User login
Unexpected skin necrosis of the thighs
A 62-YEAR-OLD WOMAN sought care at our clinic for painful skin lesions that had developed on her thighs 5 days earlier. She had received ongoing treatment at our clinic over the past few years for diabetes, hyperlipidemia, hypertension, and sarcoidosis. In the last 2 years, she’d had 2 hospitalizations for acute renal failure, with a creatinine value as high as 3.8 mg/dL and a persistent glomerular filtration rate consistent with stage 3 chronic kidney disease.
The medications she was taking included glyburide, pravastatin, and lisinopril. During the 2 years prior to her recent clinic visit, she’d had some intermittently elevated calcium readings. Repeat calcium levels each time were normal. In addition, her parathyroid hormone levels fluctuated between low, high, and normal. Her technetium sestamibi scan was negative for hyperparathyroidism. The patient was unemployed and gave no history of recent travel, injuries, or exposure to animals.
On examination, we noted large, poorly demarcated, warm, indurated erythematous lesions on her lateral thighs. She was given a diagnosis of cellulitis and treated with trimethoprim/sulfa-methoxazole 160/800 mg twice daily for 10 days. During follow-up visits 3 and 7 days later, she indicated that the lesions were less painful and they appeared to be less swollen.
Three weeks later, the patient returned to the clinic with skin sloughing that had produced necrotic lesions with black es-char on the bases (FIGURE 1). In addition, new lesions appeared on her anterior thighs. An initial punch biopsy of the lesions revealed no significant pathologic abnormality.
FIGURE 1
What started as indurated plaques…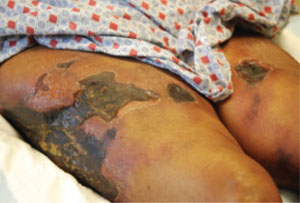
The patient initially came in for the treatment of indurated plaques, which developed into ulcerative skin lesions with erythematous edges and eschar on the bases.
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Calciphylaxis
Calciphylaxis is an uncommon disorder of vascular calcification and thrombosis resulting in skin necrosis.1 It most commonly occurs in people with end-stage renal disease (ESRD) on hemodialysis, but in nonuremic patients the most frequent cause is primary hyperparathyroidism.2,3 Similar vascular calcifications may be observed in milk alkali syndrome, rickets, collagen diseases, and hypervitaminosis D. Progression to necrosis in these cases is extremely rare.1 There are only a few documented cases of calciphylaxis associated with sarcoidosis, hypercalcemia, and non-ESRD.4
Female sex and diabetes appear to be risk factors.2 The presence of autoimmune disorders is a major feature in patients without ESRD.2,5 Although this patient did not have a previously diagnosed autoimmune disorder, an antinuclear antibody (ANA) test and lupus anticoagulant values were later found to be positive. In patients with autoimmune disorders, prednisone administration is associated with an increased risk of calciphylaxis.5 A hypercoagulable state can also underlie development of calciphylaxis. Our patient did have a mild protein C and S deficiency.
The prognosis of patients diagnosed with calciphylaxis is very poor. The mortality rate is reported to be as high as 60% to 80%.6
4 other possibilities comprise the differential diagnosis
Several conditions may present with erythema or necrosis similar to that of calciphylaxis (TABLE).
Warfarin-induced skin necrosis may produce hemorrhagic bullae and necrotic eschar, but generally presents within 3 to 10 days of initiating warfarin therapy.7 Severe dermatologic manifestations tend to affect the breasts, buttocks, and thighs.
Cutaneous anthrax causes painless necrotic lesions with black eschar, but is linked to bioterrorism or contact with infected animals. Constitutional symptoms such as fever, chills, and malaise are often present. Skin lesions are located primarily on the face, neck, and upper extremities.
Cholesterol embolization results from cholesterol crystals detaching and obstructing smaller arteries. Skin involvement includes livedo reticularis, petechiae, purpura, and ulcerations.
Vasculitis can affect all sizes of blood vessels. It can occur as a complication of connective tissue disorders, viral infections such as hepatitis B and C, or hypersensitivity reactions to medications such as penicillins and cephalosporins. Systemic symptoms are common, as is palpable purpura. Tissue biopsy is important for diagnosis and reveals blood vessel inflammation, not vessel wall calcification.
TABLE
Is it calciphylaxis or something else?1,3,7-9
| Condition | Characteristics |
|---|---|
| Warfarin-induced skin necrosis | Painful, erythematous, edematous lesions; rapidly progressive; petechiae, hemorrhagic bullae, then necrotic eschar |
| Cutaneous anthrax | Small painless, pruritic papules; advances to bullae; finally erodes to painless necrotic lesions with black eschar |
| Cholesterol embolization | Majority with livedo reticularis, cyanosis, or gangrene; smaller percentage with cutaneous ulceration, purpura, petechiae, or painful, firm erythematous nodules |
| Vasculitis | Palpable purpura; biopsy of most affected area is necessary for diagnosis |
| Calciphylaxis | Painful erythematous papules, plaques, nodules, or ulcerations in areas with high adiposity; may progress to necrosis |
What to do when the biopsy isn’t helpful
This case points out an important pathologic rule: If the biopsy doesn’t correlate with the observed disease, additional biopsies are indicated. Calciphylaxis is diagnosed on tissue microscopy, but the initial punch biopsy of the lesion revealed no significant pathologic abnormality. However, a subsequent deep-tissue biopsy showed extensive vascular wall calcification and septal fibrosis with subcutaneous fat necrosis.
Repeating abnormal laboratory testing is often appropriate, too. However, in this patient’s case, it probably would not have been helpful because she had intermittently elevated calcium levels over the years.
Wound cultures are often inaccurate in identifying a causative agent and this patient did not appear to have acute infection.
Management is mainly supportive
If you have a patient with calciphylaxis, address predisposing conditions such as hyperparathyroidism, hypercalcemia, and renal dysfunction5 (strength of recommendation [SOR]: C). In addition, discontinue calcium and vitamin D supplementation6 (SOR: C).
Finally, the patient will need meticulous wound care with adequate pain control; special attention to prevention of secondary infection is essential1,6 (SOR: C).
Our patient was one of the lucky ones
We treated this patient’s hypercalcemia, which was noted on admission to the hospital, with zoledronate and corrected her hypophosphatemia. Her renal function significantly improved with aggressive hydration.
With correction of electrolytes and normalization of kidney function, lesion progression was arrested. Granulation tissue developed in the lesions and split-thickness expanded skin grafts were performed on the large lesions (FIGURE 2). Fortunately, this patient survived despite the usual high rate of mortality. JFP
FIGURE 2
Good granulation beds, followed by closure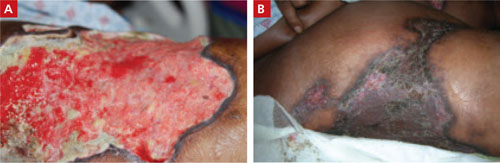
After aggressive treatment of renal dysfunction, correction of electrolyte abnormalities, and meticulous wound care, the patient’s lesions developed good granulation beds and showed signs of healing (A). The second image (B), taken 9 months after the patient first sought treatment for the lesions, shows the wounds after skin grafting.
CORRESPONDENCE
E.J. Mayeaux, Jr, MD, DABFP, FAAFP, Louisiana State University Health Sciences Center, 1501 Kings Highway, Shreveport, LA 71130; [email protected]
1. Kent RB 3rd, Lylerly RT. Systemic calciphylaxis. South Med J. 1994;87:278-281.
2. Nigwekar SU, Wolf M, Sterns RH, et al. Calciphylaxis from nonuremic causes. Clin J Am Soc Nephrol. 2008;3:1139-1143.
3. Fine A, Zacharias J. Calciphylaxis is usually non-ulcerating. Kidney Int. 2002;61:2210-2217.
4. Swanson AM, Desai SR, Jackson JD, et al. Calciphylaxis associated with chronic inflammatory conditions, immunosuppression therapy, and normal renal function: a report of 2 cases. Arch Dermatol. 2009;145:723-725.
5. Weenig RH, Sewell LD, Davis MD, et al. Calciphylaxis: natural history, risk factor analysis, and outcome. J Am Acad Dermatol. 2007;56:569-579.
6. Al-Hwiesh AK. Calciphylaxis of both proximal and distal distribution. Saudi J Kidney Dis Transpl. 2008;19:82-86.
7. Renick AM Jr. Anticoagulant-induced necrosis of skin and sub-cutaneous tissues. South Med J. 1976;69:775-778, 804.
8. Wenner KA, Kenner JR. Anthrax. Dermatol Clin. 2004;22:247-256.
9. Falanga V, Fine MJ, Kapoor WN. The cutaneous manifestations of cholesterol crystal embolization. Arch Dermatol. 1986;122:1194-1198.
A 62-YEAR-OLD WOMAN sought care at our clinic for painful skin lesions that had developed on her thighs 5 days earlier. She had received ongoing treatment at our clinic over the past few years for diabetes, hyperlipidemia, hypertension, and sarcoidosis. In the last 2 years, she’d had 2 hospitalizations for acute renal failure, with a creatinine value as high as 3.8 mg/dL and a persistent glomerular filtration rate consistent with stage 3 chronic kidney disease.
The medications she was taking included glyburide, pravastatin, and lisinopril. During the 2 years prior to her recent clinic visit, she’d had some intermittently elevated calcium readings. Repeat calcium levels each time were normal. In addition, her parathyroid hormone levels fluctuated between low, high, and normal. Her technetium sestamibi scan was negative for hyperparathyroidism. The patient was unemployed and gave no history of recent travel, injuries, or exposure to animals.
On examination, we noted large, poorly demarcated, warm, indurated erythematous lesions on her lateral thighs. She was given a diagnosis of cellulitis and treated with trimethoprim/sulfa-methoxazole 160/800 mg twice daily for 10 days. During follow-up visits 3 and 7 days later, she indicated that the lesions were less painful and they appeared to be less swollen.
Three weeks later, the patient returned to the clinic with skin sloughing that had produced necrotic lesions with black es-char on the bases (FIGURE 1). In addition, new lesions appeared on her anterior thighs. An initial punch biopsy of the lesions revealed no significant pathologic abnormality.
FIGURE 1
What started as indurated plaques…
The patient initially came in for the treatment of indurated plaques, which developed into ulcerative skin lesions with erythematous edges and eschar on the bases.
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Calciphylaxis
Calciphylaxis is an uncommon disorder of vascular calcification and thrombosis resulting in skin necrosis.1 It most commonly occurs in people with end-stage renal disease (ESRD) on hemodialysis, but in nonuremic patients the most frequent cause is primary hyperparathyroidism.2,3 Similar vascular calcifications may be observed in milk alkali syndrome, rickets, collagen diseases, and hypervitaminosis D. Progression to necrosis in these cases is extremely rare.1 There are only a few documented cases of calciphylaxis associated with sarcoidosis, hypercalcemia, and non-ESRD.4
Female sex and diabetes appear to be risk factors.2 The presence of autoimmune disorders is a major feature in patients without ESRD.2,5 Although this patient did not have a previously diagnosed autoimmune disorder, an antinuclear antibody (ANA) test and lupus anticoagulant values were later found to be positive. In patients with autoimmune disorders, prednisone administration is associated with an increased risk of calciphylaxis.5 A hypercoagulable state can also underlie development of calciphylaxis. Our patient did have a mild protein C and S deficiency.
The prognosis of patients diagnosed with calciphylaxis is very poor. The mortality rate is reported to be as high as 60% to 80%.6
4 other possibilities comprise the differential diagnosis
Several conditions may present with erythema or necrosis similar to that of calciphylaxis (TABLE).
Warfarin-induced skin necrosis may produce hemorrhagic bullae and necrotic eschar, but generally presents within 3 to 10 days of initiating warfarin therapy.7 Severe dermatologic manifestations tend to affect the breasts, buttocks, and thighs.
Cutaneous anthrax causes painless necrotic lesions with black eschar, but is linked to bioterrorism or contact with infected animals. Constitutional symptoms such as fever, chills, and malaise are often present. Skin lesions are located primarily on the face, neck, and upper extremities.
Cholesterol embolization results from cholesterol crystals detaching and obstructing smaller arteries. Skin involvement includes livedo reticularis, petechiae, purpura, and ulcerations.
Vasculitis can affect all sizes of blood vessels. It can occur as a complication of connective tissue disorders, viral infections such as hepatitis B and C, or hypersensitivity reactions to medications such as penicillins and cephalosporins. Systemic symptoms are common, as is palpable purpura. Tissue biopsy is important for diagnosis and reveals blood vessel inflammation, not vessel wall calcification.
TABLE
Is it calciphylaxis or something else?1,3,7-9
| Condition | Characteristics |
|---|---|
| Warfarin-induced skin necrosis | Painful, erythematous, edematous lesions; rapidly progressive; petechiae, hemorrhagic bullae, then necrotic eschar |
| Cutaneous anthrax | Small painless, pruritic papules; advances to bullae; finally erodes to painless necrotic lesions with black eschar |
| Cholesterol embolization | Majority with livedo reticularis, cyanosis, or gangrene; smaller percentage with cutaneous ulceration, purpura, petechiae, or painful, firm erythematous nodules |
| Vasculitis | Palpable purpura; biopsy of most affected area is necessary for diagnosis |
| Calciphylaxis | Painful erythematous papules, plaques, nodules, or ulcerations in areas with high adiposity; may progress to necrosis |
What to do when the biopsy isn’t helpful
This case points out an important pathologic rule: If the biopsy doesn’t correlate with the observed disease, additional biopsies are indicated. Calciphylaxis is diagnosed on tissue microscopy, but the initial punch biopsy of the lesion revealed no significant pathologic abnormality. However, a subsequent deep-tissue biopsy showed extensive vascular wall calcification and septal fibrosis with subcutaneous fat necrosis.
Repeating abnormal laboratory testing is often appropriate, too. However, in this patient’s case, it probably would not have been helpful because she had intermittently elevated calcium levels over the years.
Wound cultures are often inaccurate in identifying a causative agent and this patient did not appear to have acute infection.
Management is mainly supportive
If you have a patient with calciphylaxis, address predisposing conditions such as hyperparathyroidism, hypercalcemia, and renal dysfunction5 (strength of recommendation [SOR]: C). In addition, discontinue calcium and vitamin D supplementation6 (SOR: C).
Finally, the patient will need meticulous wound care with adequate pain control; special attention to prevention of secondary infection is essential1,6 (SOR: C).
Our patient was one of the lucky ones
We treated this patient’s hypercalcemia, which was noted on admission to the hospital, with zoledronate and corrected her hypophosphatemia. Her renal function significantly improved with aggressive hydration.
With correction of electrolytes and normalization of kidney function, lesion progression was arrested. Granulation tissue developed in the lesions and split-thickness expanded skin grafts were performed on the large lesions (FIGURE 2). Fortunately, this patient survived despite the usual high rate of mortality. JFP
FIGURE 2
Good granulation beds, followed by closure
After aggressive treatment of renal dysfunction, correction of electrolyte abnormalities, and meticulous wound care, the patient’s lesions developed good granulation beds and showed signs of healing (A). The second image (B), taken 9 months after the patient first sought treatment for the lesions, shows the wounds after skin grafting.
CORRESPONDENCE
E.J. Mayeaux, Jr, MD, DABFP, FAAFP, Louisiana State University Health Sciences Center, 1501 Kings Highway, Shreveport, LA 71130; [email protected]
A 62-YEAR-OLD WOMAN sought care at our clinic for painful skin lesions that had developed on her thighs 5 days earlier. She had received ongoing treatment at our clinic over the past few years for diabetes, hyperlipidemia, hypertension, and sarcoidosis. In the last 2 years, she’d had 2 hospitalizations for acute renal failure, with a creatinine value as high as 3.8 mg/dL and a persistent glomerular filtration rate consistent with stage 3 chronic kidney disease.
The medications she was taking included glyburide, pravastatin, and lisinopril. During the 2 years prior to her recent clinic visit, she’d had some intermittently elevated calcium readings. Repeat calcium levels each time were normal. In addition, her parathyroid hormone levels fluctuated between low, high, and normal. Her technetium sestamibi scan was negative for hyperparathyroidism. The patient was unemployed and gave no history of recent travel, injuries, or exposure to animals.
On examination, we noted large, poorly demarcated, warm, indurated erythematous lesions on her lateral thighs. She was given a diagnosis of cellulitis and treated with trimethoprim/sulfa-methoxazole 160/800 mg twice daily for 10 days. During follow-up visits 3 and 7 days later, she indicated that the lesions were less painful and they appeared to be less swollen.
Three weeks later, the patient returned to the clinic with skin sloughing that had produced necrotic lesions with black es-char on the bases (FIGURE 1). In addition, new lesions appeared on her anterior thighs. An initial punch biopsy of the lesions revealed no significant pathologic abnormality.
FIGURE 1
What started as indurated plaques…
The patient initially came in for the treatment of indurated plaques, which developed into ulcerative skin lesions with erythematous edges and eschar on the bases.
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Calciphylaxis
Calciphylaxis is an uncommon disorder of vascular calcification and thrombosis resulting in skin necrosis.1 It most commonly occurs in people with end-stage renal disease (ESRD) on hemodialysis, but in nonuremic patients the most frequent cause is primary hyperparathyroidism.2,3 Similar vascular calcifications may be observed in milk alkali syndrome, rickets, collagen diseases, and hypervitaminosis D. Progression to necrosis in these cases is extremely rare.1 There are only a few documented cases of calciphylaxis associated with sarcoidosis, hypercalcemia, and non-ESRD.4
Female sex and diabetes appear to be risk factors.2 The presence of autoimmune disorders is a major feature in patients without ESRD.2,5 Although this patient did not have a previously diagnosed autoimmune disorder, an antinuclear antibody (ANA) test and lupus anticoagulant values were later found to be positive. In patients with autoimmune disorders, prednisone administration is associated with an increased risk of calciphylaxis.5 A hypercoagulable state can also underlie development of calciphylaxis. Our patient did have a mild protein C and S deficiency.
The prognosis of patients diagnosed with calciphylaxis is very poor. The mortality rate is reported to be as high as 60% to 80%.6
4 other possibilities comprise the differential diagnosis
Several conditions may present with erythema or necrosis similar to that of calciphylaxis (TABLE).
Warfarin-induced skin necrosis may produce hemorrhagic bullae and necrotic eschar, but generally presents within 3 to 10 days of initiating warfarin therapy.7 Severe dermatologic manifestations tend to affect the breasts, buttocks, and thighs.
Cutaneous anthrax causes painless necrotic lesions with black eschar, but is linked to bioterrorism or contact with infected animals. Constitutional symptoms such as fever, chills, and malaise are often present. Skin lesions are located primarily on the face, neck, and upper extremities.
Cholesterol embolization results from cholesterol crystals detaching and obstructing smaller arteries. Skin involvement includes livedo reticularis, petechiae, purpura, and ulcerations.
Vasculitis can affect all sizes of blood vessels. It can occur as a complication of connective tissue disorders, viral infections such as hepatitis B and C, or hypersensitivity reactions to medications such as penicillins and cephalosporins. Systemic symptoms are common, as is palpable purpura. Tissue biopsy is important for diagnosis and reveals blood vessel inflammation, not vessel wall calcification.
TABLE
Is it calciphylaxis or something else?1,3,7-9
| Condition | Characteristics |
|---|---|
| Warfarin-induced skin necrosis | Painful, erythematous, edematous lesions; rapidly progressive; petechiae, hemorrhagic bullae, then necrotic eschar |
| Cutaneous anthrax | Small painless, pruritic papules; advances to bullae; finally erodes to painless necrotic lesions with black eschar |
| Cholesterol embolization | Majority with livedo reticularis, cyanosis, or gangrene; smaller percentage with cutaneous ulceration, purpura, petechiae, or painful, firm erythematous nodules |
| Vasculitis | Palpable purpura; biopsy of most affected area is necessary for diagnosis |
| Calciphylaxis | Painful erythematous papules, plaques, nodules, or ulcerations in areas with high adiposity; may progress to necrosis |
What to do when the biopsy isn’t helpful
This case points out an important pathologic rule: If the biopsy doesn’t correlate with the observed disease, additional biopsies are indicated. Calciphylaxis is diagnosed on tissue microscopy, but the initial punch biopsy of the lesion revealed no significant pathologic abnormality. However, a subsequent deep-tissue biopsy showed extensive vascular wall calcification and septal fibrosis with subcutaneous fat necrosis.
Repeating abnormal laboratory testing is often appropriate, too. However, in this patient’s case, it probably would not have been helpful because she had intermittently elevated calcium levels over the years.
Wound cultures are often inaccurate in identifying a causative agent and this patient did not appear to have acute infection.
Management is mainly supportive
If you have a patient with calciphylaxis, address predisposing conditions such as hyperparathyroidism, hypercalcemia, and renal dysfunction5 (strength of recommendation [SOR]: C). In addition, discontinue calcium and vitamin D supplementation6 (SOR: C).
Finally, the patient will need meticulous wound care with adequate pain control; special attention to prevention of secondary infection is essential1,6 (SOR: C).
Our patient was one of the lucky ones
We treated this patient’s hypercalcemia, which was noted on admission to the hospital, with zoledronate and corrected her hypophosphatemia. Her renal function significantly improved with aggressive hydration.
With correction of electrolytes and normalization of kidney function, lesion progression was arrested. Granulation tissue developed in the lesions and split-thickness expanded skin grafts were performed on the large lesions (FIGURE 2). Fortunately, this patient survived despite the usual high rate of mortality. JFP
FIGURE 2
Good granulation beds, followed by closure
After aggressive treatment of renal dysfunction, correction of electrolyte abnormalities, and meticulous wound care, the patient’s lesions developed good granulation beds and showed signs of healing (A). The second image (B), taken 9 months after the patient first sought treatment for the lesions, shows the wounds after skin grafting.
CORRESPONDENCE
E.J. Mayeaux, Jr, MD, DABFP, FAAFP, Louisiana State University Health Sciences Center, 1501 Kings Highway, Shreveport, LA 71130; [email protected]
1. Kent RB 3rd, Lylerly RT. Systemic calciphylaxis. South Med J. 1994;87:278-281.
2. Nigwekar SU, Wolf M, Sterns RH, et al. Calciphylaxis from nonuremic causes. Clin J Am Soc Nephrol. 2008;3:1139-1143.
3. Fine A, Zacharias J. Calciphylaxis is usually non-ulcerating. Kidney Int. 2002;61:2210-2217.
4. Swanson AM, Desai SR, Jackson JD, et al. Calciphylaxis associated with chronic inflammatory conditions, immunosuppression therapy, and normal renal function: a report of 2 cases. Arch Dermatol. 2009;145:723-725.
5. Weenig RH, Sewell LD, Davis MD, et al. Calciphylaxis: natural history, risk factor analysis, and outcome. J Am Acad Dermatol. 2007;56:569-579.
6. Al-Hwiesh AK. Calciphylaxis of both proximal and distal distribution. Saudi J Kidney Dis Transpl. 2008;19:82-86.
7. Renick AM Jr. Anticoagulant-induced necrosis of skin and sub-cutaneous tissues. South Med J. 1976;69:775-778, 804.
8. Wenner KA, Kenner JR. Anthrax. Dermatol Clin. 2004;22:247-256.
9. Falanga V, Fine MJ, Kapoor WN. The cutaneous manifestations of cholesterol crystal embolization. Arch Dermatol. 1986;122:1194-1198.
1. Kent RB 3rd, Lylerly RT. Systemic calciphylaxis. South Med J. 1994;87:278-281.
2. Nigwekar SU, Wolf M, Sterns RH, et al. Calciphylaxis from nonuremic causes. Clin J Am Soc Nephrol. 2008;3:1139-1143.
3. Fine A, Zacharias J. Calciphylaxis is usually non-ulcerating. Kidney Int. 2002;61:2210-2217.
4. Swanson AM, Desai SR, Jackson JD, et al. Calciphylaxis associated with chronic inflammatory conditions, immunosuppression therapy, and normal renal function: a report of 2 cases. Arch Dermatol. 2009;145:723-725.
5. Weenig RH, Sewell LD, Davis MD, et al. Calciphylaxis: natural history, risk factor analysis, and outcome. J Am Acad Dermatol. 2007;56:569-579.
6. Al-Hwiesh AK. Calciphylaxis of both proximal and distal distribution. Saudi J Kidney Dis Transpl. 2008;19:82-86.
7. Renick AM Jr. Anticoagulant-induced necrosis of skin and sub-cutaneous tissues. South Med J. 1976;69:775-778, 804.
8. Wenner KA, Kenner JR. Anthrax. Dermatol Clin. 2004;22:247-256.
9. Falanga V, Fine MJ, Kapoor WN. The cutaneous manifestations of cholesterol crystal embolization. Arch Dermatol. 1986;122:1194-1198.
Opiates and psychotropics: Pharmacokinetics for practitioners
• When choosing pharmacologic therapy, make sure that all medications your patient takes are documented, consider drug-drug interactions, and instruct the patient to notify you of any new medications.
• In addition to toxicity, loss of efficacy of some opiate drugs may occur as a result of metabolic inhibition or induction by psychotropic medications.
• Collaborate with the physician who is prescribing the opioid if psychotropic choices are limited. The patient’s pain may be treated adequately with another analgesic that does not interact with the psychotropic that has been chosen.
As prescribed by his internist, Mr. G, age 44, takes 10 mg of methadone every 4 hours for chronic back pain secondary to a work-related injury 3 years ago. He experiences minimal sedation. Mr. G presents for psychiatric evaluation with complaints of increasing irritability, poor focus, low energy, and lack of interest in usual activities. The psychiatrist diagnoses him with depressive disorder not otherwise specified, and prescribes fluoxetine, 20 mg/d. Three weeks later, Mr. G’s wife contacts the psychiatrist reporting that her husband seems “overmedicated” and describes excess drowsiness and slowed thought processing.
After discussion with Mr. G’s internist and pharmacist, the psychiatrist decides that this oversedation may represent a drug-drug interaction between methadone and fluoxetine resulting in higher-than-expected methadone serum levels. Mr. G is instructed to stop fluoxetine with no taper, and his methadone dose is lowered with good results. Over the next 2 weeks Mr. G is titrated back to his original methadone dose and is re-evaluated by the psychiatrist to discuss medication options to address his depression.
Psychiatrists commonly encounter patients who receive opiate medications for chronic pain. Being aware of potential drug-drug interactions between opiate medications and psychotropics can help avoid adverse effects and combinations that may affect the efficacy of either drug. Pharmacokinetic interactions may affect your choice of psychiatric medication and should be taken into account when addressing adverse effects in any patient who takes opiates and psychotropics.
Metabolic pathways
The primary metabolic pathways for opiate metabolism are the cytochrome P450 (CYP) 2D6 and 3A4 isoenzymes. Depending on the agent used, prescribers may need to consider interactions for both pathways (Table 11,2 and Table 21). For example, oxycodone is metabolized via 2D6 and 3A4 isoenzymes and is a potent analgesic with oxymorphone and noroxycodone as its active metabolites. These metabolites, however, make a negligible contribution to oxycodone’s analgesic effect.3,4 Metabolism by the 3A4 isoenzyme is the principal oxidative pathway and the 2D6 site accounts for approximately 10% of oxycodone metabolism. A randomized, placebo-controlled, crossover study showed that 2D6 inhibition by paroxetine had no significant effect on oxycodone levels; however, a combination of paroxetine and itraconazole, a potent 3A4 inhibitor, resulted in substantial increases in oxycodone plasma levels.5 Remain vigilant for possible opiate toxicity when administering oxycodone with 3A4 inhibitors.
Methadone and meperidine also involve dual pathways. Methadone is metabolized primarily by 3A4 and 2B6, with 2D6 playing a smaller role.6 CYP2D6 seems to play an important part in metabolizing the R-enantiomer of methadone, which is largely responsible for the drug’s opiate effects, such as analgesia and respiratory depression.7,8 Induction of the 3A4 isoenzyme may result in methadone withdrawal, and inhibition may cause methadone toxicity.9 Inducers of 3A4, such as carbamazepine, and inhibitors, such as fluoxetine and fluvoxamine, should be avoided or used very cautiously in patients taking methadone. The 2B6 and 2D6 isoenzymes also may increase or decrease methadone levels and should be treated similarly. In Mr. G’s case, fluoxetine inhibited all 3 isoenzymes that are primarily responsible for methadone metabolism. A better antidepressant choice for Mr. G may have been venlafaxine, which is known to only mildly inhibit 2D6, or mirtazapine, which does not seem to inhibit the major CYP isoforms to an appreciable degree.10
Although the full scope of meperidine metabolism has not been identified,9 an in vitro test demonstrated that 2B6 and 3A4 play important roles in metabolizing meperidine to normeperidine, its major metabolite.11 Normeperidine does not provide analgesia and is associated with neurotoxicity, including anxiety, tremor, muscle twitching, and seizure.12 Agents that induce 3A4—such as carbamazepine or St. John’s wort—may contribute to neurotoxicity.9 Inhibition of these isoenzymes may increase meperidine levels and lead to anticholinergic toxicity or respiratory and central nervous system depression.13,14
Opiates metabolized by the 2D6 isoenzyme include codeine, hydrocodone, and tramadol. The analgesic effect of codeine seems dependent on 2D6 metabolism. Via this pathway, codeine is converted into morphine, which has a 300-times stronger affinity for the μ opioid receptor compared with codeine. 2D6 poor metabolizers have shown codeine intolerance and toxicity.3 Psychotropics known to strongly inhibit 2D6 isoenzyme processes—such as paroxetine, fluoxetine, and bupropion—should be avoided in patients taking codeine to prevent adverse effects and potential loss of efficacy. Better antidepressant choices include citalopram or venlafaxine, which inhibit 2D6 to a lesser degree.
Hydrocodone may be a viable option for patients taking 2D6 inhibitors. Hydrocodone is metabolized by 2D6 into hydromorphone, which is 7 to 33 times more potent than hydrocodone.15 Unlike codeine, 2D6 inhibition may have little effect on hydrocodone’s analgesic properties. Animal studies have shown that inhibition of the CYP analog to 2D6 does not affect analgesic response. In humans, 2D6 inhibition does not seem to affect hydrocodone’s abuse liability.16 Two case reports describe known 2D6 poor metabolizers who showed at least a partial response to hydrocodone.15,16
Tramadol’s analgesic properties may be related to serotonin and norepinephrine reuptake inhibition. It is less potent than codeine but is metabolized via the 2D6 isoenzyme into 0-desmethyltramadol, which is up to 200 times stronger than its parent compound.17 Clinicians should be aware that tramadol’s efficacy may be decreased when coadministered with 2D6 inhibitors. In a randomized, placebo-controlled trial, paroxetine, a potent 2D6 inhibitor, was shown to lessen the analgesic effect of tramadol.18
The 3A4 site is the primary pathway for fentanyl metabolism. Agents that inhibit 3A4 could increase fentanyl plasma concentration, leading to respiratory depression.19 Examples of 3A4 inhibitors include fluoxetine and fluvoxamine.
Psychotropics may inhibit or induce P450 isoenzymes to varying degrees. For example, paroxetine and citalopram are known to inhibit 2D6 but paroxetine is a stronger inhibitor; therefore, a significant drug-drug interaction is more likely with paroxetine and a 2D6 substrate than the same substrate administered with citalopram.
Table 1
Cytochrome P450 isoenzymes inhibited and induced by psychotropics
| Isoenzyme | Potency | Psychotropic(s) |
|---|---|---|
| 2B6 inducer | Moderate | Carbamazepine |
| 2B6 inhibitors | Mild to moderate | Fluoxetine, fluvoxamine |
| Moderate | Sertraline | |
| Potent | Paroxetine | |
| 2D6 inhibitors | Mild | Venlafaxine |
| Mild to moderate | Citalopram, escitalopram, fluvoxamine, risperidone | |
| Moderate | Duloxetine | |
| Moderate to potent | Bupropion | |
| Potent | Fluoxetine, haloperidol, paroxetine | |
| Dose-dependent | Sertraline | |
| 3A4 inducer | Potent | Carbamazepine |
| 3A4 inhibitors | Mild | Sertraline |
| Mild to moderate | Fluoxetine, fluvoxamine | |
| Source: References 1,2 | ||
Table 2
Cytochrome P450 isoenzymes inhibiting and inducing opiate metabolism
| Isoenzyme | Opiates |
|---|---|
| 2B6 inducer | Methadone |
| 2B6 inhibitors | Meperidine, methadone |
| 2D6 inhibitors | Codeine (may involve loss of efficacy as well as toxicity), methadone, tramadol (may involve loss of efficacy) |
| 3A4 inducer | Meperidine, methadone |
| 3A4 inhibitors | Fentanyl, oxycodone, meperidine, methadone |
| Source: Reference 1 | |
Other considerations
In addition to pharmacokinetic interactions, it is important to consider synergistic effects of some opiates and psychotropics. Examples include:
- additive effect on respiratory depression by benzodiazepines and opiates
- increased risk of serotonin syndrome and seizure when using tramadol with selective serotonin reuptake inhibitors or tricyclic antidepressants
- additive prolongation of the QTc interval by methadone when used with psychotropics known to prolong the QTc, such as ziprasidone.9,17,20
Careful attention to these interactions and collaboration among providers can ensure the best outcome for our patients. In Mr. G’s case, collaboration with his internist would be in order, particularly if antidepressant choices are limited. In consultation with the psychiatrist, the internist might choose another opiate to treat Mr. G’s pain that would not interact with fluoxetine. If Mr. G and his physician have struggled to manage his pain and if he is stable on the current regimen, selecting a different antidepressant may be warranted.
Related Resources
- Indiana University School of Medicine drug interactions: cytochrome P450 drug interaction table. http://medicine.iupui.edu/clinpharm/ddis/table.asp.
- Ferrando SJ, Levenson JL, Owen JA, eds. Clinical manual of psychopharmacology in the medically ill. Arlington, VA: American Psychiatric Publishing, Inc; 2010.
Drug Brand Names
- Bupropion • Wellbutrin, Zyban
- Carbamazepine • Tegretol
- Citalopram • Celexa
- Duloxetine • Cymbalta
- Escitalopram • Lexapro
- Fentanyl • Duragesic, Actiq
- Fluoxetine • Prozac
- Fluvoxamine • Luvox
- Haloperidol • Haldol
- Hydrocodone • Lortab, Vicodin, others
- Itraconazole • Sporanox
- Meperidine • Demerol
- Methadone • Dolophine, Methadose
- Mirtazapine • Remeron
- Morphine • Avinza, Duramorph, others
- Oxycodone • OxyContin, Roxicodone
- Paroxetine • Paxil
- Risperidone • Risperdal
- Sertraline • Zoloft
- Tramadol • Ultram
- Venlafaxine • Effexor
- Ziprasidone • Geodon
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Sandson NB, Armstrong SC, Cozza KL. An overview of psychotropic drug-drug interactions. Psychosomatics. 2005;46:464-494.
2. Faucette SR, Wang H, Hamilton GA. Regulation of CYP2B6 in primary human hepatocytes by prototypical inducers. Drug Metab Dispos. 2004;32(3):348-358.
3. Smith HS. Opioid metabolism. Mayo Clin Proc. 2009;84:613-624.
4. Armstrong SC, Cozza KL. Pharmacokinetic drug interactions of morphine codeine, and their derivatives: theory and clinical reality, part II. Psychosomatics. 2003;44:515-520.
5. Grönlund J, Saari TI, Hagelberg NM, et al. Exposure to oral oxycodone is increased by concomitant inhibition of CYP2D6 and 3A4 pathways, but not by inhibition of CYP2D6 alone. Br J Clin Pharmacol. 2010;70:78-87.
6. Leavitt SB. Methadone-drug* interactions. (*medications illicit drugs, and other substances). 3rd ed. Mundelein, IL: Addiction Treatment Forum; 2005.
7. Pérez de los Cobos J, Siñol N, Trujols J, et al. Association of CYP2D6 ultrarapid metabolizer genotype with deficient patient satisfaction regarding methadone maintenance treatment. Drug Alcohol Depend. 2007;89:190-194.
8. Kristensen K, Christensen CB, Christrup LL. The mu1 mu2, delta, kappa opioid receptor binding profiles of methadone stereoisomers and morphine. Life Sci. 1995;56:PL45-50.
9. Armstrong SC, Wynn GH, Sandson NB. Pharmacokinetic drug interactions of synthetic opiate analgesics. Psychosomatics. 2009;50:169-176.
10. Spina E, Santoro V, D’Arrigo C. Clinically relevant pharmacokinetic drug interactions with second-generation antidepressants: an update. Clin Ther. 2008;30:1206-1227.
11. Ramírez J, Innocenti F, Schuetz EG, et al. CYP2B6, CYP3A4, and CYP2C19 are responsible for the in vitro N-demethylation of meperidine in human liver microsomes. Drug Metab Dispos. 2004;32:930-936.
12. Kaiko RF, Foley KM, Grabinski PY, et al. Central nervous system excitatory effects of meperidine in cancer patients. Ann Neurol. 1983;13:180-185.
13. Chalverus C. Clinically important meperidine toxicities. Journal of Pharmaceutical Care in Pain and Symptom Control. 2001;9:37-55.
14. Beckwith MC, Fox ER, Chandramouli J. Removing meperidine from the health-system formulary—frequently asked questions. J Pain Palliat Care Pharmacother. 2002;16:45-59.
15. Foster A, Mobley E, Wang Z. Complicated pain management in a CYP450 2D6 poor metabolizer. Pain Pract. 2007;7:352-356.
16. Susce MT, Murray-Carmichael E, de Leon J. Response to hydrocodone codeine and oxycodone in a CYP2D6 poor metabolizer. Prog Neuropsychopharmacol Biol Psychiatry. 2006;30:1356-1358.
17. Sansone RA, Sansone LA. Tramadol: seizures serotonin syndrome, and coadministered antidepressants. Psychiatry (Edgmont). 2009;6:17-21.
18. Laugesen S, Enggaard TP, Pedersen RS, et al. Paroxetine, a cytochrome P450 2D6 inhibitor, diminishes the stereoselective O-demethylation and reduces the hypoalgesic effect of tramadol. Clin Pharmacol Ther. 2005;77:312-323.
19. Duragesic [package insert]. Raritan NJ: Ortho-McNeil-Janssen Pharmaceuticals, Inc; 2009.
20. Caplehorn JR, Drummer OH. Fatal methadone toxicity: signs and circumstances and the role of benzodiazepines. Aust N Z J Public Health. 2002;26:358-362;discussion 362–363.
• When choosing pharmacologic therapy, make sure that all medications your patient takes are documented, consider drug-drug interactions, and instruct the patient to notify you of any new medications.
• In addition to toxicity, loss of efficacy of some opiate drugs may occur as a result of metabolic inhibition or induction by psychotropic medications.
• Collaborate with the physician who is prescribing the opioid if psychotropic choices are limited. The patient’s pain may be treated adequately with another analgesic that does not interact with the psychotropic that has been chosen.
As prescribed by his internist, Mr. G, age 44, takes 10 mg of methadone every 4 hours for chronic back pain secondary to a work-related injury 3 years ago. He experiences minimal sedation. Mr. G presents for psychiatric evaluation with complaints of increasing irritability, poor focus, low energy, and lack of interest in usual activities. The psychiatrist diagnoses him with depressive disorder not otherwise specified, and prescribes fluoxetine, 20 mg/d. Three weeks later, Mr. G’s wife contacts the psychiatrist reporting that her husband seems “overmedicated” and describes excess drowsiness and slowed thought processing.
After discussion with Mr. G’s internist and pharmacist, the psychiatrist decides that this oversedation may represent a drug-drug interaction between methadone and fluoxetine resulting in higher-than-expected methadone serum levels. Mr. G is instructed to stop fluoxetine with no taper, and his methadone dose is lowered with good results. Over the next 2 weeks Mr. G is titrated back to his original methadone dose and is re-evaluated by the psychiatrist to discuss medication options to address his depression.
Psychiatrists commonly encounter patients who receive opiate medications for chronic pain. Being aware of potential drug-drug interactions between opiate medications and psychotropics can help avoid adverse effects and combinations that may affect the efficacy of either drug. Pharmacokinetic interactions may affect your choice of psychiatric medication and should be taken into account when addressing adverse effects in any patient who takes opiates and psychotropics.
Metabolic pathways
The primary metabolic pathways for opiate metabolism are the cytochrome P450 (CYP) 2D6 and 3A4 isoenzymes. Depending on the agent used, prescribers may need to consider interactions for both pathways (Table 11,2 and Table 21). For example, oxycodone is metabolized via 2D6 and 3A4 isoenzymes and is a potent analgesic with oxymorphone and noroxycodone as its active metabolites. These metabolites, however, make a negligible contribution to oxycodone’s analgesic effect.3,4 Metabolism by the 3A4 isoenzyme is the principal oxidative pathway and the 2D6 site accounts for approximately 10% of oxycodone metabolism. A randomized, placebo-controlled, crossover study showed that 2D6 inhibition by paroxetine had no significant effect on oxycodone levels; however, a combination of paroxetine and itraconazole, a potent 3A4 inhibitor, resulted in substantial increases in oxycodone plasma levels.5 Remain vigilant for possible opiate toxicity when administering oxycodone with 3A4 inhibitors.
Methadone and meperidine also involve dual pathways. Methadone is metabolized primarily by 3A4 and 2B6, with 2D6 playing a smaller role.6 CYP2D6 seems to play an important part in metabolizing the R-enantiomer of methadone, which is largely responsible for the drug’s opiate effects, such as analgesia and respiratory depression.7,8 Induction of the 3A4 isoenzyme may result in methadone withdrawal, and inhibition may cause methadone toxicity.9 Inducers of 3A4, such as carbamazepine, and inhibitors, such as fluoxetine and fluvoxamine, should be avoided or used very cautiously in patients taking methadone. The 2B6 and 2D6 isoenzymes also may increase or decrease methadone levels and should be treated similarly. In Mr. G’s case, fluoxetine inhibited all 3 isoenzymes that are primarily responsible for methadone metabolism. A better antidepressant choice for Mr. G may have been venlafaxine, which is known to only mildly inhibit 2D6, or mirtazapine, which does not seem to inhibit the major CYP isoforms to an appreciable degree.10
Although the full scope of meperidine metabolism has not been identified,9 an in vitro test demonstrated that 2B6 and 3A4 play important roles in metabolizing meperidine to normeperidine, its major metabolite.11 Normeperidine does not provide analgesia and is associated with neurotoxicity, including anxiety, tremor, muscle twitching, and seizure.12 Agents that induce 3A4—such as carbamazepine or St. John’s wort—may contribute to neurotoxicity.9 Inhibition of these isoenzymes may increase meperidine levels and lead to anticholinergic toxicity or respiratory and central nervous system depression.13,14
Opiates metabolized by the 2D6 isoenzyme include codeine, hydrocodone, and tramadol. The analgesic effect of codeine seems dependent on 2D6 metabolism. Via this pathway, codeine is converted into morphine, which has a 300-times stronger affinity for the μ opioid receptor compared with codeine. 2D6 poor metabolizers have shown codeine intolerance and toxicity.3 Psychotropics known to strongly inhibit 2D6 isoenzyme processes—such as paroxetine, fluoxetine, and bupropion—should be avoided in patients taking codeine to prevent adverse effects and potential loss of efficacy. Better antidepressant choices include citalopram or venlafaxine, which inhibit 2D6 to a lesser degree.
Hydrocodone may be a viable option for patients taking 2D6 inhibitors. Hydrocodone is metabolized by 2D6 into hydromorphone, which is 7 to 33 times more potent than hydrocodone.15 Unlike codeine, 2D6 inhibition may have little effect on hydrocodone’s analgesic properties. Animal studies have shown that inhibition of the CYP analog to 2D6 does not affect analgesic response. In humans, 2D6 inhibition does not seem to affect hydrocodone’s abuse liability.16 Two case reports describe known 2D6 poor metabolizers who showed at least a partial response to hydrocodone.15,16
Tramadol’s analgesic properties may be related to serotonin and norepinephrine reuptake inhibition. It is less potent than codeine but is metabolized via the 2D6 isoenzyme into 0-desmethyltramadol, which is up to 200 times stronger than its parent compound.17 Clinicians should be aware that tramadol’s efficacy may be decreased when coadministered with 2D6 inhibitors. In a randomized, placebo-controlled trial, paroxetine, a potent 2D6 inhibitor, was shown to lessen the analgesic effect of tramadol.18
The 3A4 site is the primary pathway for fentanyl metabolism. Agents that inhibit 3A4 could increase fentanyl plasma concentration, leading to respiratory depression.19 Examples of 3A4 inhibitors include fluoxetine and fluvoxamine.
Psychotropics may inhibit or induce P450 isoenzymes to varying degrees. For example, paroxetine and citalopram are known to inhibit 2D6 but paroxetine is a stronger inhibitor; therefore, a significant drug-drug interaction is more likely with paroxetine and a 2D6 substrate than the same substrate administered with citalopram.
Table 1
Cytochrome P450 isoenzymes inhibited and induced by psychotropics
| Isoenzyme | Potency | Psychotropic(s) |
|---|---|---|
| 2B6 inducer | Moderate | Carbamazepine |
| 2B6 inhibitors | Mild to moderate | Fluoxetine, fluvoxamine |
| Moderate | Sertraline | |
| Potent | Paroxetine | |
| 2D6 inhibitors | Mild | Venlafaxine |
| Mild to moderate | Citalopram, escitalopram, fluvoxamine, risperidone | |
| Moderate | Duloxetine | |
| Moderate to potent | Bupropion | |
| Potent | Fluoxetine, haloperidol, paroxetine | |
| Dose-dependent | Sertraline | |
| 3A4 inducer | Potent | Carbamazepine |
| 3A4 inhibitors | Mild | Sertraline |
| Mild to moderate | Fluoxetine, fluvoxamine | |
| Source: References 1,2 | ||
Table 2
Cytochrome P450 isoenzymes inhibiting and inducing opiate metabolism
| Isoenzyme | Opiates |
|---|---|
| 2B6 inducer | Methadone |
| 2B6 inhibitors | Meperidine, methadone |
| 2D6 inhibitors | Codeine (may involve loss of efficacy as well as toxicity), methadone, tramadol (may involve loss of efficacy) |
| 3A4 inducer | Meperidine, methadone |
| 3A4 inhibitors | Fentanyl, oxycodone, meperidine, methadone |
| Source: Reference 1 | |
Other considerations
In addition to pharmacokinetic interactions, it is important to consider synergistic effects of some opiates and psychotropics. Examples include:
- additive effect on respiratory depression by benzodiazepines and opiates
- increased risk of serotonin syndrome and seizure when using tramadol with selective serotonin reuptake inhibitors or tricyclic antidepressants
- additive prolongation of the QTc interval by methadone when used with psychotropics known to prolong the QTc, such as ziprasidone.9,17,20
Careful attention to these interactions and collaboration among providers can ensure the best outcome for our patients. In Mr. G’s case, collaboration with his internist would be in order, particularly if antidepressant choices are limited. In consultation with the psychiatrist, the internist might choose another opiate to treat Mr. G’s pain that would not interact with fluoxetine. If Mr. G and his physician have struggled to manage his pain and if he is stable on the current regimen, selecting a different antidepressant may be warranted.
Related Resources
- Indiana University School of Medicine drug interactions: cytochrome P450 drug interaction table. http://medicine.iupui.edu/clinpharm/ddis/table.asp.
- Ferrando SJ, Levenson JL, Owen JA, eds. Clinical manual of psychopharmacology in the medically ill. Arlington, VA: American Psychiatric Publishing, Inc; 2010.
Drug Brand Names
- Bupropion • Wellbutrin, Zyban
- Carbamazepine • Tegretol
- Citalopram • Celexa
- Duloxetine • Cymbalta
- Escitalopram • Lexapro
- Fentanyl • Duragesic, Actiq
- Fluoxetine • Prozac
- Fluvoxamine • Luvox
- Haloperidol • Haldol
- Hydrocodone • Lortab, Vicodin, others
- Itraconazole • Sporanox
- Meperidine • Demerol
- Methadone • Dolophine, Methadose
- Mirtazapine • Remeron
- Morphine • Avinza, Duramorph, others
- Oxycodone • OxyContin, Roxicodone
- Paroxetine • Paxil
- Risperidone • Risperdal
- Sertraline • Zoloft
- Tramadol • Ultram
- Venlafaxine • Effexor
- Ziprasidone • Geodon
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
• When choosing pharmacologic therapy, make sure that all medications your patient takes are documented, consider drug-drug interactions, and instruct the patient to notify you of any new medications.
• In addition to toxicity, loss of efficacy of some opiate drugs may occur as a result of metabolic inhibition or induction by psychotropic medications.
• Collaborate with the physician who is prescribing the opioid if psychotropic choices are limited. The patient’s pain may be treated adequately with another analgesic that does not interact with the psychotropic that has been chosen.
As prescribed by his internist, Mr. G, age 44, takes 10 mg of methadone every 4 hours for chronic back pain secondary to a work-related injury 3 years ago. He experiences minimal sedation. Mr. G presents for psychiatric evaluation with complaints of increasing irritability, poor focus, low energy, and lack of interest in usual activities. The psychiatrist diagnoses him with depressive disorder not otherwise specified, and prescribes fluoxetine, 20 mg/d. Three weeks later, Mr. G’s wife contacts the psychiatrist reporting that her husband seems “overmedicated” and describes excess drowsiness and slowed thought processing.
After discussion with Mr. G’s internist and pharmacist, the psychiatrist decides that this oversedation may represent a drug-drug interaction between methadone and fluoxetine resulting in higher-than-expected methadone serum levels. Mr. G is instructed to stop fluoxetine with no taper, and his methadone dose is lowered with good results. Over the next 2 weeks Mr. G is titrated back to his original methadone dose and is re-evaluated by the psychiatrist to discuss medication options to address his depression.
Psychiatrists commonly encounter patients who receive opiate medications for chronic pain. Being aware of potential drug-drug interactions between opiate medications and psychotropics can help avoid adverse effects and combinations that may affect the efficacy of either drug. Pharmacokinetic interactions may affect your choice of psychiatric medication and should be taken into account when addressing adverse effects in any patient who takes opiates and psychotropics.
Metabolic pathways
The primary metabolic pathways for opiate metabolism are the cytochrome P450 (CYP) 2D6 and 3A4 isoenzymes. Depending on the agent used, prescribers may need to consider interactions for both pathways (Table 11,2 and Table 21). For example, oxycodone is metabolized via 2D6 and 3A4 isoenzymes and is a potent analgesic with oxymorphone and noroxycodone as its active metabolites. These metabolites, however, make a negligible contribution to oxycodone’s analgesic effect.3,4 Metabolism by the 3A4 isoenzyme is the principal oxidative pathway and the 2D6 site accounts for approximately 10% of oxycodone metabolism. A randomized, placebo-controlled, crossover study showed that 2D6 inhibition by paroxetine had no significant effect on oxycodone levels; however, a combination of paroxetine and itraconazole, a potent 3A4 inhibitor, resulted in substantial increases in oxycodone plasma levels.5 Remain vigilant for possible opiate toxicity when administering oxycodone with 3A4 inhibitors.
Methadone and meperidine also involve dual pathways. Methadone is metabolized primarily by 3A4 and 2B6, with 2D6 playing a smaller role.6 CYP2D6 seems to play an important part in metabolizing the R-enantiomer of methadone, which is largely responsible for the drug’s opiate effects, such as analgesia and respiratory depression.7,8 Induction of the 3A4 isoenzyme may result in methadone withdrawal, and inhibition may cause methadone toxicity.9 Inducers of 3A4, such as carbamazepine, and inhibitors, such as fluoxetine and fluvoxamine, should be avoided or used very cautiously in patients taking methadone. The 2B6 and 2D6 isoenzymes also may increase or decrease methadone levels and should be treated similarly. In Mr. G’s case, fluoxetine inhibited all 3 isoenzymes that are primarily responsible for methadone metabolism. A better antidepressant choice for Mr. G may have been venlafaxine, which is known to only mildly inhibit 2D6, or mirtazapine, which does not seem to inhibit the major CYP isoforms to an appreciable degree.10
Although the full scope of meperidine metabolism has not been identified,9 an in vitro test demonstrated that 2B6 and 3A4 play important roles in metabolizing meperidine to normeperidine, its major metabolite.11 Normeperidine does not provide analgesia and is associated with neurotoxicity, including anxiety, tremor, muscle twitching, and seizure.12 Agents that induce 3A4—such as carbamazepine or St. John’s wort—may contribute to neurotoxicity.9 Inhibition of these isoenzymes may increase meperidine levels and lead to anticholinergic toxicity or respiratory and central nervous system depression.13,14
Opiates metabolized by the 2D6 isoenzyme include codeine, hydrocodone, and tramadol. The analgesic effect of codeine seems dependent on 2D6 metabolism. Via this pathway, codeine is converted into morphine, which has a 300-times stronger affinity for the μ opioid receptor compared with codeine. 2D6 poor metabolizers have shown codeine intolerance and toxicity.3 Psychotropics known to strongly inhibit 2D6 isoenzyme processes—such as paroxetine, fluoxetine, and bupropion—should be avoided in patients taking codeine to prevent adverse effects and potential loss of efficacy. Better antidepressant choices include citalopram or venlafaxine, which inhibit 2D6 to a lesser degree.
Hydrocodone may be a viable option for patients taking 2D6 inhibitors. Hydrocodone is metabolized by 2D6 into hydromorphone, which is 7 to 33 times more potent than hydrocodone.15 Unlike codeine, 2D6 inhibition may have little effect on hydrocodone’s analgesic properties. Animal studies have shown that inhibition of the CYP analog to 2D6 does not affect analgesic response. In humans, 2D6 inhibition does not seem to affect hydrocodone’s abuse liability.16 Two case reports describe known 2D6 poor metabolizers who showed at least a partial response to hydrocodone.15,16
Tramadol’s analgesic properties may be related to serotonin and norepinephrine reuptake inhibition. It is less potent than codeine but is metabolized via the 2D6 isoenzyme into 0-desmethyltramadol, which is up to 200 times stronger than its parent compound.17 Clinicians should be aware that tramadol’s efficacy may be decreased when coadministered with 2D6 inhibitors. In a randomized, placebo-controlled trial, paroxetine, a potent 2D6 inhibitor, was shown to lessen the analgesic effect of tramadol.18
The 3A4 site is the primary pathway for fentanyl metabolism. Agents that inhibit 3A4 could increase fentanyl plasma concentration, leading to respiratory depression.19 Examples of 3A4 inhibitors include fluoxetine and fluvoxamine.
Psychotropics may inhibit or induce P450 isoenzymes to varying degrees. For example, paroxetine and citalopram are known to inhibit 2D6 but paroxetine is a stronger inhibitor; therefore, a significant drug-drug interaction is more likely with paroxetine and a 2D6 substrate than the same substrate administered with citalopram.
Table 1
Cytochrome P450 isoenzymes inhibited and induced by psychotropics
| Isoenzyme | Potency | Psychotropic(s) |
|---|---|---|
| 2B6 inducer | Moderate | Carbamazepine |
| 2B6 inhibitors | Mild to moderate | Fluoxetine, fluvoxamine |
| Moderate | Sertraline | |
| Potent | Paroxetine | |
| 2D6 inhibitors | Mild | Venlafaxine |
| Mild to moderate | Citalopram, escitalopram, fluvoxamine, risperidone | |
| Moderate | Duloxetine | |
| Moderate to potent | Bupropion | |
| Potent | Fluoxetine, haloperidol, paroxetine | |
| Dose-dependent | Sertraline | |
| 3A4 inducer | Potent | Carbamazepine |
| 3A4 inhibitors | Mild | Sertraline |
| Mild to moderate | Fluoxetine, fluvoxamine | |
| Source: References 1,2 | ||
Table 2
Cytochrome P450 isoenzymes inhibiting and inducing opiate metabolism
| Isoenzyme | Opiates |
|---|---|
| 2B6 inducer | Methadone |
| 2B6 inhibitors | Meperidine, methadone |
| 2D6 inhibitors | Codeine (may involve loss of efficacy as well as toxicity), methadone, tramadol (may involve loss of efficacy) |
| 3A4 inducer | Meperidine, methadone |
| 3A4 inhibitors | Fentanyl, oxycodone, meperidine, methadone |
| Source: Reference 1 | |
Other considerations
In addition to pharmacokinetic interactions, it is important to consider synergistic effects of some opiates and psychotropics. Examples include:
- additive effect on respiratory depression by benzodiazepines and opiates
- increased risk of serotonin syndrome and seizure when using tramadol with selective serotonin reuptake inhibitors or tricyclic antidepressants
- additive prolongation of the QTc interval by methadone when used with psychotropics known to prolong the QTc, such as ziprasidone.9,17,20
Careful attention to these interactions and collaboration among providers can ensure the best outcome for our patients. In Mr. G’s case, collaboration with his internist would be in order, particularly if antidepressant choices are limited. In consultation with the psychiatrist, the internist might choose another opiate to treat Mr. G’s pain that would not interact with fluoxetine. If Mr. G and his physician have struggled to manage his pain and if he is stable on the current regimen, selecting a different antidepressant may be warranted.
Related Resources
- Indiana University School of Medicine drug interactions: cytochrome P450 drug interaction table. http://medicine.iupui.edu/clinpharm/ddis/table.asp.
- Ferrando SJ, Levenson JL, Owen JA, eds. Clinical manual of psychopharmacology in the medically ill. Arlington, VA: American Psychiatric Publishing, Inc; 2010.
Drug Brand Names
- Bupropion • Wellbutrin, Zyban
- Carbamazepine • Tegretol
- Citalopram • Celexa
- Duloxetine • Cymbalta
- Escitalopram • Lexapro
- Fentanyl • Duragesic, Actiq
- Fluoxetine • Prozac
- Fluvoxamine • Luvox
- Haloperidol • Haldol
- Hydrocodone • Lortab, Vicodin, others
- Itraconazole • Sporanox
- Meperidine • Demerol
- Methadone • Dolophine, Methadose
- Mirtazapine • Remeron
- Morphine • Avinza, Duramorph, others
- Oxycodone • OxyContin, Roxicodone
- Paroxetine • Paxil
- Risperidone • Risperdal
- Sertraline • Zoloft
- Tramadol • Ultram
- Venlafaxine • Effexor
- Ziprasidone • Geodon
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Sandson NB, Armstrong SC, Cozza KL. An overview of psychotropic drug-drug interactions. Psychosomatics. 2005;46:464-494.
2. Faucette SR, Wang H, Hamilton GA. Regulation of CYP2B6 in primary human hepatocytes by prototypical inducers. Drug Metab Dispos. 2004;32(3):348-358.
3. Smith HS. Opioid metabolism. Mayo Clin Proc. 2009;84:613-624.
4. Armstrong SC, Cozza KL. Pharmacokinetic drug interactions of morphine codeine, and their derivatives: theory and clinical reality, part II. Psychosomatics. 2003;44:515-520.
5. Grönlund J, Saari TI, Hagelberg NM, et al. Exposure to oral oxycodone is increased by concomitant inhibition of CYP2D6 and 3A4 pathways, but not by inhibition of CYP2D6 alone. Br J Clin Pharmacol. 2010;70:78-87.
6. Leavitt SB. Methadone-drug* interactions. (*medications illicit drugs, and other substances). 3rd ed. Mundelein, IL: Addiction Treatment Forum; 2005.
7. Pérez de los Cobos J, Siñol N, Trujols J, et al. Association of CYP2D6 ultrarapid metabolizer genotype with deficient patient satisfaction regarding methadone maintenance treatment. Drug Alcohol Depend. 2007;89:190-194.
8. Kristensen K, Christensen CB, Christrup LL. The mu1 mu2, delta, kappa opioid receptor binding profiles of methadone stereoisomers and morphine. Life Sci. 1995;56:PL45-50.
9. Armstrong SC, Wynn GH, Sandson NB. Pharmacokinetic drug interactions of synthetic opiate analgesics. Psychosomatics. 2009;50:169-176.
10. Spina E, Santoro V, D’Arrigo C. Clinically relevant pharmacokinetic drug interactions with second-generation antidepressants: an update. Clin Ther. 2008;30:1206-1227.
11. Ramírez J, Innocenti F, Schuetz EG, et al. CYP2B6, CYP3A4, and CYP2C19 are responsible for the in vitro N-demethylation of meperidine in human liver microsomes. Drug Metab Dispos. 2004;32:930-936.
12. Kaiko RF, Foley KM, Grabinski PY, et al. Central nervous system excitatory effects of meperidine in cancer patients. Ann Neurol. 1983;13:180-185.
13. Chalverus C. Clinically important meperidine toxicities. Journal of Pharmaceutical Care in Pain and Symptom Control. 2001;9:37-55.
14. Beckwith MC, Fox ER, Chandramouli J. Removing meperidine from the health-system formulary—frequently asked questions. J Pain Palliat Care Pharmacother. 2002;16:45-59.
15. Foster A, Mobley E, Wang Z. Complicated pain management in a CYP450 2D6 poor metabolizer. Pain Pract. 2007;7:352-356.
16. Susce MT, Murray-Carmichael E, de Leon J. Response to hydrocodone codeine and oxycodone in a CYP2D6 poor metabolizer. Prog Neuropsychopharmacol Biol Psychiatry. 2006;30:1356-1358.
17. Sansone RA, Sansone LA. Tramadol: seizures serotonin syndrome, and coadministered antidepressants. Psychiatry (Edgmont). 2009;6:17-21.
18. Laugesen S, Enggaard TP, Pedersen RS, et al. Paroxetine, a cytochrome P450 2D6 inhibitor, diminishes the stereoselective O-demethylation and reduces the hypoalgesic effect of tramadol. Clin Pharmacol Ther. 2005;77:312-323.
19. Duragesic [package insert]. Raritan NJ: Ortho-McNeil-Janssen Pharmaceuticals, Inc; 2009.
20. Caplehorn JR, Drummer OH. Fatal methadone toxicity: signs and circumstances and the role of benzodiazepines. Aust N Z J Public Health. 2002;26:358-362;discussion 362–363.
1. Sandson NB, Armstrong SC, Cozza KL. An overview of psychotropic drug-drug interactions. Psychosomatics. 2005;46:464-494.
2. Faucette SR, Wang H, Hamilton GA. Regulation of CYP2B6 in primary human hepatocytes by prototypical inducers. Drug Metab Dispos. 2004;32(3):348-358.
3. Smith HS. Opioid metabolism. Mayo Clin Proc. 2009;84:613-624.
4. Armstrong SC, Cozza KL. Pharmacokinetic drug interactions of morphine codeine, and their derivatives: theory and clinical reality, part II. Psychosomatics. 2003;44:515-520.
5. Grönlund J, Saari TI, Hagelberg NM, et al. Exposure to oral oxycodone is increased by concomitant inhibition of CYP2D6 and 3A4 pathways, but not by inhibition of CYP2D6 alone. Br J Clin Pharmacol. 2010;70:78-87.
6. Leavitt SB. Methadone-drug* interactions. (*medications illicit drugs, and other substances). 3rd ed. Mundelein, IL: Addiction Treatment Forum; 2005.
7. Pérez de los Cobos J, Siñol N, Trujols J, et al. Association of CYP2D6 ultrarapid metabolizer genotype with deficient patient satisfaction regarding methadone maintenance treatment. Drug Alcohol Depend. 2007;89:190-194.
8. Kristensen K, Christensen CB, Christrup LL. The mu1 mu2, delta, kappa opioid receptor binding profiles of methadone stereoisomers and morphine. Life Sci. 1995;56:PL45-50.
9. Armstrong SC, Wynn GH, Sandson NB. Pharmacokinetic drug interactions of synthetic opiate analgesics. Psychosomatics. 2009;50:169-176.
10. Spina E, Santoro V, D’Arrigo C. Clinically relevant pharmacokinetic drug interactions with second-generation antidepressants: an update. Clin Ther. 2008;30:1206-1227.
11. Ramírez J, Innocenti F, Schuetz EG, et al. CYP2B6, CYP3A4, and CYP2C19 are responsible for the in vitro N-demethylation of meperidine in human liver microsomes. Drug Metab Dispos. 2004;32:930-936.
12. Kaiko RF, Foley KM, Grabinski PY, et al. Central nervous system excitatory effects of meperidine in cancer patients. Ann Neurol. 1983;13:180-185.
13. Chalverus C. Clinically important meperidine toxicities. Journal of Pharmaceutical Care in Pain and Symptom Control. 2001;9:37-55.
14. Beckwith MC, Fox ER, Chandramouli J. Removing meperidine from the health-system formulary—frequently asked questions. J Pain Palliat Care Pharmacother. 2002;16:45-59.
15. Foster A, Mobley E, Wang Z. Complicated pain management in a CYP450 2D6 poor metabolizer. Pain Pract. 2007;7:352-356.
16. Susce MT, Murray-Carmichael E, de Leon J. Response to hydrocodone codeine and oxycodone in a CYP2D6 poor metabolizer. Prog Neuropsychopharmacol Biol Psychiatry. 2006;30:1356-1358.
17. Sansone RA, Sansone LA. Tramadol: seizures serotonin syndrome, and coadministered antidepressants. Psychiatry (Edgmont). 2009;6:17-21.
18. Laugesen S, Enggaard TP, Pedersen RS, et al. Paroxetine, a cytochrome P450 2D6 inhibitor, diminishes the stereoselective O-demethylation and reduces the hypoalgesic effect of tramadol. Clin Pharmacol Ther. 2005;77:312-323.
19. Duragesic [package insert]. Raritan NJ: Ortho-McNeil-Janssen Pharmaceuticals, Inc; 2009.
20. Caplehorn JR, Drummer OH. Fatal methadone toxicity: signs and circumstances and the role of benzodiazepines. Aust N Z J Public Health. 2002;26:358-362;discussion 362–363.
Should you prescribe medications for family and friends?
Dear Dr. Mossman:
On a recent golf outing, my buddy Mike told me about his trouble staying “focused” while studying for his grad school exams. He asked me to write him a prescription for methylphenidate, which he had taken in high school and college. I want to help Mike, but I’m worried about my liability if something goes wrong. What should I do?—Submitted by “Dr. C”
Doctors learn early in their careers that family, friends, or coworkers often seek informal medical advice and ask for prescriptions. Also, doctors commonly diagnose and medicate themselves rather than seek care from other professionals.1,2
In this article, we use the phrase “casual prescribing” to describe activities related to prescribing drugs for individuals such as Mike, a friend who has sought medication outside Dr. C’s customary practice setting. Despite having good intentions, you’re probably increasing your malpractice liability whenever you casually prescribe medication. Even more serious, if you casually prescribe controlled substances (eg, stimulants), you risk investigation and potential sanction by your state medical licensing agency.
- Submit your malpractice-related questions to Dr. Mossman at [email protected].
- Include your name, address, and practice location. If your question is chosen for publication, your name can be withheld by request.
To decide whether, how, and when you may prescribe drugs for yourself, family members, colleagues, or friends, you need to:
- anticipate being asked to casually prescribe
- understand the emotions and forces that drive casual prescribing
- know your state medical board’s rules and regulations
- be prepared with an appropriate response.
After we explore these points, we’ll consider what Dr. C might do.
A common request
People often seek medical advice outside doctors’ offices. Playing a sport together, sitting on an airplane, or sharing other social activities strips away the veneer of formality, lets people relax, makes doctors seem more approachable, and allows medical concerns to come forth more easily.3
Access to medical care is a problem for lay people and doctors alike. In many locales, simply getting an appointment with a primary care physician or psychiatrist is difficult.4,5 Navigating health insurance rules and referral lists is frustrating. When people find a provider, they may feel guilty about taking a slot from someone else. Job expectations or a tough economy can make employees reluctant to take time off work6,7 or concerned that they’ll miss productivity goals because of illness.1
Doctors often self-prescribe to avoid facing the stigma of being ill. Although doctors should know better, many of us don’t want to experience the vulnerability that comes with being sick and needing health care. Some doctors fear colleagues’ scrutiny if their serious mental illness (eg, depression) becomes known, or they would rather treat themselves than seek professional help.1 The most formidable obstacle physicians face is time—or lack of it. Many doctors work >60 hours per week, and their dedication and altruism causes them to neglect their own health until illness interferes with their professional lives.8
Emotional factors
Doctors pride themselves on knowing how to help people, and when loved ones or colleagues ask for our help, it’s gratifying and flattering.3 Such feelings may help explain why the largest numbers of prescriptions written for non-patients are for family members and friends, followed by prescriptions written by residents for fellow house officers.9
The circumstances surrounding casual prescribing usually make it difficult to maintain objectivity, avoid substandard care, uphold ethical principles, and handle discomfort. Your professional objectivity and clinical judgment likely are compromised when a close friend, an immediate family member, or you yourself are the patient.10 Treating loved ones and close friends can make it awkward to ask about sensitive matters (eg, “How much alcohol do you drink?”) or to perform intimate parts of a physical examination. Physicians who want to “go the extra mile” for family members or friends may try to treat problems that are beyond their expertise or training—a setup for failing to meet your legal and medical obligations to conform to the prevailing standard of care.11
State medical board rules
The American Medical Association, British Medical Association, and Canadian Medical Association all discourage physicians from prescribing for themselves or family members.2Table 110,12-16 gives examples of states’ comments and guidelines relevant to casual prescribing. Overwhelmingly, state medical boards tell you that casual prescribing is ill-advised. However, in emergencies or in isolated settings where no other qualified physician is readily available, you should provide needed treatment for yourself, family, friends, or colleagues until another physician can assume care. Physicians should not be the primary or regular care providers for their immediate family members, but giving routine care for short-term, minor problems may be acceptable.14 Although state medical boards use differing language, all agree that casual prescribing requires assessment and documentation similar to what you do for patients seen in your regular practice setting. Table 2 summarizes appropriate casual prescribing practices, but you should also know the boards’ rules in the locales where you work.
Restrictions and rules for prescribing controlled substances are stricter, despite many doctors’ sometime-lax attitudes. State medical boards tell doctors not to prescribe controlled substances for friends, family, or themselves except in emergencies. Yet studies have found that house officers often write prescriptions for psychoactive drugs (including narcotics) for family members, friends, and colleagues9 and that residents are willing to prescribe codeine for a hypothetical colleague with pain from a fractured finger.17
Table 1
Selected state medical board rules and comments on casual prescribing
| State | Rules |
|---|---|
| California12 | ‘[E]valuating, diagnosing, treating, or prescribing to family members, co-workers, or friends…is discouraged’ and requires ‘the same practice/protocol for any patient in which medications are prescribed,’ including a ‘good faith exam’ and documentation that justifies the prescription |
| Montana13 | Although prescribing for one’s family or oneself is not prohibited, doing so ‘arguably…does not meet the general accepted standards of practice, and is therefore unprofessional conduct [that] may subject the physician to license discipline’ |
| New Hampshire14 | ‘Physicians generally should not treat themselves or members of their immediate families.…Except in emergencies, it is not appropriate for physicians to write prescriptions for controlled substances for themselves or immediate family members’ |
| Ohio15 | ‘[I]t is almost always a bad idea to treat a family member’s chronic condition, serious illness, or psychiatric/emotional problems’ |
| South Carolina10 | Treating immediate family members may produce less than optimal care. ‘[P]rescribing controlled substances for family members is outside the scope of good medical practice except for a bona fide emergency situation’ |
| Virginia16 | Prescriptions ‘must be based on a bona fide practitioner-patient relationship. Practitioners should obtain a medical or drug history, provide information about risks, benefits, and side effects, perform an exam, and initiate follow-up care. Practitioners should not prescribe controlled substances for themselves or family members except in emergencies, isolated settings, or for a single episode of an acute illness’ |
Table 2
Cautions and recommendations for casual prescribing
| Avoid doing it in non-emergencies |
| Obtain a medical and drug history |
| Perform an appropriate, good-faith exam |
| Create a medical record that documents the need for a prescription |
| Prescribe controlled substances only in emergencies or isolated settings |
| Inform your patient about risks, benefits, and side effects |
| Initiate needed additional interventions and follow-up care |
| Maintain confidentiality and respect HIPAA rules |
| Ask yourself, ‘Can I avoid this—is there another option?’ If the answer is ‘yes,’ don’t do it |
| HIPAA: Health Insurance Portability and Accountability Act |
Liability risk
Most residents are unaware of federal or state regulations addressing the appropriateness of prescription writing for non-patients.18 A survey of U.S. internal medicine and family practice residents at a teaching hospital found that less than a quarter believed that ethical guidelines on prescription writing existed.17 Such deficits can make malpractice liability more likely if something “goes wrong” with your casually prescribed treatment. Friends and relatives do sue doctors whom they have consulted informally,18 and casual prescribing can be hard to defend in court because it usually looks suspicious and is not well documented.
Revisiting Mike’s case
Understandably, Dr. C wants to help Mike and may even think he has a condition (eg, adult attention-deficit/hyperactivity disorder) for which a stimulant would be appropriate. But respect for Mike’s humanity—the paramount value in medical practice19—suggests that his treatment should occur after and because of a careful medical assessment rather than a golf game. Moreover, prescribing a controlled substance in a non-emergency likely would violate standards of practice promulgated by Dr. C’s medical board. Dr. C should tell Mike that his problem deserves thoughtful evaluation and suggest that Mike see his primary physician. Dr. C also could recommend psychiatrists whom Mike might consult.
Related Resource
- Aboff B, Collier V, Farber N. Residents’ prescription writing for nonpatients. JAMA. 2002;288:381-385.
Drug Brand Names
- Codeine • Tylenol with Codeine, others
- Methylphenidate • Ritalin
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Be prepared to be asked for advice and prescriptions in casual settings. When this happens, it’s fine to provide general medical information, but it’s best not to give specific advice or engage in “casual prescribing.” You can maintain social connections, be caring, and avoid boundary violations by responding with tact, referral information, and good judgment.19,20
1. Balon R. Psychiatrist attitudes toward self-treatment of their own depression. Psychother Psychosom. 2007;76:306-310.
2. Walter JK, Lang CW, Ross LF. When physicians forego the doctor-patient relationship should they elect to self-prescribe or curbside? An empirical and ethical analysis. J Med Ethics. 2010;36:19-23.
3. Reynolds H. Medical ear in the early morning tennis group—when to advise and what to say. Pharos Alpha Omega Alpha Honor Med Soc. 2010;73:14-15 discussion 16.
4. Sataline S, Wang SS. Medical schools can’t keep up: as ranks of insured expand nation faces shortage of 150,000 doctors in 15 years. Available at: http://online.wsj.com/article/SB10001424052702304506904575180331528424238.html. Accessed March 21, 2011.
5. Steinberg S. Of medical specialties demand for psychiatrists growing fastest. USA Today. July 1, 2010:6D.
6. Leonhardt D. A labor market punishing to mothers. New York Times. August 4 2010:B1.
7. Madden K. Reluctant to go on vacation? Available at: http://www.cnn.com/2010/LIVING/08/04/cb.reluctant.to.take.vacation/index.html. Accessed March 20 2011.
8. Meier DE, Back AL, Morrison RS. The inner life of physicians and care of the seriously ill. JAMA. 2001;286:3007-3014.
9. Clark A, Kau J. Patterns of psychoactive drug prescriptions by house officers for nonpatients. J Med Educ. 1988;63:44-50.
10. State Medical Board of South Carolina. Prescribing for family members. Available at: http://www.llr.state.sc.us/pol/medical/index.asp?file=Policies/MEPRESCRIBEFAM.HTM. Accessed March 20 2011.
11. Dietz LH, Jacobs A, Leming TL, et al. Physicians, surgeons, and other healers, §§130, 216-218. In: American jurisprudence. vol 61. 2nd ed. New York, NY: Thomson Reuters; 2010.
12. Medical Board of California. General office practices/protocols-frequently asked questions. Available at: http://www.medbd.ca.gov/consumer/complaint_info_questions_practice.html#13. Accessed March 20 2011.
13. Montana Board of Medical Examiners. Statement of physician prescribing for self or members of the physician’s immediate family. Available at: http://bsd.dli.mt.gov/license/bsd_boards/med_board/pdf/prescribing_self.pdf. Accessed March 20 2011.
14. New Hampshire Medical Board. Guidelines for self-prescribing and prescribing for family members. Available at: http://www.nh.gov/medicine/aboutus/self_presc.htm. Accessed March 21 2011.
15. State Medical Board of Ohio. Frequently asked questions. Available at: http://www.med.ohio.gov/professional_guidelines.htm. Accessed March 20 2011.
16. Virginia Board of Medicine. Can I prescribe for my family and myself? Available at: http://www.dhp.virginia.gov/Medicine/medicine_faq.htm#Prescribe. Accessed March 20 2011.
17. Aboff B, Collier V, Farber N. Residents’ prescription writing for nonpatients. JAMA. 2002;288:381-385.
18. Johnson LJ. Malpractice consult. Should you give informal medical advice? Med Econ. 2007;84:36.-
19. Nisselle P. Danger zone: when boundaries are crossed in the doctor-patient relationship. Aust Family Physician. 2000;29:541-544.
20. Eastwood GL. When relatives and friends ask physicians for medical advice: ethical legal, and practical considerations. J Gen Intern Med. 2009;24:1333-1335.
Dear Dr. Mossman:
On a recent golf outing, my buddy Mike told me about his trouble staying “focused” while studying for his grad school exams. He asked me to write him a prescription for methylphenidate, which he had taken in high school and college. I want to help Mike, but I’m worried about my liability if something goes wrong. What should I do?—Submitted by “Dr. C”
Doctors learn early in their careers that family, friends, or coworkers often seek informal medical advice and ask for prescriptions. Also, doctors commonly diagnose and medicate themselves rather than seek care from other professionals.1,2
In this article, we use the phrase “casual prescribing” to describe activities related to prescribing drugs for individuals such as Mike, a friend who has sought medication outside Dr. C’s customary practice setting. Despite having good intentions, you’re probably increasing your malpractice liability whenever you casually prescribe medication. Even more serious, if you casually prescribe controlled substances (eg, stimulants), you risk investigation and potential sanction by your state medical licensing agency.
- Submit your malpractice-related questions to Dr. Mossman at [email protected].
- Include your name, address, and practice location. If your question is chosen for publication, your name can be withheld by request.
To decide whether, how, and when you may prescribe drugs for yourself, family members, colleagues, or friends, you need to:
- anticipate being asked to casually prescribe
- understand the emotions and forces that drive casual prescribing
- know your state medical board’s rules and regulations
- be prepared with an appropriate response.
After we explore these points, we’ll consider what Dr. C might do.
A common request
People often seek medical advice outside doctors’ offices. Playing a sport together, sitting on an airplane, or sharing other social activities strips away the veneer of formality, lets people relax, makes doctors seem more approachable, and allows medical concerns to come forth more easily.3
Access to medical care is a problem for lay people and doctors alike. In many locales, simply getting an appointment with a primary care physician or psychiatrist is difficult.4,5 Navigating health insurance rules and referral lists is frustrating. When people find a provider, they may feel guilty about taking a slot from someone else. Job expectations or a tough economy can make employees reluctant to take time off work6,7 or concerned that they’ll miss productivity goals because of illness.1
Doctors often self-prescribe to avoid facing the stigma of being ill. Although doctors should know better, many of us don’t want to experience the vulnerability that comes with being sick and needing health care. Some doctors fear colleagues’ scrutiny if their serious mental illness (eg, depression) becomes known, or they would rather treat themselves than seek professional help.1 The most formidable obstacle physicians face is time—or lack of it. Many doctors work >60 hours per week, and their dedication and altruism causes them to neglect their own health until illness interferes with their professional lives.8
Emotional factors
Doctors pride themselves on knowing how to help people, and when loved ones or colleagues ask for our help, it’s gratifying and flattering.3 Such feelings may help explain why the largest numbers of prescriptions written for non-patients are for family members and friends, followed by prescriptions written by residents for fellow house officers.9
The circumstances surrounding casual prescribing usually make it difficult to maintain objectivity, avoid substandard care, uphold ethical principles, and handle discomfort. Your professional objectivity and clinical judgment likely are compromised when a close friend, an immediate family member, or you yourself are the patient.10 Treating loved ones and close friends can make it awkward to ask about sensitive matters (eg, “How much alcohol do you drink?”) or to perform intimate parts of a physical examination. Physicians who want to “go the extra mile” for family members or friends may try to treat problems that are beyond their expertise or training—a setup for failing to meet your legal and medical obligations to conform to the prevailing standard of care.11
State medical board rules
The American Medical Association, British Medical Association, and Canadian Medical Association all discourage physicians from prescribing for themselves or family members.2Table 110,12-16 gives examples of states’ comments and guidelines relevant to casual prescribing. Overwhelmingly, state medical boards tell you that casual prescribing is ill-advised. However, in emergencies or in isolated settings where no other qualified physician is readily available, you should provide needed treatment for yourself, family, friends, or colleagues until another physician can assume care. Physicians should not be the primary or regular care providers for their immediate family members, but giving routine care for short-term, minor problems may be acceptable.14 Although state medical boards use differing language, all agree that casual prescribing requires assessment and documentation similar to what you do for patients seen in your regular practice setting. Table 2 summarizes appropriate casual prescribing practices, but you should also know the boards’ rules in the locales where you work.
Restrictions and rules for prescribing controlled substances are stricter, despite many doctors’ sometime-lax attitudes. State medical boards tell doctors not to prescribe controlled substances for friends, family, or themselves except in emergencies. Yet studies have found that house officers often write prescriptions for psychoactive drugs (including narcotics) for family members, friends, and colleagues9 and that residents are willing to prescribe codeine for a hypothetical colleague with pain from a fractured finger.17
Table 1
Selected state medical board rules and comments on casual prescribing
| State | Rules |
|---|---|
| California12 | ‘[E]valuating, diagnosing, treating, or prescribing to family members, co-workers, or friends…is discouraged’ and requires ‘the same practice/protocol for any patient in which medications are prescribed,’ including a ‘good faith exam’ and documentation that justifies the prescription |
| Montana13 | Although prescribing for one’s family or oneself is not prohibited, doing so ‘arguably…does not meet the general accepted standards of practice, and is therefore unprofessional conduct [that] may subject the physician to license discipline’ |
| New Hampshire14 | ‘Physicians generally should not treat themselves or members of their immediate families.…Except in emergencies, it is not appropriate for physicians to write prescriptions for controlled substances for themselves or immediate family members’ |
| Ohio15 | ‘[I]t is almost always a bad idea to treat a family member’s chronic condition, serious illness, or psychiatric/emotional problems’ |
| South Carolina10 | Treating immediate family members may produce less than optimal care. ‘[P]rescribing controlled substances for family members is outside the scope of good medical practice except for a bona fide emergency situation’ |
| Virginia16 | Prescriptions ‘must be based on a bona fide practitioner-patient relationship. Practitioners should obtain a medical or drug history, provide information about risks, benefits, and side effects, perform an exam, and initiate follow-up care. Practitioners should not prescribe controlled substances for themselves or family members except in emergencies, isolated settings, or for a single episode of an acute illness’ |
Table 2
Cautions and recommendations for casual prescribing
| Avoid doing it in non-emergencies |
| Obtain a medical and drug history |
| Perform an appropriate, good-faith exam |
| Create a medical record that documents the need for a prescription |
| Prescribe controlled substances only in emergencies or isolated settings |
| Inform your patient about risks, benefits, and side effects |
| Initiate needed additional interventions and follow-up care |
| Maintain confidentiality and respect HIPAA rules |
| Ask yourself, ‘Can I avoid this—is there another option?’ If the answer is ‘yes,’ don’t do it |
| HIPAA: Health Insurance Portability and Accountability Act |
Liability risk
Most residents are unaware of federal or state regulations addressing the appropriateness of prescription writing for non-patients.18 A survey of U.S. internal medicine and family practice residents at a teaching hospital found that less than a quarter believed that ethical guidelines on prescription writing existed.17 Such deficits can make malpractice liability more likely if something “goes wrong” with your casually prescribed treatment. Friends and relatives do sue doctors whom they have consulted informally,18 and casual prescribing can be hard to defend in court because it usually looks suspicious and is not well documented.
Revisiting Mike’s case
Understandably, Dr. C wants to help Mike and may even think he has a condition (eg, adult attention-deficit/hyperactivity disorder) for which a stimulant would be appropriate. But respect for Mike’s humanity—the paramount value in medical practice19—suggests that his treatment should occur after and because of a careful medical assessment rather than a golf game. Moreover, prescribing a controlled substance in a non-emergency likely would violate standards of practice promulgated by Dr. C’s medical board. Dr. C should tell Mike that his problem deserves thoughtful evaluation and suggest that Mike see his primary physician. Dr. C also could recommend psychiatrists whom Mike might consult.
Related Resource
- Aboff B, Collier V, Farber N. Residents’ prescription writing for nonpatients. JAMA. 2002;288:381-385.
Drug Brand Names
- Codeine • Tylenol with Codeine, others
- Methylphenidate • Ritalin
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Be prepared to be asked for advice and prescriptions in casual settings. When this happens, it’s fine to provide general medical information, but it’s best not to give specific advice or engage in “casual prescribing.” You can maintain social connections, be caring, and avoid boundary violations by responding with tact, referral information, and good judgment.19,20
Dear Dr. Mossman:
On a recent golf outing, my buddy Mike told me about his trouble staying “focused” while studying for his grad school exams. He asked me to write him a prescription for methylphenidate, which he had taken in high school and college. I want to help Mike, but I’m worried about my liability if something goes wrong. What should I do?—Submitted by “Dr. C”
Doctors learn early in their careers that family, friends, or coworkers often seek informal medical advice and ask for prescriptions. Also, doctors commonly diagnose and medicate themselves rather than seek care from other professionals.1,2
In this article, we use the phrase “casual prescribing” to describe activities related to prescribing drugs for individuals such as Mike, a friend who has sought medication outside Dr. C’s customary practice setting. Despite having good intentions, you’re probably increasing your malpractice liability whenever you casually prescribe medication. Even more serious, if you casually prescribe controlled substances (eg, stimulants), you risk investigation and potential sanction by your state medical licensing agency.
- Submit your malpractice-related questions to Dr. Mossman at [email protected].
- Include your name, address, and practice location. If your question is chosen for publication, your name can be withheld by request.
To decide whether, how, and when you may prescribe drugs for yourself, family members, colleagues, or friends, you need to:
- anticipate being asked to casually prescribe
- understand the emotions and forces that drive casual prescribing
- know your state medical board’s rules and regulations
- be prepared with an appropriate response.
After we explore these points, we’ll consider what Dr. C might do.
A common request
People often seek medical advice outside doctors’ offices. Playing a sport together, sitting on an airplane, or sharing other social activities strips away the veneer of formality, lets people relax, makes doctors seem more approachable, and allows medical concerns to come forth more easily.3
Access to medical care is a problem for lay people and doctors alike. In many locales, simply getting an appointment with a primary care physician or psychiatrist is difficult.4,5 Navigating health insurance rules and referral lists is frustrating. When people find a provider, they may feel guilty about taking a slot from someone else. Job expectations or a tough economy can make employees reluctant to take time off work6,7 or concerned that they’ll miss productivity goals because of illness.1
Doctors often self-prescribe to avoid facing the stigma of being ill. Although doctors should know better, many of us don’t want to experience the vulnerability that comes with being sick and needing health care. Some doctors fear colleagues’ scrutiny if their serious mental illness (eg, depression) becomes known, or they would rather treat themselves than seek professional help.1 The most formidable obstacle physicians face is time—or lack of it. Many doctors work >60 hours per week, and their dedication and altruism causes them to neglect their own health until illness interferes with their professional lives.8
Emotional factors
Doctors pride themselves on knowing how to help people, and when loved ones or colleagues ask for our help, it’s gratifying and flattering.3 Such feelings may help explain why the largest numbers of prescriptions written for non-patients are for family members and friends, followed by prescriptions written by residents for fellow house officers.9
The circumstances surrounding casual prescribing usually make it difficult to maintain objectivity, avoid substandard care, uphold ethical principles, and handle discomfort. Your professional objectivity and clinical judgment likely are compromised when a close friend, an immediate family member, or you yourself are the patient.10 Treating loved ones and close friends can make it awkward to ask about sensitive matters (eg, “How much alcohol do you drink?”) or to perform intimate parts of a physical examination. Physicians who want to “go the extra mile” for family members or friends may try to treat problems that are beyond their expertise or training—a setup for failing to meet your legal and medical obligations to conform to the prevailing standard of care.11
State medical board rules
The American Medical Association, British Medical Association, and Canadian Medical Association all discourage physicians from prescribing for themselves or family members.2Table 110,12-16 gives examples of states’ comments and guidelines relevant to casual prescribing. Overwhelmingly, state medical boards tell you that casual prescribing is ill-advised. However, in emergencies or in isolated settings where no other qualified physician is readily available, you should provide needed treatment for yourself, family, friends, or colleagues until another physician can assume care. Physicians should not be the primary or regular care providers for their immediate family members, but giving routine care for short-term, minor problems may be acceptable.14 Although state medical boards use differing language, all agree that casual prescribing requires assessment and documentation similar to what you do for patients seen in your regular practice setting. Table 2 summarizes appropriate casual prescribing practices, but you should also know the boards’ rules in the locales where you work.
Restrictions and rules for prescribing controlled substances are stricter, despite many doctors’ sometime-lax attitudes. State medical boards tell doctors not to prescribe controlled substances for friends, family, or themselves except in emergencies. Yet studies have found that house officers often write prescriptions for psychoactive drugs (including narcotics) for family members, friends, and colleagues9 and that residents are willing to prescribe codeine for a hypothetical colleague with pain from a fractured finger.17
Table 1
Selected state medical board rules and comments on casual prescribing
| State | Rules |
|---|---|
| California12 | ‘[E]valuating, diagnosing, treating, or prescribing to family members, co-workers, or friends…is discouraged’ and requires ‘the same practice/protocol for any patient in which medications are prescribed,’ including a ‘good faith exam’ and documentation that justifies the prescription |
| Montana13 | Although prescribing for one’s family or oneself is not prohibited, doing so ‘arguably…does not meet the general accepted standards of practice, and is therefore unprofessional conduct [that] may subject the physician to license discipline’ |
| New Hampshire14 | ‘Physicians generally should not treat themselves or members of their immediate families.…Except in emergencies, it is not appropriate for physicians to write prescriptions for controlled substances for themselves or immediate family members’ |
| Ohio15 | ‘[I]t is almost always a bad idea to treat a family member’s chronic condition, serious illness, or psychiatric/emotional problems’ |
| South Carolina10 | Treating immediate family members may produce less than optimal care. ‘[P]rescribing controlled substances for family members is outside the scope of good medical practice except for a bona fide emergency situation’ |
| Virginia16 | Prescriptions ‘must be based on a bona fide practitioner-patient relationship. Practitioners should obtain a medical or drug history, provide information about risks, benefits, and side effects, perform an exam, and initiate follow-up care. Practitioners should not prescribe controlled substances for themselves or family members except in emergencies, isolated settings, or for a single episode of an acute illness’ |
Table 2
Cautions and recommendations for casual prescribing
| Avoid doing it in non-emergencies |
| Obtain a medical and drug history |
| Perform an appropriate, good-faith exam |
| Create a medical record that documents the need for a prescription |
| Prescribe controlled substances only in emergencies or isolated settings |
| Inform your patient about risks, benefits, and side effects |
| Initiate needed additional interventions and follow-up care |
| Maintain confidentiality and respect HIPAA rules |
| Ask yourself, ‘Can I avoid this—is there another option?’ If the answer is ‘yes,’ don’t do it |
| HIPAA: Health Insurance Portability and Accountability Act |
Liability risk
Most residents are unaware of federal or state regulations addressing the appropriateness of prescription writing for non-patients.18 A survey of U.S. internal medicine and family practice residents at a teaching hospital found that less than a quarter believed that ethical guidelines on prescription writing existed.17 Such deficits can make malpractice liability more likely if something “goes wrong” with your casually prescribed treatment. Friends and relatives do sue doctors whom they have consulted informally,18 and casual prescribing can be hard to defend in court because it usually looks suspicious and is not well documented.
Revisiting Mike’s case
Understandably, Dr. C wants to help Mike and may even think he has a condition (eg, adult attention-deficit/hyperactivity disorder) for which a stimulant would be appropriate. But respect for Mike’s humanity—the paramount value in medical practice19—suggests that his treatment should occur after and because of a careful medical assessment rather than a golf game. Moreover, prescribing a controlled substance in a non-emergency likely would violate standards of practice promulgated by Dr. C’s medical board. Dr. C should tell Mike that his problem deserves thoughtful evaluation and suggest that Mike see his primary physician. Dr. C also could recommend psychiatrists whom Mike might consult.
Related Resource
- Aboff B, Collier V, Farber N. Residents’ prescription writing for nonpatients. JAMA. 2002;288:381-385.
Drug Brand Names
- Codeine • Tylenol with Codeine, others
- Methylphenidate • Ritalin
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Be prepared to be asked for advice and prescriptions in casual settings. When this happens, it’s fine to provide general medical information, but it’s best not to give specific advice or engage in “casual prescribing.” You can maintain social connections, be caring, and avoid boundary violations by responding with tact, referral information, and good judgment.19,20
1. Balon R. Psychiatrist attitudes toward self-treatment of their own depression. Psychother Psychosom. 2007;76:306-310.
2. Walter JK, Lang CW, Ross LF. When physicians forego the doctor-patient relationship should they elect to self-prescribe or curbside? An empirical and ethical analysis. J Med Ethics. 2010;36:19-23.
3. Reynolds H. Medical ear in the early morning tennis group—when to advise and what to say. Pharos Alpha Omega Alpha Honor Med Soc. 2010;73:14-15 discussion 16.
4. Sataline S, Wang SS. Medical schools can’t keep up: as ranks of insured expand nation faces shortage of 150,000 doctors in 15 years. Available at: http://online.wsj.com/article/SB10001424052702304506904575180331528424238.html. Accessed March 21, 2011.
5. Steinberg S. Of medical specialties demand for psychiatrists growing fastest. USA Today. July 1, 2010:6D.
6. Leonhardt D. A labor market punishing to mothers. New York Times. August 4 2010:B1.
7. Madden K. Reluctant to go on vacation? Available at: http://www.cnn.com/2010/LIVING/08/04/cb.reluctant.to.take.vacation/index.html. Accessed March 20 2011.
8. Meier DE, Back AL, Morrison RS. The inner life of physicians and care of the seriously ill. JAMA. 2001;286:3007-3014.
9. Clark A, Kau J. Patterns of psychoactive drug prescriptions by house officers for nonpatients. J Med Educ. 1988;63:44-50.
10. State Medical Board of South Carolina. Prescribing for family members. Available at: http://www.llr.state.sc.us/pol/medical/index.asp?file=Policies/MEPRESCRIBEFAM.HTM. Accessed March 20 2011.
11. Dietz LH, Jacobs A, Leming TL, et al. Physicians, surgeons, and other healers, §§130, 216-218. In: American jurisprudence. vol 61. 2nd ed. New York, NY: Thomson Reuters; 2010.
12. Medical Board of California. General office practices/protocols-frequently asked questions. Available at: http://www.medbd.ca.gov/consumer/complaint_info_questions_practice.html#13. Accessed March 20 2011.
13. Montana Board of Medical Examiners. Statement of physician prescribing for self or members of the physician’s immediate family. Available at: http://bsd.dli.mt.gov/license/bsd_boards/med_board/pdf/prescribing_self.pdf. Accessed March 20 2011.
14. New Hampshire Medical Board. Guidelines for self-prescribing and prescribing for family members. Available at: http://www.nh.gov/medicine/aboutus/self_presc.htm. Accessed March 21 2011.
15. State Medical Board of Ohio. Frequently asked questions. Available at: http://www.med.ohio.gov/professional_guidelines.htm. Accessed March 20 2011.
16. Virginia Board of Medicine. Can I prescribe for my family and myself? Available at: http://www.dhp.virginia.gov/Medicine/medicine_faq.htm#Prescribe. Accessed March 20 2011.
17. Aboff B, Collier V, Farber N. Residents’ prescription writing for nonpatients. JAMA. 2002;288:381-385.
18. Johnson LJ. Malpractice consult. Should you give informal medical advice? Med Econ. 2007;84:36.-
19. Nisselle P. Danger zone: when boundaries are crossed in the doctor-patient relationship. Aust Family Physician. 2000;29:541-544.
20. Eastwood GL. When relatives and friends ask physicians for medical advice: ethical legal, and practical considerations. J Gen Intern Med. 2009;24:1333-1335.
1. Balon R. Psychiatrist attitudes toward self-treatment of their own depression. Psychother Psychosom. 2007;76:306-310.
2. Walter JK, Lang CW, Ross LF. When physicians forego the doctor-patient relationship should they elect to self-prescribe or curbside? An empirical and ethical analysis. J Med Ethics. 2010;36:19-23.
3. Reynolds H. Medical ear in the early morning tennis group—when to advise and what to say. Pharos Alpha Omega Alpha Honor Med Soc. 2010;73:14-15 discussion 16.
4. Sataline S, Wang SS. Medical schools can’t keep up: as ranks of insured expand nation faces shortage of 150,000 doctors in 15 years. Available at: http://online.wsj.com/article/SB10001424052702304506904575180331528424238.html. Accessed March 21, 2011.
5. Steinberg S. Of medical specialties demand for psychiatrists growing fastest. USA Today. July 1, 2010:6D.
6. Leonhardt D. A labor market punishing to mothers. New York Times. August 4 2010:B1.
7. Madden K. Reluctant to go on vacation? Available at: http://www.cnn.com/2010/LIVING/08/04/cb.reluctant.to.take.vacation/index.html. Accessed March 20 2011.
8. Meier DE, Back AL, Morrison RS. The inner life of physicians and care of the seriously ill. JAMA. 2001;286:3007-3014.
9. Clark A, Kau J. Patterns of psychoactive drug prescriptions by house officers for nonpatients. J Med Educ. 1988;63:44-50.
10. State Medical Board of South Carolina. Prescribing for family members. Available at: http://www.llr.state.sc.us/pol/medical/index.asp?file=Policies/MEPRESCRIBEFAM.HTM. Accessed March 20 2011.
11. Dietz LH, Jacobs A, Leming TL, et al. Physicians, surgeons, and other healers, §§130, 216-218. In: American jurisprudence. vol 61. 2nd ed. New York, NY: Thomson Reuters; 2010.
12. Medical Board of California. General office practices/protocols-frequently asked questions. Available at: http://www.medbd.ca.gov/consumer/complaint_info_questions_practice.html#13. Accessed March 20 2011.
13. Montana Board of Medical Examiners. Statement of physician prescribing for self or members of the physician’s immediate family. Available at: http://bsd.dli.mt.gov/license/bsd_boards/med_board/pdf/prescribing_self.pdf. Accessed March 20 2011.
14. New Hampshire Medical Board. Guidelines for self-prescribing and prescribing for family members. Available at: http://www.nh.gov/medicine/aboutus/self_presc.htm. Accessed March 21 2011.
15. State Medical Board of Ohio. Frequently asked questions. Available at: http://www.med.ohio.gov/professional_guidelines.htm. Accessed March 20 2011.
16. Virginia Board of Medicine. Can I prescribe for my family and myself? Available at: http://www.dhp.virginia.gov/Medicine/medicine_faq.htm#Prescribe. Accessed March 20 2011.
17. Aboff B, Collier V, Farber N. Residents’ prescription writing for nonpatients. JAMA. 2002;288:381-385.
18. Johnson LJ. Malpractice consult. Should you give informal medical advice? Med Econ. 2007;84:36.-
19. Nisselle P. Danger zone: when boundaries are crossed in the doctor-patient relationship. Aust Family Physician. 2000;29:541-544.
20. Eastwood GL. When relatives and friends ask physicians for medical advice: ethical legal, and practical considerations. J Gen Intern Med. 2009;24:1333-1335.
Dissociative identity disorder: No excuse for criminal activity
Formerly called multiple personality disorder, dissociative identity disorder (DID) is a controversial diagnosis that challenges forensic psychiatrists, other mental health clinicians, legal professionals, the media, and the public. DID cases often present in the criminal justice system rather than in the mental health system, and the illness perplexes experts in both professions.
Individuals may commit criminal acts while in a dissociated state. A study that tracked 21 reported DID cases found that 47% of men and 35% of women reported engaging in criminal activity, including 19% of men and 7% of women who committed homicide.1 Defendants occasionally use DID as a basis for pleading not guilty by reason of insanity (NGRI). Controversy over the DID diagnosis has contributed to debates about the disorder’s role in criminal responsibility.
The DID diagnosis
An American Psychiatric Association Work Group has proposed new diagnostic criteria for DID for DSM-5, which is scheduled to be published in May 2013.2 Presently, DID is listed in DSM-IV-TR as an axis I disorder.3 Criteria for DID include the presence of ≥2 distinctive identities or personality states that recurrently take control of an individual’s behavior (Table 1).3 This is accompanied by an inability to recall important personal information to an extent that cannot be explained by ordinary forgetfulness. Patients with DID typically have a primary identity that is passive, dependent, guilty, and depressed, and alternate identities with characteristics that differ from the primary identity, commonly in reported age and gender, vocabulary, general knowledge, or predominant affect.3
Dissociative pathology may result from trauma, comorbid mental illness, or other medical issues, including complex partial seizures. Developmental theorists have proposed that severe sexual, physical, or psychological trauma in childhood predisposes an individual to develop DID.4 Theoretically, harm by a trusted caregiver forces a child to split off awareness and memory of the trauma to survive in the relationship. Later these memories and feelings are experienced as a separate personality. Because this process happens repeatedly, the patient develops multiple personalities; each has different memories and performs different functions, which may be helpful or destructive. Later, dissociation becomes a coping mechanism when individuals face stressful situations.5
Personality traits that may predispose patients to develop a dissociative disorder include mental absorption, suggestibility, ability to be easily hypnotized, and tendency to fantasize.6 Patients with dissociation also may meet criteria for posttraumatic stress disorder, borderline personality disorder, somatoform disorder, eating disorder, or substance use disorders.7
Table 1
DSM-IV-TR criteria for dissociative identity disorder
| The presence of ≥2 distinct identities or personality states, each with its own relatively enduring pattern of perceiving, relating to, and thinking about the environment and self |
| At least 2 of these identities or personality states must recurrently take control of the person’s behavior |
| An inability to recall important personal information that is too extensive to be explained by ordinary forgetfulness |
| The disturbance is not due to the direct physiological effects of substance or a general medical condition. In children, the symptoms are not attributable to imaginary playmates or other fantasy play |
| Source: Reference 3 |
DID and NGRI
An insanity defense is raised in <1% of felony cases, and is successful in only a fraction of those.8 A criminal defendant who claims NGRI asserts that he committed the offense and asks the court to find him not culpable because of his mental state when the offense occurred.
The legal approach used by the defense in cases of NGRI due to DID will be determined by the jurisdiction in which the case is tried. The “Alter-in-control” approach considers the key issue as which “alter” (personality) was in control at the time of the offense and whether he or she met the insanity standard, the “Each-alter” approach considers whether each personality met the insanity standard, and the “Host-alter” approach considers whether the dominant or primary personality met the insanity standard.9
Legal and mental health professionals are divided on whether DID warrants an acquittal for insanity. The first time DID was recognized as a mental disorder that could excuse criminal responsibility occurred in State v Milligan (1978).10 The court declared serial rapist Billy Milligan insane due to lack of one integrated personality and therefore not culpable of the crimes he committed. Public outrage was extraordinary. Since this case, most DID defenses have not been successful (Table 2).10-16
In a 1980 court case,11 a defendant charged with murder pleaded NGRI due to having multiple personalities. The court found that having alter personalities was not necessarily a mental disease that would preclude responsibility for the murder.
In State v Grimsley (1982),12 the defense used NGRI due to multiple personalities in a drunk driving case. The court ruled that it is immaterial what state of consciousness or personality the defendant was in as long as the personality controlling the behavior was conscious and aware of his or her actions.
In Kirkland v State (1983),13 attorneys for a woman who committed a bank robbery asserted an insanity defense based on a psychogenic fugue, which is sudden, unexpected travel away from home accompanied by inability to recall one’s past and confusion about identity or assumption of a new identity.3 The court found that the law adjudges criminal liability according to the person’s state of mind at the time of the act and will not inquire whether an individual possesses other personalities, fugues, or even moods in which he would not have performed the crime.
In State v Jones (1988),14 the court found the defendant guilty of murdering a woman he met at a bar despite expert testimony that his multiple personalities “paralyzed” him from knowing right from wrong.
More recently, courts have rejected the admissibility of DID evidence, including expert testimony, because the scientific evidence failed to meet reliability standards, and therefore is not ultimately useful to the judge or jury. In State v Greene (1998),15 the defendant claimed that 1 of his 24 alters was responsible for killing his therapist. The Supreme Court of Washington affirmed that evidence of Mr. Greene’s DID, including expert testimony, was not reliable and not admissible.
Similarly, in State v Lockhart (2000),16 Mr. Lockhart contested his conviction of first degree sexual assault on the basis that he was not permitted to present evidence of DID to support his insanity defense. The West Virginia Court held that the diagnosis of DID was speculative and therefore did not meet reliability standards for evidence.
Table 2
Using dissociative identity disorder* as a basis for not guilty by reason of insanity
| Case | Year | Charge | Defense | Court ruling |
|---|---|---|---|---|
| State v Milligan10 | 1978 | Rape | NGRI-MPD | Lack of an integrated personality meant the defendant was not culpable |
| State v Darnall11 | 1980 | Murder | NGRI-MPD | Multiple personalities do not preclude criminal responsibility |
| State v Grimsley12 | 1982 | Drunk driving | NGRI-MPD; primary personality had no control over the ‘alter’ | State of consciousness or personality of defendant is immaterial |
| Kirkland v State13 | 1983 | Bank robbery | NGRI-psychogenic fugue | Law does not inquire about other personalities, fugue states, or moods in cases of criminal liability |
| State v Jones14 | 1988 | Murder | NGRI-MPD | Alter personalities will not be an excuse for inability to distinguish right from wrong |
| State v Greene15 | 1998 | Murder | NGRI-DID; primary personality was ‘unconscious’ | Evidence of DID, including expert testimony, was not admissible because it did not meet reliability standards |
| State v Lockhart16 | 2000 | Sexual assault | NGRI-DID | DID was not allowed into evidence by the West Virginia Court due to lack of scientific evidence |
| *Dissociative identity disorder formerly was referred to as multiple personality disorder DID: dissociative identity disorder; MPD: multiple personality disorder; NGRI: not guilty by reason of insanity. | ||||
Evaluating DID
Because the courts may ask psychiatrists to provide expert opinion on DID to assist with legal rulings, clinicians must remain vigilant to the possibility of DID as well as to defendants who may malinger multiple personalities to evade punishment. In such situations, factors to consider include the mental status examination, data and history collection, collateral information, criminal background, mental health history, history of abuse, and objective assessment tools.
Extensive field testing has shown that the Structured Clinical Interview for Dissociative Disorders (SCID-D) has good reliability and excellent validity.17 The SCID-D allows a trained interviewer to assess the severity of 5 dissociative symptoms: amnesia, depersonalization, derealization, identity confusion, and identity alteration.17 Other tools that may help assess a patient with suspected DID are listed in Table 3.
Patients who commit criminal acts while in a dissociated state may assert a defense of NGRI due to DID, but rarely has this defense been successful. Although a patient may have distinct personalities that control his or her behavior, this condition does not preclude criminal responsibility.
The role of hypnosis in evaluating DID is controversial. The introduction of pseudo-memories and potential for iatrogenic DID may complicate the clinical presentation and subsequent diagnosis.18
Table 3
Tools for diagnosing dissociative identity disorder
| Structured Clinical Interview for Dissociative Disorders |
| Dissociative Disorder Interview Schedule |
| Dissociative Experiences Scale |
| Childhood Trauma Questionnaire |
Related Resource
- West S, Noffsinger S. Is this patient not guilty by reason of insanity? Current Psychiatry. 2006;5(8):54-62.
Disclosure
Dr. Farrell reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Putnam. Diagnosis and treatment of multiple personality disorder. New York, NY: The Guilford Press; 1989.
2. American Psychiatric Association. DSM-5 Development. 300. 14. Dissociative identity disorder. Proposed revision. Available at: http://www.dsm5.org/ProposedRevisions/Pages/proposedrevision.aspx?rid=57. Accessed April 22 2011.
3. Diagnostic and statistical manual of mental disorders. 4th ed, text rev. Washington DC: American Psychiatric Association; 2000.
4. Sadock BJ, Sadock VA. eds. Dissociative disorders. In: Sadock BJ, Sadock VA, eds. Kaplan & Sadock’s comprehensive textbook of psychiatry. 7th ed. New York, NY: Lippincott Williams & Wilkins; 2000:1544–1576.
5. Lyons-Ruth K, Dutra L, Schuder MR, et al. From infant attachment disorganization to adult dissociation: relational adaptations or traumatic experiences? Psychiatr Clin North Am. 2006;29(1):63-86, viii.
6. Brahams D. Automatism and post-traumatic stress disorder. Lancet. 1990;335(8701):1333.-
7. Simeon D, Guralnik O, Knutelska M, et al. Personality factors associated with dissociation: temperament, defenses, and cognitive schemata. Am J Psychiatry. 2002;159(3):489-491.
8. Perlin M. The jurisprudence of the insanity defense. Durham NC: Carolina Academic Press; 1994.
9. Steinberg M, Bancroft J, Buchanan J. Multiple personality disorder in criminal law. Bull Am Acad Psychiatry Law. 1993;21(3):345-356.
10. State v Milligan, No 77-CR-11-2908 (Franklin County, Ohio, December 4 1978).
11. State v Darnall, 47 Or App 161, 614 P2d 120 (1980).
12. State v Grimsley, 3 Ohio App 3d 165 444 NE2d 1071 (1982).
13. Kirkland v State, 166 Ga App 478, 304 SE2d 561 (1983).
14. State v Jones, 743 P2d 176 (Wash Ct App 1987) aff’d, 759 P2d 1183, 1185 (Wash 1998).
15. State v Greene, 92 Wn App 80, 960 P2d 980 (1998).
16. State v Lockhart, 208 W Va 622 (2000).
17. Steinberg M, Rounsaville B, Cicchetti D. Detection of dissociative disorders in psychiatric patients by a screening instrument and a structured diagnostic interview. Am J Psychiatry. 1991;148(8):1050-1054.
18. Putnam FW. Dissociative phenomena. In: Tasman A ed. Annual review of psychiatry. Washington, DC: American Psychiatric Press; 1991:145–160.
Formerly called multiple personality disorder, dissociative identity disorder (DID) is a controversial diagnosis that challenges forensic psychiatrists, other mental health clinicians, legal professionals, the media, and the public. DID cases often present in the criminal justice system rather than in the mental health system, and the illness perplexes experts in both professions.
Individuals may commit criminal acts while in a dissociated state. A study that tracked 21 reported DID cases found that 47% of men and 35% of women reported engaging in criminal activity, including 19% of men and 7% of women who committed homicide.1 Defendants occasionally use DID as a basis for pleading not guilty by reason of insanity (NGRI). Controversy over the DID diagnosis has contributed to debates about the disorder’s role in criminal responsibility.
The DID diagnosis
An American Psychiatric Association Work Group has proposed new diagnostic criteria for DID for DSM-5, which is scheduled to be published in May 2013.2 Presently, DID is listed in DSM-IV-TR as an axis I disorder.3 Criteria for DID include the presence of ≥2 distinctive identities or personality states that recurrently take control of an individual’s behavior (Table 1).3 This is accompanied by an inability to recall important personal information to an extent that cannot be explained by ordinary forgetfulness. Patients with DID typically have a primary identity that is passive, dependent, guilty, and depressed, and alternate identities with characteristics that differ from the primary identity, commonly in reported age and gender, vocabulary, general knowledge, or predominant affect.3
Dissociative pathology may result from trauma, comorbid mental illness, or other medical issues, including complex partial seizures. Developmental theorists have proposed that severe sexual, physical, or psychological trauma in childhood predisposes an individual to develop DID.4 Theoretically, harm by a trusted caregiver forces a child to split off awareness and memory of the trauma to survive in the relationship. Later these memories and feelings are experienced as a separate personality. Because this process happens repeatedly, the patient develops multiple personalities; each has different memories and performs different functions, which may be helpful or destructive. Later, dissociation becomes a coping mechanism when individuals face stressful situations.5
Personality traits that may predispose patients to develop a dissociative disorder include mental absorption, suggestibility, ability to be easily hypnotized, and tendency to fantasize.6 Patients with dissociation also may meet criteria for posttraumatic stress disorder, borderline personality disorder, somatoform disorder, eating disorder, or substance use disorders.7
Table 1
DSM-IV-TR criteria for dissociative identity disorder
| The presence of ≥2 distinct identities or personality states, each with its own relatively enduring pattern of perceiving, relating to, and thinking about the environment and self |
| At least 2 of these identities or personality states must recurrently take control of the person’s behavior |
| An inability to recall important personal information that is too extensive to be explained by ordinary forgetfulness |
| The disturbance is not due to the direct physiological effects of substance or a general medical condition. In children, the symptoms are not attributable to imaginary playmates or other fantasy play |
| Source: Reference 3 |
DID and NGRI
An insanity defense is raised in <1% of felony cases, and is successful in only a fraction of those.8 A criminal defendant who claims NGRI asserts that he committed the offense and asks the court to find him not culpable because of his mental state when the offense occurred.
The legal approach used by the defense in cases of NGRI due to DID will be determined by the jurisdiction in which the case is tried. The “Alter-in-control” approach considers the key issue as which “alter” (personality) was in control at the time of the offense and whether he or she met the insanity standard, the “Each-alter” approach considers whether each personality met the insanity standard, and the “Host-alter” approach considers whether the dominant or primary personality met the insanity standard.9
Legal and mental health professionals are divided on whether DID warrants an acquittal for insanity. The first time DID was recognized as a mental disorder that could excuse criminal responsibility occurred in State v Milligan (1978).10 The court declared serial rapist Billy Milligan insane due to lack of one integrated personality and therefore not culpable of the crimes he committed. Public outrage was extraordinary. Since this case, most DID defenses have not been successful (Table 2).10-16
In a 1980 court case,11 a defendant charged with murder pleaded NGRI due to having multiple personalities. The court found that having alter personalities was not necessarily a mental disease that would preclude responsibility for the murder.
In State v Grimsley (1982),12 the defense used NGRI due to multiple personalities in a drunk driving case. The court ruled that it is immaterial what state of consciousness or personality the defendant was in as long as the personality controlling the behavior was conscious and aware of his or her actions.
In Kirkland v State (1983),13 attorneys for a woman who committed a bank robbery asserted an insanity defense based on a psychogenic fugue, which is sudden, unexpected travel away from home accompanied by inability to recall one’s past and confusion about identity or assumption of a new identity.3 The court found that the law adjudges criminal liability according to the person’s state of mind at the time of the act and will not inquire whether an individual possesses other personalities, fugues, or even moods in which he would not have performed the crime.
In State v Jones (1988),14 the court found the defendant guilty of murdering a woman he met at a bar despite expert testimony that his multiple personalities “paralyzed” him from knowing right from wrong.
More recently, courts have rejected the admissibility of DID evidence, including expert testimony, because the scientific evidence failed to meet reliability standards, and therefore is not ultimately useful to the judge or jury. In State v Greene (1998),15 the defendant claimed that 1 of his 24 alters was responsible for killing his therapist. The Supreme Court of Washington affirmed that evidence of Mr. Greene’s DID, including expert testimony, was not reliable and not admissible.
Similarly, in State v Lockhart (2000),16 Mr. Lockhart contested his conviction of first degree sexual assault on the basis that he was not permitted to present evidence of DID to support his insanity defense. The West Virginia Court held that the diagnosis of DID was speculative and therefore did not meet reliability standards for evidence.
Table 2
Using dissociative identity disorder* as a basis for not guilty by reason of insanity
| Case | Year | Charge | Defense | Court ruling |
|---|---|---|---|---|
| State v Milligan10 | 1978 | Rape | NGRI-MPD | Lack of an integrated personality meant the defendant was not culpable |
| State v Darnall11 | 1980 | Murder | NGRI-MPD | Multiple personalities do not preclude criminal responsibility |
| State v Grimsley12 | 1982 | Drunk driving | NGRI-MPD; primary personality had no control over the ‘alter’ | State of consciousness or personality of defendant is immaterial |
| Kirkland v State13 | 1983 | Bank robbery | NGRI-psychogenic fugue | Law does not inquire about other personalities, fugue states, or moods in cases of criminal liability |
| State v Jones14 | 1988 | Murder | NGRI-MPD | Alter personalities will not be an excuse for inability to distinguish right from wrong |
| State v Greene15 | 1998 | Murder | NGRI-DID; primary personality was ‘unconscious’ | Evidence of DID, including expert testimony, was not admissible because it did not meet reliability standards |
| State v Lockhart16 | 2000 | Sexual assault | NGRI-DID | DID was not allowed into evidence by the West Virginia Court due to lack of scientific evidence |
| *Dissociative identity disorder formerly was referred to as multiple personality disorder DID: dissociative identity disorder; MPD: multiple personality disorder; NGRI: not guilty by reason of insanity. | ||||
Evaluating DID
Because the courts may ask psychiatrists to provide expert opinion on DID to assist with legal rulings, clinicians must remain vigilant to the possibility of DID as well as to defendants who may malinger multiple personalities to evade punishment. In such situations, factors to consider include the mental status examination, data and history collection, collateral information, criminal background, mental health history, history of abuse, and objective assessment tools.
Extensive field testing has shown that the Structured Clinical Interview for Dissociative Disorders (SCID-D) has good reliability and excellent validity.17 The SCID-D allows a trained interviewer to assess the severity of 5 dissociative symptoms: amnesia, depersonalization, derealization, identity confusion, and identity alteration.17 Other tools that may help assess a patient with suspected DID are listed in Table 3.
Patients who commit criminal acts while in a dissociated state may assert a defense of NGRI due to DID, but rarely has this defense been successful. Although a patient may have distinct personalities that control his or her behavior, this condition does not preclude criminal responsibility.
The role of hypnosis in evaluating DID is controversial. The introduction of pseudo-memories and potential for iatrogenic DID may complicate the clinical presentation and subsequent diagnosis.18
Table 3
Tools for diagnosing dissociative identity disorder
| Structured Clinical Interview for Dissociative Disorders |
| Dissociative Disorder Interview Schedule |
| Dissociative Experiences Scale |
| Childhood Trauma Questionnaire |
Related Resource
- West S, Noffsinger S. Is this patient not guilty by reason of insanity? Current Psychiatry. 2006;5(8):54-62.
Disclosure
Dr. Farrell reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Formerly called multiple personality disorder, dissociative identity disorder (DID) is a controversial diagnosis that challenges forensic psychiatrists, other mental health clinicians, legal professionals, the media, and the public. DID cases often present in the criminal justice system rather than in the mental health system, and the illness perplexes experts in both professions.
Individuals may commit criminal acts while in a dissociated state. A study that tracked 21 reported DID cases found that 47% of men and 35% of women reported engaging in criminal activity, including 19% of men and 7% of women who committed homicide.1 Defendants occasionally use DID as a basis for pleading not guilty by reason of insanity (NGRI). Controversy over the DID diagnosis has contributed to debates about the disorder’s role in criminal responsibility.
The DID diagnosis
An American Psychiatric Association Work Group has proposed new diagnostic criteria for DID for DSM-5, which is scheduled to be published in May 2013.2 Presently, DID is listed in DSM-IV-TR as an axis I disorder.3 Criteria for DID include the presence of ≥2 distinctive identities or personality states that recurrently take control of an individual’s behavior (Table 1).3 This is accompanied by an inability to recall important personal information to an extent that cannot be explained by ordinary forgetfulness. Patients with DID typically have a primary identity that is passive, dependent, guilty, and depressed, and alternate identities with characteristics that differ from the primary identity, commonly in reported age and gender, vocabulary, general knowledge, or predominant affect.3
Dissociative pathology may result from trauma, comorbid mental illness, or other medical issues, including complex partial seizures. Developmental theorists have proposed that severe sexual, physical, or psychological trauma in childhood predisposes an individual to develop DID.4 Theoretically, harm by a trusted caregiver forces a child to split off awareness and memory of the trauma to survive in the relationship. Later these memories and feelings are experienced as a separate personality. Because this process happens repeatedly, the patient develops multiple personalities; each has different memories and performs different functions, which may be helpful or destructive. Later, dissociation becomes a coping mechanism when individuals face stressful situations.5
Personality traits that may predispose patients to develop a dissociative disorder include mental absorption, suggestibility, ability to be easily hypnotized, and tendency to fantasize.6 Patients with dissociation also may meet criteria for posttraumatic stress disorder, borderline personality disorder, somatoform disorder, eating disorder, or substance use disorders.7
Table 1
DSM-IV-TR criteria for dissociative identity disorder
| The presence of ≥2 distinct identities or personality states, each with its own relatively enduring pattern of perceiving, relating to, and thinking about the environment and self |
| At least 2 of these identities or personality states must recurrently take control of the person’s behavior |
| An inability to recall important personal information that is too extensive to be explained by ordinary forgetfulness |
| The disturbance is not due to the direct physiological effects of substance or a general medical condition. In children, the symptoms are not attributable to imaginary playmates or other fantasy play |
| Source: Reference 3 |
DID and NGRI
An insanity defense is raised in <1% of felony cases, and is successful in only a fraction of those.8 A criminal defendant who claims NGRI asserts that he committed the offense and asks the court to find him not culpable because of his mental state when the offense occurred.
The legal approach used by the defense in cases of NGRI due to DID will be determined by the jurisdiction in which the case is tried. The “Alter-in-control” approach considers the key issue as which “alter” (personality) was in control at the time of the offense and whether he or she met the insanity standard, the “Each-alter” approach considers whether each personality met the insanity standard, and the “Host-alter” approach considers whether the dominant or primary personality met the insanity standard.9
Legal and mental health professionals are divided on whether DID warrants an acquittal for insanity. The first time DID was recognized as a mental disorder that could excuse criminal responsibility occurred in State v Milligan (1978).10 The court declared serial rapist Billy Milligan insane due to lack of one integrated personality and therefore not culpable of the crimes he committed. Public outrage was extraordinary. Since this case, most DID defenses have not been successful (Table 2).10-16
In a 1980 court case,11 a defendant charged with murder pleaded NGRI due to having multiple personalities. The court found that having alter personalities was not necessarily a mental disease that would preclude responsibility for the murder.
In State v Grimsley (1982),12 the defense used NGRI due to multiple personalities in a drunk driving case. The court ruled that it is immaterial what state of consciousness or personality the defendant was in as long as the personality controlling the behavior was conscious and aware of his or her actions.
In Kirkland v State (1983),13 attorneys for a woman who committed a bank robbery asserted an insanity defense based on a psychogenic fugue, which is sudden, unexpected travel away from home accompanied by inability to recall one’s past and confusion about identity or assumption of a new identity.3 The court found that the law adjudges criminal liability according to the person’s state of mind at the time of the act and will not inquire whether an individual possesses other personalities, fugues, or even moods in which he would not have performed the crime.
In State v Jones (1988),14 the court found the defendant guilty of murdering a woman he met at a bar despite expert testimony that his multiple personalities “paralyzed” him from knowing right from wrong.
More recently, courts have rejected the admissibility of DID evidence, including expert testimony, because the scientific evidence failed to meet reliability standards, and therefore is not ultimately useful to the judge or jury. In State v Greene (1998),15 the defendant claimed that 1 of his 24 alters was responsible for killing his therapist. The Supreme Court of Washington affirmed that evidence of Mr. Greene’s DID, including expert testimony, was not reliable and not admissible.
Similarly, in State v Lockhart (2000),16 Mr. Lockhart contested his conviction of first degree sexual assault on the basis that he was not permitted to present evidence of DID to support his insanity defense. The West Virginia Court held that the diagnosis of DID was speculative and therefore did not meet reliability standards for evidence.
Table 2
Using dissociative identity disorder* as a basis for not guilty by reason of insanity
| Case | Year | Charge | Defense | Court ruling |
|---|---|---|---|---|
| State v Milligan10 | 1978 | Rape | NGRI-MPD | Lack of an integrated personality meant the defendant was not culpable |
| State v Darnall11 | 1980 | Murder | NGRI-MPD | Multiple personalities do not preclude criminal responsibility |
| State v Grimsley12 | 1982 | Drunk driving | NGRI-MPD; primary personality had no control over the ‘alter’ | State of consciousness or personality of defendant is immaterial |
| Kirkland v State13 | 1983 | Bank robbery | NGRI-psychogenic fugue | Law does not inquire about other personalities, fugue states, or moods in cases of criminal liability |
| State v Jones14 | 1988 | Murder | NGRI-MPD | Alter personalities will not be an excuse for inability to distinguish right from wrong |
| State v Greene15 | 1998 | Murder | NGRI-DID; primary personality was ‘unconscious’ | Evidence of DID, including expert testimony, was not admissible because it did not meet reliability standards |
| State v Lockhart16 | 2000 | Sexual assault | NGRI-DID | DID was not allowed into evidence by the West Virginia Court due to lack of scientific evidence |
| *Dissociative identity disorder formerly was referred to as multiple personality disorder DID: dissociative identity disorder; MPD: multiple personality disorder; NGRI: not guilty by reason of insanity. | ||||
Evaluating DID
Because the courts may ask psychiatrists to provide expert opinion on DID to assist with legal rulings, clinicians must remain vigilant to the possibility of DID as well as to defendants who may malinger multiple personalities to evade punishment. In such situations, factors to consider include the mental status examination, data and history collection, collateral information, criminal background, mental health history, history of abuse, and objective assessment tools.
Extensive field testing has shown that the Structured Clinical Interview for Dissociative Disorders (SCID-D) has good reliability and excellent validity.17 The SCID-D allows a trained interviewer to assess the severity of 5 dissociative symptoms: amnesia, depersonalization, derealization, identity confusion, and identity alteration.17 Other tools that may help assess a patient with suspected DID are listed in Table 3.
Patients who commit criminal acts while in a dissociated state may assert a defense of NGRI due to DID, but rarely has this defense been successful. Although a patient may have distinct personalities that control his or her behavior, this condition does not preclude criminal responsibility.
The role of hypnosis in evaluating DID is controversial. The introduction of pseudo-memories and potential for iatrogenic DID may complicate the clinical presentation and subsequent diagnosis.18
Table 3
Tools for diagnosing dissociative identity disorder
| Structured Clinical Interview for Dissociative Disorders |
| Dissociative Disorder Interview Schedule |
| Dissociative Experiences Scale |
| Childhood Trauma Questionnaire |
Related Resource
- West S, Noffsinger S. Is this patient not guilty by reason of insanity? Current Psychiatry. 2006;5(8):54-62.
Disclosure
Dr. Farrell reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Putnam. Diagnosis and treatment of multiple personality disorder. New York, NY: The Guilford Press; 1989.
2. American Psychiatric Association. DSM-5 Development. 300. 14. Dissociative identity disorder. Proposed revision. Available at: http://www.dsm5.org/ProposedRevisions/Pages/proposedrevision.aspx?rid=57. Accessed April 22 2011.
3. Diagnostic and statistical manual of mental disorders. 4th ed, text rev. Washington DC: American Psychiatric Association; 2000.
4. Sadock BJ, Sadock VA. eds. Dissociative disorders. In: Sadock BJ, Sadock VA, eds. Kaplan & Sadock’s comprehensive textbook of psychiatry. 7th ed. New York, NY: Lippincott Williams & Wilkins; 2000:1544–1576.
5. Lyons-Ruth K, Dutra L, Schuder MR, et al. From infant attachment disorganization to adult dissociation: relational adaptations or traumatic experiences? Psychiatr Clin North Am. 2006;29(1):63-86, viii.
6. Brahams D. Automatism and post-traumatic stress disorder. Lancet. 1990;335(8701):1333.-
7. Simeon D, Guralnik O, Knutelska M, et al. Personality factors associated with dissociation: temperament, defenses, and cognitive schemata. Am J Psychiatry. 2002;159(3):489-491.
8. Perlin M. The jurisprudence of the insanity defense. Durham NC: Carolina Academic Press; 1994.
9. Steinberg M, Bancroft J, Buchanan J. Multiple personality disorder in criminal law. Bull Am Acad Psychiatry Law. 1993;21(3):345-356.
10. State v Milligan, No 77-CR-11-2908 (Franklin County, Ohio, December 4 1978).
11. State v Darnall, 47 Or App 161, 614 P2d 120 (1980).
12. State v Grimsley, 3 Ohio App 3d 165 444 NE2d 1071 (1982).
13. Kirkland v State, 166 Ga App 478, 304 SE2d 561 (1983).
14. State v Jones, 743 P2d 176 (Wash Ct App 1987) aff’d, 759 P2d 1183, 1185 (Wash 1998).
15. State v Greene, 92 Wn App 80, 960 P2d 980 (1998).
16. State v Lockhart, 208 W Va 622 (2000).
17. Steinberg M, Rounsaville B, Cicchetti D. Detection of dissociative disorders in psychiatric patients by a screening instrument and a structured diagnostic interview. Am J Psychiatry. 1991;148(8):1050-1054.
18. Putnam FW. Dissociative phenomena. In: Tasman A ed. Annual review of psychiatry. Washington, DC: American Psychiatric Press; 1991:145–160.
1. Putnam. Diagnosis and treatment of multiple personality disorder. New York, NY: The Guilford Press; 1989.
2. American Psychiatric Association. DSM-5 Development. 300. 14. Dissociative identity disorder. Proposed revision. Available at: http://www.dsm5.org/ProposedRevisions/Pages/proposedrevision.aspx?rid=57. Accessed April 22 2011.
3. Diagnostic and statistical manual of mental disorders. 4th ed, text rev. Washington DC: American Psychiatric Association; 2000.
4. Sadock BJ, Sadock VA. eds. Dissociative disorders. In: Sadock BJ, Sadock VA, eds. Kaplan & Sadock’s comprehensive textbook of psychiatry. 7th ed. New York, NY: Lippincott Williams & Wilkins; 2000:1544–1576.
5. Lyons-Ruth K, Dutra L, Schuder MR, et al. From infant attachment disorganization to adult dissociation: relational adaptations or traumatic experiences? Psychiatr Clin North Am. 2006;29(1):63-86, viii.
6. Brahams D. Automatism and post-traumatic stress disorder. Lancet. 1990;335(8701):1333.-
7. Simeon D, Guralnik O, Knutelska M, et al. Personality factors associated with dissociation: temperament, defenses, and cognitive schemata. Am J Psychiatry. 2002;159(3):489-491.
8. Perlin M. The jurisprudence of the insanity defense. Durham NC: Carolina Academic Press; 1994.
9. Steinberg M, Bancroft J, Buchanan J. Multiple personality disorder in criminal law. Bull Am Acad Psychiatry Law. 1993;21(3):345-356.
10. State v Milligan, No 77-CR-11-2908 (Franklin County, Ohio, December 4 1978).
11. State v Darnall, 47 Or App 161, 614 P2d 120 (1980).
12. State v Grimsley, 3 Ohio App 3d 165 444 NE2d 1071 (1982).
13. Kirkland v State, 166 Ga App 478, 304 SE2d 561 (1983).
14. State v Jones, 743 P2d 176 (Wash Ct App 1987) aff’d, 759 P2d 1183, 1185 (Wash 1998).
15. State v Greene, 92 Wn App 80, 960 P2d 980 (1998).
16. State v Lockhart, 208 W Va 622 (2000).
17. Steinberg M, Rounsaville B, Cicchetti D. Detection of dissociative disorders in psychiatric patients by a screening instrument and a structured diagnostic interview. Am J Psychiatry. 1991;148(8):1050-1054.
18. Putnam FW. Dissociative phenomena. In: Tasman A ed. Annual review of psychiatry. Washington, DC: American Psychiatric Press; 1991:145–160.
The Benefits of Robot-Assisted Myomectomy
Myomectomy offers an alternative to hysterectomy for the treatment of uterine fibroids whether or not future fertility is an issue. While many women chose a uterine-sparing approach to maintain their fertility options, there still are many women who prefer myomectomy for reasons other than fertility preservation.
The procedure is an important one for gynecologic surgeons and their patients, as it conveys a high rate of symptom resolution: Eighty-one percent of women who undergo a myomectomy experience complete resolution of their symptoms (Fertil. Steril. 1981;36:433-45).
Robot-assisted laparoscopic myomectomy was first described in 2004 by Dr. Arnold P. Advincula and his colleagues (J. Am. Assoc. Gynecol. Laparosc. 2004;11:511-8).
Their report played a pivotal role in the Food and Drug Administration's approval in 2005 for use of the da Vinci Surgical System (Intuitive Surgical, Sunnyvale, Calif.) for gynecologic surgical procedures.
While myomectomy still is most commonly performed via laparotomy, a significant number of surgeons have adopted the robotic approach. According to data from Solucient, a health care information company managed by Thomson Reuters, approximately 4,000 robotic myomectomies were performed in the United States in 2010. This represents 10% of the approximately 40,000 myomectomies performed each year, a significant proportion considering that robotics had been introduced to gynecology only 5 years earlier.
Myomectomy is a suture-intensive procedure, and suturing by a conventional laparoscopic approach has proved to be extremely challenging. The robotic platform gives surgeons greater capability of successfully repairing deep hysterotomy defects and provides them with a more achievable minimally invasive option to offer patients.
Interestingly, utilization of the laparoscopic approach for hysterectomy also has increased with the introduction of robotics. Current statistics show that only 16% of all hysterectomy procedures performed in the United States are done via conventional laparoscopy (20 years, approximately, after the techniques were developed), while another 20% are now being performed with robot assistance. A new AAGL position statement saying that surgeons who offer hysterectomy should be able to perform either vaginal hysterectomy (the preferred approach) or laparoscopic hysterectomy (the second best approach) – or refer their patients to a surgeon who can (J. Minim. Invasive Gynecol. 2011;18:1-3) – is indicative of the growing belief that the benefits of minimally invasive surgery over open procedures should be considered where possible in aspects of gynecologic surgery.
At our institution, we saw a significant improvement in operative time after the first 20 cases of robot-assisted myomectomy and hysterectomy. Our operative time went from a mean of 212 min. for cases 1–20 to a mean of 151 min. for cases 21–40 (Int. J. Med. Robot. 2008;4:114-20).
Others have reported similar findings on the learning curve for robot-assisted gynecologic surgery: Another case series published several years ago, for instance, showed operative times for various surgical procedures for benign gynecologic problems stabilizing within 50 cases (J. Minim. Invasive Gynecol. 2008;15:589-94). In general, these data are indicative of a significantly shorter learning curve than seen with traditional laparoscopic surgery.
Incorporation of MRI
The main drawback to robotics always has been the absence of haptics or tactile feedback. This limitation has, however, spurred the development of creative techniques to compensate, including the use of real-time magnetic resonance imaging.
MR images can now be incorporated in a real-time, 3-dimensional fashion into the surgeon's console for use in mapping, detecting, locating, and enucleating myomas. All three views – axial, coronal, and sagittal – can be seen during the surgery. This enables the surgeon both to overcome the haptic limitations and to remove multiple fibroids. (See images 1 and 2.)
Certainly, the gynecologic surgeon employing this technique must be comfortable reading and interpreting MR images. The necessary comfort level can be achieved, on an individual basis, with time spent reviewing series of pelvic MR images with a radiologist.
MR imaging also has proved, of course, to be an excellent preoperative tool for determining ahead of time the size, number, and location of myomas, and for ruling out adenomyosis. In my experience, MR imaging can be useful preoperatively in conjunction with pelvic exams to effectively screen for patients who are likely to have successful outcomes with robotic myomectomy.
For example, a patient with a 12- to 14-week-size uterus may not be a good candidate for robotic myomectomy if on the MR image the uterus has innumerable myomas without a clearly defined cleavage plane between the tumors. A woman with a significantly larger uterus may be an excellent candidate, on the other hand, if the number and location of leiomyomas is determined by MRI.
Set-Up, Technique
The three basic components of the da Vinci system are a patient-side cart, a vision system, and a surgeon's console. The patient-side cart has four robotic arms that are attached or “docked” to trocars that are placed in the abdomen in strategic locations. One arm holds the endoscope (either an 8.5-mm or 12-mm diameter, with a 0-degree or 30-degree configuration) and the other three arms hold miniaturized 8-mm (or 5-mm) instruments. Some surgeons employ only two of these arms. The vision system delivers a high-definition 3D image to the viewer in the surgeon's console, and 2D images to other monitors in the operating room.
From the console, the surgeon uses hand controllers and foot pedals to move the instrument and camera robotic arms of the patient cart via a process of computer algorithms that reduce tremor and employ motion scaling to deliver precise movements within the surgical field. The robotic instruments have seven degrees of freedom that replicate or surpass the motions of the human hand, allowing the surgeon to essentially perform open surgery through laparoscopic access.
A uterine manipulator is typically used for traditional laparoscopic myomectomy procedures, and robotic myomectomy is no exception. I typically use a standard HUMI manipulator (Harris-Kronner Uterine Manipulator Injector by CooperSurgical), and I dock the patient-side cart between the patient's legs rather than on the side. This placement of the patient cart enables me to employ a four-arm approach for robotic myomectomy, which I prefer, rather than a three-arm approach. With this configuration, I can use one of the instrument arms to manipulate the uterus instead of relying on a bedside assistant having vaginal access to do this task.
One arm, at or above the umbilicus, holds the endoscope. At the beginning of the procedure, an instrument arm on the left side holds a bipolar device (a PK Dissector that is made by Gyrus ACMI for Intuitive Surgical), and one of two instrument arms on the right side holds the robotic scissors (the da Vinci HotShears). The other right-handed instrument arm holds tenaculum forceps, which can be used to manipulate the uterus or fibroid in any direction. At the end of the procedure, for closure of the hysterotomy incision, needle drivers may be substituted for the PK Dissector and HotShears and the ProGrasp (part of the da Vinci Surgical System) substituted for the tenaculum.
The ports or trocar sites are placed after establishing pneumoperitoneum, typically starting with a Veress needle at the primary or camera site. The camera site is chosen based on the size of the uterus, and an attempt is made to keep at least 10 cm (one handbreadth) between the fundus or top of the presenting fibroid and the camera trocar site.
The left lower quadrant port is placed at least 4–5 cm (three fingerbreadth) directly cephalad to the anterior superior iliac spine. The right lower quadrant port is similarly placed, and then the right upper quadrant port, with the distance between the two right ports being at least one handbreadth (10 cm) in a medial direction. The assistant's port is placed in the left upper quadrant near Palmer's point (the point 3 cm below the last rib in the left midclavicular line). (See image 3.)
One can also “side dock” the patient cart using this configuration to provide more access to the vagina when necessary, and the ports can be adjusted higher or lower on the abdomen depending on the size of the uterus. Clearly, there is a limit to how high one may traverse on the abdomen before entering the thoracic cavity using these principles. There are cases, though, in which the camera port may end up below the fundus of the uterus.
Spacing of the arms also can be negatively affected by a lower body mass index (BMI), but every attempt should be made to obtain at least 8–10 cm of spacing between the robotic port sites to minimize or prevent collision of the instrument and camera arms externally and internally. Caution also must be employed to place the trocars perpendicular to the plane of the abdominal wall; this prevents tunneling of the port, which would defeat the purpose of the strategic placement of the arms externally.
The use of two robotic instruments on the patient's right side is key. Having two right-handed instruments gives the surgeon the ability, at any point in the operation, to manipulate the uterus or the fibroid(s) with two graspers, and to be fairly self-sufficient in enucleating and retracting the fibroid(s) as well as in closing the myometrium.
Prior to the hysterotomy, a vasopressin solution of 20 U diluted in 60 cc of normal saline is injected transcutaneously into the myometrium surrounding the myomas using a 22-gauge 3½-inch or 7-inch spinal needle. This is done by direct vision under endoscopic guidance while using MR imagery. (See image 4.)
An incision is then made over the serosa overlying the fibroid to the level of the pseudocapsule. Whenever possible, and especially when the woman plans to have children, we make a transverse incision, as cesarean-section data of vertical versus low transverse incisions demonstrate that the strongest closure is obtained from transverse incisions. (See image 5.)
The myoma is grasped with the robotic tenaculum, and traction/counter-traction is then used to enucleate the myoma, with the tenaculum pulling away from a push-spread motion created with the scissor and a curved bipolar device in the opposite direction. The push-spread technique is preferable over significant use of cautery for two reasons: It reduces the amount of necrosis that occurs within the myometrium as a result of excessive thermal injury, and it promotes healing within the myometrium after the surgery is completed. Any vessels present at the base of the myoma can be addressed with use of the bipolar device. (See image 6.)
Indigo carmine dye may be injected through the uterine manipulator to help discern the location of the endometrial cavity, but the presence of the inflated balloon of the HUMI manipulator is also sufficient for that purpose.
The removed myoma is stored in the cul-de-sac or in the right upper quadrant, and must be counted upon removal just as any other sponge or instrument would be counted. Alternatively, the myomas can be attached on a suture, as a string of pearls, using a needle introduced laparoscopically.
Robotic needle drivers, one standard large and one Mega SutureCut, are then placed. Closure of the hysterotomy incision can currently be achieved with the use of barbed suture, a recently developed type of product that enables consistent tension on the suture line and does not need to be tied. Closure of the deep hysterotomy defect should be done in layers, especially if the defect is greater than 4–5 cm, using at least a 2–0 barbed suture. The myomas are subsequently removed from the abdomen by a process of morcellation. (See images 7 and 8.)
I recommend not using barbed suture on the serosa, but instead using a monofilament, nonbarbed suture of a smaller gauge such as 3–0. This is because exposure of the barbs on the serosa of the uterus may lead to adhesion formation by catching bowel or omentum.
Closure of the serosa can be achieved with either a running, imbricating stitch, or a baseball stitch. Morcellation is performed under direct vision (after undocking the robotic patient side-cart) using a 15-mm mechanical device placed either in the camera port or the left upper quadrant assistant port. A traditional 5-mm laparoscope or a robotic 8.5-mm endoscope can be used to facilitate this process.
Patients and Outcomes
Based on the published literature to date, and on MRI mapping, I recommend that the number of myomas removed not exceed five, and that the uterus be no larger than a 20-week gestational size. One can certainly exceed these limits, but these criteria are advisable for a surgeon with an average level of experience with robotics.
Although the cost of robotic myomectomy may be greater than that of myomectomy performed by laparotomy, a standardization of the type and number of instruments used, as well as a reduction in the number of disposables used per case, may result in significant cost savings in an institution that already has a robotic system.
Regarding pregnancies achieved after robotic myomectomies, preliminary data have been positive. We will report studies of long-term experience this fall.
Download a mobile quick response (QR) code reader from your smartphone's app store to view a video by Dr. Pitter, or visit
Image 1 (left): Below the endoscopic view of the fibroid uterus (top), MR images are superimposed in the surgeon's console viewer. The upper left MR image is a sagittal pelvic view indicating the presence of fibroids (left to right) in the posterior fundal, submucosal, and posterior midportion of the uterus. The upper right and lower left images are axial views showing fibroids (top to bottom) in the anterior left, submucosal left midportion, and posterior midportion of the uterus. In Image 2, the relative size of the images are adjusted to the surgeon's needs.
Source Images courtesy Dr. Michael C. Pitter
Image 3 shows robotic trocar placement in a 4-arm approach.
Image 4 (top): A spinal needle injects a dilute vasopressin solution into the fibroid pseudocapsule. Image 5: An initial incision is made to find the fibroid, using the PK Dissector (left) and HotShears (right).
Image 6 (left): The fibroid is enucleated using three robotic instruments and a suction irrigator from the assistant port. Clockwise from 12 o'clock are HotShears, robotic tenaculum, laparoscopic suction irrigator, and PK Dissector. Image 7 (middle): Closure of the hysterotomy incision is done using a 2–0 V-Loc suture to close the myometrium in two layers. Instruments (from left, upward, to right) are the standard large robotic needle driver; the Prograsp, which holds the suture and supports the uterus while the layers are being closed; and the Mega SutureCut needle driver, used to drive the needle through the myometrium. Image 8 (right): The final layer is closed with a monofilament suture, with optimal leveraging of all three robotic instruments.
Source Images courtesy Dr. Michael C. Pitter
Myomectomy – The Robotic Way
As noted in my AAGL Presidential Address in 2008, while cholecystectomies, hernia repairs, and bariatric surgeries are generally performed via minimally invasive techniques, only a small percentage of hysterectomies are executed by a laparoscopic technique. As pointed out in the text of this edition of the Master Class in gynecologic surgery by guest author Dr. Michael C. Pitter, there has been a recent increase in the percentage of minimally invasive hysterectomies due to robotic assistance.
Even more difficult to master laparoscopically than hysterectomy is myomectomy. Despite numerous opportunities for gynecologists to learn the technique of laparoscopic suturing, laparoscopic myomectomy remains in the domain of a few minimally invasive gynecologic surgeons worldwide. As Dr. Pitter so ably demonstrates in his discourse, for the gynecologist who is challenged by a pure laparoscopic approach, myomectomy can still be performed in a minimally invasive manner with use of robotic assistance. The difficulty of suturing at bedside is simplified with use of the robot due to 3-D visualization and articulating instrumentation.
Dr. Pitter is the chief of gynecologic robotic and minimally invasive surgery and a clinical assistant professor of obstetrics and gynecology at Newark (N.J.) Beth Israel Medical Center. Dr. Pitter is vice chair of the Robotics Special Interest Group of the AAGL and is a charter member of the Society of Robotic Surgery. He has publications both on establishing training criteria in robotic assisted gynecologic surgery, as well as robotic assisted hysterectomy in patients with large uteri. It is a pleasure and honor to welcome Dr. Pitter to this edition of the Master Class in gynecologic surgery.
Myomectomy offers an alternative to hysterectomy for the treatment of uterine fibroids whether or not future fertility is an issue. While many women chose a uterine-sparing approach to maintain their fertility options, there still are many women who prefer myomectomy for reasons other than fertility preservation.
The procedure is an important one for gynecologic surgeons and their patients, as it conveys a high rate of symptom resolution: Eighty-one percent of women who undergo a myomectomy experience complete resolution of their symptoms (Fertil. Steril. 1981;36:433-45).
Robot-assisted laparoscopic myomectomy was first described in 2004 by Dr. Arnold P. Advincula and his colleagues (J. Am. Assoc. Gynecol. Laparosc. 2004;11:511-8).
Their report played a pivotal role in the Food and Drug Administration's approval in 2005 for use of the da Vinci Surgical System (Intuitive Surgical, Sunnyvale, Calif.) for gynecologic surgical procedures.
While myomectomy still is most commonly performed via laparotomy, a significant number of surgeons have adopted the robotic approach. According to data from Solucient, a health care information company managed by Thomson Reuters, approximately 4,000 robotic myomectomies were performed in the United States in 2010. This represents 10% of the approximately 40,000 myomectomies performed each year, a significant proportion considering that robotics had been introduced to gynecology only 5 years earlier.
Myomectomy is a suture-intensive procedure, and suturing by a conventional laparoscopic approach has proved to be extremely challenging. The robotic platform gives surgeons greater capability of successfully repairing deep hysterotomy defects and provides them with a more achievable minimally invasive option to offer patients.
Interestingly, utilization of the laparoscopic approach for hysterectomy also has increased with the introduction of robotics. Current statistics show that only 16% of all hysterectomy procedures performed in the United States are done via conventional laparoscopy (20 years, approximately, after the techniques were developed), while another 20% are now being performed with robot assistance. A new AAGL position statement saying that surgeons who offer hysterectomy should be able to perform either vaginal hysterectomy (the preferred approach) or laparoscopic hysterectomy (the second best approach) – or refer their patients to a surgeon who can (J. Minim. Invasive Gynecol. 2011;18:1-3) – is indicative of the growing belief that the benefits of minimally invasive surgery over open procedures should be considered where possible in aspects of gynecologic surgery.
At our institution, we saw a significant improvement in operative time after the first 20 cases of robot-assisted myomectomy and hysterectomy. Our operative time went from a mean of 212 min. for cases 1–20 to a mean of 151 min. for cases 21–40 (Int. J. Med. Robot. 2008;4:114-20).
Others have reported similar findings on the learning curve for robot-assisted gynecologic surgery: Another case series published several years ago, for instance, showed operative times for various surgical procedures for benign gynecologic problems stabilizing within 50 cases (J. Minim. Invasive Gynecol. 2008;15:589-94). In general, these data are indicative of a significantly shorter learning curve than seen with traditional laparoscopic surgery.
Incorporation of MRI
The main drawback to robotics always has been the absence of haptics or tactile feedback. This limitation has, however, spurred the development of creative techniques to compensate, including the use of real-time magnetic resonance imaging.
MR images can now be incorporated in a real-time, 3-dimensional fashion into the surgeon's console for use in mapping, detecting, locating, and enucleating myomas. All three views – axial, coronal, and sagittal – can be seen during the surgery. This enables the surgeon both to overcome the haptic limitations and to remove multiple fibroids. (See images 1 and 2.)
Certainly, the gynecologic surgeon employing this technique must be comfortable reading and interpreting MR images. The necessary comfort level can be achieved, on an individual basis, with time spent reviewing series of pelvic MR images with a radiologist.
MR imaging also has proved, of course, to be an excellent preoperative tool for determining ahead of time the size, number, and location of myomas, and for ruling out adenomyosis. In my experience, MR imaging can be useful preoperatively in conjunction with pelvic exams to effectively screen for patients who are likely to have successful outcomes with robotic myomectomy.
For example, a patient with a 12- to 14-week-size uterus may not be a good candidate for robotic myomectomy if on the MR image the uterus has innumerable myomas without a clearly defined cleavage plane between the tumors. A woman with a significantly larger uterus may be an excellent candidate, on the other hand, if the number and location of leiomyomas is determined by MRI.
Set-Up, Technique
The three basic components of the da Vinci system are a patient-side cart, a vision system, and a surgeon's console. The patient-side cart has four robotic arms that are attached or “docked” to trocars that are placed in the abdomen in strategic locations. One arm holds the endoscope (either an 8.5-mm or 12-mm diameter, with a 0-degree or 30-degree configuration) and the other three arms hold miniaturized 8-mm (or 5-mm) instruments. Some surgeons employ only two of these arms. The vision system delivers a high-definition 3D image to the viewer in the surgeon's console, and 2D images to other monitors in the operating room.
From the console, the surgeon uses hand controllers and foot pedals to move the instrument and camera robotic arms of the patient cart via a process of computer algorithms that reduce tremor and employ motion scaling to deliver precise movements within the surgical field. The robotic instruments have seven degrees of freedom that replicate or surpass the motions of the human hand, allowing the surgeon to essentially perform open surgery through laparoscopic access.
A uterine manipulator is typically used for traditional laparoscopic myomectomy procedures, and robotic myomectomy is no exception. I typically use a standard HUMI manipulator (Harris-Kronner Uterine Manipulator Injector by CooperSurgical), and I dock the patient-side cart between the patient's legs rather than on the side. This placement of the patient cart enables me to employ a four-arm approach for robotic myomectomy, which I prefer, rather than a three-arm approach. With this configuration, I can use one of the instrument arms to manipulate the uterus instead of relying on a bedside assistant having vaginal access to do this task.
One arm, at or above the umbilicus, holds the endoscope. At the beginning of the procedure, an instrument arm on the left side holds a bipolar device (a PK Dissector that is made by Gyrus ACMI for Intuitive Surgical), and one of two instrument arms on the right side holds the robotic scissors (the da Vinci HotShears). The other right-handed instrument arm holds tenaculum forceps, which can be used to manipulate the uterus or fibroid in any direction. At the end of the procedure, for closure of the hysterotomy incision, needle drivers may be substituted for the PK Dissector and HotShears and the ProGrasp (part of the da Vinci Surgical System) substituted for the tenaculum.
The ports or trocar sites are placed after establishing pneumoperitoneum, typically starting with a Veress needle at the primary or camera site. The camera site is chosen based on the size of the uterus, and an attempt is made to keep at least 10 cm (one handbreadth) between the fundus or top of the presenting fibroid and the camera trocar site.
The left lower quadrant port is placed at least 4–5 cm (three fingerbreadth) directly cephalad to the anterior superior iliac spine. The right lower quadrant port is similarly placed, and then the right upper quadrant port, with the distance between the two right ports being at least one handbreadth (10 cm) in a medial direction. The assistant's port is placed in the left upper quadrant near Palmer's point (the point 3 cm below the last rib in the left midclavicular line). (See image 3.)
One can also “side dock” the patient cart using this configuration to provide more access to the vagina when necessary, and the ports can be adjusted higher or lower on the abdomen depending on the size of the uterus. Clearly, there is a limit to how high one may traverse on the abdomen before entering the thoracic cavity using these principles. There are cases, though, in which the camera port may end up below the fundus of the uterus.
Spacing of the arms also can be negatively affected by a lower body mass index (BMI), but every attempt should be made to obtain at least 8–10 cm of spacing between the robotic port sites to minimize or prevent collision of the instrument and camera arms externally and internally. Caution also must be employed to place the trocars perpendicular to the plane of the abdominal wall; this prevents tunneling of the port, which would defeat the purpose of the strategic placement of the arms externally.
The use of two robotic instruments on the patient's right side is key. Having two right-handed instruments gives the surgeon the ability, at any point in the operation, to manipulate the uterus or the fibroid(s) with two graspers, and to be fairly self-sufficient in enucleating and retracting the fibroid(s) as well as in closing the myometrium.
Prior to the hysterotomy, a vasopressin solution of 20 U diluted in 60 cc of normal saline is injected transcutaneously into the myometrium surrounding the myomas using a 22-gauge 3½-inch or 7-inch spinal needle. This is done by direct vision under endoscopic guidance while using MR imagery. (See image 4.)
An incision is then made over the serosa overlying the fibroid to the level of the pseudocapsule. Whenever possible, and especially when the woman plans to have children, we make a transverse incision, as cesarean-section data of vertical versus low transverse incisions demonstrate that the strongest closure is obtained from transverse incisions. (See image 5.)
The myoma is grasped with the robotic tenaculum, and traction/counter-traction is then used to enucleate the myoma, with the tenaculum pulling away from a push-spread motion created with the scissor and a curved bipolar device in the opposite direction. The push-spread technique is preferable over significant use of cautery for two reasons: It reduces the amount of necrosis that occurs within the myometrium as a result of excessive thermal injury, and it promotes healing within the myometrium after the surgery is completed. Any vessels present at the base of the myoma can be addressed with use of the bipolar device. (See image 6.)
Indigo carmine dye may be injected through the uterine manipulator to help discern the location of the endometrial cavity, but the presence of the inflated balloon of the HUMI manipulator is also sufficient for that purpose.
The removed myoma is stored in the cul-de-sac or in the right upper quadrant, and must be counted upon removal just as any other sponge or instrument would be counted. Alternatively, the myomas can be attached on a suture, as a string of pearls, using a needle introduced laparoscopically.
Robotic needle drivers, one standard large and one Mega SutureCut, are then placed. Closure of the hysterotomy incision can currently be achieved with the use of barbed suture, a recently developed type of product that enables consistent tension on the suture line and does not need to be tied. Closure of the deep hysterotomy defect should be done in layers, especially if the defect is greater than 4–5 cm, using at least a 2–0 barbed suture. The myomas are subsequently removed from the abdomen by a process of morcellation. (See images 7 and 8.)
I recommend not using barbed suture on the serosa, but instead using a monofilament, nonbarbed suture of a smaller gauge such as 3–0. This is because exposure of the barbs on the serosa of the uterus may lead to adhesion formation by catching bowel or omentum.
Closure of the serosa can be achieved with either a running, imbricating stitch, or a baseball stitch. Morcellation is performed under direct vision (after undocking the robotic patient side-cart) using a 15-mm mechanical device placed either in the camera port or the left upper quadrant assistant port. A traditional 5-mm laparoscope or a robotic 8.5-mm endoscope can be used to facilitate this process.
Patients and Outcomes
Based on the published literature to date, and on MRI mapping, I recommend that the number of myomas removed not exceed five, and that the uterus be no larger than a 20-week gestational size. One can certainly exceed these limits, but these criteria are advisable for a surgeon with an average level of experience with robotics.
Although the cost of robotic myomectomy may be greater than that of myomectomy performed by laparotomy, a standardization of the type and number of instruments used, as well as a reduction in the number of disposables used per case, may result in significant cost savings in an institution that already has a robotic system.
Regarding pregnancies achieved after robotic myomectomies, preliminary data have been positive. We will report studies of long-term experience this fall.
Download a mobile quick response (QR) code reader from your smartphone's app store to view a video by Dr. Pitter, or visit
Image 1 (left): Below the endoscopic view of the fibroid uterus (top), MR images are superimposed in the surgeon's console viewer. The upper left MR image is a sagittal pelvic view indicating the presence of fibroids (left to right) in the posterior fundal, submucosal, and posterior midportion of the uterus. The upper right and lower left images are axial views showing fibroids (top to bottom) in the anterior left, submucosal left midportion, and posterior midportion of the uterus. In Image 2, the relative size of the images are adjusted to the surgeon's needs.
Source Images courtesy Dr. Michael C. Pitter
Image 3 shows robotic trocar placement in a 4-arm approach.
Image 4 (top): A spinal needle injects a dilute vasopressin solution into the fibroid pseudocapsule. Image 5: An initial incision is made to find the fibroid, using the PK Dissector (left) and HotShears (right).
Image 6 (left): The fibroid is enucleated using three robotic instruments and a suction irrigator from the assistant port. Clockwise from 12 o'clock are HotShears, robotic tenaculum, laparoscopic suction irrigator, and PK Dissector. Image 7 (middle): Closure of the hysterotomy incision is done using a 2–0 V-Loc suture to close the myometrium in two layers. Instruments (from left, upward, to right) are the standard large robotic needle driver; the Prograsp, which holds the suture and supports the uterus while the layers are being closed; and the Mega SutureCut needle driver, used to drive the needle through the myometrium. Image 8 (right): The final layer is closed with a monofilament suture, with optimal leveraging of all three robotic instruments.
Source Images courtesy Dr. Michael C. Pitter
Myomectomy – The Robotic Way
As noted in my AAGL Presidential Address in 2008, while cholecystectomies, hernia repairs, and bariatric surgeries are generally performed via minimally invasive techniques, only a small percentage of hysterectomies are executed by a laparoscopic technique. As pointed out in the text of this edition of the Master Class in gynecologic surgery by guest author Dr. Michael C. Pitter, there has been a recent increase in the percentage of minimally invasive hysterectomies due to robotic assistance.
Even more difficult to master laparoscopically than hysterectomy is myomectomy. Despite numerous opportunities for gynecologists to learn the technique of laparoscopic suturing, laparoscopic myomectomy remains in the domain of a few minimally invasive gynecologic surgeons worldwide. As Dr. Pitter so ably demonstrates in his discourse, for the gynecologist who is challenged by a pure laparoscopic approach, myomectomy can still be performed in a minimally invasive manner with use of robotic assistance. The difficulty of suturing at bedside is simplified with use of the robot due to 3-D visualization and articulating instrumentation.
Dr. Pitter is the chief of gynecologic robotic and minimally invasive surgery and a clinical assistant professor of obstetrics and gynecology at Newark (N.J.) Beth Israel Medical Center. Dr. Pitter is vice chair of the Robotics Special Interest Group of the AAGL and is a charter member of the Society of Robotic Surgery. He has publications both on establishing training criteria in robotic assisted gynecologic surgery, as well as robotic assisted hysterectomy in patients with large uteri. It is a pleasure and honor to welcome Dr. Pitter to this edition of the Master Class in gynecologic surgery.
Myomectomy offers an alternative to hysterectomy for the treatment of uterine fibroids whether or not future fertility is an issue. While many women chose a uterine-sparing approach to maintain their fertility options, there still are many women who prefer myomectomy for reasons other than fertility preservation.
The procedure is an important one for gynecologic surgeons and their patients, as it conveys a high rate of symptom resolution: Eighty-one percent of women who undergo a myomectomy experience complete resolution of their symptoms (Fertil. Steril. 1981;36:433-45).
Robot-assisted laparoscopic myomectomy was first described in 2004 by Dr. Arnold P. Advincula and his colleagues (J. Am. Assoc. Gynecol. Laparosc. 2004;11:511-8).
Their report played a pivotal role in the Food and Drug Administration's approval in 2005 for use of the da Vinci Surgical System (Intuitive Surgical, Sunnyvale, Calif.) for gynecologic surgical procedures.
While myomectomy still is most commonly performed via laparotomy, a significant number of surgeons have adopted the robotic approach. According to data from Solucient, a health care information company managed by Thomson Reuters, approximately 4,000 robotic myomectomies were performed in the United States in 2010. This represents 10% of the approximately 40,000 myomectomies performed each year, a significant proportion considering that robotics had been introduced to gynecology only 5 years earlier.
Myomectomy is a suture-intensive procedure, and suturing by a conventional laparoscopic approach has proved to be extremely challenging. The robotic platform gives surgeons greater capability of successfully repairing deep hysterotomy defects and provides them with a more achievable minimally invasive option to offer patients.
Interestingly, utilization of the laparoscopic approach for hysterectomy also has increased with the introduction of robotics. Current statistics show that only 16% of all hysterectomy procedures performed in the United States are done via conventional laparoscopy (20 years, approximately, after the techniques were developed), while another 20% are now being performed with robot assistance. A new AAGL position statement saying that surgeons who offer hysterectomy should be able to perform either vaginal hysterectomy (the preferred approach) or laparoscopic hysterectomy (the second best approach) – or refer their patients to a surgeon who can (J. Minim. Invasive Gynecol. 2011;18:1-3) – is indicative of the growing belief that the benefits of minimally invasive surgery over open procedures should be considered where possible in aspects of gynecologic surgery.
At our institution, we saw a significant improvement in operative time after the first 20 cases of robot-assisted myomectomy and hysterectomy. Our operative time went from a mean of 212 min. for cases 1–20 to a mean of 151 min. for cases 21–40 (Int. J. Med. Robot. 2008;4:114-20).
Others have reported similar findings on the learning curve for robot-assisted gynecologic surgery: Another case series published several years ago, for instance, showed operative times for various surgical procedures for benign gynecologic problems stabilizing within 50 cases (J. Minim. Invasive Gynecol. 2008;15:589-94). In general, these data are indicative of a significantly shorter learning curve than seen with traditional laparoscopic surgery.
Incorporation of MRI
The main drawback to robotics always has been the absence of haptics or tactile feedback. This limitation has, however, spurred the development of creative techniques to compensate, including the use of real-time magnetic resonance imaging.
MR images can now be incorporated in a real-time, 3-dimensional fashion into the surgeon's console for use in mapping, detecting, locating, and enucleating myomas. All three views – axial, coronal, and sagittal – can be seen during the surgery. This enables the surgeon both to overcome the haptic limitations and to remove multiple fibroids. (See images 1 and 2.)
Certainly, the gynecologic surgeon employing this technique must be comfortable reading and interpreting MR images. The necessary comfort level can be achieved, on an individual basis, with time spent reviewing series of pelvic MR images with a radiologist.
MR imaging also has proved, of course, to be an excellent preoperative tool for determining ahead of time the size, number, and location of myomas, and for ruling out adenomyosis. In my experience, MR imaging can be useful preoperatively in conjunction with pelvic exams to effectively screen for patients who are likely to have successful outcomes with robotic myomectomy.
For example, a patient with a 12- to 14-week-size uterus may not be a good candidate for robotic myomectomy if on the MR image the uterus has innumerable myomas without a clearly defined cleavage plane between the tumors. A woman with a significantly larger uterus may be an excellent candidate, on the other hand, if the number and location of leiomyomas is determined by MRI.
Set-Up, Technique
The three basic components of the da Vinci system are a patient-side cart, a vision system, and a surgeon's console. The patient-side cart has four robotic arms that are attached or “docked” to trocars that are placed in the abdomen in strategic locations. One arm holds the endoscope (either an 8.5-mm or 12-mm diameter, with a 0-degree or 30-degree configuration) and the other three arms hold miniaturized 8-mm (or 5-mm) instruments. Some surgeons employ only two of these arms. The vision system delivers a high-definition 3D image to the viewer in the surgeon's console, and 2D images to other monitors in the operating room.
From the console, the surgeon uses hand controllers and foot pedals to move the instrument and camera robotic arms of the patient cart via a process of computer algorithms that reduce tremor and employ motion scaling to deliver precise movements within the surgical field. The robotic instruments have seven degrees of freedom that replicate or surpass the motions of the human hand, allowing the surgeon to essentially perform open surgery through laparoscopic access.
A uterine manipulator is typically used for traditional laparoscopic myomectomy procedures, and robotic myomectomy is no exception. I typically use a standard HUMI manipulator (Harris-Kronner Uterine Manipulator Injector by CooperSurgical), and I dock the patient-side cart between the patient's legs rather than on the side. This placement of the patient cart enables me to employ a four-arm approach for robotic myomectomy, which I prefer, rather than a three-arm approach. With this configuration, I can use one of the instrument arms to manipulate the uterus instead of relying on a bedside assistant having vaginal access to do this task.
One arm, at or above the umbilicus, holds the endoscope. At the beginning of the procedure, an instrument arm on the left side holds a bipolar device (a PK Dissector that is made by Gyrus ACMI for Intuitive Surgical), and one of two instrument arms on the right side holds the robotic scissors (the da Vinci HotShears). The other right-handed instrument arm holds tenaculum forceps, which can be used to manipulate the uterus or fibroid in any direction. At the end of the procedure, for closure of the hysterotomy incision, needle drivers may be substituted for the PK Dissector and HotShears and the ProGrasp (part of the da Vinci Surgical System) substituted for the tenaculum.
The ports or trocar sites are placed after establishing pneumoperitoneum, typically starting with a Veress needle at the primary or camera site. The camera site is chosen based on the size of the uterus, and an attempt is made to keep at least 10 cm (one handbreadth) between the fundus or top of the presenting fibroid and the camera trocar site.
The left lower quadrant port is placed at least 4–5 cm (three fingerbreadth) directly cephalad to the anterior superior iliac spine. The right lower quadrant port is similarly placed, and then the right upper quadrant port, with the distance between the two right ports being at least one handbreadth (10 cm) in a medial direction. The assistant's port is placed in the left upper quadrant near Palmer's point (the point 3 cm below the last rib in the left midclavicular line). (See image 3.)
One can also “side dock” the patient cart using this configuration to provide more access to the vagina when necessary, and the ports can be adjusted higher or lower on the abdomen depending on the size of the uterus. Clearly, there is a limit to how high one may traverse on the abdomen before entering the thoracic cavity using these principles. There are cases, though, in which the camera port may end up below the fundus of the uterus.
Spacing of the arms also can be negatively affected by a lower body mass index (BMI), but every attempt should be made to obtain at least 8–10 cm of spacing between the robotic port sites to minimize or prevent collision of the instrument and camera arms externally and internally. Caution also must be employed to place the trocars perpendicular to the plane of the abdominal wall; this prevents tunneling of the port, which would defeat the purpose of the strategic placement of the arms externally.
The use of two robotic instruments on the patient's right side is key. Having two right-handed instruments gives the surgeon the ability, at any point in the operation, to manipulate the uterus or the fibroid(s) with two graspers, and to be fairly self-sufficient in enucleating and retracting the fibroid(s) as well as in closing the myometrium.
Prior to the hysterotomy, a vasopressin solution of 20 U diluted in 60 cc of normal saline is injected transcutaneously into the myometrium surrounding the myomas using a 22-gauge 3½-inch or 7-inch spinal needle. This is done by direct vision under endoscopic guidance while using MR imagery. (See image 4.)
An incision is then made over the serosa overlying the fibroid to the level of the pseudocapsule. Whenever possible, and especially when the woman plans to have children, we make a transverse incision, as cesarean-section data of vertical versus low transverse incisions demonstrate that the strongest closure is obtained from transverse incisions. (See image 5.)
The myoma is grasped with the robotic tenaculum, and traction/counter-traction is then used to enucleate the myoma, with the tenaculum pulling away from a push-spread motion created with the scissor and a curved bipolar device in the opposite direction. The push-spread technique is preferable over significant use of cautery for two reasons: It reduces the amount of necrosis that occurs within the myometrium as a result of excessive thermal injury, and it promotes healing within the myometrium after the surgery is completed. Any vessels present at the base of the myoma can be addressed with use of the bipolar device. (See image 6.)
Indigo carmine dye may be injected through the uterine manipulator to help discern the location of the endometrial cavity, but the presence of the inflated balloon of the HUMI manipulator is also sufficient for that purpose.
The removed myoma is stored in the cul-de-sac or in the right upper quadrant, and must be counted upon removal just as any other sponge or instrument would be counted. Alternatively, the myomas can be attached on a suture, as a string of pearls, using a needle introduced laparoscopically.
Robotic needle drivers, one standard large and one Mega SutureCut, are then placed. Closure of the hysterotomy incision can currently be achieved with the use of barbed suture, a recently developed type of product that enables consistent tension on the suture line and does not need to be tied. Closure of the deep hysterotomy defect should be done in layers, especially if the defect is greater than 4–5 cm, using at least a 2–0 barbed suture. The myomas are subsequently removed from the abdomen by a process of morcellation. (See images 7 and 8.)
I recommend not using barbed suture on the serosa, but instead using a monofilament, nonbarbed suture of a smaller gauge such as 3–0. This is because exposure of the barbs on the serosa of the uterus may lead to adhesion formation by catching bowel or omentum.
Closure of the serosa can be achieved with either a running, imbricating stitch, or a baseball stitch. Morcellation is performed under direct vision (after undocking the robotic patient side-cart) using a 15-mm mechanical device placed either in the camera port or the left upper quadrant assistant port. A traditional 5-mm laparoscope or a robotic 8.5-mm endoscope can be used to facilitate this process.
Patients and Outcomes
Based on the published literature to date, and on MRI mapping, I recommend that the number of myomas removed not exceed five, and that the uterus be no larger than a 20-week gestational size. One can certainly exceed these limits, but these criteria are advisable for a surgeon with an average level of experience with robotics.
Although the cost of robotic myomectomy may be greater than that of myomectomy performed by laparotomy, a standardization of the type and number of instruments used, as well as a reduction in the number of disposables used per case, may result in significant cost savings in an institution that already has a robotic system.
Regarding pregnancies achieved after robotic myomectomies, preliminary data have been positive. We will report studies of long-term experience this fall.
Download a mobile quick response (QR) code reader from your smartphone's app store to view a video by Dr. Pitter, or visit
Image 1 (left): Below the endoscopic view of the fibroid uterus (top), MR images are superimposed in the surgeon's console viewer. The upper left MR image is a sagittal pelvic view indicating the presence of fibroids (left to right) in the posterior fundal, submucosal, and posterior midportion of the uterus. The upper right and lower left images are axial views showing fibroids (top to bottom) in the anterior left, submucosal left midportion, and posterior midportion of the uterus. In Image 2, the relative size of the images are adjusted to the surgeon's needs.
Source Images courtesy Dr. Michael C. Pitter
Image 3 shows robotic trocar placement in a 4-arm approach.
Image 4 (top): A spinal needle injects a dilute vasopressin solution into the fibroid pseudocapsule. Image 5: An initial incision is made to find the fibroid, using the PK Dissector (left) and HotShears (right).
Image 6 (left): The fibroid is enucleated using three robotic instruments and a suction irrigator from the assistant port. Clockwise from 12 o'clock are HotShears, robotic tenaculum, laparoscopic suction irrigator, and PK Dissector. Image 7 (middle): Closure of the hysterotomy incision is done using a 2–0 V-Loc suture to close the myometrium in two layers. Instruments (from left, upward, to right) are the standard large robotic needle driver; the Prograsp, which holds the suture and supports the uterus while the layers are being closed; and the Mega SutureCut needle driver, used to drive the needle through the myometrium. Image 8 (right): The final layer is closed with a monofilament suture, with optimal leveraging of all three robotic instruments.
Source Images courtesy Dr. Michael C. Pitter
Myomectomy – The Robotic Way
As noted in my AAGL Presidential Address in 2008, while cholecystectomies, hernia repairs, and bariatric surgeries are generally performed via minimally invasive techniques, only a small percentage of hysterectomies are executed by a laparoscopic technique. As pointed out in the text of this edition of the Master Class in gynecologic surgery by guest author Dr. Michael C. Pitter, there has been a recent increase in the percentage of minimally invasive hysterectomies due to robotic assistance.
Even more difficult to master laparoscopically than hysterectomy is myomectomy. Despite numerous opportunities for gynecologists to learn the technique of laparoscopic suturing, laparoscopic myomectomy remains in the domain of a few minimally invasive gynecologic surgeons worldwide. As Dr. Pitter so ably demonstrates in his discourse, for the gynecologist who is challenged by a pure laparoscopic approach, myomectomy can still be performed in a minimally invasive manner with use of robotic assistance. The difficulty of suturing at bedside is simplified with use of the robot due to 3-D visualization and articulating instrumentation.
Dr. Pitter is the chief of gynecologic robotic and minimally invasive surgery and a clinical assistant professor of obstetrics and gynecology at Newark (N.J.) Beth Israel Medical Center. Dr. Pitter is vice chair of the Robotics Special Interest Group of the AAGL and is a charter member of the Society of Robotic Surgery. He has publications both on establishing training criteria in robotic assisted gynecologic surgery, as well as robotic assisted hysterectomy in patients with large uteri. It is a pleasure and honor to welcome Dr. Pitter to this edition of the Master Class in gynecologic surgery.
On Transcatheter Aortic Valves
The natural history and pathology of aortic stenosis has been well described since the mid-18th century by John Baptist Morgagni. Its latency period usually runs 6-7 decades before expressing its classic symptoms. Once the symptoms of heart failure, angina, and syncope occur, the life span of patients is measured in 1-2 years.
Because of the increased number of octogenarians around these days, aortic stenosis has become a larger therapeutic problem to cardiologists. Unfortunately, when octogenarians come to the doctor with the symptoms of aortic stenosis, they usually bring a number of other comorbidities, such as coronary artery disease, diabetes, pulmonary insufficiency, and renal dysfunction, just to name a few. Surgical intervention in these patients carries high risk and both the patient and surgeon are reluctant to proceed with high-risk surgery in such a complex medical environment.
The recent development of a percutaneous aortic valve that can be implanted either transvenously or transapically has provided interesting options for these elderly patients. Several transcatheter aortic valves are now available in Europe, but until the last few months there have been no randomized clinical trials evaluating there efficacy.
The two most recent trials, the PARTNER trials, using a SAPIEN heart valve system (Edwards Lifesciences) have provided an opportunity to consider the potential benefits of transcatheter aortic-valve replacement (TAVR). The first reported trial compared TAVR to standard medical therapy in patients with severe aortic stenosis deemed inoperable for traditional aortic valve replacement (AVR). A second group of patient with severe aortic stenosis was randomized to either TAVR or AVR. Both studies have provided optimism that these percutaneous devices can provided significant benefit.
The initial PARTNER study randomized 358 stenosis patients who were considered to be inoperable, to either TAVR or standard medical therapy including in some case balloon aortic valvulotomy (N. Engl. J. Med. 2010; 363:1597-607). That trial reported a 30-day mortality of 5.0% and 2.8% and a 1-year mortality of 30.7% and 50.7% in the TAVR and standard medical therapy groups, respectively. Associated with this improvement in mortality, there was both symptomatic improvement and decrease in hospitalization in the TAVR treated patients. There was, however, an increase occurrence of major strokes, at 5.0% in the TAVR patients compared with 1.1% in the medical patients.
The most recent PARTNER trial reported at the annual meeting of the American Cardiology compared TAVR to standard surgical AVR in patients with severe aortic stenosis. In that trial, 699 patients with mean aortic valve area of 0.6-0.7 cm
The device used in PARTNER is currently approved for use in Europe and soon to be available in the United States. Several other transcatheter valve systems are currently in development by device companies, and one, the CoreValve (Medtronics) is currently undergoing clinical trials in the United States. The devices included in the early trials have been improved upon and investigators using the Edwards Lifesciences device are currently testing the fourth generation of that valve, which is smaller and easier to pass through the femoral artery.
In addition, protection devices are being developed to deal with the observed increased stroke morbidity. Although stroke remains a problem, emboli have not been limited to the brain but some reports suggest that, there is evidence for intracoronary embolism.
The development of these valves are obviously on the fast track but unfortunately little is known about their long-term durability. There are some follow-up data from Europe where the valve has been in use for about 2 years. When weighed against the years of experience and the excellent durability of the current AVR there should be some reticence to the application of these valves in patients at better surgical risks.
Although the operative risks for either TAVR or AVR are acceptable, considering the natural history of the disease, unfortunately the long-term risks of the elderly patients with aortic stenosis remains high even after successful valve replacement.
The natural history and pathology of aortic stenosis has been well described since the mid-18th century by John Baptist Morgagni. Its latency period usually runs 6-7 decades before expressing its classic symptoms. Once the symptoms of heart failure, angina, and syncope occur, the life span of patients is measured in 1-2 years.
Because of the increased number of octogenarians around these days, aortic stenosis has become a larger therapeutic problem to cardiologists. Unfortunately, when octogenarians come to the doctor with the symptoms of aortic stenosis, they usually bring a number of other comorbidities, such as coronary artery disease, diabetes, pulmonary insufficiency, and renal dysfunction, just to name a few. Surgical intervention in these patients carries high risk and both the patient and surgeon are reluctant to proceed with high-risk surgery in such a complex medical environment.
The recent development of a percutaneous aortic valve that can be implanted either transvenously or transapically has provided interesting options for these elderly patients. Several transcatheter aortic valves are now available in Europe, but until the last few months there have been no randomized clinical trials evaluating there efficacy.
The two most recent trials, the PARTNER trials, using a SAPIEN heart valve system (Edwards Lifesciences) have provided an opportunity to consider the potential benefits of transcatheter aortic-valve replacement (TAVR). The first reported trial compared TAVR to standard medical therapy in patients with severe aortic stenosis deemed inoperable for traditional aortic valve replacement (AVR). A second group of patient with severe aortic stenosis was randomized to either TAVR or AVR. Both studies have provided optimism that these percutaneous devices can provided significant benefit.
The initial PARTNER study randomized 358 stenosis patients who were considered to be inoperable, to either TAVR or standard medical therapy including in some case balloon aortic valvulotomy (N. Engl. J. Med. 2010; 363:1597-607). That trial reported a 30-day mortality of 5.0% and 2.8% and a 1-year mortality of 30.7% and 50.7% in the TAVR and standard medical therapy groups, respectively. Associated with this improvement in mortality, there was both symptomatic improvement and decrease in hospitalization in the TAVR treated patients. There was, however, an increase occurrence of major strokes, at 5.0% in the TAVR patients compared with 1.1% in the medical patients.
The most recent PARTNER trial reported at the annual meeting of the American Cardiology compared TAVR to standard surgical AVR in patients with severe aortic stenosis. In that trial, 699 patients with mean aortic valve area of 0.6-0.7 cm
The device used in PARTNER is currently approved for use in Europe and soon to be available in the United States. Several other transcatheter valve systems are currently in development by device companies, and one, the CoreValve (Medtronics) is currently undergoing clinical trials in the United States. The devices included in the early trials have been improved upon and investigators using the Edwards Lifesciences device are currently testing the fourth generation of that valve, which is smaller and easier to pass through the femoral artery.
In addition, protection devices are being developed to deal with the observed increased stroke morbidity. Although stroke remains a problem, emboli have not been limited to the brain but some reports suggest that, there is evidence for intracoronary embolism.
The development of these valves are obviously on the fast track but unfortunately little is known about their long-term durability. There are some follow-up data from Europe where the valve has been in use for about 2 years. When weighed against the years of experience and the excellent durability of the current AVR there should be some reticence to the application of these valves in patients at better surgical risks.
Although the operative risks for either TAVR or AVR are acceptable, considering the natural history of the disease, unfortunately the long-term risks of the elderly patients with aortic stenosis remains high even after successful valve replacement.
The natural history and pathology of aortic stenosis has been well described since the mid-18th century by John Baptist Morgagni. Its latency period usually runs 6-7 decades before expressing its classic symptoms. Once the symptoms of heart failure, angina, and syncope occur, the life span of patients is measured in 1-2 years.
Because of the increased number of octogenarians around these days, aortic stenosis has become a larger therapeutic problem to cardiologists. Unfortunately, when octogenarians come to the doctor with the symptoms of aortic stenosis, they usually bring a number of other comorbidities, such as coronary artery disease, diabetes, pulmonary insufficiency, and renal dysfunction, just to name a few. Surgical intervention in these patients carries high risk and both the patient and surgeon are reluctant to proceed with high-risk surgery in such a complex medical environment.
The recent development of a percutaneous aortic valve that can be implanted either transvenously or transapically has provided interesting options for these elderly patients. Several transcatheter aortic valves are now available in Europe, but until the last few months there have been no randomized clinical trials evaluating there efficacy.
The two most recent trials, the PARTNER trials, using a SAPIEN heart valve system (Edwards Lifesciences) have provided an opportunity to consider the potential benefits of transcatheter aortic-valve replacement (TAVR). The first reported trial compared TAVR to standard medical therapy in patients with severe aortic stenosis deemed inoperable for traditional aortic valve replacement (AVR). A second group of patient with severe aortic stenosis was randomized to either TAVR or AVR. Both studies have provided optimism that these percutaneous devices can provided significant benefit.
The initial PARTNER study randomized 358 stenosis patients who were considered to be inoperable, to either TAVR or standard medical therapy including in some case balloon aortic valvulotomy (N. Engl. J. Med. 2010; 363:1597-607). That trial reported a 30-day mortality of 5.0% and 2.8% and a 1-year mortality of 30.7% and 50.7% in the TAVR and standard medical therapy groups, respectively. Associated with this improvement in mortality, there was both symptomatic improvement and decrease in hospitalization in the TAVR treated patients. There was, however, an increase occurrence of major strokes, at 5.0% in the TAVR patients compared with 1.1% in the medical patients.
The most recent PARTNER trial reported at the annual meeting of the American Cardiology compared TAVR to standard surgical AVR in patients with severe aortic stenosis. In that trial, 699 patients with mean aortic valve area of 0.6-0.7 cm
The device used in PARTNER is currently approved for use in Europe and soon to be available in the United States. Several other transcatheter valve systems are currently in development by device companies, and one, the CoreValve (Medtronics) is currently undergoing clinical trials in the United States. The devices included in the early trials have been improved upon and investigators using the Edwards Lifesciences device are currently testing the fourth generation of that valve, which is smaller and easier to pass through the femoral artery.
In addition, protection devices are being developed to deal with the observed increased stroke morbidity. Although stroke remains a problem, emboli have not been limited to the brain but some reports suggest that, there is evidence for intracoronary embolism.
The development of these valves are obviously on the fast track but unfortunately little is known about their long-term durability. There are some follow-up data from Europe where the valve has been in use for about 2 years. When weighed against the years of experience and the excellent durability of the current AVR there should be some reticence to the application of these valves in patients at better surgical risks.
Although the operative risks for either TAVR or AVR are acceptable, considering the natural history of the disease, unfortunately the long-term risks of the elderly patients with aortic stenosis remains high even after successful valve replacement.
Vision Screening, Examination, and Referral
Screen for vision concerns at every well-child visit. I recommend a consistent method of screening so you and your staff develop a skill set and yield consistent results. The screening method varies by the age and cooperation of the child, so it is useful to establish protocols for the preverbal child and for an older child who can actively participate in visual acuity testing.
The American Academy of Pediatrics has a wonderful publication with vision screening recommendations for pediatricians. The AAP's policy statement on “Eye Examination and Vision Screening in Infants, Children, and Young Adults” contains consensus-driven information of high value to pediatricians (http://tinyurl.com/3o6papp
Importantly, keep in mind that many children old enough to participate in vision testing often perform poorly on initial testing.
There is a large learning curve, and a child who performs poorly the first time will often do very well on the second test. So it's a good idea to retest before referring a child to a specialist for poor vision.
If the child is cooperative, you can retest during the same visit and save the patient a return trip to your office. If the child fails visual acuity testing twice, that is when I would refer to an eye specialist.
Pediatricians are essentially looking for reduced vision, misalignment of the eye, and any anatomic abnormalities of the eye.
More specifically, you are screening for amblyopia, which can occur in up to 4% of the population; strabismus or eye misalignment; and anatomic concerns including ptosis, abnormal size of the eye, or a white pupil that suggests a cataract or a retinoblastoma.
A positive finding on almost every aspect of screening indicates need for referral of the patient to a specialist.
In contrast, acute abnormalities such as redness of the eye, minor injuries, and allergic conjunctivitis can be managed well in your office.
Keep in mind that failure to respond to initial treatment is an indication for referral. If you treat a child for red eye, for example, and the eye does not improve quickly, referral is warranted. Importantly, it is not usually an indication to change their antibiotic drop or switch them to another treatment. Although most of the time a simple problem may be the culprit, a red eye also can signal a more serious condition.
Referral of any child who screens positive for an eye concern or fails to initially respond to treatment generally requires no additional evaluation in the primary care setting. Just send the child along with a note explaining your concern and outlining any special circumstances that might not emerge on routine history taking with the parents. We'll take it from there.
In addition to the AAP guidelines, I recommend two mnemonics to assist primary care physicians during eye evaluation. A stretched version of MVP, the MVPea mnemonic can guide assessment and ensure that a quick examination is complete:
▸ M stands for motility. Are the eyes straight and do they move normally?
▸ V is vision assessment.
▸ P is pupil assessment. Are the pupils equal, round, and reactive? Do you see an afferent defect (decreased pupillary response to light in the affected eye), or an abnormality such as a white pupil or pupillary asymmetry?
▸ e is external exam. Assess surrounding structures, including the eyelids and eyelashes, for any abnormality.
▸ a is for the anterior segment. Evaluate the cornea, the lens, and other structures.
These are the critical components of a pediatrician's regular eye examination. These steps should take you a very short amount of time.
If you have a particular interest in eye disorders and some practice and skill at ocular examinations, you can consider adding three supplementary components to your assessment. I call these the ophthalmic version of CPR for the pediatrician:
▸ C is for confrontational visual field testing.
▸ P is for pressure.
▸ R is for retina.
Screen for vision concerns at every well-child visit. I recommend a consistent method of screening so you and your staff develop a skill set and yield consistent results. The screening method varies by the age and cooperation of the child, so it is useful to establish protocols for the preverbal child and for an older child who can actively participate in visual acuity testing.
The American Academy of Pediatrics has a wonderful publication with vision screening recommendations for pediatricians. The AAP's policy statement on “Eye Examination and Vision Screening in Infants, Children, and Young Adults” contains consensus-driven information of high value to pediatricians (http://tinyurl.com/3o6papp
Importantly, keep in mind that many children old enough to participate in vision testing often perform poorly on initial testing.
There is a large learning curve, and a child who performs poorly the first time will often do very well on the second test. So it's a good idea to retest before referring a child to a specialist for poor vision.
If the child is cooperative, you can retest during the same visit and save the patient a return trip to your office. If the child fails visual acuity testing twice, that is when I would refer to an eye specialist.
Pediatricians are essentially looking for reduced vision, misalignment of the eye, and any anatomic abnormalities of the eye.
More specifically, you are screening for amblyopia, which can occur in up to 4% of the population; strabismus or eye misalignment; and anatomic concerns including ptosis, abnormal size of the eye, or a white pupil that suggests a cataract or a retinoblastoma.
A positive finding on almost every aspect of screening indicates need for referral of the patient to a specialist.
In contrast, acute abnormalities such as redness of the eye, minor injuries, and allergic conjunctivitis can be managed well in your office.
Keep in mind that failure to respond to initial treatment is an indication for referral. If you treat a child for red eye, for example, and the eye does not improve quickly, referral is warranted. Importantly, it is not usually an indication to change their antibiotic drop or switch them to another treatment. Although most of the time a simple problem may be the culprit, a red eye also can signal a more serious condition.
Referral of any child who screens positive for an eye concern or fails to initially respond to treatment generally requires no additional evaluation in the primary care setting. Just send the child along with a note explaining your concern and outlining any special circumstances that might not emerge on routine history taking with the parents. We'll take it from there.
In addition to the AAP guidelines, I recommend two mnemonics to assist primary care physicians during eye evaluation. A stretched version of MVP, the MVPea mnemonic can guide assessment and ensure that a quick examination is complete:
▸ M stands for motility. Are the eyes straight and do they move normally?
▸ V is vision assessment.
▸ P is pupil assessment. Are the pupils equal, round, and reactive? Do you see an afferent defect (decreased pupillary response to light in the affected eye), or an abnormality such as a white pupil or pupillary asymmetry?
▸ e is external exam. Assess surrounding structures, including the eyelids and eyelashes, for any abnormality.
▸ a is for the anterior segment. Evaluate the cornea, the lens, and other structures.
These are the critical components of a pediatrician's regular eye examination. These steps should take you a very short amount of time.
If you have a particular interest in eye disorders and some practice and skill at ocular examinations, you can consider adding three supplementary components to your assessment. I call these the ophthalmic version of CPR for the pediatrician:
▸ C is for confrontational visual field testing.
▸ P is for pressure.
▸ R is for retina.
Screen for vision concerns at every well-child visit. I recommend a consistent method of screening so you and your staff develop a skill set and yield consistent results. The screening method varies by the age and cooperation of the child, so it is useful to establish protocols for the preverbal child and for an older child who can actively participate in visual acuity testing.
The American Academy of Pediatrics has a wonderful publication with vision screening recommendations for pediatricians. The AAP's policy statement on “Eye Examination and Vision Screening in Infants, Children, and Young Adults” contains consensus-driven information of high value to pediatricians (http://tinyurl.com/3o6papp
Importantly, keep in mind that many children old enough to participate in vision testing often perform poorly on initial testing.
There is a large learning curve, and a child who performs poorly the first time will often do very well on the second test. So it's a good idea to retest before referring a child to a specialist for poor vision.
If the child is cooperative, you can retest during the same visit and save the patient a return trip to your office. If the child fails visual acuity testing twice, that is when I would refer to an eye specialist.
Pediatricians are essentially looking for reduced vision, misalignment of the eye, and any anatomic abnormalities of the eye.
More specifically, you are screening for amblyopia, which can occur in up to 4% of the population; strabismus or eye misalignment; and anatomic concerns including ptosis, abnormal size of the eye, or a white pupil that suggests a cataract or a retinoblastoma.
A positive finding on almost every aspect of screening indicates need for referral of the patient to a specialist.
In contrast, acute abnormalities such as redness of the eye, minor injuries, and allergic conjunctivitis can be managed well in your office.
Keep in mind that failure to respond to initial treatment is an indication for referral. If you treat a child for red eye, for example, and the eye does not improve quickly, referral is warranted. Importantly, it is not usually an indication to change their antibiotic drop or switch them to another treatment. Although most of the time a simple problem may be the culprit, a red eye also can signal a more serious condition.
Referral of any child who screens positive for an eye concern or fails to initially respond to treatment generally requires no additional evaluation in the primary care setting. Just send the child along with a note explaining your concern and outlining any special circumstances that might not emerge on routine history taking with the parents. We'll take it from there.
In addition to the AAP guidelines, I recommend two mnemonics to assist primary care physicians during eye evaluation. A stretched version of MVP, the MVPea mnemonic can guide assessment and ensure that a quick examination is complete:
▸ M stands for motility. Are the eyes straight and do they move normally?
▸ V is vision assessment.
▸ P is pupil assessment. Are the pupils equal, round, and reactive? Do you see an afferent defect (decreased pupillary response to light in the affected eye), or an abnormality such as a white pupil or pupillary asymmetry?
▸ e is external exam. Assess surrounding structures, including the eyelids and eyelashes, for any abnormality.
▸ a is for the anterior segment. Evaluate the cornea, the lens, and other structures.
These are the critical components of a pediatrician's regular eye examination. These steps should take you a very short amount of time.
If you have a particular interest in eye disorders and some practice and skill at ocular examinations, you can consider adding three supplementary components to your assessment. I call these the ophthalmic version of CPR for the pediatrician:
▸ C is for confrontational visual field testing.
▸ P is for pressure.
▸ R is for retina.
NHLBI Halts Niacin Study Early; No Added Reduction in CV Events
The National Heart, Lung, and Blood Institute has stopped the AIM-HIGH clinical trial 18 months earlier than planned because the addition of high-dose, extended-release niacin has not reduced the risk of cardiovascular events in patients with a history of cardiovascular disease who were taking a statin to lower LDL cholesterol.
At the time the trial was stopped, the yearly rate of cardiovascular events (fatal or nonfatal MI, strokes, hospitalization for acute coronary syndrome, or revascularization procedures) was 5.6% in the control arm and 5.8% in the niacin arm. The news comes from a telebriefing held by the NHLBI on May 26.
"Although high-dose niacin effectively raised the participants’ HDL cholesterol, it did not affect the overall rate of cardiovascular events," said Dr. Susan B. Shurin, acting director of NHLBI.
"The trial was stopped because the data showed that there was a less than 1 in 10,000 chance that the trial would ever show the expected benefit planned in the protocol on the primary outcome measure," added co–primary investigator Dr. Jeffrey Probstfield of the University of Washington, Seattle.
The investigators cautioned nonetheless that individuals who take niacin should not stop without consulting their physician. They also noted that the findings should not alter clinical practice, and should apply only to participants of the AIM-HIGH trial for now.
In late April 2011, the study’s data safety and monitoring board concluded that high-dose, extended-release niacin offered no benefits beyond statin therapy alone in reducing cardiovascular events. The board also noted a small, statistically significant but unexplained increase in ischemic stroke rates in the high-dose, extended-release niacin group.
During the 32-month follow-up period, there were 28 strokes (1.6%) among patients in the niacin group, compared with 12 strokes (0.7%) reported in the control group. In particular, roughly a third (32%) of the strokes in the niacin group occurred in participants who had discontinued the drug at least 2 months and up to 4 years earlier. This finding contributed to the NHLBI acting director’s decision to stop the trial early. Previous studies have not suggested that stroke is a potential complication of niacin. It’s unclear whether this trend seen in this study was due to chance or related to niacin administration or some other issue.
For the AIM-HIGH (Atherothrombosis Intervention in Metabolic Syndrome With Low HDL/High Triglycerides: Impact on Global Health Outcomes) trial, researchers enrolled 3,414 participants in the United States and Canada who had a history of cardiovascular disease and who were taking a statin drug to keep their LDL cholesterol levels low. Study participants also had low HDL cholesterol and high triglyceride levels, which meant that they were at significant risk of experiencing future cardiovascular events.
Niacin is known to raise HDL cholesterol and lower triglycerides. Study participants were randomly assigned to either high-dose extended-release niacin (Niaspan) in gradually increasing doses up to 2,000 mg/day (1,718 people) or a placebo treatment (1,696 people). All participants were prescribed simvastatin (Zocor), and 515 participants were given a second LDL cholesterol–lowering drug, ezetimibe (Zetia), in order to maintain LDL cholesterol levels in the target range of 40-80 mg/dL.
Importantly, this trial excluded individuals with acute coronary syndrome and recent MI or percutaneous coronary intervention. The investigators highlighted that the results of this trial apply only to patients in this specific population and can’t be extrapolated to other groups.
Earlier studies of niacin had shown more favorable results. However, the earlier studies were not designed specifically to evaluate the impact of raising HDL cholesterol on the risk of cardiovascular events while maintaining excellent LDL cholesterol control, as in the AIM-HIGH study. Several other trials testing this hypothesis – including a large international trial of high-dose, extended-release niacin – are still ongoing.
The niacin tested in the study is a proprietary formulation manufactured by Abbott Laboratories. The Food and Drug Administration regulates the use of high doses of niacin (more than 500 mg) by prescription for helping treat low HDL cholesterol and/or high triglycerides. At prescription-level doses, flushing can occur. The extended-release formulation of niacin that was tested in AIM-HIGH was intended to help reduce the likelihood of flushing.
All AIM-HIGH study participants have been informed of the results and will be followed for an additional 12-18 months. The study investigators will focus on completing data collection and analysis. The preliminary outcomes of the study are expected to be reported at scientific meetings in the fall of 2011.
The NHLBI funded the AIM-HIGH study with additional support from Abbott Laboratories. Abbott also provided Niaspan, and Merck Pharmaceuticals provided Zocor.
The National Heart, Lung, and Blood Institute has stopped the AIM-HIGH clinical trial 18 months earlier than planned because the addition of high-dose, extended-release niacin has not reduced the risk of cardiovascular events in patients with a history of cardiovascular disease who were taking a statin to lower LDL cholesterol.
At the time the trial was stopped, the yearly rate of cardiovascular events (fatal or nonfatal MI, strokes, hospitalization for acute coronary syndrome, or revascularization procedures) was 5.6% in the control arm and 5.8% in the niacin arm. The news comes from a telebriefing held by the NHLBI on May 26.
"Although high-dose niacin effectively raised the participants’ HDL cholesterol, it did not affect the overall rate of cardiovascular events," said Dr. Susan B. Shurin, acting director of NHLBI.
"The trial was stopped because the data showed that there was a less than 1 in 10,000 chance that the trial would ever show the expected benefit planned in the protocol on the primary outcome measure," added co–primary investigator Dr. Jeffrey Probstfield of the University of Washington, Seattle.
The investigators cautioned nonetheless that individuals who take niacin should not stop without consulting their physician. They also noted that the findings should not alter clinical practice, and should apply only to participants of the AIM-HIGH trial for now.
In late April 2011, the study’s data safety and monitoring board concluded that high-dose, extended-release niacin offered no benefits beyond statin therapy alone in reducing cardiovascular events. The board also noted a small, statistically significant but unexplained increase in ischemic stroke rates in the high-dose, extended-release niacin group.
During the 32-month follow-up period, there were 28 strokes (1.6%) among patients in the niacin group, compared with 12 strokes (0.7%) reported in the control group. In particular, roughly a third (32%) of the strokes in the niacin group occurred in participants who had discontinued the drug at least 2 months and up to 4 years earlier. This finding contributed to the NHLBI acting director’s decision to stop the trial early. Previous studies have not suggested that stroke is a potential complication of niacin. It’s unclear whether this trend seen in this study was due to chance or related to niacin administration or some other issue.
For the AIM-HIGH (Atherothrombosis Intervention in Metabolic Syndrome With Low HDL/High Triglycerides: Impact on Global Health Outcomes) trial, researchers enrolled 3,414 participants in the United States and Canada who had a history of cardiovascular disease and who were taking a statin drug to keep their LDL cholesterol levels low. Study participants also had low HDL cholesterol and high triglyceride levels, which meant that they were at significant risk of experiencing future cardiovascular events.
Niacin is known to raise HDL cholesterol and lower triglycerides. Study participants were randomly assigned to either high-dose extended-release niacin (Niaspan) in gradually increasing doses up to 2,000 mg/day (1,718 people) or a placebo treatment (1,696 people). All participants were prescribed simvastatin (Zocor), and 515 participants were given a second LDL cholesterol–lowering drug, ezetimibe (Zetia), in order to maintain LDL cholesterol levels in the target range of 40-80 mg/dL.
Importantly, this trial excluded individuals with acute coronary syndrome and recent MI or percutaneous coronary intervention. The investigators highlighted that the results of this trial apply only to patients in this specific population and can’t be extrapolated to other groups.
Earlier studies of niacin had shown more favorable results. However, the earlier studies were not designed specifically to evaluate the impact of raising HDL cholesterol on the risk of cardiovascular events while maintaining excellent LDL cholesterol control, as in the AIM-HIGH study. Several other trials testing this hypothesis – including a large international trial of high-dose, extended-release niacin – are still ongoing.
The niacin tested in the study is a proprietary formulation manufactured by Abbott Laboratories. The Food and Drug Administration regulates the use of high doses of niacin (more than 500 mg) by prescription for helping treat low HDL cholesterol and/or high triglycerides. At prescription-level doses, flushing can occur. The extended-release formulation of niacin that was tested in AIM-HIGH was intended to help reduce the likelihood of flushing.
All AIM-HIGH study participants have been informed of the results and will be followed for an additional 12-18 months. The study investigators will focus on completing data collection and analysis. The preliminary outcomes of the study are expected to be reported at scientific meetings in the fall of 2011.
The NHLBI funded the AIM-HIGH study with additional support from Abbott Laboratories. Abbott also provided Niaspan, and Merck Pharmaceuticals provided Zocor.
The National Heart, Lung, and Blood Institute has stopped the AIM-HIGH clinical trial 18 months earlier than planned because the addition of high-dose, extended-release niacin has not reduced the risk of cardiovascular events in patients with a history of cardiovascular disease who were taking a statin to lower LDL cholesterol.
At the time the trial was stopped, the yearly rate of cardiovascular events (fatal or nonfatal MI, strokes, hospitalization for acute coronary syndrome, or revascularization procedures) was 5.6% in the control arm and 5.8% in the niacin arm. The news comes from a telebriefing held by the NHLBI on May 26.
"Although high-dose niacin effectively raised the participants’ HDL cholesterol, it did not affect the overall rate of cardiovascular events," said Dr. Susan B. Shurin, acting director of NHLBI.
"The trial was stopped because the data showed that there was a less than 1 in 10,000 chance that the trial would ever show the expected benefit planned in the protocol on the primary outcome measure," added co–primary investigator Dr. Jeffrey Probstfield of the University of Washington, Seattle.
The investigators cautioned nonetheless that individuals who take niacin should not stop without consulting their physician. They also noted that the findings should not alter clinical practice, and should apply only to participants of the AIM-HIGH trial for now.
In late April 2011, the study’s data safety and monitoring board concluded that high-dose, extended-release niacin offered no benefits beyond statin therapy alone in reducing cardiovascular events. The board also noted a small, statistically significant but unexplained increase in ischemic stroke rates in the high-dose, extended-release niacin group.
During the 32-month follow-up period, there were 28 strokes (1.6%) among patients in the niacin group, compared with 12 strokes (0.7%) reported in the control group. In particular, roughly a third (32%) of the strokes in the niacin group occurred in participants who had discontinued the drug at least 2 months and up to 4 years earlier. This finding contributed to the NHLBI acting director’s decision to stop the trial early. Previous studies have not suggested that stroke is a potential complication of niacin. It’s unclear whether this trend seen in this study was due to chance or related to niacin administration or some other issue.
For the AIM-HIGH (Atherothrombosis Intervention in Metabolic Syndrome With Low HDL/High Triglycerides: Impact on Global Health Outcomes) trial, researchers enrolled 3,414 participants in the United States and Canada who had a history of cardiovascular disease and who were taking a statin drug to keep their LDL cholesterol levels low. Study participants also had low HDL cholesterol and high triglyceride levels, which meant that they were at significant risk of experiencing future cardiovascular events.
Niacin is known to raise HDL cholesterol and lower triglycerides. Study participants were randomly assigned to either high-dose extended-release niacin (Niaspan) in gradually increasing doses up to 2,000 mg/day (1,718 people) or a placebo treatment (1,696 people). All participants were prescribed simvastatin (Zocor), and 515 participants were given a second LDL cholesterol–lowering drug, ezetimibe (Zetia), in order to maintain LDL cholesterol levels in the target range of 40-80 mg/dL.
Importantly, this trial excluded individuals with acute coronary syndrome and recent MI or percutaneous coronary intervention. The investigators highlighted that the results of this trial apply only to patients in this specific population and can’t be extrapolated to other groups.
Earlier studies of niacin had shown more favorable results. However, the earlier studies were not designed specifically to evaluate the impact of raising HDL cholesterol on the risk of cardiovascular events while maintaining excellent LDL cholesterol control, as in the AIM-HIGH study. Several other trials testing this hypothesis – including a large international trial of high-dose, extended-release niacin – are still ongoing.
The niacin tested in the study is a proprietary formulation manufactured by Abbott Laboratories. The Food and Drug Administration regulates the use of high doses of niacin (more than 500 mg) by prescription for helping treat low HDL cholesterol and/or high triglycerides. At prescription-level doses, flushing can occur. The extended-release formulation of niacin that was tested in AIM-HIGH was intended to help reduce the likelihood of flushing.
All AIM-HIGH study participants have been informed of the results and will be followed for an additional 12-18 months. The study investigators will focus on completing data collection and analysis. The preliminary outcomes of the study are expected to be reported at scientific meetings in the fall of 2011.
The NHLBI funded the AIM-HIGH study with additional support from Abbott Laboratories. Abbott also provided Niaspan, and Merck Pharmaceuticals provided Zocor.
FROM NHLBI
Major Finding: High-dose, extended-release niacin did not reduce the risk of cardiovascular events in patients who were taking a statin to lower LDL cholesterol.
Data Source: The AIM-HIGH clinical trial, which included 3,414 participants from the U.S. and Canada.
Disclosures: The NHLBI funded the AIM-HIGH study with additional support from Abbott Laboratories. Abbott also provided Niaspan, and Merck Pharmaceuticals provided Zocor.
Teamwork Works
Quality-improvement (QI) initiatives should be viewed through the prism of systems change, not just as one-off checklists that show some uptick against core measures, says a researcher whose work was published in the Journal of Hospital Medicine this month.
Ted Speroff, PhD, professor at the VA Tennessee Valley Healthcare System's Center for Health Services Research, led a team of researchers who found that using a collaborative approach to preventing central-line-associated bloodstream infections (CLABSI) and ventilator-associated pneumonias (VAP) worked better than simply using toolkits.
The study, "Quality Improvement Projects Targeting Healthcare-Associated Infections: Comparing Virtual Collaborative and Toolkit Approaches," found that 83% of ICUs using the collaborative approach implemented all CLABSI interventions, versus 64% of those in the toolkit group (P=0.13). The study further reported that 86% of the "collaborative group" implemented the VAP bundle, compared with 64% of the "toolkit group" (P=0.06). There was no statistically significant difference in patient outcomes.
"The key point is that quality improvement has a cultural and psychological component to it," Dr. Speroff says. "It's not just a task force of a subcommittee that you set up to achieve one tactical objective."
The study refers repeatedly to continuous quality improvement (CQI), which Dr. Speroff says HM leaders are in position to spearhead as many hospitalists already are viewed as QI leaders in their institutions.
But reform can only happen if HM and other specialties buy into the concept, and administrators don’t view it in terms of purely a cost-benefit analysis.
"There has to be a will to go that that road or some sense of urgency," Dr. Speroff says.
Quality-improvement (QI) initiatives should be viewed through the prism of systems change, not just as one-off checklists that show some uptick against core measures, says a researcher whose work was published in the Journal of Hospital Medicine this month.
Ted Speroff, PhD, professor at the VA Tennessee Valley Healthcare System's Center for Health Services Research, led a team of researchers who found that using a collaborative approach to preventing central-line-associated bloodstream infections (CLABSI) and ventilator-associated pneumonias (VAP) worked better than simply using toolkits.
The study, "Quality Improvement Projects Targeting Healthcare-Associated Infections: Comparing Virtual Collaborative and Toolkit Approaches," found that 83% of ICUs using the collaborative approach implemented all CLABSI interventions, versus 64% of those in the toolkit group (P=0.13). The study further reported that 86% of the "collaborative group" implemented the VAP bundle, compared with 64% of the "toolkit group" (P=0.06). There was no statistically significant difference in patient outcomes.
"The key point is that quality improvement has a cultural and psychological component to it," Dr. Speroff says. "It's not just a task force of a subcommittee that you set up to achieve one tactical objective."
The study refers repeatedly to continuous quality improvement (CQI), which Dr. Speroff says HM leaders are in position to spearhead as many hospitalists already are viewed as QI leaders in their institutions.
But reform can only happen if HM and other specialties buy into the concept, and administrators don’t view it in terms of purely a cost-benefit analysis.
"There has to be a will to go that that road or some sense of urgency," Dr. Speroff says.
Quality-improvement (QI) initiatives should be viewed through the prism of systems change, not just as one-off checklists that show some uptick against core measures, says a researcher whose work was published in the Journal of Hospital Medicine this month.
Ted Speroff, PhD, professor at the VA Tennessee Valley Healthcare System's Center for Health Services Research, led a team of researchers who found that using a collaborative approach to preventing central-line-associated bloodstream infections (CLABSI) and ventilator-associated pneumonias (VAP) worked better than simply using toolkits.
The study, "Quality Improvement Projects Targeting Healthcare-Associated Infections: Comparing Virtual Collaborative and Toolkit Approaches," found that 83% of ICUs using the collaborative approach implemented all CLABSI interventions, versus 64% of those in the toolkit group (P=0.13). The study further reported that 86% of the "collaborative group" implemented the VAP bundle, compared with 64% of the "toolkit group" (P=0.06). There was no statistically significant difference in patient outcomes.
"The key point is that quality improvement has a cultural and psychological component to it," Dr. Speroff says. "It's not just a task force of a subcommittee that you set up to achieve one tactical objective."
The study refers repeatedly to continuous quality improvement (CQI), which Dr. Speroff says HM leaders are in position to spearhead as many hospitalists already are viewed as QI leaders in their institutions.
But reform can only happen if HM and other specialties buy into the concept, and administrators don’t view it in terms of purely a cost-benefit analysis.
"There has to be a will to go that that road or some sense of urgency," Dr. Speroff says.
Patient-Safety Professionals Form Society
The National Patient Safety Foundation (NPSF) recently announced the launch of the American Society of Professionals in Patient Safety (ASPPS), a multidisciplinary membership organization designed to elevate patient safety to the level of a unique healthcare discipline. The new group has set as an early priority the development of a cross-disciplinary certification program for patient-safety professionals by 2012.
NPSF was formed in 1997 following a meeting of major health organizations to discuss patient safety, explains president Diane Pinakiewicz, MBA, who also heads ASPPS.
"When we first started, issues of medical errors and patient safety were not as widely recognized," she says.
The translation of error-prevention strategies from other fields (e.g. aviation) into healthcare continues to grow, and today there are more tools and resources to help healthcare professionals. "But they often have a hard time prioritizing it," Pinakiewicz says. "The challenge is to identify where are the biggest levers for the field and the discipline of patient safety."
Increasingly, transitions of care are being recognized as critical to safe and effective patient flow, and NPSF included a focus on preventing readmissions in its promotion of Patient Safety Awareness Week, March 6-12.
More than 300 health professionals have joined ASPPS, about 15% of them physicians, including many chief medical officers and patient safety officers, Pinakiewicz says. Although it is not known how many of these physicians are working hospitalists, "from my perspective, the hospitalist is in an incredibly pivotal seat."
The National Patient Safety Foundation (NPSF) recently announced the launch of the American Society of Professionals in Patient Safety (ASPPS), a multidisciplinary membership organization designed to elevate patient safety to the level of a unique healthcare discipline. The new group has set as an early priority the development of a cross-disciplinary certification program for patient-safety professionals by 2012.
NPSF was formed in 1997 following a meeting of major health organizations to discuss patient safety, explains president Diane Pinakiewicz, MBA, who also heads ASPPS.
"When we first started, issues of medical errors and patient safety were not as widely recognized," she says.
The translation of error-prevention strategies from other fields (e.g. aviation) into healthcare continues to grow, and today there are more tools and resources to help healthcare professionals. "But they often have a hard time prioritizing it," Pinakiewicz says. "The challenge is to identify where are the biggest levers for the field and the discipline of patient safety."
Increasingly, transitions of care are being recognized as critical to safe and effective patient flow, and NPSF included a focus on preventing readmissions in its promotion of Patient Safety Awareness Week, March 6-12.
More than 300 health professionals have joined ASPPS, about 15% of them physicians, including many chief medical officers and patient safety officers, Pinakiewicz says. Although it is not known how many of these physicians are working hospitalists, "from my perspective, the hospitalist is in an incredibly pivotal seat."
The National Patient Safety Foundation (NPSF) recently announced the launch of the American Society of Professionals in Patient Safety (ASPPS), a multidisciplinary membership organization designed to elevate patient safety to the level of a unique healthcare discipline. The new group has set as an early priority the development of a cross-disciplinary certification program for patient-safety professionals by 2012.
NPSF was formed in 1997 following a meeting of major health organizations to discuss patient safety, explains president Diane Pinakiewicz, MBA, who also heads ASPPS.
"When we first started, issues of medical errors and patient safety were not as widely recognized," she says.
The translation of error-prevention strategies from other fields (e.g. aviation) into healthcare continues to grow, and today there are more tools and resources to help healthcare professionals. "But they often have a hard time prioritizing it," Pinakiewicz says. "The challenge is to identify where are the biggest levers for the field and the discipline of patient safety."
Increasingly, transitions of care are being recognized as critical to safe and effective patient flow, and NPSF included a focus on preventing readmissions in its promotion of Patient Safety Awareness Week, March 6-12.
More than 300 health professionals have joined ASPPS, about 15% of them physicians, including many chief medical officers and patient safety officers, Pinakiewicz says. Although it is not known how many of these physicians are working hospitalists, "from my perspective, the hospitalist is in an incredibly pivotal seat."

