User login
Visceral angioedema due to angiotensin-converting enzyme inhibitor therapy
A 57-year-old black woman presented to the emergency department with severe, dull abdominal pain associated with nonbilious vomiting and nausea. She had diabetes mellitus and hypertension, for which she had been taking metformin (Glucophage) 500 mg twice a day and lisinopril (available as Prinivil and Zestril) 20 mg daily for the last 4 years.
Multiple admissions in the past 4 years
The patient started taking lisinopril 10 mg daily in 2005, and she presented to her medical provider 2 weeks later with abdominal discomfort. Colonoscopy was performed, which revealed a benign polyp. She continued taking her medications, including lisinopril.
She continued to occasionally have abdominal pain of variable severity, but it was tolerable until 6 months later, when she presented to the emergency department with severe recurrent abdominal pain.
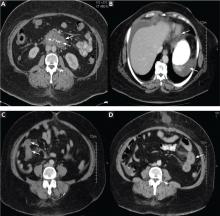
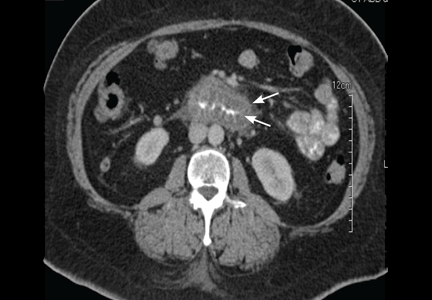
In view of the clinical picture, her physicians decided to treat her for small bowel obstruction, and an exploratory laparotomy was performed. The surgeons noted that she had moderate ascites, adhesions on the omentum, and a thickened high loop of the small bowel that was unequivocally viable and hyperemic, with thickening of the mesentery. Ascitic fluid was evacuated, adhesions were lysed, and the abdomen was closed. She was discharged with the same medications, including lisinopril; the dose was subsequently increased for better control of her hypertension.
The woman was admitted three more times within the same year for the same symptoms and underwent multiple workups for pancreatitis, gastritis, small-bowel obstruction, and other common gastrointestinal diseases.
Present admission
On review of systems, she denied any dry cough, weight loss or gain, food allergies, new medications, or hematochezia.
On physical examination, she had hypoactive bowel sounds and diffuse tenderness with guarding around the epigastric area.
Laboratory tests did not reveal any abnormalities; in particular, her C1 esterase concentration was normal. Stool studies were negative for infectious diseases.
Plain radiography of the abdomen showed a nonobstructive bowel-gas pattern.
She was diagnosed with gastrointestinal angioedema secondary to angiotensin-converting enzyme (ACE) inhibitor therapy. Her lisinopril was discontinued, and the symptoms resolved completely in 24 hours. On follow-up 8 weeks and 16 months later, her symptoms had not returned.
A RARE COMPLICATION OF ACE-INHIBITOR THERAPY
Angioedema occurs in 0.1% to 0.7% of patients taking ACE inhibitors, and it can affect about 1 of 2,500 patients during the first week of exposure.1–3 It usually manifests as swelling of the face, tongue, and lips, and in rare cases, the gastrointestinal wall. Thus, visceral angioedema is a rare complication of ACE-inhibitor therapy.
Because angioedema is less obvious when it involves abdominal organs, it presents a diagnostic challenge. It is placed lower in the differential diagnosis, as other, more common, and occasionally more high-risk medical conditions are generally considered first. Most of the time, the diagnosis is missed. Some physicians may not be aware of this problem, since only a few case reports have been published. Nevertheless, this potential complication needs to be considered when any patient receiving ACE inhibitors for treatment of hypertension, myocardial infarction, heart failure, or diabetic nephropathy presents with diffuse abdominal pain, diarrhea, or edema of the upper airways.4–8
If a high level of suspicion is applied along with good clinical judgment, then hospitalizations, unnecessary procedures, patient discomfort, and unnecessary health care costs can be prevented.
A MEDLINE SEARCH
To investigate the characteristics associated with this unusual presentation, including the time of symptom onset, the types of symptoms, and the diagnostic studies performed on the patients with visceral angioedema, we performed a MEDLINE search to identify case reports and case series published in English from 1980 to 2010 on the topic of abdominal or visceral angioedema. The search terms used were “visceral,” “intestinal angioedema,” “ACE-inhibitor side effects,” and the names of various ACE inhibitors.
Pertinent articles were identified, and clinical characteristics were collected, including demographics, onset of symptoms, the drug’s name, and others. In our summary below, data are presented as the mean and standard deviation for continuous variables and percentages for categorical variables.
SUMMARY OF REPORTED CASES
Our search revealed 27 reported cases of visceral angioedema associated with ACE inhibitors (a table summarizing our findings is available).9–34 The drug most often involved was lisinopril (11 cases), followed by enalapril (Vasotec) (8 cases).
Twenty-three (82%) of the cases were in women. The mean age of the patients was 49.5 ± 12.2 years (range 29–77 years); the mean age was 46.7 ± 11.7 years in women and 57 ± 13 years in men. Unfortunately, the race and ethnicity of the patients was documented in only some cases.
In 15 (54%) of the cases, the patient presented to a physician or emergency department within 72 hours (41.1 ± 17.4) of starting therapy, and in 8 cases the patient presented between 2 weeks and 18 months.
In 10 cases (including the case we are reporting here), the patients were kept on ACE inhibitors from 2 to 9 years after the initial presentation, as the diagnosis was missed.9,12,14,18,20,31,32 In 2 cases, the dose of the ACE inhibitor had been increased after the patient presented with the abdominal pain.
All of the patients were hospitalized for further diagnostic workup.
As for the presenting symptoms, all the patients had abdominal pain, 24 (86%) had emesis, 14 (50%) had diarrhea, and 20 (71%) had ascites. Laboratory results were mostly nonspecific. Twelve (44%) of the patients had leukocytosis. The C1 esterase inhibitor concentration was measured in 18 patients, and the results were normal in all of them.
Twenty-four (86%) of the patients underwent abdominal and pelvic CT or ultrasonography as part of the initial diagnostic evaluation, and intestinal wall-thickening was found in 21 (87.5%) of them.
Either surgery or gastrointestinal biopsy was performed in 16 (57%) of the patients; the surgical procedures included 2 cholecystectomies and 1 bone marrow biopsy. Only 1 case was diagnosed on the basis of clinical suspicion and abdominal radiographs alone.
The combination of intestinal and stomach angioedema was found in only 2 cases.
Two patients were kept on an ACE inhibitor in spite of symptoms and intestinal wall edema that showed a migratory pattern on imaging after chronic exposure.
The thickening involved the jejunum in 14 patients (50%), the ileum in 8 (29%), the duodenum in 5 (18%), the stomach in 2, and the sigmoid colon in 1.
In 12 cases (43%), visceral angioedema and its symptoms resolved within 48 hours of stopping the ACE inhibitor.
A DIAGNOSIS TO KEEP IN MIND
As we have seen, the diagnosis of visceral angioedema needs to be kept in mind when a patient—especially a middle-aged woman—taking an ACE inhibitor presents with abdominal pain, vomiting, diarrhea, leukocytosis, ascites, and wall-thickening of the small bowel on imaging studies.9,35,36
The diagnosis is hard to establish, and in the interim the patient may undergo invasive and unnecessary procedures, which can be avoided by a heightened awareness of this complication. In all of the reported cases, the patients required hospitalization because of the severity of symptoms and attempts to exclude other possible diseases.36
POSSIBLY DUE TO BRADYKININ
Several theories have been proposed to explain how visceral angioedema is induced by ACE inhibitors. The possible mechanisms that have been described include the following:
- The accumulation of bradykinin and substance P secondary to the effect of the ACE inhibitor, which may lead to the inflammatory response, therefore increasing permeability of the vascular compartment
- Deficiency of complement and the enzymes carboxypeptidase N and alpha-1 antitrypsin
- An antibody-antigen reaction37
- Hormones such as estrogen and progesterone (suggested by the greater number of women represented38)
- Contrast media used for imaging39
- Genetic predisposition
- Inflammation due to acute-phase proteins
- C1-inhibitor deficiency or dysfunction (however, the levels of C1/C4 and the C1-esterase inhibitor functional activity usually are normal2,10,40).
Many other theories are being explored.11,12,38,41–53
The most plausible mechanism is an increase in the levels of bradykinin and its metabolites.45 The absence of ACE can lead to breakdown of bradykinin to des-Arg bradykinin via the minor pathway, which can lead to more pronounced vasodilation and vascular permeability.54,55 During an acute attack of angioedema secondary to ACE inhibition, the bradykinin concentration can increase to more than 10 times the normal level.56
Moreover, C-reactive protein levels were higher (mean 4.42 mg/dL ± 0.15 mg/dL) in patients with ACE-inhibitor-induced angioedema than in those with other causes of angioedema (P < .0001).52 The patients taking ACE inhibitors without any previous angioedema had normal C-reactive protein levels (0.39 mg/dL ± 0.1 mg/dL).52
INCIDENCE RATES
In our review of the literature, all of the patients were taking an ACE inhibitor, and some were taking both an ACE inhibitor and an angiotensin-receptor blocker (ARB).
Initially, the incidence rate of angioedema was thought to be 0.1% to 0.2%, but recently the Omapatrilat Cardiovascular Treatment Assessment vs Enalapril (OCTAVE) trial had more than 12,000 patients on enalapril and reported the incidence of angioedema to be 0.68%,57 with a higher risk in women than in men (0.84% vs 0.54%)58 and a relative risk of 3.03 for blacks compared with whites.59
Even though ARBs seem to be safer, angioedema can recur in up to one-third of patients who switch from an ACE inhibitor to an ARB.60–63
Moreover, one study in the United States found that the frequency of hospital admission of patients with angioedema increased from 8,839 per year in 1998 to 11,925 in 2005, and the cost was estimated to be close to $123 million in 2005.64
Interestingly, when angioedema involved the face, it developed within the first week in 60% of cases,65 whereas when visceral angioedema developed, it did so within the first week in 59% of cases. Therefore, the timing of the onset is similar regardless of the body area involved.
Smokers who developed ACE-inhibitor-induced cough had a higher risk of ACE-inhibitor-induced angioedema in a retrospective cohort study by Morimoto,66 but no relationship to the area of involvement was made.
ON IMAGING, A THICKENED BOWEL WALL
Computed tomography can reveal bowel edema and ascites more reliably than plain radiography or barium studies. Edema thickens the bowel wall, with increased contrast enhancement that makes mesenteric vessels show up on the study. In some instances edema is so significant that edematous submucosa can be differentiated from the serosa due to impressive thickening of the mucosal wall.15,16 Oral contrast can be seen in the middle of the lumen, giving it a target-sign appearance. Edema of the small bowel and ascites can lead to fluid sequestration in the abdomen, resulting in a presentation with shock.67
Magnetic resonance imaging can be even more useful in identifying gastrointestinal angioedema, but it would not be cost-effective, and based on our study, CT and ultrasonography of the abdomen were diagnostic in most cases.
AVOIDING UNNECESSARY TESTING
Hemodynamic instability and abdominal pain usually trigger a surgical consult and a more extensive workup, but with a good clinical approach, unnecessary testing and invasive diagnostic procedures can be avoided under the right circumstances.
Numerous surgical procedures have been reported in patients presenting with visceral angioedema secondary to ACE inhibitors.67 Although a thorough history and physical examination can give us a clue in the diagnosis of drug-induced gastrointestinal angioedema, CT is extremely helpful, as it shows dilated loops, thickened mucosal folds, perihepatic fluid, ascites, mesenteric edema, and a “doughnut” or “stacked coin” appearance.17,68
So far, there have been only two reports of angioedema of the stomach (the case reported by Shahzad et al10 and the current report). Angioedema can affect any visceral organ, but we usually see involvement of the jejunum followed by the ileum and duodenum.40
FINDINGS ON ENDOSCOPY
Usually, endoscopic examination of the upper and lower gastrointestinal tract does not reveal any specific pathology, but endoscopy and biopsy can rule out other causes of abdominal pain, such as Crohn disease, ulcerative colitis, infection, malignancy, granuloma, and vasculitis. Also, hereditary or acquired C1-esterase deficiency and other autoimmune disorders should be considered in the workup.18,69 In the reported cases, endoscopy revealed petechial bleeding with generalized edema.19
Biopsy often demonstrates an expanded edematous submucosal layer with inflammatory cell infiltration and protrusion of the proper muscular layer into the submucosal layer.15 A proper muscular layer and an edematous submucosal layer can produce edema so severe as to obstruct the intestine.15
Ultrasonography or CT provides essential information as to location, structure, and size, and it rules out other diagnoses. Therefore, consideration should be given to noninvasive imaging studies and laboratory testing (C1-esterase inhibitor, complement, antinuclear antibody, complete metabolic panel, complete blood cell count) before resorting to endoscopy or exploratory laparotomy.20,70 In three case reports,29,30,32 abdominal ultrasonography did not show any thickening of the small-bowel wall. Several cases have been diagnosed with the help of endoscopy.
Symptoms usually resolve when the ACE inhibitor is stopped
There is no standard treatment for ACE-inhibitor-induced visceral angioedema. In most patients, stopping the drug, giving nothing by mouth, and giving intravenous fluids to prevent dehydration are sufficient. Symptoms usually resolve within 48 hours.
In several case reports, fresh-frozen plasma was used to increase the levels of kininase II, which can degrade high levels of bradykinin.51,71,72 However, no randomized controlled trial of fresh-frozen plasma for ACE-inhibitor-induced angioedema has been published.
Drugs for hereditary angioedema—eg, recombinant C1-INH, the kallikrein inhibitor ecallantide (Kalbitor), and the BKR-2-antagonist icatibant (Firazyr)73—have not been prospectively studied in gastrointestinal angioedema associated with ACE inhibitors. Icatibant has been shown to be effective in the treatment of hereditary angioedema and could be promising in treating angioedema secondary to ACE inhibitors.8 Rosenberg et al21 described a patient who was on prednisone when she developed intestinal angioedema, thus calling into question the efficacy of steroids in the treatment of visceral angioedema.
RAISING AWARENESS
More than 40 million patients are currently taking ACE inhibitors or ARBs.9 Therefore, we suggest that patients with a known history of angioedema in response to these drugs should wear an identification bracelet to increase awareness and to prevent recurrence of angioedema.
- Brown NJ, Snowden M, Griffin MR. Recurrent angiotensin-converting enzyme inhibitor–associated angioedema. JAMA 1997; 278:232–233.
- Israili ZH, Hall WD. Cough and angioneurotic edema associated with angiotensin-converting enzyme inhibitor therapy. A review of the literature and pathophysiology. Ann Intern Med 1992; 117:234–242.
- Messerli FH, Nussberger J. Vasopeptidase inhibition and angiooedema. Lancet 2000; 356:608–609.
- Jessup M, Brozena S. Heart failure. N Engl J Med 2003; 348:2007–2018.
- Jessup M. The less familiar face of heart failure. J Am Coll Cardiol 2003; 41:224–226.
- Chobanian AV. Clinical practice. Isolated systolic hypertension in the elderly. N Engl J Med 2007; 357:789–796.
- Casas JP, Chua W, Loukogeorgakis S, et al. Effect of inhibitors of the renin-angiotensin system and other antihypertensive drugs on renal outcomes: systematic review and meta-analysis. Lancet 2005; 366:2026–2033.
- Weber MA, Messerli FH. Angiotensin-converting enzyme inhibitors and angioedema: estimating the risk. Hypertension 2008; 51:1465–1467.
- Oudit G, Girgrah N, Allard J. ACE inhibitor-induced angioedema of the intestine: Case report, incidence, pathophysiology, diagnosis and management. Can J Gastroenterol 2001; 15:827–832.
- Shahzad G, Korsten MA, Blatt C, Motwani P. Angiotensin-converting enzyme (ACE) inhibitor-associated angioedema of the stomach and small intestine: a case report. Mt Sinai J Med 2006; 73:1123–1125.
- Chase MP, Fiarman GS, Scholz FJ, MacDermott RP. Angioedema of the small bowel due to an angiotensin-converting enzyme inhibitor. J Clin Gastroenterol 2000; 31:254–257.
- Mullins RJ, Shanahan TM, Dobson RT. Visceral angioedema related to treatment with an ACE inhibitor. Med J Aust 1996; 165:319–321.
- Schmidt TD, McGrath KM. Angiotensin-converting enzyme inhibitor angioedema of the intestine: a case report and review of the literature. Am J Med Sci 2002; 324:106–108.
- Smoger SH, Sayed MA. Simultaneous mucosal and small bowel angioedema due to captopril. South Med J 1998; 91:1060–1063.
- Tojo A, Onozato ML, Fujita T. Repeated subileus due to angioedema during renin-angiotensin system blockade. Am J Med Sci 2006; 332:36–38.
- De Backer AI, De Schepper AM, Vandevenne JE, Schoeters P, Michielsen P, Stevens WJ. CT of angioedema of the small bowel. AJR Am J Roentgenol 2001; 176:649–652.
- Marmery H, Mirvis SE. Angiotensin-converting enzyme inhibitor-induced visceral angioedema. Clin Radiol 2006; 61:979–982.
- Orr KK, Myers JR. Intermittent visceral edema induced by long-term enalapril administration. Ann Pharmacother 2004; 38:825–827.
- Spahn TW, Grosse-Thie W, Mueller MK. Endoscopic visualization of angiotensin-converting enzyme inhibitor-induced small bowel angioedema as a cause of relapsing abdominal pain using double-balloon enteroscopy. Dig Dis Sci 2008; 53:1257–1260.
- Byrne TJ, Douglas DD, Landis ME, Heppell JP. Isolated visceral angioedema: an underdiagnosed complication of ACE inhibitors? Mayo Clin Proc 2000; 75:1201–1204.
- Rosenberg EI, Mishra G, Abdelmalek MF. Angiotensin-converting enzyme inhibitor-induced isolated visceral angioedema in a liver transplant recipient. Transplantation 2003; 75:730–732.
- Salloum H, Locher C, Chenard A, et al. [Small bowel angioedema due to perindopril]. Gastroenterol Clin Biol 2005; 29:1180–1181.
- Arakawa M, Murata Y, Rikimaru Y, Sasaki Y. Drug-induced isolated visceral angioneurotic edema. Intern Med 2005; 44:975–978.
- Abdelmalek MF, Douglas DD. Lisinopril-induced isolated visceral angioedema: review of ACE-inhibitor-induced small bowel angioedema. Dig Dis Sci 1997; 42:847–850.
- Gregory KW, Davis RC. Images in clinical medicine. Angioedema of the intestine. N Engl J Med 1996; 334:1641.
- Farraye FA, Peppercorn MA, Steer ML, Joffe N, Rees M. Acute small-bowel mucosal edema following enalapril use. JAMA 1988; 259:3131.
- Jacobs RL, Hoberman LJ, Goldstein HM. Angioedema of the small bowel caused by an angiotensin-converting enzyme inhibitor. Am J Gastroenterol 1994; 89:127–128.
- Herman L, Jocums SB, Coleman MD. A 29-year-old woman with crampy abdominal pain. Tenn Med 1999; 92:272–273.
- Guy C, Cathébras P, Rousset H. Suspected angioedema of abdominal viscera. Ann Intern Med 1994; 121:900.
- Dupasquier E. [A rare clinical form of angioneurotic edema caused by enalapril: acute abdomen]. Arch Mal Coeur Vaiss 1994; 87:1371–1374.
- Jardine DL, Anderson JC, McClintock AD. Delayed diagnosis of recurrent visceral angio-oedema secondary to ACE inhibitor therapy. Aust N Z J Med 1999; 29:377–378.
- Matsumura M, Haruki K, Kajinami K, Takada T. Angioedema likely related to angiotensin converting enzyme inhibitors. Intern Med 1993; 32:424–426.
- Khan MU, Baig MA, Javed RA, et al. Benazepril induced isolated visceral angioedema: a rare and under diagnosed adverse effect of angiotensin converting enzyme inhibitors. Int J Cardiol 2007; 118:e68–e69.
- Adhikari SP, Schneider JI. An unusual cause of abdominal pain and hypotension: angioedema of the bowel. J Emerg Med 2009; 36:23–25.
- Gibbs CR, Lip GY, Beevers DG. Angioedema due to ACE inhibitors: increased risk in patients of African origin. Br J Clin Pharmacol 1999; 48:861–865.
- Johnsen SP, Jacobsen J, Monster TB, Friis S, McLaughlin JK, Sørensen HT. Risk of first-time hospitalization for angioedema among users of ACE inhibitors and angiotensin receptor antagonists. Am J Med 2005; 118:1428–1329.
- Bi CK, Soltani K, Sloan JB, Weber RR, Elliott WJ, Murphy MB. Tissue-specific autoantibodies induced by captopril. Clin Res 1987; 35:922A.
- Bork K, Dewald G. Hereditary angioedema type III, angioedema associated with angiotensin II receptor antagonists, and female sex. Am J Med 2004; 116:644–645.
- Witten DM, Hirsch FD, Hartman GW. Acute reactions to urographic contrast medium: incidence, clinical characteristics and relationship to history of hypersensitivity states. Am J Roentgenol Radium Ther Nucl Med 1973; 119:832–840.
- Eck SL, Morse JH, Janssen DA, Emerson SG, Markovitz DM. Angioedema presenting as chronic gastrointestinal symptoms. Am J Gastroenterol 1993; 88:436–439.
- Coleman JW, Yeung JH, Roberts DH, Breckenridge AM, Park BK. Drug-specific antibodies in patients receiving captopril. Br J Clin Pharmacol 1986; 22:161–165.
- Kallenberg CG. Autoantibodies during captopril treatment. Arthritis Rheum 1985; 28:597–598.
- Inman WH, Rawson NS, Wilton LV, Pearce GL, Speirs CJ. Postmarketing surveillance of enalapril. I: Results of prescription-event monitoring. BMJ 1988; 297:826–829.
- Lefebvre J, Murphey LJ, Hartert TV, Jiao Shan R, Simmons WH, Brown NJ. Dipeptidyl peptidase IV activity in patients with ACE-inhibitor-associated angioedema. Hypertension 2002; 39:460–464.
- Molinaro G, Cugno M, Perez M, et al. Angiotensin-converting enzyme inhibitor-associated angioedema is characterized by a slower degradation of des-arginine(9)-bradykinin. J Pharmacol Exp Ther 2002; 303:232–237.
- Adam A, Cugno M, Molinaro G, Perez M, Lepage Y, Agostoni A. Aminopeptidase P in individuals with a history of angiooedema on ACE inhibitors. Lancet 2002; 359:2088–2089.
- Binkley KE, Davis A. Clinical, biochemical, and genetic characterization of a novel estrogen-dependent inherited form of angioedema. J Allergy Clin Immunol 2000; 106:546–550.
- Yeung JH, Coleman JW, Park BK. Drug-protein conjugates—IX. Immunogenicity of captopril-protein conjugates. Biochem Pharmacol 1985; 34:4005–4012.
- Abbosh J, Anderson JA, Levine AB, Kupin WL. Angiotensin converting enzyme inhibitor-induced angioedema more prevalent in transplant patients. Ann Allergy Asthma Immunol 1999; 82:473–476.
- Pichler WJ, Lehner R, Späth PJ. Recurrent angioedema associated with hypogonadism or anti-androgen therapy. Ann Allergy 1989; 63:301–305.
- Bass G, Honan D. Octaplas is not equivalent to fresh frozen plasma in the treatment of acute angioedema. Eur J Anaesthesiol 2007; 24:1062–1063.
- Bas M, Hoffmann TK, Bier H, Kojda G. Increased C-reactive protein in ACE-inhibitor-induced angioedema. Br J Clin Pharmacol 2005; 59:233–238.
- Herman AG. Differences in structure of angiotensin-converting enzyme inhibitors might predict differences in action. Am J Cardiol 1992; 70:102C–108C.
- Cunnion KM, Lee JC, Frank MM. Capsule production and growth phase influence binding of complement to Staphylococcus aureus. Infect Immunol 2001; 69:6796–6803.
- Cunnion KM, Wagner E, Frank MM. Complement and kinins. In:Parlow TG, Stites DP, Imboden JB, editors. Medical Immunology. 10th ed. New York, NY: Lange Medical Books; 2001:186–188.
- Pellacani A, Brunner HR, Nussberger J. Plasma kinins increase after angiotensin-converting enzyme inhibition in human subjects. Clin Sci (Lond) 1994; 87:567–574.
- Bristol-Myers Squibb Pharmaceutical Research Institute. FDA Advisory Committee Briefing Book for OMAPATRILAT Tablets NDA 21-188. www.fda.gov/ohrms/dockets/ac/02/briefing/3877B2_01_BristolMeyersSquibb.pdf. Accessed 2/4/2011.
- Kostis JB, Kim HJ, Rusnak J, et al. Incidence and characteristics of angioedema associated with enalapril. Arch Intern Med 2005; 165:1637–1642.
- Mahoney EJ, Devaiah AK. Angioedema and angiotensin-converting enzyme inhibitors: are demographics a risk? Otolaryngol Head Neck Surg 2008; 139:105–108.
- Warner KK, Visconti JA, Tschampel MM. Angiotensin II receptor blockers in patients with ACE inhibitor-induced angioedema. Ann Pharmacother 2000; 34:526–528.
- Kyrmizakis DE, Papadakis CE, Liolios AD, et al. Angiotensin-converting enzyme inhibitors and angiotensin II receptor antagonists. Arch Otolaryngol Head Neck Surg 2004; 130:1416–1419.
- MacLean JA, Hannaway PJ. Angioedema and AT1 receptor blockers: proceed with caution. Arch Intern Med 2003; 163:1488–1489,
- Abdi R, Dong VM, Lee CJ, Ntoso KA. Angiotensin II receptor blocker-associated angioedema: on the heels of ACE inhibitor angioedema. Pharmacotherapy 2002; 22:1173–1175.
- Lin RY, Shah SN. Increasing hospitalizations due to angioedema in the United States. Ann Allergy Asthma Immunol 2008; 101:185–192.
- Slater EE, Merrill DD, Guess HA, et al. Clinical profile of angioedema associated with angiotensin converting-enzyme inhibition. JAMA 1988; 260:967–970.
- Morimoto T, Gandhi TK, Fiskio JM, et al. An evaluation of risk factors for adverse drug events associated with angiotensin-converting enzyme inhibitors. J Eval Clin Pract 2004; 10:499–509.
- Cohen N, Sharon A, Golik A, Zaidenstein R, Modai D. Hereditary angioneurotic edema with severe hypovolemic shock. J Clin Gastroenterol 1993; 16:237–239.
- Ciaccia D, Brazer SR, Baker ME. Acquired C1 esterase inhibitor deficiency causing intestinal angioedema: CT appearance. AJR Am J Roentgenol 1993; 161:1215–1216.
- Malcolm A, Prather CM. Intestinal angioedema mimicking Crohn’s disease. Med J Aust 1999; 171:418–420.
- Schmidt TD, McGrath KM. Angiotensin-converting enzyme inhibitor angioedema of the intestine: a case report and review of the literature. Am J Med Sci 2002; 324:106–108.
- Karim MY, Masood A. Fresh-frozen plasma as a treatment for life-threatening ACE-inhibitor angioedema. J Allergy Clin Immunol 2002; 109:370–371.
- Warrier MR, Copilevitz CA, Dykewicz MS, Slavin RG. Fresh frozen plasma in the treatment of resistant angiotensin-converting enzyme inhibitor angioedema. Ann Allergy Asthma Immunol 2004; 92:573–575.
- Bas M, Adams V, Suvorava T, Niehues T, Hoffmann TK, Kojda G. Nonallergic angioedema: role of bradykinin. Allergy 2007; 62:842–856.
- Agostoni A, Cicardi M, Cugno M, Zingale LC, Gioffré D, Nussberger J. Angioedema due to angiotensin-converting enzyme inhibitors. Immunopharmacology 1999; 44:21–25.
A 57-year-old black woman presented to the emergency department with severe, dull abdominal pain associated with nonbilious vomiting and nausea. She had diabetes mellitus and hypertension, for which she had been taking metformin (Glucophage) 500 mg twice a day and lisinopril (available as Prinivil and Zestril) 20 mg daily for the last 4 years.
Multiple admissions in the past 4 years
The patient started taking lisinopril 10 mg daily in 2005, and she presented to her medical provider 2 weeks later with abdominal discomfort. Colonoscopy was performed, which revealed a benign polyp. She continued taking her medications, including lisinopril.
She continued to occasionally have abdominal pain of variable severity, but it was tolerable until 6 months later, when she presented to the emergency department with severe recurrent abdominal pain.
In view of the clinical picture, her physicians decided to treat her for small bowel obstruction, and an exploratory laparotomy was performed. The surgeons noted that she had moderate ascites, adhesions on the omentum, and a thickened high loop of the small bowel that was unequivocally viable and hyperemic, with thickening of the mesentery. Ascitic fluid was evacuated, adhesions were lysed, and the abdomen was closed. She was discharged with the same medications, including lisinopril; the dose was subsequently increased for better control of her hypertension.
The woman was admitted three more times within the same year for the same symptoms and underwent multiple workups for pancreatitis, gastritis, small-bowel obstruction, and other common gastrointestinal diseases.
Present admission
On review of systems, she denied any dry cough, weight loss or gain, food allergies, new medications, or hematochezia.
On physical examination, she had hypoactive bowel sounds and diffuse tenderness with guarding around the epigastric area.
Laboratory tests did not reveal any abnormalities; in particular, her C1 esterase concentration was normal. Stool studies were negative for infectious diseases.
Plain radiography of the abdomen showed a nonobstructive bowel-gas pattern.
She was diagnosed with gastrointestinal angioedema secondary to angiotensin-converting enzyme (ACE) inhibitor therapy. Her lisinopril was discontinued, and the symptoms resolved completely in 24 hours. On follow-up 8 weeks and 16 months later, her symptoms had not returned.
A RARE COMPLICATION OF ACE-INHIBITOR THERAPY
Angioedema occurs in 0.1% to 0.7% of patients taking ACE inhibitors, and it can affect about 1 of 2,500 patients during the first week of exposure.1–3 It usually manifests as swelling of the face, tongue, and lips, and in rare cases, the gastrointestinal wall. Thus, visceral angioedema is a rare complication of ACE-inhibitor therapy.
Because angioedema is less obvious when it involves abdominal organs, it presents a diagnostic challenge. It is placed lower in the differential diagnosis, as other, more common, and occasionally more high-risk medical conditions are generally considered first. Most of the time, the diagnosis is missed. Some physicians may not be aware of this problem, since only a few case reports have been published. Nevertheless, this potential complication needs to be considered when any patient receiving ACE inhibitors for treatment of hypertension, myocardial infarction, heart failure, or diabetic nephropathy presents with diffuse abdominal pain, diarrhea, or edema of the upper airways.4–8
If a high level of suspicion is applied along with good clinical judgment, then hospitalizations, unnecessary procedures, patient discomfort, and unnecessary health care costs can be prevented.
A MEDLINE SEARCH
To investigate the characteristics associated with this unusual presentation, including the time of symptom onset, the types of symptoms, and the diagnostic studies performed on the patients with visceral angioedema, we performed a MEDLINE search to identify case reports and case series published in English from 1980 to 2010 on the topic of abdominal or visceral angioedema. The search terms used were “visceral,” “intestinal angioedema,” “ACE-inhibitor side effects,” and the names of various ACE inhibitors.
Pertinent articles were identified, and clinical characteristics were collected, including demographics, onset of symptoms, the drug’s name, and others. In our summary below, data are presented as the mean and standard deviation for continuous variables and percentages for categorical variables.
SUMMARY OF REPORTED CASES
Our search revealed 27 reported cases of visceral angioedema associated with ACE inhibitors (a table summarizing our findings is available).9–34 The drug most often involved was lisinopril (11 cases), followed by enalapril (Vasotec) (8 cases).
Twenty-three (82%) of the cases were in women. The mean age of the patients was 49.5 ± 12.2 years (range 29–77 years); the mean age was 46.7 ± 11.7 years in women and 57 ± 13 years in men. Unfortunately, the race and ethnicity of the patients was documented in only some cases.
In 15 (54%) of the cases, the patient presented to a physician or emergency department within 72 hours (41.1 ± 17.4) of starting therapy, and in 8 cases the patient presented between 2 weeks and 18 months.
In 10 cases (including the case we are reporting here), the patients were kept on ACE inhibitors from 2 to 9 years after the initial presentation, as the diagnosis was missed.9,12,14,18,20,31,32 In 2 cases, the dose of the ACE inhibitor had been increased after the patient presented with the abdominal pain.
All of the patients were hospitalized for further diagnostic workup.
As for the presenting symptoms, all the patients had abdominal pain, 24 (86%) had emesis, 14 (50%) had diarrhea, and 20 (71%) had ascites. Laboratory results were mostly nonspecific. Twelve (44%) of the patients had leukocytosis. The C1 esterase inhibitor concentration was measured in 18 patients, and the results were normal in all of them.
Twenty-four (86%) of the patients underwent abdominal and pelvic CT or ultrasonography as part of the initial diagnostic evaluation, and intestinal wall-thickening was found in 21 (87.5%) of them.
Either surgery or gastrointestinal biopsy was performed in 16 (57%) of the patients; the surgical procedures included 2 cholecystectomies and 1 bone marrow biopsy. Only 1 case was diagnosed on the basis of clinical suspicion and abdominal radiographs alone.
The combination of intestinal and stomach angioedema was found in only 2 cases.
Two patients were kept on an ACE inhibitor in spite of symptoms and intestinal wall edema that showed a migratory pattern on imaging after chronic exposure.
The thickening involved the jejunum in 14 patients (50%), the ileum in 8 (29%), the duodenum in 5 (18%), the stomach in 2, and the sigmoid colon in 1.
In 12 cases (43%), visceral angioedema and its symptoms resolved within 48 hours of stopping the ACE inhibitor.
A DIAGNOSIS TO KEEP IN MIND
As we have seen, the diagnosis of visceral angioedema needs to be kept in mind when a patient—especially a middle-aged woman—taking an ACE inhibitor presents with abdominal pain, vomiting, diarrhea, leukocytosis, ascites, and wall-thickening of the small bowel on imaging studies.9,35,36
The diagnosis is hard to establish, and in the interim the patient may undergo invasive and unnecessary procedures, which can be avoided by a heightened awareness of this complication. In all of the reported cases, the patients required hospitalization because of the severity of symptoms and attempts to exclude other possible diseases.36
POSSIBLY DUE TO BRADYKININ
Several theories have been proposed to explain how visceral angioedema is induced by ACE inhibitors. The possible mechanisms that have been described include the following:
- The accumulation of bradykinin and substance P secondary to the effect of the ACE inhibitor, which may lead to the inflammatory response, therefore increasing permeability of the vascular compartment
- Deficiency of complement and the enzymes carboxypeptidase N and alpha-1 antitrypsin
- An antibody-antigen reaction37
- Hormones such as estrogen and progesterone (suggested by the greater number of women represented38)
- Contrast media used for imaging39
- Genetic predisposition
- Inflammation due to acute-phase proteins
- C1-inhibitor deficiency or dysfunction (however, the levels of C1/C4 and the C1-esterase inhibitor functional activity usually are normal2,10,40).
Many other theories are being explored.11,12,38,41–53
The most plausible mechanism is an increase in the levels of bradykinin and its metabolites.45 The absence of ACE can lead to breakdown of bradykinin to des-Arg bradykinin via the minor pathway, which can lead to more pronounced vasodilation and vascular permeability.54,55 During an acute attack of angioedema secondary to ACE inhibition, the bradykinin concentration can increase to more than 10 times the normal level.56
Moreover, C-reactive protein levels were higher (mean 4.42 mg/dL ± 0.15 mg/dL) in patients with ACE-inhibitor-induced angioedema than in those with other causes of angioedema (P < .0001).52 The patients taking ACE inhibitors without any previous angioedema had normal C-reactive protein levels (0.39 mg/dL ± 0.1 mg/dL).52
INCIDENCE RATES
In our review of the literature, all of the patients were taking an ACE inhibitor, and some were taking both an ACE inhibitor and an angiotensin-receptor blocker (ARB).
Initially, the incidence rate of angioedema was thought to be 0.1% to 0.2%, but recently the Omapatrilat Cardiovascular Treatment Assessment vs Enalapril (OCTAVE) trial had more than 12,000 patients on enalapril and reported the incidence of angioedema to be 0.68%,57 with a higher risk in women than in men (0.84% vs 0.54%)58 and a relative risk of 3.03 for blacks compared with whites.59
Even though ARBs seem to be safer, angioedema can recur in up to one-third of patients who switch from an ACE inhibitor to an ARB.60–63
Moreover, one study in the United States found that the frequency of hospital admission of patients with angioedema increased from 8,839 per year in 1998 to 11,925 in 2005, and the cost was estimated to be close to $123 million in 2005.64
Interestingly, when angioedema involved the face, it developed within the first week in 60% of cases,65 whereas when visceral angioedema developed, it did so within the first week in 59% of cases. Therefore, the timing of the onset is similar regardless of the body area involved.
Smokers who developed ACE-inhibitor-induced cough had a higher risk of ACE-inhibitor-induced angioedema in a retrospective cohort study by Morimoto,66 but no relationship to the area of involvement was made.
ON IMAGING, A THICKENED BOWEL WALL
Computed tomography can reveal bowel edema and ascites more reliably than plain radiography or barium studies. Edema thickens the bowel wall, with increased contrast enhancement that makes mesenteric vessels show up on the study. In some instances edema is so significant that edematous submucosa can be differentiated from the serosa due to impressive thickening of the mucosal wall.15,16 Oral contrast can be seen in the middle of the lumen, giving it a target-sign appearance. Edema of the small bowel and ascites can lead to fluid sequestration in the abdomen, resulting in a presentation with shock.67
Magnetic resonance imaging can be even more useful in identifying gastrointestinal angioedema, but it would not be cost-effective, and based on our study, CT and ultrasonography of the abdomen were diagnostic in most cases.
AVOIDING UNNECESSARY TESTING
Hemodynamic instability and abdominal pain usually trigger a surgical consult and a more extensive workup, but with a good clinical approach, unnecessary testing and invasive diagnostic procedures can be avoided under the right circumstances.
Numerous surgical procedures have been reported in patients presenting with visceral angioedema secondary to ACE inhibitors.67 Although a thorough history and physical examination can give us a clue in the diagnosis of drug-induced gastrointestinal angioedema, CT is extremely helpful, as it shows dilated loops, thickened mucosal folds, perihepatic fluid, ascites, mesenteric edema, and a “doughnut” or “stacked coin” appearance.17,68
So far, there have been only two reports of angioedema of the stomach (the case reported by Shahzad et al10 and the current report). Angioedema can affect any visceral organ, but we usually see involvement of the jejunum followed by the ileum and duodenum.40
FINDINGS ON ENDOSCOPY
Usually, endoscopic examination of the upper and lower gastrointestinal tract does not reveal any specific pathology, but endoscopy and biopsy can rule out other causes of abdominal pain, such as Crohn disease, ulcerative colitis, infection, malignancy, granuloma, and vasculitis. Also, hereditary or acquired C1-esterase deficiency and other autoimmune disorders should be considered in the workup.18,69 In the reported cases, endoscopy revealed petechial bleeding with generalized edema.19
Biopsy often demonstrates an expanded edematous submucosal layer with inflammatory cell infiltration and protrusion of the proper muscular layer into the submucosal layer.15 A proper muscular layer and an edematous submucosal layer can produce edema so severe as to obstruct the intestine.15
Ultrasonography or CT provides essential information as to location, structure, and size, and it rules out other diagnoses. Therefore, consideration should be given to noninvasive imaging studies and laboratory testing (C1-esterase inhibitor, complement, antinuclear antibody, complete metabolic panel, complete blood cell count) before resorting to endoscopy or exploratory laparotomy.20,70 In three case reports,29,30,32 abdominal ultrasonography did not show any thickening of the small-bowel wall. Several cases have been diagnosed with the help of endoscopy.
Symptoms usually resolve when the ACE inhibitor is stopped
There is no standard treatment for ACE-inhibitor-induced visceral angioedema. In most patients, stopping the drug, giving nothing by mouth, and giving intravenous fluids to prevent dehydration are sufficient. Symptoms usually resolve within 48 hours.
In several case reports, fresh-frozen plasma was used to increase the levels of kininase II, which can degrade high levels of bradykinin.51,71,72 However, no randomized controlled trial of fresh-frozen plasma for ACE-inhibitor-induced angioedema has been published.
Drugs for hereditary angioedema—eg, recombinant C1-INH, the kallikrein inhibitor ecallantide (Kalbitor), and the BKR-2-antagonist icatibant (Firazyr)73—have not been prospectively studied in gastrointestinal angioedema associated with ACE inhibitors. Icatibant has been shown to be effective in the treatment of hereditary angioedema and could be promising in treating angioedema secondary to ACE inhibitors.8 Rosenberg et al21 described a patient who was on prednisone when she developed intestinal angioedema, thus calling into question the efficacy of steroids in the treatment of visceral angioedema.
RAISING AWARENESS
More than 40 million patients are currently taking ACE inhibitors or ARBs.9 Therefore, we suggest that patients with a known history of angioedema in response to these drugs should wear an identification bracelet to increase awareness and to prevent recurrence of angioedema.
A 57-year-old black woman presented to the emergency department with severe, dull abdominal pain associated with nonbilious vomiting and nausea. She had diabetes mellitus and hypertension, for which she had been taking metformin (Glucophage) 500 mg twice a day and lisinopril (available as Prinivil and Zestril) 20 mg daily for the last 4 years.
Multiple admissions in the past 4 years
The patient started taking lisinopril 10 mg daily in 2005, and she presented to her medical provider 2 weeks later with abdominal discomfort. Colonoscopy was performed, which revealed a benign polyp. She continued taking her medications, including lisinopril.
She continued to occasionally have abdominal pain of variable severity, but it was tolerable until 6 months later, when she presented to the emergency department with severe recurrent abdominal pain.
In view of the clinical picture, her physicians decided to treat her for small bowel obstruction, and an exploratory laparotomy was performed. The surgeons noted that she had moderate ascites, adhesions on the omentum, and a thickened high loop of the small bowel that was unequivocally viable and hyperemic, with thickening of the mesentery. Ascitic fluid was evacuated, adhesions were lysed, and the abdomen was closed. She was discharged with the same medications, including lisinopril; the dose was subsequently increased for better control of her hypertension.
The woman was admitted three more times within the same year for the same symptoms and underwent multiple workups for pancreatitis, gastritis, small-bowel obstruction, and other common gastrointestinal diseases.
Present admission
On review of systems, she denied any dry cough, weight loss or gain, food allergies, new medications, or hematochezia.
On physical examination, she had hypoactive bowel sounds and diffuse tenderness with guarding around the epigastric area.
Laboratory tests did not reveal any abnormalities; in particular, her C1 esterase concentration was normal. Stool studies were negative for infectious diseases.
Plain radiography of the abdomen showed a nonobstructive bowel-gas pattern.
She was diagnosed with gastrointestinal angioedema secondary to angiotensin-converting enzyme (ACE) inhibitor therapy. Her lisinopril was discontinued, and the symptoms resolved completely in 24 hours. On follow-up 8 weeks and 16 months later, her symptoms had not returned.
A RARE COMPLICATION OF ACE-INHIBITOR THERAPY
Angioedema occurs in 0.1% to 0.7% of patients taking ACE inhibitors, and it can affect about 1 of 2,500 patients during the first week of exposure.1–3 It usually manifests as swelling of the face, tongue, and lips, and in rare cases, the gastrointestinal wall. Thus, visceral angioedema is a rare complication of ACE-inhibitor therapy.
Because angioedema is less obvious when it involves abdominal organs, it presents a diagnostic challenge. It is placed lower in the differential diagnosis, as other, more common, and occasionally more high-risk medical conditions are generally considered first. Most of the time, the diagnosis is missed. Some physicians may not be aware of this problem, since only a few case reports have been published. Nevertheless, this potential complication needs to be considered when any patient receiving ACE inhibitors for treatment of hypertension, myocardial infarction, heart failure, or diabetic nephropathy presents with diffuse abdominal pain, diarrhea, or edema of the upper airways.4–8
If a high level of suspicion is applied along with good clinical judgment, then hospitalizations, unnecessary procedures, patient discomfort, and unnecessary health care costs can be prevented.
A MEDLINE SEARCH
To investigate the characteristics associated with this unusual presentation, including the time of symptom onset, the types of symptoms, and the diagnostic studies performed on the patients with visceral angioedema, we performed a MEDLINE search to identify case reports and case series published in English from 1980 to 2010 on the topic of abdominal or visceral angioedema. The search terms used were “visceral,” “intestinal angioedema,” “ACE-inhibitor side effects,” and the names of various ACE inhibitors.
Pertinent articles were identified, and clinical characteristics were collected, including demographics, onset of symptoms, the drug’s name, and others. In our summary below, data are presented as the mean and standard deviation for continuous variables and percentages for categorical variables.
SUMMARY OF REPORTED CASES
Our search revealed 27 reported cases of visceral angioedema associated with ACE inhibitors (a table summarizing our findings is available).9–34 The drug most often involved was lisinopril (11 cases), followed by enalapril (Vasotec) (8 cases).
Twenty-three (82%) of the cases were in women. The mean age of the patients was 49.5 ± 12.2 years (range 29–77 years); the mean age was 46.7 ± 11.7 years in women and 57 ± 13 years in men. Unfortunately, the race and ethnicity of the patients was documented in only some cases.
In 15 (54%) of the cases, the patient presented to a physician or emergency department within 72 hours (41.1 ± 17.4) of starting therapy, and in 8 cases the patient presented between 2 weeks and 18 months.
In 10 cases (including the case we are reporting here), the patients were kept on ACE inhibitors from 2 to 9 years after the initial presentation, as the diagnosis was missed.9,12,14,18,20,31,32 In 2 cases, the dose of the ACE inhibitor had been increased after the patient presented with the abdominal pain.
All of the patients were hospitalized for further diagnostic workup.
As for the presenting symptoms, all the patients had abdominal pain, 24 (86%) had emesis, 14 (50%) had diarrhea, and 20 (71%) had ascites. Laboratory results were mostly nonspecific. Twelve (44%) of the patients had leukocytosis. The C1 esterase inhibitor concentration was measured in 18 patients, and the results were normal in all of them.
Twenty-four (86%) of the patients underwent abdominal and pelvic CT or ultrasonography as part of the initial diagnostic evaluation, and intestinal wall-thickening was found in 21 (87.5%) of them.
Either surgery or gastrointestinal biopsy was performed in 16 (57%) of the patients; the surgical procedures included 2 cholecystectomies and 1 bone marrow biopsy. Only 1 case was diagnosed on the basis of clinical suspicion and abdominal radiographs alone.
The combination of intestinal and stomach angioedema was found in only 2 cases.
Two patients were kept on an ACE inhibitor in spite of symptoms and intestinal wall edema that showed a migratory pattern on imaging after chronic exposure.
The thickening involved the jejunum in 14 patients (50%), the ileum in 8 (29%), the duodenum in 5 (18%), the stomach in 2, and the sigmoid colon in 1.
In 12 cases (43%), visceral angioedema and its symptoms resolved within 48 hours of stopping the ACE inhibitor.
A DIAGNOSIS TO KEEP IN MIND
As we have seen, the diagnosis of visceral angioedema needs to be kept in mind when a patient—especially a middle-aged woman—taking an ACE inhibitor presents with abdominal pain, vomiting, diarrhea, leukocytosis, ascites, and wall-thickening of the small bowel on imaging studies.9,35,36
The diagnosis is hard to establish, and in the interim the patient may undergo invasive and unnecessary procedures, which can be avoided by a heightened awareness of this complication. In all of the reported cases, the patients required hospitalization because of the severity of symptoms and attempts to exclude other possible diseases.36
POSSIBLY DUE TO BRADYKININ
Several theories have been proposed to explain how visceral angioedema is induced by ACE inhibitors. The possible mechanisms that have been described include the following:
- The accumulation of bradykinin and substance P secondary to the effect of the ACE inhibitor, which may lead to the inflammatory response, therefore increasing permeability of the vascular compartment
- Deficiency of complement and the enzymes carboxypeptidase N and alpha-1 antitrypsin
- An antibody-antigen reaction37
- Hormones such as estrogen and progesterone (suggested by the greater number of women represented38)
- Contrast media used for imaging39
- Genetic predisposition
- Inflammation due to acute-phase proteins
- C1-inhibitor deficiency or dysfunction (however, the levels of C1/C4 and the C1-esterase inhibitor functional activity usually are normal2,10,40).
Many other theories are being explored.11,12,38,41–53
The most plausible mechanism is an increase in the levels of bradykinin and its metabolites.45 The absence of ACE can lead to breakdown of bradykinin to des-Arg bradykinin via the minor pathway, which can lead to more pronounced vasodilation and vascular permeability.54,55 During an acute attack of angioedema secondary to ACE inhibition, the bradykinin concentration can increase to more than 10 times the normal level.56
Moreover, C-reactive protein levels were higher (mean 4.42 mg/dL ± 0.15 mg/dL) in patients with ACE-inhibitor-induced angioedema than in those with other causes of angioedema (P < .0001).52 The patients taking ACE inhibitors without any previous angioedema had normal C-reactive protein levels (0.39 mg/dL ± 0.1 mg/dL).52
INCIDENCE RATES
In our review of the literature, all of the patients were taking an ACE inhibitor, and some were taking both an ACE inhibitor and an angiotensin-receptor blocker (ARB).
Initially, the incidence rate of angioedema was thought to be 0.1% to 0.2%, but recently the Omapatrilat Cardiovascular Treatment Assessment vs Enalapril (OCTAVE) trial had more than 12,000 patients on enalapril and reported the incidence of angioedema to be 0.68%,57 with a higher risk in women than in men (0.84% vs 0.54%)58 and a relative risk of 3.03 for blacks compared with whites.59
Even though ARBs seem to be safer, angioedema can recur in up to one-third of patients who switch from an ACE inhibitor to an ARB.60–63
Moreover, one study in the United States found that the frequency of hospital admission of patients with angioedema increased from 8,839 per year in 1998 to 11,925 in 2005, and the cost was estimated to be close to $123 million in 2005.64
Interestingly, when angioedema involved the face, it developed within the first week in 60% of cases,65 whereas when visceral angioedema developed, it did so within the first week in 59% of cases. Therefore, the timing of the onset is similar regardless of the body area involved.
Smokers who developed ACE-inhibitor-induced cough had a higher risk of ACE-inhibitor-induced angioedema in a retrospective cohort study by Morimoto,66 but no relationship to the area of involvement was made.
ON IMAGING, A THICKENED BOWEL WALL
Computed tomography can reveal bowel edema and ascites more reliably than plain radiography or barium studies. Edema thickens the bowel wall, with increased contrast enhancement that makes mesenteric vessels show up on the study. In some instances edema is so significant that edematous submucosa can be differentiated from the serosa due to impressive thickening of the mucosal wall.15,16 Oral contrast can be seen in the middle of the lumen, giving it a target-sign appearance. Edema of the small bowel and ascites can lead to fluid sequestration in the abdomen, resulting in a presentation with shock.67
Magnetic resonance imaging can be even more useful in identifying gastrointestinal angioedema, but it would not be cost-effective, and based on our study, CT and ultrasonography of the abdomen were diagnostic in most cases.
AVOIDING UNNECESSARY TESTING
Hemodynamic instability and abdominal pain usually trigger a surgical consult and a more extensive workup, but with a good clinical approach, unnecessary testing and invasive diagnostic procedures can be avoided under the right circumstances.
Numerous surgical procedures have been reported in patients presenting with visceral angioedema secondary to ACE inhibitors.67 Although a thorough history and physical examination can give us a clue in the diagnosis of drug-induced gastrointestinal angioedema, CT is extremely helpful, as it shows dilated loops, thickened mucosal folds, perihepatic fluid, ascites, mesenteric edema, and a “doughnut” or “stacked coin” appearance.17,68
So far, there have been only two reports of angioedema of the stomach (the case reported by Shahzad et al10 and the current report). Angioedema can affect any visceral organ, but we usually see involvement of the jejunum followed by the ileum and duodenum.40
FINDINGS ON ENDOSCOPY
Usually, endoscopic examination of the upper and lower gastrointestinal tract does not reveal any specific pathology, but endoscopy and biopsy can rule out other causes of abdominal pain, such as Crohn disease, ulcerative colitis, infection, malignancy, granuloma, and vasculitis. Also, hereditary or acquired C1-esterase deficiency and other autoimmune disorders should be considered in the workup.18,69 In the reported cases, endoscopy revealed petechial bleeding with generalized edema.19
Biopsy often demonstrates an expanded edematous submucosal layer with inflammatory cell infiltration and protrusion of the proper muscular layer into the submucosal layer.15 A proper muscular layer and an edematous submucosal layer can produce edema so severe as to obstruct the intestine.15
Ultrasonography or CT provides essential information as to location, structure, and size, and it rules out other diagnoses. Therefore, consideration should be given to noninvasive imaging studies and laboratory testing (C1-esterase inhibitor, complement, antinuclear antibody, complete metabolic panel, complete blood cell count) before resorting to endoscopy or exploratory laparotomy.20,70 In three case reports,29,30,32 abdominal ultrasonography did not show any thickening of the small-bowel wall. Several cases have been diagnosed with the help of endoscopy.
Symptoms usually resolve when the ACE inhibitor is stopped
There is no standard treatment for ACE-inhibitor-induced visceral angioedema. In most patients, stopping the drug, giving nothing by mouth, and giving intravenous fluids to prevent dehydration are sufficient. Symptoms usually resolve within 48 hours.
In several case reports, fresh-frozen plasma was used to increase the levels of kininase II, which can degrade high levels of bradykinin.51,71,72 However, no randomized controlled trial of fresh-frozen plasma for ACE-inhibitor-induced angioedema has been published.
Drugs for hereditary angioedema—eg, recombinant C1-INH, the kallikrein inhibitor ecallantide (Kalbitor), and the BKR-2-antagonist icatibant (Firazyr)73—have not been prospectively studied in gastrointestinal angioedema associated with ACE inhibitors. Icatibant has been shown to be effective in the treatment of hereditary angioedema and could be promising in treating angioedema secondary to ACE inhibitors.8 Rosenberg et al21 described a patient who was on prednisone when she developed intestinal angioedema, thus calling into question the efficacy of steroids in the treatment of visceral angioedema.
RAISING AWARENESS
More than 40 million patients are currently taking ACE inhibitors or ARBs.9 Therefore, we suggest that patients with a known history of angioedema in response to these drugs should wear an identification bracelet to increase awareness and to prevent recurrence of angioedema.
- Brown NJ, Snowden M, Griffin MR. Recurrent angiotensin-converting enzyme inhibitor–associated angioedema. JAMA 1997; 278:232–233.
- Israili ZH, Hall WD. Cough and angioneurotic edema associated with angiotensin-converting enzyme inhibitor therapy. A review of the literature and pathophysiology. Ann Intern Med 1992; 117:234–242.
- Messerli FH, Nussberger J. Vasopeptidase inhibition and angiooedema. Lancet 2000; 356:608–609.
- Jessup M, Brozena S. Heart failure. N Engl J Med 2003; 348:2007–2018.
- Jessup M. The less familiar face of heart failure. J Am Coll Cardiol 2003; 41:224–226.
- Chobanian AV. Clinical practice. Isolated systolic hypertension in the elderly. N Engl J Med 2007; 357:789–796.
- Casas JP, Chua W, Loukogeorgakis S, et al. Effect of inhibitors of the renin-angiotensin system and other antihypertensive drugs on renal outcomes: systematic review and meta-analysis. Lancet 2005; 366:2026–2033.
- Weber MA, Messerli FH. Angiotensin-converting enzyme inhibitors and angioedema: estimating the risk. Hypertension 2008; 51:1465–1467.
- Oudit G, Girgrah N, Allard J. ACE inhibitor-induced angioedema of the intestine: Case report, incidence, pathophysiology, diagnosis and management. Can J Gastroenterol 2001; 15:827–832.
- Shahzad G, Korsten MA, Blatt C, Motwani P. Angiotensin-converting enzyme (ACE) inhibitor-associated angioedema of the stomach and small intestine: a case report. Mt Sinai J Med 2006; 73:1123–1125.
- Chase MP, Fiarman GS, Scholz FJ, MacDermott RP. Angioedema of the small bowel due to an angiotensin-converting enzyme inhibitor. J Clin Gastroenterol 2000; 31:254–257.
- Mullins RJ, Shanahan TM, Dobson RT. Visceral angioedema related to treatment with an ACE inhibitor. Med J Aust 1996; 165:319–321.
- Schmidt TD, McGrath KM. Angiotensin-converting enzyme inhibitor angioedema of the intestine: a case report and review of the literature. Am J Med Sci 2002; 324:106–108.
- Smoger SH, Sayed MA. Simultaneous mucosal and small bowel angioedema due to captopril. South Med J 1998; 91:1060–1063.
- Tojo A, Onozato ML, Fujita T. Repeated subileus due to angioedema during renin-angiotensin system blockade. Am J Med Sci 2006; 332:36–38.
- De Backer AI, De Schepper AM, Vandevenne JE, Schoeters P, Michielsen P, Stevens WJ. CT of angioedema of the small bowel. AJR Am J Roentgenol 2001; 176:649–652.
- Marmery H, Mirvis SE. Angiotensin-converting enzyme inhibitor-induced visceral angioedema. Clin Radiol 2006; 61:979–982.
- Orr KK, Myers JR. Intermittent visceral edema induced by long-term enalapril administration. Ann Pharmacother 2004; 38:825–827.
- Spahn TW, Grosse-Thie W, Mueller MK. Endoscopic visualization of angiotensin-converting enzyme inhibitor-induced small bowel angioedema as a cause of relapsing abdominal pain using double-balloon enteroscopy. Dig Dis Sci 2008; 53:1257–1260.
- Byrne TJ, Douglas DD, Landis ME, Heppell JP. Isolated visceral angioedema: an underdiagnosed complication of ACE inhibitors? Mayo Clin Proc 2000; 75:1201–1204.
- Rosenberg EI, Mishra G, Abdelmalek MF. Angiotensin-converting enzyme inhibitor-induced isolated visceral angioedema in a liver transplant recipient. Transplantation 2003; 75:730–732.
- Salloum H, Locher C, Chenard A, et al. [Small bowel angioedema due to perindopril]. Gastroenterol Clin Biol 2005; 29:1180–1181.
- Arakawa M, Murata Y, Rikimaru Y, Sasaki Y. Drug-induced isolated visceral angioneurotic edema. Intern Med 2005; 44:975–978.
- Abdelmalek MF, Douglas DD. Lisinopril-induced isolated visceral angioedema: review of ACE-inhibitor-induced small bowel angioedema. Dig Dis Sci 1997; 42:847–850.
- Gregory KW, Davis RC. Images in clinical medicine. Angioedema of the intestine. N Engl J Med 1996; 334:1641.
- Farraye FA, Peppercorn MA, Steer ML, Joffe N, Rees M. Acute small-bowel mucosal edema following enalapril use. JAMA 1988; 259:3131.
- Jacobs RL, Hoberman LJ, Goldstein HM. Angioedema of the small bowel caused by an angiotensin-converting enzyme inhibitor. Am J Gastroenterol 1994; 89:127–128.
- Herman L, Jocums SB, Coleman MD. A 29-year-old woman with crampy abdominal pain. Tenn Med 1999; 92:272–273.
- Guy C, Cathébras P, Rousset H. Suspected angioedema of abdominal viscera. Ann Intern Med 1994; 121:900.
- Dupasquier E. [A rare clinical form of angioneurotic edema caused by enalapril: acute abdomen]. Arch Mal Coeur Vaiss 1994; 87:1371–1374.
- Jardine DL, Anderson JC, McClintock AD. Delayed diagnosis of recurrent visceral angio-oedema secondary to ACE inhibitor therapy. Aust N Z J Med 1999; 29:377–378.
- Matsumura M, Haruki K, Kajinami K, Takada T. Angioedema likely related to angiotensin converting enzyme inhibitors. Intern Med 1993; 32:424–426.
- Khan MU, Baig MA, Javed RA, et al. Benazepril induced isolated visceral angioedema: a rare and under diagnosed adverse effect of angiotensin converting enzyme inhibitors. Int J Cardiol 2007; 118:e68–e69.
- Adhikari SP, Schneider JI. An unusual cause of abdominal pain and hypotension: angioedema of the bowel. J Emerg Med 2009; 36:23–25.
- Gibbs CR, Lip GY, Beevers DG. Angioedema due to ACE inhibitors: increased risk in patients of African origin. Br J Clin Pharmacol 1999; 48:861–865.
- Johnsen SP, Jacobsen J, Monster TB, Friis S, McLaughlin JK, Sørensen HT. Risk of first-time hospitalization for angioedema among users of ACE inhibitors and angiotensin receptor antagonists. Am J Med 2005; 118:1428–1329.
- Bi CK, Soltani K, Sloan JB, Weber RR, Elliott WJ, Murphy MB. Tissue-specific autoantibodies induced by captopril. Clin Res 1987; 35:922A.
- Bork K, Dewald G. Hereditary angioedema type III, angioedema associated with angiotensin II receptor antagonists, and female sex. Am J Med 2004; 116:644–645.
- Witten DM, Hirsch FD, Hartman GW. Acute reactions to urographic contrast medium: incidence, clinical characteristics and relationship to history of hypersensitivity states. Am J Roentgenol Radium Ther Nucl Med 1973; 119:832–840.
- Eck SL, Morse JH, Janssen DA, Emerson SG, Markovitz DM. Angioedema presenting as chronic gastrointestinal symptoms. Am J Gastroenterol 1993; 88:436–439.
- Coleman JW, Yeung JH, Roberts DH, Breckenridge AM, Park BK. Drug-specific antibodies in patients receiving captopril. Br J Clin Pharmacol 1986; 22:161–165.
- Kallenberg CG. Autoantibodies during captopril treatment. Arthritis Rheum 1985; 28:597–598.
- Inman WH, Rawson NS, Wilton LV, Pearce GL, Speirs CJ. Postmarketing surveillance of enalapril. I: Results of prescription-event monitoring. BMJ 1988; 297:826–829.
- Lefebvre J, Murphey LJ, Hartert TV, Jiao Shan R, Simmons WH, Brown NJ. Dipeptidyl peptidase IV activity in patients with ACE-inhibitor-associated angioedema. Hypertension 2002; 39:460–464.
- Molinaro G, Cugno M, Perez M, et al. Angiotensin-converting enzyme inhibitor-associated angioedema is characterized by a slower degradation of des-arginine(9)-bradykinin. J Pharmacol Exp Ther 2002; 303:232–237.
- Adam A, Cugno M, Molinaro G, Perez M, Lepage Y, Agostoni A. Aminopeptidase P in individuals with a history of angiooedema on ACE inhibitors. Lancet 2002; 359:2088–2089.
- Binkley KE, Davis A. Clinical, biochemical, and genetic characterization of a novel estrogen-dependent inherited form of angioedema. J Allergy Clin Immunol 2000; 106:546–550.
- Yeung JH, Coleman JW, Park BK. Drug-protein conjugates—IX. Immunogenicity of captopril-protein conjugates. Biochem Pharmacol 1985; 34:4005–4012.
- Abbosh J, Anderson JA, Levine AB, Kupin WL. Angiotensin converting enzyme inhibitor-induced angioedema more prevalent in transplant patients. Ann Allergy Asthma Immunol 1999; 82:473–476.
- Pichler WJ, Lehner R, Späth PJ. Recurrent angioedema associated with hypogonadism or anti-androgen therapy. Ann Allergy 1989; 63:301–305.
- Bass G, Honan D. Octaplas is not equivalent to fresh frozen plasma in the treatment of acute angioedema. Eur J Anaesthesiol 2007; 24:1062–1063.
- Bas M, Hoffmann TK, Bier H, Kojda G. Increased C-reactive protein in ACE-inhibitor-induced angioedema. Br J Clin Pharmacol 2005; 59:233–238.
- Herman AG. Differences in structure of angiotensin-converting enzyme inhibitors might predict differences in action. Am J Cardiol 1992; 70:102C–108C.
- Cunnion KM, Lee JC, Frank MM. Capsule production and growth phase influence binding of complement to Staphylococcus aureus. Infect Immunol 2001; 69:6796–6803.
- Cunnion KM, Wagner E, Frank MM. Complement and kinins. In:Parlow TG, Stites DP, Imboden JB, editors. Medical Immunology. 10th ed. New York, NY: Lange Medical Books; 2001:186–188.
- Pellacani A, Brunner HR, Nussberger J. Plasma kinins increase after angiotensin-converting enzyme inhibition in human subjects. Clin Sci (Lond) 1994; 87:567–574.
- Bristol-Myers Squibb Pharmaceutical Research Institute. FDA Advisory Committee Briefing Book for OMAPATRILAT Tablets NDA 21-188. www.fda.gov/ohrms/dockets/ac/02/briefing/3877B2_01_BristolMeyersSquibb.pdf. Accessed 2/4/2011.
- Kostis JB, Kim HJ, Rusnak J, et al. Incidence and characteristics of angioedema associated with enalapril. Arch Intern Med 2005; 165:1637–1642.
- Mahoney EJ, Devaiah AK. Angioedema and angiotensin-converting enzyme inhibitors: are demographics a risk? Otolaryngol Head Neck Surg 2008; 139:105–108.
- Warner KK, Visconti JA, Tschampel MM. Angiotensin II receptor blockers in patients with ACE inhibitor-induced angioedema. Ann Pharmacother 2000; 34:526–528.
- Kyrmizakis DE, Papadakis CE, Liolios AD, et al. Angiotensin-converting enzyme inhibitors and angiotensin II receptor antagonists. Arch Otolaryngol Head Neck Surg 2004; 130:1416–1419.
- MacLean JA, Hannaway PJ. Angioedema and AT1 receptor blockers: proceed with caution. Arch Intern Med 2003; 163:1488–1489,
- Abdi R, Dong VM, Lee CJ, Ntoso KA. Angiotensin II receptor blocker-associated angioedema: on the heels of ACE inhibitor angioedema. Pharmacotherapy 2002; 22:1173–1175.
- Lin RY, Shah SN. Increasing hospitalizations due to angioedema in the United States. Ann Allergy Asthma Immunol 2008; 101:185–192.
- Slater EE, Merrill DD, Guess HA, et al. Clinical profile of angioedema associated with angiotensin converting-enzyme inhibition. JAMA 1988; 260:967–970.
- Morimoto T, Gandhi TK, Fiskio JM, et al. An evaluation of risk factors for adverse drug events associated with angiotensin-converting enzyme inhibitors. J Eval Clin Pract 2004; 10:499–509.
- Cohen N, Sharon A, Golik A, Zaidenstein R, Modai D. Hereditary angioneurotic edema with severe hypovolemic shock. J Clin Gastroenterol 1993; 16:237–239.
- Ciaccia D, Brazer SR, Baker ME. Acquired C1 esterase inhibitor deficiency causing intestinal angioedema: CT appearance. AJR Am J Roentgenol 1993; 161:1215–1216.
- Malcolm A, Prather CM. Intestinal angioedema mimicking Crohn’s disease. Med J Aust 1999; 171:418–420.
- Schmidt TD, McGrath KM. Angiotensin-converting enzyme inhibitor angioedema of the intestine: a case report and review of the literature. Am J Med Sci 2002; 324:106–108.
- Karim MY, Masood A. Fresh-frozen plasma as a treatment for life-threatening ACE-inhibitor angioedema. J Allergy Clin Immunol 2002; 109:370–371.
- Warrier MR, Copilevitz CA, Dykewicz MS, Slavin RG. Fresh frozen plasma in the treatment of resistant angiotensin-converting enzyme inhibitor angioedema. Ann Allergy Asthma Immunol 2004; 92:573–575.
- Bas M, Adams V, Suvorava T, Niehues T, Hoffmann TK, Kojda G. Nonallergic angioedema: role of bradykinin. Allergy 2007; 62:842–856.
- Agostoni A, Cicardi M, Cugno M, Zingale LC, Gioffré D, Nussberger J. Angioedema due to angiotensin-converting enzyme inhibitors. Immunopharmacology 1999; 44:21–25.
- Brown NJ, Snowden M, Griffin MR. Recurrent angiotensin-converting enzyme inhibitor–associated angioedema. JAMA 1997; 278:232–233.
- Israili ZH, Hall WD. Cough and angioneurotic edema associated with angiotensin-converting enzyme inhibitor therapy. A review of the literature and pathophysiology. Ann Intern Med 1992; 117:234–242.
- Messerli FH, Nussberger J. Vasopeptidase inhibition and angiooedema. Lancet 2000; 356:608–609.
- Jessup M, Brozena S. Heart failure. N Engl J Med 2003; 348:2007–2018.
- Jessup M. The less familiar face of heart failure. J Am Coll Cardiol 2003; 41:224–226.
- Chobanian AV. Clinical practice. Isolated systolic hypertension in the elderly. N Engl J Med 2007; 357:789–796.
- Casas JP, Chua W, Loukogeorgakis S, et al. Effect of inhibitors of the renin-angiotensin system and other antihypertensive drugs on renal outcomes: systematic review and meta-analysis. Lancet 2005; 366:2026–2033.
- Weber MA, Messerli FH. Angiotensin-converting enzyme inhibitors and angioedema: estimating the risk. Hypertension 2008; 51:1465–1467.
- Oudit G, Girgrah N, Allard J. ACE inhibitor-induced angioedema of the intestine: Case report, incidence, pathophysiology, diagnosis and management. Can J Gastroenterol 2001; 15:827–832.
- Shahzad G, Korsten MA, Blatt C, Motwani P. Angiotensin-converting enzyme (ACE) inhibitor-associated angioedema of the stomach and small intestine: a case report. Mt Sinai J Med 2006; 73:1123–1125.
- Chase MP, Fiarman GS, Scholz FJ, MacDermott RP. Angioedema of the small bowel due to an angiotensin-converting enzyme inhibitor. J Clin Gastroenterol 2000; 31:254–257.
- Mullins RJ, Shanahan TM, Dobson RT. Visceral angioedema related to treatment with an ACE inhibitor. Med J Aust 1996; 165:319–321.
- Schmidt TD, McGrath KM. Angiotensin-converting enzyme inhibitor angioedema of the intestine: a case report and review of the literature. Am J Med Sci 2002; 324:106–108.
- Smoger SH, Sayed MA. Simultaneous mucosal and small bowel angioedema due to captopril. South Med J 1998; 91:1060–1063.
- Tojo A, Onozato ML, Fujita T. Repeated subileus due to angioedema during renin-angiotensin system blockade. Am J Med Sci 2006; 332:36–38.
- De Backer AI, De Schepper AM, Vandevenne JE, Schoeters P, Michielsen P, Stevens WJ. CT of angioedema of the small bowel. AJR Am J Roentgenol 2001; 176:649–652.
- Marmery H, Mirvis SE. Angiotensin-converting enzyme inhibitor-induced visceral angioedema. Clin Radiol 2006; 61:979–982.
- Orr KK, Myers JR. Intermittent visceral edema induced by long-term enalapril administration. Ann Pharmacother 2004; 38:825–827.
- Spahn TW, Grosse-Thie W, Mueller MK. Endoscopic visualization of angiotensin-converting enzyme inhibitor-induced small bowel angioedema as a cause of relapsing abdominal pain using double-balloon enteroscopy. Dig Dis Sci 2008; 53:1257–1260.
- Byrne TJ, Douglas DD, Landis ME, Heppell JP. Isolated visceral angioedema: an underdiagnosed complication of ACE inhibitors? Mayo Clin Proc 2000; 75:1201–1204.
- Rosenberg EI, Mishra G, Abdelmalek MF. Angiotensin-converting enzyme inhibitor-induced isolated visceral angioedema in a liver transplant recipient. Transplantation 2003; 75:730–732.
- Salloum H, Locher C, Chenard A, et al. [Small bowel angioedema due to perindopril]. Gastroenterol Clin Biol 2005; 29:1180–1181.
- Arakawa M, Murata Y, Rikimaru Y, Sasaki Y. Drug-induced isolated visceral angioneurotic edema. Intern Med 2005; 44:975–978.
- Abdelmalek MF, Douglas DD. Lisinopril-induced isolated visceral angioedema: review of ACE-inhibitor-induced small bowel angioedema. Dig Dis Sci 1997; 42:847–850.
- Gregory KW, Davis RC. Images in clinical medicine. Angioedema of the intestine. N Engl J Med 1996; 334:1641.
- Farraye FA, Peppercorn MA, Steer ML, Joffe N, Rees M. Acute small-bowel mucosal edema following enalapril use. JAMA 1988; 259:3131.
- Jacobs RL, Hoberman LJ, Goldstein HM. Angioedema of the small bowel caused by an angiotensin-converting enzyme inhibitor. Am J Gastroenterol 1994; 89:127–128.
- Herman L, Jocums SB, Coleman MD. A 29-year-old woman with crampy abdominal pain. Tenn Med 1999; 92:272–273.
- Guy C, Cathébras P, Rousset H. Suspected angioedema of abdominal viscera. Ann Intern Med 1994; 121:900.
- Dupasquier E. [A rare clinical form of angioneurotic edema caused by enalapril: acute abdomen]. Arch Mal Coeur Vaiss 1994; 87:1371–1374.
- Jardine DL, Anderson JC, McClintock AD. Delayed diagnosis of recurrent visceral angio-oedema secondary to ACE inhibitor therapy. Aust N Z J Med 1999; 29:377–378.
- Matsumura M, Haruki K, Kajinami K, Takada T. Angioedema likely related to angiotensin converting enzyme inhibitors. Intern Med 1993; 32:424–426.
- Khan MU, Baig MA, Javed RA, et al. Benazepril induced isolated visceral angioedema: a rare and under diagnosed adverse effect of angiotensin converting enzyme inhibitors. Int J Cardiol 2007; 118:e68–e69.
- Adhikari SP, Schneider JI. An unusual cause of abdominal pain and hypotension: angioedema of the bowel. J Emerg Med 2009; 36:23–25.
- Gibbs CR, Lip GY, Beevers DG. Angioedema due to ACE inhibitors: increased risk in patients of African origin. Br J Clin Pharmacol 1999; 48:861–865.
- Johnsen SP, Jacobsen J, Monster TB, Friis S, McLaughlin JK, Sørensen HT. Risk of first-time hospitalization for angioedema among users of ACE inhibitors and angiotensin receptor antagonists. Am J Med 2005; 118:1428–1329.
- Bi CK, Soltani K, Sloan JB, Weber RR, Elliott WJ, Murphy MB. Tissue-specific autoantibodies induced by captopril. Clin Res 1987; 35:922A.
- Bork K, Dewald G. Hereditary angioedema type III, angioedema associated with angiotensin II receptor antagonists, and female sex. Am J Med 2004; 116:644–645.
- Witten DM, Hirsch FD, Hartman GW. Acute reactions to urographic contrast medium: incidence, clinical characteristics and relationship to history of hypersensitivity states. Am J Roentgenol Radium Ther Nucl Med 1973; 119:832–840.
- Eck SL, Morse JH, Janssen DA, Emerson SG, Markovitz DM. Angioedema presenting as chronic gastrointestinal symptoms. Am J Gastroenterol 1993; 88:436–439.
- Coleman JW, Yeung JH, Roberts DH, Breckenridge AM, Park BK. Drug-specific antibodies in patients receiving captopril. Br J Clin Pharmacol 1986; 22:161–165.
- Kallenberg CG. Autoantibodies during captopril treatment. Arthritis Rheum 1985; 28:597–598.
- Inman WH, Rawson NS, Wilton LV, Pearce GL, Speirs CJ. Postmarketing surveillance of enalapril. I: Results of prescription-event monitoring. BMJ 1988; 297:826–829.
- Lefebvre J, Murphey LJ, Hartert TV, Jiao Shan R, Simmons WH, Brown NJ. Dipeptidyl peptidase IV activity in patients with ACE-inhibitor-associated angioedema. Hypertension 2002; 39:460–464.
- Molinaro G, Cugno M, Perez M, et al. Angiotensin-converting enzyme inhibitor-associated angioedema is characterized by a slower degradation of des-arginine(9)-bradykinin. J Pharmacol Exp Ther 2002; 303:232–237.
- Adam A, Cugno M, Molinaro G, Perez M, Lepage Y, Agostoni A. Aminopeptidase P in individuals with a history of angiooedema on ACE inhibitors. Lancet 2002; 359:2088–2089.
- Binkley KE, Davis A. Clinical, biochemical, and genetic characterization of a novel estrogen-dependent inherited form of angioedema. J Allergy Clin Immunol 2000; 106:546–550.
- Yeung JH, Coleman JW, Park BK. Drug-protein conjugates—IX. Immunogenicity of captopril-protein conjugates. Biochem Pharmacol 1985; 34:4005–4012.
- Abbosh J, Anderson JA, Levine AB, Kupin WL. Angiotensin converting enzyme inhibitor-induced angioedema more prevalent in transplant patients. Ann Allergy Asthma Immunol 1999; 82:473–476.
- Pichler WJ, Lehner R, Späth PJ. Recurrent angioedema associated with hypogonadism or anti-androgen therapy. Ann Allergy 1989; 63:301–305.
- Bass G, Honan D. Octaplas is not equivalent to fresh frozen plasma in the treatment of acute angioedema. Eur J Anaesthesiol 2007; 24:1062–1063.
- Bas M, Hoffmann TK, Bier H, Kojda G. Increased C-reactive protein in ACE-inhibitor-induced angioedema. Br J Clin Pharmacol 2005; 59:233–238.
- Herman AG. Differences in structure of angiotensin-converting enzyme inhibitors might predict differences in action. Am J Cardiol 1992; 70:102C–108C.
- Cunnion KM, Lee JC, Frank MM. Capsule production and growth phase influence binding of complement to Staphylococcus aureus. Infect Immunol 2001; 69:6796–6803.
- Cunnion KM, Wagner E, Frank MM. Complement and kinins. In:Parlow TG, Stites DP, Imboden JB, editors. Medical Immunology. 10th ed. New York, NY: Lange Medical Books; 2001:186–188.
- Pellacani A, Brunner HR, Nussberger J. Plasma kinins increase after angiotensin-converting enzyme inhibition in human subjects. Clin Sci (Lond) 1994; 87:567–574.
- Bristol-Myers Squibb Pharmaceutical Research Institute. FDA Advisory Committee Briefing Book for OMAPATRILAT Tablets NDA 21-188. www.fda.gov/ohrms/dockets/ac/02/briefing/3877B2_01_BristolMeyersSquibb.pdf. Accessed 2/4/2011.
- Kostis JB, Kim HJ, Rusnak J, et al. Incidence and characteristics of angioedema associated with enalapril. Arch Intern Med 2005; 165:1637–1642.
- Mahoney EJ, Devaiah AK. Angioedema and angiotensin-converting enzyme inhibitors: are demographics a risk? Otolaryngol Head Neck Surg 2008; 139:105–108.
- Warner KK, Visconti JA, Tschampel MM. Angiotensin II receptor blockers in patients with ACE inhibitor-induced angioedema. Ann Pharmacother 2000; 34:526–528.
- Kyrmizakis DE, Papadakis CE, Liolios AD, et al. Angiotensin-converting enzyme inhibitors and angiotensin II receptor antagonists. Arch Otolaryngol Head Neck Surg 2004; 130:1416–1419.
- MacLean JA, Hannaway PJ. Angioedema and AT1 receptor blockers: proceed with caution. Arch Intern Med 2003; 163:1488–1489,
- Abdi R, Dong VM, Lee CJ, Ntoso KA. Angiotensin II receptor blocker-associated angioedema: on the heels of ACE inhibitor angioedema. Pharmacotherapy 2002; 22:1173–1175.
- Lin RY, Shah SN. Increasing hospitalizations due to angioedema in the United States. Ann Allergy Asthma Immunol 2008; 101:185–192.
- Slater EE, Merrill DD, Guess HA, et al. Clinical profile of angioedema associated with angiotensin converting-enzyme inhibition. JAMA 1988; 260:967–970.
- Morimoto T, Gandhi TK, Fiskio JM, et al. An evaluation of risk factors for adverse drug events associated with angiotensin-converting enzyme inhibitors. J Eval Clin Pract 2004; 10:499–509.
- Cohen N, Sharon A, Golik A, Zaidenstein R, Modai D. Hereditary angioneurotic edema with severe hypovolemic shock. J Clin Gastroenterol 1993; 16:237–239.
- Ciaccia D, Brazer SR, Baker ME. Acquired C1 esterase inhibitor deficiency causing intestinal angioedema: CT appearance. AJR Am J Roentgenol 1993; 161:1215–1216.
- Malcolm A, Prather CM. Intestinal angioedema mimicking Crohn’s disease. Med J Aust 1999; 171:418–420.
- Schmidt TD, McGrath KM. Angiotensin-converting enzyme inhibitor angioedema of the intestine: a case report and review of the literature. Am J Med Sci 2002; 324:106–108.
- Karim MY, Masood A. Fresh-frozen plasma as a treatment for life-threatening ACE-inhibitor angioedema. J Allergy Clin Immunol 2002; 109:370–371.
- Warrier MR, Copilevitz CA, Dykewicz MS, Slavin RG. Fresh frozen plasma in the treatment of resistant angiotensin-converting enzyme inhibitor angioedema. Ann Allergy Asthma Immunol 2004; 92:573–575.
- Bas M, Adams V, Suvorava T, Niehues T, Hoffmann TK, Kojda G. Nonallergic angioedema: role of bradykinin. Allergy 2007; 62:842–856.
- Agostoni A, Cicardi M, Cugno M, Zingale LC, Gioffré D, Nussberger J. Angioedema due to angiotensin-converting enzyme inhibitors. Immunopharmacology 1999; 44:21–25.
KEY POINTS
- Visceral angioedema due to ACE-inhibitor therapy can easily be diagnosed by clinical suspicion and abdominal computed tomography (CT).
- Many physicians are not aware of this condition and so may subject patients to unnecessary invasive procedures, including surgery and endoscopy.
- If a middle-aged woman taking an ACE inhibitor presents with abdominal pain and emesis, the differential diagnosis should include visceral angioedema, and CT should be strongly considered.
In reply: Coadministration of clopidogrel and proton pump inhibitors
In Reply: I thank Dr. Keller for his interest in my review on the side effects and drug interactions of proton pump inhibitors (PPIs).1 In particular, the concern about the potentially increased risk of a cardiovascular event in patients taking a PPI while on clopidogrel is a matter of active research. Since the prevention of death, myocardial infarction, or stroke is the desired outcome in patients receiving antiplatelet therapy, any reduction in the antiplatelet effect of clopidogrel could put patients at increased risk. Because of the enormous number of patients on both PPIs and clopidogrel, investigators are studying the effect of PPIs on clopidogrel to determine the true significance in day-to-day practice. We should expect that the data will continue to evolve in the coming years as more research is done on this important interaction.
The FDA Web site that Dr. Keller brings up2 was posted a few months after the submission of my manuscript. But even with the FDA’s cautionary words, it is important to realize that the risk that purportedly exists with the interaction of omeprazole and clopidogrel and the suggestion for the alternative use of pantoprazole are both based on pharmacokinetic, pharmacodynamic, and epidemiologic studies, not on clinical outcome data.
As much as we would like to rely on such studies, pharmacokinetic and pharmacodynamic studies do not address clinical outcomes, and observational studies cannot account for every confounder, because patients in these studies are not randomly assigned to the intervention, which is the rationale behind the necessity for a prospective trial. The Clopidogrel and the Optimization of Gastrointestinal Events (COGENT) study,3 a prospective randomized controlled trial with 3,761 analyzed patients, found no differences in adjudicated cardiovascular outcomes between groups who received a clopidogrel plus omeprazole vs clopidogrel alone.3 Although the COGENT study ended prematurely because of bankruptcy of the funding source, these outcomes represent the only randomized prospective data that can be found to date on PubMed. With such large numbers of patients in each group (1,876 and 1,885, respectively) and no differences in outcomes, it stands to reason that only a study with massive sample sizes would be able to detect a statistically significant difference. Differences between clopidogrel-treated patients taking and not taking omeprazole are likely be found in a well-designed prospective trial; however, it would be virtually impossible to find differences among PPIs.
To make matters even less convincing that therapy should be altered, the Working Group on High On-treatment Platelet Reactivity stated in their recent consensus paper that there are “limited data to support that alteration of therapy based on platelet function measurements actually improves outcomes.” 4 Additionally, a recent multisociety Expert Consensus Document discussing the concomitant use of PPIs and thienopyridine drugs to reduce gastrointestinal complications further supports this argument.5 Therefore, it is difficult to justify a marked increase in cost of the PPI selected (pantoprazole costs nearly seven times more per dose than omeprazole, according to one Web site6) for a benefit that is supported only by theoretical and observational data, not by outcome data.
As Dr. Keller also mentions, Aggrenox can be used for secondary stroke prophylaxis, but a discussion about a therapeutic exchange between clopidogrel and other antiplatelet agents was beyond the scope of my review. A recently published joint guideline of the American Heart Association and the American Stroke Association guideline should be consulted for further information.7
Other gastroprotective therapies are available. However, misoprostol (as mentioned) is associated with significant gastrointestinal side effects and must be taken four times a day. H2-receptor antagonists are not considered to be as effective as PPIs.8,9
- Madanick RD. Proton pump inhibitor side effects and drug interactions: much ado about nothing? Cleve Clin J Med 2011; 78:39–49.
- US Food and Drug Administration. FDA reminder to avoid concomitant use of Plavix (clopidogrel) and omeprazole. www.fda.gov/Drugs/DrugSafety/ucm231161.htm. Accessed March 23, 2011.
- Bhatt DL, Cryer BL, Contant CF, et al. Clopidogrel with or without omeprazole in coronary artery disease. N Engl J Med 2010; 363:1909–1917.
- Bonello L, Tantry US, Marcucci R, et al. Consensus and future directions on the definition of high on-treatment platelet reactivity to adenosine diphosphate. J Am Coll Cardiol 2010; 56:919–33.
- Abraham NS, Hlatky MA, Antman EM, et al. ACCF/ACG/AHA 2010 expert consensus document on the concomitant use of proton pump inhibitors and thienopyridines:a focused update of the ACCF/ACG/AHA 2008 expert consensus document on reducing the gastrointestinal risks of antiplatelet therapy and NSAID use. Am J Gastroenterol 2010; 105:2533–2549.
- HealthWarehouse. www.healthwarehouse.com. Accessed March 23, 2011.
- Furie KL, Kasner SE, Adams RJ, et al. Guidelines for the prevention of stroke in patients with stroke or transient ischemic attack: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2011; 42:227–276.
- Bhatt DL, Scheiman J, Abraham NS, et al. ACCF/ACG/AHA 2008 expert consensus document on reducing the gastrointestinal risks of antiplatelet therapy and NSAID use: a report of the American College of Cardiology Foundation task force on clinical expert consensus documents.Circulation 2008; 118:1894–1909.
- Lanza FL, Chan FK, Quigley EM, et al. Guidelines forprevention of NSAID-related ulcer complications. Am JGastroenterol 2009; 104:728–738.
In Reply: I thank Dr. Keller for his interest in my review on the side effects and drug interactions of proton pump inhibitors (PPIs).1 In particular, the concern about the potentially increased risk of a cardiovascular event in patients taking a PPI while on clopidogrel is a matter of active research. Since the prevention of death, myocardial infarction, or stroke is the desired outcome in patients receiving antiplatelet therapy, any reduction in the antiplatelet effect of clopidogrel could put patients at increased risk. Because of the enormous number of patients on both PPIs and clopidogrel, investigators are studying the effect of PPIs on clopidogrel to determine the true significance in day-to-day practice. We should expect that the data will continue to evolve in the coming years as more research is done on this important interaction.
The FDA Web site that Dr. Keller brings up2 was posted a few months after the submission of my manuscript. But even with the FDA’s cautionary words, it is important to realize that the risk that purportedly exists with the interaction of omeprazole and clopidogrel and the suggestion for the alternative use of pantoprazole are both based on pharmacokinetic, pharmacodynamic, and epidemiologic studies, not on clinical outcome data.
As much as we would like to rely on such studies, pharmacokinetic and pharmacodynamic studies do not address clinical outcomes, and observational studies cannot account for every confounder, because patients in these studies are not randomly assigned to the intervention, which is the rationale behind the necessity for a prospective trial. The Clopidogrel and the Optimization of Gastrointestinal Events (COGENT) study,3 a prospective randomized controlled trial with 3,761 analyzed patients, found no differences in adjudicated cardiovascular outcomes between groups who received a clopidogrel plus omeprazole vs clopidogrel alone.3 Although the COGENT study ended prematurely because of bankruptcy of the funding source, these outcomes represent the only randomized prospective data that can be found to date on PubMed. With such large numbers of patients in each group (1,876 and 1,885, respectively) and no differences in outcomes, it stands to reason that only a study with massive sample sizes would be able to detect a statistically significant difference. Differences between clopidogrel-treated patients taking and not taking omeprazole are likely be found in a well-designed prospective trial; however, it would be virtually impossible to find differences among PPIs.
To make matters even less convincing that therapy should be altered, the Working Group on High On-treatment Platelet Reactivity stated in their recent consensus paper that there are “limited data to support that alteration of therapy based on platelet function measurements actually improves outcomes.” 4 Additionally, a recent multisociety Expert Consensus Document discussing the concomitant use of PPIs and thienopyridine drugs to reduce gastrointestinal complications further supports this argument.5 Therefore, it is difficult to justify a marked increase in cost of the PPI selected (pantoprazole costs nearly seven times more per dose than omeprazole, according to one Web site6) for a benefit that is supported only by theoretical and observational data, not by outcome data.
As Dr. Keller also mentions, Aggrenox can be used for secondary stroke prophylaxis, but a discussion about a therapeutic exchange between clopidogrel and other antiplatelet agents was beyond the scope of my review. A recently published joint guideline of the American Heart Association and the American Stroke Association guideline should be consulted for further information.7
Other gastroprotective therapies are available. However, misoprostol (as mentioned) is associated with significant gastrointestinal side effects and must be taken four times a day. H2-receptor antagonists are not considered to be as effective as PPIs.8,9
In Reply: I thank Dr. Keller for his interest in my review on the side effects and drug interactions of proton pump inhibitors (PPIs).1 In particular, the concern about the potentially increased risk of a cardiovascular event in patients taking a PPI while on clopidogrel is a matter of active research. Since the prevention of death, myocardial infarction, or stroke is the desired outcome in patients receiving antiplatelet therapy, any reduction in the antiplatelet effect of clopidogrel could put patients at increased risk. Because of the enormous number of patients on both PPIs and clopidogrel, investigators are studying the effect of PPIs on clopidogrel to determine the true significance in day-to-day practice. We should expect that the data will continue to evolve in the coming years as more research is done on this important interaction.
The FDA Web site that Dr. Keller brings up2 was posted a few months after the submission of my manuscript. But even with the FDA’s cautionary words, it is important to realize that the risk that purportedly exists with the interaction of omeprazole and clopidogrel and the suggestion for the alternative use of pantoprazole are both based on pharmacokinetic, pharmacodynamic, and epidemiologic studies, not on clinical outcome data.
As much as we would like to rely on such studies, pharmacokinetic and pharmacodynamic studies do not address clinical outcomes, and observational studies cannot account for every confounder, because patients in these studies are not randomly assigned to the intervention, which is the rationale behind the necessity for a prospective trial. The Clopidogrel and the Optimization of Gastrointestinal Events (COGENT) study,3 a prospective randomized controlled trial with 3,761 analyzed patients, found no differences in adjudicated cardiovascular outcomes between groups who received a clopidogrel plus omeprazole vs clopidogrel alone.3 Although the COGENT study ended prematurely because of bankruptcy of the funding source, these outcomes represent the only randomized prospective data that can be found to date on PubMed. With such large numbers of patients in each group (1,876 and 1,885, respectively) and no differences in outcomes, it stands to reason that only a study with massive sample sizes would be able to detect a statistically significant difference. Differences between clopidogrel-treated patients taking and not taking omeprazole are likely be found in a well-designed prospective trial; however, it would be virtually impossible to find differences among PPIs.
To make matters even less convincing that therapy should be altered, the Working Group on High On-treatment Platelet Reactivity stated in their recent consensus paper that there are “limited data to support that alteration of therapy based on platelet function measurements actually improves outcomes.” 4 Additionally, a recent multisociety Expert Consensus Document discussing the concomitant use of PPIs and thienopyridine drugs to reduce gastrointestinal complications further supports this argument.5 Therefore, it is difficult to justify a marked increase in cost of the PPI selected (pantoprazole costs nearly seven times more per dose than omeprazole, according to one Web site6) for a benefit that is supported only by theoretical and observational data, not by outcome data.
As Dr. Keller also mentions, Aggrenox can be used for secondary stroke prophylaxis, but a discussion about a therapeutic exchange between clopidogrel and other antiplatelet agents was beyond the scope of my review. A recently published joint guideline of the American Heart Association and the American Stroke Association guideline should be consulted for further information.7
Other gastroprotective therapies are available. However, misoprostol (as mentioned) is associated with significant gastrointestinal side effects and must be taken four times a day. H2-receptor antagonists are not considered to be as effective as PPIs.8,9
- Madanick RD. Proton pump inhibitor side effects and drug interactions: much ado about nothing? Cleve Clin J Med 2011; 78:39–49.
- US Food and Drug Administration. FDA reminder to avoid concomitant use of Plavix (clopidogrel) and omeprazole. www.fda.gov/Drugs/DrugSafety/ucm231161.htm. Accessed March 23, 2011.
- Bhatt DL, Cryer BL, Contant CF, et al. Clopidogrel with or without omeprazole in coronary artery disease. N Engl J Med 2010; 363:1909–1917.
- Bonello L, Tantry US, Marcucci R, et al. Consensus and future directions on the definition of high on-treatment platelet reactivity to adenosine diphosphate. J Am Coll Cardiol 2010; 56:919–33.
- Abraham NS, Hlatky MA, Antman EM, et al. ACCF/ACG/AHA 2010 expert consensus document on the concomitant use of proton pump inhibitors and thienopyridines:a focused update of the ACCF/ACG/AHA 2008 expert consensus document on reducing the gastrointestinal risks of antiplatelet therapy and NSAID use. Am J Gastroenterol 2010; 105:2533–2549.
- HealthWarehouse. www.healthwarehouse.com. Accessed March 23, 2011.
- Furie KL, Kasner SE, Adams RJ, et al. Guidelines for the prevention of stroke in patients with stroke or transient ischemic attack: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2011; 42:227–276.
- Bhatt DL, Scheiman J, Abraham NS, et al. ACCF/ACG/AHA 2008 expert consensus document on reducing the gastrointestinal risks of antiplatelet therapy and NSAID use: a report of the American College of Cardiology Foundation task force on clinical expert consensus documents.Circulation 2008; 118:1894–1909.
- Lanza FL, Chan FK, Quigley EM, et al. Guidelines forprevention of NSAID-related ulcer complications. Am JGastroenterol 2009; 104:728–738.
- Madanick RD. Proton pump inhibitor side effects and drug interactions: much ado about nothing? Cleve Clin J Med 2011; 78:39–49.
- US Food and Drug Administration. FDA reminder to avoid concomitant use of Plavix (clopidogrel) and omeprazole. www.fda.gov/Drugs/DrugSafety/ucm231161.htm. Accessed March 23, 2011.
- Bhatt DL, Cryer BL, Contant CF, et al. Clopidogrel with or without omeprazole in coronary artery disease. N Engl J Med 2010; 363:1909–1917.
- Bonello L, Tantry US, Marcucci R, et al. Consensus and future directions on the definition of high on-treatment platelet reactivity to adenosine diphosphate. J Am Coll Cardiol 2010; 56:919–33.
- Abraham NS, Hlatky MA, Antman EM, et al. ACCF/ACG/AHA 2010 expert consensus document on the concomitant use of proton pump inhibitors and thienopyridines:a focused update of the ACCF/ACG/AHA 2008 expert consensus document on reducing the gastrointestinal risks of antiplatelet therapy and NSAID use. Am J Gastroenterol 2010; 105:2533–2549.
- HealthWarehouse. www.healthwarehouse.com. Accessed March 23, 2011.
- Furie KL, Kasner SE, Adams RJ, et al. Guidelines for the prevention of stroke in patients with stroke or transient ischemic attack: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2011; 42:227–276.
- Bhatt DL, Scheiman J, Abraham NS, et al. ACCF/ACG/AHA 2008 expert consensus document on reducing the gastrointestinal risks of antiplatelet therapy and NSAID use: a report of the American College of Cardiology Foundation task force on clinical expert consensus documents.Circulation 2008; 118:1894–1909.
- Lanza FL, Chan FK, Quigley EM, et al. Guidelines forprevention of NSAID-related ulcer complications. Am JGastroenterol 2009; 104:728–738.
Coadministration of clopidogrel and proton pump inhibitors
To the Editor: Thank you for the excellent review on proton pump inhibitors (PPIs) in the January 2011 issue.1 I would like to make the following comments about Dr. Madanick’s suggested algorithm (see Figure 2 in the article) for deciding whether to use a PPI in patients requiring clopidogrel:
A posting dated October 27, 2010, on the Web site of the US Food and Drug Administration (FDA) states the following: “With regard to the proton pump inhibitor (PPI) drug class, this recommendation [against the concomitant use of Plavix (clopidogrel) and omeprazole (Prilosec)] applies only to omeprazole and not to all PPIs. Not all PPIs have the same inhibitory effect on the enzyme [CYP2C19] that is crucial for conversion of Plavix into its active form. Pantoprazole (Protonix) may be an alternative PPI for consideration. It is a weak inhibitor of CYP2C19 and has less effect on the pharmacological activity of Plavix than omeprazole.”2 Thus, when it is deemed necessary to coadminister clopidogrel with a PPI, pantoprazole appears to be the preferred PPI.
If the patient is taking clopidogrel for stroke prophylaxis, one can consider switching to Aggrenox (aspirin plus extended-release dipyridamole), which has no warnings regarding coadministration with PPIs.
Patients taking aspirin plus clopidogrel may benefit by the addition of misoprostol (Cytotec), which is indicated for reducing the risk of aspirin-induced gastric ulcers in patients at high risk of complications from gastric ulcer.
- Madanick RD. Proton pump inhibitor side effects and drug interactions: much ado about nothing? Cleve Clin J Med 2011; 78:39–49.
- US Food and Drug Administration. FDA reminder to avoid concomitant use of Plavix (clopidogrel) and omeprazole. www.fda.gov/Drugs/DrugSafety/ucm231161.htm. Accessed March 23, 2011.
To the Editor: Thank you for the excellent review on proton pump inhibitors (PPIs) in the January 2011 issue.1 I would like to make the following comments about Dr. Madanick’s suggested algorithm (see Figure 2 in the article) for deciding whether to use a PPI in patients requiring clopidogrel:
A posting dated October 27, 2010, on the Web site of the US Food and Drug Administration (FDA) states the following: “With regard to the proton pump inhibitor (PPI) drug class, this recommendation [against the concomitant use of Plavix (clopidogrel) and omeprazole (Prilosec)] applies only to omeprazole and not to all PPIs. Not all PPIs have the same inhibitory effect on the enzyme [CYP2C19] that is crucial for conversion of Plavix into its active form. Pantoprazole (Protonix) may be an alternative PPI for consideration. It is a weak inhibitor of CYP2C19 and has less effect on the pharmacological activity of Plavix than omeprazole.”2 Thus, when it is deemed necessary to coadminister clopidogrel with a PPI, pantoprazole appears to be the preferred PPI.
If the patient is taking clopidogrel for stroke prophylaxis, one can consider switching to Aggrenox (aspirin plus extended-release dipyridamole), which has no warnings regarding coadministration with PPIs.
Patients taking aspirin plus clopidogrel may benefit by the addition of misoprostol (Cytotec), which is indicated for reducing the risk of aspirin-induced gastric ulcers in patients at high risk of complications from gastric ulcer.
To the Editor: Thank you for the excellent review on proton pump inhibitors (PPIs) in the January 2011 issue.1 I would like to make the following comments about Dr. Madanick’s suggested algorithm (see Figure 2 in the article) for deciding whether to use a PPI in patients requiring clopidogrel:
A posting dated October 27, 2010, on the Web site of the US Food and Drug Administration (FDA) states the following: “With regard to the proton pump inhibitor (PPI) drug class, this recommendation [against the concomitant use of Plavix (clopidogrel) and omeprazole (Prilosec)] applies only to omeprazole and not to all PPIs. Not all PPIs have the same inhibitory effect on the enzyme [CYP2C19] that is crucial for conversion of Plavix into its active form. Pantoprazole (Protonix) may be an alternative PPI for consideration. It is a weak inhibitor of CYP2C19 and has less effect on the pharmacological activity of Plavix than omeprazole.”2 Thus, when it is deemed necessary to coadminister clopidogrel with a PPI, pantoprazole appears to be the preferred PPI.
If the patient is taking clopidogrel for stroke prophylaxis, one can consider switching to Aggrenox (aspirin plus extended-release dipyridamole), which has no warnings regarding coadministration with PPIs.
Patients taking aspirin plus clopidogrel may benefit by the addition of misoprostol (Cytotec), which is indicated for reducing the risk of aspirin-induced gastric ulcers in patients at high risk of complications from gastric ulcer.
- Madanick RD. Proton pump inhibitor side effects and drug interactions: much ado about nothing? Cleve Clin J Med 2011; 78:39–49.
- US Food and Drug Administration. FDA reminder to avoid concomitant use of Plavix (clopidogrel) and omeprazole. www.fda.gov/Drugs/DrugSafety/ucm231161.htm. Accessed March 23, 2011.
- Madanick RD. Proton pump inhibitor side effects and drug interactions: much ado about nothing? Cleve Clin J Med 2011; 78:39–49.
- US Food and Drug Administration. FDA reminder to avoid concomitant use of Plavix (clopidogrel) and omeprazole. www.fda.gov/Drugs/DrugSafety/ucm231161.htm. Accessed March 23, 2011.
Fear and Loathing on the Interview Trail
As we strive to provide the most advanced care possible to our patients with vascular disease, there is one area of our practice that stands out as being as archaic as cellophane wrapping of aneurysms: the process of finding a job.
Instead of an enlightening quest to find the perfect practice, many graduating vascular fellows have found it to be a tiring and laborious process. The excitement around the opportunity to finally do what we have trained so long and hard to do has been eclipsed in frustration by a disorganized and antiquated interview process.
The business side of medicine has very much been on display in the popular media with the institution of new healthcare reform legislation. No financially viable business would fly a client halfway across the country to find out if they're a 'good guy' prior to discussing a potential deal. This should not be the routine in medicine, either.
I implore vascular surgeons to utilize technology to modernize the process of expanding their practice. Replace the 'good old boy' and device rep networks with posts on the Society for Vascular Surgery (SVS) Job bank. Loose the recruiters and write a concise description of your current practice and what you have to offer. Replace the mandatory meet and greet first interview with a video conference via SKYPE. This will obviate the need to clear your schedule for the day and shelve the awkward interview in the operating theater.
A colleague of mine has equated interviewing for a vascular surgery job with trying to buy a used car without knowing the price. Even if it requires a non-disclosure agreement, opening the books to a prospective partner early in the interview process is vital. In addition to demonstrating integrity and building good will, it validates your case mix and volume. Without this knowledge, an applicant cannot make an informed decision to join a practice.
Most importantly, be professional. If a candidate is not what you're looking for, be straightforward and tactful. Communicate; don't string someone along in case another prospect falls through.
For those of you who are getting ready to or are still looking for your first job- do your homework. Converse with your faculty members, get in touch with the local device reps, and talk to former fellows about your job prospects. On the interview don't hesitate to pull the ancillary staff aside for their opinion of the group. One of my co-fellows did this and learned that the surgeon had not booked a case in the operating room for six months. The hospital administrators confessed that they were interviewing to replace the surgeon, not hire on a partner.
There are a multitude of resources available to arm yourself when it comes to contract negotiations. Read "The Physician's Comprehensive Guide to Negotiating" (SEAK, Inc.) by Steven Babitsky and James J. Mangraviti, Jr. I obtained a copy through interlibrary loan and read it in two days between cases. It is well worth the small time investment.
Call your institution's physician contract liaison to review a sample contract and gain access to the MGMA physician salary survey. Remember these data includes responses from vascular surgeons in both private practice and academics and may not reflect your true market value. Talk to the fellows who graduated before you to get an idea of what range of offers are out there.
Attend regional and national vascular surgery conferences to network and gain leads. The SVS young surgeon's forum at the annual meeting is a good introduction into the job search process. The most comprehensive review to date is the Mote Vascular Symposium put on by Dr. Russell H. Samson's group out of Sarasota, FL. Beg, borrow, or steal the weekend off to attend this meeting.
Our goal as graduating fellows is to not only provide exceptional care to the patients with vascular disease in the communities we join, but to modernize the practice of our partners. Whether this means an aggressive endovascular approach to aneurysmal disease or overseeing the redesign of the practice's website, by virtue of training in the information age we are well prepared.
Every page of this newspaper is geared toward making us better vascular surgeons. So please, let's start by bringing the interview process in to the 21st century.
Christopher Everett, M.D., is a Vascular Surgery Fellow, year two, at the Greenville University Hospital Medical Center, Greenville, SC.
As we strive to provide the most advanced care possible to our patients with vascular disease, there is one area of our practice that stands out as being as archaic as cellophane wrapping of aneurysms: the process of finding a job.
Instead of an enlightening quest to find the perfect practice, many graduating vascular fellows have found it to be a tiring and laborious process. The excitement around the opportunity to finally do what we have trained so long and hard to do has been eclipsed in frustration by a disorganized and antiquated interview process.
The business side of medicine has very much been on display in the popular media with the institution of new healthcare reform legislation. No financially viable business would fly a client halfway across the country to find out if they're a 'good guy' prior to discussing a potential deal. This should not be the routine in medicine, either.
I implore vascular surgeons to utilize technology to modernize the process of expanding their practice. Replace the 'good old boy' and device rep networks with posts on the Society for Vascular Surgery (SVS) Job bank. Loose the recruiters and write a concise description of your current practice and what you have to offer. Replace the mandatory meet and greet first interview with a video conference via SKYPE. This will obviate the need to clear your schedule for the day and shelve the awkward interview in the operating theater.
A colleague of mine has equated interviewing for a vascular surgery job with trying to buy a used car without knowing the price. Even if it requires a non-disclosure agreement, opening the books to a prospective partner early in the interview process is vital. In addition to demonstrating integrity and building good will, it validates your case mix and volume. Without this knowledge, an applicant cannot make an informed decision to join a practice.
Most importantly, be professional. If a candidate is not what you're looking for, be straightforward and tactful. Communicate; don't string someone along in case another prospect falls through.
For those of you who are getting ready to or are still looking for your first job- do your homework. Converse with your faculty members, get in touch with the local device reps, and talk to former fellows about your job prospects. On the interview don't hesitate to pull the ancillary staff aside for their opinion of the group. One of my co-fellows did this and learned that the surgeon had not booked a case in the operating room for six months. The hospital administrators confessed that they were interviewing to replace the surgeon, not hire on a partner.
There are a multitude of resources available to arm yourself when it comes to contract negotiations. Read "The Physician's Comprehensive Guide to Negotiating" (SEAK, Inc.) by Steven Babitsky and James J. Mangraviti, Jr. I obtained a copy through interlibrary loan and read it in two days between cases. It is well worth the small time investment.
Call your institution's physician contract liaison to review a sample contract and gain access to the MGMA physician salary survey. Remember these data includes responses from vascular surgeons in both private practice and academics and may not reflect your true market value. Talk to the fellows who graduated before you to get an idea of what range of offers are out there.
Attend regional and national vascular surgery conferences to network and gain leads. The SVS young surgeon's forum at the annual meeting is a good introduction into the job search process. The most comprehensive review to date is the Mote Vascular Symposium put on by Dr. Russell H. Samson's group out of Sarasota, FL. Beg, borrow, or steal the weekend off to attend this meeting.
Our goal as graduating fellows is to not only provide exceptional care to the patients with vascular disease in the communities we join, but to modernize the practice of our partners. Whether this means an aggressive endovascular approach to aneurysmal disease or overseeing the redesign of the practice's website, by virtue of training in the information age we are well prepared.
Every page of this newspaper is geared toward making us better vascular surgeons. So please, let's start by bringing the interview process in to the 21st century.
Christopher Everett, M.D., is a Vascular Surgery Fellow, year two, at the Greenville University Hospital Medical Center, Greenville, SC.
As we strive to provide the most advanced care possible to our patients with vascular disease, there is one area of our practice that stands out as being as archaic as cellophane wrapping of aneurysms: the process of finding a job.
Instead of an enlightening quest to find the perfect practice, many graduating vascular fellows have found it to be a tiring and laborious process. The excitement around the opportunity to finally do what we have trained so long and hard to do has been eclipsed in frustration by a disorganized and antiquated interview process.
The business side of medicine has very much been on display in the popular media with the institution of new healthcare reform legislation. No financially viable business would fly a client halfway across the country to find out if they're a 'good guy' prior to discussing a potential deal. This should not be the routine in medicine, either.
I implore vascular surgeons to utilize technology to modernize the process of expanding their practice. Replace the 'good old boy' and device rep networks with posts on the Society for Vascular Surgery (SVS) Job bank. Loose the recruiters and write a concise description of your current practice and what you have to offer. Replace the mandatory meet and greet first interview with a video conference via SKYPE. This will obviate the need to clear your schedule for the day and shelve the awkward interview in the operating theater.
A colleague of mine has equated interviewing for a vascular surgery job with trying to buy a used car without knowing the price. Even if it requires a non-disclosure agreement, opening the books to a prospective partner early in the interview process is vital. In addition to demonstrating integrity and building good will, it validates your case mix and volume. Without this knowledge, an applicant cannot make an informed decision to join a practice.
Most importantly, be professional. If a candidate is not what you're looking for, be straightforward and tactful. Communicate; don't string someone along in case another prospect falls through.
For those of you who are getting ready to or are still looking for your first job- do your homework. Converse with your faculty members, get in touch with the local device reps, and talk to former fellows about your job prospects. On the interview don't hesitate to pull the ancillary staff aside for their opinion of the group. One of my co-fellows did this and learned that the surgeon had not booked a case in the operating room for six months. The hospital administrators confessed that they were interviewing to replace the surgeon, not hire on a partner.
There are a multitude of resources available to arm yourself when it comes to contract negotiations. Read "The Physician's Comprehensive Guide to Negotiating" (SEAK, Inc.) by Steven Babitsky and James J. Mangraviti, Jr. I obtained a copy through interlibrary loan and read it in two days between cases. It is well worth the small time investment.
Call your institution's physician contract liaison to review a sample contract and gain access to the MGMA physician salary survey. Remember these data includes responses from vascular surgeons in both private practice and academics and may not reflect your true market value. Talk to the fellows who graduated before you to get an idea of what range of offers are out there.
Attend regional and national vascular surgery conferences to network and gain leads. The SVS young surgeon's forum at the annual meeting is a good introduction into the job search process. The most comprehensive review to date is the Mote Vascular Symposium put on by Dr. Russell H. Samson's group out of Sarasota, FL. Beg, borrow, or steal the weekend off to attend this meeting.
Our goal as graduating fellows is to not only provide exceptional care to the patients with vascular disease in the communities we join, but to modernize the practice of our partners. Whether this means an aggressive endovascular approach to aneurysmal disease or overseeing the redesign of the practice's website, by virtue of training in the information age we are well prepared.
Every page of this newspaper is geared toward making us better vascular surgeons. So please, let's start by bringing the interview process in to the 21st century.
Christopher Everett, M.D., is a Vascular Surgery Fellow, year two, at the Greenville University Hospital Medical Center, Greenville, SC.
About The Residents' Forum
With this issue, we begin a new column which we have chosen to call Residents' Forum. We hope that Vascular Residents, Fellows, and others interested in entering this exciting field will consider submitting an essay on topics important to them related to vascular surgery and their careers.
-George Andros, M.D., Medical Editor
With this issue, we begin a new column which we have chosen to call Residents' Forum. We hope that Vascular Residents, Fellows, and others interested in entering this exciting field will consider submitting an essay on topics important to them related to vascular surgery and their careers.
-George Andros, M.D., Medical Editor
With this issue, we begin a new column which we have chosen to call Residents' Forum. We hope that Vascular Residents, Fellows, and others interested in entering this exciting field will consider submitting an essay on topics important to them related to vascular surgery and their careers.
-George Andros, M.D., Medical Editor
Presentation, Not Treatment, May Determine Cost in CLI
LAKE BUENA VISTA, FLA. - Endovascular treatment for critical limb ischemia offered no significant cost savings over open repair in a recent analysis of outcomes in 137 patients.
Of the 148 patients included in the retrospective review, 42% were treated with an endovascular procedure, 47% with an open procedure, and the remaining with a hybrid of the two. The mean costs were $49,802 for an endovascular hospitalization and $45,832 for an open repair hospitalization; these amounts were not significantly different, Dr. Nicholas Gargiulo III reported at the annual meeting of the Society for Clinical Vascular Surgery.
The mean lengths of stay were also similar, at 9.3 days and 10.4 days in the endovascular and open repair groups, respectively, said Dr. Gargiulo of Montefiore Medical Center, New York.
The percentage of patients discharged to a skilled nursing facility was 35% vs. 44% of endovascular and open repair patients, respectively. Although endovascular repair was associated with slightly increased likelihood of recovering enough function to be released to home upon hospital discharge, this difference also did not reach statistical significance.
Rates of readmission within 90 days were similar at 12% and 13% for the endovascular repair and open repair patients, respectively.
For hybrid repair patients, the hospitalization costs ($27,922) and length of stay (9.8 days) were lower, compared with the other groups. However, the readmission rate was much higher, at 50%, and the percentage discharged to home initially was lower at 28%.
The study included all of those patients who presented with critical limb ischemia at Montefiore Medical Center from January 1, 2007, through December 2007, for whom complete data were available. The patients, who had a mean age of 67 years and Rutherford Class 4 or 5 disease, underwent initial diagnostic evaluation with conventional arteriography, and the treatment approach was based on the anatomic TransAtlantic InterSociety Consensus II classification and adequate runoff.
A variety of endovascular interventions and open procedures were used. The perioperative mortality rate was 2.7%, and amputation-free survival was 94.6% at 1 year.
Most of the patients had hypertension and diabetes; a large variety of other comorbidities were seen as well.
About two-thirds of the cohort presented with rest pain, and the remainder presented with gangrene or ulceration. The hospitalization costs were higher in those who presented with gangrene and ulceration, Dr. Gargiulo said. Over the past year, more patients have been presenting with gangrene than with rest pain, and this is a concern, he added.
“Interestingly, the only thing that was different is that those with rest pain cost less than the patients with gangrene and ulceration … gangrene and ulceration increase the length of stay, increase readmission, and of course increase supplies and nursing services, resulting in an overall increase in mean cost,” Dr. Gargiulo said.
Possible cost-cutting measures include educational programs, new alliances with podiatry colleagues, prevention, and new trials, he added.
“In conclusion, endovascular and open procedures were equally cost effective in this diverse ethnic population of patients with critical limb ischemia, and patients with gangrene and ulceration have increased health care costs. It appears it's not the type of procedure which incurs cost, but the clinical presentation,” he said.
Dr. Gargiulo had no disclosures.
LAKE BUENA VISTA, FLA. - Endovascular treatment for critical limb ischemia offered no significant cost savings over open repair in a recent analysis of outcomes in 137 patients.
Of the 148 patients included in the retrospective review, 42% were treated with an endovascular procedure, 47% with an open procedure, and the remaining with a hybrid of the two. The mean costs were $49,802 for an endovascular hospitalization and $45,832 for an open repair hospitalization; these amounts were not significantly different, Dr. Nicholas Gargiulo III reported at the annual meeting of the Society for Clinical Vascular Surgery.
The mean lengths of stay were also similar, at 9.3 days and 10.4 days in the endovascular and open repair groups, respectively, said Dr. Gargiulo of Montefiore Medical Center, New York.
The percentage of patients discharged to a skilled nursing facility was 35% vs. 44% of endovascular and open repair patients, respectively. Although endovascular repair was associated with slightly increased likelihood of recovering enough function to be released to home upon hospital discharge, this difference also did not reach statistical significance.
Rates of readmission within 90 days were similar at 12% and 13% for the endovascular repair and open repair patients, respectively.
For hybrid repair patients, the hospitalization costs ($27,922) and length of stay (9.8 days) were lower, compared with the other groups. However, the readmission rate was much higher, at 50%, and the percentage discharged to home initially was lower at 28%.
The study included all of those patients who presented with critical limb ischemia at Montefiore Medical Center from January 1, 2007, through December 2007, for whom complete data were available. The patients, who had a mean age of 67 years and Rutherford Class 4 or 5 disease, underwent initial diagnostic evaluation with conventional arteriography, and the treatment approach was based on the anatomic TransAtlantic InterSociety Consensus II classification and adequate runoff.
A variety of endovascular interventions and open procedures were used. The perioperative mortality rate was 2.7%, and amputation-free survival was 94.6% at 1 year.
Most of the patients had hypertension and diabetes; a large variety of other comorbidities were seen as well.
About two-thirds of the cohort presented with rest pain, and the remainder presented with gangrene or ulceration. The hospitalization costs were higher in those who presented with gangrene and ulceration, Dr. Gargiulo said. Over the past year, more patients have been presenting with gangrene than with rest pain, and this is a concern, he added.
“Interestingly, the only thing that was different is that those with rest pain cost less than the patients with gangrene and ulceration … gangrene and ulceration increase the length of stay, increase readmission, and of course increase supplies and nursing services, resulting in an overall increase in mean cost,” Dr. Gargiulo said.
Possible cost-cutting measures include educational programs, new alliances with podiatry colleagues, prevention, and new trials, he added.
“In conclusion, endovascular and open procedures were equally cost effective in this diverse ethnic population of patients with critical limb ischemia, and patients with gangrene and ulceration have increased health care costs. It appears it's not the type of procedure which incurs cost, but the clinical presentation,” he said.
Dr. Gargiulo had no disclosures.
LAKE BUENA VISTA, FLA. - Endovascular treatment for critical limb ischemia offered no significant cost savings over open repair in a recent analysis of outcomes in 137 patients.
Of the 148 patients included in the retrospective review, 42% were treated with an endovascular procedure, 47% with an open procedure, and the remaining with a hybrid of the two. The mean costs were $49,802 for an endovascular hospitalization and $45,832 for an open repair hospitalization; these amounts were not significantly different, Dr. Nicholas Gargiulo III reported at the annual meeting of the Society for Clinical Vascular Surgery.
The mean lengths of stay were also similar, at 9.3 days and 10.4 days in the endovascular and open repair groups, respectively, said Dr. Gargiulo of Montefiore Medical Center, New York.
The percentage of patients discharged to a skilled nursing facility was 35% vs. 44% of endovascular and open repair patients, respectively. Although endovascular repair was associated with slightly increased likelihood of recovering enough function to be released to home upon hospital discharge, this difference also did not reach statistical significance.
Rates of readmission within 90 days were similar at 12% and 13% for the endovascular repair and open repair patients, respectively.
For hybrid repair patients, the hospitalization costs ($27,922) and length of stay (9.8 days) were lower, compared with the other groups. However, the readmission rate was much higher, at 50%, and the percentage discharged to home initially was lower at 28%.
The study included all of those patients who presented with critical limb ischemia at Montefiore Medical Center from January 1, 2007, through December 2007, for whom complete data were available. The patients, who had a mean age of 67 years and Rutherford Class 4 or 5 disease, underwent initial diagnostic evaluation with conventional arteriography, and the treatment approach was based on the anatomic TransAtlantic InterSociety Consensus II classification and adequate runoff.
A variety of endovascular interventions and open procedures were used. The perioperative mortality rate was 2.7%, and amputation-free survival was 94.6% at 1 year.
Most of the patients had hypertension and diabetes; a large variety of other comorbidities were seen as well.
About two-thirds of the cohort presented with rest pain, and the remainder presented with gangrene or ulceration. The hospitalization costs were higher in those who presented with gangrene and ulceration, Dr. Gargiulo said. Over the past year, more patients have been presenting with gangrene than with rest pain, and this is a concern, he added.
“Interestingly, the only thing that was different is that those with rest pain cost less than the patients with gangrene and ulceration … gangrene and ulceration increase the length of stay, increase readmission, and of course increase supplies and nursing services, resulting in an overall increase in mean cost,” Dr. Gargiulo said.
Possible cost-cutting measures include educational programs, new alliances with podiatry colleagues, prevention, and new trials, he added.
“In conclusion, endovascular and open procedures were equally cost effective in this diverse ethnic population of patients with critical limb ischemia, and patients with gangrene and ulceration have increased health care costs. It appears it's not the type of procedure which incurs cost, but the clinical presentation,” he said.
Dr. Gargiulo had no disclosures.
Foam Sclerotherapy: As Effective as Surgery?
SAN DIEGO - At 5 years of follow-up, patients who had ultrasound-guided foam sclerotherapy combined with saphenofemoral ligation had equally good clinical results compared with patients who underwent surgical treatment of varicose veins, based on a randomized controlled trial.
"Since surgery may not provide a definitive treatment, foam sclerotherapy could be offered like a dental care treatment model: treating as and when the problem appears," Dr. Evi Kalodiki said at the annual meeting of the American Venous Forum.
Dr. Kalodiki, a vascular surgeon at Ealing Hospital and Imperial College, London, and her associates evaluated 82 legs that were treated in 73 patients. The mean age of the patients was 48 years, and 75% were female.
The researchers randomized the cases to two treatments: 39 legs underwent saphenofemoral ligation, stripping, and multiple phlebectomies under general anesthesia (surgery group) and 43 underwent saphenofemoral ligation under local anesthesia with concurrent foam sclerotherapy (foam group).
Assessments included ultrasound, the CEAP (clinical, etiologic, anatomical, pathophysiologic) classification, the Venous Clinical Severity Score, the Aberdeen Varicose Veins Questionnaire (AVVQ), and the Short Form-36 Health Survey (SF-36). Follow-ups were conducted annually to a median of 5.12 years.
Dr. Kalodiki reported that the CEAP classification was similar for the two groups, as was the percentage of legs that required additional foam sessions (40% of legs in the surgery group, with a mean volume of 11 mL for each case, vs. 48% of legs in the foam group, with a mean volume of 9 mL for each case.
Preoperative Venous Clinical Severity Scores (VCSS) were similar for the two groups (median of 5 in the surgery group vs. 4 in the foam group), as were post-treatment VCSS, at a median of 1 in each group.
Median changes in VCSS from baseline to 5 years were similar in the two groups: 3 in the surgery group vs. 3.5 in the foam group. Absolute VCSS values at 5 years were a median of 1 in each group.
The AVVQ score also improved in both groups. In the surgery group, the median AVVQ score improved from 16.32 preoperatively to 8.94 at 3 years, while in the foam group the median AVVQ score improved from 12.28 preoperatively to 4.97 at 3 years. These results were maintained at 5 years.
Scores on the mental component of the SF-36 worsened in the surgery group. There was no change in the physical scores of the SF-36 in either group over 3 or 5 years.
There were no significant differences between the groups or within the groups regarding obliteration or reflux above the knee or below the knee at 3 or 5 years. In the majority of cases, the reflux was asymptomatic and was only detected because of the follow-up duplex examination.
The study was funded by STD Pharmaceutical. Dr. Kalodiki said that she had no relevant financial disclosures.
This industry-funded study appears to have selected a population of relatively typical patients with symptomatic primary varicose veins. It is striking that fully 40% of patients in the surgical group had at least one follow-up foam session and not as surprising that the ligation and foam group required follow-up session(s). It would be interesting to know how many patients in each group required more than one postoperative foam session. The implication that we should approach foam therapy of veins "like a dental treatment model" suggests that treatment of neither group was particularly satisfactory. One also wonders about how variables beyond therapeutic efficacy, such as the fluid relationship between patient expectations and availability of services (syringes at the ready), affect findings of studies like these. Use of standardized outcome metrics and the SF-36 is laudable and can only help us find our way through the weeds and biases intrinsic to all clinical studies.
Magruder C. Donaldson, M.D., is chair of surgery at Metrowest Medical Center in Framingham, Mass. He is also an associate medical editor for Vascular Specialist.
This industry-funded study appears to have selected a population of relatively typical patients with symptomatic primary varicose veins. It is striking that fully 40% of patients in the surgical group had at least one follow-up foam session and not as surprising that the ligation and foam group required follow-up session(s). It would be interesting to know how many patients in each group required more than one postoperative foam session. The implication that we should approach foam therapy of veins "like a dental treatment model" suggests that treatment of neither group was particularly satisfactory. One also wonders about how variables beyond therapeutic efficacy, such as the fluid relationship between patient expectations and availability of services (syringes at the ready), affect findings of studies like these. Use of standardized outcome metrics and the SF-36 is laudable and can only help us find our way through the weeds and biases intrinsic to all clinical studies.
Magruder C. Donaldson, M.D., is chair of surgery at Metrowest Medical Center in Framingham, Mass. He is also an associate medical editor for Vascular Specialist.
This industry-funded study appears to have selected a population of relatively typical patients with symptomatic primary varicose veins. It is striking that fully 40% of patients in the surgical group had at least one follow-up foam session and not as surprising that the ligation and foam group required follow-up session(s). It would be interesting to know how many patients in each group required more than one postoperative foam session. The implication that we should approach foam therapy of veins "like a dental treatment model" suggests that treatment of neither group was particularly satisfactory. One also wonders about how variables beyond therapeutic efficacy, such as the fluid relationship between patient expectations and availability of services (syringes at the ready), affect findings of studies like these. Use of standardized outcome metrics and the SF-36 is laudable and can only help us find our way through the weeds and biases intrinsic to all clinical studies.
Magruder C. Donaldson, M.D., is chair of surgery at Metrowest Medical Center in Framingham, Mass. He is also an associate medical editor for Vascular Specialist.
SAN DIEGO - At 5 years of follow-up, patients who had ultrasound-guided foam sclerotherapy combined with saphenofemoral ligation had equally good clinical results compared with patients who underwent surgical treatment of varicose veins, based on a randomized controlled trial.
"Since surgery may not provide a definitive treatment, foam sclerotherapy could be offered like a dental care treatment model: treating as and when the problem appears," Dr. Evi Kalodiki said at the annual meeting of the American Venous Forum.
Dr. Kalodiki, a vascular surgeon at Ealing Hospital and Imperial College, London, and her associates evaluated 82 legs that were treated in 73 patients. The mean age of the patients was 48 years, and 75% were female.
The researchers randomized the cases to two treatments: 39 legs underwent saphenofemoral ligation, stripping, and multiple phlebectomies under general anesthesia (surgery group) and 43 underwent saphenofemoral ligation under local anesthesia with concurrent foam sclerotherapy (foam group).
Assessments included ultrasound, the CEAP (clinical, etiologic, anatomical, pathophysiologic) classification, the Venous Clinical Severity Score, the Aberdeen Varicose Veins Questionnaire (AVVQ), and the Short Form-36 Health Survey (SF-36). Follow-ups were conducted annually to a median of 5.12 years.
Dr. Kalodiki reported that the CEAP classification was similar for the two groups, as was the percentage of legs that required additional foam sessions (40% of legs in the surgery group, with a mean volume of 11 mL for each case, vs. 48% of legs in the foam group, with a mean volume of 9 mL for each case.
Preoperative Venous Clinical Severity Scores (VCSS) were similar for the two groups (median of 5 in the surgery group vs. 4 in the foam group), as were post-treatment VCSS, at a median of 1 in each group.
Median changes in VCSS from baseline to 5 years were similar in the two groups: 3 in the surgery group vs. 3.5 in the foam group. Absolute VCSS values at 5 years were a median of 1 in each group.
The AVVQ score also improved in both groups. In the surgery group, the median AVVQ score improved from 16.32 preoperatively to 8.94 at 3 years, while in the foam group the median AVVQ score improved from 12.28 preoperatively to 4.97 at 3 years. These results were maintained at 5 years.
Scores on the mental component of the SF-36 worsened in the surgery group. There was no change in the physical scores of the SF-36 in either group over 3 or 5 years.
There were no significant differences between the groups or within the groups regarding obliteration or reflux above the knee or below the knee at 3 or 5 years. In the majority of cases, the reflux was asymptomatic and was only detected because of the follow-up duplex examination.
The study was funded by STD Pharmaceutical. Dr. Kalodiki said that she had no relevant financial disclosures.
SAN DIEGO - At 5 years of follow-up, patients who had ultrasound-guided foam sclerotherapy combined with saphenofemoral ligation had equally good clinical results compared with patients who underwent surgical treatment of varicose veins, based on a randomized controlled trial.
"Since surgery may not provide a definitive treatment, foam sclerotherapy could be offered like a dental care treatment model: treating as and when the problem appears," Dr. Evi Kalodiki said at the annual meeting of the American Venous Forum.
Dr. Kalodiki, a vascular surgeon at Ealing Hospital and Imperial College, London, and her associates evaluated 82 legs that were treated in 73 patients. The mean age of the patients was 48 years, and 75% were female.
The researchers randomized the cases to two treatments: 39 legs underwent saphenofemoral ligation, stripping, and multiple phlebectomies under general anesthesia (surgery group) and 43 underwent saphenofemoral ligation under local anesthesia with concurrent foam sclerotherapy (foam group).
Assessments included ultrasound, the CEAP (clinical, etiologic, anatomical, pathophysiologic) classification, the Venous Clinical Severity Score, the Aberdeen Varicose Veins Questionnaire (AVVQ), and the Short Form-36 Health Survey (SF-36). Follow-ups were conducted annually to a median of 5.12 years.
Dr. Kalodiki reported that the CEAP classification was similar for the two groups, as was the percentage of legs that required additional foam sessions (40% of legs in the surgery group, with a mean volume of 11 mL for each case, vs. 48% of legs in the foam group, with a mean volume of 9 mL for each case.
Preoperative Venous Clinical Severity Scores (VCSS) were similar for the two groups (median of 5 in the surgery group vs. 4 in the foam group), as were post-treatment VCSS, at a median of 1 in each group.
Median changes in VCSS from baseline to 5 years were similar in the two groups: 3 in the surgery group vs. 3.5 in the foam group. Absolute VCSS values at 5 years were a median of 1 in each group.
The AVVQ score also improved in both groups. In the surgery group, the median AVVQ score improved from 16.32 preoperatively to 8.94 at 3 years, while in the foam group the median AVVQ score improved from 12.28 preoperatively to 4.97 at 3 years. These results were maintained at 5 years.
Scores on the mental component of the SF-36 worsened in the surgery group. There was no change in the physical scores of the SF-36 in either group over 3 or 5 years.
There were no significant differences between the groups or within the groups regarding obliteration or reflux above the knee or below the knee at 3 or 5 years. In the majority of cases, the reflux was asymptomatic and was only detected because of the follow-up duplex examination.
The study was funded by STD Pharmaceutical. Dr. Kalodiki said that she had no relevant financial disclosures.
Venous Surgery Compared To 'Conservative' Therapy
SAN DIEGO - Patients with chronic venous disease who were treated surgically were significantly more likely to experience relief of symptoms than were those who underwent conservative therapy, results from a single-center study showed.
“The interventional treatment of varicose veins is considered to be medically necessary by insurance policies if the patient remains symptomatic after compression stockings or conservative therapy,” Dr. Fedor Lurie said at the annual meeting of the American Venous Forum. “This is despite the fact that little is known about symptoms of chronic venous disease. Even less known is how the symptoms of chronic venous disease react to treatment.”
Dr. Lurie and his colleague, Dr. Robert L. Kistner, prospectively studied 150 patients (59 men, 91 women; mean age 55 years) with CEAP class 2-4 primary chronic venous disease who were treated at the Kistner Vein Clinic in Honolulu during a 12-month period. (CEAP is a classification system based on the elements of Clinical severity, Etiology or cause, Anatomy, and Pathophysiology.) Initial treatment consisted of compression stockings and other conservative measures, after which patients chose whether to continue conservative treatment or undergo surgery.
Patients completed the Specific Quality of Life and Outcome Response-Venous (SQOR-V) assessment prior to the initial visit, after completion of conservative treatment, and at 1- and 12-month follow-up visits after surgical treatment. The quality of life and symptom score components of this tool were analyzed separately.
Following conservative treatment, symptom scores improved in 85 patients (57%), while quality of life scores improved in 111 patients (74%). Despite this improvement, 121 patients (81%) chose to undergo surgical treatment.
During the 1-month follow-up after surgical treatment, symptom scores improved in 97 patients (80%), while quality of life scores improved in 114 patients (94%).
Dr. Lurie also reported that 51 of 65 patients who did not improve after conservative treatment were treated surgically. Of those 51, only 30 (59%) improved after surgery.
Patients whose symptom scores improved after conservative treatment were 15.2 times more likely to have symptom relief at 1 month and 21.3 times more likely to have symptom relief at 1 year than were those who had no improvement in symptom scores. They were also 9.4 times more likely to have improved quality of life at 1 month and 4.3 times more likely to have improved quality of life at 1 year.
“The relief of symptoms by conservative therapy is a good predictor of successful surgical treatment,” said Dr. Lurie, who is also a clinical professor of vascular surgery at the University of Hawaii. “These findings contradict the present practice of insurance policies that interpret the success of conservative measures as a contraindication to surgical treatment, and provide evidence that those who respond favorably to conservative treatment are the ones who will benefit greatly from surgical elimination of the venous reflux.”
He concluded by noting that when the treatment goal is relief of symptoms, “success of conservative therapy should be considered an indication for surgical treatment, and the failure of conservative therapy should be an indication for reconsideration of the true cause of the symptoms.”
Dr. Lurie and Dr. Kistner said that they had no relevant financial disclosures.
This is an interesting study from the point of view of insurance reimbursement for the treatment of varicose veins. It confirms that conservative therapy with stockings will improve symptoms in many patients but they still want to undergo more definitive therapy. The reasons for this are not explained but I would suggest that most patients find wearing stockings inconvenient and also they just do not appreciate the cosmetic issues associated with stocking therapy. Further, I would suggest that although many patients complain about symptoms the predominant issue that drives them to undergo varicose veins removal remains cosmetic. As such, one wonders why insurance carriers should pay for cosmetic vein removal. In this age of skyrocketing health care costs I believe it behooves the vein specialist to truly evaluate their patients to decide whether the patient is using “symptoms” to get insurance to reimburse them for cosmetic vein treatments. If cosmetic issues are predominant then perhaps the patient should bear the cost themselves.
Russell H. Samson, M.D., is a physician in the practice of Samson, Showalter, Lepore, and Nair, and clinical associate professor of surgery, Florida State University.
This is an interesting study from the point of view of insurance reimbursement for the treatment of varicose veins. It confirms that conservative therapy with stockings will improve symptoms in many patients but they still want to undergo more definitive therapy. The reasons for this are not explained but I would suggest that most patients find wearing stockings inconvenient and also they just do not appreciate the cosmetic issues associated with stocking therapy. Further, I would suggest that although many patients complain about symptoms the predominant issue that drives them to undergo varicose veins removal remains cosmetic. As such, one wonders why insurance carriers should pay for cosmetic vein removal. In this age of skyrocketing health care costs I believe it behooves the vein specialist to truly evaluate their patients to decide whether the patient is using “symptoms” to get insurance to reimburse them for cosmetic vein treatments. If cosmetic issues are predominant then perhaps the patient should bear the cost themselves.
Russell H. Samson, M.D., is a physician in the practice of Samson, Showalter, Lepore, and Nair, and clinical associate professor of surgery, Florida State University.
This is an interesting study from the point of view of insurance reimbursement for the treatment of varicose veins. It confirms that conservative therapy with stockings will improve symptoms in many patients but they still want to undergo more definitive therapy. The reasons for this are not explained but I would suggest that most patients find wearing stockings inconvenient and also they just do not appreciate the cosmetic issues associated with stocking therapy. Further, I would suggest that although many patients complain about symptoms the predominant issue that drives them to undergo varicose veins removal remains cosmetic. As such, one wonders why insurance carriers should pay for cosmetic vein removal. In this age of skyrocketing health care costs I believe it behooves the vein specialist to truly evaluate their patients to decide whether the patient is using “symptoms” to get insurance to reimburse them for cosmetic vein treatments. If cosmetic issues are predominant then perhaps the patient should bear the cost themselves.
Russell H. Samson, M.D., is a physician in the practice of Samson, Showalter, Lepore, and Nair, and clinical associate professor of surgery, Florida State University.
SAN DIEGO - Patients with chronic venous disease who were treated surgically were significantly more likely to experience relief of symptoms than were those who underwent conservative therapy, results from a single-center study showed.
“The interventional treatment of varicose veins is considered to be medically necessary by insurance policies if the patient remains symptomatic after compression stockings or conservative therapy,” Dr. Fedor Lurie said at the annual meeting of the American Venous Forum. “This is despite the fact that little is known about symptoms of chronic venous disease. Even less known is how the symptoms of chronic venous disease react to treatment.”
Dr. Lurie and his colleague, Dr. Robert L. Kistner, prospectively studied 150 patients (59 men, 91 women; mean age 55 years) with CEAP class 2-4 primary chronic venous disease who were treated at the Kistner Vein Clinic in Honolulu during a 12-month period. (CEAP is a classification system based on the elements of Clinical severity, Etiology or cause, Anatomy, and Pathophysiology.) Initial treatment consisted of compression stockings and other conservative measures, after which patients chose whether to continue conservative treatment or undergo surgery.
Patients completed the Specific Quality of Life and Outcome Response-Venous (SQOR-V) assessment prior to the initial visit, after completion of conservative treatment, and at 1- and 12-month follow-up visits after surgical treatment. The quality of life and symptom score components of this tool were analyzed separately.
Following conservative treatment, symptom scores improved in 85 patients (57%), while quality of life scores improved in 111 patients (74%). Despite this improvement, 121 patients (81%) chose to undergo surgical treatment.
During the 1-month follow-up after surgical treatment, symptom scores improved in 97 patients (80%), while quality of life scores improved in 114 patients (94%).
Dr. Lurie also reported that 51 of 65 patients who did not improve after conservative treatment were treated surgically. Of those 51, only 30 (59%) improved after surgery.
Patients whose symptom scores improved after conservative treatment were 15.2 times more likely to have symptom relief at 1 month and 21.3 times more likely to have symptom relief at 1 year than were those who had no improvement in symptom scores. They were also 9.4 times more likely to have improved quality of life at 1 month and 4.3 times more likely to have improved quality of life at 1 year.
“The relief of symptoms by conservative therapy is a good predictor of successful surgical treatment,” said Dr. Lurie, who is also a clinical professor of vascular surgery at the University of Hawaii. “These findings contradict the present practice of insurance policies that interpret the success of conservative measures as a contraindication to surgical treatment, and provide evidence that those who respond favorably to conservative treatment are the ones who will benefit greatly from surgical elimination of the venous reflux.”
He concluded by noting that when the treatment goal is relief of symptoms, “success of conservative therapy should be considered an indication for surgical treatment, and the failure of conservative therapy should be an indication for reconsideration of the true cause of the symptoms.”
Dr. Lurie and Dr. Kistner said that they had no relevant financial disclosures.
SAN DIEGO - Patients with chronic venous disease who were treated surgically were significantly more likely to experience relief of symptoms than were those who underwent conservative therapy, results from a single-center study showed.
“The interventional treatment of varicose veins is considered to be medically necessary by insurance policies if the patient remains symptomatic after compression stockings or conservative therapy,” Dr. Fedor Lurie said at the annual meeting of the American Venous Forum. “This is despite the fact that little is known about symptoms of chronic venous disease. Even less known is how the symptoms of chronic venous disease react to treatment.”
Dr. Lurie and his colleague, Dr. Robert L. Kistner, prospectively studied 150 patients (59 men, 91 women; mean age 55 years) with CEAP class 2-4 primary chronic venous disease who were treated at the Kistner Vein Clinic in Honolulu during a 12-month period. (CEAP is a classification system based on the elements of Clinical severity, Etiology or cause, Anatomy, and Pathophysiology.) Initial treatment consisted of compression stockings and other conservative measures, after which patients chose whether to continue conservative treatment or undergo surgery.
Patients completed the Specific Quality of Life and Outcome Response-Venous (SQOR-V) assessment prior to the initial visit, after completion of conservative treatment, and at 1- and 12-month follow-up visits after surgical treatment. The quality of life and symptom score components of this tool were analyzed separately.
Following conservative treatment, symptom scores improved in 85 patients (57%), while quality of life scores improved in 111 patients (74%). Despite this improvement, 121 patients (81%) chose to undergo surgical treatment.
During the 1-month follow-up after surgical treatment, symptom scores improved in 97 patients (80%), while quality of life scores improved in 114 patients (94%).
Dr. Lurie also reported that 51 of 65 patients who did not improve after conservative treatment were treated surgically. Of those 51, only 30 (59%) improved after surgery.
Patients whose symptom scores improved after conservative treatment were 15.2 times more likely to have symptom relief at 1 month and 21.3 times more likely to have symptom relief at 1 year than were those who had no improvement in symptom scores. They were also 9.4 times more likely to have improved quality of life at 1 month and 4.3 times more likely to have improved quality of life at 1 year.
“The relief of symptoms by conservative therapy is a good predictor of successful surgical treatment,” said Dr. Lurie, who is also a clinical professor of vascular surgery at the University of Hawaii. “These findings contradict the present practice of insurance policies that interpret the success of conservative measures as a contraindication to surgical treatment, and provide evidence that those who respond favorably to conservative treatment are the ones who will benefit greatly from surgical elimination of the venous reflux.”
He concluded by noting that when the treatment goal is relief of symptoms, “success of conservative therapy should be considered an indication for surgical treatment, and the failure of conservative therapy should be an indication for reconsideration of the true cause of the symptoms.”
Dr. Lurie and Dr. Kistner said that they had no relevant financial disclosures.
TAMARIS Trial: Gene Therapy Ineffective In CLI
CHICAGO - Gene therapy proved ineffective in critical limb ischemia patients with ischemic skin lesions unsuitable for revascularization in the largest-ever clinical trial of gene therapy for this application.
Results of the phase III, randomized, double-blind, 525-patient TAMARIS study showed a 37% rate of death or major amputation at 1 year in recipients of the gene controlling for the expression of angiogenic fibroblast growth factor 1, compared with a 33% rate in placebo-treated controls.
“This was a definitively negative trial,” Dr. William R. Hiatt declared in presenting the TAMARIS results at the annual scientific sessions of the American Heart Association.
The TAMARIS findings are particularly disappointing in light of the earlier, highly impressive results of the phase II TALISMAN study. In that 125-patient study, the same gene delivery system, known as NV1FGF, resulted in a 56% reduction in the combined end point of death or major amputation at 1 year, said Dr. Hiatt, professor of medicine and cardiology at the University of Colorado, Denver.
The delivered gene, FGF1, codes for heparin-binding growth factor 1, which is a known angiogenic factor.
“I would merely say that this confirms the importance of large, well-conducted, randomized controlled trials. You really shouldn't make therapeutic decisions based on phase II trials,” he said.
Gene therapy recipients received four 4-mg intramuscular injections of NV1FGF over a 6-week period.
Dr. Hiatt said the concept of therapeutic angiogenesis “remains alive,” but the focus should shift to stem cell-based therapies, which show great promise.
Indeed, elsewhere at the meeting Dr. Douglas W. Losordo presented the results of a phase II study of autologous CD34+ cells for critical limb ischemia unsuitable for surgical or percutaneous revascularization. In that 28-patient study, cell therapy recipients had a 74% greater amputation-free survival rate at 1 year than did placebo-treated controls.
Dr. Losordo, the designated discussant for the TAMARIS trial, said one possible explanation for the divergent results relative to the earlier phase II TALISMAN study is that TAMARIS was conducted in 30 countries on multiple continents, whereas TALISMAN was carried out in a more genetically homogeneous European population. It's possible, he theorized, that there are genetic differences in critical limb ischemia and in the response to gene therapy.
New treatments for critical limb ischemia with tissue loss are desperately needed. There are 100,000 amputations per year in the United States for this condition. The amputation rate hasn't changed in 3 decades, noted Dr. Losordo, professor of medicine and director of the Feinberg Cardiovascular Research Institute at Northwestern University, Chicago.
Dr. Hiatt disclosed that he has received a research grant from Sanofi-Aventis, which funded the TAMARIS trial. The CD34+ cell therapy study was sponsored by Baxter Healthcare. Dr. Losordo declared that he was previously a paid consultant to Baxter.
New treatments for patients with critical limb ischemiaand tissue loss unsuitable for revascularizationare desperately needed. Unfortunately the present study fails to demonstrate a significant benefit from gene therapy utilizing gene controlling for expression of angiogenic fibroblast growth factor-1. Although it is suggested in this article that the focus should shift to stem cell-based therapies, this would also require confirmation with aphase -III, randomized double- blinded study. Certainly future research is very important to this challenging population of patients.
Charles Andersen, M.D., is chief of vascular/endo-vascular surgery at the Madigan Army Medical Center. He is also an associate medical editor for Vascular Specialist.
New treatments for patients with critical limb ischemiaand tissue loss unsuitable for revascularizationare desperately needed. Unfortunately the present study fails to demonstrate a significant benefit from gene therapy utilizing gene controlling for expression of angiogenic fibroblast growth factor-1. Although it is suggested in this article that the focus should shift to stem cell-based therapies, this would also require confirmation with aphase -III, randomized double- blinded study. Certainly future research is very important to this challenging population of patients.
Charles Andersen, M.D., is chief of vascular/endo-vascular surgery at the Madigan Army Medical Center. He is also an associate medical editor for Vascular Specialist.
New treatments for patients with critical limb ischemiaand tissue loss unsuitable for revascularizationare desperately needed. Unfortunately the present study fails to demonstrate a significant benefit from gene therapy utilizing gene controlling for expression of angiogenic fibroblast growth factor-1. Although it is suggested in this article that the focus should shift to stem cell-based therapies, this would also require confirmation with aphase -III, randomized double- blinded study. Certainly future research is very important to this challenging population of patients.
Charles Andersen, M.D., is chief of vascular/endo-vascular surgery at the Madigan Army Medical Center. He is also an associate medical editor for Vascular Specialist.
CHICAGO - Gene therapy proved ineffective in critical limb ischemia patients with ischemic skin lesions unsuitable for revascularization in the largest-ever clinical trial of gene therapy for this application.
Results of the phase III, randomized, double-blind, 525-patient TAMARIS study showed a 37% rate of death or major amputation at 1 year in recipients of the gene controlling for the expression of angiogenic fibroblast growth factor 1, compared with a 33% rate in placebo-treated controls.
“This was a definitively negative trial,” Dr. William R. Hiatt declared in presenting the TAMARIS results at the annual scientific sessions of the American Heart Association.
The TAMARIS findings are particularly disappointing in light of the earlier, highly impressive results of the phase II TALISMAN study. In that 125-patient study, the same gene delivery system, known as NV1FGF, resulted in a 56% reduction in the combined end point of death or major amputation at 1 year, said Dr. Hiatt, professor of medicine and cardiology at the University of Colorado, Denver.
The delivered gene, FGF1, codes for heparin-binding growth factor 1, which is a known angiogenic factor.
“I would merely say that this confirms the importance of large, well-conducted, randomized controlled trials. You really shouldn't make therapeutic decisions based on phase II trials,” he said.
Gene therapy recipients received four 4-mg intramuscular injections of NV1FGF over a 6-week period.
Dr. Hiatt said the concept of therapeutic angiogenesis “remains alive,” but the focus should shift to stem cell-based therapies, which show great promise.
Indeed, elsewhere at the meeting Dr. Douglas W. Losordo presented the results of a phase II study of autologous CD34+ cells for critical limb ischemia unsuitable for surgical or percutaneous revascularization. In that 28-patient study, cell therapy recipients had a 74% greater amputation-free survival rate at 1 year than did placebo-treated controls.
Dr. Losordo, the designated discussant for the TAMARIS trial, said one possible explanation for the divergent results relative to the earlier phase II TALISMAN study is that TAMARIS was conducted in 30 countries on multiple continents, whereas TALISMAN was carried out in a more genetically homogeneous European population. It's possible, he theorized, that there are genetic differences in critical limb ischemia and in the response to gene therapy.
New treatments for critical limb ischemia with tissue loss are desperately needed. There are 100,000 amputations per year in the United States for this condition. The amputation rate hasn't changed in 3 decades, noted Dr. Losordo, professor of medicine and director of the Feinberg Cardiovascular Research Institute at Northwestern University, Chicago.
Dr. Hiatt disclosed that he has received a research grant from Sanofi-Aventis, which funded the TAMARIS trial. The CD34+ cell therapy study was sponsored by Baxter Healthcare. Dr. Losordo declared that he was previously a paid consultant to Baxter.
CHICAGO - Gene therapy proved ineffective in critical limb ischemia patients with ischemic skin lesions unsuitable for revascularization in the largest-ever clinical trial of gene therapy for this application.
Results of the phase III, randomized, double-blind, 525-patient TAMARIS study showed a 37% rate of death or major amputation at 1 year in recipients of the gene controlling for the expression of angiogenic fibroblast growth factor 1, compared with a 33% rate in placebo-treated controls.
“This was a definitively negative trial,” Dr. William R. Hiatt declared in presenting the TAMARIS results at the annual scientific sessions of the American Heart Association.
The TAMARIS findings are particularly disappointing in light of the earlier, highly impressive results of the phase II TALISMAN study. In that 125-patient study, the same gene delivery system, known as NV1FGF, resulted in a 56% reduction in the combined end point of death or major amputation at 1 year, said Dr. Hiatt, professor of medicine and cardiology at the University of Colorado, Denver.
The delivered gene, FGF1, codes for heparin-binding growth factor 1, which is a known angiogenic factor.
“I would merely say that this confirms the importance of large, well-conducted, randomized controlled trials. You really shouldn't make therapeutic decisions based on phase II trials,” he said.
Gene therapy recipients received four 4-mg intramuscular injections of NV1FGF over a 6-week period.
Dr. Hiatt said the concept of therapeutic angiogenesis “remains alive,” but the focus should shift to stem cell-based therapies, which show great promise.
Indeed, elsewhere at the meeting Dr. Douglas W. Losordo presented the results of a phase II study of autologous CD34+ cells for critical limb ischemia unsuitable for surgical or percutaneous revascularization. In that 28-patient study, cell therapy recipients had a 74% greater amputation-free survival rate at 1 year than did placebo-treated controls.
Dr. Losordo, the designated discussant for the TAMARIS trial, said one possible explanation for the divergent results relative to the earlier phase II TALISMAN study is that TAMARIS was conducted in 30 countries on multiple continents, whereas TALISMAN was carried out in a more genetically homogeneous European population. It's possible, he theorized, that there are genetic differences in critical limb ischemia and in the response to gene therapy.
New treatments for critical limb ischemia with tissue loss are desperately needed. There are 100,000 amputations per year in the United States for this condition. The amputation rate hasn't changed in 3 decades, noted Dr. Losordo, professor of medicine and director of the Feinberg Cardiovascular Research Institute at Northwestern University, Chicago.
Dr. Hiatt disclosed that he has received a research grant from Sanofi-Aventis, which funded the TAMARIS trial. The CD34+ cell therapy study was sponsored by Baxter Healthcare. Dr. Losordo declared that he was previously a paid consultant to Baxter.