User login
The shrinking woman
A 45-year-old woman who has been undergoing hemodialysis for 20 years presents with diffuse bone pain, pruritic skin, muscle weakness, and disability. About 8 years ago, she was diagnosed with uremic hyperparathyroidism and underwent two parathyroidectomy procedures and eight sessions of percutaneous alcohol ablation of the parathyroid gland.
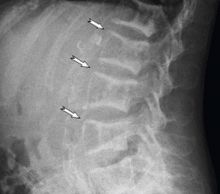
A technetium-99m sestamibi radionuclide scan shows bilateral parathyroid hyperplasia with no ectopic parathyroid adenomas. Although a surgeon she consulted 1 month ago declined to perform another parathyroidectomy for technical reasons, another surgeon agreed to do it at this time. Parathyroidectomy was successfully performed, after which the parathyroid hormone level decreased drastically, to 85 pg/mL.
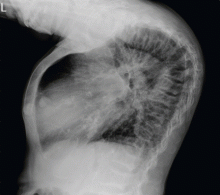
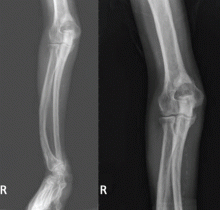
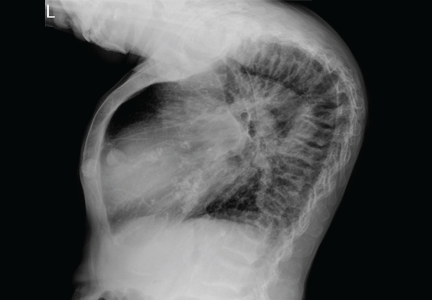
To our surprise, her total body length increased by 6 to 7 cm after surgery, with partial straightening of the back and legs noted about 4 to 5 weeks after surgery. Furthermore, she was able to walk a short distance just a few weeks after surgery. Unfortunately, she died from sepsis the next year.
SEVERE UREMIC HYPERPARATHYROIDISM: A CLINICAL DILEMMA
The clinical appearance of reduced body length and diffuse bony deformity leading to “shrinking” as a consequence of prolonged severe uremic hyperparathyroidism has only rarely been reported.1 However, it is not uncommon for surgeons to decide against parathyroidectomy because of concerns of extensive subcutaneous fibrosis and recurrent laryngeal nerve damage associated with previous operations. The result is that the patient’s uremic hyperparathyroidism goes untreated, increasing the risk of long-term complications, as in this patient.
TYPICAL RADIOGRAPHIC FEATURES
Why the body length increases after parathyroidectomy is not yet known, but plausible mechanisms are the effects of a recovery of muscle strength and the vigorous bony remineralization that strengthens weight-bearing bones after resolution of uremic hyperparathyroidism.
THE DANGERS OF DELAYED TREATMENT
Delaying parathyroidectomy may induce prolonged and severe uremic hyperparathyroidism, as in this patient. Nevertheless, despite the delay, surgery was able to partially ameliorate the symptoms of hyperparathyroidism and improve the extreme bone deformity. However, the patient’s informed consent, a detailed preoperative evaluation, and exclusion of ectopic parathyroid adenomas are imperative before surgical treatment.
- Horensten ML, Boner G, Rosenfeld JB. The shrinking man. A manifestation of severe renal osteodystrophy. JAMA 1980; 244:267–268.
- Jevtic V. Imaging of renal osteodystrophy. Eur J Radiol 2003; 46:85–95.
- Ferreira MA. Diagnosis of renal osteodystrophy: when and how to use biochemical markers and non-invasive methods; when bone biopsy is needed. Nephrol Dial Transplant 2000; 15(suppl 5):8–14.
A 45-year-old woman who has been undergoing hemodialysis for 20 years presents with diffuse bone pain, pruritic skin, muscle weakness, and disability. About 8 years ago, she was diagnosed with uremic hyperparathyroidism and underwent two parathyroidectomy procedures and eight sessions of percutaneous alcohol ablation of the parathyroid gland.
A technetium-99m sestamibi radionuclide scan shows bilateral parathyroid hyperplasia with no ectopic parathyroid adenomas. Although a surgeon she consulted 1 month ago declined to perform another parathyroidectomy for technical reasons, another surgeon agreed to do it at this time. Parathyroidectomy was successfully performed, after which the parathyroid hormone level decreased drastically, to 85 pg/mL.
To our surprise, her total body length increased by 6 to 7 cm after surgery, with partial straightening of the back and legs noted about 4 to 5 weeks after surgery. Furthermore, she was able to walk a short distance just a few weeks after surgery. Unfortunately, she died from sepsis the next year.
SEVERE UREMIC HYPERPARATHYROIDISM: A CLINICAL DILEMMA
The clinical appearance of reduced body length and diffuse bony deformity leading to “shrinking” as a consequence of prolonged severe uremic hyperparathyroidism has only rarely been reported.1 However, it is not uncommon for surgeons to decide against parathyroidectomy because of concerns of extensive subcutaneous fibrosis and recurrent laryngeal nerve damage associated with previous operations. The result is that the patient’s uremic hyperparathyroidism goes untreated, increasing the risk of long-term complications, as in this patient.
TYPICAL RADIOGRAPHIC FEATURES
Why the body length increases after parathyroidectomy is not yet known, but plausible mechanisms are the effects of a recovery of muscle strength and the vigorous bony remineralization that strengthens weight-bearing bones after resolution of uremic hyperparathyroidism.
THE DANGERS OF DELAYED TREATMENT
Delaying parathyroidectomy may induce prolonged and severe uremic hyperparathyroidism, as in this patient. Nevertheless, despite the delay, surgery was able to partially ameliorate the symptoms of hyperparathyroidism and improve the extreme bone deformity. However, the patient’s informed consent, a detailed preoperative evaluation, and exclusion of ectopic parathyroid adenomas are imperative before surgical treatment.
A 45-year-old woman who has been undergoing hemodialysis for 20 years presents with diffuse bone pain, pruritic skin, muscle weakness, and disability. About 8 years ago, she was diagnosed with uremic hyperparathyroidism and underwent two parathyroidectomy procedures and eight sessions of percutaneous alcohol ablation of the parathyroid gland.
A technetium-99m sestamibi radionuclide scan shows bilateral parathyroid hyperplasia with no ectopic parathyroid adenomas. Although a surgeon she consulted 1 month ago declined to perform another parathyroidectomy for technical reasons, another surgeon agreed to do it at this time. Parathyroidectomy was successfully performed, after which the parathyroid hormone level decreased drastically, to 85 pg/mL.
To our surprise, her total body length increased by 6 to 7 cm after surgery, with partial straightening of the back and legs noted about 4 to 5 weeks after surgery. Furthermore, she was able to walk a short distance just a few weeks after surgery. Unfortunately, she died from sepsis the next year.
SEVERE UREMIC HYPERPARATHYROIDISM: A CLINICAL DILEMMA
The clinical appearance of reduced body length and diffuse bony deformity leading to “shrinking” as a consequence of prolonged severe uremic hyperparathyroidism has only rarely been reported.1 However, it is not uncommon for surgeons to decide against parathyroidectomy because of concerns of extensive subcutaneous fibrosis and recurrent laryngeal nerve damage associated with previous operations. The result is that the patient’s uremic hyperparathyroidism goes untreated, increasing the risk of long-term complications, as in this patient.
TYPICAL RADIOGRAPHIC FEATURES
Why the body length increases after parathyroidectomy is not yet known, but plausible mechanisms are the effects of a recovery of muscle strength and the vigorous bony remineralization that strengthens weight-bearing bones after resolution of uremic hyperparathyroidism.
THE DANGERS OF DELAYED TREATMENT
Delaying parathyroidectomy may induce prolonged and severe uremic hyperparathyroidism, as in this patient. Nevertheless, despite the delay, surgery was able to partially ameliorate the symptoms of hyperparathyroidism and improve the extreme bone deformity. However, the patient’s informed consent, a detailed preoperative evaluation, and exclusion of ectopic parathyroid adenomas are imperative before surgical treatment.
- Horensten ML, Boner G, Rosenfeld JB. The shrinking man. A manifestation of severe renal osteodystrophy. JAMA 1980; 244:267–268.
- Jevtic V. Imaging of renal osteodystrophy. Eur J Radiol 2003; 46:85–95.
- Ferreira MA. Diagnosis of renal osteodystrophy: when and how to use biochemical markers and non-invasive methods; when bone biopsy is needed. Nephrol Dial Transplant 2000; 15(suppl 5):8–14.
- Horensten ML, Boner G, Rosenfeld JB. The shrinking man. A manifestation of severe renal osteodystrophy. JAMA 1980; 244:267–268.
- Jevtic V. Imaging of renal osteodystrophy. Eur J Radiol 2003; 46:85–95.
- Ferreira MA. Diagnosis of renal osteodystrophy: when and how to use biochemical markers and non-invasive methods; when bone biopsy is needed. Nephrol Dial Transplant 2000; 15(suppl 5):8–14.
Are antibiotics indicated for the treatment of aspiration pneumonia?
Antibiotics are indicated for primary bacterial aspiration pneumonia and secondary bacterial infection of aspiration (chemical) pneumonitis, but not for uncomplicated chemical pneumonitis.
THREE TYPES OF ‘ASPIRATION PNEUMONIA’
Aspiration pneumonia is a broad and vague term mainly used to refer to the pulmonary consequences of abnormal entry of exogenous or endogenous substances into the lower airways. It can be classified as:
- Aspiration (chemical) pneumonitis
- Primary bacterial aspiration pneumonia
- Secondary bacterial infection of chemical pneumonitis.
These three are sometimes difficult to differentiate, as their signs and symptoms can overlap.
CHEMICAL PNEUMONITIS
Aspiration of stomach contents is relatively common, even in healthy people, and usually has no clinical consequences.1 However, it has also been closely related to community-acquired and nosocomial pneumonia in some studies.2,3
Chemical pneumonitis is usually a consequence of the aspiration of a large volume (≥ 4 mL/kg) of sterile acidic (pH < 2.5) gastric contents into the lower airways (Mendelson syndrome).4,5 The clinical picture varies from asymptomatic to signs of severe dyspnea, hypoxia, cough, and low-grade fever; these signs and symptoms may develop rapidly, within minutes to hours after a witnessed or suspected episode of aspiration.2,6,7 However, they represent an inflammatory reaction to the gastric acid rather than a reaction to bacterial infection.8–10
Chemical pneumonitis affects the most dependent regions of the lungs
Chest radiography shows infiltrates in the most dependent regions of the lung. If aspiration occurs while the patient is supine, the posterior segments of the upper lobes and the apical segments of the lower lobes are most affected. The basal segments of the lower lobes are usually affected if aspiration occurs while the patient is standing or upright.1,2,11,12
Clinical course varies
The clinical course varies. In almost 60% of cases, the patient’s condition improves and the lung infiltrates resolve rapidly, within 2 to 4 days. On the other hand, in about 15% of cases, the patient’s condition deteriorates quickly, within 24 to 36 hours, and progresses to hypoxic respiratory failure and acute respiratory distress syndrome.
In the other 25% of cases, the patient’s condition may improve initially but then worsen as a secondary bacterial infection sets in. The death rate in these patients is almost three times higher than the rate in patients with uncomplicated chemical pneumonitis.11,13
Treatment of uncomplicated cases is mainly supportive
The treatment of uncomplicated chemical pneumonitis involves supportive measures such as airway clearance, oxygen supplementation, and positive pressure ventilation if needed. An obstructing foreign body may need to be removed.12,14 Corticosteroids have been tried, without success.11–13,15
Empiric antibiotic treatment is controversial
Chemical pneumonitis can be difficult to differentiate from bacterial aspiration pneumonia, and whether to give antibiotics is controversial. 16 A survey of current practices among intensivists showed that antimicrobial therapy was often given empirically for noninfectious chemical pneumonitis.17 This practice raises concerns of higher treatment costs and antibiotic resistance.16,18,19 Additionally, antibiotics do not seem to alter the clinical outcome, including radiographic resolution, duration of hospitalization, or death rate, nor do they influence the subsequent development of infection.1,11,13,20
In cases of witnessed or strongly suspected aspiration of gastric contents, antibiotics are not warranted since bacterial infection is not likely to be the cause of any signs or symptoms. 2,7,16 However, to detect secondary infection early, the patient’s respiratory status should be monitored carefully and chest radiography should be repeated.
In less clear-cut cases, ie, if it is not clear whether the patient actually has chemical pneumonitis or primary bacterial aspiration pneumonia, it is prudent to start antibiotics empirically after obtaining lower-respiratory-tract secretions for stains and cultures, and then to reassess within 48 to 72 hours. The antibiotics can be discontinued if the patient has rapid clinical and radiographic improvement and negative cultures. Those whose condition does not improve or who have positive cultures should receive a full course of antibiotics.21,22
PRIMARY BACTERIAL ASPIRATION PNEUMONIA
Primary bacterial aspiration pneumonia—ie, caused by bacteria residing in the upper airways and stomach gaining access to lower airways through aspiration in small or large amounts—is the most common form of aspiration pneumonia, although the actual episode of aspiration is seldom observed.
Signs of bacterial pneumonia
Primary bacterial aspiration pneumonia bears the hallmarks of bacterial pneumonia.12 The clinical picture is more indolent than chemical pneumonitis and includes cough, fever, and putrid sputum, mainly in patients who have clinical conditions predisposing to aspiration (eg, coma, stroke, alcoholism, poor dentition, tube feedings).1,12,20
The characteristic signs on chest radiography are infiltrates involving mainly the lung bases (the right more then the left). If untreated or inadequately treated, complications such as lung abscess, empyema, bronchiectasis, and broncopleural fistula are common.23
Are aerobic organisms replacing anaerobic ones in the community?
The causative organisms in community-acquired aspiration pneumonia are still debated despite abundant research. Older studies1,24,25 found mostly anaerobic organisms (pepto-streptococci, peptococci, Fusobacterium, Prevotela, Bacteroides) as the underlying pathogens, whereas more recent studies16,26,27 found mostly aerobic organisms (Streptococcus pneumoniae, Haemophilus influenzae, Staphylococcus aureus, Enterobacteriaceae) and failed to recover anaerobic organisms. These discrepancies may be the result of different techniques used to isolate organisms: older studies used transtracheal sampling, and transtracheal aspirates may be easily contaminated or colonized by oropharyngeal flora; more recent studies used protected specimen brushes to collect lower-airway specimens.2
In addition, the pathogenic organisms that predominate in community-acquired aspiration pneumonia, as listed above, are different from those most often found in nosocomial cases; gram-negative bacilli (Pseudomonas aeruginosa, Klebsiella pneumoniae, Escherichia coli) are most often isolated in patients with aspiration pneumonia acquired in hospitals and nursing homes.16,27,28S aureus also is an important causative organism in nosocomial cases.16,28
Knowing the causative organisms in bacterial aspiration pneumonia is important for guiding antimicrobial therapy.
Antibiotics are required for bacterial aspiration pneumonia
A course of antibiotics is required for bacterial aspiration pneumonia. While there are no definitive recommendations for the duration of treatment, 7 to 8 days is probably appropriate in uncomplicated cases (ie, no lung abscess, empyema, bronchopleural fistula).22,29 Patients who have complications may need drainage of abscesses or empyema along with a longer duration of antibiotic therapy until clinical and radiographic signs improve.
For community-acquired cases of aspiration pneumonia, a number of antibiotics have proven effective:
- Clindamycin (Cleocin) is still the agent most commonly used, although it lacks gram-negative bacterial coverage.
- Beta-lactam penicillins and newer quinolones have been used successfully.2,29–31 In addition to covering the previously mentioned bacteria, these antibiotics have the added benefit of covering anaerobic bacteria.
- Metronidazole (Flagyl) should not be used alone because it has a higher clinical failure rate.32,33
For nosocomial aspiration pneumonia, giving a broad-spectrum antibiotic empirically is warranted. Beta-lactam penicillins with extended gram-negative coverage, carbapenems, or monobactams in combination with an anti-staphylococcal drug have been advocated for nosocomial aspiration.2,22 A strategy of broad-spectrum coverage followed by narrowing or de-escalating coverage according to lower respiratory tract cultures is encouraged.21,22,34
SECONDARY BACTERIAL INFECTION OF CHEMICAL PNEUMONITIS
Nearly 25% of patients with chemical pneumonitis improve initially, then show clinical deterioration secondary to superimposed bacterial infection.13 Chest radiographs show worsening of initial infiltrates or the development of new ones. The causative organisms and treatment depend on whether the superimposed infection is community-acquired or nosocomial, as is the case in primary bacterial aspiration pneumonia.
PREVENTING ASPIRATION
Measures should be taken to prevent aspiration pneumonia and chemical pneumonitis, especially in institutionalized patients at high risk.12
Elevation of the head of the bed while feeding, dental prophylaxis, and good oral hygiene are known to reduce the incidence of these problems.35–37
A swallowing evaluation for patients with dysphagia can identify those at higher risk of aspiration. These patients may be candidates for postural adjustments, diet modification, strengthening, and other measures offered by the speech and language pathology teams to improve swallowing physiology, biomechanics, safety, and endurance.2,35
Although percutaneous endoscopic gastrostomy tubes are often placed in patients who have aspirated or who are at high risk of aspiration, they do not protect against aspiration, nor do orogastric or nasogastric tubes.38
To date, we have no evidence that prophylactic antibiotic therapy prevents bacterial aspiration pneumonia. In addition, this practice encourages the development of resistant organisms.19,39,40
- Bartlett JG, Gorbach SL. The triple threat of aspiration pneumonia. Chest 1975; 68:560–566.
- Marik PE. Aspiration pneumonitis and aspiration pneumonia. N Engl J Med 2001; 344:665–671.
- Kikuchi R, Watabe N, Konno T, Mishina N, Sekizawa K, Sasaki H. High incidence of silent aspiration in elderly patients with community-acquired pneumonia. Am J Respir Crit Care Med 1994; 150:251–253.
- Mendelson CL. The aspiration of stomach contents into lungs during obstetric anesthesia. Am J Obstet Gynecol 1946; 52:191–205.
- Cameron JL, Caldini P, Toung JK, Zuidema GD. Aspiration pneumonia: physiologic data following experimental aspiration. Surgery 1972; 72:238–245.
- Warner MA, Warner ME, Weber JG. Clinical significance of pulmonary aspiration during the perioperative period. Anesthesiology 1993; 78:56–62.
- DePaso WJ. Aspiration pneumonia. Clin Chest Med 1991; 12:269–284.
- Folkesson HG, Matthay MA, Hébert CA, Broaddus VC. Acid aspiration-induced lung injury in rabbits is mediated by interleukin-8-dependent mechanisms. J Clin Invest 1995; 96:107–116.
- Goldman G, Welbourn R, Kobzik L, Valeri CR, Shepro D, Hechtman HB. Tumor necrosis factor-alpha mediates acid aspiration-induced systemic organ injury. Ann Surg 1990; 212:513–519.
- LeFrock JL, Clark TS, Davies B, Klainer AS. Aspiration pneumonia: a ten-year review. Am Surg 1979; 45:305–313.
- Cameron JL, Mitchell WH, Zuidema GD. Aspiration pneumonia. Clinical outcome following documented aspiration. Arch Surg 1973; 106:49–52.
- Arms RA, Dines DE, Tinstman TC. Aspiration pneumonia. Chest 1974; 65:136–139.
- Bynum LJ, Pierce AK. Pulmonary aspiration of gastric contents. Am Rev Respir Dis 1976; 114:1129–1136.
- Merchant SN, Kirtane MV, Shah KL, Karnik PP. Foreign bodies in the bronchi (a 10 year review of 132 cases). J Postgrad Med 1984; 30:219–223.
- Wolfe JE, Bone RC, Ruth WE. Effects of corticosteroids in the treatment of patients with gastric aspiration. Am J Med 1977; 63:719–722.
- Kane-Gill SL, Olsen KM, Rebuck JA, et al; Aspiration Evaluation Group of the Clinical Pharmacy and Pharmacology Section. Multicenter treatment and outcome evaluation of aspiration syndromes in critically ill patients. Ann Pharmacother 2007; 41:549–555.
- Rebuck JA, Rasmussen JR, Olsen KM. Clinical aspiration-related practice patterns in the intensive care unit: a physician survey. Crit Care Med 2001; 29:2239–2244.
- Singh N, Rogers P, Atwood CW, Wagener MM, Yu VL. Short-course empiric antibiotic therapy for patients with pulmonary infiltrates in the intensive care unit. A proposed solution for indiscriminate antibiotic prescription. Am J Respir Crit Care Med 2000; 162:505–511.
- Kollef MH, Fraser VJ. Antibiotic resistance in the intensive care unit. Ann Intern Med 2001; 134:298–314.
- Lewis RT, Burgess JH, Hampson LG. Cardiorespiratory studies in critical illness. Changes in aspiration pneumonitis. Arch Surg 1971; 103:335–340.
- Rello J. Importance of appropriate initial antibiotic therapy and de-escalation in the treatment of nosocomial pneumonia. Eur Respir Rev 2007; 16:33–39.
- American Thoracic Society. Guidelines for the management of adults with hospital-acquired, ventilator-associated, and healthcare-associated pneumonia. Am J Respir Crit Care Med 2005; 171:388–416.
- Bartlett JG. Anaerobic bacterial infections of the lung and pleural space. Clin Infect Dis 1993; 16(suppl 4):S248–S255.
- Lorber B, Swenson RM. Bacteriology of aspiration pneumonia. A prospective study of community- and hospital-acquired cases. Ann Intern Med 1974; 81:329–331.
- Bartlett JG, Gorbach SL, Finegold SM. The bacteriology of aspiration pneumonia. Am J Med 1974; 56:202–207.
- Mier L, Dreyfuss D, Darchy B, et al. Is penicillin G an adequate initial treatment for aspiration pneumonia? A prospective evaluation using a protected specimen brush and quantitative cultures. Intensive Care Med 1993; 19:279–284.
- Marik PE, Careau P. The role of anaerobes in patients with ventilator-associated pneumonia and aspiration pneumonia: a prospective study. Chest 1999; 115:178–183.
- El-Solh AA, Pietrantoni C, Bhat A, et al. Microbiology of severe aspiration pneumonia in institutionalized elderly. Am J Respir Crit Care Med 2003; 167:1650–1654.
- Mandell LA, Wunderink RG, Anzueto A, et al; Infectious Diseases Society of America. Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community-acquired pneumonia in adults. Clin Infect Dis 2007; 44(suppl 2):S27–S72.
- Kadowaki M, Demura Y, Mizuno S, et al. Reappraisal of clindamycin IV monotherapy for treatment of mild-to-moderate aspiration pneumonia in elderly patients. Chest 2005; 127:1276–1282.
- Ott SR, Allewelt M, Lorenz J, Reimnitz P, Lode H; German Lung Abscess Study Group. Moxifloxacin vs ampicillin/sulbactam in aspiration pneumonia and primary lung abscess. Infection 2008; 36:23–30.
- Perlino CA. Metronidazole vs clindamycin treatment of anerobic pulmonary infection. Failure of metronidazole therapy. Arch Intern Med 1981; 141:1424–1427.
- Sanders CV, Hanna BJ, Lewis AC. Metronidazole in the treatment of anaerobic infections. Am Rev Respir Dis 1979; 120:337–343.
- Alvarez-Lerma F, Alvarez B, Luque P, et al; ADANN Study Group. Empiric broad-spectrum antibiotic therapy of nosocomial pneumonia in the intensive care unit: a prospective observational study. Crit Care 2006; 10:R78.
- Johnson JL, Hirsch CS. Aspiration pneumonia. Recognizing and managing a potentially growing disorder. Postgrad Med 2003; 113:99–112.
- Scolapio JS. Methods for decreasing risk of aspiration pneumonia in critically ill patients. JPEN J Parenter Enteral Nutr 2002; 26(suppl 6):S58–S61.
- Orozco-Levi M, Torres A, Ferrer M, et al. Semirecumbent position protects from pulmonary aspiration but not completely from gastroesophageal reflux in mechanically ventilated patients. Am J Respir Crit Care Med 1995; 152:1387–1390.
- Park RH, Allison MC, Lang J, et al. Randomised comparison of percutaneous endoscopic gastrostomy and nasogastric tube feeding in patients with persisting neurological dysphagia. BMJ 1992; 304( 6839):1406–1409.
- Donskey CJ, Chowdhry TK, Hecker MT, et al. Effect of antibiotic therapy on the density of vancomycin-resistant enterococci in the stool of colonized patients. N Engl J Med 2000; 343:1925–1932.
- Mouw DR, Langlois JP, Turner LF, Neher JO. Clinical inquiries. Are antibiotics effective in preventing pneumonia for nursing home patients? J Fam Pract 2004; 53:994–996.
Antibiotics are indicated for primary bacterial aspiration pneumonia and secondary bacterial infection of aspiration (chemical) pneumonitis, but not for uncomplicated chemical pneumonitis.
THREE TYPES OF ‘ASPIRATION PNEUMONIA’
Aspiration pneumonia is a broad and vague term mainly used to refer to the pulmonary consequences of abnormal entry of exogenous or endogenous substances into the lower airways. It can be classified as:
- Aspiration (chemical) pneumonitis
- Primary bacterial aspiration pneumonia
- Secondary bacterial infection of chemical pneumonitis.
These three are sometimes difficult to differentiate, as their signs and symptoms can overlap.
CHEMICAL PNEUMONITIS
Aspiration of stomach contents is relatively common, even in healthy people, and usually has no clinical consequences.1 However, it has also been closely related to community-acquired and nosocomial pneumonia in some studies.2,3
Chemical pneumonitis is usually a consequence of the aspiration of a large volume (≥ 4 mL/kg) of sterile acidic (pH < 2.5) gastric contents into the lower airways (Mendelson syndrome).4,5 The clinical picture varies from asymptomatic to signs of severe dyspnea, hypoxia, cough, and low-grade fever; these signs and symptoms may develop rapidly, within minutes to hours after a witnessed or suspected episode of aspiration.2,6,7 However, they represent an inflammatory reaction to the gastric acid rather than a reaction to bacterial infection.8–10
Chemical pneumonitis affects the most dependent regions of the lungs
Chest radiography shows infiltrates in the most dependent regions of the lung. If aspiration occurs while the patient is supine, the posterior segments of the upper lobes and the apical segments of the lower lobes are most affected. The basal segments of the lower lobes are usually affected if aspiration occurs while the patient is standing or upright.1,2,11,12
Clinical course varies
The clinical course varies. In almost 60% of cases, the patient’s condition improves and the lung infiltrates resolve rapidly, within 2 to 4 days. On the other hand, in about 15% of cases, the patient’s condition deteriorates quickly, within 24 to 36 hours, and progresses to hypoxic respiratory failure and acute respiratory distress syndrome.
In the other 25% of cases, the patient’s condition may improve initially but then worsen as a secondary bacterial infection sets in. The death rate in these patients is almost three times higher than the rate in patients with uncomplicated chemical pneumonitis.11,13
Treatment of uncomplicated cases is mainly supportive
The treatment of uncomplicated chemical pneumonitis involves supportive measures such as airway clearance, oxygen supplementation, and positive pressure ventilation if needed. An obstructing foreign body may need to be removed.12,14 Corticosteroids have been tried, without success.11–13,15
Empiric antibiotic treatment is controversial
Chemical pneumonitis can be difficult to differentiate from bacterial aspiration pneumonia, and whether to give antibiotics is controversial. 16 A survey of current practices among intensivists showed that antimicrobial therapy was often given empirically for noninfectious chemical pneumonitis.17 This practice raises concerns of higher treatment costs and antibiotic resistance.16,18,19 Additionally, antibiotics do not seem to alter the clinical outcome, including radiographic resolution, duration of hospitalization, or death rate, nor do they influence the subsequent development of infection.1,11,13,20
In cases of witnessed or strongly suspected aspiration of gastric contents, antibiotics are not warranted since bacterial infection is not likely to be the cause of any signs or symptoms. 2,7,16 However, to detect secondary infection early, the patient’s respiratory status should be monitored carefully and chest radiography should be repeated.
In less clear-cut cases, ie, if it is not clear whether the patient actually has chemical pneumonitis or primary bacterial aspiration pneumonia, it is prudent to start antibiotics empirically after obtaining lower-respiratory-tract secretions for stains and cultures, and then to reassess within 48 to 72 hours. The antibiotics can be discontinued if the patient has rapid clinical and radiographic improvement and negative cultures. Those whose condition does not improve or who have positive cultures should receive a full course of antibiotics.21,22
PRIMARY BACTERIAL ASPIRATION PNEUMONIA
Primary bacterial aspiration pneumonia—ie, caused by bacteria residing in the upper airways and stomach gaining access to lower airways through aspiration in small or large amounts—is the most common form of aspiration pneumonia, although the actual episode of aspiration is seldom observed.
Signs of bacterial pneumonia
Primary bacterial aspiration pneumonia bears the hallmarks of bacterial pneumonia.12 The clinical picture is more indolent than chemical pneumonitis and includes cough, fever, and putrid sputum, mainly in patients who have clinical conditions predisposing to aspiration (eg, coma, stroke, alcoholism, poor dentition, tube feedings).1,12,20
The characteristic signs on chest radiography are infiltrates involving mainly the lung bases (the right more then the left). If untreated or inadequately treated, complications such as lung abscess, empyema, bronchiectasis, and broncopleural fistula are common.23
Are aerobic organisms replacing anaerobic ones in the community?
The causative organisms in community-acquired aspiration pneumonia are still debated despite abundant research. Older studies1,24,25 found mostly anaerobic organisms (pepto-streptococci, peptococci, Fusobacterium, Prevotela, Bacteroides) as the underlying pathogens, whereas more recent studies16,26,27 found mostly aerobic organisms (Streptococcus pneumoniae, Haemophilus influenzae, Staphylococcus aureus, Enterobacteriaceae) and failed to recover anaerobic organisms. These discrepancies may be the result of different techniques used to isolate organisms: older studies used transtracheal sampling, and transtracheal aspirates may be easily contaminated or colonized by oropharyngeal flora; more recent studies used protected specimen brushes to collect lower-airway specimens.2
In addition, the pathogenic organisms that predominate in community-acquired aspiration pneumonia, as listed above, are different from those most often found in nosocomial cases; gram-negative bacilli (Pseudomonas aeruginosa, Klebsiella pneumoniae, Escherichia coli) are most often isolated in patients with aspiration pneumonia acquired in hospitals and nursing homes.16,27,28S aureus also is an important causative organism in nosocomial cases.16,28
Knowing the causative organisms in bacterial aspiration pneumonia is important for guiding antimicrobial therapy.
Antibiotics are required for bacterial aspiration pneumonia
A course of antibiotics is required for bacterial aspiration pneumonia. While there are no definitive recommendations for the duration of treatment, 7 to 8 days is probably appropriate in uncomplicated cases (ie, no lung abscess, empyema, bronchopleural fistula).22,29 Patients who have complications may need drainage of abscesses or empyema along with a longer duration of antibiotic therapy until clinical and radiographic signs improve.
For community-acquired cases of aspiration pneumonia, a number of antibiotics have proven effective:
- Clindamycin (Cleocin) is still the agent most commonly used, although it lacks gram-negative bacterial coverage.
- Beta-lactam penicillins and newer quinolones have been used successfully.2,29–31 In addition to covering the previously mentioned bacteria, these antibiotics have the added benefit of covering anaerobic bacteria.
- Metronidazole (Flagyl) should not be used alone because it has a higher clinical failure rate.32,33
For nosocomial aspiration pneumonia, giving a broad-spectrum antibiotic empirically is warranted. Beta-lactam penicillins with extended gram-negative coverage, carbapenems, or monobactams in combination with an anti-staphylococcal drug have been advocated for nosocomial aspiration.2,22 A strategy of broad-spectrum coverage followed by narrowing or de-escalating coverage according to lower respiratory tract cultures is encouraged.21,22,34
SECONDARY BACTERIAL INFECTION OF CHEMICAL PNEUMONITIS
Nearly 25% of patients with chemical pneumonitis improve initially, then show clinical deterioration secondary to superimposed bacterial infection.13 Chest radiographs show worsening of initial infiltrates or the development of new ones. The causative organisms and treatment depend on whether the superimposed infection is community-acquired or nosocomial, as is the case in primary bacterial aspiration pneumonia.
PREVENTING ASPIRATION
Measures should be taken to prevent aspiration pneumonia and chemical pneumonitis, especially in institutionalized patients at high risk.12
Elevation of the head of the bed while feeding, dental prophylaxis, and good oral hygiene are known to reduce the incidence of these problems.35–37
A swallowing evaluation for patients with dysphagia can identify those at higher risk of aspiration. These patients may be candidates for postural adjustments, diet modification, strengthening, and other measures offered by the speech and language pathology teams to improve swallowing physiology, biomechanics, safety, and endurance.2,35
Although percutaneous endoscopic gastrostomy tubes are often placed in patients who have aspirated or who are at high risk of aspiration, they do not protect against aspiration, nor do orogastric or nasogastric tubes.38
To date, we have no evidence that prophylactic antibiotic therapy prevents bacterial aspiration pneumonia. In addition, this practice encourages the development of resistant organisms.19,39,40
Antibiotics are indicated for primary bacterial aspiration pneumonia and secondary bacterial infection of aspiration (chemical) pneumonitis, but not for uncomplicated chemical pneumonitis.
THREE TYPES OF ‘ASPIRATION PNEUMONIA’
Aspiration pneumonia is a broad and vague term mainly used to refer to the pulmonary consequences of abnormal entry of exogenous or endogenous substances into the lower airways. It can be classified as:
- Aspiration (chemical) pneumonitis
- Primary bacterial aspiration pneumonia
- Secondary bacterial infection of chemical pneumonitis.
These three are sometimes difficult to differentiate, as their signs and symptoms can overlap.
CHEMICAL PNEUMONITIS
Aspiration of stomach contents is relatively common, even in healthy people, and usually has no clinical consequences.1 However, it has also been closely related to community-acquired and nosocomial pneumonia in some studies.2,3
Chemical pneumonitis is usually a consequence of the aspiration of a large volume (≥ 4 mL/kg) of sterile acidic (pH < 2.5) gastric contents into the lower airways (Mendelson syndrome).4,5 The clinical picture varies from asymptomatic to signs of severe dyspnea, hypoxia, cough, and low-grade fever; these signs and symptoms may develop rapidly, within minutes to hours after a witnessed or suspected episode of aspiration.2,6,7 However, they represent an inflammatory reaction to the gastric acid rather than a reaction to bacterial infection.8–10
Chemical pneumonitis affects the most dependent regions of the lungs
Chest radiography shows infiltrates in the most dependent regions of the lung. If aspiration occurs while the patient is supine, the posterior segments of the upper lobes and the apical segments of the lower lobes are most affected. The basal segments of the lower lobes are usually affected if aspiration occurs while the patient is standing or upright.1,2,11,12
Clinical course varies
The clinical course varies. In almost 60% of cases, the patient’s condition improves and the lung infiltrates resolve rapidly, within 2 to 4 days. On the other hand, in about 15% of cases, the patient’s condition deteriorates quickly, within 24 to 36 hours, and progresses to hypoxic respiratory failure and acute respiratory distress syndrome.
In the other 25% of cases, the patient’s condition may improve initially but then worsen as a secondary bacterial infection sets in. The death rate in these patients is almost three times higher than the rate in patients with uncomplicated chemical pneumonitis.11,13
Treatment of uncomplicated cases is mainly supportive
The treatment of uncomplicated chemical pneumonitis involves supportive measures such as airway clearance, oxygen supplementation, and positive pressure ventilation if needed. An obstructing foreign body may need to be removed.12,14 Corticosteroids have been tried, without success.11–13,15
Empiric antibiotic treatment is controversial
Chemical pneumonitis can be difficult to differentiate from bacterial aspiration pneumonia, and whether to give antibiotics is controversial. 16 A survey of current practices among intensivists showed that antimicrobial therapy was often given empirically for noninfectious chemical pneumonitis.17 This practice raises concerns of higher treatment costs and antibiotic resistance.16,18,19 Additionally, antibiotics do not seem to alter the clinical outcome, including radiographic resolution, duration of hospitalization, or death rate, nor do they influence the subsequent development of infection.1,11,13,20
In cases of witnessed or strongly suspected aspiration of gastric contents, antibiotics are not warranted since bacterial infection is not likely to be the cause of any signs or symptoms. 2,7,16 However, to detect secondary infection early, the patient’s respiratory status should be monitored carefully and chest radiography should be repeated.
In less clear-cut cases, ie, if it is not clear whether the patient actually has chemical pneumonitis or primary bacterial aspiration pneumonia, it is prudent to start antibiotics empirically after obtaining lower-respiratory-tract secretions for stains and cultures, and then to reassess within 48 to 72 hours. The antibiotics can be discontinued if the patient has rapid clinical and radiographic improvement and negative cultures. Those whose condition does not improve or who have positive cultures should receive a full course of antibiotics.21,22
PRIMARY BACTERIAL ASPIRATION PNEUMONIA
Primary bacterial aspiration pneumonia—ie, caused by bacteria residing in the upper airways and stomach gaining access to lower airways through aspiration in small or large amounts—is the most common form of aspiration pneumonia, although the actual episode of aspiration is seldom observed.
Signs of bacterial pneumonia
Primary bacterial aspiration pneumonia bears the hallmarks of bacterial pneumonia.12 The clinical picture is more indolent than chemical pneumonitis and includes cough, fever, and putrid sputum, mainly in patients who have clinical conditions predisposing to aspiration (eg, coma, stroke, alcoholism, poor dentition, tube feedings).1,12,20
The characteristic signs on chest radiography are infiltrates involving mainly the lung bases (the right more then the left). If untreated or inadequately treated, complications such as lung abscess, empyema, bronchiectasis, and broncopleural fistula are common.23
Are aerobic organisms replacing anaerobic ones in the community?
The causative organisms in community-acquired aspiration pneumonia are still debated despite abundant research. Older studies1,24,25 found mostly anaerobic organisms (pepto-streptococci, peptococci, Fusobacterium, Prevotela, Bacteroides) as the underlying pathogens, whereas more recent studies16,26,27 found mostly aerobic organisms (Streptococcus pneumoniae, Haemophilus influenzae, Staphylococcus aureus, Enterobacteriaceae) and failed to recover anaerobic organisms. These discrepancies may be the result of different techniques used to isolate organisms: older studies used transtracheal sampling, and transtracheal aspirates may be easily contaminated or colonized by oropharyngeal flora; more recent studies used protected specimen brushes to collect lower-airway specimens.2
In addition, the pathogenic organisms that predominate in community-acquired aspiration pneumonia, as listed above, are different from those most often found in nosocomial cases; gram-negative bacilli (Pseudomonas aeruginosa, Klebsiella pneumoniae, Escherichia coli) are most often isolated in patients with aspiration pneumonia acquired in hospitals and nursing homes.16,27,28S aureus also is an important causative organism in nosocomial cases.16,28
Knowing the causative organisms in bacterial aspiration pneumonia is important for guiding antimicrobial therapy.
Antibiotics are required for bacterial aspiration pneumonia
A course of antibiotics is required for bacterial aspiration pneumonia. While there are no definitive recommendations for the duration of treatment, 7 to 8 days is probably appropriate in uncomplicated cases (ie, no lung abscess, empyema, bronchopleural fistula).22,29 Patients who have complications may need drainage of abscesses or empyema along with a longer duration of antibiotic therapy until clinical and radiographic signs improve.
For community-acquired cases of aspiration pneumonia, a number of antibiotics have proven effective:
- Clindamycin (Cleocin) is still the agent most commonly used, although it lacks gram-negative bacterial coverage.
- Beta-lactam penicillins and newer quinolones have been used successfully.2,29–31 In addition to covering the previously mentioned bacteria, these antibiotics have the added benefit of covering anaerobic bacteria.
- Metronidazole (Flagyl) should not be used alone because it has a higher clinical failure rate.32,33
For nosocomial aspiration pneumonia, giving a broad-spectrum antibiotic empirically is warranted. Beta-lactam penicillins with extended gram-negative coverage, carbapenems, or monobactams in combination with an anti-staphylococcal drug have been advocated for nosocomial aspiration.2,22 A strategy of broad-spectrum coverage followed by narrowing or de-escalating coverage according to lower respiratory tract cultures is encouraged.21,22,34
SECONDARY BACTERIAL INFECTION OF CHEMICAL PNEUMONITIS
Nearly 25% of patients with chemical pneumonitis improve initially, then show clinical deterioration secondary to superimposed bacterial infection.13 Chest radiographs show worsening of initial infiltrates or the development of new ones. The causative organisms and treatment depend on whether the superimposed infection is community-acquired or nosocomial, as is the case in primary bacterial aspiration pneumonia.
PREVENTING ASPIRATION
Measures should be taken to prevent aspiration pneumonia and chemical pneumonitis, especially in institutionalized patients at high risk.12
Elevation of the head of the bed while feeding, dental prophylaxis, and good oral hygiene are known to reduce the incidence of these problems.35–37
A swallowing evaluation for patients with dysphagia can identify those at higher risk of aspiration. These patients may be candidates for postural adjustments, diet modification, strengthening, and other measures offered by the speech and language pathology teams to improve swallowing physiology, biomechanics, safety, and endurance.2,35
Although percutaneous endoscopic gastrostomy tubes are often placed in patients who have aspirated or who are at high risk of aspiration, they do not protect against aspiration, nor do orogastric or nasogastric tubes.38
To date, we have no evidence that prophylactic antibiotic therapy prevents bacterial aspiration pneumonia. In addition, this practice encourages the development of resistant organisms.19,39,40
- Bartlett JG, Gorbach SL. The triple threat of aspiration pneumonia. Chest 1975; 68:560–566.
- Marik PE. Aspiration pneumonitis and aspiration pneumonia. N Engl J Med 2001; 344:665–671.
- Kikuchi R, Watabe N, Konno T, Mishina N, Sekizawa K, Sasaki H. High incidence of silent aspiration in elderly patients with community-acquired pneumonia. Am J Respir Crit Care Med 1994; 150:251–253.
- Mendelson CL. The aspiration of stomach contents into lungs during obstetric anesthesia. Am J Obstet Gynecol 1946; 52:191–205.
- Cameron JL, Caldini P, Toung JK, Zuidema GD. Aspiration pneumonia: physiologic data following experimental aspiration. Surgery 1972; 72:238–245.
- Warner MA, Warner ME, Weber JG. Clinical significance of pulmonary aspiration during the perioperative period. Anesthesiology 1993; 78:56–62.
- DePaso WJ. Aspiration pneumonia. Clin Chest Med 1991; 12:269–284.
- Folkesson HG, Matthay MA, Hébert CA, Broaddus VC. Acid aspiration-induced lung injury in rabbits is mediated by interleukin-8-dependent mechanisms. J Clin Invest 1995; 96:107–116.
- Goldman G, Welbourn R, Kobzik L, Valeri CR, Shepro D, Hechtman HB. Tumor necrosis factor-alpha mediates acid aspiration-induced systemic organ injury. Ann Surg 1990; 212:513–519.
- LeFrock JL, Clark TS, Davies B, Klainer AS. Aspiration pneumonia: a ten-year review. Am Surg 1979; 45:305–313.
- Cameron JL, Mitchell WH, Zuidema GD. Aspiration pneumonia. Clinical outcome following documented aspiration. Arch Surg 1973; 106:49–52.
- Arms RA, Dines DE, Tinstman TC. Aspiration pneumonia. Chest 1974; 65:136–139.
- Bynum LJ, Pierce AK. Pulmonary aspiration of gastric contents. Am Rev Respir Dis 1976; 114:1129–1136.
- Merchant SN, Kirtane MV, Shah KL, Karnik PP. Foreign bodies in the bronchi (a 10 year review of 132 cases). J Postgrad Med 1984; 30:219–223.
- Wolfe JE, Bone RC, Ruth WE. Effects of corticosteroids in the treatment of patients with gastric aspiration. Am J Med 1977; 63:719–722.
- Kane-Gill SL, Olsen KM, Rebuck JA, et al; Aspiration Evaluation Group of the Clinical Pharmacy and Pharmacology Section. Multicenter treatment and outcome evaluation of aspiration syndromes in critically ill patients. Ann Pharmacother 2007; 41:549–555.
- Rebuck JA, Rasmussen JR, Olsen KM. Clinical aspiration-related practice patterns in the intensive care unit: a physician survey. Crit Care Med 2001; 29:2239–2244.
- Singh N, Rogers P, Atwood CW, Wagener MM, Yu VL. Short-course empiric antibiotic therapy for patients with pulmonary infiltrates in the intensive care unit. A proposed solution for indiscriminate antibiotic prescription. Am J Respir Crit Care Med 2000; 162:505–511.
- Kollef MH, Fraser VJ. Antibiotic resistance in the intensive care unit. Ann Intern Med 2001; 134:298–314.
- Lewis RT, Burgess JH, Hampson LG. Cardiorespiratory studies in critical illness. Changes in aspiration pneumonitis. Arch Surg 1971; 103:335–340.
- Rello J. Importance of appropriate initial antibiotic therapy and de-escalation in the treatment of nosocomial pneumonia. Eur Respir Rev 2007; 16:33–39.
- American Thoracic Society. Guidelines for the management of adults with hospital-acquired, ventilator-associated, and healthcare-associated pneumonia. Am J Respir Crit Care Med 2005; 171:388–416.
- Bartlett JG. Anaerobic bacterial infections of the lung and pleural space. Clin Infect Dis 1993; 16(suppl 4):S248–S255.
- Lorber B, Swenson RM. Bacteriology of aspiration pneumonia. A prospective study of community- and hospital-acquired cases. Ann Intern Med 1974; 81:329–331.
- Bartlett JG, Gorbach SL, Finegold SM. The bacteriology of aspiration pneumonia. Am J Med 1974; 56:202–207.
- Mier L, Dreyfuss D, Darchy B, et al. Is penicillin G an adequate initial treatment for aspiration pneumonia? A prospective evaluation using a protected specimen brush and quantitative cultures. Intensive Care Med 1993; 19:279–284.
- Marik PE, Careau P. The role of anaerobes in patients with ventilator-associated pneumonia and aspiration pneumonia: a prospective study. Chest 1999; 115:178–183.
- El-Solh AA, Pietrantoni C, Bhat A, et al. Microbiology of severe aspiration pneumonia in institutionalized elderly. Am J Respir Crit Care Med 2003; 167:1650–1654.
- Mandell LA, Wunderink RG, Anzueto A, et al; Infectious Diseases Society of America. Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community-acquired pneumonia in adults. Clin Infect Dis 2007; 44(suppl 2):S27–S72.
- Kadowaki M, Demura Y, Mizuno S, et al. Reappraisal of clindamycin IV monotherapy for treatment of mild-to-moderate aspiration pneumonia in elderly patients. Chest 2005; 127:1276–1282.
- Ott SR, Allewelt M, Lorenz J, Reimnitz P, Lode H; German Lung Abscess Study Group. Moxifloxacin vs ampicillin/sulbactam in aspiration pneumonia and primary lung abscess. Infection 2008; 36:23–30.
- Perlino CA. Metronidazole vs clindamycin treatment of anerobic pulmonary infection. Failure of metronidazole therapy. Arch Intern Med 1981; 141:1424–1427.
- Sanders CV, Hanna BJ, Lewis AC. Metronidazole in the treatment of anaerobic infections. Am Rev Respir Dis 1979; 120:337–343.
- Alvarez-Lerma F, Alvarez B, Luque P, et al; ADANN Study Group. Empiric broad-spectrum antibiotic therapy of nosocomial pneumonia in the intensive care unit: a prospective observational study. Crit Care 2006; 10:R78.
- Johnson JL, Hirsch CS. Aspiration pneumonia. Recognizing and managing a potentially growing disorder. Postgrad Med 2003; 113:99–112.
- Scolapio JS. Methods for decreasing risk of aspiration pneumonia in critically ill patients. JPEN J Parenter Enteral Nutr 2002; 26(suppl 6):S58–S61.
- Orozco-Levi M, Torres A, Ferrer M, et al. Semirecumbent position protects from pulmonary aspiration but not completely from gastroesophageal reflux in mechanically ventilated patients. Am J Respir Crit Care Med 1995; 152:1387–1390.
- Park RH, Allison MC, Lang J, et al. Randomised comparison of percutaneous endoscopic gastrostomy and nasogastric tube feeding in patients with persisting neurological dysphagia. BMJ 1992; 304( 6839):1406–1409.
- Donskey CJ, Chowdhry TK, Hecker MT, et al. Effect of antibiotic therapy on the density of vancomycin-resistant enterococci in the stool of colonized patients. N Engl J Med 2000; 343:1925–1932.
- Mouw DR, Langlois JP, Turner LF, Neher JO. Clinical inquiries. Are antibiotics effective in preventing pneumonia for nursing home patients? J Fam Pract 2004; 53:994–996.
- Bartlett JG, Gorbach SL. The triple threat of aspiration pneumonia. Chest 1975; 68:560–566.
- Marik PE. Aspiration pneumonitis and aspiration pneumonia. N Engl J Med 2001; 344:665–671.
- Kikuchi R, Watabe N, Konno T, Mishina N, Sekizawa K, Sasaki H. High incidence of silent aspiration in elderly patients with community-acquired pneumonia. Am J Respir Crit Care Med 1994; 150:251–253.
- Mendelson CL. The aspiration of stomach contents into lungs during obstetric anesthesia. Am J Obstet Gynecol 1946; 52:191–205.
- Cameron JL, Caldini P, Toung JK, Zuidema GD. Aspiration pneumonia: physiologic data following experimental aspiration. Surgery 1972; 72:238–245.
- Warner MA, Warner ME, Weber JG. Clinical significance of pulmonary aspiration during the perioperative period. Anesthesiology 1993; 78:56–62.
- DePaso WJ. Aspiration pneumonia. Clin Chest Med 1991; 12:269–284.
- Folkesson HG, Matthay MA, Hébert CA, Broaddus VC. Acid aspiration-induced lung injury in rabbits is mediated by interleukin-8-dependent mechanisms. J Clin Invest 1995; 96:107–116.
- Goldman G, Welbourn R, Kobzik L, Valeri CR, Shepro D, Hechtman HB. Tumor necrosis factor-alpha mediates acid aspiration-induced systemic organ injury. Ann Surg 1990; 212:513–519.
- LeFrock JL, Clark TS, Davies B, Klainer AS. Aspiration pneumonia: a ten-year review. Am Surg 1979; 45:305–313.
- Cameron JL, Mitchell WH, Zuidema GD. Aspiration pneumonia. Clinical outcome following documented aspiration. Arch Surg 1973; 106:49–52.
- Arms RA, Dines DE, Tinstman TC. Aspiration pneumonia. Chest 1974; 65:136–139.
- Bynum LJ, Pierce AK. Pulmonary aspiration of gastric contents. Am Rev Respir Dis 1976; 114:1129–1136.
- Merchant SN, Kirtane MV, Shah KL, Karnik PP. Foreign bodies in the bronchi (a 10 year review of 132 cases). J Postgrad Med 1984; 30:219–223.
- Wolfe JE, Bone RC, Ruth WE. Effects of corticosteroids in the treatment of patients with gastric aspiration. Am J Med 1977; 63:719–722.
- Kane-Gill SL, Olsen KM, Rebuck JA, et al; Aspiration Evaluation Group of the Clinical Pharmacy and Pharmacology Section. Multicenter treatment and outcome evaluation of aspiration syndromes in critically ill patients. Ann Pharmacother 2007; 41:549–555.
- Rebuck JA, Rasmussen JR, Olsen KM. Clinical aspiration-related practice patterns in the intensive care unit: a physician survey. Crit Care Med 2001; 29:2239–2244.
- Singh N, Rogers P, Atwood CW, Wagener MM, Yu VL. Short-course empiric antibiotic therapy for patients with pulmonary infiltrates in the intensive care unit. A proposed solution for indiscriminate antibiotic prescription. Am J Respir Crit Care Med 2000; 162:505–511.
- Kollef MH, Fraser VJ. Antibiotic resistance in the intensive care unit. Ann Intern Med 2001; 134:298–314.
- Lewis RT, Burgess JH, Hampson LG. Cardiorespiratory studies in critical illness. Changes in aspiration pneumonitis. Arch Surg 1971; 103:335–340.
- Rello J. Importance of appropriate initial antibiotic therapy and de-escalation in the treatment of nosocomial pneumonia. Eur Respir Rev 2007; 16:33–39.
- American Thoracic Society. Guidelines for the management of adults with hospital-acquired, ventilator-associated, and healthcare-associated pneumonia. Am J Respir Crit Care Med 2005; 171:388–416.
- Bartlett JG. Anaerobic bacterial infections of the lung and pleural space. Clin Infect Dis 1993; 16(suppl 4):S248–S255.
- Lorber B, Swenson RM. Bacteriology of aspiration pneumonia. A prospective study of community- and hospital-acquired cases. Ann Intern Med 1974; 81:329–331.
- Bartlett JG, Gorbach SL, Finegold SM. The bacteriology of aspiration pneumonia. Am J Med 1974; 56:202–207.
- Mier L, Dreyfuss D, Darchy B, et al. Is penicillin G an adequate initial treatment for aspiration pneumonia? A prospective evaluation using a protected specimen brush and quantitative cultures. Intensive Care Med 1993; 19:279–284.
- Marik PE, Careau P. The role of anaerobes in patients with ventilator-associated pneumonia and aspiration pneumonia: a prospective study. Chest 1999; 115:178–183.
- El-Solh AA, Pietrantoni C, Bhat A, et al. Microbiology of severe aspiration pneumonia in institutionalized elderly. Am J Respir Crit Care Med 2003; 167:1650–1654.
- Mandell LA, Wunderink RG, Anzueto A, et al; Infectious Diseases Society of America. Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community-acquired pneumonia in adults. Clin Infect Dis 2007; 44(suppl 2):S27–S72.
- Kadowaki M, Demura Y, Mizuno S, et al. Reappraisal of clindamycin IV monotherapy for treatment of mild-to-moderate aspiration pneumonia in elderly patients. Chest 2005; 127:1276–1282.
- Ott SR, Allewelt M, Lorenz J, Reimnitz P, Lode H; German Lung Abscess Study Group. Moxifloxacin vs ampicillin/sulbactam in aspiration pneumonia and primary lung abscess. Infection 2008; 36:23–30.
- Perlino CA. Metronidazole vs clindamycin treatment of anerobic pulmonary infection. Failure of metronidazole therapy. Arch Intern Med 1981; 141:1424–1427.
- Sanders CV, Hanna BJ, Lewis AC. Metronidazole in the treatment of anaerobic infections. Am Rev Respir Dis 1979; 120:337–343.
- Alvarez-Lerma F, Alvarez B, Luque P, et al; ADANN Study Group. Empiric broad-spectrum antibiotic therapy of nosocomial pneumonia in the intensive care unit: a prospective observational study. Crit Care 2006; 10:R78.
- Johnson JL, Hirsch CS. Aspiration pneumonia. Recognizing and managing a potentially growing disorder. Postgrad Med 2003; 113:99–112.
- Scolapio JS. Methods for decreasing risk of aspiration pneumonia in critically ill patients. JPEN J Parenter Enteral Nutr 2002; 26(suppl 6):S58–S61.
- Orozco-Levi M, Torres A, Ferrer M, et al. Semirecumbent position protects from pulmonary aspiration but not completely from gastroesophageal reflux in mechanically ventilated patients. Am J Respir Crit Care Med 1995; 152:1387–1390.
- Park RH, Allison MC, Lang J, et al. Randomised comparison of percutaneous endoscopic gastrostomy and nasogastric tube feeding in patients with persisting neurological dysphagia. BMJ 1992; 304( 6839):1406–1409.
- Donskey CJ, Chowdhry TK, Hecker MT, et al. Effect of antibiotic therapy on the density of vancomycin-resistant enterococci in the stool of colonized patients. N Engl J Med 2000; 343:1925–1932.
- Mouw DR, Langlois JP, Turner LF, Neher JO. Clinical inquiries. Are antibiotics effective in preventing pneumonia for nursing home patients? J Fam Pract 2004; 53:994–996.
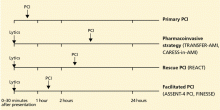
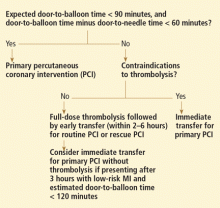
Timeliness of treatment is more important than choice of reperfusion therapy
Reperfusion therapy decreases morbidity and mortality rates in patients with ST-segment elevation myocardial infarction (MI). Primary percutaneous coronary intervention (PCI) is preferred over fibrinolytic therapy as a reperfusion strategy when the delay in the time to treatment is short and the patient presents to a high-volume, well-equipped center with expert interventional cardiologists.
Compared with fibrinolytic therapy in randomized clinical trials, primary PCI produces higher rates of infarct artery patency, complete reperfusion (grade 3 by the criteria of the Thrombolysis in Myocardial Infarction [TIMI] study), and access-site bleeding. It also produces lower rates of recurrent ischemia, reinfarction, emergency repeat revascularization procedures, intracranial hemorrhage, and death.1 If performed early and successfully, primary PCI also greatly decreases the rates of complications of ST-elevation MI that result from longer ischemic times or unsuccessful fibrinolytic therapy, allowing earlier hospital discharge and resumption of daily activities. Primary PCI is also the best reperfusion option in patients who present late after the onset of symptoms and in patients with cardiogenic shock, and it is the only option in patients who have contraindications to fibrinolytic therapy because of bleeding risk.
However, most hospitals do not have PCI capability. Two options at these hospitals are to transfer the patient to a PCI center quickly for primary PCI or to keep the patient on site and give fibrinolytic therapy, with its limitations. Earlier trials suggested that the transfer strategy was superior, but they had limitations: most patients received streptokinase, an inferior fibrinolytic agent, and door-to-door-to-balloon times were rapid, averaging only 95 minutes because of excellent logistical protocols and careful patient selection.2 Most importantly, rescue PCI and routine PCI were seldom performed in patients receiving fibrinolytics, so fibrinolytic therapy was being tested as monotherapy.
In the real world, however, treatment delays are much longer, and fibrinolytic therapy has evolved into a strategy that includes crossover to rescue PCI or routine PCI. Therefore, the initial trials of transfer for primary PCI do not reflect current practice. In fact, recent registry data suggest that prehospital fibrinolytic therapy followed by early angiography is superior to primary PCI because most patients can be treated within 2 hours of symptom onset; they also suggest that on-site fibrinolytic therapy followed by early angiography is equal in efficacy to primary PCI as long as rescue PCI and routine PCI can be performed.3,4
The most important modifiable predictor of outcome in ST-elevation MI is the time to treatment, a biological truth that continues to be supported by clinical evidence despite ideologic arguments by some interventional cardiology enthusiasts who claim that primary PCI is always superior to the fibrinolytic strategy, regardless of delays.
SURPRISINGLY, OUTCOMES WERE WORSE WITH FACILITATED PCI
It made sense, then, to conclude that the perfect strategy for hospitals without PCI capability would be a combined strategy of immediate fibrinolytic therapy to decrease the time delay associated with organizing PCI, and rapid transfer for immediate PCI to improve the limited reperfusion rates associated with fibrinolytic therapy.
Surprisingly, though, randomized trials found worse outcomes with this “facilitated PCI” strategy.5
Again, limitations in trial design might explain the lack of benefit in the trials. Inadequate anticoagulant and antiplatelet therapy were given to the fibrinolytic patients, and primary PCI patients had relatively short treatment delays, with many patients enrolled at hospitals with PCI capability.
PROGRESS IN REPERFUSION THERAPY
Great strides have been made in reperfusion therapy in recent years. Adjunctive therapy with clopidogrel (Plavix) and enoxaparin (Lovenox) has been shown to improve outcomes with fibrinolytic therapy. Bivalirudin (Angiomax) and stents have improved primary PCI’s performance. Reducing bleeding complications has become a clinical priority, with increasing emphasis on adjusting some drug doses according to renal function and using the radial artery for cardiac catheterization.
The American College of Cardiology initiative, “Door-to-Balloon (D2B): An Alliance for Quality,” focused much attention on organizing in-hospital systems of care for primary PCI, thus increasing the national rate of achieving a door-to-balloon time within 90 minutes from 50% to over 75% in patients who presented to hospitals with PCI capability.6
The American Heart Association has launched “Mission: Lifeline,” a national campaign to organize prehospital systems of care with their program,7 working within communities to address their unique needs, resources, and barriers to implementing systems of care for ST-elevation MI. The key aspect of this effort is to help geographic regions develop local solutions, an explicit recognition that there is no one-size-fits-all solution. Early triage by emergency medical services, rapid diagnosis with prehospital electrocardiography, destination and interhospital transfer protocols, and prehospital activation of the cardiac catheterization laboratory can greatly streamline emergency care and decrease treatment delays for primary PCI.
FOR OUTLYING HOSPITALS, A PHARMACOINVASIVE STRATEGY
So what about hospitals without PCI capability that cannot routinely transfer patients to a hospital with PCI capability within 90 minutes?
Lessons learned from the experiences with immediate PCI, rescue PCI, and facilitated PCI have evolved into the “pharmacoinvasive strategy.” Patients with ST-elevation MI are treated as rapidly as possible with a bolus of a fibrinolytic drug, eg, tenecteplase (TNKase) or reteplase (Retavase), and are also given aspirin, clopidogrel, and enoxaparin. Then, they are rapidly transferred to a PCI-capable hospital so that emergency PCI can be performed if reperfusion is not clinically apparent or if the patient develops pulmonary edema or cardiogenic shock. If the clinical signs suggest that reperfusion has been achieved (relief of chest pain, rapid resolution of ST-segment elevation, bursts of accelerated idioventricular rhythm), coronary angiography (and PCI, if indicated) can be performed within 3 to 24 hours of fibrinolytic therapy. This time frame allows the initial fibrinolytic effect to dissipate, while the antiplatelet and anticoagulant drugs achieve therapeutic levels.
Today, the goal is to treat every patient with the best reperfusion strategy available, given the limitations in resources and the geographic location of some centers, and to maximize the possibility of sustained patency of the infarct-related artery by implanting a stent, even if it takes several hours and transfer to another hospital to perform PCI.8 The pharmacoinvasive strategy of rapid administration of fibrinolytic therapy followed by PCI within 24 hours would be practical in most hospitals without PCI capability where treatment delays prohibit performance of primary PCI within 90 minutes of first medical contact.9
THE ‘STREAM’ TRIAL IS UNDER WAY
As proof of concept, the Strategic Reperfusion Early After Myocardial Infarction (STREAM) trial is enrolling 2,000 patients with ST-elevation MI presenting within 3 hours of symptom onset if primary PCI is not feasible within 60 minutes of first medical contact.10 Patients will be randomized to either of the following:
- Receive prehospital therapy with tenecteplase, aspirin, clopidogrel, and enoxaparin and undergo cardiac catheterization in 6 to 24 hours (or rescue PCI if reperfusion fails within 90 minutes of fibrinolysis)
- Undergo primary PCI performed according to local guidelines.
The primary measure of efficacy will be the composite rate of death, cardiogenic shock, heart failure, and reinfarction at 30 days. Measures of safety include the rates of ischemic stroke, intracranial hemorrhage, and major nonintracranial bleeding.
- Keeley EC, Boura JA, Grines CL. Primary angioplasty versus intravenous thrombolytic therapy for acute myocardial infarction: a quantitative review of 23 randomised trials. Lancet 2003; 361:13–20.
- Dalby M, Bouzamondo A, Lechat P, Montalescot G. Transfer for primary angioplasty versus immediate thrombolysis in acute myocardial infarction: a meta-analysis. Circulation 2003; 108:1809–1814.
- Danchin N, Coste P, Ferrières J, et al; FAST-MI Investigators. Comparison of thrombolysis followed by broad use of percutaneous coronary intervention with primary percutaneous coronary intervention for ST-segment-elevation acute myocardial infarction: data from the French registry on Acute ST-elevation Myocardial Infarction (FASTMI). Circulation 2008; 118:268–276.
- Lambert L, Brown K, Segal E, Brophy J, Rodes-Cabau J, Bogaty P. Association between timeliness of reperfusion therapy and clinical outcomes in ST-elevation myocardial infarction. JAMA 2010; 303:2148–2155.
- Keeley EC, Boura JA, Grines CL. Comparison of primary and facilitated percutaneous coronary interventions for ST-elevation myocardial infarction: quantitative review of randomised trials. Lancet 2006; 367:579–588.
- Krumholz HM, Bradley EH, Nallamothu BK, et al. A campaign to improve the timeliness of primary percutaneous coronary intervention: Door-to-Balloon: An Alliance for Quality. JACC Cardiovasc Interv. 2008; 1:97–104.
- Jacobs AK, Antman EM, Faxon DP, Gregory T, Solis P. Development of systems of care for ST-elevation myocardial infarction patients: executive summary. Circulation 2007; 116:217–230.
- Kushner FG, Hand M, Smith SC, et al; American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. 2009 Focused Updates: ACC/AHA Guidelines for the Management of Patients With ST-Elevation Myocardial Infarction (updating the 2004 Guideline and 2007 Focused Update) and ACC/AHA/SCAI Guidelines on Percutaneous Coronary Intervention (updating the 2005 Guideline and 2007 Focused Update): a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation 2009; 120:2271–2306.
- Bates ER, Nallamothu BK. Commentary: the role of percutaneous coronary intervention in ST-segment-elevation myocardial infarction. Circulation 2008; 118:567–573.
- Armstrong PW, Gershlick A, Goldstein P, et al; STREAM Steering Committee. The Strategic Reperfusion Early After Myocardial Infarction (STREAM) study. Am Heart J 2010; 160:30–35.
Reperfusion therapy decreases morbidity and mortality rates in patients with ST-segment elevation myocardial infarction (MI). Primary percutaneous coronary intervention (PCI) is preferred over fibrinolytic therapy as a reperfusion strategy when the delay in the time to treatment is short and the patient presents to a high-volume, well-equipped center with expert interventional cardiologists.
Compared with fibrinolytic therapy in randomized clinical trials, primary PCI produces higher rates of infarct artery patency, complete reperfusion (grade 3 by the criteria of the Thrombolysis in Myocardial Infarction [TIMI] study), and access-site bleeding. It also produces lower rates of recurrent ischemia, reinfarction, emergency repeat revascularization procedures, intracranial hemorrhage, and death.1 If performed early and successfully, primary PCI also greatly decreases the rates of complications of ST-elevation MI that result from longer ischemic times or unsuccessful fibrinolytic therapy, allowing earlier hospital discharge and resumption of daily activities. Primary PCI is also the best reperfusion option in patients who present late after the onset of symptoms and in patients with cardiogenic shock, and it is the only option in patients who have contraindications to fibrinolytic therapy because of bleeding risk.
However, most hospitals do not have PCI capability. Two options at these hospitals are to transfer the patient to a PCI center quickly for primary PCI or to keep the patient on site and give fibrinolytic therapy, with its limitations. Earlier trials suggested that the transfer strategy was superior, but they had limitations: most patients received streptokinase, an inferior fibrinolytic agent, and door-to-door-to-balloon times were rapid, averaging only 95 minutes because of excellent logistical protocols and careful patient selection.2 Most importantly, rescue PCI and routine PCI were seldom performed in patients receiving fibrinolytics, so fibrinolytic therapy was being tested as monotherapy.
In the real world, however, treatment delays are much longer, and fibrinolytic therapy has evolved into a strategy that includes crossover to rescue PCI or routine PCI. Therefore, the initial trials of transfer for primary PCI do not reflect current practice. In fact, recent registry data suggest that prehospital fibrinolytic therapy followed by early angiography is superior to primary PCI because most patients can be treated within 2 hours of symptom onset; they also suggest that on-site fibrinolytic therapy followed by early angiography is equal in efficacy to primary PCI as long as rescue PCI and routine PCI can be performed.3,4
The most important modifiable predictor of outcome in ST-elevation MI is the time to treatment, a biological truth that continues to be supported by clinical evidence despite ideologic arguments by some interventional cardiology enthusiasts who claim that primary PCI is always superior to the fibrinolytic strategy, regardless of delays.
SURPRISINGLY, OUTCOMES WERE WORSE WITH FACILITATED PCI
It made sense, then, to conclude that the perfect strategy for hospitals without PCI capability would be a combined strategy of immediate fibrinolytic therapy to decrease the time delay associated with organizing PCI, and rapid transfer for immediate PCI to improve the limited reperfusion rates associated with fibrinolytic therapy.
Surprisingly, though, randomized trials found worse outcomes with this “facilitated PCI” strategy.5
Again, limitations in trial design might explain the lack of benefit in the trials. Inadequate anticoagulant and antiplatelet therapy were given to the fibrinolytic patients, and primary PCI patients had relatively short treatment delays, with many patients enrolled at hospitals with PCI capability.
PROGRESS IN REPERFUSION THERAPY
Great strides have been made in reperfusion therapy in recent years. Adjunctive therapy with clopidogrel (Plavix) and enoxaparin (Lovenox) has been shown to improve outcomes with fibrinolytic therapy. Bivalirudin (Angiomax) and stents have improved primary PCI’s performance. Reducing bleeding complications has become a clinical priority, with increasing emphasis on adjusting some drug doses according to renal function and using the radial artery for cardiac catheterization.
The American College of Cardiology initiative, “Door-to-Balloon (D2B): An Alliance for Quality,” focused much attention on organizing in-hospital systems of care for primary PCI, thus increasing the national rate of achieving a door-to-balloon time within 90 minutes from 50% to over 75% in patients who presented to hospitals with PCI capability.6
The American Heart Association has launched “Mission: Lifeline,” a national campaign to organize prehospital systems of care with their program,7 working within communities to address their unique needs, resources, and barriers to implementing systems of care for ST-elevation MI. The key aspect of this effort is to help geographic regions develop local solutions, an explicit recognition that there is no one-size-fits-all solution. Early triage by emergency medical services, rapid diagnosis with prehospital electrocardiography, destination and interhospital transfer protocols, and prehospital activation of the cardiac catheterization laboratory can greatly streamline emergency care and decrease treatment delays for primary PCI.
FOR OUTLYING HOSPITALS, A PHARMACOINVASIVE STRATEGY
So what about hospitals without PCI capability that cannot routinely transfer patients to a hospital with PCI capability within 90 minutes?
Lessons learned from the experiences with immediate PCI, rescue PCI, and facilitated PCI have evolved into the “pharmacoinvasive strategy.” Patients with ST-elevation MI are treated as rapidly as possible with a bolus of a fibrinolytic drug, eg, tenecteplase (TNKase) or reteplase (Retavase), and are also given aspirin, clopidogrel, and enoxaparin. Then, they are rapidly transferred to a PCI-capable hospital so that emergency PCI can be performed if reperfusion is not clinically apparent or if the patient develops pulmonary edema or cardiogenic shock. If the clinical signs suggest that reperfusion has been achieved (relief of chest pain, rapid resolution of ST-segment elevation, bursts of accelerated idioventricular rhythm), coronary angiography (and PCI, if indicated) can be performed within 3 to 24 hours of fibrinolytic therapy. This time frame allows the initial fibrinolytic effect to dissipate, while the antiplatelet and anticoagulant drugs achieve therapeutic levels.
Today, the goal is to treat every patient with the best reperfusion strategy available, given the limitations in resources and the geographic location of some centers, and to maximize the possibility of sustained patency of the infarct-related artery by implanting a stent, even if it takes several hours and transfer to another hospital to perform PCI.8 The pharmacoinvasive strategy of rapid administration of fibrinolytic therapy followed by PCI within 24 hours would be practical in most hospitals without PCI capability where treatment delays prohibit performance of primary PCI within 90 minutes of first medical contact.9
THE ‘STREAM’ TRIAL IS UNDER WAY
As proof of concept, the Strategic Reperfusion Early After Myocardial Infarction (STREAM) trial is enrolling 2,000 patients with ST-elevation MI presenting within 3 hours of symptom onset if primary PCI is not feasible within 60 minutes of first medical contact.10 Patients will be randomized to either of the following:
- Receive prehospital therapy with tenecteplase, aspirin, clopidogrel, and enoxaparin and undergo cardiac catheterization in 6 to 24 hours (or rescue PCI if reperfusion fails within 90 minutes of fibrinolysis)
- Undergo primary PCI performed according to local guidelines.
The primary measure of efficacy will be the composite rate of death, cardiogenic shock, heart failure, and reinfarction at 30 days. Measures of safety include the rates of ischemic stroke, intracranial hemorrhage, and major nonintracranial bleeding.
Reperfusion therapy decreases morbidity and mortality rates in patients with ST-segment elevation myocardial infarction (MI). Primary percutaneous coronary intervention (PCI) is preferred over fibrinolytic therapy as a reperfusion strategy when the delay in the time to treatment is short and the patient presents to a high-volume, well-equipped center with expert interventional cardiologists.
Compared with fibrinolytic therapy in randomized clinical trials, primary PCI produces higher rates of infarct artery patency, complete reperfusion (grade 3 by the criteria of the Thrombolysis in Myocardial Infarction [TIMI] study), and access-site bleeding. It also produces lower rates of recurrent ischemia, reinfarction, emergency repeat revascularization procedures, intracranial hemorrhage, and death.1 If performed early and successfully, primary PCI also greatly decreases the rates of complications of ST-elevation MI that result from longer ischemic times or unsuccessful fibrinolytic therapy, allowing earlier hospital discharge and resumption of daily activities. Primary PCI is also the best reperfusion option in patients who present late after the onset of symptoms and in patients with cardiogenic shock, and it is the only option in patients who have contraindications to fibrinolytic therapy because of bleeding risk.
However, most hospitals do not have PCI capability. Two options at these hospitals are to transfer the patient to a PCI center quickly for primary PCI or to keep the patient on site and give fibrinolytic therapy, with its limitations. Earlier trials suggested that the transfer strategy was superior, but they had limitations: most patients received streptokinase, an inferior fibrinolytic agent, and door-to-door-to-balloon times were rapid, averaging only 95 minutes because of excellent logistical protocols and careful patient selection.2 Most importantly, rescue PCI and routine PCI were seldom performed in patients receiving fibrinolytics, so fibrinolytic therapy was being tested as monotherapy.
In the real world, however, treatment delays are much longer, and fibrinolytic therapy has evolved into a strategy that includes crossover to rescue PCI or routine PCI. Therefore, the initial trials of transfer for primary PCI do not reflect current practice. In fact, recent registry data suggest that prehospital fibrinolytic therapy followed by early angiography is superior to primary PCI because most patients can be treated within 2 hours of symptom onset; they also suggest that on-site fibrinolytic therapy followed by early angiography is equal in efficacy to primary PCI as long as rescue PCI and routine PCI can be performed.3,4
The most important modifiable predictor of outcome in ST-elevation MI is the time to treatment, a biological truth that continues to be supported by clinical evidence despite ideologic arguments by some interventional cardiology enthusiasts who claim that primary PCI is always superior to the fibrinolytic strategy, regardless of delays.
SURPRISINGLY, OUTCOMES WERE WORSE WITH FACILITATED PCI
It made sense, then, to conclude that the perfect strategy for hospitals without PCI capability would be a combined strategy of immediate fibrinolytic therapy to decrease the time delay associated with organizing PCI, and rapid transfer for immediate PCI to improve the limited reperfusion rates associated with fibrinolytic therapy.
Surprisingly, though, randomized trials found worse outcomes with this “facilitated PCI” strategy.5
Again, limitations in trial design might explain the lack of benefit in the trials. Inadequate anticoagulant and antiplatelet therapy were given to the fibrinolytic patients, and primary PCI patients had relatively short treatment delays, with many patients enrolled at hospitals with PCI capability.
PROGRESS IN REPERFUSION THERAPY
Great strides have been made in reperfusion therapy in recent years. Adjunctive therapy with clopidogrel (Plavix) and enoxaparin (Lovenox) has been shown to improve outcomes with fibrinolytic therapy. Bivalirudin (Angiomax) and stents have improved primary PCI’s performance. Reducing bleeding complications has become a clinical priority, with increasing emphasis on adjusting some drug doses according to renal function and using the radial artery for cardiac catheterization.
The American College of Cardiology initiative, “Door-to-Balloon (D2B): An Alliance for Quality,” focused much attention on organizing in-hospital systems of care for primary PCI, thus increasing the national rate of achieving a door-to-balloon time within 90 minutes from 50% to over 75% in patients who presented to hospitals with PCI capability.6
The American Heart Association has launched “Mission: Lifeline,” a national campaign to organize prehospital systems of care with their program,7 working within communities to address their unique needs, resources, and barriers to implementing systems of care for ST-elevation MI. The key aspect of this effort is to help geographic regions develop local solutions, an explicit recognition that there is no one-size-fits-all solution. Early triage by emergency medical services, rapid diagnosis with prehospital electrocardiography, destination and interhospital transfer protocols, and prehospital activation of the cardiac catheterization laboratory can greatly streamline emergency care and decrease treatment delays for primary PCI.
FOR OUTLYING HOSPITALS, A PHARMACOINVASIVE STRATEGY
So what about hospitals without PCI capability that cannot routinely transfer patients to a hospital with PCI capability within 90 minutes?
Lessons learned from the experiences with immediate PCI, rescue PCI, and facilitated PCI have evolved into the “pharmacoinvasive strategy.” Patients with ST-elevation MI are treated as rapidly as possible with a bolus of a fibrinolytic drug, eg, tenecteplase (TNKase) or reteplase (Retavase), and are also given aspirin, clopidogrel, and enoxaparin. Then, they are rapidly transferred to a PCI-capable hospital so that emergency PCI can be performed if reperfusion is not clinically apparent or if the patient develops pulmonary edema or cardiogenic shock. If the clinical signs suggest that reperfusion has been achieved (relief of chest pain, rapid resolution of ST-segment elevation, bursts of accelerated idioventricular rhythm), coronary angiography (and PCI, if indicated) can be performed within 3 to 24 hours of fibrinolytic therapy. This time frame allows the initial fibrinolytic effect to dissipate, while the antiplatelet and anticoagulant drugs achieve therapeutic levels.
Today, the goal is to treat every patient with the best reperfusion strategy available, given the limitations in resources and the geographic location of some centers, and to maximize the possibility of sustained patency of the infarct-related artery by implanting a stent, even if it takes several hours and transfer to another hospital to perform PCI.8 The pharmacoinvasive strategy of rapid administration of fibrinolytic therapy followed by PCI within 24 hours would be practical in most hospitals without PCI capability where treatment delays prohibit performance of primary PCI within 90 minutes of first medical contact.9
THE ‘STREAM’ TRIAL IS UNDER WAY
As proof of concept, the Strategic Reperfusion Early After Myocardial Infarction (STREAM) trial is enrolling 2,000 patients with ST-elevation MI presenting within 3 hours of symptom onset if primary PCI is not feasible within 60 minutes of first medical contact.10 Patients will be randomized to either of the following:
- Receive prehospital therapy with tenecteplase, aspirin, clopidogrel, and enoxaparin and undergo cardiac catheterization in 6 to 24 hours (or rescue PCI if reperfusion fails within 90 minutes of fibrinolysis)
- Undergo primary PCI performed according to local guidelines.
The primary measure of efficacy will be the composite rate of death, cardiogenic shock, heart failure, and reinfarction at 30 days. Measures of safety include the rates of ischemic stroke, intracranial hemorrhage, and major nonintracranial bleeding.
- Keeley EC, Boura JA, Grines CL. Primary angioplasty versus intravenous thrombolytic therapy for acute myocardial infarction: a quantitative review of 23 randomised trials. Lancet 2003; 361:13–20.
- Dalby M, Bouzamondo A, Lechat P, Montalescot G. Transfer for primary angioplasty versus immediate thrombolysis in acute myocardial infarction: a meta-analysis. Circulation 2003; 108:1809–1814.
- Danchin N, Coste P, Ferrières J, et al; FAST-MI Investigators. Comparison of thrombolysis followed by broad use of percutaneous coronary intervention with primary percutaneous coronary intervention for ST-segment-elevation acute myocardial infarction: data from the French registry on Acute ST-elevation Myocardial Infarction (FASTMI). Circulation 2008; 118:268–276.
- Lambert L, Brown K, Segal E, Brophy J, Rodes-Cabau J, Bogaty P. Association between timeliness of reperfusion therapy and clinical outcomes in ST-elevation myocardial infarction. JAMA 2010; 303:2148–2155.
- Keeley EC, Boura JA, Grines CL. Comparison of primary and facilitated percutaneous coronary interventions for ST-elevation myocardial infarction: quantitative review of randomised trials. Lancet 2006; 367:579–588.
- Krumholz HM, Bradley EH, Nallamothu BK, et al. A campaign to improve the timeliness of primary percutaneous coronary intervention: Door-to-Balloon: An Alliance for Quality. JACC Cardiovasc Interv. 2008; 1:97–104.
- Jacobs AK, Antman EM, Faxon DP, Gregory T, Solis P. Development of systems of care for ST-elevation myocardial infarction patients: executive summary. Circulation 2007; 116:217–230.
- Kushner FG, Hand M, Smith SC, et al; American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. 2009 Focused Updates: ACC/AHA Guidelines for the Management of Patients With ST-Elevation Myocardial Infarction (updating the 2004 Guideline and 2007 Focused Update) and ACC/AHA/SCAI Guidelines on Percutaneous Coronary Intervention (updating the 2005 Guideline and 2007 Focused Update): a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation 2009; 120:2271–2306.
- Bates ER, Nallamothu BK. Commentary: the role of percutaneous coronary intervention in ST-segment-elevation myocardial infarction. Circulation 2008; 118:567–573.
- Armstrong PW, Gershlick A, Goldstein P, et al; STREAM Steering Committee. The Strategic Reperfusion Early After Myocardial Infarction (STREAM) study. Am Heart J 2010; 160:30–35.
- Keeley EC, Boura JA, Grines CL. Primary angioplasty versus intravenous thrombolytic therapy for acute myocardial infarction: a quantitative review of 23 randomised trials. Lancet 2003; 361:13–20.
- Dalby M, Bouzamondo A, Lechat P, Montalescot G. Transfer for primary angioplasty versus immediate thrombolysis in acute myocardial infarction: a meta-analysis. Circulation 2003; 108:1809–1814.
- Danchin N, Coste P, Ferrières J, et al; FAST-MI Investigators. Comparison of thrombolysis followed by broad use of percutaneous coronary intervention with primary percutaneous coronary intervention for ST-segment-elevation acute myocardial infarction: data from the French registry on Acute ST-elevation Myocardial Infarction (FASTMI). Circulation 2008; 118:268–276.
- Lambert L, Brown K, Segal E, Brophy J, Rodes-Cabau J, Bogaty P. Association between timeliness of reperfusion therapy and clinical outcomes in ST-elevation myocardial infarction. JAMA 2010; 303:2148–2155.
- Keeley EC, Boura JA, Grines CL. Comparison of primary and facilitated percutaneous coronary interventions for ST-elevation myocardial infarction: quantitative review of randomised trials. Lancet 2006; 367:579–588.
- Krumholz HM, Bradley EH, Nallamothu BK, et al. A campaign to improve the timeliness of primary percutaneous coronary intervention: Door-to-Balloon: An Alliance for Quality. JACC Cardiovasc Interv. 2008; 1:97–104.
- Jacobs AK, Antman EM, Faxon DP, Gregory T, Solis P. Development of systems of care for ST-elevation myocardial infarction patients: executive summary. Circulation 2007; 116:217–230.
- Kushner FG, Hand M, Smith SC, et al; American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. 2009 Focused Updates: ACC/AHA Guidelines for the Management of Patients With ST-Elevation Myocardial Infarction (updating the 2004 Guideline and 2007 Focused Update) and ACC/AHA/SCAI Guidelines on Percutaneous Coronary Intervention (updating the 2005 Guideline and 2007 Focused Update): a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation 2009; 120:2271–2306.
- Bates ER, Nallamothu BK. Commentary: the role of percutaneous coronary intervention in ST-segment-elevation myocardial infarction. Circulation 2008; 118:567–573.
- Armstrong PW, Gershlick A, Goldstein P, et al; STREAM Steering Committee. The Strategic Reperfusion Early After Myocardial Infarction (STREAM) study. Am Heart J 2010; 160:30–35.
Combined reperfusion strategies in ST-segment elevation MI: Rationale and current role
Effective and rapid reperfusion is crucial in patients with acute ST-segment elevation myocardial infarction (MI). The preferred strategy for reperfusion—when it can be performed in a timely fashion at an experienced facility—is primary percutaneous coronary intervention (PCI), which produces outcomes superior to those of pharmacologic thrombolysis.1
Unfortunately, in the United States about half of patients present to hospitals that do not have PCI capability,2 and in one analysis, 91% of transferred patients had a door-to-balloon time greater than the recommended 90 minutes, with a mean of 152 minutes.3 (In this case, the door-to-balloon time was the time that elapsed between entry into the first hospital and inflation of the PCI balloon at the second hospital.)
In situations such as these, a combined approach may be appropriate, with thrombolysis delivered by paramedics or at a local facility, followed by transfer to a PCI facility and performance of PCI within a few hours. However, this is feasible only if standardized community-based or regional protocols for prompt transfer and reperfusion are in place.
In this paper we discuss the rationale and the clinical data behind several approaches to combined reperfusion, as well as experiences with community-based care protocols.
WITHIN 3 HOURS OF SYMPTOM ONSET, THROMBOLYSIS IS AS GOOD AS PCI
The PRAGUE-2 Trial
In the randomized PRAGUE-2 trial,4 patients with ST-elevation MI who presented to a non-PCI facility had better outcomes if they were transferred promptly for PCI (median door-to-balloon time 97 minutes), as opposed to receiving local therapy with streptokinase. However, for patients presenting within 3 hours of symptom onset, the mortality rates were comparable with either strategy.4
See the glossary of clinical trial names below
The CAPTIM trial
In the CAPTIM trial,5 patients who presented within 2 hours of symptom onset and who were randomized to receive prehospital thrombolysis had outcomes similar to those of patients treated with primary PCI, despite a short door-to-balloon time (82 minutes).
The Vienna STEMI Registry
In the Vienna STEMI Registry,6 the mortality rates with primary PCI and with thrombolysis were similar when patients presented within 2 hours of symptom onset. However, as the time from symptom onset increased, primary PCI appeared to offer an increasing survival benefit compared with thrombolysis.
Comments: Thrombolysis is effective mostly in the first 2 to 3 hours, with some benefit up to 12 hours
Previous studies have shown that the sooner thrombolysis is given after symptom onset, the more effective it is. If it is given within an hour of symptom onset, the relative reduction in the mortality rate is 50% and the absolute reduction is 6.5% compared with no reperfusion therapy. If it is started in the second hour, the absolute reduction in the mortality rate drops to 4%, and a lesser benefit extends to patients presenting up to 12 hours after symptom onset.7 This time-dependent benefit is due to the fact that very early reperfusion of the occluded coronary artery may lead to full recovery of ischemic tissue and thus prevent necrosis. In addition, thrombolysis in the first 2 hours is highly efficacious in lysing a fresh thrombus.
These data support the current guidelines of the American College of Cardiology (ACC) and the American Heart Association (AHA), which state no preference for either thrombolytic therapy or PCI in ST-elevation MI if the presentation is less than 3 hours after symptom onset.8
Of note, in the CAPTIM trial and in the Vienna STEMI Registry, rescue PCI was available and was in fact used after thrombolysis in about 25% of patients, which might have contributed to the benefit of early thrombolysis.

PRIMARY PCI MAY NOT BE SUPERIOR IF TRANSFER TIME IS LONG
Another time-related factor to consider is the PCI-related delay, ie, the theoretical difference between the expected time from first medical contact to balloon inflation (if the patient undergoes primary PCI) and the time from first medical contact to the start of thrombolytic therapy (if the patient undergoes primary thrombolysis).
A meta-analysis of 13 trials comparing PCI and thrombolysis showed that a PCI-related delay of more than 60 minutes might negate the potential advantage of primary PCI over immediate thrombolysis in terms of deaths.9
This observation has been further refined by data from the National Registry of Myocardial Infarction.10 In this analysis, patient factors, including age, duration of symptoms, and infarct location, significantly affected the point at which the PCI-related delay negated the survival advantage of primary PCI. The survival advantage of primary PCI was lost more rapidly—with a PCI-related delay as short as 40 minutes—in patients who presented sooner, were younger, or had anterior MI. Primary PCI maintained its survival advantage even with a PCI-related delay longer than 100 minutes in older patients or patients with nonanterior MI presenting more than 3 hours after symptom onset. Given that median door-to-balloon times in the United States may exceed 150 minutes when transfer is involved, 3 primary PCI may be no better than primary thrombolysis in transferred patients who present early or who have large infarcts.
Although these results were derived from a post hoc analysis of a registry and the delay times reported were sometimes inaccurate, they suggest that both the PCI-related delay time and patient characteristics should be considered when selecting a reperfusion strategy. Thrombolytic therapy before and in conjunction with primary PCI was considered a potential solution to these concerns.
In addition, while the benefit of any reperfusion strategy depends on the time of presentation, the loss in benefit by later presentation is less pronounced with primary PCI than with thrombolysis, making thrombolysis less attractive in later presentations (> 3 hours).11
Also, while thrombolytic therapy in patients older than 75 years was associated with a lower mortality rate compared with no therapy in a large Swedish registry,12 this benefit was less striking than in younger patients. A meta-analysis of thrombolysis trials failed to show a similar benefit in patients over age 75 vs younger patients,13 whereas primary PCI remained effective and superior to thrombolysis in the elderly, with more absolute reduction in mortality rates in the elderly subgroup than with younger patients. 14 This makes thrombolysis less attractive in the elderly, either as a stand-alone therapy or in conjunction with PCI. Studies of combined thrombolysis and PCI included very few patients over age 75.15–17
THREE COMBINATION REPERFUSION STRATEGIES
Facilitated PCI is a strategy of thrombolysis immediately followed by PCI, with a planned door-to-balloon time of 90 to 120 minutes.
Pharmacoinvasive therapy means giving thrombolysis at a non-PCI facility and then promptly and systematically transferring the patient to a PCI facility, where PCI is performed 2 to 24 hours after the start of thrombolytic therapy, regardless of whether thrombolysis results in successful reperfusion. 15 Thus, the time to PCI is longer than with facilitated PCI. Facilitated PCI addresses the value of pretreatment with thrombolytics or glycoprotein IIb/IIIa inhibitors in patients otherwise eligible for primary PCI, whereas pharmacoinvasive therapy addresses the value of routine early PCI after thrombolysis in patients who are not eligible for primary PCI.16
Rescue PCI refers to PCI that is performed urgently if thrombolysis fails, failure being defined as persistent hemodynamic or electrical instability, persistent ischemic symptoms, or failure to achieve at least a 50% to 70% resolution of the maximal ST-segment elevation 90 minutes after the infusion is started.
FACILITATED PCI: NEGATIVE RESULTS IN CLINICAL TRIALS
ASSENT-4 PCI trial
In the ASSENT-4 PCI trial,18 patients receiving full thrombolytic therapy before PCI had a higher rate of in-hospital death, bleeding, and cardiovascular events at 90 days than patients treated with primary PCI.
This trial recruited patients arriving at hospitals with or without PCI capability. The door-to-balloon time was about 110 minutes in both groups, which might not have been prolonged enough to show a benefit from a timely addition of thrombolysis. In addition, antiplatelet therapy was limited in these patients: glycoprotein IIb/IIIa inhibitors were not given, and clopidogrel (Plavix) was not appropriately preloaded, and this might have offset the potential benefit of early PCI. In fact, data suggest that platelet activation and aggregation are heightened after thrombolysis, 21–23 and that glycoprotein IIb/IIIa antagonists can inhibit these effects.23
The FINESSE trial
In the FINESSE trial,19 patients were randomized to undergo primary PCI, to undergo PCI facilitated (ie, preceded) by abciximab (Reo-Pro), or to undergo PCI facilitated by half-dose reteplase (Retavase) and full-dose abciximab. Despite a median door-to-balloon time of 132 minutes, the three strategies were associated with similar rates of death, heart failure, or ischemic outcome at 90 days. Even though the dosage of heparin was weight-adjusted, more major bleeding events occurred with the facilitated strategies.
Comments: Some subgroups may still benefit from facilitated PCI
The results of ASSENT-4 PCI and FINESSE led to the conclusion that PCI facilitated by full-dose thrombolysis should be avoided, and called into question the value of PCI facilitation using glycoprotein IIb/IIIa inhibitors with or without half-dose thrombolytic therapy.
However, subgroup analyses of these trials identified some subgroups that may benefit from a facilitated strategy. In ASSENT-4 PCI, 45% of patients were enrolled at PCI hospitals with a minimal PCI-related delay time. These patients had the worst outcome with the facilitated strategy. In contrast, patients who had a short time from pain onset to thrombolysis (2 to 3 hours) and who were given prehospital thrombolysis had a trend toward better outcomes with facilitated PCI.24 And in FINESSE, 60% of patients were enrolled at centers with PCI capability. Analysis of a small subgroup of patients with a Thrombolysis in Myocardial Infarction study (TIMI) risk score of 3 or greater presenting to non-PCI hospitals within 4 hours of symptom onset suggested a potential reduction of ischemic events with the facilitated strategy in these patients.25
Thus, for patients seen in the first 2 to 3 hours after symptom onset, immediate thrombolysis is recommended if PCI will likely be delayed, with or without plans for subsequent early PCI. “Time is muscle,” especially during the first 3 hours.
PHARMACOINVASIVE STRATEGY: GOOD RESULTS IN HIGH-RISK PATIENTS
A number of randomized studies during the last 10 years have examined the value of a pharmacoinvasive strategy.15,16,26–29
The TRANSFER-AMI trial
The TRANSFER-AMI trial15 randomized 1,059 patients with high-risk ST-elevation MI (ie, anterior or high-risk inferior) at non-PCI centers to undergo either pharmacoinvasive care, ie, full-dose tenecteplase (TNKase) with immediate transfer for PCI or standard care, ie, tenecteplase with transfer for rescue PCI if the patient had persistent ST-segment elevation, chest pain, or hemodynamic instability.15 The goal was to perform PCI within 6 hours of thrombolysis, and the median time to PCI was 3.9 hours (range 2–6 hours). In the standard-care group, 35% of patients needed to be transferred for rescue PCI. Unlike in the ASSENT-4 trial, over 80% of patients received aggressive antiplatelet therapy with both 300 mg of clopidogrel and glycoprotein IIb/IIIa inhibitors.
The rate of cardiovascular events at 30 days was significantly lower with pharmacoinvasive therapy than with standard care and rescue PCI (11% vs 17%, P = .004). This difference was driven by lower rates of recurrent ischemia, reinfarction, and heart failure.
The CARESS-in-AMI study
The CARESS-in-AMI study16 found a similar improvement in ischemic outcomes in 600 patients with high-risk ST-elevation MI arriving at non-PCI centers if they had received pharmacoinvasive therapy. Patients received half-dose reteplase and abciximab and were randomized either to be immediately transferred for PCI (median time to PCI 2.25 hours) or to be transferred only if they had persistent ST-segment elevation or clinical deterioration.16 The event rate was low with pharmacoinvasive therapy, comparable to that achieved in primary PCI trials.
Interestingly, no significant increase was seen in the risk of major and minor bleeding in these two trials despite the use of a femoral approach for PCI in over 80% of the cases; this is probably due to the delays between thrombolytic administration and PCI and to the use of a highly fibrin-specific thrombolytic agent and adjusted-dose heparin.
Meta-analysis of pharmacoinvasive trials
A meta-analysis29 of studies of systematic early PCI (mainly with stenting) within 24 hours of thrombolysis showed a reduction in the rates of mortality and reinfarction with this strategy, without an increase in the risk of major or intracranial bleeding.30 In contrast to the results of the trials of facilitated PCI, a pharmacoinvasive strategy improved outcomes in these trials because the delay between thrombolysis and PCI was more than 2 hours, ie, long enough to prevent bleeding complications, and because most patients randomized in these trials presented within 2 to 3 hours of symptom onset, when the time to reperfusion is critical. After 3 hours, the PCI-mediated myocardial salvage is less time-dependent. Moreover, trials of pharmacoinvasive strategy used aggressive antiplatelet therapy with clopidogrel and glycoprotein IIb/IIIa inhibitors.
Comment: Pharmacoinvasive strategy in the guidelines
These results and those of the subgroup analysis from the FINESSE trial suggest that patients with high-risk ST-elevation MI treated at non-PCI hospitals have better outcomes without an increase in major bleeding events when given thrombolysis and then immediately transferred for routine PCI, rather than being transferred only if reperfusion fails.
Hence, the 2009 update of the ACC/AHA guidelines31 gives a class IIa recommendation for transferring patients with anterior ST-elevation MI or high-risk inferior ST-elevation MI treated with thrombolysis to a PCI-capable facility where PCI is performed as part of a pharmacoinvasive or rescue strategy soon after thrombolysis.
This strategy has been particularly studied in patients younger than 75 years presenting with high-risk types of ST-elevation MI early (< 3 hours) after symptom onset. If not at high risk, the patient may be transferred to a PCI facility after receiving thrombolysis or observed in the initial facility (class IIb recommendation). Consideration should be given to starting anticoagulant and antiplatelet therapy before and during transfer—ie, 300 mg of clopidogrel before transfer for PCI and glycoprotein IIb/IIIa inhibitor therapy during PCI.
The European Society of Cardiology (ESC) guidelines32 recommend early routine angiography 3 to 24 hours after successful thrombolysis. This time window was selected to avoid PCI during the prothrombotic period in the first few hours after thrombolysis and to minimize the risk of reocclusion with PCI delays of more than 24 hours (class IIa recommendation).
Larger randomized trials are still needed to establish whether the pharmacoinvasive strategy confers a survival benefit, to determine its usefulness in low-risk inferior or lateral ST-elevation MI, and to further refine the time window when PCI is both safe and beneficial after thrombolysis.33
RESCUE PCI REDUCES MORTALITY RATES
Rescue PCI is the most accepted form of thrombolysis-PCI combination.
The REACT trial
The REACT trial20 showed that rescue PCI performed at a mean of 4.5 hours after failed thrombolysis reduces the rate of adverse cardiovascular events by more than 50% at 6 to 12 months and reduces the 5-year mortality rate by more than 50% compared with conservative management.20 As in the pharmacoinvasive strategy, aggressive antiplatelet regimens were used in the REACT trial.
A meta-analysis of rescue PCI trials
A meta-analysis of rescue PCI trials34 confirmed these results, showing a reduction in heart failure and reinfarction and a trend toward a lower mortality rate with rescue PCI.34 After thrombolysis, 40% of patients do not achieve grade 3 TIMI flow, which explains why in modern clinical trials 30% of patients treated with thrombolysis require rescue PCI.5,15,16,35
For patients with high-risk ST-elevation MI, current ACC/AHA guidelines assign a class IIa recommendation to rescue PCI.31
WHEN PATIENTS WITH ST-ELEVATION MI PRESENT TO A NON-PCI HOSPITAL
Transfer for primary PCI vs thrombolysis at the non-PCI hospital
The DANAMI-2 trial36 found that immediate transfer for PCI was superior to onsite thrombolytic therapy, as measured by a reduction in the rate of ischemic events (composite of death, myocardial infarction, or stroke at 30 days): 8.5% vs 14.2% (P < .001). There were no deaths during transfer.3
The PRAGUE-2 trial4 showed similar results for patients presenting 3 to 12 hours after symptom onset (30-day mortality rate 6% with immediate transfer vs 15.3% with on-site thrombolysis, P < .002), whereas patients presenting within 3 hours of symptom onset had a similar mortality rate with either therapy.4
Comment. These trials showed that transfer for primary PCI is superior to thrombolytic therapy when performed in a timely fashion. However, they were done in countries with established transfer networks and short distances between community hospitals and PCI centers, with a PCI-related delay of only 44 minutes and a door-to-balloon time of 90 minutes despite transfer. The large-scale application of this prompt transfer policy is not practical in most regions in the United States. Thus, a strategy of local thrombolysis followed by routine early transfer for routine or rescue PCI seems warranted when the door-to-balloon time or the PCI-related delay time is expected to be too long.
Experiences with community-based systems of care and prehospital thrombolysis
In Minnesota, Henry et al37 developed a PCI-based treatment system and an integrated transfer program for ST-elevation MI involving 30 hospitals within 210 miles of the Minneapolis Heart Institute. Participating hospitals were divided into two zones: zone 1 hospitals were within 60 miles, and zone 2 facilities were between 60 and 210 miles from the Heart Institute. Zone 2 patients received half-dose tenecteplase (if thrombolytic therapy was not contraindicated) in anticipation of a lengthy transfer time.
The median door-to-balloon time for zone 1 patients was 95 minutes (interquartile range 82 and 116 minutes) and for zone 2 patients 120 minutes (interquartile range 100 and 145 minutes). The diagnosis of ST-elevation MI was made by the emergency department physician, who activated the system with a phone call. The patient was then directly transferred to the catheterization laboratory, most often by helicopter.
The in-hospital death rate for patients who presented to the PCI center and for patients in zones 1 and 2 was similarly low (about 5%).37
In France, the FAST-MI registry,17 which collected outcome data for different reperfusion strategies, found that thrombolysis yielded in-hospital and midterm results that were comparable to those of primary PCI. Of note, thrombolysis was started early after symptom onset (about 2 hours), and was started in the ambulance in two-thirds of cases. Nearly all patients underwent a pharmacoinvasive strategy that combined thrombolysis with coronary angiography and PCI within 24 hours of symptom onset. These findings suggest that timely thrombolysis followed by semiurgent transfer for PCI is an alternative to primary PCI for patients presenting to hospitals with no PCI capability, and that this alternative offers similar benefit to that of primary PCI.
Five centers in the United States have reported their experience with half-dose thrombolysis in the prehospital setting (in the field or during transfer) or at a non-PCI hospital, followed by prompt transfer to a PCI facility. In this registry of almost 3,000 patients,38 patients treated with thrombolysis had better outcomes than patients directly transferred for primary PCI, with a significantly lower 30-day mortality rate (3.8% vs from 6.4%), and no increase in bleeding.38,39 The mean door-to-balloon time was long (168 minutes in the primary PCI group and 196 minutes in the thrombolysis-PCI group), which might explain the benefit achieved with prompt thrombolysis.
CARDIOGENIC SHOCK
Patients presenting with left ventricular cardiogenic shock derive a large mortality benefit from revascularization, whether they are transferred or directly admitted to a PCI center. 40 Moreover, in the SHOCK registry, patients with predominant right ventricular cardiogenic shock had an in-hospital mortality rate similar to that of patients with predominant left ventricular cardiogenic shock, and revascularization (PCI or surgical revascularization) was associated with a strikingly lower mortality rate in both groups.41
Thus, all patients with left or right cardiogenic shock should be revascularized on an emergency basis, either surgically or percutaneously.
While trials of pharmacoinvasive therapy excluded patients with cardiogenic shock,15,16 thrombolytic therapy was associated with improved outcomes in the drug-therapy group of the SHOCK trial and in hypotensive patients randomized in the early thrombolysis trials.13 Thus, the ACC/AHA guidelines recommend thrombolytic therapy before transfer if a patient presents in shock within 3 to 6 hours of onset of the MI and delays in transport and intervention are anticipated.8
PUTTING IT ALL TOGETHER: MANAGEMENT STRATEGIES
If an effective transfer system is in place, primary PCI not preceded by thrombolytic therapy or glycoprotein IIb/IIIa inhibitor therapy is the preferred approach, according to ACC/AHA and ESC guidelines.31,32 Giving thrombolytics immediately before PCI is harmful and thus should be avoided when the expected door-to-balloon time is 90 minutes or less.
All hospitals (whether or not they offer PCI) and regional emergency medical services should participate in a community-based system of care for ST-elevation MI, with protocols for expeditious transfer as defined and coordinated by the American Heart Association initiative “Mission: Lifeline.” In addition, a system of field triage and direct transport to the catheterization laboratory of a PCI facility after field activation significantly reduces door-to-balloon times and improves outcomes.42
If such a system is not in place, then a pharmacoinvasive strategy seems best: ie, local full-dose thrombolysis (if not contraindicated) followed by transfer to a PCI facility and routine performance of PCI 2 to 6 hours after thrombolysis—in conjunction with aggressive early dual oral antiplatelet therapy and “downstream” glycoprotein IIb/IIIa inhibition. This approach is associated with outcomes similar to those of primary PCI.15–17,37
Prehospital thrombolysis delivered by paramedics and followed by early transfer to a PCI facility has been associated with further reduction in mortality rates compared with in-hospital thrombolysis (as in the Swedish registry43), and a reduction in death rate comparable to that of primary PCI in patients presenting early. This is an adequate strategy in regions where such a system can be established.5,17,38,43,44
Patients presenting more than 3 to 4 hours after symptom onset, older patients, and patients with lower-risk MI or a higher risk of bleeding may still be suited for primary PCI even when the door-to-balloon time is 90 to 120 minutes, as stated by the European guidelines,32 or when the PCI-related delay time is as long as 100 minutes. 10 On the other hand, while the ACC/AHA guidelines recognize that in these patients the mortality advantage of primary PCI vs thrombolytic therapy is maintained with more prolonged door-to-balloon times, they nevertheless state that the focus should be on developing systems of care to increase the number of patients with access to primary PCI in less than 90 minutes rather than extending the acceptable window for door-to-balloon time.
In conclusion, for patients presenting with ST-elevation MI who cannot undergo timely primary PCI, the best approach seems to be prehospital thrombolysis delivered by paramedics or local thrombolysis at the non-PCI hospital followed by transferring the patient and performing PCI within a few hours. This is especially important in patients with high-risk ST-elevation MI who present early after symptom onset, when the extent of myocardial necrosis associated with delayed primary PCI is largest.
In addition, every community should develop a coordinated transfer strategy between non-PCI and PCI hospitals.
- Keeley EC, Boura JA, Grines CL. Primary angioplasty versus intravenous thrombolytic therapy for acute myocardial infarction: a quantitative review of 23 randomised trials. Lancet 2003; 361:13–20.
- Waters RE, Singh KP, Roe MT, et al. Rationale and strategies for implementing community-based transfer protocols for primary percutaneous coronary intervention for acute ST-segment elevation myocardial infarction. J Am Coll Cardiol 2004; 43:2153–2159.
- Chakrabarti A, Krumholz HM, Wang Y, Rumsfeld JS, Nallamothu BK; National Cardiovascular Data Registry. Time-to-reperfusion in patients undergoing interhospital transfer for primary percutaneous coronary intervention in the U.S: an analysis of 2005 and 2006 data from the National Cardiovascular Data Registry. J Am Coll Cardiol 2008; 51:2442–2443.
- Widimský P, Budesínský T, Vorác D, et al; ‘PRAGUE’ Study Group Investigators. Long distance transport for primary angioplasty vs immediate thrombolysis in acute myocardial infarction. Final results of the randomized national multicentre trial—PRAGUE-2. Eur Heart J 2003; 24:94–104.
- Steg PG, Bonnefoy E, Chabaud S, et al; Comparison of Angioplasty and Prehospital Thrombolysis in Acute Myocardial infarction (CAPTIM) Investigators. Impact of time to treatment on mortality after prehospital fibrinolysis or primary angioplasty: data from the CAPTIM randomized clinical trial. Circulation 2003; 108:2851–2856.
- Kalla K, Christ G, Karnik R, et al; Vienna STEMI Registry Group. Implementation of guidelines improves the standard of care: the Viennese registry on reperfusion strategies in ST-elevation myocardial infarction (Vienna STEMI registry). Circulation 2006; 113:2398–2405.
- Boersma E, Maas AC, Deckers JW, Simoons ML. Early thrombolytic treatment in acute myocardial infarction: reappraisal of the golden hour. Lancet 1996; 348:771–775.
- Antman EM, Anbe DT, Armstrong PW, et al; American College of Cardiology; American Heart Association Task Force on Practice Guidelines; Canadian Cardiovascular Society. ACC/AHA guidelines for the management of patients with ST-elevation myocardial infarction: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee to Revise the 1999 Guidelines for the Management of Patients with Acute Myocardial Infarction). Circulation 2004; 110:e82–e292.
- Nallamothu BK, Bates ER. Percutaneous coronary intervention versus fibrinolytic therapy in acute myocardial infarction: is timing (almost) everything? Am J Cardiol 2003; 92:824–826.
- Pinto DS, Kirtane AJ, Nallamothu BK, et al. Hospital delays in reperfusion for ST-elevation myocardial infarction: implications when selecting a reperfusion strategy. Circulation 2006; 114:2019–2025.
- Boersma E; Primary Coronary Angioplasty vs Thrombolysis Group. Does time matter? A pooled analysis of randomized clinical trials comparing primary percutaneous coronary intervention and in-hospital fibrinolysis in acute myocardial infarction patients. Eur Heart J 2006; 27:779–788.
- Stenestrand U, Wallentin L; Register of Information and Knowledge About Swedish Heart Intensive Care Admissions (RIKS-HIA). Fibrinolytic therapy in patients 75 years and older with ST-segment-elevation myocardial infarction: one-year follow-up of a large prospective cohort. Arch Intern Med 2003; 163:965–971.
- Indications for fibrinolytic therapy in suspected acute myocardial infarction: collaborative overview of early mortality and major morbidity results from all randomised trials of more than 1000 patients. Fibrinolytic Therapy Trialists’ (FTT) Collaborative Group. Lancet 1994; 343:311–322.
- Grines CL, Browne KF, Marco J, et al. A comparison of immediate angioplasty with thrombolytic therapy for acute myocardial infarction. The Primary Angioplasty in Myocardial Infarction Study Group. N Engl J Med 1993; 328:673–679.
- Cantor WJ, Fitchett D, Borgundvaag B, et al; TRANSFER-AMI Trial Investigators. Routine early angioplasty after fibrinolysis for acute myocardial infarction. N Engl J Med 2009; 360:2705–2718.
- Di Mario C, Dudek D, Piscione F, et al; CARESS-in-AMI (Combined Abciximab RE-teplase Stent Study in Acute Myocardial Infarction) Investigators. Immediate angioplasty versus standard therapy with rescue angioplasty after thrombolysis in the Combined Abciximab REteplase Stent Study in Acute Myocardial Infarction (CARESS-in-AMI): an open, prospective, randomised, multicentre trial. Lancet 2008; 371:559–568.
- Danchin N, Coste P, Ferrières J, et al; FAST-MI Investigators. Comparison of thrombolysis followed by broad use of percutaneous coronary intervention with primary percutaneous coronary intervention for ST-segment-elevation acute myocardial infarction: data from the French registry on Acute ST-elevation Myocardial Infarction (FAST-MI). Circulation 2008; 118:268–276.
- Assessment of the Safety and Efficacy of a New Treatment Strategy with Percutaneous Coronary Intervention (ASSENT-4 PCI) investigators. Primary versus tenecteplase-facilitated percutaneous coronary intervention in patients with ST-segment elevation acute myocardial infarction (ASSENT-4 PCI): randomised trial. Lancet 2006; 367:569–578.
- Ellis SG, Tendera M, de Belder MA, et al; FINESSE Investigators. Facilitated PCI in patients with ST-elevation myocardial infarction. N Engl J Med 2008; 358:2205–2217.
- Carver A, Rafelt S, Gershlick AH, Fairbrother KL, Hughes S, Wilcox R; REACT Investigators. Longer-term follow-up of patients recruited to the REACT (Rescue Angioplasty Versus Conservative Treatment or Repeat Thrombolysis) trial. J Am Coll Cardiol 2009; 54:118–126.
- Rasmanis G, Vesterqvist O, Gréen K, Edhag O, Henriksson P. Evidence of increased platelet activation after thrombolysis in patients with acute myocardial infarction. Br Heart J 1992; 68:374–376.
- Gurbel PA, Serebruany VL, Shustov AR, et al. Effects of reteplase and alteplase on platelet aggregation and major receptor expression during the first 24 hours of acute myocardial infarction treatment. GUSTO-III Investigators. Global Use of Strategies to Open Occluded Coronary Arteries. J Am Coll Cardiol 1998; 31:1466–1473.
- Coulter SA, Cannon CP, Ault KA, et al. High levels of platelet inhibition with abciximab despite heightened platelet activation and aggregation during thrombolysis for acute myocardial infarction: results from TIMI (thrombolysis in myocardial infarction) 14. Circulation 2000; 101:2690–2695.
- Ross AM, Huber K, Zeymer U, et al. The impact of place of enrollment and delay to reperfusion on 90-day post-infarction mortality in the ASSENT-4 PCI trial: assessment of the safety and efficacy of a new treatment strategy with percutaneous coronary intervention. JACC Cardiovasc Interv 2009; 2:925–930.
- Herrmann HC, Lu J, Brodie BR, et al; FINESSE Investigators. Benefit of facilitated percutaneous coronary intervention in high-risk ST-segment elevation myocardial infarction patients presenting to nonpercutaneous coronary intervention hospitals. JACC Cardiovasc Interv 2009; 2:917–924.
- Scheller B, Hennen B, Hammer B, et al; SIAM III Study Group. Beneficial effects of immediate stenting after thrombolysis in acute myocardial infarction. J Am Coll Cardiol 2003; 42:634–641.
- Fernandez-Avilés F, Alonso JJ, Castro-Beiras A, et al; GRACIA (Grupo de Análisis de la Cardiopatía Isquémica Aguda) Group. Routine invasive strategy within 24 hours of thrombolysis versus ischaemiaguided conservative approach for acute myocardial infarction with ST-segment elevation (GRACIA-1): a randomised controlled trial. Lancet 2004; 364:1045–1053.
- Le May MR, Wells GA, Labinaz M, et al. Combined angioplasty and pharmacological intervention versus thrombolysis alone in acute myocardial infarction (CAPITAL AMI study). J Am Coll Cardiol 2005; 46:417–424.
- Bøhmer E, Hoffmann P, Abdelnoor M, Arnesen H, Halvorsen S. Efficacy and safety of immediate angioplasty versus ischemia-guided management after thrombolysis in acute myocardial infarction in areas with very long transfer distances results of the NORDISTEMI (NORwegian study on DIstrict treatment of ST-elevation myocardial infarction). J Am Coll Cardiol 2010; 55:102–110.
- Wijeysundera HC, You JJ, Nallamothu BK, Krumholz HM, Cantor WJ, Ko DT. An early invasive strategy versus ischemia-guided management after fibrinolytic therapy for ST-segment elevation myocardial infarction: a meta-analysis of contemporary randomized controlled trials. Am Heart J 2008; 156:564–572,572.e1–e2.
- Kushner FG, Hand M, Smith SC, et al. 2009 focused updates: ACC/AHA guidelines for the management of patients with ST-elevation myocardial infarction (updating the 2004 guideline and 2007 focused update) and ACC/AHA/SCAI guidelines on percutaneous coronary intervention (updating the 2005 guideline and 2007 focused update) a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2009; 54:2205–2241.
- Van de Werf F, Bax J, Betriu A, et al. Management of acute myocardial infarction in patients presenting with persistent ST-segment elevation: the Task Force on the Management of ST-Segment Elevation Acute Myocardial Infarction of the European Society of Cardiology. Eur Heart J 2008; 29:2909–2945.
- Mukherjee D, Moliterno DJ. The timely coupling of mechanical revascularization following thrombolysis for myocardial infarction. Cardiology 2007; 107:337–339.
- Wijeysundera HC, Vijayaraghavan R, Nallamothu BK, et al. Rescue angioplasty or repeat fibrinolysis after failed fibrinolytic therapy for ST-segment myocardial infarction: a meta-analysis of randomized trials. J Am Coll Cardiol 2007; 49:422–430.
- The GUSTO Angiographic Investigators. The effects of tissue plasminogen activator, streptokinase, or both on coronary-artery patency, ventricular function, and survival after acute myocardial infarction. N Engl J Med 1993; 329:1615–1622.
- Andersen HR, Nielsen TT, Rasmussen K, et al; DANAMI-2 Investigators. A comparison of coronary angioplasty with fibrinolytic therapy in acute myocardial infarction. N Engl J Med 2003; 349:733–742.
- Henry TD, Sharkey SW, Burke MN, et al. A regional system to provide timely access to percutaneous coronary intervention for ST-elevation myocardial infarction. Circulation 2007; 116:721–728.
- Denktas AE, Athar H, Henry TD, et al. Reduced-dose fibrinolytic acceleration of ST-segment elevation myocardial infarction treatment coupled with urgent percutaneous coronary intervention compared to primary percutaneous coronary intervention alone results of the AMICO (Alliance for Myocardial Infarction Care Optimization) Registry. JACC Cardiovasc Interv 2008; 1:504–510.
- Smalling RW. Ischemic time: the new gold standard for ST-segment elevation myocardial infarction care. J Am Coll Cardiol 2009; 54:2154–2156.
- Hochman JS, Sleeper LA, White HD, et al; SHOCK Investigators. Should We Emergently Revascularize Occluded Coronaries for Cardiogenic Shock. One-year survival following early revascularization for cardiogenic shock. JAMA 2001; 285:190–192.
- Jacobs AK, Leopold JA, Bates E, et al. Cardiogenic shock caused by right ventricular infarction: a report from the SHOCK registry. J Am Coll Cardiol 2003; 41:1273–1279.
- Pedersen SH, Galatius S, Hansen PR, et al. Field triage reduces treatment delay and improves long-term clinical outcome in patients with acute ST-segment elevation myocardial infarction treated with primary percutaneous coronary intervention. J Am Coll Cardiol 2009; 54:2296–2302.
- Björklund E, Stenestrand U, Lindbäck J, Svensson L, Wallentin L, Lindahl B. Pre-hospital thrombolysis delivered by paramedics is associated with reduced time delay and mortality in ambulance-transported real-life patients with ST-elevation myocardial infarction. Eur Heart J 2006; 27:1146–1152.
- The European Myocardial Infarction Project Group. Prehospital thrombolytic therapy in patients with suspected acute myocardial infarction. N Engl J Med 1993; 329:383–389.
Effective and rapid reperfusion is crucial in patients with acute ST-segment elevation myocardial infarction (MI). The preferred strategy for reperfusion—when it can be performed in a timely fashion at an experienced facility—is primary percutaneous coronary intervention (PCI), which produces outcomes superior to those of pharmacologic thrombolysis.1
Unfortunately, in the United States about half of patients present to hospitals that do not have PCI capability,2 and in one analysis, 91% of transferred patients had a door-to-balloon time greater than the recommended 90 minutes, with a mean of 152 minutes.3 (In this case, the door-to-balloon time was the time that elapsed between entry into the first hospital and inflation of the PCI balloon at the second hospital.)
In situations such as these, a combined approach may be appropriate, with thrombolysis delivered by paramedics or at a local facility, followed by transfer to a PCI facility and performance of PCI within a few hours. However, this is feasible only if standardized community-based or regional protocols for prompt transfer and reperfusion are in place.
In this paper we discuss the rationale and the clinical data behind several approaches to combined reperfusion, as well as experiences with community-based care protocols.
WITHIN 3 HOURS OF SYMPTOM ONSET, THROMBOLYSIS IS AS GOOD AS PCI
The PRAGUE-2 Trial
In the randomized PRAGUE-2 trial,4 patients with ST-elevation MI who presented to a non-PCI facility had better outcomes if they were transferred promptly for PCI (median door-to-balloon time 97 minutes), as opposed to receiving local therapy with streptokinase. However, for patients presenting within 3 hours of symptom onset, the mortality rates were comparable with either strategy.4
See the glossary of clinical trial names below
The CAPTIM trial
In the CAPTIM trial,5 patients who presented within 2 hours of symptom onset and who were randomized to receive prehospital thrombolysis had outcomes similar to those of patients treated with primary PCI, despite a short door-to-balloon time (82 minutes).
The Vienna STEMI Registry
In the Vienna STEMI Registry,6 the mortality rates with primary PCI and with thrombolysis were similar when patients presented within 2 hours of symptom onset. However, as the time from symptom onset increased, primary PCI appeared to offer an increasing survival benefit compared with thrombolysis.
Comments: Thrombolysis is effective mostly in the first 2 to 3 hours, with some benefit up to 12 hours
Previous studies have shown that the sooner thrombolysis is given after symptom onset, the more effective it is. If it is given within an hour of symptom onset, the relative reduction in the mortality rate is 50% and the absolute reduction is 6.5% compared with no reperfusion therapy. If it is started in the second hour, the absolute reduction in the mortality rate drops to 4%, and a lesser benefit extends to patients presenting up to 12 hours after symptom onset.7 This time-dependent benefit is due to the fact that very early reperfusion of the occluded coronary artery may lead to full recovery of ischemic tissue and thus prevent necrosis. In addition, thrombolysis in the first 2 hours is highly efficacious in lysing a fresh thrombus.
These data support the current guidelines of the American College of Cardiology (ACC) and the American Heart Association (AHA), which state no preference for either thrombolytic therapy or PCI in ST-elevation MI if the presentation is less than 3 hours after symptom onset.8
Of note, in the CAPTIM trial and in the Vienna STEMI Registry, rescue PCI was available and was in fact used after thrombolysis in about 25% of patients, which might have contributed to the benefit of early thrombolysis.

PRIMARY PCI MAY NOT BE SUPERIOR IF TRANSFER TIME IS LONG
Another time-related factor to consider is the PCI-related delay, ie, the theoretical difference between the expected time from first medical contact to balloon inflation (if the patient undergoes primary PCI) and the time from first medical contact to the start of thrombolytic therapy (if the patient undergoes primary thrombolysis).
A meta-analysis of 13 trials comparing PCI and thrombolysis showed that a PCI-related delay of more than 60 minutes might negate the potential advantage of primary PCI over immediate thrombolysis in terms of deaths.9
This observation has been further refined by data from the National Registry of Myocardial Infarction.10 In this analysis, patient factors, including age, duration of symptoms, and infarct location, significantly affected the point at which the PCI-related delay negated the survival advantage of primary PCI. The survival advantage of primary PCI was lost more rapidly—with a PCI-related delay as short as 40 minutes—in patients who presented sooner, were younger, or had anterior MI. Primary PCI maintained its survival advantage even with a PCI-related delay longer than 100 minutes in older patients or patients with nonanterior MI presenting more than 3 hours after symptom onset. Given that median door-to-balloon times in the United States may exceed 150 minutes when transfer is involved, 3 primary PCI may be no better than primary thrombolysis in transferred patients who present early or who have large infarcts.
Although these results were derived from a post hoc analysis of a registry and the delay times reported were sometimes inaccurate, they suggest that both the PCI-related delay time and patient characteristics should be considered when selecting a reperfusion strategy. Thrombolytic therapy before and in conjunction with primary PCI was considered a potential solution to these concerns.
In addition, while the benefit of any reperfusion strategy depends on the time of presentation, the loss in benefit by later presentation is less pronounced with primary PCI than with thrombolysis, making thrombolysis less attractive in later presentations (> 3 hours).11
Also, while thrombolytic therapy in patients older than 75 years was associated with a lower mortality rate compared with no therapy in a large Swedish registry,12 this benefit was less striking than in younger patients. A meta-analysis of thrombolysis trials failed to show a similar benefit in patients over age 75 vs younger patients,13 whereas primary PCI remained effective and superior to thrombolysis in the elderly, with more absolute reduction in mortality rates in the elderly subgroup than with younger patients. 14 This makes thrombolysis less attractive in the elderly, either as a stand-alone therapy or in conjunction with PCI. Studies of combined thrombolysis and PCI included very few patients over age 75.15–17
THREE COMBINATION REPERFUSION STRATEGIES
Facilitated PCI is a strategy of thrombolysis immediately followed by PCI, with a planned door-to-balloon time of 90 to 120 minutes.
Pharmacoinvasive therapy means giving thrombolysis at a non-PCI facility and then promptly and systematically transferring the patient to a PCI facility, where PCI is performed 2 to 24 hours after the start of thrombolytic therapy, regardless of whether thrombolysis results in successful reperfusion. 15 Thus, the time to PCI is longer than with facilitated PCI. Facilitated PCI addresses the value of pretreatment with thrombolytics or glycoprotein IIb/IIIa inhibitors in patients otherwise eligible for primary PCI, whereas pharmacoinvasive therapy addresses the value of routine early PCI after thrombolysis in patients who are not eligible for primary PCI.16
Rescue PCI refers to PCI that is performed urgently if thrombolysis fails, failure being defined as persistent hemodynamic or electrical instability, persistent ischemic symptoms, or failure to achieve at least a 50% to 70% resolution of the maximal ST-segment elevation 90 minutes after the infusion is started.
FACILITATED PCI: NEGATIVE RESULTS IN CLINICAL TRIALS
ASSENT-4 PCI trial
In the ASSENT-4 PCI trial,18 patients receiving full thrombolytic therapy before PCI had a higher rate of in-hospital death, bleeding, and cardiovascular events at 90 days than patients treated with primary PCI.
This trial recruited patients arriving at hospitals with or without PCI capability. The door-to-balloon time was about 110 minutes in both groups, which might not have been prolonged enough to show a benefit from a timely addition of thrombolysis. In addition, antiplatelet therapy was limited in these patients: glycoprotein IIb/IIIa inhibitors were not given, and clopidogrel (Plavix) was not appropriately preloaded, and this might have offset the potential benefit of early PCI. In fact, data suggest that platelet activation and aggregation are heightened after thrombolysis, 21–23 and that glycoprotein IIb/IIIa antagonists can inhibit these effects.23
The FINESSE trial
In the FINESSE trial,19 patients were randomized to undergo primary PCI, to undergo PCI facilitated (ie, preceded) by abciximab (Reo-Pro), or to undergo PCI facilitated by half-dose reteplase (Retavase) and full-dose abciximab. Despite a median door-to-balloon time of 132 minutes, the three strategies were associated with similar rates of death, heart failure, or ischemic outcome at 90 days. Even though the dosage of heparin was weight-adjusted, more major bleeding events occurred with the facilitated strategies.
Comments: Some subgroups may still benefit from facilitated PCI
The results of ASSENT-4 PCI and FINESSE led to the conclusion that PCI facilitated by full-dose thrombolysis should be avoided, and called into question the value of PCI facilitation using glycoprotein IIb/IIIa inhibitors with or without half-dose thrombolytic therapy.
However, subgroup analyses of these trials identified some subgroups that may benefit from a facilitated strategy. In ASSENT-4 PCI, 45% of patients were enrolled at PCI hospitals with a minimal PCI-related delay time. These patients had the worst outcome with the facilitated strategy. In contrast, patients who had a short time from pain onset to thrombolysis (2 to 3 hours) and who were given prehospital thrombolysis had a trend toward better outcomes with facilitated PCI.24 And in FINESSE, 60% of patients were enrolled at centers with PCI capability. Analysis of a small subgroup of patients with a Thrombolysis in Myocardial Infarction study (TIMI) risk score of 3 or greater presenting to non-PCI hospitals within 4 hours of symptom onset suggested a potential reduction of ischemic events with the facilitated strategy in these patients.25
Thus, for patients seen in the first 2 to 3 hours after symptom onset, immediate thrombolysis is recommended if PCI will likely be delayed, with or without plans for subsequent early PCI. “Time is muscle,” especially during the first 3 hours.
PHARMACOINVASIVE STRATEGY: GOOD RESULTS IN HIGH-RISK PATIENTS
A number of randomized studies during the last 10 years have examined the value of a pharmacoinvasive strategy.15,16,26–29
The TRANSFER-AMI trial
The TRANSFER-AMI trial15 randomized 1,059 patients with high-risk ST-elevation MI (ie, anterior or high-risk inferior) at non-PCI centers to undergo either pharmacoinvasive care, ie, full-dose tenecteplase (TNKase) with immediate transfer for PCI or standard care, ie, tenecteplase with transfer for rescue PCI if the patient had persistent ST-segment elevation, chest pain, or hemodynamic instability.15 The goal was to perform PCI within 6 hours of thrombolysis, and the median time to PCI was 3.9 hours (range 2–6 hours). In the standard-care group, 35% of patients needed to be transferred for rescue PCI. Unlike in the ASSENT-4 trial, over 80% of patients received aggressive antiplatelet therapy with both 300 mg of clopidogrel and glycoprotein IIb/IIIa inhibitors.
The rate of cardiovascular events at 30 days was significantly lower with pharmacoinvasive therapy than with standard care and rescue PCI (11% vs 17%, P = .004). This difference was driven by lower rates of recurrent ischemia, reinfarction, and heart failure.
The CARESS-in-AMI study
The CARESS-in-AMI study16 found a similar improvement in ischemic outcomes in 600 patients with high-risk ST-elevation MI arriving at non-PCI centers if they had received pharmacoinvasive therapy. Patients received half-dose reteplase and abciximab and were randomized either to be immediately transferred for PCI (median time to PCI 2.25 hours) or to be transferred only if they had persistent ST-segment elevation or clinical deterioration.16 The event rate was low with pharmacoinvasive therapy, comparable to that achieved in primary PCI trials.
Interestingly, no significant increase was seen in the risk of major and minor bleeding in these two trials despite the use of a femoral approach for PCI in over 80% of the cases; this is probably due to the delays between thrombolytic administration and PCI and to the use of a highly fibrin-specific thrombolytic agent and adjusted-dose heparin.
Meta-analysis of pharmacoinvasive trials
A meta-analysis29 of studies of systematic early PCI (mainly with stenting) within 24 hours of thrombolysis showed a reduction in the rates of mortality and reinfarction with this strategy, without an increase in the risk of major or intracranial bleeding.30 In contrast to the results of the trials of facilitated PCI, a pharmacoinvasive strategy improved outcomes in these trials because the delay between thrombolysis and PCI was more than 2 hours, ie, long enough to prevent bleeding complications, and because most patients randomized in these trials presented within 2 to 3 hours of symptom onset, when the time to reperfusion is critical. After 3 hours, the PCI-mediated myocardial salvage is less time-dependent. Moreover, trials of pharmacoinvasive strategy used aggressive antiplatelet therapy with clopidogrel and glycoprotein IIb/IIIa inhibitors.
Comment: Pharmacoinvasive strategy in the guidelines
These results and those of the subgroup analysis from the FINESSE trial suggest that patients with high-risk ST-elevation MI treated at non-PCI hospitals have better outcomes without an increase in major bleeding events when given thrombolysis and then immediately transferred for routine PCI, rather than being transferred only if reperfusion fails.
Hence, the 2009 update of the ACC/AHA guidelines31 gives a class IIa recommendation for transferring patients with anterior ST-elevation MI or high-risk inferior ST-elevation MI treated with thrombolysis to a PCI-capable facility where PCI is performed as part of a pharmacoinvasive or rescue strategy soon after thrombolysis.
This strategy has been particularly studied in patients younger than 75 years presenting with high-risk types of ST-elevation MI early (< 3 hours) after symptom onset. If not at high risk, the patient may be transferred to a PCI facility after receiving thrombolysis or observed in the initial facility (class IIb recommendation). Consideration should be given to starting anticoagulant and antiplatelet therapy before and during transfer—ie, 300 mg of clopidogrel before transfer for PCI and glycoprotein IIb/IIIa inhibitor therapy during PCI.
The European Society of Cardiology (ESC) guidelines32 recommend early routine angiography 3 to 24 hours after successful thrombolysis. This time window was selected to avoid PCI during the prothrombotic period in the first few hours after thrombolysis and to minimize the risk of reocclusion with PCI delays of more than 24 hours (class IIa recommendation).
Larger randomized trials are still needed to establish whether the pharmacoinvasive strategy confers a survival benefit, to determine its usefulness in low-risk inferior or lateral ST-elevation MI, and to further refine the time window when PCI is both safe and beneficial after thrombolysis.33
RESCUE PCI REDUCES MORTALITY RATES
Rescue PCI is the most accepted form of thrombolysis-PCI combination.
The REACT trial
The REACT trial20 showed that rescue PCI performed at a mean of 4.5 hours after failed thrombolysis reduces the rate of adverse cardiovascular events by more than 50% at 6 to 12 months and reduces the 5-year mortality rate by more than 50% compared with conservative management.20 As in the pharmacoinvasive strategy, aggressive antiplatelet regimens were used in the REACT trial.
A meta-analysis of rescue PCI trials
A meta-analysis of rescue PCI trials34 confirmed these results, showing a reduction in heart failure and reinfarction and a trend toward a lower mortality rate with rescue PCI.34 After thrombolysis, 40% of patients do not achieve grade 3 TIMI flow, which explains why in modern clinical trials 30% of patients treated with thrombolysis require rescue PCI.5,15,16,35
For patients with high-risk ST-elevation MI, current ACC/AHA guidelines assign a class IIa recommendation to rescue PCI.31
WHEN PATIENTS WITH ST-ELEVATION MI PRESENT TO A NON-PCI HOSPITAL
Transfer for primary PCI vs thrombolysis at the non-PCI hospital
The DANAMI-2 trial36 found that immediate transfer for PCI was superior to onsite thrombolytic therapy, as measured by a reduction in the rate of ischemic events (composite of death, myocardial infarction, or stroke at 30 days): 8.5% vs 14.2% (P < .001). There were no deaths during transfer.3
The PRAGUE-2 trial4 showed similar results for patients presenting 3 to 12 hours after symptom onset (30-day mortality rate 6% with immediate transfer vs 15.3% with on-site thrombolysis, P < .002), whereas patients presenting within 3 hours of symptom onset had a similar mortality rate with either therapy.4
Comment. These trials showed that transfer for primary PCI is superior to thrombolytic therapy when performed in a timely fashion. However, they were done in countries with established transfer networks and short distances between community hospitals and PCI centers, with a PCI-related delay of only 44 minutes and a door-to-balloon time of 90 minutes despite transfer. The large-scale application of this prompt transfer policy is not practical in most regions in the United States. Thus, a strategy of local thrombolysis followed by routine early transfer for routine or rescue PCI seems warranted when the door-to-balloon time or the PCI-related delay time is expected to be too long.
Experiences with community-based systems of care and prehospital thrombolysis
In Minnesota, Henry et al37 developed a PCI-based treatment system and an integrated transfer program for ST-elevation MI involving 30 hospitals within 210 miles of the Minneapolis Heart Institute. Participating hospitals were divided into two zones: zone 1 hospitals were within 60 miles, and zone 2 facilities were between 60 and 210 miles from the Heart Institute. Zone 2 patients received half-dose tenecteplase (if thrombolytic therapy was not contraindicated) in anticipation of a lengthy transfer time.
The median door-to-balloon time for zone 1 patients was 95 minutes (interquartile range 82 and 116 minutes) and for zone 2 patients 120 minutes (interquartile range 100 and 145 minutes). The diagnosis of ST-elevation MI was made by the emergency department physician, who activated the system with a phone call. The patient was then directly transferred to the catheterization laboratory, most often by helicopter.
The in-hospital death rate for patients who presented to the PCI center and for patients in zones 1 and 2 was similarly low (about 5%).37
In France, the FAST-MI registry,17 which collected outcome data for different reperfusion strategies, found that thrombolysis yielded in-hospital and midterm results that were comparable to those of primary PCI. Of note, thrombolysis was started early after symptom onset (about 2 hours), and was started in the ambulance in two-thirds of cases. Nearly all patients underwent a pharmacoinvasive strategy that combined thrombolysis with coronary angiography and PCI within 24 hours of symptom onset. These findings suggest that timely thrombolysis followed by semiurgent transfer for PCI is an alternative to primary PCI for patients presenting to hospitals with no PCI capability, and that this alternative offers similar benefit to that of primary PCI.
Five centers in the United States have reported their experience with half-dose thrombolysis in the prehospital setting (in the field or during transfer) or at a non-PCI hospital, followed by prompt transfer to a PCI facility. In this registry of almost 3,000 patients,38 patients treated with thrombolysis had better outcomes than patients directly transferred for primary PCI, with a significantly lower 30-day mortality rate (3.8% vs from 6.4%), and no increase in bleeding.38,39 The mean door-to-balloon time was long (168 minutes in the primary PCI group and 196 minutes in the thrombolysis-PCI group), which might explain the benefit achieved with prompt thrombolysis.
CARDIOGENIC SHOCK
Patients presenting with left ventricular cardiogenic shock derive a large mortality benefit from revascularization, whether they are transferred or directly admitted to a PCI center. 40 Moreover, in the SHOCK registry, patients with predominant right ventricular cardiogenic shock had an in-hospital mortality rate similar to that of patients with predominant left ventricular cardiogenic shock, and revascularization (PCI or surgical revascularization) was associated with a strikingly lower mortality rate in both groups.41
Thus, all patients with left or right cardiogenic shock should be revascularized on an emergency basis, either surgically or percutaneously.
While trials of pharmacoinvasive therapy excluded patients with cardiogenic shock,15,16 thrombolytic therapy was associated with improved outcomes in the drug-therapy group of the SHOCK trial and in hypotensive patients randomized in the early thrombolysis trials.13 Thus, the ACC/AHA guidelines recommend thrombolytic therapy before transfer if a patient presents in shock within 3 to 6 hours of onset of the MI and delays in transport and intervention are anticipated.8
PUTTING IT ALL TOGETHER: MANAGEMENT STRATEGIES
If an effective transfer system is in place, primary PCI not preceded by thrombolytic therapy or glycoprotein IIb/IIIa inhibitor therapy is the preferred approach, according to ACC/AHA and ESC guidelines.31,32 Giving thrombolytics immediately before PCI is harmful and thus should be avoided when the expected door-to-balloon time is 90 minutes or less.
All hospitals (whether or not they offer PCI) and regional emergency medical services should participate in a community-based system of care for ST-elevation MI, with protocols for expeditious transfer as defined and coordinated by the American Heart Association initiative “Mission: Lifeline.” In addition, a system of field triage and direct transport to the catheterization laboratory of a PCI facility after field activation significantly reduces door-to-balloon times and improves outcomes.42
If such a system is not in place, then a pharmacoinvasive strategy seems best: ie, local full-dose thrombolysis (if not contraindicated) followed by transfer to a PCI facility and routine performance of PCI 2 to 6 hours after thrombolysis—in conjunction with aggressive early dual oral antiplatelet therapy and “downstream” glycoprotein IIb/IIIa inhibition. This approach is associated with outcomes similar to those of primary PCI.15–17,37
Prehospital thrombolysis delivered by paramedics and followed by early transfer to a PCI facility has been associated with further reduction in mortality rates compared with in-hospital thrombolysis (as in the Swedish registry43), and a reduction in death rate comparable to that of primary PCI in patients presenting early. This is an adequate strategy in regions where such a system can be established.5,17,38,43,44
Patients presenting more than 3 to 4 hours after symptom onset, older patients, and patients with lower-risk MI or a higher risk of bleeding may still be suited for primary PCI even when the door-to-balloon time is 90 to 120 minutes, as stated by the European guidelines,32 or when the PCI-related delay time is as long as 100 minutes. 10 On the other hand, while the ACC/AHA guidelines recognize that in these patients the mortality advantage of primary PCI vs thrombolytic therapy is maintained with more prolonged door-to-balloon times, they nevertheless state that the focus should be on developing systems of care to increase the number of patients with access to primary PCI in less than 90 minutes rather than extending the acceptable window for door-to-balloon time.
In conclusion, for patients presenting with ST-elevation MI who cannot undergo timely primary PCI, the best approach seems to be prehospital thrombolysis delivered by paramedics or local thrombolysis at the non-PCI hospital followed by transferring the patient and performing PCI within a few hours. This is especially important in patients with high-risk ST-elevation MI who present early after symptom onset, when the extent of myocardial necrosis associated with delayed primary PCI is largest.
In addition, every community should develop a coordinated transfer strategy between non-PCI and PCI hospitals.
Effective and rapid reperfusion is crucial in patients with acute ST-segment elevation myocardial infarction (MI). The preferred strategy for reperfusion—when it can be performed in a timely fashion at an experienced facility—is primary percutaneous coronary intervention (PCI), which produces outcomes superior to those of pharmacologic thrombolysis.1
Unfortunately, in the United States about half of patients present to hospitals that do not have PCI capability,2 and in one analysis, 91% of transferred patients had a door-to-balloon time greater than the recommended 90 minutes, with a mean of 152 minutes.3 (In this case, the door-to-balloon time was the time that elapsed between entry into the first hospital and inflation of the PCI balloon at the second hospital.)
In situations such as these, a combined approach may be appropriate, with thrombolysis delivered by paramedics or at a local facility, followed by transfer to a PCI facility and performance of PCI within a few hours. However, this is feasible only if standardized community-based or regional protocols for prompt transfer and reperfusion are in place.
In this paper we discuss the rationale and the clinical data behind several approaches to combined reperfusion, as well as experiences with community-based care protocols.
WITHIN 3 HOURS OF SYMPTOM ONSET, THROMBOLYSIS IS AS GOOD AS PCI
The PRAGUE-2 Trial
In the randomized PRAGUE-2 trial,4 patients with ST-elevation MI who presented to a non-PCI facility had better outcomes if they were transferred promptly for PCI (median door-to-balloon time 97 minutes), as opposed to receiving local therapy with streptokinase. However, for patients presenting within 3 hours of symptom onset, the mortality rates were comparable with either strategy.4
See the glossary of clinical trial names below
The CAPTIM trial
In the CAPTIM trial,5 patients who presented within 2 hours of symptom onset and who were randomized to receive prehospital thrombolysis had outcomes similar to those of patients treated with primary PCI, despite a short door-to-balloon time (82 minutes).
The Vienna STEMI Registry
In the Vienna STEMI Registry,6 the mortality rates with primary PCI and with thrombolysis were similar when patients presented within 2 hours of symptom onset. However, as the time from symptom onset increased, primary PCI appeared to offer an increasing survival benefit compared with thrombolysis.
Comments: Thrombolysis is effective mostly in the first 2 to 3 hours, with some benefit up to 12 hours
Previous studies have shown that the sooner thrombolysis is given after symptom onset, the more effective it is. If it is given within an hour of symptom onset, the relative reduction in the mortality rate is 50% and the absolute reduction is 6.5% compared with no reperfusion therapy. If it is started in the second hour, the absolute reduction in the mortality rate drops to 4%, and a lesser benefit extends to patients presenting up to 12 hours after symptom onset.7 This time-dependent benefit is due to the fact that very early reperfusion of the occluded coronary artery may lead to full recovery of ischemic tissue and thus prevent necrosis. In addition, thrombolysis in the first 2 hours is highly efficacious in lysing a fresh thrombus.
These data support the current guidelines of the American College of Cardiology (ACC) and the American Heart Association (AHA), which state no preference for either thrombolytic therapy or PCI in ST-elevation MI if the presentation is less than 3 hours after symptom onset.8
Of note, in the CAPTIM trial and in the Vienna STEMI Registry, rescue PCI was available and was in fact used after thrombolysis in about 25% of patients, which might have contributed to the benefit of early thrombolysis.

PRIMARY PCI MAY NOT BE SUPERIOR IF TRANSFER TIME IS LONG
Another time-related factor to consider is the PCI-related delay, ie, the theoretical difference between the expected time from first medical contact to balloon inflation (if the patient undergoes primary PCI) and the time from first medical contact to the start of thrombolytic therapy (if the patient undergoes primary thrombolysis).
A meta-analysis of 13 trials comparing PCI and thrombolysis showed that a PCI-related delay of more than 60 minutes might negate the potential advantage of primary PCI over immediate thrombolysis in terms of deaths.9
This observation has been further refined by data from the National Registry of Myocardial Infarction.10 In this analysis, patient factors, including age, duration of symptoms, and infarct location, significantly affected the point at which the PCI-related delay negated the survival advantage of primary PCI. The survival advantage of primary PCI was lost more rapidly—with a PCI-related delay as short as 40 minutes—in patients who presented sooner, were younger, or had anterior MI. Primary PCI maintained its survival advantage even with a PCI-related delay longer than 100 minutes in older patients or patients with nonanterior MI presenting more than 3 hours after symptom onset. Given that median door-to-balloon times in the United States may exceed 150 minutes when transfer is involved, 3 primary PCI may be no better than primary thrombolysis in transferred patients who present early or who have large infarcts.
Although these results were derived from a post hoc analysis of a registry and the delay times reported were sometimes inaccurate, they suggest that both the PCI-related delay time and patient characteristics should be considered when selecting a reperfusion strategy. Thrombolytic therapy before and in conjunction with primary PCI was considered a potential solution to these concerns.
In addition, while the benefit of any reperfusion strategy depends on the time of presentation, the loss in benefit by later presentation is less pronounced with primary PCI than with thrombolysis, making thrombolysis less attractive in later presentations (> 3 hours).11
Also, while thrombolytic therapy in patients older than 75 years was associated with a lower mortality rate compared with no therapy in a large Swedish registry,12 this benefit was less striking than in younger patients. A meta-analysis of thrombolysis trials failed to show a similar benefit in patients over age 75 vs younger patients,13 whereas primary PCI remained effective and superior to thrombolysis in the elderly, with more absolute reduction in mortality rates in the elderly subgroup than with younger patients. 14 This makes thrombolysis less attractive in the elderly, either as a stand-alone therapy or in conjunction with PCI. Studies of combined thrombolysis and PCI included very few patients over age 75.15–17
THREE COMBINATION REPERFUSION STRATEGIES
Facilitated PCI is a strategy of thrombolysis immediately followed by PCI, with a planned door-to-balloon time of 90 to 120 minutes.
Pharmacoinvasive therapy means giving thrombolysis at a non-PCI facility and then promptly and systematically transferring the patient to a PCI facility, where PCI is performed 2 to 24 hours after the start of thrombolytic therapy, regardless of whether thrombolysis results in successful reperfusion. 15 Thus, the time to PCI is longer than with facilitated PCI. Facilitated PCI addresses the value of pretreatment with thrombolytics or glycoprotein IIb/IIIa inhibitors in patients otherwise eligible for primary PCI, whereas pharmacoinvasive therapy addresses the value of routine early PCI after thrombolysis in patients who are not eligible for primary PCI.16
Rescue PCI refers to PCI that is performed urgently if thrombolysis fails, failure being defined as persistent hemodynamic or electrical instability, persistent ischemic symptoms, or failure to achieve at least a 50% to 70% resolution of the maximal ST-segment elevation 90 minutes after the infusion is started.
FACILITATED PCI: NEGATIVE RESULTS IN CLINICAL TRIALS
ASSENT-4 PCI trial
In the ASSENT-4 PCI trial,18 patients receiving full thrombolytic therapy before PCI had a higher rate of in-hospital death, bleeding, and cardiovascular events at 90 days than patients treated with primary PCI.
This trial recruited patients arriving at hospitals with or without PCI capability. The door-to-balloon time was about 110 minutes in both groups, which might not have been prolonged enough to show a benefit from a timely addition of thrombolysis. In addition, antiplatelet therapy was limited in these patients: glycoprotein IIb/IIIa inhibitors were not given, and clopidogrel (Plavix) was not appropriately preloaded, and this might have offset the potential benefit of early PCI. In fact, data suggest that platelet activation and aggregation are heightened after thrombolysis, 21–23 and that glycoprotein IIb/IIIa antagonists can inhibit these effects.23
The FINESSE trial
In the FINESSE trial,19 patients were randomized to undergo primary PCI, to undergo PCI facilitated (ie, preceded) by abciximab (Reo-Pro), or to undergo PCI facilitated by half-dose reteplase (Retavase) and full-dose abciximab. Despite a median door-to-balloon time of 132 minutes, the three strategies were associated with similar rates of death, heart failure, or ischemic outcome at 90 days. Even though the dosage of heparin was weight-adjusted, more major bleeding events occurred with the facilitated strategies.
Comments: Some subgroups may still benefit from facilitated PCI
The results of ASSENT-4 PCI and FINESSE led to the conclusion that PCI facilitated by full-dose thrombolysis should be avoided, and called into question the value of PCI facilitation using glycoprotein IIb/IIIa inhibitors with or without half-dose thrombolytic therapy.
However, subgroup analyses of these trials identified some subgroups that may benefit from a facilitated strategy. In ASSENT-4 PCI, 45% of patients were enrolled at PCI hospitals with a minimal PCI-related delay time. These patients had the worst outcome with the facilitated strategy. In contrast, patients who had a short time from pain onset to thrombolysis (2 to 3 hours) and who were given prehospital thrombolysis had a trend toward better outcomes with facilitated PCI.24 And in FINESSE, 60% of patients were enrolled at centers with PCI capability. Analysis of a small subgroup of patients with a Thrombolysis in Myocardial Infarction study (TIMI) risk score of 3 or greater presenting to non-PCI hospitals within 4 hours of symptom onset suggested a potential reduction of ischemic events with the facilitated strategy in these patients.25
Thus, for patients seen in the first 2 to 3 hours after symptom onset, immediate thrombolysis is recommended if PCI will likely be delayed, with or without plans for subsequent early PCI. “Time is muscle,” especially during the first 3 hours.
PHARMACOINVASIVE STRATEGY: GOOD RESULTS IN HIGH-RISK PATIENTS
A number of randomized studies during the last 10 years have examined the value of a pharmacoinvasive strategy.15,16,26–29
The TRANSFER-AMI trial
The TRANSFER-AMI trial15 randomized 1,059 patients with high-risk ST-elevation MI (ie, anterior or high-risk inferior) at non-PCI centers to undergo either pharmacoinvasive care, ie, full-dose tenecteplase (TNKase) with immediate transfer for PCI or standard care, ie, tenecteplase with transfer for rescue PCI if the patient had persistent ST-segment elevation, chest pain, or hemodynamic instability.15 The goal was to perform PCI within 6 hours of thrombolysis, and the median time to PCI was 3.9 hours (range 2–6 hours). In the standard-care group, 35% of patients needed to be transferred for rescue PCI. Unlike in the ASSENT-4 trial, over 80% of patients received aggressive antiplatelet therapy with both 300 mg of clopidogrel and glycoprotein IIb/IIIa inhibitors.
The rate of cardiovascular events at 30 days was significantly lower with pharmacoinvasive therapy than with standard care and rescue PCI (11% vs 17%, P = .004). This difference was driven by lower rates of recurrent ischemia, reinfarction, and heart failure.
The CARESS-in-AMI study
The CARESS-in-AMI study16 found a similar improvement in ischemic outcomes in 600 patients with high-risk ST-elevation MI arriving at non-PCI centers if they had received pharmacoinvasive therapy. Patients received half-dose reteplase and abciximab and were randomized either to be immediately transferred for PCI (median time to PCI 2.25 hours) or to be transferred only if they had persistent ST-segment elevation or clinical deterioration.16 The event rate was low with pharmacoinvasive therapy, comparable to that achieved in primary PCI trials.
Interestingly, no significant increase was seen in the risk of major and minor bleeding in these two trials despite the use of a femoral approach for PCI in over 80% of the cases; this is probably due to the delays between thrombolytic administration and PCI and to the use of a highly fibrin-specific thrombolytic agent and adjusted-dose heparin.
Meta-analysis of pharmacoinvasive trials
A meta-analysis29 of studies of systematic early PCI (mainly with stenting) within 24 hours of thrombolysis showed a reduction in the rates of mortality and reinfarction with this strategy, without an increase in the risk of major or intracranial bleeding.30 In contrast to the results of the trials of facilitated PCI, a pharmacoinvasive strategy improved outcomes in these trials because the delay between thrombolysis and PCI was more than 2 hours, ie, long enough to prevent bleeding complications, and because most patients randomized in these trials presented within 2 to 3 hours of symptom onset, when the time to reperfusion is critical. After 3 hours, the PCI-mediated myocardial salvage is less time-dependent. Moreover, trials of pharmacoinvasive strategy used aggressive antiplatelet therapy with clopidogrel and glycoprotein IIb/IIIa inhibitors.
Comment: Pharmacoinvasive strategy in the guidelines
These results and those of the subgroup analysis from the FINESSE trial suggest that patients with high-risk ST-elevation MI treated at non-PCI hospitals have better outcomes without an increase in major bleeding events when given thrombolysis and then immediately transferred for routine PCI, rather than being transferred only if reperfusion fails.
Hence, the 2009 update of the ACC/AHA guidelines31 gives a class IIa recommendation for transferring patients with anterior ST-elevation MI or high-risk inferior ST-elevation MI treated with thrombolysis to a PCI-capable facility where PCI is performed as part of a pharmacoinvasive or rescue strategy soon after thrombolysis.
This strategy has been particularly studied in patients younger than 75 years presenting with high-risk types of ST-elevation MI early (< 3 hours) after symptom onset. If not at high risk, the patient may be transferred to a PCI facility after receiving thrombolysis or observed in the initial facility (class IIb recommendation). Consideration should be given to starting anticoagulant and antiplatelet therapy before and during transfer—ie, 300 mg of clopidogrel before transfer for PCI and glycoprotein IIb/IIIa inhibitor therapy during PCI.
The European Society of Cardiology (ESC) guidelines32 recommend early routine angiography 3 to 24 hours after successful thrombolysis. This time window was selected to avoid PCI during the prothrombotic period in the first few hours after thrombolysis and to minimize the risk of reocclusion with PCI delays of more than 24 hours (class IIa recommendation).
Larger randomized trials are still needed to establish whether the pharmacoinvasive strategy confers a survival benefit, to determine its usefulness in low-risk inferior or lateral ST-elevation MI, and to further refine the time window when PCI is both safe and beneficial after thrombolysis.33
RESCUE PCI REDUCES MORTALITY RATES
Rescue PCI is the most accepted form of thrombolysis-PCI combination.
The REACT trial
The REACT trial20 showed that rescue PCI performed at a mean of 4.5 hours after failed thrombolysis reduces the rate of adverse cardiovascular events by more than 50% at 6 to 12 months and reduces the 5-year mortality rate by more than 50% compared with conservative management.20 As in the pharmacoinvasive strategy, aggressive antiplatelet regimens were used in the REACT trial.
A meta-analysis of rescue PCI trials
A meta-analysis of rescue PCI trials34 confirmed these results, showing a reduction in heart failure and reinfarction and a trend toward a lower mortality rate with rescue PCI.34 After thrombolysis, 40% of patients do not achieve grade 3 TIMI flow, which explains why in modern clinical trials 30% of patients treated with thrombolysis require rescue PCI.5,15,16,35
For patients with high-risk ST-elevation MI, current ACC/AHA guidelines assign a class IIa recommendation to rescue PCI.31
WHEN PATIENTS WITH ST-ELEVATION MI PRESENT TO A NON-PCI HOSPITAL
Transfer for primary PCI vs thrombolysis at the non-PCI hospital
The DANAMI-2 trial36 found that immediate transfer for PCI was superior to onsite thrombolytic therapy, as measured by a reduction in the rate of ischemic events (composite of death, myocardial infarction, or stroke at 30 days): 8.5% vs 14.2% (P < .001). There were no deaths during transfer.3
The PRAGUE-2 trial4 showed similar results for patients presenting 3 to 12 hours after symptom onset (30-day mortality rate 6% with immediate transfer vs 15.3% with on-site thrombolysis, P < .002), whereas patients presenting within 3 hours of symptom onset had a similar mortality rate with either therapy.4
Comment. These trials showed that transfer for primary PCI is superior to thrombolytic therapy when performed in a timely fashion. However, they were done in countries with established transfer networks and short distances between community hospitals and PCI centers, with a PCI-related delay of only 44 minutes and a door-to-balloon time of 90 minutes despite transfer. The large-scale application of this prompt transfer policy is not practical in most regions in the United States. Thus, a strategy of local thrombolysis followed by routine early transfer for routine or rescue PCI seems warranted when the door-to-balloon time or the PCI-related delay time is expected to be too long.
Experiences with community-based systems of care and prehospital thrombolysis
In Minnesota, Henry et al37 developed a PCI-based treatment system and an integrated transfer program for ST-elevation MI involving 30 hospitals within 210 miles of the Minneapolis Heart Institute. Participating hospitals were divided into two zones: zone 1 hospitals were within 60 miles, and zone 2 facilities were between 60 and 210 miles from the Heart Institute. Zone 2 patients received half-dose tenecteplase (if thrombolytic therapy was not contraindicated) in anticipation of a lengthy transfer time.
The median door-to-balloon time for zone 1 patients was 95 minutes (interquartile range 82 and 116 minutes) and for zone 2 patients 120 minutes (interquartile range 100 and 145 minutes). The diagnosis of ST-elevation MI was made by the emergency department physician, who activated the system with a phone call. The patient was then directly transferred to the catheterization laboratory, most often by helicopter.
The in-hospital death rate for patients who presented to the PCI center and for patients in zones 1 and 2 was similarly low (about 5%).37
In France, the FAST-MI registry,17 which collected outcome data for different reperfusion strategies, found that thrombolysis yielded in-hospital and midterm results that were comparable to those of primary PCI. Of note, thrombolysis was started early after symptom onset (about 2 hours), and was started in the ambulance in two-thirds of cases. Nearly all patients underwent a pharmacoinvasive strategy that combined thrombolysis with coronary angiography and PCI within 24 hours of symptom onset. These findings suggest that timely thrombolysis followed by semiurgent transfer for PCI is an alternative to primary PCI for patients presenting to hospitals with no PCI capability, and that this alternative offers similar benefit to that of primary PCI.
Five centers in the United States have reported their experience with half-dose thrombolysis in the prehospital setting (in the field or during transfer) or at a non-PCI hospital, followed by prompt transfer to a PCI facility. In this registry of almost 3,000 patients,38 patients treated with thrombolysis had better outcomes than patients directly transferred for primary PCI, with a significantly lower 30-day mortality rate (3.8% vs from 6.4%), and no increase in bleeding.38,39 The mean door-to-balloon time was long (168 minutes in the primary PCI group and 196 minutes in the thrombolysis-PCI group), which might explain the benefit achieved with prompt thrombolysis.
CARDIOGENIC SHOCK
Patients presenting with left ventricular cardiogenic shock derive a large mortality benefit from revascularization, whether they are transferred or directly admitted to a PCI center. 40 Moreover, in the SHOCK registry, patients with predominant right ventricular cardiogenic shock had an in-hospital mortality rate similar to that of patients with predominant left ventricular cardiogenic shock, and revascularization (PCI or surgical revascularization) was associated with a strikingly lower mortality rate in both groups.41
Thus, all patients with left or right cardiogenic shock should be revascularized on an emergency basis, either surgically or percutaneously.
While trials of pharmacoinvasive therapy excluded patients with cardiogenic shock,15,16 thrombolytic therapy was associated with improved outcomes in the drug-therapy group of the SHOCK trial and in hypotensive patients randomized in the early thrombolysis trials.13 Thus, the ACC/AHA guidelines recommend thrombolytic therapy before transfer if a patient presents in shock within 3 to 6 hours of onset of the MI and delays in transport and intervention are anticipated.8
PUTTING IT ALL TOGETHER: MANAGEMENT STRATEGIES
If an effective transfer system is in place, primary PCI not preceded by thrombolytic therapy or glycoprotein IIb/IIIa inhibitor therapy is the preferred approach, according to ACC/AHA and ESC guidelines.31,32 Giving thrombolytics immediately before PCI is harmful and thus should be avoided when the expected door-to-balloon time is 90 minutes or less.
All hospitals (whether or not they offer PCI) and regional emergency medical services should participate in a community-based system of care for ST-elevation MI, with protocols for expeditious transfer as defined and coordinated by the American Heart Association initiative “Mission: Lifeline.” In addition, a system of field triage and direct transport to the catheterization laboratory of a PCI facility after field activation significantly reduces door-to-balloon times and improves outcomes.42
If such a system is not in place, then a pharmacoinvasive strategy seems best: ie, local full-dose thrombolysis (if not contraindicated) followed by transfer to a PCI facility and routine performance of PCI 2 to 6 hours after thrombolysis—in conjunction with aggressive early dual oral antiplatelet therapy and “downstream” glycoprotein IIb/IIIa inhibition. This approach is associated with outcomes similar to those of primary PCI.15–17,37
Prehospital thrombolysis delivered by paramedics and followed by early transfer to a PCI facility has been associated with further reduction in mortality rates compared with in-hospital thrombolysis (as in the Swedish registry43), and a reduction in death rate comparable to that of primary PCI in patients presenting early. This is an adequate strategy in regions where such a system can be established.5,17,38,43,44
Patients presenting more than 3 to 4 hours after symptom onset, older patients, and patients with lower-risk MI or a higher risk of bleeding may still be suited for primary PCI even when the door-to-balloon time is 90 to 120 minutes, as stated by the European guidelines,32 or when the PCI-related delay time is as long as 100 minutes. 10 On the other hand, while the ACC/AHA guidelines recognize that in these patients the mortality advantage of primary PCI vs thrombolytic therapy is maintained with more prolonged door-to-balloon times, they nevertheless state that the focus should be on developing systems of care to increase the number of patients with access to primary PCI in less than 90 minutes rather than extending the acceptable window for door-to-balloon time.
In conclusion, for patients presenting with ST-elevation MI who cannot undergo timely primary PCI, the best approach seems to be prehospital thrombolysis delivered by paramedics or local thrombolysis at the non-PCI hospital followed by transferring the patient and performing PCI within a few hours. This is especially important in patients with high-risk ST-elevation MI who present early after symptom onset, when the extent of myocardial necrosis associated with delayed primary PCI is largest.
In addition, every community should develop a coordinated transfer strategy between non-PCI and PCI hospitals.
- Keeley EC, Boura JA, Grines CL. Primary angioplasty versus intravenous thrombolytic therapy for acute myocardial infarction: a quantitative review of 23 randomised trials. Lancet 2003; 361:13–20.
- Waters RE, Singh KP, Roe MT, et al. Rationale and strategies for implementing community-based transfer protocols for primary percutaneous coronary intervention for acute ST-segment elevation myocardial infarction. J Am Coll Cardiol 2004; 43:2153–2159.
- Chakrabarti A, Krumholz HM, Wang Y, Rumsfeld JS, Nallamothu BK; National Cardiovascular Data Registry. Time-to-reperfusion in patients undergoing interhospital transfer for primary percutaneous coronary intervention in the U.S: an analysis of 2005 and 2006 data from the National Cardiovascular Data Registry. J Am Coll Cardiol 2008; 51:2442–2443.
- Widimský P, Budesínský T, Vorác D, et al; ‘PRAGUE’ Study Group Investigators. Long distance transport for primary angioplasty vs immediate thrombolysis in acute myocardial infarction. Final results of the randomized national multicentre trial—PRAGUE-2. Eur Heart J 2003; 24:94–104.
- Steg PG, Bonnefoy E, Chabaud S, et al; Comparison of Angioplasty and Prehospital Thrombolysis in Acute Myocardial infarction (CAPTIM) Investigators. Impact of time to treatment on mortality after prehospital fibrinolysis or primary angioplasty: data from the CAPTIM randomized clinical trial. Circulation 2003; 108:2851–2856.
- Kalla K, Christ G, Karnik R, et al; Vienna STEMI Registry Group. Implementation of guidelines improves the standard of care: the Viennese registry on reperfusion strategies in ST-elevation myocardial infarction (Vienna STEMI registry). Circulation 2006; 113:2398–2405.
- Boersma E, Maas AC, Deckers JW, Simoons ML. Early thrombolytic treatment in acute myocardial infarction: reappraisal of the golden hour. Lancet 1996; 348:771–775.
- Antman EM, Anbe DT, Armstrong PW, et al; American College of Cardiology; American Heart Association Task Force on Practice Guidelines; Canadian Cardiovascular Society. ACC/AHA guidelines for the management of patients with ST-elevation myocardial infarction: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee to Revise the 1999 Guidelines for the Management of Patients with Acute Myocardial Infarction). Circulation 2004; 110:e82–e292.
- Nallamothu BK, Bates ER. Percutaneous coronary intervention versus fibrinolytic therapy in acute myocardial infarction: is timing (almost) everything? Am J Cardiol 2003; 92:824–826.
- Pinto DS, Kirtane AJ, Nallamothu BK, et al. Hospital delays in reperfusion for ST-elevation myocardial infarction: implications when selecting a reperfusion strategy. Circulation 2006; 114:2019–2025.
- Boersma E; Primary Coronary Angioplasty vs Thrombolysis Group. Does time matter? A pooled analysis of randomized clinical trials comparing primary percutaneous coronary intervention and in-hospital fibrinolysis in acute myocardial infarction patients. Eur Heart J 2006; 27:779–788.
- Stenestrand U, Wallentin L; Register of Information and Knowledge About Swedish Heart Intensive Care Admissions (RIKS-HIA). Fibrinolytic therapy in patients 75 years and older with ST-segment-elevation myocardial infarction: one-year follow-up of a large prospective cohort. Arch Intern Med 2003; 163:965–971.
- Indications for fibrinolytic therapy in suspected acute myocardial infarction: collaborative overview of early mortality and major morbidity results from all randomised trials of more than 1000 patients. Fibrinolytic Therapy Trialists’ (FTT) Collaborative Group. Lancet 1994; 343:311–322.
- Grines CL, Browne KF, Marco J, et al. A comparison of immediate angioplasty with thrombolytic therapy for acute myocardial infarction. The Primary Angioplasty in Myocardial Infarction Study Group. N Engl J Med 1993; 328:673–679.
- Cantor WJ, Fitchett D, Borgundvaag B, et al; TRANSFER-AMI Trial Investigators. Routine early angioplasty after fibrinolysis for acute myocardial infarction. N Engl J Med 2009; 360:2705–2718.
- Di Mario C, Dudek D, Piscione F, et al; CARESS-in-AMI (Combined Abciximab RE-teplase Stent Study in Acute Myocardial Infarction) Investigators. Immediate angioplasty versus standard therapy with rescue angioplasty after thrombolysis in the Combined Abciximab REteplase Stent Study in Acute Myocardial Infarction (CARESS-in-AMI): an open, prospective, randomised, multicentre trial. Lancet 2008; 371:559–568.
- Danchin N, Coste P, Ferrières J, et al; FAST-MI Investigators. Comparison of thrombolysis followed by broad use of percutaneous coronary intervention with primary percutaneous coronary intervention for ST-segment-elevation acute myocardial infarction: data from the French registry on Acute ST-elevation Myocardial Infarction (FAST-MI). Circulation 2008; 118:268–276.
- Assessment of the Safety and Efficacy of a New Treatment Strategy with Percutaneous Coronary Intervention (ASSENT-4 PCI) investigators. Primary versus tenecteplase-facilitated percutaneous coronary intervention in patients with ST-segment elevation acute myocardial infarction (ASSENT-4 PCI): randomised trial. Lancet 2006; 367:569–578.
- Ellis SG, Tendera M, de Belder MA, et al; FINESSE Investigators. Facilitated PCI in patients with ST-elevation myocardial infarction. N Engl J Med 2008; 358:2205–2217.
- Carver A, Rafelt S, Gershlick AH, Fairbrother KL, Hughes S, Wilcox R; REACT Investigators. Longer-term follow-up of patients recruited to the REACT (Rescue Angioplasty Versus Conservative Treatment or Repeat Thrombolysis) trial. J Am Coll Cardiol 2009; 54:118–126.
- Rasmanis G, Vesterqvist O, Gréen K, Edhag O, Henriksson P. Evidence of increased platelet activation after thrombolysis in patients with acute myocardial infarction. Br Heart J 1992; 68:374–376.
- Gurbel PA, Serebruany VL, Shustov AR, et al. Effects of reteplase and alteplase on platelet aggregation and major receptor expression during the first 24 hours of acute myocardial infarction treatment. GUSTO-III Investigators. Global Use of Strategies to Open Occluded Coronary Arteries. J Am Coll Cardiol 1998; 31:1466–1473.
- Coulter SA, Cannon CP, Ault KA, et al. High levels of platelet inhibition with abciximab despite heightened platelet activation and aggregation during thrombolysis for acute myocardial infarction: results from TIMI (thrombolysis in myocardial infarction) 14. Circulation 2000; 101:2690–2695.
- Ross AM, Huber K, Zeymer U, et al. The impact of place of enrollment and delay to reperfusion on 90-day post-infarction mortality in the ASSENT-4 PCI trial: assessment of the safety and efficacy of a new treatment strategy with percutaneous coronary intervention. JACC Cardiovasc Interv 2009; 2:925–930.
- Herrmann HC, Lu J, Brodie BR, et al; FINESSE Investigators. Benefit of facilitated percutaneous coronary intervention in high-risk ST-segment elevation myocardial infarction patients presenting to nonpercutaneous coronary intervention hospitals. JACC Cardiovasc Interv 2009; 2:917–924.
- Scheller B, Hennen B, Hammer B, et al; SIAM III Study Group. Beneficial effects of immediate stenting after thrombolysis in acute myocardial infarction. J Am Coll Cardiol 2003; 42:634–641.
- Fernandez-Avilés F, Alonso JJ, Castro-Beiras A, et al; GRACIA (Grupo de Análisis de la Cardiopatía Isquémica Aguda) Group. Routine invasive strategy within 24 hours of thrombolysis versus ischaemiaguided conservative approach for acute myocardial infarction with ST-segment elevation (GRACIA-1): a randomised controlled trial. Lancet 2004; 364:1045–1053.
- Le May MR, Wells GA, Labinaz M, et al. Combined angioplasty and pharmacological intervention versus thrombolysis alone in acute myocardial infarction (CAPITAL AMI study). J Am Coll Cardiol 2005; 46:417–424.
- Bøhmer E, Hoffmann P, Abdelnoor M, Arnesen H, Halvorsen S. Efficacy and safety of immediate angioplasty versus ischemia-guided management after thrombolysis in acute myocardial infarction in areas with very long transfer distances results of the NORDISTEMI (NORwegian study on DIstrict treatment of ST-elevation myocardial infarction). J Am Coll Cardiol 2010; 55:102–110.
- Wijeysundera HC, You JJ, Nallamothu BK, Krumholz HM, Cantor WJ, Ko DT. An early invasive strategy versus ischemia-guided management after fibrinolytic therapy for ST-segment elevation myocardial infarction: a meta-analysis of contemporary randomized controlled trials. Am Heart J 2008; 156:564–572,572.e1–e2.
- Kushner FG, Hand M, Smith SC, et al. 2009 focused updates: ACC/AHA guidelines for the management of patients with ST-elevation myocardial infarction (updating the 2004 guideline and 2007 focused update) and ACC/AHA/SCAI guidelines on percutaneous coronary intervention (updating the 2005 guideline and 2007 focused update) a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2009; 54:2205–2241.
- Van de Werf F, Bax J, Betriu A, et al. Management of acute myocardial infarction in patients presenting with persistent ST-segment elevation: the Task Force on the Management of ST-Segment Elevation Acute Myocardial Infarction of the European Society of Cardiology. Eur Heart J 2008; 29:2909–2945.
- Mukherjee D, Moliterno DJ. The timely coupling of mechanical revascularization following thrombolysis for myocardial infarction. Cardiology 2007; 107:337–339.
- Wijeysundera HC, Vijayaraghavan R, Nallamothu BK, et al. Rescue angioplasty or repeat fibrinolysis after failed fibrinolytic therapy for ST-segment myocardial infarction: a meta-analysis of randomized trials. J Am Coll Cardiol 2007; 49:422–430.
- The GUSTO Angiographic Investigators. The effects of tissue plasminogen activator, streptokinase, or both on coronary-artery patency, ventricular function, and survival after acute myocardial infarction. N Engl J Med 1993; 329:1615–1622.
- Andersen HR, Nielsen TT, Rasmussen K, et al; DANAMI-2 Investigators. A comparison of coronary angioplasty with fibrinolytic therapy in acute myocardial infarction. N Engl J Med 2003; 349:733–742.
- Henry TD, Sharkey SW, Burke MN, et al. A regional system to provide timely access to percutaneous coronary intervention for ST-elevation myocardial infarction. Circulation 2007; 116:721–728.
- Denktas AE, Athar H, Henry TD, et al. Reduced-dose fibrinolytic acceleration of ST-segment elevation myocardial infarction treatment coupled with urgent percutaneous coronary intervention compared to primary percutaneous coronary intervention alone results of the AMICO (Alliance for Myocardial Infarction Care Optimization) Registry. JACC Cardiovasc Interv 2008; 1:504–510.
- Smalling RW. Ischemic time: the new gold standard for ST-segment elevation myocardial infarction care. J Am Coll Cardiol 2009; 54:2154–2156.
- Hochman JS, Sleeper LA, White HD, et al; SHOCK Investigators. Should We Emergently Revascularize Occluded Coronaries for Cardiogenic Shock. One-year survival following early revascularization for cardiogenic shock. JAMA 2001; 285:190–192.
- Jacobs AK, Leopold JA, Bates E, et al. Cardiogenic shock caused by right ventricular infarction: a report from the SHOCK registry. J Am Coll Cardiol 2003; 41:1273–1279.
- Pedersen SH, Galatius S, Hansen PR, et al. Field triage reduces treatment delay and improves long-term clinical outcome in patients with acute ST-segment elevation myocardial infarction treated with primary percutaneous coronary intervention. J Am Coll Cardiol 2009; 54:2296–2302.
- Björklund E, Stenestrand U, Lindbäck J, Svensson L, Wallentin L, Lindahl B. Pre-hospital thrombolysis delivered by paramedics is associated with reduced time delay and mortality in ambulance-transported real-life patients with ST-elevation myocardial infarction. Eur Heart J 2006; 27:1146–1152.
- The European Myocardial Infarction Project Group. Prehospital thrombolytic therapy in patients with suspected acute myocardial infarction. N Engl J Med 1993; 329:383–389.
- Keeley EC, Boura JA, Grines CL. Primary angioplasty versus intravenous thrombolytic therapy for acute myocardial infarction: a quantitative review of 23 randomised trials. Lancet 2003; 361:13–20.
- Waters RE, Singh KP, Roe MT, et al. Rationale and strategies for implementing community-based transfer protocols for primary percutaneous coronary intervention for acute ST-segment elevation myocardial infarction. J Am Coll Cardiol 2004; 43:2153–2159.
- Chakrabarti A, Krumholz HM, Wang Y, Rumsfeld JS, Nallamothu BK; National Cardiovascular Data Registry. Time-to-reperfusion in patients undergoing interhospital transfer for primary percutaneous coronary intervention in the U.S: an analysis of 2005 and 2006 data from the National Cardiovascular Data Registry. J Am Coll Cardiol 2008; 51:2442–2443.
- Widimský P, Budesínský T, Vorác D, et al; ‘PRAGUE’ Study Group Investigators. Long distance transport for primary angioplasty vs immediate thrombolysis in acute myocardial infarction. Final results of the randomized national multicentre trial—PRAGUE-2. Eur Heart J 2003; 24:94–104.
- Steg PG, Bonnefoy E, Chabaud S, et al; Comparison of Angioplasty and Prehospital Thrombolysis in Acute Myocardial infarction (CAPTIM) Investigators. Impact of time to treatment on mortality after prehospital fibrinolysis or primary angioplasty: data from the CAPTIM randomized clinical trial. Circulation 2003; 108:2851–2856.
- Kalla K, Christ G, Karnik R, et al; Vienna STEMI Registry Group. Implementation of guidelines improves the standard of care: the Viennese registry on reperfusion strategies in ST-elevation myocardial infarction (Vienna STEMI registry). Circulation 2006; 113:2398–2405.
- Boersma E, Maas AC, Deckers JW, Simoons ML. Early thrombolytic treatment in acute myocardial infarction: reappraisal of the golden hour. Lancet 1996; 348:771–775.
- Antman EM, Anbe DT, Armstrong PW, et al; American College of Cardiology; American Heart Association Task Force on Practice Guidelines; Canadian Cardiovascular Society. ACC/AHA guidelines for the management of patients with ST-elevation myocardial infarction: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee to Revise the 1999 Guidelines for the Management of Patients with Acute Myocardial Infarction). Circulation 2004; 110:e82–e292.
- Nallamothu BK, Bates ER. Percutaneous coronary intervention versus fibrinolytic therapy in acute myocardial infarction: is timing (almost) everything? Am J Cardiol 2003; 92:824–826.
- Pinto DS, Kirtane AJ, Nallamothu BK, et al. Hospital delays in reperfusion for ST-elevation myocardial infarction: implications when selecting a reperfusion strategy. Circulation 2006; 114:2019–2025.
- Boersma E; Primary Coronary Angioplasty vs Thrombolysis Group. Does time matter? A pooled analysis of randomized clinical trials comparing primary percutaneous coronary intervention and in-hospital fibrinolysis in acute myocardial infarction patients. Eur Heart J 2006; 27:779–788.
- Stenestrand U, Wallentin L; Register of Information and Knowledge About Swedish Heart Intensive Care Admissions (RIKS-HIA). Fibrinolytic therapy in patients 75 years and older with ST-segment-elevation myocardial infarction: one-year follow-up of a large prospective cohort. Arch Intern Med 2003; 163:965–971.
- Indications for fibrinolytic therapy in suspected acute myocardial infarction: collaborative overview of early mortality and major morbidity results from all randomised trials of more than 1000 patients. Fibrinolytic Therapy Trialists’ (FTT) Collaborative Group. Lancet 1994; 343:311–322.
- Grines CL, Browne KF, Marco J, et al. A comparison of immediate angioplasty with thrombolytic therapy for acute myocardial infarction. The Primary Angioplasty in Myocardial Infarction Study Group. N Engl J Med 1993; 328:673–679.
- Cantor WJ, Fitchett D, Borgundvaag B, et al; TRANSFER-AMI Trial Investigators. Routine early angioplasty after fibrinolysis for acute myocardial infarction. N Engl J Med 2009; 360:2705–2718.
- Di Mario C, Dudek D, Piscione F, et al; CARESS-in-AMI (Combined Abciximab RE-teplase Stent Study in Acute Myocardial Infarction) Investigators. Immediate angioplasty versus standard therapy with rescue angioplasty after thrombolysis in the Combined Abciximab REteplase Stent Study in Acute Myocardial Infarction (CARESS-in-AMI): an open, prospective, randomised, multicentre trial. Lancet 2008; 371:559–568.
- Danchin N, Coste P, Ferrières J, et al; FAST-MI Investigators. Comparison of thrombolysis followed by broad use of percutaneous coronary intervention with primary percutaneous coronary intervention for ST-segment-elevation acute myocardial infarction: data from the French registry on Acute ST-elevation Myocardial Infarction (FAST-MI). Circulation 2008; 118:268–276.
- Assessment of the Safety and Efficacy of a New Treatment Strategy with Percutaneous Coronary Intervention (ASSENT-4 PCI) investigators. Primary versus tenecteplase-facilitated percutaneous coronary intervention in patients with ST-segment elevation acute myocardial infarction (ASSENT-4 PCI): randomised trial. Lancet 2006; 367:569–578.
- Ellis SG, Tendera M, de Belder MA, et al; FINESSE Investigators. Facilitated PCI in patients with ST-elevation myocardial infarction. N Engl J Med 2008; 358:2205–2217.
- Carver A, Rafelt S, Gershlick AH, Fairbrother KL, Hughes S, Wilcox R; REACT Investigators. Longer-term follow-up of patients recruited to the REACT (Rescue Angioplasty Versus Conservative Treatment or Repeat Thrombolysis) trial. J Am Coll Cardiol 2009; 54:118–126.
- Rasmanis G, Vesterqvist O, Gréen K, Edhag O, Henriksson P. Evidence of increased platelet activation after thrombolysis in patients with acute myocardial infarction. Br Heart J 1992; 68:374–376.
- Gurbel PA, Serebruany VL, Shustov AR, et al. Effects of reteplase and alteplase on platelet aggregation and major receptor expression during the first 24 hours of acute myocardial infarction treatment. GUSTO-III Investigators. Global Use of Strategies to Open Occluded Coronary Arteries. J Am Coll Cardiol 1998; 31:1466–1473.
- Coulter SA, Cannon CP, Ault KA, et al. High levels of platelet inhibition with abciximab despite heightened platelet activation and aggregation during thrombolysis for acute myocardial infarction: results from TIMI (thrombolysis in myocardial infarction) 14. Circulation 2000; 101:2690–2695.
- Ross AM, Huber K, Zeymer U, et al. The impact of place of enrollment and delay to reperfusion on 90-day post-infarction mortality in the ASSENT-4 PCI trial: assessment of the safety and efficacy of a new treatment strategy with percutaneous coronary intervention. JACC Cardiovasc Interv 2009; 2:925–930.
- Herrmann HC, Lu J, Brodie BR, et al; FINESSE Investigators. Benefit of facilitated percutaneous coronary intervention in high-risk ST-segment elevation myocardial infarction patients presenting to nonpercutaneous coronary intervention hospitals. JACC Cardiovasc Interv 2009; 2:917–924.
- Scheller B, Hennen B, Hammer B, et al; SIAM III Study Group. Beneficial effects of immediate stenting after thrombolysis in acute myocardial infarction. J Am Coll Cardiol 2003; 42:634–641.
- Fernandez-Avilés F, Alonso JJ, Castro-Beiras A, et al; GRACIA (Grupo de Análisis de la Cardiopatía Isquémica Aguda) Group. Routine invasive strategy within 24 hours of thrombolysis versus ischaemiaguided conservative approach for acute myocardial infarction with ST-segment elevation (GRACIA-1): a randomised controlled trial. Lancet 2004; 364:1045–1053.
- Le May MR, Wells GA, Labinaz M, et al. Combined angioplasty and pharmacological intervention versus thrombolysis alone in acute myocardial infarction (CAPITAL AMI study). J Am Coll Cardiol 2005; 46:417–424.
- Bøhmer E, Hoffmann P, Abdelnoor M, Arnesen H, Halvorsen S. Efficacy and safety of immediate angioplasty versus ischemia-guided management after thrombolysis in acute myocardial infarction in areas with very long transfer distances results of the NORDISTEMI (NORwegian study on DIstrict treatment of ST-elevation myocardial infarction). J Am Coll Cardiol 2010; 55:102–110.
- Wijeysundera HC, You JJ, Nallamothu BK, Krumholz HM, Cantor WJ, Ko DT. An early invasive strategy versus ischemia-guided management after fibrinolytic therapy for ST-segment elevation myocardial infarction: a meta-analysis of contemporary randomized controlled trials. Am Heart J 2008; 156:564–572,572.e1–e2.
- Kushner FG, Hand M, Smith SC, et al. 2009 focused updates: ACC/AHA guidelines for the management of patients with ST-elevation myocardial infarction (updating the 2004 guideline and 2007 focused update) and ACC/AHA/SCAI guidelines on percutaneous coronary intervention (updating the 2005 guideline and 2007 focused update) a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2009; 54:2205–2241.
- Van de Werf F, Bax J, Betriu A, et al. Management of acute myocardial infarction in patients presenting with persistent ST-segment elevation: the Task Force on the Management of ST-Segment Elevation Acute Myocardial Infarction of the European Society of Cardiology. Eur Heart J 2008; 29:2909–2945.
- Mukherjee D, Moliterno DJ. The timely coupling of mechanical revascularization following thrombolysis for myocardial infarction. Cardiology 2007; 107:337–339.
- Wijeysundera HC, Vijayaraghavan R, Nallamothu BK, et al. Rescue angioplasty or repeat fibrinolysis after failed fibrinolytic therapy for ST-segment myocardial infarction: a meta-analysis of randomized trials. J Am Coll Cardiol 2007; 49:422–430.
- The GUSTO Angiographic Investigators. The effects of tissue plasminogen activator, streptokinase, or both on coronary-artery patency, ventricular function, and survival after acute myocardial infarction. N Engl J Med 1993; 329:1615–1622.
- Andersen HR, Nielsen TT, Rasmussen K, et al; DANAMI-2 Investigators. A comparison of coronary angioplasty with fibrinolytic therapy in acute myocardial infarction. N Engl J Med 2003; 349:733–742.
- Henry TD, Sharkey SW, Burke MN, et al. A regional system to provide timely access to percutaneous coronary intervention for ST-elevation myocardial infarction. Circulation 2007; 116:721–728.
- Denktas AE, Athar H, Henry TD, et al. Reduced-dose fibrinolytic acceleration of ST-segment elevation myocardial infarction treatment coupled with urgent percutaneous coronary intervention compared to primary percutaneous coronary intervention alone results of the AMICO (Alliance for Myocardial Infarction Care Optimization) Registry. JACC Cardiovasc Interv 2008; 1:504–510.
- Smalling RW. Ischemic time: the new gold standard for ST-segment elevation myocardial infarction care. J Am Coll Cardiol 2009; 54:2154–2156.
- Hochman JS, Sleeper LA, White HD, et al; SHOCK Investigators. Should We Emergently Revascularize Occluded Coronaries for Cardiogenic Shock. One-year survival following early revascularization for cardiogenic shock. JAMA 2001; 285:190–192.
- Jacobs AK, Leopold JA, Bates E, et al. Cardiogenic shock caused by right ventricular infarction: a report from the SHOCK registry. J Am Coll Cardiol 2003; 41:1273–1279.
- Pedersen SH, Galatius S, Hansen PR, et al. Field triage reduces treatment delay and improves long-term clinical outcome in patients with acute ST-segment elevation myocardial infarction treated with primary percutaneous coronary intervention. J Am Coll Cardiol 2009; 54:2296–2302.
- Björklund E, Stenestrand U, Lindbäck J, Svensson L, Wallentin L, Lindahl B. Pre-hospital thrombolysis delivered by paramedics is associated with reduced time delay and mortality in ambulance-transported real-life patients with ST-elevation myocardial infarction. Eur Heart J 2006; 27:1146–1152.
- The European Myocardial Infarction Project Group. Prehospital thrombolytic therapy in patients with suspected acute myocardial infarction. N Engl J Med 1993; 329:383–389.
KEY POINTS
- When the expected door-to-balloon time is less than 90 minutes and the door-to-balloon time minus the door-to-needle time is less than 60 minutes, the preferred approach is PCI not preceded by thrombolysis.
- Evidence suggests that routine early (but not immediate) PCI—ie, 2 to 6 hours after thrombolysis—is beneficial, particularly in patients with high-risk ST-elevation MI.
- Hospitals and emergency services should participate in community-based and regional systems of care, with standardized protocols to ensure expeditious transfer and prompt reperfusion.
- Prehospital thrombolysis followed by early transfer to a PCI facility as part of a community-based system of care may further improve outcomes of patients with very long transfer times.
Exchanging the skin bleb for the test tube
Detecting latent tuberculosis is important for public health reasons, since reactivated tuberculosis can lead to infection of vulnerable populations, including those with some degree of immunosuppression due to aging, comorbid disease, or immunosuppressive therapy such as corticosteroids or anti-tumor necrosis factor-alpha agents.
The widely used tuberculin skin test is safe and relatively inexpensive, although it is unwieldy because it must be done by a person skilled in its technique, and the patient must make a second trip to a health care provider 48 to 72 hours later to have it interpreted. A previous effort to make testing more user-friendly, the tine test, proved to be less reliable.
In this issue of the Journal, Drs. Cyndee Miranda, J. Walton Tomford, and Steven M. Gordon describe the relatively new ex vivo interferon-gamma-release assays, which have received the full support of the US Centers for Disease Control and Prevention and are beginning to supplant the tuberculin skin test.
Besides solving some of the logistic problems, these newer tests have additional benefits. The skin tests detect prior exposure to several nontuberculous mycobacterial species, including Mycobacterium bovis, the strain used in the bacille Calmette-Guérin (BCG) vaccine given in many countries around the world. Because many areas where BCG is given also have a high prevalence of tuberculosis and nontuberculous mycobacterial infection, this limited specificity can cause confusion when immigrants from these areas enter the United States and undergo skin testing. Many people unnecessarily receive antibiotic therapy for assumed latent tuberculosis, due to a false-positive tuberculin skin test.
The interferon-gamma-release assays utilize more limited mycobacterial material obtained from M tuberculosis and thus have a greater specificity but a similar sensitivity.
These tests are not perfect. There are challenges with the interpretation of some results, the assay kits are relatively costly, and laboratory technicians must handle the samples and assay kits with great care. Nonetheless, I believe that these tests are a positive step towards accurate recognition and treatment of patients with latent tuberculosis.
Detecting latent tuberculosis is important for public health reasons, since reactivated tuberculosis can lead to infection of vulnerable populations, including those with some degree of immunosuppression due to aging, comorbid disease, or immunosuppressive therapy such as corticosteroids or anti-tumor necrosis factor-alpha agents.
The widely used tuberculin skin test is safe and relatively inexpensive, although it is unwieldy because it must be done by a person skilled in its technique, and the patient must make a second trip to a health care provider 48 to 72 hours later to have it interpreted. A previous effort to make testing more user-friendly, the tine test, proved to be less reliable.
In this issue of the Journal, Drs. Cyndee Miranda, J. Walton Tomford, and Steven M. Gordon describe the relatively new ex vivo interferon-gamma-release assays, which have received the full support of the US Centers for Disease Control and Prevention and are beginning to supplant the tuberculin skin test.
Besides solving some of the logistic problems, these newer tests have additional benefits. The skin tests detect prior exposure to several nontuberculous mycobacterial species, including Mycobacterium bovis, the strain used in the bacille Calmette-Guérin (BCG) vaccine given in many countries around the world. Because many areas where BCG is given also have a high prevalence of tuberculosis and nontuberculous mycobacterial infection, this limited specificity can cause confusion when immigrants from these areas enter the United States and undergo skin testing. Many people unnecessarily receive antibiotic therapy for assumed latent tuberculosis, due to a false-positive tuberculin skin test.
The interferon-gamma-release assays utilize more limited mycobacterial material obtained from M tuberculosis and thus have a greater specificity but a similar sensitivity.
These tests are not perfect. There are challenges with the interpretation of some results, the assay kits are relatively costly, and laboratory technicians must handle the samples and assay kits with great care. Nonetheless, I believe that these tests are a positive step towards accurate recognition and treatment of patients with latent tuberculosis.
Detecting latent tuberculosis is important for public health reasons, since reactivated tuberculosis can lead to infection of vulnerable populations, including those with some degree of immunosuppression due to aging, comorbid disease, or immunosuppressive therapy such as corticosteroids or anti-tumor necrosis factor-alpha agents.
The widely used tuberculin skin test is safe and relatively inexpensive, although it is unwieldy because it must be done by a person skilled in its technique, and the patient must make a second trip to a health care provider 48 to 72 hours later to have it interpreted. A previous effort to make testing more user-friendly, the tine test, proved to be less reliable.
In this issue of the Journal, Drs. Cyndee Miranda, J. Walton Tomford, and Steven M. Gordon describe the relatively new ex vivo interferon-gamma-release assays, which have received the full support of the US Centers for Disease Control and Prevention and are beginning to supplant the tuberculin skin test.
Besides solving some of the logistic problems, these newer tests have additional benefits. The skin tests detect prior exposure to several nontuberculous mycobacterial species, including Mycobacterium bovis, the strain used in the bacille Calmette-Guérin (BCG) vaccine given in many countries around the world. Because many areas where BCG is given also have a high prevalence of tuberculosis and nontuberculous mycobacterial infection, this limited specificity can cause confusion when immigrants from these areas enter the United States and undergo skin testing. Many people unnecessarily receive antibiotic therapy for assumed latent tuberculosis, due to a false-positive tuberculin skin test.
The interferon-gamma-release assays utilize more limited mycobacterial material obtained from M tuberculosis and thus have a greater specificity but a similar sensitivity.
These tests are not perfect. There are challenges with the interpretation of some results, the assay kits are relatively costly, and laboratory technicians must handle the samples and assay kits with great care. Nonetheless, I believe that these tests are a positive step towards accurate recognition and treatment of patients with latent tuberculosis.
Interferon-gamma-release assays: Better than tuberculin skin testing?
Tuberculin skin testing, long the standard method for detecting latent tuberculosis,1,2 has well-known limitations. Prior vaccination with bacille Calmette-Guérin (BCG) or exposure to other nontuberculous mycobacterial species can cause false-positive results.1,3 Errors can occur in the intradermal placement and the reading of the test. The patient must return in 48 to 72 hours for an accurate reading of the test. False-negative results can occur in severe illness or immunosuppression. And a “booster response” can occur, in which immunologic memory of an earlier skin test can provoke a false-positive response.1,3–5
Interferon-gamma-release assays are an alternative. The QuantiFERON-TB Gold test (Cellestis, Carnegie, Australia) was approved by the US Food and Drug Administration in 2001. Subsequently, two other tests were approved and are now commercially available:
- QuantiFERON-TB Gold In-Tube (QFTGIT) (Cellestis)
- T-SPOT.TB (Oxford Immunotec, Marlborough, MA).
We discuss how these tests work, focusing mainly on the QFT-GIT, and we present several cases to illustrate how they are used in preemployment screening and in sequential-testing surveillance programs for health care workers, and potential challenges in interpreting the results.
HOW THE NEW ASSAYS COMPARE WITH TUBERCULIN SKIN TESTING
Unlike tuberculin skin testing, interferongamma-release assays are blood tests.1
Either whole blood (in the QuantiFERON tests) or peripheral blood mononuclear cells (in the T-SPOT.TB test) are incubated with various tuberculosis-specific antigens. In response to the antigens, effector T cells produce interferon-gamma, which is measured quantitatively and qualitatively by either enzyme-linked immunosorbent assay (in the QuantiFERON tests) or enzymelinked immunospot assay (in the T-SPOT. TB test).1,6,7
The kit for the QFT-GIT test,6 which we use, contains three heparinized tubes for blood collection:
- A control (“nil”) tube, which contains no antigens. The purpose of this tube is to determine the patient’s “baseline” level of interferon gamma.
- A tube containing tuberculin antigens (ESAT-6, CFP-10, and TB7.7). When blood from patients who were previously exposed to Mycobacterium tuberculosis is incubated in this tube, the T cells recognizing the tuberculin antigen produce significant amounts of interferon gamma, and levels go up above that in the control tube. The level should not increase in patients not exposed to this organism.
- A tube containing mitogen, a nonspecific stimulant of interferon gamma production. This tube represents a “positive” control.
These tests appear to be unaffected by previous BCG vaccination, unlike tuberculin skin testing. A meta-analysis in 2008 reported a pooled specificity of 98% for the QuantiFERON tests: 99% in patients not vaccinated with BCG, and 96% in BCG-vaccinated patients. 8 The analysis also concluded that the T-SPOT.TB test appears to be more sensitive for latent tuberculosis than the QuantiFERON tests or tuberculin skin testing.8
HOW SHOULD THESE NEW TESTS BE USED?
In 2005 and in 2010, the US Centers for Disease Control and Prevention (CDC) recommended that interferon-gamma-release assays be used in all situations in which the skin test is currently used, “including contact investigations, evaluation of recent immigrants, and sequential-testing surveillance programs for infection control,”9 such as for health care workers. The UK National Institute for Clinical Excellence has taken a more conservative approach, suggesting that they be used only as adjuvants to tuberculin skin testing.10
In 2007, Cleveland Clinic began using the QFT-GIT test instead of the skin test for preemployment screening of health care workers for latent tuberculosis, and these workers will continue to be screened once a year with this test. Employees hired before 2007 are still being screened every year by skin testing. The number of health care workers with latent tuberculosis infection accepting isoniazid treatment for it increased when assay testing was implemented along with a process for counseling and providing treatment.11
Converting from tuberculin skin testing to interferon-gamma-release assays poses challenges. Phlebotomists need to be trained in how to collect and process the blood. Specimens must be received in the laboratory within 16 hours of collection, which may require courier service.12 Other considerations include availability of a laboratory that can process the assays.1 Also, these tests cost substantially more than the tuberculin skin test. However, one recent cost-benefit analysis13 found that in screening programs for healthcare workers, using interferon gamma release assays was clinically superior and more cost-effective than skin testing.
In the following sections, we present cases that illustrate how these new tests are used in the diagnosis of latent tuberculosis, and potential challenges in interpretation of results. We will not discuss their use for diagnosing active tuberculosis.
CASE 1: A FOREIGN-BORN HEALTH CARE WORKER WITH A POSITIVE RESULT
A 30-year-old woman, an immigrant from the Philippines, is applying for a position as a registered nurse. On preemployment screening, her QFT-GIT test is positive: 8.1 IU/mL in the tuberculin antigen tube minus 0.6 IU/mL in the nil tube, for a tuberculin response of 7.5 IU/mL. Her medical record shows that previous tuberculin skin tests were positive. Her current screening examination and chest radiograph are normal. She received BCG vaccination as a child.
Comment. This case illustrates how the assays are useful in diagnosing latent tuberculosis in foreign-born health care workers. Whereas this patient’s previous positive skin tests may have been falsely positive because of her childhood BCG vaccination, BCG vaccination does not affect the results of interferon-gamma-release assays, and thus a positive QFT-GIT test is likely to indicate latent tuberculosis.
Case continued
We believe our patient has latent tuberculosis, and we recommend isoniazid therapy. However, she does not want to take isoniazid: she says she underwent a tuberculin skin test 2 days before the QFT-GIT test, and she thinks that may have affected her QFT-GIT test result.
Comment. Can tuberculin skin testing influence the results of interferon-gamma-release assays? The question is important, considering that the UK National Institute for Health and Clinical Excellence recommends a two-step procedure, with tuberculin skin testing first, then an interferon-gamma-release assay if the skin test is positive.10
Studies have found conflicting results.14 However, van Zyl-Smit et al14 obtained blood samples for QFT-GIT and T-SPOT.TB testing in 26 South Africans at 21, 14, and 7 days before tuberculin skin testing, and also on the day of the test and at 3, 7, 28, and 84 days after. They observed higher interferon-gamma responses after tuberculin skin testing, greater than the within-subject variability. This “boosting” effect was evident on day 7 but not on day 3, leading the investigators to conclude that interferon-gamma-release assays should ideally be performed no more than 3 days after a skin test.
The Canadian guidelines15 recommend an interferon-gamma-release assay on or before the day the skin test is read if both types of tests will be used. It is important to note that interferon-gamma-release assay testing does not boost subsequent test results,9 such as when used for serial or periodic testing.
For our patient in this case, isoniazid therapy is still recommended.
CASE 2: A MAN AT LOW RISK WITH A POSITIVE RESULT
A 26-year-old man applying for a position in health data services has a positive QFT-GIT test on preemployment health screening. He was born and raised in the United States, and has no known contacts with tuberculosis. He has never had a tuberculin skin test. A chest radiograph shows no evidence of tuberculosis, and he has no symptoms. His quantitative result (ie, the interferon-gamma level in his blood incubated with tuberculin antigens, minus the interferon-gamma level in his blood cultured without antigens) is 0.37 IU/mL.
Comment. QFT-GIT results are considered positive if the tuberculin response (tuberculin antigen tube minus nil tube) is 0.35 IU/mL or higher, and at least 25% higher than in the nil sample (Table 1), so this man’s result is just above the cutoff. T-cell responses can vary from time to time in the same person and from person to person, and this variation is reflected in the 15% variance accepted by the FDA.16 Given the applicant’s history, he is unlikely to have latent tuberculosis or to need isoniazid treatment.
This case shows the importance of having the actual quantitative interferon-gamma value when evaluating a patient with a positive interferon-gamma-release assay, particularly a patient at low risk of tuberculosis.
CASE 3: SEROCONVERSION
A 59-year-old woman, born and raised in the United States and working in the hospital environmental services department, has a positive QFT-GIT result on routine annual screening. Previous tuberculin skin tests were negative, and her first QFT-GIT test result on annual screening was negative. Her chest radiograph is negative, and she has no symptoms. One year ago her QFT-GIT value (tuberculin antigen tube minus nil tube) was 0.09 IU/mL; now it is 0.61 IU/mL. A tuberculin skin test is placed and is negative.
Comment. This case illustrates “QFT-GIT conversion,” ie, a positive test result in a person who previously had negative results.17 However, as with the man in case 2, 0.61 IU/mL can also be considered a weakly positive result. If the QFT-GIT result is weakly positive and the skin test is negative, results must be interpreted with caution. Nonspecific variations can occur with serial testing, and weakly positive responses may fluctuate over time.18
Veerapathran et al18 studied the shortterm reproducibility of the QFT-GIT test in 14 health care workers who underwent serial testing; discordance was mostly noted in those who had interferon-gamma values around the cutoff point. They suggested that a QFT-GIT conversion should be defined as a change from a negative to a positive result and at least a 30% increase in the baseline interferon-gamma response.17
Also, a small prospective series in a highrisk US immigrant population showed that the QFT-GIT test had inconsistent results in 13% of those tested, particularly in those with low positive responses (< 0.69 IU/mL).19
For clinicians, the question remains whether we need to use another cutoff to distinguish new infection from nonspecific variations, and whether the cutoff should vary depending on risk of infection.
CASE 4: AN INDETERMINATE RESULT IN A WOMAN AT LOW RISK
A 65-year-old woman, also from the United States, has an indeterminate QFT-GIT result on preemployment screening. She has no known contacts with tuberculosis.
Comment. An indeterminate result can mean either that the person is immunosuppressed (in which case her blood would show a low response to mitogen; Table 1), or that there could have been errors in the performance of the test, such as improper transport, handling, or storage of the blood specimen.6 Previously at our institution, 8% of the results in our health care workers were indeterminate, a finding that led to changes in specimen collection and laboratory analysis that significantly decreased the number of indeterminate results.12 We also found that using the newer QuantiFERON test, ie, the QFT-GIT, further decreased the indeterminate rate.12
A person with an indeterminate result should be tested again and be evaluated by a physician for underlying immunosuppression or to rule out active tuberculosis (eg, via chest radiography).
There are only limited data on the use of interferon-gamma-release assays in immunosuppressed people, such as patients with human immunodeficiency virus (HIV) infection. False-negative and indeterminate results are increasingly more common in HIV patients with declining CD4 counts.20 In immunocompromised patients at high risk of infection, use of both an assay and skin testing may be reasonable.16
CASE 5: SCREENING THE CONTACTS OF A MAN WITH ACTIVE TUBERCULOSIS
A 39-year-old male health care worker is diagnosed with active tuberculosis. The QFT-GIT test is then used to determine exposure in all possible contacts.
Comment. The CDC guidelines recommend using QuantiFERON tests in all circumstances in which the tuberculin skin test has been used, including contact investigation screening.9 The QFT-GIT test can be used to screen possible contacts of infected health care workers at baseline, and it is recommended that the test be repeated 8 to 10 weeks after the exposure.9 In our experience, contact investigation has been more efficient and easier to conduct with the use of the QFT-GIT than with the tuberculin skin test.21
THE FUTURE OF TUBERCULOSIS TESTING
Given the wide availability of interferon-gamma-release assays and laboratories that process them, more tuberculosis control programs will probably start using them rather than tuberculin skin testing. Successful implementation requires education of everyone involved—phlebotomists, laboratory personnel, occupational health workers, and clinicians. Further study is needed to evaluate the feasibility, utility, cost-effectiveness, and value of using these new tests.
- Menzies D, Pai M, Comstock G. Meta-analysis: new tests for the diagnosis of latent tuberculosis infection: areas of uncertainty and recommendations for research. Ann Intern Med 2007; 146:340–354.
- Lalvani A. Diagnosing tuberculosis infection in the 21st century: new tools to tackle an old enemy. Chest 2007; 131:1898–1906.
- Andersen P, Munk ME, Pollock JM, Doherty TM. Specific immune-based diagnosis of tuberculosis. Lancet 2000; 356:1099–1104.
- Madariaga MG, Jalali Z, Swindells S. Clinical utility of interferon gamma assay in the diagnosis of tuberculosis. J Am Board Fam Med 2007; 20:540–547.
- Dewan PK, Grinsdale J, Liska S, Wong E, Fallstad R, Kawamura LM. Feasibility, acceptability, and cost of tuberculosis testing by whole-blood interferon-gamma assay. BMC Infect Dis 2006; 6:47.
- QuantiFERON®-TB GOLD (In-Tube Method) Package Insert. http://www.cellestis.com/IRM/Company/ShowPage.aspx?CPID=1023. Accessed August 11, 2010.
- T-SPOT.TB. www.oxfordimmunotec.com. Accessed August 11, 2010.
- Pai M, Zwerling A, Menzies D. Systematic review: T-cell-based assays for the diagnosis of latent tuberculosis infection: an update. Ann Intern Med 2008; 149:177–184.
- Mazurek GH, Jereb J, Vernon A, LoBue P, Goldberg S, Castro K; Division of Tuberculosis Elimination, National Center for HIV, STD, and TB Prevention, Centers for Disease Control and Prevention (CDC). Updated guidelines for using interferon gamma release assays to detect Mycobacterium tuberculosis infection. MMWR Recomm Rep 2010; 59:1–25.
- National Institute for Health and Clinical Excellence. Tuberculosis: clinical diagnosis and management of tuberculosis, and measures for its prevention and control. CG33. http://www.evidence.nhs.uk/search.aspx?t=CG33. Accessed June 10, 2010.
- Sahni R, Miranda C, Yen-Lieberman B, et al. Does the implementation of an interferon-gamma release assay in lieu of a tuberculin skin test increase acceptance of preventive therapy for latent tuberculosis among healthcare workers? Infect Control Hosp Epidemiol 2009; 30:197–199.
- Miranda C, Yen-Lieberman B, Terpeluk P, Tomford JW, Gordon S. Reducing the rates of indeterminate results of the QuantiFERON-TB Gold In-Tube test during routine preemployment screening for latent tuberculosis infection among healthcare personnel. Infect Control Hosp Epidemiol 2009; 30:296–298.
- de Perio MA, Tsevat J, Roselle GA, Kralovic SM, Eckman MH. Cost-effectiveness of interferon gamma release assays vs tuberculin skin tests in health care workers. Arch Intern Med 2009; 169:179–187.
- van Zyl-Smit RN, Pai M, Peprah K, et al. Within-subject variability and boosting of T-cell interferon-gamma responses after tuberculin skin testing. Am J Respir Crit Care Med 2009; 180:49–58.
- Canadian Tuberculosis Committee (CTC). Updated recommendations on interferon gamma release assays for latent tuberculosis infection. An Advisory Committee Statement (ACS). Can Commun Dis Rep 2008; 34:1–13.
- Nyendak MR, Lewinsohn DA, Lewinsohn DM. New diagnostic methods for tuberculosis. Curr Opin Infect Dis 2009; 22:174–182.
- Jensen PA, Lambert LA, Iademarco MF, Ridzon RCDC. Guidelines for preventing the transmission of Mycobacterium tuberculosis in health-care settings, 2005. MMWR Recomm Rep 2005; 54:1–141.
- Veerapathran A, Joshi R, Goswami K, et al. T-cell assays for tuberculosis infection: deriving cut-offs for conversions using reproducibility data. PLoS One 2008; 3:e1850.
- Perry S, Sanchez L, Yang S, Agarwal Z, Hurst P, Parsonnet J. Reproducibility of QuantiFERON-TB Gold In-Tube assay. Clin Vaccine Immunol 2008; 15:425–432.
- Lalvani A, Pareek M. A 100-year update on diagnosis of tuberculosis infection. Br Med Bull 2010; 93:69–84.
- Miranda C, Schnellinger P, Scarpeli M, Tomford JW, Fraser TG, Gordon SM. Use of interferon gamma release assay (IGRA) for contact investigation in coworkers of a fast food worker with pulmonary tuberculosis (abstract). Presented at the Annual Scientific Meeting of the Society for Healthcare Epidemiology of America; Atlanta, GA, March 18–21, 2010.
Tuberculin skin testing, long the standard method for detecting latent tuberculosis,1,2 has well-known limitations. Prior vaccination with bacille Calmette-Guérin (BCG) or exposure to other nontuberculous mycobacterial species can cause false-positive results.1,3 Errors can occur in the intradermal placement and the reading of the test. The patient must return in 48 to 72 hours for an accurate reading of the test. False-negative results can occur in severe illness or immunosuppression. And a “booster response” can occur, in which immunologic memory of an earlier skin test can provoke a false-positive response.1,3–5
Interferon-gamma-release assays are an alternative. The QuantiFERON-TB Gold test (Cellestis, Carnegie, Australia) was approved by the US Food and Drug Administration in 2001. Subsequently, two other tests were approved and are now commercially available:
- QuantiFERON-TB Gold In-Tube (QFTGIT) (Cellestis)
- T-SPOT.TB (Oxford Immunotec, Marlborough, MA).
We discuss how these tests work, focusing mainly on the QFT-GIT, and we present several cases to illustrate how they are used in preemployment screening and in sequential-testing surveillance programs for health care workers, and potential challenges in interpreting the results.
HOW THE NEW ASSAYS COMPARE WITH TUBERCULIN SKIN TESTING
Unlike tuberculin skin testing, interferongamma-release assays are blood tests.1
Either whole blood (in the QuantiFERON tests) or peripheral blood mononuclear cells (in the T-SPOT.TB test) are incubated with various tuberculosis-specific antigens. In response to the antigens, effector T cells produce interferon-gamma, which is measured quantitatively and qualitatively by either enzyme-linked immunosorbent assay (in the QuantiFERON tests) or enzymelinked immunospot assay (in the T-SPOT. TB test).1,6,7
The kit for the QFT-GIT test,6 which we use, contains three heparinized tubes for blood collection:
- A control (“nil”) tube, which contains no antigens. The purpose of this tube is to determine the patient’s “baseline” level of interferon gamma.
- A tube containing tuberculin antigens (ESAT-6, CFP-10, and TB7.7). When blood from patients who were previously exposed to Mycobacterium tuberculosis is incubated in this tube, the T cells recognizing the tuberculin antigen produce significant amounts of interferon gamma, and levels go up above that in the control tube. The level should not increase in patients not exposed to this organism.
- A tube containing mitogen, a nonspecific stimulant of interferon gamma production. This tube represents a “positive” control.
These tests appear to be unaffected by previous BCG vaccination, unlike tuberculin skin testing. A meta-analysis in 2008 reported a pooled specificity of 98% for the QuantiFERON tests: 99% in patients not vaccinated with BCG, and 96% in BCG-vaccinated patients. 8 The analysis also concluded that the T-SPOT.TB test appears to be more sensitive for latent tuberculosis than the QuantiFERON tests or tuberculin skin testing.8
HOW SHOULD THESE NEW TESTS BE USED?
In 2005 and in 2010, the US Centers for Disease Control and Prevention (CDC) recommended that interferon-gamma-release assays be used in all situations in which the skin test is currently used, “including contact investigations, evaluation of recent immigrants, and sequential-testing surveillance programs for infection control,”9 such as for health care workers. The UK National Institute for Clinical Excellence has taken a more conservative approach, suggesting that they be used only as adjuvants to tuberculin skin testing.10
In 2007, Cleveland Clinic began using the QFT-GIT test instead of the skin test for preemployment screening of health care workers for latent tuberculosis, and these workers will continue to be screened once a year with this test. Employees hired before 2007 are still being screened every year by skin testing. The number of health care workers with latent tuberculosis infection accepting isoniazid treatment for it increased when assay testing was implemented along with a process for counseling and providing treatment.11
Converting from tuberculin skin testing to interferon-gamma-release assays poses challenges. Phlebotomists need to be trained in how to collect and process the blood. Specimens must be received in the laboratory within 16 hours of collection, which may require courier service.12 Other considerations include availability of a laboratory that can process the assays.1 Also, these tests cost substantially more than the tuberculin skin test. However, one recent cost-benefit analysis13 found that in screening programs for healthcare workers, using interferon gamma release assays was clinically superior and more cost-effective than skin testing.
In the following sections, we present cases that illustrate how these new tests are used in the diagnosis of latent tuberculosis, and potential challenges in interpretation of results. We will not discuss their use for diagnosing active tuberculosis.
CASE 1: A FOREIGN-BORN HEALTH CARE WORKER WITH A POSITIVE RESULT
A 30-year-old woman, an immigrant from the Philippines, is applying for a position as a registered nurse. On preemployment screening, her QFT-GIT test is positive: 8.1 IU/mL in the tuberculin antigen tube minus 0.6 IU/mL in the nil tube, for a tuberculin response of 7.5 IU/mL. Her medical record shows that previous tuberculin skin tests were positive. Her current screening examination and chest radiograph are normal. She received BCG vaccination as a child.
Comment. This case illustrates how the assays are useful in diagnosing latent tuberculosis in foreign-born health care workers. Whereas this patient’s previous positive skin tests may have been falsely positive because of her childhood BCG vaccination, BCG vaccination does not affect the results of interferon-gamma-release assays, and thus a positive QFT-GIT test is likely to indicate latent tuberculosis.
Case continued
We believe our patient has latent tuberculosis, and we recommend isoniazid therapy. However, she does not want to take isoniazid: she says she underwent a tuberculin skin test 2 days before the QFT-GIT test, and she thinks that may have affected her QFT-GIT test result.
Comment. Can tuberculin skin testing influence the results of interferon-gamma-release assays? The question is important, considering that the UK National Institute for Health and Clinical Excellence recommends a two-step procedure, with tuberculin skin testing first, then an interferon-gamma-release assay if the skin test is positive.10
Studies have found conflicting results.14 However, van Zyl-Smit et al14 obtained blood samples for QFT-GIT and T-SPOT.TB testing in 26 South Africans at 21, 14, and 7 days before tuberculin skin testing, and also on the day of the test and at 3, 7, 28, and 84 days after. They observed higher interferon-gamma responses after tuberculin skin testing, greater than the within-subject variability. This “boosting” effect was evident on day 7 but not on day 3, leading the investigators to conclude that interferon-gamma-release assays should ideally be performed no more than 3 days after a skin test.
The Canadian guidelines15 recommend an interferon-gamma-release assay on or before the day the skin test is read if both types of tests will be used. It is important to note that interferon-gamma-release assay testing does not boost subsequent test results,9 such as when used for serial or periodic testing.
For our patient in this case, isoniazid therapy is still recommended.
CASE 2: A MAN AT LOW RISK WITH A POSITIVE RESULT
A 26-year-old man applying for a position in health data services has a positive QFT-GIT test on preemployment health screening. He was born and raised in the United States, and has no known contacts with tuberculosis. He has never had a tuberculin skin test. A chest radiograph shows no evidence of tuberculosis, and he has no symptoms. His quantitative result (ie, the interferon-gamma level in his blood incubated with tuberculin antigens, minus the interferon-gamma level in his blood cultured without antigens) is 0.37 IU/mL.
Comment. QFT-GIT results are considered positive if the tuberculin response (tuberculin antigen tube minus nil tube) is 0.35 IU/mL or higher, and at least 25% higher than in the nil sample (Table 1), so this man’s result is just above the cutoff. T-cell responses can vary from time to time in the same person and from person to person, and this variation is reflected in the 15% variance accepted by the FDA.16 Given the applicant’s history, he is unlikely to have latent tuberculosis or to need isoniazid treatment.
This case shows the importance of having the actual quantitative interferon-gamma value when evaluating a patient with a positive interferon-gamma-release assay, particularly a patient at low risk of tuberculosis.
CASE 3: SEROCONVERSION
A 59-year-old woman, born and raised in the United States and working in the hospital environmental services department, has a positive QFT-GIT result on routine annual screening. Previous tuberculin skin tests were negative, and her first QFT-GIT test result on annual screening was negative. Her chest radiograph is negative, and she has no symptoms. One year ago her QFT-GIT value (tuberculin antigen tube minus nil tube) was 0.09 IU/mL; now it is 0.61 IU/mL. A tuberculin skin test is placed and is negative.
Comment. This case illustrates “QFT-GIT conversion,” ie, a positive test result in a person who previously had negative results.17 However, as with the man in case 2, 0.61 IU/mL can also be considered a weakly positive result. If the QFT-GIT result is weakly positive and the skin test is negative, results must be interpreted with caution. Nonspecific variations can occur with serial testing, and weakly positive responses may fluctuate over time.18
Veerapathran et al18 studied the shortterm reproducibility of the QFT-GIT test in 14 health care workers who underwent serial testing; discordance was mostly noted in those who had interferon-gamma values around the cutoff point. They suggested that a QFT-GIT conversion should be defined as a change from a negative to a positive result and at least a 30% increase in the baseline interferon-gamma response.17
Also, a small prospective series in a highrisk US immigrant population showed that the QFT-GIT test had inconsistent results in 13% of those tested, particularly in those with low positive responses (< 0.69 IU/mL).19
For clinicians, the question remains whether we need to use another cutoff to distinguish new infection from nonspecific variations, and whether the cutoff should vary depending on risk of infection.
CASE 4: AN INDETERMINATE RESULT IN A WOMAN AT LOW RISK
A 65-year-old woman, also from the United States, has an indeterminate QFT-GIT result on preemployment screening. She has no known contacts with tuberculosis.
Comment. An indeterminate result can mean either that the person is immunosuppressed (in which case her blood would show a low response to mitogen; Table 1), or that there could have been errors in the performance of the test, such as improper transport, handling, or storage of the blood specimen.6 Previously at our institution, 8% of the results in our health care workers were indeterminate, a finding that led to changes in specimen collection and laboratory analysis that significantly decreased the number of indeterminate results.12 We also found that using the newer QuantiFERON test, ie, the QFT-GIT, further decreased the indeterminate rate.12
A person with an indeterminate result should be tested again and be evaluated by a physician for underlying immunosuppression or to rule out active tuberculosis (eg, via chest radiography).
There are only limited data on the use of interferon-gamma-release assays in immunosuppressed people, such as patients with human immunodeficiency virus (HIV) infection. False-negative and indeterminate results are increasingly more common in HIV patients with declining CD4 counts.20 In immunocompromised patients at high risk of infection, use of both an assay and skin testing may be reasonable.16
CASE 5: SCREENING THE CONTACTS OF A MAN WITH ACTIVE TUBERCULOSIS
A 39-year-old male health care worker is diagnosed with active tuberculosis. The QFT-GIT test is then used to determine exposure in all possible contacts.
Comment. The CDC guidelines recommend using QuantiFERON tests in all circumstances in which the tuberculin skin test has been used, including contact investigation screening.9 The QFT-GIT test can be used to screen possible contacts of infected health care workers at baseline, and it is recommended that the test be repeated 8 to 10 weeks after the exposure.9 In our experience, contact investigation has been more efficient and easier to conduct with the use of the QFT-GIT than with the tuberculin skin test.21
THE FUTURE OF TUBERCULOSIS TESTING
Given the wide availability of interferon-gamma-release assays and laboratories that process them, more tuberculosis control programs will probably start using them rather than tuberculin skin testing. Successful implementation requires education of everyone involved—phlebotomists, laboratory personnel, occupational health workers, and clinicians. Further study is needed to evaluate the feasibility, utility, cost-effectiveness, and value of using these new tests.
Tuberculin skin testing, long the standard method for detecting latent tuberculosis,1,2 has well-known limitations. Prior vaccination with bacille Calmette-Guérin (BCG) or exposure to other nontuberculous mycobacterial species can cause false-positive results.1,3 Errors can occur in the intradermal placement and the reading of the test. The patient must return in 48 to 72 hours for an accurate reading of the test. False-negative results can occur in severe illness or immunosuppression. And a “booster response” can occur, in which immunologic memory of an earlier skin test can provoke a false-positive response.1,3–5
Interferon-gamma-release assays are an alternative. The QuantiFERON-TB Gold test (Cellestis, Carnegie, Australia) was approved by the US Food and Drug Administration in 2001. Subsequently, two other tests were approved and are now commercially available:
- QuantiFERON-TB Gold In-Tube (QFTGIT) (Cellestis)
- T-SPOT.TB (Oxford Immunotec, Marlborough, MA).
We discuss how these tests work, focusing mainly on the QFT-GIT, and we present several cases to illustrate how they are used in preemployment screening and in sequential-testing surveillance programs for health care workers, and potential challenges in interpreting the results.
HOW THE NEW ASSAYS COMPARE WITH TUBERCULIN SKIN TESTING
Unlike tuberculin skin testing, interferongamma-release assays are blood tests.1
Either whole blood (in the QuantiFERON tests) or peripheral blood mononuclear cells (in the T-SPOT.TB test) are incubated with various tuberculosis-specific antigens. In response to the antigens, effector T cells produce interferon-gamma, which is measured quantitatively and qualitatively by either enzyme-linked immunosorbent assay (in the QuantiFERON tests) or enzymelinked immunospot assay (in the T-SPOT. TB test).1,6,7
The kit for the QFT-GIT test,6 which we use, contains three heparinized tubes for blood collection:
- A control (“nil”) tube, which contains no antigens. The purpose of this tube is to determine the patient’s “baseline” level of interferon gamma.
- A tube containing tuberculin antigens (ESAT-6, CFP-10, and TB7.7). When blood from patients who were previously exposed to Mycobacterium tuberculosis is incubated in this tube, the T cells recognizing the tuberculin antigen produce significant amounts of interferon gamma, and levels go up above that in the control tube. The level should not increase in patients not exposed to this organism.
- A tube containing mitogen, a nonspecific stimulant of interferon gamma production. This tube represents a “positive” control.
These tests appear to be unaffected by previous BCG vaccination, unlike tuberculin skin testing. A meta-analysis in 2008 reported a pooled specificity of 98% for the QuantiFERON tests: 99% in patients not vaccinated with BCG, and 96% in BCG-vaccinated patients. 8 The analysis also concluded that the T-SPOT.TB test appears to be more sensitive for latent tuberculosis than the QuantiFERON tests or tuberculin skin testing.8
HOW SHOULD THESE NEW TESTS BE USED?
In 2005 and in 2010, the US Centers for Disease Control and Prevention (CDC) recommended that interferon-gamma-release assays be used in all situations in which the skin test is currently used, “including contact investigations, evaluation of recent immigrants, and sequential-testing surveillance programs for infection control,”9 such as for health care workers. The UK National Institute for Clinical Excellence has taken a more conservative approach, suggesting that they be used only as adjuvants to tuberculin skin testing.10
In 2007, Cleveland Clinic began using the QFT-GIT test instead of the skin test for preemployment screening of health care workers for latent tuberculosis, and these workers will continue to be screened once a year with this test. Employees hired before 2007 are still being screened every year by skin testing. The number of health care workers with latent tuberculosis infection accepting isoniazid treatment for it increased when assay testing was implemented along with a process for counseling and providing treatment.11
Converting from tuberculin skin testing to interferon-gamma-release assays poses challenges. Phlebotomists need to be trained in how to collect and process the blood. Specimens must be received in the laboratory within 16 hours of collection, which may require courier service.12 Other considerations include availability of a laboratory that can process the assays.1 Also, these tests cost substantially more than the tuberculin skin test. However, one recent cost-benefit analysis13 found that in screening programs for healthcare workers, using interferon gamma release assays was clinically superior and more cost-effective than skin testing.
In the following sections, we present cases that illustrate how these new tests are used in the diagnosis of latent tuberculosis, and potential challenges in interpretation of results. We will not discuss their use for diagnosing active tuberculosis.
CASE 1: A FOREIGN-BORN HEALTH CARE WORKER WITH A POSITIVE RESULT
A 30-year-old woman, an immigrant from the Philippines, is applying for a position as a registered nurse. On preemployment screening, her QFT-GIT test is positive: 8.1 IU/mL in the tuberculin antigen tube minus 0.6 IU/mL in the nil tube, for a tuberculin response of 7.5 IU/mL. Her medical record shows that previous tuberculin skin tests were positive. Her current screening examination and chest radiograph are normal. She received BCG vaccination as a child.
Comment. This case illustrates how the assays are useful in diagnosing latent tuberculosis in foreign-born health care workers. Whereas this patient’s previous positive skin tests may have been falsely positive because of her childhood BCG vaccination, BCG vaccination does not affect the results of interferon-gamma-release assays, and thus a positive QFT-GIT test is likely to indicate latent tuberculosis.
Case continued
We believe our patient has latent tuberculosis, and we recommend isoniazid therapy. However, she does not want to take isoniazid: she says she underwent a tuberculin skin test 2 days before the QFT-GIT test, and she thinks that may have affected her QFT-GIT test result.
Comment. Can tuberculin skin testing influence the results of interferon-gamma-release assays? The question is important, considering that the UK National Institute for Health and Clinical Excellence recommends a two-step procedure, with tuberculin skin testing first, then an interferon-gamma-release assay if the skin test is positive.10
Studies have found conflicting results.14 However, van Zyl-Smit et al14 obtained blood samples for QFT-GIT and T-SPOT.TB testing in 26 South Africans at 21, 14, and 7 days before tuberculin skin testing, and also on the day of the test and at 3, 7, 28, and 84 days after. They observed higher interferon-gamma responses after tuberculin skin testing, greater than the within-subject variability. This “boosting” effect was evident on day 7 but not on day 3, leading the investigators to conclude that interferon-gamma-release assays should ideally be performed no more than 3 days after a skin test.
The Canadian guidelines15 recommend an interferon-gamma-release assay on or before the day the skin test is read if both types of tests will be used. It is important to note that interferon-gamma-release assay testing does not boost subsequent test results,9 such as when used for serial or periodic testing.
For our patient in this case, isoniazid therapy is still recommended.
CASE 2: A MAN AT LOW RISK WITH A POSITIVE RESULT
A 26-year-old man applying for a position in health data services has a positive QFT-GIT test on preemployment health screening. He was born and raised in the United States, and has no known contacts with tuberculosis. He has never had a tuberculin skin test. A chest radiograph shows no evidence of tuberculosis, and he has no symptoms. His quantitative result (ie, the interferon-gamma level in his blood incubated with tuberculin antigens, minus the interferon-gamma level in his blood cultured without antigens) is 0.37 IU/mL.
Comment. QFT-GIT results are considered positive if the tuberculin response (tuberculin antigen tube minus nil tube) is 0.35 IU/mL or higher, and at least 25% higher than in the nil sample (Table 1), so this man’s result is just above the cutoff. T-cell responses can vary from time to time in the same person and from person to person, and this variation is reflected in the 15% variance accepted by the FDA.16 Given the applicant’s history, he is unlikely to have latent tuberculosis or to need isoniazid treatment.
This case shows the importance of having the actual quantitative interferon-gamma value when evaluating a patient with a positive interferon-gamma-release assay, particularly a patient at low risk of tuberculosis.
CASE 3: SEROCONVERSION
A 59-year-old woman, born and raised in the United States and working in the hospital environmental services department, has a positive QFT-GIT result on routine annual screening. Previous tuberculin skin tests were negative, and her first QFT-GIT test result on annual screening was negative. Her chest radiograph is negative, and she has no symptoms. One year ago her QFT-GIT value (tuberculin antigen tube minus nil tube) was 0.09 IU/mL; now it is 0.61 IU/mL. A tuberculin skin test is placed and is negative.
Comment. This case illustrates “QFT-GIT conversion,” ie, a positive test result in a person who previously had negative results.17 However, as with the man in case 2, 0.61 IU/mL can also be considered a weakly positive result. If the QFT-GIT result is weakly positive and the skin test is negative, results must be interpreted with caution. Nonspecific variations can occur with serial testing, and weakly positive responses may fluctuate over time.18
Veerapathran et al18 studied the shortterm reproducibility of the QFT-GIT test in 14 health care workers who underwent serial testing; discordance was mostly noted in those who had interferon-gamma values around the cutoff point. They suggested that a QFT-GIT conversion should be defined as a change from a negative to a positive result and at least a 30% increase in the baseline interferon-gamma response.17
Also, a small prospective series in a highrisk US immigrant population showed that the QFT-GIT test had inconsistent results in 13% of those tested, particularly in those with low positive responses (< 0.69 IU/mL).19
For clinicians, the question remains whether we need to use another cutoff to distinguish new infection from nonspecific variations, and whether the cutoff should vary depending on risk of infection.
CASE 4: AN INDETERMINATE RESULT IN A WOMAN AT LOW RISK
A 65-year-old woman, also from the United States, has an indeterminate QFT-GIT result on preemployment screening. She has no known contacts with tuberculosis.
Comment. An indeterminate result can mean either that the person is immunosuppressed (in which case her blood would show a low response to mitogen; Table 1), or that there could have been errors in the performance of the test, such as improper transport, handling, or storage of the blood specimen.6 Previously at our institution, 8% of the results in our health care workers were indeterminate, a finding that led to changes in specimen collection and laboratory analysis that significantly decreased the number of indeterminate results.12 We also found that using the newer QuantiFERON test, ie, the QFT-GIT, further decreased the indeterminate rate.12
A person with an indeterminate result should be tested again and be evaluated by a physician for underlying immunosuppression or to rule out active tuberculosis (eg, via chest radiography).
There are only limited data on the use of interferon-gamma-release assays in immunosuppressed people, such as patients with human immunodeficiency virus (HIV) infection. False-negative and indeterminate results are increasingly more common in HIV patients with declining CD4 counts.20 In immunocompromised patients at high risk of infection, use of both an assay and skin testing may be reasonable.16
CASE 5: SCREENING THE CONTACTS OF A MAN WITH ACTIVE TUBERCULOSIS
A 39-year-old male health care worker is diagnosed with active tuberculosis. The QFT-GIT test is then used to determine exposure in all possible contacts.
Comment. The CDC guidelines recommend using QuantiFERON tests in all circumstances in which the tuberculin skin test has been used, including contact investigation screening.9 The QFT-GIT test can be used to screen possible contacts of infected health care workers at baseline, and it is recommended that the test be repeated 8 to 10 weeks after the exposure.9 In our experience, contact investigation has been more efficient and easier to conduct with the use of the QFT-GIT than with the tuberculin skin test.21
THE FUTURE OF TUBERCULOSIS TESTING
Given the wide availability of interferon-gamma-release assays and laboratories that process them, more tuberculosis control programs will probably start using them rather than tuberculin skin testing. Successful implementation requires education of everyone involved—phlebotomists, laboratory personnel, occupational health workers, and clinicians. Further study is needed to evaluate the feasibility, utility, cost-effectiveness, and value of using these new tests.
- Menzies D, Pai M, Comstock G. Meta-analysis: new tests for the diagnosis of latent tuberculosis infection: areas of uncertainty and recommendations for research. Ann Intern Med 2007; 146:340–354.
- Lalvani A. Diagnosing tuberculosis infection in the 21st century: new tools to tackle an old enemy. Chest 2007; 131:1898–1906.
- Andersen P, Munk ME, Pollock JM, Doherty TM. Specific immune-based diagnosis of tuberculosis. Lancet 2000; 356:1099–1104.
- Madariaga MG, Jalali Z, Swindells S. Clinical utility of interferon gamma assay in the diagnosis of tuberculosis. J Am Board Fam Med 2007; 20:540–547.
- Dewan PK, Grinsdale J, Liska S, Wong E, Fallstad R, Kawamura LM. Feasibility, acceptability, and cost of tuberculosis testing by whole-blood interferon-gamma assay. BMC Infect Dis 2006; 6:47.
- QuantiFERON®-TB GOLD (In-Tube Method) Package Insert. http://www.cellestis.com/IRM/Company/ShowPage.aspx?CPID=1023. Accessed August 11, 2010.
- T-SPOT.TB. www.oxfordimmunotec.com. Accessed August 11, 2010.
- Pai M, Zwerling A, Menzies D. Systematic review: T-cell-based assays for the diagnosis of latent tuberculosis infection: an update. Ann Intern Med 2008; 149:177–184.
- Mazurek GH, Jereb J, Vernon A, LoBue P, Goldberg S, Castro K; Division of Tuberculosis Elimination, National Center for HIV, STD, and TB Prevention, Centers for Disease Control and Prevention (CDC). Updated guidelines for using interferon gamma release assays to detect Mycobacterium tuberculosis infection. MMWR Recomm Rep 2010; 59:1–25.
- National Institute for Health and Clinical Excellence. Tuberculosis: clinical diagnosis and management of tuberculosis, and measures for its prevention and control. CG33. http://www.evidence.nhs.uk/search.aspx?t=CG33. Accessed June 10, 2010.
- Sahni R, Miranda C, Yen-Lieberman B, et al. Does the implementation of an interferon-gamma release assay in lieu of a tuberculin skin test increase acceptance of preventive therapy for latent tuberculosis among healthcare workers? Infect Control Hosp Epidemiol 2009; 30:197–199.
- Miranda C, Yen-Lieberman B, Terpeluk P, Tomford JW, Gordon S. Reducing the rates of indeterminate results of the QuantiFERON-TB Gold In-Tube test during routine preemployment screening for latent tuberculosis infection among healthcare personnel. Infect Control Hosp Epidemiol 2009; 30:296–298.
- de Perio MA, Tsevat J, Roselle GA, Kralovic SM, Eckman MH. Cost-effectiveness of interferon gamma release assays vs tuberculin skin tests in health care workers. Arch Intern Med 2009; 169:179–187.
- van Zyl-Smit RN, Pai M, Peprah K, et al. Within-subject variability and boosting of T-cell interferon-gamma responses after tuberculin skin testing. Am J Respir Crit Care Med 2009; 180:49–58.
- Canadian Tuberculosis Committee (CTC). Updated recommendations on interferon gamma release assays for latent tuberculosis infection. An Advisory Committee Statement (ACS). Can Commun Dis Rep 2008; 34:1–13.
- Nyendak MR, Lewinsohn DA, Lewinsohn DM. New diagnostic methods for tuberculosis. Curr Opin Infect Dis 2009; 22:174–182.
- Jensen PA, Lambert LA, Iademarco MF, Ridzon RCDC. Guidelines for preventing the transmission of Mycobacterium tuberculosis in health-care settings, 2005. MMWR Recomm Rep 2005; 54:1–141.
- Veerapathran A, Joshi R, Goswami K, et al. T-cell assays for tuberculosis infection: deriving cut-offs for conversions using reproducibility data. PLoS One 2008; 3:e1850.
- Perry S, Sanchez L, Yang S, Agarwal Z, Hurst P, Parsonnet J. Reproducibility of QuantiFERON-TB Gold In-Tube assay. Clin Vaccine Immunol 2008; 15:425–432.
- Lalvani A, Pareek M. A 100-year update on diagnosis of tuberculosis infection. Br Med Bull 2010; 93:69–84.
- Miranda C, Schnellinger P, Scarpeli M, Tomford JW, Fraser TG, Gordon SM. Use of interferon gamma release assay (IGRA) for contact investigation in coworkers of a fast food worker with pulmonary tuberculosis (abstract). Presented at the Annual Scientific Meeting of the Society for Healthcare Epidemiology of America; Atlanta, GA, March 18–21, 2010.
- Menzies D, Pai M, Comstock G. Meta-analysis: new tests for the diagnosis of latent tuberculosis infection: areas of uncertainty and recommendations for research. Ann Intern Med 2007; 146:340–354.
- Lalvani A. Diagnosing tuberculosis infection in the 21st century: new tools to tackle an old enemy. Chest 2007; 131:1898–1906.
- Andersen P, Munk ME, Pollock JM, Doherty TM. Specific immune-based diagnosis of tuberculosis. Lancet 2000; 356:1099–1104.
- Madariaga MG, Jalali Z, Swindells S. Clinical utility of interferon gamma assay in the diagnosis of tuberculosis. J Am Board Fam Med 2007; 20:540–547.
- Dewan PK, Grinsdale J, Liska S, Wong E, Fallstad R, Kawamura LM. Feasibility, acceptability, and cost of tuberculosis testing by whole-blood interferon-gamma assay. BMC Infect Dis 2006; 6:47.
- QuantiFERON®-TB GOLD (In-Tube Method) Package Insert. http://www.cellestis.com/IRM/Company/ShowPage.aspx?CPID=1023. Accessed August 11, 2010.
- T-SPOT.TB. www.oxfordimmunotec.com. Accessed August 11, 2010.
- Pai M, Zwerling A, Menzies D. Systematic review: T-cell-based assays for the diagnosis of latent tuberculosis infection: an update. Ann Intern Med 2008; 149:177–184.
- Mazurek GH, Jereb J, Vernon A, LoBue P, Goldberg S, Castro K; Division of Tuberculosis Elimination, National Center for HIV, STD, and TB Prevention, Centers for Disease Control and Prevention (CDC). Updated guidelines for using interferon gamma release assays to detect Mycobacterium tuberculosis infection. MMWR Recomm Rep 2010; 59:1–25.
- National Institute for Health and Clinical Excellence. Tuberculosis: clinical diagnosis and management of tuberculosis, and measures for its prevention and control. CG33. http://www.evidence.nhs.uk/search.aspx?t=CG33. Accessed June 10, 2010.
- Sahni R, Miranda C, Yen-Lieberman B, et al. Does the implementation of an interferon-gamma release assay in lieu of a tuberculin skin test increase acceptance of preventive therapy for latent tuberculosis among healthcare workers? Infect Control Hosp Epidemiol 2009; 30:197–199.
- Miranda C, Yen-Lieberman B, Terpeluk P, Tomford JW, Gordon S. Reducing the rates of indeterminate results of the QuantiFERON-TB Gold In-Tube test during routine preemployment screening for latent tuberculosis infection among healthcare personnel. Infect Control Hosp Epidemiol 2009; 30:296–298.
- de Perio MA, Tsevat J, Roselle GA, Kralovic SM, Eckman MH. Cost-effectiveness of interferon gamma release assays vs tuberculin skin tests in health care workers. Arch Intern Med 2009; 169:179–187.
- van Zyl-Smit RN, Pai M, Peprah K, et al. Within-subject variability and boosting of T-cell interferon-gamma responses after tuberculin skin testing. Am J Respir Crit Care Med 2009; 180:49–58.
- Canadian Tuberculosis Committee (CTC). Updated recommendations on interferon gamma release assays for latent tuberculosis infection. An Advisory Committee Statement (ACS). Can Commun Dis Rep 2008; 34:1–13.
- Nyendak MR, Lewinsohn DA, Lewinsohn DM. New diagnostic methods for tuberculosis. Curr Opin Infect Dis 2009; 22:174–182.
- Jensen PA, Lambert LA, Iademarco MF, Ridzon RCDC. Guidelines for preventing the transmission of Mycobacterium tuberculosis in health-care settings, 2005. MMWR Recomm Rep 2005; 54:1–141.
- Veerapathran A, Joshi R, Goswami K, et al. T-cell assays for tuberculosis infection: deriving cut-offs for conversions using reproducibility data. PLoS One 2008; 3:e1850.
- Perry S, Sanchez L, Yang S, Agarwal Z, Hurst P, Parsonnet J. Reproducibility of QuantiFERON-TB Gold In-Tube assay. Clin Vaccine Immunol 2008; 15:425–432.
- Lalvani A, Pareek M. A 100-year update on diagnosis of tuberculosis infection. Br Med Bull 2010; 93:69–84.
- Miranda C, Schnellinger P, Scarpeli M, Tomford JW, Fraser TG, Gordon SM. Use of interferon gamma release assay (IGRA) for contact investigation in coworkers of a fast food worker with pulmonary tuberculosis (abstract). Presented at the Annual Scientific Meeting of the Society for Healthcare Epidemiology of America; Atlanta, GA, March 18–21, 2010.
KEY POINTS
- Prior vaccination with bacille Calmette-Guérin can cause the results of skin testing to be falsely positive, but it does not affect interferon-gamma-release assays.
- In 2005, the US Centers for Disease Control and Prevention recommended that interferon-gamma-release assays be used in all situations in which skin testing is currently used. Updated guidelines were published on June 25, 2010.
- Successful implementation of interferon-gamma-release assay testing requires education of everyone involved—phlebotomists, laboratory personnel, occupational health workers, and clinicians.
Open Mesenteric Approach Still Useful
SCOTTSDALE, ARIZ. -- Despite the fact that open surgery for patients with chronic mesenteric ischemia tends to be reserved for sicker, more complicated patients who aren't good candidates for endovascular revascularization, outcomes have not declined over time.
A single-center review of 116 patients with 203 obstructed mesenteric arteries who underwent open repairs since 1998 found no significant differences in outcomes in 58 patients treated before endovascular treatment became the norm and 58 treated with open surgery during the endovascular era. "We believe that open revascularization still plays an important role in the treatment of this disease," Dr. Evan Ryer and his associates reported at the annual meeting of the Society for Clinical Vascular Surgery.
All patients were symptomatic and had open surgery at the Mayo Clinic, Rochester, Minn., which adopted endovascular treatment in 2002 for most cases of chronic mesenteric ischemia. Starting in that year, approximately 70% of patients with the disease were treated using endovascular revascularization.
"Since 2002, open mesenteric revascularization has been used in only 58 of 176 patients (33%) treated for chronic mesenteric ischemia at the Mayo Clinic," Dr. Ryer said in an interview. "Endovascular revascularization, which is our primary modality of treatment in most patients with suitable lesions, was not performed in these cases because it had failed previously or the anatomy was considered unfavorable because of chronic occlusion, severe calcification, or long-segment stenosis," he explained.
Patients in the pre-endovascular era (1998-2001) and post-endovascular era (2002-2009) who underwent open surgery reported similar durations of symptoms and degrees of weight loss. Compared with the pre-endovascular era patients, however, the post-endovascular era group had significantly higher rates of hypertension (86% vs. 66%), hyperlipidemia (76% vs. 36%), coronary artery disease requiring intervention (29% vs. 14%), cardiac dysrhythmias (28% vs. 7%), postprandial pain (88% vs. 72%), food fear (71% vs. 45%), and need for total parenteral nutrition (10% vs. 2%), as well as higher Society for Vascular Surgery comorbidity severity scores (7 vs. 5).
The extent of disease was greater in post-endovascular era patients, who were more likely to have three-vessel disease (79% vs. 59%) and occluded superior mesenteric arteries (67% vs. 45%) compared with pre-endovascular era patients, Dr. Ryer said. Two-vessel disease accounted for 81% of cases in the pre-endovascular era and 69% of cases in the post-endovascular era. Only 1% of patients in the post-endovascular era and none in the earlier time period had single-vessel disease. The differences between eras in two- and single-vessel disease rates were not significant.
The two time periods did not differ significantly in the technical details of the open procedures or in any outcomes, he added.
In the pre- and post-endovascular eras, patients averaged 4 and 5 days in the intensive care unit, respectively, and 13 and 12 days in the hospital. Among short-term outcomes, symptoms improved in 56% and 54% of patients treated in the pre- and post-endovascular eras, respectively. Two patients in the earlier era and three patients in the more recent era died, and major complications developed in 17 and 21 patients, respectively. These differences between groups were not significant.
After 5 years of follow-up, survival rates were 84% for pre-endovascular era patients and 78% for those in the post-endovascular era. Recurrence-free survival rates were 84% and 76%, respectively, primary patency rates were 82% and 81%, and secondary patency rates were 86% and 82%. None of the differences in outcomes were significant between groups.
The investigators declared they had no conflicts.
SCOTTSDALE, ARIZ. -- Despite the fact that open surgery for patients with chronic mesenteric ischemia tends to be reserved for sicker, more complicated patients who aren't good candidates for endovascular revascularization, outcomes have not declined over time.
A single-center review of 116 patients with 203 obstructed mesenteric arteries who underwent open repairs since 1998 found no significant differences in outcomes in 58 patients treated before endovascular treatment became the norm and 58 treated with open surgery during the endovascular era. "We believe that open revascularization still plays an important role in the treatment of this disease," Dr. Evan Ryer and his associates reported at the annual meeting of the Society for Clinical Vascular Surgery.
All patients were symptomatic and had open surgery at the Mayo Clinic, Rochester, Minn., which adopted endovascular treatment in 2002 for most cases of chronic mesenteric ischemia. Starting in that year, approximately 70% of patients with the disease were treated using endovascular revascularization.
"Since 2002, open mesenteric revascularization has been used in only 58 of 176 patients (33%) treated for chronic mesenteric ischemia at the Mayo Clinic," Dr. Ryer said in an interview. "Endovascular revascularization, which is our primary modality of treatment in most patients with suitable lesions, was not performed in these cases because it had failed previously or the anatomy was considered unfavorable because of chronic occlusion, severe calcification, or long-segment stenosis," he explained.
Patients in the pre-endovascular era (1998-2001) and post-endovascular era (2002-2009) who underwent open surgery reported similar durations of symptoms and degrees of weight loss. Compared with the pre-endovascular era patients, however, the post-endovascular era group had significantly higher rates of hypertension (86% vs. 66%), hyperlipidemia (76% vs. 36%), coronary artery disease requiring intervention (29% vs. 14%), cardiac dysrhythmias (28% vs. 7%), postprandial pain (88% vs. 72%), food fear (71% vs. 45%), and need for total parenteral nutrition (10% vs. 2%), as well as higher Society for Vascular Surgery comorbidity severity scores (7 vs. 5).
The extent of disease was greater in post-endovascular era patients, who were more likely to have three-vessel disease (79% vs. 59%) and occluded superior mesenteric arteries (67% vs. 45%) compared with pre-endovascular era patients, Dr. Ryer said. Two-vessel disease accounted for 81% of cases in the pre-endovascular era and 69% of cases in the post-endovascular era. Only 1% of patients in the post-endovascular era and none in the earlier time period had single-vessel disease. The differences between eras in two- and single-vessel disease rates were not significant.
The two time periods did not differ significantly in the technical details of the open procedures or in any outcomes, he added.
In the pre- and post-endovascular eras, patients averaged 4 and 5 days in the intensive care unit, respectively, and 13 and 12 days in the hospital. Among short-term outcomes, symptoms improved in 56% and 54% of patients treated in the pre- and post-endovascular eras, respectively. Two patients in the earlier era and three patients in the more recent era died, and major complications developed in 17 and 21 patients, respectively. These differences between groups were not significant.
After 5 years of follow-up, survival rates were 84% for pre-endovascular era patients and 78% for those in the post-endovascular era. Recurrence-free survival rates were 84% and 76%, respectively, primary patency rates were 82% and 81%, and secondary patency rates were 86% and 82%. None of the differences in outcomes were significant between groups.
The investigators declared they had no conflicts.
SCOTTSDALE, ARIZ. -- Despite the fact that open surgery for patients with chronic mesenteric ischemia tends to be reserved for sicker, more complicated patients who aren't good candidates for endovascular revascularization, outcomes have not declined over time.
A single-center review of 116 patients with 203 obstructed mesenteric arteries who underwent open repairs since 1998 found no significant differences in outcomes in 58 patients treated before endovascular treatment became the norm and 58 treated with open surgery during the endovascular era. "We believe that open revascularization still plays an important role in the treatment of this disease," Dr. Evan Ryer and his associates reported at the annual meeting of the Society for Clinical Vascular Surgery.
All patients were symptomatic and had open surgery at the Mayo Clinic, Rochester, Minn., which adopted endovascular treatment in 2002 for most cases of chronic mesenteric ischemia. Starting in that year, approximately 70% of patients with the disease were treated using endovascular revascularization.
"Since 2002, open mesenteric revascularization has been used in only 58 of 176 patients (33%) treated for chronic mesenteric ischemia at the Mayo Clinic," Dr. Ryer said in an interview. "Endovascular revascularization, which is our primary modality of treatment in most patients with suitable lesions, was not performed in these cases because it had failed previously or the anatomy was considered unfavorable because of chronic occlusion, severe calcification, or long-segment stenosis," he explained.
Patients in the pre-endovascular era (1998-2001) and post-endovascular era (2002-2009) who underwent open surgery reported similar durations of symptoms and degrees of weight loss. Compared with the pre-endovascular era patients, however, the post-endovascular era group had significantly higher rates of hypertension (86% vs. 66%), hyperlipidemia (76% vs. 36%), coronary artery disease requiring intervention (29% vs. 14%), cardiac dysrhythmias (28% vs. 7%), postprandial pain (88% vs. 72%), food fear (71% vs. 45%), and need for total parenteral nutrition (10% vs. 2%), as well as higher Society for Vascular Surgery comorbidity severity scores (7 vs. 5).
The extent of disease was greater in post-endovascular era patients, who were more likely to have three-vessel disease (79% vs. 59%) and occluded superior mesenteric arteries (67% vs. 45%) compared with pre-endovascular era patients, Dr. Ryer said. Two-vessel disease accounted for 81% of cases in the pre-endovascular era and 69% of cases in the post-endovascular era. Only 1% of patients in the post-endovascular era and none in the earlier time period had single-vessel disease. The differences between eras in two- and single-vessel disease rates were not significant.
The two time periods did not differ significantly in the technical details of the open procedures or in any outcomes, he added.
In the pre- and post-endovascular eras, patients averaged 4 and 5 days in the intensive care unit, respectively, and 13 and 12 days in the hospital. Among short-term outcomes, symptoms improved in 56% and 54% of patients treated in the pre- and post-endovascular eras, respectively. Two patients in the earlier era and three patients in the more recent era died, and major complications developed in 17 and 21 patients, respectively. These differences between groups were not significant.
After 5 years of follow-up, survival rates were 84% for pre-endovascular era patients and 78% for those in the post-endovascular era. Recurrence-free survival rates were 84% and 76%, respectively, primary patency rates were 82% and 81%, and secondary patency rates were 86% and 82%. None of the differences in outcomes were significant between groups.
The investigators declared they had no conflicts.
Update on Pediatric Psoriasis, Part 1: Clinical Features and Demographics
Excessive opioids blamed for respiratory arrest…A rising PSA, but no evaluation…A hemorrhoid…or something else?
Excessive opioids blamed for respiratory arrest
A MIDNIGHT VISIT TO THE HOSPITAL prompted by abdominal pain, nausea, and vomiting led to a diagnosis of acute pancreatitis and secondary conditions in a 67-year-old woman. She was admitted to the intensive care unit (ICU) and given pain medication, including Demerol, morphine, and a fentanyl transdermal patch, despite the fact that she was opioid naïve, with no tolerance to strong opioid-based medications. A black box warning for fentanyl specifies that it should not be administered to opioid-naïve patients for acute or short-term pain.
During her stay in the ICU, the patient received increasing amounts of pain medication. On the third day, a physician prescribed almost 10 times the dose given on the previous day. The patient subsequently suffered respiratory arrest, resulting in brain damage that left her with no short-term memory and in need of full-time care.
PLAINTIFF’S CLAIM Excessive administration of opioids caused respiratory arrest and brain damage.
THE DEFENSE Respiratory arrest resulted from the patient’s underlying illnesses, not opioid overdose. The patient did not show typical signs of overdose, such as slowed heart rate and decreased breathing, and was, in fact, agitated up to the time she went into respiratory arrest.
VERDICT Confidential Missouri settlement.
COMMENT I’m seeing many malpractice suits involving the prescription of opioids. Caution and due diligence are essential.
A rising PSA, but no evaluation
A 59-YEAR-OLD MAN received a prostate-specific antigen (PSA) score of 2.0 in 2003. In 2006, his score was 5.26. His primary care physician didn’t evaluate him for prostate cancer.
A year later, the patient complained of frequent, slow urination. A digital rectal examination revealed a hardened, nodular prostate. The patient’s PSA was 209. A biopsy showed stage 4 terminal prostate cancer. Computed tomography and bone scans of the abdomen and pelvis indicated metastasis to lymph nodes and bones. The patient wasn’t a candidate for surgery or radiation.
PLAINTIFF’S CLAIM The patient had been diagnosed with benign prostatic hypertrophy in 2005 and 2006, but had received no further evaluation. A biopsy should have been performed in 2003, at the time of the initial PSA test. If the cancer had been diagnosed and treated with radiation then, the patient’s condition wouldn’t have become terminal.
THE DEFENSE No information about the defense is available.
VERDICT $500,000 California settlement.
COMMENT We may disagree with the assessment that more aggressive evaluation would have been lifesaving. Nonetheless, the lack of follow-up and discussion with the patient makes for a very unfortunate situation.
A hemorrhoid…or something else?
WHILE GIVING BIRTH TO HER SECOND CHILD, a 35-year-old woman sustained a second-degree vaginal tear that required repair. The physician who performed the repair noticed a large hemorrhoid and told a nurse midwife to have it evaluated with a possible gastroenterological consult to rule out a mass. The next day, another doctor and midwife examined the patient. They agreed with the patient to defer a gastroenterology consult and have the patient follow up with her primary care physician in a few weeks.
When the patient saw her primary care physician 3 weeks after delivery, her exam revealed no hemorrhoids; she was instructed to call back if the hemorrhoids recurred. The hemorrhoids didn’t recur, and the patient didn’t follow up with her primary care physician.
During the next 4 years, the patient received care from her gynecologist that didn’t include rectal examinations. Five years after delivery, the patient went to her primary care physician complaining of rectal bleeding with bowel movements. The physician found no external hemorrhoids but noted a rectal mass.
He referred the patient for a gastroenterology consult and biopsy, which revealed intramucosal adenocarcinoma. A computed tomography (CT) scan of the chest showed a nodule in the lower lobe of the right lung, which was suspected to be a metastasis. An abdominal CT scan and a positron-emission tomography scan indicated likely liver metastasis. A liver biopsy confi rmed adenocarcinoma.
The patient underwent chemotherapy and chemoradiation followed several months later by abdominal perineal resection, left lateral segmentectomy of the liver, cholecystectomy, and appendectomy. At the time of the settlement, she was doing well and receiving no cancer treatment.
PLAINTIFF’S CLAIM The primary care physician should have followed up on the rectal finding, which would have led to earlier diagnosis and treatment of the cancer.
THE DEFENSE The finding made at the time of the delivery was a simple hemorrhoid, which went away after delivery. The absence of symptoms for 4½ years indicated that the cancer couldn’t have been present at the time of delivery. The diagnosed cancer was in a different place than the original hemorrhoid.
VERDICT $1 million Massachusetts settlement.
COMMENT The folly of the failed hand off. One of the most common root causes of litigation is poor communication that results in a bad outcome. How many lives could be saved simply by phone calls between physicians?
Excessive opioids blamed for respiratory arrest
A MIDNIGHT VISIT TO THE HOSPITAL prompted by abdominal pain, nausea, and vomiting led to a diagnosis of acute pancreatitis and secondary conditions in a 67-year-old woman. She was admitted to the intensive care unit (ICU) and given pain medication, including Demerol, morphine, and a fentanyl transdermal patch, despite the fact that she was opioid naïve, with no tolerance to strong opioid-based medications. A black box warning for fentanyl specifies that it should not be administered to opioid-naïve patients for acute or short-term pain.
During her stay in the ICU, the patient received increasing amounts of pain medication. On the third day, a physician prescribed almost 10 times the dose given on the previous day. The patient subsequently suffered respiratory arrest, resulting in brain damage that left her with no short-term memory and in need of full-time care.
PLAINTIFF’S CLAIM Excessive administration of opioids caused respiratory arrest and brain damage.
THE DEFENSE Respiratory arrest resulted from the patient’s underlying illnesses, not opioid overdose. The patient did not show typical signs of overdose, such as slowed heart rate and decreased breathing, and was, in fact, agitated up to the time she went into respiratory arrest.
VERDICT Confidential Missouri settlement.
COMMENT I’m seeing many malpractice suits involving the prescription of opioids. Caution and due diligence are essential.
A rising PSA, but no evaluation
A 59-YEAR-OLD MAN received a prostate-specific antigen (PSA) score of 2.0 in 2003. In 2006, his score was 5.26. His primary care physician didn’t evaluate him for prostate cancer.
A year later, the patient complained of frequent, slow urination. A digital rectal examination revealed a hardened, nodular prostate. The patient’s PSA was 209. A biopsy showed stage 4 terminal prostate cancer. Computed tomography and bone scans of the abdomen and pelvis indicated metastasis to lymph nodes and bones. The patient wasn’t a candidate for surgery or radiation.
PLAINTIFF’S CLAIM The patient had been diagnosed with benign prostatic hypertrophy in 2005 and 2006, but had received no further evaluation. A biopsy should have been performed in 2003, at the time of the initial PSA test. If the cancer had been diagnosed and treated with radiation then, the patient’s condition wouldn’t have become terminal.
THE DEFENSE No information about the defense is available.
VERDICT $500,000 California settlement.
COMMENT We may disagree with the assessment that more aggressive evaluation would have been lifesaving. Nonetheless, the lack of follow-up and discussion with the patient makes for a very unfortunate situation.
A hemorrhoid…or something else?
WHILE GIVING BIRTH TO HER SECOND CHILD, a 35-year-old woman sustained a second-degree vaginal tear that required repair. The physician who performed the repair noticed a large hemorrhoid and told a nurse midwife to have it evaluated with a possible gastroenterological consult to rule out a mass. The next day, another doctor and midwife examined the patient. They agreed with the patient to defer a gastroenterology consult and have the patient follow up with her primary care physician in a few weeks.
When the patient saw her primary care physician 3 weeks after delivery, her exam revealed no hemorrhoids; she was instructed to call back if the hemorrhoids recurred. The hemorrhoids didn’t recur, and the patient didn’t follow up with her primary care physician.
During the next 4 years, the patient received care from her gynecologist that didn’t include rectal examinations. Five years after delivery, the patient went to her primary care physician complaining of rectal bleeding with bowel movements. The physician found no external hemorrhoids but noted a rectal mass.
He referred the patient for a gastroenterology consult and biopsy, which revealed intramucosal adenocarcinoma. A computed tomography (CT) scan of the chest showed a nodule in the lower lobe of the right lung, which was suspected to be a metastasis. An abdominal CT scan and a positron-emission tomography scan indicated likely liver metastasis. A liver biopsy confi rmed adenocarcinoma.
The patient underwent chemotherapy and chemoradiation followed several months later by abdominal perineal resection, left lateral segmentectomy of the liver, cholecystectomy, and appendectomy. At the time of the settlement, she was doing well and receiving no cancer treatment.
PLAINTIFF’S CLAIM The primary care physician should have followed up on the rectal finding, which would have led to earlier diagnosis and treatment of the cancer.
THE DEFENSE The finding made at the time of the delivery was a simple hemorrhoid, which went away after delivery. The absence of symptoms for 4½ years indicated that the cancer couldn’t have been present at the time of delivery. The diagnosed cancer was in a different place than the original hemorrhoid.
VERDICT $1 million Massachusetts settlement.
COMMENT The folly of the failed hand off. One of the most common root causes of litigation is poor communication that results in a bad outcome. How many lives could be saved simply by phone calls between physicians?
Excessive opioids blamed for respiratory arrest
A MIDNIGHT VISIT TO THE HOSPITAL prompted by abdominal pain, nausea, and vomiting led to a diagnosis of acute pancreatitis and secondary conditions in a 67-year-old woman. She was admitted to the intensive care unit (ICU) and given pain medication, including Demerol, morphine, and a fentanyl transdermal patch, despite the fact that she was opioid naïve, with no tolerance to strong opioid-based medications. A black box warning for fentanyl specifies that it should not be administered to opioid-naïve patients for acute or short-term pain.
During her stay in the ICU, the patient received increasing amounts of pain medication. On the third day, a physician prescribed almost 10 times the dose given on the previous day. The patient subsequently suffered respiratory arrest, resulting in brain damage that left her with no short-term memory and in need of full-time care.
PLAINTIFF’S CLAIM Excessive administration of opioids caused respiratory arrest and brain damage.
THE DEFENSE Respiratory arrest resulted from the patient’s underlying illnesses, not opioid overdose. The patient did not show typical signs of overdose, such as slowed heart rate and decreased breathing, and was, in fact, agitated up to the time she went into respiratory arrest.
VERDICT Confidential Missouri settlement.
COMMENT I’m seeing many malpractice suits involving the prescription of opioids. Caution and due diligence are essential.
A rising PSA, but no evaluation
A 59-YEAR-OLD MAN received a prostate-specific antigen (PSA) score of 2.0 in 2003. In 2006, his score was 5.26. His primary care physician didn’t evaluate him for prostate cancer.
A year later, the patient complained of frequent, slow urination. A digital rectal examination revealed a hardened, nodular prostate. The patient’s PSA was 209. A biopsy showed stage 4 terminal prostate cancer. Computed tomography and bone scans of the abdomen and pelvis indicated metastasis to lymph nodes and bones. The patient wasn’t a candidate for surgery or radiation.
PLAINTIFF’S CLAIM The patient had been diagnosed with benign prostatic hypertrophy in 2005 and 2006, but had received no further evaluation. A biopsy should have been performed in 2003, at the time of the initial PSA test. If the cancer had been diagnosed and treated with radiation then, the patient’s condition wouldn’t have become terminal.
THE DEFENSE No information about the defense is available.
VERDICT $500,000 California settlement.
COMMENT We may disagree with the assessment that more aggressive evaluation would have been lifesaving. Nonetheless, the lack of follow-up and discussion with the patient makes for a very unfortunate situation.
A hemorrhoid…or something else?
WHILE GIVING BIRTH TO HER SECOND CHILD, a 35-year-old woman sustained a second-degree vaginal tear that required repair. The physician who performed the repair noticed a large hemorrhoid and told a nurse midwife to have it evaluated with a possible gastroenterological consult to rule out a mass. The next day, another doctor and midwife examined the patient. They agreed with the patient to defer a gastroenterology consult and have the patient follow up with her primary care physician in a few weeks.
When the patient saw her primary care physician 3 weeks after delivery, her exam revealed no hemorrhoids; she was instructed to call back if the hemorrhoids recurred. The hemorrhoids didn’t recur, and the patient didn’t follow up with her primary care physician.
During the next 4 years, the patient received care from her gynecologist that didn’t include rectal examinations. Five years after delivery, the patient went to her primary care physician complaining of rectal bleeding with bowel movements. The physician found no external hemorrhoids but noted a rectal mass.
He referred the patient for a gastroenterology consult and biopsy, which revealed intramucosal adenocarcinoma. A computed tomography (CT) scan of the chest showed a nodule in the lower lobe of the right lung, which was suspected to be a metastasis. An abdominal CT scan and a positron-emission tomography scan indicated likely liver metastasis. A liver biopsy confi rmed adenocarcinoma.
The patient underwent chemotherapy and chemoradiation followed several months later by abdominal perineal resection, left lateral segmentectomy of the liver, cholecystectomy, and appendectomy. At the time of the settlement, she was doing well and receiving no cancer treatment.
PLAINTIFF’S CLAIM The primary care physician should have followed up on the rectal finding, which would have led to earlier diagnosis and treatment of the cancer.
THE DEFENSE The finding made at the time of the delivery was a simple hemorrhoid, which went away after delivery. The absence of symptoms for 4½ years indicated that the cancer couldn’t have been present at the time of delivery. The diagnosed cancer was in a different place than the original hemorrhoid.
VERDICT $1 million Massachusetts settlement.
COMMENT The folly of the failed hand off. One of the most common root causes of litigation is poor communication that results in a bad outcome. How many lives could be saved simply by phone calls between physicians?
Another option for patients with liver disease
Consider prescribing rifaximin for patients with hepatic encephalopathy, not only as a treatment for acute episodes but also to prevent a recurrence.1
STRENGTH OF RECOMMENDATION:
A: Based on a high-quality randomized controlled trial (RCT)
Bass NM, Mullen KD, Sanyal A, et al. Rifaximin treatment in hepatic encephalopathy. N Engl J Med. 2010;362:1071-1081.
ILLUSTRATIVE CASE
A 64-year-old patient with chronic liver disease has been hospitalized on 3 occasions for hepatic encephalopathy, all while he was taking lactulose. He is still taking it, but wonders if there are other ways to prevent future episodes of hepatic encephalopathy. What can you tell him?
Characterized by periods of impaired cognition of varying severity, hepatic encephalopathy is a common complication of chronic liver disease—and a frequent cause of hospitalization, morbidity, and mortality in this patient population. Up to 70% of patients with cirrhosis may have some degree of hepatic encephalopathy,2 which can occur without provocation or be triggered by gastrointestinal (GI) bleeding, infection, kidney disease, electrolyte abnormalities, shunt placement, respiratory disease, or anemia. Hepatic encephalopathy is thought to be caused by elevated ammonia levels.
Current first-line treatment is not problem-free
Patients with chronic liver disease and hepatic encephalopathy are often placed on nonabsorbable disaccharides, such as lactulose, to prevent recurrent hepatic encephalopathy. However, disaccharides’ effectiveness as prophylaxis is unproven.3 In addition, many patients have difficulty tolerating lactulose because of its taste and GI side effects.
A 2004 Cochrane review examined the effectiveness of lactulose in preventing hepatic encephalopathy.3 The reviewers also compared the effectiveness of an oral antibiotic, rifaximin, with lactulose for this purpose. Rifaximin, like lactu-lose, is believed to work by reducing ammonia in the gut. The antibiotic is a well-established treatment for acute hepatic encephalopathy, but not widely used for preventive purposes.
The reviewers found rifaximin to be more effective compared with lactulose at preventing recurrent episodes of hepatic encephalopathy (number needed to treat [NNT]=11).3 Other studies have also suggested that the antibiotic, which has minimal systemic absorption, may be as effective as, or more effective than, lactu-lose in preventing recurrences.4,5 The new RCT detailed in this PURL took another look at rifaximin’s usefulness as prophylaxis.
STUDY SUMMARY: Patients on rifaximin had better outcomes
The study by Bass et al was a double-blinded RCT enrolling 299 patients with chronic liver disease.1 Criteria for inclusion were age ≥18 years, a minimum of 2 prior episodes of hepatic encephalopathy, remission from hepatic encephalopathy at the time of enrollment, and mild to moderate liver disease severity, defined as a score ≤25 on the Model for End-Stage Liver Disease (MELD) scale.6 (The scale ranges from 6 to 40, with higher numbers indicating more severe disease.) The researchers excluded patients for whom liver transplant was imminent and those with conditions that precipitate hepatic encephalopathy, as described earlier.
Patients were assigned to either rifaximin 550 mg twice a day (140 patients) or placebo (159 patients) for 6 months. Both groups had similar baseline characteristics, including a high percentage of subjects (>90%) with concomitant lactulose use. The researchers assessed the patients at clinic visits every 2 weeks, both by their Conn score (the scale commonly used to grade hepatic encephalopathy) and grade of asterixis, and during telephone calls on alternate weeks. Analysis was by intention-to-treat.
The primary endpoint was the mean time to the first episode of hepatic encephalopathy, which was 130.0 (±56.5) days in the rifaximin group and 105.7 (±62.7) days in the control group. During the 6-month study period, 22% of patients in the rifaximin group experienced a breakthrough hepatic encephalopathy event, vs 45.9% of the placebo group (95% confidence interval, 0.28-0.64; P<0.001; hazard ratio=0.42; NNT=9). Both groups had high rates of compliance (~84%) and high rates of adverse events (80%). Two patients receiving rifaximin experienced Clostridium difficile infections, from which they recovered. Death rates were similar in both groups, and were attributed to liver disease progression.
WHAT’S NEW?: FDA approves rifaximin to prevent recurrence
This trial adds further support for the use of rifaximin in the prevention of recurrent episodes of hepatic encephalopathy. In addition, the US Food and Drug Administration approved the antibiotic for that purpose in March of this year.7 Given the lack of proven, well-tolerated treatments to prevent hepatic encephalopathy in patients with liver disease and the significant morbidity and mortality associated with this complication, family physicians should consider prescribing rifaximin for patients with prior episodes of hepatic encephalopathy. Rifaximin resistance is not common and, because its activity is concentrated in the gut, resistance is unlikely to become a significant issue.
CAVEATS: Long-term safety has not been established
Because of this study’s short duration (6 months) and relatively small sample size, we cannot be certain of its long-term effects or safety. However, patients with advanced liver disease and recurrent hepatic encephalopathy have a poor prognosis, and a treatment that is effective, even if just for 6 months, is meaningful.
Also, because this study excluded patients with more severe liver disease (MELD score >25), we have no data to guide the use of rifaximin in this patient population. However, the mechanism of action and risk of adverse effects are likely to be similar.
Finally, the manufacturer of the drug was involved in the study design, data collection, data analysis, and manuscript preparation.
CHALLENGES TO IMPLEMENTATION: Drug cost and coverage are potential barriers
Rifaximin is available in the United States in 200- and 550-mg tablets, so it can be dosed at 1100 or 1200 mg per day in divided doses. The drug is not generic, however, and is costly: A month’s supply of the 550-mg tablets is about $1300 (a supply of the 200-mg tablets is even more expensive),8 and the drug may not be covered by insurance.
Acknowledgement
The PURls Surveillance System is supported in part by Grant number UL1RR024999 from the National Center for Research Resources; the grant was a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources or the National Institutes of Health.
Click here to view PURL METHODOLOGY
1. Bass NM, Mullen KD, Sanyal A, et al. Rifaximin treatment in hepatic encephalopathy. N Engl J Med. 2010;362:1071-1081.
2. Riordan SM, Williams R. Treatment of hepatic encephalopathy. N Engl J Med. 1997;337:473-479.
3. Als-Nielsen B, Gluud LL, Gluud C. Nonabsorbable disaccharides for hepatic encephalopathy. Cochrane Database Syst Rev. 2004;(2):CD003044.-
4. Paik YH, Lee KS, Han KH, et al. Comparison of rifaximin and lactulose for the treatment of hepatic encephalopathy: a prospective randomized study. Yonsei Med J. 2005;46:399-407.
5. Lawrence KR, Klee JA. Rifaximin for the treatment of hepatic encephalopathy. Pharmacotherapy. 2008;28:1019-1032.
6. Mayo Clinic. The MELD model, UNOS modification. Available at: http://www.mayoclinic.org/meld/mayomodel6.html. Accessed August 16, 2010.
7. US Food and Drug Administration. FDA approves new use of Xifaxan for patients with liver disease. Available at: http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm206104.htm. Updated March 26, 2010. Accessed July 7, 2010.
8. Drugstore.com. Available at: http://www.drugstore.com/. Accessed August 20, 2010.
Consider prescribing rifaximin for patients with hepatic encephalopathy, not only as a treatment for acute episodes but also to prevent a recurrence.1
STRENGTH OF RECOMMENDATION:
A: Based on a high-quality randomized controlled trial (RCT)
Bass NM, Mullen KD, Sanyal A, et al. Rifaximin treatment in hepatic encephalopathy. N Engl J Med. 2010;362:1071-1081.
ILLUSTRATIVE CASE
A 64-year-old patient with chronic liver disease has been hospitalized on 3 occasions for hepatic encephalopathy, all while he was taking lactulose. He is still taking it, but wonders if there are other ways to prevent future episodes of hepatic encephalopathy. What can you tell him?
Characterized by periods of impaired cognition of varying severity, hepatic encephalopathy is a common complication of chronic liver disease—and a frequent cause of hospitalization, morbidity, and mortality in this patient population. Up to 70% of patients with cirrhosis may have some degree of hepatic encephalopathy,2 which can occur without provocation or be triggered by gastrointestinal (GI) bleeding, infection, kidney disease, electrolyte abnormalities, shunt placement, respiratory disease, or anemia. Hepatic encephalopathy is thought to be caused by elevated ammonia levels.
Current first-line treatment is not problem-free
Patients with chronic liver disease and hepatic encephalopathy are often placed on nonabsorbable disaccharides, such as lactulose, to prevent recurrent hepatic encephalopathy. However, disaccharides’ effectiveness as prophylaxis is unproven.3 In addition, many patients have difficulty tolerating lactulose because of its taste and GI side effects.
A 2004 Cochrane review examined the effectiveness of lactulose in preventing hepatic encephalopathy.3 The reviewers also compared the effectiveness of an oral antibiotic, rifaximin, with lactulose for this purpose. Rifaximin, like lactu-lose, is believed to work by reducing ammonia in the gut. The antibiotic is a well-established treatment for acute hepatic encephalopathy, but not widely used for preventive purposes.
The reviewers found rifaximin to be more effective compared with lactulose at preventing recurrent episodes of hepatic encephalopathy (number needed to treat [NNT]=11).3 Other studies have also suggested that the antibiotic, which has minimal systemic absorption, may be as effective as, or more effective than, lactu-lose in preventing recurrences.4,5 The new RCT detailed in this PURL took another look at rifaximin’s usefulness as prophylaxis.
STUDY SUMMARY: Patients on rifaximin had better outcomes
The study by Bass et al was a double-blinded RCT enrolling 299 patients with chronic liver disease.1 Criteria for inclusion were age ≥18 years, a minimum of 2 prior episodes of hepatic encephalopathy, remission from hepatic encephalopathy at the time of enrollment, and mild to moderate liver disease severity, defined as a score ≤25 on the Model for End-Stage Liver Disease (MELD) scale.6 (The scale ranges from 6 to 40, with higher numbers indicating more severe disease.) The researchers excluded patients for whom liver transplant was imminent and those with conditions that precipitate hepatic encephalopathy, as described earlier.
Patients were assigned to either rifaximin 550 mg twice a day (140 patients) or placebo (159 patients) for 6 months. Both groups had similar baseline characteristics, including a high percentage of subjects (>90%) with concomitant lactulose use. The researchers assessed the patients at clinic visits every 2 weeks, both by their Conn score (the scale commonly used to grade hepatic encephalopathy) and grade of asterixis, and during telephone calls on alternate weeks. Analysis was by intention-to-treat.
The primary endpoint was the mean time to the first episode of hepatic encephalopathy, which was 130.0 (±56.5) days in the rifaximin group and 105.7 (±62.7) days in the control group. During the 6-month study period, 22% of patients in the rifaximin group experienced a breakthrough hepatic encephalopathy event, vs 45.9% of the placebo group (95% confidence interval, 0.28-0.64; P<0.001; hazard ratio=0.42; NNT=9). Both groups had high rates of compliance (~84%) and high rates of adverse events (80%). Two patients receiving rifaximin experienced Clostridium difficile infections, from which they recovered. Death rates were similar in both groups, and were attributed to liver disease progression.
WHAT’S NEW?: FDA approves rifaximin to prevent recurrence
This trial adds further support for the use of rifaximin in the prevention of recurrent episodes of hepatic encephalopathy. In addition, the US Food and Drug Administration approved the antibiotic for that purpose in March of this year.7 Given the lack of proven, well-tolerated treatments to prevent hepatic encephalopathy in patients with liver disease and the significant morbidity and mortality associated with this complication, family physicians should consider prescribing rifaximin for patients with prior episodes of hepatic encephalopathy. Rifaximin resistance is not common and, because its activity is concentrated in the gut, resistance is unlikely to become a significant issue.
CAVEATS: Long-term safety has not been established
Because of this study’s short duration (6 months) and relatively small sample size, we cannot be certain of its long-term effects or safety. However, patients with advanced liver disease and recurrent hepatic encephalopathy have a poor prognosis, and a treatment that is effective, even if just for 6 months, is meaningful.
Also, because this study excluded patients with more severe liver disease (MELD score >25), we have no data to guide the use of rifaximin in this patient population. However, the mechanism of action and risk of adverse effects are likely to be similar.
Finally, the manufacturer of the drug was involved in the study design, data collection, data analysis, and manuscript preparation.
CHALLENGES TO IMPLEMENTATION: Drug cost and coverage are potential barriers
Rifaximin is available in the United States in 200- and 550-mg tablets, so it can be dosed at 1100 or 1200 mg per day in divided doses. The drug is not generic, however, and is costly: A month’s supply of the 550-mg tablets is about $1300 (a supply of the 200-mg tablets is even more expensive),8 and the drug may not be covered by insurance.
Acknowledgement
The PURls Surveillance System is supported in part by Grant number UL1RR024999 from the National Center for Research Resources; the grant was a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources or the National Institutes of Health.
Click here to view PURL METHODOLOGY
Consider prescribing rifaximin for patients with hepatic encephalopathy, not only as a treatment for acute episodes but also to prevent a recurrence.1
STRENGTH OF RECOMMENDATION:
A: Based on a high-quality randomized controlled trial (RCT)
Bass NM, Mullen KD, Sanyal A, et al. Rifaximin treatment in hepatic encephalopathy. N Engl J Med. 2010;362:1071-1081.
ILLUSTRATIVE CASE
A 64-year-old patient with chronic liver disease has been hospitalized on 3 occasions for hepatic encephalopathy, all while he was taking lactulose. He is still taking it, but wonders if there are other ways to prevent future episodes of hepatic encephalopathy. What can you tell him?
Characterized by periods of impaired cognition of varying severity, hepatic encephalopathy is a common complication of chronic liver disease—and a frequent cause of hospitalization, morbidity, and mortality in this patient population. Up to 70% of patients with cirrhosis may have some degree of hepatic encephalopathy,2 which can occur without provocation or be triggered by gastrointestinal (GI) bleeding, infection, kidney disease, electrolyte abnormalities, shunt placement, respiratory disease, or anemia. Hepatic encephalopathy is thought to be caused by elevated ammonia levels.
Current first-line treatment is not problem-free
Patients with chronic liver disease and hepatic encephalopathy are often placed on nonabsorbable disaccharides, such as lactulose, to prevent recurrent hepatic encephalopathy. However, disaccharides’ effectiveness as prophylaxis is unproven.3 In addition, many patients have difficulty tolerating lactulose because of its taste and GI side effects.
A 2004 Cochrane review examined the effectiveness of lactulose in preventing hepatic encephalopathy.3 The reviewers also compared the effectiveness of an oral antibiotic, rifaximin, with lactulose for this purpose. Rifaximin, like lactu-lose, is believed to work by reducing ammonia in the gut. The antibiotic is a well-established treatment for acute hepatic encephalopathy, but not widely used for preventive purposes.
The reviewers found rifaximin to be more effective compared with lactulose at preventing recurrent episodes of hepatic encephalopathy (number needed to treat [NNT]=11).3 Other studies have also suggested that the antibiotic, which has minimal systemic absorption, may be as effective as, or more effective than, lactu-lose in preventing recurrences.4,5 The new RCT detailed in this PURL took another look at rifaximin’s usefulness as prophylaxis.
STUDY SUMMARY: Patients on rifaximin had better outcomes
The study by Bass et al was a double-blinded RCT enrolling 299 patients with chronic liver disease.1 Criteria for inclusion were age ≥18 years, a minimum of 2 prior episodes of hepatic encephalopathy, remission from hepatic encephalopathy at the time of enrollment, and mild to moderate liver disease severity, defined as a score ≤25 on the Model for End-Stage Liver Disease (MELD) scale.6 (The scale ranges from 6 to 40, with higher numbers indicating more severe disease.) The researchers excluded patients for whom liver transplant was imminent and those with conditions that precipitate hepatic encephalopathy, as described earlier.
Patients were assigned to either rifaximin 550 mg twice a day (140 patients) or placebo (159 patients) for 6 months. Both groups had similar baseline characteristics, including a high percentage of subjects (>90%) with concomitant lactulose use. The researchers assessed the patients at clinic visits every 2 weeks, both by their Conn score (the scale commonly used to grade hepatic encephalopathy) and grade of asterixis, and during telephone calls on alternate weeks. Analysis was by intention-to-treat.
The primary endpoint was the mean time to the first episode of hepatic encephalopathy, which was 130.0 (±56.5) days in the rifaximin group and 105.7 (±62.7) days in the control group. During the 6-month study period, 22% of patients in the rifaximin group experienced a breakthrough hepatic encephalopathy event, vs 45.9% of the placebo group (95% confidence interval, 0.28-0.64; P<0.001; hazard ratio=0.42; NNT=9). Both groups had high rates of compliance (~84%) and high rates of adverse events (80%). Two patients receiving rifaximin experienced Clostridium difficile infections, from which they recovered. Death rates were similar in both groups, and were attributed to liver disease progression.
WHAT’S NEW?: FDA approves rifaximin to prevent recurrence
This trial adds further support for the use of rifaximin in the prevention of recurrent episodes of hepatic encephalopathy. In addition, the US Food and Drug Administration approved the antibiotic for that purpose in March of this year.7 Given the lack of proven, well-tolerated treatments to prevent hepatic encephalopathy in patients with liver disease and the significant morbidity and mortality associated with this complication, family physicians should consider prescribing rifaximin for patients with prior episodes of hepatic encephalopathy. Rifaximin resistance is not common and, because its activity is concentrated in the gut, resistance is unlikely to become a significant issue.
CAVEATS: Long-term safety has not been established
Because of this study’s short duration (6 months) and relatively small sample size, we cannot be certain of its long-term effects or safety. However, patients with advanced liver disease and recurrent hepatic encephalopathy have a poor prognosis, and a treatment that is effective, even if just for 6 months, is meaningful.
Also, because this study excluded patients with more severe liver disease (MELD score >25), we have no data to guide the use of rifaximin in this patient population. However, the mechanism of action and risk of adverse effects are likely to be similar.
Finally, the manufacturer of the drug was involved in the study design, data collection, data analysis, and manuscript preparation.
CHALLENGES TO IMPLEMENTATION: Drug cost and coverage are potential barriers
Rifaximin is available in the United States in 200- and 550-mg tablets, so it can be dosed at 1100 or 1200 mg per day in divided doses. The drug is not generic, however, and is costly: A month’s supply of the 550-mg tablets is about $1300 (a supply of the 200-mg tablets is even more expensive),8 and the drug may not be covered by insurance.
Acknowledgement
The PURls Surveillance System is supported in part by Grant number UL1RR024999 from the National Center for Research Resources; the grant was a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources or the National Institutes of Health.
Click here to view PURL METHODOLOGY
1. Bass NM, Mullen KD, Sanyal A, et al. Rifaximin treatment in hepatic encephalopathy. N Engl J Med. 2010;362:1071-1081.
2. Riordan SM, Williams R. Treatment of hepatic encephalopathy. N Engl J Med. 1997;337:473-479.
3. Als-Nielsen B, Gluud LL, Gluud C. Nonabsorbable disaccharides for hepatic encephalopathy. Cochrane Database Syst Rev. 2004;(2):CD003044.-
4. Paik YH, Lee KS, Han KH, et al. Comparison of rifaximin and lactulose for the treatment of hepatic encephalopathy: a prospective randomized study. Yonsei Med J. 2005;46:399-407.
5. Lawrence KR, Klee JA. Rifaximin for the treatment of hepatic encephalopathy. Pharmacotherapy. 2008;28:1019-1032.
6. Mayo Clinic. The MELD model, UNOS modification. Available at: http://www.mayoclinic.org/meld/mayomodel6.html. Accessed August 16, 2010.
7. US Food and Drug Administration. FDA approves new use of Xifaxan for patients with liver disease. Available at: http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm206104.htm. Updated March 26, 2010. Accessed July 7, 2010.
8. Drugstore.com. Available at: http://www.drugstore.com/. Accessed August 20, 2010.
1. Bass NM, Mullen KD, Sanyal A, et al. Rifaximin treatment in hepatic encephalopathy. N Engl J Med. 2010;362:1071-1081.
2. Riordan SM, Williams R. Treatment of hepatic encephalopathy. N Engl J Med. 1997;337:473-479.
3. Als-Nielsen B, Gluud LL, Gluud C. Nonabsorbable disaccharides for hepatic encephalopathy. Cochrane Database Syst Rev. 2004;(2):CD003044.-
4. Paik YH, Lee KS, Han KH, et al. Comparison of rifaximin and lactulose for the treatment of hepatic encephalopathy: a prospective randomized study. Yonsei Med J. 2005;46:399-407.
5. Lawrence KR, Klee JA. Rifaximin for the treatment of hepatic encephalopathy. Pharmacotherapy. 2008;28:1019-1032.
6. Mayo Clinic. The MELD model, UNOS modification. Available at: http://www.mayoclinic.org/meld/mayomodel6.html. Accessed August 16, 2010.
7. US Food and Drug Administration. FDA approves new use of Xifaxan for patients with liver disease. Available at: http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm206104.htm. Updated March 26, 2010. Accessed July 7, 2010.
8. Drugstore.com. Available at: http://www.drugstore.com/. Accessed August 20, 2010.
Copyright © 2010 The Family Physicians Inquiries Network.
All rights reserved.