User login
Treatment of Complicated Pneumonia
Community‐acquired pneumonia, the most common serious bacterial infection in childhood, may be complicated by parapneumonic effusion (ie, complicated pneumonia).1 Children with complicated pneumonia require prolonged hospitalization and frequently undergo multiple pleural fluid drainage procedures.2 Additionally, the incidence of complicated pneumonia has increased,37 making the need to define appropriate therapy even more pressing. Defining appropriate therapy is challenging for the individual physician as a result of inconsistent and insufficient evidence, and wide variation in treatment practices.2, 8
Historically, thoracotomy was performed only if initial chest tube placement did not lead to clinical improvement.9, 10 Several authors, noting the rapid resolution of symptoms in children undergoing earlier thoracotomy, advocated for the use of thoracotomy as initial therapy rather than as a procedure of last resort.114 The advent of less invasive techniques such as video‐assisted thoracoscopic surgery (VATS) has served as an additional impetus to consider surgical drainage as the initial treatment strategy.1518 Few well‐designed studies have examined the relative efficacy of these interventions.2, 1922 Published randomized trials were single center, enrolled few patients, and arrived at different conclusions.19, 21, 22 In addition, these trials did not examine other important outcomes such as requirement for additional pleural fluid drainage procedures and hospital readmission. Two large retrospective multicenter studies found modest reductions in length of stay (LOS) and substantial decreases in the requirement for additional pleural fluid drainage procedures in children undergoing initial VATS compared with initial chest tube placement.2, 20 However, Shah et al2 included relatively few patients undergoing VATS. Li et al20 combined patients undergoing initial thoracentesis, initial chest tube placement, late pleural fluid drainage (by any method), and no pleural fluid drainage into a single non‐operative management category, precluding conclusions about the relative benefits of chest tube placement compared with VATS. Neither study2, 20 examined the role of chemical fibrinolysis, a therapy which has been associated with outcomes comparable to VATS in two small randomized trials.21, 22
The objectives of this multicenter study were to describe the variation in the initial management strategy along with associated outcomes of complicated pneumonia in childhood and to determine the comparative effectiveness of different pleural fluid drainage procedures.
Methods
Data Source
The Pediatric Health Information System (PHIS), which contains resource utilization data from 40 freestanding children's hospitals, provided data for this multicenter retrospective cohort study. Participating hospitals are located in noncompeting markets of 27 states plus the District of Columbia. The PHIS database includes patient demographics, diagnoses, and procedures as well as data for all drugs, radiologic studies, laboratory tests, and supplies charged to each patient. Data are de‐identified, however encrypted medical record numbers allow for tracking individual patients across admissions. The Child Health Corporation of America (Shawnee Mission, KS) and participating hospitals jointly assure data quality and reliability as described previously.23, 24 The Children's Hospital of Philadelphia Institutional Review Board reviewed and approved this study.
Patients
Children 18 years of age receiving a pleural drainage procedure for complicated pneumonia were eligible if they were discharged from participating hospitals between January 1, 2004 and June 30, 2009. Study participants met the following criteria: 1) discharge diagnosis of pneumonia (International Classification of Diseases, 9th revision [ICD‐9] discharge diagnosis codes 480.x‐483.x, 485.x‐487.x), 2) discharge diagnosis of pleural effusion (ICD‐9 codes 510.0, 510.9, 511.0, 511.1, or 511.9), and 3) billing charge for antibiotics on the first day of hospitalization. Additionally, the primary discharge diagnosis had to be either pneumonia or pleural effusion. Patients were excluded if they did not undergo pleural fluid drainage or if their initial pleural fluid drainage procedure was thoracentesis.
Study Definitions
Pleural drainage procedures were identified using ICD‐9 procedure codes for thoracentesis (34.91), chest tube placement (34.04), VATS (34.21), and thoracotomy (34.02 or 34.09). Fibrinolysis was defined as receipt of urokinase, streptokinase, or alteplase within two days of initial chest tube placement.
Acute conditions or complications included influenza (487, 487.0, 487.1, 487.8, 488, or V04.81) and hemolytic‐uremic syndrome (283.11). Chronic comorbid conditions (CCCs) (eg, malignancy) were identified using a previously reported classification scheme.25 Billing data were used to classify receipt of mechanical ventilation and medications on the first day of hospitalization.
Measured Outcomes
The primary outcomes were hospital LOS (both overall and post‐initial procedure), requirement for additional pleural drainage procedures, total cost for index hospitalization, all‐cause readmission within 14 days after index hospital discharge, and total cost of the episode (accounting for the cost of readmissions).
Measured Exposures
The primary exposure of interest was the initial pleural fluid drainage procedure, classified as chest tube placement without fibrinolysis, chest tube placement with fibrinolysis, VATS, or thoracotomy.
Statistical Analysis
Variables were summarized using frequencies and percentages for categorical variables, and median, interquartile range (IQR), and range for continuous variables. Outcomes by initial pleural drainage procedure were compared using chi‐squared tests for categorical variables and Kruskal‐Wallis tests for continuous variables.
Multivariable analysis was performed to account for potential confounding by observed baseline variables. For dichotomous outcome variables, modeling consisted of logistic regression using generalized estimating equations to account for hospital clustering. For continuous variables, a mixed model approach was used, treating hospital as a random effect. Log transformation was applied to the right‐skewed outcome variables (LOS and cost). Cost outcomes remained skewed following log transformation, thus gamma mixed models were applied.2629 Odds ratios and 95% confidence intervals (CIs) were reported for comparison of dichotomous outcomes and the adjusted means and 95% CIs were reported for continuous outcomes after appropriate back transformation.
Additional analyses addressed the potential impact of confounding by indication inherent in any observational study. First, patients with an underlying CCC were excluded to ensure that our results would be generalizable to otherwise healthy children with community‐acquired pneumonia. Second, patients undergoing pleural drainage >2 days after hospitalization were excluded to minimize the effect of residual confounding related to differences in timing of the initial drainage procedure. Third, the analysis was repeated using a generalized propensity score as an additional method to account for confounding by indication for the initial drainage procedure.30 Propensity scores, constructed using a multivariable generalized logit model, included all variables listed in Table 1. The inverse of the propensity score was included as a weight in each multivariable model described previously. Only the primary multivariable analyses are presented as the results of the propensity score analysis were nearly identical to the primary analyses.
| Overall | Chest Tube Without Fibrinolysis | Chest Tube With Fibrinolysis | Thoracotomy | VATS | P Value | |
|---|---|---|---|---|---|---|
| ||||||
| N | 3500 | 1672 (47.8) | 623 (17.8) | 797 (22.8) | 408 (11.7) | |
| Age | ||||||
| <1 year | 335 (9.6) | 176 (10.5) | 56 (9.0) | 78 (9.8) | 25 (6.1) | |
| 1 year | 475 (13.6) | 238 (14.2) | 98 (15.7) | 92 (11.5) | 47 (11.5) | 0.003 |
| 24 years | 1230 (35.1) | 548 (32.8) | 203 (32.6) | 310 (38.9) | 169 (41.4) | |
| 59 years | 897 (25.6) | 412 (24.6) | 170 (27.3) | 199 (25.0) | 116 (28.4) | |
| 1014 years | 324 (9.3) | 167 (10.0) | 61 (9.8) | 65 (8.2) | 31 (7.6) | |
| 1518 years | 193 (5.5) | 106 (6.3) | 29 (4.6) | 40 (5.0) | 18 (4.4) | |
| >18 years | 46 (1.3) | 25 (1.5) | 6 (0.96) | 13 (1.6) | 2 (0.5) | |
| Comorbid Conditions | ||||||
| Cardiac | 69 (2.0) | 43 (2.6) | 14 (2.3) | 12 (1.5) | 0 (0.0) | 0.006 |
| Malignancy | 81 (2.3) | 31 (1.9) | 18 (2.9) | 21 (2.6) | 11 (2.7) | 0.375 |
| Neurological | 138 (3.9) | 73 (4.4) | 20 (3.2) | 34 (4.3) | 11 (2.7) | 0.313 |
| Any Other Condition | 202 (5.8) | 96 (5.7) | 40 (6.4) | 47 (5.9) | 19 (4.7) | 0.696 |
| Payer | ||||||
| Government | 1240 (35.6) | 630 (37.8) | 224 (36.0) | 259 (32.7) | 127 (31.3) | <0.001 |
| Private | 1383 (39.7) | 607 (36.4) | 283 (45.4) | 310 (39.2) | 183 (45.07) | |
| Other | 864 (24.8) | 430 (25.8) | 116 (18.6) | 222 (28.1) | 96 (23.65) | |
| Race | ||||||
| Non‐Hispanic White | 1746 (51.9) | 838 (51.6) | 358 (59.7) | 361 (47.8) | 189 (48.7) | <0.001 |
| Non‐Hispanic Black | 601 (17.9) | 318 (19.6) | 90 (15.0) | 128 (17.0) | 65 (16.8) | |
| Hispanic | 588 (17.5) | 280 (17.3) | 73 (12.2) | 155 (20.5) | 80 (20.6) | |
| Asian | 117 (3.5) | 47 (2.9) | 20 (3.3) | 37 (4.9) | 13 (3.4) | |
| Other | 314 (9.3) | 140 (8.6) | 59 (9.8) | 74 (9.8) | 41 (10.6) | |
| Male Sex | 1912 (54.6) | 923 (55.2) | 336 (53.9) | 439 (55.1) | 214 (52.5) | 0.755 |
| Radiology | ||||||
| CT, no US | 1200 (34.3) | 600 (35.9) | 184 (29.5) | 280 (35.1) | 136 (33.3) | <0.001 |
| CT and US | 221 (6.3) | 84 (5.0) | 53 (8.5) | 61 (7.7) | 23 (5.6) | |
| US, no CT | 799 (22.8) | 324 (19.4) | 178 (28.6) | 200 (25.1) | 97 (23.8) | |
| No US, no CT | 1280 (36.6) | 664 (39.7) | 208 (33.4) | 256 (32.1) | 152 (37.3) | |
| Empiric Antibiotic Regimen | ||||||
| Cephalosporins alone | 448 (12.8) | 181 (10.83) | 126 (20.2) | 73 (9.2) | 68 (16.7) | <0.001 |
| Cephalosporin and clindamycin | 797 (22.8) | 359 (21.5) | 145 (23.3) | 184 (23.1) | 109 (26.7) | |
| Other antibiotic combination | 167 (4.8) | 82 (4.9) | 30 (4.8) | 38 (4.8) | 17 (4.2) | |
| Cephalosporin and vancomycin | 2088 (59.7) | 1050 (62.8) | 322 (51.7) | 502 (63.0) | 214 (52.5) | |
| Mechanical ventilation | 494 (14.1) | 251 (15.0) | 75 (12.0) | 114 (14.3) | 54 (13.2) | 0.307 |
| Corticosteroids | 520 (14.9) | 291 (17.4) | 72 (11.6) | 114 (14.3) | 43 (10.5) | <0.001 |
| Blood product transfusionsb | 761 (21.7) | 387 (23.2) | 145 (23.3) | 161 (20.2) | 68 (16.7) | 0.018 |
| Vasoactive infusionsc | 381 (10.9) | 223 (13.3) | 63 (10.1) | 72 (9.0) | 23 (5.6) | <0.001 |
| Admission to intensive care | 1397 (39.9) | 731 (43.7) | 234 (37.6) | 296 (37.1) | 136 (33.3) | <0.001 |
| Extracorporeal membranous oxygenation | 18 (0.5) | 13 (0.8) | 2 (0.3) | 3 (0.4) | 0 (0.0) | 0.163 |
| Hemolytic‐uremic syndrome | 31 (0.9) | 15 (0.9) | 6 (1.0) | 7 (0.9) | 3 (0.7) | 0.985 |
| Influenza | 108 (3.1) | 53 (3.2) | 27 (4.3) | 23 (2.9) | 5 (1.2) | 0.044 |
| Arterial blood gas measurements | 0 (0,1) | 0 (0, 2) | 0 (0,1) | 0 (0, 1) | 0 (0, 1) | <0.001 |
| Days to first procedure | 1 (0, 3) | 1 (0, 2) | 1 (1, 3) | 1 (1, 3) | 1 (1, 3) | <0.001 |
Medical records of a randomly selected subset of subjects from 6 hospitals were reviewed to determine the accuracy of our algorithm in identifying patients with complicated pneumonia; these subjects represented 1% of the study population. For the purposes of medical record review, complicated pneumonia was defined by the following: 1) radiologically‐confirmed lung infiltrate; 2) moderate or large pleural effusion; and 3) signs and symptoms of lower respiratory tract infection. Complicated pneumonia was identified in 118 of 120 reviewed subjects for a positive predictive value of 98.3%.
All analyses were clustered by hospital. Analyses were performed using SAS version 9.2 (SAS Institute, Cary, NC). A two‐tailed P < 0.05 was considered statistically significant.
Results
Patient Characteristics
During the study period, 9,680 subjects had complicated pneumonia. Subjects were excluded if they did not have a pleural drainage procedure (n = 5798), or if thoracentesis was the first pleural fluid drainage procedure performed (n = 382). The remaining 3500 patients were included. Demographic characteristics are summarized in Table 1. The median patient age was 4.1 years (IQR: 2.17.2 years). An underlying CCC was present in 424 (12.1%) patients. There was no association between type of drainage procedure and mechanical ventilation. However, factors associated with more severe systemic illness, such as blood product transfusion, were more common among those undergoing initial chest tube placement with or without fibrinolysis (Table 1).
Initial Pleural Fluid Drainage Procedures
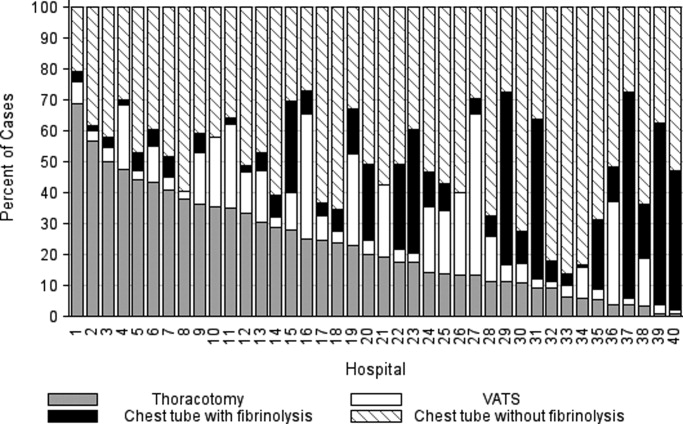
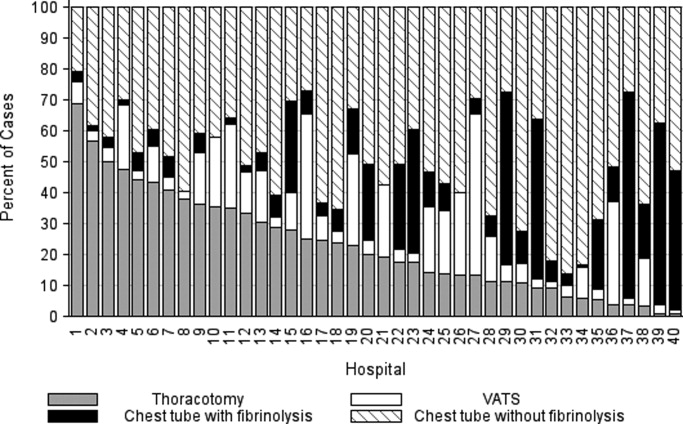
The primary procedures included chest tube without fibrinolysis (47.8%); chest tube with fibrinolysis (17.8%); thoracotomy (22.8%); and VATS (11.7%) (Table 1). The proportion of patients undergoing primary chest tube placement with fibrinolysis increased over time from 14.2% in 2004 to 30.0% in 2009 (P < 0.001; chi‐squared test for trend). The initial procedure varied by hospital with the greatest proportion of patients undergoing primary chest tube placement without fibrinolysis at 28 (70.0%) hospitals, chest tube placement with fibrinolysis at 5 (12.5%) hospitals, thoracotomy at 5 (12.5%) hospitals, and VATS at 2 (5.0%) hospitals (Figure 1). The median proportion of patients undergoing primary VATS across all hospitals was 11.5% (IQR: 3.9%‐26.5%) (Figure 1). The median time to first procedure was 1 day (IQR: 03 days).
Outcome Measures
Variation in outcomes occurred across hospitals. Additional pleural drainage procedures were performed in a median of 20.9% of patients with a range of 6.8% to 44.8% (IQR: 14.5%‐25.3%) of patients across all hospitals. Median LOS was 10 days with a range of 714 days (IQR: 8.511 days) and the median LOS following the initial pleural fluid drainage procedure was 8 days with a range of 6 to 13 days (IQR: 78 days). Variation in timing of the initial pleural fluid drainage procedure explained 9.6% of the variability in LOS (Spearman rho, 0.31; P < 0.001).
Overall, 118 (3.4%) patients were readmitted within 14 days of index discharge; the median readmission rate was 3.8% with a range of 0.8% to 33.3% (IQR: 2.1%‐5.8%) across hospitals. The median total cost of the index hospitalization was $19,574 (IQR: $13,791‐$31,063). The total cost for the index hospitalization exceeded $54,215 for 10% of patients and the total cost of the episode exceeded $55,208 for 10% of patients. Unadjusted outcomes, stratified by primary pleural fluid drainage procedure, are summarized in Table 2.
| Overall | Chest Tube Without Fibrinolysis | Chest Tube With Fibrinolysis | Thoracotomy | VATS | P Valueb | |
|---|---|---|---|---|---|---|
| ||||||
| Additional Procedure | 716 (20.5) | 331 (19.8) | 144 (23.1) | 197 (24.7) | 44 (10.8) | <0.001 |
| Readmission within 14 days | 118 (3.4) | 54 (3.3) | 13 (2.1) | 32 (4.0) | 19 (4.7) | 0.096 |
| Total LOS (days) | 10 (7, 14) | 10 (7, 14) | 9 (7, 13) | 10 (7, 14) | 9 (7, 12) | <.001 |
| Post‐initial Procedure LOS (days) | 8 (5, 12) | 8 (6, 12) | 7 (5, 10) | 8 (5, 12) | 7 (5, 10) | <0.001 |
| Total Cost, Index Hospitalization ($)e | 19319 (13358, 30955) | 19951 (13576, 32018)c | 19565 (13209, 32778)d | 20352 (14351, 31343) | 17918 (13531, 25166) | 0.016 |
| Total Cost, Episode of Illness ($)e | 19831 (13927, 31749) | 20151 (13764, 32653) | 19593 (13210, 32861) | 20573 (14419, 31753) | 18344 (13835, 25462) | 0.029 |
In multivariable analysis, differences in total LOS and post‐procedure LOS were not significant (Table 3). The odds of additional drainage procedures were higher for all drainage procedures compared with initial VATS (Table 3). Patients undergoing initial chest tube placement with fibrinolysis were less likely to require readmission compared with patients undergoing initial VATS (Table 3). The total cost for the episode of illness (including the cost of readmission) was significantly less for those undergoing primary chest tube placement without fibrinolysis compared with primary VATS. The results of subanalyses excluding patients with an underlying CCC (Supporting Appendix online, Table 4) and restricting the cohort to patients undergoing pleural drainage within two days of admission (Supporting Appendix online, Table 5) were similar to the results of our primary analysis with one exception; in the latter subanalysis, children undergoing initial chest tube placement without fibrinolysis were also less likely to require readmission compared with patients undergoing initial VATS.
| Adjusted OR (95% CI)a | P Value | |
|---|---|---|
| ||
| Additional pleural drainage procedure | ||
| Chest tube without fibrinolysis | 1.82 (1.103.00) | .019 |
| Chest tube with fibrinolysis | 2.31 (1.443.72) | <0.001 |
| Thoracotomy | 2.59 (1.624.14) | <0.001 |
| VATS | Reference | |
| Readmission within 14 days | ||
| Chest tube without fibrinolysis | 0.61 (0.361.05) | .077 |
| Chest tube with fibrinolysis | 0.45 (0.230.86) | .015 |
| Thoracotomy | 0.85 (0.521.39) | .521 |
| VATS | Reference | |
| Adjusted Mean (95% CI)a | P Value | |
| Total LOS (days) | ||
| Chest tube without fibrinolysis | 8.0 (7.88.2) | .339 |
| Chest tube with fibrinolysis | 8.1 (7.98.3) | .812 |
| Thoracotomy | 8.1 (7.98.3) | .632 |
| VATS | 8.1 (7.98.3) | Ref |
| Post‐initial procedure LOS (days) | ||
| Chest tube without fibrinolysis | 7.3 (7.07.5) | .512 |
| Chest tube with fibrinolysis | 7.5 (7.27.8) | .239 |
| Thoracotomy | 7.3 (7.07.6) | .841 |
| VATS | 7.3 (7.17.6) | Reference |
| Total cost, index hospitalization ($) | ||
| Chest tube without fibrinolysis | 22928 (2200023895 | .012 |
| Chest tube with fibrinolysis | 23621 (2263124655) | .657 |
| Thoracotomy | 23386 (2241924395 | .262 |
| VATS | 23820 (2280824878) | Reference |
| Total cost, episode of illness ($) | ||
| Chest tube without fibrinolysis | 23218 (2227824199) | .004 |
| Chest tube with fibrinolysis | 23749 (2275224790) | .253 |
| Thoracotomy | 23673 (2269324696) | .131 |
| VATS | 24280 (2324425362) | Reference |
Discussion
This multicenter study is the largest to evaluate the management of children hospitalized with complicated pneumonia. We found considerable variation in initial management and outcomes across hospitals. Differences in timing of the initial drainage procedure explained only a small amount of the variability in outcomes. Children undergoing initial VATS less commonly required additional drainage procedures while children undergoing initial chest tube placement with fibrinolysis less commonly required readmission. Differences in total and post‐procedure LOS were not statistically significant. Differences in cost, while statistically significant, were of marginal relevance.
Previous studies have also shown significant variation in treatment and outcomes of children with complicated pneumonia across hospitals.2, 8 Our study provides data from additional hospitals, includes a substantially larger number of patients undergoing initial VATS, distinguishes between fibrinolysis recipients and nonrecipients, and is the first to compare outcomes between four different initial drainage strategies. The creation of national consensus guidelines might reduce variability in initial management strategies, although the variability in outcomes across hospitals in the current study could not be explained simply by differences in the type or timing of the initial drainage procedure. Thus, future studies examining hospital‐level factors may play an important role in improving quality of care for children with complicated pneumonia.
Patients with initial thoracotomy or chest tube placement with or without fibrinolysis more commonly received additional drainage procedures than patients with initial VATS. This difference remained when patients with CCCs were excluded from the analysis and when the analysis was limited to patients undergoing pleural fluid drainage within 2 days of hospitalization. Several small, randomized trials demonstrated conflicting results when comparing initial chest tube placement with fibrinolysis and VATS. St. Peter et al22 reported that 3 (17%) of 18 patients undergoing initial chest tube placement with fibrinolysis and none of the 18 patients undergoing initial VATS received additional pleural drainage procedures. Sonnappa et al21 found no differences between the two groups. Kurt et al19 did not state the proportion of patients receiving additional procedures. However, the mean number of drainage procedures was 2.25 among the 8 patients undergoing initial chest tube placement while none of the 10 patients with VATS received additional drainage.19
Thoracotomy is often perceived as a definitive procedure for treatment of complicated pneumonia. However, several possibilities exist to explain why additional procedures were performed less frequently in patients undergoing initial VATS compared with initial thoracotomy. The limited visual field in thoracotomy may lead to greater residual disease post‐operatively in those receiving thoracotomy compared with VATS.31 Additionally, thoracotomy substantially disrupts the integrity of the chest wall and is consequently associated with complications such as bleeding and air leak into the pleural cavity more often than VATS.31, 32 It is thus possible that some of the additional procedures in patients receiving initial thoracotomy were necessary for management of thoracotomy‐associated complications rather than for failure of the initial drainage procedure.
Similar to the randomized trials by Sonnappa et al21 and St. Peter et al,22 differences in the overall and post‐procedure LOS were not significant among patients undergoing initial VATS compared with initial chest tube placement with fibrinolysis. However, chest tube placement without fibrinolysis did not result in significant differences in LOS compared with initial VATS. In the only pediatric randomized trial, the 29 intrapleural urokinase recipients had a 2 day shorter LOS compared with the 29 intrapleural saline recipients.33 Several small, randomized controlled trials of adults with complicated pneumonia reported improved pleural fluid drainage among intrapleural fibrinolysis recipients compared with non‐recipients.3436 However, a large multicenter randomized trial in adults found no differences in mortality, requirement for surgical drainage, or LOS between intrapleural streptokinase and placebo recipients.37 Subsequent meta‐analyses of randomized trials in adults also demonstrated no benefit to fibrinolysis.38, 39 In the context of the increasing use of intrapleural fibrinolysis in children with complicated pneumonia, our results highlight the need for a large, multicenter randomized controlled trial to determine whether chest tube with fibrinolysis is superior to chest tube alone.
Two small randomized trials21, 22 and a decision analysis40 identified chest tube with fibrinolysis as the most economical approach to children with complicated pneumonia. However, the costs did not differ significantly between patients undergoing initial VATS or initial chest tube placement with fibrinolysis in our study. The least costly approach was initial chest tube placement without fibrinolysis. Unlike the randomized controlled trials, we considered costs associated with readmissions in determining the total costs. Shah et al41 found no difference in total charges for patients undergoing initial VATS compared with initial chest tube placement; however, patients undergoing initial VATS were concentrated in a few centers, making it difficult to determine the relative importance of procedural and hospital factors.
This multicenter observational study has several limitations. First, discharge diagnosis coding may be unreliable for specific diseases. However, our rigorous definition of complicated pneumonia, supported by the high positive predictive value as verified by medical record review, minimizes the likelihood of misclassification.
Second, unmeasured confounding or residual confounding by indication for the method of pleural drainage may occur, potentially influencing our results in two disparate ways. If patients with more severe systemic illness were too unstable for operative interventions, then our results would be biased towards worse outcomes for children undergoing initial chest tube placement. We adjusted for several variables associated with a greater systemic severity of illness, including intensive care unit admission, making this possibility less likely. We also could not account for some factors associated with more severe local disease such as the size and character of the effusion. We suspect that patients with more extensive local disease (ie, loculated effusions) would have worse outcomes than other patients, regardless of initial procedure, and that these patients would also be more likely to undergo primary surgical drainage. Thus, this study may have underestimated the benefit of initial surgical drainage (eg, VATS) compared with nonsurgical drainage (ie, chest tube placement).
Third, misclassification of the method of initial pleural drainage may have occurred. Patients transferred from another institution following chest tube placement could either be classified as not receiving pleural drainage and thus excluded from the study or classified as having initial VATS or thoracotomy if the reason for transfer was chest tube treatment failure. Additionally, we could not distinguish routine use of fibrinolysis from fibrinolysis to maintain chest tube patency. Whether such misclassification would falsely minimize or maximize differences in outcomes between the various groups remains uncertain. Fourth, because this study only included tertiary care children's hospitals, these data are not generalizable to community settings. VATS requires specialized surgical training that may be unavailable in some areas. Finally, this study demonstrates the relative efficacy of various pleural fluid drainage procedures on short‐term clinical outcomes and resource utilization. However, long‐term functional outcomes should be measured in future prospective studies.
Conclusions
In conclusion, emphasis on evidence driven treatment to optimize care has led to an increasing examination of unwarranted practice variation.42 The lack of evidence for best practice makes it difficult to define unwarranted variation in the treatment of complicated pneumonia. Our study demonstrates the large variability in practice and raises additional questions regarding the optimal drainage strategies. Published randomized trials have focused on comparisons between chest tube placement with fibrinolysis and VATS. However, our data suggest that future randomized trials should include chest tube placement without fibrinolysis as a treatment strategy. In determining the current best treatment for patients with complicated pneumonia, a clinician must weigh the impact of needing an additional procedure in approximately one‐quarter of patients undergoing initial chest tube placement (with or without fibrinolysis) with the risks of general anesthesia and readmission in patients undergoing initial VATS.
Acknowledgements
Dr. Hall had full access to all the data in the study and takes responsibility for the integrity of the data and accuracy of the analysis.
- ,.Parapneumonic pleural effusion and empyema in children. Review of a 19‐year experience, 1962–1980.Clin Pediatr (Phila).1983;22:414–419.
- ,,,,.Primary early thoracoscopy and reduction in length of hospital stay and additional procedures among children with complicated pneumonia: Results of a multicenter retrospective cohort study.Arch Pediatr Adolesc Med.2008;162:675–681.
- ,.Empyema hospitalizations increased in US children despite pneumococcal conjugate vaccine.Pediatrics.2010;125:26–33.
- ,,, et al.Impact of the pneumococcal conjugate vaccine on pneumococcal parapneumonic empyema.Pediatr Infect Dis J.2006;25:250–254.
- ,,,,.Five‐fold increase in pediatric parapneumonic empyema since introduction of pneumococcal conjugate vaccine.Pediatric Infect Dis J.2008;27:1030–1032.
- ,,,.Increasing incidence of empyema complicating childhood community‐acquired pneumonia in the United States.Clin Infect Dis.2010;50:805–813.
- ,,,,.National hospitalization trends for pediatric pneumonia and associated complications.Pediatrics.2010;126:204–213.
- ,,, et al.Empyema associated with community‐acquired pneumonia: A Pediatric Investigator's Collaborative Network on Infections in Canada (PICNIC) study.BMC Infect Dis.2008;8:129.
- ,,,,.Pleural empyema in children.Ann Thorac Surg.1970;10:37–44.
- ,,.Management of streptococcal empyema.Ann Thorac Surg.1966;2:658–664.
- ,.Thoracoscopy in the management of empyema in children.J Pediatr Surg.1993;28:1128–1132.
- ,,,.Surgical treatment of parapneumonic empyema.Pediatr Pulmonol.1996;22:348–356.
- ,.The controversial role of decortication in the management of pediatric empyema.J Thorac Cardiovasc Surg.1988;96:166–170.
- ,,,,,.Postpneumonic empyema in children treated by early decortication.Eur J Pediatr Surg.1997;7:135–137.
- ,.Video‐assisted thoracoscopic surgery in the management of pediatric empyema.JSLS.1997;1:251–3.
- ,,,.Early video‐assisted thoracic surgery in the management of empyema.Pediatrics.1999;103:e63.
- ,,,,.Early definitive intervention by thoracoscopy in pediatric empyema.J Pediatr Surg.1999;34:178–180; discussion80–81.
- ,,,,.Thoracoscopy in the management of pediatric empyema.J Pediatr Surg.1995;30:1211–1215.
- ,,,,.Therapy of parapneumonic effusions in children: Video‐assisted thoracoscopic surgery versus conventional thoracostomy drainage.Pediatrics.2006;118:e547–e553.
- ,.Primary operative management for pediatric empyema: Decreases in hospital length of stay and charges in a national sample.Arch Pediatr Adolesc Med.2008;162:44–48.
- ,,, et al.Comparison of urokinase and video‐assisted thoracoscopic surgery for treatment of childhood empyema.Am J Respir Crit Care Med.2006;174:221–227.
- ,,, et al.Thoracoscopic decortication vs tube thoracostomy with fibrinolysis for empyema in children: A prospective, randomized trial.J Pediatr Surg.2009;44:106–111; discussion11.
- ,,,.Corticosteroids and mortality in children with bacterial meningitis.JAMA.2008;299:2048–2055.
- ,,,,.Intravenous immunoglobulin in children with streptococcal toxic shock syndrome.Clin Infect Dis.2009;49:1369–1376.
- ,,, et al.Deaths attributed to pediatric complex chronic conditions: National trends and implications for supportive care services.Pediatrics.2001;107:e99.
- ,.Multiple regression of cost data: Use of generalised linear models.J Health Serv Res Policy.2004;9:197–204.
- ,,,.A robustified modeling approach to analyze pediatric length of stay.Ann Epidemiol.2005;15:673–677.
- ,,.Correlates of length of stay, cost of care, and mortality among patients hospitalized for necrotizing fasciitis.Epidemiol Infect.2007;135:868–876.
- ,,,,,.Health care costs of adults treated for attention‐deficit/hyperactivity disorder who received alternative drug therapies.J Manag Care Pharm.2007;13:561–569.
- .The role of the propensity score in estimating dose‐response functions.Biometrika.2000;87:706–710.
- ,,,,.Experience with video‐assisted thoracoscopic surgery in the management of complicated pneumonia in children.J Pediatr Surg.2001;36:316–319.
- ,,, et al.VATS debridement versus thoracotomy in the treatment of loculated postpneumonia empyema.Ann Thorac Surg.1996;61:1626–1630.
- ,,,,.Randomised trial of intrapleural urokinase in the treatment of childhood empyema.Thorax.2002;57:343–347.
- ,,,,,.Intrapleural urokinase versus normal saline in the treatment of complicated parapneumonic effusions and empyema. A randomized, double‐blind study.Am J Respir Crit Care Med.1999;159:37–42.
- ,,.Randomised controlled trial of intrapleural streptokinase in community acquired pleural infection.Thorax.1997;52:416–421.
- ,,,,.Intrapleural streptokinase for empyema and complicated parapneumonic effusions.Am J Respir Crit Care Med.2004;170:49–53.
- ,,, et al.U.K. Controlled trial of intrapleural streptokinase for pleural infection.N Engl J Med.2005;352:865–874.
- ,.Intra‐pleural fibrinolytic therapy versus conservative management in the treatment of adult parapneumonic effusions and empyema.Cochrane Database Syst Rev.2008:CD002312.
- ,,,.Intrapleural fibrinolytic agents for empyema and complicated parapneumonic effusions: A meta‐analysis.Chest.2006;129:783–790.
- ,,.Cost‐effectiveness of competing strategies for the treatment of pediatric empyema.Pediatrics.2008;121:e1250–e1257.
- ,,.Costs of treating children with complicated pneumonia: A comparison of primary video‐assisted thoracoscopic surgery and chest tube placement.Pediatr Pulmonol.2010;45:71–77.
- .Unwarranted variation in pediatric medical care.Pediatr Clin North Am.2009;56:745–755.
Community‐acquired pneumonia, the most common serious bacterial infection in childhood, may be complicated by parapneumonic effusion (ie, complicated pneumonia).1 Children with complicated pneumonia require prolonged hospitalization and frequently undergo multiple pleural fluid drainage procedures.2 Additionally, the incidence of complicated pneumonia has increased,37 making the need to define appropriate therapy even more pressing. Defining appropriate therapy is challenging for the individual physician as a result of inconsistent and insufficient evidence, and wide variation in treatment practices.2, 8
Historically, thoracotomy was performed only if initial chest tube placement did not lead to clinical improvement.9, 10 Several authors, noting the rapid resolution of symptoms in children undergoing earlier thoracotomy, advocated for the use of thoracotomy as initial therapy rather than as a procedure of last resort.114 The advent of less invasive techniques such as video‐assisted thoracoscopic surgery (VATS) has served as an additional impetus to consider surgical drainage as the initial treatment strategy.1518 Few well‐designed studies have examined the relative efficacy of these interventions.2, 1922 Published randomized trials were single center, enrolled few patients, and arrived at different conclusions.19, 21, 22 In addition, these trials did not examine other important outcomes such as requirement for additional pleural fluid drainage procedures and hospital readmission. Two large retrospective multicenter studies found modest reductions in length of stay (LOS) and substantial decreases in the requirement for additional pleural fluid drainage procedures in children undergoing initial VATS compared with initial chest tube placement.2, 20 However, Shah et al2 included relatively few patients undergoing VATS. Li et al20 combined patients undergoing initial thoracentesis, initial chest tube placement, late pleural fluid drainage (by any method), and no pleural fluid drainage into a single non‐operative management category, precluding conclusions about the relative benefits of chest tube placement compared with VATS. Neither study2, 20 examined the role of chemical fibrinolysis, a therapy which has been associated with outcomes comparable to VATS in two small randomized trials.21, 22
The objectives of this multicenter study were to describe the variation in the initial management strategy along with associated outcomes of complicated pneumonia in childhood and to determine the comparative effectiveness of different pleural fluid drainage procedures.
Methods
Data Source
The Pediatric Health Information System (PHIS), which contains resource utilization data from 40 freestanding children's hospitals, provided data for this multicenter retrospective cohort study. Participating hospitals are located in noncompeting markets of 27 states plus the District of Columbia. The PHIS database includes patient demographics, diagnoses, and procedures as well as data for all drugs, radiologic studies, laboratory tests, and supplies charged to each patient. Data are de‐identified, however encrypted medical record numbers allow for tracking individual patients across admissions. The Child Health Corporation of America (Shawnee Mission, KS) and participating hospitals jointly assure data quality and reliability as described previously.23, 24 The Children's Hospital of Philadelphia Institutional Review Board reviewed and approved this study.
Patients
Children 18 years of age receiving a pleural drainage procedure for complicated pneumonia were eligible if they were discharged from participating hospitals between January 1, 2004 and June 30, 2009. Study participants met the following criteria: 1) discharge diagnosis of pneumonia (International Classification of Diseases, 9th revision [ICD‐9] discharge diagnosis codes 480.x‐483.x, 485.x‐487.x), 2) discharge diagnosis of pleural effusion (ICD‐9 codes 510.0, 510.9, 511.0, 511.1, or 511.9), and 3) billing charge for antibiotics on the first day of hospitalization. Additionally, the primary discharge diagnosis had to be either pneumonia or pleural effusion. Patients were excluded if they did not undergo pleural fluid drainage or if their initial pleural fluid drainage procedure was thoracentesis.
Study Definitions
Pleural drainage procedures were identified using ICD‐9 procedure codes for thoracentesis (34.91), chest tube placement (34.04), VATS (34.21), and thoracotomy (34.02 or 34.09). Fibrinolysis was defined as receipt of urokinase, streptokinase, or alteplase within two days of initial chest tube placement.
Acute conditions or complications included influenza (487, 487.0, 487.1, 487.8, 488, or V04.81) and hemolytic‐uremic syndrome (283.11). Chronic comorbid conditions (CCCs) (eg, malignancy) were identified using a previously reported classification scheme.25 Billing data were used to classify receipt of mechanical ventilation and medications on the first day of hospitalization.
Measured Outcomes
The primary outcomes were hospital LOS (both overall and post‐initial procedure), requirement for additional pleural drainage procedures, total cost for index hospitalization, all‐cause readmission within 14 days after index hospital discharge, and total cost of the episode (accounting for the cost of readmissions).
Measured Exposures
The primary exposure of interest was the initial pleural fluid drainage procedure, classified as chest tube placement without fibrinolysis, chest tube placement with fibrinolysis, VATS, or thoracotomy.
Statistical Analysis
Variables were summarized using frequencies and percentages for categorical variables, and median, interquartile range (IQR), and range for continuous variables. Outcomes by initial pleural drainage procedure were compared using chi‐squared tests for categorical variables and Kruskal‐Wallis tests for continuous variables.
Multivariable analysis was performed to account for potential confounding by observed baseline variables. For dichotomous outcome variables, modeling consisted of logistic regression using generalized estimating equations to account for hospital clustering. For continuous variables, a mixed model approach was used, treating hospital as a random effect. Log transformation was applied to the right‐skewed outcome variables (LOS and cost). Cost outcomes remained skewed following log transformation, thus gamma mixed models were applied.2629 Odds ratios and 95% confidence intervals (CIs) were reported for comparison of dichotomous outcomes and the adjusted means and 95% CIs were reported for continuous outcomes after appropriate back transformation.
Additional analyses addressed the potential impact of confounding by indication inherent in any observational study. First, patients with an underlying CCC were excluded to ensure that our results would be generalizable to otherwise healthy children with community‐acquired pneumonia. Second, patients undergoing pleural drainage >2 days after hospitalization were excluded to minimize the effect of residual confounding related to differences in timing of the initial drainage procedure. Third, the analysis was repeated using a generalized propensity score as an additional method to account for confounding by indication for the initial drainage procedure.30 Propensity scores, constructed using a multivariable generalized logit model, included all variables listed in Table 1. The inverse of the propensity score was included as a weight in each multivariable model described previously. Only the primary multivariable analyses are presented as the results of the propensity score analysis were nearly identical to the primary analyses.
| Overall | Chest Tube Without Fibrinolysis | Chest Tube With Fibrinolysis | Thoracotomy | VATS | P Value | |
|---|---|---|---|---|---|---|
| ||||||
| N | 3500 | 1672 (47.8) | 623 (17.8) | 797 (22.8) | 408 (11.7) | |
| Age | ||||||
| <1 year | 335 (9.6) | 176 (10.5) | 56 (9.0) | 78 (9.8) | 25 (6.1) | |
| 1 year | 475 (13.6) | 238 (14.2) | 98 (15.7) | 92 (11.5) | 47 (11.5) | 0.003 |
| 24 years | 1230 (35.1) | 548 (32.8) | 203 (32.6) | 310 (38.9) | 169 (41.4) | |
| 59 years | 897 (25.6) | 412 (24.6) | 170 (27.3) | 199 (25.0) | 116 (28.4) | |
| 1014 years | 324 (9.3) | 167 (10.0) | 61 (9.8) | 65 (8.2) | 31 (7.6) | |
| 1518 years | 193 (5.5) | 106 (6.3) | 29 (4.6) | 40 (5.0) | 18 (4.4) | |
| >18 years | 46 (1.3) | 25 (1.5) | 6 (0.96) | 13 (1.6) | 2 (0.5) | |
| Comorbid Conditions | ||||||
| Cardiac | 69 (2.0) | 43 (2.6) | 14 (2.3) | 12 (1.5) | 0 (0.0) | 0.006 |
| Malignancy | 81 (2.3) | 31 (1.9) | 18 (2.9) | 21 (2.6) | 11 (2.7) | 0.375 |
| Neurological | 138 (3.9) | 73 (4.4) | 20 (3.2) | 34 (4.3) | 11 (2.7) | 0.313 |
| Any Other Condition | 202 (5.8) | 96 (5.7) | 40 (6.4) | 47 (5.9) | 19 (4.7) | 0.696 |
| Payer | ||||||
| Government | 1240 (35.6) | 630 (37.8) | 224 (36.0) | 259 (32.7) | 127 (31.3) | <0.001 |
| Private | 1383 (39.7) | 607 (36.4) | 283 (45.4) | 310 (39.2) | 183 (45.07) | |
| Other | 864 (24.8) | 430 (25.8) | 116 (18.6) | 222 (28.1) | 96 (23.65) | |
| Race | ||||||
| Non‐Hispanic White | 1746 (51.9) | 838 (51.6) | 358 (59.7) | 361 (47.8) | 189 (48.7) | <0.001 |
| Non‐Hispanic Black | 601 (17.9) | 318 (19.6) | 90 (15.0) | 128 (17.0) | 65 (16.8) | |
| Hispanic | 588 (17.5) | 280 (17.3) | 73 (12.2) | 155 (20.5) | 80 (20.6) | |
| Asian | 117 (3.5) | 47 (2.9) | 20 (3.3) | 37 (4.9) | 13 (3.4) | |
| Other | 314 (9.3) | 140 (8.6) | 59 (9.8) | 74 (9.8) | 41 (10.6) | |
| Male Sex | 1912 (54.6) | 923 (55.2) | 336 (53.9) | 439 (55.1) | 214 (52.5) | 0.755 |
| Radiology | ||||||
| CT, no US | 1200 (34.3) | 600 (35.9) | 184 (29.5) | 280 (35.1) | 136 (33.3) | <0.001 |
| CT and US | 221 (6.3) | 84 (5.0) | 53 (8.5) | 61 (7.7) | 23 (5.6) | |
| US, no CT | 799 (22.8) | 324 (19.4) | 178 (28.6) | 200 (25.1) | 97 (23.8) | |
| No US, no CT | 1280 (36.6) | 664 (39.7) | 208 (33.4) | 256 (32.1) | 152 (37.3) | |
| Empiric Antibiotic Regimen | ||||||
| Cephalosporins alone | 448 (12.8) | 181 (10.83) | 126 (20.2) | 73 (9.2) | 68 (16.7) | <0.001 |
| Cephalosporin and clindamycin | 797 (22.8) | 359 (21.5) | 145 (23.3) | 184 (23.1) | 109 (26.7) | |
| Other antibiotic combination | 167 (4.8) | 82 (4.9) | 30 (4.8) | 38 (4.8) | 17 (4.2) | |
| Cephalosporin and vancomycin | 2088 (59.7) | 1050 (62.8) | 322 (51.7) | 502 (63.0) | 214 (52.5) | |
| Mechanical ventilation | 494 (14.1) | 251 (15.0) | 75 (12.0) | 114 (14.3) | 54 (13.2) | 0.307 |
| Corticosteroids | 520 (14.9) | 291 (17.4) | 72 (11.6) | 114 (14.3) | 43 (10.5) | <0.001 |
| Blood product transfusionsb | 761 (21.7) | 387 (23.2) | 145 (23.3) | 161 (20.2) | 68 (16.7) | 0.018 |
| Vasoactive infusionsc | 381 (10.9) | 223 (13.3) | 63 (10.1) | 72 (9.0) | 23 (5.6) | <0.001 |
| Admission to intensive care | 1397 (39.9) | 731 (43.7) | 234 (37.6) | 296 (37.1) | 136 (33.3) | <0.001 |
| Extracorporeal membranous oxygenation | 18 (0.5) | 13 (0.8) | 2 (0.3) | 3 (0.4) | 0 (0.0) | 0.163 |
| Hemolytic‐uremic syndrome | 31 (0.9) | 15 (0.9) | 6 (1.0) | 7 (0.9) | 3 (0.7) | 0.985 |
| Influenza | 108 (3.1) | 53 (3.2) | 27 (4.3) | 23 (2.9) | 5 (1.2) | 0.044 |
| Arterial blood gas measurements | 0 (0,1) | 0 (0, 2) | 0 (0,1) | 0 (0, 1) | 0 (0, 1) | <0.001 |
| Days to first procedure | 1 (0, 3) | 1 (0, 2) | 1 (1, 3) | 1 (1, 3) | 1 (1, 3) | <0.001 |
Medical records of a randomly selected subset of subjects from 6 hospitals were reviewed to determine the accuracy of our algorithm in identifying patients with complicated pneumonia; these subjects represented 1% of the study population. For the purposes of medical record review, complicated pneumonia was defined by the following: 1) radiologically‐confirmed lung infiltrate; 2) moderate or large pleural effusion; and 3) signs and symptoms of lower respiratory tract infection. Complicated pneumonia was identified in 118 of 120 reviewed subjects for a positive predictive value of 98.3%.
All analyses were clustered by hospital. Analyses were performed using SAS version 9.2 (SAS Institute, Cary, NC). A two‐tailed P < 0.05 was considered statistically significant.
Results
Patient Characteristics
During the study period, 9,680 subjects had complicated pneumonia. Subjects were excluded if they did not have a pleural drainage procedure (n = 5798), or if thoracentesis was the first pleural fluid drainage procedure performed (n = 382). The remaining 3500 patients were included. Demographic characteristics are summarized in Table 1. The median patient age was 4.1 years (IQR: 2.17.2 years). An underlying CCC was present in 424 (12.1%) patients. There was no association between type of drainage procedure and mechanical ventilation. However, factors associated with more severe systemic illness, such as blood product transfusion, were more common among those undergoing initial chest tube placement with or without fibrinolysis (Table 1).
Initial Pleural Fluid Drainage Procedures
The primary procedures included chest tube without fibrinolysis (47.8%); chest tube with fibrinolysis (17.8%); thoracotomy (22.8%); and VATS (11.7%) (Table 1). The proportion of patients undergoing primary chest tube placement with fibrinolysis increased over time from 14.2% in 2004 to 30.0% in 2009 (P < 0.001; chi‐squared test for trend). The initial procedure varied by hospital with the greatest proportion of patients undergoing primary chest tube placement without fibrinolysis at 28 (70.0%) hospitals, chest tube placement with fibrinolysis at 5 (12.5%) hospitals, thoracotomy at 5 (12.5%) hospitals, and VATS at 2 (5.0%) hospitals (Figure 1). The median proportion of patients undergoing primary VATS across all hospitals was 11.5% (IQR: 3.9%‐26.5%) (Figure 1). The median time to first procedure was 1 day (IQR: 03 days).

Outcome Measures
Variation in outcomes occurred across hospitals. Additional pleural drainage procedures were performed in a median of 20.9% of patients with a range of 6.8% to 44.8% (IQR: 14.5%‐25.3%) of patients across all hospitals. Median LOS was 10 days with a range of 714 days (IQR: 8.511 days) and the median LOS following the initial pleural fluid drainage procedure was 8 days with a range of 6 to 13 days (IQR: 78 days). Variation in timing of the initial pleural fluid drainage procedure explained 9.6% of the variability in LOS (Spearman rho, 0.31; P < 0.001).
Overall, 118 (3.4%) patients were readmitted within 14 days of index discharge; the median readmission rate was 3.8% with a range of 0.8% to 33.3% (IQR: 2.1%‐5.8%) across hospitals. The median total cost of the index hospitalization was $19,574 (IQR: $13,791‐$31,063). The total cost for the index hospitalization exceeded $54,215 for 10% of patients and the total cost of the episode exceeded $55,208 for 10% of patients. Unadjusted outcomes, stratified by primary pleural fluid drainage procedure, are summarized in Table 2.
| Overall | Chest Tube Without Fibrinolysis | Chest Tube With Fibrinolysis | Thoracotomy | VATS | P Valueb | |
|---|---|---|---|---|---|---|
| ||||||
| Additional Procedure | 716 (20.5) | 331 (19.8) | 144 (23.1) | 197 (24.7) | 44 (10.8) | <0.001 |
| Readmission within 14 days | 118 (3.4) | 54 (3.3) | 13 (2.1) | 32 (4.0) | 19 (4.7) | 0.096 |
| Total LOS (days) | 10 (7, 14) | 10 (7, 14) | 9 (7, 13) | 10 (7, 14) | 9 (7, 12) | <.001 |
| Post‐initial Procedure LOS (days) | 8 (5, 12) | 8 (6, 12) | 7 (5, 10) | 8 (5, 12) | 7 (5, 10) | <0.001 |
| Total Cost, Index Hospitalization ($)e | 19319 (13358, 30955) | 19951 (13576, 32018)c | 19565 (13209, 32778)d | 20352 (14351, 31343) | 17918 (13531, 25166) | 0.016 |
| Total Cost, Episode of Illness ($)e | 19831 (13927, 31749) | 20151 (13764, 32653) | 19593 (13210, 32861) | 20573 (14419, 31753) | 18344 (13835, 25462) | 0.029 |
In multivariable analysis, differences in total LOS and post‐procedure LOS were not significant (Table 3). The odds of additional drainage procedures were higher for all drainage procedures compared with initial VATS (Table 3). Patients undergoing initial chest tube placement with fibrinolysis were less likely to require readmission compared with patients undergoing initial VATS (Table 3). The total cost for the episode of illness (including the cost of readmission) was significantly less for those undergoing primary chest tube placement without fibrinolysis compared with primary VATS. The results of subanalyses excluding patients with an underlying CCC (Supporting Appendix online, Table 4) and restricting the cohort to patients undergoing pleural drainage within two days of admission (Supporting Appendix online, Table 5) were similar to the results of our primary analysis with one exception; in the latter subanalysis, children undergoing initial chest tube placement without fibrinolysis were also less likely to require readmission compared with patients undergoing initial VATS.
| Adjusted OR (95% CI)a | P Value | |
|---|---|---|
| ||
| Additional pleural drainage procedure | ||
| Chest tube without fibrinolysis | 1.82 (1.103.00) | .019 |
| Chest tube with fibrinolysis | 2.31 (1.443.72) | <0.001 |
| Thoracotomy | 2.59 (1.624.14) | <0.001 |
| VATS | Reference | |
| Readmission within 14 days | ||
| Chest tube without fibrinolysis | 0.61 (0.361.05) | .077 |
| Chest tube with fibrinolysis | 0.45 (0.230.86) | .015 |
| Thoracotomy | 0.85 (0.521.39) | .521 |
| VATS | Reference | |
| Adjusted Mean (95% CI)a | P Value | |
| Total LOS (days) | ||
| Chest tube without fibrinolysis | 8.0 (7.88.2) | .339 |
| Chest tube with fibrinolysis | 8.1 (7.98.3) | .812 |
| Thoracotomy | 8.1 (7.98.3) | .632 |
| VATS | 8.1 (7.98.3) | Ref |
| Post‐initial procedure LOS (days) | ||
| Chest tube without fibrinolysis | 7.3 (7.07.5) | .512 |
| Chest tube with fibrinolysis | 7.5 (7.27.8) | .239 |
| Thoracotomy | 7.3 (7.07.6) | .841 |
| VATS | 7.3 (7.17.6) | Reference |
| Total cost, index hospitalization ($) | ||
| Chest tube without fibrinolysis | 22928 (2200023895 | .012 |
| Chest tube with fibrinolysis | 23621 (2263124655) | .657 |
| Thoracotomy | 23386 (2241924395 | .262 |
| VATS | 23820 (2280824878) | Reference |
| Total cost, episode of illness ($) | ||
| Chest tube without fibrinolysis | 23218 (2227824199) | .004 |
| Chest tube with fibrinolysis | 23749 (2275224790) | .253 |
| Thoracotomy | 23673 (2269324696) | .131 |
| VATS | 24280 (2324425362) | Reference |
Discussion
This multicenter study is the largest to evaluate the management of children hospitalized with complicated pneumonia. We found considerable variation in initial management and outcomes across hospitals. Differences in timing of the initial drainage procedure explained only a small amount of the variability in outcomes. Children undergoing initial VATS less commonly required additional drainage procedures while children undergoing initial chest tube placement with fibrinolysis less commonly required readmission. Differences in total and post‐procedure LOS were not statistically significant. Differences in cost, while statistically significant, were of marginal relevance.
Previous studies have also shown significant variation in treatment and outcomes of children with complicated pneumonia across hospitals.2, 8 Our study provides data from additional hospitals, includes a substantially larger number of patients undergoing initial VATS, distinguishes between fibrinolysis recipients and nonrecipients, and is the first to compare outcomes between four different initial drainage strategies. The creation of national consensus guidelines might reduce variability in initial management strategies, although the variability in outcomes across hospitals in the current study could not be explained simply by differences in the type or timing of the initial drainage procedure. Thus, future studies examining hospital‐level factors may play an important role in improving quality of care for children with complicated pneumonia.
Patients with initial thoracotomy or chest tube placement with or without fibrinolysis more commonly received additional drainage procedures than patients with initial VATS. This difference remained when patients with CCCs were excluded from the analysis and when the analysis was limited to patients undergoing pleural fluid drainage within 2 days of hospitalization. Several small, randomized trials demonstrated conflicting results when comparing initial chest tube placement with fibrinolysis and VATS. St. Peter et al22 reported that 3 (17%) of 18 patients undergoing initial chest tube placement with fibrinolysis and none of the 18 patients undergoing initial VATS received additional pleural drainage procedures. Sonnappa et al21 found no differences between the two groups. Kurt et al19 did not state the proportion of patients receiving additional procedures. However, the mean number of drainage procedures was 2.25 among the 8 patients undergoing initial chest tube placement while none of the 10 patients with VATS received additional drainage.19
Thoracotomy is often perceived as a definitive procedure for treatment of complicated pneumonia. However, several possibilities exist to explain why additional procedures were performed less frequently in patients undergoing initial VATS compared with initial thoracotomy. The limited visual field in thoracotomy may lead to greater residual disease post‐operatively in those receiving thoracotomy compared with VATS.31 Additionally, thoracotomy substantially disrupts the integrity of the chest wall and is consequently associated with complications such as bleeding and air leak into the pleural cavity more often than VATS.31, 32 It is thus possible that some of the additional procedures in patients receiving initial thoracotomy were necessary for management of thoracotomy‐associated complications rather than for failure of the initial drainage procedure.
Similar to the randomized trials by Sonnappa et al21 and St. Peter et al,22 differences in the overall and post‐procedure LOS were not significant among patients undergoing initial VATS compared with initial chest tube placement with fibrinolysis. However, chest tube placement without fibrinolysis did not result in significant differences in LOS compared with initial VATS. In the only pediatric randomized trial, the 29 intrapleural urokinase recipients had a 2 day shorter LOS compared with the 29 intrapleural saline recipients.33 Several small, randomized controlled trials of adults with complicated pneumonia reported improved pleural fluid drainage among intrapleural fibrinolysis recipients compared with non‐recipients.3436 However, a large multicenter randomized trial in adults found no differences in mortality, requirement for surgical drainage, or LOS between intrapleural streptokinase and placebo recipients.37 Subsequent meta‐analyses of randomized trials in adults also demonstrated no benefit to fibrinolysis.38, 39 In the context of the increasing use of intrapleural fibrinolysis in children with complicated pneumonia, our results highlight the need for a large, multicenter randomized controlled trial to determine whether chest tube with fibrinolysis is superior to chest tube alone.
Two small randomized trials21, 22 and a decision analysis40 identified chest tube with fibrinolysis as the most economical approach to children with complicated pneumonia. However, the costs did not differ significantly between patients undergoing initial VATS or initial chest tube placement with fibrinolysis in our study. The least costly approach was initial chest tube placement without fibrinolysis. Unlike the randomized controlled trials, we considered costs associated with readmissions in determining the total costs. Shah et al41 found no difference in total charges for patients undergoing initial VATS compared with initial chest tube placement; however, patients undergoing initial VATS were concentrated in a few centers, making it difficult to determine the relative importance of procedural and hospital factors.
This multicenter observational study has several limitations. First, discharge diagnosis coding may be unreliable for specific diseases. However, our rigorous definition of complicated pneumonia, supported by the high positive predictive value as verified by medical record review, minimizes the likelihood of misclassification.
Second, unmeasured confounding or residual confounding by indication for the method of pleural drainage may occur, potentially influencing our results in two disparate ways. If patients with more severe systemic illness were too unstable for operative interventions, then our results would be biased towards worse outcomes for children undergoing initial chest tube placement. We adjusted for several variables associated with a greater systemic severity of illness, including intensive care unit admission, making this possibility less likely. We also could not account for some factors associated with more severe local disease such as the size and character of the effusion. We suspect that patients with more extensive local disease (ie, loculated effusions) would have worse outcomes than other patients, regardless of initial procedure, and that these patients would also be more likely to undergo primary surgical drainage. Thus, this study may have underestimated the benefit of initial surgical drainage (eg, VATS) compared with nonsurgical drainage (ie, chest tube placement).
Third, misclassification of the method of initial pleural drainage may have occurred. Patients transferred from another institution following chest tube placement could either be classified as not receiving pleural drainage and thus excluded from the study or classified as having initial VATS or thoracotomy if the reason for transfer was chest tube treatment failure. Additionally, we could not distinguish routine use of fibrinolysis from fibrinolysis to maintain chest tube patency. Whether such misclassification would falsely minimize or maximize differences in outcomes between the various groups remains uncertain. Fourth, because this study only included tertiary care children's hospitals, these data are not generalizable to community settings. VATS requires specialized surgical training that may be unavailable in some areas. Finally, this study demonstrates the relative efficacy of various pleural fluid drainage procedures on short‐term clinical outcomes and resource utilization. However, long‐term functional outcomes should be measured in future prospective studies.
Conclusions
In conclusion, emphasis on evidence driven treatment to optimize care has led to an increasing examination of unwarranted practice variation.42 The lack of evidence for best practice makes it difficult to define unwarranted variation in the treatment of complicated pneumonia. Our study demonstrates the large variability in practice and raises additional questions regarding the optimal drainage strategies. Published randomized trials have focused on comparisons between chest tube placement with fibrinolysis and VATS. However, our data suggest that future randomized trials should include chest tube placement without fibrinolysis as a treatment strategy. In determining the current best treatment for patients with complicated pneumonia, a clinician must weigh the impact of needing an additional procedure in approximately one‐quarter of patients undergoing initial chest tube placement (with or without fibrinolysis) with the risks of general anesthesia and readmission in patients undergoing initial VATS.
Acknowledgements
Dr. Hall had full access to all the data in the study and takes responsibility for the integrity of the data and accuracy of the analysis.
Community‐acquired pneumonia, the most common serious bacterial infection in childhood, may be complicated by parapneumonic effusion (ie, complicated pneumonia).1 Children with complicated pneumonia require prolonged hospitalization and frequently undergo multiple pleural fluid drainage procedures.2 Additionally, the incidence of complicated pneumonia has increased,37 making the need to define appropriate therapy even more pressing. Defining appropriate therapy is challenging for the individual physician as a result of inconsistent and insufficient evidence, and wide variation in treatment practices.2, 8
Historically, thoracotomy was performed only if initial chest tube placement did not lead to clinical improvement.9, 10 Several authors, noting the rapid resolution of symptoms in children undergoing earlier thoracotomy, advocated for the use of thoracotomy as initial therapy rather than as a procedure of last resort.114 The advent of less invasive techniques such as video‐assisted thoracoscopic surgery (VATS) has served as an additional impetus to consider surgical drainage as the initial treatment strategy.1518 Few well‐designed studies have examined the relative efficacy of these interventions.2, 1922 Published randomized trials were single center, enrolled few patients, and arrived at different conclusions.19, 21, 22 In addition, these trials did not examine other important outcomes such as requirement for additional pleural fluid drainage procedures and hospital readmission. Two large retrospective multicenter studies found modest reductions in length of stay (LOS) and substantial decreases in the requirement for additional pleural fluid drainage procedures in children undergoing initial VATS compared with initial chest tube placement.2, 20 However, Shah et al2 included relatively few patients undergoing VATS. Li et al20 combined patients undergoing initial thoracentesis, initial chest tube placement, late pleural fluid drainage (by any method), and no pleural fluid drainage into a single non‐operative management category, precluding conclusions about the relative benefits of chest tube placement compared with VATS. Neither study2, 20 examined the role of chemical fibrinolysis, a therapy which has been associated with outcomes comparable to VATS in two small randomized trials.21, 22
The objectives of this multicenter study were to describe the variation in the initial management strategy along with associated outcomes of complicated pneumonia in childhood and to determine the comparative effectiveness of different pleural fluid drainage procedures.
Methods
Data Source
The Pediatric Health Information System (PHIS), which contains resource utilization data from 40 freestanding children's hospitals, provided data for this multicenter retrospective cohort study. Participating hospitals are located in noncompeting markets of 27 states plus the District of Columbia. The PHIS database includes patient demographics, diagnoses, and procedures as well as data for all drugs, radiologic studies, laboratory tests, and supplies charged to each patient. Data are de‐identified, however encrypted medical record numbers allow for tracking individual patients across admissions. The Child Health Corporation of America (Shawnee Mission, KS) and participating hospitals jointly assure data quality and reliability as described previously.23, 24 The Children's Hospital of Philadelphia Institutional Review Board reviewed and approved this study.
Patients
Children 18 years of age receiving a pleural drainage procedure for complicated pneumonia were eligible if they were discharged from participating hospitals between January 1, 2004 and June 30, 2009. Study participants met the following criteria: 1) discharge diagnosis of pneumonia (International Classification of Diseases, 9th revision [ICD‐9] discharge diagnosis codes 480.x‐483.x, 485.x‐487.x), 2) discharge diagnosis of pleural effusion (ICD‐9 codes 510.0, 510.9, 511.0, 511.1, or 511.9), and 3) billing charge for antibiotics on the first day of hospitalization. Additionally, the primary discharge diagnosis had to be either pneumonia or pleural effusion. Patients were excluded if they did not undergo pleural fluid drainage or if their initial pleural fluid drainage procedure was thoracentesis.
Study Definitions
Pleural drainage procedures were identified using ICD‐9 procedure codes for thoracentesis (34.91), chest tube placement (34.04), VATS (34.21), and thoracotomy (34.02 or 34.09). Fibrinolysis was defined as receipt of urokinase, streptokinase, or alteplase within two days of initial chest tube placement.
Acute conditions or complications included influenza (487, 487.0, 487.1, 487.8, 488, or V04.81) and hemolytic‐uremic syndrome (283.11). Chronic comorbid conditions (CCCs) (eg, malignancy) were identified using a previously reported classification scheme.25 Billing data were used to classify receipt of mechanical ventilation and medications on the first day of hospitalization.
Measured Outcomes
The primary outcomes were hospital LOS (both overall and post‐initial procedure), requirement for additional pleural drainage procedures, total cost for index hospitalization, all‐cause readmission within 14 days after index hospital discharge, and total cost of the episode (accounting for the cost of readmissions).
Measured Exposures
The primary exposure of interest was the initial pleural fluid drainage procedure, classified as chest tube placement without fibrinolysis, chest tube placement with fibrinolysis, VATS, or thoracotomy.
Statistical Analysis
Variables were summarized using frequencies and percentages for categorical variables, and median, interquartile range (IQR), and range for continuous variables. Outcomes by initial pleural drainage procedure were compared using chi‐squared tests for categorical variables and Kruskal‐Wallis tests for continuous variables.
Multivariable analysis was performed to account for potential confounding by observed baseline variables. For dichotomous outcome variables, modeling consisted of logistic regression using generalized estimating equations to account for hospital clustering. For continuous variables, a mixed model approach was used, treating hospital as a random effect. Log transformation was applied to the right‐skewed outcome variables (LOS and cost). Cost outcomes remained skewed following log transformation, thus gamma mixed models were applied.2629 Odds ratios and 95% confidence intervals (CIs) were reported for comparison of dichotomous outcomes and the adjusted means and 95% CIs were reported for continuous outcomes after appropriate back transformation.
Additional analyses addressed the potential impact of confounding by indication inherent in any observational study. First, patients with an underlying CCC were excluded to ensure that our results would be generalizable to otherwise healthy children with community‐acquired pneumonia. Second, patients undergoing pleural drainage >2 days after hospitalization were excluded to minimize the effect of residual confounding related to differences in timing of the initial drainage procedure. Third, the analysis was repeated using a generalized propensity score as an additional method to account for confounding by indication for the initial drainage procedure.30 Propensity scores, constructed using a multivariable generalized logit model, included all variables listed in Table 1. The inverse of the propensity score was included as a weight in each multivariable model described previously. Only the primary multivariable analyses are presented as the results of the propensity score analysis were nearly identical to the primary analyses.
| Overall | Chest Tube Without Fibrinolysis | Chest Tube With Fibrinolysis | Thoracotomy | VATS | P Value | |
|---|---|---|---|---|---|---|
| ||||||
| N | 3500 | 1672 (47.8) | 623 (17.8) | 797 (22.8) | 408 (11.7) | |
| Age | ||||||
| <1 year | 335 (9.6) | 176 (10.5) | 56 (9.0) | 78 (9.8) | 25 (6.1) | |
| 1 year | 475 (13.6) | 238 (14.2) | 98 (15.7) | 92 (11.5) | 47 (11.5) | 0.003 |
| 24 years | 1230 (35.1) | 548 (32.8) | 203 (32.6) | 310 (38.9) | 169 (41.4) | |
| 59 years | 897 (25.6) | 412 (24.6) | 170 (27.3) | 199 (25.0) | 116 (28.4) | |
| 1014 years | 324 (9.3) | 167 (10.0) | 61 (9.8) | 65 (8.2) | 31 (7.6) | |
| 1518 years | 193 (5.5) | 106 (6.3) | 29 (4.6) | 40 (5.0) | 18 (4.4) | |
| >18 years | 46 (1.3) | 25 (1.5) | 6 (0.96) | 13 (1.6) | 2 (0.5) | |
| Comorbid Conditions | ||||||
| Cardiac | 69 (2.0) | 43 (2.6) | 14 (2.3) | 12 (1.5) | 0 (0.0) | 0.006 |
| Malignancy | 81 (2.3) | 31 (1.9) | 18 (2.9) | 21 (2.6) | 11 (2.7) | 0.375 |
| Neurological | 138 (3.9) | 73 (4.4) | 20 (3.2) | 34 (4.3) | 11 (2.7) | 0.313 |
| Any Other Condition | 202 (5.8) | 96 (5.7) | 40 (6.4) | 47 (5.9) | 19 (4.7) | 0.696 |
| Payer | ||||||
| Government | 1240 (35.6) | 630 (37.8) | 224 (36.0) | 259 (32.7) | 127 (31.3) | <0.001 |
| Private | 1383 (39.7) | 607 (36.4) | 283 (45.4) | 310 (39.2) | 183 (45.07) | |
| Other | 864 (24.8) | 430 (25.8) | 116 (18.6) | 222 (28.1) | 96 (23.65) | |
| Race | ||||||
| Non‐Hispanic White | 1746 (51.9) | 838 (51.6) | 358 (59.7) | 361 (47.8) | 189 (48.7) | <0.001 |
| Non‐Hispanic Black | 601 (17.9) | 318 (19.6) | 90 (15.0) | 128 (17.0) | 65 (16.8) | |
| Hispanic | 588 (17.5) | 280 (17.3) | 73 (12.2) | 155 (20.5) | 80 (20.6) | |
| Asian | 117 (3.5) | 47 (2.9) | 20 (3.3) | 37 (4.9) | 13 (3.4) | |
| Other | 314 (9.3) | 140 (8.6) | 59 (9.8) | 74 (9.8) | 41 (10.6) | |
| Male Sex | 1912 (54.6) | 923 (55.2) | 336 (53.9) | 439 (55.1) | 214 (52.5) | 0.755 |
| Radiology | ||||||
| CT, no US | 1200 (34.3) | 600 (35.9) | 184 (29.5) | 280 (35.1) | 136 (33.3) | <0.001 |
| CT and US | 221 (6.3) | 84 (5.0) | 53 (8.5) | 61 (7.7) | 23 (5.6) | |
| US, no CT | 799 (22.8) | 324 (19.4) | 178 (28.6) | 200 (25.1) | 97 (23.8) | |
| No US, no CT | 1280 (36.6) | 664 (39.7) | 208 (33.4) | 256 (32.1) | 152 (37.3) | |
| Empiric Antibiotic Regimen | ||||||
| Cephalosporins alone | 448 (12.8) | 181 (10.83) | 126 (20.2) | 73 (9.2) | 68 (16.7) | <0.001 |
| Cephalosporin and clindamycin | 797 (22.8) | 359 (21.5) | 145 (23.3) | 184 (23.1) | 109 (26.7) | |
| Other antibiotic combination | 167 (4.8) | 82 (4.9) | 30 (4.8) | 38 (4.8) | 17 (4.2) | |
| Cephalosporin and vancomycin | 2088 (59.7) | 1050 (62.8) | 322 (51.7) | 502 (63.0) | 214 (52.5) | |
| Mechanical ventilation | 494 (14.1) | 251 (15.0) | 75 (12.0) | 114 (14.3) | 54 (13.2) | 0.307 |
| Corticosteroids | 520 (14.9) | 291 (17.4) | 72 (11.6) | 114 (14.3) | 43 (10.5) | <0.001 |
| Blood product transfusionsb | 761 (21.7) | 387 (23.2) | 145 (23.3) | 161 (20.2) | 68 (16.7) | 0.018 |
| Vasoactive infusionsc | 381 (10.9) | 223 (13.3) | 63 (10.1) | 72 (9.0) | 23 (5.6) | <0.001 |
| Admission to intensive care | 1397 (39.9) | 731 (43.7) | 234 (37.6) | 296 (37.1) | 136 (33.3) | <0.001 |
| Extracorporeal membranous oxygenation | 18 (0.5) | 13 (0.8) | 2 (0.3) | 3 (0.4) | 0 (0.0) | 0.163 |
| Hemolytic‐uremic syndrome | 31 (0.9) | 15 (0.9) | 6 (1.0) | 7 (0.9) | 3 (0.7) | 0.985 |
| Influenza | 108 (3.1) | 53 (3.2) | 27 (4.3) | 23 (2.9) | 5 (1.2) | 0.044 |
| Arterial blood gas measurements | 0 (0,1) | 0 (0, 2) | 0 (0,1) | 0 (0, 1) | 0 (0, 1) | <0.001 |
| Days to first procedure | 1 (0, 3) | 1 (0, 2) | 1 (1, 3) | 1 (1, 3) | 1 (1, 3) | <0.001 |
Medical records of a randomly selected subset of subjects from 6 hospitals were reviewed to determine the accuracy of our algorithm in identifying patients with complicated pneumonia; these subjects represented 1% of the study population. For the purposes of medical record review, complicated pneumonia was defined by the following: 1) radiologically‐confirmed lung infiltrate; 2) moderate or large pleural effusion; and 3) signs and symptoms of lower respiratory tract infection. Complicated pneumonia was identified in 118 of 120 reviewed subjects for a positive predictive value of 98.3%.
All analyses were clustered by hospital. Analyses were performed using SAS version 9.2 (SAS Institute, Cary, NC). A two‐tailed P < 0.05 was considered statistically significant.
Results
Patient Characteristics
During the study period, 9,680 subjects had complicated pneumonia. Subjects were excluded if they did not have a pleural drainage procedure (n = 5798), or if thoracentesis was the first pleural fluid drainage procedure performed (n = 382). The remaining 3500 patients were included. Demographic characteristics are summarized in Table 1. The median patient age was 4.1 years (IQR: 2.17.2 years). An underlying CCC was present in 424 (12.1%) patients. There was no association between type of drainage procedure and mechanical ventilation. However, factors associated with more severe systemic illness, such as blood product transfusion, were more common among those undergoing initial chest tube placement with or without fibrinolysis (Table 1).
Initial Pleural Fluid Drainage Procedures
The primary procedures included chest tube without fibrinolysis (47.8%); chest tube with fibrinolysis (17.8%); thoracotomy (22.8%); and VATS (11.7%) (Table 1). The proportion of patients undergoing primary chest tube placement with fibrinolysis increased over time from 14.2% in 2004 to 30.0% in 2009 (P < 0.001; chi‐squared test for trend). The initial procedure varied by hospital with the greatest proportion of patients undergoing primary chest tube placement without fibrinolysis at 28 (70.0%) hospitals, chest tube placement with fibrinolysis at 5 (12.5%) hospitals, thoracotomy at 5 (12.5%) hospitals, and VATS at 2 (5.0%) hospitals (Figure 1). The median proportion of patients undergoing primary VATS across all hospitals was 11.5% (IQR: 3.9%‐26.5%) (Figure 1). The median time to first procedure was 1 day (IQR: 03 days).

Outcome Measures
Variation in outcomes occurred across hospitals. Additional pleural drainage procedures were performed in a median of 20.9% of patients with a range of 6.8% to 44.8% (IQR: 14.5%‐25.3%) of patients across all hospitals. Median LOS was 10 days with a range of 714 days (IQR: 8.511 days) and the median LOS following the initial pleural fluid drainage procedure was 8 days with a range of 6 to 13 days (IQR: 78 days). Variation in timing of the initial pleural fluid drainage procedure explained 9.6% of the variability in LOS (Spearman rho, 0.31; P < 0.001).
Overall, 118 (3.4%) patients were readmitted within 14 days of index discharge; the median readmission rate was 3.8% with a range of 0.8% to 33.3% (IQR: 2.1%‐5.8%) across hospitals. The median total cost of the index hospitalization was $19,574 (IQR: $13,791‐$31,063). The total cost for the index hospitalization exceeded $54,215 for 10% of patients and the total cost of the episode exceeded $55,208 for 10% of patients. Unadjusted outcomes, stratified by primary pleural fluid drainage procedure, are summarized in Table 2.
| Overall | Chest Tube Without Fibrinolysis | Chest Tube With Fibrinolysis | Thoracotomy | VATS | P Valueb | |
|---|---|---|---|---|---|---|
| ||||||
| Additional Procedure | 716 (20.5) | 331 (19.8) | 144 (23.1) | 197 (24.7) | 44 (10.8) | <0.001 |
| Readmission within 14 days | 118 (3.4) | 54 (3.3) | 13 (2.1) | 32 (4.0) | 19 (4.7) | 0.096 |
| Total LOS (days) | 10 (7, 14) | 10 (7, 14) | 9 (7, 13) | 10 (7, 14) | 9 (7, 12) | <.001 |
| Post‐initial Procedure LOS (days) | 8 (5, 12) | 8 (6, 12) | 7 (5, 10) | 8 (5, 12) | 7 (5, 10) | <0.001 |
| Total Cost, Index Hospitalization ($)e | 19319 (13358, 30955) | 19951 (13576, 32018)c | 19565 (13209, 32778)d | 20352 (14351, 31343) | 17918 (13531, 25166) | 0.016 |
| Total Cost, Episode of Illness ($)e | 19831 (13927, 31749) | 20151 (13764, 32653) | 19593 (13210, 32861) | 20573 (14419, 31753) | 18344 (13835, 25462) | 0.029 |
In multivariable analysis, differences in total LOS and post‐procedure LOS were not significant (Table 3). The odds of additional drainage procedures were higher for all drainage procedures compared with initial VATS (Table 3). Patients undergoing initial chest tube placement with fibrinolysis were less likely to require readmission compared with patients undergoing initial VATS (Table 3). The total cost for the episode of illness (including the cost of readmission) was significantly less for those undergoing primary chest tube placement without fibrinolysis compared with primary VATS. The results of subanalyses excluding patients with an underlying CCC (Supporting Appendix online, Table 4) and restricting the cohort to patients undergoing pleural drainage within two days of admission (Supporting Appendix online, Table 5) were similar to the results of our primary analysis with one exception; in the latter subanalysis, children undergoing initial chest tube placement without fibrinolysis were also less likely to require readmission compared with patients undergoing initial VATS.
| Adjusted OR (95% CI)a | P Value | |
|---|---|---|
| ||
| Additional pleural drainage procedure | ||
| Chest tube without fibrinolysis | 1.82 (1.103.00) | .019 |
| Chest tube with fibrinolysis | 2.31 (1.443.72) | <0.001 |
| Thoracotomy | 2.59 (1.624.14) | <0.001 |
| VATS | Reference | |
| Readmission within 14 days | ||
| Chest tube without fibrinolysis | 0.61 (0.361.05) | .077 |
| Chest tube with fibrinolysis | 0.45 (0.230.86) | .015 |
| Thoracotomy | 0.85 (0.521.39) | .521 |
| VATS | Reference | |
| Adjusted Mean (95% CI)a | P Value | |
| Total LOS (days) | ||
| Chest tube without fibrinolysis | 8.0 (7.88.2) | .339 |
| Chest tube with fibrinolysis | 8.1 (7.98.3) | .812 |
| Thoracotomy | 8.1 (7.98.3) | .632 |
| VATS | 8.1 (7.98.3) | Ref |
| Post‐initial procedure LOS (days) | ||
| Chest tube without fibrinolysis | 7.3 (7.07.5) | .512 |
| Chest tube with fibrinolysis | 7.5 (7.27.8) | .239 |
| Thoracotomy | 7.3 (7.07.6) | .841 |
| VATS | 7.3 (7.17.6) | Reference |
| Total cost, index hospitalization ($) | ||
| Chest tube without fibrinolysis | 22928 (2200023895 | .012 |
| Chest tube with fibrinolysis | 23621 (2263124655) | .657 |
| Thoracotomy | 23386 (2241924395 | .262 |
| VATS | 23820 (2280824878) | Reference |
| Total cost, episode of illness ($) | ||
| Chest tube without fibrinolysis | 23218 (2227824199) | .004 |
| Chest tube with fibrinolysis | 23749 (2275224790) | .253 |
| Thoracotomy | 23673 (2269324696) | .131 |
| VATS | 24280 (2324425362) | Reference |
Discussion
This multicenter study is the largest to evaluate the management of children hospitalized with complicated pneumonia. We found considerable variation in initial management and outcomes across hospitals. Differences in timing of the initial drainage procedure explained only a small amount of the variability in outcomes. Children undergoing initial VATS less commonly required additional drainage procedures while children undergoing initial chest tube placement with fibrinolysis less commonly required readmission. Differences in total and post‐procedure LOS were not statistically significant. Differences in cost, while statistically significant, were of marginal relevance.
Previous studies have also shown significant variation in treatment and outcomes of children with complicated pneumonia across hospitals.2, 8 Our study provides data from additional hospitals, includes a substantially larger number of patients undergoing initial VATS, distinguishes between fibrinolysis recipients and nonrecipients, and is the first to compare outcomes between four different initial drainage strategies. The creation of national consensus guidelines might reduce variability in initial management strategies, although the variability in outcomes across hospitals in the current study could not be explained simply by differences in the type or timing of the initial drainage procedure. Thus, future studies examining hospital‐level factors may play an important role in improving quality of care for children with complicated pneumonia.
Patients with initial thoracotomy or chest tube placement with or without fibrinolysis more commonly received additional drainage procedures than patients with initial VATS. This difference remained when patients with CCCs were excluded from the analysis and when the analysis was limited to patients undergoing pleural fluid drainage within 2 days of hospitalization. Several small, randomized trials demonstrated conflicting results when comparing initial chest tube placement with fibrinolysis and VATS. St. Peter et al22 reported that 3 (17%) of 18 patients undergoing initial chest tube placement with fibrinolysis and none of the 18 patients undergoing initial VATS received additional pleural drainage procedures. Sonnappa et al21 found no differences between the two groups. Kurt et al19 did not state the proportion of patients receiving additional procedures. However, the mean number of drainage procedures was 2.25 among the 8 patients undergoing initial chest tube placement while none of the 10 patients with VATS received additional drainage.19
Thoracotomy is often perceived as a definitive procedure for treatment of complicated pneumonia. However, several possibilities exist to explain why additional procedures were performed less frequently in patients undergoing initial VATS compared with initial thoracotomy. The limited visual field in thoracotomy may lead to greater residual disease post‐operatively in those receiving thoracotomy compared with VATS.31 Additionally, thoracotomy substantially disrupts the integrity of the chest wall and is consequently associated with complications such as bleeding and air leak into the pleural cavity more often than VATS.31, 32 It is thus possible that some of the additional procedures in patients receiving initial thoracotomy were necessary for management of thoracotomy‐associated complications rather than for failure of the initial drainage procedure.
Similar to the randomized trials by Sonnappa et al21 and St. Peter et al,22 differences in the overall and post‐procedure LOS were not significant among patients undergoing initial VATS compared with initial chest tube placement with fibrinolysis. However, chest tube placement without fibrinolysis did not result in significant differences in LOS compared with initial VATS. In the only pediatric randomized trial, the 29 intrapleural urokinase recipients had a 2 day shorter LOS compared with the 29 intrapleural saline recipients.33 Several small, randomized controlled trials of adults with complicated pneumonia reported improved pleural fluid drainage among intrapleural fibrinolysis recipients compared with non‐recipients.3436 However, a large multicenter randomized trial in adults found no differences in mortality, requirement for surgical drainage, or LOS between intrapleural streptokinase and placebo recipients.37 Subsequent meta‐analyses of randomized trials in adults also demonstrated no benefit to fibrinolysis.38, 39 In the context of the increasing use of intrapleural fibrinolysis in children with complicated pneumonia, our results highlight the need for a large, multicenter randomized controlled trial to determine whether chest tube with fibrinolysis is superior to chest tube alone.
Two small randomized trials21, 22 and a decision analysis40 identified chest tube with fibrinolysis as the most economical approach to children with complicated pneumonia. However, the costs did not differ significantly between patients undergoing initial VATS or initial chest tube placement with fibrinolysis in our study. The least costly approach was initial chest tube placement without fibrinolysis. Unlike the randomized controlled trials, we considered costs associated with readmissions in determining the total costs. Shah et al41 found no difference in total charges for patients undergoing initial VATS compared with initial chest tube placement; however, patients undergoing initial VATS were concentrated in a few centers, making it difficult to determine the relative importance of procedural and hospital factors.
This multicenter observational study has several limitations. First, discharge diagnosis coding may be unreliable for specific diseases. However, our rigorous definition of complicated pneumonia, supported by the high positive predictive value as verified by medical record review, minimizes the likelihood of misclassification.
Second, unmeasured confounding or residual confounding by indication for the method of pleural drainage may occur, potentially influencing our results in two disparate ways. If patients with more severe systemic illness were too unstable for operative interventions, then our results would be biased towards worse outcomes for children undergoing initial chest tube placement. We adjusted for several variables associated with a greater systemic severity of illness, including intensive care unit admission, making this possibility less likely. We also could not account for some factors associated with more severe local disease such as the size and character of the effusion. We suspect that patients with more extensive local disease (ie, loculated effusions) would have worse outcomes than other patients, regardless of initial procedure, and that these patients would also be more likely to undergo primary surgical drainage. Thus, this study may have underestimated the benefit of initial surgical drainage (eg, VATS) compared with nonsurgical drainage (ie, chest tube placement).
Third, misclassification of the method of initial pleural drainage may have occurred. Patients transferred from another institution following chest tube placement could either be classified as not receiving pleural drainage and thus excluded from the study or classified as having initial VATS or thoracotomy if the reason for transfer was chest tube treatment failure. Additionally, we could not distinguish routine use of fibrinolysis from fibrinolysis to maintain chest tube patency. Whether such misclassification would falsely minimize or maximize differences in outcomes between the various groups remains uncertain. Fourth, because this study only included tertiary care children's hospitals, these data are not generalizable to community settings. VATS requires specialized surgical training that may be unavailable in some areas. Finally, this study demonstrates the relative efficacy of various pleural fluid drainage procedures on short‐term clinical outcomes and resource utilization. However, long‐term functional outcomes should be measured in future prospective studies.
Conclusions
In conclusion, emphasis on evidence driven treatment to optimize care has led to an increasing examination of unwarranted practice variation.42 The lack of evidence for best practice makes it difficult to define unwarranted variation in the treatment of complicated pneumonia. Our study demonstrates the large variability in practice and raises additional questions regarding the optimal drainage strategies. Published randomized trials have focused on comparisons between chest tube placement with fibrinolysis and VATS. However, our data suggest that future randomized trials should include chest tube placement without fibrinolysis as a treatment strategy. In determining the current best treatment for patients with complicated pneumonia, a clinician must weigh the impact of needing an additional procedure in approximately one‐quarter of patients undergoing initial chest tube placement (with or without fibrinolysis) with the risks of general anesthesia and readmission in patients undergoing initial VATS.
Acknowledgements
Dr. Hall had full access to all the data in the study and takes responsibility for the integrity of the data and accuracy of the analysis.
- ,.Parapneumonic pleural effusion and empyema in children. Review of a 19‐year experience, 1962–1980.Clin Pediatr (Phila).1983;22:414–419.
- ,,,,.Primary early thoracoscopy and reduction in length of hospital stay and additional procedures among children with complicated pneumonia: Results of a multicenter retrospective cohort study.Arch Pediatr Adolesc Med.2008;162:675–681.
- ,.Empyema hospitalizations increased in US children despite pneumococcal conjugate vaccine.Pediatrics.2010;125:26–33.
- ,,, et al.Impact of the pneumococcal conjugate vaccine on pneumococcal parapneumonic empyema.Pediatr Infect Dis J.2006;25:250–254.
- ,,,,.Five‐fold increase in pediatric parapneumonic empyema since introduction of pneumococcal conjugate vaccine.Pediatric Infect Dis J.2008;27:1030–1032.
- ,,,.Increasing incidence of empyema complicating childhood community‐acquired pneumonia in the United States.Clin Infect Dis.2010;50:805–813.
- ,,,,.National hospitalization trends for pediatric pneumonia and associated complications.Pediatrics.2010;126:204–213.
- ,,, et al.Empyema associated with community‐acquired pneumonia: A Pediatric Investigator's Collaborative Network on Infections in Canada (PICNIC) study.BMC Infect Dis.2008;8:129.
- ,,,,.Pleural empyema in children.Ann Thorac Surg.1970;10:37–44.
- ,,.Management of streptococcal empyema.Ann Thorac Surg.1966;2:658–664.
- ,.Thoracoscopy in the management of empyema in children.J Pediatr Surg.1993;28:1128–1132.
- ,,,.Surgical treatment of parapneumonic empyema.Pediatr Pulmonol.1996;22:348–356.
- ,.The controversial role of decortication in the management of pediatric empyema.J Thorac Cardiovasc Surg.1988;96:166–170.
- ,,,,,.Postpneumonic empyema in children treated by early decortication.Eur J Pediatr Surg.1997;7:135–137.
- ,.Video‐assisted thoracoscopic surgery in the management of pediatric empyema.JSLS.1997;1:251–3.
- ,,,.Early video‐assisted thoracic surgery in the management of empyema.Pediatrics.1999;103:e63.
- ,,,,.Early definitive intervention by thoracoscopy in pediatric empyema.J Pediatr Surg.1999;34:178–180; discussion80–81.
- ,,,,.Thoracoscopy in the management of pediatric empyema.J Pediatr Surg.1995;30:1211–1215.
- ,,,,.Therapy of parapneumonic effusions in children: Video‐assisted thoracoscopic surgery versus conventional thoracostomy drainage.Pediatrics.2006;118:e547–e553.
- ,.Primary operative management for pediatric empyema: Decreases in hospital length of stay and charges in a national sample.Arch Pediatr Adolesc Med.2008;162:44–48.
- ,,, et al.Comparison of urokinase and video‐assisted thoracoscopic surgery for treatment of childhood empyema.Am J Respir Crit Care Med.2006;174:221–227.
- ,,, et al.Thoracoscopic decortication vs tube thoracostomy with fibrinolysis for empyema in children: A prospective, randomized trial.J Pediatr Surg.2009;44:106–111; discussion11.
- ,,,.Corticosteroids and mortality in children with bacterial meningitis.JAMA.2008;299:2048–2055.
- ,,,,.Intravenous immunoglobulin in children with streptococcal toxic shock syndrome.Clin Infect Dis.2009;49:1369–1376.
- ,,, et al.Deaths attributed to pediatric complex chronic conditions: National trends and implications for supportive care services.Pediatrics.2001;107:e99.
- ,.Multiple regression of cost data: Use of generalised linear models.J Health Serv Res Policy.2004;9:197–204.
- ,,,.A robustified modeling approach to analyze pediatric length of stay.Ann Epidemiol.2005;15:673–677.
- ,,.Correlates of length of stay, cost of care, and mortality among patients hospitalized for necrotizing fasciitis.Epidemiol Infect.2007;135:868–876.
- ,,,,,.Health care costs of adults treated for attention‐deficit/hyperactivity disorder who received alternative drug therapies.J Manag Care Pharm.2007;13:561–569.
- .The role of the propensity score in estimating dose‐response functions.Biometrika.2000;87:706–710.
- ,,,,.Experience with video‐assisted thoracoscopic surgery in the management of complicated pneumonia in children.J Pediatr Surg.2001;36:316–319.
- ,,, et al.VATS debridement versus thoracotomy in the treatment of loculated postpneumonia empyema.Ann Thorac Surg.1996;61:1626–1630.
- ,,,,.Randomised trial of intrapleural urokinase in the treatment of childhood empyema.Thorax.2002;57:343–347.
- ,,,,,.Intrapleural urokinase versus normal saline in the treatment of complicated parapneumonic effusions and empyema. A randomized, double‐blind study.Am J Respir Crit Care Med.1999;159:37–42.
- ,,.Randomised controlled trial of intrapleural streptokinase in community acquired pleural infection.Thorax.1997;52:416–421.
- ,,,,.Intrapleural streptokinase for empyema and complicated parapneumonic effusions.Am J Respir Crit Care Med.2004;170:49–53.
- ,,, et al.U.K. Controlled trial of intrapleural streptokinase for pleural infection.N Engl J Med.2005;352:865–874.
- ,.Intra‐pleural fibrinolytic therapy versus conservative management in the treatment of adult parapneumonic effusions and empyema.Cochrane Database Syst Rev.2008:CD002312.
- ,,,.Intrapleural fibrinolytic agents for empyema and complicated parapneumonic effusions: A meta‐analysis.Chest.2006;129:783–790.
- ,,.Cost‐effectiveness of competing strategies for the treatment of pediatric empyema.Pediatrics.2008;121:e1250–e1257.
- ,,.Costs of treating children with complicated pneumonia: A comparison of primary video‐assisted thoracoscopic surgery and chest tube placement.Pediatr Pulmonol.2010;45:71–77.
- .Unwarranted variation in pediatric medical care.Pediatr Clin North Am.2009;56:745–755.
- ,.Parapneumonic pleural effusion and empyema in children. Review of a 19‐year experience, 1962–1980.Clin Pediatr (Phila).1983;22:414–419.
- ,,,,.Primary early thoracoscopy and reduction in length of hospital stay and additional procedures among children with complicated pneumonia: Results of a multicenter retrospective cohort study.Arch Pediatr Adolesc Med.2008;162:675–681.
- ,.Empyema hospitalizations increased in US children despite pneumococcal conjugate vaccine.Pediatrics.2010;125:26–33.
- ,,, et al.Impact of the pneumococcal conjugate vaccine on pneumococcal parapneumonic empyema.Pediatr Infect Dis J.2006;25:250–254.
- ,,,,.Five‐fold increase in pediatric parapneumonic empyema since introduction of pneumococcal conjugate vaccine.Pediatric Infect Dis J.2008;27:1030–1032.
- ,,,.Increasing incidence of empyema complicating childhood community‐acquired pneumonia in the United States.Clin Infect Dis.2010;50:805–813.
- ,,,,.National hospitalization trends for pediatric pneumonia and associated complications.Pediatrics.2010;126:204–213.
- ,,, et al.Empyema associated with community‐acquired pneumonia: A Pediatric Investigator's Collaborative Network on Infections in Canada (PICNIC) study.BMC Infect Dis.2008;8:129.
- ,,,,.Pleural empyema in children.Ann Thorac Surg.1970;10:37–44.
- ,,.Management of streptococcal empyema.Ann Thorac Surg.1966;2:658–664.
- ,.Thoracoscopy in the management of empyema in children.J Pediatr Surg.1993;28:1128–1132.
- ,,,.Surgical treatment of parapneumonic empyema.Pediatr Pulmonol.1996;22:348–356.
- ,.The controversial role of decortication in the management of pediatric empyema.J Thorac Cardiovasc Surg.1988;96:166–170.
- ,,,,,.Postpneumonic empyema in children treated by early decortication.Eur J Pediatr Surg.1997;7:135–137.
- ,.Video‐assisted thoracoscopic surgery in the management of pediatric empyema.JSLS.1997;1:251–3.
- ,,,.Early video‐assisted thoracic surgery in the management of empyema.Pediatrics.1999;103:e63.
- ,,,,.Early definitive intervention by thoracoscopy in pediatric empyema.J Pediatr Surg.1999;34:178–180; discussion80–81.
- ,,,,.Thoracoscopy in the management of pediatric empyema.J Pediatr Surg.1995;30:1211–1215.
- ,,,,.Therapy of parapneumonic effusions in children: Video‐assisted thoracoscopic surgery versus conventional thoracostomy drainage.Pediatrics.2006;118:e547–e553.
- ,.Primary operative management for pediatric empyema: Decreases in hospital length of stay and charges in a national sample.Arch Pediatr Adolesc Med.2008;162:44–48.
- ,,, et al.Comparison of urokinase and video‐assisted thoracoscopic surgery for treatment of childhood empyema.Am J Respir Crit Care Med.2006;174:221–227.
- ,,, et al.Thoracoscopic decortication vs tube thoracostomy with fibrinolysis for empyema in children: A prospective, randomized trial.J Pediatr Surg.2009;44:106–111; discussion11.
- ,,,.Corticosteroids and mortality in children with bacterial meningitis.JAMA.2008;299:2048–2055.
- ,,,,.Intravenous immunoglobulin in children with streptococcal toxic shock syndrome.Clin Infect Dis.2009;49:1369–1376.
- ,,, et al.Deaths attributed to pediatric complex chronic conditions: National trends and implications for supportive care services.Pediatrics.2001;107:e99.
- ,.Multiple regression of cost data: Use of generalised linear models.J Health Serv Res Policy.2004;9:197–204.
- ,,,.A robustified modeling approach to analyze pediatric length of stay.Ann Epidemiol.2005;15:673–677.
- ,,.Correlates of length of stay, cost of care, and mortality among patients hospitalized for necrotizing fasciitis.Epidemiol Infect.2007;135:868–876.
- ,,,,,.Health care costs of adults treated for attention‐deficit/hyperactivity disorder who received alternative drug therapies.J Manag Care Pharm.2007;13:561–569.
- .The role of the propensity score in estimating dose‐response functions.Biometrika.2000;87:706–710.
- ,,,,.Experience with video‐assisted thoracoscopic surgery in the management of complicated pneumonia in children.J Pediatr Surg.2001;36:316–319.
- ,,, et al.VATS debridement versus thoracotomy in the treatment of loculated postpneumonia empyema.Ann Thorac Surg.1996;61:1626–1630.
- ,,,,.Randomised trial of intrapleural urokinase in the treatment of childhood empyema.Thorax.2002;57:343–347.
- ,,,,,.Intrapleural urokinase versus normal saline in the treatment of complicated parapneumonic effusions and empyema. A randomized, double‐blind study.Am J Respir Crit Care Med.1999;159:37–42.
- ,,.Randomised controlled trial of intrapleural streptokinase in community acquired pleural infection.Thorax.1997;52:416–421.
- ,,,,.Intrapleural streptokinase for empyema and complicated parapneumonic effusions.Am J Respir Crit Care Med.2004;170:49–53.
- ,,, et al.U.K. Controlled trial of intrapleural streptokinase for pleural infection.N Engl J Med.2005;352:865–874.
- ,.Intra‐pleural fibrinolytic therapy versus conservative management in the treatment of adult parapneumonic effusions and empyema.Cochrane Database Syst Rev.2008:CD002312.
- ,,,.Intrapleural fibrinolytic agents for empyema and complicated parapneumonic effusions: A meta‐analysis.Chest.2006;129:783–790.
- ,,.Cost‐effectiveness of competing strategies for the treatment of pediatric empyema.Pediatrics.2008;121:e1250–e1257.
- ,,.Costs of treating children with complicated pneumonia: A comparison of primary video‐assisted thoracoscopic surgery and chest tube placement.Pediatr Pulmonol.2010;45:71–77.
- .Unwarranted variation in pediatric medical care.Pediatr Clin North Am.2009;56:745–755.
Copyright © 2011 Society of Hospital Medicine
Intervention Progress
A new study of patients with ischemic stroke (JAMA. 2011;305:373-380) found that those admitted to hospitals certified as primary stroke centers had a modestly lower risk of death and serious disability.
Ying Xian, MD, PhD, of the Duke Clinical Research Institute in Durham, N.C., and colleagues compared mortality rates for 30,000 stroke patients in a New York state database, half of them admitted to certified stroke centers and the rest to nondesignated hospitals. Overall 30-day, all-cause mortality was 10.1% for the former group, 12.5% for the latter.
But this modestly lower death rate is still important, says Mark J. Alberts, MD, director of the stroke program at Northwestern University Feinberg School of Medicine in Chicago. "There aren't that many interventions we do in modern medical care that actually prevent death."
In an editorial accompanying the JAMA stroke study, Dr. Alberts portrays an emerging, multitiered system of stroke care, "with the comprehensive stroke center at the top of the pyramid, the primary stroke center in the middle, and the acute stroke-ready hospital at the base." He compares this emerging system to trauma care, which has Level 1 trauma centers at the top of its pyramid.
Not every hospital within a region might be able to pursue stroke center certification, or even become more stroke-ready, he says. But hospitals could work collaboratively to create regional stroke referral networks based on the distribution of patients and resources. Hospitalists can help promote such networks (see The Hospitalist, December 2009). "I would start by knowing your patient population, how many stroke patients present at your hospital each year," he explains.
"The overriding concept is to get stroke patients as efficiently and safely as possible to the hospital that can provide them with the most appropriate level of care," Dr. Alberts says.
More than 800 U.S. hospitals are certified as primary stroke centers by the Joint Commission. While there are not yet formal standards or requirements for an acute-stroke-ready hospital, the term suggests mobilizing resources, capabilities and expertise to receive stroke patients, stabilize them, and send them to the most appropriate facilities based on their medical needs.
A new study of patients with ischemic stroke (JAMA. 2011;305:373-380) found that those admitted to hospitals certified as primary stroke centers had a modestly lower risk of death and serious disability.
Ying Xian, MD, PhD, of the Duke Clinical Research Institute in Durham, N.C., and colleagues compared mortality rates for 30,000 stroke patients in a New York state database, half of them admitted to certified stroke centers and the rest to nondesignated hospitals. Overall 30-day, all-cause mortality was 10.1% for the former group, 12.5% for the latter.
But this modestly lower death rate is still important, says Mark J. Alberts, MD, director of the stroke program at Northwestern University Feinberg School of Medicine in Chicago. "There aren't that many interventions we do in modern medical care that actually prevent death."
In an editorial accompanying the JAMA stroke study, Dr. Alberts portrays an emerging, multitiered system of stroke care, "with the comprehensive stroke center at the top of the pyramid, the primary stroke center in the middle, and the acute stroke-ready hospital at the base." He compares this emerging system to trauma care, which has Level 1 trauma centers at the top of its pyramid.
Not every hospital within a region might be able to pursue stroke center certification, or even become more stroke-ready, he says. But hospitals could work collaboratively to create regional stroke referral networks based on the distribution of patients and resources. Hospitalists can help promote such networks (see The Hospitalist, December 2009). "I would start by knowing your patient population, how many stroke patients present at your hospital each year," he explains.
"The overriding concept is to get stroke patients as efficiently and safely as possible to the hospital that can provide them with the most appropriate level of care," Dr. Alberts says.
More than 800 U.S. hospitals are certified as primary stroke centers by the Joint Commission. While there are not yet formal standards or requirements for an acute-stroke-ready hospital, the term suggests mobilizing resources, capabilities and expertise to receive stroke patients, stabilize them, and send them to the most appropriate facilities based on their medical needs.
A new study of patients with ischemic stroke (JAMA. 2011;305:373-380) found that those admitted to hospitals certified as primary stroke centers had a modestly lower risk of death and serious disability.
Ying Xian, MD, PhD, of the Duke Clinical Research Institute in Durham, N.C., and colleagues compared mortality rates for 30,000 stroke patients in a New York state database, half of them admitted to certified stroke centers and the rest to nondesignated hospitals. Overall 30-day, all-cause mortality was 10.1% for the former group, 12.5% for the latter.
But this modestly lower death rate is still important, says Mark J. Alberts, MD, director of the stroke program at Northwestern University Feinberg School of Medicine in Chicago. "There aren't that many interventions we do in modern medical care that actually prevent death."
In an editorial accompanying the JAMA stroke study, Dr. Alberts portrays an emerging, multitiered system of stroke care, "with the comprehensive stroke center at the top of the pyramid, the primary stroke center in the middle, and the acute stroke-ready hospital at the base." He compares this emerging system to trauma care, which has Level 1 trauma centers at the top of its pyramid.
Not every hospital within a region might be able to pursue stroke center certification, or even become more stroke-ready, he says. But hospitals could work collaboratively to create regional stroke referral networks based on the distribution of patients and resources. Hospitalists can help promote such networks (see The Hospitalist, December 2009). "I would start by knowing your patient population, how many stroke patients present at your hospital each year," he explains.
"The overriding concept is to get stroke patients as efficiently and safely as possible to the hospital that can provide them with the most appropriate level of care," Dr. Alberts says.
More than 800 U.S. hospitals are certified as primary stroke centers by the Joint Commission. While there are not yet formal standards or requirements for an acute-stroke-ready hospital, the term suggests mobilizing resources, capabilities and expertise to receive stroke patients, stabilize them, and send them to the most appropriate facilities based on their medical needs.
The Facebook of Medical Records
A California hospitalist has launched a website that he envisions as an electronic health records (EHR) portal for physicians and patients alike.
MDblackbox aims to blend the interactive familiarity of such social networks as Facebook with the institutional-grade security necessary to comply with the Health Insurance Portability and Accountability Act (HIPPA), according to its inventor, Sami Bogale, MD, a hospitalist at Mills-Peninsula Medical Center in Burlingame, Calif.
"The idea is for any doctor to sign up, nationwide, and [the site] will provide uninterrupted communication" both between doctors and between physicians and patients, says Dr. Bogale, CEO of MDblackbox Inc. "The idea from the patient side is that if a patient goes out of town or goes to a new physician, they have a real copy of their medical record right there."
Dr. Bogale says he's been working on the site for the better part of three years and has spent $250,000 or so on its development. (He jokes that he doesn’t want to know how much time he’s spent on it.) He decided to launch the site this month as he saw other entrepreneurs and physicians looking to take advantage of the momentum behind EHR, buzz attributable in large part to health reform. In fact, the Centers for Medicare & Medicaid Services announced in January that registration had begun for applications to garner a piece of the $20 billion the federal government has set aside for doctors and hospitals that adopt new technologies.
Dr. Bogale continues to look for venture capitalists to back his site, which includes records management, appointment scheduling and reminder, lab orders and voice recordings that can be attached to medical files. Most services are free.
"The idea is to have a nationwide system where every doctor could pretty much have their own personal page and interact with doctors and patients," Dr. Bogale says. "I could see it really growing."
A California hospitalist has launched a website that he envisions as an electronic health records (EHR) portal for physicians and patients alike.
MDblackbox aims to blend the interactive familiarity of such social networks as Facebook with the institutional-grade security necessary to comply with the Health Insurance Portability and Accountability Act (HIPPA), according to its inventor, Sami Bogale, MD, a hospitalist at Mills-Peninsula Medical Center in Burlingame, Calif.
"The idea is for any doctor to sign up, nationwide, and [the site] will provide uninterrupted communication" both between doctors and between physicians and patients, says Dr. Bogale, CEO of MDblackbox Inc. "The idea from the patient side is that if a patient goes out of town or goes to a new physician, they have a real copy of their medical record right there."
Dr. Bogale says he's been working on the site for the better part of three years and has spent $250,000 or so on its development. (He jokes that he doesn’t want to know how much time he’s spent on it.) He decided to launch the site this month as he saw other entrepreneurs and physicians looking to take advantage of the momentum behind EHR, buzz attributable in large part to health reform. In fact, the Centers for Medicare & Medicaid Services announced in January that registration had begun for applications to garner a piece of the $20 billion the federal government has set aside for doctors and hospitals that adopt new technologies.
Dr. Bogale continues to look for venture capitalists to back his site, which includes records management, appointment scheduling and reminder, lab orders and voice recordings that can be attached to medical files. Most services are free.
"The idea is to have a nationwide system where every doctor could pretty much have their own personal page and interact with doctors and patients," Dr. Bogale says. "I could see it really growing."
A California hospitalist has launched a website that he envisions as an electronic health records (EHR) portal for physicians and patients alike.
MDblackbox aims to blend the interactive familiarity of such social networks as Facebook with the institutional-grade security necessary to comply with the Health Insurance Portability and Accountability Act (HIPPA), according to its inventor, Sami Bogale, MD, a hospitalist at Mills-Peninsula Medical Center in Burlingame, Calif.
"The idea is for any doctor to sign up, nationwide, and [the site] will provide uninterrupted communication" both between doctors and between physicians and patients, says Dr. Bogale, CEO of MDblackbox Inc. "The idea from the patient side is that if a patient goes out of town or goes to a new physician, they have a real copy of their medical record right there."
Dr. Bogale says he's been working on the site for the better part of three years and has spent $250,000 or so on its development. (He jokes that he doesn’t want to know how much time he’s spent on it.) He decided to launch the site this month as he saw other entrepreneurs and physicians looking to take advantage of the momentum behind EHR, buzz attributable in large part to health reform. In fact, the Centers for Medicare & Medicaid Services announced in January that registration had begun for applications to garner a piece of the $20 billion the federal government has set aside for doctors and hospitals that adopt new technologies.
Dr. Bogale continues to look for venture capitalists to back his site, which includes records management, appointment scheduling and reminder, lab orders and voice recordings that can be attached to medical files. Most services are free.
"The idea is to have a nationwide system where every doctor could pretty much have their own personal page and interact with doctors and patients," Dr. Bogale says. "I could see it really growing."
The Locums Litmus Test: Is Per Diem Work for You?
Family-medicine-trained hospitalist Benjamin Craig Hamilton, MD,mightrepresent the ideal locum tenens candidate. He’s single, enjoys the challenge of working in new environments, and has is licensed in six states. Currently based in Knoxville, Tenn., he values the professional and monetary rewards of per diem work.
“The beauty is that you can work three months and take three months off,” says Dr. Hamilton, one of thousands of physicians who contract to provide temporary coverage to fill staffing gaps at HM programs around the country. Dr. Hamilton works for Locum Leaders LLC, a national locums firm headquartered in Alpharetta, Ga., that focuses on hospitalists. “If you’re willing to go, you’re never without work. It’s the ultimate flexibility.”
Are You Suited?
Flexibilitytops the list of qualities locum tenens recruiters look for. “The more they [the candidates] can say ‘yes,’ the more likely that we can find a good fit for them,” says Andrea Oldendorf, physician recruiter for Nashville, Tenn.-based Cogent Healthcare. Oldendorfhandles locum tenens placements in the company’s central and western regions.
“We’re a very team-oriented company,” Oldendorf says, noting her objective is to integrate a locum physician into an HM group. “If someone comes in with a lot of limitations, such as ‘I won’t work nights’ or ‘I won’t cover ER admissions,’ that starts to narrow the pool of places where we can use them.”
Every HM program is a little different, according to Robert W. Harrington, MD, SFHM, chief medical officer at Locum Leaders LLC and chair of SHM’s Family Medicine Task Force. “Being able to work with new people, new systems, new computers, and new nursing staff is very important,” he says. Interestingly, the credentialing process provides his recruiters an avenue for gaugingcandidates’ flexibility.“There is so much paperwork, and there are certain parts of it that we can’t do. By the time they get through credentialing, we know whether they’re flexible or not.”
Temporary Work = Test Run
The monetary rewards of locums work often are cited as a major advantage. Locum physicians can gross 30% to 40% more per year (about $280,000 to $300,000) for the same number of shifts as a typical FTE hospitalist (median compensation for non-teaching hospitalists is $215,000 per year, according to the 2010 MGMA-SHM State of Hospital Medicine report). As contracted employees, locum physicians are self-employed and must report their own income and pay their own taxes; many times locum physicians need to hire an accountant.
Working locum tenens also offers a chance to sample a variety of hospital settings. “There’s a big difference between a small rural hospital and a big teaching hospital with a closed ICU,” points out Karen Belote, a physician recruiter for Locum Leaders. “[Working locums] is a great way to try some different locations and different hospitals before settling into a permanent position.”
Pitfalls and Cautions
The only pitfall Dr. Hamilton has observed is perception.“Some people have an initial gut reflex that a locums doctor is either running from something or is not of the same caliber as a permanent physician,” he says. But that notion is quickly dispelled, he says, when he or she demonstrates flexibility, competency, and eagerness to work with the team.
Dr. Hamilton has found a great fit with his current assignments and has no immediate plans to take a permanent position. “I’m learning every day,” he says. “Remember, you are the boss of your life.”
Gretchen Henkel is a freelance writer based in California.
Increase your hirability
Thinking of taking a locum tenens assignment? Consider acquiring additional procedural competencies during your third year of residency, Dr. Harrington advises.
“We are getting more and more requests for people who are comfortable with procedural skills, such as intubations, placing central lines, and vent management,” he says.
Your locum tenens agency might underwrite a procedures course, as Locum Leaders did for Dr. Hamilton. Just make sure you don’t misrepresent your abilities or your comfort level, he cautions. “Know your limits and realize that you are building your career every month,” he says.
SHM will offer a pre-course, “Medical Procedures for the Hospitalist,” at HM11 (May 9, 2011 in Grapevine, Texas), and at many of the HM regional meetings.—GH
Family-medicine-trained hospitalist Benjamin Craig Hamilton, MD,mightrepresent the ideal locum tenens candidate. He’s single, enjoys the challenge of working in new environments, and has is licensed in six states. Currently based in Knoxville, Tenn., he values the professional and monetary rewards of per diem work.
“The beauty is that you can work three months and take three months off,” says Dr. Hamilton, one of thousands of physicians who contract to provide temporary coverage to fill staffing gaps at HM programs around the country. Dr. Hamilton works for Locum Leaders LLC, a national locums firm headquartered in Alpharetta, Ga., that focuses on hospitalists. “If you’re willing to go, you’re never without work. It’s the ultimate flexibility.”
Are You Suited?
Flexibilitytops the list of qualities locum tenens recruiters look for. “The more they [the candidates] can say ‘yes,’ the more likely that we can find a good fit for them,” says Andrea Oldendorf, physician recruiter for Nashville, Tenn.-based Cogent Healthcare. Oldendorfhandles locum tenens placements in the company’s central and western regions.
“We’re a very team-oriented company,” Oldendorf says, noting her objective is to integrate a locum physician into an HM group. “If someone comes in with a lot of limitations, such as ‘I won’t work nights’ or ‘I won’t cover ER admissions,’ that starts to narrow the pool of places where we can use them.”
Every HM program is a little different, according to Robert W. Harrington, MD, SFHM, chief medical officer at Locum Leaders LLC and chair of SHM’s Family Medicine Task Force. “Being able to work with new people, new systems, new computers, and new nursing staff is very important,” he says. Interestingly, the credentialing process provides his recruiters an avenue for gaugingcandidates’ flexibility.“There is so much paperwork, and there are certain parts of it that we can’t do. By the time they get through credentialing, we know whether they’re flexible or not.”
Temporary Work = Test Run
The monetary rewards of locums work often are cited as a major advantage. Locum physicians can gross 30% to 40% more per year (about $280,000 to $300,000) for the same number of shifts as a typical FTE hospitalist (median compensation for non-teaching hospitalists is $215,000 per year, according to the 2010 MGMA-SHM State of Hospital Medicine report). As contracted employees, locum physicians are self-employed and must report their own income and pay their own taxes; many times locum physicians need to hire an accountant.
Working locum tenens also offers a chance to sample a variety of hospital settings. “There’s a big difference between a small rural hospital and a big teaching hospital with a closed ICU,” points out Karen Belote, a physician recruiter for Locum Leaders. “[Working locums] is a great way to try some different locations and different hospitals before settling into a permanent position.”
Pitfalls and Cautions
The only pitfall Dr. Hamilton has observed is perception.“Some people have an initial gut reflex that a locums doctor is either running from something or is not of the same caliber as a permanent physician,” he says. But that notion is quickly dispelled, he says, when he or she demonstrates flexibility, competency, and eagerness to work with the team.
Dr. Hamilton has found a great fit with his current assignments and has no immediate plans to take a permanent position. “I’m learning every day,” he says. “Remember, you are the boss of your life.”
Gretchen Henkel is a freelance writer based in California.
Increase your hirability
Thinking of taking a locum tenens assignment? Consider acquiring additional procedural competencies during your third year of residency, Dr. Harrington advises.
“We are getting more and more requests for people who are comfortable with procedural skills, such as intubations, placing central lines, and vent management,” he says.
Your locum tenens agency might underwrite a procedures course, as Locum Leaders did for Dr. Hamilton. Just make sure you don’t misrepresent your abilities or your comfort level, he cautions. “Know your limits and realize that you are building your career every month,” he says.
SHM will offer a pre-course, “Medical Procedures for the Hospitalist,” at HM11 (May 9, 2011 in Grapevine, Texas), and at many of the HM regional meetings.—GH
Family-medicine-trained hospitalist Benjamin Craig Hamilton, MD,mightrepresent the ideal locum tenens candidate. He’s single, enjoys the challenge of working in new environments, and has is licensed in six states. Currently based in Knoxville, Tenn., he values the professional and monetary rewards of per diem work.
“The beauty is that you can work three months and take three months off,” says Dr. Hamilton, one of thousands of physicians who contract to provide temporary coverage to fill staffing gaps at HM programs around the country. Dr. Hamilton works for Locum Leaders LLC, a national locums firm headquartered in Alpharetta, Ga., that focuses on hospitalists. “If you’re willing to go, you’re never without work. It’s the ultimate flexibility.”
Are You Suited?
Flexibilitytops the list of qualities locum tenens recruiters look for. “The more they [the candidates] can say ‘yes,’ the more likely that we can find a good fit for them,” says Andrea Oldendorf, physician recruiter for Nashville, Tenn.-based Cogent Healthcare. Oldendorfhandles locum tenens placements in the company’s central and western regions.
“We’re a very team-oriented company,” Oldendorf says, noting her objective is to integrate a locum physician into an HM group. “If someone comes in with a lot of limitations, such as ‘I won’t work nights’ or ‘I won’t cover ER admissions,’ that starts to narrow the pool of places where we can use them.”
Every HM program is a little different, according to Robert W. Harrington, MD, SFHM, chief medical officer at Locum Leaders LLC and chair of SHM’s Family Medicine Task Force. “Being able to work with new people, new systems, new computers, and new nursing staff is very important,” he says. Interestingly, the credentialing process provides his recruiters an avenue for gaugingcandidates’ flexibility.“There is so much paperwork, and there are certain parts of it that we can’t do. By the time they get through credentialing, we know whether they’re flexible or not.”
Temporary Work = Test Run
The monetary rewards of locums work often are cited as a major advantage. Locum physicians can gross 30% to 40% more per year (about $280,000 to $300,000) for the same number of shifts as a typical FTE hospitalist (median compensation for non-teaching hospitalists is $215,000 per year, according to the 2010 MGMA-SHM State of Hospital Medicine report). As contracted employees, locum physicians are self-employed and must report their own income and pay their own taxes; many times locum physicians need to hire an accountant.
Working locum tenens also offers a chance to sample a variety of hospital settings. “There’s a big difference between a small rural hospital and a big teaching hospital with a closed ICU,” points out Karen Belote, a physician recruiter for Locum Leaders. “[Working locums] is a great way to try some different locations and different hospitals before settling into a permanent position.”
Pitfalls and Cautions
The only pitfall Dr. Hamilton has observed is perception.“Some people have an initial gut reflex that a locums doctor is either running from something or is not of the same caliber as a permanent physician,” he says. But that notion is quickly dispelled, he says, when he or she demonstrates flexibility, competency, and eagerness to work with the team.
Dr. Hamilton has found a great fit with his current assignments and has no immediate plans to take a permanent position. “I’m learning every day,” he says. “Remember, you are the boss of your life.”
Gretchen Henkel is a freelance writer based in California.
Increase your hirability
Thinking of taking a locum tenens assignment? Consider acquiring additional procedural competencies during your third year of residency, Dr. Harrington advises.
“We are getting more and more requests for people who are comfortable with procedural skills, such as intubations, placing central lines, and vent management,” he says.
Your locum tenens agency might underwrite a procedures course, as Locum Leaders did for Dr. Hamilton. Just make sure you don’t misrepresent your abilities or your comfort level, he cautions. “Know your limits and realize that you are building your career every month,” he says.
SHM will offer a pre-course, “Medical Procedures for the Hospitalist,” at HM11 (May 9, 2011 in Grapevine, Texas), and at many of the HM regional meetings.—GH
Health Reform Turns 1
As America’s love-hate relationship with healthcare reform approaches its first anniversary, the law is proving just as divisive now as it was during the midterm elections. Fittingly, the Patient Protection and Affordable Care Act of 2010 (ACA), which has polarized the country, is moving forward along three separate tracks.
“Usually, at this point of the game one would only be worrying about the implementation,” says Leighton Ku, a health-policy analyst at George Washington University. “But, obviously, there’s been enough discord that the political route and the legal route are now equally important.”
Here’s a look at where the ACA stands from practical, political, and legal standpoints, along with the major players involved in the ongoing tussle.
The Battle of Public Perception
America is hopelessly divided. Despite pollsters’ best efforts to break the stalemate, the collective numbers still suggest that roughly equal numbers of respondents favor and oppose healthcare reform (with a slight advantage to opponents). It’s a trend line that has barely budged since the bill’s enactment last March.
In January, the Republican-led House of Representatives voted to repeal the entire reform act in what analysts have called a largely symbolic gesture, given that the repeal effort subsequently failed in the Democratic-controlled Senate. Even so, Ku says, the vote fulfills a Republican campaign promise and sends a strong signal to the party’s political base. “What’s driving the Republicans is that their constituencies really don’t like it,” agrees Robert J. Blendon, a professor of health policy and political analysis at the Harvard School of Public Health. “Almost all Republican congressmen who ran had on their website, ‘I will repeal this bill if elected.’ ”
But opposition doesn’t necessarily mean voters want everything repealed, a caveat also borne out by recent polling. “Where the public stands is a little more ambiguous than what the campaign rhetoric is,” Ku says. That ambiguity could present an opportunity for both parties to reframe the debate in the coming months in an effort to win over a clear majority of the public. With the economy of paramount concern, Republicans have cast healthcare reform as a “job-destroying” act that will speed the country’s descent into bankruptcy.
If the economy improves, however, opposition to the law is likely to soften. And with their “no” vote behind them, Republicans in the House will be expected to craft a coherent alternative to the legislation. “Now comes the tough part,” Ku says.
Democrats, meanwhile, have largely regrouped and are being more vocal about the law’s necessity—after a campaign season in which many conservative Democrats largely avoided talking about it, or even touted their opposition to it, and were beaten anyway.
In the absence of wholesale repeal, a few individual provisions might be stripped away. Most key elements cannot be defunded, although Republicans could cut funding streams to Health and Human Services (HHS) or the IRS to hamper implementation. Congress also could choose not to appropriate money to the estimated $106 billion worth of new spending authorizations. A sizable percentage of that pool covers popular pre-existing programs, however, which makes a “no” vote politically more risky.
A Matter of Time
The nonpartisan Congressional Budget Office has predicted that repealing the healthcare reform legislation would increase the federal deficit by $230 billion over the next decade. Even so, the law’s supporters are finding little traction among voters who have heard repeated claims by Republicans that the act itself will push the country deeper into debt (many Republicans say the CBO estimate is based on faulty numbers provided by the law’s supporters). One big reason why: The tanking economy has eroded public trust in the government. “The ratings of trust in the federal government are so low,” Blendon says, “you need a stethoscope to try to hear them.”
And then there’s the matter of time. Because the act’s biggest provisions don’t go into effect until 2014, there are no made-for-media moments—like the large numbers of previously uninsured receiving health insurance cards—to counter the dire predictions that patients will lose their doctors. Instead, the White House has tried to make the most of smaller provisions now in effect, such as one that allows children to stay on a parent’s insurance until their 26th birthday, another that lifts the lifetime caps on insurance coverage, and a third that bans insurers from dropping children with pre-existing conditions (a video explaining what the ACA does and doesn’t do, produced by the Kaiser Family Foundation, is available at http://healthreform.kff.org/The Animation.aspx).
In mid-January, on the eve of the House vote to repeal the entire act, the White House released an HHS study to bolster its contention that the law will eventually aid tens of millions, and, conversely, that any repeal would harm them (www.healthcare.gov/center/reports/preexisting.html). The study estimates that 50 million to 129 million Americans under the age of 65 have pre-existing conditions that would, theoretically, make it harder for them to buy insurance in the absence of regulations requiring coverage. But the study also reports that up to 82 million of these people already have employer-provided insurance, meaning they wouldn’t be affected either way unless they switch jobs or become unemployed.
The White House’s case has been made harder by the confluence of a poor economy, growing concern over the deficit, and the ongoing battle over whether and how to fix the Medicare reimbursement rate paid to doctors, according to Blendon. When the rate paid to doctors temporarily nosedived last June, stories about doctors refusing to see Medicare beneficiaries proliferated among alarmed seniors (Congress eventually passed another short-term patch). The memory of that lack of medical access is now being conflated with the potential side effects of the new law by the constituency most likely to vote (seniors) and most skeptical in general about healthcare reform.
Legal Limbo
More than half the states have now joined lawsuits challenging the ACA’s constitutionality. In the first of what observers expect to be a multitude of legal decisions, federal judges in two cases upheld the law, and the individual mandate requiring people to buy health insurance was ruled unconstitutional in a third.
Ultimately, most experts believe the Supreme Court will have the final say, likely before the 2012 elections. Ku says analysts already are talking about a possible 5-4 decision, with Justice Anthony Kennedy as the potential swing vote—though so far, he’s given no clear hints about which way he may be leaning. Even if the individual mandate component is struck down, Ku says, the court could uphold everything else, changing its overall impact but not the implementation of most provisions. TH
Bryn Nelson is a freelance medical writer based in Seattle.
As America’s love-hate relationship with healthcare reform approaches its first anniversary, the law is proving just as divisive now as it was during the midterm elections. Fittingly, the Patient Protection and Affordable Care Act of 2010 (ACA), which has polarized the country, is moving forward along three separate tracks.
“Usually, at this point of the game one would only be worrying about the implementation,” says Leighton Ku, a health-policy analyst at George Washington University. “But, obviously, there’s been enough discord that the political route and the legal route are now equally important.”
Here’s a look at where the ACA stands from practical, political, and legal standpoints, along with the major players involved in the ongoing tussle.
The Battle of Public Perception
America is hopelessly divided. Despite pollsters’ best efforts to break the stalemate, the collective numbers still suggest that roughly equal numbers of respondents favor and oppose healthcare reform (with a slight advantage to opponents). It’s a trend line that has barely budged since the bill’s enactment last March.
In January, the Republican-led House of Representatives voted to repeal the entire reform act in what analysts have called a largely symbolic gesture, given that the repeal effort subsequently failed in the Democratic-controlled Senate. Even so, Ku says, the vote fulfills a Republican campaign promise and sends a strong signal to the party’s political base. “What’s driving the Republicans is that their constituencies really don’t like it,” agrees Robert J. Blendon, a professor of health policy and political analysis at the Harvard School of Public Health. “Almost all Republican congressmen who ran had on their website, ‘I will repeal this bill if elected.’ ”
But opposition doesn’t necessarily mean voters want everything repealed, a caveat also borne out by recent polling. “Where the public stands is a little more ambiguous than what the campaign rhetoric is,” Ku says. That ambiguity could present an opportunity for both parties to reframe the debate in the coming months in an effort to win over a clear majority of the public. With the economy of paramount concern, Republicans have cast healthcare reform as a “job-destroying” act that will speed the country’s descent into bankruptcy.
If the economy improves, however, opposition to the law is likely to soften. And with their “no” vote behind them, Republicans in the House will be expected to craft a coherent alternative to the legislation. “Now comes the tough part,” Ku says.
Democrats, meanwhile, have largely regrouped and are being more vocal about the law’s necessity—after a campaign season in which many conservative Democrats largely avoided talking about it, or even touted their opposition to it, and were beaten anyway.
In the absence of wholesale repeal, a few individual provisions might be stripped away. Most key elements cannot be defunded, although Republicans could cut funding streams to Health and Human Services (HHS) or the IRS to hamper implementation. Congress also could choose not to appropriate money to the estimated $106 billion worth of new spending authorizations. A sizable percentage of that pool covers popular pre-existing programs, however, which makes a “no” vote politically more risky.
A Matter of Time
The nonpartisan Congressional Budget Office has predicted that repealing the healthcare reform legislation would increase the federal deficit by $230 billion over the next decade. Even so, the law’s supporters are finding little traction among voters who have heard repeated claims by Republicans that the act itself will push the country deeper into debt (many Republicans say the CBO estimate is based on faulty numbers provided by the law’s supporters). One big reason why: The tanking economy has eroded public trust in the government. “The ratings of trust in the federal government are so low,” Blendon says, “you need a stethoscope to try to hear them.”
And then there’s the matter of time. Because the act’s biggest provisions don’t go into effect until 2014, there are no made-for-media moments—like the large numbers of previously uninsured receiving health insurance cards—to counter the dire predictions that patients will lose their doctors. Instead, the White House has tried to make the most of smaller provisions now in effect, such as one that allows children to stay on a parent’s insurance until their 26th birthday, another that lifts the lifetime caps on insurance coverage, and a third that bans insurers from dropping children with pre-existing conditions (a video explaining what the ACA does and doesn’t do, produced by the Kaiser Family Foundation, is available at http://healthreform.kff.org/The Animation.aspx).
In mid-January, on the eve of the House vote to repeal the entire act, the White House released an HHS study to bolster its contention that the law will eventually aid tens of millions, and, conversely, that any repeal would harm them (www.healthcare.gov/center/reports/preexisting.html). The study estimates that 50 million to 129 million Americans under the age of 65 have pre-existing conditions that would, theoretically, make it harder for them to buy insurance in the absence of regulations requiring coverage. But the study also reports that up to 82 million of these people already have employer-provided insurance, meaning they wouldn’t be affected either way unless they switch jobs or become unemployed.
The White House’s case has been made harder by the confluence of a poor economy, growing concern over the deficit, and the ongoing battle over whether and how to fix the Medicare reimbursement rate paid to doctors, according to Blendon. When the rate paid to doctors temporarily nosedived last June, stories about doctors refusing to see Medicare beneficiaries proliferated among alarmed seniors (Congress eventually passed another short-term patch). The memory of that lack of medical access is now being conflated with the potential side effects of the new law by the constituency most likely to vote (seniors) and most skeptical in general about healthcare reform.
Legal Limbo
More than half the states have now joined lawsuits challenging the ACA’s constitutionality. In the first of what observers expect to be a multitude of legal decisions, federal judges in two cases upheld the law, and the individual mandate requiring people to buy health insurance was ruled unconstitutional in a third.
Ultimately, most experts believe the Supreme Court will have the final say, likely before the 2012 elections. Ku says analysts already are talking about a possible 5-4 decision, with Justice Anthony Kennedy as the potential swing vote—though so far, he’s given no clear hints about which way he may be leaning. Even if the individual mandate component is struck down, Ku says, the court could uphold everything else, changing its overall impact but not the implementation of most provisions. TH
Bryn Nelson is a freelance medical writer based in Seattle.
As America’s love-hate relationship with healthcare reform approaches its first anniversary, the law is proving just as divisive now as it was during the midterm elections. Fittingly, the Patient Protection and Affordable Care Act of 2010 (ACA), which has polarized the country, is moving forward along three separate tracks.
“Usually, at this point of the game one would only be worrying about the implementation,” says Leighton Ku, a health-policy analyst at George Washington University. “But, obviously, there’s been enough discord that the political route and the legal route are now equally important.”
Here’s a look at where the ACA stands from practical, political, and legal standpoints, along with the major players involved in the ongoing tussle.
The Battle of Public Perception
America is hopelessly divided. Despite pollsters’ best efforts to break the stalemate, the collective numbers still suggest that roughly equal numbers of respondents favor and oppose healthcare reform (with a slight advantage to opponents). It’s a trend line that has barely budged since the bill’s enactment last March.
In January, the Republican-led House of Representatives voted to repeal the entire reform act in what analysts have called a largely symbolic gesture, given that the repeal effort subsequently failed in the Democratic-controlled Senate. Even so, Ku says, the vote fulfills a Republican campaign promise and sends a strong signal to the party’s political base. “What’s driving the Republicans is that their constituencies really don’t like it,” agrees Robert J. Blendon, a professor of health policy and political analysis at the Harvard School of Public Health. “Almost all Republican congressmen who ran had on their website, ‘I will repeal this bill if elected.’ ”
But opposition doesn’t necessarily mean voters want everything repealed, a caveat also borne out by recent polling. “Where the public stands is a little more ambiguous than what the campaign rhetoric is,” Ku says. That ambiguity could present an opportunity for both parties to reframe the debate in the coming months in an effort to win over a clear majority of the public. With the economy of paramount concern, Republicans have cast healthcare reform as a “job-destroying” act that will speed the country’s descent into bankruptcy.
If the economy improves, however, opposition to the law is likely to soften. And with their “no” vote behind them, Republicans in the House will be expected to craft a coherent alternative to the legislation. “Now comes the tough part,” Ku says.
Democrats, meanwhile, have largely regrouped and are being more vocal about the law’s necessity—after a campaign season in which many conservative Democrats largely avoided talking about it, or even touted their opposition to it, and were beaten anyway.
In the absence of wholesale repeal, a few individual provisions might be stripped away. Most key elements cannot be defunded, although Republicans could cut funding streams to Health and Human Services (HHS) or the IRS to hamper implementation. Congress also could choose not to appropriate money to the estimated $106 billion worth of new spending authorizations. A sizable percentage of that pool covers popular pre-existing programs, however, which makes a “no” vote politically more risky.
A Matter of Time
The nonpartisan Congressional Budget Office has predicted that repealing the healthcare reform legislation would increase the federal deficit by $230 billion over the next decade. Even so, the law’s supporters are finding little traction among voters who have heard repeated claims by Republicans that the act itself will push the country deeper into debt (many Republicans say the CBO estimate is based on faulty numbers provided by the law’s supporters). One big reason why: The tanking economy has eroded public trust in the government. “The ratings of trust in the federal government are so low,” Blendon says, “you need a stethoscope to try to hear them.”
And then there’s the matter of time. Because the act’s biggest provisions don’t go into effect until 2014, there are no made-for-media moments—like the large numbers of previously uninsured receiving health insurance cards—to counter the dire predictions that patients will lose their doctors. Instead, the White House has tried to make the most of smaller provisions now in effect, such as one that allows children to stay on a parent’s insurance until their 26th birthday, another that lifts the lifetime caps on insurance coverage, and a third that bans insurers from dropping children with pre-existing conditions (a video explaining what the ACA does and doesn’t do, produced by the Kaiser Family Foundation, is available at http://healthreform.kff.org/The Animation.aspx).
In mid-January, on the eve of the House vote to repeal the entire act, the White House released an HHS study to bolster its contention that the law will eventually aid tens of millions, and, conversely, that any repeal would harm them (www.healthcare.gov/center/reports/preexisting.html). The study estimates that 50 million to 129 million Americans under the age of 65 have pre-existing conditions that would, theoretically, make it harder for them to buy insurance in the absence of regulations requiring coverage. But the study also reports that up to 82 million of these people already have employer-provided insurance, meaning they wouldn’t be affected either way unless they switch jobs or become unemployed.
The White House’s case has been made harder by the confluence of a poor economy, growing concern over the deficit, and the ongoing battle over whether and how to fix the Medicare reimbursement rate paid to doctors, according to Blendon. When the rate paid to doctors temporarily nosedived last June, stories about doctors refusing to see Medicare beneficiaries proliferated among alarmed seniors (Congress eventually passed another short-term patch). The memory of that lack of medical access is now being conflated with the potential side effects of the new law by the constituency most likely to vote (seniors) and most skeptical in general about healthcare reform.
Legal Limbo
More than half the states have now joined lawsuits challenging the ACA’s constitutionality. In the first of what observers expect to be a multitude of legal decisions, federal judges in two cases upheld the law, and the individual mandate requiring people to buy health insurance was ruled unconstitutional in a third.
Ultimately, most experts believe the Supreme Court will have the final say, likely before the 2012 elections. Ku says analysts already are talking about a possible 5-4 decision, with Justice Anthony Kennedy as the potential swing vote—though so far, he’s given no clear hints about which way he may be leaning. Even if the individual mandate component is struck down, Ku says, the court could uphold everything else, changing its overall impact but not the implementation of most provisions. TH
Bryn Nelson is a freelance medical writer based in Seattle.
Call of Duty
Dave Bowman, MD, doesn’t run anywhere anymore; it’s more of a fast walk. He doesn’t consider himself political, yet he does his civic duty and votes in every election. He’s not a big fan of vegetables, but he eats them to appease his wife and his conscience. Most important, Dr. Bowman doesn’t consider himself a hero. In fact, he doesn’t consider what he did that day any different from what he does every day in the hospital.
On the morning of Jan. 8, Dr. Bowman and his wife, Nancy, were thrust into the epicenter of one of the worst shooting rampages in American history. Dr. Bowman, a hospitalist, was the first physician on the scene outside the Tucson, Ariz., grocery store where a lone gunman killed six people and injured 13 others, including U.S. Representative Gabrielle Giffords (D-Ariz.).
“A hero is somebody, to me, who steps out of their element, steps up to the task that is needed, a task that is completely foreign to them, and steps up and helps people,” says Dr. Bowman, 61, executive director for IPC The Hospitalist Co.’s Tucson region. “A hero is not somebody who should be able to help in some manner and should be expected to help. … I don’t think nurses, doctors, firemen, EMTs get to be labeled as heroes. It’s what we should do, and gladly do, and in many cases took an oath to do.”
Even so, Dr. Bowman’s recollection of that frightful morning is a story laced with tragedy, courage, and hope. He remembers those who are “no longer with us.” He remembers his wife administering CPR and calling victims’ loved ones on their cellphones. And he remembers the brave men and women who not only subdued the shooter, but who worked together, selflessly, and in many cases with no medical training, to assist their injured neighbors.
“It was a pretty traumatic scene. More for others than for me; I am supposed to be the doctor and can handle all that,” he says, pausing. “But they don’t teach this course in medical school.”
Shots Ring Out, First Thought Is Help
Born and raised 100 miles south of Tucson and internal-medicine-trained at the University of Arizona, Dr. Bowman became a hospitalist in 1998 and began working with IPC in 2000. He supervises a staff of about 90 providers, including more than 50 full-time hospitalists, serving two large community hospitals, rehabilitations centers, and skilled nursing facilities in the Tucson area.
Yet he knows who the real boss is in the Bowman household. He and Nancy, an ICU nurse, had just finished a brisk walk, eaten breakfast at McDonald’s, and stopped at Safeway to pick up some vegetables on the morning of Jan. 8. “I had the oatmeal. I was so proud of myself,” Dr. Bowman says. “And, as wives will want to do, she pushes the envelope and says she wants to stop by and get some Brussels sprouts. She hadn’t had them in 20 years, and I hadn’t had them in 45 years—since my mom stunk up the house with them. So we ended up at the Safeway, because the two things that keeps a marriage together are those two words: ‘Yes, dear.’ ”
The Bowmans passed by Rep. Giffords, her staff, and about 25 people in front of the grocery store entrance. They went to the produce department and had not been in the store for more than three or four minutes before shots rang out.
“I was 150 percent sure they were gunshots,” Dr. Bowman says. “I said to my wife, ‘Let’s go, Nancy,’ and she didn’t hear me. I thought she was right behind me. But she had gone over to the Brussels sprouts and I was still in mushrooms.”
Walking to the door, Dr. Bowman saw a woman rushing in and shouting, “They shot her. They shot Congresswoman Giffords.”
“I stepped out and stood behind a pillar until no more gunshots,” he says. “They’d actually already taken down the shooter as soon as he ran out of bullets. I looked around the corner to the carnage, as you’d expect, with Congresswoman Giffords the first person I saw right at the head of the line near the front door.
“Quite frankly, I stepped over people who were no longer with us to get to her. I got her turned around and moved off to the window; she wasn’t breathing real well. I worked on her airway, cleaning her airway with the young man [Rep. Giffords’ intern, Daniel Hernandez], who then held her for the next 15 to 20 minutes until the paramedics got her ready to go to the hospital.”
From that moment, Dr. Bowman says his training and instincts took over. More important, he went into field-triage mode, “which means you can’t do anything for that one. Can’t do anything there, this one is breathing and talking, not bleeding bad, good; still breathing, good, stay right there, I will be back. You go all the way up the line from person to person, seeing who you can and can’t help.
“The problem with field triage is that you really can’t stop and do a lot, like CPR, because then the rest of the people don’t have anybody looking at them. So you just keep moving,” he explains.
He says that while it was only minutes from the first gunshot to the time police arrived and secured the scene to the time paramedics were allowed to assist with the injured, it seemed like hours. Additionally, in his haste to help, Dr. Bowman had lost track of Nancy.
“I looked back and she was not there,” he says, adding she’d been swept to the back of the grocery store. “I kept working and I looked back and she had pushed [aside] the 17-year-old sacker who was acting as a security guard. … She came outside and started doing CPR on the first person she saw. I got up the line and there was a doctor doing CPR on the young girl [Christina-Taylor Green]. He was in the parking lot when the shots rang out; he threw his wife in the car and ran up to the little girl.”
Instinct and Autopilot
In a little more than 10 seconds, the gunman had fired more than 30 rounds and killed or injured nearly 20 people.
After he’d checked on all of the wounded, Dr. Bowman says he came upon four people holding the suspected shooter down. “One of them, I didn’t know it, was one of my colleagues, Dr. Stephen Rayle,” Dr. Bowman says. “I said, ‘Hold him, and I will send the first officer over here.’ ”
From there, Dr. Bowman says, he went back up the line of injured, checking for breathing sounds and bleeding, making sure every injured person was being attended to.
“People shot in the leg were holding a hand over a chest wound on the man or woman next to them,” he says. “It was one of those chaotic scenes that you try to make some kind of order to, by deciding who you can help and who you can’t.”
When the first paramedics arrived, Dr. Bowman directed them to Rep. Giffords and the 9-year-old girl. Soon after, there were enough EMTs on scene to attend to each of the victims.
“You are just on automatic,” Dr. Bowman explains. “Actually, after I dealt with the first two victims pretty quickly, Giffords taking the most time to just get her situated, I did stop and look out in the parking lot just to see if anybody was aiming anything at us.”
The most difficult part of field triage, Dr. Bowman says, is staying calm, organized, and “not losing it emotionally.” In fact, he ushered some shell-shocked bystanders away from the scene. “Some people will step right forward and say, ‘Can I help? I know CPR.’ Other people will just stand there and scream.”
Even tougher, he says, was watching his wife try to save the life of U.S. District Judge John Roll (see “Remembering the Dead,” above). “I finally had to go to my wife, pull her off of him, and say, ‘Honey, he’s gone. I need help with this lady who has been shot in the chest laying in the street,’ ” Dr. Bowman says. “Pulling them away is pretty hard. ... It was harder for her and the bystanders, because they were the ones getting right down close with the patient, talking to them, telling patients to ‘keep looking at me.’ That is a very close bond that develops. Field triage, you just keep moving.”
Human Spirit, Cooperation, and Hope
One month after the shooting, Rep. Giffords was moving, talking, thinking, and recuperating at what her doctors at Houston’s TIRR Hermann Memorial deemed “lightning speed.” For Dr. Bowman, such news brings more than a sigh of relief.
“In truth, when I was first with her, she wasn’t responding,” he says. “She was breathing, although with a compromised airway. We got that straightened away and she had a good pulse. Daniel Hernandez, the young man with her, had some nursing training and was comfortable being with her.
“It was amazing to me, the second or third time down the line, just to look over to this guy, and he was watching for me, and he would nod and say, ‘We’re OK. She’s breathing, pulse is OK.’ I didn’t have to go back, and the third time down he said, ‘She’s moving, she’s squeezing my hand.’ ”
It was at that moment that Dr. Bowman felt the congresswoman, in spite of her severe head trauma, had a fighting chance. Others miracles were happening all around him. Each memory ceases to amaze the veteran physician.
“People who were injured holding somebody’s head in their lap because they were hit in the head and this person was hit in the chest,” he says. “It gives you hope for the human race. Those people, to me, were the heroes.”
Looking back, Dr. Bowman says, he’s played back the moment in his mind more than a few times, wondering if he did the right thing for every victim. He wonders how much he and his wife, the other doctors at the scene, and the bystanders really helped. Then, after a brief moment, he has the answer.
“It really took two weeks to really say that, when all the victims that had expired were dead at the scene, and everybody that got taken away made it and out of the hospital, it sunk in that we had made the right decisions,” he says. “Nobody died on the operating table because we didn’t pick up the gunshot wound to the back. It took two weeks to realize we did the right thing.”
Some of those answers will take many more weeks. Rep. Giffords, although she is on a positive path, still has a long road to a full recovery, and doctors are making no promises. “I don’t know how far she will make it or how long it is going to take, but there are some miracles out there,” Dr. Bowman says.
You’re a Hospitalist? You’re Ready
As a physician in the middle of a mass-casualty event, the media called upon Dr. Bowman to recount the events of the Tucson shooting. In more than a dozen interviews with local and national media, not once was Dr. Bowman referred to as a hospitalist.
“At the scene, when I kneel down, I am a doctor. Can you tell me what’s going on, can you talk to me? It goes no farther than that,” he says. “In talking with one of the interviewers, she asked, ‘What kind of doctor are you?’ I said, ‘I am an internist and a hospitalist, which is a doctor who works just in the hospital.’ They weren’t interested in that. When the cameras rolled, she said, ‘I understand you are an intern?’ I said, ‘No, I am an internist. Turn that thing off and start again.’
“That’s the level of knowledge you are dealing with, and that was a national anchorperson I was dealing with.”
It’s an all-too-common refrain for hospitalists around the country, but one Dr. Bowman and others like him have endured for years. It doesn’t bother him, and he says it shouldn’t bother others.
“Doctor or nurse was as far as it got. I can certainly understand that,” he says. “We explain what a hospitalist is every day. We’ve been doing it for 12, 13, 14 years, and people still don’t understand. It’s OK.”
What isn’t OK is hospitalist unpreparedness. In fact, Dr. Bowman says, his training as an internist and his years of HM experience played a pivotal role in managing the scene of the Tucson shooting. The first thing to do, in addition to remaining calm, is to keep your priorities straight and remember your ABCs.

“It’s airway, airway, airway,” he says. “Without an airway, people don’t live. Then you are looking for bleeding, bleeding, bleeding. Then if they are talking, not a lot of bleeding and have a pulse, that is good enough for right now. So, it’s ‘Lady, just keep pushing on the chest right there on that wound.’ … I think, if you remember your ABCs, that’s all you can do in a field triage situation.”
Although the circumstances are less stressful, hospitalists are faced with make-or-break decisions every day, Dr. Bowman says. For example, it’s 4 p.m. and the day shift physician in the ED calls and says he has six admissions he’s been working on for the past three hours. “You, based on the info given to you, have to decide, Well, who is sickest, who do I have to get to first, who is going to the ICU?” Dr. Bowman says. “You do this triage thing in your mind as you walk to the ED. If there is any corollary, it’s the fact that you, as the hospitalist, get hit with a slew of patients all at once. They don’t come in one every 15 minutes like in your office for a blood-pressure check.
“Be ready. And, by the way, you are ready,” he adds. “You take care of a stroke patient in room one, take care of a gangrenous leg in room two, a diabetic with ketoacidosis in room three. The broadness, the generality of your training, you are ready to take care of a variety of things. You’re going to be able to help. Just be ready.” TH
Jason Carris is editor of The Hospitalist.
Dave Bowman, MD, doesn’t run anywhere anymore; it’s more of a fast walk. He doesn’t consider himself political, yet he does his civic duty and votes in every election. He’s not a big fan of vegetables, but he eats them to appease his wife and his conscience. Most important, Dr. Bowman doesn’t consider himself a hero. In fact, he doesn’t consider what he did that day any different from what he does every day in the hospital.
On the morning of Jan. 8, Dr. Bowman and his wife, Nancy, were thrust into the epicenter of one of the worst shooting rampages in American history. Dr. Bowman, a hospitalist, was the first physician on the scene outside the Tucson, Ariz., grocery store where a lone gunman killed six people and injured 13 others, including U.S. Representative Gabrielle Giffords (D-Ariz.).
“A hero is somebody, to me, who steps out of their element, steps up to the task that is needed, a task that is completely foreign to them, and steps up and helps people,” says Dr. Bowman, 61, executive director for IPC The Hospitalist Co.’s Tucson region. “A hero is not somebody who should be able to help in some manner and should be expected to help. … I don’t think nurses, doctors, firemen, EMTs get to be labeled as heroes. It’s what we should do, and gladly do, and in many cases took an oath to do.”
Even so, Dr. Bowman’s recollection of that frightful morning is a story laced with tragedy, courage, and hope. He remembers those who are “no longer with us.” He remembers his wife administering CPR and calling victims’ loved ones on their cellphones. And he remembers the brave men and women who not only subdued the shooter, but who worked together, selflessly, and in many cases with no medical training, to assist their injured neighbors.
“It was a pretty traumatic scene. More for others than for me; I am supposed to be the doctor and can handle all that,” he says, pausing. “But they don’t teach this course in medical school.”
Shots Ring Out, First Thought Is Help
Born and raised 100 miles south of Tucson and internal-medicine-trained at the University of Arizona, Dr. Bowman became a hospitalist in 1998 and began working with IPC in 2000. He supervises a staff of about 90 providers, including more than 50 full-time hospitalists, serving two large community hospitals, rehabilitations centers, and skilled nursing facilities in the Tucson area.
Yet he knows who the real boss is in the Bowman household. He and Nancy, an ICU nurse, had just finished a brisk walk, eaten breakfast at McDonald’s, and stopped at Safeway to pick up some vegetables on the morning of Jan. 8. “I had the oatmeal. I was so proud of myself,” Dr. Bowman says. “And, as wives will want to do, she pushes the envelope and says she wants to stop by and get some Brussels sprouts. She hadn’t had them in 20 years, and I hadn’t had them in 45 years—since my mom stunk up the house with them. So we ended up at the Safeway, because the two things that keeps a marriage together are those two words: ‘Yes, dear.’ ”
The Bowmans passed by Rep. Giffords, her staff, and about 25 people in front of the grocery store entrance. They went to the produce department and had not been in the store for more than three or four minutes before shots rang out.
“I was 150 percent sure they were gunshots,” Dr. Bowman says. “I said to my wife, ‘Let’s go, Nancy,’ and she didn’t hear me. I thought she was right behind me. But she had gone over to the Brussels sprouts and I was still in mushrooms.”
Walking to the door, Dr. Bowman saw a woman rushing in and shouting, “They shot her. They shot Congresswoman Giffords.”
“I stepped out and stood behind a pillar until no more gunshots,” he says. “They’d actually already taken down the shooter as soon as he ran out of bullets. I looked around the corner to the carnage, as you’d expect, with Congresswoman Giffords the first person I saw right at the head of the line near the front door.
“Quite frankly, I stepped over people who were no longer with us to get to her. I got her turned around and moved off to the window; she wasn’t breathing real well. I worked on her airway, cleaning her airway with the young man [Rep. Giffords’ intern, Daniel Hernandez], who then held her for the next 15 to 20 minutes until the paramedics got her ready to go to the hospital.”
From that moment, Dr. Bowman says his training and instincts took over. More important, he went into field-triage mode, “which means you can’t do anything for that one. Can’t do anything there, this one is breathing and talking, not bleeding bad, good; still breathing, good, stay right there, I will be back. You go all the way up the line from person to person, seeing who you can and can’t help.
“The problem with field triage is that you really can’t stop and do a lot, like CPR, because then the rest of the people don’t have anybody looking at them. So you just keep moving,” he explains.
He says that while it was only minutes from the first gunshot to the time police arrived and secured the scene to the time paramedics were allowed to assist with the injured, it seemed like hours. Additionally, in his haste to help, Dr. Bowman had lost track of Nancy.
“I looked back and she was not there,” he says, adding she’d been swept to the back of the grocery store. “I kept working and I looked back and she had pushed [aside] the 17-year-old sacker who was acting as a security guard. … She came outside and started doing CPR on the first person she saw. I got up the line and there was a doctor doing CPR on the young girl [Christina-Taylor Green]. He was in the parking lot when the shots rang out; he threw his wife in the car and ran up to the little girl.”
Instinct and Autopilot
In a little more than 10 seconds, the gunman had fired more than 30 rounds and killed or injured nearly 20 people.
After he’d checked on all of the wounded, Dr. Bowman says he came upon four people holding the suspected shooter down. “One of them, I didn’t know it, was one of my colleagues, Dr. Stephen Rayle,” Dr. Bowman says. “I said, ‘Hold him, and I will send the first officer over here.’ ”
From there, Dr. Bowman says, he went back up the line of injured, checking for breathing sounds and bleeding, making sure every injured person was being attended to.
“People shot in the leg were holding a hand over a chest wound on the man or woman next to them,” he says. “It was one of those chaotic scenes that you try to make some kind of order to, by deciding who you can help and who you can’t.”
When the first paramedics arrived, Dr. Bowman directed them to Rep. Giffords and the 9-year-old girl. Soon after, there were enough EMTs on scene to attend to each of the victims.
“You are just on automatic,” Dr. Bowman explains. “Actually, after I dealt with the first two victims pretty quickly, Giffords taking the most time to just get her situated, I did stop and look out in the parking lot just to see if anybody was aiming anything at us.”
The most difficult part of field triage, Dr. Bowman says, is staying calm, organized, and “not losing it emotionally.” In fact, he ushered some shell-shocked bystanders away from the scene. “Some people will step right forward and say, ‘Can I help? I know CPR.’ Other people will just stand there and scream.”
Even tougher, he says, was watching his wife try to save the life of U.S. District Judge John Roll (see “Remembering the Dead,” above). “I finally had to go to my wife, pull her off of him, and say, ‘Honey, he’s gone. I need help with this lady who has been shot in the chest laying in the street,’ ” Dr. Bowman says. “Pulling them away is pretty hard. ... It was harder for her and the bystanders, because they were the ones getting right down close with the patient, talking to them, telling patients to ‘keep looking at me.’ That is a very close bond that develops. Field triage, you just keep moving.”
Human Spirit, Cooperation, and Hope
One month after the shooting, Rep. Giffords was moving, talking, thinking, and recuperating at what her doctors at Houston’s TIRR Hermann Memorial deemed “lightning speed.” For Dr. Bowman, such news brings more than a sigh of relief.
“In truth, when I was first with her, she wasn’t responding,” he says. “She was breathing, although with a compromised airway. We got that straightened away and she had a good pulse. Daniel Hernandez, the young man with her, had some nursing training and was comfortable being with her.
“It was amazing to me, the second or third time down the line, just to look over to this guy, and he was watching for me, and he would nod and say, ‘We’re OK. She’s breathing, pulse is OK.’ I didn’t have to go back, and the third time down he said, ‘She’s moving, she’s squeezing my hand.’ ”
It was at that moment that Dr. Bowman felt the congresswoman, in spite of her severe head trauma, had a fighting chance. Others miracles were happening all around him. Each memory ceases to amaze the veteran physician.
“People who were injured holding somebody’s head in their lap because they were hit in the head and this person was hit in the chest,” he says. “It gives you hope for the human race. Those people, to me, were the heroes.”
Looking back, Dr. Bowman says, he’s played back the moment in his mind more than a few times, wondering if he did the right thing for every victim. He wonders how much he and his wife, the other doctors at the scene, and the bystanders really helped. Then, after a brief moment, he has the answer.
“It really took two weeks to really say that, when all the victims that had expired were dead at the scene, and everybody that got taken away made it and out of the hospital, it sunk in that we had made the right decisions,” he says. “Nobody died on the operating table because we didn’t pick up the gunshot wound to the back. It took two weeks to realize we did the right thing.”
Some of those answers will take many more weeks. Rep. Giffords, although she is on a positive path, still has a long road to a full recovery, and doctors are making no promises. “I don’t know how far she will make it or how long it is going to take, but there are some miracles out there,” Dr. Bowman says.
You’re a Hospitalist? You’re Ready
As a physician in the middle of a mass-casualty event, the media called upon Dr. Bowman to recount the events of the Tucson shooting. In more than a dozen interviews with local and national media, not once was Dr. Bowman referred to as a hospitalist.
“At the scene, when I kneel down, I am a doctor. Can you tell me what’s going on, can you talk to me? It goes no farther than that,” he says. “In talking with one of the interviewers, she asked, ‘What kind of doctor are you?’ I said, ‘I am an internist and a hospitalist, which is a doctor who works just in the hospital.’ They weren’t interested in that. When the cameras rolled, she said, ‘I understand you are an intern?’ I said, ‘No, I am an internist. Turn that thing off and start again.’
“That’s the level of knowledge you are dealing with, and that was a national anchorperson I was dealing with.”
It’s an all-too-common refrain for hospitalists around the country, but one Dr. Bowman and others like him have endured for years. It doesn’t bother him, and he says it shouldn’t bother others.
“Doctor or nurse was as far as it got. I can certainly understand that,” he says. “We explain what a hospitalist is every day. We’ve been doing it for 12, 13, 14 years, and people still don’t understand. It’s OK.”
What isn’t OK is hospitalist unpreparedness. In fact, Dr. Bowman says, his training as an internist and his years of HM experience played a pivotal role in managing the scene of the Tucson shooting. The first thing to do, in addition to remaining calm, is to keep your priorities straight and remember your ABCs.

“It’s airway, airway, airway,” he says. “Without an airway, people don’t live. Then you are looking for bleeding, bleeding, bleeding. Then if they are talking, not a lot of bleeding and have a pulse, that is good enough for right now. So, it’s ‘Lady, just keep pushing on the chest right there on that wound.’ … I think, if you remember your ABCs, that’s all you can do in a field triage situation.”
Although the circumstances are less stressful, hospitalists are faced with make-or-break decisions every day, Dr. Bowman says. For example, it’s 4 p.m. and the day shift physician in the ED calls and says he has six admissions he’s been working on for the past three hours. “You, based on the info given to you, have to decide, Well, who is sickest, who do I have to get to first, who is going to the ICU?” Dr. Bowman says. “You do this triage thing in your mind as you walk to the ED. If there is any corollary, it’s the fact that you, as the hospitalist, get hit with a slew of patients all at once. They don’t come in one every 15 minutes like in your office for a blood-pressure check.
“Be ready. And, by the way, you are ready,” he adds. “You take care of a stroke patient in room one, take care of a gangrenous leg in room two, a diabetic with ketoacidosis in room three. The broadness, the generality of your training, you are ready to take care of a variety of things. You’re going to be able to help. Just be ready.” TH
Jason Carris is editor of The Hospitalist.
Dave Bowman, MD, doesn’t run anywhere anymore; it’s more of a fast walk. He doesn’t consider himself political, yet he does his civic duty and votes in every election. He’s not a big fan of vegetables, but he eats them to appease his wife and his conscience. Most important, Dr. Bowman doesn’t consider himself a hero. In fact, he doesn’t consider what he did that day any different from what he does every day in the hospital.
On the morning of Jan. 8, Dr. Bowman and his wife, Nancy, were thrust into the epicenter of one of the worst shooting rampages in American history. Dr. Bowman, a hospitalist, was the first physician on the scene outside the Tucson, Ariz., grocery store where a lone gunman killed six people and injured 13 others, including U.S. Representative Gabrielle Giffords (D-Ariz.).
“A hero is somebody, to me, who steps out of their element, steps up to the task that is needed, a task that is completely foreign to them, and steps up and helps people,” says Dr. Bowman, 61, executive director for IPC The Hospitalist Co.’s Tucson region. “A hero is not somebody who should be able to help in some manner and should be expected to help. … I don’t think nurses, doctors, firemen, EMTs get to be labeled as heroes. It’s what we should do, and gladly do, and in many cases took an oath to do.”
Even so, Dr. Bowman’s recollection of that frightful morning is a story laced with tragedy, courage, and hope. He remembers those who are “no longer with us.” He remembers his wife administering CPR and calling victims’ loved ones on their cellphones. And he remembers the brave men and women who not only subdued the shooter, but who worked together, selflessly, and in many cases with no medical training, to assist their injured neighbors.
“It was a pretty traumatic scene. More for others than for me; I am supposed to be the doctor and can handle all that,” he says, pausing. “But they don’t teach this course in medical school.”
Shots Ring Out, First Thought Is Help
Born and raised 100 miles south of Tucson and internal-medicine-trained at the University of Arizona, Dr. Bowman became a hospitalist in 1998 and began working with IPC in 2000. He supervises a staff of about 90 providers, including more than 50 full-time hospitalists, serving two large community hospitals, rehabilitations centers, and skilled nursing facilities in the Tucson area.
Yet he knows who the real boss is in the Bowman household. He and Nancy, an ICU nurse, had just finished a brisk walk, eaten breakfast at McDonald’s, and stopped at Safeway to pick up some vegetables on the morning of Jan. 8. “I had the oatmeal. I was so proud of myself,” Dr. Bowman says. “And, as wives will want to do, she pushes the envelope and says she wants to stop by and get some Brussels sprouts. She hadn’t had them in 20 years, and I hadn’t had them in 45 years—since my mom stunk up the house with them. So we ended up at the Safeway, because the two things that keeps a marriage together are those two words: ‘Yes, dear.’ ”
The Bowmans passed by Rep. Giffords, her staff, and about 25 people in front of the grocery store entrance. They went to the produce department and had not been in the store for more than three or four minutes before shots rang out.
“I was 150 percent sure they were gunshots,” Dr. Bowman says. “I said to my wife, ‘Let’s go, Nancy,’ and she didn’t hear me. I thought she was right behind me. But she had gone over to the Brussels sprouts and I was still in mushrooms.”
Walking to the door, Dr. Bowman saw a woman rushing in and shouting, “They shot her. They shot Congresswoman Giffords.”
“I stepped out and stood behind a pillar until no more gunshots,” he says. “They’d actually already taken down the shooter as soon as he ran out of bullets. I looked around the corner to the carnage, as you’d expect, with Congresswoman Giffords the first person I saw right at the head of the line near the front door.
“Quite frankly, I stepped over people who were no longer with us to get to her. I got her turned around and moved off to the window; she wasn’t breathing real well. I worked on her airway, cleaning her airway with the young man [Rep. Giffords’ intern, Daniel Hernandez], who then held her for the next 15 to 20 minutes until the paramedics got her ready to go to the hospital.”
From that moment, Dr. Bowman says his training and instincts took over. More important, he went into field-triage mode, “which means you can’t do anything for that one. Can’t do anything there, this one is breathing and talking, not bleeding bad, good; still breathing, good, stay right there, I will be back. You go all the way up the line from person to person, seeing who you can and can’t help.
“The problem with field triage is that you really can’t stop and do a lot, like CPR, because then the rest of the people don’t have anybody looking at them. So you just keep moving,” he explains.
He says that while it was only minutes from the first gunshot to the time police arrived and secured the scene to the time paramedics were allowed to assist with the injured, it seemed like hours. Additionally, in his haste to help, Dr. Bowman had lost track of Nancy.
“I looked back and she was not there,” he says, adding she’d been swept to the back of the grocery store. “I kept working and I looked back and she had pushed [aside] the 17-year-old sacker who was acting as a security guard. … She came outside and started doing CPR on the first person she saw. I got up the line and there was a doctor doing CPR on the young girl [Christina-Taylor Green]. He was in the parking lot when the shots rang out; he threw his wife in the car and ran up to the little girl.”
Instinct and Autopilot
In a little more than 10 seconds, the gunman had fired more than 30 rounds and killed or injured nearly 20 people.
After he’d checked on all of the wounded, Dr. Bowman says he came upon four people holding the suspected shooter down. “One of them, I didn’t know it, was one of my colleagues, Dr. Stephen Rayle,” Dr. Bowman says. “I said, ‘Hold him, and I will send the first officer over here.’ ”
From there, Dr. Bowman says, he went back up the line of injured, checking for breathing sounds and bleeding, making sure every injured person was being attended to.
“People shot in the leg were holding a hand over a chest wound on the man or woman next to them,” he says. “It was one of those chaotic scenes that you try to make some kind of order to, by deciding who you can help and who you can’t.”
When the first paramedics arrived, Dr. Bowman directed them to Rep. Giffords and the 9-year-old girl. Soon after, there were enough EMTs on scene to attend to each of the victims.
“You are just on automatic,” Dr. Bowman explains. “Actually, after I dealt with the first two victims pretty quickly, Giffords taking the most time to just get her situated, I did stop and look out in the parking lot just to see if anybody was aiming anything at us.”
The most difficult part of field triage, Dr. Bowman says, is staying calm, organized, and “not losing it emotionally.” In fact, he ushered some shell-shocked bystanders away from the scene. “Some people will step right forward and say, ‘Can I help? I know CPR.’ Other people will just stand there and scream.”
Even tougher, he says, was watching his wife try to save the life of U.S. District Judge John Roll (see “Remembering the Dead,” above). “I finally had to go to my wife, pull her off of him, and say, ‘Honey, he’s gone. I need help with this lady who has been shot in the chest laying in the street,’ ” Dr. Bowman says. “Pulling them away is pretty hard. ... It was harder for her and the bystanders, because they were the ones getting right down close with the patient, talking to them, telling patients to ‘keep looking at me.’ That is a very close bond that develops. Field triage, you just keep moving.”
Human Spirit, Cooperation, and Hope
One month after the shooting, Rep. Giffords was moving, talking, thinking, and recuperating at what her doctors at Houston’s TIRR Hermann Memorial deemed “lightning speed.” For Dr. Bowman, such news brings more than a sigh of relief.
“In truth, when I was first with her, she wasn’t responding,” he says. “She was breathing, although with a compromised airway. We got that straightened away and she had a good pulse. Daniel Hernandez, the young man with her, had some nursing training and was comfortable being with her.
“It was amazing to me, the second or third time down the line, just to look over to this guy, and he was watching for me, and he would nod and say, ‘We’re OK. She’s breathing, pulse is OK.’ I didn’t have to go back, and the third time down he said, ‘She’s moving, she’s squeezing my hand.’ ”
It was at that moment that Dr. Bowman felt the congresswoman, in spite of her severe head trauma, had a fighting chance. Others miracles were happening all around him. Each memory ceases to amaze the veteran physician.
“People who were injured holding somebody’s head in their lap because they were hit in the head and this person was hit in the chest,” he says. “It gives you hope for the human race. Those people, to me, were the heroes.”
Looking back, Dr. Bowman says, he’s played back the moment in his mind more than a few times, wondering if he did the right thing for every victim. He wonders how much he and his wife, the other doctors at the scene, and the bystanders really helped. Then, after a brief moment, he has the answer.
“It really took two weeks to really say that, when all the victims that had expired were dead at the scene, and everybody that got taken away made it and out of the hospital, it sunk in that we had made the right decisions,” he says. “Nobody died on the operating table because we didn’t pick up the gunshot wound to the back. It took two weeks to realize we did the right thing.”
Some of those answers will take many more weeks. Rep. Giffords, although she is on a positive path, still has a long road to a full recovery, and doctors are making no promises. “I don’t know how far she will make it or how long it is going to take, but there are some miracles out there,” Dr. Bowman says.
You’re a Hospitalist? You’re Ready
As a physician in the middle of a mass-casualty event, the media called upon Dr. Bowman to recount the events of the Tucson shooting. In more than a dozen interviews with local and national media, not once was Dr. Bowman referred to as a hospitalist.
“At the scene, when I kneel down, I am a doctor. Can you tell me what’s going on, can you talk to me? It goes no farther than that,” he says. “In talking with one of the interviewers, she asked, ‘What kind of doctor are you?’ I said, ‘I am an internist and a hospitalist, which is a doctor who works just in the hospital.’ They weren’t interested in that. When the cameras rolled, she said, ‘I understand you are an intern?’ I said, ‘No, I am an internist. Turn that thing off and start again.’
“That’s the level of knowledge you are dealing with, and that was a national anchorperson I was dealing with.”
It’s an all-too-common refrain for hospitalists around the country, but one Dr. Bowman and others like him have endured for years. It doesn’t bother him, and he says it shouldn’t bother others.
“Doctor or nurse was as far as it got. I can certainly understand that,” he says. “We explain what a hospitalist is every day. We’ve been doing it for 12, 13, 14 years, and people still don’t understand. It’s OK.”
What isn’t OK is hospitalist unpreparedness. In fact, Dr. Bowman says, his training as an internist and his years of HM experience played a pivotal role in managing the scene of the Tucson shooting. The first thing to do, in addition to remaining calm, is to keep your priorities straight and remember your ABCs.

“It’s airway, airway, airway,” he says. “Without an airway, people don’t live. Then you are looking for bleeding, bleeding, bleeding. Then if they are talking, not a lot of bleeding and have a pulse, that is good enough for right now. So, it’s ‘Lady, just keep pushing on the chest right there on that wound.’ … I think, if you remember your ABCs, that’s all you can do in a field triage situation.”
Although the circumstances are less stressful, hospitalists are faced with make-or-break decisions every day, Dr. Bowman says. For example, it’s 4 p.m. and the day shift physician in the ED calls and says he has six admissions he’s been working on for the past three hours. “You, based on the info given to you, have to decide, Well, who is sickest, who do I have to get to first, who is going to the ICU?” Dr. Bowman says. “You do this triage thing in your mind as you walk to the ED. If there is any corollary, it’s the fact that you, as the hospitalist, get hit with a slew of patients all at once. They don’t come in one every 15 minutes like in your office for a blood-pressure check.
“Be ready. And, by the way, you are ready,” he adds. “You take care of a stroke patient in room one, take care of a gangrenous leg in room two, a diabetic with ketoacidosis in room three. The broadness, the generality of your training, you are ready to take care of a variety of things. You’re going to be able to help. Just be ready.” TH
Jason Carris is editor of The Hospitalist.
ONLINE EXCLUSIVE: Listen to IPC hospitalist Dave Bowman recount the Arizona shooting
The Future is Near
Satish Misra, MD, a first-year internal-medicine resident at Johns Hopkins School of Medicine in Baltimore, used to carry a guidebook—many schools refer to it as their Red Book—around the hospital; it served as a tutorial on how to handle a litany of common medical problems. Now, Dr. Misra mostly scans his iPhone.
Henry Feldman, MD, a hospitalist at Beth Israel Deaconess Medical Center (BIDMC) in Boston who also serves as chief information architect for Harvard Medical Faculty Physicians, used to lug around a bulky copy of Netter’s Anatomy if he wanted to visually explain to a patient how their endoscopic retrograde cholangiopancreatography (ERCP) would work. Now, he pulls up the medical illustrations via an application on his iPad.
In an increasingly technological society in which there is an “app” for nearly everything, healthcare—and HM in particular—is no exception. The growing prevalence of touchscreen technology, mostly via smartphones and tablet computers, already has had an impact on how some hospitalists do their jobs. That upward trend should continue in the coming years, as both hardware and software technology become even more sophisticated and easy to use.
Of course, there are roadblocks. Patient privacy, wireless security, and the well-known reticence of healthcare as an industry to adopt information technology (IT) changes have—and will continue to—slowed the spread of the new technologies. However, with potential or practical usage already being forged in the arenas of patient interaction, billing and coding, and quality and patient safety initiatives, the integration of interactive devices into a physician’s daily workflow could become as commonplace in 10 years as the presence of hospitalists is today.
Still, the CEO of one software company points out that the presence of innovation alone does not translate to efficacy. The value of mobile and touchscreen technology to hospitalists—both from the hardware and the software perspectives—lies in how much a physician chooses to incorporate it into their daily practice.
“The number-one factor in these things being adopted is: Can you improve the quality of documentation … without negatively impacting a physician’s interaction with the patient?” says Todd Johnson, president of Salar Inc., a Baltimore-based firm that develops software applications for clinical documentation. Touchscreen technology “absolutely does help meet that goal, but it depends on the providers. It truly is different strokes for different folks.”
Steven Peskin, MD, MBA, FACP, executive vice president and CMO of Yardley, Pa.-based MediMedia USA, has long preached the value of digital technology for inpatient care, particularly for hospitalists. He categorizes the latest wave of technology into five silos:
- Smartphones: Powered by operating systems that turn them into pocket-size mini-computers, the smallest and most mobile of these technologies are ubiquitous in society and hospitals alike (see Table 1, right).
- Tablet PCs: Led by the iPad’s debut in April 2010, the product is a larger version of the smartphone; the oversized screen makes it practical to use as a virtual chalkboard to explain topics to patients.
- Peripherals: From blood pressure cuffs produced by iHealth Labs (www.ihealth99.com) and Withings (www.withings.com/en/bloodpressuremonitor) to Mobisante’s prototype plug-in ultrasound probe (www.technologyreview.com/biomedicine/), there is a burgeoning marketplace for devices that serve as accessories to a smartphone or tablet, effectively turning those devices into handheld versions of costly machines. Most are connected to a mobile device via simple plug-in cables.
- Applications: According to Dr. Feldman, “It’s not the mobile device that’s the gate to any of this. It’s the applications you interact with.” App stores already feature medical specialty sections, and the number of offerings is expected to grow exponentially in the coming years.
- Cloud computing: A cloud is a metaphorical moniker for the interactivity and interoperability of different devices, systems, and servers to provide immediate connectivity and access to remote data and processes (http://csrc.nist.gov/groups/SNS/cloud-computing/).

“There’s tremendous potential and power of medical computing systems out there, but the stumbling block is they’re bulky or not effective,” says Larry Nathanson, MD, director of emergency medical informatics for BIDMC’s Department of Emergency Medicine, who served as architect and programmer of the ED Dashboard, the information system that is used at BIDMC and a number of other hospitals. “By improving the user interface, the systems become easier to use and the systems become revolutionary.”
Impact: Cloudy, Optimistic
Experts agree that the exact role mobile and touchscreen technologies will play in hospitalist groups around the country remains murky because the field is still a novel one, mostly devoid of evidence-based conclusions. In one of the first planned research studies, the two-year-old University of Central Florida College of Medicine in Orlando has provided iPads to each student in order to research the use of technology in medical education.
Regardless, physicians and tablet manufacturers alike agree that the point-of-service efficiency offered by mobile devices inherently allows their users to be more efficient. Several hospitalists have taken to the Internet, touting how mobile devices have streamlined their efficiency. One popular (and anonymous) blogger, The Happy Hospitalist (http://thehappyhospitalist.blogspot.com/), noted in two recent posts how they were able to round on 16 patients in less than 4 1/2 hours using an iPhone or iPad. On one of those days, the blogger discharged 13 of those patients.
“I no longer have to walk back and forth between patient rooms and nursing stations,” according to The Happy Hospitalist. “I can just drink my coffee at the bedside. I don’t have to fight with other doctors and nurses to log into a paucity of computers that are often way too slow and way too unpredictable. I just sync my iPhone with the patient database app on my iPhone screen and I’m up and running with a real-time update of all my patient’s information.”
The mobile devices allow faster, possibly better, interactions with patients, Dr. Feldman says. For example, a patient tells their hospitalist they need a change to their pain medication. Having a handheld touchscreen device linked to other technologies allows the order to be placed instantly. It even can send the nursing station an alert to the change. The sloppiness of a handwritten note is taken out of play; plus, rounding never misses a beat. “I’m terrible at remembering what I wrote down six patients ago,” Dr. Feldman admits. “Ultimately, for saving money, if I can get things done sooner, theoretically, length of stay can be reduced. That hasn’t been studied, but it is common sense.”
Dr. Feldman, who describes himself as a “hardcore code jockey,” says hospitalists would do well to work closely with their IT staffs to help conceptualize and design in-house applications and interoperability that would make their jobs easier. In institutions with an informatics department, that conversation could be as simple as a one-on-one conversation between an HM group leader and the IT department head.
In other hospitals, a field trip can help. “We will take IT staff out on the wards,” Dr. Feldman explains. “Come observe the process you’re automating. When they come back, they’re very sobered.”
Dr. Misra, the Johns Hopkins intern, notes that mobile devices are perfect hosts for checklists. Their ease of use can even be viewed as a potential motivator to ensure that those checklists are completed, particularly for younger physicians who have either grown up with or started their careers with more exposure to technology than previous generations.
“The biggest strength of touchscreen technology is it’s interactive,” Dr. Misra says. “It’s fun to use, much more fun than checking off boxes on a piece of paper or on a computer screen.
“It’s portable, it’s lightweight, it’s where you are.”
Trouble Spots
The virtually limitless boundaries for touchscreen technology to replace functions in the hospitalist’s workflow is, of course, limited in one glaring respect: privacy. The security of devices, applications, or peripherals must be paramount to their effectiveness, Dr. Feldman says, adding patient information must “remain sacrosanct.”
At BIDMC, digital security is accomplished in part via a bifurcated wireless network that allows physicians access to a secure connection while simultaneously and transparently maintaining a free wireless network for patients and visitors. Not all hospitals can afford the infrastructure necessary for such a setup. And even for health systems that have separate wireless systems, the connectivity cuts both ways, says Mike Stinson, vice president of marketing for Motion in Computing, an Austin, Texas, firm that produces tablet computers for multiple industries, including healthcare.
“Are you willing to have every file on your personal system viewable and accessible by the IT guys so they can make sure you don’t have access to something you shouldn’t have access to?” Stinson asks. “It seems easy and appealing, but there are larger issues.”
Stinson says the privacy and safety concerns of the technology can be addressed. Even potential fears regarding the sterility of the equipment might be simply solved. To wit, a column in the Journal of Surgical Radiology in January found that the device worked well when put in an X-ray cassette sealed off with a hemostat.1
Dr. Nathanson, an ED physician who has worked closely with hospitalists at BIDMC in the past, says it’s clear to him that making the technology easy enough to use in a medical setting is no longer the hurdle. It’s the systemic timidity of physicians who are slow to endorse and incorporate cutting-edge technology into entrenched work patterns.
“In medicine, it tends to take a long time,” he says. “The adoption of technology in medicine can be very challenging. If nothing else, we’re very early in the process.” TH
Richard Quinn is a freelance writer based in New Jersey.
Reference
- Wodajo, FM. The iPad in the hospital and operating room. Journal of Surgical Radiology website. Available at: www.surgisphere.com/SurgRad/issues/volume-2/1-january-2011—pages-1-112/152-column-the-ipad-in-the-hospital-and-operating-room.html. Accessed Jan. 3, 2011.
Satish Misra, MD, a first-year internal-medicine resident at Johns Hopkins School of Medicine in Baltimore, used to carry a guidebook—many schools refer to it as their Red Book—around the hospital; it served as a tutorial on how to handle a litany of common medical problems. Now, Dr. Misra mostly scans his iPhone.
Henry Feldman, MD, a hospitalist at Beth Israel Deaconess Medical Center (BIDMC) in Boston who also serves as chief information architect for Harvard Medical Faculty Physicians, used to lug around a bulky copy of Netter’s Anatomy if he wanted to visually explain to a patient how their endoscopic retrograde cholangiopancreatography (ERCP) would work. Now, he pulls up the medical illustrations via an application on his iPad.
In an increasingly technological society in which there is an “app” for nearly everything, healthcare—and HM in particular—is no exception. The growing prevalence of touchscreen technology, mostly via smartphones and tablet computers, already has had an impact on how some hospitalists do their jobs. That upward trend should continue in the coming years, as both hardware and software technology become even more sophisticated and easy to use.
Of course, there are roadblocks. Patient privacy, wireless security, and the well-known reticence of healthcare as an industry to adopt information technology (IT) changes have—and will continue to—slowed the spread of the new technologies. However, with potential or practical usage already being forged in the arenas of patient interaction, billing and coding, and quality and patient safety initiatives, the integration of interactive devices into a physician’s daily workflow could become as commonplace in 10 years as the presence of hospitalists is today.
Still, the CEO of one software company points out that the presence of innovation alone does not translate to efficacy. The value of mobile and touchscreen technology to hospitalists—both from the hardware and the software perspectives—lies in how much a physician chooses to incorporate it into their daily practice.
“The number-one factor in these things being adopted is: Can you improve the quality of documentation … without negatively impacting a physician’s interaction with the patient?” says Todd Johnson, president of Salar Inc., a Baltimore-based firm that develops software applications for clinical documentation. Touchscreen technology “absolutely does help meet that goal, but it depends on the providers. It truly is different strokes for different folks.”
Steven Peskin, MD, MBA, FACP, executive vice president and CMO of Yardley, Pa.-based MediMedia USA, has long preached the value of digital technology for inpatient care, particularly for hospitalists. He categorizes the latest wave of technology into five silos:
- Smartphones: Powered by operating systems that turn them into pocket-size mini-computers, the smallest and most mobile of these technologies are ubiquitous in society and hospitals alike (see Table 1, right).
- Tablet PCs: Led by the iPad’s debut in April 2010, the product is a larger version of the smartphone; the oversized screen makes it practical to use as a virtual chalkboard to explain topics to patients.
- Peripherals: From blood pressure cuffs produced by iHealth Labs (www.ihealth99.com) and Withings (www.withings.com/en/bloodpressuremonitor) to Mobisante’s prototype plug-in ultrasound probe (www.technologyreview.com/biomedicine/), there is a burgeoning marketplace for devices that serve as accessories to a smartphone or tablet, effectively turning those devices into handheld versions of costly machines. Most are connected to a mobile device via simple plug-in cables.
- Applications: According to Dr. Feldman, “It’s not the mobile device that’s the gate to any of this. It’s the applications you interact with.” App stores already feature medical specialty sections, and the number of offerings is expected to grow exponentially in the coming years.
- Cloud computing: A cloud is a metaphorical moniker for the interactivity and interoperability of different devices, systems, and servers to provide immediate connectivity and access to remote data and processes (http://csrc.nist.gov/groups/SNS/cloud-computing/).

“There’s tremendous potential and power of medical computing systems out there, but the stumbling block is they’re bulky or not effective,” says Larry Nathanson, MD, director of emergency medical informatics for BIDMC’s Department of Emergency Medicine, who served as architect and programmer of the ED Dashboard, the information system that is used at BIDMC and a number of other hospitals. “By improving the user interface, the systems become easier to use and the systems become revolutionary.”
Impact: Cloudy, Optimistic
Experts agree that the exact role mobile and touchscreen technologies will play in hospitalist groups around the country remains murky because the field is still a novel one, mostly devoid of evidence-based conclusions. In one of the first planned research studies, the two-year-old University of Central Florida College of Medicine in Orlando has provided iPads to each student in order to research the use of technology in medical education.
Regardless, physicians and tablet manufacturers alike agree that the point-of-service efficiency offered by mobile devices inherently allows their users to be more efficient. Several hospitalists have taken to the Internet, touting how mobile devices have streamlined their efficiency. One popular (and anonymous) blogger, The Happy Hospitalist (http://thehappyhospitalist.blogspot.com/), noted in two recent posts how they were able to round on 16 patients in less than 4 1/2 hours using an iPhone or iPad. On one of those days, the blogger discharged 13 of those patients.
“I no longer have to walk back and forth between patient rooms and nursing stations,” according to The Happy Hospitalist. “I can just drink my coffee at the bedside. I don’t have to fight with other doctors and nurses to log into a paucity of computers that are often way too slow and way too unpredictable. I just sync my iPhone with the patient database app on my iPhone screen and I’m up and running with a real-time update of all my patient’s information.”
The mobile devices allow faster, possibly better, interactions with patients, Dr. Feldman says. For example, a patient tells their hospitalist they need a change to their pain medication. Having a handheld touchscreen device linked to other technologies allows the order to be placed instantly. It even can send the nursing station an alert to the change. The sloppiness of a handwritten note is taken out of play; plus, rounding never misses a beat. “I’m terrible at remembering what I wrote down six patients ago,” Dr. Feldman admits. “Ultimately, for saving money, if I can get things done sooner, theoretically, length of stay can be reduced. That hasn’t been studied, but it is common sense.”
Dr. Feldman, who describes himself as a “hardcore code jockey,” says hospitalists would do well to work closely with their IT staffs to help conceptualize and design in-house applications and interoperability that would make their jobs easier. In institutions with an informatics department, that conversation could be as simple as a one-on-one conversation between an HM group leader and the IT department head.
In other hospitals, a field trip can help. “We will take IT staff out on the wards,” Dr. Feldman explains. “Come observe the process you’re automating. When they come back, they’re very sobered.”
Dr. Misra, the Johns Hopkins intern, notes that mobile devices are perfect hosts for checklists. Their ease of use can even be viewed as a potential motivator to ensure that those checklists are completed, particularly for younger physicians who have either grown up with or started their careers with more exposure to technology than previous generations.
“The biggest strength of touchscreen technology is it’s interactive,” Dr. Misra says. “It’s fun to use, much more fun than checking off boxes on a piece of paper or on a computer screen.
“It’s portable, it’s lightweight, it’s where you are.”
Trouble Spots
The virtually limitless boundaries for touchscreen technology to replace functions in the hospitalist’s workflow is, of course, limited in one glaring respect: privacy. The security of devices, applications, or peripherals must be paramount to their effectiveness, Dr. Feldman says, adding patient information must “remain sacrosanct.”
At BIDMC, digital security is accomplished in part via a bifurcated wireless network that allows physicians access to a secure connection while simultaneously and transparently maintaining a free wireless network for patients and visitors. Not all hospitals can afford the infrastructure necessary for such a setup. And even for health systems that have separate wireless systems, the connectivity cuts both ways, says Mike Stinson, vice president of marketing for Motion in Computing, an Austin, Texas, firm that produces tablet computers for multiple industries, including healthcare.
“Are you willing to have every file on your personal system viewable and accessible by the IT guys so they can make sure you don’t have access to something you shouldn’t have access to?” Stinson asks. “It seems easy and appealing, but there are larger issues.”
Stinson says the privacy and safety concerns of the technology can be addressed. Even potential fears regarding the sterility of the equipment might be simply solved. To wit, a column in the Journal of Surgical Radiology in January found that the device worked well when put in an X-ray cassette sealed off with a hemostat.1
Dr. Nathanson, an ED physician who has worked closely with hospitalists at BIDMC in the past, says it’s clear to him that making the technology easy enough to use in a medical setting is no longer the hurdle. It’s the systemic timidity of physicians who are slow to endorse and incorporate cutting-edge technology into entrenched work patterns.
“In medicine, it tends to take a long time,” he says. “The adoption of technology in medicine can be very challenging. If nothing else, we’re very early in the process.” TH
Richard Quinn is a freelance writer based in New Jersey.
Reference
- Wodajo, FM. The iPad in the hospital and operating room. Journal of Surgical Radiology website. Available at: www.surgisphere.com/SurgRad/issues/volume-2/1-january-2011—pages-1-112/152-column-the-ipad-in-the-hospital-and-operating-room.html. Accessed Jan. 3, 2011.
Satish Misra, MD, a first-year internal-medicine resident at Johns Hopkins School of Medicine in Baltimore, used to carry a guidebook—many schools refer to it as their Red Book—around the hospital; it served as a tutorial on how to handle a litany of common medical problems. Now, Dr. Misra mostly scans his iPhone.
Henry Feldman, MD, a hospitalist at Beth Israel Deaconess Medical Center (BIDMC) in Boston who also serves as chief information architect for Harvard Medical Faculty Physicians, used to lug around a bulky copy of Netter’s Anatomy if he wanted to visually explain to a patient how their endoscopic retrograde cholangiopancreatography (ERCP) would work. Now, he pulls up the medical illustrations via an application on his iPad.
In an increasingly technological society in which there is an “app” for nearly everything, healthcare—and HM in particular—is no exception. The growing prevalence of touchscreen technology, mostly via smartphones and tablet computers, already has had an impact on how some hospitalists do their jobs. That upward trend should continue in the coming years, as both hardware and software technology become even more sophisticated and easy to use.
Of course, there are roadblocks. Patient privacy, wireless security, and the well-known reticence of healthcare as an industry to adopt information technology (IT) changes have—and will continue to—slowed the spread of the new technologies. However, with potential or practical usage already being forged in the arenas of patient interaction, billing and coding, and quality and patient safety initiatives, the integration of interactive devices into a physician’s daily workflow could become as commonplace in 10 years as the presence of hospitalists is today.
Still, the CEO of one software company points out that the presence of innovation alone does not translate to efficacy. The value of mobile and touchscreen technology to hospitalists—both from the hardware and the software perspectives—lies in how much a physician chooses to incorporate it into their daily practice.
“The number-one factor in these things being adopted is: Can you improve the quality of documentation … without negatively impacting a physician’s interaction with the patient?” says Todd Johnson, president of Salar Inc., a Baltimore-based firm that develops software applications for clinical documentation. Touchscreen technology “absolutely does help meet that goal, but it depends on the providers. It truly is different strokes for different folks.”
Steven Peskin, MD, MBA, FACP, executive vice president and CMO of Yardley, Pa.-based MediMedia USA, has long preached the value of digital technology for inpatient care, particularly for hospitalists. He categorizes the latest wave of technology into five silos:
- Smartphones: Powered by operating systems that turn them into pocket-size mini-computers, the smallest and most mobile of these technologies are ubiquitous in society and hospitals alike (see Table 1, right).
- Tablet PCs: Led by the iPad’s debut in April 2010, the product is a larger version of the smartphone; the oversized screen makes it practical to use as a virtual chalkboard to explain topics to patients.
- Peripherals: From blood pressure cuffs produced by iHealth Labs (www.ihealth99.com) and Withings (www.withings.com/en/bloodpressuremonitor) to Mobisante’s prototype plug-in ultrasound probe (www.technologyreview.com/biomedicine/), there is a burgeoning marketplace for devices that serve as accessories to a smartphone or tablet, effectively turning those devices into handheld versions of costly machines. Most are connected to a mobile device via simple plug-in cables.
- Applications: According to Dr. Feldman, “It’s not the mobile device that’s the gate to any of this. It’s the applications you interact with.” App stores already feature medical specialty sections, and the number of offerings is expected to grow exponentially in the coming years.
- Cloud computing: A cloud is a metaphorical moniker for the interactivity and interoperability of different devices, systems, and servers to provide immediate connectivity and access to remote data and processes (http://csrc.nist.gov/groups/SNS/cloud-computing/).

“There’s tremendous potential and power of medical computing systems out there, but the stumbling block is they’re bulky or not effective,” says Larry Nathanson, MD, director of emergency medical informatics for BIDMC’s Department of Emergency Medicine, who served as architect and programmer of the ED Dashboard, the information system that is used at BIDMC and a number of other hospitals. “By improving the user interface, the systems become easier to use and the systems become revolutionary.”
Impact: Cloudy, Optimistic
Experts agree that the exact role mobile and touchscreen technologies will play in hospitalist groups around the country remains murky because the field is still a novel one, mostly devoid of evidence-based conclusions. In one of the first planned research studies, the two-year-old University of Central Florida College of Medicine in Orlando has provided iPads to each student in order to research the use of technology in medical education.
Regardless, physicians and tablet manufacturers alike agree that the point-of-service efficiency offered by mobile devices inherently allows their users to be more efficient. Several hospitalists have taken to the Internet, touting how mobile devices have streamlined their efficiency. One popular (and anonymous) blogger, The Happy Hospitalist (http://thehappyhospitalist.blogspot.com/), noted in two recent posts how they were able to round on 16 patients in less than 4 1/2 hours using an iPhone or iPad. On one of those days, the blogger discharged 13 of those patients.
“I no longer have to walk back and forth between patient rooms and nursing stations,” according to The Happy Hospitalist. “I can just drink my coffee at the bedside. I don’t have to fight with other doctors and nurses to log into a paucity of computers that are often way too slow and way too unpredictable. I just sync my iPhone with the patient database app on my iPhone screen and I’m up and running with a real-time update of all my patient’s information.”
The mobile devices allow faster, possibly better, interactions with patients, Dr. Feldman says. For example, a patient tells their hospitalist they need a change to their pain medication. Having a handheld touchscreen device linked to other technologies allows the order to be placed instantly. It even can send the nursing station an alert to the change. The sloppiness of a handwritten note is taken out of play; plus, rounding never misses a beat. “I’m terrible at remembering what I wrote down six patients ago,” Dr. Feldman admits. “Ultimately, for saving money, if I can get things done sooner, theoretically, length of stay can be reduced. That hasn’t been studied, but it is common sense.”
Dr. Feldman, who describes himself as a “hardcore code jockey,” says hospitalists would do well to work closely with their IT staffs to help conceptualize and design in-house applications and interoperability that would make their jobs easier. In institutions with an informatics department, that conversation could be as simple as a one-on-one conversation between an HM group leader and the IT department head.
In other hospitals, a field trip can help. “We will take IT staff out on the wards,” Dr. Feldman explains. “Come observe the process you’re automating. When they come back, they’re very sobered.”
Dr. Misra, the Johns Hopkins intern, notes that mobile devices are perfect hosts for checklists. Their ease of use can even be viewed as a potential motivator to ensure that those checklists are completed, particularly for younger physicians who have either grown up with or started their careers with more exposure to technology than previous generations.
“The biggest strength of touchscreen technology is it’s interactive,” Dr. Misra says. “It’s fun to use, much more fun than checking off boxes on a piece of paper or on a computer screen.
“It’s portable, it’s lightweight, it’s where you are.”
Trouble Spots
The virtually limitless boundaries for touchscreen technology to replace functions in the hospitalist’s workflow is, of course, limited in one glaring respect: privacy. The security of devices, applications, or peripherals must be paramount to their effectiveness, Dr. Feldman says, adding patient information must “remain sacrosanct.”
At BIDMC, digital security is accomplished in part via a bifurcated wireless network that allows physicians access to a secure connection while simultaneously and transparently maintaining a free wireless network for patients and visitors. Not all hospitals can afford the infrastructure necessary for such a setup. And even for health systems that have separate wireless systems, the connectivity cuts both ways, says Mike Stinson, vice president of marketing for Motion in Computing, an Austin, Texas, firm that produces tablet computers for multiple industries, including healthcare.
“Are you willing to have every file on your personal system viewable and accessible by the IT guys so they can make sure you don’t have access to something you shouldn’t have access to?” Stinson asks. “It seems easy and appealing, but there are larger issues.”
Stinson says the privacy and safety concerns of the technology can be addressed. Even potential fears regarding the sterility of the equipment might be simply solved. To wit, a column in the Journal of Surgical Radiology in January found that the device worked well when put in an X-ray cassette sealed off with a hemostat.1
Dr. Nathanson, an ED physician who has worked closely with hospitalists at BIDMC in the past, says it’s clear to him that making the technology easy enough to use in a medical setting is no longer the hurdle. It’s the systemic timidity of physicians who are slow to endorse and incorporate cutting-edge technology into entrenched work patterns.
“In medicine, it tends to take a long time,” he says. “The adoption of technology in medicine can be very challenging. If nothing else, we’re very early in the process.” TH
Richard Quinn is a freelance writer based in New Jersey.
Reference
- Wodajo, FM. The iPad in the hospital and operating room. Journal of Surgical Radiology website. Available at: www.surgisphere.com/SurgRad/issues/volume-2/1-january-2011—pages-1-112/152-column-the-ipad-in-the-hospital-and-operating-room.html. Accessed Jan. 3, 2011.
Managerial Muscle
Ajay Kharbanda, MBA, CMPE, is regional director of Arlington-based Texas Health Resources, a nonprofit healthcare system that serves 16 counties in North and Central Texas with 4,100 beds at 24 acute-care and short-stay hospitals. Kharbanda, chair of SHM’s Administrators Task Force, chatted with The Hospitalist about his work, his involvement in SHM leadership, and how administrators can work with HM to improve the healthcare delivery.
Question: How would you characterize your role?
Answer: I work closely with the medical director of the hospitalist group to support physician practice operations for employed hospitalist physicians.
Q: What do you like most about your job as an administrator?
A: I serve professionals who make a difference in people’s lives, and I work with a specialty that is making a difference in how healthcare is being delivered in the country. Additionally, I work for a health system that has the mission to improve the health of the people in the communities we serve.

Q: What motivated you to join—and lead—SHM’s Administrators Task Force?
A: I have been a member of both MGMA (Medical Group Management Association) and SHM for many years, and I have seen SHM mature in the sense of meeting needs of nonclinicians who are looking for an avenue to network and seek answers to our common issues. I remember going to annual meetings, looking for familiar faces, and seeking out peers among the stream of physicians attending the event. Several of us saw the need for an avenue, especially at the annual meetings, for administrators to huddle and brainstorm.
As nonphysician administrators, we send a powerful message about our commitment to the specialty of hospital medicine by becoming a member of the society, and we do need to remember that this is a community unique to our needs as hospital medicine practice administrators.
Q: How is the task force moving HM forward?
A: The Administrators Task Force (ATF) is helping to develop initiatives and programs that promote and define the role of nonphysician practice administrators in hospital medicine. The ATF is charged with facilitating and enhancing the integration of administrators into the society. We strive to strengthen the society’s ability to fulfill its mission by developing and using the talents of current and future administrative leaders.
Q: How is the ATF helping hospitals improve patient care?
A: I believe it is by strengthening the role of the society. ATF has reached out to administrators nationwide to build awareness of the value of SHM resources, and we advised on the practice management [curriculum] for HM10. Plus, we have created the Web-based Practice Administrators’ Roundtable Series. These quarterly events provide an opportunity to discuss issues of common concern and share best practices around various topics. Following a brief formal presentation, participants are encouraged to take part in the discussion.
We all know that SHM offers a remarkable avenue for clinical knowledge, and we are helping to build an avenue for nonclinicians.
Q: Are there ways for other hospitalists and administrators to get involved with SHM?
A: First, visit the Practice Management Institute Web page at www.hospitalmedicine.org. It has information about the Practice Administrators’ Roundtable Series and resources on staffing and scheduling, career satisfaction, and coding and documentation.
Second, come to HM11, SHM’s annual meeting. We will be hosting a special-interest forum specifically designed for administrators. TH
Brendon Shank is vice president of communications for SHM.
Ajay Kharbanda, MBA, CMPE, is regional director of Arlington-based Texas Health Resources, a nonprofit healthcare system that serves 16 counties in North and Central Texas with 4,100 beds at 24 acute-care and short-stay hospitals. Kharbanda, chair of SHM’s Administrators Task Force, chatted with The Hospitalist about his work, his involvement in SHM leadership, and how administrators can work with HM to improve the healthcare delivery.
Question: How would you characterize your role?
Answer: I work closely with the medical director of the hospitalist group to support physician practice operations for employed hospitalist physicians.
Q: What do you like most about your job as an administrator?
A: I serve professionals who make a difference in people’s lives, and I work with a specialty that is making a difference in how healthcare is being delivered in the country. Additionally, I work for a health system that has the mission to improve the health of the people in the communities we serve.

Q: What motivated you to join—and lead—SHM’s Administrators Task Force?
A: I have been a member of both MGMA (Medical Group Management Association) and SHM for many years, and I have seen SHM mature in the sense of meeting needs of nonclinicians who are looking for an avenue to network and seek answers to our common issues. I remember going to annual meetings, looking for familiar faces, and seeking out peers among the stream of physicians attending the event. Several of us saw the need for an avenue, especially at the annual meetings, for administrators to huddle and brainstorm.
As nonphysician administrators, we send a powerful message about our commitment to the specialty of hospital medicine by becoming a member of the society, and we do need to remember that this is a community unique to our needs as hospital medicine practice administrators.
Q: How is the task force moving HM forward?
A: The Administrators Task Force (ATF) is helping to develop initiatives and programs that promote and define the role of nonphysician practice administrators in hospital medicine. The ATF is charged with facilitating and enhancing the integration of administrators into the society. We strive to strengthen the society’s ability to fulfill its mission by developing and using the talents of current and future administrative leaders.
Q: How is the ATF helping hospitals improve patient care?
A: I believe it is by strengthening the role of the society. ATF has reached out to administrators nationwide to build awareness of the value of SHM resources, and we advised on the practice management [curriculum] for HM10. Plus, we have created the Web-based Practice Administrators’ Roundtable Series. These quarterly events provide an opportunity to discuss issues of common concern and share best practices around various topics. Following a brief formal presentation, participants are encouraged to take part in the discussion.
We all know that SHM offers a remarkable avenue for clinical knowledge, and we are helping to build an avenue for nonclinicians.
Q: Are there ways for other hospitalists and administrators to get involved with SHM?
A: First, visit the Practice Management Institute Web page at www.hospitalmedicine.org. It has information about the Practice Administrators’ Roundtable Series and resources on staffing and scheduling, career satisfaction, and coding and documentation.
Second, come to HM11, SHM’s annual meeting. We will be hosting a special-interest forum specifically designed for administrators. TH
Brendon Shank is vice president of communications for SHM.
Ajay Kharbanda, MBA, CMPE, is regional director of Arlington-based Texas Health Resources, a nonprofit healthcare system that serves 16 counties in North and Central Texas with 4,100 beds at 24 acute-care and short-stay hospitals. Kharbanda, chair of SHM’s Administrators Task Force, chatted with The Hospitalist about his work, his involvement in SHM leadership, and how administrators can work with HM to improve the healthcare delivery.
Question: How would you characterize your role?
Answer: I work closely with the medical director of the hospitalist group to support physician practice operations for employed hospitalist physicians.
Q: What do you like most about your job as an administrator?
A: I serve professionals who make a difference in people’s lives, and I work with a specialty that is making a difference in how healthcare is being delivered in the country. Additionally, I work for a health system that has the mission to improve the health of the people in the communities we serve.

Q: What motivated you to join—and lead—SHM’s Administrators Task Force?
A: I have been a member of both MGMA (Medical Group Management Association) and SHM for many years, and I have seen SHM mature in the sense of meeting needs of nonclinicians who are looking for an avenue to network and seek answers to our common issues. I remember going to annual meetings, looking for familiar faces, and seeking out peers among the stream of physicians attending the event. Several of us saw the need for an avenue, especially at the annual meetings, for administrators to huddle and brainstorm.
As nonphysician administrators, we send a powerful message about our commitment to the specialty of hospital medicine by becoming a member of the society, and we do need to remember that this is a community unique to our needs as hospital medicine practice administrators.
Q: How is the task force moving HM forward?
A: The Administrators Task Force (ATF) is helping to develop initiatives and programs that promote and define the role of nonphysician practice administrators in hospital medicine. The ATF is charged with facilitating and enhancing the integration of administrators into the society. We strive to strengthen the society’s ability to fulfill its mission by developing and using the talents of current and future administrative leaders.
Q: How is the ATF helping hospitals improve patient care?
A: I believe it is by strengthening the role of the society. ATF has reached out to administrators nationwide to build awareness of the value of SHM resources, and we advised on the practice management [curriculum] for HM10. Plus, we have created the Web-based Practice Administrators’ Roundtable Series. These quarterly events provide an opportunity to discuss issues of common concern and share best practices around various topics. Following a brief formal presentation, participants are encouraged to take part in the discussion.
We all know that SHM offers a remarkable avenue for clinical knowledge, and we are helping to build an avenue for nonclinicians.
Q: Are there ways for other hospitalists and administrators to get involved with SHM?
A: First, visit the Practice Management Institute Web page at www.hospitalmedicine.org. It has information about the Practice Administrators’ Roundtable Series and resources on staffing and scheduling, career satisfaction, and coding and documentation.
Second, come to HM11, SHM’s annual meeting. We will be hosting a special-interest forum specifically designed for administrators. TH
Brendon Shank is vice president of communications for SHM.
NEW MEMBERS
Enter text here
Enter text here
Enter text here