User login
First Fellow
Pediatric hospitalist Patrick Conway, MD, MSc, has become the first hospitalist accepted into The White House Fellows Program, a spokeswoman for the program says.
Dr. Conway had just reported to a new job at Cincinnati Children’s Hospital Medical Center when he found out he’d been accepted into the White House program. He’s serving with 14 other fellows, including one other medical professional, until August. He’ll return to Cincinnati with a deeper understanding of how physicians can affect federal health policy.
“It’s a once-in-a-lifetime learning experience, to see policy setting at the highest level of government,” Dr. Conway says.
Throughout the year, Dr. Conway, 33, will work in the office of Michael Leavitt, secretary of Health and Human Services (HHS), and with Carolyn Clancy, MD, director of the Agency for Healthcare Research and Quality (AHRQ).
“He’s an amazingly sharp physician who brings a great wealth of expertise, both because of the research he’s done but also in a clinical sense,” says Dr. Clancy, one of Dr. Conway’s mentors in the program. “He has a great grasp of policy.”
Dr. Conway has had to hit the ground running in his new role.
“We’re involving him directly in a number of very high-priority areas” including the improvement of healthcare quality and value, Dr. Clancy says. “He’ll be doing some research and a lot of trying to distill what we know from research to try and influence policy.”
The year will also bring another achievement: Dr. Conway will become a dad for the first time; his wife, Heather, is due March 30.
“I’m sort of peripherally involved,” he says ruefully. “I haven’t made it to any OB appointments.”
Dr. Conway, originally from College Station, Texas, received a master’s in health services research from the University of Pennsylvania. He earned his MD from Baylor College of Medicine and did his pediatrics residency at Children’s Hospital Boston, the primary pediatric teaching hospital for Harvard Medical School. He worked with healthcare clients as a management consultant at McKinsey & Company in Chicago, and he was a Robert Wood Johnson Clinical Scholar from 2005 to 2007. He’s done volunteer work in Nicaragua, the Dominican Republic, Bolivia, and Ghana. When he returns to Cincinnati Children’s, he’ll resume work as assistant professor in the Center for Health Care Quality and the Division of General Pediatrics.
On the Radar
While a Robert Wood Johnson scholar, Dr. Conway’s primary mentor was Ron Keren, MD, MPH, attending physician and director of the General Pediatrics Fellowship Program at the Children’s Hospital of Philadelphia. They worked together on a study of the use of prophylactic antibiotics in recurrent urinary tract infections in children, published last summer in the Journal of the American Medical Association (JAMA). The study found that, contrary to expectations, prophylactic antibiotics are not associated with a lower risk of recurrent infections and are associated with a higher risk of resistant infections.1
Dr. Keren was one of the people who recommended Dr. Conway on his application for the White House fellowship.
“Patrick … is intense,” says Dr. Keren, laughing. “But not in an obvious way. He’s very mild-mannered and polite and easy going on the outside. But when he starts a project, he is pretty aggressive as far as setting a very ambitious timeline, pushing things forward, and working as hard as possible. I just had to point him in the right direction every now and then, and he got the job done.”
Dr. Conway, lead author of the JAMA study, also contributed to a video news release and podcasts about its results. He impressed some of Dr. Clancy’s colleagues at the AHRQ with his ability to make the information easy to understand for people without a clinical medical background, Dr. Clancy says. That skill made him a good candidate for the fellowship.
“My goal is that he would get a lot of exposure to how healthcare policy is made, and that he would go back to Cincinnati Children’s understanding how physicians can play a more vital role in making sure that we get health policy right,” Dr. Clancy says. “To do that, you’ve got to be bilingual both in policy and in medicine—and there aren’t enough people who have that skill.”
And there’s something else to look forward to. “Supposedly we get the opportunity to ride mountain bikes with the president if we’re good enough,” Dr. Conway says. “I bike, but not extensively, so I’m working up to that. I need to make sure I don’t embarrass myself.”
Hospitalist Goals
Dr. Conway says one of his focuses is on the implementation of health information technology that better serves physicians and patients.
“We are interested in the alignment of incentive payments to physicians who use information technology to improve the care delivered to patients,” including electronic medical records and interoperability of data, he says. “In the last five years or so, there’s been increased interest in pay for performance, and now we’re moving toward thinking about how to structure these programs to pay for and enable quality improvement and the effective utilization of information technology.
“From a hospitalist perspective, I think one of the important issues is that many of these quality measures are directly related to the care delivered in hospitals by, primarily, hospitalists, so therefore it’s important for hospitalists to be involved in these processes.”
He’s also working with HHS on a value-driven healthcare initiative, intended “to bring transparency around quality and cost in healthcare and to enable quality improvement,” Dr. Conway says. “In this case, transparency for all stakeholders, so for consumers, for providers, for payers. We can criticize the process from the outside or we can get involved. We need to get involved.”
He has had a clearer idea than most about his career plan from the start, said Chris Landrigan, MD, MPH, research and fellowship director, inpatient pediatrics service, and assistant professor of pediatrics at Children’s Hospital Boston, where Dr. Conway interned. The two found they had a lot in common: Both were interested in the operations of the health system and in finding ways to improve it through clinical work, research, and policy, Dr. Landrigan says.
“Most of our work together has revolved around looking at the variations in care in hospital systems,” Dr. Landrigan says. “Some of my work has been in trying to set up a research network for pediatric hospitalists, and to try and improve the care of hospitalized children.”
Dr. Landrigan was surveying pediatric hospitalists about how they treat several common conditions, looking for variations, when Dr. Conway arrived at Children’s.
“He immediately said, ‘How do we know how this compares to what pediatricians do?’ ” Dr. Landrigan says. “I said, ‘Well, we don’t,’ So he set out on a project and asked a random sample of pediatricians around the country.”
Dr. Conway’s work revealed greater variations of care among pediatricians than among pediatric hospitalists—a finding Dr. Conway brought all the way to publication.2
HHS and AHRQ “have been very focused on issues that are near and dear to Patrick’s heart,” Dr. Landrigan says. “I think he’s got the experience and the intelligence to really make substantial contributions there. There’s no question in my mind that he’ll end up a leader in healthcare.”
One of those contributions has been to educate high-level decision-makers on a vital question.
“I have to explain every time I meet somebody what a hospitalist is,” Dr. Conway says. “We meet with everybody, from President Bush to Cabinet secretaries, and at all those meetings I say, ‘I practice generally as a pediatric hospitalist,’ at which point they say, ‘What’s a hospitalist?’ ”
That’s not likely to remain a problem as more hospitalists get involved at high levels.
“I would fully expect that we’re going to see hospitalists play a major role in assessing patient care and quality, and I hope that Patrick’s being named a White House fellow is a harbinger of that,” Dr. Clancy says. “We’re thrilled to have him here, and I hope to see more physicians taking a very serious interest in healthcare policy.” TH
Liz Tascio is a journalist based in New York.
References
- Conway PH, Cnann A, Zaoutis T, et al. Recurrent Urinary Tract Infections in Children: Risk Factors and Association With Prophylactic Antimicrobials. JAMA. 2007 July 11;298(2):179-186.
- Conway PH, Edwards S, Stucky ER, et al. Variations in management of common inpatient pediatric illnesses: hospitalists and community pediatricians. Pediatrics. 2006 Aug;118(2):441-447.
Pediatric hospitalist Patrick Conway, MD, MSc, has become the first hospitalist accepted into The White House Fellows Program, a spokeswoman for the program says.
Dr. Conway had just reported to a new job at Cincinnati Children’s Hospital Medical Center when he found out he’d been accepted into the White House program. He’s serving with 14 other fellows, including one other medical professional, until August. He’ll return to Cincinnati with a deeper understanding of how physicians can affect federal health policy.
“It’s a once-in-a-lifetime learning experience, to see policy setting at the highest level of government,” Dr. Conway says.
Throughout the year, Dr. Conway, 33, will work in the office of Michael Leavitt, secretary of Health and Human Services (HHS), and with Carolyn Clancy, MD, director of the Agency for Healthcare Research and Quality (AHRQ).
“He’s an amazingly sharp physician who brings a great wealth of expertise, both because of the research he’s done but also in a clinical sense,” says Dr. Clancy, one of Dr. Conway’s mentors in the program. “He has a great grasp of policy.”
Dr. Conway has had to hit the ground running in his new role.
“We’re involving him directly in a number of very high-priority areas” including the improvement of healthcare quality and value, Dr. Clancy says. “He’ll be doing some research and a lot of trying to distill what we know from research to try and influence policy.”
The year will also bring another achievement: Dr. Conway will become a dad for the first time; his wife, Heather, is due March 30.
“I’m sort of peripherally involved,” he says ruefully. “I haven’t made it to any OB appointments.”
Dr. Conway, originally from College Station, Texas, received a master’s in health services research from the University of Pennsylvania. He earned his MD from Baylor College of Medicine and did his pediatrics residency at Children’s Hospital Boston, the primary pediatric teaching hospital for Harvard Medical School. He worked with healthcare clients as a management consultant at McKinsey & Company in Chicago, and he was a Robert Wood Johnson Clinical Scholar from 2005 to 2007. He’s done volunteer work in Nicaragua, the Dominican Republic, Bolivia, and Ghana. When he returns to Cincinnati Children’s, he’ll resume work as assistant professor in the Center for Health Care Quality and the Division of General Pediatrics.
On the Radar
While a Robert Wood Johnson scholar, Dr. Conway’s primary mentor was Ron Keren, MD, MPH, attending physician and director of the General Pediatrics Fellowship Program at the Children’s Hospital of Philadelphia. They worked together on a study of the use of prophylactic antibiotics in recurrent urinary tract infections in children, published last summer in the Journal of the American Medical Association (JAMA). The study found that, contrary to expectations, prophylactic antibiotics are not associated with a lower risk of recurrent infections and are associated with a higher risk of resistant infections.1
Dr. Keren was one of the people who recommended Dr. Conway on his application for the White House fellowship.
“Patrick … is intense,” says Dr. Keren, laughing. “But not in an obvious way. He’s very mild-mannered and polite and easy going on the outside. But when he starts a project, he is pretty aggressive as far as setting a very ambitious timeline, pushing things forward, and working as hard as possible. I just had to point him in the right direction every now and then, and he got the job done.”
Dr. Conway, lead author of the JAMA study, also contributed to a video news release and podcasts about its results. He impressed some of Dr. Clancy’s colleagues at the AHRQ with his ability to make the information easy to understand for people without a clinical medical background, Dr. Clancy says. That skill made him a good candidate for the fellowship.
“My goal is that he would get a lot of exposure to how healthcare policy is made, and that he would go back to Cincinnati Children’s understanding how physicians can play a more vital role in making sure that we get health policy right,” Dr. Clancy says. “To do that, you’ve got to be bilingual both in policy and in medicine—and there aren’t enough people who have that skill.”
And there’s something else to look forward to. “Supposedly we get the opportunity to ride mountain bikes with the president if we’re good enough,” Dr. Conway says. “I bike, but not extensively, so I’m working up to that. I need to make sure I don’t embarrass myself.”
Hospitalist Goals
Dr. Conway says one of his focuses is on the implementation of health information technology that better serves physicians and patients.
“We are interested in the alignment of incentive payments to physicians who use information technology to improve the care delivered to patients,” including electronic medical records and interoperability of data, he says. “In the last five years or so, there’s been increased interest in pay for performance, and now we’re moving toward thinking about how to structure these programs to pay for and enable quality improvement and the effective utilization of information technology.
“From a hospitalist perspective, I think one of the important issues is that many of these quality measures are directly related to the care delivered in hospitals by, primarily, hospitalists, so therefore it’s important for hospitalists to be involved in these processes.”
He’s also working with HHS on a value-driven healthcare initiative, intended “to bring transparency around quality and cost in healthcare and to enable quality improvement,” Dr. Conway says. “In this case, transparency for all stakeholders, so for consumers, for providers, for payers. We can criticize the process from the outside or we can get involved. We need to get involved.”
He has had a clearer idea than most about his career plan from the start, said Chris Landrigan, MD, MPH, research and fellowship director, inpatient pediatrics service, and assistant professor of pediatrics at Children’s Hospital Boston, where Dr. Conway interned. The two found they had a lot in common: Both were interested in the operations of the health system and in finding ways to improve it through clinical work, research, and policy, Dr. Landrigan says.
“Most of our work together has revolved around looking at the variations in care in hospital systems,” Dr. Landrigan says. “Some of my work has been in trying to set up a research network for pediatric hospitalists, and to try and improve the care of hospitalized children.”
Dr. Landrigan was surveying pediatric hospitalists about how they treat several common conditions, looking for variations, when Dr. Conway arrived at Children’s.
“He immediately said, ‘How do we know how this compares to what pediatricians do?’ ” Dr. Landrigan says. “I said, ‘Well, we don’t,’ So he set out on a project and asked a random sample of pediatricians around the country.”
Dr. Conway’s work revealed greater variations of care among pediatricians than among pediatric hospitalists—a finding Dr. Conway brought all the way to publication.2
HHS and AHRQ “have been very focused on issues that are near and dear to Patrick’s heart,” Dr. Landrigan says. “I think he’s got the experience and the intelligence to really make substantial contributions there. There’s no question in my mind that he’ll end up a leader in healthcare.”
One of those contributions has been to educate high-level decision-makers on a vital question.
“I have to explain every time I meet somebody what a hospitalist is,” Dr. Conway says. “We meet with everybody, from President Bush to Cabinet secretaries, and at all those meetings I say, ‘I practice generally as a pediatric hospitalist,’ at which point they say, ‘What’s a hospitalist?’ ”
That’s not likely to remain a problem as more hospitalists get involved at high levels.
“I would fully expect that we’re going to see hospitalists play a major role in assessing patient care and quality, and I hope that Patrick’s being named a White House fellow is a harbinger of that,” Dr. Clancy says. “We’re thrilled to have him here, and I hope to see more physicians taking a very serious interest in healthcare policy.” TH
Liz Tascio is a journalist based in New York.
References
- Conway PH, Cnann A, Zaoutis T, et al. Recurrent Urinary Tract Infections in Children: Risk Factors and Association With Prophylactic Antimicrobials. JAMA. 2007 July 11;298(2):179-186.
- Conway PH, Edwards S, Stucky ER, et al. Variations in management of common inpatient pediatric illnesses: hospitalists and community pediatricians. Pediatrics. 2006 Aug;118(2):441-447.
Pediatric hospitalist Patrick Conway, MD, MSc, has become the first hospitalist accepted into The White House Fellows Program, a spokeswoman for the program says.
Dr. Conway had just reported to a new job at Cincinnati Children’s Hospital Medical Center when he found out he’d been accepted into the White House program. He’s serving with 14 other fellows, including one other medical professional, until August. He’ll return to Cincinnati with a deeper understanding of how physicians can affect federal health policy.
“It’s a once-in-a-lifetime learning experience, to see policy setting at the highest level of government,” Dr. Conway says.
Throughout the year, Dr. Conway, 33, will work in the office of Michael Leavitt, secretary of Health and Human Services (HHS), and with Carolyn Clancy, MD, director of the Agency for Healthcare Research and Quality (AHRQ).
“He’s an amazingly sharp physician who brings a great wealth of expertise, both because of the research he’s done but also in a clinical sense,” says Dr. Clancy, one of Dr. Conway’s mentors in the program. “He has a great grasp of policy.”
Dr. Conway has had to hit the ground running in his new role.
“We’re involving him directly in a number of very high-priority areas” including the improvement of healthcare quality and value, Dr. Clancy says. “He’ll be doing some research and a lot of trying to distill what we know from research to try and influence policy.”
The year will also bring another achievement: Dr. Conway will become a dad for the first time; his wife, Heather, is due March 30.
“I’m sort of peripherally involved,” he says ruefully. “I haven’t made it to any OB appointments.”
Dr. Conway, originally from College Station, Texas, received a master’s in health services research from the University of Pennsylvania. He earned his MD from Baylor College of Medicine and did his pediatrics residency at Children’s Hospital Boston, the primary pediatric teaching hospital for Harvard Medical School. He worked with healthcare clients as a management consultant at McKinsey & Company in Chicago, and he was a Robert Wood Johnson Clinical Scholar from 2005 to 2007. He’s done volunteer work in Nicaragua, the Dominican Republic, Bolivia, and Ghana. When he returns to Cincinnati Children’s, he’ll resume work as assistant professor in the Center for Health Care Quality and the Division of General Pediatrics.
On the Radar
While a Robert Wood Johnson scholar, Dr. Conway’s primary mentor was Ron Keren, MD, MPH, attending physician and director of the General Pediatrics Fellowship Program at the Children’s Hospital of Philadelphia. They worked together on a study of the use of prophylactic antibiotics in recurrent urinary tract infections in children, published last summer in the Journal of the American Medical Association (JAMA). The study found that, contrary to expectations, prophylactic antibiotics are not associated with a lower risk of recurrent infections and are associated with a higher risk of resistant infections.1
Dr. Keren was one of the people who recommended Dr. Conway on his application for the White House fellowship.
“Patrick … is intense,” says Dr. Keren, laughing. “But not in an obvious way. He’s very mild-mannered and polite and easy going on the outside. But when he starts a project, he is pretty aggressive as far as setting a very ambitious timeline, pushing things forward, and working as hard as possible. I just had to point him in the right direction every now and then, and he got the job done.”
Dr. Conway, lead author of the JAMA study, also contributed to a video news release and podcasts about its results. He impressed some of Dr. Clancy’s colleagues at the AHRQ with his ability to make the information easy to understand for people without a clinical medical background, Dr. Clancy says. That skill made him a good candidate for the fellowship.
“My goal is that he would get a lot of exposure to how healthcare policy is made, and that he would go back to Cincinnati Children’s understanding how physicians can play a more vital role in making sure that we get health policy right,” Dr. Clancy says. “To do that, you’ve got to be bilingual both in policy and in medicine—and there aren’t enough people who have that skill.”
And there’s something else to look forward to. “Supposedly we get the opportunity to ride mountain bikes with the president if we’re good enough,” Dr. Conway says. “I bike, but not extensively, so I’m working up to that. I need to make sure I don’t embarrass myself.”
Hospitalist Goals
Dr. Conway says one of his focuses is on the implementation of health information technology that better serves physicians and patients.
“We are interested in the alignment of incentive payments to physicians who use information technology to improve the care delivered to patients,” including electronic medical records and interoperability of data, he says. “In the last five years or so, there’s been increased interest in pay for performance, and now we’re moving toward thinking about how to structure these programs to pay for and enable quality improvement and the effective utilization of information technology.
“From a hospitalist perspective, I think one of the important issues is that many of these quality measures are directly related to the care delivered in hospitals by, primarily, hospitalists, so therefore it’s important for hospitalists to be involved in these processes.”
He’s also working with HHS on a value-driven healthcare initiative, intended “to bring transparency around quality and cost in healthcare and to enable quality improvement,” Dr. Conway says. “In this case, transparency for all stakeholders, so for consumers, for providers, for payers. We can criticize the process from the outside or we can get involved. We need to get involved.”
He has had a clearer idea than most about his career plan from the start, said Chris Landrigan, MD, MPH, research and fellowship director, inpatient pediatrics service, and assistant professor of pediatrics at Children’s Hospital Boston, where Dr. Conway interned. The two found they had a lot in common: Both were interested in the operations of the health system and in finding ways to improve it through clinical work, research, and policy, Dr. Landrigan says.
“Most of our work together has revolved around looking at the variations in care in hospital systems,” Dr. Landrigan says. “Some of my work has been in trying to set up a research network for pediatric hospitalists, and to try and improve the care of hospitalized children.”
Dr. Landrigan was surveying pediatric hospitalists about how they treat several common conditions, looking for variations, when Dr. Conway arrived at Children’s.
“He immediately said, ‘How do we know how this compares to what pediatricians do?’ ” Dr. Landrigan says. “I said, ‘Well, we don’t,’ So he set out on a project and asked a random sample of pediatricians around the country.”
Dr. Conway’s work revealed greater variations of care among pediatricians than among pediatric hospitalists—a finding Dr. Conway brought all the way to publication.2
HHS and AHRQ “have been very focused on issues that are near and dear to Patrick’s heart,” Dr. Landrigan says. “I think he’s got the experience and the intelligence to really make substantial contributions there. There’s no question in my mind that he’ll end up a leader in healthcare.”
One of those contributions has been to educate high-level decision-makers on a vital question.
“I have to explain every time I meet somebody what a hospitalist is,” Dr. Conway says. “We meet with everybody, from President Bush to Cabinet secretaries, and at all those meetings I say, ‘I practice generally as a pediatric hospitalist,’ at which point they say, ‘What’s a hospitalist?’ ”
That’s not likely to remain a problem as more hospitalists get involved at high levels.
“I would fully expect that we’re going to see hospitalists play a major role in assessing patient care and quality, and I hope that Patrick’s being named a White House fellow is a harbinger of that,” Dr. Clancy says. “We’re thrilled to have him here, and I hope to see more physicians taking a very serious interest in healthcare policy.” TH
Liz Tascio is a journalist based in New York.
References
- Conway PH, Cnann A, Zaoutis T, et al. Recurrent Urinary Tract Infections in Children: Risk Factors and Association With Prophylactic Antimicrobials. JAMA. 2007 July 11;298(2):179-186.
- Conway PH, Edwards S, Stucky ER, et al. Variations in management of common inpatient pediatric illnesses: hospitalists and community pediatricians. Pediatrics. 2006 Aug;118(2):441-447.
What pre-operative cardiac evaluation of patients undergoing intermediate-risk surgery is most appropriate?
Case
The orthopedic service asks you to evaluate a 76-year-old woman with a hip fracture. She has diabetes, hypertension, and hyperlipidemia but no known coronary artery disease (CAD). She says she can carry a bag of groceries up one flight of stairs without chest symptoms.
Her physical exam is significant only for a shortened, internally rotated right hip. Her blood pressure is 160/88 mm/hg, her pulse is 75 beats per minute, and her respiratory rate is 16 breaths a minute with an oxygen saturation of 95% on one liter. Her creatinine is 1.2 mg/dL, and her fasting glucose is 106 mg/dL. An electrocardiogram reveals normal sinus rhythm without evidence of prior myocardial infarction (MI).
Her medications are lisinopril, atorvastatin, aspirin, fluoxetine, and diazepam. She is scheduled for the operating room tomorrow. What is the best strategy to evaluate and minimize her perioperative cardiac risk, and does it include a beta-blocker?
Overview
There are many ways to identify patients at risk for perioperative cardiac complications—but few simple, safe, evidence-based means of mitigating risk.1
Over the past 10 years, the general approach has been that preoperative revascularization is beneficial in a limited number of clinical scenarios. Further, beta-blockers reduce risk in nearly all other high- and intermediate-risk patients. Unfortunately, routine perioperative administration of beta-blockers to intermediate-risk patients is not supported by trial evidence and may expose these patients to increased risk of adverse outcomes—including death and stroke.
Review of the Data
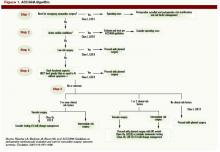
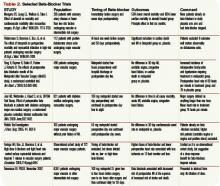
Intermediate-risk patients: Inter-mediate risk patients have recently been redefined as patients with a Revised Cardiac Risk Index (RCRI) score of two or one (See Table 1, p. 27).2,3 Older guidelines suggested noninvasive testing for such patients if they had poor functional capacity (less than four metabolic equivalents [METS]) and were undergoing intermediate-risk surgery, including orthopedic, peritoneal, and thoracic procedures.
Unfortunately, this situation is common, leading to frequent testing and unclear benefit to patients. Omission of a noninvasive evaluation in intermediate-risk orthopedic surgery patients is not associated with an increase in perioperative cardiac events.4 Most events occur in patients who did not meet criteria for preoperative testing.
The 2007 ACC/AHA Guidelines for Perioperative Evaluation and Care address this by recommending noninvasive testing only “if it will change management.” But they offer little guidance in unclear clinical situations, such as the urgent hip-fracture repair needed by our patient.
Preoperative revascularization: While it makes intuitive sense that preoperative revascularization of high-risk patients would decrease their risk of perioperative cardiac complications, evidence countering this idea is nearly definitive. In a study by McFalls, revascularization prior to major vascular surgery did not decrease the risk of perioperative MI or 30-day mortality; however, it delayed the surgical procedure, even in patients with high-risk noninvasive test results.5,6 It is generally accepted that if these high-risk patients can safely undergo major vascular surgery without revascularization, a lower-risk patient such as ours can do so at even lower risk.
In these trials, revascularization occurred in addition to medical management of coronary disease, including aspirin, statin, and—particularly in the study by Poldermans,6 where beta-blockers were started and titrated well before surgery—beta-blocker therapy.
Patients with active cardiac symptoms or signs or uncharacterized anginal symptoms should have elective surgery delayed. However, delay is rarely an option for the hospitalist, who is typically asked to address a patient’s risk shortly before urgent or emergent surgery. These difficult situations require one to weigh the cardiac risk of surgery in a patient who is not optimized versus the risk of delaying surgery to address the more urgent cardiac situation.
Timing of perioperative percutaneous intervention: For patients with coronary artery disease (CAD) or coronary lesions, the interval between percutaneous revascularization (via stent or percutaneous transluminal coronary angioplasty [PTCA]) and surgery affects rates of postoperative cardiac events.7
The recommended interval between stent placement and noncardiac surgery for patients receiving bare-metal and drug-eluting stents is six weeks and one year, respectively.8 Surgery within two weeks of stent placement can carry mortality rates as high as 40%, and this risk appears to decrease out to one year.9,10 If a new stent is in place, any potential benefit appears to be offset by the increased risk of in-stent thrombosis with subsequent MI and possible death. PTCA may not be a safe alternative, although some recommend using PTCA if the patient has unstable cardiac symptoms and needs urgent/emergent surgery.11
Perioperative discontinuation of dual antiplatelet agents (e.g., clopidogrel and aspirin) is common and appears to increase thrombosis risk. This presents a challenge when patients with recent stent placement present for urgent surgery. Minimizing the interruption of dual antiplatelet therapy is the most important intervention a hospitalist can perform. Interruption is associated with increased risk of stent thrombosis, MI, and death. If clopidogrel must be discontinued in the perioperative period, continuation of aspirin is recommended and intravenous glycoprotein 2b/3a inhibitors can be considered.12
Perioperative beta-blocker: Studies on the outcomes of perioperative beta blockade strongly suggested benefits initially. But a number of randomized trials in the past three years have not shown a positive effect.
In a landmark study published in 1996, Mangano showed that initiation of beta blockade just prior to surgery reduced perioperative MI and cardiac death in a mixed surgical population.13 Similar findings were seen with initiation of beta-blocker one month prior to vascular surgery.14 Additionally, higher doses of beta-blocker and lower heart rates in the perioperative period seem to be associated with decreased troponin release.15 Finally, perioperative beta blockade was associated with decreased mortality in high-risk patients (RCRI of three or greater), but higher mortality in lower-risk patients (e.g., RCRI of zero or one).16
More recent data reveal less benefit for perioperative beta blockade. Yang, et al., suggested that initiation of beta-blockers just prior to surgery did not decrease postoperative cardiac complications in vascular surgery patients.17 Similar results were found in a cohort of diabetic patients undergoing major surgery.18 A subsequent meta-analysis concluded that, in the aggregate, perioperative beta blockade was neither beneficial nor harmful.19
Further data have shown increased mortality with perioperative beta blockade in low-risk patients. Most recently, an abstract from the largest randomized controlled trial to date, the POISE study, suggested that preoperative beta blockade decreased MI and cardiac death, but increased the risk of stroke and produced higher overall mortality.20
It is challenging to reconcile this newer evidence with the previous data. While it seems intuitive that blunting the catecholamine response would minimize cardiac workload and therefore decrease perioperative infarcts, surgical patients are also at risk for poor pain control, sepsis, hypovolemia, and venous thromboembolism. Beta blockade can obscure their clinical manifestations, delaying diagnosis or complicating therapy. Inconsistencies among studies and published guidelines make them difficult to apply broadly, particularly with the intermediate-risk patient. Finally, perioperative beta blockade is poorly defined in terms of timing of initiation, target heart rate, and duration of postoperative use.
Until more definitive trial data are published, it seems most prudent to continue beta-blockers in patients already using them. Start them as far in advance of surgery as possible in patients with high-risk features (such as a positive stress test). After surgery, pay close attention to volume status, pain, signs of sepsis, or other noncardiac complications.
Back to the Case
As per the 2007 ACC/AHA guidelines, this patient with one clinical risk factor (diabetes) and good functional capacity can proceed to the operating room without further intervention. While it is likely a patient with diabetes and hyperlipidemia has some degree of CAD, including possible vulnerable plaques, the best medical evidence offers little to decrease her operative cardiac risk. Perioperative beta blockade is not indicated at her level of risk (RCRI of one) given the inconsistent benefits and possible harm to patients like this seen in trials to date.
If she were limited in terms of functional capacity (i.e., less than four METS), the 2007 ACC/AHA algorithm suggests preoperative noninvasive testing “if it would change management.”
How might a positive stress test change management in this case? Revascularization with stenting in close proximity to noncardiac surgery is not safe, and there appears to be no benefit to preoperative revascularization before high-risk vascular surgery. However, ischemia on preoperative testing is an indication for a beta-blocker. A brief delay in her surgery to allow dose titration and use of telemetry monitoring after surgery would increase the safety of beta-blockers after surgery. How long to continue beta-blockers is an open question, but at least 30 days would seem adequate, tapering rather than abruptly discontinuing the dose. TH
Dr. Carter is an assistant professor of medicine at the University of Colorado Denver in the Section of Hospital Medicine, where he directs the Medicine Consult Service. Dr. Auerbach is an associate professor of medicine in residence, associate director of the general medicine research fellowship, director of quality improvement for the UCSF Department of Medicine, and director of the surgical care Improvement program at UCSF. His research interests include perioperative medicine and quality improvement.
References
- Lee TH, Marcantonio ER, Mangione CM, et al. Derivation and prospective validation of a simple index for prediction of cardiac risk of major noncardiac surgery. Circulation. 1999;100(10):1043-1049.
- Eagle KA, Berger PB, Calkins H, et al. ACC/AHA Guideline update for perioperative cardiovascular evaluation for noncardiac surgery—executive summary. Circulation 2002;105:1257-1267.
- Fleischer LA, Beckman JA, Brown KA, et al. ACC/AHA Guidelines on Perioperative Cardiovascular Evaluation and Care for noncardiac surgery: executive summary. Circulation. 2007;116:1971-1996.
- Salerno SM, Carlson DW, Soh EK, et al. Impact of perioperative cardiac assessment guidelines on management of orthopedic surgery patients. Am J Med. 2007;120(2):185.
- McFalls EO, Ward HB, Moritz TE, et al. Coronary artery revascularization before elective major vascular surgery. N Engl J Med. 2004;351:2795-2804.
- Poldermans D, Schouten O, Vidakovic R, et al. A clinical randomized trial to evaluate the safety of a noninvasive approach in high-risk patients undergoing major vascular surgery: The DECREASE-V Pilot Study. J Am Coll Cardiol. 2007;49(17):1763-1769.
- Wilson, SH, Fasseas P, Orford JL, et al. Clinical outcomes of patients undergoing non-cardiac surgery in the two months following coronary stenting. J Am Coll Cardiol. 2003;42(2):234-240.
- Grines, CL, Bonow RO, Casey DE Jr, et al. Prevention of premature discontinuation of dual antiplatelet therapy in patients with coronary artery stents. Circulation. 2007; 115:813-818.
- Kaluza GL, Joseph J, Lee JR, et al. Catastrophic outcomes of noncardiac surgery soon after coronary stenting. Am J Coll Cardiol. 2000;35(5):1288-1294.
- Schouten O, Bax JJ, Damen J, et al. Coronary stent placement immediately before non cardiac surgery: a potential risk? Anesthesiology 106(5);2007:1067.
- Leibowitz D, Cohen M, Planer D, et al. Comparison of cardiovascular risk of noncardiac surgery following coronary angioplasty with versus without stenting. Am J Cardiol. 2006;97(8):1188-1191.
- Auerbach A, Goldman L. Assessing and reducing the cardiac risk of noncardiac surgery. Circulation 2006;113:1361-1376.
- Mangano DT, Layug EL, Wallace A, et al. Effect of atenolol on mortality and cardiovascular morbidity after noncardiac surgery. N Engl J Med. 1996;335(23):1713-1720.
- Poldermans D, Boersma E, Bax JJ, et al. The effect of bisoprolol on perioperative mortality and myocardial infarction in high-risk patients undergoing vascular surgery. N Engl J Med. 1999;341(24):1789-1794.
- Feringa HH, Bax JJ, Boersma E, et al. High dose b-blockers and tight heart rate control reduce myocardial ischemia and troponin T release in vascular surgery patients. Circulation. 2006;114(supp):I344.
- Lindenauer PK Pekow P, Wang K, et al. Perioperative beta-blocker therapy and mortality after major noncardiac surgery. N Engl J Med. 2005;353(4):349-361.
- Yang H, Raymer K, Butler R, et al. The effects of perioperative beta blockade: results of the Metoprolol after Vascular Surgery (MaVS) study, a randomized controlled trial. Am Heart J. 2006;152(5):983-990.
- Juul AB, Wetterslev J, Gluud C, et al. Effect of perioperative ß blockade in patients with diabetes undergoing major non-cardiac surgery: randomized placebo controlled, blinded multicentre trial. BMJ. 2006 June;332:1482.
- Devereaux PJ, Beattie WS, Choi PT, et al. How strong is the evidence for the use of perioperative ß blockers in noncardiac surgery? Systematic review and meta-analysis of randomized controlled trials. BMJ. 2005;331:313.
- Devereaux PJ. POISE Abstract. American Heart Association Annual Scientific Session, Orlando, Fla., November 2007.
Case
The orthopedic service asks you to evaluate a 76-year-old woman with a hip fracture. She has diabetes, hypertension, and hyperlipidemia but no known coronary artery disease (CAD). She says she can carry a bag of groceries up one flight of stairs without chest symptoms.
Her physical exam is significant only for a shortened, internally rotated right hip. Her blood pressure is 160/88 mm/hg, her pulse is 75 beats per minute, and her respiratory rate is 16 breaths a minute with an oxygen saturation of 95% on one liter. Her creatinine is 1.2 mg/dL, and her fasting glucose is 106 mg/dL. An electrocardiogram reveals normal sinus rhythm without evidence of prior myocardial infarction (MI).
Her medications are lisinopril, atorvastatin, aspirin, fluoxetine, and diazepam. She is scheduled for the operating room tomorrow. What is the best strategy to evaluate and minimize her perioperative cardiac risk, and does it include a beta-blocker?
Overview
There are many ways to identify patients at risk for perioperative cardiac complications—but few simple, safe, evidence-based means of mitigating risk.1
Over the past 10 years, the general approach has been that preoperative revascularization is beneficial in a limited number of clinical scenarios. Further, beta-blockers reduce risk in nearly all other high- and intermediate-risk patients. Unfortunately, routine perioperative administration of beta-blockers to intermediate-risk patients is not supported by trial evidence and may expose these patients to increased risk of adverse outcomes—including death and stroke.
Review of the Data
Intermediate-risk patients: Inter-mediate risk patients have recently been redefined as patients with a Revised Cardiac Risk Index (RCRI) score of two or one (See Table 1, p. 27).2,3 Older guidelines suggested noninvasive testing for such patients if they had poor functional capacity (less than four metabolic equivalents [METS]) and were undergoing intermediate-risk surgery, including orthopedic, peritoneal, and thoracic procedures.
Unfortunately, this situation is common, leading to frequent testing and unclear benefit to patients. Omission of a noninvasive evaluation in intermediate-risk orthopedic surgery patients is not associated with an increase in perioperative cardiac events.4 Most events occur in patients who did not meet criteria for preoperative testing.
The 2007 ACC/AHA Guidelines for Perioperative Evaluation and Care address this by recommending noninvasive testing only “if it will change management.” But they offer little guidance in unclear clinical situations, such as the urgent hip-fracture repair needed by our patient.
Preoperative revascularization: While it makes intuitive sense that preoperative revascularization of high-risk patients would decrease their risk of perioperative cardiac complications, evidence countering this idea is nearly definitive. In a study by McFalls, revascularization prior to major vascular surgery did not decrease the risk of perioperative MI or 30-day mortality; however, it delayed the surgical procedure, even in patients with high-risk noninvasive test results.5,6 It is generally accepted that if these high-risk patients can safely undergo major vascular surgery without revascularization, a lower-risk patient such as ours can do so at even lower risk.
In these trials, revascularization occurred in addition to medical management of coronary disease, including aspirin, statin, and—particularly in the study by Poldermans,6 where beta-blockers were started and titrated well before surgery—beta-blocker therapy.
Patients with active cardiac symptoms or signs or uncharacterized anginal symptoms should have elective surgery delayed. However, delay is rarely an option for the hospitalist, who is typically asked to address a patient’s risk shortly before urgent or emergent surgery. These difficult situations require one to weigh the cardiac risk of surgery in a patient who is not optimized versus the risk of delaying surgery to address the more urgent cardiac situation.
Timing of perioperative percutaneous intervention: For patients with coronary artery disease (CAD) or coronary lesions, the interval between percutaneous revascularization (via stent or percutaneous transluminal coronary angioplasty [PTCA]) and surgery affects rates of postoperative cardiac events.7
The recommended interval between stent placement and noncardiac surgery for patients receiving bare-metal and drug-eluting stents is six weeks and one year, respectively.8 Surgery within two weeks of stent placement can carry mortality rates as high as 40%, and this risk appears to decrease out to one year.9,10 If a new stent is in place, any potential benefit appears to be offset by the increased risk of in-stent thrombosis with subsequent MI and possible death. PTCA may not be a safe alternative, although some recommend using PTCA if the patient has unstable cardiac symptoms and needs urgent/emergent surgery.11
Perioperative discontinuation of dual antiplatelet agents (e.g., clopidogrel and aspirin) is common and appears to increase thrombosis risk. This presents a challenge when patients with recent stent placement present for urgent surgery. Minimizing the interruption of dual antiplatelet therapy is the most important intervention a hospitalist can perform. Interruption is associated with increased risk of stent thrombosis, MI, and death. If clopidogrel must be discontinued in the perioperative period, continuation of aspirin is recommended and intravenous glycoprotein 2b/3a inhibitors can be considered.12
Perioperative beta-blocker: Studies on the outcomes of perioperative beta blockade strongly suggested benefits initially. But a number of randomized trials in the past three years have not shown a positive effect.
In a landmark study published in 1996, Mangano showed that initiation of beta blockade just prior to surgery reduced perioperative MI and cardiac death in a mixed surgical population.13 Similar findings were seen with initiation of beta-blocker one month prior to vascular surgery.14 Additionally, higher doses of beta-blocker and lower heart rates in the perioperative period seem to be associated with decreased troponin release.15 Finally, perioperative beta blockade was associated with decreased mortality in high-risk patients (RCRI of three or greater), but higher mortality in lower-risk patients (e.g., RCRI of zero or one).16
More recent data reveal less benefit for perioperative beta blockade. Yang, et al., suggested that initiation of beta-blockers just prior to surgery did not decrease postoperative cardiac complications in vascular surgery patients.17 Similar results were found in a cohort of diabetic patients undergoing major surgery.18 A subsequent meta-analysis concluded that, in the aggregate, perioperative beta blockade was neither beneficial nor harmful.19
Further data have shown increased mortality with perioperative beta blockade in low-risk patients. Most recently, an abstract from the largest randomized controlled trial to date, the POISE study, suggested that preoperative beta blockade decreased MI and cardiac death, but increased the risk of stroke and produced higher overall mortality.20
It is challenging to reconcile this newer evidence with the previous data. While it seems intuitive that blunting the catecholamine response would minimize cardiac workload and therefore decrease perioperative infarcts, surgical patients are also at risk for poor pain control, sepsis, hypovolemia, and venous thromboembolism. Beta blockade can obscure their clinical manifestations, delaying diagnosis or complicating therapy. Inconsistencies among studies and published guidelines make them difficult to apply broadly, particularly with the intermediate-risk patient. Finally, perioperative beta blockade is poorly defined in terms of timing of initiation, target heart rate, and duration of postoperative use.
Until more definitive trial data are published, it seems most prudent to continue beta-blockers in patients already using them. Start them as far in advance of surgery as possible in patients with high-risk features (such as a positive stress test). After surgery, pay close attention to volume status, pain, signs of sepsis, or other noncardiac complications.
Back to the Case
As per the 2007 ACC/AHA guidelines, this patient with one clinical risk factor (diabetes) and good functional capacity can proceed to the operating room without further intervention. While it is likely a patient with diabetes and hyperlipidemia has some degree of CAD, including possible vulnerable plaques, the best medical evidence offers little to decrease her operative cardiac risk. Perioperative beta blockade is not indicated at her level of risk (RCRI of one) given the inconsistent benefits and possible harm to patients like this seen in trials to date.
If she were limited in terms of functional capacity (i.e., less than four METS), the 2007 ACC/AHA algorithm suggests preoperative noninvasive testing “if it would change management.”
How might a positive stress test change management in this case? Revascularization with stenting in close proximity to noncardiac surgery is not safe, and there appears to be no benefit to preoperative revascularization before high-risk vascular surgery. However, ischemia on preoperative testing is an indication for a beta-blocker. A brief delay in her surgery to allow dose titration and use of telemetry monitoring after surgery would increase the safety of beta-blockers after surgery. How long to continue beta-blockers is an open question, but at least 30 days would seem adequate, tapering rather than abruptly discontinuing the dose. TH
Dr. Carter is an assistant professor of medicine at the University of Colorado Denver in the Section of Hospital Medicine, where he directs the Medicine Consult Service. Dr. Auerbach is an associate professor of medicine in residence, associate director of the general medicine research fellowship, director of quality improvement for the UCSF Department of Medicine, and director of the surgical care Improvement program at UCSF. His research interests include perioperative medicine and quality improvement.
References
- Lee TH, Marcantonio ER, Mangione CM, et al. Derivation and prospective validation of a simple index for prediction of cardiac risk of major noncardiac surgery. Circulation. 1999;100(10):1043-1049.
- Eagle KA, Berger PB, Calkins H, et al. ACC/AHA Guideline update for perioperative cardiovascular evaluation for noncardiac surgery—executive summary. Circulation 2002;105:1257-1267.
- Fleischer LA, Beckman JA, Brown KA, et al. ACC/AHA Guidelines on Perioperative Cardiovascular Evaluation and Care for noncardiac surgery: executive summary. Circulation. 2007;116:1971-1996.
- Salerno SM, Carlson DW, Soh EK, et al. Impact of perioperative cardiac assessment guidelines on management of orthopedic surgery patients. Am J Med. 2007;120(2):185.
- McFalls EO, Ward HB, Moritz TE, et al. Coronary artery revascularization before elective major vascular surgery. N Engl J Med. 2004;351:2795-2804.
- Poldermans D, Schouten O, Vidakovic R, et al. A clinical randomized trial to evaluate the safety of a noninvasive approach in high-risk patients undergoing major vascular surgery: The DECREASE-V Pilot Study. J Am Coll Cardiol. 2007;49(17):1763-1769.
- Wilson, SH, Fasseas P, Orford JL, et al. Clinical outcomes of patients undergoing non-cardiac surgery in the two months following coronary stenting. J Am Coll Cardiol. 2003;42(2):234-240.
- Grines, CL, Bonow RO, Casey DE Jr, et al. Prevention of premature discontinuation of dual antiplatelet therapy in patients with coronary artery stents. Circulation. 2007; 115:813-818.
- Kaluza GL, Joseph J, Lee JR, et al. Catastrophic outcomes of noncardiac surgery soon after coronary stenting. Am J Coll Cardiol. 2000;35(5):1288-1294.
- Schouten O, Bax JJ, Damen J, et al. Coronary stent placement immediately before non cardiac surgery: a potential risk? Anesthesiology 106(5);2007:1067.
- Leibowitz D, Cohen M, Planer D, et al. Comparison of cardiovascular risk of noncardiac surgery following coronary angioplasty with versus without stenting. Am J Cardiol. 2006;97(8):1188-1191.
- Auerbach A, Goldman L. Assessing and reducing the cardiac risk of noncardiac surgery. Circulation 2006;113:1361-1376.
- Mangano DT, Layug EL, Wallace A, et al. Effect of atenolol on mortality and cardiovascular morbidity after noncardiac surgery. N Engl J Med. 1996;335(23):1713-1720.
- Poldermans D, Boersma E, Bax JJ, et al. The effect of bisoprolol on perioperative mortality and myocardial infarction in high-risk patients undergoing vascular surgery. N Engl J Med. 1999;341(24):1789-1794.
- Feringa HH, Bax JJ, Boersma E, et al. High dose b-blockers and tight heart rate control reduce myocardial ischemia and troponin T release in vascular surgery patients. Circulation. 2006;114(supp):I344.
- Lindenauer PK Pekow P, Wang K, et al. Perioperative beta-blocker therapy and mortality after major noncardiac surgery. N Engl J Med. 2005;353(4):349-361.
- Yang H, Raymer K, Butler R, et al. The effects of perioperative beta blockade: results of the Metoprolol after Vascular Surgery (MaVS) study, a randomized controlled trial. Am Heart J. 2006;152(5):983-990.
- Juul AB, Wetterslev J, Gluud C, et al. Effect of perioperative ß blockade in patients with diabetes undergoing major non-cardiac surgery: randomized placebo controlled, blinded multicentre trial. BMJ. 2006 June;332:1482.
- Devereaux PJ, Beattie WS, Choi PT, et al. How strong is the evidence for the use of perioperative ß blockers in noncardiac surgery? Systematic review and meta-analysis of randomized controlled trials. BMJ. 2005;331:313.
- Devereaux PJ. POISE Abstract. American Heart Association Annual Scientific Session, Orlando, Fla., November 2007.
Case
The orthopedic service asks you to evaluate a 76-year-old woman with a hip fracture. She has diabetes, hypertension, and hyperlipidemia but no known coronary artery disease (CAD). She says she can carry a bag of groceries up one flight of stairs without chest symptoms.
Her physical exam is significant only for a shortened, internally rotated right hip. Her blood pressure is 160/88 mm/hg, her pulse is 75 beats per minute, and her respiratory rate is 16 breaths a minute with an oxygen saturation of 95% on one liter. Her creatinine is 1.2 mg/dL, and her fasting glucose is 106 mg/dL. An electrocardiogram reveals normal sinus rhythm without evidence of prior myocardial infarction (MI).
Her medications are lisinopril, atorvastatin, aspirin, fluoxetine, and diazepam. She is scheduled for the operating room tomorrow. What is the best strategy to evaluate and minimize her perioperative cardiac risk, and does it include a beta-blocker?
Overview
There are many ways to identify patients at risk for perioperative cardiac complications—but few simple, safe, evidence-based means of mitigating risk.1
Over the past 10 years, the general approach has been that preoperative revascularization is beneficial in a limited number of clinical scenarios. Further, beta-blockers reduce risk in nearly all other high- and intermediate-risk patients. Unfortunately, routine perioperative administration of beta-blockers to intermediate-risk patients is not supported by trial evidence and may expose these patients to increased risk of adverse outcomes—including death and stroke.
Review of the Data
Intermediate-risk patients: Inter-mediate risk patients have recently been redefined as patients with a Revised Cardiac Risk Index (RCRI) score of two or one (See Table 1, p. 27).2,3 Older guidelines suggested noninvasive testing for such patients if they had poor functional capacity (less than four metabolic equivalents [METS]) and were undergoing intermediate-risk surgery, including orthopedic, peritoneal, and thoracic procedures.
Unfortunately, this situation is common, leading to frequent testing and unclear benefit to patients. Omission of a noninvasive evaluation in intermediate-risk orthopedic surgery patients is not associated with an increase in perioperative cardiac events.4 Most events occur in patients who did not meet criteria for preoperative testing.
The 2007 ACC/AHA Guidelines for Perioperative Evaluation and Care address this by recommending noninvasive testing only “if it will change management.” But they offer little guidance in unclear clinical situations, such as the urgent hip-fracture repair needed by our patient.
Preoperative revascularization: While it makes intuitive sense that preoperative revascularization of high-risk patients would decrease their risk of perioperative cardiac complications, evidence countering this idea is nearly definitive. In a study by McFalls, revascularization prior to major vascular surgery did not decrease the risk of perioperative MI or 30-day mortality; however, it delayed the surgical procedure, even in patients with high-risk noninvasive test results.5,6 It is generally accepted that if these high-risk patients can safely undergo major vascular surgery without revascularization, a lower-risk patient such as ours can do so at even lower risk.
In these trials, revascularization occurred in addition to medical management of coronary disease, including aspirin, statin, and—particularly in the study by Poldermans,6 where beta-blockers were started and titrated well before surgery—beta-blocker therapy.
Patients with active cardiac symptoms or signs or uncharacterized anginal symptoms should have elective surgery delayed. However, delay is rarely an option for the hospitalist, who is typically asked to address a patient’s risk shortly before urgent or emergent surgery. These difficult situations require one to weigh the cardiac risk of surgery in a patient who is not optimized versus the risk of delaying surgery to address the more urgent cardiac situation.
Timing of perioperative percutaneous intervention: For patients with coronary artery disease (CAD) or coronary lesions, the interval between percutaneous revascularization (via stent or percutaneous transluminal coronary angioplasty [PTCA]) and surgery affects rates of postoperative cardiac events.7
The recommended interval between stent placement and noncardiac surgery for patients receiving bare-metal and drug-eluting stents is six weeks and one year, respectively.8 Surgery within two weeks of stent placement can carry mortality rates as high as 40%, and this risk appears to decrease out to one year.9,10 If a new stent is in place, any potential benefit appears to be offset by the increased risk of in-stent thrombosis with subsequent MI and possible death. PTCA may not be a safe alternative, although some recommend using PTCA if the patient has unstable cardiac symptoms and needs urgent/emergent surgery.11
Perioperative discontinuation of dual antiplatelet agents (e.g., clopidogrel and aspirin) is common and appears to increase thrombosis risk. This presents a challenge when patients with recent stent placement present for urgent surgery. Minimizing the interruption of dual antiplatelet therapy is the most important intervention a hospitalist can perform. Interruption is associated with increased risk of stent thrombosis, MI, and death. If clopidogrel must be discontinued in the perioperative period, continuation of aspirin is recommended and intravenous glycoprotein 2b/3a inhibitors can be considered.12
Perioperative beta-blocker: Studies on the outcomes of perioperative beta blockade strongly suggested benefits initially. But a number of randomized trials in the past three years have not shown a positive effect.
In a landmark study published in 1996, Mangano showed that initiation of beta blockade just prior to surgery reduced perioperative MI and cardiac death in a mixed surgical population.13 Similar findings were seen with initiation of beta-blocker one month prior to vascular surgery.14 Additionally, higher doses of beta-blocker and lower heart rates in the perioperative period seem to be associated with decreased troponin release.15 Finally, perioperative beta blockade was associated with decreased mortality in high-risk patients (RCRI of three or greater), but higher mortality in lower-risk patients (e.g., RCRI of zero or one).16
More recent data reveal less benefit for perioperative beta blockade. Yang, et al., suggested that initiation of beta-blockers just prior to surgery did not decrease postoperative cardiac complications in vascular surgery patients.17 Similar results were found in a cohort of diabetic patients undergoing major surgery.18 A subsequent meta-analysis concluded that, in the aggregate, perioperative beta blockade was neither beneficial nor harmful.19
Further data have shown increased mortality with perioperative beta blockade in low-risk patients. Most recently, an abstract from the largest randomized controlled trial to date, the POISE study, suggested that preoperative beta blockade decreased MI and cardiac death, but increased the risk of stroke and produced higher overall mortality.20
It is challenging to reconcile this newer evidence with the previous data. While it seems intuitive that blunting the catecholamine response would minimize cardiac workload and therefore decrease perioperative infarcts, surgical patients are also at risk for poor pain control, sepsis, hypovolemia, and venous thromboembolism. Beta blockade can obscure their clinical manifestations, delaying diagnosis or complicating therapy. Inconsistencies among studies and published guidelines make them difficult to apply broadly, particularly with the intermediate-risk patient. Finally, perioperative beta blockade is poorly defined in terms of timing of initiation, target heart rate, and duration of postoperative use.
Until more definitive trial data are published, it seems most prudent to continue beta-blockers in patients already using them. Start them as far in advance of surgery as possible in patients with high-risk features (such as a positive stress test). After surgery, pay close attention to volume status, pain, signs of sepsis, or other noncardiac complications.
Back to the Case
As per the 2007 ACC/AHA guidelines, this patient with one clinical risk factor (diabetes) and good functional capacity can proceed to the operating room without further intervention. While it is likely a patient with diabetes and hyperlipidemia has some degree of CAD, including possible vulnerable plaques, the best medical evidence offers little to decrease her operative cardiac risk. Perioperative beta blockade is not indicated at her level of risk (RCRI of one) given the inconsistent benefits and possible harm to patients like this seen in trials to date.
If she were limited in terms of functional capacity (i.e., less than four METS), the 2007 ACC/AHA algorithm suggests preoperative noninvasive testing “if it would change management.”
How might a positive stress test change management in this case? Revascularization with stenting in close proximity to noncardiac surgery is not safe, and there appears to be no benefit to preoperative revascularization before high-risk vascular surgery. However, ischemia on preoperative testing is an indication for a beta-blocker. A brief delay in her surgery to allow dose titration and use of telemetry monitoring after surgery would increase the safety of beta-blockers after surgery. How long to continue beta-blockers is an open question, but at least 30 days would seem adequate, tapering rather than abruptly discontinuing the dose. TH
Dr. Carter is an assistant professor of medicine at the University of Colorado Denver in the Section of Hospital Medicine, where he directs the Medicine Consult Service. Dr. Auerbach is an associate professor of medicine in residence, associate director of the general medicine research fellowship, director of quality improvement for the UCSF Department of Medicine, and director of the surgical care Improvement program at UCSF. His research interests include perioperative medicine and quality improvement.
References
- Lee TH, Marcantonio ER, Mangione CM, et al. Derivation and prospective validation of a simple index for prediction of cardiac risk of major noncardiac surgery. Circulation. 1999;100(10):1043-1049.
- Eagle KA, Berger PB, Calkins H, et al. ACC/AHA Guideline update for perioperative cardiovascular evaluation for noncardiac surgery—executive summary. Circulation 2002;105:1257-1267.
- Fleischer LA, Beckman JA, Brown KA, et al. ACC/AHA Guidelines on Perioperative Cardiovascular Evaluation and Care for noncardiac surgery: executive summary. Circulation. 2007;116:1971-1996.
- Salerno SM, Carlson DW, Soh EK, et al. Impact of perioperative cardiac assessment guidelines on management of orthopedic surgery patients. Am J Med. 2007;120(2):185.
- McFalls EO, Ward HB, Moritz TE, et al. Coronary artery revascularization before elective major vascular surgery. N Engl J Med. 2004;351:2795-2804.
- Poldermans D, Schouten O, Vidakovic R, et al. A clinical randomized trial to evaluate the safety of a noninvasive approach in high-risk patients undergoing major vascular surgery: The DECREASE-V Pilot Study. J Am Coll Cardiol. 2007;49(17):1763-1769.
- Wilson, SH, Fasseas P, Orford JL, et al. Clinical outcomes of patients undergoing non-cardiac surgery in the two months following coronary stenting. J Am Coll Cardiol. 2003;42(2):234-240.
- Grines, CL, Bonow RO, Casey DE Jr, et al. Prevention of premature discontinuation of dual antiplatelet therapy in patients with coronary artery stents. Circulation. 2007; 115:813-818.
- Kaluza GL, Joseph J, Lee JR, et al. Catastrophic outcomes of noncardiac surgery soon after coronary stenting. Am J Coll Cardiol. 2000;35(5):1288-1294.
- Schouten O, Bax JJ, Damen J, et al. Coronary stent placement immediately before non cardiac surgery: a potential risk? Anesthesiology 106(5);2007:1067.
- Leibowitz D, Cohen M, Planer D, et al. Comparison of cardiovascular risk of noncardiac surgery following coronary angioplasty with versus without stenting. Am J Cardiol. 2006;97(8):1188-1191.
- Auerbach A, Goldman L. Assessing and reducing the cardiac risk of noncardiac surgery. Circulation 2006;113:1361-1376.
- Mangano DT, Layug EL, Wallace A, et al. Effect of atenolol on mortality and cardiovascular morbidity after noncardiac surgery. N Engl J Med. 1996;335(23):1713-1720.
- Poldermans D, Boersma E, Bax JJ, et al. The effect of bisoprolol on perioperative mortality and myocardial infarction in high-risk patients undergoing vascular surgery. N Engl J Med. 1999;341(24):1789-1794.
- Feringa HH, Bax JJ, Boersma E, et al. High dose b-blockers and tight heart rate control reduce myocardial ischemia and troponin T release in vascular surgery patients. Circulation. 2006;114(supp):I344.
- Lindenauer PK Pekow P, Wang K, et al. Perioperative beta-blocker therapy and mortality after major noncardiac surgery. N Engl J Med. 2005;353(4):349-361.
- Yang H, Raymer K, Butler R, et al. The effects of perioperative beta blockade: results of the Metoprolol after Vascular Surgery (MaVS) study, a randomized controlled trial. Am Heart J. 2006;152(5):983-990.
- Juul AB, Wetterslev J, Gluud C, et al. Effect of perioperative ß blockade in patients with diabetes undergoing major non-cardiac surgery: randomized placebo controlled, blinded multicentre trial. BMJ. 2006 June;332:1482.
- Devereaux PJ, Beattie WS, Choi PT, et al. How strong is the evidence for the use of perioperative ß blockers in noncardiac surgery? Systematic review and meta-analysis of randomized controlled trials. BMJ. 2005;331:313.
- Devereaux PJ. POISE Abstract. American Heart Association Annual Scientific Session, Orlando, Fla., November 2007.
Know Your Neurology
Although hospitalists may work alongside neurological specialists, they are increasingly on their own when responding to neurological emergencies, such as strokes, in hospitalized patients.
There are times the neurologist may be in the clinic, out of the hospital after hours, or otherwise unavailable, so responsibility for managing neurological conditions falls back on the hospitalist. But he or she may not have received sufficient exposure to neurology during medical training.
S. Andrew Josephson, MD, of the neurovascular division, director of the neurohospitalist program and assistant professor of neurology at the University of California-San Francisco (UCSF), regularly speaks on neurological issues to hospitalist audiences.
“I ask how many hospitalists in the room are primary caregivers for stroke in their hospital, and a surprising proportion raise their hands,” he says. “We do a good job of teaching neurology residents and fellows how to treat strokes. But it is important that we train internal medicine doctors as well, as they are seeing the majority of these patients nationwide.”
Depending on the setting, there may be wide variation in the hospitalist’s responsibility for neurological cases. “Here at UCSF, hospitalists almost never see stroke patients because we have a dedicated stroke service staffed by neurology attendings and residents,” Dr. Josephson says. “But at many community hospitals, they [care for neurological patients] all the time.”
David Likosky, MD, director of the stroke program at Evergreen Hospital Medical Center in Kirkland, Wash., concurs. “Neurology training in internal medicine residencies can be fairly limited,” he says. “After entering practice, these doctors are on the front lines in the hospital managing patients, many times without readily available neurologist backup.”
Dr. Likosky’s colleague at Evergreen, hospitalist Tony Yen, MD, says there are several neurological issues hospitalists are likely to encounter on a regular basis.
“Often the first responder to a stroke is the emergency department [ED] doctor or the hospitalist,” notes Dr. Yen. “Strokes are a time-critical, high-volume condition for our community hospitalist practice.”
Another important diagnosis is uncontrolled seizure (status epilepticus) that is unremitting for 10 minutes or more. Prompt response is critical.
Dr. Yen recalls the case of a young woman who collapsed while playing soccer. She was brought to the hospital and found to have suffered a brain-stem stroke. Physicians had three hours from the onset of symptoms to decide whether the patient was a candidate for tissue plasminogen activator (t-PA), a thrombolytic clot buster.
“I worked alongside the interventional radiologist and neurologist,” Dr. Yen recalls. “We were able to quickly establish a definitive diagnosis and then treat with intra-arterial t-PA.” The patient had a prolonged stay in intensive care and was on a ventilator for a couple of weeks but eventually recovered and walked out of the hospital.
Common Conditions
Stroke: The most common neurological emergency hospitalists are likely to see, whether on the floor or through the ED, is acute stroke, Dr. Josephson notes. “The evaluation of stroke requires a non-contrast computed tomography (CT) scan of the head to exclude intracerebral hemorrhage,” he says. “You can’t tell by looking at the patient whether it’s an ischemic stroke, the more common variety, or hemorrhagic stroke. But the difference is crucial because drugs to treat ischemic stroke can make hemorrhage worse. We view stroke as such a time-sensitive emergency that it always gets priority in the radiology department.”
It is also important to ascertain, as much as possible, when symptoms first began or when the patient was last observed to be normal. The treatment of choice in the first three hours following an ischemic stroke is intravenous t-PA. From hours three through six or eight, endovascular therapies (intra-arterial thrombolysis or mechanical clot retrieval) are an option. Signs suggesting a possible stroke include a new unilateral weakness, one-sided numbness, vertigo or imbalance, visual changes, inability to talk, and new headaches—although indications of a stroke can be subtle. The National Institutes of Health has issued a stroke scale, with training modules, accessible at www.strokecenter.org/trials/scales/nihss.html.
Seizures: Prolonged seizures that don’t resolve on their own within a reasonable amount of time require attention because the longer they last, the more likely they are to cause brain damage, Dr. Josephson says. Medications to treat the seizure work more effectively the earlier they are administered. He recommends a protocol for treating status epilepticus that starts with lorazepam (Ativan), proceeds to fosphenytoin (Cerebyx), and is followed by a general anesthetic such as midazolam (Versed) or propofol (Diprivan).
Intracranial pressure (ICP): This could be the result of a stroke or hemorrhage, brain tumor, or trauma. Fast action to control ICP is important because permanent brain injury can result. “I emphasize to hospitalists who are used to targeting ICP that it is better to look at cerebral perfusion pressure (CPP),” Dr. Josephson says, offering the following equation: CPP equals mean arterial pressure minus ICP. He also emphasizes raising the head of the patient’s bed, hyperventilation in early stages of treatment, and using osmotic agents such as mannitol to remove water from the brain.
Neuro-muscular emergencies: Acute disorders of the peripheral nerves, including Guillain-Barre Syndrome (an autoimmune neuropathy often triggered by infection), present a subacute onset of weakness and numbness. “We have good treatments for Guillain-Barre, such as plasmapheresis and administration of intravenous immunoglobulin,” Dr. Josephson says. “But recognition is important because the breathing may be affected. If the disorder reaches the diaphragm, it could kill the patient.” Disorders such as Guillain-Barre commonly present with ascending weakness, from the toes up.
A lumbar puncture (demonstrating few if any cells with an elevated protein) or an electromyogram (EMG) may be required for diagnosis. Hospitalists also are urged to watch for impending respiratory weakness, which can be measured by forced vital capacity or mean inspiratory flow. “Consider this diagnosis for anyone presenting with general weakness,” he says.
Exams on the Run
There is a standard technique for assessing and diagnosing neurological conditions, called the neurological examination. Unfortunately, a full, detailed neurological exam can be time-consuming and unrealistic, given caseload demands and field judgments required from the working hospitalist.
“As a hospitalist, you don’t have to perform an hourlong neurological examination,” Dr. Josephson says. “But for patients presenting neurological symptoms, you need to do a screening examination tied to their specific complaint. Your hypothesis-driven exam can be done in a few minutes if you know which elements are high-yield screening tests.”
These brief screening tests can be part of a routine assessment of the patient, Dr. Likosky adds.
Hospitalists can learn a lot just by walking into the patient’s room. “The bulk of such a neurological exam can be performed while talking to the patient, if you pay attention,” he notes. “There may be subtle signs of weakness. For example, when the patient is lying in bed, the feet should point straight up.” Note if one foot points to the side, or if the patient uses both sides of the face equally when talking.
“You can do sensory exams and test reflexes very briefly, as well,” Dr. Likosky says. “If those issues are on your radar screen, you can do much of the screening work in a stepwise fashion. The rest depends on clinical observation.”
There is not a huge spectrum of neurological disorders likely to confront the hospitalist, but it is important to know about the most common conditions and remember that time is of the essence, Dr. Likosky says. “Most neurological conditions are garden variety, but keep in mind the differential diagnoses, for example, for weakness and headache—common conditions that may rarely have an uncommon cause.”
Beef Up Training
Heather A. Harris, MD, a hospitalist at UCSF, illustrates the divide between academic medical centers and community hospitals when it comes to management of neurological diseases. She did her internal medicine training at UCSF and in 2003 went to a community hospital, Eden Medical Center in suburban Castro Valley, Calif., to help establish a hospitalist group. Suddenly, she was seeing lots of neurological cases.
“I’ll be frank: My internal medicine training at a wonderful medical institution had not prepared me for the reality that many new hospitalists face regarding neurological disorders,” says Dr. Harris. “You may see strokes as a resident, but it’s very different when you are the physician primarily managing strokes as they roll in. Yes, you may have a neurologist back-up, but they can’t always come in right away. The first time you see a patient with a stroke, it can be quite intimidating. You’re really learning on the fly. Plus, stroke management has advanced substantially in the last few years and there may be controversy, for example, over the use of t-PA in a community hospital setting.”
Feeling that her exposure to neurology was insufficient, Dr. Harris sought additional training at SHM meetings and talked to hospitalist colleagues in other community settings. “Hospitalists like me were trying to beef up our neurological knowledge and skill set.”
Dr. Harris developed a keen personal interest in neurology. In 2007, she returned to UCSF, where many of the hospitalists rarely see neurological patients. But she joined a new co-management service where hospitalists work alongside neuro-surgeons, helping manage the inevitable medical issues that arise in these patients.
Based on her first-hand appreciation for what hospitalists in community settings need to learn, Dr. Harris is also part of a team developing a new, hands-on training curriculum at UCSF for working hospitalists from community settings. That team is making sure neurology is adequately covered in UCSF’s curriculum.
“My overall experience is that if you’re going to be a hospitalist in a community setting, you’ll have to face a wide range of neurological emergencies,” Dr. Harris concludes. “It behooves us as hospitalists to learn the skill sets to manage these issues. There are also medical-legal issues that may put hospitalists out on a limb for doing too much too far outside of their knowledge and training. These are issues for SHM and our specialty to address.” TH
Larry Beresford is a regular contributor to The Hospitalist.
Although hospitalists may work alongside neurological specialists, they are increasingly on their own when responding to neurological emergencies, such as strokes, in hospitalized patients.
There are times the neurologist may be in the clinic, out of the hospital after hours, or otherwise unavailable, so responsibility for managing neurological conditions falls back on the hospitalist. But he or she may not have received sufficient exposure to neurology during medical training.
S. Andrew Josephson, MD, of the neurovascular division, director of the neurohospitalist program and assistant professor of neurology at the University of California-San Francisco (UCSF), regularly speaks on neurological issues to hospitalist audiences.
“I ask how many hospitalists in the room are primary caregivers for stroke in their hospital, and a surprising proportion raise their hands,” he says. “We do a good job of teaching neurology residents and fellows how to treat strokes. But it is important that we train internal medicine doctors as well, as they are seeing the majority of these patients nationwide.”
Depending on the setting, there may be wide variation in the hospitalist’s responsibility for neurological cases. “Here at UCSF, hospitalists almost never see stroke patients because we have a dedicated stroke service staffed by neurology attendings and residents,” Dr. Josephson says. “But at many community hospitals, they [care for neurological patients] all the time.”
David Likosky, MD, director of the stroke program at Evergreen Hospital Medical Center in Kirkland, Wash., concurs. “Neurology training in internal medicine residencies can be fairly limited,” he says. “After entering practice, these doctors are on the front lines in the hospital managing patients, many times without readily available neurologist backup.”
Dr. Likosky’s colleague at Evergreen, hospitalist Tony Yen, MD, says there are several neurological issues hospitalists are likely to encounter on a regular basis.
“Often the first responder to a stroke is the emergency department [ED] doctor or the hospitalist,” notes Dr. Yen. “Strokes are a time-critical, high-volume condition for our community hospitalist practice.”
Another important diagnosis is uncontrolled seizure (status epilepticus) that is unremitting for 10 minutes or more. Prompt response is critical.
Dr. Yen recalls the case of a young woman who collapsed while playing soccer. She was brought to the hospital and found to have suffered a brain-stem stroke. Physicians had three hours from the onset of symptoms to decide whether the patient was a candidate for tissue plasminogen activator (t-PA), a thrombolytic clot buster.
“I worked alongside the interventional radiologist and neurologist,” Dr. Yen recalls. “We were able to quickly establish a definitive diagnosis and then treat with intra-arterial t-PA.” The patient had a prolonged stay in intensive care and was on a ventilator for a couple of weeks but eventually recovered and walked out of the hospital.
Common Conditions
Stroke: The most common neurological emergency hospitalists are likely to see, whether on the floor or through the ED, is acute stroke, Dr. Josephson notes. “The evaluation of stroke requires a non-contrast computed tomography (CT) scan of the head to exclude intracerebral hemorrhage,” he says. “You can’t tell by looking at the patient whether it’s an ischemic stroke, the more common variety, or hemorrhagic stroke. But the difference is crucial because drugs to treat ischemic stroke can make hemorrhage worse. We view stroke as such a time-sensitive emergency that it always gets priority in the radiology department.”
It is also important to ascertain, as much as possible, when symptoms first began or when the patient was last observed to be normal. The treatment of choice in the first three hours following an ischemic stroke is intravenous t-PA. From hours three through six or eight, endovascular therapies (intra-arterial thrombolysis or mechanical clot retrieval) are an option. Signs suggesting a possible stroke include a new unilateral weakness, one-sided numbness, vertigo or imbalance, visual changes, inability to talk, and new headaches—although indications of a stroke can be subtle. The National Institutes of Health has issued a stroke scale, with training modules, accessible at www.strokecenter.org/trials/scales/nihss.html.
Seizures: Prolonged seizures that don’t resolve on their own within a reasonable amount of time require attention because the longer they last, the more likely they are to cause brain damage, Dr. Josephson says. Medications to treat the seizure work more effectively the earlier they are administered. He recommends a protocol for treating status epilepticus that starts with lorazepam (Ativan), proceeds to fosphenytoin (Cerebyx), and is followed by a general anesthetic such as midazolam (Versed) or propofol (Diprivan).
Intracranial pressure (ICP): This could be the result of a stroke or hemorrhage, brain tumor, or trauma. Fast action to control ICP is important because permanent brain injury can result. “I emphasize to hospitalists who are used to targeting ICP that it is better to look at cerebral perfusion pressure (CPP),” Dr. Josephson says, offering the following equation: CPP equals mean arterial pressure minus ICP. He also emphasizes raising the head of the patient’s bed, hyperventilation in early stages of treatment, and using osmotic agents such as mannitol to remove water from the brain.
Neuro-muscular emergencies: Acute disorders of the peripheral nerves, including Guillain-Barre Syndrome (an autoimmune neuropathy often triggered by infection), present a subacute onset of weakness and numbness. “We have good treatments for Guillain-Barre, such as plasmapheresis and administration of intravenous immunoglobulin,” Dr. Josephson says. “But recognition is important because the breathing may be affected. If the disorder reaches the diaphragm, it could kill the patient.” Disorders such as Guillain-Barre commonly present with ascending weakness, from the toes up.
A lumbar puncture (demonstrating few if any cells with an elevated protein) or an electromyogram (EMG) may be required for diagnosis. Hospitalists also are urged to watch for impending respiratory weakness, which can be measured by forced vital capacity or mean inspiratory flow. “Consider this diagnosis for anyone presenting with general weakness,” he says.
Exams on the Run
There is a standard technique for assessing and diagnosing neurological conditions, called the neurological examination. Unfortunately, a full, detailed neurological exam can be time-consuming and unrealistic, given caseload demands and field judgments required from the working hospitalist.
“As a hospitalist, you don’t have to perform an hourlong neurological examination,” Dr. Josephson says. “But for patients presenting neurological symptoms, you need to do a screening examination tied to their specific complaint. Your hypothesis-driven exam can be done in a few minutes if you know which elements are high-yield screening tests.”
These brief screening tests can be part of a routine assessment of the patient, Dr. Likosky adds.
Hospitalists can learn a lot just by walking into the patient’s room. “The bulk of such a neurological exam can be performed while talking to the patient, if you pay attention,” he notes. “There may be subtle signs of weakness. For example, when the patient is lying in bed, the feet should point straight up.” Note if one foot points to the side, or if the patient uses both sides of the face equally when talking.
“You can do sensory exams and test reflexes very briefly, as well,” Dr. Likosky says. “If those issues are on your radar screen, you can do much of the screening work in a stepwise fashion. The rest depends on clinical observation.”
There is not a huge spectrum of neurological disorders likely to confront the hospitalist, but it is important to know about the most common conditions and remember that time is of the essence, Dr. Likosky says. “Most neurological conditions are garden variety, but keep in mind the differential diagnoses, for example, for weakness and headache—common conditions that may rarely have an uncommon cause.”
Beef Up Training
Heather A. Harris, MD, a hospitalist at UCSF, illustrates the divide between academic medical centers and community hospitals when it comes to management of neurological diseases. She did her internal medicine training at UCSF and in 2003 went to a community hospital, Eden Medical Center in suburban Castro Valley, Calif., to help establish a hospitalist group. Suddenly, she was seeing lots of neurological cases.
“I’ll be frank: My internal medicine training at a wonderful medical institution had not prepared me for the reality that many new hospitalists face regarding neurological disorders,” says Dr. Harris. “You may see strokes as a resident, but it’s very different when you are the physician primarily managing strokes as they roll in. Yes, you may have a neurologist back-up, but they can’t always come in right away. The first time you see a patient with a stroke, it can be quite intimidating. You’re really learning on the fly. Plus, stroke management has advanced substantially in the last few years and there may be controversy, for example, over the use of t-PA in a community hospital setting.”
Feeling that her exposure to neurology was insufficient, Dr. Harris sought additional training at SHM meetings and talked to hospitalist colleagues in other community settings. “Hospitalists like me were trying to beef up our neurological knowledge and skill set.”
Dr. Harris developed a keen personal interest in neurology. In 2007, she returned to UCSF, where many of the hospitalists rarely see neurological patients. But she joined a new co-management service where hospitalists work alongside neuro-surgeons, helping manage the inevitable medical issues that arise in these patients.
Based on her first-hand appreciation for what hospitalists in community settings need to learn, Dr. Harris is also part of a team developing a new, hands-on training curriculum at UCSF for working hospitalists from community settings. That team is making sure neurology is adequately covered in UCSF’s curriculum.
“My overall experience is that if you’re going to be a hospitalist in a community setting, you’ll have to face a wide range of neurological emergencies,” Dr. Harris concludes. “It behooves us as hospitalists to learn the skill sets to manage these issues. There are also medical-legal issues that may put hospitalists out on a limb for doing too much too far outside of their knowledge and training. These are issues for SHM and our specialty to address.” TH
Larry Beresford is a regular contributor to The Hospitalist.
Although hospitalists may work alongside neurological specialists, they are increasingly on their own when responding to neurological emergencies, such as strokes, in hospitalized patients.
There are times the neurologist may be in the clinic, out of the hospital after hours, or otherwise unavailable, so responsibility for managing neurological conditions falls back on the hospitalist. But he or she may not have received sufficient exposure to neurology during medical training.
S. Andrew Josephson, MD, of the neurovascular division, director of the neurohospitalist program and assistant professor of neurology at the University of California-San Francisco (UCSF), regularly speaks on neurological issues to hospitalist audiences.
“I ask how many hospitalists in the room are primary caregivers for stroke in their hospital, and a surprising proportion raise their hands,” he says. “We do a good job of teaching neurology residents and fellows how to treat strokes. But it is important that we train internal medicine doctors as well, as they are seeing the majority of these patients nationwide.”
Depending on the setting, there may be wide variation in the hospitalist’s responsibility for neurological cases. “Here at UCSF, hospitalists almost never see stroke patients because we have a dedicated stroke service staffed by neurology attendings and residents,” Dr. Josephson says. “But at many community hospitals, they [care for neurological patients] all the time.”
David Likosky, MD, director of the stroke program at Evergreen Hospital Medical Center in Kirkland, Wash., concurs. “Neurology training in internal medicine residencies can be fairly limited,” he says. “After entering practice, these doctors are on the front lines in the hospital managing patients, many times without readily available neurologist backup.”
Dr. Likosky’s colleague at Evergreen, hospitalist Tony Yen, MD, says there are several neurological issues hospitalists are likely to encounter on a regular basis.
“Often the first responder to a stroke is the emergency department [ED] doctor or the hospitalist,” notes Dr. Yen. “Strokes are a time-critical, high-volume condition for our community hospitalist practice.”
Another important diagnosis is uncontrolled seizure (status epilepticus) that is unremitting for 10 minutes or more. Prompt response is critical.
Dr. Yen recalls the case of a young woman who collapsed while playing soccer. She was brought to the hospital and found to have suffered a brain-stem stroke. Physicians had three hours from the onset of symptoms to decide whether the patient was a candidate for tissue plasminogen activator (t-PA), a thrombolytic clot buster.
“I worked alongside the interventional radiologist and neurologist,” Dr. Yen recalls. “We were able to quickly establish a definitive diagnosis and then treat with intra-arterial t-PA.” The patient had a prolonged stay in intensive care and was on a ventilator for a couple of weeks but eventually recovered and walked out of the hospital.
Common Conditions
Stroke: The most common neurological emergency hospitalists are likely to see, whether on the floor or through the ED, is acute stroke, Dr. Josephson notes. “The evaluation of stroke requires a non-contrast computed tomography (CT) scan of the head to exclude intracerebral hemorrhage,” he says. “You can’t tell by looking at the patient whether it’s an ischemic stroke, the more common variety, or hemorrhagic stroke. But the difference is crucial because drugs to treat ischemic stroke can make hemorrhage worse. We view stroke as such a time-sensitive emergency that it always gets priority in the radiology department.”
It is also important to ascertain, as much as possible, when symptoms first began or when the patient was last observed to be normal. The treatment of choice in the first three hours following an ischemic stroke is intravenous t-PA. From hours three through six or eight, endovascular therapies (intra-arterial thrombolysis or mechanical clot retrieval) are an option. Signs suggesting a possible stroke include a new unilateral weakness, one-sided numbness, vertigo or imbalance, visual changes, inability to talk, and new headaches—although indications of a stroke can be subtle. The National Institutes of Health has issued a stroke scale, with training modules, accessible at www.strokecenter.org/trials/scales/nihss.html.
Seizures: Prolonged seizures that don’t resolve on their own within a reasonable amount of time require attention because the longer they last, the more likely they are to cause brain damage, Dr. Josephson says. Medications to treat the seizure work more effectively the earlier they are administered. He recommends a protocol for treating status epilepticus that starts with lorazepam (Ativan), proceeds to fosphenytoin (Cerebyx), and is followed by a general anesthetic such as midazolam (Versed) or propofol (Diprivan).
Intracranial pressure (ICP): This could be the result of a stroke or hemorrhage, brain tumor, or trauma. Fast action to control ICP is important because permanent brain injury can result. “I emphasize to hospitalists who are used to targeting ICP that it is better to look at cerebral perfusion pressure (CPP),” Dr. Josephson says, offering the following equation: CPP equals mean arterial pressure minus ICP. He also emphasizes raising the head of the patient’s bed, hyperventilation in early stages of treatment, and using osmotic agents such as mannitol to remove water from the brain.
Neuro-muscular emergencies: Acute disorders of the peripheral nerves, including Guillain-Barre Syndrome (an autoimmune neuropathy often triggered by infection), present a subacute onset of weakness and numbness. “We have good treatments for Guillain-Barre, such as plasmapheresis and administration of intravenous immunoglobulin,” Dr. Josephson says. “But recognition is important because the breathing may be affected. If the disorder reaches the diaphragm, it could kill the patient.” Disorders such as Guillain-Barre commonly present with ascending weakness, from the toes up.
A lumbar puncture (demonstrating few if any cells with an elevated protein) or an electromyogram (EMG) may be required for diagnosis. Hospitalists also are urged to watch for impending respiratory weakness, which can be measured by forced vital capacity or mean inspiratory flow. “Consider this diagnosis for anyone presenting with general weakness,” he says.
Exams on the Run
There is a standard technique for assessing and diagnosing neurological conditions, called the neurological examination. Unfortunately, a full, detailed neurological exam can be time-consuming and unrealistic, given caseload demands and field judgments required from the working hospitalist.
“As a hospitalist, you don’t have to perform an hourlong neurological examination,” Dr. Josephson says. “But for patients presenting neurological symptoms, you need to do a screening examination tied to their specific complaint. Your hypothesis-driven exam can be done in a few minutes if you know which elements are high-yield screening tests.”
These brief screening tests can be part of a routine assessment of the patient, Dr. Likosky adds.
Hospitalists can learn a lot just by walking into the patient’s room. “The bulk of such a neurological exam can be performed while talking to the patient, if you pay attention,” he notes. “There may be subtle signs of weakness. For example, when the patient is lying in bed, the feet should point straight up.” Note if one foot points to the side, or if the patient uses both sides of the face equally when talking.
“You can do sensory exams and test reflexes very briefly, as well,” Dr. Likosky says. “If those issues are on your radar screen, you can do much of the screening work in a stepwise fashion. The rest depends on clinical observation.”
There is not a huge spectrum of neurological disorders likely to confront the hospitalist, but it is important to know about the most common conditions and remember that time is of the essence, Dr. Likosky says. “Most neurological conditions are garden variety, but keep in mind the differential diagnoses, for example, for weakness and headache—common conditions that may rarely have an uncommon cause.”
Beef Up Training
Heather A. Harris, MD, a hospitalist at UCSF, illustrates the divide between academic medical centers and community hospitals when it comes to management of neurological diseases. She did her internal medicine training at UCSF and in 2003 went to a community hospital, Eden Medical Center in suburban Castro Valley, Calif., to help establish a hospitalist group. Suddenly, she was seeing lots of neurological cases.
“I’ll be frank: My internal medicine training at a wonderful medical institution had not prepared me for the reality that many new hospitalists face regarding neurological disorders,” says Dr. Harris. “You may see strokes as a resident, but it’s very different when you are the physician primarily managing strokes as they roll in. Yes, you may have a neurologist back-up, but they can’t always come in right away. The first time you see a patient with a stroke, it can be quite intimidating. You’re really learning on the fly. Plus, stroke management has advanced substantially in the last few years and there may be controversy, for example, over the use of t-PA in a community hospital setting.”
Feeling that her exposure to neurology was insufficient, Dr. Harris sought additional training at SHM meetings and talked to hospitalist colleagues in other community settings. “Hospitalists like me were trying to beef up our neurological knowledge and skill set.”
Dr. Harris developed a keen personal interest in neurology. In 2007, she returned to UCSF, where many of the hospitalists rarely see neurological patients. But she joined a new co-management service where hospitalists work alongside neuro-surgeons, helping manage the inevitable medical issues that arise in these patients.
Based on her first-hand appreciation for what hospitalists in community settings need to learn, Dr. Harris is also part of a team developing a new, hands-on training curriculum at UCSF for working hospitalists from community settings. That team is making sure neurology is adequately covered in UCSF’s curriculum.
“My overall experience is that if you’re going to be a hospitalist in a community setting, you’ll have to face a wide range of neurological emergencies,” Dr. Harris concludes. “It behooves us as hospitalists to learn the skill sets to manage these issues. There are also medical-legal issues that may put hospitalists out on a limb for doing too much too far outside of their knowledge and training. These are issues for SHM and our specialty to address.” TH
Larry Beresford is a regular contributor to The Hospitalist.
Deposition Minefield
One day, you’re sitting in your office when a stranger appears and asks, “Are you Dr. Smith?” When you say yes, the stranger hands you a sheaf of papers. You open the papers and see you’ve been “commanded” to attend a deposition at a lawyer’s office next week. How do you prepare?
The Basics
Black’s Law Dictionary gives a long definition of a deposition. But the shorter, more practical definition is that a deposition is a witness’s sworn out-of-court testimony. When a physician gives a deposition in a lawyer’s office, this testimony has the same legal effect as though the physician were testifying in court.
Lawyers typically view depositions as one of two types:
- Discovery depositions: These allow lawyers to discover the substance of a witness’s testimony before trial. They can touch upon a number of subjects that seem tangential to the case. A lawyer taking a discovery deposition is putting together the pieces of the case and may or may not ask the witness to testify at trial; and
- Perpetuation depositions: These let lawyers present the testimony of a witness who cannot appear at trial. Perpetuation depositions substitute for the examinations and cross-examinations that would normally occur in the courtroom. Perpetuation depositions are generally shorter and more focused than discovery depositions.
In all depositions, lawyers ask questions of the witness and can object to legally improper questions. The lawyers can ask the witness to refer to documents or other exhibits during the deposition. A court reporter will transcribe the questions and answers and condense them into a written transcript. A judge is normally not present for a deposition but can be called during the deposition to make rulings.
Know Your Role
Perhaps the most important thing you can do in preparing for a deposition is understand your role in the lawsuit. Generally, physicians serve in one of three potential roles as deponents:
Medical malpractice defendant: When a patient sues a physician for malpractice, the patient’s attorney normally will take the physician’s deposition. In this highly adversarial process, the patient’s attorney attempts to demonstrate that the physician’s negligence injured the patient. A physician being deposed as a defendant must prepare by meeting with his attorney and reviewing the issues likely to arise during the proceedings. If you are a defendant in a lawsuit, you must set aside adequate time to prepare for the deposition with your attorney;
Retained expert witness: The rules of evidence allow people with specialized knowledge to testify as experts in fields normally beyond the average juror’s experience. Because they have specialized knowledge, experts are allowed to state opinions in their testimony, such as whether a physician’s conduct complied with the applicable standards of care. Attorneys generally hire expert witnesses to present opinions in a case and will provide a summary of the expert’s testimony before the deposition; and
Treating physician: Many physicians are deposed concerning the care they provided to a patient in lawsuits that implicate the patient’s health (auto accident, work injury, disability suit). These depositions focus on the substance of treatment, the patient’s medical condition, and the patient’s prognosis. The physician normally does not have any interest in how the lawsuit is resolved. A treating physician is often compensated for his time in the deposition, even though he was not retained as an expert to testify in the lawsuit.
Golden Rules
Because depositions are stressful, lawyers ask witnesses to remember only three rules.
Tell the truth: Your only job as a witness is to tell the truth. If you follow this rule, you have discharged your obligation to the legal system.
However, keep some things in mind when telling the truth. In particular, your ability to tell the truth is subject to the limitations of your memory and the fact that your deposition may be occurring several years after you provided care. “I don’t know” and “I don’t remember” are absolutely acceptable answers in a deposition. In fact, they are preferable to inaccurate or untruthful testimony. If reviewing a document (such as the patient’s medical records) will help you provide accurate and truthful testimony, don’t be shy about asking to review them. In any situation where you are guessing or providing your best recollection, make sure the lawyer knows you are doing your best but that you can’t remember all the details.
Make sure you understand the question: This rule seems self-evident, but many lawyers ask convoluted or compound questions. Lawyers may also use language unfamiliar to you as an outsider to the legal process. For example, when lawyers use the phrase “standard of care,” it has a fairly precise definition (it is an action a reasonably careful physician would undertake under the same or similar circumstances). Ask for clarification of any question that is not clear. It’s the lawyer’s job to ask an understandable question, not the physician’s job to answer a question that doesn’t make sense. Be extra careful when the opposing lawyer objects to a question. While the lawyer’s objection does not relieve you from answering, it should signal you that the question is potentially flawed or beyond the scope of your knowledge.
Answer only what you’re asked: Invariably, physicians struggle most when they don’t focus their answers on the question posed to them.
The majority of questions in a deposition can be answered “Yes,” “No,” “I don’t know,” and “I don’t remember.” Yet many physicians tend to volunteer additional information to explain their answers. Because lawyers are trained to recognize and follow up on nonresponsive answers, the physician’s deposition becomes longer and more challenging. To provide a better answer, don’t think out loud. Ponder the question and mentally prepare your answer. Doing so lets you respond more precisely. Answer only the question you are asked. If there is an area that needs more explanation, the other party’s attorney (or your attorney) will have an opportunity to allow you to clarify the record.
To help you follow the rules, use this decision tree during your deposition (see Figure 1, left).
Regardless of the purpose of a deposition or your perceived role in it, consult with an attorney before being deposed. Even if you believe you are being deposed only as a treating provider, a deposition could lead to potential claims or raise concerns about your records. If served with a subpoena, contact your insurance company, which may retain an attorney to assist you. TH
Patrick O’Rourke works in the Office of University Counsel, Department of Litigation, University of Colorado, Denver.
One day, you’re sitting in your office when a stranger appears and asks, “Are you Dr. Smith?” When you say yes, the stranger hands you a sheaf of papers. You open the papers and see you’ve been “commanded” to attend a deposition at a lawyer’s office next week. How do you prepare?
The Basics
Black’s Law Dictionary gives a long definition of a deposition. But the shorter, more practical definition is that a deposition is a witness’s sworn out-of-court testimony. When a physician gives a deposition in a lawyer’s office, this testimony has the same legal effect as though the physician were testifying in court.
Lawyers typically view depositions as one of two types:
- Discovery depositions: These allow lawyers to discover the substance of a witness’s testimony before trial. They can touch upon a number of subjects that seem tangential to the case. A lawyer taking a discovery deposition is putting together the pieces of the case and may or may not ask the witness to testify at trial; and
- Perpetuation depositions: These let lawyers present the testimony of a witness who cannot appear at trial. Perpetuation depositions substitute for the examinations and cross-examinations that would normally occur in the courtroom. Perpetuation depositions are generally shorter and more focused than discovery depositions.
In all depositions, lawyers ask questions of the witness and can object to legally improper questions. The lawyers can ask the witness to refer to documents or other exhibits during the deposition. A court reporter will transcribe the questions and answers and condense them into a written transcript. A judge is normally not present for a deposition but can be called during the deposition to make rulings.
Know Your Role
Perhaps the most important thing you can do in preparing for a deposition is understand your role in the lawsuit. Generally, physicians serve in one of three potential roles as deponents:
Medical malpractice defendant: When a patient sues a physician for malpractice, the patient’s attorney normally will take the physician’s deposition. In this highly adversarial process, the patient’s attorney attempts to demonstrate that the physician’s negligence injured the patient. A physician being deposed as a defendant must prepare by meeting with his attorney and reviewing the issues likely to arise during the proceedings. If you are a defendant in a lawsuit, you must set aside adequate time to prepare for the deposition with your attorney;
Retained expert witness: The rules of evidence allow people with specialized knowledge to testify as experts in fields normally beyond the average juror’s experience. Because they have specialized knowledge, experts are allowed to state opinions in their testimony, such as whether a physician’s conduct complied with the applicable standards of care. Attorneys generally hire expert witnesses to present opinions in a case and will provide a summary of the expert’s testimony before the deposition; and
Treating physician: Many physicians are deposed concerning the care they provided to a patient in lawsuits that implicate the patient’s health (auto accident, work injury, disability suit). These depositions focus on the substance of treatment, the patient’s medical condition, and the patient’s prognosis. The physician normally does not have any interest in how the lawsuit is resolved. A treating physician is often compensated for his time in the deposition, even though he was not retained as an expert to testify in the lawsuit.
Golden Rules
Because depositions are stressful, lawyers ask witnesses to remember only three rules.
Tell the truth: Your only job as a witness is to tell the truth. If you follow this rule, you have discharged your obligation to the legal system.
However, keep some things in mind when telling the truth. In particular, your ability to tell the truth is subject to the limitations of your memory and the fact that your deposition may be occurring several years after you provided care. “I don’t know” and “I don’t remember” are absolutely acceptable answers in a deposition. In fact, they are preferable to inaccurate or untruthful testimony. If reviewing a document (such as the patient’s medical records) will help you provide accurate and truthful testimony, don’t be shy about asking to review them. In any situation where you are guessing or providing your best recollection, make sure the lawyer knows you are doing your best but that you can’t remember all the details.
Make sure you understand the question: This rule seems self-evident, but many lawyers ask convoluted or compound questions. Lawyers may also use language unfamiliar to you as an outsider to the legal process. For example, when lawyers use the phrase “standard of care,” it has a fairly precise definition (it is an action a reasonably careful physician would undertake under the same or similar circumstances). Ask for clarification of any question that is not clear. It’s the lawyer’s job to ask an understandable question, not the physician’s job to answer a question that doesn’t make sense. Be extra careful when the opposing lawyer objects to a question. While the lawyer’s objection does not relieve you from answering, it should signal you that the question is potentially flawed or beyond the scope of your knowledge.
Answer only what you’re asked: Invariably, physicians struggle most when they don’t focus their answers on the question posed to them.
The majority of questions in a deposition can be answered “Yes,” “No,” “I don’t know,” and “I don’t remember.” Yet many physicians tend to volunteer additional information to explain their answers. Because lawyers are trained to recognize and follow up on nonresponsive answers, the physician’s deposition becomes longer and more challenging. To provide a better answer, don’t think out loud. Ponder the question and mentally prepare your answer. Doing so lets you respond more precisely. Answer only the question you are asked. If there is an area that needs more explanation, the other party’s attorney (or your attorney) will have an opportunity to allow you to clarify the record.
To help you follow the rules, use this decision tree during your deposition (see Figure 1, left).
Regardless of the purpose of a deposition or your perceived role in it, consult with an attorney before being deposed. Even if you believe you are being deposed only as a treating provider, a deposition could lead to potential claims or raise concerns about your records. If served with a subpoena, contact your insurance company, which may retain an attorney to assist you. TH
Patrick O’Rourke works in the Office of University Counsel, Department of Litigation, University of Colorado, Denver.
One day, you’re sitting in your office when a stranger appears and asks, “Are you Dr. Smith?” When you say yes, the stranger hands you a sheaf of papers. You open the papers and see you’ve been “commanded” to attend a deposition at a lawyer’s office next week. How do you prepare?
The Basics
Black’s Law Dictionary gives a long definition of a deposition. But the shorter, more practical definition is that a deposition is a witness’s sworn out-of-court testimony. When a physician gives a deposition in a lawyer’s office, this testimony has the same legal effect as though the physician were testifying in court.
Lawyers typically view depositions as one of two types:
- Discovery depositions: These allow lawyers to discover the substance of a witness’s testimony before trial. They can touch upon a number of subjects that seem tangential to the case. A lawyer taking a discovery deposition is putting together the pieces of the case and may or may not ask the witness to testify at trial; and
- Perpetuation depositions: These let lawyers present the testimony of a witness who cannot appear at trial. Perpetuation depositions substitute for the examinations and cross-examinations that would normally occur in the courtroom. Perpetuation depositions are generally shorter and more focused than discovery depositions.
In all depositions, lawyers ask questions of the witness and can object to legally improper questions. The lawyers can ask the witness to refer to documents or other exhibits during the deposition. A court reporter will transcribe the questions and answers and condense them into a written transcript. A judge is normally not present for a deposition but can be called during the deposition to make rulings.
Know Your Role
Perhaps the most important thing you can do in preparing for a deposition is understand your role in the lawsuit. Generally, physicians serve in one of three potential roles as deponents:
Medical malpractice defendant: When a patient sues a physician for malpractice, the patient’s attorney normally will take the physician’s deposition. In this highly adversarial process, the patient’s attorney attempts to demonstrate that the physician’s negligence injured the patient. A physician being deposed as a defendant must prepare by meeting with his attorney and reviewing the issues likely to arise during the proceedings. If you are a defendant in a lawsuit, you must set aside adequate time to prepare for the deposition with your attorney;
Retained expert witness: The rules of evidence allow people with specialized knowledge to testify as experts in fields normally beyond the average juror’s experience. Because they have specialized knowledge, experts are allowed to state opinions in their testimony, such as whether a physician’s conduct complied with the applicable standards of care. Attorneys generally hire expert witnesses to present opinions in a case and will provide a summary of the expert’s testimony before the deposition; and
Treating physician: Many physicians are deposed concerning the care they provided to a patient in lawsuits that implicate the patient’s health (auto accident, work injury, disability suit). These depositions focus on the substance of treatment, the patient’s medical condition, and the patient’s prognosis. The physician normally does not have any interest in how the lawsuit is resolved. A treating physician is often compensated for his time in the deposition, even though he was not retained as an expert to testify in the lawsuit.
Golden Rules
Because depositions are stressful, lawyers ask witnesses to remember only three rules.
Tell the truth: Your only job as a witness is to tell the truth. If you follow this rule, you have discharged your obligation to the legal system.
However, keep some things in mind when telling the truth. In particular, your ability to tell the truth is subject to the limitations of your memory and the fact that your deposition may be occurring several years after you provided care. “I don’t know” and “I don’t remember” are absolutely acceptable answers in a deposition. In fact, they are preferable to inaccurate or untruthful testimony. If reviewing a document (such as the patient’s medical records) will help you provide accurate and truthful testimony, don’t be shy about asking to review them. In any situation where you are guessing or providing your best recollection, make sure the lawyer knows you are doing your best but that you can’t remember all the details.
Make sure you understand the question: This rule seems self-evident, but many lawyers ask convoluted or compound questions. Lawyers may also use language unfamiliar to you as an outsider to the legal process. For example, when lawyers use the phrase “standard of care,” it has a fairly precise definition (it is an action a reasonably careful physician would undertake under the same or similar circumstances). Ask for clarification of any question that is not clear. It’s the lawyer’s job to ask an understandable question, not the physician’s job to answer a question that doesn’t make sense. Be extra careful when the opposing lawyer objects to a question. While the lawyer’s objection does not relieve you from answering, it should signal you that the question is potentially flawed or beyond the scope of your knowledge.
Answer only what you’re asked: Invariably, physicians struggle most when they don’t focus their answers on the question posed to them.
The majority of questions in a deposition can be answered “Yes,” “No,” “I don’t know,” and “I don’t remember.” Yet many physicians tend to volunteer additional information to explain their answers. Because lawyers are trained to recognize and follow up on nonresponsive answers, the physician’s deposition becomes longer and more challenging. To provide a better answer, don’t think out loud. Ponder the question and mentally prepare your answer. Doing so lets you respond more precisely. Answer only the question you are asked. If there is an area that needs more explanation, the other party’s attorney (or your attorney) will have an opportunity to allow you to clarify the record.
To help you follow the rules, use this decision tree during your deposition (see Figure 1, left).
Regardless of the purpose of a deposition or your perceived role in it, consult with an attorney before being deposed. Even if you believe you are being deposed only as a treating provider, a deposition could lead to potential claims or raise concerns about your records. If served with a subpoena, contact your insurance company, which may retain an attorney to assist you. TH
Patrick O’Rourke works in the Office of University Counsel, Department of Litigation, University of Colorado, Denver.
Plan for Discharge
Discharge planning typically begins at the time of admission. Physicians and hospital staff manage the patient’s acute issues throughout the stay while simultaneously trying to anticipate the patient’s discharge needs. Physicians capture these associated efforts by reporting discharge day management codes 99238 or 99239.
Code Use
Use of discharge day management codes 99238-99239 is reserved for the admitting physician/group, unless a formal transfer of care occurs (e.g., patient is transferred from the intensive care unit by the critical care physician to the medical-surgical floor on the hospitalist’s service).
Report one discharge code per hospitalization, but only when the service occurs after the initial date of admission. Codes 99238 or 99239 are not permitted for use when the patient is admitted and discharged on the same calendar date. When this occurs, the physician selects from 99221-99223 (initial inpatient care) or 99234-99236 (admission and discharge on the same day). Choose 99234-99238 when the patient stay is eight or more hours on the same calendar day and the insurer accepts these codes.
Documentation must also reflect two components of service: the corresponding elements of both the admission and discharge. Alternately, if the patient stays less than eight hours, or the insurer does not recognize 99234-99236 (admission and discharge on the same day), report 9922x (initial inpatient care) as appropriate.
Don’t mistakenly report discharge services for merely dictating the discharge summary. Discharge day management, as with most payable evaluation and management (E/M) services, requires a face-to-face visit between the physician and the patient on discharge day.
The entire visit need not take place at the bedside and may include other discharge-related elements performed on the patient’s unit/floor such as discussions with other healthcare professionals, patient/caregiver instruction and coordination of follow-up care. The discharge code description indicates that a final examination of the patient is included, but only “as appropriate.” In other words, an exam may not occur, or may not be documented, yet this does not preclude the physician from reporting 99238-99239. However, inclusion of the exam in the discharge day documentation is the best way to justify that a face-to-face service occurred on discharge day. This may be included in the discharge summary or a separate progress note in the medical record.
Time-Based Service
Discharge day management codes reflect the time accumulated on a calendar date, ending when the patient physically leaves the hospital. Services performed in a location other than the patient’s unit/floor (e.g., dictating the discharge summary from the outpatient office), do not count toward the cumulative time. Additionally, discharge-related services performed by residents, students or ancillary staff (i.e., registered nurses), such as reviewing instructions with the patient, do not count toward the discharge service time.
To support the discharge day management claim, documentation should reference the discharge status and other clinically relevant information. Time is not required when documenting 99238 because this service code constitutes any amount of time up to and including 30 minutes. When reporting 99239, documentation must include the physician’s cumulative service time (more than 30 minutes).
Medicare currently initiates a prepayment review (i.e., request for documentation to review the service prior to any payment consideration) for claims involving 99239. Failure to respond to the prepayment request or failure to include the time component in the documentation often results in claim denial. Payment can be recovered only through the appeal process or claim correction, when applicable.
Rules For Surgery
Surgeons are prohibited from separately reporting inpatient postoperative services related to the surgery, including discharge day management (99238-99239). Additionally, when the surgeon admits a patient to the hospital and discharge services are performed postoperatively by the hospitalist, discharge day management is included in the surgical package.
The reasons are two-fold: If the surgeon transfers the remaining inpatient care to the hospitalist, these discharge services are considered part of the global surgical package.
If no transfer occurs (as the surgeon is typically responsible and paid for all care up to 90 days following surgery), only the admitting physician/group (i.e., the surgeon) may report discharge day management codes 99238-99239.
In the latter scenario, the hospitalist reports subsequent hospital care (99231-99233) for all medically necessary services involving the patient’s medical management, even if provided on the day of discharge.
Pronouncement of Death
One of the most underreported services involves pronouncement of death. A physician who performs this service may qualify to report discharge day management code 99238-99239. To pronounce death, the physician must examine the patient, thus satisfying the face-to-face visit requirement.
Additionally, the physician may have to coordinate the necessary services, speak with family members or other healthcare providers, and fill out the necessary documentation.
If performed on the patient’s unit/floor, these services count toward the cumulative discharge service time. Documentation must include the time (if reporting 99239) as well as the patient’s discharge status and clinically relevant information. Completion of the death certificate alone is not sufficient for billing. TH
Carol Pohlig is a billing and coding expert with the University of Pennsylvania Medical Center, Philadelphia. She is also on the faculty of SHM’s inpatient coding course.
Discharge planning typically begins at the time of admission. Physicians and hospital staff manage the patient’s acute issues throughout the stay while simultaneously trying to anticipate the patient’s discharge needs. Physicians capture these associated efforts by reporting discharge day management codes 99238 or 99239.
Code Use
Use of discharge day management codes 99238-99239 is reserved for the admitting physician/group, unless a formal transfer of care occurs (e.g., patient is transferred from the intensive care unit by the critical care physician to the medical-surgical floor on the hospitalist’s service).
Report one discharge code per hospitalization, but only when the service occurs after the initial date of admission. Codes 99238 or 99239 are not permitted for use when the patient is admitted and discharged on the same calendar date. When this occurs, the physician selects from 99221-99223 (initial inpatient care) or 99234-99236 (admission and discharge on the same day). Choose 99234-99238 when the patient stay is eight or more hours on the same calendar day and the insurer accepts these codes.
Documentation must also reflect two components of service: the corresponding elements of both the admission and discharge. Alternately, if the patient stays less than eight hours, or the insurer does not recognize 99234-99236 (admission and discharge on the same day), report 9922x (initial inpatient care) as appropriate.
Don’t mistakenly report discharge services for merely dictating the discharge summary. Discharge day management, as with most payable evaluation and management (E/M) services, requires a face-to-face visit between the physician and the patient on discharge day.
The entire visit need not take place at the bedside and may include other discharge-related elements performed on the patient’s unit/floor such as discussions with other healthcare professionals, patient/caregiver instruction and coordination of follow-up care. The discharge code description indicates that a final examination of the patient is included, but only “as appropriate.” In other words, an exam may not occur, or may not be documented, yet this does not preclude the physician from reporting 99238-99239. However, inclusion of the exam in the discharge day documentation is the best way to justify that a face-to-face service occurred on discharge day. This may be included in the discharge summary or a separate progress note in the medical record.
Time-Based Service
Discharge day management codes reflect the time accumulated on a calendar date, ending when the patient physically leaves the hospital. Services performed in a location other than the patient’s unit/floor (e.g., dictating the discharge summary from the outpatient office), do not count toward the cumulative time. Additionally, discharge-related services performed by residents, students or ancillary staff (i.e., registered nurses), such as reviewing instructions with the patient, do not count toward the discharge service time.
To support the discharge day management claim, documentation should reference the discharge status and other clinically relevant information. Time is not required when documenting 99238 because this service code constitutes any amount of time up to and including 30 minutes. When reporting 99239, documentation must include the physician’s cumulative service time (more than 30 minutes).
Medicare currently initiates a prepayment review (i.e., request for documentation to review the service prior to any payment consideration) for claims involving 99239. Failure to respond to the prepayment request or failure to include the time component in the documentation often results in claim denial. Payment can be recovered only through the appeal process or claim correction, when applicable.
Rules For Surgery
Surgeons are prohibited from separately reporting inpatient postoperative services related to the surgery, including discharge day management (99238-99239). Additionally, when the surgeon admits a patient to the hospital and discharge services are performed postoperatively by the hospitalist, discharge day management is included in the surgical package.
The reasons are two-fold: If the surgeon transfers the remaining inpatient care to the hospitalist, these discharge services are considered part of the global surgical package.
If no transfer occurs (as the surgeon is typically responsible and paid for all care up to 90 days following surgery), only the admitting physician/group (i.e., the surgeon) may report discharge day management codes 99238-99239.
In the latter scenario, the hospitalist reports subsequent hospital care (99231-99233) for all medically necessary services involving the patient’s medical management, even if provided on the day of discharge.
Pronouncement of Death
One of the most underreported services involves pronouncement of death. A physician who performs this service may qualify to report discharge day management code 99238-99239. To pronounce death, the physician must examine the patient, thus satisfying the face-to-face visit requirement.
Additionally, the physician may have to coordinate the necessary services, speak with family members or other healthcare providers, and fill out the necessary documentation.
If performed on the patient’s unit/floor, these services count toward the cumulative discharge service time. Documentation must include the time (if reporting 99239) as well as the patient’s discharge status and clinically relevant information. Completion of the death certificate alone is not sufficient for billing. TH
Carol Pohlig is a billing and coding expert with the University of Pennsylvania Medical Center, Philadelphia. She is also on the faculty of SHM’s inpatient coding course.
Discharge planning typically begins at the time of admission. Physicians and hospital staff manage the patient’s acute issues throughout the stay while simultaneously trying to anticipate the patient’s discharge needs. Physicians capture these associated efforts by reporting discharge day management codes 99238 or 99239.
Code Use
Use of discharge day management codes 99238-99239 is reserved for the admitting physician/group, unless a formal transfer of care occurs (e.g., patient is transferred from the intensive care unit by the critical care physician to the medical-surgical floor on the hospitalist’s service).
Report one discharge code per hospitalization, but only when the service occurs after the initial date of admission. Codes 99238 or 99239 are not permitted for use when the patient is admitted and discharged on the same calendar date. When this occurs, the physician selects from 99221-99223 (initial inpatient care) or 99234-99236 (admission and discharge on the same day). Choose 99234-99238 when the patient stay is eight or more hours on the same calendar day and the insurer accepts these codes.
Documentation must also reflect two components of service: the corresponding elements of both the admission and discharge. Alternately, if the patient stays less than eight hours, or the insurer does not recognize 99234-99236 (admission and discharge on the same day), report 9922x (initial inpatient care) as appropriate.
Don’t mistakenly report discharge services for merely dictating the discharge summary. Discharge day management, as with most payable evaluation and management (E/M) services, requires a face-to-face visit between the physician and the patient on discharge day.
The entire visit need not take place at the bedside and may include other discharge-related elements performed on the patient’s unit/floor such as discussions with other healthcare professionals, patient/caregiver instruction and coordination of follow-up care. The discharge code description indicates that a final examination of the patient is included, but only “as appropriate.” In other words, an exam may not occur, or may not be documented, yet this does not preclude the physician from reporting 99238-99239. However, inclusion of the exam in the discharge day documentation is the best way to justify that a face-to-face service occurred on discharge day. This may be included in the discharge summary or a separate progress note in the medical record.
Time-Based Service
Discharge day management codes reflect the time accumulated on a calendar date, ending when the patient physically leaves the hospital. Services performed in a location other than the patient’s unit/floor (e.g., dictating the discharge summary from the outpatient office), do not count toward the cumulative time. Additionally, discharge-related services performed by residents, students or ancillary staff (i.e., registered nurses), such as reviewing instructions with the patient, do not count toward the discharge service time.
To support the discharge day management claim, documentation should reference the discharge status and other clinically relevant information. Time is not required when documenting 99238 because this service code constitutes any amount of time up to and including 30 minutes. When reporting 99239, documentation must include the physician’s cumulative service time (more than 30 minutes).
Medicare currently initiates a prepayment review (i.e., request for documentation to review the service prior to any payment consideration) for claims involving 99239. Failure to respond to the prepayment request or failure to include the time component in the documentation often results in claim denial. Payment can be recovered only through the appeal process or claim correction, when applicable.
Rules For Surgery
Surgeons are prohibited from separately reporting inpatient postoperative services related to the surgery, including discharge day management (99238-99239). Additionally, when the surgeon admits a patient to the hospital and discharge services are performed postoperatively by the hospitalist, discharge day management is included in the surgical package.
The reasons are two-fold: If the surgeon transfers the remaining inpatient care to the hospitalist, these discharge services are considered part of the global surgical package.
If no transfer occurs (as the surgeon is typically responsible and paid for all care up to 90 days following surgery), only the admitting physician/group (i.e., the surgeon) may report discharge day management codes 99238-99239.
In the latter scenario, the hospitalist reports subsequent hospital care (99231-99233) for all medically necessary services involving the patient’s medical management, even if provided on the day of discharge.
Pronouncement of Death
One of the most underreported services involves pronouncement of death. A physician who performs this service may qualify to report discharge day management code 99238-99239. To pronounce death, the physician must examine the patient, thus satisfying the face-to-face visit requirement.
Additionally, the physician may have to coordinate the necessary services, speak with family members or other healthcare providers, and fill out the necessary documentation.
If performed on the patient’s unit/floor, these services count toward the cumulative discharge service time. Documentation must include the time (if reporting 99239) as well as the patient’s discharge status and clinically relevant information. Completion of the death certificate alone is not sufficient for billing. TH
Carol Pohlig is a billing and coding expert with the University of Pennsylvania Medical Center, Philadelphia. She is also on the faculty of SHM’s inpatient coding course.
AHRQ in the Lead
What exactly is the Agency for Healthcare Research and Quality (AHRQ), and why are hospitalists urged to increase its portion of the federal budget pie each year?
According to its mission statement, the AHRQ is “the lead federal agency charged with improving the quality, safety, efficiency, and effectiveness of healthcare for all Americans.” This includes supporting high-quality, impartial research that specifically improves healthcare quality, reduces costs, advances patient safety, decreases medical errors, eliminates healthcare disparities, and broadens access to essential services.
“Supporting AHRQ is supporting an unbiased government organization that’s clearly on the side of patient safety, and that gets important information out fast,” says Andrew Fishmann, MD, FCCP, FACP, a member of AHRQ’s National Advisory Council and director of intensive care at Good Samaritan Hospital in Los Angeles. “Where’s the argument?”
Fight over Funding
The argument is over money, plain and simple. Each year, medical associations like SHM push for increased federal funding for AHRQ so the agency’s research can be expanded. And each year, Congress refuses those increases. Lawmakers have granted a slight boost in funding: Since 2002, AHRQ’s budget has increased by $2 million, or 6.7%.
Proponents of AHRQ believe precarious funding levels threaten the agency’s ability to achieve its essential mission. Last year, SHM lobbied for an increase in federal funding for AHRQ to $350 million in fiscal year 2008—$31 million more than the agency’s fiscal 2007 budget. By late 2007, Congress was weighing an increase of $329 million, plus $5 million targeted for comparative-effectiveness research.
“Think of AHRQ compared to the $28 billion that NIH gets,” says Dr. Fishmann. “[AHRQ’s] is a small budget relative to what they do.”
How much does AHRQ need to provide adequate research information? The answer is, apparently, as much as they can get. There are countless areas in healthcare the agency could address.
“If they got $500 million, could they spend it?” asks Dr. Fishmann. “Yes. They could look at the top 20 diseases instead of the top 10.”
What AHRQ Does
Regardless of the final budget amount they receive, AHRQ spends roughly 80% on grants and contracts focused on improving healthcare.
“AHRQ doesn’t do its own research or create its own data,” explains Dr. Fishmann. Rather, AHRQ conducts and supports health services research in leading academic institutions, hospitals, and other settings. In 2005, two hospitalists received separate grants for projects that have already had an effect on hospital medicine. Greg Maynard, MD, MS, division chief of hospital medicine at University of California San Diego School of Medicine, used AHRQ funds for an intervention project to prevent hospital-acquired venous thromboembolism (VTE). Dr. Maynard’s project continued to grow since that grant and has yielded key findings such as a risk-assessment model for VTE. Data and lessons learned are available in the VTE Resource Room on SHM’s Web site at www.hospitalmedicine.org/ResourceRoomRedesign/RR_LandingPage.cfm.
Asked why he went after AHRQ funding, Dr. Maynard explains: “AHRQ is one of the few [funding] agencies that focuses on the realm of implementation—that impact the patient immediately. It was a perfect marriage of what we wanted to do.” The other AHRQ-funded hospital medicine project was conducted by Mark V. Williams, MD, FACP, professor and chief, division of hospital medicine at Northwestern University Feinberg School of Medicine in Chicago, and editor of the Journal of Hospital Medicine. Working for Emory University’s hospital medicine program in Atlanta at the time, Dr. Williams used the grant to create a “discharge bundle” of patient safety interventions such as medication reconciliation and patient-centered education to improve patient safety transitions out of the hospital setting.
“We would not have been able to conduct the study without the support of AHRQ,” says Dr. Williams. “We certainly need more research funds such as this. AHRQ is the primary federal agency funding health services research—however, they receive less than 5% of the funding that goes to NIH and fund more basic science-oriented research. As few as one in 10 grants submitted to AHRQ are actually funded.”
Like Dr. Maynard’s work on VTE prevention, the injection of AHRQ funds also allowed Dr. Williams’ project to continue and grow. “With support from the Society of Hospital Medicine, we have been quite fortunate to utilize the momentum from the AHRQ Patient Safe-[Discharge] grant to obtain a $1.4 million grant from the John A. Hartford Grant to develop a discharge toolkit and facilitate implementation of it at hundreds of hospital,” he explains. “The BOOST [Better Outcomes for Older adults through Safe Transitions] project aims to improve care delivery to older adults at hospitals across America as they transition from the hospital to home.”
Additional research is developed in AHRQ’s Centers for Education and Research in Therapeutics (CERTS). Each of the 11 CERTS has a specific charge and gathers data on the benefits, risks, and cost-effectiveness of therapeutic products such as drugs, medical devices, and biological products.
AHRQ disseminates current healthcare data quickly and more effectively than private channels. “They look at healthcare as a whole,” explains Dr. Fishmann. “For five years, they’ve published the annual National Quality Report and the National Disparity Report. They try to zero in on information to share with the public and with physicians, including all issues related to patient safety. They allow anyone access to the information: One market is hospitalists.”
AHRQ and Hospitalists
Of course, the research and information that AHRQ provides is vital to all physicians. But Dr. Fishmann believes hospitalists find the agency particularly valuable.
“SHM perceives AHRQ as their champion,” he says. “It’s a great partnership: AHRQ documents the value of having hospitalists. SHM provides an efficient way to disseminate new information relevant to hospitals.”
Many essential data and resources for physicians can be found on AHRQ’s Web site at www.ahrq.gov.
“The average hospitalist already uses this site, but I don’t think the average resident does,” says Dr. Fishmann. “I hope everyone knows about it.” TH
Jane Jerrard has written for The Hospitalist since 2005.
What exactly is the Agency for Healthcare Research and Quality (AHRQ), and why are hospitalists urged to increase its portion of the federal budget pie each year?
According to its mission statement, the AHRQ is “the lead federal agency charged with improving the quality, safety, efficiency, and effectiveness of healthcare for all Americans.” This includes supporting high-quality, impartial research that specifically improves healthcare quality, reduces costs, advances patient safety, decreases medical errors, eliminates healthcare disparities, and broadens access to essential services.
“Supporting AHRQ is supporting an unbiased government organization that’s clearly on the side of patient safety, and that gets important information out fast,” says Andrew Fishmann, MD, FCCP, FACP, a member of AHRQ’s National Advisory Council and director of intensive care at Good Samaritan Hospital in Los Angeles. “Where’s the argument?”
Fight over Funding
The argument is over money, plain and simple. Each year, medical associations like SHM push for increased federal funding for AHRQ so the agency’s research can be expanded. And each year, Congress refuses those increases. Lawmakers have granted a slight boost in funding: Since 2002, AHRQ’s budget has increased by $2 million, or 6.7%.
Proponents of AHRQ believe precarious funding levels threaten the agency’s ability to achieve its essential mission. Last year, SHM lobbied for an increase in federal funding for AHRQ to $350 million in fiscal year 2008—$31 million more than the agency’s fiscal 2007 budget. By late 2007, Congress was weighing an increase of $329 million, plus $5 million targeted for comparative-effectiveness research.
“Think of AHRQ compared to the $28 billion that NIH gets,” says Dr. Fishmann. “[AHRQ’s] is a small budget relative to what they do.”
How much does AHRQ need to provide adequate research information? The answer is, apparently, as much as they can get. There are countless areas in healthcare the agency could address.
“If they got $500 million, could they spend it?” asks Dr. Fishmann. “Yes. They could look at the top 20 diseases instead of the top 10.”
What AHRQ Does
Regardless of the final budget amount they receive, AHRQ spends roughly 80% on grants and contracts focused on improving healthcare.
“AHRQ doesn’t do its own research or create its own data,” explains Dr. Fishmann. Rather, AHRQ conducts and supports health services research in leading academic institutions, hospitals, and other settings. In 2005, two hospitalists received separate grants for projects that have already had an effect on hospital medicine. Greg Maynard, MD, MS, division chief of hospital medicine at University of California San Diego School of Medicine, used AHRQ funds for an intervention project to prevent hospital-acquired venous thromboembolism (VTE). Dr. Maynard’s project continued to grow since that grant and has yielded key findings such as a risk-assessment model for VTE. Data and lessons learned are available in the VTE Resource Room on SHM’s Web site at www.hospitalmedicine.org/ResourceRoomRedesign/RR_LandingPage.cfm.
Asked why he went after AHRQ funding, Dr. Maynard explains: “AHRQ is one of the few [funding] agencies that focuses on the realm of implementation—that impact the patient immediately. It was a perfect marriage of what we wanted to do.” The other AHRQ-funded hospital medicine project was conducted by Mark V. Williams, MD, FACP, professor and chief, division of hospital medicine at Northwestern University Feinberg School of Medicine in Chicago, and editor of the Journal of Hospital Medicine. Working for Emory University’s hospital medicine program in Atlanta at the time, Dr. Williams used the grant to create a “discharge bundle” of patient safety interventions such as medication reconciliation and patient-centered education to improve patient safety transitions out of the hospital setting.
“We would not have been able to conduct the study without the support of AHRQ,” says Dr. Williams. “We certainly need more research funds such as this. AHRQ is the primary federal agency funding health services research—however, they receive less than 5% of the funding that goes to NIH and fund more basic science-oriented research. As few as one in 10 grants submitted to AHRQ are actually funded.”
Like Dr. Maynard’s work on VTE prevention, the injection of AHRQ funds also allowed Dr. Williams’ project to continue and grow. “With support from the Society of Hospital Medicine, we have been quite fortunate to utilize the momentum from the AHRQ Patient Safe-[Discharge] grant to obtain a $1.4 million grant from the John A. Hartford Grant to develop a discharge toolkit and facilitate implementation of it at hundreds of hospital,” he explains. “The BOOST [Better Outcomes for Older adults through Safe Transitions] project aims to improve care delivery to older adults at hospitals across America as they transition from the hospital to home.”
Additional research is developed in AHRQ’s Centers for Education and Research in Therapeutics (CERTS). Each of the 11 CERTS has a specific charge and gathers data on the benefits, risks, and cost-effectiveness of therapeutic products such as drugs, medical devices, and biological products.
AHRQ disseminates current healthcare data quickly and more effectively than private channels. “They look at healthcare as a whole,” explains Dr. Fishmann. “For five years, they’ve published the annual National Quality Report and the National Disparity Report. They try to zero in on information to share with the public and with physicians, including all issues related to patient safety. They allow anyone access to the information: One market is hospitalists.”
AHRQ and Hospitalists
Of course, the research and information that AHRQ provides is vital to all physicians. But Dr. Fishmann believes hospitalists find the agency particularly valuable.
“SHM perceives AHRQ as their champion,” he says. “It’s a great partnership: AHRQ documents the value of having hospitalists. SHM provides an efficient way to disseminate new information relevant to hospitals.”
Many essential data and resources for physicians can be found on AHRQ’s Web site at www.ahrq.gov.
“The average hospitalist already uses this site, but I don’t think the average resident does,” says Dr. Fishmann. “I hope everyone knows about it.” TH
Jane Jerrard has written for The Hospitalist since 2005.
What exactly is the Agency for Healthcare Research and Quality (AHRQ), and why are hospitalists urged to increase its portion of the federal budget pie each year?
According to its mission statement, the AHRQ is “the lead federal agency charged with improving the quality, safety, efficiency, and effectiveness of healthcare for all Americans.” This includes supporting high-quality, impartial research that specifically improves healthcare quality, reduces costs, advances patient safety, decreases medical errors, eliminates healthcare disparities, and broadens access to essential services.
“Supporting AHRQ is supporting an unbiased government organization that’s clearly on the side of patient safety, and that gets important information out fast,” says Andrew Fishmann, MD, FCCP, FACP, a member of AHRQ’s National Advisory Council and director of intensive care at Good Samaritan Hospital in Los Angeles. “Where’s the argument?”
Fight over Funding
The argument is over money, plain and simple. Each year, medical associations like SHM push for increased federal funding for AHRQ so the agency’s research can be expanded. And each year, Congress refuses those increases. Lawmakers have granted a slight boost in funding: Since 2002, AHRQ’s budget has increased by $2 million, or 6.7%.
Proponents of AHRQ believe precarious funding levels threaten the agency’s ability to achieve its essential mission. Last year, SHM lobbied for an increase in federal funding for AHRQ to $350 million in fiscal year 2008—$31 million more than the agency’s fiscal 2007 budget. By late 2007, Congress was weighing an increase of $329 million, plus $5 million targeted for comparative-effectiveness research.
“Think of AHRQ compared to the $28 billion that NIH gets,” says Dr. Fishmann. “[AHRQ’s] is a small budget relative to what they do.”
How much does AHRQ need to provide adequate research information? The answer is, apparently, as much as they can get. There are countless areas in healthcare the agency could address.
“If they got $500 million, could they spend it?” asks Dr. Fishmann. “Yes. They could look at the top 20 diseases instead of the top 10.”
What AHRQ Does
Regardless of the final budget amount they receive, AHRQ spends roughly 80% on grants and contracts focused on improving healthcare.
“AHRQ doesn’t do its own research or create its own data,” explains Dr. Fishmann. Rather, AHRQ conducts and supports health services research in leading academic institutions, hospitals, and other settings. In 2005, two hospitalists received separate grants for projects that have already had an effect on hospital medicine. Greg Maynard, MD, MS, division chief of hospital medicine at University of California San Diego School of Medicine, used AHRQ funds for an intervention project to prevent hospital-acquired venous thromboembolism (VTE). Dr. Maynard’s project continued to grow since that grant and has yielded key findings such as a risk-assessment model for VTE. Data and lessons learned are available in the VTE Resource Room on SHM’s Web site at www.hospitalmedicine.org/ResourceRoomRedesign/RR_LandingPage.cfm.
Asked why he went after AHRQ funding, Dr. Maynard explains: “AHRQ is one of the few [funding] agencies that focuses on the realm of implementation—that impact the patient immediately. It was a perfect marriage of what we wanted to do.” The other AHRQ-funded hospital medicine project was conducted by Mark V. Williams, MD, FACP, professor and chief, division of hospital medicine at Northwestern University Feinberg School of Medicine in Chicago, and editor of the Journal of Hospital Medicine. Working for Emory University’s hospital medicine program in Atlanta at the time, Dr. Williams used the grant to create a “discharge bundle” of patient safety interventions such as medication reconciliation and patient-centered education to improve patient safety transitions out of the hospital setting.
“We would not have been able to conduct the study without the support of AHRQ,” says Dr. Williams. “We certainly need more research funds such as this. AHRQ is the primary federal agency funding health services research—however, they receive less than 5% of the funding that goes to NIH and fund more basic science-oriented research. As few as one in 10 grants submitted to AHRQ are actually funded.”
Like Dr. Maynard’s work on VTE prevention, the injection of AHRQ funds also allowed Dr. Williams’ project to continue and grow. “With support from the Society of Hospital Medicine, we have been quite fortunate to utilize the momentum from the AHRQ Patient Safe-[Discharge] grant to obtain a $1.4 million grant from the John A. Hartford Grant to develop a discharge toolkit and facilitate implementation of it at hundreds of hospital,” he explains. “The BOOST [Better Outcomes for Older adults through Safe Transitions] project aims to improve care delivery to older adults at hospitals across America as they transition from the hospital to home.”
Additional research is developed in AHRQ’s Centers for Education and Research in Therapeutics (CERTS). Each of the 11 CERTS has a specific charge and gathers data on the benefits, risks, and cost-effectiveness of therapeutic products such as drugs, medical devices, and biological products.
AHRQ disseminates current healthcare data quickly and more effectively than private channels. “They look at healthcare as a whole,” explains Dr. Fishmann. “For five years, they’ve published the annual National Quality Report and the National Disparity Report. They try to zero in on information to share with the public and with physicians, including all issues related to patient safety. They allow anyone access to the information: One market is hospitalists.”
AHRQ and Hospitalists
Of course, the research and information that AHRQ provides is vital to all physicians. But Dr. Fishmann believes hospitalists find the agency particularly valuable.
“SHM perceives AHRQ as their champion,” he says. “It’s a great partnership: AHRQ documents the value of having hospitalists. SHM provides an efficient way to disseminate new information relevant to hospitals.”
Many essential data and resources for physicians can be found on AHRQ’s Web site at www.ahrq.gov.
“The average hospitalist already uses this site, but I don’t think the average resident does,” says Dr. Fishmann. “I hope everyone knows about it.” TH
Jane Jerrard has written for The Hospitalist since 2005.
Medical Mediation
How many conflicts do you witness during your average shift? How many are you embroiled in? Are any of them resolved amicably? How many can you resolve?
“Everyone does conflict resolution on some level,” says Leonard J. Marcus, PhD, director of the Program for Health Care Negotiation and Conflict Resolution, Harvard School of Public Health in Cambridge, Mass. “If you’re in a relationship, if you have kids—we all do it. The difference is that hospitalists do it as part of their professional work, and that requires a different level of complexity.” Dr. Marcus teaches conflict resolution skills in SHM’s Leadership Academy.
Everyone’s Best Interest
Dr. Marcus’ colleague and associate director of his program is Barry C. Dorn, MD, MHCM. Dr. Dorn clarifies: “Conflict is not bad. But unresolved conflict can be costly.”
A good leader can—and should—resolve problems on his team for the sake of the team, the project or work, and the hospital. Understanding that is easy—it’s how to end the problem that can be tricky.
“There are two poles to conflict resolution,” explains Dr. Marcus. “Positional bargaining is the adversarial win-lose approach to problem solving, and many people believe that’s the only way to resolve a conflict. However, interest-based negotiation focuses on what different people want to accomplish. It’s what we call a collaborative, cooperative approach—it’s gain-gain negotiating.”
How Hard Can It Be?
As Dr. Marcus says, everyone resolves conflicts. So is training in how to go about it really necessary?
“Absolutely,” asserts Dr. Dorn. “[Hospitalist leaders] need some sort of training, though a lot of it can be self-taught. I think this training is the most important thing a physician can do. The stresses and rapid changes in healthcare today make people crazy. It’s not just hospitalists—all physicians have conflicts with other groups. That conflict takes its toll; it’s a tremendous waste of time and energy. And it’s very costly to an institution to have people constantly at odds.”
Dr. Marcus agrees. “Given the role of the hospitalist, having specific skills training in conflict resolution is a huge plus,” he says. “They regularly face challenges in engaging other departments and other physicians, which can lead to turf wars and territoriality. They have to go beyond the simple ability to resolve conflicts and get to the core of the issue quickly. That’s where the training comes in.”
Conflicts among Peers
To further complicate the conflicts they face, hospitalists often find themselves managing a group of peers as committee chairs or lead researchers. They don’t have the title or authority to tell fellow hospitalists, other physicians, hospital staff, and administrators what to do. This can lead to some delicate conflict resolution.
“They’re dealing with people who lead individual silos within the healthcare system,” says Dr. Dorn. “And when someone else wants to step into their silo, it makes you and them uncomfortable. Leaders have to make others feel comfortable and learn to speak their language. Hospitalists have to lead across silos as well as within their own silo [of hospital medicine]; then they have to lead up, because hospital administrators have a lot of control. There are many nuances to leadership.”
As group leaders, hospitalists may face a wide range of conflicts, says Dr. Marcus, “from differences of opinion to resistance to downright draw-the-line-in-the-sand and get out of my way. The other piece is that some issues are clinical, whether between physicians, between physician and patient or family member, and some are administrative or managerial. Hospitalists are at the hub of all those issues; they serve as the fulcrum.”
According to Dr. Dorn, physician-physician conflicts can be disagreements of opinion, of training, of personality, and of reimbursement issues. “Physicians are very concerned with reimbursement—they want to know what it is going to cost them in time and money,” he explains.
For a hospitalist serving as a committee chair, says Dr. Dorn, “The critical thing is that when they assume these positions without authority, the only way to make it work is to increase their level of influence. The level of influence over authority is the indication of a good leader.”
You can acquire the necessary influence by learning solid conflict resolution skills.
Resolve to Study
There are a number of resources available for hospitalists interested in studying conflict resolution. Drs. Dorn and Marcus have co-written a book on the subject, Renegotiating Health Care: Resolving Conflict to Build Collaboration. “The conflicts we deal with [in the book] are right at the core of what’s going on in healthcare right now,” Dr. Marcus says.
Dr. Dorn also recommends some general books on resolving conflict. “Most of conflict resolution is interest-based negotiation, and the father of interest-based negotiation is Roger Fisher,” he says. “With Bill Ury, he wrote Getting to Yes. I think a better book for physicians is Getting Past No. It’s very simple and concise. These are basic books on conflict resolution.”
For a more detailed textbook, Dr. Dorn suggests The Mediation Process: Practical Strategies for Resolving Conflicts by Chris Moore. “This is the definitive text,” he says. “I also like Difficult Conversations by Stone, Patton, and Heen.”
Whether you’re in a leadership role or a hospitalist doing straight clinical work, successfully resolving conflicts on the job can be a much-appreciated skill. “[Conflict resolution training] will make your life so much easier, so much more pleasant,” promises Dr. Dorn. TH
Jane Jerrard writes “Public Policy” for The Hospitalist.
How many conflicts do you witness during your average shift? How many are you embroiled in? Are any of them resolved amicably? How many can you resolve?
“Everyone does conflict resolution on some level,” says Leonard J. Marcus, PhD, director of the Program for Health Care Negotiation and Conflict Resolution, Harvard School of Public Health in Cambridge, Mass. “If you’re in a relationship, if you have kids—we all do it. The difference is that hospitalists do it as part of their professional work, and that requires a different level of complexity.” Dr. Marcus teaches conflict resolution skills in SHM’s Leadership Academy.
Everyone’s Best Interest
Dr. Marcus’ colleague and associate director of his program is Barry C. Dorn, MD, MHCM. Dr. Dorn clarifies: “Conflict is not bad. But unresolved conflict can be costly.”
A good leader can—and should—resolve problems on his team for the sake of the team, the project or work, and the hospital. Understanding that is easy—it’s how to end the problem that can be tricky.
“There are two poles to conflict resolution,” explains Dr. Marcus. “Positional bargaining is the adversarial win-lose approach to problem solving, and many people believe that’s the only way to resolve a conflict. However, interest-based negotiation focuses on what different people want to accomplish. It’s what we call a collaborative, cooperative approach—it’s gain-gain negotiating.”
How Hard Can It Be?
As Dr. Marcus says, everyone resolves conflicts. So is training in how to go about it really necessary?
“Absolutely,” asserts Dr. Dorn. “[Hospitalist leaders] need some sort of training, though a lot of it can be self-taught. I think this training is the most important thing a physician can do. The stresses and rapid changes in healthcare today make people crazy. It’s not just hospitalists—all physicians have conflicts with other groups. That conflict takes its toll; it’s a tremendous waste of time and energy. And it’s very costly to an institution to have people constantly at odds.”
Dr. Marcus agrees. “Given the role of the hospitalist, having specific skills training in conflict resolution is a huge plus,” he says. “They regularly face challenges in engaging other departments and other physicians, which can lead to turf wars and territoriality. They have to go beyond the simple ability to resolve conflicts and get to the core of the issue quickly. That’s where the training comes in.”
Conflicts among Peers
To further complicate the conflicts they face, hospitalists often find themselves managing a group of peers as committee chairs or lead researchers. They don’t have the title or authority to tell fellow hospitalists, other physicians, hospital staff, and administrators what to do. This can lead to some delicate conflict resolution.
“They’re dealing with people who lead individual silos within the healthcare system,” says Dr. Dorn. “And when someone else wants to step into their silo, it makes you and them uncomfortable. Leaders have to make others feel comfortable and learn to speak their language. Hospitalists have to lead across silos as well as within their own silo [of hospital medicine]; then they have to lead up, because hospital administrators have a lot of control. There are many nuances to leadership.”
As group leaders, hospitalists may face a wide range of conflicts, says Dr. Marcus, “from differences of opinion to resistance to downright draw-the-line-in-the-sand and get out of my way. The other piece is that some issues are clinical, whether between physicians, between physician and patient or family member, and some are administrative or managerial. Hospitalists are at the hub of all those issues; they serve as the fulcrum.”
According to Dr. Dorn, physician-physician conflicts can be disagreements of opinion, of training, of personality, and of reimbursement issues. “Physicians are very concerned with reimbursement—they want to know what it is going to cost them in time and money,” he explains.
For a hospitalist serving as a committee chair, says Dr. Dorn, “The critical thing is that when they assume these positions without authority, the only way to make it work is to increase their level of influence. The level of influence over authority is the indication of a good leader.”
You can acquire the necessary influence by learning solid conflict resolution skills.
Resolve to Study
There are a number of resources available for hospitalists interested in studying conflict resolution. Drs. Dorn and Marcus have co-written a book on the subject, Renegotiating Health Care: Resolving Conflict to Build Collaboration. “The conflicts we deal with [in the book] are right at the core of what’s going on in healthcare right now,” Dr. Marcus says.
Dr. Dorn also recommends some general books on resolving conflict. “Most of conflict resolution is interest-based negotiation, and the father of interest-based negotiation is Roger Fisher,” he says. “With Bill Ury, he wrote Getting to Yes. I think a better book for physicians is Getting Past No. It’s very simple and concise. These are basic books on conflict resolution.”
For a more detailed textbook, Dr. Dorn suggests The Mediation Process: Practical Strategies for Resolving Conflicts by Chris Moore. “This is the definitive text,” he says. “I also like Difficult Conversations by Stone, Patton, and Heen.”
Whether you’re in a leadership role or a hospitalist doing straight clinical work, successfully resolving conflicts on the job can be a much-appreciated skill. “[Conflict resolution training] will make your life so much easier, so much more pleasant,” promises Dr. Dorn. TH
Jane Jerrard writes “Public Policy” for The Hospitalist.
How many conflicts do you witness during your average shift? How many are you embroiled in? Are any of them resolved amicably? How many can you resolve?
“Everyone does conflict resolution on some level,” says Leonard J. Marcus, PhD, director of the Program for Health Care Negotiation and Conflict Resolution, Harvard School of Public Health in Cambridge, Mass. “If you’re in a relationship, if you have kids—we all do it. The difference is that hospitalists do it as part of their professional work, and that requires a different level of complexity.” Dr. Marcus teaches conflict resolution skills in SHM’s Leadership Academy.
Everyone’s Best Interest
Dr. Marcus’ colleague and associate director of his program is Barry C. Dorn, MD, MHCM. Dr. Dorn clarifies: “Conflict is not bad. But unresolved conflict can be costly.”
A good leader can—and should—resolve problems on his team for the sake of the team, the project or work, and the hospital. Understanding that is easy—it’s how to end the problem that can be tricky.
“There are two poles to conflict resolution,” explains Dr. Marcus. “Positional bargaining is the adversarial win-lose approach to problem solving, and many people believe that’s the only way to resolve a conflict. However, interest-based negotiation focuses on what different people want to accomplish. It’s what we call a collaborative, cooperative approach—it’s gain-gain negotiating.”
How Hard Can It Be?
As Dr. Marcus says, everyone resolves conflicts. So is training in how to go about it really necessary?
“Absolutely,” asserts Dr. Dorn. “[Hospitalist leaders] need some sort of training, though a lot of it can be self-taught. I think this training is the most important thing a physician can do. The stresses and rapid changes in healthcare today make people crazy. It’s not just hospitalists—all physicians have conflicts with other groups. That conflict takes its toll; it’s a tremendous waste of time and energy. And it’s very costly to an institution to have people constantly at odds.”
Dr. Marcus agrees. “Given the role of the hospitalist, having specific skills training in conflict resolution is a huge plus,” he says. “They regularly face challenges in engaging other departments and other physicians, which can lead to turf wars and territoriality. They have to go beyond the simple ability to resolve conflicts and get to the core of the issue quickly. That’s where the training comes in.”
Conflicts among Peers
To further complicate the conflicts they face, hospitalists often find themselves managing a group of peers as committee chairs or lead researchers. They don’t have the title or authority to tell fellow hospitalists, other physicians, hospital staff, and administrators what to do. This can lead to some delicate conflict resolution.
“They’re dealing with people who lead individual silos within the healthcare system,” says Dr. Dorn. “And when someone else wants to step into their silo, it makes you and them uncomfortable. Leaders have to make others feel comfortable and learn to speak their language. Hospitalists have to lead across silos as well as within their own silo [of hospital medicine]; then they have to lead up, because hospital administrators have a lot of control. There are many nuances to leadership.”
As group leaders, hospitalists may face a wide range of conflicts, says Dr. Marcus, “from differences of opinion to resistance to downright draw-the-line-in-the-sand and get out of my way. The other piece is that some issues are clinical, whether between physicians, between physician and patient or family member, and some are administrative or managerial. Hospitalists are at the hub of all those issues; they serve as the fulcrum.”
According to Dr. Dorn, physician-physician conflicts can be disagreements of opinion, of training, of personality, and of reimbursement issues. “Physicians are very concerned with reimbursement—they want to know what it is going to cost them in time and money,” he explains.
For a hospitalist serving as a committee chair, says Dr. Dorn, “The critical thing is that when they assume these positions without authority, the only way to make it work is to increase their level of influence. The level of influence over authority is the indication of a good leader.”
You can acquire the necessary influence by learning solid conflict resolution skills.
Resolve to Study
There are a number of resources available for hospitalists interested in studying conflict resolution. Drs. Dorn and Marcus have co-written a book on the subject, Renegotiating Health Care: Resolving Conflict to Build Collaboration. “The conflicts we deal with [in the book] are right at the core of what’s going on in healthcare right now,” Dr. Marcus says.
Dr. Dorn also recommends some general books on resolving conflict. “Most of conflict resolution is interest-based negotiation, and the father of interest-based negotiation is Roger Fisher,” he says. “With Bill Ury, he wrote Getting to Yes. I think a better book for physicians is Getting Past No. It’s very simple and concise. These are basic books on conflict resolution.”
For a more detailed textbook, Dr. Dorn suggests The Mediation Process: Practical Strategies for Resolving Conflicts by Chris Moore. “This is the definitive text,” he says. “I also like Difficult Conversations by Stone, Patton, and Heen.”
Whether you’re in a leadership role or a hospitalist doing straight clinical work, successfully resolving conflicts on the job can be a much-appreciated skill. “[Conflict resolution training] will make your life so much easier, so much more pleasant,” promises Dr. Dorn. TH
Jane Jerrard writes “Public Policy” for The Hospitalist.
Hail Fellows
Because hospital medicine is still a new specialty, the finer points of hospitalist education and training are being developed. According to two papers in this month’s Journal of Hospital Medicine, students, hospitalists, employers—even patients—are eager for programs that allow hospitalists to hone their skills.
In one study, investigators at the University of California, San Francisco (UCSF), designed a program for third-year medical students and pharmacy graduate students emphasizing the issues involved in making the transition from inpatient to outpatient care.
Patients often are overwhelmed by the change, and the situation is ripe for miscommunication and error. Students accustomed to seeing these individuals only in the hospital often underestimate the challenges patients confront when they leave the hospital with a bewildering array of instructions and medications.
Medical students also receive little exposure to the roles of caregivers from other fields, yet good transitional care involves professionals from several disciplines. The authors, led by Cindy Lai, MD, assistant clinical professor of medicine at UCSF, reason that “training students in interdisciplinary collaboration may improve their ability to provide quality care.”
They designed an inpatient medicine clerkship curriculum in which teams of medical and pharmacy students paid a home visit to a patient they had cared for in the hospital. After the visits, the students wrote summary letters to each patient’s primary care physician.
The home visits lasted one to two hours and in general consisted of an introduction to the patient’s living quarters, a review of symptoms and medication, a brief physical examination, and a home tour to check for relevant issues such as safety hazards or the patient’s ability to function independently. Students quickly discovered the visits consisted of much more than that.
“Across the board, the response that came back was the ability to view the patient as a person,” says Heather Nye, MD, PhD, assistant clinical professor of internal medicine and one of the authors of the study. Students found it inspiring to see patients as people in control of their surroundings and also were surprised how well or poorly some people did away from the hospital.
They learned how to maximize their interaction with the pharmacy students and how to anticipate problems patients might encounter at home, such as taking medicines appropriately or scheduling and keeping follow-up appointments.
Apparently, the lessons went both ways, with some patients inviting students to stay for dinner or dessert. “That human aspect was one of the most profound features of the visits,” Dr. Nye says.
She acknowledges that scheduling home visits regularly would require a commitment of time and money that is simply not feasible in today’s environment, especially after medical school. But she urged that instruction in transitional and interdisciplinary care be incorporated into the curriculum whenever possible. “We all understand that safe discharges require multiple disciplines,” she said. “It’s never too early to start teaching about transitional care.”
Medical school training is especially important because students and residents who specialize in hospital medicine will find fellowships in short supply. The few that exist function more to train educators rather than practicing physicians.
—Philip Goodman, MD, professor of internal medicine and biomedical engineering, University of Nevada, Reno, School of Medicine
This despite the fact hospital medicine has grown at a near-exponential pace, from 2000 practitioners in 1998 to 15,000 in 2005, with 30,000 projected by 2010.
Practicing hospitalists and residents Philip Goodman, MD, MS, and Andrius Januska, BS, of the University of Nevada, Reno, School of Medicine set about to gauge the value of and interest in a practical fellowship in hospital medicine to employers. They sent questionnaires to employers and practicing hospitalists. Of 103 employers, two-thirds indicated they would offer fellowship graduates a signing bonus or salary premium ranging from $10,000 to more than $20,000.
Of 101 practicing hospitalists, 58% felt a clinical fellowship probably or strongly would be a good career move. Further, 91% said it would at least possibly be a good move. And 57% of the residents thinking of a career in hospital medicine said they would consider a one-year clinical fellowship if one were available.
“I was surprised at how strongly practicing hospitalists, most of whom are not academics, supported the value of an intense year of clinical hospital medicine fellowship training,” says Dr. Goodman, professor of internal medicine and biomedical engineering. “Most felt that graduating internal medicine residents ‘probably’ or ‘strongly’ should consider such fellowship training. I had expected a more neutral response, reflecting a balanced response bias of those with strong feelings at either extreme.”
Such training can offer new physicians a chance to develop expertise and leadership capabilities that might otherwise require years of on-the-job experience, he explains. Fellowship training also might elevate hospitalists to a level of prestige equaling that of other subspecialties, he says.
Ironically, the specialty’s rapid growth is probably slowing the establishment of fellowship programs, because residents can command annual salaries of $160,000 to $200,000 upon graduation with no special fellowship training. But a few months into it, they often realize a fellowship would have helped them master some of the unique aspects of hospital medicine, such as process of care, communication, productivity and medicolegal insight, and quality improvement, Dr. Goodman notes.
The University of Nevada will start training its first six hospitalist fellows next year. “I wouldn’t be surprised if most applicants were those who had recently taken hospitalist positions but realized the professional impact a year of polishing school can provide,” he says. TH
Norra MacReady is a medical writer based in California.
Because hospital medicine is still a new specialty, the finer points of hospitalist education and training are being developed. According to two papers in this month’s Journal of Hospital Medicine, students, hospitalists, employers—even patients—are eager for programs that allow hospitalists to hone their skills.
In one study, investigators at the University of California, San Francisco (UCSF), designed a program for third-year medical students and pharmacy graduate students emphasizing the issues involved in making the transition from inpatient to outpatient care.
Patients often are overwhelmed by the change, and the situation is ripe for miscommunication and error. Students accustomed to seeing these individuals only in the hospital often underestimate the challenges patients confront when they leave the hospital with a bewildering array of instructions and medications.
Medical students also receive little exposure to the roles of caregivers from other fields, yet good transitional care involves professionals from several disciplines. The authors, led by Cindy Lai, MD, assistant clinical professor of medicine at UCSF, reason that “training students in interdisciplinary collaboration may improve their ability to provide quality care.”
They designed an inpatient medicine clerkship curriculum in which teams of medical and pharmacy students paid a home visit to a patient they had cared for in the hospital. After the visits, the students wrote summary letters to each patient’s primary care physician.
The home visits lasted one to two hours and in general consisted of an introduction to the patient’s living quarters, a review of symptoms and medication, a brief physical examination, and a home tour to check for relevant issues such as safety hazards or the patient’s ability to function independently. Students quickly discovered the visits consisted of much more than that.
“Across the board, the response that came back was the ability to view the patient as a person,” says Heather Nye, MD, PhD, assistant clinical professor of internal medicine and one of the authors of the study. Students found it inspiring to see patients as people in control of their surroundings and also were surprised how well or poorly some people did away from the hospital.
They learned how to maximize their interaction with the pharmacy students and how to anticipate problems patients might encounter at home, such as taking medicines appropriately or scheduling and keeping follow-up appointments.
Apparently, the lessons went both ways, with some patients inviting students to stay for dinner or dessert. “That human aspect was one of the most profound features of the visits,” Dr. Nye says.
She acknowledges that scheduling home visits regularly would require a commitment of time and money that is simply not feasible in today’s environment, especially after medical school. But she urged that instruction in transitional and interdisciplinary care be incorporated into the curriculum whenever possible. “We all understand that safe discharges require multiple disciplines,” she said. “It’s never too early to start teaching about transitional care.”
Medical school training is especially important because students and residents who specialize in hospital medicine will find fellowships in short supply. The few that exist function more to train educators rather than practicing physicians.
—Philip Goodman, MD, professor of internal medicine and biomedical engineering, University of Nevada, Reno, School of Medicine
This despite the fact hospital medicine has grown at a near-exponential pace, from 2000 practitioners in 1998 to 15,000 in 2005, with 30,000 projected by 2010.
Practicing hospitalists and residents Philip Goodman, MD, MS, and Andrius Januska, BS, of the University of Nevada, Reno, School of Medicine set about to gauge the value of and interest in a practical fellowship in hospital medicine to employers. They sent questionnaires to employers and practicing hospitalists. Of 103 employers, two-thirds indicated they would offer fellowship graduates a signing bonus or salary premium ranging from $10,000 to more than $20,000.
Of 101 practicing hospitalists, 58% felt a clinical fellowship probably or strongly would be a good career move. Further, 91% said it would at least possibly be a good move. And 57% of the residents thinking of a career in hospital medicine said they would consider a one-year clinical fellowship if one were available.
“I was surprised at how strongly practicing hospitalists, most of whom are not academics, supported the value of an intense year of clinical hospital medicine fellowship training,” says Dr. Goodman, professor of internal medicine and biomedical engineering. “Most felt that graduating internal medicine residents ‘probably’ or ‘strongly’ should consider such fellowship training. I had expected a more neutral response, reflecting a balanced response bias of those with strong feelings at either extreme.”
Such training can offer new physicians a chance to develop expertise and leadership capabilities that might otherwise require years of on-the-job experience, he explains. Fellowship training also might elevate hospitalists to a level of prestige equaling that of other subspecialties, he says.
Ironically, the specialty’s rapid growth is probably slowing the establishment of fellowship programs, because residents can command annual salaries of $160,000 to $200,000 upon graduation with no special fellowship training. But a few months into it, they often realize a fellowship would have helped them master some of the unique aspects of hospital medicine, such as process of care, communication, productivity and medicolegal insight, and quality improvement, Dr. Goodman notes.
The University of Nevada will start training its first six hospitalist fellows next year. “I wouldn’t be surprised if most applicants were those who had recently taken hospitalist positions but realized the professional impact a year of polishing school can provide,” he says. TH
Norra MacReady is a medical writer based in California.
Because hospital medicine is still a new specialty, the finer points of hospitalist education and training are being developed. According to two papers in this month’s Journal of Hospital Medicine, students, hospitalists, employers—even patients—are eager for programs that allow hospitalists to hone their skills.
In one study, investigators at the University of California, San Francisco (UCSF), designed a program for third-year medical students and pharmacy graduate students emphasizing the issues involved in making the transition from inpatient to outpatient care.
Patients often are overwhelmed by the change, and the situation is ripe for miscommunication and error. Students accustomed to seeing these individuals only in the hospital often underestimate the challenges patients confront when they leave the hospital with a bewildering array of instructions and medications.
Medical students also receive little exposure to the roles of caregivers from other fields, yet good transitional care involves professionals from several disciplines. The authors, led by Cindy Lai, MD, assistant clinical professor of medicine at UCSF, reason that “training students in interdisciplinary collaboration may improve their ability to provide quality care.”
They designed an inpatient medicine clerkship curriculum in which teams of medical and pharmacy students paid a home visit to a patient they had cared for in the hospital. After the visits, the students wrote summary letters to each patient’s primary care physician.
The home visits lasted one to two hours and in general consisted of an introduction to the patient’s living quarters, a review of symptoms and medication, a brief physical examination, and a home tour to check for relevant issues such as safety hazards or the patient’s ability to function independently. Students quickly discovered the visits consisted of much more than that.
“Across the board, the response that came back was the ability to view the patient as a person,” says Heather Nye, MD, PhD, assistant clinical professor of internal medicine and one of the authors of the study. Students found it inspiring to see patients as people in control of their surroundings and also were surprised how well or poorly some people did away from the hospital.
They learned how to maximize their interaction with the pharmacy students and how to anticipate problems patients might encounter at home, such as taking medicines appropriately or scheduling and keeping follow-up appointments.
Apparently, the lessons went both ways, with some patients inviting students to stay for dinner or dessert. “That human aspect was one of the most profound features of the visits,” Dr. Nye says.
She acknowledges that scheduling home visits regularly would require a commitment of time and money that is simply not feasible in today’s environment, especially after medical school. But she urged that instruction in transitional and interdisciplinary care be incorporated into the curriculum whenever possible. “We all understand that safe discharges require multiple disciplines,” she said. “It’s never too early to start teaching about transitional care.”
Medical school training is especially important because students and residents who specialize in hospital medicine will find fellowships in short supply. The few that exist function more to train educators rather than practicing physicians.
—Philip Goodman, MD, professor of internal medicine and biomedical engineering, University of Nevada, Reno, School of Medicine
This despite the fact hospital medicine has grown at a near-exponential pace, from 2000 practitioners in 1998 to 15,000 in 2005, with 30,000 projected by 2010.
Practicing hospitalists and residents Philip Goodman, MD, MS, and Andrius Januska, BS, of the University of Nevada, Reno, School of Medicine set about to gauge the value of and interest in a practical fellowship in hospital medicine to employers. They sent questionnaires to employers and practicing hospitalists. Of 103 employers, two-thirds indicated they would offer fellowship graduates a signing bonus or salary premium ranging from $10,000 to more than $20,000.
Of 101 practicing hospitalists, 58% felt a clinical fellowship probably or strongly would be a good career move. Further, 91% said it would at least possibly be a good move. And 57% of the residents thinking of a career in hospital medicine said they would consider a one-year clinical fellowship if one were available.
“I was surprised at how strongly practicing hospitalists, most of whom are not academics, supported the value of an intense year of clinical hospital medicine fellowship training,” says Dr. Goodman, professor of internal medicine and biomedical engineering. “Most felt that graduating internal medicine residents ‘probably’ or ‘strongly’ should consider such fellowship training. I had expected a more neutral response, reflecting a balanced response bias of those with strong feelings at either extreme.”
Such training can offer new physicians a chance to develop expertise and leadership capabilities that might otherwise require years of on-the-job experience, he explains. Fellowship training also might elevate hospitalists to a level of prestige equaling that of other subspecialties, he says.
Ironically, the specialty’s rapid growth is probably slowing the establishment of fellowship programs, because residents can command annual salaries of $160,000 to $200,000 upon graduation with no special fellowship training. But a few months into it, they often realize a fellowship would have helped them master some of the unique aspects of hospital medicine, such as process of care, communication, productivity and medicolegal insight, and quality improvement, Dr. Goodman notes.
The University of Nevada will start training its first six hospitalist fellows next year. “I wouldn’t be surprised if most applicants were those who had recently taken hospitalist positions but realized the professional impact a year of polishing school can provide,” he says. TH
Norra MacReady is a medical writer based in California.
Liver Risks Abound
Drug-induced liver injury (DILI) or hepatotoxicity accounts for more than 50% of all cases of acute liver failure. DILI, often life-threatening, is the leading cause of patient referral for liver transplantation.1,2
DILI is an important diagnostic challenge for the treating clinician because of its presentation, which is often a diagnosis of exclusion. Determination of all potential causes of hepatic injury need to be assessed through onset of symptoms and a careful drug history (including prescription and over-the-counter medication, dietary supplements, and complementary and alternative therapies).3
DILI has brought an increase in Food and Drug Administration (FDA) “black box” warnings. Among the drugs affected are ketoconazole, pemoline, tolcapone, valproate sodium, and zalcitabine.4
A number of drugs have been withdrawn from the U.S. market after DILI or interactions with those that are hepatically metabolized, such as:
- Astemizole (cardiotoxicity);
- Bromfenac sodium, cisapride (cardiotoxicity);
- Felbamate, mibefradil (cardiotoxicity);
- Temafloxacin (abnormal liver function tests, as well as renal failure and other serious adverse events);
- Terfenadine (cardiotoxicity);
- Troglitazone; and
- Trovafloxacin mesylate.
One of the most common causes of DILI is intentional or unintentional overdose with acetaminophen.
DILI has been classified into two major types: cholestatic and hepatocellular, or cytolytic injury. In cholestatic liver injury, the serum alkaline phosphatase (ALP) is elevated; total bilirubin level (TBL) and the alanine aminotransferase (ALT) may also be elevated. In hepatocellular injury, initial elevation is noted in the ALT.
There may also be overlap in the pattern of injury (mixed-pattern injury) whereby ALP and ALT are elevated. These patterns of injury may be defined further by the degree of enzyme elevation, such as an ALT level of three or more times the upper limit of normal (ULN), an ALP level two or more times ULN, and TBL two or more times ULN if associated with an elevation of ALP or ALT.
Hepatotoxicity is often predictable—but not always. When predictable, the reaction is usually dose-dependent, such as in the case of acetaminophen. These reactions usually occur shortly after a threshold for toxicity has been reached. Unpredictable reactions can occur days or months after exposure, usually without warning. Hypersensitivity reactions are often delayed and occur upon repeated exposure to the agent. Symptoms of immunologic injury may include rash, fever, or eosinophilia. More severe forms include Stevens-Johnson syndrome, toxic epidermal necrolysis, or cytopenias. Reactions are more severe upon repeat exposure or rechallenge of the offending agent.
Symptomatology
Patients usually present with vague symptoms that may include nausea, anorexia, fatigue, right upper-quadrant discomfort, jaundice, or dark urine. Patients with cholestatic liver disease may also present with pruritus.
Any of these along with laboratory evidence of liver injury should indicate further investigation into possible DILI. Impaired hepatic function such as increased prothrombin time and encephalopathy (signs of acute liver failure) indicate severe hepatic injury.
The Agents
Common causes of hepatocellular injury include: acetaminophen, fluoxetine, highly active antiretroviral therapies, kava kava (other herbal products), non-steroidal anti-inflammatory drugs, paroxetine, rifampin, risperidone, statins, trazodone, and troglitazone.
Common causes of cholestatic injury include: ampicillin-clavulanic acid, anabolic steroids, chlorpromazine, clopidogrel, estrogens, mirtazapine, terbinafine, and tricyclics.
Common causes of mixed-pattern injury include: amitriptyline, azathioprine, captopril, carbamazepine, enalapril, erythromycins, flutamide, phenytoin, sulfonamides, trazodone, and trimethoprim-sulfamethoxazole.
Most cases of non-fulminant hepatitis will improve upon cessation of offending or potentially offending agent(s). Assess hepatic injury immediately via continuously obtained biochemical tests.
Consult a hepatologist or gastroenterologist immediately if jaundice, impaired hepatic function or clinical signs of acute hepatic failure (e.g., encephalopathy) are evident.
Report all cases of potential DILI to the FDA’s adverse events reporting program, Medwatch, at www.fda.gov/ medwatch or by calling (800) 332-1088. For patients receiving potentially hepatotoxic agents, liver function test monitoring is recommended following a baseline assessment. Some agents require monthly rather than periodic monitoring. For a list of some agents that require hepatic monitoring and the recommended frequency, visit www.factsandcomparisons.com/assets/hospitalpharm/feb2002_HepSp.pdf.5 TH
Michele B. Kaufman is a freelance medical writer based in New York City.
References
- Nathwani RA, Kaplowitz N. Drug hepatotoxicity. Clin Liver Dis. 2006;10:207-217.
- Navarro VJ, Senior JR. Drug-related hepatotoxicity. N Engl J Med. 2006;354(7):731-739.
- Hepatic Toxicity Possibly Associated with Kava-Containing Products—United States, Germany, and Switzerland, 1999-2002. MMWR Weekly, Nov. 29, 2002/51(47):1065-1067. Available at: www.cdc.gov/MMWR/previews/mmwrhtml/mm5147a1.htm. Last accessed Nov. 5, 2007.
- Lasser KE, Allen PD, Woolhandler SJ, et al. Timing of new black box warnings and withdrawals for prescription medications. JAMA. 2002;287:17:2215-2220.
- Tice SA, Parr D. Medications that require hepatic monitoring. Hospital Pharmacy 2004;39(6):595-606.
Drug-induced liver injury (DILI) or hepatotoxicity accounts for more than 50% of all cases of acute liver failure. DILI, often life-threatening, is the leading cause of patient referral for liver transplantation.1,2
DILI is an important diagnostic challenge for the treating clinician because of its presentation, which is often a diagnosis of exclusion. Determination of all potential causes of hepatic injury need to be assessed through onset of symptoms and a careful drug history (including prescription and over-the-counter medication, dietary supplements, and complementary and alternative therapies).3
DILI has brought an increase in Food and Drug Administration (FDA) “black box” warnings. Among the drugs affected are ketoconazole, pemoline, tolcapone, valproate sodium, and zalcitabine.4
A number of drugs have been withdrawn from the U.S. market after DILI or interactions with those that are hepatically metabolized, such as:
- Astemizole (cardiotoxicity);
- Bromfenac sodium, cisapride (cardiotoxicity);
- Felbamate, mibefradil (cardiotoxicity);
- Temafloxacin (abnormal liver function tests, as well as renal failure and other serious adverse events);
- Terfenadine (cardiotoxicity);
- Troglitazone; and
- Trovafloxacin mesylate.
One of the most common causes of DILI is intentional or unintentional overdose with acetaminophen.
DILI has been classified into two major types: cholestatic and hepatocellular, or cytolytic injury. In cholestatic liver injury, the serum alkaline phosphatase (ALP) is elevated; total bilirubin level (TBL) and the alanine aminotransferase (ALT) may also be elevated. In hepatocellular injury, initial elevation is noted in the ALT.
There may also be overlap in the pattern of injury (mixed-pattern injury) whereby ALP and ALT are elevated. These patterns of injury may be defined further by the degree of enzyme elevation, such as an ALT level of three or more times the upper limit of normal (ULN), an ALP level two or more times ULN, and TBL two or more times ULN if associated with an elevation of ALP or ALT.
Hepatotoxicity is often predictable—but not always. When predictable, the reaction is usually dose-dependent, such as in the case of acetaminophen. These reactions usually occur shortly after a threshold for toxicity has been reached. Unpredictable reactions can occur days or months after exposure, usually without warning. Hypersensitivity reactions are often delayed and occur upon repeated exposure to the agent. Symptoms of immunologic injury may include rash, fever, or eosinophilia. More severe forms include Stevens-Johnson syndrome, toxic epidermal necrolysis, or cytopenias. Reactions are more severe upon repeat exposure or rechallenge of the offending agent.
Symptomatology
Patients usually present with vague symptoms that may include nausea, anorexia, fatigue, right upper-quadrant discomfort, jaundice, or dark urine. Patients with cholestatic liver disease may also present with pruritus.
Any of these along with laboratory evidence of liver injury should indicate further investigation into possible DILI. Impaired hepatic function such as increased prothrombin time and encephalopathy (signs of acute liver failure) indicate severe hepatic injury.
The Agents
Common causes of hepatocellular injury include: acetaminophen, fluoxetine, highly active antiretroviral therapies, kava kava (other herbal products), non-steroidal anti-inflammatory drugs, paroxetine, rifampin, risperidone, statins, trazodone, and troglitazone.
Common causes of cholestatic injury include: ampicillin-clavulanic acid, anabolic steroids, chlorpromazine, clopidogrel, estrogens, mirtazapine, terbinafine, and tricyclics.
Common causes of mixed-pattern injury include: amitriptyline, azathioprine, captopril, carbamazepine, enalapril, erythromycins, flutamide, phenytoin, sulfonamides, trazodone, and trimethoprim-sulfamethoxazole.
Most cases of non-fulminant hepatitis will improve upon cessation of offending or potentially offending agent(s). Assess hepatic injury immediately via continuously obtained biochemical tests.
Consult a hepatologist or gastroenterologist immediately if jaundice, impaired hepatic function or clinical signs of acute hepatic failure (e.g., encephalopathy) are evident.
Report all cases of potential DILI to the FDA’s adverse events reporting program, Medwatch, at www.fda.gov/ medwatch or by calling (800) 332-1088. For patients receiving potentially hepatotoxic agents, liver function test monitoring is recommended following a baseline assessment. Some agents require monthly rather than periodic monitoring. For a list of some agents that require hepatic monitoring and the recommended frequency, visit www.factsandcomparisons.com/assets/hospitalpharm/feb2002_HepSp.pdf.5 TH
Michele B. Kaufman is a freelance medical writer based in New York City.
References
- Nathwani RA, Kaplowitz N. Drug hepatotoxicity. Clin Liver Dis. 2006;10:207-217.
- Navarro VJ, Senior JR. Drug-related hepatotoxicity. N Engl J Med. 2006;354(7):731-739.
- Hepatic Toxicity Possibly Associated with Kava-Containing Products—United States, Germany, and Switzerland, 1999-2002. MMWR Weekly, Nov. 29, 2002/51(47):1065-1067. Available at: www.cdc.gov/MMWR/previews/mmwrhtml/mm5147a1.htm. Last accessed Nov. 5, 2007.
- Lasser KE, Allen PD, Woolhandler SJ, et al. Timing of new black box warnings and withdrawals for prescription medications. JAMA. 2002;287:17:2215-2220.
- Tice SA, Parr D. Medications that require hepatic monitoring. Hospital Pharmacy 2004;39(6):595-606.
Drug-induced liver injury (DILI) or hepatotoxicity accounts for more than 50% of all cases of acute liver failure. DILI, often life-threatening, is the leading cause of patient referral for liver transplantation.1,2
DILI is an important diagnostic challenge for the treating clinician because of its presentation, which is often a diagnosis of exclusion. Determination of all potential causes of hepatic injury need to be assessed through onset of symptoms and a careful drug history (including prescription and over-the-counter medication, dietary supplements, and complementary and alternative therapies).3
DILI has brought an increase in Food and Drug Administration (FDA) “black box” warnings. Among the drugs affected are ketoconazole, pemoline, tolcapone, valproate sodium, and zalcitabine.4
A number of drugs have been withdrawn from the U.S. market after DILI or interactions with those that are hepatically metabolized, such as:
- Astemizole (cardiotoxicity);
- Bromfenac sodium, cisapride (cardiotoxicity);
- Felbamate, mibefradil (cardiotoxicity);
- Temafloxacin (abnormal liver function tests, as well as renal failure and other serious adverse events);
- Terfenadine (cardiotoxicity);
- Troglitazone; and
- Trovafloxacin mesylate.
One of the most common causes of DILI is intentional or unintentional overdose with acetaminophen.
DILI has been classified into two major types: cholestatic and hepatocellular, or cytolytic injury. In cholestatic liver injury, the serum alkaline phosphatase (ALP) is elevated; total bilirubin level (TBL) and the alanine aminotransferase (ALT) may also be elevated. In hepatocellular injury, initial elevation is noted in the ALT.
There may also be overlap in the pattern of injury (mixed-pattern injury) whereby ALP and ALT are elevated. These patterns of injury may be defined further by the degree of enzyme elevation, such as an ALT level of three or more times the upper limit of normal (ULN), an ALP level two or more times ULN, and TBL two or more times ULN if associated with an elevation of ALP or ALT.
Hepatotoxicity is often predictable—but not always. When predictable, the reaction is usually dose-dependent, such as in the case of acetaminophen. These reactions usually occur shortly after a threshold for toxicity has been reached. Unpredictable reactions can occur days or months after exposure, usually without warning. Hypersensitivity reactions are often delayed and occur upon repeated exposure to the agent. Symptoms of immunologic injury may include rash, fever, or eosinophilia. More severe forms include Stevens-Johnson syndrome, toxic epidermal necrolysis, or cytopenias. Reactions are more severe upon repeat exposure or rechallenge of the offending agent.
Symptomatology
Patients usually present with vague symptoms that may include nausea, anorexia, fatigue, right upper-quadrant discomfort, jaundice, or dark urine. Patients with cholestatic liver disease may also present with pruritus.
Any of these along with laboratory evidence of liver injury should indicate further investigation into possible DILI. Impaired hepatic function such as increased prothrombin time and encephalopathy (signs of acute liver failure) indicate severe hepatic injury.
The Agents
Common causes of hepatocellular injury include: acetaminophen, fluoxetine, highly active antiretroviral therapies, kava kava (other herbal products), non-steroidal anti-inflammatory drugs, paroxetine, rifampin, risperidone, statins, trazodone, and troglitazone.
Common causes of cholestatic injury include: ampicillin-clavulanic acid, anabolic steroids, chlorpromazine, clopidogrel, estrogens, mirtazapine, terbinafine, and tricyclics.
Common causes of mixed-pattern injury include: amitriptyline, azathioprine, captopril, carbamazepine, enalapril, erythromycins, flutamide, phenytoin, sulfonamides, trazodone, and trimethoprim-sulfamethoxazole.
Most cases of non-fulminant hepatitis will improve upon cessation of offending or potentially offending agent(s). Assess hepatic injury immediately via continuously obtained biochemical tests.
Consult a hepatologist or gastroenterologist immediately if jaundice, impaired hepatic function or clinical signs of acute hepatic failure (e.g., encephalopathy) are evident.
Report all cases of potential DILI to the FDA’s adverse events reporting program, Medwatch, at www.fda.gov/ medwatch or by calling (800) 332-1088. For patients receiving potentially hepatotoxic agents, liver function test monitoring is recommended following a baseline assessment. Some agents require monthly rather than periodic monitoring. For a list of some agents that require hepatic monitoring and the recommended frequency, visit www.factsandcomparisons.com/assets/hospitalpharm/feb2002_HepSp.pdf.5 TH
Michele B. Kaufman is a freelance medical writer based in New York City.
References
- Nathwani RA, Kaplowitz N. Drug hepatotoxicity. Clin Liver Dis. 2006;10:207-217.
- Navarro VJ, Senior JR. Drug-related hepatotoxicity. N Engl J Med. 2006;354(7):731-739.
- Hepatic Toxicity Possibly Associated with Kava-Containing Products—United States, Germany, and Switzerland, 1999-2002. MMWR Weekly, Nov. 29, 2002/51(47):1065-1067. Available at: www.cdc.gov/MMWR/previews/mmwrhtml/mm5147a1.htm. Last accessed Nov. 5, 2007.
- Lasser KE, Allen PD, Woolhandler SJ, et al. Timing of new black box warnings and withdrawals for prescription medications. JAMA. 2002;287:17:2215-2220.
- Tice SA, Parr D. Medications that require hepatic monitoring. Hospital Pharmacy 2004;39(6):595-606.
Preventing and managing diabetic complications in elderly patients
In elderly patients, as in all patients, diabetes is much more than the blood glucose level. However, in elderly patients the disease accelerates other common conditions of that population and markedly complicates their management.
Hypertension, coronary artery disease, and cerebrovascular attacks are more common in patients with diabetes.1 Longitudinal studies of elderly and middle-aged people with diabetes show increased rates of cognitive decline and dementia.2–4 Depression, urinary incontinence, and falls are also more common in elderly patients with diabetes. Physical disability is also increased: women with diabetes are half as likely to be able to manage ordinary physical tasks such as walking, climbing stairs, and doing housework as women without diabetes.5
In an earlier paper in this journal,6 we reviewed the management of diabetes per se in elderly patients. In the pages that follow, we review the management of its associated conditions.
HEART RISK TRUMPS BLOOD SUGAR
Coronary artery disease is by far the leading cause of death in elderly people with diabetes: 40% to 50% of patients with type 2 diabetes die of cardiac disease.7–9 The conventional risk factors—hypertension, hyperlipidemia, smoking, and diabetes—remain risk factors throughout old age. Risk reduction should focus on treating hypertension and dyslipidemia, smoking cessation, aspirin therapy, and exercise. While glycemic control reduces the risk of microvascular complications (eg, diabetic retinopathy and nephropathy) after about 8 years of treatment, benefits from control of elevated blood pressure and cholesterol occur after only 2 to 3 years.
Tight control of hypertension confers significant benefit
The United Kingdom Prospective Diabetes Study (UKPDS)10 found that patients who had tight control of blood pressure (mean treated blood pressure 144/82 mm Hg) had 24% fewer diabetes-related end points, 32% fewer diabetes-related deaths, 44% fewer strokes, a 34% reduced risk of deterioration of retinopathy, and a 47% reduced risk of visual deterioration than patients who had usual control (mean treated blood pressure 157/87 mm Hg). The benefit of treating hypertension outweighed the benefits of tight glycemic control.
A strong focus on blood pressure control should be a major focus of any treatment program. The American Geriatrics Society goal for blood pressure is less than 140/80 mm Hg if tolerated. Others have proposed more stringent targets.
Lipid control
Lipid control is integral to managing elderly patients with diabetes. In the Cholesterol and Recurrent Events trial11 and the Heart Protection Study,12 the cardiovascular benefits of reducing serum low-density lipoprotein cholesterol (LDL-C) levels were similar in elderly and younger patients with diabetes. In a meta-analysis of secondary prevention trials, absolute risk reduction was greatest in subjects older than 65 years with either diabetes or diastolic hypertension.
The American Diabetes Association,13 the American Geriatrics Society,14 and the Department of Veterans Affairs15,16 have all set a goal for serum LDL-C of less than 100 mg/dL. In addition, the American Diabetes Association has set goal levels for triglycerides (< 150 mg/dL) and high-density lipoprotein cholesterol (> 40 mg/dL).
Glycemic control
The importance of tight glycemic control in preventing coronary heart disease in the elderly is somewhat controversial. Treatment guidelines for elderly patients with diabetes are mainly extrapolated from the UKPDS, in which patients were a mean of 54 years old at the start of the study. After 10 years, the mean hemoglobin A1c levels were 7.9% in patients receiving conventional control and 7.0% in patients with intensive therapy. Every 1% reduction in hemoglobin A1c was associated with a 37% decline in microvascular complications of diabetes, a 14% decline in myocardial infarctions, and a 21% decline in any diabetes-related outcome.17
In the original trial,18 the rate of myocardial infarction was 17.4% in the conventional treatment group vs 14.7% in the intensive group (P = .052), and the risk of stroke did not differ. No thresholds for realizing benefits from reducing fasting glucose or hemoglobin A1c levels were detected.
A recent cohort study involving about 10,000 participants aged 45 to 79 years found that the risk of cardiovascular disease and death from any cause increased continuously with increasing hemoglobin A1c levels in people with or without diabetes.19 However, the impact of treatment remains to be clarified. The Action to Control Cardiovascular Risk in Diabetes trial will address this question (and others), but results will not be available for several years.
RETINOPATHY IS A MAJOR CAUSE OF BLINDNESS
Diabetic retinopathy, a leading cause of blindness in the United States, is perhaps the most threatening of the chronic microvascular complications of diabetes for elderly patients. The strongest predictor of retinopathy is the duration of diabetes.20–22 Retinopathy is classified as being nonproliferative, preproliferative, or proliferative.
Ischemia is believed to be the major cause of diabetic retinopathy, and glucose control has been shown to be of major benefit. A study of young adults with type 1 diabetes found that intensive therapy reduced the risk of developing retinopathy by 76% and slowed the progression of retinopathy by 54%. Comparable data for patients with type 2 diabetes are lacking.
Of some concern is a study in which retinopathy progressed more rapidly during the first year of aggressive insulin therapy in elderly patients with diabetes and baseline retinopathy.23 Further research is needed to identify which subgroups would benefit most from aggressive glycemic control.
In addition to specific ophthalmologic treatment, managing cardiovascular risk factors may reduce the progression of retinopathy: each cardiovascular risk factor has been found to also be a risk factor for retinopathy. Hypertension is an independent risk factor for any retinopathy, and its tight control reduces progression.20,24 Aspirin therapy has not been found to confer either risk or benefit.25,26
Although guidelines typically call for yearly ophthalmic examinations to screen for retinopathy, whether this is cost-effective has been questioned.27,28 But people older than 65 years with diabetes also have twice the risk of developing cataracts and three times the risk of developing glaucoma than those without diabetes. Considering the effects of visual loss on quality of life as well as the subsequent higher risk of accidents, eye examinations by an ophthalmologist at the time of diagnosis and annually thereafter are recommended. Tight glycemic and blood pressure control remains the cornerstone in the primary prevention of diabetic retinopathy. Panretinal and focal retinal laser photocoagulation reduces the risk of visual loss in patients with severe retinopathy and macular edema, respectively.29
NEUROPATHY PRESENTS IN MANY FORMS
Neuropathy is a particularly distressing complication and can lead to loss of sleep, limitation of activity, and depression.26,30,31 Diabetic neuropathies include focal neuropathies (entrapment syndromes and mono-neuropathies), polyneuropathy, and autonomic neuropathy.
Distal symmetric polyneuropathy (“glove and stocking” sensory symptoms) is the most common neuropathy of elderly people with diabetes. Pain, which can interrupt sleep and limit activity, can be treated with the anticonvulsants gabapentin (Gabarone, Neurontin), phenytoin (Dilantin, Phenytek) and carbamazepine (Carbatrol, Epitol, Equetro, Tegretol), and with tricyclic antidepressants. However, the anticholinergic effects of tricyclic antidepressants limit their use in older patients. Newer agents, such as duloxetine (Cymbalta) and pregabalin (Lyrica) show promise.30,31 Dysesthesia of a burning quality is sometimes treated with topical capsaicin or with oral mexiletine (Mexitil), although their role in treating older patients is not well established.
Patients with distal sensory polyneuropathy are predisposed to develop Charcot joints, which may mimic gout or degenerative joint disease. Plain radiography of the foot can help differentiate these diseases. Distal sensory polyneuropathy also predisposes patients to neuropathic foot ulcer, the leading cause of foot amputation in the United States.32
Feet should be inspected at each office visit. Testing sensation with a monofilament detects sensory neuropathy. Patients should be encouraged to examine their feet daily. Therapeutic shoes, prescribed by a podiatrist and individually designed to prevent blisters, calluses, and ulcers, are covered by Medicare for peripheral neuropathy if any of the following are also present: callus formation, poor circulation, foot deformity, or a history of foot callus, ulcer, or amputation (partial or complete). Medicare will pay for one pair of shoes plus three pairs of inserts per year.
Proximal motor neuropathy (diabetic amyotrophy) primarily affects elderly patients. It begins with unilateral thigh pain, which becomes bilateral and progresses to proximal muscle weakness and wasting. Distal symmetric polyneuropathy may also be present. Treatment includes glycemic control (usually with insulin) and physical therapy. Some forms of amyotrophy respond to immunotherapy.
Autonomic neuropathy, although not painful, can be the most life-threatening form of diabetic neuropathy.33 Tachycardia increases the risk of sudden death, while postural hypotension increases the risk of syncope, falling, and injury. Other forms of autonomic neuropathy include neurogenic bladder, sexual dysfunction, gastropathy (which is particularly sensitive to glycemic control), enteropathy, and gustatory sweating. Patients with autonomic neuropathy are more likely to have hypoglycemic unawareness.
NEPHROPATHY CAN PROGRESS RAPIDLY
Elderly patients with diabetes are especially at risk of developing nephropathy, which progresses from microalbuminuria to overt proteinuria to renal insufficiency and end-stage renal disease. Nephropathy may develop over a shorter time than the typical 10 to 20 years in younger patients. Independent risk factors for proteinuria and renal insufficiency include poor glycemic control over many years, hypertension, longer duration of diabetes, male sex, high serum total cholesterol levels, and smoking. Elderly patients are also at risk of renal insults such as receiving intravenous iodinated contrast agents in the course of radiologic procedures, nephrotoxic drugs, and comorbid illness such as congestive heart failure.
The diagnosis of diabetic nephropathy is usually made clinically and not by renal biopsy. Diabetic nephropathy can be diagnosed with almost 100% specificity in type 1 diabetes and more than 85% specificity in type 2 diabetes by a urinary albumin excretion of more than 300 mg per day and an appropriate time course in the absence of other obvious causes of renal disease. The urinary albumin-to-creatinine ratio can be used to screen for microalbuminuria (the precursor of frank proteinuria and renal insufficiency). A value of more than 30 mg of albumin per gram of creatinine suggests that albumin excretion exceeds 30 mg and that microalbuminuria is present.
Prevention is a cornerstone of management. Good glycemic control reduces the risk of microalbuminuria, the progression of albuminuria, and the development of renal insufficiency. Lowering blood pressure reduces the decline in glomerular filtration rate and albuminuria. Angiotensin-converting enzyme (ACE) inhibitors reduce the rate of progression of proteinuria and reduce the rate of end-stage renal disease, although the data are stronger in patients with type 1 diabetes.34 When side effects such as cough limit the use of ACE inhibitors, angiotensin receptor blockers can be used as an alternative. Blood pressure should be controlled to reduce stroke and cardiovascular complications, regardless of whether microalbuminuria is present.35
End-stage renal disease in elderly patients with diabetes is becoming increasingly frequent. Nephropathy in older patients is different from that in younger patients. In elderly patients, the pathologic findings may suggest ischemia and hypertension, and the classic Kimmelstiel-Wilson lesions may be absent. Patients may present with end-stage renal disease following an episode of acute renal failure that does not resolve, which may occur after a radiologic procedure involving an iodinated contrast agent.
NONKETOTIC HYPEROSMOLAR COMA
Nonketotic hyperosmolar coma occurs predominantly in elderly patients with type 2 diabetes. Predisposing factors include dementia, infection, stroke, and myocardial infarction. Coma results from osmotic diuresis due to hyperglycemia and consequent dehydration. A drop in the glomerular filtration rate promotes further hyperglycemia and dehydration in a vicious circle. Glucose levels commonly reach 600 mg/dL or more, and serum osmolality often exceeds 320 mOsm/L. A fluid deficit of 5 to 10 L is typical.
Fluid replacement is the mainstay of treatment. Because free water is typically lost in an osmotic diuresis, 0.9% (normal) saline is usually given if hemodynamic instability is present or 0.45% (half-normal) saline otherwise. Insulin is also required, as is specific treatment of the precipitating cause, eg, infection. Ketoacidosis may also occur in the elderly.
Recovery from coma or improvement in mental status may lag behind correction of the serum osmolality and may take several days. Mortality rates can be high: severe hyperosmolarity, advanced age, and nursing home residence are the major risk factors for death.
INFECTIONS: SEVERE AND UNUSUAL
Elderly patients with diabetes are at increased risk of developing severe and unusual infections, particularly malignant external otitis. Necrotizing Pseudomonas aeruginosa infection initially involves the external ear canal and progresses to the mastoid air cells, the skull base, or temporal bone. The clinical presentation consists of fever, otalgia, otorrhea, and less commonly, cranial nerve palsy. Treatment involves surgical debridement and antibiotics.
Other infections associated with diabetes include rhinocerebral mucormycosis, necrotizing fasciitis, emphysematous cholecystitis, and emphysematous pyelonephritis. An elderly patient with diabetes is also at increased risk of renal papillary necrosis, which presents as insidious renal failure.
COGNITIVE IMPAIRMENT
Elderly people with diabetes are at increased risk of cognitive impairment, which poses a barrier to taking medications appropriately and performing other tasks of self-management.
Because dementia may go undetected, particularly in the early stages, cognitive function should be assessed in elderly patients when they fail to take therapy correctly or have frequent episodes of hypoglycemia, or if glycemic control deteriorates without an obvious explanation. Caregivers play a critical role in detecting and reporting early cognitive impairment.
DEPRESSION IS OFTEN UNDETECTED
Elderly patients with diabetes have a higher rate of depression than do age-matched controls, but it is commonly underdetected and undertreated.5,36 Depression has been associated with poor glycemic control, and treatment of depression is associated with improved control. Routine screening for depression should be performed; a variety of diagnostic instruments are available. Particular attention should be given to medications that are associated with depression.
POLYPHARMACY
Many elderly patients take multiple medications. Polypharmacy increases the risk of drug side effects, interactions, and nonadherence to taking medications.37–39 This problem is increased in diabetes, in which several medications are necessary to manage hyper-glycemia, hyperlipidemia, hypertension, and other associated conditions.
Patients should keep accurate medication lists, including over-the-counter medications, herbs, and nutritional supplements. Physicians should carefully review each medication to check if it is appropriate and used correctly.
FALLS
Elderly patients with diabetes mellitus are at increased risk of injurious falls, which are associated with high rates of complications, death, and functional decline.40,41 Risk factors include frailty and functional disability, visual impairment, peripheral or autonomic neuropathy, hypoglycemia, and polypharmacy.
Elderly patients should be screened for their risk of falls, and appropriate measures should be instituted. The American Geriatrics Society has guidelines for preventing falls in the elderly.41
URINARY INCONTINENCE
Elderly women with diabetes are at increased risk of developing urinary incontinence. Risk factors include autonomic neuropathy (causing either neurogenic bladder or fecal impaction), polyuria due to hyperglycemia, and urinary tract and vaginal infections. Although evidence is lacking that urinary incontinence affects glycemic control, assessing and treating the condition improves quality of life.
SUMMARY
Diabetes is a common problem in the elderly, accounting for considerable morbidity and mortality. In a large longitudinal analysis (> 50,000 patients), elderly persons newly diagnosed as having diabetes experienced high rates of complications during 10-year follow-up, far in excess of elderly persons without diabetes.42 Diabetes is underdiagnosed in the elderly and is frequently undertreated. Management of the elderly with diabetes presents unique challenges because of associated comorbidities, but with attention to detail and individualized approaches, quality and duration of life can be optimized. The greatest attention should be given to reduction of overall cardiovascular risk. Glycemic goals and the treatment regimens to achieve those goals should be individualized and chosen to control hyperglycemic symptoms and achieve the maximal glycemic control possible while minimizing the risk of hypoglycemia. Diabetes will continue to be a challenge to the patient, the physician, the care team, and the health care system.
- Gregg EW, Engelgau MM, Narayan V. Complications of diabetes in elderly people. BMJ 2002; 325:916–917.
- Knopman D, Boland LL, Mosley T, et al. Cardiovascular risk factors and cognitive decline in middle-aged adults. Neurology 2001; 56:42–48.
- Ott A, Stolk RP, van Harskamp F, Pols HA, Hofman A, Breteler MM. Diabetes mellitus and the risk of dementia: The Rotterdam Study. Neurology 1999; 53:1937–1942.
- Fontbonne A, Berr C, Ducimetiere P, Alperovitch A. Changes in cognitive abilities over a 4-year period are unfavorably affected in elderly diabetic subjects: results of the Epidemiology of Vascular Aging Study. Diabetes Care 2001; 24:366–370.
- Gregg EW, Mangione CM, Cauley JA, et al. Diabetes and incidence of functional disability in older women. Diabetes Care 2002; 25:61–67.
- Hornick T, Aron DC. Managing diabetes in the elderly: go easy, individualize. Cleve Clin J Med 2008; 75:70–78.
- Haffner SM, Lehto S, Ronnemaa T, Pyorala K, Laakso M. Mortality from coronary heart disease in subjects with type 2 diabetes and in nondiabetic subjects with and without prior myocardial infarction. N Engl J Med 1998; 339:229–234.
- Bertoni AG, Krop JS, Anderson GF, Brancati FL. Diabetes-related morbidity and mortality in a national sample of U.S. elders. Diabetes Care 2002; 25:471–475.
- Bertoni AG, Kirk JK, Goff DC, Wagenknecht LE. Excess mortality related to diabetes mellitus in elderly Medicare beneficiaries. Ann Epidemiol 2004; 14:362–367.
- UK Prospective Diabetes Study Group. Tight blood pressure control and risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 38. BMJ 1998; 317:703–713. Erratum in: BMJ 1999; 318:29.
- Goldberg RB, Mellies MJ, Sacks FM, et al. Cardiovascular events and their reduction with pravastatin in diabetic and glucose-intolerant myocardial infarction survivors with average cholesterol levels: subgroup analyses in the Cholesterol and Recurrent Events (CARE) trial. The CARE Investigators. Circulation 1998; 98:2513–2519.
- Collins R, Armitage J, Parish S, Sleigh P, Peto R. MRC/BHF Heart Protection Study of cholesterol-lowering with simvastatin in 5963 people with diabetes. Lancet 2003; 361:2005–2016.
- American Diabetes Association. Standards of medical care in diabetes. Diabetes Care 2005; 28:S4–S36.
- Brown AF, Mangione CM, Saliba D, Sarkisian CA California Healthcare Foundation/American Geriatrics Society Panel on Improving Care for Elders with Diabetes. Guidelines for improving the care of the older person with diabetes mellitus. J Am Geriatr Soc 2003; 51:S265–S280.
- VA/DoD Clinical Practice Guideline for the Management of Diabetes Mellitus in the Primary Care Setting 2003. Accessed January 4, 2008. www.oqp.med.va.gov/cpg/dm/DM3_cpg/content/introduction.htm.
- Pogach LM, Brietzke SA, Cowan CL, Conlin P, Walder DJ, Sawin CT VA/DoD Diabetes Guideline Development Group. Development of evidence-based clinical practice guidelines for diabetes: the Department of Veterans Affairs/Department of Defense guidelines initiative. Diabetes Care 2004; 27:B82–B89.
- Stratton IM, Asler AI, Neil HA, et al. Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. BMJ 2000; 321:405–412.
- UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet 1998; 352:837–853. Erratum in: Lancet 1999; 354:602.
- Khaw KT, Wareham N, Bingham S, Luben R, Welch A, Day N. Association of hemoglobin A1c with cardiovascular disease and mortality in adults: the European prospective investigation into cancer in Norfolk. Ann Intern Med 2004; 141:413–420.
- Matthews DR, Stratton IM, Aldington SJ, Holman RR, Kohner EM UK Prospective Diabetes Study Group. Risks of progression of retinopathy and vision loss related to tight blood pressure control in type 2 diabetes mellitus: UKPDS 69. Arch Ophthalmol 2004; 122:1631–1640.
- Cahill M, Halley A, Codd M, et al. Prevalence of diabetic retinopathy in patients with diabetic mellitus diagnosed after the age of 70 years. Br J Opthalmol 1997; 81:218–222.
- Hirvela H, Laatikainen L. Diabetic retinopathy in people aged 70 years or older. The Oulu Eye Study. Br J Ophthalmol 1997; 81:214–217.
- Tovi J, Ingemansson SO, Engfeldt P. Insulin treatment of elderly type 2 diabetic patients: effects on retinopathy. Diabetes Metab 1998; 24:442–447.
- Schrier RW, Estacio RO, Esler A, Mehler P. Effects of aggressive blood pressure control in normotensive type 2 diabetic patients on albuminuria, retinopathy and strokes. Kidney Int 2002; 61:1086–1097.
- Kohner EM. Aspirin for diabetic retinopathy. BMJ 2003; 327:1060–1061.
- Greene DA, Stevens MJ, Feldman EL. Diabetic neuropathy: scope of the syndrome. Am J Med 1999; 107:2S–8S.
- Hutchinson A, McIntosh A, Peters J, et al. Effectiveness of screening and monitoring tests for diabetic retinopathy—a systematic review. Diabet Med 2000; 17:495–506.
- Vijan S, Hofer TP, Hayward RA. Cost-utility analysis of screening intervals for diabetic retinopathy in patients with type 2 diabetes mellitus. JAMA 2000; 283:889–896.
- Mohamed Q, Gillies MC, Wong TY. Management of diabetic retinopathy: a systematic review. JAMA 2007; 298:902–916.
- Argoff CE, Cole BE, Fishbain DA, Irving GA. Diabetic peripheral neuropathic pain: clinical and quality-of-life issues. Mayo Clin Proc 2006; 81:S3–S11.
- Wong MC, Chung JW, Wong TK. Effects of treatments for symptoms of painful diabetic neuropathy: systematic review. BMJ 2007; 335:87: epubl June 11, 2007.
- Bild DE, Selby JV, Sinnock P, Browner WS, Braveman P, Showstack JA. Lower-extremity amputation in people with diabetes. Epidemiology and prevention. Diabetes Care 1989; 12:24–31.
- Wheeler SG, Ahroni JH, Boyko EJ. Prospective study of autonomic neuropathy as a predictor of mortality in patients with diabetes. Diabetes Res Clin Pract 2002; 58:131–138.
- Brenner BM, Cooper ME, de Zeeuw D RENAAL Study Investigators. Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med 2001; 345:861–869.
- UK Prospective Diabetes Study Group. Efficacy of atenolol and captopril in reducing risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 39. BMJ 1998; 317:713–720.
- Sinclair AJ, Girling AJ, Bayer AJ. Cognitive dysfunction in older subjects with diabetes mellitus: impact on diabetes self-management and use of care services. All Wales Research into Elderly (AWARE) Study. Diabetes Res Clin Pract 2000; 50:203–212.
- Moisan J, Gaudet M, Gregoire JP, Bouchard R. Non-compliance with drug treatment and reading difficulties with regard to prescription labelling among seniors. Gerontology 2002; 48:44–51.
- Boyd CM, Darer J, Boult C, Fried LP, Boult L, Wu AW. Clinical practice guidelines and quality of care for older patients with multiple comorbid diseases: implications for pay for performance. JAMA 2005; 294:716–724.
- Jackevicius CA, Mamdani M, Tu JV. Adherence with statin therapy in elderly patients with and without acute coronary syndromes. JAMA 2002; 288:462–467.
- Schwartz AV, Hillier TA, Sellmeyer DE, et al. Older women with diabetes have a higher risk of falls: a prospective study. Diabetes Care 2002; 25:1749–1754.
- American Geriatrics Society, British Geriatrics Society, and American Academy of Orthopaedic Surgeons Panel on Falls Prevention. Guideline for the prevention of falls in older persons. J Am Geriatr Soc 2001; 49:664–672.
- Bethel MA, Sloan FA, Belsky D, Feinglos MN. Longitudinal incidence and prevalence of adverse outcomes of diabetes mellitus in elderly patients. Arch Intern Med 2007; 167:921–927.
In elderly patients, as in all patients, diabetes is much more than the blood glucose level. However, in elderly patients the disease accelerates other common conditions of that population and markedly complicates their management.
Hypertension, coronary artery disease, and cerebrovascular attacks are more common in patients with diabetes.1 Longitudinal studies of elderly and middle-aged people with diabetes show increased rates of cognitive decline and dementia.2–4 Depression, urinary incontinence, and falls are also more common in elderly patients with diabetes. Physical disability is also increased: women with diabetes are half as likely to be able to manage ordinary physical tasks such as walking, climbing stairs, and doing housework as women without diabetes.5
In an earlier paper in this journal,6 we reviewed the management of diabetes per se in elderly patients. In the pages that follow, we review the management of its associated conditions.
HEART RISK TRUMPS BLOOD SUGAR
Coronary artery disease is by far the leading cause of death in elderly people with diabetes: 40% to 50% of patients with type 2 diabetes die of cardiac disease.7–9 The conventional risk factors—hypertension, hyperlipidemia, smoking, and diabetes—remain risk factors throughout old age. Risk reduction should focus on treating hypertension and dyslipidemia, smoking cessation, aspirin therapy, and exercise. While glycemic control reduces the risk of microvascular complications (eg, diabetic retinopathy and nephropathy) after about 8 years of treatment, benefits from control of elevated blood pressure and cholesterol occur after only 2 to 3 years.
Tight control of hypertension confers significant benefit
The United Kingdom Prospective Diabetes Study (UKPDS)10 found that patients who had tight control of blood pressure (mean treated blood pressure 144/82 mm Hg) had 24% fewer diabetes-related end points, 32% fewer diabetes-related deaths, 44% fewer strokes, a 34% reduced risk of deterioration of retinopathy, and a 47% reduced risk of visual deterioration than patients who had usual control (mean treated blood pressure 157/87 mm Hg). The benefit of treating hypertension outweighed the benefits of tight glycemic control.
A strong focus on blood pressure control should be a major focus of any treatment program. The American Geriatrics Society goal for blood pressure is less than 140/80 mm Hg if tolerated. Others have proposed more stringent targets.
Lipid control
Lipid control is integral to managing elderly patients with diabetes. In the Cholesterol and Recurrent Events trial11 and the Heart Protection Study,12 the cardiovascular benefits of reducing serum low-density lipoprotein cholesterol (LDL-C) levels were similar in elderly and younger patients with diabetes. In a meta-analysis of secondary prevention trials, absolute risk reduction was greatest in subjects older than 65 years with either diabetes or diastolic hypertension.
The American Diabetes Association,13 the American Geriatrics Society,14 and the Department of Veterans Affairs15,16 have all set a goal for serum LDL-C of less than 100 mg/dL. In addition, the American Diabetes Association has set goal levels for triglycerides (< 150 mg/dL) and high-density lipoprotein cholesterol (> 40 mg/dL).
Glycemic control
The importance of tight glycemic control in preventing coronary heart disease in the elderly is somewhat controversial. Treatment guidelines for elderly patients with diabetes are mainly extrapolated from the UKPDS, in which patients were a mean of 54 years old at the start of the study. After 10 years, the mean hemoglobin A1c levels were 7.9% in patients receiving conventional control and 7.0% in patients with intensive therapy. Every 1% reduction in hemoglobin A1c was associated with a 37% decline in microvascular complications of diabetes, a 14% decline in myocardial infarctions, and a 21% decline in any diabetes-related outcome.17
In the original trial,18 the rate of myocardial infarction was 17.4% in the conventional treatment group vs 14.7% in the intensive group (P = .052), and the risk of stroke did not differ. No thresholds for realizing benefits from reducing fasting glucose or hemoglobin A1c levels were detected.
A recent cohort study involving about 10,000 participants aged 45 to 79 years found that the risk of cardiovascular disease and death from any cause increased continuously with increasing hemoglobin A1c levels in people with or without diabetes.19 However, the impact of treatment remains to be clarified. The Action to Control Cardiovascular Risk in Diabetes trial will address this question (and others), but results will not be available for several years.
RETINOPATHY IS A MAJOR CAUSE OF BLINDNESS
Diabetic retinopathy, a leading cause of blindness in the United States, is perhaps the most threatening of the chronic microvascular complications of diabetes for elderly patients. The strongest predictor of retinopathy is the duration of diabetes.20–22 Retinopathy is classified as being nonproliferative, preproliferative, or proliferative.
Ischemia is believed to be the major cause of diabetic retinopathy, and glucose control has been shown to be of major benefit. A study of young adults with type 1 diabetes found that intensive therapy reduced the risk of developing retinopathy by 76% and slowed the progression of retinopathy by 54%. Comparable data for patients with type 2 diabetes are lacking.
Of some concern is a study in which retinopathy progressed more rapidly during the first year of aggressive insulin therapy in elderly patients with diabetes and baseline retinopathy.23 Further research is needed to identify which subgroups would benefit most from aggressive glycemic control.
In addition to specific ophthalmologic treatment, managing cardiovascular risk factors may reduce the progression of retinopathy: each cardiovascular risk factor has been found to also be a risk factor for retinopathy. Hypertension is an independent risk factor for any retinopathy, and its tight control reduces progression.20,24 Aspirin therapy has not been found to confer either risk or benefit.25,26
Although guidelines typically call for yearly ophthalmic examinations to screen for retinopathy, whether this is cost-effective has been questioned.27,28 But people older than 65 years with diabetes also have twice the risk of developing cataracts and three times the risk of developing glaucoma than those without diabetes. Considering the effects of visual loss on quality of life as well as the subsequent higher risk of accidents, eye examinations by an ophthalmologist at the time of diagnosis and annually thereafter are recommended. Tight glycemic and blood pressure control remains the cornerstone in the primary prevention of diabetic retinopathy. Panretinal and focal retinal laser photocoagulation reduces the risk of visual loss in patients with severe retinopathy and macular edema, respectively.29
NEUROPATHY PRESENTS IN MANY FORMS
Neuropathy is a particularly distressing complication and can lead to loss of sleep, limitation of activity, and depression.26,30,31 Diabetic neuropathies include focal neuropathies (entrapment syndromes and mono-neuropathies), polyneuropathy, and autonomic neuropathy.
Distal symmetric polyneuropathy (“glove and stocking” sensory symptoms) is the most common neuropathy of elderly people with diabetes. Pain, which can interrupt sleep and limit activity, can be treated with the anticonvulsants gabapentin (Gabarone, Neurontin), phenytoin (Dilantin, Phenytek) and carbamazepine (Carbatrol, Epitol, Equetro, Tegretol), and with tricyclic antidepressants. However, the anticholinergic effects of tricyclic antidepressants limit their use in older patients. Newer agents, such as duloxetine (Cymbalta) and pregabalin (Lyrica) show promise.30,31 Dysesthesia of a burning quality is sometimes treated with topical capsaicin or with oral mexiletine (Mexitil), although their role in treating older patients is not well established.
Patients with distal sensory polyneuropathy are predisposed to develop Charcot joints, which may mimic gout or degenerative joint disease. Plain radiography of the foot can help differentiate these diseases. Distal sensory polyneuropathy also predisposes patients to neuropathic foot ulcer, the leading cause of foot amputation in the United States.32
Feet should be inspected at each office visit. Testing sensation with a monofilament detects sensory neuropathy. Patients should be encouraged to examine their feet daily. Therapeutic shoes, prescribed by a podiatrist and individually designed to prevent blisters, calluses, and ulcers, are covered by Medicare for peripheral neuropathy if any of the following are also present: callus formation, poor circulation, foot deformity, or a history of foot callus, ulcer, or amputation (partial or complete). Medicare will pay for one pair of shoes plus three pairs of inserts per year.
Proximal motor neuropathy (diabetic amyotrophy) primarily affects elderly patients. It begins with unilateral thigh pain, which becomes bilateral and progresses to proximal muscle weakness and wasting. Distal symmetric polyneuropathy may also be present. Treatment includes glycemic control (usually with insulin) and physical therapy. Some forms of amyotrophy respond to immunotherapy.
Autonomic neuropathy, although not painful, can be the most life-threatening form of diabetic neuropathy.33 Tachycardia increases the risk of sudden death, while postural hypotension increases the risk of syncope, falling, and injury. Other forms of autonomic neuropathy include neurogenic bladder, sexual dysfunction, gastropathy (which is particularly sensitive to glycemic control), enteropathy, and gustatory sweating. Patients with autonomic neuropathy are more likely to have hypoglycemic unawareness.
NEPHROPATHY CAN PROGRESS RAPIDLY
Elderly patients with diabetes are especially at risk of developing nephropathy, which progresses from microalbuminuria to overt proteinuria to renal insufficiency and end-stage renal disease. Nephropathy may develop over a shorter time than the typical 10 to 20 years in younger patients. Independent risk factors for proteinuria and renal insufficiency include poor glycemic control over many years, hypertension, longer duration of diabetes, male sex, high serum total cholesterol levels, and smoking. Elderly patients are also at risk of renal insults such as receiving intravenous iodinated contrast agents in the course of radiologic procedures, nephrotoxic drugs, and comorbid illness such as congestive heart failure.
The diagnosis of diabetic nephropathy is usually made clinically and not by renal biopsy. Diabetic nephropathy can be diagnosed with almost 100% specificity in type 1 diabetes and more than 85% specificity in type 2 diabetes by a urinary albumin excretion of more than 300 mg per day and an appropriate time course in the absence of other obvious causes of renal disease. The urinary albumin-to-creatinine ratio can be used to screen for microalbuminuria (the precursor of frank proteinuria and renal insufficiency). A value of more than 30 mg of albumin per gram of creatinine suggests that albumin excretion exceeds 30 mg and that microalbuminuria is present.
Prevention is a cornerstone of management. Good glycemic control reduces the risk of microalbuminuria, the progression of albuminuria, and the development of renal insufficiency. Lowering blood pressure reduces the decline in glomerular filtration rate and albuminuria. Angiotensin-converting enzyme (ACE) inhibitors reduce the rate of progression of proteinuria and reduce the rate of end-stage renal disease, although the data are stronger in patients with type 1 diabetes.34 When side effects such as cough limit the use of ACE inhibitors, angiotensin receptor blockers can be used as an alternative. Blood pressure should be controlled to reduce stroke and cardiovascular complications, regardless of whether microalbuminuria is present.35
End-stage renal disease in elderly patients with diabetes is becoming increasingly frequent. Nephropathy in older patients is different from that in younger patients. In elderly patients, the pathologic findings may suggest ischemia and hypertension, and the classic Kimmelstiel-Wilson lesions may be absent. Patients may present with end-stage renal disease following an episode of acute renal failure that does not resolve, which may occur after a radiologic procedure involving an iodinated contrast agent.
NONKETOTIC HYPEROSMOLAR COMA
Nonketotic hyperosmolar coma occurs predominantly in elderly patients with type 2 diabetes. Predisposing factors include dementia, infection, stroke, and myocardial infarction. Coma results from osmotic diuresis due to hyperglycemia and consequent dehydration. A drop in the glomerular filtration rate promotes further hyperglycemia and dehydration in a vicious circle. Glucose levels commonly reach 600 mg/dL or more, and serum osmolality often exceeds 320 mOsm/L. A fluid deficit of 5 to 10 L is typical.
Fluid replacement is the mainstay of treatment. Because free water is typically lost in an osmotic diuresis, 0.9% (normal) saline is usually given if hemodynamic instability is present or 0.45% (half-normal) saline otherwise. Insulin is also required, as is specific treatment of the precipitating cause, eg, infection. Ketoacidosis may also occur in the elderly.
Recovery from coma or improvement in mental status may lag behind correction of the serum osmolality and may take several days. Mortality rates can be high: severe hyperosmolarity, advanced age, and nursing home residence are the major risk factors for death.
INFECTIONS: SEVERE AND UNUSUAL
Elderly patients with diabetes are at increased risk of developing severe and unusual infections, particularly malignant external otitis. Necrotizing Pseudomonas aeruginosa infection initially involves the external ear canal and progresses to the mastoid air cells, the skull base, or temporal bone. The clinical presentation consists of fever, otalgia, otorrhea, and less commonly, cranial nerve palsy. Treatment involves surgical debridement and antibiotics.
Other infections associated with diabetes include rhinocerebral mucormycosis, necrotizing fasciitis, emphysematous cholecystitis, and emphysematous pyelonephritis. An elderly patient with diabetes is also at increased risk of renal papillary necrosis, which presents as insidious renal failure.
COGNITIVE IMPAIRMENT
Elderly people with diabetes are at increased risk of cognitive impairment, which poses a barrier to taking medications appropriately and performing other tasks of self-management.
Because dementia may go undetected, particularly in the early stages, cognitive function should be assessed in elderly patients when they fail to take therapy correctly or have frequent episodes of hypoglycemia, or if glycemic control deteriorates without an obvious explanation. Caregivers play a critical role in detecting and reporting early cognitive impairment.
DEPRESSION IS OFTEN UNDETECTED
Elderly patients with diabetes have a higher rate of depression than do age-matched controls, but it is commonly underdetected and undertreated.5,36 Depression has been associated with poor glycemic control, and treatment of depression is associated with improved control. Routine screening for depression should be performed; a variety of diagnostic instruments are available. Particular attention should be given to medications that are associated with depression.
POLYPHARMACY
Many elderly patients take multiple medications. Polypharmacy increases the risk of drug side effects, interactions, and nonadherence to taking medications.37–39 This problem is increased in diabetes, in which several medications are necessary to manage hyper-glycemia, hyperlipidemia, hypertension, and other associated conditions.
Patients should keep accurate medication lists, including over-the-counter medications, herbs, and nutritional supplements. Physicians should carefully review each medication to check if it is appropriate and used correctly.
FALLS
Elderly patients with diabetes mellitus are at increased risk of injurious falls, which are associated with high rates of complications, death, and functional decline.40,41 Risk factors include frailty and functional disability, visual impairment, peripheral or autonomic neuropathy, hypoglycemia, and polypharmacy.
Elderly patients should be screened for their risk of falls, and appropriate measures should be instituted. The American Geriatrics Society has guidelines for preventing falls in the elderly.41
URINARY INCONTINENCE
Elderly women with diabetes are at increased risk of developing urinary incontinence. Risk factors include autonomic neuropathy (causing either neurogenic bladder or fecal impaction), polyuria due to hyperglycemia, and urinary tract and vaginal infections. Although evidence is lacking that urinary incontinence affects glycemic control, assessing and treating the condition improves quality of life.
SUMMARY
Diabetes is a common problem in the elderly, accounting for considerable morbidity and mortality. In a large longitudinal analysis (> 50,000 patients), elderly persons newly diagnosed as having diabetes experienced high rates of complications during 10-year follow-up, far in excess of elderly persons without diabetes.42 Diabetes is underdiagnosed in the elderly and is frequently undertreated. Management of the elderly with diabetes presents unique challenges because of associated comorbidities, but with attention to detail and individualized approaches, quality and duration of life can be optimized. The greatest attention should be given to reduction of overall cardiovascular risk. Glycemic goals and the treatment regimens to achieve those goals should be individualized and chosen to control hyperglycemic symptoms and achieve the maximal glycemic control possible while minimizing the risk of hypoglycemia. Diabetes will continue to be a challenge to the patient, the physician, the care team, and the health care system.
In elderly patients, as in all patients, diabetes is much more than the blood glucose level. However, in elderly patients the disease accelerates other common conditions of that population and markedly complicates their management.
Hypertension, coronary artery disease, and cerebrovascular attacks are more common in patients with diabetes.1 Longitudinal studies of elderly and middle-aged people with diabetes show increased rates of cognitive decline and dementia.2–4 Depression, urinary incontinence, and falls are also more common in elderly patients with diabetes. Physical disability is also increased: women with diabetes are half as likely to be able to manage ordinary physical tasks such as walking, climbing stairs, and doing housework as women without diabetes.5
In an earlier paper in this journal,6 we reviewed the management of diabetes per se in elderly patients. In the pages that follow, we review the management of its associated conditions.
HEART RISK TRUMPS BLOOD SUGAR
Coronary artery disease is by far the leading cause of death in elderly people with diabetes: 40% to 50% of patients with type 2 diabetes die of cardiac disease.7–9 The conventional risk factors—hypertension, hyperlipidemia, smoking, and diabetes—remain risk factors throughout old age. Risk reduction should focus on treating hypertension and dyslipidemia, smoking cessation, aspirin therapy, and exercise. While glycemic control reduces the risk of microvascular complications (eg, diabetic retinopathy and nephropathy) after about 8 years of treatment, benefits from control of elevated blood pressure and cholesterol occur after only 2 to 3 years.
Tight control of hypertension confers significant benefit
The United Kingdom Prospective Diabetes Study (UKPDS)10 found that patients who had tight control of blood pressure (mean treated blood pressure 144/82 mm Hg) had 24% fewer diabetes-related end points, 32% fewer diabetes-related deaths, 44% fewer strokes, a 34% reduced risk of deterioration of retinopathy, and a 47% reduced risk of visual deterioration than patients who had usual control (mean treated blood pressure 157/87 mm Hg). The benefit of treating hypertension outweighed the benefits of tight glycemic control.
A strong focus on blood pressure control should be a major focus of any treatment program. The American Geriatrics Society goal for blood pressure is less than 140/80 mm Hg if tolerated. Others have proposed more stringent targets.
Lipid control
Lipid control is integral to managing elderly patients with diabetes. In the Cholesterol and Recurrent Events trial11 and the Heart Protection Study,12 the cardiovascular benefits of reducing serum low-density lipoprotein cholesterol (LDL-C) levels were similar in elderly and younger patients with diabetes. In a meta-analysis of secondary prevention trials, absolute risk reduction was greatest in subjects older than 65 years with either diabetes or diastolic hypertension.
The American Diabetes Association,13 the American Geriatrics Society,14 and the Department of Veterans Affairs15,16 have all set a goal for serum LDL-C of less than 100 mg/dL. In addition, the American Diabetes Association has set goal levels for triglycerides (< 150 mg/dL) and high-density lipoprotein cholesterol (> 40 mg/dL).
Glycemic control
The importance of tight glycemic control in preventing coronary heart disease in the elderly is somewhat controversial. Treatment guidelines for elderly patients with diabetes are mainly extrapolated from the UKPDS, in which patients were a mean of 54 years old at the start of the study. After 10 years, the mean hemoglobin A1c levels were 7.9% in patients receiving conventional control and 7.0% in patients with intensive therapy. Every 1% reduction in hemoglobin A1c was associated with a 37% decline in microvascular complications of diabetes, a 14% decline in myocardial infarctions, and a 21% decline in any diabetes-related outcome.17
In the original trial,18 the rate of myocardial infarction was 17.4% in the conventional treatment group vs 14.7% in the intensive group (P = .052), and the risk of stroke did not differ. No thresholds for realizing benefits from reducing fasting glucose or hemoglobin A1c levels were detected.
A recent cohort study involving about 10,000 participants aged 45 to 79 years found that the risk of cardiovascular disease and death from any cause increased continuously with increasing hemoglobin A1c levels in people with or without diabetes.19 However, the impact of treatment remains to be clarified. The Action to Control Cardiovascular Risk in Diabetes trial will address this question (and others), but results will not be available for several years.
RETINOPATHY IS A MAJOR CAUSE OF BLINDNESS
Diabetic retinopathy, a leading cause of blindness in the United States, is perhaps the most threatening of the chronic microvascular complications of diabetes for elderly patients. The strongest predictor of retinopathy is the duration of diabetes.20–22 Retinopathy is classified as being nonproliferative, preproliferative, or proliferative.
Ischemia is believed to be the major cause of diabetic retinopathy, and glucose control has been shown to be of major benefit. A study of young adults with type 1 diabetes found that intensive therapy reduced the risk of developing retinopathy by 76% and slowed the progression of retinopathy by 54%. Comparable data for patients with type 2 diabetes are lacking.
Of some concern is a study in which retinopathy progressed more rapidly during the first year of aggressive insulin therapy in elderly patients with diabetes and baseline retinopathy.23 Further research is needed to identify which subgroups would benefit most from aggressive glycemic control.
In addition to specific ophthalmologic treatment, managing cardiovascular risk factors may reduce the progression of retinopathy: each cardiovascular risk factor has been found to also be a risk factor for retinopathy. Hypertension is an independent risk factor for any retinopathy, and its tight control reduces progression.20,24 Aspirin therapy has not been found to confer either risk or benefit.25,26
Although guidelines typically call for yearly ophthalmic examinations to screen for retinopathy, whether this is cost-effective has been questioned.27,28 But people older than 65 years with diabetes also have twice the risk of developing cataracts and three times the risk of developing glaucoma than those without diabetes. Considering the effects of visual loss on quality of life as well as the subsequent higher risk of accidents, eye examinations by an ophthalmologist at the time of diagnosis and annually thereafter are recommended. Tight glycemic and blood pressure control remains the cornerstone in the primary prevention of diabetic retinopathy. Panretinal and focal retinal laser photocoagulation reduces the risk of visual loss in patients with severe retinopathy and macular edema, respectively.29
NEUROPATHY PRESENTS IN MANY FORMS
Neuropathy is a particularly distressing complication and can lead to loss of sleep, limitation of activity, and depression.26,30,31 Diabetic neuropathies include focal neuropathies (entrapment syndromes and mono-neuropathies), polyneuropathy, and autonomic neuropathy.
Distal symmetric polyneuropathy (“glove and stocking” sensory symptoms) is the most common neuropathy of elderly people with diabetes. Pain, which can interrupt sleep and limit activity, can be treated with the anticonvulsants gabapentin (Gabarone, Neurontin), phenytoin (Dilantin, Phenytek) and carbamazepine (Carbatrol, Epitol, Equetro, Tegretol), and with tricyclic antidepressants. However, the anticholinergic effects of tricyclic antidepressants limit their use in older patients. Newer agents, such as duloxetine (Cymbalta) and pregabalin (Lyrica) show promise.30,31 Dysesthesia of a burning quality is sometimes treated with topical capsaicin or with oral mexiletine (Mexitil), although their role in treating older patients is not well established.
Patients with distal sensory polyneuropathy are predisposed to develop Charcot joints, which may mimic gout or degenerative joint disease. Plain radiography of the foot can help differentiate these diseases. Distal sensory polyneuropathy also predisposes patients to neuropathic foot ulcer, the leading cause of foot amputation in the United States.32
Feet should be inspected at each office visit. Testing sensation with a monofilament detects sensory neuropathy. Patients should be encouraged to examine their feet daily. Therapeutic shoes, prescribed by a podiatrist and individually designed to prevent blisters, calluses, and ulcers, are covered by Medicare for peripheral neuropathy if any of the following are also present: callus formation, poor circulation, foot deformity, or a history of foot callus, ulcer, or amputation (partial or complete). Medicare will pay for one pair of shoes plus three pairs of inserts per year.
Proximal motor neuropathy (diabetic amyotrophy) primarily affects elderly patients. It begins with unilateral thigh pain, which becomes bilateral and progresses to proximal muscle weakness and wasting. Distal symmetric polyneuropathy may also be present. Treatment includes glycemic control (usually with insulin) and physical therapy. Some forms of amyotrophy respond to immunotherapy.
Autonomic neuropathy, although not painful, can be the most life-threatening form of diabetic neuropathy.33 Tachycardia increases the risk of sudden death, while postural hypotension increases the risk of syncope, falling, and injury. Other forms of autonomic neuropathy include neurogenic bladder, sexual dysfunction, gastropathy (which is particularly sensitive to glycemic control), enteropathy, and gustatory sweating. Patients with autonomic neuropathy are more likely to have hypoglycemic unawareness.
NEPHROPATHY CAN PROGRESS RAPIDLY
Elderly patients with diabetes are especially at risk of developing nephropathy, which progresses from microalbuminuria to overt proteinuria to renal insufficiency and end-stage renal disease. Nephropathy may develop over a shorter time than the typical 10 to 20 years in younger patients. Independent risk factors for proteinuria and renal insufficiency include poor glycemic control over many years, hypertension, longer duration of diabetes, male sex, high serum total cholesterol levels, and smoking. Elderly patients are also at risk of renal insults such as receiving intravenous iodinated contrast agents in the course of radiologic procedures, nephrotoxic drugs, and comorbid illness such as congestive heart failure.
The diagnosis of diabetic nephropathy is usually made clinically and not by renal biopsy. Diabetic nephropathy can be diagnosed with almost 100% specificity in type 1 diabetes and more than 85% specificity in type 2 diabetes by a urinary albumin excretion of more than 300 mg per day and an appropriate time course in the absence of other obvious causes of renal disease. The urinary albumin-to-creatinine ratio can be used to screen for microalbuminuria (the precursor of frank proteinuria and renal insufficiency). A value of more than 30 mg of albumin per gram of creatinine suggests that albumin excretion exceeds 30 mg and that microalbuminuria is present.
Prevention is a cornerstone of management. Good glycemic control reduces the risk of microalbuminuria, the progression of albuminuria, and the development of renal insufficiency. Lowering blood pressure reduces the decline in glomerular filtration rate and albuminuria. Angiotensin-converting enzyme (ACE) inhibitors reduce the rate of progression of proteinuria and reduce the rate of end-stage renal disease, although the data are stronger in patients with type 1 diabetes.34 When side effects such as cough limit the use of ACE inhibitors, angiotensin receptor blockers can be used as an alternative. Blood pressure should be controlled to reduce stroke and cardiovascular complications, regardless of whether microalbuminuria is present.35
End-stage renal disease in elderly patients with diabetes is becoming increasingly frequent. Nephropathy in older patients is different from that in younger patients. In elderly patients, the pathologic findings may suggest ischemia and hypertension, and the classic Kimmelstiel-Wilson lesions may be absent. Patients may present with end-stage renal disease following an episode of acute renal failure that does not resolve, which may occur after a radiologic procedure involving an iodinated contrast agent.
NONKETOTIC HYPEROSMOLAR COMA
Nonketotic hyperosmolar coma occurs predominantly in elderly patients with type 2 diabetes. Predisposing factors include dementia, infection, stroke, and myocardial infarction. Coma results from osmotic diuresis due to hyperglycemia and consequent dehydration. A drop in the glomerular filtration rate promotes further hyperglycemia and dehydration in a vicious circle. Glucose levels commonly reach 600 mg/dL or more, and serum osmolality often exceeds 320 mOsm/L. A fluid deficit of 5 to 10 L is typical.
Fluid replacement is the mainstay of treatment. Because free water is typically lost in an osmotic diuresis, 0.9% (normal) saline is usually given if hemodynamic instability is present or 0.45% (half-normal) saline otherwise. Insulin is also required, as is specific treatment of the precipitating cause, eg, infection. Ketoacidosis may also occur in the elderly.
Recovery from coma or improvement in mental status may lag behind correction of the serum osmolality and may take several days. Mortality rates can be high: severe hyperosmolarity, advanced age, and nursing home residence are the major risk factors for death.
INFECTIONS: SEVERE AND UNUSUAL
Elderly patients with diabetes are at increased risk of developing severe and unusual infections, particularly malignant external otitis. Necrotizing Pseudomonas aeruginosa infection initially involves the external ear canal and progresses to the mastoid air cells, the skull base, or temporal bone. The clinical presentation consists of fever, otalgia, otorrhea, and less commonly, cranial nerve palsy. Treatment involves surgical debridement and antibiotics.
Other infections associated with diabetes include rhinocerebral mucormycosis, necrotizing fasciitis, emphysematous cholecystitis, and emphysematous pyelonephritis. An elderly patient with diabetes is also at increased risk of renal papillary necrosis, which presents as insidious renal failure.
COGNITIVE IMPAIRMENT
Elderly people with diabetes are at increased risk of cognitive impairment, which poses a barrier to taking medications appropriately and performing other tasks of self-management.
Because dementia may go undetected, particularly in the early stages, cognitive function should be assessed in elderly patients when they fail to take therapy correctly or have frequent episodes of hypoglycemia, or if glycemic control deteriorates without an obvious explanation. Caregivers play a critical role in detecting and reporting early cognitive impairment.
DEPRESSION IS OFTEN UNDETECTED
Elderly patients with diabetes have a higher rate of depression than do age-matched controls, but it is commonly underdetected and undertreated.5,36 Depression has been associated with poor glycemic control, and treatment of depression is associated with improved control. Routine screening for depression should be performed; a variety of diagnostic instruments are available. Particular attention should be given to medications that are associated with depression.
POLYPHARMACY
Many elderly patients take multiple medications. Polypharmacy increases the risk of drug side effects, interactions, and nonadherence to taking medications.37–39 This problem is increased in diabetes, in which several medications are necessary to manage hyper-glycemia, hyperlipidemia, hypertension, and other associated conditions.
Patients should keep accurate medication lists, including over-the-counter medications, herbs, and nutritional supplements. Physicians should carefully review each medication to check if it is appropriate and used correctly.
FALLS
Elderly patients with diabetes mellitus are at increased risk of injurious falls, which are associated with high rates of complications, death, and functional decline.40,41 Risk factors include frailty and functional disability, visual impairment, peripheral or autonomic neuropathy, hypoglycemia, and polypharmacy.
Elderly patients should be screened for their risk of falls, and appropriate measures should be instituted. The American Geriatrics Society has guidelines for preventing falls in the elderly.41
URINARY INCONTINENCE
Elderly women with diabetes are at increased risk of developing urinary incontinence. Risk factors include autonomic neuropathy (causing either neurogenic bladder or fecal impaction), polyuria due to hyperglycemia, and urinary tract and vaginal infections. Although evidence is lacking that urinary incontinence affects glycemic control, assessing and treating the condition improves quality of life.
SUMMARY
Diabetes is a common problem in the elderly, accounting for considerable morbidity and mortality. In a large longitudinal analysis (> 50,000 patients), elderly persons newly diagnosed as having diabetes experienced high rates of complications during 10-year follow-up, far in excess of elderly persons without diabetes.42 Diabetes is underdiagnosed in the elderly and is frequently undertreated. Management of the elderly with diabetes presents unique challenges because of associated comorbidities, but with attention to detail and individualized approaches, quality and duration of life can be optimized. The greatest attention should be given to reduction of overall cardiovascular risk. Glycemic goals and the treatment regimens to achieve those goals should be individualized and chosen to control hyperglycemic symptoms and achieve the maximal glycemic control possible while minimizing the risk of hypoglycemia. Diabetes will continue to be a challenge to the patient, the physician, the care team, and the health care system.
- Gregg EW, Engelgau MM, Narayan V. Complications of diabetes in elderly people. BMJ 2002; 325:916–917.
- Knopman D, Boland LL, Mosley T, et al. Cardiovascular risk factors and cognitive decline in middle-aged adults. Neurology 2001; 56:42–48.
- Ott A, Stolk RP, van Harskamp F, Pols HA, Hofman A, Breteler MM. Diabetes mellitus and the risk of dementia: The Rotterdam Study. Neurology 1999; 53:1937–1942.
- Fontbonne A, Berr C, Ducimetiere P, Alperovitch A. Changes in cognitive abilities over a 4-year period are unfavorably affected in elderly diabetic subjects: results of the Epidemiology of Vascular Aging Study. Diabetes Care 2001; 24:366–370.
- Gregg EW, Mangione CM, Cauley JA, et al. Diabetes and incidence of functional disability in older women. Diabetes Care 2002; 25:61–67.
- Hornick T, Aron DC. Managing diabetes in the elderly: go easy, individualize. Cleve Clin J Med 2008; 75:70–78.
- Haffner SM, Lehto S, Ronnemaa T, Pyorala K, Laakso M. Mortality from coronary heart disease in subjects with type 2 diabetes and in nondiabetic subjects with and without prior myocardial infarction. N Engl J Med 1998; 339:229–234.
- Bertoni AG, Krop JS, Anderson GF, Brancati FL. Diabetes-related morbidity and mortality in a national sample of U.S. elders. Diabetes Care 2002; 25:471–475.
- Bertoni AG, Kirk JK, Goff DC, Wagenknecht LE. Excess mortality related to diabetes mellitus in elderly Medicare beneficiaries. Ann Epidemiol 2004; 14:362–367.
- UK Prospective Diabetes Study Group. Tight blood pressure control and risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 38. BMJ 1998; 317:703–713. Erratum in: BMJ 1999; 318:29.
- Goldberg RB, Mellies MJ, Sacks FM, et al. Cardiovascular events and their reduction with pravastatin in diabetic and glucose-intolerant myocardial infarction survivors with average cholesterol levels: subgroup analyses in the Cholesterol and Recurrent Events (CARE) trial. The CARE Investigators. Circulation 1998; 98:2513–2519.
- Collins R, Armitage J, Parish S, Sleigh P, Peto R. MRC/BHF Heart Protection Study of cholesterol-lowering with simvastatin in 5963 people with diabetes. Lancet 2003; 361:2005–2016.
- American Diabetes Association. Standards of medical care in diabetes. Diabetes Care 2005; 28:S4–S36.
- Brown AF, Mangione CM, Saliba D, Sarkisian CA California Healthcare Foundation/American Geriatrics Society Panel on Improving Care for Elders with Diabetes. Guidelines for improving the care of the older person with diabetes mellitus. J Am Geriatr Soc 2003; 51:S265–S280.
- VA/DoD Clinical Practice Guideline for the Management of Diabetes Mellitus in the Primary Care Setting 2003. Accessed January 4, 2008. www.oqp.med.va.gov/cpg/dm/DM3_cpg/content/introduction.htm.
- Pogach LM, Brietzke SA, Cowan CL, Conlin P, Walder DJ, Sawin CT VA/DoD Diabetes Guideline Development Group. Development of evidence-based clinical practice guidelines for diabetes: the Department of Veterans Affairs/Department of Defense guidelines initiative. Diabetes Care 2004; 27:B82–B89.
- Stratton IM, Asler AI, Neil HA, et al. Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. BMJ 2000; 321:405–412.
- UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet 1998; 352:837–853. Erratum in: Lancet 1999; 354:602.
- Khaw KT, Wareham N, Bingham S, Luben R, Welch A, Day N. Association of hemoglobin A1c with cardiovascular disease and mortality in adults: the European prospective investigation into cancer in Norfolk. Ann Intern Med 2004; 141:413–420.
- Matthews DR, Stratton IM, Aldington SJ, Holman RR, Kohner EM UK Prospective Diabetes Study Group. Risks of progression of retinopathy and vision loss related to tight blood pressure control in type 2 diabetes mellitus: UKPDS 69. Arch Ophthalmol 2004; 122:1631–1640.
- Cahill M, Halley A, Codd M, et al. Prevalence of diabetic retinopathy in patients with diabetic mellitus diagnosed after the age of 70 years. Br J Opthalmol 1997; 81:218–222.
- Hirvela H, Laatikainen L. Diabetic retinopathy in people aged 70 years or older. The Oulu Eye Study. Br J Ophthalmol 1997; 81:214–217.
- Tovi J, Ingemansson SO, Engfeldt P. Insulin treatment of elderly type 2 diabetic patients: effects on retinopathy. Diabetes Metab 1998; 24:442–447.
- Schrier RW, Estacio RO, Esler A, Mehler P. Effects of aggressive blood pressure control in normotensive type 2 diabetic patients on albuminuria, retinopathy and strokes. Kidney Int 2002; 61:1086–1097.
- Kohner EM. Aspirin for diabetic retinopathy. BMJ 2003; 327:1060–1061.
- Greene DA, Stevens MJ, Feldman EL. Diabetic neuropathy: scope of the syndrome. Am J Med 1999; 107:2S–8S.
- Hutchinson A, McIntosh A, Peters J, et al. Effectiveness of screening and monitoring tests for diabetic retinopathy—a systematic review. Diabet Med 2000; 17:495–506.
- Vijan S, Hofer TP, Hayward RA. Cost-utility analysis of screening intervals for diabetic retinopathy in patients with type 2 diabetes mellitus. JAMA 2000; 283:889–896.
- Mohamed Q, Gillies MC, Wong TY. Management of diabetic retinopathy: a systematic review. JAMA 2007; 298:902–916.
- Argoff CE, Cole BE, Fishbain DA, Irving GA. Diabetic peripheral neuropathic pain: clinical and quality-of-life issues. Mayo Clin Proc 2006; 81:S3–S11.
- Wong MC, Chung JW, Wong TK. Effects of treatments for symptoms of painful diabetic neuropathy: systematic review. BMJ 2007; 335:87: epubl June 11, 2007.
- Bild DE, Selby JV, Sinnock P, Browner WS, Braveman P, Showstack JA. Lower-extremity amputation in people with diabetes. Epidemiology and prevention. Diabetes Care 1989; 12:24–31.
- Wheeler SG, Ahroni JH, Boyko EJ. Prospective study of autonomic neuropathy as a predictor of mortality in patients with diabetes. Diabetes Res Clin Pract 2002; 58:131–138.
- Brenner BM, Cooper ME, de Zeeuw D RENAAL Study Investigators. Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med 2001; 345:861–869.
- UK Prospective Diabetes Study Group. Efficacy of atenolol and captopril in reducing risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 39. BMJ 1998; 317:713–720.
- Sinclair AJ, Girling AJ, Bayer AJ. Cognitive dysfunction in older subjects with diabetes mellitus: impact on diabetes self-management and use of care services. All Wales Research into Elderly (AWARE) Study. Diabetes Res Clin Pract 2000; 50:203–212.
- Moisan J, Gaudet M, Gregoire JP, Bouchard R. Non-compliance with drug treatment and reading difficulties with regard to prescription labelling among seniors. Gerontology 2002; 48:44–51.
- Boyd CM, Darer J, Boult C, Fried LP, Boult L, Wu AW. Clinical practice guidelines and quality of care for older patients with multiple comorbid diseases: implications for pay for performance. JAMA 2005; 294:716–724.
- Jackevicius CA, Mamdani M, Tu JV. Adherence with statin therapy in elderly patients with and without acute coronary syndromes. JAMA 2002; 288:462–467.
- Schwartz AV, Hillier TA, Sellmeyer DE, et al. Older women with diabetes have a higher risk of falls: a prospective study. Diabetes Care 2002; 25:1749–1754.
- American Geriatrics Society, British Geriatrics Society, and American Academy of Orthopaedic Surgeons Panel on Falls Prevention. Guideline for the prevention of falls in older persons. J Am Geriatr Soc 2001; 49:664–672.
- Bethel MA, Sloan FA, Belsky D, Feinglos MN. Longitudinal incidence and prevalence of adverse outcomes of diabetes mellitus in elderly patients. Arch Intern Med 2007; 167:921–927.
- Gregg EW, Engelgau MM, Narayan V. Complications of diabetes in elderly people. BMJ 2002; 325:916–917.
- Knopman D, Boland LL, Mosley T, et al. Cardiovascular risk factors and cognitive decline in middle-aged adults. Neurology 2001; 56:42–48.
- Ott A, Stolk RP, van Harskamp F, Pols HA, Hofman A, Breteler MM. Diabetes mellitus and the risk of dementia: The Rotterdam Study. Neurology 1999; 53:1937–1942.
- Fontbonne A, Berr C, Ducimetiere P, Alperovitch A. Changes in cognitive abilities over a 4-year period are unfavorably affected in elderly diabetic subjects: results of the Epidemiology of Vascular Aging Study. Diabetes Care 2001; 24:366–370.
- Gregg EW, Mangione CM, Cauley JA, et al. Diabetes and incidence of functional disability in older women. Diabetes Care 2002; 25:61–67.
- Hornick T, Aron DC. Managing diabetes in the elderly: go easy, individualize. Cleve Clin J Med 2008; 75:70–78.
- Haffner SM, Lehto S, Ronnemaa T, Pyorala K, Laakso M. Mortality from coronary heart disease in subjects with type 2 diabetes and in nondiabetic subjects with and without prior myocardial infarction. N Engl J Med 1998; 339:229–234.
- Bertoni AG, Krop JS, Anderson GF, Brancati FL. Diabetes-related morbidity and mortality in a national sample of U.S. elders. Diabetes Care 2002; 25:471–475.
- Bertoni AG, Kirk JK, Goff DC, Wagenknecht LE. Excess mortality related to diabetes mellitus in elderly Medicare beneficiaries. Ann Epidemiol 2004; 14:362–367.
- UK Prospective Diabetes Study Group. Tight blood pressure control and risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 38. BMJ 1998; 317:703–713. Erratum in: BMJ 1999; 318:29.
- Goldberg RB, Mellies MJ, Sacks FM, et al. Cardiovascular events and their reduction with pravastatin in diabetic and glucose-intolerant myocardial infarction survivors with average cholesterol levels: subgroup analyses in the Cholesterol and Recurrent Events (CARE) trial. The CARE Investigators. Circulation 1998; 98:2513–2519.
- Collins R, Armitage J, Parish S, Sleigh P, Peto R. MRC/BHF Heart Protection Study of cholesterol-lowering with simvastatin in 5963 people with diabetes. Lancet 2003; 361:2005–2016.
- American Diabetes Association. Standards of medical care in diabetes. Diabetes Care 2005; 28:S4–S36.
- Brown AF, Mangione CM, Saliba D, Sarkisian CA California Healthcare Foundation/American Geriatrics Society Panel on Improving Care for Elders with Diabetes. Guidelines for improving the care of the older person with diabetes mellitus. J Am Geriatr Soc 2003; 51:S265–S280.
- VA/DoD Clinical Practice Guideline for the Management of Diabetes Mellitus in the Primary Care Setting 2003. Accessed January 4, 2008. www.oqp.med.va.gov/cpg/dm/DM3_cpg/content/introduction.htm.
- Pogach LM, Brietzke SA, Cowan CL, Conlin P, Walder DJ, Sawin CT VA/DoD Diabetes Guideline Development Group. Development of evidence-based clinical practice guidelines for diabetes: the Department of Veterans Affairs/Department of Defense guidelines initiative. Diabetes Care 2004; 27:B82–B89.
- Stratton IM, Asler AI, Neil HA, et al. Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. BMJ 2000; 321:405–412.
- UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet 1998; 352:837–853. Erratum in: Lancet 1999; 354:602.
- Khaw KT, Wareham N, Bingham S, Luben R, Welch A, Day N. Association of hemoglobin A1c with cardiovascular disease and mortality in adults: the European prospective investigation into cancer in Norfolk. Ann Intern Med 2004; 141:413–420.
- Matthews DR, Stratton IM, Aldington SJ, Holman RR, Kohner EM UK Prospective Diabetes Study Group. Risks of progression of retinopathy and vision loss related to tight blood pressure control in type 2 diabetes mellitus: UKPDS 69. Arch Ophthalmol 2004; 122:1631–1640.
- Cahill M, Halley A, Codd M, et al. Prevalence of diabetic retinopathy in patients with diabetic mellitus diagnosed after the age of 70 years. Br J Opthalmol 1997; 81:218–222.
- Hirvela H, Laatikainen L. Diabetic retinopathy in people aged 70 years or older. The Oulu Eye Study. Br J Ophthalmol 1997; 81:214–217.
- Tovi J, Ingemansson SO, Engfeldt P. Insulin treatment of elderly type 2 diabetic patients: effects on retinopathy. Diabetes Metab 1998; 24:442–447.
- Schrier RW, Estacio RO, Esler A, Mehler P. Effects of aggressive blood pressure control in normotensive type 2 diabetic patients on albuminuria, retinopathy and strokes. Kidney Int 2002; 61:1086–1097.
- Kohner EM. Aspirin for diabetic retinopathy. BMJ 2003; 327:1060–1061.
- Greene DA, Stevens MJ, Feldman EL. Diabetic neuropathy: scope of the syndrome. Am J Med 1999; 107:2S–8S.
- Hutchinson A, McIntosh A, Peters J, et al. Effectiveness of screening and monitoring tests for diabetic retinopathy—a systematic review. Diabet Med 2000; 17:495–506.
- Vijan S, Hofer TP, Hayward RA. Cost-utility analysis of screening intervals for diabetic retinopathy in patients with type 2 diabetes mellitus. JAMA 2000; 283:889–896.
- Mohamed Q, Gillies MC, Wong TY. Management of diabetic retinopathy: a systematic review. JAMA 2007; 298:902–916.
- Argoff CE, Cole BE, Fishbain DA, Irving GA. Diabetic peripheral neuropathic pain: clinical and quality-of-life issues. Mayo Clin Proc 2006; 81:S3–S11.
- Wong MC, Chung JW, Wong TK. Effects of treatments for symptoms of painful diabetic neuropathy: systematic review. BMJ 2007; 335:87: epubl June 11, 2007.
- Bild DE, Selby JV, Sinnock P, Browner WS, Braveman P, Showstack JA. Lower-extremity amputation in people with diabetes. Epidemiology and prevention. Diabetes Care 1989; 12:24–31.
- Wheeler SG, Ahroni JH, Boyko EJ. Prospective study of autonomic neuropathy as a predictor of mortality in patients with diabetes. Diabetes Res Clin Pract 2002; 58:131–138.
- Brenner BM, Cooper ME, de Zeeuw D RENAAL Study Investigators. Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med 2001; 345:861–869.
- UK Prospective Diabetes Study Group. Efficacy of atenolol and captopril in reducing risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 39. BMJ 1998; 317:713–720.
- Sinclair AJ, Girling AJ, Bayer AJ. Cognitive dysfunction in older subjects with diabetes mellitus: impact on diabetes self-management and use of care services. All Wales Research into Elderly (AWARE) Study. Diabetes Res Clin Pract 2000; 50:203–212.
- Moisan J, Gaudet M, Gregoire JP, Bouchard R. Non-compliance with drug treatment and reading difficulties with regard to prescription labelling among seniors. Gerontology 2002; 48:44–51.
- Boyd CM, Darer J, Boult C, Fried LP, Boult L, Wu AW. Clinical practice guidelines and quality of care for older patients with multiple comorbid diseases: implications for pay for performance. JAMA 2005; 294:716–724.
- Jackevicius CA, Mamdani M, Tu JV. Adherence with statin therapy in elderly patients with and without acute coronary syndromes. JAMA 2002; 288:462–467.
- Schwartz AV, Hillier TA, Sellmeyer DE, et al. Older women with diabetes have a higher risk of falls: a prospective study. Diabetes Care 2002; 25:1749–1754.
- American Geriatrics Society, British Geriatrics Society, and American Academy of Orthopaedic Surgeons Panel on Falls Prevention. Guideline for the prevention of falls in older persons. J Am Geriatr Soc 2001; 49:664–672.
- Bethel MA, Sloan FA, Belsky D, Feinglos MN. Longitudinal incidence and prevalence of adverse outcomes of diabetes mellitus in elderly patients. Arch Intern Med 2007; 167:921–927.
KEY POINTS
- Compared with strict glycemic control, treating cardiovascular risk factors offers more benefit in a shorter time and should be a higher priority.
- Diabetic retinopathy is a leading cause of blindness. Yearly eye examinations are recommended.
- Elderly patients with diabetes are prone to rapidly progressive nephropathy, especially after receiving iodinated contrast agents. Good glycemic control and control of blood pressure, especially with angiotensin-converting enzyme inhibitors, reduce the risk and the rate of progression.
- Elderly patients with diabetes are at higher risk of cognitive decline, depression, and polypharmacy, all of which impede good diabetes management.