User login
Annual Meeting Mariner
It’s been one of those days. It all started at 4:30 this morning, when my 3-year-old son crawled into our bed, naked except for the diarrhea dripping down his leg. Turns out, this was his way—quite effective, I might add—of telling my wife and me that he had had an “accident.” After an hour of carrying soiled sheets to the washer, child-bathing, and Weimaraner coat-scrubbing, we relaxed to the sound of our 1-year-old daughter’s blood-curdling screams.
Upon examination, we found that the night had mysteriously transformed our precious little button-nosed bundle of joy into a tangle-haired, snot-nosed bundle of melancholy. Where her face used to be, there now hung something approximating the mask from that Scream movie. Additionally, her throat was raw, olive-sized lymph nodes populated her neck, and her nose had taken to perpetual booger-manufacturing. A rapid strep swab would later reveal the culprit, but at the moment, our differential tilted toward demonic possession.
That Dripping Feeling
Moments later, my wife and I picked 6:15 a.m. as the time to discover that we both had 7 a.m. meetings and no time to drop the kids off at daycare, especially when factoring in the 10-minute “discussion” we had about who was going to drop the kids off at daycare. All of this preceded my 7:10 a.m. arrival time for the 7 o’clock meeting with a hospital executive team to discuss our HM group funding for the next year—an encounter that left me feeling as my son must have just prior to crawling into bed with us that morning.
Now 8 a.m., I had to meet with a surgeon eager to unveil his “great idea” for our hospitalists to admit all of his patients. “It solves our problem of no interns, and allows you to play a meaningful role in the hospital!” he exclaimed.
“The meaningful role of intern?” I replied. Again, I had that dripping feeling.
It was 8:30 a.m. and I was ready to round on my patients. The first patient, a lovely woman, was stricken with un-insure-ia and a deep-seated belief that the inequitable health system that rendered her unable to get her surgery was clearly the result of some moral failing on my part. Next up was a spectacularly intoxicated male who welcomed my caring touch by belching a bit of breakfast burrito onto my cheek. Then it was a floridly bipolar patient whose apparent life mission was to drop her pants to show me her new mesh thong.
Burnout, Respect, Satisfaction
And so it continued until 1 p.m., when I had a meeting with a resident mentee of mine. It turns out that he wanted to tell me that despite his desire to be a hospitalist since his fourth year of medical school, he instead was going to apply to a rheumatology fellowship. After talking to several practicing hospitalists, he’d decided it just wasn’t for him—discussions he summarized as too much burnout, too little respect, and not enough satisfaction. Again, that dripping feeling.
Stuffing my face with a vending-machine carb-load that doubled as both breakfast and lunch, I sat down for a few minutes of e-mail. First up, a journal rejection of a research paper we’d recently submitted. Oh, the fulfillment of academics. Next were two e-mails that enzymatically trebled my “to do” list for the day. Sandwiched between those e-mails and one from a friend reminding me not to be late for a dinner that night that I was clearly going to be late for was an e-mail from a nice-appearing Nigerian man wanting to give me millions of dollars; at last, my day was turning around.
Alas, this was not the case. Checking my voicemail, I found out that my uncle was in the hospital, my dog’s lab tests were abnormal, my mom was angry about something, and I had missed a dentist appointment that morning. Finally, our group assistant came with a message that our prized hospitalist recruit had accepted a job at another institution. Drip … drip … drip…
Self-Reflection
Now 2:30 p.m., I took stock of my day and reflected on what my resident mentee had said about hospitalists. Trying to balance the rigors of patient care, academic requirements, life, friends, family, and being a boss, I was most definitely feeling a bit downtrodden, unsated, and crispy around the edges. What, exactly, did I like about this job? Was this what I wanted professionally? Would I ever find balance? Perhaps, too, a rheumatology application could salve my problems.
It was at that point that the notice for the SHM annual meeting appeared, oracle-like, on my desk. Picking it up, I realized this, not a two-year sojourn through the world of creaky joints, was the tonic to my problems.
Meeting Hierarchy
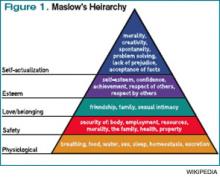
Every year since I began going to the SHM annual meeting in 2003, the meeting has helped me rejuvenate and grow. Much like Maslow’s Hierarchy of Needs, which posits that humans develop in stages that build on each other, I’ve found stepwise growth in the annual meeting.
Before my first meeting, I had been wandering nomadically through a hospitalist job for three years, wondering what, exactly, I was doing. I was the only hospitalist in my group, had few days off, with no support system around me. I had just agreed to take a job at another institution to build a new 10-person hospitalist group and had no idea how to do this. In Maslow terms, I was trying to satisfy my “physiologic needs” to survive. I needed to find, metaphorically, “food, water, clothes, and shelter.”
I found them at the annual meeting. The practice-management pre-course taught me how to build a hospitalist group, the mentorship breakfast introduced me to a veteran I still turn to, and the educational offerings helped improve my patient-care skills. I had conquered the base of Maslow’s pyramid.
The next year, I became involved in an SHM committee, and our gathering at the annual meeting helped set the course for our group’s future endeavors. I also met up with many friends I hadn’t seen since medical school and even recruited a person to my new group. In Maslow-speak, the meeting was helping me achieve my “safety needs” by providing control, well-being, and predictability.
By the third year, I was beginning to look forward to meeting up with national colleagues I had met at prior annual meetings, fulfilling Maslow’s third-stage need of “belonging.” During the ensuing years, I presented research projects, gave talks, and helped develop and lead forums and summits, thus quenching Maslow’s “self-esteem” need.
I wonder, as I leave my office to go back to see my afternoon complement of new patients, what my ninth annual meeting will bring. I’m not sure if I’ll ever achieve Maslow’s final phase of “self-actualization,” mostly because I’m not entirely sure what that means. However, I do know this: This job can be tough. We all feel it regardless of our age, gender, or practice setting. It is easy to get knocked out of balance, to get beaten down, to lose our focus. It is at those times that we all need a mariner to right the course. To remind us why we do this, to allow us to recharge, to facilitate our growth, to fulfill our needs.
For me, that mariner is the annual meeting. I look forward to seeing you all in Dallas next month. TH
Dr. Glasheen is associate professor of medicine at the University of Colorado at Denver, where he serves as director of the Hospital Medicine Program and the Hospitalist Training Program, and as associate program director of the Internal Medicine Residency Program.
It’s been one of those days. It all started at 4:30 this morning, when my 3-year-old son crawled into our bed, naked except for the diarrhea dripping down his leg. Turns out, this was his way—quite effective, I might add—of telling my wife and me that he had had an “accident.” After an hour of carrying soiled sheets to the washer, child-bathing, and Weimaraner coat-scrubbing, we relaxed to the sound of our 1-year-old daughter’s blood-curdling screams.
Upon examination, we found that the night had mysteriously transformed our precious little button-nosed bundle of joy into a tangle-haired, snot-nosed bundle of melancholy. Where her face used to be, there now hung something approximating the mask from that Scream movie. Additionally, her throat was raw, olive-sized lymph nodes populated her neck, and her nose had taken to perpetual booger-manufacturing. A rapid strep swab would later reveal the culprit, but at the moment, our differential tilted toward demonic possession.
That Dripping Feeling
Moments later, my wife and I picked 6:15 a.m. as the time to discover that we both had 7 a.m. meetings and no time to drop the kids off at daycare, especially when factoring in the 10-minute “discussion” we had about who was going to drop the kids off at daycare. All of this preceded my 7:10 a.m. arrival time for the 7 o’clock meeting with a hospital executive team to discuss our HM group funding for the next year—an encounter that left me feeling as my son must have just prior to crawling into bed with us that morning.
Now 8 a.m., I had to meet with a surgeon eager to unveil his “great idea” for our hospitalists to admit all of his patients. “It solves our problem of no interns, and allows you to play a meaningful role in the hospital!” he exclaimed.
“The meaningful role of intern?” I replied. Again, I had that dripping feeling.
It was 8:30 a.m. and I was ready to round on my patients. The first patient, a lovely woman, was stricken with un-insure-ia and a deep-seated belief that the inequitable health system that rendered her unable to get her surgery was clearly the result of some moral failing on my part. Next up was a spectacularly intoxicated male who welcomed my caring touch by belching a bit of breakfast burrito onto my cheek. Then it was a floridly bipolar patient whose apparent life mission was to drop her pants to show me her new mesh thong.
Burnout, Respect, Satisfaction
And so it continued until 1 p.m., when I had a meeting with a resident mentee of mine. It turns out that he wanted to tell me that despite his desire to be a hospitalist since his fourth year of medical school, he instead was going to apply to a rheumatology fellowship. After talking to several practicing hospitalists, he’d decided it just wasn’t for him—discussions he summarized as too much burnout, too little respect, and not enough satisfaction. Again, that dripping feeling.
Stuffing my face with a vending-machine carb-load that doubled as both breakfast and lunch, I sat down for a few minutes of e-mail. First up, a journal rejection of a research paper we’d recently submitted. Oh, the fulfillment of academics. Next were two e-mails that enzymatically trebled my “to do” list for the day. Sandwiched between those e-mails and one from a friend reminding me not to be late for a dinner that night that I was clearly going to be late for was an e-mail from a nice-appearing Nigerian man wanting to give me millions of dollars; at last, my day was turning around.
Alas, this was not the case. Checking my voicemail, I found out that my uncle was in the hospital, my dog’s lab tests were abnormal, my mom was angry about something, and I had missed a dentist appointment that morning. Finally, our group assistant came with a message that our prized hospitalist recruit had accepted a job at another institution. Drip … drip … drip…
Self-Reflection
Now 2:30 p.m., I took stock of my day and reflected on what my resident mentee had said about hospitalists. Trying to balance the rigors of patient care, academic requirements, life, friends, family, and being a boss, I was most definitely feeling a bit downtrodden, unsated, and crispy around the edges. What, exactly, did I like about this job? Was this what I wanted professionally? Would I ever find balance? Perhaps, too, a rheumatology application could salve my problems.
It was at that point that the notice for the SHM annual meeting appeared, oracle-like, on my desk. Picking it up, I realized this, not a two-year sojourn through the world of creaky joints, was the tonic to my problems.
Meeting Hierarchy
Every year since I began going to the SHM annual meeting in 2003, the meeting has helped me rejuvenate and grow. Much like Maslow’s Hierarchy of Needs, which posits that humans develop in stages that build on each other, I’ve found stepwise growth in the annual meeting.
Before my first meeting, I had been wandering nomadically through a hospitalist job for three years, wondering what, exactly, I was doing. I was the only hospitalist in my group, had few days off, with no support system around me. I had just agreed to take a job at another institution to build a new 10-person hospitalist group and had no idea how to do this. In Maslow terms, I was trying to satisfy my “physiologic needs” to survive. I needed to find, metaphorically, “food, water, clothes, and shelter.”
I found them at the annual meeting. The practice-management pre-course taught me how to build a hospitalist group, the mentorship breakfast introduced me to a veteran I still turn to, and the educational offerings helped improve my patient-care skills. I had conquered the base of Maslow’s pyramid.
The next year, I became involved in an SHM committee, and our gathering at the annual meeting helped set the course for our group’s future endeavors. I also met up with many friends I hadn’t seen since medical school and even recruited a person to my new group. In Maslow-speak, the meeting was helping me achieve my “safety needs” by providing control, well-being, and predictability.
By the third year, I was beginning to look forward to meeting up with national colleagues I had met at prior annual meetings, fulfilling Maslow’s third-stage need of “belonging.” During the ensuing years, I presented research projects, gave talks, and helped develop and lead forums and summits, thus quenching Maslow’s “self-esteem” need.
I wonder, as I leave my office to go back to see my afternoon complement of new patients, what my ninth annual meeting will bring. I’m not sure if I’ll ever achieve Maslow’s final phase of “self-actualization,” mostly because I’m not entirely sure what that means. However, I do know this: This job can be tough. We all feel it regardless of our age, gender, or practice setting. It is easy to get knocked out of balance, to get beaten down, to lose our focus. It is at those times that we all need a mariner to right the course. To remind us why we do this, to allow us to recharge, to facilitate our growth, to fulfill our needs.
For me, that mariner is the annual meeting. I look forward to seeing you all in Dallas next month. TH
Dr. Glasheen is associate professor of medicine at the University of Colorado at Denver, where he serves as director of the Hospital Medicine Program and the Hospitalist Training Program, and as associate program director of the Internal Medicine Residency Program.
It’s been one of those days. It all started at 4:30 this morning, when my 3-year-old son crawled into our bed, naked except for the diarrhea dripping down his leg. Turns out, this was his way—quite effective, I might add—of telling my wife and me that he had had an “accident.” After an hour of carrying soiled sheets to the washer, child-bathing, and Weimaraner coat-scrubbing, we relaxed to the sound of our 1-year-old daughter’s blood-curdling screams.
Upon examination, we found that the night had mysteriously transformed our precious little button-nosed bundle of joy into a tangle-haired, snot-nosed bundle of melancholy. Where her face used to be, there now hung something approximating the mask from that Scream movie. Additionally, her throat was raw, olive-sized lymph nodes populated her neck, and her nose had taken to perpetual booger-manufacturing. A rapid strep swab would later reveal the culprit, but at the moment, our differential tilted toward demonic possession.
That Dripping Feeling
Moments later, my wife and I picked 6:15 a.m. as the time to discover that we both had 7 a.m. meetings and no time to drop the kids off at daycare, especially when factoring in the 10-minute “discussion” we had about who was going to drop the kids off at daycare. All of this preceded my 7:10 a.m. arrival time for the 7 o’clock meeting with a hospital executive team to discuss our HM group funding for the next year—an encounter that left me feeling as my son must have just prior to crawling into bed with us that morning.
Now 8 a.m., I had to meet with a surgeon eager to unveil his “great idea” for our hospitalists to admit all of his patients. “It solves our problem of no interns, and allows you to play a meaningful role in the hospital!” he exclaimed.
“The meaningful role of intern?” I replied. Again, I had that dripping feeling.
It was 8:30 a.m. and I was ready to round on my patients. The first patient, a lovely woman, was stricken with un-insure-ia and a deep-seated belief that the inequitable health system that rendered her unable to get her surgery was clearly the result of some moral failing on my part. Next up was a spectacularly intoxicated male who welcomed my caring touch by belching a bit of breakfast burrito onto my cheek. Then it was a floridly bipolar patient whose apparent life mission was to drop her pants to show me her new mesh thong.
Burnout, Respect, Satisfaction
And so it continued until 1 p.m., when I had a meeting with a resident mentee of mine. It turns out that he wanted to tell me that despite his desire to be a hospitalist since his fourth year of medical school, he instead was going to apply to a rheumatology fellowship. After talking to several practicing hospitalists, he’d decided it just wasn’t for him—discussions he summarized as too much burnout, too little respect, and not enough satisfaction. Again, that dripping feeling.
Stuffing my face with a vending-machine carb-load that doubled as both breakfast and lunch, I sat down for a few minutes of e-mail. First up, a journal rejection of a research paper we’d recently submitted. Oh, the fulfillment of academics. Next were two e-mails that enzymatically trebled my “to do” list for the day. Sandwiched between those e-mails and one from a friend reminding me not to be late for a dinner that night that I was clearly going to be late for was an e-mail from a nice-appearing Nigerian man wanting to give me millions of dollars; at last, my day was turning around.
Alas, this was not the case. Checking my voicemail, I found out that my uncle was in the hospital, my dog’s lab tests were abnormal, my mom was angry about something, and I had missed a dentist appointment that morning. Finally, our group assistant came with a message that our prized hospitalist recruit had accepted a job at another institution. Drip … drip … drip…
Self-Reflection
Now 2:30 p.m., I took stock of my day and reflected on what my resident mentee had said about hospitalists. Trying to balance the rigors of patient care, academic requirements, life, friends, family, and being a boss, I was most definitely feeling a bit downtrodden, unsated, and crispy around the edges. What, exactly, did I like about this job? Was this what I wanted professionally? Would I ever find balance? Perhaps, too, a rheumatology application could salve my problems.
It was at that point that the notice for the SHM annual meeting appeared, oracle-like, on my desk. Picking it up, I realized this, not a two-year sojourn through the world of creaky joints, was the tonic to my problems.
Meeting Hierarchy
Every year since I began going to the SHM annual meeting in 2003, the meeting has helped me rejuvenate and grow. Much like Maslow’s Hierarchy of Needs, which posits that humans develop in stages that build on each other, I’ve found stepwise growth in the annual meeting.
Before my first meeting, I had been wandering nomadically through a hospitalist job for three years, wondering what, exactly, I was doing. I was the only hospitalist in my group, had few days off, with no support system around me. I had just agreed to take a job at another institution to build a new 10-person hospitalist group and had no idea how to do this. In Maslow terms, I was trying to satisfy my “physiologic needs” to survive. I needed to find, metaphorically, “food, water, clothes, and shelter.”
I found them at the annual meeting. The practice-management pre-course taught me how to build a hospitalist group, the mentorship breakfast introduced me to a veteran I still turn to, and the educational offerings helped improve my patient-care skills. I had conquered the base of Maslow’s pyramid.
The next year, I became involved in an SHM committee, and our gathering at the annual meeting helped set the course for our group’s future endeavors. I also met up with many friends I hadn’t seen since medical school and even recruited a person to my new group. In Maslow-speak, the meeting was helping me achieve my “safety needs” by providing control, well-being, and predictability.
By the third year, I was beginning to look forward to meeting up with national colleagues I had met at prior annual meetings, fulfilling Maslow’s third-stage need of “belonging.” During the ensuing years, I presented research projects, gave talks, and helped develop and lead forums and summits, thus quenching Maslow’s “self-esteem” need.
I wonder, as I leave my office to go back to see my afternoon complement of new patients, what my ninth annual meeting will bring. I’m not sure if I’ll ever achieve Maslow’s final phase of “self-actualization,” mostly because I’m not entirely sure what that means. However, I do know this: This job can be tough. We all feel it regardless of our age, gender, or practice setting. It is easy to get knocked out of balance, to get beaten down, to lose our focus. It is at those times that we all need a mariner to right the course. To remind us why we do this, to allow us to recharge, to facilitate our growth, to fulfill our needs.
For me, that mariner is the annual meeting. I look forward to seeing you all in Dallas next month. TH
Dr. Glasheen is associate professor of medicine at the University of Colorado at Denver, where he serves as director of the Hospital Medicine Program and the Hospitalist Training Program, and as associate program director of the Internal Medicine Residency Program.
The To-Don’t List
Last month, I wrote about the attributes of hospitalist practices that I associate with success. This month, I’ll do the opposite. That is, I’ll write about strategies your practice could, or even should, do without. Of course, all of these things are open to debate, and some thoughtful people might (and in my experience, probably will) arrive at different conclusions.
So I offer my list as food for thought, and if your practice relies on some of these strategies, you shouldn’t feel threatened by my opinion. But you might want to think about whether they’ve been made part of your practice by design, or if things just evolved this way without careful consideration of alternatives. I’ve listed them in no particular order.
Fixed-duration day shifts. My sense is that the majority of practices have a day shift with a predetermined start and end. That is, the hospitalist is expected to arrive and depart at the same time each day.
This seems to make a lot of sense, but it ignores the dramatic variations in workload a practice will have. For example, a practice that is appropriately staffed with four daytime hospitalists, and schedules each of them to work a 12-hour shift, provides 48 hours of daytime hospitalist manpower each day. But that will turn out to be precisely the right level of staffing only a few days a year. On all other days, daytime staffing will be optimal with a different number of hours. So it would make sense for the doctors to work more or less on those days.
Telling doctors that their shift always starts at the same time has significant lifestyle advantages. But it can inhibit the doctors who would be happy to start earlier to address more discharges early in the day and potentially go home earlier. So, just like most other doctors at your hospital have, why not let the doctors have significant latitude in when they start and stop working each day? In most cases, it might be necessary to have a time by which every doctor must be available to respond to pages (and one who must be on-site before the night doctor leaves), but they should feel free to actually arrive and start working when they choose. Most will make good choices and will likely feel a little more empowered and happy with their work.
And, at the end of the day, it might be reasonable to allow some of the day-shift doctors to leave when their work is done, and allow the others to stay to handle admissions until the night shift takes over. Those who leave early might still be required to respond to pages until a specified time.
Shifts that don’t involve rounding on “continuity” patients, such as night and evening (“swing”) shifts, usually should be arranged with predetermined start. I wrote in more detail on this topic in January 2007 and October 2010.
Contractual vacation provisions. Hospitalists should have significant amounts of time off. We work a lot of evenings, nights, and weekends, and we must have liberal amounts of time away from work. But for many practices, there is no advantage in classifying this time as vacation (or CME, etc.) time. In most cases, it makes the most sense to simply specify how much work (e.g. number of shifts) a doctor is to do each year and not specify a number of days or hours of vacation time. For more detail, read “The Vacation Conundrum” from March 2007.
If your practice has a vacation system that works well, then stick with it. But if you or your administrators are going nuts trying to categorize nonworking days between vacation and days the doctor simply wasn’t scheduled, then it might be best to stop trying. Just settle on the number of shifts (or some other metric) that a doctor is to work each year.
Tenure-based salary increases. It makes a lot of sense to pay doctors in most specialties an increasing salary based on his or her tenure with the practice. As they build a patient population and a referral stream, they generate more revenue and should benefit accordingly. But a new hospitalist who joins an existing group almost never has to build the referrals. In most cases, the group hired the doctor because the referrals are already coming and the practice needs more help, or the new doctor is replacing a departing one. So paying a new hospitalist a lower salary that increases automatically every few years isn’t really a raise earned by the doctor’s improved financial performance. Usually it’s just a system of withholding money that could be available for compensation for the doctor’s first few years in the practice. This lower starting salary might adversely impact recruiting. For more, see “Compensation Conundrum” from December 2009.
Poor roles for nonphysician providers (NPPs). I’ve worked with a lot of practices that have NPs and PAs (and, in some cases, RNs) who are doing what amounts to clerical work. They’re faxing discharge summaries, making calls to schedule patient appointments, dividing up the overnight admissions for the day rounders, etc.
Don’t make this mistake. Hire a secretary for that sort of work. And be sure that the roles occupied by trained clinicians (PAs, NPs, RNs, etc.) are professionally satisfying and will position them to make an effective contribution to the practice.
For more on this topic, see “The 411 on NPPs” from September 2008 and “Role Refinement” from September 2009; the latter features the perspective of Ryan Genzink, a thoughtful PA-C from Michigan.
Blinded performance reporting. First, make sure your practice provides regular, meaningful reports on each doctor’s performance and the group as a whole. This usually takes the form of a dashboard or report card. In my experience, too few practices do this. Make sure your group isn’t in that category.
Groups that do provide performance data often allow each doctor to see only his or her data. If data about other individuals in the group are provided, the names have often been removed. With exception of certain human resources issues (e.g. counseling a doctor to prevent termination), I think all performance data in the group should be shared by name with the whole group. In most practices, everyone should know by name which doctors are the high and low producers, each doctor’s compensation, and CPT coding practices (e.g. the portion of discharges coded at the high level).
When clinical performance can be attributed to individual providers, report those metrics openly, too. This usually creates greater cohesion within the group and helps foster a mentality of practice ownership. TH
Dr. Nelson has been a practicing hospitalist since 1988 and is co-founder and past president of SHM. He is a principal in Nelson Flores Hospital Medicine Consultants a national hospitalist practice management consulting firm (www.nelsonflores.com). He is course co-director and faculty for SHM’s “Best Practices in Managing a Hospital Medicine Program.” This column represents his views and is not intended to reflect an official position of SHM.
Last month, I wrote about the attributes of hospitalist practices that I associate with success. This month, I’ll do the opposite. That is, I’ll write about strategies your practice could, or even should, do without. Of course, all of these things are open to debate, and some thoughtful people might (and in my experience, probably will) arrive at different conclusions.
So I offer my list as food for thought, and if your practice relies on some of these strategies, you shouldn’t feel threatened by my opinion. But you might want to think about whether they’ve been made part of your practice by design, or if things just evolved this way without careful consideration of alternatives. I’ve listed them in no particular order.
Fixed-duration day shifts. My sense is that the majority of practices have a day shift with a predetermined start and end. That is, the hospitalist is expected to arrive and depart at the same time each day.
This seems to make a lot of sense, but it ignores the dramatic variations in workload a practice will have. For example, a practice that is appropriately staffed with four daytime hospitalists, and schedules each of them to work a 12-hour shift, provides 48 hours of daytime hospitalist manpower each day. But that will turn out to be precisely the right level of staffing only a few days a year. On all other days, daytime staffing will be optimal with a different number of hours. So it would make sense for the doctors to work more or less on those days.
Telling doctors that their shift always starts at the same time has significant lifestyle advantages. But it can inhibit the doctors who would be happy to start earlier to address more discharges early in the day and potentially go home earlier. So, just like most other doctors at your hospital have, why not let the doctors have significant latitude in when they start and stop working each day? In most cases, it might be necessary to have a time by which every doctor must be available to respond to pages (and one who must be on-site before the night doctor leaves), but they should feel free to actually arrive and start working when they choose. Most will make good choices and will likely feel a little more empowered and happy with their work.
And, at the end of the day, it might be reasonable to allow some of the day-shift doctors to leave when their work is done, and allow the others to stay to handle admissions until the night shift takes over. Those who leave early might still be required to respond to pages until a specified time.
Shifts that don’t involve rounding on “continuity” patients, such as night and evening (“swing”) shifts, usually should be arranged with predetermined start. I wrote in more detail on this topic in January 2007 and October 2010.
Contractual vacation provisions. Hospitalists should have significant amounts of time off. We work a lot of evenings, nights, and weekends, and we must have liberal amounts of time away from work. But for many practices, there is no advantage in classifying this time as vacation (or CME, etc.) time. In most cases, it makes the most sense to simply specify how much work (e.g. number of shifts) a doctor is to do each year and not specify a number of days or hours of vacation time. For more detail, read “The Vacation Conundrum” from March 2007.
If your practice has a vacation system that works well, then stick with it. But if you or your administrators are going nuts trying to categorize nonworking days between vacation and days the doctor simply wasn’t scheduled, then it might be best to stop trying. Just settle on the number of shifts (or some other metric) that a doctor is to work each year.
Tenure-based salary increases. It makes a lot of sense to pay doctors in most specialties an increasing salary based on his or her tenure with the practice. As they build a patient population and a referral stream, they generate more revenue and should benefit accordingly. But a new hospitalist who joins an existing group almost never has to build the referrals. In most cases, the group hired the doctor because the referrals are already coming and the practice needs more help, or the new doctor is replacing a departing one. So paying a new hospitalist a lower salary that increases automatically every few years isn’t really a raise earned by the doctor’s improved financial performance. Usually it’s just a system of withholding money that could be available for compensation for the doctor’s first few years in the practice. This lower starting salary might adversely impact recruiting. For more, see “Compensation Conundrum” from December 2009.
Poor roles for nonphysician providers (NPPs). I’ve worked with a lot of practices that have NPs and PAs (and, in some cases, RNs) who are doing what amounts to clerical work. They’re faxing discharge summaries, making calls to schedule patient appointments, dividing up the overnight admissions for the day rounders, etc.
Don’t make this mistake. Hire a secretary for that sort of work. And be sure that the roles occupied by trained clinicians (PAs, NPs, RNs, etc.) are professionally satisfying and will position them to make an effective contribution to the practice.
For more on this topic, see “The 411 on NPPs” from September 2008 and “Role Refinement” from September 2009; the latter features the perspective of Ryan Genzink, a thoughtful PA-C from Michigan.
Blinded performance reporting. First, make sure your practice provides regular, meaningful reports on each doctor’s performance and the group as a whole. This usually takes the form of a dashboard or report card. In my experience, too few practices do this. Make sure your group isn’t in that category.
Groups that do provide performance data often allow each doctor to see only his or her data. If data about other individuals in the group are provided, the names have often been removed. With exception of certain human resources issues (e.g. counseling a doctor to prevent termination), I think all performance data in the group should be shared by name with the whole group. In most practices, everyone should know by name which doctors are the high and low producers, each doctor’s compensation, and CPT coding practices (e.g. the portion of discharges coded at the high level).
When clinical performance can be attributed to individual providers, report those metrics openly, too. This usually creates greater cohesion within the group and helps foster a mentality of practice ownership. TH
Dr. Nelson has been a practicing hospitalist since 1988 and is co-founder and past president of SHM. He is a principal in Nelson Flores Hospital Medicine Consultants a national hospitalist practice management consulting firm (www.nelsonflores.com). He is course co-director and faculty for SHM’s “Best Practices in Managing a Hospital Medicine Program.” This column represents his views and is not intended to reflect an official position of SHM.
Last month, I wrote about the attributes of hospitalist practices that I associate with success. This month, I’ll do the opposite. That is, I’ll write about strategies your practice could, or even should, do without. Of course, all of these things are open to debate, and some thoughtful people might (and in my experience, probably will) arrive at different conclusions.
So I offer my list as food for thought, and if your practice relies on some of these strategies, you shouldn’t feel threatened by my opinion. But you might want to think about whether they’ve been made part of your practice by design, or if things just evolved this way without careful consideration of alternatives. I’ve listed them in no particular order.
Fixed-duration day shifts. My sense is that the majority of practices have a day shift with a predetermined start and end. That is, the hospitalist is expected to arrive and depart at the same time each day.
This seems to make a lot of sense, but it ignores the dramatic variations in workload a practice will have. For example, a practice that is appropriately staffed with four daytime hospitalists, and schedules each of them to work a 12-hour shift, provides 48 hours of daytime hospitalist manpower each day. But that will turn out to be precisely the right level of staffing only a few days a year. On all other days, daytime staffing will be optimal with a different number of hours. So it would make sense for the doctors to work more or less on those days.
Telling doctors that their shift always starts at the same time has significant lifestyle advantages. But it can inhibit the doctors who would be happy to start earlier to address more discharges early in the day and potentially go home earlier. So, just like most other doctors at your hospital have, why not let the doctors have significant latitude in when they start and stop working each day? In most cases, it might be necessary to have a time by which every doctor must be available to respond to pages (and one who must be on-site before the night doctor leaves), but they should feel free to actually arrive and start working when they choose. Most will make good choices and will likely feel a little more empowered and happy with their work.
And, at the end of the day, it might be reasonable to allow some of the day-shift doctors to leave when their work is done, and allow the others to stay to handle admissions until the night shift takes over. Those who leave early might still be required to respond to pages until a specified time.
Shifts that don’t involve rounding on “continuity” patients, such as night and evening (“swing”) shifts, usually should be arranged with predetermined start. I wrote in more detail on this topic in January 2007 and October 2010.
Contractual vacation provisions. Hospitalists should have significant amounts of time off. We work a lot of evenings, nights, and weekends, and we must have liberal amounts of time away from work. But for many practices, there is no advantage in classifying this time as vacation (or CME, etc.) time. In most cases, it makes the most sense to simply specify how much work (e.g. number of shifts) a doctor is to do each year and not specify a number of days or hours of vacation time. For more detail, read “The Vacation Conundrum” from March 2007.
If your practice has a vacation system that works well, then stick with it. But if you or your administrators are going nuts trying to categorize nonworking days between vacation and days the doctor simply wasn’t scheduled, then it might be best to stop trying. Just settle on the number of shifts (or some other metric) that a doctor is to work each year.
Tenure-based salary increases. It makes a lot of sense to pay doctors in most specialties an increasing salary based on his or her tenure with the practice. As they build a patient population and a referral stream, they generate more revenue and should benefit accordingly. But a new hospitalist who joins an existing group almost never has to build the referrals. In most cases, the group hired the doctor because the referrals are already coming and the practice needs more help, or the new doctor is replacing a departing one. So paying a new hospitalist a lower salary that increases automatically every few years isn’t really a raise earned by the doctor’s improved financial performance. Usually it’s just a system of withholding money that could be available for compensation for the doctor’s first few years in the practice. This lower starting salary might adversely impact recruiting. For more, see “Compensation Conundrum” from December 2009.
Poor roles for nonphysician providers (NPPs). I’ve worked with a lot of practices that have NPs and PAs (and, in some cases, RNs) who are doing what amounts to clerical work. They’re faxing discharge summaries, making calls to schedule patient appointments, dividing up the overnight admissions for the day rounders, etc.
Don’t make this mistake. Hire a secretary for that sort of work. And be sure that the roles occupied by trained clinicians (PAs, NPs, RNs, etc.) are professionally satisfying and will position them to make an effective contribution to the practice.
For more on this topic, see “The 411 on NPPs” from September 2008 and “Role Refinement” from September 2009; the latter features the perspective of Ryan Genzink, a thoughtful PA-C from Michigan.
Blinded performance reporting. First, make sure your practice provides regular, meaningful reports on each doctor’s performance and the group as a whole. This usually takes the form of a dashboard or report card. In my experience, too few practices do this. Make sure your group isn’t in that category.
Groups that do provide performance data often allow each doctor to see only his or her data. If data about other individuals in the group are provided, the names have often been removed. With exception of certain human resources issues (e.g. counseling a doctor to prevent termination), I think all performance data in the group should be shared by name with the whole group. In most practices, everyone should know by name which doctors are the high and low producers, each doctor’s compensation, and CPT coding practices (e.g. the portion of discharges coded at the high level).
When clinical performance can be attributed to individual providers, report those metrics openly, too. This usually creates greater cohesion within the group and helps foster a mentality of practice ownership. TH
Dr. Nelson has been a practicing hospitalist since 1988 and is co-founder and past president of SHM. He is a principal in Nelson Flores Hospital Medicine Consultants a national hospitalist practice management consulting firm (www.nelsonflores.com). He is course co-director and faculty for SHM’s “Best Practices in Managing a Hospital Medicine Program.” This column represents his views and is not intended to reflect an official position of SHM.
FDA Mandates Enrollment for Erythropoesis Stimulating Agents
I heard from my hospital’s pharmacist director that soon I will not be able to prescribe Epogen unless I get some additional certification. Is this true? Can you explain what is going on?
Ritesh Magge, MD,
Detroit
Dr. Hospitalist responds: Your pharmacist is referring to the new FDA-mandated requirements, which went into effect in February, that will affect who can prescribe erythropoesis-stimulating agents (ESAs), including epoetin (Epogen, Procrit) and darbepoetin (Aranesp). This is part of a broader FDA strategy to improve patient safety as it relates to medications.
According to the FDA website, “The Food and Drug Administration Amendments Act of 2007 gave FDA the authority to require a Risk Evaluation and Mitigation Strategy (REMS) from manufacturers to ensure that the benefits of a drug or biological product outweigh its risks.”
The ESAs are not the only class of drug that will require a REM. More than 150 drugs already have varying requirements. For a complete list of these medications, please visit the FDA website (www.fda.gov) and search “drug safety.” The FDA plans to create additional REMs for additional drugs and biologic agents.
If you are a provider who will prescribe a medication with a REM, you will be obligated to adhere to the FDA REM associated with that medication. The drug manufacturers are working with the FDA to set up REMs for their drugs. For example, if you plan to prescribe an ESA for a patient with cancer, you will first need to enroll in the ESA APPRISE (Assisting Providers and Cancer Patients with Risk Information for the Safe Use of ESAs) Oncology Program, which was established by ESA drug manufacturers. Once enrolled in the program, you will be assigned a unique ESA APPRISE ID number. ESA prescribers will be required to provide and review the ESA Medication Guide and counsel each patient on the risks and benefits of ESAs before each course of therapy. This counseling must be done no more than every 30 days after the first dose. (At the same time, prescribing ESAs to non-cancer patients basically only entails patient education.)
Providers also will be required to fill out the ESA APPRISE Oncology Program Patient and Healthcare Professional Acknowledgment form, which documents that the risk-benefit discussion occurred. This form is in triplicate and includes both the patient and the provider signature. One copy is given to the patient, another copy placed in the chart, and the third copy is stored for potential future audit.
For more information about the ESA APPRISE Oncology Program, please visit www.esaapprise.com/ESAAppriseUI/ESAAppriseUI/default.jsp. TH
I heard from my hospital’s pharmacist director that soon I will not be able to prescribe Epogen unless I get some additional certification. Is this true? Can you explain what is going on?
Ritesh Magge, MD,
Detroit
Dr. Hospitalist responds: Your pharmacist is referring to the new FDA-mandated requirements, which went into effect in February, that will affect who can prescribe erythropoesis-stimulating agents (ESAs), including epoetin (Epogen, Procrit) and darbepoetin (Aranesp). This is part of a broader FDA strategy to improve patient safety as it relates to medications.
According to the FDA website, “The Food and Drug Administration Amendments Act of 2007 gave FDA the authority to require a Risk Evaluation and Mitigation Strategy (REMS) from manufacturers to ensure that the benefits of a drug or biological product outweigh its risks.”
The ESAs are not the only class of drug that will require a REM. More than 150 drugs already have varying requirements. For a complete list of these medications, please visit the FDA website (www.fda.gov) and search “drug safety.” The FDA plans to create additional REMs for additional drugs and biologic agents.
If you are a provider who will prescribe a medication with a REM, you will be obligated to adhere to the FDA REM associated with that medication. The drug manufacturers are working with the FDA to set up REMs for their drugs. For example, if you plan to prescribe an ESA for a patient with cancer, you will first need to enroll in the ESA APPRISE (Assisting Providers and Cancer Patients with Risk Information for the Safe Use of ESAs) Oncology Program, which was established by ESA drug manufacturers. Once enrolled in the program, you will be assigned a unique ESA APPRISE ID number. ESA prescribers will be required to provide and review the ESA Medication Guide and counsel each patient on the risks and benefits of ESAs before each course of therapy. This counseling must be done no more than every 30 days after the first dose. (At the same time, prescribing ESAs to non-cancer patients basically only entails patient education.)
Providers also will be required to fill out the ESA APPRISE Oncology Program Patient and Healthcare Professional Acknowledgment form, which documents that the risk-benefit discussion occurred. This form is in triplicate and includes both the patient and the provider signature. One copy is given to the patient, another copy placed in the chart, and the third copy is stored for potential future audit.
For more information about the ESA APPRISE Oncology Program, please visit www.esaapprise.com/ESAAppriseUI/ESAAppriseUI/default.jsp. TH
I heard from my hospital’s pharmacist director that soon I will not be able to prescribe Epogen unless I get some additional certification. Is this true? Can you explain what is going on?
Ritesh Magge, MD,
Detroit
Dr. Hospitalist responds: Your pharmacist is referring to the new FDA-mandated requirements, which went into effect in February, that will affect who can prescribe erythropoesis-stimulating agents (ESAs), including epoetin (Epogen, Procrit) and darbepoetin (Aranesp). This is part of a broader FDA strategy to improve patient safety as it relates to medications.
According to the FDA website, “The Food and Drug Administration Amendments Act of 2007 gave FDA the authority to require a Risk Evaluation and Mitigation Strategy (REMS) from manufacturers to ensure that the benefits of a drug or biological product outweigh its risks.”
The ESAs are not the only class of drug that will require a REM. More than 150 drugs already have varying requirements. For a complete list of these medications, please visit the FDA website (www.fda.gov) and search “drug safety.” The FDA plans to create additional REMs for additional drugs and biologic agents.
If you are a provider who will prescribe a medication with a REM, you will be obligated to adhere to the FDA REM associated with that medication. The drug manufacturers are working with the FDA to set up REMs for their drugs. For example, if you plan to prescribe an ESA for a patient with cancer, you will first need to enroll in the ESA APPRISE (Assisting Providers and Cancer Patients with Risk Information for the Safe Use of ESAs) Oncology Program, which was established by ESA drug manufacturers. Once enrolled in the program, you will be assigned a unique ESA APPRISE ID number. ESA prescribers will be required to provide and review the ESA Medication Guide and counsel each patient on the risks and benefits of ESAs before each course of therapy. This counseling must be done no more than every 30 days after the first dose. (At the same time, prescribing ESAs to non-cancer patients basically only entails patient education.)
Providers also will be required to fill out the ESA APPRISE Oncology Program Patient and Healthcare Professional Acknowledgment form, which documents that the risk-benefit discussion occurred. This form is in triplicate and includes both the patient and the provider signature. One copy is given to the patient, another copy placed in the chart, and the third copy is stored for potential future audit.
For more information about the ESA APPRISE Oncology Program, please visit www.esaapprise.com/ESAAppriseUI/ESAAppriseUI/default.jsp. TH
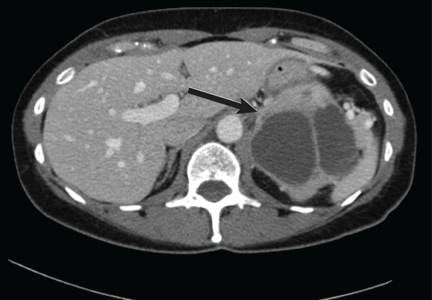
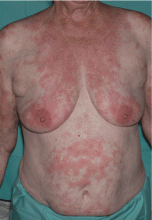
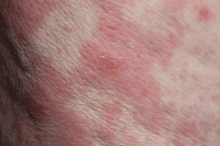
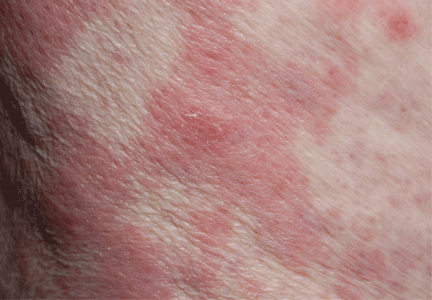
Emerging research on biomarkers that may help clarify diagnosis
Improving Medication Adherence in Chronic Disease Management
The magnitude of medication nonadherence and the consequent negative health impact are great and should create a sense of urgency among clinicians to address this serious problem.
The magnitude of medication nonadherence and the consequent negative health impact are great and should create a sense of urgency among clinicians to address this serious problem.
The magnitude of medication nonadherence and the consequent negative health impact are great and should create a sense of urgency among clinicians to address this serious problem.
Giant cell arteritis: Suspect it, treat it promptly
Giant cell arteritis is the most common primary systemic vasculitis. The disease occurs almost exclusively in people over age 50, with an annual incidence of 15 to 25 per 100,000.1 Incidence rates vary significantly depending on ethnicity. The highest rates are in whites, particularly those of North European descent.2 Incidence rates progressively increase after age 50. The disease is more prevalent in women. Its cause is unknown; both genetic and environmental factors are thought to play a role.
INFLAMED ARTERIES
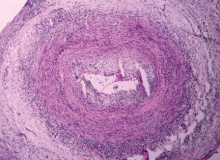
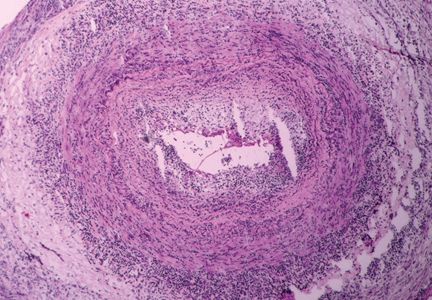
Giant cell arteritis is characterized by a granulomatous inflammatory infiltrate affecting large and medium-size arteries. Not all vessels are equally affected: the most susceptible are the cranial arteries, the aorta, and the aorta’s primary branches, particularly those in the upper extremities.
The disease is usually associated with an intense acute-phase response. Vessel wall inflammation results in intimal hyperplasia, luminal occlusion, and tissue ischemia. Typical histologic features include a mononuclear inflammatory infiltrate primarily composed of CD4+ T cells and activated macrophages. Multinucleated giant cells are seen in only about 50% of positive biopsies; therefore, their presence is not essential for the diagnosis.3
FOUR MAIN PHENOTYPES
Some of the possible symptoms of giant cell arteritis readily point to the correct diagnosis, eg, those due to cranial artery involvement, such as temporal headache, claudication of masticatory muscles, and visual changes. However, the clinical presentation can be quite varied.
There are four predominant clinical phenotypes, which may be present at the onset of disease or appear later as the disease progresses. Although they will be described separately in this review, these clinical presentations often overlap.
Cranial arteritis
Cranial arteritis is the clinical presentation most readily associated with giant cell arteritis. Clinical features result from involvement of branches of the external or internal carotid artery.
Headache, the most frequent symptom, is typically but not exclusively localized to the temporal areas.
Visual loss is due to involvement of the branches of the ophthalmic or posterior ciliary arteries, resulting in ischemia of the optic nerve or its tracts. It occurs in up to 20% of patients.4,5
Other symptoms and complications from cranial arteritis include tenderness of the scalp and temporal areas, claudication of the tongue or jaw muscles, stroke, and more rarely, tongue infarction.
Polymyalgia rheumatica
Polymyalgia rheumatica is a clinical syndrome that can occur by itself or in conjunction with giant cell arteritis. It may occur independently of giant cell arteritis, but also occurs in about 40% of patients with giant cell arteritis. It may precede, develop simultaneously with, or develop later during the course of the giant cell arteritis.6,7 It is a common clinical manifestation in relapses of giant cell arteritis, even in those who did not have symptoms of polymyalgia rheumatica at the time giant cell arteritis was diagnosed.
Polymyalgia rheumatica is characterized by aching of the shoulder and hip girdle, with morning stiffness. Fatigue and malaise are often present and may be severe. Some patients with polymyalgia rheumatica may also present with peripheral joint synovitis, which may be mistakenly diagnosed as rheumatoid arthritis.8 Muscle weakness and elevated muscle enzymes are not associated with polymyalgia rheumatica.
Polymyalgia rheumatica is a clinical diagnosis. Approximately 80% of patients with polymyalgia rheumatica have an elevated erythrocyte sedimentation rate or an elevated C-reactive protein level.9 When it occurs in the absence of giant cell arteritis, it is treated differently, with less intense doses of corticosteroids. All patients with polymyalgia rheumatica should be routinely questioned regarding symptoms of giant cell arteritis.
Nonspecific systemic inflammatory disease
Some patients with giant cell arteritis may present with a nonspecific systemic inflammatory disease characterized by some combination of fever, night sweats, fatigue, malaise, and weight loss. In these patients, the diagnosis may be delayed by the lack of localizing symptoms.
Laboratory tests typically show anemia, leukocytosis, and thrombocytosis. The erythrocyte sedimentation rate and the C-reactive protein level are usually very high.
Giant cell arteritis should be in the differential diagnosis when these signs and symptoms are found in patients over age 50.
Large-vessel vasculitis
Although thoracic aortic aneurysm and dissection have been described as late complications of giant cell arteritis, large-vessel vasculitis may precede or occur concomitantly with cranial arteritis early in the disease.10,11
Population-based surveys have shown that large-vessel vasculitis is extremely frequent in patients with giant cell arteritis. In a postmortem study of 11 patients with giant cell arteritis, all of them had evidence of arteritis involving the subclavian artery, the carotid artery, and the aorta.12
Patients may have no symptoms or may present with symptoms or signs of tissue ischemia such as claudication of the extremities, carotid artery tenderness, decreased or absent pulses, and large-vessel bruits on physical examination.
CONSIDER THE DIAGNOSIS IN OLDER PATIENTS
Giant cell arteritis should always be considered in patients over age 50 who have any of the clinical features described above. It is therefore very important to be familiar with its symptoms and signs.
A complete and detailed history and a detailed but focused physical examination that includes a comprehensive vascular examination are the first and most important steps in establishing the diagnosis. The vascular examination includes measuring the blood pressure in all four extremities, palpating the peripheral pulses, listening for bruits, and palpating the temporal arteries.
Temporal artery biopsy: The gold standard
Confirming the diagnosis of giant cell arteritis requires histologic findings of inflammation in the temporal artery. Superficial temporal artery biopsy is recommended for diagnostic confirmation in patients who have cranial symptoms and other signs suggesting the disease.
The biopsy should be performed on the same side as the symptoms or abnormal findings on examination. Performing a biopsy in both temporal arteries may increase the diagnostic yield but may not need to be done routinely.13
Although some experts recommend temporal artery biopsy in all patients in whom giant cell arteritis is suspected, biopsy has a lower diagnostic yield in patients who have no cranial symptoms. Interestingly, 5% to 15% of temporal artery biopsies performed in patients who had isolated polymyalgia rheumatica were found to be positive.14,15 Patients with polymyalgia rheumatica and no clinical symptoms to suggest giant cell arteritis generally are not biopsied.
The segmental nature of the inflammation involving the temporal artery in giant cell arteritis may result in negative biopsy results in patients with giant cell arteritis. A temporal artery biopsy length of 5 mm or less has a very low (8%) rate of positive results, whereas a length longer than 20 mm exceeds a 50% rate of positive results. Although the optimal length of a temporal artery specimen is still debated, a longer biopsy specimen should be obtained to increase the chance of arterial specimens showing inflammatory changes.16,17
Laboratory studies: Acute-phase reactants may be elevated
High levels of acute-phase reactants should increase one’s suspicion of giant cell arteritis. Elevations in the erythrocyte sedimentation rate and C-reactive protein and interleukin 6 levels reflect the inflammatory process in this disease.19 However, not all patients with giant cell arteritis have a high sedimentation rate, and as many as 20% of patients with biopsy-proven giant cell arteritis have a normal sedimentation rate before therapy.20 Therefore, a normal sedimentation rate does not exclude the diagnosis of giant cell arteritis and should not delay its diagnosis and treatment.
As a result of systemic inflammation, the patient may also present with normochromic normocytic anemia, leukocytosis, and thrombocytosis.
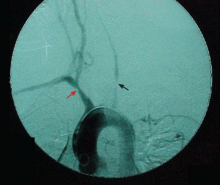
Imaging studies are controversial
Imaging studies are potentially useful diagnostic tools in large-vessel vasculitis but are still the subject of significant controversy.
Ultrasonography of the temporal artery has been a controversial subject in many studies.21,22 Color duplex ultrasonography of the temporal artery has been reported to be helpful in the diagnosis of giant cell arteritis (showing a “halo” around the arterial lumen), but further studies are needed to establish its clinical utility.
At this time, temporal artery biopsy remains the gold standard diagnostic test for giant cell arteritis, and ultrasonography is neither a substitute for biopsy nor a screening test for this disease.23 Some have suggested, however, that ultrasonography may help to identify the best site for biopsy of the temporal artery in some patients.
Arteriography is an accurate technique for evaluating the vessel lumen and allows for measuring central blood pressure and performing vascular interventions. However, because of potential complications, it has been largely replaced by noninvasive angiographic imaging to delineate vascular anatomy.
TREATMENT
Glucocorticoid therapy remains the standard of care
Once the diagnosis of giant cell arteritis is established, glucocorticoid treatment should be started. Glucocorticoids are the standard therapy, and they usually bring about a prompt clinical response. Although never evaluated in placebo-controlled trials, these drugs have been shown to prevent progression of visual loss in a retrospective study.26
In patients with visual symptoms or imminent visual loss, therapy should be started promptly once suspicion of giant cell arteritis is raised; ie, one should not wait until the diagnosis is confirmed by biopsy.
Ideally, a glucocorticoid should be started after a temporal artery biopsy is done, but treatment should not be delayed, as it rapidly suppresses the inflammatory response and may prevent complications from tissue ischemia, such as blindness. Visual loss is usually irreversible.
There is still a role for obtaining a temporal artery biopsy up to several weeks after glucocorticoid therapy is started, as the pathological abnormalities of arteritis do not rapidly resolve.27
Glucocorticoid therapy is highly effective in inducing disease remission in patients with giant cell arteritis. Nearly all patients respond to 1 mg/kg (40–60 mg) per day of prednisone or its equivalent.
The initial dosing is usually maintained for 4 weeks and then decreased slowly. The duration of therapy varies; most patients remain on therapy for at least 1 year, and some cannot stop it completely without recurrence of symptoms.
If a patient is about to lose his or her vision or has lost all or some vision in one eye, a higher initial dose of a glucocorticoid is usually used (ie, a pulse of 500 or 1,000 mg of intravenous methylprednisolone) and may be beneficial.28
Although a rapid clinical response to therapy is usually seen within 48 hours, some patients may have a more delayed clinical improvement.
Alternate-day therapy was compared with daily therapy and was found to be less effective, and as a result it is not recommended.29
Glucocorticoid therapy can cause significant toxicity in patients with giant cell arteritis, as they commonly must take these drugs for long periods. The rate of relapse in those who discontinue therapy is quite high—as high as 77% within 12 months.30
Given the concern about glucocorticoid toxicity, several studies have evaluated alternative strategies and other immunosuppressive drugs. However, no study has concluded that other medications are effective in the treatment of giant cell arteritis.
Mazlumzadeh et al31 evaluated the initial use of intravenous pulse methylprednisolone therapy (15 mg/kg ideal body weight on 3 consecutive days) in an attempt to decrease the glucocorticoid requirement. Although the group receiving this therapy had a lower relapse rate than in the placebo group, and their cumulative dose of glucocorticoid was lower (all patients also received oral prednisone), there was no reduction in the rate of glucocorticoid-associated toxicity.31 Care must be taken to prevent and monitor for corticosteroid complications such as osteoporosis, glaucoma, diabetes mellitus, and hypertension.
Methotrexate: Mixed results in clinical trials
Methotrexate has been evaluated in three prospective randomized trials,30,32,33 with mixed results.
Spiera et al32 enrolled 21 patients in a double-blind placebo-controlled trial: 12 patients received low-dose methotrexate (7.5 mg/week) and 9 received placebo. In addition, all 21 received a glucocorticoid. There was no significant difference between the methotrexate- and placebo-treated patients in the cumulative dose of glucocorticoid, duration of glucocorticoid therapy, time to taper off the glucocorticoid to less than 10 mg of prednisone per day, or glucocorticoidrelated adverse effects.
Jover et al,33 in another double-blind placebo-controlled trial, studied 42 patients with giant cell arteritis, half of whom were randomized to receive methotrexate 10 mg/week, while the other half received placebo. All patients received prednisone. Patients in the methotrexate group had fewer relapses and a 25% lower cumulative dose of prednisone during follow-up. However, the incidence of adverse events was similar in both groups. Methotrexate was discontinued in 3 patients who developed drug-related adverse events.
Hoffman et al30 randomized 98 patients to receive either methotrexate (up to 15 mg/week) or placebo in a double-blind fashion. All patients also received prednisone at an initial dose of 1 mg/kg/day (up to 60 mg/day). At completion of the study, no differences between the groups were noted in the rates of relapse or treatment-related morbidity or in the cumulative dose of glucocorticoid. However, treatment with methotrexate appeared to lower the rate of recurrence of isolated polymyalgia rheumatica in a small number of patients.30
Comment. Differences in the results of these trials may be attributed to several factors, including different definitions of relapses and different glucocorticoid doses and tapering regimens.
A meta-analysis of these three trials34 showed a reduction in the risk of relapse: 4 patients would have to be treated to prevent one first relapse, 5 would have to be treated to prevent one second relapse, and 11 would need to be treated to prevent one first relapse of cranial symptoms in the first 48 weeks. However, the main goal of methotrexate therapy is to decrease the frequency of adverse events from glucocorticoids, and this meta-analysis found no difference in rates of glucocorticoid-related adverse events in patients treated with methotrexate.
The study raises the question of whether methotrexate should be further evaluated in in different patient populations and at higher doses.34
Infliximab is not recommended
In a prospective study, patients with giant cell arteritis were randomly assigned to receive either infliximab (Remicade) 5 mg/kg every 8 weeks or placebo, in addition to standard glucocorticoid therapy. The study showed no significant difference in the relapse rate and a higher rate of infection in the infliximab group (71%) than in the placebo group (56%). Given the lack of any benefit observed in this study, infliximab is not recommended in the treatment of patients with giant cell arteritis.35
Aspirin is recommended
Daily low-dose aspirin therapy has been shown in several studies to be effective in preventing ischemic complications of giant cell arteritis, including stroke and visual loss. It is currently recommended that all patients with giant cell arteritis without a major contraindication take aspirin 81 mg daily.36–38
- Salvarani C, Gabriel SE, O’Fallon WM, Hunder GG. The incidence of giant cell arteritis in Olmsted County, Minnesota: apparent fluctuations in a cyclic pattern. Ann Intern Med 1995; 123:192–194.
- Baldursson O, Steinsson K, Björnsson J, Lie JT. Giant cell arteritis in Iceland. An epidemiologic and histopathologic analysis. Arthritis Rheum 1994; 37:1007–1012.
- Weyand CM, Goronzy JJ. Medium- and large-vessel vasculitis. N Engl J Med 2003; 349:160–169.
- Aiello PD, Trautmann JC, McPhee TJ, Kunselman AR, Hunder GG. Visual prognosis in giant cell arteritis. Ophthalmology 1993; 100:550–555.
- Salvarani C, Cimino L, Macchioni P, et al. Risk factors for visual loss in an Italian population-based cohort of patients with giant cell arteritis. Arthritis Rheum 2005; 53:293–297.
- Bahlas S, Ramos-Remus C, Davis P. Clinical outcome of 149 patients with polymyalgia rheumatica and giant cell arteritis. J Rheumatol 1998; 25:99–104.
- Gonzalez-Gay MA, Barros S, Lopez-Diaz MJ, Garcia-Porrua C, Sanchez-Andrade A, Llorca J. Giant cell arteritis: disease patterns of clinical presentation in a series of 240 patients. Medicine (Baltimore) 2005; 84:269–276.
- Salvarani C, Cantini F, Macchioni P, et al. Distal musculoskeletal manifestations in polymyalgia rheumatica: a prospective followup study. Arthritis Rheum 1998; 41:1221–1226.
- Salvarani C, Cantini F, Boiardi L, Hunder GG. Polymyalgia rheumatica and giant-cell arteritis. N Engl J Med 2002; 347:261–271.
- Lie JT. Aortic and extracranial large vessel giant cell arteritis: a review of 72 cases with histopathologic documentation. Semin Arthritis Rheum 1995; 24:422–431.
- Evans JM, O’Fallon WM, Hunder GG. Increased incidence of aortic aneurysm and dissection in giant cell (temporal) arteritis. A population-based study. Ann Intern Med 1995; 122:502–507.
- Ostberg G. An arteritis with special reference to polymyalgia arteritica. Acta Pathol Microbiol Scand Suppl 1973; 237(suppl 237):1–59.
- Boyev LR, Miller NR, Green WR. Efficacy of unilateral versus bilateral temporal artery biopsies for the diagnosis of giant cell arteritis. Am J Ophthalmol 1999; 128:211–215.
- González-Gay MA, Garcia-Porrua C, Rivas MJ, Rodriguez-Ledo P, Llorca J. Epidemiology of biopsy proven giant cell arteritis in northwestern Spain: trend over an 18 year period. Ann Rheum Dis 2001; 60:367–371.
- Rodriguez-Valverde V, Sarabia JM, González-Gay MA, et al. Risk factors and predictive models of giant cell arteritis in polymyalgia rheumatica. Am J Med 1997; 102:331–336.
- Mahr A, Saba M, Kambouchner M, et al. Temporal artery biopsy for diagnosing giant cell arteritis: the longer, the better? Ann Rheum Dis 2006; 65:826–828.
- Breuer GS, Nesher R, Nesher G. Effect of biopsy length on the rate of positive temporal artery biopsies. Clin Exp Rheumatol 2009; 27(1 suppl 52):S10–S13.
- Weyand CM, Goronzy JJ. Giant-cell arteritis and polymyalgia rheumatica. Ann Intern Med 2003; 139:505–515.
- Salvarani C, Cantini F, Boiardi L, Hunder GG. Laboratory investigations useful in giant cell arteritis and Takayasu’s arteritis. Clin Exp Rheumatol 2003; 21(6 suppl 32):S23–S28.
- Salvarani C, Hunder GG. Giant cell arteritis with low erythrocyte sedimentation rate: frequency of occurence in a population-based study. Arthritis Rheum 2001; 45:140–145.
- Schmidt WA, Kraft HE, Vorpahl K, Völker L, Gromnica-Ihle EJ. Color duplex ultrasonography in the diagnosis of temporal arteritis. N Engl J Med 1997; 337:1336–1342.
- Karassa FB, Matsagas MI, Schmidt WA, Ioannidis JP. Meta-analysis: test performance of ultrasonography for giant-cell arteritis. Ann Intern Med 2005; 142:359–369.
- Maldini C, Dépinay-Dhellemmes C, Tra TT, et al. Limited value of temporal artery ultrasonography examinations for diagnosis of giant cell arteritis: analysis of 77 subjects. J Rheumatol 2010; Epub ahead of print.
- Both M, Ahmadi-Simab K, Reuter M, et al. MRI and FDG-PET in the assessment of inflammatory aortic arch syndrome in complicated courses of giant cell arteritis. Ann Rheum Dis 2008; 67:1030–1033.
- Tso E, Flamm SD, White RD, Schvartzman PR, Mascha E, Hoffman GS. Takayasu arteritis: utility and limitations of magnetic resonance imaging in diagnosis and treatment. Arthritis Rheum 2002; 46:1634–1642.
- Birkhead NC, Wagener HP, Shick RM. Treatment of temporal arteritis with adrenal corticosteroids; results in fifty-five cases in which lesion was proved at biopsy. J Am Med Assoc 1957; 163:821–827.
- Ray-Chaudhuri N, Kiné DA, Tijani SO, et al. Effect of prior steroid treatment on temporal artery biopsy findings in giant cell arteritis. Br J Ophthalmol 2002; 86:530–532.
- Chan CC, Paine M, O’Day J. Steroid management in giant cell arteritis. Br J Ophthalmol 2001; 85:1061–1064.
- Hunder GG, Sheps SG, Allen GL, Joyce JW. Daily and alternate-day corticosteroid regimens in treatment of giant cell arteritis: comparison in a prospective study. Ann Intern Med 1975; 82:613–618.
- Hoffman GS, Cid MC, Hellmann DB, et al; International Network for the Study of Systemic Vasculitides. A multicenter, randomized, double-blind, placebo-controlled trial of adjuvant methotrexate treatment for giant cell arteritis. Arthritis Rheum 2002; 46:1309–1318.
- Mazlumzadeh M, Hunder GG, Easley KA, et al. Treatment of giant cell arteritis using induction therapy with high-dose glucocorticoids: a double-blind, placebo-controlled, randomized prospective clinical trial. Arthritis Rheum 2006; 54:3310–3318.
- Spiera RF, Mitnick HJ, Kupersmith M, et al. A prospective, doubleblind, randomized, placebo controlled trial of methotrexate in the treatment of giant cell arteritis (GCA). Clin Exp Rheumatol 2001; 19:495–501.
- Jover JA, Hernández-García C, Morado IC, Vargas E, Bañares A, Fernández-Gutiérrez B. Combined treatment of giant-cell arteritis with methotrexate and prednisone. a randomized, double-blind, placebo-controlled trial. Ann Intern Med 2001; 134:106–114.
- Mahr AD, Jover JA, Spiera RF, et al. Adjunctive methotrexate for treatment of giant cell arteritis: an individual patient data meta-analysis. Arthritis Rheum 2007; 56:2789–2797.
- Hoffman GS, Cid MC, Rendt-Zagar KE, et al; Infliximab-GCA Study Group. Infliximab for maintenance of glucocorticosteroid-induced remission of giant cell arteritis: a randomized trial. Ann Intern Med 2007; 146:621–630.
- Weyand CM, Kaiser M, Yang H, Younge B, Goronzy JJ. Therapeutic effects of acetylsalicylic acid in giant cell arteritis. Arthritis Rheum 2002; 46:457–466.
- Nesher G, Berkun Y, Mates M, Baras M, Rubinow A, Sonnenblick M. Low-dose aspirin and prevention of cranial ischemic complications in giant cell arteritis. Arthritis Rheum 2004; 50:1332–1337.
- Lee MS, Smith SD, Galor A, Hoffman GS. Antiplatelet and anticoagulant therapy in patients with giant cell arteritis. Arthritis Rheum 2006; 54:3306–3309.
Giant cell arteritis is the most common primary systemic vasculitis. The disease occurs almost exclusively in people over age 50, with an annual incidence of 15 to 25 per 100,000.1 Incidence rates vary significantly depending on ethnicity. The highest rates are in whites, particularly those of North European descent.2 Incidence rates progressively increase after age 50. The disease is more prevalent in women. Its cause is unknown; both genetic and environmental factors are thought to play a role.
INFLAMED ARTERIES
Giant cell arteritis is characterized by a granulomatous inflammatory infiltrate affecting large and medium-size arteries. Not all vessels are equally affected: the most susceptible are the cranial arteries, the aorta, and the aorta’s primary branches, particularly those in the upper extremities.
The disease is usually associated with an intense acute-phase response. Vessel wall inflammation results in intimal hyperplasia, luminal occlusion, and tissue ischemia. Typical histologic features include a mononuclear inflammatory infiltrate primarily composed of CD4+ T cells and activated macrophages. Multinucleated giant cells are seen in only about 50% of positive biopsies; therefore, their presence is not essential for the diagnosis.3
FOUR MAIN PHENOTYPES
Some of the possible symptoms of giant cell arteritis readily point to the correct diagnosis, eg, those due to cranial artery involvement, such as temporal headache, claudication of masticatory muscles, and visual changes. However, the clinical presentation can be quite varied.
There are four predominant clinical phenotypes, which may be present at the onset of disease or appear later as the disease progresses. Although they will be described separately in this review, these clinical presentations often overlap.
Cranial arteritis
Cranial arteritis is the clinical presentation most readily associated with giant cell arteritis. Clinical features result from involvement of branches of the external or internal carotid artery.
Headache, the most frequent symptom, is typically but not exclusively localized to the temporal areas.
Visual loss is due to involvement of the branches of the ophthalmic or posterior ciliary arteries, resulting in ischemia of the optic nerve or its tracts. It occurs in up to 20% of patients.4,5
Other symptoms and complications from cranial arteritis include tenderness of the scalp and temporal areas, claudication of the tongue or jaw muscles, stroke, and more rarely, tongue infarction.
Polymyalgia rheumatica
Polymyalgia rheumatica is a clinical syndrome that can occur by itself or in conjunction with giant cell arteritis. It may occur independently of giant cell arteritis, but also occurs in about 40% of patients with giant cell arteritis. It may precede, develop simultaneously with, or develop later during the course of the giant cell arteritis.6,7 It is a common clinical manifestation in relapses of giant cell arteritis, even in those who did not have symptoms of polymyalgia rheumatica at the time giant cell arteritis was diagnosed.
Polymyalgia rheumatica is characterized by aching of the shoulder and hip girdle, with morning stiffness. Fatigue and malaise are often present and may be severe. Some patients with polymyalgia rheumatica may also present with peripheral joint synovitis, which may be mistakenly diagnosed as rheumatoid arthritis.8 Muscle weakness and elevated muscle enzymes are not associated with polymyalgia rheumatica.
Polymyalgia rheumatica is a clinical diagnosis. Approximately 80% of patients with polymyalgia rheumatica have an elevated erythrocyte sedimentation rate or an elevated C-reactive protein level.9 When it occurs in the absence of giant cell arteritis, it is treated differently, with less intense doses of corticosteroids. All patients with polymyalgia rheumatica should be routinely questioned regarding symptoms of giant cell arteritis.
Nonspecific systemic inflammatory disease
Some patients with giant cell arteritis may present with a nonspecific systemic inflammatory disease characterized by some combination of fever, night sweats, fatigue, malaise, and weight loss. In these patients, the diagnosis may be delayed by the lack of localizing symptoms.
Laboratory tests typically show anemia, leukocytosis, and thrombocytosis. The erythrocyte sedimentation rate and the C-reactive protein level are usually very high.
Giant cell arteritis should be in the differential diagnosis when these signs and symptoms are found in patients over age 50.
Large-vessel vasculitis
Although thoracic aortic aneurysm and dissection have been described as late complications of giant cell arteritis, large-vessel vasculitis may precede or occur concomitantly with cranial arteritis early in the disease.10,11
Population-based surveys have shown that large-vessel vasculitis is extremely frequent in patients with giant cell arteritis. In a postmortem study of 11 patients with giant cell arteritis, all of them had evidence of arteritis involving the subclavian artery, the carotid artery, and the aorta.12
Patients may have no symptoms or may present with symptoms or signs of tissue ischemia such as claudication of the extremities, carotid artery tenderness, decreased or absent pulses, and large-vessel bruits on physical examination.
CONSIDER THE DIAGNOSIS IN OLDER PATIENTS
Giant cell arteritis should always be considered in patients over age 50 who have any of the clinical features described above. It is therefore very important to be familiar with its symptoms and signs.
A complete and detailed history and a detailed but focused physical examination that includes a comprehensive vascular examination are the first and most important steps in establishing the diagnosis. The vascular examination includes measuring the blood pressure in all four extremities, palpating the peripheral pulses, listening for bruits, and palpating the temporal arteries.
Temporal artery biopsy: The gold standard
Confirming the diagnosis of giant cell arteritis requires histologic findings of inflammation in the temporal artery. Superficial temporal artery biopsy is recommended for diagnostic confirmation in patients who have cranial symptoms and other signs suggesting the disease.
The biopsy should be performed on the same side as the symptoms or abnormal findings on examination. Performing a biopsy in both temporal arteries may increase the diagnostic yield but may not need to be done routinely.13
Although some experts recommend temporal artery biopsy in all patients in whom giant cell arteritis is suspected, biopsy has a lower diagnostic yield in patients who have no cranial symptoms. Interestingly, 5% to 15% of temporal artery biopsies performed in patients who had isolated polymyalgia rheumatica were found to be positive.14,15 Patients with polymyalgia rheumatica and no clinical symptoms to suggest giant cell arteritis generally are not biopsied.
The segmental nature of the inflammation involving the temporal artery in giant cell arteritis may result in negative biopsy results in patients with giant cell arteritis. A temporal artery biopsy length of 5 mm or less has a very low (8%) rate of positive results, whereas a length longer than 20 mm exceeds a 50% rate of positive results. Although the optimal length of a temporal artery specimen is still debated, a longer biopsy specimen should be obtained to increase the chance of arterial specimens showing inflammatory changes.16,17
Laboratory studies: Acute-phase reactants may be elevated
High levels of acute-phase reactants should increase one’s suspicion of giant cell arteritis. Elevations in the erythrocyte sedimentation rate and C-reactive protein and interleukin 6 levels reflect the inflammatory process in this disease.19 However, not all patients with giant cell arteritis have a high sedimentation rate, and as many as 20% of patients with biopsy-proven giant cell arteritis have a normal sedimentation rate before therapy.20 Therefore, a normal sedimentation rate does not exclude the diagnosis of giant cell arteritis and should not delay its diagnosis and treatment.
As a result of systemic inflammation, the patient may also present with normochromic normocytic anemia, leukocytosis, and thrombocytosis.
Imaging studies are controversial
Imaging studies are potentially useful diagnostic tools in large-vessel vasculitis but are still the subject of significant controversy.
Ultrasonography of the temporal artery has been a controversial subject in many studies.21,22 Color duplex ultrasonography of the temporal artery has been reported to be helpful in the diagnosis of giant cell arteritis (showing a “halo” around the arterial lumen), but further studies are needed to establish its clinical utility.
At this time, temporal artery biopsy remains the gold standard diagnostic test for giant cell arteritis, and ultrasonography is neither a substitute for biopsy nor a screening test for this disease.23 Some have suggested, however, that ultrasonography may help to identify the best site for biopsy of the temporal artery in some patients.
Arteriography is an accurate technique for evaluating the vessel lumen and allows for measuring central blood pressure and performing vascular interventions. However, because of potential complications, it has been largely replaced by noninvasive angiographic imaging to delineate vascular anatomy.
TREATMENT
Glucocorticoid therapy remains the standard of care
Once the diagnosis of giant cell arteritis is established, glucocorticoid treatment should be started. Glucocorticoids are the standard therapy, and they usually bring about a prompt clinical response. Although never evaluated in placebo-controlled trials, these drugs have been shown to prevent progression of visual loss in a retrospective study.26
In patients with visual symptoms or imminent visual loss, therapy should be started promptly once suspicion of giant cell arteritis is raised; ie, one should not wait until the diagnosis is confirmed by biopsy.
Ideally, a glucocorticoid should be started after a temporal artery biopsy is done, but treatment should not be delayed, as it rapidly suppresses the inflammatory response and may prevent complications from tissue ischemia, such as blindness. Visual loss is usually irreversible.
There is still a role for obtaining a temporal artery biopsy up to several weeks after glucocorticoid therapy is started, as the pathological abnormalities of arteritis do not rapidly resolve.27
Glucocorticoid therapy is highly effective in inducing disease remission in patients with giant cell arteritis. Nearly all patients respond to 1 mg/kg (40–60 mg) per day of prednisone or its equivalent.
The initial dosing is usually maintained for 4 weeks and then decreased slowly. The duration of therapy varies; most patients remain on therapy for at least 1 year, and some cannot stop it completely without recurrence of symptoms.
If a patient is about to lose his or her vision or has lost all or some vision in one eye, a higher initial dose of a glucocorticoid is usually used (ie, a pulse of 500 or 1,000 mg of intravenous methylprednisolone) and may be beneficial.28
Although a rapid clinical response to therapy is usually seen within 48 hours, some patients may have a more delayed clinical improvement.
Alternate-day therapy was compared with daily therapy and was found to be less effective, and as a result it is not recommended.29
Glucocorticoid therapy can cause significant toxicity in patients with giant cell arteritis, as they commonly must take these drugs for long periods. The rate of relapse in those who discontinue therapy is quite high—as high as 77% within 12 months.30
Given the concern about glucocorticoid toxicity, several studies have evaluated alternative strategies and other immunosuppressive drugs. However, no study has concluded that other medications are effective in the treatment of giant cell arteritis.
Mazlumzadeh et al31 evaluated the initial use of intravenous pulse methylprednisolone therapy (15 mg/kg ideal body weight on 3 consecutive days) in an attempt to decrease the glucocorticoid requirement. Although the group receiving this therapy had a lower relapse rate than in the placebo group, and their cumulative dose of glucocorticoid was lower (all patients also received oral prednisone), there was no reduction in the rate of glucocorticoid-associated toxicity.31 Care must be taken to prevent and monitor for corticosteroid complications such as osteoporosis, glaucoma, diabetes mellitus, and hypertension.
Methotrexate: Mixed results in clinical trials
Methotrexate has been evaluated in three prospective randomized trials,30,32,33 with mixed results.
Spiera et al32 enrolled 21 patients in a double-blind placebo-controlled trial: 12 patients received low-dose methotrexate (7.5 mg/week) and 9 received placebo. In addition, all 21 received a glucocorticoid. There was no significant difference between the methotrexate- and placebo-treated patients in the cumulative dose of glucocorticoid, duration of glucocorticoid therapy, time to taper off the glucocorticoid to less than 10 mg of prednisone per day, or glucocorticoidrelated adverse effects.
Jover et al,33 in another double-blind placebo-controlled trial, studied 42 patients with giant cell arteritis, half of whom were randomized to receive methotrexate 10 mg/week, while the other half received placebo. All patients received prednisone. Patients in the methotrexate group had fewer relapses and a 25% lower cumulative dose of prednisone during follow-up. However, the incidence of adverse events was similar in both groups. Methotrexate was discontinued in 3 patients who developed drug-related adverse events.
Hoffman et al30 randomized 98 patients to receive either methotrexate (up to 15 mg/week) or placebo in a double-blind fashion. All patients also received prednisone at an initial dose of 1 mg/kg/day (up to 60 mg/day). At completion of the study, no differences between the groups were noted in the rates of relapse or treatment-related morbidity or in the cumulative dose of glucocorticoid. However, treatment with methotrexate appeared to lower the rate of recurrence of isolated polymyalgia rheumatica in a small number of patients.30
Comment. Differences in the results of these trials may be attributed to several factors, including different definitions of relapses and different glucocorticoid doses and tapering regimens.
A meta-analysis of these three trials34 showed a reduction in the risk of relapse: 4 patients would have to be treated to prevent one first relapse, 5 would have to be treated to prevent one second relapse, and 11 would need to be treated to prevent one first relapse of cranial symptoms in the first 48 weeks. However, the main goal of methotrexate therapy is to decrease the frequency of adverse events from glucocorticoids, and this meta-analysis found no difference in rates of glucocorticoid-related adverse events in patients treated with methotrexate.
The study raises the question of whether methotrexate should be further evaluated in in different patient populations and at higher doses.34
Infliximab is not recommended
In a prospective study, patients with giant cell arteritis were randomly assigned to receive either infliximab (Remicade) 5 mg/kg every 8 weeks or placebo, in addition to standard glucocorticoid therapy. The study showed no significant difference in the relapse rate and a higher rate of infection in the infliximab group (71%) than in the placebo group (56%). Given the lack of any benefit observed in this study, infliximab is not recommended in the treatment of patients with giant cell arteritis.35
Aspirin is recommended
Daily low-dose aspirin therapy has been shown in several studies to be effective in preventing ischemic complications of giant cell arteritis, including stroke and visual loss. It is currently recommended that all patients with giant cell arteritis without a major contraindication take aspirin 81 mg daily.36–38
Giant cell arteritis is the most common primary systemic vasculitis. The disease occurs almost exclusively in people over age 50, with an annual incidence of 15 to 25 per 100,000.1 Incidence rates vary significantly depending on ethnicity. The highest rates are in whites, particularly those of North European descent.2 Incidence rates progressively increase after age 50. The disease is more prevalent in women. Its cause is unknown; both genetic and environmental factors are thought to play a role.
INFLAMED ARTERIES
Giant cell arteritis is characterized by a granulomatous inflammatory infiltrate affecting large and medium-size arteries. Not all vessels are equally affected: the most susceptible are the cranial arteries, the aorta, and the aorta’s primary branches, particularly those in the upper extremities.
The disease is usually associated with an intense acute-phase response. Vessel wall inflammation results in intimal hyperplasia, luminal occlusion, and tissue ischemia. Typical histologic features include a mononuclear inflammatory infiltrate primarily composed of CD4+ T cells and activated macrophages. Multinucleated giant cells are seen in only about 50% of positive biopsies; therefore, their presence is not essential for the diagnosis.3
FOUR MAIN PHENOTYPES
Some of the possible symptoms of giant cell arteritis readily point to the correct diagnosis, eg, those due to cranial artery involvement, such as temporal headache, claudication of masticatory muscles, and visual changes. However, the clinical presentation can be quite varied.
There are four predominant clinical phenotypes, which may be present at the onset of disease or appear later as the disease progresses. Although they will be described separately in this review, these clinical presentations often overlap.
Cranial arteritis
Cranial arteritis is the clinical presentation most readily associated with giant cell arteritis. Clinical features result from involvement of branches of the external or internal carotid artery.
Headache, the most frequent symptom, is typically but not exclusively localized to the temporal areas.
Visual loss is due to involvement of the branches of the ophthalmic or posterior ciliary arteries, resulting in ischemia of the optic nerve or its tracts. It occurs in up to 20% of patients.4,5
Other symptoms and complications from cranial arteritis include tenderness of the scalp and temporal areas, claudication of the tongue or jaw muscles, stroke, and more rarely, tongue infarction.
Polymyalgia rheumatica
Polymyalgia rheumatica is a clinical syndrome that can occur by itself or in conjunction with giant cell arteritis. It may occur independently of giant cell arteritis, but also occurs in about 40% of patients with giant cell arteritis. It may precede, develop simultaneously with, or develop later during the course of the giant cell arteritis.6,7 It is a common clinical manifestation in relapses of giant cell arteritis, even in those who did not have symptoms of polymyalgia rheumatica at the time giant cell arteritis was diagnosed.
Polymyalgia rheumatica is characterized by aching of the shoulder and hip girdle, with morning stiffness. Fatigue and malaise are often present and may be severe. Some patients with polymyalgia rheumatica may also present with peripheral joint synovitis, which may be mistakenly diagnosed as rheumatoid arthritis.8 Muscle weakness and elevated muscle enzymes are not associated with polymyalgia rheumatica.
Polymyalgia rheumatica is a clinical diagnosis. Approximately 80% of patients with polymyalgia rheumatica have an elevated erythrocyte sedimentation rate or an elevated C-reactive protein level.9 When it occurs in the absence of giant cell arteritis, it is treated differently, with less intense doses of corticosteroids. All patients with polymyalgia rheumatica should be routinely questioned regarding symptoms of giant cell arteritis.
Nonspecific systemic inflammatory disease
Some patients with giant cell arteritis may present with a nonspecific systemic inflammatory disease characterized by some combination of fever, night sweats, fatigue, malaise, and weight loss. In these patients, the diagnosis may be delayed by the lack of localizing symptoms.
Laboratory tests typically show anemia, leukocytosis, and thrombocytosis. The erythrocyte sedimentation rate and the C-reactive protein level are usually very high.
Giant cell arteritis should be in the differential diagnosis when these signs and symptoms are found in patients over age 50.
Large-vessel vasculitis
Although thoracic aortic aneurysm and dissection have been described as late complications of giant cell arteritis, large-vessel vasculitis may precede or occur concomitantly with cranial arteritis early in the disease.10,11
Population-based surveys have shown that large-vessel vasculitis is extremely frequent in patients with giant cell arteritis. In a postmortem study of 11 patients with giant cell arteritis, all of them had evidence of arteritis involving the subclavian artery, the carotid artery, and the aorta.12
Patients may have no symptoms or may present with symptoms or signs of tissue ischemia such as claudication of the extremities, carotid artery tenderness, decreased or absent pulses, and large-vessel bruits on physical examination.
CONSIDER THE DIAGNOSIS IN OLDER PATIENTS
Giant cell arteritis should always be considered in patients over age 50 who have any of the clinical features described above. It is therefore very important to be familiar with its symptoms and signs.
A complete and detailed history and a detailed but focused physical examination that includes a comprehensive vascular examination are the first and most important steps in establishing the diagnosis. The vascular examination includes measuring the blood pressure in all four extremities, palpating the peripheral pulses, listening for bruits, and palpating the temporal arteries.
Temporal artery biopsy: The gold standard
Confirming the diagnosis of giant cell arteritis requires histologic findings of inflammation in the temporal artery. Superficial temporal artery biopsy is recommended for diagnostic confirmation in patients who have cranial symptoms and other signs suggesting the disease.
The biopsy should be performed on the same side as the symptoms or abnormal findings on examination. Performing a biopsy in both temporal arteries may increase the diagnostic yield but may not need to be done routinely.13
Although some experts recommend temporal artery biopsy in all patients in whom giant cell arteritis is suspected, biopsy has a lower diagnostic yield in patients who have no cranial symptoms. Interestingly, 5% to 15% of temporal artery biopsies performed in patients who had isolated polymyalgia rheumatica were found to be positive.14,15 Patients with polymyalgia rheumatica and no clinical symptoms to suggest giant cell arteritis generally are not biopsied.
The segmental nature of the inflammation involving the temporal artery in giant cell arteritis may result in negative biopsy results in patients with giant cell arteritis. A temporal artery biopsy length of 5 mm or less has a very low (8%) rate of positive results, whereas a length longer than 20 mm exceeds a 50% rate of positive results. Although the optimal length of a temporal artery specimen is still debated, a longer biopsy specimen should be obtained to increase the chance of arterial specimens showing inflammatory changes.16,17
Laboratory studies: Acute-phase reactants may be elevated
High levels of acute-phase reactants should increase one’s suspicion of giant cell arteritis. Elevations in the erythrocyte sedimentation rate and C-reactive protein and interleukin 6 levels reflect the inflammatory process in this disease.19 However, not all patients with giant cell arteritis have a high sedimentation rate, and as many as 20% of patients with biopsy-proven giant cell arteritis have a normal sedimentation rate before therapy.20 Therefore, a normal sedimentation rate does not exclude the diagnosis of giant cell arteritis and should not delay its diagnosis and treatment.
As a result of systemic inflammation, the patient may also present with normochromic normocytic anemia, leukocytosis, and thrombocytosis.
Imaging studies are controversial
Imaging studies are potentially useful diagnostic tools in large-vessel vasculitis but are still the subject of significant controversy.
Ultrasonography of the temporal artery has been a controversial subject in many studies.21,22 Color duplex ultrasonography of the temporal artery has been reported to be helpful in the diagnosis of giant cell arteritis (showing a “halo” around the arterial lumen), but further studies are needed to establish its clinical utility.
At this time, temporal artery biopsy remains the gold standard diagnostic test for giant cell arteritis, and ultrasonography is neither a substitute for biopsy nor a screening test for this disease.23 Some have suggested, however, that ultrasonography may help to identify the best site for biopsy of the temporal artery in some patients.
Arteriography is an accurate technique for evaluating the vessel lumen and allows for measuring central blood pressure and performing vascular interventions. However, because of potential complications, it has been largely replaced by noninvasive angiographic imaging to delineate vascular anatomy.
TREATMENT
Glucocorticoid therapy remains the standard of care
Once the diagnosis of giant cell arteritis is established, glucocorticoid treatment should be started. Glucocorticoids are the standard therapy, and they usually bring about a prompt clinical response. Although never evaluated in placebo-controlled trials, these drugs have been shown to prevent progression of visual loss in a retrospective study.26
In patients with visual symptoms or imminent visual loss, therapy should be started promptly once suspicion of giant cell arteritis is raised; ie, one should not wait until the diagnosis is confirmed by biopsy.
Ideally, a glucocorticoid should be started after a temporal artery biopsy is done, but treatment should not be delayed, as it rapidly suppresses the inflammatory response and may prevent complications from tissue ischemia, such as blindness. Visual loss is usually irreversible.
There is still a role for obtaining a temporal artery biopsy up to several weeks after glucocorticoid therapy is started, as the pathological abnormalities of arteritis do not rapidly resolve.27
Glucocorticoid therapy is highly effective in inducing disease remission in patients with giant cell arteritis. Nearly all patients respond to 1 mg/kg (40–60 mg) per day of prednisone or its equivalent.
The initial dosing is usually maintained for 4 weeks and then decreased slowly. The duration of therapy varies; most patients remain on therapy for at least 1 year, and some cannot stop it completely without recurrence of symptoms.
If a patient is about to lose his or her vision or has lost all or some vision in one eye, a higher initial dose of a glucocorticoid is usually used (ie, a pulse of 500 or 1,000 mg of intravenous methylprednisolone) and may be beneficial.28
Although a rapid clinical response to therapy is usually seen within 48 hours, some patients may have a more delayed clinical improvement.
Alternate-day therapy was compared with daily therapy and was found to be less effective, and as a result it is not recommended.29
Glucocorticoid therapy can cause significant toxicity in patients with giant cell arteritis, as they commonly must take these drugs for long periods. The rate of relapse in those who discontinue therapy is quite high—as high as 77% within 12 months.30
Given the concern about glucocorticoid toxicity, several studies have evaluated alternative strategies and other immunosuppressive drugs. However, no study has concluded that other medications are effective in the treatment of giant cell arteritis.
Mazlumzadeh et al31 evaluated the initial use of intravenous pulse methylprednisolone therapy (15 mg/kg ideal body weight on 3 consecutive days) in an attempt to decrease the glucocorticoid requirement. Although the group receiving this therapy had a lower relapse rate than in the placebo group, and their cumulative dose of glucocorticoid was lower (all patients also received oral prednisone), there was no reduction in the rate of glucocorticoid-associated toxicity.31 Care must be taken to prevent and monitor for corticosteroid complications such as osteoporosis, glaucoma, diabetes mellitus, and hypertension.
Methotrexate: Mixed results in clinical trials
Methotrexate has been evaluated in three prospective randomized trials,30,32,33 with mixed results.
Spiera et al32 enrolled 21 patients in a double-blind placebo-controlled trial: 12 patients received low-dose methotrexate (7.5 mg/week) and 9 received placebo. In addition, all 21 received a glucocorticoid. There was no significant difference between the methotrexate- and placebo-treated patients in the cumulative dose of glucocorticoid, duration of glucocorticoid therapy, time to taper off the glucocorticoid to less than 10 mg of prednisone per day, or glucocorticoidrelated adverse effects.
Jover et al,33 in another double-blind placebo-controlled trial, studied 42 patients with giant cell arteritis, half of whom were randomized to receive methotrexate 10 mg/week, while the other half received placebo. All patients received prednisone. Patients in the methotrexate group had fewer relapses and a 25% lower cumulative dose of prednisone during follow-up. However, the incidence of adverse events was similar in both groups. Methotrexate was discontinued in 3 patients who developed drug-related adverse events.
Hoffman et al30 randomized 98 patients to receive either methotrexate (up to 15 mg/week) or placebo in a double-blind fashion. All patients also received prednisone at an initial dose of 1 mg/kg/day (up to 60 mg/day). At completion of the study, no differences between the groups were noted in the rates of relapse or treatment-related morbidity or in the cumulative dose of glucocorticoid. However, treatment with methotrexate appeared to lower the rate of recurrence of isolated polymyalgia rheumatica in a small number of patients.30
Comment. Differences in the results of these trials may be attributed to several factors, including different definitions of relapses and different glucocorticoid doses and tapering regimens.
A meta-analysis of these three trials34 showed a reduction in the risk of relapse: 4 patients would have to be treated to prevent one first relapse, 5 would have to be treated to prevent one second relapse, and 11 would need to be treated to prevent one first relapse of cranial symptoms in the first 48 weeks. However, the main goal of methotrexate therapy is to decrease the frequency of adverse events from glucocorticoids, and this meta-analysis found no difference in rates of glucocorticoid-related adverse events in patients treated with methotrexate.
The study raises the question of whether methotrexate should be further evaluated in in different patient populations and at higher doses.34
Infliximab is not recommended
In a prospective study, patients with giant cell arteritis were randomly assigned to receive either infliximab (Remicade) 5 mg/kg every 8 weeks or placebo, in addition to standard glucocorticoid therapy. The study showed no significant difference in the relapse rate and a higher rate of infection in the infliximab group (71%) than in the placebo group (56%). Given the lack of any benefit observed in this study, infliximab is not recommended in the treatment of patients with giant cell arteritis.35
Aspirin is recommended
Daily low-dose aspirin therapy has been shown in several studies to be effective in preventing ischemic complications of giant cell arteritis, including stroke and visual loss. It is currently recommended that all patients with giant cell arteritis without a major contraindication take aspirin 81 mg daily.36–38
- Salvarani C, Gabriel SE, O’Fallon WM, Hunder GG. The incidence of giant cell arteritis in Olmsted County, Minnesota: apparent fluctuations in a cyclic pattern. Ann Intern Med 1995; 123:192–194.
- Baldursson O, Steinsson K, Björnsson J, Lie JT. Giant cell arteritis in Iceland. An epidemiologic and histopathologic analysis. Arthritis Rheum 1994; 37:1007–1012.
- Weyand CM, Goronzy JJ. Medium- and large-vessel vasculitis. N Engl J Med 2003; 349:160–169.
- Aiello PD, Trautmann JC, McPhee TJ, Kunselman AR, Hunder GG. Visual prognosis in giant cell arteritis. Ophthalmology 1993; 100:550–555.
- Salvarani C, Cimino L, Macchioni P, et al. Risk factors for visual loss in an Italian population-based cohort of patients with giant cell arteritis. Arthritis Rheum 2005; 53:293–297.
- Bahlas S, Ramos-Remus C, Davis P. Clinical outcome of 149 patients with polymyalgia rheumatica and giant cell arteritis. J Rheumatol 1998; 25:99–104.
- Gonzalez-Gay MA, Barros S, Lopez-Diaz MJ, Garcia-Porrua C, Sanchez-Andrade A, Llorca J. Giant cell arteritis: disease patterns of clinical presentation in a series of 240 patients. Medicine (Baltimore) 2005; 84:269–276.
- Salvarani C, Cantini F, Macchioni P, et al. Distal musculoskeletal manifestations in polymyalgia rheumatica: a prospective followup study. Arthritis Rheum 1998; 41:1221–1226.
- Salvarani C, Cantini F, Boiardi L, Hunder GG. Polymyalgia rheumatica and giant-cell arteritis. N Engl J Med 2002; 347:261–271.
- Lie JT. Aortic and extracranial large vessel giant cell arteritis: a review of 72 cases with histopathologic documentation. Semin Arthritis Rheum 1995; 24:422–431.
- Evans JM, O’Fallon WM, Hunder GG. Increased incidence of aortic aneurysm and dissection in giant cell (temporal) arteritis. A population-based study. Ann Intern Med 1995; 122:502–507.
- Ostberg G. An arteritis with special reference to polymyalgia arteritica. Acta Pathol Microbiol Scand Suppl 1973; 237(suppl 237):1–59.
- Boyev LR, Miller NR, Green WR. Efficacy of unilateral versus bilateral temporal artery biopsies for the diagnosis of giant cell arteritis. Am J Ophthalmol 1999; 128:211–215.
- González-Gay MA, Garcia-Porrua C, Rivas MJ, Rodriguez-Ledo P, Llorca J. Epidemiology of biopsy proven giant cell arteritis in northwestern Spain: trend over an 18 year period. Ann Rheum Dis 2001; 60:367–371.
- Rodriguez-Valverde V, Sarabia JM, González-Gay MA, et al. Risk factors and predictive models of giant cell arteritis in polymyalgia rheumatica. Am J Med 1997; 102:331–336.
- Mahr A, Saba M, Kambouchner M, et al. Temporal artery biopsy for diagnosing giant cell arteritis: the longer, the better? Ann Rheum Dis 2006; 65:826–828.
- Breuer GS, Nesher R, Nesher G. Effect of biopsy length on the rate of positive temporal artery biopsies. Clin Exp Rheumatol 2009; 27(1 suppl 52):S10–S13.
- Weyand CM, Goronzy JJ. Giant-cell arteritis and polymyalgia rheumatica. Ann Intern Med 2003; 139:505–515.
- Salvarani C, Cantini F, Boiardi L, Hunder GG. Laboratory investigations useful in giant cell arteritis and Takayasu’s arteritis. Clin Exp Rheumatol 2003; 21(6 suppl 32):S23–S28.
- Salvarani C, Hunder GG. Giant cell arteritis with low erythrocyte sedimentation rate: frequency of occurence in a population-based study. Arthritis Rheum 2001; 45:140–145.
- Schmidt WA, Kraft HE, Vorpahl K, Völker L, Gromnica-Ihle EJ. Color duplex ultrasonography in the diagnosis of temporal arteritis. N Engl J Med 1997; 337:1336–1342.
- Karassa FB, Matsagas MI, Schmidt WA, Ioannidis JP. Meta-analysis: test performance of ultrasonography for giant-cell arteritis. Ann Intern Med 2005; 142:359–369.
- Maldini C, Dépinay-Dhellemmes C, Tra TT, et al. Limited value of temporal artery ultrasonography examinations for diagnosis of giant cell arteritis: analysis of 77 subjects. J Rheumatol 2010; Epub ahead of print.
- Both M, Ahmadi-Simab K, Reuter M, et al. MRI and FDG-PET in the assessment of inflammatory aortic arch syndrome in complicated courses of giant cell arteritis. Ann Rheum Dis 2008; 67:1030–1033.
- Tso E, Flamm SD, White RD, Schvartzman PR, Mascha E, Hoffman GS. Takayasu arteritis: utility and limitations of magnetic resonance imaging in diagnosis and treatment. Arthritis Rheum 2002; 46:1634–1642.
- Birkhead NC, Wagener HP, Shick RM. Treatment of temporal arteritis with adrenal corticosteroids; results in fifty-five cases in which lesion was proved at biopsy. J Am Med Assoc 1957; 163:821–827.
- Ray-Chaudhuri N, Kiné DA, Tijani SO, et al. Effect of prior steroid treatment on temporal artery biopsy findings in giant cell arteritis. Br J Ophthalmol 2002; 86:530–532.
- Chan CC, Paine M, O’Day J. Steroid management in giant cell arteritis. Br J Ophthalmol 2001; 85:1061–1064.
- Hunder GG, Sheps SG, Allen GL, Joyce JW. Daily and alternate-day corticosteroid regimens in treatment of giant cell arteritis: comparison in a prospective study. Ann Intern Med 1975; 82:613–618.
- Hoffman GS, Cid MC, Hellmann DB, et al; International Network for the Study of Systemic Vasculitides. A multicenter, randomized, double-blind, placebo-controlled trial of adjuvant methotrexate treatment for giant cell arteritis. Arthritis Rheum 2002; 46:1309–1318.
- Mazlumzadeh M, Hunder GG, Easley KA, et al. Treatment of giant cell arteritis using induction therapy with high-dose glucocorticoids: a double-blind, placebo-controlled, randomized prospective clinical trial. Arthritis Rheum 2006; 54:3310–3318.
- Spiera RF, Mitnick HJ, Kupersmith M, et al. A prospective, doubleblind, randomized, placebo controlled trial of methotrexate in the treatment of giant cell arteritis (GCA). Clin Exp Rheumatol 2001; 19:495–501.
- Jover JA, Hernández-García C, Morado IC, Vargas E, Bañares A, Fernández-Gutiérrez B. Combined treatment of giant-cell arteritis with methotrexate and prednisone. a randomized, double-blind, placebo-controlled trial. Ann Intern Med 2001; 134:106–114.
- Mahr AD, Jover JA, Spiera RF, et al. Adjunctive methotrexate for treatment of giant cell arteritis: an individual patient data meta-analysis. Arthritis Rheum 2007; 56:2789–2797.
- Hoffman GS, Cid MC, Rendt-Zagar KE, et al; Infliximab-GCA Study Group. Infliximab for maintenance of glucocorticosteroid-induced remission of giant cell arteritis: a randomized trial. Ann Intern Med 2007; 146:621–630.
- Weyand CM, Kaiser M, Yang H, Younge B, Goronzy JJ. Therapeutic effects of acetylsalicylic acid in giant cell arteritis. Arthritis Rheum 2002; 46:457–466.
- Nesher G, Berkun Y, Mates M, Baras M, Rubinow A, Sonnenblick M. Low-dose aspirin and prevention of cranial ischemic complications in giant cell arteritis. Arthritis Rheum 2004; 50:1332–1337.
- Lee MS, Smith SD, Galor A, Hoffman GS. Antiplatelet and anticoagulant therapy in patients with giant cell arteritis. Arthritis Rheum 2006; 54:3306–3309.
- Salvarani C, Gabriel SE, O’Fallon WM, Hunder GG. The incidence of giant cell arteritis in Olmsted County, Minnesota: apparent fluctuations in a cyclic pattern. Ann Intern Med 1995; 123:192–194.
- Baldursson O, Steinsson K, Björnsson J, Lie JT. Giant cell arteritis in Iceland. An epidemiologic and histopathologic analysis. Arthritis Rheum 1994; 37:1007–1012.
- Weyand CM, Goronzy JJ. Medium- and large-vessel vasculitis. N Engl J Med 2003; 349:160–169.
- Aiello PD, Trautmann JC, McPhee TJ, Kunselman AR, Hunder GG. Visual prognosis in giant cell arteritis. Ophthalmology 1993; 100:550–555.
- Salvarani C, Cimino L, Macchioni P, et al. Risk factors for visual loss in an Italian population-based cohort of patients with giant cell arteritis. Arthritis Rheum 2005; 53:293–297.
- Bahlas S, Ramos-Remus C, Davis P. Clinical outcome of 149 patients with polymyalgia rheumatica and giant cell arteritis. J Rheumatol 1998; 25:99–104.
- Gonzalez-Gay MA, Barros S, Lopez-Diaz MJ, Garcia-Porrua C, Sanchez-Andrade A, Llorca J. Giant cell arteritis: disease patterns of clinical presentation in a series of 240 patients. Medicine (Baltimore) 2005; 84:269–276.
- Salvarani C, Cantini F, Macchioni P, et al. Distal musculoskeletal manifestations in polymyalgia rheumatica: a prospective followup study. Arthritis Rheum 1998; 41:1221–1226.
- Salvarani C, Cantini F, Boiardi L, Hunder GG. Polymyalgia rheumatica and giant-cell arteritis. N Engl J Med 2002; 347:261–271.
- Lie JT. Aortic and extracranial large vessel giant cell arteritis: a review of 72 cases with histopathologic documentation. Semin Arthritis Rheum 1995; 24:422–431.
- Evans JM, O’Fallon WM, Hunder GG. Increased incidence of aortic aneurysm and dissection in giant cell (temporal) arteritis. A population-based study. Ann Intern Med 1995; 122:502–507.
- Ostberg G. An arteritis with special reference to polymyalgia arteritica. Acta Pathol Microbiol Scand Suppl 1973; 237(suppl 237):1–59.
- Boyev LR, Miller NR, Green WR. Efficacy of unilateral versus bilateral temporal artery biopsies for the diagnosis of giant cell arteritis. Am J Ophthalmol 1999; 128:211–215.
- González-Gay MA, Garcia-Porrua C, Rivas MJ, Rodriguez-Ledo P, Llorca J. Epidemiology of biopsy proven giant cell arteritis in northwestern Spain: trend over an 18 year period. Ann Rheum Dis 2001; 60:367–371.
- Rodriguez-Valverde V, Sarabia JM, González-Gay MA, et al. Risk factors and predictive models of giant cell arteritis in polymyalgia rheumatica. Am J Med 1997; 102:331–336.
- Mahr A, Saba M, Kambouchner M, et al. Temporal artery biopsy for diagnosing giant cell arteritis: the longer, the better? Ann Rheum Dis 2006; 65:826–828.
- Breuer GS, Nesher R, Nesher G. Effect of biopsy length on the rate of positive temporal artery biopsies. Clin Exp Rheumatol 2009; 27(1 suppl 52):S10–S13.
- Weyand CM, Goronzy JJ. Giant-cell arteritis and polymyalgia rheumatica. Ann Intern Med 2003; 139:505–515.
- Salvarani C, Cantini F, Boiardi L, Hunder GG. Laboratory investigations useful in giant cell arteritis and Takayasu’s arteritis. Clin Exp Rheumatol 2003; 21(6 suppl 32):S23–S28.
- Salvarani C, Hunder GG. Giant cell arteritis with low erythrocyte sedimentation rate: frequency of occurence in a population-based study. Arthritis Rheum 2001; 45:140–145.
- Schmidt WA, Kraft HE, Vorpahl K, Völker L, Gromnica-Ihle EJ. Color duplex ultrasonography in the diagnosis of temporal arteritis. N Engl J Med 1997; 337:1336–1342.
- Karassa FB, Matsagas MI, Schmidt WA, Ioannidis JP. Meta-analysis: test performance of ultrasonography for giant-cell arteritis. Ann Intern Med 2005; 142:359–369.
- Maldini C, Dépinay-Dhellemmes C, Tra TT, et al. Limited value of temporal artery ultrasonography examinations for diagnosis of giant cell arteritis: analysis of 77 subjects. J Rheumatol 2010; Epub ahead of print.
- Both M, Ahmadi-Simab K, Reuter M, et al. MRI and FDG-PET in the assessment of inflammatory aortic arch syndrome in complicated courses of giant cell arteritis. Ann Rheum Dis 2008; 67:1030–1033.
- Tso E, Flamm SD, White RD, Schvartzman PR, Mascha E, Hoffman GS. Takayasu arteritis: utility and limitations of magnetic resonance imaging in diagnosis and treatment. Arthritis Rheum 2002; 46:1634–1642.
- Birkhead NC, Wagener HP, Shick RM. Treatment of temporal arteritis with adrenal corticosteroids; results in fifty-five cases in which lesion was proved at biopsy. J Am Med Assoc 1957; 163:821–827.
- Ray-Chaudhuri N, Kiné DA, Tijani SO, et al. Effect of prior steroid treatment on temporal artery biopsy findings in giant cell arteritis. Br J Ophthalmol 2002; 86:530–532.
- Chan CC, Paine M, O’Day J. Steroid management in giant cell arteritis. Br J Ophthalmol 2001; 85:1061–1064.
- Hunder GG, Sheps SG, Allen GL, Joyce JW. Daily and alternate-day corticosteroid regimens in treatment of giant cell arteritis: comparison in a prospective study. Ann Intern Med 1975; 82:613–618.
- Hoffman GS, Cid MC, Hellmann DB, et al; International Network for the Study of Systemic Vasculitides. A multicenter, randomized, double-blind, placebo-controlled trial of adjuvant methotrexate treatment for giant cell arteritis. Arthritis Rheum 2002; 46:1309–1318.
- Mazlumzadeh M, Hunder GG, Easley KA, et al. Treatment of giant cell arteritis using induction therapy with high-dose glucocorticoids: a double-blind, placebo-controlled, randomized prospective clinical trial. Arthritis Rheum 2006; 54:3310–3318.
- Spiera RF, Mitnick HJ, Kupersmith M, et al. A prospective, doubleblind, randomized, placebo controlled trial of methotrexate in the treatment of giant cell arteritis (GCA). Clin Exp Rheumatol 2001; 19:495–501.
- Jover JA, Hernández-García C, Morado IC, Vargas E, Bañares A, Fernández-Gutiérrez B. Combined treatment of giant-cell arteritis with methotrexate and prednisone. a randomized, double-blind, placebo-controlled trial. Ann Intern Med 2001; 134:106–114.
- Mahr AD, Jover JA, Spiera RF, et al. Adjunctive methotrexate for treatment of giant cell arteritis: an individual patient data meta-analysis. Arthritis Rheum 2007; 56:2789–2797.
- Hoffman GS, Cid MC, Rendt-Zagar KE, et al; Infliximab-GCA Study Group. Infliximab for maintenance of glucocorticosteroid-induced remission of giant cell arteritis: a randomized trial. Ann Intern Med 2007; 146:621–630.
- Weyand CM, Kaiser M, Yang H, Younge B, Goronzy JJ. Therapeutic effects of acetylsalicylic acid in giant cell arteritis. Arthritis Rheum 2002; 46:457–466.
- Nesher G, Berkun Y, Mates M, Baras M, Rubinow A, Sonnenblick M. Low-dose aspirin and prevention of cranial ischemic complications in giant cell arteritis. Arthritis Rheum 2004; 50:1332–1337.
- Lee MS, Smith SD, Galor A, Hoffman GS. Antiplatelet and anticoagulant therapy in patients with giant cell arteritis. Arthritis Rheum 2006; 54:3306–3309.
KEY POINTS
- Giant cell arteritis is often associated with an intense acute-phase response and cranial symptoms.
- Large-vessel involvement is commonly present and may result in serious complications such as visual loss, stroke, limb claudication, and aortic aneurysm.
- The diagnosis is usually confirmed by an abnormal temporal artery biopsy.
- Symptoms of giant cell arteritis usually respond rapidly and completely to glucocorticoid therapy, still the mainstay of treatment. Most patients need prolonged therapy.
- Several studies have evaluated alternative drugs in an attempt to avoid toxicity from long-term use of glucocorticoids. Results have been mixed, and further study is needed.
Nausea, vomiting, and panic attacks in a 50-year-old woman
A 50-year-old woman presents to the emergency department because of repeated episodes of vomiting over the past 12 hours. She reports eight episodes of non-bloody, nonbilious emesis associated with palpitations and feelings of anxiety, but with no fever or diarrhea. She has not traveled recently and does not have any sick contacts.
She reports that she never had health problems until 6 months ago, when she began having panic attacks that woke her from sleep. The episodes first occurred once or twice per week, usually at night, and involved palpitations and feelings of anxiety that lasted 2 to 4 hours, but no other associated symptoms. After a month, the episodes began to occur more regularly during the day and were accompanied by a pounding headache that began in the back of her neck and extended up and over her head. Her primary care physician prescribed sertraline (Zoloft) and referred her to a neurologist to evaluate the headaches. The neurologic workup included brain magnetic resonance imaging and electroencephalography, both of which were normal.
After 8 weeks on sertraline, the episodes continued to increase in frequency and severity, and her physician switched her to paroxetine (Paxil) and added lorazepam (Ativan), which did not improve her symptoms. Over the past 2 months, during which time she has not been taking any medications, the episodes began to involve nausea and, more recently, vomiting, with episodes occurring as often as once or twice daily, and with intermittent symptom-free days. None of the prior episodes was accompanied by symptoms as severe as those she is currently experiencing.
She is otherwise healthy with no chronic diseases. Her surgical history includes resection of an angiolipoma from her right arm and dilation and curettage for endometrial polyps. She has no personal or family history of psychiatric illness.
PHYSICAL EXAMINATION
The patient is slender and tremulous but does not appear diaphoretic. Her blood pressure is 176/92 mm Hg, pulse 98, temperature 36.5°C (97.7°F), and respiratory rate 20 per minute. Oxygen saturation by pulse oximetry is 98% on room air. She has dry mucus membranes and orthostatic hypotension, but her physical examination is otherwise normal. Electrocardiography (ECG) shows a normal sinus rhythm with a prolonged QTc of 571 ms and peaked P and T waves.
LABORATORY VALUES
- Hemoglobin 15.6 g/dL (reference range 11.5–15.5)
- Hematocrit 47.2% (36.0–46.0)
- Platelet count 448 × 109/L (150–400)
- White cell count 18.65 × 109/L (3.70–11.00)
- Potassium 2.5 mmol/L (3.5–4.0)
- Chloride 97 mmol/L (98–110)
- Bicarbonate 21 mmol/L (23–32)
- Anion gap 20 mmol/L (0–15)
- Glucose 233 mg/dL (65–100).
Sodium, blood urea nitrogen, and creatinine levels are all within normal limits. Urinalysis suggests a urinary tract infection.
IS THIS A PANIC ATTACK?
1. Which of the following is not characteristic of a panic attack?
- Nausea and vomiting
- Onset during sleep
- Palpitations
- Chest pain or discomfort
- Headache
- Trembling or shaking
According to the Diagnostic and Statistical Manual of Mental Disorders (Fourth Edition) (DSM-IV), the diagnosis of panic attack requires the presence of intense fear or discomfort and four or more other symptoms that may come from any of six domains:
- Cardiovascular: palpitations, pounding heart, tachycardia, and chest pain or discomfort
- Autonomic: sweating, chills or hot flushes, and trembling or shaking
- Pulmonary: shortness of breath or a smothering sensation
- Neurologic: dizziness or light-headedness and paresthesias
- Gastrointestinal: choking and nausea or abdominal distress
- Psychological: compass derealization, depersonalization, and the fear of losing control or “going crazy.”1
Two aspects of the patient’s history may be misinterpreted by those unfamiliar with the symptomatology of panic attack. First, although panic disorder carries an increased risk of many comorbidities, including migraine, headache is not typically associated with the panic attacks themselves.2 Second, while not a part of the diagnostic criteria, sleep disturbances are common in patients with panic disorder, and 30% to 45% of patients with the disorder experience recurrent nocturnal panic attacks.3 Therefore, the correct answer is headache.
THE DIFFERENTIAL DIAGNOSIS
When considering a diagnosis of panic attack or panic disorder, the DSM-IV mandates that medical causes of the symptoms must be excluded. Common conditions causing a similar spectrum of symptoms include hyperthyroidism, caffeine and stimulant use or abuse, asthma, cardiac arrhythmias, alcohol withdrawal, and, more rarely, complex partial seizures and pheochromocytoma.2,4 Many of these conditions can be ruled out by the history alone in a reliable patient.
Our patient’s electrocardiogram showed no evidence of ischemia or arrhythmias. Also, her recent negative neurologic workup makes seizure activity less likely.
Many of this patient’s laboratory abnormalities are easily explained by her repeated bouts of vomiting. Specifically, her elevated hemoglobin level and hematocrit are likely secondary to volume contraction, while hypochloremia is seen following losses of HCl with emesis. Typically, however, patients with vomiting have a hypochloremic metabolic alkalosis, and her low serum bicarbonate level is inconsistent with the history.
Three factors might be contributing to this patient’s hypokalemia. First, in a volume-depleted state, the cortical collecting tubules secrete potassium in exchange for increased sodium reabsorption in an attempt to correct volume status. Second, the alkalotic state caused by losses of acid with vomiting results in a transcellular shift of potassium ions into cells in exchange for hydrogen ions. Third, increased levels of epinephrine also cause a shift of potassium ions into cells.5 Potassium is not lost directly through nausea and vomiting.
A state of catecholamine excess, such as during a severe panic attack or in the presence of a catecholamine-secreting tumor, could explain many of her abnormalities. In addition to causing hypokalemia, epinephrine has a gluconeogenic effect, whereas norepinephrine inhibits insulin release, providing a potential explanation for hyperglycemia in a patient with no risk factors for diabetes. Finally, catecholamine excess contributes to lactic acidosis, which could help to explain the low serum bicarbonate level and the elevated anion gap, but unless we take arterial blood gas measurements, the patient’s acid-base status cannot be determined.
While panic attacks do stimulate the sympathetic nervous system, certain elements of her history raise the clinical suspicion for another process. First, the severity of the electrolyte abnormalities is suspicious. Second, a typical panic attack peaks at 10 minutes and begins to subside, whereas this woman’s symptoms have persisted for 12 hours. Finally, the clinical history, in particular the prominence of headaches associated with the symptoms, is inconsistent with classic panic attack. Consequently, an alternative diagnosis, such as pheochromocytoma, deserves more careful evaluation.
Whenever laboratory results do not fit with the clinical scenario or patient, however, one final possibility should always be considered—laboratory error. Errors can be preanalytical (eg, patient misidentification), analytical, or postanalytical. In aggregate, the frequency of errors in laboratory results is 1 in 214 to 8,316.6 Given that even the more conservative estimates show an incidence higher than that of many of the rare diseases for which clinicians may be testing, laboratory error always deserves consideration.
COULD THIS BE PHEOCHROMOCYTOMA?
Pheochromocytoma is a neuroendocrine tumor most commonly arising from the chromaffin cells of the adrenal medulla. However, extra-adrenal pheochromocytoma, generally paraganglioma, accounts for 15% to 20% of these tumors. Although the condition is generally considered very rare, autopsy studies have demonstrated a prevalence of 0.05%, suggesting that many tumors are either missed or are not clinically significant.
The diagnosis is most often sought in hypertensive patients, a population in which pheochromocytoma has a prevalence of 0.1% to 0.6%.7
2. What is the most common presenting symptom of pheochromocytoma?
- Paroxysmal hypertension
- Sustained hypertension
- Nausea
- Cardiomyopathy
- Headache
- Hemorrhagic shock
- Psychological symptoms such as anxiety or panic
Although 50% to 60% of patients with pheochromocytoma have sustained hypertension, it may be absent in patients with primarily epinephrine-secreting tumors or large tumors that degrade catecholamines, leading to normal or low blood pressure.
Cardiomyopathy is a rare consequence of untreated pheochromocytoma, caused by the effects of excess circulating catecholamines over a long period of time.8 As seen in this patient, a prolonged QTc on ECG associated with elevated levels of norepinephrine and normetanephrine may be the only red flag.9
Pheochromocytoma is typically an extremely well-vascularized tumor, and rupture or hemorrhage is a rare but often fatal complication.
IMPORTANT FAMILY HISTORY
The classic “rule of 10s” suggests that 10% of pheochromocytomas are hereditary, but in fact the number may be higher. In a large cohort of patients with apparently sporadic pheochromocytoma, 25% were found to have germ-line mutations.10 This finding highlights the importance not only of obtaining a thorough family history, but also of genetic testing and counseling once the diagnosis has been made.
3. Which hereditary syndrome is not associated with pheochromocytoma?
- Von Hippel-Lindau syndrome
- Neurofibromatosis type 1
- Neurofibromatosis type 2
- Multiple endocrine neoplasia type 2
- Paraganglioma syndromes
Germ-line mutations in five genes related to three hereditary syndromes (von Hippel-Lindau, neurofibromatosis type 1, and multiple endocrine neoplasia type 2) and in two genes related to paraganglioma syndromes are known to be associated with pheochromocytoma.7
Von Hippel-Lindau syndrome
Von Hippel-Lindau syndrome affects 1 in 36,000 live births. It is caused by a mutation of the von Hippel-Lindau gene on chromosome 3, and 10% to 20% of patients with the syndrome have pheochromocytoma. Other associated problems include renal clear-cell carcinomas and cysts, central nervous system and retinal hemangioblastomas, pancreatic tumors and cysts, endolymphatic tumors, and epididymal cysts.
Neurofibromatosis type 1
Neurofibromatosis type 1 affects 1 in 2,500 to 3,000 individuals and is caused by a mutation of the neurofibromatosis type 1 gene on chromosome 17. The disease is diagnosed by the presence of café-au-lait macules, axillary or inguinal freckling (or both), dermal or plexiform neurofibromas, Lisch nodules, or osseous lesions, but the condition is associated with many other pathologic findings, including optic pathway gliomas, cardiovascular abnormalities, and, in up to 5.7% of patients, pheochromocytoma.11
Neurofibromatosis type 2
Neurofibromatosis type 2 affects 1 in 25,000 live births and is caused by a mutation of the neurofibromatosis type 2 gene on chromosome 22. Patients often develop nervous system tumors, ophthalmologic pathology, and cutaneous lesions, but the condition is not associated with pheochromocytoma.12
Multiple endocrine neoplasia type 2
Multiple endocrine neoplasia type 2 affects 1 in 35,000 individuals and is caused by an activating mutation of the RET proto-oncogene on chromosome 21. The syndrome is most worrisome because of the 95% lifetime risk of medullary thyroid carcinoma in affected patients, but it is also associated with a 50% risk of pheochromocytoma and a 20% to 30% risk of primary hyperparathyroidism. Pheochromocytoma is the presenting clinical problem in 10% to 30% of patients.13
Paraganglioma syndromes
Paraganglioma syndromes are caused by mutations in the three genes encoding subunits of the succinate dehydrogenase enzyme. These mutations affect 1 in 30,000 to 100,000 individuals and incur a 70% lifetime risk of developing pheochromocytoma or paraganglioma.14
TESTING FOR AND MANAGING PHEOCHROMOCYTOMA
The consequences of untreated pheochromocytoma are potentially devastating and include progression to metastatic disease, hypertensive crises, cardiomyopathy, and adrenal hemorrhage. Nevertheless, the average patient goes 3 years before receiving the correct diagnosis.7 Consequently, heightened suspicion and tests with both high sensitivity and specificity are needed.
4. Which test for pheochromocytoma has the highest sensitivity?
- Plasma free metanephrines
- Plasma catecholamines
- Urine total metanephrines
- Urine fractionated metanephrines
- Urine catecholamines
- Urine vanillylmandelic acid
Some researchers have also examined plasma total metanephrines and found that any one of these three biochemical markers at a value two times greater than the upper limit of normal provides specificity of around 95%.16
Further laboratory tests in our patient
- Serum dopamine 70 pg/mL (reference range 0–20)
- Norepinephrine 2,018 pg/mL (80–520)
- Epinephrine 2,479 pg/mL (10–200)
- Free normetanephrine 12 pg/mL (< 0.9)
- Free metanephrine 17.8 pg/mL (< 0.5).
VALUE OF IMAGING STUDIES
Computed tomography has a sensitivity of up to 95% for detecting adrenal tumors and is able to detect tumors larger than 0.5 cm, but its specificity may be as low as 50%.17 Studies utilizing modern imaging equipment report a prevalence of adrenal incidentaloma of 4%, of which only 1.5% to 11% are pheochromocytoma.18 Thus, while the simultaneous occurrence of pheochromocytoma-like symptoms and an incidentaloma is not common, the potential for unnecessary surgery precludes diagnosis and treatment based on symptoms and imaging alone.
Magnetic resonance imaging has similar sensitivity and specificity but can better characterize the tumor’s blood supply and relationship to other structures.
Iodine 131 metaiodobenzylguanidine (MIBG) scanning is a physiologic study that uses a radiolabeled amine. Since it can identify pheochromocytoma regardless of location, MIBG scanning is typically used when pheochromocytoma is diagnosed by biochemical testing but CT and MRI fail to locate the lesion, or as a follow-up test in patients in whom recurrence or metastasis is suspected or documented.
The specificity of MIBG scanning is 95% to 100%, but the need to protect the thyroid from ablation and the potential need to repeat scans for up to 72 hours make it a poor choice for the initial evaluation.17
5. What is the next best step in our patient’s management?
- Treat her hypertension with a beta-blocker
- Begin a course of alpha-blockade
- Urgent surgery
- Observation
Because of the high concentration of circulating catecholamines and the instability of the tumor to physical manipulation, appropriate medical management before surgical resection is of paramount importance.
Beta-blockade can lead to malignant hypertension due to the unopposed alpha stimulation and must not be begun until alpha-blockade has been started. The standard of care is to give an alpha-blocker or calcium channel blocker 10 to 14 days before surgery. Typically, oral phenoxybenzamine (Dibenzyline) 10 mg twice daily is started and titrated upward daily by 10 to 20 mg until a target seated blood pressure of 120/80 mm Hg is obtained. Selective alpha-1 blockers such as prazosin (Minipress) and terazosin (Hytrin) have also been used and have the benefit of a preserved alpha-2 catecholamine reuptake mechanism.17
After several days, a beta-blocker may be added, particularly for patients with arrhythmias.7 In patients with refractory hypertension, metyrosine (Demser) can be useful.
During surgery, the patient’s hemodynamic stability and glucose levels can fluctuate rapidly from sudden releases of catecholamines during manipulation of the tumor, as well as from the sudden loss of catecholamines after ligation of draining vessels. Advances in medical care have reduced the perioperative death rate from 50% to less than 3%.7,19
CASE CONCLUSION AND FOLLOW-UP
Two months after her initial presentation, the patient underwent open surgery and had the mass removed without complications. She reports that the “panic attacks” have ceased completely.
The recurrence rate of pheochromocytoma is 13% in patients with sporadic disease and 33% in patients with familial syndromes. The overall recurrence rate with long-term follow-up is 17%, half of recurrences being malignant disease. All patients should therefore be followed in the clinic annually for at least 10 years to identify and treat recurrences early,7 and many experts recommend lifelong follow-up, even for patients without hereditary syndromes.17
Nearly every diagnosis in the DSM-IV includes the caveat that medical causes of disease must be excluded before psychiatric labels can be applied. Although panic disorder and panic attack are far more common than pheochromocytoma, just as essential hypertension is far more common than pheochromocytoma, physicians need to remember that pheochromocytoma can cause symptoms common to both illnesses. Thus, while rare conditions are rare, atypical presentations of common conditions may deserve a second glance.
- Yates WR. Phenomenology and epidemiology of panic disorder. Ann Clin Psychiatry 2009; 21:95–102.
- Katon WJ. Clinical practice. Panic disorder. N Engl J Med 2006; 354:2360–2367.
- Craske MG, Tsao JC. Assessment and treatment of nocturnal panic attacks. Sleep Med Rev 2005; 9:173–184.
- Roy-Byrne PP, Craske MG, Stein MB. Panic disorder. Lancet 2006; 368:1023–1032.
- Beal AL, Deuser WE, Beilman GJ. A role for epinephrine in post-traumatic hypokalemia. Shock 2007; 27:358–363.
- Kalra J. Medical errors: impact on clinical laboratories and other critical areas. Clin Biochem 2004; 37:1052–1062.
- Lenders JW, Eisenhofer G, Mannelli M, Pacak K. Phaeochromocytoma. Lancet 2005; 366:665–675.
- Leissner KB, Mahmood F, Aragam JR, Amouzgar A, Ortega R. Catecholamine-induced cardiomyopathy and pheochromocytoma. Anesth Analg 2008; 107:410–412.
- Yu R, Furmark L, Wong C. Cardiac abnormalities associated with pheochromocytoma and other adrenal tumors. Endocr Pract 2009; 15:10–16.
- Neumann HP, Bausch B, McWhinney SR, et al; Freiburg-Warsaw-Columbus Pheochromocytoma Study Group. Germ-line mutations in nonsyndromic pheochromocytoma. N Engl J Med 2002; 346:1459–1466.
- Williams VC, Lucas J, Babcock MA, Gutmann DH, Korf B, Maria BL. Neurofibromatosis type 1 revisited. Pediatrics 2009; 123:124–133.
- Asthagiri AR, Parry DM, Butman JA, et al. Neurofibromatosis type 2. Lancet 2009; 373:1974–1986.
- Callender GG, Rich TA, Perrier ND. Multiple endocrine neoplasia syndromes. Surg Clin North Am 2008; 88:863–895.
- Pasini B, Stratakis CA. SDH mutations in tumorigenesis and inherited endocrine tumours: lesson from the phaeochromocytoma-paraganglioma syndromes. J Intern Med 2009; 266:19–42.
- Yu R, Nissen NN, Chopra P, Dhall D, Phillips E, Wei M. Diagnosis and treatment of pheochromocytoma in an academic hospital from 1997 to 2007. Am J Med 2009; 122:85–95.
- Grouzmann E, Drouard-Troalen L, Baudin E, et al. Diagnostic accuracy of free and total metanephrines in plasma and fractionated metanephrines in urine of patients with pheochromocytoma. Eur J Endocrinol 2010; 162:951–960.
- Mittendorf EA, Evans DB, Lee JE, Perrier ND. Pheochromocytoma: advances in genetics, diagnosis, localization, and treatment. Hematol Oncol Clin North Am 2007; 21:509–525.
- Singh PK, Buch HN. Adrenal incidentaloma: evaluation and management. J Clin Pathol 2008; 61:1168–1173.
- Kasturi S, Kutikov A, Guzzo TJ, Smith AL, Wein AJ. Modern management of pheochromocytoma. Nat Clin Pract Urol 2007; 4:630–633.
A 50-year-old woman presents to the emergency department because of repeated episodes of vomiting over the past 12 hours. She reports eight episodes of non-bloody, nonbilious emesis associated with palpitations and feelings of anxiety, but with no fever or diarrhea. She has not traveled recently and does not have any sick contacts.
She reports that she never had health problems until 6 months ago, when she began having panic attacks that woke her from sleep. The episodes first occurred once or twice per week, usually at night, and involved palpitations and feelings of anxiety that lasted 2 to 4 hours, but no other associated symptoms. After a month, the episodes began to occur more regularly during the day and were accompanied by a pounding headache that began in the back of her neck and extended up and over her head. Her primary care physician prescribed sertraline (Zoloft) and referred her to a neurologist to evaluate the headaches. The neurologic workup included brain magnetic resonance imaging and electroencephalography, both of which were normal.
After 8 weeks on sertraline, the episodes continued to increase in frequency and severity, and her physician switched her to paroxetine (Paxil) and added lorazepam (Ativan), which did not improve her symptoms. Over the past 2 months, during which time she has not been taking any medications, the episodes began to involve nausea and, more recently, vomiting, with episodes occurring as often as once or twice daily, and with intermittent symptom-free days. None of the prior episodes was accompanied by symptoms as severe as those she is currently experiencing.
She is otherwise healthy with no chronic diseases. Her surgical history includes resection of an angiolipoma from her right arm and dilation and curettage for endometrial polyps. She has no personal or family history of psychiatric illness.
PHYSICAL EXAMINATION
The patient is slender and tremulous but does not appear diaphoretic. Her blood pressure is 176/92 mm Hg, pulse 98, temperature 36.5°C (97.7°F), and respiratory rate 20 per minute. Oxygen saturation by pulse oximetry is 98% on room air. She has dry mucus membranes and orthostatic hypotension, but her physical examination is otherwise normal. Electrocardiography (ECG) shows a normal sinus rhythm with a prolonged QTc of 571 ms and peaked P and T waves.
LABORATORY VALUES
- Hemoglobin 15.6 g/dL (reference range 11.5–15.5)
- Hematocrit 47.2% (36.0–46.0)
- Platelet count 448 × 109/L (150–400)
- White cell count 18.65 × 109/L (3.70–11.00)
- Potassium 2.5 mmol/L (3.5–4.0)
- Chloride 97 mmol/L (98–110)
- Bicarbonate 21 mmol/L (23–32)
- Anion gap 20 mmol/L (0–15)
- Glucose 233 mg/dL (65–100).
Sodium, blood urea nitrogen, and creatinine levels are all within normal limits. Urinalysis suggests a urinary tract infection.
IS THIS A PANIC ATTACK?
1. Which of the following is not characteristic of a panic attack?
- Nausea and vomiting
- Onset during sleep
- Palpitations
- Chest pain or discomfort
- Headache
- Trembling or shaking
According to the Diagnostic and Statistical Manual of Mental Disorders (Fourth Edition) (DSM-IV), the diagnosis of panic attack requires the presence of intense fear or discomfort and four or more other symptoms that may come from any of six domains:
- Cardiovascular: palpitations, pounding heart, tachycardia, and chest pain or discomfort
- Autonomic: sweating, chills or hot flushes, and trembling or shaking
- Pulmonary: shortness of breath or a smothering sensation
- Neurologic: dizziness or light-headedness and paresthesias
- Gastrointestinal: choking and nausea or abdominal distress
- Psychological: compass derealization, depersonalization, and the fear of losing control or “going crazy.”1
Two aspects of the patient’s history may be misinterpreted by those unfamiliar with the symptomatology of panic attack. First, although panic disorder carries an increased risk of many comorbidities, including migraine, headache is not typically associated with the panic attacks themselves.2 Second, while not a part of the diagnostic criteria, sleep disturbances are common in patients with panic disorder, and 30% to 45% of patients with the disorder experience recurrent nocturnal panic attacks.3 Therefore, the correct answer is headache.
THE DIFFERENTIAL DIAGNOSIS
When considering a diagnosis of panic attack or panic disorder, the DSM-IV mandates that medical causes of the symptoms must be excluded. Common conditions causing a similar spectrum of symptoms include hyperthyroidism, caffeine and stimulant use or abuse, asthma, cardiac arrhythmias, alcohol withdrawal, and, more rarely, complex partial seizures and pheochromocytoma.2,4 Many of these conditions can be ruled out by the history alone in a reliable patient.
Our patient’s electrocardiogram showed no evidence of ischemia or arrhythmias. Also, her recent negative neurologic workup makes seizure activity less likely.
Many of this patient’s laboratory abnormalities are easily explained by her repeated bouts of vomiting. Specifically, her elevated hemoglobin level and hematocrit are likely secondary to volume contraction, while hypochloremia is seen following losses of HCl with emesis. Typically, however, patients with vomiting have a hypochloremic metabolic alkalosis, and her low serum bicarbonate level is inconsistent with the history.
Three factors might be contributing to this patient’s hypokalemia. First, in a volume-depleted state, the cortical collecting tubules secrete potassium in exchange for increased sodium reabsorption in an attempt to correct volume status. Second, the alkalotic state caused by losses of acid with vomiting results in a transcellular shift of potassium ions into cells in exchange for hydrogen ions. Third, increased levels of epinephrine also cause a shift of potassium ions into cells.5 Potassium is not lost directly through nausea and vomiting.
A state of catecholamine excess, such as during a severe panic attack or in the presence of a catecholamine-secreting tumor, could explain many of her abnormalities. In addition to causing hypokalemia, epinephrine has a gluconeogenic effect, whereas norepinephrine inhibits insulin release, providing a potential explanation for hyperglycemia in a patient with no risk factors for diabetes. Finally, catecholamine excess contributes to lactic acidosis, which could help to explain the low serum bicarbonate level and the elevated anion gap, but unless we take arterial blood gas measurements, the patient’s acid-base status cannot be determined.
While panic attacks do stimulate the sympathetic nervous system, certain elements of her history raise the clinical suspicion for another process. First, the severity of the electrolyte abnormalities is suspicious. Second, a typical panic attack peaks at 10 minutes and begins to subside, whereas this woman’s symptoms have persisted for 12 hours. Finally, the clinical history, in particular the prominence of headaches associated with the symptoms, is inconsistent with classic panic attack. Consequently, an alternative diagnosis, such as pheochromocytoma, deserves more careful evaluation.
Whenever laboratory results do not fit with the clinical scenario or patient, however, one final possibility should always be considered—laboratory error. Errors can be preanalytical (eg, patient misidentification), analytical, or postanalytical. In aggregate, the frequency of errors in laboratory results is 1 in 214 to 8,316.6 Given that even the more conservative estimates show an incidence higher than that of many of the rare diseases for which clinicians may be testing, laboratory error always deserves consideration.
COULD THIS BE PHEOCHROMOCYTOMA?
Pheochromocytoma is a neuroendocrine tumor most commonly arising from the chromaffin cells of the adrenal medulla. However, extra-adrenal pheochromocytoma, generally paraganglioma, accounts for 15% to 20% of these tumors. Although the condition is generally considered very rare, autopsy studies have demonstrated a prevalence of 0.05%, suggesting that many tumors are either missed or are not clinically significant.
The diagnosis is most often sought in hypertensive patients, a population in which pheochromocytoma has a prevalence of 0.1% to 0.6%.7
2. What is the most common presenting symptom of pheochromocytoma?
- Paroxysmal hypertension
- Sustained hypertension
- Nausea
- Cardiomyopathy
- Headache
- Hemorrhagic shock
- Psychological symptoms such as anxiety or panic
Although 50% to 60% of patients with pheochromocytoma have sustained hypertension, it may be absent in patients with primarily epinephrine-secreting tumors or large tumors that degrade catecholamines, leading to normal or low blood pressure.
Cardiomyopathy is a rare consequence of untreated pheochromocytoma, caused by the effects of excess circulating catecholamines over a long period of time.8 As seen in this patient, a prolonged QTc on ECG associated with elevated levels of norepinephrine and normetanephrine may be the only red flag.9
Pheochromocytoma is typically an extremely well-vascularized tumor, and rupture or hemorrhage is a rare but often fatal complication.
IMPORTANT FAMILY HISTORY
The classic “rule of 10s” suggests that 10% of pheochromocytomas are hereditary, but in fact the number may be higher. In a large cohort of patients with apparently sporadic pheochromocytoma, 25% were found to have germ-line mutations.10 This finding highlights the importance not only of obtaining a thorough family history, but also of genetic testing and counseling once the diagnosis has been made.
3. Which hereditary syndrome is not associated with pheochromocytoma?
- Von Hippel-Lindau syndrome
- Neurofibromatosis type 1
- Neurofibromatosis type 2
- Multiple endocrine neoplasia type 2
- Paraganglioma syndromes
Germ-line mutations in five genes related to three hereditary syndromes (von Hippel-Lindau, neurofibromatosis type 1, and multiple endocrine neoplasia type 2) and in two genes related to paraganglioma syndromes are known to be associated with pheochromocytoma.7
Von Hippel-Lindau syndrome
Von Hippel-Lindau syndrome affects 1 in 36,000 live births. It is caused by a mutation of the von Hippel-Lindau gene on chromosome 3, and 10% to 20% of patients with the syndrome have pheochromocytoma. Other associated problems include renal clear-cell carcinomas and cysts, central nervous system and retinal hemangioblastomas, pancreatic tumors and cysts, endolymphatic tumors, and epididymal cysts.
Neurofibromatosis type 1
Neurofibromatosis type 1 affects 1 in 2,500 to 3,000 individuals and is caused by a mutation of the neurofibromatosis type 1 gene on chromosome 17. The disease is diagnosed by the presence of café-au-lait macules, axillary or inguinal freckling (or both), dermal or plexiform neurofibromas, Lisch nodules, or osseous lesions, but the condition is associated with many other pathologic findings, including optic pathway gliomas, cardiovascular abnormalities, and, in up to 5.7% of patients, pheochromocytoma.11
Neurofibromatosis type 2
Neurofibromatosis type 2 affects 1 in 25,000 live births and is caused by a mutation of the neurofibromatosis type 2 gene on chromosome 22. Patients often develop nervous system tumors, ophthalmologic pathology, and cutaneous lesions, but the condition is not associated with pheochromocytoma.12
Multiple endocrine neoplasia type 2
Multiple endocrine neoplasia type 2 affects 1 in 35,000 individuals and is caused by an activating mutation of the RET proto-oncogene on chromosome 21. The syndrome is most worrisome because of the 95% lifetime risk of medullary thyroid carcinoma in affected patients, but it is also associated with a 50% risk of pheochromocytoma and a 20% to 30% risk of primary hyperparathyroidism. Pheochromocytoma is the presenting clinical problem in 10% to 30% of patients.13
Paraganglioma syndromes
Paraganglioma syndromes are caused by mutations in the three genes encoding subunits of the succinate dehydrogenase enzyme. These mutations affect 1 in 30,000 to 100,000 individuals and incur a 70% lifetime risk of developing pheochromocytoma or paraganglioma.14
TESTING FOR AND MANAGING PHEOCHROMOCYTOMA
The consequences of untreated pheochromocytoma are potentially devastating and include progression to metastatic disease, hypertensive crises, cardiomyopathy, and adrenal hemorrhage. Nevertheless, the average patient goes 3 years before receiving the correct diagnosis.7 Consequently, heightened suspicion and tests with both high sensitivity and specificity are needed.
4. Which test for pheochromocytoma has the highest sensitivity?
- Plasma free metanephrines
- Plasma catecholamines
- Urine total metanephrines
- Urine fractionated metanephrines
- Urine catecholamines
- Urine vanillylmandelic acid
Some researchers have also examined plasma total metanephrines and found that any one of these three biochemical markers at a value two times greater than the upper limit of normal provides specificity of around 95%.16
Further laboratory tests in our patient
- Serum dopamine 70 pg/mL (reference range 0–20)
- Norepinephrine 2,018 pg/mL (80–520)
- Epinephrine 2,479 pg/mL (10–200)
- Free normetanephrine 12 pg/mL (< 0.9)
- Free metanephrine 17.8 pg/mL (< 0.5).
VALUE OF IMAGING STUDIES
Computed tomography has a sensitivity of up to 95% for detecting adrenal tumors and is able to detect tumors larger than 0.5 cm, but its specificity may be as low as 50%.17 Studies utilizing modern imaging equipment report a prevalence of adrenal incidentaloma of 4%, of which only 1.5% to 11% are pheochromocytoma.18 Thus, while the simultaneous occurrence of pheochromocytoma-like symptoms and an incidentaloma is not common, the potential for unnecessary surgery precludes diagnosis and treatment based on symptoms and imaging alone.
Magnetic resonance imaging has similar sensitivity and specificity but can better characterize the tumor’s blood supply and relationship to other structures.
Iodine 131 metaiodobenzylguanidine (MIBG) scanning is a physiologic study that uses a radiolabeled amine. Since it can identify pheochromocytoma regardless of location, MIBG scanning is typically used when pheochromocytoma is diagnosed by biochemical testing but CT and MRI fail to locate the lesion, or as a follow-up test in patients in whom recurrence or metastasis is suspected or documented.
The specificity of MIBG scanning is 95% to 100%, but the need to protect the thyroid from ablation and the potential need to repeat scans for up to 72 hours make it a poor choice for the initial evaluation.17
5. What is the next best step in our patient’s management?
- Treat her hypertension with a beta-blocker
- Begin a course of alpha-blockade
- Urgent surgery
- Observation
Because of the high concentration of circulating catecholamines and the instability of the tumor to physical manipulation, appropriate medical management before surgical resection is of paramount importance.
Beta-blockade can lead to malignant hypertension due to the unopposed alpha stimulation and must not be begun until alpha-blockade has been started. The standard of care is to give an alpha-blocker or calcium channel blocker 10 to 14 days before surgery. Typically, oral phenoxybenzamine (Dibenzyline) 10 mg twice daily is started and titrated upward daily by 10 to 20 mg until a target seated blood pressure of 120/80 mm Hg is obtained. Selective alpha-1 blockers such as prazosin (Minipress) and terazosin (Hytrin) have also been used and have the benefit of a preserved alpha-2 catecholamine reuptake mechanism.17
After several days, a beta-blocker may be added, particularly for patients with arrhythmias.7 In patients with refractory hypertension, metyrosine (Demser) can be useful.
During surgery, the patient’s hemodynamic stability and glucose levels can fluctuate rapidly from sudden releases of catecholamines during manipulation of the tumor, as well as from the sudden loss of catecholamines after ligation of draining vessels. Advances in medical care have reduced the perioperative death rate from 50% to less than 3%.7,19
CASE CONCLUSION AND FOLLOW-UP
Two months after her initial presentation, the patient underwent open surgery and had the mass removed without complications. She reports that the “panic attacks” have ceased completely.
The recurrence rate of pheochromocytoma is 13% in patients with sporadic disease and 33% in patients with familial syndromes. The overall recurrence rate with long-term follow-up is 17%, half of recurrences being malignant disease. All patients should therefore be followed in the clinic annually for at least 10 years to identify and treat recurrences early,7 and many experts recommend lifelong follow-up, even for patients without hereditary syndromes.17
Nearly every diagnosis in the DSM-IV includes the caveat that medical causes of disease must be excluded before psychiatric labels can be applied. Although panic disorder and panic attack are far more common than pheochromocytoma, just as essential hypertension is far more common than pheochromocytoma, physicians need to remember that pheochromocytoma can cause symptoms common to both illnesses. Thus, while rare conditions are rare, atypical presentations of common conditions may deserve a second glance.
A 50-year-old woman presents to the emergency department because of repeated episodes of vomiting over the past 12 hours. She reports eight episodes of non-bloody, nonbilious emesis associated with palpitations and feelings of anxiety, but with no fever or diarrhea. She has not traveled recently and does not have any sick contacts.
She reports that she never had health problems until 6 months ago, when she began having panic attacks that woke her from sleep. The episodes first occurred once or twice per week, usually at night, and involved palpitations and feelings of anxiety that lasted 2 to 4 hours, but no other associated symptoms. After a month, the episodes began to occur more regularly during the day and were accompanied by a pounding headache that began in the back of her neck and extended up and over her head. Her primary care physician prescribed sertraline (Zoloft) and referred her to a neurologist to evaluate the headaches. The neurologic workup included brain magnetic resonance imaging and electroencephalography, both of which were normal.
After 8 weeks on sertraline, the episodes continued to increase in frequency and severity, and her physician switched her to paroxetine (Paxil) and added lorazepam (Ativan), which did not improve her symptoms. Over the past 2 months, during which time she has not been taking any medications, the episodes began to involve nausea and, more recently, vomiting, with episodes occurring as often as once or twice daily, and with intermittent symptom-free days. None of the prior episodes was accompanied by symptoms as severe as those she is currently experiencing.
She is otherwise healthy with no chronic diseases. Her surgical history includes resection of an angiolipoma from her right arm and dilation and curettage for endometrial polyps. She has no personal or family history of psychiatric illness.
PHYSICAL EXAMINATION
The patient is slender and tremulous but does not appear diaphoretic. Her blood pressure is 176/92 mm Hg, pulse 98, temperature 36.5°C (97.7°F), and respiratory rate 20 per minute. Oxygen saturation by pulse oximetry is 98% on room air. She has dry mucus membranes and orthostatic hypotension, but her physical examination is otherwise normal. Electrocardiography (ECG) shows a normal sinus rhythm with a prolonged QTc of 571 ms and peaked P and T waves.
LABORATORY VALUES
- Hemoglobin 15.6 g/dL (reference range 11.5–15.5)
- Hematocrit 47.2% (36.0–46.0)
- Platelet count 448 × 109/L (150–400)
- White cell count 18.65 × 109/L (3.70–11.00)
- Potassium 2.5 mmol/L (3.5–4.0)
- Chloride 97 mmol/L (98–110)
- Bicarbonate 21 mmol/L (23–32)
- Anion gap 20 mmol/L (0–15)
- Glucose 233 mg/dL (65–100).
Sodium, blood urea nitrogen, and creatinine levels are all within normal limits. Urinalysis suggests a urinary tract infection.
IS THIS A PANIC ATTACK?
1. Which of the following is not characteristic of a panic attack?
- Nausea and vomiting
- Onset during sleep
- Palpitations
- Chest pain or discomfort
- Headache
- Trembling or shaking
According to the Diagnostic and Statistical Manual of Mental Disorders (Fourth Edition) (DSM-IV), the diagnosis of panic attack requires the presence of intense fear or discomfort and four or more other symptoms that may come from any of six domains:
- Cardiovascular: palpitations, pounding heart, tachycardia, and chest pain or discomfort
- Autonomic: sweating, chills or hot flushes, and trembling or shaking
- Pulmonary: shortness of breath or a smothering sensation
- Neurologic: dizziness or light-headedness and paresthesias
- Gastrointestinal: choking and nausea or abdominal distress
- Psychological: compass derealization, depersonalization, and the fear of losing control or “going crazy.”1
Two aspects of the patient’s history may be misinterpreted by those unfamiliar with the symptomatology of panic attack. First, although panic disorder carries an increased risk of many comorbidities, including migraine, headache is not typically associated with the panic attacks themselves.2 Second, while not a part of the diagnostic criteria, sleep disturbances are common in patients with panic disorder, and 30% to 45% of patients with the disorder experience recurrent nocturnal panic attacks.3 Therefore, the correct answer is headache.
THE DIFFERENTIAL DIAGNOSIS
When considering a diagnosis of panic attack or panic disorder, the DSM-IV mandates that medical causes of the symptoms must be excluded. Common conditions causing a similar spectrum of symptoms include hyperthyroidism, caffeine and stimulant use or abuse, asthma, cardiac arrhythmias, alcohol withdrawal, and, more rarely, complex partial seizures and pheochromocytoma.2,4 Many of these conditions can be ruled out by the history alone in a reliable patient.
Our patient’s electrocardiogram showed no evidence of ischemia or arrhythmias. Also, her recent negative neurologic workup makes seizure activity less likely.
Many of this patient’s laboratory abnormalities are easily explained by her repeated bouts of vomiting. Specifically, her elevated hemoglobin level and hematocrit are likely secondary to volume contraction, while hypochloremia is seen following losses of HCl with emesis. Typically, however, patients with vomiting have a hypochloremic metabolic alkalosis, and her low serum bicarbonate level is inconsistent with the history.
Three factors might be contributing to this patient’s hypokalemia. First, in a volume-depleted state, the cortical collecting tubules secrete potassium in exchange for increased sodium reabsorption in an attempt to correct volume status. Second, the alkalotic state caused by losses of acid with vomiting results in a transcellular shift of potassium ions into cells in exchange for hydrogen ions. Third, increased levels of epinephrine also cause a shift of potassium ions into cells.5 Potassium is not lost directly through nausea and vomiting.
A state of catecholamine excess, such as during a severe panic attack or in the presence of a catecholamine-secreting tumor, could explain many of her abnormalities. In addition to causing hypokalemia, epinephrine has a gluconeogenic effect, whereas norepinephrine inhibits insulin release, providing a potential explanation for hyperglycemia in a patient with no risk factors for diabetes. Finally, catecholamine excess contributes to lactic acidosis, which could help to explain the low serum bicarbonate level and the elevated anion gap, but unless we take arterial blood gas measurements, the patient’s acid-base status cannot be determined.
While panic attacks do stimulate the sympathetic nervous system, certain elements of her history raise the clinical suspicion for another process. First, the severity of the electrolyte abnormalities is suspicious. Second, a typical panic attack peaks at 10 minutes and begins to subside, whereas this woman’s symptoms have persisted for 12 hours. Finally, the clinical history, in particular the prominence of headaches associated with the symptoms, is inconsistent with classic panic attack. Consequently, an alternative diagnosis, such as pheochromocytoma, deserves more careful evaluation.
Whenever laboratory results do not fit with the clinical scenario or patient, however, one final possibility should always be considered—laboratory error. Errors can be preanalytical (eg, patient misidentification), analytical, or postanalytical. In aggregate, the frequency of errors in laboratory results is 1 in 214 to 8,316.6 Given that even the more conservative estimates show an incidence higher than that of many of the rare diseases for which clinicians may be testing, laboratory error always deserves consideration.
COULD THIS BE PHEOCHROMOCYTOMA?
Pheochromocytoma is a neuroendocrine tumor most commonly arising from the chromaffin cells of the adrenal medulla. However, extra-adrenal pheochromocytoma, generally paraganglioma, accounts for 15% to 20% of these tumors. Although the condition is generally considered very rare, autopsy studies have demonstrated a prevalence of 0.05%, suggesting that many tumors are either missed or are not clinically significant.
The diagnosis is most often sought in hypertensive patients, a population in which pheochromocytoma has a prevalence of 0.1% to 0.6%.7
2. What is the most common presenting symptom of pheochromocytoma?
- Paroxysmal hypertension
- Sustained hypertension
- Nausea
- Cardiomyopathy
- Headache
- Hemorrhagic shock
- Psychological symptoms such as anxiety or panic
Although 50% to 60% of patients with pheochromocytoma have sustained hypertension, it may be absent in patients with primarily epinephrine-secreting tumors or large tumors that degrade catecholamines, leading to normal or low blood pressure.
Cardiomyopathy is a rare consequence of untreated pheochromocytoma, caused by the effects of excess circulating catecholamines over a long period of time.8 As seen in this patient, a prolonged QTc on ECG associated with elevated levels of norepinephrine and normetanephrine may be the only red flag.9
Pheochromocytoma is typically an extremely well-vascularized tumor, and rupture or hemorrhage is a rare but often fatal complication.
IMPORTANT FAMILY HISTORY
The classic “rule of 10s” suggests that 10% of pheochromocytomas are hereditary, but in fact the number may be higher. In a large cohort of patients with apparently sporadic pheochromocytoma, 25% were found to have germ-line mutations.10 This finding highlights the importance not only of obtaining a thorough family history, but also of genetic testing and counseling once the diagnosis has been made.
3. Which hereditary syndrome is not associated with pheochromocytoma?
- Von Hippel-Lindau syndrome
- Neurofibromatosis type 1
- Neurofibromatosis type 2
- Multiple endocrine neoplasia type 2
- Paraganglioma syndromes
Germ-line mutations in five genes related to three hereditary syndromes (von Hippel-Lindau, neurofibromatosis type 1, and multiple endocrine neoplasia type 2) and in two genes related to paraganglioma syndromes are known to be associated with pheochromocytoma.7
Von Hippel-Lindau syndrome
Von Hippel-Lindau syndrome affects 1 in 36,000 live births. It is caused by a mutation of the von Hippel-Lindau gene on chromosome 3, and 10% to 20% of patients with the syndrome have pheochromocytoma. Other associated problems include renal clear-cell carcinomas and cysts, central nervous system and retinal hemangioblastomas, pancreatic tumors and cysts, endolymphatic tumors, and epididymal cysts.
Neurofibromatosis type 1
Neurofibromatosis type 1 affects 1 in 2,500 to 3,000 individuals and is caused by a mutation of the neurofibromatosis type 1 gene on chromosome 17. The disease is diagnosed by the presence of café-au-lait macules, axillary or inguinal freckling (or both), dermal or plexiform neurofibromas, Lisch nodules, or osseous lesions, but the condition is associated with many other pathologic findings, including optic pathway gliomas, cardiovascular abnormalities, and, in up to 5.7% of patients, pheochromocytoma.11
Neurofibromatosis type 2
Neurofibromatosis type 2 affects 1 in 25,000 live births and is caused by a mutation of the neurofibromatosis type 2 gene on chromosome 22. Patients often develop nervous system tumors, ophthalmologic pathology, and cutaneous lesions, but the condition is not associated with pheochromocytoma.12
Multiple endocrine neoplasia type 2
Multiple endocrine neoplasia type 2 affects 1 in 35,000 individuals and is caused by an activating mutation of the RET proto-oncogene on chromosome 21. The syndrome is most worrisome because of the 95% lifetime risk of medullary thyroid carcinoma in affected patients, but it is also associated with a 50% risk of pheochromocytoma and a 20% to 30% risk of primary hyperparathyroidism. Pheochromocytoma is the presenting clinical problem in 10% to 30% of patients.13
Paraganglioma syndromes
Paraganglioma syndromes are caused by mutations in the three genes encoding subunits of the succinate dehydrogenase enzyme. These mutations affect 1 in 30,000 to 100,000 individuals and incur a 70% lifetime risk of developing pheochromocytoma or paraganglioma.14
TESTING FOR AND MANAGING PHEOCHROMOCYTOMA
The consequences of untreated pheochromocytoma are potentially devastating and include progression to metastatic disease, hypertensive crises, cardiomyopathy, and adrenal hemorrhage. Nevertheless, the average patient goes 3 years before receiving the correct diagnosis.7 Consequently, heightened suspicion and tests with both high sensitivity and specificity are needed.
4. Which test for pheochromocytoma has the highest sensitivity?
- Plasma free metanephrines
- Plasma catecholamines
- Urine total metanephrines
- Urine fractionated metanephrines
- Urine catecholamines
- Urine vanillylmandelic acid
Some researchers have also examined plasma total metanephrines and found that any one of these three biochemical markers at a value two times greater than the upper limit of normal provides specificity of around 95%.16
Further laboratory tests in our patient
- Serum dopamine 70 pg/mL (reference range 0–20)
- Norepinephrine 2,018 pg/mL (80–520)
- Epinephrine 2,479 pg/mL (10–200)
- Free normetanephrine 12 pg/mL (< 0.9)
- Free metanephrine 17.8 pg/mL (< 0.5).
VALUE OF IMAGING STUDIES
Computed tomography has a sensitivity of up to 95% for detecting adrenal tumors and is able to detect tumors larger than 0.5 cm, but its specificity may be as low as 50%.17 Studies utilizing modern imaging equipment report a prevalence of adrenal incidentaloma of 4%, of which only 1.5% to 11% are pheochromocytoma.18 Thus, while the simultaneous occurrence of pheochromocytoma-like symptoms and an incidentaloma is not common, the potential for unnecessary surgery precludes diagnosis and treatment based on symptoms and imaging alone.
Magnetic resonance imaging has similar sensitivity and specificity but can better characterize the tumor’s blood supply and relationship to other structures.
Iodine 131 metaiodobenzylguanidine (MIBG) scanning is a physiologic study that uses a radiolabeled amine. Since it can identify pheochromocytoma regardless of location, MIBG scanning is typically used when pheochromocytoma is diagnosed by biochemical testing but CT and MRI fail to locate the lesion, or as a follow-up test in patients in whom recurrence or metastasis is suspected or documented.
The specificity of MIBG scanning is 95% to 100%, but the need to protect the thyroid from ablation and the potential need to repeat scans for up to 72 hours make it a poor choice for the initial evaluation.17
5. What is the next best step in our patient’s management?
- Treat her hypertension with a beta-blocker
- Begin a course of alpha-blockade
- Urgent surgery
- Observation
Because of the high concentration of circulating catecholamines and the instability of the tumor to physical manipulation, appropriate medical management before surgical resection is of paramount importance.
Beta-blockade can lead to malignant hypertension due to the unopposed alpha stimulation and must not be begun until alpha-blockade has been started. The standard of care is to give an alpha-blocker or calcium channel blocker 10 to 14 days before surgery. Typically, oral phenoxybenzamine (Dibenzyline) 10 mg twice daily is started and titrated upward daily by 10 to 20 mg until a target seated blood pressure of 120/80 mm Hg is obtained. Selective alpha-1 blockers such as prazosin (Minipress) and terazosin (Hytrin) have also been used and have the benefit of a preserved alpha-2 catecholamine reuptake mechanism.17
After several days, a beta-blocker may be added, particularly for patients with arrhythmias.7 In patients with refractory hypertension, metyrosine (Demser) can be useful.
During surgery, the patient’s hemodynamic stability and glucose levels can fluctuate rapidly from sudden releases of catecholamines during manipulation of the tumor, as well as from the sudden loss of catecholamines after ligation of draining vessels. Advances in medical care have reduced the perioperative death rate from 50% to less than 3%.7,19
CASE CONCLUSION AND FOLLOW-UP
Two months after her initial presentation, the patient underwent open surgery and had the mass removed without complications. She reports that the “panic attacks” have ceased completely.
The recurrence rate of pheochromocytoma is 13% in patients with sporadic disease and 33% in patients with familial syndromes. The overall recurrence rate with long-term follow-up is 17%, half of recurrences being malignant disease. All patients should therefore be followed in the clinic annually for at least 10 years to identify and treat recurrences early,7 and many experts recommend lifelong follow-up, even for patients without hereditary syndromes.17
Nearly every diagnosis in the DSM-IV includes the caveat that medical causes of disease must be excluded before psychiatric labels can be applied. Although panic disorder and panic attack are far more common than pheochromocytoma, just as essential hypertension is far more common than pheochromocytoma, physicians need to remember that pheochromocytoma can cause symptoms common to both illnesses. Thus, while rare conditions are rare, atypical presentations of common conditions may deserve a second glance.
- Yates WR. Phenomenology and epidemiology of panic disorder. Ann Clin Psychiatry 2009; 21:95–102.
- Katon WJ. Clinical practice. Panic disorder. N Engl J Med 2006; 354:2360–2367.
- Craske MG, Tsao JC. Assessment and treatment of nocturnal panic attacks. Sleep Med Rev 2005; 9:173–184.
- Roy-Byrne PP, Craske MG, Stein MB. Panic disorder. Lancet 2006; 368:1023–1032.
- Beal AL, Deuser WE, Beilman GJ. A role for epinephrine in post-traumatic hypokalemia. Shock 2007; 27:358–363.
- Kalra J. Medical errors: impact on clinical laboratories and other critical areas. Clin Biochem 2004; 37:1052–1062.
- Lenders JW, Eisenhofer G, Mannelli M, Pacak K. Phaeochromocytoma. Lancet 2005; 366:665–675.
- Leissner KB, Mahmood F, Aragam JR, Amouzgar A, Ortega R. Catecholamine-induced cardiomyopathy and pheochromocytoma. Anesth Analg 2008; 107:410–412.
- Yu R, Furmark L, Wong C. Cardiac abnormalities associated with pheochromocytoma and other adrenal tumors. Endocr Pract 2009; 15:10–16.
- Neumann HP, Bausch B, McWhinney SR, et al; Freiburg-Warsaw-Columbus Pheochromocytoma Study Group. Germ-line mutations in nonsyndromic pheochromocytoma. N Engl J Med 2002; 346:1459–1466.
- Williams VC, Lucas J, Babcock MA, Gutmann DH, Korf B, Maria BL. Neurofibromatosis type 1 revisited. Pediatrics 2009; 123:124–133.
- Asthagiri AR, Parry DM, Butman JA, et al. Neurofibromatosis type 2. Lancet 2009; 373:1974–1986.
- Callender GG, Rich TA, Perrier ND. Multiple endocrine neoplasia syndromes. Surg Clin North Am 2008; 88:863–895.
- Pasini B, Stratakis CA. SDH mutations in tumorigenesis and inherited endocrine tumours: lesson from the phaeochromocytoma-paraganglioma syndromes. J Intern Med 2009; 266:19–42.
- Yu R, Nissen NN, Chopra P, Dhall D, Phillips E, Wei M. Diagnosis and treatment of pheochromocytoma in an academic hospital from 1997 to 2007. Am J Med 2009; 122:85–95.
- Grouzmann E, Drouard-Troalen L, Baudin E, et al. Diagnostic accuracy of free and total metanephrines in plasma and fractionated metanephrines in urine of patients with pheochromocytoma. Eur J Endocrinol 2010; 162:951–960.
- Mittendorf EA, Evans DB, Lee JE, Perrier ND. Pheochromocytoma: advances in genetics, diagnosis, localization, and treatment. Hematol Oncol Clin North Am 2007; 21:509–525.
- Singh PK, Buch HN. Adrenal incidentaloma: evaluation and management. J Clin Pathol 2008; 61:1168–1173.
- Kasturi S, Kutikov A, Guzzo TJ, Smith AL, Wein AJ. Modern management of pheochromocytoma. Nat Clin Pract Urol 2007; 4:630–633.
- Yates WR. Phenomenology and epidemiology of panic disorder. Ann Clin Psychiatry 2009; 21:95–102.
- Katon WJ. Clinical practice. Panic disorder. N Engl J Med 2006; 354:2360–2367.
- Craske MG, Tsao JC. Assessment and treatment of nocturnal panic attacks. Sleep Med Rev 2005; 9:173–184.
- Roy-Byrne PP, Craske MG, Stein MB. Panic disorder. Lancet 2006; 368:1023–1032.
- Beal AL, Deuser WE, Beilman GJ. A role for epinephrine in post-traumatic hypokalemia. Shock 2007; 27:358–363.
- Kalra J. Medical errors: impact on clinical laboratories and other critical areas. Clin Biochem 2004; 37:1052–1062.
- Lenders JW, Eisenhofer G, Mannelli M, Pacak K. Phaeochromocytoma. Lancet 2005; 366:665–675.
- Leissner KB, Mahmood F, Aragam JR, Amouzgar A, Ortega R. Catecholamine-induced cardiomyopathy and pheochromocytoma. Anesth Analg 2008; 107:410–412.
- Yu R, Furmark L, Wong C. Cardiac abnormalities associated with pheochromocytoma and other adrenal tumors. Endocr Pract 2009; 15:10–16.
- Neumann HP, Bausch B, McWhinney SR, et al; Freiburg-Warsaw-Columbus Pheochromocytoma Study Group. Germ-line mutations in nonsyndromic pheochromocytoma. N Engl J Med 2002; 346:1459–1466.
- Williams VC, Lucas J, Babcock MA, Gutmann DH, Korf B, Maria BL. Neurofibromatosis type 1 revisited. Pediatrics 2009; 123:124–133.
- Asthagiri AR, Parry DM, Butman JA, et al. Neurofibromatosis type 2. Lancet 2009; 373:1974–1986.
- Callender GG, Rich TA, Perrier ND. Multiple endocrine neoplasia syndromes. Surg Clin North Am 2008; 88:863–895.
- Pasini B, Stratakis CA. SDH mutations in tumorigenesis and inherited endocrine tumours: lesson from the phaeochromocytoma-paraganglioma syndromes. J Intern Med 2009; 266:19–42.
- Yu R, Nissen NN, Chopra P, Dhall D, Phillips E, Wei M. Diagnosis and treatment of pheochromocytoma in an academic hospital from 1997 to 2007. Am J Med 2009; 122:85–95.
- Grouzmann E, Drouard-Troalen L, Baudin E, et al. Diagnostic accuracy of free and total metanephrines in plasma and fractionated metanephrines in urine of patients with pheochromocytoma. Eur J Endocrinol 2010; 162:951–960.
- Mittendorf EA, Evans DB, Lee JE, Perrier ND. Pheochromocytoma: advances in genetics, diagnosis, localization, and treatment. Hematol Oncol Clin North Am 2007; 21:509–525.
- Singh PK, Buch HN. Adrenal incidentaloma: evaluation and management. J Clin Pathol 2008; 61:1168–1173.
- Kasturi S, Kutikov A, Guzzo TJ, Smith AL, Wein AJ. Modern management of pheochromocytoma. Nat Clin Pract Urol 2007; 4:630–633.
Leukemia cutis
Q: What is the most likely diagnosis?
- Leukemia cutis
- Drug reaction
- Sweet syndrome
- Erythema multiforme
- Urticaria
A: The correct answer is leukemia cutis, defined as a cutaneous infiltration by neoplastic leukocytes.1 When the leukocytes are primarily granulocytic precursors, the terms myeloid sarcoma, granulocytic sarcoma, chloroma, and primary extramedullary leukemia have been used.2 The term monoblastic sarcoma has been used when the cutaneous infiltrate is composed of neoplastic monocytic precursors.2
Leukemia cutis most commonly manifests as erythematous papules and nodules, single or multiple, of varying sizes, and afflicting one or more body sites; it typically is asymptomatic.3 It occurs in 10% to 15% of patients with acute myeloid leukemia4 and is itself a poor prognostic sign.5 The cutaneous changes may pre-date the hematologic manifestations and may even herald a relapse.6
In patients presenting with extramedullary leukemia and no bone marrow or blood involvement, the importance of preemptive chemotherapy for acute myelogenous leukemia has recently been emphasized.7
CASE CONTINUED
The patient undergoes further testing with bone marrow aspiration and trephination, which are diagnostic of acute myeloid leukemia with myelodysplastic changes: the studies reveal a clear excess of myeloblasts (accounting for 40% to 50% of nucleated cells) and clearly dysplastic erythropoiesis and myelopoiesis. Bone marrow cytogenetic analysis reveals a complex abnormal female karyotype with multiple numerical and structural abnormalities, in particular deletion of the long arm of chromosome 5, suggestive of a poor prognosis.
Skin biopsy reveals a normal epidermis but dermal perivascular involvement with a reactive T-lymphocyte infiltrate associated with immature myeloid elements, characterized by positive staining to myeloperoxidase, in keeping with leukemia cutis. It should be noted that histopathologic confirmation of leukemia cutis can be challenging, as the condition can adopt a variety of patterns, and clinicopathologic correlation is often warranted.
The patient is treated with a cycle of cytarabine-based chemotherapy, and her skin eruption transiently improves. However, her clinical condition subsequently deteriorates; she has a relapse of leukemia, with the rash returning more florid and angry-looking than previously. She is subsequently managed palliatively and passes away 3 weeks later.
THE OTHER DIAGNOSTIC CHOICES
Sweet syndrome or acute febrile neutrophilic dermatosis is often seen in association with hematologic malignancies, but the lesions are typically tender, and histopathology reveals an intense dermal neutrophilic infiltrate.6
Erythema multiforme is associated with malignancy, but its characteristic concentric “target” lesions are typically acral and symmetrical in their distribution; their histopathology is inflammatory.8
The patient had not been on any regular medications and her rash could not have been medication-induced.
Urticaria presents with pruritic evanescent wheals, which rarely last more than 12 hours.9 Our patient had a fixed and entirely asymptomatic rash, which in addition did not have the histopathologic features of urticaria—namely, dermal edema involved with an infiltrate made of lymphocytes and eosinophils.9
- Strutton G. Cutaneous infiltrates: lymphomatous and leukemic. In:Weedon D, editor. Skin Pathology, 2nd ed. New York, NY: Churchill Livingstone, 2002:1118–1120.
- Brunning RD, Matutes E, Flandria F, et al. In: Jaffe ES, Harris NL, Stein H, Vardiman JW, editors. World Health Organization Classification of Tumours: Pathology and Genetics of Tumours of Haematopoietic and Lymphoid Tissues. Lyon, France: IARC Press, 2001:104–105.
- Watson KM, Mufti G, Salisbury JR, du Vivier AW, Creamer D. Spectrum of clinical presentation, treatment and prognosis in a series of eight patients with leukaemia cutis. Clin Exp Dermatol 2006; 31:218–221.
- Agis H, Weltermann A, Fonatsch C, et al. A comparative study on demographic, hematological, and cytogenetic findings and prognosis in acute myeloid leukemia with and without leukemia cutis. Ann Hematol 2002; 81:90–95.
- Kaddu S, Zenahlik P, Beham-Schmid C, Kerl H, Cerroni L. Specific cutaneous infiltrates in patients with myelogenous leukemia: a clinicopathologic study of 26 patients with assessment of diagnostic criteria. J Am Acad Dermatol 1999; 40:966–978.
- Cutaneous aspects of leukemia. In: Braun-Falco O, Plewig G, Wolff HH, Burgdorf WHC, editors. Dermatology, 2nd ed. Berlin: Springer, 2000:1640–1648.
- Tsimberidou AM, Kantarjian HM, Wen S, et al. Myeloid sarcoma is associated with superior event-free survival and overall survival compared with acute myeloid leukemia. Cancer 2008; 113:1370–1378.
- Erythemato-papulo-squamous diseases. In: Braun-Falco O, Plewig G, Wolff HH, Burgdorf WHC. Dermatology, 2nd ed. Berlin: Springer, 2000.
- Urticaria, angioedema and anaphylaxis. In: Braun-Falco O, Plewig G, Wolff HH, Burgdorf WHC. Dermatology, 2nd ed. Berlin: Springer, 2000.
Q: What is the most likely diagnosis?
- Leukemia cutis
- Drug reaction
- Sweet syndrome
- Erythema multiforme
- Urticaria
A: The correct answer is leukemia cutis, defined as a cutaneous infiltration by neoplastic leukocytes.1 When the leukocytes are primarily granulocytic precursors, the terms myeloid sarcoma, granulocytic sarcoma, chloroma, and primary extramedullary leukemia have been used.2 The term monoblastic sarcoma has been used when the cutaneous infiltrate is composed of neoplastic monocytic precursors.2
Leukemia cutis most commonly manifests as erythematous papules and nodules, single or multiple, of varying sizes, and afflicting one or more body sites; it typically is asymptomatic.3 It occurs in 10% to 15% of patients with acute myeloid leukemia4 and is itself a poor prognostic sign.5 The cutaneous changes may pre-date the hematologic manifestations and may even herald a relapse.6
In patients presenting with extramedullary leukemia and no bone marrow or blood involvement, the importance of preemptive chemotherapy for acute myelogenous leukemia has recently been emphasized.7
CASE CONTINUED
The patient undergoes further testing with bone marrow aspiration and trephination, which are diagnostic of acute myeloid leukemia with myelodysplastic changes: the studies reveal a clear excess of myeloblasts (accounting for 40% to 50% of nucleated cells) and clearly dysplastic erythropoiesis and myelopoiesis. Bone marrow cytogenetic analysis reveals a complex abnormal female karyotype with multiple numerical and structural abnormalities, in particular deletion of the long arm of chromosome 5, suggestive of a poor prognosis.
Skin biopsy reveals a normal epidermis but dermal perivascular involvement with a reactive T-lymphocyte infiltrate associated with immature myeloid elements, characterized by positive staining to myeloperoxidase, in keeping with leukemia cutis. It should be noted that histopathologic confirmation of leukemia cutis can be challenging, as the condition can adopt a variety of patterns, and clinicopathologic correlation is often warranted.
The patient is treated with a cycle of cytarabine-based chemotherapy, and her skin eruption transiently improves. However, her clinical condition subsequently deteriorates; she has a relapse of leukemia, with the rash returning more florid and angry-looking than previously. She is subsequently managed palliatively and passes away 3 weeks later.
THE OTHER DIAGNOSTIC CHOICES
Sweet syndrome or acute febrile neutrophilic dermatosis is often seen in association with hematologic malignancies, but the lesions are typically tender, and histopathology reveals an intense dermal neutrophilic infiltrate.6
Erythema multiforme is associated with malignancy, but its characteristic concentric “target” lesions are typically acral and symmetrical in their distribution; their histopathology is inflammatory.8
The patient had not been on any regular medications and her rash could not have been medication-induced.
Urticaria presents with pruritic evanescent wheals, which rarely last more than 12 hours.9 Our patient had a fixed and entirely asymptomatic rash, which in addition did not have the histopathologic features of urticaria—namely, dermal edema involved with an infiltrate made of lymphocytes and eosinophils.9
Q: What is the most likely diagnosis?
- Leukemia cutis
- Drug reaction
- Sweet syndrome
- Erythema multiforme
- Urticaria
A: The correct answer is leukemia cutis, defined as a cutaneous infiltration by neoplastic leukocytes.1 When the leukocytes are primarily granulocytic precursors, the terms myeloid sarcoma, granulocytic sarcoma, chloroma, and primary extramedullary leukemia have been used.2 The term monoblastic sarcoma has been used when the cutaneous infiltrate is composed of neoplastic monocytic precursors.2
Leukemia cutis most commonly manifests as erythematous papules and nodules, single or multiple, of varying sizes, and afflicting one or more body sites; it typically is asymptomatic.3 It occurs in 10% to 15% of patients with acute myeloid leukemia4 and is itself a poor prognostic sign.5 The cutaneous changes may pre-date the hematologic manifestations and may even herald a relapse.6
In patients presenting with extramedullary leukemia and no bone marrow or blood involvement, the importance of preemptive chemotherapy for acute myelogenous leukemia has recently been emphasized.7
CASE CONTINUED
The patient undergoes further testing with bone marrow aspiration and trephination, which are diagnostic of acute myeloid leukemia with myelodysplastic changes: the studies reveal a clear excess of myeloblasts (accounting for 40% to 50% of nucleated cells) and clearly dysplastic erythropoiesis and myelopoiesis. Bone marrow cytogenetic analysis reveals a complex abnormal female karyotype with multiple numerical and structural abnormalities, in particular deletion of the long arm of chromosome 5, suggestive of a poor prognosis.
Skin biopsy reveals a normal epidermis but dermal perivascular involvement with a reactive T-lymphocyte infiltrate associated with immature myeloid elements, characterized by positive staining to myeloperoxidase, in keeping with leukemia cutis. It should be noted that histopathologic confirmation of leukemia cutis can be challenging, as the condition can adopt a variety of patterns, and clinicopathologic correlation is often warranted.
The patient is treated with a cycle of cytarabine-based chemotherapy, and her skin eruption transiently improves. However, her clinical condition subsequently deteriorates; she has a relapse of leukemia, with the rash returning more florid and angry-looking than previously. She is subsequently managed palliatively and passes away 3 weeks later.
THE OTHER DIAGNOSTIC CHOICES
Sweet syndrome or acute febrile neutrophilic dermatosis is often seen in association with hematologic malignancies, but the lesions are typically tender, and histopathology reveals an intense dermal neutrophilic infiltrate.6
Erythema multiforme is associated with malignancy, but its characteristic concentric “target” lesions are typically acral and symmetrical in their distribution; their histopathology is inflammatory.8
The patient had not been on any regular medications and her rash could not have been medication-induced.
Urticaria presents with pruritic evanescent wheals, which rarely last more than 12 hours.9 Our patient had a fixed and entirely asymptomatic rash, which in addition did not have the histopathologic features of urticaria—namely, dermal edema involved with an infiltrate made of lymphocytes and eosinophils.9
- Strutton G. Cutaneous infiltrates: lymphomatous and leukemic. In:Weedon D, editor. Skin Pathology, 2nd ed. New York, NY: Churchill Livingstone, 2002:1118–1120.
- Brunning RD, Matutes E, Flandria F, et al. In: Jaffe ES, Harris NL, Stein H, Vardiman JW, editors. World Health Organization Classification of Tumours: Pathology and Genetics of Tumours of Haematopoietic and Lymphoid Tissues. Lyon, France: IARC Press, 2001:104–105.
- Watson KM, Mufti G, Salisbury JR, du Vivier AW, Creamer D. Spectrum of clinical presentation, treatment and prognosis in a series of eight patients with leukaemia cutis. Clin Exp Dermatol 2006; 31:218–221.
- Agis H, Weltermann A, Fonatsch C, et al. A comparative study on demographic, hematological, and cytogenetic findings and prognosis in acute myeloid leukemia with and without leukemia cutis. Ann Hematol 2002; 81:90–95.
- Kaddu S, Zenahlik P, Beham-Schmid C, Kerl H, Cerroni L. Specific cutaneous infiltrates in patients with myelogenous leukemia: a clinicopathologic study of 26 patients with assessment of diagnostic criteria. J Am Acad Dermatol 1999; 40:966–978.
- Cutaneous aspects of leukemia. In: Braun-Falco O, Plewig G, Wolff HH, Burgdorf WHC, editors. Dermatology, 2nd ed. Berlin: Springer, 2000:1640–1648.
- Tsimberidou AM, Kantarjian HM, Wen S, et al. Myeloid sarcoma is associated with superior event-free survival and overall survival compared with acute myeloid leukemia. Cancer 2008; 113:1370–1378.
- Erythemato-papulo-squamous diseases. In: Braun-Falco O, Plewig G, Wolff HH, Burgdorf WHC. Dermatology, 2nd ed. Berlin: Springer, 2000.
- Urticaria, angioedema and anaphylaxis. In: Braun-Falco O, Plewig G, Wolff HH, Burgdorf WHC. Dermatology, 2nd ed. Berlin: Springer, 2000.
- Strutton G. Cutaneous infiltrates: lymphomatous and leukemic. In:Weedon D, editor. Skin Pathology, 2nd ed. New York, NY: Churchill Livingstone, 2002:1118–1120.
- Brunning RD, Matutes E, Flandria F, et al. In: Jaffe ES, Harris NL, Stein H, Vardiman JW, editors. World Health Organization Classification of Tumours: Pathology and Genetics of Tumours of Haematopoietic and Lymphoid Tissues. Lyon, France: IARC Press, 2001:104–105.
- Watson KM, Mufti G, Salisbury JR, du Vivier AW, Creamer D. Spectrum of clinical presentation, treatment and prognosis in a series of eight patients with leukaemia cutis. Clin Exp Dermatol 2006; 31:218–221.
- Agis H, Weltermann A, Fonatsch C, et al. A comparative study on demographic, hematological, and cytogenetic findings and prognosis in acute myeloid leukemia with and without leukemia cutis. Ann Hematol 2002; 81:90–95.
- Kaddu S, Zenahlik P, Beham-Schmid C, Kerl H, Cerroni L. Specific cutaneous infiltrates in patients with myelogenous leukemia: a clinicopathologic study of 26 patients with assessment of diagnostic criteria. J Am Acad Dermatol 1999; 40:966–978.
- Cutaneous aspects of leukemia. In: Braun-Falco O, Plewig G, Wolff HH, Burgdorf WHC, editors. Dermatology, 2nd ed. Berlin: Springer, 2000:1640–1648.
- Tsimberidou AM, Kantarjian HM, Wen S, et al. Myeloid sarcoma is associated with superior event-free survival and overall survival compared with acute myeloid leukemia. Cancer 2008; 113:1370–1378.
- Erythemato-papulo-squamous diseases. In: Braun-Falco O, Plewig G, Wolff HH, Burgdorf WHC. Dermatology, 2nd ed. Berlin: Springer, 2000.
- Urticaria, angioedema and anaphylaxis. In: Braun-Falco O, Plewig G, Wolff HH, Burgdorf WHC. Dermatology, 2nd ed. Berlin: Springer, 2000.
Ending LGBT invisibility in health care: The first step in ensuring equitable care
In speaking about lesbian, gay, bisexual, and transgender (LGBT) health, it is not uncommon for me to be asked what is so unique about the health care needs of lesbians, gay men, bisexuals, and transgender individuals that it warrants focused attention in the training of health professionals and while providing care.1 Although it is true that most health issues affecting LGBT individuals parallel those of the general population, people who are LGBT have been shown to have unique health needs and to experience disparities in care.
There is a growing if limited number of good studies of LGBT health. The Institute of Medicine2 reported on lesbian health in 1999, concluding that enough evidence of disparities exists to support more research and to develop better methods of conducting the research. Healthy People 2020 actually recognizes significant health care disparities.3 Finally, the Institute of Medicine recently formed a committee on LGBT health issues to identify gaps in our knowledge and priorities for research. Their findings were expected to be published in late March 2011, after this article went to press.
MAKING A DIFFERENCE
While this article will not attempt to discuss all the disparities, the focus will be on how physicians can take the first critical step to helping LGBT individuals feel comfortable seeking care, ie, by being proactive in taking a history that includes discussion of sexual orientation and gender identity. Only by knowing this about patients will clinicians appropriately care for specific health needs, and will patients feel comfortable discussing their concerns in clinical settings.
While some feel this is relevant only in select areas of the country, recent data show that the LGBT population is both spread throughout the country and diverse in how they might present themselves in clinical settings.1,4 In the United States, 1.4% to 4.1% of people identify themselves as lesbian, gay, or bisexual.5 About 3% of women and 4% of men say they have had a same-sex sexual contact in the last year, and 4% to 11% of women and 6% to 9% of men report having ever had one.
Everyone who practices clinical medicine needs to understand whether patients are LGBT and how to engage in conversation about sexual orientation and gender identity.
GETTING TO KNOW LGBT PATIENTS
What questions should a clinician ask to get this information? In thinking about what to ask, it helps to realize that patients generally do not mind being questioned about personal matters if the provider approaches the topic and the patient with genuine respect, empathy, and even curiosity.
On the other hand, providers often feel ill-prepared to discuss intimate issues, or feel uncomfortable doing so. Successfully achieving a change in clinical practice involves learning an approach to doing so and becoming comfortable with discussions that may follow. One question to consider is how you will feel and how you will follow up if a patient tells you that he or she is LGBT.
The core comprehensive history for LGBT patients is the same as for all patients, keeping in mind the unique LGBT health risks and issues. Clinicians may begin by getting to know each patient as a person (eg, ask about partners, children, and jobs). I like to begin a session with a patient who is otherwise in good health with an open-ended question such as “Tell me a bit about yourself.” This provides an opportunity for patients to raise a range of issues without any additional focused questions being asked. In this context, if a patient brings up issues regarding sexual orientation or gender identity, ask permission to include this information in the medical record and assure the patient of its importance and that it will be confidential.
If these issues do not come up in response to general questions, they can be embedded in the sexual history, which should be more than a history of risk behaviors and should include a discussion of sexual health, sexual orientation (including identity, behavior, and desire), and gender identity. One can start by simply asking, “Do you have any concerns or questions about your sexuality, sexual orientation, or sexual desires?”
When it is necessary to ask more directed questions, it helps to provide some context so patients do not wonder why you are asking questions they may never have been asked by a physician before. It is best to explain that these are questions you ask all patients, as the information can be important in providing quality care. Patients should be told that discussion of sexual identity, behavior, and desire, as well as gender identity, is routine and confidential. For example, you might say: “I am going to ask you some questions about your sexual health and sexuality that I ask all my patients. The answers to these questions are important for me to know to help keep you healthy. Like the rest of this visit, this information is strictly confidential.”
One usually need not be too probing to get answers; people are often very forthcoming. During such conversations, patients often tell me that it is the first time a doctor has shown any interest in talking about these topics.
In having these conversations, initially it is best to use gender-neutral terms and pronouns when referring to partners until you know which to use: for example, “Do you have a partner or a spouse?” “Are you currently in a relationship?” “What do you call your partner?” Even if you make an incorrect assumption, and the patient corrects you, you can always apologize if a mistake is made and ask which term the patient prefers. Once you know it, use the pronoun that matches a person’s gender identity.
In order to get more information from the patient, the physician can engage in a series of questions, such as:
- Are you sexually active?
- When was the last time you had sex?
- When you have sex, do you do so with men, women, or both?
- How many sexual partners have you had during the last year?
- Do you have any desires regarding sexual intimacy that you would like to discuss?
In general, it is best to mirror the patient’s language. If patients use the term “gay” or “lesbian” to describe themselves, it would be off-putting to the patient to use a more clinical term, such as homosexual, in response. Some patients may use terms such as “queer” to indicate that they do not choose to identify as gay or straight. If terms like this are unclear to you, you may simply ask what this term means to the patient.
ASSESS SEXUAL BEHAVIOR TO DETERMINE RISK
In taking a history, it is important to distinguish sexual identity from sexual behavior. Physicians need to discuss sexual behavior with patients regardless of their sexual identity in order to do a risk-assessment, ascertaining what activities they engage in and to learn what they do to prevent transmission of sexually transmitted disease. In a 2006 study of more than 4,000 men in New York City,4 9.4% of those who identified themselves as straight had had sex with a man in the previous year. These men were more likely to be either foreign-born or from minority racial and ethnic groups with lower socioeconomic status. They were also less likely to have used a condom. A study of lesbians reported that 77% to 91% had at least one prior sexual experience with men, and 8% reported having had sex with a man in the previous year.6
Once you understand more about a patient’s sexual behavior, it is important to ask how patients protect themselves from human immunodeficiency virus (HIV) and other sexually transmitted diseases. If they use condoms or latex dams, they should be asked whether they do so consistently. Many patients have the misconception that they are practicing safe sex by only engaging in oral sex and do not realize that although it is probably protective against HIV infection, it does not protect against gonorrhea, syphilis, and other sexually transmitted diseases. Although most sexually transmitted diseases are treatable, their presence increases the risk of transmission of HIV.
Counseling on safer sex should include behavioral risk-reduction approaches. Depending on what behaviors a patient already engages in and what counseling he or she would be willing to accept, one could counsel abstinence, monogamy with an uninfected partner, reducing the number of partners, low-risk sexual practices, consistent and correct use of barrier methods, ceasing to engage in at least one high-risk activity, and avoiding excessive substance abuse. Physicians should advise patients to have a proactive plan to protect themselves and their partners. Patients should also be counseled on the correct use of barrier protection and on what is available for prophylaxis in case of high-risk HIV exposure (eg, a condom breaking or postcoital HIV disclosure). Another important question is, “Do you use alcohol or drugs when you have sex, and does your partner?” because these behaviors are often associated with unsafe sexual practices.
A new dimension of care will be biomedical prevention. While there are many ongoing studies of vaginal and anal microbicides to prevent HIV infection, there are also ongoing studies of antiretroviral therapies to do so.
One important new study demonstrated the effectiveness a biomedical intervention using antiretroviral therapy to prevent HIV infection in high-risk individuals.7 The study showed that men who were assigned to take a combination antiretroviral medication orally on a daily basis decreased their HIV risk by almost half compared with those assigned to take a placebo. The therapy was given along with intensive behavioral counseling. While this study was done in men who have sex with men, it is a major breakthrough and suggests there will be many new approaches to preventing HIV in the future.
A guide for clinicians has not been published by any government agency at this point, but guidance for clinicians is available from the Fenway Institute at www.fenwayhealth.org.
ASSESS GENDER-IDENTITY ISSUES
One should also routinely ask about whether patients are transgender or have gender-identity concerns. Psychologists start the conversation with the following example, which can also be used by general clinicians:
“Because so many people are impacted by gender issues, I have begun to ask everyone if they have any concerns about their gender. Anything you say about gender issues will be kept confidential. If this topic isn’t relevant to you, tell me and I’ll move on.”8
It is important to open the door to conversation, because many transgender people see a doctor for years and the topic never comes up. When they realize that they want to change their life, no one has ever helped them deal with the issues.
If appropriate, one can also say:
“Out of respect for my clients’ right to self-identify, I ask all clients what gender pronoun they’d prefer I use for them. What pronoun would you like me to use for you?”
Once these issues have been raised, it is important to support transgender people and help them explore a number of choices, including whether they wish to undergo hormone treatment, cosmetic surgery, and genital surgery. This may not be easy for many clinicians, so it will be important to learn about resources to care for transgender individuals in your community. Resources that can be very helpful for primary care clinicians include the following:
- The World Professional Association for Transgender Health (www.wpath.org) is the oldest and most traditional source for establishing standards of care.
- Vancouver Coastal Health published a series of monographs online (http://transhealth.vch.ca) that were developed by the University of British Columbia so that transgender people could be cared for in the community by primary care clinicians.
- The Endocrine Society in the United States published guidelines in 2009.9
PROVIDE SUPPORT FOR ‘COMING OUT’
We should also be understanding of people’s desires and support those who are “coming out.” The desire to reveal sexual orientation to others can happen at any age, including in childhood and among those who appear to have a traditional life because they are married and have children. Sometimes people do not know how to come out and would like to discuss such issues with their doctor.
MENTAL HEALTH CONCERNS
Given the marginalization and stigma that LGBT people face throughout their lives, it is not surprising that mental health problems are more prevalent in this population than in the general population. Gay and bisexual men have more depression, panic attacks, suicidal ideation, psychological distress, and body image and eating disorders than do heterosexual men. Lesbian and bisexual women are at greater risk of generalized anxiety disorder, depression, antidepressant use, and psychological distress.10 Care providers should screen for mental health disorders, assess comfort with sexual identity, and ask about social support.
FAMILY LIFE
Gays and lesbians increasingly want to discuss commitment, marriage, having children, parenting, and legal issues. A lot of research is being conducted on the sexual orientation of children raised by gay parents, and evidence shows that they are not more likely to be gay or lesbian than children raised by straight parents.
Elderly same-sex couples face special difficulties. They are less likely to feel comfortable “out of the closet” than are younger people. Fewer family and community supports are available to them, and they are often unable to live together in an assisted living facility. They particularly need to have advanced directives because they do not have the legal protections of other couples.
JUST A BEGINNING
While the points made above are relatively straightforward, they will open the door for many patients to have more meaningful conversations about their lives with their health care providers. It may only be a first step, but it can make a world of difference helping LGBT people feel comfortable accessing health care and receiving appropriate preventive care and treatment. Beyond the interaction with clinicians, health care providers should consider their overall environment and ensure that it is welcoming to LGBT individuals who come there for care.11
RESOURCES
Family Acceptance Project. familyproject.sfsu.edu
Gay & Lesbian Medical Association. www.glma.org
Human Rights Campaign. HRC.org
Parents, Families and Friends of Lesbians and Gays. PFLAG.org
World Professional Association for Transgender Health. www.wpath.org
Youth Resource (website by and for LGBT youth). Youthresource.com
- Makadon HJ. Improving health care for the lesbian and gay communities. N Engl J Med 2006; 354:895–897.
- Solarz AL, editor. Committee on Lesbian Health Research Priorities, Institute of Medicine. Lesbian Health: Current Assessment and Directions for the Future. Washington, DC: National Academy Press; 1999.
- Healthy People 2020. Lesbian, gay, bisexuaal, and transgender health. http://www.healthypeople.gov/2020/topicsobjectives2020/overview.aspx?topicid=25. Accessed 3/10/2011.
- Pathela P, Hajat A, Schillinger J, Blank S, Sell R, Mostashari F. Discordance between sexual behavior and self-reported sexual identity: a population-based survey of New York City men. Ann Intern Med 2006; 145:416–425. Erratum in: Ann Intern Med 2006; 145:936.
- Mosher WD, Chandra A, Jones J. Sexual behavior and selected health measures: men and women 15–44 years of age, United States, 2002. Adv Data 2005; 362:1–55.
- O’Hanlan KA, Robertson PA, Cabaj R, Schatz B, Nemrow P. A review of the medical consequences of homophobia with suggestions for resolution. Journal of the Gay and Lesbian Medical Association 1997; 1( 1):25–39.
- Grant RM, Lama JR, Anderson PL, et al. Preexposure chemoprophylaxis for HIV prevention in men who have sex with men. N Engl J Med 2010; 363:2587–2599.
- Feldman J, Goldberg JM. Transgender Primary Medical Care: Suggested Guidelines for Clinicians in British Columbia. Vancouver, BC: Vancouver Coastal Health Authority, 2006.
- Hembree WC, Cohen-Kettenis P, Delemarre-van de Waal HA, et al; Endocrine Society. Endocrine treatment of transsexual persons: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab 2009; 94:3132–3154.
- Cochran SD, Mays VM, Sullivan JG. Prevalence of mental disorders, psychological distress, and mental health services use among lesbian, gay, and bisexual adults in the United States. J Consult Clin Psychol 2003; 71:53–61.
- Human Rights Campaign Foundation. Healthcare equality index 2010. www.hrc.org/hei.
In speaking about lesbian, gay, bisexual, and transgender (LGBT) health, it is not uncommon for me to be asked what is so unique about the health care needs of lesbians, gay men, bisexuals, and transgender individuals that it warrants focused attention in the training of health professionals and while providing care.1 Although it is true that most health issues affecting LGBT individuals parallel those of the general population, people who are LGBT have been shown to have unique health needs and to experience disparities in care.
There is a growing if limited number of good studies of LGBT health. The Institute of Medicine2 reported on lesbian health in 1999, concluding that enough evidence of disparities exists to support more research and to develop better methods of conducting the research. Healthy People 2020 actually recognizes significant health care disparities.3 Finally, the Institute of Medicine recently formed a committee on LGBT health issues to identify gaps in our knowledge and priorities for research. Their findings were expected to be published in late March 2011, after this article went to press.
MAKING A DIFFERENCE
While this article will not attempt to discuss all the disparities, the focus will be on how physicians can take the first critical step to helping LGBT individuals feel comfortable seeking care, ie, by being proactive in taking a history that includes discussion of sexual orientation and gender identity. Only by knowing this about patients will clinicians appropriately care for specific health needs, and will patients feel comfortable discussing their concerns in clinical settings.
While some feel this is relevant only in select areas of the country, recent data show that the LGBT population is both spread throughout the country and diverse in how they might present themselves in clinical settings.1,4 In the United States, 1.4% to 4.1% of people identify themselves as lesbian, gay, or bisexual.5 About 3% of women and 4% of men say they have had a same-sex sexual contact in the last year, and 4% to 11% of women and 6% to 9% of men report having ever had one.
Everyone who practices clinical medicine needs to understand whether patients are LGBT and how to engage in conversation about sexual orientation and gender identity.
GETTING TO KNOW LGBT PATIENTS
What questions should a clinician ask to get this information? In thinking about what to ask, it helps to realize that patients generally do not mind being questioned about personal matters if the provider approaches the topic and the patient with genuine respect, empathy, and even curiosity.
On the other hand, providers often feel ill-prepared to discuss intimate issues, or feel uncomfortable doing so. Successfully achieving a change in clinical practice involves learning an approach to doing so and becoming comfortable with discussions that may follow. One question to consider is how you will feel and how you will follow up if a patient tells you that he or she is LGBT.
The core comprehensive history for LGBT patients is the same as for all patients, keeping in mind the unique LGBT health risks and issues. Clinicians may begin by getting to know each patient as a person (eg, ask about partners, children, and jobs). I like to begin a session with a patient who is otherwise in good health with an open-ended question such as “Tell me a bit about yourself.” This provides an opportunity for patients to raise a range of issues without any additional focused questions being asked. In this context, if a patient brings up issues regarding sexual orientation or gender identity, ask permission to include this information in the medical record and assure the patient of its importance and that it will be confidential.
If these issues do not come up in response to general questions, they can be embedded in the sexual history, which should be more than a history of risk behaviors and should include a discussion of sexual health, sexual orientation (including identity, behavior, and desire), and gender identity. One can start by simply asking, “Do you have any concerns or questions about your sexuality, sexual orientation, or sexual desires?”
When it is necessary to ask more directed questions, it helps to provide some context so patients do not wonder why you are asking questions they may never have been asked by a physician before. It is best to explain that these are questions you ask all patients, as the information can be important in providing quality care. Patients should be told that discussion of sexual identity, behavior, and desire, as well as gender identity, is routine and confidential. For example, you might say: “I am going to ask you some questions about your sexual health and sexuality that I ask all my patients. The answers to these questions are important for me to know to help keep you healthy. Like the rest of this visit, this information is strictly confidential.”
One usually need not be too probing to get answers; people are often very forthcoming. During such conversations, patients often tell me that it is the first time a doctor has shown any interest in talking about these topics.
In having these conversations, initially it is best to use gender-neutral terms and pronouns when referring to partners until you know which to use: for example, “Do you have a partner or a spouse?” “Are you currently in a relationship?” “What do you call your partner?” Even if you make an incorrect assumption, and the patient corrects you, you can always apologize if a mistake is made and ask which term the patient prefers. Once you know it, use the pronoun that matches a person’s gender identity.
In order to get more information from the patient, the physician can engage in a series of questions, such as:
- Are you sexually active?
- When was the last time you had sex?
- When you have sex, do you do so with men, women, or both?
- How many sexual partners have you had during the last year?
- Do you have any desires regarding sexual intimacy that you would like to discuss?
In general, it is best to mirror the patient’s language. If patients use the term “gay” or “lesbian” to describe themselves, it would be off-putting to the patient to use a more clinical term, such as homosexual, in response. Some patients may use terms such as “queer” to indicate that they do not choose to identify as gay or straight. If terms like this are unclear to you, you may simply ask what this term means to the patient.
ASSESS SEXUAL BEHAVIOR TO DETERMINE RISK
In taking a history, it is important to distinguish sexual identity from sexual behavior. Physicians need to discuss sexual behavior with patients regardless of their sexual identity in order to do a risk-assessment, ascertaining what activities they engage in and to learn what they do to prevent transmission of sexually transmitted disease. In a 2006 study of more than 4,000 men in New York City,4 9.4% of those who identified themselves as straight had had sex with a man in the previous year. These men were more likely to be either foreign-born or from minority racial and ethnic groups with lower socioeconomic status. They were also less likely to have used a condom. A study of lesbians reported that 77% to 91% had at least one prior sexual experience with men, and 8% reported having had sex with a man in the previous year.6
Once you understand more about a patient’s sexual behavior, it is important to ask how patients protect themselves from human immunodeficiency virus (HIV) and other sexually transmitted diseases. If they use condoms or latex dams, they should be asked whether they do so consistently. Many patients have the misconception that they are practicing safe sex by only engaging in oral sex and do not realize that although it is probably protective against HIV infection, it does not protect against gonorrhea, syphilis, and other sexually transmitted diseases. Although most sexually transmitted diseases are treatable, their presence increases the risk of transmission of HIV.
Counseling on safer sex should include behavioral risk-reduction approaches. Depending on what behaviors a patient already engages in and what counseling he or she would be willing to accept, one could counsel abstinence, monogamy with an uninfected partner, reducing the number of partners, low-risk sexual practices, consistent and correct use of barrier methods, ceasing to engage in at least one high-risk activity, and avoiding excessive substance abuse. Physicians should advise patients to have a proactive plan to protect themselves and their partners. Patients should also be counseled on the correct use of barrier protection and on what is available for prophylaxis in case of high-risk HIV exposure (eg, a condom breaking or postcoital HIV disclosure). Another important question is, “Do you use alcohol or drugs when you have sex, and does your partner?” because these behaviors are often associated with unsafe sexual practices.
A new dimension of care will be biomedical prevention. While there are many ongoing studies of vaginal and anal microbicides to prevent HIV infection, there are also ongoing studies of antiretroviral therapies to do so.
One important new study demonstrated the effectiveness a biomedical intervention using antiretroviral therapy to prevent HIV infection in high-risk individuals.7 The study showed that men who were assigned to take a combination antiretroviral medication orally on a daily basis decreased their HIV risk by almost half compared with those assigned to take a placebo. The therapy was given along with intensive behavioral counseling. While this study was done in men who have sex with men, it is a major breakthrough and suggests there will be many new approaches to preventing HIV in the future.
A guide for clinicians has not been published by any government agency at this point, but guidance for clinicians is available from the Fenway Institute at www.fenwayhealth.org.
ASSESS GENDER-IDENTITY ISSUES
One should also routinely ask about whether patients are transgender or have gender-identity concerns. Psychologists start the conversation with the following example, which can also be used by general clinicians:
“Because so many people are impacted by gender issues, I have begun to ask everyone if they have any concerns about their gender. Anything you say about gender issues will be kept confidential. If this topic isn’t relevant to you, tell me and I’ll move on.”8
It is important to open the door to conversation, because many transgender people see a doctor for years and the topic never comes up. When they realize that they want to change their life, no one has ever helped them deal with the issues.
If appropriate, one can also say:
“Out of respect for my clients’ right to self-identify, I ask all clients what gender pronoun they’d prefer I use for them. What pronoun would you like me to use for you?”
Once these issues have been raised, it is important to support transgender people and help them explore a number of choices, including whether they wish to undergo hormone treatment, cosmetic surgery, and genital surgery. This may not be easy for many clinicians, so it will be important to learn about resources to care for transgender individuals in your community. Resources that can be very helpful for primary care clinicians include the following:
- The World Professional Association for Transgender Health (www.wpath.org) is the oldest and most traditional source for establishing standards of care.
- Vancouver Coastal Health published a series of monographs online (http://transhealth.vch.ca) that were developed by the University of British Columbia so that transgender people could be cared for in the community by primary care clinicians.
- The Endocrine Society in the United States published guidelines in 2009.9
PROVIDE SUPPORT FOR ‘COMING OUT’
We should also be understanding of people’s desires and support those who are “coming out.” The desire to reveal sexual orientation to others can happen at any age, including in childhood and among those who appear to have a traditional life because they are married and have children. Sometimes people do not know how to come out and would like to discuss such issues with their doctor.
MENTAL HEALTH CONCERNS
Given the marginalization and stigma that LGBT people face throughout their lives, it is not surprising that mental health problems are more prevalent in this population than in the general population. Gay and bisexual men have more depression, panic attacks, suicidal ideation, psychological distress, and body image and eating disorders than do heterosexual men. Lesbian and bisexual women are at greater risk of generalized anxiety disorder, depression, antidepressant use, and psychological distress.10 Care providers should screen for mental health disorders, assess comfort with sexual identity, and ask about social support.
FAMILY LIFE
Gays and lesbians increasingly want to discuss commitment, marriage, having children, parenting, and legal issues. A lot of research is being conducted on the sexual orientation of children raised by gay parents, and evidence shows that they are not more likely to be gay or lesbian than children raised by straight parents.
Elderly same-sex couples face special difficulties. They are less likely to feel comfortable “out of the closet” than are younger people. Fewer family and community supports are available to them, and they are often unable to live together in an assisted living facility. They particularly need to have advanced directives because they do not have the legal protections of other couples.
JUST A BEGINNING
While the points made above are relatively straightforward, they will open the door for many patients to have more meaningful conversations about their lives with their health care providers. It may only be a first step, but it can make a world of difference helping LGBT people feel comfortable accessing health care and receiving appropriate preventive care and treatment. Beyond the interaction with clinicians, health care providers should consider their overall environment and ensure that it is welcoming to LGBT individuals who come there for care.11
RESOURCES
Family Acceptance Project. familyproject.sfsu.edu
Gay & Lesbian Medical Association. www.glma.org
Human Rights Campaign. HRC.org
Parents, Families and Friends of Lesbians and Gays. PFLAG.org
World Professional Association for Transgender Health. www.wpath.org
Youth Resource (website by and for LGBT youth). Youthresource.com
In speaking about lesbian, gay, bisexual, and transgender (LGBT) health, it is not uncommon for me to be asked what is so unique about the health care needs of lesbians, gay men, bisexuals, and transgender individuals that it warrants focused attention in the training of health professionals and while providing care.1 Although it is true that most health issues affecting LGBT individuals parallel those of the general population, people who are LGBT have been shown to have unique health needs and to experience disparities in care.
There is a growing if limited number of good studies of LGBT health. The Institute of Medicine2 reported on lesbian health in 1999, concluding that enough evidence of disparities exists to support more research and to develop better methods of conducting the research. Healthy People 2020 actually recognizes significant health care disparities.3 Finally, the Institute of Medicine recently formed a committee on LGBT health issues to identify gaps in our knowledge and priorities for research. Their findings were expected to be published in late March 2011, after this article went to press.
MAKING A DIFFERENCE
While this article will not attempt to discuss all the disparities, the focus will be on how physicians can take the first critical step to helping LGBT individuals feel comfortable seeking care, ie, by being proactive in taking a history that includes discussion of sexual orientation and gender identity. Only by knowing this about patients will clinicians appropriately care for specific health needs, and will patients feel comfortable discussing their concerns in clinical settings.
While some feel this is relevant only in select areas of the country, recent data show that the LGBT population is both spread throughout the country and diverse in how they might present themselves in clinical settings.1,4 In the United States, 1.4% to 4.1% of people identify themselves as lesbian, gay, or bisexual.5 About 3% of women and 4% of men say they have had a same-sex sexual contact in the last year, and 4% to 11% of women and 6% to 9% of men report having ever had one.
Everyone who practices clinical medicine needs to understand whether patients are LGBT and how to engage in conversation about sexual orientation and gender identity.
GETTING TO KNOW LGBT PATIENTS
What questions should a clinician ask to get this information? In thinking about what to ask, it helps to realize that patients generally do not mind being questioned about personal matters if the provider approaches the topic and the patient with genuine respect, empathy, and even curiosity.
On the other hand, providers often feel ill-prepared to discuss intimate issues, or feel uncomfortable doing so. Successfully achieving a change in clinical practice involves learning an approach to doing so and becoming comfortable with discussions that may follow. One question to consider is how you will feel and how you will follow up if a patient tells you that he or she is LGBT.
The core comprehensive history for LGBT patients is the same as for all patients, keeping in mind the unique LGBT health risks and issues. Clinicians may begin by getting to know each patient as a person (eg, ask about partners, children, and jobs). I like to begin a session with a patient who is otherwise in good health with an open-ended question such as “Tell me a bit about yourself.” This provides an opportunity for patients to raise a range of issues without any additional focused questions being asked. In this context, if a patient brings up issues regarding sexual orientation or gender identity, ask permission to include this information in the medical record and assure the patient of its importance and that it will be confidential.
If these issues do not come up in response to general questions, they can be embedded in the sexual history, which should be more than a history of risk behaviors and should include a discussion of sexual health, sexual orientation (including identity, behavior, and desire), and gender identity. One can start by simply asking, “Do you have any concerns or questions about your sexuality, sexual orientation, or sexual desires?”
When it is necessary to ask more directed questions, it helps to provide some context so patients do not wonder why you are asking questions they may never have been asked by a physician before. It is best to explain that these are questions you ask all patients, as the information can be important in providing quality care. Patients should be told that discussion of sexual identity, behavior, and desire, as well as gender identity, is routine and confidential. For example, you might say: “I am going to ask you some questions about your sexual health and sexuality that I ask all my patients. The answers to these questions are important for me to know to help keep you healthy. Like the rest of this visit, this information is strictly confidential.”
One usually need not be too probing to get answers; people are often very forthcoming. During such conversations, patients often tell me that it is the first time a doctor has shown any interest in talking about these topics.
In having these conversations, initially it is best to use gender-neutral terms and pronouns when referring to partners until you know which to use: for example, “Do you have a partner or a spouse?” “Are you currently in a relationship?” “What do you call your partner?” Even if you make an incorrect assumption, and the patient corrects you, you can always apologize if a mistake is made and ask which term the patient prefers. Once you know it, use the pronoun that matches a person’s gender identity.
In order to get more information from the patient, the physician can engage in a series of questions, such as:
- Are you sexually active?
- When was the last time you had sex?
- When you have sex, do you do so with men, women, or both?
- How many sexual partners have you had during the last year?
- Do you have any desires regarding sexual intimacy that you would like to discuss?
In general, it is best to mirror the patient’s language. If patients use the term “gay” or “lesbian” to describe themselves, it would be off-putting to the patient to use a more clinical term, such as homosexual, in response. Some patients may use terms such as “queer” to indicate that they do not choose to identify as gay or straight. If terms like this are unclear to you, you may simply ask what this term means to the patient.
ASSESS SEXUAL BEHAVIOR TO DETERMINE RISK
In taking a history, it is important to distinguish sexual identity from sexual behavior. Physicians need to discuss sexual behavior with patients regardless of their sexual identity in order to do a risk-assessment, ascertaining what activities they engage in and to learn what they do to prevent transmission of sexually transmitted disease. In a 2006 study of more than 4,000 men in New York City,4 9.4% of those who identified themselves as straight had had sex with a man in the previous year. These men were more likely to be either foreign-born or from minority racial and ethnic groups with lower socioeconomic status. They were also less likely to have used a condom. A study of lesbians reported that 77% to 91% had at least one prior sexual experience with men, and 8% reported having had sex with a man in the previous year.6
Once you understand more about a patient’s sexual behavior, it is important to ask how patients protect themselves from human immunodeficiency virus (HIV) and other sexually transmitted diseases. If they use condoms or latex dams, they should be asked whether they do so consistently. Many patients have the misconception that they are practicing safe sex by only engaging in oral sex and do not realize that although it is probably protective against HIV infection, it does not protect against gonorrhea, syphilis, and other sexually transmitted diseases. Although most sexually transmitted diseases are treatable, their presence increases the risk of transmission of HIV.
Counseling on safer sex should include behavioral risk-reduction approaches. Depending on what behaviors a patient already engages in and what counseling he or she would be willing to accept, one could counsel abstinence, monogamy with an uninfected partner, reducing the number of partners, low-risk sexual practices, consistent and correct use of barrier methods, ceasing to engage in at least one high-risk activity, and avoiding excessive substance abuse. Physicians should advise patients to have a proactive plan to protect themselves and their partners. Patients should also be counseled on the correct use of barrier protection and on what is available for prophylaxis in case of high-risk HIV exposure (eg, a condom breaking or postcoital HIV disclosure). Another important question is, “Do you use alcohol or drugs when you have sex, and does your partner?” because these behaviors are often associated with unsafe sexual practices.
A new dimension of care will be biomedical prevention. While there are many ongoing studies of vaginal and anal microbicides to prevent HIV infection, there are also ongoing studies of antiretroviral therapies to do so.
One important new study demonstrated the effectiveness a biomedical intervention using antiretroviral therapy to prevent HIV infection in high-risk individuals.7 The study showed that men who were assigned to take a combination antiretroviral medication orally on a daily basis decreased their HIV risk by almost half compared with those assigned to take a placebo. The therapy was given along with intensive behavioral counseling. While this study was done in men who have sex with men, it is a major breakthrough and suggests there will be many new approaches to preventing HIV in the future.
A guide for clinicians has not been published by any government agency at this point, but guidance for clinicians is available from the Fenway Institute at www.fenwayhealth.org.
ASSESS GENDER-IDENTITY ISSUES
One should also routinely ask about whether patients are transgender or have gender-identity concerns. Psychologists start the conversation with the following example, which can also be used by general clinicians:
“Because so many people are impacted by gender issues, I have begun to ask everyone if they have any concerns about their gender. Anything you say about gender issues will be kept confidential. If this topic isn’t relevant to you, tell me and I’ll move on.”8
It is important to open the door to conversation, because many transgender people see a doctor for years and the topic never comes up. When they realize that they want to change their life, no one has ever helped them deal with the issues.
If appropriate, one can also say:
“Out of respect for my clients’ right to self-identify, I ask all clients what gender pronoun they’d prefer I use for them. What pronoun would you like me to use for you?”
Once these issues have been raised, it is important to support transgender people and help them explore a number of choices, including whether they wish to undergo hormone treatment, cosmetic surgery, and genital surgery. This may not be easy for many clinicians, so it will be important to learn about resources to care for transgender individuals in your community. Resources that can be very helpful for primary care clinicians include the following:
- The World Professional Association for Transgender Health (www.wpath.org) is the oldest and most traditional source for establishing standards of care.
- Vancouver Coastal Health published a series of monographs online (http://transhealth.vch.ca) that were developed by the University of British Columbia so that transgender people could be cared for in the community by primary care clinicians.
- The Endocrine Society in the United States published guidelines in 2009.9
PROVIDE SUPPORT FOR ‘COMING OUT’
We should also be understanding of people’s desires and support those who are “coming out.” The desire to reveal sexual orientation to others can happen at any age, including in childhood and among those who appear to have a traditional life because they are married and have children. Sometimes people do not know how to come out and would like to discuss such issues with their doctor.
MENTAL HEALTH CONCERNS
Given the marginalization and stigma that LGBT people face throughout their lives, it is not surprising that mental health problems are more prevalent in this population than in the general population. Gay and bisexual men have more depression, panic attacks, suicidal ideation, psychological distress, and body image and eating disorders than do heterosexual men. Lesbian and bisexual women are at greater risk of generalized anxiety disorder, depression, antidepressant use, and psychological distress.10 Care providers should screen for mental health disorders, assess comfort with sexual identity, and ask about social support.
FAMILY LIFE
Gays and lesbians increasingly want to discuss commitment, marriage, having children, parenting, and legal issues. A lot of research is being conducted on the sexual orientation of children raised by gay parents, and evidence shows that they are not more likely to be gay or lesbian than children raised by straight parents.
Elderly same-sex couples face special difficulties. They are less likely to feel comfortable “out of the closet” than are younger people. Fewer family and community supports are available to them, and they are often unable to live together in an assisted living facility. They particularly need to have advanced directives because they do not have the legal protections of other couples.
JUST A BEGINNING
While the points made above are relatively straightforward, they will open the door for many patients to have more meaningful conversations about their lives with their health care providers. It may only be a first step, but it can make a world of difference helping LGBT people feel comfortable accessing health care and receiving appropriate preventive care and treatment. Beyond the interaction with clinicians, health care providers should consider their overall environment and ensure that it is welcoming to LGBT individuals who come there for care.11
RESOURCES
Family Acceptance Project. familyproject.sfsu.edu
Gay & Lesbian Medical Association. www.glma.org
Human Rights Campaign. HRC.org
Parents, Families and Friends of Lesbians and Gays. PFLAG.org
World Professional Association for Transgender Health. www.wpath.org
Youth Resource (website by and for LGBT youth). Youthresource.com
- Makadon HJ. Improving health care for the lesbian and gay communities. N Engl J Med 2006; 354:895–897.
- Solarz AL, editor. Committee on Lesbian Health Research Priorities, Institute of Medicine. Lesbian Health: Current Assessment and Directions for the Future. Washington, DC: National Academy Press; 1999.
- Healthy People 2020. Lesbian, gay, bisexuaal, and transgender health. http://www.healthypeople.gov/2020/topicsobjectives2020/overview.aspx?topicid=25. Accessed 3/10/2011.
- Pathela P, Hajat A, Schillinger J, Blank S, Sell R, Mostashari F. Discordance between sexual behavior and self-reported sexual identity: a population-based survey of New York City men. Ann Intern Med 2006; 145:416–425. Erratum in: Ann Intern Med 2006; 145:936.
- Mosher WD, Chandra A, Jones J. Sexual behavior and selected health measures: men and women 15–44 years of age, United States, 2002. Adv Data 2005; 362:1–55.
- O’Hanlan KA, Robertson PA, Cabaj R, Schatz B, Nemrow P. A review of the medical consequences of homophobia with suggestions for resolution. Journal of the Gay and Lesbian Medical Association 1997; 1( 1):25–39.
- Grant RM, Lama JR, Anderson PL, et al. Preexposure chemoprophylaxis for HIV prevention in men who have sex with men. N Engl J Med 2010; 363:2587–2599.
- Feldman J, Goldberg JM. Transgender Primary Medical Care: Suggested Guidelines for Clinicians in British Columbia. Vancouver, BC: Vancouver Coastal Health Authority, 2006.
- Hembree WC, Cohen-Kettenis P, Delemarre-van de Waal HA, et al; Endocrine Society. Endocrine treatment of transsexual persons: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab 2009; 94:3132–3154.
- Cochran SD, Mays VM, Sullivan JG. Prevalence of mental disorders, psychological distress, and mental health services use among lesbian, gay, and bisexual adults in the United States. J Consult Clin Psychol 2003; 71:53–61.
- Human Rights Campaign Foundation. Healthcare equality index 2010. www.hrc.org/hei.
- Makadon HJ. Improving health care for the lesbian and gay communities. N Engl J Med 2006; 354:895–897.
- Solarz AL, editor. Committee on Lesbian Health Research Priorities, Institute of Medicine. Lesbian Health: Current Assessment and Directions for the Future. Washington, DC: National Academy Press; 1999.
- Healthy People 2020. Lesbian, gay, bisexuaal, and transgender health. http://www.healthypeople.gov/2020/topicsobjectives2020/overview.aspx?topicid=25. Accessed 3/10/2011.
- Pathela P, Hajat A, Schillinger J, Blank S, Sell R, Mostashari F. Discordance between sexual behavior and self-reported sexual identity: a population-based survey of New York City men. Ann Intern Med 2006; 145:416–425. Erratum in: Ann Intern Med 2006; 145:936.
- Mosher WD, Chandra A, Jones J. Sexual behavior and selected health measures: men and women 15–44 years of age, United States, 2002. Adv Data 2005; 362:1–55.
- O’Hanlan KA, Robertson PA, Cabaj R, Schatz B, Nemrow P. A review of the medical consequences of homophobia with suggestions for resolution. Journal of the Gay and Lesbian Medical Association 1997; 1( 1):25–39.
- Grant RM, Lama JR, Anderson PL, et al. Preexposure chemoprophylaxis for HIV prevention in men who have sex with men. N Engl J Med 2010; 363:2587–2599.
- Feldman J, Goldberg JM. Transgender Primary Medical Care: Suggested Guidelines for Clinicians in British Columbia. Vancouver, BC: Vancouver Coastal Health Authority, 2006.
- Hembree WC, Cohen-Kettenis P, Delemarre-van de Waal HA, et al; Endocrine Society. Endocrine treatment of transsexual persons: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab 2009; 94:3132–3154.
- Cochran SD, Mays VM, Sullivan JG. Prevalence of mental disorders, psychological distress, and mental health services use among lesbian, gay, and bisexual adults in the United States. J Consult Clin Psychol 2003; 71:53–61.
- Human Rights Campaign Foundation. Healthcare equality index 2010. www.hrc.org/hei.
KEY POINTS
- LGBT people are represented in most medical practices, and their health issues, including sexually transmitted diseases such as human immunodeficiency virus, can generally be managed in traditional health care settings rather than in special clinics.
- Physicians need to become more comfortable asking patients about sexual health, identity, and behavior, and make such queries more routine.
- Sexual behavior is not always congruent with routine understanding of sexual identity. For example, many men who do not identify themselves as gay occasionally have sex with men, as do many self-identified lesbians. It is important to know this to provide appropriate preventive screening and care.
Gene-based, rational drug-dosing: An evolving, complex opportunity
For some drugs there is a key step in metabolism, often in a rate-limiting pathway, with an enzyme that has known and detectable polymorphisms that differ dramatically in their ability to affect the drug’s degradation. In theory, by determining the patient’s specific genotype ahead of time, the initial dose of the drug can be determined more rationally. In this issue of the Journal, Kitzmiller et al describe several drugs for which this may be true.
However, for this approach to be practical and cost-effective, several conditions should be met. The drug must be one that needs to be dosed to its therapeutic level rapidly: if there is time to titrate slowly, then there is little need for the extra expense associated with genotyping in order to titrate it more rapidly. Also, it should be proven that dosing based on advance knowledge of the genotype of the target actually results in safer or more efficacious dosing.
For carbamazepine (Tegretol, Equetro) and allopurinol (Zyloprim), specific human leukocyte antigen haplotypes are associated with a strikingly increased frequency of serious hypersensitivity reactions. In some patients, these should be checked before giving the drug.
But the concept of pharmacogenomics is broad, and it may yet explain many vagaries of drug-responsiveness in individual patients. Polymorphisms in renal anion transporters may dictate the level of anionic drugs. Drug-receptor polymorphisms may determine the affinity of a drug for its target and, hence, its efficacy. Cell-membrane transporters, which may have functionally different stable alleles or polymorphisms, may regulate intracellular drug levels by pumping the drug into or out of cells with different efficiencies.
As the entire human genome is dissected and analyzed, and as more and more genes (with their polymorphisms) are linked to specific functions readily detectable in specific patients, we will have more opportunities to match the right drug and dose to the right patient. We are not there yet, but that day is coming.
For some drugs there is a key step in metabolism, often in a rate-limiting pathway, with an enzyme that has known and detectable polymorphisms that differ dramatically in their ability to affect the drug’s degradation. In theory, by determining the patient’s specific genotype ahead of time, the initial dose of the drug can be determined more rationally. In this issue of the Journal, Kitzmiller et al describe several drugs for which this may be true.
However, for this approach to be practical and cost-effective, several conditions should be met. The drug must be one that needs to be dosed to its therapeutic level rapidly: if there is time to titrate slowly, then there is little need for the extra expense associated with genotyping in order to titrate it more rapidly. Also, it should be proven that dosing based on advance knowledge of the genotype of the target actually results in safer or more efficacious dosing.
For carbamazepine (Tegretol, Equetro) and allopurinol (Zyloprim), specific human leukocyte antigen haplotypes are associated with a strikingly increased frequency of serious hypersensitivity reactions. In some patients, these should be checked before giving the drug.
But the concept of pharmacogenomics is broad, and it may yet explain many vagaries of drug-responsiveness in individual patients. Polymorphisms in renal anion transporters may dictate the level of anionic drugs. Drug-receptor polymorphisms may determine the affinity of a drug for its target and, hence, its efficacy. Cell-membrane transporters, which may have functionally different stable alleles or polymorphisms, may regulate intracellular drug levels by pumping the drug into or out of cells with different efficiencies.
As the entire human genome is dissected and analyzed, and as more and more genes (with their polymorphisms) are linked to specific functions readily detectable in specific patients, we will have more opportunities to match the right drug and dose to the right patient. We are not there yet, but that day is coming.
For some drugs there is a key step in metabolism, often in a rate-limiting pathway, with an enzyme that has known and detectable polymorphisms that differ dramatically in their ability to affect the drug’s degradation. In theory, by determining the patient’s specific genotype ahead of time, the initial dose of the drug can be determined more rationally. In this issue of the Journal, Kitzmiller et al describe several drugs for which this may be true.
However, for this approach to be practical and cost-effective, several conditions should be met. The drug must be one that needs to be dosed to its therapeutic level rapidly: if there is time to titrate slowly, then there is little need for the extra expense associated with genotyping in order to titrate it more rapidly. Also, it should be proven that dosing based on advance knowledge of the genotype of the target actually results in safer or more efficacious dosing.
For carbamazepine (Tegretol, Equetro) and allopurinol (Zyloprim), specific human leukocyte antigen haplotypes are associated with a strikingly increased frequency of serious hypersensitivity reactions. In some patients, these should be checked before giving the drug.
But the concept of pharmacogenomics is broad, and it may yet explain many vagaries of drug-responsiveness in individual patients. Polymorphisms in renal anion transporters may dictate the level of anionic drugs. Drug-receptor polymorphisms may determine the affinity of a drug for its target and, hence, its efficacy. Cell-membrane transporters, which may have functionally different stable alleles or polymorphisms, may regulate intracellular drug levels by pumping the drug into or out of cells with different efficiencies.
As the entire human genome is dissected and analyzed, and as more and more genes (with their polymorphisms) are linked to specific functions readily detectable in specific patients, we will have more opportunities to match the right drug and dose to the right patient. We are not there yet, but that day is coming.