User login
Information Continuity on Outcomes
Hospitalists are common in North America.1, 2 Hospitalists have been associated with a range of beneficial outcomes including decreased length of stay.3, 4 A primary concern of the hospitalist model is its potential detrimental effect on continuity of care5 partly because patients are often not seen by their hospitalists after discharge.
Continuity of care6 is primarily composed of provider continuity (an ongoing relationship between a patient and a particular provider over time) and information continuity (availability of data from prior events for subsequent patient encounters).6 The association between continuity of care and patient outcomes has been quantified in many studies.720 However, the relationship of continuity and outcomes is especially relevant after discharge from the hospital since this is a time when patients have a high risk of poor patient outcomes21 and poor provider22 and information continuity.2325
The association between continuity and outcomes after hospital discharge has been directly quantified in 2 studies. One found that patients seen by a physician who treated them in the hospital had a significant adjusted relative risk reduction in 30‐day death or readmission of 5% and 3%, respectively.22 The other study found that patients discharged from a general medicine ward were less likely to be readmitted if they were seen by physicians who had access to their discharge summary.23 However, neither of these studies concurrently measured the influence of provider and information continuity on patient outcomes.
Determining whether and how continuity of care influences patient outcomes after hospital discharge is essential to improve health care in an evidence‐based fashion. In addition, the influence that hospital physician follow‐up has on patient outcomes can best be determined by measuring provider and information continuity in patients after hospital discharge. This study sought to measure the independent association of several provider and information continuity measures on death or urgent readmission after hospital discharge.
Methods
Study Design
This was a multicenter prospective cohort study of consecutive patients discharged to the community from the medical or surgical services of 11 Ontario hospitals (6 university‐affiliated hospitals and 5 community hospitals) in 5 cities after an elective or emergency hospitalization. Patients were invited to participate in the study if they were cognitively intact, had a telephone, and provided written informed consent. Patients were excluded if they were less than 18 years old, were discharged to nursing homes, or were not proficient in English and did not have someone to help communicate with study staff. Enrolled patients were excluded from the analysis if they had less than 2 physician visits prior to one of the study's outcomes or the end of patient observation (which was 6 months postdischarge). This final exclusion criterion was necessary since 2 continuity measures (including postdischarge physician continuity and postdischarge information continuity) were incalculable with less than 2 physician visits during follow‐up (Supporting information). The study was approved by the research ethics board of each participating hospital.
Data Collection
Prior to hospital discharge, patients were interviewed by study personnel to identify their baseline functional status, their living conditions, all physicians who regularly treated the patient prior to admission (including both family physicians and consultants), and chronic medical conditions. The latter were confirmed by a review of the patient's chart and hospital discharge summary, when available. Patients also provided principal contacts whom we could contact in the event patients could not be reached. The chart and discharge summary were also used to identify diagnoses in hospitalincluding complications (diagnoses arising in the hospital)and medications at discharge.
Patients or their designated contacts were telephoned 1, 3, and 6 months after hospital discharge to identify the date and the physician of all postdischarge physician visits. For each postdischarge physician visit, we determined whether the physician had access to a discharge summary for the index hospitalization. We also determined the availability of information from all previous postdischarge visits that the patient had with other physicians. The methods used to collect these data were previously detailed.26 Briefly, we used three complementary methods to elicit this information from each follow‐up physician. First, patients gave the physician a survey on which the physician listed all prior visits with other doctors for which they had information. If this survey was not returned, we faxed the survey to the physician. If the faxed survey was not returned, we telephoned the physician or their office staff and administered the survey over the telephone.
Continuity Measures
We measured components of both provider and information continuity. For the posthospitalization period, we measured provider continuity for physicians who had provided patient care during three distinct phases: the prehospital period; the hospital period; and the postdischarge period. Prehospital physicians were those classified by the patient as their regular physician(s) (defined as physiciansboth family physicians and consultantsthat they had seen in the past and were likely to see again in the future). Hospital provider continuity was divided into 2 components: hospital physician continuity (ie, the most responsible physician in the hospital); and hospital consultant continuity (ie, another physician who consulted on the patient during admission). Information continuity was divided into discharge summary continuity and postdischarge visit information continuity.
We quantified provider and information continuity using Breslau's Usual Provider of Continuity (UPC)27 measure. It is a widely used and validated continuity measure whose values are meaningful and interpretable.6 The UPC measures the proportion of visits with the physician of interest (for provider continuity) or the proportion of visits having the information of interest (for information continuity). The UPC was calculated as: $${\rm UPC} = {\rm n}_{\rm i} / {\rm N}$$
As the formulae in the supporting information suggest, all continuity measures were incalculable prior to the first postdischarge visit and all continuity measures changed value at each visit during patient observation. In addition, a particular physician visit could increase multiple continuity measures simultaneously. For example, a visit with a physician who was the hospital physician and who regularly treated the patient prior to the hospitalization would increase both hospital and prehospital provider continuity. If the patient had previously seen the physician after discharge, the visit would also increase postdischarge physician continuity.
Study Outcomes
Outcomes for the study included time to all‐cause death and time to all‐cause, urgent readmission. To be classified as urgent, readmissions could not be arranged when the patient was originally discharged from hospital or more than 4 weeks prior to the readmission. All hospital admissions meeting these criteria during the 6 month study period were labeled in this study as urgent readmissions even if they were unrelated to the index admission.
Principal contacts were called if we were unable to reach the patient to determine their outcomes. If the patient's vital status remained unclear, we contacted the Office of the Provincial Registrar to determine if and when the patient died during the 6 months after discharge from hospital.
Analysis
Outcome incidence densities and 95% confidence intervals [CIs] were calculated using PROC GENMOD in SAS to account for clustering of patients in hospitals. We used multivariate proportional hazards modeling to determine the independent association of provider and information continuity measures with time to death and time to urgent readmission. Patient observation started when patients were discharged from the hospital. Patient observation ended at the earliest of the following: death; urgent readmission to the hospital; end of follow‐up (which was 6 months after discharge from the hospital) or loss to follow‐up. Because hospital consultant continuity was very highly skewed (95.6% of patients had a value of 0; mean value of 0.016; skewness 6.9), it was not included in the primary regression models but was included in a sensitivity analysis.
To adjust for potential confounders in the association between continuity and the outcomes, our model included all factors that were independently associated with either the outcome or any continuity measure. Factors associated with death or urgent readmission were summarized using the LACE index.29 This index combines a patient's hospital length of stay, admission acuity, patient comorbidity (measured with the Charlson Score30 using updated disease category weights by Schneeweiss et al.),31 and emergency room utilization (measured as the number of visits in the 6 months prior to admission) into a single number ranging from 0 to 19. The LACE index was moderately discriminative and highly accurate at predicting 30‐day death or urgent readmission.29 In a separate study,28 we found that the following factors were independently associated with at least one of the continuity measures: patient age; patient sex; number of admissions in previous 6 months; number of regular treating physicians prior to admission; hospital service (medicine vs. surgery); and number of complications in the hospital (defined as new problems arising after admission to hospital). By including all factors that were independently associated with either the outcome or continuity, we controlled for all measured factors that could act as confounders in the association between continuity and outcomes. We accounted for the clustered study design by using conditional proportional hazards models that stratified by hospitals.32 Analytical details are given in the supporting information.
Results
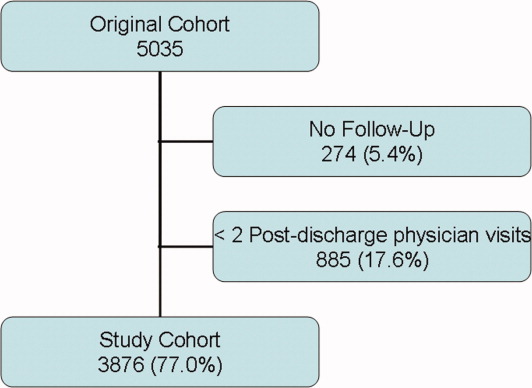
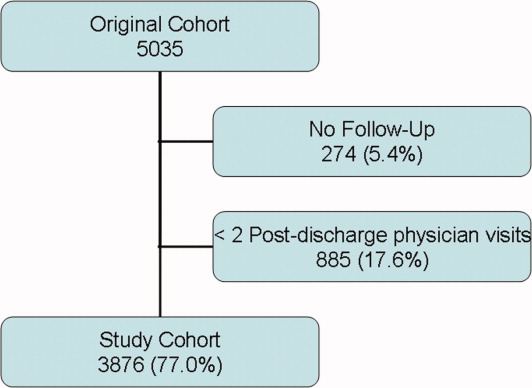
Between October 2002 and July 2006, we enrolled 5035 patients from 11 hospitals (Figure 1). Of the 5035 patients, 274 (5.4%) had no follow up interview with study personnel. A total of 885 (17.6%) had fewer than 2 post discharge physician visits and were not included in the continuity analyses. This left 3876 patients for this analysis (77.0% of the original cohort), of which 3727 had complete follow up (96.1% of the study cohort). A total of 531 patients (10.6% of the original cohort) had incomplete follow‐up because: 342 (6.8%) were lost to follow‐up; 172 (3.4%) refused participation; and 24 (0.5%) were transferred into a nursing home during the first month of observation.
The 3876 study patients are described in Table 1. Overall, these people had a mean age of 62 and most commonly had no physical limitations. Almost a third of patients had been admitted to the hospital in the previous 6 months. A total of 7.6% of patients had no regular prehospital physician while 5.8% had more than one regular prehospital physician. Patients were evenly split between acute and elective admissions and 12% had a complication during their admission. They were discharged after a median of 4 days on a median of 4 medications.
| Factor | Value | Death or Urgent Readmission | All (n = 3876) | |
|---|---|---|---|---|
| No (n = 3491) | Yes (n = 385) | |||
| ||||
| Mean patient age, years (SD) | 61.59 16.16 | 67.70 15.53 | 62.19 16.20 | |
| Female (%) | 1838 (52.6) | 217 (56.4) | 2055 (53.0) | |
| Lives alone (%) | 791 (22.7) | 107 (27.8) | 898 (23.2) | |
| # activities of daily living requiring aids (%) | 0 | 3277 (93.9) | 354 (91.9) | 3631 (93.7) |
| 1 | 125 (3.6) | 20 (5.2) | 145 (3.7) | |
| >1 | 89 (2.5) | 11 (2.8) | 100 (2.8) | |
| # physicians who see patient regularly (%) | 0 | 241 (6.9) | 22 (5.7) | 263 (6.8) |
| 1 | 3060 (87.7) | 333 (86.5) | 3393 (87.5) | |
| 2 | 150 (4.3) | 21 (5.5) | 171 (4.4) | |
| >2 | 281 (8.0) | 31 (8.0) | 312 (8.0) | |
| # admissions in previous 6 months (%) | 0 | 2420 (69.3) | 222 (57.7) | 2642 (68.2) |
| 1 | 833 (23.9) | 103 (26.8) | 936 (24.1) | |
| >1 | 238 (6.8) | 60 (15.6) | 298 (7.7) | |
| Index hospitalization description | ||||
| Number of discharge medications (IQR) | 4 (2‐7) | 6 (3‐9) | 4 (2‐7) | |
| Admitted to medical service (%) | 1440 (41.2) | 231 (60.0) | 1671 (43.1) | |
| Acute diagnoses: | ||||
| CAD (%) | 238 (6.8) | 23 (6.0) | 261 (6.7) | |
| Neoplasm of unspecified nature (%) | 196 (5.6) | 35 (9.1) | 231 (6.0) | |
| Heart failure (%) | 127 (3.6) | 38 (9.9) | 165 (4.3) | |
| Acute procedures | ||||
| CABG (%) | 182 (5.2) | 14 (3.6) | 196 (5.1) | |
| Total knee arthoplasty (%) | 173 (5.0) | 10 (2.6) | 183 (4.7) | |
| Total hip arthroplasty (%) | 118 (3.4) | (0.5) | 120 (3.1) | |
| Complication during admission (%) | 403 (11.5) | 63 (16.4) | 466 (12.0) | |
| LACE index: mean (SD) | 8.0 (3.6) | 10.3 (3.8) | 8.2 (3.7) | |
| Length of stay in days: median (IQR) | 4 (2‐7) | 6 (3‐10) | 4 (2‐8) | |
| Acute/emergent admission (%) | 1851 (53.0) | 272 (70.6) | 2123 (54.8) | |
| Charlson score (%) | 0 | 2771 (79.4) | 241 (62.6) | 3012 (77.7) |
| 1 | 103 (3.0) | 17 (4.4) | 120 (3.1) | |
| 2 | 446 (12.8) | 86 (22.3) | 532 (13.7) | |
| >2 | 171 (4.9) | 41 (10.6) | 212 (5.5) | |
| Emergency room use (# visits/ year) (%) | 0 | 2342 (67.1) | 190 (49.4) | 2532 (65.3) |
| 1 | 761 (21.8) | 101 (26.2) | 862 (22.2) | |
| >1 | 388 (11.1) | 94 (24.4) | 482 (12.4) | |
Patients were observed in the study for a median of 175 days (interquartile range [IQR] 175‐178). During this time they had a median of 4 physician visits (IQR 3‐6). The first postdischarge physician visit occurred a median of 10 days (IQR 6‐18) after discharge from hospital.
Continuity Measures
Table 2 summarizes all continuity scores. Since continuity scores varied significantly over time,28 Table 2 provides continuity scores on the last day of patient observation. Preadmission provider, postdischarge provider, and discharge summary continuity all had similar values and distributions with median values ranging between 0.444 and 0.571. 1797 (46.4%) patients had a hospital physician provider continuity scorae of 0.
| Minimum | 25th Percentile | Median | 75th Percentile | Maximum | |
|---|---|---|---|---|---|
| Provider continuity | |||||
| A: Pre‐admission physician | 0 | 0.143 | 0.444 | 0.667 | 1.000 |
| B: Hospital physician | 0 | 0 | 0.143 | 0.400 | 1.000 |
| C: Post‐discharge physician | 0 | 0.333 | 0.571 | 0.750 | 1.000 |
| Information continuity | |||||
| D: Discharge summary | 0 | 0.095 | 0.500 | 0.800 | 1.000 |
| E: Post‐discharge information | 0 | 0 | 0.182 | 0.500 | 1.000 |
Study Outcomes
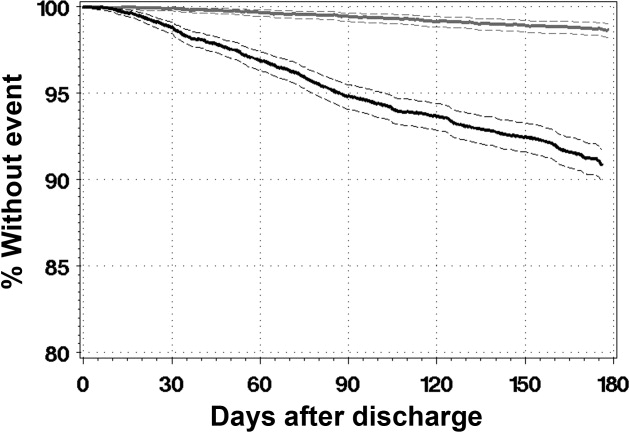
During a median of 175 days of observation, 45 patients died (event rate 2.6 events per 100 patient‐years observation [95% CI 2.0‐3.4]) and 340 patients were urgently readmitted (event rate 19.6 events per 100 patient‐years observation [95% CI 15.9‐24.3]). Figure 2 presents the survival curves for time to death and time to urgent readmission. The hazard of death was consistent through the observation period but the risk of urgent readmission decreased slightly after 90 days postdischarge.
Association Between Continuity and Outcomes
Table 3 summarizes the association between provider and information continuity with study outcomes. No continuity measure was associated with time to death by itself (Table 3, column A) or with the other continuity measures (Table 3, column B). Preadmission physician continuity was associated with a significantly decreased risk of urgent readmission. When the proportion of postdischarge visits with a prehospital physician increased by 10%, the adjusted risk of urgent readmission decreased by 6% (adjusted hazards ratio (adj‐HR)) of 0.94 (95% CI, 0.91‐0.98). None of the other continuity measuresincluding hospital physicianwere significantly associated with urgent readmission either by themselves (Table 3, column A) or after adjusting for other continuity measures (Table 3, column B).
| Outcome | ||||||||
|---|---|---|---|---|---|---|---|---|
| Death (95% CI) | Urgent Readmission (95% CI) | |||||||
| A: Adjusted for Other Confounders Only | B: Adjusted for Other Confounders and Continuity Measures | A: Adjusted for Other Confounders Only | B: Adjusted for Other Confounders and Continuity Measures | |||||
| ||||||||
| Provider continuity | ||||||||
| A: Pre‐admission physician | 1.03 | (0.95, 1.12) | 1.06 | (0.95, 1.18) | 0.95 | (0.92, 0.98) | 0.94 | (0.91, 0.98) |
| B: Hospital physician | 0.87 | (0.74, 1.02) | 0.86 | (0.70, 1.03) | 0.98 | (0.94, 1.02) | 0.97 | (0.92, 1.01) |
| C: Post‐discharge physician | 0.97 | (0.89, 1.06) | 0.93 | (0.84, 1.04) | 0.98 | (0.95, 1.01) | 0.98 | (0.94, 1.02) |
| Information continuity | ||||||||
| D: Discharge Summary | 0.96 | (0.89, 1.04) | 0.94 | (0.87, 1.03) | 1.01 | (0.98, 1.04) | 1.02 | (0.99, 1.05) |
| E: Post‐discharge information | 1.01 | (0.94, 1.08) | 1.03 | (0.95, 1.11) | 1.00 | (0.97, 1.03) | 1.03 | (0.95, 1.11) |
| Other confounders | ||||||||
| Patient age in decades* | 1.43 | (1.13, 1.82) | 1.18 | (1.10, 1.28) | ||||
| Female | 1.50 | (0.81, 2.77) | 1.16 | (0.94, 1.44) | ||||
| # physicians who see patient regularly | ||||||||
| 1 | 1.46 | (0.92, 2.34) | ||||||
| 2 | 2.17 | (1.11, 4.26) | ||||||
| >2 | 3.71 | (1.55, 8.88) | ||||||
| Complications during admission | ||||||||
| 1 | 1.38 | (0.61, 3.10) | 0.81 | (0.55, 1.17) | ||||
| >1 | 1.01 | (0.28, 3.58) | 0.91 | (0.56, 1.48) | ||||
| # admissions in previous 6 months | ||||||||
| 1 | 1.27 | (0.59, 2.70) | 1.34 | (1.02, 1.76) | ||||
| >1 | 1.42 | (0.55, 3.67) | 1.78 | (1.26, 2.51) | ||||
| LACE index* | 1.16 | (1.06, 1.26) | 1.10 | (1.07, 1.14) | ||||
Increased patient age and increased LACE index score were both strongly associated with an increased risk of death (adj‐HR 1.43 [1.13‐1.82] and 1.16 [1.06‐1.26], respectively) and urgent readmission (adj‐HR 1.18 [1.10‐1.28] and 1.10 [1.07‐1.14], respectively). Hospitalization in the 6 months prior to admission significantly increased the risk of urgent readmission but not death. The risk of urgent readmission increased significantly as the number of regular prehospital physicians increased.
Sensitivity Analyses
Our study conclusions did not change in the sensitivity analyses. The number of postdischarge physician visits (expressed as a time‐dependent covariate) was not associated with either death or with urgent readmission and preadmission physician continuity remained significantly associated with time to urgent readmission (supporting information). Adding consultant continuity to the model also did not change our results (supporting information). In‐hospital consultant continuity was associated with an increased risk of urgent readmission (adj‐HR 1.10, 95% CI, 1.01‐1.20). The association between pre‐admission physician continuity and time to urgent readmission did not interact significantly with patient age, LACE index score, or number of previous admissions.
Discussion
This large, prospective cohort study measured the independent association of several provider and information continuity measures with important outcomes in patients discharged from hospital. After adjusting for potential confounders, we found that increased continuity with physicians who regularly cared for the patient prior to the admission was significantly and independently associated with a decreased risk of urgent readmission. Our data suggest that continuity with the hospital physician did not independently influence the risk of patient death or urgent readmission after discharge.
Although hospital physician continuity did not significantly change patient outcomes, we found that follow‐up with a physician who regularly treated the patient prior to their admission was associated with a significantly decreased risk of urgent readmission. This could reflect the important role that a patient's regular physician plays in their health care. Other studies have shown a positive association between continuity with a regular physician and improved outcomes including decreased emergency room utilization7, 8 and decreased hospitalization.10, 11
We were somewhat disappointed that information continuity was not independently associated with improved patient outcomes. Information continuity is likely more amenable to modification than is provider continuity. Of course, our study findings do not mean that information continuity does not improve patient outcomes, as in other studies.23, 33 Instead, our results could reflect that we solely measured the availability of information to physicians. Future studies that measure the quality, relevance, and actual utilization of patient information will be better able to discern the influence of information continuity on patient outcomes.
We believe that our study was methodologically strong and unique. We captured both provider and information continuity in a large group of representative patients using a broad range of measures that captured continuity's diverse components including both provider and information continuity. The continuity measures were expressed and properly analyzed as time‐dependent variables in a multivariate model.34 Our analysis controlled for important potential confounders. Our follow‐up and data collection was rigorous with 96.1% of our study group having complete follow‐up. Finally, the analysis used multiple imputation to appropriately handle missing data in the one incomplete variable (post‐discharge information continuity).3537
Several limitations of our study should be kept in mind. We are uncertain how our results might generalize to patients discharged from obstetrical or psychiatric services or people in other health systems. Our analysis had to exclude patients with less than two physician visits after discharge since this was the minimum required to calculate postdischarge physician and information continuity. Data collection for postdischarge information continuity was incomplete with data missing for 19.0% of all 15 401 visits in the original cohort.38 However, a response rate of 81.0% is very good39 when compared to other survey‐based studies40 and we accounted for the missing data using multiple imputation methods. The primary outcomes of our studytime to death or urgent readmissionmay be relatively insensitive to modification of quality of care, which is presumably improved by increased continuity.41 For example, Clarke found that the majority of readmissions in all patient groups were unavoidable with 94% of medical readmissions 1 month postdischarge judged to be unavoidable.42 Future studies regarding the effects of continuity could focus on its association with other outcomes that are more reflective of quality of care such as the risk of adverse events or medical error.21 Such outcomes would presumably be more sensitive to improved quality of care from increased continuity.
We believe that our study's major limitation was its inability to establish a causal association between continuity and patient outcomes. Our finding that increased consultant continuity was associated with an increased risk of poor outcomes highlights this concern. Presumably, patient follow‐up with a hospital consultant indicates a disease status with a high risk of bad patient outcomesa risk that is not entirely accounted for by the covariates used in this study. If we accept that unresolved confounding explains this association, the same could also apply to the association between preadmission physician continuity and improved outcomes. Perhaps patients who are doing well after discharge from hospital are able to return to their regular physician. Our analysis would therefore identify an association between increased preadmission physician continuity and improved patient outcomes. Analyses could also incorporate more discriminative measures of severity of hospital illness, such as those developed by Escobar et al.43 Since patients may experience health events after their discharge from hospital that could influence outcomes, recording these and expressing them in the study model as time‐dependent covariates will be important. Finally, similar to the classic study by Wasson et al.44 in 1984, a proper randomized trial that measures the effect of a continuity‐building intervention on both continuity of care and patient outcomes would help determine how continuity influences outcomes.
In conclusion, after discharge from hospital, increased continuity with physicians who routinely care for the patient is significantly and independently associated with a decreased risk of urgent readmission. Continuity with the hospital physician after discharge did not independently influence the risk of patient death or urgent readmission in our study. Further research is required to determine the causal association between preadmission physician continuity and improved outcomes. Until that time, clinicians should strive to optimize continuity with physicians their patients have seen prior to the hospitalization.
- Society of Hospital Medicine.2009.Ref Type: Internet Communication.
- ,,,.The status of hospital medicine groups in the United States.J Hosp Med.2006;1:75–80.
- ,.The hospitalist movement 5 years later. [see comment].JAMA.2002;287:487–494. [Review]
- ,,,.Hospitalists and the practice of inpatient medicine: results of a survey of the National Association of Inpatient Physicians. [see comment].Ann Intern Med.1999;130:343–349.
- ,,,.Primary care physician attitudes regarding communication with hospitalists.Am J Med.2001;111:15S–20S.
- ,,.Defusing the confusion: concepts and measures of continuity of healthcare.Ottawa,Canadian Health Services Research Foundation. Ref Type: Report.2002;1–50.
- ,,,,.Association between infant continuity of care and pediatric emergency department utilization.Pediatrics.2004;113:738–741.
- ,,,,.Is greater continuity of care associated with less emergency department utilization?Pediatrics.1999;103:738–742.
- ,,,,.Association of lower continuity of care with greater risk of emergency department use and hospitalization in children.Pediatrics.2001;107:524–529.
- ,,The role of provider continuity in preventing hospitalizations.Arch Fam Med.1998;7:352–357.
- ,.The importance of continuity of care in the likelihood of future hospitalization: is site of care equivalent to a primary clinician?Am J Public Health.1998;88:1539–1541.
- ,,,.Exploration of the relationship between continuity, trust in regular doctors and patient satisfaction with consultations with family doctors.Scand J Prim Health Care.2003;21:27–32.
- ,,,,.Longitudinal continuity of care is associated with high patient satisfaction with physical therapy.Phys Ther.2005;85:1046–1052.
- ,,,,.Provider continuity and outcomes of care for persons with schizophrenia.Ment Health Serv Res.2000;V2:201–211.
- ,,,,.Continuity of care is associated with well‐coordinated care.Ambul Pediatr.2003;3:82–86.
- ,,.The impact of insurance type and forced discontinuity on the delivery of primary care. [see comments.].J Fam Pract.1997;45:129–135.
- .Measuring attributes of primary care: development of a new instrument.J Fam Pract.1997;45:64–74.
- .Continuity of care during pregnancy: the effect of provider continuity on outcome.J Fam Pract.1985;21:375–380.
- ,,,,,.Physician‐patient relationship and medication compliance: a primary care investigation.Ann Fam Med.2004;2:455–461.
- ,,,.Continuity of care and cardiovascular risk factor management: does care by a single clinician add to informational continuity provided by electronic medical records?Am J Manag Care.2005;11:689–696.
- ,,,,.The incidence and severity of adverse events affecting patients after discharge from the hospital.Ann Intern Med.2003;138:161–167.
- ,,,.Continuity of care and patient outcomes after hospital discharge.J Gen lntern Med.2004;19:624–645.
- ,,,.Effect of discharge summary availability during post‐discharge visits on hospital readmission.J Gen Intern Med.2002;17:186–192.
- ,,, et al.Association of communication between hospital‐based physicians and primary care providers with patient outcomes.[see comment].J Gen Intern Med2009;24(3):381–386.
- ,,,,,.Deficits in communication and information transfer between hospital‐based and primary care physicians: implications for patient safety and continuity of care.JAMA.2007;297:831–841.
- ,,, et al.Information exchange among physicians caring for the same patient in the community.Can Med Assoc J.2008;179:1013–1018.
- ,.Continuity of care in a university‐based practice.J Med Educ.1975;965–969.
- ,,, et al.Provider and information continuity after discharge from hospital: a prospective cohort study.2009. Ref Type: Unpublished Work.
- ,,, et al.Derivation and validation of the LACE index to predict early death or unplanned readmission after discharge from hospital to the community.CMAJ. (In press)
- ,,,.A new method of classifying prognostic comorbidity in longitudinal studies: development and validation.J Chronic Dis.1987;40:373–383.
- ,,,.Improved comorbidity adjustment for predicting mortality in Medicare populations.Health Serv Res.2003;38(4):1103–1120.
- ,.Modelling clustered survival data from multicentre clinical trials.Stat Med.2004;23:369–388.
- ,,,.Prevalence of information gaps in the emergency department and the effect on patient outcomes.CMAJ.2003;169:1023–1028.
- ,,,.Time‐dependent bias due to improper analytical methodology is common in prominent medical journals.J Clin Epidemiol.2004;57:672–682.
- .What do we do with missing data? Some options for analysis of incomplete data.Annu Rev Public Health.2004;25:99–117.
- ,,,.Survival estimates of a prognostic classification depended more on year of treatment than on imputation of missing values.J Clin Epidemiol.2006;59:246–253. [Review]
- .Bias arising from missing data in predictive models.[see comment].J Clin Epidemiol.2006;59:1115–1123.
- ,,, et al.Information exchange among physicians caring for the same patient in the community.CMAJ.2008;179:1013–1018.
- .Survey Research Methods.2nd ed.,Beverly Hills:Sage;1993.
- ,,.Response rates to mail surveys published in medical journals.J Clin Epidemiol.1997;50:1129–1136.
- .Readmission of patients to hospital: still ill defined and poorly understood.Int J Qual Health Care.2001;13:177–179.
- .Are readmissions avoidable?Br Med J.1990;301:1136–1138.
- ,,,,,.Risk‐adjusting hospital inpatient mortality using automated inpatient, outpatient, and laboratory databases.Med Care.2008;46:232–239.
- ,,, et al.Continuity of outpatient medical care in elderly men. A randomized trial.JAMA.1984;252:2413–2417.
Hospitalists are common in North America.1, 2 Hospitalists have been associated with a range of beneficial outcomes including decreased length of stay.3, 4 A primary concern of the hospitalist model is its potential detrimental effect on continuity of care5 partly because patients are often not seen by their hospitalists after discharge.
Continuity of care6 is primarily composed of provider continuity (an ongoing relationship between a patient and a particular provider over time) and information continuity (availability of data from prior events for subsequent patient encounters).6 The association between continuity of care and patient outcomes has been quantified in many studies.720 However, the relationship of continuity and outcomes is especially relevant after discharge from the hospital since this is a time when patients have a high risk of poor patient outcomes21 and poor provider22 and information continuity.2325
The association between continuity and outcomes after hospital discharge has been directly quantified in 2 studies. One found that patients seen by a physician who treated them in the hospital had a significant adjusted relative risk reduction in 30‐day death or readmission of 5% and 3%, respectively.22 The other study found that patients discharged from a general medicine ward were less likely to be readmitted if they were seen by physicians who had access to their discharge summary.23 However, neither of these studies concurrently measured the influence of provider and information continuity on patient outcomes.
Determining whether and how continuity of care influences patient outcomes after hospital discharge is essential to improve health care in an evidence‐based fashion. In addition, the influence that hospital physician follow‐up has on patient outcomes can best be determined by measuring provider and information continuity in patients after hospital discharge. This study sought to measure the independent association of several provider and information continuity measures on death or urgent readmission after hospital discharge.
Methods
Study Design
This was a multicenter prospective cohort study of consecutive patients discharged to the community from the medical or surgical services of 11 Ontario hospitals (6 university‐affiliated hospitals and 5 community hospitals) in 5 cities after an elective or emergency hospitalization. Patients were invited to participate in the study if they were cognitively intact, had a telephone, and provided written informed consent. Patients were excluded if they were less than 18 years old, were discharged to nursing homes, or were not proficient in English and did not have someone to help communicate with study staff. Enrolled patients were excluded from the analysis if they had less than 2 physician visits prior to one of the study's outcomes or the end of patient observation (which was 6 months postdischarge). This final exclusion criterion was necessary since 2 continuity measures (including postdischarge physician continuity and postdischarge information continuity) were incalculable with less than 2 physician visits during follow‐up (Supporting information). The study was approved by the research ethics board of each participating hospital.
Data Collection
Prior to hospital discharge, patients were interviewed by study personnel to identify their baseline functional status, their living conditions, all physicians who regularly treated the patient prior to admission (including both family physicians and consultants), and chronic medical conditions. The latter were confirmed by a review of the patient's chart and hospital discharge summary, when available. Patients also provided principal contacts whom we could contact in the event patients could not be reached. The chart and discharge summary were also used to identify diagnoses in hospitalincluding complications (diagnoses arising in the hospital)and medications at discharge.
Patients or their designated contacts were telephoned 1, 3, and 6 months after hospital discharge to identify the date and the physician of all postdischarge physician visits. For each postdischarge physician visit, we determined whether the physician had access to a discharge summary for the index hospitalization. We also determined the availability of information from all previous postdischarge visits that the patient had with other physicians. The methods used to collect these data were previously detailed.26 Briefly, we used three complementary methods to elicit this information from each follow‐up physician. First, patients gave the physician a survey on which the physician listed all prior visits with other doctors for which they had information. If this survey was not returned, we faxed the survey to the physician. If the faxed survey was not returned, we telephoned the physician or their office staff and administered the survey over the telephone.
Continuity Measures
We measured components of both provider and information continuity. For the posthospitalization period, we measured provider continuity for physicians who had provided patient care during three distinct phases: the prehospital period; the hospital period; and the postdischarge period. Prehospital physicians were those classified by the patient as their regular physician(s) (defined as physiciansboth family physicians and consultantsthat they had seen in the past and were likely to see again in the future). Hospital provider continuity was divided into 2 components: hospital physician continuity (ie, the most responsible physician in the hospital); and hospital consultant continuity (ie, another physician who consulted on the patient during admission). Information continuity was divided into discharge summary continuity and postdischarge visit information continuity.
We quantified provider and information continuity using Breslau's Usual Provider of Continuity (UPC)27 measure. It is a widely used and validated continuity measure whose values are meaningful and interpretable.6 The UPC measures the proportion of visits with the physician of interest (for provider continuity) or the proportion of visits having the information of interest (for information continuity). The UPC was calculated as: $${\rm UPC} = {\rm n}_{\rm i} / {\rm N}$$
As the formulae in the supporting information suggest, all continuity measures were incalculable prior to the first postdischarge visit and all continuity measures changed value at each visit during patient observation. In addition, a particular physician visit could increase multiple continuity measures simultaneously. For example, a visit with a physician who was the hospital physician and who regularly treated the patient prior to the hospitalization would increase both hospital and prehospital provider continuity. If the patient had previously seen the physician after discharge, the visit would also increase postdischarge physician continuity.
Study Outcomes
Outcomes for the study included time to all‐cause death and time to all‐cause, urgent readmission. To be classified as urgent, readmissions could not be arranged when the patient was originally discharged from hospital or more than 4 weeks prior to the readmission. All hospital admissions meeting these criteria during the 6 month study period were labeled in this study as urgent readmissions even if they were unrelated to the index admission.
Principal contacts were called if we were unable to reach the patient to determine their outcomes. If the patient's vital status remained unclear, we contacted the Office of the Provincial Registrar to determine if and when the patient died during the 6 months after discharge from hospital.
Analysis
Outcome incidence densities and 95% confidence intervals [CIs] were calculated using PROC GENMOD in SAS to account for clustering of patients in hospitals. We used multivariate proportional hazards modeling to determine the independent association of provider and information continuity measures with time to death and time to urgent readmission. Patient observation started when patients were discharged from the hospital. Patient observation ended at the earliest of the following: death; urgent readmission to the hospital; end of follow‐up (which was 6 months after discharge from the hospital) or loss to follow‐up. Because hospital consultant continuity was very highly skewed (95.6% of patients had a value of 0; mean value of 0.016; skewness 6.9), it was not included in the primary regression models but was included in a sensitivity analysis.
To adjust for potential confounders in the association between continuity and the outcomes, our model included all factors that were independently associated with either the outcome or any continuity measure. Factors associated with death or urgent readmission were summarized using the LACE index.29 This index combines a patient's hospital length of stay, admission acuity, patient comorbidity (measured with the Charlson Score30 using updated disease category weights by Schneeweiss et al.),31 and emergency room utilization (measured as the number of visits in the 6 months prior to admission) into a single number ranging from 0 to 19. The LACE index was moderately discriminative and highly accurate at predicting 30‐day death or urgent readmission.29 In a separate study,28 we found that the following factors were independently associated with at least one of the continuity measures: patient age; patient sex; number of admissions in previous 6 months; number of regular treating physicians prior to admission; hospital service (medicine vs. surgery); and number of complications in the hospital (defined as new problems arising after admission to hospital). By including all factors that were independently associated with either the outcome or continuity, we controlled for all measured factors that could act as confounders in the association between continuity and outcomes. We accounted for the clustered study design by using conditional proportional hazards models that stratified by hospitals.32 Analytical details are given in the supporting information.
Results
Between October 2002 and July 2006, we enrolled 5035 patients from 11 hospitals (Figure 1). Of the 5035 patients, 274 (5.4%) had no follow up interview with study personnel. A total of 885 (17.6%) had fewer than 2 post discharge physician visits and were not included in the continuity analyses. This left 3876 patients for this analysis (77.0% of the original cohort), of which 3727 had complete follow up (96.1% of the study cohort). A total of 531 patients (10.6% of the original cohort) had incomplete follow‐up because: 342 (6.8%) were lost to follow‐up; 172 (3.4%) refused participation; and 24 (0.5%) were transferred into a nursing home during the first month of observation.


The 3876 study patients are described in Table 1. Overall, these people had a mean age of 62 and most commonly had no physical limitations. Almost a third of patients had been admitted to the hospital in the previous 6 months. A total of 7.6% of patients had no regular prehospital physician while 5.8% had more than one regular prehospital physician. Patients were evenly split between acute and elective admissions and 12% had a complication during their admission. They were discharged after a median of 4 days on a median of 4 medications.
| Factor | Value | Death or Urgent Readmission | All (n = 3876) | |
|---|---|---|---|---|
| No (n = 3491) | Yes (n = 385) | |||
| ||||
| Mean patient age, years (SD) | 61.59 16.16 | 67.70 15.53 | 62.19 16.20 | |
| Female (%) | 1838 (52.6) | 217 (56.4) | 2055 (53.0) | |
| Lives alone (%) | 791 (22.7) | 107 (27.8) | 898 (23.2) | |
| # activities of daily living requiring aids (%) | 0 | 3277 (93.9) | 354 (91.9) | 3631 (93.7) |
| 1 | 125 (3.6) | 20 (5.2) | 145 (3.7) | |
| >1 | 89 (2.5) | 11 (2.8) | 100 (2.8) | |
| # physicians who see patient regularly (%) | 0 | 241 (6.9) | 22 (5.7) | 263 (6.8) |
| 1 | 3060 (87.7) | 333 (86.5) | 3393 (87.5) | |
| 2 | 150 (4.3) | 21 (5.5) | 171 (4.4) | |
| >2 | 281 (8.0) | 31 (8.0) | 312 (8.0) | |
| # admissions in previous 6 months (%) | 0 | 2420 (69.3) | 222 (57.7) | 2642 (68.2) |
| 1 | 833 (23.9) | 103 (26.8) | 936 (24.1) | |
| >1 | 238 (6.8) | 60 (15.6) | 298 (7.7) | |
| Index hospitalization description | ||||
| Number of discharge medications (IQR) | 4 (2‐7) | 6 (3‐9) | 4 (2‐7) | |
| Admitted to medical service (%) | 1440 (41.2) | 231 (60.0) | 1671 (43.1) | |
| Acute diagnoses: | ||||
| CAD (%) | 238 (6.8) | 23 (6.0) | 261 (6.7) | |
| Neoplasm of unspecified nature (%) | 196 (5.6) | 35 (9.1) | 231 (6.0) | |
| Heart failure (%) | 127 (3.6) | 38 (9.9) | 165 (4.3) | |
| Acute procedures | ||||
| CABG (%) | 182 (5.2) | 14 (3.6) | 196 (5.1) | |
| Total knee arthoplasty (%) | 173 (5.0) | 10 (2.6) | 183 (4.7) | |
| Total hip arthroplasty (%) | 118 (3.4) | (0.5) | 120 (3.1) | |
| Complication during admission (%) | 403 (11.5) | 63 (16.4) | 466 (12.0) | |
| LACE index: mean (SD) | 8.0 (3.6) | 10.3 (3.8) | 8.2 (3.7) | |
| Length of stay in days: median (IQR) | 4 (2‐7) | 6 (3‐10) | 4 (2‐8) | |
| Acute/emergent admission (%) | 1851 (53.0) | 272 (70.6) | 2123 (54.8) | |
| Charlson score (%) | 0 | 2771 (79.4) | 241 (62.6) | 3012 (77.7) |
| 1 | 103 (3.0) | 17 (4.4) | 120 (3.1) | |
| 2 | 446 (12.8) | 86 (22.3) | 532 (13.7) | |
| >2 | 171 (4.9) | 41 (10.6) | 212 (5.5) | |
| Emergency room use (# visits/ year) (%) | 0 | 2342 (67.1) | 190 (49.4) | 2532 (65.3) |
| 1 | 761 (21.8) | 101 (26.2) | 862 (22.2) | |
| >1 | 388 (11.1) | 94 (24.4) | 482 (12.4) | |
Patients were observed in the study for a median of 175 days (interquartile range [IQR] 175‐178). During this time they had a median of 4 physician visits (IQR 3‐6). The first postdischarge physician visit occurred a median of 10 days (IQR 6‐18) after discharge from hospital.
Continuity Measures
Table 2 summarizes all continuity scores. Since continuity scores varied significantly over time,28 Table 2 provides continuity scores on the last day of patient observation. Preadmission provider, postdischarge provider, and discharge summary continuity all had similar values and distributions with median values ranging between 0.444 and 0.571. 1797 (46.4%) patients had a hospital physician provider continuity scorae of 0.
| Minimum | 25th Percentile | Median | 75th Percentile | Maximum | |
|---|---|---|---|---|---|
| Provider continuity | |||||
| A: Pre‐admission physician | 0 | 0.143 | 0.444 | 0.667 | 1.000 |
| B: Hospital physician | 0 | 0 | 0.143 | 0.400 | 1.000 |
| C: Post‐discharge physician | 0 | 0.333 | 0.571 | 0.750 | 1.000 |
| Information continuity | |||||
| D: Discharge summary | 0 | 0.095 | 0.500 | 0.800 | 1.000 |
| E: Post‐discharge information | 0 | 0 | 0.182 | 0.500 | 1.000 |
Study Outcomes
During a median of 175 days of observation, 45 patients died (event rate 2.6 events per 100 patient‐years observation [95% CI 2.0‐3.4]) and 340 patients were urgently readmitted (event rate 19.6 events per 100 patient‐years observation [95% CI 15.9‐24.3]). Figure 2 presents the survival curves for time to death and time to urgent readmission. The hazard of death was consistent through the observation period but the risk of urgent readmission decreased slightly after 90 days postdischarge.
Association Between Continuity and Outcomes
Table 3 summarizes the association between provider and information continuity with study outcomes. No continuity measure was associated with time to death by itself (Table 3, column A) or with the other continuity measures (Table 3, column B). Preadmission physician continuity was associated with a significantly decreased risk of urgent readmission. When the proportion of postdischarge visits with a prehospital physician increased by 10%, the adjusted risk of urgent readmission decreased by 6% (adjusted hazards ratio (adj‐HR)) of 0.94 (95% CI, 0.91‐0.98). None of the other continuity measuresincluding hospital physicianwere significantly associated with urgent readmission either by themselves (Table 3, column A) or after adjusting for other continuity measures (Table 3, column B).
| Outcome | ||||||||
|---|---|---|---|---|---|---|---|---|
| Death (95% CI) | Urgent Readmission (95% CI) | |||||||
| A: Adjusted for Other Confounders Only | B: Adjusted for Other Confounders and Continuity Measures | A: Adjusted for Other Confounders Only | B: Adjusted for Other Confounders and Continuity Measures | |||||
| ||||||||
| Provider continuity | ||||||||
| A: Pre‐admission physician | 1.03 | (0.95, 1.12) | 1.06 | (0.95, 1.18) | 0.95 | (0.92, 0.98) | 0.94 | (0.91, 0.98) |
| B: Hospital physician | 0.87 | (0.74, 1.02) | 0.86 | (0.70, 1.03) | 0.98 | (0.94, 1.02) | 0.97 | (0.92, 1.01) |
| C: Post‐discharge physician | 0.97 | (0.89, 1.06) | 0.93 | (0.84, 1.04) | 0.98 | (0.95, 1.01) | 0.98 | (0.94, 1.02) |
| Information continuity | ||||||||
| D: Discharge Summary | 0.96 | (0.89, 1.04) | 0.94 | (0.87, 1.03) | 1.01 | (0.98, 1.04) | 1.02 | (0.99, 1.05) |
| E: Post‐discharge information | 1.01 | (0.94, 1.08) | 1.03 | (0.95, 1.11) | 1.00 | (0.97, 1.03) | 1.03 | (0.95, 1.11) |
| Other confounders | ||||||||
| Patient age in decades* | 1.43 | (1.13, 1.82) | 1.18 | (1.10, 1.28) | ||||
| Female | 1.50 | (0.81, 2.77) | 1.16 | (0.94, 1.44) | ||||
| # physicians who see patient regularly | ||||||||
| 1 | 1.46 | (0.92, 2.34) | ||||||
| 2 | 2.17 | (1.11, 4.26) | ||||||
| >2 | 3.71 | (1.55, 8.88) | ||||||
| Complications during admission | ||||||||
| 1 | 1.38 | (0.61, 3.10) | 0.81 | (0.55, 1.17) | ||||
| >1 | 1.01 | (0.28, 3.58) | 0.91 | (0.56, 1.48) | ||||
| # admissions in previous 6 months | ||||||||
| 1 | 1.27 | (0.59, 2.70) | 1.34 | (1.02, 1.76) | ||||
| >1 | 1.42 | (0.55, 3.67) | 1.78 | (1.26, 2.51) | ||||
| LACE index* | 1.16 | (1.06, 1.26) | 1.10 | (1.07, 1.14) | ||||
Increased patient age and increased LACE index score were both strongly associated with an increased risk of death (adj‐HR 1.43 [1.13‐1.82] and 1.16 [1.06‐1.26], respectively) and urgent readmission (adj‐HR 1.18 [1.10‐1.28] and 1.10 [1.07‐1.14], respectively). Hospitalization in the 6 months prior to admission significantly increased the risk of urgent readmission but not death. The risk of urgent readmission increased significantly as the number of regular prehospital physicians increased.
Sensitivity Analyses
Our study conclusions did not change in the sensitivity analyses. The number of postdischarge physician visits (expressed as a time‐dependent covariate) was not associated with either death or with urgent readmission and preadmission physician continuity remained significantly associated with time to urgent readmission (supporting information). Adding consultant continuity to the model also did not change our results (supporting information). In‐hospital consultant continuity was associated with an increased risk of urgent readmission (adj‐HR 1.10, 95% CI, 1.01‐1.20). The association between pre‐admission physician continuity and time to urgent readmission did not interact significantly with patient age, LACE index score, or number of previous admissions.
Discussion
This large, prospective cohort study measured the independent association of several provider and information continuity measures with important outcomes in patients discharged from hospital. After adjusting for potential confounders, we found that increased continuity with physicians who regularly cared for the patient prior to the admission was significantly and independently associated with a decreased risk of urgent readmission. Our data suggest that continuity with the hospital physician did not independently influence the risk of patient death or urgent readmission after discharge.
Although hospital physician continuity did not significantly change patient outcomes, we found that follow‐up with a physician who regularly treated the patient prior to their admission was associated with a significantly decreased risk of urgent readmission. This could reflect the important role that a patient's regular physician plays in their health care. Other studies have shown a positive association between continuity with a regular physician and improved outcomes including decreased emergency room utilization7, 8 and decreased hospitalization.10, 11
We were somewhat disappointed that information continuity was not independently associated with improved patient outcomes. Information continuity is likely more amenable to modification than is provider continuity. Of course, our study findings do not mean that information continuity does not improve patient outcomes, as in other studies.23, 33 Instead, our results could reflect that we solely measured the availability of information to physicians. Future studies that measure the quality, relevance, and actual utilization of patient information will be better able to discern the influence of information continuity on patient outcomes.
We believe that our study was methodologically strong and unique. We captured both provider and information continuity in a large group of representative patients using a broad range of measures that captured continuity's diverse components including both provider and information continuity. The continuity measures were expressed and properly analyzed as time‐dependent variables in a multivariate model.34 Our analysis controlled for important potential confounders. Our follow‐up and data collection was rigorous with 96.1% of our study group having complete follow‐up. Finally, the analysis used multiple imputation to appropriately handle missing data in the one incomplete variable (post‐discharge information continuity).3537
Several limitations of our study should be kept in mind. We are uncertain how our results might generalize to patients discharged from obstetrical or psychiatric services or people in other health systems. Our analysis had to exclude patients with less than two physician visits after discharge since this was the minimum required to calculate postdischarge physician and information continuity. Data collection for postdischarge information continuity was incomplete with data missing for 19.0% of all 15 401 visits in the original cohort.38 However, a response rate of 81.0% is very good39 when compared to other survey‐based studies40 and we accounted for the missing data using multiple imputation methods. The primary outcomes of our studytime to death or urgent readmissionmay be relatively insensitive to modification of quality of care, which is presumably improved by increased continuity.41 For example, Clarke found that the majority of readmissions in all patient groups were unavoidable with 94% of medical readmissions 1 month postdischarge judged to be unavoidable.42 Future studies regarding the effects of continuity could focus on its association with other outcomes that are more reflective of quality of care such as the risk of adverse events or medical error.21 Such outcomes would presumably be more sensitive to improved quality of care from increased continuity.
We believe that our study's major limitation was its inability to establish a causal association between continuity and patient outcomes. Our finding that increased consultant continuity was associated with an increased risk of poor outcomes highlights this concern. Presumably, patient follow‐up with a hospital consultant indicates a disease status with a high risk of bad patient outcomesa risk that is not entirely accounted for by the covariates used in this study. If we accept that unresolved confounding explains this association, the same could also apply to the association between preadmission physician continuity and improved outcomes. Perhaps patients who are doing well after discharge from hospital are able to return to their regular physician. Our analysis would therefore identify an association between increased preadmission physician continuity and improved patient outcomes. Analyses could also incorporate more discriminative measures of severity of hospital illness, such as those developed by Escobar et al.43 Since patients may experience health events after their discharge from hospital that could influence outcomes, recording these and expressing them in the study model as time‐dependent covariates will be important. Finally, similar to the classic study by Wasson et al.44 in 1984, a proper randomized trial that measures the effect of a continuity‐building intervention on both continuity of care and patient outcomes would help determine how continuity influences outcomes.
In conclusion, after discharge from hospital, increased continuity with physicians who routinely care for the patient is significantly and independently associated with a decreased risk of urgent readmission. Continuity with the hospital physician after discharge did not independently influence the risk of patient death or urgent readmission in our study. Further research is required to determine the causal association between preadmission physician continuity and improved outcomes. Until that time, clinicians should strive to optimize continuity with physicians their patients have seen prior to the hospitalization.
Hospitalists are common in North America.1, 2 Hospitalists have been associated with a range of beneficial outcomes including decreased length of stay.3, 4 A primary concern of the hospitalist model is its potential detrimental effect on continuity of care5 partly because patients are often not seen by their hospitalists after discharge.
Continuity of care6 is primarily composed of provider continuity (an ongoing relationship between a patient and a particular provider over time) and information continuity (availability of data from prior events for subsequent patient encounters).6 The association between continuity of care and patient outcomes has been quantified in many studies.720 However, the relationship of continuity and outcomes is especially relevant after discharge from the hospital since this is a time when patients have a high risk of poor patient outcomes21 and poor provider22 and information continuity.2325
The association between continuity and outcomes after hospital discharge has been directly quantified in 2 studies. One found that patients seen by a physician who treated them in the hospital had a significant adjusted relative risk reduction in 30‐day death or readmission of 5% and 3%, respectively.22 The other study found that patients discharged from a general medicine ward were less likely to be readmitted if they were seen by physicians who had access to their discharge summary.23 However, neither of these studies concurrently measured the influence of provider and information continuity on patient outcomes.
Determining whether and how continuity of care influences patient outcomes after hospital discharge is essential to improve health care in an evidence‐based fashion. In addition, the influence that hospital physician follow‐up has on patient outcomes can best be determined by measuring provider and information continuity in patients after hospital discharge. This study sought to measure the independent association of several provider and information continuity measures on death or urgent readmission after hospital discharge.
Methods
Study Design
This was a multicenter prospective cohort study of consecutive patients discharged to the community from the medical or surgical services of 11 Ontario hospitals (6 university‐affiliated hospitals and 5 community hospitals) in 5 cities after an elective or emergency hospitalization. Patients were invited to participate in the study if they were cognitively intact, had a telephone, and provided written informed consent. Patients were excluded if they were less than 18 years old, were discharged to nursing homes, or were not proficient in English and did not have someone to help communicate with study staff. Enrolled patients were excluded from the analysis if they had less than 2 physician visits prior to one of the study's outcomes or the end of patient observation (which was 6 months postdischarge). This final exclusion criterion was necessary since 2 continuity measures (including postdischarge physician continuity and postdischarge information continuity) were incalculable with less than 2 physician visits during follow‐up (Supporting information). The study was approved by the research ethics board of each participating hospital.
Data Collection
Prior to hospital discharge, patients were interviewed by study personnel to identify their baseline functional status, their living conditions, all physicians who regularly treated the patient prior to admission (including both family physicians and consultants), and chronic medical conditions. The latter were confirmed by a review of the patient's chart and hospital discharge summary, when available. Patients also provided principal contacts whom we could contact in the event patients could not be reached. The chart and discharge summary were also used to identify diagnoses in hospitalincluding complications (diagnoses arising in the hospital)and medications at discharge.
Patients or their designated contacts were telephoned 1, 3, and 6 months after hospital discharge to identify the date and the physician of all postdischarge physician visits. For each postdischarge physician visit, we determined whether the physician had access to a discharge summary for the index hospitalization. We also determined the availability of information from all previous postdischarge visits that the patient had with other physicians. The methods used to collect these data were previously detailed.26 Briefly, we used three complementary methods to elicit this information from each follow‐up physician. First, patients gave the physician a survey on which the physician listed all prior visits with other doctors for which they had information. If this survey was not returned, we faxed the survey to the physician. If the faxed survey was not returned, we telephoned the physician or their office staff and administered the survey over the telephone.
Continuity Measures
We measured components of both provider and information continuity. For the posthospitalization period, we measured provider continuity for physicians who had provided patient care during three distinct phases: the prehospital period; the hospital period; and the postdischarge period. Prehospital physicians were those classified by the patient as their regular physician(s) (defined as physiciansboth family physicians and consultantsthat they had seen in the past and were likely to see again in the future). Hospital provider continuity was divided into 2 components: hospital physician continuity (ie, the most responsible physician in the hospital); and hospital consultant continuity (ie, another physician who consulted on the patient during admission). Information continuity was divided into discharge summary continuity and postdischarge visit information continuity.
We quantified provider and information continuity using Breslau's Usual Provider of Continuity (UPC)27 measure. It is a widely used and validated continuity measure whose values are meaningful and interpretable.6 The UPC measures the proportion of visits with the physician of interest (for provider continuity) or the proportion of visits having the information of interest (for information continuity). The UPC was calculated as: $${\rm UPC} = {\rm n}_{\rm i} / {\rm N}$$
As the formulae in the supporting information suggest, all continuity measures were incalculable prior to the first postdischarge visit and all continuity measures changed value at each visit during patient observation. In addition, a particular physician visit could increase multiple continuity measures simultaneously. For example, a visit with a physician who was the hospital physician and who regularly treated the patient prior to the hospitalization would increase both hospital and prehospital provider continuity. If the patient had previously seen the physician after discharge, the visit would also increase postdischarge physician continuity.
Study Outcomes
Outcomes for the study included time to all‐cause death and time to all‐cause, urgent readmission. To be classified as urgent, readmissions could not be arranged when the patient was originally discharged from hospital or more than 4 weeks prior to the readmission. All hospital admissions meeting these criteria during the 6 month study period were labeled in this study as urgent readmissions even if they were unrelated to the index admission.
Principal contacts were called if we were unable to reach the patient to determine their outcomes. If the patient's vital status remained unclear, we contacted the Office of the Provincial Registrar to determine if and when the patient died during the 6 months after discharge from hospital.
Analysis
Outcome incidence densities and 95% confidence intervals [CIs] were calculated using PROC GENMOD in SAS to account for clustering of patients in hospitals. We used multivariate proportional hazards modeling to determine the independent association of provider and information continuity measures with time to death and time to urgent readmission. Patient observation started when patients were discharged from the hospital. Patient observation ended at the earliest of the following: death; urgent readmission to the hospital; end of follow‐up (which was 6 months after discharge from the hospital) or loss to follow‐up. Because hospital consultant continuity was very highly skewed (95.6% of patients had a value of 0; mean value of 0.016; skewness 6.9), it was not included in the primary regression models but was included in a sensitivity analysis.
To adjust for potential confounders in the association between continuity and the outcomes, our model included all factors that were independently associated with either the outcome or any continuity measure. Factors associated with death or urgent readmission were summarized using the LACE index.29 This index combines a patient's hospital length of stay, admission acuity, patient comorbidity (measured with the Charlson Score30 using updated disease category weights by Schneeweiss et al.),31 and emergency room utilization (measured as the number of visits in the 6 months prior to admission) into a single number ranging from 0 to 19. The LACE index was moderately discriminative and highly accurate at predicting 30‐day death or urgent readmission.29 In a separate study,28 we found that the following factors were independently associated with at least one of the continuity measures: patient age; patient sex; number of admissions in previous 6 months; number of regular treating physicians prior to admission; hospital service (medicine vs. surgery); and number of complications in the hospital (defined as new problems arising after admission to hospital). By including all factors that were independently associated with either the outcome or continuity, we controlled for all measured factors that could act as confounders in the association between continuity and outcomes. We accounted for the clustered study design by using conditional proportional hazards models that stratified by hospitals.32 Analytical details are given in the supporting information.
Results
Between October 2002 and July 2006, we enrolled 5035 patients from 11 hospitals (Figure 1). Of the 5035 patients, 274 (5.4%) had no follow up interview with study personnel. A total of 885 (17.6%) had fewer than 2 post discharge physician visits and were not included in the continuity analyses. This left 3876 patients for this analysis (77.0% of the original cohort), of which 3727 had complete follow up (96.1% of the study cohort). A total of 531 patients (10.6% of the original cohort) had incomplete follow‐up because: 342 (6.8%) were lost to follow‐up; 172 (3.4%) refused participation; and 24 (0.5%) were transferred into a nursing home during the first month of observation.


The 3876 study patients are described in Table 1. Overall, these people had a mean age of 62 and most commonly had no physical limitations. Almost a third of patients had been admitted to the hospital in the previous 6 months. A total of 7.6% of patients had no regular prehospital physician while 5.8% had more than one regular prehospital physician. Patients were evenly split between acute and elective admissions and 12% had a complication during their admission. They were discharged after a median of 4 days on a median of 4 medications.
| Factor | Value | Death or Urgent Readmission | All (n = 3876) | |
|---|---|---|---|---|
| No (n = 3491) | Yes (n = 385) | |||
| ||||
| Mean patient age, years (SD) | 61.59 16.16 | 67.70 15.53 | 62.19 16.20 | |
| Female (%) | 1838 (52.6) | 217 (56.4) | 2055 (53.0) | |
| Lives alone (%) | 791 (22.7) | 107 (27.8) | 898 (23.2) | |
| # activities of daily living requiring aids (%) | 0 | 3277 (93.9) | 354 (91.9) | 3631 (93.7) |
| 1 | 125 (3.6) | 20 (5.2) | 145 (3.7) | |
| >1 | 89 (2.5) | 11 (2.8) | 100 (2.8) | |
| # physicians who see patient regularly (%) | 0 | 241 (6.9) | 22 (5.7) | 263 (6.8) |
| 1 | 3060 (87.7) | 333 (86.5) | 3393 (87.5) | |
| 2 | 150 (4.3) | 21 (5.5) | 171 (4.4) | |
| >2 | 281 (8.0) | 31 (8.0) | 312 (8.0) | |
| # admissions in previous 6 months (%) | 0 | 2420 (69.3) | 222 (57.7) | 2642 (68.2) |
| 1 | 833 (23.9) | 103 (26.8) | 936 (24.1) | |
| >1 | 238 (6.8) | 60 (15.6) | 298 (7.7) | |
| Index hospitalization description | ||||
| Number of discharge medications (IQR) | 4 (2‐7) | 6 (3‐9) | 4 (2‐7) | |
| Admitted to medical service (%) | 1440 (41.2) | 231 (60.0) | 1671 (43.1) | |
| Acute diagnoses: | ||||
| CAD (%) | 238 (6.8) | 23 (6.0) | 261 (6.7) | |
| Neoplasm of unspecified nature (%) | 196 (5.6) | 35 (9.1) | 231 (6.0) | |
| Heart failure (%) | 127 (3.6) | 38 (9.9) | 165 (4.3) | |
| Acute procedures | ||||
| CABG (%) | 182 (5.2) | 14 (3.6) | 196 (5.1) | |
| Total knee arthoplasty (%) | 173 (5.0) | 10 (2.6) | 183 (4.7) | |
| Total hip arthroplasty (%) | 118 (3.4) | (0.5) | 120 (3.1) | |
| Complication during admission (%) | 403 (11.5) | 63 (16.4) | 466 (12.0) | |
| LACE index: mean (SD) | 8.0 (3.6) | 10.3 (3.8) | 8.2 (3.7) | |
| Length of stay in days: median (IQR) | 4 (2‐7) | 6 (3‐10) | 4 (2‐8) | |
| Acute/emergent admission (%) | 1851 (53.0) | 272 (70.6) | 2123 (54.8) | |
| Charlson score (%) | 0 | 2771 (79.4) | 241 (62.6) | 3012 (77.7) |
| 1 | 103 (3.0) | 17 (4.4) | 120 (3.1) | |
| 2 | 446 (12.8) | 86 (22.3) | 532 (13.7) | |
| >2 | 171 (4.9) | 41 (10.6) | 212 (5.5) | |
| Emergency room use (# visits/ year) (%) | 0 | 2342 (67.1) | 190 (49.4) | 2532 (65.3) |
| 1 | 761 (21.8) | 101 (26.2) | 862 (22.2) | |
| >1 | 388 (11.1) | 94 (24.4) | 482 (12.4) | |
Patients were observed in the study for a median of 175 days (interquartile range [IQR] 175‐178). During this time they had a median of 4 physician visits (IQR 3‐6). The first postdischarge physician visit occurred a median of 10 days (IQR 6‐18) after discharge from hospital.
Continuity Measures
Table 2 summarizes all continuity scores. Since continuity scores varied significantly over time,28 Table 2 provides continuity scores on the last day of patient observation. Preadmission provider, postdischarge provider, and discharge summary continuity all had similar values and distributions with median values ranging between 0.444 and 0.571. 1797 (46.4%) patients had a hospital physician provider continuity scorae of 0.
| Minimum | 25th Percentile | Median | 75th Percentile | Maximum | |
|---|---|---|---|---|---|
| Provider continuity | |||||
| A: Pre‐admission physician | 0 | 0.143 | 0.444 | 0.667 | 1.000 |
| B: Hospital physician | 0 | 0 | 0.143 | 0.400 | 1.000 |
| C: Post‐discharge physician | 0 | 0.333 | 0.571 | 0.750 | 1.000 |
| Information continuity | |||||
| D: Discharge summary | 0 | 0.095 | 0.500 | 0.800 | 1.000 |
| E: Post‐discharge information | 0 | 0 | 0.182 | 0.500 | 1.000 |
Study Outcomes
During a median of 175 days of observation, 45 patients died (event rate 2.6 events per 100 patient‐years observation [95% CI 2.0‐3.4]) and 340 patients were urgently readmitted (event rate 19.6 events per 100 patient‐years observation [95% CI 15.9‐24.3]). Figure 2 presents the survival curves for time to death and time to urgent readmission. The hazard of death was consistent through the observation period but the risk of urgent readmission decreased slightly after 90 days postdischarge.
Association Between Continuity and Outcomes
Table 3 summarizes the association between provider and information continuity with study outcomes. No continuity measure was associated with time to death by itself (Table 3, column A) or with the other continuity measures (Table 3, column B). Preadmission physician continuity was associated with a significantly decreased risk of urgent readmission. When the proportion of postdischarge visits with a prehospital physician increased by 10%, the adjusted risk of urgent readmission decreased by 6% (adjusted hazards ratio (adj‐HR)) of 0.94 (95% CI, 0.91‐0.98). None of the other continuity measuresincluding hospital physicianwere significantly associated with urgent readmission either by themselves (Table 3, column A) or after adjusting for other continuity measures (Table 3, column B).
| Outcome | ||||||||
|---|---|---|---|---|---|---|---|---|
| Death (95% CI) | Urgent Readmission (95% CI) | |||||||
| A: Adjusted for Other Confounders Only | B: Adjusted for Other Confounders and Continuity Measures | A: Adjusted for Other Confounders Only | B: Adjusted for Other Confounders and Continuity Measures | |||||
| ||||||||
| Provider continuity | ||||||||
| A: Pre‐admission physician | 1.03 | (0.95, 1.12) | 1.06 | (0.95, 1.18) | 0.95 | (0.92, 0.98) | 0.94 | (0.91, 0.98) |
| B: Hospital physician | 0.87 | (0.74, 1.02) | 0.86 | (0.70, 1.03) | 0.98 | (0.94, 1.02) | 0.97 | (0.92, 1.01) |
| C: Post‐discharge physician | 0.97 | (0.89, 1.06) | 0.93 | (0.84, 1.04) | 0.98 | (0.95, 1.01) | 0.98 | (0.94, 1.02) |
| Information continuity | ||||||||
| D: Discharge Summary | 0.96 | (0.89, 1.04) | 0.94 | (0.87, 1.03) | 1.01 | (0.98, 1.04) | 1.02 | (0.99, 1.05) |
| E: Post‐discharge information | 1.01 | (0.94, 1.08) | 1.03 | (0.95, 1.11) | 1.00 | (0.97, 1.03) | 1.03 | (0.95, 1.11) |
| Other confounders | ||||||||
| Patient age in decades* | 1.43 | (1.13, 1.82) | 1.18 | (1.10, 1.28) | ||||
| Female | 1.50 | (0.81, 2.77) | 1.16 | (0.94, 1.44) | ||||
| # physicians who see patient regularly | ||||||||
| 1 | 1.46 | (0.92, 2.34) | ||||||
| 2 | 2.17 | (1.11, 4.26) | ||||||
| >2 | 3.71 | (1.55, 8.88) | ||||||
| Complications during admission | ||||||||
| 1 | 1.38 | (0.61, 3.10) | 0.81 | (0.55, 1.17) | ||||
| >1 | 1.01 | (0.28, 3.58) | 0.91 | (0.56, 1.48) | ||||
| # admissions in previous 6 months | ||||||||
| 1 | 1.27 | (0.59, 2.70) | 1.34 | (1.02, 1.76) | ||||
| >1 | 1.42 | (0.55, 3.67) | 1.78 | (1.26, 2.51) | ||||
| LACE index* | 1.16 | (1.06, 1.26) | 1.10 | (1.07, 1.14) | ||||
Increased patient age and increased LACE index score were both strongly associated with an increased risk of death (adj‐HR 1.43 [1.13‐1.82] and 1.16 [1.06‐1.26], respectively) and urgent readmission (adj‐HR 1.18 [1.10‐1.28] and 1.10 [1.07‐1.14], respectively). Hospitalization in the 6 months prior to admission significantly increased the risk of urgent readmission but not death. The risk of urgent readmission increased significantly as the number of regular prehospital physicians increased.
Sensitivity Analyses
Our study conclusions did not change in the sensitivity analyses. The number of postdischarge physician visits (expressed as a time‐dependent covariate) was not associated with either death or with urgent readmission and preadmission physician continuity remained significantly associated with time to urgent readmission (supporting information). Adding consultant continuity to the model also did not change our results (supporting information). In‐hospital consultant continuity was associated with an increased risk of urgent readmission (adj‐HR 1.10, 95% CI, 1.01‐1.20). The association between pre‐admission physician continuity and time to urgent readmission did not interact significantly with patient age, LACE index score, or number of previous admissions.
Discussion
This large, prospective cohort study measured the independent association of several provider and information continuity measures with important outcomes in patients discharged from hospital. After adjusting for potential confounders, we found that increased continuity with physicians who regularly cared for the patient prior to the admission was significantly and independently associated with a decreased risk of urgent readmission. Our data suggest that continuity with the hospital physician did not independently influence the risk of patient death or urgent readmission after discharge.
Although hospital physician continuity did not significantly change patient outcomes, we found that follow‐up with a physician who regularly treated the patient prior to their admission was associated with a significantly decreased risk of urgent readmission. This could reflect the important role that a patient's regular physician plays in their health care. Other studies have shown a positive association between continuity with a regular physician and improved outcomes including decreased emergency room utilization7, 8 and decreased hospitalization.10, 11
We were somewhat disappointed that information continuity was not independently associated with improved patient outcomes. Information continuity is likely more amenable to modification than is provider continuity. Of course, our study findings do not mean that information continuity does not improve patient outcomes, as in other studies.23, 33 Instead, our results could reflect that we solely measured the availability of information to physicians. Future studies that measure the quality, relevance, and actual utilization of patient information will be better able to discern the influence of information continuity on patient outcomes.
We believe that our study was methodologically strong and unique. We captured both provider and information continuity in a large group of representative patients using a broad range of measures that captured continuity's diverse components including both provider and information continuity. The continuity measures were expressed and properly analyzed as time‐dependent variables in a multivariate model.34 Our analysis controlled for important potential confounders. Our follow‐up and data collection was rigorous with 96.1% of our study group having complete follow‐up. Finally, the analysis used multiple imputation to appropriately handle missing data in the one incomplete variable (post‐discharge information continuity).3537
Several limitations of our study should be kept in mind. We are uncertain how our results might generalize to patients discharged from obstetrical or psychiatric services or people in other health systems. Our analysis had to exclude patients with less than two physician visits after discharge since this was the minimum required to calculate postdischarge physician and information continuity. Data collection for postdischarge information continuity was incomplete with data missing for 19.0% of all 15 401 visits in the original cohort.38 However, a response rate of 81.0% is very good39 when compared to other survey‐based studies40 and we accounted for the missing data using multiple imputation methods. The primary outcomes of our studytime to death or urgent readmissionmay be relatively insensitive to modification of quality of care, which is presumably improved by increased continuity.41 For example, Clarke found that the majority of readmissions in all patient groups were unavoidable with 94% of medical readmissions 1 month postdischarge judged to be unavoidable.42 Future studies regarding the effects of continuity could focus on its association with other outcomes that are more reflective of quality of care such as the risk of adverse events or medical error.21 Such outcomes would presumably be more sensitive to improved quality of care from increased continuity.
We believe that our study's major limitation was its inability to establish a causal association between continuity and patient outcomes. Our finding that increased consultant continuity was associated with an increased risk of poor outcomes highlights this concern. Presumably, patient follow‐up with a hospital consultant indicates a disease status with a high risk of bad patient outcomesa risk that is not entirely accounted for by the covariates used in this study. If we accept that unresolved confounding explains this association, the same could also apply to the association between preadmission physician continuity and improved outcomes. Perhaps patients who are doing well after discharge from hospital are able to return to their regular physician. Our analysis would therefore identify an association between increased preadmission physician continuity and improved patient outcomes. Analyses could also incorporate more discriminative measures of severity of hospital illness, such as those developed by Escobar et al.43 Since patients may experience health events after their discharge from hospital that could influence outcomes, recording these and expressing them in the study model as time‐dependent covariates will be important. Finally, similar to the classic study by Wasson et al.44 in 1984, a proper randomized trial that measures the effect of a continuity‐building intervention on both continuity of care and patient outcomes would help determine how continuity influences outcomes.
In conclusion, after discharge from hospital, increased continuity with physicians who routinely care for the patient is significantly and independently associated with a decreased risk of urgent readmission. Continuity with the hospital physician after discharge did not independently influence the risk of patient death or urgent readmission in our study. Further research is required to determine the causal association between preadmission physician continuity and improved outcomes. Until that time, clinicians should strive to optimize continuity with physicians their patients have seen prior to the hospitalization.
- Society of Hospital Medicine.2009.Ref Type: Internet Communication.
- ,,,.The status of hospital medicine groups in the United States.J Hosp Med.2006;1:75–80.
- ,.The hospitalist movement 5 years later. [see comment].JAMA.2002;287:487–494. [Review]
- ,,,.Hospitalists and the practice of inpatient medicine: results of a survey of the National Association of Inpatient Physicians. [see comment].Ann Intern Med.1999;130:343–349.
- ,,,.Primary care physician attitudes regarding communication with hospitalists.Am J Med.2001;111:15S–20S.
- ,,.Defusing the confusion: concepts and measures of continuity of healthcare.Ottawa,Canadian Health Services Research Foundation. Ref Type: Report.2002;1–50.
- ,,,,.Association between infant continuity of care and pediatric emergency department utilization.Pediatrics.2004;113:738–741.
- ,,,,.Is greater continuity of care associated with less emergency department utilization?Pediatrics.1999;103:738–742.
- ,,,,.Association of lower continuity of care with greater risk of emergency department use and hospitalization in children.Pediatrics.2001;107:524–529.
- ,,The role of provider continuity in preventing hospitalizations.Arch Fam Med.1998;7:352–357.
- ,.The importance of continuity of care in the likelihood of future hospitalization: is site of care equivalent to a primary clinician?Am J Public Health.1998;88:1539–1541.
- ,,,.Exploration of the relationship between continuity, trust in regular doctors and patient satisfaction with consultations with family doctors.Scand J Prim Health Care.2003;21:27–32.
- ,,,,.Longitudinal continuity of care is associated with high patient satisfaction with physical therapy.Phys Ther.2005;85:1046–1052.
- ,,,,.Provider continuity and outcomes of care for persons with schizophrenia.Ment Health Serv Res.2000;V2:201–211.
- ,,,,.Continuity of care is associated with well‐coordinated care.Ambul Pediatr.2003;3:82–86.
- ,,.The impact of insurance type and forced discontinuity on the delivery of primary care. [see comments.].J Fam Pract.1997;45:129–135.
- .Measuring attributes of primary care: development of a new instrument.J Fam Pract.1997;45:64–74.
- .Continuity of care during pregnancy: the effect of provider continuity on outcome.J Fam Pract.1985;21:375–380.
- ,,,,,.Physician‐patient relationship and medication compliance: a primary care investigation.Ann Fam Med.2004;2:455–461.
- ,,,.Continuity of care and cardiovascular risk factor management: does care by a single clinician add to informational continuity provided by electronic medical records?Am J Manag Care.2005;11:689–696.
- ,,,,.The incidence and severity of adverse events affecting patients after discharge from the hospital.Ann Intern Med.2003;138:161–167.
- ,,,.Continuity of care and patient outcomes after hospital discharge.J Gen lntern Med.2004;19:624–645.
- ,,,.Effect of discharge summary availability during post‐discharge visits on hospital readmission.J Gen Intern Med.2002;17:186–192.
- ,,, et al.Association of communication between hospital‐based physicians and primary care providers with patient outcomes.[see comment].J Gen Intern Med2009;24(3):381–386.
- ,,,,,.Deficits in communication and information transfer between hospital‐based and primary care physicians: implications for patient safety and continuity of care.JAMA.2007;297:831–841.
- ,,, et al.Information exchange among physicians caring for the same patient in the community.Can Med Assoc J.2008;179:1013–1018.
- ,.Continuity of care in a university‐based practice.J Med Educ.1975;965–969.
- ,,, et al.Provider and information continuity after discharge from hospital: a prospective cohort study.2009. Ref Type: Unpublished Work.
- ,,, et al.Derivation and validation of the LACE index to predict early death or unplanned readmission after discharge from hospital to the community.CMAJ. (In press)
- ,,,.A new method of classifying prognostic comorbidity in longitudinal studies: development and validation.J Chronic Dis.1987;40:373–383.
- ,,,.Improved comorbidity adjustment for predicting mortality in Medicare populations.Health Serv Res.2003;38(4):1103–1120.
- ,.Modelling clustered survival data from multicentre clinical trials.Stat Med.2004;23:369–388.
- ,,,.Prevalence of information gaps in the emergency department and the effect on patient outcomes.CMAJ.2003;169:1023–1028.
- ,,,.Time‐dependent bias due to improper analytical methodology is common in prominent medical journals.J Clin Epidemiol.2004;57:672–682.
- .What do we do with missing data? Some options for analysis of incomplete data.Annu Rev Public Health.2004;25:99–117.
- ,,,.Survival estimates of a prognostic classification depended more on year of treatment than on imputation of missing values.J Clin Epidemiol.2006;59:246–253. [Review]
- .Bias arising from missing data in predictive models.[see comment].J Clin Epidemiol.2006;59:1115–1123.
- ,,, et al.Information exchange among physicians caring for the same patient in the community.CMAJ.2008;179:1013–1018.
- .Survey Research Methods.2nd ed.,Beverly Hills:Sage;1993.
- ,,.Response rates to mail surveys published in medical journals.J Clin Epidemiol.1997;50:1129–1136.
- .Readmission of patients to hospital: still ill defined and poorly understood.Int J Qual Health Care.2001;13:177–179.
- .Are readmissions avoidable?Br Med J.1990;301:1136–1138.
- ,,,,,.Risk‐adjusting hospital inpatient mortality using automated inpatient, outpatient, and laboratory databases.Med Care.2008;46:232–239.
- ,,, et al.Continuity of outpatient medical care in elderly men. A randomized trial.JAMA.1984;252:2413–2417.
- Society of Hospital Medicine.2009.Ref Type: Internet Communication.
- ,,,.The status of hospital medicine groups in the United States.J Hosp Med.2006;1:75–80.
- ,.The hospitalist movement 5 years later. [see comment].JAMA.2002;287:487–494. [Review]
- ,,,.Hospitalists and the practice of inpatient medicine: results of a survey of the National Association of Inpatient Physicians. [see comment].Ann Intern Med.1999;130:343–349.
- ,,,.Primary care physician attitudes regarding communication with hospitalists.Am J Med.2001;111:15S–20S.
- ,,.Defusing the confusion: concepts and measures of continuity of healthcare.Ottawa,Canadian Health Services Research Foundation. Ref Type: Report.2002;1–50.
- ,,,,.Association between infant continuity of care and pediatric emergency department utilization.Pediatrics.2004;113:738–741.
- ,,,,.Is greater continuity of care associated with less emergency department utilization?Pediatrics.1999;103:738–742.
- ,,,,.Association of lower continuity of care with greater risk of emergency department use and hospitalization in children.Pediatrics.2001;107:524–529.
- ,,The role of provider continuity in preventing hospitalizations.Arch Fam Med.1998;7:352–357.
- ,.The importance of continuity of care in the likelihood of future hospitalization: is site of care equivalent to a primary clinician?Am J Public Health.1998;88:1539–1541.
- ,,,.Exploration of the relationship between continuity, trust in regular doctors and patient satisfaction with consultations with family doctors.Scand J Prim Health Care.2003;21:27–32.
- ,,,,.Longitudinal continuity of care is associated with high patient satisfaction with physical therapy.Phys Ther.2005;85:1046–1052.
- ,,,,.Provider continuity and outcomes of care for persons with schizophrenia.Ment Health Serv Res.2000;V2:201–211.
- ,,,,.Continuity of care is associated with well‐coordinated care.Ambul Pediatr.2003;3:82–86.
- ,,.The impact of insurance type and forced discontinuity on the delivery of primary care. [see comments.].J Fam Pract.1997;45:129–135.
- .Measuring attributes of primary care: development of a new instrument.J Fam Pract.1997;45:64–74.
- .Continuity of care during pregnancy: the effect of provider continuity on outcome.J Fam Pract.1985;21:375–380.
- ,,,,,.Physician‐patient relationship and medication compliance: a primary care investigation.Ann Fam Med.2004;2:455–461.
- ,,,.Continuity of care and cardiovascular risk factor management: does care by a single clinician add to informational continuity provided by electronic medical records?Am J Manag Care.2005;11:689–696.
- ,,,,.The incidence and severity of adverse events affecting patients after discharge from the hospital.Ann Intern Med.2003;138:161–167.
- ,,,.Continuity of care and patient outcomes after hospital discharge.J Gen lntern Med.2004;19:624–645.
- ,,,.Effect of discharge summary availability during post‐discharge visits on hospital readmission.J Gen Intern Med.2002;17:186–192.
- ,,, et al.Association of communication between hospital‐based physicians and primary care providers with patient outcomes.[see comment].J Gen Intern Med2009;24(3):381–386.
- ,,,,,.Deficits in communication and information transfer between hospital‐based and primary care physicians: implications for patient safety and continuity of care.JAMA.2007;297:831–841.
- ,,, et al.Information exchange among physicians caring for the same patient in the community.Can Med Assoc J.2008;179:1013–1018.
- ,.Continuity of care in a university‐based practice.J Med Educ.1975;965–969.
- ,,, et al.Provider and information continuity after discharge from hospital: a prospective cohort study.2009. Ref Type: Unpublished Work.
- ,,, et al.Derivation and validation of the LACE index to predict early death or unplanned readmission after discharge from hospital to the community.CMAJ. (In press)
- ,,,.A new method of classifying prognostic comorbidity in longitudinal studies: development and validation.J Chronic Dis.1987;40:373–383.
- ,,,.Improved comorbidity adjustment for predicting mortality in Medicare populations.Health Serv Res.2003;38(4):1103–1120.
- ,.Modelling clustered survival data from multicentre clinical trials.Stat Med.2004;23:369–388.
- ,,,.Prevalence of information gaps in the emergency department and the effect on patient outcomes.CMAJ.2003;169:1023–1028.
- ,,,.Time‐dependent bias due to improper analytical methodology is common in prominent medical journals.J Clin Epidemiol.2004;57:672–682.
- .What do we do with missing data? Some options for analysis of incomplete data.Annu Rev Public Health.2004;25:99–117.
- ,,,.Survival estimates of a prognostic classification depended more on year of treatment than on imputation of missing values.J Clin Epidemiol.2006;59:246–253. [Review]
- .Bias arising from missing data in predictive models.[see comment].J Clin Epidemiol.2006;59:1115–1123.
- ,,, et al.Information exchange among physicians caring for the same patient in the community.CMAJ.2008;179:1013–1018.
- .Survey Research Methods.2nd ed.,Beverly Hills:Sage;1993.
- ,,.Response rates to mail surveys published in medical journals.J Clin Epidemiol.1997;50:1129–1136.
- .Readmission of patients to hospital: still ill defined and poorly understood.Int J Qual Health Care.2001;13:177–179.
- .Are readmissions avoidable?Br Med J.1990;301:1136–1138.
- ,,,,,.Risk‐adjusting hospital inpatient mortality using automated inpatient, outpatient, and laboratory databases.Med Care.2008;46:232–239.
- ,,, et al.Continuity of outpatient medical care in elderly men. A randomized trial.JAMA.1984;252:2413–2417.
Copyright © 2010 Society of Hospital Medicine
Know Your Numbers, Your Market, Yourself
A self-described “numbers” guy, Troy Ahlstrom, MD, FHM, is always glad to get his hands on new data. As the CFO of Traverse City-based Hospitalists of Northern Michigan, he is a seasoned veteran of contract negotiations with new recruits or hospital administrators.
Dr. Ahlstrom encourages HM group leaders to understand their local markets, their competitors, and their hospital culture. Use that information, along with benchmarks from national surveys, to formulate expectations for your providers, he says.
“Oftentimes you are measured against the guy next door,” Dr. Ahlstrom says. “You have to know the numbers, because [administrators] are going to know the numbers.”
That’s good to know when new data are dropped on your desk. On Friday, HM group leaders will have access to the State of Hospital Medicine: 2010 Report Based on 2009 Data. The new report shows national median compensation is $215,000 for adult hospitalists; median compensation was $183,900 per adult hospitalist, according to SHM’s 2007-2008 report.
The national median for work RVUs per hospitalist FTE is 4,107, according to the new data. The national median for wRVUs per encounter is 1.86, and collections per work RVU is $45.57. (Visit the-hospitalist.org for more about the 2010 report and benchmarking your practice.)
The report, which offers new metrics, new layers of detail, and new tools to help group leaders analyze the data, compiled data from 4,211 hospitalists in 443 groups, a 30% increase in respondents over SHM’s 2007-2008 report. Dr. Ahlstrom, a member of SHM’s Practice Analysis committee, offers these tips for incorporating benchmarking data into your practice:
- Know your local market. “If you keep in mind your local needs, then you can look at the data and start to evaluate what parts are going to help you better formulate a practice that brings on the right people, does the right work, and continues to produce the amount of workload and compensation that makes sure they are happy in the future.”
- Evaluate how applicable the data is. “Pay attention to the total number of survey respondents in each category, and the standard deviation around the mean. … Find data sets that are most applicable to your practice.”
- Don’t focus on isolated data. “It’s important to look at trends in the data over time, and pick out where those trends are going to go.”
- Involve your people. “The more we are involved in understanding the trends in HM, the better we are going to plan where we are going in the future.”
A self-described “numbers” guy, Troy Ahlstrom, MD, FHM, is always glad to get his hands on new data. As the CFO of Traverse City-based Hospitalists of Northern Michigan, he is a seasoned veteran of contract negotiations with new recruits or hospital administrators.
Dr. Ahlstrom encourages HM group leaders to understand their local markets, their competitors, and their hospital culture. Use that information, along with benchmarks from national surveys, to formulate expectations for your providers, he says.
“Oftentimes you are measured against the guy next door,” Dr. Ahlstrom says. “You have to know the numbers, because [administrators] are going to know the numbers.”
That’s good to know when new data are dropped on your desk. On Friday, HM group leaders will have access to the State of Hospital Medicine: 2010 Report Based on 2009 Data. The new report shows national median compensation is $215,000 for adult hospitalists; median compensation was $183,900 per adult hospitalist, according to SHM’s 2007-2008 report.
The national median for work RVUs per hospitalist FTE is 4,107, according to the new data. The national median for wRVUs per encounter is 1.86, and collections per work RVU is $45.57. (Visit the-hospitalist.org for more about the 2010 report and benchmarking your practice.)
The report, which offers new metrics, new layers of detail, and new tools to help group leaders analyze the data, compiled data from 4,211 hospitalists in 443 groups, a 30% increase in respondents over SHM’s 2007-2008 report. Dr. Ahlstrom, a member of SHM’s Practice Analysis committee, offers these tips for incorporating benchmarking data into your practice:
- Know your local market. “If you keep in mind your local needs, then you can look at the data and start to evaluate what parts are going to help you better formulate a practice that brings on the right people, does the right work, and continues to produce the amount of workload and compensation that makes sure they are happy in the future.”
- Evaluate how applicable the data is. “Pay attention to the total number of survey respondents in each category, and the standard deviation around the mean. … Find data sets that are most applicable to your practice.”
- Don’t focus on isolated data. “It’s important to look at trends in the data over time, and pick out where those trends are going to go.”
- Involve your people. “The more we are involved in understanding the trends in HM, the better we are going to plan where we are going in the future.”
A self-described “numbers” guy, Troy Ahlstrom, MD, FHM, is always glad to get his hands on new data. As the CFO of Traverse City-based Hospitalists of Northern Michigan, he is a seasoned veteran of contract negotiations with new recruits or hospital administrators.
Dr. Ahlstrom encourages HM group leaders to understand their local markets, their competitors, and their hospital culture. Use that information, along with benchmarks from national surveys, to formulate expectations for your providers, he says.
“Oftentimes you are measured against the guy next door,” Dr. Ahlstrom says. “You have to know the numbers, because [administrators] are going to know the numbers.”
That’s good to know when new data are dropped on your desk. On Friday, HM group leaders will have access to the State of Hospital Medicine: 2010 Report Based on 2009 Data. The new report shows national median compensation is $215,000 for adult hospitalists; median compensation was $183,900 per adult hospitalist, according to SHM’s 2007-2008 report.
The national median for work RVUs per hospitalist FTE is 4,107, according to the new data. The national median for wRVUs per encounter is 1.86, and collections per work RVU is $45.57. (Visit the-hospitalist.org for more about the 2010 report and benchmarking your practice.)
The report, which offers new metrics, new layers of detail, and new tools to help group leaders analyze the data, compiled data from 4,211 hospitalists in 443 groups, a 30% increase in respondents over SHM’s 2007-2008 report. Dr. Ahlstrom, a member of SHM’s Practice Analysis committee, offers these tips for incorporating benchmarking data into your practice:
- Know your local market. “If you keep in mind your local needs, then you can look at the data and start to evaluate what parts are going to help you better formulate a practice that brings on the right people, does the right work, and continues to produce the amount of workload and compensation that makes sure they are happy in the future.”
- Evaluate how applicable the data is. “Pay attention to the total number of survey respondents in each category, and the standard deviation around the mean. … Find data sets that are most applicable to your practice.”
- Don’t focus on isolated data. “It’s important to look at trends in the data over time, and pick out where those trends are going to go.”
- Involve your people. “The more we are involved in understanding the trends in HM, the better we are going to plan where we are going in the future.”
Hospitalist Searches for Missing Link
Contemporary management of infection in acute inflammatory diseases is focused on the infectious agent—and it might be missing something, says hospitalist Kirsten Kangelaris, MD, MAS, an assistant clinical professor at the University of California at San Francisco.
Since receiving one of SHM’s first Junior Faculty Development Awards in April, Dr. Kangelaris has been researching the missing link: the genetic and biological risk factors in non-critically-ill patients with acute lung injury. So far, the $50,000 grant has helped her to uncover a chemokine receptor gene variant that appears almost exclusively in African-Americans. She hopes to use this information to improve risk-prediction algorithms, treatments, and prevention strategies.
Dr. Kangelaris spoke with the TH eWire about her new role as a hospitalist-researcher.
Question: How did you get involved in researching clinical and biological genetic risk-prediction algorithms?
Answer: In my clinical work … I was struck by how two similarly appearing patients, admitted with complications of infections like sepsis and pneumonia, could have very different outcomes in spite of excellent care in the hospital. I was learning firsthand from my patients that we still have a lot to learn about how individual host response to infection affects outcomes.
Q: What kind of training did you receive that prepared you for your research?
A: I did a two-year masters in clinical research at UCSF, which gave me skills in epidemiology and biostatistics. I had advanced training in multivariable analysis and advanced training in clinical epidemiology and epidemiological methods. I also had training in health disparities.
Q: What do you recommend for hospitalists who are interested in research?
A: A research fellowship gave me the tools and the time to embark on a research career in translational hospital medicine. I think it is difficult to begin a traditional research career without this kind of training. The field of hospital medicine has so much potential to improve human health; it is a fertile ground for research interests ranging from translational work to quality improvement and patient safety.
Contemporary management of infection in acute inflammatory diseases is focused on the infectious agent—and it might be missing something, says hospitalist Kirsten Kangelaris, MD, MAS, an assistant clinical professor at the University of California at San Francisco.
Since receiving one of SHM’s first Junior Faculty Development Awards in April, Dr. Kangelaris has been researching the missing link: the genetic and biological risk factors in non-critically-ill patients with acute lung injury. So far, the $50,000 grant has helped her to uncover a chemokine receptor gene variant that appears almost exclusively in African-Americans. She hopes to use this information to improve risk-prediction algorithms, treatments, and prevention strategies.
Dr. Kangelaris spoke with the TH eWire about her new role as a hospitalist-researcher.
Question: How did you get involved in researching clinical and biological genetic risk-prediction algorithms?
Answer: In my clinical work … I was struck by how two similarly appearing patients, admitted with complications of infections like sepsis and pneumonia, could have very different outcomes in spite of excellent care in the hospital. I was learning firsthand from my patients that we still have a lot to learn about how individual host response to infection affects outcomes.
Q: What kind of training did you receive that prepared you for your research?
A: I did a two-year masters in clinical research at UCSF, which gave me skills in epidemiology and biostatistics. I had advanced training in multivariable analysis and advanced training in clinical epidemiology and epidemiological methods. I also had training in health disparities.
Q: What do you recommend for hospitalists who are interested in research?
A: A research fellowship gave me the tools and the time to embark on a research career in translational hospital medicine. I think it is difficult to begin a traditional research career without this kind of training. The field of hospital medicine has so much potential to improve human health; it is a fertile ground for research interests ranging from translational work to quality improvement and patient safety.
Contemporary management of infection in acute inflammatory diseases is focused on the infectious agent—and it might be missing something, says hospitalist Kirsten Kangelaris, MD, MAS, an assistant clinical professor at the University of California at San Francisco.
Since receiving one of SHM’s first Junior Faculty Development Awards in April, Dr. Kangelaris has been researching the missing link: the genetic and biological risk factors in non-critically-ill patients with acute lung injury. So far, the $50,000 grant has helped her to uncover a chemokine receptor gene variant that appears almost exclusively in African-Americans. She hopes to use this information to improve risk-prediction algorithms, treatments, and prevention strategies.
Dr. Kangelaris spoke with the TH eWire about her new role as a hospitalist-researcher.
Question: How did you get involved in researching clinical and biological genetic risk-prediction algorithms?
Answer: In my clinical work … I was struck by how two similarly appearing patients, admitted with complications of infections like sepsis and pneumonia, could have very different outcomes in spite of excellent care in the hospital. I was learning firsthand from my patients that we still have a lot to learn about how individual host response to infection affects outcomes.
Q: What kind of training did you receive that prepared you for your research?
A: I did a two-year masters in clinical research at UCSF, which gave me skills in epidemiology and biostatistics. I had advanced training in multivariable analysis and advanced training in clinical epidemiology and epidemiological methods. I also had training in health disparities.
Q: What do you recommend for hospitalists who are interested in research?
A: A research fellowship gave me the tools and the time to embark on a research career in translational hospital medicine. I think it is difficult to begin a traditional research career without this kind of training. The field of hospital medicine has so much potential to improve human health; it is a fertile ground for research interests ranging from translational work to quality improvement and patient safety.
Analyze This
Editors note: This article features interactive region-by-region breakdowns and Team Hospitalist analysis of the latest compensation and productivity data from SHM and MGMA. Click here to open the interactive feature.
Every January, William “Tex” Landis, MD, FHM, sits in a conference room with key members of his hospital’s administration and presents what he affectionately refers to as the “state of the union” for his hospitalist group. The bar graphs, pie charts, and commentary have changed little in the past decade, Dr. Landis admits, but the information and analysis he has available to him as he begins crafting his 2011 presentation is better than ever.
Dr. Landis, medical director of Wellspan Hospitalists in York, Pa., and hospitalist group leaders across the country will have access to the State of Hospital Medicine: 2010 Report Based on 2009 Data this budget cycle. The new report, which will be available Sept. 10, offers new compensation and productivity information, new layers of detail, and new tools to help group leaders analyze the data.
“This data reflects the best numbers we have in our business,” says Dr. Landis, the chair of SHM’s Practice Analysis Committee. “We have better participation and better quality data analysis than we have ever had before. It’s a more standardized approach, and we are just going to be able to continue to build upon this. It sets the standard for moving forward, as far as I am concerned.”
The new report, which replaces SHM’s biannual survey, is the result of a partnership between SHM and the Medical Group Management Association (MGMA), an industry leader in practice-management resources. The report compiled data about 4,211 hospitalists in 443 groups, a 30% increase in survey respondents over SHM’s 2007-2008 report.
“The collaboration is really driven at providing a single set of benchmarks to the HM community,” says David Litzau, systems analyst at MGMA. “It provides a viewpoint of what’s happening elsewhere in the industry.”
What’s happening is that hospitalists continue to see increases in compensation. The new report, which uses some different data definitions and survey methodologies, and is based on a new population, shows that median compensation for adult hospitalists is $215,000 per year, a number that doesn’t take into account benefits. Hospitalist median compensation was $183,900, according to SHM’s 2007-2008 survey, and $171,000 in SHM’s 2005-2006 survey. MGMA’s 2009 report on physician compensation showed median compensation at $210,250 per internal medicine hospitalist.
And while the compensation numbers are higher than in previous surveys, the new report also shows adult hospitalists are increasing productivity, are seeing more patients per year (reversing a somewhat declining trend), and are collecting more per encounter.
The Numbers
Although compensation is the most popular survey metric, it’s not the only number worth investigating. A handful of key productivity measures seem to be on the rise, too, according to the new report.
The national median (the midpoint of all survey respondents) for work RVUs per adult hospitalist FTE is 4,107, according to the new data. SHM’s 2007-2008 survey reported wRVUs at 3,715 per adult hospitalist.
The national median for hospitalist wRVUs per encounter is 1.86. That same figure was reported at 1.53 wRVUs per encounter in 2008 and 1.37 in 2006.
Collections per wRVU is $45.57, according to the 2010 report. The 2008 survey showed collections at $44.97 per wRVU; the 2006 survey did not report the metric.
One thing the new metrics have in common is that they show hospitalists across the nation are becoming more efficient. “The numbers essentially reaffirm the overall trends for hospital medicine, in that the productivity continues to increase and the compensation paid to a provider continues to increase,” says Troy Ahlstrom, MD, FHM, CFO of Traverse City-based Hospitalists of Northern Michigan, which has nearly 50 hospitalists supporting three hospitals. “When you dig into the numbers, hospitalists are producing more work and more RVUs per encounter than they had been in the past.”
Financial support per hospitalist FTE, another key practice-management metric, parallels the compensation growth. Practices receive a median of $98,253 of support per hospitalist FTE, according to 2010 data. The 2008 report did not provide a median figure for support; instead, it published a mean figure of $97,375 of support per FTE. The 2010 mean (average) is $111,486.
Pediatric HM also shows signs of growth; median compensation is $160,038 in the new report. The 2008 report had pediatric hospitalist median compensation at $144,600.
The new data show a spike in HM groups providing “on site” care of patients 24 hours a day, seven days a week. More than 68% have on-site care with a physician, nurse practitioner, or physician assistant. Only 53% of groups had 24/7 coverage in the 2008 report; 51% had round-the-clock coverage in the 2006 report.
Dr. Ahlstrom, a veteran member of SHM’s Practice Analysis Committee, says he expects that trend to continue, especially with the large numbers of young hospitalists in the field interested in set schedules and work-life balance. “That’s the trend,” he says. “Younger physicians are more interested in seeing that split, where the days and nights are clearly set off. Older physicians are more than happy to have a nocturnist around, just as long as it’s not going to cost them a lot of money or productivity.”
A Word of Caution, and Unintended Benefits
The new report is based on a supplemental set of questions specifically directed at hospitalist practices in MGMA’s annual Physician Compensation and Productivity Survey. The survey is voluntary and is not audited, but it is the “best data” available for hospitalists, according to practice-management experts.
“So many people assume this data is what you should do,” says John Nelson, MD, MHM, co-founder and past president of SHM and a principal in hospitalist-consulting firm Nelson Flores Hospital Medicine Consultants. “It’s not. It is a survey of what’s happening. It’s a starting point, a frame of reference. It is the best data there is, no doubt. But you should not build your practice by trying to match the medians. You might have local data that deviates. You might be starting a program or be in a competitive situation.”
The same experts warn that the new survey population and methodologies will make it difficult to draw direct comparisons to data from previous surveys. For example, the 2007-2008 SHM survey included roughly a quarter of respondents from academic settings; the 2010 report has barely 1% of its respondents from academic settings (see Figure 1, p. 14). Traditionally, compensation and productivity levels for academic hospitalists are lower than nonacademic hospitalists. Most experts agree the “filtering” effect of the survey population factors heavily into the across- the-board increases in compensation and productivity in the 2010 report.
“The survey instrument that we use has been used historically for nonacademic physicians,” Litzau explains. “We also have an academic survey that is performed in the fall [Sept. 13 through Nov. 5], where we collect data specifically for academic faculty. We see very different trends within those two types of practice. It is difficult to draw clear comparisons between the two.”
Dr. Landis refers to the new report as a “baseline” and advises hospitalist leaders to review the caveats and cautions section (see “Survey Stipulation: Only Fools Rush In,” p. 16) before jumping right to the numbers. “This is a new set of numbers. Probably the more important comparison will be this set of numbers compared with the next set of data, next year,” he adds.
Even so, the “filtering” effect should provide nonacademic hospitalist groups a more accurate picture of compensation and productivity trends. One hospitalist leader says it’s a “win-win” for both academic and nonacademic practice leaders.
“As a community-based hospitalist, I always had to drill into those organizations that were similar to me. Being able to have more filtered information, it allows us to drill into the areas that are more important and then present that information to our CEO, CFO, VPMA,” says William D. Atchley Jr., MD, FACP, SFHM, chief of hospital medicine at Sentara Medical Group in Norfolk, Va., and a member of Team Hospitalist.
New Info, Deeper Analysis
In addition to a larger response rate and more filtered approach, the new report will offer greater frequency (annually), new data points, and in-depth breakdowns of key productivity metrics. Some of the new metrics reported include:
- Staff per FTE hospitalist physician;
- Staff turnover;
- Retirement benefits;
- Compensation to collections ratio;
- Compensation per encounter;
- Compensation per wRVU;
- Collections per encounter;
- Collections per wRVU; and
- Work RVUs per encounter.
The report will be available every fall, as compared to biannually for past SHM surveys. It also will offer more “cuts” of the data, including median, mean, 25th percentile, 75th percentile, and 90th percentile reports, along with regional breakdowns for many compensation and productivity metrics.
Practical Applications

—William “Tex” Landis, MD, FHM, medical director, Wellspan Hospitalists, York, Pa., SHM Practice Analysis Committee chair
Benchmarking data are used to set productivity goals and compensation levels in hospitalist practices throughout the country, and most administrators use multiple sources of data to make those decisions.
“If we are showing our hospitalists are generating 5,000 wRVUs per year, and the national median is 4,100, you can do the math. I can say, ‘We need to bring on another hospitalist. The timing is right, and we need to be recruiting,’ ” says Dr. Atchley, who has worked with benchmarking data for 15 years and currently supervises 45 full-time hospitalists who service five hospitals in southeast Virginia. “It’s always good to have national benchmarks to compare to, because that is always the question that is going to be asked. [Hospital administrators] want regional and national comparisons.”
Regional information and well-adapted data from national surveys guide James Gardner, MD, chief medical executive for Pro Health Care Inc., a two-hospital system just west of Milwaukee, when he’s hiring new hospitalists at 300-bed Waukesha Memorial Hospital or launching a new HM program at the system’s smaller, rural facility. In fact, Dr. Gardner currently is weighing options to expand the HM service at 80-bed Oconomowoc Hospital, less than a year after the program started.
“We like to look at a number of sources of data. The MGMA and SHM survey data, historically, have been two of our preferred sources,” Dr. Gardner says. “I think we tend to look at more regional data from the Midwest because the national data varies so much.
“We try to get a sense as to what our local market is.”
Dr. Gardner says he’d like to see a “couple years” to confirm the validity of the new SHM-MGMA report. That said, he says he knows how useful the data can be in regard to benchmarking hospitalist productivity.
“It’s been very helpful; it helps us know where we are at,” Dr. Gardner explains. “It’s one of the guideposts to decide when we are approaching the need for additional resources, whether that is midlevel providers or full-time hospitalists.”
Advice From a Numbers Guy
A self-described “numbers” guy, Dr. Ahlstrom agrees regional data is just as important as, if not more important than, the national numbers. He stresses knowing your market, your competitors, your hospital culture—and using that information along with the benchmarking data to formulate expectations for your group.
“Oftentimes you are measured against the guy next door,” Dr. Ahlstrom says. “You have to know the numbers, because [administrators] are going to know the numbers.”
Dr. Ahlstrom offers these tips for incorporating benchmarking data into your practice:
- Know your local market. “If you keep in mind your local needs, then you can look at the data and start to evaluate what parts are going to help you better formulate a practice that brings on the right people, does the right work, and continues to produce the amount of workload and compensation that makes sure they are happy in the future,” he says.
- Evaluate how applicable the data is. Pay attention to the total number of survey respondents in each category, and the standard deviation around the mean. “In other words, what is the central tendency of the data? You might find data in subsections that you find interesting, but it might not be data that has a central tendency,” he says. “Find data sets that are most applicable to your practice while assessing variations from the larger data sets. Consider how and why your practice might vary from the report as part of your evaluation.”
- Pick out trends and look at them in total. The key is to avoid looking at data points in isolation. “It’s important to look at trends in the data over time, and pick out where those trends are going to go,” he says.
- Involve your people. “I think that this data being available from the [provider] side and management side is a good thing,” Dr. Ahlstrom explains. “The more we are involved in understanding the trends in HM, the better we are going to plan where we are going in the future.” TH
Jason Carris is editor of The Hospitalist.
Editors note: This article features interactive region-by-region breakdowns and Team Hospitalist analysis of the latest compensation and productivity data from SHM and MGMA. Click here to open the interactive feature.
Every January, William “Tex” Landis, MD, FHM, sits in a conference room with key members of his hospital’s administration and presents what he affectionately refers to as the “state of the union” for his hospitalist group. The bar graphs, pie charts, and commentary have changed little in the past decade, Dr. Landis admits, but the information and analysis he has available to him as he begins crafting his 2011 presentation is better than ever.
Dr. Landis, medical director of Wellspan Hospitalists in York, Pa., and hospitalist group leaders across the country will have access to the State of Hospital Medicine: 2010 Report Based on 2009 Data this budget cycle. The new report, which will be available Sept. 10, offers new compensation and productivity information, new layers of detail, and new tools to help group leaders analyze the data.
“This data reflects the best numbers we have in our business,” says Dr. Landis, the chair of SHM’s Practice Analysis Committee. “We have better participation and better quality data analysis than we have ever had before. It’s a more standardized approach, and we are just going to be able to continue to build upon this. It sets the standard for moving forward, as far as I am concerned.”
The new report, which replaces SHM’s biannual survey, is the result of a partnership between SHM and the Medical Group Management Association (MGMA), an industry leader in practice-management resources. The report compiled data about 4,211 hospitalists in 443 groups, a 30% increase in survey respondents over SHM’s 2007-2008 report.
“The collaboration is really driven at providing a single set of benchmarks to the HM community,” says David Litzau, systems analyst at MGMA. “It provides a viewpoint of what’s happening elsewhere in the industry.”
What’s happening is that hospitalists continue to see increases in compensation. The new report, which uses some different data definitions and survey methodologies, and is based on a new population, shows that median compensation for adult hospitalists is $215,000 per year, a number that doesn’t take into account benefits. Hospitalist median compensation was $183,900, according to SHM’s 2007-2008 survey, and $171,000 in SHM’s 2005-2006 survey. MGMA’s 2009 report on physician compensation showed median compensation at $210,250 per internal medicine hospitalist.
And while the compensation numbers are higher than in previous surveys, the new report also shows adult hospitalists are increasing productivity, are seeing more patients per year (reversing a somewhat declining trend), and are collecting more per encounter.
The Numbers
Although compensation is the most popular survey metric, it’s not the only number worth investigating. A handful of key productivity measures seem to be on the rise, too, according to the new report.
The national median (the midpoint of all survey respondents) for work RVUs per adult hospitalist FTE is 4,107, according to the new data. SHM’s 2007-2008 survey reported wRVUs at 3,715 per adult hospitalist.
The national median for hospitalist wRVUs per encounter is 1.86. That same figure was reported at 1.53 wRVUs per encounter in 2008 and 1.37 in 2006.
Collections per wRVU is $45.57, according to the 2010 report. The 2008 survey showed collections at $44.97 per wRVU; the 2006 survey did not report the metric.
One thing the new metrics have in common is that they show hospitalists across the nation are becoming more efficient. “The numbers essentially reaffirm the overall trends for hospital medicine, in that the productivity continues to increase and the compensation paid to a provider continues to increase,” says Troy Ahlstrom, MD, FHM, CFO of Traverse City-based Hospitalists of Northern Michigan, which has nearly 50 hospitalists supporting three hospitals. “When you dig into the numbers, hospitalists are producing more work and more RVUs per encounter than they had been in the past.”
Financial support per hospitalist FTE, another key practice-management metric, parallels the compensation growth. Practices receive a median of $98,253 of support per hospitalist FTE, according to 2010 data. The 2008 report did not provide a median figure for support; instead, it published a mean figure of $97,375 of support per FTE. The 2010 mean (average) is $111,486.
Pediatric HM also shows signs of growth; median compensation is $160,038 in the new report. The 2008 report had pediatric hospitalist median compensation at $144,600.
The new data show a spike in HM groups providing “on site” care of patients 24 hours a day, seven days a week. More than 68% have on-site care with a physician, nurse practitioner, or physician assistant. Only 53% of groups had 24/7 coverage in the 2008 report; 51% had round-the-clock coverage in the 2006 report.
Dr. Ahlstrom, a veteran member of SHM’s Practice Analysis Committee, says he expects that trend to continue, especially with the large numbers of young hospitalists in the field interested in set schedules and work-life balance. “That’s the trend,” he says. “Younger physicians are more interested in seeing that split, where the days and nights are clearly set off. Older physicians are more than happy to have a nocturnist around, just as long as it’s not going to cost them a lot of money or productivity.”
A Word of Caution, and Unintended Benefits
The new report is based on a supplemental set of questions specifically directed at hospitalist practices in MGMA’s annual Physician Compensation and Productivity Survey. The survey is voluntary and is not audited, but it is the “best data” available for hospitalists, according to practice-management experts.
“So many people assume this data is what you should do,” says John Nelson, MD, MHM, co-founder and past president of SHM and a principal in hospitalist-consulting firm Nelson Flores Hospital Medicine Consultants. “It’s not. It is a survey of what’s happening. It’s a starting point, a frame of reference. It is the best data there is, no doubt. But you should not build your practice by trying to match the medians. You might have local data that deviates. You might be starting a program or be in a competitive situation.”
The same experts warn that the new survey population and methodologies will make it difficult to draw direct comparisons to data from previous surveys. For example, the 2007-2008 SHM survey included roughly a quarter of respondents from academic settings; the 2010 report has barely 1% of its respondents from academic settings (see Figure 1, p. 14). Traditionally, compensation and productivity levels for academic hospitalists are lower than nonacademic hospitalists. Most experts agree the “filtering” effect of the survey population factors heavily into the across- the-board increases in compensation and productivity in the 2010 report.
“The survey instrument that we use has been used historically for nonacademic physicians,” Litzau explains. “We also have an academic survey that is performed in the fall [Sept. 13 through Nov. 5], where we collect data specifically for academic faculty. We see very different trends within those two types of practice. It is difficult to draw clear comparisons between the two.”
Dr. Landis refers to the new report as a “baseline” and advises hospitalist leaders to review the caveats and cautions section (see “Survey Stipulation: Only Fools Rush In,” p. 16) before jumping right to the numbers. “This is a new set of numbers. Probably the more important comparison will be this set of numbers compared with the next set of data, next year,” he adds.
Even so, the “filtering” effect should provide nonacademic hospitalist groups a more accurate picture of compensation and productivity trends. One hospitalist leader says it’s a “win-win” for both academic and nonacademic practice leaders.
“As a community-based hospitalist, I always had to drill into those organizations that were similar to me. Being able to have more filtered information, it allows us to drill into the areas that are more important and then present that information to our CEO, CFO, VPMA,” says William D. Atchley Jr., MD, FACP, SFHM, chief of hospital medicine at Sentara Medical Group in Norfolk, Va., and a member of Team Hospitalist.
New Info, Deeper Analysis
In addition to a larger response rate and more filtered approach, the new report will offer greater frequency (annually), new data points, and in-depth breakdowns of key productivity metrics. Some of the new metrics reported include:
- Staff per FTE hospitalist physician;
- Staff turnover;
- Retirement benefits;
- Compensation to collections ratio;
- Compensation per encounter;
- Compensation per wRVU;
- Collections per encounter;
- Collections per wRVU; and
- Work RVUs per encounter.
The report will be available every fall, as compared to biannually for past SHM surveys. It also will offer more “cuts” of the data, including median, mean, 25th percentile, 75th percentile, and 90th percentile reports, along with regional breakdowns for many compensation and productivity metrics.
Practical Applications

—William “Tex” Landis, MD, FHM, medical director, Wellspan Hospitalists, York, Pa., SHM Practice Analysis Committee chair
Benchmarking data are used to set productivity goals and compensation levels in hospitalist practices throughout the country, and most administrators use multiple sources of data to make those decisions.
“If we are showing our hospitalists are generating 5,000 wRVUs per year, and the national median is 4,100, you can do the math. I can say, ‘We need to bring on another hospitalist. The timing is right, and we need to be recruiting,’ ” says Dr. Atchley, who has worked with benchmarking data for 15 years and currently supervises 45 full-time hospitalists who service five hospitals in southeast Virginia. “It’s always good to have national benchmarks to compare to, because that is always the question that is going to be asked. [Hospital administrators] want regional and national comparisons.”
Regional information and well-adapted data from national surveys guide James Gardner, MD, chief medical executive for Pro Health Care Inc., a two-hospital system just west of Milwaukee, when he’s hiring new hospitalists at 300-bed Waukesha Memorial Hospital or launching a new HM program at the system’s smaller, rural facility. In fact, Dr. Gardner currently is weighing options to expand the HM service at 80-bed Oconomowoc Hospital, less than a year after the program started.
“We like to look at a number of sources of data. The MGMA and SHM survey data, historically, have been two of our preferred sources,” Dr. Gardner says. “I think we tend to look at more regional data from the Midwest because the national data varies so much.
“We try to get a sense as to what our local market is.”
Dr. Gardner says he’d like to see a “couple years” to confirm the validity of the new SHM-MGMA report. That said, he says he knows how useful the data can be in regard to benchmarking hospitalist productivity.
“It’s been very helpful; it helps us know where we are at,” Dr. Gardner explains. “It’s one of the guideposts to decide when we are approaching the need for additional resources, whether that is midlevel providers or full-time hospitalists.”
Advice From a Numbers Guy
A self-described “numbers” guy, Dr. Ahlstrom agrees regional data is just as important as, if not more important than, the national numbers. He stresses knowing your market, your competitors, your hospital culture—and using that information along with the benchmarking data to formulate expectations for your group.
“Oftentimes you are measured against the guy next door,” Dr. Ahlstrom says. “You have to know the numbers, because [administrators] are going to know the numbers.”
Dr. Ahlstrom offers these tips for incorporating benchmarking data into your practice:
- Know your local market. “If you keep in mind your local needs, then you can look at the data and start to evaluate what parts are going to help you better formulate a practice that brings on the right people, does the right work, and continues to produce the amount of workload and compensation that makes sure they are happy in the future,” he says.
- Evaluate how applicable the data is. Pay attention to the total number of survey respondents in each category, and the standard deviation around the mean. “In other words, what is the central tendency of the data? You might find data in subsections that you find interesting, but it might not be data that has a central tendency,” he says. “Find data sets that are most applicable to your practice while assessing variations from the larger data sets. Consider how and why your practice might vary from the report as part of your evaluation.”
- Pick out trends and look at them in total. The key is to avoid looking at data points in isolation. “It’s important to look at trends in the data over time, and pick out where those trends are going to go,” he says.
- Involve your people. “I think that this data being available from the [provider] side and management side is a good thing,” Dr. Ahlstrom explains. “The more we are involved in understanding the trends in HM, the better we are going to plan where we are going in the future.” TH
Jason Carris is editor of The Hospitalist.
Editors note: This article features interactive region-by-region breakdowns and Team Hospitalist analysis of the latest compensation and productivity data from SHM and MGMA. Click here to open the interactive feature.
Every January, William “Tex” Landis, MD, FHM, sits in a conference room with key members of his hospital’s administration and presents what he affectionately refers to as the “state of the union” for his hospitalist group. The bar graphs, pie charts, and commentary have changed little in the past decade, Dr. Landis admits, but the information and analysis he has available to him as he begins crafting his 2011 presentation is better than ever.
Dr. Landis, medical director of Wellspan Hospitalists in York, Pa., and hospitalist group leaders across the country will have access to the State of Hospital Medicine: 2010 Report Based on 2009 Data this budget cycle. The new report, which will be available Sept. 10, offers new compensation and productivity information, new layers of detail, and new tools to help group leaders analyze the data.
“This data reflects the best numbers we have in our business,” says Dr. Landis, the chair of SHM’s Practice Analysis Committee. “We have better participation and better quality data analysis than we have ever had before. It’s a more standardized approach, and we are just going to be able to continue to build upon this. It sets the standard for moving forward, as far as I am concerned.”
The new report, which replaces SHM’s biannual survey, is the result of a partnership between SHM and the Medical Group Management Association (MGMA), an industry leader in practice-management resources. The report compiled data about 4,211 hospitalists in 443 groups, a 30% increase in survey respondents over SHM’s 2007-2008 report.
“The collaboration is really driven at providing a single set of benchmarks to the HM community,” says David Litzau, systems analyst at MGMA. “It provides a viewpoint of what’s happening elsewhere in the industry.”
What’s happening is that hospitalists continue to see increases in compensation. The new report, which uses some different data definitions and survey methodologies, and is based on a new population, shows that median compensation for adult hospitalists is $215,000 per year, a number that doesn’t take into account benefits. Hospitalist median compensation was $183,900, according to SHM’s 2007-2008 survey, and $171,000 in SHM’s 2005-2006 survey. MGMA’s 2009 report on physician compensation showed median compensation at $210,250 per internal medicine hospitalist.
And while the compensation numbers are higher than in previous surveys, the new report also shows adult hospitalists are increasing productivity, are seeing more patients per year (reversing a somewhat declining trend), and are collecting more per encounter.
The Numbers
Although compensation is the most popular survey metric, it’s not the only number worth investigating. A handful of key productivity measures seem to be on the rise, too, according to the new report.
The national median (the midpoint of all survey respondents) for work RVUs per adult hospitalist FTE is 4,107, according to the new data. SHM’s 2007-2008 survey reported wRVUs at 3,715 per adult hospitalist.
The national median for hospitalist wRVUs per encounter is 1.86. That same figure was reported at 1.53 wRVUs per encounter in 2008 and 1.37 in 2006.
Collections per wRVU is $45.57, according to the 2010 report. The 2008 survey showed collections at $44.97 per wRVU; the 2006 survey did not report the metric.
One thing the new metrics have in common is that they show hospitalists across the nation are becoming more efficient. “The numbers essentially reaffirm the overall trends for hospital medicine, in that the productivity continues to increase and the compensation paid to a provider continues to increase,” says Troy Ahlstrom, MD, FHM, CFO of Traverse City-based Hospitalists of Northern Michigan, which has nearly 50 hospitalists supporting three hospitals. “When you dig into the numbers, hospitalists are producing more work and more RVUs per encounter than they had been in the past.”
Financial support per hospitalist FTE, another key practice-management metric, parallels the compensation growth. Practices receive a median of $98,253 of support per hospitalist FTE, according to 2010 data. The 2008 report did not provide a median figure for support; instead, it published a mean figure of $97,375 of support per FTE. The 2010 mean (average) is $111,486.
Pediatric HM also shows signs of growth; median compensation is $160,038 in the new report. The 2008 report had pediatric hospitalist median compensation at $144,600.
The new data show a spike in HM groups providing “on site” care of patients 24 hours a day, seven days a week. More than 68% have on-site care with a physician, nurse practitioner, or physician assistant. Only 53% of groups had 24/7 coverage in the 2008 report; 51% had round-the-clock coverage in the 2006 report.
Dr. Ahlstrom, a veteran member of SHM’s Practice Analysis Committee, says he expects that trend to continue, especially with the large numbers of young hospitalists in the field interested in set schedules and work-life balance. “That’s the trend,” he says. “Younger physicians are more interested in seeing that split, where the days and nights are clearly set off. Older physicians are more than happy to have a nocturnist around, just as long as it’s not going to cost them a lot of money or productivity.”
A Word of Caution, and Unintended Benefits
The new report is based on a supplemental set of questions specifically directed at hospitalist practices in MGMA’s annual Physician Compensation and Productivity Survey. The survey is voluntary and is not audited, but it is the “best data” available for hospitalists, according to practice-management experts.
“So many people assume this data is what you should do,” says John Nelson, MD, MHM, co-founder and past president of SHM and a principal in hospitalist-consulting firm Nelson Flores Hospital Medicine Consultants. “It’s not. It is a survey of what’s happening. It’s a starting point, a frame of reference. It is the best data there is, no doubt. But you should not build your practice by trying to match the medians. You might have local data that deviates. You might be starting a program or be in a competitive situation.”
The same experts warn that the new survey population and methodologies will make it difficult to draw direct comparisons to data from previous surveys. For example, the 2007-2008 SHM survey included roughly a quarter of respondents from academic settings; the 2010 report has barely 1% of its respondents from academic settings (see Figure 1, p. 14). Traditionally, compensation and productivity levels for academic hospitalists are lower than nonacademic hospitalists. Most experts agree the “filtering” effect of the survey population factors heavily into the across- the-board increases in compensation and productivity in the 2010 report.
“The survey instrument that we use has been used historically for nonacademic physicians,” Litzau explains. “We also have an academic survey that is performed in the fall [Sept. 13 through Nov. 5], where we collect data specifically for academic faculty. We see very different trends within those two types of practice. It is difficult to draw clear comparisons between the two.”
Dr. Landis refers to the new report as a “baseline” and advises hospitalist leaders to review the caveats and cautions section (see “Survey Stipulation: Only Fools Rush In,” p. 16) before jumping right to the numbers. “This is a new set of numbers. Probably the more important comparison will be this set of numbers compared with the next set of data, next year,” he adds.
Even so, the “filtering” effect should provide nonacademic hospitalist groups a more accurate picture of compensation and productivity trends. One hospitalist leader says it’s a “win-win” for both academic and nonacademic practice leaders.
“As a community-based hospitalist, I always had to drill into those organizations that were similar to me. Being able to have more filtered information, it allows us to drill into the areas that are more important and then present that information to our CEO, CFO, VPMA,” says William D. Atchley Jr., MD, FACP, SFHM, chief of hospital medicine at Sentara Medical Group in Norfolk, Va., and a member of Team Hospitalist.
New Info, Deeper Analysis
In addition to a larger response rate and more filtered approach, the new report will offer greater frequency (annually), new data points, and in-depth breakdowns of key productivity metrics. Some of the new metrics reported include:
- Staff per FTE hospitalist physician;
- Staff turnover;
- Retirement benefits;
- Compensation to collections ratio;
- Compensation per encounter;
- Compensation per wRVU;
- Collections per encounter;
- Collections per wRVU; and
- Work RVUs per encounter.
The report will be available every fall, as compared to biannually for past SHM surveys. It also will offer more “cuts” of the data, including median, mean, 25th percentile, 75th percentile, and 90th percentile reports, along with regional breakdowns for many compensation and productivity metrics.
Practical Applications

—William “Tex” Landis, MD, FHM, medical director, Wellspan Hospitalists, York, Pa., SHM Practice Analysis Committee chair
Benchmarking data are used to set productivity goals and compensation levels in hospitalist practices throughout the country, and most administrators use multiple sources of data to make those decisions.
“If we are showing our hospitalists are generating 5,000 wRVUs per year, and the national median is 4,100, you can do the math. I can say, ‘We need to bring on another hospitalist. The timing is right, and we need to be recruiting,’ ” says Dr. Atchley, who has worked with benchmarking data for 15 years and currently supervises 45 full-time hospitalists who service five hospitals in southeast Virginia. “It’s always good to have national benchmarks to compare to, because that is always the question that is going to be asked. [Hospital administrators] want regional and national comparisons.”
Regional information and well-adapted data from national surveys guide James Gardner, MD, chief medical executive for Pro Health Care Inc., a two-hospital system just west of Milwaukee, when he’s hiring new hospitalists at 300-bed Waukesha Memorial Hospital or launching a new HM program at the system’s smaller, rural facility. In fact, Dr. Gardner currently is weighing options to expand the HM service at 80-bed Oconomowoc Hospital, less than a year after the program started.
“We like to look at a number of sources of data. The MGMA and SHM survey data, historically, have been two of our preferred sources,” Dr. Gardner says. “I think we tend to look at more regional data from the Midwest because the national data varies so much.
“We try to get a sense as to what our local market is.”
Dr. Gardner says he’d like to see a “couple years” to confirm the validity of the new SHM-MGMA report. That said, he says he knows how useful the data can be in regard to benchmarking hospitalist productivity.
“It’s been very helpful; it helps us know where we are at,” Dr. Gardner explains. “It’s one of the guideposts to decide when we are approaching the need for additional resources, whether that is midlevel providers or full-time hospitalists.”
Advice From a Numbers Guy
A self-described “numbers” guy, Dr. Ahlstrom agrees regional data is just as important as, if not more important than, the national numbers. He stresses knowing your market, your competitors, your hospital culture—and using that information along with the benchmarking data to formulate expectations for your group.
“Oftentimes you are measured against the guy next door,” Dr. Ahlstrom says. “You have to know the numbers, because [administrators] are going to know the numbers.”
Dr. Ahlstrom offers these tips for incorporating benchmarking data into your practice:
- Know your local market. “If you keep in mind your local needs, then you can look at the data and start to evaluate what parts are going to help you better formulate a practice that brings on the right people, does the right work, and continues to produce the amount of workload and compensation that makes sure they are happy in the future,” he says.
- Evaluate how applicable the data is. Pay attention to the total number of survey respondents in each category, and the standard deviation around the mean. “In other words, what is the central tendency of the data? You might find data in subsections that you find interesting, but it might not be data that has a central tendency,” he says. “Find data sets that are most applicable to your practice while assessing variations from the larger data sets. Consider how and why your practice might vary from the report as part of your evaluation.”
- Pick out trends and look at them in total. The key is to avoid looking at data points in isolation. “It’s important to look at trends in the data over time, and pick out where those trends are going to go,” he says.
- Involve your people. “I think that this data being available from the [provider] side and management side is a good thing,” Dr. Ahlstrom explains. “The more we are involved in understanding the trends in HM, the better we are going to plan where we are going in the future.” TH
Jason Carris is editor of The Hospitalist.
Playground Politics
Baseball, kick the can, Russian roulette—pick your game. Chances are good that it has worked its way into a metaphor to illustrate the infuriating, perplexing, and altogether frustrating inability of Congress to step up to the plate and pass a long-term fix to the broken sustainable growth rate (SGR) formula used to determine Medicare reimbursement rates.
On June 24, legislators avoided catastrophe by temporarily rescinding a 21.3% rate cut that went into effect June 1. The after-the-fact patch meant that some Medicare claims had to be reprocessed to recoup the full value, creating an administrative mess. The accompanying 2.2% rate increase expires Nov. 30. The reimbursement cut could reach nearly 30% next year unless Congress intervenes again.
“Obviously, there’s a lot of frustration around the issue, especially on the membership side,” says Ron Greeno, MD, FACP, SFHM, a member of SHM’s Public Policy and Leadership committees, and chief medical officer for Brentwood, Tenn.-based Cogent Healthcare. For hospitalists in many small private practices, he says, a major percentage of income comes from Medicare. “It’s a tremendous headache,” he says of the uncertainty. “It’s very hard to plan for. You’re trying to budget and you don’t know what the policy is going to be literally from week to week.”
The Blame Game
Despite the widespread sentiment among doctors that a permanent reimbursement rate fix should have been included in the healthcare reform legislation, skittishness over the price tag led legislators to drop it from the package. Based on last fall’s estimates, the total cost of a reform bill that scrapped the SGR would have ballooned by roughly $250 billion over 10 years, which would have threatened the bill’s passage.
But Congress has since been unable to pass a permanent fix as standalone legislation amid mounting concern over the national debt, and the price of inaction continues to rise. On April 30, the Congressional Budget Office (CBO) estimated that the cost of jettisoning the SGR formula and freezing rates at current levels had grown to $276 billion over 10 years.
Any serious consideration of lasting alternatives has now been pushed back to the lame-duck session, after the midterm elections. The can has been kicked down the road so many times, Dr. Greeno and others say, that most Congressional members have boot marks all over them. “So now you have a bigger problem at a more crucial time, when money is tighter than ever in a poor economy,” Dr. Greeno says. “And I just think it’s been a failure of our politicians.”
Other healthcare industry leaders have been just as critical. “Delaying the problem is not a solution,” said AMA President Cecil B. Wilson, MD, in a prepared statement after Congress passed the latest six-month reprieve in June. “It doesn’t solve the Medicare mess Congress has created with a long series of short-term Medicare patches over the last decade—including four to avert the 2010 cut alone.”
AMA-sponsored print ads have reminded legislators that delaying a fix until 2013 will again increase its cost, to $396 billion over 10 years. And the association’s June press release asserted that “Congress is playing a dangerous game of Russian roulette with seniors’ healthcare.”
Perhaps a game of “chicken” would be more apt.
Republicans have dared Democrats to spend the billions for a more lasting solution—in the absence of any cuts elsewhere in the healthcare delivery system—and be labeled as fiscally irresponsible. In turn, Democrats have dared Republicans to let the rate cut take effect and be labeled heartless as Medicare beneficiaries lose access to their healthcare providers.
Both parties blinked, resorting to almost unanimous short-term fixes that have allowed legislators to save face while putting off politically risky votes until after the November elections.
Lynne M. Allen, MN, ARNP, who works as a part-time hospitalist in hematology-oncology at 188-bed Kadlec Regional Medical Center in Richland, Wash., says she and other colleagues were initially hopeful that the Obama administration would make Congress work together to find a lasting solution. “There’s a sense of frustration because instead of that happening from our legislators, they’re playing a lot of games with the funding,” says Allen, a member of Team Hospitalist. “They’re not willing to step up to the plate, as they say, and make a decision that will allow us to go forward smoothly.”
The result, Allen says, has been a “roller-coaster ride” of uncertainty over reimbursements. Because Washington’s Tri-Cities region has a relatively high percentage of patients with private insurance, her hospital is somewhat cushioned from a precipitous drop in Medicare fees. But if CMS is ever forced to cut back on its rates, she fully expects private insurers to follow the same downward track.
Practical Concerns
Barbara Hartley, MD, a part-time hospitalist at 22-bed Benson Hospital in Benson, Ariz., says the town’s healthcare facility is somewhat protected from potential Medicare rate cuts through its official status as a Critical Access Hospital. Instead of being reimbursed through diagnosis-related group (DRG) codes, the rural hospital is repaid by Medicare for its total cost per day per patient.
The arrangement is a stable one at the moment, but not enough to dispel Dr. Hartley’s uneasy question: If the economy worsens, will Medicare be able to retain its commitment to rural hospitals? If not, the pain might be felt acutely in communities like Benson, where Dr. Hartley estimates that as much as 75% of the hospital’s in-patient business is through either Medicare or a Medicare Advantage plan.
Kirk Mathews, CEO of St. Louis-based Inpatient Management Inc. and a member of SHM’s Public Policy and Practice Management committees, says Medicare rate cuts also could significantly reduce the leverage of hospitalists during contract negotiations.
“Even if we’re employed by the hospital, but our professional fees that the hospital can recoup for our services are dramatically affected, it will affect how those future contracts go,” Mathews says. “We might be insulated temporarily by the strength of our current contract. But if the formula—however that works out—dramatically impacts the hospitalist reimbursement on the professional fee side, the hospital will feel that, and then hospitalists will eventually feel that as well.” In other words, it could strengthen the bargaining hand of the hospital at the expense of the hospitalist. “Therein lies the long-term threat,” he points out.
Independent Solution?
Some of the authority over physician payments might eventually be depoliticized via language in the reform legislation that empowers a new entity, the Independent Payment Advisory Board, to create policy on such critical monetary issues as reimbursement rates. Congress could still override the board’s policy decisions, but only if the Congressional alternative saves just as much money.
In the meantime, the money for a fix still has to come from somewhere, and no consensus has emerged. Advocates likewise refuse to coalesce around any single alternative. Some experts favor a new formula based on the Medicare economic index, which measures inflation in healthcare delivery costs. But the CBO estimates that per-beneficiary spending under such a formula would be 30% more by 2016 than under the current formula. Other proposals call for temporarily increasing rates, then reverting to annual GDP growth, plus a bit more to cover physician costs.
No matter how the crisis is resolved, experts say, doctors almost certainly will have to make do with less. “When healthcare reform is finally fully implemented, there are going to be less dollars to pay for more services. It’s inevitable,” Mathews says. “And whether it takes the form of SGR or some other form, I’m afraid physicians are going to have to get used to having less money in the pool of money that’s allocated to pay providers.”
It could be a whole new ballgame. TH
Bryn Nelson, PhD, is a freelance medical writer based in Seattle.
Baseball, kick the can, Russian roulette—pick your game. Chances are good that it has worked its way into a metaphor to illustrate the infuriating, perplexing, and altogether frustrating inability of Congress to step up to the plate and pass a long-term fix to the broken sustainable growth rate (SGR) formula used to determine Medicare reimbursement rates.
On June 24, legislators avoided catastrophe by temporarily rescinding a 21.3% rate cut that went into effect June 1. The after-the-fact patch meant that some Medicare claims had to be reprocessed to recoup the full value, creating an administrative mess. The accompanying 2.2% rate increase expires Nov. 30. The reimbursement cut could reach nearly 30% next year unless Congress intervenes again.
“Obviously, there’s a lot of frustration around the issue, especially on the membership side,” says Ron Greeno, MD, FACP, SFHM, a member of SHM’s Public Policy and Leadership committees, and chief medical officer for Brentwood, Tenn.-based Cogent Healthcare. For hospitalists in many small private practices, he says, a major percentage of income comes from Medicare. “It’s a tremendous headache,” he says of the uncertainty. “It’s very hard to plan for. You’re trying to budget and you don’t know what the policy is going to be literally from week to week.”
The Blame Game
Despite the widespread sentiment among doctors that a permanent reimbursement rate fix should have been included in the healthcare reform legislation, skittishness over the price tag led legislators to drop it from the package. Based on last fall’s estimates, the total cost of a reform bill that scrapped the SGR would have ballooned by roughly $250 billion over 10 years, which would have threatened the bill’s passage.
But Congress has since been unable to pass a permanent fix as standalone legislation amid mounting concern over the national debt, and the price of inaction continues to rise. On April 30, the Congressional Budget Office (CBO) estimated that the cost of jettisoning the SGR formula and freezing rates at current levels had grown to $276 billion over 10 years.
Any serious consideration of lasting alternatives has now been pushed back to the lame-duck session, after the midterm elections. The can has been kicked down the road so many times, Dr. Greeno and others say, that most Congressional members have boot marks all over them. “So now you have a bigger problem at a more crucial time, when money is tighter than ever in a poor economy,” Dr. Greeno says. “And I just think it’s been a failure of our politicians.”
Other healthcare industry leaders have been just as critical. “Delaying the problem is not a solution,” said AMA President Cecil B. Wilson, MD, in a prepared statement after Congress passed the latest six-month reprieve in June. “It doesn’t solve the Medicare mess Congress has created with a long series of short-term Medicare patches over the last decade—including four to avert the 2010 cut alone.”
AMA-sponsored print ads have reminded legislators that delaying a fix until 2013 will again increase its cost, to $396 billion over 10 years. And the association’s June press release asserted that “Congress is playing a dangerous game of Russian roulette with seniors’ healthcare.”
Perhaps a game of “chicken” would be more apt.
Republicans have dared Democrats to spend the billions for a more lasting solution—in the absence of any cuts elsewhere in the healthcare delivery system—and be labeled as fiscally irresponsible. In turn, Democrats have dared Republicans to let the rate cut take effect and be labeled heartless as Medicare beneficiaries lose access to their healthcare providers.
Both parties blinked, resorting to almost unanimous short-term fixes that have allowed legislators to save face while putting off politically risky votes until after the November elections.
Lynne M. Allen, MN, ARNP, who works as a part-time hospitalist in hematology-oncology at 188-bed Kadlec Regional Medical Center in Richland, Wash., says she and other colleagues were initially hopeful that the Obama administration would make Congress work together to find a lasting solution. “There’s a sense of frustration because instead of that happening from our legislators, they’re playing a lot of games with the funding,” says Allen, a member of Team Hospitalist. “They’re not willing to step up to the plate, as they say, and make a decision that will allow us to go forward smoothly.”
The result, Allen says, has been a “roller-coaster ride” of uncertainty over reimbursements. Because Washington’s Tri-Cities region has a relatively high percentage of patients with private insurance, her hospital is somewhat cushioned from a precipitous drop in Medicare fees. But if CMS is ever forced to cut back on its rates, she fully expects private insurers to follow the same downward track.
Practical Concerns
Barbara Hartley, MD, a part-time hospitalist at 22-bed Benson Hospital in Benson, Ariz., says the town’s healthcare facility is somewhat protected from potential Medicare rate cuts through its official status as a Critical Access Hospital. Instead of being reimbursed through diagnosis-related group (DRG) codes, the rural hospital is repaid by Medicare for its total cost per day per patient.
The arrangement is a stable one at the moment, but not enough to dispel Dr. Hartley’s uneasy question: If the economy worsens, will Medicare be able to retain its commitment to rural hospitals? If not, the pain might be felt acutely in communities like Benson, where Dr. Hartley estimates that as much as 75% of the hospital’s in-patient business is through either Medicare or a Medicare Advantage plan.
Kirk Mathews, CEO of St. Louis-based Inpatient Management Inc. and a member of SHM’s Public Policy and Practice Management committees, says Medicare rate cuts also could significantly reduce the leverage of hospitalists during contract negotiations.
“Even if we’re employed by the hospital, but our professional fees that the hospital can recoup for our services are dramatically affected, it will affect how those future contracts go,” Mathews says. “We might be insulated temporarily by the strength of our current contract. But if the formula—however that works out—dramatically impacts the hospitalist reimbursement on the professional fee side, the hospital will feel that, and then hospitalists will eventually feel that as well.” In other words, it could strengthen the bargaining hand of the hospital at the expense of the hospitalist. “Therein lies the long-term threat,” he points out.
Independent Solution?
Some of the authority over physician payments might eventually be depoliticized via language in the reform legislation that empowers a new entity, the Independent Payment Advisory Board, to create policy on such critical monetary issues as reimbursement rates. Congress could still override the board’s policy decisions, but only if the Congressional alternative saves just as much money.
In the meantime, the money for a fix still has to come from somewhere, and no consensus has emerged. Advocates likewise refuse to coalesce around any single alternative. Some experts favor a new formula based on the Medicare economic index, which measures inflation in healthcare delivery costs. But the CBO estimates that per-beneficiary spending under such a formula would be 30% more by 2016 than under the current formula. Other proposals call for temporarily increasing rates, then reverting to annual GDP growth, plus a bit more to cover physician costs.
No matter how the crisis is resolved, experts say, doctors almost certainly will have to make do with less. “When healthcare reform is finally fully implemented, there are going to be less dollars to pay for more services. It’s inevitable,” Mathews says. “And whether it takes the form of SGR or some other form, I’m afraid physicians are going to have to get used to having less money in the pool of money that’s allocated to pay providers.”
It could be a whole new ballgame. TH
Bryn Nelson, PhD, is a freelance medical writer based in Seattle.
Baseball, kick the can, Russian roulette—pick your game. Chances are good that it has worked its way into a metaphor to illustrate the infuriating, perplexing, and altogether frustrating inability of Congress to step up to the plate and pass a long-term fix to the broken sustainable growth rate (SGR) formula used to determine Medicare reimbursement rates.
On June 24, legislators avoided catastrophe by temporarily rescinding a 21.3% rate cut that went into effect June 1. The after-the-fact patch meant that some Medicare claims had to be reprocessed to recoup the full value, creating an administrative mess. The accompanying 2.2% rate increase expires Nov. 30. The reimbursement cut could reach nearly 30% next year unless Congress intervenes again.
“Obviously, there’s a lot of frustration around the issue, especially on the membership side,” says Ron Greeno, MD, FACP, SFHM, a member of SHM’s Public Policy and Leadership committees, and chief medical officer for Brentwood, Tenn.-based Cogent Healthcare. For hospitalists in many small private practices, he says, a major percentage of income comes from Medicare. “It’s a tremendous headache,” he says of the uncertainty. “It’s very hard to plan for. You’re trying to budget and you don’t know what the policy is going to be literally from week to week.”
The Blame Game
Despite the widespread sentiment among doctors that a permanent reimbursement rate fix should have been included in the healthcare reform legislation, skittishness over the price tag led legislators to drop it from the package. Based on last fall’s estimates, the total cost of a reform bill that scrapped the SGR would have ballooned by roughly $250 billion over 10 years, which would have threatened the bill’s passage.
But Congress has since been unable to pass a permanent fix as standalone legislation amid mounting concern over the national debt, and the price of inaction continues to rise. On April 30, the Congressional Budget Office (CBO) estimated that the cost of jettisoning the SGR formula and freezing rates at current levels had grown to $276 billion over 10 years.
Any serious consideration of lasting alternatives has now been pushed back to the lame-duck session, after the midterm elections. The can has been kicked down the road so many times, Dr. Greeno and others say, that most Congressional members have boot marks all over them. “So now you have a bigger problem at a more crucial time, when money is tighter than ever in a poor economy,” Dr. Greeno says. “And I just think it’s been a failure of our politicians.”
Other healthcare industry leaders have been just as critical. “Delaying the problem is not a solution,” said AMA President Cecil B. Wilson, MD, in a prepared statement after Congress passed the latest six-month reprieve in June. “It doesn’t solve the Medicare mess Congress has created with a long series of short-term Medicare patches over the last decade—including four to avert the 2010 cut alone.”
AMA-sponsored print ads have reminded legislators that delaying a fix until 2013 will again increase its cost, to $396 billion over 10 years. And the association’s June press release asserted that “Congress is playing a dangerous game of Russian roulette with seniors’ healthcare.”
Perhaps a game of “chicken” would be more apt.
Republicans have dared Democrats to spend the billions for a more lasting solution—in the absence of any cuts elsewhere in the healthcare delivery system—and be labeled as fiscally irresponsible. In turn, Democrats have dared Republicans to let the rate cut take effect and be labeled heartless as Medicare beneficiaries lose access to their healthcare providers.
Both parties blinked, resorting to almost unanimous short-term fixes that have allowed legislators to save face while putting off politically risky votes until after the November elections.
Lynne M. Allen, MN, ARNP, who works as a part-time hospitalist in hematology-oncology at 188-bed Kadlec Regional Medical Center in Richland, Wash., says she and other colleagues were initially hopeful that the Obama administration would make Congress work together to find a lasting solution. “There’s a sense of frustration because instead of that happening from our legislators, they’re playing a lot of games with the funding,” says Allen, a member of Team Hospitalist. “They’re not willing to step up to the plate, as they say, and make a decision that will allow us to go forward smoothly.”
The result, Allen says, has been a “roller-coaster ride” of uncertainty over reimbursements. Because Washington’s Tri-Cities region has a relatively high percentage of patients with private insurance, her hospital is somewhat cushioned from a precipitous drop in Medicare fees. But if CMS is ever forced to cut back on its rates, she fully expects private insurers to follow the same downward track.
Practical Concerns
Barbara Hartley, MD, a part-time hospitalist at 22-bed Benson Hospital in Benson, Ariz., says the town’s healthcare facility is somewhat protected from potential Medicare rate cuts through its official status as a Critical Access Hospital. Instead of being reimbursed through diagnosis-related group (DRG) codes, the rural hospital is repaid by Medicare for its total cost per day per patient.
The arrangement is a stable one at the moment, but not enough to dispel Dr. Hartley’s uneasy question: If the economy worsens, will Medicare be able to retain its commitment to rural hospitals? If not, the pain might be felt acutely in communities like Benson, where Dr. Hartley estimates that as much as 75% of the hospital’s in-patient business is through either Medicare or a Medicare Advantage plan.
Kirk Mathews, CEO of St. Louis-based Inpatient Management Inc. and a member of SHM’s Public Policy and Practice Management committees, says Medicare rate cuts also could significantly reduce the leverage of hospitalists during contract negotiations.
“Even if we’re employed by the hospital, but our professional fees that the hospital can recoup for our services are dramatically affected, it will affect how those future contracts go,” Mathews says. “We might be insulated temporarily by the strength of our current contract. But if the formula—however that works out—dramatically impacts the hospitalist reimbursement on the professional fee side, the hospital will feel that, and then hospitalists will eventually feel that as well.” In other words, it could strengthen the bargaining hand of the hospital at the expense of the hospitalist. “Therein lies the long-term threat,” he points out.
Independent Solution?
Some of the authority over physician payments might eventually be depoliticized via language in the reform legislation that empowers a new entity, the Independent Payment Advisory Board, to create policy on such critical monetary issues as reimbursement rates. Congress could still override the board’s policy decisions, but only if the Congressional alternative saves just as much money.
In the meantime, the money for a fix still has to come from somewhere, and no consensus has emerged. Advocates likewise refuse to coalesce around any single alternative. Some experts favor a new formula based on the Medicare economic index, which measures inflation in healthcare delivery costs. But the CBO estimates that per-beneficiary spending under such a formula would be 30% more by 2016 than under the current formula. Other proposals call for temporarily increasing rates, then reverting to annual GDP growth, plus a bit more to cover physician costs.
No matter how the crisis is resolved, experts say, doctors almost certainly will have to make do with less. “When healthcare reform is finally fully implemented, there are going to be less dollars to pay for more services. It’s inevitable,” Mathews says. “And whether it takes the form of SGR or some other form, I’m afraid physicians are going to have to get used to having less money in the pool of money that’s allocated to pay providers.”
It could be a whole new ballgame. TH
Bryn Nelson, PhD, is a freelance medical writer based in Seattle.
Time to Get a Move On
Hospitalists might want to incorporate questions about how much time a patient spends sitting into their diagnostic interview—then consider the same question for themselves.
A study published this spring in Medicine & Science in Sports & Exercise reports that time spent watching TV or riding in a car “were significant cardiovascular disease (CVD) mortality predictors” (Med Sci Sports Exerc. 2010;42(5):879-885). The research gained traction in medical publications and was highlighted in The New York Times. But for one of the researchers at the University of South Carolina, more work is needed.
“This is sort of a new way of looking at the equation,” says Steven Hooker, PhD, director of the university's Prevention Research Center. “We really don’t have any formal guidelines or recommendations on limiting sedentary behavior, although I think at some point in time we’ll get to that stage.”
Until then, hospitalists are in a prime position to determine a patient’s lifestyle—sedentary or active—via routine checklist questions they ask upon admission. Although some HM groups already ask questions about how often a patient exercises, Dr. Hooker suspects only a few groups ask how often a patient breaks up long periods of sitting.
Further, he suggests that while there are no standard recommendations, hospitalists would serve their patients well by incorporating helpful hints in discharge instructions or admission interviews.
“Encourage a person to build in standing or slight walking breaks in a daily routine, recommend they stand periodically while attending long meetings or during long periods of travel,” Dr. Hooker says. “Common sense reigns here.”
On the flip side, hospitalists would do well to remember that while their workday might include a high level of light-intensity activity, they face the same pitfalls as their patients: commuting, sitting in meetings, long periods of time in front of the computer, TV, or PlayStation.
“We have to get the public looking at physical activity and physical inactivity as two completely separate things,” says study first author Tatiana Warren, MS, a doctoral student in the department of exercise science at the University of South Carolina's Arnold School of Public Health. “We have to continue to do more research and get the word out.”
Hospitalists might want to incorporate questions about how much time a patient spends sitting into their diagnostic interview—then consider the same question for themselves.
A study published this spring in Medicine & Science in Sports & Exercise reports that time spent watching TV or riding in a car “were significant cardiovascular disease (CVD) mortality predictors” (Med Sci Sports Exerc. 2010;42(5):879-885). The research gained traction in medical publications and was highlighted in The New York Times. But for one of the researchers at the University of South Carolina, more work is needed.
“This is sort of a new way of looking at the equation,” says Steven Hooker, PhD, director of the university's Prevention Research Center. “We really don’t have any formal guidelines or recommendations on limiting sedentary behavior, although I think at some point in time we’ll get to that stage.”
Until then, hospitalists are in a prime position to determine a patient’s lifestyle—sedentary or active—via routine checklist questions they ask upon admission. Although some HM groups already ask questions about how often a patient exercises, Dr. Hooker suspects only a few groups ask how often a patient breaks up long periods of sitting.
Further, he suggests that while there are no standard recommendations, hospitalists would serve their patients well by incorporating helpful hints in discharge instructions or admission interviews.
“Encourage a person to build in standing or slight walking breaks in a daily routine, recommend they stand periodically while attending long meetings or during long periods of travel,” Dr. Hooker says. “Common sense reigns here.”
On the flip side, hospitalists would do well to remember that while their workday might include a high level of light-intensity activity, they face the same pitfalls as their patients: commuting, sitting in meetings, long periods of time in front of the computer, TV, or PlayStation.
“We have to get the public looking at physical activity and physical inactivity as two completely separate things,” says study first author Tatiana Warren, MS, a doctoral student in the department of exercise science at the University of South Carolina's Arnold School of Public Health. “We have to continue to do more research and get the word out.”
Hospitalists might want to incorporate questions about how much time a patient spends sitting into their diagnostic interview—then consider the same question for themselves.
A study published this spring in Medicine & Science in Sports & Exercise reports that time spent watching TV or riding in a car “were significant cardiovascular disease (CVD) mortality predictors” (Med Sci Sports Exerc. 2010;42(5):879-885). The research gained traction in medical publications and was highlighted in The New York Times. But for one of the researchers at the University of South Carolina, more work is needed.
“This is sort of a new way of looking at the equation,” says Steven Hooker, PhD, director of the university's Prevention Research Center. “We really don’t have any formal guidelines or recommendations on limiting sedentary behavior, although I think at some point in time we’ll get to that stage.”
Until then, hospitalists are in a prime position to determine a patient’s lifestyle—sedentary or active—via routine checklist questions they ask upon admission. Although some HM groups already ask questions about how often a patient exercises, Dr. Hooker suspects only a few groups ask how often a patient breaks up long periods of sitting.
Further, he suggests that while there are no standard recommendations, hospitalists would serve their patients well by incorporating helpful hints in discharge instructions or admission interviews.
“Encourage a person to build in standing or slight walking breaks in a daily routine, recommend they stand periodically while attending long meetings or during long periods of travel,” Dr. Hooker says. “Common sense reigns here.”
On the flip side, hospitalists would do well to remember that while their workday might include a high level of light-intensity activity, they face the same pitfalls as their patients: commuting, sitting in meetings, long periods of time in front of the computer, TV, or PlayStation.
“We have to get the public looking at physical activity and physical inactivity as two completely separate things,” says study first author Tatiana Warren, MS, a doctoral student in the department of exercise science at the University of South Carolina's Arnold School of Public Health. “We have to continue to do more research and get the word out.”
N.C. Hospital Names Hospitalist Its Physician of the Year
Suzanne Wilson, MD, is a self-proclaimed “outdoor-sy girl”—so much so that when the opportunity arose to work as a hospitalist in the golf mecca of Pinehurst, N.C., she didn’t think twice.
“My husband and I wanted to live somewhere we wanted to be,” says Dr. Wilson, a hospitalist for 10 years.
Pinehurst is home to one of the world’s most elegant and famous golf resorts, but it was at Moore Regional Hospital that Dr. Wilson was recently named Physician of the Year. She is Moore Regional’s first hospitalist to receive the honor.
The award process begins with the nurses, who are in charge of nominations. An independent board “reads the stories” about the nominees and their outstanding care, then chooses the winner.
Although Dr. Wilson says she was “stunned” when she was told she received the award, her colleagues were not. “Dr. Wilson is a consistent professional. It’s the way she relates to patients; her manner is very comfortable and easy going, and her ability to communicate with them—it’s like going home,” says Katherine Marsh, an oncology nurse at Moore Regional.
Dan Barnes, MD, director of the 23-hospitalist program at Moore Regional, says Dr. Wilson’s extensive interaction with the hospital’s nurses has helped improve the relationship between doctors and other staff.
“She is a true advocate for quality improvement and is always willing to help with that,” Dr. Barnes says, citing a soon-to-be-live Computerized Physician Order Entry (CPOE) initiative at the hospital, a project for which Dr. Wilson serves as officer.
Dr. Wilson’s focus on forging relationships between varying medical staff can be traced back to her early exposure to medicine. She grew up in southern Indiana with an RN mother and medical photographer father. She says that combination made for “interesting dinner conversations” as a child.
“We have to keep asking ourselves, ‘How can we do this better?'” Dr. Wilson says. “It’s all about the patient.”
Suzanne Wilson, MD, is a self-proclaimed “outdoor-sy girl”—so much so that when the opportunity arose to work as a hospitalist in the golf mecca of Pinehurst, N.C., she didn’t think twice.
“My husband and I wanted to live somewhere we wanted to be,” says Dr. Wilson, a hospitalist for 10 years.
Pinehurst is home to one of the world’s most elegant and famous golf resorts, but it was at Moore Regional Hospital that Dr. Wilson was recently named Physician of the Year. She is Moore Regional’s first hospitalist to receive the honor.
The award process begins with the nurses, who are in charge of nominations. An independent board “reads the stories” about the nominees and their outstanding care, then chooses the winner.
Although Dr. Wilson says she was “stunned” when she was told she received the award, her colleagues were not. “Dr. Wilson is a consistent professional. It’s the way she relates to patients; her manner is very comfortable and easy going, and her ability to communicate with them—it’s like going home,” says Katherine Marsh, an oncology nurse at Moore Regional.
Dan Barnes, MD, director of the 23-hospitalist program at Moore Regional, says Dr. Wilson’s extensive interaction with the hospital’s nurses has helped improve the relationship between doctors and other staff.
“She is a true advocate for quality improvement and is always willing to help with that,” Dr. Barnes says, citing a soon-to-be-live Computerized Physician Order Entry (CPOE) initiative at the hospital, a project for which Dr. Wilson serves as officer.
Dr. Wilson’s focus on forging relationships between varying medical staff can be traced back to her early exposure to medicine. She grew up in southern Indiana with an RN mother and medical photographer father. She says that combination made for “interesting dinner conversations” as a child.
“We have to keep asking ourselves, ‘How can we do this better?'” Dr. Wilson says. “It’s all about the patient.”
Suzanne Wilson, MD, is a self-proclaimed “outdoor-sy girl”—so much so that when the opportunity arose to work as a hospitalist in the golf mecca of Pinehurst, N.C., she didn’t think twice.
“My husband and I wanted to live somewhere we wanted to be,” says Dr. Wilson, a hospitalist for 10 years.
Pinehurst is home to one of the world’s most elegant and famous golf resorts, but it was at Moore Regional Hospital that Dr. Wilson was recently named Physician of the Year. She is Moore Regional’s first hospitalist to receive the honor.
The award process begins with the nurses, who are in charge of nominations. An independent board “reads the stories” about the nominees and their outstanding care, then chooses the winner.
Although Dr. Wilson says she was “stunned” when she was told she received the award, her colleagues were not. “Dr. Wilson is a consistent professional. It’s the way she relates to patients; her manner is very comfortable and easy going, and her ability to communicate with them—it’s like going home,” says Katherine Marsh, an oncology nurse at Moore Regional.
Dan Barnes, MD, director of the 23-hospitalist program at Moore Regional, says Dr. Wilson’s extensive interaction with the hospital’s nurses has helped improve the relationship between doctors and other staff.
“She is a true advocate for quality improvement and is always willing to help with that,” Dr. Barnes says, citing a soon-to-be-live Computerized Physician Order Entry (CPOE) initiative at the hospital, a project for which Dr. Wilson serves as officer.
Dr. Wilson’s focus on forging relationships between varying medical staff can be traced back to her early exposure to medicine. She grew up in southern Indiana with an RN mother and medical photographer father. She says that combination made for “interesting dinner conversations” as a child.
“We have to keep asking ourselves, ‘How can we do this better?'” Dr. Wilson says. “It’s all about the patient.”
Innovators Descend on Annual Pediatric HM Conference
More than 400 people attended the Pediatric Hospital Medicine annual conference July 22-25 in Minneapolis. The annual meeting is the premier networking and educational event for pediatric hospitalists and is sponsored by the American Academy of Pediatrics (AAP), SHM, and the Academic Pediatric Association (APA).
Innovation and improvement were popular topics throughout the conference. Keynote speaker George Buckley, CEO of manufacturing and technology conglomerate 3M, spoke about inspiring innovation, and a large percentage of the sessions and posters had quality-improvement (QI) themes. Experts from Cincinnati Children’s Hospital, led by Steve Muething, MD, assistant vice president of patient safety, and Shannon Phillips, MD, MPH, Cleveland Clinic’s patient safety officer, guided several popular sessions on QI.
A major innovation announced at the conference was the planned launch of a journal of pediatric hospital medicine, which will be sponsored by the AAP. (Update 09.14.2010--The journal has yet to officially announce an editor).
Research presentations have continued to increase in this young field, and the meeting was full of poster and platform presentations in the areas of clinical research, QI, educational research, and health services research. Vineeta Mittal, MD, of the University of Texas Southwestern and Children’s Medical Center in Dallas presented research on family-centered rounds, which was recently published in Pediatrics and picked up by the National Association of Children’s Hospitals (NACHRI) for dissemination.1 Patrick Brady, MD, of Cincinnati Children’s Hospital presented his research on short- versus long-course IV therapy for pediatric urinary tract infections, also published in Pediatrics.2
Other buzzed-about sessions included Vanderbilt University pediatric hospitalist Dr. Paul Hain’s ambitious attempt to create a PHM performance dashboard, and a case of “situational” epilepsy presented by Dr. Lisa Zaoutis of CHOP.
As in years past, the hottest ticket was for the luncheon presentation of the “Top Articles in Pediatric Hospital Medicine,” paneled this year by Drs. John Pope, Kris Rehm, and Brian Alverson. Raj Srivastava, MD, of Primary Children’s Medical Center in Salt Lake City and chairperson of the Pediatric Research in Inpatient Settings network, announced that the network had been awarded major grant funding.
Dan Rauch, MD, chair of the AAP’s Section on Hospital Medicine, dropped the biggest bombshell of all: He announced that the American Board of Pediatrics will support the development of pediatric HM as a full-fledged subspecialty in the near future. TH
Dr. Ralston is associate professor of pediatrics and chief of the division of inpatient pediatrics at the University of Texas Health Science Center in San Antonio, and medical director of inpatient services at Christus Santa Rosa Children’s Hospital.
References
- Mittal VS, Sigrest T, Ottolini MC, et al. Family-centered rounds on pediatric wards: a PRIS network survey of U.S. and Canadian hospitalists. Pediatrics. 2010;126(1):37-43.
- Brady PW, Conway PH, Goudie A. Length of intravenous antibiotic therapy and treatment failure in infants with urinary track infections. Pediatrics. 2010;126(2):196-203.
More than 400 people attended the Pediatric Hospital Medicine annual conference July 22-25 in Minneapolis. The annual meeting is the premier networking and educational event for pediatric hospitalists and is sponsored by the American Academy of Pediatrics (AAP), SHM, and the Academic Pediatric Association (APA).
Innovation and improvement were popular topics throughout the conference. Keynote speaker George Buckley, CEO of manufacturing and technology conglomerate 3M, spoke about inspiring innovation, and a large percentage of the sessions and posters had quality-improvement (QI) themes. Experts from Cincinnati Children’s Hospital, led by Steve Muething, MD, assistant vice president of patient safety, and Shannon Phillips, MD, MPH, Cleveland Clinic’s patient safety officer, guided several popular sessions on QI.
A major innovation announced at the conference was the planned launch of a journal of pediatric hospital medicine, which will be sponsored by the AAP. (Update 09.14.2010--The journal has yet to officially announce an editor).
Research presentations have continued to increase in this young field, and the meeting was full of poster and platform presentations in the areas of clinical research, QI, educational research, and health services research. Vineeta Mittal, MD, of the University of Texas Southwestern and Children’s Medical Center in Dallas presented research on family-centered rounds, which was recently published in Pediatrics and picked up by the National Association of Children’s Hospitals (NACHRI) for dissemination.1 Patrick Brady, MD, of Cincinnati Children’s Hospital presented his research on short- versus long-course IV therapy for pediatric urinary tract infections, also published in Pediatrics.2
Other buzzed-about sessions included Vanderbilt University pediatric hospitalist Dr. Paul Hain’s ambitious attempt to create a PHM performance dashboard, and a case of “situational” epilepsy presented by Dr. Lisa Zaoutis of CHOP.
As in years past, the hottest ticket was for the luncheon presentation of the “Top Articles in Pediatric Hospital Medicine,” paneled this year by Drs. John Pope, Kris Rehm, and Brian Alverson. Raj Srivastava, MD, of Primary Children’s Medical Center in Salt Lake City and chairperson of the Pediatric Research in Inpatient Settings network, announced that the network had been awarded major grant funding.
Dan Rauch, MD, chair of the AAP’s Section on Hospital Medicine, dropped the biggest bombshell of all: He announced that the American Board of Pediatrics will support the development of pediatric HM as a full-fledged subspecialty in the near future. TH
Dr. Ralston is associate professor of pediatrics and chief of the division of inpatient pediatrics at the University of Texas Health Science Center in San Antonio, and medical director of inpatient services at Christus Santa Rosa Children’s Hospital.
References
- Mittal VS, Sigrest T, Ottolini MC, et al. Family-centered rounds on pediatric wards: a PRIS network survey of U.S. and Canadian hospitalists. Pediatrics. 2010;126(1):37-43.
- Brady PW, Conway PH, Goudie A. Length of intravenous antibiotic therapy and treatment failure in infants with urinary track infections. Pediatrics. 2010;126(2):196-203.
More than 400 people attended the Pediatric Hospital Medicine annual conference July 22-25 in Minneapolis. The annual meeting is the premier networking and educational event for pediatric hospitalists and is sponsored by the American Academy of Pediatrics (AAP), SHM, and the Academic Pediatric Association (APA).
Innovation and improvement were popular topics throughout the conference. Keynote speaker George Buckley, CEO of manufacturing and technology conglomerate 3M, spoke about inspiring innovation, and a large percentage of the sessions and posters had quality-improvement (QI) themes. Experts from Cincinnati Children’s Hospital, led by Steve Muething, MD, assistant vice president of patient safety, and Shannon Phillips, MD, MPH, Cleveland Clinic’s patient safety officer, guided several popular sessions on QI.
A major innovation announced at the conference was the planned launch of a journal of pediatric hospital medicine, which will be sponsored by the AAP. (Update 09.14.2010--The journal has yet to officially announce an editor).
Research presentations have continued to increase in this young field, and the meeting was full of poster and platform presentations in the areas of clinical research, QI, educational research, and health services research. Vineeta Mittal, MD, of the University of Texas Southwestern and Children’s Medical Center in Dallas presented research on family-centered rounds, which was recently published in Pediatrics and picked up by the National Association of Children’s Hospitals (NACHRI) for dissemination.1 Patrick Brady, MD, of Cincinnati Children’s Hospital presented his research on short- versus long-course IV therapy for pediatric urinary tract infections, also published in Pediatrics.2
Other buzzed-about sessions included Vanderbilt University pediatric hospitalist Dr. Paul Hain’s ambitious attempt to create a PHM performance dashboard, and a case of “situational” epilepsy presented by Dr. Lisa Zaoutis of CHOP.
As in years past, the hottest ticket was for the luncheon presentation of the “Top Articles in Pediatric Hospital Medicine,” paneled this year by Drs. John Pope, Kris Rehm, and Brian Alverson. Raj Srivastava, MD, of Primary Children’s Medical Center in Salt Lake City and chairperson of the Pediatric Research in Inpatient Settings network, announced that the network had been awarded major grant funding.
Dan Rauch, MD, chair of the AAP’s Section on Hospital Medicine, dropped the biggest bombshell of all: He announced that the American Board of Pediatrics will support the development of pediatric HM as a full-fledged subspecialty in the near future. TH
Dr. Ralston is associate professor of pediatrics and chief of the division of inpatient pediatrics at the University of Texas Health Science Center in San Antonio, and medical director of inpatient services at Christus Santa Rosa Children’s Hospital.
References
- Mittal VS, Sigrest T, Ottolini MC, et al. Family-centered rounds on pediatric wards: a PRIS network survey of U.S. and Canadian hospitalists. Pediatrics. 2010;126(1):37-43.
- Brady PW, Conway PH, Goudie A. Length of intravenous antibiotic therapy and treatment failure in infants with urinary track infections. Pediatrics. 2010;126(2):196-203.
Hospitalist/Intensivist Model Lowers Costs, Maintains Quality of Care
As the field of HM continues to mature, branch out, and is called upon to lead in the care of a larger cross-section of hospitalized patients, it is only natural that this includes the critically ill patient. Hospitalists already care for—and are the attending of record for—this patient population in most U.S. hospitals. It is my position that a technically proficient hospitalist service, which is facility-exclusive and offers 24/7 coverage, is able to offer the same quality of care as an intensivist group. An important feature of this model is the inclusion and “buy in” from community pulmonologists in order to provide backup and consultative assistance when warranted.
Our program at Westside Regional Medical Center in Plantation, Fla., has made great strides as we continue to integrate this model in the hospital. We are actively tracking ICU length of stay and throughput, incidence of ventilator-associated pneumonia (VAP), central-line infection rates, and ICU mortality.
I believe that a clinically competent and aggressive HM service is able to drive down costs and generate revenue by establishing clinically beneficial quality-improvement (QI) protocols; drive down ICU length of stay; provide effective and timely procedural services; and incur a lower cost burden (i.e., hospitalists cost less than intensivists). And I believe all of these benefits are available without sacrificing quality or patient care.
Leadership from medical staff and administration is imperative to establish the appropriate vision and drive toward hospitalist/intensivist implementation. Finding the right supporting physicians who bring excitement and energy is equally as important. Establishing expectations for skill sets, as well as the opportunity and mechanism by which these skill sets might be acquired and refined, is a must. The following technical skills should be required of hospitalist/intensivists:
- Ultrasound-guided central line insertion;
- PICC line insertion;
- Endotracheal intubation;
- Advanced airway management;
- Thoracostomy tube insertion;
- Arterial-line insertion;
- Transvenous pacing wire insertion;
- Lumbar puncture;
- Thoracentesis; and
- Paracentesis.
An important starting point is the identification of skill sets for each hospitalist. Once this information is ascertained, the next step is to understand what the credentialing requirements for the individual procedures are. This usually consists of a certain number of “logged” cases, which must be put forward for review by the medical staff leadership. Most physicians completing residency are required to keep a procedural log where cases are documented. Any deficiencies within the log can be supplemented by establishing a practice log where proctored cases are documented until the recommended number of cases are completed and put forward for credentialing.
Obtaining buy-in from the medical staff is important. They can serve as allies in many areas, specifically as proctors in the credentialing process. The key to successful interface is in awakening them to the beneficial impact a service such as this can have on patients and on the lifestyle of providers.
As an example, before our group started the hybrid model at Westside, the nursing staff would call anesthesia to evaluate patients for endotracheal intubation. This system took anesthesia away from its OR cases, causing delays and frustration. After a conversation, the anesthesia director realized the benefits that would come with assisting the hospitalists in becoming more proficient with intubations. This same scenario has been true in our experience with ED physicians, cardiothoracic surgeons (chest tubes), and so on.
Other resources for hospitalists include the National Procedure Institute, which offers CME credit and certification toward “Hospitalist Procedures.” Additionally, difficult airway or advanced airway courses provide certification.
Hospitalists have long been called on to provide emergency services for unstable patients via rapid response or codes. In many facilities hospitalists serve as the lead physicians in the management of critically ill patients. Our hospitalist model serves as a great launching pad for the development and evolution of this new breed of physician.
There exists no clinical evidence to assert inferiority between the care provided by an in-house, 24/7 hospitalist group with assistance from pulmonary medicine versus an intensivist group. It is my belief that if the appropriate infrastructure, fostered skill sets, pulmonologist partnership, and QI protocols are implemented, there will be no measurable difference in scope of care or outcomes.
The inpatient management of critically ill and unstable patients continues to be a significant and important subgroup of hospital patient populations. As patients continue to live longer with debilitating chronic diseases, the fallout from decompensation can be devastating. Many facilities have hospitalists leading the charge in the care of these patients. It is undeniable that the next evolution in HM will require a more proactive inpatient physician, with both the clinical and technical acumen to manage all patients across the hospital spectrum.
Ulises A. Perez, MD,
medical director, hospitalist division,
Westside Regional Medical Center, Plantation, Fla.,
Kendall Regional Medical Center, Miami
As the field of HM continues to mature, branch out, and is called upon to lead in the care of a larger cross-section of hospitalized patients, it is only natural that this includes the critically ill patient. Hospitalists already care for—and are the attending of record for—this patient population in most U.S. hospitals. It is my position that a technically proficient hospitalist service, which is facility-exclusive and offers 24/7 coverage, is able to offer the same quality of care as an intensivist group. An important feature of this model is the inclusion and “buy in” from community pulmonologists in order to provide backup and consultative assistance when warranted.
Our program at Westside Regional Medical Center in Plantation, Fla., has made great strides as we continue to integrate this model in the hospital. We are actively tracking ICU length of stay and throughput, incidence of ventilator-associated pneumonia (VAP), central-line infection rates, and ICU mortality.
I believe that a clinically competent and aggressive HM service is able to drive down costs and generate revenue by establishing clinically beneficial quality-improvement (QI) protocols; drive down ICU length of stay; provide effective and timely procedural services; and incur a lower cost burden (i.e., hospitalists cost less than intensivists). And I believe all of these benefits are available without sacrificing quality or patient care.
Leadership from medical staff and administration is imperative to establish the appropriate vision and drive toward hospitalist/intensivist implementation. Finding the right supporting physicians who bring excitement and energy is equally as important. Establishing expectations for skill sets, as well as the opportunity and mechanism by which these skill sets might be acquired and refined, is a must. The following technical skills should be required of hospitalist/intensivists:
- Ultrasound-guided central line insertion;
- PICC line insertion;
- Endotracheal intubation;
- Advanced airway management;
- Thoracostomy tube insertion;
- Arterial-line insertion;
- Transvenous pacing wire insertion;
- Lumbar puncture;
- Thoracentesis; and
- Paracentesis.
An important starting point is the identification of skill sets for each hospitalist. Once this information is ascertained, the next step is to understand what the credentialing requirements for the individual procedures are. This usually consists of a certain number of “logged” cases, which must be put forward for review by the medical staff leadership. Most physicians completing residency are required to keep a procedural log where cases are documented. Any deficiencies within the log can be supplemented by establishing a practice log where proctored cases are documented until the recommended number of cases are completed and put forward for credentialing.
Obtaining buy-in from the medical staff is important. They can serve as allies in many areas, specifically as proctors in the credentialing process. The key to successful interface is in awakening them to the beneficial impact a service such as this can have on patients and on the lifestyle of providers.
As an example, before our group started the hybrid model at Westside, the nursing staff would call anesthesia to evaluate patients for endotracheal intubation. This system took anesthesia away from its OR cases, causing delays and frustration. After a conversation, the anesthesia director realized the benefits that would come with assisting the hospitalists in becoming more proficient with intubations. This same scenario has been true in our experience with ED physicians, cardiothoracic surgeons (chest tubes), and so on.
Other resources for hospitalists include the National Procedure Institute, which offers CME credit and certification toward “Hospitalist Procedures.” Additionally, difficult airway or advanced airway courses provide certification.
Hospitalists have long been called on to provide emergency services for unstable patients via rapid response or codes. In many facilities hospitalists serve as the lead physicians in the management of critically ill patients. Our hospitalist model serves as a great launching pad for the development and evolution of this new breed of physician.
There exists no clinical evidence to assert inferiority between the care provided by an in-house, 24/7 hospitalist group with assistance from pulmonary medicine versus an intensivist group. It is my belief that if the appropriate infrastructure, fostered skill sets, pulmonologist partnership, and QI protocols are implemented, there will be no measurable difference in scope of care or outcomes.
The inpatient management of critically ill and unstable patients continues to be a significant and important subgroup of hospital patient populations. As patients continue to live longer with debilitating chronic diseases, the fallout from decompensation can be devastating. Many facilities have hospitalists leading the charge in the care of these patients. It is undeniable that the next evolution in HM will require a more proactive inpatient physician, with both the clinical and technical acumen to manage all patients across the hospital spectrum.
Ulises A. Perez, MD,
medical director, hospitalist division,
Westside Regional Medical Center, Plantation, Fla.,
Kendall Regional Medical Center, Miami
As the field of HM continues to mature, branch out, and is called upon to lead in the care of a larger cross-section of hospitalized patients, it is only natural that this includes the critically ill patient. Hospitalists already care for—and are the attending of record for—this patient population in most U.S. hospitals. It is my position that a technically proficient hospitalist service, which is facility-exclusive and offers 24/7 coverage, is able to offer the same quality of care as an intensivist group. An important feature of this model is the inclusion and “buy in” from community pulmonologists in order to provide backup and consultative assistance when warranted.
Our program at Westside Regional Medical Center in Plantation, Fla., has made great strides as we continue to integrate this model in the hospital. We are actively tracking ICU length of stay and throughput, incidence of ventilator-associated pneumonia (VAP), central-line infection rates, and ICU mortality.
I believe that a clinically competent and aggressive HM service is able to drive down costs and generate revenue by establishing clinically beneficial quality-improvement (QI) protocols; drive down ICU length of stay; provide effective and timely procedural services; and incur a lower cost burden (i.e., hospitalists cost less than intensivists). And I believe all of these benefits are available without sacrificing quality or patient care.
Leadership from medical staff and administration is imperative to establish the appropriate vision and drive toward hospitalist/intensivist implementation. Finding the right supporting physicians who bring excitement and energy is equally as important. Establishing expectations for skill sets, as well as the opportunity and mechanism by which these skill sets might be acquired and refined, is a must. The following technical skills should be required of hospitalist/intensivists:
- Ultrasound-guided central line insertion;
- PICC line insertion;
- Endotracheal intubation;
- Advanced airway management;
- Thoracostomy tube insertion;
- Arterial-line insertion;
- Transvenous pacing wire insertion;
- Lumbar puncture;
- Thoracentesis; and
- Paracentesis.
An important starting point is the identification of skill sets for each hospitalist. Once this information is ascertained, the next step is to understand what the credentialing requirements for the individual procedures are. This usually consists of a certain number of “logged” cases, which must be put forward for review by the medical staff leadership. Most physicians completing residency are required to keep a procedural log where cases are documented. Any deficiencies within the log can be supplemented by establishing a practice log where proctored cases are documented until the recommended number of cases are completed and put forward for credentialing.
Obtaining buy-in from the medical staff is important. They can serve as allies in many areas, specifically as proctors in the credentialing process. The key to successful interface is in awakening them to the beneficial impact a service such as this can have on patients and on the lifestyle of providers.
As an example, before our group started the hybrid model at Westside, the nursing staff would call anesthesia to evaluate patients for endotracheal intubation. This system took anesthesia away from its OR cases, causing delays and frustration. After a conversation, the anesthesia director realized the benefits that would come with assisting the hospitalists in becoming more proficient with intubations. This same scenario has been true in our experience with ED physicians, cardiothoracic surgeons (chest tubes), and so on.
Other resources for hospitalists include the National Procedure Institute, which offers CME credit and certification toward “Hospitalist Procedures.” Additionally, difficult airway or advanced airway courses provide certification.
Hospitalists have long been called on to provide emergency services for unstable patients via rapid response or codes. In many facilities hospitalists serve as the lead physicians in the management of critically ill patients. Our hospitalist model serves as a great launching pad for the development and evolution of this new breed of physician.
There exists no clinical evidence to assert inferiority between the care provided by an in-house, 24/7 hospitalist group with assistance from pulmonary medicine versus an intensivist group. It is my belief that if the appropriate infrastructure, fostered skill sets, pulmonologist partnership, and QI protocols are implemented, there will be no measurable difference in scope of care or outcomes.
The inpatient management of critically ill and unstable patients continues to be a significant and important subgroup of hospital patient populations. As patients continue to live longer with debilitating chronic diseases, the fallout from decompensation can be devastating. Many facilities have hospitalists leading the charge in the care of these patients. It is undeniable that the next evolution in HM will require a more proactive inpatient physician, with both the clinical and technical acumen to manage all patients across the hospital spectrum.
Ulises A. Perez, MD,
medical director, hospitalist division,
Westside Regional Medical Center, Plantation, Fla.,
Kendall Regional Medical Center, Miami
SHM+MGMA = Better Survey
As HM continues to grow, the need for clear and accurate data about the specialty will only become more intense. Hospitalists and HM group leaders use survey information to better understand how they compare to other practices across the country, in terms of size and practice characteristics, as well as compensation and productivity.
Increasingly, healthcare executives are turning to survey data—either independently or via their hospitalist group leaders—to get a grasp on the best practices in the industry.
That’s why SHM teamed up with the Medical Group Management Association (MGMA), the industry leader for professional administrators and leaders of medical group practices, to research and develop the State of Hospital Medicine: 2010 Report Based on 2009 Data.
Previously, SHM created and fielded a biannual survey, then analyzed the results independently.

—Leslie A. Flores, MHA, SHM senior advisor for practice management
“Our partnership with MGMA expands our survey population, delivers more information, and brings MGMA’s 90 years of industry credibility in the medical practice management field,” says Leslie A. Flores, MHA, SHM senior advisor for practice management. “The 2010 survey gives hospitalists and hospital administrators an unprecedented snapshot of the state of hospital medicine.”
The 2010 report will be available this month in the “Practice Resources” section of the SHM website (www.hospitalmedicine.org).
The print version will be available to SHM members for $125; for $175, members receive both the print version and the report on CD-ROM.
“This is a first-ever opportunity for hospitalists, group leaders, and healthcare executives to get the clearest picture possible of a rapidly changing industry,” Flores says. TH
Brendon Shank is a freelance writer based in Philadelphia.
SHM Adopts Strict Code for Industry Relations
A long with nearly 20 other organizations, SHM has adopted the Code for Industry Relations (http://cmss.org/) established by the Council on Medical Specialty Societies (CMSS). In an era of digital communication, SHM has created a Web area (www.hospital medicine.org/industry) to continuously update its policies toward industry, display its current partnerships, and disclose the potential conflicts of interest of its board or directors, editors, and CEO.
The message from SHM leadership: SHM is committed to being a leader in an era of transparency and disclosure.
Transparency serves an important role for medical specialty societies, says Norman B. Kahn Jr., MD, executive vice president and CEO of CMSS. The code developed by CMSS and adopted by SHM “assures in interactions with industry that the patients’ needs come first,” Dr. Kahn says. “The bottom line is that this is all about protecting the independence of societies from industry without abrogating the relationship.”
From its early days, SHM has been aware of the need to balance the responsibilities of speaking for HM and the need to disclose any potential conflicts of interests. In 2000, SHM developed its Principles of Organizational Relationships (www.hospitalmedicine.org/OrgRelationships), which have guided the society’s efforts.
The principles call for a clear, bright line that is a barrier between the support of a partner and SHM’s control of content. Among other activities, SHM has applied those principles to meetings and educational initiatives, quality-improvement (QI) projects, and publications.
The principles also were the foundation for the tough conflict-of-interest policies (www.hospital medicine.org/interestpolicies) the board approved in 2005.
Over the last decade, as HM has grown, national hospitalist leaders have become the experts on a wide range of topics and are asked to speak, write, or advise government agencies, foundations, and industry.
As SHM has developed its resources to help hospitalists improve glycemic control, reduce unnecessary DVTs and PEs, and improve the transitions of care, SHM has engaged in partnerships with government agencies, foundations, and industry as well.
“SHM’s leadership recognizes that it has a fiduciary responsibility to its members—a responsibility to provide expert direction, and necessary resources, to enable the hospitalist to ensure the best possible care of his or her patients, and to advance the quality of the hospital system,” says SHM President Jeff Wiese, MD, SFHM. “But SHM cannot do this alone, and when external partnerships are established, it is the organization’s responsibility to enter into these partnerships judiciously, and to be fully transparent to the membership with respect to the arrangements of these partnerships.
“We are confident that no other organization has a more robust disclosure policy than SHM.”
Today’s Nominations, Tomorrow’s Leaders
HM leaders aren’t born that way—they’re nominated.
SHM is accepting nominations for its board election; new members will take office in May at HM11 in Dallas.
The nomination deadline is Oct. 31. Online ballots will be available to all SHM members in late 2010. The results of the election will be announced online in early 2011.
Nominees must be SHM members in good standing. SHM members may nominate themselves or be nominated by another SHM member. Nominations must include a letter of nomination, a one-page CV, and a recent photo.
The nomination committee considers candidates based on length of SHM membership, activity as a hospitalist and SHM member, the prominence of the candidate within the specialty, and a number of other factors.
Board members serve a three-year term and normally serve on one or more committees.
“Participating in SHM’s leadership is one of the best ways to help guide the future of hospital medicine,” says Larry Wellikson, MD, SFHM, CEO of SHM. “That begins by submitting a board nomination to SHM this year.”
Chapter Updates
Chicago
The Chicago chapter met May 19 at Sullivan’s Steakhouse. Twenty-five hospitalists from the Chicago area, including hospitalists at Loyola Hospital, Lutheran General Hospital, Illinois Masonic Medical Center, Trinity Hospital, Silver Cross, and Evanston Hospital, as well as hospitalist groups like Cogent and Vista, attended the meeting.
Dr. Robin Ross of Season Hospice and Palliative Care, which sponsored the event, presented clinical pearls for end-of-life care for the busy hospitalist. The meeting also featured a town-hall discussion, with topics relevant to everyday hospitalist practice—coding, consultations, and the use of observation units. Notification of chapter elections were summated to all chapter members in July and August.
Harrisburg/South Central Pennsylvania
Thirty hospitalists representing six HM programs attended the Harrisburg/South Central Pennsylvania chapter of SHM June 9 at Passage to India in Harrisburg.
Eric Kupersmith, MD, SFHM, division head of the hospitalist program at Cooper University Hospital in Camden, N.J., led an open discussion regarding “Transition of Care and the Hospitalist’s Role.” Chapter members had the opportunity to discuss how each individual program actively seeks to decrease readmission rates. Discharge-planning specifics generated group discussion, and a handful of hospitalists offered testimonials about what is working in their practices.
The meeting was sponsored by Merck.
A BOOST for all seasons: Discharge improvement resources now available year round
When Project BOOST (Better Outcomes for Older Adults through Safer Transitions), SHM’s groundbreaking program to reduce readmissions, first began in 2008, hospital sites applied to participate in a yearlong program of one-on-one mentorships, regular sessions to share best practices, and a resource toolkit.
Since then, Project BOOST has grown and evolved. Some BOOST iterations now include third parties, such as the University of Michigan, Blue Cross/Blue Shield of Michigan, and the California HealthCare Foundation. SHM also recently introduced a nationwide, tuition-based version of the BOOST initiative.
Now, SHM is announcing that new resources are available to all hospitals, regardless of their participation in Project BOOST, all year. Some resources were previously available only to Project BOOST participants; others are brand-new materials available to any hospital or hospitalist trying to reduce unplanned readmissions to their hospital.
“No matter where you are in the hospital, you have an opportunity to improve discharge,” says Tina Budnitz, MPH, senior advisor for quality improvement. “The response to Project BOOST has been overwhelmingly positive. That’s why we’re so excited to make these new materials available to anyone responsible for lowering readmissions.”
Individuals can download the Project BOOST implementation course, a new training program specifically designed to help nurses use the proven “teachback” method, and the Project BOOST patient PASS form.
Budnitz and Project BOOST organizers also plan to launch supplemental products for self-implementers, including access to Project BOOST e-mail listservs, data centers, and webinars.
Prepare Now for Flu Season
With most of the country still enjoying warm weather, it’s easy to forget that flu season is right around the corner. Hospitals can either be part of the solution—or part of the problem.
Compared to friends and family outside of the hospital, patients in the hospital are especially at risk. The chances of contracting the flu are higher, given decreased immune responses and increased proximity to caregivers and other potentially infected patients. Plus, the impact of the flu on healthcare providers can be significantly more severe.
By now, most hospitals have protocols for preparing for flu season and isolating infected patients, says Danielle Scheurer, MD, MSc, SFHM, assistant professor of medicine at Harvard Medical School in Boston and director of general medical service at Brigham and Women’s Hospitalist Service. Hospitalists play a special role within those protocols, she adds.
“Hospitalists can be instrumental in preventing the spread of flu within the hospital by having a low threshold for diagnostic testing of patients, immediate isolation of those patients, and strict adherence to infection-control measures in those with suspected influenza,” Dr. Scheurer says.
Dr. Scheurer also emphasizes the fact that flu prevention doesn’t end with better clinical practices. Patient education is key.
“Hospitalists can be vital in educating patients about how to avoid symptomatic contacts,” she says, “and how to advocate for themselves in insisting that all care providers use strict handwashing protocols to avoid transmitting influenza among patients.” TH
As HM continues to grow, the need for clear and accurate data about the specialty will only become more intense. Hospitalists and HM group leaders use survey information to better understand how they compare to other practices across the country, in terms of size and practice characteristics, as well as compensation and productivity.
Increasingly, healthcare executives are turning to survey data—either independently or via their hospitalist group leaders—to get a grasp on the best practices in the industry.
That’s why SHM teamed up with the Medical Group Management Association (MGMA), the industry leader for professional administrators and leaders of medical group practices, to research and develop the State of Hospital Medicine: 2010 Report Based on 2009 Data.
Previously, SHM created and fielded a biannual survey, then analyzed the results independently.

—Leslie A. Flores, MHA, SHM senior advisor for practice management
“Our partnership with MGMA expands our survey population, delivers more information, and brings MGMA’s 90 years of industry credibility in the medical practice management field,” says Leslie A. Flores, MHA, SHM senior advisor for practice management. “The 2010 survey gives hospitalists and hospital administrators an unprecedented snapshot of the state of hospital medicine.”
The 2010 report will be available this month in the “Practice Resources” section of the SHM website (www.hospitalmedicine.org).
The print version will be available to SHM members for $125; for $175, members receive both the print version and the report on CD-ROM.
“This is a first-ever opportunity for hospitalists, group leaders, and healthcare executives to get the clearest picture possible of a rapidly changing industry,” Flores says. TH
Brendon Shank is a freelance writer based in Philadelphia.
SHM Adopts Strict Code for Industry Relations
A long with nearly 20 other organizations, SHM has adopted the Code for Industry Relations (http://cmss.org/) established by the Council on Medical Specialty Societies (CMSS). In an era of digital communication, SHM has created a Web area (www.hospital medicine.org/industry) to continuously update its policies toward industry, display its current partnerships, and disclose the potential conflicts of interest of its board or directors, editors, and CEO.
The message from SHM leadership: SHM is committed to being a leader in an era of transparency and disclosure.
Transparency serves an important role for medical specialty societies, says Norman B. Kahn Jr., MD, executive vice president and CEO of CMSS. The code developed by CMSS and adopted by SHM “assures in interactions with industry that the patients’ needs come first,” Dr. Kahn says. “The bottom line is that this is all about protecting the independence of societies from industry without abrogating the relationship.”
From its early days, SHM has been aware of the need to balance the responsibilities of speaking for HM and the need to disclose any potential conflicts of interests. In 2000, SHM developed its Principles of Organizational Relationships (www.hospitalmedicine.org/OrgRelationships), which have guided the society’s efforts.
The principles call for a clear, bright line that is a barrier between the support of a partner and SHM’s control of content. Among other activities, SHM has applied those principles to meetings and educational initiatives, quality-improvement (QI) projects, and publications.
The principles also were the foundation for the tough conflict-of-interest policies (www.hospital medicine.org/interestpolicies) the board approved in 2005.
Over the last decade, as HM has grown, national hospitalist leaders have become the experts on a wide range of topics and are asked to speak, write, or advise government agencies, foundations, and industry.
As SHM has developed its resources to help hospitalists improve glycemic control, reduce unnecessary DVTs and PEs, and improve the transitions of care, SHM has engaged in partnerships with government agencies, foundations, and industry as well.
“SHM’s leadership recognizes that it has a fiduciary responsibility to its members—a responsibility to provide expert direction, and necessary resources, to enable the hospitalist to ensure the best possible care of his or her patients, and to advance the quality of the hospital system,” says SHM President Jeff Wiese, MD, SFHM. “But SHM cannot do this alone, and when external partnerships are established, it is the organization’s responsibility to enter into these partnerships judiciously, and to be fully transparent to the membership with respect to the arrangements of these partnerships.
“We are confident that no other organization has a more robust disclosure policy than SHM.”
Today’s Nominations, Tomorrow’s Leaders
HM leaders aren’t born that way—they’re nominated.
SHM is accepting nominations for its board election; new members will take office in May at HM11 in Dallas.
The nomination deadline is Oct. 31. Online ballots will be available to all SHM members in late 2010. The results of the election will be announced online in early 2011.
Nominees must be SHM members in good standing. SHM members may nominate themselves or be nominated by another SHM member. Nominations must include a letter of nomination, a one-page CV, and a recent photo.
The nomination committee considers candidates based on length of SHM membership, activity as a hospitalist and SHM member, the prominence of the candidate within the specialty, and a number of other factors.
Board members serve a three-year term and normally serve on one or more committees.
“Participating in SHM’s leadership is one of the best ways to help guide the future of hospital medicine,” says Larry Wellikson, MD, SFHM, CEO of SHM. “That begins by submitting a board nomination to SHM this year.”
Chapter Updates
Chicago
The Chicago chapter met May 19 at Sullivan’s Steakhouse. Twenty-five hospitalists from the Chicago area, including hospitalists at Loyola Hospital, Lutheran General Hospital, Illinois Masonic Medical Center, Trinity Hospital, Silver Cross, and Evanston Hospital, as well as hospitalist groups like Cogent and Vista, attended the meeting.
Dr. Robin Ross of Season Hospice and Palliative Care, which sponsored the event, presented clinical pearls for end-of-life care for the busy hospitalist. The meeting also featured a town-hall discussion, with topics relevant to everyday hospitalist practice—coding, consultations, and the use of observation units. Notification of chapter elections were summated to all chapter members in July and August.
Harrisburg/South Central Pennsylvania
Thirty hospitalists representing six HM programs attended the Harrisburg/South Central Pennsylvania chapter of SHM June 9 at Passage to India in Harrisburg.
Eric Kupersmith, MD, SFHM, division head of the hospitalist program at Cooper University Hospital in Camden, N.J., led an open discussion regarding “Transition of Care and the Hospitalist’s Role.” Chapter members had the opportunity to discuss how each individual program actively seeks to decrease readmission rates. Discharge-planning specifics generated group discussion, and a handful of hospitalists offered testimonials about what is working in their practices.
The meeting was sponsored by Merck.
A BOOST for all seasons: Discharge improvement resources now available year round
When Project BOOST (Better Outcomes for Older Adults through Safer Transitions), SHM’s groundbreaking program to reduce readmissions, first began in 2008, hospital sites applied to participate in a yearlong program of one-on-one mentorships, regular sessions to share best practices, and a resource toolkit.
Since then, Project BOOST has grown and evolved. Some BOOST iterations now include third parties, such as the University of Michigan, Blue Cross/Blue Shield of Michigan, and the California HealthCare Foundation. SHM also recently introduced a nationwide, tuition-based version of the BOOST initiative.
Now, SHM is announcing that new resources are available to all hospitals, regardless of their participation in Project BOOST, all year. Some resources were previously available only to Project BOOST participants; others are brand-new materials available to any hospital or hospitalist trying to reduce unplanned readmissions to their hospital.
“No matter where you are in the hospital, you have an opportunity to improve discharge,” says Tina Budnitz, MPH, senior advisor for quality improvement. “The response to Project BOOST has been overwhelmingly positive. That’s why we’re so excited to make these new materials available to anyone responsible for lowering readmissions.”
Individuals can download the Project BOOST implementation course, a new training program specifically designed to help nurses use the proven “teachback” method, and the Project BOOST patient PASS form.
Budnitz and Project BOOST organizers also plan to launch supplemental products for self-implementers, including access to Project BOOST e-mail listservs, data centers, and webinars.
Prepare Now for Flu Season
With most of the country still enjoying warm weather, it’s easy to forget that flu season is right around the corner. Hospitals can either be part of the solution—or part of the problem.
Compared to friends and family outside of the hospital, patients in the hospital are especially at risk. The chances of contracting the flu are higher, given decreased immune responses and increased proximity to caregivers and other potentially infected patients. Plus, the impact of the flu on healthcare providers can be significantly more severe.
By now, most hospitals have protocols for preparing for flu season and isolating infected patients, says Danielle Scheurer, MD, MSc, SFHM, assistant professor of medicine at Harvard Medical School in Boston and director of general medical service at Brigham and Women’s Hospitalist Service. Hospitalists play a special role within those protocols, she adds.
“Hospitalists can be instrumental in preventing the spread of flu within the hospital by having a low threshold for diagnostic testing of patients, immediate isolation of those patients, and strict adherence to infection-control measures in those with suspected influenza,” Dr. Scheurer says.
Dr. Scheurer also emphasizes the fact that flu prevention doesn’t end with better clinical practices. Patient education is key.
“Hospitalists can be vital in educating patients about how to avoid symptomatic contacts,” she says, “and how to advocate for themselves in insisting that all care providers use strict handwashing protocols to avoid transmitting influenza among patients.” TH
As HM continues to grow, the need for clear and accurate data about the specialty will only become more intense. Hospitalists and HM group leaders use survey information to better understand how they compare to other practices across the country, in terms of size and practice characteristics, as well as compensation and productivity.
Increasingly, healthcare executives are turning to survey data—either independently or via their hospitalist group leaders—to get a grasp on the best practices in the industry.
That’s why SHM teamed up with the Medical Group Management Association (MGMA), the industry leader for professional administrators and leaders of medical group practices, to research and develop the State of Hospital Medicine: 2010 Report Based on 2009 Data.
Previously, SHM created and fielded a biannual survey, then analyzed the results independently.

—Leslie A. Flores, MHA, SHM senior advisor for practice management
“Our partnership with MGMA expands our survey population, delivers more information, and brings MGMA’s 90 years of industry credibility in the medical practice management field,” says Leslie A. Flores, MHA, SHM senior advisor for practice management. “The 2010 survey gives hospitalists and hospital administrators an unprecedented snapshot of the state of hospital medicine.”
The 2010 report will be available this month in the “Practice Resources” section of the SHM website (www.hospitalmedicine.org).
The print version will be available to SHM members for $125; for $175, members receive both the print version and the report on CD-ROM.
“This is a first-ever opportunity for hospitalists, group leaders, and healthcare executives to get the clearest picture possible of a rapidly changing industry,” Flores says. TH
Brendon Shank is a freelance writer based in Philadelphia.
SHM Adopts Strict Code for Industry Relations
A long with nearly 20 other organizations, SHM has adopted the Code for Industry Relations (http://cmss.org/) established by the Council on Medical Specialty Societies (CMSS). In an era of digital communication, SHM has created a Web area (www.hospital medicine.org/industry) to continuously update its policies toward industry, display its current partnerships, and disclose the potential conflicts of interest of its board or directors, editors, and CEO.
The message from SHM leadership: SHM is committed to being a leader in an era of transparency and disclosure.
Transparency serves an important role for medical specialty societies, says Norman B. Kahn Jr., MD, executive vice president and CEO of CMSS. The code developed by CMSS and adopted by SHM “assures in interactions with industry that the patients’ needs come first,” Dr. Kahn says. “The bottom line is that this is all about protecting the independence of societies from industry without abrogating the relationship.”
From its early days, SHM has been aware of the need to balance the responsibilities of speaking for HM and the need to disclose any potential conflicts of interests. In 2000, SHM developed its Principles of Organizational Relationships (www.hospitalmedicine.org/OrgRelationships), which have guided the society’s efforts.
The principles call for a clear, bright line that is a barrier between the support of a partner and SHM’s control of content. Among other activities, SHM has applied those principles to meetings and educational initiatives, quality-improvement (QI) projects, and publications.
The principles also were the foundation for the tough conflict-of-interest policies (www.hospital medicine.org/interestpolicies) the board approved in 2005.
Over the last decade, as HM has grown, national hospitalist leaders have become the experts on a wide range of topics and are asked to speak, write, or advise government agencies, foundations, and industry.
As SHM has developed its resources to help hospitalists improve glycemic control, reduce unnecessary DVTs and PEs, and improve the transitions of care, SHM has engaged in partnerships with government agencies, foundations, and industry as well.
“SHM’s leadership recognizes that it has a fiduciary responsibility to its members—a responsibility to provide expert direction, and necessary resources, to enable the hospitalist to ensure the best possible care of his or her patients, and to advance the quality of the hospital system,” says SHM President Jeff Wiese, MD, SFHM. “But SHM cannot do this alone, and when external partnerships are established, it is the organization’s responsibility to enter into these partnerships judiciously, and to be fully transparent to the membership with respect to the arrangements of these partnerships.
“We are confident that no other organization has a more robust disclosure policy than SHM.”
Today’s Nominations, Tomorrow’s Leaders
HM leaders aren’t born that way—they’re nominated.
SHM is accepting nominations for its board election; new members will take office in May at HM11 in Dallas.
The nomination deadline is Oct. 31. Online ballots will be available to all SHM members in late 2010. The results of the election will be announced online in early 2011.
Nominees must be SHM members in good standing. SHM members may nominate themselves or be nominated by another SHM member. Nominations must include a letter of nomination, a one-page CV, and a recent photo.
The nomination committee considers candidates based on length of SHM membership, activity as a hospitalist and SHM member, the prominence of the candidate within the specialty, and a number of other factors.
Board members serve a three-year term and normally serve on one or more committees.
“Participating in SHM’s leadership is one of the best ways to help guide the future of hospital medicine,” says Larry Wellikson, MD, SFHM, CEO of SHM. “That begins by submitting a board nomination to SHM this year.”
Chapter Updates
Chicago
The Chicago chapter met May 19 at Sullivan’s Steakhouse. Twenty-five hospitalists from the Chicago area, including hospitalists at Loyola Hospital, Lutheran General Hospital, Illinois Masonic Medical Center, Trinity Hospital, Silver Cross, and Evanston Hospital, as well as hospitalist groups like Cogent and Vista, attended the meeting.
Dr. Robin Ross of Season Hospice and Palliative Care, which sponsored the event, presented clinical pearls for end-of-life care for the busy hospitalist. The meeting also featured a town-hall discussion, with topics relevant to everyday hospitalist practice—coding, consultations, and the use of observation units. Notification of chapter elections were summated to all chapter members in July and August.
Harrisburg/South Central Pennsylvania
Thirty hospitalists representing six HM programs attended the Harrisburg/South Central Pennsylvania chapter of SHM June 9 at Passage to India in Harrisburg.
Eric Kupersmith, MD, SFHM, division head of the hospitalist program at Cooper University Hospital in Camden, N.J., led an open discussion regarding “Transition of Care and the Hospitalist’s Role.” Chapter members had the opportunity to discuss how each individual program actively seeks to decrease readmission rates. Discharge-planning specifics generated group discussion, and a handful of hospitalists offered testimonials about what is working in their practices.
The meeting was sponsored by Merck.
A BOOST for all seasons: Discharge improvement resources now available year round
When Project BOOST (Better Outcomes for Older Adults through Safer Transitions), SHM’s groundbreaking program to reduce readmissions, first began in 2008, hospital sites applied to participate in a yearlong program of one-on-one mentorships, regular sessions to share best practices, and a resource toolkit.
Since then, Project BOOST has grown and evolved. Some BOOST iterations now include third parties, such as the University of Michigan, Blue Cross/Blue Shield of Michigan, and the California HealthCare Foundation. SHM also recently introduced a nationwide, tuition-based version of the BOOST initiative.
Now, SHM is announcing that new resources are available to all hospitals, regardless of their participation in Project BOOST, all year. Some resources were previously available only to Project BOOST participants; others are brand-new materials available to any hospital or hospitalist trying to reduce unplanned readmissions to their hospital.
“No matter where you are in the hospital, you have an opportunity to improve discharge,” says Tina Budnitz, MPH, senior advisor for quality improvement. “The response to Project BOOST has been overwhelmingly positive. That’s why we’re so excited to make these new materials available to anyone responsible for lowering readmissions.”
Individuals can download the Project BOOST implementation course, a new training program specifically designed to help nurses use the proven “teachback” method, and the Project BOOST patient PASS form.
Budnitz and Project BOOST organizers also plan to launch supplemental products for self-implementers, including access to Project BOOST e-mail listservs, data centers, and webinars.
Prepare Now for Flu Season
With most of the country still enjoying warm weather, it’s easy to forget that flu season is right around the corner. Hospitals can either be part of the solution—or part of the problem.
Compared to friends and family outside of the hospital, patients in the hospital are especially at risk. The chances of contracting the flu are higher, given decreased immune responses and increased proximity to caregivers and other potentially infected patients. Plus, the impact of the flu on healthcare providers can be significantly more severe.
By now, most hospitals have protocols for preparing for flu season and isolating infected patients, says Danielle Scheurer, MD, MSc, SFHM, assistant professor of medicine at Harvard Medical School in Boston and director of general medical service at Brigham and Women’s Hospitalist Service. Hospitalists play a special role within those protocols, she adds.
“Hospitalists can be instrumental in preventing the spread of flu within the hospital by having a low threshold for diagnostic testing of patients, immediate isolation of those patients, and strict adherence to infection-control measures in those with suspected influenza,” Dr. Scheurer says.
Dr. Scheurer also emphasizes the fact that flu prevention doesn’t end with better clinical practices. Patient education is key.
“Hospitalists can be vital in educating patients about how to avoid symptomatic contacts,” she says, “and how to advocate for themselves in insisting that all care providers use strict handwashing protocols to avoid transmitting influenza among patients.” TH