User login
Continuing Medical Education Program in
If you wish to receive credit for this activity, which beginson the next page, please refer to the website:
Accreditation and Designation Statement
Blackwell Futura Media Services designates this educational activity for a 1 AMA PRA Category 1 Credit. Physicians should only claim credit commensurate with the extent of their participation in the activity.
Blackwell Futura Media Services is accredited by the Accreditation Council for Continuing Medical Education to provide continuing medical education for physicians.
Educational Objectives
Upon completion of this educational activity, participants will be better able to:
-
Identify the approximate 30‐day readmission rate of Medicare patient hospitalized initially for pneumonia.
-
Distinguish which variables were accounted and unaccounted for in the development of a pneumonia readmission model.
Continuous participation in the Journal of Hospital Medicine CME program will enable learners to be better able to:
-
Interpret clinical guidelines and their applications for higher quality and more efficient care for all hospitalized patients.
-
Describe the standard of care for common illnesses and conditions treated in the hospital; such as pneumonia, COPD exacerbation, acute coronary syndrome, HF exacerbation, glycemic control, venous thromboembolic disease, stroke, etc.
-
Discuss evidence‐based recommendations involving transitions of care, including the hospital discharge process.
-
Gain insights into the roles of hospitalists as medical educators, researchers, medical ethicists, palliative care providers, and hospital‐based geriatricians.
-
Incorporate best practices for hospitalist administration, including quality improvement, patient safety, practice management, leadership, and demonstrating hospitalist value.
-
Identify evidence‐based best practices and trends for both adult and pediatric hospital medicine.
Instructions on Receiving Credit
For information on applicability and acceptance of continuing medical education credit for this activity, please consult your professional licensing board.
This activity is designed to be completed within the time designated on the title page; physicians should claim only those credits that reflect the time actually spent in the activity. To successfully earn credit, participants must complete the activity during the valid credit period that is noted on the title page.
Follow these steps to earn credit:
-
Log on to
www.blackwellpublishing.com/cme . -
Read the target audience, learning objectives, and author disclosures.
-
Read the article in print or online format.
-
Reflect on the article.
-
Access the CME Exam, and choose the best answer to each question.
-
Complete the required evaluation component of the activity.
If you wish to receive credit for this activity, which beginson the next page, please refer to the website:
Accreditation and Designation Statement
Blackwell Futura Media Services designates this educational activity for a 1 AMA PRA Category 1 Credit. Physicians should only claim credit commensurate with the extent of their participation in the activity.
Blackwell Futura Media Services is accredited by the Accreditation Council for Continuing Medical Education to provide continuing medical education for physicians.
Educational Objectives
Upon completion of this educational activity, participants will be better able to:
-
Identify the approximate 30‐day readmission rate of Medicare patient hospitalized initially for pneumonia.
-
Distinguish which variables were accounted and unaccounted for in the development of a pneumonia readmission model.
Continuous participation in the Journal of Hospital Medicine CME program will enable learners to be better able to:
-
Interpret clinical guidelines and their applications for higher quality and more efficient care for all hospitalized patients.
-
Describe the standard of care for common illnesses and conditions treated in the hospital; such as pneumonia, COPD exacerbation, acute coronary syndrome, HF exacerbation, glycemic control, venous thromboembolic disease, stroke, etc.
-
Discuss evidence‐based recommendations involving transitions of care, including the hospital discharge process.
-
Gain insights into the roles of hospitalists as medical educators, researchers, medical ethicists, palliative care providers, and hospital‐based geriatricians.
-
Incorporate best practices for hospitalist administration, including quality improvement, patient safety, practice management, leadership, and demonstrating hospitalist value.
-
Identify evidence‐based best practices and trends for both adult and pediatric hospital medicine.
Instructions on Receiving Credit
For information on applicability and acceptance of continuing medical education credit for this activity, please consult your professional licensing board.
This activity is designed to be completed within the time designated on the title page; physicians should claim only those credits that reflect the time actually spent in the activity. To successfully earn credit, participants must complete the activity during the valid credit period that is noted on the title page.
Follow these steps to earn credit:
-
Log on to
www.blackwellpublishing.com/cme . -
Read the target audience, learning objectives, and author disclosures.
-
Read the article in print or online format.
-
Reflect on the article.
-
Access the CME Exam, and choose the best answer to each question.
-
Complete the required evaluation component of the activity.
If you wish to receive credit for this activity, which beginson the next page, please refer to the website:
Accreditation and Designation Statement
Blackwell Futura Media Services designates this educational activity for a 1 AMA PRA Category 1 Credit. Physicians should only claim credit commensurate with the extent of their participation in the activity.
Blackwell Futura Media Services is accredited by the Accreditation Council for Continuing Medical Education to provide continuing medical education for physicians.
Educational Objectives
Upon completion of this educational activity, participants will be better able to:
-
Identify the approximate 30‐day readmission rate of Medicare patient hospitalized initially for pneumonia.
-
Distinguish which variables were accounted and unaccounted for in the development of a pneumonia readmission model.
Continuous participation in the Journal of Hospital Medicine CME program will enable learners to be better able to:
-
Interpret clinical guidelines and their applications for higher quality and more efficient care for all hospitalized patients.
-
Describe the standard of care for common illnesses and conditions treated in the hospital; such as pneumonia, COPD exacerbation, acute coronary syndrome, HF exacerbation, glycemic control, venous thromboembolic disease, stroke, etc.
-
Discuss evidence‐based recommendations involving transitions of care, including the hospital discharge process.
-
Gain insights into the roles of hospitalists as medical educators, researchers, medical ethicists, palliative care providers, and hospital‐based geriatricians.
-
Incorporate best practices for hospitalist administration, including quality improvement, patient safety, practice management, leadership, and demonstrating hospitalist value.
-
Identify evidence‐based best practices and trends for both adult and pediatric hospital medicine.
Instructions on Receiving Credit
For information on applicability and acceptance of continuing medical education credit for this activity, please consult your professional licensing board.
This activity is designed to be completed within the time designated on the title page; physicians should claim only those credits that reflect the time actually spent in the activity. To successfully earn credit, participants must complete the activity during the valid credit period that is noted on the title page.
Follow these steps to earn credit:
-
Log on to
www.blackwellpublishing.com/cme . -
Read the target audience, learning objectives, and author disclosures.
-
Read the article in print or online format.
-
Reflect on the article.
-
Access the CME Exam, and choose the best answer to each question.
-
Complete the required evaluation component of the activity.
SPS in Hyperkalemia
Hyperkalemia is a common and potentially life‐threatening problem encountered in many clinical settings. The incidence of hyperkalemia in hospitalized patients has been estimated to be between 1% and 10% in the United States each year.16 Medications are thought to contribute to the development of hyperkalemia in 35% to 75% of hospitalized patients.12, 9 Medications known to cause hyperkalemia include angiotensin‐converting enzyme (ACE) inhibitors, angiotensin II receptor antagonists (ARBs), potassium‐sparing diuretics, nonsteroidal anti‐inflammatory drugs (NSAIDs), potassium supplements, and antibiotics such as trimethoprim/sulfamethoxazole. Patients with hyperkalemia may develop neuromuscular symptoms such as fatigue, muscle weakness, tingling, numbness, and cramping. The most deleterious effect of hyperkalemia is cardiac toxicity, including life‐threatening arrhythmias.3, 711
Sodium polystyrene sulfonate (SPS) or Kayexalate is a cation‐exchange resin that is commonly employed to lower total body potassium in patients with mild to moderate hyperkalemia.1214 It removes potassium from the gut in exchange for sodium. SPS can be given orally or as an enema. When given orally, it is commonly administered with sorbitol to promote diarrhea. The onset of action is within 1 to 2 hours and lasts approximately 4 to 6 hours. The recommended average daily dose is 15 gm to 60 gm given as a single dose or in divided doses. An in vitro study indicated that each gram of resin binds to approximately 3.1 mEq of potassium.14 However, the majority of the exchange capacity is utilized for cations other than potassium. Therefore, the in vivo exchange capacity is thought to be no greater than 1 mEq of potassium per gram of resin.
There are conflicting data regarding the effectiveness of exchange resins.1, 13 In 1998, Guy‐Kapral et al.15 found that a 30‐gm dose of SPS failed to lower serum potassium concentration in end‐stage renal disease patients who were normokalemic. In contrast, Flinn et al.16 demonstrated effective lowering of serum potassium over a period of several days in which repeated doses of SPS were given to hyperkalemic patients. Moreover, in another study, serum potassium concentration was reduced by 1.0 mEq/L in 24 hours.17 However, this study was limited since subjects also received other potassium‐lowering agents such as insulin/glucose or sodium bicarbonate.
In 2004, Mikrut and Brockmiller‐Sell18 studied the dose response between SPS and serum potassium concentration. Their data demonstrated an average reduction in potassium concentration of approximately 1 mmol/L and 1.48 mmol/L following a 30 gm and a 60 gm dose of SPS, respectively. However, the study was small (n = 39) and only evaluated 2 dosages. In clinical practice, there is wide variability in SPS dosing. Furthermore, studies assessing the effects of different doses of SPS are lacking. Often, the dose chosen is dependent on comorbid conditions, concomitant medications and provider experience in treating hyperkalemia. The aim of our study was to examine the single‐dose effect of SPS on potassium concentration in hospitalized, hyperkalemic patients and to compare the potassium lowering effects of SPS doses commonly used.
Methods
Study Population
A retrospective cohort study involving a review of electronic medical records of inpatients receiving SPS for the treatment of hyperkalemia was conducted at the Jesse Brown Veteran Affairs Medical Center, between January 1, 2006 and December 31, 2006. Hyperkalemia was defined as a serum potassium concentration >5.1 mmol/L, as this concentration corresponded to the upper limit of normal for serum potassium at our hospital. The patient list was generated from prescription databases. Exclusion criteria included: chronic SPS use, rectal administration of SPS, multiple doses of SPS spaced less than 6 hours apart, a hemolyzed laboratory specimen, current hemodialysis, lack of a follow‐up serum potassium concentration, or other treatments started for hyperkalemia at the time of the event, specifically insulin, dextrose, albuterol, and/or furosemide. This study protocol received approval from the local Institutional Review Board and the hospital's research and development committee.
Data Collection
The Computerized Patient Record System (CPRS), the electronic medical record system used at Jesse Brown VA Medical Center (JBVAMC), was utilized to gather patient baseline demographic information consisting of age, gender, weight, and height. Data on comorbid conditions such as hypertension (HTN), diabetes mellitus (DM), chronic heart failure (CHF), chronic kidney disease (CKD), and acute renal failure (ARF) was identified from the patient's problem list in the medical record. Additionally, the authors abstracted information about concomitant hyperkalemia‐precipitating medications including ACE inhibitors, ARBs, potassium‐sparing diuretics, aldosterone antagonists, NSAIDs, potassium supplements, and antibiotics such as trimethoprim/sulfamethoxazole. Progress notes and discharge summaries were reviewed for documentation of ARF. Laboratory information including serum creatinine, blood urea nitrogen, potassium, sodium, and calcium concentrations before and after SPS administration was recorded. The date and time of laboratory draws were also collected. Data on SPS administration regarding time and date, as well as dosing information, was obtained from the medication administration records.
Outcomes
The primary outcome were the mean change in serum potassium after a single dose of SPS compared to the baseline. The data were also evaluated based on the dose of SPS that was administered, 15 gm, 30 gm, 45 gm, or 60 gm doses of SPS. Secondary outcomes were the mean change in sodium and calcium concentrations.
Statistical Analysis
In order to complete the statistical analysis for the study, continuous variables were summarized as mean standard deviation, and categorical variables were summarized as frequency counts and proportions. Group comparisons for categorical data in baseline characteristics were performed using the Fisher's exact test and the chi‐square test. The comparisons for continuous data in baseline characteristics were performed using analysis of variance (ANOVA). A paired t‐test was then used to compare changes in potassium, calcium, and sodium concentrations and again to compare the changes in potassium concentrations by different dosage groups. Subset analysis also required the paired t‐test. The differences in the change of potassium concentration between dosage groups were analyzed by ANOVA. Post hoc comparisons were performed to identify which groups were statistically different from each other. A P value less than 0.05 was considered statistically significant. The effect of dosage on primary outcome was also assessed by adjusting baseline characteristics using analysis of covariance (ANCOVA). Least square means (LSMEAN) of the primary outcome were calculated using the ANCOVA model with the SPS dosage groups as the main categorical independent variable and the baseline characteristics in Table 1 as the covariates. The LSMEAN are group means after controlling for covariates (they are the predicted group means, predicted as the population mean values of the covariates in the model). All statistical analyses were done using SAS software, version 9.1 (Cary, NC).
| All (n = 122) | 15 gm (n = 30) | 30 gm (n = 60) | 45 gm (n = 19) | 60 gm (n = 13) | P Value | |
|---|---|---|---|---|---|---|
| ||||||
| Male, n (%) | 121 (99) | 30 (100) | 60 (100) | 18 (95) | 13 (100) | 0.14 |
| Age (year) | 69.0 11.4 | 70.71 0.5 | 70.2 11.5 | 68.0 1.7 | 63.8 12.2 | 0.25 |
| Height (inch) | 69.0 3.5 | 69.2 4.6 | 68.8 3.3 | 68.2 3.0 | 70.5 1.3 | 0.31 |
| Weight (kg) | 81.4 20.7 | 77.3 15.0 | 83.1 23.3 | 82.7 21.7 | 81.6 18.1 | 0.64 |
| Hypertension, n (%) | 96 (79) | 29*(97) | 47 (78) | 12 (63) | 8 (62) | 0.01* |
| Diabetes Mellitus, n (%) | 41 (34) | 10 (33) | 21 (35) | 6 (32) | 4 (31) | 0.99 |
| Chronic kidney disease, n (%) | 46 (38) | 10 (33) | 21 (35) | 8 (42) | 7 (54) | 0.57 |
| Heart failure, n (%) | 32 (26) | 12 (40) | 13 (22) | 6 (32) | 1 (8) | 0.10 |
| Serum creatinine (mg/dL) | 2.57 2.4 | 2.09 1.5 | 2.93 3.0 | 2.37 1.2 | 2.29 2.1 | 0.41 |
| Acute renal failure, n (%) | 84 (69) | 19 (63) | 45 (75) | 12 (63) | 8 (62) | 0.55 |
| Concomitant hyperkalemia‐precipitating medications, n (%) | 60 (49) | 19 (63) | 23 (38) | 10 (53) | 8 (61) | 0.11 |
Results
Patient Characteristics
Study subjects consisted of patients who received SPS in the hospital for treatment of hyperkalemia. A total of 140 patients were identified and 122 met the inclusion criteria. Eighteen patients were excluded for current hemodialysis, receiving other treatments for hyperkalemia, and specimen hemolysis. Of those who were included in the study analysis, 30 patients received 15 gm of SPS, 60 patients received 30 gm, 19 patients received 45 gm, and 13 patients received 60 gm. Baseline characteristics of included patients are shown in Table 1. The majority of patients were male (99%) and had comorbid conditions.
At baseline, the mean serum creatinine concentration was 2.57 2.36 mg/dL. Documented acute renal dysfunction was present at the time of the hyperkalemic event in 69% of patients. Forty‐nine percent of patients were taking at least one medication that can precipitate hyperkalemia. The most common medications were ACE inhibitors (52%). All baseline characteristics were similar among the different cohorts except for HTN. There were significantly more patients with HTN in the 15 gm dosage group compared to the others. There was no effect of baseline characteristics on the primary or secondary outcomes.
Clinical Outcome
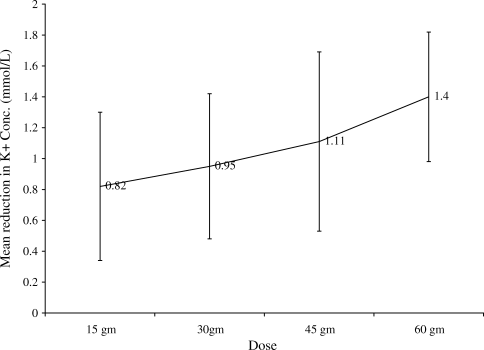
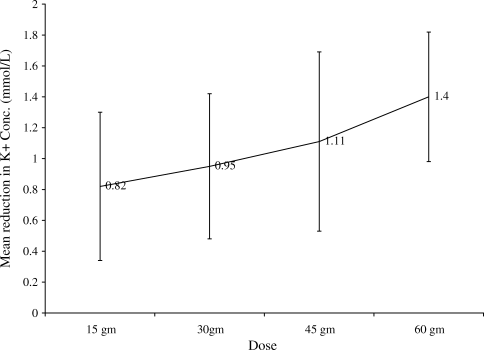
A total of 115 patients (94%) achieved normalization of potassium concentration with a single dose of SPS. The mean SPS dose given was 31.84 13.58 gm. The changes in mean electrolyte values for all patients are shown in Table 2. The mean reduction in potassium concentration was 0.99 0.51 mmol/L (P < 0.0001). The follow‐up potassium concentration was obtained on an average of 10 hours after administration of SPS. The mean reduction in calcium concentration was 0.07 0.53 mg/dL (P = 0.13). Further, the mean reduction in sodium concentration was 0.09 3.33 mg/dL (P = 0.004). Figure 1 depicts the change in serum potassium concentration in each of the SPS dosage groups. The mean change in potassium concentrations compared to baseline was statistically significant in all dosage groups. The mean reduction in potassium concentration achieved statistical significance between the 15 gm and 60 gm groups (P < 0.05) and between the 30 gm and 60 gm groups (P < 0.05) (Table 3). To adjust for the potential covariate effects in these group mean comparisons, we further calculated the LSMEAN. Table 3 presents the least square mean changes in serum potassium concentration in each of the SPS dosage groups. As shown in Table 4, the least square mean changes in potassium concentrations compared to the baseline were also statistically significant in all dosage groups. These group differences remain significant, even after the adjustment by the baseline variables.
| Electrolytes | Pre‐SPS | Post‐SPS | Mean Change | P Value |
|---|---|---|---|---|
| ||||
| K+(mmol/L) | 5.57 0.35 | 4.59 0.46 | 0.99 0.51 | <0.0001 |
| Ca2+(mg/dL) | 8.54 0.81 | 8.46 0.64 | 0.07 0.53 | 0.13 |
| Na+(mmol/L) | 136.7 4.66 | 137.6 4.65 | 0.89 3.33 | 0.004 |
| Dose (gm) | Difference Between Means | CI for Difference Between Means |
|---|---|---|
| ||
| 15‐30 | 0.13 | 0.43, 0.16 |
| 15‐45 | 0.27 | 0.66, 0.11 |
| 15‐60* | 0.64 | 1.09, 0.19 |
| 30‐45 | 0.14 | 0.48, 0.20 |
| 30‐60* | 0.50 | 0.91, 0.10 |
| 45‐60 | 0.36 | 0.82, 0.10 |
| SPS Dose | LSMEAN of Potassium Reduction | 95% Confidence Limits | |
|---|---|---|---|
| |||
| 15 gm | 0.676566 | 0.119091 | 1.234041 |
| 30 gm | 0.808860 | 0.260418 | 1.357302 |
| 45 gm | 0.951205 | 0.431039 | 1.471371 |
| 60 gm | 1.313806 | 0.718793 | 1.908818 |
Subset Analysis
To address cointervention bias, an analysis of patients who were on at least 1 medication that predisposed them to hyperkalemia such as ACE inhibitors, ARBs, potassium sparing diuretics, aldosterone antagonists, NSAIDs, potassium supplements, and antibiotics such as trimethoprim/sulfamethoxazole (n = 60) were reviewed. Of the 60 patients, 30 received SPS and had hyperkalemia‐precipitating medications adjusted and 29 patients received SPS alone. Of those who had medications adjusted, the majority of them (83%) had these medications discontinued. The mean potassium concentration before the intervention was approximately 5.6 mmol/L in both groups. The mean SPS dose given was also similar as well as the mean potassium reduction of approximately 1 mmol/L.
Discussion
In this study, we retrospectively examined inpatients who received a single dose of SPS for the treatment of hyperkalemia. In addition, we established a possible dose response relationship in the treatment of mild to moderate hyperkalemia with SPS monotherapy. Our data revealed that 94% of patients achieved normalization of serum potassium concentration with a single dose of SPS. None of the patients experienced hypokalemia as a result of SPS administration. Moreover, when compared to the work by Mikrut and Brockmiller‐Sell,18 we found a similar reduction in serum potassium associated with the 30 gm (0.95 mmol/L vs. 1 mmol/L) and 60 gm (1.40 vs. 1.48 mEq/L) doses of SPS.
As expected, patients with higher serum potassium concentrations received higher SPS doses. Our data illustrated a possible direct, dose‐response relationship between the SPS dose and the reduction in serum potassium concentration. All of the dosage groups produced a statistically significant reduction in potassium concentrations compared to baseline. Moreover, a statistically significant difference was found in the 60 gm group when compared to the 15 gm and 30 gm groups. The mean increase change in sodium of 0.89 3.33 mmol/L was found to be statistically significant (P = 0.004) but not felt to be clinically relevant. The mean change in calcium concentration of 0.07 0.53 was not statistically significant (P = 0.13).
As with any retrospective chart review, incomplete documentation limits data collection. This may have led to an underestimation of the presence of ARF or other risk factors for hyperkalemia at the time of the event. We excluded patients who received other treatment for hyperkalemia in order to truly assess the effects of SPS on potassium concentration. However, we did not account for all potential confounding variables that are likely to affect potassium concentration such as acid‐base status and glycemic control at the time of the hyperkalemic episode. Also, dietary intake of potassium was not ascertained. Absence of a control group is also a limitation of the study, as it does not allow for complete causality to be established. The sample size, although significantly larger than previous studies, was small especially in regards to the subset analysis. Results cannot be generalized to women since the sample of females was very small or to those who were excluded from the analysis by design. Finally, this study evaluated hospitalized patients and direct extrapolation of the results to outpatient settings should be done cautiously, particularly in light of nutritional considerations.
Our study demonstrates a possible dose‐response relationship in lowering potassium concentrations with SPS. The data presented in this paper can be used to develop a potential dosing guideline for the use of SPS in the clinical management of hyperkalemia in a variety of clinical settings. It provides a basis for a much needed prospective study to finalize a dosing scheme for the use of SPS. Having a dosing scheme would serve as a resource for determining what dose of SPS to give for varying degrees of hyperkalemia while being mindful that individual responses to potassium lowering effect of SPS might be variable.
Acknowledgements
The authors thank the editors and reviewers of The Journal of Hospital Medicine for their comments and suggestions. Jaclyn Ng and Kathya Valdez worked equally on manuscript as second authors.
- ,Hyperkalemia: a review.J Intensive Care Med.2005;20(5):272–290.
- Drug‐induced hyperkalemia: old culprits and new offenders.Am J Med.2000;109(4):307–314.
- ,Hyperkalemia.Am Fam Physician.2006;73(2):283–290.
- ,Indications for hospitalization of patients with hyperkalemia.Arch Intern Med.2000;160(11):1605–1611.
- Hyperkalemia: a potentially lethal clinical condition.Acta Clin Croat.2001;40(3):215–225.
- Hypokalemia and hyperkalemia.Med Clin North Am.1997;81(3):611–639.
- ,,,,Hyperkalemia in patients in hospital.BMJ.1983;286(6372):1189–1192.
- ,,,Treatment of electrolyte disorders in adult patients in the intensive care unit.Am J Health Syst Pharm.2005;62(16):1663–1682.
- ,,,Hyperkalemia revisited.Tex Heart Inst J.2006;33(1):40–47.
- Disorders of potassium homeostasis.Hypokalemia and hyperkalemia. Crit Care Clin.2002;18(2):273–288.
- ,,,Drug‐induced hyperkalemia.Medicine.1985;64(6):357–370.
- ,Therapeutic approach to hyperkalemia.Nephron.2002;92(1):33–40.
- ,Controversial issues in the treatment of hyperkalemia.Nephrol Dial Transplant.2003;18(11):2215–2218.
- Sodium polystyrene sulfonate: a cation exchange resin used in treating hyperkalemia.ANNA J.1993;20(1):93–95.
- ,,,,,Effect of single dose resin‐cathartic therapy on serum potassium concentration in patients with end‐stage renal disease.J Am Soc Nephrol.1998;9(10):1924–1930.
- ,,Treatment of the oliguric patient with a new sodium‐exchange resin and sorbitol, a preliminary report.N Engl J Med.1961;264(3):111–115.
- ,,Management of hyperkalemia with a cation exchange resin.N Engl J Med.1961;264(3):115–119.
- ,Sodium polystyrene sulfonate dosing guidelines for the treatment of adult hyperkalemia.Hosp Pharm.2004;39(8):765–771.
Hyperkalemia is a common and potentially life‐threatening problem encountered in many clinical settings. The incidence of hyperkalemia in hospitalized patients has been estimated to be between 1% and 10% in the United States each year.16 Medications are thought to contribute to the development of hyperkalemia in 35% to 75% of hospitalized patients.12, 9 Medications known to cause hyperkalemia include angiotensin‐converting enzyme (ACE) inhibitors, angiotensin II receptor antagonists (ARBs), potassium‐sparing diuretics, nonsteroidal anti‐inflammatory drugs (NSAIDs), potassium supplements, and antibiotics such as trimethoprim/sulfamethoxazole. Patients with hyperkalemia may develop neuromuscular symptoms such as fatigue, muscle weakness, tingling, numbness, and cramping. The most deleterious effect of hyperkalemia is cardiac toxicity, including life‐threatening arrhythmias.3, 711
Sodium polystyrene sulfonate (SPS) or Kayexalate is a cation‐exchange resin that is commonly employed to lower total body potassium in patients with mild to moderate hyperkalemia.1214 It removes potassium from the gut in exchange for sodium. SPS can be given orally or as an enema. When given orally, it is commonly administered with sorbitol to promote diarrhea. The onset of action is within 1 to 2 hours and lasts approximately 4 to 6 hours. The recommended average daily dose is 15 gm to 60 gm given as a single dose or in divided doses. An in vitro study indicated that each gram of resin binds to approximately 3.1 mEq of potassium.14 However, the majority of the exchange capacity is utilized for cations other than potassium. Therefore, the in vivo exchange capacity is thought to be no greater than 1 mEq of potassium per gram of resin.
There are conflicting data regarding the effectiveness of exchange resins.1, 13 In 1998, Guy‐Kapral et al.15 found that a 30‐gm dose of SPS failed to lower serum potassium concentration in end‐stage renal disease patients who were normokalemic. In contrast, Flinn et al.16 demonstrated effective lowering of serum potassium over a period of several days in which repeated doses of SPS were given to hyperkalemic patients. Moreover, in another study, serum potassium concentration was reduced by 1.0 mEq/L in 24 hours.17 However, this study was limited since subjects also received other potassium‐lowering agents such as insulin/glucose or sodium bicarbonate.
In 2004, Mikrut and Brockmiller‐Sell18 studied the dose response between SPS and serum potassium concentration. Their data demonstrated an average reduction in potassium concentration of approximately 1 mmol/L and 1.48 mmol/L following a 30 gm and a 60 gm dose of SPS, respectively. However, the study was small (n = 39) and only evaluated 2 dosages. In clinical practice, there is wide variability in SPS dosing. Furthermore, studies assessing the effects of different doses of SPS are lacking. Often, the dose chosen is dependent on comorbid conditions, concomitant medications and provider experience in treating hyperkalemia. The aim of our study was to examine the single‐dose effect of SPS on potassium concentration in hospitalized, hyperkalemic patients and to compare the potassium lowering effects of SPS doses commonly used.
Methods
Study Population
A retrospective cohort study involving a review of electronic medical records of inpatients receiving SPS for the treatment of hyperkalemia was conducted at the Jesse Brown Veteran Affairs Medical Center, between January 1, 2006 and December 31, 2006. Hyperkalemia was defined as a serum potassium concentration >5.1 mmol/L, as this concentration corresponded to the upper limit of normal for serum potassium at our hospital. The patient list was generated from prescription databases. Exclusion criteria included: chronic SPS use, rectal administration of SPS, multiple doses of SPS spaced less than 6 hours apart, a hemolyzed laboratory specimen, current hemodialysis, lack of a follow‐up serum potassium concentration, or other treatments started for hyperkalemia at the time of the event, specifically insulin, dextrose, albuterol, and/or furosemide. This study protocol received approval from the local Institutional Review Board and the hospital's research and development committee.
Data Collection
The Computerized Patient Record System (CPRS), the electronic medical record system used at Jesse Brown VA Medical Center (JBVAMC), was utilized to gather patient baseline demographic information consisting of age, gender, weight, and height. Data on comorbid conditions such as hypertension (HTN), diabetes mellitus (DM), chronic heart failure (CHF), chronic kidney disease (CKD), and acute renal failure (ARF) was identified from the patient's problem list in the medical record. Additionally, the authors abstracted information about concomitant hyperkalemia‐precipitating medications including ACE inhibitors, ARBs, potassium‐sparing diuretics, aldosterone antagonists, NSAIDs, potassium supplements, and antibiotics such as trimethoprim/sulfamethoxazole. Progress notes and discharge summaries were reviewed for documentation of ARF. Laboratory information including serum creatinine, blood urea nitrogen, potassium, sodium, and calcium concentrations before and after SPS administration was recorded. The date and time of laboratory draws were also collected. Data on SPS administration regarding time and date, as well as dosing information, was obtained from the medication administration records.
Outcomes
The primary outcome were the mean change in serum potassium after a single dose of SPS compared to the baseline. The data were also evaluated based on the dose of SPS that was administered, 15 gm, 30 gm, 45 gm, or 60 gm doses of SPS. Secondary outcomes were the mean change in sodium and calcium concentrations.
Statistical Analysis
In order to complete the statistical analysis for the study, continuous variables were summarized as mean standard deviation, and categorical variables were summarized as frequency counts and proportions. Group comparisons for categorical data in baseline characteristics were performed using the Fisher's exact test and the chi‐square test. The comparisons for continuous data in baseline characteristics were performed using analysis of variance (ANOVA). A paired t‐test was then used to compare changes in potassium, calcium, and sodium concentrations and again to compare the changes in potassium concentrations by different dosage groups. Subset analysis also required the paired t‐test. The differences in the change of potassium concentration between dosage groups were analyzed by ANOVA. Post hoc comparisons were performed to identify which groups were statistically different from each other. A P value less than 0.05 was considered statistically significant. The effect of dosage on primary outcome was also assessed by adjusting baseline characteristics using analysis of covariance (ANCOVA). Least square means (LSMEAN) of the primary outcome were calculated using the ANCOVA model with the SPS dosage groups as the main categorical independent variable and the baseline characteristics in Table 1 as the covariates. The LSMEAN are group means after controlling for covariates (they are the predicted group means, predicted as the population mean values of the covariates in the model). All statistical analyses were done using SAS software, version 9.1 (Cary, NC).
| All (n = 122) | 15 gm (n = 30) | 30 gm (n = 60) | 45 gm (n = 19) | 60 gm (n = 13) | P Value | |
|---|---|---|---|---|---|---|
| ||||||
| Male, n (%) | 121 (99) | 30 (100) | 60 (100) | 18 (95) | 13 (100) | 0.14 |
| Age (year) | 69.0 11.4 | 70.71 0.5 | 70.2 11.5 | 68.0 1.7 | 63.8 12.2 | 0.25 |
| Height (inch) | 69.0 3.5 | 69.2 4.6 | 68.8 3.3 | 68.2 3.0 | 70.5 1.3 | 0.31 |
| Weight (kg) | 81.4 20.7 | 77.3 15.0 | 83.1 23.3 | 82.7 21.7 | 81.6 18.1 | 0.64 |
| Hypertension, n (%) | 96 (79) | 29*(97) | 47 (78) | 12 (63) | 8 (62) | 0.01* |
| Diabetes Mellitus, n (%) | 41 (34) | 10 (33) | 21 (35) | 6 (32) | 4 (31) | 0.99 |
| Chronic kidney disease, n (%) | 46 (38) | 10 (33) | 21 (35) | 8 (42) | 7 (54) | 0.57 |
| Heart failure, n (%) | 32 (26) | 12 (40) | 13 (22) | 6 (32) | 1 (8) | 0.10 |
| Serum creatinine (mg/dL) | 2.57 2.4 | 2.09 1.5 | 2.93 3.0 | 2.37 1.2 | 2.29 2.1 | 0.41 |
| Acute renal failure, n (%) | 84 (69) | 19 (63) | 45 (75) | 12 (63) | 8 (62) | 0.55 |
| Concomitant hyperkalemia‐precipitating medications, n (%) | 60 (49) | 19 (63) | 23 (38) | 10 (53) | 8 (61) | 0.11 |
Results
Patient Characteristics
Study subjects consisted of patients who received SPS in the hospital for treatment of hyperkalemia. A total of 140 patients were identified and 122 met the inclusion criteria. Eighteen patients were excluded for current hemodialysis, receiving other treatments for hyperkalemia, and specimen hemolysis. Of those who were included in the study analysis, 30 patients received 15 gm of SPS, 60 patients received 30 gm, 19 patients received 45 gm, and 13 patients received 60 gm. Baseline characteristics of included patients are shown in Table 1. The majority of patients were male (99%) and had comorbid conditions.
At baseline, the mean serum creatinine concentration was 2.57 2.36 mg/dL. Documented acute renal dysfunction was present at the time of the hyperkalemic event in 69% of patients. Forty‐nine percent of patients were taking at least one medication that can precipitate hyperkalemia. The most common medications were ACE inhibitors (52%). All baseline characteristics were similar among the different cohorts except for HTN. There were significantly more patients with HTN in the 15 gm dosage group compared to the others. There was no effect of baseline characteristics on the primary or secondary outcomes.
Clinical Outcome
A total of 115 patients (94%) achieved normalization of potassium concentration with a single dose of SPS. The mean SPS dose given was 31.84 13.58 gm. The changes in mean electrolyte values for all patients are shown in Table 2. The mean reduction in potassium concentration was 0.99 0.51 mmol/L (P < 0.0001). The follow‐up potassium concentration was obtained on an average of 10 hours after administration of SPS. The mean reduction in calcium concentration was 0.07 0.53 mg/dL (P = 0.13). Further, the mean reduction in sodium concentration was 0.09 3.33 mg/dL (P = 0.004). Figure 1 depicts the change in serum potassium concentration in each of the SPS dosage groups. The mean change in potassium concentrations compared to baseline was statistically significant in all dosage groups. The mean reduction in potassium concentration achieved statistical significance between the 15 gm and 60 gm groups (P < 0.05) and between the 30 gm and 60 gm groups (P < 0.05) (Table 3). To adjust for the potential covariate effects in these group mean comparisons, we further calculated the LSMEAN. Table 3 presents the least square mean changes in serum potassium concentration in each of the SPS dosage groups. As shown in Table 4, the least square mean changes in potassium concentrations compared to the baseline were also statistically significant in all dosage groups. These group differences remain significant, even after the adjustment by the baseline variables.

| Electrolytes | Pre‐SPS | Post‐SPS | Mean Change | P Value |
|---|---|---|---|---|
| ||||
| K+(mmol/L) | 5.57 0.35 | 4.59 0.46 | 0.99 0.51 | <0.0001 |
| Ca2+(mg/dL) | 8.54 0.81 | 8.46 0.64 | 0.07 0.53 | 0.13 |
| Na+(mmol/L) | 136.7 4.66 | 137.6 4.65 | 0.89 3.33 | 0.004 |
| Dose (gm) | Difference Between Means | CI for Difference Between Means |
|---|---|---|
| ||
| 15‐30 | 0.13 | 0.43, 0.16 |
| 15‐45 | 0.27 | 0.66, 0.11 |
| 15‐60* | 0.64 | 1.09, 0.19 |
| 30‐45 | 0.14 | 0.48, 0.20 |
| 30‐60* | 0.50 | 0.91, 0.10 |
| 45‐60 | 0.36 | 0.82, 0.10 |
| SPS Dose | LSMEAN of Potassium Reduction | 95% Confidence Limits | |
|---|---|---|---|
| |||
| 15 gm | 0.676566 | 0.119091 | 1.234041 |
| 30 gm | 0.808860 | 0.260418 | 1.357302 |
| 45 gm | 0.951205 | 0.431039 | 1.471371 |
| 60 gm | 1.313806 | 0.718793 | 1.908818 |
Subset Analysis
To address cointervention bias, an analysis of patients who were on at least 1 medication that predisposed them to hyperkalemia such as ACE inhibitors, ARBs, potassium sparing diuretics, aldosterone antagonists, NSAIDs, potassium supplements, and antibiotics such as trimethoprim/sulfamethoxazole (n = 60) were reviewed. Of the 60 patients, 30 received SPS and had hyperkalemia‐precipitating medications adjusted and 29 patients received SPS alone. Of those who had medications adjusted, the majority of them (83%) had these medications discontinued. The mean potassium concentration before the intervention was approximately 5.6 mmol/L in both groups. The mean SPS dose given was also similar as well as the mean potassium reduction of approximately 1 mmol/L.
Discussion
In this study, we retrospectively examined inpatients who received a single dose of SPS for the treatment of hyperkalemia. In addition, we established a possible dose response relationship in the treatment of mild to moderate hyperkalemia with SPS monotherapy. Our data revealed that 94% of patients achieved normalization of serum potassium concentration with a single dose of SPS. None of the patients experienced hypokalemia as a result of SPS administration. Moreover, when compared to the work by Mikrut and Brockmiller‐Sell,18 we found a similar reduction in serum potassium associated with the 30 gm (0.95 mmol/L vs. 1 mmol/L) and 60 gm (1.40 vs. 1.48 mEq/L) doses of SPS.
As expected, patients with higher serum potassium concentrations received higher SPS doses. Our data illustrated a possible direct, dose‐response relationship between the SPS dose and the reduction in serum potassium concentration. All of the dosage groups produced a statistically significant reduction in potassium concentrations compared to baseline. Moreover, a statistically significant difference was found in the 60 gm group when compared to the 15 gm and 30 gm groups. The mean increase change in sodium of 0.89 3.33 mmol/L was found to be statistically significant (P = 0.004) but not felt to be clinically relevant. The mean change in calcium concentration of 0.07 0.53 was not statistically significant (P = 0.13).
As with any retrospective chart review, incomplete documentation limits data collection. This may have led to an underestimation of the presence of ARF or other risk factors for hyperkalemia at the time of the event. We excluded patients who received other treatment for hyperkalemia in order to truly assess the effects of SPS on potassium concentration. However, we did not account for all potential confounding variables that are likely to affect potassium concentration such as acid‐base status and glycemic control at the time of the hyperkalemic episode. Also, dietary intake of potassium was not ascertained. Absence of a control group is also a limitation of the study, as it does not allow for complete causality to be established. The sample size, although significantly larger than previous studies, was small especially in regards to the subset analysis. Results cannot be generalized to women since the sample of females was very small or to those who were excluded from the analysis by design. Finally, this study evaluated hospitalized patients and direct extrapolation of the results to outpatient settings should be done cautiously, particularly in light of nutritional considerations.
Our study demonstrates a possible dose‐response relationship in lowering potassium concentrations with SPS. The data presented in this paper can be used to develop a potential dosing guideline for the use of SPS in the clinical management of hyperkalemia in a variety of clinical settings. It provides a basis for a much needed prospective study to finalize a dosing scheme for the use of SPS. Having a dosing scheme would serve as a resource for determining what dose of SPS to give for varying degrees of hyperkalemia while being mindful that individual responses to potassium lowering effect of SPS might be variable.
Acknowledgements
The authors thank the editors and reviewers of The Journal of Hospital Medicine for their comments and suggestions. Jaclyn Ng and Kathya Valdez worked equally on manuscript as second authors.
Hyperkalemia is a common and potentially life‐threatening problem encountered in many clinical settings. The incidence of hyperkalemia in hospitalized patients has been estimated to be between 1% and 10% in the United States each year.16 Medications are thought to contribute to the development of hyperkalemia in 35% to 75% of hospitalized patients.12, 9 Medications known to cause hyperkalemia include angiotensin‐converting enzyme (ACE) inhibitors, angiotensin II receptor antagonists (ARBs), potassium‐sparing diuretics, nonsteroidal anti‐inflammatory drugs (NSAIDs), potassium supplements, and antibiotics such as trimethoprim/sulfamethoxazole. Patients with hyperkalemia may develop neuromuscular symptoms such as fatigue, muscle weakness, tingling, numbness, and cramping. The most deleterious effect of hyperkalemia is cardiac toxicity, including life‐threatening arrhythmias.3, 711
Sodium polystyrene sulfonate (SPS) or Kayexalate is a cation‐exchange resin that is commonly employed to lower total body potassium in patients with mild to moderate hyperkalemia.1214 It removes potassium from the gut in exchange for sodium. SPS can be given orally or as an enema. When given orally, it is commonly administered with sorbitol to promote diarrhea. The onset of action is within 1 to 2 hours and lasts approximately 4 to 6 hours. The recommended average daily dose is 15 gm to 60 gm given as a single dose or in divided doses. An in vitro study indicated that each gram of resin binds to approximately 3.1 mEq of potassium.14 However, the majority of the exchange capacity is utilized for cations other than potassium. Therefore, the in vivo exchange capacity is thought to be no greater than 1 mEq of potassium per gram of resin.
There are conflicting data regarding the effectiveness of exchange resins.1, 13 In 1998, Guy‐Kapral et al.15 found that a 30‐gm dose of SPS failed to lower serum potassium concentration in end‐stage renal disease patients who were normokalemic. In contrast, Flinn et al.16 demonstrated effective lowering of serum potassium over a period of several days in which repeated doses of SPS were given to hyperkalemic patients. Moreover, in another study, serum potassium concentration was reduced by 1.0 mEq/L in 24 hours.17 However, this study was limited since subjects also received other potassium‐lowering agents such as insulin/glucose or sodium bicarbonate.
In 2004, Mikrut and Brockmiller‐Sell18 studied the dose response between SPS and serum potassium concentration. Their data demonstrated an average reduction in potassium concentration of approximately 1 mmol/L and 1.48 mmol/L following a 30 gm and a 60 gm dose of SPS, respectively. However, the study was small (n = 39) and only evaluated 2 dosages. In clinical practice, there is wide variability in SPS dosing. Furthermore, studies assessing the effects of different doses of SPS are lacking. Often, the dose chosen is dependent on comorbid conditions, concomitant medications and provider experience in treating hyperkalemia. The aim of our study was to examine the single‐dose effect of SPS on potassium concentration in hospitalized, hyperkalemic patients and to compare the potassium lowering effects of SPS doses commonly used.
Methods
Study Population
A retrospective cohort study involving a review of electronic medical records of inpatients receiving SPS for the treatment of hyperkalemia was conducted at the Jesse Brown Veteran Affairs Medical Center, between January 1, 2006 and December 31, 2006. Hyperkalemia was defined as a serum potassium concentration >5.1 mmol/L, as this concentration corresponded to the upper limit of normal for serum potassium at our hospital. The patient list was generated from prescription databases. Exclusion criteria included: chronic SPS use, rectal administration of SPS, multiple doses of SPS spaced less than 6 hours apart, a hemolyzed laboratory specimen, current hemodialysis, lack of a follow‐up serum potassium concentration, or other treatments started for hyperkalemia at the time of the event, specifically insulin, dextrose, albuterol, and/or furosemide. This study protocol received approval from the local Institutional Review Board and the hospital's research and development committee.
Data Collection
The Computerized Patient Record System (CPRS), the electronic medical record system used at Jesse Brown VA Medical Center (JBVAMC), was utilized to gather patient baseline demographic information consisting of age, gender, weight, and height. Data on comorbid conditions such as hypertension (HTN), diabetes mellitus (DM), chronic heart failure (CHF), chronic kidney disease (CKD), and acute renal failure (ARF) was identified from the patient's problem list in the medical record. Additionally, the authors abstracted information about concomitant hyperkalemia‐precipitating medications including ACE inhibitors, ARBs, potassium‐sparing diuretics, aldosterone antagonists, NSAIDs, potassium supplements, and antibiotics such as trimethoprim/sulfamethoxazole. Progress notes and discharge summaries were reviewed for documentation of ARF. Laboratory information including serum creatinine, blood urea nitrogen, potassium, sodium, and calcium concentrations before and after SPS administration was recorded. The date and time of laboratory draws were also collected. Data on SPS administration regarding time and date, as well as dosing information, was obtained from the medication administration records.
Outcomes
The primary outcome were the mean change in serum potassium after a single dose of SPS compared to the baseline. The data were also evaluated based on the dose of SPS that was administered, 15 gm, 30 gm, 45 gm, or 60 gm doses of SPS. Secondary outcomes were the mean change in sodium and calcium concentrations.
Statistical Analysis
In order to complete the statistical analysis for the study, continuous variables were summarized as mean standard deviation, and categorical variables were summarized as frequency counts and proportions. Group comparisons for categorical data in baseline characteristics were performed using the Fisher's exact test and the chi‐square test. The comparisons for continuous data in baseline characteristics were performed using analysis of variance (ANOVA). A paired t‐test was then used to compare changes in potassium, calcium, and sodium concentrations and again to compare the changes in potassium concentrations by different dosage groups. Subset analysis also required the paired t‐test. The differences in the change of potassium concentration between dosage groups were analyzed by ANOVA. Post hoc comparisons were performed to identify which groups were statistically different from each other. A P value less than 0.05 was considered statistically significant. The effect of dosage on primary outcome was also assessed by adjusting baseline characteristics using analysis of covariance (ANCOVA). Least square means (LSMEAN) of the primary outcome were calculated using the ANCOVA model with the SPS dosage groups as the main categorical independent variable and the baseline characteristics in Table 1 as the covariates. The LSMEAN are group means after controlling for covariates (they are the predicted group means, predicted as the population mean values of the covariates in the model). All statistical analyses were done using SAS software, version 9.1 (Cary, NC).
| All (n = 122) | 15 gm (n = 30) | 30 gm (n = 60) | 45 gm (n = 19) | 60 gm (n = 13) | P Value | |
|---|---|---|---|---|---|---|
| ||||||
| Male, n (%) | 121 (99) | 30 (100) | 60 (100) | 18 (95) | 13 (100) | 0.14 |
| Age (year) | 69.0 11.4 | 70.71 0.5 | 70.2 11.5 | 68.0 1.7 | 63.8 12.2 | 0.25 |
| Height (inch) | 69.0 3.5 | 69.2 4.6 | 68.8 3.3 | 68.2 3.0 | 70.5 1.3 | 0.31 |
| Weight (kg) | 81.4 20.7 | 77.3 15.0 | 83.1 23.3 | 82.7 21.7 | 81.6 18.1 | 0.64 |
| Hypertension, n (%) | 96 (79) | 29*(97) | 47 (78) | 12 (63) | 8 (62) | 0.01* |
| Diabetes Mellitus, n (%) | 41 (34) | 10 (33) | 21 (35) | 6 (32) | 4 (31) | 0.99 |
| Chronic kidney disease, n (%) | 46 (38) | 10 (33) | 21 (35) | 8 (42) | 7 (54) | 0.57 |
| Heart failure, n (%) | 32 (26) | 12 (40) | 13 (22) | 6 (32) | 1 (8) | 0.10 |
| Serum creatinine (mg/dL) | 2.57 2.4 | 2.09 1.5 | 2.93 3.0 | 2.37 1.2 | 2.29 2.1 | 0.41 |
| Acute renal failure, n (%) | 84 (69) | 19 (63) | 45 (75) | 12 (63) | 8 (62) | 0.55 |
| Concomitant hyperkalemia‐precipitating medications, n (%) | 60 (49) | 19 (63) | 23 (38) | 10 (53) | 8 (61) | 0.11 |
Results
Patient Characteristics
Study subjects consisted of patients who received SPS in the hospital for treatment of hyperkalemia. A total of 140 patients were identified and 122 met the inclusion criteria. Eighteen patients were excluded for current hemodialysis, receiving other treatments for hyperkalemia, and specimen hemolysis. Of those who were included in the study analysis, 30 patients received 15 gm of SPS, 60 patients received 30 gm, 19 patients received 45 gm, and 13 patients received 60 gm. Baseline characteristics of included patients are shown in Table 1. The majority of patients were male (99%) and had comorbid conditions.
At baseline, the mean serum creatinine concentration was 2.57 2.36 mg/dL. Documented acute renal dysfunction was present at the time of the hyperkalemic event in 69% of patients. Forty‐nine percent of patients were taking at least one medication that can precipitate hyperkalemia. The most common medications were ACE inhibitors (52%). All baseline characteristics were similar among the different cohorts except for HTN. There were significantly more patients with HTN in the 15 gm dosage group compared to the others. There was no effect of baseline characteristics on the primary or secondary outcomes.
Clinical Outcome
A total of 115 patients (94%) achieved normalization of potassium concentration with a single dose of SPS. The mean SPS dose given was 31.84 13.58 gm. The changes in mean electrolyte values for all patients are shown in Table 2. The mean reduction in potassium concentration was 0.99 0.51 mmol/L (P < 0.0001). The follow‐up potassium concentration was obtained on an average of 10 hours after administration of SPS. The mean reduction in calcium concentration was 0.07 0.53 mg/dL (P = 0.13). Further, the mean reduction in sodium concentration was 0.09 3.33 mg/dL (P = 0.004). Figure 1 depicts the change in serum potassium concentration in each of the SPS dosage groups. The mean change in potassium concentrations compared to baseline was statistically significant in all dosage groups. The mean reduction in potassium concentration achieved statistical significance between the 15 gm and 60 gm groups (P < 0.05) and between the 30 gm and 60 gm groups (P < 0.05) (Table 3). To adjust for the potential covariate effects in these group mean comparisons, we further calculated the LSMEAN. Table 3 presents the least square mean changes in serum potassium concentration in each of the SPS dosage groups. As shown in Table 4, the least square mean changes in potassium concentrations compared to the baseline were also statistically significant in all dosage groups. These group differences remain significant, even after the adjustment by the baseline variables.

| Electrolytes | Pre‐SPS | Post‐SPS | Mean Change | P Value |
|---|---|---|---|---|
| ||||
| K+(mmol/L) | 5.57 0.35 | 4.59 0.46 | 0.99 0.51 | <0.0001 |
| Ca2+(mg/dL) | 8.54 0.81 | 8.46 0.64 | 0.07 0.53 | 0.13 |
| Na+(mmol/L) | 136.7 4.66 | 137.6 4.65 | 0.89 3.33 | 0.004 |
| Dose (gm) | Difference Between Means | CI for Difference Between Means |
|---|---|---|
| ||
| 15‐30 | 0.13 | 0.43, 0.16 |
| 15‐45 | 0.27 | 0.66, 0.11 |
| 15‐60* | 0.64 | 1.09, 0.19 |
| 30‐45 | 0.14 | 0.48, 0.20 |
| 30‐60* | 0.50 | 0.91, 0.10 |
| 45‐60 | 0.36 | 0.82, 0.10 |
| SPS Dose | LSMEAN of Potassium Reduction | 95% Confidence Limits | |
|---|---|---|---|
| |||
| 15 gm | 0.676566 | 0.119091 | 1.234041 |
| 30 gm | 0.808860 | 0.260418 | 1.357302 |
| 45 gm | 0.951205 | 0.431039 | 1.471371 |
| 60 gm | 1.313806 | 0.718793 | 1.908818 |
Subset Analysis
To address cointervention bias, an analysis of patients who were on at least 1 medication that predisposed them to hyperkalemia such as ACE inhibitors, ARBs, potassium sparing diuretics, aldosterone antagonists, NSAIDs, potassium supplements, and antibiotics such as trimethoprim/sulfamethoxazole (n = 60) were reviewed. Of the 60 patients, 30 received SPS and had hyperkalemia‐precipitating medications adjusted and 29 patients received SPS alone. Of those who had medications adjusted, the majority of them (83%) had these medications discontinued. The mean potassium concentration before the intervention was approximately 5.6 mmol/L in both groups. The mean SPS dose given was also similar as well as the mean potassium reduction of approximately 1 mmol/L.
Discussion
In this study, we retrospectively examined inpatients who received a single dose of SPS for the treatment of hyperkalemia. In addition, we established a possible dose response relationship in the treatment of mild to moderate hyperkalemia with SPS monotherapy. Our data revealed that 94% of patients achieved normalization of serum potassium concentration with a single dose of SPS. None of the patients experienced hypokalemia as a result of SPS administration. Moreover, when compared to the work by Mikrut and Brockmiller‐Sell,18 we found a similar reduction in serum potassium associated with the 30 gm (0.95 mmol/L vs. 1 mmol/L) and 60 gm (1.40 vs. 1.48 mEq/L) doses of SPS.
As expected, patients with higher serum potassium concentrations received higher SPS doses. Our data illustrated a possible direct, dose‐response relationship between the SPS dose and the reduction in serum potassium concentration. All of the dosage groups produced a statistically significant reduction in potassium concentrations compared to baseline. Moreover, a statistically significant difference was found in the 60 gm group when compared to the 15 gm and 30 gm groups. The mean increase change in sodium of 0.89 3.33 mmol/L was found to be statistically significant (P = 0.004) but not felt to be clinically relevant. The mean change in calcium concentration of 0.07 0.53 was not statistically significant (P = 0.13).
As with any retrospective chart review, incomplete documentation limits data collection. This may have led to an underestimation of the presence of ARF or other risk factors for hyperkalemia at the time of the event. We excluded patients who received other treatment for hyperkalemia in order to truly assess the effects of SPS on potassium concentration. However, we did not account for all potential confounding variables that are likely to affect potassium concentration such as acid‐base status and glycemic control at the time of the hyperkalemic episode. Also, dietary intake of potassium was not ascertained. Absence of a control group is also a limitation of the study, as it does not allow for complete causality to be established. The sample size, although significantly larger than previous studies, was small especially in regards to the subset analysis. Results cannot be generalized to women since the sample of females was very small or to those who were excluded from the analysis by design. Finally, this study evaluated hospitalized patients and direct extrapolation of the results to outpatient settings should be done cautiously, particularly in light of nutritional considerations.
Our study demonstrates a possible dose‐response relationship in lowering potassium concentrations with SPS. The data presented in this paper can be used to develop a potential dosing guideline for the use of SPS in the clinical management of hyperkalemia in a variety of clinical settings. It provides a basis for a much needed prospective study to finalize a dosing scheme for the use of SPS. Having a dosing scheme would serve as a resource for determining what dose of SPS to give for varying degrees of hyperkalemia while being mindful that individual responses to potassium lowering effect of SPS might be variable.
Acknowledgements
The authors thank the editors and reviewers of The Journal of Hospital Medicine for their comments and suggestions. Jaclyn Ng and Kathya Valdez worked equally on manuscript as second authors.
- ,Hyperkalemia: a review.J Intensive Care Med.2005;20(5):272–290.
- Drug‐induced hyperkalemia: old culprits and new offenders.Am J Med.2000;109(4):307–314.
- ,Hyperkalemia.Am Fam Physician.2006;73(2):283–290.
- ,Indications for hospitalization of patients with hyperkalemia.Arch Intern Med.2000;160(11):1605–1611.
- Hyperkalemia: a potentially lethal clinical condition.Acta Clin Croat.2001;40(3):215–225.
- Hypokalemia and hyperkalemia.Med Clin North Am.1997;81(3):611–639.
- ,,,,Hyperkalemia in patients in hospital.BMJ.1983;286(6372):1189–1192.
- ,,,Treatment of electrolyte disorders in adult patients in the intensive care unit.Am J Health Syst Pharm.2005;62(16):1663–1682.
- ,,,Hyperkalemia revisited.Tex Heart Inst J.2006;33(1):40–47.
- Disorders of potassium homeostasis.Hypokalemia and hyperkalemia. Crit Care Clin.2002;18(2):273–288.
- ,,,Drug‐induced hyperkalemia.Medicine.1985;64(6):357–370.
- ,Therapeutic approach to hyperkalemia.Nephron.2002;92(1):33–40.
- ,Controversial issues in the treatment of hyperkalemia.Nephrol Dial Transplant.2003;18(11):2215–2218.
- Sodium polystyrene sulfonate: a cation exchange resin used in treating hyperkalemia.ANNA J.1993;20(1):93–95.
- ,,,,,Effect of single dose resin‐cathartic therapy on serum potassium concentration in patients with end‐stage renal disease.J Am Soc Nephrol.1998;9(10):1924–1930.
- ,,Treatment of the oliguric patient with a new sodium‐exchange resin and sorbitol, a preliminary report.N Engl J Med.1961;264(3):111–115.
- ,,Management of hyperkalemia with a cation exchange resin.N Engl J Med.1961;264(3):115–119.
- ,Sodium polystyrene sulfonate dosing guidelines for the treatment of adult hyperkalemia.Hosp Pharm.2004;39(8):765–771.
- ,Hyperkalemia: a review.J Intensive Care Med.2005;20(5):272–290.
- Drug‐induced hyperkalemia: old culprits and new offenders.Am J Med.2000;109(4):307–314.
- ,Hyperkalemia.Am Fam Physician.2006;73(2):283–290.
- ,Indications for hospitalization of patients with hyperkalemia.Arch Intern Med.2000;160(11):1605–1611.
- Hyperkalemia: a potentially lethal clinical condition.Acta Clin Croat.2001;40(3):215–225.
- Hypokalemia and hyperkalemia.Med Clin North Am.1997;81(3):611–639.
- ,,,,Hyperkalemia in patients in hospital.BMJ.1983;286(6372):1189–1192.
- ,,,Treatment of electrolyte disorders in adult patients in the intensive care unit.Am J Health Syst Pharm.2005;62(16):1663–1682.
- ,,,Hyperkalemia revisited.Tex Heart Inst J.2006;33(1):40–47.
- Disorders of potassium homeostasis.Hypokalemia and hyperkalemia. Crit Care Clin.2002;18(2):273–288.
- ,,,Drug‐induced hyperkalemia.Medicine.1985;64(6):357–370.
- ,Therapeutic approach to hyperkalemia.Nephron.2002;92(1):33–40.
- ,Controversial issues in the treatment of hyperkalemia.Nephrol Dial Transplant.2003;18(11):2215–2218.
- Sodium polystyrene sulfonate: a cation exchange resin used in treating hyperkalemia.ANNA J.1993;20(1):93–95.
- ,,,,,Effect of single dose resin‐cathartic therapy on serum potassium concentration in patients with end‐stage renal disease.J Am Soc Nephrol.1998;9(10):1924–1930.
- ,,Treatment of the oliguric patient with a new sodium‐exchange resin and sorbitol, a preliminary report.N Engl J Med.1961;264(3):111–115.
- ,,Management of hyperkalemia with a cation exchange resin.N Engl J Med.1961;264(3):115–119.
- ,Sodium polystyrene sulfonate dosing guidelines for the treatment of adult hyperkalemia.Hosp Pharm.2004;39(8):765–771.
Copyright © 2011 Society of Hospital Medicine
Faculty Development for Hospitalists
The growth of hospitalists nationally continues at an unprecedented pace.1 In academic medical centers, the development of hospital medicine groups either as independent divisions or as part of divisions of general internal medicine (DGIM) reflects this trend. Drivers for growth in the academic setting include housestaff work hour restrictions, increased need for oversight on teaching services, development of nonhousestaff services, surgical comanagement, and greater emphasis on efficiency, quality, and safety.26 These drivers have created tremendous opportunities for hospitalists, but the rapid growth has also created challenges to achieving traditional academic success.7, 8
While hospitalists feel the traditional academic pressures to produce new knowledge and teach, the extraordinary need to expand clinical services has resulted in a young hospitalist workforce, with most lacking fellowship training. At the same time, there are few senior mentors available. Taken together, many academic hospital medicine (AHM) programs find themselves populated by large cadres of junior faculty without the support, training, and mentoring they need to succeed in a faculty career.9 For hospital medicine groups, the risk to faculty recruitment, retention, productivity, and morale is high.
In this article, we describe the development and implementation of a multifaceted Faculty Development (FD) program whose goal was to provide our faculty with clinical, educational, leadership, and scholarly skills that would promote academic output and foster work satisfaction.
Methods
Problem Identification
The University of California, San Francisco (UCSF) Medical Center operates nearly 800 beds across 2 hospitals (Parnassus and Mount Zion campuses). The UCSF Division of Hospital Medicine (DHM) provides care on the teaching service (90% of all ward months covered by a hospitalist faculty), a nonhousestaff medical service based at Mount Zion,4 a palliative care service,10 a medical consultation service, a neurosurgical comanagement service, a procedure service, and comanagement on advanced heart failure and cancer services. Like many AHM groups, ours has experienced explosive growth, more than doubling in faculty size in 3 years (50+ faculty by July 2010).
In addition, many of our new faculty joined the division directly after residency training whereas our early hospitalists were mostly former chief residents and/or fellowship‐trained. During a 2‐year period, our division lost several faculty to burnout from clinically heavy positions or because they felt their ultimate academic success was in doubt. During a 2008 divisional retreat, the single greatest need identified was to invest in the development of our first‐year faculty who were felt to be at greatest risk for burnout, dissatisfaction, and failing to integrate into the divisional mission. Based on this result, we set out to develop a program to meet this pressing need.
Needs Assessment
We formed a FD steering committee comprised of faculty from all ranks and career paths in our division (eg, educator, administrator, and investigator), with overrepresentation of recent hires to ascertain how best to meet their needs. Information from the division retreat provided the basis for the program and its priorities. The FD steering committee then outlined ideas that guided program development, which included:
New faculty should be required to meet regularly with assigned faculty mentors during their first year, and expectations for that relationship should be outlined for both parties
New faculty should be required to attend dedicated sessions that build their teaching skills
New faculty should receive a specially designed first year curriculum to provide learnings focused on high‐yield and relevant topics
New faculty should receive a set of goals, or scholarly expectations, for their first year that would foster a partnership between individual faculty and the division to meet those goals
The division should create new structures for FD that promote collaboration, sharing of personal and professional growth and challenges, and a culture of continuous learning
All of the activities that comprise our new FD program must be aligned with our stated mission: to provide the highest quality clinical care, education, system improvements, and research that benefit our patients and trainees by developing successful academic hospitalist faculty.
Program Goals and Objectives
Our DHM FD program established the goal to provide our new faculty with clinical, educational, leadership, and scholarly skills that would promote academic output and foster work satisfaction. From a broader divisional standpoint, the goal was simply to create new FD structures that fostered the division's commitment to the program. The primary objectives of the program were for new faculty to:
Increase their knowledge, skills, and attitudes about key academic hospitalist domains following participation in the program;
Demonstrate successful production of scholarly output, participation in a hospital committee, and participation in a quality or safety improvement initiative by the end of their first year;
Report high levels of satisfaction with the FD program and their first year on faculty.
Program Development Principles
We began by conducting a literature review to draw on the successes and lessons learned from existing FD programs, particularly in large departments, academic centers, and the hospitalist field.1115 We focused our program development on a set of FD principles, which included instructional improvement, organizational development, the development of professional academic skills, and the teaching of specific content.11 Furthermore, whereas many FD programs traditionally focus on mentoring or a longitudinal set of seminars, we believed a multifaceted approach could help shift our culture towards one that prioritized FD and generated a sense of community. We hoped this cultural shift would create an environment that increased faculty satisfaction with their work, with their colleagues, and in our division.
This context drove us to build programmatic activities that not only targeted new faculty, the initial focus of our planning efforts, but also the division more broadly. We wanted to adopt known strategies (eg, mentoring relationships, teaching methods for FD, and grand rounds) but also weave in new ones that targeted AHM and our Division. It was clear that successful programs used a variety of instructional methods, and often combined methods, to create active and engaged faculty. We similarly wanted to create venues for didactic and small‐group learning, but also opportunities for peer learning and facilitated discussions around important topics. Allowing new faculty to learn from each other, and having them observe more senior faculty do the same, would be an important and explicit programmatic element.
Program Description and Implementation
All new faculty meet with Divisional leadership (RMW/BAS), administrative staff (they receive an orientation binder that highlights frequently asked questions and provides service‐specific orientation documents), and the Director of FD (NLS). The latter introduces the DHM FD Program and provides the road map for their first year (Supporting Information). The checklist serves to orient, guide, and emphasize the various programmatic goals, expectations, and logistics. Discussion focuses on the activities targeted to new faculty followed by wider divisional offerings. New faculty activities include:
Coaching Program
Rather than having new faculty independently seek out an appropriate mentor, we explicitly paired each with a more senior hospitalist (eg, 3 years on faculty). We provided explicit goals and expectations for the faculty coach and used a similar road map to guide their role (Supporting Information). We chose to call them coaches rather than mentors because in the first year, we felt a new faculty member needed nuts and bolts support from a big sibling more than they needed formal academic mentoring. We placed the burden of organizing the coaching sessions on the faculty coach and provided them with periodic reminders and suggestions for topics to discuss over the course the year, including supporting the junior faculty's performance against their scholarly benchmarks. Finally, we also organized a peer mentoring session for new facultydesigned to create additional peer support and shared learnings, and establish the importance of these relationships moving forward.
Core Seminars
We created a 12‐hour curriculum to cover a broad range of relevant AHM topics (Table 1). The choice of topics was informed by our needs assessment, suggestions of the FD Steering Committee, and the new faculty themselves. The sessions included a few didactic presentations, but they were largely interactive in a workshop‐style format to allow new faculty to engage the content. For instance, a session on quality improvement asked new faculty to bring a project idea and then work through creating a project plan. We coupled three half‐day sessions with a divisional social activity and made every attempt to ensure new faculty were not distracted by clinical responsibilities (eg, not on a clinical service or coverage was provided).
|
| Core Seminars |
| Being an academic hospitalist: The nuts & bolts |
| Tools for the master clinician |
| Documentation pearls & practices: Clinical, billing, and medico‐legal issues |
| Preparing your first talk: From topic selection to power point presentation |
| Choosing a case and writing it up for a clinical vignette abstract submission |
| Searching for clinical answers: An interactive computer‐lab workshop |
| Introduction to quality improvement |
| Leadership 101: Self‐awareness, your Myers‐Briggs, and leading change |
| Project Management: An exercise in team building |
| Thinking about systems and creating a culture of safety |
| Lunch Seminars |
| Managing and updating your academic CV |
| What to do when a patient on your service dies? |
| Evaluating students & housestaffAnd giving feedback |
| Being an effective ward attending |
| Medical‐legal consultative work & being an expert witness |
| Getting involved in professional societies |
| Understanding the promotion tracks: Practical tips and career preparation |
| Getting involved in hospital committee work |
| Caring for sick family members & navigating the healthcare system as a physician |
| Retirement planning 101: Life after UCSF |
| Time management & creating scholarly work |
| Teaching medical students on the wards |
| Clinical resources: What do you use to find answers? |
Teaching Course
One of our faculty (BAS) delivered the Stanford Faculty Development Clinical Teaching program16 (a train the trainer model designed to teach faculty how to become more effective teachers) to all new faculty. The program consisted of 14 hours of highly interactive curricula, video review, and role plays. The course was offered after hours (4 PM or 5 PM) and with input from the new faculty to ensure availability and participation.
Feedback and Observation
Each new faculty received directed feedback about their teaching and supervision on the housestaff service following their first rotation. Feedback was based on housestaff evaluations and direct observation of the new faculty during patient care and teaching rounds. One of our faculty (BAS) observed each new faculty member during rounds, and met with them individually to provide feedback and generate a discussion about teaching style and improvement opportunities.
Scholarly Expectations
We developed a set of scholarly expectations for new faculty. These helped inform the coach‐new faculty meetings and our selection of content for the Core Seminars. We initially had concerns that these expectations could overwhelm new faculty, but those junior faculty (years 2‐4) on the FD steering committee urged this practice, wishing they had similar guidance in their first year.
From the divisional perspective, we also added a number of new structures.
Grand Rounds
We established a monthly continuing medical education (CME) credit‐granting DHM Grand Rounds that combined a 10‐minute Hospital Medicine Update with a 45‐minute didactic presentation. The updates were presented by new faculty in order to provide them with an opportunity to receive feedback on their teaching and presentation skills (eg, how to give a talk, make PowerPoint slides, etc.). Didactic presentations were given by senior DHM faculty as well as subspecialty colleagues or ones from other departments (eg, dermatology or neurology), disciplines (eg, risk management), or campuses.
FD Lunch Seminar Series
Our division traditionally meets each Monday over the lunch hour to talk about service or academic issues. With a growing division, we believed there was an opportunity to better organize the content of these meetings. Once monthly, we dedicated a lunch session to a Faculty Development Seminar with topics that spanned a variety of interest areas, were driven by faculty suggestions, and were focused on being facilitated discussions rather than didactics. Table 1 provides examples of these seminar topics.
| Survey Statements Reporting Level of Comfort With(% responding somewhat agree or agree) | Previous Faculty, % (n = 11) | New Faculty, % (n = 6) |
|---|---|---|
| Identifying important resources within the School of Medicine | 64 | 83 |
| Identifying important resources within the Department of Medicine | 63 | 100 |
| Identifying important resources within the Division of Hospital Medicine | 90 | 100 |
| Identifying important resources within UCSF Medical Center | 72 | 67 |
| Having a system to effectively manage my email | 64 | 67 |
| Having a system to keep my CV updated | 64 | 84 |
| Using my non‐clinical time for academic success | 54 | 67 |
| Best practices for clinical/medico‐legal documentation | 54 | 67 |
| Best practices for billing documentation | 62 | 84 |
| Being an effective supervising ward attending | 90 | 84 |
| Being an effective teacher | 90 | 84 |
| Evaluating students and housestaff performance | 90 | 83 |
| Providing feedback to students and housestaff | 90 | 100 |
| Getting involved in professional societies | 27 | 100 |
| Understanding the difference between promotion pathways | 36 | 67 |
| Getting involved in hospital committee work | 54 | 84 |
| Choosing a good case for a clinical vignette submission to a regional/national meeting | 54 | 83 |
| Creating a poster for presentation at a regional/national meeting | 36 | 84 |
| Giving a lecture to students or residents | 64 | 84 |
| Developing a PowerPoint presentation for a lecture | 45 | 100 |
| Describing my personality type and how it relates to my work | 45 | 100 |
| Understanding important aspects of being a leader | 54 | 100 |
| Explaining the basic principles of quality improvement | 45 | 84 |
| Participating and contributing to a quality improvement project | 54 | 67 |
| Explaining the basic principles of patient safety | 45 | 67 |
| Understanding the factors that contribute to medical errors | 36 | 84 |
| Creating scholarly products from my work | 27 | 50 |
| Identifying what kind of mentors I need for the future | 45 | 100 |
| Category (% completed during first year) | Previous Faculty, % (n = 11) | New Faculty, % (n = 6) |
|---|---|---|
| Medical student teaching | 90 | 100 |
| Talk for trainees | 45 | 100 |
| Hospital committee involvement | 63 | 100 |
| Participation in a quality or safety project | 33 | 67 |
| Abstract submission | 27 | 50 |
| Identified mentor for year 2 | 63 | 83 |
Quality and Safety Lunch Seminars
In addition to our FD seminars, we also used one lunch session each month to provide updates on performance measures, ongoing quality or safety improvement initiatives, or a broader quality or safety topic. Speakers were either divisional or outside experts, depending on the topic, and organized by our director for quality and safety.
Incubator Sessions
Our director of research (AA) organized a weekly works in progress meeting, to which faculty and fellows brought ideas, grant applications, early manuscript drafts, or other potential scholarship products to obtain feedback and further group mentorship.
Divisional Retreats
We began alternating annual full‐day and mini half‐day retreats as a method to bring the division together, build camaraderie, set strategic priorities, identify divisional goals, and assess needs. These helped guide the creation of additional FD opportunities as well as our overall division's strategy to achieve our academic mission. The outcomes of these retreats led to many significant initiatives and policies, such as changes in compensation models, new scheduling processes, and decisions to spend resources on areas such as quality improvement.
Program Evaluation
Our evaluation focused on measuring the FD program's impact on our new faculty. We tracked their success in completing the stated scholarly expectations and surveyed them about their satisfaction with the programmatic activities, their first year on faculty, and their preparation for year 2. Prior to implementing the program, we surveyed the previous 2 years of new faculty to provide a comparison.
Results
Seven faculty participated in the inaugural program. We compared their scholarly output and experiences (6 faculty completed the survey; 87% response rate) with that of 11 more senior faculty who completed the comparison survey. Of note, the response rate of the comparison group was 69% (5 faculty who departed from our division during the previous 2 years were not surveyed). New faculty were surveyed at the start of the academic year with the follow‐up survey completed the following June. The more senior faculty completed the survey once at the same time as the baseline survey for the new faculty. All new faculty participated in each of the Core Seminars, the Teaching Course, the required number of Coaching sessions, and the observed teaching activity. We did not track their attendance at Divisional activities such as Grand Rounds or the Lunch Seminars.
Overall, the FD programmatic offerings were rated highly by new faculty (on a scale of 1 [lowest] to 5 [highest] for a global rating of each FD activity): Core Seminars 4.83 0.41, Coaching Program 4.5 0.84, Teaching Course 4.5 0.55, Grand Rounds 4.83 0.41, and Lunch Seminars 4.5 0.84. Table 2, which compares responses to a series of end of the year statements posed to new faculty, highlights notable differences in their level of comfort with specific skills and resource awareness. Given the small sample size, statistical significance was not calculated. Table 3 illustrates similar comparisons focused on academic output, which demonstrate that new faculty gave more talks to trainees, had greater involvement in hospital committees, more actively participated in quality and safety projects, and submitted more abstracts to regional or national meetings. New faculty also responded differently to which part of the FD program was most influential with 1 suggesting the Coaching Program, 2 the Core Seminars, 2 the entire program efforts, and 1 did not specify.
Table 4 illustrates comparison responses to a series of directed statements. New faculty all reported greater degrees of satisfaction overall, measured by the above responses, compared to previous faculty.
| Categories | Previous Faculty, % (n = 11) | New Faculty, % (n = 6) |
|---|---|---|
| ||
| Success: To what degree do you feel successful as an academic hospitalist at the end of your first year? (% responding successful or very successful) | 27 | 67 |
| Prepared: To what degree do you feel prepared for academic success moving into your second year on faculty? (% responding prepared or very prepared) | 27 | 100 |
| Part of DHM: I felt like an integral part of our division after my first year on faculty (% responding somewhat agree or agree) | 45 | 84 |
| Expectations: My first year on faculty exceeded my expectations (% responding somewhat agree or agree) | 27 | 84 |
Discussion
We implemented an FD program to foster the academic development of new faculty, and to mitigate the effects of growing clinical demands and a rapid group expansion on our academic mission. The impact of the program was measured by increased work satisfaction and academic output in first year faculty, greater self‐reported comfort in a variety of skills and knowledge of resources, and an improvement in our sense of purpose behind our academic mission. Though the program is only in its second year, we believe the model is of value for other AHM groups, and perhaps even nonacademic groups, all of whom may use such an investment in their hospitalists as a method to improve recruitment, job satisfaction, and retention.
Reviewing our program's first year suggests there were at least 3 keys to our success. First, we benefited tremendously from the time spent crafting a vision for the program and relying heavily on input from the target audience of junior faculty. Moreover, we made every effort to leverage existing resources (eg, using faculty who already taught about a given topic) and time commitments (eg, reshaping our existing Monday lunch meeting). Finally, we increasingly used our FD venues to connect and build networks with colleagues outside our division and within the hospital. This was a deliberate effort to create opportunities for individual faculty to be exposed to and collaborate with nonhospitalists for academic output.
Our research has some limitations, most notably the small sample size in evaluating the program for statistical significance, and the incomplete survey return rates. However, the results were quite consistent and the nonresponses of departed faculty would tend to bias our results toward the null. We also acknowledge the possibility of other confounding factors (eg, changes in clinical compensation models) that may have played a role, although compensation changes were relatively minor during the period studied and faculty did attribute many of the benefits in job satisfaction and skill building to the FD program itself.
Hospital medicine is an unusual field in that there is low barrier to entry and exit. Providers can change jobs without having to say goodbye to a large panel of patients, and in the continued mismatch between available positions and hospitalists, alternative positions can easily and quickly be found if they are dissatisfied.17 In the academic arena, even as hospitalists are hired to fill clinical gaps, they still have to perform under more traditional academic rules in order to be promoted and receive the support and kudos of colleagues and trainees. For both these reasons, early nurturing and socialization is critical to retention and academic success. While some opportunities for FD will be offered by national organizations,18 groups also have local responsibilities to support, mentor, and develop their junior faculty. Not only is such support crucial for the junior faculty themselves, but in our young field, the mentored very quickly become the mentors. Our decision to invest in both mentees and mentors reinforced the importance of mentorship for academic success and retention while planting the seeds for continued success and growth.1923 A recent study suggested that the environment for mentoring may be as important as the mentoring itself, a finding we did not specifically measure, but would support based our anecdotal experiences.24 This orientation toward future needs and creating the right milieu is crucial because demands for continued hospitalist growth are likely to remain.
Moving into year 2 of our FD program and reflecting on the lessons learned from year 1, we've adopted the same multifaceted approach with only minor adjustments to the curriculum, greater expansion of faculty involved in teaching and coaching, and a continued focus on building a sense of community around our academic mission. For the Core Seminars, we moved away from the 3 half‐day sessions and chose to host 2‐hour sessions every other month. This allowed for the same curriculum to be delivered but was much easier to logistically orchestrate. It also had the intended effect of bringing the new faculty together more regularly. In addition, we created dedicated sessions in preparation for our national meeting to allow faculty to bring abstract submissions for review and later, posters and oral presentations for feedback. These added sessions came partly as a suggestion from new faculty in our first year program, and seemed to further energize junior faculty around converting their projects into scholarship. Finally, we continue to further develop coaching and mentoring relationships in our division, partly a result of successful new facultycoach pairings.
In conclusion, our FD program had a noted impact on our new faculty and had a meaningful impact on our division in terms of camaraderie and cohesion, a shared commitment to an academic mission, and a mechanism for recruitment and retention. We hope our practical description for development and implementation of an FD program, including our specific tools, are useful to other groups considering such an initiative.
Acknowledgements
The authors thank Katherine Li for her invaluable assistance in coordinating the DHM FD program. They are also indebted to their faculty colleagues for their time and roles in teaching and mentoring within the program. Dr. Sehgal partly developed this program as part of a project during his California Healthcare Foundation Leadership Fellowship. Dr. Sharpe delivered the teaching workshops at UCSF after completing the Stanford Faculty Development Teaching Program.
- ,,,.The status of Hospital Medicine Groups in the United States.J Hosp Med.2006;1(2):75–80.
- Accreditation Council for Graduate Medical Education: information related to the ACGME's effort to address resident duty hours and other relevant resource materials. Available at: http://www.acgme.org/acWebsite/dutyHours/dh_index.asp. Accessed August 2010.
- ,,, et al.Effects of work hour reduction on residents' lives: a systematic review.JAMA.2005;294(9):1088–1100.
- ,,,,.Non‐housestaff medicine services in academic centers: models and challenges.J Hosp Med.2008;3(3):247–255.
- ,.Surgical comanagement: a natural evolution of hospitalist practice.J Hosp Med.2008;3(5):394–397.
- ,.The expanding role of hospitalists in the United States.Swiss Med Wkly.2006;136(37–38):591–596.
- 2007–08 Hospital Medicine Survey. Society of Hospital Medicine. Available at: http://www.hospitalmedicine.org/AM/Template.cfm?Section=Survey19(4):392–393.
- ,,, et al.Challenges and opportunities in Academic Hospital Medicine: report from the Academic Hospital Medicine Summit.J Hosp Med.2009;4(4):240–246.
- .Palliative care and hospitalists: a partnership for hope.J Hosp Med.2006;1(1):5–6.
- ,.Faculty development: principles and practices.J Vet Med Educ.2006;33(3):317–324.
- ,,, et al.A systematic review of faculty development initiatives designed to improve teaching effectiveness in medical education: BEME Guide No. 8.Med Teach.2006;28(6):497–526.
- ,.A successful approach to faculty development at an independent academic medical center.Med Teach.2008;30:e10–e14.
- ,,,.An innovative approach to supporting hospitalist physicians towards academic success.J Hosp Med.2008;3(4):314–318.
- ,,, et al.The curriculum for the Hospitalized Aging Medical Patient program: a collaborative faculty development program for hospitalists, general internists, and geriatricians.J Hosp Med.2008;3(5):384–393.
- Stanford Faculty Development Clinical Teaching Program. Available at: http://www.stanford.edu/group/SFDP. Accessed August 2010.
- ,,, et al.Trends in market demand for internal medicine 1999–2004: an analysis of physician job advertisements.J Gen Intern Med.2006;21(10):1079–1085.
- The Academic Hospitalist Academy. Available at: http://www.sgim.org/index.cfm?pageId=815. Accessed August 2010.
- ,,.Mentoring in academic medicine: a systematic review.JAMA.2006;296(9):1103–1115.
- ,,.Facilitating faculty success: outcomes and cost benefit of the UCSD National Center of Leadership in Academic Medicine.Acad Med.2004;79(10 Suppl):S9–S11.
- ,,, et al.Retention of junior faculty in academic medicine at the University of California, San Diego.Acad Med.2009;84(1):37–41.
- ,,,.Helping medical school faculty realize their dreams: an innovative, collaborative mentoring program.Acad Med.2002;77:377–384.
- ,,, et al.Physicians' perceptions of institutional and leadership factors influencing their job satisfaction at one academic medical center.Acad Med.2002;77:1235–1240.
- ,,.A systematic review of qualitative research on the meaning and characteristics of mentoring in academic medicine.J Gen Intern Med.2010;25(1);72–78.
The growth of hospitalists nationally continues at an unprecedented pace.1 In academic medical centers, the development of hospital medicine groups either as independent divisions or as part of divisions of general internal medicine (DGIM) reflects this trend. Drivers for growth in the academic setting include housestaff work hour restrictions, increased need for oversight on teaching services, development of nonhousestaff services, surgical comanagement, and greater emphasis on efficiency, quality, and safety.26 These drivers have created tremendous opportunities for hospitalists, but the rapid growth has also created challenges to achieving traditional academic success.7, 8
While hospitalists feel the traditional academic pressures to produce new knowledge and teach, the extraordinary need to expand clinical services has resulted in a young hospitalist workforce, with most lacking fellowship training. At the same time, there are few senior mentors available. Taken together, many academic hospital medicine (AHM) programs find themselves populated by large cadres of junior faculty without the support, training, and mentoring they need to succeed in a faculty career.9 For hospital medicine groups, the risk to faculty recruitment, retention, productivity, and morale is high.
In this article, we describe the development and implementation of a multifaceted Faculty Development (FD) program whose goal was to provide our faculty with clinical, educational, leadership, and scholarly skills that would promote academic output and foster work satisfaction.
Methods
Problem Identification
The University of California, San Francisco (UCSF) Medical Center operates nearly 800 beds across 2 hospitals (Parnassus and Mount Zion campuses). The UCSF Division of Hospital Medicine (DHM) provides care on the teaching service (90% of all ward months covered by a hospitalist faculty), a nonhousestaff medical service based at Mount Zion,4 a palliative care service,10 a medical consultation service, a neurosurgical comanagement service, a procedure service, and comanagement on advanced heart failure and cancer services. Like many AHM groups, ours has experienced explosive growth, more than doubling in faculty size in 3 years (50+ faculty by July 2010).
In addition, many of our new faculty joined the division directly after residency training whereas our early hospitalists were mostly former chief residents and/or fellowship‐trained. During a 2‐year period, our division lost several faculty to burnout from clinically heavy positions or because they felt their ultimate academic success was in doubt. During a 2008 divisional retreat, the single greatest need identified was to invest in the development of our first‐year faculty who were felt to be at greatest risk for burnout, dissatisfaction, and failing to integrate into the divisional mission. Based on this result, we set out to develop a program to meet this pressing need.
Needs Assessment
We formed a FD steering committee comprised of faculty from all ranks and career paths in our division (eg, educator, administrator, and investigator), with overrepresentation of recent hires to ascertain how best to meet their needs. Information from the division retreat provided the basis for the program and its priorities. The FD steering committee then outlined ideas that guided program development, which included:
New faculty should be required to meet regularly with assigned faculty mentors during their first year, and expectations for that relationship should be outlined for both parties
New faculty should be required to attend dedicated sessions that build their teaching skills
New faculty should receive a specially designed first year curriculum to provide learnings focused on high‐yield and relevant topics
New faculty should receive a set of goals, or scholarly expectations, for their first year that would foster a partnership between individual faculty and the division to meet those goals
The division should create new structures for FD that promote collaboration, sharing of personal and professional growth and challenges, and a culture of continuous learning
All of the activities that comprise our new FD program must be aligned with our stated mission: to provide the highest quality clinical care, education, system improvements, and research that benefit our patients and trainees by developing successful academic hospitalist faculty.
Program Goals and Objectives
Our DHM FD program established the goal to provide our new faculty with clinical, educational, leadership, and scholarly skills that would promote academic output and foster work satisfaction. From a broader divisional standpoint, the goal was simply to create new FD structures that fostered the division's commitment to the program. The primary objectives of the program were for new faculty to:
Increase their knowledge, skills, and attitudes about key academic hospitalist domains following participation in the program;
Demonstrate successful production of scholarly output, participation in a hospital committee, and participation in a quality or safety improvement initiative by the end of their first year;
Report high levels of satisfaction with the FD program and their first year on faculty.
Program Development Principles
We began by conducting a literature review to draw on the successes and lessons learned from existing FD programs, particularly in large departments, academic centers, and the hospitalist field.1115 We focused our program development on a set of FD principles, which included instructional improvement, organizational development, the development of professional academic skills, and the teaching of specific content.11 Furthermore, whereas many FD programs traditionally focus on mentoring or a longitudinal set of seminars, we believed a multifaceted approach could help shift our culture towards one that prioritized FD and generated a sense of community. We hoped this cultural shift would create an environment that increased faculty satisfaction with their work, with their colleagues, and in our division.
This context drove us to build programmatic activities that not only targeted new faculty, the initial focus of our planning efforts, but also the division more broadly. We wanted to adopt known strategies (eg, mentoring relationships, teaching methods for FD, and grand rounds) but also weave in new ones that targeted AHM and our Division. It was clear that successful programs used a variety of instructional methods, and often combined methods, to create active and engaged faculty. We similarly wanted to create venues for didactic and small‐group learning, but also opportunities for peer learning and facilitated discussions around important topics. Allowing new faculty to learn from each other, and having them observe more senior faculty do the same, would be an important and explicit programmatic element.
Program Description and Implementation
All new faculty meet with Divisional leadership (RMW/BAS), administrative staff (they receive an orientation binder that highlights frequently asked questions and provides service‐specific orientation documents), and the Director of FD (NLS). The latter introduces the DHM FD Program and provides the road map for their first year (Supporting Information). The checklist serves to orient, guide, and emphasize the various programmatic goals, expectations, and logistics. Discussion focuses on the activities targeted to new faculty followed by wider divisional offerings. New faculty activities include:
Coaching Program
Rather than having new faculty independently seek out an appropriate mentor, we explicitly paired each with a more senior hospitalist (eg, 3 years on faculty). We provided explicit goals and expectations for the faculty coach and used a similar road map to guide their role (Supporting Information). We chose to call them coaches rather than mentors because in the first year, we felt a new faculty member needed nuts and bolts support from a big sibling more than they needed formal academic mentoring. We placed the burden of organizing the coaching sessions on the faculty coach and provided them with periodic reminders and suggestions for topics to discuss over the course the year, including supporting the junior faculty's performance against their scholarly benchmarks. Finally, we also organized a peer mentoring session for new facultydesigned to create additional peer support and shared learnings, and establish the importance of these relationships moving forward.
Core Seminars
We created a 12‐hour curriculum to cover a broad range of relevant AHM topics (Table 1). The choice of topics was informed by our needs assessment, suggestions of the FD Steering Committee, and the new faculty themselves. The sessions included a few didactic presentations, but they were largely interactive in a workshop‐style format to allow new faculty to engage the content. For instance, a session on quality improvement asked new faculty to bring a project idea and then work through creating a project plan. We coupled three half‐day sessions with a divisional social activity and made every attempt to ensure new faculty were not distracted by clinical responsibilities (eg, not on a clinical service or coverage was provided).
|
| Core Seminars |
| Being an academic hospitalist: The nuts & bolts |
| Tools for the master clinician |
| Documentation pearls & practices: Clinical, billing, and medico‐legal issues |
| Preparing your first talk: From topic selection to power point presentation |
| Choosing a case and writing it up for a clinical vignette abstract submission |
| Searching for clinical answers: An interactive computer‐lab workshop |
| Introduction to quality improvement |
| Leadership 101: Self‐awareness, your Myers‐Briggs, and leading change |
| Project Management: An exercise in team building |
| Thinking about systems and creating a culture of safety |
| Lunch Seminars |
| Managing and updating your academic CV |
| What to do when a patient on your service dies? |
| Evaluating students & housestaffAnd giving feedback |
| Being an effective ward attending |
| Medical‐legal consultative work & being an expert witness |
| Getting involved in professional societies |
| Understanding the promotion tracks: Practical tips and career preparation |
| Getting involved in hospital committee work |
| Caring for sick family members & navigating the healthcare system as a physician |
| Retirement planning 101: Life after UCSF |
| Time management & creating scholarly work |
| Teaching medical students on the wards |
| Clinical resources: What do you use to find answers? |
Teaching Course
One of our faculty (BAS) delivered the Stanford Faculty Development Clinical Teaching program16 (a train the trainer model designed to teach faculty how to become more effective teachers) to all new faculty. The program consisted of 14 hours of highly interactive curricula, video review, and role plays. The course was offered after hours (4 PM or 5 PM) and with input from the new faculty to ensure availability and participation.
Feedback and Observation
Each new faculty received directed feedback about their teaching and supervision on the housestaff service following their first rotation. Feedback was based on housestaff evaluations and direct observation of the new faculty during patient care and teaching rounds. One of our faculty (BAS) observed each new faculty member during rounds, and met with them individually to provide feedback and generate a discussion about teaching style and improvement opportunities.
Scholarly Expectations
We developed a set of scholarly expectations for new faculty. These helped inform the coach‐new faculty meetings and our selection of content for the Core Seminars. We initially had concerns that these expectations could overwhelm new faculty, but those junior faculty (years 2‐4) on the FD steering committee urged this practice, wishing they had similar guidance in their first year.
From the divisional perspective, we also added a number of new structures.
Grand Rounds
We established a monthly continuing medical education (CME) credit‐granting DHM Grand Rounds that combined a 10‐minute Hospital Medicine Update with a 45‐minute didactic presentation. The updates were presented by new faculty in order to provide them with an opportunity to receive feedback on their teaching and presentation skills (eg, how to give a talk, make PowerPoint slides, etc.). Didactic presentations were given by senior DHM faculty as well as subspecialty colleagues or ones from other departments (eg, dermatology or neurology), disciplines (eg, risk management), or campuses.
FD Lunch Seminar Series
Our division traditionally meets each Monday over the lunch hour to talk about service or academic issues. With a growing division, we believed there was an opportunity to better organize the content of these meetings. Once monthly, we dedicated a lunch session to a Faculty Development Seminar with topics that spanned a variety of interest areas, were driven by faculty suggestions, and were focused on being facilitated discussions rather than didactics. Table 1 provides examples of these seminar topics.
| Survey Statements Reporting Level of Comfort With(% responding somewhat agree or agree) | Previous Faculty, % (n = 11) | New Faculty, % (n = 6) |
|---|---|---|
| Identifying important resources within the School of Medicine | 64 | 83 |
| Identifying important resources within the Department of Medicine | 63 | 100 |
| Identifying important resources within the Division of Hospital Medicine | 90 | 100 |
| Identifying important resources within UCSF Medical Center | 72 | 67 |
| Having a system to effectively manage my email | 64 | 67 |
| Having a system to keep my CV updated | 64 | 84 |
| Using my non‐clinical time for academic success | 54 | 67 |
| Best practices for clinical/medico‐legal documentation | 54 | 67 |
| Best practices for billing documentation | 62 | 84 |
| Being an effective supervising ward attending | 90 | 84 |
| Being an effective teacher | 90 | 84 |
| Evaluating students and housestaff performance | 90 | 83 |
| Providing feedback to students and housestaff | 90 | 100 |
| Getting involved in professional societies | 27 | 100 |
| Understanding the difference between promotion pathways | 36 | 67 |
| Getting involved in hospital committee work | 54 | 84 |
| Choosing a good case for a clinical vignette submission to a regional/national meeting | 54 | 83 |
| Creating a poster for presentation at a regional/national meeting | 36 | 84 |
| Giving a lecture to students or residents | 64 | 84 |
| Developing a PowerPoint presentation for a lecture | 45 | 100 |
| Describing my personality type and how it relates to my work | 45 | 100 |
| Understanding important aspects of being a leader | 54 | 100 |
| Explaining the basic principles of quality improvement | 45 | 84 |
| Participating and contributing to a quality improvement project | 54 | 67 |
| Explaining the basic principles of patient safety | 45 | 67 |
| Understanding the factors that contribute to medical errors | 36 | 84 |
| Creating scholarly products from my work | 27 | 50 |
| Identifying what kind of mentors I need for the future | 45 | 100 |
| Category (% completed during first year) | Previous Faculty, % (n = 11) | New Faculty, % (n = 6) |
|---|---|---|
| Medical student teaching | 90 | 100 |
| Talk for trainees | 45 | 100 |
| Hospital committee involvement | 63 | 100 |
| Participation in a quality or safety project | 33 | 67 |
| Abstract submission | 27 | 50 |
| Identified mentor for year 2 | 63 | 83 |
Quality and Safety Lunch Seminars
In addition to our FD seminars, we also used one lunch session each month to provide updates on performance measures, ongoing quality or safety improvement initiatives, or a broader quality or safety topic. Speakers were either divisional or outside experts, depending on the topic, and organized by our director for quality and safety.
Incubator Sessions
Our director of research (AA) organized a weekly works in progress meeting, to which faculty and fellows brought ideas, grant applications, early manuscript drafts, or other potential scholarship products to obtain feedback and further group mentorship.
Divisional Retreats
We began alternating annual full‐day and mini half‐day retreats as a method to bring the division together, build camaraderie, set strategic priorities, identify divisional goals, and assess needs. These helped guide the creation of additional FD opportunities as well as our overall division's strategy to achieve our academic mission. The outcomes of these retreats led to many significant initiatives and policies, such as changes in compensation models, new scheduling processes, and decisions to spend resources on areas such as quality improvement.
Program Evaluation
Our evaluation focused on measuring the FD program's impact on our new faculty. We tracked their success in completing the stated scholarly expectations and surveyed them about their satisfaction with the programmatic activities, their first year on faculty, and their preparation for year 2. Prior to implementing the program, we surveyed the previous 2 years of new faculty to provide a comparison.
Results
Seven faculty participated in the inaugural program. We compared their scholarly output and experiences (6 faculty completed the survey; 87% response rate) with that of 11 more senior faculty who completed the comparison survey. Of note, the response rate of the comparison group was 69% (5 faculty who departed from our division during the previous 2 years were not surveyed). New faculty were surveyed at the start of the academic year with the follow‐up survey completed the following June. The more senior faculty completed the survey once at the same time as the baseline survey for the new faculty. All new faculty participated in each of the Core Seminars, the Teaching Course, the required number of Coaching sessions, and the observed teaching activity. We did not track their attendance at Divisional activities such as Grand Rounds or the Lunch Seminars.
Overall, the FD programmatic offerings were rated highly by new faculty (on a scale of 1 [lowest] to 5 [highest] for a global rating of each FD activity): Core Seminars 4.83 0.41, Coaching Program 4.5 0.84, Teaching Course 4.5 0.55, Grand Rounds 4.83 0.41, and Lunch Seminars 4.5 0.84. Table 2, which compares responses to a series of end of the year statements posed to new faculty, highlights notable differences in their level of comfort with specific skills and resource awareness. Given the small sample size, statistical significance was not calculated. Table 3 illustrates similar comparisons focused on academic output, which demonstrate that new faculty gave more talks to trainees, had greater involvement in hospital committees, more actively participated in quality and safety projects, and submitted more abstracts to regional or national meetings. New faculty also responded differently to which part of the FD program was most influential with 1 suggesting the Coaching Program, 2 the Core Seminars, 2 the entire program efforts, and 1 did not specify.
Table 4 illustrates comparison responses to a series of directed statements. New faculty all reported greater degrees of satisfaction overall, measured by the above responses, compared to previous faculty.
| Categories | Previous Faculty, % (n = 11) | New Faculty, % (n = 6) |
|---|---|---|
| ||
| Success: To what degree do you feel successful as an academic hospitalist at the end of your first year? (% responding successful or very successful) | 27 | 67 |
| Prepared: To what degree do you feel prepared for academic success moving into your second year on faculty? (% responding prepared or very prepared) | 27 | 100 |
| Part of DHM: I felt like an integral part of our division after my first year on faculty (% responding somewhat agree or agree) | 45 | 84 |
| Expectations: My first year on faculty exceeded my expectations (% responding somewhat agree or agree) | 27 | 84 |
Discussion
We implemented an FD program to foster the academic development of new faculty, and to mitigate the effects of growing clinical demands and a rapid group expansion on our academic mission. The impact of the program was measured by increased work satisfaction and academic output in first year faculty, greater self‐reported comfort in a variety of skills and knowledge of resources, and an improvement in our sense of purpose behind our academic mission. Though the program is only in its second year, we believe the model is of value for other AHM groups, and perhaps even nonacademic groups, all of whom may use such an investment in their hospitalists as a method to improve recruitment, job satisfaction, and retention.
Reviewing our program's first year suggests there were at least 3 keys to our success. First, we benefited tremendously from the time spent crafting a vision for the program and relying heavily on input from the target audience of junior faculty. Moreover, we made every effort to leverage existing resources (eg, using faculty who already taught about a given topic) and time commitments (eg, reshaping our existing Monday lunch meeting). Finally, we increasingly used our FD venues to connect and build networks with colleagues outside our division and within the hospital. This was a deliberate effort to create opportunities for individual faculty to be exposed to and collaborate with nonhospitalists for academic output.
Our research has some limitations, most notably the small sample size in evaluating the program for statistical significance, and the incomplete survey return rates. However, the results were quite consistent and the nonresponses of departed faculty would tend to bias our results toward the null. We also acknowledge the possibility of other confounding factors (eg, changes in clinical compensation models) that may have played a role, although compensation changes were relatively minor during the period studied and faculty did attribute many of the benefits in job satisfaction and skill building to the FD program itself.
Hospital medicine is an unusual field in that there is low barrier to entry and exit. Providers can change jobs without having to say goodbye to a large panel of patients, and in the continued mismatch between available positions and hospitalists, alternative positions can easily and quickly be found if they are dissatisfied.17 In the academic arena, even as hospitalists are hired to fill clinical gaps, they still have to perform under more traditional academic rules in order to be promoted and receive the support and kudos of colleagues and trainees. For both these reasons, early nurturing and socialization is critical to retention and academic success. While some opportunities for FD will be offered by national organizations,18 groups also have local responsibilities to support, mentor, and develop their junior faculty. Not only is such support crucial for the junior faculty themselves, but in our young field, the mentored very quickly become the mentors. Our decision to invest in both mentees and mentors reinforced the importance of mentorship for academic success and retention while planting the seeds for continued success and growth.1923 A recent study suggested that the environment for mentoring may be as important as the mentoring itself, a finding we did not specifically measure, but would support based our anecdotal experiences.24 This orientation toward future needs and creating the right milieu is crucial because demands for continued hospitalist growth are likely to remain.
Moving into year 2 of our FD program and reflecting on the lessons learned from year 1, we've adopted the same multifaceted approach with only minor adjustments to the curriculum, greater expansion of faculty involved in teaching and coaching, and a continued focus on building a sense of community around our academic mission. For the Core Seminars, we moved away from the 3 half‐day sessions and chose to host 2‐hour sessions every other month. This allowed for the same curriculum to be delivered but was much easier to logistically orchestrate. It also had the intended effect of bringing the new faculty together more regularly. In addition, we created dedicated sessions in preparation for our national meeting to allow faculty to bring abstract submissions for review and later, posters and oral presentations for feedback. These added sessions came partly as a suggestion from new faculty in our first year program, and seemed to further energize junior faculty around converting their projects into scholarship. Finally, we continue to further develop coaching and mentoring relationships in our division, partly a result of successful new facultycoach pairings.
In conclusion, our FD program had a noted impact on our new faculty and had a meaningful impact on our division in terms of camaraderie and cohesion, a shared commitment to an academic mission, and a mechanism for recruitment and retention. We hope our practical description for development and implementation of an FD program, including our specific tools, are useful to other groups considering such an initiative.
Acknowledgements
The authors thank Katherine Li for her invaluable assistance in coordinating the DHM FD program. They are also indebted to their faculty colleagues for their time and roles in teaching and mentoring within the program. Dr. Sehgal partly developed this program as part of a project during his California Healthcare Foundation Leadership Fellowship. Dr. Sharpe delivered the teaching workshops at UCSF after completing the Stanford Faculty Development Teaching Program.
The growth of hospitalists nationally continues at an unprecedented pace.1 In academic medical centers, the development of hospital medicine groups either as independent divisions or as part of divisions of general internal medicine (DGIM) reflects this trend. Drivers for growth in the academic setting include housestaff work hour restrictions, increased need for oversight on teaching services, development of nonhousestaff services, surgical comanagement, and greater emphasis on efficiency, quality, and safety.26 These drivers have created tremendous opportunities for hospitalists, but the rapid growth has also created challenges to achieving traditional academic success.7, 8
While hospitalists feel the traditional academic pressures to produce new knowledge and teach, the extraordinary need to expand clinical services has resulted in a young hospitalist workforce, with most lacking fellowship training. At the same time, there are few senior mentors available. Taken together, many academic hospital medicine (AHM) programs find themselves populated by large cadres of junior faculty without the support, training, and mentoring they need to succeed in a faculty career.9 For hospital medicine groups, the risk to faculty recruitment, retention, productivity, and morale is high.
In this article, we describe the development and implementation of a multifaceted Faculty Development (FD) program whose goal was to provide our faculty with clinical, educational, leadership, and scholarly skills that would promote academic output and foster work satisfaction.
Methods
Problem Identification
The University of California, San Francisco (UCSF) Medical Center operates nearly 800 beds across 2 hospitals (Parnassus and Mount Zion campuses). The UCSF Division of Hospital Medicine (DHM) provides care on the teaching service (90% of all ward months covered by a hospitalist faculty), a nonhousestaff medical service based at Mount Zion,4 a palliative care service,10 a medical consultation service, a neurosurgical comanagement service, a procedure service, and comanagement on advanced heart failure and cancer services. Like many AHM groups, ours has experienced explosive growth, more than doubling in faculty size in 3 years (50+ faculty by July 2010).
In addition, many of our new faculty joined the division directly after residency training whereas our early hospitalists were mostly former chief residents and/or fellowship‐trained. During a 2‐year period, our division lost several faculty to burnout from clinically heavy positions or because they felt their ultimate academic success was in doubt. During a 2008 divisional retreat, the single greatest need identified was to invest in the development of our first‐year faculty who were felt to be at greatest risk for burnout, dissatisfaction, and failing to integrate into the divisional mission. Based on this result, we set out to develop a program to meet this pressing need.
Needs Assessment
We formed a FD steering committee comprised of faculty from all ranks and career paths in our division (eg, educator, administrator, and investigator), with overrepresentation of recent hires to ascertain how best to meet their needs. Information from the division retreat provided the basis for the program and its priorities. The FD steering committee then outlined ideas that guided program development, which included:
New faculty should be required to meet regularly with assigned faculty mentors during their first year, and expectations for that relationship should be outlined for both parties
New faculty should be required to attend dedicated sessions that build their teaching skills
New faculty should receive a specially designed first year curriculum to provide learnings focused on high‐yield and relevant topics
New faculty should receive a set of goals, or scholarly expectations, for their first year that would foster a partnership between individual faculty and the division to meet those goals
The division should create new structures for FD that promote collaboration, sharing of personal and professional growth and challenges, and a culture of continuous learning
All of the activities that comprise our new FD program must be aligned with our stated mission: to provide the highest quality clinical care, education, system improvements, and research that benefit our patients and trainees by developing successful academic hospitalist faculty.
Program Goals and Objectives
Our DHM FD program established the goal to provide our new faculty with clinical, educational, leadership, and scholarly skills that would promote academic output and foster work satisfaction. From a broader divisional standpoint, the goal was simply to create new FD structures that fostered the division's commitment to the program. The primary objectives of the program were for new faculty to:
Increase their knowledge, skills, and attitudes about key academic hospitalist domains following participation in the program;
Demonstrate successful production of scholarly output, participation in a hospital committee, and participation in a quality or safety improvement initiative by the end of their first year;
Report high levels of satisfaction with the FD program and their first year on faculty.
Program Development Principles
We began by conducting a literature review to draw on the successes and lessons learned from existing FD programs, particularly in large departments, academic centers, and the hospitalist field.1115 We focused our program development on a set of FD principles, which included instructional improvement, organizational development, the development of professional academic skills, and the teaching of specific content.11 Furthermore, whereas many FD programs traditionally focus on mentoring or a longitudinal set of seminars, we believed a multifaceted approach could help shift our culture towards one that prioritized FD and generated a sense of community. We hoped this cultural shift would create an environment that increased faculty satisfaction with their work, with their colleagues, and in our division.
This context drove us to build programmatic activities that not only targeted new faculty, the initial focus of our planning efforts, but also the division more broadly. We wanted to adopt known strategies (eg, mentoring relationships, teaching methods for FD, and grand rounds) but also weave in new ones that targeted AHM and our Division. It was clear that successful programs used a variety of instructional methods, and often combined methods, to create active and engaged faculty. We similarly wanted to create venues for didactic and small‐group learning, but also opportunities for peer learning and facilitated discussions around important topics. Allowing new faculty to learn from each other, and having them observe more senior faculty do the same, would be an important and explicit programmatic element.
Program Description and Implementation
All new faculty meet with Divisional leadership (RMW/BAS), administrative staff (they receive an orientation binder that highlights frequently asked questions and provides service‐specific orientation documents), and the Director of FD (NLS). The latter introduces the DHM FD Program and provides the road map for their first year (Supporting Information). The checklist serves to orient, guide, and emphasize the various programmatic goals, expectations, and logistics. Discussion focuses on the activities targeted to new faculty followed by wider divisional offerings. New faculty activities include:
Coaching Program
Rather than having new faculty independently seek out an appropriate mentor, we explicitly paired each with a more senior hospitalist (eg, 3 years on faculty). We provided explicit goals and expectations for the faculty coach and used a similar road map to guide their role (Supporting Information). We chose to call them coaches rather than mentors because in the first year, we felt a new faculty member needed nuts and bolts support from a big sibling more than they needed formal academic mentoring. We placed the burden of organizing the coaching sessions on the faculty coach and provided them with periodic reminders and suggestions for topics to discuss over the course the year, including supporting the junior faculty's performance against their scholarly benchmarks. Finally, we also organized a peer mentoring session for new facultydesigned to create additional peer support and shared learnings, and establish the importance of these relationships moving forward.
Core Seminars
We created a 12‐hour curriculum to cover a broad range of relevant AHM topics (Table 1). The choice of topics was informed by our needs assessment, suggestions of the FD Steering Committee, and the new faculty themselves. The sessions included a few didactic presentations, but they were largely interactive in a workshop‐style format to allow new faculty to engage the content. For instance, a session on quality improvement asked new faculty to bring a project idea and then work through creating a project plan. We coupled three half‐day sessions with a divisional social activity and made every attempt to ensure new faculty were not distracted by clinical responsibilities (eg, not on a clinical service or coverage was provided).
|
| Core Seminars |
| Being an academic hospitalist: The nuts & bolts |
| Tools for the master clinician |
| Documentation pearls & practices: Clinical, billing, and medico‐legal issues |
| Preparing your first talk: From topic selection to power point presentation |
| Choosing a case and writing it up for a clinical vignette abstract submission |
| Searching for clinical answers: An interactive computer‐lab workshop |
| Introduction to quality improvement |
| Leadership 101: Self‐awareness, your Myers‐Briggs, and leading change |
| Project Management: An exercise in team building |
| Thinking about systems and creating a culture of safety |
| Lunch Seminars |
| Managing and updating your academic CV |
| What to do when a patient on your service dies? |
| Evaluating students & housestaffAnd giving feedback |
| Being an effective ward attending |
| Medical‐legal consultative work & being an expert witness |
| Getting involved in professional societies |
| Understanding the promotion tracks: Practical tips and career preparation |
| Getting involved in hospital committee work |
| Caring for sick family members & navigating the healthcare system as a physician |
| Retirement planning 101: Life after UCSF |
| Time management & creating scholarly work |
| Teaching medical students on the wards |
| Clinical resources: What do you use to find answers? |
Teaching Course
One of our faculty (BAS) delivered the Stanford Faculty Development Clinical Teaching program16 (a train the trainer model designed to teach faculty how to become more effective teachers) to all new faculty. The program consisted of 14 hours of highly interactive curricula, video review, and role plays. The course was offered after hours (4 PM or 5 PM) and with input from the new faculty to ensure availability and participation.
Feedback and Observation
Each new faculty received directed feedback about their teaching and supervision on the housestaff service following their first rotation. Feedback was based on housestaff evaluations and direct observation of the new faculty during patient care and teaching rounds. One of our faculty (BAS) observed each new faculty member during rounds, and met with them individually to provide feedback and generate a discussion about teaching style and improvement opportunities.
Scholarly Expectations
We developed a set of scholarly expectations for new faculty. These helped inform the coach‐new faculty meetings and our selection of content for the Core Seminars. We initially had concerns that these expectations could overwhelm new faculty, but those junior faculty (years 2‐4) on the FD steering committee urged this practice, wishing they had similar guidance in their first year.
From the divisional perspective, we also added a number of new structures.
Grand Rounds
We established a monthly continuing medical education (CME) credit‐granting DHM Grand Rounds that combined a 10‐minute Hospital Medicine Update with a 45‐minute didactic presentation. The updates were presented by new faculty in order to provide them with an opportunity to receive feedback on their teaching and presentation skills (eg, how to give a talk, make PowerPoint slides, etc.). Didactic presentations were given by senior DHM faculty as well as subspecialty colleagues or ones from other departments (eg, dermatology or neurology), disciplines (eg, risk management), or campuses.
FD Lunch Seminar Series
Our division traditionally meets each Monday over the lunch hour to talk about service or academic issues. With a growing division, we believed there was an opportunity to better organize the content of these meetings. Once monthly, we dedicated a lunch session to a Faculty Development Seminar with topics that spanned a variety of interest areas, were driven by faculty suggestions, and were focused on being facilitated discussions rather than didactics. Table 1 provides examples of these seminar topics.
| Survey Statements Reporting Level of Comfort With(% responding somewhat agree or agree) | Previous Faculty, % (n = 11) | New Faculty, % (n = 6) |
|---|---|---|
| Identifying important resources within the School of Medicine | 64 | 83 |
| Identifying important resources within the Department of Medicine | 63 | 100 |
| Identifying important resources within the Division of Hospital Medicine | 90 | 100 |
| Identifying important resources within UCSF Medical Center | 72 | 67 |
| Having a system to effectively manage my email | 64 | 67 |
| Having a system to keep my CV updated | 64 | 84 |
| Using my non‐clinical time for academic success | 54 | 67 |
| Best practices for clinical/medico‐legal documentation | 54 | 67 |
| Best practices for billing documentation | 62 | 84 |
| Being an effective supervising ward attending | 90 | 84 |
| Being an effective teacher | 90 | 84 |
| Evaluating students and housestaff performance | 90 | 83 |
| Providing feedback to students and housestaff | 90 | 100 |
| Getting involved in professional societies | 27 | 100 |
| Understanding the difference between promotion pathways | 36 | 67 |
| Getting involved in hospital committee work | 54 | 84 |
| Choosing a good case for a clinical vignette submission to a regional/national meeting | 54 | 83 |
| Creating a poster for presentation at a regional/national meeting | 36 | 84 |
| Giving a lecture to students or residents | 64 | 84 |
| Developing a PowerPoint presentation for a lecture | 45 | 100 |
| Describing my personality type and how it relates to my work | 45 | 100 |
| Understanding important aspects of being a leader | 54 | 100 |
| Explaining the basic principles of quality improvement | 45 | 84 |
| Participating and contributing to a quality improvement project | 54 | 67 |
| Explaining the basic principles of patient safety | 45 | 67 |
| Understanding the factors that contribute to medical errors | 36 | 84 |
| Creating scholarly products from my work | 27 | 50 |
| Identifying what kind of mentors I need for the future | 45 | 100 |
| Category (% completed during first year) | Previous Faculty, % (n = 11) | New Faculty, % (n = 6) |
|---|---|---|
| Medical student teaching | 90 | 100 |
| Talk for trainees | 45 | 100 |
| Hospital committee involvement | 63 | 100 |
| Participation in a quality or safety project | 33 | 67 |
| Abstract submission | 27 | 50 |
| Identified mentor for year 2 | 63 | 83 |
Quality and Safety Lunch Seminars
In addition to our FD seminars, we also used one lunch session each month to provide updates on performance measures, ongoing quality or safety improvement initiatives, or a broader quality or safety topic. Speakers were either divisional or outside experts, depending on the topic, and organized by our director for quality and safety.
Incubator Sessions
Our director of research (AA) organized a weekly works in progress meeting, to which faculty and fellows brought ideas, grant applications, early manuscript drafts, or other potential scholarship products to obtain feedback and further group mentorship.
Divisional Retreats
We began alternating annual full‐day and mini half‐day retreats as a method to bring the division together, build camaraderie, set strategic priorities, identify divisional goals, and assess needs. These helped guide the creation of additional FD opportunities as well as our overall division's strategy to achieve our academic mission. The outcomes of these retreats led to many significant initiatives and policies, such as changes in compensation models, new scheduling processes, and decisions to spend resources on areas such as quality improvement.
Program Evaluation
Our evaluation focused on measuring the FD program's impact on our new faculty. We tracked their success in completing the stated scholarly expectations and surveyed them about their satisfaction with the programmatic activities, their first year on faculty, and their preparation for year 2. Prior to implementing the program, we surveyed the previous 2 years of new faculty to provide a comparison.
Results
Seven faculty participated in the inaugural program. We compared their scholarly output and experiences (6 faculty completed the survey; 87% response rate) with that of 11 more senior faculty who completed the comparison survey. Of note, the response rate of the comparison group was 69% (5 faculty who departed from our division during the previous 2 years were not surveyed). New faculty were surveyed at the start of the academic year with the follow‐up survey completed the following June. The more senior faculty completed the survey once at the same time as the baseline survey for the new faculty. All new faculty participated in each of the Core Seminars, the Teaching Course, the required number of Coaching sessions, and the observed teaching activity. We did not track their attendance at Divisional activities such as Grand Rounds or the Lunch Seminars.
Overall, the FD programmatic offerings were rated highly by new faculty (on a scale of 1 [lowest] to 5 [highest] for a global rating of each FD activity): Core Seminars 4.83 0.41, Coaching Program 4.5 0.84, Teaching Course 4.5 0.55, Grand Rounds 4.83 0.41, and Lunch Seminars 4.5 0.84. Table 2, which compares responses to a series of end of the year statements posed to new faculty, highlights notable differences in their level of comfort with specific skills and resource awareness. Given the small sample size, statistical significance was not calculated. Table 3 illustrates similar comparisons focused on academic output, which demonstrate that new faculty gave more talks to trainees, had greater involvement in hospital committees, more actively participated in quality and safety projects, and submitted more abstracts to regional or national meetings. New faculty also responded differently to which part of the FD program was most influential with 1 suggesting the Coaching Program, 2 the Core Seminars, 2 the entire program efforts, and 1 did not specify.
Table 4 illustrates comparison responses to a series of directed statements. New faculty all reported greater degrees of satisfaction overall, measured by the above responses, compared to previous faculty.
| Categories | Previous Faculty, % (n = 11) | New Faculty, % (n = 6) |
|---|---|---|
| ||
| Success: To what degree do you feel successful as an academic hospitalist at the end of your first year? (% responding successful or very successful) | 27 | 67 |
| Prepared: To what degree do you feel prepared for academic success moving into your second year on faculty? (% responding prepared or very prepared) | 27 | 100 |
| Part of DHM: I felt like an integral part of our division after my first year on faculty (% responding somewhat agree or agree) | 45 | 84 |
| Expectations: My first year on faculty exceeded my expectations (% responding somewhat agree or agree) | 27 | 84 |
Discussion
We implemented an FD program to foster the academic development of new faculty, and to mitigate the effects of growing clinical demands and a rapid group expansion on our academic mission. The impact of the program was measured by increased work satisfaction and academic output in first year faculty, greater self‐reported comfort in a variety of skills and knowledge of resources, and an improvement in our sense of purpose behind our academic mission. Though the program is only in its second year, we believe the model is of value for other AHM groups, and perhaps even nonacademic groups, all of whom may use such an investment in their hospitalists as a method to improve recruitment, job satisfaction, and retention.
Reviewing our program's first year suggests there were at least 3 keys to our success. First, we benefited tremendously from the time spent crafting a vision for the program and relying heavily on input from the target audience of junior faculty. Moreover, we made every effort to leverage existing resources (eg, using faculty who already taught about a given topic) and time commitments (eg, reshaping our existing Monday lunch meeting). Finally, we increasingly used our FD venues to connect and build networks with colleagues outside our division and within the hospital. This was a deliberate effort to create opportunities for individual faculty to be exposed to and collaborate with nonhospitalists for academic output.
Our research has some limitations, most notably the small sample size in evaluating the program for statistical significance, and the incomplete survey return rates. However, the results were quite consistent and the nonresponses of departed faculty would tend to bias our results toward the null. We also acknowledge the possibility of other confounding factors (eg, changes in clinical compensation models) that may have played a role, although compensation changes were relatively minor during the period studied and faculty did attribute many of the benefits in job satisfaction and skill building to the FD program itself.
Hospital medicine is an unusual field in that there is low barrier to entry and exit. Providers can change jobs without having to say goodbye to a large panel of patients, and in the continued mismatch between available positions and hospitalists, alternative positions can easily and quickly be found if they are dissatisfied.17 In the academic arena, even as hospitalists are hired to fill clinical gaps, they still have to perform under more traditional academic rules in order to be promoted and receive the support and kudos of colleagues and trainees. For both these reasons, early nurturing and socialization is critical to retention and academic success. While some opportunities for FD will be offered by national organizations,18 groups also have local responsibilities to support, mentor, and develop their junior faculty. Not only is such support crucial for the junior faculty themselves, but in our young field, the mentored very quickly become the mentors. Our decision to invest in both mentees and mentors reinforced the importance of mentorship for academic success and retention while planting the seeds for continued success and growth.1923 A recent study suggested that the environment for mentoring may be as important as the mentoring itself, a finding we did not specifically measure, but would support based our anecdotal experiences.24 This orientation toward future needs and creating the right milieu is crucial because demands for continued hospitalist growth are likely to remain.
Moving into year 2 of our FD program and reflecting on the lessons learned from year 1, we've adopted the same multifaceted approach with only minor adjustments to the curriculum, greater expansion of faculty involved in teaching and coaching, and a continued focus on building a sense of community around our academic mission. For the Core Seminars, we moved away from the 3 half‐day sessions and chose to host 2‐hour sessions every other month. This allowed for the same curriculum to be delivered but was much easier to logistically orchestrate. It also had the intended effect of bringing the new faculty together more regularly. In addition, we created dedicated sessions in preparation for our national meeting to allow faculty to bring abstract submissions for review and later, posters and oral presentations for feedback. These added sessions came partly as a suggestion from new faculty in our first year program, and seemed to further energize junior faculty around converting their projects into scholarship. Finally, we continue to further develop coaching and mentoring relationships in our division, partly a result of successful new facultycoach pairings.
In conclusion, our FD program had a noted impact on our new faculty and had a meaningful impact on our division in terms of camaraderie and cohesion, a shared commitment to an academic mission, and a mechanism for recruitment and retention. We hope our practical description for development and implementation of an FD program, including our specific tools, are useful to other groups considering such an initiative.
Acknowledgements
The authors thank Katherine Li for her invaluable assistance in coordinating the DHM FD program. They are also indebted to their faculty colleagues for their time and roles in teaching and mentoring within the program. Dr. Sehgal partly developed this program as part of a project during his California Healthcare Foundation Leadership Fellowship. Dr. Sharpe delivered the teaching workshops at UCSF after completing the Stanford Faculty Development Teaching Program.
- ,,,.The status of Hospital Medicine Groups in the United States.J Hosp Med.2006;1(2):75–80.
- Accreditation Council for Graduate Medical Education: information related to the ACGME's effort to address resident duty hours and other relevant resource materials. Available at: http://www.acgme.org/acWebsite/dutyHours/dh_index.asp. Accessed August 2010.
- ,,, et al.Effects of work hour reduction on residents' lives: a systematic review.JAMA.2005;294(9):1088–1100.
- ,,,,.Non‐housestaff medicine services in academic centers: models and challenges.J Hosp Med.2008;3(3):247–255.
- ,.Surgical comanagement: a natural evolution of hospitalist practice.J Hosp Med.2008;3(5):394–397.
- ,.The expanding role of hospitalists in the United States.Swiss Med Wkly.2006;136(37–38):591–596.
- 2007–08 Hospital Medicine Survey. Society of Hospital Medicine. Available at: http://www.hospitalmedicine.org/AM/Template.cfm?Section=Survey19(4):392–393.
- ,,, et al.Challenges and opportunities in Academic Hospital Medicine: report from the Academic Hospital Medicine Summit.J Hosp Med.2009;4(4):240–246.
- .Palliative care and hospitalists: a partnership for hope.J Hosp Med.2006;1(1):5–6.
- ,.Faculty development: principles and practices.J Vet Med Educ.2006;33(3):317–324.
- ,,, et al.A systematic review of faculty development initiatives designed to improve teaching effectiveness in medical education: BEME Guide No. 8.Med Teach.2006;28(6):497–526.
- ,.A successful approach to faculty development at an independent academic medical center.Med Teach.2008;30:e10–e14.
- ,,,.An innovative approach to supporting hospitalist physicians towards academic success.J Hosp Med.2008;3(4):314–318.
- ,,, et al.The curriculum for the Hospitalized Aging Medical Patient program: a collaborative faculty development program for hospitalists, general internists, and geriatricians.J Hosp Med.2008;3(5):384–393.
- Stanford Faculty Development Clinical Teaching Program. Available at: http://www.stanford.edu/group/SFDP. Accessed August 2010.
- ,,, et al.Trends in market demand for internal medicine 1999–2004: an analysis of physician job advertisements.J Gen Intern Med.2006;21(10):1079–1085.
- The Academic Hospitalist Academy. Available at: http://www.sgim.org/index.cfm?pageId=815. Accessed August 2010.
- ,,.Mentoring in academic medicine: a systematic review.JAMA.2006;296(9):1103–1115.
- ,,.Facilitating faculty success: outcomes and cost benefit of the UCSD National Center of Leadership in Academic Medicine.Acad Med.2004;79(10 Suppl):S9–S11.
- ,,, et al.Retention of junior faculty in academic medicine at the University of California, San Diego.Acad Med.2009;84(1):37–41.
- ,,,.Helping medical school faculty realize their dreams: an innovative, collaborative mentoring program.Acad Med.2002;77:377–384.
- ,,, et al.Physicians' perceptions of institutional and leadership factors influencing their job satisfaction at one academic medical center.Acad Med.2002;77:1235–1240.
- ,,.A systematic review of qualitative research on the meaning and characteristics of mentoring in academic medicine.J Gen Intern Med.2010;25(1);72–78.
- ,,,.The status of Hospital Medicine Groups in the United States.J Hosp Med.2006;1(2):75–80.
- Accreditation Council for Graduate Medical Education: information related to the ACGME's effort to address resident duty hours and other relevant resource materials. Available at: http://www.acgme.org/acWebsite/dutyHours/dh_index.asp. Accessed August 2010.
- ,,, et al.Effects of work hour reduction on residents' lives: a systematic review.JAMA.2005;294(9):1088–1100.
- ,,,,.Non‐housestaff medicine services in academic centers: models and challenges.J Hosp Med.2008;3(3):247–255.
- ,.Surgical comanagement: a natural evolution of hospitalist practice.J Hosp Med.2008;3(5):394–397.
- ,.The expanding role of hospitalists in the United States.Swiss Med Wkly.2006;136(37–38):591–596.
- 2007–08 Hospital Medicine Survey. Society of Hospital Medicine. Available at: http://www.hospitalmedicine.org/AM/Template.cfm?Section=Survey19(4):392–393.
- ,,, et al.Challenges and opportunities in Academic Hospital Medicine: report from the Academic Hospital Medicine Summit.J Hosp Med.2009;4(4):240–246.
- .Palliative care and hospitalists: a partnership for hope.J Hosp Med.2006;1(1):5–6.
- ,.Faculty development: principles and practices.J Vet Med Educ.2006;33(3):317–324.
- ,,, et al.A systematic review of faculty development initiatives designed to improve teaching effectiveness in medical education: BEME Guide No. 8.Med Teach.2006;28(6):497–526.
- ,.A successful approach to faculty development at an independent academic medical center.Med Teach.2008;30:e10–e14.
- ,,,.An innovative approach to supporting hospitalist physicians towards academic success.J Hosp Med.2008;3(4):314–318.
- ,,, et al.The curriculum for the Hospitalized Aging Medical Patient program: a collaborative faculty development program for hospitalists, general internists, and geriatricians.J Hosp Med.2008;3(5):384–393.
- Stanford Faculty Development Clinical Teaching Program. Available at: http://www.stanford.edu/group/SFDP. Accessed August 2010.
- ,,, et al.Trends in market demand for internal medicine 1999–2004: an analysis of physician job advertisements.J Gen Intern Med.2006;21(10):1079–1085.
- The Academic Hospitalist Academy. Available at: http://www.sgim.org/index.cfm?pageId=815. Accessed August 2010.
- ,,.Mentoring in academic medicine: a systematic review.JAMA.2006;296(9):1103–1115.
- ,,.Facilitating faculty success: outcomes and cost benefit of the UCSD National Center of Leadership in Academic Medicine.Acad Med.2004;79(10 Suppl):S9–S11.
- ,,, et al.Retention of junior faculty in academic medicine at the University of California, San Diego.Acad Med.2009;84(1):37–41.
- ,,,.Helping medical school faculty realize their dreams: an innovative, collaborative mentoring program.Acad Med.2002;77:377–384.
- ,,, et al.Physicians' perceptions of institutional and leadership factors influencing their job satisfaction at one academic medical center.Acad Med.2002;77:1235–1240.
- ,,.A systematic review of qualitative research on the meaning and characteristics of mentoring in academic medicine.J Gen Intern Med.2010;25(1);72–78.
Copyright © 2011 Society of Hospital Medicine
His Mother
Bertha Johnson is back with pneumonia again. The ED doctor on the telephone sounded both matter‐of‐fact and mildly bored when I answered her page about another admission to the hospitalist service. I hadn't met Mrs. Johnson previously, but came to know her and Douglas, her only son, well over the next few days.
The Johnsons were facing a difficult choice. Bertha was now bedbound and quadriplegic following a 40‐year battle with multiple sclerosis and gradually mounting disability. She was cognitively intact and had a solid grasp of medical realities, but was hard of hearing and quite dysarthric. Forming even short phrases and sentences took great effort. However tenuous, this ability to speak allowed her to communicate with those she loved. She had been admitted thrice in the past year with aspiration pneumonia, as she was unable to clear her secretions reliably. Repeated bronchoscopies demonstrated an inability to protect her airway. Douglas, who was also her health care proxy, favored proceeding with the tracheostomy suggested by the pulmonary team. On a prior admission, he had been distressed when his mother refused this intervention. Now she was back with the identical problem and had been given the same recommendation by her doctors. It was particularly difficult for me to discuss these sensitive issues when I had not previously met either Bertha or her son. I spoke to her primary care physician over the phone and he agreed with the need for tracheostomy. The pulmonary team had been involved in discussions about tracheostomy in all of her hospital admissions, providing continuity of care in the process. Ultimately, it was my responsibility to help Bertha and Douglas come to a decision.
After multiple discussions between the Johnsons and me, a consensus emerged to proceed with the tracheostomy. We recognized that the procedure would increase her care needs and arranged for a stay in a skilled nursing facility to provide access to round‐the‐clock suctioning. The evening prior to the tracheostomy, the floor nurse and I reviewed the procedure to ensure that Bertha was fully prepared. What followed resulted in a drastic change of plan. Bertha emphasized that she did not want to lose her only means of communication, even if the surgery would prolong her life. She admitted that she reluctantly agreed to the procedure only to please her son and doctors, because they believed it to be in her best interest. Her fear of the prospect of death from drowning in her own secretions was much less than her fear of silence and isolation that would result from her loss of speech. I shared her misgivings with Douglas, and she admitted to him that she had only agreed to the procedure for his sake. We cancelled the surgery.
Douglas later revealed that he also had been ambivalent about the procedure for sometime, as it would necessitate a nursing home stay and the loss of the caretakers who had cared for his mother so wonderfully for many years. Bertha lived alone in her own home with the help of visiting nurses and patient care assistants Douglas paid for out‐of‐pocket. Douglas lived and worked in a city over a hundred miles away, but managed to visit several times a month to facilitate his mother's care. He had supported the procedure only because the doctors had said it was the only way to avoid future pneumonias. The idea of a tracheostomy was definitively abandoned once and for all.
The Johnsons wanted Bertha to return to her home, but hospital case managers felt this would be an unsafe plan of care as she was alone for several hours a day. She was largely immobile and unable to escape if there were a fire or other emergency. Also, the caretakers were not trained to use the suction equipment, and the visiting nurses would only be available intermittently. The home care staff felt they could no longer meet her needs and declined to resume her care. Douglas became very frustrated by the delays and protracted negotiations, enough so that he threatened to sue the institution for taking over my mother's life. The threat of litigation is usually a cry for help that reflects either miscommunication or the suffering of a conflicted family.
As their hospitalist, I hoped to advocate for both the patient and Douglas while coordinating the overall care plan. I had always received consistent responses from the Johnsons, but other staff members noted that Douglas had expressed shifting views on the best site of care for his mother. At Bertha's request, I convened a meeting with her, Douglas, the social worker, case manager, visiting and staff nurses, the palliative care nurse, and floor manager. Prior to this, I met with all involved health care providers to ensure we understood each other's abilities and limitations regarding Bertha's care. As I entered the room for the family meeting, I knew it was ultimately the patient's choice whether she wanted to return home or notas long as she understood the risks involved.
During the meeting, all the team members explained the dilemmas they faced in planning for a safe disposition. Douglas's response illuminated his devotion and love for his ailing mother. He had known all along that it would be less expensive and burdensome for him for Bertha to be placed in a facility. However, he feared nursing home admission represented giving up and failing to fulfill my duty to my mother. Tears ran down the face of this otherwise well composed, immaculately dressed, articulate man in his late forties. He had assumed the responsibility for his mother's care while still a child and had carried this self‐imposed moral burden his entire life. This meeting was his first opportunity to voice explicitly to the medical team his immense love and concern for his dear mother.
I gently probed to clarify Bertha's values and goals. On a brief, prior nursing home stay, Bertha had found the experience to be scary and unfamiliar. However, as her functional abilities continued to decline, her feelings had changed. She now felt lonely and anxious at home when her caretakers were absent. She actually wanted to go to a nursing home, where there would always be company and support available! She had not told Douglas this because she knew he cared for her deeply and she didn't want to hurt his feelings; he seemed committed to caring for her the same way he had for so many years.
In short, Douglas knew it would be easier for him if his mother were in a nursing facility, but assumed she wanted to stay in her home. Oddly, Bertha was only remaining at home because she believed that was what her son wished. A few days later, Bertha was transferred to a nursing home near several relatives who would visit her regularly. Douglas was again selfless in not seeking to move her closer to him. He didn't want to uproot her more than was unavoidable.
Day‐to‐day practice reveals many examples of love and dedication, but I have never seen such blinding and unquestioning commitment as exemplified by this mother‐son duo. From them, I learned the importance of attentive and active listening. Our polite patients may only subtly hint at matters of the deepest import. If we cannot truly hear their unspoken emotions, we risk harming them and misinterpreting their words and actions. Some healthcare providers had seen Douglas as aggressive and demanding with his threat of a lawsuit, whereas Bertha had been described as unrealistic or in denial. These views distorted a much more complex reality. Time and attention to careful communication between the healthcare providers, the patient, and her son bore fruit in this case. The procedure that was really needed was the family meeting and not the tracheostomy! An undesired and invasive procedure was avoided, goals of care were clarified, quality of life maximized, a safe discharge arranged, and a new mutual understanding achieved. I was humbled, and reminded of the importance of team‐based care and the need to approach each patient and family member in a receptive, nonjudgmental, and open manner. Douglas and Bertha Johnson were linked with a profound and abiding bond that would only be severed at death.
Bertha Johnson is back with pneumonia again. The ED doctor on the telephone sounded both matter‐of‐fact and mildly bored when I answered her page about another admission to the hospitalist service. I hadn't met Mrs. Johnson previously, but came to know her and Douglas, her only son, well over the next few days.
The Johnsons were facing a difficult choice. Bertha was now bedbound and quadriplegic following a 40‐year battle with multiple sclerosis and gradually mounting disability. She was cognitively intact and had a solid grasp of medical realities, but was hard of hearing and quite dysarthric. Forming even short phrases and sentences took great effort. However tenuous, this ability to speak allowed her to communicate with those she loved. She had been admitted thrice in the past year with aspiration pneumonia, as she was unable to clear her secretions reliably. Repeated bronchoscopies demonstrated an inability to protect her airway. Douglas, who was also her health care proxy, favored proceeding with the tracheostomy suggested by the pulmonary team. On a prior admission, he had been distressed when his mother refused this intervention. Now she was back with the identical problem and had been given the same recommendation by her doctors. It was particularly difficult for me to discuss these sensitive issues when I had not previously met either Bertha or her son. I spoke to her primary care physician over the phone and he agreed with the need for tracheostomy. The pulmonary team had been involved in discussions about tracheostomy in all of her hospital admissions, providing continuity of care in the process. Ultimately, it was my responsibility to help Bertha and Douglas come to a decision.
After multiple discussions between the Johnsons and me, a consensus emerged to proceed with the tracheostomy. We recognized that the procedure would increase her care needs and arranged for a stay in a skilled nursing facility to provide access to round‐the‐clock suctioning. The evening prior to the tracheostomy, the floor nurse and I reviewed the procedure to ensure that Bertha was fully prepared. What followed resulted in a drastic change of plan. Bertha emphasized that she did not want to lose her only means of communication, even if the surgery would prolong her life. She admitted that she reluctantly agreed to the procedure only to please her son and doctors, because they believed it to be in her best interest. Her fear of the prospect of death from drowning in her own secretions was much less than her fear of silence and isolation that would result from her loss of speech. I shared her misgivings with Douglas, and she admitted to him that she had only agreed to the procedure for his sake. We cancelled the surgery.
Douglas later revealed that he also had been ambivalent about the procedure for sometime, as it would necessitate a nursing home stay and the loss of the caretakers who had cared for his mother so wonderfully for many years. Bertha lived alone in her own home with the help of visiting nurses and patient care assistants Douglas paid for out‐of‐pocket. Douglas lived and worked in a city over a hundred miles away, but managed to visit several times a month to facilitate his mother's care. He had supported the procedure only because the doctors had said it was the only way to avoid future pneumonias. The idea of a tracheostomy was definitively abandoned once and for all.
The Johnsons wanted Bertha to return to her home, but hospital case managers felt this would be an unsafe plan of care as she was alone for several hours a day. She was largely immobile and unable to escape if there were a fire or other emergency. Also, the caretakers were not trained to use the suction equipment, and the visiting nurses would only be available intermittently. The home care staff felt they could no longer meet her needs and declined to resume her care. Douglas became very frustrated by the delays and protracted negotiations, enough so that he threatened to sue the institution for taking over my mother's life. The threat of litigation is usually a cry for help that reflects either miscommunication or the suffering of a conflicted family.
As their hospitalist, I hoped to advocate for both the patient and Douglas while coordinating the overall care plan. I had always received consistent responses from the Johnsons, but other staff members noted that Douglas had expressed shifting views on the best site of care for his mother. At Bertha's request, I convened a meeting with her, Douglas, the social worker, case manager, visiting and staff nurses, the palliative care nurse, and floor manager. Prior to this, I met with all involved health care providers to ensure we understood each other's abilities and limitations regarding Bertha's care. As I entered the room for the family meeting, I knew it was ultimately the patient's choice whether she wanted to return home or notas long as she understood the risks involved.
During the meeting, all the team members explained the dilemmas they faced in planning for a safe disposition. Douglas's response illuminated his devotion and love for his ailing mother. He had known all along that it would be less expensive and burdensome for him for Bertha to be placed in a facility. However, he feared nursing home admission represented giving up and failing to fulfill my duty to my mother. Tears ran down the face of this otherwise well composed, immaculately dressed, articulate man in his late forties. He had assumed the responsibility for his mother's care while still a child and had carried this self‐imposed moral burden his entire life. This meeting was his first opportunity to voice explicitly to the medical team his immense love and concern for his dear mother.
I gently probed to clarify Bertha's values and goals. On a brief, prior nursing home stay, Bertha had found the experience to be scary and unfamiliar. However, as her functional abilities continued to decline, her feelings had changed. She now felt lonely and anxious at home when her caretakers were absent. She actually wanted to go to a nursing home, where there would always be company and support available! She had not told Douglas this because she knew he cared for her deeply and she didn't want to hurt his feelings; he seemed committed to caring for her the same way he had for so many years.
In short, Douglas knew it would be easier for him if his mother were in a nursing facility, but assumed she wanted to stay in her home. Oddly, Bertha was only remaining at home because she believed that was what her son wished. A few days later, Bertha was transferred to a nursing home near several relatives who would visit her regularly. Douglas was again selfless in not seeking to move her closer to him. He didn't want to uproot her more than was unavoidable.
Day‐to‐day practice reveals many examples of love and dedication, but I have never seen such blinding and unquestioning commitment as exemplified by this mother‐son duo. From them, I learned the importance of attentive and active listening. Our polite patients may only subtly hint at matters of the deepest import. If we cannot truly hear their unspoken emotions, we risk harming them and misinterpreting their words and actions. Some healthcare providers had seen Douglas as aggressive and demanding with his threat of a lawsuit, whereas Bertha had been described as unrealistic or in denial. These views distorted a much more complex reality. Time and attention to careful communication between the healthcare providers, the patient, and her son bore fruit in this case. The procedure that was really needed was the family meeting and not the tracheostomy! An undesired and invasive procedure was avoided, goals of care were clarified, quality of life maximized, a safe discharge arranged, and a new mutual understanding achieved. I was humbled, and reminded of the importance of team‐based care and the need to approach each patient and family member in a receptive, nonjudgmental, and open manner. Douglas and Bertha Johnson were linked with a profound and abiding bond that would only be severed at death.
Bertha Johnson is back with pneumonia again. The ED doctor on the telephone sounded both matter‐of‐fact and mildly bored when I answered her page about another admission to the hospitalist service. I hadn't met Mrs. Johnson previously, but came to know her and Douglas, her only son, well over the next few days.
The Johnsons were facing a difficult choice. Bertha was now bedbound and quadriplegic following a 40‐year battle with multiple sclerosis and gradually mounting disability. She was cognitively intact and had a solid grasp of medical realities, but was hard of hearing and quite dysarthric. Forming even short phrases and sentences took great effort. However tenuous, this ability to speak allowed her to communicate with those she loved. She had been admitted thrice in the past year with aspiration pneumonia, as she was unable to clear her secretions reliably. Repeated bronchoscopies demonstrated an inability to protect her airway. Douglas, who was also her health care proxy, favored proceeding with the tracheostomy suggested by the pulmonary team. On a prior admission, he had been distressed when his mother refused this intervention. Now she was back with the identical problem and had been given the same recommendation by her doctors. It was particularly difficult for me to discuss these sensitive issues when I had not previously met either Bertha or her son. I spoke to her primary care physician over the phone and he agreed with the need for tracheostomy. The pulmonary team had been involved in discussions about tracheostomy in all of her hospital admissions, providing continuity of care in the process. Ultimately, it was my responsibility to help Bertha and Douglas come to a decision.
After multiple discussions between the Johnsons and me, a consensus emerged to proceed with the tracheostomy. We recognized that the procedure would increase her care needs and arranged for a stay in a skilled nursing facility to provide access to round‐the‐clock suctioning. The evening prior to the tracheostomy, the floor nurse and I reviewed the procedure to ensure that Bertha was fully prepared. What followed resulted in a drastic change of plan. Bertha emphasized that she did not want to lose her only means of communication, even if the surgery would prolong her life. She admitted that she reluctantly agreed to the procedure only to please her son and doctors, because they believed it to be in her best interest. Her fear of the prospect of death from drowning in her own secretions was much less than her fear of silence and isolation that would result from her loss of speech. I shared her misgivings with Douglas, and she admitted to him that she had only agreed to the procedure for his sake. We cancelled the surgery.
Douglas later revealed that he also had been ambivalent about the procedure for sometime, as it would necessitate a nursing home stay and the loss of the caretakers who had cared for his mother so wonderfully for many years. Bertha lived alone in her own home with the help of visiting nurses and patient care assistants Douglas paid for out‐of‐pocket. Douglas lived and worked in a city over a hundred miles away, but managed to visit several times a month to facilitate his mother's care. He had supported the procedure only because the doctors had said it was the only way to avoid future pneumonias. The idea of a tracheostomy was definitively abandoned once and for all.
The Johnsons wanted Bertha to return to her home, but hospital case managers felt this would be an unsafe plan of care as she was alone for several hours a day. She was largely immobile and unable to escape if there were a fire or other emergency. Also, the caretakers were not trained to use the suction equipment, and the visiting nurses would only be available intermittently. The home care staff felt they could no longer meet her needs and declined to resume her care. Douglas became very frustrated by the delays and protracted negotiations, enough so that he threatened to sue the institution for taking over my mother's life. The threat of litigation is usually a cry for help that reflects either miscommunication or the suffering of a conflicted family.
As their hospitalist, I hoped to advocate for both the patient and Douglas while coordinating the overall care plan. I had always received consistent responses from the Johnsons, but other staff members noted that Douglas had expressed shifting views on the best site of care for his mother. At Bertha's request, I convened a meeting with her, Douglas, the social worker, case manager, visiting and staff nurses, the palliative care nurse, and floor manager. Prior to this, I met with all involved health care providers to ensure we understood each other's abilities and limitations regarding Bertha's care. As I entered the room for the family meeting, I knew it was ultimately the patient's choice whether she wanted to return home or notas long as she understood the risks involved.
During the meeting, all the team members explained the dilemmas they faced in planning for a safe disposition. Douglas's response illuminated his devotion and love for his ailing mother. He had known all along that it would be less expensive and burdensome for him for Bertha to be placed in a facility. However, he feared nursing home admission represented giving up and failing to fulfill my duty to my mother. Tears ran down the face of this otherwise well composed, immaculately dressed, articulate man in his late forties. He had assumed the responsibility for his mother's care while still a child and had carried this self‐imposed moral burden his entire life. This meeting was his first opportunity to voice explicitly to the medical team his immense love and concern for his dear mother.
I gently probed to clarify Bertha's values and goals. On a brief, prior nursing home stay, Bertha had found the experience to be scary and unfamiliar. However, as her functional abilities continued to decline, her feelings had changed. She now felt lonely and anxious at home when her caretakers were absent. She actually wanted to go to a nursing home, where there would always be company and support available! She had not told Douglas this because she knew he cared for her deeply and she didn't want to hurt his feelings; he seemed committed to caring for her the same way he had for so many years.
In short, Douglas knew it would be easier for him if his mother were in a nursing facility, but assumed she wanted to stay in her home. Oddly, Bertha was only remaining at home because she believed that was what her son wished. A few days later, Bertha was transferred to a nursing home near several relatives who would visit her regularly. Douglas was again selfless in not seeking to move her closer to him. He didn't want to uproot her more than was unavoidable.
Day‐to‐day practice reveals many examples of love and dedication, but I have never seen such blinding and unquestioning commitment as exemplified by this mother‐son duo. From them, I learned the importance of attentive and active listening. Our polite patients may only subtly hint at matters of the deepest import. If we cannot truly hear their unspoken emotions, we risk harming them and misinterpreting their words and actions. Some healthcare providers had seen Douglas as aggressive and demanding with his threat of a lawsuit, whereas Bertha had been described as unrealistic or in denial. These views distorted a much more complex reality. Time and attention to careful communication between the healthcare providers, the patient, and her son bore fruit in this case. The procedure that was really needed was the family meeting and not the tracheostomy! An undesired and invasive procedure was avoided, goals of care were clarified, quality of life maximized, a safe discharge arranged, and a new mutual understanding achieved. I was humbled, and reminded of the importance of team‐based care and the need to approach each patient and family member in a receptive, nonjudgmental, and open manner. Douglas and Bertha Johnson were linked with a profound and abiding bond that would only be severed at death.
Mapping Out Diagnosis
A 19‐year‐old Japanese man was admitted to a hospital near Kyoto, Japan, because of fever and rash. Two weeks prior to admission, he developed mild headache and low‐grade fever; a rapid test for influenza was negative. His symptoms transiently improved with acetaminophen, but 8 days prior to admission, he developed fever to 38.5C and a pruritic maculopapular rash over his back that spread to his limbs. Six days prior to admission, a chest radiograph was clear; clarithromycin was prescribed for presumed upper respiratory infection. He visited the emergency department the day before admission because of continued fever of greater than 39C, fatigue, and headache. Because there was no jolt accentuation of the headache (ie, worsening with rapid horizontal rotation), or neck pain with extreme neck flexion, he was discharged on acetaminophen. He returned the next day with worsening fatigue and was admitted. He denied chills, rigor, weight loss, photosensitivity, sore throat, neck pain, cough, dyspnea, chest pain, nausea, vomiting, diarrhea, abdominal pain, back pain, and arthralgia.
Fever and diffuse rash are often due to infection, although drugs, autoimmune processes, and cancer must be considered. The presence of headache does not focus the differential diagnosis substantially, because many of the candidate diagnoses can be accompanied by meningitis or encephalitis, or even more frequently, nonspecific headaches. In one small study, jolt‐induced aggravation of headache was shown to be a sensitive indicator of cerebrospinal fluid pleocytosis. The absence of neck stiffness and the 2‐week duration makes bacterial meningitis unlikely, but a more indolent form of aseptic meningitis may need to be evaluated with a lumbar puncture.
The 2‐week illness without rapid deterioration makes some serious causes of fever and rash, such as toxic shock syndrome, disseminated meningococcal infection, or toxic epidermal necrolysis unlikely. A viral exanthema is possible, although the 2‐week duration is longer than usual. Given his youth, however, his immunization history should be queried, and acute infection with human immunodeficiency virus (HIV) should be considered. A more indolent infection, such as subacute bacterial endocarditis, disseminated gonococcal infection, or syphilis is plausible. Among autoimmune etiologies, systemic lupus erythematosus (SLE) and Behcet's disease (which is prevalent in Japan) can involve the central nervous system and cause fever. A careful inquiry directed at prescribed, complementary, and illicit drugs is required.
The patient's past medical history was notable only for mumps at the age of 10. His medications included acetaminophen, clarithromycin, and an herbal medicine, which he had been taking for the prior several days. He reported no tobacco or illicit drug use and rarely drank alcohol. He had never been sexually active. He worked in a factory and reported occasional contact with silver. He lived with his parents; there was no family history of tuberculosis or connective tissue diseases. His father was from Kyushu (the southernmost major island in Japan) and had chronic hepatitis C. The patient denied recent animal exposure or recent travel. His childhood vaccinations were said to be up to date.
Mumps at age 10 might signal general lack of immunization, in which case childhood viral exanthema‐like measles (characterized by fever, headache, and diffuse rash) would warrant consideration. The listed medications had been started after the onset of illness and therefore are unlikely to be causal. Silver causes at least 2 skin conditionscontact dermatitis and argyriabut not the systemic illness seen here. Human T lymphotropic virus‐1 (HTLV‐1) is endemic in southern Japan, but only a minority of infected humans are afflicted with associated adult T cell leukemia/lymphoma or myelopathy. Leukemia and lymphoma are the most likely cancers to cause fever, rash, and central nervous system involvement (with T cell disorders demonstrating a particular tropism for the skin). Overall, however, the differential has not changed substantially.
On physical examination, the patient was mildly overweight and appeared acutely ill. His blood pressure was 136/78 mm Hg, pulse rate was 76 and regular, temperature was 39.2C and respiratory rate was 20 with an oxygen saturation of 98% on room air. A diffuse but nonconfluent erythematous maculopapular rash was present over his chest wall, back, medial aspects of both thighs, and around the knees. There was no jolt‐induced headache. His eyes, nose, oral cavity, and throat were all clear. The neck was supple. There were palpable lymph nodes, each about 1 cm in size, which were firm and moderately tender, in his left neck and left axilla. Lungs and heart were normal. The abdomen was soft, nontender, with normal bowel sounds and no hepatosplenomegaly. His genitalia were normal. Rectal examination revealed no masses or tenderness and a scant amount of brown stool that was negative for occult blood. Neurologic examination was unremarkable.
The multifocal lymphadenopathy does not help distinguish among the categories of disease under consideration. The diffuse maculopapular rash is similarly nonspecific, occurring more frequently with infection and drug reaction than malignancy and autoimmunity. Acute HIV, Epstein‐Barr virus (EBV), syphilis, SLE, drug exposure, or a hematologic malignancy would all be suitable explanations for fever, headache, diffuse rash, and disseminated lymphadenopathy in a previously healthy young man.
Laboratory data obtained on admission was notable for a white blood cell (WBC) count of 2100/L with 72% neutrophils, 19% lymphocytes, and 9% monocytes. Hemoglobin was 13.5 mg/dL with a mean corpuscular volume of 85 fL. Platelet count was 136,000/L. Erythrocyte sedimentation rate was 26 mm/hour. Serum chemistries revealed a sodium level of 135 mEq/L, potassium level of 3.6 mEq/L, chloride level of 100 mEq/L, blood urea nitrogen of 9.8 mg/dL, creatinine level of 1.0 mg/dL, glucose level of 101 mg/dL, calcium level of 8.8 mg/dL, albumin of 4.6 mg/dL, total protein of 8.4 mg/dL, aspartate aminotransferase of 42 IU/L (normal 35 IU/L), alanine aminotransferase of 27 IU/L, total bilirubin of 0.5 mg/dL, and lactate dehydrogenase (LDH) level of 463 IU/L (normal 260 IU/L). Chest radiography and electrocardiogram were normal.
A mild elevation in LDH is nonspecific, but without hemolysis or infarction of the kidney, lung, or muscle, it suggests a lymphoproliferative process. Leukopenia with thrombocytopenia can be seen in a number of disorders, most commonly infections including viruses (e.g., EBV, HIV, dengue), malaria, Rocky Mountain spotted fever, or ehrlichiosis/anaplasmosis. Confirmation of his lack of travel could help prioritize those considerations. An invasive bone marrow disorder cannot be excluded, although the near‐normal hemoglobin argues against it. Autoimmune cytopenias are seen in SLE. Given his age, lymphadenopathy, LDH elevation, and absence of infectious exposures, lymphoma rises to the top of the list.
Noninvasive measures should include examination of the peripheral smear, HIV testing (including HIV RNA for acute infection), EBV serologies, and tests for syphilis and SLE. Lumbar puncture (for evaluation of aseptic meningitis) and lymph node biopsy would be informative. Skin biopsy may be helpful to evaluate for aggressive T cell lymphoproliferative disorder, but this can await the results of initial testing.
The patient was given intravenous fluids and acetaminophen as needed. Blood cultures, urine culture, cytomegalovirus and EBV serologies, hepatitis B surface antigen, hepatitis C virus antibody, HIV antibody, antinuclear antibody, complement and ferritin levels, and quantiferon‐TB were ordered. The urine was normal and a urinary antigen test for Legionella was negative. Contrast‐enhanced computed tomography scan of the chest and abdomen was normal except for mild splenomegaly and an enlarged left axillary lymph node.
The ferritin may have been ordered to help evaluate for Still's disease, which is characterized by sustained fever, lymphadenopathy, and transient rash; however, the characteristic leukocytosis and arthralgias are absent. The computed tomography findings are most notable for the absence of generalized lymphadenopathy or significant hepatosplenomegaly that is seen in lymphoma, leukemia, and lymphotropic processes such as acute EBV infection. The localization of disease to the skin (where the predominant lymphocytes are of T cell origin) with relatively modest lymphadenopathy suggests a T cell lymphoma, perhaps of an indolent variety. Vertical transmission of HTLV‐1 decades ago would make adult T cell leukemia or lymphoma a major consideration.
On the third hospital day, WBC count was 1800/L with 67% neutrophils, 22% lymphocytes, and 1% atypical lymphocytes; LDH rose to 623 IU/L. He had continued fatigue and high fever while the rash gradually faded with oral antihistamines and steroid ointment. On hospital day 4, bone marrow biopsy and skin biopsy of his left thigh were performed.
The further decline in WBC and rise in LDH are modest and therefore do not significantly modify the differential diagnosis. Likewise, 1% atypical lymphocytosis is too low to pinpoint an etiology. Because unremitting fevers start to extend into their third week without a clear source of infection, the probability of malignancy and autoimmunity rise. Improvement with oral antihistamines and topical steroids frequently suggests an underlying allergic process, but the remainder of the clinical picture is not in keeping with atopy or allergy. Cutaneous lymphomas (eg, mycosis fungoides) can have waxing and waning skin manifestations, and can be temporarily or definitively treated by topical steroids. The persistence of his fatigue is of concern given the absence of anemia, cardiopulmonary involvement, or motor weakness.
Bone marrow biopsy showed normocellular marrow with no abnormal cells and some activated macrophages with hemophagocytic activity. Skin biopsy failed to show specific pathology.
His left cervical lymph nodes gradually enlarged. Ultrasound of the neck showed multiple enlarged lymph nodes (left side dominant) with dimension of 17 mm 9 mm 31 mm. Blood and urine cultures returned negative, as did HIV antibody. cytomegalovirus and EBV serologies were consistent with previous infection and the ferritin level was 578 ng/mL (normal, 39‐340 ng/mL). Toxoplasma serology and HTLV‐1 antibody were ordered.
The absence of malignant cells on bone marrow biopsy does not exclude lymphoma, but makes a myelophthisic cause of the cytopenias less likely. The macrophage hemophagocytosis reflects immune activation, which in turn is usually caused by the same viral infections, autoimmune conditions, and lymphoproliferative disorders which constitute the current differential diagnosis.
Bone marrow and skin biopsies are both subject to sampling error, and detection of cutaneous T cell lymphoma is notoriously difficult. However, taken together, the absence of cancer on 2 specimens reduces that possibility.
Sustained unilateral cervical lymphadenopathy with fever in a young Japanese man without any histologic evidence of lymphoma points to Kikuchi's disease, ie, lymphadenitis of unknown etiology associated with varying degrees of systemic manifestations. Fever is a frequent feature, we believe, but diffuse sustained rash, cytopenias, and headache are less common or are seen in severe forms of the disease. The diagnosis of Kikuchi's requires the diligent exclusion of SLE and lymphoma. Examination of the peripheral smear and a lymph node biopsy are required.
Of note, there is also a localized form of Castleman's disease, a nonmalignant lymphoproliferative disorder, that similarly is characterized by focal lymphadenopathy. In distinction to Kikuchi's, however, localized Castleman's is largely asymptomatic and responds marvelously to excision.
On hospital day 9, an excisional biopsy of his left anterior cervical lymph nodes was performed, which revealed paracortical foci with necrosis and a histiocytic cellular infiltrate consistent with subacute necrotizing lymphadenitis (Kikuchi‐Fujimoto disease). Antinuclear antibody, Toxoplasma, and HTLV‐1 antibodies returned negative.
There is no treatment for Kikuchi's. It is usually self‐limited, but steroids are sometimes given for symptomatic control.
His condition began to improve after hospital day 9 without specific treatment, including his WBC count and LDH level. He was discharged home on hospital day 15. In the outpatient clinic 1 and 3 months later, he was well and active without recurrences of any symptoms or laboratory abnormalities. His WBC count was 6600/L and LDH was 268 IU/L.
Commentary
Kikuchi‐Fujimoto disease (KFD), also called Kikuchi's disease, is a benign histiocytic necrotizing lymphadenitis described by both Kikuchi and Fujimoto in 1972.1, 2 It is rare in the United States, but seems more common in Asia, especially Japan, where at least 143 cases have been reported since 1972. The etiology has not been determined, but a viral causeincluding EBV, and human herpesvirus 6 and 8has been suggested.3 An autoimmune etiology is also implicated because of infrequent association with SLE. In general, young women are most likely to be affected. In a review of 244 cases by Kucukardali and colleagues, 77% of patients were female and the mean age was 25; 70% were younger than 30 years of age.4
The common presentation is low‐grade fever with unilateral cervical lymphadenopathy.4 Although generalized lymphadenopathy can occur, it is rare. Other common clinical manifestations include malaise, joint pain, rash, arthritis, and hepatosplenomegaly. No specific laboratory tests for diagnosis are available, but leukopenia (seen in 43% of patients), increased erythrocyte sedimentation rate (40%), and anemia (23%) may be observed.4 In this case, atypical lymphocytes were seen, and are reported in one‐third of patients.5 KFD is generally diagnosed by lymph node biopsy, which typically shows irregular paracortical areas of coagulation necrosis that can distort the nodal architecture, while different types of histiocytes are observed at the margin of necrotic areas.
Other diseases in the differential diagnosisseveral of which were considered by the discussantinclude lymphoma, tuberculosis, SLE, and even metastatic adenocarcinoma. KFD is self‐limited; symptoms typically resolve within 1 to 4 months. Patients with severe manifestations have been treated with anti‐inflammatory drugs and glucocorticosteroids. A recurrence rate of 3% to 4% has been reported.6
The clinicians taking care of this patient initially focused on ruling out those infections occasionally resulting in prolonged fever in a previously healthy young man, such as viruses from the herpes family, HIV, viral hepatitis, tuberculosis, syphilis, infective endocarditis, and intra‐abdominal abscess. Physical examination, specifically lymphadenopathy and mild splenomegaly, made Herpesviridae infections, tuberculosis, syphilis, and lymphoma difficult to exclude. Once the initial evaluation ruled out common infections, attention focused on malignancy and histiocytic necrotizing lymphadenitis, given his ethnicity and geographic location.
The discussant was similarly concerned about infection, malignancy, and noninfectious inflammatory diseases, such as SLE, as possible causes. As evidence of these treatable diseases failed to accumulate, the discussant, an American physician with teaching and clinical experience in Japan, considered endemic diseases such as Behcet's, HTLV‐1, and KFD because they fit the unfolding pattern. Given our global society, clinicians will increasingly benefit from becoming familiar with the less common diseases that afflict the various populations around the world.
Teaching Points
-
The combination of fever, lymphadenopathy, and leukopenia in young adults suggests SLE, lymphoma, and HIV. Clinicians should also consider KFD in patients from Japan and neighboring countries.
-
Lymph node biopsy is usually diagnostic of KFD, although interpretation of histopathology can be difficult and sometimes leads to confusion with SLE and lymphoma.
-
KFD typically resolves without specific treatment.
The approach to clinical conundrums by an expert clinician is revealed through the presentation of an actual patient's case in an approach typical of a morning report. Similarly to patient care, sequential pieces of information are provided to the clinician, who is unfamiliar with the case. The focus is on the thought processes of both the clinical team caring for the patient and the discussant.
- .Lymphadenitis showing focal reticulum cell hyperplasia with nuclear debris and phagocytes: a clinicopathological study.Acta Hematol Jpn.1972;35:379–380.
- ,,.Cervical subacute necrotizing lymphadenitis: a new clinicopathological agent.Naika.1972;20:920–927.
- ,,,.Enigmatic Kikuchi‐Fujimoto disease: a comprehensive review.Am J Clin Pathol.2004;122:141–152.
- ,,,,,.Kikuchi‐Fujimoto Disease: analysis of 244 cases.Clin Rheumatol.2007;26:50–54.
- ,,,,.Kikuchi's disease: A review and analysis of 61 cases.Otolaryngol Head Neck Surg.2003;128:650–653.
- .Histiocytic necrotizing lymphadenitis of Kikuchi and Fujimoto.Arch Pathol Lab Med.1987;11:1026–1029.
A 19‐year‐old Japanese man was admitted to a hospital near Kyoto, Japan, because of fever and rash. Two weeks prior to admission, he developed mild headache and low‐grade fever; a rapid test for influenza was negative. His symptoms transiently improved with acetaminophen, but 8 days prior to admission, he developed fever to 38.5C and a pruritic maculopapular rash over his back that spread to his limbs. Six days prior to admission, a chest radiograph was clear; clarithromycin was prescribed for presumed upper respiratory infection. He visited the emergency department the day before admission because of continued fever of greater than 39C, fatigue, and headache. Because there was no jolt accentuation of the headache (ie, worsening with rapid horizontal rotation), or neck pain with extreme neck flexion, he was discharged on acetaminophen. He returned the next day with worsening fatigue and was admitted. He denied chills, rigor, weight loss, photosensitivity, sore throat, neck pain, cough, dyspnea, chest pain, nausea, vomiting, diarrhea, abdominal pain, back pain, and arthralgia.
Fever and diffuse rash are often due to infection, although drugs, autoimmune processes, and cancer must be considered. The presence of headache does not focus the differential diagnosis substantially, because many of the candidate diagnoses can be accompanied by meningitis or encephalitis, or even more frequently, nonspecific headaches. In one small study, jolt‐induced aggravation of headache was shown to be a sensitive indicator of cerebrospinal fluid pleocytosis. The absence of neck stiffness and the 2‐week duration makes bacterial meningitis unlikely, but a more indolent form of aseptic meningitis may need to be evaluated with a lumbar puncture.
The 2‐week illness without rapid deterioration makes some serious causes of fever and rash, such as toxic shock syndrome, disseminated meningococcal infection, or toxic epidermal necrolysis unlikely. A viral exanthema is possible, although the 2‐week duration is longer than usual. Given his youth, however, his immunization history should be queried, and acute infection with human immunodeficiency virus (HIV) should be considered. A more indolent infection, such as subacute bacterial endocarditis, disseminated gonococcal infection, or syphilis is plausible. Among autoimmune etiologies, systemic lupus erythematosus (SLE) and Behcet's disease (which is prevalent in Japan) can involve the central nervous system and cause fever. A careful inquiry directed at prescribed, complementary, and illicit drugs is required.
The patient's past medical history was notable only for mumps at the age of 10. His medications included acetaminophen, clarithromycin, and an herbal medicine, which he had been taking for the prior several days. He reported no tobacco or illicit drug use and rarely drank alcohol. He had never been sexually active. He worked in a factory and reported occasional contact with silver. He lived with his parents; there was no family history of tuberculosis or connective tissue diseases. His father was from Kyushu (the southernmost major island in Japan) and had chronic hepatitis C. The patient denied recent animal exposure or recent travel. His childhood vaccinations were said to be up to date.
Mumps at age 10 might signal general lack of immunization, in which case childhood viral exanthema‐like measles (characterized by fever, headache, and diffuse rash) would warrant consideration. The listed medications had been started after the onset of illness and therefore are unlikely to be causal. Silver causes at least 2 skin conditionscontact dermatitis and argyriabut not the systemic illness seen here. Human T lymphotropic virus‐1 (HTLV‐1) is endemic in southern Japan, but only a minority of infected humans are afflicted with associated adult T cell leukemia/lymphoma or myelopathy. Leukemia and lymphoma are the most likely cancers to cause fever, rash, and central nervous system involvement (with T cell disorders demonstrating a particular tropism for the skin). Overall, however, the differential has not changed substantially.
On physical examination, the patient was mildly overweight and appeared acutely ill. His blood pressure was 136/78 mm Hg, pulse rate was 76 and regular, temperature was 39.2C and respiratory rate was 20 with an oxygen saturation of 98% on room air. A diffuse but nonconfluent erythematous maculopapular rash was present over his chest wall, back, medial aspects of both thighs, and around the knees. There was no jolt‐induced headache. His eyes, nose, oral cavity, and throat were all clear. The neck was supple. There were palpable lymph nodes, each about 1 cm in size, which were firm and moderately tender, in his left neck and left axilla. Lungs and heart were normal. The abdomen was soft, nontender, with normal bowel sounds and no hepatosplenomegaly. His genitalia were normal. Rectal examination revealed no masses or tenderness and a scant amount of brown stool that was negative for occult blood. Neurologic examination was unremarkable.
The multifocal lymphadenopathy does not help distinguish among the categories of disease under consideration. The diffuse maculopapular rash is similarly nonspecific, occurring more frequently with infection and drug reaction than malignancy and autoimmunity. Acute HIV, Epstein‐Barr virus (EBV), syphilis, SLE, drug exposure, or a hematologic malignancy would all be suitable explanations for fever, headache, diffuse rash, and disseminated lymphadenopathy in a previously healthy young man.
Laboratory data obtained on admission was notable for a white blood cell (WBC) count of 2100/L with 72% neutrophils, 19% lymphocytes, and 9% monocytes. Hemoglobin was 13.5 mg/dL with a mean corpuscular volume of 85 fL. Platelet count was 136,000/L. Erythrocyte sedimentation rate was 26 mm/hour. Serum chemistries revealed a sodium level of 135 mEq/L, potassium level of 3.6 mEq/L, chloride level of 100 mEq/L, blood urea nitrogen of 9.8 mg/dL, creatinine level of 1.0 mg/dL, glucose level of 101 mg/dL, calcium level of 8.8 mg/dL, albumin of 4.6 mg/dL, total protein of 8.4 mg/dL, aspartate aminotransferase of 42 IU/L (normal 35 IU/L), alanine aminotransferase of 27 IU/L, total bilirubin of 0.5 mg/dL, and lactate dehydrogenase (LDH) level of 463 IU/L (normal 260 IU/L). Chest radiography and electrocardiogram were normal.
A mild elevation in LDH is nonspecific, but without hemolysis or infarction of the kidney, lung, or muscle, it suggests a lymphoproliferative process. Leukopenia with thrombocytopenia can be seen in a number of disorders, most commonly infections including viruses (e.g., EBV, HIV, dengue), malaria, Rocky Mountain spotted fever, or ehrlichiosis/anaplasmosis. Confirmation of his lack of travel could help prioritize those considerations. An invasive bone marrow disorder cannot be excluded, although the near‐normal hemoglobin argues against it. Autoimmune cytopenias are seen in SLE. Given his age, lymphadenopathy, LDH elevation, and absence of infectious exposures, lymphoma rises to the top of the list.
Noninvasive measures should include examination of the peripheral smear, HIV testing (including HIV RNA for acute infection), EBV serologies, and tests for syphilis and SLE. Lumbar puncture (for evaluation of aseptic meningitis) and lymph node biopsy would be informative. Skin biopsy may be helpful to evaluate for aggressive T cell lymphoproliferative disorder, but this can await the results of initial testing.
The patient was given intravenous fluids and acetaminophen as needed. Blood cultures, urine culture, cytomegalovirus and EBV serologies, hepatitis B surface antigen, hepatitis C virus antibody, HIV antibody, antinuclear antibody, complement and ferritin levels, and quantiferon‐TB were ordered. The urine was normal and a urinary antigen test for Legionella was negative. Contrast‐enhanced computed tomography scan of the chest and abdomen was normal except for mild splenomegaly and an enlarged left axillary lymph node.
The ferritin may have been ordered to help evaluate for Still's disease, which is characterized by sustained fever, lymphadenopathy, and transient rash; however, the characteristic leukocytosis and arthralgias are absent. The computed tomography findings are most notable for the absence of generalized lymphadenopathy or significant hepatosplenomegaly that is seen in lymphoma, leukemia, and lymphotropic processes such as acute EBV infection. The localization of disease to the skin (where the predominant lymphocytes are of T cell origin) with relatively modest lymphadenopathy suggests a T cell lymphoma, perhaps of an indolent variety. Vertical transmission of HTLV‐1 decades ago would make adult T cell leukemia or lymphoma a major consideration.
On the third hospital day, WBC count was 1800/L with 67% neutrophils, 22% lymphocytes, and 1% atypical lymphocytes; LDH rose to 623 IU/L. He had continued fatigue and high fever while the rash gradually faded with oral antihistamines and steroid ointment. On hospital day 4, bone marrow biopsy and skin biopsy of his left thigh were performed.
The further decline in WBC and rise in LDH are modest and therefore do not significantly modify the differential diagnosis. Likewise, 1% atypical lymphocytosis is too low to pinpoint an etiology. Because unremitting fevers start to extend into their third week without a clear source of infection, the probability of malignancy and autoimmunity rise. Improvement with oral antihistamines and topical steroids frequently suggests an underlying allergic process, but the remainder of the clinical picture is not in keeping with atopy or allergy. Cutaneous lymphomas (eg, mycosis fungoides) can have waxing and waning skin manifestations, and can be temporarily or definitively treated by topical steroids. The persistence of his fatigue is of concern given the absence of anemia, cardiopulmonary involvement, or motor weakness.
Bone marrow biopsy showed normocellular marrow with no abnormal cells and some activated macrophages with hemophagocytic activity. Skin biopsy failed to show specific pathology.
His left cervical lymph nodes gradually enlarged. Ultrasound of the neck showed multiple enlarged lymph nodes (left side dominant) with dimension of 17 mm 9 mm 31 mm. Blood and urine cultures returned negative, as did HIV antibody. cytomegalovirus and EBV serologies were consistent with previous infection and the ferritin level was 578 ng/mL (normal, 39‐340 ng/mL). Toxoplasma serology and HTLV‐1 antibody were ordered.
The absence of malignant cells on bone marrow biopsy does not exclude lymphoma, but makes a myelophthisic cause of the cytopenias less likely. The macrophage hemophagocytosis reflects immune activation, which in turn is usually caused by the same viral infections, autoimmune conditions, and lymphoproliferative disorders which constitute the current differential diagnosis.
Bone marrow and skin biopsies are both subject to sampling error, and detection of cutaneous T cell lymphoma is notoriously difficult. However, taken together, the absence of cancer on 2 specimens reduces that possibility.
Sustained unilateral cervical lymphadenopathy with fever in a young Japanese man without any histologic evidence of lymphoma points to Kikuchi's disease, ie, lymphadenitis of unknown etiology associated with varying degrees of systemic manifestations. Fever is a frequent feature, we believe, but diffuse sustained rash, cytopenias, and headache are less common or are seen in severe forms of the disease. The diagnosis of Kikuchi's requires the diligent exclusion of SLE and lymphoma. Examination of the peripheral smear and a lymph node biopsy are required.
Of note, there is also a localized form of Castleman's disease, a nonmalignant lymphoproliferative disorder, that similarly is characterized by focal lymphadenopathy. In distinction to Kikuchi's, however, localized Castleman's is largely asymptomatic and responds marvelously to excision.
On hospital day 9, an excisional biopsy of his left anterior cervical lymph nodes was performed, which revealed paracortical foci with necrosis and a histiocytic cellular infiltrate consistent with subacute necrotizing lymphadenitis (Kikuchi‐Fujimoto disease). Antinuclear antibody, Toxoplasma, and HTLV‐1 antibodies returned negative.
There is no treatment for Kikuchi's. It is usually self‐limited, but steroids are sometimes given for symptomatic control.
His condition began to improve after hospital day 9 without specific treatment, including his WBC count and LDH level. He was discharged home on hospital day 15. In the outpatient clinic 1 and 3 months later, he was well and active without recurrences of any symptoms or laboratory abnormalities. His WBC count was 6600/L and LDH was 268 IU/L.
Commentary
Kikuchi‐Fujimoto disease (KFD), also called Kikuchi's disease, is a benign histiocytic necrotizing lymphadenitis described by both Kikuchi and Fujimoto in 1972.1, 2 It is rare in the United States, but seems more common in Asia, especially Japan, where at least 143 cases have been reported since 1972. The etiology has not been determined, but a viral causeincluding EBV, and human herpesvirus 6 and 8has been suggested.3 An autoimmune etiology is also implicated because of infrequent association with SLE. In general, young women are most likely to be affected. In a review of 244 cases by Kucukardali and colleagues, 77% of patients were female and the mean age was 25; 70% were younger than 30 years of age.4
The common presentation is low‐grade fever with unilateral cervical lymphadenopathy.4 Although generalized lymphadenopathy can occur, it is rare. Other common clinical manifestations include malaise, joint pain, rash, arthritis, and hepatosplenomegaly. No specific laboratory tests for diagnosis are available, but leukopenia (seen in 43% of patients), increased erythrocyte sedimentation rate (40%), and anemia (23%) may be observed.4 In this case, atypical lymphocytes were seen, and are reported in one‐third of patients.5 KFD is generally diagnosed by lymph node biopsy, which typically shows irregular paracortical areas of coagulation necrosis that can distort the nodal architecture, while different types of histiocytes are observed at the margin of necrotic areas.
Other diseases in the differential diagnosisseveral of which were considered by the discussantinclude lymphoma, tuberculosis, SLE, and even metastatic adenocarcinoma. KFD is self‐limited; symptoms typically resolve within 1 to 4 months. Patients with severe manifestations have been treated with anti‐inflammatory drugs and glucocorticosteroids. A recurrence rate of 3% to 4% has been reported.6
The clinicians taking care of this patient initially focused on ruling out those infections occasionally resulting in prolonged fever in a previously healthy young man, such as viruses from the herpes family, HIV, viral hepatitis, tuberculosis, syphilis, infective endocarditis, and intra‐abdominal abscess. Physical examination, specifically lymphadenopathy and mild splenomegaly, made Herpesviridae infections, tuberculosis, syphilis, and lymphoma difficult to exclude. Once the initial evaluation ruled out common infections, attention focused on malignancy and histiocytic necrotizing lymphadenitis, given his ethnicity and geographic location.
The discussant was similarly concerned about infection, malignancy, and noninfectious inflammatory diseases, such as SLE, as possible causes. As evidence of these treatable diseases failed to accumulate, the discussant, an American physician with teaching and clinical experience in Japan, considered endemic diseases such as Behcet's, HTLV‐1, and KFD because they fit the unfolding pattern. Given our global society, clinicians will increasingly benefit from becoming familiar with the less common diseases that afflict the various populations around the world.
Teaching Points
-
The combination of fever, lymphadenopathy, and leukopenia in young adults suggests SLE, lymphoma, and HIV. Clinicians should also consider KFD in patients from Japan and neighboring countries.
-
Lymph node biopsy is usually diagnostic of KFD, although interpretation of histopathology can be difficult and sometimes leads to confusion with SLE and lymphoma.
-
KFD typically resolves without specific treatment.
The approach to clinical conundrums by an expert clinician is revealed through the presentation of an actual patient's case in an approach typical of a morning report. Similarly to patient care, sequential pieces of information are provided to the clinician, who is unfamiliar with the case. The focus is on the thought processes of both the clinical team caring for the patient and the discussant.
A 19‐year‐old Japanese man was admitted to a hospital near Kyoto, Japan, because of fever and rash. Two weeks prior to admission, he developed mild headache and low‐grade fever; a rapid test for influenza was negative. His symptoms transiently improved with acetaminophen, but 8 days prior to admission, he developed fever to 38.5C and a pruritic maculopapular rash over his back that spread to his limbs. Six days prior to admission, a chest radiograph was clear; clarithromycin was prescribed for presumed upper respiratory infection. He visited the emergency department the day before admission because of continued fever of greater than 39C, fatigue, and headache. Because there was no jolt accentuation of the headache (ie, worsening with rapid horizontal rotation), or neck pain with extreme neck flexion, he was discharged on acetaminophen. He returned the next day with worsening fatigue and was admitted. He denied chills, rigor, weight loss, photosensitivity, sore throat, neck pain, cough, dyspnea, chest pain, nausea, vomiting, diarrhea, abdominal pain, back pain, and arthralgia.
Fever and diffuse rash are often due to infection, although drugs, autoimmune processes, and cancer must be considered. The presence of headache does not focus the differential diagnosis substantially, because many of the candidate diagnoses can be accompanied by meningitis or encephalitis, or even more frequently, nonspecific headaches. In one small study, jolt‐induced aggravation of headache was shown to be a sensitive indicator of cerebrospinal fluid pleocytosis. The absence of neck stiffness and the 2‐week duration makes bacterial meningitis unlikely, but a more indolent form of aseptic meningitis may need to be evaluated with a lumbar puncture.
The 2‐week illness without rapid deterioration makes some serious causes of fever and rash, such as toxic shock syndrome, disseminated meningococcal infection, or toxic epidermal necrolysis unlikely. A viral exanthema is possible, although the 2‐week duration is longer than usual. Given his youth, however, his immunization history should be queried, and acute infection with human immunodeficiency virus (HIV) should be considered. A more indolent infection, such as subacute bacterial endocarditis, disseminated gonococcal infection, or syphilis is plausible. Among autoimmune etiologies, systemic lupus erythematosus (SLE) and Behcet's disease (which is prevalent in Japan) can involve the central nervous system and cause fever. A careful inquiry directed at prescribed, complementary, and illicit drugs is required.
The patient's past medical history was notable only for mumps at the age of 10. His medications included acetaminophen, clarithromycin, and an herbal medicine, which he had been taking for the prior several days. He reported no tobacco or illicit drug use and rarely drank alcohol. He had never been sexually active. He worked in a factory and reported occasional contact with silver. He lived with his parents; there was no family history of tuberculosis or connective tissue diseases. His father was from Kyushu (the southernmost major island in Japan) and had chronic hepatitis C. The patient denied recent animal exposure or recent travel. His childhood vaccinations were said to be up to date.
Mumps at age 10 might signal general lack of immunization, in which case childhood viral exanthema‐like measles (characterized by fever, headache, and diffuse rash) would warrant consideration. The listed medications had been started after the onset of illness and therefore are unlikely to be causal. Silver causes at least 2 skin conditionscontact dermatitis and argyriabut not the systemic illness seen here. Human T lymphotropic virus‐1 (HTLV‐1) is endemic in southern Japan, but only a minority of infected humans are afflicted with associated adult T cell leukemia/lymphoma or myelopathy. Leukemia and lymphoma are the most likely cancers to cause fever, rash, and central nervous system involvement (with T cell disorders demonstrating a particular tropism for the skin). Overall, however, the differential has not changed substantially.
On physical examination, the patient was mildly overweight and appeared acutely ill. His blood pressure was 136/78 mm Hg, pulse rate was 76 and regular, temperature was 39.2C and respiratory rate was 20 with an oxygen saturation of 98% on room air. A diffuse but nonconfluent erythematous maculopapular rash was present over his chest wall, back, medial aspects of both thighs, and around the knees. There was no jolt‐induced headache. His eyes, nose, oral cavity, and throat were all clear. The neck was supple. There were palpable lymph nodes, each about 1 cm in size, which were firm and moderately tender, in his left neck and left axilla. Lungs and heart were normal. The abdomen was soft, nontender, with normal bowel sounds and no hepatosplenomegaly. His genitalia were normal. Rectal examination revealed no masses or tenderness and a scant amount of brown stool that was negative for occult blood. Neurologic examination was unremarkable.
The multifocal lymphadenopathy does not help distinguish among the categories of disease under consideration. The diffuse maculopapular rash is similarly nonspecific, occurring more frequently with infection and drug reaction than malignancy and autoimmunity. Acute HIV, Epstein‐Barr virus (EBV), syphilis, SLE, drug exposure, or a hematologic malignancy would all be suitable explanations for fever, headache, diffuse rash, and disseminated lymphadenopathy in a previously healthy young man.
Laboratory data obtained on admission was notable for a white blood cell (WBC) count of 2100/L with 72% neutrophils, 19% lymphocytes, and 9% monocytes. Hemoglobin was 13.5 mg/dL with a mean corpuscular volume of 85 fL. Platelet count was 136,000/L. Erythrocyte sedimentation rate was 26 mm/hour. Serum chemistries revealed a sodium level of 135 mEq/L, potassium level of 3.6 mEq/L, chloride level of 100 mEq/L, blood urea nitrogen of 9.8 mg/dL, creatinine level of 1.0 mg/dL, glucose level of 101 mg/dL, calcium level of 8.8 mg/dL, albumin of 4.6 mg/dL, total protein of 8.4 mg/dL, aspartate aminotransferase of 42 IU/L (normal 35 IU/L), alanine aminotransferase of 27 IU/L, total bilirubin of 0.5 mg/dL, and lactate dehydrogenase (LDH) level of 463 IU/L (normal 260 IU/L). Chest radiography and electrocardiogram were normal.
A mild elevation in LDH is nonspecific, but without hemolysis or infarction of the kidney, lung, or muscle, it suggests a lymphoproliferative process. Leukopenia with thrombocytopenia can be seen in a number of disorders, most commonly infections including viruses (e.g., EBV, HIV, dengue), malaria, Rocky Mountain spotted fever, or ehrlichiosis/anaplasmosis. Confirmation of his lack of travel could help prioritize those considerations. An invasive bone marrow disorder cannot be excluded, although the near‐normal hemoglobin argues against it. Autoimmune cytopenias are seen in SLE. Given his age, lymphadenopathy, LDH elevation, and absence of infectious exposures, lymphoma rises to the top of the list.
Noninvasive measures should include examination of the peripheral smear, HIV testing (including HIV RNA for acute infection), EBV serologies, and tests for syphilis and SLE. Lumbar puncture (for evaluation of aseptic meningitis) and lymph node biopsy would be informative. Skin biopsy may be helpful to evaluate for aggressive T cell lymphoproliferative disorder, but this can await the results of initial testing.
The patient was given intravenous fluids and acetaminophen as needed. Blood cultures, urine culture, cytomegalovirus and EBV serologies, hepatitis B surface antigen, hepatitis C virus antibody, HIV antibody, antinuclear antibody, complement and ferritin levels, and quantiferon‐TB were ordered. The urine was normal and a urinary antigen test for Legionella was negative. Contrast‐enhanced computed tomography scan of the chest and abdomen was normal except for mild splenomegaly and an enlarged left axillary lymph node.
The ferritin may have been ordered to help evaluate for Still's disease, which is characterized by sustained fever, lymphadenopathy, and transient rash; however, the characteristic leukocytosis and arthralgias are absent. The computed tomography findings are most notable for the absence of generalized lymphadenopathy or significant hepatosplenomegaly that is seen in lymphoma, leukemia, and lymphotropic processes such as acute EBV infection. The localization of disease to the skin (where the predominant lymphocytes are of T cell origin) with relatively modest lymphadenopathy suggests a T cell lymphoma, perhaps of an indolent variety. Vertical transmission of HTLV‐1 decades ago would make adult T cell leukemia or lymphoma a major consideration.
On the third hospital day, WBC count was 1800/L with 67% neutrophils, 22% lymphocytes, and 1% atypical lymphocytes; LDH rose to 623 IU/L. He had continued fatigue and high fever while the rash gradually faded with oral antihistamines and steroid ointment. On hospital day 4, bone marrow biopsy and skin biopsy of his left thigh were performed.
The further decline in WBC and rise in LDH are modest and therefore do not significantly modify the differential diagnosis. Likewise, 1% atypical lymphocytosis is too low to pinpoint an etiology. Because unremitting fevers start to extend into their third week without a clear source of infection, the probability of malignancy and autoimmunity rise. Improvement with oral antihistamines and topical steroids frequently suggests an underlying allergic process, but the remainder of the clinical picture is not in keeping with atopy or allergy. Cutaneous lymphomas (eg, mycosis fungoides) can have waxing and waning skin manifestations, and can be temporarily or definitively treated by topical steroids. The persistence of his fatigue is of concern given the absence of anemia, cardiopulmonary involvement, or motor weakness.
Bone marrow biopsy showed normocellular marrow with no abnormal cells and some activated macrophages with hemophagocytic activity. Skin biopsy failed to show specific pathology.
His left cervical lymph nodes gradually enlarged. Ultrasound of the neck showed multiple enlarged lymph nodes (left side dominant) with dimension of 17 mm 9 mm 31 mm. Blood and urine cultures returned negative, as did HIV antibody. cytomegalovirus and EBV serologies were consistent with previous infection and the ferritin level was 578 ng/mL (normal, 39‐340 ng/mL). Toxoplasma serology and HTLV‐1 antibody were ordered.
The absence of malignant cells on bone marrow biopsy does not exclude lymphoma, but makes a myelophthisic cause of the cytopenias less likely. The macrophage hemophagocytosis reflects immune activation, which in turn is usually caused by the same viral infections, autoimmune conditions, and lymphoproliferative disorders which constitute the current differential diagnosis.
Bone marrow and skin biopsies are both subject to sampling error, and detection of cutaneous T cell lymphoma is notoriously difficult. However, taken together, the absence of cancer on 2 specimens reduces that possibility.
Sustained unilateral cervical lymphadenopathy with fever in a young Japanese man without any histologic evidence of lymphoma points to Kikuchi's disease, ie, lymphadenitis of unknown etiology associated with varying degrees of systemic manifestations. Fever is a frequent feature, we believe, but diffuse sustained rash, cytopenias, and headache are less common or are seen in severe forms of the disease. The diagnosis of Kikuchi's requires the diligent exclusion of SLE and lymphoma. Examination of the peripheral smear and a lymph node biopsy are required.
Of note, there is also a localized form of Castleman's disease, a nonmalignant lymphoproliferative disorder, that similarly is characterized by focal lymphadenopathy. In distinction to Kikuchi's, however, localized Castleman's is largely asymptomatic and responds marvelously to excision.
On hospital day 9, an excisional biopsy of his left anterior cervical lymph nodes was performed, which revealed paracortical foci with necrosis and a histiocytic cellular infiltrate consistent with subacute necrotizing lymphadenitis (Kikuchi‐Fujimoto disease). Antinuclear antibody, Toxoplasma, and HTLV‐1 antibodies returned negative.
There is no treatment for Kikuchi's. It is usually self‐limited, but steroids are sometimes given for symptomatic control.
His condition began to improve after hospital day 9 without specific treatment, including his WBC count and LDH level. He was discharged home on hospital day 15. In the outpatient clinic 1 and 3 months later, he was well and active without recurrences of any symptoms or laboratory abnormalities. His WBC count was 6600/L and LDH was 268 IU/L.
Commentary
Kikuchi‐Fujimoto disease (KFD), also called Kikuchi's disease, is a benign histiocytic necrotizing lymphadenitis described by both Kikuchi and Fujimoto in 1972.1, 2 It is rare in the United States, but seems more common in Asia, especially Japan, where at least 143 cases have been reported since 1972. The etiology has not been determined, but a viral causeincluding EBV, and human herpesvirus 6 and 8has been suggested.3 An autoimmune etiology is also implicated because of infrequent association with SLE. In general, young women are most likely to be affected. In a review of 244 cases by Kucukardali and colleagues, 77% of patients were female and the mean age was 25; 70% were younger than 30 years of age.4
The common presentation is low‐grade fever with unilateral cervical lymphadenopathy.4 Although generalized lymphadenopathy can occur, it is rare. Other common clinical manifestations include malaise, joint pain, rash, arthritis, and hepatosplenomegaly. No specific laboratory tests for diagnosis are available, but leukopenia (seen in 43% of patients), increased erythrocyte sedimentation rate (40%), and anemia (23%) may be observed.4 In this case, atypical lymphocytes were seen, and are reported in one‐third of patients.5 KFD is generally diagnosed by lymph node biopsy, which typically shows irregular paracortical areas of coagulation necrosis that can distort the nodal architecture, while different types of histiocytes are observed at the margin of necrotic areas.
Other diseases in the differential diagnosisseveral of which were considered by the discussantinclude lymphoma, tuberculosis, SLE, and even metastatic adenocarcinoma. KFD is self‐limited; symptoms typically resolve within 1 to 4 months. Patients with severe manifestations have been treated with anti‐inflammatory drugs and glucocorticosteroids. A recurrence rate of 3% to 4% has been reported.6
The clinicians taking care of this patient initially focused on ruling out those infections occasionally resulting in prolonged fever in a previously healthy young man, such as viruses from the herpes family, HIV, viral hepatitis, tuberculosis, syphilis, infective endocarditis, and intra‐abdominal abscess. Physical examination, specifically lymphadenopathy and mild splenomegaly, made Herpesviridae infections, tuberculosis, syphilis, and lymphoma difficult to exclude. Once the initial evaluation ruled out common infections, attention focused on malignancy and histiocytic necrotizing lymphadenitis, given his ethnicity and geographic location.
The discussant was similarly concerned about infection, malignancy, and noninfectious inflammatory diseases, such as SLE, as possible causes. As evidence of these treatable diseases failed to accumulate, the discussant, an American physician with teaching and clinical experience in Japan, considered endemic diseases such as Behcet's, HTLV‐1, and KFD because they fit the unfolding pattern. Given our global society, clinicians will increasingly benefit from becoming familiar with the less common diseases that afflict the various populations around the world.
Teaching Points
-
The combination of fever, lymphadenopathy, and leukopenia in young adults suggests SLE, lymphoma, and HIV. Clinicians should also consider KFD in patients from Japan and neighboring countries.
-
Lymph node biopsy is usually diagnostic of KFD, although interpretation of histopathology can be difficult and sometimes leads to confusion with SLE and lymphoma.
-
KFD typically resolves without specific treatment.
The approach to clinical conundrums by an expert clinician is revealed through the presentation of an actual patient's case in an approach typical of a morning report. Similarly to patient care, sequential pieces of information are provided to the clinician, who is unfamiliar with the case. The focus is on the thought processes of both the clinical team caring for the patient and the discussant.
- .Lymphadenitis showing focal reticulum cell hyperplasia with nuclear debris and phagocytes: a clinicopathological study.Acta Hematol Jpn.1972;35:379–380.
- ,,.Cervical subacute necrotizing lymphadenitis: a new clinicopathological agent.Naika.1972;20:920–927.
- ,,,.Enigmatic Kikuchi‐Fujimoto disease: a comprehensive review.Am J Clin Pathol.2004;122:141–152.
- ,,,,,.Kikuchi‐Fujimoto Disease: analysis of 244 cases.Clin Rheumatol.2007;26:50–54.
- ,,,,.Kikuchi's disease: A review and analysis of 61 cases.Otolaryngol Head Neck Surg.2003;128:650–653.
- .Histiocytic necrotizing lymphadenitis of Kikuchi and Fujimoto.Arch Pathol Lab Med.1987;11:1026–1029.
- .Lymphadenitis showing focal reticulum cell hyperplasia with nuclear debris and phagocytes: a clinicopathological study.Acta Hematol Jpn.1972;35:379–380.
- ,,.Cervical subacute necrotizing lymphadenitis: a new clinicopathological agent.Naika.1972;20:920–927.
- ,,,.Enigmatic Kikuchi‐Fujimoto disease: a comprehensive review.Am J Clin Pathol.2004;122:141–152.
- ,,,,,.Kikuchi‐Fujimoto Disease: analysis of 244 cases.Clin Rheumatol.2007;26:50–54.
- ,,,,.Kikuchi's disease: A review and analysis of 61 cases.Otolaryngol Head Neck Surg.2003;128:650–653.
- .Histiocytic necrotizing lymphadenitis of Kikuchi and Fujimoto.Arch Pathol Lab Med.1987;11:1026–1029.
Physician Assistant‐Based General Medical Inpatient Care
In 2003 the Accreditation Council for Graduate Medical Education (ACGME) prescribed residency reform in the form of work hour restrictions without prescribing alternatives to resident based care.1 As a response, many academic medical centers have developed innovative models for providing inpatient care, some of which incorporate Physician Assistants (PAs).2 With further restrictions in resident work hours possible,3 teaching hospitals may increase use of these alternate models to provide inpatient care. Widespread implementation of such new and untested models could impact the care of the approximately 20 million hospitalizations that occur every year in US teaching hospitals.4
Few reports have compared the care delivered by these alternate models with the care provided by traditional resident‐based models of care.58 Roy et al.8 have provided the only recent comparison of a PA‐based model of care with a resident‐based model. They showed lower adjusted costs of inpatient care associated with PA based care but other outcomes were similar to resident‐based teams.
The objective of this study is to provide a valid and usable comparison of the outcomes of a hospitalist‐PA (H‐PA) model of inpatient care with the traditional resident‐based model. This will add to the quantity and quality of the limited research on PA‐based inpatient care, and informs the anticipated increase in the involvement of PAs in this arena.
Methods
Study Design and Setting
We conducted a retrospective cohort study at a 430‐bed urban academic medical center in the Midwestern United States.
Models of General Medical (GM) Inpatient Care at the Study Hospital During the Study Period
In November 2004, as a response to the ACGME‐mandated work hour regulations, we formed 2 Hospitalist‐PA teams (H‐PA) to supplement the 6 preexisting general medicine resident teams (RES).
The H‐PA and RES teams differed in staffing, admitting times and weekend/overnight cross coverage structure (Table 1). There were no predesigned differences between the teams in the ward location of their patients, availability of laboratory/radiology services, specialty consultation, social services/case management resources, nursing resources or documentation requirements for admission, daily care, and discharge.
| H‐PA Teams | RES Teams | |
|---|---|---|
| Attending physician | Always a hospitalist | Hospitalist, non‐hospitalist general internist or rarely a specialist |
| Attending physician role | Supervisory for some patients (about half) and sole care provider for others. | Supervisory for all patients |
| Team composition | One attending paired with 1 PA | Attending + senior resident + (2) interns + (2‐3) medical students |
| Rotation schedule | ||
| Attending | Every 2 weeks | Every 2 weeks |
| Physician assistant | Off on weekends | |
| House staff & medical students | Every month | |
| Weekend | No new admissions & hospitalist manages all patients | Accept new admissions |
| Admission times (weekdays) | 7 AM to 3 PM | Noon to 7 AM |
| Source of admissions | Emergency room, clinics, other hospitals | Emergency room, clinics, other hospitals |
| Number of admissions (weekdays) | 4‐6 patients per day per team | Noon to 5 PM: 2 teams admit a maximum of 9 patients total |
| 5 PM to 7 AM: 3 teams admit a maximum 5 patients each. | ||
| Overnight coverageroles and responsibilities | One in‐house faculty | 3 on call interns |
| Cross‐covering 2 H‐PA teams | Cross‐covering 2 teams each | |
| Performing triage | Admitting up to 5 patients each | |
| Admitting patients if necessary | ||
| Assisting residents if necessary | ||
| General medical consultation |
Admission Schedule for H‐PA or RES Teams
The admitting schedule was designed to decrease the workload of the house staff and to do so specifically during the periods of peak educational activity (morning report, attending‐led teaching rounds, and noon report). A faculty admitting medical officer (AMO) assigned patients strictly based on the time an admission was requested. Importantly, the request for admission preceded the time of actual admission recorded when the patient reached the ward. The time difference between request for admission and actual admission depended on the source of admission and the delay associated with assigning a patient room. The AMO assigned 8 to 12 new patients to the H‐PA teams every weekday between 7 AM and 3 PM and to the RES teams between noon and 7 AM the next day. There was a designed period of overlap from noon to 3 PM during which both H‐PA and RES teams could admit patients. This period allowed for flexibility in assigning patients to either type of team depending on their workload. The AMO did not use patient complexity or teaching value to assign patients.
Exceptions to Admission Schedule
Patients admitted overnight after the on call RES had reached their admission limits were assigned to H‐PA teams the next morning. In addition, recently discharged patients who were readmitted while the discharging hospitalist (H‐PA teams) or the discharging resident (RES teams) was still scheduled for inpatient duties, were assigned back to the discharging team irrespective of the admitting schedule.
The same medicine team cared for a patient from admission to discharge but on transfer to the intensive care unit (ICU), an intensivist led critical care team assumed care. On transfer out of the ICU these patients were assigned back to the original team irrespective of admitting schedulethe so called bounce back rule to promote inpatient continuity of care. But if the residents (RES teams) or the hospitalist (H‐PA teams) had changedthe bounce back rule was no longer in effect and these patients were assigned to a team according to the admission schedule.
Study Population and Study Period
We included all hospitalizations of adult patients to GM teams if both their date of admission and their date of discharge fell within the study period (January 1, 2005 to December 31, 2006). We excluded hospitalizations with admissions during the weekendwhen H‐PA teams did not admit patients; hospitalizations to GM services with transfer to nonGM service (excluding ICU) and hospitalizations involving comanagement with specialty servicesas the contribution of GM teams for these was variable; and hospitalizations of private patients.
Data Collection and Team Assignment
We collected patient data from our hospital's discharge abstract database. This database did not contain team information so to assign teams we matched the discharging attending and the day of discharge to the type of team that the discharging attending was leading that day.
We collected patient age, gender, race, insurance status, zip‐code, primary care provider, source of admission, ward type, time and day of admission, and time and day of discharge for use as independent variables. The time of admission captured in the database was the time of actual admission and not the time the admission was requested.
We grouped the principal diagnosis International Statistical Classification of Diseases and Related Health Problems, 9th edition (ICD‐9) codes into clinically relevant categories using the Clinical Classification Software.9 We created comorbidity measures using Healthcare Cost and Utilization Project Comorbidity Software, version 3.4.10
Outcome Measures
We used length of stay (LOS), charges, readmissions within 7, 14, and 30 days and inpatient mortality as our outcome measures. We calculated LOS by subtracting the discharge day and time from the admission day and time. The LOS included time spent in the ICU. We summed all charges accrued during the entire hospitalization including any stay in the ICU but did not include professional fees. We considered any repeat hospitalization to our hospital within 7, 14, and 30 days following a discharge to be a readmission except that we excluded readmissions for a planned procedure or for inpatient rehabilitation.
Statistical Analysis
Descriptive Analysis
We performed unadjusted descriptive statistics at the level of an individual hospitalization using medians and interquartile ranges for continuous data and frequencies and percentages for categorical data. We used chi‐square tests of association and KruskalWallis analysis of variance to compare H‐PA and RES teams.
Missing Data
Because we lacked data on whether a primary outpatient care provider was available for 284 (2.9%) of our study hospitalizations, we dropped them from our multivariable analyses. We used an arbitrary discharge time of noon for the 11 hospitalizations which did not have a discharge time recorded.
Multivariable Analysis
We used multivariable mixed models to risk adjust for a wide variety of variables. We included age, gender, race, insurance, presence of primary care physician, and total number of comorbidities as fixed effects in all models because of the high face validity of these variables. We then added admission source, ward, time, day of week, discharge day of week, and comorbidity measures one by one as fixed effects, including them only if significant at P < 0.01. For assessing LOS, charges, and readmissions, we added a variable identifying each patient as a random effect to account for multiple admissions for the same patient. We then added variables identifying attending physician, principal diagnostic group, and ZIP code of residence as random effects to account for clustering of hospitalizations within these categories, including them only if significant at P < 0.01. For the model assessing mortality we included variables for attending physician, principal diagnostic group, and ZIP code of residence as random effects if significant at P < 0.01. We log transformed LOS and charges because they were extremely skewed in nature. Readmissions were analyzed after excluding patients who died or were discharged alive within 7, 14, or 30 days of the end of the study period.
Sensitivity Analyses
To assess the influence of LOS outliers, we changed LOS to 6 hours if it was less than 6 hours, and 45 days if it was more than 45 daysa process called winsorizing. We consider winsorizing superior to dropping outliers because it acknowledges that outliers contribute information, but prevent them from being too influential. We chose the 6 hour cut off because we believed that was the minimum time required to admit and then discharge a patient. We chose the upper limit of 45 days on reviewing the frequency distribution for outliers. Similarly, we winsorized charges at the first and 99th percentile after reviewing the frequency distribution for outliers. We then log transformed the winsorized data before analysis.
Inpatient deaths reduce the LOS and charges associated with a hospitalization. Thus excess mortality may provide a false concession in terms of lower LOS or charges. To check if this occurred in our study we repeated the analyses after excluding inpatient deaths.
ICU stays are associated with higher LOS, charges, and mortality. In our model of care, some patients transferred to the ICU are not cared for by the original team on transfer out. Moreover, care in the ICU is not controlled by the team that discharges them. Since this might obscure differences in outcomes achieved by RES vs. H‐PA teams, we repeated these analyses after excluding hospitalizations with an ICU stay.
Since mortality can only occur during 1 hospitalization per patient, we repeated the mortality analysis using only each patient's first admission or last admission and using a randomly selected single admission for each patient.
Subgroup Analysis
To limit the effect of different physician characteristics on H‐PA and RES teams we separately analyzed the hospitalizations under the care of hospitalists who served on both H‐PA and RES teams.
To limit the effect of different admission schedules of H‐PA and RES teams we analyzed the hospitalizations with admission times between 11.00 AM and 4.00 PM. Such hospitalizations were likely to be assigned during the noon to 3 PM period when they could be assigned to either an H‐PA or RES team.
Interactions
Finally we explored interactions between the type of team and the fixed effect variables included in each model.
Statistical Software
We performed the statistical analysis using SAS software version 9.0 for UNIX (SAS Institute, Inc., Cary, NC) and R software (The R Project for Statistical Computing).
This study protocol was approved by the hospital's institutional review board.
Results
Study Population
Of the 52,391 hospitalizations to our hospital during the study period, 13,058 were admitted to general medicine. We excluded 3102 weekend admissions and 209 who met other exclusion criteria. We could not determine the team assignment for 66. Of the remaining 9681 hospitalizations, we assigned 2171 to H‐PA teams and 7510 to RES teams (Figure 1).

Descriptive Analysis
We compare patients assigned to H‐PA and RES teams in Table 2. They were similar in age, gender, race, having a primary care provider or not, and insurance status. Clinically, they had similar comorbidities and a similar distribution of common principal diagnoses. Consistent with their admitting schedule, H‐PA teams admitted and discharged more patients earlier in the day and admitted more patients earlier in the work week. Patients cared for by H‐PA teams were admitted from the Emergency Room (ER) less often and were more likely to reside on wards designated as nonmedicine by nursing specialty. Hospitalizations to H‐PA teams more often included an ICU stay.
| H‐PA (n = 2171) | RES (n = 7510) | P Value | |
|---|---|---|---|
| |||
| Age | |||
| Mean | 56.80 | 57.04 | |
| Median | 56 | 56 | 0.15 |
| Interquartile range | 43‐72 | 43‐73 | |
| Age group (years), n (%) | |||
| < 20 | 10 (0.5) | 57 (0.8) | |
| 20‐29 | 186 (8.6) | 632 (8.7) | |
| 30‐39 | 221 (10.2) | 766 (10.3) | |
| 40‐49 | 387 (17.8) | 1341 (18.1) | |
| 50‐59 | 434 (20.0) | 1492 (20.2) | 0.28 |
| 60‐69 | 325 (15.0) | 974 (12.8) | |
| 70‐79 | 271 (12.5) | 1035 (13.6) | |
| 80‐89 | 262 (12.0) | 951(12.3) | |
| 90< | 75 (3.5) | 262 (3.4) | |
| Female, n (%) | 1175 (54.1) | 4138 (55.1) | 0.42 |
| Race, n (%) | |||
| White | 1282 (59.1) | 4419 (58.9) | |
| Black | 793 (36.5) | 2754 (36.7) | 0.98 |
| Other | 96 (4.4) | 337 (4.5) | |
| Primary care provider, n (%) | 0.16 | ||
| Yes | 1537 (73.2) | 5451 (74.7) | |
| Missing: 284 | 71 (3.3) | 213 (2.8) | |
| Insurance status, n (%) | |||
| Commercial/worker's comp | 440 (20.3) | 1442 (19.2) | |
| Medicare | 1017 (46.8) | 3589 (47.8) | 0.52 |
| Medicaid/others | 714 (32.9) | 2479 (33.0) | |
| Time of admission, n (%) | |||
| 0000‐0259 | 167 (7.7) | 1068 (14.2) | |
| 0300‐0559 | 244 (11.2) | 485 (6.5) | |
| 0600‐0859 | 456 (21.0) | 270 (3.6) | |
| 0900‐1159 | 782 (36.0) | 1146 (15.3) | <0.001 |
| 1200‐1459 | 299 (13.8) | 1750 (23.3) | |
| 1500‐1759 | 155 (7.1) | 1676 (22.3) | |
| 1800‐2359 | 68 (3.1) | 1115 (14.9) | |
| Time of discharge, n (%) | |||
| 2100‐0859 | 36 (1.7) | 174 (2.3) | |
| 0900‐1159 | 275 (12.7) | 495 (6.6) | |
| 1200‐1459 | 858 (39.6) | 2608 (34.8) | <0.001 |
| 1500‐1759 | 749 (34.6) | 3122 (41.6) | |
| 1800‐2059 | 249 (11.5) | 1104 (14.7) | |
| Missing | 4 | 7 | |
| Day of week of admission, n (%) | |||
| Monday | 462 (21.3) | 1549 (20.6) | |
| Tuesday | 499 (23.0) | 1470 (19.6) | |
| Wednesday | 430 (19.8) | 1479 (19.7) | 0.001 |
| Thursday | 400 (18.4) | 1482 (19.7) | |
| Friday | 380 (17.5) | 1530 (20.4) | |
| Day of week of discharge, n (%) | |||
| Monday | 207 (9.5) | 829 (11.0) | |
| Tuesday | 268 (12.3) | 973 (13.0) | |
| Wednesday | 334 (15.4) | 1142 (15.2) | |
| Thursday | 362 (16.7) | 1297 (17.3) | 0.16 |
| Friday | 485 (22.3) | 1523 (20.3) | |
| Saturday | 330 (15.2) | 1165 (15.5) | |
| Sunday | 185 (8.5) | 581 (7.7) | |
| Admit to non‐medicine wards, n (%) | 1332 (61.4) | 2624 (34.9) | <0.001 |
| Transfer to ICU (at least once), n (%) | 299 (13.8) | 504 (6.7) | <0.001 |
| Admit from ER No (%) | 1663 (76.6) | 6063 (80.7) | <0.001 |
| 10 most frequent diagnosis (%) | Pneumonia (4.9) | Pneumonia (5.5) | |
| Congestive heart failure; nonhypertensive (4.2) | Congestive heart failure; nonhypertensive (3.9) | ||
| Sickle cell anemia (3.9) | Nonspecific chest pain (3.7) | ||
| Chronic obstructive pulmonary disease and Bronchiectasis (3.3) | Urinary tract infections(3.6) | ||
| Diabetes mellitus with complications (3.2) | Skin and subcutaneous tissue infections (3.3) | ||
| Urinary tract infections (3.2) | Sickle cell anemia (3.3) | ||
| Asthma (3.0) | Pancreatic disorders (not diabetes) (2.8) | ||
| Nonspecific chest pain (3.0) | Asthma (2.8) | ||
| Pancreatic disorders (not diabetes) (2.9) | Chronic obstructive pulmonary disease and Bronchiectasis (2.6) | ||
| Septicemia (2.2) | Diabetes mellitus with complications (2.6) | ||
| Average number of comorbidities mean (95% CI) | 0.39 (0.37‐0.42) | 0.38 (0.36‐0.39) | 0.23 |
In unadjusted comparisons of outcomes (Table 3), hospitalizations on H‐PA teams had higher lengths of stay and charges than hospitalizations on RES teams, possibly higher inpatient mortality rates but similar unadjusted readmission rates at 7, 14, and 30 days
| H‐PA (n = 2171) | RES (n = 7150) | % Difference* (CI) | P Value | |
|---|---|---|---|---|
| ||||
| LOS | Median (IQR) | Median (IQR) | ||
| Days | 3.17 (2.03‐5.30) | 2.99 (1.80‐5.08) | +8.9% (4.71‐13.29%) | <0.001 |
| Charges | ||||
| US Dollars | 9390 (6196‐16,239) | 9044 (6106‐14,805) | +5.56% (1.96‐9.28%) | 0.002 |
| Readmissions | n (%) | n (%) | Odds Ratio (CI) | |
| Within 7 days | 147 (6.96) | 571 (7.78) | 0.88 (0.73‐1.06) | 0.19 |
| Within14 days | 236 (11.34) | 924 (12.76) | 0.87 (0.75‐1.01) | 0.07 |
| Within 30 days | 383 (18.91) | 1436 (20.31) | 0.91 (0.80‐1.03) | 0.14 |
| Inpatient deaths | 39 (1.8) | 95 (1.3) | 1.36 (0.90‐2.00) | 0.06 |
Multivariable Analysis
LOS
Hospitalizations to H‐PA teams were associated with a 6.73% longer LOS (P = 0.005) (Table 4). This difference persisted when we used the winsorized data (6.45% increase, P = 0.006), excluded inpatient deaths (6.81% increase, P = 0.005), or excluded hospitalizations that involved an ICU stay (6.40%increase, P = 0.011) (Table 5).
| Overall | Subgroup: Restricted to Physicians Attending on Both H‐PA and RES Teams* | Subgroup: Restricted to Hospitalizations Between 11.00 AM and 4.00 PM | ||||
|---|---|---|---|---|---|---|
| % Difference (CI) | P Value | % Difference (CI) | P Value | % Difference (CI) | P Value | |
| ||||||
| LOS | 6.73% (1.99% to 11.70%) | 0.005 | 5.44% (0.65% to 11.91%) | 0.08 | 2.97% (4.47% to 10.98%) | 0.44 |
| Charges | 2.75% (1.30% to 6.97%) | 0.19 | 1.55% (3.76% to 7.16%) | 0.57 | 6.45% (0.62% to 14.03%) | 0.07 |
| Risk of Readmission | Adjusted OR (95%CI) | P Value | Adjusted OR (95% CI) | P Value | Adjusted OR (95% CI) | P Value |
| Within 7 days | 0.88 (0.64‐1.20) | 0.42 | 0.74 (0.40‐1.35) | 0.32 | 0.90 (0.40‐2.00) | 0.78 |
| Within14 days | 0.90 (0.69‐1.19) | 0.46 | 0.71 (0.51‐0.99) | 0.05 | 0.87 (0.36‐2.13) | 0.77 |
| Within 30 days | 0.89 (0.75‐1.06) | 0.20 | 0.75 (0.51‐1.08) | 0.12 | 0.92 (0.55‐1.54) | 0.75 |
| Inpatient mortality | 1.27 (0.82‐1.97) | 0.28 | 1.46 (0.67‐3.17) | 0.33 | 1.14 (0.47‐2.74) | 0.77 |
| Analysis With Winsorized Data | Analysis After Excluding Inpatient Deaths | Analysis After Excluding Patients With ICU Stays | ||||
|---|---|---|---|---|---|---|
| % Difference (CI) | P Value | % Difference (CI) | P Value | % Difference (CI) | P Value | |
| ||||||
| LOS | 6.45% (4.04 to 8.91%) | 0.006 | 6.81% (2.03 to 11.80%) | 0.005 | 6.40% (1.46 to 11.58%) | 0.011 |
| Charges | 2.67 (1.27 to 6.76%) | 0.187 | 2.89% (1.16 to 7.11%) | 0.164 | 0.74% (3.11 to 4.76%) | 0.710 |
Charges
Hospitalizations to H‐PA and RES teams were associated with similar charges (Table 4). The results were similar when we used winsorized data, excluded inpatient deaths or excluded hospitalizations involving an ICU stay (Table 5).
Readmissions
The risk of readmission at 7, 14, and 30 days was similar between hospitalizations to H‐PA and RES teams (Table 4).
Mortality
The risk of inpatient death was similar between all hospitalizations to H‐PA and RES teams or only hospitalizations without an ICU stay (Table 4). The results also remained the same in analyses restricted to first admissions, last admissions, or 1 randomly selected admission per patient.
Sub‐Group Analysis
On restricting the multivariable analyses to the subset of hospitalists who staffed both types of teams (Table 4), the increase in LOS associated with H‐PA care was no longer significant (5.44% higher, P = 0.081). The charges, risk of readmission at 7 and 30 days, and risk of inpatient mortality remained similar. The risk of readmission at 14 days was slightly lower following hospitalizations to H‐PA teams (odds ratio 0.71, 95% confidence interval [CI] 0.51‐0.99).
The increase in LOS associated with H‐PA care was further attenuated in analyses of the subset of admissions between 11.00 AM and 4.00 PM (2.97% higher, P = 0.444). The difference in charges approached significance (6.45% higher, P = 0.07), but risk of readmission at 7, 14, and 30 days and risk of inpatient mortality were no different (Table 4).
Interactions
On adding interaction terms between the team assignment and the fixed effect variables in each model we detected that the effect of H‐PA care on LOS (P < 0.001) and charges (P < 0.001) varied by time of admission (Figure 2a and b). Hospitalizations to H‐PA teams from 6.00 PM to 6.00 AM had greater relative increases in LOS as compared to hospitalizations to RES teams during those times. Similarly, hospitalizations during the period 3.00 PM to 3.00 AM had relatively higher charges associated with H‐PA care compared to RES care.
Discussion
We found that hospitalizations to our H‐PA teams had longer LOS but similar charges, readmission rates, and mortality as compared to traditional resident‐based teams. These findings were robust to multiple sensitivity and subgroup analyses but when we examined times when both types of teams could receive admissions, the difference in LOS was markedly attenuated and nonsignificant.
We note that most prior reports comparing PA‐based models of inpatient care predate the ACGME work hour regulations. In a randomized control trial (1987‐1988) Simmer et al.5 showed lower lengths of stay and charges but possibly higher risk of readmission for PA based teams as compared to resident based teams. Van Rhee et al.7 conducted a nonrandomized retrospective cohort study (1994‐1995) using administrative data which showed lower resource utilization for PA‐based inpatient care. Our results from 2005 to 2006 reflect the important changes in the organization and delivery of inpatient care since these previous investigations.
Roy et al.8 report the only previously published comparison of PA and resident based GM inpatient care after the ACGME mandated work hour regulations. They found PA‐based care was associated with lower costs, whereas we found similar charges for admissions to RES and H‐PA teams. They also found that LOS was similar for PA and resident‐based care, while we found a higher LOS for admissions to our H‐PA team. We note that although the design of Roy's study was similar to our own, patients cared for by PA‐based teams were geographically localized in their model. This may contribute to the differences in results noted between our studies.
Despite no designed differences in patients assigned to either type of team other than time of admission we noted some differences between the H‐PA and RES teams in the descriptive analysis. These differences, such as a higher proportion of hospitalizations to H‐PA teams being admitted from the ER, residing on nonmedicine wards or having an ICU stay are likely a result of our system of assigning admissions to H‐PA teams early during the workday. For example patients on H‐PA teams were more often located on nonmedicine wards as a result of later discharges and bed availability on medicine wards. The difference that deserves special comment is the much higher proportion (13.8% vs. 6.7%) of hospitalizations with an ICU stay on the H‐PA teams. Hospitalizations directly to the ICU were excluded from our study which means that the hospitalizations with an ICU stay in our study were initially admitted to either H‐PA or RES teams and then transferred to the ICU. Transfers out of the ICU usually occur early in the workday when H‐PA teams accepted patients per our admission schedule. These patients may have been preferentially assigned to H‐PA teams, if on returning from the ICU the original team's resident had changed (and the bounce back rule was not in effect). Importantly, the conclusions of our research are not altered on controlling for this difference in the teams by excluding hospitalizations with an ICU stay.
Hospitalizations to H‐PA teams were associated with higher resource utilization if they occurred later in the day or overnight (Figure 2a and b). During these times a transition of care occurred shortly after admission. For a late day admission the H‐PA teams would transfer care for overnight cross cover soon after the admission and for patients admitted overnight as overflow they would assume care of a patient from the nighttime covering physician performing the admission. On the other hand, on RES teams, interns admitting patients overnight continued to care for their patients for part of the following day (30‐hour call). Similar findings of higher resource utilization associated with transfer of care after admission in the daytime11 and nighttime12 have been previously reported. An alternative hypothesis for our findings is that the hospital maybe busier and thus less efficient during times when H‐PA teams had to admit later in the day or accept patients admitted overnight as overflow. Future research to determine the cause of this significant interaction between team assignment and time of admission on resource utilization is important as the large increases in LOS (up to 30%) and charges (up to 50%) noted, could have a potentially large impact if a higher proportion of hospitalizations were affected by this phenomenon.
Our H‐PA teams were assigned equally complex patients as our RES teams, in contrast to previous reports.8, 13 This was accomplished while improving the resident's educational experience and we have previously reported increases in our resident's board pass rates and in‐service training exam scores with that introduction of our H‐PA teams.14 We thus believe that selection of less complex patients to H‐PA teams such as ours is unnecessary and may give them a second tier status in academic settings.
Our report has limitations. It is a retrospective, nonrandomized investigation using a single institution's administrative database and has the limitations of not being able to account for unmeasured confounders, severity of illness, errors in the database, selection bias and has limited generalizability. We measured charges not actual costs,15 but we feel charges are a true reflection of relative resource use when compared between similar patients within a single institution. We also did not account for the readmissions that occur to other hospitals16 and our results do not reflect resource utilization for the healthcare system in total. For example, we could not tell if higher LOS on H‐PA teams resulted in lower readmissions for their patients in all hospitals in the region, which may reveal an overall resource savings. Additionally, we measured in‐hospital mortality and could not capture deaths related to hospital care that may occur shortly after discharge.
ACGME has proposed revised standards that may further restrict resident duty hours when they take effect in July 2011.3 This may lead to further decreases in resident‐based inpatient care. Teaching hospitals will need to continue to develop alternate models for inpatient care that do not depend on house staff. Our findings provide important evidence to inform the development of such models. Our study shows that one such model: PAs paired with hospitalists, accepting admissions early in the workday, with hospitalist coverage over the weekend and nights can care for GM inpatients as complex as those cared for by resident‐based teams without increasing readmission rates, inpatient mortality, or charges but at the cost of slightly higher LOS.
- ACGME‐Common Program Requirements for Resident Duty Hours. Available at: http://www.acgme.org/acWebsite/dutyHours/dh_ComProgrRequirmentsDutyHours0707.pdf. Accessed July 2010.
- ,,,,.Non‐housestaff medicine services in academic centers: models and challenges.J Hosp Med.2008;3(3):247–255.
- ACGME. Duty Hours: Proposed Standards for Review and comment. Available at: http://acgme‐2010standards.org/pdf/Proposed_Standards. pdf. Accessed July 22,2010.
- Agency for Health Care Policy and Research. HCUPnet: A tool for identifying, tracking, and analyzing national hospital statistics. Available at: http://hcup.ahrq.gov/HCUPnet.asp. Accessed July2010.
- ,,,,.A randomized, controlled trial of an attending staff service in general internal medicine.Med Care.1991;29(7 suppl):JS31–JS40.
- ,.Replacing an academic internal medicine residency program with a physician assistant‐‐hospitalist model: a Comparative Analysis Study.Am J Med Qual.2009;24(2):132–139.
- ,,.Resource use by physician assistant services versus teaching services.JAAPA.2002;15(1):33–42.
- ,,, et al.Implementation of a physician assistant/hospitalist service in an academic medical center: impact on efficiency and patient outcomes.J Hosp Med.2008;3(5):361–368.
- AHRQ. Clinical Classifications Software (CCS) for ICD‐9‐CM. Available at: http://www.hcup‐us.ahrq.gov/toolssoftware/ccs/ccs.jsp#overview. Accessed July2010.
- AHRQ. HCUP: Comorbidity Software, Version 3.4.;Available at: http://www.hcup‐us.ahrq.gov/toolssoftware/comorbidity/comorbidity.jsp. Accessed July2010.
- ,,, et al.Effect of short call admission on length of stay and quality of care for acute decompensated heart failure.Circulation.2008;117(20):2637–2644.
- ,,,.Post‐call transfer of resident responsibility: its effect on patient care.J Gen Intern Med.1990;5(6):501–505.
- ,,,,,.The effect of nonteaching services on the distribution of inpatient cases for internal medicine residents.Acad Med.2009:84(2):220–225.
- ,,, et al.Allowing for better resident education and improving patient care: hospitalist‐physician assistant teams fill in the gaps.J Hosp Med.2007;2[S2]:1–39.
- .The distinction between cost and charges.Ann Intern Med.1982;96(1):102–109.
- ,,.Rehospitalizations among patients in the Medicare Fee‐for‐Service Program.N Engl J Med.2009;360(14):1418–1428.
In 2003 the Accreditation Council for Graduate Medical Education (ACGME) prescribed residency reform in the form of work hour restrictions without prescribing alternatives to resident based care.1 As a response, many academic medical centers have developed innovative models for providing inpatient care, some of which incorporate Physician Assistants (PAs).2 With further restrictions in resident work hours possible,3 teaching hospitals may increase use of these alternate models to provide inpatient care. Widespread implementation of such new and untested models could impact the care of the approximately 20 million hospitalizations that occur every year in US teaching hospitals.4
Few reports have compared the care delivered by these alternate models with the care provided by traditional resident‐based models of care.58 Roy et al.8 have provided the only recent comparison of a PA‐based model of care with a resident‐based model. They showed lower adjusted costs of inpatient care associated with PA based care but other outcomes were similar to resident‐based teams.
The objective of this study is to provide a valid and usable comparison of the outcomes of a hospitalist‐PA (H‐PA) model of inpatient care with the traditional resident‐based model. This will add to the quantity and quality of the limited research on PA‐based inpatient care, and informs the anticipated increase in the involvement of PAs in this arena.
Methods
Study Design and Setting
We conducted a retrospective cohort study at a 430‐bed urban academic medical center in the Midwestern United States.
Models of General Medical (GM) Inpatient Care at the Study Hospital During the Study Period
In November 2004, as a response to the ACGME‐mandated work hour regulations, we formed 2 Hospitalist‐PA teams (H‐PA) to supplement the 6 preexisting general medicine resident teams (RES).
The H‐PA and RES teams differed in staffing, admitting times and weekend/overnight cross coverage structure (Table 1). There were no predesigned differences between the teams in the ward location of their patients, availability of laboratory/radiology services, specialty consultation, social services/case management resources, nursing resources or documentation requirements for admission, daily care, and discharge.
| H‐PA Teams | RES Teams | |
|---|---|---|
| Attending physician | Always a hospitalist | Hospitalist, non‐hospitalist general internist or rarely a specialist |
| Attending physician role | Supervisory for some patients (about half) and sole care provider for others. | Supervisory for all patients |
| Team composition | One attending paired with 1 PA | Attending + senior resident + (2) interns + (2‐3) medical students |
| Rotation schedule | ||
| Attending | Every 2 weeks | Every 2 weeks |
| Physician assistant | Off on weekends | |
| House staff & medical students | Every month | |
| Weekend | No new admissions & hospitalist manages all patients | Accept new admissions |
| Admission times (weekdays) | 7 AM to 3 PM | Noon to 7 AM |
| Source of admissions | Emergency room, clinics, other hospitals | Emergency room, clinics, other hospitals |
| Number of admissions (weekdays) | 4‐6 patients per day per team | Noon to 5 PM: 2 teams admit a maximum of 9 patients total |
| 5 PM to 7 AM: 3 teams admit a maximum 5 patients each. | ||
| Overnight coverageroles and responsibilities | One in‐house faculty | 3 on call interns |
| Cross‐covering 2 H‐PA teams | Cross‐covering 2 teams each | |
| Performing triage | Admitting up to 5 patients each | |
| Admitting patients if necessary | ||
| Assisting residents if necessary | ||
| General medical consultation |
Admission Schedule for H‐PA or RES Teams
The admitting schedule was designed to decrease the workload of the house staff and to do so specifically during the periods of peak educational activity (morning report, attending‐led teaching rounds, and noon report). A faculty admitting medical officer (AMO) assigned patients strictly based on the time an admission was requested. Importantly, the request for admission preceded the time of actual admission recorded when the patient reached the ward. The time difference between request for admission and actual admission depended on the source of admission and the delay associated with assigning a patient room. The AMO assigned 8 to 12 new patients to the H‐PA teams every weekday between 7 AM and 3 PM and to the RES teams between noon and 7 AM the next day. There was a designed period of overlap from noon to 3 PM during which both H‐PA and RES teams could admit patients. This period allowed for flexibility in assigning patients to either type of team depending on their workload. The AMO did not use patient complexity or teaching value to assign patients.
Exceptions to Admission Schedule
Patients admitted overnight after the on call RES had reached their admission limits were assigned to H‐PA teams the next morning. In addition, recently discharged patients who were readmitted while the discharging hospitalist (H‐PA teams) or the discharging resident (RES teams) was still scheduled for inpatient duties, were assigned back to the discharging team irrespective of the admitting schedule.
The same medicine team cared for a patient from admission to discharge but on transfer to the intensive care unit (ICU), an intensivist led critical care team assumed care. On transfer out of the ICU these patients were assigned back to the original team irrespective of admitting schedulethe so called bounce back rule to promote inpatient continuity of care. But if the residents (RES teams) or the hospitalist (H‐PA teams) had changedthe bounce back rule was no longer in effect and these patients were assigned to a team according to the admission schedule.
Study Population and Study Period
We included all hospitalizations of adult patients to GM teams if both their date of admission and their date of discharge fell within the study period (January 1, 2005 to December 31, 2006). We excluded hospitalizations with admissions during the weekendwhen H‐PA teams did not admit patients; hospitalizations to GM services with transfer to nonGM service (excluding ICU) and hospitalizations involving comanagement with specialty servicesas the contribution of GM teams for these was variable; and hospitalizations of private patients.
Data Collection and Team Assignment
We collected patient data from our hospital's discharge abstract database. This database did not contain team information so to assign teams we matched the discharging attending and the day of discharge to the type of team that the discharging attending was leading that day.
We collected patient age, gender, race, insurance status, zip‐code, primary care provider, source of admission, ward type, time and day of admission, and time and day of discharge for use as independent variables. The time of admission captured in the database was the time of actual admission and not the time the admission was requested.
We grouped the principal diagnosis International Statistical Classification of Diseases and Related Health Problems, 9th edition (ICD‐9) codes into clinically relevant categories using the Clinical Classification Software.9 We created comorbidity measures using Healthcare Cost and Utilization Project Comorbidity Software, version 3.4.10
Outcome Measures
We used length of stay (LOS), charges, readmissions within 7, 14, and 30 days and inpatient mortality as our outcome measures. We calculated LOS by subtracting the discharge day and time from the admission day and time. The LOS included time spent in the ICU. We summed all charges accrued during the entire hospitalization including any stay in the ICU but did not include professional fees. We considered any repeat hospitalization to our hospital within 7, 14, and 30 days following a discharge to be a readmission except that we excluded readmissions for a planned procedure or for inpatient rehabilitation.
Statistical Analysis
Descriptive Analysis
We performed unadjusted descriptive statistics at the level of an individual hospitalization using medians and interquartile ranges for continuous data and frequencies and percentages for categorical data. We used chi‐square tests of association and KruskalWallis analysis of variance to compare H‐PA and RES teams.
Missing Data
Because we lacked data on whether a primary outpatient care provider was available for 284 (2.9%) of our study hospitalizations, we dropped them from our multivariable analyses. We used an arbitrary discharge time of noon for the 11 hospitalizations which did not have a discharge time recorded.
Multivariable Analysis
We used multivariable mixed models to risk adjust for a wide variety of variables. We included age, gender, race, insurance, presence of primary care physician, and total number of comorbidities as fixed effects in all models because of the high face validity of these variables. We then added admission source, ward, time, day of week, discharge day of week, and comorbidity measures one by one as fixed effects, including them only if significant at P < 0.01. For assessing LOS, charges, and readmissions, we added a variable identifying each patient as a random effect to account for multiple admissions for the same patient. We then added variables identifying attending physician, principal diagnostic group, and ZIP code of residence as random effects to account for clustering of hospitalizations within these categories, including them only if significant at P < 0.01. For the model assessing mortality we included variables for attending physician, principal diagnostic group, and ZIP code of residence as random effects if significant at P < 0.01. We log transformed LOS and charges because they were extremely skewed in nature. Readmissions were analyzed after excluding patients who died or were discharged alive within 7, 14, or 30 days of the end of the study period.
Sensitivity Analyses
To assess the influence of LOS outliers, we changed LOS to 6 hours if it was less than 6 hours, and 45 days if it was more than 45 daysa process called winsorizing. We consider winsorizing superior to dropping outliers because it acknowledges that outliers contribute information, but prevent them from being too influential. We chose the 6 hour cut off because we believed that was the minimum time required to admit and then discharge a patient. We chose the upper limit of 45 days on reviewing the frequency distribution for outliers. Similarly, we winsorized charges at the first and 99th percentile after reviewing the frequency distribution for outliers. We then log transformed the winsorized data before analysis.
Inpatient deaths reduce the LOS and charges associated with a hospitalization. Thus excess mortality may provide a false concession in terms of lower LOS or charges. To check if this occurred in our study we repeated the analyses after excluding inpatient deaths.
ICU stays are associated with higher LOS, charges, and mortality. In our model of care, some patients transferred to the ICU are not cared for by the original team on transfer out. Moreover, care in the ICU is not controlled by the team that discharges them. Since this might obscure differences in outcomes achieved by RES vs. H‐PA teams, we repeated these analyses after excluding hospitalizations with an ICU stay.
Since mortality can only occur during 1 hospitalization per patient, we repeated the mortality analysis using only each patient's first admission or last admission and using a randomly selected single admission for each patient.
Subgroup Analysis
To limit the effect of different physician characteristics on H‐PA and RES teams we separately analyzed the hospitalizations under the care of hospitalists who served on both H‐PA and RES teams.
To limit the effect of different admission schedules of H‐PA and RES teams we analyzed the hospitalizations with admission times between 11.00 AM and 4.00 PM. Such hospitalizations were likely to be assigned during the noon to 3 PM period when they could be assigned to either an H‐PA or RES team.
Interactions
Finally we explored interactions between the type of team and the fixed effect variables included in each model.
Statistical Software
We performed the statistical analysis using SAS software version 9.0 for UNIX (SAS Institute, Inc., Cary, NC) and R software (The R Project for Statistical Computing).
This study protocol was approved by the hospital's institutional review board.
Results
Study Population
Of the 52,391 hospitalizations to our hospital during the study period, 13,058 were admitted to general medicine. We excluded 3102 weekend admissions and 209 who met other exclusion criteria. We could not determine the team assignment for 66. Of the remaining 9681 hospitalizations, we assigned 2171 to H‐PA teams and 7510 to RES teams (Figure 1).

Descriptive Analysis
We compare patients assigned to H‐PA and RES teams in Table 2. They were similar in age, gender, race, having a primary care provider or not, and insurance status. Clinically, they had similar comorbidities and a similar distribution of common principal diagnoses. Consistent with their admitting schedule, H‐PA teams admitted and discharged more patients earlier in the day and admitted more patients earlier in the work week. Patients cared for by H‐PA teams were admitted from the Emergency Room (ER) less often and were more likely to reside on wards designated as nonmedicine by nursing specialty. Hospitalizations to H‐PA teams more often included an ICU stay.
| H‐PA (n = 2171) | RES (n = 7510) | P Value | |
|---|---|---|---|
| |||
| Age | |||
| Mean | 56.80 | 57.04 | |
| Median | 56 | 56 | 0.15 |
| Interquartile range | 43‐72 | 43‐73 | |
| Age group (years), n (%) | |||
| < 20 | 10 (0.5) | 57 (0.8) | |
| 20‐29 | 186 (8.6) | 632 (8.7) | |
| 30‐39 | 221 (10.2) | 766 (10.3) | |
| 40‐49 | 387 (17.8) | 1341 (18.1) | |
| 50‐59 | 434 (20.0) | 1492 (20.2) | 0.28 |
| 60‐69 | 325 (15.0) | 974 (12.8) | |
| 70‐79 | 271 (12.5) | 1035 (13.6) | |
| 80‐89 | 262 (12.0) | 951(12.3) | |
| 90< | 75 (3.5) | 262 (3.4) | |
| Female, n (%) | 1175 (54.1) | 4138 (55.1) | 0.42 |
| Race, n (%) | |||
| White | 1282 (59.1) | 4419 (58.9) | |
| Black | 793 (36.5) | 2754 (36.7) | 0.98 |
| Other | 96 (4.4) | 337 (4.5) | |
| Primary care provider, n (%) | 0.16 | ||
| Yes | 1537 (73.2) | 5451 (74.7) | |
| Missing: 284 | 71 (3.3) | 213 (2.8) | |
| Insurance status, n (%) | |||
| Commercial/worker's comp | 440 (20.3) | 1442 (19.2) | |
| Medicare | 1017 (46.8) | 3589 (47.8) | 0.52 |
| Medicaid/others | 714 (32.9) | 2479 (33.0) | |
| Time of admission, n (%) | |||
| 0000‐0259 | 167 (7.7) | 1068 (14.2) | |
| 0300‐0559 | 244 (11.2) | 485 (6.5) | |
| 0600‐0859 | 456 (21.0) | 270 (3.6) | |
| 0900‐1159 | 782 (36.0) | 1146 (15.3) | <0.001 |
| 1200‐1459 | 299 (13.8) | 1750 (23.3) | |
| 1500‐1759 | 155 (7.1) | 1676 (22.3) | |
| 1800‐2359 | 68 (3.1) | 1115 (14.9) | |
| Time of discharge, n (%) | |||
| 2100‐0859 | 36 (1.7) | 174 (2.3) | |
| 0900‐1159 | 275 (12.7) | 495 (6.6) | |
| 1200‐1459 | 858 (39.6) | 2608 (34.8) | <0.001 |
| 1500‐1759 | 749 (34.6) | 3122 (41.6) | |
| 1800‐2059 | 249 (11.5) | 1104 (14.7) | |
| Missing | 4 | 7 | |
| Day of week of admission, n (%) | |||
| Monday | 462 (21.3) | 1549 (20.6) | |
| Tuesday | 499 (23.0) | 1470 (19.6) | |
| Wednesday | 430 (19.8) | 1479 (19.7) | 0.001 |
| Thursday | 400 (18.4) | 1482 (19.7) | |
| Friday | 380 (17.5) | 1530 (20.4) | |
| Day of week of discharge, n (%) | |||
| Monday | 207 (9.5) | 829 (11.0) | |
| Tuesday | 268 (12.3) | 973 (13.0) | |
| Wednesday | 334 (15.4) | 1142 (15.2) | |
| Thursday | 362 (16.7) | 1297 (17.3) | 0.16 |
| Friday | 485 (22.3) | 1523 (20.3) | |
| Saturday | 330 (15.2) | 1165 (15.5) | |
| Sunday | 185 (8.5) | 581 (7.7) | |
| Admit to non‐medicine wards, n (%) | 1332 (61.4) | 2624 (34.9) | <0.001 |
| Transfer to ICU (at least once), n (%) | 299 (13.8) | 504 (6.7) | <0.001 |
| Admit from ER No (%) | 1663 (76.6) | 6063 (80.7) | <0.001 |
| 10 most frequent diagnosis (%) | Pneumonia (4.9) | Pneumonia (5.5) | |
| Congestive heart failure; nonhypertensive (4.2) | Congestive heart failure; nonhypertensive (3.9) | ||
| Sickle cell anemia (3.9) | Nonspecific chest pain (3.7) | ||
| Chronic obstructive pulmonary disease and Bronchiectasis (3.3) | Urinary tract infections(3.6) | ||
| Diabetes mellitus with complications (3.2) | Skin and subcutaneous tissue infections (3.3) | ||
| Urinary tract infections (3.2) | Sickle cell anemia (3.3) | ||
| Asthma (3.0) | Pancreatic disorders (not diabetes) (2.8) | ||
| Nonspecific chest pain (3.0) | Asthma (2.8) | ||
| Pancreatic disorders (not diabetes) (2.9) | Chronic obstructive pulmonary disease and Bronchiectasis (2.6) | ||
| Septicemia (2.2) | Diabetes mellitus with complications (2.6) | ||
| Average number of comorbidities mean (95% CI) | 0.39 (0.37‐0.42) | 0.38 (0.36‐0.39) | 0.23 |
In unadjusted comparisons of outcomes (Table 3), hospitalizations on H‐PA teams had higher lengths of stay and charges than hospitalizations on RES teams, possibly higher inpatient mortality rates but similar unadjusted readmission rates at 7, 14, and 30 days
| H‐PA (n = 2171) | RES (n = 7150) | % Difference* (CI) | P Value | |
|---|---|---|---|---|
| ||||
| LOS | Median (IQR) | Median (IQR) | ||
| Days | 3.17 (2.03‐5.30) | 2.99 (1.80‐5.08) | +8.9% (4.71‐13.29%) | <0.001 |
| Charges | ||||
| US Dollars | 9390 (6196‐16,239) | 9044 (6106‐14,805) | +5.56% (1.96‐9.28%) | 0.002 |
| Readmissions | n (%) | n (%) | Odds Ratio (CI) | |
| Within 7 days | 147 (6.96) | 571 (7.78) | 0.88 (0.73‐1.06) | 0.19 |
| Within14 days | 236 (11.34) | 924 (12.76) | 0.87 (0.75‐1.01) | 0.07 |
| Within 30 days | 383 (18.91) | 1436 (20.31) | 0.91 (0.80‐1.03) | 0.14 |
| Inpatient deaths | 39 (1.8) | 95 (1.3) | 1.36 (0.90‐2.00) | 0.06 |
Multivariable Analysis
LOS
Hospitalizations to H‐PA teams were associated with a 6.73% longer LOS (P = 0.005) (Table 4). This difference persisted when we used the winsorized data (6.45% increase, P = 0.006), excluded inpatient deaths (6.81% increase, P = 0.005), or excluded hospitalizations that involved an ICU stay (6.40%increase, P = 0.011) (Table 5).
| Overall | Subgroup: Restricted to Physicians Attending on Both H‐PA and RES Teams* | Subgroup: Restricted to Hospitalizations Between 11.00 AM and 4.00 PM | ||||
|---|---|---|---|---|---|---|
| % Difference (CI) | P Value | % Difference (CI) | P Value | % Difference (CI) | P Value | |
| ||||||
| LOS | 6.73% (1.99% to 11.70%) | 0.005 | 5.44% (0.65% to 11.91%) | 0.08 | 2.97% (4.47% to 10.98%) | 0.44 |
| Charges | 2.75% (1.30% to 6.97%) | 0.19 | 1.55% (3.76% to 7.16%) | 0.57 | 6.45% (0.62% to 14.03%) | 0.07 |
| Risk of Readmission | Adjusted OR (95%CI) | P Value | Adjusted OR (95% CI) | P Value | Adjusted OR (95% CI) | P Value |
| Within 7 days | 0.88 (0.64‐1.20) | 0.42 | 0.74 (0.40‐1.35) | 0.32 | 0.90 (0.40‐2.00) | 0.78 |
| Within14 days | 0.90 (0.69‐1.19) | 0.46 | 0.71 (0.51‐0.99) | 0.05 | 0.87 (0.36‐2.13) | 0.77 |
| Within 30 days | 0.89 (0.75‐1.06) | 0.20 | 0.75 (0.51‐1.08) | 0.12 | 0.92 (0.55‐1.54) | 0.75 |
| Inpatient mortality | 1.27 (0.82‐1.97) | 0.28 | 1.46 (0.67‐3.17) | 0.33 | 1.14 (0.47‐2.74) | 0.77 |
| Analysis With Winsorized Data | Analysis After Excluding Inpatient Deaths | Analysis After Excluding Patients With ICU Stays | ||||
|---|---|---|---|---|---|---|
| % Difference (CI) | P Value | % Difference (CI) | P Value | % Difference (CI) | P Value | |
| ||||||
| LOS | 6.45% (4.04 to 8.91%) | 0.006 | 6.81% (2.03 to 11.80%) | 0.005 | 6.40% (1.46 to 11.58%) | 0.011 |
| Charges | 2.67 (1.27 to 6.76%) | 0.187 | 2.89% (1.16 to 7.11%) | 0.164 | 0.74% (3.11 to 4.76%) | 0.710 |
Charges
Hospitalizations to H‐PA and RES teams were associated with similar charges (Table 4). The results were similar when we used winsorized data, excluded inpatient deaths or excluded hospitalizations involving an ICU stay (Table 5).
Readmissions
The risk of readmission at 7, 14, and 30 days was similar between hospitalizations to H‐PA and RES teams (Table 4).
Mortality
The risk of inpatient death was similar between all hospitalizations to H‐PA and RES teams or only hospitalizations without an ICU stay (Table 4). The results also remained the same in analyses restricted to first admissions, last admissions, or 1 randomly selected admission per patient.
Sub‐Group Analysis
On restricting the multivariable analyses to the subset of hospitalists who staffed both types of teams (Table 4), the increase in LOS associated with H‐PA care was no longer significant (5.44% higher, P = 0.081). The charges, risk of readmission at 7 and 30 days, and risk of inpatient mortality remained similar. The risk of readmission at 14 days was slightly lower following hospitalizations to H‐PA teams (odds ratio 0.71, 95% confidence interval [CI] 0.51‐0.99).
The increase in LOS associated with H‐PA care was further attenuated in analyses of the subset of admissions between 11.00 AM and 4.00 PM (2.97% higher, P = 0.444). The difference in charges approached significance (6.45% higher, P = 0.07), but risk of readmission at 7, 14, and 30 days and risk of inpatient mortality were no different (Table 4).
Interactions
On adding interaction terms between the team assignment and the fixed effect variables in each model we detected that the effect of H‐PA care on LOS (P < 0.001) and charges (P < 0.001) varied by time of admission (Figure 2a and b). Hospitalizations to H‐PA teams from 6.00 PM to 6.00 AM had greater relative increases in LOS as compared to hospitalizations to RES teams during those times. Similarly, hospitalizations during the period 3.00 PM to 3.00 AM had relatively higher charges associated with H‐PA care compared to RES care.

Discussion
We found that hospitalizations to our H‐PA teams had longer LOS but similar charges, readmission rates, and mortality as compared to traditional resident‐based teams. These findings were robust to multiple sensitivity and subgroup analyses but when we examined times when both types of teams could receive admissions, the difference in LOS was markedly attenuated and nonsignificant.
We note that most prior reports comparing PA‐based models of inpatient care predate the ACGME work hour regulations. In a randomized control trial (1987‐1988) Simmer et al.5 showed lower lengths of stay and charges but possibly higher risk of readmission for PA based teams as compared to resident based teams. Van Rhee et al.7 conducted a nonrandomized retrospective cohort study (1994‐1995) using administrative data which showed lower resource utilization for PA‐based inpatient care. Our results from 2005 to 2006 reflect the important changes in the organization and delivery of inpatient care since these previous investigations.
Roy et al.8 report the only previously published comparison of PA and resident based GM inpatient care after the ACGME mandated work hour regulations. They found PA‐based care was associated with lower costs, whereas we found similar charges for admissions to RES and H‐PA teams. They also found that LOS was similar for PA and resident‐based care, while we found a higher LOS for admissions to our H‐PA team. We note that although the design of Roy's study was similar to our own, patients cared for by PA‐based teams were geographically localized in their model. This may contribute to the differences in results noted between our studies.
Despite no designed differences in patients assigned to either type of team other than time of admission we noted some differences between the H‐PA and RES teams in the descriptive analysis. These differences, such as a higher proportion of hospitalizations to H‐PA teams being admitted from the ER, residing on nonmedicine wards or having an ICU stay are likely a result of our system of assigning admissions to H‐PA teams early during the workday. For example patients on H‐PA teams were more often located on nonmedicine wards as a result of later discharges and bed availability on medicine wards. The difference that deserves special comment is the much higher proportion (13.8% vs. 6.7%) of hospitalizations with an ICU stay on the H‐PA teams. Hospitalizations directly to the ICU were excluded from our study which means that the hospitalizations with an ICU stay in our study were initially admitted to either H‐PA or RES teams and then transferred to the ICU. Transfers out of the ICU usually occur early in the workday when H‐PA teams accepted patients per our admission schedule. These patients may have been preferentially assigned to H‐PA teams, if on returning from the ICU the original team's resident had changed (and the bounce back rule was not in effect). Importantly, the conclusions of our research are not altered on controlling for this difference in the teams by excluding hospitalizations with an ICU stay.
Hospitalizations to H‐PA teams were associated with higher resource utilization if they occurred later in the day or overnight (Figure 2a and b). During these times a transition of care occurred shortly after admission. For a late day admission the H‐PA teams would transfer care for overnight cross cover soon after the admission and for patients admitted overnight as overflow they would assume care of a patient from the nighttime covering physician performing the admission. On the other hand, on RES teams, interns admitting patients overnight continued to care for their patients for part of the following day (30‐hour call). Similar findings of higher resource utilization associated with transfer of care after admission in the daytime11 and nighttime12 have been previously reported. An alternative hypothesis for our findings is that the hospital maybe busier and thus less efficient during times when H‐PA teams had to admit later in the day or accept patients admitted overnight as overflow. Future research to determine the cause of this significant interaction between team assignment and time of admission on resource utilization is important as the large increases in LOS (up to 30%) and charges (up to 50%) noted, could have a potentially large impact if a higher proportion of hospitalizations were affected by this phenomenon.
Our H‐PA teams were assigned equally complex patients as our RES teams, in contrast to previous reports.8, 13 This was accomplished while improving the resident's educational experience and we have previously reported increases in our resident's board pass rates and in‐service training exam scores with that introduction of our H‐PA teams.14 We thus believe that selection of less complex patients to H‐PA teams such as ours is unnecessary and may give them a second tier status in academic settings.
Our report has limitations. It is a retrospective, nonrandomized investigation using a single institution's administrative database and has the limitations of not being able to account for unmeasured confounders, severity of illness, errors in the database, selection bias and has limited generalizability. We measured charges not actual costs,15 but we feel charges are a true reflection of relative resource use when compared between similar patients within a single institution. We also did not account for the readmissions that occur to other hospitals16 and our results do not reflect resource utilization for the healthcare system in total. For example, we could not tell if higher LOS on H‐PA teams resulted in lower readmissions for their patients in all hospitals in the region, which may reveal an overall resource savings. Additionally, we measured in‐hospital mortality and could not capture deaths related to hospital care that may occur shortly after discharge.
ACGME has proposed revised standards that may further restrict resident duty hours when they take effect in July 2011.3 This may lead to further decreases in resident‐based inpatient care. Teaching hospitals will need to continue to develop alternate models for inpatient care that do not depend on house staff. Our findings provide important evidence to inform the development of such models. Our study shows that one such model: PAs paired with hospitalists, accepting admissions early in the workday, with hospitalist coverage over the weekend and nights can care for GM inpatients as complex as those cared for by resident‐based teams without increasing readmission rates, inpatient mortality, or charges but at the cost of slightly higher LOS.
In 2003 the Accreditation Council for Graduate Medical Education (ACGME) prescribed residency reform in the form of work hour restrictions without prescribing alternatives to resident based care.1 As a response, many academic medical centers have developed innovative models for providing inpatient care, some of which incorporate Physician Assistants (PAs).2 With further restrictions in resident work hours possible,3 teaching hospitals may increase use of these alternate models to provide inpatient care. Widespread implementation of such new and untested models could impact the care of the approximately 20 million hospitalizations that occur every year in US teaching hospitals.4
Few reports have compared the care delivered by these alternate models with the care provided by traditional resident‐based models of care.58 Roy et al.8 have provided the only recent comparison of a PA‐based model of care with a resident‐based model. They showed lower adjusted costs of inpatient care associated with PA based care but other outcomes were similar to resident‐based teams.
The objective of this study is to provide a valid and usable comparison of the outcomes of a hospitalist‐PA (H‐PA) model of inpatient care with the traditional resident‐based model. This will add to the quantity and quality of the limited research on PA‐based inpatient care, and informs the anticipated increase in the involvement of PAs in this arena.
Methods
Study Design and Setting
We conducted a retrospective cohort study at a 430‐bed urban academic medical center in the Midwestern United States.
Models of General Medical (GM) Inpatient Care at the Study Hospital During the Study Period
In November 2004, as a response to the ACGME‐mandated work hour regulations, we formed 2 Hospitalist‐PA teams (H‐PA) to supplement the 6 preexisting general medicine resident teams (RES).
The H‐PA and RES teams differed in staffing, admitting times and weekend/overnight cross coverage structure (Table 1). There were no predesigned differences between the teams in the ward location of their patients, availability of laboratory/radiology services, specialty consultation, social services/case management resources, nursing resources or documentation requirements for admission, daily care, and discharge.
| H‐PA Teams | RES Teams | |
|---|---|---|
| Attending physician | Always a hospitalist | Hospitalist, non‐hospitalist general internist or rarely a specialist |
| Attending physician role | Supervisory for some patients (about half) and sole care provider for others. | Supervisory for all patients |
| Team composition | One attending paired with 1 PA | Attending + senior resident + (2) interns + (2‐3) medical students |
| Rotation schedule | ||
| Attending | Every 2 weeks | Every 2 weeks |
| Physician assistant | Off on weekends | |
| House staff & medical students | Every month | |
| Weekend | No new admissions & hospitalist manages all patients | Accept new admissions |
| Admission times (weekdays) | 7 AM to 3 PM | Noon to 7 AM |
| Source of admissions | Emergency room, clinics, other hospitals | Emergency room, clinics, other hospitals |
| Number of admissions (weekdays) | 4‐6 patients per day per team | Noon to 5 PM: 2 teams admit a maximum of 9 patients total |
| 5 PM to 7 AM: 3 teams admit a maximum 5 patients each. | ||
| Overnight coverageroles and responsibilities | One in‐house faculty | 3 on call interns |
| Cross‐covering 2 H‐PA teams | Cross‐covering 2 teams each | |
| Performing triage | Admitting up to 5 patients each | |
| Admitting patients if necessary | ||
| Assisting residents if necessary | ||
| General medical consultation |
Admission Schedule for H‐PA or RES Teams
The admitting schedule was designed to decrease the workload of the house staff and to do so specifically during the periods of peak educational activity (morning report, attending‐led teaching rounds, and noon report). A faculty admitting medical officer (AMO) assigned patients strictly based on the time an admission was requested. Importantly, the request for admission preceded the time of actual admission recorded when the patient reached the ward. The time difference between request for admission and actual admission depended on the source of admission and the delay associated with assigning a patient room. The AMO assigned 8 to 12 new patients to the H‐PA teams every weekday between 7 AM and 3 PM and to the RES teams between noon and 7 AM the next day. There was a designed period of overlap from noon to 3 PM during which both H‐PA and RES teams could admit patients. This period allowed for flexibility in assigning patients to either type of team depending on their workload. The AMO did not use patient complexity or teaching value to assign patients.
Exceptions to Admission Schedule
Patients admitted overnight after the on call RES had reached their admission limits were assigned to H‐PA teams the next morning. In addition, recently discharged patients who were readmitted while the discharging hospitalist (H‐PA teams) or the discharging resident (RES teams) was still scheduled for inpatient duties, were assigned back to the discharging team irrespective of the admitting schedule.
The same medicine team cared for a patient from admission to discharge but on transfer to the intensive care unit (ICU), an intensivist led critical care team assumed care. On transfer out of the ICU these patients were assigned back to the original team irrespective of admitting schedulethe so called bounce back rule to promote inpatient continuity of care. But if the residents (RES teams) or the hospitalist (H‐PA teams) had changedthe bounce back rule was no longer in effect and these patients were assigned to a team according to the admission schedule.
Study Population and Study Period
We included all hospitalizations of adult patients to GM teams if both their date of admission and their date of discharge fell within the study period (January 1, 2005 to December 31, 2006). We excluded hospitalizations with admissions during the weekendwhen H‐PA teams did not admit patients; hospitalizations to GM services with transfer to nonGM service (excluding ICU) and hospitalizations involving comanagement with specialty servicesas the contribution of GM teams for these was variable; and hospitalizations of private patients.
Data Collection and Team Assignment
We collected patient data from our hospital's discharge abstract database. This database did not contain team information so to assign teams we matched the discharging attending and the day of discharge to the type of team that the discharging attending was leading that day.
We collected patient age, gender, race, insurance status, zip‐code, primary care provider, source of admission, ward type, time and day of admission, and time and day of discharge for use as independent variables. The time of admission captured in the database was the time of actual admission and not the time the admission was requested.
We grouped the principal diagnosis International Statistical Classification of Diseases and Related Health Problems, 9th edition (ICD‐9) codes into clinically relevant categories using the Clinical Classification Software.9 We created comorbidity measures using Healthcare Cost and Utilization Project Comorbidity Software, version 3.4.10
Outcome Measures
We used length of stay (LOS), charges, readmissions within 7, 14, and 30 days and inpatient mortality as our outcome measures. We calculated LOS by subtracting the discharge day and time from the admission day and time. The LOS included time spent in the ICU. We summed all charges accrued during the entire hospitalization including any stay in the ICU but did not include professional fees. We considered any repeat hospitalization to our hospital within 7, 14, and 30 days following a discharge to be a readmission except that we excluded readmissions for a planned procedure or for inpatient rehabilitation.
Statistical Analysis
Descriptive Analysis
We performed unadjusted descriptive statistics at the level of an individual hospitalization using medians and interquartile ranges for continuous data and frequencies and percentages for categorical data. We used chi‐square tests of association and KruskalWallis analysis of variance to compare H‐PA and RES teams.
Missing Data
Because we lacked data on whether a primary outpatient care provider was available for 284 (2.9%) of our study hospitalizations, we dropped them from our multivariable analyses. We used an arbitrary discharge time of noon for the 11 hospitalizations which did not have a discharge time recorded.
Multivariable Analysis
We used multivariable mixed models to risk adjust for a wide variety of variables. We included age, gender, race, insurance, presence of primary care physician, and total number of comorbidities as fixed effects in all models because of the high face validity of these variables. We then added admission source, ward, time, day of week, discharge day of week, and comorbidity measures one by one as fixed effects, including them only if significant at P < 0.01. For assessing LOS, charges, and readmissions, we added a variable identifying each patient as a random effect to account for multiple admissions for the same patient. We then added variables identifying attending physician, principal diagnostic group, and ZIP code of residence as random effects to account for clustering of hospitalizations within these categories, including them only if significant at P < 0.01. For the model assessing mortality we included variables for attending physician, principal diagnostic group, and ZIP code of residence as random effects if significant at P < 0.01. We log transformed LOS and charges because they were extremely skewed in nature. Readmissions were analyzed after excluding patients who died or were discharged alive within 7, 14, or 30 days of the end of the study period.
Sensitivity Analyses
To assess the influence of LOS outliers, we changed LOS to 6 hours if it was less than 6 hours, and 45 days if it was more than 45 daysa process called winsorizing. We consider winsorizing superior to dropping outliers because it acknowledges that outliers contribute information, but prevent them from being too influential. We chose the 6 hour cut off because we believed that was the minimum time required to admit and then discharge a patient. We chose the upper limit of 45 days on reviewing the frequency distribution for outliers. Similarly, we winsorized charges at the first and 99th percentile after reviewing the frequency distribution for outliers. We then log transformed the winsorized data before analysis.
Inpatient deaths reduce the LOS and charges associated with a hospitalization. Thus excess mortality may provide a false concession in terms of lower LOS or charges. To check if this occurred in our study we repeated the analyses after excluding inpatient deaths.
ICU stays are associated with higher LOS, charges, and mortality. In our model of care, some patients transferred to the ICU are not cared for by the original team on transfer out. Moreover, care in the ICU is not controlled by the team that discharges them. Since this might obscure differences in outcomes achieved by RES vs. H‐PA teams, we repeated these analyses after excluding hospitalizations with an ICU stay.
Since mortality can only occur during 1 hospitalization per patient, we repeated the mortality analysis using only each patient's first admission or last admission and using a randomly selected single admission for each patient.
Subgroup Analysis
To limit the effect of different physician characteristics on H‐PA and RES teams we separately analyzed the hospitalizations under the care of hospitalists who served on both H‐PA and RES teams.
To limit the effect of different admission schedules of H‐PA and RES teams we analyzed the hospitalizations with admission times between 11.00 AM and 4.00 PM. Such hospitalizations were likely to be assigned during the noon to 3 PM period when they could be assigned to either an H‐PA or RES team.
Interactions
Finally we explored interactions between the type of team and the fixed effect variables included in each model.
Statistical Software
We performed the statistical analysis using SAS software version 9.0 for UNIX (SAS Institute, Inc., Cary, NC) and R software (The R Project for Statistical Computing).
This study protocol was approved by the hospital's institutional review board.
Results
Study Population
Of the 52,391 hospitalizations to our hospital during the study period, 13,058 were admitted to general medicine. We excluded 3102 weekend admissions and 209 who met other exclusion criteria. We could not determine the team assignment for 66. Of the remaining 9681 hospitalizations, we assigned 2171 to H‐PA teams and 7510 to RES teams (Figure 1).

Descriptive Analysis
We compare patients assigned to H‐PA and RES teams in Table 2. They were similar in age, gender, race, having a primary care provider or not, and insurance status. Clinically, they had similar comorbidities and a similar distribution of common principal diagnoses. Consistent with their admitting schedule, H‐PA teams admitted and discharged more patients earlier in the day and admitted more patients earlier in the work week. Patients cared for by H‐PA teams were admitted from the Emergency Room (ER) less often and were more likely to reside on wards designated as nonmedicine by nursing specialty. Hospitalizations to H‐PA teams more often included an ICU stay.
| H‐PA (n = 2171) | RES (n = 7510) | P Value | |
|---|---|---|---|
| |||
| Age | |||
| Mean | 56.80 | 57.04 | |
| Median | 56 | 56 | 0.15 |
| Interquartile range | 43‐72 | 43‐73 | |
| Age group (years), n (%) | |||
| < 20 | 10 (0.5) | 57 (0.8) | |
| 20‐29 | 186 (8.6) | 632 (8.7) | |
| 30‐39 | 221 (10.2) | 766 (10.3) | |
| 40‐49 | 387 (17.8) | 1341 (18.1) | |
| 50‐59 | 434 (20.0) | 1492 (20.2) | 0.28 |
| 60‐69 | 325 (15.0) | 974 (12.8) | |
| 70‐79 | 271 (12.5) | 1035 (13.6) | |
| 80‐89 | 262 (12.0) | 951(12.3) | |
| 90< | 75 (3.5) | 262 (3.4) | |
| Female, n (%) | 1175 (54.1) | 4138 (55.1) | 0.42 |
| Race, n (%) | |||
| White | 1282 (59.1) | 4419 (58.9) | |
| Black | 793 (36.5) | 2754 (36.7) | 0.98 |
| Other | 96 (4.4) | 337 (4.5) | |
| Primary care provider, n (%) | 0.16 | ||
| Yes | 1537 (73.2) | 5451 (74.7) | |
| Missing: 284 | 71 (3.3) | 213 (2.8) | |
| Insurance status, n (%) | |||
| Commercial/worker's comp | 440 (20.3) | 1442 (19.2) | |
| Medicare | 1017 (46.8) | 3589 (47.8) | 0.52 |
| Medicaid/others | 714 (32.9) | 2479 (33.0) | |
| Time of admission, n (%) | |||
| 0000‐0259 | 167 (7.7) | 1068 (14.2) | |
| 0300‐0559 | 244 (11.2) | 485 (6.5) | |
| 0600‐0859 | 456 (21.0) | 270 (3.6) | |
| 0900‐1159 | 782 (36.0) | 1146 (15.3) | <0.001 |
| 1200‐1459 | 299 (13.8) | 1750 (23.3) | |
| 1500‐1759 | 155 (7.1) | 1676 (22.3) | |
| 1800‐2359 | 68 (3.1) | 1115 (14.9) | |
| Time of discharge, n (%) | |||
| 2100‐0859 | 36 (1.7) | 174 (2.3) | |
| 0900‐1159 | 275 (12.7) | 495 (6.6) | |
| 1200‐1459 | 858 (39.6) | 2608 (34.8) | <0.001 |
| 1500‐1759 | 749 (34.6) | 3122 (41.6) | |
| 1800‐2059 | 249 (11.5) | 1104 (14.7) | |
| Missing | 4 | 7 | |
| Day of week of admission, n (%) | |||
| Monday | 462 (21.3) | 1549 (20.6) | |
| Tuesday | 499 (23.0) | 1470 (19.6) | |
| Wednesday | 430 (19.8) | 1479 (19.7) | 0.001 |
| Thursday | 400 (18.4) | 1482 (19.7) | |
| Friday | 380 (17.5) | 1530 (20.4) | |
| Day of week of discharge, n (%) | |||
| Monday | 207 (9.5) | 829 (11.0) | |
| Tuesday | 268 (12.3) | 973 (13.0) | |
| Wednesday | 334 (15.4) | 1142 (15.2) | |
| Thursday | 362 (16.7) | 1297 (17.3) | 0.16 |
| Friday | 485 (22.3) | 1523 (20.3) | |
| Saturday | 330 (15.2) | 1165 (15.5) | |
| Sunday | 185 (8.5) | 581 (7.7) | |
| Admit to non‐medicine wards, n (%) | 1332 (61.4) | 2624 (34.9) | <0.001 |
| Transfer to ICU (at least once), n (%) | 299 (13.8) | 504 (6.7) | <0.001 |
| Admit from ER No (%) | 1663 (76.6) | 6063 (80.7) | <0.001 |
| 10 most frequent diagnosis (%) | Pneumonia (4.9) | Pneumonia (5.5) | |
| Congestive heart failure; nonhypertensive (4.2) | Congestive heart failure; nonhypertensive (3.9) | ||
| Sickle cell anemia (3.9) | Nonspecific chest pain (3.7) | ||
| Chronic obstructive pulmonary disease and Bronchiectasis (3.3) | Urinary tract infections(3.6) | ||
| Diabetes mellitus with complications (3.2) | Skin and subcutaneous tissue infections (3.3) | ||
| Urinary tract infections (3.2) | Sickle cell anemia (3.3) | ||
| Asthma (3.0) | Pancreatic disorders (not diabetes) (2.8) | ||
| Nonspecific chest pain (3.0) | Asthma (2.8) | ||
| Pancreatic disorders (not diabetes) (2.9) | Chronic obstructive pulmonary disease and Bronchiectasis (2.6) | ||
| Septicemia (2.2) | Diabetes mellitus with complications (2.6) | ||
| Average number of comorbidities mean (95% CI) | 0.39 (0.37‐0.42) | 0.38 (0.36‐0.39) | 0.23 |
In unadjusted comparisons of outcomes (Table 3), hospitalizations on H‐PA teams had higher lengths of stay and charges than hospitalizations on RES teams, possibly higher inpatient mortality rates but similar unadjusted readmission rates at 7, 14, and 30 days
| H‐PA (n = 2171) | RES (n = 7150) | % Difference* (CI) | P Value | |
|---|---|---|---|---|
| ||||
| LOS | Median (IQR) | Median (IQR) | ||
| Days | 3.17 (2.03‐5.30) | 2.99 (1.80‐5.08) | +8.9% (4.71‐13.29%) | <0.001 |
| Charges | ||||
| US Dollars | 9390 (6196‐16,239) | 9044 (6106‐14,805) | +5.56% (1.96‐9.28%) | 0.002 |
| Readmissions | n (%) | n (%) | Odds Ratio (CI) | |
| Within 7 days | 147 (6.96) | 571 (7.78) | 0.88 (0.73‐1.06) | 0.19 |
| Within14 days | 236 (11.34) | 924 (12.76) | 0.87 (0.75‐1.01) | 0.07 |
| Within 30 days | 383 (18.91) | 1436 (20.31) | 0.91 (0.80‐1.03) | 0.14 |
| Inpatient deaths | 39 (1.8) | 95 (1.3) | 1.36 (0.90‐2.00) | 0.06 |
Multivariable Analysis
LOS
Hospitalizations to H‐PA teams were associated with a 6.73% longer LOS (P = 0.005) (Table 4). This difference persisted when we used the winsorized data (6.45% increase, P = 0.006), excluded inpatient deaths (6.81% increase, P = 0.005), or excluded hospitalizations that involved an ICU stay (6.40%increase, P = 0.011) (Table 5).
| Overall | Subgroup: Restricted to Physicians Attending on Both H‐PA and RES Teams* | Subgroup: Restricted to Hospitalizations Between 11.00 AM and 4.00 PM | ||||
|---|---|---|---|---|---|---|
| % Difference (CI) | P Value | % Difference (CI) | P Value | % Difference (CI) | P Value | |
| ||||||
| LOS | 6.73% (1.99% to 11.70%) | 0.005 | 5.44% (0.65% to 11.91%) | 0.08 | 2.97% (4.47% to 10.98%) | 0.44 |
| Charges | 2.75% (1.30% to 6.97%) | 0.19 | 1.55% (3.76% to 7.16%) | 0.57 | 6.45% (0.62% to 14.03%) | 0.07 |
| Risk of Readmission | Adjusted OR (95%CI) | P Value | Adjusted OR (95% CI) | P Value | Adjusted OR (95% CI) | P Value |
| Within 7 days | 0.88 (0.64‐1.20) | 0.42 | 0.74 (0.40‐1.35) | 0.32 | 0.90 (0.40‐2.00) | 0.78 |
| Within14 days | 0.90 (0.69‐1.19) | 0.46 | 0.71 (0.51‐0.99) | 0.05 | 0.87 (0.36‐2.13) | 0.77 |
| Within 30 days | 0.89 (0.75‐1.06) | 0.20 | 0.75 (0.51‐1.08) | 0.12 | 0.92 (0.55‐1.54) | 0.75 |
| Inpatient mortality | 1.27 (0.82‐1.97) | 0.28 | 1.46 (0.67‐3.17) | 0.33 | 1.14 (0.47‐2.74) | 0.77 |
| Analysis With Winsorized Data | Analysis After Excluding Inpatient Deaths | Analysis After Excluding Patients With ICU Stays | ||||
|---|---|---|---|---|---|---|
| % Difference (CI) | P Value | % Difference (CI) | P Value | % Difference (CI) | P Value | |
| ||||||
| LOS | 6.45% (4.04 to 8.91%) | 0.006 | 6.81% (2.03 to 11.80%) | 0.005 | 6.40% (1.46 to 11.58%) | 0.011 |
| Charges | 2.67 (1.27 to 6.76%) | 0.187 | 2.89% (1.16 to 7.11%) | 0.164 | 0.74% (3.11 to 4.76%) | 0.710 |
Charges
Hospitalizations to H‐PA and RES teams were associated with similar charges (Table 4). The results were similar when we used winsorized data, excluded inpatient deaths or excluded hospitalizations involving an ICU stay (Table 5).
Readmissions
The risk of readmission at 7, 14, and 30 days was similar between hospitalizations to H‐PA and RES teams (Table 4).
Mortality
The risk of inpatient death was similar between all hospitalizations to H‐PA and RES teams or only hospitalizations without an ICU stay (Table 4). The results also remained the same in analyses restricted to first admissions, last admissions, or 1 randomly selected admission per patient.
Sub‐Group Analysis
On restricting the multivariable analyses to the subset of hospitalists who staffed both types of teams (Table 4), the increase in LOS associated with H‐PA care was no longer significant (5.44% higher, P = 0.081). The charges, risk of readmission at 7 and 30 days, and risk of inpatient mortality remained similar. The risk of readmission at 14 days was slightly lower following hospitalizations to H‐PA teams (odds ratio 0.71, 95% confidence interval [CI] 0.51‐0.99).
The increase in LOS associated with H‐PA care was further attenuated in analyses of the subset of admissions between 11.00 AM and 4.00 PM (2.97% higher, P = 0.444). The difference in charges approached significance (6.45% higher, P = 0.07), but risk of readmission at 7, 14, and 30 days and risk of inpatient mortality were no different (Table 4).
Interactions
On adding interaction terms between the team assignment and the fixed effect variables in each model we detected that the effect of H‐PA care on LOS (P < 0.001) and charges (P < 0.001) varied by time of admission (Figure 2a and b). Hospitalizations to H‐PA teams from 6.00 PM to 6.00 AM had greater relative increases in LOS as compared to hospitalizations to RES teams during those times. Similarly, hospitalizations during the period 3.00 PM to 3.00 AM had relatively higher charges associated with H‐PA care compared to RES care.

Discussion
We found that hospitalizations to our H‐PA teams had longer LOS but similar charges, readmission rates, and mortality as compared to traditional resident‐based teams. These findings were robust to multiple sensitivity and subgroup analyses but when we examined times when both types of teams could receive admissions, the difference in LOS was markedly attenuated and nonsignificant.
We note that most prior reports comparing PA‐based models of inpatient care predate the ACGME work hour regulations. In a randomized control trial (1987‐1988) Simmer et al.5 showed lower lengths of stay and charges but possibly higher risk of readmission for PA based teams as compared to resident based teams. Van Rhee et al.7 conducted a nonrandomized retrospective cohort study (1994‐1995) using administrative data which showed lower resource utilization for PA‐based inpatient care. Our results from 2005 to 2006 reflect the important changes in the organization and delivery of inpatient care since these previous investigations.
Roy et al.8 report the only previously published comparison of PA and resident based GM inpatient care after the ACGME mandated work hour regulations. They found PA‐based care was associated with lower costs, whereas we found similar charges for admissions to RES and H‐PA teams. They also found that LOS was similar for PA and resident‐based care, while we found a higher LOS for admissions to our H‐PA team. We note that although the design of Roy's study was similar to our own, patients cared for by PA‐based teams were geographically localized in their model. This may contribute to the differences in results noted between our studies.
Despite no designed differences in patients assigned to either type of team other than time of admission we noted some differences between the H‐PA and RES teams in the descriptive analysis. These differences, such as a higher proportion of hospitalizations to H‐PA teams being admitted from the ER, residing on nonmedicine wards or having an ICU stay are likely a result of our system of assigning admissions to H‐PA teams early during the workday. For example patients on H‐PA teams were more often located on nonmedicine wards as a result of later discharges and bed availability on medicine wards. The difference that deserves special comment is the much higher proportion (13.8% vs. 6.7%) of hospitalizations with an ICU stay on the H‐PA teams. Hospitalizations directly to the ICU were excluded from our study which means that the hospitalizations with an ICU stay in our study were initially admitted to either H‐PA or RES teams and then transferred to the ICU. Transfers out of the ICU usually occur early in the workday when H‐PA teams accepted patients per our admission schedule. These patients may have been preferentially assigned to H‐PA teams, if on returning from the ICU the original team's resident had changed (and the bounce back rule was not in effect). Importantly, the conclusions of our research are not altered on controlling for this difference in the teams by excluding hospitalizations with an ICU stay.
Hospitalizations to H‐PA teams were associated with higher resource utilization if they occurred later in the day or overnight (Figure 2a and b). During these times a transition of care occurred shortly after admission. For a late day admission the H‐PA teams would transfer care for overnight cross cover soon after the admission and for patients admitted overnight as overflow they would assume care of a patient from the nighttime covering physician performing the admission. On the other hand, on RES teams, interns admitting patients overnight continued to care for their patients for part of the following day (30‐hour call). Similar findings of higher resource utilization associated with transfer of care after admission in the daytime11 and nighttime12 have been previously reported. An alternative hypothesis for our findings is that the hospital maybe busier and thus less efficient during times when H‐PA teams had to admit later in the day or accept patients admitted overnight as overflow. Future research to determine the cause of this significant interaction between team assignment and time of admission on resource utilization is important as the large increases in LOS (up to 30%) and charges (up to 50%) noted, could have a potentially large impact if a higher proportion of hospitalizations were affected by this phenomenon.
Our H‐PA teams were assigned equally complex patients as our RES teams, in contrast to previous reports.8, 13 This was accomplished while improving the resident's educational experience and we have previously reported increases in our resident's board pass rates and in‐service training exam scores with that introduction of our H‐PA teams.14 We thus believe that selection of less complex patients to H‐PA teams such as ours is unnecessary and may give them a second tier status in academic settings.
Our report has limitations. It is a retrospective, nonrandomized investigation using a single institution's administrative database and has the limitations of not being able to account for unmeasured confounders, severity of illness, errors in the database, selection bias and has limited generalizability. We measured charges not actual costs,15 but we feel charges are a true reflection of relative resource use when compared between similar patients within a single institution. We also did not account for the readmissions that occur to other hospitals16 and our results do not reflect resource utilization for the healthcare system in total. For example, we could not tell if higher LOS on H‐PA teams resulted in lower readmissions for their patients in all hospitals in the region, which may reveal an overall resource savings. Additionally, we measured in‐hospital mortality and could not capture deaths related to hospital care that may occur shortly after discharge.
ACGME has proposed revised standards that may further restrict resident duty hours when they take effect in July 2011.3 This may lead to further decreases in resident‐based inpatient care. Teaching hospitals will need to continue to develop alternate models for inpatient care that do not depend on house staff. Our findings provide important evidence to inform the development of such models. Our study shows that one such model: PAs paired with hospitalists, accepting admissions early in the workday, with hospitalist coverage over the weekend and nights can care for GM inpatients as complex as those cared for by resident‐based teams without increasing readmission rates, inpatient mortality, or charges but at the cost of slightly higher LOS.
- ACGME‐Common Program Requirements for Resident Duty Hours. Available at: http://www.acgme.org/acWebsite/dutyHours/dh_ComProgrRequirmentsDutyHours0707.pdf. Accessed July 2010.
- ,,,,.Non‐housestaff medicine services in academic centers: models and challenges.J Hosp Med.2008;3(3):247–255.
- ACGME. Duty Hours: Proposed Standards for Review and comment. Available at: http://acgme‐2010standards.org/pdf/Proposed_Standards. pdf. Accessed July 22,2010.
- Agency for Health Care Policy and Research. HCUPnet: A tool for identifying, tracking, and analyzing national hospital statistics. Available at: http://hcup.ahrq.gov/HCUPnet.asp. Accessed July2010.
- ,,,,.A randomized, controlled trial of an attending staff service in general internal medicine.Med Care.1991;29(7 suppl):JS31–JS40.
- ,.Replacing an academic internal medicine residency program with a physician assistant‐‐hospitalist model: a Comparative Analysis Study.Am J Med Qual.2009;24(2):132–139.
- ,,.Resource use by physician assistant services versus teaching services.JAAPA.2002;15(1):33–42.
- ,,, et al.Implementation of a physician assistant/hospitalist service in an academic medical center: impact on efficiency and patient outcomes.J Hosp Med.2008;3(5):361–368.
- AHRQ. Clinical Classifications Software (CCS) for ICD‐9‐CM. Available at: http://www.hcup‐us.ahrq.gov/toolssoftware/ccs/ccs.jsp#overview. Accessed July2010.
- AHRQ. HCUP: Comorbidity Software, Version 3.4.;Available at: http://www.hcup‐us.ahrq.gov/toolssoftware/comorbidity/comorbidity.jsp. Accessed July2010.
- ,,, et al.Effect of short call admission on length of stay and quality of care for acute decompensated heart failure.Circulation.2008;117(20):2637–2644.
- ,,,.Post‐call transfer of resident responsibility: its effect on patient care.J Gen Intern Med.1990;5(6):501–505.
- ,,,,,.The effect of nonteaching services on the distribution of inpatient cases for internal medicine residents.Acad Med.2009:84(2):220–225.
- ,,, et al.Allowing for better resident education and improving patient care: hospitalist‐physician assistant teams fill in the gaps.J Hosp Med.2007;2[S2]:1–39.
- .The distinction between cost and charges.Ann Intern Med.1982;96(1):102–109.
- ,,.Rehospitalizations among patients in the Medicare Fee‐for‐Service Program.N Engl J Med.2009;360(14):1418–1428.
- ACGME‐Common Program Requirements for Resident Duty Hours. Available at: http://www.acgme.org/acWebsite/dutyHours/dh_ComProgrRequirmentsDutyHours0707.pdf. Accessed July 2010.
- ,,,,.Non‐housestaff medicine services in academic centers: models and challenges.J Hosp Med.2008;3(3):247–255.
- ACGME. Duty Hours: Proposed Standards for Review and comment. Available at: http://acgme‐2010standards.org/pdf/Proposed_Standards. pdf. Accessed July 22,2010.
- Agency for Health Care Policy and Research. HCUPnet: A tool for identifying, tracking, and analyzing national hospital statistics. Available at: http://hcup.ahrq.gov/HCUPnet.asp. Accessed July2010.
- ,,,,.A randomized, controlled trial of an attending staff service in general internal medicine.Med Care.1991;29(7 suppl):JS31–JS40.
- ,.Replacing an academic internal medicine residency program with a physician assistant‐‐hospitalist model: a Comparative Analysis Study.Am J Med Qual.2009;24(2):132–139.
- ,,.Resource use by physician assistant services versus teaching services.JAAPA.2002;15(1):33–42.
- ,,, et al.Implementation of a physician assistant/hospitalist service in an academic medical center: impact on efficiency and patient outcomes.J Hosp Med.2008;3(5):361–368.
- AHRQ. Clinical Classifications Software (CCS) for ICD‐9‐CM. Available at: http://www.hcup‐us.ahrq.gov/toolssoftware/ccs/ccs.jsp#overview. Accessed July2010.
- AHRQ. HCUP: Comorbidity Software, Version 3.4.;Available at: http://www.hcup‐us.ahrq.gov/toolssoftware/comorbidity/comorbidity.jsp. Accessed July2010.
- ,,, et al.Effect of short call admission on length of stay and quality of care for acute decompensated heart failure.Circulation.2008;117(20):2637–2644.
- ,,,.Post‐call transfer of resident responsibility: its effect on patient care.J Gen Intern Med.1990;5(6):501–505.
- ,,,,,.The effect of nonteaching services on the distribution of inpatient cases for internal medicine residents.Acad Med.2009:84(2):220–225.
- ,,, et al.Allowing for better resident education and improving patient care: hospitalist‐physician assistant teams fill in the gaps.J Hosp Med.2007;2[S2]:1–39.
- .The distinction between cost and charges.Ann Intern Med.1982;96(1):102–109.
- ,,.Rehospitalizations among patients in the Medicare Fee‐for‐Service Program.N Engl J Med.2009;360(14):1418–1428.
Copyright © 2011 Society of Hospital Medicine
Chronic Constipation in the Elderly
A supplement to Family Practice News supported by Takeda Pharmaceuticals North America, Inc. The supplement is based on a faculty interview.

To view the supplement, click the image above.
A supplement to Family Practice News supported by Takeda Pharmaceuticals North America, Inc. The supplement is based on a faculty interview.

To view the supplement, click the image above.
A supplement to Family Practice News supported by Takeda Pharmaceuticals North America, Inc. The supplement is based on a faculty interview.

To view the supplement, click the image above.
Recognition and Management of Nighttime Reflux Symptoms
A supplement to Family Practice News supported by Santarus, Inc. The supplement is based on a faculty interview.

To view the supplement, click the image above.
A supplement to Family Practice News supported by Santarus, Inc. The supplement is based on a faculty interview.

To view the supplement, click the image above.
A supplement to Family Practice News supported by Santarus, Inc. The supplement is based on a faculty interview.

To view the supplement, click the image above.
Plastic Surgery Groups Remove Cancer-Implant Webinar After Complaints
Two plastic surgery professional organizations have removed a members-only webinar in the wake of complaints by an advocacy group that the program downplayed the risk of anaplastic large-cell lymphoma (ALCL) in women who have breast implants.
Public Citizens Health Research Group wrote to the Food and Drug Administration on Feb. 17 to urge the agency to take action against the American Society of Plastic Surgeons (ASPS) and the American Society of Aesthetic Plastic Surgery (ASAPS).
The Washington-based nonprofit said that the groups held the webinar in the wake of the FDA’s Jan. 26 announcement that there were a growing number of cases of ALCL in women with implants.
Dr. Jeffrey Shuren, director of the FDA’s Center for Devices and Radiological Health, said in a Feb. 28 letter (pdf) to Public Citizen that it had viewed the webinar and "spoke with representatives of both organizations." Dr. Shuren added that, "They informed us of their plans to remove the webinar from their Web site."
Both organizations said that they were not instructed by the FDA to take the webinar down, but that it was a voluntary decision.
In a March 2 statement, the ASAPS said that it removed the webinar "as newer information became available a week ago." That information, according to ASAPS president Felmont F. Eaves III, is "an independent, systematic review of ALCL, which will be published in an upcoming edition of Plastic and Reconstructive Surgery."
Dr. Eaves said in an interview that the Rand Corp. conducted the review and that it is his understanding that the article will be available some time in June. For the time being, the advanced copy of the article is available only to ASAPS members.
The ASPS said in a March 2 statement, "It was never our intention to downplay the risk of a very rarely occurring cancer associated with breast implants." Rather, said the Society, "We did not want to unnecessarily alarm patients when the risk of ALCL associated with breast implants is so low."
According to Public Citizen, ASPS president Phil Haeck explained in the webinar that ALCL should not be referred to as a tumor or a malignancy, but as a "condition." Dr. Haeck said, "I would recommend that you use the same terms with your patients rather than disturb them by saying this is a cancer, this is a malignancy. The best word is this is a condition," according to Public Citizen. Dr. Haeck added, "And I think you are certainly justified, with what we know now, in downplaying the malignant potential of these."
Public Citizen also objected to the webinar telling members that "surgery was curative," for ALCL.
In his response to Public Citizen, the FDA’s Dr. Shuren said, "the FDA believes the optimal treatment regimen has not been established and that additional data collection is needed to fully understand the possible relationship between ALCL and breast implants, as well as the risk factors, optimal treatment plan, and prognosis."
The FDA is asking health care providers to report confirmed cases of ALCL. The agency also notes that ASPS and others are collaborating with the agency to develop a registry tracking ALCL and implants. ASAPS said in late January that it also is supporting the registry. Details are still being worked out.
Two plastic surgery professional organizations have removed a members-only webinar in the wake of complaints by an advocacy group that the program downplayed the risk of anaplastic large-cell lymphoma (ALCL) in women who have breast implants.
Public Citizens Health Research Group wrote to the Food and Drug Administration on Feb. 17 to urge the agency to take action against the American Society of Plastic Surgeons (ASPS) and the American Society of Aesthetic Plastic Surgery (ASAPS).
The Washington-based nonprofit said that the groups held the webinar in the wake of the FDA’s Jan. 26 announcement that there were a growing number of cases of ALCL in women with implants.
Dr. Jeffrey Shuren, director of the FDA’s Center for Devices and Radiological Health, said in a Feb. 28 letter (pdf) to Public Citizen that it had viewed the webinar and "spoke with representatives of both organizations." Dr. Shuren added that, "They informed us of their plans to remove the webinar from their Web site."
Both organizations said that they were not instructed by the FDA to take the webinar down, but that it was a voluntary decision.
In a March 2 statement, the ASAPS said that it removed the webinar "as newer information became available a week ago." That information, according to ASAPS president Felmont F. Eaves III, is "an independent, systematic review of ALCL, which will be published in an upcoming edition of Plastic and Reconstructive Surgery."
Dr. Eaves said in an interview that the Rand Corp. conducted the review and that it is his understanding that the article will be available some time in June. For the time being, the advanced copy of the article is available only to ASAPS members.
The ASPS said in a March 2 statement, "It was never our intention to downplay the risk of a very rarely occurring cancer associated with breast implants." Rather, said the Society, "We did not want to unnecessarily alarm patients when the risk of ALCL associated with breast implants is so low."
According to Public Citizen, ASPS president Phil Haeck explained in the webinar that ALCL should not be referred to as a tumor or a malignancy, but as a "condition." Dr. Haeck said, "I would recommend that you use the same terms with your patients rather than disturb them by saying this is a cancer, this is a malignancy. The best word is this is a condition," according to Public Citizen. Dr. Haeck added, "And I think you are certainly justified, with what we know now, in downplaying the malignant potential of these."
Public Citizen also objected to the webinar telling members that "surgery was curative," for ALCL.
In his response to Public Citizen, the FDA’s Dr. Shuren said, "the FDA believes the optimal treatment regimen has not been established and that additional data collection is needed to fully understand the possible relationship between ALCL and breast implants, as well as the risk factors, optimal treatment plan, and prognosis."
The FDA is asking health care providers to report confirmed cases of ALCL. The agency also notes that ASPS and others are collaborating with the agency to develop a registry tracking ALCL and implants. ASAPS said in late January that it also is supporting the registry. Details are still being worked out.
Two plastic surgery professional organizations have removed a members-only webinar in the wake of complaints by an advocacy group that the program downplayed the risk of anaplastic large-cell lymphoma (ALCL) in women who have breast implants.
Public Citizens Health Research Group wrote to the Food and Drug Administration on Feb. 17 to urge the agency to take action against the American Society of Plastic Surgeons (ASPS) and the American Society of Aesthetic Plastic Surgery (ASAPS).
The Washington-based nonprofit said that the groups held the webinar in the wake of the FDA’s Jan. 26 announcement that there were a growing number of cases of ALCL in women with implants.
Dr. Jeffrey Shuren, director of the FDA’s Center for Devices and Radiological Health, said in a Feb. 28 letter (pdf) to Public Citizen that it had viewed the webinar and "spoke with representatives of both organizations." Dr. Shuren added that, "They informed us of their plans to remove the webinar from their Web site."
Both organizations said that they were not instructed by the FDA to take the webinar down, but that it was a voluntary decision.
In a March 2 statement, the ASAPS said that it removed the webinar "as newer information became available a week ago." That information, according to ASAPS president Felmont F. Eaves III, is "an independent, systematic review of ALCL, which will be published in an upcoming edition of Plastic and Reconstructive Surgery."
Dr. Eaves said in an interview that the Rand Corp. conducted the review and that it is his understanding that the article will be available some time in June. For the time being, the advanced copy of the article is available only to ASAPS members.
The ASPS said in a March 2 statement, "It was never our intention to downplay the risk of a very rarely occurring cancer associated with breast implants." Rather, said the Society, "We did not want to unnecessarily alarm patients when the risk of ALCL associated with breast implants is so low."
According to Public Citizen, ASPS president Phil Haeck explained in the webinar that ALCL should not be referred to as a tumor or a malignancy, but as a "condition." Dr. Haeck said, "I would recommend that you use the same terms with your patients rather than disturb them by saying this is a cancer, this is a malignancy. The best word is this is a condition," according to Public Citizen. Dr. Haeck added, "And I think you are certainly justified, with what we know now, in downplaying the malignant potential of these."
Public Citizen also objected to the webinar telling members that "surgery was curative," for ALCL.
In his response to Public Citizen, the FDA’s Dr. Shuren said, "the FDA believes the optimal treatment regimen has not been established and that additional data collection is needed to fully understand the possible relationship between ALCL and breast implants, as well as the risk factors, optimal treatment plan, and prognosis."
The FDA is asking health care providers to report confirmed cases of ALCL. The agency also notes that ASPS and others are collaborating with the agency to develop a registry tracking ALCL and implants. ASAPS said in late January that it also is supporting the registry. Details are still being worked out.
Triple Therapy
Dual antiplatelet therapy (DAPT) (aspirin plus a thienopyridine: clopidogrel or prasugrel) has become the standard treatment for patients with acute coronary syndromes (ACS) and after coronary stent placement (Table 1). Anticoagulant therapy with warfarin is indicated for stroke prevention in atrial fibrillation (AF), profound left ventricular dysfunction, and after mechanical heart valve replacement, as well as for treatment of deep venous thrombosis and pulmonary embolism (Table 2). It is estimated that 41% of the U.S. population over age 40 years is on some form of antiplatelet therapy,6 and 2.5 million patients, mostly elderly, are on long‐term warfarin therapy.7 More specifically, 5% of patients undergoing percutaneous coronary interventions (PCIs) also have an indication for warfarin.8 With widespread use of drug‐eluting stents (DES), the need for a longer duration of DAPT, and the increased age and complexity of hospitalized patients, the safety and challenges of triple therapy (combined DAPT and warfarin) have become more important to the practice of hospital medicine. Triple therapy may increase hospitalization rates, as the risk of major bleeding is four to five times higher than with DAPT.911 In contrast, DAPT is much less effective than warfarin alone in preventing embolic events in AF,12 and warfarin alone or in combination with aspirin (ASA) is inadequate therapy to prevent stent thrombosis. Even fewer data exist on the efficacy and safety of triple therapy in patients with mechanical valves or left ventricular dysfunction.
| Class Recommendations | Level of Evidence | |
|---|---|---|
| ||
| DAPT after PCI/stenting1 | ||
| ASA | ||
| Class I | ASA 325 mg/d after PCI for 1 mo (up to 6 mo depending on type of stent implanted) and then 7562 mg/d indefinitely | B |
| Class IIa | ASA 75‐325 mg/d indefinitely after brachytherapy unless risk of bleeding is significant | C |
| In patients at risk of bleeding, a lower dose of 75‐162 mg/d is reasonable after stent implantation | C | |
| Thienopyridine | ||
| Class I | Clopidogrel 75 mg/d after BMS for at least 1 mo and ideally up to 12 mo unless increased risk of bleeding (at least 2 wk) | B |
| Clopidogrel 75 mg/d after DES for at least 12 mo if not at high risk for bleeding | B | |
| 2009 focus update2: Clopidogrel 75 mg daily or prasugrel 10 mg daily for at least 12 mo after BMS or DES for ACS | B | |
| Class IIa | Clopidogrel 75 mg/d indefinitely after brachytherapy unless risk of bleeding is significant | C |
| Class IIb | In patients with potential for lethal or catastrophic stent thrombosis, consider platelet aggregation studies and increase clopidogrel dose to 150 mg/d if 50% inhibition of platelet aggregation is seen | C |
| Continuation of clopidogrel 75 mg/day beyond 12 mo is reasonable after DES | C | |
| 2009 focus update2: consider continuation of clopidogrel or prasugrel beyond 15 mo after DES placement | C | |
| DAPT for UA/NSTEMI without stenting3 | ||
| ASA | ||
| Class I | Continue ASA (75 to 162 mg/d) indefinitely | A |
| Clopidogrel | ||
| Class 1 | Clopidogrel (75 mg/d) for at least 1 mo (A) and ideally for up to 1 y | B |
| Dipyridamole | ||
| Class III | Dipyridamole is not recommended because it has not been shown to be effective | A |
| |
| Condition | Risk (%) |
| Atrial fibrillation (without anticoagulation)4 | |
| Low‐risk atrial fibrillation (CHADS2 score 0) | 1.9 |
| Intermediate‐risk atrial fibrillation (CHADS2 score 1) | 2.8 |
| High‐risk atrial fibrillation (CHADS2 score 2‐6) | 418 |
| Mechanical heart valve5b | |
| Mechanical heart valve (without anticoagulation) | 8.6c |
| Mechanical heart valve (treated with ASA alone) | 7.5c |
| Mechanical heart valve (treated with warfarin) | 1.8c |
| Mechanical aortic valve (treated with warfarin) | 1.1c |
| Mechanical mitral valve (treated with warfarin) | 2.7c |
Hospitalists commonly care for patients on triple therapy; certain indications are appropriate and supported from the available literature while others lack evidence. Knowledge of existing practice guidelines and of supporting research studies leads to optimal management of these complicated patients, and minimizes excessive morbidity from bleeding complications or thromboembolic events such as strokes and stent thrombosis.
In the first part of this article, we present the evidence that supports current recommendations for DAPT or warfarin in specific medical conditions. We also address controversies and unanswered questions. The second part of this review focuses on the available data and provides guidance on the optimal care of patients on triple therapy.
Dual Antiplatelet Therapy Following Acute Coronary Syndromes
Table 3 summarizes key randomized trials of DAPT versus ASA alone in several clinical scenarios. The addition of clopidogrel to ASA in patients with nonST‐elevation ACS reduced the risk of adverse ischemic outcomes in the clopidogrel in unstable angina to prevent recurrent events (CURE) trial,15 as well as in its substudy, the PCI‐CURE (patients with ACS who have undergone stenting).17 In the main CURE study, the study groups diverged within the first 30 days after randomization and the benefit of DAPT persisted throughout the 12 months of the study period. DAPT is also superior to ASA in patients with ST‐elevation myocardial infarction (MI) (CLARITYTIMI 28 and COMMIT trials).13, 14 On the basis of these findings, DAPT has become the standard of care for patients with ACS. The American College of Cardiology (ACC)/American Heart Association (AHA)3 and the European Society of Cardiology18 recommend ASA treatment indefinitely for patients with ACS whether or not they underwent PCI. Clopidogrel is recommended for at least 12 months following ACS, especially for patients who receive a coronary stent.
| Trial | Endpoints | Results |
|---|---|---|
| ||
| ST elevation MI | ||
| CLARITY‐TIMI13 | Incidence of death, infarct‐related artery occlusion, or recurrent MI | 36% reduction (95% CI 2447); P .001 |
| COMMIT14 | Incidence of death, MI, or stroke | 9% reduction (95% CI 314); P .002 |
| ACS without ST elevation | ||
| CURE15 | Incidence of death, MI, or stroke | 20% reduction (RR 0.80 [0.720.90]); P .001 |
| Bare‐metal stent placement | ||
| CREDO16 | Incidence of death, MI, or stroke | 27% reduction (95% CI 3.944.4); P .02 |
| PCI‐CURE17 | Incidence of death, MI, or urgent TVR | 30% reduction (RR 0.70 [0.500.97]); P .03 |
Despite the proven efficacy of DAPT in ACS, about 15% of patients die or experience reinfarction within 30 days of diagnosis.19 The continued risk for thrombotic events could be due to delayed onset of platelet inhibition and to patient heterogeneity in responsiveness to therapy with ASA and/or clopidogrel.20 Consequently, the optimum dose for clopidogrel and ASA following ACS is uncertain. The CURRENT‐OASIS 7 trial evaluated the efficacy and safety of high‐dose clopidogrel (600‐mg loading dose, 150 mg once daily for 7 days, followed by 75 mg/d) versus standard‐dose clopidogrel (300‐mg loading dose, followed by 75 mg/d) and ASA (75‐100 mg versus 300‐325 mg/d) in patients with ACS who were treated medically, with or without stenting.21 In the overall study population as well as in patients who did not receive stenting, there was no significant difference in the combined rate of death from cardiovascular causes, MI, and stroke between patients receiving the high‐dose and the standard‐dose clopidogrel (4.2% vs 4.4%; P = .37) and high‐dose versus low‐dose ASA (4.2% vs 4.4%; P = .47). There were no significant differences in bleeding complications between the two clopidogrel treatment arms or between the high‐dose and low‐dose ASA groups.
The ACC/AHA guidelines recommend ASA, 75‐162 mg/d indefinitely after medical therapy without stenting (class I, level of evidence: A)3 and clopidogrel 75 mg/d for at least 1 month (class IA) and optimally for 1 year (class IB). Clopidogrel monotherapy is appropriate for patients with ACS who are unable to tolerate ASA due to either hypersensitivity or recent significant gastrointestinal bleeding.
As is the case after coronary stenting, interruption of DAPT soon after ACS may subject patients to high recurrence of cardiovascular events, although few data are available to support this observation. Interruption of DAPT due to bleeding complications or surgical procedures more than 1 month after ACS may be reasonable for a patient who did not receive a stent. Clinicians should restart DAPT after the surgical procedure once the bleeding risk becomes acceptable.
Dual Antiplatelet Therapy Following Coronary Stenting
Following Bare Metal Stents
Stent thrombosis occurs in approximately 20% of patients who receive bare metal stents (BMS) without DAPT22; the risk is highest in the first 30 days after implantation. The clinical presentation of stent thrombosis is often catastrophic: MI or sudden death occurs in over 60% of cases. DAPT reduces the incidence of stent thrombosis to a clinically acceptable level.22
In the ISAR trial of 517 patients treated with BMS for MI, suboptimal angioplasty, or other high‐risk clinical and anatomic features,23 patients were randomly assigned to treatment with ASA plus ticlopidine or ASA plus anticoagulation with heparin and warfarin. The primary endpoint of cardiac death, MI, coronary bypass surgery, or repeat angioplasty occurred in 1.5% of patients assigned to DAPT and 6.2% of those assigned to anticoagulant therapy (relative risk [RR], 0.25; 95% confidence interval [CI], 0.06‐0.77). The PCI‐CURE study evaluated patients who received BMS after ACS.17 The primary endpoint was a composite of cardiovascular death, MI, or urgent target‐vessel revascularization within 30 days of PCI. Long‐term administration of clopidogrel (8 months) conferred a lower rate of cardiovascular death, MI, or any revascularization (P = .03), with no significant difference in major bleeding between the groups (P = .64). In the CREDO trial,16 investigators evaluated 2116 patients undergoing PCI at 99 North American centers. Subjects received either a 300‐mg loading dose of clopidogrel or placebo 3‐24 hours before PCI. All patients then received clopidogrel 75 mg/d through day 28. For the following 12 months, patients in the loading dose group received clopidogrel, and those in the control group received placebo. All patients received ASA throughout the study. At 1 year, loading dose plus long‐term clopidogrel therapy conferred a 27% RR reduction (3% absolute risk reduction) in the combined endpoint of death, MI, or stroke (P = .02).
Based on these trials, the ACC and AHA recommend clopidogrel (75 mg/d) for a minimum of 1 month and optimally 12 months after BMS (class 1B).2 For patients at increased risk of bleeding, the ACC/AHA recommends a minimum of 2 weeks of clopidogrel. Although lifelong therapy with ASA is recommended, the optimal dose of ASA after BMS is unknown. However, on the basis of clinical trial protocols (no randomized data), guidelines recommend ASA 162 mg‐325 mg/d for at least 1 month, followed by indefinite use at a dose of 75‐162 mg. In patients for whom there is concern about bleeding, lower doses of ASA (75‐162 mg) are acceptable for the initial period after stent implantation.
Following Drug‐Eluting Stents
Drug‐eluting stents have become the standard percutaneous treatment for patients with symptomatic coronary artery disease. In 2005, a sampling of 140 US hospitals indicated that 94% of patients treated with a stent received at least one DES.24 Compared with BMS, restenosis and the need for revascularization are significantly less frequent. In contrast, unanticipated high rates of very late (>1 year) stent thrombosis have complicated DES.25 Because of the potentially lethal consequences of stent thrombosis, several authors have questioned the long‐term safety of DES2635 and examined the role of extended DAPT in reducing this delayed complication.27, 31, 36 Although the initial pivotal randomized trials of DES mandated clopidogrel use for only 3 months after sirolimus‐eluting stent and 6 months after paclitaxel‐eluting stent,37, 38 current guidelines recommend DAPT for at least 12 months after DES placement for patients who are not at high risk of bleeding.1
Although multiple studies have confirmed the benefit of DAPT, controversy remains regarding the extended use for more than 1 year. The only randomized trial that addressed this issue was nonblinded and underpowered.39 In this study of patients from two ongoing trials, the REAL‐LATE and ZEST‐LATE, extended duration DAPT (>12 months, median duration 19.2 months), did not reduce the incidence of MI and cardiac death.39 The rate of the primary endpoint was less than 25% of that expected (underpowered), and patients had already received clopidogrel for up to 24 months before enrollment.
The results from small, nonrandomized trials regarding this issue have been contradictory. Banerjee and colleagues studied 530 consecutive patients who underwent PCI (85% received a DES), were free of cardiovascular events for 6 months after PCI, and had follow‐up available for >12 months.26 In a multivariate analysis, clopidogrel use for 1 year was associated with lower mortality (hazard ratio [HR], 0.28; 95% CI, 0.140.59); this effect was independent of traditional cardiovascular risk factors, clinical presentation, and DES use. In a study at the Duke Heart Center40 among patients with DES (n = 528) who were event‐free at 12 months, continued clopidogrel use conferred lower rates of death (0% versus 3.5%; difference, 3.5%; 95% CI, 5.9% to 1.1%; P = .004) and death or MI (0% versus 4.5%; difference, 4.5%; 95% CI, 7.1% to 1.9%; P .001) at 24 months. In the TYCOON registry,35 patients with DES receiving clopidogrel for 2 years had a rate of stent thrombosis (0.4%) that was similar to those with BMS (0.7%) but significantly lower than patients with DES and 1‐year DAPT (2.9%).
In contrast, Roy and colleagues33 found that clopidogrel cessation at 12 months did not predict stent thrombosis, and Park and colleagues32 reported that clopidogrel continuation beyond 1 year did not appear to decrease stent thrombosis or clinical events after DES implantation. Similarly, Stone et al.34 performed a landmark analysis on the basis of the prospective, double‐blind TAXUS‐II SR, TAXUS‐IV, and TAXUS‐V trials. The authors found that thienopyridine use beyond 1 year after DES may reduce stent thrombosis over the subsequent 12‐month period, but did not reduce rates of death and MI at 2 and 5 years after either DES or BMS.
Current guidelines recommend ASA 162‐325 mg/d for at least 3‐6 months, followed by treatment indefinitely at a dose of 75‐162 mg daily. Clopidogrel, on the other hand, is given at 75 mg/d for at least 12 months.
Warfarin After Acute Coronary Syndromes
Warfarin with different international normalized ratio (INR) goals alone or in combination with ASA has been evaluated after ACS. In an early trial, patients with recent (mean interval 27 days) MI were treated with warfarin alone versus placebo.41 Warfarin conferred a relative risk reduction in mortality of 24% (95% CI, 4‐44%; P = .027) at the expense of major bleeding rates of 0.6%/y. In the ASPECT trial,42 moderate to high intensity anticoagulation after MI resulted in a 53% and 40% reduction in the relative risk of reinfarction (annual incidence 2.3% versus 5.1%) and cerebrovascular events (annual incidence 0.7% versus 1.2%), respectively. In the WARIS II43 and ASPECT‐244 trials, moderate intensity warfarin (INR 2.0‐2.5) in combination with low‐dose ASA, compared with ASA alone, reduced the composite occurrence of death or nonfatal reinfarction, as well as recurrent coronary occlusion after ST‐segment elevation MI. High‐intensity warfarin therapy alone (INR 3.0‐4.0 for ASPECT, 2.8‐4.2 for WARISII) reduced ischemic vascular events compared with ASA alone. Not unexpectedly, major bleeding episodes were more common among patients receiving warfarin.
No randomized trials have compared DAPT with warfarin plus ASA for patients with ACS who did not receive stents. The ACC/AHA guidelines recommend warfarin for secondary prevention following ACS (class IIb). High‐intensity warfarin alone (INR 2.5‐3.5) or moderate intensity (INR 2.0‐2.5) with low‐dose ASA (75‐81 mg/d) may be reasonable for patients at high ischemic and low bleeding risk who are intolerant of clopidogrel (level of evidence: B). Fixed dose warfarin is not recommended by the ACC/AHA primarily on the basis of the Coumadin Aspirin Reinfarction Study (CARS) results. This study of patients following MI was discontinued prematurely because of a lack of incremental benefit of reduced‐dose ASA (80 mg/d) combined with either 1 or 3 mg of warfarin daily when compared with 160 mg/d of ASA alone.
Triple Therapy for PCI and Atrial Fibrillation
AF is the most frequent indication (70%) for long‐term therapy with warfarin in patients scheduled for stent placement.10 Clinical trials have shown that warfarin alone is superior to ASA, clopidogrel, or DAPT for prevention of stroke in patients with AF.45, 46 Although warfarin is indispensable in these settings, DAPT is similarly necessary after stent implantation. As triple therapy increases the risk of bleeding, the management of patients with AF and who have received stents remains controversial. This situation is particularly problematic among patients who have received DES and may benefit from extended DAPT. No randomized trials exist to clarify the optimal treatment in these patients; and the feasibility of such studies is questionable. Small, mostly retrospective, studies (Table 4) provide limited guidance on this issue; most studies focus on bleeding events rather than the cardiovascular efficacy of triple therapy. Because of these limitations, cardiovascular societies give IIb recommendation for either triple therapy or the combination of warfarin and clopidogrel in this setting and the level of evidence is C.1, 59, 60
| Author | Year | Type | No. | Major Bleeding, % (range) | Thrombotic Events | Comments |
|---|---|---|---|---|---|---|
| ||||||
| Studies of one group (triple therapy group) | ||||||
| Orford et al.47 | 2004 | Obs | 66 | 4.5 (0.211.2) | N/A | Bleeding occurred only with suboptimal control of INR and/or pre‐existing GI disease. |
| Porter et al.48 | 2006 | Obs | 180 | 1.6 (0.04.2) | N/A | Bleeding rates were acceptable with short‐term TT after PCI. |
| Rubboli et al.49 | 2007 | Obs | 49 | 18 (4.436.9) | N/A | Most hemorrhages occurred during TT. |
| Rogacka et al.50 | 2008 | Obs | 127 | 4.7 | N/A | One‐half of bleeding episodes were lethal and 67% occurred within the first month. |
| Studies comparing triple therapy with dual antiplatelet therapy | ||||||
| Mattichak et al.51 | 2005 | Obs | 82 | 21 vs. 3.5 (P = .028)a | Reinfarction (29% vs. 9%, P = .15) | TT did not reduce reinfarction after stenting for MI but increased rates of GI bleeding and transfusions. |
| Khurram et al.11 | 2006 | Matched cohort | 214 | 6.6 vs. 0 (P = .03) | N/A | Higher bleeding rates for TT than DAPT. INR range or ASA dosage did not influence the bleeding risk. |
| DeEugenio et al.9 | 2007 | Matched cohort | 194 | OR 5.0 (1.417.8, P = .012) | N/A | ASA dose, age, sex, BMI, DM, hypertension, and procedural anticoagulant type or use did not influence risk of major bleeding. |
| Ruiz‐Nodar et al.52 | 2008 | Obs | 426 | 14.9 vs. 9.0 (P = .19) | Mortality: OR 3.43 (1.617.54, P = .002)b MACE: OR 4.9 (2.1711.1, P .01)b | TT was associated with a nonsignificant increase in major bleeding but lower all‐cause mortality and fewer MACE. |
| Sarafoff et al.53 | 2008 | Prosp | 515 | 1.4 vs. 3.1 (P = .34). | MACCE: OR 0.76 (0.481.21, P = .25) | No difference in MACCE or bleeding at 2 y. Stent thrombosis did not differ between groups. |
| Rossini et al.54 | 2008 | Prosp | 204 | 10.8 vs. 4.9 (P = .1) | MACE: 5.8% vs. 4.9% (P = .7) | INR was targeted to the lower range (2.0‐2.5). No significant difference in bleeding rates for TT versus DAPT at 18 mo. Less bleeding for patients whose INR was within target (4.9 versus 33%, P = .00019). No significant differences in MACE between groups. |
| Uchida et al.55 | 2010 | Obs | 575 | 18 vs. 2.7 (P .001) | MACE (P = .108) | No differences in MACE rates. More bleeding for patients on TT. |
| Studies comparing triple therapy versus dual antiplatelet therapy versus wararin and single antiplatelet agent | ||||||
| Karjalainen et al.10 | 2007 | Matched cohort | 239 | OR 3.3 (1.38.6, P = .014)c | MACE: OR 1.7 (1.0‐3.0, P = 0.05)c | This study compared patients on warfarin at baseline with those not on warfarinall undergoing stenting. Patients on warfarin at baseline were treated with a variety of strategies. Baseline warfarin use increased both major bleeding and MACE at 1 y. ASA plus warfarin was inadequate to prevent stent thrombosis, and premature warfarin cessation was associated with stroke. |
| Manzano‐Fernandez et al.56 | 2008 | Obs | 104 | EB (5.8 vs. 11.3, P = .33) LB (21.6 vs. 3.8, P = .006)d | MACE: 25.5% vs. 21.0% (P = .53)d | No difference in MACE rates between TT and non‐TT (WAA or DAPT). TT conferred higher late bleeding (>48 h). |
| Gao et al.57 | 2010 | Prosp | 622 | 2.9 vs. 1.8 vs. 2.5 (P = .725)e | MACCE: 8.8% vs. 20.1% vs. 14.9% (P = .010)e | Target INR was set as 1.8‐2.5. Lower stroke and MACCE rates for TT as compared with DAPT or WAA; no difference in bleeding. |
| Studies comparing triple therapy with warfarin and single antiplatelet agent | ||||||
| Nguyen et al.58 | 2007 | Obs | 800 | 5.9 vs. 46 (P = .46) | Death: 5.1% vs. 6.5% (P = .47) Stroke: 0.7% vs. 3.4% (P = .02) MI: 3.3% vs. 4.5% (P = .49) | TT and WAA lead to similar 6‐mo bleeding, death, and MI. Fewer strokes with TT (caveat: low event rate). |
In the largest study to date, Nguyen et al.58 evaluated 800 patients who underwent stenting for ACS and were discharged on warfarin plus single antiplatelet agent or triple therapy as part of the GRACE registry. At 6 months, triple therapy conferred a significant reduction in stroke (0.7% versus 3.4%, P = .02) but not in death or MI. There were no differences in in‐hospital major bleeding events between the two groups (5.9% versus 4.6%; P = .46). Similarly, Sarafoff et al.53 reported no significant differences in the combined endpoint (death, MI, stent thrombosis or stroke) or bleeding complications among patients who received triple therapy or DAPT at 2 years of follow‐up. In contrast, Ruiz‐Nodar et al.52 showed that triple therapy, compared with DAPT, at discharge reduced the incidence of death (17.8% versus 27.8%; adjusted HR = 3.43; 95% CI, 1.617.54; P = .002) and major adverse cardiac events (26.5% versus 38.7%; adjusted HR = 4.9; 95% CI, 2.1711.1; P = .01), without a substantial increase in major bleeding events.
The value of combination antiplatelet therapy to prevent stent thrombosis in these patients is clearer in the study reported by Karjalainen et al.10 This case‐control study of 239 patients receiving warfarin at baseline who underwent PCI evaluated a primary endpoint of death, MI, target‐vessel revascularization, or stent thrombosis and a secondary endpoint of major bleeding and stroke to 12 months of follow‐up. Forty‐eight percent of patients received triple therapy, whereas 15.5% were discharged on DAPT. The remaining patients received warfarin plus a single antiplatelet agent. Stent thrombosis occurred more frequently among patients receiving warfarin plus ASA (15.2%) than among those receiving triple therapy (1.9%). As expected, stroke was more frequent in patients treated with DAPT (8.8%) than among those receiving triple therapy (2.8%). Major bleeding was similar between groups. Therapy with warfarin was an independent predictor of both major bleeding and major cardiac events at 1 year. This observation illustrates that the outcome of PCI in patients on chronic warfarin therapy is unsatisfactory irrespective of the antithrombotic combinations used, highlighting the need for better strategies to treat these patients.
Choice of Therapy and Management of Patients Eligible for Triple Therapy
Current guidelines for PCI do not provide guidance for patients with an indication for triple therapy due to a paucity of published evidence. Several ongoing prospective trials aim to address the management of these patients (AFCAS, ISAR‐TRIPLE). Pending further study, clinicians should consider the embolic risk (CHADS2 score), target INR, type of stent, bleeding risk, and duration of treatment when determining the appropriate antiplatelet/anticoagulant combinations. The CHADS2 score (Table 2) stratifies the risk for stroke among patients with AF,4 while the Outpatient Bleeding Risk Index (OBRI) allows estimation of bleeding risk.12, 61 The OBRI considers age > 65 years, prior stroke, prior gastrointestinal bleeding, and any of four comorbidities (recent MI, anemia, diabetes, or renal insufficiency) in order to stratify patients into three risk groups.61 Patients with three to four risk factors have a high risk of bleeding (23% at 3 months and 48% at 12 months) whereas patients with no risk factors have only a 3% risk of bleeding at 12 months. Unfortunately, advanced age and prior stroke appear in both OBRI and CHADS.
For patients with AF who are at high risk for embolic stroke (>3% per year), we recommend triple therapy for the shortest time possible, followed by warfarin and ASA indefinitely. In case of BMS, it is acceptable to shorten triple therapy duration to 1 month. The optimal duration of triple therapy for patients with DES is uncertain; recommended durations range from 3 months to 1 year.62 If the potential consequences of stent thrombosis are high due to a large amount of myocardium at risk, an extended period of triple therapy might be justified. For patients whose stroke risk is lower (CHADS2 score of 0‐1), the risk for bleeding likely outweighs any benefit from stroke prevention. In this instance, it is reasonable to use DAPT with ASA and clopidogrel for 1 month after BMS and 12 months after DES, followed by ASA, with or without warfarin, indefinitely. In a recently published study, patients with AF and a CHADS2 score of 1 had a yearly stroke risk of 1.25% while taking DAPT63; the risk of major bleeding for triple therapy is 6.1% per year.64
For patients who have a high bleeding risk, BMS are the preferred stent type as the duration of triple therapy might be limited to 4 weeks. To our knowledge, no randomized study has evaluated the outcome of patients with BMS compared with DES who also have an indication for warfarin. Because studies have suggested that clopidogrel is more effective than aspirin in preventing stent thrombosis and in reducing death or MI after coronary stenting,40, 65 warfarin and single antiplatelet therapy with clopidogrel might be a reasonable treatment option in patients with high bleeding risk. The WOEST study (NCT00769938), currently recruiting participants, is the first randomized study specifically designed to test this hypothesis.
Since gastrointestinal bleeding accounts for approximately 30‐40% of hemorrhagic events in patients on combined ASA and anticoagulant therapy, an expert consensus document recommended concomitant treatment with proton pump inhibitors (PPIs) to reduce this risk.66 In contrast, the 2009 Focused Updates of the ACC/AHA/SCAI Guidelines did not recommend the use of PPIs with DAPT in the setting of ACS.2 This is because of studies that show inhibition of platelet activation,67 and potential clinical harm,68 when clopidogrel is combined with certain PPIs that inhibit the CYP2C19 enzyme. However, to date there are no convincing randomized clinical trial data documenting an important clinical drug‐drug interaction. The U.S. Food and Drug Administration (FDA) advises that physicians avoid the use of clopidogrel in patients with impaired CYP2C19 function due to known genetic variation or due to concomitant use of drugs that inhibit CYP2C19 activity. More specifically, the FDA recommends avoiding the use of omeprazole and esomeprazole in patients taking clopidogrel.69
In particular, elderly patients have an increased risk of bleeding while receiving triple therapy. In a study of patients over age 65, 2.5% were hospitalized for bleeding in the first year after PCI, and the use of triple therapy was the strongest predictor of bleeding (more than threefold increase).70 One in five patients suffered death or MI at 1 year after hospitalization for bleeding.70 The basis for poor outcomes after hospitalization for bleeding in this population is multifactorial and may be due to the location of bleeding, associated hypercoagulable state, potential adverse impact of blood transfusion, withdrawal of warfarin therapy in patients with AF and PCI, and the premature discontinuation of DAPT. The use of nonsteroidal anti‐inflammatory drugs (NSAIDs) is common among the elderly and conferred a doubling of bleeding risk.70 Limiting the use of NSAID, the use of low‐dose ASA beyond 30 days after stent implantation, greater use of BMS, and maintaining INR at the lowest possible level (INR of 22.5) will reduce the risk for bleeding.57, 71
New Anticoagulants
Due to the high risk for bleeding with warfarin and the challenges inherent in INR monitoring, researchers have developed several novel anticoagulants whose advantages include fixed daily dosing and no need for monitoring. Dabigatran is a direct oral thrombin inhibitor that is already licensed in Europe and Canada for thromboprophylaxis after hip or knee surgery. It has also been studied in patients with AF. In the RE‐LY trial, patients with AF who received dabigatran 110 mg daily had rates of stroke and systemic embolism that were similar to those with warfarin, as well as lower rates of major hemorrhage.72 The randomized ReDEEM trial, reported at the AHA 2009 Scientific Sessions, was aimed at finding a dosage of dabigatran that achieves a good balance between clinical effectiveness and bleeding risk when combined with aspirin and clopidogrel after acute MI. Dosages ranging from 50 mg twice daily to 150 mg twice daily were all associated with 6‐month rates of bleeding lower than 2%. Hospitalists should view these encouraging results cautiously until the publication of ReDEEM trial results in a peer‐reviewed journal.
A variety of oral Xa antagonists are also being evaluated in patients with AF or ACS. These trials offer insight into triple therapy regimens that include ASA, clopidogrel, and an Xa antagonist. In a recent study of the oral Xa antagonist rivaroxaban, investigators stratified 3491 subjects with ACS according to whether they received concomitant ASA alone or ASA and clopidogrel.73 Subjects receiving ASA plus rivaroxaban had a modest increase in bleeding. Triple therapy, however, increased the composite bleeding rate from 3.5% in the DAPT group to approximately 6‐15% (low‐dose or high‐dose rivaroxaban, respectively). Rivaroxaban is currently under review by the FDA.
These novel agents might eventually replace warfarin for many or most indications for anticoagulation. It is imperative that future research compare the efficacy and risk of bleeding between triple therapy using these new agents and triple therapy with warfarin.
Conclusions
The management of patients on long‐term anticoagulation who require DAPT because of ACS or coronary stenting is challenging. DAPT may safely substitute for warfarin only for patients at low risk for a thromboembolic event (ie, low‐risk AF with low CHADS2 score). Clinicians should not interrupt warfarin in patients at higher risk (ie, intermediate to high‐risk AF, mechanical valves, or recent venous thromboembolism), even in the presence of DAPT. In these patients, triple therapy is the optimal approach following coronary stenting (and possibly during the initial period after ACS without stenting). As this approach confers a fivefold increase in bleeding complications compared with DAPT, careful monitoring of the INR, the addition of PPIs, and the exclusion of elderly patients who are at the highest risk for bleeding complications74 is recommended. The preferred duration of triple therapy after BMS in patients who require long‐term anticoagulation is 1 month, whereas the optimal duration after ACS or DES remains unresolved.
- ,,, et al.2007 Focused Update of the ACC/AHA/SCAI 2005 Guideline Update for Percutaneous Coronary Intervention: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines: 2007 Writing Group to Review New Evidence and Update the ACC/AHA/SCAI 2005 Guideline Update for Percutaneous Coronary Intervention, Writing on Behalf of the 2005 Writing Committee.Circulation2008;117:261–295.
- ,,, et al.2009 Focused Updates: ACC/AHA Guidelines for the Management of Patients With ST‐Elevation Myocardial Infarction (updating the 2004 Guideline and 2007 Focused Update) and ACC/AHA/SCAI Guidelines on Percutaneous Coronary Intervention (updating the 2005 Guideline and 2007 Focused Update): a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines.Circulation2009;120:2271–2306.
- ,,, et al.ACC/AHA 2007 guidelines for the management of patients with unstable angina/non‐ST‐Elevation myocardial infarction: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the 2002 Guidelines for the Management of Patients With Unstable Angina/Non‐ST‐Elevation Myocardial Infarction) developed in collaboration with the American College of Emergency Physicians, the Society for Cardiovascular Angiography and Interventions, and the Society of Thoracic Surgeons endorsed by the American Association of Cardiovascular and Pulmonary Rehabilitation and the Society for Academic Emergency Medicine.J Am Coll Cardiol2007;50:e1–e157.
- ,,, et al.Selecting patients with atrial fibrillation for anticoagulation: stroke risk stratification in patients taking aspirin.Circulation2004;110:2287–2292.
- ,,.Thromboembolic and bleeding complications in patients with mechanical heart valve prostheses.Circulation1994;89:635–641.
- ,,,,.Aspirin use among adults aged 40 and older in the United States: results of a national survey.Am J Prev Med2007;32:403–407.
- ,,, et al.The perioperative management of antithrombotic therapy: American College of Chest Physicians Evidence‐Based Clinical Practice Guidelines (8th Edition).Chest2008;133:299S–339S.
- ,,, et al.Discharge antithrombotic strategies among patients with acute coronary syndrome previously on warfarin anticoagulation: physician practice in the CRUSADE registry.Am Heart J2008;155:361–368.
- ,,, et al.Risk of major bleeding with concomitant dual antiplatelet therapy after percutaneous coronary intervention in patients receiving long‐term warfarin therapy.Pharmacotherapy2007;27:691–696.
- ,,, et al.Safety and efficacy of combined antiplatelet‐warfarin therapy after coronary stenting.Eur Heart J2007;28:726–732.
- ,,, et al.Combination therapy with aspirin, clopidogrel and warfarin following coronary stenting is associated with a significant risk of bleeding.J Invasive Cardiol2006;18:162–164.
- ,,,,,.Development of a contemporary bleeding risk model for elderly warfarin recipients.Chest2006;130:1390–1396.
- ,,, et al.Addition of clopidogrel to aspirin and fibrinolytic therapy for myocardial infarction with ST‐segment elevation.N Engl J Med2005;352:1179–1189.
- ,,, et al.Addition of clopidogrel to aspirin in 45,852 patients with acute myocardial infarction: randomised placebo‐controlled trial.Lancet2005;366:1607–1621.
- ,,,,,.Effects of clopidogrel in addition to aspirin in patients with acute coronary syndromes without ST‐segment elevation.N Engl J Med2001;345:494–502.
- ,,, et al.Early and sustained dual oral antiplatelet therapy following percutaneous coronary intervention: a randomized controlled trial.JAMA2002;288:2411–2420.
- ,,, et al.Effects of pretreatment with clopidogrel and aspirin followed by long‐term therapy in patients undergoing percutaneous coronary intervention: the PCI‐CURE study.Lancet2001;358:527–533.
- ,,, et al.Guidelines for the diagnosis and treatment of non‐ST‐segment elevation acute coronary syndromes.Eur Heart J2007;28:1598–1660.
- .Burden of disease: medical and economic impact of acute coronary syndromes.Am J Manag Care2006;12:S430–S434.
- ,,,,,.Variability in platelet responsiveness to clopidogrel among 544 individuals.J Am Coll Cardiol2005;45:246–251.
- ,,, et al.Dose comparisons of clopidogrel and aspirin in acute coronary syndromes.N Engl J Med2010;363:930–942.
- ,,, et al.A clinical trial comparing three antithrombotic‐drug regimens after coronary‐artery stenting. Stent Anticoagulation Restenosis Study Investigators.N Engl J Med1998;339:1665–1671.
- ,,, et al.A randomized comparison of antiplatelet and anticoagulant therapy after the placement of coronary‐artery stents.N Engl J Med1996;334:1084–1089.
- ,,.Outcomes of 6906 patients undergoing percutaneous coronary intervention in the era of drug‐eluting stents: report of the DEScover Registry.Circulation2006;114:2154–2162.
- ,,, et al.Safety and efficacy of sirolimus‐ and paclitaxel‐eluting coronary stents.N Engl J Med2007;356:998–1008.
- ,,, et al.Comparison of the impact of short (1 year) and long‐term (> or =1 year) clopidogrel use following percutaneous coronary intervention on mortality.Am J Cardiol2008;102:1159–1162.
- ,,.Stent thrombosis late after implantation of first‐generation drug‐eluting stents: a cause for concern.Circulation2007;115:1440–1455; discussion 55.
- ,,, et al.Early and late coronary stent thrombosis of sirolimus‐eluting and paclitaxel‐eluting stents in routine clinical practice: data from a large two‐institutional cohort study.Lancet2007;369:667–678.
- ,,, et al.Analysis of 14 trials comparing sirolimus‐eluting stents with bare‐metal stents.N Engl J Med2007;356:1030–1039.
- ,,,,,.Long‐term outcomes with drug‐eluting stents versus bare‐metal stents in Sweden.N Engl J Med2007;356:1009–1019.
- ,,,,,.Stent thrombosis in randomized clinical trials of drug‐eluting stents.N Engl J Med2007;356:1020–1029.
- ,,, et al.Stent thrombosis, clinical events, and influence of prolonged clopidogrel use after placement of drug‐eluting stent data from an observational cohort study of drug‐eluting versus bare‐metal stents.JACC Cardiovasc Interv2008;1:494–503.
- ,,, et al.Temporal relation between Clopidogrel cessation and stent thrombosis after drug‐eluting stent implantation.Am J Cardiol2009;103:801–805.
- ,,, et al.Effect of prolonged thienopyridine use after drug‐eluting stent implantation (from the TAXUS landmark trials data).Am J Cardiol2008;102:1017–1022.
- ,,, et al.Effectiveness of two‐year clopidogrel + aspirin in abolishing the risk of very late thrombosis after drug‐eluting stent implantation (from the TYCOON [two‐year ClOpidOgrel need] study).Am J Cardiol2009;104:1357–1361.
- ,,.Mortality in randomized controlled trials comparing drug‐eluting vs. bare metal stents in coronary artery disease: a meta‐analysis.Eur Heart J2006;27:2784–2814.
- ,,, et al.Sirolimus‐eluting stents versus standard stents in patients with stenosis in a native coronary artery.N Engl J Med2003;349:1315–1323.
- ,,, et al.A polymer‐based, paclitaxel‐eluting stent in patients with coronary artery disease.N Engl J Med2004;350:221–231.
- ,,, et al.Duration of dual antiplatelet therapy after implantation of drug‐eluting stents.N Engl J Med2010;362:1374–1382.
- ,,, et al.Clopidogrel use and long‐term clinical outcomes after drug‐eluting stent implantation.JAMA2007;297:159–168.
- ,,.The effect of warfarin on mortality and reinfarction after myocardial infarction.N Engl J Med1990;323:147–152.
- Effect of long‐term oral anticoagulant treatment on mortality and cardiovascular morbidity after myocardial infarction.Anticoagulants in the Secondary Prevention of Events in Coronary Thrombosis (ASPECT) Research Group.Lancet1994;343:499–503.
- ,,,,.Warfarin, aspirin, or both after myocardial infarction.N Engl J Med2002;347:969–974.
- ,,,,.Aspirin and coumadin after acute coronary syndromes (the ASPECT‐2 study): a randomised controlled trial.Lancet2002;360:109–113.
- ,,, et al.Clopidogrel plus aspirin versus oral anticoagulation for atrial fibrillation in the Atrial fibrillation Clopidogrel Trial with Irbesartan for prevention of Vascular Events (ACTIVE W): a randomised controlled trial.Lancet2006;367:1903–1912.
- ,,,.Antithrombotic therapy to prevent stroke in patients with atrial fibrillation: a meta‐analysis.Ann Intern Med1999;131:492–501.
- ,,, et al.Safety and efficacy of aspirin, clopidogrel, and warfarin after coronary stent placement in patients with an indication for anticoagulation.Am Heart J2004;147:463–467.
- ,,,,,.Short‐term triple therapy with aspirin, warfarin, and a thienopyridine among patients undergoing percutaneous coronary intervention.Catheter Cardiovasc Interv2006;68:56–61.
- ,,,,.Periprocedural and medium‐term antithrombotic strategies in patients with an indication for long‐term anticoagulation undergoing coronary angiography and intervention.Coron Artery Dis2007;18:193–199.
- ,,, et al.Dual antiplatelet therapy after percutaneous coronary intervention with stent implantation in patients taking chronic oral anticoagulation.JACC Cardiovasc Interv2008;1:56–61.
- ,,,,,.Evaluation of safety of warfarin in combination with antiplatelet therapy for patients treated with coronary stents for acute myocardial infarction.J Interv Cardiol2005;18:163–166.
- ,,, et al.Anticoagulant and antiplatelet therapy use in 426 patients with atrial fibrillation undergoing percutaneous coronary intervention and stent implantation implications for bleeding risk and prognosis.J Am Coll Cardiol2008;51:818–825.
- ,,, et al.Aspirin and clopidogrel with or without phenprocoumon after drug eluting coronary stent placement in patients on chronic oral anticoagulation.J Intern Med2008;264:472–480.
- ,,, et al.Long‐term outcomes in patients undergoing coronary stenting on dual oral antiplatelet treatment requiring oral anticoagulant therapy.Am J Cardiol2008;102:1618–1623.
- ,,,,.Impact of anticoagulant therapy with dual antiplatelet therapy on prognosis after treatment with drug‐eluting coronary stents.J Cardiol2010;55:362–369.
- ,,, et al.Increased major bleeding complications related to triple antithrombotic therapy usage in patients with atrial fibrillation undergoing percutaneous coronary artery stenting.Chest2008;134:559–567.
- ,,, et al.Comparison of different antithrombotic regimens for patients with atrial fibrillation undergoing drug‐eluting stent implantation.Circ J2010;74:701–708.
- ,,, et al.Combining warfarin and antiplatelet therapy after coronary stenting in the Global Registry of Acute Coronary Events: is it safe and effective to use just one antiplatelet agent?Eur Heart J2007;28:1717–1722.
- ,,, et al.2007 focused update of the ACC/AHA 2004 guidelines for the management of patients with ST‐elevation myocardial infarction: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines.J Am Coll Cardiol2008;51:210–247.
- ,,, et al.Guidelines for percutaneous coronary interventions. The Task Force for Percutaneous Coronary Interventions of the European Society of Cardiology.Eur Heart J2005;26:804–847.
- ,,.Prospective evaluation of an index for predicting the risk of major bleeding in outpatients treated with warfarin.Am J Med1998;105:91–99.
- ,,,.Coronary stents and chronic anticoagulation.Circulation2009;119:1682–1688.
- ,,, et al.Risks and benefits of oral anticoagulation compared with clopidogrel plus aspirin in patients with atrial fibrillation according to stroke risk: the atrial fibrillation clopidogrel trial with irbesartan for prevention of vascular events (ACTIVE‐W).Stroke2008;39:1482–1486.
- ,,, et al.Early and late increased bleeding rates after angioplasty and stenting due to combined antiplatelet and anticoagulanttherapy.EuroIntervention2009;5:425–431.
- ,,, et al.Late clinical events after clopidogrel discontinuation may limit the benefit of drug‐eluting stents: an observational study of drug‐eluting versus bare‐metal stents.J Am Coll Cardiol2006;48:2584–2591.
- ,,, et al.ACCF/ACG/AHA 2008 expert consensus document on reducing the gastrointestinal risks of antiplatelet therapy and NSAID use: a report of the American College of Cardiology Foundation Task Force on Clinical Expert Consensus Documents.Circulation2008;118:1894–1909.
- ,,, et al.Influence of omeprazole on the antiplatelet action of clopidogrel associated with aspirin: the randomized, double‐blind OCLA (Omeprazole CLopidogrel Aspirin) study.J Am Coll Cardiol2008;51:256–260.
- ,,, et al.Risk of adverse outcomes associated with concomitant use of clopidogrel and proton pump inhibitors following acute coronary syndrome.JAMA2009;301:937–944.
- Follow‐Up to the January 26,2009, Early Communication about an Ongoing Safety Review of Clopidogrel Bisulfate (marketed as Plavix) and Omeprazole (marketed as Prilosec and Prilosec OTC). 11/17/2009. (Accessed at http://www.fda.gov/Drugs/DrugSafety/Postmarket DrugSafetyInformationforPatientsandProviders/DrugSafetyInformationfor HeathcareProfessionals/ucm190784.htm.)
- ,,, et al.Incidence, predictors, and prognostic implications of hospitalization for late bleeding after percutaneous coronary intervention for patients older than 65 years.Circ Cardiovasc Interv2010;3:140–147.
- ,,, et al.Antithrombotic therapy in patients treated with oral anticoagulation undergoing coronary artery stenting. An expert consensus document with focus on atrial fibrillation.Ann Med2008;40:428–436.
- ,,, et al.Dabigatran versus warfarin in patients with atrial fibrillation.N Engl J Med2009;361:1139–1151.
- ,,, et al.Rivaroxaban versus placebo in patients with acute coronary syndromes (ATLAS ACS‐TIMI 46): a randomised, double‐blind, phase II trial.Lancet2009;374:29–38.
- ,,,.Bleeding complications associated with combinations of aspirin, thienopyridine derivatives, and warfarin in elderly patients following acute myocardial infarction.Arch Intern Med2005;165:784–789.
Dual antiplatelet therapy (DAPT) (aspirin plus a thienopyridine: clopidogrel or prasugrel) has become the standard treatment for patients with acute coronary syndromes (ACS) and after coronary stent placement (Table 1). Anticoagulant therapy with warfarin is indicated for stroke prevention in atrial fibrillation (AF), profound left ventricular dysfunction, and after mechanical heart valve replacement, as well as for treatment of deep venous thrombosis and pulmonary embolism (Table 2). It is estimated that 41% of the U.S. population over age 40 years is on some form of antiplatelet therapy,6 and 2.5 million patients, mostly elderly, are on long‐term warfarin therapy.7 More specifically, 5% of patients undergoing percutaneous coronary interventions (PCIs) also have an indication for warfarin.8 With widespread use of drug‐eluting stents (DES), the need for a longer duration of DAPT, and the increased age and complexity of hospitalized patients, the safety and challenges of triple therapy (combined DAPT and warfarin) have become more important to the practice of hospital medicine. Triple therapy may increase hospitalization rates, as the risk of major bleeding is four to five times higher than with DAPT.911 In contrast, DAPT is much less effective than warfarin alone in preventing embolic events in AF,12 and warfarin alone or in combination with aspirin (ASA) is inadequate therapy to prevent stent thrombosis. Even fewer data exist on the efficacy and safety of triple therapy in patients with mechanical valves or left ventricular dysfunction.
| Class Recommendations | Level of Evidence | |
|---|---|---|
| ||
| DAPT after PCI/stenting1 | ||
| ASA | ||
| Class I | ASA 325 mg/d after PCI for 1 mo (up to 6 mo depending on type of stent implanted) and then 7562 mg/d indefinitely | B |
| Class IIa | ASA 75‐325 mg/d indefinitely after brachytherapy unless risk of bleeding is significant | C |
| In patients at risk of bleeding, a lower dose of 75‐162 mg/d is reasonable after stent implantation | C | |
| Thienopyridine | ||
| Class I | Clopidogrel 75 mg/d after BMS for at least 1 mo and ideally up to 12 mo unless increased risk of bleeding (at least 2 wk) | B |
| Clopidogrel 75 mg/d after DES for at least 12 mo if not at high risk for bleeding | B | |
| 2009 focus update2: Clopidogrel 75 mg daily or prasugrel 10 mg daily for at least 12 mo after BMS or DES for ACS | B | |
| Class IIa | Clopidogrel 75 mg/d indefinitely after brachytherapy unless risk of bleeding is significant | C |
| Class IIb | In patients with potential for lethal or catastrophic stent thrombosis, consider platelet aggregation studies and increase clopidogrel dose to 150 mg/d if 50% inhibition of platelet aggregation is seen | C |
| Continuation of clopidogrel 75 mg/day beyond 12 mo is reasonable after DES | C | |
| 2009 focus update2: consider continuation of clopidogrel or prasugrel beyond 15 mo after DES placement | C | |
| DAPT for UA/NSTEMI without stenting3 | ||
| ASA | ||
| Class I | Continue ASA (75 to 162 mg/d) indefinitely | A |
| Clopidogrel | ||
| Class 1 | Clopidogrel (75 mg/d) for at least 1 mo (A) and ideally for up to 1 y | B |
| Dipyridamole | ||
| Class III | Dipyridamole is not recommended because it has not been shown to be effective | A |
| |
| Condition | Risk (%) |
| Atrial fibrillation (without anticoagulation)4 | |
| Low‐risk atrial fibrillation (CHADS2 score 0) | 1.9 |
| Intermediate‐risk atrial fibrillation (CHADS2 score 1) | 2.8 |
| High‐risk atrial fibrillation (CHADS2 score 2‐6) | 418 |
| Mechanical heart valve5b | |
| Mechanical heart valve (without anticoagulation) | 8.6c |
| Mechanical heart valve (treated with ASA alone) | 7.5c |
| Mechanical heart valve (treated with warfarin) | 1.8c |
| Mechanical aortic valve (treated with warfarin) | 1.1c |
| Mechanical mitral valve (treated with warfarin) | 2.7c |
Hospitalists commonly care for patients on triple therapy; certain indications are appropriate and supported from the available literature while others lack evidence. Knowledge of existing practice guidelines and of supporting research studies leads to optimal management of these complicated patients, and minimizes excessive morbidity from bleeding complications or thromboembolic events such as strokes and stent thrombosis.
In the first part of this article, we present the evidence that supports current recommendations for DAPT or warfarin in specific medical conditions. We also address controversies and unanswered questions. The second part of this review focuses on the available data and provides guidance on the optimal care of patients on triple therapy.
Dual Antiplatelet Therapy Following Acute Coronary Syndromes
Table 3 summarizes key randomized trials of DAPT versus ASA alone in several clinical scenarios. The addition of clopidogrel to ASA in patients with nonST‐elevation ACS reduced the risk of adverse ischemic outcomes in the clopidogrel in unstable angina to prevent recurrent events (CURE) trial,15 as well as in its substudy, the PCI‐CURE (patients with ACS who have undergone stenting).17 In the main CURE study, the study groups diverged within the first 30 days after randomization and the benefit of DAPT persisted throughout the 12 months of the study period. DAPT is also superior to ASA in patients with ST‐elevation myocardial infarction (MI) (CLARITYTIMI 28 and COMMIT trials).13, 14 On the basis of these findings, DAPT has become the standard of care for patients with ACS. The American College of Cardiology (ACC)/American Heart Association (AHA)3 and the European Society of Cardiology18 recommend ASA treatment indefinitely for patients with ACS whether or not they underwent PCI. Clopidogrel is recommended for at least 12 months following ACS, especially for patients who receive a coronary stent.
| Trial | Endpoints | Results |
|---|---|---|
| ||
| ST elevation MI | ||
| CLARITY‐TIMI13 | Incidence of death, infarct‐related artery occlusion, or recurrent MI | 36% reduction (95% CI 2447); P .001 |
| COMMIT14 | Incidence of death, MI, or stroke | 9% reduction (95% CI 314); P .002 |
| ACS without ST elevation | ||
| CURE15 | Incidence of death, MI, or stroke | 20% reduction (RR 0.80 [0.720.90]); P .001 |
| Bare‐metal stent placement | ||
| CREDO16 | Incidence of death, MI, or stroke | 27% reduction (95% CI 3.944.4); P .02 |
| PCI‐CURE17 | Incidence of death, MI, or urgent TVR | 30% reduction (RR 0.70 [0.500.97]); P .03 |
Despite the proven efficacy of DAPT in ACS, about 15% of patients die or experience reinfarction within 30 days of diagnosis.19 The continued risk for thrombotic events could be due to delayed onset of platelet inhibition and to patient heterogeneity in responsiveness to therapy with ASA and/or clopidogrel.20 Consequently, the optimum dose for clopidogrel and ASA following ACS is uncertain. The CURRENT‐OASIS 7 trial evaluated the efficacy and safety of high‐dose clopidogrel (600‐mg loading dose, 150 mg once daily for 7 days, followed by 75 mg/d) versus standard‐dose clopidogrel (300‐mg loading dose, followed by 75 mg/d) and ASA (75‐100 mg versus 300‐325 mg/d) in patients with ACS who were treated medically, with or without stenting.21 In the overall study population as well as in patients who did not receive stenting, there was no significant difference in the combined rate of death from cardiovascular causes, MI, and stroke between patients receiving the high‐dose and the standard‐dose clopidogrel (4.2% vs 4.4%; P = .37) and high‐dose versus low‐dose ASA (4.2% vs 4.4%; P = .47). There were no significant differences in bleeding complications between the two clopidogrel treatment arms or between the high‐dose and low‐dose ASA groups.
The ACC/AHA guidelines recommend ASA, 75‐162 mg/d indefinitely after medical therapy without stenting (class I, level of evidence: A)3 and clopidogrel 75 mg/d for at least 1 month (class IA) and optimally for 1 year (class IB). Clopidogrel monotherapy is appropriate for patients with ACS who are unable to tolerate ASA due to either hypersensitivity or recent significant gastrointestinal bleeding.
As is the case after coronary stenting, interruption of DAPT soon after ACS may subject patients to high recurrence of cardiovascular events, although few data are available to support this observation. Interruption of DAPT due to bleeding complications or surgical procedures more than 1 month after ACS may be reasonable for a patient who did not receive a stent. Clinicians should restart DAPT after the surgical procedure once the bleeding risk becomes acceptable.
Dual Antiplatelet Therapy Following Coronary Stenting
Following Bare Metal Stents
Stent thrombosis occurs in approximately 20% of patients who receive bare metal stents (BMS) without DAPT22; the risk is highest in the first 30 days after implantation. The clinical presentation of stent thrombosis is often catastrophic: MI or sudden death occurs in over 60% of cases. DAPT reduces the incidence of stent thrombosis to a clinically acceptable level.22
In the ISAR trial of 517 patients treated with BMS for MI, suboptimal angioplasty, or other high‐risk clinical and anatomic features,23 patients were randomly assigned to treatment with ASA plus ticlopidine or ASA plus anticoagulation with heparin and warfarin. The primary endpoint of cardiac death, MI, coronary bypass surgery, or repeat angioplasty occurred in 1.5% of patients assigned to DAPT and 6.2% of those assigned to anticoagulant therapy (relative risk [RR], 0.25; 95% confidence interval [CI], 0.06‐0.77). The PCI‐CURE study evaluated patients who received BMS after ACS.17 The primary endpoint was a composite of cardiovascular death, MI, or urgent target‐vessel revascularization within 30 days of PCI. Long‐term administration of clopidogrel (8 months) conferred a lower rate of cardiovascular death, MI, or any revascularization (P = .03), with no significant difference in major bleeding between the groups (P = .64). In the CREDO trial,16 investigators evaluated 2116 patients undergoing PCI at 99 North American centers. Subjects received either a 300‐mg loading dose of clopidogrel or placebo 3‐24 hours before PCI. All patients then received clopidogrel 75 mg/d through day 28. For the following 12 months, patients in the loading dose group received clopidogrel, and those in the control group received placebo. All patients received ASA throughout the study. At 1 year, loading dose plus long‐term clopidogrel therapy conferred a 27% RR reduction (3% absolute risk reduction) in the combined endpoint of death, MI, or stroke (P = .02).
Based on these trials, the ACC and AHA recommend clopidogrel (75 mg/d) for a minimum of 1 month and optimally 12 months after BMS (class 1B).2 For patients at increased risk of bleeding, the ACC/AHA recommends a minimum of 2 weeks of clopidogrel. Although lifelong therapy with ASA is recommended, the optimal dose of ASA after BMS is unknown. However, on the basis of clinical trial protocols (no randomized data), guidelines recommend ASA 162 mg‐325 mg/d for at least 1 month, followed by indefinite use at a dose of 75‐162 mg. In patients for whom there is concern about bleeding, lower doses of ASA (75‐162 mg) are acceptable for the initial period after stent implantation.
Following Drug‐Eluting Stents
Drug‐eluting stents have become the standard percutaneous treatment for patients with symptomatic coronary artery disease. In 2005, a sampling of 140 US hospitals indicated that 94% of patients treated with a stent received at least one DES.24 Compared with BMS, restenosis and the need for revascularization are significantly less frequent. In contrast, unanticipated high rates of very late (>1 year) stent thrombosis have complicated DES.25 Because of the potentially lethal consequences of stent thrombosis, several authors have questioned the long‐term safety of DES2635 and examined the role of extended DAPT in reducing this delayed complication.27, 31, 36 Although the initial pivotal randomized trials of DES mandated clopidogrel use for only 3 months after sirolimus‐eluting stent and 6 months after paclitaxel‐eluting stent,37, 38 current guidelines recommend DAPT for at least 12 months after DES placement for patients who are not at high risk of bleeding.1
Although multiple studies have confirmed the benefit of DAPT, controversy remains regarding the extended use for more than 1 year. The only randomized trial that addressed this issue was nonblinded and underpowered.39 In this study of patients from two ongoing trials, the REAL‐LATE and ZEST‐LATE, extended duration DAPT (>12 months, median duration 19.2 months), did not reduce the incidence of MI and cardiac death.39 The rate of the primary endpoint was less than 25% of that expected (underpowered), and patients had already received clopidogrel for up to 24 months before enrollment.
The results from small, nonrandomized trials regarding this issue have been contradictory. Banerjee and colleagues studied 530 consecutive patients who underwent PCI (85% received a DES), were free of cardiovascular events for 6 months after PCI, and had follow‐up available for >12 months.26 In a multivariate analysis, clopidogrel use for 1 year was associated with lower mortality (hazard ratio [HR], 0.28; 95% CI, 0.140.59); this effect was independent of traditional cardiovascular risk factors, clinical presentation, and DES use. In a study at the Duke Heart Center40 among patients with DES (n = 528) who were event‐free at 12 months, continued clopidogrel use conferred lower rates of death (0% versus 3.5%; difference, 3.5%; 95% CI, 5.9% to 1.1%; P = .004) and death or MI (0% versus 4.5%; difference, 4.5%; 95% CI, 7.1% to 1.9%; P .001) at 24 months. In the TYCOON registry,35 patients with DES receiving clopidogrel for 2 years had a rate of stent thrombosis (0.4%) that was similar to those with BMS (0.7%) but significantly lower than patients with DES and 1‐year DAPT (2.9%).
In contrast, Roy and colleagues33 found that clopidogrel cessation at 12 months did not predict stent thrombosis, and Park and colleagues32 reported that clopidogrel continuation beyond 1 year did not appear to decrease stent thrombosis or clinical events after DES implantation. Similarly, Stone et al.34 performed a landmark analysis on the basis of the prospective, double‐blind TAXUS‐II SR, TAXUS‐IV, and TAXUS‐V trials. The authors found that thienopyridine use beyond 1 year after DES may reduce stent thrombosis over the subsequent 12‐month period, but did not reduce rates of death and MI at 2 and 5 years after either DES or BMS.
Current guidelines recommend ASA 162‐325 mg/d for at least 3‐6 months, followed by treatment indefinitely at a dose of 75‐162 mg daily. Clopidogrel, on the other hand, is given at 75 mg/d for at least 12 months.
Warfarin After Acute Coronary Syndromes
Warfarin with different international normalized ratio (INR) goals alone or in combination with ASA has been evaluated after ACS. In an early trial, patients with recent (mean interval 27 days) MI were treated with warfarin alone versus placebo.41 Warfarin conferred a relative risk reduction in mortality of 24% (95% CI, 4‐44%; P = .027) at the expense of major bleeding rates of 0.6%/y. In the ASPECT trial,42 moderate to high intensity anticoagulation after MI resulted in a 53% and 40% reduction in the relative risk of reinfarction (annual incidence 2.3% versus 5.1%) and cerebrovascular events (annual incidence 0.7% versus 1.2%), respectively. In the WARIS II43 and ASPECT‐244 trials, moderate intensity warfarin (INR 2.0‐2.5) in combination with low‐dose ASA, compared with ASA alone, reduced the composite occurrence of death or nonfatal reinfarction, as well as recurrent coronary occlusion after ST‐segment elevation MI. High‐intensity warfarin therapy alone (INR 3.0‐4.0 for ASPECT, 2.8‐4.2 for WARISII) reduced ischemic vascular events compared with ASA alone. Not unexpectedly, major bleeding episodes were more common among patients receiving warfarin.
No randomized trials have compared DAPT with warfarin plus ASA for patients with ACS who did not receive stents. The ACC/AHA guidelines recommend warfarin for secondary prevention following ACS (class IIb). High‐intensity warfarin alone (INR 2.5‐3.5) or moderate intensity (INR 2.0‐2.5) with low‐dose ASA (75‐81 mg/d) may be reasonable for patients at high ischemic and low bleeding risk who are intolerant of clopidogrel (level of evidence: B). Fixed dose warfarin is not recommended by the ACC/AHA primarily on the basis of the Coumadin Aspirin Reinfarction Study (CARS) results. This study of patients following MI was discontinued prematurely because of a lack of incremental benefit of reduced‐dose ASA (80 mg/d) combined with either 1 or 3 mg of warfarin daily when compared with 160 mg/d of ASA alone.
Triple Therapy for PCI and Atrial Fibrillation
AF is the most frequent indication (70%) for long‐term therapy with warfarin in patients scheduled for stent placement.10 Clinical trials have shown that warfarin alone is superior to ASA, clopidogrel, or DAPT for prevention of stroke in patients with AF.45, 46 Although warfarin is indispensable in these settings, DAPT is similarly necessary after stent implantation. As triple therapy increases the risk of bleeding, the management of patients with AF and who have received stents remains controversial. This situation is particularly problematic among patients who have received DES and may benefit from extended DAPT. No randomized trials exist to clarify the optimal treatment in these patients; and the feasibility of such studies is questionable. Small, mostly retrospective, studies (Table 4) provide limited guidance on this issue; most studies focus on bleeding events rather than the cardiovascular efficacy of triple therapy. Because of these limitations, cardiovascular societies give IIb recommendation for either triple therapy or the combination of warfarin and clopidogrel in this setting and the level of evidence is C.1, 59, 60
| Author | Year | Type | No. | Major Bleeding, % (range) | Thrombotic Events | Comments |
|---|---|---|---|---|---|---|
| ||||||
| Studies of one group (triple therapy group) | ||||||
| Orford et al.47 | 2004 | Obs | 66 | 4.5 (0.211.2) | N/A | Bleeding occurred only with suboptimal control of INR and/or pre‐existing GI disease. |
| Porter et al.48 | 2006 | Obs | 180 | 1.6 (0.04.2) | N/A | Bleeding rates were acceptable with short‐term TT after PCI. |
| Rubboli et al.49 | 2007 | Obs | 49 | 18 (4.436.9) | N/A | Most hemorrhages occurred during TT. |
| Rogacka et al.50 | 2008 | Obs | 127 | 4.7 | N/A | One‐half of bleeding episodes were lethal and 67% occurred within the first month. |
| Studies comparing triple therapy with dual antiplatelet therapy | ||||||
| Mattichak et al.51 | 2005 | Obs | 82 | 21 vs. 3.5 (P = .028)a | Reinfarction (29% vs. 9%, P = .15) | TT did not reduce reinfarction after stenting for MI but increased rates of GI bleeding and transfusions. |
| Khurram et al.11 | 2006 | Matched cohort | 214 | 6.6 vs. 0 (P = .03) | N/A | Higher bleeding rates for TT than DAPT. INR range or ASA dosage did not influence the bleeding risk. |
| DeEugenio et al.9 | 2007 | Matched cohort | 194 | OR 5.0 (1.417.8, P = .012) | N/A | ASA dose, age, sex, BMI, DM, hypertension, and procedural anticoagulant type or use did not influence risk of major bleeding. |
| Ruiz‐Nodar et al.52 | 2008 | Obs | 426 | 14.9 vs. 9.0 (P = .19) | Mortality: OR 3.43 (1.617.54, P = .002)b MACE: OR 4.9 (2.1711.1, P .01)b | TT was associated with a nonsignificant increase in major bleeding but lower all‐cause mortality and fewer MACE. |
| Sarafoff et al.53 | 2008 | Prosp | 515 | 1.4 vs. 3.1 (P = .34). | MACCE: OR 0.76 (0.481.21, P = .25) | No difference in MACCE or bleeding at 2 y. Stent thrombosis did not differ between groups. |
| Rossini et al.54 | 2008 | Prosp | 204 | 10.8 vs. 4.9 (P = .1) | MACE: 5.8% vs. 4.9% (P = .7) | INR was targeted to the lower range (2.0‐2.5). No significant difference in bleeding rates for TT versus DAPT at 18 mo. Less bleeding for patients whose INR was within target (4.9 versus 33%, P = .00019). No significant differences in MACE between groups. |
| Uchida et al.55 | 2010 | Obs | 575 | 18 vs. 2.7 (P .001) | MACE (P = .108) | No differences in MACE rates. More bleeding for patients on TT. |
| Studies comparing triple therapy versus dual antiplatelet therapy versus wararin and single antiplatelet agent | ||||||
| Karjalainen et al.10 | 2007 | Matched cohort | 239 | OR 3.3 (1.38.6, P = .014)c | MACE: OR 1.7 (1.0‐3.0, P = 0.05)c | This study compared patients on warfarin at baseline with those not on warfarinall undergoing stenting. Patients on warfarin at baseline were treated with a variety of strategies. Baseline warfarin use increased both major bleeding and MACE at 1 y. ASA plus warfarin was inadequate to prevent stent thrombosis, and premature warfarin cessation was associated with stroke. |
| Manzano‐Fernandez et al.56 | 2008 | Obs | 104 | EB (5.8 vs. 11.3, P = .33) LB (21.6 vs. 3.8, P = .006)d | MACE: 25.5% vs. 21.0% (P = .53)d | No difference in MACE rates between TT and non‐TT (WAA or DAPT). TT conferred higher late bleeding (>48 h). |
| Gao et al.57 | 2010 | Prosp | 622 | 2.9 vs. 1.8 vs. 2.5 (P = .725)e | MACCE: 8.8% vs. 20.1% vs. 14.9% (P = .010)e | Target INR was set as 1.8‐2.5. Lower stroke and MACCE rates for TT as compared with DAPT or WAA; no difference in bleeding. |
| Studies comparing triple therapy with warfarin and single antiplatelet agent | ||||||
| Nguyen et al.58 | 2007 | Obs | 800 | 5.9 vs. 46 (P = .46) | Death: 5.1% vs. 6.5% (P = .47) Stroke: 0.7% vs. 3.4% (P = .02) MI: 3.3% vs. 4.5% (P = .49) | TT and WAA lead to similar 6‐mo bleeding, death, and MI. Fewer strokes with TT (caveat: low event rate). |
In the largest study to date, Nguyen et al.58 evaluated 800 patients who underwent stenting for ACS and were discharged on warfarin plus single antiplatelet agent or triple therapy as part of the GRACE registry. At 6 months, triple therapy conferred a significant reduction in stroke (0.7% versus 3.4%, P = .02) but not in death or MI. There were no differences in in‐hospital major bleeding events between the two groups (5.9% versus 4.6%; P = .46). Similarly, Sarafoff et al.53 reported no significant differences in the combined endpoint (death, MI, stent thrombosis or stroke) or bleeding complications among patients who received triple therapy or DAPT at 2 years of follow‐up. In contrast, Ruiz‐Nodar et al.52 showed that triple therapy, compared with DAPT, at discharge reduced the incidence of death (17.8% versus 27.8%; adjusted HR = 3.43; 95% CI, 1.617.54; P = .002) and major adverse cardiac events (26.5% versus 38.7%; adjusted HR = 4.9; 95% CI, 2.1711.1; P = .01), without a substantial increase in major bleeding events.
The value of combination antiplatelet therapy to prevent stent thrombosis in these patients is clearer in the study reported by Karjalainen et al.10 This case‐control study of 239 patients receiving warfarin at baseline who underwent PCI evaluated a primary endpoint of death, MI, target‐vessel revascularization, or stent thrombosis and a secondary endpoint of major bleeding and stroke to 12 months of follow‐up. Forty‐eight percent of patients received triple therapy, whereas 15.5% were discharged on DAPT. The remaining patients received warfarin plus a single antiplatelet agent. Stent thrombosis occurred more frequently among patients receiving warfarin plus ASA (15.2%) than among those receiving triple therapy (1.9%). As expected, stroke was more frequent in patients treated with DAPT (8.8%) than among those receiving triple therapy (2.8%). Major bleeding was similar between groups. Therapy with warfarin was an independent predictor of both major bleeding and major cardiac events at 1 year. This observation illustrates that the outcome of PCI in patients on chronic warfarin therapy is unsatisfactory irrespective of the antithrombotic combinations used, highlighting the need for better strategies to treat these patients.
Choice of Therapy and Management of Patients Eligible for Triple Therapy
Current guidelines for PCI do not provide guidance for patients with an indication for triple therapy due to a paucity of published evidence. Several ongoing prospective trials aim to address the management of these patients (AFCAS, ISAR‐TRIPLE). Pending further study, clinicians should consider the embolic risk (CHADS2 score), target INR, type of stent, bleeding risk, and duration of treatment when determining the appropriate antiplatelet/anticoagulant combinations. The CHADS2 score (Table 2) stratifies the risk for stroke among patients with AF,4 while the Outpatient Bleeding Risk Index (OBRI) allows estimation of bleeding risk.12, 61 The OBRI considers age > 65 years, prior stroke, prior gastrointestinal bleeding, and any of four comorbidities (recent MI, anemia, diabetes, or renal insufficiency) in order to stratify patients into three risk groups.61 Patients with three to four risk factors have a high risk of bleeding (23% at 3 months and 48% at 12 months) whereas patients with no risk factors have only a 3% risk of bleeding at 12 months. Unfortunately, advanced age and prior stroke appear in both OBRI and CHADS.
For patients with AF who are at high risk for embolic stroke (>3% per year), we recommend triple therapy for the shortest time possible, followed by warfarin and ASA indefinitely. In case of BMS, it is acceptable to shorten triple therapy duration to 1 month. The optimal duration of triple therapy for patients with DES is uncertain; recommended durations range from 3 months to 1 year.62 If the potential consequences of stent thrombosis are high due to a large amount of myocardium at risk, an extended period of triple therapy might be justified. For patients whose stroke risk is lower (CHADS2 score of 0‐1), the risk for bleeding likely outweighs any benefit from stroke prevention. In this instance, it is reasonable to use DAPT with ASA and clopidogrel for 1 month after BMS and 12 months after DES, followed by ASA, with or without warfarin, indefinitely. In a recently published study, patients with AF and a CHADS2 score of 1 had a yearly stroke risk of 1.25% while taking DAPT63; the risk of major bleeding for triple therapy is 6.1% per year.64
For patients who have a high bleeding risk, BMS are the preferred stent type as the duration of triple therapy might be limited to 4 weeks. To our knowledge, no randomized study has evaluated the outcome of patients with BMS compared with DES who also have an indication for warfarin. Because studies have suggested that clopidogrel is more effective than aspirin in preventing stent thrombosis and in reducing death or MI after coronary stenting,40, 65 warfarin and single antiplatelet therapy with clopidogrel might be a reasonable treatment option in patients with high bleeding risk. The WOEST study (NCT00769938), currently recruiting participants, is the first randomized study specifically designed to test this hypothesis.
Since gastrointestinal bleeding accounts for approximately 30‐40% of hemorrhagic events in patients on combined ASA and anticoagulant therapy, an expert consensus document recommended concomitant treatment with proton pump inhibitors (PPIs) to reduce this risk.66 In contrast, the 2009 Focused Updates of the ACC/AHA/SCAI Guidelines did not recommend the use of PPIs with DAPT in the setting of ACS.2 This is because of studies that show inhibition of platelet activation,67 and potential clinical harm,68 when clopidogrel is combined with certain PPIs that inhibit the CYP2C19 enzyme. However, to date there are no convincing randomized clinical trial data documenting an important clinical drug‐drug interaction. The U.S. Food and Drug Administration (FDA) advises that physicians avoid the use of clopidogrel in patients with impaired CYP2C19 function due to known genetic variation or due to concomitant use of drugs that inhibit CYP2C19 activity. More specifically, the FDA recommends avoiding the use of omeprazole and esomeprazole in patients taking clopidogrel.69
In particular, elderly patients have an increased risk of bleeding while receiving triple therapy. In a study of patients over age 65, 2.5% were hospitalized for bleeding in the first year after PCI, and the use of triple therapy was the strongest predictor of bleeding (more than threefold increase).70 One in five patients suffered death or MI at 1 year after hospitalization for bleeding.70 The basis for poor outcomes after hospitalization for bleeding in this population is multifactorial and may be due to the location of bleeding, associated hypercoagulable state, potential adverse impact of blood transfusion, withdrawal of warfarin therapy in patients with AF and PCI, and the premature discontinuation of DAPT. The use of nonsteroidal anti‐inflammatory drugs (NSAIDs) is common among the elderly and conferred a doubling of bleeding risk.70 Limiting the use of NSAID, the use of low‐dose ASA beyond 30 days after stent implantation, greater use of BMS, and maintaining INR at the lowest possible level (INR of 22.5) will reduce the risk for bleeding.57, 71
New Anticoagulants
Due to the high risk for bleeding with warfarin and the challenges inherent in INR monitoring, researchers have developed several novel anticoagulants whose advantages include fixed daily dosing and no need for monitoring. Dabigatran is a direct oral thrombin inhibitor that is already licensed in Europe and Canada for thromboprophylaxis after hip or knee surgery. It has also been studied in patients with AF. In the RE‐LY trial, patients with AF who received dabigatran 110 mg daily had rates of stroke and systemic embolism that were similar to those with warfarin, as well as lower rates of major hemorrhage.72 The randomized ReDEEM trial, reported at the AHA 2009 Scientific Sessions, was aimed at finding a dosage of dabigatran that achieves a good balance between clinical effectiveness and bleeding risk when combined with aspirin and clopidogrel after acute MI. Dosages ranging from 50 mg twice daily to 150 mg twice daily were all associated with 6‐month rates of bleeding lower than 2%. Hospitalists should view these encouraging results cautiously until the publication of ReDEEM trial results in a peer‐reviewed journal.
A variety of oral Xa antagonists are also being evaluated in patients with AF or ACS. These trials offer insight into triple therapy regimens that include ASA, clopidogrel, and an Xa antagonist. In a recent study of the oral Xa antagonist rivaroxaban, investigators stratified 3491 subjects with ACS according to whether they received concomitant ASA alone or ASA and clopidogrel.73 Subjects receiving ASA plus rivaroxaban had a modest increase in bleeding. Triple therapy, however, increased the composite bleeding rate from 3.5% in the DAPT group to approximately 6‐15% (low‐dose or high‐dose rivaroxaban, respectively). Rivaroxaban is currently under review by the FDA.
These novel agents might eventually replace warfarin for many or most indications for anticoagulation. It is imperative that future research compare the efficacy and risk of bleeding between triple therapy using these new agents and triple therapy with warfarin.
Conclusions
The management of patients on long‐term anticoagulation who require DAPT because of ACS or coronary stenting is challenging. DAPT may safely substitute for warfarin only for patients at low risk for a thromboembolic event (ie, low‐risk AF with low CHADS2 score). Clinicians should not interrupt warfarin in patients at higher risk (ie, intermediate to high‐risk AF, mechanical valves, or recent venous thromboembolism), even in the presence of DAPT. In these patients, triple therapy is the optimal approach following coronary stenting (and possibly during the initial period after ACS without stenting). As this approach confers a fivefold increase in bleeding complications compared with DAPT, careful monitoring of the INR, the addition of PPIs, and the exclusion of elderly patients who are at the highest risk for bleeding complications74 is recommended. The preferred duration of triple therapy after BMS in patients who require long‐term anticoagulation is 1 month, whereas the optimal duration after ACS or DES remains unresolved.
Dual antiplatelet therapy (DAPT) (aspirin plus a thienopyridine: clopidogrel or prasugrel) has become the standard treatment for patients with acute coronary syndromes (ACS) and after coronary stent placement (Table 1). Anticoagulant therapy with warfarin is indicated for stroke prevention in atrial fibrillation (AF), profound left ventricular dysfunction, and after mechanical heart valve replacement, as well as for treatment of deep venous thrombosis and pulmonary embolism (Table 2). It is estimated that 41% of the U.S. population over age 40 years is on some form of antiplatelet therapy,6 and 2.5 million patients, mostly elderly, are on long‐term warfarin therapy.7 More specifically, 5% of patients undergoing percutaneous coronary interventions (PCIs) also have an indication for warfarin.8 With widespread use of drug‐eluting stents (DES), the need for a longer duration of DAPT, and the increased age and complexity of hospitalized patients, the safety and challenges of triple therapy (combined DAPT and warfarin) have become more important to the practice of hospital medicine. Triple therapy may increase hospitalization rates, as the risk of major bleeding is four to five times higher than with DAPT.911 In contrast, DAPT is much less effective than warfarin alone in preventing embolic events in AF,12 and warfarin alone or in combination with aspirin (ASA) is inadequate therapy to prevent stent thrombosis. Even fewer data exist on the efficacy and safety of triple therapy in patients with mechanical valves or left ventricular dysfunction.
| Class Recommendations | Level of Evidence | |
|---|---|---|
| ||
| DAPT after PCI/stenting1 | ||
| ASA | ||
| Class I | ASA 325 mg/d after PCI for 1 mo (up to 6 mo depending on type of stent implanted) and then 7562 mg/d indefinitely | B |
| Class IIa | ASA 75‐325 mg/d indefinitely after brachytherapy unless risk of bleeding is significant | C |
| In patients at risk of bleeding, a lower dose of 75‐162 mg/d is reasonable after stent implantation | C | |
| Thienopyridine | ||
| Class I | Clopidogrel 75 mg/d after BMS for at least 1 mo and ideally up to 12 mo unless increased risk of bleeding (at least 2 wk) | B |
| Clopidogrel 75 mg/d after DES for at least 12 mo if not at high risk for bleeding | B | |
| 2009 focus update2: Clopidogrel 75 mg daily or prasugrel 10 mg daily for at least 12 mo after BMS or DES for ACS | B | |
| Class IIa | Clopidogrel 75 mg/d indefinitely after brachytherapy unless risk of bleeding is significant | C |
| Class IIb | In patients with potential for lethal or catastrophic stent thrombosis, consider platelet aggregation studies and increase clopidogrel dose to 150 mg/d if 50% inhibition of platelet aggregation is seen | C |
| Continuation of clopidogrel 75 mg/day beyond 12 mo is reasonable after DES | C | |
| 2009 focus update2: consider continuation of clopidogrel or prasugrel beyond 15 mo after DES placement | C | |
| DAPT for UA/NSTEMI without stenting3 | ||
| ASA | ||
| Class I | Continue ASA (75 to 162 mg/d) indefinitely | A |
| Clopidogrel | ||
| Class 1 | Clopidogrel (75 mg/d) for at least 1 mo (A) and ideally for up to 1 y | B |
| Dipyridamole | ||
| Class III | Dipyridamole is not recommended because it has not been shown to be effective | A |
| |
| Condition | Risk (%) |
| Atrial fibrillation (without anticoagulation)4 | |
| Low‐risk atrial fibrillation (CHADS2 score 0) | 1.9 |
| Intermediate‐risk atrial fibrillation (CHADS2 score 1) | 2.8 |
| High‐risk atrial fibrillation (CHADS2 score 2‐6) | 418 |
| Mechanical heart valve5b | |
| Mechanical heart valve (without anticoagulation) | 8.6c |
| Mechanical heart valve (treated with ASA alone) | 7.5c |
| Mechanical heart valve (treated with warfarin) | 1.8c |
| Mechanical aortic valve (treated with warfarin) | 1.1c |
| Mechanical mitral valve (treated with warfarin) | 2.7c |
Hospitalists commonly care for patients on triple therapy; certain indications are appropriate and supported from the available literature while others lack evidence. Knowledge of existing practice guidelines and of supporting research studies leads to optimal management of these complicated patients, and minimizes excessive morbidity from bleeding complications or thromboembolic events such as strokes and stent thrombosis.
In the first part of this article, we present the evidence that supports current recommendations for DAPT or warfarin in specific medical conditions. We also address controversies and unanswered questions. The second part of this review focuses on the available data and provides guidance on the optimal care of patients on triple therapy.
Dual Antiplatelet Therapy Following Acute Coronary Syndromes
Table 3 summarizes key randomized trials of DAPT versus ASA alone in several clinical scenarios. The addition of clopidogrel to ASA in patients with nonST‐elevation ACS reduced the risk of adverse ischemic outcomes in the clopidogrel in unstable angina to prevent recurrent events (CURE) trial,15 as well as in its substudy, the PCI‐CURE (patients with ACS who have undergone stenting).17 In the main CURE study, the study groups diverged within the first 30 days after randomization and the benefit of DAPT persisted throughout the 12 months of the study period. DAPT is also superior to ASA in patients with ST‐elevation myocardial infarction (MI) (CLARITYTIMI 28 and COMMIT trials).13, 14 On the basis of these findings, DAPT has become the standard of care for patients with ACS. The American College of Cardiology (ACC)/American Heart Association (AHA)3 and the European Society of Cardiology18 recommend ASA treatment indefinitely for patients with ACS whether or not they underwent PCI. Clopidogrel is recommended for at least 12 months following ACS, especially for patients who receive a coronary stent.
| Trial | Endpoints | Results |
|---|---|---|
| ||
| ST elevation MI | ||
| CLARITY‐TIMI13 | Incidence of death, infarct‐related artery occlusion, or recurrent MI | 36% reduction (95% CI 2447); P .001 |
| COMMIT14 | Incidence of death, MI, or stroke | 9% reduction (95% CI 314); P .002 |
| ACS without ST elevation | ||
| CURE15 | Incidence of death, MI, or stroke | 20% reduction (RR 0.80 [0.720.90]); P .001 |
| Bare‐metal stent placement | ||
| CREDO16 | Incidence of death, MI, or stroke | 27% reduction (95% CI 3.944.4); P .02 |
| PCI‐CURE17 | Incidence of death, MI, or urgent TVR | 30% reduction (RR 0.70 [0.500.97]); P .03 |
Despite the proven efficacy of DAPT in ACS, about 15% of patients die or experience reinfarction within 30 days of diagnosis.19 The continued risk for thrombotic events could be due to delayed onset of platelet inhibition and to patient heterogeneity in responsiveness to therapy with ASA and/or clopidogrel.20 Consequently, the optimum dose for clopidogrel and ASA following ACS is uncertain. The CURRENT‐OASIS 7 trial evaluated the efficacy and safety of high‐dose clopidogrel (600‐mg loading dose, 150 mg once daily for 7 days, followed by 75 mg/d) versus standard‐dose clopidogrel (300‐mg loading dose, followed by 75 mg/d) and ASA (75‐100 mg versus 300‐325 mg/d) in patients with ACS who were treated medically, with or without stenting.21 In the overall study population as well as in patients who did not receive stenting, there was no significant difference in the combined rate of death from cardiovascular causes, MI, and stroke between patients receiving the high‐dose and the standard‐dose clopidogrel (4.2% vs 4.4%; P = .37) and high‐dose versus low‐dose ASA (4.2% vs 4.4%; P = .47). There were no significant differences in bleeding complications between the two clopidogrel treatment arms or between the high‐dose and low‐dose ASA groups.
The ACC/AHA guidelines recommend ASA, 75‐162 mg/d indefinitely after medical therapy without stenting (class I, level of evidence: A)3 and clopidogrel 75 mg/d for at least 1 month (class IA) and optimally for 1 year (class IB). Clopidogrel monotherapy is appropriate for patients with ACS who are unable to tolerate ASA due to either hypersensitivity or recent significant gastrointestinal bleeding.
As is the case after coronary stenting, interruption of DAPT soon after ACS may subject patients to high recurrence of cardiovascular events, although few data are available to support this observation. Interruption of DAPT due to bleeding complications or surgical procedures more than 1 month after ACS may be reasonable for a patient who did not receive a stent. Clinicians should restart DAPT after the surgical procedure once the bleeding risk becomes acceptable.
Dual Antiplatelet Therapy Following Coronary Stenting
Following Bare Metal Stents
Stent thrombosis occurs in approximately 20% of patients who receive bare metal stents (BMS) without DAPT22; the risk is highest in the first 30 days after implantation. The clinical presentation of stent thrombosis is often catastrophic: MI or sudden death occurs in over 60% of cases. DAPT reduces the incidence of stent thrombosis to a clinically acceptable level.22
In the ISAR trial of 517 patients treated with BMS for MI, suboptimal angioplasty, or other high‐risk clinical and anatomic features,23 patients were randomly assigned to treatment with ASA plus ticlopidine or ASA plus anticoagulation with heparin and warfarin. The primary endpoint of cardiac death, MI, coronary bypass surgery, or repeat angioplasty occurred in 1.5% of patients assigned to DAPT and 6.2% of those assigned to anticoagulant therapy (relative risk [RR], 0.25; 95% confidence interval [CI], 0.06‐0.77). The PCI‐CURE study evaluated patients who received BMS after ACS.17 The primary endpoint was a composite of cardiovascular death, MI, or urgent target‐vessel revascularization within 30 days of PCI. Long‐term administration of clopidogrel (8 months) conferred a lower rate of cardiovascular death, MI, or any revascularization (P = .03), with no significant difference in major bleeding between the groups (P = .64). In the CREDO trial,16 investigators evaluated 2116 patients undergoing PCI at 99 North American centers. Subjects received either a 300‐mg loading dose of clopidogrel or placebo 3‐24 hours before PCI. All patients then received clopidogrel 75 mg/d through day 28. For the following 12 months, patients in the loading dose group received clopidogrel, and those in the control group received placebo. All patients received ASA throughout the study. At 1 year, loading dose plus long‐term clopidogrel therapy conferred a 27% RR reduction (3% absolute risk reduction) in the combined endpoint of death, MI, or stroke (P = .02).
Based on these trials, the ACC and AHA recommend clopidogrel (75 mg/d) for a minimum of 1 month and optimally 12 months after BMS (class 1B).2 For patients at increased risk of bleeding, the ACC/AHA recommends a minimum of 2 weeks of clopidogrel. Although lifelong therapy with ASA is recommended, the optimal dose of ASA after BMS is unknown. However, on the basis of clinical trial protocols (no randomized data), guidelines recommend ASA 162 mg‐325 mg/d for at least 1 month, followed by indefinite use at a dose of 75‐162 mg. In patients for whom there is concern about bleeding, lower doses of ASA (75‐162 mg) are acceptable for the initial period after stent implantation.
Following Drug‐Eluting Stents
Drug‐eluting stents have become the standard percutaneous treatment for patients with symptomatic coronary artery disease. In 2005, a sampling of 140 US hospitals indicated that 94% of patients treated with a stent received at least one DES.24 Compared with BMS, restenosis and the need for revascularization are significantly less frequent. In contrast, unanticipated high rates of very late (>1 year) stent thrombosis have complicated DES.25 Because of the potentially lethal consequences of stent thrombosis, several authors have questioned the long‐term safety of DES2635 and examined the role of extended DAPT in reducing this delayed complication.27, 31, 36 Although the initial pivotal randomized trials of DES mandated clopidogrel use for only 3 months after sirolimus‐eluting stent and 6 months after paclitaxel‐eluting stent,37, 38 current guidelines recommend DAPT for at least 12 months after DES placement for patients who are not at high risk of bleeding.1
Although multiple studies have confirmed the benefit of DAPT, controversy remains regarding the extended use for more than 1 year. The only randomized trial that addressed this issue was nonblinded and underpowered.39 In this study of patients from two ongoing trials, the REAL‐LATE and ZEST‐LATE, extended duration DAPT (>12 months, median duration 19.2 months), did not reduce the incidence of MI and cardiac death.39 The rate of the primary endpoint was less than 25% of that expected (underpowered), and patients had already received clopidogrel for up to 24 months before enrollment.
The results from small, nonrandomized trials regarding this issue have been contradictory. Banerjee and colleagues studied 530 consecutive patients who underwent PCI (85% received a DES), were free of cardiovascular events for 6 months after PCI, and had follow‐up available for >12 months.26 In a multivariate analysis, clopidogrel use for 1 year was associated with lower mortality (hazard ratio [HR], 0.28; 95% CI, 0.140.59); this effect was independent of traditional cardiovascular risk factors, clinical presentation, and DES use. In a study at the Duke Heart Center40 among patients with DES (n = 528) who were event‐free at 12 months, continued clopidogrel use conferred lower rates of death (0% versus 3.5%; difference, 3.5%; 95% CI, 5.9% to 1.1%; P = .004) and death or MI (0% versus 4.5%; difference, 4.5%; 95% CI, 7.1% to 1.9%; P .001) at 24 months. In the TYCOON registry,35 patients with DES receiving clopidogrel for 2 years had a rate of stent thrombosis (0.4%) that was similar to those with BMS (0.7%) but significantly lower than patients with DES and 1‐year DAPT (2.9%).
In contrast, Roy and colleagues33 found that clopidogrel cessation at 12 months did not predict stent thrombosis, and Park and colleagues32 reported that clopidogrel continuation beyond 1 year did not appear to decrease stent thrombosis or clinical events after DES implantation. Similarly, Stone et al.34 performed a landmark analysis on the basis of the prospective, double‐blind TAXUS‐II SR, TAXUS‐IV, and TAXUS‐V trials. The authors found that thienopyridine use beyond 1 year after DES may reduce stent thrombosis over the subsequent 12‐month period, but did not reduce rates of death and MI at 2 and 5 years after either DES or BMS.
Current guidelines recommend ASA 162‐325 mg/d for at least 3‐6 months, followed by treatment indefinitely at a dose of 75‐162 mg daily. Clopidogrel, on the other hand, is given at 75 mg/d for at least 12 months.
Warfarin After Acute Coronary Syndromes
Warfarin with different international normalized ratio (INR) goals alone or in combination with ASA has been evaluated after ACS. In an early trial, patients with recent (mean interval 27 days) MI were treated with warfarin alone versus placebo.41 Warfarin conferred a relative risk reduction in mortality of 24% (95% CI, 4‐44%; P = .027) at the expense of major bleeding rates of 0.6%/y. In the ASPECT trial,42 moderate to high intensity anticoagulation after MI resulted in a 53% and 40% reduction in the relative risk of reinfarction (annual incidence 2.3% versus 5.1%) and cerebrovascular events (annual incidence 0.7% versus 1.2%), respectively. In the WARIS II43 and ASPECT‐244 trials, moderate intensity warfarin (INR 2.0‐2.5) in combination with low‐dose ASA, compared with ASA alone, reduced the composite occurrence of death or nonfatal reinfarction, as well as recurrent coronary occlusion after ST‐segment elevation MI. High‐intensity warfarin therapy alone (INR 3.0‐4.0 for ASPECT, 2.8‐4.2 for WARISII) reduced ischemic vascular events compared with ASA alone. Not unexpectedly, major bleeding episodes were more common among patients receiving warfarin.
No randomized trials have compared DAPT with warfarin plus ASA for patients with ACS who did not receive stents. The ACC/AHA guidelines recommend warfarin for secondary prevention following ACS (class IIb). High‐intensity warfarin alone (INR 2.5‐3.5) or moderate intensity (INR 2.0‐2.5) with low‐dose ASA (75‐81 mg/d) may be reasonable for patients at high ischemic and low bleeding risk who are intolerant of clopidogrel (level of evidence: B). Fixed dose warfarin is not recommended by the ACC/AHA primarily on the basis of the Coumadin Aspirin Reinfarction Study (CARS) results. This study of patients following MI was discontinued prematurely because of a lack of incremental benefit of reduced‐dose ASA (80 mg/d) combined with either 1 or 3 mg of warfarin daily when compared with 160 mg/d of ASA alone.
Triple Therapy for PCI and Atrial Fibrillation
AF is the most frequent indication (70%) for long‐term therapy with warfarin in patients scheduled for stent placement.10 Clinical trials have shown that warfarin alone is superior to ASA, clopidogrel, or DAPT for prevention of stroke in patients with AF.45, 46 Although warfarin is indispensable in these settings, DAPT is similarly necessary after stent implantation. As triple therapy increases the risk of bleeding, the management of patients with AF and who have received stents remains controversial. This situation is particularly problematic among patients who have received DES and may benefit from extended DAPT. No randomized trials exist to clarify the optimal treatment in these patients; and the feasibility of such studies is questionable. Small, mostly retrospective, studies (Table 4) provide limited guidance on this issue; most studies focus on bleeding events rather than the cardiovascular efficacy of triple therapy. Because of these limitations, cardiovascular societies give IIb recommendation for either triple therapy or the combination of warfarin and clopidogrel in this setting and the level of evidence is C.1, 59, 60
| Author | Year | Type | No. | Major Bleeding, % (range) | Thrombotic Events | Comments |
|---|---|---|---|---|---|---|
| ||||||
| Studies of one group (triple therapy group) | ||||||
| Orford et al.47 | 2004 | Obs | 66 | 4.5 (0.211.2) | N/A | Bleeding occurred only with suboptimal control of INR and/or pre‐existing GI disease. |
| Porter et al.48 | 2006 | Obs | 180 | 1.6 (0.04.2) | N/A | Bleeding rates were acceptable with short‐term TT after PCI. |
| Rubboli et al.49 | 2007 | Obs | 49 | 18 (4.436.9) | N/A | Most hemorrhages occurred during TT. |
| Rogacka et al.50 | 2008 | Obs | 127 | 4.7 | N/A | One‐half of bleeding episodes were lethal and 67% occurred within the first month. |
| Studies comparing triple therapy with dual antiplatelet therapy | ||||||
| Mattichak et al.51 | 2005 | Obs | 82 | 21 vs. 3.5 (P = .028)a | Reinfarction (29% vs. 9%, P = .15) | TT did not reduce reinfarction after stenting for MI but increased rates of GI bleeding and transfusions. |
| Khurram et al.11 | 2006 | Matched cohort | 214 | 6.6 vs. 0 (P = .03) | N/A | Higher bleeding rates for TT than DAPT. INR range or ASA dosage did not influence the bleeding risk. |
| DeEugenio et al.9 | 2007 | Matched cohort | 194 | OR 5.0 (1.417.8, P = .012) | N/A | ASA dose, age, sex, BMI, DM, hypertension, and procedural anticoagulant type or use did not influence risk of major bleeding. |
| Ruiz‐Nodar et al.52 | 2008 | Obs | 426 | 14.9 vs. 9.0 (P = .19) | Mortality: OR 3.43 (1.617.54, P = .002)b MACE: OR 4.9 (2.1711.1, P .01)b | TT was associated with a nonsignificant increase in major bleeding but lower all‐cause mortality and fewer MACE. |
| Sarafoff et al.53 | 2008 | Prosp | 515 | 1.4 vs. 3.1 (P = .34). | MACCE: OR 0.76 (0.481.21, P = .25) | No difference in MACCE or bleeding at 2 y. Stent thrombosis did not differ between groups. |
| Rossini et al.54 | 2008 | Prosp | 204 | 10.8 vs. 4.9 (P = .1) | MACE: 5.8% vs. 4.9% (P = .7) | INR was targeted to the lower range (2.0‐2.5). No significant difference in bleeding rates for TT versus DAPT at 18 mo. Less bleeding for patients whose INR was within target (4.9 versus 33%, P = .00019). No significant differences in MACE between groups. |
| Uchida et al.55 | 2010 | Obs | 575 | 18 vs. 2.7 (P .001) | MACE (P = .108) | No differences in MACE rates. More bleeding for patients on TT. |
| Studies comparing triple therapy versus dual antiplatelet therapy versus wararin and single antiplatelet agent | ||||||
| Karjalainen et al.10 | 2007 | Matched cohort | 239 | OR 3.3 (1.38.6, P = .014)c | MACE: OR 1.7 (1.0‐3.0, P = 0.05)c | This study compared patients on warfarin at baseline with those not on warfarinall undergoing stenting. Patients on warfarin at baseline were treated with a variety of strategies. Baseline warfarin use increased both major bleeding and MACE at 1 y. ASA plus warfarin was inadequate to prevent stent thrombosis, and premature warfarin cessation was associated with stroke. |
| Manzano‐Fernandez et al.56 | 2008 | Obs | 104 | EB (5.8 vs. 11.3, P = .33) LB (21.6 vs. 3.8, P = .006)d | MACE: 25.5% vs. 21.0% (P = .53)d | No difference in MACE rates between TT and non‐TT (WAA or DAPT). TT conferred higher late bleeding (>48 h). |
| Gao et al.57 | 2010 | Prosp | 622 | 2.9 vs. 1.8 vs. 2.5 (P = .725)e | MACCE: 8.8% vs. 20.1% vs. 14.9% (P = .010)e | Target INR was set as 1.8‐2.5. Lower stroke and MACCE rates for TT as compared with DAPT or WAA; no difference in bleeding. |
| Studies comparing triple therapy with warfarin and single antiplatelet agent | ||||||
| Nguyen et al.58 | 2007 | Obs | 800 | 5.9 vs. 46 (P = .46) | Death: 5.1% vs. 6.5% (P = .47) Stroke: 0.7% vs. 3.4% (P = .02) MI: 3.3% vs. 4.5% (P = .49) | TT and WAA lead to similar 6‐mo bleeding, death, and MI. Fewer strokes with TT (caveat: low event rate). |
In the largest study to date, Nguyen et al.58 evaluated 800 patients who underwent stenting for ACS and were discharged on warfarin plus single antiplatelet agent or triple therapy as part of the GRACE registry. At 6 months, triple therapy conferred a significant reduction in stroke (0.7% versus 3.4%, P = .02) but not in death or MI. There were no differences in in‐hospital major bleeding events between the two groups (5.9% versus 4.6%; P = .46). Similarly, Sarafoff et al.53 reported no significant differences in the combined endpoint (death, MI, stent thrombosis or stroke) or bleeding complications among patients who received triple therapy or DAPT at 2 years of follow‐up. In contrast, Ruiz‐Nodar et al.52 showed that triple therapy, compared with DAPT, at discharge reduced the incidence of death (17.8% versus 27.8%; adjusted HR = 3.43; 95% CI, 1.617.54; P = .002) and major adverse cardiac events (26.5% versus 38.7%; adjusted HR = 4.9; 95% CI, 2.1711.1; P = .01), without a substantial increase in major bleeding events.
The value of combination antiplatelet therapy to prevent stent thrombosis in these patients is clearer in the study reported by Karjalainen et al.10 This case‐control study of 239 patients receiving warfarin at baseline who underwent PCI evaluated a primary endpoint of death, MI, target‐vessel revascularization, or stent thrombosis and a secondary endpoint of major bleeding and stroke to 12 months of follow‐up. Forty‐eight percent of patients received triple therapy, whereas 15.5% were discharged on DAPT. The remaining patients received warfarin plus a single antiplatelet agent. Stent thrombosis occurred more frequently among patients receiving warfarin plus ASA (15.2%) than among those receiving triple therapy (1.9%). As expected, stroke was more frequent in patients treated with DAPT (8.8%) than among those receiving triple therapy (2.8%). Major bleeding was similar between groups. Therapy with warfarin was an independent predictor of both major bleeding and major cardiac events at 1 year. This observation illustrates that the outcome of PCI in patients on chronic warfarin therapy is unsatisfactory irrespective of the antithrombotic combinations used, highlighting the need for better strategies to treat these patients.
Choice of Therapy and Management of Patients Eligible for Triple Therapy
Current guidelines for PCI do not provide guidance for patients with an indication for triple therapy due to a paucity of published evidence. Several ongoing prospective trials aim to address the management of these patients (AFCAS, ISAR‐TRIPLE). Pending further study, clinicians should consider the embolic risk (CHADS2 score), target INR, type of stent, bleeding risk, and duration of treatment when determining the appropriate antiplatelet/anticoagulant combinations. The CHADS2 score (Table 2) stratifies the risk for stroke among patients with AF,4 while the Outpatient Bleeding Risk Index (OBRI) allows estimation of bleeding risk.12, 61 The OBRI considers age > 65 years, prior stroke, prior gastrointestinal bleeding, and any of four comorbidities (recent MI, anemia, diabetes, or renal insufficiency) in order to stratify patients into three risk groups.61 Patients with three to four risk factors have a high risk of bleeding (23% at 3 months and 48% at 12 months) whereas patients with no risk factors have only a 3% risk of bleeding at 12 months. Unfortunately, advanced age and prior stroke appear in both OBRI and CHADS.
For patients with AF who are at high risk for embolic stroke (>3% per year), we recommend triple therapy for the shortest time possible, followed by warfarin and ASA indefinitely. In case of BMS, it is acceptable to shorten triple therapy duration to 1 month. The optimal duration of triple therapy for patients with DES is uncertain; recommended durations range from 3 months to 1 year.62 If the potential consequences of stent thrombosis are high due to a large amount of myocardium at risk, an extended period of triple therapy might be justified. For patients whose stroke risk is lower (CHADS2 score of 0‐1), the risk for bleeding likely outweighs any benefit from stroke prevention. In this instance, it is reasonable to use DAPT with ASA and clopidogrel for 1 month after BMS and 12 months after DES, followed by ASA, with or without warfarin, indefinitely. In a recently published study, patients with AF and a CHADS2 score of 1 had a yearly stroke risk of 1.25% while taking DAPT63; the risk of major bleeding for triple therapy is 6.1% per year.64
For patients who have a high bleeding risk, BMS are the preferred stent type as the duration of triple therapy might be limited to 4 weeks. To our knowledge, no randomized study has evaluated the outcome of patients with BMS compared with DES who also have an indication for warfarin. Because studies have suggested that clopidogrel is more effective than aspirin in preventing stent thrombosis and in reducing death or MI after coronary stenting,40, 65 warfarin and single antiplatelet therapy with clopidogrel might be a reasonable treatment option in patients with high bleeding risk. The WOEST study (NCT00769938), currently recruiting participants, is the first randomized study specifically designed to test this hypothesis.
Since gastrointestinal bleeding accounts for approximately 30‐40% of hemorrhagic events in patients on combined ASA and anticoagulant therapy, an expert consensus document recommended concomitant treatment with proton pump inhibitors (PPIs) to reduce this risk.66 In contrast, the 2009 Focused Updates of the ACC/AHA/SCAI Guidelines did not recommend the use of PPIs with DAPT in the setting of ACS.2 This is because of studies that show inhibition of platelet activation,67 and potential clinical harm,68 when clopidogrel is combined with certain PPIs that inhibit the CYP2C19 enzyme. However, to date there are no convincing randomized clinical trial data documenting an important clinical drug‐drug interaction. The U.S. Food and Drug Administration (FDA) advises that physicians avoid the use of clopidogrel in patients with impaired CYP2C19 function due to known genetic variation or due to concomitant use of drugs that inhibit CYP2C19 activity. More specifically, the FDA recommends avoiding the use of omeprazole and esomeprazole in patients taking clopidogrel.69
In particular, elderly patients have an increased risk of bleeding while receiving triple therapy. In a study of patients over age 65, 2.5% were hospitalized for bleeding in the first year after PCI, and the use of triple therapy was the strongest predictor of bleeding (more than threefold increase).70 One in five patients suffered death or MI at 1 year after hospitalization for bleeding.70 The basis for poor outcomes after hospitalization for bleeding in this population is multifactorial and may be due to the location of bleeding, associated hypercoagulable state, potential adverse impact of blood transfusion, withdrawal of warfarin therapy in patients with AF and PCI, and the premature discontinuation of DAPT. The use of nonsteroidal anti‐inflammatory drugs (NSAIDs) is common among the elderly and conferred a doubling of bleeding risk.70 Limiting the use of NSAID, the use of low‐dose ASA beyond 30 days after stent implantation, greater use of BMS, and maintaining INR at the lowest possible level (INR of 22.5) will reduce the risk for bleeding.57, 71
New Anticoagulants
Due to the high risk for bleeding with warfarin and the challenges inherent in INR monitoring, researchers have developed several novel anticoagulants whose advantages include fixed daily dosing and no need for monitoring. Dabigatran is a direct oral thrombin inhibitor that is already licensed in Europe and Canada for thromboprophylaxis after hip or knee surgery. It has also been studied in patients with AF. In the RE‐LY trial, patients with AF who received dabigatran 110 mg daily had rates of stroke and systemic embolism that were similar to those with warfarin, as well as lower rates of major hemorrhage.72 The randomized ReDEEM trial, reported at the AHA 2009 Scientific Sessions, was aimed at finding a dosage of dabigatran that achieves a good balance between clinical effectiveness and bleeding risk when combined with aspirin and clopidogrel after acute MI. Dosages ranging from 50 mg twice daily to 150 mg twice daily were all associated with 6‐month rates of bleeding lower than 2%. Hospitalists should view these encouraging results cautiously until the publication of ReDEEM trial results in a peer‐reviewed journal.
A variety of oral Xa antagonists are also being evaluated in patients with AF or ACS. These trials offer insight into triple therapy regimens that include ASA, clopidogrel, and an Xa antagonist. In a recent study of the oral Xa antagonist rivaroxaban, investigators stratified 3491 subjects with ACS according to whether they received concomitant ASA alone or ASA and clopidogrel.73 Subjects receiving ASA plus rivaroxaban had a modest increase in bleeding. Triple therapy, however, increased the composite bleeding rate from 3.5% in the DAPT group to approximately 6‐15% (low‐dose or high‐dose rivaroxaban, respectively). Rivaroxaban is currently under review by the FDA.
These novel agents might eventually replace warfarin for many or most indications for anticoagulation. It is imperative that future research compare the efficacy and risk of bleeding between triple therapy using these new agents and triple therapy with warfarin.
Conclusions
The management of patients on long‐term anticoagulation who require DAPT because of ACS or coronary stenting is challenging. DAPT may safely substitute for warfarin only for patients at low risk for a thromboembolic event (ie, low‐risk AF with low CHADS2 score). Clinicians should not interrupt warfarin in patients at higher risk (ie, intermediate to high‐risk AF, mechanical valves, or recent venous thromboembolism), even in the presence of DAPT. In these patients, triple therapy is the optimal approach following coronary stenting (and possibly during the initial period after ACS without stenting). As this approach confers a fivefold increase in bleeding complications compared with DAPT, careful monitoring of the INR, the addition of PPIs, and the exclusion of elderly patients who are at the highest risk for bleeding complications74 is recommended. The preferred duration of triple therapy after BMS in patients who require long‐term anticoagulation is 1 month, whereas the optimal duration after ACS or DES remains unresolved.
- ,,, et al.2007 Focused Update of the ACC/AHA/SCAI 2005 Guideline Update for Percutaneous Coronary Intervention: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines: 2007 Writing Group to Review New Evidence and Update the ACC/AHA/SCAI 2005 Guideline Update for Percutaneous Coronary Intervention, Writing on Behalf of the 2005 Writing Committee.Circulation2008;117:261–295.
- ,,, et al.2009 Focused Updates: ACC/AHA Guidelines for the Management of Patients With ST‐Elevation Myocardial Infarction (updating the 2004 Guideline and 2007 Focused Update) and ACC/AHA/SCAI Guidelines on Percutaneous Coronary Intervention (updating the 2005 Guideline and 2007 Focused Update): a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines.Circulation2009;120:2271–2306.
- ,,, et al.ACC/AHA 2007 guidelines for the management of patients with unstable angina/non‐ST‐Elevation myocardial infarction: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the 2002 Guidelines for the Management of Patients With Unstable Angina/Non‐ST‐Elevation Myocardial Infarction) developed in collaboration with the American College of Emergency Physicians, the Society for Cardiovascular Angiography and Interventions, and the Society of Thoracic Surgeons endorsed by the American Association of Cardiovascular and Pulmonary Rehabilitation and the Society for Academic Emergency Medicine.J Am Coll Cardiol2007;50:e1–e157.
- ,,, et al.Selecting patients with atrial fibrillation for anticoagulation: stroke risk stratification in patients taking aspirin.Circulation2004;110:2287–2292.
- ,,.Thromboembolic and bleeding complications in patients with mechanical heart valve prostheses.Circulation1994;89:635–641.
- ,,,,.Aspirin use among adults aged 40 and older in the United States: results of a national survey.Am J Prev Med2007;32:403–407.
- ,,, et al.The perioperative management of antithrombotic therapy: American College of Chest Physicians Evidence‐Based Clinical Practice Guidelines (8th Edition).Chest2008;133:299S–339S.
- ,,, et al.Discharge antithrombotic strategies among patients with acute coronary syndrome previously on warfarin anticoagulation: physician practice in the CRUSADE registry.Am Heart J2008;155:361–368.
- ,,, et al.Risk of major bleeding with concomitant dual antiplatelet therapy after percutaneous coronary intervention in patients receiving long‐term warfarin therapy.Pharmacotherapy2007;27:691–696.
- ,,, et al.Safety and efficacy of combined antiplatelet‐warfarin therapy after coronary stenting.Eur Heart J2007;28:726–732.
- ,,, et al.Combination therapy with aspirin, clopidogrel and warfarin following coronary stenting is associated with a significant risk of bleeding.J Invasive Cardiol2006;18:162–164.
- ,,,,,.Development of a contemporary bleeding risk model for elderly warfarin recipients.Chest2006;130:1390–1396.
- ,,, et al.Addition of clopidogrel to aspirin and fibrinolytic therapy for myocardial infarction with ST‐segment elevation.N Engl J Med2005;352:1179–1189.
- ,,, et al.Addition of clopidogrel to aspirin in 45,852 patients with acute myocardial infarction: randomised placebo‐controlled trial.Lancet2005;366:1607–1621.
- ,,,,,.Effects of clopidogrel in addition to aspirin in patients with acute coronary syndromes without ST‐segment elevation.N Engl J Med2001;345:494–502.
- ,,, et al.Early and sustained dual oral antiplatelet therapy following percutaneous coronary intervention: a randomized controlled trial.JAMA2002;288:2411–2420.
- ,,, et al.Effects of pretreatment with clopidogrel and aspirin followed by long‐term therapy in patients undergoing percutaneous coronary intervention: the PCI‐CURE study.Lancet2001;358:527–533.
- ,,, et al.Guidelines for the diagnosis and treatment of non‐ST‐segment elevation acute coronary syndromes.Eur Heart J2007;28:1598–1660.
- .Burden of disease: medical and economic impact of acute coronary syndromes.Am J Manag Care2006;12:S430–S434.
- ,,,,,.Variability in platelet responsiveness to clopidogrel among 544 individuals.J Am Coll Cardiol2005;45:246–251.
- ,,, et al.Dose comparisons of clopidogrel and aspirin in acute coronary syndromes.N Engl J Med2010;363:930–942.
- ,,, et al.A clinical trial comparing three antithrombotic‐drug regimens after coronary‐artery stenting. Stent Anticoagulation Restenosis Study Investigators.N Engl J Med1998;339:1665–1671.
- ,,, et al.A randomized comparison of antiplatelet and anticoagulant therapy after the placement of coronary‐artery stents.N Engl J Med1996;334:1084–1089.
- ,,.Outcomes of 6906 patients undergoing percutaneous coronary intervention in the era of drug‐eluting stents: report of the DEScover Registry.Circulation2006;114:2154–2162.
- ,,, et al.Safety and efficacy of sirolimus‐ and paclitaxel‐eluting coronary stents.N Engl J Med2007;356:998–1008.
- ,,, et al.Comparison of the impact of short (1 year) and long‐term (> or =1 year) clopidogrel use following percutaneous coronary intervention on mortality.Am J Cardiol2008;102:1159–1162.
- ,,.Stent thrombosis late after implantation of first‐generation drug‐eluting stents: a cause for concern.Circulation2007;115:1440–1455; discussion 55.
- ,,, et al.Early and late coronary stent thrombosis of sirolimus‐eluting and paclitaxel‐eluting stents in routine clinical practice: data from a large two‐institutional cohort study.Lancet2007;369:667–678.
- ,,, et al.Analysis of 14 trials comparing sirolimus‐eluting stents with bare‐metal stents.N Engl J Med2007;356:1030–1039.
- ,,,,,.Long‐term outcomes with drug‐eluting stents versus bare‐metal stents in Sweden.N Engl J Med2007;356:1009–1019.
- ,,,,,.Stent thrombosis in randomized clinical trials of drug‐eluting stents.N Engl J Med2007;356:1020–1029.
- ,,, et al.Stent thrombosis, clinical events, and influence of prolonged clopidogrel use after placement of drug‐eluting stent data from an observational cohort study of drug‐eluting versus bare‐metal stents.JACC Cardiovasc Interv2008;1:494–503.
- ,,, et al.Temporal relation between Clopidogrel cessation and stent thrombosis after drug‐eluting stent implantation.Am J Cardiol2009;103:801–805.
- ,,, et al.Effect of prolonged thienopyridine use after drug‐eluting stent implantation (from the TAXUS landmark trials data).Am J Cardiol2008;102:1017–1022.
- ,,, et al.Effectiveness of two‐year clopidogrel + aspirin in abolishing the risk of very late thrombosis after drug‐eluting stent implantation (from the TYCOON [two‐year ClOpidOgrel need] study).Am J Cardiol2009;104:1357–1361.
- ,,.Mortality in randomized controlled trials comparing drug‐eluting vs. bare metal stents in coronary artery disease: a meta‐analysis.Eur Heart J2006;27:2784–2814.
- ,,, et al.Sirolimus‐eluting stents versus standard stents in patients with stenosis in a native coronary artery.N Engl J Med2003;349:1315–1323.
- ,,, et al.A polymer‐based, paclitaxel‐eluting stent in patients with coronary artery disease.N Engl J Med2004;350:221–231.
- ,,, et al.Duration of dual antiplatelet therapy after implantation of drug‐eluting stents.N Engl J Med2010;362:1374–1382.
- ,,, et al.Clopidogrel use and long‐term clinical outcomes after drug‐eluting stent implantation.JAMA2007;297:159–168.
- ,,.The effect of warfarin on mortality and reinfarction after myocardial infarction.N Engl J Med1990;323:147–152.
- Effect of long‐term oral anticoagulant treatment on mortality and cardiovascular morbidity after myocardial infarction.Anticoagulants in the Secondary Prevention of Events in Coronary Thrombosis (ASPECT) Research Group.Lancet1994;343:499–503.
- ,,,,.Warfarin, aspirin, or both after myocardial infarction.N Engl J Med2002;347:969–974.
- ,,,,.Aspirin and coumadin after acute coronary syndromes (the ASPECT‐2 study): a randomised controlled trial.Lancet2002;360:109–113.
- ,,, et al.Clopidogrel plus aspirin versus oral anticoagulation for atrial fibrillation in the Atrial fibrillation Clopidogrel Trial with Irbesartan for prevention of Vascular Events (ACTIVE W): a randomised controlled trial.Lancet2006;367:1903–1912.
- ,,,.Antithrombotic therapy to prevent stroke in patients with atrial fibrillation: a meta‐analysis.Ann Intern Med1999;131:492–501.
- ,,, et al.Safety and efficacy of aspirin, clopidogrel, and warfarin after coronary stent placement in patients with an indication for anticoagulation.Am Heart J2004;147:463–467.
- ,,,,,.Short‐term triple therapy with aspirin, warfarin, and a thienopyridine among patients undergoing percutaneous coronary intervention.Catheter Cardiovasc Interv2006;68:56–61.
- ,,,,.Periprocedural and medium‐term antithrombotic strategies in patients with an indication for long‐term anticoagulation undergoing coronary angiography and intervention.Coron Artery Dis2007;18:193–199.
- ,,, et al.Dual antiplatelet therapy after percutaneous coronary intervention with stent implantation in patients taking chronic oral anticoagulation.JACC Cardiovasc Interv2008;1:56–61.
- ,,,,,.Evaluation of safety of warfarin in combination with antiplatelet therapy for patients treated with coronary stents for acute myocardial infarction.J Interv Cardiol2005;18:163–166.
- ,,, et al.Anticoagulant and antiplatelet therapy use in 426 patients with atrial fibrillation undergoing percutaneous coronary intervention and stent implantation implications for bleeding risk and prognosis.J Am Coll Cardiol2008;51:818–825.
- ,,, et al.Aspirin and clopidogrel with or without phenprocoumon after drug eluting coronary stent placement in patients on chronic oral anticoagulation.J Intern Med2008;264:472–480.
- ,,, et al.Long‐term outcomes in patients undergoing coronary stenting on dual oral antiplatelet treatment requiring oral anticoagulant therapy.Am J Cardiol2008;102:1618–1623.
- ,,,,.Impact of anticoagulant therapy with dual antiplatelet therapy on prognosis after treatment with drug‐eluting coronary stents.J Cardiol2010;55:362–369.
- ,,, et al.Increased major bleeding complications related to triple antithrombotic therapy usage in patients with atrial fibrillation undergoing percutaneous coronary artery stenting.Chest2008;134:559–567.
- ,,, et al.Comparison of different antithrombotic regimens for patients with atrial fibrillation undergoing drug‐eluting stent implantation.Circ J2010;74:701–708.
- ,,, et al.Combining warfarin and antiplatelet therapy after coronary stenting in the Global Registry of Acute Coronary Events: is it safe and effective to use just one antiplatelet agent?Eur Heart J2007;28:1717–1722.
- ,,, et al.2007 focused update of the ACC/AHA 2004 guidelines for the management of patients with ST‐elevation myocardial infarction: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines.J Am Coll Cardiol2008;51:210–247.
- ,,, et al.Guidelines for percutaneous coronary interventions. The Task Force for Percutaneous Coronary Interventions of the European Society of Cardiology.Eur Heart J2005;26:804–847.
- ,,.Prospective evaluation of an index for predicting the risk of major bleeding in outpatients treated with warfarin.Am J Med1998;105:91–99.
- ,,,.Coronary stents and chronic anticoagulation.Circulation2009;119:1682–1688.
- ,,, et al.Risks and benefits of oral anticoagulation compared with clopidogrel plus aspirin in patients with atrial fibrillation according to stroke risk: the atrial fibrillation clopidogrel trial with irbesartan for prevention of vascular events (ACTIVE‐W).Stroke2008;39:1482–1486.
- ,,, et al.Early and late increased bleeding rates after angioplasty and stenting due to combined antiplatelet and anticoagulanttherapy.EuroIntervention2009;5:425–431.
- ,,, et al.Late clinical events after clopidogrel discontinuation may limit the benefit of drug‐eluting stents: an observational study of drug‐eluting versus bare‐metal stents.J Am Coll Cardiol2006;48:2584–2591.
- ,,, et al.ACCF/ACG/AHA 2008 expert consensus document on reducing the gastrointestinal risks of antiplatelet therapy and NSAID use: a report of the American College of Cardiology Foundation Task Force on Clinical Expert Consensus Documents.Circulation2008;118:1894–1909.
- ,,, et al.Influence of omeprazole on the antiplatelet action of clopidogrel associated with aspirin: the randomized, double‐blind OCLA (Omeprazole CLopidogrel Aspirin) study.J Am Coll Cardiol2008;51:256–260.
- ,,, et al.Risk of adverse outcomes associated with concomitant use of clopidogrel and proton pump inhibitors following acute coronary syndrome.JAMA2009;301:937–944.
- Follow‐Up to the January 26,2009, Early Communication about an Ongoing Safety Review of Clopidogrel Bisulfate (marketed as Plavix) and Omeprazole (marketed as Prilosec and Prilosec OTC). 11/17/2009. (Accessed at http://www.fda.gov/Drugs/DrugSafety/Postmarket DrugSafetyInformationforPatientsandProviders/DrugSafetyInformationfor HeathcareProfessionals/ucm190784.htm.)
- ,,, et al.Incidence, predictors, and prognostic implications of hospitalization for late bleeding after percutaneous coronary intervention for patients older than 65 years.Circ Cardiovasc Interv2010;3:140–147.
- ,,, et al.Antithrombotic therapy in patients treated with oral anticoagulation undergoing coronary artery stenting. An expert consensus document with focus on atrial fibrillation.Ann Med2008;40:428–436.
- ,,, et al.Dabigatran versus warfarin in patients with atrial fibrillation.N Engl J Med2009;361:1139–1151.
- ,,, et al.Rivaroxaban versus placebo in patients with acute coronary syndromes (ATLAS ACS‐TIMI 46): a randomised, double‐blind, phase II trial.Lancet2009;374:29–38.
- ,,,.Bleeding complications associated with combinations of aspirin, thienopyridine derivatives, and warfarin in elderly patients following acute myocardial infarction.Arch Intern Med2005;165:784–789.
- ,,, et al.2007 Focused Update of the ACC/AHA/SCAI 2005 Guideline Update for Percutaneous Coronary Intervention: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines: 2007 Writing Group to Review New Evidence and Update the ACC/AHA/SCAI 2005 Guideline Update for Percutaneous Coronary Intervention, Writing on Behalf of the 2005 Writing Committee.Circulation2008;117:261–295.
- ,,, et al.2009 Focused Updates: ACC/AHA Guidelines for the Management of Patients With ST‐Elevation Myocardial Infarction (updating the 2004 Guideline and 2007 Focused Update) and ACC/AHA/SCAI Guidelines on Percutaneous Coronary Intervention (updating the 2005 Guideline and 2007 Focused Update): a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines.Circulation2009;120:2271–2306.
- ,,, et al.ACC/AHA 2007 guidelines for the management of patients with unstable angina/non‐ST‐Elevation myocardial infarction: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the 2002 Guidelines for the Management of Patients With Unstable Angina/Non‐ST‐Elevation Myocardial Infarction) developed in collaboration with the American College of Emergency Physicians, the Society for Cardiovascular Angiography and Interventions, and the Society of Thoracic Surgeons endorsed by the American Association of Cardiovascular and Pulmonary Rehabilitation and the Society for Academic Emergency Medicine.J Am Coll Cardiol2007;50:e1–e157.
- ,,, et al.Selecting patients with atrial fibrillation for anticoagulation: stroke risk stratification in patients taking aspirin.Circulation2004;110:2287–2292.
- ,,.Thromboembolic and bleeding complications in patients with mechanical heart valve prostheses.Circulation1994;89:635–641.
- ,,,,.Aspirin use among adults aged 40 and older in the United States: results of a national survey.Am J Prev Med2007;32:403–407.
- ,,, et al.The perioperative management of antithrombotic therapy: American College of Chest Physicians Evidence‐Based Clinical Practice Guidelines (8th Edition).Chest2008;133:299S–339S.
- ,,, et al.Discharge antithrombotic strategies among patients with acute coronary syndrome previously on warfarin anticoagulation: physician practice in the CRUSADE registry.Am Heart J2008;155:361–368.
- ,,, et al.Risk of major bleeding with concomitant dual antiplatelet therapy after percutaneous coronary intervention in patients receiving long‐term warfarin therapy.Pharmacotherapy2007;27:691–696.
- ,,, et al.Safety and efficacy of combined antiplatelet‐warfarin therapy after coronary stenting.Eur Heart J2007;28:726–732.
- ,,, et al.Combination therapy with aspirin, clopidogrel and warfarin following coronary stenting is associated with a significant risk of bleeding.J Invasive Cardiol2006;18:162–164.
- ,,,,,.Development of a contemporary bleeding risk model for elderly warfarin recipients.Chest2006;130:1390–1396.
- ,,, et al.Addition of clopidogrel to aspirin and fibrinolytic therapy for myocardial infarction with ST‐segment elevation.N Engl J Med2005;352:1179–1189.
- ,,, et al.Addition of clopidogrel to aspirin in 45,852 patients with acute myocardial infarction: randomised placebo‐controlled trial.Lancet2005;366:1607–1621.
- ,,,,,.Effects of clopidogrel in addition to aspirin in patients with acute coronary syndromes without ST‐segment elevation.N Engl J Med2001;345:494–502.
- ,,, et al.Early and sustained dual oral antiplatelet therapy following percutaneous coronary intervention: a randomized controlled trial.JAMA2002;288:2411–2420.
- ,,, et al.Effects of pretreatment with clopidogrel and aspirin followed by long‐term therapy in patients undergoing percutaneous coronary intervention: the PCI‐CURE study.Lancet2001;358:527–533.
- ,,, et al.Guidelines for the diagnosis and treatment of non‐ST‐segment elevation acute coronary syndromes.Eur Heart J2007;28:1598–1660.
- .Burden of disease: medical and economic impact of acute coronary syndromes.Am J Manag Care2006;12:S430–S434.
- ,,,,,.Variability in platelet responsiveness to clopidogrel among 544 individuals.J Am Coll Cardiol2005;45:246–251.
- ,,, et al.Dose comparisons of clopidogrel and aspirin in acute coronary syndromes.N Engl J Med2010;363:930–942.
- ,,, et al.A clinical trial comparing three antithrombotic‐drug regimens after coronary‐artery stenting. Stent Anticoagulation Restenosis Study Investigators.N Engl J Med1998;339:1665–1671.
- ,,, et al.A randomized comparison of antiplatelet and anticoagulant therapy after the placement of coronary‐artery stents.N Engl J Med1996;334:1084–1089.
- ,,.Outcomes of 6906 patients undergoing percutaneous coronary intervention in the era of drug‐eluting stents: report of the DEScover Registry.Circulation2006;114:2154–2162.
- ,,, et al.Safety and efficacy of sirolimus‐ and paclitaxel‐eluting coronary stents.N Engl J Med2007;356:998–1008.
- ,,, et al.Comparison of the impact of short (1 year) and long‐term (> or =1 year) clopidogrel use following percutaneous coronary intervention on mortality.Am J Cardiol2008;102:1159–1162.
- ,,.Stent thrombosis late after implantation of first‐generation drug‐eluting stents: a cause for concern.Circulation2007;115:1440–1455; discussion 55.
- ,,, et al.Early and late coronary stent thrombosis of sirolimus‐eluting and paclitaxel‐eluting stents in routine clinical practice: data from a large two‐institutional cohort study.Lancet2007;369:667–678.
- ,,, et al.Analysis of 14 trials comparing sirolimus‐eluting stents with bare‐metal stents.N Engl J Med2007;356:1030–1039.
- ,,,,,.Long‐term outcomes with drug‐eluting stents versus bare‐metal stents in Sweden.N Engl J Med2007;356:1009–1019.
- ,,,,,.Stent thrombosis in randomized clinical trials of drug‐eluting stents.N Engl J Med2007;356:1020–1029.
- ,,, et al.Stent thrombosis, clinical events, and influence of prolonged clopidogrel use after placement of drug‐eluting stent data from an observational cohort study of drug‐eluting versus bare‐metal stents.JACC Cardiovasc Interv2008;1:494–503.
- ,,, et al.Temporal relation between Clopidogrel cessation and stent thrombosis after drug‐eluting stent implantation.Am J Cardiol2009;103:801–805.
- ,,, et al.Effect of prolonged thienopyridine use after drug‐eluting stent implantation (from the TAXUS landmark trials data).Am J Cardiol2008;102:1017–1022.
- ,,, et al.Effectiveness of two‐year clopidogrel + aspirin in abolishing the risk of very late thrombosis after drug‐eluting stent implantation (from the TYCOON [two‐year ClOpidOgrel need] study).Am J Cardiol2009;104:1357–1361.
- ,,.Mortality in randomized controlled trials comparing drug‐eluting vs. bare metal stents in coronary artery disease: a meta‐analysis.Eur Heart J2006;27:2784–2814.
- ,,, et al.Sirolimus‐eluting stents versus standard stents in patients with stenosis in a native coronary artery.N Engl J Med2003;349:1315–1323.
- ,,, et al.A polymer‐based, paclitaxel‐eluting stent in patients with coronary artery disease.N Engl J Med2004;350:221–231.
- ,,, et al.Duration of dual antiplatelet therapy after implantation of drug‐eluting stents.N Engl J Med2010;362:1374–1382.
- ,,, et al.Clopidogrel use and long‐term clinical outcomes after drug‐eluting stent implantation.JAMA2007;297:159–168.
- ,,.The effect of warfarin on mortality and reinfarction after myocardial infarction.N Engl J Med1990;323:147–152.
- Effect of long‐term oral anticoagulant treatment on mortality and cardiovascular morbidity after myocardial infarction.Anticoagulants in the Secondary Prevention of Events in Coronary Thrombosis (ASPECT) Research Group.Lancet1994;343:499–503.
- ,,,,.Warfarin, aspirin, or both after myocardial infarction.N Engl J Med2002;347:969–974.
- ,,,,.Aspirin and coumadin after acute coronary syndromes (the ASPECT‐2 study): a randomised controlled trial.Lancet2002;360:109–113.
- ,,, et al.Clopidogrel plus aspirin versus oral anticoagulation for atrial fibrillation in the Atrial fibrillation Clopidogrel Trial with Irbesartan for prevention of Vascular Events (ACTIVE W): a randomised controlled trial.Lancet2006;367:1903–1912.
- ,,,.Antithrombotic therapy to prevent stroke in patients with atrial fibrillation: a meta‐analysis.Ann Intern Med1999;131:492–501.
- ,,, et al.Safety and efficacy of aspirin, clopidogrel, and warfarin after coronary stent placement in patients with an indication for anticoagulation.Am Heart J2004;147:463–467.
- ,,,,,.Short‐term triple therapy with aspirin, warfarin, and a thienopyridine among patients undergoing percutaneous coronary intervention.Catheter Cardiovasc Interv2006;68:56–61.
- ,,,,.Periprocedural and medium‐term antithrombotic strategies in patients with an indication for long‐term anticoagulation undergoing coronary angiography and intervention.Coron Artery Dis2007;18:193–199.
- ,,, et al.Dual antiplatelet therapy after percutaneous coronary intervention with stent implantation in patients taking chronic oral anticoagulation.JACC Cardiovasc Interv2008;1:56–61.
- ,,,,,.Evaluation of safety of warfarin in combination with antiplatelet therapy for patients treated with coronary stents for acute myocardial infarction.J Interv Cardiol2005;18:163–166.
- ,,, et al.Anticoagulant and antiplatelet therapy use in 426 patients with atrial fibrillation undergoing percutaneous coronary intervention and stent implantation implications for bleeding risk and prognosis.J Am Coll Cardiol2008;51:818–825.
- ,,, et al.Aspirin and clopidogrel with or without phenprocoumon after drug eluting coronary stent placement in patients on chronic oral anticoagulation.J Intern Med2008;264:472–480.
- ,,, et al.Long‐term outcomes in patients undergoing coronary stenting on dual oral antiplatelet treatment requiring oral anticoagulant therapy.Am J Cardiol2008;102:1618–1623.
- ,,,,.Impact of anticoagulant therapy with dual antiplatelet therapy on prognosis after treatment with drug‐eluting coronary stents.J Cardiol2010;55:362–369.
- ,,, et al.Increased major bleeding complications related to triple antithrombotic therapy usage in patients with atrial fibrillation undergoing percutaneous coronary artery stenting.Chest2008;134:559–567.
- ,,, et al.Comparison of different antithrombotic regimens for patients with atrial fibrillation undergoing drug‐eluting stent implantation.Circ J2010;74:701–708.
- ,,, et al.Combining warfarin and antiplatelet therapy after coronary stenting in the Global Registry of Acute Coronary Events: is it safe and effective to use just one antiplatelet agent?Eur Heart J2007;28:1717–1722.
- ,,, et al.2007 focused update of the ACC/AHA 2004 guidelines for the management of patients with ST‐elevation myocardial infarction: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines.J Am Coll Cardiol2008;51:210–247.
- ,,, et al.Guidelines for percutaneous coronary interventions. The Task Force for Percutaneous Coronary Interventions of the European Society of Cardiology.Eur Heart J2005;26:804–847.
- ,,.Prospective evaluation of an index for predicting the risk of major bleeding in outpatients treated with warfarin.Am J Med1998;105:91–99.
- ,,,.Coronary stents and chronic anticoagulation.Circulation2009;119:1682–1688.
- ,,, et al.Risks and benefits of oral anticoagulation compared with clopidogrel plus aspirin in patients with atrial fibrillation according to stroke risk: the atrial fibrillation clopidogrel trial with irbesartan for prevention of vascular events (ACTIVE‐W).Stroke2008;39:1482–1486.
- ,,, et al.Early and late increased bleeding rates after angioplasty and stenting due to combined antiplatelet and anticoagulanttherapy.EuroIntervention2009;5:425–431.
- ,,, et al.Late clinical events after clopidogrel discontinuation may limit the benefit of drug‐eluting stents: an observational study of drug‐eluting versus bare‐metal stents.J Am Coll Cardiol2006;48:2584–2591.
- ,,, et al.ACCF/ACG/AHA 2008 expert consensus document on reducing the gastrointestinal risks of antiplatelet therapy and NSAID use: a report of the American College of Cardiology Foundation Task Force on Clinical Expert Consensus Documents.Circulation2008;118:1894–1909.
- ,,, et al.Influence of omeprazole on the antiplatelet action of clopidogrel associated with aspirin: the randomized, double‐blind OCLA (Omeprazole CLopidogrel Aspirin) study.J Am Coll Cardiol2008;51:256–260.
- ,,, et al.Risk of adverse outcomes associated with concomitant use of clopidogrel and proton pump inhibitors following acute coronary syndrome.JAMA2009;301:937–944.
- Follow‐Up to the January 26,2009, Early Communication about an Ongoing Safety Review of Clopidogrel Bisulfate (marketed as Plavix) and Omeprazole (marketed as Prilosec and Prilosec OTC). 11/17/2009. (Accessed at http://www.fda.gov/Drugs/DrugSafety/Postmarket DrugSafetyInformationforPatientsandProviders/DrugSafetyInformationfor HeathcareProfessionals/ucm190784.htm.)
- ,,, et al.Incidence, predictors, and prognostic implications of hospitalization for late bleeding after percutaneous coronary intervention for patients older than 65 years.Circ Cardiovasc Interv2010;3:140–147.
- ,,, et al.Antithrombotic therapy in patients treated with oral anticoagulation undergoing coronary artery stenting. An expert consensus document with focus on atrial fibrillation.Ann Med2008;40:428–436.
- ,,, et al.Dabigatran versus warfarin in patients with atrial fibrillation.N Engl J Med2009;361:1139–1151.
- ,,, et al.Rivaroxaban versus placebo in patients with acute coronary syndromes (ATLAS ACS‐TIMI 46): a randomised, double‐blind, phase II trial.Lancet2009;374:29–38.
- ,,,.Bleeding complications associated with combinations of aspirin, thienopyridine derivatives, and warfarin in elderly patients following acute myocardial infarction.Arch Intern Med2005;165:784–789.