User login
Irregularly shaped abdominal mass
A 46-year-old man sought care at our clinic for an abdominal mass, fatigue, and shortness of breath. He also indicated that he was feeling depressed.
Four years earlier, he’d had a prolonged hospitalization for severe cor pulmonale, during which he suffered a perforated cecum. He had multiple abdominal surgeries, including a right hemicolectomy. His postoperative course was complicated by multi-system organ failure and several nosocomial infections.
In the wake of his recovery, he developed an anterior midline abdominal mass that slowly enlarged over the following years (FIGURE 1). He sought a surgical consultation, but was deferred because of his high-risk operative profile.
Our examination of the patient revealed an anterior, midline, irregularly shaped mass measuring 14 × 20 in. The nontender mass was hollow to percussion and was not as prominent when the patient was supine.
FIGURE 1
Abdominal mass measuring 14 × 20 in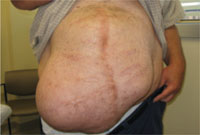
Four years earlier, this 46-year-old patient had undergone multiple abdominal surgeries. On this visit, he sought care for a nontender mass that was hollow to percussion.
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Ventral hernia
An abdominal ultrasound revealed subcutaneous, peristalsing bowel loops consistent with a ventral hernia (FIGURE 2). A small amount of ascites was also found.
Most abdominal wall hernias occur in the inguinal region, but in 2003 there were 360,000 ventral hernia repairs performed in the United States.1 Ventral hernias can be further classified as primary or incisional (depending on patient history) and according to their location—midline (epigastric and umbilical) or lateral (Spighelian and lumbar).2
An abdominal hernia typically presents as a nontender, protruding mass that is either stable in size or gradually expands. The mass may be pulsatile, depending on the contents of the hernia and their activity. Hernias may be reducible, meaning that the contents are able to return to the abdominal cavity with external pressure or if the patient is supine. If a hernia is not reducible, then incarceration becomes a significant risk. Compromised blood supply to the incarcerated organ(s) can lead to tissue necrosis and viscous perforation. Epigastric hernias, in particular, carry a high risk of incarceration.3
FIGURE 2
Another view of the ventral hernia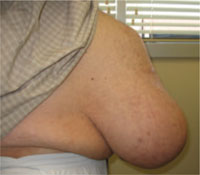
3 conditions comprise the differential
The differential diagnosis includes diastasis recti, ascites, and lipoma.
Diastasis recti is a separation of the rectus abdominus muscles at the linea alba. It is seen almost exclusively in pregnant women and newborns. In this condition, the flat abdominal wall muscles remain intact, and thus abdominal contents would not protrude.
Ascites is the collection of fluid in the abdominal cavity, secondary to conditions such as cirrhosis or congestive heart failure. In ascites, the abdomen is dull to percussion, with no discrete, irregular mass.
Lipoma is a solid benign tumor composed of fatty tissue. A lipoma of this size is rare, and would be solid to percussion. Also, it would not be reducible with the patient supine.
Ultrasound or CT scan is diagnostic
After a thorough history and physical examination, ultrasonography or CT often helps differentiate a ventral hernia from other abdominal wall defects. In patients with a ventral hernia, either imaging modality will demonstrate prolapsed loops of hollow viscus.
A CT scan was not an option for our patient because none of the local machines could accommodate the size and shape of his body. He had an abdominal ultrasound instead.
Surgery sets things right
Treatment of a ventral hernia involves either an open or laparoscopic surgical correction, often with the placement of a supportive mesh4 (SOR: B, inconsistent or limited-quality patient-oriented evidence). Repair of epigastric hernias is crucial even in asymptomatic patients due to the high rate of incarceration.3
Our patient was referred to a hernia specialty clinic at a nationally recognized medical center. He moved out of state shortly thereafter and was lost to follow-up.
CORRESPONDENCE
William Murdoch, MD, WSU/Crittenton Family Medicine Residency, 1135 West University Drive, Suite #250, Rochester Hills, MI 48307; [email protected]
1. Park AE, et al. Abdominal wall hernia. Curr Probl Surg. 2006;43:326-375.
2. Muysoms FE, et al. Classification of primary and incisional abdominal wall hernias. Hernia. 2009;13:407-414.
3. Salameh JR. Primary and unusual abdominal wall hernias. Surg Clin North Am. 2008;88:45-60.
4. Bencini L, et al. Comparison of laparoscopic and open repair for primary ventral hernias. Surg Laparosc Endosc Percutan Tech. 2009;19:341-344.
A 46-year-old man sought care at our clinic for an abdominal mass, fatigue, and shortness of breath. He also indicated that he was feeling depressed.
Four years earlier, he’d had a prolonged hospitalization for severe cor pulmonale, during which he suffered a perforated cecum. He had multiple abdominal surgeries, including a right hemicolectomy. His postoperative course was complicated by multi-system organ failure and several nosocomial infections.
In the wake of his recovery, he developed an anterior midline abdominal mass that slowly enlarged over the following years (FIGURE 1). He sought a surgical consultation, but was deferred because of his high-risk operative profile.
Our examination of the patient revealed an anterior, midline, irregularly shaped mass measuring 14 × 20 in. The nontender mass was hollow to percussion and was not as prominent when the patient was supine.
FIGURE 1
Abdominal mass measuring 14 × 20 in
Four years earlier, this 46-year-old patient had undergone multiple abdominal surgeries. On this visit, he sought care for a nontender mass that was hollow to percussion.
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Ventral hernia
An abdominal ultrasound revealed subcutaneous, peristalsing bowel loops consistent with a ventral hernia (FIGURE 2). A small amount of ascites was also found.
Most abdominal wall hernias occur in the inguinal region, but in 2003 there were 360,000 ventral hernia repairs performed in the United States.1 Ventral hernias can be further classified as primary or incisional (depending on patient history) and according to their location—midline (epigastric and umbilical) or lateral (Spighelian and lumbar).2
An abdominal hernia typically presents as a nontender, protruding mass that is either stable in size or gradually expands. The mass may be pulsatile, depending on the contents of the hernia and their activity. Hernias may be reducible, meaning that the contents are able to return to the abdominal cavity with external pressure or if the patient is supine. If a hernia is not reducible, then incarceration becomes a significant risk. Compromised blood supply to the incarcerated organ(s) can lead to tissue necrosis and viscous perforation. Epigastric hernias, in particular, carry a high risk of incarceration.3
FIGURE 2
Another view of the ventral hernia
3 conditions comprise the differential
The differential diagnosis includes diastasis recti, ascites, and lipoma.
Diastasis recti is a separation of the rectus abdominus muscles at the linea alba. It is seen almost exclusively in pregnant women and newborns. In this condition, the flat abdominal wall muscles remain intact, and thus abdominal contents would not protrude.
Ascites is the collection of fluid in the abdominal cavity, secondary to conditions such as cirrhosis or congestive heart failure. In ascites, the abdomen is dull to percussion, with no discrete, irregular mass.
Lipoma is a solid benign tumor composed of fatty tissue. A lipoma of this size is rare, and would be solid to percussion. Also, it would not be reducible with the patient supine.
Ultrasound or CT scan is diagnostic
After a thorough history and physical examination, ultrasonography or CT often helps differentiate a ventral hernia from other abdominal wall defects. In patients with a ventral hernia, either imaging modality will demonstrate prolapsed loops of hollow viscus.
A CT scan was not an option for our patient because none of the local machines could accommodate the size and shape of his body. He had an abdominal ultrasound instead.
Surgery sets things right
Treatment of a ventral hernia involves either an open or laparoscopic surgical correction, often with the placement of a supportive mesh4 (SOR: B, inconsistent or limited-quality patient-oriented evidence). Repair of epigastric hernias is crucial even in asymptomatic patients due to the high rate of incarceration.3
Our patient was referred to a hernia specialty clinic at a nationally recognized medical center. He moved out of state shortly thereafter and was lost to follow-up.
CORRESPONDENCE
William Murdoch, MD, WSU/Crittenton Family Medicine Residency, 1135 West University Drive, Suite #250, Rochester Hills, MI 48307; [email protected]
A 46-year-old man sought care at our clinic for an abdominal mass, fatigue, and shortness of breath. He also indicated that he was feeling depressed.
Four years earlier, he’d had a prolonged hospitalization for severe cor pulmonale, during which he suffered a perforated cecum. He had multiple abdominal surgeries, including a right hemicolectomy. His postoperative course was complicated by multi-system organ failure and several nosocomial infections.
In the wake of his recovery, he developed an anterior midline abdominal mass that slowly enlarged over the following years (FIGURE 1). He sought a surgical consultation, but was deferred because of his high-risk operative profile.
Our examination of the patient revealed an anterior, midline, irregularly shaped mass measuring 14 × 20 in. The nontender mass was hollow to percussion and was not as prominent when the patient was supine.
FIGURE 1
Abdominal mass measuring 14 × 20 in
Four years earlier, this 46-year-old patient had undergone multiple abdominal surgeries. On this visit, he sought care for a nontender mass that was hollow to percussion.
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Ventral hernia
An abdominal ultrasound revealed subcutaneous, peristalsing bowel loops consistent with a ventral hernia (FIGURE 2). A small amount of ascites was also found.
Most abdominal wall hernias occur in the inguinal region, but in 2003 there were 360,000 ventral hernia repairs performed in the United States.1 Ventral hernias can be further classified as primary or incisional (depending on patient history) and according to their location—midline (epigastric and umbilical) or lateral (Spighelian and lumbar).2
An abdominal hernia typically presents as a nontender, protruding mass that is either stable in size or gradually expands. The mass may be pulsatile, depending on the contents of the hernia and their activity. Hernias may be reducible, meaning that the contents are able to return to the abdominal cavity with external pressure or if the patient is supine. If a hernia is not reducible, then incarceration becomes a significant risk. Compromised blood supply to the incarcerated organ(s) can lead to tissue necrosis and viscous perforation. Epigastric hernias, in particular, carry a high risk of incarceration.3
FIGURE 2
Another view of the ventral hernia
3 conditions comprise the differential
The differential diagnosis includes diastasis recti, ascites, and lipoma.
Diastasis recti is a separation of the rectus abdominus muscles at the linea alba. It is seen almost exclusively in pregnant women and newborns. In this condition, the flat abdominal wall muscles remain intact, and thus abdominal contents would not protrude.
Ascites is the collection of fluid in the abdominal cavity, secondary to conditions such as cirrhosis or congestive heart failure. In ascites, the abdomen is dull to percussion, with no discrete, irregular mass.
Lipoma is a solid benign tumor composed of fatty tissue. A lipoma of this size is rare, and would be solid to percussion. Also, it would not be reducible with the patient supine.
Ultrasound or CT scan is diagnostic
After a thorough history and physical examination, ultrasonography or CT often helps differentiate a ventral hernia from other abdominal wall defects. In patients with a ventral hernia, either imaging modality will demonstrate prolapsed loops of hollow viscus.
A CT scan was not an option for our patient because none of the local machines could accommodate the size and shape of his body. He had an abdominal ultrasound instead.
Surgery sets things right
Treatment of a ventral hernia involves either an open or laparoscopic surgical correction, often with the placement of a supportive mesh4 (SOR: B, inconsistent or limited-quality patient-oriented evidence). Repair of epigastric hernias is crucial even in asymptomatic patients due to the high rate of incarceration.3
Our patient was referred to a hernia specialty clinic at a nationally recognized medical center. He moved out of state shortly thereafter and was lost to follow-up.
CORRESPONDENCE
William Murdoch, MD, WSU/Crittenton Family Medicine Residency, 1135 West University Drive, Suite #250, Rochester Hills, MI 48307; [email protected]
1. Park AE, et al. Abdominal wall hernia. Curr Probl Surg. 2006;43:326-375.
2. Muysoms FE, et al. Classification of primary and incisional abdominal wall hernias. Hernia. 2009;13:407-414.
3. Salameh JR. Primary and unusual abdominal wall hernias. Surg Clin North Am. 2008;88:45-60.
4. Bencini L, et al. Comparison of laparoscopic and open repair for primary ventral hernias. Surg Laparosc Endosc Percutan Tech. 2009;19:341-344.
1. Park AE, et al. Abdominal wall hernia. Curr Probl Surg. 2006;43:326-375.
2. Muysoms FE, et al. Classification of primary and incisional abdominal wall hernias. Hernia. 2009;13:407-414.
3. Salameh JR. Primary and unusual abdominal wall hernias. Surg Clin North Am. 2008;88:45-60.
4. Bencini L, et al. Comparison of laparoscopic and open repair for primary ventral hernias. Surg Laparosc Endosc Percutan Tech. 2009;19:341-344.
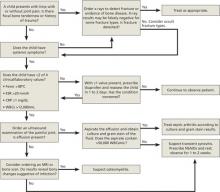
Ureteral calculi: What should you consider before intervening?
THE SIZE OF THE CALCULI, their location, and complicating factors such as infection should all be considered.
Most ureteral calculi smaller than 5 mm pass spontaneously, as do approximately half of calculi between 5 and 10 mm. Calculi larger than 10 mm are unlikely to pass without intervention. Distal calculi are more likely to pass spontaneously than calculi in mid- or proximal ureteral locations; most spontaneous passage occurs within 4 to 6 weeks (strength of recommendation [SOR]: A, prospective cohort studies).
All patients with calculi complicated by such factors as obstruction, infection, renal injury, or a single kidney require surgical consultation (SOR: C, expert opinion).
Medical expulsion therapy with alpha-blockers (usually tamsulosin) and nifedipine improves passage rates, including for some calculi larger than 10 mm (SOR: A, meta-analysis of prospective cohort studies).
Evidence summary
A meta-analysis of 5 prospective cohort studies evaluated the rate of spontaneous passage of ureteral calculi according to size. Calculi smaller than 5 mm passed spontaneously in 68% of patients (5 studies, N=224). Calculi between 5 and 10 mm passed spontaneously in 47% of patients (3 studies, N=104).1
A prospective cohort study evaluated spontaneous passage rates of ureteral calculi by size in 172 patients who were diagnosed by unenhanced helical computed tomography.2 Investigators found spontaneous passage rates of 87% for 1-mm calculi, 76% for 2- to 4-mm calculi, 60% for 5- to 7-mm calculi, 48% for 7- to 9-mm calculi, and 25% for calculi larger than 9 mm.
Spontaneous passage rates differed significantly for calculi 1 to 4 mm in size compared with calculi 5 to 7 mm in size (P<.001) and for calculi 5 to 7 mm in size compared with calculi 8 mm or larger (P<.001). Calculi in either the distal ureter or ureterovesicular junction were more likely to pass that those in the mid- or proximal ureter (75% to 79% vs 48% to 60%; P<.001).
Most smaller calculi pass in 4 to 6 weeks
Another prospective cohort study (N=75) found that most calculi pass spontaneously within 4 to 6 weeks. In 95% of patients, calculi passed within 31 days (2 mm or smaller), 40 days (2-4 mm), or 39 days (4-6 mm).3
Some cases require prompt surgery
The American Urological Association (AUA) expert panel recommends early surgical intervention, regardless of calculus size, under the following circumstances: obstruction with high-grade hydronephrosis, infection, impending renal deterioration, intractable pain, nausea and vomiting, or obstruction in a solitary or transplanted kidney.1
Medical expulsion therapy trumps waiting for distal calculi to pass
A meta-analysis comparing rates of calculus passage found that medical expulsion therapy was more effective than expectant management for patients with distal ureteral calculi. Sixteen RCTs (N=1235) evaluated alpha-antagonists (mostly tamsulosin), and 9 RCTs (N=686) evaluated nifedipine. Treat ment periods for medical expulsion therapy ranged from 30 to 60 days.
Alpha-antagonists increased expulsion rates over expectant management for calculi ranging in size from 3 to 18 mm with a mean diameter greater than 5 mm (relative risk [RR]=1.59; 95% confidence interval [CI], 1.44-1.75; number needed to treat [NNT]=3). The mean time until passage ranged from 2.7 to 14.2 days. Nifedipine also increased expulsion rates for calculi with a mean diameter larger than 5 mm, ranging in size from 3.9 to 12.8 mm (RR=1.50; 95% CI, 1.34-1.68; NNT=4).4
Recommendations
The Joint European Association of Urology/ AUA Nephrolithiasis Guideline Panel recommends observation with periodic evaluation for patients newly diagnosed with ureteral calculi smaller than 10 mm.1 Patients may be offered medical expulsion therapy to facilitate calculus passage. Surveillance should be maintained until calculi pass; intervention should be considered if calculi don’t pass spontaneously within about 30 days.
The Panel states that patients with ureteral calculi larger than 10 mm could be observed (with or without medical expulsion therapy); however, most cases will require surgical intervention.1
Acknowledgements
The opinions and assertions contained herein are the private views of the authors and are not to be construed as official or as reflecting the views of the Medical Department of the United States Army or the US Army Service at large.
1. European Association of Urology/American Urology Association Nephrolithiasis Guideline Panel. 2007 Guideline for the management of ureteral calculi. Available at: www.auanet.org/content/guidelines-and-quality-care/clinical-guidelines.cfm?sub=uc. Accessed August 16, 2010.
2. Coll DM, Varanelli MJ, Smith RC. Relationship of spontaneous passage of ureteral calculi to calculus size and location as revealed by unenhanced helical CT. Am J Roentgenol. 2002;178:101-103.
3. Miller OF, Kane CJ. Time to calculus passage for observed ureteral calculi: a guide for patient education. J Urol. 1999;162:688-691.
4. Singh A, Alter HJ, Littlepage A. A systematic review of medical therapy to facilitate passage of ureteral calculi. Ann Emerg Med. 2007;50:552-563.
THE SIZE OF THE CALCULI, their location, and complicating factors such as infection should all be considered.
Most ureteral calculi smaller than 5 mm pass spontaneously, as do approximately half of calculi between 5 and 10 mm. Calculi larger than 10 mm are unlikely to pass without intervention. Distal calculi are more likely to pass spontaneously than calculi in mid- or proximal ureteral locations; most spontaneous passage occurs within 4 to 6 weeks (strength of recommendation [SOR]: A, prospective cohort studies).
All patients with calculi complicated by such factors as obstruction, infection, renal injury, or a single kidney require surgical consultation (SOR: C, expert opinion).
Medical expulsion therapy with alpha-blockers (usually tamsulosin) and nifedipine improves passage rates, including for some calculi larger than 10 mm (SOR: A, meta-analysis of prospective cohort studies).
Evidence summary
A meta-analysis of 5 prospective cohort studies evaluated the rate of spontaneous passage of ureteral calculi according to size. Calculi smaller than 5 mm passed spontaneously in 68% of patients (5 studies, N=224). Calculi between 5 and 10 mm passed spontaneously in 47% of patients (3 studies, N=104).1
A prospective cohort study evaluated spontaneous passage rates of ureteral calculi by size in 172 patients who were diagnosed by unenhanced helical computed tomography.2 Investigators found spontaneous passage rates of 87% for 1-mm calculi, 76% for 2- to 4-mm calculi, 60% for 5- to 7-mm calculi, 48% for 7- to 9-mm calculi, and 25% for calculi larger than 9 mm.
Spontaneous passage rates differed significantly for calculi 1 to 4 mm in size compared with calculi 5 to 7 mm in size (P<.001) and for calculi 5 to 7 mm in size compared with calculi 8 mm or larger (P<.001). Calculi in either the distal ureter or ureterovesicular junction were more likely to pass that those in the mid- or proximal ureter (75% to 79% vs 48% to 60%; P<.001).
Most smaller calculi pass in 4 to 6 weeks
Another prospective cohort study (N=75) found that most calculi pass spontaneously within 4 to 6 weeks. In 95% of patients, calculi passed within 31 days (2 mm or smaller), 40 days (2-4 mm), or 39 days (4-6 mm).3
Some cases require prompt surgery
The American Urological Association (AUA) expert panel recommends early surgical intervention, regardless of calculus size, under the following circumstances: obstruction with high-grade hydronephrosis, infection, impending renal deterioration, intractable pain, nausea and vomiting, or obstruction in a solitary or transplanted kidney.1
Medical expulsion therapy trumps waiting for distal calculi to pass
A meta-analysis comparing rates of calculus passage found that medical expulsion therapy was more effective than expectant management for patients with distal ureteral calculi. Sixteen RCTs (N=1235) evaluated alpha-antagonists (mostly tamsulosin), and 9 RCTs (N=686) evaluated nifedipine. Treat ment periods for medical expulsion therapy ranged from 30 to 60 days.
Alpha-antagonists increased expulsion rates over expectant management for calculi ranging in size from 3 to 18 mm with a mean diameter greater than 5 mm (relative risk [RR]=1.59; 95% confidence interval [CI], 1.44-1.75; number needed to treat [NNT]=3). The mean time until passage ranged from 2.7 to 14.2 days. Nifedipine also increased expulsion rates for calculi with a mean diameter larger than 5 mm, ranging in size from 3.9 to 12.8 mm (RR=1.50; 95% CI, 1.34-1.68; NNT=4).4
Recommendations
The Joint European Association of Urology/ AUA Nephrolithiasis Guideline Panel recommends observation with periodic evaluation for patients newly diagnosed with ureteral calculi smaller than 10 mm.1 Patients may be offered medical expulsion therapy to facilitate calculus passage. Surveillance should be maintained until calculi pass; intervention should be considered if calculi don’t pass spontaneously within about 30 days.
The Panel states that patients with ureteral calculi larger than 10 mm could be observed (with or without medical expulsion therapy); however, most cases will require surgical intervention.1
Acknowledgements
The opinions and assertions contained herein are the private views of the authors and are not to be construed as official or as reflecting the views of the Medical Department of the United States Army or the US Army Service at large.
THE SIZE OF THE CALCULI, their location, and complicating factors such as infection should all be considered.
Most ureteral calculi smaller than 5 mm pass spontaneously, as do approximately half of calculi between 5 and 10 mm. Calculi larger than 10 mm are unlikely to pass without intervention. Distal calculi are more likely to pass spontaneously than calculi in mid- or proximal ureteral locations; most spontaneous passage occurs within 4 to 6 weeks (strength of recommendation [SOR]: A, prospective cohort studies).
All patients with calculi complicated by such factors as obstruction, infection, renal injury, or a single kidney require surgical consultation (SOR: C, expert opinion).
Medical expulsion therapy with alpha-blockers (usually tamsulosin) and nifedipine improves passage rates, including for some calculi larger than 10 mm (SOR: A, meta-analysis of prospective cohort studies).
Evidence summary
A meta-analysis of 5 prospective cohort studies evaluated the rate of spontaneous passage of ureteral calculi according to size. Calculi smaller than 5 mm passed spontaneously in 68% of patients (5 studies, N=224). Calculi between 5 and 10 mm passed spontaneously in 47% of patients (3 studies, N=104).1
A prospective cohort study evaluated spontaneous passage rates of ureteral calculi by size in 172 patients who were diagnosed by unenhanced helical computed tomography.2 Investigators found spontaneous passage rates of 87% for 1-mm calculi, 76% for 2- to 4-mm calculi, 60% for 5- to 7-mm calculi, 48% for 7- to 9-mm calculi, and 25% for calculi larger than 9 mm.
Spontaneous passage rates differed significantly for calculi 1 to 4 mm in size compared with calculi 5 to 7 mm in size (P<.001) and for calculi 5 to 7 mm in size compared with calculi 8 mm or larger (P<.001). Calculi in either the distal ureter or ureterovesicular junction were more likely to pass that those in the mid- or proximal ureter (75% to 79% vs 48% to 60%; P<.001).
Most smaller calculi pass in 4 to 6 weeks
Another prospective cohort study (N=75) found that most calculi pass spontaneously within 4 to 6 weeks. In 95% of patients, calculi passed within 31 days (2 mm or smaller), 40 days (2-4 mm), or 39 days (4-6 mm).3
Some cases require prompt surgery
The American Urological Association (AUA) expert panel recommends early surgical intervention, regardless of calculus size, under the following circumstances: obstruction with high-grade hydronephrosis, infection, impending renal deterioration, intractable pain, nausea and vomiting, or obstruction in a solitary or transplanted kidney.1
Medical expulsion therapy trumps waiting for distal calculi to pass
A meta-analysis comparing rates of calculus passage found that medical expulsion therapy was more effective than expectant management for patients with distal ureteral calculi. Sixteen RCTs (N=1235) evaluated alpha-antagonists (mostly tamsulosin), and 9 RCTs (N=686) evaluated nifedipine. Treat ment periods for medical expulsion therapy ranged from 30 to 60 days.
Alpha-antagonists increased expulsion rates over expectant management for calculi ranging in size from 3 to 18 mm with a mean diameter greater than 5 mm (relative risk [RR]=1.59; 95% confidence interval [CI], 1.44-1.75; number needed to treat [NNT]=3). The mean time until passage ranged from 2.7 to 14.2 days. Nifedipine also increased expulsion rates for calculi with a mean diameter larger than 5 mm, ranging in size from 3.9 to 12.8 mm (RR=1.50; 95% CI, 1.34-1.68; NNT=4).4
Recommendations
The Joint European Association of Urology/ AUA Nephrolithiasis Guideline Panel recommends observation with periodic evaluation for patients newly diagnosed with ureteral calculi smaller than 10 mm.1 Patients may be offered medical expulsion therapy to facilitate calculus passage. Surveillance should be maintained until calculi pass; intervention should be considered if calculi don’t pass spontaneously within about 30 days.
The Panel states that patients with ureteral calculi larger than 10 mm could be observed (with or without medical expulsion therapy); however, most cases will require surgical intervention.1
Acknowledgements
The opinions and assertions contained herein are the private views of the authors and are not to be construed as official or as reflecting the views of the Medical Department of the United States Army or the US Army Service at large.
1. European Association of Urology/American Urology Association Nephrolithiasis Guideline Panel. 2007 Guideline for the management of ureteral calculi. Available at: www.auanet.org/content/guidelines-and-quality-care/clinical-guidelines.cfm?sub=uc. Accessed August 16, 2010.
2. Coll DM, Varanelli MJ, Smith RC. Relationship of spontaneous passage of ureteral calculi to calculus size and location as revealed by unenhanced helical CT. Am J Roentgenol. 2002;178:101-103.
3. Miller OF, Kane CJ. Time to calculus passage for observed ureteral calculi: a guide for patient education. J Urol. 1999;162:688-691.
4. Singh A, Alter HJ, Littlepage A. A systematic review of medical therapy to facilitate passage of ureteral calculi. Ann Emerg Med. 2007;50:552-563.
1. European Association of Urology/American Urology Association Nephrolithiasis Guideline Panel. 2007 Guideline for the management of ureteral calculi. Available at: www.auanet.org/content/guidelines-and-quality-care/clinical-guidelines.cfm?sub=uc. Accessed August 16, 2010.
2. Coll DM, Varanelli MJ, Smith RC. Relationship of spontaneous passage of ureteral calculi to calculus size and location as revealed by unenhanced helical CT. Am J Roentgenol. 2002;178:101-103.
3. Miller OF, Kane CJ. Time to calculus passage for observed ureteral calculi: a guide for patient education. J Urol. 1999;162:688-691.
4. Singh A, Alter HJ, Littlepage A. A systematic review of medical therapy to facilitate passage of ureteral calculi. Ann Emerg Med. 2007;50:552-563.
Evidence-based answers from the Family Physicians Inquiries Network
Weight-loss talks: What works (and what doesn’t)
Background In primary care encounters, it is unknown whether physician advice on weight-related matters leads to patient weight loss. To examine this issue, we analyzed physician weight loss advice and measured corresponding changes in patients’ dietary intake, physical activity, and weight.
Methods Using audio-recorded primary care encounters between 40 physicians and 461 of their overweight or obese patients, we coded weight-related advice as nonspecific, specific nutritional, specific exercise, or specific weight. Physicians and patients were told the study was about preventive health, not weight. We used mixed models (SAS Proc Mixed), controlled for physician clustering and baseline covariates, to assess changes in diet, exercise, and measured weight, both pre-encounter and 3 months post-encounter.
Results When discussing weight, physicians typically provided a combination of specific weight, nutrition, and physical activity advice to their patients (34%). Combined advice resulted in patients reducing their dietary fat intake (P=.02). However, when physicians provided physical activity advice only, patients were significantly (P=.02) more likely to gain weight (+1.41 kg) compared with those who received no advice.
Conclusion When giving weight-related advice, most physicians provided a combination of lifestyle recommendations. Combining advice may help patients reduce their fat in-take. Physical activity advice alone may not be particularly helpful.
The US Preventive Services Task Force (USPSTF) recommends that physicians screen patients for obesity and offer intensive counseling and behavioral interventions to promote sustained weight loss.1 Evidence suggests that physician counseling, including advice, can help patients to lose weight, increase physical activity, and improve diet.2-9 However, little is known about what specific types of weight loss advice physicians give to patients, and whether some types are more effective than others at influencing behavior change.
We analyzed physician weight loss advice delivered in primary care visits and measured changes in patients’ dietary intake, physical activity, and body weight. We examined both the type of weight loss advice delivered and the impact of type of advice on weight and behavior change.
Methods
This study analyzed audio recordings from Project CHAT – Communicating Health: Analyzing Talk. The project was approved by the Duke University Medical Center Institutional Review Board.
Recruitment Physicians. We obtained consent from 40 primary care physicians in community-based practices and told them the study would examine communication around preventive health topics, not weight specifically.
Patients. We identified potential participants by reviewing scheduled appointments 3 weeks in advance. Eligible participants were at least 18 years of age, English-speaking, overweight or obese (body mass index [BMI] ≥25 kg/m2), cognitively competent, and not pregnant. After we obtained consent, a remotely located research assistant started a digital audio recorder as the patient entered the exam room. Immediately after the encounter, the research assistant administered a post-encounter survey to the patient and recorded the patient’s vital signs (N=461). Three months later, the research assistant met with the participant to record vital signs and administer a survey assessing changes in dietary fat intake and exercise (N=426).
Data coding
We coded advice into 4 broad categories: (1) nutrition advice, (2) physical activity advice, (3) specific weight loss advice, and (4) nonspecific weight loss/weight-related advice. We transcribed each piece of advice verbatim.
Nutrition advice consisted of 9 sub-categories: calorie/portion control, meal timing/planning, commercial diet plans, negative diet plans, increase fruits/vegetables, reduce sugar/carbohydrates, reduce fat/cholesterol, other micronutrient recommendations, and specific food items from multiple categories.
Physical activity advice consisted of 6 subcategories: walking, aerobic exercise, anaerobic exercise, exercise intensity, exercise duration, and exercise for comorbid conditions.
Specific weight loss advice consisted of 3 categories: weight loss behavior, weight loss for comorbid conditions, and referrals.
Nonspecific weight loss advice also consisted of 3 subcategories in which physicians provided no details about the general topics of nutrition, physical activity, or weight loss.
Two independent coders (CBT and MEC) assessed each piece of advice and double coded 20% of conversations for reliability. Cohen’s kappa was used to calculate inter-rater reliability for each code using Landis and Koch’s classification (0.21-0.40=fair agreement; 0.41-0.60=moderate agreement; 0.61-0.80=substantial agreement; 0.81-1.0=near-perfect agreement).10 Three advice categories achieved near perfect agreement: nutrition (kappa= 0.94; 95% confidence interval [CI] 0.82-1.0; 99.2% agreement), physical activity (kappa=0.91; 95% CI, 0.84-0.99; 98.6% agreement), and weight loss (kappa=0.95; 95% CI, 0.82-1.0; 99.7% agreement). The nonspecific weight loss advice category had slightly lower agreement but still achieved near-perfect agreement (kappa=0.82; 95% CI, 0.62-1.0; 99.2% agreement).
After all advice was coded, we placed conversations into 1 of 6 categories: (1) no advice given; (2) nonspecific advice only; (3) nutrition only; (4) physical activity only; (5) weight loss only; or (6) combination of nutrition, physical activity, and/or weight loss.
Measures
Dietary fat and fiber intake. We assessed dietary fat intake at baseline and at 3 months using the 22-item Fat- and Fiber-Related Diet Behavior Questionnaire.11,12 Questions about frequency of food selections included, “When you ate dessert, how often did you eat only fruit?” and “When you ate chicken, how often did you take off the skin?” We averaged responses into a total score wherein 1 reflected higher fiber, lower fat food choices; a score of 4 reflected lower fiber, higher fat choices (α=0.74 at baseline and α=0.77 at 3-month follow-up).
Physical activity. We measured physical activity (baseline, 3 months) using the Framingham Physical Activity Index.13 Participants recalled the average number of hours spent engaged in various daily activities (sleeping, working, leisure) and the level of activity for each (sedentary, slight, moderate, or heavy). The composite score accounts for activity duration and intensity.
Anthropometrics. We measured patient weight (baseline, 3 months) and height (baseline only) using a calibrated scale and portable stadiometer. Patients removed shoes, outerwear, and belongings from their pockets before being weighed.
Analysis
We analyzed data using SAS (SAS Institute, Inc., Cary, NC). We assessed the association between type of advice and weight loss, improvement in dietary fat intake behaviors, and increase in physical activity between baseline and the 3-month follow-up visit. We used PROC MIXED to fit general linear models; we incorporated responses into these models from all participants who provided measurements for at least one time point. This modeling framework yields unbiased estimates when missing data are unrelated to the observed variable.14
Primary predictors: (1) type of advice (none, nonspecific, nutrition, physical activity, weight loss, and combination), (2) time since baseline visit, and (3) time by type of advice interaction. All models included a priori defined patient, physician, and visit-related covariates that were theoretically or empirically related to changes in the outcomes (weight, physical activity, or dietary fat in-take). The 14 patient covariates were sex; age; race; high school education; economic security (enough money to pay monthly bills); over-weight (BMI, 25-29.9 kg/m2) or obese (BMI ≥30 kg/m2); actively trying to lose weight (yes/ no); motivated to lose weight (Likert scale 1-7); comfortable discussing weight (Likert scale 1-5); confident about losing weight (Likert scale 1-5); and patient-reported comorbid conditions of diabetes, hypertension, arthritis, and hyperlipidemia.
The 9 physician covariates were sex; race; years since medical school graduation; specialty (family vs internal medicine); self-efficacy (Likert scale 1-5); barriers for weight counseling (Likert scale 1-5); comfort discussing weight (Likert scale 1-5); insurance reimbursement concerns (Likert scale 1-5); and prior training in behavioral counseling (yes/no). Finally, 2 visit-level covariates were included: minutes spent addressing weight issues and visit type (preventive vs chronic).
Results
Sample characteristics
Of the 40 physicians, 19 were family physicians and 21 were internists. More than half of the physicians were female (60%), and 85% were white. Mean age was 47.2 years and mean BMI was 24.9 kg/m2. Of the 461 patients, 66% were female, 65% were white, 35% were African American, and two-thirds had post-high school education (TABLE 1). Mean patient age was 59.8 years; only 4% of the patients were new to their physicians.
TABLE 1
Patient characteristics (N=461)
| % or mean (SD) | |
|---|---|
| Race | |
| White/Asian/Pacific Islander | 65% |
| African American | 35% |
| Female | 66% |
| Age, y (missing=1)* | 59.8 (13.9) |
| BMI, kg/m2 (missing=1)* | 33.1 (7.1) |
| Education (missing=1)* | |
| Post-high school | 67% |
| Income (missing=37)* | |
| $45,000 or less | 48% |
| High financial burden (missing=13)* | |
| Pay bills with trouble | 14% |
| Diagnosed with: | |
| Diabetes | 31% |
| Hypertension (missing=1)* | 69% |
| Hyperlipidemia (missing=1)* | 56% |
| Arthritis | 47% |
| New patient | 4% |
| BMI, body mass index; SD, standard deviation. * Missing data at baseline. | |
Frequency of advice
Physicians gave some type of weight-related advice in 63% of the encounters. They combined types of advice in 34% of all conversations, provided physical activity advice only in 13%, nutrition advice only in 8%, nonspecific advice in 5%, and weight loss advice only in 3%. Many times when physicians gave advice, it was centered on self (eg, “I need you to do X” or “What will it take for me to get you to do Y?”).
Nutrition advice most commonly pertained to specific food items from multiple categories (27% of conversations). Physicians also advised patients to reduce sugar/carbohydrates, control calories and portions, add other micronutrients, eat more fruits/vegetables, and eat meals more frequently.
Walking was the physical activity topic discussed most frequently, followed by exercise duration, exercise for comorbidities, aerobic activities, exercise intensity, and anaerobic exercise. The most common specific weight loss topic was weight loss behavioral advice, followed by weight loss for comorbid conditions. Physicians rarely provided referrals to weight-loss programs.
Effect of type of advice on fat and fiber diet behavior score
Receipt of nutrition advice only was not associated with reduction in fat intake (P=.43, TABLE 2). However, those who received combined types of advice exhibited a significantly greater reduction of fat intake compared with those who received no advice (Fat- and Fiber-Related Diet Behavior Questionnaire score reduction of 0.15 vs 0.05; P=.02).
TABLE 2
How types of physician advice affected dietary fat intake, physical activity, and weight
| Type of advice | ||||||
|---|---|---|---|---|---|---|
| None | Nutrition only | Physical activity only | Weight loss only | Combined advice | Nonspecific | |
| Dietary fat change in Fat- and Fiber-Related Diet Behavior Questionnaire score differences | ||||||
| At 3 months from baseline (95% CI) | -0.05 (-0.11 to 0.004) | -0.10 (-0.22 to 0.01) | -0.07 (-0.16 to 0.02) | -0.08 (-0.26 to 0.09) | -0.15 (-0.20 to -0.09) | 0.03 (0.11 to 0.18) |
| P value* | .43 | .75 | .73 | .02 | .31 | |
| Physical activity score (change in MET hours) | ||||||
| At 3 months from baseline (95% CI) | 0.48 (-0.17 to 1.11) | 0.83 (-0.51 to 2.14) | 0.69 (-0.33 to 1.69) | -0.72 (-2.66 to 1.21) | 0.24 (-0.40 to 0.86) | -0.07 (-1.74 to 1.59) |
| P value* | .64 | .73 | .25 | .60 | .55 | |
| Weight change (kg) | ||||||
| At 3 months from baseline (95% CI) | -0.18 (-0.39 to 0.75) | -0.18 (-1.38 to 1.02) | 1.41 (0.51 to 2.31) | -0.26 (-1.99 to 1.47) | -0.55 (-1.12 to 0.02) | -0.62 (-2.11 to 0.87) |
| P value* | .59 | .02 | .63 | .08 | .32 | |
| CI, confidence interval; MET, metabolic equivalent tasks. *Test of difference between advice given and no advice given. | ||||||
Effect of type of advice on Framingham Physical Activity score
No type of advice, including physical activity advice, led to a change in Framingham Physical Activity scores at the 3-month visit (overall P=.76; TABLE 2).
Effect of type of advice on weight loss
Patients who received physical activity advice gained significantly more weight than patients who received no advice (1.41 kg gained vs 0.18 kg lost; P=.02). Patients who received combined advice lost more weight than patients who received no advice, but the difference did not reach statistical significance (0.55 kg lost vs 0.18 kg lost; P=.08).
Discussion
Physicians typically took an “all or nothing” approach to weight-related issues, giving no advice (37%) or a combination of nutrition, physical activity, and weight loss advice (34%). It seems when physicians do give advice, most of them follow the USPSTF guidelines by addressing nutrition and physical activity together.15
Providing advice alone did not predict a change in patient behavior. For instance, we found no significant association between dietary fat reduction and having received only nutrition advice. Possible explanations include the following:
- Although physicians advised patients to reduce fat/cholesterol intake in 28% of conversations, they did so mostly in combination with other types of advice. Nutrition-only advice occurred in only 8% of conversations. Thus, there may have been insufficient power to detect the impact of this specific type of advice.
- With nutrition-only advice, the most common recommendation was to reduce carbohydrates/sweets, which should not affect fat intake.
Advising patients solely on physical activity led to unintended weight gain overall. Other data have shown that exercise without dietary changes, though beneficial in many ways, is not substantially effective for weight loss.15 People may eat more when they exercise, either to reward themselves or to satiate increased appetite from increased energy expenditure. Or, if physicians recommend the standard goal of 150 minutes of intensive physical activity per week, normally sedentary patients may see that as unattainable and become too discouraged to try.1,16,17
Combining types of advice seemed to help patients reduce their fat intake. Overall, however, simple, brief advice from a physician may not be enough to promote healthy lifestyle changes.
Also notable was that physicians rarely provided referrals, even though this is a strong recommendation from the National Institutes of Health, the American Diabetes Association, and the USPSTF.1,16,17 It could be that many physicians believe referrals are not covered by insurance. Yet, the low frequency of referrals may suggest an important missing component of weight loss therapy, especially given that physician advice alone seems an inadequate intervention.
Avoid physician-centered appeals. Advice was often given in a physician-centered way. There are 3 possible explanations for such phrasing:
- In the absence of clear evidence about how to deliver weight loss advice, physicians may be formulating advice based on their personal or clinical experiences.
- Physicians either assume or sense that patients lack internal motivation to make lifestyle changes for themselves and instead request that patients make changes for the doctor-patient relationship.
- Physicians might be trying to invoke authority in the hope that patients will respond accordingly.
Whatever the reason, the literature on self-centered physician talk indicates that patients are less satisfied when physicians make the visit more about themselves than about patients.18 A better strategy might be to use Motivational Interviewing19 that supports patient autonomy and attempts to elicit and build on internal motivation.
The take-away message is that behavior change is complex and that knowledge is a necessary but insufficient agent for change. Following the tenets of Social Cognitive Theory,20 physicians might also need to address patient motivation, confidence, outcome expectations, and skills to help promote behavior change.
Strengths and limitations of this study
We recorded conversations rather than relying on physician or patient recall. Additionally, these primary care patients were not enrolled in a weight-loss trial and, therefore, were not self-selected to be highly motivated to lose weight. Because of this, and the large and ethnically diverse sample, our results should be generalizable to many clinical settings.
One limitation is that few younger, lower-income patients were included in the sample, which limits generalizability to those populations. Also, the study was observational. Although we adjusted for a broad set of patient, physician, and visit covariates, unmeasured confounding variables may still account for at least part of the observed associations. The analysis is limited by the use of self-reported dietary fat intake and physical activity measures. A food diary and accelerometer would have been more accurate; however, such involved measures could invoke changes in behavior, which would have confounded our ability to assess the effect of physician advice on weight loss.
Acknowledgements
The authors thank all of the physicians and patients who participated in this study, the study project managers Gretchen Yonish and Iguehi Esoimeme, and research assistant Justin Manusov.
CORRESPONDENCE
Stewart C. Alexander, PhD, Department of Medicine, Duke University School of Medicine, P.O. Box 3140 Medical Center, Durham, NC 27710; [email protected]
1. US Preventive Services Task Force. Screening for obesity in adults: recommendations and rationale. Ann Intern Med. 2003;139:930-932.
2. Nawaz H, Adams ML, Katz DL. Physician-patient interactions regarding diet, exercise, and smoking. Prev Med. 2000;31:652-657.
3. Sciamanna CN, Tate DF, Lang W, et al. Who reports receiving advice to lose weight? Results from a multistate survey. Arch Intern Med. 2000;160:2334-2339.
4. Galuska DA, Will JC, Serdula MK, et al. Are health care professionals advising obese patients to lose weight? JAMA. 1999;282:1576-1578.
5. Evans E. Why should obesity be managed? The obese individual’s perspective. Int J Obes Relat Metab Disord. 1999;23(suppl 4):S3-S6.
6. Mehrotra C, Naimi TS, Serdula M, et al. Arthritis, body mass index, and professional advice to lose weight: implications for clinical medicine and public health. Am J Prev Med. 2004;27:16-21.
7. Loureiro ML, Nayga RM Jr. Obesity, weight loss, and physician’s advice. Soc Sci Med. 2006;62:2458-2468.
8. Flocke SA, Clark A, Schlessman K, et al. Exercise, diet, and weight loss advice in the family medicine outpatient setting. Fam Med. 2005;37:415-421.
9. Alexander SC, Cox ME, Østbye T, et al. Do the 5 A’s work for weight-loss counseling? Presented at: International Conference on Communication in Healthcare; October 4–7, 2009; Miami, Fla.
10. McGinn T, Wyer PC, Newman TB, et al. Tips for learners of evidence-based medicine: 3. Measures of observer variability (kappa statistic). CMAJ. 2004;171:1369-1373.
11. Shannon J, Kristal AR, Curry SJ, et al. Application of a behavioral approach to measuring dietary change: the fat- and fiber-related diet behavior questionnaire. Cancer Epidemiol Biomarkers Prev. 1997;6:355-361.
12. Kristal AR, Shattuck AL, Henry HJ. Patterns of dietary behavior associated with selecting diets low in fat: reliability and validity of a behavioral approach to dietary assessment. J Am Diet Assoc. 1990;90:214-220.
13. Kannel WB, Sorlie P. Some health benefits of physical activity: the Framingham study. Arch Intern Med. 1979;139:857-861.
14. Little RJA, Rubin DB. Statistical Analysis With Missing Data. New York, NY: John Wiley & Sons; 2002.
15. Franz MJ, VanWormer JJ, Crain AL, et al. Weight-loss outcomes: a systematic review and meta-analysis of weight-loss clinical trials with a minimum 1-year follow-up. J Am Diet Assoc. 2007;107:1755-1767.
16. National Institutes of Health. The practical guide: identification, evaluation, and treatment of overweight and obesity in adults. NIH publication 00-4084. 2000. Available at: http://www.nhlbi.nih.gov/guidelines/obesity/prctgd_c.pdf. Accessed August 17, 2009.
17. American Diabetes Association. Standards of medical care in diabetes–2010. Diabetes Care. 2010;33(suppl 1):S11-S61.
18. Beach MC, Roter DL. Interpersonal expectations in the patient-physician relationship. J Gen Intern Med. 2000;15:825-827.
19. Miller WR, Rollnick SP, Miller WR. Motivational Interviewing: Preparing People for Change. 2nd ed. New York, NY: Guilford Press; 2002.
20. Bandura A. Social Foundations of Thought and Action: A Social Cognitive Theory. Englewood Cliffs, NJ: Prentice-Hall; 1986.
Background In primary care encounters, it is unknown whether physician advice on weight-related matters leads to patient weight loss. To examine this issue, we analyzed physician weight loss advice and measured corresponding changes in patients’ dietary intake, physical activity, and weight.
Methods Using audio-recorded primary care encounters between 40 physicians and 461 of their overweight or obese patients, we coded weight-related advice as nonspecific, specific nutritional, specific exercise, or specific weight. Physicians and patients were told the study was about preventive health, not weight. We used mixed models (SAS Proc Mixed), controlled for physician clustering and baseline covariates, to assess changes in diet, exercise, and measured weight, both pre-encounter and 3 months post-encounter.
Results When discussing weight, physicians typically provided a combination of specific weight, nutrition, and physical activity advice to their patients (34%). Combined advice resulted in patients reducing their dietary fat intake (P=.02). However, when physicians provided physical activity advice only, patients were significantly (P=.02) more likely to gain weight (+1.41 kg) compared with those who received no advice.
Conclusion When giving weight-related advice, most physicians provided a combination of lifestyle recommendations. Combining advice may help patients reduce their fat in-take. Physical activity advice alone may not be particularly helpful.
The US Preventive Services Task Force (USPSTF) recommends that physicians screen patients for obesity and offer intensive counseling and behavioral interventions to promote sustained weight loss.1 Evidence suggests that physician counseling, including advice, can help patients to lose weight, increase physical activity, and improve diet.2-9 However, little is known about what specific types of weight loss advice physicians give to patients, and whether some types are more effective than others at influencing behavior change.
We analyzed physician weight loss advice delivered in primary care visits and measured changes in patients’ dietary intake, physical activity, and body weight. We examined both the type of weight loss advice delivered and the impact of type of advice on weight and behavior change.
Methods
This study analyzed audio recordings from Project CHAT – Communicating Health: Analyzing Talk. The project was approved by the Duke University Medical Center Institutional Review Board.
Recruitment Physicians. We obtained consent from 40 primary care physicians in community-based practices and told them the study would examine communication around preventive health topics, not weight specifically.
Patients. We identified potential participants by reviewing scheduled appointments 3 weeks in advance. Eligible participants were at least 18 years of age, English-speaking, overweight or obese (body mass index [BMI] ≥25 kg/m2), cognitively competent, and not pregnant. After we obtained consent, a remotely located research assistant started a digital audio recorder as the patient entered the exam room. Immediately after the encounter, the research assistant administered a post-encounter survey to the patient and recorded the patient’s vital signs (N=461). Three months later, the research assistant met with the participant to record vital signs and administer a survey assessing changes in dietary fat intake and exercise (N=426).
Data coding
We coded advice into 4 broad categories: (1) nutrition advice, (2) physical activity advice, (3) specific weight loss advice, and (4) nonspecific weight loss/weight-related advice. We transcribed each piece of advice verbatim.
Nutrition advice consisted of 9 sub-categories: calorie/portion control, meal timing/planning, commercial diet plans, negative diet plans, increase fruits/vegetables, reduce sugar/carbohydrates, reduce fat/cholesterol, other micronutrient recommendations, and specific food items from multiple categories.
Physical activity advice consisted of 6 subcategories: walking, aerobic exercise, anaerobic exercise, exercise intensity, exercise duration, and exercise for comorbid conditions.
Specific weight loss advice consisted of 3 categories: weight loss behavior, weight loss for comorbid conditions, and referrals.
Nonspecific weight loss advice also consisted of 3 subcategories in which physicians provided no details about the general topics of nutrition, physical activity, or weight loss.
Two independent coders (CBT and MEC) assessed each piece of advice and double coded 20% of conversations for reliability. Cohen’s kappa was used to calculate inter-rater reliability for each code using Landis and Koch’s classification (0.21-0.40=fair agreement; 0.41-0.60=moderate agreement; 0.61-0.80=substantial agreement; 0.81-1.0=near-perfect agreement).10 Three advice categories achieved near perfect agreement: nutrition (kappa= 0.94; 95% confidence interval [CI] 0.82-1.0; 99.2% agreement), physical activity (kappa=0.91; 95% CI, 0.84-0.99; 98.6% agreement), and weight loss (kappa=0.95; 95% CI, 0.82-1.0; 99.7% agreement). The nonspecific weight loss advice category had slightly lower agreement but still achieved near-perfect agreement (kappa=0.82; 95% CI, 0.62-1.0; 99.2% agreement).
After all advice was coded, we placed conversations into 1 of 6 categories: (1) no advice given; (2) nonspecific advice only; (3) nutrition only; (4) physical activity only; (5) weight loss only; or (6) combination of nutrition, physical activity, and/or weight loss.
Measures
Dietary fat and fiber intake. We assessed dietary fat intake at baseline and at 3 months using the 22-item Fat- and Fiber-Related Diet Behavior Questionnaire.11,12 Questions about frequency of food selections included, “When you ate dessert, how often did you eat only fruit?” and “When you ate chicken, how often did you take off the skin?” We averaged responses into a total score wherein 1 reflected higher fiber, lower fat food choices; a score of 4 reflected lower fiber, higher fat choices (α=0.74 at baseline and α=0.77 at 3-month follow-up).
Physical activity. We measured physical activity (baseline, 3 months) using the Framingham Physical Activity Index.13 Participants recalled the average number of hours spent engaged in various daily activities (sleeping, working, leisure) and the level of activity for each (sedentary, slight, moderate, or heavy). The composite score accounts for activity duration and intensity.
Anthropometrics. We measured patient weight (baseline, 3 months) and height (baseline only) using a calibrated scale and portable stadiometer. Patients removed shoes, outerwear, and belongings from their pockets before being weighed.
Analysis
We analyzed data using SAS (SAS Institute, Inc., Cary, NC). We assessed the association between type of advice and weight loss, improvement in dietary fat intake behaviors, and increase in physical activity between baseline and the 3-month follow-up visit. We used PROC MIXED to fit general linear models; we incorporated responses into these models from all participants who provided measurements for at least one time point. This modeling framework yields unbiased estimates when missing data are unrelated to the observed variable.14
Primary predictors: (1) type of advice (none, nonspecific, nutrition, physical activity, weight loss, and combination), (2) time since baseline visit, and (3) time by type of advice interaction. All models included a priori defined patient, physician, and visit-related covariates that were theoretically or empirically related to changes in the outcomes (weight, physical activity, or dietary fat in-take). The 14 patient covariates were sex; age; race; high school education; economic security (enough money to pay monthly bills); over-weight (BMI, 25-29.9 kg/m2) or obese (BMI ≥30 kg/m2); actively trying to lose weight (yes/ no); motivated to lose weight (Likert scale 1-7); comfortable discussing weight (Likert scale 1-5); confident about losing weight (Likert scale 1-5); and patient-reported comorbid conditions of diabetes, hypertension, arthritis, and hyperlipidemia.
The 9 physician covariates were sex; race; years since medical school graduation; specialty (family vs internal medicine); self-efficacy (Likert scale 1-5); barriers for weight counseling (Likert scale 1-5); comfort discussing weight (Likert scale 1-5); insurance reimbursement concerns (Likert scale 1-5); and prior training in behavioral counseling (yes/no). Finally, 2 visit-level covariates were included: minutes spent addressing weight issues and visit type (preventive vs chronic).
Results
Sample characteristics
Of the 40 physicians, 19 were family physicians and 21 were internists. More than half of the physicians were female (60%), and 85% were white. Mean age was 47.2 years and mean BMI was 24.9 kg/m2. Of the 461 patients, 66% were female, 65% were white, 35% were African American, and two-thirds had post-high school education (TABLE 1). Mean patient age was 59.8 years; only 4% of the patients were new to their physicians.
TABLE 1
Patient characteristics (N=461)
| % or mean (SD) | |
|---|---|
| Race | |
| White/Asian/Pacific Islander | 65% |
| African American | 35% |
| Female | 66% |
| Age, y (missing=1)* | 59.8 (13.9) |
| BMI, kg/m2 (missing=1)* | 33.1 (7.1) |
| Education (missing=1)* | |
| Post-high school | 67% |
| Income (missing=37)* | |
| $45,000 or less | 48% |
| High financial burden (missing=13)* | |
| Pay bills with trouble | 14% |
| Diagnosed with: | |
| Diabetes | 31% |
| Hypertension (missing=1)* | 69% |
| Hyperlipidemia (missing=1)* | 56% |
| Arthritis | 47% |
| New patient | 4% |
| BMI, body mass index; SD, standard deviation. * Missing data at baseline. | |
Frequency of advice
Physicians gave some type of weight-related advice in 63% of the encounters. They combined types of advice in 34% of all conversations, provided physical activity advice only in 13%, nutrition advice only in 8%, nonspecific advice in 5%, and weight loss advice only in 3%. Many times when physicians gave advice, it was centered on self (eg, “I need you to do X” or “What will it take for me to get you to do Y?”).
Nutrition advice most commonly pertained to specific food items from multiple categories (27% of conversations). Physicians also advised patients to reduce sugar/carbohydrates, control calories and portions, add other micronutrients, eat more fruits/vegetables, and eat meals more frequently.
Walking was the physical activity topic discussed most frequently, followed by exercise duration, exercise for comorbidities, aerobic activities, exercise intensity, and anaerobic exercise. The most common specific weight loss topic was weight loss behavioral advice, followed by weight loss for comorbid conditions. Physicians rarely provided referrals to weight-loss programs.
Effect of type of advice on fat and fiber diet behavior score
Receipt of nutrition advice only was not associated with reduction in fat intake (P=.43, TABLE 2). However, those who received combined types of advice exhibited a significantly greater reduction of fat intake compared with those who received no advice (Fat- and Fiber-Related Diet Behavior Questionnaire score reduction of 0.15 vs 0.05; P=.02).
TABLE 2
How types of physician advice affected dietary fat intake, physical activity, and weight
| Type of advice | ||||||
|---|---|---|---|---|---|---|
| None | Nutrition only | Physical activity only | Weight loss only | Combined advice | Nonspecific | |
| Dietary fat change in Fat- and Fiber-Related Diet Behavior Questionnaire score differences | ||||||
| At 3 months from baseline (95% CI) | -0.05 (-0.11 to 0.004) | -0.10 (-0.22 to 0.01) | -0.07 (-0.16 to 0.02) | -0.08 (-0.26 to 0.09) | -0.15 (-0.20 to -0.09) | 0.03 (0.11 to 0.18) |
| P value* | .43 | .75 | .73 | .02 | .31 | |
| Physical activity score (change in MET hours) | ||||||
| At 3 months from baseline (95% CI) | 0.48 (-0.17 to 1.11) | 0.83 (-0.51 to 2.14) | 0.69 (-0.33 to 1.69) | -0.72 (-2.66 to 1.21) | 0.24 (-0.40 to 0.86) | -0.07 (-1.74 to 1.59) |
| P value* | .64 | .73 | .25 | .60 | .55 | |
| Weight change (kg) | ||||||
| At 3 months from baseline (95% CI) | -0.18 (-0.39 to 0.75) | -0.18 (-1.38 to 1.02) | 1.41 (0.51 to 2.31) | -0.26 (-1.99 to 1.47) | -0.55 (-1.12 to 0.02) | -0.62 (-2.11 to 0.87) |
| P value* | .59 | .02 | .63 | .08 | .32 | |
| CI, confidence interval; MET, metabolic equivalent tasks. *Test of difference between advice given and no advice given. | ||||||
Effect of type of advice on Framingham Physical Activity score
No type of advice, including physical activity advice, led to a change in Framingham Physical Activity scores at the 3-month visit (overall P=.76; TABLE 2).
Effect of type of advice on weight loss
Patients who received physical activity advice gained significantly more weight than patients who received no advice (1.41 kg gained vs 0.18 kg lost; P=.02). Patients who received combined advice lost more weight than patients who received no advice, but the difference did not reach statistical significance (0.55 kg lost vs 0.18 kg lost; P=.08).
Discussion
Physicians typically took an “all or nothing” approach to weight-related issues, giving no advice (37%) or a combination of nutrition, physical activity, and weight loss advice (34%). It seems when physicians do give advice, most of them follow the USPSTF guidelines by addressing nutrition and physical activity together.15
Providing advice alone did not predict a change in patient behavior. For instance, we found no significant association between dietary fat reduction and having received only nutrition advice. Possible explanations include the following:
- Although physicians advised patients to reduce fat/cholesterol intake in 28% of conversations, they did so mostly in combination with other types of advice. Nutrition-only advice occurred in only 8% of conversations. Thus, there may have been insufficient power to detect the impact of this specific type of advice.
- With nutrition-only advice, the most common recommendation was to reduce carbohydrates/sweets, which should not affect fat intake.
Advising patients solely on physical activity led to unintended weight gain overall. Other data have shown that exercise without dietary changes, though beneficial in many ways, is not substantially effective for weight loss.15 People may eat more when they exercise, either to reward themselves or to satiate increased appetite from increased energy expenditure. Or, if physicians recommend the standard goal of 150 minutes of intensive physical activity per week, normally sedentary patients may see that as unattainable and become too discouraged to try.1,16,17
Combining types of advice seemed to help patients reduce their fat intake. Overall, however, simple, brief advice from a physician may not be enough to promote healthy lifestyle changes.
Also notable was that physicians rarely provided referrals, even though this is a strong recommendation from the National Institutes of Health, the American Diabetes Association, and the USPSTF.1,16,17 It could be that many physicians believe referrals are not covered by insurance. Yet, the low frequency of referrals may suggest an important missing component of weight loss therapy, especially given that physician advice alone seems an inadequate intervention.
Avoid physician-centered appeals. Advice was often given in a physician-centered way. There are 3 possible explanations for such phrasing:
- In the absence of clear evidence about how to deliver weight loss advice, physicians may be formulating advice based on their personal or clinical experiences.
- Physicians either assume or sense that patients lack internal motivation to make lifestyle changes for themselves and instead request that patients make changes for the doctor-patient relationship.
- Physicians might be trying to invoke authority in the hope that patients will respond accordingly.
Whatever the reason, the literature on self-centered physician talk indicates that patients are less satisfied when physicians make the visit more about themselves than about patients.18 A better strategy might be to use Motivational Interviewing19 that supports patient autonomy and attempts to elicit and build on internal motivation.
The take-away message is that behavior change is complex and that knowledge is a necessary but insufficient agent for change. Following the tenets of Social Cognitive Theory,20 physicians might also need to address patient motivation, confidence, outcome expectations, and skills to help promote behavior change.
Strengths and limitations of this study
We recorded conversations rather than relying on physician or patient recall. Additionally, these primary care patients were not enrolled in a weight-loss trial and, therefore, were not self-selected to be highly motivated to lose weight. Because of this, and the large and ethnically diverse sample, our results should be generalizable to many clinical settings.
One limitation is that few younger, lower-income patients were included in the sample, which limits generalizability to those populations. Also, the study was observational. Although we adjusted for a broad set of patient, physician, and visit covariates, unmeasured confounding variables may still account for at least part of the observed associations. The analysis is limited by the use of self-reported dietary fat intake and physical activity measures. A food diary and accelerometer would have been more accurate; however, such involved measures could invoke changes in behavior, which would have confounded our ability to assess the effect of physician advice on weight loss.
Acknowledgements
The authors thank all of the physicians and patients who participated in this study, the study project managers Gretchen Yonish and Iguehi Esoimeme, and research assistant Justin Manusov.
CORRESPONDENCE
Stewart C. Alexander, PhD, Department of Medicine, Duke University School of Medicine, P.O. Box 3140 Medical Center, Durham, NC 27710; [email protected]
Background In primary care encounters, it is unknown whether physician advice on weight-related matters leads to patient weight loss. To examine this issue, we analyzed physician weight loss advice and measured corresponding changes in patients’ dietary intake, physical activity, and weight.
Methods Using audio-recorded primary care encounters between 40 physicians and 461 of their overweight or obese patients, we coded weight-related advice as nonspecific, specific nutritional, specific exercise, or specific weight. Physicians and patients were told the study was about preventive health, not weight. We used mixed models (SAS Proc Mixed), controlled for physician clustering and baseline covariates, to assess changes in diet, exercise, and measured weight, both pre-encounter and 3 months post-encounter.
Results When discussing weight, physicians typically provided a combination of specific weight, nutrition, and physical activity advice to their patients (34%). Combined advice resulted in patients reducing their dietary fat intake (P=.02). However, when physicians provided physical activity advice only, patients were significantly (P=.02) more likely to gain weight (+1.41 kg) compared with those who received no advice.
Conclusion When giving weight-related advice, most physicians provided a combination of lifestyle recommendations. Combining advice may help patients reduce their fat in-take. Physical activity advice alone may not be particularly helpful.
The US Preventive Services Task Force (USPSTF) recommends that physicians screen patients for obesity and offer intensive counseling and behavioral interventions to promote sustained weight loss.1 Evidence suggests that physician counseling, including advice, can help patients to lose weight, increase physical activity, and improve diet.2-9 However, little is known about what specific types of weight loss advice physicians give to patients, and whether some types are more effective than others at influencing behavior change.
We analyzed physician weight loss advice delivered in primary care visits and measured changes in patients’ dietary intake, physical activity, and body weight. We examined both the type of weight loss advice delivered and the impact of type of advice on weight and behavior change.
Methods
This study analyzed audio recordings from Project CHAT – Communicating Health: Analyzing Talk. The project was approved by the Duke University Medical Center Institutional Review Board.
Recruitment Physicians. We obtained consent from 40 primary care physicians in community-based practices and told them the study would examine communication around preventive health topics, not weight specifically.
Patients. We identified potential participants by reviewing scheduled appointments 3 weeks in advance. Eligible participants were at least 18 years of age, English-speaking, overweight or obese (body mass index [BMI] ≥25 kg/m2), cognitively competent, and not pregnant. After we obtained consent, a remotely located research assistant started a digital audio recorder as the patient entered the exam room. Immediately after the encounter, the research assistant administered a post-encounter survey to the patient and recorded the patient’s vital signs (N=461). Three months later, the research assistant met with the participant to record vital signs and administer a survey assessing changes in dietary fat intake and exercise (N=426).
Data coding
We coded advice into 4 broad categories: (1) nutrition advice, (2) physical activity advice, (3) specific weight loss advice, and (4) nonspecific weight loss/weight-related advice. We transcribed each piece of advice verbatim.
Nutrition advice consisted of 9 sub-categories: calorie/portion control, meal timing/planning, commercial diet plans, negative diet plans, increase fruits/vegetables, reduce sugar/carbohydrates, reduce fat/cholesterol, other micronutrient recommendations, and specific food items from multiple categories.
Physical activity advice consisted of 6 subcategories: walking, aerobic exercise, anaerobic exercise, exercise intensity, exercise duration, and exercise for comorbid conditions.
Specific weight loss advice consisted of 3 categories: weight loss behavior, weight loss for comorbid conditions, and referrals.
Nonspecific weight loss advice also consisted of 3 subcategories in which physicians provided no details about the general topics of nutrition, physical activity, or weight loss.
Two independent coders (CBT and MEC) assessed each piece of advice and double coded 20% of conversations for reliability. Cohen’s kappa was used to calculate inter-rater reliability for each code using Landis and Koch’s classification (0.21-0.40=fair agreement; 0.41-0.60=moderate agreement; 0.61-0.80=substantial agreement; 0.81-1.0=near-perfect agreement).10 Three advice categories achieved near perfect agreement: nutrition (kappa= 0.94; 95% confidence interval [CI] 0.82-1.0; 99.2% agreement), physical activity (kappa=0.91; 95% CI, 0.84-0.99; 98.6% agreement), and weight loss (kappa=0.95; 95% CI, 0.82-1.0; 99.7% agreement). The nonspecific weight loss advice category had slightly lower agreement but still achieved near-perfect agreement (kappa=0.82; 95% CI, 0.62-1.0; 99.2% agreement).
After all advice was coded, we placed conversations into 1 of 6 categories: (1) no advice given; (2) nonspecific advice only; (3) nutrition only; (4) physical activity only; (5) weight loss only; or (6) combination of nutrition, physical activity, and/or weight loss.
Measures
Dietary fat and fiber intake. We assessed dietary fat intake at baseline and at 3 months using the 22-item Fat- and Fiber-Related Diet Behavior Questionnaire.11,12 Questions about frequency of food selections included, “When you ate dessert, how often did you eat only fruit?” and “When you ate chicken, how often did you take off the skin?” We averaged responses into a total score wherein 1 reflected higher fiber, lower fat food choices; a score of 4 reflected lower fiber, higher fat choices (α=0.74 at baseline and α=0.77 at 3-month follow-up).
Physical activity. We measured physical activity (baseline, 3 months) using the Framingham Physical Activity Index.13 Participants recalled the average number of hours spent engaged in various daily activities (sleeping, working, leisure) and the level of activity for each (sedentary, slight, moderate, or heavy). The composite score accounts for activity duration and intensity.
Anthropometrics. We measured patient weight (baseline, 3 months) and height (baseline only) using a calibrated scale and portable stadiometer. Patients removed shoes, outerwear, and belongings from their pockets before being weighed.
Analysis
We analyzed data using SAS (SAS Institute, Inc., Cary, NC). We assessed the association between type of advice and weight loss, improvement in dietary fat intake behaviors, and increase in physical activity between baseline and the 3-month follow-up visit. We used PROC MIXED to fit general linear models; we incorporated responses into these models from all participants who provided measurements for at least one time point. This modeling framework yields unbiased estimates when missing data are unrelated to the observed variable.14
Primary predictors: (1) type of advice (none, nonspecific, nutrition, physical activity, weight loss, and combination), (2) time since baseline visit, and (3) time by type of advice interaction. All models included a priori defined patient, physician, and visit-related covariates that were theoretically or empirically related to changes in the outcomes (weight, physical activity, or dietary fat in-take). The 14 patient covariates were sex; age; race; high school education; economic security (enough money to pay monthly bills); over-weight (BMI, 25-29.9 kg/m2) or obese (BMI ≥30 kg/m2); actively trying to lose weight (yes/ no); motivated to lose weight (Likert scale 1-7); comfortable discussing weight (Likert scale 1-5); confident about losing weight (Likert scale 1-5); and patient-reported comorbid conditions of diabetes, hypertension, arthritis, and hyperlipidemia.
The 9 physician covariates were sex; race; years since medical school graduation; specialty (family vs internal medicine); self-efficacy (Likert scale 1-5); barriers for weight counseling (Likert scale 1-5); comfort discussing weight (Likert scale 1-5); insurance reimbursement concerns (Likert scale 1-5); and prior training in behavioral counseling (yes/no). Finally, 2 visit-level covariates were included: minutes spent addressing weight issues and visit type (preventive vs chronic).
Results
Sample characteristics
Of the 40 physicians, 19 were family physicians and 21 were internists. More than half of the physicians were female (60%), and 85% were white. Mean age was 47.2 years and mean BMI was 24.9 kg/m2. Of the 461 patients, 66% were female, 65% were white, 35% were African American, and two-thirds had post-high school education (TABLE 1). Mean patient age was 59.8 years; only 4% of the patients were new to their physicians.
TABLE 1
Patient characteristics (N=461)
| % or mean (SD) | |
|---|---|
| Race | |
| White/Asian/Pacific Islander | 65% |
| African American | 35% |
| Female | 66% |
| Age, y (missing=1)* | 59.8 (13.9) |
| BMI, kg/m2 (missing=1)* | 33.1 (7.1) |
| Education (missing=1)* | |
| Post-high school | 67% |
| Income (missing=37)* | |
| $45,000 or less | 48% |
| High financial burden (missing=13)* | |
| Pay bills with trouble | 14% |
| Diagnosed with: | |
| Diabetes | 31% |
| Hypertension (missing=1)* | 69% |
| Hyperlipidemia (missing=1)* | 56% |
| Arthritis | 47% |
| New patient | 4% |
| BMI, body mass index; SD, standard deviation. * Missing data at baseline. | |
Frequency of advice
Physicians gave some type of weight-related advice in 63% of the encounters. They combined types of advice in 34% of all conversations, provided physical activity advice only in 13%, nutrition advice only in 8%, nonspecific advice in 5%, and weight loss advice only in 3%. Many times when physicians gave advice, it was centered on self (eg, “I need you to do X” or “What will it take for me to get you to do Y?”).
Nutrition advice most commonly pertained to specific food items from multiple categories (27% of conversations). Physicians also advised patients to reduce sugar/carbohydrates, control calories and portions, add other micronutrients, eat more fruits/vegetables, and eat meals more frequently.
Walking was the physical activity topic discussed most frequently, followed by exercise duration, exercise for comorbidities, aerobic activities, exercise intensity, and anaerobic exercise. The most common specific weight loss topic was weight loss behavioral advice, followed by weight loss for comorbid conditions. Physicians rarely provided referrals to weight-loss programs.
Effect of type of advice on fat and fiber diet behavior score
Receipt of nutrition advice only was not associated with reduction in fat intake (P=.43, TABLE 2). However, those who received combined types of advice exhibited a significantly greater reduction of fat intake compared with those who received no advice (Fat- and Fiber-Related Diet Behavior Questionnaire score reduction of 0.15 vs 0.05; P=.02).
TABLE 2
How types of physician advice affected dietary fat intake, physical activity, and weight
| Type of advice | ||||||
|---|---|---|---|---|---|---|
| None | Nutrition only | Physical activity only | Weight loss only | Combined advice | Nonspecific | |
| Dietary fat change in Fat- and Fiber-Related Diet Behavior Questionnaire score differences | ||||||
| At 3 months from baseline (95% CI) | -0.05 (-0.11 to 0.004) | -0.10 (-0.22 to 0.01) | -0.07 (-0.16 to 0.02) | -0.08 (-0.26 to 0.09) | -0.15 (-0.20 to -0.09) | 0.03 (0.11 to 0.18) |
| P value* | .43 | .75 | .73 | .02 | .31 | |
| Physical activity score (change in MET hours) | ||||||
| At 3 months from baseline (95% CI) | 0.48 (-0.17 to 1.11) | 0.83 (-0.51 to 2.14) | 0.69 (-0.33 to 1.69) | -0.72 (-2.66 to 1.21) | 0.24 (-0.40 to 0.86) | -0.07 (-1.74 to 1.59) |
| P value* | .64 | .73 | .25 | .60 | .55 | |
| Weight change (kg) | ||||||
| At 3 months from baseline (95% CI) | -0.18 (-0.39 to 0.75) | -0.18 (-1.38 to 1.02) | 1.41 (0.51 to 2.31) | -0.26 (-1.99 to 1.47) | -0.55 (-1.12 to 0.02) | -0.62 (-2.11 to 0.87) |
| P value* | .59 | .02 | .63 | .08 | .32 | |
| CI, confidence interval; MET, metabolic equivalent tasks. *Test of difference between advice given and no advice given. | ||||||
Effect of type of advice on Framingham Physical Activity score
No type of advice, including physical activity advice, led to a change in Framingham Physical Activity scores at the 3-month visit (overall P=.76; TABLE 2).
Effect of type of advice on weight loss
Patients who received physical activity advice gained significantly more weight than patients who received no advice (1.41 kg gained vs 0.18 kg lost; P=.02). Patients who received combined advice lost more weight than patients who received no advice, but the difference did not reach statistical significance (0.55 kg lost vs 0.18 kg lost; P=.08).
Discussion
Physicians typically took an “all or nothing” approach to weight-related issues, giving no advice (37%) or a combination of nutrition, physical activity, and weight loss advice (34%). It seems when physicians do give advice, most of them follow the USPSTF guidelines by addressing nutrition and physical activity together.15
Providing advice alone did not predict a change in patient behavior. For instance, we found no significant association between dietary fat reduction and having received only nutrition advice. Possible explanations include the following:
- Although physicians advised patients to reduce fat/cholesterol intake in 28% of conversations, they did so mostly in combination with other types of advice. Nutrition-only advice occurred in only 8% of conversations. Thus, there may have been insufficient power to detect the impact of this specific type of advice.
- With nutrition-only advice, the most common recommendation was to reduce carbohydrates/sweets, which should not affect fat intake.
Advising patients solely on physical activity led to unintended weight gain overall. Other data have shown that exercise without dietary changes, though beneficial in many ways, is not substantially effective for weight loss.15 People may eat more when they exercise, either to reward themselves or to satiate increased appetite from increased energy expenditure. Or, if physicians recommend the standard goal of 150 minutes of intensive physical activity per week, normally sedentary patients may see that as unattainable and become too discouraged to try.1,16,17
Combining types of advice seemed to help patients reduce their fat intake. Overall, however, simple, brief advice from a physician may not be enough to promote healthy lifestyle changes.
Also notable was that physicians rarely provided referrals, even though this is a strong recommendation from the National Institutes of Health, the American Diabetes Association, and the USPSTF.1,16,17 It could be that many physicians believe referrals are not covered by insurance. Yet, the low frequency of referrals may suggest an important missing component of weight loss therapy, especially given that physician advice alone seems an inadequate intervention.
Avoid physician-centered appeals. Advice was often given in a physician-centered way. There are 3 possible explanations for such phrasing:
- In the absence of clear evidence about how to deliver weight loss advice, physicians may be formulating advice based on their personal or clinical experiences.
- Physicians either assume or sense that patients lack internal motivation to make lifestyle changes for themselves and instead request that patients make changes for the doctor-patient relationship.
- Physicians might be trying to invoke authority in the hope that patients will respond accordingly.
Whatever the reason, the literature on self-centered physician talk indicates that patients are less satisfied when physicians make the visit more about themselves than about patients.18 A better strategy might be to use Motivational Interviewing19 that supports patient autonomy and attempts to elicit and build on internal motivation.
The take-away message is that behavior change is complex and that knowledge is a necessary but insufficient agent for change. Following the tenets of Social Cognitive Theory,20 physicians might also need to address patient motivation, confidence, outcome expectations, and skills to help promote behavior change.
Strengths and limitations of this study
We recorded conversations rather than relying on physician or patient recall. Additionally, these primary care patients were not enrolled in a weight-loss trial and, therefore, were not self-selected to be highly motivated to lose weight. Because of this, and the large and ethnically diverse sample, our results should be generalizable to many clinical settings.
One limitation is that few younger, lower-income patients were included in the sample, which limits generalizability to those populations. Also, the study was observational. Although we adjusted for a broad set of patient, physician, and visit covariates, unmeasured confounding variables may still account for at least part of the observed associations. The analysis is limited by the use of self-reported dietary fat intake and physical activity measures. A food diary and accelerometer would have been more accurate; however, such involved measures could invoke changes in behavior, which would have confounded our ability to assess the effect of physician advice on weight loss.
Acknowledgements
The authors thank all of the physicians and patients who participated in this study, the study project managers Gretchen Yonish and Iguehi Esoimeme, and research assistant Justin Manusov.
CORRESPONDENCE
Stewart C. Alexander, PhD, Department of Medicine, Duke University School of Medicine, P.O. Box 3140 Medical Center, Durham, NC 27710; [email protected]
1. US Preventive Services Task Force. Screening for obesity in adults: recommendations and rationale. Ann Intern Med. 2003;139:930-932.
2. Nawaz H, Adams ML, Katz DL. Physician-patient interactions regarding diet, exercise, and smoking. Prev Med. 2000;31:652-657.
3. Sciamanna CN, Tate DF, Lang W, et al. Who reports receiving advice to lose weight? Results from a multistate survey. Arch Intern Med. 2000;160:2334-2339.
4. Galuska DA, Will JC, Serdula MK, et al. Are health care professionals advising obese patients to lose weight? JAMA. 1999;282:1576-1578.
5. Evans E. Why should obesity be managed? The obese individual’s perspective. Int J Obes Relat Metab Disord. 1999;23(suppl 4):S3-S6.
6. Mehrotra C, Naimi TS, Serdula M, et al. Arthritis, body mass index, and professional advice to lose weight: implications for clinical medicine and public health. Am J Prev Med. 2004;27:16-21.
7. Loureiro ML, Nayga RM Jr. Obesity, weight loss, and physician’s advice. Soc Sci Med. 2006;62:2458-2468.
8. Flocke SA, Clark A, Schlessman K, et al. Exercise, diet, and weight loss advice in the family medicine outpatient setting. Fam Med. 2005;37:415-421.
9. Alexander SC, Cox ME, Østbye T, et al. Do the 5 A’s work for weight-loss counseling? Presented at: International Conference on Communication in Healthcare; October 4–7, 2009; Miami, Fla.
10. McGinn T, Wyer PC, Newman TB, et al. Tips for learners of evidence-based medicine: 3. Measures of observer variability (kappa statistic). CMAJ. 2004;171:1369-1373.
11. Shannon J, Kristal AR, Curry SJ, et al. Application of a behavioral approach to measuring dietary change: the fat- and fiber-related diet behavior questionnaire. Cancer Epidemiol Biomarkers Prev. 1997;6:355-361.
12. Kristal AR, Shattuck AL, Henry HJ. Patterns of dietary behavior associated with selecting diets low in fat: reliability and validity of a behavioral approach to dietary assessment. J Am Diet Assoc. 1990;90:214-220.
13. Kannel WB, Sorlie P. Some health benefits of physical activity: the Framingham study. Arch Intern Med. 1979;139:857-861.
14. Little RJA, Rubin DB. Statistical Analysis With Missing Data. New York, NY: John Wiley & Sons; 2002.
15. Franz MJ, VanWormer JJ, Crain AL, et al. Weight-loss outcomes: a systematic review and meta-analysis of weight-loss clinical trials with a minimum 1-year follow-up. J Am Diet Assoc. 2007;107:1755-1767.
16. National Institutes of Health. The practical guide: identification, evaluation, and treatment of overweight and obesity in adults. NIH publication 00-4084. 2000. Available at: http://www.nhlbi.nih.gov/guidelines/obesity/prctgd_c.pdf. Accessed August 17, 2009.
17. American Diabetes Association. Standards of medical care in diabetes–2010. Diabetes Care. 2010;33(suppl 1):S11-S61.
18. Beach MC, Roter DL. Interpersonal expectations in the patient-physician relationship. J Gen Intern Med. 2000;15:825-827.
19. Miller WR, Rollnick SP, Miller WR. Motivational Interviewing: Preparing People for Change. 2nd ed. New York, NY: Guilford Press; 2002.
20. Bandura A. Social Foundations of Thought and Action: A Social Cognitive Theory. Englewood Cliffs, NJ: Prentice-Hall; 1986.
1. US Preventive Services Task Force. Screening for obesity in adults: recommendations and rationale. Ann Intern Med. 2003;139:930-932.
2. Nawaz H, Adams ML, Katz DL. Physician-patient interactions regarding diet, exercise, and smoking. Prev Med. 2000;31:652-657.
3. Sciamanna CN, Tate DF, Lang W, et al. Who reports receiving advice to lose weight? Results from a multistate survey. Arch Intern Med. 2000;160:2334-2339.
4. Galuska DA, Will JC, Serdula MK, et al. Are health care professionals advising obese patients to lose weight? JAMA. 1999;282:1576-1578.
5. Evans E. Why should obesity be managed? The obese individual’s perspective. Int J Obes Relat Metab Disord. 1999;23(suppl 4):S3-S6.
6. Mehrotra C, Naimi TS, Serdula M, et al. Arthritis, body mass index, and professional advice to lose weight: implications for clinical medicine and public health. Am J Prev Med. 2004;27:16-21.
7. Loureiro ML, Nayga RM Jr. Obesity, weight loss, and physician’s advice. Soc Sci Med. 2006;62:2458-2468.
8. Flocke SA, Clark A, Schlessman K, et al. Exercise, diet, and weight loss advice in the family medicine outpatient setting. Fam Med. 2005;37:415-421.
9. Alexander SC, Cox ME, Østbye T, et al. Do the 5 A’s work for weight-loss counseling? Presented at: International Conference on Communication in Healthcare; October 4–7, 2009; Miami, Fla.
10. McGinn T, Wyer PC, Newman TB, et al. Tips for learners of evidence-based medicine: 3. Measures of observer variability (kappa statistic). CMAJ. 2004;171:1369-1373.
11. Shannon J, Kristal AR, Curry SJ, et al. Application of a behavioral approach to measuring dietary change: the fat- and fiber-related diet behavior questionnaire. Cancer Epidemiol Biomarkers Prev. 1997;6:355-361.
12. Kristal AR, Shattuck AL, Henry HJ. Patterns of dietary behavior associated with selecting diets low in fat: reliability and validity of a behavioral approach to dietary assessment. J Am Diet Assoc. 1990;90:214-220.
13. Kannel WB, Sorlie P. Some health benefits of physical activity: the Framingham study. Arch Intern Med. 1979;139:857-861.
14. Little RJA, Rubin DB. Statistical Analysis With Missing Data. New York, NY: John Wiley & Sons; 2002.
15. Franz MJ, VanWormer JJ, Crain AL, et al. Weight-loss outcomes: a systematic review and meta-analysis of weight-loss clinical trials with a minimum 1-year follow-up. J Am Diet Assoc. 2007;107:1755-1767.
16. National Institutes of Health. The practical guide: identification, evaluation, and treatment of overweight and obesity in adults. NIH publication 00-4084. 2000. Available at: http://www.nhlbi.nih.gov/guidelines/obesity/prctgd_c.pdf. Accessed August 17, 2009.
17. American Diabetes Association. Standards of medical care in diabetes–2010. Diabetes Care. 2010;33(suppl 1):S11-S61.
18. Beach MC, Roter DL. Interpersonal expectations in the patient-physician relationship. J Gen Intern Med. 2000;15:825-827.
19. Miller WR, Rollnick SP, Miller WR. Motivational Interviewing: Preparing People for Change. 2nd ed. New York, NY: Guilford Press; 2002.
20. Bandura A. Social Foundations of Thought and Action: A Social Cognitive Theory. Englewood Cliffs, NJ: Prentice-Hall; 1986.
Opioids for osteoarthritis? Weighing benefits and risks: A Cochrane Musculoskeletal Group review
Osteoarthritis (OA) affects nearly 27 million Americans, or about 12% of US adults.1 As the average age of the population increases, the prevalence and burden of this debilitating disorder continue to rise.2
The American College of Rheumatology (ACR)’s guidelines for the medical management of OA of the hip and knee, last updated in 2000,3 focus on controlling pain and improving function and health-related quality of life while minimizing the toxic effects of therapy. The guidelines recommend tramadol—an atypical opioid with 2 distinct mechanisms of action4—for moderate-to-severe pain in OA patients who either have contraindications to COX-2 inhibitors and non steroidal anti-inflammatory drugs (NSAIDs) or have failed to respond to previous oral therapy. Patients with severe pain who don’t respond to or are unable to tolerate tramadol may be candidates for more traditional opioid therapy, the guidelines indicate.3
In recent years, however, the use (and abuse) of opioids has skyrocketed. Between 1997 and 2007, US per capita retail purchases of hydrocodone and oxycodone increased 4-fold and 9-fold, respectively.5 In a similar time frame (1996-2006), the number of deaths from opioid overdose more than tripled, going from 4000 to 13,800 annually.6 Not surprisingly, the use of narcotics for noncancer pain remains controversial.7,8 But inadequately treated pain continues to be a serious public health problem, as well.9
This is the third in a series of articles based on the findings of the Cochrane Musculoskeletal Group (CMSG). One of the largest groups in the Cochrane Collaboration, the CMSG synthesizes the results of clinical trials to determine whether interventions for the prevention, treatment, and rehabilitation of musculoskeletal disorders are safe and effective. In this installment, the reviewers use detailed analysis, as well as a case study, to bring their findings to the attention of family physicians in a practical, clinically relevant context.
In 2006 and 2009, respectively, the Cochrane Collaboration published systematic reviews of tramadol (for OA in any joint)10 and other oral and transdermal opioids (for OA of the hip or knee).11 The reviewers’ findings, presented here along with data from more recent trials, can help ensure that you prescribe opioids for patients with OA only when their use is clinically appropriate and evidence-based. We’ve also included a case study (see page 211), so you can assess your knowledge and clinical skills.
CASE Carol J, an active 72-year-old, was diagnosed with OA in her right hip 5 years ago. Now she reports that the pain is getting progressively worse, making it harder and harder to turn over in bed at night or get in and out of the car. The pain is particularly bad at night, Carol says, and she’s had interrupted sleep for months. The patient has taken acetaminophen for the pain since her OA diagnosis, but now finds the analgesic is ineffective, even at the maximum dose of 4 g per day.
Carol has hypertension, which was difficult to manage until she began taking a combination ACE inhibitor/diuretic. She also has moderate renal impairment and mild chronic obstructive pulmonary disease, which limits her exercise tolerance. Nonetheless, she continues to smoke. The patient lives with and cares for her husband, who has Alzheimer’s disease, and worries about her ability to continue to care for him.
What are her treatment options?
Full-dose acetaminophen is no longer helping Carol, and NSAIDs are contraindicated because she takes an ACE inhibitor/diuretic and has moderate renal impairment. Increasing exercise will be a challenge. You strongly encourage her to stop smoking, emphasizing that this is particularly important to reduce the risk involved with any future joint replacement surgery.
Oral dosing options for the patient include:
- prescribing tramadol, starting with a low-dose immediate-release formulation taken one hour before bedtime (The controlled-release formulation is not advisable, given her age and renal function.) or
- adding a traditional opioid, eg, codeine 30 to 60 mg every 6 hours as needed, to her regular acetaminophen regimen.
Codeine and hydrocodone are available in combination preparations with acetaminophen, which may be convenient for some patients. However, hydrocodone was not one of the opioids tested in the trials included in the Cochrane reviews, and evidence of its use in OA is lacking.
Intra-articular corticosteroid injection, performed under imaging guidance, is another option for Carol. You explain that although there have been no studies of intra-articular corticosteroid injections for OA of the hip, these are used occasionally and may provide short-term symptom relief.7
You emphasize that surgery is likely to give her the best long-term outcome. In view of the patient’s circumstances and the need to care for her husband, however, you prescribe tramadol 50 mg at night. (Because of Carol’s age, renal impairment, and the possible adverse effects, it’s wise to start with a low dose and titrate upwards.) You warn her of the risks associated with opioids and advise her to alert your office staff if she experiences any adverse effects.
Before the patient leaves, you arrange an orthopedic consult and schedule a return visit for the following week. At your urging, she agrees to look into respite options for her husband.
Tramadol produces modest results—or none at all
The tramadol review10 included 11 randomized controlled trials (RCTs) with a total of 1019 participants who took tramadol or tramadol/acetaminophen (paracetamol) and 920 controls. In 6 of the 11 studies, the controls received placebo; the remaining 5 trials used “active controls,” with the control group for each RCT receiving a different analgesic. (To learn more about the methodology, see “How the reviews were conducted”.)
Placebo-controlled trials. Compared with patients on placebo, those receiving tramadol had an average absolute reduction in pain of 8.5 mm on a 0-100 mm visual analog scale (VAS) (95% confidence interval [CI], -12.05 to -4.9). That small benefit, however, did not reach the level defined as the minimal perceptible clinical improvement—a reduction of 9.7 mm on Western Ontario and McMaster Universities (WOMAC)’s OA pain subscale.12
Active-controlled trials. In the 5 RCTs comparing tramadol with another active agent, tramadol proved to be no better than the control drug. In fact, in a study of tramadol vs acetaminophen, 500 mg acetaminophen 3 times a day provided more pain relief than 50 mg tramadol 3 times a day.13 Although this was a small (N=20), short-term (7-day) study, this finding is notable because participants took less than the usual acetaminophen dose of 1 g up to 4 times a day.
Nor was tramadol superior to the agents it was compared with in the 4 other active-controlled trials—dihydrocodeine,14 dextropropoxyphene,15 pentazocine,16 and diclofenac17—in reducing pain intensity. It is important to keep in mind, however, that in each of these studies, both the quantity and quality of the evidence was limited. (Two studies did not use numerical scales,14,16 for example; all had methodological issues; and none lasted longer than 28 days.)
The Cochrane Musculoskeletal Group conducted a review of tramadol and a review of other oral opioids and transdermal fentanyl for the treatment of osteoarthritis (OA). Both reviews featured pain, function, and safety as primary outcomes. The tramadol review included randomized controlled trials (RCTs) for OA in any joint, while the oral and transdermal opioid review included randomized and quasi-randomized trials of treatment for OA of the hip or knee. Other parameters follow:
The tramadol review included 11 RCTs, with a total of 1019 participants receiving either tramadol alone or tramadol/acetaminophen (paracetamol) and 920 controls. In 6 of the 11 studies, the controls received placebo; the remaining 5 studies featured “active control.” That is, the control groups received acetaminophen 500 mg 3 times daily, diclofenac (25-50 mg up to 3 times daily on demand), dihydrocodeine 60 mg twice daily, dextropropoxyphene 100 mg 3 times daily, or pentazocine 50 mg 4 times per day. Because each of these agents was used in only one trial, the reviewers could not reach definitive conclusions about tramadol’s performance relative to other medications. The average number of participants in the tramadol and control groups was 91 and 80, respectively. The average length of follow-up was 35 days.
The 11 RCTs included in this review used a variety of pain scales to assess the results of tramadol, active control medications, and placebo. For comparative purposes, the reviewers pooled the results from studies that used numerical scales (0 to 100 and 0 to 10) to assess pain intensity. As a reference, we have used 9.7 and 9.3, respectively, determined by other researchers to be the minimal perceptible clinical improvements on the Western Ontario and McMaster Universities (WOMAC) pain and physical function 0-100 mm visual analog scales.12
The review of oral and transdermal opioids included 10 studies, with a total of 1541 patients receiving opioids and 727 receiving placebo.17 There were 3 trials of codeine (in 2 of the 3, a simple analgesic [acetaminophen 3000 mg/d or ibuprofen 1200 mg/d] was co-administered to both the treatment and control groups); other opioids included in the trials were oxycodone (4 trials), oxymorphone (2 trials), morphine (1 trial), and transdermal fentanyl (1 trial).
A modest boost in well-being
The reviewers measured function in 2 ways, focusing on both global improvement and improvement in physical function.
Global assessment. For the global assessment, the reviewers defined a treatment response as achieving at least a moderate improvement. By that standard, tramadol may improve overall well-being more than placebo. In the placebo-controlled trials, the number needed to treat (NNT) to elicit one treatment response was 6.
Three of the trials with active controls included global/functional assessments, and the results—bearing in mind the reduced quality and quantity of the evidence—were mixed. In a comparison of tramadol with dextropropoxyphene, tramadol increased the likelihood of moderate improvement by 38% (relative risk, 1.38 (95% CI, 1.15-1.67).10 In a trial of tramadol vs pentazocine, tramadol was more effective in reducing the duration of morning stiffness (by about 10 minutes), but not its severity. Tramadol was comparable with pentazocine in the 7 other measures of OA and function.16 In the tramadol-diclofenac study, both drugs were equally effective.17
Physical function. Four of the 6 placebo-controlled tramadol studies included in the Cochrane review used the WOMAC Index score, which included the physical function subscale. The tramadol group had a larger reduction in the score than the placebo group, by 0.34 mm (95% CI, -0.49 to -0.19). While this was equivalent to an 8.5% relative reduction in mean baseline score, it is still small compared with the minimal perceptible clinical improvement level of 9.3 mm on a 0-100 scale needed for the WOMAC physical function subscale. A similar improvement was reported for those taking tramadol compared with diclofenac—the only one of the active-controlled studies to report on physical function.17
Other opioids relieve pain, improve function—but how much?
The review of oral and transdermal opioids for OA11 encompassed 10 trials, with a total of 1541 patients receiving opioids and 727 on placebo. The opioids used in the trials were codeine, oxycodone, oxymorphone, morphine, and transdermal fentanyl. (For more details, see “How the reviews were conducted”.)
Pain. The trials included in the review used a variety of scales to measure pain, so the reviewers gauged results by the proportion of patients responding to treatment. Response was defined as a 50% improvement in pain score.
In the overall analysis, 35% of patients taking opioids responded to treatment, vs 31% of those on placebo—or 4 more patients in 100. That represents an NNT of 25. (A subgroup analysis did not demonstrate any significant differences in effect size among the opioids tested. In addition, the effect size was similar regardless of the potency of the opioid or the administration route.)
Function. Seven of the 10 trials (1794 participants, including both the treatment groups and controls) used validated function scores to measure physical function after 4 weeks of treatment. Here, too, the reviewers defined a treatment response as a 50% improvement in score.
Their finding? Opioids had a greater effect on function compared with placebo, equaling 0.7 on a WOMAC disability scale of 1 to 10. This means that about 3 more patients in 100 responded to treatment with opioids vs placebo—an NNT of 30.
But what about safety?
Opioids, including tramadol, are associated with adverse events (AEs), which may be minor or major. To determine when, or whether, the benefits outweigh the risks for treating patients with OA, both reviews reported on AEs and the number of participants who stopped taking the drug because of AEs.
AEs limit tramadol’s usefulness
While tramadol was more effective than placebo at reducing pain intensity, relieving symptoms, and improving function, the benefits were small—with an overall NNT of 6 (TABLE 1). This is similar to acetaminophen (NNT, 4-16),18 but with a greater downside.
Minor AEs. Four placebo-controlled trials reported on minor AEs.19-22 Those most commonly reported by patients taking tramadol were nausea, vomiting, dizziness, constipation, somnolence, tiredness, and headache.
Overall, 39% of those who received tramadol experienced minor AEs, compared with 18% of patients receiving placebo—an NNH of 5.10 Thus, tramadol’s NNH for minor AEs is equivalent to its NNT for pain relief. In active-controlled studies, there was a higher risk of minor AEs in those receiving tramadol compared with diclofenac or dextropropoxyphene, but a lower risk compared with those taking pentazocine.10
Major AEs. An analysis of the placebo-controlled trials revealed that 21% of those who received tramadol had major AEs—defined as an event that resulted in cessation of treatment—compared with 8% of those taking placebo. By this measure, the NNH was 8: One in 8 patients stopped taking tramadol because of a major AE.10
Among the active-controlled trials, participants taking tramadol were more likely to report a major AE compared with those receiving either diclofenac or dextropropoxyphene (NNH=5), but less likely compared with patients taking pentazocine. In a trial that compared tramadol alone with paracetamol, 2 out of 10 in the tramadol group discontinued treatment; none in the paracetamol group did.13
TABLE 1
Tramadol and other opioids for OA pain: NNT and NNH
| Treatment | NNT | NNH |
|---|---|---|
| Tramadol10 | 6 | 5 |
| Opioids (overall)11 | 25 | 12 |
| NNH, number needed to harm; NNT, number needed to treat; OA, osteoarthritis. | ||
Post-review RCTs provide further evidence
We identified 4 double-blind RCTs of tramadol for the treatment of OA that were of at least 6 weeks’ duration,19-22 published after the 2006 review. The results of these studies (TABLE 2) were broadly consistent with those of the systematic review. Two of the 4 studies had active controls, with one comparing tramadol with diclofenac19 and the other with celecoxib.21 Tramadol and diclofenac were found to be equally effective; celecoxib appeared to be superior in terms of pain relief, global improvement, and physical function, but no statistical comparisons were reported.
TABLE 2
Tramadol for OA: Post-review RCTs are consistent with meta-analysis
| Study duration (N) | Intervention groups | Primary outcome measures | Improvement in | Adverse effects | ||
|---|---|---|---|---|---|---|
| Pain | Global assessment | Function | ||||
| Gana*20 12 wk (1020) | Tramadol ER Placebo | WOMAC OA index (pain and physical function subscales) 100-mm VAS: Subject global disease | Treatment groups, 35% Placebo, 25% | Treatment groups, 32%-36% Placebo, 24% | Treatment groups, 31%-33% Placebo, 22% | ≥1 AE Withdrawals due to AEs |
| Delemos*21 12 wk (1001) | Tramadol ER Placebo | WOMAC OA index (pain and physical function subscales) 100-mm VAS: Subject global disease | Tramadol, 27%-39% Celecoxib, 45% Placebo, 32% | Tramadol, 28%-40% Celecoxib, 44% Placebo, 30% | Tramadol, 26%-35% Celecoxib, 43% Placebo, 28% | ≥1 AE Withdrawals due to AEs |
| Burch†22 12 wk (646) | Tramadol (Contramid OAD) 100 mg titrating to 300 mg Placebo | Pain intensity (11-point numerical scale) Physician/patient global impressions of change (7-point scale) | Treatment group, 40% Placebo, 33% | Treatment group, 80% Placebo, 69% | NA | AEs Placebo: Nausea, 5.6%; constipation, 4.2%; dizziness/vertigo, 3.7%; somnolence, 3.7% Withdrawals due to AEs |
| Beaulieu*19 6 wk (128) | Tramadol CR 200 mg titrating to 400 mg Diclofenac SR 75 mg titrating to 150 mg | WOMAC OA index (pain and physical function subscales) 100-mm VAS: Pain intensity Subject global disease Physician/patient global impressions of change (7-point scale) | Both groups, ~29% | Tramadol, 67%‡ Diclofenac, 54%‡ | Tramadol, 29%‡ Diclofenac, 29%‡ | Withdrawals due to AEs Tramadol, 16% Diclofenac, 15% |
| *Hip or knee osteoarthritis. †Knee osteoarthritis. ‡Not statistically significant. AEs, adverse events; CR, controlled release; ER, extended release; NA, not assessed; OA, osteoarthritis; OAD, once a day; RCTs, randomized controlled trials; SR, sustained release; VAS, visual analog scale; WOMAC, Western Ontario and McMaster Universities. | ||||||
Oral and transdermal opioids: Pain relief but high risk
Among the patients with OA of the hip or knee—the study population for the review of oral and transdermal opioids—all the opioids tested were more effective than placebo. The benefits, however, were small to moderate, and were off set by large increases in the risk of AEs and a high dropout rate.
Four of the 10 trials reported the number of patients experiencing any AE: 23% of those taking opioids vs 15% of patients on placebo.11 This represents an NNH of 12 (TABLE 1). All 10 trials reported the number of patients who withdrew due to AEs. Those receiving opioids were 4 times as likely to withdraw due to AEs, compared with those taking placebo. The NNH to cause one additional withdrawal was 19 (95% CI, 13-29).
Bottom line
The data highlight both the limited role of opioids (including tramadol) in OA treatment and—when they are being considered for this patient population—the importance of making patients aware that the risks may outweigh the benefits. Used judiciously and with adequate patient counseling, tramadol may be an option when COX-2-specific inhibitors and NSAIDs fail or cannot be tolerated. Although the small-to-moderate benefits of non-tramadol opioids are generally outweighed by large increases in the risk of AEs, their use may be considered for severe OA pain if tramadol is ineffective or causes intolerable AEs.
CORRESPONDENCE
Faline Howes, BMedSci, MBBS, MPH, FRACGP, Menzies Research Institute Tasmania, Private Bag 23, University of Tasmania, Hobart, Tasmania, Australia 7001; Faline.Howes@ utas.edu.au
1. Lawrence RC, Felson DT, Helmick CG, et al. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. Part II. Arthritis Rheum. 2008;58:26-35.
2. Bitton R. The economic burden of osteoarthritis. Am J Manag Care. 2009;15(suppl):S230-S235.
3. Altman RD, Hochberg MC, Moskowitz RW, et al. Recommendations for the medical management of osteoarthritis of the hip and knee. Arthritis Rheum. 2000;43:1905-1915.
4. Gibson TP. Pharmacokinetics, efficacy, and safety of analgesia with a focus on tramadol HCl. Am J Med. 1996;101(suppl 1A):47S-53S.
5. Hall AJ, Logan JE, Toblin RL, et al. Patterns of abuse among unintentional pharmaceutical overdose fatalities. JAMA. 2008;300:2613-2620.
6. Warner M, Chen LH, Makuc DM. Increase in fatal poisonings involving opioid analgesics in the United States, 1999-2006. NCHS data brief, no 22. Hyattsville, MD: National Center for Health Statistics; 2009.
7. Von Korff M, Deyo RA. Potent opioids for chronic musculoskeletal pain: flying blind? Pain. 2004;109:207-209.
8. Zhang W, Moskowitz RW, Nuki G, et al. OARSI recommendations for the management of hip and knee osteoarthritis, Part II: OARSI evidence-based, expert consensus guidelines. Osteoarthritis Cartilage. 2008;16:137-167.
9. Pletcher MJ, Kertesz SG, Kohn MA, et al. Trends in opioid prescribing by race/ethnicity for patients seeking care in US emergency departments. JAMA. 2008;299:70-78.
10. Cepeda MS, Camargo F, Zea C, et al. Tramadol for osteoarthritis. Cochrane Database Syst Rev. 2006;(3):CD005522.-
11. Nuesch E, Rutjes AW, Husni E, et al. Oral or transdermal opioids for osteoarthritis of the knee or hip. Cochrane Database Syst Rev. 2009;(4):CD003115.-
12. Ehrich EW, Davies GM, Watson DJ, et al. Minimal perceptible clinical improvement with the Western Ontario and McMaster Universities osteoarthritis index questionnaire and global assessments in patients with osteoarthritis. J Rheumatol. 2000;27:2635-2641.
13. Bianchi M, Broggini M, Balzarini P, et al. Effects of tramadol on synovial fluid concentrations of substance P and interleukin-6 in patients with knee osteoarthritis: comparison with paracetamol. Int Immunopharm. 2003;3:1901-1908.
14. Wilder-Smith C, Hill L, Spargo K, et al. Treatment of severe pain from osteoarthritis with slow-release tramadol or dihydrocodeine in combination with NSAIDs: a randomised study comparing analgesia, antinociception and gastrointestinal effects. Pain. 2001;91:23-31.
15. Jensen E, Ginsberg F. Tramadol versus dextropropoxyphene in the treatment of osteoarthritis: a short-term double-blind study. Drug Invest. 1994;8:211-218.
16. Bird H, Hill J, Stratford M, et al. A double-blind cross-over study comparing the analgesic efficacy of tramadol with pentazocine in patients with osteoarthritis. J Drug Dev Clin Pract. 1995;7:181-188.
17. Pavelka K, Peliskova Z, Stehlikova H, et al. Intraindividual differences in pain relief and functional improvement in osteoarthritis with diclofenac or tramadol. Clin Drug Invest. 1998;16:421-429.
18. Townheed TE, Maxwell L, Judd MG, et al. Acetaminophen for osteoarthritis. Cochrane Database Syst Rev. 2006;(1):CD004257.-
19. Beaulieu AD, Peloso PM, Haraoui B, et al. Once-daily, controlled-release tramadol and sustained-release diclofenac relieve chronic pain due to osteoarthritis: a randomized controlled trial. Pain Res Manag. 2008;13:103-110.
20. Gana TJ, Pascual ML, Fleming RR, et al. Extended-release tramadol in the treatment of osteoarthritis: a multicenter, randomized, double-blind, placebo-controlled clinical trial. Curr Med Res Opin. 2006;22:1391-1401.
21. Delemos BP, Xiang J, Benson C, et al. Tramadol hydrochloride extended-release once-daily in the treatment of osteoarthritis of the knee and/or hip: a double-blind, randomized, dose-ranging trial. Am J Ther. 2010 Mar 3 [Epub ahead of print].
22. Burch F, Fishman R, Messina N, et al. A comparison of the analgesic efficacy of Tramadol Contramid OAD versus placebo in patients with pain due to osteoarthritis. J Pain Symptom Manage. 2007;34:328-338.
Osteoarthritis (OA) affects nearly 27 million Americans, or about 12% of US adults.1 As the average age of the population increases, the prevalence and burden of this debilitating disorder continue to rise.2
The American College of Rheumatology (ACR)’s guidelines for the medical management of OA of the hip and knee, last updated in 2000,3 focus on controlling pain and improving function and health-related quality of life while minimizing the toxic effects of therapy. The guidelines recommend tramadol—an atypical opioid with 2 distinct mechanisms of action4—for moderate-to-severe pain in OA patients who either have contraindications to COX-2 inhibitors and non steroidal anti-inflammatory drugs (NSAIDs) or have failed to respond to previous oral therapy. Patients with severe pain who don’t respond to or are unable to tolerate tramadol may be candidates for more traditional opioid therapy, the guidelines indicate.3
In recent years, however, the use (and abuse) of opioids has skyrocketed. Between 1997 and 2007, US per capita retail purchases of hydrocodone and oxycodone increased 4-fold and 9-fold, respectively.5 In a similar time frame (1996-2006), the number of deaths from opioid overdose more than tripled, going from 4000 to 13,800 annually.6 Not surprisingly, the use of narcotics for noncancer pain remains controversial.7,8 But inadequately treated pain continues to be a serious public health problem, as well.9
This is the third in a series of articles based on the findings of the Cochrane Musculoskeletal Group (CMSG). One of the largest groups in the Cochrane Collaboration, the CMSG synthesizes the results of clinical trials to determine whether interventions for the prevention, treatment, and rehabilitation of musculoskeletal disorders are safe and effective. In this installment, the reviewers use detailed analysis, as well as a case study, to bring their findings to the attention of family physicians in a practical, clinically relevant context.
In 2006 and 2009, respectively, the Cochrane Collaboration published systematic reviews of tramadol (for OA in any joint)10 and other oral and transdermal opioids (for OA of the hip or knee).11 The reviewers’ findings, presented here along with data from more recent trials, can help ensure that you prescribe opioids for patients with OA only when their use is clinically appropriate and evidence-based. We’ve also included a case study (see page 211), so you can assess your knowledge and clinical skills.
CASE Carol J, an active 72-year-old, was diagnosed with OA in her right hip 5 years ago. Now she reports that the pain is getting progressively worse, making it harder and harder to turn over in bed at night or get in and out of the car. The pain is particularly bad at night, Carol says, and she’s had interrupted sleep for months. The patient has taken acetaminophen for the pain since her OA diagnosis, but now finds the analgesic is ineffective, even at the maximum dose of 4 g per day.
Carol has hypertension, which was difficult to manage until she began taking a combination ACE inhibitor/diuretic. She also has moderate renal impairment and mild chronic obstructive pulmonary disease, which limits her exercise tolerance. Nonetheless, she continues to smoke. The patient lives with and cares for her husband, who has Alzheimer’s disease, and worries about her ability to continue to care for him.
What are her treatment options?
Full-dose acetaminophen is no longer helping Carol, and NSAIDs are contraindicated because she takes an ACE inhibitor/diuretic and has moderate renal impairment. Increasing exercise will be a challenge. You strongly encourage her to stop smoking, emphasizing that this is particularly important to reduce the risk involved with any future joint replacement surgery.
Oral dosing options for the patient include:
- prescribing tramadol, starting with a low-dose immediate-release formulation taken one hour before bedtime (The controlled-release formulation is not advisable, given her age and renal function.) or
- adding a traditional opioid, eg, codeine 30 to 60 mg every 6 hours as needed, to her regular acetaminophen regimen.
Codeine and hydrocodone are available in combination preparations with acetaminophen, which may be convenient for some patients. However, hydrocodone was not one of the opioids tested in the trials included in the Cochrane reviews, and evidence of its use in OA is lacking.
Intra-articular corticosteroid injection, performed under imaging guidance, is another option for Carol. You explain that although there have been no studies of intra-articular corticosteroid injections for OA of the hip, these are used occasionally and may provide short-term symptom relief.7
You emphasize that surgery is likely to give her the best long-term outcome. In view of the patient’s circumstances and the need to care for her husband, however, you prescribe tramadol 50 mg at night. (Because of Carol’s age, renal impairment, and the possible adverse effects, it’s wise to start with a low dose and titrate upwards.) You warn her of the risks associated with opioids and advise her to alert your office staff if she experiences any adverse effects.
Before the patient leaves, you arrange an orthopedic consult and schedule a return visit for the following week. At your urging, she agrees to look into respite options for her husband.
Tramadol produces modest results—or none at all
The tramadol review10 included 11 randomized controlled trials (RCTs) with a total of 1019 participants who took tramadol or tramadol/acetaminophen (paracetamol) and 920 controls. In 6 of the 11 studies, the controls received placebo; the remaining 5 trials used “active controls,” with the control group for each RCT receiving a different analgesic. (To learn more about the methodology, see “How the reviews were conducted”.)
Placebo-controlled trials. Compared with patients on placebo, those receiving tramadol had an average absolute reduction in pain of 8.5 mm on a 0-100 mm visual analog scale (VAS) (95% confidence interval [CI], -12.05 to -4.9). That small benefit, however, did not reach the level defined as the minimal perceptible clinical improvement—a reduction of 9.7 mm on Western Ontario and McMaster Universities (WOMAC)’s OA pain subscale.12
Active-controlled trials. In the 5 RCTs comparing tramadol with another active agent, tramadol proved to be no better than the control drug. In fact, in a study of tramadol vs acetaminophen, 500 mg acetaminophen 3 times a day provided more pain relief than 50 mg tramadol 3 times a day.13 Although this was a small (N=20), short-term (7-day) study, this finding is notable because participants took less than the usual acetaminophen dose of 1 g up to 4 times a day.
Nor was tramadol superior to the agents it was compared with in the 4 other active-controlled trials—dihydrocodeine,14 dextropropoxyphene,15 pentazocine,16 and diclofenac17—in reducing pain intensity. It is important to keep in mind, however, that in each of these studies, both the quantity and quality of the evidence was limited. (Two studies did not use numerical scales,14,16 for example; all had methodological issues; and none lasted longer than 28 days.)
The Cochrane Musculoskeletal Group conducted a review of tramadol and a review of other oral opioids and transdermal fentanyl for the treatment of osteoarthritis (OA). Both reviews featured pain, function, and safety as primary outcomes. The tramadol review included randomized controlled trials (RCTs) for OA in any joint, while the oral and transdermal opioid review included randomized and quasi-randomized trials of treatment for OA of the hip or knee. Other parameters follow:
The tramadol review included 11 RCTs, with a total of 1019 participants receiving either tramadol alone or tramadol/acetaminophen (paracetamol) and 920 controls. In 6 of the 11 studies, the controls received placebo; the remaining 5 studies featured “active control.” That is, the control groups received acetaminophen 500 mg 3 times daily, diclofenac (25-50 mg up to 3 times daily on demand), dihydrocodeine 60 mg twice daily, dextropropoxyphene 100 mg 3 times daily, or pentazocine 50 mg 4 times per day. Because each of these agents was used in only one trial, the reviewers could not reach definitive conclusions about tramadol’s performance relative to other medications. The average number of participants in the tramadol and control groups was 91 and 80, respectively. The average length of follow-up was 35 days.
The 11 RCTs included in this review used a variety of pain scales to assess the results of tramadol, active control medications, and placebo. For comparative purposes, the reviewers pooled the results from studies that used numerical scales (0 to 100 and 0 to 10) to assess pain intensity. As a reference, we have used 9.7 and 9.3, respectively, determined by other researchers to be the minimal perceptible clinical improvements on the Western Ontario and McMaster Universities (WOMAC) pain and physical function 0-100 mm visual analog scales.12
The review of oral and transdermal opioids included 10 studies, with a total of 1541 patients receiving opioids and 727 receiving placebo.17 There were 3 trials of codeine (in 2 of the 3, a simple analgesic [acetaminophen 3000 mg/d or ibuprofen 1200 mg/d] was co-administered to both the treatment and control groups); other opioids included in the trials were oxycodone (4 trials), oxymorphone (2 trials), morphine (1 trial), and transdermal fentanyl (1 trial).
A modest boost in well-being
The reviewers measured function in 2 ways, focusing on both global improvement and improvement in physical function.
Global assessment. For the global assessment, the reviewers defined a treatment response as achieving at least a moderate improvement. By that standard, tramadol may improve overall well-being more than placebo. In the placebo-controlled trials, the number needed to treat (NNT) to elicit one treatment response was 6.
Three of the trials with active controls included global/functional assessments, and the results—bearing in mind the reduced quality and quantity of the evidence—were mixed. In a comparison of tramadol with dextropropoxyphene, tramadol increased the likelihood of moderate improvement by 38% (relative risk, 1.38 (95% CI, 1.15-1.67).10 In a trial of tramadol vs pentazocine, tramadol was more effective in reducing the duration of morning stiffness (by about 10 minutes), but not its severity. Tramadol was comparable with pentazocine in the 7 other measures of OA and function.16 In the tramadol-diclofenac study, both drugs were equally effective.17
Physical function. Four of the 6 placebo-controlled tramadol studies included in the Cochrane review used the WOMAC Index score, which included the physical function subscale. The tramadol group had a larger reduction in the score than the placebo group, by 0.34 mm (95% CI, -0.49 to -0.19). While this was equivalent to an 8.5% relative reduction in mean baseline score, it is still small compared with the minimal perceptible clinical improvement level of 9.3 mm on a 0-100 scale needed for the WOMAC physical function subscale. A similar improvement was reported for those taking tramadol compared with diclofenac—the only one of the active-controlled studies to report on physical function.17
Other opioids relieve pain, improve function—but how much?
The review of oral and transdermal opioids for OA11 encompassed 10 trials, with a total of 1541 patients receiving opioids and 727 on placebo. The opioids used in the trials were codeine, oxycodone, oxymorphone, morphine, and transdermal fentanyl. (For more details, see “How the reviews were conducted”.)
Pain. The trials included in the review used a variety of scales to measure pain, so the reviewers gauged results by the proportion of patients responding to treatment. Response was defined as a 50% improvement in pain score.
In the overall analysis, 35% of patients taking opioids responded to treatment, vs 31% of those on placebo—or 4 more patients in 100. That represents an NNT of 25. (A subgroup analysis did not demonstrate any significant differences in effect size among the opioids tested. In addition, the effect size was similar regardless of the potency of the opioid or the administration route.)
Function. Seven of the 10 trials (1794 participants, including both the treatment groups and controls) used validated function scores to measure physical function after 4 weeks of treatment. Here, too, the reviewers defined a treatment response as a 50% improvement in score.
Their finding? Opioids had a greater effect on function compared with placebo, equaling 0.7 on a WOMAC disability scale of 1 to 10. This means that about 3 more patients in 100 responded to treatment with opioids vs placebo—an NNT of 30.
But what about safety?
Opioids, including tramadol, are associated with adverse events (AEs), which may be minor or major. To determine when, or whether, the benefits outweigh the risks for treating patients with OA, both reviews reported on AEs and the number of participants who stopped taking the drug because of AEs.
AEs limit tramadol’s usefulness
While tramadol was more effective than placebo at reducing pain intensity, relieving symptoms, and improving function, the benefits were small—with an overall NNT of 6 (TABLE 1). This is similar to acetaminophen (NNT, 4-16),18 but with a greater downside.
Minor AEs. Four placebo-controlled trials reported on minor AEs.19-22 Those most commonly reported by patients taking tramadol were nausea, vomiting, dizziness, constipation, somnolence, tiredness, and headache.
Overall, 39% of those who received tramadol experienced minor AEs, compared with 18% of patients receiving placebo—an NNH of 5.10 Thus, tramadol’s NNH for minor AEs is equivalent to its NNT for pain relief. In active-controlled studies, there was a higher risk of minor AEs in those receiving tramadol compared with diclofenac or dextropropoxyphene, but a lower risk compared with those taking pentazocine.10
Major AEs. An analysis of the placebo-controlled trials revealed that 21% of those who received tramadol had major AEs—defined as an event that resulted in cessation of treatment—compared with 8% of those taking placebo. By this measure, the NNH was 8: One in 8 patients stopped taking tramadol because of a major AE.10
Among the active-controlled trials, participants taking tramadol were more likely to report a major AE compared with those receiving either diclofenac or dextropropoxyphene (NNH=5), but less likely compared with patients taking pentazocine. In a trial that compared tramadol alone with paracetamol, 2 out of 10 in the tramadol group discontinued treatment; none in the paracetamol group did.13
TABLE 1
Tramadol and other opioids for OA pain: NNT and NNH
| Treatment | NNT | NNH |
|---|---|---|
| Tramadol10 | 6 | 5 |
| Opioids (overall)11 | 25 | 12 |
| NNH, number needed to harm; NNT, number needed to treat; OA, osteoarthritis. | ||
Post-review RCTs provide further evidence
We identified 4 double-blind RCTs of tramadol for the treatment of OA that were of at least 6 weeks’ duration,19-22 published after the 2006 review. The results of these studies (TABLE 2) were broadly consistent with those of the systematic review. Two of the 4 studies had active controls, with one comparing tramadol with diclofenac19 and the other with celecoxib.21 Tramadol and diclofenac were found to be equally effective; celecoxib appeared to be superior in terms of pain relief, global improvement, and physical function, but no statistical comparisons were reported.
TABLE 2
Tramadol for OA: Post-review RCTs are consistent with meta-analysis
| Study duration (N) | Intervention groups | Primary outcome measures | Improvement in | Adverse effects | ||
|---|---|---|---|---|---|---|
| Pain | Global assessment | Function | ||||
| Gana*20 12 wk (1020) | Tramadol ER Placebo | WOMAC OA index (pain and physical function subscales) 100-mm VAS: Subject global disease | Treatment groups, 35% Placebo, 25% | Treatment groups, 32%-36% Placebo, 24% | Treatment groups, 31%-33% Placebo, 22% | ≥1 AE Withdrawals due to AEs |
| Delemos*21 12 wk (1001) | Tramadol ER Placebo | WOMAC OA index (pain and physical function subscales) 100-mm VAS: Subject global disease | Tramadol, 27%-39% Celecoxib, 45% Placebo, 32% | Tramadol, 28%-40% Celecoxib, 44% Placebo, 30% | Tramadol, 26%-35% Celecoxib, 43% Placebo, 28% | ≥1 AE Withdrawals due to AEs |
| Burch†22 12 wk (646) | Tramadol (Contramid OAD) 100 mg titrating to 300 mg Placebo | Pain intensity (11-point numerical scale) Physician/patient global impressions of change (7-point scale) | Treatment group, 40% Placebo, 33% | Treatment group, 80% Placebo, 69% | NA | AEs Placebo: Nausea, 5.6%; constipation, 4.2%; dizziness/vertigo, 3.7%; somnolence, 3.7% Withdrawals due to AEs |
| Beaulieu*19 6 wk (128) | Tramadol CR 200 mg titrating to 400 mg Diclofenac SR 75 mg titrating to 150 mg | WOMAC OA index (pain and physical function subscales) 100-mm VAS: Pain intensity Subject global disease Physician/patient global impressions of change (7-point scale) | Both groups, ~29% | Tramadol, 67%‡ Diclofenac, 54%‡ | Tramadol, 29%‡ Diclofenac, 29%‡ | Withdrawals due to AEs Tramadol, 16% Diclofenac, 15% |
| *Hip or knee osteoarthritis. †Knee osteoarthritis. ‡Not statistically significant. AEs, adverse events; CR, controlled release; ER, extended release; NA, not assessed; OA, osteoarthritis; OAD, once a day; RCTs, randomized controlled trials; SR, sustained release; VAS, visual analog scale; WOMAC, Western Ontario and McMaster Universities. | ||||||
Oral and transdermal opioids: Pain relief but high risk
Among the patients with OA of the hip or knee—the study population for the review of oral and transdermal opioids—all the opioids tested were more effective than placebo. The benefits, however, were small to moderate, and were off set by large increases in the risk of AEs and a high dropout rate.
Four of the 10 trials reported the number of patients experiencing any AE: 23% of those taking opioids vs 15% of patients on placebo.11 This represents an NNH of 12 (TABLE 1). All 10 trials reported the number of patients who withdrew due to AEs. Those receiving opioids were 4 times as likely to withdraw due to AEs, compared with those taking placebo. The NNH to cause one additional withdrawal was 19 (95% CI, 13-29).
Bottom line
The data highlight both the limited role of opioids (including tramadol) in OA treatment and—when they are being considered for this patient population—the importance of making patients aware that the risks may outweigh the benefits. Used judiciously and with adequate patient counseling, tramadol may be an option when COX-2-specific inhibitors and NSAIDs fail or cannot be tolerated. Although the small-to-moderate benefits of non-tramadol opioids are generally outweighed by large increases in the risk of AEs, their use may be considered for severe OA pain if tramadol is ineffective or causes intolerable AEs.
CORRESPONDENCE
Faline Howes, BMedSci, MBBS, MPH, FRACGP, Menzies Research Institute Tasmania, Private Bag 23, University of Tasmania, Hobart, Tasmania, Australia 7001; Faline.Howes@ utas.edu.au
Osteoarthritis (OA) affects nearly 27 million Americans, or about 12% of US adults.1 As the average age of the population increases, the prevalence and burden of this debilitating disorder continue to rise.2
The American College of Rheumatology (ACR)’s guidelines for the medical management of OA of the hip and knee, last updated in 2000,3 focus on controlling pain and improving function and health-related quality of life while minimizing the toxic effects of therapy. The guidelines recommend tramadol—an atypical opioid with 2 distinct mechanisms of action4—for moderate-to-severe pain in OA patients who either have contraindications to COX-2 inhibitors and non steroidal anti-inflammatory drugs (NSAIDs) or have failed to respond to previous oral therapy. Patients with severe pain who don’t respond to or are unable to tolerate tramadol may be candidates for more traditional opioid therapy, the guidelines indicate.3
In recent years, however, the use (and abuse) of opioids has skyrocketed. Between 1997 and 2007, US per capita retail purchases of hydrocodone and oxycodone increased 4-fold and 9-fold, respectively.5 In a similar time frame (1996-2006), the number of deaths from opioid overdose more than tripled, going from 4000 to 13,800 annually.6 Not surprisingly, the use of narcotics for noncancer pain remains controversial.7,8 But inadequately treated pain continues to be a serious public health problem, as well.9
This is the third in a series of articles based on the findings of the Cochrane Musculoskeletal Group (CMSG). One of the largest groups in the Cochrane Collaboration, the CMSG synthesizes the results of clinical trials to determine whether interventions for the prevention, treatment, and rehabilitation of musculoskeletal disorders are safe and effective. In this installment, the reviewers use detailed analysis, as well as a case study, to bring their findings to the attention of family physicians in a practical, clinically relevant context.
In 2006 and 2009, respectively, the Cochrane Collaboration published systematic reviews of tramadol (for OA in any joint)10 and other oral and transdermal opioids (for OA of the hip or knee).11 The reviewers’ findings, presented here along with data from more recent trials, can help ensure that you prescribe opioids for patients with OA only when their use is clinically appropriate and evidence-based. We’ve also included a case study (see page 211), so you can assess your knowledge and clinical skills.
CASE Carol J, an active 72-year-old, was diagnosed with OA in her right hip 5 years ago. Now she reports that the pain is getting progressively worse, making it harder and harder to turn over in bed at night or get in and out of the car. The pain is particularly bad at night, Carol says, and she’s had interrupted sleep for months. The patient has taken acetaminophen for the pain since her OA diagnosis, but now finds the analgesic is ineffective, even at the maximum dose of 4 g per day.
Carol has hypertension, which was difficult to manage until she began taking a combination ACE inhibitor/diuretic. She also has moderate renal impairment and mild chronic obstructive pulmonary disease, which limits her exercise tolerance. Nonetheless, she continues to smoke. The patient lives with and cares for her husband, who has Alzheimer’s disease, and worries about her ability to continue to care for him.
What are her treatment options?
Full-dose acetaminophen is no longer helping Carol, and NSAIDs are contraindicated because she takes an ACE inhibitor/diuretic and has moderate renal impairment. Increasing exercise will be a challenge. You strongly encourage her to stop smoking, emphasizing that this is particularly important to reduce the risk involved with any future joint replacement surgery.
Oral dosing options for the patient include:
- prescribing tramadol, starting with a low-dose immediate-release formulation taken one hour before bedtime (The controlled-release formulation is not advisable, given her age and renal function.) or
- adding a traditional opioid, eg, codeine 30 to 60 mg every 6 hours as needed, to her regular acetaminophen regimen.
Codeine and hydrocodone are available in combination preparations with acetaminophen, which may be convenient for some patients. However, hydrocodone was not one of the opioids tested in the trials included in the Cochrane reviews, and evidence of its use in OA is lacking.
Intra-articular corticosteroid injection, performed under imaging guidance, is another option for Carol. You explain that although there have been no studies of intra-articular corticosteroid injections for OA of the hip, these are used occasionally and may provide short-term symptom relief.7
You emphasize that surgery is likely to give her the best long-term outcome. In view of the patient’s circumstances and the need to care for her husband, however, you prescribe tramadol 50 mg at night. (Because of Carol’s age, renal impairment, and the possible adverse effects, it’s wise to start with a low dose and titrate upwards.) You warn her of the risks associated with opioids and advise her to alert your office staff if she experiences any adverse effects.
Before the patient leaves, you arrange an orthopedic consult and schedule a return visit for the following week. At your urging, she agrees to look into respite options for her husband.
Tramadol produces modest results—or none at all
The tramadol review10 included 11 randomized controlled trials (RCTs) with a total of 1019 participants who took tramadol or tramadol/acetaminophen (paracetamol) and 920 controls. In 6 of the 11 studies, the controls received placebo; the remaining 5 trials used “active controls,” with the control group for each RCT receiving a different analgesic. (To learn more about the methodology, see “How the reviews were conducted”.)
Placebo-controlled trials. Compared with patients on placebo, those receiving tramadol had an average absolute reduction in pain of 8.5 mm on a 0-100 mm visual analog scale (VAS) (95% confidence interval [CI], -12.05 to -4.9). That small benefit, however, did not reach the level defined as the minimal perceptible clinical improvement—a reduction of 9.7 mm on Western Ontario and McMaster Universities (WOMAC)’s OA pain subscale.12
Active-controlled trials. In the 5 RCTs comparing tramadol with another active agent, tramadol proved to be no better than the control drug. In fact, in a study of tramadol vs acetaminophen, 500 mg acetaminophen 3 times a day provided more pain relief than 50 mg tramadol 3 times a day.13 Although this was a small (N=20), short-term (7-day) study, this finding is notable because participants took less than the usual acetaminophen dose of 1 g up to 4 times a day.
Nor was tramadol superior to the agents it was compared with in the 4 other active-controlled trials—dihydrocodeine,14 dextropropoxyphene,15 pentazocine,16 and diclofenac17—in reducing pain intensity. It is important to keep in mind, however, that in each of these studies, both the quantity and quality of the evidence was limited. (Two studies did not use numerical scales,14,16 for example; all had methodological issues; and none lasted longer than 28 days.)
The Cochrane Musculoskeletal Group conducted a review of tramadol and a review of other oral opioids and transdermal fentanyl for the treatment of osteoarthritis (OA). Both reviews featured pain, function, and safety as primary outcomes. The tramadol review included randomized controlled trials (RCTs) for OA in any joint, while the oral and transdermal opioid review included randomized and quasi-randomized trials of treatment for OA of the hip or knee. Other parameters follow:
The tramadol review included 11 RCTs, with a total of 1019 participants receiving either tramadol alone or tramadol/acetaminophen (paracetamol) and 920 controls. In 6 of the 11 studies, the controls received placebo; the remaining 5 studies featured “active control.” That is, the control groups received acetaminophen 500 mg 3 times daily, diclofenac (25-50 mg up to 3 times daily on demand), dihydrocodeine 60 mg twice daily, dextropropoxyphene 100 mg 3 times daily, or pentazocine 50 mg 4 times per day. Because each of these agents was used in only one trial, the reviewers could not reach definitive conclusions about tramadol’s performance relative to other medications. The average number of participants in the tramadol and control groups was 91 and 80, respectively. The average length of follow-up was 35 days.
The 11 RCTs included in this review used a variety of pain scales to assess the results of tramadol, active control medications, and placebo. For comparative purposes, the reviewers pooled the results from studies that used numerical scales (0 to 100 and 0 to 10) to assess pain intensity. As a reference, we have used 9.7 and 9.3, respectively, determined by other researchers to be the minimal perceptible clinical improvements on the Western Ontario and McMaster Universities (WOMAC) pain and physical function 0-100 mm visual analog scales.12
The review of oral and transdermal opioids included 10 studies, with a total of 1541 patients receiving opioids and 727 receiving placebo.17 There were 3 trials of codeine (in 2 of the 3, a simple analgesic [acetaminophen 3000 mg/d or ibuprofen 1200 mg/d] was co-administered to both the treatment and control groups); other opioids included in the trials were oxycodone (4 trials), oxymorphone (2 trials), morphine (1 trial), and transdermal fentanyl (1 trial).
A modest boost in well-being
The reviewers measured function in 2 ways, focusing on both global improvement and improvement in physical function.
Global assessment. For the global assessment, the reviewers defined a treatment response as achieving at least a moderate improvement. By that standard, tramadol may improve overall well-being more than placebo. In the placebo-controlled trials, the number needed to treat (NNT) to elicit one treatment response was 6.
Three of the trials with active controls included global/functional assessments, and the results—bearing in mind the reduced quality and quantity of the evidence—were mixed. In a comparison of tramadol with dextropropoxyphene, tramadol increased the likelihood of moderate improvement by 38% (relative risk, 1.38 (95% CI, 1.15-1.67).10 In a trial of tramadol vs pentazocine, tramadol was more effective in reducing the duration of morning stiffness (by about 10 minutes), but not its severity. Tramadol was comparable with pentazocine in the 7 other measures of OA and function.16 In the tramadol-diclofenac study, both drugs were equally effective.17
Physical function. Four of the 6 placebo-controlled tramadol studies included in the Cochrane review used the WOMAC Index score, which included the physical function subscale. The tramadol group had a larger reduction in the score than the placebo group, by 0.34 mm (95% CI, -0.49 to -0.19). While this was equivalent to an 8.5% relative reduction in mean baseline score, it is still small compared with the minimal perceptible clinical improvement level of 9.3 mm on a 0-100 scale needed for the WOMAC physical function subscale. A similar improvement was reported for those taking tramadol compared with diclofenac—the only one of the active-controlled studies to report on physical function.17
Other opioids relieve pain, improve function—but how much?
The review of oral and transdermal opioids for OA11 encompassed 10 trials, with a total of 1541 patients receiving opioids and 727 on placebo. The opioids used in the trials were codeine, oxycodone, oxymorphone, morphine, and transdermal fentanyl. (For more details, see “How the reviews were conducted”.)
Pain. The trials included in the review used a variety of scales to measure pain, so the reviewers gauged results by the proportion of patients responding to treatment. Response was defined as a 50% improvement in pain score.
In the overall analysis, 35% of patients taking opioids responded to treatment, vs 31% of those on placebo—or 4 more patients in 100. That represents an NNT of 25. (A subgroup analysis did not demonstrate any significant differences in effect size among the opioids tested. In addition, the effect size was similar regardless of the potency of the opioid or the administration route.)
Function. Seven of the 10 trials (1794 participants, including both the treatment groups and controls) used validated function scores to measure physical function after 4 weeks of treatment. Here, too, the reviewers defined a treatment response as a 50% improvement in score.
Their finding? Opioids had a greater effect on function compared with placebo, equaling 0.7 on a WOMAC disability scale of 1 to 10. This means that about 3 more patients in 100 responded to treatment with opioids vs placebo—an NNT of 30.
But what about safety?
Opioids, including tramadol, are associated with adverse events (AEs), which may be minor or major. To determine when, or whether, the benefits outweigh the risks for treating patients with OA, both reviews reported on AEs and the number of participants who stopped taking the drug because of AEs.
AEs limit tramadol’s usefulness
While tramadol was more effective than placebo at reducing pain intensity, relieving symptoms, and improving function, the benefits were small—with an overall NNT of 6 (TABLE 1). This is similar to acetaminophen (NNT, 4-16),18 but with a greater downside.
Minor AEs. Four placebo-controlled trials reported on minor AEs.19-22 Those most commonly reported by patients taking tramadol were nausea, vomiting, dizziness, constipation, somnolence, tiredness, and headache.
Overall, 39% of those who received tramadol experienced minor AEs, compared with 18% of patients receiving placebo—an NNH of 5.10 Thus, tramadol’s NNH for minor AEs is equivalent to its NNT for pain relief. In active-controlled studies, there was a higher risk of minor AEs in those receiving tramadol compared with diclofenac or dextropropoxyphene, but a lower risk compared with those taking pentazocine.10
Major AEs. An analysis of the placebo-controlled trials revealed that 21% of those who received tramadol had major AEs—defined as an event that resulted in cessation of treatment—compared with 8% of those taking placebo. By this measure, the NNH was 8: One in 8 patients stopped taking tramadol because of a major AE.10
Among the active-controlled trials, participants taking tramadol were more likely to report a major AE compared with those receiving either diclofenac or dextropropoxyphene (NNH=5), but less likely compared with patients taking pentazocine. In a trial that compared tramadol alone with paracetamol, 2 out of 10 in the tramadol group discontinued treatment; none in the paracetamol group did.13
TABLE 1
Tramadol and other opioids for OA pain: NNT and NNH
| Treatment | NNT | NNH |
|---|---|---|
| Tramadol10 | 6 | 5 |
| Opioids (overall)11 | 25 | 12 |
| NNH, number needed to harm; NNT, number needed to treat; OA, osteoarthritis. | ||
Post-review RCTs provide further evidence
We identified 4 double-blind RCTs of tramadol for the treatment of OA that were of at least 6 weeks’ duration,19-22 published after the 2006 review. The results of these studies (TABLE 2) were broadly consistent with those of the systematic review. Two of the 4 studies had active controls, with one comparing tramadol with diclofenac19 and the other with celecoxib.21 Tramadol and diclofenac were found to be equally effective; celecoxib appeared to be superior in terms of pain relief, global improvement, and physical function, but no statistical comparisons were reported.
TABLE 2
Tramadol for OA: Post-review RCTs are consistent with meta-analysis
| Study duration (N) | Intervention groups | Primary outcome measures | Improvement in | Adverse effects | ||
|---|---|---|---|---|---|---|
| Pain | Global assessment | Function | ||||
| Gana*20 12 wk (1020) | Tramadol ER Placebo | WOMAC OA index (pain and physical function subscales) 100-mm VAS: Subject global disease | Treatment groups, 35% Placebo, 25% | Treatment groups, 32%-36% Placebo, 24% | Treatment groups, 31%-33% Placebo, 22% | ≥1 AE Withdrawals due to AEs |
| Delemos*21 12 wk (1001) | Tramadol ER Placebo | WOMAC OA index (pain and physical function subscales) 100-mm VAS: Subject global disease | Tramadol, 27%-39% Celecoxib, 45% Placebo, 32% | Tramadol, 28%-40% Celecoxib, 44% Placebo, 30% | Tramadol, 26%-35% Celecoxib, 43% Placebo, 28% | ≥1 AE Withdrawals due to AEs |
| Burch†22 12 wk (646) | Tramadol (Contramid OAD) 100 mg titrating to 300 mg Placebo | Pain intensity (11-point numerical scale) Physician/patient global impressions of change (7-point scale) | Treatment group, 40% Placebo, 33% | Treatment group, 80% Placebo, 69% | NA | AEs Placebo: Nausea, 5.6%; constipation, 4.2%; dizziness/vertigo, 3.7%; somnolence, 3.7% Withdrawals due to AEs |
| Beaulieu*19 6 wk (128) | Tramadol CR 200 mg titrating to 400 mg Diclofenac SR 75 mg titrating to 150 mg | WOMAC OA index (pain and physical function subscales) 100-mm VAS: Pain intensity Subject global disease Physician/patient global impressions of change (7-point scale) | Both groups, ~29% | Tramadol, 67%‡ Diclofenac, 54%‡ | Tramadol, 29%‡ Diclofenac, 29%‡ | Withdrawals due to AEs Tramadol, 16% Diclofenac, 15% |
| *Hip or knee osteoarthritis. †Knee osteoarthritis. ‡Not statistically significant. AEs, adverse events; CR, controlled release; ER, extended release; NA, not assessed; OA, osteoarthritis; OAD, once a day; RCTs, randomized controlled trials; SR, sustained release; VAS, visual analog scale; WOMAC, Western Ontario and McMaster Universities. | ||||||
Oral and transdermal opioids: Pain relief but high risk
Among the patients with OA of the hip or knee—the study population for the review of oral and transdermal opioids—all the opioids tested were more effective than placebo. The benefits, however, were small to moderate, and were off set by large increases in the risk of AEs and a high dropout rate.
Four of the 10 trials reported the number of patients experiencing any AE: 23% of those taking opioids vs 15% of patients on placebo.11 This represents an NNH of 12 (TABLE 1). All 10 trials reported the number of patients who withdrew due to AEs. Those receiving opioids were 4 times as likely to withdraw due to AEs, compared with those taking placebo. The NNH to cause one additional withdrawal was 19 (95% CI, 13-29).
Bottom line
The data highlight both the limited role of opioids (including tramadol) in OA treatment and—when they are being considered for this patient population—the importance of making patients aware that the risks may outweigh the benefits. Used judiciously and with adequate patient counseling, tramadol may be an option when COX-2-specific inhibitors and NSAIDs fail or cannot be tolerated. Although the small-to-moderate benefits of non-tramadol opioids are generally outweighed by large increases in the risk of AEs, their use may be considered for severe OA pain if tramadol is ineffective or causes intolerable AEs.
CORRESPONDENCE
Faline Howes, BMedSci, MBBS, MPH, FRACGP, Menzies Research Institute Tasmania, Private Bag 23, University of Tasmania, Hobart, Tasmania, Australia 7001; Faline.Howes@ utas.edu.au
1. Lawrence RC, Felson DT, Helmick CG, et al. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. Part II. Arthritis Rheum. 2008;58:26-35.
2. Bitton R. The economic burden of osteoarthritis. Am J Manag Care. 2009;15(suppl):S230-S235.
3. Altman RD, Hochberg MC, Moskowitz RW, et al. Recommendations for the medical management of osteoarthritis of the hip and knee. Arthritis Rheum. 2000;43:1905-1915.
4. Gibson TP. Pharmacokinetics, efficacy, and safety of analgesia with a focus on tramadol HCl. Am J Med. 1996;101(suppl 1A):47S-53S.
5. Hall AJ, Logan JE, Toblin RL, et al. Patterns of abuse among unintentional pharmaceutical overdose fatalities. JAMA. 2008;300:2613-2620.
6. Warner M, Chen LH, Makuc DM. Increase in fatal poisonings involving opioid analgesics in the United States, 1999-2006. NCHS data brief, no 22. Hyattsville, MD: National Center for Health Statistics; 2009.
7. Von Korff M, Deyo RA. Potent opioids for chronic musculoskeletal pain: flying blind? Pain. 2004;109:207-209.
8. Zhang W, Moskowitz RW, Nuki G, et al. OARSI recommendations for the management of hip and knee osteoarthritis, Part II: OARSI evidence-based, expert consensus guidelines. Osteoarthritis Cartilage. 2008;16:137-167.
9. Pletcher MJ, Kertesz SG, Kohn MA, et al. Trends in opioid prescribing by race/ethnicity for patients seeking care in US emergency departments. JAMA. 2008;299:70-78.
10. Cepeda MS, Camargo F, Zea C, et al. Tramadol for osteoarthritis. Cochrane Database Syst Rev. 2006;(3):CD005522.-
11. Nuesch E, Rutjes AW, Husni E, et al. Oral or transdermal opioids for osteoarthritis of the knee or hip. Cochrane Database Syst Rev. 2009;(4):CD003115.-
12. Ehrich EW, Davies GM, Watson DJ, et al. Minimal perceptible clinical improvement with the Western Ontario and McMaster Universities osteoarthritis index questionnaire and global assessments in patients with osteoarthritis. J Rheumatol. 2000;27:2635-2641.
13. Bianchi M, Broggini M, Balzarini P, et al. Effects of tramadol on synovial fluid concentrations of substance P and interleukin-6 in patients with knee osteoarthritis: comparison with paracetamol. Int Immunopharm. 2003;3:1901-1908.
14. Wilder-Smith C, Hill L, Spargo K, et al. Treatment of severe pain from osteoarthritis with slow-release tramadol or dihydrocodeine in combination with NSAIDs: a randomised study comparing analgesia, antinociception and gastrointestinal effects. Pain. 2001;91:23-31.
15. Jensen E, Ginsberg F. Tramadol versus dextropropoxyphene in the treatment of osteoarthritis: a short-term double-blind study. Drug Invest. 1994;8:211-218.
16. Bird H, Hill J, Stratford M, et al. A double-blind cross-over study comparing the analgesic efficacy of tramadol with pentazocine in patients with osteoarthritis. J Drug Dev Clin Pract. 1995;7:181-188.
17. Pavelka K, Peliskova Z, Stehlikova H, et al. Intraindividual differences in pain relief and functional improvement in osteoarthritis with diclofenac or tramadol. Clin Drug Invest. 1998;16:421-429.
18. Townheed TE, Maxwell L, Judd MG, et al. Acetaminophen for osteoarthritis. Cochrane Database Syst Rev. 2006;(1):CD004257.-
19. Beaulieu AD, Peloso PM, Haraoui B, et al. Once-daily, controlled-release tramadol and sustained-release diclofenac relieve chronic pain due to osteoarthritis: a randomized controlled trial. Pain Res Manag. 2008;13:103-110.
20. Gana TJ, Pascual ML, Fleming RR, et al. Extended-release tramadol in the treatment of osteoarthritis: a multicenter, randomized, double-blind, placebo-controlled clinical trial. Curr Med Res Opin. 2006;22:1391-1401.
21. Delemos BP, Xiang J, Benson C, et al. Tramadol hydrochloride extended-release once-daily in the treatment of osteoarthritis of the knee and/or hip: a double-blind, randomized, dose-ranging trial. Am J Ther. 2010 Mar 3 [Epub ahead of print].
22. Burch F, Fishman R, Messina N, et al. A comparison of the analgesic efficacy of Tramadol Contramid OAD versus placebo in patients with pain due to osteoarthritis. J Pain Symptom Manage. 2007;34:328-338.
1. Lawrence RC, Felson DT, Helmick CG, et al. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. Part II. Arthritis Rheum. 2008;58:26-35.
2. Bitton R. The economic burden of osteoarthritis. Am J Manag Care. 2009;15(suppl):S230-S235.
3. Altman RD, Hochberg MC, Moskowitz RW, et al. Recommendations for the medical management of osteoarthritis of the hip and knee. Arthritis Rheum. 2000;43:1905-1915.
4. Gibson TP. Pharmacokinetics, efficacy, and safety of analgesia with a focus on tramadol HCl. Am J Med. 1996;101(suppl 1A):47S-53S.
5. Hall AJ, Logan JE, Toblin RL, et al. Patterns of abuse among unintentional pharmaceutical overdose fatalities. JAMA. 2008;300:2613-2620.
6. Warner M, Chen LH, Makuc DM. Increase in fatal poisonings involving opioid analgesics in the United States, 1999-2006. NCHS data brief, no 22. Hyattsville, MD: National Center for Health Statistics; 2009.
7. Von Korff M, Deyo RA. Potent opioids for chronic musculoskeletal pain: flying blind? Pain. 2004;109:207-209.
8. Zhang W, Moskowitz RW, Nuki G, et al. OARSI recommendations for the management of hip and knee osteoarthritis, Part II: OARSI evidence-based, expert consensus guidelines. Osteoarthritis Cartilage. 2008;16:137-167.
9. Pletcher MJ, Kertesz SG, Kohn MA, et al. Trends in opioid prescribing by race/ethnicity for patients seeking care in US emergency departments. JAMA. 2008;299:70-78.
10. Cepeda MS, Camargo F, Zea C, et al. Tramadol for osteoarthritis. Cochrane Database Syst Rev. 2006;(3):CD005522.-
11. Nuesch E, Rutjes AW, Husni E, et al. Oral or transdermal opioids for osteoarthritis of the knee or hip. Cochrane Database Syst Rev. 2009;(4):CD003115.-
12. Ehrich EW, Davies GM, Watson DJ, et al. Minimal perceptible clinical improvement with the Western Ontario and McMaster Universities osteoarthritis index questionnaire and global assessments in patients with osteoarthritis. J Rheumatol. 2000;27:2635-2641.
13. Bianchi M, Broggini M, Balzarini P, et al. Effects of tramadol on synovial fluid concentrations of substance P and interleukin-6 in patients with knee osteoarthritis: comparison with paracetamol. Int Immunopharm. 2003;3:1901-1908.
14. Wilder-Smith C, Hill L, Spargo K, et al. Treatment of severe pain from osteoarthritis with slow-release tramadol or dihydrocodeine in combination with NSAIDs: a randomised study comparing analgesia, antinociception and gastrointestinal effects. Pain. 2001;91:23-31.
15. Jensen E, Ginsberg F. Tramadol versus dextropropoxyphene in the treatment of osteoarthritis: a short-term double-blind study. Drug Invest. 1994;8:211-218.
16. Bird H, Hill J, Stratford M, et al. A double-blind cross-over study comparing the analgesic efficacy of tramadol with pentazocine in patients with osteoarthritis. J Drug Dev Clin Pract. 1995;7:181-188.
17. Pavelka K, Peliskova Z, Stehlikova H, et al. Intraindividual differences in pain relief and functional improvement in osteoarthritis with diclofenac or tramadol. Clin Drug Invest. 1998;16:421-429.
18. Townheed TE, Maxwell L, Judd MG, et al. Acetaminophen for osteoarthritis. Cochrane Database Syst Rev. 2006;(1):CD004257.-
19. Beaulieu AD, Peloso PM, Haraoui B, et al. Once-daily, controlled-release tramadol and sustained-release diclofenac relieve chronic pain due to osteoarthritis: a randomized controlled trial. Pain Res Manag. 2008;13:103-110.
20. Gana TJ, Pascual ML, Fleming RR, et al. Extended-release tramadol in the treatment of osteoarthritis: a multicenter, randomized, double-blind, placebo-controlled clinical trial. Curr Med Res Opin. 2006;22:1391-1401.
21. Delemos BP, Xiang J, Benson C, et al. Tramadol hydrochloride extended-release once-daily in the treatment of osteoarthritis of the knee and/or hip: a double-blind, randomized, dose-ranging trial. Am J Ther. 2010 Mar 3 [Epub ahead of print].
22. Burch F, Fishman R, Messina N, et al. A comparison of the analgesic efficacy of Tramadol Contramid OAD versus placebo in patients with pain due to osteoarthritis. J Pain Symptom Manage. 2007;34:328-338.
Limp in children: Differentiating benign from dire causes
• Use radiographs to identify bone changes from disease (as well as fracture) when evaluating a limp. C
• Consider growth plate injuries as well as toddler’s fracture; both may be radiographically occult and require immobilization for treatment. C
• Consider child abuse if the patient has an isolated mid-shaft tibial fracture. C
• Assess for fever, elevated sedimentation rate, elevated C-reactive protein, and leukocytosis when radiographs are unrevealing or when a patient has systemic symptoms associated with limp. These factors are predictors of septic arthritis. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
A mother brings her 4-year-old son to the office because he has been limping. She isn’t aware of a specific trauma. But the boy and his twin brother, while recovering from “colds,” were rough-housing in their room when this son complained of pain. He is afebrile and points to his knee as the area of pain.
Although limping in children is common—the incidence is roughly 2 per 10001—it is never normal. It indicates pain, weakness, or structural abnormality.2 Most cases result from trauma.1 Limp usually resolves with little intervention and no sequelae. However, the differential diagnosis is broad and daunting (TABLE 1), and some causes of limp are associated with significant morbidity.
TABLE 1
Possible causes of limp in a child1-3,17
| Traumatic/mechanical Fractures, stress fractures Muscle injuries Sprains/strains Contusions Developmental dysplasia of the hip Slipped capital femoral epiphysis Tarsal coalition Child abuse Overuse injuries Leg length discrepancy |
| Infectious Septic arthritis Osteomyelitis Lyme disease Psoas abscess Diskitis |
| Inflammatory Transient synovitis Juvenile rheumatoid arthritis Ankylosing spondylitis Reiter syndrome Lupus |
| Vascular Legg-Calve-Perthes disease Osteonecrosis Hemoglobinopathies (sickle cell disease) |
| Neoplastic Leukemia, lymphoma Malignant/lytic tumors (Ewing sarcoma, osteogenic sarcoma, etc.) |
| Metabolic Rickets Hyperparathyroidism |
| Neuromuscular Muscular dystrophy Cerebral palsy Peripheral neuropathy |
Helpful tips for your initial assessment
Many textbook authors have described some causes of limp as “painless.” However, truly painless limp is rare, seldom acute, and usually the result of mechanical or neuromuscular disorders.1 A more likely explanation for acute “painless” limp is that a young child with pain is unable to express pain or accurately identify its location. Further, the child may instinctively avoid painful positions or movements and, thus, may present only with decreased movement of an extremity or refusal to bear weight.3
With a child who has knee pain, remember the pediatrics maxim: “Knee pain equals hip pain,”3 underscoring the diagnostic difficulty with limp.
Also bear in mind that children of different ages tend to have different etiologies of limp (TABLE 2). For example, septic arthritis, osteomyelitis, and transient synovitis occur more commonly in children under 10 years. Legg-Calve-Perthes disease and leukemia are more common in children between the ages of 4 and 10. Slipped capital femoral epiphysis (SCFE) is more common in boys over the age of 11.
TABLE 2
Common causes of limp according to child’s age1
| < 3 years | 3-10 years | 11-18 years |
|---|---|---|
| Foreign body | Legg-Calve-Perthes disease | Juvenile arthritis |
| Osteomyelitis | Osteomyelitis | Slipped capital femoral epiphysis |
| Septic arthritis | Septic arthritis | Trauma (physeal fracture) |
| Toddler’s fracture | Transient synovitis | Tumor |
| Transient synovitis | Trauma (physeal fracture) | |
| Tumor | Tumor |
Fracture
Fracture is a possibility across all age ranges, necessitating radiographs if suspected. Beyond detecting fractures, x-ray films can identify bony changes associated with disease (eg, Legg-Calve-Perthes disease, SCFE). Radiographs can also identify a clinically significant joint effusion at the hip.4 However, x-ray results may be falsely negative for some fracture types.
Salter-Harris Type I fractures are transverse fractures through the growth plate with epiphyseal separation from the metaphysis.5 Typical findings are a history of trauma and point tenderness over the epiphyseal plate. Type I fractures are radiographically occult, making the injury easy to mistake as a sprain. Nonetheless, growth plate injuries are common in children, requiring immobilization.
Toddler’s fracture was first described as a spiral, oblique undisplaced fracture of the distal tibial shaft in children from 9 months to 3 years of age.6 It results from a rotational or twisting force through the tibia while the leg rotates internally on a planted foot.7,8 This is the most common tibial fracture in infants and young children.9 The incidence has been reported as 0.6 to 2.5 per 1000 pediatric visits.10 Accurate diagnosis is important because current treatment recommendations suggest a long leg cast for 3 to 5 weeks, followed by a short leg cast for a total of 6 weeks.11
Despite being the most common tibial fracture, toddler’s fracture is easily missed. Initial radiographs are only 53% sensitive.7,10 This implies that nearly 50% of children with tibial fracture will have an initially negative x-ray result. However, nearly 94% of children with a confirmed toddler’s fracture have been unable to bear weight.12 Evidence suggests that despite negative radiographs, patients with point tenderness over the tibia and an inability to bear weight should be treated for presumed toddler’s fracture.12
Another confusing aspect of toddler’s fracture is that the causative injury is often considered insignificant by parents—eg, tripping, falling from a modest height, or a twisting motion.7,8 These events may occur countless times during the average day of a toddler. Often parents do not witness the injury and are unable to describe the mechanism of injury.7
When to suspect child abuse. When a child presents with fracture after an unwitnessed trauma and the story does not match the injury pattern, consider child abuse. With tibial fractures, the location of the fracture can help distinguish a result of abuse from a toddler’s fracture. Toddler’s fracture is classically described as a distal tibial fracture. In contrast, a midshaft tibial fracture often suggests child abuse.8,13 In a small retrospective study of 37 children diagnosed with toddler’s fracture, 4 midshaft tibial fractures were found.8 Child abuse was confirmed in 2 of these cases.8 However, other authors, including Dr. Dunbar in his sentinel article,6 assert that toddler’s fracture may occasionally extend into the midshaft of the tibia. Consequently, a midshaft tibial fracture is not pathognomonic for child abuse. But the diagnosis should be considered. Perform a careful examination for other signs of abuse or neglect, and do not hesitate to report suspected child abuse to the proper local and state authorities.14
Transient synovitis vs septic arthritis
A child who limps or refuses to bear weight on a limb often has associated symptoms of acute illness. In these cases, or when radiographs have ruled out apparent abnormalities such as Legg-Calve-Perthes disease, SCFE, and fracture, consider septic arthritis or transient synovitis (FIGURE). Both may present with limp and fever as well as pain, decreased range of motion, bone tenderness, swelling, and warmth.15
Transient synovitis is the most common cause of hip pain in children up to 10 years of age, with a 3% risk of occurrence through childhood.16,17 Its cause is unclear, but many experts have proposed a viral agent.17 Transient synovitis universally resolves without sequelae in 1 to 2 weeks. Therefore, prescribe rest and nonsteroidal anti-inflammatory drugs (NSAIDs) for symptomatic relief, and reassure parents.16
Septic arthritis, although often similar in presentation to transient synovitis, requires hospitalization, operative drainage, and parenteral antibiotics.18 A delay in diagnosis is associated with poor outcome, including osteonecrosis, growth arrest, permanent loss of joint function, and sepsis.3,18
Several studies have shown children with septic arthritis usually appear more acutely ill than those with transient synovitis.4,18-21 They are described as toxic-appearing, and have leukocytosis, a high erythrocyte sedimentation rate (ESR), and a high fever.19 However, no single marker or specific laboratory value consistently identifies septic arthritis. Many studies have been performed in an effort to identify a collection of factors, or an algorithm, that can predict the probability of septic arthritis.
Fever, an elevated ESR, and leukocytosis are independent multivariate clinical predictors for septic arthritis. The prediction algorithm published by Jung et al is the only study to have included C-reactive protein (CRP) as a predictive factor,4 which happens to be an excellent independent predictor of septic arthritis. Specifically, with a normal CRP <1 mg/dL, the probability of a patientnot having septic arthritis is 87%.22
While no predictive algorithm has been conclusively validated, the fact that the same clinical and laboratory predictors are consistently identified can be useful. Simply, if a patient presents with joint pain and 2 or more of the 4 predictors, septic arthritis must be fully evaluated. The presence of 2 of 4 predictors suggests a risk of septic arthritis between 10% and 40%.4,18,20 A single predictor is associated with a risk of 1% to 10%.4,18,20 Yet, you must interpret these clinical predictors in light of the full clinical picture, as septic arthritis is still possible in patients with only 1 predictor. Such possibilities require cautious management and close follow-up.
With 2 of 4 predictors present, suspect septic arthritis and order an ultrasound of the affected joint. If effusion is present, aspirate the joint. Some authors suggest that all patients with hip pain should undergo ultrasound, and that those with a joint effusion should undergo aspiration.15 However, joint aspiration, particularly of the hip, can be associated with multiple complications and should be avoided if possible.22 Effusion is also possible with transient synovitis and noninfectious causes of joint pain, but the aspirate will have a negative culture and normal gram stain findings. Ultrasound has been shown to be 100% accurate in predicting the presence of effusion.23
FIGURE
Diagnostic algorithm for pediatric limp3,4,6,8-12,15
CRP, C-reactive protein; ESR, erythrocyte sedimentation rate; MRI, magnetic resonance imaging; NSAIDs, nonsteroidal anti-inflammatory drugs; WBCs, white blood cells.
How the opening case resolved
The boy avoided weight-bearing on the affected leg, but had no focal bone tenderness. Moving the hip, but not the knee, reproduced pain. Radiographs were negative for fracture or changes typical of Legg-Calve-Perthes disease. He was afebrile in the office, but the mother described a fever at home. The child appeared ill, but stable. We decided to obtain a blood sample.
Results for CRP, ESR, and white blood cell count were normal. With this information, we reassured the mother that the diagnosis was likely transient synovitis. We advised a weight-appropriate dose of ibuprofen and scheduled a follow-up appointment for 2 days later.
CORRESPONDENCE John Whiteside, MD, St. Mary’s Family Medicine Residency, 1160 Patterson Road, Grand Junction, CO 81506; [email protected]
1. Abbassian A. The limping child: a clinical approach to diagnosis. Br J Hosp Med. 2007;68:246-250.
2. Leung AK, Lemay JF. The limping child. J Ped Health Care. 2004;18:219-223.
3. Frick SL. Evaluation of the child who has hip pain. Orthop Clin North Am. 2006;37:133-140.
4. Jung ST, Rowe SM, Moon ES, et al. Significance of laboratory and radiologic findings for differentiating between septic arthritis and transient synovitis of the hip. J Pediatr Orthop. 2003;23:368-372.
5. Brown JH, DeLuca SA. Growth plate injuries: Salter-Harris classification. Am Fam Physician. 1992;46:1180-1184.
6. Dunbar JS, Owen HF, Nogrady MB, et al. Obscure tibial fracture of infants–the toddler’s fracture. J Can Assoc Radiol. 1964;15:136-144.
7. Miller JH, Sanderson RA. Scintigraphy of toddler’s fracture. J Nucl Med. 1988;29:2001-2003.
8. Tenenbein M, Reed MH, Black GB. The toddler’s fracture revisited. Am J Emerg Med. 1990;8:208-211.
9. Tschoepe EJ, John SD, Swischuk LE. Tibial fractures in infants and children: emphasis on subtle injuries. Emerg Radiol. 1998;5:245-252.
10. Clancy J, Pieterse J, Roberston P, et al. Toddler’s fracture. J Accid Emerg Med. 1996;13:366-367.
11. Wheeless CR. Cast treatment of tibial fractures. In:Wheeless’ Textbook of Orthopaedics. 2011. Available at:http://www.wheelessonline.com/ortho/cast_treatment_of_tibial_fractures. Accessed March 11, 2011.
12. Halsey MF, Finzel KC, Carrion WV, et al. Toddler’s fracture: presumptive diagnosis and treatment. J Pediatr Orthop. 2001;21:152-156.
13. Mellick LB, Milker L, Egsieker E. Childhood accidental spiral tibial (CAST) fractures. Ped Emerg Care. 1999;15:307-309.
14. Jenny C. Committee on Child Abuse and Neglect. Evaluating infants and young children with multiple fractures. Pediatrics. 2006;118:1299-1303.
15. Dabney KW, Lipton G. Evaluation of limp in children. Curr Opin Pediatr. 1995;7:88-94.
16. Sherry DD. Limb pain in childhood. Pediatr Rev. 1990;12:39-46.
17. Do TT. Transient synovitis as a cause of painful limps in children. Curr Opin Pediatr. 2000;12:48-51.
18. Kocher MS, Zurakowski D, Kasser JR. Differentiating between septic arthritis and transient synovitis of the hip in children: an evidence-based clinical prediction algorithm. J Bone Joint Surg Am. 1999;81:1662-1670.
19. Luhmann SJ, Jones A, Schoolman M, et al. Differentiation between septic arthritis and transient synovitis of the hip in children with clinical prediction algorithms. J Bone Joint Surg Am. 2004;86-A:956-962.
20. Kocher MS, Mandiga R, Zurakowski D, et al. Validation of a clinical prediction rule for the differentiation between septic arthritis and transient synovitis of the hip in children. J Bone Joint Surg Am. 2004;86-A:1629-1635.
21. Delaney RA, Lenehan B, O’Sullivan L, et al. The limping child: an algorithm to outrule musculoskeletal sepsis. Ir J Med Sci. 2007;176:181-187.
22. Levine MJ, McGuire KJ, McGowan KL, et al. Assessment of the test characteristics of C-reactive protein for septic arthritis in children. J Pediatr Orthop. 2003;23:373-377.
23. Alexander JE, Seibert JJ, Glasier CM, et al. High-resolution hip ultrasound in the limping child. J Clin Ultrasound. 1989;17:19-24.
• Use radiographs to identify bone changes from disease (as well as fracture) when evaluating a limp. C
• Consider growth plate injuries as well as toddler’s fracture; both may be radiographically occult and require immobilization for treatment. C
• Consider child abuse if the patient has an isolated mid-shaft tibial fracture. C
• Assess for fever, elevated sedimentation rate, elevated C-reactive protein, and leukocytosis when radiographs are unrevealing or when a patient has systemic symptoms associated with limp. These factors are predictors of septic arthritis. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
A mother brings her 4-year-old son to the office because he has been limping. She isn’t aware of a specific trauma. But the boy and his twin brother, while recovering from “colds,” were rough-housing in their room when this son complained of pain. He is afebrile and points to his knee as the area of pain.
Although limping in children is common—the incidence is roughly 2 per 10001—it is never normal. It indicates pain, weakness, or structural abnormality.2 Most cases result from trauma.1 Limp usually resolves with little intervention and no sequelae. However, the differential diagnosis is broad and daunting (TABLE 1), and some causes of limp are associated with significant morbidity.
TABLE 1
Possible causes of limp in a child1-3,17
| Traumatic/mechanical Fractures, stress fractures Muscle injuries Sprains/strains Contusions Developmental dysplasia of the hip Slipped capital femoral epiphysis Tarsal coalition Child abuse Overuse injuries Leg length discrepancy |
| Infectious Septic arthritis Osteomyelitis Lyme disease Psoas abscess Diskitis |
| Inflammatory Transient synovitis Juvenile rheumatoid arthritis Ankylosing spondylitis Reiter syndrome Lupus |
| Vascular Legg-Calve-Perthes disease Osteonecrosis Hemoglobinopathies (sickle cell disease) |
| Neoplastic Leukemia, lymphoma Malignant/lytic tumors (Ewing sarcoma, osteogenic sarcoma, etc.) |
| Metabolic Rickets Hyperparathyroidism |
| Neuromuscular Muscular dystrophy Cerebral palsy Peripheral neuropathy |
Helpful tips for your initial assessment
Many textbook authors have described some causes of limp as “painless.” However, truly painless limp is rare, seldom acute, and usually the result of mechanical or neuromuscular disorders.1 A more likely explanation for acute “painless” limp is that a young child with pain is unable to express pain or accurately identify its location. Further, the child may instinctively avoid painful positions or movements and, thus, may present only with decreased movement of an extremity or refusal to bear weight.3
With a child who has knee pain, remember the pediatrics maxim: “Knee pain equals hip pain,”3 underscoring the diagnostic difficulty with limp.
Also bear in mind that children of different ages tend to have different etiologies of limp (TABLE 2). For example, septic arthritis, osteomyelitis, and transient synovitis occur more commonly in children under 10 years. Legg-Calve-Perthes disease and leukemia are more common in children between the ages of 4 and 10. Slipped capital femoral epiphysis (SCFE) is more common in boys over the age of 11.
TABLE 2
Common causes of limp according to child’s age1
| < 3 years | 3-10 years | 11-18 years |
|---|---|---|
| Foreign body | Legg-Calve-Perthes disease | Juvenile arthritis |
| Osteomyelitis | Osteomyelitis | Slipped capital femoral epiphysis |
| Septic arthritis | Septic arthritis | Trauma (physeal fracture) |
| Toddler’s fracture | Transient synovitis | Tumor |
| Transient synovitis | Trauma (physeal fracture) | |
| Tumor | Tumor |
Fracture
Fracture is a possibility across all age ranges, necessitating radiographs if suspected. Beyond detecting fractures, x-ray films can identify bony changes associated with disease (eg, Legg-Calve-Perthes disease, SCFE). Radiographs can also identify a clinically significant joint effusion at the hip.4 However, x-ray results may be falsely negative for some fracture types.
Salter-Harris Type I fractures are transverse fractures through the growth plate with epiphyseal separation from the metaphysis.5 Typical findings are a history of trauma and point tenderness over the epiphyseal plate. Type I fractures are radiographically occult, making the injury easy to mistake as a sprain. Nonetheless, growth plate injuries are common in children, requiring immobilization.
Toddler’s fracture was first described as a spiral, oblique undisplaced fracture of the distal tibial shaft in children from 9 months to 3 years of age.6 It results from a rotational or twisting force through the tibia while the leg rotates internally on a planted foot.7,8 This is the most common tibial fracture in infants and young children.9 The incidence has been reported as 0.6 to 2.5 per 1000 pediatric visits.10 Accurate diagnosis is important because current treatment recommendations suggest a long leg cast for 3 to 5 weeks, followed by a short leg cast for a total of 6 weeks.11
Despite being the most common tibial fracture, toddler’s fracture is easily missed. Initial radiographs are only 53% sensitive.7,10 This implies that nearly 50% of children with tibial fracture will have an initially negative x-ray result. However, nearly 94% of children with a confirmed toddler’s fracture have been unable to bear weight.12 Evidence suggests that despite negative radiographs, patients with point tenderness over the tibia and an inability to bear weight should be treated for presumed toddler’s fracture.12
Another confusing aspect of toddler’s fracture is that the causative injury is often considered insignificant by parents—eg, tripping, falling from a modest height, or a twisting motion.7,8 These events may occur countless times during the average day of a toddler. Often parents do not witness the injury and are unable to describe the mechanism of injury.7
When to suspect child abuse. When a child presents with fracture after an unwitnessed trauma and the story does not match the injury pattern, consider child abuse. With tibial fractures, the location of the fracture can help distinguish a result of abuse from a toddler’s fracture. Toddler’s fracture is classically described as a distal tibial fracture. In contrast, a midshaft tibial fracture often suggests child abuse.8,13 In a small retrospective study of 37 children diagnosed with toddler’s fracture, 4 midshaft tibial fractures were found.8 Child abuse was confirmed in 2 of these cases.8 However, other authors, including Dr. Dunbar in his sentinel article,6 assert that toddler’s fracture may occasionally extend into the midshaft of the tibia. Consequently, a midshaft tibial fracture is not pathognomonic for child abuse. But the diagnosis should be considered. Perform a careful examination for other signs of abuse or neglect, and do not hesitate to report suspected child abuse to the proper local and state authorities.14
Transient synovitis vs septic arthritis
A child who limps or refuses to bear weight on a limb often has associated symptoms of acute illness. In these cases, or when radiographs have ruled out apparent abnormalities such as Legg-Calve-Perthes disease, SCFE, and fracture, consider septic arthritis or transient synovitis (FIGURE). Both may present with limp and fever as well as pain, decreased range of motion, bone tenderness, swelling, and warmth.15
Transient synovitis is the most common cause of hip pain in children up to 10 years of age, with a 3% risk of occurrence through childhood.16,17 Its cause is unclear, but many experts have proposed a viral agent.17 Transient synovitis universally resolves without sequelae in 1 to 2 weeks. Therefore, prescribe rest and nonsteroidal anti-inflammatory drugs (NSAIDs) for symptomatic relief, and reassure parents.16
Septic arthritis, although often similar in presentation to transient synovitis, requires hospitalization, operative drainage, and parenteral antibiotics.18 A delay in diagnosis is associated with poor outcome, including osteonecrosis, growth arrest, permanent loss of joint function, and sepsis.3,18
Several studies have shown children with septic arthritis usually appear more acutely ill than those with transient synovitis.4,18-21 They are described as toxic-appearing, and have leukocytosis, a high erythrocyte sedimentation rate (ESR), and a high fever.19 However, no single marker or specific laboratory value consistently identifies septic arthritis. Many studies have been performed in an effort to identify a collection of factors, or an algorithm, that can predict the probability of septic arthritis.
Fever, an elevated ESR, and leukocytosis are independent multivariate clinical predictors for septic arthritis. The prediction algorithm published by Jung et al is the only study to have included C-reactive protein (CRP) as a predictive factor,4 which happens to be an excellent independent predictor of septic arthritis. Specifically, with a normal CRP <1 mg/dL, the probability of a patientnot having septic arthritis is 87%.22
While no predictive algorithm has been conclusively validated, the fact that the same clinical and laboratory predictors are consistently identified can be useful. Simply, if a patient presents with joint pain and 2 or more of the 4 predictors, septic arthritis must be fully evaluated. The presence of 2 of 4 predictors suggests a risk of septic arthritis between 10% and 40%.4,18,20 A single predictor is associated with a risk of 1% to 10%.4,18,20 Yet, you must interpret these clinical predictors in light of the full clinical picture, as septic arthritis is still possible in patients with only 1 predictor. Such possibilities require cautious management and close follow-up.
With 2 of 4 predictors present, suspect septic arthritis and order an ultrasound of the affected joint. If effusion is present, aspirate the joint. Some authors suggest that all patients with hip pain should undergo ultrasound, and that those with a joint effusion should undergo aspiration.15 However, joint aspiration, particularly of the hip, can be associated with multiple complications and should be avoided if possible.22 Effusion is also possible with transient synovitis and noninfectious causes of joint pain, but the aspirate will have a negative culture and normal gram stain findings. Ultrasound has been shown to be 100% accurate in predicting the presence of effusion.23
FIGURE
Diagnostic algorithm for pediatric limp3,4,6,8-12,15
CRP, C-reactive protein; ESR, erythrocyte sedimentation rate; MRI, magnetic resonance imaging; NSAIDs, nonsteroidal anti-inflammatory drugs; WBCs, white blood cells.
How the opening case resolved
The boy avoided weight-bearing on the affected leg, but had no focal bone tenderness. Moving the hip, but not the knee, reproduced pain. Radiographs were negative for fracture or changes typical of Legg-Calve-Perthes disease. He was afebrile in the office, but the mother described a fever at home. The child appeared ill, but stable. We decided to obtain a blood sample.
Results for CRP, ESR, and white blood cell count were normal. With this information, we reassured the mother that the diagnosis was likely transient synovitis. We advised a weight-appropriate dose of ibuprofen and scheduled a follow-up appointment for 2 days later.
CORRESPONDENCE John Whiteside, MD, St. Mary’s Family Medicine Residency, 1160 Patterson Road, Grand Junction, CO 81506; [email protected]
• Use radiographs to identify bone changes from disease (as well as fracture) when evaluating a limp. C
• Consider growth plate injuries as well as toddler’s fracture; both may be radiographically occult and require immobilization for treatment. C
• Consider child abuse if the patient has an isolated mid-shaft tibial fracture. C
• Assess for fever, elevated sedimentation rate, elevated C-reactive protein, and leukocytosis when radiographs are unrevealing or when a patient has systemic symptoms associated with limp. These factors are predictors of septic arthritis. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
A mother brings her 4-year-old son to the office because he has been limping. She isn’t aware of a specific trauma. But the boy and his twin brother, while recovering from “colds,” were rough-housing in their room when this son complained of pain. He is afebrile and points to his knee as the area of pain.
Although limping in children is common—the incidence is roughly 2 per 10001—it is never normal. It indicates pain, weakness, or structural abnormality.2 Most cases result from trauma.1 Limp usually resolves with little intervention and no sequelae. However, the differential diagnosis is broad and daunting (TABLE 1), and some causes of limp are associated with significant morbidity.
TABLE 1
Possible causes of limp in a child1-3,17
| Traumatic/mechanical Fractures, stress fractures Muscle injuries Sprains/strains Contusions Developmental dysplasia of the hip Slipped capital femoral epiphysis Tarsal coalition Child abuse Overuse injuries Leg length discrepancy |
| Infectious Septic arthritis Osteomyelitis Lyme disease Psoas abscess Diskitis |
| Inflammatory Transient synovitis Juvenile rheumatoid arthritis Ankylosing spondylitis Reiter syndrome Lupus |
| Vascular Legg-Calve-Perthes disease Osteonecrosis Hemoglobinopathies (sickle cell disease) |
| Neoplastic Leukemia, lymphoma Malignant/lytic tumors (Ewing sarcoma, osteogenic sarcoma, etc.) |
| Metabolic Rickets Hyperparathyroidism |
| Neuromuscular Muscular dystrophy Cerebral palsy Peripheral neuropathy |
Helpful tips for your initial assessment
Many textbook authors have described some causes of limp as “painless.” However, truly painless limp is rare, seldom acute, and usually the result of mechanical or neuromuscular disorders.1 A more likely explanation for acute “painless” limp is that a young child with pain is unable to express pain or accurately identify its location. Further, the child may instinctively avoid painful positions or movements and, thus, may present only with decreased movement of an extremity or refusal to bear weight.3
With a child who has knee pain, remember the pediatrics maxim: “Knee pain equals hip pain,”3 underscoring the diagnostic difficulty with limp.
Also bear in mind that children of different ages tend to have different etiologies of limp (TABLE 2). For example, septic arthritis, osteomyelitis, and transient synovitis occur more commonly in children under 10 years. Legg-Calve-Perthes disease and leukemia are more common in children between the ages of 4 and 10. Slipped capital femoral epiphysis (SCFE) is more common in boys over the age of 11.
TABLE 2
Common causes of limp according to child’s age1
| < 3 years | 3-10 years | 11-18 years |
|---|---|---|
| Foreign body | Legg-Calve-Perthes disease | Juvenile arthritis |
| Osteomyelitis | Osteomyelitis | Slipped capital femoral epiphysis |
| Septic arthritis | Septic arthritis | Trauma (physeal fracture) |
| Toddler’s fracture | Transient synovitis | Tumor |
| Transient synovitis | Trauma (physeal fracture) | |
| Tumor | Tumor |
Fracture
Fracture is a possibility across all age ranges, necessitating radiographs if suspected. Beyond detecting fractures, x-ray films can identify bony changes associated with disease (eg, Legg-Calve-Perthes disease, SCFE). Radiographs can also identify a clinically significant joint effusion at the hip.4 However, x-ray results may be falsely negative for some fracture types.
Salter-Harris Type I fractures are transverse fractures through the growth plate with epiphyseal separation from the metaphysis.5 Typical findings are a history of trauma and point tenderness over the epiphyseal plate. Type I fractures are radiographically occult, making the injury easy to mistake as a sprain. Nonetheless, growth plate injuries are common in children, requiring immobilization.
Toddler’s fracture was first described as a spiral, oblique undisplaced fracture of the distal tibial shaft in children from 9 months to 3 years of age.6 It results from a rotational or twisting force through the tibia while the leg rotates internally on a planted foot.7,8 This is the most common tibial fracture in infants and young children.9 The incidence has been reported as 0.6 to 2.5 per 1000 pediatric visits.10 Accurate diagnosis is important because current treatment recommendations suggest a long leg cast for 3 to 5 weeks, followed by a short leg cast for a total of 6 weeks.11
Despite being the most common tibial fracture, toddler’s fracture is easily missed. Initial radiographs are only 53% sensitive.7,10 This implies that nearly 50% of children with tibial fracture will have an initially negative x-ray result. However, nearly 94% of children with a confirmed toddler’s fracture have been unable to bear weight.12 Evidence suggests that despite negative radiographs, patients with point tenderness over the tibia and an inability to bear weight should be treated for presumed toddler’s fracture.12
Another confusing aspect of toddler’s fracture is that the causative injury is often considered insignificant by parents—eg, tripping, falling from a modest height, or a twisting motion.7,8 These events may occur countless times during the average day of a toddler. Often parents do not witness the injury and are unable to describe the mechanism of injury.7
When to suspect child abuse. When a child presents with fracture after an unwitnessed trauma and the story does not match the injury pattern, consider child abuse. With tibial fractures, the location of the fracture can help distinguish a result of abuse from a toddler’s fracture. Toddler’s fracture is classically described as a distal tibial fracture. In contrast, a midshaft tibial fracture often suggests child abuse.8,13 In a small retrospective study of 37 children diagnosed with toddler’s fracture, 4 midshaft tibial fractures were found.8 Child abuse was confirmed in 2 of these cases.8 However, other authors, including Dr. Dunbar in his sentinel article,6 assert that toddler’s fracture may occasionally extend into the midshaft of the tibia. Consequently, a midshaft tibial fracture is not pathognomonic for child abuse. But the diagnosis should be considered. Perform a careful examination for other signs of abuse or neglect, and do not hesitate to report suspected child abuse to the proper local and state authorities.14
Transient synovitis vs septic arthritis
A child who limps or refuses to bear weight on a limb often has associated symptoms of acute illness. In these cases, or when radiographs have ruled out apparent abnormalities such as Legg-Calve-Perthes disease, SCFE, and fracture, consider septic arthritis or transient synovitis (FIGURE). Both may present with limp and fever as well as pain, decreased range of motion, bone tenderness, swelling, and warmth.15
Transient synovitis is the most common cause of hip pain in children up to 10 years of age, with a 3% risk of occurrence through childhood.16,17 Its cause is unclear, but many experts have proposed a viral agent.17 Transient synovitis universally resolves without sequelae in 1 to 2 weeks. Therefore, prescribe rest and nonsteroidal anti-inflammatory drugs (NSAIDs) for symptomatic relief, and reassure parents.16
Septic arthritis, although often similar in presentation to transient synovitis, requires hospitalization, operative drainage, and parenteral antibiotics.18 A delay in diagnosis is associated with poor outcome, including osteonecrosis, growth arrest, permanent loss of joint function, and sepsis.3,18
Several studies have shown children with septic arthritis usually appear more acutely ill than those with transient synovitis.4,18-21 They are described as toxic-appearing, and have leukocytosis, a high erythrocyte sedimentation rate (ESR), and a high fever.19 However, no single marker or specific laboratory value consistently identifies septic arthritis. Many studies have been performed in an effort to identify a collection of factors, or an algorithm, that can predict the probability of septic arthritis.
Fever, an elevated ESR, and leukocytosis are independent multivariate clinical predictors for septic arthritis. The prediction algorithm published by Jung et al is the only study to have included C-reactive protein (CRP) as a predictive factor,4 which happens to be an excellent independent predictor of septic arthritis. Specifically, with a normal CRP <1 mg/dL, the probability of a patientnot having septic arthritis is 87%.22
While no predictive algorithm has been conclusively validated, the fact that the same clinical and laboratory predictors are consistently identified can be useful. Simply, if a patient presents with joint pain and 2 or more of the 4 predictors, septic arthritis must be fully evaluated. The presence of 2 of 4 predictors suggests a risk of septic arthritis between 10% and 40%.4,18,20 A single predictor is associated with a risk of 1% to 10%.4,18,20 Yet, you must interpret these clinical predictors in light of the full clinical picture, as septic arthritis is still possible in patients with only 1 predictor. Such possibilities require cautious management and close follow-up.
With 2 of 4 predictors present, suspect septic arthritis and order an ultrasound of the affected joint. If effusion is present, aspirate the joint. Some authors suggest that all patients with hip pain should undergo ultrasound, and that those with a joint effusion should undergo aspiration.15 However, joint aspiration, particularly of the hip, can be associated with multiple complications and should be avoided if possible.22 Effusion is also possible with transient synovitis and noninfectious causes of joint pain, but the aspirate will have a negative culture and normal gram stain findings. Ultrasound has been shown to be 100% accurate in predicting the presence of effusion.23
FIGURE
Diagnostic algorithm for pediatric limp3,4,6,8-12,15
CRP, C-reactive protein; ESR, erythrocyte sedimentation rate; MRI, magnetic resonance imaging; NSAIDs, nonsteroidal anti-inflammatory drugs; WBCs, white blood cells.
How the opening case resolved
The boy avoided weight-bearing on the affected leg, but had no focal bone tenderness. Moving the hip, but not the knee, reproduced pain. Radiographs were negative for fracture or changes typical of Legg-Calve-Perthes disease. He was afebrile in the office, but the mother described a fever at home. The child appeared ill, but stable. We decided to obtain a blood sample.
Results for CRP, ESR, and white blood cell count were normal. With this information, we reassured the mother that the diagnosis was likely transient synovitis. We advised a weight-appropriate dose of ibuprofen and scheduled a follow-up appointment for 2 days later.
CORRESPONDENCE John Whiteside, MD, St. Mary’s Family Medicine Residency, 1160 Patterson Road, Grand Junction, CO 81506; [email protected]
1. Abbassian A. The limping child: a clinical approach to diagnosis. Br J Hosp Med. 2007;68:246-250.
2. Leung AK, Lemay JF. The limping child. J Ped Health Care. 2004;18:219-223.
3. Frick SL. Evaluation of the child who has hip pain. Orthop Clin North Am. 2006;37:133-140.
4. Jung ST, Rowe SM, Moon ES, et al. Significance of laboratory and radiologic findings for differentiating between septic arthritis and transient synovitis of the hip. J Pediatr Orthop. 2003;23:368-372.
5. Brown JH, DeLuca SA. Growth plate injuries: Salter-Harris classification. Am Fam Physician. 1992;46:1180-1184.
6. Dunbar JS, Owen HF, Nogrady MB, et al. Obscure tibial fracture of infants–the toddler’s fracture. J Can Assoc Radiol. 1964;15:136-144.
7. Miller JH, Sanderson RA. Scintigraphy of toddler’s fracture. J Nucl Med. 1988;29:2001-2003.
8. Tenenbein M, Reed MH, Black GB. The toddler’s fracture revisited. Am J Emerg Med. 1990;8:208-211.
9. Tschoepe EJ, John SD, Swischuk LE. Tibial fractures in infants and children: emphasis on subtle injuries. Emerg Radiol. 1998;5:245-252.
10. Clancy J, Pieterse J, Roberston P, et al. Toddler’s fracture. J Accid Emerg Med. 1996;13:366-367.
11. Wheeless CR. Cast treatment of tibial fractures. In:Wheeless’ Textbook of Orthopaedics. 2011. Available at:http://www.wheelessonline.com/ortho/cast_treatment_of_tibial_fractures. Accessed March 11, 2011.
12. Halsey MF, Finzel KC, Carrion WV, et al. Toddler’s fracture: presumptive diagnosis and treatment. J Pediatr Orthop. 2001;21:152-156.
13. Mellick LB, Milker L, Egsieker E. Childhood accidental spiral tibial (CAST) fractures. Ped Emerg Care. 1999;15:307-309.
14. Jenny C. Committee on Child Abuse and Neglect. Evaluating infants and young children with multiple fractures. Pediatrics. 2006;118:1299-1303.
15. Dabney KW, Lipton G. Evaluation of limp in children. Curr Opin Pediatr. 1995;7:88-94.
16. Sherry DD. Limb pain in childhood. Pediatr Rev. 1990;12:39-46.
17. Do TT. Transient synovitis as a cause of painful limps in children. Curr Opin Pediatr. 2000;12:48-51.
18. Kocher MS, Zurakowski D, Kasser JR. Differentiating between septic arthritis and transient synovitis of the hip in children: an evidence-based clinical prediction algorithm. J Bone Joint Surg Am. 1999;81:1662-1670.
19. Luhmann SJ, Jones A, Schoolman M, et al. Differentiation between septic arthritis and transient synovitis of the hip in children with clinical prediction algorithms. J Bone Joint Surg Am. 2004;86-A:956-962.
20. Kocher MS, Mandiga R, Zurakowski D, et al. Validation of a clinical prediction rule for the differentiation between septic arthritis and transient synovitis of the hip in children. J Bone Joint Surg Am. 2004;86-A:1629-1635.
21. Delaney RA, Lenehan B, O’Sullivan L, et al. The limping child: an algorithm to outrule musculoskeletal sepsis. Ir J Med Sci. 2007;176:181-187.
22. Levine MJ, McGuire KJ, McGowan KL, et al. Assessment of the test characteristics of C-reactive protein for septic arthritis in children. J Pediatr Orthop. 2003;23:373-377.
23. Alexander JE, Seibert JJ, Glasier CM, et al. High-resolution hip ultrasound in the limping child. J Clin Ultrasound. 1989;17:19-24.
1. Abbassian A. The limping child: a clinical approach to diagnosis. Br J Hosp Med. 2007;68:246-250.
2. Leung AK, Lemay JF. The limping child. J Ped Health Care. 2004;18:219-223.
3. Frick SL. Evaluation of the child who has hip pain. Orthop Clin North Am. 2006;37:133-140.
4. Jung ST, Rowe SM, Moon ES, et al. Significance of laboratory and radiologic findings for differentiating between septic arthritis and transient synovitis of the hip. J Pediatr Orthop. 2003;23:368-372.
5. Brown JH, DeLuca SA. Growth plate injuries: Salter-Harris classification. Am Fam Physician. 1992;46:1180-1184.
6. Dunbar JS, Owen HF, Nogrady MB, et al. Obscure tibial fracture of infants–the toddler’s fracture. J Can Assoc Radiol. 1964;15:136-144.
7. Miller JH, Sanderson RA. Scintigraphy of toddler’s fracture. J Nucl Med. 1988;29:2001-2003.
8. Tenenbein M, Reed MH, Black GB. The toddler’s fracture revisited. Am J Emerg Med. 1990;8:208-211.
9. Tschoepe EJ, John SD, Swischuk LE. Tibial fractures in infants and children: emphasis on subtle injuries. Emerg Radiol. 1998;5:245-252.
10. Clancy J, Pieterse J, Roberston P, et al. Toddler’s fracture. J Accid Emerg Med. 1996;13:366-367.
11. Wheeless CR. Cast treatment of tibial fractures. In:Wheeless’ Textbook of Orthopaedics. 2011. Available at:http://www.wheelessonline.com/ortho/cast_treatment_of_tibial_fractures. Accessed March 11, 2011.
12. Halsey MF, Finzel KC, Carrion WV, et al. Toddler’s fracture: presumptive diagnosis and treatment. J Pediatr Orthop. 2001;21:152-156.
13. Mellick LB, Milker L, Egsieker E. Childhood accidental spiral tibial (CAST) fractures. Ped Emerg Care. 1999;15:307-309.
14. Jenny C. Committee on Child Abuse and Neglect. Evaluating infants and young children with multiple fractures. Pediatrics. 2006;118:1299-1303.
15. Dabney KW, Lipton G. Evaluation of limp in children. Curr Opin Pediatr. 1995;7:88-94.
16. Sherry DD. Limb pain in childhood. Pediatr Rev. 1990;12:39-46.
17. Do TT. Transient synovitis as a cause of painful limps in children. Curr Opin Pediatr. 2000;12:48-51.
18. Kocher MS, Zurakowski D, Kasser JR. Differentiating between septic arthritis and transient synovitis of the hip in children: an evidence-based clinical prediction algorithm. J Bone Joint Surg Am. 1999;81:1662-1670.
19. Luhmann SJ, Jones A, Schoolman M, et al. Differentiation between septic arthritis and transient synovitis of the hip in children with clinical prediction algorithms. J Bone Joint Surg Am. 2004;86-A:956-962.
20. Kocher MS, Mandiga R, Zurakowski D, et al. Validation of a clinical prediction rule for the differentiation between septic arthritis and transient synovitis of the hip in children. J Bone Joint Surg Am. 2004;86-A:1629-1635.
21. Delaney RA, Lenehan B, O’Sullivan L, et al. The limping child: an algorithm to outrule musculoskeletal sepsis. Ir J Med Sci. 2007;176:181-187.
22. Levine MJ, McGuire KJ, McGowan KL, et al. Assessment of the test characteristics of C-reactive protein for septic arthritis in children. J Pediatr Orthop. 2003;23:373-377.
23. Alexander JE, Seibert JJ, Glasier CM, et al. High-resolution hip ultrasound in the limping child. J Clin Ultrasound. 1989;17:19-24.
Statin neuropathy?
It took 13 years before an 82-year-old patient learned what had caused the pain and tingling in his feet that he’d been living with all those years.
In 1996 he had a coronary stent insertion, and after the procedure, began taking a beta-blocker and atorvastatin. He subsequently noticed a sensory change in his toes bilaterally. This slowly progressed to paresthesia in the anterior segments of both feet on the plantar and dorsal surfaces.
A nerve conduction study (TABLE) confirmed the presence of a sensorimotor polyneuropathy, despite the fact that he did not have diabetes, or any other condition known to predispose him to polyneuropathy. The patient’s left sural peak latency and amplitude, a measure of sensory nerve action potential (SNAP), was absent. The right sural SNAP demonstrated a mild decrease of the amplitude with a normal distal latency. The left peroneal F wave response (a measure of nerve conduction velocity) was within the upper limits of normal. The left tibial F wave response was normal. The left peroneal and left tibial CMAPs (compound muscle action potentials) were normal.
A nerve biopsy was not considered for this patient because its main use is in the identification of specific lesions that are generally lacking in acquired, distal, symmetrical sensory neuropathy. (Plus, biopsy gives no more information than electrophysiological tests.)1
TABLE
A look at the patient’s nerve conduction results
| Nerve and site | Peak latency (ms) | Amplitude (mV) | Segment | Latency difference (ms) | Distance (mm) | Conduction velocity (m/s) |
|---|---|---|---|---|---|---|
| Sensory nerve conduction | ||||||
| Sural nerve (left) Lower leg | 0.0 | 0.0 | N/A | N/A | N/A | |
| Sural nerve (right) Lower leg | 4.0 | 2 | N/A | N/A | N/A | |
| Motor nerve conduction | ||||||
| Peroneal nerve (left) Ankle | 4.3 | 1.9 | N/A | N/A | N/A | |
| Fibular head | 13.2 | 1.6 | Ankle-fibular head | 8.9 | 358 | 40 |
| Knee | 16.2 | 1.5 | Fibular head-knee | 3.0 | 115 | 38 |
| Tibial nerve (left) Ankle | 4.2 | 2.9 | N/A | N/A | N/A | |
| Popliteal fossa | 15.4 | 2.6 | Ankle-popliteal fossa | 11.2 | 450 | 40 |
| ms, millisecond; m/s, meters/second; mV, millivolt; N/A, not applicable. | ||||||
Connecting the dots years later
Neither the patient’s cardiologist, nor his general physician, was aware of any connection between statins and neuropathy, but the patient stopped taking the drug in 2003. And while the neuropathy never went away, it did subside slightly to a fairly constant level.
In August 2009, because of suboptimal levels of low-density lipoprotein (LDL), high-density lipoprotein (HDL), and C-reactive protein, his cardiologist prescribed simvastatin 5 mg daily.
On the third day, the patient experienced a marked increase of the neuropathy, which extended above his ankles. Cutaneous sensory loss became more extensive and pronounced. He stopped the statin that day, but the paresthesia did not lessen. In addition, he developed intermittent pins and needles in both hands and some instability in his gait. To date, there has been no improvement in his symptoms. Nerve conduction studies were not repeated.
Discussion: The various causes of neuropathy
In 2003, this journal published a question, “Do statins cause myopathy?”2 The item concluded that if they did, the risk was very low, although isolated case reports suggested a myopathy risk for all statins, ranging from benign myalgia to fatal rhabdomyolysis.
It is now widely acknowledged that statins can cause myopathy in as many as 10% of patients taking these drugs.3
The involvement of peripheral nerves bilaterally, usually affecting distal axons of the feet and legs, is the most common form of polyneuropathy and its presentation generally excludes consideration of other forms of neuropathy, such as the mononeuropathies and neuritis. Affected nerves may be sensory, motor, or autonomic.
Symptoms include all varieties of paresthesia, sensory loss, muscle weakness, and pain. The most common cause is diabetes mellitus, which must be the first condition to be excluded. Other conditions, such as vitamin deficiencies, have also been linked with this complication.
Laboratory work-up, aside from blood glucose testing for diabetes, should include routine complete blood count and SMA-12, as well as thyroid profile and vitamin deficiency status (particularly vitamins B12 and B1).
Is a medication—perhaps a statin— to blame?
Numerous drugs are known to be associated with neuropathy.4 These include chemotherapy agents (cisplatin, taxoids), certain antibiotics, nucleoside analogs, dapsone, metronidazole, and certain cardiovascular drugs (amiodarone, hydralazine, statins).4 Recent work has indicated that simvastatin inhibits central nervous system remyelination by blocking progenitor cell differentiation.5 By extension, it probably inhibits progenitor cells in the peripheral nervous system.
The possibility of an association between statins and peripheral neuropathy has expanded from several case reports to a population-based study involving 465,000 subjects.6 More recently, a review of the literature7 concluded that exposure to statins may increase the risk of polyneuropathy and that statins should be considered the cause when other etiologies have been excluded. The authors suggested that the incidence of peripheral neuropathy due to statins is approximately 1 person/14,000 person-years of treatment.
An exposure, a “break,” and another exposure
The reappearance or aggravation of symptoms after cessation of statin therapy and subsequent second exposure has been described in the literature.8 In the case described here, the time between re-exposure and symptoms was suggestive of a T-cell-mediated hypersensitivity reaction. It has been proposed that tumor necrosis factor (TNF)-alpha released by T cells may contribute to the pathogenesis of demyelinating neuropathy.9
Managing this patient’s lipid levels going forward
The patient described in this report is now receiving ezetimibe 10 mg daily, which reduces the absorption of cholesterol from the diet, and niacin 2 g daily, which he can tolerate. His most recent fasting lipid panel showed the following results: cholesterol, 171 mg/dL; LDL cholesterol, calculated, 113 mg/dL; HDL cholesterol, 37 mg/dL; triglycerides, 106 mg/dL; and non-HDL cholesterol, 134 mg/dL.
Controlling the patient’s pain was another matter. Drugs commonly used for paresthesia and pain (including opiates) did not provide relief. Pregabalin (Lyrica) also had little effect. Transcutaneous electrical nerve stimulation did not perceptibly lessen his symptoms, and was also discontinued.
At the present time, this patient is not on any specific treatment for his neuropathy.
CORRESPONDENCE
Walter F. Coulson, MD, Department of Pathology, UCLA, CHS, Los Angeles, CA 90095-1732; [email protected]
1. Said G. Indications and usefulness of nerve biopsy. Arch Neurol. 2002;59:1532-1535.
2. Daugird AJ, Crowell K. Do statins cause myopathy? J Fam Pract. 2003;52:973-976.
3. Joy TR, Hegele RA. Narrative review: Statin-related myopathy. Ann Intern Med. 2009;150:858-868.
4. Weimer LH. Medication-induced peripheral neuropathy. Curr Neurosci Rep. 2003;3:86-92.
5. Miron VE, Zehntner SP, Kuhlmann T, et al. Statin therapy inhibits remyelination in the central nervous system. Am J Pathol. 2009;174:1880-1890.
6. Gaist D, Jeppesen U, Andersen M, et al. Statins and risk of polyneuropathy: a case-control study. Neurology. 2002;58:1333-1337.
7. Chong PH, Boskovich A, Stevkovic N, et al. Statin-associated peripheral neuropathy: review of the literature. Pharmacotherapy. 2004;24:1194-1203.
8. Phan T, McLeod JG, Pollard JD, et al. Peripheral neuropathy associated with simvastatin. J Neurol Neurosurg Psychiatry. 1995;58:625-628.
9. Stübgen JP. Tumor necrosis factor–alpha antagonists and neuropathy. Muscle Nerve. 2008;37:281-292.
It took 13 years before an 82-year-old patient learned what had caused the pain and tingling in his feet that he’d been living with all those years.
In 1996 he had a coronary stent insertion, and after the procedure, began taking a beta-blocker and atorvastatin. He subsequently noticed a sensory change in his toes bilaterally. This slowly progressed to paresthesia in the anterior segments of both feet on the plantar and dorsal surfaces.
A nerve conduction study (TABLE) confirmed the presence of a sensorimotor polyneuropathy, despite the fact that he did not have diabetes, or any other condition known to predispose him to polyneuropathy. The patient’s left sural peak latency and amplitude, a measure of sensory nerve action potential (SNAP), was absent. The right sural SNAP demonstrated a mild decrease of the amplitude with a normal distal latency. The left peroneal F wave response (a measure of nerve conduction velocity) was within the upper limits of normal. The left tibial F wave response was normal. The left peroneal and left tibial CMAPs (compound muscle action potentials) were normal.
A nerve biopsy was not considered for this patient because its main use is in the identification of specific lesions that are generally lacking in acquired, distal, symmetrical sensory neuropathy. (Plus, biopsy gives no more information than electrophysiological tests.)1
TABLE
A look at the patient’s nerve conduction results
| Nerve and site | Peak latency (ms) | Amplitude (mV) | Segment | Latency difference (ms) | Distance (mm) | Conduction velocity (m/s) |
|---|---|---|---|---|---|---|
| Sensory nerve conduction | ||||||
| Sural nerve (left) Lower leg | 0.0 | 0.0 | N/A | N/A | N/A | |
| Sural nerve (right) Lower leg | 4.0 | 2 | N/A | N/A | N/A | |
| Motor nerve conduction | ||||||
| Peroneal nerve (left) Ankle | 4.3 | 1.9 | N/A | N/A | N/A | |
| Fibular head | 13.2 | 1.6 | Ankle-fibular head | 8.9 | 358 | 40 |
| Knee | 16.2 | 1.5 | Fibular head-knee | 3.0 | 115 | 38 |
| Tibial nerve (left) Ankle | 4.2 | 2.9 | N/A | N/A | N/A | |
| Popliteal fossa | 15.4 | 2.6 | Ankle-popliteal fossa | 11.2 | 450 | 40 |
| ms, millisecond; m/s, meters/second; mV, millivolt; N/A, not applicable. | ||||||
Connecting the dots years later
Neither the patient’s cardiologist, nor his general physician, was aware of any connection between statins and neuropathy, but the patient stopped taking the drug in 2003. And while the neuropathy never went away, it did subside slightly to a fairly constant level.
In August 2009, because of suboptimal levels of low-density lipoprotein (LDL), high-density lipoprotein (HDL), and C-reactive protein, his cardiologist prescribed simvastatin 5 mg daily.
On the third day, the patient experienced a marked increase of the neuropathy, which extended above his ankles. Cutaneous sensory loss became more extensive and pronounced. He stopped the statin that day, but the paresthesia did not lessen. In addition, he developed intermittent pins and needles in both hands and some instability in his gait. To date, there has been no improvement in his symptoms. Nerve conduction studies were not repeated.
Discussion: The various causes of neuropathy
In 2003, this journal published a question, “Do statins cause myopathy?”2 The item concluded that if they did, the risk was very low, although isolated case reports suggested a myopathy risk for all statins, ranging from benign myalgia to fatal rhabdomyolysis.
It is now widely acknowledged that statins can cause myopathy in as many as 10% of patients taking these drugs.3
The involvement of peripheral nerves bilaterally, usually affecting distal axons of the feet and legs, is the most common form of polyneuropathy and its presentation generally excludes consideration of other forms of neuropathy, such as the mononeuropathies and neuritis. Affected nerves may be sensory, motor, or autonomic.
Symptoms include all varieties of paresthesia, sensory loss, muscle weakness, and pain. The most common cause is diabetes mellitus, which must be the first condition to be excluded. Other conditions, such as vitamin deficiencies, have also been linked with this complication.
Laboratory work-up, aside from blood glucose testing for diabetes, should include routine complete blood count and SMA-12, as well as thyroid profile and vitamin deficiency status (particularly vitamins B12 and B1).
Is a medication—perhaps a statin— to blame?
Numerous drugs are known to be associated with neuropathy.4 These include chemotherapy agents (cisplatin, taxoids), certain antibiotics, nucleoside analogs, dapsone, metronidazole, and certain cardiovascular drugs (amiodarone, hydralazine, statins).4 Recent work has indicated that simvastatin inhibits central nervous system remyelination by blocking progenitor cell differentiation.5 By extension, it probably inhibits progenitor cells in the peripheral nervous system.
The possibility of an association between statins and peripheral neuropathy has expanded from several case reports to a population-based study involving 465,000 subjects.6 More recently, a review of the literature7 concluded that exposure to statins may increase the risk of polyneuropathy and that statins should be considered the cause when other etiologies have been excluded. The authors suggested that the incidence of peripheral neuropathy due to statins is approximately 1 person/14,000 person-years of treatment.
An exposure, a “break,” and another exposure
The reappearance or aggravation of symptoms after cessation of statin therapy and subsequent second exposure has been described in the literature.8 In the case described here, the time between re-exposure and symptoms was suggestive of a T-cell-mediated hypersensitivity reaction. It has been proposed that tumor necrosis factor (TNF)-alpha released by T cells may contribute to the pathogenesis of demyelinating neuropathy.9
Managing this patient’s lipid levels going forward
The patient described in this report is now receiving ezetimibe 10 mg daily, which reduces the absorption of cholesterol from the diet, and niacin 2 g daily, which he can tolerate. His most recent fasting lipid panel showed the following results: cholesterol, 171 mg/dL; LDL cholesterol, calculated, 113 mg/dL; HDL cholesterol, 37 mg/dL; triglycerides, 106 mg/dL; and non-HDL cholesterol, 134 mg/dL.
Controlling the patient’s pain was another matter. Drugs commonly used for paresthesia and pain (including opiates) did not provide relief. Pregabalin (Lyrica) also had little effect. Transcutaneous electrical nerve stimulation did not perceptibly lessen his symptoms, and was also discontinued.
At the present time, this patient is not on any specific treatment for his neuropathy.
CORRESPONDENCE
Walter F. Coulson, MD, Department of Pathology, UCLA, CHS, Los Angeles, CA 90095-1732; [email protected]
It took 13 years before an 82-year-old patient learned what had caused the pain and tingling in his feet that he’d been living with all those years.
In 1996 he had a coronary stent insertion, and after the procedure, began taking a beta-blocker and atorvastatin. He subsequently noticed a sensory change in his toes bilaterally. This slowly progressed to paresthesia in the anterior segments of both feet on the plantar and dorsal surfaces.
A nerve conduction study (TABLE) confirmed the presence of a sensorimotor polyneuropathy, despite the fact that he did not have diabetes, or any other condition known to predispose him to polyneuropathy. The patient’s left sural peak latency and amplitude, a measure of sensory nerve action potential (SNAP), was absent. The right sural SNAP demonstrated a mild decrease of the amplitude with a normal distal latency. The left peroneal F wave response (a measure of nerve conduction velocity) was within the upper limits of normal. The left tibial F wave response was normal. The left peroneal and left tibial CMAPs (compound muscle action potentials) were normal.
A nerve biopsy was not considered for this patient because its main use is in the identification of specific lesions that are generally lacking in acquired, distal, symmetrical sensory neuropathy. (Plus, biopsy gives no more information than electrophysiological tests.)1
TABLE
A look at the patient’s nerve conduction results
| Nerve and site | Peak latency (ms) | Amplitude (mV) | Segment | Latency difference (ms) | Distance (mm) | Conduction velocity (m/s) |
|---|---|---|---|---|---|---|
| Sensory nerve conduction | ||||||
| Sural nerve (left) Lower leg | 0.0 | 0.0 | N/A | N/A | N/A | |
| Sural nerve (right) Lower leg | 4.0 | 2 | N/A | N/A | N/A | |
| Motor nerve conduction | ||||||
| Peroneal nerve (left) Ankle | 4.3 | 1.9 | N/A | N/A | N/A | |
| Fibular head | 13.2 | 1.6 | Ankle-fibular head | 8.9 | 358 | 40 |
| Knee | 16.2 | 1.5 | Fibular head-knee | 3.0 | 115 | 38 |
| Tibial nerve (left) Ankle | 4.2 | 2.9 | N/A | N/A | N/A | |
| Popliteal fossa | 15.4 | 2.6 | Ankle-popliteal fossa | 11.2 | 450 | 40 |
| ms, millisecond; m/s, meters/second; mV, millivolt; N/A, not applicable. | ||||||
Connecting the dots years later
Neither the patient’s cardiologist, nor his general physician, was aware of any connection between statins and neuropathy, but the patient stopped taking the drug in 2003. And while the neuropathy never went away, it did subside slightly to a fairly constant level.
In August 2009, because of suboptimal levels of low-density lipoprotein (LDL), high-density lipoprotein (HDL), and C-reactive protein, his cardiologist prescribed simvastatin 5 mg daily.
On the third day, the patient experienced a marked increase of the neuropathy, which extended above his ankles. Cutaneous sensory loss became more extensive and pronounced. He stopped the statin that day, but the paresthesia did not lessen. In addition, he developed intermittent pins and needles in both hands and some instability in his gait. To date, there has been no improvement in his symptoms. Nerve conduction studies were not repeated.
Discussion: The various causes of neuropathy
In 2003, this journal published a question, “Do statins cause myopathy?”2 The item concluded that if they did, the risk was very low, although isolated case reports suggested a myopathy risk for all statins, ranging from benign myalgia to fatal rhabdomyolysis.
It is now widely acknowledged that statins can cause myopathy in as many as 10% of patients taking these drugs.3
The involvement of peripheral nerves bilaterally, usually affecting distal axons of the feet and legs, is the most common form of polyneuropathy and its presentation generally excludes consideration of other forms of neuropathy, such as the mononeuropathies and neuritis. Affected nerves may be sensory, motor, or autonomic.
Symptoms include all varieties of paresthesia, sensory loss, muscle weakness, and pain. The most common cause is diabetes mellitus, which must be the first condition to be excluded. Other conditions, such as vitamin deficiencies, have also been linked with this complication.
Laboratory work-up, aside from blood glucose testing for diabetes, should include routine complete blood count and SMA-12, as well as thyroid profile and vitamin deficiency status (particularly vitamins B12 and B1).
Is a medication—perhaps a statin— to blame?
Numerous drugs are known to be associated with neuropathy.4 These include chemotherapy agents (cisplatin, taxoids), certain antibiotics, nucleoside analogs, dapsone, metronidazole, and certain cardiovascular drugs (amiodarone, hydralazine, statins).4 Recent work has indicated that simvastatin inhibits central nervous system remyelination by blocking progenitor cell differentiation.5 By extension, it probably inhibits progenitor cells in the peripheral nervous system.
The possibility of an association between statins and peripheral neuropathy has expanded from several case reports to a population-based study involving 465,000 subjects.6 More recently, a review of the literature7 concluded that exposure to statins may increase the risk of polyneuropathy and that statins should be considered the cause when other etiologies have been excluded. The authors suggested that the incidence of peripheral neuropathy due to statins is approximately 1 person/14,000 person-years of treatment.
An exposure, a “break,” and another exposure
The reappearance or aggravation of symptoms after cessation of statin therapy and subsequent second exposure has been described in the literature.8 In the case described here, the time between re-exposure and symptoms was suggestive of a T-cell-mediated hypersensitivity reaction. It has been proposed that tumor necrosis factor (TNF)-alpha released by T cells may contribute to the pathogenesis of demyelinating neuropathy.9
Managing this patient’s lipid levels going forward
The patient described in this report is now receiving ezetimibe 10 mg daily, which reduces the absorption of cholesterol from the diet, and niacin 2 g daily, which he can tolerate. His most recent fasting lipid panel showed the following results: cholesterol, 171 mg/dL; LDL cholesterol, calculated, 113 mg/dL; HDL cholesterol, 37 mg/dL; triglycerides, 106 mg/dL; and non-HDL cholesterol, 134 mg/dL.
Controlling the patient’s pain was another matter. Drugs commonly used for paresthesia and pain (including opiates) did not provide relief. Pregabalin (Lyrica) also had little effect. Transcutaneous electrical nerve stimulation did not perceptibly lessen his symptoms, and was also discontinued.
At the present time, this patient is not on any specific treatment for his neuropathy.
CORRESPONDENCE
Walter F. Coulson, MD, Department of Pathology, UCLA, CHS, Los Angeles, CA 90095-1732; [email protected]
1. Said G. Indications and usefulness of nerve biopsy. Arch Neurol. 2002;59:1532-1535.
2. Daugird AJ, Crowell K. Do statins cause myopathy? J Fam Pract. 2003;52:973-976.
3. Joy TR, Hegele RA. Narrative review: Statin-related myopathy. Ann Intern Med. 2009;150:858-868.
4. Weimer LH. Medication-induced peripheral neuropathy. Curr Neurosci Rep. 2003;3:86-92.
5. Miron VE, Zehntner SP, Kuhlmann T, et al. Statin therapy inhibits remyelination in the central nervous system. Am J Pathol. 2009;174:1880-1890.
6. Gaist D, Jeppesen U, Andersen M, et al. Statins and risk of polyneuropathy: a case-control study. Neurology. 2002;58:1333-1337.
7. Chong PH, Boskovich A, Stevkovic N, et al. Statin-associated peripheral neuropathy: review of the literature. Pharmacotherapy. 2004;24:1194-1203.
8. Phan T, McLeod JG, Pollard JD, et al. Peripheral neuropathy associated with simvastatin. J Neurol Neurosurg Psychiatry. 1995;58:625-628.
9. Stübgen JP. Tumor necrosis factor–alpha antagonists and neuropathy. Muscle Nerve. 2008;37:281-292.
1. Said G. Indications and usefulness of nerve biopsy. Arch Neurol. 2002;59:1532-1535.
2. Daugird AJ, Crowell K. Do statins cause myopathy? J Fam Pract. 2003;52:973-976.
3. Joy TR, Hegele RA. Narrative review: Statin-related myopathy. Ann Intern Med. 2009;150:858-868.
4. Weimer LH. Medication-induced peripheral neuropathy. Curr Neurosci Rep. 2003;3:86-92.
5. Miron VE, Zehntner SP, Kuhlmann T, et al. Statin therapy inhibits remyelination in the central nervous system. Am J Pathol. 2009;174:1880-1890.
6. Gaist D, Jeppesen U, Andersen M, et al. Statins and risk of polyneuropathy: a case-control study. Neurology. 2002;58:1333-1337.
7. Chong PH, Boskovich A, Stevkovic N, et al. Statin-associated peripheral neuropathy: review of the literature. Pharmacotherapy. 2004;24:1194-1203.
8. Phan T, McLeod JG, Pollard JD, et al. Peripheral neuropathy associated with simvastatin. J Neurol Neurosurg Psychiatry. 1995;58:625-628.
9. Stübgen JP. Tumor necrosis factor–alpha antagonists and neuropathy. Muscle Nerve. 2008;37:281-292.
The Ever-Changing Laparoscopic Myomectomy
A successful laparoscopic myomectomy begins with the correct assessment of the size, number, and location of the myomata inside the uterus. In the past, I have recommended multiple techniques for evaluation, including hysteroscopy, two-dimensional (2-D) ultrasound (transvaginal, transabdominal), 3-D ultrasound (transvaginal, transabdominal), the 2-D saline infusion sonohysterogram (2-D SIS), the 3-D SIS, and magnetic resonance imaging (MRI).
At this juncture, because of improved diagnostic acumen, I now recommend MRI or saline infusion sonography. MRI (
In my estimation, the 3-D saline infusion sonogram is superior to 2-D evaluation. The ability to render a three-dimensional image – and thus manipulate the ability to visualize the saline infusion sonogram image further – enhances fibroid mapping.
Although the saline infusion sonohysterogram is far better for evaluating uterine leiomyomata than is the hysterosalpingogram, the technique does not allow evaluation of the fallopian tubes. Recently, I helped launch Femasys Inc.'s Femvue System (
This testing does not, however, diminish the importance of physician examination prior to surgery. Through the physical exam, the minimally invasive gynecologic surgeon is able to determine how large the uterus/leiomyomata complex is, relative to the patient's size, and therefore where ports should be placed, as well as the potential difficulty of surgery. If the surgeon considers the uterus/leiomyomata complex too large, or if anemia is noted, a gonadotropin-releasing hormone (GnRH) agonist can be given for 3 months to attempt shrinkage of the leiomyomata or to enable hemoglobin to rise (through the resultant amenorrhea) prior to surgery.
Laparoscopic Myomectomy
The laparoscopic surgery is scheduled in the proliferative phase of the cycle to avoid thickened endometrium. This is especially important in the case of removal of a type II submucosal leiomyomata or one that is impinging on the endometrial cavity.
On the day of surgery, prior to the laparoscopic myomectomy and after the patient has been placed into the dorsal lithotomy position and a Foley catheter has been placed in the bladder, hysteroscopy is performed to treat any abnormalities that are seen within the endometrial cavity. This may include hysteroscopic myomectomy on a leiomyomata previously believed to be located away from the endometrial cavity.
Once hysteroscopy has been completed, a uterine manipulator must be placed inside the uterine cavity. It is imperative to utilize a manipulator that can be placed deep enough into the cavity to enable anterior/posterior and lateral uterus flexion. I consider this function to be so important for the success of laparoscopic myomectomy that a surgical assistant, standing between the patient's legs, continues to manipulate the uterus throughout the duration of the procedure.
Generally, the 5-mm laparoscope is placed initially through the umbilicus, unless periumbilical adhesions are anticipated. In this latter case, I proceed to make a left-upper-quadrant incision. Lateral ports are then placed under direct visualization. These ports must be placed above and lateral to the uterus fibroid complex (See
To minimize blood loss, a dilute solution of vasopressin (30 U of vasopressin in 100 cc of normal saline) is placed in the myoma bed via an 18-gauge spinal needle placed percutaneously through a small skin nick. (See
If the myoma is pedunculated, on a broad base, the vasopressin should not be placed into the pedicle itself, as bleeding can be excessive; rather, the vasopressin is placed in the uterus around the pedicle. It is imperative to aspirate prior to injection of vasopressin in order to prevent inadvertent intravascular injection of the vasopressin.
If possible, to reduce the risk of adhesions, make an anterior incision in the uterus and try to remove as many fibroids through the single incision as possible. For years, my instrument of choice has been the curved blade of Ethicon Endo-Surgery Inc.'s Harmonic Scalpel. Harmonic energy allows excellent cutting with minimal tissue desiccation. Moreover, the curve of the blade allows easier dissection between the myoma and myometrium. When a posterior incision is required, I use a vertical incision to decrease risk of adhesion formation near the adnexa.
If multiple fibroids are removed, I place a #1 nylon suture with a Keith needle transcutaneously into the pelvis. The numerous leiomyomata are then strung on this suture to avoid losing a myoma in the abdomen or pelvis. (See
Although suturing in the “vertical zone” (with two ports placed on the same side of the pelvis) has become a popular technique, I continue to profess cross-table suturing. When the surgeon stands cephalad to the incisions, the repair is quite comfortable to perform. Furthermore, the ports can be placed higher on the abdomen to accommodate the very large uterus, and can be positioned more widely apart to improve triangulation.
I have always recommended multiple-layer closure of the uterus to minimize hematoma formation, and have advised skimming the myometrium rather than taking deep bites of tissue in order to minimize tissue destruction. When I began to perform laparoscopic myomectomy in earnest more than 20 years ago, closure of the uterine cavity was performed with Ethicon Inc.'s nonbraided PDS II 3-0 suture placed in an interrupted or mattress style using a “knot pusher.”
Even now, when the endometrial cavity is entered at the time of myomectomy, this is the technique I currently recommend, with the interrupted or mattress sutures placed immediately above the endometrium. During the past 15 years, I have advised repairing the uterus via a running-suture technique. After multiple layers are placed, the two suture ends are tied together via an intracorporeal suture technique. This has not only proved to be more efficient, but also allows the various layers to collapse upon themselves. Ultimately, the serosa is repaired via a baseball closure (suture placed in to out, in to out, and so on). (See
In my opinion, the recent introduction of barbed sutures has served as a monumental advance in our ability to repair the uterus in multiple layers. Both Covidien's V-Loc and Angiotech Pharmaceuticals Inc.'s Quill sutures do not have to be tied. Moreover, the barbs enable consistent tension on the suture line. In order to secure the suture from slipping, the Quill uses a bidirectional barb (See
My current barbed suture of choice is the 3-0 V-Loc, which is created from 2-0 suture. When a barbed suture is used, it is imperative that the physician “hide” the suture as much as possible and thus use a baseball closure; theoretically, the barbs could catch bowel or omentum, leading to adhesion formation.
To allow for a better cosmetic repair and to minimize the risk of postoperative hernia, I recommend utilizing a larger umbilical incision for tissue extraction – I use a 12-mm umbilical port – while maintaining other ports at 5 mm. At the conclusion of the uterine repair and after placement of an antiadhesive barrier (Ethicon Inc.'s Interceed), the umbilical port is removed. Large cervical dilators are then used to stretch the umbilical incision to allow direct placement of the 15-mm morcellator. Currently, I use Karl Storz Endoscopy America Inc.'s Storz Rotocut Morcellation System. This morcellator is reusable to decrease costs, and it has a beveled tip to enhance the “apple peel” shaving of the fibroid, a very durable blade to maximize cutting ability, and variable speed to enhance the morcellation procedure.
With this laparoscopic technique, I utilize laparotomy in fewer than 1% percent of more than 200 myomectomy cases per year, of which more than 30% involve fibroids greater than 8 cm and of which nearly 20% involve five or more fibroids.
Major complication rates continue to be fewer than 1% percent, and heterologous transfusions occur in fewer than 0.5% of cases.
More than 20 years after its inception, laparoscopic myomectomy continues to be an evolving procedure – one that, especially with current advancements, should become a more common technique in the armamentarium of the minimally invasive gynecologic surgeon.
Dr. Miller disclosed that he is a consultant for Covidien and Femasys Inc., and a consultant and speaker for Ethicon Endo-Surgery Inc.
Laparoscopic Myomectomy
In this month's installment of the Master Class in Gynecologic Surgery, we are taking an interesting twist and featuring the expertise of our own medical editor, Dr. Charles E. Miller, an internationally renowned expert in minimally invasive gynecologic surgery.
When Dr. Miller inaugurated this column more than 7 years ago with a feature on “Maximizing Myomectomy” (
In his opening Master Class feature, Dr. Miller detailed the advantages of laparoscopic myomectomy and shared some pearls he acquired from a retrospective study of almost 300 laparoscopic myomectomy patients whom he had managed. He advised us on patient selection, presurgery planning, port placement, equipment, and key components of surgical technique.
At this point, laparoscopic myomectomy is a procedure that Dr. Miller has been performing for more than 20 years. And as he tells us here, it is a procedure that is still evolving and one that – even more so than in the past – should become a more common technique in the armamentarium of the minimally invasive gynecologic surgeon.
Laparoscopic myomectomy is one of Dr. Miller's key research and practice concerns. In this Master Class, he gives us a valuable update. He explains how he has honed his selection of diagnostic tools for preoperative evaluation, and details how to minimize blood loss and the risk of adhesions and hematoma formation. He also provides some suturing pearls and weighs in on the role and use of recently introduced barbed sutures.
A successful laparoscopic myomectomy begins with the correct assessment of the size, number, and location of the myomata inside the uterus. In the past, I have recommended multiple techniques for evaluation, including hysteroscopy, two-dimensional (2-D) ultrasound (transvaginal, transabdominal), 3-D ultrasound (transvaginal, transabdominal), the 2-D saline infusion sonohysterogram (2-D SIS), the 3-D SIS, and magnetic resonance imaging (MRI).
At this juncture, because of improved diagnostic acumen, I now recommend MRI or saline infusion sonography. MRI (
In my estimation, the 3-D saline infusion sonogram is superior to 2-D evaluation. The ability to render a three-dimensional image – and thus manipulate the ability to visualize the saline infusion sonogram image further – enhances fibroid mapping.
Although the saline infusion sonohysterogram is far better for evaluating uterine leiomyomata than is the hysterosalpingogram, the technique does not allow evaluation of the fallopian tubes. Recently, I helped launch Femasys Inc.'s Femvue System (
This testing does not, however, diminish the importance of physician examination prior to surgery. Through the physical exam, the minimally invasive gynecologic surgeon is able to determine how large the uterus/leiomyomata complex is, relative to the patient's size, and therefore where ports should be placed, as well as the potential difficulty of surgery. If the surgeon considers the uterus/leiomyomata complex too large, or if anemia is noted, a gonadotropin-releasing hormone (GnRH) agonist can be given for 3 months to attempt shrinkage of the leiomyomata or to enable hemoglobin to rise (through the resultant amenorrhea) prior to surgery.
Laparoscopic Myomectomy
The laparoscopic surgery is scheduled in the proliferative phase of the cycle to avoid thickened endometrium. This is especially important in the case of removal of a type II submucosal leiomyomata or one that is impinging on the endometrial cavity.
On the day of surgery, prior to the laparoscopic myomectomy and after the patient has been placed into the dorsal lithotomy position and a Foley catheter has been placed in the bladder, hysteroscopy is performed to treat any abnormalities that are seen within the endometrial cavity. This may include hysteroscopic myomectomy on a leiomyomata previously believed to be located away from the endometrial cavity.
Once hysteroscopy has been completed, a uterine manipulator must be placed inside the uterine cavity. It is imperative to utilize a manipulator that can be placed deep enough into the cavity to enable anterior/posterior and lateral uterus flexion. I consider this function to be so important for the success of laparoscopic myomectomy that a surgical assistant, standing between the patient's legs, continues to manipulate the uterus throughout the duration of the procedure.
Generally, the 5-mm laparoscope is placed initially through the umbilicus, unless periumbilical adhesions are anticipated. In this latter case, I proceed to make a left-upper-quadrant incision. Lateral ports are then placed under direct visualization. These ports must be placed above and lateral to the uterus fibroid complex (See
To minimize blood loss, a dilute solution of vasopressin (30 U of vasopressin in 100 cc of normal saline) is placed in the myoma bed via an 18-gauge spinal needle placed percutaneously through a small skin nick. (See
If the myoma is pedunculated, on a broad base, the vasopressin should not be placed into the pedicle itself, as bleeding can be excessive; rather, the vasopressin is placed in the uterus around the pedicle. It is imperative to aspirate prior to injection of vasopressin in order to prevent inadvertent intravascular injection of the vasopressin.
If possible, to reduce the risk of adhesions, make an anterior incision in the uterus and try to remove as many fibroids through the single incision as possible. For years, my instrument of choice has been the curved blade of Ethicon Endo-Surgery Inc.'s Harmonic Scalpel. Harmonic energy allows excellent cutting with minimal tissue desiccation. Moreover, the curve of the blade allows easier dissection between the myoma and myometrium. When a posterior incision is required, I use a vertical incision to decrease risk of adhesion formation near the adnexa.
If multiple fibroids are removed, I place a #1 nylon suture with a Keith needle transcutaneously into the pelvis. The numerous leiomyomata are then strung on this suture to avoid losing a myoma in the abdomen or pelvis. (See
Although suturing in the “vertical zone” (with two ports placed on the same side of the pelvis) has become a popular technique, I continue to profess cross-table suturing. When the surgeon stands cephalad to the incisions, the repair is quite comfortable to perform. Furthermore, the ports can be placed higher on the abdomen to accommodate the very large uterus, and can be positioned more widely apart to improve triangulation.
I have always recommended multiple-layer closure of the uterus to minimize hematoma formation, and have advised skimming the myometrium rather than taking deep bites of tissue in order to minimize tissue destruction. When I began to perform laparoscopic myomectomy in earnest more than 20 years ago, closure of the uterine cavity was performed with Ethicon Inc.'s nonbraided PDS II 3-0 suture placed in an interrupted or mattress style using a “knot pusher.”
Even now, when the endometrial cavity is entered at the time of myomectomy, this is the technique I currently recommend, with the interrupted or mattress sutures placed immediately above the endometrium. During the past 15 years, I have advised repairing the uterus via a running-suture technique. After multiple layers are placed, the two suture ends are tied together via an intracorporeal suture technique. This has not only proved to be more efficient, but also allows the various layers to collapse upon themselves. Ultimately, the serosa is repaired via a baseball closure (suture placed in to out, in to out, and so on). (See
In my opinion, the recent introduction of barbed sutures has served as a monumental advance in our ability to repair the uterus in multiple layers. Both Covidien's V-Loc and Angiotech Pharmaceuticals Inc.'s Quill sutures do not have to be tied. Moreover, the barbs enable consistent tension on the suture line. In order to secure the suture from slipping, the Quill uses a bidirectional barb (See
My current barbed suture of choice is the 3-0 V-Loc, which is created from 2-0 suture. When a barbed suture is used, it is imperative that the physician “hide” the suture as much as possible and thus use a baseball closure; theoretically, the barbs could catch bowel or omentum, leading to adhesion formation.
To allow for a better cosmetic repair and to minimize the risk of postoperative hernia, I recommend utilizing a larger umbilical incision for tissue extraction – I use a 12-mm umbilical port – while maintaining other ports at 5 mm. At the conclusion of the uterine repair and after placement of an antiadhesive barrier (Ethicon Inc.'s Interceed), the umbilical port is removed. Large cervical dilators are then used to stretch the umbilical incision to allow direct placement of the 15-mm morcellator. Currently, I use Karl Storz Endoscopy America Inc.'s Storz Rotocut Morcellation System. This morcellator is reusable to decrease costs, and it has a beveled tip to enhance the “apple peel” shaving of the fibroid, a very durable blade to maximize cutting ability, and variable speed to enhance the morcellation procedure.
With this laparoscopic technique, I utilize laparotomy in fewer than 1% percent of more than 200 myomectomy cases per year, of which more than 30% involve fibroids greater than 8 cm and of which nearly 20% involve five or more fibroids.
Major complication rates continue to be fewer than 1% percent, and heterologous transfusions occur in fewer than 0.5% of cases.
More than 20 years after its inception, laparoscopic myomectomy continues to be an evolving procedure – one that, especially with current advancements, should become a more common technique in the armamentarium of the minimally invasive gynecologic surgeon.
Dr. Miller disclosed that he is a consultant for Covidien and Femasys Inc., and a consultant and speaker for Ethicon Endo-Surgery Inc.
Laparoscopic Myomectomy
In this month's installment of the Master Class in Gynecologic Surgery, we are taking an interesting twist and featuring the expertise of our own medical editor, Dr. Charles E. Miller, an internationally renowned expert in minimally invasive gynecologic surgery.
When Dr. Miller inaugurated this column more than 7 years ago with a feature on “Maximizing Myomectomy” (
In his opening Master Class feature, Dr. Miller detailed the advantages of laparoscopic myomectomy and shared some pearls he acquired from a retrospective study of almost 300 laparoscopic myomectomy patients whom he had managed. He advised us on patient selection, presurgery planning, port placement, equipment, and key components of surgical technique.
At this point, laparoscopic myomectomy is a procedure that Dr. Miller has been performing for more than 20 years. And as he tells us here, it is a procedure that is still evolving and one that – even more so than in the past – should become a more common technique in the armamentarium of the minimally invasive gynecologic surgeon.
Laparoscopic myomectomy is one of Dr. Miller's key research and practice concerns. In this Master Class, he gives us a valuable update. He explains how he has honed his selection of diagnostic tools for preoperative evaluation, and details how to minimize blood loss and the risk of adhesions and hematoma formation. He also provides some suturing pearls and weighs in on the role and use of recently introduced barbed sutures.
A successful laparoscopic myomectomy begins with the correct assessment of the size, number, and location of the myomata inside the uterus. In the past, I have recommended multiple techniques for evaluation, including hysteroscopy, two-dimensional (2-D) ultrasound (transvaginal, transabdominal), 3-D ultrasound (transvaginal, transabdominal), the 2-D saline infusion sonohysterogram (2-D SIS), the 3-D SIS, and magnetic resonance imaging (MRI).
At this juncture, because of improved diagnostic acumen, I now recommend MRI or saline infusion sonography. MRI (
In my estimation, the 3-D saline infusion sonogram is superior to 2-D evaluation. The ability to render a three-dimensional image – and thus manipulate the ability to visualize the saline infusion sonogram image further – enhances fibroid mapping.
Although the saline infusion sonohysterogram is far better for evaluating uterine leiomyomata than is the hysterosalpingogram, the technique does not allow evaluation of the fallopian tubes. Recently, I helped launch Femasys Inc.'s Femvue System (
This testing does not, however, diminish the importance of physician examination prior to surgery. Through the physical exam, the minimally invasive gynecologic surgeon is able to determine how large the uterus/leiomyomata complex is, relative to the patient's size, and therefore where ports should be placed, as well as the potential difficulty of surgery. If the surgeon considers the uterus/leiomyomata complex too large, or if anemia is noted, a gonadotropin-releasing hormone (GnRH) agonist can be given for 3 months to attempt shrinkage of the leiomyomata or to enable hemoglobin to rise (through the resultant amenorrhea) prior to surgery.
Laparoscopic Myomectomy
The laparoscopic surgery is scheduled in the proliferative phase of the cycle to avoid thickened endometrium. This is especially important in the case of removal of a type II submucosal leiomyomata or one that is impinging on the endometrial cavity.
On the day of surgery, prior to the laparoscopic myomectomy and after the patient has been placed into the dorsal lithotomy position and a Foley catheter has been placed in the bladder, hysteroscopy is performed to treat any abnormalities that are seen within the endometrial cavity. This may include hysteroscopic myomectomy on a leiomyomata previously believed to be located away from the endometrial cavity.
Once hysteroscopy has been completed, a uterine manipulator must be placed inside the uterine cavity. It is imperative to utilize a manipulator that can be placed deep enough into the cavity to enable anterior/posterior and lateral uterus flexion. I consider this function to be so important for the success of laparoscopic myomectomy that a surgical assistant, standing between the patient's legs, continues to manipulate the uterus throughout the duration of the procedure.
Generally, the 5-mm laparoscope is placed initially through the umbilicus, unless periumbilical adhesions are anticipated. In this latter case, I proceed to make a left-upper-quadrant incision. Lateral ports are then placed under direct visualization. These ports must be placed above and lateral to the uterus fibroid complex (See
To minimize blood loss, a dilute solution of vasopressin (30 U of vasopressin in 100 cc of normal saline) is placed in the myoma bed via an 18-gauge spinal needle placed percutaneously through a small skin nick. (See
If the myoma is pedunculated, on a broad base, the vasopressin should not be placed into the pedicle itself, as bleeding can be excessive; rather, the vasopressin is placed in the uterus around the pedicle. It is imperative to aspirate prior to injection of vasopressin in order to prevent inadvertent intravascular injection of the vasopressin.
If possible, to reduce the risk of adhesions, make an anterior incision in the uterus and try to remove as many fibroids through the single incision as possible. For years, my instrument of choice has been the curved blade of Ethicon Endo-Surgery Inc.'s Harmonic Scalpel. Harmonic energy allows excellent cutting with minimal tissue desiccation. Moreover, the curve of the blade allows easier dissection between the myoma and myometrium. When a posterior incision is required, I use a vertical incision to decrease risk of adhesion formation near the adnexa.
If multiple fibroids are removed, I place a #1 nylon suture with a Keith needle transcutaneously into the pelvis. The numerous leiomyomata are then strung on this suture to avoid losing a myoma in the abdomen or pelvis. (See
Although suturing in the “vertical zone” (with two ports placed on the same side of the pelvis) has become a popular technique, I continue to profess cross-table suturing. When the surgeon stands cephalad to the incisions, the repair is quite comfortable to perform. Furthermore, the ports can be placed higher on the abdomen to accommodate the very large uterus, and can be positioned more widely apart to improve triangulation.
I have always recommended multiple-layer closure of the uterus to minimize hematoma formation, and have advised skimming the myometrium rather than taking deep bites of tissue in order to minimize tissue destruction. When I began to perform laparoscopic myomectomy in earnest more than 20 years ago, closure of the uterine cavity was performed with Ethicon Inc.'s nonbraided PDS II 3-0 suture placed in an interrupted or mattress style using a “knot pusher.”
Even now, when the endometrial cavity is entered at the time of myomectomy, this is the technique I currently recommend, with the interrupted or mattress sutures placed immediately above the endometrium. During the past 15 years, I have advised repairing the uterus via a running-suture technique. After multiple layers are placed, the two suture ends are tied together via an intracorporeal suture technique. This has not only proved to be more efficient, but also allows the various layers to collapse upon themselves. Ultimately, the serosa is repaired via a baseball closure (suture placed in to out, in to out, and so on). (See
In my opinion, the recent introduction of barbed sutures has served as a monumental advance in our ability to repair the uterus in multiple layers. Both Covidien's V-Loc and Angiotech Pharmaceuticals Inc.'s Quill sutures do not have to be tied. Moreover, the barbs enable consistent tension on the suture line. In order to secure the suture from slipping, the Quill uses a bidirectional barb (See
My current barbed suture of choice is the 3-0 V-Loc, which is created from 2-0 suture. When a barbed suture is used, it is imperative that the physician “hide” the suture as much as possible and thus use a baseball closure; theoretically, the barbs could catch bowel or omentum, leading to adhesion formation.
To allow for a better cosmetic repair and to minimize the risk of postoperative hernia, I recommend utilizing a larger umbilical incision for tissue extraction – I use a 12-mm umbilical port – while maintaining other ports at 5 mm. At the conclusion of the uterine repair and after placement of an antiadhesive barrier (Ethicon Inc.'s Interceed), the umbilical port is removed. Large cervical dilators are then used to stretch the umbilical incision to allow direct placement of the 15-mm morcellator. Currently, I use Karl Storz Endoscopy America Inc.'s Storz Rotocut Morcellation System. This morcellator is reusable to decrease costs, and it has a beveled tip to enhance the “apple peel” shaving of the fibroid, a very durable blade to maximize cutting ability, and variable speed to enhance the morcellation procedure.
With this laparoscopic technique, I utilize laparotomy in fewer than 1% percent of more than 200 myomectomy cases per year, of which more than 30% involve fibroids greater than 8 cm and of which nearly 20% involve five or more fibroids.
Major complication rates continue to be fewer than 1% percent, and heterologous transfusions occur in fewer than 0.5% of cases.
More than 20 years after its inception, laparoscopic myomectomy continues to be an evolving procedure – one that, especially with current advancements, should become a more common technique in the armamentarium of the minimally invasive gynecologic surgeon.
Dr. Miller disclosed that he is a consultant for Covidien and Femasys Inc., and a consultant and speaker for Ethicon Endo-Surgery Inc.
Laparoscopic Myomectomy
In this month's installment of the Master Class in Gynecologic Surgery, we are taking an interesting twist and featuring the expertise of our own medical editor, Dr. Charles E. Miller, an internationally renowned expert in minimally invasive gynecologic surgery.
When Dr. Miller inaugurated this column more than 7 years ago with a feature on “Maximizing Myomectomy” (
In his opening Master Class feature, Dr. Miller detailed the advantages of laparoscopic myomectomy and shared some pearls he acquired from a retrospective study of almost 300 laparoscopic myomectomy patients whom he had managed. He advised us on patient selection, presurgery planning, port placement, equipment, and key components of surgical technique.
At this point, laparoscopic myomectomy is a procedure that Dr. Miller has been performing for more than 20 years. And as he tells us here, it is a procedure that is still evolving and one that – even more so than in the past – should become a more common technique in the armamentarium of the minimally invasive gynecologic surgeon.
Laparoscopic myomectomy is one of Dr. Miller's key research and practice concerns. In this Master Class, he gives us a valuable update. He explains how he has honed his selection of diagnostic tools for preoperative evaluation, and details how to minimize blood loss and the risk of adhesions and hematoma formation. He also provides some suturing pearls and weighs in on the role and use of recently introduced barbed sutures.
Doctor Shortage and Caribbean Medical Schools
Thirty years ago, the Graduate Medical Education National Advisory Committee predicted a surplus of 145,000 physicians, including cardiologists, by the year 2000, and recommended a limitation of the number of entering positions in U.S. medical schools and the number of international graduates coming to the United States.
Although there was no restriction placed on international graduates coming to the United States, the number of positions available for students to enter U.S. medical schools has remained static until the last 2 years. This obstruction to medical school entry led many students to seek education at offshore medical schools (OMS), particularly in the Caribbean.
The flawed predictions of a surplus of doctors were made in anticipation of an expanded role of health maintenance organizations as gatekeepers for access to both family and specialty doctors. GMENAC also failed to foresee the expansion of the elderly population as a result of the baby boomer generation and the increased availability of new diagnostic and therapeutic technologies.
It is now estimated that by 2020 or 2025 there will be a shortage of almost 200,000 doctors in the United States (J. Gen. Intern. Med. 2007;22:264–8). U.S. medical schools are now projected to graduate 16,000 doctors annually, and that number is expected to increase by 30% in 2015, unless the proposed restrictions to education budgets by Congress come into place. However, this increase will continue to fall short of national requirements if physician retirement is factored into the estimates.
I recently had an opportunity to visit one of the Caribbean medical schools and to observe the students in the classroom. I also learned a great deal about the role that the OMS play in mitigating the doctor shortage in the United States. The students in these schools are clearly different from those who attend American medical schools. They are distinguished, not exclusively by their MCAT scores, as though that really matters, but also by being very motivated to become doctors. Many had been out of undergraduate programs for sometime – some as long 15 years – and had tested other careers and come to the realization that medicine is what they really wanted.
Most of these students will spend 2 years in the Caribbean and then move to clinical training in hospitals throughout the United States, ultimately entering residency programs and practice in mainland America.
One of the first hurdles that the OMS students will face is passing the United States Medical Licensing Examination taken by both U.S. and International Medical Graduates (IMGs). Measured against U.S. medical school graduates, who have a first-time passing rate of about 95%, they unfortunately fall short: The rate for non-U.S. IMGs is 73%, and that for American IMGs is lower still, at 60% (Health Aff. 2009;28:1226–33).
Upon the completion of their training, although they may go into subspecialties as do U.S. students, more of the Caribbean students enter family practice, a fact that has not been lost on health planners.
There have been some recent attempts to limit the number of training slots available for OMS students in New York City hospitals because of the presumed lack of total residency positions.
However, the state legislators, aware of current needs, have been reluctant to erect any barriers for physicians interested in family practice.
Currently there are 40 OMS in the Caribbean basin including Mexico, 24 of which were started in the last 10 years, which graduate more than 4,000 students annually in three classes, which vary in size between 60 and 600 students. Tuition is similar to that of U.S. schools and ranges from $47,500 to $186,085 for the 4 years. U.S. medical schools must be accredited by the Liaison Committee on Medical Education, but there is no accreditation process for OMS.
The LCME is now partnering with the Caribbean Accreditation Authority for Education in Medicine and Other Health Professions to establish similar accreditation processes. Federally supported scholarships are available to U.S. citizens in the OMS just as they are for students enrolled in U.S. schools. As a result of the high tuition and relatively low overhead, some of these schools have been targets for venture capitalists.
Of the 800,000 actively practicing doctors in the United States, 23.7% are IMGs, a percentage that is sure to increase. Approximately 60% of the IMGs are from the offshore medical schools.
It is clear that the United States has become increasingly dependent on OMS to meet our doctor supply. It is also clear that a vigorous attempt to improve the certification process for OMS would go a long way to ensure the quality of our future doctors.
Thirty years ago, the Graduate Medical Education National Advisory Committee predicted a surplus of 145,000 physicians, including cardiologists, by the year 2000, and recommended a limitation of the number of entering positions in U.S. medical schools and the number of international graduates coming to the United States.
Although there was no restriction placed on international graduates coming to the United States, the number of positions available for students to enter U.S. medical schools has remained static until the last 2 years. This obstruction to medical school entry led many students to seek education at offshore medical schools (OMS), particularly in the Caribbean.
The flawed predictions of a surplus of doctors were made in anticipation of an expanded role of health maintenance organizations as gatekeepers for access to both family and specialty doctors. GMENAC also failed to foresee the expansion of the elderly population as a result of the baby boomer generation and the increased availability of new diagnostic and therapeutic technologies.
It is now estimated that by 2020 or 2025 there will be a shortage of almost 200,000 doctors in the United States (J. Gen. Intern. Med. 2007;22:264–8). U.S. medical schools are now projected to graduate 16,000 doctors annually, and that number is expected to increase by 30% in 2015, unless the proposed restrictions to education budgets by Congress come into place. However, this increase will continue to fall short of national requirements if physician retirement is factored into the estimates.
I recently had an opportunity to visit one of the Caribbean medical schools and to observe the students in the classroom. I also learned a great deal about the role that the OMS play in mitigating the doctor shortage in the United States. The students in these schools are clearly different from those who attend American medical schools. They are distinguished, not exclusively by their MCAT scores, as though that really matters, but also by being very motivated to become doctors. Many had been out of undergraduate programs for sometime – some as long 15 years – and had tested other careers and come to the realization that medicine is what they really wanted.
Most of these students will spend 2 years in the Caribbean and then move to clinical training in hospitals throughout the United States, ultimately entering residency programs and practice in mainland America.
One of the first hurdles that the OMS students will face is passing the United States Medical Licensing Examination taken by both U.S. and International Medical Graduates (IMGs). Measured against U.S. medical school graduates, who have a first-time passing rate of about 95%, they unfortunately fall short: The rate for non-U.S. IMGs is 73%, and that for American IMGs is lower still, at 60% (Health Aff. 2009;28:1226–33).
Upon the completion of their training, although they may go into subspecialties as do U.S. students, more of the Caribbean students enter family practice, a fact that has not been lost on health planners.
There have been some recent attempts to limit the number of training slots available for OMS students in New York City hospitals because of the presumed lack of total residency positions.
However, the state legislators, aware of current needs, have been reluctant to erect any barriers for physicians interested in family practice.
Currently there are 40 OMS in the Caribbean basin including Mexico, 24 of which were started in the last 10 years, which graduate more than 4,000 students annually in three classes, which vary in size between 60 and 600 students. Tuition is similar to that of U.S. schools and ranges from $47,500 to $186,085 for the 4 years. U.S. medical schools must be accredited by the Liaison Committee on Medical Education, but there is no accreditation process for OMS.
The LCME is now partnering with the Caribbean Accreditation Authority for Education in Medicine and Other Health Professions to establish similar accreditation processes. Federally supported scholarships are available to U.S. citizens in the OMS just as they are for students enrolled in U.S. schools. As a result of the high tuition and relatively low overhead, some of these schools have been targets for venture capitalists.
Of the 800,000 actively practicing doctors in the United States, 23.7% are IMGs, a percentage that is sure to increase. Approximately 60% of the IMGs are from the offshore medical schools.
It is clear that the United States has become increasingly dependent on OMS to meet our doctor supply. It is also clear that a vigorous attempt to improve the certification process for OMS would go a long way to ensure the quality of our future doctors.
Thirty years ago, the Graduate Medical Education National Advisory Committee predicted a surplus of 145,000 physicians, including cardiologists, by the year 2000, and recommended a limitation of the number of entering positions in U.S. medical schools and the number of international graduates coming to the United States.
Although there was no restriction placed on international graduates coming to the United States, the number of positions available for students to enter U.S. medical schools has remained static until the last 2 years. This obstruction to medical school entry led many students to seek education at offshore medical schools (OMS), particularly in the Caribbean.
The flawed predictions of a surplus of doctors were made in anticipation of an expanded role of health maintenance organizations as gatekeepers for access to both family and specialty doctors. GMENAC also failed to foresee the expansion of the elderly population as a result of the baby boomer generation and the increased availability of new diagnostic and therapeutic technologies.
It is now estimated that by 2020 or 2025 there will be a shortage of almost 200,000 doctors in the United States (J. Gen. Intern. Med. 2007;22:264–8). U.S. medical schools are now projected to graduate 16,000 doctors annually, and that number is expected to increase by 30% in 2015, unless the proposed restrictions to education budgets by Congress come into place. However, this increase will continue to fall short of national requirements if physician retirement is factored into the estimates.
I recently had an opportunity to visit one of the Caribbean medical schools and to observe the students in the classroom. I also learned a great deal about the role that the OMS play in mitigating the doctor shortage in the United States. The students in these schools are clearly different from those who attend American medical schools. They are distinguished, not exclusively by their MCAT scores, as though that really matters, but also by being very motivated to become doctors. Many had been out of undergraduate programs for sometime – some as long 15 years – and had tested other careers and come to the realization that medicine is what they really wanted.
Most of these students will spend 2 years in the Caribbean and then move to clinical training in hospitals throughout the United States, ultimately entering residency programs and practice in mainland America.
One of the first hurdles that the OMS students will face is passing the United States Medical Licensing Examination taken by both U.S. and International Medical Graduates (IMGs). Measured against U.S. medical school graduates, who have a first-time passing rate of about 95%, they unfortunately fall short: The rate for non-U.S. IMGs is 73%, and that for American IMGs is lower still, at 60% (Health Aff. 2009;28:1226–33).
Upon the completion of their training, although they may go into subspecialties as do U.S. students, more of the Caribbean students enter family practice, a fact that has not been lost on health planners.
There have been some recent attempts to limit the number of training slots available for OMS students in New York City hospitals because of the presumed lack of total residency positions.
However, the state legislators, aware of current needs, have been reluctant to erect any barriers for physicians interested in family practice.
Currently there are 40 OMS in the Caribbean basin including Mexico, 24 of which were started in the last 10 years, which graduate more than 4,000 students annually in three classes, which vary in size between 60 and 600 students. Tuition is similar to that of U.S. schools and ranges from $47,500 to $186,085 for the 4 years. U.S. medical schools must be accredited by the Liaison Committee on Medical Education, but there is no accreditation process for OMS.
The LCME is now partnering with the Caribbean Accreditation Authority for Education in Medicine and Other Health Professions to establish similar accreditation processes. Federally supported scholarships are available to U.S. citizens in the OMS just as they are for students enrolled in U.S. schools. As a result of the high tuition and relatively low overhead, some of these schools have been targets for venture capitalists.
Of the 800,000 actively practicing doctors in the United States, 23.7% are IMGs, a percentage that is sure to increase. Approximately 60% of the IMGs are from the offshore medical schools.
It is clear that the United States has become increasingly dependent on OMS to meet our doctor supply. It is also clear that a vigorous attempt to improve the certification process for OMS would go a long way to ensure the quality of our future doctors.
Treating Sports Overuse Injuries
Overuse injuries are very common in children and teenagers, especially among kids who play sports throughout the year.
A high volume of sports puts your patients at higher risk for an overuse injury. Ask which sports they play, how often they play them, and how many teams they play for when taking the patient history. It is more and more common now that kids play on multiple teams at the same time or that sports seasons overlap. Here in the South, for example, baseball can start in January or February, while basketball – a winter sport – is still going on.
Year-round participation in multiple sports has an advantage as well – it becomes a form of built-in cross training. Your patients will be using the same muscles but developing them in different ways.
Encourage your athletic patients to play different sports and discourage “early specialization.” You can counsel patients regularly about sports diversification – during well-child visits and school or sports physical examinations. Patients who play football or soccer in the fall; basketball or wrestling in the winter; and then softball or lacrosse in the spring generally are at a lower risk for overuse injuries.
In contrast, specialization in the same sport throughout the year increases the risk for overuse injuries as well as “burnout.” For example, a child who starts at age 7 or 8 years and plays the same sport for years might find participation becomes less fun by age 13 or 14 years. In some cases, parents get enthusiastic, pay for private lessons to extend the “season” to 12 months, and the kids just never have a time to rest.
For some families, it seems like success of the team or success on the playing field becomes more important than the health of the child. You can face a dilemma if you recommend rest for a child about to play a big game or tournament. The best way I found around that is to spend sufficient time to explain why you are making your recommendations. If you just say, “His knee hurts, and he shouldn't play,” the patient and parents are less likely to be compliant.
We give advice. We rarely forbid a kid from playing. But you can explain what could happen if they don't follow recommendations. You might say something like, “Here is what I think you have, here is what I think you should do, and here's why. If you don't, the risk of making this a stress fracture is higher.” You can also explain that a nonsurgical elbow injury could become surgical if you continue to throw, play, or tumble.
Pediatricians can manage most overuse injuries. Watch for signs that can warrant referral, however, such as a swollen joint, limitation of joint movement, or symptoms of trauma/acute injury. Consider consulting a subspecialist when the child cannot completely bend or extend the elbow, for example. These findings suggest something worse than just overuse.
In general, the best way to treat an overuse injury is to underuse the affected area. Apply the PRICEMM techniques (protection, rest, ice, compression, elevation, medication, and [physical therapy] modalities) for 2 or 3 days. If there is no improvement, expand your differential diagnosis. Overuse injuries should improve quickly if patients start underusing the affected area in addition to modifying their workouts and using ice and anti-inflammatory medications.
Recommend the patient back off after you identify the likely source of pain. If a baseball player presents with elbow pain, for example, he might improve by pitching less or switching from shortstop to first base. Rarely do children need to stop playing altogether. Modification of the workout a little bit might be all it takes to give the body a chance to adapt. You could recommend a child play only part of the soccer game or avoid particular conditioning drills during practice, for example.
An overuse injury is defined as repetitive, submaximal stress applied to a tissue that occurs when the adaptive capability of the tissue is exceeded and injury results. A blister is a perfect example. If you put on a new shoe that starts rubbing your foot too much, eventually the skin breaks down. But if you wear the new shoes for a little bit, then switch to sandals, then boots, and finally put your new shoes back on, you slowly introduce those stresses. This way, the body has a chance to adapt, the skin will become callused, and you won't develop a blister.
Acute trauma is another reason to consider referring the child to a sports medicine specialist. If a child comes to you with instant pain from a jump off the monkey bars or a slide into home, she should be referred to rule out something more serious, such as a fracture or a cartilage or a ligament tear.
Another time to refer is anytime you feel uncomfortable. If you sense something isn't right, you will never be faulted for referring the patient to a specialist. So, when in doubt, go ahead and refer.
Typically, a good history and physical examination will be sufficient, with or without x-rays, for a pediatrician to determine the best recommendations for the patient.
Although x-rays are a necessity for evaluation of most orthopedic or sports injuries, it is preferable to refer the child and have the subspecialist order imaging tests. This avoids duplication of radiation exposure for the child and the unnecessary time and expense of repeated x-rays. In addition, laboratory assays typically do not help in the evaluation of a suspected overuse injury, unless you suspect a comorbid condition such as arthritis or joint infection.
Overuse injuries are very common in children and teenagers, especially among kids who play sports throughout the year.
A high volume of sports puts your patients at higher risk for an overuse injury. Ask which sports they play, how often they play them, and how many teams they play for when taking the patient history. It is more and more common now that kids play on multiple teams at the same time or that sports seasons overlap. Here in the South, for example, baseball can start in January or February, while basketball – a winter sport – is still going on.
Year-round participation in multiple sports has an advantage as well – it becomes a form of built-in cross training. Your patients will be using the same muscles but developing them in different ways.
Encourage your athletic patients to play different sports and discourage “early specialization.” You can counsel patients regularly about sports diversification – during well-child visits and school or sports physical examinations. Patients who play football or soccer in the fall; basketball or wrestling in the winter; and then softball or lacrosse in the spring generally are at a lower risk for overuse injuries.
In contrast, specialization in the same sport throughout the year increases the risk for overuse injuries as well as “burnout.” For example, a child who starts at age 7 or 8 years and plays the same sport for years might find participation becomes less fun by age 13 or 14 years. In some cases, parents get enthusiastic, pay for private lessons to extend the “season” to 12 months, and the kids just never have a time to rest.
For some families, it seems like success of the team or success on the playing field becomes more important than the health of the child. You can face a dilemma if you recommend rest for a child about to play a big game or tournament. The best way I found around that is to spend sufficient time to explain why you are making your recommendations. If you just say, “His knee hurts, and he shouldn't play,” the patient and parents are less likely to be compliant.
We give advice. We rarely forbid a kid from playing. But you can explain what could happen if they don't follow recommendations. You might say something like, “Here is what I think you have, here is what I think you should do, and here's why. If you don't, the risk of making this a stress fracture is higher.” You can also explain that a nonsurgical elbow injury could become surgical if you continue to throw, play, or tumble.
Pediatricians can manage most overuse injuries. Watch for signs that can warrant referral, however, such as a swollen joint, limitation of joint movement, or symptoms of trauma/acute injury. Consider consulting a subspecialist when the child cannot completely bend or extend the elbow, for example. These findings suggest something worse than just overuse.
In general, the best way to treat an overuse injury is to underuse the affected area. Apply the PRICEMM techniques (protection, rest, ice, compression, elevation, medication, and [physical therapy] modalities) for 2 or 3 days. If there is no improvement, expand your differential diagnosis. Overuse injuries should improve quickly if patients start underusing the affected area in addition to modifying their workouts and using ice and anti-inflammatory medications.
Recommend the patient back off after you identify the likely source of pain. If a baseball player presents with elbow pain, for example, he might improve by pitching less or switching from shortstop to first base. Rarely do children need to stop playing altogether. Modification of the workout a little bit might be all it takes to give the body a chance to adapt. You could recommend a child play only part of the soccer game or avoid particular conditioning drills during practice, for example.
An overuse injury is defined as repetitive, submaximal stress applied to a tissue that occurs when the adaptive capability of the tissue is exceeded and injury results. A blister is a perfect example. If you put on a new shoe that starts rubbing your foot too much, eventually the skin breaks down. But if you wear the new shoes for a little bit, then switch to sandals, then boots, and finally put your new shoes back on, you slowly introduce those stresses. This way, the body has a chance to adapt, the skin will become callused, and you won't develop a blister.
Acute trauma is another reason to consider referring the child to a sports medicine specialist. If a child comes to you with instant pain from a jump off the monkey bars or a slide into home, she should be referred to rule out something more serious, such as a fracture or a cartilage or a ligament tear.
Another time to refer is anytime you feel uncomfortable. If you sense something isn't right, you will never be faulted for referring the patient to a specialist. So, when in doubt, go ahead and refer.
Typically, a good history and physical examination will be sufficient, with or without x-rays, for a pediatrician to determine the best recommendations for the patient.
Although x-rays are a necessity for evaluation of most orthopedic or sports injuries, it is preferable to refer the child and have the subspecialist order imaging tests. This avoids duplication of radiation exposure for the child and the unnecessary time and expense of repeated x-rays. In addition, laboratory assays typically do not help in the evaluation of a suspected overuse injury, unless you suspect a comorbid condition such as arthritis or joint infection.
Overuse injuries are very common in children and teenagers, especially among kids who play sports throughout the year.
A high volume of sports puts your patients at higher risk for an overuse injury. Ask which sports they play, how often they play them, and how many teams they play for when taking the patient history. It is more and more common now that kids play on multiple teams at the same time or that sports seasons overlap. Here in the South, for example, baseball can start in January or February, while basketball – a winter sport – is still going on.
Year-round participation in multiple sports has an advantage as well – it becomes a form of built-in cross training. Your patients will be using the same muscles but developing them in different ways.
Encourage your athletic patients to play different sports and discourage “early specialization.” You can counsel patients regularly about sports diversification – during well-child visits and school or sports physical examinations. Patients who play football or soccer in the fall; basketball or wrestling in the winter; and then softball or lacrosse in the spring generally are at a lower risk for overuse injuries.
In contrast, specialization in the same sport throughout the year increases the risk for overuse injuries as well as “burnout.” For example, a child who starts at age 7 or 8 years and plays the same sport for years might find participation becomes less fun by age 13 or 14 years. In some cases, parents get enthusiastic, pay for private lessons to extend the “season” to 12 months, and the kids just never have a time to rest.
For some families, it seems like success of the team or success on the playing field becomes more important than the health of the child. You can face a dilemma if you recommend rest for a child about to play a big game or tournament. The best way I found around that is to spend sufficient time to explain why you are making your recommendations. If you just say, “His knee hurts, and he shouldn't play,” the patient and parents are less likely to be compliant.
We give advice. We rarely forbid a kid from playing. But you can explain what could happen if they don't follow recommendations. You might say something like, “Here is what I think you have, here is what I think you should do, and here's why. If you don't, the risk of making this a stress fracture is higher.” You can also explain that a nonsurgical elbow injury could become surgical if you continue to throw, play, or tumble.
Pediatricians can manage most overuse injuries. Watch for signs that can warrant referral, however, such as a swollen joint, limitation of joint movement, or symptoms of trauma/acute injury. Consider consulting a subspecialist when the child cannot completely bend or extend the elbow, for example. These findings suggest something worse than just overuse.
In general, the best way to treat an overuse injury is to underuse the affected area. Apply the PRICEMM techniques (protection, rest, ice, compression, elevation, medication, and [physical therapy] modalities) for 2 or 3 days. If there is no improvement, expand your differential diagnosis. Overuse injuries should improve quickly if patients start underusing the affected area in addition to modifying their workouts and using ice and anti-inflammatory medications.
Recommend the patient back off after you identify the likely source of pain. If a baseball player presents with elbow pain, for example, he might improve by pitching less or switching from shortstop to first base. Rarely do children need to stop playing altogether. Modification of the workout a little bit might be all it takes to give the body a chance to adapt. You could recommend a child play only part of the soccer game or avoid particular conditioning drills during practice, for example.
An overuse injury is defined as repetitive, submaximal stress applied to a tissue that occurs when the adaptive capability of the tissue is exceeded and injury results. A blister is a perfect example. If you put on a new shoe that starts rubbing your foot too much, eventually the skin breaks down. But if you wear the new shoes for a little bit, then switch to sandals, then boots, and finally put your new shoes back on, you slowly introduce those stresses. This way, the body has a chance to adapt, the skin will become callused, and you won't develop a blister.
Acute trauma is another reason to consider referring the child to a sports medicine specialist. If a child comes to you with instant pain from a jump off the monkey bars or a slide into home, she should be referred to rule out something more serious, such as a fracture or a cartilage or a ligament tear.
Another time to refer is anytime you feel uncomfortable. If you sense something isn't right, you will never be faulted for referring the patient to a specialist. So, when in doubt, go ahead and refer.
Typically, a good history and physical examination will be sufficient, with or without x-rays, for a pediatrician to determine the best recommendations for the patient.
Although x-rays are a necessity for evaluation of most orthopedic or sports injuries, it is preferable to refer the child and have the subspecialist order imaging tests. This avoids duplication of radiation exposure for the child and the unnecessary time and expense of repeated x-rays. In addition, laboratory assays typically do not help in the evaluation of a suspected overuse injury, unless you suspect a comorbid condition such as arthritis or joint infection.
Managing ADHD in a Young Child
Some parents of very young children – those 6 years old and younger – will come to you exhausted, feeling inadequate as parents, and even angry at their children.
Parents will report that their children with attention-deficit/hyperactivity disorder are very difficult at home and in preschool. Probably when their children were around age 2, 3, or 4 years old, their parents began wondering if the youngsters were immature, were more impulsive, and had a shorter attention span, compared with peers.
Pediatricians will recognize this pattern as likely ADHD and, in addition to making an accurate diagnosis, know that much is at stake in guiding and supporting parents. A critical relationship is at risk as the child's behavior evokes criticism and a negative tone from parents. Pediatricians should help parents set reasonable expectations and focus on behaviors and activities that build self-esteem. For some families, this work can be difficult and time consuming, requiring counseling and behavioral reward training – probably better delegated to a mental health specialist.
Attention span, impulsivity, and motoric hyperactivity are active concerns every waking hour of the child's day. Certain demands in preschool, at a longer dinner, or in church may exacerbate the symptoms, whereas playing in the park or playing a fun-filled computer game may ease the symptoms. The pediatrician can sort through a typical day and recommend approaches that are consistent with developmentally reasonable expectations, and modified for the child's ADHD symptoms.
Family history is another consideration. About 30% of children with ADHD come from fathers who had or have the disorder. Reminding a father of his difficulties growing up or any ongoing ADHD symptoms can be helpful in eliciting some empathy from him for his child's suffering.
Beyond family life, ADHD will affect the choice of school and activities. Based on what works for the child, consider how many hours a child should spend in preschool and how much structure is helpful. How will the culture of the school fit the child's style? Remember that the last thing a child with ADHD needs is an early school experience characterized by criticism and a sense of not being able to please teachers. A school with more recess and activity opportunities, as well as after-school programs, might be a good choice, and might offer some respite for parents.
As part of building self-esteem, ask parents if there is anything the child is really good at. For example, I treated a 6-year-old with ADHD who was gifted with computers. He was able to teach his peers and play games with friends, and he felt genuine pride working with a machine that was tolerant and nonjudgmental, and could be reset as needed. Might this not lead to a path of an after-school computer club or computer summer camp (that of course would include other activities)? Other young children may show strength in music, art, or a sport, and these activities are at least as important as remediating weaknesses.
Awareness of the different ADHD subtypes is important in general, but also can guide you in when to refer these children. Some kids with ADHD are more moody or depressed, some are more anxious, and others are more physically aggressive. Consider referral to a child and adolescent psychiatrist if one of these subtypes becomes more difficult to manage. A mental health consultation can help these higher-risk children.
Some children with ADHD also have learning disabilities, and diagnosis at a young age, before school failure, is invaluable. If you suspect this in a particular patient, you might want to recommend some early testing through the schools to avoid creation of unrealistic expectations in the classroom.
Parents may tell you their children are impulsive. While other kids are more predictable when playing in the sandbox, their children with ADHD may do something unpredictable. They might jump out of the sandbox or grab a toy from another child, for example. A mother of a 3-year-old with ADHD will stay closer to the sandbox because she doesn't know what that child is going to do next.
Typically, the child also will have a shorter attention span. The parents cannot relax because they know the sandbox, or a particular project in the sandbox, won't hold their child as long. Other children may be occupied for 15, 20, or even 30 minutes, but their young child with ADHD might last only 3 or 4 minutes and then need to move on. That, as you can imagine, is going to make the parenting demands much higher. Remember this doesn't happen for just 1 or 2 hours per day; children with true ADHD are going to be like this from the time they get up until the time they go to sleep.
All these behaviors associated with ADHD set these children up for a fair amount of criticism. The parents are tempted, especially if they don't understand the disorder, to say: “Don't do that!”; “Put that down!”; “I just bought you this – why don't you want to play with it?”; “Why can't you play like your friend Johnny does?”; “Why can't you sit still for a minute while Mommy fixes dinner?”
These children are subject to a lot of negative feedback from their environment. The world is not very tolerant of a young child, or even an older child, with ADHD. My guess is if these children are in preschool, the teacher is having the same issues with their behaviors. They may get criticized during circle time or while doing a certain project. Except for recess and lunch, they are going to be under a lot of scrutiny and most of the feedback is going to be negative.
We can see how children with ADHD, in a typical day, can hear 10, if not 25, negative comments. That is about two to three per hour. That degree of criticism begins to become part of how they see themselves, and they become fairly self-critical.
One of the key risks from ADHD at this young age is that it's hard for these children to differentiate if what they are doing is bad or if they are bad. Their self-esteem is very vulnerable. One principle that guides a lot of my management of these youngsters with ADHD is figuring out how to protect or enhance their self-esteem. Therefore, one of the initial things I ask parents to do is to think about how much negative criticism their child is hearing. Next, I ask them to think about what are reasonable expectations for that particular child.
Any opportunity to build self-esteem and build a sense of success based on reasonable expectations is worthwhile. A lot of parents will start sports for their children when they are 4 or 5. Kids with ADHD don't do very well in the outfield of T-ball because they are distracted. They don't stand out there waiting for the hit, and then they get yelled at for missing the ball. Help parents choose a sport that fits their children. I've seen some ADHD kids be goalies because they have to pay attention for a few seconds when the ball is coming, and then when the ball is somewhere else they can daydream with impunity. A lot of children with ADHD do well with swimming, for example, because there are fewer rules and they have a little more freedom. Others thrive with the structure and sense of accomplishment that comes from the “belt” system of karate.
Clearly one of the most effective treatments for the symptoms of ADHD is medication. Medication will increase attention span in school, church, or at dinner. Of course, every parent has concerns about how young to start children on medication, or whether to use medication at all. For those families, the first set of efforts may be directed to setting reasonable expectations and reviewing daily activities.
This focus will help, but will not be enough, and medication will be a critical part of treatment. Medication adds some risk, but the benefits to the child's functioning and self-esteem often outweigh these risks.
One of the things that medication probably does best is reduce the amount of negative feedback because the child will not be as impulsive and will appear to have a longer attention span. Again, you can ask too much of a child, but you will see higher expectations if the child is taking medication that is working correctly. Once ADHD is diagnosed in a young child, the pediatrician has a key role in trying to protect and enhance the child's self-esteem, advising on the child's day-to-day functioning, and supporting the overall care with appropriate use of medications.
Some parents of very young children – those 6 years old and younger – will come to you exhausted, feeling inadequate as parents, and even angry at their children.
Parents will report that their children with attention-deficit/hyperactivity disorder are very difficult at home and in preschool. Probably when their children were around age 2, 3, or 4 years old, their parents began wondering if the youngsters were immature, were more impulsive, and had a shorter attention span, compared with peers.
Pediatricians will recognize this pattern as likely ADHD and, in addition to making an accurate diagnosis, know that much is at stake in guiding and supporting parents. A critical relationship is at risk as the child's behavior evokes criticism and a negative tone from parents. Pediatricians should help parents set reasonable expectations and focus on behaviors and activities that build self-esteem. For some families, this work can be difficult and time consuming, requiring counseling and behavioral reward training – probably better delegated to a mental health specialist.
Attention span, impulsivity, and motoric hyperactivity are active concerns every waking hour of the child's day. Certain demands in preschool, at a longer dinner, or in church may exacerbate the symptoms, whereas playing in the park or playing a fun-filled computer game may ease the symptoms. The pediatrician can sort through a typical day and recommend approaches that are consistent with developmentally reasonable expectations, and modified for the child's ADHD symptoms.
Family history is another consideration. About 30% of children with ADHD come from fathers who had or have the disorder. Reminding a father of his difficulties growing up or any ongoing ADHD symptoms can be helpful in eliciting some empathy from him for his child's suffering.
Beyond family life, ADHD will affect the choice of school and activities. Based on what works for the child, consider how many hours a child should spend in preschool and how much structure is helpful. How will the culture of the school fit the child's style? Remember that the last thing a child with ADHD needs is an early school experience characterized by criticism and a sense of not being able to please teachers. A school with more recess and activity opportunities, as well as after-school programs, might be a good choice, and might offer some respite for parents.
As part of building self-esteem, ask parents if there is anything the child is really good at. For example, I treated a 6-year-old with ADHD who was gifted with computers. He was able to teach his peers and play games with friends, and he felt genuine pride working with a machine that was tolerant and nonjudgmental, and could be reset as needed. Might this not lead to a path of an after-school computer club or computer summer camp (that of course would include other activities)? Other young children may show strength in music, art, or a sport, and these activities are at least as important as remediating weaknesses.
Awareness of the different ADHD subtypes is important in general, but also can guide you in when to refer these children. Some kids with ADHD are more moody or depressed, some are more anxious, and others are more physically aggressive. Consider referral to a child and adolescent psychiatrist if one of these subtypes becomes more difficult to manage. A mental health consultation can help these higher-risk children.
Some children with ADHD also have learning disabilities, and diagnosis at a young age, before school failure, is invaluable. If you suspect this in a particular patient, you might want to recommend some early testing through the schools to avoid creation of unrealistic expectations in the classroom.
Parents may tell you their children are impulsive. While other kids are more predictable when playing in the sandbox, their children with ADHD may do something unpredictable. They might jump out of the sandbox or grab a toy from another child, for example. A mother of a 3-year-old with ADHD will stay closer to the sandbox because she doesn't know what that child is going to do next.
Typically, the child also will have a shorter attention span. The parents cannot relax because they know the sandbox, or a particular project in the sandbox, won't hold their child as long. Other children may be occupied for 15, 20, or even 30 minutes, but their young child with ADHD might last only 3 or 4 minutes and then need to move on. That, as you can imagine, is going to make the parenting demands much higher. Remember this doesn't happen for just 1 or 2 hours per day; children with true ADHD are going to be like this from the time they get up until the time they go to sleep.
All these behaviors associated with ADHD set these children up for a fair amount of criticism. The parents are tempted, especially if they don't understand the disorder, to say: “Don't do that!”; “Put that down!”; “I just bought you this – why don't you want to play with it?”; “Why can't you play like your friend Johnny does?”; “Why can't you sit still for a minute while Mommy fixes dinner?”
These children are subject to a lot of negative feedback from their environment. The world is not very tolerant of a young child, or even an older child, with ADHD. My guess is if these children are in preschool, the teacher is having the same issues with their behaviors. They may get criticized during circle time or while doing a certain project. Except for recess and lunch, they are going to be under a lot of scrutiny and most of the feedback is going to be negative.
We can see how children with ADHD, in a typical day, can hear 10, if not 25, negative comments. That is about two to three per hour. That degree of criticism begins to become part of how they see themselves, and they become fairly self-critical.
One of the key risks from ADHD at this young age is that it's hard for these children to differentiate if what they are doing is bad or if they are bad. Their self-esteem is very vulnerable. One principle that guides a lot of my management of these youngsters with ADHD is figuring out how to protect or enhance their self-esteem. Therefore, one of the initial things I ask parents to do is to think about how much negative criticism their child is hearing. Next, I ask them to think about what are reasonable expectations for that particular child.
Any opportunity to build self-esteem and build a sense of success based on reasonable expectations is worthwhile. A lot of parents will start sports for their children when they are 4 or 5. Kids with ADHD don't do very well in the outfield of T-ball because they are distracted. They don't stand out there waiting for the hit, and then they get yelled at for missing the ball. Help parents choose a sport that fits their children. I've seen some ADHD kids be goalies because they have to pay attention for a few seconds when the ball is coming, and then when the ball is somewhere else they can daydream with impunity. A lot of children with ADHD do well with swimming, for example, because there are fewer rules and they have a little more freedom. Others thrive with the structure and sense of accomplishment that comes from the “belt” system of karate.
Clearly one of the most effective treatments for the symptoms of ADHD is medication. Medication will increase attention span in school, church, or at dinner. Of course, every parent has concerns about how young to start children on medication, or whether to use medication at all. For those families, the first set of efforts may be directed to setting reasonable expectations and reviewing daily activities.
This focus will help, but will not be enough, and medication will be a critical part of treatment. Medication adds some risk, but the benefits to the child's functioning and self-esteem often outweigh these risks.
One of the things that medication probably does best is reduce the amount of negative feedback because the child will not be as impulsive and will appear to have a longer attention span. Again, you can ask too much of a child, but you will see higher expectations if the child is taking medication that is working correctly. Once ADHD is diagnosed in a young child, the pediatrician has a key role in trying to protect and enhance the child's self-esteem, advising on the child's day-to-day functioning, and supporting the overall care with appropriate use of medications.
Some parents of very young children – those 6 years old and younger – will come to you exhausted, feeling inadequate as parents, and even angry at their children.
Parents will report that their children with attention-deficit/hyperactivity disorder are very difficult at home and in preschool. Probably when their children were around age 2, 3, or 4 years old, their parents began wondering if the youngsters were immature, were more impulsive, and had a shorter attention span, compared with peers.
Pediatricians will recognize this pattern as likely ADHD and, in addition to making an accurate diagnosis, know that much is at stake in guiding and supporting parents. A critical relationship is at risk as the child's behavior evokes criticism and a negative tone from parents. Pediatricians should help parents set reasonable expectations and focus on behaviors and activities that build self-esteem. For some families, this work can be difficult and time consuming, requiring counseling and behavioral reward training – probably better delegated to a mental health specialist.
Attention span, impulsivity, and motoric hyperactivity are active concerns every waking hour of the child's day. Certain demands in preschool, at a longer dinner, or in church may exacerbate the symptoms, whereas playing in the park or playing a fun-filled computer game may ease the symptoms. The pediatrician can sort through a typical day and recommend approaches that are consistent with developmentally reasonable expectations, and modified for the child's ADHD symptoms.
Family history is another consideration. About 30% of children with ADHD come from fathers who had or have the disorder. Reminding a father of his difficulties growing up or any ongoing ADHD symptoms can be helpful in eliciting some empathy from him for his child's suffering.
Beyond family life, ADHD will affect the choice of school and activities. Based on what works for the child, consider how many hours a child should spend in preschool and how much structure is helpful. How will the culture of the school fit the child's style? Remember that the last thing a child with ADHD needs is an early school experience characterized by criticism and a sense of not being able to please teachers. A school with more recess and activity opportunities, as well as after-school programs, might be a good choice, and might offer some respite for parents.
As part of building self-esteem, ask parents if there is anything the child is really good at. For example, I treated a 6-year-old with ADHD who was gifted with computers. He was able to teach his peers and play games with friends, and he felt genuine pride working with a machine that was tolerant and nonjudgmental, and could be reset as needed. Might this not lead to a path of an after-school computer club or computer summer camp (that of course would include other activities)? Other young children may show strength in music, art, or a sport, and these activities are at least as important as remediating weaknesses.
Awareness of the different ADHD subtypes is important in general, but also can guide you in when to refer these children. Some kids with ADHD are more moody or depressed, some are more anxious, and others are more physically aggressive. Consider referral to a child and adolescent psychiatrist if one of these subtypes becomes more difficult to manage. A mental health consultation can help these higher-risk children.
Some children with ADHD also have learning disabilities, and diagnosis at a young age, before school failure, is invaluable. If you suspect this in a particular patient, you might want to recommend some early testing through the schools to avoid creation of unrealistic expectations in the classroom.
Parents may tell you their children are impulsive. While other kids are more predictable when playing in the sandbox, their children with ADHD may do something unpredictable. They might jump out of the sandbox or grab a toy from another child, for example. A mother of a 3-year-old with ADHD will stay closer to the sandbox because she doesn't know what that child is going to do next.
Typically, the child also will have a shorter attention span. The parents cannot relax because they know the sandbox, or a particular project in the sandbox, won't hold their child as long. Other children may be occupied for 15, 20, or even 30 minutes, but their young child with ADHD might last only 3 or 4 minutes and then need to move on. That, as you can imagine, is going to make the parenting demands much higher. Remember this doesn't happen for just 1 or 2 hours per day; children with true ADHD are going to be like this from the time they get up until the time they go to sleep.
All these behaviors associated with ADHD set these children up for a fair amount of criticism. The parents are tempted, especially if they don't understand the disorder, to say: “Don't do that!”; “Put that down!”; “I just bought you this – why don't you want to play with it?”; “Why can't you play like your friend Johnny does?”; “Why can't you sit still for a minute while Mommy fixes dinner?”
These children are subject to a lot of negative feedback from their environment. The world is not very tolerant of a young child, or even an older child, with ADHD. My guess is if these children are in preschool, the teacher is having the same issues with their behaviors. They may get criticized during circle time or while doing a certain project. Except for recess and lunch, they are going to be under a lot of scrutiny and most of the feedback is going to be negative.
We can see how children with ADHD, in a typical day, can hear 10, if not 25, negative comments. That is about two to three per hour. That degree of criticism begins to become part of how they see themselves, and they become fairly self-critical.
One of the key risks from ADHD at this young age is that it's hard for these children to differentiate if what they are doing is bad or if they are bad. Their self-esteem is very vulnerable. One principle that guides a lot of my management of these youngsters with ADHD is figuring out how to protect or enhance their self-esteem. Therefore, one of the initial things I ask parents to do is to think about how much negative criticism their child is hearing. Next, I ask them to think about what are reasonable expectations for that particular child.
Any opportunity to build self-esteem and build a sense of success based on reasonable expectations is worthwhile. A lot of parents will start sports for their children when they are 4 or 5. Kids with ADHD don't do very well in the outfield of T-ball because they are distracted. They don't stand out there waiting for the hit, and then they get yelled at for missing the ball. Help parents choose a sport that fits their children. I've seen some ADHD kids be goalies because they have to pay attention for a few seconds when the ball is coming, and then when the ball is somewhere else they can daydream with impunity. A lot of children with ADHD do well with swimming, for example, because there are fewer rules and they have a little more freedom. Others thrive with the structure and sense of accomplishment that comes from the “belt” system of karate.
Clearly one of the most effective treatments for the symptoms of ADHD is medication. Medication will increase attention span in school, church, or at dinner. Of course, every parent has concerns about how young to start children on medication, or whether to use medication at all. For those families, the first set of efforts may be directed to setting reasonable expectations and reviewing daily activities.
This focus will help, but will not be enough, and medication will be a critical part of treatment. Medication adds some risk, but the benefits to the child's functioning and self-esteem often outweigh these risks.
One of the things that medication probably does best is reduce the amount of negative feedback because the child will not be as impulsive and will appear to have a longer attention span. Again, you can ask too much of a child, but you will see higher expectations if the child is taking medication that is working correctly. Once ADHD is diagnosed in a young child, the pediatrician has a key role in trying to protect and enhance the child's self-esteem, advising on the child's day-to-day functioning, and supporting the overall care with appropriate use of medications.