User login
SHM Board Adds First Family-Medicine-Trained Member
Hospitalist Robert Harrington Jr., MD, SFHM, chief medical officer for Alpharetta, Ga.-based Locum Leaders, a national physician recruiting and staffing company for hospitalists and other specialties, was recently elected to an at-large seat on SHM's Board of Directors. Dr. Harrington, the first family-medicine-trained hospitalist to serve on the board, says he brings a particular focus on the community-based hospitalist's perspective and will work to make SHM better for all HM providers.
"I regularly interact with community hospitals and physicians. I have also practiced in community settings," he says. "Being an advocate for community-based physicians, I'm a big believer in the 'big tent' philosophy of SHM's recent discussions about its future."
Those discussions about SHM's future have highlighted the variety of "hyphenated" and specialized hospitalists that have emerged in recent years. Dr. Harrington says that SHM should nurture specialist HM groups. "My goal on the board is to continue to push SHM to make it a home for all members of the patient care team," he says. "I continue to believe that hospital medicine is truly a team sport, with quality care delivered by inpatient care teams.”
Dr. Harrington chairs SHM's Family Medicine Task Force and recently was honored as a Senior Fellow in Hospital Medicine. He attended medical school at Temple University in Philadelphia and completed his residency at the Medical Center of Delaware in Wilmington, followed by a stint in the U.S. Air Force Medical Corps. He will commence his three-year term on the board at HM11 in Grapevine, Texas, in May.
Hospitalist Robert Harrington Jr., MD, SFHM, chief medical officer for Alpharetta, Ga.-based Locum Leaders, a national physician recruiting and staffing company for hospitalists and other specialties, was recently elected to an at-large seat on SHM's Board of Directors. Dr. Harrington, the first family-medicine-trained hospitalist to serve on the board, says he brings a particular focus on the community-based hospitalist's perspective and will work to make SHM better for all HM providers.
"I regularly interact with community hospitals and physicians. I have also practiced in community settings," he says. "Being an advocate for community-based physicians, I'm a big believer in the 'big tent' philosophy of SHM's recent discussions about its future."
Those discussions about SHM's future have highlighted the variety of "hyphenated" and specialized hospitalists that have emerged in recent years. Dr. Harrington says that SHM should nurture specialist HM groups. "My goal on the board is to continue to push SHM to make it a home for all members of the patient care team," he says. "I continue to believe that hospital medicine is truly a team sport, with quality care delivered by inpatient care teams.”
Dr. Harrington chairs SHM's Family Medicine Task Force and recently was honored as a Senior Fellow in Hospital Medicine. He attended medical school at Temple University in Philadelphia and completed his residency at the Medical Center of Delaware in Wilmington, followed by a stint in the U.S. Air Force Medical Corps. He will commence his three-year term on the board at HM11 in Grapevine, Texas, in May.
Hospitalist Robert Harrington Jr., MD, SFHM, chief medical officer for Alpharetta, Ga.-based Locum Leaders, a national physician recruiting and staffing company for hospitalists and other specialties, was recently elected to an at-large seat on SHM's Board of Directors. Dr. Harrington, the first family-medicine-trained hospitalist to serve on the board, says he brings a particular focus on the community-based hospitalist's perspective and will work to make SHM better for all HM providers.
"I regularly interact with community hospitals and physicians. I have also practiced in community settings," he says. "Being an advocate for community-based physicians, I'm a big believer in the 'big tent' philosophy of SHM's recent discussions about its future."
Those discussions about SHM's future have highlighted the variety of "hyphenated" and specialized hospitalists that have emerged in recent years. Dr. Harrington says that SHM should nurture specialist HM groups. "My goal on the board is to continue to push SHM to make it a home for all members of the patient care team," he says. "I continue to believe that hospital medicine is truly a team sport, with quality care delivered by inpatient care teams.”
Dr. Harrington chairs SHM's Family Medicine Task Force and recently was honored as a Senior Fellow in Hospital Medicine. He attended medical school at Temple University in Philadelphia and completed his residency at the Medical Center of Delaware in Wilmington, followed by a stint in the U.S. Air Force Medical Corps. He will commence his three-year term on the board at HM11 in Grapevine, Texas, in May.
Benefits of Using Lipid-Lowering Agents Persist After Trials End
NEW ORLEANS – In major clinical trials of lipid-lowering agents, the mortality benefit derived from medical therapy persists long after the studies end, according to a meta-analysis presented at the annual meeting of the American College of Cardiology.
Furthermore, placebo recipients who cross over to lipid-lowering therapy in the open-label phases of the studies demonstrate survival benefits as well, but never attain the protection achieved by being randomized to active treatment earlier on, according to Dr. William J. Kostis of Massachusetts General Hospital, Boston. "Persons with risk factors for coronary artery disease should be treated early," Dr. Kostis said in an interview. "The sooner you treat, the better."
He and his colleagues searched several major databases to identify randomized trials of lipid-lowering therapies that also contained an analysis of patient outcomes after the randomized portion of the trials had ended and an open-label phase had begun. Active treatment in the trials involved statins, niacin, cholestyramine, or gemfibrozil.
The analysis included eight clinical trials involving 44,255 patients, of whom 8,144 died during follow-up.
The average patient remained on the assigned treatment for approximately 5 years and was on the lipid-lowering agent in the open-label phase for approximately 6 years.
During the randomized phase of the trials, the mean all-cause mortality was significantly lower for the active treatment group (odds ratio, 0.84; P = .0006), as was cardiovascular mortality (0.72; P less than .001). The lower mortality among those initially receiving active therapy persisted during the open-label follow-up phase (OR, 0.90; P = .0035), as did the reduction in cardiovascular mortality (OR, 0.82; P = .0014).
"Being treated with a beneficial medication for a longer period of time is better, possibly because we are arresting pathophysiology at an earlier stage," Dr. Kostis proposed. He added that statins may be reducing the size of infarcts in patients who have myocardial infarctions.
Dr. Patrick Moriarty, a lipid specialist and a professor of medicine at the University of Kansas in Kansas City, agreed. "We need to start lipid-lowering therapy early to get the most benefit," and this includes interventions in children when necessary, he added.
"We treat pediatric patients all the time," he said, "not only those with familial hyperlipidemias but also those with metabolic syndrome. ... The future emphasis will be, ‘the sooner the better.’ "
Dr. Kostis and Dr. Moriarty reported having no relevant conflicts of interest.
NEW ORLEANS – In major clinical trials of lipid-lowering agents, the mortality benefit derived from medical therapy persists long after the studies end, according to a meta-analysis presented at the annual meeting of the American College of Cardiology.
Furthermore, placebo recipients who cross over to lipid-lowering therapy in the open-label phases of the studies demonstrate survival benefits as well, but never attain the protection achieved by being randomized to active treatment earlier on, according to Dr. William J. Kostis of Massachusetts General Hospital, Boston. "Persons with risk factors for coronary artery disease should be treated early," Dr. Kostis said in an interview. "The sooner you treat, the better."
He and his colleagues searched several major databases to identify randomized trials of lipid-lowering therapies that also contained an analysis of patient outcomes after the randomized portion of the trials had ended and an open-label phase had begun. Active treatment in the trials involved statins, niacin, cholestyramine, or gemfibrozil.
The analysis included eight clinical trials involving 44,255 patients, of whom 8,144 died during follow-up.
The average patient remained on the assigned treatment for approximately 5 years and was on the lipid-lowering agent in the open-label phase for approximately 6 years.
During the randomized phase of the trials, the mean all-cause mortality was significantly lower for the active treatment group (odds ratio, 0.84; P = .0006), as was cardiovascular mortality (0.72; P less than .001). The lower mortality among those initially receiving active therapy persisted during the open-label follow-up phase (OR, 0.90; P = .0035), as did the reduction in cardiovascular mortality (OR, 0.82; P = .0014).
"Being treated with a beneficial medication for a longer period of time is better, possibly because we are arresting pathophysiology at an earlier stage," Dr. Kostis proposed. He added that statins may be reducing the size of infarcts in patients who have myocardial infarctions.
Dr. Patrick Moriarty, a lipid specialist and a professor of medicine at the University of Kansas in Kansas City, agreed. "We need to start lipid-lowering therapy early to get the most benefit," and this includes interventions in children when necessary, he added.
"We treat pediatric patients all the time," he said, "not only those with familial hyperlipidemias but also those with metabolic syndrome. ... The future emphasis will be, ‘the sooner the better.’ "
Dr. Kostis and Dr. Moriarty reported having no relevant conflicts of interest.
NEW ORLEANS – In major clinical trials of lipid-lowering agents, the mortality benefit derived from medical therapy persists long after the studies end, according to a meta-analysis presented at the annual meeting of the American College of Cardiology.
Furthermore, placebo recipients who cross over to lipid-lowering therapy in the open-label phases of the studies demonstrate survival benefits as well, but never attain the protection achieved by being randomized to active treatment earlier on, according to Dr. William J. Kostis of Massachusetts General Hospital, Boston. "Persons with risk factors for coronary artery disease should be treated early," Dr. Kostis said in an interview. "The sooner you treat, the better."
He and his colleagues searched several major databases to identify randomized trials of lipid-lowering therapies that also contained an analysis of patient outcomes after the randomized portion of the trials had ended and an open-label phase had begun. Active treatment in the trials involved statins, niacin, cholestyramine, or gemfibrozil.
The analysis included eight clinical trials involving 44,255 patients, of whom 8,144 died during follow-up.
The average patient remained on the assigned treatment for approximately 5 years and was on the lipid-lowering agent in the open-label phase for approximately 6 years.
During the randomized phase of the trials, the mean all-cause mortality was significantly lower for the active treatment group (odds ratio, 0.84; P = .0006), as was cardiovascular mortality (0.72; P less than .001). The lower mortality among those initially receiving active therapy persisted during the open-label follow-up phase (OR, 0.90; P = .0035), as did the reduction in cardiovascular mortality (OR, 0.82; P = .0014).
"Being treated with a beneficial medication for a longer period of time is better, possibly because we are arresting pathophysiology at an earlier stage," Dr. Kostis proposed. He added that statins may be reducing the size of infarcts in patients who have myocardial infarctions.
Dr. Patrick Moriarty, a lipid specialist and a professor of medicine at the University of Kansas in Kansas City, agreed. "We need to start lipid-lowering therapy early to get the most benefit," and this includes interventions in children when necessary, he added.
"We treat pediatric patients all the time," he said, "not only those with familial hyperlipidemias but also those with metabolic syndrome. ... The future emphasis will be, ‘the sooner the better.’ "
Dr. Kostis and Dr. Moriarty reported having no relevant conflicts of interest.
FROM THE ANNUAL MEETING OF THE AMERICAN COLLEGE OF CARDIOLOGY
Major Finding: During the open-label phase of randomized trials studied, the lower mortality among those who initially received active therapy persisted (odds ratio, 0.90; P = .0035), as did the reduction in cardiovascular mortality (OR, 0.82; P = .0014).
Data Source: A meta-analysis involving 44,255 patients in eight clinical trials of lipid-lowering therapy. All trials involved an open-label phase after the randomized treatment period ended.
Disclosures: Dr. Kostis and Dr. Moriarty reported having no relevant conflicts of interest.
ONLINE EXCLUSIVE: TKTK
Enter text here
Enter text here
Enter text here
How to Find a Mentor in the Mentorship Gap
Looking for a mentor to help guide your career development? HM presents young physicians with unique challenges and opportunities.
As a relatively young field, HM hasn't yet accrued a depth of senior leaders. Protected time is in shorter supply in academic medicine, as hospitalists bridge the Accreditation Council for Graduate Medical Education (ACGME) duty-hour coverage gap. In community hospitals, hospitalists are expressly hired to provide clinical coverage, and rewards for career advancement might not be included in the hospital's contract with the program. (For more on career advancement, listen to experts talk about why hospitalists make great hospital leaders.)

—Jeff Wiese, MD, SFHM, professor, associate dean for Graduate Medical Education, Tulane University Health Sciences Center, New Orleans
Each is a reason Steven B. Deitelzweig, MD, MMM, FACP, SFHM, vice president of medical affairs and system chairman of the Department of Hospital Medicine at Ochsner Health System in New Orleans, concludes: "If you rely on hospital medicine to teach you hospital medicine, you're only going to get so far in this day and age."
Big Community Ops
Working a week on/week off schedule might be the preferred goal for some hospitalists in the community setting, says Erik DeLue, MD, MBA, SFHM, hospitalist medical director for the Virtua healthcare system, which runs four hospitals in southern New Jersey. But if you find yourself asking, "Is there more out there for me?" community settings offer lots of opportunities to explore.
For example, one PhD physician in Dr. DeLue's group is parlaying her interest in research to the next level by submitting an abstract to the SHM annual meeting's poster competition regarding care coordination and fragmentation rates. Two other hospitalists share a clinical position (each works 0.5 FTE). On their off time, one works in information technology (IT) for Virtua, which pays the other half of his salary; the other does IT work for two hospital systems in New York City.
Click here to read the full story.
Searching for a new opportunity? View hundreds of jobs at SHM's Career Center.
Looking for a mentor to help guide your career development? HM presents young physicians with unique challenges and opportunities.
As a relatively young field, HM hasn't yet accrued a depth of senior leaders. Protected time is in shorter supply in academic medicine, as hospitalists bridge the Accreditation Council for Graduate Medical Education (ACGME) duty-hour coverage gap. In community hospitals, hospitalists are expressly hired to provide clinical coverage, and rewards for career advancement might not be included in the hospital's contract with the program. (For more on career advancement, listen to experts talk about why hospitalists make great hospital leaders.)

—Jeff Wiese, MD, SFHM, professor, associate dean for Graduate Medical Education, Tulane University Health Sciences Center, New Orleans
Each is a reason Steven B. Deitelzweig, MD, MMM, FACP, SFHM, vice president of medical affairs and system chairman of the Department of Hospital Medicine at Ochsner Health System in New Orleans, concludes: "If you rely on hospital medicine to teach you hospital medicine, you're only going to get so far in this day and age."
Big Community Ops
Working a week on/week off schedule might be the preferred goal for some hospitalists in the community setting, says Erik DeLue, MD, MBA, SFHM, hospitalist medical director for the Virtua healthcare system, which runs four hospitals in southern New Jersey. But if you find yourself asking, "Is there more out there for me?" community settings offer lots of opportunities to explore.
For example, one PhD physician in Dr. DeLue's group is parlaying her interest in research to the next level by submitting an abstract to the SHM annual meeting's poster competition regarding care coordination and fragmentation rates. Two other hospitalists share a clinical position (each works 0.5 FTE). On their off time, one works in information technology (IT) for Virtua, which pays the other half of his salary; the other does IT work for two hospital systems in New York City.
Click here to read the full story.
Searching for a new opportunity? View hundreds of jobs at SHM's Career Center.
Looking for a mentor to help guide your career development? HM presents young physicians with unique challenges and opportunities.
As a relatively young field, HM hasn't yet accrued a depth of senior leaders. Protected time is in shorter supply in academic medicine, as hospitalists bridge the Accreditation Council for Graduate Medical Education (ACGME) duty-hour coverage gap. In community hospitals, hospitalists are expressly hired to provide clinical coverage, and rewards for career advancement might not be included in the hospital's contract with the program. (For more on career advancement, listen to experts talk about why hospitalists make great hospital leaders.)

—Jeff Wiese, MD, SFHM, professor, associate dean for Graduate Medical Education, Tulane University Health Sciences Center, New Orleans
Each is a reason Steven B. Deitelzweig, MD, MMM, FACP, SFHM, vice president of medical affairs and system chairman of the Department of Hospital Medicine at Ochsner Health System in New Orleans, concludes: "If you rely on hospital medicine to teach you hospital medicine, you're only going to get so far in this day and age."
Big Community Ops
Working a week on/week off schedule might be the preferred goal for some hospitalists in the community setting, says Erik DeLue, MD, MBA, SFHM, hospitalist medical director for the Virtua healthcare system, which runs four hospitals in southern New Jersey. But if you find yourself asking, "Is there more out there for me?" community settings offer lots of opportunities to explore.
For example, one PhD physician in Dr. DeLue's group is parlaying her interest in research to the next level by submitting an abstract to the SHM annual meeting's poster competition regarding care coordination and fragmentation rates. Two other hospitalists share a clinical position (each works 0.5 FTE). On their off time, one works in information technology (IT) for Virtua, which pays the other half of his salary; the other does IT work for two hospital systems in New York City.
Click here to read the full story.
Searching for a new opportunity? View hundreds of jobs at SHM's Career Center.
Survey: Academic Hospitalists Earn $173K Annually
Academic hospitalists earn less than their nonacademic counterparts, but they appear to earn more per work RVU, according to new data from SHM and the Medical Group Management Association (MGMA).
Compensation and productivity information, dubbed the 2011 Academic Practice Compensation and Production Module, is currently available via MGMA's website, but a more detailed review of academic hospitalists will be included in the annual State of Hospital Medicine report this summer.
The recently released data show the national median salary for an academic hospitalist in internal medicine is $173,113. National median productivity for all academic faculty, standardized to 100% billable clinical activity, is 3,365 wRVUs.
By comparison, median compensation for community hospitalists is $215,000 annually, according to the State of Hospital Medicine: 2010 Report Based on 2009 Data. The 2010 report also pegged the median number of work RVUs at 4,107 per hospitalist per year.
"It doesn't surprise me salaries are lower," says Grace Huang, MD, staff hospitalist at Beth Israel Deaconess and assistant professor of medicine at Harvard Medical School, both in Boston. "I knew that choosing the life of an academic hospitalist."
In fact, Dr. Huang notes, capturing productivity for academicians is particularly tricky as activities like mentorship are difficult to quantify. She also cautions against reading too much into statistics, as "I'm a measurement person, so you can always make the data look different."
"When I look at my job as an academic hospitalist, clinical care is just part of it," adds Dr. Huang, who is among the group of hospitalists helping SHM scrub the data to be released this summer. "We have very different aspects of the job that are not easily represented in RVUs."
Academic hospitalists earn less than their nonacademic counterparts, but they appear to earn more per work RVU, according to new data from SHM and the Medical Group Management Association (MGMA).
Compensation and productivity information, dubbed the 2011 Academic Practice Compensation and Production Module, is currently available via MGMA's website, but a more detailed review of academic hospitalists will be included in the annual State of Hospital Medicine report this summer.
The recently released data show the national median salary for an academic hospitalist in internal medicine is $173,113. National median productivity for all academic faculty, standardized to 100% billable clinical activity, is 3,365 wRVUs.
By comparison, median compensation for community hospitalists is $215,000 annually, according to the State of Hospital Medicine: 2010 Report Based on 2009 Data. The 2010 report also pegged the median number of work RVUs at 4,107 per hospitalist per year.
"It doesn't surprise me salaries are lower," says Grace Huang, MD, staff hospitalist at Beth Israel Deaconess and assistant professor of medicine at Harvard Medical School, both in Boston. "I knew that choosing the life of an academic hospitalist."
In fact, Dr. Huang notes, capturing productivity for academicians is particularly tricky as activities like mentorship are difficult to quantify. She also cautions against reading too much into statistics, as "I'm a measurement person, so you can always make the data look different."
"When I look at my job as an academic hospitalist, clinical care is just part of it," adds Dr. Huang, who is among the group of hospitalists helping SHM scrub the data to be released this summer. "We have very different aspects of the job that are not easily represented in RVUs."
Academic hospitalists earn less than their nonacademic counterparts, but they appear to earn more per work RVU, according to new data from SHM and the Medical Group Management Association (MGMA).
Compensation and productivity information, dubbed the 2011 Academic Practice Compensation and Production Module, is currently available via MGMA's website, but a more detailed review of academic hospitalists will be included in the annual State of Hospital Medicine report this summer.
The recently released data show the national median salary for an academic hospitalist in internal medicine is $173,113. National median productivity for all academic faculty, standardized to 100% billable clinical activity, is 3,365 wRVUs.
By comparison, median compensation for community hospitalists is $215,000 annually, according to the State of Hospital Medicine: 2010 Report Based on 2009 Data. The 2010 report also pegged the median number of work RVUs at 4,107 per hospitalist per year.
"It doesn't surprise me salaries are lower," says Grace Huang, MD, staff hospitalist at Beth Israel Deaconess and assistant professor of medicine at Harvard Medical School, both in Boston. "I knew that choosing the life of an academic hospitalist."
In fact, Dr. Huang notes, capturing productivity for academicians is particularly tricky as activities like mentorship are difficult to quantify. She also cautions against reading too much into statistics, as "I'm a measurement person, so you can always make the data look different."
"When I look at my job as an academic hospitalist, clinical care is just part of it," adds Dr. Huang, who is among the group of hospitalists helping SHM scrub the data to be released this summer. "We have very different aspects of the job that are not easily represented in RVUs."
Set the Bar High
Newly elected board member Erin Stucky Fisher, MD, MHM, says that SHM should set its sights high when it comes to the quality of hospital care and the leadership role of hospitalists in improving the healthcare system.
A board-certified pediatrician and hospitalist at Rady Children's Hospital of San Diego, Dr. Fisher was elected via online voting in January to fill an at-large seat on SHM's 12-member board and starts her three-year term at SHM's annual meeting in Dallas in May.
"My vision of SHM is as the go-to medical society for clinical effectiveness in the hospital and for quality improvement and collaboration," Dr. Fisher says. "SHM represents systems, predominantly but not exclusively physician-based, and that's a huge strength for us. We have a different kind of opportunity because of what and who the society represents."
Key to SHM's leadership is promoting education, from workshops and conferences to supporting the Focused Practice in Hospital Medicine recertification process for hospitalists and, eventually, specific credentialing for pediatric hospitalists, she says.
"Working within this robust body, we need to leverage the great products and tools that are out there," she says. "How do we make sure we're offering more? How do we create that expectation from our members? We need to be hard on ourselves as leaders, and prove that we deserve to lead."
Dr. Fisher, a graduate of the University of California at San Francisco School of Medicine, completed her residency at UC San Diego and today serves there as professor of clinical pediatrics and director of a pediatric hospital medicine fellowship. She was awarded Masters in Hospital Medicine earlier this year and, in 2006, its recognition for outstanding service in hospital medicine.
Newly elected board member Erin Stucky Fisher, MD, MHM, says that SHM should set its sights high when it comes to the quality of hospital care and the leadership role of hospitalists in improving the healthcare system.
A board-certified pediatrician and hospitalist at Rady Children's Hospital of San Diego, Dr. Fisher was elected via online voting in January to fill an at-large seat on SHM's 12-member board and starts her three-year term at SHM's annual meeting in Dallas in May.
"My vision of SHM is as the go-to medical society for clinical effectiveness in the hospital and for quality improvement and collaboration," Dr. Fisher says. "SHM represents systems, predominantly but not exclusively physician-based, and that's a huge strength for us. We have a different kind of opportunity because of what and who the society represents."
Key to SHM's leadership is promoting education, from workshops and conferences to supporting the Focused Practice in Hospital Medicine recertification process for hospitalists and, eventually, specific credentialing for pediatric hospitalists, she says.
"Working within this robust body, we need to leverage the great products and tools that are out there," she says. "How do we make sure we're offering more? How do we create that expectation from our members? We need to be hard on ourselves as leaders, and prove that we deserve to lead."
Dr. Fisher, a graduate of the University of California at San Francisco School of Medicine, completed her residency at UC San Diego and today serves there as professor of clinical pediatrics and director of a pediatric hospital medicine fellowship. She was awarded Masters in Hospital Medicine earlier this year and, in 2006, its recognition for outstanding service in hospital medicine.
Newly elected board member Erin Stucky Fisher, MD, MHM, says that SHM should set its sights high when it comes to the quality of hospital care and the leadership role of hospitalists in improving the healthcare system.
A board-certified pediatrician and hospitalist at Rady Children's Hospital of San Diego, Dr. Fisher was elected via online voting in January to fill an at-large seat on SHM's 12-member board and starts her three-year term at SHM's annual meeting in Dallas in May.
"My vision of SHM is as the go-to medical society for clinical effectiveness in the hospital and for quality improvement and collaboration," Dr. Fisher says. "SHM represents systems, predominantly but not exclusively physician-based, and that's a huge strength for us. We have a different kind of opportunity because of what and who the society represents."
Key to SHM's leadership is promoting education, from workshops and conferences to supporting the Focused Practice in Hospital Medicine recertification process for hospitalists and, eventually, specific credentialing for pediatric hospitalists, she says.
"Working within this robust body, we need to leverage the great products and tools that are out there," she says. "How do we make sure we're offering more? How do we create that expectation from our members? We need to be hard on ourselves as leaders, and prove that we deserve to lead."
Dr. Fisher, a graduate of the University of California at San Francisco School of Medicine, completed her residency at UC San Diego and today serves there as professor of clinical pediatrics and director of a pediatric hospital medicine fellowship. She was awarded Masters in Hospital Medicine earlier this year and, in 2006, its recognition for outstanding service in hospital medicine.
AHA Targets Elevated Triglycerides; Sees Fructose Among Culprits
Triglyceride levels, which play a large role in both atherosclerotic risk and metabolic health, are highly responsive to decreases in dietary sugar intake and saturated and trans fat intake, along with increases in omega-3 acid intake and exercise, according to a scientific statement from the American Heart Association.
"What’s new is that we point out that triglycerides might be considered a marker for metabolic health," said Dr. Neil J. Stone of Northwestern University, Chicago, vice chair of the statement’s writing group, in an interview. "If you have a country where you’re seeing more obesity and more diabetes, it becomes important for people to start asking themselves ‘are there signs that I should be doing something different?’ and this is one," he said.
The scientific advisory, published online April 18 in the journal Circulation and citing some 528 sources, was not presented as a clinical guideline so much as a distillation of 30 years worth of evidence on the complex relationship among lifestyle factors, triglycerides, and cardiovascular and metabolic health (Circulation 2011 [doi:10.1161/ CIR.0b013e3182160726]).
However, the statement’s authors, led by Dr. Michael Miller, director of the Center for Preventive Cardiology at the University of Maryland, Baltimore, included a number of recommendations on diagnosing and treating hypertriglyceridemia, focusing on dietary and lifestyle changes.
The statement emphasizes the "increasingly crucial role" of triglycerides in the evaluation and management of cardiovascular disease, and the importance of diet – including consumption of sugars common in beverages – in contributing to unhealthy triglyceride levels.
Reductions of 50% or more are achievable without the use of medication – indeed medication is not a widely accepted strategy for reducing triglycerides except among people with extremely high values of greater than 500 mg/dL. "The subject of medication and triglycerides is still lacking crucial clinical trial evidence," Dr. Miller and colleagues wrote in their analysis, noting that certain medications, including hormonal treatments, can also contribute to elevated triglycerides.
About a third of American adults have elevated triglyceride levels, which are defined as fasting triglyceride of 150 mg/dL or higher. The authors recommended that optimal fasting triglyceride levels now be defined as 100 mg/dL – and that clinicians screen initially for nonfasting triglyceride, defining normal at below 200 mg/dL. People with higher nonfasting levels may then be further screened for fasting triglyceride.
The new dietary recommendations include restricting added dietary sugar to 5%-10% percent of calories consumed. In support of this, the authors cited a study of 6,113 U.S. adults showing that the lowest triglyceride levels were observed when added sugar represented less than 10% of total energy, and that higher triglyceride levels corresponded with added sugar accounting for a greater proportion of energy intake (JAMA 2010;303:1490-7).
The authors singled out fructose, a type of dietary sugar increasingly common in processed foods and soft drinks, as particularly problematic. Fructose in excess of 100 g/day, and possibly in excess of 50 g/day, has been associated with raised triglyceride levels. A typical can of cola or lemon-lime soda contains more than 20 grams of fructose, the authors noted.
Dr. Miller and his colleagues advocated weight loss of 5%-10% of body weight, which is associated with a 20% reduction in triglycerides, and regular aerobic exercise, to reduce triglyceride levels closer to optimal.
They also promoted increasing dietary fiber, keeping saturated fat below 7% of calories, eliminating trans fat from the diet, and increasing omega-3 polyunsaturated fatty acid consumption in the form of marine fish, though the authors said more research was needed to determine whether supplementing with fish-oil capsules provided equivalent benefits. Complete abstinence from alcohol was also recommended for people with very high triglycerides.
"Overall, optimization of nutrition-related practices can result in a marked triglyceride-lowering effect that ranges between 20% and 50%," they concluded.
Funding for the scientific advisory statement was provided by the American Heart Association. Dr. Miller declared no conflicts of interest affecting the drafting of the statement. However, Dr. Stone and the report’s third author, Dr. Christie Ballantyne of Baylor College of Medicine in Houston, disclosed support from pharmaceutical industry sources. Other coauthors and some reviewers disclosed additional support from pharmaceutical and agricultural firms.
Triglyceride levels, which play a large role in both atherosclerotic risk and metabolic health, are highly responsive to decreases in dietary sugar intake and saturated and trans fat intake, along with increases in omega-3 acid intake and exercise, according to a scientific statement from the American Heart Association.
"What’s new is that we point out that triglycerides might be considered a marker for metabolic health," said Dr. Neil J. Stone of Northwestern University, Chicago, vice chair of the statement’s writing group, in an interview. "If you have a country where you’re seeing more obesity and more diabetes, it becomes important for people to start asking themselves ‘are there signs that I should be doing something different?’ and this is one," he said.
The scientific advisory, published online April 18 in the journal Circulation and citing some 528 sources, was not presented as a clinical guideline so much as a distillation of 30 years worth of evidence on the complex relationship among lifestyle factors, triglycerides, and cardiovascular and metabolic health (Circulation 2011 [doi:10.1161/ CIR.0b013e3182160726]).
However, the statement’s authors, led by Dr. Michael Miller, director of the Center for Preventive Cardiology at the University of Maryland, Baltimore, included a number of recommendations on diagnosing and treating hypertriglyceridemia, focusing on dietary and lifestyle changes.
The statement emphasizes the "increasingly crucial role" of triglycerides in the evaluation and management of cardiovascular disease, and the importance of diet – including consumption of sugars common in beverages – in contributing to unhealthy triglyceride levels.
Reductions of 50% or more are achievable without the use of medication – indeed medication is not a widely accepted strategy for reducing triglycerides except among people with extremely high values of greater than 500 mg/dL. "The subject of medication and triglycerides is still lacking crucial clinical trial evidence," Dr. Miller and colleagues wrote in their analysis, noting that certain medications, including hormonal treatments, can also contribute to elevated triglycerides.
About a third of American adults have elevated triglyceride levels, which are defined as fasting triglyceride of 150 mg/dL or higher. The authors recommended that optimal fasting triglyceride levels now be defined as 100 mg/dL – and that clinicians screen initially for nonfasting triglyceride, defining normal at below 200 mg/dL. People with higher nonfasting levels may then be further screened for fasting triglyceride.
The new dietary recommendations include restricting added dietary sugar to 5%-10% percent of calories consumed. In support of this, the authors cited a study of 6,113 U.S. adults showing that the lowest triglyceride levels were observed when added sugar represented less than 10% of total energy, and that higher triglyceride levels corresponded with added sugar accounting for a greater proportion of energy intake (JAMA 2010;303:1490-7).
The authors singled out fructose, a type of dietary sugar increasingly common in processed foods and soft drinks, as particularly problematic. Fructose in excess of 100 g/day, and possibly in excess of 50 g/day, has been associated with raised triglyceride levels. A typical can of cola or lemon-lime soda contains more than 20 grams of fructose, the authors noted.
Dr. Miller and his colleagues advocated weight loss of 5%-10% of body weight, which is associated with a 20% reduction in triglycerides, and regular aerobic exercise, to reduce triglyceride levels closer to optimal.
They also promoted increasing dietary fiber, keeping saturated fat below 7% of calories, eliminating trans fat from the diet, and increasing omega-3 polyunsaturated fatty acid consumption in the form of marine fish, though the authors said more research was needed to determine whether supplementing with fish-oil capsules provided equivalent benefits. Complete abstinence from alcohol was also recommended for people with very high triglycerides.
"Overall, optimization of nutrition-related practices can result in a marked triglyceride-lowering effect that ranges between 20% and 50%," they concluded.
Funding for the scientific advisory statement was provided by the American Heart Association. Dr. Miller declared no conflicts of interest affecting the drafting of the statement. However, Dr. Stone and the report’s third author, Dr. Christie Ballantyne of Baylor College of Medicine in Houston, disclosed support from pharmaceutical industry sources. Other coauthors and some reviewers disclosed additional support from pharmaceutical and agricultural firms.
Triglyceride levels, which play a large role in both atherosclerotic risk and metabolic health, are highly responsive to decreases in dietary sugar intake and saturated and trans fat intake, along with increases in omega-3 acid intake and exercise, according to a scientific statement from the American Heart Association.
"What’s new is that we point out that triglycerides might be considered a marker for metabolic health," said Dr. Neil J. Stone of Northwestern University, Chicago, vice chair of the statement’s writing group, in an interview. "If you have a country where you’re seeing more obesity and more diabetes, it becomes important for people to start asking themselves ‘are there signs that I should be doing something different?’ and this is one," he said.
The scientific advisory, published online April 18 in the journal Circulation and citing some 528 sources, was not presented as a clinical guideline so much as a distillation of 30 years worth of evidence on the complex relationship among lifestyle factors, triglycerides, and cardiovascular and metabolic health (Circulation 2011 [doi:10.1161/ CIR.0b013e3182160726]).
However, the statement’s authors, led by Dr. Michael Miller, director of the Center for Preventive Cardiology at the University of Maryland, Baltimore, included a number of recommendations on diagnosing and treating hypertriglyceridemia, focusing on dietary and lifestyle changes.
The statement emphasizes the "increasingly crucial role" of triglycerides in the evaluation and management of cardiovascular disease, and the importance of diet – including consumption of sugars common in beverages – in contributing to unhealthy triglyceride levels.
Reductions of 50% or more are achievable without the use of medication – indeed medication is not a widely accepted strategy for reducing triglycerides except among people with extremely high values of greater than 500 mg/dL. "The subject of medication and triglycerides is still lacking crucial clinical trial evidence," Dr. Miller and colleagues wrote in their analysis, noting that certain medications, including hormonal treatments, can also contribute to elevated triglycerides.
About a third of American adults have elevated triglyceride levels, which are defined as fasting triglyceride of 150 mg/dL or higher. The authors recommended that optimal fasting triglyceride levels now be defined as 100 mg/dL – and that clinicians screen initially for nonfasting triglyceride, defining normal at below 200 mg/dL. People with higher nonfasting levels may then be further screened for fasting triglyceride.
The new dietary recommendations include restricting added dietary sugar to 5%-10% percent of calories consumed. In support of this, the authors cited a study of 6,113 U.S. adults showing that the lowest triglyceride levels were observed when added sugar represented less than 10% of total energy, and that higher triglyceride levels corresponded with added sugar accounting for a greater proportion of energy intake (JAMA 2010;303:1490-7).
The authors singled out fructose, a type of dietary sugar increasingly common in processed foods and soft drinks, as particularly problematic. Fructose in excess of 100 g/day, and possibly in excess of 50 g/day, has been associated with raised triglyceride levels. A typical can of cola or lemon-lime soda contains more than 20 grams of fructose, the authors noted.
Dr. Miller and his colleagues advocated weight loss of 5%-10% of body weight, which is associated with a 20% reduction in triglycerides, and regular aerobic exercise, to reduce triglyceride levels closer to optimal.
They also promoted increasing dietary fiber, keeping saturated fat below 7% of calories, eliminating trans fat from the diet, and increasing omega-3 polyunsaturated fatty acid consumption in the form of marine fish, though the authors said more research was needed to determine whether supplementing with fish-oil capsules provided equivalent benefits. Complete abstinence from alcohol was also recommended for people with very high triglycerides.
"Overall, optimization of nutrition-related practices can result in a marked triglyceride-lowering effect that ranges between 20% and 50%," they concluded.
Funding for the scientific advisory statement was provided by the American Heart Association. Dr. Miller declared no conflicts of interest affecting the drafting of the statement. However, Dr. Stone and the report’s third author, Dr. Christie Ballantyne of Baylor College of Medicine in Houston, disclosed support from pharmaceutical industry sources. Other coauthors and some reviewers disclosed additional support from pharmaceutical and agricultural firms.
FROM CIRCULATION
FDA explains dabigatran dose approval

Credit: Andre E.X. Brown
The medical community was surprised when the Food and Drug Administration (FDA) approved the higher dose of dabigatran and not the lower dose last October for patients with atrial fibrillation.
Now three reviewers from the FDA’s Center for Drug Evaluation and Research explain how the data gave them no other choice. Their account is published as a Perspective piece in the April 13 issue of The New England Journal of Medicine.
The FDA based its approval on the multicenter, active-control Randomized Evaluation of Long-term Anticoagulation Therapy (RE-LY) trial. It was designed to show the noninferiority of dabigatran, either 110 mg or 150 mg, compared to warfarin in reducing the risk of stroke and systemic embolism in patients with nonvalvular atrial fibrillation.
The study investigators found both dosages to be noninferior to warfarin. However, the 150-mg regimen was significantly superior to both warfarin (P=0.0001) and the 110-mg dose (P=0.004).
The FDA review team attempted to identify subsets within the RE-LY patient population that might benefit more from the lower dose. They focused on elderly patients, patients with renal function impairment, and those with previous bleeding episodes.
And in fact, the FDA reviewers said they were “unable to find any population for whom the availability of a lower dose would improve dabigatran’s benefit-risk profile.”
In the elderly, the rate of stroke or embolism was lower with the 150-mg dose, although the rate of major bleeding was higher. However, the reviewers assumed that “most people would agree...that the irreversible effects of strokes and emboli have greater clinical significance than nonfatal bleeding.”
In the subset of patients with moderate renal impairment, the rate of stroke or embolism with 150-mg was about half that of the lower-dose group and the rate of bleeding was similar. Patients with severe renal impairment were excluded from the trial.
And for those patients at higher risk for bleeding because of previous hemorrhage, they experienced similar rates of hemorrhage in both dabigatran groups and the warfarin group.
The review team commented, “[I]t appeared clear that most, if not all, patients should receive the higher dose.”
The review team pointed out that the noninferiority of the lower dose of dabigatran was less strong when it was compared with warfarin dosing that was well managed, which, they added, “is not always achieved.”
“Ultimately,” they said, the decision to approve only the higher dose “was based on our inability to identify any subgroup in which use of the lower dose would not represent a substantial disadvantage.”
The Perspective piece was written by B. Nhi Beasley, PharmD, Ellis F. Unger, MD, and Robert Temple, MD. ![]()

Credit: Andre E.X. Brown
The medical community was surprised when the Food and Drug Administration (FDA) approved the higher dose of dabigatran and not the lower dose last October for patients with atrial fibrillation.
Now three reviewers from the FDA’s Center for Drug Evaluation and Research explain how the data gave them no other choice. Their account is published as a Perspective piece in the April 13 issue of The New England Journal of Medicine.
The FDA based its approval on the multicenter, active-control Randomized Evaluation of Long-term Anticoagulation Therapy (RE-LY) trial. It was designed to show the noninferiority of dabigatran, either 110 mg or 150 mg, compared to warfarin in reducing the risk of stroke and systemic embolism in patients with nonvalvular atrial fibrillation.
The study investigators found both dosages to be noninferior to warfarin. However, the 150-mg regimen was significantly superior to both warfarin (P=0.0001) and the 110-mg dose (P=0.004).
The FDA review team attempted to identify subsets within the RE-LY patient population that might benefit more from the lower dose. They focused on elderly patients, patients with renal function impairment, and those with previous bleeding episodes.
And in fact, the FDA reviewers said they were “unable to find any population for whom the availability of a lower dose would improve dabigatran’s benefit-risk profile.”
In the elderly, the rate of stroke or embolism was lower with the 150-mg dose, although the rate of major bleeding was higher. However, the reviewers assumed that “most people would agree...that the irreversible effects of strokes and emboli have greater clinical significance than nonfatal bleeding.”
In the subset of patients with moderate renal impairment, the rate of stroke or embolism with 150-mg was about half that of the lower-dose group and the rate of bleeding was similar. Patients with severe renal impairment were excluded from the trial.
And for those patients at higher risk for bleeding because of previous hemorrhage, they experienced similar rates of hemorrhage in both dabigatran groups and the warfarin group.
The review team commented, “[I]t appeared clear that most, if not all, patients should receive the higher dose.”
The review team pointed out that the noninferiority of the lower dose of dabigatran was less strong when it was compared with warfarin dosing that was well managed, which, they added, “is not always achieved.”
“Ultimately,” they said, the decision to approve only the higher dose “was based on our inability to identify any subgroup in which use of the lower dose would not represent a substantial disadvantage.”
The Perspective piece was written by B. Nhi Beasley, PharmD, Ellis F. Unger, MD, and Robert Temple, MD. ![]()

Credit: Andre E.X. Brown
The medical community was surprised when the Food and Drug Administration (FDA) approved the higher dose of dabigatran and not the lower dose last October for patients with atrial fibrillation.
Now three reviewers from the FDA’s Center for Drug Evaluation and Research explain how the data gave them no other choice. Their account is published as a Perspective piece in the April 13 issue of The New England Journal of Medicine.
The FDA based its approval on the multicenter, active-control Randomized Evaluation of Long-term Anticoagulation Therapy (RE-LY) trial. It was designed to show the noninferiority of dabigatran, either 110 mg or 150 mg, compared to warfarin in reducing the risk of stroke and systemic embolism in patients with nonvalvular atrial fibrillation.
The study investigators found both dosages to be noninferior to warfarin. However, the 150-mg regimen was significantly superior to both warfarin (P=0.0001) and the 110-mg dose (P=0.004).
The FDA review team attempted to identify subsets within the RE-LY patient population that might benefit more from the lower dose. They focused on elderly patients, patients with renal function impairment, and those with previous bleeding episodes.
And in fact, the FDA reviewers said they were “unable to find any population for whom the availability of a lower dose would improve dabigatran’s benefit-risk profile.”
In the elderly, the rate of stroke or embolism was lower with the 150-mg dose, although the rate of major bleeding was higher. However, the reviewers assumed that “most people would agree...that the irreversible effects of strokes and emboli have greater clinical significance than nonfatal bleeding.”
In the subset of patients with moderate renal impairment, the rate of stroke or embolism with 150-mg was about half that of the lower-dose group and the rate of bleeding was similar. Patients with severe renal impairment were excluded from the trial.
And for those patients at higher risk for bleeding because of previous hemorrhage, they experienced similar rates of hemorrhage in both dabigatran groups and the warfarin group.
The review team commented, “[I]t appeared clear that most, if not all, patients should receive the higher dose.”
The review team pointed out that the noninferiority of the lower dose of dabigatran was less strong when it was compared with warfarin dosing that was well managed, which, they added, “is not always achieved.”
“Ultimately,” they said, the decision to approve only the higher dose “was based on our inability to identify any subgroup in which use of the lower dose would not represent a substantial disadvantage.”
The Perspective piece was written by B. Nhi Beasley, PharmD, Ellis F. Unger, MD, and Robert Temple, MD. ![]()
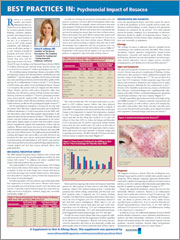
BEST PRACTICES IN: Psychosocial Impact of Rosacea
A supplement Family Practice News®. This supplement was sponsored by Galderma Laboratories, L.P.
To view the supplement, click the image above.
Topics
- NRS Digital Perception Survey
- Presentation And Diagnosis
- Treatment Strategies
Faculty/Faculty Disclosure
Debra B. Luftman, MD
Coauthor of The Beauty Prescription:
The Complete Formula for Looking and Feeling Beautiful
Calabasas, California
Dr Luftman has received funding for clinical grants from and is a consultant for Galderma Laboratories, L.P.
Copyright © 2011 Elsevier Inc.
A supplement Family Practice News®. This supplement was sponsored by Galderma Laboratories, L.P.
To view the supplement, click the image above.
Topics
- NRS Digital Perception Survey
- Presentation And Diagnosis
- Treatment Strategies
Faculty/Faculty Disclosure
Debra B. Luftman, MD
Coauthor of The Beauty Prescription:
The Complete Formula for Looking and Feeling Beautiful
Calabasas, California
Dr Luftman has received funding for clinical grants from and is a consultant for Galderma Laboratories, L.P.
Copyright © 2011 Elsevier Inc.
A supplement Family Practice News®. This supplement was sponsored by Galderma Laboratories, L.P.
To view the supplement, click the image above.
Topics
- NRS Digital Perception Survey
- Presentation And Diagnosis
- Treatment Strategies
Faculty/Faculty Disclosure
Debra B. Luftman, MD
Coauthor of The Beauty Prescription:
The Complete Formula for Looking and Feeling Beautiful
Calabasas, California
Dr Luftman has received funding for clinical grants from and is a consultant for Galderma Laboratories, L.P.
Copyright © 2011 Elsevier Inc.
Rare Lymphoma Reports Continue in Young Patients on TNF Blockers
Cases of a rare, aggressive, and usually fatal lymphoma continue to be reported in people being treated with tumor necrosis factor blockers, azathioprine, and/or mercaptopurine, the Food and Drug Administration announced in an April 14 statement.
The reports of the lymphoma, hepatosplenic T-cell lymphoma (HSTCL), have primarily involved adolescents and young adults being treated with these agents for Crohn’s disease or ulcerative colitis. One patient, however, was being treated for psoriasis, and two others for rheumatoid arthritis.
Most patients were on a combination of treatments that are known to suppress the immune system, but there have been cases in patients taking azathioprine or mercaptopurine alone, the statement said.
"The risks and benefits of using TNF blockers, azathioprine, and/or mercaptopurine should be carefully weighed when prescribing these drugs to children and young adults, especially for the treatment of Crohn’s disease and ulcerative colitis," according to the FDA.
The statement recommends that health care professionals monitor patients on these treatments for malignancies and educate patients and their caregivers about the signs and symptoms of HSTCL, which can include splenomegaly, hepatomegaly, abdominal pain, persistent fever, night sweats, and weight loss.
The statement also notes that people with rheumatoid arthritis, Crohn’s, ankylosing spondylitis, psoriatic arthritis, and plaque psoriasis "may be more likely to develop lymphoma," compared with the general U.S. population, making it difficult to estimate the increased risk of malignancies associated with TNF blockers, azathioprine and/or mercaptopurine.
As of Dec. 31, 2010, the FDA’s Adverse Event Reporting System (AERS), the medical literature, and the Cancer Survivors Network had received the following unduplicated reports of HSTCL:
20 cases in patients taking infliximab (Remicade), including 18 patients also taking mercaptopurine or azathioprine.
• 1 case in a patient taking etanercept (Enbrel).
• 2 cases in patients taking adalimumab (Humira).
• 5 cases in patients taking a combination of infliximab and adalimumab (including 4 patients also taking mercaptopurine or azathioprine).
• 12 cases in patients taking azathioprine.
• 3 cases in patients taking mercaptopurine.
No cases have been reported in the TNF blockers certolizumab pegol (Cimzia) and golimumab (Simponi).
Reports of serious adverse events associated with these and other drugs should be reported online to the FDA’s MedWatch program or by phone to 800-332-1088.
Cases of a rare, aggressive, and usually fatal lymphoma continue to be reported in people being treated with tumor necrosis factor blockers, azathioprine, and/or mercaptopurine, the Food and Drug Administration announced in an April 14 statement.
The reports of the lymphoma, hepatosplenic T-cell lymphoma (HSTCL), have primarily involved adolescents and young adults being treated with these agents for Crohn’s disease or ulcerative colitis. One patient, however, was being treated for psoriasis, and two others for rheumatoid arthritis.
Most patients were on a combination of treatments that are known to suppress the immune system, but there have been cases in patients taking azathioprine or mercaptopurine alone, the statement said.
"The risks and benefits of using TNF blockers, azathioprine, and/or mercaptopurine should be carefully weighed when prescribing these drugs to children and young adults, especially for the treatment of Crohn’s disease and ulcerative colitis," according to the FDA.
The statement recommends that health care professionals monitor patients on these treatments for malignancies and educate patients and their caregivers about the signs and symptoms of HSTCL, which can include splenomegaly, hepatomegaly, abdominal pain, persistent fever, night sweats, and weight loss.
The statement also notes that people with rheumatoid arthritis, Crohn’s, ankylosing spondylitis, psoriatic arthritis, and plaque psoriasis "may be more likely to develop lymphoma," compared with the general U.S. population, making it difficult to estimate the increased risk of malignancies associated with TNF blockers, azathioprine and/or mercaptopurine.
As of Dec. 31, 2010, the FDA’s Adverse Event Reporting System (AERS), the medical literature, and the Cancer Survivors Network had received the following unduplicated reports of HSTCL:
20 cases in patients taking infliximab (Remicade), including 18 patients also taking mercaptopurine or azathioprine.
• 1 case in a patient taking etanercept (Enbrel).
• 2 cases in patients taking adalimumab (Humira).
• 5 cases in patients taking a combination of infliximab and adalimumab (including 4 patients also taking mercaptopurine or azathioprine).
• 12 cases in patients taking azathioprine.
• 3 cases in patients taking mercaptopurine.
No cases have been reported in the TNF blockers certolizumab pegol (Cimzia) and golimumab (Simponi).
Reports of serious adverse events associated with these and other drugs should be reported online to the FDA’s MedWatch program or by phone to 800-332-1088.
Cases of a rare, aggressive, and usually fatal lymphoma continue to be reported in people being treated with tumor necrosis factor blockers, azathioprine, and/or mercaptopurine, the Food and Drug Administration announced in an April 14 statement.
The reports of the lymphoma, hepatosplenic T-cell lymphoma (HSTCL), have primarily involved adolescents and young adults being treated with these agents for Crohn’s disease or ulcerative colitis. One patient, however, was being treated for psoriasis, and two others for rheumatoid arthritis.
Most patients were on a combination of treatments that are known to suppress the immune system, but there have been cases in patients taking azathioprine or mercaptopurine alone, the statement said.
"The risks and benefits of using TNF blockers, azathioprine, and/or mercaptopurine should be carefully weighed when prescribing these drugs to children and young adults, especially for the treatment of Crohn’s disease and ulcerative colitis," according to the FDA.
The statement recommends that health care professionals monitor patients on these treatments for malignancies and educate patients and their caregivers about the signs and symptoms of HSTCL, which can include splenomegaly, hepatomegaly, abdominal pain, persistent fever, night sweats, and weight loss.
The statement also notes that people with rheumatoid arthritis, Crohn’s, ankylosing spondylitis, psoriatic arthritis, and plaque psoriasis "may be more likely to develop lymphoma," compared with the general U.S. population, making it difficult to estimate the increased risk of malignancies associated with TNF blockers, azathioprine and/or mercaptopurine.
As of Dec. 31, 2010, the FDA’s Adverse Event Reporting System (AERS), the medical literature, and the Cancer Survivors Network had received the following unduplicated reports of HSTCL:
20 cases in patients taking infliximab (Remicade), including 18 patients also taking mercaptopurine or azathioprine.
• 1 case in a patient taking etanercept (Enbrel).
• 2 cases in patients taking adalimumab (Humira).
• 5 cases in patients taking a combination of infliximab and adalimumab (including 4 patients also taking mercaptopurine or azathioprine).
• 12 cases in patients taking azathioprine.
• 3 cases in patients taking mercaptopurine.
No cases have been reported in the TNF blockers certolizumab pegol (Cimzia) and golimumab (Simponi).
Reports of serious adverse events associated with these and other drugs should be reported online to the FDA’s MedWatch program or by phone to 800-332-1088.