User login
Outcomes Discrepancy
A new study in the Journal of Hospital Medicine that reports insured hospitalized patients from ages 18-64 have 50% higher odds of surviving a heart attack or stroke than their uninsured counterparts should be a wake-up call to HM leaders looking to improve standards of care, one hospitalist says.
“It’s almost startling and embarrassing when you see the statistics on paper,” says Danielle Scheurer, MD, MSc, SFHM, assistant professor of medicine at Harvard Medical School and director of Boston-based Brigham and Women’s Hospital’s general medicine service. “If you’re going to assign a specialty to address the problem, it’s a hospital medicine problem.”
The researchers retrospectively analyzed 150,000 discharges among patients hospitalized for acute myocardial infarction (MI), stroke, or pneumonia (DOI: 10.1002/jhm.687). Compared with the privately insured, the study reported "in-hospital mortality among AMI and stroke patients was significantly higher for the uninsured (adjusted odds ratio [OR] 1.52, 95% confidence interval [CI], 1.24-1.85 for AMI and 1.49 OR, 1.29-1.72 for stroke) and among pneumonia patients was significantly higher for Medicaid recipients (1.21 OR, 1.01-1.45)." The lead author was Omar Hasan, MBBS, MPH, an instructor at Harvard Medical School and a hospitalist at Brigham and Women’s.
Dr. Scheurer cautions that the subject raised by her colleague is a multidimensional problem with no easy solution. Physicians may unconsciously make triage decisions that feed into the difference of care, she says, while insured patients who more actively engage their doctors could also skew the numbers.
She thinks, however, that “systematically creating protocols, policies, and procedures” could result in clinical-care delivery that helps reduce the disparity.
“Part of [the importance of the study] is having an open dialogue,” Dr. Scheurer says. “This is real. There is this disparity.”
A new study in the Journal of Hospital Medicine that reports insured hospitalized patients from ages 18-64 have 50% higher odds of surviving a heart attack or stroke than their uninsured counterparts should be a wake-up call to HM leaders looking to improve standards of care, one hospitalist says.
“It’s almost startling and embarrassing when you see the statistics on paper,” says Danielle Scheurer, MD, MSc, SFHM, assistant professor of medicine at Harvard Medical School and director of Boston-based Brigham and Women’s Hospital’s general medicine service. “If you’re going to assign a specialty to address the problem, it’s a hospital medicine problem.”
The researchers retrospectively analyzed 150,000 discharges among patients hospitalized for acute myocardial infarction (MI), stroke, or pneumonia (DOI: 10.1002/jhm.687). Compared with the privately insured, the study reported "in-hospital mortality among AMI and stroke patients was significantly higher for the uninsured (adjusted odds ratio [OR] 1.52, 95% confidence interval [CI], 1.24-1.85 for AMI and 1.49 OR, 1.29-1.72 for stroke) and among pneumonia patients was significantly higher for Medicaid recipients (1.21 OR, 1.01-1.45)." The lead author was Omar Hasan, MBBS, MPH, an instructor at Harvard Medical School and a hospitalist at Brigham and Women’s.
Dr. Scheurer cautions that the subject raised by her colleague is a multidimensional problem with no easy solution. Physicians may unconsciously make triage decisions that feed into the difference of care, she says, while insured patients who more actively engage their doctors could also skew the numbers.
She thinks, however, that “systematically creating protocols, policies, and procedures” could result in clinical-care delivery that helps reduce the disparity.
“Part of [the importance of the study] is having an open dialogue,” Dr. Scheurer says. “This is real. There is this disparity.”
A new study in the Journal of Hospital Medicine that reports insured hospitalized patients from ages 18-64 have 50% higher odds of surviving a heart attack or stroke than their uninsured counterparts should be a wake-up call to HM leaders looking to improve standards of care, one hospitalist says.
“It’s almost startling and embarrassing when you see the statistics on paper,” says Danielle Scheurer, MD, MSc, SFHM, assistant professor of medicine at Harvard Medical School and director of Boston-based Brigham and Women’s Hospital’s general medicine service. “If you’re going to assign a specialty to address the problem, it’s a hospital medicine problem.”
The researchers retrospectively analyzed 150,000 discharges among patients hospitalized for acute myocardial infarction (MI), stroke, or pneumonia (DOI: 10.1002/jhm.687). Compared with the privately insured, the study reported "in-hospital mortality among AMI and stroke patients was significantly higher for the uninsured (adjusted odds ratio [OR] 1.52, 95% confidence interval [CI], 1.24-1.85 for AMI and 1.49 OR, 1.29-1.72 for stroke) and among pneumonia patients was significantly higher for Medicaid recipients (1.21 OR, 1.01-1.45)." The lead author was Omar Hasan, MBBS, MPH, an instructor at Harvard Medical School and a hospitalist at Brigham and Women’s.
Dr. Scheurer cautions that the subject raised by her colleague is a multidimensional problem with no easy solution. Physicians may unconsciously make triage decisions that feed into the difference of care, she says, while insured patients who more actively engage their doctors could also skew the numbers.
She thinks, however, that “systematically creating protocols, policies, and procedures” could result in clinical-care delivery that helps reduce the disparity.
“Part of [the importance of the study] is having an open dialogue,” Dr. Scheurer says. “This is real. There is this disparity.”
In the Literature: Research You Need to Know
Clinical question: What is the prevalence of silent pulmonary embolism (PE) in patients with deep venous thrombosis (DVT)?
Background: Pulmonary embolism was undiagnosed or unsuspected in approximately 80% to 93% patients antemortem who were found to have a PE at autopsy. The extent to which silent pulmonary embolism explains the undiagnosed or unsuspected pulmonary emboli at autopsy is not certain. Prior studies have demonstrated the association of silent PE in living patients with DVT.
Study design: Systematic review.
Setting: Published trials performed worldwide.
Synopsis: A systematic review of published trials addressing the prevalence of silent pulmonary embolism in patients with deep vein thrombosis was performed. Studies were included if methods of diagnosis of PE were described, if it was an asymptomatic PE, and if raw data were presented. Twenty-eight studies were identified and were stratified according to how the PE was diagnosed (Tier 1: high-probability VQ scan based on PIOPED criteria, CTA, angiography; Tier 2: VQ scans based on non-PIOPED criteria).
Among Tier 1 studies, silent PE was detected among 27% of patients with DVT. Among Tier 2 studies, silent PE was detected among 37% of patients with DVT. Combined, silent PE was diagnosed in 1,665 of 5,233 patients (32%) with DVT. Further analysis showed that the prevalence of silent PE in patients with proximal DVT was higher in those with distal DVT, and that there was a trend toward increased prevalence of silent PE with increased age.
A limitation of this study includes the heterogeneity in the methods used for diagnosis of silent pulmonary embolism.
Bottom line: Silent pulmonary embolism occurs in one-third of patients with deep venous thrombosis, and routine screening should be considered.
Citation: Stein P, Matta F, Musani MH, Diaczok B. Silent pulmonary embolism in patients with deep venous thrombosis: a systematic review. Am J Med. 2010;123(5):426-431.
Reviewed for TH eWire by Alexander R. Carbo, MD, SFHM, Lauren Doctoroff, MD, John Fani Srour, MD, Matthew Hill, MD, Nancy Torres-Finnerty, MD, FHM, Anita Vanka, MD, Hospital Medicine Program, Beth Israel Deaconess Medical Center.
For more physician reviews of HM-related research, visit our website.
Clinical question: What is the prevalence of silent pulmonary embolism (PE) in patients with deep venous thrombosis (DVT)?
Background: Pulmonary embolism was undiagnosed or unsuspected in approximately 80% to 93% patients antemortem who were found to have a PE at autopsy. The extent to which silent pulmonary embolism explains the undiagnosed or unsuspected pulmonary emboli at autopsy is not certain. Prior studies have demonstrated the association of silent PE in living patients with DVT.
Study design: Systematic review.
Setting: Published trials performed worldwide.
Synopsis: A systematic review of published trials addressing the prevalence of silent pulmonary embolism in patients with deep vein thrombosis was performed. Studies were included if methods of diagnosis of PE were described, if it was an asymptomatic PE, and if raw data were presented. Twenty-eight studies were identified and were stratified according to how the PE was diagnosed (Tier 1: high-probability VQ scan based on PIOPED criteria, CTA, angiography; Tier 2: VQ scans based on non-PIOPED criteria).
Among Tier 1 studies, silent PE was detected among 27% of patients with DVT. Among Tier 2 studies, silent PE was detected among 37% of patients with DVT. Combined, silent PE was diagnosed in 1,665 of 5,233 patients (32%) with DVT. Further analysis showed that the prevalence of silent PE in patients with proximal DVT was higher in those with distal DVT, and that there was a trend toward increased prevalence of silent PE with increased age.
A limitation of this study includes the heterogeneity in the methods used for diagnosis of silent pulmonary embolism.
Bottom line: Silent pulmonary embolism occurs in one-third of patients with deep venous thrombosis, and routine screening should be considered.
Citation: Stein P, Matta F, Musani MH, Diaczok B. Silent pulmonary embolism in patients with deep venous thrombosis: a systematic review. Am J Med. 2010;123(5):426-431.
Reviewed for TH eWire by Alexander R. Carbo, MD, SFHM, Lauren Doctoroff, MD, John Fani Srour, MD, Matthew Hill, MD, Nancy Torres-Finnerty, MD, FHM, Anita Vanka, MD, Hospital Medicine Program, Beth Israel Deaconess Medical Center.
For more physician reviews of HM-related research, visit our website.
Clinical question: What is the prevalence of silent pulmonary embolism (PE) in patients with deep venous thrombosis (DVT)?
Background: Pulmonary embolism was undiagnosed or unsuspected in approximately 80% to 93% patients antemortem who were found to have a PE at autopsy. The extent to which silent pulmonary embolism explains the undiagnosed or unsuspected pulmonary emboli at autopsy is not certain. Prior studies have demonstrated the association of silent PE in living patients with DVT.
Study design: Systematic review.
Setting: Published trials performed worldwide.
Synopsis: A systematic review of published trials addressing the prevalence of silent pulmonary embolism in patients with deep vein thrombosis was performed. Studies were included if methods of diagnosis of PE were described, if it was an asymptomatic PE, and if raw data were presented. Twenty-eight studies were identified and were stratified according to how the PE was diagnosed (Tier 1: high-probability VQ scan based on PIOPED criteria, CTA, angiography; Tier 2: VQ scans based on non-PIOPED criteria).
Among Tier 1 studies, silent PE was detected among 27% of patients with DVT. Among Tier 2 studies, silent PE was detected among 37% of patients with DVT. Combined, silent PE was diagnosed in 1,665 of 5,233 patients (32%) with DVT. Further analysis showed that the prevalence of silent PE in patients with proximal DVT was higher in those with distal DVT, and that there was a trend toward increased prevalence of silent PE with increased age.
A limitation of this study includes the heterogeneity in the methods used for diagnosis of silent pulmonary embolism.
Bottom line: Silent pulmonary embolism occurs in one-third of patients with deep venous thrombosis, and routine screening should be considered.
Citation: Stein P, Matta F, Musani MH, Diaczok B. Silent pulmonary embolism in patients with deep venous thrombosis: a systematic review. Am J Med. 2010;123(5):426-431.
Reviewed for TH eWire by Alexander R. Carbo, MD, SFHM, Lauren Doctoroff, MD, John Fani Srour, MD, Matthew Hill, MD, Nancy Torres-Finnerty, MD, FHM, Anita Vanka, MD, Hospital Medicine Program, Beth Israel Deaconess Medical Center.
For more physician reviews of HM-related research, visit our website.
Age‐Specific CSF Protein Reference Values
Emergency department evaluation of a febrile neonate or young infant routinely includes lumbar puncture and cerebrospinal fluid (CSF) analysis to diagnose meningitis or encephalitis. In addition to CSF Gram stain and culture, clinicians generally request a laboratory report for the CSF cell count, glucose content and protein concentration. Interpretation of these ancillary tests requires knowledge of normal reference values. In adult medicine, the accepted reference value for CSF protein concentration at the level of the lumbar spine is 15 mg/dL to45 mg/dL.1 There is general consensus among reference texts and published original studies dating back to Widell2 in 1958 that adult CSF protein reference values are not valid in the pediatric population. A healthy neonate's CSF protein concentration is normally twice to 3 times that of an adult, and declines with age from birth to early childhood. The most rapid rate of decline is thought to occur in the first 6 months of life as the infant's blood‐CSF barrier matures.3 However, published studies47 differ in the reported rate, timing, and magnitude of this decline; on close review these studies have significant limitations which call into question the appropriateness of using these values in clinical practice. Perhaps in recognition of the limited evidence, textbooks of general pediatrics,810 hospital medicine,1113 emergency medicine,14, 15 infectious diseases,16, 17 neonatology,18 and neurology19, 20 frequently publish norms for pediatric CSF protein concentration without reference to any original research studies.
Because ethical considerations prohibit subjecting young infants to a potentially painfully procedure (ie, lumbar puncture) before they are able to assent, we sought to define a study population that approximates a group of healthy infants. Our objectives were to quantify age‐related declines in CSF protein concentration and to determine accurate, age‐specific reference values for CSF protein concentration in a population of neonates and young infants who presented for medical care with an indication for lumbar puncture and were subsequently found to have no condition associated with elevated or depressed CSF protein concentration.
Methods
Study Design and Setting
This cross‐sectional study was performed at The Children's Hospital of Philadelphia (Philadelphia, PA), an urban, tertiary‐care children's hospital. The Committees for the Protection of Human Subjects approved this study with a waiver of informed consent.
Study Participants
Infants 56 days of age or younger were eligible for inclusion if they had a lumbar puncture performed as part of their emergency department evaluation between January 1, 2005 and June 30, 2007. Children in this age range were selected as they routinely undergo lumbar puncture when presenting with fever at our institution.21, 22 Patients undergoing lumbar puncture in the emergency department were identified using 2 different data sources to ensure accurate identification of all eligible infants: (1) Emergency department computerized order entry records identified all infants with CSF testing (including CSF Gram stain, culture, cell count, glucose, or protein) performed during the study period, and (2) Clinical Virology Laboratory records identified all infants in whom CSF herpes simplex virus or enterovirus testing was performed. Medical records of infants identified by these 2 sources were reviewed to determine study eligibility.
Subjects with conditions known or suspected to cause abnormal CSF protein concentration were systematically excluded from the final analysis. Exclusion criteria included traumatic lumbar puncture (defined as CSF sample with >500 red blood cells per mm3), serious bacterial infection (including meningitis, urinary tract infection, bacteremia, pneumonia, osteomyelitis, or septic arthritis), congenital infection, CSF positive for enterovirus by polymerase chain reaction (PCR) testing, seizure prior to presentation, presence of a ventricular shunt device, elevated serum bilirubin, and absent CSF protein measurements or CSF red blood cell counts. The presence of lysed red blood cells in the CSF secondary to a traumatic lumbar puncture or subarachnoid hemorrhage alters the CSF protein.23 We also excluded subjects who had CSF assays done on samples drawn by accessing a ventricular shunt device, as there may be up to a 300% regional difference in CSF protein concentration between the cranial and caudal ends of the neuroaxis.1 Bilirubin in the CSF sample at a concentration of 5 mg/dL biases the CSF protein concentration measurement by an average of 13.7 mg/dL.24 Quantitative protein assay was performed on the institution's standard Vitros chemistry system; the protein assay is a modified biuret reaction.
Study Definitions
CSF pleocytosis was defined as a CSF white blood cell count (WBC) >22/mm3 (for infants age 28 days) or >15/mm3 (for infants 2956 days of age).25 Bacterial meningitis was defined as isolation of a bacterial pathogen from the CSF. Bacteremia was defined as isolation of a bacterial pathogen from blood culture, excluding isolates that reflected commensal skin flora. Bacterial pneumonia was defined as a new discrete infiltrate on chest radiograph as documented by an attending pediatric radiologist in conjunction with growth of a respiratory bacterial pathogen from blood culture. Urinary tract infection was defined as growth of a single known pathogen in culture as follows: (1) 1000 colony‐forming units/mL for cultures obtained by suprapubic aspiration, (2) 50,000 cfu/mL from a catheterized specimen, or (3) 10,000 cfu/mL from catheterized specimen in conjunction with a positive urinalysis.26 Positive urinalysis was defined as trace or greater leukocyte esterase by dip stick, or >9 WBC per high‐power filed on standard microscopic exam of centrifuged urine, or >10 WBC/mm3 by hemocytometer count of uncentrifuged urine.27, 28 We defined osteomyelitis as growth of pathogenic bacteria from blood, bone, or subperiosteal aspirate culture in a subject with fever and localized tenderness, edema or erythema at the site of bony infection, and compatible imaging; and septic arthritis as growth of pathogenic bacteria from synovial fluid or blood culture from a subject with purulent synovial fluid or positive Gram stain of synovial fluid.
A temperature 38.0C by any method qualified as fever. Prematurity was defined as a gestational age less than 37 weeks. Seizure included any clinical description of the event within 48 hours of presentation to the Emergency Department, or documented seizure activity on electroencephalogram. Enterovirus season was defined as June 1st to October 31st of each year.29
Data Collection and Statistical Analysis
Information collected included the following: demographics, vital signs, history of present illness, birth history, clinical findings, results of laboratory testing and imaging within 48 hours of presentation, antibiotics administered, and duration of visit to the Emergency Department or admission to the hospital.
Categorical data were described using frequencies and percents, and continuous variables were described using mean, median, interquartile range, and 90th and 95th percentile values. Linear regression was used to determine the association between age and CSF protein concentration. Because the CSF protein concentrations had a skewed distribution (P < 0.001, Shapiro‐Wilk test), our analyses were performed using logarithmically transformed CSF protein values as the dependent variable. The resulting beta‐coefficients were transformed to reflect the percent change in CSF protein with increasing age. Two‐sample Wilcoxon rank‐sum tests were subsequently used to compare the distribution of CSF protein concentrations amongst four predefined age categories to facilitate implementation of our results into clinical practice: 014 days, 1528 days, 2942 days, and 4356 days. The analyses were repeated while excluding preterm infants, patients receiving antibiotics before lumbar puncture, and patients with CSF pleocytosis to determine the impact of these factors on CSF protein concentrations. Data were analyzed using STATA v10 (Stata Corporation, College Station, TX). Two‐tailed P values < 0.05 were considered statistically significant.
Results
During the study period, 1064 infants age 56 days of age or younger underwent lumbar puncture in the emergency department. Of these, 689 (65%) met sequential exclusion criteria as follows: traumatic lumbar puncture (n = 330); transported from an outside medical facility (n = 90); bacterial meningitis (n = 6); noncentral nervous system serious bacterial infections (n = 135); CSF positive for herpes simplex virus by PCR (n = 2); CSF positive for enterovirus by PCR (n = 45); congenital syphilis (n = 1); seizures (n = 28); abnormal central nervous system imaging (n = 2); and ventricular shunt device (n = 1). An additional 44 patients had lumbar puncture and CSF testing but the protein assay was never done or never reported and 5 patients did not have a CSF red blood cell count available. No cases were excluded for elevated serum bilirubin. Infants may have met multiple exclusion criteria. The remaining 375 (35%) subjects were included in the final analysis. The median patient age was 36 days (interquartile range: 2247 days); 139 (37%) were 28 days of age or younger. Overall, 205 (55%) were male, 211 (56%) were black, and 145 (39%) presented during enterovirus season. Most (43 of 57) preterm infants were born between 34 weeks to 37 weeks gestation. Antibiotics were administered before lumbar puncture to 42 (11%) infants and 312 (83%) infants had fever.
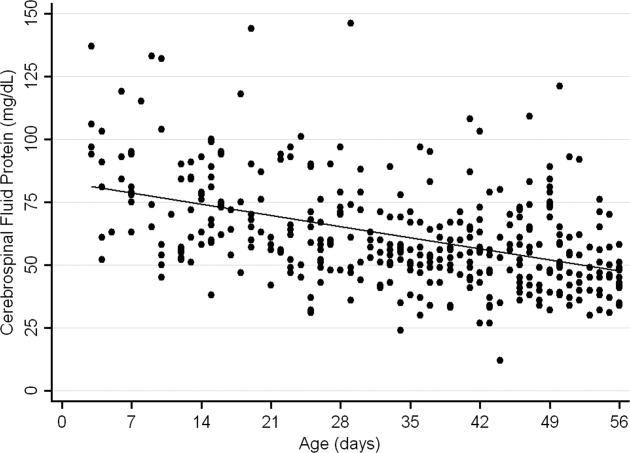
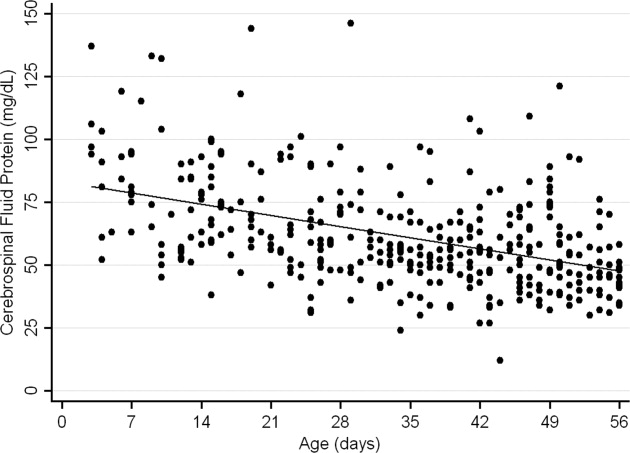
The median CSF protein value was 58 mg/dL (interquartile range: 4872 mg/dL). There was an age‐related declined in CSF protein concentration (Figure 1). In linear regression, the CSF protein concentration decreased 6.8% (95% confidence interval [CI], 5.48.1%; P < 0.001) for each 1‐week increase in age.0
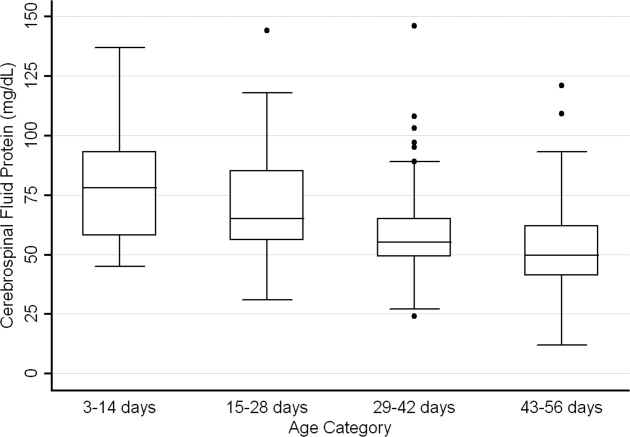
CSF protein concentrations were higher for infants 28 days of age than for infants 2956 days of age (P < 0.001, Wilcoxon rank‐sum test). The median CSF protein concentrations were 68 mg/dL (95th percentile value, 115 mg/dL) for infants 28 days of age and 54 mg/dL (95th percentile value, 89 mg/dL) for infants 2956 days. CSF protein concentrations by 2‐week age intervals are shown in Table 1. The 95th percentile CSF protein concentrations were as follows: ages 014 days, 132 mg/dL; ages 1528 days, 100 mg/dL; ages 2942 days, 89 mg/dL; and ages 4356 days, 83 mg/dL (Table 1). CSF protein concentration decreased significantly across each age interval when compared with infants in the next highest age category (P < 0.02 for all pair‐wise comparisons, Wilcoxon rank‐sum test).
| Value | 014 days (n = 52) | 1528 days (n = 87) | 2942 days (n = 110) | 4356 days (n = 126) | All Infants (n = 375) |
|---|---|---|---|---|---|
| |||||
| Mean (SD) | 79 (23) | 69 (20) | 58 (17) | 53 (17) | 62 (21) |
| Median (IQR) | 78 (5893) | 65 (5685) | 55 (4965) | 50 (4162) | 58 (4872) |
| 90th percentile | 106 | 95 | 79 | 75 | 91 |
| 95th percentile | 132 | 100 | 89 | 83 | 99 |
| 95th percentile* | 132 | 101 | 89 | 82 | 97 |
| 95th percentile | 132 | 100 | 87 | 74 | 97 |
Age‐specific 95th percentile CSF protein values changed by <1% when infants receiving antibiotics before lumbar puncture were excluded (Table 1). Age‐specific CSF protein values changed minimally when preterm infants were excluded with the exception of infants 4356 days of age where the 95th percentile value was 9.7% lower than when all infants were included (Table 1); the 90th percentile values in this age group were more comparable at 75 mg/dL and 71 mg/dL, respectively, in the subgroups with and without preterm infants. Age‐specific 95th percentile CSF protein values changes by <1% when patients with CSF pleocytosis were excluded.
Discussion
We examined CSF protein values in neonates and young infants to establish reference values and to bring the literature up to date at a time when molecular tools are commonly used in clinical practice. We also quantified the age‐related decline in CSF protein concentrations over the first two months of life. Our findings provide age‐specific reference ranges for CSF protein concentrations in neonates and young infants. These findings are particularly important because a variety of infectious (eg, herpes simplex virus infection) and noninfectious (eg, subarachnoid or intraventricular hemorrhage) conditions may occur in the absence of appreciable elevations in the CSF WBC.
CSF protein concentrations depend on serum protein concentrations and on the permeability of the blood‐CSF barrier. Immaturity of the blood‐CSF barrier is thought to result in higher CSF protein concentrations for neonates and young infants compared with older children and adults. Though previous studies agree that CSF protein concentrations depend on age, the reported age‐specific values and rates of decline vary considerably.47, 3032 Additionally, these prior studies are limited by (1) small sample size, (2) variable inclusion and exclusion criteria, (3) variable laboratory techniques to quantify protein concentration in a CSF sample, and (4) presentation of mean, standard deviation, and range values rather than the 75th, 90th, or 95th percentile values necessary to define a clinically meaningful reference range.
The median and mean values found in this study were generally comparable to previously published values (Table 2). In addition, we have quantified the age‐related decline in CSF protein concentrations identified in previous studies. While our large sample size allowed us to define narrower reference intervals than most previous studies, direct comparison of values used to define reference ranges was hampered by lack of consistent reporting of data across studies. Ahmed et al.5 and Bonadio et al.4 reported only mean and standard deviation values. When data are skewed, as is the case for CSF protein values, the standard deviation will be grossly inflated, making extrapolation to percentile values unreliable. The 90th percentile value of 87 mg/dL reported by Wong et al.7 for infants 060 days of age was similar to the value of 91 mg/dL for infants 56 days of age and younger found in this study. Biou et al.6 reported the following 95th percentile values: ages 18 days, 108 mg/dL; ages 830 days, 90 mg/dL; and ages 12 months, 77 mg/dL. These values are lower than those reported in our study. The reason for such differences is not clear. The exclusion criteria were similar between the two studies though Biou et al.6 did not include preterm infants. When we excluded preterm infants from our analysis, no age‐specific result decreased by more than 5%, making the inclusion of this population an unlikely explanation for the differences between the two studies.
| Author | Year | Number of Infants | Age (days) | Median (mg/dL) | Mean SD (mg/dL) |
|---|---|---|---|---|---|
| |||||
| Bonadio et al.4 | 1992 | 35 | 030 | 84 45 | |
| 40 | 3060 | 59 25 | |||
| Ahmed et al.5 | 1996 | 17 | 07 | 81 31 | |
| 33 | 814 | 69 23 | |||
| 25 | 1521 | 60 23 | |||
| 33 | 2230 | 54 16 | |||
| Biou et al.6 | 2000 | 26 | 18 | 71 | |
| 76 | 830 | 59 | |||
| 155 | 3060 | 47 | |||
| Wong et al.7 | 2000 | 99 | 060 | 60 | 59 21 |
CSF protein concentration is a method‐dependent value; the results depend a great deal on what technique the laboratory uses. Two common methods used in the past few decades are Biuret Colorimetry and Turbidimetric; reported values are approximately 25% higher with the Biuret method compared with the Turbidimetric method.33 A CSF protein reference value is only clinically useful if the method used to define the norm is specified and equivalent to currently used methods. Similar to our study, Biou et al.6 and Wong et al.7 used the Biuret (Vitros) method. The method of protein measurement was not specified by other studies.4, 5
This study had several limitations that could cause us to overestimate the upper bound of the reference range. First, spectrum bias is possible in this observational study. Individual physicians determined whether lumbar puncture was warranted, a limitation that could potentially lead to the disproportionate inclusion of infants with conditions associated with higher CSF protein concentrations. We do not believe that this limitation would meaningfully affect our results because febrile infants 56 days of age or younger routinely undergo lumbar puncture at our institution, regardless of illness severity, and patients diagnosed with conditions known or suspected to increase CSF protein concentrations were excluded. Second, infants with aseptic meningitisa condition that can be associated with elevated CSF protein concentrationsmay have been misclassified as uninfected. Though we excluded patients with positive CSF enteroviral PCR tests, some infants were not tested and other viruses (eg, parechoviruses)34 not detected by the enterovirus PCR may also cause aseptic meningitis. Third, certain antibiotics including ampicillin and vancomycin are known to interfere with the CSF protein assay used in our laboratory.24 Forty‐two of the 375 subjects included in our final analysis received antibiotics prior to lumbar puncture. When receiving antibiotics prior to lumbar puncture were excluded from analysis, the CSF protein concentrations were within 1% of the overall study population, suggesting that antibiotic administration before lumbar puncture did not influence our results in any meaningful way. We would not expect any of these limitations to disproportionately affect patients in 1 particular age category.
In conclusion, the CSF protein concentration values reported here represent the largest series to‐date for this young age group. Our study quantifies the age‐related decline in CSF protein concentration from birth to 56 days of life. Our work designing this study, specifically the exclusion criteria, refines the approach to defining normal CSF protein values in children. As CSF protein values decline steadily with increasing age, the selection of reference values is a balance of accuracy and convenience. Age‐specific reference values by 2‐week increments would be most accurate. However, considering reference values by month of age, as is the convention for CSF WBCs, is far more practical. The 95th percentile values by age category in our study were as follows: ages 014 days, 132 mg/dL; ages 1528 days, 100 mg/dL; ages 2942 days, 89 mg/dL; and ages 4356 days, 83 mg/dL. The 95th percentile values were 115 mg/dL for infants 28 days and 89 mg/dL for infants 2956 days. We feel that either approach is reasonable. These values can be used to accurately interpret the results of CSF studies in neonates and young infants.
- ,.Henry's Clinical Diagnosis and Management by Laboratory Methods.21st ed.Philadelphia, PA:W.B. Saunders, Inc.;2006.
- .On the cerebrospinal fluid in normal children and in patients with acute abacterial meningo‐encephalitis.Acta Paediatr Suppl.1958;47(Suppl 115):1–102.
- ,.Development of the blood‐CSF barrier.Dev Med Child Neurol.1983;25(2):152–161.
- ,,,,.Reference values of normal cerebrospinal fluid composition in infants ages 0 to 8 weeks.Pediatr Infect Dis J.1992;11(7):589–591.
- ,,, et al.Cerebrospinal fluid values in the term neonate.Pediatr Infect Dis J.1996;15(4):298–303.
- ,,,,,.Cerebrospinal fluid protein concentrations in children: age‐related values in patients without disorders of the central nervous system.Clin Chem.2000;46(3):399–403.
- ,,,,.Cerebrospinal fluid protein concentration in pediatric patients: defining clinically relevant reference values.Arch Pediatr Adolesc Med.2000;154(8):827–831.
- ,,.Nelson Textbook of Pediatrics.17th ed.Philadelphia, PA:Saunders;2004.
- ,,,.Oski's pediatrics : principles 2006.
- Robertson J, Shilkofski N, eds.Johns Hopkins: The Harriet Lane Handbook: A Manual for Pediatric House Officers.17 ed.Philadelphia, PA:Elsevier Mosby;2005.
- ,,,.The Philadelphia Guide: Inpatient Pediatrics.Philadelphia, PA:Lippincott Williams 2005.
- ,,,.Pediatric Hospital Medicine: Textbook of Inpatient Management.Philadelphia, PA:Lippincott Williams 2008.
- ,.Comprehensive pediatric hospital medicine.Philadelphia, PA:Mosby Elsevier;2007.
- ,,.Textbook of Pediatric Emergency Medicine.5th ed.Philadelphia, PA:Lippincott Williams 2006.
- ,,,.Pediatric Emergency Medicine.Philadelphia, PA:Saunders Elsevier;2008.
- ,,,.Textbook of Pediatric Infectious Diseases.5th ed.Philadelphia, PA:Saunders;2004.
- ,.Infectious Diseases of the Fetus and Newborn Infant.6th ed.Philadelphia, PA:Elsevier Saunders;2006.
- ,.Avery's diseases of the newborn.7th ed.Philadelphia, PA:Saunders;1998.
- ,.Child Neurology.6th ed.Philadelphia, PA:Lippincott Williams 2000.
- ,.Pediatric Neurology: Principles and Practice.3rd ed.St. Louis, MO:Mosby;1999.
- ,.Unpredictability of serious bacterial illness in febrile infants from birth to 1 month of age.Arch Pediatr Adolesc Med.1999;153(5):508–511.
- ,,.The efficacy of routine outpatient management without antibiotics of fever in selected infants.Pediatrics.1999;103(3):627–631.
- ,,.Bacterial sepsis and meningitis. In: Remington JS, Klein JO, Wilson CB, Baker CJ, eds.Infectious Diseases of the Fetus and Newborn Infant.6th ed.Philadelphia, PA:Elsevier, Inc.;2006:247–295.
- NCCLS.Interference testing in Clinical Chemistry, NCCLS Document EP7.Wayne, PA:NCCLS;1986.
- ,,,.Lack of cerebrospinal fluid pleocytosis in young infants with enterovirus infections of the central nervous system.Pediatr Emerg Care.2010;26(2):77–81.
- ,,, et al.Clinical and demographic factors associated with urinary tract infection in young febrile infants.Pediatrics.2005;116(3):644–648.
- ,,,,.Enhanced urinalysis as a screening test for urinary tract infection.Pediatrics.1993;91(6):1196–1199.
- ,,,.Screening for urinary tract infection in infants in the emergency department: which test is best?Pediatrics.1998;101(6):E1.
- ,,,,,.Routine cerebrospinal fluid enterovirus polymerase chain reaction testing reduces hospitalization and antibiotic use for infants 90 days of age or younger.Pediatrics.2007;120(3):489–496.
- .The normal cerebro‐spinal fluid in children.Archf Dis Child.1928:96–108.
- .The cerebrospinal fluid in the healthy newborn infant.S Afr Med J.1968;42(35):933–935.
- ,,.Cerebrospinal fluid evaluation in neonates: comparison of high‐risk infants with and without meningitis.J Pediatr.1976;88(3):473–477.
- ,.Estimation of reference intervals for total protein in cerebrospinal fluid.Clin Chem.1989;35(8):1766–1770.
- ,,,,,.Severe neonatal parechovirus infection and similarity with enterovirus infection.Pediatr Infect Dis J.2008;27(3):241–245.
Emergency department evaluation of a febrile neonate or young infant routinely includes lumbar puncture and cerebrospinal fluid (CSF) analysis to diagnose meningitis or encephalitis. In addition to CSF Gram stain and culture, clinicians generally request a laboratory report for the CSF cell count, glucose content and protein concentration. Interpretation of these ancillary tests requires knowledge of normal reference values. In adult medicine, the accepted reference value for CSF protein concentration at the level of the lumbar spine is 15 mg/dL to45 mg/dL.1 There is general consensus among reference texts and published original studies dating back to Widell2 in 1958 that adult CSF protein reference values are not valid in the pediatric population. A healthy neonate's CSF protein concentration is normally twice to 3 times that of an adult, and declines with age from birth to early childhood. The most rapid rate of decline is thought to occur in the first 6 months of life as the infant's blood‐CSF barrier matures.3 However, published studies47 differ in the reported rate, timing, and magnitude of this decline; on close review these studies have significant limitations which call into question the appropriateness of using these values in clinical practice. Perhaps in recognition of the limited evidence, textbooks of general pediatrics,810 hospital medicine,1113 emergency medicine,14, 15 infectious diseases,16, 17 neonatology,18 and neurology19, 20 frequently publish norms for pediatric CSF protein concentration without reference to any original research studies.
Because ethical considerations prohibit subjecting young infants to a potentially painfully procedure (ie, lumbar puncture) before they are able to assent, we sought to define a study population that approximates a group of healthy infants. Our objectives were to quantify age‐related declines in CSF protein concentration and to determine accurate, age‐specific reference values for CSF protein concentration in a population of neonates and young infants who presented for medical care with an indication for lumbar puncture and were subsequently found to have no condition associated with elevated or depressed CSF protein concentration.
Methods
Study Design and Setting
This cross‐sectional study was performed at The Children's Hospital of Philadelphia (Philadelphia, PA), an urban, tertiary‐care children's hospital. The Committees for the Protection of Human Subjects approved this study with a waiver of informed consent.
Study Participants
Infants 56 days of age or younger were eligible for inclusion if they had a lumbar puncture performed as part of their emergency department evaluation between January 1, 2005 and June 30, 2007. Children in this age range were selected as they routinely undergo lumbar puncture when presenting with fever at our institution.21, 22 Patients undergoing lumbar puncture in the emergency department were identified using 2 different data sources to ensure accurate identification of all eligible infants: (1) Emergency department computerized order entry records identified all infants with CSF testing (including CSF Gram stain, culture, cell count, glucose, or protein) performed during the study period, and (2) Clinical Virology Laboratory records identified all infants in whom CSF herpes simplex virus or enterovirus testing was performed. Medical records of infants identified by these 2 sources were reviewed to determine study eligibility.
Subjects with conditions known or suspected to cause abnormal CSF protein concentration were systematically excluded from the final analysis. Exclusion criteria included traumatic lumbar puncture (defined as CSF sample with >500 red blood cells per mm3), serious bacterial infection (including meningitis, urinary tract infection, bacteremia, pneumonia, osteomyelitis, or septic arthritis), congenital infection, CSF positive for enterovirus by polymerase chain reaction (PCR) testing, seizure prior to presentation, presence of a ventricular shunt device, elevated serum bilirubin, and absent CSF protein measurements or CSF red blood cell counts. The presence of lysed red blood cells in the CSF secondary to a traumatic lumbar puncture or subarachnoid hemorrhage alters the CSF protein.23 We also excluded subjects who had CSF assays done on samples drawn by accessing a ventricular shunt device, as there may be up to a 300% regional difference in CSF protein concentration between the cranial and caudal ends of the neuroaxis.1 Bilirubin in the CSF sample at a concentration of 5 mg/dL biases the CSF protein concentration measurement by an average of 13.7 mg/dL.24 Quantitative protein assay was performed on the institution's standard Vitros chemistry system; the protein assay is a modified biuret reaction.
Study Definitions
CSF pleocytosis was defined as a CSF white blood cell count (WBC) >22/mm3 (for infants age 28 days) or >15/mm3 (for infants 2956 days of age).25 Bacterial meningitis was defined as isolation of a bacterial pathogen from the CSF. Bacteremia was defined as isolation of a bacterial pathogen from blood culture, excluding isolates that reflected commensal skin flora. Bacterial pneumonia was defined as a new discrete infiltrate on chest radiograph as documented by an attending pediatric radiologist in conjunction with growth of a respiratory bacterial pathogen from blood culture. Urinary tract infection was defined as growth of a single known pathogen in culture as follows: (1) 1000 colony‐forming units/mL for cultures obtained by suprapubic aspiration, (2) 50,000 cfu/mL from a catheterized specimen, or (3) 10,000 cfu/mL from catheterized specimen in conjunction with a positive urinalysis.26 Positive urinalysis was defined as trace or greater leukocyte esterase by dip stick, or >9 WBC per high‐power filed on standard microscopic exam of centrifuged urine, or >10 WBC/mm3 by hemocytometer count of uncentrifuged urine.27, 28 We defined osteomyelitis as growth of pathogenic bacteria from blood, bone, or subperiosteal aspirate culture in a subject with fever and localized tenderness, edema or erythema at the site of bony infection, and compatible imaging; and septic arthritis as growth of pathogenic bacteria from synovial fluid or blood culture from a subject with purulent synovial fluid or positive Gram stain of synovial fluid.
A temperature 38.0C by any method qualified as fever. Prematurity was defined as a gestational age less than 37 weeks. Seizure included any clinical description of the event within 48 hours of presentation to the Emergency Department, or documented seizure activity on electroencephalogram. Enterovirus season was defined as June 1st to October 31st of each year.29
Data Collection and Statistical Analysis
Information collected included the following: demographics, vital signs, history of present illness, birth history, clinical findings, results of laboratory testing and imaging within 48 hours of presentation, antibiotics administered, and duration of visit to the Emergency Department or admission to the hospital.
Categorical data were described using frequencies and percents, and continuous variables were described using mean, median, interquartile range, and 90th and 95th percentile values. Linear regression was used to determine the association between age and CSF protein concentration. Because the CSF protein concentrations had a skewed distribution (P < 0.001, Shapiro‐Wilk test), our analyses were performed using logarithmically transformed CSF protein values as the dependent variable. The resulting beta‐coefficients were transformed to reflect the percent change in CSF protein with increasing age. Two‐sample Wilcoxon rank‐sum tests were subsequently used to compare the distribution of CSF protein concentrations amongst four predefined age categories to facilitate implementation of our results into clinical practice: 014 days, 1528 days, 2942 days, and 4356 days. The analyses were repeated while excluding preterm infants, patients receiving antibiotics before lumbar puncture, and patients with CSF pleocytosis to determine the impact of these factors on CSF protein concentrations. Data were analyzed using STATA v10 (Stata Corporation, College Station, TX). Two‐tailed P values < 0.05 were considered statistically significant.
Results
During the study period, 1064 infants age 56 days of age or younger underwent lumbar puncture in the emergency department. Of these, 689 (65%) met sequential exclusion criteria as follows: traumatic lumbar puncture (n = 330); transported from an outside medical facility (n = 90); bacterial meningitis (n = 6); noncentral nervous system serious bacterial infections (n = 135); CSF positive for herpes simplex virus by PCR (n = 2); CSF positive for enterovirus by PCR (n = 45); congenital syphilis (n = 1); seizures (n = 28); abnormal central nervous system imaging (n = 2); and ventricular shunt device (n = 1). An additional 44 patients had lumbar puncture and CSF testing but the protein assay was never done or never reported and 5 patients did not have a CSF red blood cell count available. No cases were excluded for elevated serum bilirubin. Infants may have met multiple exclusion criteria. The remaining 375 (35%) subjects were included in the final analysis. The median patient age was 36 days (interquartile range: 2247 days); 139 (37%) were 28 days of age or younger. Overall, 205 (55%) were male, 211 (56%) were black, and 145 (39%) presented during enterovirus season. Most (43 of 57) preterm infants were born between 34 weeks to 37 weeks gestation. Antibiotics were administered before lumbar puncture to 42 (11%) infants and 312 (83%) infants had fever.
The median CSF protein value was 58 mg/dL (interquartile range: 4872 mg/dL). There was an age‐related declined in CSF protein concentration (Figure 1). In linear regression, the CSF protein concentration decreased 6.8% (95% confidence interval [CI], 5.48.1%; P < 0.001) for each 1‐week increase in age.0


CSF protein concentrations were higher for infants 28 days of age than for infants 2956 days of age (P < 0.001, Wilcoxon rank‐sum test). The median CSF protein concentrations were 68 mg/dL (95th percentile value, 115 mg/dL) for infants 28 days of age and 54 mg/dL (95th percentile value, 89 mg/dL) for infants 2956 days. CSF protein concentrations by 2‐week age intervals are shown in Table 1. The 95th percentile CSF protein concentrations were as follows: ages 014 days, 132 mg/dL; ages 1528 days, 100 mg/dL; ages 2942 days, 89 mg/dL; and ages 4356 days, 83 mg/dL (Table 1). CSF protein concentration decreased significantly across each age interval when compared with infants in the next highest age category (P < 0.02 for all pair‐wise comparisons, Wilcoxon rank‐sum test).
| Value | 014 days (n = 52) | 1528 days (n = 87) | 2942 days (n = 110) | 4356 days (n = 126) | All Infants (n = 375) |
|---|---|---|---|---|---|
| |||||
| Mean (SD) | 79 (23) | 69 (20) | 58 (17) | 53 (17) | 62 (21) |
| Median (IQR) | 78 (5893) | 65 (5685) | 55 (4965) | 50 (4162) | 58 (4872) |
| 90th percentile | 106 | 95 | 79 | 75 | 91 |
| 95th percentile | 132 | 100 | 89 | 83 | 99 |
| 95th percentile* | 132 | 101 | 89 | 82 | 97 |
| 95th percentile | 132 | 100 | 87 | 74 | 97 |
Age‐specific 95th percentile CSF protein values changed by <1% when infants receiving antibiotics before lumbar puncture were excluded (Table 1). Age‐specific CSF protein values changed minimally when preterm infants were excluded with the exception of infants 4356 days of age where the 95th percentile value was 9.7% lower than when all infants were included (Table 1); the 90th percentile values in this age group were more comparable at 75 mg/dL and 71 mg/dL, respectively, in the subgroups with and without preterm infants. Age‐specific 95th percentile CSF protein values changes by <1% when patients with CSF pleocytosis were excluded.
Discussion
We examined CSF protein values in neonates and young infants to establish reference values and to bring the literature up to date at a time when molecular tools are commonly used in clinical practice. We also quantified the age‐related decline in CSF protein concentrations over the first two months of life. Our findings provide age‐specific reference ranges for CSF protein concentrations in neonates and young infants. These findings are particularly important because a variety of infectious (eg, herpes simplex virus infection) and noninfectious (eg, subarachnoid or intraventricular hemorrhage) conditions may occur in the absence of appreciable elevations in the CSF WBC.
CSF protein concentrations depend on serum protein concentrations and on the permeability of the blood‐CSF barrier. Immaturity of the blood‐CSF barrier is thought to result in higher CSF protein concentrations for neonates and young infants compared with older children and adults. Though previous studies agree that CSF protein concentrations depend on age, the reported age‐specific values and rates of decline vary considerably.47, 3032 Additionally, these prior studies are limited by (1) small sample size, (2) variable inclusion and exclusion criteria, (3) variable laboratory techniques to quantify protein concentration in a CSF sample, and (4) presentation of mean, standard deviation, and range values rather than the 75th, 90th, or 95th percentile values necessary to define a clinically meaningful reference range.
The median and mean values found in this study were generally comparable to previously published values (Table 2). In addition, we have quantified the age‐related decline in CSF protein concentrations identified in previous studies. While our large sample size allowed us to define narrower reference intervals than most previous studies, direct comparison of values used to define reference ranges was hampered by lack of consistent reporting of data across studies. Ahmed et al.5 and Bonadio et al.4 reported only mean and standard deviation values. When data are skewed, as is the case for CSF protein values, the standard deviation will be grossly inflated, making extrapolation to percentile values unreliable. The 90th percentile value of 87 mg/dL reported by Wong et al.7 for infants 060 days of age was similar to the value of 91 mg/dL for infants 56 days of age and younger found in this study. Biou et al.6 reported the following 95th percentile values: ages 18 days, 108 mg/dL; ages 830 days, 90 mg/dL; and ages 12 months, 77 mg/dL. These values are lower than those reported in our study. The reason for such differences is not clear. The exclusion criteria were similar between the two studies though Biou et al.6 did not include preterm infants. When we excluded preterm infants from our analysis, no age‐specific result decreased by more than 5%, making the inclusion of this population an unlikely explanation for the differences between the two studies.
| Author | Year | Number of Infants | Age (days) | Median (mg/dL) | Mean SD (mg/dL) |
|---|---|---|---|---|---|
| |||||
| Bonadio et al.4 | 1992 | 35 | 030 | 84 45 | |
| 40 | 3060 | 59 25 | |||
| Ahmed et al.5 | 1996 | 17 | 07 | 81 31 | |
| 33 | 814 | 69 23 | |||
| 25 | 1521 | 60 23 | |||
| 33 | 2230 | 54 16 | |||
| Biou et al.6 | 2000 | 26 | 18 | 71 | |
| 76 | 830 | 59 | |||
| 155 | 3060 | 47 | |||
| Wong et al.7 | 2000 | 99 | 060 | 60 | 59 21 |
CSF protein concentration is a method‐dependent value; the results depend a great deal on what technique the laboratory uses. Two common methods used in the past few decades are Biuret Colorimetry and Turbidimetric; reported values are approximately 25% higher with the Biuret method compared with the Turbidimetric method.33 A CSF protein reference value is only clinically useful if the method used to define the norm is specified and equivalent to currently used methods. Similar to our study, Biou et al.6 and Wong et al.7 used the Biuret (Vitros) method. The method of protein measurement was not specified by other studies.4, 5
This study had several limitations that could cause us to overestimate the upper bound of the reference range. First, spectrum bias is possible in this observational study. Individual physicians determined whether lumbar puncture was warranted, a limitation that could potentially lead to the disproportionate inclusion of infants with conditions associated with higher CSF protein concentrations. We do not believe that this limitation would meaningfully affect our results because febrile infants 56 days of age or younger routinely undergo lumbar puncture at our institution, regardless of illness severity, and patients diagnosed with conditions known or suspected to increase CSF protein concentrations were excluded. Second, infants with aseptic meningitisa condition that can be associated with elevated CSF protein concentrationsmay have been misclassified as uninfected. Though we excluded patients with positive CSF enteroviral PCR tests, some infants were not tested and other viruses (eg, parechoviruses)34 not detected by the enterovirus PCR may also cause aseptic meningitis. Third, certain antibiotics including ampicillin and vancomycin are known to interfere with the CSF protein assay used in our laboratory.24 Forty‐two of the 375 subjects included in our final analysis received antibiotics prior to lumbar puncture. When receiving antibiotics prior to lumbar puncture were excluded from analysis, the CSF protein concentrations were within 1% of the overall study population, suggesting that antibiotic administration before lumbar puncture did not influence our results in any meaningful way. We would not expect any of these limitations to disproportionately affect patients in 1 particular age category.
In conclusion, the CSF protein concentration values reported here represent the largest series to‐date for this young age group. Our study quantifies the age‐related decline in CSF protein concentration from birth to 56 days of life. Our work designing this study, specifically the exclusion criteria, refines the approach to defining normal CSF protein values in children. As CSF protein values decline steadily with increasing age, the selection of reference values is a balance of accuracy and convenience. Age‐specific reference values by 2‐week increments would be most accurate. However, considering reference values by month of age, as is the convention for CSF WBCs, is far more practical. The 95th percentile values by age category in our study were as follows: ages 014 days, 132 mg/dL; ages 1528 days, 100 mg/dL; ages 2942 days, 89 mg/dL; and ages 4356 days, 83 mg/dL. The 95th percentile values were 115 mg/dL for infants 28 days and 89 mg/dL for infants 2956 days. We feel that either approach is reasonable. These values can be used to accurately interpret the results of CSF studies in neonates and young infants.
Emergency department evaluation of a febrile neonate or young infant routinely includes lumbar puncture and cerebrospinal fluid (CSF) analysis to diagnose meningitis or encephalitis. In addition to CSF Gram stain and culture, clinicians generally request a laboratory report for the CSF cell count, glucose content and protein concentration. Interpretation of these ancillary tests requires knowledge of normal reference values. In adult medicine, the accepted reference value for CSF protein concentration at the level of the lumbar spine is 15 mg/dL to45 mg/dL.1 There is general consensus among reference texts and published original studies dating back to Widell2 in 1958 that adult CSF protein reference values are not valid in the pediatric population. A healthy neonate's CSF protein concentration is normally twice to 3 times that of an adult, and declines with age from birth to early childhood. The most rapid rate of decline is thought to occur in the first 6 months of life as the infant's blood‐CSF barrier matures.3 However, published studies47 differ in the reported rate, timing, and magnitude of this decline; on close review these studies have significant limitations which call into question the appropriateness of using these values in clinical practice. Perhaps in recognition of the limited evidence, textbooks of general pediatrics,810 hospital medicine,1113 emergency medicine,14, 15 infectious diseases,16, 17 neonatology,18 and neurology19, 20 frequently publish norms for pediatric CSF protein concentration without reference to any original research studies.
Because ethical considerations prohibit subjecting young infants to a potentially painfully procedure (ie, lumbar puncture) before they are able to assent, we sought to define a study population that approximates a group of healthy infants. Our objectives were to quantify age‐related declines in CSF protein concentration and to determine accurate, age‐specific reference values for CSF protein concentration in a population of neonates and young infants who presented for medical care with an indication for lumbar puncture and were subsequently found to have no condition associated with elevated or depressed CSF protein concentration.
Methods
Study Design and Setting
This cross‐sectional study was performed at The Children's Hospital of Philadelphia (Philadelphia, PA), an urban, tertiary‐care children's hospital. The Committees for the Protection of Human Subjects approved this study with a waiver of informed consent.
Study Participants
Infants 56 days of age or younger were eligible for inclusion if they had a lumbar puncture performed as part of their emergency department evaluation between January 1, 2005 and June 30, 2007. Children in this age range were selected as they routinely undergo lumbar puncture when presenting with fever at our institution.21, 22 Patients undergoing lumbar puncture in the emergency department were identified using 2 different data sources to ensure accurate identification of all eligible infants: (1) Emergency department computerized order entry records identified all infants with CSF testing (including CSF Gram stain, culture, cell count, glucose, or protein) performed during the study period, and (2) Clinical Virology Laboratory records identified all infants in whom CSF herpes simplex virus or enterovirus testing was performed. Medical records of infants identified by these 2 sources were reviewed to determine study eligibility.
Subjects with conditions known or suspected to cause abnormal CSF protein concentration were systematically excluded from the final analysis. Exclusion criteria included traumatic lumbar puncture (defined as CSF sample with >500 red blood cells per mm3), serious bacterial infection (including meningitis, urinary tract infection, bacteremia, pneumonia, osteomyelitis, or septic arthritis), congenital infection, CSF positive for enterovirus by polymerase chain reaction (PCR) testing, seizure prior to presentation, presence of a ventricular shunt device, elevated serum bilirubin, and absent CSF protein measurements or CSF red blood cell counts. The presence of lysed red blood cells in the CSF secondary to a traumatic lumbar puncture or subarachnoid hemorrhage alters the CSF protein.23 We also excluded subjects who had CSF assays done on samples drawn by accessing a ventricular shunt device, as there may be up to a 300% regional difference in CSF protein concentration between the cranial and caudal ends of the neuroaxis.1 Bilirubin in the CSF sample at a concentration of 5 mg/dL biases the CSF protein concentration measurement by an average of 13.7 mg/dL.24 Quantitative protein assay was performed on the institution's standard Vitros chemistry system; the protein assay is a modified biuret reaction.
Study Definitions
CSF pleocytosis was defined as a CSF white blood cell count (WBC) >22/mm3 (for infants age 28 days) or >15/mm3 (for infants 2956 days of age).25 Bacterial meningitis was defined as isolation of a bacterial pathogen from the CSF. Bacteremia was defined as isolation of a bacterial pathogen from blood culture, excluding isolates that reflected commensal skin flora. Bacterial pneumonia was defined as a new discrete infiltrate on chest radiograph as documented by an attending pediatric radiologist in conjunction with growth of a respiratory bacterial pathogen from blood culture. Urinary tract infection was defined as growth of a single known pathogen in culture as follows: (1) 1000 colony‐forming units/mL for cultures obtained by suprapubic aspiration, (2) 50,000 cfu/mL from a catheterized specimen, or (3) 10,000 cfu/mL from catheterized specimen in conjunction with a positive urinalysis.26 Positive urinalysis was defined as trace or greater leukocyte esterase by dip stick, or >9 WBC per high‐power filed on standard microscopic exam of centrifuged urine, or >10 WBC/mm3 by hemocytometer count of uncentrifuged urine.27, 28 We defined osteomyelitis as growth of pathogenic bacteria from blood, bone, or subperiosteal aspirate culture in a subject with fever and localized tenderness, edema or erythema at the site of bony infection, and compatible imaging; and septic arthritis as growth of pathogenic bacteria from synovial fluid or blood culture from a subject with purulent synovial fluid or positive Gram stain of synovial fluid.
A temperature 38.0C by any method qualified as fever. Prematurity was defined as a gestational age less than 37 weeks. Seizure included any clinical description of the event within 48 hours of presentation to the Emergency Department, or documented seizure activity on electroencephalogram. Enterovirus season was defined as June 1st to October 31st of each year.29
Data Collection and Statistical Analysis
Information collected included the following: demographics, vital signs, history of present illness, birth history, clinical findings, results of laboratory testing and imaging within 48 hours of presentation, antibiotics administered, and duration of visit to the Emergency Department or admission to the hospital.
Categorical data were described using frequencies and percents, and continuous variables were described using mean, median, interquartile range, and 90th and 95th percentile values. Linear regression was used to determine the association between age and CSF protein concentration. Because the CSF protein concentrations had a skewed distribution (P < 0.001, Shapiro‐Wilk test), our analyses were performed using logarithmically transformed CSF protein values as the dependent variable. The resulting beta‐coefficients were transformed to reflect the percent change in CSF protein with increasing age. Two‐sample Wilcoxon rank‐sum tests were subsequently used to compare the distribution of CSF protein concentrations amongst four predefined age categories to facilitate implementation of our results into clinical practice: 014 days, 1528 days, 2942 days, and 4356 days. The analyses were repeated while excluding preterm infants, patients receiving antibiotics before lumbar puncture, and patients with CSF pleocytosis to determine the impact of these factors on CSF protein concentrations. Data were analyzed using STATA v10 (Stata Corporation, College Station, TX). Two‐tailed P values < 0.05 were considered statistically significant.
Results
During the study period, 1064 infants age 56 days of age or younger underwent lumbar puncture in the emergency department. Of these, 689 (65%) met sequential exclusion criteria as follows: traumatic lumbar puncture (n = 330); transported from an outside medical facility (n = 90); bacterial meningitis (n = 6); noncentral nervous system serious bacterial infections (n = 135); CSF positive for herpes simplex virus by PCR (n = 2); CSF positive for enterovirus by PCR (n = 45); congenital syphilis (n = 1); seizures (n = 28); abnormal central nervous system imaging (n = 2); and ventricular shunt device (n = 1). An additional 44 patients had lumbar puncture and CSF testing but the protein assay was never done or never reported and 5 patients did not have a CSF red blood cell count available. No cases were excluded for elevated serum bilirubin. Infants may have met multiple exclusion criteria. The remaining 375 (35%) subjects were included in the final analysis. The median patient age was 36 days (interquartile range: 2247 days); 139 (37%) were 28 days of age or younger. Overall, 205 (55%) were male, 211 (56%) were black, and 145 (39%) presented during enterovirus season. Most (43 of 57) preterm infants were born between 34 weeks to 37 weeks gestation. Antibiotics were administered before lumbar puncture to 42 (11%) infants and 312 (83%) infants had fever.
The median CSF protein value was 58 mg/dL (interquartile range: 4872 mg/dL). There was an age‐related declined in CSF protein concentration (Figure 1). In linear regression, the CSF protein concentration decreased 6.8% (95% confidence interval [CI], 5.48.1%; P < 0.001) for each 1‐week increase in age.0


CSF protein concentrations were higher for infants 28 days of age than for infants 2956 days of age (P < 0.001, Wilcoxon rank‐sum test). The median CSF protein concentrations were 68 mg/dL (95th percentile value, 115 mg/dL) for infants 28 days of age and 54 mg/dL (95th percentile value, 89 mg/dL) for infants 2956 days. CSF protein concentrations by 2‐week age intervals are shown in Table 1. The 95th percentile CSF protein concentrations were as follows: ages 014 days, 132 mg/dL; ages 1528 days, 100 mg/dL; ages 2942 days, 89 mg/dL; and ages 4356 days, 83 mg/dL (Table 1). CSF protein concentration decreased significantly across each age interval when compared with infants in the next highest age category (P < 0.02 for all pair‐wise comparisons, Wilcoxon rank‐sum test).
| Value | 014 days (n = 52) | 1528 days (n = 87) | 2942 days (n = 110) | 4356 days (n = 126) | All Infants (n = 375) |
|---|---|---|---|---|---|
| |||||
| Mean (SD) | 79 (23) | 69 (20) | 58 (17) | 53 (17) | 62 (21) |
| Median (IQR) | 78 (5893) | 65 (5685) | 55 (4965) | 50 (4162) | 58 (4872) |
| 90th percentile | 106 | 95 | 79 | 75 | 91 |
| 95th percentile | 132 | 100 | 89 | 83 | 99 |
| 95th percentile* | 132 | 101 | 89 | 82 | 97 |
| 95th percentile | 132 | 100 | 87 | 74 | 97 |
Age‐specific 95th percentile CSF protein values changed by <1% when infants receiving antibiotics before lumbar puncture were excluded (Table 1). Age‐specific CSF protein values changed minimally when preterm infants were excluded with the exception of infants 4356 days of age where the 95th percentile value was 9.7% lower than when all infants were included (Table 1); the 90th percentile values in this age group were more comparable at 75 mg/dL and 71 mg/dL, respectively, in the subgroups with and without preterm infants. Age‐specific 95th percentile CSF protein values changes by <1% when patients with CSF pleocytosis were excluded.
Discussion
We examined CSF protein values in neonates and young infants to establish reference values and to bring the literature up to date at a time when molecular tools are commonly used in clinical practice. We also quantified the age‐related decline in CSF protein concentrations over the first two months of life. Our findings provide age‐specific reference ranges for CSF protein concentrations in neonates and young infants. These findings are particularly important because a variety of infectious (eg, herpes simplex virus infection) and noninfectious (eg, subarachnoid or intraventricular hemorrhage) conditions may occur in the absence of appreciable elevations in the CSF WBC.
CSF protein concentrations depend on serum protein concentrations and on the permeability of the blood‐CSF barrier. Immaturity of the blood‐CSF barrier is thought to result in higher CSF protein concentrations for neonates and young infants compared with older children and adults. Though previous studies agree that CSF protein concentrations depend on age, the reported age‐specific values and rates of decline vary considerably.47, 3032 Additionally, these prior studies are limited by (1) small sample size, (2) variable inclusion and exclusion criteria, (3) variable laboratory techniques to quantify protein concentration in a CSF sample, and (4) presentation of mean, standard deviation, and range values rather than the 75th, 90th, or 95th percentile values necessary to define a clinically meaningful reference range.
The median and mean values found in this study were generally comparable to previously published values (Table 2). In addition, we have quantified the age‐related decline in CSF protein concentrations identified in previous studies. While our large sample size allowed us to define narrower reference intervals than most previous studies, direct comparison of values used to define reference ranges was hampered by lack of consistent reporting of data across studies. Ahmed et al.5 and Bonadio et al.4 reported only mean and standard deviation values. When data are skewed, as is the case for CSF protein values, the standard deviation will be grossly inflated, making extrapolation to percentile values unreliable. The 90th percentile value of 87 mg/dL reported by Wong et al.7 for infants 060 days of age was similar to the value of 91 mg/dL for infants 56 days of age and younger found in this study. Biou et al.6 reported the following 95th percentile values: ages 18 days, 108 mg/dL; ages 830 days, 90 mg/dL; and ages 12 months, 77 mg/dL. These values are lower than those reported in our study. The reason for such differences is not clear. The exclusion criteria were similar between the two studies though Biou et al.6 did not include preterm infants. When we excluded preterm infants from our analysis, no age‐specific result decreased by more than 5%, making the inclusion of this population an unlikely explanation for the differences between the two studies.
| Author | Year | Number of Infants | Age (days) | Median (mg/dL) | Mean SD (mg/dL) |
|---|---|---|---|---|---|
| |||||
| Bonadio et al.4 | 1992 | 35 | 030 | 84 45 | |
| 40 | 3060 | 59 25 | |||
| Ahmed et al.5 | 1996 | 17 | 07 | 81 31 | |
| 33 | 814 | 69 23 | |||
| 25 | 1521 | 60 23 | |||
| 33 | 2230 | 54 16 | |||
| Biou et al.6 | 2000 | 26 | 18 | 71 | |
| 76 | 830 | 59 | |||
| 155 | 3060 | 47 | |||
| Wong et al.7 | 2000 | 99 | 060 | 60 | 59 21 |
CSF protein concentration is a method‐dependent value; the results depend a great deal on what technique the laboratory uses. Two common methods used in the past few decades are Biuret Colorimetry and Turbidimetric; reported values are approximately 25% higher with the Biuret method compared with the Turbidimetric method.33 A CSF protein reference value is only clinically useful if the method used to define the norm is specified and equivalent to currently used methods. Similar to our study, Biou et al.6 and Wong et al.7 used the Biuret (Vitros) method. The method of protein measurement was not specified by other studies.4, 5
This study had several limitations that could cause us to overestimate the upper bound of the reference range. First, spectrum bias is possible in this observational study. Individual physicians determined whether lumbar puncture was warranted, a limitation that could potentially lead to the disproportionate inclusion of infants with conditions associated with higher CSF protein concentrations. We do not believe that this limitation would meaningfully affect our results because febrile infants 56 days of age or younger routinely undergo lumbar puncture at our institution, regardless of illness severity, and patients diagnosed with conditions known or suspected to increase CSF protein concentrations were excluded. Second, infants with aseptic meningitisa condition that can be associated with elevated CSF protein concentrationsmay have been misclassified as uninfected. Though we excluded patients with positive CSF enteroviral PCR tests, some infants were not tested and other viruses (eg, parechoviruses)34 not detected by the enterovirus PCR may also cause aseptic meningitis. Third, certain antibiotics including ampicillin and vancomycin are known to interfere with the CSF protein assay used in our laboratory.24 Forty‐two of the 375 subjects included in our final analysis received antibiotics prior to lumbar puncture. When receiving antibiotics prior to lumbar puncture were excluded from analysis, the CSF protein concentrations were within 1% of the overall study population, suggesting that antibiotic administration before lumbar puncture did not influence our results in any meaningful way. We would not expect any of these limitations to disproportionately affect patients in 1 particular age category.
In conclusion, the CSF protein concentration values reported here represent the largest series to‐date for this young age group. Our study quantifies the age‐related decline in CSF protein concentration from birth to 56 days of life. Our work designing this study, specifically the exclusion criteria, refines the approach to defining normal CSF protein values in children. As CSF protein values decline steadily with increasing age, the selection of reference values is a balance of accuracy and convenience. Age‐specific reference values by 2‐week increments would be most accurate. However, considering reference values by month of age, as is the convention for CSF WBCs, is far more practical. The 95th percentile values by age category in our study were as follows: ages 014 days, 132 mg/dL; ages 1528 days, 100 mg/dL; ages 2942 days, 89 mg/dL; and ages 4356 days, 83 mg/dL. The 95th percentile values were 115 mg/dL for infants 28 days and 89 mg/dL for infants 2956 days. We feel that either approach is reasonable. These values can be used to accurately interpret the results of CSF studies in neonates and young infants.
- ,.Henry's Clinical Diagnosis and Management by Laboratory Methods.21st ed.Philadelphia, PA:W.B. Saunders, Inc.;2006.
- .On the cerebrospinal fluid in normal children and in patients with acute abacterial meningo‐encephalitis.Acta Paediatr Suppl.1958;47(Suppl 115):1–102.
- ,.Development of the blood‐CSF barrier.Dev Med Child Neurol.1983;25(2):152–161.
- ,,,,.Reference values of normal cerebrospinal fluid composition in infants ages 0 to 8 weeks.Pediatr Infect Dis J.1992;11(7):589–591.
- ,,, et al.Cerebrospinal fluid values in the term neonate.Pediatr Infect Dis J.1996;15(4):298–303.
- ,,,,,.Cerebrospinal fluid protein concentrations in children: age‐related values in patients without disorders of the central nervous system.Clin Chem.2000;46(3):399–403.
- ,,,,.Cerebrospinal fluid protein concentration in pediatric patients: defining clinically relevant reference values.Arch Pediatr Adolesc Med.2000;154(8):827–831.
- ,,.Nelson Textbook of Pediatrics.17th ed.Philadelphia, PA:Saunders;2004.
- ,,,.Oski's pediatrics : principles 2006.
- Robertson J, Shilkofski N, eds.Johns Hopkins: The Harriet Lane Handbook: A Manual for Pediatric House Officers.17 ed.Philadelphia, PA:Elsevier Mosby;2005.
- ,,,.The Philadelphia Guide: Inpatient Pediatrics.Philadelphia, PA:Lippincott Williams 2005.
- ,,,.Pediatric Hospital Medicine: Textbook of Inpatient Management.Philadelphia, PA:Lippincott Williams 2008.
- ,.Comprehensive pediatric hospital medicine.Philadelphia, PA:Mosby Elsevier;2007.
- ,,.Textbook of Pediatric Emergency Medicine.5th ed.Philadelphia, PA:Lippincott Williams 2006.
- ,,,.Pediatric Emergency Medicine.Philadelphia, PA:Saunders Elsevier;2008.
- ,,,.Textbook of Pediatric Infectious Diseases.5th ed.Philadelphia, PA:Saunders;2004.
- ,.Infectious Diseases of the Fetus and Newborn Infant.6th ed.Philadelphia, PA:Elsevier Saunders;2006.
- ,.Avery's diseases of the newborn.7th ed.Philadelphia, PA:Saunders;1998.
- ,.Child Neurology.6th ed.Philadelphia, PA:Lippincott Williams 2000.
- ,.Pediatric Neurology: Principles and Practice.3rd ed.St. Louis, MO:Mosby;1999.
- ,.Unpredictability of serious bacterial illness in febrile infants from birth to 1 month of age.Arch Pediatr Adolesc Med.1999;153(5):508–511.
- ,,.The efficacy of routine outpatient management without antibiotics of fever in selected infants.Pediatrics.1999;103(3):627–631.
- ,,.Bacterial sepsis and meningitis. In: Remington JS, Klein JO, Wilson CB, Baker CJ, eds.Infectious Diseases of the Fetus and Newborn Infant.6th ed.Philadelphia, PA:Elsevier, Inc.;2006:247–295.
- NCCLS.Interference testing in Clinical Chemistry, NCCLS Document EP7.Wayne, PA:NCCLS;1986.
- ,,,.Lack of cerebrospinal fluid pleocytosis in young infants with enterovirus infections of the central nervous system.Pediatr Emerg Care.2010;26(2):77–81.
- ,,, et al.Clinical and demographic factors associated with urinary tract infection in young febrile infants.Pediatrics.2005;116(3):644–648.
- ,,,,.Enhanced urinalysis as a screening test for urinary tract infection.Pediatrics.1993;91(6):1196–1199.
- ,,,.Screening for urinary tract infection in infants in the emergency department: which test is best?Pediatrics.1998;101(6):E1.
- ,,,,,.Routine cerebrospinal fluid enterovirus polymerase chain reaction testing reduces hospitalization and antibiotic use for infants 90 days of age or younger.Pediatrics.2007;120(3):489–496.
- .The normal cerebro‐spinal fluid in children.Archf Dis Child.1928:96–108.
- .The cerebrospinal fluid in the healthy newborn infant.S Afr Med J.1968;42(35):933–935.
- ,,.Cerebrospinal fluid evaluation in neonates: comparison of high‐risk infants with and without meningitis.J Pediatr.1976;88(3):473–477.
- ,.Estimation of reference intervals for total protein in cerebrospinal fluid.Clin Chem.1989;35(8):1766–1770.
- ,,,,,.Severe neonatal parechovirus infection and similarity with enterovirus infection.Pediatr Infect Dis J.2008;27(3):241–245.
- ,.Henry's Clinical Diagnosis and Management by Laboratory Methods.21st ed.Philadelphia, PA:W.B. Saunders, Inc.;2006.
- .On the cerebrospinal fluid in normal children and in patients with acute abacterial meningo‐encephalitis.Acta Paediatr Suppl.1958;47(Suppl 115):1–102.
- ,.Development of the blood‐CSF barrier.Dev Med Child Neurol.1983;25(2):152–161.
- ,,,,.Reference values of normal cerebrospinal fluid composition in infants ages 0 to 8 weeks.Pediatr Infect Dis J.1992;11(7):589–591.
- ,,, et al.Cerebrospinal fluid values in the term neonate.Pediatr Infect Dis J.1996;15(4):298–303.
- ,,,,,.Cerebrospinal fluid protein concentrations in children: age‐related values in patients without disorders of the central nervous system.Clin Chem.2000;46(3):399–403.
- ,,,,.Cerebrospinal fluid protein concentration in pediatric patients: defining clinically relevant reference values.Arch Pediatr Adolesc Med.2000;154(8):827–831.
- ,,.Nelson Textbook of Pediatrics.17th ed.Philadelphia, PA:Saunders;2004.
- ,,,.Oski's pediatrics : principles 2006.
- Robertson J, Shilkofski N, eds.Johns Hopkins: The Harriet Lane Handbook: A Manual for Pediatric House Officers.17 ed.Philadelphia, PA:Elsevier Mosby;2005.
- ,,,.The Philadelphia Guide: Inpatient Pediatrics.Philadelphia, PA:Lippincott Williams 2005.
- ,,,.Pediatric Hospital Medicine: Textbook of Inpatient Management.Philadelphia, PA:Lippincott Williams 2008.
- ,.Comprehensive pediatric hospital medicine.Philadelphia, PA:Mosby Elsevier;2007.
- ,,.Textbook of Pediatric Emergency Medicine.5th ed.Philadelphia, PA:Lippincott Williams 2006.
- ,,,.Pediatric Emergency Medicine.Philadelphia, PA:Saunders Elsevier;2008.
- ,,,.Textbook of Pediatric Infectious Diseases.5th ed.Philadelphia, PA:Saunders;2004.
- ,.Infectious Diseases of the Fetus and Newborn Infant.6th ed.Philadelphia, PA:Elsevier Saunders;2006.
- ,.Avery's diseases of the newborn.7th ed.Philadelphia, PA:Saunders;1998.
- ,.Child Neurology.6th ed.Philadelphia, PA:Lippincott Williams 2000.
- ,.Pediatric Neurology: Principles and Practice.3rd ed.St. Louis, MO:Mosby;1999.
- ,.Unpredictability of serious bacterial illness in febrile infants from birth to 1 month of age.Arch Pediatr Adolesc Med.1999;153(5):508–511.
- ,,.The efficacy of routine outpatient management without antibiotics of fever in selected infants.Pediatrics.1999;103(3):627–631.
- ,,.Bacterial sepsis and meningitis. In: Remington JS, Klein JO, Wilson CB, Baker CJ, eds.Infectious Diseases of the Fetus and Newborn Infant.6th ed.Philadelphia, PA:Elsevier, Inc.;2006:247–295.
- NCCLS.Interference testing in Clinical Chemistry, NCCLS Document EP7.Wayne, PA:NCCLS;1986.
- ,,,.Lack of cerebrospinal fluid pleocytosis in young infants with enterovirus infections of the central nervous system.Pediatr Emerg Care.2010;26(2):77–81.
- ,,, et al.Clinical and demographic factors associated with urinary tract infection in young febrile infants.Pediatrics.2005;116(3):644–648.
- ,,,,.Enhanced urinalysis as a screening test for urinary tract infection.Pediatrics.1993;91(6):1196–1199.
- ,,,.Screening for urinary tract infection in infants in the emergency department: which test is best?Pediatrics.1998;101(6):E1.
- ,,,,,.Routine cerebrospinal fluid enterovirus polymerase chain reaction testing reduces hospitalization and antibiotic use for infants 90 days of age or younger.Pediatrics.2007;120(3):489–496.
- .The normal cerebro‐spinal fluid in children.Archf Dis Child.1928:96–108.
- .The cerebrospinal fluid in the healthy newborn infant.S Afr Med J.1968;42(35):933–935.
- ,,.Cerebrospinal fluid evaluation in neonates: comparison of high‐risk infants with and without meningitis.J Pediatr.1976;88(3):473–477.
- ,.Estimation of reference intervals for total protein in cerebrospinal fluid.Clin Chem.1989;35(8):1766–1770.
- ,,,,,.Severe neonatal parechovirus infection and similarity with enterovirus infection.Pediatr Infect Dis J.2008;27(3):241–245.
Copyright © 2010 Society of Hospital Medicine
Improving Teamwork with SIDR
Communication among hospital care providers is critically important to provide safe and effective care.15 Yet, studies in operating rooms, intensive care units (ICUs), and general medical units have revealed widely discrepant views on the quality of collaboration and communication between physicians and nurses.68 Although physicians consistently gave high ratings to the quality of collaboration with nurses, nurses rated the quality of collaboration with physicians relatively poorly.
A significant barrier to communication among providers on patient care units is the fluidity and geographic dispersion of team members.8 Physicians, nurses, and other hospital care providers have difficulty finding a way to discuss the care of their patients in person. Research has shown that nurses and physicians on patient care units do not communicate consistently and frequently are not in agreement about their patients' plans of care9, 10
Interdisciplinary Rounds (IDR) have been used as a means to assemble patient care unit team members and improve collaboration on the plan of care.1114 Prior research has demonstrated improved ratings of collaboration on the part of physicians,13, 14 but the effect of IDR on nurses' ratings of collaboration and teamwork has not been adequately assessed. One IDR study did not assess nurses' perceptions,13 while others used instruments not previously described and/or validated in the literature.12, 14 Regarding more concrete outcomes, research indicates variable effects of IDR on length of stay (LOS) and cost. Although 2 studies documented a reduction in LOS and cost with the use of IDR,12, 13 another study showed no effect.15 Furthermore, prior studies evaluated the use of IDR on resident‐covered teaching services. The effect IDR has on collaboration, LOS, and cost in a nonteaching hospitalist service setting is not known.
This study had 3 aims. The first was to assess the impact of an intervention, Structured Inter‐Disciplinary Rounds (SIDR), on nurses' ratings of collaboration and teamwork. The second was to assess the feasibility and sustainability of the intervention. The third was to assess the impact of the intervention on hospital LOS and cost.
Methods
Setting and Study Design
The study was conducted at Northwestern Memorial Hospital (NMH), an 897‐bed tertiary care teaching hospital in Chicago, IL, and was approved by the Institutional Review Board of Northwestern University. The study was a controlled trial of an intervention, SIDR, on collaboration and teamwork on patient care units. One of 2 similar hospitalist service units was randomly selected for the intervention, while the other served as a control unit. SIDR was implemented in August 2008 and data were collected over a 24 week study period.
Each hospitalist service unit consisted of 30 beds and was equipped with continuous cardiac telemetry monitoring. Units were also identical in structure and staffing of nonphysician personnel. The intervention unit included a heart failure‐hospitalist comanagement service. Patients followed at the Center for Heart Failure in the Bluhm Cardiovascular Institute of Northwestern were preferentially admitted to this service. All other patients were admitted to units based on bed availability in a quasi‐randomized fashion. Hospitalists worked 7 consecutive days while on service and cared for patients primarily on the units involved in this study. Therefore, hospitalists cared for patients on both the intervention and control units during their weeks on service. Hospitalists cared for patients independently without the assistance of resident physicians or mid‐level providers (ie, physician assistants or nurse practitioners).
Intervention
SIDR combined a structured format for communication with a forum for regular interdisciplinary meetings. A working group, consisting of nurses, hospitalists, and the unit pharmacist, social worker, and case manager, met weekly for 12 weeks prior to implementation. The working group determined the optimal timing, frequency, and location for SIDR. Additionally, the working group finalized the content of a structured communication tool (Supporting Information) to be used during SIDR. The structured communication tool was modeled after prior research demonstrating the benefit of daily goals of care forms16, 17 and ensured that important elements of the daily plan of care were discussed. Based on the working group's recommendation, SIDR took place each weekday at 11:00 AM in the unit conference room and lasted approximately 30 minutes. The nurse manager and a unit medical director co‐led rounds each day. SIDR was attended by all nurses and hospitalists caring for patients on the unit, as well as the pharmacist, social worker, and case manager assigned to the unit.
Provider Survey
Nurses working on the intervention and control units during the study period were administered a survey 16 weeks to 20 weeks after implementation of SIDR to assess ratings of collaboration and teamwork. The first portion of the survey was based on previously published surveys assessing teamwork attitudes among providers.6, 7 We asked nurses to rate the quality of communication and collaboration they had experienced with hospitalists using a 5‐point ordinal scale (1 = very low, 2 = low, 3 = adequate, 4 = high, 5 = very high). The second portion of the survey assessed teamwork and safety climate using the teamwork and safety domains of the Safety Attitudes Questionnaire (SAQ) developed by Sexton et al.18 The SAQ is based on previous research in aviation and medicine and has been validated in clinical settings.19, 20 Because hospitalists worked with nurses on both units, and in light of our prior research demonstrating that hospitalists rate the quality of collaboration with nurses highly,8 we did not assess hospitalists' ratings of collaboration. A final portion of the survey assessed nurses' perceptions of whether SIDR improved efficiency of communication, collaboration among team members, and patient care using a 5‐point Likert scale (1 = strongly disagree; 2 = disagree; 3 = neutral; 4 = agree; 5 = strongly agree). Hospitalists also received this portion of the survey at the completion of each clinical rotation. All surveys were administered in a web‐based format using an internet link (
SIDR Characteristics and Attendance
The unit medical director recorded the duration of SIDR, the number of patients on the unit, and the number of patients discussed each day. Attendance for each discipline was also recorded each day during the study period.
Data Analysis
Provider demographic data were obtained from completed surveys and group comparisons were done using chi‐square and t tests. The percentage of nurses on each unit rating of the quality of communication and collaboration with hospitalist physicians as high or very high was compared using chi‐square. Teamwork and safety climate scores were compared using the Mann Whitney U test.
Patient data were obtained from administrative databases for both the control and intervention unit during the study period as well as for the intervention unit in the 24 weeks preceding the study period. Demographic data were compared using chi‐square and t tests. Primary discharge diagnosis ICD‐9 codes were grouped into diagnosis clusters using the Healthcare Cost and Utilization Project system of the Agency for Healthcare Research and Quality.21 Diagnosis clusters were then analyzed using the chi‐square test. Because of case mix differences between patients on the intervention and control units, we analyzed LOS and cost using a concurrent control as well as an historic control. Unadjusted LOS and costs were compared using the Mann Whitney U test. We then conducted multivariable linear regression analyses to assess the impact of SIDR on LOS and cost. To satisfy normality requirements and distribution of residuals, we explored 2 methods of transforming skewed data on LOS and cost: logarithmic conversion and truncation at the mean LOS + 3 standard deviations (SDs). Since both techniques yielded similar results, we chose to present results by using truncation. Covariates for multivariable analyses included age, gender, race, payor, admission source, case‐mix, discharge disposition, presence of ICU stay during hospitalization, and Medicare Severity‐Diagnosis Related Group (MS‐DRG) weight. We used standard errors robust to the clustering of patients within each physician. All analyses were conducted using Stata version 10.0 (College Station, TX).
Results
Characteristics of Providers, Patients, and SIDR
Forty‐nine of 58 (84%) nurses completed the survey. Eighty‐eight of 96 (92%) surveys were completed by hospitalists at the end of their week on service. Hospitalist surveys represented 33 different hospitalists because individuals may have worked on study units more than once during the study period. Nurses were a mean 35.0 10.4 years of age and had been working at the hospital for a mean 5.0 6.3 years. Hospitalists were a mean 32.8 2.8 years of age and had been working at the hospital for a mean 2.6 1.9 years.
Patient characteristics are shown in Table 1. Intervention unit patients were admitted from the Emergency Department slightly more often in the postSIDR period. Patient case mix differed between the control and intervention unit, but was similar when comparing the intervention unit preSIDR and postSIDR. Intervention unit MS‐DRG weight was lower in the postSIDR period.
| Control Unit (n = 815) | Intervention Unit Pre‐SIDR (n = 722) | Intervention Unit Post‐SIDR (n = 684) | P Value for Comparison of Intervention Unit Post‐SIDR vs. Control | P Value for Comparison of Intervention Unit Post‐ vs. Pre‐SIDR | |
|---|---|---|---|---|---|
| |||||
| Mean age, years (SD) | 63.8 (16.0) | 64.2 (16.3) | 64.1 (17.2) | 0.74 | 0.92 |
| Women, n (%) | 403 (49) | 347 (48) | 336 (49) | 0.90 | 0.69 |
| Ethnicity, n (%) | 0.22 | 0.71 | |||
| White | 438 (54) | 350 (48) | 334 (49) | ||
| Black | 269 (33) | 266 (37) | 264 (39) | ||
| Hispanic | 48 (6) | 40 (6) | 34 (5) | ||
| Asian | 6 (1) | 8 (1) | 4 (1) | ||
| Other | 54 (7) | 58 (8) | 48 (7) | ||
| Payor, n (%) | 0.07 | 0.67 | |||
| Medicare | 456 (56) | 436 (60) | 399 (58) | ||
| Private | 261 (32) | 176 (24) | 182 (27) | ||
| Medicaid | 67 (8) | 75 (10) | 65 (10) | ||
| Self pay | 31 (4) | 35 (5) | 38 (6) | ||
| Admission source, n (%) | 0.51 | 0.03 | |||
| Emergency department | 695 (85) | 590 (82) | 593 (87) | ||
| Direct admission | 92 (11) | 99 (14) | 65 (10) | ||
| Transfer | 28 (3) | 33 (5) | 26 (4) | ||
| Case mix, n (%) | |||||
| Congestive heart failure | 78 (10) | 164 (23) | 144 (21) | <0.01 | 0.45 |
| Cardiac dysrhythmia | 167 (20) | 69 (10) | 81 (12) | <0.01 | 0.17 |
| Chest pain | 100 (12) | 47 (7) | 59 (9) | 0.02 | 0.13 |
| Coronary atherosclerosis | 52 (6) | 19 (3) | 19 (3) | <0.01 | 0.87 |
| Hypertension | 24 (3) | 38 (5) | 24 (4) | 0.54 | 0.11 |
| Syncope | 27 (3) | 23 (3) | 26 (4) | 0.61 | 0.53 |
| Fluid or electrolyte disorder | 11 (1) | 25 (3) | 23 (3) | 0.01 | 0.92 |
| Pneumonia | 14 (2) | 13 (2) | 22 (3) | 0.06 | 0.09 |
| Pulmonary heart disease | 16 (2) | 13 (2) | 14 (2) | 0.91 | 0.74 |
| Intervertebral disc or other back problem | 32 (4) | 3 (0) | 6 (1) | <0.01 | 0.28 |
| Other diagnosis | 294 (36) | 308 (43) | 266 (39) | 0.26 | 0.15 |
| Cardiovascular procedure during admission | 151 (19) | 95 (13) | 86 (13) | <0.01 | 0.74 |
| Intensive care unit stay during admission, n (%) | 39 (5) | 44 (6) | 27 (4) | 0.43 | 0.07 |
| Discharge disposition, n (%) | |||||
| Home | 736 (90) | 646 (89) | 610 (89) | 0.88 | 0.82 |
| Skilled nursing facility or rehabilitation | 66 (8) | 61 (8) | 63 (9) | ||
| Other facility | 9 (1) | 11 (2) | 7 (1) | ||
| Expired | 4 (0) | 4 (1) | 4 (1) | ||
| Mean Medicare severity ‐diagnosis related group weight (SD) | 1.08 (0.73) | 1.14 (0.76) | 1.06 (0.72) | 0.61 | 0.04 |
SIDR occurred each weekday (with the exception of holidays) on the intervention unit and lasted a mean 27.7 4.6 minutes. The unit had a mean 27 patients per day and 86% of patients on the unit were discussed each day. Attendance exceeded 85% for each discipline (hospitalists, nurses, and the unit pharmacist, social worker, and case manager).
Ratings of Teamwork and Perceptions of SIDR
As shown in Figure 1, a larger percentage of nurses rated the quality of communication and collaboration with hospitalists as high or very high on the intervention unit compared to the control unit (80% vs. 54%; P = 0.05).
Nurses' ratings of the teamwork and safety climate are summarized in Table 2. The median teamwork climate score was 85.7 (interquartile range [IQR], 75.092.9) for the intervention unit as compared to 61.6 (IQR, 48.283.9) for the control unit (P = 0.008). The median safety climate score was 75.0 (IQR, 70.581.3) for the intervention unit as compared to 61.1 (IQR, 30.281.3) for the control unit (P = 0.03).
| Control Unit, n = 24 | Intervention Unit, n = 25 | P Value | |
|---|---|---|---|
| |||
| Median Teamwork Climate Score (IQR) | 75.0 (70.581.3) | 61.6 (48.283.9) | 0.008 |
| Median Safety Climate Score (IQR) | 85.7 (75.092.9) | 61.1 (30.281.3) | 0.03 |
Sixty‐five of 88 (74%) hospitalists and 18 of 24 (75%) nurses agreed that SIDR improved the efficiency of their work day. Eighty of 88 (91%) hospitalists and 18 of 24 (75%) nurses agreed that SIDR improved team collaboration. Seventy‐six of 88 (86%) hospitalists and 18 of 24 (75%) nurses agreed that SIDR improved patient care. Sixty‐seven of 88 (76%) hospitalists and 22 of 25 (88%) nurses indicated that they wanted SIDR to continue indefinitely.
SIDR Impact on LOS and Cost
The unadjusted mean LOS was significantly higher for the intervention unit postSIDR as compared to the control unit (4.0 3.4 vs. 3.7 3.3 days; P = 0.03). However, the unadjusted mean LOS was not significantly different for the intervention unit postSIDR as compared to the intervention unit preSIDR (4.0 3.4 vs. 4.26 3.5 days; P = 0.10). The unadjusted cost was lower for the intervention unit postSIDR as compared to the control unit ($7,513.23 7,085.10 vs. $8,588.66 7,381.03; P < 0.001). The unadjusted mean cost was not significantly different for the invention unit postSIDR as compared to the intervention unit preSIDR ($7,513.23 7,085.10 vs. $7,937.00 7,512.23; P = 0.19).
Multivariable analyses of LOS and cost are summarized in Table 3. The adjusted LOS was not significantly different when comparing the intervention unit postSIDR to either the control unit or the intervention unit preSIDR. The adjusted cost for the intervention unit postSIDR was $739.55 less than the control unit (P = 0.02). The adjusted cost was not significantly different when comparing the intervention unit postSIDR to the intervention unit preSIDR.
| Adjusted Difference for Intervention Unit Post‐SIDR vs. Control | P Value for Adjusted Difference for Intervention Unit Post‐SIDR vs. Control | Adjusted Difference for Intervention Unit Post‐ vs. Pre‐SIDR | P Value for Adjusted Difference for Intervention Unit Post‐ vs. Pre‐SIDR | |
|---|---|---|---|---|
| ||||
| Length of stay | 0.05 | 0.75 | 0.04 | 0.83 |
| Cost | 739.55 | 0.02 | 302.94 | 0.34 |
Discussion
We found that nurses working on a unit using SIDR rated the quality of communication and collaboration with hospitalists significantly higher as compared to a control unit. Notably, because hospitalists worked on both the intervention and control unit during their weeks on service, nurses on each unit were rating the quality of collaboration with the same hospitalists. Nurses also rated the teamwork and safety climate higher on the intervention unit. These findings are important because prior research has shown that nurses are often dissatisfied with the quality of collaboration and teamwork with physicians.68 Potential explanations include fundamental differences between nurses and physicians with regard to status/authority, gender, training, and patient care responsibilities.6 Unfortunately, a culture of poor teamwork may lead to a workplace in which team members feel unable to approach certain individuals and uncomfortable raising concerns. Not surprisingly, higher ratings of teamwork culture have been associated with nurse retention.22, 23 SIDR provided a facilitated forum for interdisciplinary discussion, exchange of critical clinical information, and collaboration on the plan of care.
Our findings are also important because poor communication represents a major etiology of preventable adverse events in hospitals.15 Higher ratings of collaboration and teamwork have been associated with better patient outcomes in observational studies.2426 Further research should evaluate the impact of improved interdisciplinary collaboration as a result of SIDR on the safety of care delivered on inpatient medical units.
The majority of providers agreed that SIDR improved patient care and that SIDR should continue indefinitely. Importantly, providers also felt that SIDR improved the efficiency of their workday and attendance was high among all disciplines. Prior studies on IDR either did not report attendance or struggled with attendance.11 Incorporating the input of frontline providers into the design of SIDR allowed us to create a sustainable intervention which fit into daily workflow.
Our bivariate analyses found significant patient case‐mix differences between the intervention and control unit, limiting our ability to perform direct comparisons in LOS and cost. Pre‐post analyses of LOS and cost may be affected by cyclical or secular trends. Because each approach has its own limitations, we felt that analyses using both an historic as well as a concurrent control would provide a more complete assessment of the effect of the intervention. We included case mix, among other variables, in out multivariable regression analyses and found no benefit to SIDR with regard to LOS and cost. Two prior studies have shown a reduction in LOS and cost with the use of IDR.12, 13 However, one study was conducted approximately 15 years ago and included patients with a longer mean LOS.12 The second study used a pre‐post study design which may not have accounted for unmeasured confounders affecting LOS and cost.13 A third, smaller study showed no effect on LOS and cost with the use of IDR.15 No prior study has evaluated the effect of IDR on LOS and cost in a nonteaching hospitalist service setting.
Our study has several limitations. First, our study reflects the experience of an intervention unit compared to a control unit in a single hospital. Larger studies will be required to test the reproducibility and generalizability of our findings. Second, we did not conduct preintervention provider surveys for comparison ratings of collaboration and teamwork. A prior study, conducted by our research group, found that nurses gave low ratings to the teamwork climate and the quality of collaboration with hospitalists.8 Because this baseline study showed consistently low nurse ratings of collaboration and teamwork across all medical units, and because the units in the current study were identical in size, structure, and staffing of nonphysician personnel, we did not repeat nurse surveys prior to the intervention. Third, as previously mentioned, our study did not directly assess the effect of improved teamwork and collaboration on patient safety. Further study is needed to evaluate this. Although we are not aware of any other interventions to improve interdisciplinary communication on the intervention unit, it is possible that other unknown factors contributed to our findings. We believe this is unlikely due to the magnitude of the improvement in collaboration and the high ratings of SIDR by nurses and physicians on the intervention unit.
In summary, SIDR had a positive effect on nurses' ratings of collaboration and teamwork on a nonteaching hospitalist unit. Future research efforts should assess whether improved teamwork as a result of SIDR also translates into safer patient care.
- Joint Commission on Accreditation of Healthcare Organizations. Sentinel Event Statistics. Available at: http://www.jointcommission.org/SentinelEvents/Statistics. Accessed March2010.
- ,,, et al.A look into the nature and causes of human errors in the intensive care unit.Crit Care Med.1995;23(2):294–300.
- ,,, et al.The nature of adverse events in hospitalized patients. Results of the Harvard Medical Practice Study II.N Engl J Med.1991;324(6):377–384.
- ,,.Communication failures: an insidious contributor to medical mishaps.Acad Med.2004;79(2):186–194.
- ,,,,,.The quality in Australian Health Care Study.Med J Aust.1995;163(9):458–471.
- ,,, et al.Operating room teamwork among physicians and nurses: teamwork in the eye of the beholder.J Am Coll Surg.2006;202(5):746–752.
- ,,.Discrepant attitudes about teamwork among critical care nurses and physicians.Crit Care Med.2003;31(3):956–959.
- ,,,,,.Teamwork on inpatient medical units: assessing attitudes and barriers.Qual Saf Health Care2010;19(2):117–121.
- ,,,,,.Can we talk? Priorities for patient care differed among health care providers:AHRQ;2005.
- ,,, et al.Patterns of nurse—physicians communication and agreement on the plan of care.Qual Saf Health Care. In press.
- ,,, et al.The effect of a multidisciplinary hospitalist/physician and advanced practice nurse collaboration on hospital costs.J Nurs Adm.2006;36(2):79–85.
- ,,.A firm trial of interdisciplinary rounds on the inpatient medical wards: an intervention designed using continuous quality improvement.Med Care.1998;36(8 Suppl):AS4–A12.
- ,,,,.Use of multidisciplinary rounds to simultaneously improve quality outcomes, enhance resident education, and shorten length of stay.J Gen Intern Med.2007;22(8):1073–1079.
- ,,,.Effect of a multidisciplinary intervention on communication and collaboration among physicians and nurses.Am J Crit Care.2005;14(1):71–77.
- ,,,.Effects of interdisciplinary rounds on length of stay in a telemetry unit.J Public Health Manag Pract.2004;10(1):63–69.
- ,,,,.Improving nurse‐physician communication and satisfaction in the intensive care unit with a daily goals worksheet.Am J Crit Care.2006;15(2):217–222.
- ,,,,,.Improving communication in the ICU using daily goals.J Crit Care.2003;18(2):71–75.
- ,,, et al.The Safety Attitudes Questionnaire: psychometric properties, benchmarking data, and emerging research.BMC Health Serv Res.2006;6:44.
- ,,,.Safety Climate Survey: reliability of results from a multicenter ICU survey.Qual Saf Health Care.2005;14(4):273–278.
- ,,, et al.Teamwork in the operating room: frontline perspectives among hospitals and operating room personnel.Anesthesiology.2006;105(5):877–884.
- HCUP Clinical Classification Software [computer program]. Version: Agency for Healthcare Research and Quality, Rockville, MD. Available at: http://www.hcup‐us.ahrq.gov/toolssoftware/ccs/ccs.jsp. Accessed March2010.
- ,,.The influence of teamwork culture on physician and nurse resignation rates in hospitals.Health Serv Manage Res.2008;21(1):23–31.
- .Original research: nurse‐physician relationships: impact on nurse satisfaction and retention.Am J Nurs.2002;102(6):26–34.
- ,,, et al.Association between nurse‐physician collaboration and patient outcomes in three intensive care units.Crit Care Med.1999;27(9):1991–1998.
- ,,,,.Risk‐adjusted morbidity in teaching hospitals correlates with reported levels of communication and collaboration on surgical teams but not with scale measures of teamwork climate, safety climate, or working conditions.J Am Coll Surg.2007;205(6):778–784.
- ,,.The link between teamwork and patients' outcomes in intensive care units.Am J Crit Care.2003;12(6):527–534.
Communication among hospital care providers is critically important to provide safe and effective care.15 Yet, studies in operating rooms, intensive care units (ICUs), and general medical units have revealed widely discrepant views on the quality of collaboration and communication between physicians and nurses.68 Although physicians consistently gave high ratings to the quality of collaboration with nurses, nurses rated the quality of collaboration with physicians relatively poorly.
A significant barrier to communication among providers on patient care units is the fluidity and geographic dispersion of team members.8 Physicians, nurses, and other hospital care providers have difficulty finding a way to discuss the care of their patients in person. Research has shown that nurses and physicians on patient care units do not communicate consistently and frequently are not in agreement about their patients' plans of care9, 10
Interdisciplinary Rounds (IDR) have been used as a means to assemble patient care unit team members and improve collaboration on the plan of care.1114 Prior research has demonstrated improved ratings of collaboration on the part of physicians,13, 14 but the effect of IDR on nurses' ratings of collaboration and teamwork has not been adequately assessed. One IDR study did not assess nurses' perceptions,13 while others used instruments not previously described and/or validated in the literature.12, 14 Regarding more concrete outcomes, research indicates variable effects of IDR on length of stay (LOS) and cost. Although 2 studies documented a reduction in LOS and cost with the use of IDR,12, 13 another study showed no effect.15 Furthermore, prior studies evaluated the use of IDR on resident‐covered teaching services. The effect IDR has on collaboration, LOS, and cost in a nonteaching hospitalist service setting is not known.
This study had 3 aims. The first was to assess the impact of an intervention, Structured Inter‐Disciplinary Rounds (SIDR), on nurses' ratings of collaboration and teamwork. The second was to assess the feasibility and sustainability of the intervention. The third was to assess the impact of the intervention on hospital LOS and cost.
Methods
Setting and Study Design
The study was conducted at Northwestern Memorial Hospital (NMH), an 897‐bed tertiary care teaching hospital in Chicago, IL, and was approved by the Institutional Review Board of Northwestern University. The study was a controlled trial of an intervention, SIDR, on collaboration and teamwork on patient care units. One of 2 similar hospitalist service units was randomly selected for the intervention, while the other served as a control unit. SIDR was implemented in August 2008 and data were collected over a 24 week study period.
Each hospitalist service unit consisted of 30 beds and was equipped with continuous cardiac telemetry monitoring. Units were also identical in structure and staffing of nonphysician personnel. The intervention unit included a heart failure‐hospitalist comanagement service. Patients followed at the Center for Heart Failure in the Bluhm Cardiovascular Institute of Northwestern were preferentially admitted to this service. All other patients were admitted to units based on bed availability in a quasi‐randomized fashion. Hospitalists worked 7 consecutive days while on service and cared for patients primarily on the units involved in this study. Therefore, hospitalists cared for patients on both the intervention and control units during their weeks on service. Hospitalists cared for patients independently without the assistance of resident physicians or mid‐level providers (ie, physician assistants or nurse practitioners).
Intervention
SIDR combined a structured format for communication with a forum for regular interdisciplinary meetings. A working group, consisting of nurses, hospitalists, and the unit pharmacist, social worker, and case manager, met weekly for 12 weeks prior to implementation. The working group determined the optimal timing, frequency, and location for SIDR. Additionally, the working group finalized the content of a structured communication tool (Supporting Information) to be used during SIDR. The structured communication tool was modeled after prior research demonstrating the benefit of daily goals of care forms16, 17 and ensured that important elements of the daily plan of care were discussed. Based on the working group's recommendation, SIDR took place each weekday at 11:00 AM in the unit conference room and lasted approximately 30 minutes. The nurse manager and a unit medical director co‐led rounds each day. SIDR was attended by all nurses and hospitalists caring for patients on the unit, as well as the pharmacist, social worker, and case manager assigned to the unit.
Provider Survey
Nurses working on the intervention and control units during the study period were administered a survey 16 weeks to 20 weeks after implementation of SIDR to assess ratings of collaboration and teamwork. The first portion of the survey was based on previously published surveys assessing teamwork attitudes among providers.6, 7 We asked nurses to rate the quality of communication and collaboration they had experienced with hospitalists using a 5‐point ordinal scale (1 = very low, 2 = low, 3 = adequate, 4 = high, 5 = very high). The second portion of the survey assessed teamwork and safety climate using the teamwork and safety domains of the Safety Attitudes Questionnaire (SAQ) developed by Sexton et al.18 The SAQ is based on previous research in aviation and medicine and has been validated in clinical settings.19, 20 Because hospitalists worked with nurses on both units, and in light of our prior research demonstrating that hospitalists rate the quality of collaboration with nurses highly,8 we did not assess hospitalists' ratings of collaboration. A final portion of the survey assessed nurses' perceptions of whether SIDR improved efficiency of communication, collaboration among team members, and patient care using a 5‐point Likert scale (1 = strongly disagree; 2 = disagree; 3 = neutral; 4 = agree; 5 = strongly agree). Hospitalists also received this portion of the survey at the completion of each clinical rotation. All surveys were administered in a web‐based format using an internet link (
SIDR Characteristics and Attendance
The unit medical director recorded the duration of SIDR, the number of patients on the unit, and the number of patients discussed each day. Attendance for each discipline was also recorded each day during the study period.
Data Analysis
Provider demographic data were obtained from completed surveys and group comparisons were done using chi‐square and t tests. The percentage of nurses on each unit rating of the quality of communication and collaboration with hospitalist physicians as high or very high was compared using chi‐square. Teamwork and safety climate scores were compared using the Mann Whitney U test.
Patient data were obtained from administrative databases for both the control and intervention unit during the study period as well as for the intervention unit in the 24 weeks preceding the study period. Demographic data were compared using chi‐square and t tests. Primary discharge diagnosis ICD‐9 codes were grouped into diagnosis clusters using the Healthcare Cost and Utilization Project system of the Agency for Healthcare Research and Quality.21 Diagnosis clusters were then analyzed using the chi‐square test. Because of case mix differences between patients on the intervention and control units, we analyzed LOS and cost using a concurrent control as well as an historic control. Unadjusted LOS and costs were compared using the Mann Whitney U test. We then conducted multivariable linear regression analyses to assess the impact of SIDR on LOS and cost. To satisfy normality requirements and distribution of residuals, we explored 2 methods of transforming skewed data on LOS and cost: logarithmic conversion and truncation at the mean LOS + 3 standard deviations (SDs). Since both techniques yielded similar results, we chose to present results by using truncation. Covariates for multivariable analyses included age, gender, race, payor, admission source, case‐mix, discharge disposition, presence of ICU stay during hospitalization, and Medicare Severity‐Diagnosis Related Group (MS‐DRG) weight. We used standard errors robust to the clustering of patients within each physician. All analyses were conducted using Stata version 10.0 (College Station, TX).
Results
Characteristics of Providers, Patients, and SIDR
Forty‐nine of 58 (84%) nurses completed the survey. Eighty‐eight of 96 (92%) surveys were completed by hospitalists at the end of their week on service. Hospitalist surveys represented 33 different hospitalists because individuals may have worked on study units more than once during the study period. Nurses were a mean 35.0 10.4 years of age and had been working at the hospital for a mean 5.0 6.3 years. Hospitalists were a mean 32.8 2.8 years of age and had been working at the hospital for a mean 2.6 1.9 years.
Patient characteristics are shown in Table 1. Intervention unit patients were admitted from the Emergency Department slightly more often in the postSIDR period. Patient case mix differed between the control and intervention unit, but was similar when comparing the intervention unit preSIDR and postSIDR. Intervention unit MS‐DRG weight was lower in the postSIDR period.
| Control Unit (n = 815) | Intervention Unit Pre‐SIDR (n = 722) | Intervention Unit Post‐SIDR (n = 684) | P Value for Comparison of Intervention Unit Post‐SIDR vs. Control | P Value for Comparison of Intervention Unit Post‐ vs. Pre‐SIDR | |
|---|---|---|---|---|---|
| |||||
| Mean age, years (SD) | 63.8 (16.0) | 64.2 (16.3) | 64.1 (17.2) | 0.74 | 0.92 |
| Women, n (%) | 403 (49) | 347 (48) | 336 (49) | 0.90 | 0.69 |
| Ethnicity, n (%) | 0.22 | 0.71 | |||
| White | 438 (54) | 350 (48) | 334 (49) | ||
| Black | 269 (33) | 266 (37) | 264 (39) | ||
| Hispanic | 48 (6) | 40 (6) | 34 (5) | ||
| Asian | 6 (1) | 8 (1) | 4 (1) | ||
| Other | 54 (7) | 58 (8) | 48 (7) | ||
| Payor, n (%) | 0.07 | 0.67 | |||
| Medicare | 456 (56) | 436 (60) | 399 (58) | ||
| Private | 261 (32) | 176 (24) | 182 (27) | ||
| Medicaid | 67 (8) | 75 (10) | 65 (10) | ||
| Self pay | 31 (4) | 35 (5) | 38 (6) | ||
| Admission source, n (%) | 0.51 | 0.03 | |||
| Emergency department | 695 (85) | 590 (82) | 593 (87) | ||
| Direct admission | 92 (11) | 99 (14) | 65 (10) | ||
| Transfer | 28 (3) | 33 (5) | 26 (4) | ||
| Case mix, n (%) | |||||
| Congestive heart failure | 78 (10) | 164 (23) | 144 (21) | <0.01 | 0.45 |
| Cardiac dysrhythmia | 167 (20) | 69 (10) | 81 (12) | <0.01 | 0.17 |
| Chest pain | 100 (12) | 47 (7) | 59 (9) | 0.02 | 0.13 |
| Coronary atherosclerosis | 52 (6) | 19 (3) | 19 (3) | <0.01 | 0.87 |
| Hypertension | 24 (3) | 38 (5) | 24 (4) | 0.54 | 0.11 |
| Syncope | 27 (3) | 23 (3) | 26 (4) | 0.61 | 0.53 |
| Fluid or electrolyte disorder | 11 (1) | 25 (3) | 23 (3) | 0.01 | 0.92 |
| Pneumonia | 14 (2) | 13 (2) | 22 (3) | 0.06 | 0.09 |
| Pulmonary heart disease | 16 (2) | 13 (2) | 14 (2) | 0.91 | 0.74 |
| Intervertebral disc or other back problem | 32 (4) | 3 (0) | 6 (1) | <0.01 | 0.28 |
| Other diagnosis | 294 (36) | 308 (43) | 266 (39) | 0.26 | 0.15 |
| Cardiovascular procedure during admission | 151 (19) | 95 (13) | 86 (13) | <0.01 | 0.74 |
| Intensive care unit stay during admission, n (%) | 39 (5) | 44 (6) | 27 (4) | 0.43 | 0.07 |
| Discharge disposition, n (%) | |||||
| Home | 736 (90) | 646 (89) | 610 (89) | 0.88 | 0.82 |
| Skilled nursing facility or rehabilitation | 66 (8) | 61 (8) | 63 (9) | ||
| Other facility | 9 (1) | 11 (2) | 7 (1) | ||
| Expired | 4 (0) | 4 (1) | 4 (1) | ||
| Mean Medicare severity ‐diagnosis related group weight (SD) | 1.08 (0.73) | 1.14 (0.76) | 1.06 (0.72) | 0.61 | 0.04 |
SIDR occurred each weekday (with the exception of holidays) on the intervention unit and lasted a mean 27.7 4.6 minutes. The unit had a mean 27 patients per day and 86% of patients on the unit were discussed each day. Attendance exceeded 85% for each discipline (hospitalists, nurses, and the unit pharmacist, social worker, and case manager).
Ratings of Teamwork and Perceptions of SIDR
As shown in Figure 1, a larger percentage of nurses rated the quality of communication and collaboration with hospitalists as high or very high on the intervention unit compared to the control unit (80% vs. 54%; P = 0.05).

Nurses' ratings of the teamwork and safety climate are summarized in Table 2. The median teamwork climate score was 85.7 (interquartile range [IQR], 75.092.9) for the intervention unit as compared to 61.6 (IQR, 48.283.9) for the control unit (P = 0.008). The median safety climate score was 75.0 (IQR, 70.581.3) for the intervention unit as compared to 61.1 (IQR, 30.281.3) for the control unit (P = 0.03).
| Control Unit, n = 24 | Intervention Unit, n = 25 | P Value | |
|---|---|---|---|
| |||
| Median Teamwork Climate Score (IQR) | 75.0 (70.581.3) | 61.6 (48.283.9) | 0.008 |
| Median Safety Climate Score (IQR) | 85.7 (75.092.9) | 61.1 (30.281.3) | 0.03 |
Sixty‐five of 88 (74%) hospitalists and 18 of 24 (75%) nurses agreed that SIDR improved the efficiency of their work day. Eighty of 88 (91%) hospitalists and 18 of 24 (75%) nurses agreed that SIDR improved team collaboration. Seventy‐six of 88 (86%) hospitalists and 18 of 24 (75%) nurses agreed that SIDR improved patient care. Sixty‐seven of 88 (76%) hospitalists and 22 of 25 (88%) nurses indicated that they wanted SIDR to continue indefinitely.
SIDR Impact on LOS and Cost
The unadjusted mean LOS was significantly higher for the intervention unit postSIDR as compared to the control unit (4.0 3.4 vs. 3.7 3.3 days; P = 0.03). However, the unadjusted mean LOS was not significantly different for the intervention unit postSIDR as compared to the intervention unit preSIDR (4.0 3.4 vs. 4.26 3.5 days; P = 0.10). The unadjusted cost was lower for the intervention unit postSIDR as compared to the control unit ($7,513.23 7,085.10 vs. $8,588.66 7,381.03; P < 0.001). The unadjusted mean cost was not significantly different for the invention unit postSIDR as compared to the intervention unit preSIDR ($7,513.23 7,085.10 vs. $7,937.00 7,512.23; P = 0.19).
Multivariable analyses of LOS and cost are summarized in Table 3. The adjusted LOS was not significantly different when comparing the intervention unit postSIDR to either the control unit or the intervention unit preSIDR. The adjusted cost for the intervention unit postSIDR was $739.55 less than the control unit (P = 0.02). The adjusted cost was not significantly different when comparing the intervention unit postSIDR to the intervention unit preSIDR.
| Adjusted Difference for Intervention Unit Post‐SIDR vs. Control | P Value for Adjusted Difference for Intervention Unit Post‐SIDR vs. Control | Adjusted Difference for Intervention Unit Post‐ vs. Pre‐SIDR | P Value for Adjusted Difference for Intervention Unit Post‐ vs. Pre‐SIDR | |
|---|---|---|---|---|
| ||||
| Length of stay | 0.05 | 0.75 | 0.04 | 0.83 |
| Cost | 739.55 | 0.02 | 302.94 | 0.34 |
Discussion
We found that nurses working on a unit using SIDR rated the quality of communication and collaboration with hospitalists significantly higher as compared to a control unit. Notably, because hospitalists worked on both the intervention and control unit during their weeks on service, nurses on each unit were rating the quality of collaboration with the same hospitalists. Nurses also rated the teamwork and safety climate higher on the intervention unit. These findings are important because prior research has shown that nurses are often dissatisfied with the quality of collaboration and teamwork with physicians.68 Potential explanations include fundamental differences between nurses and physicians with regard to status/authority, gender, training, and patient care responsibilities.6 Unfortunately, a culture of poor teamwork may lead to a workplace in which team members feel unable to approach certain individuals and uncomfortable raising concerns. Not surprisingly, higher ratings of teamwork culture have been associated with nurse retention.22, 23 SIDR provided a facilitated forum for interdisciplinary discussion, exchange of critical clinical information, and collaboration on the plan of care.
Our findings are also important because poor communication represents a major etiology of preventable adverse events in hospitals.15 Higher ratings of collaboration and teamwork have been associated with better patient outcomes in observational studies.2426 Further research should evaluate the impact of improved interdisciplinary collaboration as a result of SIDR on the safety of care delivered on inpatient medical units.
The majority of providers agreed that SIDR improved patient care and that SIDR should continue indefinitely. Importantly, providers also felt that SIDR improved the efficiency of their workday and attendance was high among all disciplines. Prior studies on IDR either did not report attendance or struggled with attendance.11 Incorporating the input of frontline providers into the design of SIDR allowed us to create a sustainable intervention which fit into daily workflow.
Our bivariate analyses found significant patient case‐mix differences between the intervention and control unit, limiting our ability to perform direct comparisons in LOS and cost. Pre‐post analyses of LOS and cost may be affected by cyclical or secular trends. Because each approach has its own limitations, we felt that analyses using both an historic as well as a concurrent control would provide a more complete assessment of the effect of the intervention. We included case mix, among other variables, in out multivariable regression analyses and found no benefit to SIDR with regard to LOS and cost. Two prior studies have shown a reduction in LOS and cost with the use of IDR.12, 13 However, one study was conducted approximately 15 years ago and included patients with a longer mean LOS.12 The second study used a pre‐post study design which may not have accounted for unmeasured confounders affecting LOS and cost.13 A third, smaller study showed no effect on LOS and cost with the use of IDR.15 No prior study has evaluated the effect of IDR on LOS and cost in a nonteaching hospitalist service setting.
Our study has several limitations. First, our study reflects the experience of an intervention unit compared to a control unit in a single hospital. Larger studies will be required to test the reproducibility and generalizability of our findings. Second, we did not conduct preintervention provider surveys for comparison ratings of collaboration and teamwork. A prior study, conducted by our research group, found that nurses gave low ratings to the teamwork climate and the quality of collaboration with hospitalists.8 Because this baseline study showed consistently low nurse ratings of collaboration and teamwork across all medical units, and because the units in the current study were identical in size, structure, and staffing of nonphysician personnel, we did not repeat nurse surveys prior to the intervention. Third, as previously mentioned, our study did not directly assess the effect of improved teamwork and collaboration on patient safety. Further study is needed to evaluate this. Although we are not aware of any other interventions to improve interdisciplinary communication on the intervention unit, it is possible that other unknown factors contributed to our findings. We believe this is unlikely due to the magnitude of the improvement in collaboration and the high ratings of SIDR by nurses and physicians on the intervention unit.
In summary, SIDR had a positive effect on nurses' ratings of collaboration and teamwork on a nonteaching hospitalist unit. Future research efforts should assess whether improved teamwork as a result of SIDR also translates into safer patient care.
Communication among hospital care providers is critically important to provide safe and effective care.15 Yet, studies in operating rooms, intensive care units (ICUs), and general medical units have revealed widely discrepant views on the quality of collaboration and communication between physicians and nurses.68 Although physicians consistently gave high ratings to the quality of collaboration with nurses, nurses rated the quality of collaboration with physicians relatively poorly.
A significant barrier to communication among providers on patient care units is the fluidity and geographic dispersion of team members.8 Physicians, nurses, and other hospital care providers have difficulty finding a way to discuss the care of their patients in person. Research has shown that nurses and physicians on patient care units do not communicate consistently and frequently are not in agreement about their patients' plans of care9, 10
Interdisciplinary Rounds (IDR) have been used as a means to assemble patient care unit team members and improve collaboration on the plan of care.1114 Prior research has demonstrated improved ratings of collaboration on the part of physicians,13, 14 but the effect of IDR on nurses' ratings of collaboration and teamwork has not been adequately assessed. One IDR study did not assess nurses' perceptions,13 while others used instruments not previously described and/or validated in the literature.12, 14 Regarding more concrete outcomes, research indicates variable effects of IDR on length of stay (LOS) and cost. Although 2 studies documented a reduction in LOS and cost with the use of IDR,12, 13 another study showed no effect.15 Furthermore, prior studies evaluated the use of IDR on resident‐covered teaching services. The effect IDR has on collaboration, LOS, and cost in a nonteaching hospitalist service setting is not known.
This study had 3 aims. The first was to assess the impact of an intervention, Structured Inter‐Disciplinary Rounds (SIDR), on nurses' ratings of collaboration and teamwork. The second was to assess the feasibility and sustainability of the intervention. The third was to assess the impact of the intervention on hospital LOS and cost.
Methods
Setting and Study Design
The study was conducted at Northwestern Memorial Hospital (NMH), an 897‐bed tertiary care teaching hospital in Chicago, IL, and was approved by the Institutional Review Board of Northwestern University. The study was a controlled trial of an intervention, SIDR, on collaboration and teamwork on patient care units. One of 2 similar hospitalist service units was randomly selected for the intervention, while the other served as a control unit. SIDR was implemented in August 2008 and data were collected over a 24 week study period.
Each hospitalist service unit consisted of 30 beds and was equipped with continuous cardiac telemetry monitoring. Units were also identical in structure and staffing of nonphysician personnel. The intervention unit included a heart failure‐hospitalist comanagement service. Patients followed at the Center for Heart Failure in the Bluhm Cardiovascular Institute of Northwestern were preferentially admitted to this service. All other patients were admitted to units based on bed availability in a quasi‐randomized fashion. Hospitalists worked 7 consecutive days while on service and cared for patients primarily on the units involved in this study. Therefore, hospitalists cared for patients on both the intervention and control units during their weeks on service. Hospitalists cared for patients independently without the assistance of resident physicians or mid‐level providers (ie, physician assistants or nurse practitioners).
Intervention
SIDR combined a structured format for communication with a forum for regular interdisciplinary meetings. A working group, consisting of nurses, hospitalists, and the unit pharmacist, social worker, and case manager, met weekly for 12 weeks prior to implementation. The working group determined the optimal timing, frequency, and location for SIDR. Additionally, the working group finalized the content of a structured communication tool (Supporting Information) to be used during SIDR. The structured communication tool was modeled after prior research demonstrating the benefit of daily goals of care forms16, 17 and ensured that important elements of the daily plan of care were discussed. Based on the working group's recommendation, SIDR took place each weekday at 11:00 AM in the unit conference room and lasted approximately 30 minutes. The nurse manager and a unit medical director co‐led rounds each day. SIDR was attended by all nurses and hospitalists caring for patients on the unit, as well as the pharmacist, social worker, and case manager assigned to the unit.
Provider Survey
Nurses working on the intervention and control units during the study period were administered a survey 16 weeks to 20 weeks after implementation of SIDR to assess ratings of collaboration and teamwork. The first portion of the survey was based on previously published surveys assessing teamwork attitudes among providers.6, 7 We asked nurses to rate the quality of communication and collaboration they had experienced with hospitalists using a 5‐point ordinal scale (1 = very low, 2 = low, 3 = adequate, 4 = high, 5 = very high). The second portion of the survey assessed teamwork and safety climate using the teamwork and safety domains of the Safety Attitudes Questionnaire (SAQ) developed by Sexton et al.18 The SAQ is based on previous research in aviation and medicine and has been validated in clinical settings.19, 20 Because hospitalists worked with nurses on both units, and in light of our prior research demonstrating that hospitalists rate the quality of collaboration with nurses highly,8 we did not assess hospitalists' ratings of collaboration. A final portion of the survey assessed nurses' perceptions of whether SIDR improved efficiency of communication, collaboration among team members, and patient care using a 5‐point Likert scale (1 = strongly disagree; 2 = disagree; 3 = neutral; 4 = agree; 5 = strongly agree). Hospitalists also received this portion of the survey at the completion of each clinical rotation. All surveys were administered in a web‐based format using an internet link (
SIDR Characteristics and Attendance
The unit medical director recorded the duration of SIDR, the number of patients on the unit, and the number of patients discussed each day. Attendance for each discipline was also recorded each day during the study period.
Data Analysis
Provider demographic data were obtained from completed surveys and group comparisons were done using chi‐square and t tests. The percentage of nurses on each unit rating of the quality of communication and collaboration with hospitalist physicians as high or very high was compared using chi‐square. Teamwork and safety climate scores were compared using the Mann Whitney U test.
Patient data were obtained from administrative databases for both the control and intervention unit during the study period as well as for the intervention unit in the 24 weeks preceding the study period. Demographic data were compared using chi‐square and t tests. Primary discharge diagnosis ICD‐9 codes were grouped into diagnosis clusters using the Healthcare Cost and Utilization Project system of the Agency for Healthcare Research and Quality.21 Diagnosis clusters were then analyzed using the chi‐square test. Because of case mix differences between patients on the intervention and control units, we analyzed LOS and cost using a concurrent control as well as an historic control. Unadjusted LOS and costs were compared using the Mann Whitney U test. We then conducted multivariable linear regression analyses to assess the impact of SIDR on LOS and cost. To satisfy normality requirements and distribution of residuals, we explored 2 methods of transforming skewed data on LOS and cost: logarithmic conversion and truncation at the mean LOS + 3 standard deviations (SDs). Since both techniques yielded similar results, we chose to present results by using truncation. Covariates for multivariable analyses included age, gender, race, payor, admission source, case‐mix, discharge disposition, presence of ICU stay during hospitalization, and Medicare Severity‐Diagnosis Related Group (MS‐DRG) weight. We used standard errors robust to the clustering of patients within each physician. All analyses were conducted using Stata version 10.0 (College Station, TX).
Results
Characteristics of Providers, Patients, and SIDR
Forty‐nine of 58 (84%) nurses completed the survey. Eighty‐eight of 96 (92%) surveys were completed by hospitalists at the end of their week on service. Hospitalist surveys represented 33 different hospitalists because individuals may have worked on study units more than once during the study period. Nurses were a mean 35.0 10.4 years of age and had been working at the hospital for a mean 5.0 6.3 years. Hospitalists were a mean 32.8 2.8 years of age and had been working at the hospital for a mean 2.6 1.9 years.
Patient characteristics are shown in Table 1. Intervention unit patients were admitted from the Emergency Department slightly more often in the postSIDR period. Patient case mix differed between the control and intervention unit, but was similar when comparing the intervention unit preSIDR and postSIDR. Intervention unit MS‐DRG weight was lower in the postSIDR period.
| Control Unit (n = 815) | Intervention Unit Pre‐SIDR (n = 722) | Intervention Unit Post‐SIDR (n = 684) | P Value for Comparison of Intervention Unit Post‐SIDR vs. Control | P Value for Comparison of Intervention Unit Post‐ vs. Pre‐SIDR | |
|---|---|---|---|---|---|
| |||||
| Mean age, years (SD) | 63.8 (16.0) | 64.2 (16.3) | 64.1 (17.2) | 0.74 | 0.92 |
| Women, n (%) | 403 (49) | 347 (48) | 336 (49) | 0.90 | 0.69 |
| Ethnicity, n (%) | 0.22 | 0.71 | |||
| White | 438 (54) | 350 (48) | 334 (49) | ||
| Black | 269 (33) | 266 (37) | 264 (39) | ||
| Hispanic | 48 (6) | 40 (6) | 34 (5) | ||
| Asian | 6 (1) | 8 (1) | 4 (1) | ||
| Other | 54 (7) | 58 (8) | 48 (7) | ||
| Payor, n (%) | 0.07 | 0.67 | |||
| Medicare | 456 (56) | 436 (60) | 399 (58) | ||
| Private | 261 (32) | 176 (24) | 182 (27) | ||
| Medicaid | 67 (8) | 75 (10) | 65 (10) | ||
| Self pay | 31 (4) | 35 (5) | 38 (6) | ||
| Admission source, n (%) | 0.51 | 0.03 | |||
| Emergency department | 695 (85) | 590 (82) | 593 (87) | ||
| Direct admission | 92 (11) | 99 (14) | 65 (10) | ||
| Transfer | 28 (3) | 33 (5) | 26 (4) | ||
| Case mix, n (%) | |||||
| Congestive heart failure | 78 (10) | 164 (23) | 144 (21) | <0.01 | 0.45 |
| Cardiac dysrhythmia | 167 (20) | 69 (10) | 81 (12) | <0.01 | 0.17 |
| Chest pain | 100 (12) | 47 (7) | 59 (9) | 0.02 | 0.13 |
| Coronary atherosclerosis | 52 (6) | 19 (3) | 19 (3) | <0.01 | 0.87 |
| Hypertension | 24 (3) | 38 (5) | 24 (4) | 0.54 | 0.11 |
| Syncope | 27 (3) | 23 (3) | 26 (4) | 0.61 | 0.53 |
| Fluid or electrolyte disorder | 11 (1) | 25 (3) | 23 (3) | 0.01 | 0.92 |
| Pneumonia | 14 (2) | 13 (2) | 22 (3) | 0.06 | 0.09 |
| Pulmonary heart disease | 16 (2) | 13 (2) | 14 (2) | 0.91 | 0.74 |
| Intervertebral disc or other back problem | 32 (4) | 3 (0) | 6 (1) | <0.01 | 0.28 |
| Other diagnosis | 294 (36) | 308 (43) | 266 (39) | 0.26 | 0.15 |
| Cardiovascular procedure during admission | 151 (19) | 95 (13) | 86 (13) | <0.01 | 0.74 |
| Intensive care unit stay during admission, n (%) | 39 (5) | 44 (6) | 27 (4) | 0.43 | 0.07 |
| Discharge disposition, n (%) | |||||
| Home | 736 (90) | 646 (89) | 610 (89) | 0.88 | 0.82 |
| Skilled nursing facility or rehabilitation | 66 (8) | 61 (8) | 63 (9) | ||
| Other facility | 9 (1) | 11 (2) | 7 (1) | ||
| Expired | 4 (0) | 4 (1) | 4 (1) | ||
| Mean Medicare severity ‐diagnosis related group weight (SD) | 1.08 (0.73) | 1.14 (0.76) | 1.06 (0.72) | 0.61 | 0.04 |
SIDR occurred each weekday (with the exception of holidays) on the intervention unit and lasted a mean 27.7 4.6 minutes. The unit had a mean 27 patients per day and 86% of patients on the unit were discussed each day. Attendance exceeded 85% for each discipline (hospitalists, nurses, and the unit pharmacist, social worker, and case manager).
Ratings of Teamwork and Perceptions of SIDR
As shown in Figure 1, a larger percentage of nurses rated the quality of communication and collaboration with hospitalists as high or very high on the intervention unit compared to the control unit (80% vs. 54%; P = 0.05).

Nurses' ratings of the teamwork and safety climate are summarized in Table 2. The median teamwork climate score was 85.7 (interquartile range [IQR], 75.092.9) for the intervention unit as compared to 61.6 (IQR, 48.283.9) for the control unit (P = 0.008). The median safety climate score was 75.0 (IQR, 70.581.3) for the intervention unit as compared to 61.1 (IQR, 30.281.3) for the control unit (P = 0.03).
| Control Unit, n = 24 | Intervention Unit, n = 25 | P Value | |
|---|---|---|---|
| |||
| Median Teamwork Climate Score (IQR) | 75.0 (70.581.3) | 61.6 (48.283.9) | 0.008 |
| Median Safety Climate Score (IQR) | 85.7 (75.092.9) | 61.1 (30.281.3) | 0.03 |
Sixty‐five of 88 (74%) hospitalists and 18 of 24 (75%) nurses agreed that SIDR improved the efficiency of their work day. Eighty of 88 (91%) hospitalists and 18 of 24 (75%) nurses agreed that SIDR improved team collaboration. Seventy‐six of 88 (86%) hospitalists and 18 of 24 (75%) nurses agreed that SIDR improved patient care. Sixty‐seven of 88 (76%) hospitalists and 22 of 25 (88%) nurses indicated that they wanted SIDR to continue indefinitely.
SIDR Impact on LOS and Cost
The unadjusted mean LOS was significantly higher for the intervention unit postSIDR as compared to the control unit (4.0 3.4 vs. 3.7 3.3 days; P = 0.03). However, the unadjusted mean LOS was not significantly different for the intervention unit postSIDR as compared to the intervention unit preSIDR (4.0 3.4 vs. 4.26 3.5 days; P = 0.10). The unadjusted cost was lower for the intervention unit postSIDR as compared to the control unit ($7,513.23 7,085.10 vs. $8,588.66 7,381.03; P < 0.001). The unadjusted mean cost was not significantly different for the invention unit postSIDR as compared to the intervention unit preSIDR ($7,513.23 7,085.10 vs. $7,937.00 7,512.23; P = 0.19).
Multivariable analyses of LOS and cost are summarized in Table 3. The adjusted LOS was not significantly different when comparing the intervention unit postSIDR to either the control unit or the intervention unit preSIDR. The adjusted cost for the intervention unit postSIDR was $739.55 less than the control unit (P = 0.02). The adjusted cost was not significantly different when comparing the intervention unit postSIDR to the intervention unit preSIDR.
| Adjusted Difference for Intervention Unit Post‐SIDR vs. Control | P Value for Adjusted Difference for Intervention Unit Post‐SIDR vs. Control | Adjusted Difference for Intervention Unit Post‐ vs. Pre‐SIDR | P Value for Adjusted Difference for Intervention Unit Post‐ vs. Pre‐SIDR | |
|---|---|---|---|---|
| ||||
| Length of stay | 0.05 | 0.75 | 0.04 | 0.83 |
| Cost | 739.55 | 0.02 | 302.94 | 0.34 |
Discussion
We found that nurses working on a unit using SIDR rated the quality of communication and collaboration with hospitalists significantly higher as compared to a control unit. Notably, because hospitalists worked on both the intervention and control unit during their weeks on service, nurses on each unit were rating the quality of collaboration with the same hospitalists. Nurses also rated the teamwork and safety climate higher on the intervention unit. These findings are important because prior research has shown that nurses are often dissatisfied with the quality of collaboration and teamwork with physicians.68 Potential explanations include fundamental differences between nurses and physicians with regard to status/authority, gender, training, and patient care responsibilities.6 Unfortunately, a culture of poor teamwork may lead to a workplace in which team members feel unable to approach certain individuals and uncomfortable raising concerns. Not surprisingly, higher ratings of teamwork culture have been associated with nurse retention.22, 23 SIDR provided a facilitated forum for interdisciplinary discussion, exchange of critical clinical information, and collaboration on the plan of care.
Our findings are also important because poor communication represents a major etiology of preventable adverse events in hospitals.15 Higher ratings of collaboration and teamwork have been associated with better patient outcomes in observational studies.2426 Further research should evaluate the impact of improved interdisciplinary collaboration as a result of SIDR on the safety of care delivered on inpatient medical units.
The majority of providers agreed that SIDR improved patient care and that SIDR should continue indefinitely. Importantly, providers also felt that SIDR improved the efficiency of their workday and attendance was high among all disciplines. Prior studies on IDR either did not report attendance or struggled with attendance.11 Incorporating the input of frontline providers into the design of SIDR allowed us to create a sustainable intervention which fit into daily workflow.
Our bivariate analyses found significant patient case‐mix differences between the intervention and control unit, limiting our ability to perform direct comparisons in LOS and cost. Pre‐post analyses of LOS and cost may be affected by cyclical or secular trends. Because each approach has its own limitations, we felt that analyses using both an historic as well as a concurrent control would provide a more complete assessment of the effect of the intervention. We included case mix, among other variables, in out multivariable regression analyses and found no benefit to SIDR with regard to LOS and cost. Two prior studies have shown a reduction in LOS and cost with the use of IDR.12, 13 However, one study was conducted approximately 15 years ago and included patients with a longer mean LOS.12 The second study used a pre‐post study design which may not have accounted for unmeasured confounders affecting LOS and cost.13 A third, smaller study showed no effect on LOS and cost with the use of IDR.15 No prior study has evaluated the effect of IDR on LOS and cost in a nonteaching hospitalist service setting.
Our study has several limitations. First, our study reflects the experience of an intervention unit compared to a control unit in a single hospital. Larger studies will be required to test the reproducibility and generalizability of our findings. Second, we did not conduct preintervention provider surveys for comparison ratings of collaboration and teamwork. A prior study, conducted by our research group, found that nurses gave low ratings to the teamwork climate and the quality of collaboration with hospitalists.8 Because this baseline study showed consistently low nurse ratings of collaboration and teamwork across all medical units, and because the units in the current study were identical in size, structure, and staffing of nonphysician personnel, we did not repeat nurse surveys prior to the intervention. Third, as previously mentioned, our study did not directly assess the effect of improved teamwork and collaboration on patient safety. Further study is needed to evaluate this. Although we are not aware of any other interventions to improve interdisciplinary communication on the intervention unit, it is possible that other unknown factors contributed to our findings. We believe this is unlikely due to the magnitude of the improvement in collaboration and the high ratings of SIDR by nurses and physicians on the intervention unit.
In summary, SIDR had a positive effect on nurses' ratings of collaboration and teamwork on a nonteaching hospitalist unit. Future research efforts should assess whether improved teamwork as a result of SIDR also translates into safer patient care.
- Joint Commission on Accreditation of Healthcare Organizations. Sentinel Event Statistics. Available at: http://www.jointcommission.org/SentinelEvents/Statistics. Accessed March2010.
- ,,, et al.A look into the nature and causes of human errors in the intensive care unit.Crit Care Med.1995;23(2):294–300.
- ,,, et al.The nature of adverse events in hospitalized patients. Results of the Harvard Medical Practice Study II.N Engl J Med.1991;324(6):377–384.
- ,,.Communication failures: an insidious contributor to medical mishaps.Acad Med.2004;79(2):186–194.
- ,,,,,.The quality in Australian Health Care Study.Med J Aust.1995;163(9):458–471.
- ,,, et al.Operating room teamwork among physicians and nurses: teamwork in the eye of the beholder.J Am Coll Surg.2006;202(5):746–752.
- ,,.Discrepant attitudes about teamwork among critical care nurses and physicians.Crit Care Med.2003;31(3):956–959.
- ,,,,,.Teamwork on inpatient medical units: assessing attitudes and barriers.Qual Saf Health Care2010;19(2):117–121.
- ,,,,,.Can we talk? Priorities for patient care differed among health care providers:AHRQ;2005.
- ,,, et al.Patterns of nurse—physicians communication and agreement on the plan of care.Qual Saf Health Care. In press.
- ,,, et al.The effect of a multidisciplinary hospitalist/physician and advanced practice nurse collaboration on hospital costs.J Nurs Adm.2006;36(2):79–85.
- ,,.A firm trial of interdisciplinary rounds on the inpatient medical wards: an intervention designed using continuous quality improvement.Med Care.1998;36(8 Suppl):AS4–A12.
- ,,,,.Use of multidisciplinary rounds to simultaneously improve quality outcomes, enhance resident education, and shorten length of stay.J Gen Intern Med.2007;22(8):1073–1079.
- ,,,.Effect of a multidisciplinary intervention on communication and collaboration among physicians and nurses.Am J Crit Care.2005;14(1):71–77.
- ,,,.Effects of interdisciplinary rounds on length of stay in a telemetry unit.J Public Health Manag Pract.2004;10(1):63–69.
- ,,,,.Improving nurse‐physician communication and satisfaction in the intensive care unit with a daily goals worksheet.Am J Crit Care.2006;15(2):217–222.
- ,,,,,.Improving communication in the ICU using daily goals.J Crit Care.2003;18(2):71–75.
- ,,, et al.The Safety Attitudes Questionnaire: psychometric properties, benchmarking data, and emerging research.BMC Health Serv Res.2006;6:44.
- ,,,.Safety Climate Survey: reliability of results from a multicenter ICU survey.Qual Saf Health Care.2005;14(4):273–278.
- ,,, et al.Teamwork in the operating room: frontline perspectives among hospitals and operating room personnel.Anesthesiology.2006;105(5):877–884.
- HCUP Clinical Classification Software [computer program]. Version: Agency for Healthcare Research and Quality, Rockville, MD. Available at: http://www.hcup‐us.ahrq.gov/toolssoftware/ccs/ccs.jsp. Accessed March2010.
- ,,.The influence of teamwork culture on physician and nurse resignation rates in hospitals.Health Serv Manage Res.2008;21(1):23–31.
- .Original research: nurse‐physician relationships: impact on nurse satisfaction and retention.Am J Nurs.2002;102(6):26–34.
- ,,, et al.Association between nurse‐physician collaboration and patient outcomes in three intensive care units.Crit Care Med.1999;27(9):1991–1998.
- ,,,,.Risk‐adjusted morbidity in teaching hospitals correlates with reported levels of communication and collaboration on surgical teams but not with scale measures of teamwork climate, safety climate, or working conditions.J Am Coll Surg.2007;205(6):778–784.
- ,,.The link between teamwork and patients' outcomes in intensive care units.Am J Crit Care.2003;12(6):527–534.
- Joint Commission on Accreditation of Healthcare Organizations. Sentinel Event Statistics. Available at: http://www.jointcommission.org/SentinelEvents/Statistics. Accessed March2010.
- ,,, et al.A look into the nature and causes of human errors in the intensive care unit.Crit Care Med.1995;23(2):294–300.
- ,,, et al.The nature of adverse events in hospitalized patients. Results of the Harvard Medical Practice Study II.N Engl J Med.1991;324(6):377–384.
- ,,.Communication failures: an insidious contributor to medical mishaps.Acad Med.2004;79(2):186–194.
- ,,,,,.The quality in Australian Health Care Study.Med J Aust.1995;163(9):458–471.
- ,,, et al.Operating room teamwork among physicians and nurses: teamwork in the eye of the beholder.J Am Coll Surg.2006;202(5):746–752.
- ,,.Discrepant attitudes about teamwork among critical care nurses and physicians.Crit Care Med.2003;31(3):956–959.
- ,,,,,.Teamwork on inpatient medical units: assessing attitudes and barriers.Qual Saf Health Care2010;19(2):117–121.
- ,,,,,.Can we talk? Priorities for patient care differed among health care providers:AHRQ;2005.
- ,,, et al.Patterns of nurse—physicians communication and agreement on the plan of care.Qual Saf Health Care. In press.
- ,,, et al.The effect of a multidisciplinary hospitalist/physician and advanced practice nurse collaboration on hospital costs.J Nurs Adm.2006;36(2):79–85.
- ,,.A firm trial of interdisciplinary rounds on the inpatient medical wards: an intervention designed using continuous quality improvement.Med Care.1998;36(8 Suppl):AS4–A12.
- ,,,,.Use of multidisciplinary rounds to simultaneously improve quality outcomes, enhance resident education, and shorten length of stay.J Gen Intern Med.2007;22(8):1073–1079.
- ,,,.Effect of a multidisciplinary intervention on communication and collaboration among physicians and nurses.Am J Crit Care.2005;14(1):71–77.
- ,,,.Effects of interdisciplinary rounds on length of stay in a telemetry unit.J Public Health Manag Pract.2004;10(1):63–69.
- ,,,,.Improving nurse‐physician communication and satisfaction in the intensive care unit with a daily goals worksheet.Am J Crit Care.2006;15(2):217–222.
- ,,,,,.Improving communication in the ICU using daily goals.J Crit Care.2003;18(2):71–75.
- ,,, et al.The Safety Attitudes Questionnaire: psychometric properties, benchmarking data, and emerging research.BMC Health Serv Res.2006;6:44.
- ,,,.Safety Climate Survey: reliability of results from a multicenter ICU survey.Qual Saf Health Care.2005;14(4):273–278.
- ,,, et al.Teamwork in the operating room: frontline perspectives among hospitals and operating room personnel.Anesthesiology.2006;105(5):877–884.
- HCUP Clinical Classification Software [computer program]. Version: Agency for Healthcare Research and Quality, Rockville, MD. Available at: http://www.hcup‐us.ahrq.gov/toolssoftware/ccs/ccs.jsp. Accessed March2010.
- ,,.The influence of teamwork culture on physician and nurse resignation rates in hospitals.Health Serv Manage Res.2008;21(1):23–31.
- .Original research: nurse‐physician relationships: impact on nurse satisfaction and retention.Am J Nurs.2002;102(6):26–34.
- ,,, et al.Association between nurse‐physician collaboration and patient outcomes in three intensive care units.Crit Care Med.1999;27(9):1991–1998.
- ,,,,.Risk‐adjusted morbidity in teaching hospitals correlates with reported levels of communication and collaboration on surgical teams but not with scale measures of teamwork climate, safety climate, or working conditions.J Am Coll Surg.2007;205(6):778–784.
- ,,.The link between teamwork and patients' outcomes in intensive care units.Am J Crit Care.2003;12(6):527–534.
Copyright © 2010 Society of Hospital Medicine
Malignant Otitis Externa
Malignant Otitis Externa (MOE) is a necrotizing infection of the external auditory canal characterized by extension into nearby soft tissue and bony structures that can potentially lead to mastoiditis, skull base osteomyelitis, cranial nerve palsies, and rarely, intracranial complications. MOE has been classically described as a disease affecting elderly diabetics1 and has been reported in immunocompromised patients with acquired immune deficiency syndrome (AIDS), malignancy, patients receiving chemotherapy, and neutropenic children.24 The incidence of MOE in the general population is estimated to be quite low and difficult to determine.5 However, over the past decades, the number of reported cases has been increasing, suggesting increased awareness of this syndrome by primary care physicians.6
Case Report
A 56‐year‐old white male was brought to the emergency department with altered mental status including decreased level of consciousness, bizarre behavior, headaches, and nausea for several weeks. He had a history of alcohol and cocaine abuse. He was homeless and a smoker. On examination, the patient was lethargic, disoriented with respect to time, place and person. Blood pressure was 107/72 mm Hg, heart rate 85 beats per minute; respirations were 16 per minute and temperature was 97.9F. Neurological examination was significant for loss of vision of the left eye and left facial peripheral nerve palsy. Examination of the left eye showed yellowish‐greenish discharge, a lower lid ectropium with upper lid ptosis, conjunctival erythema, and an 8 mm 6 mm abrasion in the medial half of the cornea. He had purulent drainage from the left ear with small vesicular lesions on the auricle, and a 3 cm 4 cm abscess on the right forearm. The remainder of the physical examination was unremarkable.
Laboratory‐test results showed white blood cell (WBC) count; 11.27 109 cells/L with 76.6% neutrophils. A complete metabolic panel was within normal limits. Human immunodeficiency virus (HIV) and RPR testing were negative. Cerebrospinal fluid (CSF) studies demonstrated a WBC 39 cells/mm3 with lymphocytes predominance (85%), Red blood cell (RBC) 6 cells/mm3, protein 48 mg/dL, and glucose; 63 mg/dL. Computed tomographic scan of the head revealed an area of low attenuation with surrounding edema of the left temporal lobe and fracture of the temporal bone on the superior margin of the mastoid air cells extending into the left mastoid air cells (Figure 1). The fluid draining from the patient's left ear grew 2 different strains of Pseudomonas aeruginosa. Magnetic resonance imaging (MRI) of the brain demonstrated a 2.5 cm to 3.0 cm region of multiple loculations and edema in the left temporal lobe representing cerebritis with abscess and complete opacification of left mastoid air cell suggestive of mastoiditis (Figure 2). Piperacillin/tazobactam and tobramycin were initiated for suspected MOE with brain involvement. CSF and blood cultures were negative.
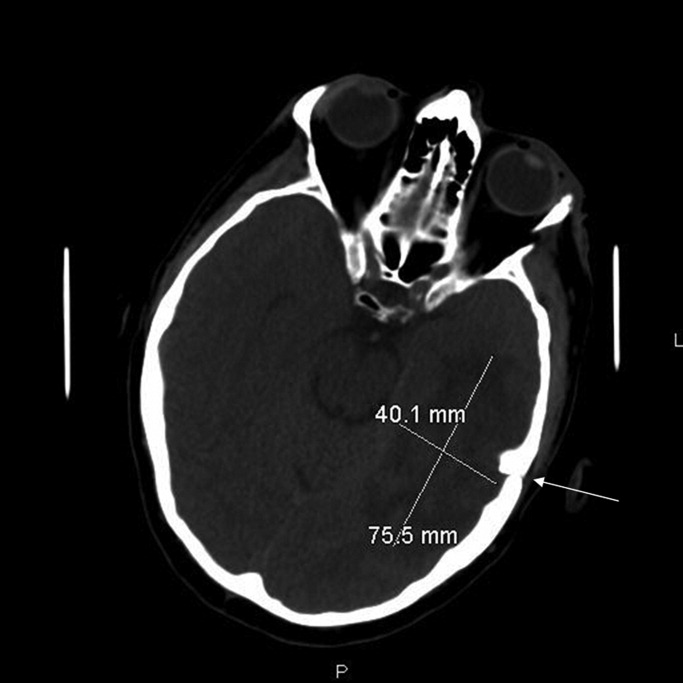
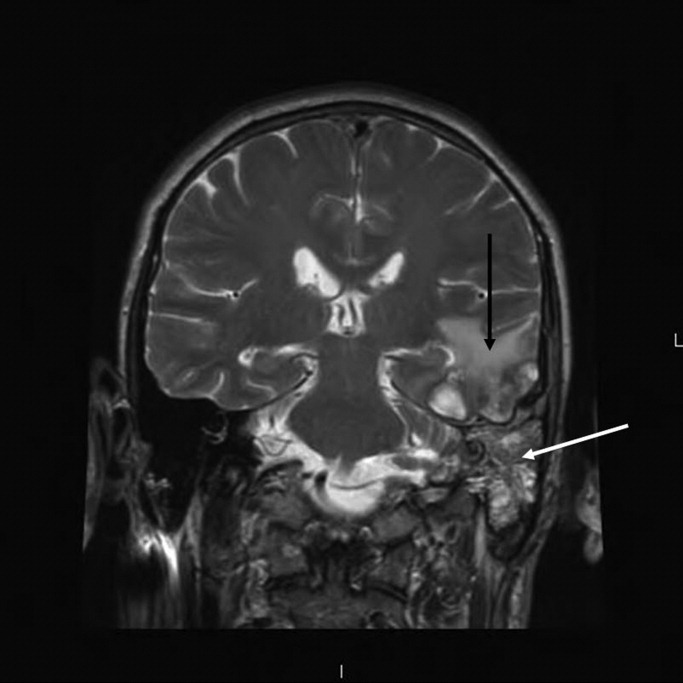
A repeat MRI at 3 weeks of therapy demonstrated interval improvement in the temporal lobe abscess and the edema surrounding the infection. At the time of discharge, after 6 weeks of antimicrobial therapy, the patient was alert and oriented with respect to person, place, and time.
Discussion
Pseudomonas aeruginosa is the most common organism cultured from MOE.4, 5 Other organisms such as Staphylococcus aureus,6 Proteus mirabilis, Klebsiella oxytocea,7 and Aspergillus species8 have been reported as well.
While the exact pathogenesis of MOE is poorly understood, accidental trauma from cotton swabs, exposure to lake water, swimming pool water, and repeated aural lavage have all been implicated as inciting factors.9 The current literature suggests that Pseudomonal otitis externa occurs due to abnormal host defense mechanisms rather than enhanced pathogen colonization.10
MOE typically presents with severe otalgia, headache, auricular tenderness, mastoid tenderness, or persistent otorrhea. The pain of MOE is usually severe, and the classic signs of infection such as fever, leukocytosis and neutrophil predominance (left shift) may not be present.5 The diagnosis can be confirmed by otoscopic exam which will demonstrate granulation tissue at the junction between the bony and cartilaginous tissues in the external auditory canal. MOE can produce certain physical findings that should raise red flags for local extension. Temporomandibular joint pain in the susceptible patient with otalgia could indicate MOE with joint invasion. Cranial nerve involvement, most commonly involves the seventh cranial nerve which results in a facial palsy. Other cranial neuropathies have been reported in MOE such as the glossopharyngeal, vagal, spinal accessory, and hypoglossal nerves.11 Confusion and nuchal rigidity should arouse suspicion of intracranial extension of the infection.
The diagnosis of MOE is usually made by a constellation of clinical, microbiological and radiological features. The first attempt at defining diagnostic criteria for MOE was in 1987, when Cohen and Friedman named several obligatory signs such as pain, exudate, edema, granulation tissue in the external ear canal, the presence of microabscesses if surgery is performed, and either a positive Tc‐99m bone scan or failure of local treatment for 1 week.12
Recent literature reviews emphasize that a positive bacterial or fungal culture of the external ear canal can help make the diagnosis of MOE.4 A recent study looking at diagnostic criteria noted that MOE may be present even without meeting all of these major criteria (clinical, microbiological, and radiological features).13 Some authors suggest that ear biopsy be considered if malignancy is a reasonable possibility.9
Other laboratory data can be within normal limits, such as a WBC count or differential and metabolic profiles.4 Erythrocyte sedimentation rate (ESR), while non‐specific, has been reported to be markedly increased in the setting of MOE.5 It is recommended that a baseline ESR be obtained, and then used to follow the response to treatment.14
Computed tomography (CT) scanning is considered the appropriate initial imaging study, however, there are mixed reports about whether a CT scan alone is enough to evaluate disease severity and its complications.15 While CT scan is quite sensitive for demonstrating bony destruction associated with MOE, MRI is better at detecting the soft tissue changes associated with MOE and more useful for following disease resolution after treatment.16
The treatment of MOE has evolved over time. Before the introduction of effective anti‐pseudomonal antibiotics, MOE had an associated mortality near 50%, and surgery was the recommended therapy.17 Currently, given the availability of effective anti‐pseudomonal therapy, there is usually no indication for surgery as a primary treatment in MOE. For sensitive pseudomonal species, oral Ciprofloxacin therapy for 6 to 8 weeks is considered the treatment of choice for MOE.18 Clinicians should be aware of the emergence of quinolone resistance in P. Aeruginosa,19 and antibiotic sensitivities should be performed on the culture to guide further treatment. In the setting of resistant or multi‐species infection, one should obtain external ear canal biopsy with wound debridement and begin long‐term intravenous therapy with an antipseudomonal beta‐lactam plus an aminoglycoside.18 In the setting of extension into the cranium causing cerebritis or abscess, it is recommended that a neurosurgeon be consulted immediately to determine whether the lesion is amenable to aspiration, excision, or watchful waiting with imaging follow up.
This case illustrates a serious complication from MOE, with ipsilateral facial nerve palsy, opthalmitis, mastoiditis, and cerebral abscess that was successfully treated with conservative medical management.
- .Malignant external otitis.Laryngoscope.1968;78:1257.
- ,,, et al.Necrotizing external otitis in patients with AIDS.Laryngoscope.1997;107:456.
- ,,,.Malignant external otitis in an infant.J Laryngol Otol.1990;104(6):488–490.
- ,,.The changing face of malignant (necrotising) external otitis: clinical, radiological, and anatomic correlations.Lancet Infect Dis.2004;4:34.
- ,.Malignant external otitis: insights into pathogenesis, clinical manifestations, diagnosis, and therapy.Am J Med.1988;85:391.
- ,,Staphylococcal malignant external otitis.Can Med Assoc J.1982;126:155.
- ,,, et al.A case of malignant external otitis involving Klebsiella oxytoca.Eur J Clin Microbiol Infect Dis.1992;11:75.
- ,.Invasive otitis externa due to Aspergillus species: case report and review.Clin Infect Dis.1994;19:866.
- ,.Necrotizing (malignant) external otitis.Am Fam Physician.2003;68(2):309–312.
- ,,,.Bacterial flora of the external canal in diabetics and non‐diabetics.Laryngoscope.1982;92:672.
- ,,.Neurologic complications of malignant external otitis.Neurology.1971;21:1077–1084.
- ,.The diagnostic criteria of malignant external otitis.J Laryngol Otol.1987;101:216–221.
- ,,,,.Stratification for malignant external otitis.Otolaryngol Head Neck Surg.2007;137(2):301–305.
- ,,,,,.Efficacy of oral ciprofloxacin plus rifampin for treatment of malignant external otitis.Arch Otolaryngol Head Neck Surg.1989;115:1063–1069.
- ,,,,,.Usefulness of CT scans in malignant external otitis: effective tool for the diagnosis, but of limited value in predicting outcome.Eur Arch Otorhinolaryngol.2008;265(1):53–56.
- ,,.Use of magnetic resonance imaging as the primary imaging modality in the diagnosis and follow‐up of malignant external otitis.J Laryngol Otol.2004;118:576.
- ,.Skull base osteomyelitis. Malignant external otitis.Otolaryngol Clin North Am.1996;29(5):795–806.
- .Ciprofloxacin: an oral quinolone for the treatment of infections with gram‐negative pathogens. Committee on Antimicrobial Agents. Canadian Infectious Disease Society.Can Med Assoc J.1994;150(5):669–676.
- ,,,.Resistance of Pseudomonas to ciprofloxacin: implications for the treatment of malignant otitis externa.J Laryngol Otol.2007;121:118–123.
Malignant Otitis Externa (MOE) is a necrotizing infection of the external auditory canal characterized by extension into nearby soft tissue and bony structures that can potentially lead to mastoiditis, skull base osteomyelitis, cranial nerve palsies, and rarely, intracranial complications. MOE has been classically described as a disease affecting elderly diabetics1 and has been reported in immunocompromised patients with acquired immune deficiency syndrome (AIDS), malignancy, patients receiving chemotherapy, and neutropenic children.24 The incidence of MOE in the general population is estimated to be quite low and difficult to determine.5 However, over the past decades, the number of reported cases has been increasing, suggesting increased awareness of this syndrome by primary care physicians.6
Case Report
A 56‐year‐old white male was brought to the emergency department with altered mental status including decreased level of consciousness, bizarre behavior, headaches, and nausea for several weeks. He had a history of alcohol and cocaine abuse. He was homeless and a smoker. On examination, the patient was lethargic, disoriented with respect to time, place and person. Blood pressure was 107/72 mm Hg, heart rate 85 beats per minute; respirations were 16 per minute and temperature was 97.9F. Neurological examination was significant for loss of vision of the left eye and left facial peripheral nerve palsy. Examination of the left eye showed yellowish‐greenish discharge, a lower lid ectropium with upper lid ptosis, conjunctival erythema, and an 8 mm 6 mm abrasion in the medial half of the cornea. He had purulent drainage from the left ear with small vesicular lesions on the auricle, and a 3 cm 4 cm abscess on the right forearm. The remainder of the physical examination was unremarkable.
Laboratory‐test results showed white blood cell (WBC) count; 11.27 109 cells/L with 76.6% neutrophils. A complete metabolic panel was within normal limits. Human immunodeficiency virus (HIV) and RPR testing were negative. Cerebrospinal fluid (CSF) studies demonstrated a WBC 39 cells/mm3 with lymphocytes predominance (85%), Red blood cell (RBC) 6 cells/mm3, protein 48 mg/dL, and glucose; 63 mg/dL. Computed tomographic scan of the head revealed an area of low attenuation with surrounding edema of the left temporal lobe and fracture of the temporal bone on the superior margin of the mastoid air cells extending into the left mastoid air cells (Figure 1). The fluid draining from the patient's left ear grew 2 different strains of Pseudomonas aeruginosa. Magnetic resonance imaging (MRI) of the brain demonstrated a 2.5 cm to 3.0 cm region of multiple loculations and edema in the left temporal lobe representing cerebritis with abscess and complete opacification of left mastoid air cell suggestive of mastoiditis (Figure 2). Piperacillin/tazobactam and tobramycin were initiated for suspected MOE with brain involvement. CSF and blood cultures were negative.


A repeat MRI at 3 weeks of therapy demonstrated interval improvement in the temporal lobe abscess and the edema surrounding the infection. At the time of discharge, after 6 weeks of antimicrobial therapy, the patient was alert and oriented with respect to person, place, and time.
Discussion
Pseudomonas aeruginosa is the most common organism cultured from MOE.4, 5 Other organisms such as Staphylococcus aureus,6 Proteus mirabilis, Klebsiella oxytocea,7 and Aspergillus species8 have been reported as well.
While the exact pathogenesis of MOE is poorly understood, accidental trauma from cotton swabs, exposure to lake water, swimming pool water, and repeated aural lavage have all been implicated as inciting factors.9 The current literature suggests that Pseudomonal otitis externa occurs due to abnormal host defense mechanisms rather than enhanced pathogen colonization.10
MOE typically presents with severe otalgia, headache, auricular tenderness, mastoid tenderness, or persistent otorrhea. The pain of MOE is usually severe, and the classic signs of infection such as fever, leukocytosis and neutrophil predominance (left shift) may not be present.5 The diagnosis can be confirmed by otoscopic exam which will demonstrate granulation tissue at the junction between the bony and cartilaginous tissues in the external auditory canal. MOE can produce certain physical findings that should raise red flags for local extension. Temporomandibular joint pain in the susceptible patient with otalgia could indicate MOE with joint invasion. Cranial nerve involvement, most commonly involves the seventh cranial nerve which results in a facial palsy. Other cranial neuropathies have been reported in MOE such as the glossopharyngeal, vagal, spinal accessory, and hypoglossal nerves.11 Confusion and nuchal rigidity should arouse suspicion of intracranial extension of the infection.
The diagnosis of MOE is usually made by a constellation of clinical, microbiological and radiological features. The first attempt at defining diagnostic criteria for MOE was in 1987, when Cohen and Friedman named several obligatory signs such as pain, exudate, edema, granulation tissue in the external ear canal, the presence of microabscesses if surgery is performed, and either a positive Tc‐99m bone scan or failure of local treatment for 1 week.12
Recent literature reviews emphasize that a positive bacterial or fungal culture of the external ear canal can help make the diagnosis of MOE.4 A recent study looking at diagnostic criteria noted that MOE may be present even without meeting all of these major criteria (clinical, microbiological, and radiological features).13 Some authors suggest that ear biopsy be considered if malignancy is a reasonable possibility.9
Other laboratory data can be within normal limits, such as a WBC count or differential and metabolic profiles.4 Erythrocyte sedimentation rate (ESR), while non‐specific, has been reported to be markedly increased in the setting of MOE.5 It is recommended that a baseline ESR be obtained, and then used to follow the response to treatment.14
Computed tomography (CT) scanning is considered the appropriate initial imaging study, however, there are mixed reports about whether a CT scan alone is enough to evaluate disease severity and its complications.15 While CT scan is quite sensitive for demonstrating bony destruction associated with MOE, MRI is better at detecting the soft tissue changes associated with MOE and more useful for following disease resolution after treatment.16
The treatment of MOE has evolved over time. Before the introduction of effective anti‐pseudomonal antibiotics, MOE had an associated mortality near 50%, and surgery was the recommended therapy.17 Currently, given the availability of effective anti‐pseudomonal therapy, there is usually no indication for surgery as a primary treatment in MOE. For sensitive pseudomonal species, oral Ciprofloxacin therapy for 6 to 8 weeks is considered the treatment of choice for MOE.18 Clinicians should be aware of the emergence of quinolone resistance in P. Aeruginosa,19 and antibiotic sensitivities should be performed on the culture to guide further treatment. In the setting of resistant or multi‐species infection, one should obtain external ear canal biopsy with wound debridement and begin long‐term intravenous therapy with an antipseudomonal beta‐lactam plus an aminoglycoside.18 In the setting of extension into the cranium causing cerebritis or abscess, it is recommended that a neurosurgeon be consulted immediately to determine whether the lesion is amenable to aspiration, excision, or watchful waiting with imaging follow up.
This case illustrates a serious complication from MOE, with ipsilateral facial nerve palsy, opthalmitis, mastoiditis, and cerebral abscess that was successfully treated with conservative medical management.
Malignant Otitis Externa (MOE) is a necrotizing infection of the external auditory canal characterized by extension into nearby soft tissue and bony structures that can potentially lead to mastoiditis, skull base osteomyelitis, cranial nerve palsies, and rarely, intracranial complications. MOE has been classically described as a disease affecting elderly diabetics1 and has been reported in immunocompromised patients with acquired immune deficiency syndrome (AIDS), malignancy, patients receiving chemotherapy, and neutropenic children.24 The incidence of MOE in the general population is estimated to be quite low and difficult to determine.5 However, over the past decades, the number of reported cases has been increasing, suggesting increased awareness of this syndrome by primary care physicians.6
Case Report
A 56‐year‐old white male was brought to the emergency department with altered mental status including decreased level of consciousness, bizarre behavior, headaches, and nausea for several weeks. He had a history of alcohol and cocaine abuse. He was homeless and a smoker. On examination, the patient was lethargic, disoriented with respect to time, place and person. Blood pressure was 107/72 mm Hg, heart rate 85 beats per minute; respirations were 16 per minute and temperature was 97.9F. Neurological examination was significant for loss of vision of the left eye and left facial peripheral nerve palsy. Examination of the left eye showed yellowish‐greenish discharge, a lower lid ectropium with upper lid ptosis, conjunctival erythema, and an 8 mm 6 mm abrasion in the medial half of the cornea. He had purulent drainage from the left ear with small vesicular lesions on the auricle, and a 3 cm 4 cm abscess on the right forearm. The remainder of the physical examination was unremarkable.
Laboratory‐test results showed white blood cell (WBC) count; 11.27 109 cells/L with 76.6% neutrophils. A complete metabolic panel was within normal limits. Human immunodeficiency virus (HIV) and RPR testing were negative. Cerebrospinal fluid (CSF) studies demonstrated a WBC 39 cells/mm3 with lymphocytes predominance (85%), Red blood cell (RBC) 6 cells/mm3, protein 48 mg/dL, and glucose; 63 mg/dL. Computed tomographic scan of the head revealed an area of low attenuation with surrounding edema of the left temporal lobe and fracture of the temporal bone on the superior margin of the mastoid air cells extending into the left mastoid air cells (Figure 1). The fluid draining from the patient's left ear grew 2 different strains of Pseudomonas aeruginosa. Magnetic resonance imaging (MRI) of the brain demonstrated a 2.5 cm to 3.0 cm region of multiple loculations and edema in the left temporal lobe representing cerebritis with abscess and complete opacification of left mastoid air cell suggestive of mastoiditis (Figure 2). Piperacillin/tazobactam and tobramycin were initiated for suspected MOE with brain involvement. CSF and blood cultures were negative.


A repeat MRI at 3 weeks of therapy demonstrated interval improvement in the temporal lobe abscess and the edema surrounding the infection. At the time of discharge, after 6 weeks of antimicrobial therapy, the patient was alert and oriented with respect to person, place, and time.
Discussion
Pseudomonas aeruginosa is the most common organism cultured from MOE.4, 5 Other organisms such as Staphylococcus aureus,6 Proteus mirabilis, Klebsiella oxytocea,7 and Aspergillus species8 have been reported as well.
While the exact pathogenesis of MOE is poorly understood, accidental trauma from cotton swabs, exposure to lake water, swimming pool water, and repeated aural lavage have all been implicated as inciting factors.9 The current literature suggests that Pseudomonal otitis externa occurs due to abnormal host defense mechanisms rather than enhanced pathogen colonization.10
MOE typically presents with severe otalgia, headache, auricular tenderness, mastoid tenderness, or persistent otorrhea. The pain of MOE is usually severe, and the classic signs of infection such as fever, leukocytosis and neutrophil predominance (left shift) may not be present.5 The diagnosis can be confirmed by otoscopic exam which will demonstrate granulation tissue at the junction between the bony and cartilaginous tissues in the external auditory canal. MOE can produce certain physical findings that should raise red flags for local extension. Temporomandibular joint pain in the susceptible patient with otalgia could indicate MOE with joint invasion. Cranial nerve involvement, most commonly involves the seventh cranial nerve which results in a facial palsy. Other cranial neuropathies have been reported in MOE such as the glossopharyngeal, vagal, spinal accessory, and hypoglossal nerves.11 Confusion and nuchal rigidity should arouse suspicion of intracranial extension of the infection.
The diagnosis of MOE is usually made by a constellation of clinical, microbiological and radiological features. The first attempt at defining diagnostic criteria for MOE was in 1987, when Cohen and Friedman named several obligatory signs such as pain, exudate, edema, granulation tissue in the external ear canal, the presence of microabscesses if surgery is performed, and either a positive Tc‐99m bone scan or failure of local treatment for 1 week.12
Recent literature reviews emphasize that a positive bacterial or fungal culture of the external ear canal can help make the diagnosis of MOE.4 A recent study looking at diagnostic criteria noted that MOE may be present even without meeting all of these major criteria (clinical, microbiological, and radiological features).13 Some authors suggest that ear biopsy be considered if malignancy is a reasonable possibility.9
Other laboratory data can be within normal limits, such as a WBC count or differential and metabolic profiles.4 Erythrocyte sedimentation rate (ESR), while non‐specific, has been reported to be markedly increased in the setting of MOE.5 It is recommended that a baseline ESR be obtained, and then used to follow the response to treatment.14
Computed tomography (CT) scanning is considered the appropriate initial imaging study, however, there are mixed reports about whether a CT scan alone is enough to evaluate disease severity and its complications.15 While CT scan is quite sensitive for demonstrating bony destruction associated with MOE, MRI is better at detecting the soft tissue changes associated with MOE and more useful for following disease resolution after treatment.16
The treatment of MOE has evolved over time. Before the introduction of effective anti‐pseudomonal antibiotics, MOE had an associated mortality near 50%, and surgery was the recommended therapy.17 Currently, given the availability of effective anti‐pseudomonal therapy, there is usually no indication for surgery as a primary treatment in MOE. For sensitive pseudomonal species, oral Ciprofloxacin therapy for 6 to 8 weeks is considered the treatment of choice for MOE.18 Clinicians should be aware of the emergence of quinolone resistance in P. Aeruginosa,19 and antibiotic sensitivities should be performed on the culture to guide further treatment. In the setting of resistant or multi‐species infection, one should obtain external ear canal biopsy with wound debridement and begin long‐term intravenous therapy with an antipseudomonal beta‐lactam plus an aminoglycoside.18 In the setting of extension into the cranium causing cerebritis or abscess, it is recommended that a neurosurgeon be consulted immediately to determine whether the lesion is amenable to aspiration, excision, or watchful waiting with imaging follow up.
This case illustrates a serious complication from MOE, with ipsilateral facial nerve palsy, opthalmitis, mastoiditis, and cerebral abscess that was successfully treated with conservative medical management.
- .Malignant external otitis.Laryngoscope.1968;78:1257.
- ,,, et al.Necrotizing external otitis in patients with AIDS.Laryngoscope.1997;107:456.
- ,,,.Malignant external otitis in an infant.J Laryngol Otol.1990;104(6):488–490.
- ,,.The changing face of malignant (necrotising) external otitis: clinical, radiological, and anatomic correlations.Lancet Infect Dis.2004;4:34.
- ,.Malignant external otitis: insights into pathogenesis, clinical manifestations, diagnosis, and therapy.Am J Med.1988;85:391.
- ,,Staphylococcal malignant external otitis.Can Med Assoc J.1982;126:155.
- ,,, et al.A case of malignant external otitis involving Klebsiella oxytoca.Eur J Clin Microbiol Infect Dis.1992;11:75.
- ,.Invasive otitis externa due to Aspergillus species: case report and review.Clin Infect Dis.1994;19:866.
- ,.Necrotizing (malignant) external otitis.Am Fam Physician.2003;68(2):309–312.
- ,,,.Bacterial flora of the external canal in diabetics and non‐diabetics.Laryngoscope.1982;92:672.
- ,,.Neurologic complications of malignant external otitis.Neurology.1971;21:1077–1084.
- ,.The diagnostic criteria of malignant external otitis.J Laryngol Otol.1987;101:216–221.
- ,,,,.Stratification for malignant external otitis.Otolaryngol Head Neck Surg.2007;137(2):301–305.
- ,,,,,.Efficacy of oral ciprofloxacin plus rifampin for treatment of malignant external otitis.Arch Otolaryngol Head Neck Surg.1989;115:1063–1069.
- ,,,,,.Usefulness of CT scans in malignant external otitis: effective tool for the diagnosis, but of limited value in predicting outcome.Eur Arch Otorhinolaryngol.2008;265(1):53–56.
- ,,.Use of magnetic resonance imaging as the primary imaging modality in the diagnosis and follow‐up of malignant external otitis.J Laryngol Otol.2004;118:576.
- ,.Skull base osteomyelitis. Malignant external otitis.Otolaryngol Clin North Am.1996;29(5):795–806.
- .Ciprofloxacin: an oral quinolone for the treatment of infections with gram‐negative pathogens. Committee on Antimicrobial Agents. Canadian Infectious Disease Society.Can Med Assoc J.1994;150(5):669–676.
- ,,,.Resistance of Pseudomonas to ciprofloxacin: implications for the treatment of malignant otitis externa.J Laryngol Otol.2007;121:118–123.
- .Malignant external otitis.Laryngoscope.1968;78:1257.
- ,,, et al.Necrotizing external otitis in patients with AIDS.Laryngoscope.1997;107:456.
- ,,,.Malignant external otitis in an infant.J Laryngol Otol.1990;104(6):488–490.
- ,,.The changing face of malignant (necrotising) external otitis: clinical, radiological, and anatomic correlations.Lancet Infect Dis.2004;4:34.
- ,.Malignant external otitis: insights into pathogenesis, clinical manifestations, diagnosis, and therapy.Am J Med.1988;85:391.
- ,,Staphylococcal malignant external otitis.Can Med Assoc J.1982;126:155.
- ,,, et al.A case of malignant external otitis involving Klebsiella oxytoca.Eur J Clin Microbiol Infect Dis.1992;11:75.
- ,.Invasive otitis externa due to Aspergillus species: case report and review.Clin Infect Dis.1994;19:866.
- ,.Necrotizing (malignant) external otitis.Am Fam Physician.2003;68(2):309–312.
- ,,,.Bacterial flora of the external canal in diabetics and non‐diabetics.Laryngoscope.1982;92:672.
- ,,.Neurologic complications of malignant external otitis.Neurology.1971;21:1077–1084.
- ,.The diagnostic criteria of malignant external otitis.J Laryngol Otol.1987;101:216–221.
- ,,,,.Stratification for malignant external otitis.Otolaryngol Head Neck Surg.2007;137(2):301–305.
- ,,,,,.Efficacy of oral ciprofloxacin plus rifampin for treatment of malignant external otitis.Arch Otolaryngol Head Neck Surg.1989;115:1063–1069.
- ,,,,,.Usefulness of CT scans in malignant external otitis: effective tool for the diagnosis, but of limited value in predicting outcome.Eur Arch Otorhinolaryngol.2008;265(1):53–56.
- ,,.Use of magnetic resonance imaging as the primary imaging modality in the diagnosis and follow‐up of malignant external otitis.J Laryngol Otol.2004;118:576.
- ,.Skull base osteomyelitis. Malignant external otitis.Otolaryngol Clin North Am.1996;29(5):795–806.
- .Ciprofloxacin: an oral quinolone for the treatment of infections with gram‐negative pathogens. Committee on Antimicrobial Agents. Canadian Infectious Disease Society.Can Med Assoc J.1994;150(5):669–676.
- ,,,.Resistance of Pseudomonas to ciprofloxacin: implications for the treatment of malignant otitis externa.J Laryngol Otol.2007;121:118–123.
Effects of blood conservation on the incidence of anemia and transfusions in pediatric parapneumonic effusion: A hospitalist perspective
Pediatric patients with pneumonia frequently develop parapneumonic effusion (PNE),1 which account for 0.4 to 6 cases per 1000 pediatric admissions.2 The effusion initially is in a transudative, free‐flowing phase that may evolve to fibrino‐purulent phase and a later organizing phase.3 Hospital management includes antibiotic therapy, pain control, fluids, nutritional support, diagnostic imaging and most important is fluid drainage. Fluid drainage could be through thoracentesis, chest tubes,4 fibrinolysis,5 video‐assisted thoracoscopic surgery (VATS),6 or thoracotomy.7
During hospitalization, repeated phlebotomy and surgical procedures result in ongoing blood losses8, 9 while systemic inflammation and nutritional compromise may blunt erythropoiesis.10, 11 Both mechanisms may result in developing anemia and subsequent need for red blood cell transfusion (RBCT). Blood transfusions are associated with multiple complications including transfusion transmitted infections, acute lung injury, hemodynamic compromise, volume overload, hemolysis, and immune compromise.12, 13 Furthermore, suboptimal benefits,14 increased resource utilization,15 and risk of mortality16 have also been reported in Pediatric intensive care unit (ICU) patients who received blood transfusions.
A Pediatric Blood Conservation program was launched at Helen DeVos Children's Hospital in 1999, and since its inception the pediatric hospitalists have implemented blood conservation guidelines (BCG) in the management of some of the patients with PNE. The BCG incorporated into the care of the intervention group (group I) were orders for:
Minimizing phlebotomy draws (without stating a specific frequency).
Use of micro sampling blood collection tubes and reinfusion of any blood drawn prior to obtaining a blood sample (waste return).
Hematinics use at managing physician's discretion (see Supporting Appendices 1 and 2 in the online version of this article).
No previous studies have addressed the impact of blood conservation strategies on the development of anemia in pediatric PNE. We hypothesized that BCG implementation resulted in smaller phlebotomy losses, lesser incidence of anemia, and lower transfusion requirements.
Methods
After obtaining an approval from the institutional review board, a retrospective medical records review was conducted of all pediatric patients (1 month‐18 years) admitted to Helen DeVos Children's Hospital with diagnoses of PNE from the period of January 1997 to December 2004.
Study Groups
Intervention group (group I) included all patients who were admitted with a diagnosis of PNE between the year 2000 to 2004 and had orders for BCG (as outlined above) written on or after admission to the hospital. That group included patients that were either solely managed by the hospitalists or comanaged by both the general pediatrician and the Hospitalist.
Simultaneous nonintervention group (group S) included patients with PNE who were admitted between 2000 to 2004 and did not have BCG orders on their record. Those patients were either managed solely by the general pediatricians or the Hospitalist may have been involved but no BCG orders were written. It was assumed that no intervention was implemented.
Historical nonintervention group (group H) included patients with PNE admitted between 1997 and 2000 prior to the implementation of the blood conservation program or the hospitalists service. Those patients were managed by the general pediatricians with the Intensivists help at times and no blood conservation measures were implemented.
Phlebotomy frequency and volume data were collected from the patient's medical record. When volume was not documented, an estimate was made based on the hospital actual practice and labs reported. If the child had central vascular access, a standard of 2 mL of blood was removed to clear the line prior to drawing the blood sample. In the S and H groups that volume was discarded and recorded as a blood loss. In Group I the blood used to clear the line was returned to the patients. Data regarding the patient's hemoglobin (Hgb) levels on admission and during hospitalization as well as RBCT frequency and volume were also collected. Other background data collected included, hospital stay, ICU admission, antibiotic use, isolated organisms, and PNE‐related interventions (thoracentesis, VATS and chest tube placement). A pediatric risk of mortality score (PRISM score17) was assigned for every patient based on data collected on the day of admission.
Statistical analysis was done using a Fisher exact test to compare qualitative data. For quantitative data Kruskal‐Wallis was used for comparison between the three groups while the Mann Whitney test was used for the pair wise comparisons. Odds Ratios and Confidence Intervals were calculated for variables associated with needing RBCTs.
Results
A total of 81 patients who were admitted to the hospital for medical and surgical management with PNE were included in the study. During the study period 24 patients with blood conservation orders on the chart were assigned to the intervention group. Another 28 patients were identified during the same period but did not have blood conservation orders and were assigned to the simultaneous no intervention group. A historical no intervention group of 29 patients were identified from a 3‐year period prior to the study period. Groups were similar in age, weight, and an overall low PRISM score. Group H tended to have lower acuity, longer hospital stay, more frequent pediatric intensive care unit (PICU) admissions, and tended to have more chest tube days compared to the (I) and (S) groups (Table 1).
| Group I (n = 24) | Group S (n = 28) | Group H (n = 29) | Kruskal‐Wallis P Value | |
|---|---|---|---|---|
| ||||
| Age, years | 6 (4) | 5 (4) | 6 (5) | 0.75 |
| Weight, kg | 26 (19) | 22 (13) | 27 (17) | 0.77 |
| PRISM Score | 2 (3) | 3 (5) | (1) | 0.007* |
| PICU admission number (%) | 3 (12) | 8 (29) | 14 (48) | 0.018 |
| Chest tube days | 7 (4) | 6 (4) | 9 (5) | 0.054 |
| Hospital stay days | 10 (4) | 13 (6) | 15 (6) | 0.008 |
| Initial Hgb, gm | 11.2 (1.7) | 11.2 (1.6) | 11.1 (1.9) | 0.95 |
| Drop in Hgb, gm | 1.7 (1.4) | 2.1 (1.2) | 2 (1.4) | 0.37 |
| Days to Hgb Nadir | 6.1 (37) | 8.5 (5.5) | 6.9 (4.3) | 0.31 |
| Number of blood draws | 7.6 (4) | 11 (9) | 12.6 (12) | 0.36 |
| Phlebotomy volume, mL/kg/day | 0.08 (0.05) | 0.14 (0.33) | 0.22 (0.24) | 0.006 |
| Number of patients transfused (%) | 2 (8.3) | 5 (17.9) | 9 (31) | 0.11 |
All groups (I, S and H) had similar initial Hgb, in‐hospital decline in Hgb levels and time to reach a Hgb nadir. There was a trend toward a lower frequency of phlebotomy and significant difference in the phlebotomy volumes drawn even when corrected for patient weight and hospital stay, (I < S < H; P = 0.006), (Table 1).
All 3 groups had a similar pretransfusion Hgb trigger (7.7 1 gm/dL), timing of transfusions (7.9 1 day), volume of packed red blood cells (PRBCs) (19 15 mL/kg), and magnitude of Hgb rise following transfusions (3.9 1.5 gm/dL). There was a strong trend toward lower transfusion need in the intervention group though it did not reach statistical significance (8.3% [I], 17.9% [S]; and 31% [H]; P = 0.11) (Table 1 and Figure 1). Being in group (S) compared to (I) carried a relative risk of transfusion of 2.14 (confidence interval [CI], 0.4610.06).
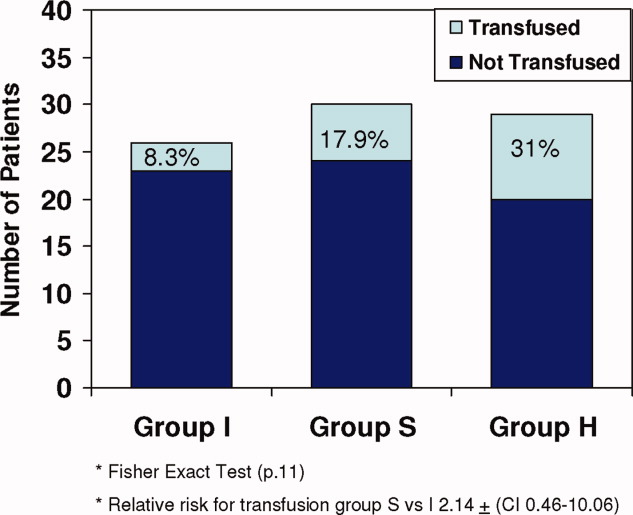
Of all study patients, 19.8% received RBCT. Compared to those who did not require transfusions they were significantly younger, smaller (P = 0.001) and had a higher severity of illness score (PRISM) (P = 0.25). Transfused children had lower initial Hgb levels, more frequent phlebotomy, greater volume of blood drawn, and longer hospital stay (P = 0.001) (Table 2 and Figure 2).
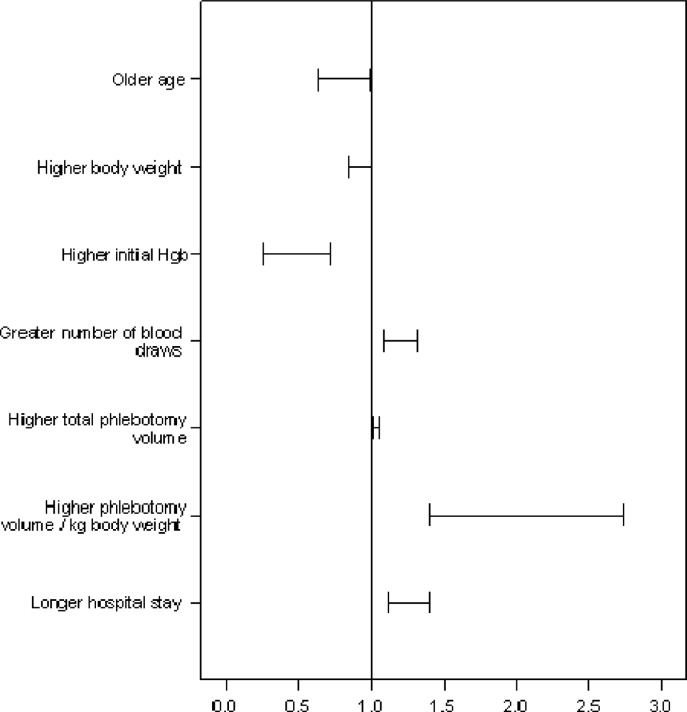
| Transfused (n = 16) | Not Transfused (n = 65) | Odds Ratio (95% CI) | |
|---|---|---|---|
| |||
| Ages, years | 3.5 (4) | 6.4 (4) | 0.8 (0.640.99)* |
| Weight, kg | 16.1 (9) | 26.9 (17) | 0.92 (0.851)* |
| Initial Hgb, gm | 9.9 (1) | 11.4 (1) | 0.43 (0.260.71)* |
| Number of blood draws | 20 (12) | 8 (6) | 1.2 (1.091.32)* |
| Total phlebotomy volume, mL | 82 (75) | 23 (25) | 1.03 (1.011.05)* |
| Total phlebotomy volume, mL/kg | 5.9 (7) | 1.1 (1) | 1.97 (1.412.74)* |
| Hospital stay days | 19 (5) | 11 (5) | 1.25 (1.121.4)* |
| PRISM score | 3.4 (6) | 1.6 (3) | P. 25 |
A total of 36% of the patients were pretreated with antibiotics prior to obtaining pleural fluid cultures. Forty‐eight patients (59%) had negative cultures, 22 (27%) grew pneumococcus, 5 patients (6%) had streptococcus A, 2 patients had streptococcus viridans, 3 patients had staphylococci aureus, and 1 patient had Haemophilus influenzae. There was no difference between the study groups regarding the site of effusion, prior antibiotics therapy or culture results.
Discussion
This retrospective study showed that children with PNE had low Hgb upon admission to the hospital and after dropping an average of 2 gm/dL over the first week of hospitalization one‐fifth of the patients required transfusion. The phlebotomy volumes significantly decreased with BCG implementation, and transfusion frequency showed a strong trend toward decline. The fact that all 3 groups had similar pretransfusion Hgb and similar Hgb decline, despite differences in the phlebotomy volumes, may implicate other factors like bone marrow suppression, malnutrition, and procedural blood losses. All of these factors have been shown to contribute to the development of anemia in the critically ill patients10 and were not accounted for in this limited retrospective review.
Transfused patients were significantly smaller, younger, and had higher illness severity scores. They had lower initial Hgb levels, more phlebotomy, and longer hospital stay than nontransfused patients.
Pretransfusion Hgb was 7.5 gm/dL to 7.7 gm/dL in all groups, consistent with Lacroix et al's18 report on the safety of lower transfusion threshold in stable PICU patients. BCG resulted in a strong trend toward less transfusion but did not reach statistical significance likely due to the small sample size. It is tempting to hypothesize that aggressive erythropoietin therapy might augment that trend; however, given the relatively short hospital stay (1014 days), erythropoietin therapy may be less efficacious in the milder case with shorter hospital stay than those with longer hospitalization. After encouraging smaller studies19 Corwin et al.20 did not find a beneficial effect for erythropoietin in adult ICU patients nor did Jacobs et al.21 in ventilated children with bronchiolitis who had comparable length of hospitalization.
A specific benefit could not be attributed to iron/folate or erythropoietin therapy as neither the dosing, duration nor timing was controlled. Furthermore, given the limitations of retrospective reviews, the relative importance of the beneficial effect of limiting phlebotomy vs. hematinics use could not be determined.
The Hospitalist is focused on improving the quality and efficiency of caring for the inpatient.2225 The initiation of the hospitalist's service at our institution coincided with that of the blood conservation service. Consequently, the Hospitalist contributed to patient care as well as daily house staff and nursing education. For those children whose care was coordinated by the hospitalists, the study data showed a trend toward lesser admissions to the PICU and lower blood utilization. This trend further emphasizes the role a Hospitalist could play in adopting and implementing useful medical strategies lowering the cost of care. In this study, a change in patient care was observed over time with the hospitalists tending to employ more blood conservation measures when compared to the pediatrician.
In summary, children with PNE are at risk for developing severe anemia requiring transfusion. This retrospective study identified the characteristics of those likely to require transfusions. Blood conservation strategies seem to decrease the need for transfusions. The hospitalists played an important role in implementing the BCG. A prospective controlled study with adequate power is needed to examine both the various mechanisms for developing anemia and the impact of the individual components of the blood conservation strategies.
- ,.Thoracic empyema.Surg Clin North Am.2002;82:643–671.
- ,.Parapneumonic Pleural effusion and empyema in children: review of 19 year experience.Clin Pediatr (Phila).1983;22:414–419.
- American Thoracic Society.Management of nontuberculosis empyema.Am Rev Respir Dis.1962;85:935–936.
- ,,, et al.Short‐term course and outcome of treatments of pleural empyema in pediatric patients: repeated ultrasound‐guided needle thoracentesis vs. chest tube drainage.Chest.2002;121:836–840.
- ,,, et al.Randomized trial of Intrapleural Urokinase in the treatment of childhood empyema.Thorax.2002;57:343–347.
- ,,,,.Therapy of parapneumonic effusion in children: video‐assisted thoracoscopic surgery versus conventional thoracostomy drainage.Pediatrics.2006;118:e547–e553.
- ,,,.Is open thoracotomy still a good treatment option for the management of Empyema in children?Ann Thorac Surg.2003;76:1854–1858.
- ,,, et al.Anemia, blood loss, and blood transfusions in North American children in the intensive care unit.Am J Respir Crit Care Med.2008;178:25–33.
- .Medical vampires.N Engl J Med.1983;314:1250–1251.
- ,,, et al.Important role of nondiagnostic blood loss and blunted erythropoietic response in the anemia of medical intensive care patients.Crit Care Med.1999;27:2630–2639.
- ,,, et al.Erythropoietin response is blunted in critically ill patients.Intensive Care Med.1997;23:159–162.
- ,.The continuing risk of transfusion‐transmitted infections.N Engl J Med.2006;355:1303–1305.
- ,.Immunomodulation by blood transfusion: an evolving scientific and clinical challenge.Am J Med.1996;101:299–308.
- ,,,.Pediatric red blood cell transfusions increase resource use.J Pediatr.2003;142:95–97.
- ,.Effect of blood transfusion on oxygen consumption in pediatric septic shock.Crit Care Med.1990;18:1087–1091.
- ,,,,.Red blood cell transfusion in critically ill children is independently associated with increased mortality.Intensive Care Med.2007;33:1414–1422.
- ,,.PRISM III: an updated pediatric risk of mortality.Crit Care Med.1996;24(5):743–752.
- ,,, et al.Transfusion strategies for patients in pediatric intensive care units.Nw Engl J Med.2007;356:1609–1619.
- ,,, et al.Efficacy of recombinant human erythropoietin in critically ill patients.JAMA.2002;288:2827–2835.
- ,,, et al.EPO Critical Care Trials Group.Efficacy and safety of epoetin alfa in criticall ill patients.Nw Engl J Med.2007;357:965–976.
- ,,.Erythropoietin therapy in children with Bronchiolitis and anemia.Pediatr Crit Care Med.2003;4(1):44–47.
- .The state of hospital medicine in 2008.Med Clin North Am.2008;92:265–273.
- ,,, et al.Impact of a Hospitalist system on length of stay and cost for children with common conditions.Pediatrics.2007;120:267–274.
- ,,,.Comparison of hospitalists and nonhospitalists regarding core measures of pneumonia care.Am J Manag Care.2007;13:129–132.
- ,,.Improved survival with hospitalists in a pediatric intensive care unit.Crit Care Med.2003;31:847–852.
Pediatric patients with pneumonia frequently develop parapneumonic effusion (PNE),1 which account for 0.4 to 6 cases per 1000 pediatric admissions.2 The effusion initially is in a transudative, free‐flowing phase that may evolve to fibrino‐purulent phase and a later organizing phase.3 Hospital management includes antibiotic therapy, pain control, fluids, nutritional support, diagnostic imaging and most important is fluid drainage. Fluid drainage could be through thoracentesis, chest tubes,4 fibrinolysis,5 video‐assisted thoracoscopic surgery (VATS),6 or thoracotomy.7
During hospitalization, repeated phlebotomy and surgical procedures result in ongoing blood losses8, 9 while systemic inflammation and nutritional compromise may blunt erythropoiesis.10, 11 Both mechanisms may result in developing anemia and subsequent need for red blood cell transfusion (RBCT). Blood transfusions are associated with multiple complications including transfusion transmitted infections, acute lung injury, hemodynamic compromise, volume overload, hemolysis, and immune compromise.12, 13 Furthermore, suboptimal benefits,14 increased resource utilization,15 and risk of mortality16 have also been reported in Pediatric intensive care unit (ICU) patients who received blood transfusions.
A Pediatric Blood Conservation program was launched at Helen DeVos Children's Hospital in 1999, and since its inception the pediatric hospitalists have implemented blood conservation guidelines (BCG) in the management of some of the patients with PNE. The BCG incorporated into the care of the intervention group (group I) were orders for:
Minimizing phlebotomy draws (without stating a specific frequency).
Use of micro sampling blood collection tubes and reinfusion of any blood drawn prior to obtaining a blood sample (waste return).
Hematinics use at managing physician's discretion (see Supporting Appendices 1 and 2 in the online version of this article).
No previous studies have addressed the impact of blood conservation strategies on the development of anemia in pediatric PNE. We hypothesized that BCG implementation resulted in smaller phlebotomy losses, lesser incidence of anemia, and lower transfusion requirements.
Methods
After obtaining an approval from the institutional review board, a retrospective medical records review was conducted of all pediatric patients (1 month‐18 years) admitted to Helen DeVos Children's Hospital with diagnoses of PNE from the period of January 1997 to December 2004.
Study Groups
Intervention group (group I) included all patients who were admitted with a diagnosis of PNE between the year 2000 to 2004 and had orders for BCG (as outlined above) written on or after admission to the hospital. That group included patients that were either solely managed by the hospitalists or comanaged by both the general pediatrician and the Hospitalist.
Simultaneous nonintervention group (group S) included patients with PNE who were admitted between 2000 to 2004 and did not have BCG orders on their record. Those patients were either managed solely by the general pediatricians or the Hospitalist may have been involved but no BCG orders were written. It was assumed that no intervention was implemented.
Historical nonintervention group (group H) included patients with PNE admitted between 1997 and 2000 prior to the implementation of the blood conservation program or the hospitalists service. Those patients were managed by the general pediatricians with the Intensivists help at times and no blood conservation measures were implemented.
Phlebotomy frequency and volume data were collected from the patient's medical record. When volume was not documented, an estimate was made based on the hospital actual practice and labs reported. If the child had central vascular access, a standard of 2 mL of blood was removed to clear the line prior to drawing the blood sample. In the S and H groups that volume was discarded and recorded as a blood loss. In Group I the blood used to clear the line was returned to the patients. Data regarding the patient's hemoglobin (Hgb) levels on admission and during hospitalization as well as RBCT frequency and volume were also collected. Other background data collected included, hospital stay, ICU admission, antibiotic use, isolated organisms, and PNE‐related interventions (thoracentesis, VATS and chest tube placement). A pediatric risk of mortality score (PRISM score17) was assigned for every patient based on data collected on the day of admission.
Statistical analysis was done using a Fisher exact test to compare qualitative data. For quantitative data Kruskal‐Wallis was used for comparison between the three groups while the Mann Whitney test was used for the pair wise comparisons. Odds Ratios and Confidence Intervals were calculated for variables associated with needing RBCTs.
Results
A total of 81 patients who were admitted to the hospital for medical and surgical management with PNE were included in the study. During the study period 24 patients with blood conservation orders on the chart were assigned to the intervention group. Another 28 patients were identified during the same period but did not have blood conservation orders and were assigned to the simultaneous no intervention group. A historical no intervention group of 29 patients were identified from a 3‐year period prior to the study period. Groups were similar in age, weight, and an overall low PRISM score. Group H tended to have lower acuity, longer hospital stay, more frequent pediatric intensive care unit (PICU) admissions, and tended to have more chest tube days compared to the (I) and (S) groups (Table 1).
| Group I (n = 24) | Group S (n = 28) | Group H (n = 29) | Kruskal‐Wallis P Value | |
|---|---|---|---|---|
| ||||
| Age, years | 6 (4) | 5 (4) | 6 (5) | 0.75 |
| Weight, kg | 26 (19) | 22 (13) | 27 (17) | 0.77 |
| PRISM Score | 2 (3) | 3 (5) | (1) | 0.007* |
| PICU admission number (%) | 3 (12) | 8 (29) | 14 (48) | 0.018 |
| Chest tube days | 7 (4) | 6 (4) | 9 (5) | 0.054 |
| Hospital stay days | 10 (4) | 13 (6) | 15 (6) | 0.008 |
| Initial Hgb, gm | 11.2 (1.7) | 11.2 (1.6) | 11.1 (1.9) | 0.95 |
| Drop in Hgb, gm | 1.7 (1.4) | 2.1 (1.2) | 2 (1.4) | 0.37 |
| Days to Hgb Nadir | 6.1 (37) | 8.5 (5.5) | 6.9 (4.3) | 0.31 |
| Number of blood draws | 7.6 (4) | 11 (9) | 12.6 (12) | 0.36 |
| Phlebotomy volume, mL/kg/day | 0.08 (0.05) | 0.14 (0.33) | 0.22 (0.24) | 0.006 |
| Number of patients transfused (%) | 2 (8.3) | 5 (17.9) | 9 (31) | 0.11 |
All groups (I, S and H) had similar initial Hgb, in‐hospital decline in Hgb levels and time to reach a Hgb nadir. There was a trend toward a lower frequency of phlebotomy and significant difference in the phlebotomy volumes drawn even when corrected for patient weight and hospital stay, (I < S < H; P = 0.006), (Table 1).
All 3 groups had a similar pretransfusion Hgb trigger (7.7 1 gm/dL), timing of transfusions (7.9 1 day), volume of packed red blood cells (PRBCs) (19 15 mL/kg), and magnitude of Hgb rise following transfusions (3.9 1.5 gm/dL). There was a strong trend toward lower transfusion need in the intervention group though it did not reach statistical significance (8.3% [I], 17.9% [S]; and 31% [H]; P = 0.11) (Table 1 and Figure 1). Being in group (S) compared to (I) carried a relative risk of transfusion of 2.14 (confidence interval [CI], 0.4610.06).

Of all study patients, 19.8% received RBCT. Compared to those who did not require transfusions they were significantly younger, smaller (P = 0.001) and had a higher severity of illness score (PRISM) (P = 0.25). Transfused children had lower initial Hgb levels, more frequent phlebotomy, greater volume of blood drawn, and longer hospital stay (P = 0.001) (Table 2 and Figure 2).

| Transfused (n = 16) | Not Transfused (n = 65) | Odds Ratio (95% CI) | |
|---|---|---|---|
| |||
| Ages, years | 3.5 (4) | 6.4 (4) | 0.8 (0.640.99)* |
| Weight, kg | 16.1 (9) | 26.9 (17) | 0.92 (0.851)* |
| Initial Hgb, gm | 9.9 (1) | 11.4 (1) | 0.43 (0.260.71)* |
| Number of blood draws | 20 (12) | 8 (6) | 1.2 (1.091.32)* |
| Total phlebotomy volume, mL | 82 (75) | 23 (25) | 1.03 (1.011.05)* |
| Total phlebotomy volume, mL/kg | 5.9 (7) | 1.1 (1) | 1.97 (1.412.74)* |
| Hospital stay days | 19 (5) | 11 (5) | 1.25 (1.121.4)* |
| PRISM score | 3.4 (6) | 1.6 (3) | P. 25 |
A total of 36% of the patients were pretreated with antibiotics prior to obtaining pleural fluid cultures. Forty‐eight patients (59%) had negative cultures, 22 (27%) grew pneumococcus, 5 patients (6%) had streptococcus A, 2 patients had streptococcus viridans, 3 patients had staphylococci aureus, and 1 patient had Haemophilus influenzae. There was no difference between the study groups regarding the site of effusion, prior antibiotics therapy or culture results.
Discussion
This retrospective study showed that children with PNE had low Hgb upon admission to the hospital and after dropping an average of 2 gm/dL over the first week of hospitalization one‐fifth of the patients required transfusion. The phlebotomy volumes significantly decreased with BCG implementation, and transfusion frequency showed a strong trend toward decline. The fact that all 3 groups had similar pretransfusion Hgb and similar Hgb decline, despite differences in the phlebotomy volumes, may implicate other factors like bone marrow suppression, malnutrition, and procedural blood losses. All of these factors have been shown to contribute to the development of anemia in the critically ill patients10 and were not accounted for in this limited retrospective review.
Transfused patients were significantly smaller, younger, and had higher illness severity scores. They had lower initial Hgb levels, more phlebotomy, and longer hospital stay than nontransfused patients.
Pretransfusion Hgb was 7.5 gm/dL to 7.7 gm/dL in all groups, consistent with Lacroix et al's18 report on the safety of lower transfusion threshold in stable PICU patients. BCG resulted in a strong trend toward less transfusion but did not reach statistical significance likely due to the small sample size. It is tempting to hypothesize that aggressive erythropoietin therapy might augment that trend; however, given the relatively short hospital stay (1014 days), erythropoietin therapy may be less efficacious in the milder case with shorter hospital stay than those with longer hospitalization. After encouraging smaller studies19 Corwin et al.20 did not find a beneficial effect for erythropoietin in adult ICU patients nor did Jacobs et al.21 in ventilated children with bronchiolitis who had comparable length of hospitalization.
A specific benefit could not be attributed to iron/folate or erythropoietin therapy as neither the dosing, duration nor timing was controlled. Furthermore, given the limitations of retrospective reviews, the relative importance of the beneficial effect of limiting phlebotomy vs. hematinics use could not be determined.
The Hospitalist is focused on improving the quality and efficiency of caring for the inpatient.2225 The initiation of the hospitalist's service at our institution coincided with that of the blood conservation service. Consequently, the Hospitalist contributed to patient care as well as daily house staff and nursing education. For those children whose care was coordinated by the hospitalists, the study data showed a trend toward lesser admissions to the PICU and lower blood utilization. This trend further emphasizes the role a Hospitalist could play in adopting and implementing useful medical strategies lowering the cost of care. In this study, a change in patient care was observed over time with the hospitalists tending to employ more blood conservation measures when compared to the pediatrician.
In summary, children with PNE are at risk for developing severe anemia requiring transfusion. This retrospective study identified the characteristics of those likely to require transfusions. Blood conservation strategies seem to decrease the need for transfusions. The hospitalists played an important role in implementing the BCG. A prospective controlled study with adequate power is needed to examine both the various mechanisms for developing anemia and the impact of the individual components of the blood conservation strategies.
Pediatric patients with pneumonia frequently develop parapneumonic effusion (PNE),1 which account for 0.4 to 6 cases per 1000 pediatric admissions.2 The effusion initially is in a transudative, free‐flowing phase that may evolve to fibrino‐purulent phase and a later organizing phase.3 Hospital management includes antibiotic therapy, pain control, fluids, nutritional support, diagnostic imaging and most important is fluid drainage. Fluid drainage could be through thoracentesis, chest tubes,4 fibrinolysis,5 video‐assisted thoracoscopic surgery (VATS),6 or thoracotomy.7
During hospitalization, repeated phlebotomy and surgical procedures result in ongoing blood losses8, 9 while systemic inflammation and nutritional compromise may blunt erythropoiesis.10, 11 Both mechanisms may result in developing anemia and subsequent need for red blood cell transfusion (RBCT). Blood transfusions are associated with multiple complications including transfusion transmitted infections, acute lung injury, hemodynamic compromise, volume overload, hemolysis, and immune compromise.12, 13 Furthermore, suboptimal benefits,14 increased resource utilization,15 and risk of mortality16 have also been reported in Pediatric intensive care unit (ICU) patients who received blood transfusions.
A Pediatric Blood Conservation program was launched at Helen DeVos Children's Hospital in 1999, and since its inception the pediatric hospitalists have implemented blood conservation guidelines (BCG) in the management of some of the patients with PNE. The BCG incorporated into the care of the intervention group (group I) were orders for:
Minimizing phlebotomy draws (without stating a specific frequency).
Use of micro sampling blood collection tubes and reinfusion of any blood drawn prior to obtaining a blood sample (waste return).
Hematinics use at managing physician's discretion (see Supporting Appendices 1 and 2 in the online version of this article).
No previous studies have addressed the impact of blood conservation strategies on the development of anemia in pediatric PNE. We hypothesized that BCG implementation resulted in smaller phlebotomy losses, lesser incidence of anemia, and lower transfusion requirements.
Methods
After obtaining an approval from the institutional review board, a retrospective medical records review was conducted of all pediatric patients (1 month‐18 years) admitted to Helen DeVos Children's Hospital with diagnoses of PNE from the period of January 1997 to December 2004.
Study Groups
Intervention group (group I) included all patients who were admitted with a diagnosis of PNE between the year 2000 to 2004 and had orders for BCG (as outlined above) written on or after admission to the hospital. That group included patients that were either solely managed by the hospitalists or comanaged by both the general pediatrician and the Hospitalist.
Simultaneous nonintervention group (group S) included patients with PNE who were admitted between 2000 to 2004 and did not have BCG orders on their record. Those patients were either managed solely by the general pediatricians or the Hospitalist may have been involved but no BCG orders were written. It was assumed that no intervention was implemented.
Historical nonintervention group (group H) included patients with PNE admitted between 1997 and 2000 prior to the implementation of the blood conservation program or the hospitalists service. Those patients were managed by the general pediatricians with the Intensivists help at times and no blood conservation measures were implemented.
Phlebotomy frequency and volume data were collected from the patient's medical record. When volume was not documented, an estimate was made based on the hospital actual practice and labs reported. If the child had central vascular access, a standard of 2 mL of blood was removed to clear the line prior to drawing the blood sample. In the S and H groups that volume was discarded and recorded as a blood loss. In Group I the blood used to clear the line was returned to the patients. Data regarding the patient's hemoglobin (Hgb) levels on admission and during hospitalization as well as RBCT frequency and volume were also collected. Other background data collected included, hospital stay, ICU admission, antibiotic use, isolated organisms, and PNE‐related interventions (thoracentesis, VATS and chest tube placement). A pediatric risk of mortality score (PRISM score17) was assigned for every patient based on data collected on the day of admission.
Statistical analysis was done using a Fisher exact test to compare qualitative data. For quantitative data Kruskal‐Wallis was used for comparison between the three groups while the Mann Whitney test was used for the pair wise comparisons. Odds Ratios and Confidence Intervals were calculated for variables associated with needing RBCTs.
Results
A total of 81 patients who were admitted to the hospital for medical and surgical management with PNE were included in the study. During the study period 24 patients with blood conservation orders on the chart were assigned to the intervention group. Another 28 patients were identified during the same period but did not have blood conservation orders and were assigned to the simultaneous no intervention group. A historical no intervention group of 29 patients were identified from a 3‐year period prior to the study period. Groups were similar in age, weight, and an overall low PRISM score. Group H tended to have lower acuity, longer hospital stay, more frequent pediatric intensive care unit (PICU) admissions, and tended to have more chest tube days compared to the (I) and (S) groups (Table 1).
| Group I (n = 24) | Group S (n = 28) | Group H (n = 29) | Kruskal‐Wallis P Value | |
|---|---|---|---|---|
| ||||
| Age, years | 6 (4) | 5 (4) | 6 (5) | 0.75 |
| Weight, kg | 26 (19) | 22 (13) | 27 (17) | 0.77 |
| PRISM Score | 2 (3) | 3 (5) | (1) | 0.007* |
| PICU admission number (%) | 3 (12) | 8 (29) | 14 (48) | 0.018 |
| Chest tube days | 7 (4) | 6 (4) | 9 (5) | 0.054 |
| Hospital stay days | 10 (4) | 13 (6) | 15 (6) | 0.008 |
| Initial Hgb, gm | 11.2 (1.7) | 11.2 (1.6) | 11.1 (1.9) | 0.95 |
| Drop in Hgb, gm | 1.7 (1.4) | 2.1 (1.2) | 2 (1.4) | 0.37 |
| Days to Hgb Nadir | 6.1 (37) | 8.5 (5.5) | 6.9 (4.3) | 0.31 |
| Number of blood draws | 7.6 (4) | 11 (9) | 12.6 (12) | 0.36 |
| Phlebotomy volume, mL/kg/day | 0.08 (0.05) | 0.14 (0.33) | 0.22 (0.24) | 0.006 |
| Number of patients transfused (%) | 2 (8.3) | 5 (17.9) | 9 (31) | 0.11 |
All groups (I, S and H) had similar initial Hgb, in‐hospital decline in Hgb levels and time to reach a Hgb nadir. There was a trend toward a lower frequency of phlebotomy and significant difference in the phlebotomy volumes drawn even when corrected for patient weight and hospital stay, (I < S < H; P = 0.006), (Table 1).
All 3 groups had a similar pretransfusion Hgb trigger (7.7 1 gm/dL), timing of transfusions (7.9 1 day), volume of packed red blood cells (PRBCs) (19 15 mL/kg), and magnitude of Hgb rise following transfusions (3.9 1.5 gm/dL). There was a strong trend toward lower transfusion need in the intervention group though it did not reach statistical significance (8.3% [I], 17.9% [S]; and 31% [H]; P = 0.11) (Table 1 and Figure 1). Being in group (S) compared to (I) carried a relative risk of transfusion of 2.14 (confidence interval [CI], 0.4610.06).

Of all study patients, 19.8% received RBCT. Compared to those who did not require transfusions they were significantly younger, smaller (P = 0.001) and had a higher severity of illness score (PRISM) (P = 0.25). Transfused children had lower initial Hgb levels, more frequent phlebotomy, greater volume of blood drawn, and longer hospital stay (P = 0.001) (Table 2 and Figure 2).

| Transfused (n = 16) | Not Transfused (n = 65) | Odds Ratio (95% CI) | |
|---|---|---|---|
| |||
| Ages, years | 3.5 (4) | 6.4 (4) | 0.8 (0.640.99)* |
| Weight, kg | 16.1 (9) | 26.9 (17) | 0.92 (0.851)* |
| Initial Hgb, gm | 9.9 (1) | 11.4 (1) | 0.43 (0.260.71)* |
| Number of blood draws | 20 (12) | 8 (6) | 1.2 (1.091.32)* |
| Total phlebotomy volume, mL | 82 (75) | 23 (25) | 1.03 (1.011.05)* |
| Total phlebotomy volume, mL/kg | 5.9 (7) | 1.1 (1) | 1.97 (1.412.74)* |
| Hospital stay days | 19 (5) | 11 (5) | 1.25 (1.121.4)* |
| PRISM score | 3.4 (6) | 1.6 (3) | P. 25 |
A total of 36% of the patients were pretreated with antibiotics prior to obtaining pleural fluid cultures. Forty‐eight patients (59%) had negative cultures, 22 (27%) grew pneumococcus, 5 patients (6%) had streptococcus A, 2 patients had streptococcus viridans, 3 patients had staphylococci aureus, and 1 patient had Haemophilus influenzae. There was no difference between the study groups regarding the site of effusion, prior antibiotics therapy or culture results.
Discussion
This retrospective study showed that children with PNE had low Hgb upon admission to the hospital and after dropping an average of 2 gm/dL over the first week of hospitalization one‐fifth of the patients required transfusion. The phlebotomy volumes significantly decreased with BCG implementation, and transfusion frequency showed a strong trend toward decline. The fact that all 3 groups had similar pretransfusion Hgb and similar Hgb decline, despite differences in the phlebotomy volumes, may implicate other factors like bone marrow suppression, malnutrition, and procedural blood losses. All of these factors have been shown to contribute to the development of anemia in the critically ill patients10 and were not accounted for in this limited retrospective review.
Transfused patients were significantly smaller, younger, and had higher illness severity scores. They had lower initial Hgb levels, more phlebotomy, and longer hospital stay than nontransfused patients.
Pretransfusion Hgb was 7.5 gm/dL to 7.7 gm/dL in all groups, consistent with Lacroix et al's18 report on the safety of lower transfusion threshold in stable PICU patients. BCG resulted in a strong trend toward less transfusion but did not reach statistical significance likely due to the small sample size. It is tempting to hypothesize that aggressive erythropoietin therapy might augment that trend; however, given the relatively short hospital stay (1014 days), erythropoietin therapy may be less efficacious in the milder case with shorter hospital stay than those with longer hospitalization. After encouraging smaller studies19 Corwin et al.20 did not find a beneficial effect for erythropoietin in adult ICU patients nor did Jacobs et al.21 in ventilated children with bronchiolitis who had comparable length of hospitalization.
A specific benefit could not be attributed to iron/folate or erythropoietin therapy as neither the dosing, duration nor timing was controlled. Furthermore, given the limitations of retrospective reviews, the relative importance of the beneficial effect of limiting phlebotomy vs. hematinics use could not be determined.
The Hospitalist is focused on improving the quality and efficiency of caring for the inpatient.2225 The initiation of the hospitalist's service at our institution coincided with that of the blood conservation service. Consequently, the Hospitalist contributed to patient care as well as daily house staff and nursing education. For those children whose care was coordinated by the hospitalists, the study data showed a trend toward lesser admissions to the PICU and lower blood utilization. This trend further emphasizes the role a Hospitalist could play in adopting and implementing useful medical strategies lowering the cost of care. In this study, a change in patient care was observed over time with the hospitalists tending to employ more blood conservation measures when compared to the pediatrician.
In summary, children with PNE are at risk for developing severe anemia requiring transfusion. This retrospective study identified the characteristics of those likely to require transfusions. Blood conservation strategies seem to decrease the need for transfusions. The hospitalists played an important role in implementing the BCG. A prospective controlled study with adequate power is needed to examine both the various mechanisms for developing anemia and the impact of the individual components of the blood conservation strategies.
- ,.Thoracic empyema.Surg Clin North Am.2002;82:643–671.
- ,.Parapneumonic Pleural effusion and empyema in children: review of 19 year experience.Clin Pediatr (Phila).1983;22:414–419.
- American Thoracic Society.Management of nontuberculosis empyema.Am Rev Respir Dis.1962;85:935–936.
- ,,, et al.Short‐term course and outcome of treatments of pleural empyema in pediatric patients: repeated ultrasound‐guided needle thoracentesis vs. chest tube drainage.Chest.2002;121:836–840.
- ,,, et al.Randomized trial of Intrapleural Urokinase in the treatment of childhood empyema.Thorax.2002;57:343–347.
- ,,,,.Therapy of parapneumonic effusion in children: video‐assisted thoracoscopic surgery versus conventional thoracostomy drainage.Pediatrics.2006;118:e547–e553.
- ,,,.Is open thoracotomy still a good treatment option for the management of Empyema in children?Ann Thorac Surg.2003;76:1854–1858.
- ,,, et al.Anemia, blood loss, and blood transfusions in North American children in the intensive care unit.Am J Respir Crit Care Med.2008;178:25–33.
- .Medical vampires.N Engl J Med.1983;314:1250–1251.
- ,,, et al.Important role of nondiagnostic blood loss and blunted erythropoietic response in the anemia of medical intensive care patients.Crit Care Med.1999;27:2630–2639.
- ,,, et al.Erythropoietin response is blunted in critically ill patients.Intensive Care Med.1997;23:159–162.
- ,.The continuing risk of transfusion‐transmitted infections.N Engl J Med.2006;355:1303–1305.
- ,.Immunomodulation by blood transfusion: an evolving scientific and clinical challenge.Am J Med.1996;101:299–308.
- ,,,.Pediatric red blood cell transfusions increase resource use.J Pediatr.2003;142:95–97.
- ,.Effect of blood transfusion on oxygen consumption in pediatric septic shock.Crit Care Med.1990;18:1087–1091.
- ,,,,.Red blood cell transfusion in critically ill children is independently associated with increased mortality.Intensive Care Med.2007;33:1414–1422.
- ,,.PRISM III: an updated pediatric risk of mortality.Crit Care Med.1996;24(5):743–752.
- ,,, et al.Transfusion strategies for patients in pediatric intensive care units.Nw Engl J Med.2007;356:1609–1619.
- ,,, et al.Efficacy of recombinant human erythropoietin in critically ill patients.JAMA.2002;288:2827–2835.
- ,,, et al.EPO Critical Care Trials Group.Efficacy and safety of epoetin alfa in criticall ill patients.Nw Engl J Med.2007;357:965–976.
- ,,.Erythropoietin therapy in children with Bronchiolitis and anemia.Pediatr Crit Care Med.2003;4(1):44–47.
- .The state of hospital medicine in 2008.Med Clin North Am.2008;92:265–273.
- ,,, et al.Impact of a Hospitalist system on length of stay and cost for children with common conditions.Pediatrics.2007;120:267–274.
- ,,,.Comparison of hospitalists and nonhospitalists regarding core measures of pneumonia care.Am J Manag Care.2007;13:129–132.
- ,,.Improved survival with hospitalists in a pediatric intensive care unit.Crit Care Med.2003;31:847–852.
- ,.Thoracic empyema.Surg Clin North Am.2002;82:643–671.
- ,.Parapneumonic Pleural effusion and empyema in children: review of 19 year experience.Clin Pediatr (Phila).1983;22:414–419.
- American Thoracic Society.Management of nontuberculosis empyema.Am Rev Respir Dis.1962;85:935–936.
- ,,, et al.Short‐term course and outcome of treatments of pleural empyema in pediatric patients: repeated ultrasound‐guided needle thoracentesis vs. chest tube drainage.Chest.2002;121:836–840.
- ,,, et al.Randomized trial of Intrapleural Urokinase in the treatment of childhood empyema.Thorax.2002;57:343–347.
- ,,,,.Therapy of parapneumonic effusion in children: video‐assisted thoracoscopic surgery versus conventional thoracostomy drainage.Pediatrics.2006;118:e547–e553.
- ,,,.Is open thoracotomy still a good treatment option for the management of Empyema in children?Ann Thorac Surg.2003;76:1854–1858.
- ,,, et al.Anemia, blood loss, and blood transfusions in North American children in the intensive care unit.Am J Respir Crit Care Med.2008;178:25–33.
- .Medical vampires.N Engl J Med.1983;314:1250–1251.
- ,,, et al.Important role of nondiagnostic blood loss and blunted erythropoietic response in the anemia of medical intensive care patients.Crit Care Med.1999;27:2630–2639.
- ,,, et al.Erythropoietin response is blunted in critically ill patients.Intensive Care Med.1997;23:159–162.
- ,.The continuing risk of transfusion‐transmitted infections.N Engl J Med.2006;355:1303–1305.
- ,.Immunomodulation by blood transfusion: an evolving scientific and clinical challenge.Am J Med.1996;101:299–308.
- ,,,.Pediatric red blood cell transfusions increase resource use.J Pediatr.2003;142:95–97.
- ,.Effect of blood transfusion on oxygen consumption in pediatric septic shock.Crit Care Med.1990;18:1087–1091.
- ,,,,.Red blood cell transfusion in critically ill children is independently associated with increased mortality.Intensive Care Med.2007;33:1414–1422.
- ,,.PRISM III: an updated pediatric risk of mortality.Crit Care Med.1996;24(5):743–752.
- ,,, et al.Transfusion strategies for patients in pediatric intensive care units.Nw Engl J Med.2007;356:1609–1619.
- ,,, et al.Efficacy of recombinant human erythropoietin in critically ill patients.JAMA.2002;288:2827–2835.
- ,,, et al.EPO Critical Care Trials Group.Efficacy and safety of epoetin alfa in criticall ill patients.Nw Engl J Med.2007;357:965–976.
- ,,.Erythropoietin therapy in children with Bronchiolitis and anemia.Pediatr Crit Care Med.2003;4(1):44–47.
- .The state of hospital medicine in 2008.Med Clin North Am.2008;92:265–273.
- ,,, et al.Impact of a Hospitalist system on length of stay and cost for children with common conditions.Pediatrics.2007;120:267–274.
- ,,,.Comparison of hospitalists and nonhospitalists regarding core measures of pneumonia care.Am J Manag Care.2007;13:129–132.
- ,,.Improved survival with hospitalists in a pediatric intensive care unit.Crit Care Med.2003;31:847–852.
Copyright © 2010 Society of Hospital Medicine
Cold case: Bedside diagnosis of Mycoplasma pneumonia
A 35‐year‐old woman with no past medical history presented to the Emergency Department (ED) with 3 weeks of worsening cough and shortness of breath. Two weeks prior to her presentation she was seen by her primary care physician for flu‐like symptoms, including myalgias, subjective fevers, nonproductive cough, and malaise. She was told that her symptoms were attributable to influenza, and she was treated supportively; however, her symptoms progressed, and she was referred to the ED for further care. Of note, she reported recent cross‐continental air travel as well as an upper respiratory illness in her young child.
On physical exam she was afebrile with normal vital signs and normal room air oxygen saturation. Her oropharynx was clear, and she had no sinus tenderness, rashes, joint swelling, or palpable lymphadenopathy. She was in moderate respiratory distress and had inspiratory crackles at both lung bases.
Complete blood count, electrolytes, and electrocardiogram (ECG) were within normal limits. A D‐dimer level was slightly elevated. Chest X‐ray showed a mild hazy opacity at the right lung base (Figure 1). Computed tomography (CT) angiography of the chest showed bilateral lower lobe infiltrates (Figure 2) but no pulmonary emboli.

A bedside cold agglutinin test, in which the patients blood is drawn into an ethylene diamine tetraacetic acid (EDTA) tube and placed on ice for 30 seconds to 60 seconds, was positive for grains of sand, suggestive of high titers of Mycoplasma pneumoniae immunoglobulin M (IgM) (Figure 3). The patient was discharged with oral antibiotics and reported marked symptomatic relief within 2 days.

The utility of culture and serology for acute diagnosis of M. pneumoniae infection is limited. Cold agglutinins are antibodiesmost commonly IgMthat cross‐react with red blood cell antigens. They develop in 50% to 75% of patients 1 week to 2 weeks following initial exposure to M. pneumoniae and their incidence decreases with age. Within the clinical context of community‐acquired pneumonia, a bedside cold agglutinin test is specific for M. pneumoniae and provides a rapid and inexpensive means for confirming a suspected diagnosis.
A 35‐year‐old woman with no past medical history presented to the Emergency Department (ED) with 3 weeks of worsening cough and shortness of breath. Two weeks prior to her presentation she was seen by her primary care physician for flu‐like symptoms, including myalgias, subjective fevers, nonproductive cough, and malaise. She was told that her symptoms were attributable to influenza, and she was treated supportively; however, her symptoms progressed, and she was referred to the ED for further care. Of note, she reported recent cross‐continental air travel as well as an upper respiratory illness in her young child.
On physical exam she was afebrile with normal vital signs and normal room air oxygen saturation. Her oropharynx was clear, and she had no sinus tenderness, rashes, joint swelling, or palpable lymphadenopathy. She was in moderate respiratory distress and had inspiratory crackles at both lung bases.
Complete blood count, electrolytes, and electrocardiogram (ECG) were within normal limits. A D‐dimer level was slightly elevated. Chest X‐ray showed a mild hazy opacity at the right lung base (Figure 1). Computed tomography (CT) angiography of the chest showed bilateral lower lobe infiltrates (Figure 2) but no pulmonary emboli.


A bedside cold agglutinin test, in which the patients blood is drawn into an ethylene diamine tetraacetic acid (EDTA) tube and placed on ice for 30 seconds to 60 seconds, was positive for grains of sand, suggestive of high titers of Mycoplasma pneumoniae immunoglobulin M (IgM) (Figure 3). The patient was discharged with oral antibiotics and reported marked symptomatic relief within 2 days.

The utility of culture and serology for acute diagnosis of M. pneumoniae infection is limited. Cold agglutinins are antibodiesmost commonly IgMthat cross‐react with red blood cell antigens. They develop in 50% to 75% of patients 1 week to 2 weeks following initial exposure to M. pneumoniae and their incidence decreases with age. Within the clinical context of community‐acquired pneumonia, a bedside cold agglutinin test is specific for M. pneumoniae and provides a rapid and inexpensive means for confirming a suspected diagnosis.
A 35‐year‐old woman with no past medical history presented to the Emergency Department (ED) with 3 weeks of worsening cough and shortness of breath. Two weeks prior to her presentation she was seen by her primary care physician for flu‐like symptoms, including myalgias, subjective fevers, nonproductive cough, and malaise. She was told that her symptoms were attributable to influenza, and she was treated supportively; however, her symptoms progressed, and she was referred to the ED for further care. Of note, she reported recent cross‐continental air travel as well as an upper respiratory illness in her young child.
On physical exam she was afebrile with normal vital signs and normal room air oxygen saturation. Her oropharynx was clear, and she had no sinus tenderness, rashes, joint swelling, or palpable lymphadenopathy. She was in moderate respiratory distress and had inspiratory crackles at both lung bases.
Complete blood count, electrolytes, and electrocardiogram (ECG) were within normal limits. A D‐dimer level was slightly elevated. Chest X‐ray showed a mild hazy opacity at the right lung base (Figure 1). Computed tomography (CT) angiography of the chest showed bilateral lower lobe infiltrates (Figure 2) but no pulmonary emboli.


A bedside cold agglutinin test, in which the patients blood is drawn into an ethylene diamine tetraacetic acid (EDTA) tube and placed on ice for 30 seconds to 60 seconds, was positive for grains of sand, suggestive of high titers of Mycoplasma pneumoniae immunoglobulin M (IgM) (Figure 3). The patient was discharged with oral antibiotics and reported marked symptomatic relief within 2 days.

The utility of culture and serology for acute diagnosis of M. pneumoniae infection is limited. Cold agglutinins are antibodiesmost commonly IgMthat cross‐react with red blood cell antigens. They develop in 50% to 75% of patients 1 week to 2 weeks following initial exposure to M. pneumoniae and their incidence decreases with age. Within the clinical context of community‐acquired pneumonia, a bedside cold agglutinin test is specific for M. pneumoniae and provides a rapid and inexpensive means for confirming a suspected diagnosis.
A Small Kindness
On August 6, 2009, my vigorously healthy 59‐year‐old brother‐in‐law, a beloved husband and father of 2 sons, developed mild right hand clumsiness and slight slurring of speech. This led to a primary care visit and the symptoms were originally felt to be related to working long hours and stress. The symptoms failed to improve and on August 11th, an magnetic resonance imaging (MRI) made a shocking discovery, my brother‐in‐law had a brain tumor. He was seen in the Neurosurgery department of a major academic center the following day and on August 13th he underwent resection of the majority of the tumor with a pathologic finding of grade IV glioblastoma multiforme (GBM).
During this initial admission, a consultation was obtained from a neuro‐oncologist who would then become the principle director of his care. My brother‐in‐law was crystal clear with his physicians; he wanted honest information about what to expect. From the outset he understood this was an incurable disease, but hoped that with aggressive treatment, he could live for months, possibly even years. He started chemotherapy almost immediately and in early October started a 6‐week course of daily radiation. The early weeks went relatively well. He saw his oncologist regularly and stayed in close contact with his oncology nurse. With his engineering background and attention to details, he followed his physician's instructions to the letter; however, despite his very best efforts at compliance and working intensively with physical therapy he was becoming progressively weaker.
In early November, a follow‐up MRI appeared to show progression of tumor and on November 12th, a second resection was undertaken. Pathologically, this seemed successful with removal of tumor bulk, but he was left even weaker, particularly on the right side. His symptoms were managed with dexamethasone; however, aphasia and hemiparesis would always reemerge with attempts to taper the drug and his functional status was too poor to allow for further chemotherapy. As his communication ability was becoming more limited, my sister‐in‐law was increasingly becoming his spokesperson at doctor visits and with phone updates to his oncology nurse.
On January 21, 2010 after working with a home physical therapist, he was making a transfer and became nonresponsive. Paramedics were called, arriving within minutes, but he was found pulseless. Despite a heroic resuscitative effort, he was pronounced dead at a nearby hospital a short time thereafter. A postmortem was not performed and the presumptive cause of death was pulmonary embolism.
His funeral was January 29th.
My brother‐in‐law and I lived 1600 miles apart and saw each other on only the rarest occasions. When the diagnosis was made, my role changed as I am the only relative within the extended family with medical expertise. Questions were directed to me via e‐mails and I did my best using UpToDate and other resources to learn about GBM and relay this information back to the family.
Recently, and in a vicarious way, I was becoming more and more deeply involved. Using the best descriptions of functional status that I could extract from e‐mail and phone calls, I estimated his Karnofsky Performance Score as being fairly poor. By way of my e‐mails to him and his wife, I was just beginning, ever so gently, to touch on the subject of hospice care at the time of his sudden death.
At the visitation and reception following the funeral, I think I was seen as more than a distant brother‐in‐law; I was also seen as a surrogate for the medical profession and for his doctors in particular. One message that I got loud and clear, from more than 1 family member was the devastated, abandoned feeling that was emerging in the 8 days since his death. On the morning following his death, my sister‐in‐law called his physician's office and told his oncology nurse that her husband had died suddenly and unexpectedly the night before. The nurse expressed sympathy and indicated she would relay this information to his doctor and she would personally call back. As of the time I left to return home, that was the last communication any family member had received from anyone involved in his care.
Although the outpouring of community support and sympathy was powerful and touching, not a single condolence card or phone call came from his doctor. I was shocked at the suddenness of his death, but I was also shocked at the complete absence of any communication, any acknowledgement of his courageous struggle against a terrible illness, or of his family's depth of caring and love over these last few months.
I am reminded of an essay by Gregory Kane, MD in CHEST,1 in which he describes a disturbing personal encounter with the following;
In a personal and memorable patient encounter, I sat and listened while a tearful patient cried at having received no contact from the physician who treated her husband for metastatic lung cancer for a treatment duration of 9 months. As I struggled to comprehend her sense of pain and abandonment, I considered offering as possible explanation that the physician may not have been on call at the time of the death and may have mistakenly believed that his partner had offered such a gesture verbally. Before I could respond, however, my patient added that her veterinarian had sent a card when the family dog died. I was speechless.1
As Hospitalists, we are gifted and privileged to work closely with patients and their loved ones struggling with the existential and eternal questions of life and death. As we can all well attest from 6 PM family meetings, the unit‐of‐care extends beyond the patient and certainly includes the loving and caring members of the patient's family and close support system. If we fail to acknowledge a family's bereavement, we run the risk of unintentionally communicating the message that the patient was not important, that their suffering did not matter or that the crushing grief the family may be bearing is somehow insignificant compared to our busy schedules.
I have asked you to join me on this short journey my brother‐in‐law has taken these last few months in the interest of raising awareness. There are many occasions when a verbal, bedside expression of condolence is very appropriate and completely adequate. There are other times when a condolence letter will better facilitate the closure of the physician‐patient‐family relationship. This may be intimidating, both the extra work involved and especially the challenge of not knowing what to say. The article quoted above by Dr. Kane is an excellent resource for guidance concerning content, style, and other writing considerations. Using examples, such as a letter from Abraham Lincoln to a girl whose father died in the Civil War, he shows that this essential communication does not have to be lengthy or difficult. Another excellent resource can be found in the New England Journal of Medicine; The Doctor's Letter of Condolence.2 See Table 1 for suggestions to help with bereavement communications.
| Handwritten on a card or stationary. |
| Timely (within 1‐2 weeks of death). |
| Use sensitive, caring language and avoid clich. |
| Acknowledge the family's grief and loss. |
| Acknowledge the patient's courage or other qualities. |
| Mention the privilege it has been to work with the patient. |
| Mention your appreciation of the family's caring. |
| Avoid sincerely yours and end on a personal note such as suggesting your thoughts are with them at this most difficult time. |
We are the profession of Hospital Medicine. It is our knowledge, our hope, our compassion, our experience and judgment that often directs care at the end‐of‐life for many of our patients. We are teammates along with the primary care provider, subspecialty consultants, palliative care specialists and other members of the care team. We have a professional obligation to extend a thoughtful condolence to surviving family members and to contact other members of the care team so that they too may have this opportunity. The responsibility for the final closure rests with us and within this responsibility is a powerful fulfillment of the promise of the practice of medicine.
The condolence note is a small kindness, a part of the art of medicine, a part of our humanness and essential to our vision of patient‐centered hospital care.
- .A Dying Art?: The Doctor's Letter of Condolence.Chest.2007;131(4):1245–1247. Permission for use obtained by direct communication with the author on January 31, 2010.
- ,,.The doctor's letter of condolence.N Engl J Med.2001;344(15):1162–1163.
On August 6, 2009, my vigorously healthy 59‐year‐old brother‐in‐law, a beloved husband and father of 2 sons, developed mild right hand clumsiness and slight slurring of speech. This led to a primary care visit and the symptoms were originally felt to be related to working long hours and stress. The symptoms failed to improve and on August 11th, an magnetic resonance imaging (MRI) made a shocking discovery, my brother‐in‐law had a brain tumor. He was seen in the Neurosurgery department of a major academic center the following day and on August 13th he underwent resection of the majority of the tumor with a pathologic finding of grade IV glioblastoma multiforme (GBM).
During this initial admission, a consultation was obtained from a neuro‐oncologist who would then become the principle director of his care. My brother‐in‐law was crystal clear with his physicians; he wanted honest information about what to expect. From the outset he understood this was an incurable disease, but hoped that with aggressive treatment, he could live for months, possibly even years. He started chemotherapy almost immediately and in early October started a 6‐week course of daily radiation. The early weeks went relatively well. He saw his oncologist regularly and stayed in close contact with his oncology nurse. With his engineering background and attention to details, he followed his physician's instructions to the letter; however, despite his very best efforts at compliance and working intensively with physical therapy he was becoming progressively weaker.
In early November, a follow‐up MRI appeared to show progression of tumor and on November 12th, a second resection was undertaken. Pathologically, this seemed successful with removal of tumor bulk, but he was left even weaker, particularly on the right side. His symptoms were managed with dexamethasone; however, aphasia and hemiparesis would always reemerge with attempts to taper the drug and his functional status was too poor to allow for further chemotherapy. As his communication ability was becoming more limited, my sister‐in‐law was increasingly becoming his spokesperson at doctor visits and with phone updates to his oncology nurse.
On January 21, 2010 after working with a home physical therapist, he was making a transfer and became nonresponsive. Paramedics were called, arriving within minutes, but he was found pulseless. Despite a heroic resuscitative effort, he was pronounced dead at a nearby hospital a short time thereafter. A postmortem was not performed and the presumptive cause of death was pulmonary embolism.
His funeral was January 29th.
My brother‐in‐law and I lived 1600 miles apart and saw each other on only the rarest occasions. When the diagnosis was made, my role changed as I am the only relative within the extended family with medical expertise. Questions were directed to me via e‐mails and I did my best using UpToDate and other resources to learn about GBM and relay this information back to the family.
Recently, and in a vicarious way, I was becoming more and more deeply involved. Using the best descriptions of functional status that I could extract from e‐mail and phone calls, I estimated his Karnofsky Performance Score as being fairly poor. By way of my e‐mails to him and his wife, I was just beginning, ever so gently, to touch on the subject of hospice care at the time of his sudden death.
At the visitation and reception following the funeral, I think I was seen as more than a distant brother‐in‐law; I was also seen as a surrogate for the medical profession and for his doctors in particular. One message that I got loud and clear, from more than 1 family member was the devastated, abandoned feeling that was emerging in the 8 days since his death. On the morning following his death, my sister‐in‐law called his physician's office and told his oncology nurse that her husband had died suddenly and unexpectedly the night before. The nurse expressed sympathy and indicated she would relay this information to his doctor and she would personally call back. As of the time I left to return home, that was the last communication any family member had received from anyone involved in his care.
Although the outpouring of community support and sympathy was powerful and touching, not a single condolence card or phone call came from his doctor. I was shocked at the suddenness of his death, but I was also shocked at the complete absence of any communication, any acknowledgement of his courageous struggle against a terrible illness, or of his family's depth of caring and love over these last few months.
I am reminded of an essay by Gregory Kane, MD in CHEST,1 in which he describes a disturbing personal encounter with the following;
In a personal and memorable patient encounter, I sat and listened while a tearful patient cried at having received no contact from the physician who treated her husband for metastatic lung cancer for a treatment duration of 9 months. As I struggled to comprehend her sense of pain and abandonment, I considered offering as possible explanation that the physician may not have been on call at the time of the death and may have mistakenly believed that his partner had offered such a gesture verbally. Before I could respond, however, my patient added that her veterinarian had sent a card when the family dog died. I was speechless.1
As Hospitalists, we are gifted and privileged to work closely with patients and their loved ones struggling with the existential and eternal questions of life and death. As we can all well attest from 6 PM family meetings, the unit‐of‐care extends beyond the patient and certainly includes the loving and caring members of the patient's family and close support system. If we fail to acknowledge a family's bereavement, we run the risk of unintentionally communicating the message that the patient was not important, that their suffering did not matter or that the crushing grief the family may be bearing is somehow insignificant compared to our busy schedules.
I have asked you to join me on this short journey my brother‐in‐law has taken these last few months in the interest of raising awareness. There are many occasions when a verbal, bedside expression of condolence is very appropriate and completely adequate. There are other times when a condolence letter will better facilitate the closure of the physician‐patient‐family relationship. This may be intimidating, both the extra work involved and especially the challenge of not knowing what to say. The article quoted above by Dr. Kane is an excellent resource for guidance concerning content, style, and other writing considerations. Using examples, such as a letter from Abraham Lincoln to a girl whose father died in the Civil War, he shows that this essential communication does not have to be lengthy or difficult. Another excellent resource can be found in the New England Journal of Medicine; The Doctor's Letter of Condolence.2 See Table 1 for suggestions to help with bereavement communications.
| Handwritten on a card or stationary. |
| Timely (within 1‐2 weeks of death). |
| Use sensitive, caring language and avoid clich. |
| Acknowledge the family's grief and loss. |
| Acknowledge the patient's courage or other qualities. |
| Mention the privilege it has been to work with the patient. |
| Mention your appreciation of the family's caring. |
| Avoid sincerely yours and end on a personal note such as suggesting your thoughts are with them at this most difficult time. |
We are the profession of Hospital Medicine. It is our knowledge, our hope, our compassion, our experience and judgment that often directs care at the end‐of‐life for many of our patients. We are teammates along with the primary care provider, subspecialty consultants, palliative care specialists and other members of the care team. We have a professional obligation to extend a thoughtful condolence to surviving family members and to contact other members of the care team so that they too may have this opportunity. The responsibility for the final closure rests with us and within this responsibility is a powerful fulfillment of the promise of the practice of medicine.
The condolence note is a small kindness, a part of the art of medicine, a part of our humanness and essential to our vision of patient‐centered hospital care.
On August 6, 2009, my vigorously healthy 59‐year‐old brother‐in‐law, a beloved husband and father of 2 sons, developed mild right hand clumsiness and slight slurring of speech. This led to a primary care visit and the symptoms were originally felt to be related to working long hours and stress. The symptoms failed to improve and on August 11th, an magnetic resonance imaging (MRI) made a shocking discovery, my brother‐in‐law had a brain tumor. He was seen in the Neurosurgery department of a major academic center the following day and on August 13th he underwent resection of the majority of the tumor with a pathologic finding of grade IV glioblastoma multiforme (GBM).
During this initial admission, a consultation was obtained from a neuro‐oncologist who would then become the principle director of his care. My brother‐in‐law was crystal clear with his physicians; he wanted honest information about what to expect. From the outset he understood this was an incurable disease, but hoped that with aggressive treatment, he could live for months, possibly even years. He started chemotherapy almost immediately and in early October started a 6‐week course of daily radiation. The early weeks went relatively well. He saw his oncologist regularly and stayed in close contact with his oncology nurse. With his engineering background and attention to details, he followed his physician's instructions to the letter; however, despite his very best efforts at compliance and working intensively with physical therapy he was becoming progressively weaker.
In early November, a follow‐up MRI appeared to show progression of tumor and on November 12th, a second resection was undertaken. Pathologically, this seemed successful with removal of tumor bulk, but he was left even weaker, particularly on the right side. His symptoms were managed with dexamethasone; however, aphasia and hemiparesis would always reemerge with attempts to taper the drug and his functional status was too poor to allow for further chemotherapy. As his communication ability was becoming more limited, my sister‐in‐law was increasingly becoming his spokesperson at doctor visits and with phone updates to his oncology nurse.
On January 21, 2010 after working with a home physical therapist, he was making a transfer and became nonresponsive. Paramedics were called, arriving within minutes, but he was found pulseless. Despite a heroic resuscitative effort, he was pronounced dead at a nearby hospital a short time thereafter. A postmortem was not performed and the presumptive cause of death was pulmonary embolism.
His funeral was January 29th.
My brother‐in‐law and I lived 1600 miles apart and saw each other on only the rarest occasions. When the diagnosis was made, my role changed as I am the only relative within the extended family with medical expertise. Questions were directed to me via e‐mails and I did my best using UpToDate and other resources to learn about GBM and relay this information back to the family.
Recently, and in a vicarious way, I was becoming more and more deeply involved. Using the best descriptions of functional status that I could extract from e‐mail and phone calls, I estimated his Karnofsky Performance Score as being fairly poor. By way of my e‐mails to him and his wife, I was just beginning, ever so gently, to touch on the subject of hospice care at the time of his sudden death.
At the visitation and reception following the funeral, I think I was seen as more than a distant brother‐in‐law; I was also seen as a surrogate for the medical profession and for his doctors in particular. One message that I got loud and clear, from more than 1 family member was the devastated, abandoned feeling that was emerging in the 8 days since his death. On the morning following his death, my sister‐in‐law called his physician's office and told his oncology nurse that her husband had died suddenly and unexpectedly the night before. The nurse expressed sympathy and indicated she would relay this information to his doctor and she would personally call back. As of the time I left to return home, that was the last communication any family member had received from anyone involved in his care.
Although the outpouring of community support and sympathy was powerful and touching, not a single condolence card or phone call came from his doctor. I was shocked at the suddenness of his death, but I was also shocked at the complete absence of any communication, any acknowledgement of his courageous struggle against a terrible illness, or of his family's depth of caring and love over these last few months.
I am reminded of an essay by Gregory Kane, MD in CHEST,1 in which he describes a disturbing personal encounter with the following;
In a personal and memorable patient encounter, I sat and listened while a tearful patient cried at having received no contact from the physician who treated her husband for metastatic lung cancer for a treatment duration of 9 months. As I struggled to comprehend her sense of pain and abandonment, I considered offering as possible explanation that the physician may not have been on call at the time of the death and may have mistakenly believed that his partner had offered such a gesture verbally. Before I could respond, however, my patient added that her veterinarian had sent a card when the family dog died. I was speechless.1
As Hospitalists, we are gifted and privileged to work closely with patients and their loved ones struggling with the existential and eternal questions of life and death. As we can all well attest from 6 PM family meetings, the unit‐of‐care extends beyond the patient and certainly includes the loving and caring members of the patient's family and close support system. If we fail to acknowledge a family's bereavement, we run the risk of unintentionally communicating the message that the patient was not important, that their suffering did not matter or that the crushing grief the family may be bearing is somehow insignificant compared to our busy schedules.
I have asked you to join me on this short journey my brother‐in‐law has taken these last few months in the interest of raising awareness. There are many occasions when a verbal, bedside expression of condolence is very appropriate and completely adequate. There are other times when a condolence letter will better facilitate the closure of the physician‐patient‐family relationship. This may be intimidating, both the extra work involved and especially the challenge of not knowing what to say. The article quoted above by Dr. Kane is an excellent resource for guidance concerning content, style, and other writing considerations. Using examples, such as a letter from Abraham Lincoln to a girl whose father died in the Civil War, he shows that this essential communication does not have to be lengthy or difficult. Another excellent resource can be found in the New England Journal of Medicine; The Doctor's Letter of Condolence.2 See Table 1 for suggestions to help with bereavement communications.
| Handwritten on a card or stationary. |
| Timely (within 1‐2 weeks of death). |
| Use sensitive, caring language and avoid clich. |
| Acknowledge the family's grief and loss. |
| Acknowledge the patient's courage or other qualities. |
| Mention the privilege it has been to work with the patient. |
| Mention your appreciation of the family's caring. |
| Avoid sincerely yours and end on a personal note such as suggesting your thoughts are with them at this most difficult time. |
We are the profession of Hospital Medicine. It is our knowledge, our hope, our compassion, our experience and judgment that often directs care at the end‐of‐life for many of our patients. We are teammates along with the primary care provider, subspecialty consultants, palliative care specialists and other members of the care team. We have a professional obligation to extend a thoughtful condolence to surviving family members and to contact other members of the care team so that they too may have this opportunity. The responsibility for the final closure rests with us and within this responsibility is a powerful fulfillment of the promise of the practice of medicine.
The condolence note is a small kindness, a part of the art of medicine, a part of our humanness and essential to our vision of patient‐centered hospital care.
- .A Dying Art?: The Doctor's Letter of Condolence.Chest.2007;131(4):1245–1247. Permission for use obtained by direct communication with the author on January 31, 2010.
- ,,.The doctor's letter of condolence.N Engl J Med.2001;344(15):1162–1163.
- .A Dying Art?: The Doctor's Letter of Condolence.Chest.2007;131(4):1245–1247. Permission for use obtained by direct communication with the author on January 31, 2010.
- ,,.The doctor's letter of condolence.N Engl J Med.2001;344(15):1162–1163.
Band-Aids Won't Fix Medicare Payment Problems
July 1 marked the debut of several features in this year’s healthcare reform legislation, including the acceptance of uninsured applicants to high-risk insurance pools. For many hospitalists and other doctors, however, the new arrivals only heightened their disappointment over the legislation’s lost opportunity: a permanent fix to the flawed sustainable growth rate, or SGR, that dictates Medicare reimbursements.
“It should have been taken care of in that bill,” says Ron Greeno, MD, SFHM, a member of SHM’s Public Policy Committee and CMO of Brentwood, Tenn.-based Cogent Healthcare. “Obviously, there’s a lot of frustration around the issue, especially on the membership side.”
June provided a stark reminder of the potential for catastrophe should the annual rate cuts to Medicare reimbursements to physicians—21.3% to date—ever go into effect. Congress eventually pushed through yet another temporary fix, delaying any cut from June 1 until Dec. 1 (after the midterm elections, of course) and even tacking on a 2.2% increase. But the House didn’t pass the extension until June 24, nearly a week after the Centers for Medicare & Medicaid Services (CMS) began processing June claims at the lower rate.
By that point, some doctors had begun refusing to see Medicare patients. Both doctors and hospitals have since had to resubmit some June claims to gain the full value, creating new headaches and expenses over the additional paperwork. “It’s wreaking havoc on the provider community,” Dr. Greeno says. “The uncertainty that has been created around these short-term fixes is very disquieting.”
Doctors and hospitals have had to resubmit some June claims to gain the full value, creating expenses over the additional paperwork. And then, of course, there’s the new Nov. 30 deadline facing a lame-duck Congress. As politicians continue dithering over a permanent fix and an accompanying price tag of $250 billion or more over a decade, CMS has announced next year’s rate cut. With a 6.1% decrease slated to take effect on Jan. 1, 2011, the combined SGR-mandated drop would reach nearly 30%. Ouch.
Find out the latest information on SGR reform and contact your legislators in support of permanent repeal through SHM's Legislative Action Center.
July 1 marked the debut of several features in this year’s healthcare reform legislation, including the acceptance of uninsured applicants to high-risk insurance pools. For many hospitalists and other doctors, however, the new arrivals only heightened their disappointment over the legislation’s lost opportunity: a permanent fix to the flawed sustainable growth rate, or SGR, that dictates Medicare reimbursements.
“It should have been taken care of in that bill,” says Ron Greeno, MD, SFHM, a member of SHM’s Public Policy Committee and CMO of Brentwood, Tenn.-based Cogent Healthcare. “Obviously, there’s a lot of frustration around the issue, especially on the membership side.”
June provided a stark reminder of the potential for catastrophe should the annual rate cuts to Medicare reimbursements to physicians—21.3% to date—ever go into effect. Congress eventually pushed through yet another temporary fix, delaying any cut from June 1 until Dec. 1 (after the midterm elections, of course) and even tacking on a 2.2% increase. But the House didn’t pass the extension until June 24, nearly a week after the Centers for Medicare & Medicaid Services (CMS) began processing June claims at the lower rate.
By that point, some doctors had begun refusing to see Medicare patients. Both doctors and hospitals have since had to resubmit some June claims to gain the full value, creating new headaches and expenses over the additional paperwork. “It’s wreaking havoc on the provider community,” Dr. Greeno says. “The uncertainty that has been created around these short-term fixes is very disquieting.”
Doctors and hospitals have had to resubmit some June claims to gain the full value, creating expenses over the additional paperwork. And then, of course, there’s the new Nov. 30 deadline facing a lame-duck Congress. As politicians continue dithering over a permanent fix and an accompanying price tag of $250 billion or more over a decade, CMS has announced next year’s rate cut. With a 6.1% decrease slated to take effect on Jan. 1, 2011, the combined SGR-mandated drop would reach nearly 30%. Ouch.
Find out the latest information on SGR reform and contact your legislators in support of permanent repeal through SHM's Legislative Action Center.
July 1 marked the debut of several features in this year’s healthcare reform legislation, including the acceptance of uninsured applicants to high-risk insurance pools. For many hospitalists and other doctors, however, the new arrivals only heightened their disappointment over the legislation’s lost opportunity: a permanent fix to the flawed sustainable growth rate, or SGR, that dictates Medicare reimbursements.
“It should have been taken care of in that bill,” says Ron Greeno, MD, SFHM, a member of SHM’s Public Policy Committee and CMO of Brentwood, Tenn.-based Cogent Healthcare. “Obviously, there’s a lot of frustration around the issue, especially on the membership side.”
June provided a stark reminder of the potential for catastrophe should the annual rate cuts to Medicare reimbursements to physicians—21.3% to date—ever go into effect. Congress eventually pushed through yet another temporary fix, delaying any cut from June 1 until Dec. 1 (after the midterm elections, of course) and even tacking on a 2.2% increase. But the House didn’t pass the extension until June 24, nearly a week after the Centers for Medicare & Medicaid Services (CMS) began processing June claims at the lower rate.
By that point, some doctors had begun refusing to see Medicare patients. Both doctors and hospitals have since had to resubmit some June claims to gain the full value, creating new headaches and expenses over the additional paperwork. “It’s wreaking havoc on the provider community,” Dr. Greeno says. “The uncertainty that has been created around these short-term fixes is very disquieting.”
Doctors and hospitals have had to resubmit some June claims to gain the full value, creating expenses over the additional paperwork. And then, of course, there’s the new Nov. 30 deadline facing a lame-duck Congress. As politicians continue dithering over a permanent fix and an accompanying price tag of $250 billion or more over a decade, CMS has announced next year’s rate cut. With a 6.1% decrease slated to take effect on Jan. 1, 2011, the combined SGR-mandated drop would reach nearly 30%. Ouch.
Find out the latest information on SGR reform and contact your legislators in support of permanent repeal through SHM's Legislative Action Center.
Handoffs Smoother in Rural Communities
Before Matthew Schreiber, MD, became chief medical officer of Piedmont Hospital in Atlanta, he was director of hospitalist services for the four-hospital Piedmont health system, and before that, a hospitalist for the system’s smallest hospital, 35-bed Piedmont Mountainside in Jasper, Ga., population 2,000, so he knows just how different transitions of care are between hospitals large and small.
In many rural communities, the hospitalist concept has only recently been introduced, and patients are accustomed to PCPs being responsible for all of their medical care. But it can be easier to achieve high-quality handoffs in rural areas because the number of physicians involved is much smaller, Dr. Schreiber says.
“At Piedmont Mountainside, only eight physicians made most of our referrals. It was possible to memorize their office numbers and their call-coverage arrangements,” he explains. Some doctors are accessible 24 hours, seven days a week, while others take their patients’ charts home overnight in case they get called. This encourages an individualized approach to communicating with them. “It makes the care feel more personal, with a different level of accountability,” Dr. Schreiber says. “You feel a connection to the patient and the doctor—and that your job isn’t done when the patient goes home.”
Rural hospitals and doctors also tend to have closer relationships with community services like home health agencies. “We can give the medication list to the home health nurse and say, ‘This is what we think the patient is taking. We want you to go in and find out what they’re actually taking and reconcile the two,’ ” Dr. Schreiber says.
However, small and rural hospitals—particularly stand-alone and critical access facilities—are less likely to have computerized tools for automating and facilitating care transitions, Dr. Schreiber says. In some cases, the rural hospitalist carries a pager and takes calls 24/7.
Dr. Schreiber says it’s important to hand patients a piece of paper that summarizes their condition, key events in the hospitalization, and new medications, all in patient-friendly language, to take home and post on the refrigerator. “In our experience, patients hold onto this document and bring it to the doctor’s office or even to the emergency room,” he says, adding that if the formal discharge summary doesn’t reach the PCP in time, this summary could be a godsend.
Visit our website for more information about local and national efforts to improve care transitions.
Check out the SHM website for information on Project BOOST (Better Outcomes for Older Adults through Safer Transitions), a national QI initiative to improve handoffs and transitions.
Before Matthew Schreiber, MD, became chief medical officer of Piedmont Hospital in Atlanta, he was director of hospitalist services for the four-hospital Piedmont health system, and before that, a hospitalist for the system’s smallest hospital, 35-bed Piedmont Mountainside in Jasper, Ga., population 2,000, so he knows just how different transitions of care are between hospitals large and small.
In many rural communities, the hospitalist concept has only recently been introduced, and patients are accustomed to PCPs being responsible for all of their medical care. But it can be easier to achieve high-quality handoffs in rural areas because the number of physicians involved is much smaller, Dr. Schreiber says.
“At Piedmont Mountainside, only eight physicians made most of our referrals. It was possible to memorize their office numbers and their call-coverage arrangements,” he explains. Some doctors are accessible 24 hours, seven days a week, while others take their patients’ charts home overnight in case they get called. This encourages an individualized approach to communicating with them. “It makes the care feel more personal, with a different level of accountability,” Dr. Schreiber says. “You feel a connection to the patient and the doctor—and that your job isn’t done when the patient goes home.”
Rural hospitals and doctors also tend to have closer relationships with community services like home health agencies. “We can give the medication list to the home health nurse and say, ‘This is what we think the patient is taking. We want you to go in and find out what they’re actually taking and reconcile the two,’ ” Dr. Schreiber says.
However, small and rural hospitals—particularly stand-alone and critical access facilities—are less likely to have computerized tools for automating and facilitating care transitions, Dr. Schreiber says. In some cases, the rural hospitalist carries a pager and takes calls 24/7.
Dr. Schreiber says it’s important to hand patients a piece of paper that summarizes their condition, key events in the hospitalization, and new medications, all in patient-friendly language, to take home and post on the refrigerator. “In our experience, patients hold onto this document and bring it to the doctor’s office or even to the emergency room,” he says, adding that if the formal discharge summary doesn’t reach the PCP in time, this summary could be a godsend.
Visit our website for more information about local and national efforts to improve care transitions.
Check out the SHM website for information on Project BOOST (Better Outcomes for Older Adults through Safer Transitions), a national QI initiative to improve handoffs and transitions.
Before Matthew Schreiber, MD, became chief medical officer of Piedmont Hospital in Atlanta, he was director of hospitalist services for the four-hospital Piedmont health system, and before that, a hospitalist for the system’s smallest hospital, 35-bed Piedmont Mountainside in Jasper, Ga., population 2,000, so he knows just how different transitions of care are between hospitals large and small.
In many rural communities, the hospitalist concept has only recently been introduced, and patients are accustomed to PCPs being responsible for all of their medical care. But it can be easier to achieve high-quality handoffs in rural areas because the number of physicians involved is much smaller, Dr. Schreiber says.
“At Piedmont Mountainside, only eight physicians made most of our referrals. It was possible to memorize their office numbers and their call-coverage arrangements,” he explains. Some doctors are accessible 24 hours, seven days a week, while others take their patients’ charts home overnight in case they get called. This encourages an individualized approach to communicating with them. “It makes the care feel more personal, with a different level of accountability,” Dr. Schreiber says. “You feel a connection to the patient and the doctor—and that your job isn’t done when the patient goes home.”
Rural hospitals and doctors also tend to have closer relationships with community services like home health agencies. “We can give the medication list to the home health nurse and say, ‘This is what we think the patient is taking. We want you to go in and find out what they’re actually taking and reconcile the two,’ ” Dr. Schreiber says.
However, small and rural hospitals—particularly stand-alone and critical access facilities—are less likely to have computerized tools for automating and facilitating care transitions, Dr. Schreiber says. In some cases, the rural hospitalist carries a pager and takes calls 24/7.
Dr. Schreiber says it’s important to hand patients a piece of paper that summarizes their condition, key events in the hospitalization, and new medications, all in patient-friendly language, to take home and post on the refrigerator. “In our experience, patients hold onto this document and bring it to the doctor’s office or even to the emergency room,” he says, adding that if the formal discharge summary doesn’t reach the PCP in time, this summary could be a godsend.
Visit our website for more information about local and national efforts to improve care transitions.
Check out the SHM website for information on Project BOOST (Better Outcomes for Older Adults through Safer Transitions), a national QI initiative to improve handoffs and transitions.