User login
Audit and Feedback Urinary Catheter Duration
The ubiquitous urinary catheter is associated with 80% of hospital‐acquired urinary tract infections (UTIs)1estimated to number one million annuallyaccounting for 40% of all nosocomial infections.2, 3 The clinical consequences of these catheter‐associated urinary tract infections (CAUTIs) are substantial and include prolonged hospital stay, bacteremia, and death.4 Despite the known risks of CAUTIs, it is estimated that 25% of hospitalized patients receive urinary catheters and that inappropriate urinary catheter use is widespread.5 Among catheterized patients, catheter duration is the most important modifiable risk factor for CAUTI. The excess risk of any bacteriuria accrues at a rate of 5% per catheter‐day beyond the first 48 hours of catheterization.6, 7 The reduction of catheter‐days for a given patient is an important component of quality improvement efforts to reduce CAUTIs. Unfortunately, as of a 2005 survey, most hospitals do not systematically track urinary catheter insertions and removals.8
The above concerns are highlighted for surgical patients among whom indwelling urinary catheter use is particularly high. In a 2001 sample of Medicare beneficiaries, 85% of major surgical patients had perioperative urinary catheters.9 In this population, postoperative catheter duration exceeded 48 hours in nearly 50%, despite concern that the risks of infection offset the benefits of continued catheterization after 24 hours to 48 hours postoperatively.4, 7, 10 Patients with catheters greater than 2 postoperative days had a 21% increased likelihood of in‐hospital UTI, increased 30‐day mortality, and decreased odds of discharge to home.9
To address the risk of CAUTI associated with excess urinary catheter days, The Centers for Medicare and Medicaid Services' (CMS) Surgical Care Improvement Project (SCIP) added catheter removal on postoperative day 1 or 2 to its process measure set beginning in October 2009. SCIP is 1 of several high‐profile surgical quality improvement programs that employs performance audit and feedback of patient‐level process or outcome measures to address deficiencies in surgical care.11, 12 In addition, audit and feedback of CAUTI rates has been used to successfully reduce CAUTIs in medical‐surgical patients.13 The goal of our study was to audit patient‐level postoperative urinary catheter duration and measure the impact of its feedback to nursing staff on postoperative catheter duration, CAUTI rates, and nurse's attitudes about CAUTI prevention.
Methods
Study Setting
The study was conducted within the orthopedic and general surgery units at the University of Colorado Hospital (UCH) Anschutz Inpatient Pavilion (AIP) in Aurora, CO. The AIP is a 425‐bed tertiary care hospital which is the major teaching affiliate of the University of Colorado Medical School. The orthopedic surgery unit has 22 beds. The general surgery unit has 18 beds.
Study Population
All postoperative patients 18 years of age admitted to the general surgery unit and orthopedic surgery units who had perioperative placement of an indwelling urinary catheter were eligible for study inclusion. Exclusion criteria included: evidence of a chronic indwelling catheter or chronic intermittent catheterization, a urologic or gynecologic surgery. For patients undergoing more than one operation in the same hospitalization, only the final operation was included in the study. For patients who were recatheterized after initial catheter removal, only the first catheterization and removal were included in the study. The registered nurses (RNs) on the study floors (n = 29 orthopedic surgery and 31 general surgery nurses) were the targets of the audit and feedback intervention with education. The baseline period was September 1, 2007 through January 31, 2008 and, the follow‐up period was April 1 through July 31, 2008.
Measures
The primary study outcome was postoperative urinary catheter duration measured in 2 ways:
Postoperative catheter duration in days defined as: the date of surgery subtracted from the date of catheter removal.
Postoperative catheter duration performance measure defined as: the number of patients with catheter removal before postoperative day three divided by the number of study eligible patients.
Both measures were calculated for each of the surgical units using data from all eligible study patients on the unit during the study period. For patients who were recatheterized in the same hospitalization, only the days to the first removal were counted. If the catheter was removed on the day of surgery, the postoperative catheter duration was zero days.
Total device days were calculated as the sum of the postoperative catheter duration for every eligible patient for each unit. Total device days/hospital days was calculated as the total device days divided by the sum of the lengths of stay for every eligible patient for each unit.
Secondary Outcome
CAUTI was the secondary outcome. CAUTI was defined as a positive urine culture (105 organisms/cc of no more than 2 microorganisms) sent 3 or more days following admission and 7 days following catheter removal. The definition for CAUTI was based on that used by the National Healthcare Safety Network for infection control surveillance purposes at the time of the study and included both symptomatic CAUTI and asymptomatic bacteriuria. CAUTI was reported as the number of infections per 1000 catheter‐days for eligible patients on each surgical unit for the baseline and follow‐up data collection periods.
Additional Descriptive Variables
Descriptive variables included the patient's name, surgical procedure, surgeon, presence of a chronic indwelling catheter, date of admission to the floor, date of surgery, date of birth, and length of stay in days.
Data Collection
A professional research assistant (PRA) identified eligible patients on the 2 surgical units of interest and collected the number of postoperative urinary catheter days per patient using daily and weekly automated electronic queries of an EHR containing all nursing documentation on medical and surgical floors at UCH. These queries identified all patients on the floors of interest with urinary bladder elimination management documentation. Those with documented indwelling catheters were included in the study unless exclusion criteria were met. During the study period, the EHR was configured to provide the following documentation of urinary output management: date and location of catheter insertion, routine assessment of urinary output and devices, and date and time of catheter removal. At UCH, catheter insertion and removal are documented in the EHR for 93% and 88% of surgical patients, respectively. When documentation of catheter insertion was missing from the EMR, the operative note was reviewed electronically for documentation of insertion. If the operative note did not reference the insertion, it was presumed to have occurred perioperatively. Likewise, if there was no documentation of catheter removal, it was presumed to have occurred prior to documentation of urinary continence. The PRA abstracted additional information (surgical procedure and date) from the discharge abstracts and operative notes using a standardized data collection sheet.
Laboratory‐based surveillance was used to determine the incidence of CAUTIs in a manner similar to that employed by infection preventionists at UCH. The microbiology laboratory provided a monthly summary of all positive urine cultures for both study units. Positive culture results were cross‐referenced with the catheter removal dates for all eligible study patients.
Validation
Validation of catheter documentation in the EHR was carried out on each of the 2 surgical units for a 2‐week period at the outset of the data collection. During each day of the validation period, the PRA compared the EHR report with the charge nurse report on each floor. Any discrepancies regarding the presence or absence of the indwelling catheter were resolved by querying the patient's nurse directly. All patients with catheters during their inpatient stay were captured by EHR documentation. The daily EHR reports had a 91% agreement with a daily nursing query (reference standard) and a Kappa (percent agreement adjusted for chance agreement) of 0.77. Instances of disagreement were generally due to a lag in EHR documentation on the part of the nursing staff.
Audit and Feedback Intervention
An educational presentation was developed to cover the following topics: the definition and epidemiology of CAUTI, harms associated with CAUTI, risk factors for CAUTI, commonly accepted indications for indwelling catheters, and alternatives to catheters. In addition, the catheter duration performance measure was defined, followed by the feedback of unit‐specific performance from the baseline data collection period. The presentation was made by the principal investigator to nursing staff on each of the 2 surgical units on 3 occasions per unit with days and times selected so as to reach as many unit nurses as possible. At the conclusion of each session, nurses were asked to brainstorm barriers to evidence‐based management of indwelling catheters. Light refreshments and a hour continuing education credit were provided regardless of participation in the brainstorming session. Additionally, participants completed brief evaluations of the sessions.
Analyses
Descriptive data are reported as means and standard deviations for continuous variables and percentages for categorical variables. Outcome measures were calculated as defined above. For these comparisons, we used t‐tests for continuous variables and chi‐square tests for dichotomous variables. We used Cochran‐Mantel‐Haenszel to test for trend for categorical variables. Confidence intervals were calculated for the incidence rate differences based on Poisson approximations. Analyses were completed using SAS Statistical Software Version 9.2.
This study was approved by the Colorado Multiple Institutional Review Board. Waivers of Health Insurance Portability and Accountability Act (HIPAA) and informed consent were obtained for study patients. Nurses participating in the educational sessions provided informed consent.
Results
During the study period there were a total of 1657 surgeries on the 2 study units during the baseline and follow‐up periods. After exclusions for urologic or gynecologic surgery (271), no indwelling catheter for surgery (505), or first surgery of 2 or more during the hospitalization (31), there were 846 eligible surgeries (51%).
Table 1 describes the population for the baseline and follow‐up periods for orthopedic and general surgery patients. Within each unit, the surgical populations were comparable during the baseline and follow‐up periods with the exception that the mean length of stay for eligible general surgery patients was significantly shorter in the baseline period as compared to the follow‐up period (6.6 vs. 8.5 days, P = 0.02). Cases on the orthopedic surgery unit were predominantly knee, hip and spine surgeries (85.9%), while those on the general surgery unit were predominantly gut and other gastrointestinal (GI) procedures (80.3%).
| Characteristic | Orthopedic Surgery | General Surgery | |||||
|---|---|---|---|---|---|---|---|
| Baseline, n = 206 | Follow‐Up, n = 290 | P Value | Baseline, n = 167 | Follow‐Up, n = 183 | P Value | ||
| |||||||
| Age in years, mean (SD) | 58.3 (15.6) | 58.1 (14.7) | 0.87 | 53.8 (16.1) | 52.7 (15.7) | 0.54 | |
| Male gender (%) | 47.1 | 45.2 | 0.67 | 43.1 | 48.6 | 0.30 | |
| Length of stay in days, mean (SD) | 4.0 (3.5) | 3.7 (2.8) | 0.22 | 6.6 (5.5) | 8.5 (8.6) | 0.02 | |
| Type of surgery (%) | |||||||
| Knee | 24.8 | 27.9 | 0.91 | Gut | 54.5 | 50.3 | 0.09 |
| Hip | 37.4 | 35.5 | Other GI | 22.8 | 32.8 | ||
| Spine | 22.8 | 23.1 | Non‐GI | 22.8 | 16.9 | ||
| Other Ortho | 5.3 | 4.1 | |||||
| Non‐Ortho | 9.7 | 9.3 | |||||
The Intervention
The educational intervention and feedback was received by two‐thirds of registered nurses on each unit and was rated highly by participants. A total of 79% of nurses agreed or strongly agreed that the information provided was relevant to their daily practice and 42% strongly agreed that they would change their practice based on the presentation. Barriers to evidence‐based use of urinary catheters identified by surgical nurses on each unit are shown in Table 2. They included the following domains: communication, patient concerns, clinical concerns, equipment, policies and procedures, and skills. General surgery and orthopedic surgery nurses identified different concerns arising from the different patient populations and surgeries cared for on each unit.
| Domain | Orthopedic Surgery | General Surgery |
|---|---|---|
| ||
| Communication | Communication among teams | Occasional need to call MD for order |
| Patient Comfort | Discomfort first overnight postop without catheter; discomfort of straight cathethers | Discomfort and embarrassment associated with straight catheters; patient request for indwelling catheter |
| Clinical concerns | Removal POD 1 too soon | Need to monitor I/Os in patients with low output |
| Equipment | Portable ultrasound on a different floor | |
| Policies and Procedures | ||
| 1. Epidural anesthesia | Duration of epidural/delay post epidural removal | |
| 2. Straight catheters | Risk of infection | Risk of trauma; infection |
| 3. Management of Urinary Retention | No standardized protocol for urinary retention. | Need for traumatic reinsertion of catheter. |
| Skills | Perineal care; catheter care | Perineal care |
BaselineFollow‐Up Comparison
Table 3 describes the measures of urinary catheter use for each surgical population for both data collection periods. On both units, measures of catheter duration were improved following the education and feedback intervention. For the orthopedic unit, mean postoperative catheter duration was reduced from 1.7 to 1.4 days (P = 0.01) and the proportion of patients with catheter removal before day 3 was increased from 86% to 92% (P = 0.04). For the general surgery unit, mean postoperative catheter duration was reduced from 2.6 to 2.2 days (P = 0.01) and the proportion of patients with catheter removal before day 3 was increased from 56% to 63% (P = 0.14). When the general surgery measures were adjusted to account for the difference in length of stay between the 2 time periods, the odds of meeting the performance measure at follow‐up compared to baseline increased from unadjusted odds of 1.38 (P = 0.14) to adjusted odds of 1.69 (P = 0.02).
| Measure | Orthopedic Surgery | General Surgery | ||||
|---|---|---|---|---|---|---|
| Baseline, n = 206 | Follow‐Up, n = 290 | P Value | Baseline, n = 167 | Follow‐Up, n = 183 | P Value | |
| ||||||
| Postoperative catheter duration in days (mean, SD) | 1.70 (1.24) | 1.44 (0.85) | 0.01 | 2.64 (1.85) | 2.19 (1.40) | 0.01 |
| Postoperative catheter duration performance measure (%) | 86 | 92 | 0.04 | 56 | 63 | 0.14 |
| Total catheter days | 350 | 418 | 441 | 401 | ||
| Catheter days/1000 hospital days | 423 | 394 | ns* | 398 | 259 | s* |
| Catheter‐associated UTIs | 3 | 0 | 3 | 3 | ||
| Catheter‐associated UTI rate (infections/1000 device days) | 8.6 | 0 | ns* | 6.8 | 7.5 | ns* |
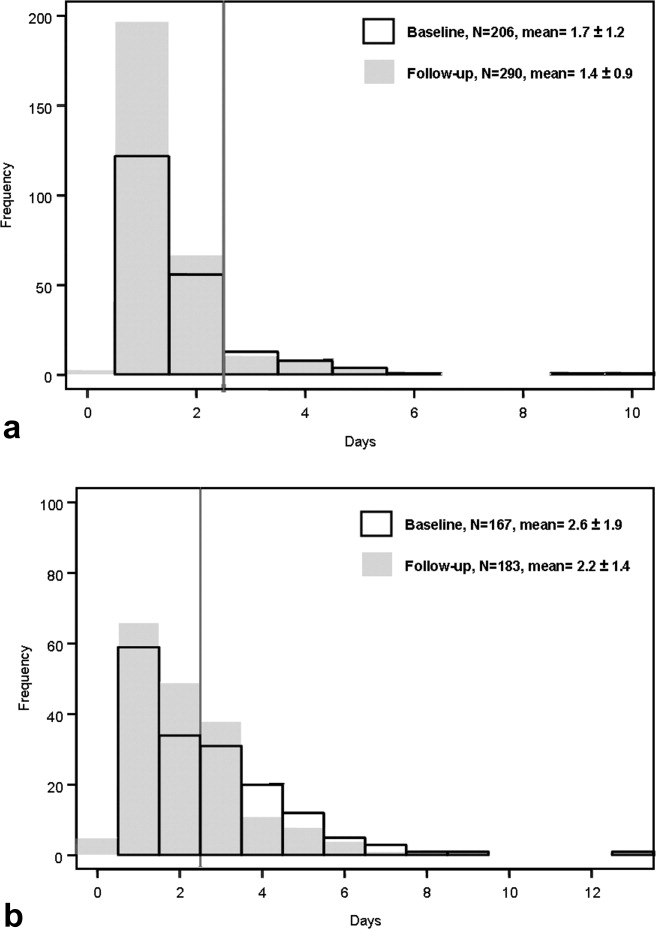
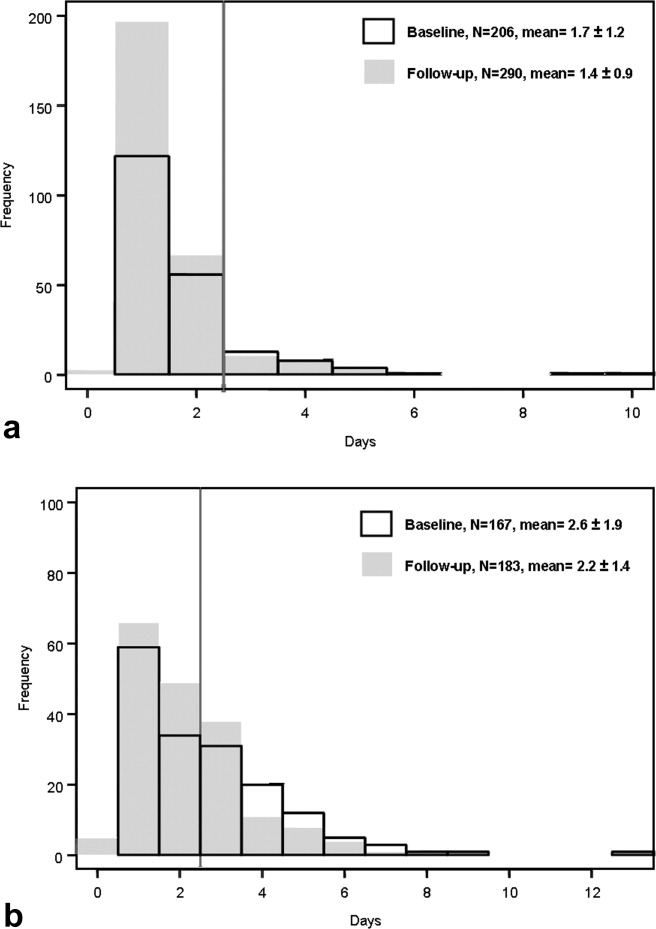
Figure 1a and b are histograms of the frequency of cases having a given postoperative catheter duration in days. The dark bars show the baseline distribution and the light bars show the follow‐up distribution. Although the number of patients in the follow‐up period is greater than for the baseline period for the orthopedic surgery cohort, the images are instructive. In both groups of patients, but most notably in the general surgery population, the reduction in catheter measures resulted from a left shift in the frequency distribution, both for the longer duration outliers (removing the tail of each plot) and for the shorter duration catheters (3 days), increasing the proportion of catheters removed on postoperative day 1.
CAUTIs
The CAUTI rate on the orthopedic surgery unit demonstrated a nonsignificant decline from 8.9 to 0 infections per 1000 device days, and on the general surgery unit the rate was constant at approximately 7 infections per 1000 device days.
Discussion
This preobservational and postobservational study found that audit and feedback of patient‐level postoperative urinary catheter duration delivered in the context of an educational intervention and brainstorming session was temporally associated with clinically meaningful reductions in urinary catheter duration. In so doing, we demonstrated the feasibility of collecting patient level urinary catheter duration, and delivering it in a manner that had utility for frontline staff. Our results are consistent with the quality improvement literature which demonstrates that audit and feedback is a successful quality improvement strategy in many contexts and may be as good as more complex interventions at increasing adherence to the performance of process measures for surgical infection prevention.14 Two large national programs, the VA National Surgical Quality Improvement Program (NSQIP) and SCIP, use audit and feedback as the backbone of their large‐scale quality improvement strategies with promising results.11, 12
Given that the 2 study units were so different in practice patterns regarding urinary catheter management and nursing‐identified barriers to evidence‐based care, this work suggests that urinary catheter management may pose unique challenges for different clinical areas and provides a caution that one‐size‐fits‐all interventions for the rationalization of urinary catheter management and reduction of CAUTIs may be of limited effectiveness in the absence of local tailoring. As such, audit and feedback is well‐suited to this purpose as more proscriptive quality improvement strategies may meet with a variety of implementation challenges.
The impact of our intervention on CAUTI rates was not significant. There are several possible explanations for this finding. First, the study was not powered to detect a difference in CAUTI rates given the low infection rate at our institution. However, we cannot exclude the possibility that a reduction in mean catheter duration of one‐third to one‐half of a day is insufficient to impact CAUTI rates in postoperative patients, particularly when many of the follow‐up patients still had postoperative catheter duration 2 daysthe timeframe beyond which bacterial colonization of the catheter begins. While both study units had similar increases in postoperative catheter duration, the UTI rate was only decreased in the orthopedic surgery group which had much higher rates of postoperative catheter duration 2 days at baseline.
In recent years, there has been a renewed focus on the eradication of hospital‐acquired infections prompted by intense interest from the public, federal and state legislators, and others.15, 16 The CMS has recently used the revamping of the Inpatient Prospective Payment System (IPPS) as an opportunity to align financial incentives so that reimbursements for claims with certain hospital‐acquired conditions, including CAUTIs, will be reduced to that of the reimbursement of the same claim without the presence of the complication.17 This move is just one of several strategies to motivate hospitals and clinicians to address the pervasive problem of hospital‐acquired infection.
As urinary catheters are intimately linked to hospital‐acquired UTI, a focus of reduction efforts on catheter use is appropriate. The National Quality Forum (NQF) endorsed a postoperative catheter duration quality measure which was incorporated by the CMS's SCIP in late 2009.18 As a result, every hospital in the country that performs surgery and participates in the Medicare program is now tasked with determining patient‐level urinary catheter duration for selected surgical patients. This move represents a departure from current recommendations from the Centers for Disease Control and Prevention (CDC) and its National Healthcare Safety Network19, 20 which endorse the measurement of a catheter utilization ratio (urinary catheter days/patient days) for patient care units, but does not endorse any patient‐level utilization measures. In this instance, the use of patient‐level data may be better suited to quality improvement interventions such as audit and feedback because of its clinical relevance to frontline providers. However, it may also increase the data collection burden on hospitals. Notably, the measurement of postoperative catheter duration in this study was semiautomated using queries of an EHR. Such an approach can significantly reduce the data collection burden for this process measure and is consistent with national initiatives to integrate EHRs with quality improvement initiatives going forward.21
Our study has several limitations. This study took place in the year following the announcement of a high profile Medicare rule change regarding payment for hospital‐acquired harms. Certainly, the uncontrolled prestudy and poststudy design does not allow for the assessment of the impact of our intervention independent of this context. We are unable, therefore, to attribute the observed reduction solely to the intervention. Additionally, we did not follow postoperative urinary catheter duration beyond the immediate follow‐up period. It is anticipated that the impact of an audit and feedback intervention may diminish over time without a mechanism for repeated feedback. Certainly the sustainability of such repeated feedback in a single institution would be improved with an appropriately configured EHR.
In addition, we have reliable data on catheter reinsertions only from the follow‐up period. While the rates of reinsertions we recorded (0.7% on orthopedic surgery and 2.7% on general surgery) were lower than expected based on the literature,22 we are unable to determine if our intervention led to increases in postoperative urinary retention.
This study was limited to 2 surgical units of a single academic medical center and therefore the urinary catheter utilization patterns may not be representative of other patient populations at other institutions. However, the urinary catheter patterns were comparable to those identified in our prior work in a national sample of Medicare patients undergoing elective surgery.9
Finally, the field of CAUTI prevention has evolved rapidly since this study was performed. In particular, the surveillance definition of CAUTI was altered twice by the CDC in December of 2008 and March of 2009. In addition, the Infectious Diseases Society of America issued a new definition of CAUTI in February of 2010.23 All of these changes highlight the difference between asymptomatic bateriuria (ASB) and symptomatic CAUTI. However, the surveillance definition in use at the time of this study did not make this distinction. Therefore, we are unable to comment on the relative occurrence of ASB versus symptomatic CAUTI under the new definitions.
Rational urinary catheter use is a central component of CAUTI prevention efforts.24 We describe the use of patient‐level urinary catheter use in an audit and feedback intervention to frontline staff that was associated with reductions in urinary catheter duration. To do so, we employed a methodology for tracking urinary catheter use patterns that can provide important data for infection preventionists and frontline providers in efforts to improve urinary output management. This promising approach merits further study as an adjunct to current efforts to rationalize urinary catheter utilization and reduce CAUTIs. In the current environment, having the right data is a powerful aide for ongoing performance improvement.
Acknowledgements
The authors acknowledge the contributions of Daniel Sandy, BA, MPN, Vivienne Smith, RN, UCH; and the insights of Michelle Barron, MD, Linda Burton, RN, Teri Hulett, RN, UCH, and Jean Kutner, MD, MSPH.
- ,,.Urinary tract etiology of bloodstream infections in hospitalized patients.J Infect Dis.1983;148:57–62.
- ,,, et al.Nosocomial infections in US hospitals, 1975–1976: estimated frequency by selected characteristics of patients.Am J Med.1981;70:947–959.
- ,,,,.The nationwide nosocomial infection rate. a new need for vital statistics.Am J Epidemiol.1985;121:159–167.
- .Clinical and economic consequences of nosocomial catheter‐related bacteriuria.Am J Infect Control.2000;28:68–75.
- ,,,.Overuse of the indwelling urinary tract catheter in hospitalized medical patients.Arch Intern Med.1995;155:1425–1429.
- .Catheter‐associated bacteriuria.Urol Clin North Am.1986;13:735.
- .Guidelines for prevention of catheter‐associated urinary tract infections.Ann Intern Med.1975;82:386.
- ,,, et al.Preventing hospital‐acquired urinary tract infection in the united states: a national study.Clin Infect Dis.2008;46:243–250.
- ,,,.Indwelling urinary catheter use in the postoperative period: analysis of the national surgical infection prevention project data.Arch Surg.2008;143:551–557.
- ,,,,.Management of urinary retention after surgical repair of hip fracture.Can Med Assoc J.1992;146:1185–1188.
- ,,.The comparative assessment and improvement of quality of surgical care in the department of veterans affairs.Arch Surg.2002;137:20–27.
- .The surgical infection prevention and surgical care improvement projects: promises and pitfalls.Am Surg.2006;72:1010–1016.
- ,,,.Feedback to nursing staff as an intervention to reduce catheter‐associated urinary tract infections.Am J Infect Control.1999;27:402–424.
- ,,, et al.The effect of a quality improvement collaborative to improve antimicrobial prophylaxis in surgical patients.Ann Intern Med.2008;149:480.
- ,.Nonpayment for harms resulting from medical care: catheter‐associated urinary tract infections.JAMA.2007;289:2782–2784.
- Kaiser Family Foundation. Hospital‐based infections reporting requirements,2008. Available at: www.Kaiser Family Foundation State Health Facts.org. Accessed August 25, 2010.
- Centers for Medicare and Medicaid Services, Department of Health and Human Services.Medicare Program: Changes to the Hospital Inpatient Prospective Payment Systems and Fiscal Year 2008 Rates. CMS‐1390‐F. 8–1‐2007.
- Centers for Medicare and Medicaid Services, Department of Health and Human Services and The Joint Commission.Specifications Manual for National Hospital Inpatient Quality Measures, Discharges 10/1/09 (4Q09) through 3/31/10 (1Q10). 5–4‐2009.
- ,,,,, andthe Healthcare Infection Control Practices Advisory Committee.Guideline for the Prevention of Catheter‐associated Urinary Tract Infections,2009. Centers for Disease Control and Prevention. 1–22‐2010.
- Centers for Disease Control and Prevention.NHSN Patient Safety Component Key Terms. 1–22‐2010.
- .Stimulating the adoption of health information technology.N Engl J Med.2010;360:1477–1479.
- ,.Management of postoperative urinary retention: a randomized trial of in‐out versus overnight catheterization.ANZ J Surg.2004;2004:658–661.
- ,,, et al.Diagnosis, prevention, and treatment of catheter‐associated urinary tract infection in adults: 2009 International Clinical Practice Guidelines from the Infectious Diseases Society of America.Clin Infect Dis.2010;50:625–663.
- ,,, et al.Strategies to prevent catheter‐associated urinary tract infections in acute care hospitals.Infect Control Hosp Epidemiol.2008;29:S41–S50.
The ubiquitous urinary catheter is associated with 80% of hospital‐acquired urinary tract infections (UTIs)1estimated to number one million annuallyaccounting for 40% of all nosocomial infections.2, 3 The clinical consequences of these catheter‐associated urinary tract infections (CAUTIs) are substantial and include prolonged hospital stay, bacteremia, and death.4 Despite the known risks of CAUTIs, it is estimated that 25% of hospitalized patients receive urinary catheters and that inappropriate urinary catheter use is widespread.5 Among catheterized patients, catheter duration is the most important modifiable risk factor for CAUTI. The excess risk of any bacteriuria accrues at a rate of 5% per catheter‐day beyond the first 48 hours of catheterization.6, 7 The reduction of catheter‐days for a given patient is an important component of quality improvement efforts to reduce CAUTIs. Unfortunately, as of a 2005 survey, most hospitals do not systematically track urinary catheter insertions and removals.8
The above concerns are highlighted for surgical patients among whom indwelling urinary catheter use is particularly high. In a 2001 sample of Medicare beneficiaries, 85% of major surgical patients had perioperative urinary catheters.9 In this population, postoperative catheter duration exceeded 48 hours in nearly 50%, despite concern that the risks of infection offset the benefits of continued catheterization after 24 hours to 48 hours postoperatively.4, 7, 10 Patients with catheters greater than 2 postoperative days had a 21% increased likelihood of in‐hospital UTI, increased 30‐day mortality, and decreased odds of discharge to home.9
To address the risk of CAUTI associated with excess urinary catheter days, The Centers for Medicare and Medicaid Services' (CMS) Surgical Care Improvement Project (SCIP) added catheter removal on postoperative day 1 or 2 to its process measure set beginning in October 2009. SCIP is 1 of several high‐profile surgical quality improvement programs that employs performance audit and feedback of patient‐level process or outcome measures to address deficiencies in surgical care.11, 12 In addition, audit and feedback of CAUTI rates has been used to successfully reduce CAUTIs in medical‐surgical patients.13 The goal of our study was to audit patient‐level postoperative urinary catheter duration and measure the impact of its feedback to nursing staff on postoperative catheter duration, CAUTI rates, and nurse's attitudes about CAUTI prevention.
Methods
Study Setting
The study was conducted within the orthopedic and general surgery units at the University of Colorado Hospital (UCH) Anschutz Inpatient Pavilion (AIP) in Aurora, CO. The AIP is a 425‐bed tertiary care hospital which is the major teaching affiliate of the University of Colorado Medical School. The orthopedic surgery unit has 22 beds. The general surgery unit has 18 beds.
Study Population
All postoperative patients 18 years of age admitted to the general surgery unit and orthopedic surgery units who had perioperative placement of an indwelling urinary catheter were eligible for study inclusion. Exclusion criteria included: evidence of a chronic indwelling catheter or chronic intermittent catheterization, a urologic or gynecologic surgery. For patients undergoing more than one operation in the same hospitalization, only the final operation was included in the study. For patients who were recatheterized after initial catheter removal, only the first catheterization and removal were included in the study. The registered nurses (RNs) on the study floors (n = 29 orthopedic surgery and 31 general surgery nurses) were the targets of the audit and feedback intervention with education. The baseline period was September 1, 2007 through January 31, 2008 and, the follow‐up period was April 1 through July 31, 2008.
Measures
The primary study outcome was postoperative urinary catheter duration measured in 2 ways:
Postoperative catheter duration in days defined as: the date of surgery subtracted from the date of catheter removal.
Postoperative catheter duration performance measure defined as: the number of patients with catheter removal before postoperative day three divided by the number of study eligible patients.
Both measures were calculated for each of the surgical units using data from all eligible study patients on the unit during the study period. For patients who were recatheterized in the same hospitalization, only the days to the first removal were counted. If the catheter was removed on the day of surgery, the postoperative catheter duration was zero days.
Total device days were calculated as the sum of the postoperative catheter duration for every eligible patient for each unit. Total device days/hospital days was calculated as the total device days divided by the sum of the lengths of stay for every eligible patient for each unit.
Secondary Outcome
CAUTI was the secondary outcome. CAUTI was defined as a positive urine culture (105 organisms/cc of no more than 2 microorganisms) sent 3 or more days following admission and 7 days following catheter removal. The definition for CAUTI was based on that used by the National Healthcare Safety Network for infection control surveillance purposes at the time of the study and included both symptomatic CAUTI and asymptomatic bacteriuria. CAUTI was reported as the number of infections per 1000 catheter‐days for eligible patients on each surgical unit for the baseline and follow‐up data collection periods.
Additional Descriptive Variables
Descriptive variables included the patient's name, surgical procedure, surgeon, presence of a chronic indwelling catheter, date of admission to the floor, date of surgery, date of birth, and length of stay in days.
Data Collection
A professional research assistant (PRA) identified eligible patients on the 2 surgical units of interest and collected the number of postoperative urinary catheter days per patient using daily and weekly automated electronic queries of an EHR containing all nursing documentation on medical and surgical floors at UCH. These queries identified all patients on the floors of interest with urinary bladder elimination management documentation. Those with documented indwelling catheters were included in the study unless exclusion criteria were met. During the study period, the EHR was configured to provide the following documentation of urinary output management: date and location of catheter insertion, routine assessment of urinary output and devices, and date and time of catheter removal. At UCH, catheter insertion and removal are documented in the EHR for 93% and 88% of surgical patients, respectively. When documentation of catheter insertion was missing from the EMR, the operative note was reviewed electronically for documentation of insertion. If the operative note did not reference the insertion, it was presumed to have occurred perioperatively. Likewise, if there was no documentation of catheter removal, it was presumed to have occurred prior to documentation of urinary continence. The PRA abstracted additional information (surgical procedure and date) from the discharge abstracts and operative notes using a standardized data collection sheet.
Laboratory‐based surveillance was used to determine the incidence of CAUTIs in a manner similar to that employed by infection preventionists at UCH. The microbiology laboratory provided a monthly summary of all positive urine cultures for both study units. Positive culture results were cross‐referenced with the catheter removal dates for all eligible study patients.
Validation
Validation of catheter documentation in the EHR was carried out on each of the 2 surgical units for a 2‐week period at the outset of the data collection. During each day of the validation period, the PRA compared the EHR report with the charge nurse report on each floor. Any discrepancies regarding the presence or absence of the indwelling catheter were resolved by querying the patient's nurse directly. All patients with catheters during their inpatient stay were captured by EHR documentation. The daily EHR reports had a 91% agreement with a daily nursing query (reference standard) and a Kappa (percent agreement adjusted for chance agreement) of 0.77. Instances of disagreement were generally due to a lag in EHR documentation on the part of the nursing staff.
Audit and Feedback Intervention
An educational presentation was developed to cover the following topics: the definition and epidemiology of CAUTI, harms associated with CAUTI, risk factors for CAUTI, commonly accepted indications for indwelling catheters, and alternatives to catheters. In addition, the catheter duration performance measure was defined, followed by the feedback of unit‐specific performance from the baseline data collection period. The presentation was made by the principal investigator to nursing staff on each of the 2 surgical units on 3 occasions per unit with days and times selected so as to reach as many unit nurses as possible. At the conclusion of each session, nurses were asked to brainstorm barriers to evidence‐based management of indwelling catheters. Light refreshments and a hour continuing education credit were provided regardless of participation in the brainstorming session. Additionally, participants completed brief evaluations of the sessions.
Analyses
Descriptive data are reported as means and standard deviations for continuous variables and percentages for categorical variables. Outcome measures were calculated as defined above. For these comparisons, we used t‐tests for continuous variables and chi‐square tests for dichotomous variables. We used Cochran‐Mantel‐Haenszel to test for trend for categorical variables. Confidence intervals were calculated for the incidence rate differences based on Poisson approximations. Analyses were completed using SAS Statistical Software Version 9.2.
This study was approved by the Colorado Multiple Institutional Review Board. Waivers of Health Insurance Portability and Accountability Act (HIPAA) and informed consent were obtained for study patients. Nurses participating in the educational sessions provided informed consent.
Results
During the study period there were a total of 1657 surgeries on the 2 study units during the baseline and follow‐up periods. After exclusions for urologic or gynecologic surgery (271), no indwelling catheter for surgery (505), or first surgery of 2 or more during the hospitalization (31), there were 846 eligible surgeries (51%).
Table 1 describes the population for the baseline and follow‐up periods for orthopedic and general surgery patients. Within each unit, the surgical populations were comparable during the baseline and follow‐up periods with the exception that the mean length of stay for eligible general surgery patients was significantly shorter in the baseline period as compared to the follow‐up period (6.6 vs. 8.5 days, P = 0.02). Cases on the orthopedic surgery unit were predominantly knee, hip and spine surgeries (85.9%), while those on the general surgery unit were predominantly gut and other gastrointestinal (GI) procedures (80.3%).
| Characteristic | Orthopedic Surgery | General Surgery | |||||
|---|---|---|---|---|---|---|---|
| Baseline, n = 206 | Follow‐Up, n = 290 | P Value | Baseline, n = 167 | Follow‐Up, n = 183 | P Value | ||
| |||||||
| Age in years, mean (SD) | 58.3 (15.6) | 58.1 (14.7) | 0.87 | 53.8 (16.1) | 52.7 (15.7) | 0.54 | |
| Male gender (%) | 47.1 | 45.2 | 0.67 | 43.1 | 48.6 | 0.30 | |
| Length of stay in days, mean (SD) | 4.0 (3.5) | 3.7 (2.8) | 0.22 | 6.6 (5.5) | 8.5 (8.6) | 0.02 | |
| Type of surgery (%) | |||||||
| Knee | 24.8 | 27.9 | 0.91 | Gut | 54.5 | 50.3 | 0.09 |
| Hip | 37.4 | 35.5 | Other GI | 22.8 | 32.8 | ||
| Spine | 22.8 | 23.1 | Non‐GI | 22.8 | 16.9 | ||
| Other Ortho | 5.3 | 4.1 | |||||
| Non‐Ortho | 9.7 | 9.3 | |||||
The Intervention
The educational intervention and feedback was received by two‐thirds of registered nurses on each unit and was rated highly by participants. A total of 79% of nurses agreed or strongly agreed that the information provided was relevant to their daily practice and 42% strongly agreed that they would change their practice based on the presentation. Barriers to evidence‐based use of urinary catheters identified by surgical nurses on each unit are shown in Table 2. They included the following domains: communication, patient concerns, clinical concerns, equipment, policies and procedures, and skills. General surgery and orthopedic surgery nurses identified different concerns arising from the different patient populations and surgeries cared for on each unit.
| Domain | Orthopedic Surgery | General Surgery |
|---|---|---|
| ||
| Communication | Communication among teams | Occasional need to call MD for order |
| Patient Comfort | Discomfort first overnight postop without catheter; discomfort of straight cathethers | Discomfort and embarrassment associated with straight catheters; patient request for indwelling catheter |
| Clinical concerns | Removal POD 1 too soon | Need to monitor I/Os in patients with low output |
| Equipment | Portable ultrasound on a different floor | |
| Policies and Procedures | ||
| 1. Epidural anesthesia | Duration of epidural/delay post epidural removal | |
| 2. Straight catheters | Risk of infection | Risk of trauma; infection |
| 3. Management of Urinary Retention | No standardized protocol for urinary retention. | Need for traumatic reinsertion of catheter. |
| Skills | Perineal care; catheter care | Perineal care |
BaselineFollow‐Up Comparison
Table 3 describes the measures of urinary catheter use for each surgical population for both data collection periods. On both units, measures of catheter duration were improved following the education and feedback intervention. For the orthopedic unit, mean postoperative catheter duration was reduced from 1.7 to 1.4 days (P = 0.01) and the proportion of patients with catheter removal before day 3 was increased from 86% to 92% (P = 0.04). For the general surgery unit, mean postoperative catheter duration was reduced from 2.6 to 2.2 days (P = 0.01) and the proportion of patients with catheter removal before day 3 was increased from 56% to 63% (P = 0.14). When the general surgery measures were adjusted to account for the difference in length of stay between the 2 time periods, the odds of meeting the performance measure at follow‐up compared to baseline increased from unadjusted odds of 1.38 (P = 0.14) to adjusted odds of 1.69 (P = 0.02).
| Measure | Orthopedic Surgery | General Surgery | ||||
|---|---|---|---|---|---|---|
| Baseline, n = 206 | Follow‐Up, n = 290 | P Value | Baseline, n = 167 | Follow‐Up, n = 183 | P Value | |
| ||||||
| Postoperative catheter duration in days (mean, SD) | 1.70 (1.24) | 1.44 (0.85) | 0.01 | 2.64 (1.85) | 2.19 (1.40) | 0.01 |
| Postoperative catheter duration performance measure (%) | 86 | 92 | 0.04 | 56 | 63 | 0.14 |
| Total catheter days | 350 | 418 | 441 | 401 | ||
| Catheter days/1000 hospital days | 423 | 394 | ns* | 398 | 259 | s* |
| Catheter‐associated UTIs | 3 | 0 | 3 | 3 | ||
| Catheter‐associated UTI rate (infections/1000 device days) | 8.6 | 0 | ns* | 6.8 | 7.5 | ns* |
Figure 1a and b are histograms of the frequency of cases having a given postoperative catheter duration in days. The dark bars show the baseline distribution and the light bars show the follow‐up distribution. Although the number of patients in the follow‐up period is greater than for the baseline period for the orthopedic surgery cohort, the images are instructive. In both groups of patients, but most notably in the general surgery population, the reduction in catheter measures resulted from a left shift in the frequency distribution, both for the longer duration outliers (removing the tail of each plot) and for the shorter duration catheters (3 days), increasing the proportion of catheters removed on postoperative day 1.

CAUTIs
The CAUTI rate on the orthopedic surgery unit demonstrated a nonsignificant decline from 8.9 to 0 infections per 1000 device days, and on the general surgery unit the rate was constant at approximately 7 infections per 1000 device days.
Discussion
This preobservational and postobservational study found that audit and feedback of patient‐level postoperative urinary catheter duration delivered in the context of an educational intervention and brainstorming session was temporally associated with clinically meaningful reductions in urinary catheter duration. In so doing, we demonstrated the feasibility of collecting patient level urinary catheter duration, and delivering it in a manner that had utility for frontline staff. Our results are consistent with the quality improvement literature which demonstrates that audit and feedback is a successful quality improvement strategy in many contexts and may be as good as more complex interventions at increasing adherence to the performance of process measures for surgical infection prevention.14 Two large national programs, the VA National Surgical Quality Improvement Program (NSQIP) and SCIP, use audit and feedback as the backbone of their large‐scale quality improvement strategies with promising results.11, 12
Given that the 2 study units were so different in practice patterns regarding urinary catheter management and nursing‐identified barriers to evidence‐based care, this work suggests that urinary catheter management may pose unique challenges for different clinical areas and provides a caution that one‐size‐fits‐all interventions for the rationalization of urinary catheter management and reduction of CAUTIs may be of limited effectiveness in the absence of local tailoring. As such, audit and feedback is well‐suited to this purpose as more proscriptive quality improvement strategies may meet with a variety of implementation challenges.
The impact of our intervention on CAUTI rates was not significant. There are several possible explanations for this finding. First, the study was not powered to detect a difference in CAUTI rates given the low infection rate at our institution. However, we cannot exclude the possibility that a reduction in mean catheter duration of one‐third to one‐half of a day is insufficient to impact CAUTI rates in postoperative patients, particularly when many of the follow‐up patients still had postoperative catheter duration 2 daysthe timeframe beyond which bacterial colonization of the catheter begins. While both study units had similar increases in postoperative catheter duration, the UTI rate was only decreased in the orthopedic surgery group which had much higher rates of postoperative catheter duration 2 days at baseline.
In recent years, there has been a renewed focus on the eradication of hospital‐acquired infections prompted by intense interest from the public, federal and state legislators, and others.15, 16 The CMS has recently used the revamping of the Inpatient Prospective Payment System (IPPS) as an opportunity to align financial incentives so that reimbursements for claims with certain hospital‐acquired conditions, including CAUTIs, will be reduced to that of the reimbursement of the same claim without the presence of the complication.17 This move is just one of several strategies to motivate hospitals and clinicians to address the pervasive problem of hospital‐acquired infection.
As urinary catheters are intimately linked to hospital‐acquired UTI, a focus of reduction efforts on catheter use is appropriate. The National Quality Forum (NQF) endorsed a postoperative catheter duration quality measure which was incorporated by the CMS's SCIP in late 2009.18 As a result, every hospital in the country that performs surgery and participates in the Medicare program is now tasked with determining patient‐level urinary catheter duration for selected surgical patients. This move represents a departure from current recommendations from the Centers for Disease Control and Prevention (CDC) and its National Healthcare Safety Network19, 20 which endorse the measurement of a catheter utilization ratio (urinary catheter days/patient days) for patient care units, but does not endorse any patient‐level utilization measures. In this instance, the use of patient‐level data may be better suited to quality improvement interventions such as audit and feedback because of its clinical relevance to frontline providers. However, it may also increase the data collection burden on hospitals. Notably, the measurement of postoperative catheter duration in this study was semiautomated using queries of an EHR. Such an approach can significantly reduce the data collection burden for this process measure and is consistent with national initiatives to integrate EHRs with quality improvement initiatives going forward.21
Our study has several limitations. This study took place in the year following the announcement of a high profile Medicare rule change regarding payment for hospital‐acquired harms. Certainly, the uncontrolled prestudy and poststudy design does not allow for the assessment of the impact of our intervention independent of this context. We are unable, therefore, to attribute the observed reduction solely to the intervention. Additionally, we did not follow postoperative urinary catheter duration beyond the immediate follow‐up period. It is anticipated that the impact of an audit and feedback intervention may diminish over time without a mechanism for repeated feedback. Certainly the sustainability of such repeated feedback in a single institution would be improved with an appropriately configured EHR.
In addition, we have reliable data on catheter reinsertions only from the follow‐up period. While the rates of reinsertions we recorded (0.7% on orthopedic surgery and 2.7% on general surgery) were lower than expected based on the literature,22 we are unable to determine if our intervention led to increases in postoperative urinary retention.
This study was limited to 2 surgical units of a single academic medical center and therefore the urinary catheter utilization patterns may not be representative of other patient populations at other institutions. However, the urinary catheter patterns were comparable to those identified in our prior work in a national sample of Medicare patients undergoing elective surgery.9
Finally, the field of CAUTI prevention has evolved rapidly since this study was performed. In particular, the surveillance definition of CAUTI was altered twice by the CDC in December of 2008 and March of 2009. In addition, the Infectious Diseases Society of America issued a new definition of CAUTI in February of 2010.23 All of these changes highlight the difference between asymptomatic bateriuria (ASB) and symptomatic CAUTI. However, the surveillance definition in use at the time of this study did not make this distinction. Therefore, we are unable to comment on the relative occurrence of ASB versus symptomatic CAUTI under the new definitions.
Rational urinary catheter use is a central component of CAUTI prevention efforts.24 We describe the use of patient‐level urinary catheter use in an audit and feedback intervention to frontline staff that was associated with reductions in urinary catheter duration. To do so, we employed a methodology for tracking urinary catheter use patterns that can provide important data for infection preventionists and frontline providers in efforts to improve urinary output management. This promising approach merits further study as an adjunct to current efforts to rationalize urinary catheter utilization and reduce CAUTIs. In the current environment, having the right data is a powerful aide for ongoing performance improvement.
Acknowledgements
The authors acknowledge the contributions of Daniel Sandy, BA, MPN, Vivienne Smith, RN, UCH; and the insights of Michelle Barron, MD, Linda Burton, RN, Teri Hulett, RN, UCH, and Jean Kutner, MD, MSPH.
The ubiquitous urinary catheter is associated with 80% of hospital‐acquired urinary tract infections (UTIs)1estimated to number one million annuallyaccounting for 40% of all nosocomial infections.2, 3 The clinical consequences of these catheter‐associated urinary tract infections (CAUTIs) are substantial and include prolonged hospital stay, bacteremia, and death.4 Despite the known risks of CAUTIs, it is estimated that 25% of hospitalized patients receive urinary catheters and that inappropriate urinary catheter use is widespread.5 Among catheterized patients, catheter duration is the most important modifiable risk factor for CAUTI. The excess risk of any bacteriuria accrues at a rate of 5% per catheter‐day beyond the first 48 hours of catheterization.6, 7 The reduction of catheter‐days for a given patient is an important component of quality improvement efforts to reduce CAUTIs. Unfortunately, as of a 2005 survey, most hospitals do not systematically track urinary catheter insertions and removals.8
The above concerns are highlighted for surgical patients among whom indwelling urinary catheter use is particularly high. In a 2001 sample of Medicare beneficiaries, 85% of major surgical patients had perioperative urinary catheters.9 In this population, postoperative catheter duration exceeded 48 hours in nearly 50%, despite concern that the risks of infection offset the benefits of continued catheterization after 24 hours to 48 hours postoperatively.4, 7, 10 Patients with catheters greater than 2 postoperative days had a 21% increased likelihood of in‐hospital UTI, increased 30‐day mortality, and decreased odds of discharge to home.9
To address the risk of CAUTI associated with excess urinary catheter days, The Centers for Medicare and Medicaid Services' (CMS) Surgical Care Improvement Project (SCIP) added catheter removal on postoperative day 1 or 2 to its process measure set beginning in October 2009. SCIP is 1 of several high‐profile surgical quality improvement programs that employs performance audit and feedback of patient‐level process or outcome measures to address deficiencies in surgical care.11, 12 In addition, audit and feedback of CAUTI rates has been used to successfully reduce CAUTIs in medical‐surgical patients.13 The goal of our study was to audit patient‐level postoperative urinary catheter duration and measure the impact of its feedback to nursing staff on postoperative catheter duration, CAUTI rates, and nurse's attitudes about CAUTI prevention.
Methods
Study Setting
The study was conducted within the orthopedic and general surgery units at the University of Colorado Hospital (UCH) Anschutz Inpatient Pavilion (AIP) in Aurora, CO. The AIP is a 425‐bed tertiary care hospital which is the major teaching affiliate of the University of Colorado Medical School. The orthopedic surgery unit has 22 beds. The general surgery unit has 18 beds.
Study Population
All postoperative patients 18 years of age admitted to the general surgery unit and orthopedic surgery units who had perioperative placement of an indwelling urinary catheter were eligible for study inclusion. Exclusion criteria included: evidence of a chronic indwelling catheter or chronic intermittent catheterization, a urologic or gynecologic surgery. For patients undergoing more than one operation in the same hospitalization, only the final operation was included in the study. For patients who were recatheterized after initial catheter removal, only the first catheterization and removal were included in the study. The registered nurses (RNs) on the study floors (n = 29 orthopedic surgery and 31 general surgery nurses) were the targets of the audit and feedback intervention with education. The baseline period was September 1, 2007 through January 31, 2008 and, the follow‐up period was April 1 through July 31, 2008.
Measures
The primary study outcome was postoperative urinary catheter duration measured in 2 ways:
Postoperative catheter duration in days defined as: the date of surgery subtracted from the date of catheter removal.
Postoperative catheter duration performance measure defined as: the number of patients with catheter removal before postoperative day three divided by the number of study eligible patients.
Both measures were calculated for each of the surgical units using data from all eligible study patients on the unit during the study period. For patients who were recatheterized in the same hospitalization, only the days to the first removal were counted. If the catheter was removed on the day of surgery, the postoperative catheter duration was zero days.
Total device days were calculated as the sum of the postoperative catheter duration for every eligible patient for each unit. Total device days/hospital days was calculated as the total device days divided by the sum of the lengths of stay for every eligible patient for each unit.
Secondary Outcome
CAUTI was the secondary outcome. CAUTI was defined as a positive urine culture (105 organisms/cc of no more than 2 microorganisms) sent 3 or more days following admission and 7 days following catheter removal. The definition for CAUTI was based on that used by the National Healthcare Safety Network for infection control surveillance purposes at the time of the study and included both symptomatic CAUTI and asymptomatic bacteriuria. CAUTI was reported as the number of infections per 1000 catheter‐days for eligible patients on each surgical unit for the baseline and follow‐up data collection periods.
Additional Descriptive Variables
Descriptive variables included the patient's name, surgical procedure, surgeon, presence of a chronic indwelling catheter, date of admission to the floor, date of surgery, date of birth, and length of stay in days.
Data Collection
A professional research assistant (PRA) identified eligible patients on the 2 surgical units of interest and collected the number of postoperative urinary catheter days per patient using daily and weekly automated electronic queries of an EHR containing all nursing documentation on medical and surgical floors at UCH. These queries identified all patients on the floors of interest with urinary bladder elimination management documentation. Those with documented indwelling catheters were included in the study unless exclusion criteria were met. During the study period, the EHR was configured to provide the following documentation of urinary output management: date and location of catheter insertion, routine assessment of urinary output and devices, and date and time of catheter removal. At UCH, catheter insertion and removal are documented in the EHR for 93% and 88% of surgical patients, respectively. When documentation of catheter insertion was missing from the EMR, the operative note was reviewed electronically for documentation of insertion. If the operative note did not reference the insertion, it was presumed to have occurred perioperatively. Likewise, if there was no documentation of catheter removal, it was presumed to have occurred prior to documentation of urinary continence. The PRA abstracted additional information (surgical procedure and date) from the discharge abstracts and operative notes using a standardized data collection sheet.
Laboratory‐based surveillance was used to determine the incidence of CAUTIs in a manner similar to that employed by infection preventionists at UCH. The microbiology laboratory provided a monthly summary of all positive urine cultures for both study units. Positive culture results were cross‐referenced with the catheter removal dates for all eligible study patients.
Validation
Validation of catheter documentation in the EHR was carried out on each of the 2 surgical units for a 2‐week period at the outset of the data collection. During each day of the validation period, the PRA compared the EHR report with the charge nurse report on each floor. Any discrepancies regarding the presence or absence of the indwelling catheter were resolved by querying the patient's nurse directly. All patients with catheters during their inpatient stay were captured by EHR documentation. The daily EHR reports had a 91% agreement with a daily nursing query (reference standard) and a Kappa (percent agreement adjusted for chance agreement) of 0.77. Instances of disagreement were generally due to a lag in EHR documentation on the part of the nursing staff.
Audit and Feedback Intervention
An educational presentation was developed to cover the following topics: the definition and epidemiology of CAUTI, harms associated with CAUTI, risk factors for CAUTI, commonly accepted indications for indwelling catheters, and alternatives to catheters. In addition, the catheter duration performance measure was defined, followed by the feedback of unit‐specific performance from the baseline data collection period. The presentation was made by the principal investigator to nursing staff on each of the 2 surgical units on 3 occasions per unit with days and times selected so as to reach as many unit nurses as possible. At the conclusion of each session, nurses were asked to brainstorm barriers to evidence‐based management of indwelling catheters. Light refreshments and a hour continuing education credit were provided regardless of participation in the brainstorming session. Additionally, participants completed brief evaluations of the sessions.
Analyses
Descriptive data are reported as means and standard deviations for continuous variables and percentages for categorical variables. Outcome measures were calculated as defined above. For these comparisons, we used t‐tests for continuous variables and chi‐square tests for dichotomous variables. We used Cochran‐Mantel‐Haenszel to test for trend for categorical variables. Confidence intervals were calculated for the incidence rate differences based on Poisson approximations. Analyses were completed using SAS Statistical Software Version 9.2.
This study was approved by the Colorado Multiple Institutional Review Board. Waivers of Health Insurance Portability and Accountability Act (HIPAA) and informed consent were obtained for study patients. Nurses participating in the educational sessions provided informed consent.
Results
During the study period there were a total of 1657 surgeries on the 2 study units during the baseline and follow‐up periods. After exclusions for urologic or gynecologic surgery (271), no indwelling catheter for surgery (505), or first surgery of 2 or more during the hospitalization (31), there were 846 eligible surgeries (51%).
Table 1 describes the population for the baseline and follow‐up periods for orthopedic and general surgery patients. Within each unit, the surgical populations were comparable during the baseline and follow‐up periods with the exception that the mean length of stay for eligible general surgery patients was significantly shorter in the baseline period as compared to the follow‐up period (6.6 vs. 8.5 days, P = 0.02). Cases on the orthopedic surgery unit were predominantly knee, hip and spine surgeries (85.9%), while those on the general surgery unit were predominantly gut and other gastrointestinal (GI) procedures (80.3%).
| Characteristic | Orthopedic Surgery | General Surgery | |||||
|---|---|---|---|---|---|---|---|
| Baseline, n = 206 | Follow‐Up, n = 290 | P Value | Baseline, n = 167 | Follow‐Up, n = 183 | P Value | ||
| |||||||
| Age in years, mean (SD) | 58.3 (15.6) | 58.1 (14.7) | 0.87 | 53.8 (16.1) | 52.7 (15.7) | 0.54 | |
| Male gender (%) | 47.1 | 45.2 | 0.67 | 43.1 | 48.6 | 0.30 | |
| Length of stay in days, mean (SD) | 4.0 (3.5) | 3.7 (2.8) | 0.22 | 6.6 (5.5) | 8.5 (8.6) | 0.02 | |
| Type of surgery (%) | |||||||
| Knee | 24.8 | 27.9 | 0.91 | Gut | 54.5 | 50.3 | 0.09 |
| Hip | 37.4 | 35.5 | Other GI | 22.8 | 32.8 | ||
| Spine | 22.8 | 23.1 | Non‐GI | 22.8 | 16.9 | ||
| Other Ortho | 5.3 | 4.1 | |||||
| Non‐Ortho | 9.7 | 9.3 | |||||
The Intervention
The educational intervention and feedback was received by two‐thirds of registered nurses on each unit and was rated highly by participants. A total of 79% of nurses agreed or strongly agreed that the information provided was relevant to their daily practice and 42% strongly agreed that they would change their practice based on the presentation. Barriers to evidence‐based use of urinary catheters identified by surgical nurses on each unit are shown in Table 2. They included the following domains: communication, patient concerns, clinical concerns, equipment, policies and procedures, and skills. General surgery and orthopedic surgery nurses identified different concerns arising from the different patient populations and surgeries cared for on each unit.
| Domain | Orthopedic Surgery | General Surgery |
|---|---|---|
| ||
| Communication | Communication among teams | Occasional need to call MD for order |
| Patient Comfort | Discomfort first overnight postop without catheter; discomfort of straight cathethers | Discomfort and embarrassment associated with straight catheters; patient request for indwelling catheter |
| Clinical concerns | Removal POD 1 too soon | Need to monitor I/Os in patients with low output |
| Equipment | Portable ultrasound on a different floor | |
| Policies and Procedures | ||
| 1. Epidural anesthesia | Duration of epidural/delay post epidural removal | |
| 2. Straight catheters | Risk of infection | Risk of trauma; infection |
| 3. Management of Urinary Retention | No standardized protocol for urinary retention. | Need for traumatic reinsertion of catheter. |
| Skills | Perineal care; catheter care | Perineal care |
BaselineFollow‐Up Comparison
Table 3 describes the measures of urinary catheter use for each surgical population for both data collection periods. On both units, measures of catheter duration were improved following the education and feedback intervention. For the orthopedic unit, mean postoperative catheter duration was reduced from 1.7 to 1.4 days (P = 0.01) and the proportion of patients with catheter removal before day 3 was increased from 86% to 92% (P = 0.04). For the general surgery unit, mean postoperative catheter duration was reduced from 2.6 to 2.2 days (P = 0.01) and the proportion of patients with catheter removal before day 3 was increased from 56% to 63% (P = 0.14). When the general surgery measures were adjusted to account for the difference in length of stay between the 2 time periods, the odds of meeting the performance measure at follow‐up compared to baseline increased from unadjusted odds of 1.38 (P = 0.14) to adjusted odds of 1.69 (P = 0.02).
| Measure | Orthopedic Surgery | General Surgery | ||||
|---|---|---|---|---|---|---|
| Baseline, n = 206 | Follow‐Up, n = 290 | P Value | Baseline, n = 167 | Follow‐Up, n = 183 | P Value | |
| ||||||
| Postoperative catheter duration in days (mean, SD) | 1.70 (1.24) | 1.44 (0.85) | 0.01 | 2.64 (1.85) | 2.19 (1.40) | 0.01 |
| Postoperative catheter duration performance measure (%) | 86 | 92 | 0.04 | 56 | 63 | 0.14 |
| Total catheter days | 350 | 418 | 441 | 401 | ||
| Catheter days/1000 hospital days | 423 | 394 | ns* | 398 | 259 | s* |
| Catheter‐associated UTIs | 3 | 0 | 3 | 3 | ||
| Catheter‐associated UTI rate (infections/1000 device days) | 8.6 | 0 | ns* | 6.8 | 7.5 | ns* |
Figure 1a and b are histograms of the frequency of cases having a given postoperative catheter duration in days. The dark bars show the baseline distribution and the light bars show the follow‐up distribution. Although the number of patients in the follow‐up period is greater than for the baseline period for the orthopedic surgery cohort, the images are instructive. In both groups of patients, but most notably in the general surgery population, the reduction in catheter measures resulted from a left shift in the frequency distribution, both for the longer duration outliers (removing the tail of each plot) and for the shorter duration catheters (3 days), increasing the proportion of catheters removed on postoperative day 1.

CAUTIs
The CAUTI rate on the orthopedic surgery unit demonstrated a nonsignificant decline from 8.9 to 0 infections per 1000 device days, and on the general surgery unit the rate was constant at approximately 7 infections per 1000 device days.
Discussion
This preobservational and postobservational study found that audit and feedback of patient‐level postoperative urinary catheter duration delivered in the context of an educational intervention and brainstorming session was temporally associated with clinically meaningful reductions in urinary catheter duration. In so doing, we demonstrated the feasibility of collecting patient level urinary catheter duration, and delivering it in a manner that had utility for frontline staff. Our results are consistent with the quality improvement literature which demonstrates that audit and feedback is a successful quality improvement strategy in many contexts and may be as good as more complex interventions at increasing adherence to the performance of process measures for surgical infection prevention.14 Two large national programs, the VA National Surgical Quality Improvement Program (NSQIP) and SCIP, use audit and feedback as the backbone of their large‐scale quality improvement strategies with promising results.11, 12
Given that the 2 study units were so different in practice patterns regarding urinary catheter management and nursing‐identified barriers to evidence‐based care, this work suggests that urinary catheter management may pose unique challenges for different clinical areas and provides a caution that one‐size‐fits‐all interventions for the rationalization of urinary catheter management and reduction of CAUTIs may be of limited effectiveness in the absence of local tailoring. As such, audit and feedback is well‐suited to this purpose as more proscriptive quality improvement strategies may meet with a variety of implementation challenges.
The impact of our intervention on CAUTI rates was not significant. There are several possible explanations for this finding. First, the study was not powered to detect a difference in CAUTI rates given the low infection rate at our institution. However, we cannot exclude the possibility that a reduction in mean catheter duration of one‐third to one‐half of a day is insufficient to impact CAUTI rates in postoperative patients, particularly when many of the follow‐up patients still had postoperative catheter duration 2 daysthe timeframe beyond which bacterial colonization of the catheter begins. While both study units had similar increases in postoperative catheter duration, the UTI rate was only decreased in the orthopedic surgery group which had much higher rates of postoperative catheter duration 2 days at baseline.
In recent years, there has been a renewed focus on the eradication of hospital‐acquired infections prompted by intense interest from the public, federal and state legislators, and others.15, 16 The CMS has recently used the revamping of the Inpatient Prospective Payment System (IPPS) as an opportunity to align financial incentives so that reimbursements for claims with certain hospital‐acquired conditions, including CAUTIs, will be reduced to that of the reimbursement of the same claim without the presence of the complication.17 This move is just one of several strategies to motivate hospitals and clinicians to address the pervasive problem of hospital‐acquired infection.
As urinary catheters are intimately linked to hospital‐acquired UTI, a focus of reduction efforts on catheter use is appropriate. The National Quality Forum (NQF) endorsed a postoperative catheter duration quality measure which was incorporated by the CMS's SCIP in late 2009.18 As a result, every hospital in the country that performs surgery and participates in the Medicare program is now tasked with determining patient‐level urinary catheter duration for selected surgical patients. This move represents a departure from current recommendations from the Centers for Disease Control and Prevention (CDC) and its National Healthcare Safety Network19, 20 which endorse the measurement of a catheter utilization ratio (urinary catheter days/patient days) for patient care units, but does not endorse any patient‐level utilization measures. In this instance, the use of patient‐level data may be better suited to quality improvement interventions such as audit and feedback because of its clinical relevance to frontline providers. However, it may also increase the data collection burden on hospitals. Notably, the measurement of postoperative catheter duration in this study was semiautomated using queries of an EHR. Such an approach can significantly reduce the data collection burden for this process measure and is consistent with national initiatives to integrate EHRs with quality improvement initiatives going forward.21
Our study has several limitations. This study took place in the year following the announcement of a high profile Medicare rule change regarding payment for hospital‐acquired harms. Certainly, the uncontrolled prestudy and poststudy design does not allow for the assessment of the impact of our intervention independent of this context. We are unable, therefore, to attribute the observed reduction solely to the intervention. Additionally, we did not follow postoperative urinary catheter duration beyond the immediate follow‐up period. It is anticipated that the impact of an audit and feedback intervention may diminish over time without a mechanism for repeated feedback. Certainly the sustainability of such repeated feedback in a single institution would be improved with an appropriately configured EHR.
In addition, we have reliable data on catheter reinsertions only from the follow‐up period. While the rates of reinsertions we recorded (0.7% on orthopedic surgery and 2.7% on general surgery) were lower than expected based on the literature,22 we are unable to determine if our intervention led to increases in postoperative urinary retention.
This study was limited to 2 surgical units of a single academic medical center and therefore the urinary catheter utilization patterns may not be representative of other patient populations at other institutions. However, the urinary catheter patterns were comparable to those identified in our prior work in a national sample of Medicare patients undergoing elective surgery.9
Finally, the field of CAUTI prevention has evolved rapidly since this study was performed. In particular, the surveillance definition of CAUTI was altered twice by the CDC in December of 2008 and March of 2009. In addition, the Infectious Diseases Society of America issued a new definition of CAUTI in February of 2010.23 All of these changes highlight the difference between asymptomatic bateriuria (ASB) and symptomatic CAUTI. However, the surveillance definition in use at the time of this study did not make this distinction. Therefore, we are unable to comment on the relative occurrence of ASB versus symptomatic CAUTI under the new definitions.
Rational urinary catheter use is a central component of CAUTI prevention efforts.24 We describe the use of patient‐level urinary catheter use in an audit and feedback intervention to frontline staff that was associated with reductions in urinary catheter duration. To do so, we employed a methodology for tracking urinary catheter use patterns that can provide important data for infection preventionists and frontline providers in efforts to improve urinary output management. This promising approach merits further study as an adjunct to current efforts to rationalize urinary catheter utilization and reduce CAUTIs. In the current environment, having the right data is a powerful aide for ongoing performance improvement.
Acknowledgements
The authors acknowledge the contributions of Daniel Sandy, BA, MPN, Vivienne Smith, RN, UCH; and the insights of Michelle Barron, MD, Linda Burton, RN, Teri Hulett, RN, UCH, and Jean Kutner, MD, MSPH.
- ,,.Urinary tract etiology of bloodstream infections in hospitalized patients.J Infect Dis.1983;148:57–62.
- ,,, et al.Nosocomial infections in US hospitals, 1975–1976: estimated frequency by selected characteristics of patients.Am J Med.1981;70:947–959.
- ,,,,.The nationwide nosocomial infection rate. a new need for vital statistics.Am J Epidemiol.1985;121:159–167.
- .Clinical and economic consequences of nosocomial catheter‐related bacteriuria.Am J Infect Control.2000;28:68–75.
- ,,,.Overuse of the indwelling urinary tract catheter in hospitalized medical patients.Arch Intern Med.1995;155:1425–1429.
- .Catheter‐associated bacteriuria.Urol Clin North Am.1986;13:735.
- .Guidelines for prevention of catheter‐associated urinary tract infections.Ann Intern Med.1975;82:386.
- ,,, et al.Preventing hospital‐acquired urinary tract infection in the united states: a national study.Clin Infect Dis.2008;46:243–250.
- ,,,.Indwelling urinary catheter use in the postoperative period: analysis of the national surgical infection prevention project data.Arch Surg.2008;143:551–557.
- ,,,,.Management of urinary retention after surgical repair of hip fracture.Can Med Assoc J.1992;146:1185–1188.
- ,,.The comparative assessment and improvement of quality of surgical care in the department of veterans affairs.Arch Surg.2002;137:20–27.
- .The surgical infection prevention and surgical care improvement projects: promises and pitfalls.Am Surg.2006;72:1010–1016.
- ,,,.Feedback to nursing staff as an intervention to reduce catheter‐associated urinary tract infections.Am J Infect Control.1999;27:402–424.
- ,,, et al.The effect of a quality improvement collaborative to improve antimicrobial prophylaxis in surgical patients.Ann Intern Med.2008;149:480.
- ,.Nonpayment for harms resulting from medical care: catheter‐associated urinary tract infections.JAMA.2007;289:2782–2784.
- Kaiser Family Foundation. Hospital‐based infections reporting requirements,2008. Available at: www.Kaiser Family Foundation State Health Facts.org. Accessed August 25, 2010.
- Centers for Medicare and Medicaid Services, Department of Health and Human Services.Medicare Program: Changes to the Hospital Inpatient Prospective Payment Systems and Fiscal Year 2008 Rates. CMS‐1390‐F. 8–1‐2007.
- Centers for Medicare and Medicaid Services, Department of Health and Human Services and The Joint Commission.Specifications Manual for National Hospital Inpatient Quality Measures, Discharges 10/1/09 (4Q09) through 3/31/10 (1Q10). 5–4‐2009.
- ,,,,, andthe Healthcare Infection Control Practices Advisory Committee.Guideline for the Prevention of Catheter‐associated Urinary Tract Infections,2009. Centers for Disease Control and Prevention. 1–22‐2010.
- Centers for Disease Control and Prevention.NHSN Patient Safety Component Key Terms. 1–22‐2010.
- .Stimulating the adoption of health information technology.N Engl J Med.2010;360:1477–1479.
- ,.Management of postoperative urinary retention: a randomized trial of in‐out versus overnight catheterization.ANZ J Surg.2004;2004:658–661.
- ,,, et al.Diagnosis, prevention, and treatment of catheter‐associated urinary tract infection in adults: 2009 International Clinical Practice Guidelines from the Infectious Diseases Society of America.Clin Infect Dis.2010;50:625–663.
- ,,, et al.Strategies to prevent catheter‐associated urinary tract infections in acute care hospitals.Infect Control Hosp Epidemiol.2008;29:S41–S50.
- ,,.Urinary tract etiology of bloodstream infections in hospitalized patients.J Infect Dis.1983;148:57–62.
- ,,, et al.Nosocomial infections in US hospitals, 1975–1976: estimated frequency by selected characteristics of patients.Am J Med.1981;70:947–959.
- ,,,,.The nationwide nosocomial infection rate. a new need for vital statistics.Am J Epidemiol.1985;121:159–167.
- .Clinical and economic consequences of nosocomial catheter‐related bacteriuria.Am J Infect Control.2000;28:68–75.
- ,,,.Overuse of the indwelling urinary tract catheter in hospitalized medical patients.Arch Intern Med.1995;155:1425–1429.
- .Catheter‐associated bacteriuria.Urol Clin North Am.1986;13:735.
- .Guidelines for prevention of catheter‐associated urinary tract infections.Ann Intern Med.1975;82:386.
- ,,, et al.Preventing hospital‐acquired urinary tract infection in the united states: a national study.Clin Infect Dis.2008;46:243–250.
- ,,,.Indwelling urinary catheter use in the postoperative period: analysis of the national surgical infection prevention project data.Arch Surg.2008;143:551–557.
- ,,,,.Management of urinary retention after surgical repair of hip fracture.Can Med Assoc J.1992;146:1185–1188.
- ,,.The comparative assessment and improvement of quality of surgical care in the department of veterans affairs.Arch Surg.2002;137:20–27.
- .The surgical infection prevention and surgical care improvement projects: promises and pitfalls.Am Surg.2006;72:1010–1016.
- ,,,.Feedback to nursing staff as an intervention to reduce catheter‐associated urinary tract infections.Am J Infect Control.1999;27:402–424.
- ,,, et al.The effect of a quality improvement collaborative to improve antimicrobial prophylaxis in surgical patients.Ann Intern Med.2008;149:480.
- ,.Nonpayment for harms resulting from medical care: catheter‐associated urinary tract infections.JAMA.2007;289:2782–2784.
- Kaiser Family Foundation. Hospital‐based infections reporting requirements,2008. Available at: www.Kaiser Family Foundation State Health Facts.org. Accessed August 25, 2010.
- Centers for Medicare and Medicaid Services, Department of Health and Human Services.Medicare Program: Changes to the Hospital Inpatient Prospective Payment Systems and Fiscal Year 2008 Rates. CMS‐1390‐F. 8–1‐2007.
- Centers for Medicare and Medicaid Services, Department of Health and Human Services and The Joint Commission.Specifications Manual for National Hospital Inpatient Quality Measures, Discharges 10/1/09 (4Q09) through 3/31/10 (1Q10). 5–4‐2009.
- ,,,,, andthe Healthcare Infection Control Practices Advisory Committee.Guideline for the Prevention of Catheter‐associated Urinary Tract Infections,2009. Centers for Disease Control and Prevention. 1–22‐2010.
- Centers for Disease Control and Prevention.NHSN Patient Safety Component Key Terms. 1–22‐2010.
- .Stimulating the adoption of health information technology.N Engl J Med.2010;360:1477–1479.
- ,.Management of postoperative urinary retention: a randomized trial of in‐out versus overnight catheterization.ANZ J Surg.2004;2004:658–661.
- ,,, et al.Diagnosis, prevention, and treatment of catheter‐associated urinary tract infection in adults: 2009 International Clinical Practice Guidelines from the Infectious Diseases Society of America.Clin Infect Dis.2010;50:625–663.
- ,,, et al.Strategies to prevent catheter‐associated urinary tract infections in acute care hospitals.Infect Control Hosp Epidemiol.2008;29:S41–S50.
Copyright © 2010 Society of Hospital Medicine
The Hospitalist Field Turns 15
Many people date the start of the hospitalist field to my 1996 New England Journal of Medicine article,1 which first introduced the concept to a broad audience. That makes 2011 the field's 15th year, andif you have kidsyou know this is a tough and exciting age. The cuteness of childhood has faded, and bad decisions can no longer be excused as youthful indiscretions.
That's an apt metaphor for our field as we celebrate our 15th birthday. We are now an established part of the health care landscape, with a clear place in the House of Medicine. All of the measures of a successful specialty are ours: a thriving professional society, high‐quality training programs, increasingly robust research, a flourishing journal, and more. The field has truly arrived.
But these successes are also tempered by several challenges that have become more evident in recent years. In this article, I'll reflect on some of these successes and challenges.
The Hospitalist Field's Successes and Growth
In our 1996 article, Goldman and I1 wrote about the forces promoting the hospitalist model:
It seems unlikelythat high value care can be delivered in the hospital by physicians who spend only a small fraction of their time in this setting. As hospital stays become shorter and inpatient care becomes more intensive, a greater premium will be placed on the skill, experience, and availability of physicians caring for inpatients.
When we cited the search for value as a driving force in 1996, we were a bit ahead of our time, since there was relatively little skin in this game at the time. Remember that when our field launched, none of these value‐promoting forces existed: robust unannounced hospital inspections by the Joint Commission, public reporting of quality data, pay for performance, no pay for errors, state reporting of sentinel events, and more. In other words, until recently, neither a hospital's income stream nor its reputation was threatened by poor performance.
But this landscape is undergoing a sea change. By 2015, fully 9% of a hospital's Medicare reimbursements will be at risk through a variety of initiatives, including value‐based purchasing and meaningful use standards. And private payers are beginning to replicate Medicare's standards, particularly when they perceive that they may lead to both improved quality and lower costs.
Hospitals and health systems increasingly recognize how indispensable hospitalists can be as they demonstrate that their presence improves value. But this is only one of the forces driving the fieldalready the fastest growing specialty in medical historyto even higher levels of growth. These others include: the exodus of primary care physicians from the hospital, the fact that the specialists have left the building, comanagement of nonmedical patients, new opportunities in systems leadership, and dealing with housestaff duty hours reductions. I'll say a word about each.
The Exodus of Primary Care Physicians
In the early days of our field, one of the major sources of pushback was the desire of many primary care doctors to continue managing their own inpatients. Beginning a decade ago, this pressure began to abate, as many primary care physicians began to recognize the potential advantages of working with hospitalists.2
Over the next several years, I predict that the growth in the patient‐centered medical home model3with the physician's new responsibilities to provide comprehensive patient‐centered carewill make it even less likely that primary care doctors will have the time to manage their own inpatients. Luckily, information systems now being installed throughout the country (fueled by federal subsidies) will lead to unprecedented connectivity between the inpatient and outpatient worlds,4 hopefully resulting in improving handoffs.
Moreover, the increasing scrutiny of, and upcoming penalties for, high readmission rates are driving hospitals and clinics into creating more robust systems of care to improve inpatientoutpatient communications. The bottom line is that the main Achilles heel of hospitalist systemsthe handoff at hospital admission and dischargeshould improve over the next few years, making it easier than ever for primary care doctors to forego hospital care without losing track of critical patient information.
The Specialists Have Left the Building
One of the more interesting phenomena in the recent history of the hospitalist field is the growth of what I call hyphenated hospitalists: neurology hospitalists, ob‐gyn hospitalists, surgical hospitalists, and the like. The forces promoting these models are similar to those that catalyzed the hospitalist model: the recognition that bifurcating inpatient and outpatient care sometimes makes sense when several conditions are met (Table 1).
|
| 1) Is the number of inpatients who require the services of that specialty (either for consults or principal care) large enough to justify having at least one doctor in the house during daytime? |
| 2) Is the specialist frequently needed to see an inpatient urgently? |
| 3) Under the usual model of mixed inpatient and outpatient care, is the specialist frequently busy in the office, operating room, or procedural suite at times where they are urgently needed in the hospital (see #2)? |
| 4) Has the field become sub‐sub specialized, such that many covering physicians are now uncomfortable managing common acute inpatient problems (i.e., the headache neurologist asked to handle an acute stroke)? |
The emergence of hyphenated hospitalists raises all sorts of questions for the hospitalist field, many of which I have addressed elsewhere.5 But the bottom line is that the growth of specialty hospitalists may help create a new hospital home teama group of dedicated inpatient physicians spanning virtually every specialty who share best practices, work together on systems improvements, and operate under similar accountabilities. This development may well be the most exciting one in the field's recent history.
Comanagement of Nonmedical Patients
The same forces that led to the emergence of the hospitalist field are also catalyzing the growth of hospitalist comanagement programs. There is a shortage of general surgeons, and in teaching hospitals, there are fewer surgical residents available to help provide floor‐based pre‐ and post‐operative care. And surgical patients are under the same value pressures as medical patients, with increasing public reporting of quality processes and outcomes and new pay for performance programs coming on line. Although the evidence of benefit is mixed,68 many hospitalists are finding that increasing parts of their work involve comanagement.
Comanagement raises several issues, all of which need to be addressed. How do we define clear boundaries between what the hospitalist does and what the specialist does? Comanagement programs, to be effective, need very clear rules of engagement and open lines of communication to work through inevitable conflicts.6 How does the money flow? Most hospitalist programs receive hospital support, but it is legitimate to wonder whether the specialists, particularly surgeons, should chip in to support the program, particularly if they continue to collect a global case rate that was predicated on their provision of pre‐ and post‐operative care. How do comanagement programs and specialty hospitalist programs interrelate, and what are the relative advantages and disadvantages of each? To my mind, programs that meet the conditions outlined in Table 1 probably would do well to start a specialty hospitalist program, assuming that they can find high‐quality specialists to staff it. But there will be myriad variations on these themes. In my hospital, for example, we have both neurohospitalists and medical hospitalists who co‐manage neurosurgery patients.
New Opportunities in Systems Leadership
The growth of the hospitalist field will partly come from individuals who begin their careers performing clinical work, but who transition over time to managerial and leadership roles. This is a natural transition: Who better than a hospitalist to help organize and deliver educational programs, manage clinical operations, implement information technology systems, or lead quality, safety, or utilization management efforts? Of course, as hospitalists assume these roles, others need to take their places covering their clinical shifts.
This might seem like a relatively unimportant driver of personnel growth, but in more advanced systems, it can become a major one. Table 2 lists the faculty in my Division of Hospital Medicine at the University of California, San Francisco (UCSF) who have major institutional (i.e., nondivisional) roles. These roles, spread across eight faculty, account for 3.7 full‐time equivalents (FTEs).
| Role | Works for Whom? | Approximate % FTE |
|---|---|---|
| ||
| Associate Chief Medical Officer | Medical Center | 80% |
| Associate Medical Director for Information Technology | Medical Center | 80% |
| Associate Chair for Quality and Safety | Department of Medicine | 50% |
| Director of Quality and Patient Safety | Department of Neurosurgery | 50% |
| Associate Medicine Residency Director (two people) | Department of Medicine | 30% (for each) |
| Director of Medical Student Clerkships | Department of Medicine | 25% |
| Director of Patient Safety/Quality Programs | Office of Graduate Medical Education, School of Medicine | 25% |
| Total FTEs: 3.7 | ||
Dealing with New ACGME Regulations
In 2003, the Accreditation Council for Graduate Medical Education (ACGME) issued its first housestaff duty hours reductions (limiting housestaff to a maximum of 80 hours per week, with no single shift lasting longer than 30 hours). This reduction led to the development of nonteaching services in most teaching hospitals; the vast majority of such programs have hospitalists at their core.
In July 2011, new ACGME regulations go into effect,10 which will further cut the availability of housestaff to cover clinical services. Although the 80‐hour weekly limit remains, intern shifts are now limited to 16 hours, meaning that the traditional long call system involving interns must be replaced by a shift‐based system. Like the earlier changes, these new regulations are leading to additional hospitalist growth in the nation's teaching hospitals. By the time the changes are fully implemented, many hospitalist programs will have half or more of their hospitalist FTEs devoted to covering patients previously cared for by residents.
Challenges for Hospitalist Programs
These powerful forces promoting the growth in the hospitalist field continue to ensure that hospitalists are in high demand. As a practical matter, this has resulted in increasing salaries and improved job conditions for hospitalists.
But this growth brings many challenges. Many hospitalist programs are poorly managed, often because the leaders lack the training and experience to effectively run such a rapidly growing and complex enterprise. One manifestation of these leadership challenges is that schedules are often created around the convenience and desires of the physicians rather than the needs of the patients. For example, the increasingly prevalent seven‐days‐on, seven‐days‐off schedule often leads to burnout and a feeling by the hospitalists that they are working too hard. Yet many groups are unwilling to consider modifications to the schedule that might decrease the intensity, if the cost is fewer days off.
On the other hand, some groups pay little attention to patient continuity in constructing their schedules. I know of programs that schedule their hospitalists in 24‐hour shifts (followed by a few days off), which means that admitted patients will see a different hospitalist every day. I see this as highly problematic, particularly because the most common complaint I hear from patients about hospitalist programs is that I saw a different doctor every day.
Many of the field's challenges stem from hospitalists' near‐total dependency on hospital funding to create sustainable job descriptions.11 While I continue to believe that this bit of financial happenstance has been good for both hospitalists and hospitalssince it has driven uncommon degrees of interdependency and alignmentit does mean that a difficult budget battle is virtually assured every year. As hospital finances become tighter, one can expect these battles to grow even more heated. Speaking for hospitalists, I am not too worried about the outcomes of these battles, since hospitalists provide a mission‐critical service at a fair price, there are no viable lower‐priced replacements (expect perhaps for nonphysician providers such as nurse practitioners for the less‐complex patients), and hospitalists are extraordinarily mobilethere are virtually no barriers for a hospitalist, or an entire group, to transfer to another institution. Nevertheless, it seems inevitable that these battles will leave scars, scars that may ultimately compromise the crucial collaboration that both hospitalists and hospitals depend on.
The Bottom Line
Even at age 15, an age at which many adolescents are irredeemably cynical, the hospitalist field retains much of its sense of limitless possibility and exuberance. This is not because things are perfectthey are not. Some hospitalist jobs are poorly constructed, some groups have poor leadership, some hospitalists are burning out, there are examples of spotty quality and collaboration, and hospitalists continue to have to work to earn the respect of colleagues and patients that other specialists take for granted.
That said, the field of hospital medicine remains uniquely exciting, in part because it is so tightly linked to the broader changes in the health care policy landscape. Many other specialties see the profound changes underway in health care as an existential threat to their professional values and incomes. Hospitalists, on the other hand, see these changes as raising the pressure on hospitals to deliver the highest quality, most satisfying, and safest care at the lowest cost. Framed this way, forward‐thinking hospitalists quite naturally see these changes as yet another catalyst for the growth and indispensability of their field.
- ,.The emerging role of “hospitalists” in the American health care system.N Engl J Med.1996;335:514–517.
- ,,,.How physicians perceive hospitalist services after implementation: Anticipation vs reality.Arch Intern Med.2003;163:2330–2336.
- ,,.Medical homes: Challenges in translating theory into practice.Med Care.2009;47:714–722.
- ,.Improving safety with information technology.N Engl J Med.2003;348:2526–2534.
- The New Home Team: The Remarkable Rise of the Hyphenated Hospitalist. Wachter's World blog, January 16,2011. Available at: http://tinyurl. com/4h2jy7e. Accessed February 12, 2011.
- ,,, et al.Comanagement of surgical patients between neurosurgeons and hospitalists.Arch Intern Med.2010;170:2004–2010.
- ,,, et al.Medical and surgical comanagement after elective hip and knee arthroplasty: A randomized, controlled trial.Ann Intern Med.2004;141:28–38.
- ,,,,.Comanagement of hospitalized surgical patients by medicine physicians in the United States.Arch Intern Med.2010;170:363–368.
- ,,.Neurohospitalists: An emerging model for inpatient neurological care.Ann Neurol.2008;63:135–140.
- ,,;ACGME Duty Hour Task Force. The new recommendations on duty hours from the ACGME Task Force.N Engl J Med.2010 Jul 8;363(2):e3. Epub 2010 Jun 23. PubMed PMID: 20573917. The website is here: http://www.nejm.org/doi/full/10.1056/NEJMsb1005800.
- .Elevated expectations.The Hospitalist. January2011. Available at: http://www.the‐hospitalist.org/details/article/972781/Elevated_Expectations.html. Accessed February 12, 2011.
Many people date the start of the hospitalist field to my 1996 New England Journal of Medicine article,1 which first introduced the concept to a broad audience. That makes 2011 the field's 15th year, andif you have kidsyou know this is a tough and exciting age. The cuteness of childhood has faded, and bad decisions can no longer be excused as youthful indiscretions.
That's an apt metaphor for our field as we celebrate our 15th birthday. We are now an established part of the health care landscape, with a clear place in the House of Medicine. All of the measures of a successful specialty are ours: a thriving professional society, high‐quality training programs, increasingly robust research, a flourishing journal, and more. The field has truly arrived.
But these successes are also tempered by several challenges that have become more evident in recent years. In this article, I'll reflect on some of these successes and challenges.
The Hospitalist Field's Successes and Growth
In our 1996 article, Goldman and I1 wrote about the forces promoting the hospitalist model:
It seems unlikelythat high value care can be delivered in the hospital by physicians who spend only a small fraction of their time in this setting. As hospital stays become shorter and inpatient care becomes more intensive, a greater premium will be placed on the skill, experience, and availability of physicians caring for inpatients.
When we cited the search for value as a driving force in 1996, we were a bit ahead of our time, since there was relatively little skin in this game at the time. Remember that when our field launched, none of these value‐promoting forces existed: robust unannounced hospital inspections by the Joint Commission, public reporting of quality data, pay for performance, no pay for errors, state reporting of sentinel events, and more. In other words, until recently, neither a hospital's income stream nor its reputation was threatened by poor performance.
But this landscape is undergoing a sea change. By 2015, fully 9% of a hospital's Medicare reimbursements will be at risk through a variety of initiatives, including value‐based purchasing and meaningful use standards. And private payers are beginning to replicate Medicare's standards, particularly when they perceive that they may lead to both improved quality and lower costs.
Hospitals and health systems increasingly recognize how indispensable hospitalists can be as they demonstrate that their presence improves value. But this is only one of the forces driving the fieldalready the fastest growing specialty in medical historyto even higher levels of growth. These others include: the exodus of primary care physicians from the hospital, the fact that the specialists have left the building, comanagement of nonmedical patients, new opportunities in systems leadership, and dealing with housestaff duty hours reductions. I'll say a word about each.
The Exodus of Primary Care Physicians
In the early days of our field, one of the major sources of pushback was the desire of many primary care doctors to continue managing their own inpatients. Beginning a decade ago, this pressure began to abate, as many primary care physicians began to recognize the potential advantages of working with hospitalists.2
Over the next several years, I predict that the growth in the patient‐centered medical home model3with the physician's new responsibilities to provide comprehensive patient‐centered carewill make it even less likely that primary care doctors will have the time to manage their own inpatients. Luckily, information systems now being installed throughout the country (fueled by federal subsidies) will lead to unprecedented connectivity between the inpatient and outpatient worlds,4 hopefully resulting in improving handoffs.
Moreover, the increasing scrutiny of, and upcoming penalties for, high readmission rates are driving hospitals and clinics into creating more robust systems of care to improve inpatientoutpatient communications. The bottom line is that the main Achilles heel of hospitalist systemsthe handoff at hospital admission and dischargeshould improve over the next few years, making it easier than ever for primary care doctors to forego hospital care without losing track of critical patient information.
The Specialists Have Left the Building
One of the more interesting phenomena in the recent history of the hospitalist field is the growth of what I call hyphenated hospitalists: neurology hospitalists, ob‐gyn hospitalists, surgical hospitalists, and the like. The forces promoting these models are similar to those that catalyzed the hospitalist model: the recognition that bifurcating inpatient and outpatient care sometimes makes sense when several conditions are met (Table 1).
|
| 1) Is the number of inpatients who require the services of that specialty (either for consults or principal care) large enough to justify having at least one doctor in the house during daytime? |
| 2) Is the specialist frequently needed to see an inpatient urgently? |
| 3) Under the usual model of mixed inpatient and outpatient care, is the specialist frequently busy in the office, operating room, or procedural suite at times where they are urgently needed in the hospital (see #2)? |
| 4) Has the field become sub‐sub specialized, such that many covering physicians are now uncomfortable managing common acute inpatient problems (i.e., the headache neurologist asked to handle an acute stroke)? |
The emergence of hyphenated hospitalists raises all sorts of questions for the hospitalist field, many of which I have addressed elsewhere.5 But the bottom line is that the growth of specialty hospitalists may help create a new hospital home teama group of dedicated inpatient physicians spanning virtually every specialty who share best practices, work together on systems improvements, and operate under similar accountabilities. This development may well be the most exciting one in the field's recent history.
Comanagement of Nonmedical Patients
The same forces that led to the emergence of the hospitalist field are also catalyzing the growth of hospitalist comanagement programs. There is a shortage of general surgeons, and in teaching hospitals, there are fewer surgical residents available to help provide floor‐based pre‐ and post‐operative care. And surgical patients are under the same value pressures as medical patients, with increasing public reporting of quality processes and outcomes and new pay for performance programs coming on line. Although the evidence of benefit is mixed,68 many hospitalists are finding that increasing parts of their work involve comanagement.
Comanagement raises several issues, all of which need to be addressed. How do we define clear boundaries between what the hospitalist does and what the specialist does? Comanagement programs, to be effective, need very clear rules of engagement and open lines of communication to work through inevitable conflicts.6 How does the money flow? Most hospitalist programs receive hospital support, but it is legitimate to wonder whether the specialists, particularly surgeons, should chip in to support the program, particularly if they continue to collect a global case rate that was predicated on their provision of pre‐ and post‐operative care. How do comanagement programs and specialty hospitalist programs interrelate, and what are the relative advantages and disadvantages of each? To my mind, programs that meet the conditions outlined in Table 1 probably would do well to start a specialty hospitalist program, assuming that they can find high‐quality specialists to staff it. But there will be myriad variations on these themes. In my hospital, for example, we have both neurohospitalists and medical hospitalists who co‐manage neurosurgery patients.
New Opportunities in Systems Leadership
The growth of the hospitalist field will partly come from individuals who begin their careers performing clinical work, but who transition over time to managerial and leadership roles. This is a natural transition: Who better than a hospitalist to help organize and deliver educational programs, manage clinical operations, implement information technology systems, or lead quality, safety, or utilization management efforts? Of course, as hospitalists assume these roles, others need to take their places covering their clinical shifts.
This might seem like a relatively unimportant driver of personnel growth, but in more advanced systems, it can become a major one. Table 2 lists the faculty in my Division of Hospital Medicine at the University of California, San Francisco (UCSF) who have major institutional (i.e., nondivisional) roles. These roles, spread across eight faculty, account for 3.7 full‐time equivalents (FTEs).
| Role | Works for Whom? | Approximate % FTE |
|---|---|---|
| ||
| Associate Chief Medical Officer | Medical Center | 80% |
| Associate Medical Director for Information Technology | Medical Center | 80% |
| Associate Chair for Quality and Safety | Department of Medicine | 50% |
| Director of Quality and Patient Safety | Department of Neurosurgery | 50% |
| Associate Medicine Residency Director (two people) | Department of Medicine | 30% (for each) |
| Director of Medical Student Clerkships | Department of Medicine | 25% |
| Director of Patient Safety/Quality Programs | Office of Graduate Medical Education, School of Medicine | 25% |
| Total FTEs: 3.7 | ||
Dealing with New ACGME Regulations
In 2003, the Accreditation Council for Graduate Medical Education (ACGME) issued its first housestaff duty hours reductions (limiting housestaff to a maximum of 80 hours per week, with no single shift lasting longer than 30 hours). This reduction led to the development of nonteaching services in most teaching hospitals; the vast majority of such programs have hospitalists at their core.
In July 2011, new ACGME regulations go into effect,10 which will further cut the availability of housestaff to cover clinical services. Although the 80‐hour weekly limit remains, intern shifts are now limited to 16 hours, meaning that the traditional long call system involving interns must be replaced by a shift‐based system. Like the earlier changes, these new regulations are leading to additional hospitalist growth in the nation's teaching hospitals. By the time the changes are fully implemented, many hospitalist programs will have half or more of their hospitalist FTEs devoted to covering patients previously cared for by residents.
Challenges for Hospitalist Programs
These powerful forces promoting the growth in the hospitalist field continue to ensure that hospitalists are in high demand. As a practical matter, this has resulted in increasing salaries and improved job conditions for hospitalists.
But this growth brings many challenges. Many hospitalist programs are poorly managed, often because the leaders lack the training and experience to effectively run such a rapidly growing and complex enterprise. One manifestation of these leadership challenges is that schedules are often created around the convenience and desires of the physicians rather than the needs of the patients. For example, the increasingly prevalent seven‐days‐on, seven‐days‐off schedule often leads to burnout and a feeling by the hospitalists that they are working too hard. Yet many groups are unwilling to consider modifications to the schedule that might decrease the intensity, if the cost is fewer days off.
On the other hand, some groups pay little attention to patient continuity in constructing their schedules. I know of programs that schedule their hospitalists in 24‐hour shifts (followed by a few days off), which means that admitted patients will see a different hospitalist every day. I see this as highly problematic, particularly because the most common complaint I hear from patients about hospitalist programs is that I saw a different doctor every day.
Many of the field's challenges stem from hospitalists' near‐total dependency on hospital funding to create sustainable job descriptions.11 While I continue to believe that this bit of financial happenstance has been good for both hospitalists and hospitalssince it has driven uncommon degrees of interdependency and alignmentit does mean that a difficult budget battle is virtually assured every year. As hospital finances become tighter, one can expect these battles to grow even more heated. Speaking for hospitalists, I am not too worried about the outcomes of these battles, since hospitalists provide a mission‐critical service at a fair price, there are no viable lower‐priced replacements (expect perhaps for nonphysician providers such as nurse practitioners for the less‐complex patients), and hospitalists are extraordinarily mobilethere are virtually no barriers for a hospitalist, or an entire group, to transfer to another institution. Nevertheless, it seems inevitable that these battles will leave scars, scars that may ultimately compromise the crucial collaboration that both hospitalists and hospitals depend on.
The Bottom Line
Even at age 15, an age at which many adolescents are irredeemably cynical, the hospitalist field retains much of its sense of limitless possibility and exuberance. This is not because things are perfectthey are not. Some hospitalist jobs are poorly constructed, some groups have poor leadership, some hospitalists are burning out, there are examples of spotty quality and collaboration, and hospitalists continue to have to work to earn the respect of colleagues and patients that other specialists take for granted.
That said, the field of hospital medicine remains uniquely exciting, in part because it is so tightly linked to the broader changes in the health care policy landscape. Many other specialties see the profound changes underway in health care as an existential threat to their professional values and incomes. Hospitalists, on the other hand, see these changes as raising the pressure on hospitals to deliver the highest quality, most satisfying, and safest care at the lowest cost. Framed this way, forward‐thinking hospitalists quite naturally see these changes as yet another catalyst for the growth and indispensability of their field.
Many people date the start of the hospitalist field to my 1996 New England Journal of Medicine article,1 which first introduced the concept to a broad audience. That makes 2011 the field's 15th year, andif you have kidsyou know this is a tough and exciting age. The cuteness of childhood has faded, and bad decisions can no longer be excused as youthful indiscretions.
That's an apt metaphor for our field as we celebrate our 15th birthday. We are now an established part of the health care landscape, with a clear place in the House of Medicine. All of the measures of a successful specialty are ours: a thriving professional society, high‐quality training programs, increasingly robust research, a flourishing journal, and more. The field has truly arrived.
But these successes are also tempered by several challenges that have become more evident in recent years. In this article, I'll reflect on some of these successes and challenges.
The Hospitalist Field's Successes and Growth
In our 1996 article, Goldman and I1 wrote about the forces promoting the hospitalist model:
It seems unlikelythat high value care can be delivered in the hospital by physicians who spend only a small fraction of their time in this setting. As hospital stays become shorter and inpatient care becomes more intensive, a greater premium will be placed on the skill, experience, and availability of physicians caring for inpatients.
When we cited the search for value as a driving force in 1996, we were a bit ahead of our time, since there was relatively little skin in this game at the time. Remember that when our field launched, none of these value‐promoting forces existed: robust unannounced hospital inspections by the Joint Commission, public reporting of quality data, pay for performance, no pay for errors, state reporting of sentinel events, and more. In other words, until recently, neither a hospital's income stream nor its reputation was threatened by poor performance.
But this landscape is undergoing a sea change. By 2015, fully 9% of a hospital's Medicare reimbursements will be at risk through a variety of initiatives, including value‐based purchasing and meaningful use standards. And private payers are beginning to replicate Medicare's standards, particularly when they perceive that they may lead to both improved quality and lower costs.
Hospitals and health systems increasingly recognize how indispensable hospitalists can be as they demonstrate that their presence improves value. But this is only one of the forces driving the fieldalready the fastest growing specialty in medical historyto even higher levels of growth. These others include: the exodus of primary care physicians from the hospital, the fact that the specialists have left the building, comanagement of nonmedical patients, new opportunities in systems leadership, and dealing with housestaff duty hours reductions. I'll say a word about each.
The Exodus of Primary Care Physicians
In the early days of our field, one of the major sources of pushback was the desire of many primary care doctors to continue managing their own inpatients. Beginning a decade ago, this pressure began to abate, as many primary care physicians began to recognize the potential advantages of working with hospitalists.2
Over the next several years, I predict that the growth in the patient‐centered medical home model3with the physician's new responsibilities to provide comprehensive patient‐centered carewill make it even less likely that primary care doctors will have the time to manage their own inpatients. Luckily, information systems now being installed throughout the country (fueled by federal subsidies) will lead to unprecedented connectivity between the inpatient and outpatient worlds,4 hopefully resulting in improving handoffs.
Moreover, the increasing scrutiny of, and upcoming penalties for, high readmission rates are driving hospitals and clinics into creating more robust systems of care to improve inpatientoutpatient communications. The bottom line is that the main Achilles heel of hospitalist systemsthe handoff at hospital admission and dischargeshould improve over the next few years, making it easier than ever for primary care doctors to forego hospital care without losing track of critical patient information.
The Specialists Have Left the Building
One of the more interesting phenomena in the recent history of the hospitalist field is the growth of what I call hyphenated hospitalists: neurology hospitalists, ob‐gyn hospitalists, surgical hospitalists, and the like. The forces promoting these models are similar to those that catalyzed the hospitalist model: the recognition that bifurcating inpatient and outpatient care sometimes makes sense when several conditions are met (Table 1).
|
| 1) Is the number of inpatients who require the services of that specialty (either for consults or principal care) large enough to justify having at least one doctor in the house during daytime? |
| 2) Is the specialist frequently needed to see an inpatient urgently? |
| 3) Under the usual model of mixed inpatient and outpatient care, is the specialist frequently busy in the office, operating room, or procedural suite at times where they are urgently needed in the hospital (see #2)? |
| 4) Has the field become sub‐sub specialized, such that many covering physicians are now uncomfortable managing common acute inpatient problems (i.e., the headache neurologist asked to handle an acute stroke)? |
The emergence of hyphenated hospitalists raises all sorts of questions for the hospitalist field, many of which I have addressed elsewhere.5 But the bottom line is that the growth of specialty hospitalists may help create a new hospital home teama group of dedicated inpatient physicians spanning virtually every specialty who share best practices, work together on systems improvements, and operate under similar accountabilities. This development may well be the most exciting one in the field's recent history.
Comanagement of Nonmedical Patients
The same forces that led to the emergence of the hospitalist field are also catalyzing the growth of hospitalist comanagement programs. There is a shortage of general surgeons, and in teaching hospitals, there are fewer surgical residents available to help provide floor‐based pre‐ and post‐operative care. And surgical patients are under the same value pressures as medical patients, with increasing public reporting of quality processes and outcomes and new pay for performance programs coming on line. Although the evidence of benefit is mixed,68 many hospitalists are finding that increasing parts of their work involve comanagement.
Comanagement raises several issues, all of which need to be addressed. How do we define clear boundaries between what the hospitalist does and what the specialist does? Comanagement programs, to be effective, need very clear rules of engagement and open lines of communication to work through inevitable conflicts.6 How does the money flow? Most hospitalist programs receive hospital support, but it is legitimate to wonder whether the specialists, particularly surgeons, should chip in to support the program, particularly if they continue to collect a global case rate that was predicated on their provision of pre‐ and post‐operative care. How do comanagement programs and specialty hospitalist programs interrelate, and what are the relative advantages and disadvantages of each? To my mind, programs that meet the conditions outlined in Table 1 probably would do well to start a specialty hospitalist program, assuming that they can find high‐quality specialists to staff it. But there will be myriad variations on these themes. In my hospital, for example, we have both neurohospitalists and medical hospitalists who co‐manage neurosurgery patients.
New Opportunities in Systems Leadership
The growth of the hospitalist field will partly come from individuals who begin their careers performing clinical work, but who transition over time to managerial and leadership roles. This is a natural transition: Who better than a hospitalist to help organize and deliver educational programs, manage clinical operations, implement information technology systems, or lead quality, safety, or utilization management efforts? Of course, as hospitalists assume these roles, others need to take their places covering their clinical shifts.
This might seem like a relatively unimportant driver of personnel growth, but in more advanced systems, it can become a major one. Table 2 lists the faculty in my Division of Hospital Medicine at the University of California, San Francisco (UCSF) who have major institutional (i.e., nondivisional) roles. These roles, spread across eight faculty, account for 3.7 full‐time equivalents (FTEs).
| Role | Works for Whom? | Approximate % FTE |
|---|---|---|
| ||
| Associate Chief Medical Officer | Medical Center | 80% |
| Associate Medical Director for Information Technology | Medical Center | 80% |
| Associate Chair for Quality and Safety | Department of Medicine | 50% |
| Director of Quality and Patient Safety | Department of Neurosurgery | 50% |
| Associate Medicine Residency Director (two people) | Department of Medicine | 30% (for each) |
| Director of Medical Student Clerkships | Department of Medicine | 25% |
| Director of Patient Safety/Quality Programs | Office of Graduate Medical Education, School of Medicine | 25% |
| Total FTEs: 3.7 | ||
Dealing with New ACGME Regulations
In 2003, the Accreditation Council for Graduate Medical Education (ACGME) issued its first housestaff duty hours reductions (limiting housestaff to a maximum of 80 hours per week, with no single shift lasting longer than 30 hours). This reduction led to the development of nonteaching services in most teaching hospitals; the vast majority of such programs have hospitalists at their core.
In July 2011, new ACGME regulations go into effect,10 which will further cut the availability of housestaff to cover clinical services. Although the 80‐hour weekly limit remains, intern shifts are now limited to 16 hours, meaning that the traditional long call system involving interns must be replaced by a shift‐based system. Like the earlier changes, these new regulations are leading to additional hospitalist growth in the nation's teaching hospitals. By the time the changes are fully implemented, many hospitalist programs will have half or more of their hospitalist FTEs devoted to covering patients previously cared for by residents.
Challenges for Hospitalist Programs
These powerful forces promoting the growth in the hospitalist field continue to ensure that hospitalists are in high demand. As a practical matter, this has resulted in increasing salaries and improved job conditions for hospitalists.
But this growth brings many challenges. Many hospitalist programs are poorly managed, often because the leaders lack the training and experience to effectively run such a rapidly growing and complex enterprise. One manifestation of these leadership challenges is that schedules are often created around the convenience and desires of the physicians rather than the needs of the patients. For example, the increasingly prevalent seven‐days‐on, seven‐days‐off schedule often leads to burnout and a feeling by the hospitalists that they are working too hard. Yet many groups are unwilling to consider modifications to the schedule that might decrease the intensity, if the cost is fewer days off.
On the other hand, some groups pay little attention to patient continuity in constructing their schedules. I know of programs that schedule their hospitalists in 24‐hour shifts (followed by a few days off), which means that admitted patients will see a different hospitalist every day. I see this as highly problematic, particularly because the most common complaint I hear from patients about hospitalist programs is that I saw a different doctor every day.
Many of the field's challenges stem from hospitalists' near‐total dependency on hospital funding to create sustainable job descriptions.11 While I continue to believe that this bit of financial happenstance has been good for both hospitalists and hospitalssince it has driven uncommon degrees of interdependency and alignmentit does mean that a difficult budget battle is virtually assured every year. As hospital finances become tighter, one can expect these battles to grow even more heated. Speaking for hospitalists, I am not too worried about the outcomes of these battles, since hospitalists provide a mission‐critical service at a fair price, there are no viable lower‐priced replacements (expect perhaps for nonphysician providers such as nurse practitioners for the less‐complex patients), and hospitalists are extraordinarily mobilethere are virtually no barriers for a hospitalist, or an entire group, to transfer to another institution. Nevertheless, it seems inevitable that these battles will leave scars, scars that may ultimately compromise the crucial collaboration that both hospitalists and hospitals depend on.
The Bottom Line
Even at age 15, an age at which many adolescents are irredeemably cynical, the hospitalist field retains much of its sense of limitless possibility and exuberance. This is not because things are perfectthey are not. Some hospitalist jobs are poorly constructed, some groups have poor leadership, some hospitalists are burning out, there are examples of spotty quality and collaboration, and hospitalists continue to have to work to earn the respect of colleagues and patients that other specialists take for granted.
That said, the field of hospital medicine remains uniquely exciting, in part because it is so tightly linked to the broader changes in the health care policy landscape. Many other specialties see the profound changes underway in health care as an existential threat to their professional values and incomes. Hospitalists, on the other hand, see these changes as raising the pressure on hospitals to deliver the highest quality, most satisfying, and safest care at the lowest cost. Framed this way, forward‐thinking hospitalists quite naturally see these changes as yet another catalyst for the growth and indispensability of their field.
- ,.The emerging role of “hospitalists” in the American health care system.N Engl J Med.1996;335:514–517.
- ,,,.How physicians perceive hospitalist services after implementation: Anticipation vs reality.Arch Intern Med.2003;163:2330–2336.
- ,,.Medical homes: Challenges in translating theory into practice.Med Care.2009;47:714–722.
- ,.Improving safety with information technology.N Engl J Med.2003;348:2526–2534.
- The New Home Team: The Remarkable Rise of the Hyphenated Hospitalist. Wachter's World blog, January 16,2011. Available at: http://tinyurl. com/4h2jy7e. Accessed February 12, 2011.
- ,,, et al.Comanagement of surgical patients between neurosurgeons and hospitalists.Arch Intern Med.2010;170:2004–2010.
- ,,, et al.Medical and surgical comanagement after elective hip and knee arthroplasty: A randomized, controlled trial.Ann Intern Med.2004;141:28–38.
- ,,,,.Comanagement of hospitalized surgical patients by medicine physicians in the United States.Arch Intern Med.2010;170:363–368.
- ,,.Neurohospitalists: An emerging model for inpatient neurological care.Ann Neurol.2008;63:135–140.
- ,,;ACGME Duty Hour Task Force. The new recommendations on duty hours from the ACGME Task Force.N Engl J Med.2010 Jul 8;363(2):e3. Epub 2010 Jun 23. PubMed PMID: 20573917. The website is here: http://www.nejm.org/doi/full/10.1056/NEJMsb1005800.
- .Elevated expectations.The Hospitalist. January2011. Available at: http://www.the‐hospitalist.org/details/article/972781/Elevated_Expectations.html. Accessed February 12, 2011.
- ,.The emerging role of “hospitalists” in the American health care system.N Engl J Med.1996;335:514–517.
- ,,,.How physicians perceive hospitalist services after implementation: Anticipation vs reality.Arch Intern Med.2003;163:2330–2336.
- ,,.Medical homes: Challenges in translating theory into practice.Med Care.2009;47:714–722.
- ,.Improving safety with information technology.N Engl J Med.2003;348:2526–2534.
- The New Home Team: The Remarkable Rise of the Hyphenated Hospitalist. Wachter's World blog, January 16,2011. Available at: http://tinyurl. com/4h2jy7e. Accessed February 12, 2011.
- ,,, et al.Comanagement of surgical patients between neurosurgeons and hospitalists.Arch Intern Med.2010;170:2004–2010.
- ,,, et al.Medical and surgical comanagement after elective hip and knee arthroplasty: A randomized, controlled trial.Ann Intern Med.2004;141:28–38.
- ,,,,.Comanagement of hospitalized surgical patients by medicine physicians in the United States.Arch Intern Med.2010;170:363–368.
- ,,.Neurohospitalists: An emerging model for inpatient neurological care.Ann Neurol.2008;63:135–140.
- ,,;ACGME Duty Hour Task Force. The new recommendations on duty hours from the ACGME Task Force.N Engl J Med.2010 Jul 8;363(2):e3. Epub 2010 Jun 23. PubMed PMID: 20573917. The website is here: http://www.nejm.org/doi/full/10.1056/NEJMsb1005800.
- .Elevated expectations.The Hospitalist. January2011. Available at: http://www.the‐hospitalist.org/details/article/972781/Elevated_Expectations.html. Accessed February 12, 2011.
Assessment Tool in the Hands of Medical Residents
Venous thromboembolic events (VTE) are a significant cause of mortality in hospitalized medical and surgical patients.1, 2 The incidence of hospital‐acquired deep vein thrombosis (DVT) is 1040% among medical or general surgical patients in the absence of VTE prophylaxis.3 Approximately 5075% of these cases are preventable with appropriate prophylaxis.3, 4 To reduce hospital‐acquired VTE, the American College of Chest Physicians (ACCP) has published VTE prevention guidelines regularly since 1986. The latest version of the ACCP guidelines recommends that every hospital have an institution‐wide policy that encourages use of VTE prevention strategies. Nevertheless, current evidence demonstrates that VTE prophylaxis remains underutilized in at‐risk patients.5, 6 For example, the ENDORSE study showed that only 39% of at‐risk medical patients received appropriate VTE prophylaxis.6 A more recent study estimated that 58% of hospital‐acquired VTEs were preventable with appropriate prophylaxis utilization.7
Such data demonstrate that a quality gap exists between VTE prophylaxis guideline recommendations and actual practice. Such a gap highlights the need to identify barriers to appropriate implementation of systems‐based strategies aimed at preventing VTE. A presumed barrier for adherence to any VTE protocol is the complexity involved in performing individualized risk assessments.810 All VTE prophylaxis strategies require the clinician to risk‐stratify each patient, identify contraindications to a prophylaxis strategy, and select an accepted strategy. While many VTE risk assessment protocols exist, they tend to fall into two categories: 1) a point‐based system, and 2) a simplified tiered system. Point‐based clinical prediction rules have been advocated by Caprini and others.1115 Such approaches require the clinician to assign points during the identification of VTE risk factors. The clinician must add the points to determine a patient's cumulative VTE risk and use the points to classify that risk as low, moderate, or high. Such point‐based systems are generally considered complex and may underestimate VTE risk, potentially leading to underutilization of prophylaxis strategies.16
Studies have demonstrated that complexity introduces variation into the decision‐making process.17 As a result, both the ACCP and SHM advocate for simplifying the VTE risk assessment process.4, 18 To date, several studies have demonstrated that attending physicians and nurses can reliably apply a VTE risk assessment tool, but none that measure how reliably residents can perform this task when using a point‐based tool.18, 19 For academic medical centers, information about the reliability of such tools is especially important, since they will often be applied by physicians‐in‐training, who have limited knowledge and experience with VTE guidelines and risk assessment. The goal of our study, therefore, was to use clinical vignettes to determine the reliability and protocol adherence of medical residents' application of an adapted point‐based VTE risk assessment tool, independent of other interventions.
Methods
Development of the Risk Assessment Tool
A multispecialty team adapted existing individualized VTE risk assessment tools based on one developed by Caprini.11 The VTE tool (Fig. 1) was designed to assist residents in making two essential determinations prior to ordering a prophylaxis plan. The first determination was the calculation of a total risk score (070 points). This score was determined by identifying and assigning a point value to all medical and surgical risk factors, and summing the points into 3 categories: low (01 point), moderate (24 points), and high (>4 points) VTE risk. The second determination was to identify any contraindications to pharmacological prophylaxis. Like other nonvalidated tools, our tool divided contraindications into absolute or relative. After making these two determinations, residents were encouraged to order 1 of 6 VTE prophylaxis plans. These plans were intended to balance VTE risk against risk factors for bleeding due to prophylaxis.
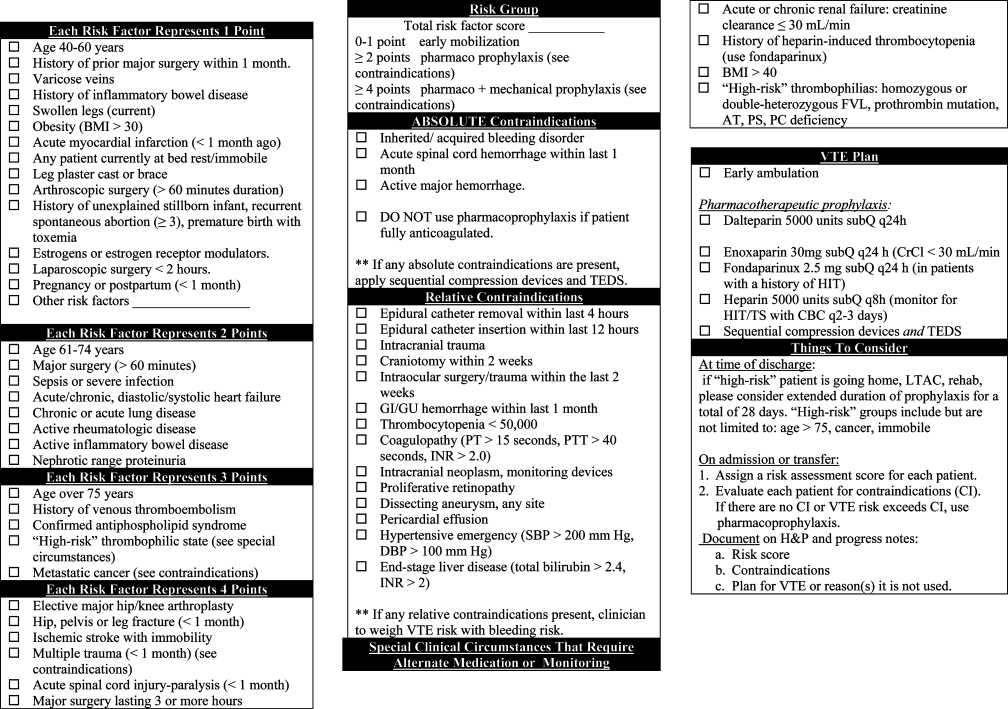
Construction of Clinical Vignettes
Approval was obtained by the Pennsylvania State University Institutional Review Board. Since previous research demonstrates the utility of clinical vignettes to study the effectiveness of guideline application and decision making, we used a series of 21 randomly selected and de‐identified clinical vignettes to portray a range of real‐world patient admission scenarios.2022 We identified individuals who had been admitted to the Hershey Medical Center using data from the inpatient electronic health record and applying the following inclusion criteria: age >17 years, and admission to a general medical service from the Emergency Department during a 14‐day period in 2008. Since more than 80% of patients admitted to our medical service are admitted through the Emergency Department and residents place all of the admission orders, our goal was to use vignettes that were typical of patients they commonly admit. We attached a paper form of our institution's VTE prophylaxis strategy (Figure 1) to each vignette.
Data Collection
A 1‐hour noon conference titled VTE Workshop was conducted by one of the authors (M.J.B.) during the first quarter of the 2008 academic year. We asked the medical residents to apply the VTE prophylaxis protocol to 21 vignettes during this session. In order to determine the appropriate time allotted to complete the vignettes, each case was completed by M.J.B. and three medical residents (one intern, one second year, and one third year) prior to conducting the VTE workshop. Based on these data, we determined that the median time to complete each vignette was 2 minutes and 15 seconds (range 30 seconds to 7 minutes). Therefore, we assumed that the 21 vignettes could be completed within 1 hour. At the beginning of the conference, the residents were provided with 10 minutes of verbal instruction about how to apply the VTE risk assessment tool. They were instructed to provide a total risk score (070 points) and, based on the total score, to classify each patient as low, moderate, or high risk for VTE. Following the risk assignment, they were instructed to document any absolute and relative contraindications. Finally, they were asked to select the most appropriate VTE prophylaxis plan according to the recommendations of the protocol. Vignettes were considered complete if they had an assessment and plan for >75% of the cases.
Prior to conducting this study, there had been no formal orientation regarding use of the VTE risk assessment tool or incorporation of it into our institution's computerized order entry system. Average attendance for the noon conference is between 20 and 30 house staff, approximately one‐third of the entire residency. Medical students were excluded from the study. All respondents voluntarily and anonymously performed the assessments, and indicated on the front of their vignette packet their level of training as PGY‐1, ‐2, or ‐3. The sessions were overseen by one of the authors to ensure that no communication occurred among the residents.
Data Analysis
We constructed a database with five variables collected from each resident's VTE risk assessment form: 1) a total risk score, 2) a risk classification (low, medium, or high), 3) the number and type of absolute contraindications to pharmacological prophylaxis, 4) the number and type of relative contraindications to pharmacological prophylaxis, and 5) a VTE prophylaxis plan. The lead author also performed these assessments of the 21 vignettes 1 month prior to the resident session. In power calculations performed prior to the session, we determined that the study would need at least 300 observations in order to calculate inter‐rater reliability.23 With the estimation that between 20 and 30 residents would attend, we determined that 21 vignettes would exceed the minimum required observations to allow for an accurate calculation of inter‐rater reliability.
The total risk score was treated as a continuous variable for which the intra‐class correlation (ICC) was calculated. The ICC is used to assess the consistency, or conformity, of measurements made by multiple observers measuring the same quantity.24 Risk stratification, presence of absolute and relative contraindications, and VTE plan were treated as categorical variables. For these, we used Cohen's kappa to assess variability in resident ratings. The kappa score has been used in other studies to determine inter‐rater reliability using similar VTE risk assessment tools.18, 19 Finally, adherence to our hospital's protocol was determined by comparing the residents' VTE plans with the lead author's VTE plans for each of the 21 vignettes. We used SAS 9.1.3 for all statistical analyses (SAS Institute, Cary, NC).
Results
Twenty‐six medical residents attended the conference. Three residents left without submitting their assessments and were excluded from the analysis. Of the 23 residents included in the analysis, 15 (65%) were interns, 5 (22%) second‐year residents, and 3 (13%) third‐year residents. A maximum of 483 observations (21 clinical vignettes and 23 residents) was possible. Six (1%) risk stratifications were missing, and 14 (3%) VTE prophylaxis plans were missing. Therefore, out of a possible 483 paired assessments and plans, complete data existed for 95% (469) of the observations. Residents risk‐stratified the vignettes as low risk for 27% of cases, moderate risk for 38%, and high risk 34%. These differed from those of the lead author, who stratified proportionately more vignettes as high risk (Table 1).
| Risk Stratification | Resident no./total (%) | Attending no./total (%) |
|---|---|---|
| Low | 130/479 (27) | 3/21 (14) |
| Moderate | 183/479 (38) | 7/21 (33) |
| High | 166/479 (34) | 11/21 (52) |
Of those vignette patients stratified as high risk, 77% (128/166) received some form of prophylaxis. Of those stratified as moderate risk, 66% (121/183) received some form of prophylaxis. Finally, of those stratified as low risk, 15% (20/130) received some form of prophylaxis. To explore the impact of the disparity in risk assessments between residents and attending physicians, we used the lead author's assessments as the standard for comparison, and determined that only 64% (309/479) of the observations were risk‐stratified correctly. To emphasize further the potential negative impact of these misclassifications, we determined that appropriate plans would have occurred only 47% of the time. Analysis of these data via risk category showed that low‐risk patients received appropriate prophylaxis 84% of the time. However, protocol adherence for moderate and high‐risk patients occurred only 33 and 40% of the time, respectively (Table 2). Making the assumption that those vignette patients at moderate and high risk who only received mechanical prophylaxis had appropriate contraindications to heparin prophylaxis, protocol adherence remained low at 54 and 58%, respectively.
| Attending Classification | Total | Appropriate Risk Assessment No. (%) | SCDs Only No. (%) | Heparin Only | Both Heparin and SCDs | Ambulation |
|---|---|---|---|---|---|---|
| ||||||
| Low risk | 115 | 86 (75) | 11 (10) | 7 (6) | 0 | 93 (84)a |
| Moderate risk | 138 | 85 (62) | 28 (21)b | 44 (33)a | 16 (12) | 47 (35) |
| High risk | 230 | 138 (60) | 39 (18)b | 69 (31) | 88 (40)a | 27 (12) |
| Total | 483 | 309 (64) | 78 (16) | 120 (26) | 104 (22) | 167 (36) |
The ICC for the total risk score was 0.66, and the kappa coefficient for risk stratification was 0.51 (95% CI 0.50, 0.53), both of which represent moderate agreement. Absolute and relative contraindications were identified 12% (57/483) and 13% (61/483) of the time, respectively. The kappa scores for absolute and relative contraindications were 0.29 and 0.23, respectively. The kappa score for the VTE plan was 0.28 and represents only fair agreement (Table 2).
Subgroup analysis of the 15 intern participants for ICC for the risk score was 0.63. The kappa scores for risk stratification and VTE plan were 0.47 and 0.23, respectively. The kappa scores for senior residents represent aggregate data of 168 observations of second‐ and third‐year residents. For senior residents, the kappa scores for risk stratification and VTE plan were 0.61 and 0.35, respectively (Table 3).
| Risk Score | Stratification | Absolute Contraindication | Relative Contraindication | VTE Plan | ||
|---|---|---|---|---|---|---|
| ||||||
| Aggregate | ICC | 0.66 | ||||
| Kappa | 0.51 | 0.29 | 0.23 | 0.28 | ||
| Intern | ICC | 0.63 | ||||
| Kappa | 0.47 | NA | NA | 0.23 | ||
| Seniora | ICC | 0.73 | ||||
| Kappa | 0.61 | NA | NA | 0.35 | ||
Discussion
We performed this study to determine how reliably our medical residents could apply a point‐based VTE risk assessment tool, similar to those published previously.11 We observed that early in the academic year, our residents were not able to use this tool reliably. While our study does not evaluate the effects of audit and feedback, reminder alerts, or educational interventions, an important first step toward quality improvement in VTE prophylaxis is to reduce variability in risk assessment and decision making. In this endeavor, our results differ markedly from those in the literature. For instance, one study used 3 trained nurses to employ a similar risk assessment tool, and found an ICC of 0.98 for overall assessments of VTE risk, but did not report protocol adherence.19 Another study found inter‐rater reliability to be high among 5 physician observers (kappa scores of 0.81 and 0.90 for risk stratification and VTE plan, respectively).18 These two studies evaluated the performance of experienced evaluators who employed different and simpler VTE risk assessment tools. Our study determined that the inter‐rater reliability of risk assessment and VTE plan between residents using a point‐based VTE risk assessment tool was significantly lower, at 0.51 and 0.28, respectively. There was marked disparity between the lead author's and residents' risk assessments of those deemed to be at low and high risk (Table 1). While both determined approximately one‐third of the patient vignettes to be at moderate risk, the residents misclassified those at high risk in comparison with the author's assessments. This underestimation of VTE risk could lead to profound underprophylaxis in at‐risk patients. To the extent that our findings represent those in other teaching hospitals, such errors could hinder VTE quality improvement efforts in such institutions.
Previous studies that successfully improved VTE prophylaxis rates coupled a risk assessment tool with provider education as well as audit‐and‐feedback interventions.25, 26 In one study, provider education occurred on the first day of every month with an orientation to the hospital's recommended VTE prevention strategies.26 Another study sought to improve the rates of VTE prophylaxis in medical intensive care (MICU) patients without performing individualized risk assessment.27 Using only weekly graphic feedback and verbal reminders to the medical team, it showed an improvement in VTE prophylaxis for 1 year. A third study improved VTE prophylaxis adherence and reduced VTE at 90 days using only reminder alerts.28 Interestingly, several studies reduced the incidence of VTE without employing any patient risk stratification.29 These studies suggest that improvement in VTE prophylaxis rates could have occurred as result of audit‐and‐feedback or reminder systems and perhaps independent of the reliable application of a risk assessment tool.29 The studies that used risk assessment tools with layered interventions make it difficult to interpret whether the tool or the layered interventions were responsible for the improvement in VTE outcomes. Ours is the first study to evaluate how reliably residents can apply a tool independent of other interventions. With only fair to moderate resident agreement in patient risk assessment and VTE plan, our study suggests that the complexity of a point‐based risk assessment protocol (as opposed to a simplified three‐tiered approach) may affect resident prescriptive behavior.
As a result, our study corroborates two things: first, in medical centers that rely on residents to perform VTE prevention using individualized risk assessment, a multilayered approach for VTE prevention must occur. Second, a passively disseminated VTE protocol in the form of a pocket card will most likely not create a sustained improvement in VTE prophylaxis rates or reduce VTE.3035
When addressing certain aspects of quality improvement and safety, teaching hospitals must recognize that their efforts largely rely on resident performance. The 2009 National Resident Matching Program data indicate that there are 22,427 intern positions available in the United States. Often it is the resident's responsibility to perform risk assessments and provide prophylaxis, possibly using a tool that is too complex to apply reliably. Several studies have determined that 65% of medical errors were committed by interns and that 35‐44% of those errors resulted from knowledge deficits.3638 In order to best improve adherence to clinical guidelines, strategies that result in changing physician behavior need to be implemented, and can include but are not limited to the ones found in Figure 2.39 Ideally, teaching centers with computerized order entry should embed the risk assessment process as part of an admission/transfer order set, with a reminder alert. The alert would be activated when at‐risk patients do not receive appropriate prophylaxis. Most alert systems require hospitals to have computerized order entry, which has achieved only 20% market penetration in US hospitals.40, 41 Therefore, some hospitals employ, or intend to employ, passively disseminated risk assessment tools in the form of pocket cards or preprinted forms. These methods are estimated to improve prophylaxis by only 50% and are therefore not considered to be highly reliable strategies.3135
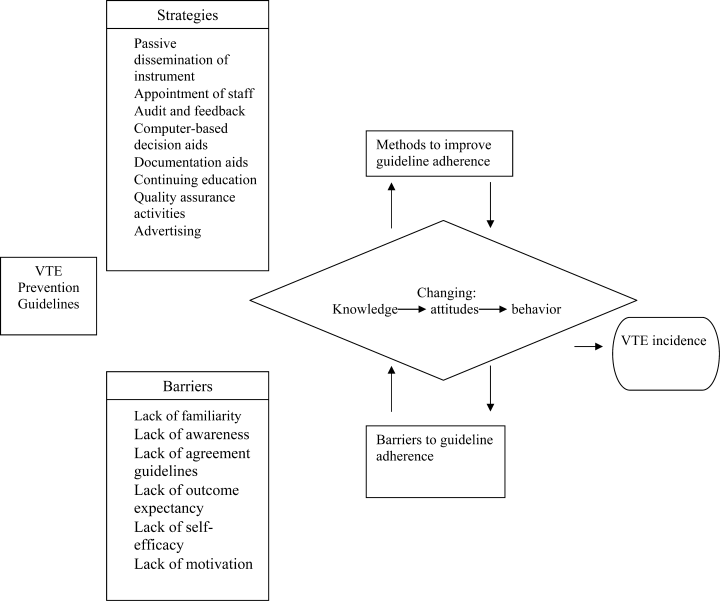
Our study demonstrates only fair to moderate reliability of a point‐based VTE risk assessment tool when used by residents independent of other strategies. It also suggests that residents underestimate those at high risk. In addition, our residents' protocol adherence was suboptimal and would have resulted in appropriate prophylaxis approximately 50% of the time in patients at moderate or high VTE risk. Therefore, when risk assessment tools such as ours are used, it is imperative that frequent education be combined with real‐time patient identification strategies as well as audit and feedback, a process called measure‐vention.13, 14 This is especially true when the risk assessment process is not linked to a reminder system as part of computer‐assisted order entry protocols.
A limitation of our study was the lack of a control group. Since all the residents in attendance received the same clinical vignettes, it would have been of interest to see how the risk assessment tool performed compared with residents who did not have access to the tool. However, based on average noon conference attendance, it would have been difficult to achieve an adequate number of observations to calculate credible ICC and kappa scores. Other limitations include the high number of interns who completed the vignettes compared with senior residents, and the lack of additional attending reviewers to score the vignettes prior to the session. Ideally, in determining the accuracy of protocol adherence, we would have compared residents' determinations with those of several experts who had used an adjudication process in the event of disagreement. In our ongoing work, we are collecting data from a representative sample of attending physicians at our hospital to compare their assessments both with each other and with those of the residents.
Another issue in our design was that the study presented only a limited amount of medical information in the vignettes. In actual clinical circumstances, the amount of historical information is greater and more complicated. One could argue that the artificiality of clinical vignettes is not an accurate representation of resident performance when ordering VTE prophylaxis. However, this approach limits case‐mix variation, so residents should have been able to reach similar conclusions with the information given. Thus the limited information should have maximized residents' intentions to prescribe VTE prophylaxis, and kappa scores would likely be lower in real clinical settings. Finally, our kappa scores were calculated based on aggregate data of interns and residents; however, interns comprised almost two‐thirds of the resident participants. As reported in the results section, intern inter‐rater reliability was slightly lower compared with the senior resident subgroup, suggesting that the variability may be a result of less clinical experience of the interns. However, the study was not powered to assess differences in kappa scores for level of training.
In conclusion, we determined the inter‐rater reliability of an individualized, point‐based VTE risk assessment tool when used by medical residents unfamiliar with its use. Our study showed that under conditions of minimal education, a point‐based VTE assessment tool achieves only fair to moderate reliability. It also suggests that as a stand‐alone tool without a reminder alert, adherence to VTE prevention guidelines is suboptimal and might result in underprophylaxis of hospitalized medical patients at moderate or high VTE risk. In fact, with appropriate prophylaxis, rates were maximally estimated to be 55% (Table 2). Because of the high percentage of interns in the study, these results approximate intern application of a VTE prevention protocol independent of other interventions. Comparing reliability data from our study with those of others raises the question of whether the observed differences in kappa score are because other studies used highly trained observers or because their protocols were less complex. However, a recent study validated a simpler method of VTE risk grouping that performs well regardless of clinical experience.20 Future studies are needed to determine whether there is improved resident inter‐rater reliability using a point‐based risk assessment tool that is embedded into a computerized order entry system with electronic reminder alerts. Finally, in actual clinical settings, the question remains of whether kappa scores correlate with protocol adherence, prophylaxis rates, and VTE reduction when using point‐based tools. If not, then the use of simplified risk‐stratification tools and VTE measure‐vention strategies should be implemented.
Acknowledgements
The authors thank Lisabeth V. Scalzi, MD, MS, Lora Moyer, Kevin McKenna MD, Hammid Al‐Mondhiry, Lucille Anderson, MD, Kathleen Williams, Kevin Larraway, Cynthia Chuang, MD, MS, the residents of the Internal Medicine and Combined Medicine/Pediatrics residencies, and the division of General Internal Medicine at Hershey Medical Center.
- ,,.Autopsy‐verified pulmonary embolism in a surgical department: analysis of the period from 1951 to 1988.Br J Surg.1991;78(7):849–852.
- , , , , et al.Fatal pulmonary embolism in hospitalised patients: a necropsy review.J Clin Pathol.2004;57(12):1254–1257.
- , , , et al.Prevention of venous thromboembolism: American College of Chest Physicians Evidence‐Based Clinical Practice Guidelines (8th edition).Chest.2008;133(6 suppl):381S–453S.
- ,.Coronary artery vasculitis and myocardial infarction with systemic lupus erythematosus.NY State J Med.1974;74(5):873–874.
- , , , et al.Venous thromboembolism prophylaxis in acutely ill hospitalized medical patients: findings from the International Medical Prevention Registry on Venous Thromboembolism.Chest.2007;132(3):936–945.
- , , , et al.Venous thromboembolism risk and prophylaxis in the acute hospital care setting (ENDORSE study): a multinational cross‐sectional study.Lancet.2008;371(9610):387–394.
- , , , .Venous thromboembolic events in hospitalised medical patients.Thromb Haemost.2009;102(3):505–510.
- ,,.Compliance with recommended prophylaxis for venous thromboembolism: improving the use and rate of uptake of clinical practice guidelines.J Thromb Haemost.2004;2(2):221–227.
- , , , et al.A systematic review of strategies to improve prophylaxis for venous thromboembolism in hospitals.Ann Surg.2005;241(3):397–415.
- ,.Prevention of venous thromboembolism: consensus, controversies, and challenges.Hematol Am Soc Hematol Educ Program.2009:286–292.
- ,,.International perspective on venous thromboembolism prophylaxis in surgery.Semin Thromb Hemost.1991;17(4):322–325.
- , , , et al.Clinical assessment of venous thromboembolic risk in surgical patients.Semin Thromb Hemost.1991;17(suppl 3):304–312.
- , et al.Risk factor assessment in the management of patients with suspected deep venous thrombosis.Int Angiol.2000;19(1):47–51.
- ,,.Effective risk stratification of surgical and nonsurgical patients for venous thromboembolic disease.Semin Hematol.2001;38(2 suppl 5):12–19.
- .Thrombosis risk assessment as a guide to quality patient care.Dis Mon.2005;51(2–3):70–78.
- ,.Designing and implementing effective venous thromboembolism prevention protocols: lessons from collaborative efforts.J Thromb Thrombolysis.2010;29(2):159–166.
- ,,.Emergency medicine practitioner knowledge and use of decision rules for the evaluation of patients with suspected pulmonary embolism: variations by practice setting and training level.Acad Emerg Med.2007;14(1):53–57.
- , , , , , , , .Optimizing prevention of hospital‐acquired (HA) venous thromboembolism (VTE): prospective validation of a VTE risk assessment model (RAM).J Hosp Med.2010;5(1):10–18..
- , , , et al.Development and testing of a DVT risk assessment tool: providing evidence of validity and reliability.Worldviews Evid Based Nurs.2007;4(1):14–20.
- , , , et al.A novel educational strategy to enhance internal medicine residents' familial colorectal cancer knowledge and risk assessment skills.Am J Gastroenterol.2005;100(3):677–684.
- ,, , , , .Impact of formal continuing medical education: do conferences, workshops, rounds, and other traditional continuing education activities change physician behavior or health care outcomes?JAMA.1999;282(9):867–874.
- , , , et al.Comparison of vignettes, standardized patients, and chart abstraction: a prospective validation study of 3 methods for measuring quality.JAMA.2000;283(13):1715–1722.
- .Psychometric Theory.New Delhi:Tate McGraw‐Hill;1981.
- ,.Intraclass correlations: uses in assessing rater reliability.Psychol Bull.1979;86(2):420–428.
- ,,.Innovative approaches to increase deep vein thrombosis prophylaxis rate resulting in a decrease in hospital‐acquired deep vein thrombosis at a tertiary‐care teaching hospital.J Hosp Med.2008;3(2):148–155.
- ,,.Improved use of thromboprophylaxis for deep vein thrombosis following an educational intervention.J Hosp Med.2006;1(6):331–338.
- , , , et al.Minimizing errors of omission: behavioural reenforcement of heparin to avert venous emboli: the BEHAVE study.Crit Care Med.2006;34(3):694–699.
- , , , et al.Electronic alerts to prevent venous thromboembolism among hospitalized patients.N Engl J Med.2005;352(10):969–977.
- , , , .Getting a validated guideline into local practice: implementation and audit of the SIGN guideline on the prevention of deep vein thrombosis in a district general hospital.Scott Med J.1998;43(1):23–25.
- ,,. Improving the Reliability of Health Care. IHI Innovation Series white paper 2004. Available at: www.IHI.org/IHI/Results/WhitePapers/Improving theReliabilityof HealthCare.htm. Accessed October 4,2010.
- , , , et al.Thrombosis prophylaxis in medical patients: a retrospective review of clinical practice patterns.Haematologica.2002;87(7):746–750; discussion 250.
- ,.Genetic epidemiology: systemic lupus erythematosus.Arthritis Res.2001;3(6):331–336.
- ,,.Missed opportunities for prevention of venous thromboembolism: an evaluation of the use of thromboprophylaxis guidelines.Chest.2001;120(6):1964–1971.
- , , , , .Underuse of venous thromboembolism prophylaxis for general surgery patients: physician practices in the community hospital setting.Arch Intern Med.1998;158(17):1909–1912.
- ,,.Venous thromboembolism prophylaxis used by consultant general surgeons in Scotland.J R Coll Surg Edinb.2001;46(6):329–333.
- ,.Ordering errors by first‐year residents: evidence of learning from mistakes.Mo Med.2004;101(2):128–131.
- ,.Prevention of medication errors: teaching and training.Br J Clin Pharmacol.2009;67(6):656–661.
- ,,.Reducing medication errors in a surgical residency training program.Am Surg.2004;70(5):467–471.
- , , , et al.Why don't physicians follow clinical practice guidelines? A framework for improvement.JAMA.1999;282(15):1458–1465.
- , , , , .Predicting computerized physician order entry system adoption in US hospitals: can the federal mandate be met?Int J Med Inform.2008;77(8):539–545.
- ,.Implementation of computerized physician order entry in seven countries.Health Aff (Millwood).2009;28(2):404–414.
Venous thromboembolic events (VTE) are a significant cause of mortality in hospitalized medical and surgical patients.1, 2 The incidence of hospital‐acquired deep vein thrombosis (DVT) is 1040% among medical or general surgical patients in the absence of VTE prophylaxis.3 Approximately 5075% of these cases are preventable with appropriate prophylaxis.3, 4 To reduce hospital‐acquired VTE, the American College of Chest Physicians (ACCP) has published VTE prevention guidelines regularly since 1986. The latest version of the ACCP guidelines recommends that every hospital have an institution‐wide policy that encourages use of VTE prevention strategies. Nevertheless, current evidence demonstrates that VTE prophylaxis remains underutilized in at‐risk patients.5, 6 For example, the ENDORSE study showed that only 39% of at‐risk medical patients received appropriate VTE prophylaxis.6 A more recent study estimated that 58% of hospital‐acquired VTEs were preventable with appropriate prophylaxis utilization.7
Such data demonstrate that a quality gap exists between VTE prophylaxis guideline recommendations and actual practice. Such a gap highlights the need to identify barriers to appropriate implementation of systems‐based strategies aimed at preventing VTE. A presumed barrier for adherence to any VTE protocol is the complexity involved in performing individualized risk assessments.810 All VTE prophylaxis strategies require the clinician to risk‐stratify each patient, identify contraindications to a prophylaxis strategy, and select an accepted strategy. While many VTE risk assessment protocols exist, they tend to fall into two categories: 1) a point‐based system, and 2) a simplified tiered system. Point‐based clinical prediction rules have been advocated by Caprini and others.1115 Such approaches require the clinician to assign points during the identification of VTE risk factors. The clinician must add the points to determine a patient's cumulative VTE risk and use the points to classify that risk as low, moderate, or high. Such point‐based systems are generally considered complex and may underestimate VTE risk, potentially leading to underutilization of prophylaxis strategies.16
Studies have demonstrated that complexity introduces variation into the decision‐making process.17 As a result, both the ACCP and SHM advocate for simplifying the VTE risk assessment process.4, 18 To date, several studies have demonstrated that attending physicians and nurses can reliably apply a VTE risk assessment tool, but none that measure how reliably residents can perform this task when using a point‐based tool.18, 19 For academic medical centers, information about the reliability of such tools is especially important, since they will often be applied by physicians‐in‐training, who have limited knowledge and experience with VTE guidelines and risk assessment. The goal of our study, therefore, was to use clinical vignettes to determine the reliability and protocol adherence of medical residents' application of an adapted point‐based VTE risk assessment tool, independent of other interventions.
Methods
Development of the Risk Assessment Tool
A multispecialty team adapted existing individualized VTE risk assessment tools based on one developed by Caprini.11 The VTE tool (Fig. 1) was designed to assist residents in making two essential determinations prior to ordering a prophylaxis plan. The first determination was the calculation of a total risk score (070 points). This score was determined by identifying and assigning a point value to all medical and surgical risk factors, and summing the points into 3 categories: low (01 point), moderate (24 points), and high (>4 points) VTE risk. The second determination was to identify any contraindications to pharmacological prophylaxis. Like other nonvalidated tools, our tool divided contraindications into absolute or relative. After making these two determinations, residents were encouraged to order 1 of 6 VTE prophylaxis plans. These plans were intended to balance VTE risk against risk factors for bleeding due to prophylaxis.

Construction of Clinical Vignettes
Approval was obtained by the Pennsylvania State University Institutional Review Board. Since previous research demonstrates the utility of clinical vignettes to study the effectiveness of guideline application and decision making, we used a series of 21 randomly selected and de‐identified clinical vignettes to portray a range of real‐world patient admission scenarios.2022 We identified individuals who had been admitted to the Hershey Medical Center using data from the inpatient electronic health record and applying the following inclusion criteria: age >17 years, and admission to a general medical service from the Emergency Department during a 14‐day period in 2008. Since more than 80% of patients admitted to our medical service are admitted through the Emergency Department and residents place all of the admission orders, our goal was to use vignettes that were typical of patients they commonly admit. We attached a paper form of our institution's VTE prophylaxis strategy (Figure 1) to each vignette.
Data Collection
A 1‐hour noon conference titled VTE Workshop was conducted by one of the authors (M.J.B.) during the first quarter of the 2008 academic year. We asked the medical residents to apply the VTE prophylaxis protocol to 21 vignettes during this session. In order to determine the appropriate time allotted to complete the vignettes, each case was completed by M.J.B. and three medical residents (one intern, one second year, and one third year) prior to conducting the VTE workshop. Based on these data, we determined that the median time to complete each vignette was 2 minutes and 15 seconds (range 30 seconds to 7 minutes). Therefore, we assumed that the 21 vignettes could be completed within 1 hour. At the beginning of the conference, the residents were provided with 10 minutes of verbal instruction about how to apply the VTE risk assessment tool. They were instructed to provide a total risk score (070 points) and, based on the total score, to classify each patient as low, moderate, or high risk for VTE. Following the risk assignment, they were instructed to document any absolute and relative contraindications. Finally, they were asked to select the most appropriate VTE prophylaxis plan according to the recommendations of the protocol. Vignettes were considered complete if they had an assessment and plan for >75% of the cases.
Prior to conducting this study, there had been no formal orientation regarding use of the VTE risk assessment tool or incorporation of it into our institution's computerized order entry system. Average attendance for the noon conference is between 20 and 30 house staff, approximately one‐third of the entire residency. Medical students were excluded from the study. All respondents voluntarily and anonymously performed the assessments, and indicated on the front of their vignette packet their level of training as PGY‐1, ‐2, or ‐3. The sessions were overseen by one of the authors to ensure that no communication occurred among the residents.
Data Analysis
We constructed a database with five variables collected from each resident's VTE risk assessment form: 1) a total risk score, 2) a risk classification (low, medium, or high), 3) the number and type of absolute contraindications to pharmacological prophylaxis, 4) the number and type of relative contraindications to pharmacological prophylaxis, and 5) a VTE prophylaxis plan. The lead author also performed these assessments of the 21 vignettes 1 month prior to the resident session. In power calculations performed prior to the session, we determined that the study would need at least 300 observations in order to calculate inter‐rater reliability.23 With the estimation that between 20 and 30 residents would attend, we determined that 21 vignettes would exceed the minimum required observations to allow for an accurate calculation of inter‐rater reliability.
The total risk score was treated as a continuous variable for which the intra‐class correlation (ICC) was calculated. The ICC is used to assess the consistency, or conformity, of measurements made by multiple observers measuring the same quantity.24 Risk stratification, presence of absolute and relative contraindications, and VTE plan were treated as categorical variables. For these, we used Cohen's kappa to assess variability in resident ratings. The kappa score has been used in other studies to determine inter‐rater reliability using similar VTE risk assessment tools.18, 19 Finally, adherence to our hospital's protocol was determined by comparing the residents' VTE plans with the lead author's VTE plans for each of the 21 vignettes. We used SAS 9.1.3 for all statistical analyses (SAS Institute, Cary, NC).
Results
Twenty‐six medical residents attended the conference. Three residents left without submitting their assessments and were excluded from the analysis. Of the 23 residents included in the analysis, 15 (65%) were interns, 5 (22%) second‐year residents, and 3 (13%) third‐year residents. A maximum of 483 observations (21 clinical vignettes and 23 residents) was possible. Six (1%) risk stratifications were missing, and 14 (3%) VTE prophylaxis plans were missing. Therefore, out of a possible 483 paired assessments and plans, complete data existed for 95% (469) of the observations. Residents risk‐stratified the vignettes as low risk for 27% of cases, moderate risk for 38%, and high risk 34%. These differed from those of the lead author, who stratified proportionately more vignettes as high risk (Table 1).
| Risk Stratification | Resident no./total (%) | Attending no./total (%) |
|---|---|---|
| Low | 130/479 (27) | 3/21 (14) |
| Moderate | 183/479 (38) | 7/21 (33) |
| High | 166/479 (34) | 11/21 (52) |
Of those vignette patients stratified as high risk, 77% (128/166) received some form of prophylaxis. Of those stratified as moderate risk, 66% (121/183) received some form of prophylaxis. Finally, of those stratified as low risk, 15% (20/130) received some form of prophylaxis. To explore the impact of the disparity in risk assessments between residents and attending physicians, we used the lead author's assessments as the standard for comparison, and determined that only 64% (309/479) of the observations were risk‐stratified correctly. To emphasize further the potential negative impact of these misclassifications, we determined that appropriate plans would have occurred only 47% of the time. Analysis of these data via risk category showed that low‐risk patients received appropriate prophylaxis 84% of the time. However, protocol adherence for moderate and high‐risk patients occurred only 33 and 40% of the time, respectively (Table 2). Making the assumption that those vignette patients at moderate and high risk who only received mechanical prophylaxis had appropriate contraindications to heparin prophylaxis, protocol adherence remained low at 54 and 58%, respectively.
| Attending Classification | Total | Appropriate Risk Assessment No. (%) | SCDs Only No. (%) | Heparin Only | Both Heparin and SCDs | Ambulation |
|---|---|---|---|---|---|---|
| ||||||
| Low risk | 115 | 86 (75) | 11 (10) | 7 (6) | 0 | 93 (84)a |
| Moderate risk | 138 | 85 (62) | 28 (21)b | 44 (33)a | 16 (12) | 47 (35) |
| High risk | 230 | 138 (60) | 39 (18)b | 69 (31) | 88 (40)a | 27 (12) |
| Total | 483 | 309 (64) | 78 (16) | 120 (26) | 104 (22) | 167 (36) |
The ICC for the total risk score was 0.66, and the kappa coefficient for risk stratification was 0.51 (95% CI 0.50, 0.53), both of which represent moderate agreement. Absolute and relative contraindications were identified 12% (57/483) and 13% (61/483) of the time, respectively. The kappa scores for absolute and relative contraindications were 0.29 and 0.23, respectively. The kappa score for the VTE plan was 0.28 and represents only fair agreement (Table 2).
Subgroup analysis of the 15 intern participants for ICC for the risk score was 0.63. The kappa scores for risk stratification and VTE plan were 0.47 and 0.23, respectively. The kappa scores for senior residents represent aggregate data of 168 observations of second‐ and third‐year residents. For senior residents, the kappa scores for risk stratification and VTE plan were 0.61 and 0.35, respectively (Table 3).
| Risk Score | Stratification | Absolute Contraindication | Relative Contraindication | VTE Plan | ||
|---|---|---|---|---|---|---|
| ||||||
| Aggregate | ICC | 0.66 | ||||
| Kappa | 0.51 | 0.29 | 0.23 | 0.28 | ||
| Intern | ICC | 0.63 | ||||
| Kappa | 0.47 | NA | NA | 0.23 | ||
| Seniora | ICC | 0.73 | ||||
| Kappa | 0.61 | NA | NA | 0.35 | ||
Discussion
We performed this study to determine how reliably our medical residents could apply a point‐based VTE risk assessment tool, similar to those published previously.11 We observed that early in the academic year, our residents were not able to use this tool reliably. While our study does not evaluate the effects of audit and feedback, reminder alerts, or educational interventions, an important first step toward quality improvement in VTE prophylaxis is to reduce variability in risk assessment and decision making. In this endeavor, our results differ markedly from those in the literature. For instance, one study used 3 trained nurses to employ a similar risk assessment tool, and found an ICC of 0.98 for overall assessments of VTE risk, but did not report protocol adherence.19 Another study found inter‐rater reliability to be high among 5 physician observers (kappa scores of 0.81 and 0.90 for risk stratification and VTE plan, respectively).18 These two studies evaluated the performance of experienced evaluators who employed different and simpler VTE risk assessment tools. Our study determined that the inter‐rater reliability of risk assessment and VTE plan between residents using a point‐based VTE risk assessment tool was significantly lower, at 0.51 and 0.28, respectively. There was marked disparity between the lead author's and residents' risk assessments of those deemed to be at low and high risk (Table 1). While both determined approximately one‐third of the patient vignettes to be at moderate risk, the residents misclassified those at high risk in comparison with the author's assessments. This underestimation of VTE risk could lead to profound underprophylaxis in at‐risk patients. To the extent that our findings represent those in other teaching hospitals, such errors could hinder VTE quality improvement efforts in such institutions.
Previous studies that successfully improved VTE prophylaxis rates coupled a risk assessment tool with provider education as well as audit‐and‐feedback interventions.25, 26 In one study, provider education occurred on the first day of every month with an orientation to the hospital's recommended VTE prevention strategies.26 Another study sought to improve the rates of VTE prophylaxis in medical intensive care (MICU) patients without performing individualized risk assessment.27 Using only weekly graphic feedback and verbal reminders to the medical team, it showed an improvement in VTE prophylaxis for 1 year. A third study improved VTE prophylaxis adherence and reduced VTE at 90 days using only reminder alerts.28 Interestingly, several studies reduced the incidence of VTE without employing any patient risk stratification.29 These studies suggest that improvement in VTE prophylaxis rates could have occurred as result of audit‐and‐feedback or reminder systems and perhaps independent of the reliable application of a risk assessment tool.29 The studies that used risk assessment tools with layered interventions make it difficult to interpret whether the tool or the layered interventions were responsible for the improvement in VTE outcomes. Ours is the first study to evaluate how reliably residents can apply a tool independent of other interventions. With only fair to moderate resident agreement in patient risk assessment and VTE plan, our study suggests that the complexity of a point‐based risk assessment protocol (as opposed to a simplified three‐tiered approach) may affect resident prescriptive behavior.
As a result, our study corroborates two things: first, in medical centers that rely on residents to perform VTE prevention using individualized risk assessment, a multilayered approach for VTE prevention must occur. Second, a passively disseminated VTE protocol in the form of a pocket card will most likely not create a sustained improvement in VTE prophylaxis rates or reduce VTE.3035
When addressing certain aspects of quality improvement and safety, teaching hospitals must recognize that their efforts largely rely on resident performance. The 2009 National Resident Matching Program data indicate that there are 22,427 intern positions available in the United States. Often it is the resident's responsibility to perform risk assessments and provide prophylaxis, possibly using a tool that is too complex to apply reliably. Several studies have determined that 65% of medical errors were committed by interns and that 35‐44% of those errors resulted from knowledge deficits.3638 In order to best improve adherence to clinical guidelines, strategies that result in changing physician behavior need to be implemented, and can include but are not limited to the ones found in Figure 2.39 Ideally, teaching centers with computerized order entry should embed the risk assessment process as part of an admission/transfer order set, with a reminder alert. The alert would be activated when at‐risk patients do not receive appropriate prophylaxis. Most alert systems require hospitals to have computerized order entry, which has achieved only 20% market penetration in US hospitals.40, 41 Therefore, some hospitals employ, or intend to employ, passively disseminated risk assessment tools in the form of pocket cards or preprinted forms. These methods are estimated to improve prophylaxis by only 50% and are therefore not considered to be highly reliable strategies.3135

Our study demonstrates only fair to moderate reliability of a point‐based VTE risk assessment tool when used by residents independent of other strategies. It also suggests that residents underestimate those at high risk. In addition, our residents' protocol adherence was suboptimal and would have resulted in appropriate prophylaxis approximately 50% of the time in patients at moderate or high VTE risk. Therefore, when risk assessment tools such as ours are used, it is imperative that frequent education be combined with real‐time patient identification strategies as well as audit and feedback, a process called measure‐vention.13, 14 This is especially true when the risk assessment process is not linked to a reminder system as part of computer‐assisted order entry protocols.
A limitation of our study was the lack of a control group. Since all the residents in attendance received the same clinical vignettes, it would have been of interest to see how the risk assessment tool performed compared with residents who did not have access to the tool. However, based on average noon conference attendance, it would have been difficult to achieve an adequate number of observations to calculate credible ICC and kappa scores. Other limitations include the high number of interns who completed the vignettes compared with senior residents, and the lack of additional attending reviewers to score the vignettes prior to the session. Ideally, in determining the accuracy of protocol adherence, we would have compared residents' determinations with those of several experts who had used an adjudication process in the event of disagreement. In our ongoing work, we are collecting data from a representative sample of attending physicians at our hospital to compare their assessments both with each other and with those of the residents.
Another issue in our design was that the study presented only a limited amount of medical information in the vignettes. In actual clinical circumstances, the amount of historical information is greater and more complicated. One could argue that the artificiality of clinical vignettes is not an accurate representation of resident performance when ordering VTE prophylaxis. However, this approach limits case‐mix variation, so residents should have been able to reach similar conclusions with the information given. Thus the limited information should have maximized residents' intentions to prescribe VTE prophylaxis, and kappa scores would likely be lower in real clinical settings. Finally, our kappa scores were calculated based on aggregate data of interns and residents; however, interns comprised almost two‐thirds of the resident participants. As reported in the results section, intern inter‐rater reliability was slightly lower compared with the senior resident subgroup, suggesting that the variability may be a result of less clinical experience of the interns. However, the study was not powered to assess differences in kappa scores for level of training.
In conclusion, we determined the inter‐rater reliability of an individualized, point‐based VTE risk assessment tool when used by medical residents unfamiliar with its use. Our study showed that under conditions of minimal education, a point‐based VTE assessment tool achieves only fair to moderate reliability. It also suggests that as a stand‐alone tool without a reminder alert, adherence to VTE prevention guidelines is suboptimal and might result in underprophylaxis of hospitalized medical patients at moderate or high VTE risk. In fact, with appropriate prophylaxis, rates were maximally estimated to be 55% (Table 2). Because of the high percentage of interns in the study, these results approximate intern application of a VTE prevention protocol independent of other interventions. Comparing reliability data from our study with those of others raises the question of whether the observed differences in kappa score are because other studies used highly trained observers or because their protocols were less complex. However, a recent study validated a simpler method of VTE risk grouping that performs well regardless of clinical experience.20 Future studies are needed to determine whether there is improved resident inter‐rater reliability using a point‐based risk assessment tool that is embedded into a computerized order entry system with electronic reminder alerts. Finally, in actual clinical settings, the question remains of whether kappa scores correlate with protocol adherence, prophylaxis rates, and VTE reduction when using point‐based tools. If not, then the use of simplified risk‐stratification tools and VTE measure‐vention strategies should be implemented.
Acknowledgements
The authors thank Lisabeth V. Scalzi, MD, MS, Lora Moyer, Kevin McKenna MD, Hammid Al‐Mondhiry, Lucille Anderson, MD, Kathleen Williams, Kevin Larraway, Cynthia Chuang, MD, MS, the residents of the Internal Medicine and Combined Medicine/Pediatrics residencies, and the division of General Internal Medicine at Hershey Medical Center.
Venous thromboembolic events (VTE) are a significant cause of mortality in hospitalized medical and surgical patients.1, 2 The incidence of hospital‐acquired deep vein thrombosis (DVT) is 1040% among medical or general surgical patients in the absence of VTE prophylaxis.3 Approximately 5075% of these cases are preventable with appropriate prophylaxis.3, 4 To reduce hospital‐acquired VTE, the American College of Chest Physicians (ACCP) has published VTE prevention guidelines regularly since 1986. The latest version of the ACCP guidelines recommends that every hospital have an institution‐wide policy that encourages use of VTE prevention strategies. Nevertheless, current evidence demonstrates that VTE prophylaxis remains underutilized in at‐risk patients.5, 6 For example, the ENDORSE study showed that only 39% of at‐risk medical patients received appropriate VTE prophylaxis.6 A more recent study estimated that 58% of hospital‐acquired VTEs were preventable with appropriate prophylaxis utilization.7
Such data demonstrate that a quality gap exists between VTE prophylaxis guideline recommendations and actual practice. Such a gap highlights the need to identify barriers to appropriate implementation of systems‐based strategies aimed at preventing VTE. A presumed barrier for adherence to any VTE protocol is the complexity involved in performing individualized risk assessments.810 All VTE prophylaxis strategies require the clinician to risk‐stratify each patient, identify contraindications to a prophylaxis strategy, and select an accepted strategy. While many VTE risk assessment protocols exist, they tend to fall into two categories: 1) a point‐based system, and 2) a simplified tiered system. Point‐based clinical prediction rules have been advocated by Caprini and others.1115 Such approaches require the clinician to assign points during the identification of VTE risk factors. The clinician must add the points to determine a patient's cumulative VTE risk and use the points to classify that risk as low, moderate, or high. Such point‐based systems are generally considered complex and may underestimate VTE risk, potentially leading to underutilization of prophylaxis strategies.16
Studies have demonstrated that complexity introduces variation into the decision‐making process.17 As a result, both the ACCP and SHM advocate for simplifying the VTE risk assessment process.4, 18 To date, several studies have demonstrated that attending physicians and nurses can reliably apply a VTE risk assessment tool, but none that measure how reliably residents can perform this task when using a point‐based tool.18, 19 For academic medical centers, information about the reliability of such tools is especially important, since they will often be applied by physicians‐in‐training, who have limited knowledge and experience with VTE guidelines and risk assessment. The goal of our study, therefore, was to use clinical vignettes to determine the reliability and protocol adherence of medical residents' application of an adapted point‐based VTE risk assessment tool, independent of other interventions.
Methods
Development of the Risk Assessment Tool
A multispecialty team adapted existing individualized VTE risk assessment tools based on one developed by Caprini.11 The VTE tool (Fig. 1) was designed to assist residents in making two essential determinations prior to ordering a prophylaxis plan. The first determination was the calculation of a total risk score (070 points). This score was determined by identifying and assigning a point value to all medical and surgical risk factors, and summing the points into 3 categories: low (01 point), moderate (24 points), and high (>4 points) VTE risk. The second determination was to identify any contraindications to pharmacological prophylaxis. Like other nonvalidated tools, our tool divided contraindications into absolute or relative. After making these two determinations, residents were encouraged to order 1 of 6 VTE prophylaxis plans. These plans were intended to balance VTE risk against risk factors for bleeding due to prophylaxis.

Construction of Clinical Vignettes
Approval was obtained by the Pennsylvania State University Institutional Review Board. Since previous research demonstrates the utility of clinical vignettes to study the effectiveness of guideline application and decision making, we used a series of 21 randomly selected and de‐identified clinical vignettes to portray a range of real‐world patient admission scenarios.2022 We identified individuals who had been admitted to the Hershey Medical Center using data from the inpatient electronic health record and applying the following inclusion criteria: age >17 years, and admission to a general medical service from the Emergency Department during a 14‐day period in 2008. Since more than 80% of patients admitted to our medical service are admitted through the Emergency Department and residents place all of the admission orders, our goal was to use vignettes that were typical of patients they commonly admit. We attached a paper form of our institution's VTE prophylaxis strategy (Figure 1) to each vignette.
Data Collection
A 1‐hour noon conference titled VTE Workshop was conducted by one of the authors (M.J.B.) during the first quarter of the 2008 academic year. We asked the medical residents to apply the VTE prophylaxis protocol to 21 vignettes during this session. In order to determine the appropriate time allotted to complete the vignettes, each case was completed by M.J.B. and three medical residents (one intern, one second year, and one third year) prior to conducting the VTE workshop. Based on these data, we determined that the median time to complete each vignette was 2 minutes and 15 seconds (range 30 seconds to 7 minutes). Therefore, we assumed that the 21 vignettes could be completed within 1 hour. At the beginning of the conference, the residents were provided with 10 minutes of verbal instruction about how to apply the VTE risk assessment tool. They were instructed to provide a total risk score (070 points) and, based on the total score, to classify each patient as low, moderate, or high risk for VTE. Following the risk assignment, they were instructed to document any absolute and relative contraindications. Finally, they were asked to select the most appropriate VTE prophylaxis plan according to the recommendations of the protocol. Vignettes were considered complete if they had an assessment and plan for >75% of the cases.
Prior to conducting this study, there had been no formal orientation regarding use of the VTE risk assessment tool or incorporation of it into our institution's computerized order entry system. Average attendance for the noon conference is between 20 and 30 house staff, approximately one‐third of the entire residency. Medical students were excluded from the study. All respondents voluntarily and anonymously performed the assessments, and indicated on the front of their vignette packet their level of training as PGY‐1, ‐2, or ‐3. The sessions were overseen by one of the authors to ensure that no communication occurred among the residents.
Data Analysis
We constructed a database with five variables collected from each resident's VTE risk assessment form: 1) a total risk score, 2) a risk classification (low, medium, or high), 3) the number and type of absolute contraindications to pharmacological prophylaxis, 4) the number and type of relative contraindications to pharmacological prophylaxis, and 5) a VTE prophylaxis plan. The lead author also performed these assessments of the 21 vignettes 1 month prior to the resident session. In power calculations performed prior to the session, we determined that the study would need at least 300 observations in order to calculate inter‐rater reliability.23 With the estimation that between 20 and 30 residents would attend, we determined that 21 vignettes would exceed the minimum required observations to allow for an accurate calculation of inter‐rater reliability.
The total risk score was treated as a continuous variable for which the intra‐class correlation (ICC) was calculated. The ICC is used to assess the consistency, or conformity, of measurements made by multiple observers measuring the same quantity.24 Risk stratification, presence of absolute and relative contraindications, and VTE plan were treated as categorical variables. For these, we used Cohen's kappa to assess variability in resident ratings. The kappa score has been used in other studies to determine inter‐rater reliability using similar VTE risk assessment tools.18, 19 Finally, adherence to our hospital's protocol was determined by comparing the residents' VTE plans with the lead author's VTE plans for each of the 21 vignettes. We used SAS 9.1.3 for all statistical analyses (SAS Institute, Cary, NC).
Results
Twenty‐six medical residents attended the conference. Three residents left without submitting their assessments and were excluded from the analysis. Of the 23 residents included in the analysis, 15 (65%) were interns, 5 (22%) second‐year residents, and 3 (13%) third‐year residents. A maximum of 483 observations (21 clinical vignettes and 23 residents) was possible. Six (1%) risk stratifications were missing, and 14 (3%) VTE prophylaxis plans were missing. Therefore, out of a possible 483 paired assessments and plans, complete data existed for 95% (469) of the observations. Residents risk‐stratified the vignettes as low risk for 27% of cases, moderate risk for 38%, and high risk 34%. These differed from those of the lead author, who stratified proportionately more vignettes as high risk (Table 1).
| Risk Stratification | Resident no./total (%) | Attending no./total (%) |
|---|---|---|
| Low | 130/479 (27) | 3/21 (14) |
| Moderate | 183/479 (38) | 7/21 (33) |
| High | 166/479 (34) | 11/21 (52) |
Of those vignette patients stratified as high risk, 77% (128/166) received some form of prophylaxis. Of those stratified as moderate risk, 66% (121/183) received some form of prophylaxis. Finally, of those stratified as low risk, 15% (20/130) received some form of prophylaxis. To explore the impact of the disparity in risk assessments between residents and attending physicians, we used the lead author's assessments as the standard for comparison, and determined that only 64% (309/479) of the observations were risk‐stratified correctly. To emphasize further the potential negative impact of these misclassifications, we determined that appropriate plans would have occurred only 47% of the time. Analysis of these data via risk category showed that low‐risk patients received appropriate prophylaxis 84% of the time. However, protocol adherence for moderate and high‐risk patients occurred only 33 and 40% of the time, respectively (Table 2). Making the assumption that those vignette patients at moderate and high risk who only received mechanical prophylaxis had appropriate contraindications to heparin prophylaxis, protocol adherence remained low at 54 and 58%, respectively.
| Attending Classification | Total | Appropriate Risk Assessment No. (%) | SCDs Only No. (%) | Heparin Only | Both Heparin and SCDs | Ambulation |
|---|---|---|---|---|---|---|
| ||||||
| Low risk | 115 | 86 (75) | 11 (10) | 7 (6) | 0 | 93 (84)a |
| Moderate risk | 138 | 85 (62) | 28 (21)b | 44 (33)a | 16 (12) | 47 (35) |
| High risk | 230 | 138 (60) | 39 (18)b | 69 (31) | 88 (40)a | 27 (12) |
| Total | 483 | 309 (64) | 78 (16) | 120 (26) | 104 (22) | 167 (36) |
The ICC for the total risk score was 0.66, and the kappa coefficient for risk stratification was 0.51 (95% CI 0.50, 0.53), both of which represent moderate agreement. Absolute and relative contraindications were identified 12% (57/483) and 13% (61/483) of the time, respectively. The kappa scores for absolute and relative contraindications were 0.29 and 0.23, respectively. The kappa score for the VTE plan was 0.28 and represents only fair agreement (Table 2).
Subgroup analysis of the 15 intern participants for ICC for the risk score was 0.63. The kappa scores for risk stratification and VTE plan were 0.47 and 0.23, respectively. The kappa scores for senior residents represent aggregate data of 168 observations of second‐ and third‐year residents. For senior residents, the kappa scores for risk stratification and VTE plan were 0.61 and 0.35, respectively (Table 3).
| Risk Score | Stratification | Absolute Contraindication | Relative Contraindication | VTE Plan | ||
|---|---|---|---|---|---|---|
| ||||||
| Aggregate | ICC | 0.66 | ||||
| Kappa | 0.51 | 0.29 | 0.23 | 0.28 | ||
| Intern | ICC | 0.63 | ||||
| Kappa | 0.47 | NA | NA | 0.23 | ||
| Seniora | ICC | 0.73 | ||||
| Kappa | 0.61 | NA | NA | 0.35 | ||
Discussion
We performed this study to determine how reliably our medical residents could apply a point‐based VTE risk assessment tool, similar to those published previously.11 We observed that early in the academic year, our residents were not able to use this tool reliably. While our study does not evaluate the effects of audit and feedback, reminder alerts, or educational interventions, an important first step toward quality improvement in VTE prophylaxis is to reduce variability in risk assessment and decision making. In this endeavor, our results differ markedly from those in the literature. For instance, one study used 3 trained nurses to employ a similar risk assessment tool, and found an ICC of 0.98 for overall assessments of VTE risk, but did not report protocol adherence.19 Another study found inter‐rater reliability to be high among 5 physician observers (kappa scores of 0.81 and 0.90 for risk stratification and VTE plan, respectively).18 These two studies evaluated the performance of experienced evaluators who employed different and simpler VTE risk assessment tools. Our study determined that the inter‐rater reliability of risk assessment and VTE plan between residents using a point‐based VTE risk assessment tool was significantly lower, at 0.51 and 0.28, respectively. There was marked disparity between the lead author's and residents' risk assessments of those deemed to be at low and high risk (Table 1). While both determined approximately one‐third of the patient vignettes to be at moderate risk, the residents misclassified those at high risk in comparison with the author's assessments. This underestimation of VTE risk could lead to profound underprophylaxis in at‐risk patients. To the extent that our findings represent those in other teaching hospitals, such errors could hinder VTE quality improvement efforts in such institutions.
Previous studies that successfully improved VTE prophylaxis rates coupled a risk assessment tool with provider education as well as audit‐and‐feedback interventions.25, 26 In one study, provider education occurred on the first day of every month with an orientation to the hospital's recommended VTE prevention strategies.26 Another study sought to improve the rates of VTE prophylaxis in medical intensive care (MICU) patients without performing individualized risk assessment.27 Using only weekly graphic feedback and verbal reminders to the medical team, it showed an improvement in VTE prophylaxis for 1 year. A third study improved VTE prophylaxis adherence and reduced VTE at 90 days using only reminder alerts.28 Interestingly, several studies reduced the incidence of VTE without employing any patient risk stratification.29 These studies suggest that improvement in VTE prophylaxis rates could have occurred as result of audit‐and‐feedback or reminder systems and perhaps independent of the reliable application of a risk assessment tool.29 The studies that used risk assessment tools with layered interventions make it difficult to interpret whether the tool or the layered interventions were responsible for the improvement in VTE outcomes. Ours is the first study to evaluate how reliably residents can apply a tool independent of other interventions. With only fair to moderate resident agreement in patient risk assessment and VTE plan, our study suggests that the complexity of a point‐based risk assessment protocol (as opposed to a simplified three‐tiered approach) may affect resident prescriptive behavior.
As a result, our study corroborates two things: first, in medical centers that rely on residents to perform VTE prevention using individualized risk assessment, a multilayered approach for VTE prevention must occur. Second, a passively disseminated VTE protocol in the form of a pocket card will most likely not create a sustained improvement in VTE prophylaxis rates or reduce VTE.3035
When addressing certain aspects of quality improvement and safety, teaching hospitals must recognize that their efforts largely rely on resident performance. The 2009 National Resident Matching Program data indicate that there are 22,427 intern positions available in the United States. Often it is the resident's responsibility to perform risk assessments and provide prophylaxis, possibly using a tool that is too complex to apply reliably. Several studies have determined that 65% of medical errors were committed by interns and that 35‐44% of those errors resulted from knowledge deficits.3638 In order to best improve adherence to clinical guidelines, strategies that result in changing physician behavior need to be implemented, and can include but are not limited to the ones found in Figure 2.39 Ideally, teaching centers with computerized order entry should embed the risk assessment process as part of an admission/transfer order set, with a reminder alert. The alert would be activated when at‐risk patients do not receive appropriate prophylaxis. Most alert systems require hospitals to have computerized order entry, which has achieved only 20% market penetration in US hospitals.40, 41 Therefore, some hospitals employ, or intend to employ, passively disseminated risk assessment tools in the form of pocket cards or preprinted forms. These methods are estimated to improve prophylaxis by only 50% and are therefore not considered to be highly reliable strategies.3135

Our study demonstrates only fair to moderate reliability of a point‐based VTE risk assessment tool when used by residents independent of other strategies. It also suggests that residents underestimate those at high risk. In addition, our residents' protocol adherence was suboptimal and would have resulted in appropriate prophylaxis approximately 50% of the time in patients at moderate or high VTE risk. Therefore, when risk assessment tools such as ours are used, it is imperative that frequent education be combined with real‐time patient identification strategies as well as audit and feedback, a process called measure‐vention.13, 14 This is especially true when the risk assessment process is not linked to a reminder system as part of computer‐assisted order entry protocols.
A limitation of our study was the lack of a control group. Since all the residents in attendance received the same clinical vignettes, it would have been of interest to see how the risk assessment tool performed compared with residents who did not have access to the tool. However, based on average noon conference attendance, it would have been difficult to achieve an adequate number of observations to calculate credible ICC and kappa scores. Other limitations include the high number of interns who completed the vignettes compared with senior residents, and the lack of additional attending reviewers to score the vignettes prior to the session. Ideally, in determining the accuracy of protocol adherence, we would have compared residents' determinations with those of several experts who had used an adjudication process in the event of disagreement. In our ongoing work, we are collecting data from a representative sample of attending physicians at our hospital to compare their assessments both with each other and with those of the residents.
Another issue in our design was that the study presented only a limited amount of medical information in the vignettes. In actual clinical circumstances, the amount of historical information is greater and more complicated. One could argue that the artificiality of clinical vignettes is not an accurate representation of resident performance when ordering VTE prophylaxis. However, this approach limits case‐mix variation, so residents should have been able to reach similar conclusions with the information given. Thus the limited information should have maximized residents' intentions to prescribe VTE prophylaxis, and kappa scores would likely be lower in real clinical settings. Finally, our kappa scores were calculated based on aggregate data of interns and residents; however, interns comprised almost two‐thirds of the resident participants. As reported in the results section, intern inter‐rater reliability was slightly lower compared with the senior resident subgroup, suggesting that the variability may be a result of less clinical experience of the interns. However, the study was not powered to assess differences in kappa scores for level of training.
In conclusion, we determined the inter‐rater reliability of an individualized, point‐based VTE risk assessment tool when used by medical residents unfamiliar with its use. Our study showed that under conditions of minimal education, a point‐based VTE assessment tool achieves only fair to moderate reliability. It also suggests that as a stand‐alone tool without a reminder alert, adherence to VTE prevention guidelines is suboptimal and might result in underprophylaxis of hospitalized medical patients at moderate or high VTE risk. In fact, with appropriate prophylaxis, rates were maximally estimated to be 55% (Table 2). Because of the high percentage of interns in the study, these results approximate intern application of a VTE prevention protocol independent of other interventions. Comparing reliability data from our study with those of others raises the question of whether the observed differences in kappa score are because other studies used highly trained observers or because their protocols were less complex. However, a recent study validated a simpler method of VTE risk grouping that performs well regardless of clinical experience.20 Future studies are needed to determine whether there is improved resident inter‐rater reliability using a point‐based risk assessment tool that is embedded into a computerized order entry system with electronic reminder alerts. Finally, in actual clinical settings, the question remains of whether kappa scores correlate with protocol adherence, prophylaxis rates, and VTE reduction when using point‐based tools. If not, then the use of simplified risk‐stratification tools and VTE measure‐vention strategies should be implemented.
Acknowledgements
The authors thank Lisabeth V. Scalzi, MD, MS, Lora Moyer, Kevin McKenna MD, Hammid Al‐Mondhiry, Lucille Anderson, MD, Kathleen Williams, Kevin Larraway, Cynthia Chuang, MD, MS, the residents of the Internal Medicine and Combined Medicine/Pediatrics residencies, and the division of General Internal Medicine at Hershey Medical Center.
- ,,.Autopsy‐verified pulmonary embolism in a surgical department: analysis of the period from 1951 to 1988.Br J Surg.1991;78(7):849–852.
- , , , , et al.Fatal pulmonary embolism in hospitalised patients: a necropsy review.J Clin Pathol.2004;57(12):1254–1257.
- , , , et al.Prevention of venous thromboembolism: American College of Chest Physicians Evidence‐Based Clinical Practice Guidelines (8th edition).Chest.2008;133(6 suppl):381S–453S.
- ,.Coronary artery vasculitis and myocardial infarction with systemic lupus erythematosus.NY State J Med.1974;74(5):873–874.
- , , , et al.Venous thromboembolism prophylaxis in acutely ill hospitalized medical patients: findings from the International Medical Prevention Registry on Venous Thromboembolism.Chest.2007;132(3):936–945.
- , , , et al.Venous thromboembolism risk and prophylaxis in the acute hospital care setting (ENDORSE study): a multinational cross‐sectional study.Lancet.2008;371(9610):387–394.
- , , , .Venous thromboembolic events in hospitalised medical patients.Thromb Haemost.2009;102(3):505–510.
- ,,.Compliance with recommended prophylaxis for venous thromboembolism: improving the use and rate of uptake of clinical practice guidelines.J Thromb Haemost.2004;2(2):221–227.
- , , , et al.A systematic review of strategies to improve prophylaxis for venous thromboembolism in hospitals.Ann Surg.2005;241(3):397–415.
- ,.Prevention of venous thromboembolism: consensus, controversies, and challenges.Hematol Am Soc Hematol Educ Program.2009:286–292.
- ,,.International perspective on venous thromboembolism prophylaxis in surgery.Semin Thromb Hemost.1991;17(4):322–325.
- , , , et al.Clinical assessment of venous thromboembolic risk in surgical patients.Semin Thromb Hemost.1991;17(suppl 3):304–312.
- , et al.Risk factor assessment in the management of patients with suspected deep venous thrombosis.Int Angiol.2000;19(1):47–51.
- ,,.Effective risk stratification of surgical and nonsurgical patients for venous thromboembolic disease.Semin Hematol.2001;38(2 suppl 5):12–19.
- .Thrombosis risk assessment as a guide to quality patient care.Dis Mon.2005;51(2–3):70–78.
- ,.Designing and implementing effective venous thromboembolism prevention protocols: lessons from collaborative efforts.J Thromb Thrombolysis.2010;29(2):159–166.
- ,,.Emergency medicine practitioner knowledge and use of decision rules for the evaluation of patients with suspected pulmonary embolism: variations by practice setting and training level.Acad Emerg Med.2007;14(1):53–57.
- , , , , , , , .Optimizing prevention of hospital‐acquired (HA) venous thromboembolism (VTE): prospective validation of a VTE risk assessment model (RAM).J Hosp Med.2010;5(1):10–18..
- , , , et al.Development and testing of a DVT risk assessment tool: providing evidence of validity and reliability.Worldviews Evid Based Nurs.2007;4(1):14–20.
- , , , et al.A novel educational strategy to enhance internal medicine residents' familial colorectal cancer knowledge and risk assessment skills.Am J Gastroenterol.2005;100(3):677–684.
- ,, , , , .Impact of formal continuing medical education: do conferences, workshops, rounds, and other traditional continuing education activities change physician behavior or health care outcomes?JAMA.1999;282(9):867–874.
- , , , et al.Comparison of vignettes, standardized patients, and chart abstraction: a prospective validation study of 3 methods for measuring quality.JAMA.2000;283(13):1715–1722.
- .Psychometric Theory.New Delhi:Tate McGraw‐Hill;1981.
- ,.Intraclass correlations: uses in assessing rater reliability.Psychol Bull.1979;86(2):420–428.
- ,,.Innovative approaches to increase deep vein thrombosis prophylaxis rate resulting in a decrease in hospital‐acquired deep vein thrombosis at a tertiary‐care teaching hospital.J Hosp Med.2008;3(2):148–155.
- ,,.Improved use of thromboprophylaxis for deep vein thrombosis following an educational intervention.J Hosp Med.2006;1(6):331–338.
- , , , et al.Minimizing errors of omission: behavioural reenforcement of heparin to avert venous emboli: the BEHAVE study.Crit Care Med.2006;34(3):694–699.
- , , , et al.Electronic alerts to prevent venous thromboembolism among hospitalized patients.N Engl J Med.2005;352(10):969–977.
- , , , .Getting a validated guideline into local practice: implementation and audit of the SIGN guideline on the prevention of deep vein thrombosis in a district general hospital.Scott Med J.1998;43(1):23–25.
- ,,. Improving the Reliability of Health Care. IHI Innovation Series white paper 2004. Available at: www.IHI.org/IHI/Results/WhitePapers/Improving theReliabilityof HealthCare.htm. Accessed October 4,2010.
- , , , et al.Thrombosis prophylaxis in medical patients: a retrospective review of clinical practice patterns.Haematologica.2002;87(7):746–750; discussion 250.
- ,.Genetic epidemiology: systemic lupus erythematosus.Arthritis Res.2001;3(6):331–336.
- ,,.Missed opportunities for prevention of venous thromboembolism: an evaluation of the use of thromboprophylaxis guidelines.Chest.2001;120(6):1964–1971.
- , , , , .Underuse of venous thromboembolism prophylaxis for general surgery patients: physician practices in the community hospital setting.Arch Intern Med.1998;158(17):1909–1912.
- ,,.Venous thromboembolism prophylaxis used by consultant general surgeons in Scotland.J R Coll Surg Edinb.2001;46(6):329–333.
- ,.Ordering errors by first‐year residents: evidence of learning from mistakes.Mo Med.2004;101(2):128–131.
- ,.Prevention of medication errors: teaching and training.Br J Clin Pharmacol.2009;67(6):656–661.
- ,,.Reducing medication errors in a surgical residency training program.Am Surg.2004;70(5):467–471.
- , , , et al.Why don't physicians follow clinical practice guidelines? A framework for improvement.JAMA.1999;282(15):1458–1465.
- , , , , .Predicting computerized physician order entry system adoption in US hospitals: can the federal mandate be met?Int J Med Inform.2008;77(8):539–545.
- ,.Implementation of computerized physician order entry in seven countries.Health Aff (Millwood).2009;28(2):404–414.
- ,,.Autopsy‐verified pulmonary embolism in a surgical department: analysis of the period from 1951 to 1988.Br J Surg.1991;78(7):849–852.
- , , , , et al.Fatal pulmonary embolism in hospitalised patients: a necropsy review.J Clin Pathol.2004;57(12):1254–1257.
- , , , et al.Prevention of venous thromboembolism: American College of Chest Physicians Evidence‐Based Clinical Practice Guidelines (8th edition).Chest.2008;133(6 suppl):381S–453S.
- ,.Coronary artery vasculitis and myocardial infarction with systemic lupus erythematosus.NY State J Med.1974;74(5):873–874.
- , , , et al.Venous thromboembolism prophylaxis in acutely ill hospitalized medical patients: findings from the International Medical Prevention Registry on Venous Thromboembolism.Chest.2007;132(3):936–945.
- , , , et al.Venous thromboembolism risk and prophylaxis in the acute hospital care setting (ENDORSE study): a multinational cross‐sectional study.Lancet.2008;371(9610):387–394.
- , , , .Venous thromboembolic events in hospitalised medical patients.Thromb Haemost.2009;102(3):505–510.
- ,,.Compliance with recommended prophylaxis for venous thromboembolism: improving the use and rate of uptake of clinical practice guidelines.J Thromb Haemost.2004;2(2):221–227.
- , , , et al.A systematic review of strategies to improve prophylaxis for venous thromboembolism in hospitals.Ann Surg.2005;241(3):397–415.
- ,.Prevention of venous thromboembolism: consensus, controversies, and challenges.Hematol Am Soc Hematol Educ Program.2009:286–292.
- ,,.International perspective on venous thromboembolism prophylaxis in surgery.Semin Thromb Hemost.1991;17(4):322–325.
- , , , et al.Clinical assessment of venous thromboembolic risk in surgical patients.Semin Thromb Hemost.1991;17(suppl 3):304–312.
- , et al.Risk factor assessment in the management of patients with suspected deep venous thrombosis.Int Angiol.2000;19(1):47–51.
- ,,.Effective risk stratification of surgical and nonsurgical patients for venous thromboembolic disease.Semin Hematol.2001;38(2 suppl 5):12–19.
- .Thrombosis risk assessment as a guide to quality patient care.Dis Mon.2005;51(2–3):70–78.
- ,.Designing and implementing effective venous thromboembolism prevention protocols: lessons from collaborative efforts.J Thromb Thrombolysis.2010;29(2):159–166.
- ,,.Emergency medicine practitioner knowledge and use of decision rules for the evaluation of patients with suspected pulmonary embolism: variations by practice setting and training level.Acad Emerg Med.2007;14(1):53–57.
- , , , , , , , .Optimizing prevention of hospital‐acquired (HA) venous thromboembolism (VTE): prospective validation of a VTE risk assessment model (RAM).J Hosp Med.2010;5(1):10–18..
- , , , et al.Development and testing of a DVT risk assessment tool: providing evidence of validity and reliability.Worldviews Evid Based Nurs.2007;4(1):14–20.
- , , , et al.A novel educational strategy to enhance internal medicine residents' familial colorectal cancer knowledge and risk assessment skills.Am J Gastroenterol.2005;100(3):677–684.
- ,, , , , .Impact of formal continuing medical education: do conferences, workshops, rounds, and other traditional continuing education activities change physician behavior or health care outcomes?JAMA.1999;282(9):867–874.
- , , , et al.Comparison of vignettes, standardized patients, and chart abstraction: a prospective validation study of 3 methods for measuring quality.JAMA.2000;283(13):1715–1722.
- .Psychometric Theory.New Delhi:Tate McGraw‐Hill;1981.
- ,.Intraclass correlations: uses in assessing rater reliability.Psychol Bull.1979;86(2):420–428.
- ,,.Innovative approaches to increase deep vein thrombosis prophylaxis rate resulting in a decrease in hospital‐acquired deep vein thrombosis at a tertiary‐care teaching hospital.J Hosp Med.2008;3(2):148–155.
- ,,.Improved use of thromboprophylaxis for deep vein thrombosis following an educational intervention.J Hosp Med.2006;1(6):331–338.
- , , , et al.Minimizing errors of omission: behavioural reenforcement of heparin to avert venous emboli: the BEHAVE study.Crit Care Med.2006;34(3):694–699.
- , , , et al.Electronic alerts to prevent venous thromboembolism among hospitalized patients.N Engl J Med.2005;352(10):969–977.
- , , , .Getting a validated guideline into local practice: implementation and audit of the SIGN guideline on the prevention of deep vein thrombosis in a district general hospital.Scott Med J.1998;43(1):23–25.
- ,,. Improving the Reliability of Health Care. IHI Innovation Series white paper 2004. Available at: www.IHI.org/IHI/Results/WhitePapers/Improving theReliabilityof HealthCare.htm. Accessed October 4,2010.
- , , , et al.Thrombosis prophylaxis in medical patients: a retrospective review of clinical practice patterns.Haematologica.2002;87(7):746–750; discussion 250.
- ,.Genetic epidemiology: systemic lupus erythematosus.Arthritis Res.2001;3(6):331–336.
- ,,.Missed opportunities for prevention of venous thromboembolism: an evaluation of the use of thromboprophylaxis guidelines.Chest.2001;120(6):1964–1971.
- , , , , .Underuse of venous thromboembolism prophylaxis for general surgery patients: physician practices in the community hospital setting.Arch Intern Med.1998;158(17):1909–1912.
- ,,.Venous thromboembolism prophylaxis used by consultant general surgeons in Scotland.J R Coll Surg Edinb.2001;46(6):329–333.
- ,.Ordering errors by first‐year residents: evidence of learning from mistakes.Mo Med.2004;101(2):128–131.
- ,.Prevention of medication errors: teaching and training.Br J Clin Pharmacol.2009;67(6):656–661.
- ,,.Reducing medication errors in a surgical residency training program.Am Surg.2004;70(5):467–471.
- , , , et al.Why don't physicians follow clinical practice guidelines? A framework for improvement.JAMA.1999;282(15):1458–1465.
- , , , , .Predicting computerized physician order entry system adoption in US hospitals: can the federal mandate be met?Int J Med Inform.2008;77(8):539–545.
- ,.Implementation of computerized physician order entry in seven countries.Health Aff (Millwood).2009;28(2):404–414.
Copyright © 2011 Society of Hospital Medicine
Making a List, Check It Twice
In October, a 36‐year‐old woman with no significant past medical history presented to the Emergency Department (ED) with a 3‐day history of headache and fever. The headache was severe, throbbing, and frontal in location. She also complained of daily fevers measured up to 103F, generalized malaise, and fatigue. She did not report neck stiffness or photophobia. She felt better after receiving intravenous fluids and was discharged home with a diagnosis of a nonspecific viral illness. Two days later, she returned to the ED with worsening headache, fever, mild photophobia, and poor oral intake. She also complained of a dry cough that made her headache worse, as did bending over. She did not report confusion, neck stiffness, shortness of breath, sore throat, runny nose, abdominal symptoms, or rash.
This patient presents a second time to the ED with worsening headache and fever raising concerns about meningitis. At the time of her first ED visit, it can be assumed that she had a nontoxic appearance because she was discharged shortly thereafter. Thus, acute bacterial meningitis seems less likely, but occasionally patients with meningococcal meningitis may not appear significantly ill until later in the process. Nonetheless, acute meningitis, possibly viral, is the initial concern. The time of the year is an important variable because many viral infections are seasonal. Enteroviruses are the most common cause of viral meningitis in the United States, particularly in the summer and fall. In contrast, mumps, measles, and varicella zoster viruses occur more commonly in winter and spring. Herpetic meningoencephalitis is a life‐threatening condition with a guarded prognosis. Therefore, early recognition and treatment is necessary to decrease morbidity and mortality. Drugs such as nonsteroidal anti‐inflammatory agents, trimethoprim‐sulfamethoxazole, amoxicillin, and rarely vaccines can also cause aseptic meningitis. Infections from fungi, spirochetes, mycobacteria, and rarely parasites also cause meningitis, but would be of greater concern in a patient with risk factors such as recent travel or an immunocompromised state.
Increased headache with bending and cough might indicate elevated intracranial pressure. However, this is a nonspecific complaint, and headache is often worse with the Valsalva maneuver. Because she reports a cough, a chest x‐ray would be useful. In addition to routine initial tests, cerebrospinal fluid (CSF) analysis and human immunodeficiency virus (HIV) testing is recommended.
Her past medical history was notable for depression. Her medications included bupropion, multivitamins, and fish oil. She was also taking milk thistle pills daily to protect her liver because she had been drinking alcohol heavily for the past 2 weeks since her husband left her. She smoked 1 pack of cigarettes daily. She had not traveled recently. She reported no recent animal or wildlife exposure but did recall falling into a midwestern river while canoeing 2 weeks prior to presentation. She worked as a hairstylist and described no sick contacts or risk factors for HIV disease.
An important new historical element is that the patient fell into a river. If she swallowed a significant amount of water during her fall overboard, meningitis from waterborne infections such as Aeromonas, Acanthamoeba, and Naegleria need to be considered. Fortunately, these are rare in the Midwest. Her canoeing history may suggest exposure to wooded areas. Certainly, tickborne infections such as ehrlichiosis, babesiosis, Lyme disease, and Rocky Mountain spotted fever can also cause meningitis. Histoplasmosis and blastomycosis are also endemic to the midwestern United States and can disseminate and cause central nervous system disease.
At this time, viral and bacterial infections are highest on the differential diagnosis. However, the microbiology laboratory needs to be alerted to the possibility of fungal or parasitic organisms depending on the initial CSF analysis results.
The patient was a Caucasian woman who appeared comfortable. Her blood pressure was 130/62 mm Hg, heart rate was 83 beats per minute, respiratory rate was 18 per minute, temperature was 100.8F, and oxygen saturation was 98% on room air. She was fully alert and oriented. Her pupils were bilaterally equal, reactive to light and accommodation with intact extraocular movement and no nystagmus. There was conjunctival injection bilaterally without noticeable pallor or icterus. Fundoscopic examination, which the patient tolerated without difficulty, was normal. Inspection of the oral cavity showed mild tonsillar enlargement. The neck was supple with no stiffness. No cervical, axillary, or inguinal lymph nodes were palpable. Faint bilateral basilar crackles were audible over the posterior chest. There was very mild right upper quadrant abdominal tenderness without guarding. The liver and spleen were normal size and bowel sounds were present. No rash, peripheral edema, or spinal tenderness was noted. A complete neurological examination was normal.
Her general appearance and vital signs seem reassuring. Conjunctival injection and mild tonsillar enlargement are nonspecific findings and may occur in systemic inflammatory states especially viral infections. Atelectasis may account for faint bilateral basilar crackles especially if associated with post‐tussive change. Her alcohol use puts her at risk of aspiration. A right lower lobe process (pneumonia) can sometimes present with right upper quadrant tenderness. However, this tenderness may also represent muscle soreness from repeated coughing, liver, or gallbladder disease. The same infectious process affecting the central nervous system and possibly her lungs, may also be affecting the liver.
A complete blood count revealed a white blood cell count of 3000/mm3 (79% neutrophils, 15% lymphocytes, 5% monocytes), hemoglobin of 11.7 g/dL, and platelets of 110,000/mm3. The serum sodium was 133 mmol/L, potassium was 3.7 mmol/L, bicarbonate was 22 mmol/L, and blood urea nitrogen was 20 mg/dL. The serum creatinine was 1.5 compared to 1.0 mg/dL on testing 2 days prior. A liver function panel showed protein of 5.1 g/dL, albumin of 3 g/dL, aspartate aminotransferase (AST) of 576 IU/L, alanine aminotransferase (ALT) of 584 IU/L, alkaline phosphatase of 282 IU/L, and total bilirubin of 1 mg/dL. The coagulation profile, creatinine phosphokinase, acetaminophen level, urine pregnancy test, urine drug screen, and urinalysis (including urine microscopy) were normal.
The CSF opening pressure was 13 cm H2O. CSF analysis showed 4 mononuclear leukocytes per high‐power field, CSF protein was 27 mg/dL, and glucose was 76 mg/dL. No organisms were noted on gram stain. A chest x‐ray showed focal airspace opacity in the left lower lobe (Figure 1) and the patient was hospitalized for further management.
The normal CSF analysis makes acute meningitis much less likely. It is interesting to note that the aminotransferase levels are nearly equal. Usually, in viral and many other causes of hepatitis, the ALT is higher than the AST, whereas the contrary is true in alcoholic hepatitis. Because the patient has been consuming significant amounts of alcohol recently, these levels may become equal in the setting of another primary liver process. The elevation in liver enzymes also raises the possibility of autoimmune hepatitis secondary to a systemic vasculitis such as systemic lupus erythematosus. Nonetheless, the focus should be on infectious causes of hepatitis such as hepatitis C, adenovirus, parvovirus, Epstein‐Barr virus (EBV), cytomegalovirus, and herpes simplex virus that can cause pneumonia either as a primary or secondary infection. Acute HIV infection can also present in this fashion, and anti‐HIV antibody testing may be negative early in the disease. In the setting of a normal urinalysis and bland urine sediment, prerenal azotemia is the most likely cause of her acute renal injury and can be confirmed by testing the urinary sodium and creatinine. A peripheral smear should be reviewed to evaluate the pancytopenia.
Severe headache, fever, conjunctival injection, pancytopenia, acute kidney injury, hepatitis, and pneumonia may occur in leptospirosis, particularly in a patient with recent freshwater exposure. Alternatively, ehrlichiosis can also account for fever, headache, pancytopenia, renal failure, hepatitis, and pneumonia, but conjunctival suffusion is not often present. At this time, treatment for community‐acquired pneumonia that includes coverage for leptospirosis should be started.
The patient was hydrated with intravenous fluids and treated with intravenous ceftriaxone and azithromycin for community‐acquired pneumonia. An abdominal ultrasound was normal. The serologic assays for acute hepatitis A, B, and C infection were negative. The following morning, she reported worsening headache, increased cough now productive of whitish‐yellow sputum, and diffuse body aches. She appeared more lethargic and toxic. Her blood pressure was 100/83 mm Hg, heart rate was 84 beats per minute, respiratory rate was 24 per minute, and temperature was 101.3F. She had increased crackles on chest auscultation bilaterally and required supplemental oxygen at 4 L/minute by nasal cannula. Examination of both legs now revealed multiple scattered, faintly erythematous, 2‐cm‐sized patches overlying tender subtle subcutaneous nodules. Additionally, a mildly pruritic, V‐shaped area of blanchable erythema was also seen on her chest. The white blood cell count was 2500/mm3 (77% neutrophils, 15% lymphocytes), serum creatinine was 1.8 mg/dL, AST was 351 IU/L, and ALT was 485 IU/L. Blood cultures showed no growth and a peripheral smear examination was unrevealing. A noncontrast chest computed tomographic scan showed findings consistent with multifocal pneumonia (Figure 2).
It would be prudent at this time to expand her antimicrobial coverage (such as with vancomycin and piperacillin‐tazobactam) for activity against methicillin‐resistant Staphylococcus aureus and Pseudomonas because of her clinical worsening. Although ceftriaxone or piperacillin would cover leptospirosis, given the possibility of ehrlichiosis, the addition of doxycycline should be strongly considered.
The description of the rash on her legs seems consistent with erythema nodosum, which is associated with a number of infections (streptococcal, fungal, syphilis, EBV, cat‐scratch disease, tuberculosis), inflammatory conditions (inflammatory bowel disease, autoimmune disease, malignancy), and pregnancy. The blanchable rash on the chest is also a cause of concern for a possible drug reaction (ceftriaxone). A Jarisch‐Herxheimer reaction is possible given her acute worsening of symptoms with initiation of antibiotic therapy.
An antineutrophil cytoplasmic antibodyassociated vasculitis or another autoimmune condition such as systemic lupus erythematosus can account for erythema nodosum, rash, pancytopenia, and hepatitis. This diagnosis might also fit if she had a vasculitic pulmonary hemorrhage that caused her lung infiltrates and worsening hypoxia. A complete antinuclear antibody panel, antineutrophil cytoplasmic antibody, and antismooth muscle antibody testing is recommended. A skin and bronchoscopic biopsy should be considered.
Her dose of ceftriaxone was increased for possible severe pneumococcal pneumonia. The dermatology consultant felt that her leg lesions were consistent with erythema nodosum and the chest rash consistent with cutaneous photodamage. Bronchoscopic examination was normal and a bronchoalveolar lavage sample showed 2905 red blood cells/mm3 and 605 white blood cells/mm3 (70% neutrophils, 7% lymphocytes, 16% histiocytes), normal cytology, and negative cultures. There was no significant clinical improvement by the fourth hospital day and oral doxycycline was started. The next day, her skin lesions had resolved and she felt better. The serologic tests for Legionella, Mycoplasma, cytomegalovirus, EBV, Toxoplasma, Chlamydophila, Ehrlichia, Leptospira, Q‐fever, parvovirus, and adenovirus were negative. A fungal serology panel, HIV polymerase chain reaction, cryoglobulin level, and several rheumatologic tests (antinuclear antibody, extractable nuclear antigen panel, rheumatoid factor, antineutrophil cytoplasmic antibody, antiproteinase 3, and antiglomerular basement membrane antibodies) were normal. Blood cultures continued to show no growth.
The apparent response to doxycycline suggests she might have ehrlichiosis. A buffy coat review for morulae should be done. It is also possible that she may have improved on her initial therapy alone before starting doxycycline and her clinical worsening (including the chest rash) was due to a Jarisch‐Herxheimer reaction. Serologic tests for leptospirosis and ehrlichiosis should be repeated in 12 weeks because such infections may not cause detectable antibody levels early in the illness.
Ceftriaxone and doxycycline were continued and she showed rapid and significant clinical improvement. She was discharged 4 days later with instructions to complete a 10‐day course of antibiotics. At her 3‐month follow‐up, she was doing well and a repeat Leptospira antibody test by the Indirect Hemagglutination Assay (MRL Diagnostics, Cypress, California; normal titer <1:50) was positive at a titer of 1:100, which is highly suggestive of leptospirosis.
Commentary
Leptospirosis is a zoonotic infection caused by spirochetes of the genus Leptospira. The infection is usually transmitted indirectly to humans through contact with water, food, or soil contaminated with the urine of infected mammals.1 Risk factors for infection include participation in recreational activities (such as freshwater swimming, canoeing, and camping), occupational exposure, and exposure to infected pets or domesticated livestock. Approximately 100200 cases are identified annually in the United States, and approximately half occur in the state of Hawaii.2 Outbreaks of leptospirosis have been reported previously in the Midwest.3 These organisms inoculate humans through contact with mucous membranes or broken skin, or enter by swallowing infected food or water. A large number of these infections remain subclinical or result in a very mild illness with spontaneous clearance by the host's immune mechanism. Following an incubation period of 230 days, infected individuals may develop clinically significant disease (Table 1). Clinical presentations may overlap as the disease progresses. Although much remains to be learned about the exact pathogenic mechanism, disruption of the cell membranes of small vessel endothelia (a toxin‐like effect), and cytokine‐mediated tissue injury are believed to cause organ hemorrhage and ischemia.4
|
| 1. Mild influenza‐like self‐remitting disease (90% of cases) |
| Undifferentiated fever (usually 100F105F), severe headache, and myalgia (especially lower limbs). |
| 2. Moderately severe disease usually requiring hospitalization (5%9% of cases) |
| Marked prostration, anorexia, nausea, and vomiting, conjunctival suffusion, transient rash, frequently abdominal pain, constipation or diarrhea, and occasionally epistaxis. |
| 3. Severe disease involving multiple organ systems (1%5% of cases) |
| Hepatorenal Syndrome (Weil's syndrome) |
| Constellation of jaundice, hemorrhagic diathesis, and acute renal failure. Hepatic failure is rarely fatal. Renal involvement is usually more severe and the common cause of death. Cardiac (myocarditis with arrhythmias) and pulmonary complications are frequent. Confusion and restlessness may occur. |
| Hemorrhagic pneumonitis |
| Usually presents as a dry cough initially but becomes blood‐streaked after 23 days. Often characterized by a rapid progression to involve extensive areas of lungs, massive intra‐alveolar hemorrhage, acute respiratory failure, and death. |
| Central nervous system involvement |
| Meningismus, meningitis, or meningoencephalitis. |
The clinical diagnosis of leptospirosis is difficult because of its protean manifestations. Although nonspecific, 2 clinical features may provide a clue to the clinical diagnosis. First, the presence of conjunctival suffusion occurs in the early stage of the disease and is often associated with subconjunctival hemorrhage. Second, severe myalgia, commonly involving the lower limbs, is also characteristically present.1, 5 In 1 series of 58 patients with acute leptospirosis, conjunctival suffusion was observed in 50% of cases, and subconjunctival hemorrhage in 29%. Body ache and muscle tenderness was described in almost all cases.6
As seen in this case, the presence of a rash may pose a clinical challenge. A transient macular, maculopapular, purpuric, or urticarial rash may be seen in acute leptospirosis, but rashes may also be representative of a complication of treatment.1 First described in 1895 in patients with syphilis treated with mercury, the Jarisch‐Herxheimer reaction typically occurs within a few hours of antimicrobial treatment of spirochete infections and often presents with a rash, headache, fever, rigors, hypotension, sweating, and worsening symptoms of the underlying illness.7 Other skin findings such as the occurrence of erythema nodosum have been previously reported in cases of leptospirosis.8
Human ehrlichiosis (HE) is caused by tickborne, obligatory intracellular bacteria that infect leukocytes. There are 3 distinct clinical conditions: human monocytic ehrlichiosis (HME, caused by Ehrlichia chaffeensis), human granulocytic anaplasmosis (HGA, caused by Anaplasma phagocytophilum), and human ewingii ehrlichiosis (HEE, caused by E. ewingii). Although most cases of HME and HEE are seen in the southeastern and south‐central United States and California, the highest incidence of HGA is reported in the northeastern and upper Midwest regions.9 As with leptospirosis, the clinical range of HE spans from asymptomatic infection to life‐threatening illness. Following an incubation period of 12 weeks, symptomatic cases usually present with nonspecific complaints such as high fevers, chills, headache, nausea, arthralgia, myalgia, and malaise.10 The majority of cases will report a tick bite or an exposure to ticks. Laboratory tests often reveal leukopenia (white blood cell count < 4000/mm3), thrombocytopenia, hyponatremia, and elevated AST and ALT. Patients with severe disease may develop renal, respiratory, and hepatic failure. Thus, differentiating ehrlichiosis from leptospirosis is often challenging for the clinician.
However, there are a few clinical clues that help distinguish between these illnesses in this case. HGA as a cause of HE would be more likely in the Midwest. Although a rash is present in one‐third of patients with HME, it is seldom present in HGA unless coinfected with Borrelia burgdorferi, the causative agent for Lyme disease. Additionally, her history of freshwater exposure and the absence of a history of a tick bite also favor leptospirosis. As noted previously, conjunctival suffusion, a characteristic clinical feature of leptospirosis, has only been described in case reports of HE.11, 12
Serologic tests are often used to establish the diagnosis of leptospirosis and ehrlichiosis. Leptospires are fastidious organisms that are difficult to isolate on inoculated growth media. The microscopic agglutination test for leptospirosis is considered the diagnostic gold standard due to its high specificity, but its use is limited by its technical complexity, lack of availability (other than in reference laboratories), and low sensitivity early in the disease (antibody levels detected by this method usually do not appear until 7 days after symptom onset).13 A variety of rapid serologic assays are also available. Although these tests have good overall sensitivity (ranging between 79% and 93%), they perform relatively poorly for acute‐phase sera (sensitivity of 38.5%52.7%).13 The high early false negative rate is believed to be a result of inadequate Leptospira antibody titers in the acute phase of the illness. Seroconversion or a 4‐fold rise between acute and convalescent‐phase antibody titers is the most definitive criterion for the diagnosis of leptospirosis. However, without paired sera samples, a single high microscopic agglutination test titer can be taken as diagnostic for leptospirosis depending on the degree of regional endemicity.14
Similarly, currently available serologic assays for ehrlichiosis produce negative results in most patients in the first week of illness, and it is important to obtain a convalescent phase serum specimen for confirmatory diagnosis of HME and HGA. Seroconversion or a 4‐fold increase in titer between acute and convalescent phase sera is considered diagnostic. The sensitivity of finding morulae (intracytoplasmic vacuolar microcolonies of Ehrlichia) on a peripheral smear is unknown, and data suggest that this finding is more common in cases of HGA compared to HME.15
Although doxycycline is the drug of choice for the treatment of ehrlichiosis, Leptospira is susceptible to a wide variety of antibiotics because it exhibits a double membrane surface architecture with components common to both gram‐negative and gram‐positive bacteria.1 Recommended treatment regimens for severe leptospirosis include the use of high‐dose intravenous penicillin or a third‐generation cephalosporin. Less severe cases can be treated with oral amoxicillin or doxycycline.16 The fact that this patient's clinical improvement appeared to lag after initiation of ceftriaxone does not necessarily indicate a lack of efficacy but perhaps a Jarisch‐Herxheimer reaction in response to appropriate antibiotic therapy.
Teaching Points
-
Establishing a diagnosis of leptospirosis is challenging and requires a high index of suspicion. Clinicians should be aware of the limitations of the diagnostic accuracy of the serologic assays for leptospirosis because they are frequently negative in the first week after symptom onset.
-
The classic finding of conjunctival suffusion is helpful in differentiating leptospirosis from human ehrlichiosis.
-
This case also highlights the importance of the clinical practice of making a list of suspected diagnoses, remaining open to these possibilities, and checking serologic tests again in convalescence to confirm the diagnosis.
The approach to clinical conundrums by an expert clinician is revealed through the presentation of an actual patient's case in an approach typical of a morning report. Similarly to patient care, sequential pieces of information are provided to the clinician, who is unfamiliar with the case. The focus is on the thought processes of both the clinical team caring for the patient and the discussant.
Acknowledgements
The authors thank Dr. Brian Harte for his valuable guidance in the preparation of this manuscript.
- ,,.Leptospirosis: an emerging global public health problem.J Biosci.2008;33:557–569.
- Centers for Disease Control and Prevention. Leptospirosis.2005. http://www.cdc.gov/ncidod/dbmd/diseaseinfo/leptospirosis_t.htm. Accessed November 15,year="2010"2010.
- Morbidity and Mortality Weekly Report.From the Centers for Disease Control and Prevention. Update: leptospirosis and unexplained acute febrile illness among athletes participating in triathlons—Illinois and Wisconsin, 1998.JAMA.1998;280:1474–1475.
- ,.Optimal treatment of leptospirosis: queries and projections.Int J Antimicrob Agents.2006;28:491–496.
- ,.Leptospirosis in the tropics and in travelers.Curr Infect Dis Rep.2006;8:51–58.
- ,,,,,.Clinico‐epidemiological study of hospitalized cases of severe leptospirosis.Indian J Med Res.1999;109:94–99.
- ,.Proposed mechanisms and preventative options of Jarisch‐Herxheimer reactions.J Clin Pharm Ther.2005;30:291–295.
- .Leptospirosis presenting with erythema nodosum.Arch Dis Child.1977;52:418–419.
- ,,.Emerging and re‐emerging tick‐transmitted rickettsial and ehrlichial infections.Med Clin N Am.2008;92:1345–1361.
- ,.Tick‐borne ehrlichiosis infection in human beings.J Vector Borne Dis.2008;45:273–280.
- ,.Ehrlichia in Tennessee.South Med J.1989;82:669.
- ,,,,,.Ehrlichial meningitis with cerebrospinal fluid morulae.Pediatr Infect Dis J.1999;18:552–555.
- ,,, et al.Evaluation of four commercially available rapid serologic tests for diagnosis of leptospirosis.J Clin Microbiol.2003;41:803–809.
- ,.Diagnosis of leptospirosis utilizing modified Faine's criteria.J Assoc Physicians India.2004;52:678–679.
- ,.Ehrlichiosis in children.J Pediatr.1997;131:184–192.
- ,World Health Organization, International Leptospirosis Society. Human leptospirosis: guidance for diagnosis, surveillance and control.Geneva, Switzerland:World Health Organization;2003.
In October, a 36‐year‐old woman with no significant past medical history presented to the Emergency Department (ED) with a 3‐day history of headache and fever. The headache was severe, throbbing, and frontal in location. She also complained of daily fevers measured up to 103F, generalized malaise, and fatigue. She did not report neck stiffness or photophobia. She felt better after receiving intravenous fluids and was discharged home with a diagnosis of a nonspecific viral illness. Two days later, she returned to the ED with worsening headache, fever, mild photophobia, and poor oral intake. She also complained of a dry cough that made her headache worse, as did bending over. She did not report confusion, neck stiffness, shortness of breath, sore throat, runny nose, abdominal symptoms, or rash.
This patient presents a second time to the ED with worsening headache and fever raising concerns about meningitis. At the time of her first ED visit, it can be assumed that she had a nontoxic appearance because she was discharged shortly thereafter. Thus, acute bacterial meningitis seems less likely, but occasionally patients with meningococcal meningitis may not appear significantly ill until later in the process. Nonetheless, acute meningitis, possibly viral, is the initial concern. The time of the year is an important variable because many viral infections are seasonal. Enteroviruses are the most common cause of viral meningitis in the United States, particularly in the summer and fall. In contrast, mumps, measles, and varicella zoster viruses occur more commonly in winter and spring. Herpetic meningoencephalitis is a life‐threatening condition with a guarded prognosis. Therefore, early recognition and treatment is necessary to decrease morbidity and mortality. Drugs such as nonsteroidal anti‐inflammatory agents, trimethoprim‐sulfamethoxazole, amoxicillin, and rarely vaccines can also cause aseptic meningitis. Infections from fungi, spirochetes, mycobacteria, and rarely parasites also cause meningitis, but would be of greater concern in a patient with risk factors such as recent travel or an immunocompromised state.
Increased headache with bending and cough might indicate elevated intracranial pressure. However, this is a nonspecific complaint, and headache is often worse with the Valsalva maneuver. Because she reports a cough, a chest x‐ray would be useful. In addition to routine initial tests, cerebrospinal fluid (CSF) analysis and human immunodeficiency virus (HIV) testing is recommended.
Her past medical history was notable for depression. Her medications included bupropion, multivitamins, and fish oil. She was also taking milk thistle pills daily to protect her liver because she had been drinking alcohol heavily for the past 2 weeks since her husband left her. She smoked 1 pack of cigarettes daily. She had not traveled recently. She reported no recent animal or wildlife exposure but did recall falling into a midwestern river while canoeing 2 weeks prior to presentation. She worked as a hairstylist and described no sick contacts or risk factors for HIV disease.
An important new historical element is that the patient fell into a river. If she swallowed a significant amount of water during her fall overboard, meningitis from waterborne infections such as Aeromonas, Acanthamoeba, and Naegleria need to be considered. Fortunately, these are rare in the Midwest. Her canoeing history may suggest exposure to wooded areas. Certainly, tickborne infections such as ehrlichiosis, babesiosis, Lyme disease, and Rocky Mountain spotted fever can also cause meningitis. Histoplasmosis and blastomycosis are also endemic to the midwestern United States and can disseminate and cause central nervous system disease.
At this time, viral and bacterial infections are highest on the differential diagnosis. However, the microbiology laboratory needs to be alerted to the possibility of fungal or parasitic organisms depending on the initial CSF analysis results.
The patient was a Caucasian woman who appeared comfortable. Her blood pressure was 130/62 mm Hg, heart rate was 83 beats per minute, respiratory rate was 18 per minute, temperature was 100.8F, and oxygen saturation was 98% on room air. She was fully alert and oriented. Her pupils were bilaterally equal, reactive to light and accommodation with intact extraocular movement and no nystagmus. There was conjunctival injection bilaterally without noticeable pallor or icterus. Fundoscopic examination, which the patient tolerated without difficulty, was normal. Inspection of the oral cavity showed mild tonsillar enlargement. The neck was supple with no stiffness. No cervical, axillary, or inguinal lymph nodes were palpable. Faint bilateral basilar crackles were audible over the posterior chest. There was very mild right upper quadrant abdominal tenderness without guarding. The liver and spleen were normal size and bowel sounds were present. No rash, peripheral edema, or spinal tenderness was noted. A complete neurological examination was normal.
Her general appearance and vital signs seem reassuring. Conjunctival injection and mild tonsillar enlargement are nonspecific findings and may occur in systemic inflammatory states especially viral infections. Atelectasis may account for faint bilateral basilar crackles especially if associated with post‐tussive change. Her alcohol use puts her at risk of aspiration. A right lower lobe process (pneumonia) can sometimes present with right upper quadrant tenderness. However, this tenderness may also represent muscle soreness from repeated coughing, liver, or gallbladder disease. The same infectious process affecting the central nervous system and possibly her lungs, may also be affecting the liver.
A complete blood count revealed a white blood cell count of 3000/mm3 (79% neutrophils, 15% lymphocytes, 5% monocytes), hemoglobin of 11.7 g/dL, and platelets of 110,000/mm3. The serum sodium was 133 mmol/L, potassium was 3.7 mmol/L, bicarbonate was 22 mmol/L, and blood urea nitrogen was 20 mg/dL. The serum creatinine was 1.5 compared to 1.0 mg/dL on testing 2 days prior. A liver function panel showed protein of 5.1 g/dL, albumin of 3 g/dL, aspartate aminotransferase (AST) of 576 IU/L, alanine aminotransferase (ALT) of 584 IU/L, alkaline phosphatase of 282 IU/L, and total bilirubin of 1 mg/dL. The coagulation profile, creatinine phosphokinase, acetaminophen level, urine pregnancy test, urine drug screen, and urinalysis (including urine microscopy) were normal.
The CSF opening pressure was 13 cm H2O. CSF analysis showed 4 mononuclear leukocytes per high‐power field, CSF protein was 27 mg/dL, and glucose was 76 mg/dL. No organisms were noted on gram stain. A chest x‐ray showed focal airspace opacity in the left lower lobe (Figure 1) and the patient was hospitalized for further management.

The normal CSF analysis makes acute meningitis much less likely. It is interesting to note that the aminotransferase levels are nearly equal. Usually, in viral and many other causes of hepatitis, the ALT is higher than the AST, whereas the contrary is true in alcoholic hepatitis. Because the patient has been consuming significant amounts of alcohol recently, these levels may become equal in the setting of another primary liver process. The elevation in liver enzymes also raises the possibility of autoimmune hepatitis secondary to a systemic vasculitis such as systemic lupus erythematosus. Nonetheless, the focus should be on infectious causes of hepatitis such as hepatitis C, adenovirus, parvovirus, Epstein‐Barr virus (EBV), cytomegalovirus, and herpes simplex virus that can cause pneumonia either as a primary or secondary infection. Acute HIV infection can also present in this fashion, and anti‐HIV antibody testing may be negative early in the disease. In the setting of a normal urinalysis and bland urine sediment, prerenal azotemia is the most likely cause of her acute renal injury and can be confirmed by testing the urinary sodium and creatinine. A peripheral smear should be reviewed to evaluate the pancytopenia.
Severe headache, fever, conjunctival injection, pancytopenia, acute kidney injury, hepatitis, and pneumonia may occur in leptospirosis, particularly in a patient with recent freshwater exposure. Alternatively, ehrlichiosis can also account for fever, headache, pancytopenia, renal failure, hepatitis, and pneumonia, but conjunctival suffusion is not often present. At this time, treatment for community‐acquired pneumonia that includes coverage for leptospirosis should be started.
The patient was hydrated with intravenous fluids and treated with intravenous ceftriaxone and azithromycin for community‐acquired pneumonia. An abdominal ultrasound was normal. The serologic assays for acute hepatitis A, B, and C infection were negative. The following morning, she reported worsening headache, increased cough now productive of whitish‐yellow sputum, and diffuse body aches. She appeared more lethargic and toxic. Her blood pressure was 100/83 mm Hg, heart rate was 84 beats per minute, respiratory rate was 24 per minute, and temperature was 101.3F. She had increased crackles on chest auscultation bilaterally and required supplemental oxygen at 4 L/minute by nasal cannula. Examination of both legs now revealed multiple scattered, faintly erythematous, 2‐cm‐sized patches overlying tender subtle subcutaneous nodules. Additionally, a mildly pruritic, V‐shaped area of blanchable erythema was also seen on her chest. The white blood cell count was 2500/mm3 (77% neutrophils, 15% lymphocytes), serum creatinine was 1.8 mg/dL, AST was 351 IU/L, and ALT was 485 IU/L. Blood cultures showed no growth and a peripheral smear examination was unrevealing. A noncontrast chest computed tomographic scan showed findings consistent with multifocal pneumonia (Figure 2).

It would be prudent at this time to expand her antimicrobial coverage (such as with vancomycin and piperacillin‐tazobactam) for activity against methicillin‐resistant Staphylococcus aureus and Pseudomonas because of her clinical worsening. Although ceftriaxone or piperacillin would cover leptospirosis, given the possibility of ehrlichiosis, the addition of doxycycline should be strongly considered.
The description of the rash on her legs seems consistent with erythema nodosum, which is associated with a number of infections (streptococcal, fungal, syphilis, EBV, cat‐scratch disease, tuberculosis), inflammatory conditions (inflammatory bowel disease, autoimmune disease, malignancy), and pregnancy. The blanchable rash on the chest is also a cause of concern for a possible drug reaction (ceftriaxone). A Jarisch‐Herxheimer reaction is possible given her acute worsening of symptoms with initiation of antibiotic therapy.
An antineutrophil cytoplasmic antibodyassociated vasculitis or another autoimmune condition such as systemic lupus erythematosus can account for erythema nodosum, rash, pancytopenia, and hepatitis. This diagnosis might also fit if she had a vasculitic pulmonary hemorrhage that caused her lung infiltrates and worsening hypoxia. A complete antinuclear antibody panel, antineutrophil cytoplasmic antibody, and antismooth muscle antibody testing is recommended. A skin and bronchoscopic biopsy should be considered.
Her dose of ceftriaxone was increased for possible severe pneumococcal pneumonia. The dermatology consultant felt that her leg lesions were consistent with erythema nodosum and the chest rash consistent with cutaneous photodamage. Bronchoscopic examination was normal and a bronchoalveolar lavage sample showed 2905 red blood cells/mm3 and 605 white blood cells/mm3 (70% neutrophils, 7% lymphocytes, 16% histiocytes), normal cytology, and negative cultures. There was no significant clinical improvement by the fourth hospital day and oral doxycycline was started. The next day, her skin lesions had resolved and she felt better. The serologic tests for Legionella, Mycoplasma, cytomegalovirus, EBV, Toxoplasma, Chlamydophila, Ehrlichia, Leptospira, Q‐fever, parvovirus, and adenovirus were negative. A fungal serology panel, HIV polymerase chain reaction, cryoglobulin level, and several rheumatologic tests (antinuclear antibody, extractable nuclear antigen panel, rheumatoid factor, antineutrophil cytoplasmic antibody, antiproteinase 3, and antiglomerular basement membrane antibodies) were normal. Blood cultures continued to show no growth.
The apparent response to doxycycline suggests she might have ehrlichiosis. A buffy coat review for morulae should be done. It is also possible that she may have improved on her initial therapy alone before starting doxycycline and her clinical worsening (including the chest rash) was due to a Jarisch‐Herxheimer reaction. Serologic tests for leptospirosis and ehrlichiosis should be repeated in 12 weeks because such infections may not cause detectable antibody levels early in the illness.
Ceftriaxone and doxycycline were continued and she showed rapid and significant clinical improvement. She was discharged 4 days later with instructions to complete a 10‐day course of antibiotics. At her 3‐month follow‐up, she was doing well and a repeat Leptospira antibody test by the Indirect Hemagglutination Assay (MRL Diagnostics, Cypress, California; normal titer <1:50) was positive at a titer of 1:100, which is highly suggestive of leptospirosis.
Commentary
Leptospirosis is a zoonotic infection caused by spirochetes of the genus Leptospira. The infection is usually transmitted indirectly to humans through contact with water, food, or soil contaminated with the urine of infected mammals.1 Risk factors for infection include participation in recreational activities (such as freshwater swimming, canoeing, and camping), occupational exposure, and exposure to infected pets or domesticated livestock. Approximately 100200 cases are identified annually in the United States, and approximately half occur in the state of Hawaii.2 Outbreaks of leptospirosis have been reported previously in the Midwest.3 These organisms inoculate humans through contact with mucous membranes or broken skin, or enter by swallowing infected food or water. A large number of these infections remain subclinical or result in a very mild illness with spontaneous clearance by the host's immune mechanism. Following an incubation period of 230 days, infected individuals may develop clinically significant disease (Table 1). Clinical presentations may overlap as the disease progresses. Although much remains to be learned about the exact pathogenic mechanism, disruption of the cell membranes of small vessel endothelia (a toxin‐like effect), and cytokine‐mediated tissue injury are believed to cause organ hemorrhage and ischemia.4
|
| 1. Mild influenza‐like self‐remitting disease (90% of cases) |
| Undifferentiated fever (usually 100F105F), severe headache, and myalgia (especially lower limbs). |
| 2. Moderately severe disease usually requiring hospitalization (5%9% of cases) |
| Marked prostration, anorexia, nausea, and vomiting, conjunctival suffusion, transient rash, frequently abdominal pain, constipation or diarrhea, and occasionally epistaxis. |
| 3. Severe disease involving multiple organ systems (1%5% of cases) |
| Hepatorenal Syndrome (Weil's syndrome) |
| Constellation of jaundice, hemorrhagic diathesis, and acute renal failure. Hepatic failure is rarely fatal. Renal involvement is usually more severe and the common cause of death. Cardiac (myocarditis with arrhythmias) and pulmonary complications are frequent. Confusion and restlessness may occur. |
| Hemorrhagic pneumonitis |
| Usually presents as a dry cough initially but becomes blood‐streaked after 23 days. Often characterized by a rapid progression to involve extensive areas of lungs, massive intra‐alveolar hemorrhage, acute respiratory failure, and death. |
| Central nervous system involvement |
| Meningismus, meningitis, or meningoencephalitis. |
The clinical diagnosis of leptospirosis is difficult because of its protean manifestations. Although nonspecific, 2 clinical features may provide a clue to the clinical diagnosis. First, the presence of conjunctival suffusion occurs in the early stage of the disease and is often associated with subconjunctival hemorrhage. Second, severe myalgia, commonly involving the lower limbs, is also characteristically present.1, 5 In 1 series of 58 patients with acute leptospirosis, conjunctival suffusion was observed in 50% of cases, and subconjunctival hemorrhage in 29%. Body ache and muscle tenderness was described in almost all cases.6
As seen in this case, the presence of a rash may pose a clinical challenge. A transient macular, maculopapular, purpuric, or urticarial rash may be seen in acute leptospirosis, but rashes may also be representative of a complication of treatment.1 First described in 1895 in patients with syphilis treated with mercury, the Jarisch‐Herxheimer reaction typically occurs within a few hours of antimicrobial treatment of spirochete infections and often presents with a rash, headache, fever, rigors, hypotension, sweating, and worsening symptoms of the underlying illness.7 Other skin findings such as the occurrence of erythema nodosum have been previously reported in cases of leptospirosis.8
Human ehrlichiosis (HE) is caused by tickborne, obligatory intracellular bacteria that infect leukocytes. There are 3 distinct clinical conditions: human monocytic ehrlichiosis (HME, caused by Ehrlichia chaffeensis), human granulocytic anaplasmosis (HGA, caused by Anaplasma phagocytophilum), and human ewingii ehrlichiosis (HEE, caused by E. ewingii). Although most cases of HME and HEE are seen in the southeastern and south‐central United States and California, the highest incidence of HGA is reported in the northeastern and upper Midwest regions.9 As with leptospirosis, the clinical range of HE spans from asymptomatic infection to life‐threatening illness. Following an incubation period of 12 weeks, symptomatic cases usually present with nonspecific complaints such as high fevers, chills, headache, nausea, arthralgia, myalgia, and malaise.10 The majority of cases will report a tick bite or an exposure to ticks. Laboratory tests often reveal leukopenia (white blood cell count < 4000/mm3), thrombocytopenia, hyponatremia, and elevated AST and ALT. Patients with severe disease may develop renal, respiratory, and hepatic failure. Thus, differentiating ehrlichiosis from leptospirosis is often challenging for the clinician.
However, there are a few clinical clues that help distinguish between these illnesses in this case. HGA as a cause of HE would be more likely in the Midwest. Although a rash is present in one‐third of patients with HME, it is seldom present in HGA unless coinfected with Borrelia burgdorferi, the causative agent for Lyme disease. Additionally, her history of freshwater exposure and the absence of a history of a tick bite also favor leptospirosis. As noted previously, conjunctival suffusion, a characteristic clinical feature of leptospirosis, has only been described in case reports of HE.11, 12
Serologic tests are often used to establish the diagnosis of leptospirosis and ehrlichiosis. Leptospires are fastidious organisms that are difficult to isolate on inoculated growth media. The microscopic agglutination test for leptospirosis is considered the diagnostic gold standard due to its high specificity, but its use is limited by its technical complexity, lack of availability (other than in reference laboratories), and low sensitivity early in the disease (antibody levels detected by this method usually do not appear until 7 days after symptom onset).13 A variety of rapid serologic assays are also available. Although these tests have good overall sensitivity (ranging between 79% and 93%), they perform relatively poorly for acute‐phase sera (sensitivity of 38.5%52.7%).13 The high early false negative rate is believed to be a result of inadequate Leptospira antibody titers in the acute phase of the illness. Seroconversion or a 4‐fold rise between acute and convalescent‐phase antibody titers is the most definitive criterion for the diagnosis of leptospirosis. However, without paired sera samples, a single high microscopic agglutination test titer can be taken as diagnostic for leptospirosis depending on the degree of regional endemicity.14
Similarly, currently available serologic assays for ehrlichiosis produce negative results in most patients in the first week of illness, and it is important to obtain a convalescent phase serum specimen for confirmatory diagnosis of HME and HGA. Seroconversion or a 4‐fold increase in titer between acute and convalescent phase sera is considered diagnostic. The sensitivity of finding morulae (intracytoplasmic vacuolar microcolonies of Ehrlichia) on a peripheral smear is unknown, and data suggest that this finding is more common in cases of HGA compared to HME.15
Although doxycycline is the drug of choice for the treatment of ehrlichiosis, Leptospira is susceptible to a wide variety of antibiotics because it exhibits a double membrane surface architecture with components common to both gram‐negative and gram‐positive bacteria.1 Recommended treatment regimens for severe leptospirosis include the use of high‐dose intravenous penicillin or a third‐generation cephalosporin. Less severe cases can be treated with oral amoxicillin or doxycycline.16 The fact that this patient's clinical improvement appeared to lag after initiation of ceftriaxone does not necessarily indicate a lack of efficacy but perhaps a Jarisch‐Herxheimer reaction in response to appropriate antibiotic therapy.
Teaching Points
-
Establishing a diagnosis of leptospirosis is challenging and requires a high index of suspicion. Clinicians should be aware of the limitations of the diagnostic accuracy of the serologic assays for leptospirosis because they are frequently negative in the first week after symptom onset.
-
The classic finding of conjunctival suffusion is helpful in differentiating leptospirosis from human ehrlichiosis.
-
This case also highlights the importance of the clinical practice of making a list of suspected diagnoses, remaining open to these possibilities, and checking serologic tests again in convalescence to confirm the diagnosis.
The approach to clinical conundrums by an expert clinician is revealed through the presentation of an actual patient's case in an approach typical of a morning report. Similarly to patient care, sequential pieces of information are provided to the clinician, who is unfamiliar with the case. The focus is on the thought processes of both the clinical team caring for the patient and the discussant.
Acknowledgements
The authors thank Dr. Brian Harte for his valuable guidance in the preparation of this manuscript.
In October, a 36‐year‐old woman with no significant past medical history presented to the Emergency Department (ED) with a 3‐day history of headache and fever. The headache was severe, throbbing, and frontal in location. She also complained of daily fevers measured up to 103F, generalized malaise, and fatigue. She did not report neck stiffness or photophobia. She felt better after receiving intravenous fluids and was discharged home with a diagnosis of a nonspecific viral illness. Two days later, she returned to the ED with worsening headache, fever, mild photophobia, and poor oral intake. She also complained of a dry cough that made her headache worse, as did bending over. She did not report confusion, neck stiffness, shortness of breath, sore throat, runny nose, abdominal symptoms, or rash.
This patient presents a second time to the ED with worsening headache and fever raising concerns about meningitis. At the time of her first ED visit, it can be assumed that she had a nontoxic appearance because she was discharged shortly thereafter. Thus, acute bacterial meningitis seems less likely, but occasionally patients with meningococcal meningitis may not appear significantly ill until later in the process. Nonetheless, acute meningitis, possibly viral, is the initial concern. The time of the year is an important variable because many viral infections are seasonal. Enteroviruses are the most common cause of viral meningitis in the United States, particularly in the summer and fall. In contrast, mumps, measles, and varicella zoster viruses occur more commonly in winter and spring. Herpetic meningoencephalitis is a life‐threatening condition with a guarded prognosis. Therefore, early recognition and treatment is necessary to decrease morbidity and mortality. Drugs such as nonsteroidal anti‐inflammatory agents, trimethoprim‐sulfamethoxazole, amoxicillin, and rarely vaccines can also cause aseptic meningitis. Infections from fungi, spirochetes, mycobacteria, and rarely parasites also cause meningitis, but would be of greater concern in a patient with risk factors such as recent travel or an immunocompromised state.
Increased headache with bending and cough might indicate elevated intracranial pressure. However, this is a nonspecific complaint, and headache is often worse with the Valsalva maneuver. Because she reports a cough, a chest x‐ray would be useful. In addition to routine initial tests, cerebrospinal fluid (CSF) analysis and human immunodeficiency virus (HIV) testing is recommended.
Her past medical history was notable for depression. Her medications included bupropion, multivitamins, and fish oil. She was also taking milk thistle pills daily to protect her liver because she had been drinking alcohol heavily for the past 2 weeks since her husband left her. She smoked 1 pack of cigarettes daily. She had not traveled recently. She reported no recent animal or wildlife exposure but did recall falling into a midwestern river while canoeing 2 weeks prior to presentation. She worked as a hairstylist and described no sick contacts or risk factors for HIV disease.
An important new historical element is that the patient fell into a river. If she swallowed a significant amount of water during her fall overboard, meningitis from waterborne infections such as Aeromonas, Acanthamoeba, and Naegleria need to be considered. Fortunately, these are rare in the Midwest. Her canoeing history may suggest exposure to wooded areas. Certainly, tickborne infections such as ehrlichiosis, babesiosis, Lyme disease, and Rocky Mountain spotted fever can also cause meningitis. Histoplasmosis and blastomycosis are also endemic to the midwestern United States and can disseminate and cause central nervous system disease.
At this time, viral and bacterial infections are highest on the differential diagnosis. However, the microbiology laboratory needs to be alerted to the possibility of fungal or parasitic organisms depending on the initial CSF analysis results.
The patient was a Caucasian woman who appeared comfortable. Her blood pressure was 130/62 mm Hg, heart rate was 83 beats per minute, respiratory rate was 18 per minute, temperature was 100.8F, and oxygen saturation was 98% on room air. She was fully alert and oriented. Her pupils were bilaterally equal, reactive to light and accommodation with intact extraocular movement and no nystagmus. There was conjunctival injection bilaterally without noticeable pallor or icterus. Fundoscopic examination, which the patient tolerated without difficulty, was normal. Inspection of the oral cavity showed mild tonsillar enlargement. The neck was supple with no stiffness. No cervical, axillary, or inguinal lymph nodes were palpable. Faint bilateral basilar crackles were audible over the posterior chest. There was very mild right upper quadrant abdominal tenderness without guarding. The liver and spleen were normal size and bowel sounds were present. No rash, peripheral edema, or spinal tenderness was noted. A complete neurological examination was normal.
Her general appearance and vital signs seem reassuring. Conjunctival injection and mild tonsillar enlargement are nonspecific findings and may occur in systemic inflammatory states especially viral infections. Atelectasis may account for faint bilateral basilar crackles especially if associated with post‐tussive change. Her alcohol use puts her at risk of aspiration. A right lower lobe process (pneumonia) can sometimes present with right upper quadrant tenderness. However, this tenderness may also represent muscle soreness from repeated coughing, liver, or gallbladder disease. The same infectious process affecting the central nervous system and possibly her lungs, may also be affecting the liver.
A complete blood count revealed a white blood cell count of 3000/mm3 (79% neutrophils, 15% lymphocytes, 5% monocytes), hemoglobin of 11.7 g/dL, and platelets of 110,000/mm3. The serum sodium was 133 mmol/L, potassium was 3.7 mmol/L, bicarbonate was 22 mmol/L, and blood urea nitrogen was 20 mg/dL. The serum creatinine was 1.5 compared to 1.0 mg/dL on testing 2 days prior. A liver function panel showed protein of 5.1 g/dL, albumin of 3 g/dL, aspartate aminotransferase (AST) of 576 IU/L, alanine aminotransferase (ALT) of 584 IU/L, alkaline phosphatase of 282 IU/L, and total bilirubin of 1 mg/dL. The coagulation profile, creatinine phosphokinase, acetaminophen level, urine pregnancy test, urine drug screen, and urinalysis (including urine microscopy) were normal.
The CSF opening pressure was 13 cm H2O. CSF analysis showed 4 mononuclear leukocytes per high‐power field, CSF protein was 27 mg/dL, and glucose was 76 mg/dL. No organisms were noted on gram stain. A chest x‐ray showed focal airspace opacity in the left lower lobe (Figure 1) and the patient was hospitalized for further management.

The normal CSF analysis makes acute meningitis much less likely. It is interesting to note that the aminotransferase levels are nearly equal. Usually, in viral and many other causes of hepatitis, the ALT is higher than the AST, whereas the contrary is true in alcoholic hepatitis. Because the patient has been consuming significant amounts of alcohol recently, these levels may become equal in the setting of another primary liver process. The elevation in liver enzymes also raises the possibility of autoimmune hepatitis secondary to a systemic vasculitis such as systemic lupus erythematosus. Nonetheless, the focus should be on infectious causes of hepatitis such as hepatitis C, adenovirus, parvovirus, Epstein‐Barr virus (EBV), cytomegalovirus, and herpes simplex virus that can cause pneumonia either as a primary or secondary infection. Acute HIV infection can also present in this fashion, and anti‐HIV antibody testing may be negative early in the disease. In the setting of a normal urinalysis and bland urine sediment, prerenal azotemia is the most likely cause of her acute renal injury and can be confirmed by testing the urinary sodium and creatinine. A peripheral smear should be reviewed to evaluate the pancytopenia.
Severe headache, fever, conjunctival injection, pancytopenia, acute kidney injury, hepatitis, and pneumonia may occur in leptospirosis, particularly in a patient with recent freshwater exposure. Alternatively, ehrlichiosis can also account for fever, headache, pancytopenia, renal failure, hepatitis, and pneumonia, but conjunctival suffusion is not often present. At this time, treatment for community‐acquired pneumonia that includes coverage for leptospirosis should be started.
The patient was hydrated with intravenous fluids and treated with intravenous ceftriaxone and azithromycin for community‐acquired pneumonia. An abdominal ultrasound was normal. The serologic assays for acute hepatitis A, B, and C infection were negative. The following morning, she reported worsening headache, increased cough now productive of whitish‐yellow sputum, and diffuse body aches. She appeared more lethargic and toxic. Her blood pressure was 100/83 mm Hg, heart rate was 84 beats per minute, respiratory rate was 24 per minute, and temperature was 101.3F. She had increased crackles on chest auscultation bilaterally and required supplemental oxygen at 4 L/minute by nasal cannula. Examination of both legs now revealed multiple scattered, faintly erythematous, 2‐cm‐sized patches overlying tender subtle subcutaneous nodules. Additionally, a mildly pruritic, V‐shaped area of blanchable erythema was also seen on her chest. The white blood cell count was 2500/mm3 (77% neutrophils, 15% lymphocytes), serum creatinine was 1.8 mg/dL, AST was 351 IU/L, and ALT was 485 IU/L. Blood cultures showed no growth and a peripheral smear examination was unrevealing. A noncontrast chest computed tomographic scan showed findings consistent with multifocal pneumonia (Figure 2).

It would be prudent at this time to expand her antimicrobial coverage (such as with vancomycin and piperacillin‐tazobactam) for activity against methicillin‐resistant Staphylococcus aureus and Pseudomonas because of her clinical worsening. Although ceftriaxone or piperacillin would cover leptospirosis, given the possibility of ehrlichiosis, the addition of doxycycline should be strongly considered.
The description of the rash on her legs seems consistent with erythema nodosum, which is associated with a number of infections (streptococcal, fungal, syphilis, EBV, cat‐scratch disease, tuberculosis), inflammatory conditions (inflammatory bowel disease, autoimmune disease, malignancy), and pregnancy. The blanchable rash on the chest is also a cause of concern for a possible drug reaction (ceftriaxone). A Jarisch‐Herxheimer reaction is possible given her acute worsening of symptoms with initiation of antibiotic therapy.
An antineutrophil cytoplasmic antibodyassociated vasculitis or another autoimmune condition such as systemic lupus erythematosus can account for erythema nodosum, rash, pancytopenia, and hepatitis. This diagnosis might also fit if she had a vasculitic pulmonary hemorrhage that caused her lung infiltrates and worsening hypoxia. A complete antinuclear antibody panel, antineutrophil cytoplasmic antibody, and antismooth muscle antibody testing is recommended. A skin and bronchoscopic biopsy should be considered.
Her dose of ceftriaxone was increased for possible severe pneumococcal pneumonia. The dermatology consultant felt that her leg lesions were consistent with erythema nodosum and the chest rash consistent with cutaneous photodamage. Bronchoscopic examination was normal and a bronchoalveolar lavage sample showed 2905 red blood cells/mm3 and 605 white blood cells/mm3 (70% neutrophils, 7% lymphocytes, 16% histiocytes), normal cytology, and negative cultures. There was no significant clinical improvement by the fourth hospital day and oral doxycycline was started. The next day, her skin lesions had resolved and she felt better. The serologic tests for Legionella, Mycoplasma, cytomegalovirus, EBV, Toxoplasma, Chlamydophila, Ehrlichia, Leptospira, Q‐fever, parvovirus, and adenovirus were negative. A fungal serology panel, HIV polymerase chain reaction, cryoglobulin level, and several rheumatologic tests (antinuclear antibody, extractable nuclear antigen panel, rheumatoid factor, antineutrophil cytoplasmic antibody, antiproteinase 3, and antiglomerular basement membrane antibodies) were normal. Blood cultures continued to show no growth.
The apparent response to doxycycline suggests she might have ehrlichiosis. A buffy coat review for morulae should be done. It is also possible that she may have improved on her initial therapy alone before starting doxycycline and her clinical worsening (including the chest rash) was due to a Jarisch‐Herxheimer reaction. Serologic tests for leptospirosis and ehrlichiosis should be repeated in 12 weeks because such infections may not cause detectable antibody levels early in the illness.
Ceftriaxone and doxycycline were continued and she showed rapid and significant clinical improvement. She was discharged 4 days later with instructions to complete a 10‐day course of antibiotics. At her 3‐month follow‐up, she was doing well and a repeat Leptospira antibody test by the Indirect Hemagglutination Assay (MRL Diagnostics, Cypress, California; normal titer <1:50) was positive at a titer of 1:100, which is highly suggestive of leptospirosis.
Commentary
Leptospirosis is a zoonotic infection caused by spirochetes of the genus Leptospira. The infection is usually transmitted indirectly to humans through contact with water, food, or soil contaminated with the urine of infected mammals.1 Risk factors for infection include participation in recreational activities (such as freshwater swimming, canoeing, and camping), occupational exposure, and exposure to infected pets or domesticated livestock. Approximately 100200 cases are identified annually in the United States, and approximately half occur in the state of Hawaii.2 Outbreaks of leptospirosis have been reported previously in the Midwest.3 These organisms inoculate humans through contact with mucous membranes or broken skin, or enter by swallowing infected food or water. A large number of these infections remain subclinical or result in a very mild illness with spontaneous clearance by the host's immune mechanism. Following an incubation period of 230 days, infected individuals may develop clinically significant disease (Table 1). Clinical presentations may overlap as the disease progresses. Although much remains to be learned about the exact pathogenic mechanism, disruption of the cell membranes of small vessel endothelia (a toxin‐like effect), and cytokine‐mediated tissue injury are believed to cause organ hemorrhage and ischemia.4
|
| 1. Mild influenza‐like self‐remitting disease (90% of cases) |
| Undifferentiated fever (usually 100F105F), severe headache, and myalgia (especially lower limbs). |
| 2. Moderately severe disease usually requiring hospitalization (5%9% of cases) |
| Marked prostration, anorexia, nausea, and vomiting, conjunctival suffusion, transient rash, frequently abdominal pain, constipation or diarrhea, and occasionally epistaxis. |
| 3. Severe disease involving multiple organ systems (1%5% of cases) |
| Hepatorenal Syndrome (Weil's syndrome) |
| Constellation of jaundice, hemorrhagic diathesis, and acute renal failure. Hepatic failure is rarely fatal. Renal involvement is usually more severe and the common cause of death. Cardiac (myocarditis with arrhythmias) and pulmonary complications are frequent. Confusion and restlessness may occur. |
| Hemorrhagic pneumonitis |
| Usually presents as a dry cough initially but becomes blood‐streaked after 23 days. Often characterized by a rapid progression to involve extensive areas of lungs, massive intra‐alveolar hemorrhage, acute respiratory failure, and death. |
| Central nervous system involvement |
| Meningismus, meningitis, or meningoencephalitis. |
The clinical diagnosis of leptospirosis is difficult because of its protean manifestations. Although nonspecific, 2 clinical features may provide a clue to the clinical diagnosis. First, the presence of conjunctival suffusion occurs in the early stage of the disease and is often associated with subconjunctival hemorrhage. Second, severe myalgia, commonly involving the lower limbs, is also characteristically present.1, 5 In 1 series of 58 patients with acute leptospirosis, conjunctival suffusion was observed in 50% of cases, and subconjunctival hemorrhage in 29%. Body ache and muscle tenderness was described in almost all cases.6
As seen in this case, the presence of a rash may pose a clinical challenge. A transient macular, maculopapular, purpuric, or urticarial rash may be seen in acute leptospirosis, but rashes may also be representative of a complication of treatment.1 First described in 1895 in patients with syphilis treated with mercury, the Jarisch‐Herxheimer reaction typically occurs within a few hours of antimicrobial treatment of spirochete infections and often presents with a rash, headache, fever, rigors, hypotension, sweating, and worsening symptoms of the underlying illness.7 Other skin findings such as the occurrence of erythema nodosum have been previously reported in cases of leptospirosis.8
Human ehrlichiosis (HE) is caused by tickborne, obligatory intracellular bacteria that infect leukocytes. There are 3 distinct clinical conditions: human monocytic ehrlichiosis (HME, caused by Ehrlichia chaffeensis), human granulocytic anaplasmosis (HGA, caused by Anaplasma phagocytophilum), and human ewingii ehrlichiosis (HEE, caused by E. ewingii). Although most cases of HME and HEE are seen in the southeastern and south‐central United States and California, the highest incidence of HGA is reported in the northeastern and upper Midwest regions.9 As with leptospirosis, the clinical range of HE spans from asymptomatic infection to life‐threatening illness. Following an incubation period of 12 weeks, symptomatic cases usually present with nonspecific complaints such as high fevers, chills, headache, nausea, arthralgia, myalgia, and malaise.10 The majority of cases will report a tick bite or an exposure to ticks. Laboratory tests often reveal leukopenia (white blood cell count < 4000/mm3), thrombocytopenia, hyponatremia, and elevated AST and ALT. Patients with severe disease may develop renal, respiratory, and hepatic failure. Thus, differentiating ehrlichiosis from leptospirosis is often challenging for the clinician.
However, there are a few clinical clues that help distinguish between these illnesses in this case. HGA as a cause of HE would be more likely in the Midwest. Although a rash is present in one‐third of patients with HME, it is seldom present in HGA unless coinfected with Borrelia burgdorferi, the causative agent for Lyme disease. Additionally, her history of freshwater exposure and the absence of a history of a tick bite also favor leptospirosis. As noted previously, conjunctival suffusion, a characteristic clinical feature of leptospirosis, has only been described in case reports of HE.11, 12
Serologic tests are often used to establish the diagnosis of leptospirosis and ehrlichiosis. Leptospires are fastidious organisms that are difficult to isolate on inoculated growth media. The microscopic agglutination test for leptospirosis is considered the diagnostic gold standard due to its high specificity, but its use is limited by its technical complexity, lack of availability (other than in reference laboratories), and low sensitivity early in the disease (antibody levels detected by this method usually do not appear until 7 days after symptom onset).13 A variety of rapid serologic assays are also available. Although these tests have good overall sensitivity (ranging between 79% and 93%), they perform relatively poorly for acute‐phase sera (sensitivity of 38.5%52.7%).13 The high early false negative rate is believed to be a result of inadequate Leptospira antibody titers in the acute phase of the illness. Seroconversion or a 4‐fold rise between acute and convalescent‐phase antibody titers is the most definitive criterion for the diagnosis of leptospirosis. However, without paired sera samples, a single high microscopic agglutination test titer can be taken as diagnostic for leptospirosis depending on the degree of regional endemicity.14
Similarly, currently available serologic assays for ehrlichiosis produce negative results in most patients in the first week of illness, and it is important to obtain a convalescent phase serum specimen for confirmatory diagnosis of HME and HGA. Seroconversion or a 4‐fold increase in titer between acute and convalescent phase sera is considered diagnostic. The sensitivity of finding morulae (intracytoplasmic vacuolar microcolonies of Ehrlichia) on a peripheral smear is unknown, and data suggest that this finding is more common in cases of HGA compared to HME.15
Although doxycycline is the drug of choice for the treatment of ehrlichiosis, Leptospira is susceptible to a wide variety of antibiotics because it exhibits a double membrane surface architecture with components common to both gram‐negative and gram‐positive bacteria.1 Recommended treatment regimens for severe leptospirosis include the use of high‐dose intravenous penicillin or a third‐generation cephalosporin. Less severe cases can be treated with oral amoxicillin or doxycycline.16 The fact that this patient's clinical improvement appeared to lag after initiation of ceftriaxone does not necessarily indicate a lack of efficacy but perhaps a Jarisch‐Herxheimer reaction in response to appropriate antibiotic therapy.
Teaching Points
-
Establishing a diagnosis of leptospirosis is challenging and requires a high index of suspicion. Clinicians should be aware of the limitations of the diagnostic accuracy of the serologic assays for leptospirosis because they are frequently negative in the first week after symptom onset.
-
The classic finding of conjunctival suffusion is helpful in differentiating leptospirosis from human ehrlichiosis.
-
This case also highlights the importance of the clinical practice of making a list of suspected diagnoses, remaining open to these possibilities, and checking serologic tests again in convalescence to confirm the diagnosis.
The approach to clinical conundrums by an expert clinician is revealed through the presentation of an actual patient's case in an approach typical of a morning report. Similarly to patient care, sequential pieces of information are provided to the clinician, who is unfamiliar with the case. The focus is on the thought processes of both the clinical team caring for the patient and the discussant.
Acknowledgements
The authors thank Dr. Brian Harte for his valuable guidance in the preparation of this manuscript.
- ,,.Leptospirosis: an emerging global public health problem.J Biosci.2008;33:557–569.
- Centers for Disease Control and Prevention. Leptospirosis.2005. http://www.cdc.gov/ncidod/dbmd/diseaseinfo/leptospirosis_t.htm. Accessed November 15,year="2010"2010.
- Morbidity and Mortality Weekly Report.From the Centers for Disease Control and Prevention. Update: leptospirosis and unexplained acute febrile illness among athletes participating in triathlons—Illinois and Wisconsin, 1998.JAMA.1998;280:1474–1475.
- ,.Optimal treatment of leptospirosis: queries and projections.Int J Antimicrob Agents.2006;28:491–496.
- ,.Leptospirosis in the tropics and in travelers.Curr Infect Dis Rep.2006;8:51–58.
- ,,,,,.Clinico‐epidemiological study of hospitalized cases of severe leptospirosis.Indian J Med Res.1999;109:94–99.
- ,.Proposed mechanisms and preventative options of Jarisch‐Herxheimer reactions.J Clin Pharm Ther.2005;30:291–295.
- .Leptospirosis presenting with erythema nodosum.Arch Dis Child.1977;52:418–419.
- ,,.Emerging and re‐emerging tick‐transmitted rickettsial and ehrlichial infections.Med Clin N Am.2008;92:1345–1361.
- ,.Tick‐borne ehrlichiosis infection in human beings.J Vector Borne Dis.2008;45:273–280.
- ,.Ehrlichia in Tennessee.South Med J.1989;82:669.
- ,,,,,.Ehrlichial meningitis with cerebrospinal fluid morulae.Pediatr Infect Dis J.1999;18:552–555.
- ,,, et al.Evaluation of four commercially available rapid serologic tests for diagnosis of leptospirosis.J Clin Microbiol.2003;41:803–809.
- ,.Diagnosis of leptospirosis utilizing modified Faine's criteria.J Assoc Physicians India.2004;52:678–679.
- ,.Ehrlichiosis in children.J Pediatr.1997;131:184–192.
- ,World Health Organization, International Leptospirosis Society. Human leptospirosis: guidance for diagnosis, surveillance and control.Geneva, Switzerland:World Health Organization;2003.
- ,,.Leptospirosis: an emerging global public health problem.J Biosci.2008;33:557–569.
- Centers for Disease Control and Prevention. Leptospirosis.2005. http://www.cdc.gov/ncidod/dbmd/diseaseinfo/leptospirosis_t.htm. Accessed November 15,year="2010"2010.
- Morbidity and Mortality Weekly Report.From the Centers for Disease Control and Prevention. Update: leptospirosis and unexplained acute febrile illness among athletes participating in triathlons—Illinois and Wisconsin, 1998.JAMA.1998;280:1474–1475.
- ,.Optimal treatment of leptospirosis: queries and projections.Int J Antimicrob Agents.2006;28:491–496.
- ,.Leptospirosis in the tropics and in travelers.Curr Infect Dis Rep.2006;8:51–58.
- ,,,,,.Clinico‐epidemiological study of hospitalized cases of severe leptospirosis.Indian J Med Res.1999;109:94–99.
- ,.Proposed mechanisms and preventative options of Jarisch‐Herxheimer reactions.J Clin Pharm Ther.2005;30:291–295.
- .Leptospirosis presenting with erythema nodosum.Arch Dis Child.1977;52:418–419.
- ,,.Emerging and re‐emerging tick‐transmitted rickettsial and ehrlichial infections.Med Clin N Am.2008;92:1345–1361.
- ,.Tick‐borne ehrlichiosis infection in human beings.J Vector Borne Dis.2008;45:273–280.
- ,.Ehrlichia in Tennessee.South Med J.1989;82:669.
- ,,,,,.Ehrlichial meningitis with cerebrospinal fluid morulae.Pediatr Infect Dis J.1999;18:552–555.
- ,,, et al.Evaluation of four commercially available rapid serologic tests for diagnosis of leptospirosis.J Clin Microbiol.2003;41:803–809.
- ,.Diagnosis of leptospirosis utilizing modified Faine's criteria.J Assoc Physicians India.2004;52:678–679.
- ,.Ehrlichiosis in children.J Pediatr.1997;131:184–192.
- ,World Health Organization, International Leptospirosis Society. Human leptospirosis: guidance for diagnosis, surveillance and control.Geneva, Switzerland:World Health Organization;2003.
Continuing Medical Education Program in
If you wish to receive credit for this activity, please refer to the website: www.wileyblackwellcme.com.
Accreditation and Designation Statement
Blackwell Futura Media Services designates this journal‐based CME activity for a maximum of 1 AMA PRA Category 1 Credit.. Physicians should only claim credit commensurate with the extent of their participation in the activity.
Blackwell Futura Media Services is accredited by the Accreditation Council for Continuing Medical Education to provide continuing medical education for physicians.
Educational Objectives
Upon completion of this educational activity, participants will be better able to:
-
Illustrate the elements of a systematic approach to successful hospital smoking cessation programs.
-
Describe the efficacy of a coordinated real world hospital smoking cessation program in a U.S. hospital.
-
Evaluate the barriers to successful hospital smoking cessation programs.
This manuscript underwent peer review in line with the standards of editorial integrity and publication ethics maintained by Journal of Hospital Medicine. The peer reviewers have no relevant financial relationships. The peer review process for Journal of Hospital Medicine is blinded. As such, the identities of the reviewers are not disclosed in line with the standard accepted practices of medical journal peer review.
Conflicts of interest have been identified and resolved in accordance with Blackwell Futura Media Services's Policy on Activity Disclosure and Conflict of Interest. The primary resolution method used was peer review and review by a non‐conflicted expert.
Instructions on Receiving Credit
For information on applicability and acceptance of CME credit for this activity, please consult your professional licensing board.
This activity is designed to be completed within an hour; physicians should claim only those credits that reflect the time actually spent in the activity. To successfully earn credit, participants must complete the activity during the valid credit period, which is up to two years from initial publication.
Follow these steps to earn credit:
-
Log on to www.wileyblackwellcme.com
-
Read the target audience, learning objectives, and author disclosures.
-
Read the article in print or online format.
-
Reflect on the article.
-
Access the CME Exam, and choose the best answer to each question.
-
Complete the required evaluation component of the activity.
This activity will be available for CME credit for twelve months following its publication date. At that time, it will be reviewed and potentially updated and extended for an additional twelve months.
If you wish to receive credit for this activity, please refer to the website: www.wileyblackwellcme.com.
Accreditation and Designation Statement
Blackwell Futura Media Services designates this journal‐based CME activity for a maximum of 1 AMA PRA Category 1 Credit.. Physicians should only claim credit commensurate with the extent of their participation in the activity.
Blackwell Futura Media Services is accredited by the Accreditation Council for Continuing Medical Education to provide continuing medical education for physicians.
Educational Objectives
Upon completion of this educational activity, participants will be better able to:
-
Illustrate the elements of a systematic approach to successful hospital smoking cessation programs.
-
Describe the efficacy of a coordinated real world hospital smoking cessation program in a U.S. hospital.
-
Evaluate the barriers to successful hospital smoking cessation programs.
This manuscript underwent peer review in line with the standards of editorial integrity and publication ethics maintained by Journal of Hospital Medicine. The peer reviewers have no relevant financial relationships. The peer review process for Journal of Hospital Medicine is blinded. As such, the identities of the reviewers are not disclosed in line with the standard accepted practices of medical journal peer review.
Conflicts of interest have been identified and resolved in accordance with Blackwell Futura Media Services's Policy on Activity Disclosure and Conflict of Interest. The primary resolution method used was peer review and review by a non‐conflicted expert.
Instructions on Receiving Credit
For information on applicability and acceptance of CME credit for this activity, please consult your professional licensing board.
This activity is designed to be completed within an hour; physicians should claim only those credits that reflect the time actually spent in the activity. To successfully earn credit, participants must complete the activity during the valid credit period, which is up to two years from initial publication.
Follow these steps to earn credit:
-
Log on to www.wileyblackwellcme.com
-
Read the target audience, learning objectives, and author disclosures.
-
Read the article in print or online format.
-
Reflect on the article.
-
Access the CME Exam, and choose the best answer to each question.
-
Complete the required evaluation component of the activity.
This activity will be available for CME credit for twelve months following its publication date. At that time, it will be reviewed and potentially updated and extended for an additional twelve months.
If you wish to receive credit for this activity, please refer to the website: www.wileyblackwellcme.com.
Accreditation and Designation Statement
Blackwell Futura Media Services designates this journal‐based CME activity for a maximum of 1 AMA PRA Category 1 Credit.. Physicians should only claim credit commensurate with the extent of their participation in the activity.
Blackwell Futura Media Services is accredited by the Accreditation Council for Continuing Medical Education to provide continuing medical education for physicians.
Educational Objectives
Upon completion of this educational activity, participants will be better able to:
-
Illustrate the elements of a systematic approach to successful hospital smoking cessation programs.
-
Describe the efficacy of a coordinated real world hospital smoking cessation program in a U.S. hospital.
-
Evaluate the barriers to successful hospital smoking cessation programs.
This manuscript underwent peer review in line with the standards of editorial integrity and publication ethics maintained by Journal of Hospital Medicine. The peer reviewers have no relevant financial relationships. The peer review process for Journal of Hospital Medicine is blinded. As such, the identities of the reviewers are not disclosed in line with the standard accepted practices of medical journal peer review.
Conflicts of interest have been identified and resolved in accordance with Blackwell Futura Media Services's Policy on Activity Disclosure and Conflict of Interest. The primary resolution method used was peer review and review by a non‐conflicted expert.
Instructions on Receiving Credit
For information on applicability and acceptance of CME credit for this activity, please consult your professional licensing board.
This activity is designed to be completed within an hour; physicians should claim only those credits that reflect the time actually spent in the activity. To successfully earn credit, participants must complete the activity during the valid credit period, which is up to two years from initial publication.
Follow these steps to earn credit:
-
Log on to www.wileyblackwellcme.com
-
Read the target audience, learning objectives, and author disclosures.
-
Read the article in print or online format.
-
Reflect on the article.
-
Access the CME Exam, and choose the best answer to each question.
-
Complete the required evaluation component of the activity.
This activity will be available for CME credit for twelve months following its publication date. At that time, it will be reviewed and potentially updated and extended for an additional twelve months.
Nonprocedural “Time Out”
Communication and teamwork failures are the most frequently cited cause of adverse events.1, 2 Strategies to improve communication have focused on implementing formal teamwork training programs and/or teaching specific communication skills.36 For instance, many institutions have adopted SBAR (Situation‐Background‐Assessment‐Recommendation) as a method for providers to deliver critical clinical information in a structured format.7 SBAR focuses on the immediate and urgent event at hand and can occur between any 2 providers. The situation is a brief description of the event (eg, Hi Dr. Smith, this is Paul from 14‐Long, I'm calling about Mrs. Jones in 1427 who is in acute respiratory distress). The background describes details relevant to the situation (eg, She was admitted with a COPD exacerbation yesterday night, and, for the past couple hours, she appears in more distress. Her vital signs are). The assessment (eg, Her breath sounds are diminished and she's moving less air) and recommendation (eg, I'd like to call respiratory therapy and would like you to come assess her now) drive toward having an action defined at the end. Given the professional silos that exist in healthcare, the advent of a shared set of communication tools helps bridge existing gaps in training, experience, and teamwork between different providers.
Regulatory agencies have been heavily invested in attempts to standardize communication in healthcare settings. In 2003, the Joint Commission elevated the concerns for wrong‐site surgery by making its prevention a National Patient Safety Goal, and the following year required compliance with a Universal Protocol (UP).8 In addition to adequate preoperative identification of the patient and marking of their surgical site, the UP called for a time out (TO) just prior to the surgery or procedure. The UP states that a TO requires active communication among all members of the surgical/procedure team, consistently initiated by a designated member of the team, conducted in a fail‐safe mode, so that the planned procedure is not started if a member of the team has concerns.8 Simply, the TO provides an opportunity to clarify plans for care and discuss events anticipated during the procedure among all members of the team (eg, surgeons, anesthesiologists, nurses, technicians). This all‐important pause point ensures that each team member is on the same page.
Whereas a TO involves many high‐risk procedural settings, a significant proportion of hospital care occurs outside of procedures. Patients are often evaluated in an emergency department, admitted to a medical/surgical ward, treated without the need for a procedure, and ultimately discharged home or transferred to another healthcare facility (eg, skilled nursing or acute rehabilitation). In this paper, we introduce the concept of Critical Conversations, a form of nonprocedural time out, as a tool, intervention, and policy that promotes communication and teamwork at the most vulnerable junctures in a patient's hospitalization.
Rationale for Critical Conversations: a Case Scenario
An 82‐year‐old man with hypertension and chronic obstructive pulmonary disease (COPD) is admitted to the hospital with community‐acquired pneumonia and an exacerbation of his COPD. The admitting physician evaluates the patient in the emergency department and completes admission orders. The patient arrives on the medical/surgical unit and the unit clerk processes the orders, stimulating a cascade of downstream events for different providers.
Nurse
The nurse reviews the medication list, notices antibiotics and bronchodilators, and wonders why aren't we administering steroids for his COPD? Do any of these medications need to be given now? Is there anything the physician is worried about? What specific things should prompt me to call the physician with an update or change in condition? I'm not sure if it's safe to send the patient down for the ordered radiographic study because he still looks pretty short of breath. I hate paging the physician several times to get these questions answered because I know that person is busy as well. I also know the patient will have questions about the care plans, which I won't be able to answer. I wonder if I should finish administering evening medications for my other patients as I'm running behind schedule on my other tasks.
Respiratory therapist
At the same time, the respiratory therapist (RT) is contacted to assist with nebulizer therapy for the patient. In reviewing the order for bronchodilators, the RT silently asks, do we think he is going to need continuous nebulizers? What is our oxygen saturation goaldo we want him at 90% or above 95%? I wonder if this patient has a history of CO2 retention and if I should have a BiPAP machine at the bedside.
Physician
After completing the orders for the patient, the physician remains in the emergency department to admit a different patient with a gastrointestinal bleed. This is the fifth admission in the past few hours. The physician feels the impact of constant paging interruptions. A unit clerk pages asking for clarification about a radiographic study that was ordered. A bedside nurse pages and asks if the physician can come and speak to the family about the diagnosis and treatment plans for an earlier admission (something the nurse is not clear about, either). A second bedside nurse pages, stating a different admission is still tachycardic after 3 liters of intravenous fluids and wants to know whether the fluids should be continued. Finally, the bedside nurse pages about whether the new COPD admission can go off the floor for the ordered chest CT or remain on continuous pulse oximetry because of shortness of breath.
Our case scenario is representative of most non‐surgical admissions to a hospital. The hypothetical questions posed from different provider perspectives are also common and often remain unanswered in a timely fashion. Partly because there is no site to mark and no anesthesia to deliver, the clinical encounter escapes attention as an opportunity for error prevention. In our experience, there are specific times during a hospitalization when communication failures are most likely to compromise patient care: the time of admission, the time of discharge,9 and any time when a patient's clinical condition changes acutely. Whereas handoff communications focus on transitions between providers (eg, shift changes), these circumstances are driven by patient transitions. Indirect communications, such as phone, email, or faxes, are suboptimal forms of communication at such times.10 We believe that there should be an expectation for direct communication at these junctures, and we define these direct communications as Critical Conversations.
Description of a Critical Conversation
In the hours that follow an admission, providers (and often the patients or their family as well) invariably exchange any number of inefficient calls or pages to clarify care plans, discuss a suspected diagnosis, anticipate concerns in the first night, and/or highlight which orders should be prioritized, such as medications or diagnostic studies. A Critical Conversation at time of admission does in this circumstance exactly what a TO attempts to provide before a procedure foster communication and teamwork as a patient is about to be placed at risk for adverse events. The exchange involves discussion of the following:
Admitting diagnosis
Immediate treatment plan
Medications ordered (particularly those new to a patient to anticipate an adverse event)
Priority for completing any admitting orders
Guidelines for physician notification when a change in patient condition occurs.
At the other end of a hospitalization, with the known complications arising from a patient's discharge,11, 12 the same process is needed. Rather than having each discipline focus on an individual role or task in getting a patient safely discharged, Critical Conversations allow the entire team, including the patient,13 to ensure that concerns have been addressed. This might help clarify simple measures around follow‐up appointments, whom to call with questions after discharge, or symptoms to watch for that may warrant a repeat evaluation. Nurses anecdotally lament that they first learn about a planned discharge only when the discharge order is written in the chart or if a patient informs them. Both scenarios reflect poorly on the teamwork required to assure patients we're working together, and that key providers are on the same page with respect to discharge planning. The exchange at discharge involves discussion of these elements:
Discharge diagnosis
Follow‐up plans
Need for education/training prior to discharge
Necessary paperwork completed
Anticipated time of discharge.
Finally, where many patients are admitted to a hospital, improve, and then return home, others develop acute changes during their hospitalization. For example, the patient in our case scenario could develop respiratory failure and require transfer to the intensive care unit (ICU). Or a different patient might have an acute change in mental status, a new fever, a new abnormal vital sign (eg, tachycardia or hypoxia), or an acute change re existing abdominal painall of which may require a battery of diagnostic tests. These circumstances define the third time for a Critical Conversation: a change in clinical condition. Such situations often require a change in the care plan, a change in priorities for delivering care at that time (for the patient in need and for other patients being cared for by the same nurse and physician), a need for additional resources (eg, respiratory therapist, phlebotomist, pharmacist), and, ultimately, a well‐orchestrated team effort to make it all happen. The specific item prompting the Critical Conversation may impact the nature of the exchange, which involves discussion of these components:
Suspected diagnosis
Immediate treatment plan
Medications ordered (particularly those new to a patient to anticipate an adverse event)
Priority for completing any new orders
Guidelines for physician notification when a change in patient condition occurs.
In addition to the above checklist for each Critical Conversation, each exchange should also address two open‐ended questions: 1) what do you anticipate happening in the next 24 hours, and 2) what questions might the patient/family have?
One may ask, and we did, why not have a direct communication daily between a physician and a bedside nurse on each patient? Most physicians and nurses know the importance of direct communication, but there are also times when each is prioritizing work in competing fashions. Adopting Critical Conversations isn't meant to deter from communications that are vital to patient care; rather, it is intended to codify distinct times when a direct communication is required for patient safety.
Lessons Learned
Table 1 provides an example of a Critical Conversation using the sample case scenario. Table 2 lists the most frequent outcomes that resulted from providers engaging in Critical Conversations. These were captured from discussions with bedside nurses and internal medicine residents on our primary medical unit. Both tables highlight how these deliberate and direct communications can create a shared understanding of the patient's medical problems, can help prioritize what tasks should take place (eg, radiology study, medication administration, calling another provider), can improve communication between providers and patients, and potentially accomplish all of these goals in a more efficient manner.
| Physician: Hi Nurse X, I'm Dr. Y, and I just wrote admission orders for Mr. Z whom, I understand, you'll be admitting. He's 82 with a history of COPD and is having an exacerbation related to a community‐acquired pneumonia. He looks comfortable right now as he's received his first dose of antibiotics, a liter of IVF, and 2 nebulizer treatments with some relief of his dyspnea. The main thing he needs up on the floor right now is to have respiratory therapy evaluate him. He's apparently been intubated before for his COPD, so I'd like to have them on board early and consider placing a BiPAP machine at the bedside for the next few hours. I don't anticipate an acute worsening of his condition given his initial improvements in the ED, but you should call me with any change in his condition. I haven't met the family yet because they were not at the bedside, but please convey the plans to them as well. I'll be up later to talk to them directly. Do you have any questions for me right now? |
| Nurse: I'll call the respiratory therapist right now and we'll make sure to contact you with any changes in his respiratory status. It looks like a chest CT was ordered, but not completed yet. Would you like him to go down for it off monitor? |
| Physician: Actually, let's watch him for a few hours to make sure he's continuing to improve. I initially ordered the chest CT to exclude a pulmonary embolus, but his history, exam, and chest x‐ray seem consistent with pneumonia. Let's reassess in a few hours. |
| Nurse: Sounds good. I'll text‐message you a set of his vital signs in 3‐4 hours to give you an update on his respiratory status. |
| General Themes | Specific Examples |
|---|---|
| Clarity on plan of care | Clear understanding of action steps at critical junctures of hospitalization |
| Goals of admission discussed rather than gleaned from chart or less direct modes of communication | |
| Discharge planning more proactive with better anticipation of timing among patients and providers | |
| Expectation for shared understanding of care plans | |
| Assistance with prioritization of tasks (as well as for competing tasks) | Allows RNs to prioritize tasks for new admissions or planned discharges, to determine whether these tasks outweigh tasks for other patients, and to provide early planning when additional resources will be required |
| Allows MDs to prioritize communications to ensure critical orders receive attention, to obtain support for care plans that require multiple disciplines, and to confirm that intended care plans are implemented with shared sense of priority | |
| Ability to communicate plans to patient and family members | Improved consistency in information provided to patients at critical hospital junctures |
| Increased engagement of patients in understanding their care plans | |
| Better model for teamwork curative for patients when providers on the same page with communication | |
| More efficient and effective use of resources | Fewer pages between admitting RN and MD with time saved from paging and waiting for responses |
| Less time trying to interpret plans of care from chart and other less direct modes of communication | |
| Improved sharing and knowledge of information with less duplication of gathering from patients and among providers | |
| Improved teamwork | Fosters a culture for direct communication and opens lines for questioning and speaking up when care plans are not clear |
Making Critical Conversations Happen
Integrating Critical Conversations into practice requires both buy‐in among providers and a plan for monitoring the interactions. We recommend beginning with educational efforts (eg, at a physician or nurse staff meeting) and reinforcing them with visual cues, such as posters on the unit (Figure 1). These actions promote awareness and generate expectations that this new clinical policy is being supported by clinical and hospital leadership. Our experiences have demonstrated tremendous learning, including numerous anecdotes about the value of Critical Conversations (Table 3). Our implementation efforts also raised a number of questions that ultimately led to improved clarity in later iterations.

| Nothing is worse than meeting a patient for the first time at admission and not being able to answer the basic question of why they were admitted or what the plan is. It gives the impression that we don't talk to each other in caring for patients. [Critical Conversations] can really minimize that interaction and reassure patients, rather than make them worried about the apparent mixed messages or lack of communication and teamwork.Bedside Nurse |
| [Critical Conversations] seemed like an additional timely responsibility, and not always a part of my workflow, when sitting in the emergency department admitting patients. But, I found that the often 60 second conversations decreased the number of pages I would get for the same patientactually saving me time.Physician |
| I don't need to have direct communications for every order written. In fact, it would be inefficient for me and the doctors. On the other hand, being engaged in a Critical Conversation provides an opportunity for me to prioritize not only my tasks for the patient in need, but also in context of the other patients I'm caring for.Bedside Nurse |
| Late in the afternoon, there will often be several admissions coming to our unit simultaneously. Prioritizing what orders need to be processed or faxed is a typically blind task based on the way charts get organizedrather than someone telling me this is a priority.Unit Clerk |
| There are so many times when I'm trying to determine what the care plans are for a new admission, and simply having a quick conversation allows me to feel part of the team, and, more importantly, allows me to reinforce education and support for the patients and their family members.Bedside Nurse |
| Discharge always seems chaotic with everyone racing to fill out forms and meet their own tasks and requirements. Invariably, you get called to fix, change, or add new information to the discharge process that would have been easily averted by actually having a brief conversation with the bedside nurse or case manager. Every time I have [a Critical Conversation], I realize its importance for patient care.Physician |
Who should be involved in a Critical Conversation?
Identifying which healthcare team members should be involved in Critical Conversations is best determined by the conversation owner. That is, we found communication was most effective when the individual initiating the Critical Conversation directed others who needed to be involved. At admission, the physician writing the admission orders is best suited to make this determination; at a minimum, he or she should engage the bedside nurse but, as in the case example presented, the physician may also need to engage other services in particularly complex situations (eg, respiratory therapy, pharmacy). At time of discharge, there should be a physiciannurse Critical Conversation; however, the owner of the discharge process may determine that other conversations should occur, and this may be inclusive of or driven by a case manager or social worker. Because local culture and practices may drive specific ownership, it's key to outline a protocol for how this should occur. For instance, at admission, we asked the admitting physicians to take responsibility in contacting the bedside nurse. In other venues, this may work more effectively if the bedside nurse pages the physician once the orders are received and reviewed.
Conclusions
We introduced Critical Conversations as an innovative tool and policy that promotes communication and teamwork in a structured format and at a consistent time. Developing formal systems that decrease communication failures in high‐risk circumstances remains a focus in patient safety, evidenced by guidelines for TOs in procedural settings, handoffs in patient care (eg, sign‐out between providers),14, 15 and transitions into and from the hospital setting.16 Furthermore, there is growing evidence that such structured times for communication and teamwork, such as with briefings, can improve efficiency and reduce delays in care.17, 18 However, handoffs, which address provider transitions, and daily multidisciplinary rounds, which bring providers together regularly, are provider‐centered rather than patient‐centered. Critical Conversations focus on times when patients require direct communication about their care plans to ensure safe and high quality outcomes.
Implementation of Critical Conversations provides an opportunity to codify a professional standard for patient‐centered communication at times when it should be expected. Critical Conversations also help build a system that supports a positive safety culture and encourages teamwork and direct communication. This is particularly true at a time when rapid adoption of information technology may have the unintended and opposite effect. For instance, as our hospital moved toward an entirely electronic health record, providers were increasingly relocating from patient care units into remote offices, corner hideaways, or designated computer rooms to complete orders and documentation. Although this may reduce many related errors in these processes and potentially improve communication via shared access to an electronic record, it does allow for less direct communicationa circumstance that traditionally occurs (even informally) when providers share the same clinical work areas. This situation is aggravated where the nurses are unit‐based and other providers (eg, physicians, therapists, case managers) are service‐based.
Integrating Critical Conversations into practice comes with expected challenges, most notably around workflow (eg, adds a step, although may save steps down the line) and the expectations concomitant with any change in standard of care (possible enforcement or auditing of their occurrence). Certain cultural barriers may also play a significant role, such as the presence of hierarchies that can hinder open communication and the related ability to speak up with concerns, as related in the TO literature. Where these cultural barriers highlight historical descriptions of the doctornurse relationship and its effect on patient care,1921 Critical Conversations provide an opportunity to improve such interdisciplinary relationships by providing a shared tool for direct communication.
In summary, we described an innovative communication tool that promotes direct communication at critical junctures during a hospitalization. With the growing complexity of hospital care and greater interdependence between teams that deliver this care, Critical Conversations provide an opportunity to further address the known communication failures that contribute to medical errors.
Acknowledgements
Critical Conversations was developed during the Triad for Optimal Patient Safety (TOPS) project, an effort focused on improving unit‐based safety culture through improved teamwork and communication. We thank the Gordon and Betty Moore Foundation for their active support and funding of the TOPS project, which was a collaboration between the Schools of Medicine, Nursing, and Pharmacy at the University of California, San Francisco.
- ,,,,.Communication failures in patient sign‐out and suggestions for improvement: a critical incident analysis.Qual Saf Health Care.2005;14(6):401–407.
- ,,.Communication failures: an insidious contributor to medical mishaps.Acad Med.2004;79(2):186–194.
- ,.TeamSTEPPS: assuring optimal teamwork in clinical settings.Am J Med Qual.2007;22(3):214–217.
- ,,,,,.Medical team training: applying crew resource management in the Veterans Health Administration.Jt Comm J Qual Patient Saf.2007;33(6):317–325.
- ,,,,,,,,,.A multidisciplinary teamwork training program: the Triad for Optimal Patient Safety (TOPS) Experience.J Gen Intern Med.2008;23(12):2053–2057.
- ,,.The human factor: the critical importance of effective teamwork and communication in providing safe care.Qual Saf Health Care.2004;13Suppl‐1:i85–90.
- ,,.SBAR: a shared mental model for improving communication between clinicians.Jt Comm J Qual Patient Saf.2006;32(3):167–175.
- The Joint Commission Universal Protocol for Preventing Wrong Site, Wrong Procedure, Wrong Person Surgery. Available at: http://www.jointcommission.org/NR/rdonlyres/E3C600EB‐043B‐4E86‐B04E‐CA4A89AD5433/0/universal_protocol.pdf. Accessed January 24, 2010.
- ,,.The hospital discharge: a review of a high risk care transition with highlights of a reengineered discharge process.J Patient Saf.2007;3:97–106.
- How do we communicate? Communication on Agile Software Projects. Available at: www.agilemodeling.com/essays/communication.htm. Accessed January 24, 2010.
- ,,,.Promoting effective transitions of care at hospital discharge: a review of key issues for hospitalists.J Hosp Med.2007;2(5):314–323.
- ,,,,,.Deficits in communication and information transfer between hospital‐based and primary care physicians: implications for patient safety and continuity of care.JAMA.2007;297(8):831–841.
- .Engaging patients at hospital discharge.J Hosp Med.2008;3(6):498–500.
- ,,,,.Managing discontinuity in academic medical centers: strategies for a safe and effective resident sign‐out.J Hosp Med.2006;1(4):257–266.
- ,.Lost in transition: challenges and opportunities for improving the quality of transitional care.Ann Intern Med.2004;141(7):533–536.
- ,,, et al.Transition of care for hospitalized elderly patients—development of a discharge checklist for hospitalists.J Hosp Med.2006;1(6):354–360.
- ,,, et al.Impact of preoperative briefings on operating room delays.Arch Surg.2008;143(11):1068–1072.
- ,,, et al.Operating room briefings: working on the same page.Jt Comm J Qual Patient Saf.2006;32(6):351–355.
- .Doctors and nurses: a troubled partnership.Ann Surg.1999;230(3):279–288.
- ,,, et al.Association between nurse‐physician collaboration and patient outcomes in three intensive care units.Crit Care Med.1999;27(9):1991–1998.
- ,,, et al.Evaluation of a preoperative checklist and team briefing among surgeons, nurses, and anesthesiologists to reduce failures in communication.Arch Surg.2008;143(1):12–17.
Communication and teamwork failures are the most frequently cited cause of adverse events.1, 2 Strategies to improve communication have focused on implementing formal teamwork training programs and/or teaching specific communication skills.36 For instance, many institutions have adopted SBAR (Situation‐Background‐Assessment‐Recommendation) as a method for providers to deliver critical clinical information in a structured format.7 SBAR focuses on the immediate and urgent event at hand and can occur between any 2 providers. The situation is a brief description of the event (eg, Hi Dr. Smith, this is Paul from 14‐Long, I'm calling about Mrs. Jones in 1427 who is in acute respiratory distress). The background describes details relevant to the situation (eg, She was admitted with a COPD exacerbation yesterday night, and, for the past couple hours, she appears in more distress. Her vital signs are). The assessment (eg, Her breath sounds are diminished and she's moving less air) and recommendation (eg, I'd like to call respiratory therapy and would like you to come assess her now) drive toward having an action defined at the end. Given the professional silos that exist in healthcare, the advent of a shared set of communication tools helps bridge existing gaps in training, experience, and teamwork between different providers.
Regulatory agencies have been heavily invested in attempts to standardize communication in healthcare settings. In 2003, the Joint Commission elevated the concerns for wrong‐site surgery by making its prevention a National Patient Safety Goal, and the following year required compliance with a Universal Protocol (UP).8 In addition to adequate preoperative identification of the patient and marking of their surgical site, the UP called for a time out (TO) just prior to the surgery or procedure. The UP states that a TO requires active communication among all members of the surgical/procedure team, consistently initiated by a designated member of the team, conducted in a fail‐safe mode, so that the planned procedure is not started if a member of the team has concerns.8 Simply, the TO provides an opportunity to clarify plans for care and discuss events anticipated during the procedure among all members of the team (eg, surgeons, anesthesiologists, nurses, technicians). This all‐important pause point ensures that each team member is on the same page.
Whereas a TO involves many high‐risk procedural settings, a significant proportion of hospital care occurs outside of procedures. Patients are often evaluated in an emergency department, admitted to a medical/surgical ward, treated without the need for a procedure, and ultimately discharged home or transferred to another healthcare facility (eg, skilled nursing or acute rehabilitation). In this paper, we introduce the concept of Critical Conversations, a form of nonprocedural time out, as a tool, intervention, and policy that promotes communication and teamwork at the most vulnerable junctures in a patient's hospitalization.
Rationale for Critical Conversations: a Case Scenario
An 82‐year‐old man with hypertension and chronic obstructive pulmonary disease (COPD) is admitted to the hospital with community‐acquired pneumonia and an exacerbation of his COPD. The admitting physician evaluates the patient in the emergency department and completes admission orders. The patient arrives on the medical/surgical unit and the unit clerk processes the orders, stimulating a cascade of downstream events for different providers.
Nurse
The nurse reviews the medication list, notices antibiotics and bronchodilators, and wonders why aren't we administering steroids for his COPD? Do any of these medications need to be given now? Is there anything the physician is worried about? What specific things should prompt me to call the physician with an update or change in condition? I'm not sure if it's safe to send the patient down for the ordered radiographic study because he still looks pretty short of breath. I hate paging the physician several times to get these questions answered because I know that person is busy as well. I also know the patient will have questions about the care plans, which I won't be able to answer. I wonder if I should finish administering evening medications for my other patients as I'm running behind schedule on my other tasks.
Respiratory therapist
At the same time, the respiratory therapist (RT) is contacted to assist with nebulizer therapy for the patient. In reviewing the order for bronchodilators, the RT silently asks, do we think he is going to need continuous nebulizers? What is our oxygen saturation goaldo we want him at 90% or above 95%? I wonder if this patient has a history of CO2 retention and if I should have a BiPAP machine at the bedside.
Physician
After completing the orders for the patient, the physician remains in the emergency department to admit a different patient with a gastrointestinal bleed. This is the fifth admission in the past few hours. The physician feels the impact of constant paging interruptions. A unit clerk pages asking for clarification about a radiographic study that was ordered. A bedside nurse pages and asks if the physician can come and speak to the family about the diagnosis and treatment plans for an earlier admission (something the nurse is not clear about, either). A second bedside nurse pages, stating a different admission is still tachycardic after 3 liters of intravenous fluids and wants to know whether the fluids should be continued. Finally, the bedside nurse pages about whether the new COPD admission can go off the floor for the ordered chest CT or remain on continuous pulse oximetry because of shortness of breath.
Our case scenario is representative of most non‐surgical admissions to a hospital. The hypothetical questions posed from different provider perspectives are also common and often remain unanswered in a timely fashion. Partly because there is no site to mark and no anesthesia to deliver, the clinical encounter escapes attention as an opportunity for error prevention. In our experience, there are specific times during a hospitalization when communication failures are most likely to compromise patient care: the time of admission, the time of discharge,9 and any time when a patient's clinical condition changes acutely. Whereas handoff communications focus on transitions between providers (eg, shift changes), these circumstances are driven by patient transitions. Indirect communications, such as phone, email, or faxes, are suboptimal forms of communication at such times.10 We believe that there should be an expectation for direct communication at these junctures, and we define these direct communications as Critical Conversations.
Description of a Critical Conversation
In the hours that follow an admission, providers (and often the patients or their family as well) invariably exchange any number of inefficient calls or pages to clarify care plans, discuss a suspected diagnosis, anticipate concerns in the first night, and/or highlight which orders should be prioritized, such as medications or diagnostic studies. A Critical Conversation at time of admission does in this circumstance exactly what a TO attempts to provide before a procedure foster communication and teamwork as a patient is about to be placed at risk for adverse events. The exchange involves discussion of the following:
Admitting diagnosis
Immediate treatment plan
Medications ordered (particularly those new to a patient to anticipate an adverse event)
Priority for completing any admitting orders
Guidelines for physician notification when a change in patient condition occurs.
At the other end of a hospitalization, with the known complications arising from a patient's discharge,11, 12 the same process is needed. Rather than having each discipline focus on an individual role or task in getting a patient safely discharged, Critical Conversations allow the entire team, including the patient,13 to ensure that concerns have been addressed. This might help clarify simple measures around follow‐up appointments, whom to call with questions after discharge, or symptoms to watch for that may warrant a repeat evaluation. Nurses anecdotally lament that they first learn about a planned discharge only when the discharge order is written in the chart or if a patient informs them. Both scenarios reflect poorly on the teamwork required to assure patients we're working together, and that key providers are on the same page with respect to discharge planning. The exchange at discharge involves discussion of these elements:
Discharge diagnosis
Follow‐up plans
Need for education/training prior to discharge
Necessary paperwork completed
Anticipated time of discharge.
Finally, where many patients are admitted to a hospital, improve, and then return home, others develop acute changes during their hospitalization. For example, the patient in our case scenario could develop respiratory failure and require transfer to the intensive care unit (ICU). Or a different patient might have an acute change in mental status, a new fever, a new abnormal vital sign (eg, tachycardia or hypoxia), or an acute change re existing abdominal painall of which may require a battery of diagnostic tests. These circumstances define the third time for a Critical Conversation: a change in clinical condition. Such situations often require a change in the care plan, a change in priorities for delivering care at that time (for the patient in need and for other patients being cared for by the same nurse and physician), a need for additional resources (eg, respiratory therapist, phlebotomist, pharmacist), and, ultimately, a well‐orchestrated team effort to make it all happen. The specific item prompting the Critical Conversation may impact the nature of the exchange, which involves discussion of these components:
Suspected diagnosis
Immediate treatment plan
Medications ordered (particularly those new to a patient to anticipate an adverse event)
Priority for completing any new orders
Guidelines for physician notification when a change in patient condition occurs.
In addition to the above checklist for each Critical Conversation, each exchange should also address two open‐ended questions: 1) what do you anticipate happening in the next 24 hours, and 2) what questions might the patient/family have?
One may ask, and we did, why not have a direct communication daily between a physician and a bedside nurse on each patient? Most physicians and nurses know the importance of direct communication, but there are also times when each is prioritizing work in competing fashions. Adopting Critical Conversations isn't meant to deter from communications that are vital to patient care; rather, it is intended to codify distinct times when a direct communication is required for patient safety.
Lessons Learned
Table 1 provides an example of a Critical Conversation using the sample case scenario. Table 2 lists the most frequent outcomes that resulted from providers engaging in Critical Conversations. These were captured from discussions with bedside nurses and internal medicine residents on our primary medical unit. Both tables highlight how these deliberate and direct communications can create a shared understanding of the patient's medical problems, can help prioritize what tasks should take place (eg, radiology study, medication administration, calling another provider), can improve communication between providers and patients, and potentially accomplish all of these goals in a more efficient manner.
| Physician: Hi Nurse X, I'm Dr. Y, and I just wrote admission orders for Mr. Z whom, I understand, you'll be admitting. He's 82 with a history of COPD and is having an exacerbation related to a community‐acquired pneumonia. He looks comfortable right now as he's received his first dose of antibiotics, a liter of IVF, and 2 nebulizer treatments with some relief of his dyspnea. The main thing he needs up on the floor right now is to have respiratory therapy evaluate him. He's apparently been intubated before for his COPD, so I'd like to have them on board early and consider placing a BiPAP machine at the bedside for the next few hours. I don't anticipate an acute worsening of his condition given his initial improvements in the ED, but you should call me with any change in his condition. I haven't met the family yet because they were not at the bedside, but please convey the plans to them as well. I'll be up later to talk to them directly. Do you have any questions for me right now? |
| Nurse: I'll call the respiratory therapist right now and we'll make sure to contact you with any changes in his respiratory status. It looks like a chest CT was ordered, but not completed yet. Would you like him to go down for it off monitor? |
| Physician: Actually, let's watch him for a few hours to make sure he's continuing to improve. I initially ordered the chest CT to exclude a pulmonary embolus, but his history, exam, and chest x‐ray seem consistent with pneumonia. Let's reassess in a few hours. |
| Nurse: Sounds good. I'll text‐message you a set of his vital signs in 3‐4 hours to give you an update on his respiratory status. |
| General Themes | Specific Examples |
|---|---|
| Clarity on plan of care | Clear understanding of action steps at critical junctures of hospitalization |
| Goals of admission discussed rather than gleaned from chart or less direct modes of communication | |
| Discharge planning more proactive with better anticipation of timing among patients and providers | |
| Expectation for shared understanding of care plans | |
| Assistance with prioritization of tasks (as well as for competing tasks) | Allows RNs to prioritize tasks for new admissions or planned discharges, to determine whether these tasks outweigh tasks for other patients, and to provide early planning when additional resources will be required |
| Allows MDs to prioritize communications to ensure critical orders receive attention, to obtain support for care plans that require multiple disciplines, and to confirm that intended care plans are implemented with shared sense of priority | |
| Ability to communicate plans to patient and family members | Improved consistency in information provided to patients at critical hospital junctures |
| Increased engagement of patients in understanding their care plans | |
| Better model for teamwork curative for patients when providers on the same page with communication | |
| More efficient and effective use of resources | Fewer pages between admitting RN and MD with time saved from paging and waiting for responses |
| Less time trying to interpret plans of care from chart and other less direct modes of communication | |
| Improved sharing and knowledge of information with less duplication of gathering from patients and among providers | |
| Improved teamwork | Fosters a culture for direct communication and opens lines for questioning and speaking up when care plans are not clear |
Making Critical Conversations Happen
Integrating Critical Conversations into practice requires both buy‐in among providers and a plan for monitoring the interactions. We recommend beginning with educational efforts (eg, at a physician or nurse staff meeting) and reinforcing them with visual cues, such as posters on the unit (Figure 1). These actions promote awareness and generate expectations that this new clinical policy is being supported by clinical and hospital leadership. Our experiences have demonstrated tremendous learning, including numerous anecdotes about the value of Critical Conversations (Table 3). Our implementation efforts also raised a number of questions that ultimately led to improved clarity in later iterations.

| Nothing is worse than meeting a patient for the first time at admission and not being able to answer the basic question of why they were admitted or what the plan is. It gives the impression that we don't talk to each other in caring for patients. [Critical Conversations] can really minimize that interaction and reassure patients, rather than make them worried about the apparent mixed messages or lack of communication and teamwork.Bedside Nurse |
| [Critical Conversations] seemed like an additional timely responsibility, and not always a part of my workflow, when sitting in the emergency department admitting patients. But, I found that the often 60 second conversations decreased the number of pages I would get for the same patientactually saving me time.Physician |
| I don't need to have direct communications for every order written. In fact, it would be inefficient for me and the doctors. On the other hand, being engaged in a Critical Conversation provides an opportunity for me to prioritize not only my tasks for the patient in need, but also in context of the other patients I'm caring for.Bedside Nurse |
| Late in the afternoon, there will often be several admissions coming to our unit simultaneously. Prioritizing what orders need to be processed or faxed is a typically blind task based on the way charts get organizedrather than someone telling me this is a priority.Unit Clerk |
| There are so many times when I'm trying to determine what the care plans are for a new admission, and simply having a quick conversation allows me to feel part of the team, and, more importantly, allows me to reinforce education and support for the patients and their family members.Bedside Nurse |
| Discharge always seems chaotic with everyone racing to fill out forms and meet their own tasks and requirements. Invariably, you get called to fix, change, or add new information to the discharge process that would have been easily averted by actually having a brief conversation with the bedside nurse or case manager. Every time I have [a Critical Conversation], I realize its importance for patient care.Physician |
Who should be involved in a Critical Conversation?
Identifying which healthcare team members should be involved in Critical Conversations is best determined by the conversation owner. That is, we found communication was most effective when the individual initiating the Critical Conversation directed others who needed to be involved. At admission, the physician writing the admission orders is best suited to make this determination; at a minimum, he or she should engage the bedside nurse but, as in the case example presented, the physician may also need to engage other services in particularly complex situations (eg, respiratory therapy, pharmacy). At time of discharge, there should be a physiciannurse Critical Conversation; however, the owner of the discharge process may determine that other conversations should occur, and this may be inclusive of or driven by a case manager or social worker. Because local culture and practices may drive specific ownership, it's key to outline a protocol for how this should occur. For instance, at admission, we asked the admitting physicians to take responsibility in contacting the bedside nurse. In other venues, this may work more effectively if the bedside nurse pages the physician once the orders are received and reviewed.
Conclusions
We introduced Critical Conversations as an innovative tool and policy that promotes communication and teamwork in a structured format and at a consistent time. Developing formal systems that decrease communication failures in high‐risk circumstances remains a focus in patient safety, evidenced by guidelines for TOs in procedural settings, handoffs in patient care (eg, sign‐out between providers),14, 15 and transitions into and from the hospital setting.16 Furthermore, there is growing evidence that such structured times for communication and teamwork, such as with briefings, can improve efficiency and reduce delays in care.17, 18 However, handoffs, which address provider transitions, and daily multidisciplinary rounds, which bring providers together regularly, are provider‐centered rather than patient‐centered. Critical Conversations focus on times when patients require direct communication about their care plans to ensure safe and high quality outcomes.
Implementation of Critical Conversations provides an opportunity to codify a professional standard for patient‐centered communication at times when it should be expected. Critical Conversations also help build a system that supports a positive safety culture and encourages teamwork and direct communication. This is particularly true at a time when rapid adoption of information technology may have the unintended and opposite effect. For instance, as our hospital moved toward an entirely electronic health record, providers were increasingly relocating from patient care units into remote offices, corner hideaways, or designated computer rooms to complete orders and documentation. Although this may reduce many related errors in these processes and potentially improve communication via shared access to an electronic record, it does allow for less direct communicationa circumstance that traditionally occurs (even informally) when providers share the same clinical work areas. This situation is aggravated where the nurses are unit‐based and other providers (eg, physicians, therapists, case managers) are service‐based.
Integrating Critical Conversations into practice comes with expected challenges, most notably around workflow (eg, adds a step, although may save steps down the line) and the expectations concomitant with any change in standard of care (possible enforcement or auditing of their occurrence). Certain cultural barriers may also play a significant role, such as the presence of hierarchies that can hinder open communication and the related ability to speak up with concerns, as related in the TO literature. Where these cultural barriers highlight historical descriptions of the doctornurse relationship and its effect on patient care,1921 Critical Conversations provide an opportunity to improve such interdisciplinary relationships by providing a shared tool for direct communication.
In summary, we described an innovative communication tool that promotes direct communication at critical junctures during a hospitalization. With the growing complexity of hospital care and greater interdependence between teams that deliver this care, Critical Conversations provide an opportunity to further address the known communication failures that contribute to medical errors.
Acknowledgements
Critical Conversations was developed during the Triad for Optimal Patient Safety (TOPS) project, an effort focused on improving unit‐based safety culture through improved teamwork and communication. We thank the Gordon and Betty Moore Foundation for their active support and funding of the TOPS project, which was a collaboration between the Schools of Medicine, Nursing, and Pharmacy at the University of California, San Francisco.
Communication and teamwork failures are the most frequently cited cause of adverse events.1, 2 Strategies to improve communication have focused on implementing formal teamwork training programs and/or teaching specific communication skills.36 For instance, many institutions have adopted SBAR (Situation‐Background‐Assessment‐Recommendation) as a method for providers to deliver critical clinical information in a structured format.7 SBAR focuses on the immediate and urgent event at hand and can occur between any 2 providers. The situation is a brief description of the event (eg, Hi Dr. Smith, this is Paul from 14‐Long, I'm calling about Mrs. Jones in 1427 who is in acute respiratory distress). The background describes details relevant to the situation (eg, She was admitted with a COPD exacerbation yesterday night, and, for the past couple hours, she appears in more distress. Her vital signs are). The assessment (eg, Her breath sounds are diminished and she's moving less air) and recommendation (eg, I'd like to call respiratory therapy and would like you to come assess her now) drive toward having an action defined at the end. Given the professional silos that exist in healthcare, the advent of a shared set of communication tools helps bridge existing gaps in training, experience, and teamwork between different providers.
Regulatory agencies have been heavily invested in attempts to standardize communication in healthcare settings. In 2003, the Joint Commission elevated the concerns for wrong‐site surgery by making its prevention a National Patient Safety Goal, and the following year required compliance with a Universal Protocol (UP).8 In addition to adequate preoperative identification of the patient and marking of their surgical site, the UP called for a time out (TO) just prior to the surgery or procedure. The UP states that a TO requires active communication among all members of the surgical/procedure team, consistently initiated by a designated member of the team, conducted in a fail‐safe mode, so that the planned procedure is not started if a member of the team has concerns.8 Simply, the TO provides an opportunity to clarify plans for care and discuss events anticipated during the procedure among all members of the team (eg, surgeons, anesthesiologists, nurses, technicians). This all‐important pause point ensures that each team member is on the same page.
Whereas a TO involves many high‐risk procedural settings, a significant proportion of hospital care occurs outside of procedures. Patients are often evaluated in an emergency department, admitted to a medical/surgical ward, treated without the need for a procedure, and ultimately discharged home or transferred to another healthcare facility (eg, skilled nursing or acute rehabilitation). In this paper, we introduce the concept of Critical Conversations, a form of nonprocedural time out, as a tool, intervention, and policy that promotes communication and teamwork at the most vulnerable junctures in a patient's hospitalization.
Rationale for Critical Conversations: a Case Scenario
An 82‐year‐old man with hypertension and chronic obstructive pulmonary disease (COPD) is admitted to the hospital with community‐acquired pneumonia and an exacerbation of his COPD. The admitting physician evaluates the patient in the emergency department and completes admission orders. The patient arrives on the medical/surgical unit and the unit clerk processes the orders, stimulating a cascade of downstream events for different providers.
Nurse
The nurse reviews the medication list, notices antibiotics and bronchodilators, and wonders why aren't we administering steroids for his COPD? Do any of these medications need to be given now? Is there anything the physician is worried about? What specific things should prompt me to call the physician with an update or change in condition? I'm not sure if it's safe to send the patient down for the ordered radiographic study because he still looks pretty short of breath. I hate paging the physician several times to get these questions answered because I know that person is busy as well. I also know the patient will have questions about the care plans, which I won't be able to answer. I wonder if I should finish administering evening medications for my other patients as I'm running behind schedule on my other tasks.
Respiratory therapist
At the same time, the respiratory therapist (RT) is contacted to assist with nebulizer therapy for the patient. In reviewing the order for bronchodilators, the RT silently asks, do we think he is going to need continuous nebulizers? What is our oxygen saturation goaldo we want him at 90% or above 95%? I wonder if this patient has a history of CO2 retention and if I should have a BiPAP machine at the bedside.
Physician
After completing the orders for the patient, the physician remains in the emergency department to admit a different patient with a gastrointestinal bleed. This is the fifth admission in the past few hours. The physician feels the impact of constant paging interruptions. A unit clerk pages asking for clarification about a radiographic study that was ordered. A bedside nurse pages and asks if the physician can come and speak to the family about the diagnosis and treatment plans for an earlier admission (something the nurse is not clear about, either). A second bedside nurse pages, stating a different admission is still tachycardic after 3 liters of intravenous fluids and wants to know whether the fluids should be continued. Finally, the bedside nurse pages about whether the new COPD admission can go off the floor for the ordered chest CT or remain on continuous pulse oximetry because of shortness of breath.
Our case scenario is representative of most non‐surgical admissions to a hospital. The hypothetical questions posed from different provider perspectives are also common and often remain unanswered in a timely fashion. Partly because there is no site to mark and no anesthesia to deliver, the clinical encounter escapes attention as an opportunity for error prevention. In our experience, there are specific times during a hospitalization when communication failures are most likely to compromise patient care: the time of admission, the time of discharge,9 and any time when a patient's clinical condition changes acutely. Whereas handoff communications focus on transitions between providers (eg, shift changes), these circumstances are driven by patient transitions. Indirect communications, such as phone, email, or faxes, are suboptimal forms of communication at such times.10 We believe that there should be an expectation for direct communication at these junctures, and we define these direct communications as Critical Conversations.
Description of a Critical Conversation
In the hours that follow an admission, providers (and often the patients or their family as well) invariably exchange any number of inefficient calls or pages to clarify care plans, discuss a suspected diagnosis, anticipate concerns in the first night, and/or highlight which orders should be prioritized, such as medications or diagnostic studies. A Critical Conversation at time of admission does in this circumstance exactly what a TO attempts to provide before a procedure foster communication and teamwork as a patient is about to be placed at risk for adverse events. The exchange involves discussion of the following:
Admitting diagnosis
Immediate treatment plan
Medications ordered (particularly those new to a patient to anticipate an adverse event)
Priority for completing any admitting orders
Guidelines for physician notification when a change in patient condition occurs.
At the other end of a hospitalization, with the known complications arising from a patient's discharge,11, 12 the same process is needed. Rather than having each discipline focus on an individual role or task in getting a patient safely discharged, Critical Conversations allow the entire team, including the patient,13 to ensure that concerns have been addressed. This might help clarify simple measures around follow‐up appointments, whom to call with questions after discharge, or symptoms to watch for that may warrant a repeat evaluation. Nurses anecdotally lament that they first learn about a planned discharge only when the discharge order is written in the chart or if a patient informs them. Both scenarios reflect poorly on the teamwork required to assure patients we're working together, and that key providers are on the same page with respect to discharge planning. The exchange at discharge involves discussion of these elements:
Discharge diagnosis
Follow‐up plans
Need for education/training prior to discharge
Necessary paperwork completed
Anticipated time of discharge.
Finally, where many patients are admitted to a hospital, improve, and then return home, others develop acute changes during their hospitalization. For example, the patient in our case scenario could develop respiratory failure and require transfer to the intensive care unit (ICU). Or a different patient might have an acute change in mental status, a new fever, a new abnormal vital sign (eg, tachycardia or hypoxia), or an acute change re existing abdominal painall of which may require a battery of diagnostic tests. These circumstances define the third time for a Critical Conversation: a change in clinical condition. Such situations often require a change in the care plan, a change in priorities for delivering care at that time (for the patient in need and for other patients being cared for by the same nurse and physician), a need for additional resources (eg, respiratory therapist, phlebotomist, pharmacist), and, ultimately, a well‐orchestrated team effort to make it all happen. The specific item prompting the Critical Conversation may impact the nature of the exchange, which involves discussion of these components:
Suspected diagnosis
Immediate treatment plan
Medications ordered (particularly those new to a patient to anticipate an adverse event)
Priority for completing any new orders
Guidelines for physician notification when a change in patient condition occurs.
In addition to the above checklist for each Critical Conversation, each exchange should also address two open‐ended questions: 1) what do you anticipate happening in the next 24 hours, and 2) what questions might the patient/family have?
One may ask, and we did, why not have a direct communication daily between a physician and a bedside nurse on each patient? Most physicians and nurses know the importance of direct communication, but there are also times when each is prioritizing work in competing fashions. Adopting Critical Conversations isn't meant to deter from communications that are vital to patient care; rather, it is intended to codify distinct times when a direct communication is required for patient safety.
Lessons Learned
Table 1 provides an example of a Critical Conversation using the sample case scenario. Table 2 lists the most frequent outcomes that resulted from providers engaging in Critical Conversations. These were captured from discussions with bedside nurses and internal medicine residents on our primary medical unit. Both tables highlight how these deliberate and direct communications can create a shared understanding of the patient's medical problems, can help prioritize what tasks should take place (eg, radiology study, medication administration, calling another provider), can improve communication between providers and patients, and potentially accomplish all of these goals in a more efficient manner.
| Physician: Hi Nurse X, I'm Dr. Y, and I just wrote admission orders for Mr. Z whom, I understand, you'll be admitting. He's 82 with a history of COPD and is having an exacerbation related to a community‐acquired pneumonia. He looks comfortable right now as he's received his first dose of antibiotics, a liter of IVF, and 2 nebulizer treatments with some relief of his dyspnea. The main thing he needs up on the floor right now is to have respiratory therapy evaluate him. He's apparently been intubated before for his COPD, so I'd like to have them on board early and consider placing a BiPAP machine at the bedside for the next few hours. I don't anticipate an acute worsening of his condition given his initial improvements in the ED, but you should call me with any change in his condition. I haven't met the family yet because they were not at the bedside, but please convey the plans to them as well. I'll be up later to talk to them directly. Do you have any questions for me right now? |
| Nurse: I'll call the respiratory therapist right now and we'll make sure to contact you with any changes in his respiratory status. It looks like a chest CT was ordered, but not completed yet. Would you like him to go down for it off monitor? |
| Physician: Actually, let's watch him for a few hours to make sure he's continuing to improve. I initially ordered the chest CT to exclude a pulmonary embolus, but his history, exam, and chest x‐ray seem consistent with pneumonia. Let's reassess in a few hours. |
| Nurse: Sounds good. I'll text‐message you a set of his vital signs in 3‐4 hours to give you an update on his respiratory status. |
| General Themes | Specific Examples |
|---|---|
| Clarity on plan of care | Clear understanding of action steps at critical junctures of hospitalization |
| Goals of admission discussed rather than gleaned from chart or less direct modes of communication | |
| Discharge planning more proactive with better anticipation of timing among patients and providers | |
| Expectation for shared understanding of care plans | |
| Assistance with prioritization of tasks (as well as for competing tasks) | Allows RNs to prioritize tasks for new admissions or planned discharges, to determine whether these tasks outweigh tasks for other patients, and to provide early planning when additional resources will be required |
| Allows MDs to prioritize communications to ensure critical orders receive attention, to obtain support for care plans that require multiple disciplines, and to confirm that intended care plans are implemented with shared sense of priority | |
| Ability to communicate plans to patient and family members | Improved consistency in information provided to patients at critical hospital junctures |
| Increased engagement of patients in understanding their care plans | |
| Better model for teamwork curative for patients when providers on the same page with communication | |
| More efficient and effective use of resources | Fewer pages between admitting RN and MD with time saved from paging and waiting for responses |
| Less time trying to interpret plans of care from chart and other less direct modes of communication | |
| Improved sharing and knowledge of information with less duplication of gathering from patients and among providers | |
| Improved teamwork | Fosters a culture for direct communication and opens lines for questioning and speaking up when care plans are not clear |
Making Critical Conversations Happen
Integrating Critical Conversations into practice requires both buy‐in among providers and a plan for monitoring the interactions. We recommend beginning with educational efforts (eg, at a physician or nurse staff meeting) and reinforcing them with visual cues, such as posters on the unit (Figure 1). These actions promote awareness and generate expectations that this new clinical policy is being supported by clinical and hospital leadership. Our experiences have demonstrated tremendous learning, including numerous anecdotes about the value of Critical Conversations (Table 3). Our implementation efforts also raised a number of questions that ultimately led to improved clarity in later iterations.

| Nothing is worse than meeting a patient for the first time at admission and not being able to answer the basic question of why they were admitted or what the plan is. It gives the impression that we don't talk to each other in caring for patients. [Critical Conversations] can really minimize that interaction and reassure patients, rather than make them worried about the apparent mixed messages or lack of communication and teamwork.Bedside Nurse |
| [Critical Conversations] seemed like an additional timely responsibility, and not always a part of my workflow, when sitting in the emergency department admitting patients. But, I found that the often 60 second conversations decreased the number of pages I would get for the same patientactually saving me time.Physician |
| I don't need to have direct communications for every order written. In fact, it would be inefficient for me and the doctors. On the other hand, being engaged in a Critical Conversation provides an opportunity for me to prioritize not only my tasks for the patient in need, but also in context of the other patients I'm caring for.Bedside Nurse |
| Late in the afternoon, there will often be several admissions coming to our unit simultaneously. Prioritizing what orders need to be processed or faxed is a typically blind task based on the way charts get organizedrather than someone telling me this is a priority.Unit Clerk |
| There are so many times when I'm trying to determine what the care plans are for a new admission, and simply having a quick conversation allows me to feel part of the team, and, more importantly, allows me to reinforce education and support for the patients and their family members.Bedside Nurse |
| Discharge always seems chaotic with everyone racing to fill out forms and meet their own tasks and requirements. Invariably, you get called to fix, change, or add new information to the discharge process that would have been easily averted by actually having a brief conversation with the bedside nurse or case manager. Every time I have [a Critical Conversation], I realize its importance for patient care.Physician |
Who should be involved in a Critical Conversation?
Identifying which healthcare team members should be involved in Critical Conversations is best determined by the conversation owner. That is, we found communication was most effective when the individual initiating the Critical Conversation directed others who needed to be involved. At admission, the physician writing the admission orders is best suited to make this determination; at a minimum, he or she should engage the bedside nurse but, as in the case example presented, the physician may also need to engage other services in particularly complex situations (eg, respiratory therapy, pharmacy). At time of discharge, there should be a physiciannurse Critical Conversation; however, the owner of the discharge process may determine that other conversations should occur, and this may be inclusive of or driven by a case manager or social worker. Because local culture and practices may drive specific ownership, it's key to outline a protocol for how this should occur. For instance, at admission, we asked the admitting physicians to take responsibility in contacting the bedside nurse. In other venues, this may work more effectively if the bedside nurse pages the physician once the orders are received and reviewed.
Conclusions
We introduced Critical Conversations as an innovative tool and policy that promotes communication and teamwork in a structured format and at a consistent time. Developing formal systems that decrease communication failures in high‐risk circumstances remains a focus in patient safety, evidenced by guidelines for TOs in procedural settings, handoffs in patient care (eg, sign‐out between providers),14, 15 and transitions into and from the hospital setting.16 Furthermore, there is growing evidence that such structured times for communication and teamwork, such as with briefings, can improve efficiency and reduce delays in care.17, 18 However, handoffs, which address provider transitions, and daily multidisciplinary rounds, which bring providers together regularly, are provider‐centered rather than patient‐centered. Critical Conversations focus on times when patients require direct communication about their care plans to ensure safe and high quality outcomes.
Implementation of Critical Conversations provides an opportunity to codify a professional standard for patient‐centered communication at times when it should be expected. Critical Conversations also help build a system that supports a positive safety culture and encourages teamwork and direct communication. This is particularly true at a time when rapid adoption of information technology may have the unintended and opposite effect. For instance, as our hospital moved toward an entirely electronic health record, providers were increasingly relocating from patient care units into remote offices, corner hideaways, or designated computer rooms to complete orders and documentation. Although this may reduce many related errors in these processes and potentially improve communication via shared access to an electronic record, it does allow for less direct communicationa circumstance that traditionally occurs (even informally) when providers share the same clinical work areas. This situation is aggravated where the nurses are unit‐based and other providers (eg, physicians, therapists, case managers) are service‐based.
Integrating Critical Conversations into practice comes with expected challenges, most notably around workflow (eg, adds a step, although may save steps down the line) and the expectations concomitant with any change in standard of care (possible enforcement or auditing of their occurrence). Certain cultural barriers may also play a significant role, such as the presence of hierarchies that can hinder open communication and the related ability to speak up with concerns, as related in the TO literature. Where these cultural barriers highlight historical descriptions of the doctornurse relationship and its effect on patient care,1921 Critical Conversations provide an opportunity to improve such interdisciplinary relationships by providing a shared tool for direct communication.
In summary, we described an innovative communication tool that promotes direct communication at critical junctures during a hospitalization. With the growing complexity of hospital care and greater interdependence between teams that deliver this care, Critical Conversations provide an opportunity to further address the known communication failures that contribute to medical errors.
Acknowledgements
Critical Conversations was developed during the Triad for Optimal Patient Safety (TOPS) project, an effort focused on improving unit‐based safety culture through improved teamwork and communication. We thank the Gordon and Betty Moore Foundation for their active support and funding of the TOPS project, which was a collaboration between the Schools of Medicine, Nursing, and Pharmacy at the University of California, San Francisco.
- ,,,,.Communication failures in patient sign‐out and suggestions for improvement: a critical incident analysis.Qual Saf Health Care.2005;14(6):401–407.
- ,,.Communication failures: an insidious contributor to medical mishaps.Acad Med.2004;79(2):186–194.
- ,.TeamSTEPPS: assuring optimal teamwork in clinical settings.Am J Med Qual.2007;22(3):214–217.
- ,,,,,.Medical team training: applying crew resource management in the Veterans Health Administration.Jt Comm J Qual Patient Saf.2007;33(6):317–325.
- ,,,,,,,,,.A multidisciplinary teamwork training program: the Triad for Optimal Patient Safety (TOPS) Experience.J Gen Intern Med.2008;23(12):2053–2057.
- ,,.The human factor: the critical importance of effective teamwork and communication in providing safe care.Qual Saf Health Care.2004;13Suppl‐1:i85–90.
- ,,.SBAR: a shared mental model for improving communication between clinicians.Jt Comm J Qual Patient Saf.2006;32(3):167–175.
- The Joint Commission Universal Protocol for Preventing Wrong Site, Wrong Procedure, Wrong Person Surgery. Available at: http://www.jointcommission.org/NR/rdonlyres/E3C600EB‐043B‐4E86‐B04E‐CA4A89AD5433/0/universal_protocol.pdf. Accessed January 24, 2010.
- ,,.The hospital discharge: a review of a high risk care transition with highlights of a reengineered discharge process.J Patient Saf.2007;3:97–106.
- How do we communicate? Communication on Agile Software Projects. Available at: www.agilemodeling.com/essays/communication.htm. Accessed January 24, 2010.
- ,,,.Promoting effective transitions of care at hospital discharge: a review of key issues for hospitalists.J Hosp Med.2007;2(5):314–323.
- ,,,,,.Deficits in communication and information transfer between hospital‐based and primary care physicians: implications for patient safety and continuity of care.JAMA.2007;297(8):831–841.
- .Engaging patients at hospital discharge.J Hosp Med.2008;3(6):498–500.
- ,,,,.Managing discontinuity in academic medical centers: strategies for a safe and effective resident sign‐out.J Hosp Med.2006;1(4):257–266.
- ,.Lost in transition: challenges and opportunities for improving the quality of transitional care.Ann Intern Med.2004;141(7):533–536.
- ,,, et al.Transition of care for hospitalized elderly patients—development of a discharge checklist for hospitalists.J Hosp Med.2006;1(6):354–360.
- ,,, et al.Impact of preoperative briefings on operating room delays.Arch Surg.2008;143(11):1068–1072.
- ,,, et al.Operating room briefings: working on the same page.Jt Comm J Qual Patient Saf.2006;32(6):351–355.
- .Doctors and nurses: a troubled partnership.Ann Surg.1999;230(3):279–288.
- ,,, et al.Association between nurse‐physician collaboration and patient outcomes in three intensive care units.Crit Care Med.1999;27(9):1991–1998.
- ,,, et al.Evaluation of a preoperative checklist and team briefing among surgeons, nurses, and anesthesiologists to reduce failures in communication.Arch Surg.2008;143(1):12–17.
- ,,,,.Communication failures in patient sign‐out and suggestions for improvement: a critical incident analysis.Qual Saf Health Care.2005;14(6):401–407.
- ,,.Communication failures: an insidious contributor to medical mishaps.Acad Med.2004;79(2):186–194.
- ,.TeamSTEPPS: assuring optimal teamwork in clinical settings.Am J Med Qual.2007;22(3):214–217.
- ,,,,,.Medical team training: applying crew resource management in the Veterans Health Administration.Jt Comm J Qual Patient Saf.2007;33(6):317–325.
- ,,,,,,,,,.A multidisciplinary teamwork training program: the Triad for Optimal Patient Safety (TOPS) Experience.J Gen Intern Med.2008;23(12):2053–2057.
- ,,.The human factor: the critical importance of effective teamwork and communication in providing safe care.Qual Saf Health Care.2004;13Suppl‐1:i85–90.
- ,,.SBAR: a shared mental model for improving communication between clinicians.Jt Comm J Qual Patient Saf.2006;32(3):167–175.
- The Joint Commission Universal Protocol for Preventing Wrong Site, Wrong Procedure, Wrong Person Surgery. Available at: http://www.jointcommission.org/NR/rdonlyres/E3C600EB‐043B‐4E86‐B04E‐CA4A89AD5433/0/universal_protocol.pdf. Accessed January 24, 2010.
- ,,.The hospital discharge: a review of a high risk care transition with highlights of a reengineered discharge process.J Patient Saf.2007;3:97–106.
- How do we communicate? Communication on Agile Software Projects. Available at: www.agilemodeling.com/essays/communication.htm. Accessed January 24, 2010.
- ,,,.Promoting effective transitions of care at hospital discharge: a review of key issues for hospitalists.J Hosp Med.2007;2(5):314–323.
- ,,,,,.Deficits in communication and information transfer between hospital‐based and primary care physicians: implications for patient safety and continuity of care.JAMA.2007;297(8):831–841.
- .Engaging patients at hospital discharge.J Hosp Med.2008;3(6):498–500.
- ,,,,.Managing discontinuity in academic medical centers: strategies for a safe and effective resident sign‐out.J Hosp Med.2006;1(4):257–266.
- ,.Lost in transition: challenges and opportunities for improving the quality of transitional care.Ann Intern Med.2004;141(7):533–536.
- ,,, et al.Transition of care for hospitalized elderly patients—development of a discharge checklist for hospitalists.J Hosp Med.2006;1(6):354–360.
- ,,, et al.Impact of preoperative briefings on operating room delays.Arch Surg.2008;143(11):1068–1072.
- ,,, et al.Operating room briefings: working on the same page.Jt Comm J Qual Patient Saf.2006;32(6):351–355.
- .Doctors and nurses: a troubled partnership.Ann Surg.1999;230(3):279–288.
- ,,, et al.Association between nurse‐physician collaboration and patient outcomes in three intensive care units.Crit Care Med.1999;27(9):1991–1998.
- ,,, et al.Evaluation of a preoperative checklist and team briefing among surgeons, nurses, and anesthesiologists to reduce failures in communication.Arch Surg.2008;143(1):12–17.
Copyright © 2011 Society of Hospital Medicine
New DVT Guidelines Prompt HM Action
The greatest impact of new guidelines from the American Heart Association (AHA) that suggest additional therapies for treatment of more serious cases of DVT might be in prodding HM leaders to take ownership of existing standards to ensure greater compliance.
The review aims to help doctors "identify the severity of these disorders and to select who might be eligible for more invasive therapies, such as clot-busting drugs, catheter-based treatments or surgery," M. Sean McMurtry, MD, PhD, co-chair of the writing group said in a prepared statement. The guidelines outline multiple treatment options, including the use of fibrinolytic drugs, catheter-based interventions, treatment with surgery to remove the blood clots and use of filters. Additional guidance for treating pediatric patients is included.
But Gregory A. Maynard, MD, SFHM, hospital medicine division chief at the University of California at San Diego, says most hospitalists deal with more routine cases of DVT and VTE than the research paper highlights. Physicians need to take more control of the existing patchwork of guidelines recommended by various research and established protocols, he adds.
"What's missing in this paper ... is how to make those things happen more reliably," Dr. Maynard says. "To me, the hospitalist needs to look at guidelines like this and say, 'How can we make them happen reliably?'"
For example, Dr. Maynard notes that for the treatment of iliofemoral DVT, it is recommended to both overlap warfarin and heparin, as well as have patients wear elastic compression stockings. Yet, he says, neither of those recommendations is routinely followed. In fact, he says of the former: "I would guess the percentage of patients getting these stockings is a distinct minority."
And while that kind of reliability is tough to guarantee, it's one of the cornerstones of SHM's VTE prevention resource room and mentored implementation program.
The greatest impact of new guidelines from the American Heart Association (AHA) that suggest additional therapies for treatment of more serious cases of DVT might be in prodding HM leaders to take ownership of existing standards to ensure greater compliance.
The review aims to help doctors "identify the severity of these disorders and to select who might be eligible for more invasive therapies, such as clot-busting drugs, catheter-based treatments or surgery," M. Sean McMurtry, MD, PhD, co-chair of the writing group said in a prepared statement. The guidelines outline multiple treatment options, including the use of fibrinolytic drugs, catheter-based interventions, treatment with surgery to remove the blood clots and use of filters. Additional guidance for treating pediatric patients is included.
But Gregory A. Maynard, MD, SFHM, hospital medicine division chief at the University of California at San Diego, says most hospitalists deal with more routine cases of DVT and VTE than the research paper highlights. Physicians need to take more control of the existing patchwork of guidelines recommended by various research and established protocols, he adds.
"What's missing in this paper ... is how to make those things happen more reliably," Dr. Maynard says. "To me, the hospitalist needs to look at guidelines like this and say, 'How can we make them happen reliably?'"
For example, Dr. Maynard notes that for the treatment of iliofemoral DVT, it is recommended to both overlap warfarin and heparin, as well as have patients wear elastic compression stockings. Yet, he says, neither of those recommendations is routinely followed. In fact, he says of the former: "I would guess the percentage of patients getting these stockings is a distinct minority."
And while that kind of reliability is tough to guarantee, it's one of the cornerstones of SHM's VTE prevention resource room and mentored implementation program.
The greatest impact of new guidelines from the American Heart Association (AHA) that suggest additional therapies for treatment of more serious cases of DVT might be in prodding HM leaders to take ownership of existing standards to ensure greater compliance.
The review aims to help doctors "identify the severity of these disorders and to select who might be eligible for more invasive therapies, such as clot-busting drugs, catheter-based treatments or surgery," M. Sean McMurtry, MD, PhD, co-chair of the writing group said in a prepared statement. The guidelines outline multiple treatment options, including the use of fibrinolytic drugs, catheter-based interventions, treatment with surgery to remove the blood clots and use of filters. Additional guidance for treating pediatric patients is included.
But Gregory A. Maynard, MD, SFHM, hospital medicine division chief at the University of California at San Diego, says most hospitalists deal with more routine cases of DVT and VTE than the research paper highlights. Physicians need to take more control of the existing patchwork of guidelines recommended by various research and established protocols, he adds.
"What's missing in this paper ... is how to make those things happen more reliably," Dr. Maynard says. "To me, the hospitalist needs to look at guidelines like this and say, 'How can we make them happen reliably?'"
For example, Dr. Maynard notes that for the treatment of iliofemoral DVT, it is recommended to both overlap warfarin and heparin, as well as have patients wear elastic compression stockings. Yet, he says, neither of those recommendations is routinely followed. In fact, he says of the former: "I would guess the percentage of patients getting these stockings is a distinct minority."
And while that kind of reliability is tough to guarantee, it's one of the cornerstones of SHM's VTE prevention resource room and mentored implementation program.
HM Company Acquires Call Center
Apollo Medical Holdings of Glendale, Calif., which provides hospitalist services in 24 California hospitals, recently took a step toward expanding its business model and diversifying its continuum of services by acquiring Los Angeles-based Aligned Healthcare Group, a provider of physician call centers and specialized care management services for health plans.
Call centers provide patients in the community with telephonic access to physicians and other health professionals for help with urgent medical questions, health assessments, and triage. The Aligned call center was developed in 2009 at the request of Anthem Blue Cross, which wanted to provide an ED alternative for its Medi-Cal members who needed access to a physician, explains Bette Jane Reese, RN, MHA, COO of Apollo's Aligned Division.
Apollo's care continuum will include follow-up calls to recently discharged patients, post-discharge calls to PCPs, and early discharge planning. The model partners a hospitalist with a care management nurse; together, they function as a virtual team across settings.
The acquisition gives Apollo a leg up on developing a continuum of care management across settings, she adds. "I believe enhanced hospitalist models will be a trend. As healthcare revenues become tighter, with more entities coming together in what's called accountable healthcare, the name of the game is coordination between settings and providers," she says.
Experts have emphasized the importance of hospitalists looking beyond the four walls of their facility and participating in "cross-continuum teams" as a key to managing care transitions and preventing rehospitalizations. Apollo hopes its new collaboration will help eliminate communication breakdowns between hospitalists and PCPs, Reese says. "By merging these functions, we get a combination of efficient hospitalist care with a bridge to the next setting, and coordination with multiple payer entities. It all wraps around the integrated hospitalist model."
HM's future in Apollo's model might include staffing outpatient clinics located on the hospital campus for patients to return for follow-up care after they are discharged, or even making home visits for patients who need additional medical oversight. "We see the issues and problems that cause patients to go back to the hospital," Reese says. "We can suggest quality improvement approaches to address the root causes of avoidable readmissions."
Apollo Medical Holdings of Glendale, Calif., which provides hospitalist services in 24 California hospitals, recently took a step toward expanding its business model and diversifying its continuum of services by acquiring Los Angeles-based Aligned Healthcare Group, a provider of physician call centers and specialized care management services for health plans.
Call centers provide patients in the community with telephonic access to physicians and other health professionals for help with urgent medical questions, health assessments, and triage. The Aligned call center was developed in 2009 at the request of Anthem Blue Cross, which wanted to provide an ED alternative for its Medi-Cal members who needed access to a physician, explains Bette Jane Reese, RN, MHA, COO of Apollo's Aligned Division.
Apollo's care continuum will include follow-up calls to recently discharged patients, post-discharge calls to PCPs, and early discharge planning. The model partners a hospitalist with a care management nurse; together, they function as a virtual team across settings.
The acquisition gives Apollo a leg up on developing a continuum of care management across settings, she adds. "I believe enhanced hospitalist models will be a trend. As healthcare revenues become tighter, with more entities coming together in what's called accountable healthcare, the name of the game is coordination between settings and providers," she says.
Experts have emphasized the importance of hospitalists looking beyond the four walls of their facility and participating in "cross-continuum teams" as a key to managing care transitions and preventing rehospitalizations. Apollo hopes its new collaboration will help eliminate communication breakdowns between hospitalists and PCPs, Reese says. "By merging these functions, we get a combination of efficient hospitalist care with a bridge to the next setting, and coordination with multiple payer entities. It all wraps around the integrated hospitalist model."
HM's future in Apollo's model might include staffing outpatient clinics located on the hospital campus for patients to return for follow-up care after they are discharged, or even making home visits for patients who need additional medical oversight. "We see the issues and problems that cause patients to go back to the hospital," Reese says. "We can suggest quality improvement approaches to address the root causes of avoidable readmissions."
Apollo Medical Holdings of Glendale, Calif., which provides hospitalist services in 24 California hospitals, recently took a step toward expanding its business model and diversifying its continuum of services by acquiring Los Angeles-based Aligned Healthcare Group, a provider of physician call centers and specialized care management services for health plans.
Call centers provide patients in the community with telephonic access to physicians and other health professionals for help with urgent medical questions, health assessments, and triage. The Aligned call center was developed in 2009 at the request of Anthem Blue Cross, which wanted to provide an ED alternative for its Medi-Cal members who needed access to a physician, explains Bette Jane Reese, RN, MHA, COO of Apollo's Aligned Division.
Apollo's care continuum will include follow-up calls to recently discharged patients, post-discharge calls to PCPs, and early discharge planning. The model partners a hospitalist with a care management nurse; together, they function as a virtual team across settings.
The acquisition gives Apollo a leg up on developing a continuum of care management across settings, she adds. "I believe enhanced hospitalist models will be a trend. As healthcare revenues become tighter, with more entities coming together in what's called accountable healthcare, the name of the game is coordination between settings and providers," she says.
Experts have emphasized the importance of hospitalists looking beyond the four walls of their facility and participating in "cross-continuum teams" as a key to managing care transitions and preventing rehospitalizations. Apollo hopes its new collaboration will help eliminate communication breakdowns between hospitalists and PCPs, Reese says. "By merging these functions, we get a combination of efficient hospitalist care with a bridge to the next setting, and coordination with multiple payer entities. It all wraps around the integrated hospitalist model."
HM's future in Apollo's model might include staffing outpatient clinics located on the hospital campus for patients to return for follow-up care after they are discharged, or even making home visits for patients who need additional medical oversight. "We see the issues and problems that cause patients to go back to the hospital," Reese says. "We can suggest quality improvement approaches to address the root causes of avoidable readmissions."
Endobronchial Dysplasia Could Be Marker for Lung Cancer Chemoprevention
ORLANDO – Endobronchial dysplasia appears useful as a biomarker for measuring the success of lung cancer chemoprevention, investigators reported at the annual meeting of the American Association for Cancer Research.
Bronchoscopies, along with biopsies of standard endobronchial sites and any other abnormal appearing areas, were performed at baseline and at 6 months after randomization to treatment with iloprost (Ventavis) or placebo in a phase II chemoprevention trial involving 152 former or current smokers with at least a 20 pack-year history.
Former smokers who received iloprost, an oral prostacyclin analog approved for the treatment of primary pulmonary hypertension, had significant improvements on several measures of endobronchial dysplasia, while current smokers had no improvement, Dr. Paul Bunn reported.
The findings demonstrate that iloprost, which has been shown to prevent the development of lung cancer in various murine models involving cigarette-smoke exposure, also might have the same effect in humans and thus deserves further study for this purpose, Dr. Bunn and his coauthors concluded.
The results also demonstrate that endobronchial dysplasia could serve as a biomarker for effectiveness of chemopreventive treatment– much as cholesterol does in patients being treated with statins to prevent cardiovascular disease, according to Dr. Bunn, executive director of the International Association for the Study of Lung Cancer. He is also the James Dudley endowed professor of lung cancer research at the cancer center at the University of Colorado, Aurora.
In the current study, baseline histology was significantly worse in current smokers than in former smokers (average biopsy scores of 3.0 vs. 2.1, respectively, with a score of 4 indicating mild dysplasia). Former smokers experienced a 0.41-point improvement in average biopsy score (P = .010), a 1.10-point improvement in their worst baseline biopsy score (P = .002), and a 12.5% improvement in dysplasia index (P = .006), which was the percentage of biopsies with a score of at least 4, said Dr. Bunn.
"The histologic improvement in the treated patients who were former smokers was larger than the magnitude of the difference between current and former smokers," he said.
For example, the baseline dysplasia index in current and former smokers was 46% and 31%, respectively, but the pre- and post-treatment dysplasia index in former smokers was 43% and 19.6%, respectively.
Study participants had an average 30 pack-year history of smoking, and at least mild cytologic atypia on sputum cytology, but no previous history of cancer. Iloprost was given in escalating doses across the 6-month treatment period and was well tolerated. The treatment and placebo groups were well-matched for age, tobacco exposure and baseline histology, and there was no difference in dropout rate or serious adverse events between the treatment and placebo groups, Dr. Bunn noted.
Although antismoking campaigns are working – and about half of all smokers have quit, those who quit remain at greater risk for developing lung cancer than are nonsmokers; about half of all cases of lung cancer are in former smokers, and it is important to find effective chemopreventive measures for these individuals, he said.
Dr. Bunn discussed off-label use of iloprost for chemoprevention of lung cancer. He had no other disclosures.
ORLANDO – Endobronchial dysplasia appears useful as a biomarker for measuring the success of lung cancer chemoprevention, investigators reported at the annual meeting of the American Association for Cancer Research.
Bronchoscopies, along with biopsies of standard endobronchial sites and any other abnormal appearing areas, were performed at baseline and at 6 months after randomization to treatment with iloprost (Ventavis) or placebo in a phase II chemoprevention trial involving 152 former or current smokers with at least a 20 pack-year history.
Former smokers who received iloprost, an oral prostacyclin analog approved for the treatment of primary pulmonary hypertension, had significant improvements on several measures of endobronchial dysplasia, while current smokers had no improvement, Dr. Paul Bunn reported.
The findings demonstrate that iloprost, which has been shown to prevent the development of lung cancer in various murine models involving cigarette-smoke exposure, also might have the same effect in humans and thus deserves further study for this purpose, Dr. Bunn and his coauthors concluded.
The results also demonstrate that endobronchial dysplasia could serve as a biomarker for effectiveness of chemopreventive treatment– much as cholesterol does in patients being treated with statins to prevent cardiovascular disease, according to Dr. Bunn, executive director of the International Association for the Study of Lung Cancer. He is also the James Dudley endowed professor of lung cancer research at the cancer center at the University of Colorado, Aurora.
In the current study, baseline histology was significantly worse in current smokers than in former smokers (average biopsy scores of 3.0 vs. 2.1, respectively, with a score of 4 indicating mild dysplasia). Former smokers experienced a 0.41-point improvement in average biopsy score (P = .010), a 1.10-point improvement in their worst baseline biopsy score (P = .002), and a 12.5% improvement in dysplasia index (P = .006), which was the percentage of biopsies with a score of at least 4, said Dr. Bunn.
"The histologic improvement in the treated patients who were former smokers was larger than the magnitude of the difference between current and former smokers," he said.
For example, the baseline dysplasia index in current and former smokers was 46% and 31%, respectively, but the pre- and post-treatment dysplasia index in former smokers was 43% and 19.6%, respectively.
Study participants had an average 30 pack-year history of smoking, and at least mild cytologic atypia on sputum cytology, but no previous history of cancer. Iloprost was given in escalating doses across the 6-month treatment period and was well tolerated. The treatment and placebo groups were well-matched for age, tobacco exposure and baseline histology, and there was no difference in dropout rate or serious adverse events between the treatment and placebo groups, Dr. Bunn noted.
Although antismoking campaigns are working – and about half of all smokers have quit, those who quit remain at greater risk for developing lung cancer than are nonsmokers; about half of all cases of lung cancer are in former smokers, and it is important to find effective chemopreventive measures for these individuals, he said.
Dr. Bunn discussed off-label use of iloprost for chemoprevention of lung cancer. He had no other disclosures.
ORLANDO – Endobronchial dysplasia appears useful as a biomarker for measuring the success of lung cancer chemoprevention, investigators reported at the annual meeting of the American Association for Cancer Research.
Bronchoscopies, along with biopsies of standard endobronchial sites and any other abnormal appearing areas, were performed at baseline and at 6 months after randomization to treatment with iloprost (Ventavis) or placebo in a phase II chemoprevention trial involving 152 former or current smokers with at least a 20 pack-year history.
Former smokers who received iloprost, an oral prostacyclin analog approved for the treatment of primary pulmonary hypertension, had significant improvements on several measures of endobronchial dysplasia, while current smokers had no improvement, Dr. Paul Bunn reported.
The findings demonstrate that iloprost, which has been shown to prevent the development of lung cancer in various murine models involving cigarette-smoke exposure, also might have the same effect in humans and thus deserves further study for this purpose, Dr. Bunn and his coauthors concluded.
The results also demonstrate that endobronchial dysplasia could serve as a biomarker for effectiveness of chemopreventive treatment– much as cholesterol does in patients being treated with statins to prevent cardiovascular disease, according to Dr. Bunn, executive director of the International Association for the Study of Lung Cancer. He is also the James Dudley endowed professor of lung cancer research at the cancer center at the University of Colorado, Aurora.
In the current study, baseline histology was significantly worse in current smokers than in former smokers (average biopsy scores of 3.0 vs. 2.1, respectively, with a score of 4 indicating mild dysplasia). Former smokers experienced a 0.41-point improvement in average biopsy score (P = .010), a 1.10-point improvement in their worst baseline biopsy score (P = .002), and a 12.5% improvement in dysplasia index (P = .006), which was the percentage of biopsies with a score of at least 4, said Dr. Bunn.
"The histologic improvement in the treated patients who were former smokers was larger than the magnitude of the difference between current and former smokers," he said.
For example, the baseline dysplasia index in current and former smokers was 46% and 31%, respectively, but the pre- and post-treatment dysplasia index in former smokers was 43% and 19.6%, respectively.
Study participants had an average 30 pack-year history of smoking, and at least mild cytologic atypia on sputum cytology, but no previous history of cancer. Iloprost was given in escalating doses across the 6-month treatment period and was well tolerated. The treatment and placebo groups were well-matched for age, tobacco exposure and baseline histology, and there was no difference in dropout rate or serious adverse events between the treatment and placebo groups, Dr. Bunn noted.
Although antismoking campaigns are working – and about half of all smokers have quit, those who quit remain at greater risk for developing lung cancer than are nonsmokers; about half of all cases of lung cancer are in former smokers, and it is important to find effective chemopreventive measures for these individuals, he said.
Dr. Bunn discussed off-label use of iloprost for chemoprevention of lung cancer. He had no other disclosures.
FROM THE ANNUAL MEETING OF THE AMERICAN ASSOCIATION FOR CANCER RESEARCH
Major Finding: Former smokers had an average 0.41-point improvement in biopsy score, an average 1.10-point improvement in their worst baseline biopsy score, and a 12.5% improvement in dysplasia index. No improvement occurred in current smokers.
Data Source: A randomized, placebo-controlled phase II trial.
Disclosures: Dr. Bunn discussed off-label use of iloprost for chemoprevention of lung cancer. He had no other disclosures.
HM Model Expands to Ears, Noses, and Throats
After five years in the department of otolaryngology/head and neck surgery at the University of California at San Francisco (UCSF), Matthew Russell, MD, is joining the faculty as an assistant professor. Normally, such a career arc is commonplace. But Dr. Russell’s new job title—ENT hospitalist—is worth noting. In fact, it could be groundbreaking.
When Dr. Russell begins work this summer, he might be the only otolaryngologist in the country whose entire patient census and surgical pipeline will be generated by admissions to his hospital. Although there are otolaryngologists around the country who spend the majority of their time working with inpatients, nearly all work an clinical outpatient service as well.
“The hospitalist model turns the traditional ENT practice on its head,” Dr. Russell says. “An otolaryngology practice we think of as being centered around the clinic, and the clinic and referrals is where we generate our operative cases and our patient load. The question really becomes: Can you sustain a practice without a clinic-based model?”
David Nielsen, MD, executive vice president and CEO of the American Academy of Otolaryngology-Head and Neck Surgery, says that while there is no current groundswell for the model, he can envision physicians being drawn to it for two reasons: an aging cohort of otolaryngologists and younger physicians looking for work-life balance.
—Matthew Russell, MD, oto-hospitalist, University of California at San Francisco
And while the otolaryngology world at large has not yet answered in unison, the presence of what some are calling an oto-hospitalist is the latest in a series of what HM pioneer Robert Wachter, MD, MHM, has termed “hyphenated hospitalists.” Dr. Wachter, chief of hospital medicine and chief of the medical service at UCSF Medical Center, a former SHM board member, and author of the Wachter’s World blog, says the needs of otolaryngology present the same set of circumstances that allowed internal-medicine-based HM to flourish.
“The forces,” Dr. Wachter wrote in January on his blog, “are the same: sick patients, highly specialized providers who may not be comfortable with all the issues that arise in the hospital, and the need to focus on system improvement.”
But just adding hospitalist to a job title is not the mark of HM’s presence.
“You can have any hyphenated medical specialist managing patients, but the question is, What are you getting out of it as a hospital or a hospitalist, or as an institution?” adds Gulshan Sharma MD, MPH, associate professor at the University of Texas Medical Branch at Galveston. “The hospitalists really have to figure out their boundaries.”
Dr. Russell says some physicians could be dismissive of the idea of an oto-hospitalist because they’re not clear about the role. They might picture a glorified resident constantly walking between wards to serve as a secondary opinion for other specialists. “There is a perception that this may not be a glamorous position,” he adds. “There’s an assumption that the position is nonsurgical.”
Dr. Russell’s workflow will include rounding and consultations across different wards, and he will assist with complex airway issues. But he also will perform surgeries and work on quality-improvement (QI) initiatives. For those who doubt the variety that a purely inpatient setting can deliver, Dr. Russell eagerly quotes statistics from a two-year pilot program UCSF ran before hiring him as a full-time ENT hospitalist:
- 300 inpatient consultations the first year, not including ED and urgent care;
- Sinonasal and laryngotracheal were the most common consults;
- 200 procedures generated billings; and
- 45% of procedures were laryngotracheal, 33% were sinonasal/anterior skull base, and 10% were otologic.
"The hospitalist movement, in general, fills a need for the acute-care setting and manages a different set of problems than is seen in the ambulatory clinics,” Dr. Russell says. “That same basic issue is found in otolaryngology. I think it’s an area that is perhaps underappreciated.”
Richard Quinn is a freelance writer based in New Jersey.
Hopkins Physician Sees Bright Future for ENT Hospitalists
In 2000, Nasir Bhatti, MD, associate professor of otolaryngology/head and neck surgery at Johns Hopkins University in Baltimore, started a program similar to the one at UCSF. At Johns Hopkins, Dr. Bhatti, and now his successors, acted primarily as an ENT hospitalist, although he maintained minimal clinic duties as well.
He says the oto-hospitalist model could work efficiently because it would allow physicians, by choice, to determine whether they wanted to focus on surgical procedures or nonsurgical medical services. Those who favor surgery and more intensive procedures could focus on those subspecialties without feeling distracted by the demands of less intensive duties, Dr. Russell adds. Also, Dr. Bhatti points out, the setup could create more revenue capture opportunities from consultations that currently are handled by nurse practitioners (NPs) and physician assistants (PAs).
“Lots of these consultations go unstaffed and, therefore, unbilled,” Dr. Bhatti says.—RQ
After five years in the department of otolaryngology/head and neck surgery at the University of California at San Francisco (UCSF), Matthew Russell, MD, is joining the faculty as an assistant professor. Normally, such a career arc is commonplace. But Dr. Russell’s new job title—ENT hospitalist—is worth noting. In fact, it could be groundbreaking.
When Dr. Russell begins work this summer, he might be the only otolaryngologist in the country whose entire patient census and surgical pipeline will be generated by admissions to his hospital. Although there are otolaryngologists around the country who spend the majority of their time working with inpatients, nearly all work an clinical outpatient service as well.
“The hospitalist model turns the traditional ENT practice on its head,” Dr. Russell says. “An otolaryngology practice we think of as being centered around the clinic, and the clinic and referrals is where we generate our operative cases and our patient load. The question really becomes: Can you sustain a practice without a clinic-based model?”
David Nielsen, MD, executive vice president and CEO of the American Academy of Otolaryngology-Head and Neck Surgery, says that while there is no current groundswell for the model, he can envision physicians being drawn to it for two reasons: an aging cohort of otolaryngologists and younger physicians looking for work-life balance.

—Matthew Russell, MD, oto-hospitalist, University of California at San Francisco
And while the otolaryngology world at large has not yet answered in unison, the presence of what some are calling an oto-hospitalist is the latest in a series of what HM pioneer Robert Wachter, MD, MHM, has termed “hyphenated hospitalists.” Dr. Wachter, chief of hospital medicine and chief of the medical service at UCSF Medical Center, a former SHM board member, and author of the Wachter’s World blog, says the needs of otolaryngology present the same set of circumstances that allowed internal-medicine-based HM to flourish.
“The forces,” Dr. Wachter wrote in January on his blog, “are the same: sick patients, highly specialized providers who may not be comfortable with all the issues that arise in the hospital, and the need to focus on system improvement.”
But just adding hospitalist to a job title is not the mark of HM’s presence.
“You can have any hyphenated medical specialist managing patients, but the question is, What are you getting out of it as a hospital or a hospitalist, or as an institution?” adds Gulshan Sharma MD, MPH, associate professor at the University of Texas Medical Branch at Galveston. “The hospitalists really have to figure out their boundaries.”
Dr. Russell says some physicians could be dismissive of the idea of an oto-hospitalist because they’re not clear about the role. They might picture a glorified resident constantly walking between wards to serve as a secondary opinion for other specialists. “There is a perception that this may not be a glamorous position,” he adds. “There’s an assumption that the position is nonsurgical.”
Dr. Russell’s workflow will include rounding and consultations across different wards, and he will assist with complex airway issues. But he also will perform surgeries and work on quality-improvement (QI) initiatives. For those who doubt the variety that a purely inpatient setting can deliver, Dr. Russell eagerly quotes statistics from a two-year pilot program UCSF ran before hiring him as a full-time ENT hospitalist:
- 300 inpatient consultations the first year, not including ED and urgent care;
- Sinonasal and laryngotracheal were the most common consults;
- 200 procedures generated billings; and
- 45% of procedures were laryngotracheal, 33% were sinonasal/anterior skull base, and 10% were otologic.
"The hospitalist movement, in general, fills a need for the acute-care setting and manages a different set of problems than is seen in the ambulatory clinics,” Dr. Russell says. “That same basic issue is found in otolaryngology. I think it’s an area that is perhaps underappreciated.”
Richard Quinn is a freelance writer based in New Jersey.
Hopkins Physician Sees Bright Future for ENT Hospitalists
In 2000, Nasir Bhatti, MD, associate professor of otolaryngology/head and neck surgery at Johns Hopkins University in Baltimore, started a program similar to the one at UCSF. At Johns Hopkins, Dr. Bhatti, and now his successors, acted primarily as an ENT hospitalist, although he maintained minimal clinic duties as well.
He says the oto-hospitalist model could work efficiently because it would allow physicians, by choice, to determine whether they wanted to focus on surgical procedures or nonsurgical medical services. Those who favor surgery and more intensive procedures could focus on those subspecialties without feeling distracted by the demands of less intensive duties, Dr. Russell adds. Also, Dr. Bhatti points out, the setup could create more revenue capture opportunities from consultations that currently are handled by nurse practitioners (NPs) and physician assistants (PAs).
“Lots of these consultations go unstaffed and, therefore, unbilled,” Dr. Bhatti says.—RQ
After five years in the department of otolaryngology/head and neck surgery at the University of California at San Francisco (UCSF), Matthew Russell, MD, is joining the faculty as an assistant professor. Normally, such a career arc is commonplace. But Dr. Russell’s new job title—ENT hospitalist—is worth noting. In fact, it could be groundbreaking.
When Dr. Russell begins work this summer, he might be the only otolaryngologist in the country whose entire patient census and surgical pipeline will be generated by admissions to his hospital. Although there are otolaryngologists around the country who spend the majority of their time working with inpatients, nearly all work an clinical outpatient service as well.
“The hospitalist model turns the traditional ENT practice on its head,” Dr. Russell says. “An otolaryngology practice we think of as being centered around the clinic, and the clinic and referrals is where we generate our operative cases and our patient load. The question really becomes: Can you sustain a practice without a clinic-based model?”
David Nielsen, MD, executive vice president and CEO of the American Academy of Otolaryngology-Head and Neck Surgery, says that while there is no current groundswell for the model, he can envision physicians being drawn to it for two reasons: an aging cohort of otolaryngologists and younger physicians looking for work-life balance.

—Matthew Russell, MD, oto-hospitalist, University of California at San Francisco
And while the otolaryngology world at large has not yet answered in unison, the presence of what some are calling an oto-hospitalist is the latest in a series of what HM pioneer Robert Wachter, MD, MHM, has termed “hyphenated hospitalists.” Dr. Wachter, chief of hospital medicine and chief of the medical service at UCSF Medical Center, a former SHM board member, and author of the Wachter’s World blog, says the needs of otolaryngology present the same set of circumstances that allowed internal-medicine-based HM to flourish.
“The forces,” Dr. Wachter wrote in January on his blog, “are the same: sick patients, highly specialized providers who may not be comfortable with all the issues that arise in the hospital, and the need to focus on system improvement.”
But just adding hospitalist to a job title is not the mark of HM’s presence.
“You can have any hyphenated medical specialist managing patients, but the question is, What are you getting out of it as a hospital or a hospitalist, or as an institution?” adds Gulshan Sharma MD, MPH, associate professor at the University of Texas Medical Branch at Galveston. “The hospitalists really have to figure out their boundaries.”
Dr. Russell says some physicians could be dismissive of the idea of an oto-hospitalist because they’re not clear about the role. They might picture a glorified resident constantly walking between wards to serve as a secondary opinion for other specialists. “There is a perception that this may not be a glamorous position,” he adds. “There’s an assumption that the position is nonsurgical.”
Dr. Russell’s workflow will include rounding and consultations across different wards, and he will assist with complex airway issues. But he also will perform surgeries and work on quality-improvement (QI) initiatives. For those who doubt the variety that a purely inpatient setting can deliver, Dr. Russell eagerly quotes statistics from a two-year pilot program UCSF ran before hiring him as a full-time ENT hospitalist:
- 300 inpatient consultations the first year, not including ED and urgent care;
- Sinonasal and laryngotracheal were the most common consults;
- 200 procedures generated billings; and
- 45% of procedures were laryngotracheal, 33% were sinonasal/anterior skull base, and 10% were otologic.
"The hospitalist movement, in general, fills a need for the acute-care setting and manages a different set of problems than is seen in the ambulatory clinics,” Dr. Russell says. “That same basic issue is found in otolaryngology. I think it’s an area that is perhaps underappreciated.”
Richard Quinn is a freelance writer based in New Jersey.
Hopkins Physician Sees Bright Future for ENT Hospitalists
In 2000, Nasir Bhatti, MD, associate professor of otolaryngology/head and neck surgery at Johns Hopkins University in Baltimore, started a program similar to the one at UCSF. At Johns Hopkins, Dr. Bhatti, and now his successors, acted primarily as an ENT hospitalist, although he maintained minimal clinic duties as well.
He says the oto-hospitalist model could work efficiently because it would allow physicians, by choice, to determine whether they wanted to focus on surgical procedures or nonsurgical medical services. Those who favor surgery and more intensive procedures could focus on those subspecialties without feeling distracted by the demands of less intensive duties, Dr. Russell adds. Also, Dr. Bhatti points out, the setup could create more revenue capture opportunities from consultations that currently are handled by nurse practitioners (NPs) and physician assistants (PAs).
“Lots of these consultations go unstaffed and, therefore, unbilled,” Dr. Bhatti says.—RQ