates are recruiting patients into the first, prospective randomized trial to directly compare proton beam therapy with IMRT in unresectable stage II /III NSCLC. The phase II trial is supported by a grant from the National Cancer Institute, and involves treatment with 74 Gy proton beam therapy or IMRT with concurrent carboplatin and paclitaxel. To date, 107 of the planned 168 patients have been enrolled in the study at the Texas institution and at the Massachusetts General Hospital in Boston, the other participating center, she said.
A recent report from the M.D. Anderson showed that higher doses of proton radiation could be delivered to lung tumors with a lower risk of esophagitis and pneumonitis than either IMRT or 3D-CRT (Cancer 2011;doi.org/10.1002/cncr.25848).
The new data presented by Dr. Komaki showed significantly reduced rates of grade 2 or higher esophagitis (P less than .0001), pneumonitis (P less than .002), hematologic toxicities (P less than .0001 for neutrophil toxicity and P less than .001 for hemoglobin and white blood cell toxicities), and fatigue (P less than.0001) in 60 patients treated with proton beam therapy compared with 75 patients treated with IMRT (Radiother. Oncol. 2011;99:S89-90, abstract 233).
Other research from the M.D. Anderson team focused on esophagitis, and examined dosimetric and clinical factors that could lead to this side effect following proton beam therapy, IMRT, or 3D-CRT for definitive NSCLC treatment (Radiother. Oncol. 2011;99[Suppl.1]:S210, abstract 518).
Dr. Daniel Gomez, a radiation oncologist at M.D. Anderson, presented data on 678 patients treated at the institution between 1999 and 2008. Dr. Gomez explained that the type of radiation therapy received had altered over the years, with 463 patients treated with 3D-CRT between 1999 and 2005, 122 with IMRT between 2005 and 2007, and 94 patients treated with proton beam therapy between 2006 and 2008.
"Esophagitis is a common toxicity in the treatment of NSCLC with definitive radiation," Dr. Gomez observed. Although studies have looked at what factors might predict this life-limiting side effect, conflicting results have been obtained.
Data show, for example, that the presence of acute toxicity is a predictor of late toxicity (Int. J. Radiat. Oncol. Biol. Phys. 2005,61:335-47), but a variety of dosimetric parameters have been noted and there does not appear to be a single threshold at which a toxic effect is or is not likely to be observed (Int. J. Radiat. Oncol. Biol. Phys. 2010;76:S86-93).
Data presented by Dr. Gomez, however, suggest that a mean delivered esophageal dose of above 40 Gy may be predictive of high-grade inflammation regardless of whether proton beams, IMRT or 3D-CRT is used. This research might eventually help develop dosing guides for clinicians to use in routine practice, he suggested.
"Patients receiving IMRT had a higher rate of esophagitis in all grades, including grade 3," Dr. Gomez said. In contrast, "patients receiving proton therapy had lower rates of esophagitis at all grades." The incidence of grade 3 or higher esophagitis was 14% (n = 65) for 3D-CRT, 27% (n = 33) for IMRT, and 6% (n = 6) for proton beam therapy.
Dr. Gomez also reported that grade 3 or higher esophagitis was more likely in patients who received concurrent chemotherapy than in those who did not (18.4% vs. 7.4%, P less than .001). The mean esophageal dose of radiation delivered to patients given concurrent chemotherapy also was, significantly higher (32.2 Gy vs. 15.8 Gy, P less than .001), however.
The M.D. Anderson investigators said they have just finished (May 12) recruiting patients into a phase III trial (Radiation Therapy Oncology Group [RTOG] 0617) that will compare conventional (60 Gy in 6 weeks) vs. high dose (74 Gy in 7.5 weeks) radiation therapy in combination with paclitaxel and carboplatin, with or without the addition of cetuximab (Erbitux) in 500 patients with NSCLC.
Although the trial is not directly comparing the type of radiation treatment used, it should still be possible to retrospectively analyze the results to determine the individual effects of the radiation modalities used at each participating center, Dr. Komaki noted.
"When we started this trial, it was not acquiring patients because some of the radiation and medical oncologists said that it was obvious that patients given 60 Gy would do worse compared to 74 Gy," she added in the interview. "When we included cetuximab based on the results of the RTOG 0324 trial, however, recruitment started to rocket." The RTOG 0324 trial showed the feasibility of combining cetuximab with chemoradiation in NSCLC (J. Clin. Oncol. 2011 May 9 [Epub ahead of print, doi: 10.1200/JCO.2010.31.7875]).
Discussing the downsides of proton beam therapy vs. IMRT, Dr. Komaki conceded that the newer method involved a lot more sophisticated planning and was more expensive. There is also concern that the sharp drop-off of radiation received with proton beam therapy might mean that important areas of the tumor are missed – although this may explain the lower rate of side effects seen with PBT to date. "There is no give," Dr. Komaki said.
As relatively few proton beam facilities are in operation, large cooperative trials are difficult to perform. The prospective phase II trial comparing proton beam therapy and IMRT now being conducted at M.D. Anderson and the Massachusetts General Hospital will be the proof that such trials are possible, and provide valuable information on the comparative safety and efficacy of the two procedures.
Dr. Komaki and Dr. Gomez said they had no financial conflicts of interest.
LONDON – Proton beam therapy for non–small cell lung cancer is associated with fewer radiation-induced side effects than are conventional radiotherapy methods when combined with chemotherapy, according to preliminary data from two retrospective studies conducted at the University of Texas M.D. Anderson Cancer Center in Houston.
Significantly less esophagitis, pneumonitis, and bone marrow toxicity were observed with proton beam therapy (PBT) than with intensity-modulated radiotherapy (IMRT), Dr. Ritsuko Komaki reported May 10, at the European Society for Therapeutic Radiation Oncology (ESTRO) Anniversary Conference.
Proton beam therapy also significantly reduced the incidence of esophagitis when compared with IMRT and three-dimensional conformal radiotherapy (3D-CRT). A mean esophageal dose of 40 Gy or higher was identified as the cut-off point for high-grade esophagitis occurring with any method.
"Radiation dose escalation improves local control but increases toxicity, especially when combined with concurrent chemotherapy for non–small cell lung cancer [NSCLC] or even small cell lung cancer," said Dr. Komaki, a professor of radiation oncology at M.D. Anderson, which opened its 94,000-square-foot Proton Therapy Center in 2006. As such, "radiation chemotherapy is a double-edged sword," she observed. "It will kill cancer cells, but it also kills normal tissues, and more targeted treatment is needed."
"One of the most important benefits of PBT is that there is no exit dose," Dr. Komaki added in an interview with Elsevier Global Medical News. "The protons stop after penetrating the tumor, and there is no dose of radiation beyond it."
This has the potential to spare surrounding cells and organs from damage, she observed. Normal tissues that might be affected by radiation therapy for NSCLC include the lungs, esophagus, heart, and bone marrow, which cannot always be avoided by the use of 3D-CRT or even IMRT.
An expensive new technology that delivers highly targeted radiation with electrically charged particles, proton beam therapy is promising but unproven, according to a 2009 review commissioned by the Agency for Healthcare Research and Quality (AHRQ). The authors found few comparative studies to establish effectiveness or safety for the technology, which is housed in a small but growing number of proton beam centers that can cost $100 million to $225 million to build (Ann. Intern. Med. 2009;151:556-65).
Dr. Komaki and her associates are recruiting patients into the first, prospective randomized trial to directly compare proton beam therapy with IMRT in unresectable stage II /III NSCLC. The phase II trial is supported by a grant from the National Cancer Institute, and involves treatment with 74 Gy proton beam therapy or IMRT with concurrent carboplatin and paclitaxel. To date, 107 of the planned 168 patients have been enrolled in the study at the Texas institution and at the Massachusetts General Hospital in Boston, the other participating center, she said.
A recent report from the M.D. Anderson showed that higher doses of proton radiation could be delivered to lung tumors with a lower risk of esophagitis and pneumonitis than either IMRT or 3D-CRT (Cancer 2011;doi.org/10.1002/cncr.25848).
The new data presented by Dr. Komaki showed significantly reduced rates of grade 2 or higher esophagitis (P less than .0001), pneumonitis (P less than .002), hematologic toxicities (P less than .0001 for neutrophil toxicity and P less than .001 for hemoglobin and white blood cell toxicities), and fatigue (P less than.0001) in 60 patients treated with proton beam therapy compared with 75 patients treated with IMRT (Radiother. Oncol. 2011;99:S89-90, abstract 233).
Other research from the M.D. Anderson team focused on esophagitis, and examined dosimetric and clinical factors that could lead to this side effect following proton beam therapy, IMRT, or 3D-CRT for definitive NSCLC treatment (Radiother. Oncol. 2011;99[Suppl.1]:S210, abstract 518).
Dr. Daniel Gomez, a radiation oncologist at M.D. Anderson, presented data on 678 patients treated at the institution between 1999 and 2008. Dr. Gomez explained that the type of radiation therapy received had altered over the years, with 463 patients treated with 3D-CRT between 1999 and 2005, 122 with IMRT between 2005 and 2007, and 94 patients treated with proton beam therapy between 2006 and 2008.
"Esophagitis is a common toxicity in the treatment of NSCLC with definitive radiation," Dr. Gomez observed. Although studies have looked at what factors might predict this life-limiting side effect, conflicting results have been obtained.
Data show, for example, that the presence of acute toxicity is a predictor of late toxicity (Int. J. Radiat. Oncol. Biol. Phys. 2005,61:335-47), but a variety of dosimetric parameters have been noted and there does not appear to be a single threshold at which a toxic effect is or is not likely to be observed (Int. J. Radiat. Oncol. Biol. Phys. 2010;76:S86-93).
Data presented by Dr. Gomez, however, suggest that a mean delivered esophageal dose of above 40 Gy may be predictive of high-grade inflammation regardless of whether proton beams, IMRT or 3D-CRT is used. This research might eventually help develop dosing guides for clinicians to use in routine practice, he suggested.
"Patients receiving IMRT had a higher rate of esophagitis in all grades, including grade 3," Dr. Gomez said. In contrast, "patients receiving proton therapy had lower rates of esophagitis at all grades." The incidence of grade 3 or higher esophagitis was 14% (n = 65) for 3D-CRT, 27% (n = 33) for IMRT, and 6% (n = 6) for proton beam therapy.
Dr. Gomez also reported that grade 3 or higher esophagitis was more likely in patients who received concurrent chemotherapy than in those who did not (18.4% vs. 7.4%, P less than .001). The mean esophageal dose of radiation delivered to patients given concurrent chemotherapy also was, significantly higher (32.2 Gy vs. 15.8 Gy, P less than .001), however.
The M.D. Anderson investigators said they have just finished (May 12) recruiting patients into a phase III trial (Radiation Therapy Oncology Group [RTOG] 0617) that will compare conventional (60 Gy in 6 weeks) vs. high dose (74 Gy in 7.5 weeks) radiation therapy in combination with paclitaxel and carboplatin, with or without the addition of cetuximab (Erbitux) in 500 patients with NSCLC.
Although the trial is not directly comparing the type of radiation treatment used, it should still be possible to retrospectively analyze the results to determine the individual effects of the radiation modalities used at each participating center, Dr. Komaki noted.
"When we started this trial, it was not acquiring patients because some of the radiation and medical oncologists said that it was obvious that patients given 60 Gy would do worse compared to 74 Gy," she added in the interview. "When we included cetuximab based on the results of the RTOG 0324 trial, however, recruitment started to rocket." The RTOG 0324 trial showed the feasibility of combining cetuximab with chemoradiation in NSCLC (J. Clin. Oncol. 2011 May 9 [Epub ahead of print, doi: 10.1200/JCO.2010.31.7875]).
Discussing the downsides of proton beam therapy vs. IMRT, Dr. Komaki conceded that the newer method involved a lot more sophisticated planning and was more expensive. There is also concern that the sharp drop-off of radiation received with proton beam therapy might mean that important areas of the tumor are missed – although this may explain the lower rate of side effects seen with PBT to date. "There is no give," Dr. Komaki said.
As relatively few proton beam facilities are in operation, large cooperative trials are difficult to perform. The prospective phase II trial comparing proton beam therapy and IMRT now being conducted at M.D. Anderson and the Massachusetts General Hospital will be the proof that such trials are possible, and provide valuable information on the comparative safety and efficacy of the two procedures.
Dr. Komaki and Dr. Gomez said they had no financial conflicts of interest.
FROM THE EUROPEAN SOCIETY FOR THERAPEUTIC RADIATION ONCOLOGY ANNIVERSARY CONFERENCE
Major Finding: PBT resulted in significantly lower rates of grade 2 or higher esophagitis (P less than .0001), pneumonitis (P less than .002), hematologic toxicities (P less than .0001 for neutrophil toxicity and P less than .001 for hemoglobin and white blood cell toxicities), and fatigue (P less than .0001) than IMRT.
Data Source: Two retrospective studies: one involving 135 patients with NSCLC treated with concurrent chemoradiation (PBT, IMRT) and one involving 678 patients with NSCLC treated with concurrent chemoradiation between 1999 and 2008.
Disclosures: Dr. Komaki and Dr. Gomez said they had no financial conflicts of interest.
EDs Look to Hospitalists to Solve Crowding
GRAPEVINE, Texas–ED crowding may not seem like the hospitalist's purview, but an HM leader speaking at HM11 says think again.
Boarding, the term for admitted patients who are held in the ED, makes the issue particularly relevant to HM, says Eric Howell, MD, SFHM, associate professor of medicine at Johns Hopkins University and director of Johns Hopkins Bayview Medical Center's HM division.
"Almost everyone is equating boarding of admitted patients now with ED crowding," says Dr. Howell, an SHM board member. "And who do you think they're going to come to for help with all the boarded patients? They're going to come to you."
Dr. Howell says that although HM leaders might think of bed management mostly as a discharge issue, hospitalists would be well served to work with their respective ED colleagues to manage emergency throughput better. He suggests two initial approaches:
1. Round on boarders. In some practices, including at Johns Hopkins Bayview, a hospitalist can be dedicated to the practice of seeing admitted patients held in the ED. If a dedicated staffer is unavailable, group members can rotate the service.
2. Add capacity. Virtual capacity can be added by initiatives that lower LOS and therefore effectively create more bed space. Capacity can be added physically via something as ambitious as the creation of a unit, or techniques as simple as placing admitted patients in hallways.
"Targeting the ED alone doesn’t work," Dr. Howell says. "Patients boarding in the ED needs to be solved. … People are looking for good hospitalists to solve the problem."
GRAPEVINE, Texas–ED crowding may not seem like the hospitalist's purview, but an HM leader speaking at HM11 says think again.
Boarding, the term for admitted patients who are held in the ED, makes the issue particularly relevant to HM, says Eric Howell, MD, SFHM, associate professor of medicine at Johns Hopkins University and director of Johns Hopkins Bayview Medical Center's HM division.
"Almost everyone is equating boarding of admitted patients now with ED crowding," says Dr. Howell, an SHM board member. "And who do you think they're going to come to for help with all the boarded patients? They're going to come to you."
Dr. Howell says that although HM leaders might think of bed management mostly as a discharge issue, hospitalists would be well served to work with their respective ED colleagues to manage emergency throughput better. He suggests two initial approaches:
1. Round on boarders. In some practices, including at Johns Hopkins Bayview, a hospitalist can be dedicated to the practice of seeing admitted patients held in the ED. If a dedicated staffer is unavailable, group members can rotate the service.
2. Add capacity. Virtual capacity can be added by initiatives that lower LOS and therefore effectively create more bed space. Capacity can be added physically via something as ambitious as the creation of a unit, or techniques as simple as placing admitted patients in hallways.
"Targeting the ED alone doesn’t work," Dr. Howell says. "Patients boarding in the ED needs to be solved. … People are looking for good hospitalists to solve the problem."
GRAPEVINE, Texas–ED crowding may not seem like the hospitalist's purview, but an HM leader speaking at HM11 says think again.
Boarding, the term for admitted patients who are held in the ED, makes the issue particularly relevant to HM, says Eric Howell, MD, SFHM, associate professor of medicine at Johns Hopkins University and director of Johns Hopkins Bayview Medical Center's HM division.
"Almost everyone is equating boarding of admitted patients now with ED crowding," says Dr. Howell, an SHM board member. "And who do you think they're going to come to for help with all the boarded patients? They're going to come to you."
Dr. Howell says that although HM leaders might think of bed management mostly as a discharge issue, hospitalists would be well served to work with their respective ED colleagues to manage emergency throughput better. He suggests two initial approaches:
1. Round on boarders. In some practices, including at Johns Hopkins Bayview, a hospitalist can be dedicated to the practice of seeing admitted patients held in the ED. If a dedicated staffer is unavailable, group members can rotate the service.
2. Add capacity. Virtual capacity can be added by initiatives that lower LOS and therefore effectively create more bed space. Capacity can be added physically via something as ambitious as the creation of a unit, or techniques as simple as placing admitted patients in hallways.
"Targeting the ED alone doesn’t work," Dr. Howell says. "Patients boarding in the ED needs to be solved. … People are looking for good hospitalists to solve the problem."