User login
A young man with acute weakness of his right arm
A 42-year-old man was working at his computer when he suddenly became disoriented and lightheaded, had difficulty concentrating, and could not move his right arm. He could walk without difficulty, but he had a tingling sensation in his right leg. He did not lose consciousness or have any associated palpitations, chest pain, shortness of breath, nausea, vomiting, headaches, or visual changes.
He called 911, and an ambulance arrived 15 minutes later. By that time his symptoms had started to resolve. Now, in the emergency department, his only residual symptom is mild numbness of his right arm and shoulder.
Until now he has been healthy except for a history of dyslipidemia. He takes no prescription or over-the-counter medications and has no drug allergies. He has smoked one pack of cigarettes daily for the past 28 years and also smokes marijuana several times each month. He drinks alcohol occasionally. His family has no history of stroke, premature coronary artery disease, or sudden cardiac death.
INITIAL EVALUATION
His heart rate is 88 beats per minute, blood pressure 142/82 mm Hg, and blood oxygen saturation 98% while breathing room air. He is alert and in no acute distress and answers questions appropriately.
His breathing sounds are normal, without crackles or wheezes. His heart has normal first and second sounds, a normal rate and rhythm, and no extra sounds or murmurs. His abdomen is normal. His extremities are warm and well perfused with normal peripheral pulses and no edema.
On neurologic examination, his cranial nerves and visual fields are normal, and his strength is normal in all muscle groups except for the right upper arm, which is slightly weaker than the left when tested against resistance. Reflexes and response to light touch and pinprick are normal.
His serum chemistry levels, renal function, and blood counts are normal. His total cholesterol level is 155 mg/dL, high-density lipoprotein cholesterol 38 mg/dL, low-density lipoprotein cholesterol 108 mg/dL, and triglycerides 1,286 mg/dL. Electrocardiography is normal with sinus rhythm at a rate of 74.
Magnetic resonance imaging (MRI) of the head and neck with magnetic resonance angiography (MRA) of the intracranial and extracranial vessels is performed. Diffusion-weighted images show a hyperintense lesion in the left insular cortex, consistent with an infarct in the distribution of a branch of the left middle cerebral artery. There is no intracranial hemorrhage. All intracranial and extracranial major vessels are patent, and no stenoses are seen.
DIFFERENTIAL DIAGNOSIS
1. Which is the most likely cause of this patient’s stroke?
- Vertebral or carotid atherosclerosis
- Cervical arterial dissection
- A hematologic disorder
- Cocaine abuse
- Cardiac embolism
Atherosclerosis
Although 85% of all strokes are ischemic, and most ischemic strokes are caused by occlusive atherosclerosis of large vessels, most ischemic strokes occur in patients older than 65 years. In patients younger than 55 years, only about 10% of strokes are caused by large-vessel atherosclerotic disease, thus lowering the initial probability that this is the cause of our patient’s stroke.1 Furthermore, our patient’s MRA study showed no carotid artery stenoses, which effectively eliminates this as the cause of his stroke, as the diagnostic sensitivity of MRA for detecting carotid stenosis is approximately 97%.
Cervical arterial dissection
Cervical arterial dissection causes up to 20% of strokes in patients younger than 45 years.2 Dissections usually involve the extracranial portion of the vessel, and involve the internal carotid arteries at least three times as often as the vertebral arteries. In many cases the dissection is preceded by mild neck trauma, which may be as minor as a vigorous cough or turning of the head.
Typical features of dissection include neck pain, headache, and Horner syndrome, followed minutes to hours later by symptoms of ocular or cerebral ischemia, usually a transient ischemic attack rather than a stroke. Neurologic symptoms are most commonly due to thrombosis at the dissection site with distal embolization. Inherited disorders that are associated with increased risk of cervical arterial dissection include Ehlers-Danlos syndrome type IV, Marfan syndrome, autosomal-dominant polycystic kidney disease, osteogenesis imperfecta type I, and fibromuscular dysplasia.3 MRA and computed tomographic angiography are the diagnostic tests of choice.
Our patient’s symptoms began suddenly, without a history of trauma or neck pain, making arterial dissection less likely as the cause of his stroke. No dissection was seen on MRA, which also minimizes its likelihood.4
Hematologic disorders
Many hematologic disorders are associated with ischemic stroke. The disorders most likely to cause ischemic stroke in patients younger than 45 years are antiphospholipid antibody syndrome, sickle cell anemia, and heparin-induced thrombocytopenia,5 which are associated with arterial thrombosis.
Most of the common hereditary hypercoagulable disorders, such as factor V Leiden/activated protein C resistance, the prothrombin gene mutation (G20210A), antithrombin III deficiency, protein C deficiency, and protein S deficiency, typically cause venous thrombosis much more often than they cause arterial thrombosis. Thus, the most typical presentations of stroke in these disorders are cerebral venous thrombosis or paradoxical embolic stroke due to a patent foramen ovale. Antithrombin III deficiency and protein C and protein S deficiency have been associated with arterial thrombosis, but so infrequently that their likelihood in this patient is extremely low.
Clues to the diagnosis of a hypercoagulable state include venous thrombosis in the past, recurrent fetal loss, thrombocytopenia, livedo reticularis, antiphospholipid antibody syndrome, and skin necrosis at the start of oral anticoagulant therapy.
Of importance: the relationship between hereditary hypercoagulable disorders and stroke is considerably weaker than their association with venous thrombosis. Several studies in clinical and general populations have failed to show an independent association between stroke and protein C deficiency, protein S deficiency, antithrombin III deficiency, factor V Leiden/activated protein C resistance, or the prothrombin G20210A mutation.6–8 Therefore, most experts do not recommend screening all stroke patients for a hypercoagulable state—only those with a personal or family history of thrombosis or young patients with unexplained stroke.
Our patient does not have historical or clinical features that would suggest a specific hypercoagulable disorder, either acquired (eg, heparin-induced thrombocytopenia) or inherited. A laboratory workup for a hypercoagulable disorder would likely be of little value in determining the cause of his stroke, and even if a hereditary disorder were identified it would be difficult to determine causation. However, if no other explanation for his stroke can be found during the workup, one could consider testing for proteins C and S, antithrombin III, activated protein C resistance (and factor V Leiden if screening for activated protein C resistance is positive), prothrombin G20210A, fibrinogen, homocysteine, D-dimers, and antiphospholipid antibodies.
Cocaine abuse
Another important cause of ischemic stroke is the use of sympathomimetic drugs such as cocaine or amphetamines. The strongest association is with cocaine, which has been seen in case series to cause cerebral vasoconstriction in a dose-dependent manner. Vasoconstriction is also related to a longer duration of cocaine use.9 Several case-control studies have found that the risk of stroke is 4.5 to 6.5 times higher in drug abusers than in controls, and that use of catecholamines or cocaine alone was associated with a significantly increased risk of stroke.10,11
It is certainly advisable to ask about the use of illicit drugs and to send serum and urine samples for appropriate drug screening in young stroke patients, particularly if another cause cannot be found or if drug use is suspected.12
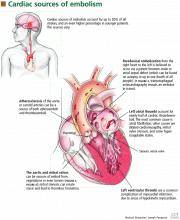
Cardiac embolism
Cardiac embolism is the most likely cause of the stroke in this patient. Up to 20% of the 500,000 strokes that occur annually in the United States are of cardiac embolic origin,13 and the prevalence is even higher in younger patients. In a registry of 428 strokes in patients 15 to 44 years of age, a cardiac source of embolism was the cause in 31.8%.14
- Masses, which include atherosclerotic plaques, cardiac tumors, and infective and noninfective valvular vegetations
- Passageways for paradoxical embolism, such as a patent foramen ovale or atrial septal defect (Figure 2)
- Stasis in the left atrium or left ventricle, with a resulting propensity for thrombosis.
Atrial thrombus is most often seen in patients with atrial fibrillation, mitral stenosis, or dilated cardiomyopathy. Echocardiography of the left atrium in patients with these conditions often reveals spontaneous echo contrast that resembles swirling “smoke,” which is thought to be produced by red blood cell aggregation due to blood stasis. This sign is strongly associated with left atrial thrombi.
Left ventricular thrombosis is one of the most common complications of myocardial infarction and is caused by blood stasis in regions of the ventricle in which the myocardium is hypokinetic or akinetic.
We cannot assume, however, that a potential cardioembolic source seen on echocardiography is the cause of a given patient’s stroke. The evidence proving a causal relationship between most potential cardiac embolic sources and stroke is less than robust. Most of the published data are from nonrandomized studies or case series, and there are no large, prospective studies available to clearly prove that a given cardioembolic source is directly related to embolic stroke.16
This being said, most studies have found high prevalence rates of cardioembolic sources in patients with embolic stroke, which suggests that a causative relationship may exist. However, many of these findings also have a relatively high prevalence among the general population without stroke, raising the possibility that the finding could be incidental and unrelated. Examples are patent foramen ovale, which exists in 27% of adults,17 and aortic arch atheroma, which is common in the elderly.
In the end, when the only potential source of embolism that can be found is in the heart (as is often the case in younger patients), the probability is much greater that it is indeed the cause of the stroke. The lack of direct evidence linking many sources of cardioembolism to stroke emphasizes the need for a thorough investigation of all possible causes of stroke.
DIAGNOSTIC EVALUATION
2. Which is the best study to evaluate for a cardiac embolic source in this patient?
- Transthoracic echocardiography (TTE)
- Transesophageal echocardiography (TEE)
- Transcranial Doppler ultrasonography
- Electrocardiography
The study of choice in this patient is TEE. Overall, TEE is better than TTE in identifying a cardiac source of embolism,18,19 mainly because the images are obtained from a probe in the esophagus, which is in close proximity to the heart, so that there is little additional soft tissue and bone between the probe and cardiac structures. In addition, higher-frequency probes can be used. Both of these result in ultrasonographic images with much greater spatial resolution than can be obtained with a transthoracic study.15
In a case series,20 TEE identified a potential cardiac source of embolism in 45 (57%) of 79 patients with cryptogenic stroke, compared with only 12 (15%) with TTE.
The main limitation of TEE is that it does not show the left ventricular apex very well, making an accurate assessment of left ventricular function or identification of a left ventricular apical thrombus much less likely.
In patients who lack evidence of atherosclerotic cerebrovascular disease, specific findings on history or physical examination could increase the chances of identifying an embolic source, such as left ventricular thrombus, on TTE. These findings could include a history of a myocardial infarction, congestive heart failure, left ventricular dysfunction, endocarditis, rheumatic heart disease, a prosthetic valve, or atrial fibrillation or flutter. TTE by itself is considered sufficient for making the diagnosis of mitral stenosis, left ventricular aneurysm, dilated cardiomyopathy, left ventricular thrombus, and mitral valve prolapse with myxomatous degeneration of the leaflets.
However, in patients without signs or symptoms of cardiac disease, the diagnostic value of TTE is significantly less. Several studies have demonstrated that in patients without evidence of cardiac disease, TTE identifies the source of embolism less than 10% of the time.21 Some series even suggest that the yield may be less than 1%.22 TEE has the advantage of being able to diagnose the above disorders and of having a higher sensitivity for identifying potential sources that may be missed by TTE, such as left atrial or left atrial appendage thrombus, aortic arch atheroma, patent foramen ovale, atrial septal aneurysm, or spontaneous echo contrast. It should be remembered, however, that TEE is a semi-invasive procedure that carries the risks of both the procedure and the sedation, eg, bronchospasm, hypoxia, arrhythmias, upper gastrointestinal trauma, and bleeding.23
Further clouding the decision are recent advances in TTE technology, such as contrast TTE with second harmonic imaging, which enhances the ability of TTE to identify potential sources of stroke such as patent foramen ovale nearly to the level of TEE.24
Unfortunately, guidelines from professional societies do not offer assistance on the best diagnostic approach. Current guidelines from the American Heart Association, American College of Cardiology, and American Society of Echocardiography do give echocardiography a class I indication in younger patients (< 45 years old) with cerebrovascular events or older patients (> 45 years old) with stroke without evidence of cerebrovascular disease or other obvious causes. However, there is no official recommendation on whether to choose TTE, TEE, or both studies.16 Given the multiple causes of cardioembolism and the variety of clinical factors that could influence the decision to choose a certain echo study, this decision is appropriately left to the individual physician.
A reasonable, evidence-based diagnostic approach in young stroke patients is to proceed to TEE when routine TTE and electrocardiography are unrevealing.25 In reality, this is the practice followed in most centers, including ours. Although TTE has a lower diagnostic yield in patients without symptoms, it has the advantages of being readily available in most centers, being noninvasive, and providing complementary information to TEE even when TTE does not reveal a potential cause of stroke.
As for the other studies:
Electrocardiography is valuable in identifying potential cardioembolic causes of stroke such as atrial fibrillation, left ventricular aneurysm, or myocardial infarction, but it is insufficient by itself to assess for many other potential sources of cardioembolism.
Transcranial Doppler ultrasonography is very sensitive for detecting patent foramen ovale and other right-to-left shunts that could be sources of cardioembolism. In this test, microbubbles from agitated saline are injected into the venous circulation and are detected in the cerebral arteries after passing through the shunt. It has no utility in identifying the other possibilities discussed above, nor can it discriminate whether these shunts are intra-cardiac or extracardiac.
Case continued
The patient undergoes TTE, which shows normal left ventricular size, wall thickness, and systolic function. His right ventricular function is normal, as are his left and right atrial size. Valvular function is normal, and no right-to-left interatrial shunt is detected with the use of agitated saline contrast.
MANAGEMENT
3. Which is the most appropriate way to manage the lesion?
- Surgical resection
- Periodic echocardiographic follow-up
- Anticoagulation and periodic echocardiographic follow-up
Cardiac papillary fibroelastomas are rare benign primary tumors of the heart. The true incidence is unknown because, when small, they can be asymptomatic and easily overlooked on gross examination. In adults, they are the second most common primary cardiac tumors, next to atrial myxoma.26
The histogenesis is not known, but the mean age at which they are detected is approximately 60 years, and most of the patients are men, likely because most of these tumors are found incidentally during echocardiography, open heart surgery, or autopsy.28
Most patients with cardiac papillary fibroelastomas have no symptoms; however, those who do have symptoms usually experience valve obstruction or embolization of tumor fragments, leading to stroke, myocardial infarction, or sudden death. Further increasing the risk of embolism, thrombus has been reported on the surface of some tumors, supporting the use of anticoagulation in patients who have experienced embolic phenomena.29
A case review of 725 patients with these tumors27 found that tumor mobility and location on the aortic valve were univariate predictors of tumor-related death and of nonfatal embolism. The only independent predictor of tumor-related death or nonfatal embolization was tumor mobility.
Surgical resection of the tumor is curative, and no recurrences have been reported, although the longest follow-up period has been 11 years.
Although no data exist to support the practice, patients with nonmobile or nonaortic valve tumors could be managed with anticoagulation and periodic echocardiographic follow-up until the tumor becomes mobile or symptomatic, but such a conservative strategy would seem inappropriate for our patient. His tumor is both mobile and located on the aortic valve, putting him at risk of death, and he has already experienced an embolic complication. Therefore, his lesion should be surgically resected.
Case continued
The patient receives anticoagulation therapy with subcutaneous enoxaparin (Lovenox) and warfarin (Coumadin). He undergoes successful surgical resection of the tumor without complication and is discharged to home on hospital day 5.
TAKE-HOME POINTS
The potential causes of stroke in patients younger than age 45 differ significantly from those in older patients. Cardiac embolism is the most frequent cause of stroke in young patients and is most often from left atrial or ventricular thrombus or from aortic atheroma.
In young patients, TEE is superior to TTE in identifying a specific source of cardiac embolism, particularly when clues from the history or physical examination are lacking and the preliminary diagnostic workup fails to identify the cause of the stroke.
Our patient’s history, physical examination, MRI, MRA, electrocardiography, and TTE all failed to disclose a probable cause of his stroke. Appropriately, TEE was performed, which confirmed the diagnosis of cardiac papillary fibroelastoma, a rare and benign primary tumor of the heart with the potential for disastrous consequences.
- Bogousslavsky J, Van Melle G, Regli F. The Lausanne Stroke Registry: analysis of 1,000 consecutive patients with first stroke. Stroke 1988; 19:1083–1092.
- Bogousslavsky J, Pierre P. Ischemic stroke in patients under age 45. Neurol Clin 1992; 10:113–124.
- Schievink WI. Spontaneous dissection of the carotid and vertebral arteries. N Engl J Med 2001; 344:898–906.
- Thanvi B, Munshi SK, Dawson SL, Ribinson TG. Carotid and vertebral artery dissection syndromes. Postgrad Med J 2005; 81:383–388.
- Levine SR. Hypercoagulable states and stroke: a selective review. CNS Spectr 2005; 10:567–578.
- Juul K, Tybjaerg-Hansen A, Steffensen R, Kofoed S, Jensen G, Nordestgaard BG. Factor V Leiden: The Copenhagen City Heart Study and 2 meta-analyses. Blood 2002; 100:3–10.
- Ridker PM, Hennekens CH, Lindpaintner K, Stampfer MJ, Eisenberg PR, Miletich JP. Mutation in the gene coding for coagulation factor V and the risk of myocardial infarction, stroke, and venous thrombosis in apparently healthy men. N Engl J Med 1995; 332:912–917.
- Hankey GJ, Eikelboom JW, van Bockxmeer FM, Lofthouse E, Staples N, Baker RI. Inherited thrombophilia in ischemic stroke and its pathogenic subtypes. Stroke 2001; 32:1793–1799.
- Kaufman MJ, Levin JM, Ross MH, et al. Cocaine-induced cerebral vasoconstriction detected in humans with magnetic resonance angiography. JAMA 1998; 279:376–380.
- Kaku DA, Lowenstein DH. Emergence of recreational drug abuse as a major risk factor for stroke in young adults. Ann Intern Med 1990; 113:821–827.
- Petitti DB, Sidney S, Quesenberry C, Bernstein A. Stroke and cocaine or amphetamine use. Epidemiology 1998; 9:596–600.
- Bruno A. Cerebrovascular complications of alcohol and sympathomimetic drug abuse. Curr Neurol Neurosci Rep 2003; 3:40–45.
- Cardiogenic brain embolism. The second report of the Cerebral Embolism Task Force. Arch Neurol 1989; 46:727–743.
- Kittner SJ, Stern BJ, Wozniak M, et al. Cerebral infarction in young adults: the Baltimore-Washington Cooperative Young Stroke Study. Neurology 1998; 50:890–894.
- Manning WJ. Role of transesophageal echocardiography in the management of thromboembolic stroke. Am J Cardiol 1997; 80 4C:19D–28D.
- Cheitlin MD, Armstrong WF, Aurigemma GP, et al American College of Cardiology; American Heart Association; American Society of Echocardiography. ACC/AHA/ASE 2003 guideline update for the clinical application of echocardiography: summary article: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (ACC/AHA/ASE Committee to Update the 1997 Guidelines for the Clinical Application of Echocardiography). Circulation 2003; 108:1146–1162.
- Kizer JR, Devereux RB. Clinical practice. Patent foramen ovale in young adults with unexplained stroke. N Engl J Med 2005; 353:2361–2372.
- Pearson AC. Transthoracic echocardiography versus transesophageal echocardiography in detecting cardiac sources of embolism. Echocardiography 1993; 10:397–403.
- DeRook FA, Comess KA, Albers GW, Popp RL. Transesophageal echocardiography in the evaluation of stroke. Ann Intern Med 1992; 117:922–932.
- Pearson AC, Labovitz AJ, Tatineni S, Gomez CR. Superiority of transesophageal echocardiography in detecting cardiac source of embolism in patients with cerebral ischemia of uncertain etiology. J Am Coll Cardiol 1991; 17:66–72.
- Rahmatullah AF, Rahko PS, Stein JH. Transesophageal echocardiography for the evaluation and management of patients with cerebral ischemia. Clin Cardiol 1999; 22:391–396.
- Come PC, Riley MF, Bivas NK. Roles of echocardiography and arrhythmia monitoring in the evaluation of patients with suspected systemic embolism. Ann Neurol 1983; 13:527–531.
- Daniel WG, Erbel R, Kasper W, et al. Safety of transesophageal echocardiography. A multicenter survey of 10,419 examinations. Circulation 1991; 83:817–821.
- Souteyrand G, Motreff P, Lusson JR, et al. Comparison of transthoracic echocardiography using second harmonic imaging, transcranial Doppler and transesophageal echocardiography for the detection of patent foramen ovale in stroke patients. Eur J Echocardiogr 2006; 7:147–154.
- Harloff A, Handke M, Reinhard M, Geibel A, Hetzel A. Therapeutic strategies after examination by transesophageal echocardiography in 503 patients with ischemic stroke. Stroke 2006; 37:859–864.
- Burke A, Virami R. Tumors of the heart and great vessels. Atlas of Tumor Pathology, 1996, 3rd Series, Fascicle 16. Washington, DC: Armed Forces Institute of Pathology.
- Gowda RM, Khan IA, Nair CK, Mehta NJ, Vasavada BC, Sacchi TJ. Cardiac papillary fibroelastoma: a comprehensive analysis of 725 cases. Am Heart J 2003; 146:404–410.
- Edwards FH, Hale D, Cohen A, Thompson L, Pezzella AT, Virmani R. Primary cardiac valve tumors. Ann Thorac Surg 1991; 52:1127–1131.
- Joffe II, Jacobs LE, Owen AN, Ioli A, Kotler MN. Rapid development of a papillary fibroelastoma with associated thrombus: the role of transthoracic and transesophageal echocardiography. Echocardiography 1997; 14:287–292.
A 42-year-old man was working at his computer when he suddenly became disoriented and lightheaded, had difficulty concentrating, and could not move his right arm. He could walk without difficulty, but he had a tingling sensation in his right leg. He did not lose consciousness or have any associated palpitations, chest pain, shortness of breath, nausea, vomiting, headaches, or visual changes.
He called 911, and an ambulance arrived 15 minutes later. By that time his symptoms had started to resolve. Now, in the emergency department, his only residual symptom is mild numbness of his right arm and shoulder.
Until now he has been healthy except for a history of dyslipidemia. He takes no prescription or over-the-counter medications and has no drug allergies. He has smoked one pack of cigarettes daily for the past 28 years and also smokes marijuana several times each month. He drinks alcohol occasionally. His family has no history of stroke, premature coronary artery disease, or sudden cardiac death.
INITIAL EVALUATION
His heart rate is 88 beats per minute, blood pressure 142/82 mm Hg, and blood oxygen saturation 98% while breathing room air. He is alert and in no acute distress and answers questions appropriately.
His breathing sounds are normal, without crackles or wheezes. His heart has normal first and second sounds, a normal rate and rhythm, and no extra sounds or murmurs. His abdomen is normal. His extremities are warm and well perfused with normal peripheral pulses and no edema.
On neurologic examination, his cranial nerves and visual fields are normal, and his strength is normal in all muscle groups except for the right upper arm, which is slightly weaker than the left when tested against resistance. Reflexes and response to light touch and pinprick are normal.
His serum chemistry levels, renal function, and blood counts are normal. His total cholesterol level is 155 mg/dL, high-density lipoprotein cholesterol 38 mg/dL, low-density lipoprotein cholesterol 108 mg/dL, and triglycerides 1,286 mg/dL. Electrocardiography is normal with sinus rhythm at a rate of 74.
Magnetic resonance imaging (MRI) of the head and neck with magnetic resonance angiography (MRA) of the intracranial and extracranial vessels is performed. Diffusion-weighted images show a hyperintense lesion in the left insular cortex, consistent with an infarct in the distribution of a branch of the left middle cerebral artery. There is no intracranial hemorrhage. All intracranial and extracranial major vessels are patent, and no stenoses are seen.
DIFFERENTIAL DIAGNOSIS
1. Which is the most likely cause of this patient’s stroke?
- Vertebral or carotid atherosclerosis
- Cervical arterial dissection
- A hematologic disorder
- Cocaine abuse
- Cardiac embolism
Atherosclerosis
Although 85% of all strokes are ischemic, and most ischemic strokes are caused by occlusive atherosclerosis of large vessels, most ischemic strokes occur in patients older than 65 years. In patients younger than 55 years, only about 10% of strokes are caused by large-vessel atherosclerotic disease, thus lowering the initial probability that this is the cause of our patient’s stroke.1 Furthermore, our patient’s MRA study showed no carotid artery stenoses, which effectively eliminates this as the cause of his stroke, as the diagnostic sensitivity of MRA for detecting carotid stenosis is approximately 97%.
Cervical arterial dissection
Cervical arterial dissection causes up to 20% of strokes in patients younger than 45 years.2 Dissections usually involve the extracranial portion of the vessel, and involve the internal carotid arteries at least three times as often as the vertebral arteries. In many cases the dissection is preceded by mild neck trauma, which may be as minor as a vigorous cough or turning of the head.
Typical features of dissection include neck pain, headache, and Horner syndrome, followed minutes to hours later by symptoms of ocular or cerebral ischemia, usually a transient ischemic attack rather than a stroke. Neurologic symptoms are most commonly due to thrombosis at the dissection site with distal embolization. Inherited disorders that are associated with increased risk of cervical arterial dissection include Ehlers-Danlos syndrome type IV, Marfan syndrome, autosomal-dominant polycystic kidney disease, osteogenesis imperfecta type I, and fibromuscular dysplasia.3 MRA and computed tomographic angiography are the diagnostic tests of choice.
Our patient’s symptoms began suddenly, without a history of trauma or neck pain, making arterial dissection less likely as the cause of his stroke. No dissection was seen on MRA, which also minimizes its likelihood.4
Hematologic disorders
Many hematologic disorders are associated with ischemic stroke. The disorders most likely to cause ischemic stroke in patients younger than 45 years are antiphospholipid antibody syndrome, sickle cell anemia, and heparin-induced thrombocytopenia,5 which are associated with arterial thrombosis.
Most of the common hereditary hypercoagulable disorders, such as factor V Leiden/activated protein C resistance, the prothrombin gene mutation (G20210A), antithrombin III deficiency, protein C deficiency, and protein S deficiency, typically cause venous thrombosis much more often than they cause arterial thrombosis. Thus, the most typical presentations of stroke in these disorders are cerebral venous thrombosis or paradoxical embolic stroke due to a patent foramen ovale. Antithrombin III deficiency and protein C and protein S deficiency have been associated with arterial thrombosis, but so infrequently that their likelihood in this patient is extremely low.
Clues to the diagnosis of a hypercoagulable state include venous thrombosis in the past, recurrent fetal loss, thrombocytopenia, livedo reticularis, antiphospholipid antibody syndrome, and skin necrosis at the start of oral anticoagulant therapy.
Of importance: the relationship between hereditary hypercoagulable disorders and stroke is considerably weaker than their association with venous thrombosis. Several studies in clinical and general populations have failed to show an independent association between stroke and protein C deficiency, protein S deficiency, antithrombin III deficiency, factor V Leiden/activated protein C resistance, or the prothrombin G20210A mutation.6–8 Therefore, most experts do not recommend screening all stroke patients for a hypercoagulable state—only those with a personal or family history of thrombosis or young patients with unexplained stroke.
Our patient does not have historical or clinical features that would suggest a specific hypercoagulable disorder, either acquired (eg, heparin-induced thrombocytopenia) or inherited. A laboratory workup for a hypercoagulable disorder would likely be of little value in determining the cause of his stroke, and even if a hereditary disorder were identified it would be difficult to determine causation. However, if no other explanation for his stroke can be found during the workup, one could consider testing for proteins C and S, antithrombin III, activated protein C resistance (and factor V Leiden if screening for activated protein C resistance is positive), prothrombin G20210A, fibrinogen, homocysteine, D-dimers, and antiphospholipid antibodies.
Cocaine abuse
Another important cause of ischemic stroke is the use of sympathomimetic drugs such as cocaine or amphetamines. The strongest association is with cocaine, which has been seen in case series to cause cerebral vasoconstriction in a dose-dependent manner. Vasoconstriction is also related to a longer duration of cocaine use.9 Several case-control studies have found that the risk of stroke is 4.5 to 6.5 times higher in drug abusers than in controls, and that use of catecholamines or cocaine alone was associated with a significantly increased risk of stroke.10,11
It is certainly advisable to ask about the use of illicit drugs and to send serum and urine samples for appropriate drug screening in young stroke patients, particularly if another cause cannot be found or if drug use is suspected.12
Cardiac embolism
Cardiac embolism is the most likely cause of the stroke in this patient. Up to 20% of the 500,000 strokes that occur annually in the United States are of cardiac embolic origin,13 and the prevalence is even higher in younger patients. In a registry of 428 strokes in patients 15 to 44 years of age, a cardiac source of embolism was the cause in 31.8%.14
- Masses, which include atherosclerotic plaques, cardiac tumors, and infective and noninfective valvular vegetations
- Passageways for paradoxical embolism, such as a patent foramen ovale or atrial septal defect (Figure 2)
- Stasis in the left atrium or left ventricle, with a resulting propensity for thrombosis.
Atrial thrombus is most often seen in patients with atrial fibrillation, mitral stenosis, or dilated cardiomyopathy. Echocardiography of the left atrium in patients with these conditions often reveals spontaneous echo contrast that resembles swirling “smoke,” which is thought to be produced by red blood cell aggregation due to blood stasis. This sign is strongly associated with left atrial thrombi.
Left ventricular thrombosis is one of the most common complications of myocardial infarction and is caused by blood stasis in regions of the ventricle in which the myocardium is hypokinetic or akinetic.
We cannot assume, however, that a potential cardioembolic source seen on echocardiography is the cause of a given patient’s stroke. The evidence proving a causal relationship between most potential cardiac embolic sources and stroke is less than robust. Most of the published data are from nonrandomized studies or case series, and there are no large, prospective studies available to clearly prove that a given cardioembolic source is directly related to embolic stroke.16
This being said, most studies have found high prevalence rates of cardioembolic sources in patients with embolic stroke, which suggests that a causative relationship may exist. However, many of these findings also have a relatively high prevalence among the general population without stroke, raising the possibility that the finding could be incidental and unrelated. Examples are patent foramen ovale, which exists in 27% of adults,17 and aortic arch atheroma, which is common in the elderly.
In the end, when the only potential source of embolism that can be found is in the heart (as is often the case in younger patients), the probability is much greater that it is indeed the cause of the stroke. The lack of direct evidence linking many sources of cardioembolism to stroke emphasizes the need for a thorough investigation of all possible causes of stroke.
DIAGNOSTIC EVALUATION
2. Which is the best study to evaluate for a cardiac embolic source in this patient?
- Transthoracic echocardiography (TTE)
- Transesophageal echocardiography (TEE)
- Transcranial Doppler ultrasonography
- Electrocardiography
The study of choice in this patient is TEE. Overall, TEE is better than TTE in identifying a cardiac source of embolism,18,19 mainly because the images are obtained from a probe in the esophagus, which is in close proximity to the heart, so that there is little additional soft tissue and bone between the probe and cardiac structures. In addition, higher-frequency probes can be used. Both of these result in ultrasonographic images with much greater spatial resolution than can be obtained with a transthoracic study.15
In a case series,20 TEE identified a potential cardiac source of embolism in 45 (57%) of 79 patients with cryptogenic stroke, compared with only 12 (15%) with TTE.
The main limitation of TEE is that it does not show the left ventricular apex very well, making an accurate assessment of left ventricular function or identification of a left ventricular apical thrombus much less likely.
In patients who lack evidence of atherosclerotic cerebrovascular disease, specific findings on history or physical examination could increase the chances of identifying an embolic source, such as left ventricular thrombus, on TTE. These findings could include a history of a myocardial infarction, congestive heart failure, left ventricular dysfunction, endocarditis, rheumatic heart disease, a prosthetic valve, or atrial fibrillation or flutter. TTE by itself is considered sufficient for making the diagnosis of mitral stenosis, left ventricular aneurysm, dilated cardiomyopathy, left ventricular thrombus, and mitral valve prolapse with myxomatous degeneration of the leaflets.
However, in patients without signs or symptoms of cardiac disease, the diagnostic value of TTE is significantly less. Several studies have demonstrated that in patients without evidence of cardiac disease, TTE identifies the source of embolism less than 10% of the time.21 Some series even suggest that the yield may be less than 1%.22 TEE has the advantage of being able to diagnose the above disorders and of having a higher sensitivity for identifying potential sources that may be missed by TTE, such as left atrial or left atrial appendage thrombus, aortic arch atheroma, patent foramen ovale, atrial septal aneurysm, or spontaneous echo contrast. It should be remembered, however, that TEE is a semi-invasive procedure that carries the risks of both the procedure and the sedation, eg, bronchospasm, hypoxia, arrhythmias, upper gastrointestinal trauma, and bleeding.23
Further clouding the decision are recent advances in TTE technology, such as contrast TTE with second harmonic imaging, which enhances the ability of TTE to identify potential sources of stroke such as patent foramen ovale nearly to the level of TEE.24
Unfortunately, guidelines from professional societies do not offer assistance on the best diagnostic approach. Current guidelines from the American Heart Association, American College of Cardiology, and American Society of Echocardiography do give echocardiography a class I indication in younger patients (< 45 years old) with cerebrovascular events or older patients (> 45 years old) with stroke without evidence of cerebrovascular disease or other obvious causes. However, there is no official recommendation on whether to choose TTE, TEE, or both studies.16 Given the multiple causes of cardioembolism and the variety of clinical factors that could influence the decision to choose a certain echo study, this decision is appropriately left to the individual physician.
A reasonable, evidence-based diagnostic approach in young stroke patients is to proceed to TEE when routine TTE and electrocardiography are unrevealing.25 In reality, this is the practice followed in most centers, including ours. Although TTE has a lower diagnostic yield in patients without symptoms, it has the advantages of being readily available in most centers, being noninvasive, and providing complementary information to TEE even when TTE does not reveal a potential cause of stroke.
As for the other studies:
Electrocardiography is valuable in identifying potential cardioembolic causes of stroke such as atrial fibrillation, left ventricular aneurysm, or myocardial infarction, but it is insufficient by itself to assess for many other potential sources of cardioembolism.
Transcranial Doppler ultrasonography is very sensitive for detecting patent foramen ovale and other right-to-left shunts that could be sources of cardioembolism. In this test, microbubbles from agitated saline are injected into the venous circulation and are detected in the cerebral arteries after passing through the shunt. It has no utility in identifying the other possibilities discussed above, nor can it discriminate whether these shunts are intra-cardiac or extracardiac.
Case continued
The patient undergoes TTE, which shows normal left ventricular size, wall thickness, and systolic function. His right ventricular function is normal, as are his left and right atrial size. Valvular function is normal, and no right-to-left interatrial shunt is detected with the use of agitated saline contrast.
MANAGEMENT
3. Which is the most appropriate way to manage the lesion?
- Surgical resection
- Periodic echocardiographic follow-up
- Anticoagulation and periodic echocardiographic follow-up
Cardiac papillary fibroelastomas are rare benign primary tumors of the heart. The true incidence is unknown because, when small, they can be asymptomatic and easily overlooked on gross examination. In adults, they are the second most common primary cardiac tumors, next to atrial myxoma.26
The histogenesis is not known, but the mean age at which they are detected is approximately 60 years, and most of the patients are men, likely because most of these tumors are found incidentally during echocardiography, open heart surgery, or autopsy.28
Most patients with cardiac papillary fibroelastomas have no symptoms; however, those who do have symptoms usually experience valve obstruction or embolization of tumor fragments, leading to stroke, myocardial infarction, or sudden death. Further increasing the risk of embolism, thrombus has been reported on the surface of some tumors, supporting the use of anticoagulation in patients who have experienced embolic phenomena.29
A case review of 725 patients with these tumors27 found that tumor mobility and location on the aortic valve were univariate predictors of tumor-related death and of nonfatal embolism. The only independent predictor of tumor-related death or nonfatal embolization was tumor mobility.
Surgical resection of the tumor is curative, and no recurrences have been reported, although the longest follow-up period has been 11 years.
Although no data exist to support the practice, patients with nonmobile or nonaortic valve tumors could be managed with anticoagulation and periodic echocardiographic follow-up until the tumor becomes mobile or symptomatic, but such a conservative strategy would seem inappropriate for our patient. His tumor is both mobile and located on the aortic valve, putting him at risk of death, and he has already experienced an embolic complication. Therefore, his lesion should be surgically resected.
Case continued
The patient receives anticoagulation therapy with subcutaneous enoxaparin (Lovenox) and warfarin (Coumadin). He undergoes successful surgical resection of the tumor without complication and is discharged to home on hospital day 5.
TAKE-HOME POINTS
The potential causes of stroke in patients younger than age 45 differ significantly from those in older patients. Cardiac embolism is the most frequent cause of stroke in young patients and is most often from left atrial or ventricular thrombus or from aortic atheroma.
In young patients, TEE is superior to TTE in identifying a specific source of cardiac embolism, particularly when clues from the history or physical examination are lacking and the preliminary diagnostic workup fails to identify the cause of the stroke.
Our patient’s history, physical examination, MRI, MRA, electrocardiography, and TTE all failed to disclose a probable cause of his stroke. Appropriately, TEE was performed, which confirmed the diagnosis of cardiac papillary fibroelastoma, a rare and benign primary tumor of the heart with the potential for disastrous consequences.
A 42-year-old man was working at his computer when he suddenly became disoriented and lightheaded, had difficulty concentrating, and could not move his right arm. He could walk without difficulty, but he had a tingling sensation in his right leg. He did not lose consciousness or have any associated palpitations, chest pain, shortness of breath, nausea, vomiting, headaches, or visual changes.
He called 911, and an ambulance arrived 15 minutes later. By that time his symptoms had started to resolve. Now, in the emergency department, his only residual symptom is mild numbness of his right arm and shoulder.
Until now he has been healthy except for a history of dyslipidemia. He takes no prescription or over-the-counter medications and has no drug allergies. He has smoked one pack of cigarettes daily for the past 28 years and also smokes marijuana several times each month. He drinks alcohol occasionally. His family has no history of stroke, premature coronary artery disease, or sudden cardiac death.
INITIAL EVALUATION
His heart rate is 88 beats per minute, blood pressure 142/82 mm Hg, and blood oxygen saturation 98% while breathing room air. He is alert and in no acute distress and answers questions appropriately.
His breathing sounds are normal, without crackles or wheezes. His heart has normal first and second sounds, a normal rate and rhythm, and no extra sounds or murmurs. His abdomen is normal. His extremities are warm and well perfused with normal peripheral pulses and no edema.
On neurologic examination, his cranial nerves and visual fields are normal, and his strength is normal in all muscle groups except for the right upper arm, which is slightly weaker than the left when tested against resistance. Reflexes and response to light touch and pinprick are normal.
His serum chemistry levels, renal function, and blood counts are normal. His total cholesterol level is 155 mg/dL, high-density lipoprotein cholesterol 38 mg/dL, low-density lipoprotein cholesterol 108 mg/dL, and triglycerides 1,286 mg/dL. Electrocardiography is normal with sinus rhythm at a rate of 74.
Magnetic resonance imaging (MRI) of the head and neck with magnetic resonance angiography (MRA) of the intracranial and extracranial vessels is performed. Diffusion-weighted images show a hyperintense lesion in the left insular cortex, consistent with an infarct in the distribution of a branch of the left middle cerebral artery. There is no intracranial hemorrhage. All intracranial and extracranial major vessels are patent, and no stenoses are seen.
DIFFERENTIAL DIAGNOSIS
1. Which is the most likely cause of this patient’s stroke?
- Vertebral or carotid atherosclerosis
- Cervical arterial dissection
- A hematologic disorder
- Cocaine abuse
- Cardiac embolism
Atherosclerosis
Although 85% of all strokes are ischemic, and most ischemic strokes are caused by occlusive atherosclerosis of large vessels, most ischemic strokes occur in patients older than 65 years. In patients younger than 55 years, only about 10% of strokes are caused by large-vessel atherosclerotic disease, thus lowering the initial probability that this is the cause of our patient’s stroke.1 Furthermore, our patient’s MRA study showed no carotid artery stenoses, which effectively eliminates this as the cause of his stroke, as the diagnostic sensitivity of MRA for detecting carotid stenosis is approximately 97%.
Cervical arterial dissection
Cervical arterial dissection causes up to 20% of strokes in patients younger than 45 years.2 Dissections usually involve the extracranial portion of the vessel, and involve the internal carotid arteries at least three times as often as the vertebral arteries. In many cases the dissection is preceded by mild neck trauma, which may be as minor as a vigorous cough or turning of the head.
Typical features of dissection include neck pain, headache, and Horner syndrome, followed minutes to hours later by symptoms of ocular or cerebral ischemia, usually a transient ischemic attack rather than a stroke. Neurologic symptoms are most commonly due to thrombosis at the dissection site with distal embolization. Inherited disorders that are associated with increased risk of cervical arterial dissection include Ehlers-Danlos syndrome type IV, Marfan syndrome, autosomal-dominant polycystic kidney disease, osteogenesis imperfecta type I, and fibromuscular dysplasia.3 MRA and computed tomographic angiography are the diagnostic tests of choice.
Our patient’s symptoms began suddenly, without a history of trauma or neck pain, making arterial dissection less likely as the cause of his stroke. No dissection was seen on MRA, which also minimizes its likelihood.4
Hematologic disorders
Many hematologic disorders are associated with ischemic stroke. The disorders most likely to cause ischemic stroke in patients younger than 45 years are antiphospholipid antibody syndrome, sickle cell anemia, and heparin-induced thrombocytopenia,5 which are associated with arterial thrombosis.
Most of the common hereditary hypercoagulable disorders, such as factor V Leiden/activated protein C resistance, the prothrombin gene mutation (G20210A), antithrombin III deficiency, protein C deficiency, and protein S deficiency, typically cause venous thrombosis much more often than they cause arterial thrombosis. Thus, the most typical presentations of stroke in these disorders are cerebral venous thrombosis or paradoxical embolic stroke due to a patent foramen ovale. Antithrombin III deficiency and protein C and protein S deficiency have been associated with arterial thrombosis, but so infrequently that their likelihood in this patient is extremely low.
Clues to the diagnosis of a hypercoagulable state include venous thrombosis in the past, recurrent fetal loss, thrombocytopenia, livedo reticularis, antiphospholipid antibody syndrome, and skin necrosis at the start of oral anticoagulant therapy.
Of importance: the relationship between hereditary hypercoagulable disorders and stroke is considerably weaker than their association with venous thrombosis. Several studies in clinical and general populations have failed to show an independent association between stroke and protein C deficiency, protein S deficiency, antithrombin III deficiency, factor V Leiden/activated protein C resistance, or the prothrombin G20210A mutation.6–8 Therefore, most experts do not recommend screening all stroke patients for a hypercoagulable state—only those with a personal or family history of thrombosis or young patients with unexplained stroke.
Our patient does not have historical or clinical features that would suggest a specific hypercoagulable disorder, either acquired (eg, heparin-induced thrombocytopenia) or inherited. A laboratory workup for a hypercoagulable disorder would likely be of little value in determining the cause of his stroke, and even if a hereditary disorder were identified it would be difficult to determine causation. However, if no other explanation for his stroke can be found during the workup, one could consider testing for proteins C and S, antithrombin III, activated protein C resistance (and factor V Leiden if screening for activated protein C resistance is positive), prothrombin G20210A, fibrinogen, homocysteine, D-dimers, and antiphospholipid antibodies.
Cocaine abuse
Another important cause of ischemic stroke is the use of sympathomimetic drugs such as cocaine or amphetamines. The strongest association is with cocaine, which has been seen in case series to cause cerebral vasoconstriction in a dose-dependent manner. Vasoconstriction is also related to a longer duration of cocaine use.9 Several case-control studies have found that the risk of stroke is 4.5 to 6.5 times higher in drug abusers than in controls, and that use of catecholamines or cocaine alone was associated with a significantly increased risk of stroke.10,11
It is certainly advisable to ask about the use of illicit drugs and to send serum and urine samples for appropriate drug screening in young stroke patients, particularly if another cause cannot be found or if drug use is suspected.12
Cardiac embolism
Cardiac embolism is the most likely cause of the stroke in this patient. Up to 20% of the 500,000 strokes that occur annually in the United States are of cardiac embolic origin,13 and the prevalence is even higher in younger patients. In a registry of 428 strokes in patients 15 to 44 years of age, a cardiac source of embolism was the cause in 31.8%.14
- Masses, which include atherosclerotic plaques, cardiac tumors, and infective and noninfective valvular vegetations
- Passageways for paradoxical embolism, such as a patent foramen ovale or atrial septal defect (Figure 2)
- Stasis in the left atrium or left ventricle, with a resulting propensity for thrombosis.
Atrial thrombus is most often seen in patients with atrial fibrillation, mitral stenosis, or dilated cardiomyopathy. Echocardiography of the left atrium in patients with these conditions often reveals spontaneous echo contrast that resembles swirling “smoke,” which is thought to be produced by red blood cell aggregation due to blood stasis. This sign is strongly associated with left atrial thrombi.
Left ventricular thrombosis is one of the most common complications of myocardial infarction and is caused by blood stasis in regions of the ventricle in which the myocardium is hypokinetic or akinetic.
We cannot assume, however, that a potential cardioembolic source seen on echocardiography is the cause of a given patient’s stroke. The evidence proving a causal relationship between most potential cardiac embolic sources and stroke is less than robust. Most of the published data are from nonrandomized studies or case series, and there are no large, prospective studies available to clearly prove that a given cardioembolic source is directly related to embolic stroke.16
This being said, most studies have found high prevalence rates of cardioembolic sources in patients with embolic stroke, which suggests that a causative relationship may exist. However, many of these findings also have a relatively high prevalence among the general population without stroke, raising the possibility that the finding could be incidental and unrelated. Examples are patent foramen ovale, which exists in 27% of adults,17 and aortic arch atheroma, which is common in the elderly.
In the end, when the only potential source of embolism that can be found is in the heart (as is often the case in younger patients), the probability is much greater that it is indeed the cause of the stroke. The lack of direct evidence linking many sources of cardioembolism to stroke emphasizes the need for a thorough investigation of all possible causes of stroke.
DIAGNOSTIC EVALUATION
2. Which is the best study to evaluate for a cardiac embolic source in this patient?
- Transthoracic echocardiography (TTE)
- Transesophageal echocardiography (TEE)
- Transcranial Doppler ultrasonography
- Electrocardiography
The study of choice in this patient is TEE. Overall, TEE is better than TTE in identifying a cardiac source of embolism,18,19 mainly because the images are obtained from a probe in the esophagus, which is in close proximity to the heart, so that there is little additional soft tissue and bone between the probe and cardiac structures. In addition, higher-frequency probes can be used. Both of these result in ultrasonographic images with much greater spatial resolution than can be obtained with a transthoracic study.15
In a case series,20 TEE identified a potential cardiac source of embolism in 45 (57%) of 79 patients with cryptogenic stroke, compared with only 12 (15%) with TTE.
The main limitation of TEE is that it does not show the left ventricular apex very well, making an accurate assessment of left ventricular function or identification of a left ventricular apical thrombus much less likely.
In patients who lack evidence of atherosclerotic cerebrovascular disease, specific findings on history or physical examination could increase the chances of identifying an embolic source, such as left ventricular thrombus, on TTE. These findings could include a history of a myocardial infarction, congestive heart failure, left ventricular dysfunction, endocarditis, rheumatic heart disease, a prosthetic valve, or atrial fibrillation or flutter. TTE by itself is considered sufficient for making the diagnosis of mitral stenosis, left ventricular aneurysm, dilated cardiomyopathy, left ventricular thrombus, and mitral valve prolapse with myxomatous degeneration of the leaflets.
However, in patients without signs or symptoms of cardiac disease, the diagnostic value of TTE is significantly less. Several studies have demonstrated that in patients without evidence of cardiac disease, TTE identifies the source of embolism less than 10% of the time.21 Some series even suggest that the yield may be less than 1%.22 TEE has the advantage of being able to diagnose the above disorders and of having a higher sensitivity for identifying potential sources that may be missed by TTE, such as left atrial or left atrial appendage thrombus, aortic arch atheroma, patent foramen ovale, atrial septal aneurysm, or spontaneous echo contrast. It should be remembered, however, that TEE is a semi-invasive procedure that carries the risks of both the procedure and the sedation, eg, bronchospasm, hypoxia, arrhythmias, upper gastrointestinal trauma, and bleeding.23
Further clouding the decision are recent advances in TTE technology, such as contrast TTE with second harmonic imaging, which enhances the ability of TTE to identify potential sources of stroke such as patent foramen ovale nearly to the level of TEE.24
Unfortunately, guidelines from professional societies do not offer assistance on the best diagnostic approach. Current guidelines from the American Heart Association, American College of Cardiology, and American Society of Echocardiography do give echocardiography a class I indication in younger patients (< 45 years old) with cerebrovascular events or older patients (> 45 years old) with stroke without evidence of cerebrovascular disease or other obvious causes. However, there is no official recommendation on whether to choose TTE, TEE, or both studies.16 Given the multiple causes of cardioembolism and the variety of clinical factors that could influence the decision to choose a certain echo study, this decision is appropriately left to the individual physician.
A reasonable, evidence-based diagnostic approach in young stroke patients is to proceed to TEE when routine TTE and electrocardiography are unrevealing.25 In reality, this is the practice followed in most centers, including ours. Although TTE has a lower diagnostic yield in patients without symptoms, it has the advantages of being readily available in most centers, being noninvasive, and providing complementary information to TEE even when TTE does not reveal a potential cause of stroke.
As for the other studies:
Electrocardiography is valuable in identifying potential cardioembolic causes of stroke such as atrial fibrillation, left ventricular aneurysm, or myocardial infarction, but it is insufficient by itself to assess for many other potential sources of cardioembolism.
Transcranial Doppler ultrasonography is very sensitive for detecting patent foramen ovale and other right-to-left shunts that could be sources of cardioembolism. In this test, microbubbles from agitated saline are injected into the venous circulation and are detected in the cerebral arteries after passing through the shunt. It has no utility in identifying the other possibilities discussed above, nor can it discriminate whether these shunts are intra-cardiac or extracardiac.
Case continued
The patient undergoes TTE, which shows normal left ventricular size, wall thickness, and systolic function. His right ventricular function is normal, as are his left and right atrial size. Valvular function is normal, and no right-to-left interatrial shunt is detected with the use of agitated saline contrast.
MANAGEMENT
3. Which is the most appropriate way to manage the lesion?
- Surgical resection
- Periodic echocardiographic follow-up
- Anticoagulation and periodic echocardiographic follow-up
Cardiac papillary fibroelastomas are rare benign primary tumors of the heart. The true incidence is unknown because, when small, they can be asymptomatic and easily overlooked on gross examination. In adults, they are the second most common primary cardiac tumors, next to atrial myxoma.26
The histogenesis is not known, but the mean age at which they are detected is approximately 60 years, and most of the patients are men, likely because most of these tumors are found incidentally during echocardiography, open heart surgery, or autopsy.28
Most patients with cardiac papillary fibroelastomas have no symptoms; however, those who do have symptoms usually experience valve obstruction or embolization of tumor fragments, leading to stroke, myocardial infarction, or sudden death. Further increasing the risk of embolism, thrombus has been reported on the surface of some tumors, supporting the use of anticoagulation in patients who have experienced embolic phenomena.29
A case review of 725 patients with these tumors27 found that tumor mobility and location on the aortic valve were univariate predictors of tumor-related death and of nonfatal embolism. The only independent predictor of tumor-related death or nonfatal embolization was tumor mobility.
Surgical resection of the tumor is curative, and no recurrences have been reported, although the longest follow-up period has been 11 years.
Although no data exist to support the practice, patients with nonmobile or nonaortic valve tumors could be managed with anticoagulation and periodic echocardiographic follow-up until the tumor becomes mobile or symptomatic, but such a conservative strategy would seem inappropriate for our patient. His tumor is both mobile and located on the aortic valve, putting him at risk of death, and he has already experienced an embolic complication. Therefore, his lesion should be surgically resected.
Case continued
The patient receives anticoagulation therapy with subcutaneous enoxaparin (Lovenox) and warfarin (Coumadin). He undergoes successful surgical resection of the tumor without complication and is discharged to home on hospital day 5.
TAKE-HOME POINTS
The potential causes of stroke in patients younger than age 45 differ significantly from those in older patients. Cardiac embolism is the most frequent cause of stroke in young patients and is most often from left atrial or ventricular thrombus or from aortic atheroma.
In young patients, TEE is superior to TTE in identifying a specific source of cardiac embolism, particularly when clues from the history or physical examination are lacking and the preliminary diagnostic workup fails to identify the cause of the stroke.
Our patient’s history, physical examination, MRI, MRA, electrocardiography, and TTE all failed to disclose a probable cause of his stroke. Appropriately, TEE was performed, which confirmed the diagnosis of cardiac papillary fibroelastoma, a rare and benign primary tumor of the heart with the potential for disastrous consequences.
- Bogousslavsky J, Van Melle G, Regli F. The Lausanne Stroke Registry: analysis of 1,000 consecutive patients with first stroke. Stroke 1988; 19:1083–1092.
- Bogousslavsky J, Pierre P. Ischemic stroke in patients under age 45. Neurol Clin 1992; 10:113–124.
- Schievink WI. Spontaneous dissection of the carotid and vertebral arteries. N Engl J Med 2001; 344:898–906.
- Thanvi B, Munshi SK, Dawson SL, Ribinson TG. Carotid and vertebral artery dissection syndromes. Postgrad Med J 2005; 81:383–388.
- Levine SR. Hypercoagulable states and stroke: a selective review. CNS Spectr 2005; 10:567–578.
- Juul K, Tybjaerg-Hansen A, Steffensen R, Kofoed S, Jensen G, Nordestgaard BG. Factor V Leiden: The Copenhagen City Heart Study and 2 meta-analyses. Blood 2002; 100:3–10.
- Ridker PM, Hennekens CH, Lindpaintner K, Stampfer MJ, Eisenberg PR, Miletich JP. Mutation in the gene coding for coagulation factor V and the risk of myocardial infarction, stroke, and venous thrombosis in apparently healthy men. N Engl J Med 1995; 332:912–917.
- Hankey GJ, Eikelboom JW, van Bockxmeer FM, Lofthouse E, Staples N, Baker RI. Inherited thrombophilia in ischemic stroke and its pathogenic subtypes. Stroke 2001; 32:1793–1799.
- Kaufman MJ, Levin JM, Ross MH, et al. Cocaine-induced cerebral vasoconstriction detected in humans with magnetic resonance angiography. JAMA 1998; 279:376–380.
- Kaku DA, Lowenstein DH. Emergence of recreational drug abuse as a major risk factor for stroke in young adults. Ann Intern Med 1990; 113:821–827.
- Petitti DB, Sidney S, Quesenberry C, Bernstein A. Stroke and cocaine or amphetamine use. Epidemiology 1998; 9:596–600.
- Bruno A. Cerebrovascular complications of alcohol and sympathomimetic drug abuse. Curr Neurol Neurosci Rep 2003; 3:40–45.
- Cardiogenic brain embolism. The second report of the Cerebral Embolism Task Force. Arch Neurol 1989; 46:727–743.
- Kittner SJ, Stern BJ, Wozniak M, et al. Cerebral infarction in young adults: the Baltimore-Washington Cooperative Young Stroke Study. Neurology 1998; 50:890–894.
- Manning WJ. Role of transesophageal echocardiography in the management of thromboembolic stroke. Am J Cardiol 1997; 80 4C:19D–28D.
- Cheitlin MD, Armstrong WF, Aurigemma GP, et al American College of Cardiology; American Heart Association; American Society of Echocardiography. ACC/AHA/ASE 2003 guideline update for the clinical application of echocardiography: summary article: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (ACC/AHA/ASE Committee to Update the 1997 Guidelines for the Clinical Application of Echocardiography). Circulation 2003; 108:1146–1162.
- Kizer JR, Devereux RB. Clinical practice. Patent foramen ovale in young adults with unexplained stroke. N Engl J Med 2005; 353:2361–2372.
- Pearson AC. Transthoracic echocardiography versus transesophageal echocardiography in detecting cardiac sources of embolism. Echocardiography 1993; 10:397–403.
- DeRook FA, Comess KA, Albers GW, Popp RL. Transesophageal echocardiography in the evaluation of stroke. Ann Intern Med 1992; 117:922–932.
- Pearson AC, Labovitz AJ, Tatineni S, Gomez CR. Superiority of transesophageal echocardiography in detecting cardiac source of embolism in patients with cerebral ischemia of uncertain etiology. J Am Coll Cardiol 1991; 17:66–72.
- Rahmatullah AF, Rahko PS, Stein JH. Transesophageal echocardiography for the evaluation and management of patients with cerebral ischemia. Clin Cardiol 1999; 22:391–396.
- Come PC, Riley MF, Bivas NK. Roles of echocardiography and arrhythmia monitoring in the evaluation of patients with suspected systemic embolism. Ann Neurol 1983; 13:527–531.
- Daniel WG, Erbel R, Kasper W, et al. Safety of transesophageal echocardiography. A multicenter survey of 10,419 examinations. Circulation 1991; 83:817–821.
- Souteyrand G, Motreff P, Lusson JR, et al. Comparison of transthoracic echocardiography using second harmonic imaging, transcranial Doppler and transesophageal echocardiography for the detection of patent foramen ovale in stroke patients. Eur J Echocardiogr 2006; 7:147–154.
- Harloff A, Handke M, Reinhard M, Geibel A, Hetzel A. Therapeutic strategies after examination by transesophageal echocardiography in 503 patients with ischemic stroke. Stroke 2006; 37:859–864.
- Burke A, Virami R. Tumors of the heart and great vessels. Atlas of Tumor Pathology, 1996, 3rd Series, Fascicle 16. Washington, DC: Armed Forces Institute of Pathology.
- Gowda RM, Khan IA, Nair CK, Mehta NJ, Vasavada BC, Sacchi TJ. Cardiac papillary fibroelastoma: a comprehensive analysis of 725 cases. Am Heart J 2003; 146:404–410.
- Edwards FH, Hale D, Cohen A, Thompson L, Pezzella AT, Virmani R. Primary cardiac valve tumors. Ann Thorac Surg 1991; 52:1127–1131.
- Joffe II, Jacobs LE, Owen AN, Ioli A, Kotler MN. Rapid development of a papillary fibroelastoma with associated thrombus: the role of transthoracic and transesophageal echocardiography. Echocardiography 1997; 14:287–292.
- Bogousslavsky J, Van Melle G, Regli F. The Lausanne Stroke Registry: analysis of 1,000 consecutive patients with first stroke. Stroke 1988; 19:1083–1092.
- Bogousslavsky J, Pierre P. Ischemic stroke in patients under age 45. Neurol Clin 1992; 10:113–124.
- Schievink WI. Spontaneous dissection of the carotid and vertebral arteries. N Engl J Med 2001; 344:898–906.
- Thanvi B, Munshi SK, Dawson SL, Ribinson TG. Carotid and vertebral artery dissection syndromes. Postgrad Med J 2005; 81:383–388.
- Levine SR. Hypercoagulable states and stroke: a selective review. CNS Spectr 2005; 10:567–578.
- Juul K, Tybjaerg-Hansen A, Steffensen R, Kofoed S, Jensen G, Nordestgaard BG. Factor V Leiden: The Copenhagen City Heart Study and 2 meta-analyses. Blood 2002; 100:3–10.
- Ridker PM, Hennekens CH, Lindpaintner K, Stampfer MJ, Eisenberg PR, Miletich JP. Mutation in the gene coding for coagulation factor V and the risk of myocardial infarction, stroke, and venous thrombosis in apparently healthy men. N Engl J Med 1995; 332:912–917.
- Hankey GJ, Eikelboom JW, van Bockxmeer FM, Lofthouse E, Staples N, Baker RI. Inherited thrombophilia in ischemic stroke and its pathogenic subtypes. Stroke 2001; 32:1793–1799.
- Kaufman MJ, Levin JM, Ross MH, et al. Cocaine-induced cerebral vasoconstriction detected in humans with magnetic resonance angiography. JAMA 1998; 279:376–380.
- Kaku DA, Lowenstein DH. Emergence of recreational drug abuse as a major risk factor for stroke in young adults. Ann Intern Med 1990; 113:821–827.
- Petitti DB, Sidney S, Quesenberry C, Bernstein A. Stroke and cocaine or amphetamine use. Epidemiology 1998; 9:596–600.
- Bruno A. Cerebrovascular complications of alcohol and sympathomimetic drug abuse. Curr Neurol Neurosci Rep 2003; 3:40–45.
- Cardiogenic brain embolism. The second report of the Cerebral Embolism Task Force. Arch Neurol 1989; 46:727–743.
- Kittner SJ, Stern BJ, Wozniak M, et al. Cerebral infarction in young adults: the Baltimore-Washington Cooperative Young Stroke Study. Neurology 1998; 50:890–894.
- Manning WJ. Role of transesophageal echocardiography in the management of thromboembolic stroke. Am J Cardiol 1997; 80 4C:19D–28D.
- Cheitlin MD, Armstrong WF, Aurigemma GP, et al American College of Cardiology; American Heart Association; American Society of Echocardiography. ACC/AHA/ASE 2003 guideline update for the clinical application of echocardiography: summary article: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (ACC/AHA/ASE Committee to Update the 1997 Guidelines for the Clinical Application of Echocardiography). Circulation 2003; 108:1146–1162.
- Kizer JR, Devereux RB. Clinical practice. Patent foramen ovale in young adults with unexplained stroke. N Engl J Med 2005; 353:2361–2372.
- Pearson AC. Transthoracic echocardiography versus transesophageal echocardiography in detecting cardiac sources of embolism. Echocardiography 1993; 10:397–403.
- DeRook FA, Comess KA, Albers GW, Popp RL. Transesophageal echocardiography in the evaluation of stroke. Ann Intern Med 1992; 117:922–932.
- Pearson AC, Labovitz AJ, Tatineni S, Gomez CR. Superiority of transesophageal echocardiography in detecting cardiac source of embolism in patients with cerebral ischemia of uncertain etiology. J Am Coll Cardiol 1991; 17:66–72.
- Rahmatullah AF, Rahko PS, Stein JH. Transesophageal echocardiography for the evaluation and management of patients with cerebral ischemia. Clin Cardiol 1999; 22:391–396.
- Come PC, Riley MF, Bivas NK. Roles of echocardiography and arrhythmia monitoring in the evaluation of patients with suspected systemic embolism. Ann Neurol 1983; 13:527–531.
- Daniel WG, Erbel R, Kasper W, et al. Safety of transesophageal echocardiography. A multicenter survey of 10,419 examinations. Circulation 1991; 83:817–821.
- Souteyrand G, Motreff P, Lusson JR, et al. Comparison of transthoracic echocardiography using second harmonic imaging, transcranial Doppler and transesophageal echocardiography for the detection of patent foramen ovale in stroke patients. Eur J Echocardiogr 2006; 7:147–154.
- Harloff A, Handke M, Reinhard M, Geibel A, Hetzel A. Therapeutic strategies after examination by transesophageal echocardiography in 503 patients with ischemic stroke. Stroke 2006; 37:859–864.
- Burke A, Virami R. Tumors of the heart and great vessels. Atlas of Tumor Pathology, 1996, 3rd Series, Fascicle 16. Washington, DC: Armed Forces Institute of Pathology.
- Gowda RM, Khan IA, Nair CK, Mehta NJ, Vasavada BC, Sacchi TJ. Cardiac papillary fibroelastoma: a comprehensive analysis of 725 cases. Am Heart J 2003; 146:404–410.
- Edwards FH, Hale D, Cohen A, Thompson L, Pezzella AT, Virmani R. Primary cardiac valve tumors. Ann Thorac Surg 1991; 52:1127–1131.
- Joffe II, Jacobs LE, Owen AN, Ioli A, Kotler MN. Rapid development of a papillary fibroelastoma with associated thrombus: the role of transthoracic and transesophageal echocardiography. Echocardiography 1997; 14:287–292.
Screen for portopulmonary hypertension, especially in liver transplant candidates
Portopulmonary hypertension poses difficulties for patients with liver disease. The elevated pulmonary artery pressure in this disorder makes liver transplantation more dangerous and in fact may rule out the procedure, although in a selected few patients, medical treatment may enable transplantation to proceed. In any event, portopulmonary hypertension should be looked for in patients with liver disease, especially if liver transplantation is being considered.
In this article we discuss the definition, pathophysiology, clinical features, diagnosis, and management of portopulmonary hypertension.
DEFINED BY HEMODYNAMIC CRITERIA
Portopulmonary hypertension—elevated pulmonary artery pressure due to increased resistance to blood flow in patients with portal hypertension—is one of several pulmonary complications of liver disease. A few others to be aware of are pleural effusions (hepatic hydrothorax), dilatation of the pulmonary vasculature with shunting and hypoxemia (hepatopulmonary syndrome), and elevation in pulmonary pressures due to the high cardiac output usually seen in liver disease (flow phenomenon).
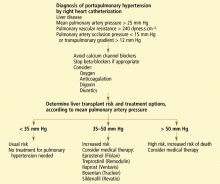
The definition of portopulmonary hypertension has evolved as the various hemodynamic profiles that occur in liver disease and their consequences have been described. Currently, it is defined by the following criteria (obtained by right heart catheterization) in a patient with portal hypertension1:
- Elevated mean pulmonary artery pressure (> 25 mm Hg at rest, > 30 mm Hg with exercise);
- Increased pulmonary vascular resistance (> 240 dynes.s.cm−5; pulmonary vascular resistance = [(mean pulmonary artery pressure minus pulmonary artery occlusion pressure) /cardiac output] times 80); and
- Normal pulmonary artery occlusion pressure (< 15 mm Hg) or an elevated transpulmonary gradient (the mean pulmonary artery pressure minus the pulmonary artery occlusion pressure; abnormal is > 12 mm Hg).
The transpulmonary gradient sometimes helps in further assessing the resistance to blood flow in cases that do not meet the other criteria.2 For example, how should we classify a patient whose mean pulmonary artery pressure is 45 mm Hg but whose pulmonary vascular resistance is only 432 dynes.s.cm−5 and whose pulmonary artery occlusion pressure is slightly high at 18 mm Hg? Although this patient does not meet the hemodynamic criteria for portopulmonary hypertension listed above, intuitively, we should not exclude the diagnosis, as the transpulmonary gradient is high at 27 mm Hg.
FLOW PHENOMENON VS TRUE PORTOPULMONARY HYPERTENSION
The cardiopulmonary hemodynamic profile is different in patients with liver disease than in those without liver disease. Understanding the “normal” hemodynamics in liver disease is paramount in understanding the abnormal hemodynamics that occur in portopulmonary hypertension. In general, patients with liver disease have a high cardiac output at baseline (high flow). They may also have an increased blood volume due to fluid shifts (elevated pulmonary artery occlusion pressure).
Right heart catheterization is necessary to make the diagnosis of portopulmonary hypertension, as pulmonary artery pressures may be increased simply from increases in cardiac output and blood volume without an increase in pulmonary vascular resistance.
Consider, for example, a patient whose mean pulmonary artery pressure is 38 mm Hg, pulmonary artery occlusion pressure 14 mm Hg, and cardiac output 8.8 L/minute. In this case, the pulmonary vascular resistance is 218 dynes.s.cm−5. About 30% to 50% of patients with cirrhosis have this type of hyperdynamic pattern, with high cardiac output, low systemic vascular resistance, and low pulmonary vascular resistance.1,3,4 These patients typically have a much better prognosis than those with portopulmonary hypertension and do well with liver transplantation.
Right heart catheterization is also helpful in assessing whether elevated pulmonary pressures are due to increased volume (increased pulmonary artery occlusion pressure), in which case the patient might benefit from more aggressive diuresis.
In true portopulmonary hypertension, the pulmonary vascular resistance is increased due to obstruction of arterial blood flow. Cardiac output may be elevated initially and then decline as pulmonary hypertension becomes more severe. These hemodynamic patterns have different treatment implications and are important when liver transplantation is being considered.5
HOW COMMON IS PORTOPULMONARY HYPERTENSION?
The incidence and prevalence of portopulmonary hypertension is difficult to assess, as many of the estimates are in patients with severe liver disease undergoing evaluation for liver transplantation. Its prevalence in patients with cirrhosis and refractory ascites has been documented at 16.1%,6 while its prevalence in patients with cirrhosis without refractory ascites has been in the range of 0.25% to 4%.7–9
Overall, about 8% of candidates for liver transplantation have portopulmonary hypertension and are at risk of its complications.10 In view of this figure, screening for it should be performed before proceeding with liver transplantation.
VASOCONSTRICTION, REMODELING, THROMBOSIS
The pathogenesis of portopulmonary hypertension is not completely understood but likely involves a complex interaction of several mechanisms, including an imbalance of vascular mediators favoring vasoconstriction,11–13 endothelial damage with vascular remodeling due to excessive pulmonary blood flow,14,15 smooth muscle proliferation, and microvascular thrombosis.16,17
The pulmonary endothelium is a complex, dynamic organ capable of influencing a variety of vascular mediators and adapting to changes in pulmonary volume as necessary. Endothelial dysfunction may initiate the vascular changes seen in portopulmonary hypertension.
Endothelin-1 (ET-1) is a potent vasoconstrictor that has been implicated in the pathogenesis of idiopathic pulmonary artery hypertension. ET-1 levels are also increased in cirrhotic patients with refractory ascites.6
Other mediators favoring vasoconstriction include serotonin, angiotensin II, and norepinephrine. Whether these mediators influence the development of portopulmonary hypertension is not clear.
At the same time, production of vasodilatory mediators such as nitric oxide and prostacyclin may be decreased in portopulmonary hypertension, facilitating vascular remodeling and a proliferative vascular response. Prostacyclin is a potent vasodilator normally found in high concentrations in the lungs. Prostacyclin synthase is the precursor enzyme for the production of prostacyclin and is decreased in the lungs of patients with portopulmonary hypertension.18
Another way that portal hypertension may influence lung vascular tone is that endotoxin, cytokines, or both, released from the splanchnic circulation, may bypass the liver and get into the lungs.19 Evidence in support of this is that patients with portosystemic shunting can develop similar pathologic changes in the pulmonary vascular bed that normalize when the shunt is reversed. To date, however, no substance has been definitively identified.
Yet another proposed mechanism is shear stress on the pulmonary endothelium from the hyperdynamic cardiac output, with resultant vascular remodeling; however, other mechanisms must be involved, as not everyone with liver disease develops portopulmonary hypertension (see below).
These changes are identical to those in idiopathic and familial pulmonary arterial hypertension,21 and indeed, the World Health Organization now classifies portopulmonary hypertension in the same category as these primary forms of pulmonary hypertension rather than in the secondary forms.3
Why doesn’t everyone with liver disease develop portopulmonary hypertension?
The severity of liver disease or degree of portal hypertension does not appear to correlate with the severity of pulmonary hypertension,4 and portopulmonary hypertension does not develop in all patients with portal hypertension. Therefore, it is likely that some patients have a genetic or environmental susceptibility or suffer a “second hit” that triggers dysregulated pulmonary vascular proliferation and contributes to the development of pulmonary hypertension.
Whether genetic mutations play a role in portopulmonary hypertension remains unknown. Such a mutation could be similar to the one identified in the bone morphogenetic protein receptor type 2 gene (BMPR2) in familial pulmonary artery hypertension or the mutation in the activin-like kinase gene (ALK1) seen in pulmonary hypertension in patients with hereditary hemorrhagic telangiectasia.22
Current studies are investigating the role that bone-marrow-derived progenitor cells might play in the pathogenesis of portopulmonary hypertension.
CLINICAL FEATURES MAY NOT BE OBVIOUS AT FIRST
In the early stages of portopulmonary hypertension, patients may have no symptoms or only symptoms of liver disease, so it is important to have a high index of suspicion and screen for pulmonary hypertension. As its severity increases, symptoms may include fatigue, dyspnea, abdominal bloating, palpitations, chest pain or pressure, and syncope. The most common presenting symptom is dyspnea on exertion.
Similarly, the findings on physical examination also depend on the severity of pulmonary hypertension. Patients with mild portopulmonary hypertension may have only signs suggesting liver disease, such as spider telangiectases, jaundice, mild lower extremity edema, and ascites. As the severity of portopulmonary hypertension increases, however, findings of right heart pressure-and-volume overload become more obvious. These include peripheral edema, elevation of the jugular venous pressure, a right ventricular lift, a loud pulmonic valve closure, increased split of the second heart sound, a pulsatile liver, or a right-sided third or fourth heart sound.
SCREEN LIVER TRANSPLANT CANDIDATES
Screening for portopulmonary hypertension should be mandatory in patients undergoing evaluation for liver transplantation. This condition increases the risk of perioperative death, so it is not acceptable to make the diagnosis in the operating room!5
Electrocardiographic abnormalities that may raise the suspicion of portopulmonary hypertension include right atrial or ventricular enlargement and a right bundle branch pattern.
Chest radiographic signs are enlarged central pulmonary arteries and cardiomegaly. These electrocardiographic and radiographic signs tend to reflect advanced pulmonary hypertension.
Pulmonary function testing is not generally helpful, but the diffusing capacity may be decreased.
B-type natriuretic peptide (BNP) measurement may be helpful. BNP is released from the ventricles when the ventricles become dilated (due to pressure or volume overload), as in left or right heart failure. BNP testing is clinically useful in monitoring the severity of disease and the efficacy of treatment in patients with pulmonary hypertension; its role in portopulmonary hypertension requires prospective study.23
Transthoracic Doppler echocardiography is an excellent screening test and should be performed in patients undergoing evaluation for liver transplantation to exclude pulmonary hypertension.1 Findings on echocardiography that suggest portopulmonary hypertension include elevation of right ventricular systolic pressure (RVSP), which is calculated from the peak tricuspid regurgitant velocity (TRV) using the modified Bernoulli equation and an estimate of right atrial pressure (RAP):
RVSP = 4(TRV)2 + RAP.
Right atrial pressure is estimated from the filling characteristics of the inferior vena cava.
Transthoracic Doppler echocardiography has a sensitivity of 97% and a specificity of 77% in diagnosing moderate to severe pulmonary hypertension in patients undergoing evaluation for liver transplantation.24 Using an RVSP cutoff of 40 mm Hg, the sensitivity of Doppler echocardiography is about 80%, specificity 96%, positive predictive value 60%, and negative predictive value 98%.25
At Mayo Clinic, patients with an estimated RVSP greater than 50 mm Hg undergo right heart catheterization (see below). Such patients should also have repeat echocardiography at 1-year intervals to monitor for increasing pulmonary artery pressures5; for those on the waiting list for liver transplantation, the interval should probably be every 6 to 12 months.
RIGHT HEART CATHETERIZATION CONFIRMS THE DIAGNOSIS
The diagnosis of portopulmonary hypertension is confirmed with right heart catheterization to accurately measure pulmonary artery pressures, pulmonary artery occlusion pressure (to exclude volume overload), cardiac output (to exclude high-output pulmonary hypertension), and pulmonary vascular resistance. One study in patients with decompensated cirrhosis and refractory ascites found that a right atrial pressure of 14 mm Hg or greater had a positive predictive value of 83% for pulmonary hypertension.6
Other, potentially treatable causes of pulmonary hypertension must be excluded before diagnosing portopulmonary hypertension. These include thromboembolic disease, interstitial lung disease, connective tissue disease, untreated obstructive sleep apnea, and elevated pulmonary artery pressures due to increased cardiac output.
Vasodilator studies are being done less frequently in patients with portopulmonary hypertension, as they generally cannot tolerate calcium channel blocker therapy. Calcium channel blocker therapy is usually started in patients with idiopathic pulmonary artery hypertension who exhibit a positive vasodilator response. A positive vasodilator response also does not predict survival with or without liver transplantation. Unlike those with idiopathic pulmonary artery hypertension, many patients with portopulmonary hypertension cannot tolerate calcium channel blockers, as some of these drugs can exacerbate edema and portal hypertension.
GENERAL MANAGEMENT
Treatment of mild portopulmonary hypertension (mean pulmonary artery pressure < 35 mm Hg) is debatable. In these cases many patients do not have any symptoms attributable to portopulmonary hypertension, but only symptoms of liver disease, and they have a good functional status. As a group, such patients have not been formally studied to date.
Anticoagulation is often contraindicated in portopulmonary hypertension because of gastroesophageal varices, thrombocytopenia, or other coagulation abnormalities related to liver disease. If contraindications to anticoagulation do not exist, it should be considered.
Diuretics are a mainstay in the treatment of portopulmonary hypertension, both for the pulmonary hypertension and for the liver disease, especially if ascites or peripheral edema is present.
Oxygen should be given to patients with hypoxemia to keep the saturation greater than 90%.
Beta-blockers: A dilemma
Beta-blockers are used in many patients with liver disease as both primary and secondary prophylaxis of variceal bleeding.
However, one study has shown that in patients with moderate to severe portopulmonary hypertension, beta-blockers are associated with significant worsening of exercise capacity and pulmonary hemodynamic measurements.26 After beta-blockers were withdrawn, the 6-minute walking distance increased in 9 of 10 patients, and cardiac output increased with no change in mean pulmonary artery pressure, resulting in a 19% decrease in pulmonary vascular resistance. The increases in cardiac output were related to a 25% increase in heart rate. Long-term follow-up was not reported, and it remains unclear whether rates of gastrointestinal bleeding may increase when beta-blockers are withdrawn.
Beta-blocker therapy in portopulmonary hypertension needs to be carefully considered and if at all possible should be avoided.
VASODILATOR THERAPY
Several vasodilating or vasomodulating drugs are available. However, much of the information about them comes from studies in patients with idiopathic pulmonary artery hypertension or pulmonary hypertension due to connective tissue disease, and no randomized controlled trials in portopulmonary hypertension have been performed.
Prostanoids
Prostanoids have been used successfully to lower pulmonary pressures in portopulmonary hypertension.
Epoprostenol (Flolan) is a pulmonary and systemic vasodilator as well as an inhibitor of platelet aggregation. It is given as a continuous intravenous infusion via an indwelling central venous catheter and a portable infusion pump. It has a very short half-life, requires mixing, and must be kept cold with ice packs, making it somewhat cumbersome to administer.
This medication has been shown to improve cardiopulmonary hemodynamics and exercise capacity in portopulmonary hypertension, although a survival advantage has not been documented to date.27 In several case series, some patients with portopulmonary hypertension treated with intravenous epoprostenol responded with a reduction in pulmonary pressures and successfully underwent liver transplantation.28–31
Complications of intravenous epoprostenol therapy include central venous catheter thrombosis, infection, and infusion pump failure; a backup pump must be available at all times. Patients with portopulmonary hypertension may also develop progressive splenomegaly and thrombocytopenia that may be due to increased blood flow in the splanchnic circulation.32
Treprostinil (Remodulin) has a longer half-life and does not have to be kept cold. It is given as a 24-hour intravenous or subcutaneous infusion, using an infusion pump that is smaller than that used with epoprostenol.
Although treprostinil is easier for patients to use, larger doses are necessary to achieve the same effect as with epoprostenol. With subcutaneous administration, the biggest drawback is site pain. Prostacyclin-related side effects include flushing, diarrhea, jaw discomfort, and lower extremity pain.
Iloprost (Ventavis) has the advantage of being given by inhalation. It is very short-acting, however, and requires six to nine inhalations per day.
Endothelin receptor blockers
Bosentan (Tracleer) is an oral agent that has been approved by the US Food and Drug Administration (FDA) for the treatment of pulmonary hypertension, including in patients with portopulmonary hypertension who have mild hepatic derangement. This medication is a dual endothelin receptor antagonist, nonselectively blocking the endothelin A and B receptors on the endothelial and vascular smooth muscle cells so that ET-1 cannot bind and cause vasoconstriction.
In approximately 10% of patients, bosentan can cause elevations in aminotransferase, alkaline phosphatase, and bilirubin levels, which therefore must be checked monthly.33 Irreversible hepatic toxicity is uncommon; in most cases, liver function abnormalities return to baseline levels when the medication is stopped. The presumed mechanism is impairment of bile-salt transporters, leading to bile-salt accumulation in the liver.34 Bosentan’s use in patients with liver disease has not been well studied, although several case reports have described its use in patients with portopulmonary hypertension.35–38
Ambrisentan (Letairis) is a selective endothelin receptor-A blocker that has just received FDA approval for the treatment of pulmonary artery hypertension. It has not yet been studied in portopulmonary hypertension. Elevations in liver enzymes and bilirubin may also occur, and monthly monitoring is indicated.
Sildenafil
Another oral agent that might be effective in portopulmonary hypertension is sildenafil (Revatio). A phosphodiesterase-5 inhibitor, it selectively inhibits the cyclic guanosine monophosphatase-specific phosphodiesterase type 5 enzyme that is found in large concentrations in pulmonary artery smooth muscle cells.
In other forms of pulmonary hypertension, sildenafil has been shown to increase cardiac output and decrease pulmonary artery pressures and pulmonary vascular resistance without serious adverse events.39–41
In one reported case, treatment with sildenafil in a patient with portopulmonary hypertension decreased the mean pulmonary artery pressure from 56 mm Hg to 28 to 31 mm Hg, and the patient underwent successful liver transplantation.42 A recent case series of 14 patients with portopulmonary hypertension treated with sildenafil documents some improvement in 6-minute walking distance, suggesting that sildenafil as monotherapy or in combination therapy might be effective in portopulmonary hypertension.43 However, in 3 of these patients, the cardiac index decreased and pulmonary vascular resistance increased.44
We must emphasize that controlled studies in portopulmonary hypertension need to be done to find the optimal therapy.
LIVER TRANSPLANTATION MAY BENEFIT A FEW PATIENTS
Liver transplantation may be beneficial in highly selected patients with portopulmonary hypertension. However, this condition increases the risk of intraoperative and immediate postoperative complications of liver transplantation, so patients should be carefully evaluated5,45 at a liver transplantation center experienced in its management, including medical treatment with well-defined protocols regarding timing of liver transplantation.
Patients with mean pulmonary artery pressures greater than 50 mm Hg should not undergo liver transplantation. Those with mean pulmonary artery pressure between 35 and 50 mm Hg also have an increased mortality rate and may benefit from prolonged treatment for pulmonary hypertension.5,46
One successful case of living-related liver transplantation in a patient with portopulmonary hypertension has been published.47 (Most other successful transplants were from unrelated cadaver donors.)
Some patients who initially cannot undergo liver transplantation owing to severe pulmonary hypertension may eventually be able to do so if they receive medical therapy that improves their pulmonary hemodynamic profile, decreasing their mean pulmonary artery pressure and pulmonary vascular resistance. This would apply to a small subset of patients with portopulmonary hypertension.
When patients without pulmonary hypertension undergo liver transplantation, right ventricular function is preserved throughout all phases of the surgery.48 Patients with portopulmonary hypertension, however, may develop hemodynamic instability during liver transplantation. The most critical times are the induction of anesthesia, during and after graft reperfusion, and the immediate postoperative period.49,50
During the surgery, patients may require vasodilators if they have worsening pulmonary hypertension, or inotropic medications if they have right ventricular dysfunction and heart failure. In one study,51 eight patients with portopulmonary hypertension diagnosed at anesthesia induction for liver transplantation all required intraoperative vasodilator therapy after graft reperfusion because of marked increases in pulmonary artery pressures and pulmonary vascular resistance.
The increase in blood flow following reperfusion or necessary fluid challenges may exacerbate pulmonary hypertension, resulting in worsening right heart function and backup into the transplanted liver. Infusion of 1 liter of crystalloid over 10 minutes has been shown to increase mean pulmonary artery pressure and pulmonary artery occlusion pressure in liver transplantation candidates without pulmonary hypertension52; this response may be exaggerated in portopulmonary hypertension.
PROGNOSIS VARIES WITH SEVERITY OF DISEASE
The natural history of untreated portopulmonary hypertension varies with the degree of liver disease and the severity of pulmonary hypertension. Transplant-free survival was 85% at 1 year and 38% at 3 years in one study.45 The cardiac index appears to be the most significant prognostic variable.20
In a retrospective study of 78 patients with portopulmonary hypertension treated conservatively (before prostanoids were available) the median survival was 6 months (range 0–84 months) from the time of diagnosis.53 Causes of death included right heart failure, sudden death, gastrointestinal bleeding, and small bowel perforation.
Most of the data on outcomes of drug treatment and liver transplantation in patients with portopulmonary hypertension come from case series and retrospective reviews; prospective trials have been lacking.
If right ventricular function is normal and pulmonary hypertension is mild (mean pulmonary artery pressure < 35 mm Hg), patients tend to do well with liver transplantation.9
Outcomes are worse if pulmonary hypertension is more severe. In a database54 from 10 liver transplant centers from 1996 to 2001, 13 (36%) of 36 patients undergoing liver transplantation died in the hospital, emphasizing the importance of accurately assessing the severity of pulmonary hypertension before attempting liver transplantation.46 The rate was even higher—92%—in those with a mean pulmonary artery pressure greater than 35 mm Hg. The cause of death in severe pulmonary hypertension was failure of the right ventricle.
However, some patients with moderate to severe portopulmonary hypertension have been bridged with medications to lower pulmonary artery pressures and pulmonary vascular resistance so that liver transplantation can be safely done, and some have even been able to discontinue medications because their pulmonary hypertension resolved.29,31,41,42,47
Unlike in hepatopulmonary syndrome, liver transplantation is not the treatment of choice for portopulmonary hypertension, and pulmonary hypertension does not always resolve after liver transplantation. Many patients continue therapy for pulmonary hypertension after liver transplantation. Pulmonary hypertension may resolve, persist, or even develop de novo after liver transplantation.1 If pulmonary hypertension resolves, it does so over a prolonged time—months to years—favoring a vascular remodeling hypothesis as opposed to simply reversing vasoconstriction.
- Rodriguez-Roisin R, Krowka MJ, Hervé P, Fallon MB; ERS Task Force Pulmonary-Hepatic Vascular Disorders (PHD) Scientific Committee. Pulmonary-hepatic vascular disorders (PHD). Eur Respir J 2004; 24:861–880.
- Krowka MJ, Swanson KL, Frantz RP, et al. Portopulmonary hypertension: results from a 10-year screening algorithm. Hepatology 2006; 44:1502–1510.
- Simonneau G, Galie N, Rubin LJ, et al. Clinical classification of pulmonary hypertension. J Am Coll Cardiol 2004; 43:5S–12S.
- Hadengue A, Benhayoun MK, Lebrec D, et al. Pulmonary hypertension complicating portal hypertension: prevalence and relation to splanchnic hemodynamics. Gastroenterology 1991; 100:520–528.
- Krowka MJ, Plevak DJ, Findlay JY, et al. Pulmonary hemodynamics and perioperative cardiopulmonary-related mortality in patients with portopulmonary hypertension undergoing liver transplantation. Liver Transplant 2000; 6:443–450.
- Benjaminov FS, Prentice M, Sniderman KW, et al. Portopulmonary hypertension in decompensated cirrhosis with refractory ascites. Gut 2003; 52:1355–1362.
- McDonnell PJ, Toye PA, Hutchins GM. Primary pulmonary hypertension and cirrhosis: are they related? Am Rev Respir Dis 1983; 127:437–441.
- Cheng EY, Woehlck H. Pulmonary artery hypertension complicating anesthesia for liver transplantation. Anesthesiology 1992; 77:375–378.
- Castro M, Krowka MJ, Schroeder DR, et al. Frequency and clinical implications of increased pulmonary artery pressures in liver transplantation. Mayo Clin Proc 1996; 71:543–551.
- Ramsay MA, Simpson BR, Nguyen AT, et al. Severe pulmonary hypertension in liver transplant candidates. Liver Transplant Surg 1997; 3:494–500.
- Kiely DG, Cargill RI, Struthers AD, et al. Cardiopulmonary effects of endothelin-1 in man. Cardiovasc Res 1997; 33:378–386.
- Panos RJ, Baker SK. Mediators, cytokines, and growth factors in liver-lung interactions. Clin Chest Med 1996; 17:151–169.
- Higgenbottam T. Pathophysiology of pulmonary hypertension. Chest 1994; 105:7S–12S.
- Krowka MJ. Hepatopulmonary syndrome and portopulmonary hypertension: distinction and dilemmas. Hepatology 1997; 25:1282–1284.
- Hongqun L, Lee SS. Cardiopulmonary dysfunction in cirrhosis. Hepatology 2000; 14:600–608.
- Lebrec D, Brenot F, Simonneau G, et al. Pulmonary arterial hypertension in portal hypertension. Eur Respir J 1998; 11:1153–1166.
- Herve P, Lebrec D, Brenot F, et al. Pulmonary vascular disorders in portal hypertension. Eur Respir J 1998; 11:1153–1166.
- Tuder RM, Cool CD, Geraci MW, et al. Prostacyclin synthase expression is decreased in lungs from patients with severe pulmonary hypertension. Am J Respir Crit Care Med 1999; 159:1925–1932.
- Hoeper MM, Krowka MJ, Strassburg CP. Portopulmonary hypertension and hepatopulmonary syndrome. Lancet 2004; 363:1461–1468.
- Edwards B, Weir K, Edwards WD, et al. Coexistent pulmonary and portal hypertension: morphologic and clinical features. J Am Coll Cardiol 1987; 10:1233–1238.
- Ramsay MAE, Simpson BR, Nguyen AT, Ramsay KJ, East C, Klintmalm GB. Severe pulmonary hypertension in liver transplant candidates. Liver Transplant Surg 1997; 3:494–500.
- Trembath RC. Clinical and molecular genetic features of pulmonary hypertension in patients with hereditary hemorrhagic telangiectasia. N Engl J Med 2001; 345:325–334.
- Leuchte HH, Holzapfel M, Baumgartner RA, et al. Clinical significance of brain natriuretic peptide in primary pulmonary hypertension. J Am Coll Cardiol 2004; 43:764–770.
- Kim WR, Krowka MJ, Plevak DJ, et al. Accuracy of Doppler echocardiography in the assessment of pulmonary hypertension in liver transplant candidates. Liver Transplant 2000; 6:453–458.
- Colle IO, Moreau R, Godinho E, et al. Diagnosis of portopulmonary hypertension in candidates for liver transplantation: a prospective study. Hepatology 2003; 37:401–409.
- Provencher S, Herve P, Jais X, et al. Deleterious effects of beta-blockers on exercise capacity and hemodynamics in patients with portopulmonary hypertension. Gastroenterology 2006; 130:120–126.
- Swanson KL, McGoon MD, Krowka MJ. Survival in patients with portopulmonary hypertension [abstract]. Am J Respir Crit Care Med 2003; 167:A693.
- Kuo PC, Johnson LB, Plotkin JS, et al. Continuous intravenous infusion of epoprostenol for the treatment of portopulmonary hypertension. Transplantation 1997; 63:604–616.
- Krowka MJ, Frantz RP, McGoon MD, et al. Improvement in pulmonary hemodynamics during intravenous epoprostenol (prostacyclin): A study of 15 patients with moderate to severe portopulmonary hypertension. Hepatology 1999; 30:641–648.
- Kähler CM, Graziadei I, Wiedermann CJ, Kneussl MP, Vogel W. Successful use of continuous intravenous prostacyclin in a patient with severe portopulmonary hypertension. Wien Klin Wochenschr 2000; 112:637–640.
- Sussman N, Kaza V, Barshes N, et al. Successful liver transplantation following medical management of portopulmonary hypertension: a single-center series. Am J Transplant 2006; 6:2177–2182.
- Findlay JY, Plevak DJ, Krowka MJ, et al. Progressive splenomegaly after epoprostenol therapy in portopulmonary hypertension. Liver Transplant Surg 1999; 5:381–387.
- Rubin LJ, Roux S. Bosentan: a dual endothelin receptor antagonist. Expert Opin Invest Drugs 2002; 11:991–1002.
- Fattinger K, Funk C, Pantze M, et al. The endothelin antagonist bosentan inhibits the canalicular bile salt export pump: a potential mechanism for hepatic adverse reactions. Clin Pharmacol Ther 2001; 69:223–231.
- Hinterhuber L, Graziadei IW, Kahler CM, et al. Endothelin-receptor anatgonist treatment of portopulmonary hypertension. Clin Gastroenterol Hepatol 2004; 2:1039–1042.
- Clift PF, Townend JN, Bramhall S, et al. Successful treatment of severe portopulmonary hypertension after liver transplantation by bosentan. Transplantation 2004; 77:1774–1775.
- Halank M, Miehlke S, Hoeffken G, et al. Use of oral endothelin-receptor antagonist bosentan in the treatment of portopulmonary hypertension. Transplantation 2004; 77:1775–1776.
- Kuntzen C, Gulberg V, Gerbes AL. Use of a mixed endothelin receptor antagonist in portopulmonary hypertension: a safe and effective therapy? Gastroenterology 2005; 128:164–168.
- Watanabe H, Ohashi K, Takeuchi K, et al. Sildenafil for primary and secondary pulmonary hypertension. Clin Pharmacol Ther 2002; 71:398–402.
- Michelakis E, Tymchak W, Lien D, et al. Oral sildenafil is an effective and specific pulmonary vasodilator in patients with pulmonary arterial hypertension: comparison with inhaled nitric oxide. Circulation 2002; 105:2398–2403.
- Ghofrani HA, Wiedemann R, Rose F, et al. Sildenafil for treatment of lung fibrosis and pulmonary hypertension: a randomised controlled trial. Lancet 2002; 360:895–900.
- Makisalo H, Koivusalo A, Vakkuri A, et al. Sildenafil for portopulmonary hypertension in a patient undergoing liver transplantation. Liver Transplant 2004; 10:945–950.
- Reichengerger F, Voswinckel R, Steveling E, et al. Sildenafil treatment for portopulmonary hypertension. Eur Respir J 2006; 28:563–567.
- Krowka MJ, Swanson KL. How should we treat portopulmonary hypertension? Eur Respir J 2006; 28:466–467.
- Kawut SM, Taichman DB, Ahya VN, et al. Hemodynamics and survival of patients with portopulmonary hypertension. Liver Transplant 2005; 11:1107–1111.
- Krowka MJ, Mandell MS, Ramsay MA, et al. Hepatopulmonary syndrome and portopulmonary hypertension: a report of the multicenter liver transplant database. Liver Transplant 2004; 10:174–182.
- Sulica R, Emre S, Poon M. Medical management of portopulmonary hypertension and right heart failure prior to living-related liver transplantation. Congest Heart Fail 2004; 10:192–194.
- De Wolf AM, Begliomini B, Gasior TA, et al. Right ventricular function during orthotopic liver transplantation. Anesthes Analges 1993; 76:562–568.
- Csete M. Intraoperative management of liver transplant patients with pulmonary hypertension. Liver Transplant Surg 1997; 3:454–455.
- Acosta F, Sansano T, Palenciano CG, et al. Portopulmonary hypertension and liver transplantation: hemodynamic consequences at reperfusion. Transplant Proc 2005; 37:3865–3866.
- Taura P, Garcia-Valdecasas JC, Beltran J, et al. Moderate primary pulmonary hypertension in patients undergoing liver transplantation. Anesthes Analges 1996; 83:675–680.
- Kuo PC, Schroeder RA, Vagelos RH, et al. Volume-mediated pulmonary responses in liver transplant candidates. Clin Transplant 1996; 10:521–527.
- Robalino BD, Moodie DS. Association between primary pulmonary hypertension and portal hypertension: analysis of its pathophysiology and clinical, laboratory and hemodynamic manifestations. J Am Coll Cardiol 1991; 17:492–498.
- Mandell MS, Krowka MJ. Formation of a national database on pulmonary hypertension and hepatopulmonary syndrome in chronic liver disease. Anesthesiology 1997; 87:450–451.
Portopulmonary hypertension poses difficulties for patients with liver disease. The elevated pulmonary artery pressure in this disorder makes liver transplantation more dangerous and in fact may rule out the procedure, although in a selected few patients, medical treatment may enable transplantation to proceed. In any event, portopulmonary hypertension should be looked for in patients with liver disease, especially if liver transplantation is being considered.
In this article we discuss the definition, pathophysiology, clinical features, diagnosis, and management of portopulmonary hypertension.
DEFINED BY HEMODYNAMIC CRITERIA
Portopulmonary hypertension—elevated pulmonary artery pressure due to increased resistance to blood flow in patients with portal hypertension—is one of several pulmonary complications of liver disease. A few others to be aware of are pleural effusions (hepatic hydrothorax), dilatation of the pulmonary vasculature with shunting and hypoxemia (hepatopulmonary syndrome), and elevation in pulmonary pressures due to the high cardiac output usually seen in liver disease (flow phenomenon).
The definition of portopulmonary hypertension has evolved as the various hemodynamic profiles that occur in liver disease and their consequences have been described. Currently, it is defined by the following criteria (obtained by right heart catheterization) in a patient with portal hypertension1:
- Elevated mean pulmonary artery pressure (> 25 mm Hg at rest, > 30 mm Hg with exercise);
- Increased pulmonary vascular resistance (> 240 dynes.s.cm−5; pulmonary vascular resistance = [(mean pulmonary artery pressure minus pulmonary artery occlusion pressure) /cardiac output] times 80); and
- Normal pulmonary artery occlusion pressure (< 15 mm Hg) or an elevated transpulmonary gradient (the mean pulmonary artery pressure minus the pulmonary artery occlusion pressure; abnormal is > 12 mm Hg).
The transpulmonary gradient sometimes helps in further assessing the resistance to blood flow in cases that do not meet the other criteria.2 For example, how should we classify a patient whose mean pulmonary artery pressure is 45 mm Hg but whose pulmonary vascular resistance is only 432 dynes.s.cm−5 and whose pulmonary artery occlusion pressure is slightly high at 18 mm Hg? Although this patient does not meet the hemodynamic criteria for portopulmonary hypertension listed above, intuitively, we should not exclude the diagnosis, as the transpulmonary gradient is high at 27 mm Hg.
FLOW PHENOMENON VS TRUE PORTOPULMONARY HYPERTENSION
The cardiopulmonary hemodynamic profile is different in patients with liver disease than in those without liver disease. Understanding the “normal” hemodynamics in liver disease is paramount in understanding the abnormal hemodynamics that occur in portopulmonary hypertension. In general, patients with liver disease have a high cardiac output at baseline (high flow). They may also have an increased blood volume due to fluid shifts (elevated pulmonary artery occlusion pressure).
Right heart catheterization is necessary to make the diagnosis of portopulmonary hypertension, as pulmonary artery pressures may be increased simply from increases in cardiac output and blood volume without an increase in pulmonary vascular resistance.
Consider, for example, a patient whose mean pulmonary artery pressure is 38 mm Hg, pulmonary artery occlusion pressure 14 mm Hg, and cardiac output 8.8 L/minute. In this case, the pulmonary vascular resistance is 218 dynes.s.cm−5. About 30% to 50% of patients with cirrhosis have this type of hyperdynamic pattern, with high cardiac output, low systemic vascular resistance, and low pulmonary vascular resistance.1,3,4 These patients typically have a much better prognosis than those with portopulmonary hypertension and do well with liver transplantation.
Right heart catheterization is also helpful in assessing whether elevated pulmonary pressures are due to increased volume (increased pulmonary artery occlusion pressure), in which case the patient might benefit from more aggressive diuresis.
In true portopulmonary hypertension, the pulmonary vascular resistance is increased due to obstruction of arterial blood flow. Cardiac output may be elevated initially and then decline as pulmonary hypertension becomes more severe. These hemodynamic patterns have different treatment implications and are important when liver transplantation is being considered.5
HOW COMMON IS PORTOPULMONARY HYPERTENSION?
The incidence and prevalence of portopulmonary hypertension is difficult to assess, as many of the estimates are in patients with severe liver disease undergoing evaluation for liver transplantation. Its prevalence in patients with cirrhosis and refractory ascites has been documented at 16.1%,6 while its prevalence in patients with cirrhosis without refractory ascites has been in the range of 0.25% to 4%.7–9
Overall, about 8% of candidates for liver transplantation have portopulmonary hypertension and are at risk of its complications.10 In view of this figure, screening for it should be performed before proceeding with liver transplantation.
VASOCONSTRICTION, REMODELING, THROMBOSIS
The pathogenesis of portopulmonary hypertension is not completely understood but likely involves a complex interaction of several mechanisms, including an imbalance of vascular mediators favoring vasoconstriction,11–13 endothelial damage with vascular remodeling due to excessive pulmonary blood flow,14,15 smooth muscle proliferation, and microvascular thrombosis.16,17
The pulmonary endothelium is a complex, dynamic organ capable of influencing a variety of vascular mediators and adapting to changes in pulmonary volume as necessary. Endothelial dysfunction may initiate the vascular changes seen in portopulmonary hypertension.
Endothelin-1 (ET-1) is a potent vasoconstrictor that has been implicated in the pathogenesis of idiopathic pulmonary artery hypertension. ET-1 levels are also increased in cirrhotic patients with refractory ascites.6
Other mediators favoring vasoconstriction include serotonin, angiotensin II, and norepinephrine. Whether these mediators influence the development of portopulmonary hypertension is not clear.
At the same time, production of vasodilatory mediators such as nitric oxide and prostacyclin may be decreased in portopulmonary hypertension, facilitating vascular remodeling and a proliferative vascular response. Prostacyclin is a potent vasodilator normally found in high concentrations in the lungs. Prostacyclin synthase is the precursor enzyme for the production of prostacyclin and is decreased in the lungs of patients with portopulmonary hypertension.18
Another way that portal hypertension may influence lung vascular tone is that endotoxin, cytokines, or both, released from the splanchnic circulation, may bypass the liver and get into the lungs.19 Evidence in support of this is that patients with portosystemic shunting can develop similar pathologic changes in the pulmonary vascular bed that normalize when the shunt is reversed. To date, however, no substance has been definitively identified.
Yet another proposed mechanism is shear stress on the pulmonary endothelium from the hyperdynamic cardiac output, with resultant vascular remodeling; however, other mechanisms must be involved, as not everyone with liver disease develops portopulmonary hypertension (see below).
These changes are identical to those in idiopathic and familial pulmonary arterial hypertension,21 and indeed, the World Health Organization now classifies portopulmonary hypertension in the same category as these primary forms of pulmonary hypertension rather than in the secondary forms.3
Why doesn’t everyone with liver disease develop portopulmonary hypertension?
The severity of liver disease or degree of portal hypertension does not appear to correlate with the severity of pulmonary hypertension,4 and portopulmonary hypertension does not develop in all patients with portal hypertension. Therefore, it is likely that some patients have a genetic or environmental susceptibility or suffer a “second hit” that triggers dysregulated pulmonary vascular proliferation and contributes to the development of pulmonary hypertension.
Whether genetic mutations play a role in portopulmonary hypertension remains unknown. Such a mutation could be similar to the one identified in the bone morphogenetic protein receptor type 2 gene (BMPR2) in familial pulmonary artery hypertension or the mutation in the activin-like kinase gene (ALK1) seen in pulmonary hypertension in patients with hereditary hemorrhagic telangiectasia.22
Current studies are investigating the role that bone-marrow-derived progenitor cells might play in the pathogenesis of portopulmonary hypertension.
CLINICAL FEATURES MAY NOT BE OBVIOUS AT FIRST
In the early stages of portopulmonary hypertension, patients may have no symptoms or only symptoms of liver disease, so it is important to have a high index of suspicion and screen for pulmonary hypertension. As its severity increases, symptoms may include fatigue, dyspnea, abdominal bloating, palpitations, chest pain or pressure, and syncope. The most common presenting symptom is dyspnea on exertion.
Similarly, the findings on physical examination also depend on the severity of pulmonary hypertension. Patients with mild portopulmonary hypertension may have only signs suggesting liver disease, such as spider telangiectases, jaundice, mild lower extremity edema, and ascites. As the severity of portopulmonary hypertension increases, however, findings of right heart pressure-and-volume overload become more obvious. These include peripheral edema, elevation of the jugular venous pressure, a right ventricular lift, a loud pulmonic valve closure, increased split of the second heart sound, a pulsatile liver, or a right-sided third or fourth heart sound.
SCREEN LIVER TRANSPLANT CANDIDATES
Screening for portopulmonary hypertension should be mandatory in patients undergoing evaluation for liver transplantation. This condition increases the risk of perioperative death, so it is not acceptable to make the diagnosis in the operating room!5
Electrocardiographic abnormalities that may raise the suspicion of portopulmonary hypertension include right atrial or ventricular enlargement and a right bundle branch pattern.
Chest radiographic signs are enlarged central pulmonary arteries and cardiomegaly. These electrocardiographic and radiographic signs tend to reflect advanced pulmonary hypertension.
Pulmonary function testing is not generally helpful, but the diffusing capacity may be decreased.
B-type natriuretic peptide (BNP) measurement may be helpful. BNP is released from the ventricles when the ventricles become dilated (due to pressure or volume overload), as in left or right heart failure. BNP testing is clinically useful in monitoring the severity of disease and the efficacy of treatment in patients with pulmonary hypertension; its role in portopulmonary hypertension requires prospective study.23
Transthoracic Doppler echocardiography is an excellent screening test and should be performed in patients undergoing evaluation for liver transplantation to exclude pulmonary hypertension.1 Findings on echocardiography that suggest portopulmonary hypertension include elevation of right ventricular systolic pressure (RVSP), which is calculated from the peak tricuspid regurgitant velocity (TRV) using the modified Bernoulli equation and an estimate of right atrial pressure (RAP):
RVSP = 4(TRV)2 + RAP.
Right atrial pressure is estimated from the filling characteristics of the inferior vena cava.
Transthoracic Doppler echocardiography has a sensitivity of 97% and a specificity of 77% in diagnosing moderate to severe pulmonary hypertension in patients undergoing evaluation for liver transplantation.24 Using an RVSP cutoff of 40 mm Hg, the sensitivity of Doppler echocardiography is about 80%, specificity 96%, positive predictive value 60%, and negative predictive value 98%.25
At Mayo Clinic, patients with an estimated RVSP greater than 50 mm Hg undergo right heart catheterization (see below). Such patients should also have repeat echocardiography at 1-year intervals to monitor for increasing pulmonary artery pressures5; for those on the waiting list for liver transplantation, the interval should probably be every 6 to 12 months.
RIGHT HEART CATHETERIZATION CONFIRMS THE DIAGNOSIS
The diagnosis of portopulmonary hypertension is confirmed with right heart catheterization to accurately measure pulmonary artery pressures, pulmonary artery occlusion pressure (to exclude volume overload), cardiac output (to exclude high-output pulmonary hypertension), and pulmonary vascular resistance. One study in patients with decompensated cirrhosis and refractory ascites found that a right atrial pressure of 14 mm Hg or greater had a positive predictive value of 83% for pulmonary hypertension.6
Other, potentially treatable causes of pulmonary hypertension must be excluded before diagnosing portopulmonary hypertension. These include thromboembolic disease, interstitial lung disease, connective tissue disease, untreated obstructive sleep apnea, and elevated pulmonary artery pressures due to increased cardiac output.
Vasodilator studies are being done less frequently in patients with portopulmonary hypertension, as they generally cannot tolerate calcium channel blocker therapy. Calcium channel blocker therapy is usually started in patients with idiopathic pulmonary artery hypertension who exhibit a positive vasodilator response. A positive vasodilator response also does not predict survival with or without liver transplantation. Unlike those with idiopathic pulmonary artery hypertension, many patients with portopulmonary hypertension cannot tolerate calcium channel blockers, as some of these drugs can exacerbate edema and portal hypertension.
GENERAL MANAGEMENT
Treatment of mild portopulmonary hypertension (mean pulmonary artery pressure < 35 mm Hg) is debatable. In these cases many patients do not have any symptoms attributable to portopulmonary hypertension, but only symptoms of liver disease, and they have a good functional status. As a group, such patients have not been formally studied to date.
Anticoagulation is often contraindicated in portopulmonary hypertension because of gastroesophageal varices, thrombocytopenia, or other coagulation abnormalities related to liver disease. If contraindications to anticoagulation do not exist, it should be considered.
Diuretics are a mainstay in the treatment of portopulmonary hypertension, both for the pulmonary hypertension and for the liver disease, especially if ascites or peripheral edema is present.
Oxygen should be given to patients with hypoxemia to keep the saturation greater than 90%.
Beta-blockers: A dilemma
Beta-blockers are used in many patients with liver disease as both primary and secondary prophylaxis of variceal bleeding.
However, one study has shown that in patients with moderate to severe portopulmonary hypertension, beta-blockers are associated with significant worsening of exercise capacity and pulmonary hemodynamic measurements.26 After beta-blockers were withdrawn, the 6-minute walking distance increased in 9 of 10 patients, and cardiac output increased with no change in mean pulmonary artery pressure, resulting in a 19% decrease in pulmonary vascular resistance. The increases in cardiac output were related to a 25% increase in heart rate. Long-term follow-up was not reported, and it remains unclear whether rates of gastrointestinal bleeding may increase when beta-blockers are withdrawn.
Beta-blocker therapy in portopulmonary hypertension needs to be carefully considered and if at all possible should be avoided.
VASODILATOR THERAPY
Several vasodilating or vasomodulating drugs are available. However, much of the information about them comes from studies in patients with idiopathic pulmonary artery hypertension or pulmonary hypertension due to connective tissue disease, and no randomized controlled trials in portopulmonary hypertension have been performed.
Prostanoids
Prostanoids have been used successfully to lower pulmonary pressures in portopulmonary hypertension.
Epoprostenol (Flolan) is a pulmonary and systemic vasodilator as well as an inhibitor of platelet aggregation. It is given as a continuous intravenous infusion via an indwelling central venous catheter and a portable infusion pump. It has a very short half-life, requires mixing, and must be kept cold with ice packs, making it somewhat cumbersome to administer.
This medication has been shown to improve cardiopulmonary hemodynamics and exercise capacity in portopulmonary hypertension, although a survival advantage has not been documented to date.27 In several case series, some patients with portopulmonary hypertension treated with intravenous epoprostenol responded with a reduction in pulmonary pressures and successfully underwent liver transplantation.28–31
Complications of intravenous epoprostenol therapy include central venous catheter thrombosis, infection, and infusion pump failure; a backup pump must be available at all times. Patients with portopulmonary hypertension may also develop progressive splenomegaly and thrombocytopenia that may be due to increased blood flow in the splanchnic circulation.32
Treprostinil (Remodulin) has a longer half-life and does not have to be kept cold. It is given as a 24-hour intravenous or subcutaneous infusion, using an infusion pump that is smaller than that used with epoprostenol.
Although treprostinil is easier for patients to use, larger doses are necessary to achieve the same effect as with epoprostenol. With subcutaneous administration, the biggest drawback is site pain. Prostacyclin-related side effects include flushing, diarrhea, jaw discomfort, and lower extremity pain.
Iloprost (Ventavis) has the advantage of being given by inhalation. It is very short-acting, however, and requires six to nine inhalations per day.
Endothelin receptor blockers
Bosentan (Tracleer) is an oral agent that has been approved by the US Food and Drug Administration (FDA) for the treatment of pulmonary hypertension, including in patients with portopulmonary hypertension who have mild hepatic derangement. This medication is a dual endothelin receptor antagonist, nonselectively blocking the endothelin A and B receptors on the endothelial and vascular smooth muscle cells so that ET-1 cannot bind and cause vasoconstriction.
In approximately 10% of patients, bosentan can cause elevations in aminotransferase, alkaline phosphatase, and bilirubin levels, which therefore must be checked monthly.33 Irreversible hepatic toxicity is uncommon; in most cases, liver function abnormalities return to baseline levels when the medication is stopped. The presumed mechanism is impairment of bile-salt transporters, leading to bile-salt accumulation in the liver.34 Bosentan’s use in patients with liver disease has not been well studied, although several case reports have described its use in patients with portopulmonary hypertension.35–38
Ambrisentan (Letairis) is a selective endothelin receptor-A blocker that has just received FDA approval for the treatment of pulmonary artery hypertension. It has not yet been studied in portopulmonary hypertension. Elevations in liver enzymes and bilirubin may also occur, and monthly monitoring is indicated.
Sildenafil
Another oral agent that might be effective in portopulmonary hypertension is sildenafil (Revatio). A phosphodiesterase-5 inhibitor, it selectively inhibits the cyclic guanosine monophosphatase-specific phosphodiesterase type 5 enzyme that is found in large concentrations in pulmonary artery smooth muscle cells.
In other forms of pulmonary hypertension, sildenafil has been shown to increase cardiac output and decrease pulmonary artery pressures and pulmonary vascular resistance without serious adverse events.39–41
In one reported case, treatment with sildenafil in a patient with portopulmonary hypertension decreased the mean pulmonary artery pressure from 56 mm Hg to 28 to 31 mm Hg, and the patient underwent successful liver transplantation.42 A recent case series of 14 patients with portopulmonary hypertension treated with sildenafil documents some improvement in 6-minute walking distance, suggesting that sildenafil as monotherapy or in combination therapy might be effective in portopulmonary hypertension.43 However, in 3 of these patients, the cardiac index decreased and pulmonary vascular resistance increased.44
We must emphasize that controlled studies in portopulmonary hypertension need to be done to find the optimal therapy.
LIVER TRANSPLANTATION MAY BENEFIT A FEW PATIENTS
Liver transplantation may be beneficial in highly selected patients with portopulmonary hypertension. However, this condition increases the risk of intraoperative and immediate postoperative complications of liver transplantation, so patients should be carefully evaluated5,45 at a liver transplantation center experienced in its management, including medical treatment with well-defined protocols regarding timing of liver transplantation.
Patients with mean pulmonary artery pressures greater than 50 mm Hg should not undergo liver transplantation. Those with mean pulmonary artery pressure between 35 and 50 mm Hg also have an increased mortality rate and may benefit from prolonged treatment for pulmonary hypertension.5,46
One successful case of living-related liver transplantation in a patient with portopulmonary hypertension has been published.47 (Most other successful transplants were from unrelated cadaver donors.)
Some patients who initially cannot undergo liver transplantation owing to severe pulmonary hypertension may eventually be able to do so if they receive medical therapy that improves their pulmonary hemodynamic profile, decreasing their mean pulmonary artery pressure and pulmonary vascular resistance. This would apply to a small subset of patients with portopulmonary hypertension.
When patients without pulmonary hypertension undergo liver transplantation, right ventricular function is preserved throughout all phases of the surgery.48 Patients with portopulmonary hypertension, however, may develop hemodynamic instability during liver transplantation. The most critical times are the induction of anesthesia, during and after graft reperfusion, and the immediate postoperative period.49,50
During the surgery, patients may require vasodilators if they have worsening pulmonary hypertension, or inotropic medications if they have right ventricular dysfunction and heart failure. In one study,51 eight patients with portopulmonary hypertension diagnosed at anesthesia induction for liver transplantation all required intraoperative vasodilator therapy after graft reperfusion because of marked increases in pulmonary artery pressures and pulmonary vascular resistance.
The increase in blood flow following reperfusion or necessary fluid challenges may exacerbate pulmonary hypertension, resulting in worsening right heart function and backup into the transplanted liver. Infusion of 1 liter of crystalloid over 10 minutes has been shown to increase mean pulmonary artery pressure and pulmonary artery occlusion pressure in liver transplantation candidates without pulmonary hypertension52; this response may be exaggerated in portopulmonary hypertension.
PROGNOSIS VARIES WITH SEVERITY OF DISEASE
The natural history of untreated portopulmonary hypertension varies with the degree of liver disease and the severity of pulmonary hypertension. Transplant-free survival was 85% at 1 year and 38% at 3 years in one study.45 The cardiac index appears to be the most significant prognostic variable.20
In a retrospective study of 78 patients with portopulmonary hypertension treated conservatively (before prostanoids were available) the median survival was 6 months (range 0–84 months) from the time of diagnosis.53 Causes of death included right heart failure, sudden death, gastrointestinal bleeding, and small bowel perforation.
Most of the data on outcomes of drug treatment and liver transplantation in patients with portopulmonary hypertension come from case series and retrospective reviews; prospective trials have been lacking.
If right ventricular function is normal and pulmonary hypertension is mild (mean pulmonary artery pressure < 35 mm Hg), patients tend to do well with liver transplantation.9
Outcomes are worse if pulmonary hypertension is more severe. In a database54 from 10 liver transplant centers from 1996 to 2001, 13 (36%) of 36 patients undergoing liver transplantation died in the hospital, emphasizing the importance of accurately assessing the severity of pulmonary hypertension before attempting liver transplantation.46 The rate was even higher—92%—in those with a mean pulmonary artery pressure greater than 35 mm Hg. The cause of death in severe pulmonary hypertension was failure of the right ventricle.
However, some patients with moderate to severe portopulmonary hypertension have been bridged with medications to lower pulmonary artery pressures and pulmonary vascular resistance so that liver transplantation can be safely done, and some have even been able to discontinue medications because their pulmonary hypertension resolved.29,31,41,42,47
Unlike in hepatopulmonary syndrome, liver transplantation is not the treatment of choice for portopulmonary hypertension, and pulmonary hypertension does not always resolve after liver transplantation. Many patients continue therapy for pulmonary hypertension after liver transplantation. Pulmonary hypertension may resolve, persist, or even develop de novo after liver transplantation.1 If pulmonary hypertension resolves, it does so over a prolonged time—months to years—favoring a vascular remodeling hypothesis as opposed to simply reversing vasoconstriction.
Portopulmonary hypertension poses difficulties for patients with liver disease. The elevated pulmonary artery pressure in this disorder makes liver transplantation more dangerous and in fact may rule out the procedure, although in a selected few patients, medical treatment may enable transplantation to proceed. In any event, portopulmonary hypertension should be looked for in patients with liver disease, especially if liver transplantation is being considered.
In this article we discuss the definition, pathophysiology, clinical features, diagnosis, and management of portopulmonary hypertension.
DEFINED BY HEMODYNAMIC CRITERIA
Portopulmonary hypertension—elevated pulmonary artery pressure due to increased resistance to blood flow in patients with portal hypertension—is one of several pulmonary complications of liver disease. A few others to be aware of are pleural effusions (hepatic hydrothorax), dilatation of the pulmonary vasculature with shunting and hypoxemia (hepatopulmonary syndrome), and elevation in pulmonary pressures due to the high cardiac output usually seen in liver disease (flow phenomenon).
The definition of portopulmonary hypertension has evolved as the various hemodynamic profiles that occur in liver disease and their consequences have been described. Currently, it is defined by the following criteria (obtained by right heart catheterization) in a patient with portal hypertension1:
- Elevated mean pulmonary artery pressure (> 25 mm Hg at rest, > 30 mm Hg with exercise);
- Increased pulmonary vascular resistance (> 240 dynes.s.cm−5; pulmonary vascular resistance = [(mean pulmonary artery pressure minus pulmonary artery occlusion pressure) /cardiac output] times 80); and
- Normal pulmonary artery occlusion pressure (< 15 mm Hg) or an elevated transpulmonary gradient (the mean pulmonary artery pressure minus the pulmonary artery occlusion pressure; abnormal is > 12 mm Hg).
The transpulmonary gradient sometimes helps in further assessing the resistance to blood flow in cases that do not meet the other criteria.2 For example, how should we classify a patient whose mean pulmonary artery pressure is 45 mm Hg but whose pulmonary vascular resistance is only 432 dynes.s.cm−5 and whose pulmonary artery occlusion pressure is slightly high at 18 mm Hg? Although this patient does not meet the hemodynamic criteria for portopulmonary hypertension listed above, intuitively, we should not exclude the diagnosis, as the transpulmonary gradient is high at 27 mm Hg.
FLOW PHENOMENON VS TRUE PORTOPULMONARY HYPERTENSION
The cardiopulmonary hemodynamic profile is different in patients with liver disease than in those without liver disease. Understanding the “normal” hemodynamics in liver disease is paramount in understanding the abnormal hemodynamics that occur in portopulmonary hypertension. In general, patients with liver disease have a high cardiac output at baseline (high flow). They may also have an increased blood volume due to fluid shifts (elevated pulmonary artery occlusion pressure).
Right heart catheterization is necessary to make the diagnosis of portopulmonary hypertension, as pulmonary artery pressures may be increased simply from increases in cardiac output and blood volume without an increase in pulmonary vascular resistance.
Consider, for example, a patient whose mean pulmonary artery pressure is 38 mm Hg, pulmonary artery occlusion pressure 14 mm Hg, and cardiac output 8.8 L/minute. In this case, the pulmonary vascular resistance is 218 dynes.s.cm−5. About 30% to 50% of patients with cirrhosis have this type of hyperdynamic pattern, with high cardiac output, low systemic vascular resistance, and low pulmonary vascular resistance.1,3,4 These patients typically have a much better prognosis than those with portopulmonary hypertension and do well with liver transplantation.
Right heart catheterization is also helpful in assessing whether elevated pulmonary pressures are due to increased volume (increased pulmonary artery occlusion pressure), in which case the patient might benefit from more aggressive diuresis.
In true portopulmonary hypertension, the pulmonary vascular resistance is increased due to obstruction of arterial blood flow. Cardiac output may be elevated initially and then decline as pulmonary hypertension becomes more severe. These hemodynamic patterns have different treatment implications and are important when liver transplantation is being considered.5
HOW COMMON IS PORTOPULMONARY HYPERTENSION?
The incidence and prevalence of portopulmonary hypertension is difficult to assess, as many of the estimates are in patients with severe liver disease undergoing evaluation for liver transplantation. Its prevalence in patients with cirrhosis and refractory ascites has been documented at 16.1%,6 while its prevalence in patients with cirrhosis without refractory ascites has been in the range of 0.25% to 4%.7–9
Overall, about 8% of candidates for liver transplantation have portopulmonary hypertension and are at risk of its complications.10 In view of this figure, screening for it should be performed before proceeding with liver transplantation.
VASOCONSTRICTION, REMODELING, THROMBOSIS
The pathogenesis of portopulmonary hypertension is not completely understood but likely involves a complex interaction of several mechanisms, including an imbalance of vascular mediators favoring vasoconstriction,11–13 endothelial damage with vascular remodeling due to excessive pulmonary blood flow,14,15 smooth muscle proliferation, and microvascular thrombosis.16,17
The pulmonary endothelium is a complex, dynamic organ capable of influencing a variety of vascular mediators and adapting to changes in pulmonary volume as necessary. Endothelial dysfunction may initiate the vascular changes seen in portopulmonary hypertension.
Endothelin-1 (ET-1) is a potent vasoconstrictor that has been implicated in the pathogenesis of idiopathic pulmonary artery hypertension. ET-1 levels are also increased in cirrhotic patients with refractory ascites.6
Other mediators favoring vasoconstriction include serotonin, angiotensin II, and norepinephrine. Whether these mediators influence the development of portopulmonary hypertension is not clear.
At the same time, production of vasodilatory mediators such as nitric oxide and prostacyclin may be decreased in portopulmonary hypertension, facilitating vascular remodeling and a proliferative vascular response. Prostacyclin is a potent vasodilator normally found in high concentrations in the lungs. Prostacyclin synthase is the precursor enzyme for the production of prostacyclin and is decreased in the lungs of patients with portopulmonary hypertension.18
Another way that portal hypertension may influence lung vascular tone is that endotoxin, cytokines, or both, released from the splanchnic circulation, may bypass the liver and get into the lungs.19 Evidence in support of this is that patients with portosystemic shunting can develop similar pathologic changes in the pulmonary vascular bed that normalize when the shunt is reversed. To date, however, no substance has been definitively identified.
Yet another proposed mechanism is shear stress on the pulmonary endothelium from the hyperdynamic cardiac output, with resultant vascular remodeling; however, other mechanisms must be involved, as not everyone with liver disease develops portopulmonary hypertension (see below).
These changes are identical to those in idiopathic and familial pulmonary arterial hypertension,21 and indeed, the World Health Organization now classifies portopulmonary hypertension in the same category as these primary forms of pulmonary hypertension rather than in the secondary forms.3
Why doesn’t everyone with liver disease develop portopulmonary hypertension?
The severity of liver disease or degree of portal hypertension does not appear to correlate with the severity of pulmonary hypertension,4 and portopulmonary hypertension does not develop in all patients with portal hypertension. Therefore, it is likely that some patients have a genetic or environmental susceptibility or suffer a “second hit” that triggers dysregulated pulmonary vascular proliferation and contributes to the development of pulmonary hypertension.
Whether genetic mutations play a role in portopulmonary hypertension remains unknown. Such a mutation could be similar to the one identified in the bone morphogenetic protein receptor type 2 gene (BMPR2) in familial pulmonary artery hypertension or the mutation in the activin-like kinase gene (ALK1) seen in pulmonary hypertension in patients with hereditary hemorrhagic telangiectasia.22
Current studies are investigating the role that bone-marrow-derived progenitor cells might play in the pathogenesis of portopulmonary hypertension.
CLINICAL FEATURES MAY NOT BE OBVIOUS AT FIRST
In the early stages of portopulmonary hypertension, patients may have no symptoms or only symptoms of liver disease, so it is important to have a high index of suspicion and screen for pulmonary hypertension. As its severity increases, symptoms may include fatigue, dyspnea, abdominal bloating, palpitations, chest pain or pressure, and syncope. The most common presenting symptom is dyspnea on exertion.
Similarly, the findings on physical examination also depend on the severity of pulmonary hypertension. Patients with mild portopulmonary hypertension may have only signs suggesting liver disease, such as spider telangiectases, jaundice, mild lower extremity edema, and ascites. As the severity of portopulmonary hypertension increases, however, findings of right heart pressure-and-volume overload become more obvious. These include peripheral edema, elevation of the jugular venous pressure, a right ventricular lift, a loud pulmonic valve closure, increased split of the second heart sound, a pulsatile liver, or a right-sided third or fourth heart sound.
SCREEN LIVER TRANSPLANT CANDIDATES
Screening for portopulmonary hypertension should be mandatory in patients undergoing evaluation for liver transplantation. This condition increases the risk of perioperative death, so it is not acceptable to make the diagnosis in the operating room!5
Electrocardiographic abnormalities that may raise the suspicion of portopulmonary hypertension include right atrial or ventricular enlargement and a right bundle branch pattern.
Chest radiographic signs are enlarged central pulmonary arteries and cardiomegaly. These electrocardiographic and radiographic signs tend to reflect advanced pulmonary hypertension.
Pulmonary function testing is not generally helpful, but the diffusing capacity may be decreased.
B-type natriuretic peptide (BNP) measurement may be helpful. BNP is released from the ventricles when the ventricles become dilated (due to pressure or volume overload), as in left or right heart failure. BNP testing is clinically useful in monitoring the severity of disease and the efficacy of treatment in patients with pulmonary hypertension; its role in portopulmonary hypertension requires prospective study.23
Transthoracic Doppler echocardiography is an excellent screening test and should be performed in patients undergoing evaluation for liver transplantation to exclude pulmonary hypertension.1 Findings on echocardiography that suggest portopulmonary hypertension include elevation of right ventricular systolic pressure (RVSP), which is calculated from the peak tricuspid regurgitant velocity (TRV) using the modified Bernoulli equation and an estimate of right atrial pressure (RAP):
RVSP = 4(TRV)2 + RAP.
Right atrial pressure is estimated from the filling characteristics of the inferior vena cava.
Transthoracic Doppler echocardiography has a sensitivity of 97% and a specificity of 77% in diagnosing moderate to severe pulmonary hypertension in patients undergoing evaluation for liver transplantation.24 Using an RVSP cutoff of 40 mm Hg, the sensitivity of Doppler echocardiography is about 80%, specificity 96%, positive predictive value 60%, and negative predictive value 98%.25
At Mayo Clinic, patients with an estimated RVSP greater than 50 mm Hg undergo right heart catheterization (see below). Such patients should also have repeat echocardiography at 1-year intervals to monitor for increasing pulmonary artery pressures5; for those on the waiting list for liver transplantation, the interval should probably be every 6 to 12 months.
RIGHT HEART CATHETERIZATION CONFIRMS THE DIAGNOSIS
The diagnosis of portopulmonary hypertension is confirmed with right heart catheterization to accurately measure pulmonary artery pressures, pulmonary artery occlusion pressure (to exclude volume overload), cardiac output (to exclude high-output pulmonary hypertension), and pulmonary vascular resistance. One study in patients with decompensated cirrhosis and refractory ascites found that a right atrial pressure of 14 mm Hg or greater had a positive predictive value of 83% for pulmonary hypertension.6
Other, potentially treatable causes of pulmonary hypertension must be excluded before diagnosing portopulmonary hypertension. These include thromboembolic disease, interstitial lung disease, connective tissue disease, untreated obstructive sleep apnea, and elevated pulmonary artery pressures due to increased cardiac output.
Vasodilator studies are being done less frequently in patients with portopulmonary hypertension, as they generally cannot tolerate calcium channel blocker therapy. Calcium channel blocker therapy is usually started in patients with idiopathic pulmonary artery hypertension who exhibit a positive vasodilator response. A positive vasodilator response also does not predict survival with or without liver transplantation. Unlike those with idiopathic pulmonary artery hypertension, many patients with portopulmonary hypertension cannot tolerate calcium channel blockers, as some of these drugs can exacerbate edema and portal hypertension.
GENERAL MANAGEMENT
Treatment of mild portopulmonary hypertension (mean pulmonary artery pressure < 35 mm Hg) is debatable. In these cases many patients do not have any symptoms attributable to portopulmonary hypertension, but only symptoms of liver disease, and they have a good functional status. As a group, such patients have not been formally studied to date.
Anticoagulation is often contraindicated in portopulmonary hypertension because of gastroesophageal varices, thrombocytopenia, or other coagulation abnormalities related to liver disease. If contraindications to anticoagulation do not exist, it should be considered.
Diuretics are a mainstay in the treatment of portopulmonary hypertension, both for the pulmonary hypertension and for the liver disease, especially if ascites or peripheral edema is present.
Oxygen should be given to patients with hypoxemia to keep the saturation greater than 90%.
Beta-blockers: A dilemma
Beta-blockers are used in many patients with liver disease as both primary and secondary prophylaxis of variceal bleeding.
However, one study has shown that in patients with moderate to severe portopulmonary hypertension, beta-blockers are associated with significant worsening of exercise capacity and pulmonary hemodynamic measurements.26 After beta-blockers were withdrawn, the 6-minute walking distance increased in 9 of 10 patients, and cardiac output increased with no change in mean pulmonary artery pressure, resulting in a 19% decrease in pulmonary vascular resistance. The increases in cardiac output were related to a 25% increase in heart rate. Long-term follow-up was not reported, and it remains unclear whether rates of gastrointestinal bleeding may increase when beta-blockers are withdrawn.
Beta-blocker therapy in portopulmonary hypertension needs to be carefully considered and if at all possible should be avoided.
VASODILATOR THERAPY
Several vasodilating or vasomodulating drugs are available. However, much of the information about them comes from studies in patients with idiopathic pulmonary artery hypertension or pulmonary hypertension due to connective tissue disease, and no randomized controlled trials in portopulmonary hypertension have been performed.
Prostanoids
Prostanoids have been used successfully to lower pulmonary pressures in portopulmonary hypertension.
Epoprostenol (Flolan) is a pulmonary and systemic vasodilator as well as an inhibitor of platelet aggregation. It is given as a continuous intravenous infusion via an indwelling central venous catheter and a portable infusion pump. It has a very short half-life, requires mixing, and must be kept cold with ice packs, making it somewhat cumbersome to administer.
This medication has been shown to improve cardiopulmonary hemodynamics and exercise capacity in portopulmonary hypertension, although a survival advantage has not been documented to date.27 In several case series, some patients with portopulmonary hypertension treated with intravenous epoprostenol responded with a reduction in pulmonary pressures and successfully underwent liver transplantation.28–31
Complications of intravenous epoprostenol therapy include central venous catheter thrombosis, infection, and infusion pump failure; a backup pump must be available at all times. Patients with portopulmonary hypertension may also develop progressive splenomegaly and thrombocytopenia that may be due to increased blood flow in the splanchnic circulation.32
Treprostinil (Remodulin) has a longer half-life and does not have to be kept cold. It is given as a 24-hour intravenous or subcutaneous infusion, using an infusion pump that is smaller than that used with epoprostenol.
Although treprostinil is easier for patients to use, larger doses are necessary to achieve the same effect as with epoprostenol. With subcutaneous administration, the biggest drawback is site pain. Prostacyclin-related side effects include flushing, diarrhea, jaw discomfort, and lower extremity pain.
Iloprost (Ventavis) has the advantage of being given by inhalation. It is very short-acting, however, and requires six to nine inhalations per day.
Endothelin receptor blockers
Bosentan (Tracleer) is an oral agent that has been approved by the US Food and Drug Administration (FDA) for the treatment of pulmonary hypertension, including in patients with portopulmonary hypertension who have mild hepatic derangement. This medication is a dual endothelin receptor antagonist, nonselectively blocking the endothelin A and B receptors on the endothelial and vascular smooth muscle cells so that ET-1 cannot bind and cause vasoconstriction.
In approximately 10% of patients, bosentan can cause elevations in aminotransferase, alkaline phosphatase, and bilirubin levels, which therefore must be checked monthly.33 Irreversible hepatic toxicity is uncommon; in most cases, liver function abnormalities return to baseline levels when the medication is stopped. The presumed mechanism is impairment of bile-salt transporters, leading to bile-salt accumulation in the liver.34 Bosentan’s use in patients with liver disease has not been well studied, although several case reports have described its use in patients with portopulmonary hypertension.35–38
Ambrisentan (Letairis) is a selective endothelin receptor-A blocker that has just received FDA approval for the treatment of pulmonary artery hypertension. It has not yet been studied in portopulmonary hypertension. Elevations in liver enzymes and bilirubin may also occur, and monthly monitoring is indicated.
Sildenafil
Another oral agent that might be effective in portopulmonary hypertension is sildenafil (Revatio). A phosphodiesterase-5 inhibitor, it selectively inhibits the cyclic guanosine monophosphatase-specific phosphodiesterase type 5 enzyme that is found in large concentrations in pulmonary artery smooth muscle cells.
In other forms of pulmonary hypertension, sildenafil has been shown to increase cardiac output and decrease pulmonary artery pressures and pulmonary vascular resistance without serious adverse events.39–41
In one reported case, treatment with sildenafil in a patient with portopulmonary hypertension decreased the mean pulmonary artery pressure from 56 mm Hg to 28 to 31 mm Hg, and the patient underwent successful liver transplantation.42 A recent case series of 14 patients with portopulmonary hypertension treated with sildenafil documents some improvement in 6-minute walking distance, suggesting that sildenafil as monotherapy or in combination therapy might be effective in portopulmonary hypertension.43 However, in 3 of these patients, the cardiac index decreased and pulmonary vascular resistance increased.44
We must emphasize that controlled studies in portopulmonary hypertension need to be done to find the optimal therapy.
LIVER TRANSPLANTATION MAY BENEFIT A FEW PATIENTS
Liver transplantation may be beneficial in highly selected patients with portopulmonary hypertension. However, this condition increases the risk of intraoperative and immediate postoperative complications of liver transplantation, so patients should be carefully evaluated5,45 at a liver transplantation center experienced in its management, including medical treatment with well-defined protocols regarding timing of liver transplantation.
Patients with mean pulmonary artery pressures greater than 50 mm Hg should not undergo liver transplantation. Those with mean pulmonary artery pressure between 35 and 50 mm Hg also have an increased mortality rate and may benefit from prolonged treatment for pulmonary hypertension.5,46
One successful case of living-related liver transplantation in a patient with portopulmonary hypertension has been published.47 (Most other successful transplants were from unrelated cadaver donors.)
Some patients who initially cannot undergo liver transplantation owing to severe pulmonary hypertension may eventually be able to do so if they receive medical therapy that improves their pulmonary hemodynamic profile, decreasing their mean pulmonary artery pressure and pulmonary vascular resistance. This would apply to a small subset of patients with portopulmonary hypertension.
When patients without pulmonary hypertension undergo liver transplantation, right ventricular function is preserved throughout all phases of the surgery.48 Patients with portopulmonary hypertension, however, may develop hemodynamic instability during liver transplantation. The most critical times are the induction of anesthesia, during and after graft reperfusion, and the immediate postoperative period.49,50
During the surgery, patients may require vasodilators if they have worsening pulmonary hypertension, or inotropic medications if they have right ventricular dysfunction and heart failure. In one study,51 eight patients with portopulmonary hypertension diagnosed at anesthesia induction for liver transplantation all required intraoperative vasodilator therapy after graft reperfusion because of marked increases in pulmonary artery pressures and pulmonary vascular resistance.
The increase in blood flow following reperfusion or necessary fluid challenges may exacerbate pulmonary hypertension, resulting in worsening right heart function and backup into the transplanted liver. Infusion of 1 liter of crystalloid over 10 minutes has been shown to increase mean pulmonary artery pressure and pulmonary artery occlusion pressure in liver transplantation candidates without pulmonary hypertension52; this response may be exaggerated in portopulmonary hypertension.
PROGNOSIS VARIES WITH SEVERITY OF DISEASE
The natural history of untreated portopulmonary hypertension varies with the degree of liver disease and the severity of pulmonary hypertension. Transplant-free survival was 85% at 1 year and 38% at 3 years in one study.45 The cardiac index appears to be the most significant prognostic variable.20
In a retrospective study of 78 patients with portopulmonary hypertension treated conservatively (before prostanoids were available) the median survival was 6 months (range 0–84 months) from the time of diagnosis.53 Causes of death included right heart failure, sudden death, gastrointestinal bleeding, and small bowel perforation.
Most of the data on outcomes of drug treatment and liver transplantation in patients with portopulmonary hypertension come from case series and retrospective reviews; prospective trials have been lacking.
If right ventricular function is normal and pulmonary hypertension is mild (mean pulmonary artery pressure < 35 mm Hg), patients tend to do well with liver transplantation.9
Outcomes are worse if pulmonary hypertension is more severe. In a database54 from 10 liver transplant centers from 1996 to 2001, 13 (36%) of 36 patients undergoing liver transplantation died in the hospital, emphasizing the importance of accurately assessing the severity of pulmonary hypertension before attempting liver transplantation.46 The rate was even higher—92%—in those with a mean pulmonary artery pressure greater than 35 mm Hg. The cause of death in severe pulmonary hypertension was failure of the right ventricle.
However, some patients with moderate to severe portopulmonary hypertension have been bridged with medications to lower pulmonary artery pressures and pulmonary vascular resistance so that liver transplantation can be safely done, and some have even been able to discontinue medications because their pulmonary hypertension resolved.29,31,41,42,47
Unlike in hepatopulmonary syndrome, liver transplantation is not the treatment of choice for portopulmonary hypertension, and pulmonary hypertension does not always resolve after liver transplantation. Many patients continue therapy for pulmonary hypertension after liver transplantation. Pulmonary hypertension may resolve, persist, or even develop de novo after liver transplantation.1 If pulmonary hypertension resolves, it does so over a prolonged time—months to years—favoring a vascular remodeling hypothesis as opposed to simply reversing vasoconstriction.
- Rodriguez-Roisin R, Krowka MJ, Hervé P, Fallon MB; ERS Task Force Pulmonary-Hepatic Vascular Disorders (PHD) Scientific Committee. Pulmonary-hepatic vascular disorders (PHD). Eur Respir J 2004; 24:861–880.
- Krowka MJ, Swanson KL, Frantz RP, et al. Portopulmonary hypertension: results from a 10-year screening algorithm. Hepatology 2006; 44:1502–1510.
- Simonneau G, Galie N, Rubin LJ, et al. Clinical classification of pulmonary hypertension. J Am Coll Cardiol 2004; 43:5S–12S.
- Hadengue A, Benhayoun MK, Lebrec D, et al. Pulmonary hypertension complicating portal hypertension: prevalence and relation to splanchnic hemodynamics. Gastroenterology 1991; 100:520–528.
- Krowka MJ, Plevak DJ, Findlay JY, et al. Pulmonary hemodynamics and perioperative cardiopulmonary-related mortality in patients with portopulmonary hypertension undergoing liver transplantation. Liver Transplant 2000; 6:443–450.
- Benjaminov FS, Prentice M, Sniderman KW, et al. Portopulmonary hypertension in decompensated cirrhosis with refractory ascites. Gut 2003; 52:1355–1362.
- McDonnell PJ, Toye PA, Hutchins GM. Primary pulmonary hypertension and cirrhosis: are they related? Am Rev Respir Dis 1983; 127:437–441.
- Cheng EY, Woehlck H. Pulmonary artery hypertension complicating anesthesia for liver transplantation. Anesthesiology 1992; 77:375–378.
- Castro M, Krowka MJ, Schroeder DR, et al. Frequency and clinical implications of increased pulmonary artery pressures in liver transplantation. Mayo Clin Proc 1996; 71:543–551.
- Ramsay MA, Simpson BR, Nguyen AT, et al. Severe pulmonary hypertension in liver transplant candidates. Liver Transplant Surg 1997; 3:494–500.
- Kiely DG, Cargill RI, Struthers AD, et al. Cardiopulmonary effects of endothelin-1 in man. Cardiovasc Res 1997; 33:378–386.
- Panos RJ, Baker SK. Mediators, cytokines, and growth factors in liver-lung interactions. Clin Chest Med 1996; 17:151–169.
- Higgenbottam T. Pathophysiology of pulmonary hypertension. Chest 1994; 105:7S–12S.
- Krowka MJ. Hepatopulmonary syndrome and portopulmonary hypertension: distinction and dilemmas. Hepatology 1997; 25:1282–1284.
- Hongqun L, Lee SS. Cardiopulmonary dysfunction in cirrhosis. Hepatology 2000; 14:600–608.
- Lebrec D, Brenot F, Simonneau G, et al. Pulmonary arterial hypertension in portal hypertension. Eur Respir J 1998; 11:1153–1166.
- Herve P, Lebrec D, Brenot F, et al. Pulmonary vascular disorders in portal hypertension. Eur Respir J 1998; 11:1153–1166.
- Tuder RM, Cool CD, Geraci MW, et al. Prostacyclin synthase expression is decreased in lungs from patients with severe pulmonary hypertension. Am J Respir Crit Care Med 1999; 159:1925–1932.
- Hoeper MM, Krowka MJ, Strassburg CP. Portopulmonary hypertension and hepatopulmonary syndrome. Lancet 2004; 363:1461–1468.
- Edwards B, Weir K, Edwards WD, et al. Coexistent pulmonary and portal hypertension: morphologic and clinical features. J Am Coll Cardiol 1987; 10:1233–1238.
- Ramsay MAE, Simpson BR, Nguyen AT, Ramsay KJ, East C, Klintmalm GB. Severe pulmonary hypertension in liver transplant candidates. Liver Transplant Surg 1997; 3:494–500.
- Trembath RC. Clinical and molecular genetic features of pulmonary hypertension in patients with hereditary hemorrhagic telangiectasia. N Engl J Med 2001; 345:325–334.
- Leuchte HH, Holzapfel M, Baumgartner RA, et al. Clinical significance of brain natriuretic peptide in primary pulmonary hypertension. J Am Coll Cardiol 2004; 43:764–770.
- Kim WR, Krowka MJ, Plevak DJ, et al. Accuracy of Doppler echocardiography in the assessment of pulmonary hypertension in liver transplant candidates. Liver Transplant 2000; 6:453–458.
- Colle IO, Moreau R, Godinho E, et al. Diagnosis of portopulmonary hypertension in candidates for liver transplantation: a prospective study. Hepatology 2003; 37:401–409.
- Provencher S, Herve P, Jais X, et al. Deleterious effects of beta-blockers on exercise capacity and hemodynamics in patients with portopulmonary hypertension. Gastroenterology 2006; 130:120–126.
- Swanson KL, McGoon MD, Krowka MJ. Survival in patients with portopulmonary hypertension [abstract]. Am J Respir Crit Care Med 2003; 167:A693.
- Kuo PC, Johnson LB, Plotkin JS, et al. Continuous intravenous infusion of epoprostenol for the treatment of portopulmonary hypertension. Transplantation 1997; 63:604–616.
- Krowka MJ, Frantz RP, McGoon MD, et al. Improvement in pulmonary hemodynamics during intravenous epoprostenol (prostacyclin): A study of 15 patients with moderate to severe portopulmonary hypertension. Hepatology 1999; 30:641–648.
- Kähler CM, Graziadei I, Wiedermann CJ, Kneussl MP, Vogel W. Successful use of continuous intravenous prostacyclin in a patient with severe portopulmonary hypertension. Wien Klin Wochenschr 2000; 112:637–640.
- Sussman N, Kaza V, Barshes N, et al. Successful liver transplantation following medical management of portopulmonary hypertension: a single-center series. Am J Transplant 2006; 6:2177–2182.
- Findlay JY, Plevak DJ, Krowka MJ, et al. Progressive splenomegaly after epoprostenol therapy in portopulmonary hypertension. Liver Transplant Surg 1999; 5:381–387.
- Rubin LJ, Roux S. Bosentan: a dual endothelin receptor antagonist. Expert Opin Invest Drugs 2002; 11:991–1002.
- Fattinger K, Funk C, Pantze M, et al. The endothelin antagonist bosentan inhibits the canalicular bile salt export pump: a potential mechanism for hepatic adverse reactions. Clin Pharmacol Ther 2001; 69:223–231.
- Hinterhuber L, Graziadei IW, Kahler CM, et al. Endothelin-receptor anatgonist treatment of portopulmonary hypertension. Clin Gastroenterol Hepatol 2004; 2:1039–1042.
- Clift PF, Townend JN, Bramhall S, et al. Successful treatment of severe portopulmonary hypertension after liver transplantation by bosentan. Transplantation 2004; 77:1774–1775.
- Halank M, Miehlke S, Hoeffken G, et al. Use of oral endothelin-receptor antagonist bosentan in the treatment of portopulmonary hypertension. Transplantation 2004; 77:1775–1776.
- Kuntzen C, Gulberg V, Gerbes AL. Use of a mixed endothelin receptor antagonist in portopulmonary hypertension: a safe and effective therapy? Gastroenterology 2005; 128:164–168.
- Watanabe H, Ohashi K, Takeuchi K, et al. Sildenafil for primary and secondary pulmonary hypertension. Clin Pharmacol Ther 2002; 71:398–402.
- Michelakis E, Tymchak W, Lien D, et al. Oral sildenafil is an effective and specific pulmonary vasodilator in patients with pulmonary arterial hypertension: comparison with inhaled nitric oxide. Circulation 2002; 105:2398–2403.
- Ghofrani HA, Wiedemann R, Rose F, et al. Sildenafil for treatment of lung fibrosis and pulmonary hypertension: a randomised controlled trial. Lancet 2002; 360:895–900.
- Makisalo H, Koivusalo A, Vakkuri A, et al. Sildenafil for portopulmonary hypertension in a patient undergoing liver transplantation. Liver Transplant 2004; 10:945–950.
- Reichengerger F, Voswinckel R, Steveling E, et al. Sildenafil treatment for portopulmonary hypertension. Eur Respir J 2006; 28:563–567.
- Krowka MJ, Swanson KL. How should we treat portopulmonary hypertension? Eur Respir J 2006; 28:466–467.
- Kawut SM, Taichman DB, Ahya VN, et al. Hemodynamics and survival of patients with portopulmonary hypertension. Liver Transplant 2005; 11:1107–1111.
- Krowka MJ, Mandell MS, Ramsay MA, et al. Hepatopulmonary syndrome and portopulmonary hypertension: a report of the multicenter liver transplant database. Liver Transplant 2004; 10:174–182.
- Sulica R, Emre S, Poon M. Medical management of portopulmonary hypertension and right heart failure prior to living-related liver transplantation. Congest Heart Fail 2004; 10:192–194.
- De Wolf AM, Begliomini B, Gasior TA, et al. Right ventricular function during orthotopic liver transplantation. Anesthes Analges 1993; 76:562–568.
- Csete M. Intraoperative management of liver transplant patients with pulmonary hypertension. Liver Transplant Surg 1997; 3:454–455.
- Acosta F, Sansano T, Palenciano CG, et al. Portopulmonary hypertension and liver transplantation: hemodynamic consequences at reperfusion. Transplant Proc 2005; 37:3865–3866.
- Taura P, Garcia-Valdecasas JC, Beltran J, et al. Moderate primary pulmonary hypertension in patients undergoing liver transplantation. Anesthes Analges 1996; 83:675–680.
- Kuo PC, Schroeder RA, Vagelos RH, et al. Volume-mediated pulmonary responses in liver transplant candidates. Clin Transplant 1996; 10:521–527.
- Robalino BD, Moodie DS. Association between primary pulmonary hypertension and portal hypertension: analysis of its pathophysiology and clinical, laboratory and hemodynamic manifestations. J Am Coll Cardiol 1991; 17:492–498.
- Mandell MS, Krowka MJ. Formation of a national database on pulmonary hypertension and hepatopulmonary syndrome in chronic liver disease. Anesthesiology 1997; 87:450–451.
- Rodriguez-Roisin R, Krowka MJ, Hervé P, Fallon MB; ERS Task Force Pulmonary-Hepatic Vascular Disorders (PHD) Scientific Committee. Pulmonary-hepatic vascular disorders (PHD). Eur Respir J 2004; 24:861–880.
- Krowka MJ, Swanson KL, Frantz RP, et al. Portopulmonary hypertension: results from a 10-year screening algorithm. Hepatology 2006; 44:1502–1510.
- Simonneau G, Galie N, Rubin LJ, et al. Clinical classification of pulmonary hypertension. J Am Coll Cardiol 2004; 43:5S–12S.
- Hadengue A, Benhayoun MK, Lebrec D, et al. Pulmonary hypertension complicating portal hypertension: prevalence and relation to splanchnic hemodynamics. Gastroenterology 1991; 100:520–528.
- Krowka MJ, Plevak DJ, Findlay JY, et al. Pulmonary hemodynamics and perioperative cardiopulmonary-related mortality in patients with portopulmonary hypertension undergoing liver transplantation. Liver Transplant 2000; 6:443–450.
- Benjaminov FS, Prentice M, Sniderman KW, et al. Portopulmonary hypertension in decompensated cirrhosis with refractory ascites. Gut 2003; 52:1355–1362.
- McDonnell PJ, Toye PA, Hutchins GM. Primary pulmonary hypertension and cirrhosis: are they related? Am Rev Respir Dis 1983; 127:437–441.
- Cheng EY, Woehlck H. Pulmonary artery hypertension complicating anesthesia for liver transplantation. Anesthesiology 1992; 77:375–378.
- Castro M, Krowka MJ, Schroeder DR, et al. Frequency and clinical implications of increased pulmonary artery pressures in liver transplantation. Mayo Clin Proc 1996; 71:543–551.
- Ramsay MA, Simpson BR, Nguyen AT, et al. Severe pulmonary hypertension in liver transplant candidates. Liver Transplant Surg 1997; 3:494–500.
- Kiely DG, Cargill RI, Struthers AD, et al. Cardiopulmonary effects of endothelin-1 in man. Cardiovasc Res 1997; 33:378–386.
- Panos RJ, Baker SK. Mediators, cytokines, and growth factors in liver-lung interactions. Clin Chest Med 1996; 17:151–169.
- Higgenbottam T. Pathophysiology of pulmonary hypertension. Chest 1994; 105:7S–12S.
- Krowka MJ. Hepatopulmonary syndrome and portopulmonary hypertension: distinction and dilemmas. Hepatology 1997; 25:1282–1284.
- Hongqun L, Lee SS. Cardiopulmonary dysfunction in cirrhosis. Hepatology 2000; 14:600–608.
- Lebrec D, Brenot F, Simonneau G, et al. Pulmonary arterial hypertension in portal hypertension. Eur Respir J 1998; 11:1153–1166.
- Herve P, Lebrec D, Brenot F, et al. Pulmonary vascular disorders in portal hypertension. Eur Respir J 1998; 11:1153–1166.
- Tuder RM, Cool CD, Geraci MW, et al. Prostacyclin synthase expression is decreased in lungs from patients with severe pulmonary hypertension. Am J Respir Crit Care Med 1999; 159:1925–1932.
- Hoeper MM, Krowka MJ, Strassburg CP. Portopulmonary hypertension and hepatopulmonary syndrome. Lancet 2004; 363:1461–1468.
- Edwards B, Weir K, Edwards WD, et al. Coexistent pulmonary and portal hypertension: morphologic and clinical features. J Am Coll Cardiol 1987; 10:1233–1238.
- Ramsay MAE, Simpson BR, Nguyen AT, Ramsay KJ, East C, Klintmalm GB. Severe pulmonary hypertension in liver transplant candidates. Liver Transplant Surg 1997; 3:494–500.
- Trembath RC. Clinical and molecular genetic features of pulmonary hypertension in patients with hereditary hemorrhagic telangiectasia. N Engl J Med 2001; 345:325–334.
- Leuchte HH, Holzapfel M, Baumgartner RA, et al. Clinical significance of brain natriuretic peptide in primary pulmonary hypertension. J Am Coll Cardiol 2004; 43:764–770.
- Kim WR, Krowka MJ, Plevak DJ, et al. Accuracy of Doppler echocardiography in the assessment of pulmonary hypertension in liver transplant candidates. Liver Transplant 2000; 6:453–458.
- Colle IO, Moreau R, Godinho E, et al. Diagnosis of portopulmonary hypertension in candidates for liver transplantation: a prospective study. Hepatology 2003; 37:401–409.
- Provencher S, Herve P, Jais X, et al. Deleterious effects of beta-blockers on exercise capacity and hemodynamics in patients with portopulmonary hypertension. Gastroenterology 2006; 130:120–126.
- Swanson KL, McGoon MD, Krowka MJ. Survival in patients with portopulmonary hypertension [abstract]. Am J Respir Crit Care Med 2003; 167:A693.
- Kuo PC, Johnson LB, Plotkin JS, et al. Continuous intravenous infusion of epoprostenol for the treatment of portopulmonary hypertension. Transplantation 1997; 63:604–616.
- Krowka MJ, Frantz RP, McGoon MD, et al. Improvement in pulmonary hemodynamics during intravenous epoprostenol (prostacyclin): A study of 15 patients with moderate to severe portopulmonary hypertension. Hepatology 1999; 30:641–648.
- Kähler CM, Graziadei I, Wiedermann CJ, Kneussl MP, Vogel W. Successful use of continuous intravenous prostacyclin in a patient with severe portopulmonary hypertension. Wien Klin Wochenschr 2000; 112:637–640.
- Sussman N, Kaza V, Barshes N, et al. Successful liver transplantation following medical management of portopulmonary hypertension: a single-center series. Am J Transplant 2006; 6:2177–2182.
- Findlay JY, Plevak DJ, Krowka MJ, et al. Progressive splenomegaly after epoprostenol therapy in portopulmonary hypertension. Liver Transplant Surg 1999; 5:381–387.
- Rubin LJ, Roux S. Bosentan: a dual endothelin receptor antagonist. Expert Opin Invest Drugs 2002; 11:991–1002.
- Fattinger K, Funk C, Pantze M, et al. The endothelin antagonist bosentan inhibits the canalicular bile salt export pump: a potential mechanism for hepatic adverse reactions. Clin Pharmacol Ther 2001; 69:223–231.
- Hinterhuber L, Graziadei IW, Kahler CM, et al. Endothelin-receptor anatgonist treatment of portopulmonary hypertension. Clin Gastroenterol Hepatol 2004; 2:1039–1042.
- Clift PF, Townend JN, Bramhall S, et al. Successful treatment of severe portopulmonary hypertension after liver transplantation by bosentan. Transplantation 2004; 77:1774–1775.
- Halank M, Miehlke S, Hoeffken G, et al. Use of oral endothelin-receptor antagonist bosentan in the treatment of portopulmonary hypertension. Transplantation 2004; 77:1775–1776.
- Kuntzen C, Gulberg V, Gerbes AL. Use of a mixed endothelin receptor antagonist in portopulmonary hypertension: a safe and effective therapy? Gastroenterology 2005; 128:164–168.
- Watanabe H, Ohashi K, Takeuchi K, et al. Sildenafil for primary and secondary pulmonary hypertension. Clin Pharmacol Ther 2002; 71:398–402.
- Michelakis E, Tymchak W, Lien D, et al. Oral sildenafil is an effective and specific pulmonary vasodilator in patients with pulmonary arterial hypertension: comparison with inhaled nitric oxide. Circulation 2002; 105:2398–2403.
- Ghofrani HA, Wiedemann R, Rose F, et al. Sildenafil for treatment of lung fibrosis and pulmonary hypertension: a randomised controlled trial. Lancet 2002; 360:895–900.
- Makisalo H, Koivusalo A, Vakkuri A, et al. Sildenafil for portopulmonary hypertension in a patient undergoing liver transplantation. Liver Transplant 2004; 10:945–950.
- Reichengerger F, Voswinckel R, Steveling E, et al. Sildenafil treatment for portopulmonary hypertension. Eur Respir J 2006; 28:563–567.
- Krowka MJ, Swanson KL. How should we treat portopulmonary hypertension? Eur Respir J 2006; 28:466–467.
- Kawut SM, Taichman DB, Ahya VN, et al. Hemodynamics and survival of patients with portopulmonary hypertension. Liver Transplant 2005; 11:1107–1111.
- Krowka MJ, Mandell MS, Ramsay MA, et al. Hepatopulmonary syndrome and portopulmonary hypertension: a report of the multicenter liver transplant database. Liver Transplant 2004; 10:174–182.
- Sulica R, Emre S, Poon M. Medical management of portopulmonary hypertension and right heart failure prior to living-related liver transplantation. Congest Heart Fail 2004; 10:192–194.
- De Wolf AM, Begliomini B, Gasior TA, et al. Right ventricular function during orthotopic liver transplantation. Anesthes Analges 1993; 76:562–568.
- Csete M. Intraoperative management of liver transplant patients with pulmonary hypertension. Liver Transplant Surg 1997; 3:454–455.
- Acosta F, Sansano T, Palenciano CG, et al. Portopulmonary hypertension and liver transplantation: hemodynamic consequences at reperfusion. Transplant Proc 2005; 37:3865–3866.
- Taura P, Garcia-Valdecasas JC, Beltran J, et al. Moderate primary pulmonary hypertension in patients undergoing liver transplantation. Anesthes Analges 1996; 83:675–680.
- Kuo PC, Schroeder RA, Vagelos RH, et al. Volume-mediated pulmonary responses in liver transplant candidates. Clin Transplant 1996; 10:521–527.
- Robalino BD, Moodie DS. Association between primary pulmonary hypertension and portal hypertension: analysis of its pathophysiology and clinical, laboratory and hemodynamic manifestations. J Am Coll Cardiol 1991; 17:492–498.
- Mandell MS, Krowka MJ. Formation of a national database on pulmonary hypertension and hepatopulmonary syndrome in chronic liver disease. Anesthesiology 1997; 87:450–451.
KEY POINTS
- In portopulmonary hypertension, the pulmonary artery pressures, pulmonary vascular resistance, and portal venous pressure are all elevated.
- All candidates for liver transplantation should undergo echocardiography to screen for portopulmonary hypertension. If the echocardiogram shows elevated pulmonary pressures, right heart catheterization must be performed to confirm the diagnosis.
- The ideal medical regimen remains to be determined. Although drug treatment may lower pulmonary artery pressures in selected patients so that liver transplantation can be safely done, morbidity and mortality rates remain higher in patients with moderate to severe portopulmonary hypertension.
- Liver transplantation is not the treatment of choice for portopulmonary hypertension.
Should all patients with chronic kidney disease take a statin?
We think some patients with chronic kidney disease should take a statin, particularly those in stages 1 through 4 (ie, not yet on dialysis1)* who have low-density lipoprotein cholesterol (LDL-C) levels higher than 100 mg/dL. However, few studies have addressed this question.
*Stages of chronic kidney disease1:
Stage 1—kidney damage with normal or high glomerular filtration rate (GFR ≥ 90 mL/min/1.73 m2)
Stage 2—kidney damage with mildly decreased GFR (60–89 mL/min/1.73 m2)
Stage 3—moderately decreased GFR (30–59 mL/min/1.73 m2)
Stage 4—severely decreased GFR (15–29 mL/min/1.73 m2)
Stage 5—kidney failure (GFR < 15 mL/min/1.73 m2 or dialysis)
The answer is murkier in patients on dialysis. Only one study has been done in this population, and it found no benefit from statin therapy. However, we would prescribe a statin for a dialysis patient who had known coronary artery disease and an LDL-C level higher than 100 mg/dL.
RATIONALE FOR STATIN USE: KIDNEY PATIENTS ARE AT RISK
Cardiovascular disease is common among patients with chronic kidney disease. While the risks of cardiovascular disease and death are highest among those requiring dialysis, earlier stages of chronic kidney disease also are associated with cardiovascular disease.2–4
The prevalence of traditional risk factors, particularly diabetes and hypertension, is high in all stages of kidney disease, and dyslipidemia is extremely common. Patients with chronic kidney disease who are not on dialysis tend to have lower levels of high-density lipoprotein cholesterol and higher levels of triglycerides, lipoprotein remnants, lipoprotein(a), and LDL-C. The lipid profile of dialysis patients is more complex, as malnutrition and inflammation in this population may lead to low cholesterol levels.
Since statins are effective for primary and secondary prevention of cardiovascular events in those in the general population with high LDL-C,5 we could expect that the same holds true for patients with chronic kidney disease. Furthermore, if kidney disease were considered a coronary heart disease equivalent, more than 85% of those with stage 3, 4, or 5 disease would qualify for lipid-lowering therapy by LDL-C criteria.6
However, compared with the large body of evidence in those without kidney disease, we have few data on the effect of statins on cardiovascular outcomes in those with kidney disease. Five of seven major trials of statins excluded patients with chronic kidney disease by using a creatinine cutoff or by excluding patients with known kidney disease.7
Renoprotective effects
Besides their cardiovascular effects, statins may slow the progression of kidney disease.
A subgroup analysis of the Greek Atorvastatin and Coronary Heart Disease Evaluation (GREACE) trial8 showed a 12% increase in creatinine clearance in the group receiving atorvastatin (Lipitor) (P = .0001). In comparison, creatinine clearance decreased by 4% in the placebo group.
A subgroup analysis of the Cholesterol and Recurrent Events (CARE) trial, a secondary prevention trial of pravastatin (Pravachol) vs placebo, showed a similar effect for patients with a glomerular filtration rate (GFR) less than 60 mL/min/1.73 m2 at baseline.9
A meta-analysis of 27 randomized trials (39,704 participants) concluded that, compared with no treatment, statins slowed the loss of GFR by a mean of 1.22 mL/min/year (95% confidence interval 0.44–2.0).10
Statins may confer this benefit independently of lipid-lowering. These drugs seem to decrease proteinuria, possibly by improving endothelial function or decreasing inflammation.11 A meta-analysis (1,384 patients) noted that 13 of 15 published studies found an antiproteinuric effect, with a greater effect in those with greater baseline proteinuria.12
The Prospective Evaluation of Proteinuria and Renal Function in Diabetic Patients With Progressive Renal Disease Trial (PLANET) will enroll 345 diabetic patients with protein-uria and hypercholesterolemia and examine the effects of rosuvastatin (Crestor) and atorvastatin on proteinuria and GFR.13
Cardioprotective effects in stages 1–4
Since patients with chronic kidney disease were excluded from most of the major statin trials, the best evidence in those with non-dialysis-dependent disease comes from post hoc analysis of data from the CARE study.14 While this trial excluded patients with more than 2+ proteinuria on dipstick analysis and those with creatinine values greater than 1.5 times the upper limit of normal, 1,711 of the initial 4,159 patients had a creatinine clearance of less than 75 mL/min; the mean creatinine clearance in this subgroup was 61. In this subgroup, pravastatin therapy was associated with a significantly lower risk of cardiovascular death or recurrent nonfatal myocardial infarction (MI) (hazard ratio 0.72, P < 0.05).
Similarly, in the 4,491 patients with chronic kidney disease (mean GFR 55 mL/min/1.73 m2) in the Pravastatin Pooling Project, the hazard of new MI, cardiovascular death, or cardiac intervention was nearly 25% lower in the pravastatin group.15
The ongoing Study of Heart and Renal Protection (SHARP),16 a randomized trial of ezetimibe/simvastatin (Vytorin) that enrolled 6,000 people with stages 3 to 4 kidney disease and 3,000 dialysis patients, will help in determining whether statin therapy prevents new vascular events. The study was launched in 2003 and has now completed enrollment. The primary outcome measure will be the time to first vascular event; secondary analyses will address whether statins decrease proteinuria or slow the progression of kidney disease.
Cardioprotective effects in dialysis patients
The only major randomized trial of statins ever conducted in dialysis patients with diabetes, the German Diabetes and Dialysis Study (4D), did not find atorvastatin 20 mg to have any benefit compared with placebo in reducing a composite end point of death from cardiac causes, stroke, and nonfatal MI over a median of 4 years of follow-up, despite a decrease in LDL-C of over 40% in the treatment group.17 Adverse events were similar in the two groups. The lack of a detectable benefit may be due to differences in the cardiovascular milieu in dialysis patients, who may have more advanced disease, with preexisting cardiac remodeling and congestive heart failure, which may not be modified to the same extent by statin therapy. Alternatively, the dose of atorvastatin may have been too low, or 4 years of treatment may not be sufficient to detect a benefit in these patients.
An ongoing prospective, randomized, placebo-controlled trial in 3,000 hemodialysis patients, called A Study to Evaluate the Use of Rosuvastatin in Subjects on Regular Haemodialysis: an Assessment of Survival and Cardiovascular Events (AURORA),18 will help to clarify the role of statins in this population.
CONCLUSION
The National Kidney Foundation guidelines1,19 note that people with chronic kidney disease are at high risk of cardiovascular disease and therefore should be treated according to guidelines for treating traditional risk factors in high-risk groups. We believe that those with dyslipidemia who are in stages 1 through 4, particularly those with other risk factors for coronary heart disease, should receive a statin, with an LDL-C target of less than 100 mg/dL, even though we have few data from large trials focused on this population and even though LDL-C may not be the only reason to consider statin use. The pleiotropic effects of statins on proteinuria and progression of kidney function loss may be of benefit in this population as well, although we would not recommend starting a statin solely for these effects until more data are available.
Despite the negative results of the 4D trial, given the relative safety of statins and the lack of any trial data suggesting harm in patients with chronic kidney disease, in our practice we treat dialysis patients with known cardiovascular disease with a statin, with a target LDL-C level less than 100 mg/dL. In dialysis patients without known cardiovascular disease, the use of a statin is even more controversial, and decisions should be made on an individual basis.
Results from the SHARP, AURORA, and PLANET trials, each of which is focused on patients with chronic kidney disease, will help determine whether statins benefit patients at this stage of disease.
- National Kidney Foundation. K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis 2002; 39 suppl 1:S1–S266.
- Shlipak MG, Sarnak MJ, Katz R, et al. Cystatin C and the risk of death and cardiovascular events among elderly persons. N Engl J Med 2005; 352:2049–2060.
- Foley RN, Parfrey PS, Sarnak MJ. Clinical epidemiology of cardiovascular disease in chronic renal disease. Am J Kidney Dis 1998; 32 suppl 3:S112–S119.
- Manjunath G, Tighiouart H, Coresh J, et al. Level of kidney function as a risk factor for cardiovascular outcomes in the elderly. Kidney Int 2003; 63:1121–1129.
- Baigent C, Keech A, Kearney PM, et al Cholesterol Treatment Trialists’ (CTT) Collaborators. Efficacy and safety of cholesterol-lowering treatment: prospective meta-analysis of data from 90,056 participants in 14 randomised trials of statins. Lancet 2005; 366:1267–1278.
- Hyre AD, Fox CS, Astor BC, Cohen AJ, Muntner P. The impact of reclassifying moderate CKD as a coronary heart disease risk equivalent on the number of US adults recommended lipid-lowering treatment. Am J Kidney Dis 2007; 49:37–45.
- Coca SG, Krumholz HM, Garg AX, Parikh CR. Underrepresentation of renal disease in randomized controlled trials of cardiovascular disease. JAMA 2006; 296:1377–1384.
- Athyros VG, Mikhailidis DP, Papageorgiou AA, et al. The effect of statins versus untreated dyslipidaemia on renal function in patients with coronary heart disease. A subgroup analysis of the Greek atorvastatin and coronary heart disease evaluation (GREACE) study. J Clin Pathol 2004; 57:728–734.
- Tonelli M, Moye L, Sacks FM, Cole T, Curhan GC Cholesterol and Recurrent Events Trial Investigators. Effect of pravastatin on loss of renal function in people with moderate chronic renal insufficiency and cardiovascular disease. J Am Soc Nephrol 2003; 14:1605–1613.
- Sandhu S, Wiebe N, Fried LF, Tonelli M. Statins for improving renal outcomes: a meta-analysis. J Am Soc Nephrol 2006; 17:2006–2016.
- Balk EM, Lau J, Goudas LC, et al. Effects of statins on nonlipid serum markers associated with cardiovascular disease: a systematic review. Ann Intern Med 2003; 139:670–682.
- Douglas K, O’Malley PG, Jackson JL. Meta-analysis: the effect of statins on albuminuria. Ann Intern Med 2006; 145:117–124.
- US National Institutes of Health. Prospective Evaluation of Proteinuria and Renal Function in Diabetic Patients with Progressive Renal Disease (PLANET 1). Accessed December 6, 2007. www.clinicaltrials.gov/ct/show/NCT00296374?order=1.
- Tonelli M, Moye L, Sacks FM, Kiberd B, Curhan G Cholesterol and Recurrent Events (CARE) Trial Investigators. Pravastatin for secondary prevention of cardiovascular events in persons with mild chronic renal insufficiency. Ann Intern Med 2003; 138:98–104.
- Tonelli M, Isles C, Curhan GC, et al. Effect of pravastatin on cardiovascular events in people with chronic kidney disease. Circulation 2004; 110:1557–1563.
- Baigent C, Landry M. Study of Heart and Renal Protection (SHARP). Kidney Int Suppl 2003; 84:S207–S210.
- Wanner C, Krane V, Marz W, et al German Diabetes and Dialysis Study Investigators. Atorvastatin in patients with type 2 diabetes mellitus undergoing hemodialysis. N Engl J Med 2005; 353:238–248.
- Fellstrom BC, Holdaas H, Jardine AG. Why do we need a statin trial in hemodialysis patients? Kidney Int 2003; 84 suppl:S204–S206.
- KDOQI Clinical Practice Guidelines and Clinical Practice Recommendations for Diabetes and Chronic Kidney Disease. Am J Kidney Dis 2007; 49 suppl 2:S12–154.
We think some patients with chronic kidney disease should take a statin, particularly those in stages 1 through 4 (ie, not yet on dialysis1)* who have low-density lipoprotein cholesterol (LDL-C) levels higher than 100 mg/dL. However, few studies have addressed this question.
*Stages of chronic kidney disease1:
Stage 1—kidney damage with normal or high glomerular filtration rate (GFR ≥ 90 mL/min/1.73 m2)
Stage 2—kidney damage with mildly decreased GFR (60–89 mL/min/1.73 m2)
Stage 3—moderately decreased GFR (30–59 mL/min/1.73 m2)
Stage 4—severely decreased GFR (15–29 mL/min/1.73 m2)
Stage 5—kidney failure (GFR < 15 mL/min/1.73 m2 or dialysis)
The answer is murkier in patients on dialysis. Only one study has been done in this population, and it found no benefit from statin therapy. However, we would prescribe a statin for a dialysis patient who had known coronary artery disease and an LDL-C level higher than 100 mg/dL.
RATIONALE FOR STATIN USE: KIDNEY PATIENTS ARE AT RISK
Cardiovascular disease is common among patients with chronic kidney disease. While the risks of cardiovascular disease and death are highest among those requiring dialysis, earlier stages of chronic kidney disease also are associated with cardiovascular disease.2–4
The prevalence of traditional risk factors, particularly diabetes and hypertension, is high in all stages of kidney disease, and dyslipidemia is extremely common. Patients with chronic kidney disease who are not on dialysis tend to have lower levels of high-density lipoprotein cholesterol and higher levels of triglycerides, lipoprotein remnants, lipoprotein(a), and LDL-C. The lipid profile of dialysis patients is more complex, as malnutrition and inflammation in this population may lead to low cholesterol levels.
Since statins are effective for primary and secondary prevention of cardiovascular events in those in the general population with high LDL-C,5 we could expect that the same holds true for patients with chronic kidney disease. Furthermore, if kidney disease were considered a coronary heart disease equivalent, more than 85% of those with stage 3, 4, or 5 disease would qualify for lipid-lowering therapy by LDL-C criteria.6
However, compared with the large body of evidence in those without kidney disease, we have few data on the effect of statins on cardiovascular outcomes in those with kidney disease. Five of seven major trials of statins excluded patients with chronic kidney disease by using a creatinine cutoff or by excluding patients with known kidney disease.7
Renoprotective effects
Besides their cardiovascular effects, statins may slow the progression of kidney disease.
A subgroup analysis of the Greek Atorvastatin and Coronary Heart Disease Evaluation (GREACE) trial8 showed a 12% increase in creatinine clearance in the group receiving atorvastatin (Lipitor) (P = .0001). In comparison, creatinine clearance decreased by 4% in the placebo group.
A subgroup analysis of the Cholesterol and Recurrent Events (CARE) trial, a secondary prevention trial of pravastatin (Pravachol) vs placebo, showed a similar effect for patients with a glomerular filtration rate (GFR) less than 60 mL/min/1.73 m2 at baseline.9
A meta-analysis of 27 randomized trials (39,704 participants) concluded that, compared with no treatment, statins slowed the loss of GFR by a mean of 1.22 mL/min/year (95% confidence interval 0.44–2.0).10
Statins may confer this benefit independently of lipid-lowering. These drugs seem to decrease proteinuria, possibly by improving endothelial function or decreasing inflammation.11 A meta-analysis (1,384 patients) noted that 13 of 15 published studies found an antiproteinuric effect, with a greater effect in those with greater baseline proteinuria.12
The Prospective Evaluation of Proteinuria and Renal Function in Diabetic Patients With Progressive Renal Disease Trial (PLANET) will enroll 345 diabetic patients with protein-uria and hypercholesterolemia and examine the effects of rosuvastatin (Crestor) and atorvastatin on proteinuria and GFR.13
Cardioprotective effects in stages 1–4
Since patients with chronic kidney disease were excluded from most of the major statin trials, the best evidence in those with non-dialysis-dependent disease comes from post hoc analysis of data from the CARE study.14 While this trial excluded patients with more than 2+ proteinuria on dipstick analysis and those with creatinine values greater than 1.5 times the upper limit of normal, 1,711 of the initial 4,159 patients had a creatinine clearance of less than 75 mL/min; the mean creatinine clearance in this subgroup was 61. In this subgroup, pravastatin therapy was associated with a significantly lower risk of cardiovascular death or recurrent nonfatal myocardial infarction (MI) (hazard ratio 0.72, P < 0.05).
Similarly, in the 4,491 patients with chronic kidney disease (mean GFR 55 mL/min/1.73 m2) in the Pravastatin Pooling Project, the hazard of new MI, cardiovascular death, or cardiac intervention was nearly 25% lower in the pravastatin group.15
The ongoing Study of Heart and Renal Protection (SHARP),16 a randomized trial of ezetimibe/simvastatin (Vytorin) that enrolled 6,000 people with stages 3 to 4 kidney disease and 3,000 dialysis patients, will help in determining whether statin therapy prevents new vascular events. The study was launched in 2003 and has now completed enrollment. The primary outcome measure will be the time to first vascular event; secondary analyses will address whether statins decrease proteinuria or slow the progression of kidney disease.
Cardioprotective effects in dialysis patients
The only major randomized trial of statins ever conducted in dialysis patients with diabetes, the German Diabetes and Dialysis Study (4D), did not find atorvastatin 20 mg to have any benefit compared with placebo in reducing a composite end point of death from cardiac causes, stroke, and nonfatal MI over a median of 4 years of follow-up, despite a decrease in LDL-C of over 40% in the treatment group.17 Adverse events were similar in the two groups. The lack of a detectable benefit may be due to differences in the cardiovascular milieu in dialysis patients, who may have more advanced disease, with preexisting cardiac remodeling and congestive heart failure, which may not be modified to the same extent by statin therapy. Alternatively, the dose of atorvastatin may have been too low, or 4 years of treatment may not be sufficient to detect a benefit in these patients.
An ongoing prospective, randomized, placebo-controlled trial in 3,000 hemodialysis patients, called A Study to Evaluate the Use of Rosuvastatin in Subjects on Regular Haemodialysis: an Assessment of Survival and Cardiovascular Events (AURORA),18 will help to clarify the role of statins in this population.
CONCLUSION
The National Kidney Foundation guidelines1,19 note that people with chronic kidney disease are at high risk of cardiovascular disease and therefore should be treated according to guidelines for treating traditional risk factors in high-risk groups. We believe that those with dyslipidemia who are in stages 1 through 4, particularly those with other risk factors for coronary heart disease, should receive a statin, with an LDL-C target of less than 100 mg/dL, even though we have few data from large trials focused on this population and even though LDL-C may not be the only reason to consider statin use. The pleiotropic effects of statins on proteinuria and progression of kidney function loss may be of benefit in this population as well, although we would not recommend starting a statin solely for these effects until more data are available.
Despite the negative results of the 4D trial, given the relative safety of statins and the lack of any trial data suggesting harm in patients with chronic kidney disease, in our practice we treat dialysis patients with known cardiovascular disease with a statin, with a target LDL-C level less than 100 mg/dL. In dialysis patients without known cardiovascular disease, the use of a statin is even more controversial, and decisions should be made on an individual basis.
Results from the SHARP, AURORA, and PLANET trials, each of which is focused on patients with chronic kidney disease, will help determine whether statins benefit patients at this stage of disease.
We think some patients with chronic kidney disease should take a statin, particularly those in stages 1 through 4 (ie, not yet on dialysis1)* who have low-density lipoprotein cholesterol (LDL-C) levels higher than 100 mg/dL. However, few studies have addressed this question.
*Stages of chronic kidney disease1:
Stage 1—kidney damage with normal or high glomerular filtration rate (GFR ≥ 90 mL/min/1.73 m2)
Stage 2—kidney damage with mildly decreased GFR (60–89 mL/min/1.73 m2)
Stage 3—moderately decreased GFR (30–59 mL/min/1.73 m2)
Stage 4—severely decreased GFR (15–29 mL/min/1.73 m2)
Stage 5—kidney failure (GFR < 15 mL/min/1.73 m2 or dialysis)
The answer is murkier in patients on dialysis. Only one study has been done in this population, and it found no benefit from statin therapy. However, we would prescribe a statin for a dialysis patient who had known coronary artery disease and an LDL-C level higher than 100 mg/dL.
RATIONALE FOR STATIN USE: KIDNEY PATIENTS ARE AT RISK
Cardiovascular disease is common among patients with chronic kidney disease. While the risks of cardiovascular disease and death are highest among those requiring dialysis, earlier stages of chronic kidney disease also are associated with cardiovascular disease.2–4
The prevalence of traditional risk factors, particularly diabetes and hypertension, is high in all stages of kidney disease, and dyslipidemia is extremely common. Patients with chronic kidney disease who are not on dialysis tend to have lower levels of high-density lipoprotein cholesterol and higher levels of triglycerides, lipoprotein remnants, lipoprotein(a), and LDL-C. The lipid profile of dialysis patients is more complex, as malnutrition and inflammation in this population may lead to low cholesterol levels.
Since statins are effective for primary and secondary prevention of cardiovascular events in those in the general population with high LDL-C,5 we could expect that the same holds true for patients with chronic kidney disease. Furthermore, if kidney disease were considered a coronary heart disease equivalent, more than 85% of those with stage 3, 4, or 5 disease would qualify for lipid-lowering therapy by LDL-C criteria.6
However, compared with the large body of evidence in those without kidney disease, we have few data on the effect of statins on cardiovascular outcomes in those with kidney disease. Five of seven major trials of statins excluded patients with chronic kidney disease by using a creatinine cutoff or by excluding patients with known kidney disease.7
Renoprotective effects
Besides their cardiovascular effects, statins may slow the progression of kidney disease.
A subgroup analysis of the Greek Atorvastatin and Coronary Heart Disease Evaluation (GREACE) trial8 showed a 12% increase in creatinine clearance in the group receiving atorvastatin (Lipitor) (P = .0001). In comparison, creatinine clearance decreased by 4% in the placebo group.
A subgroup analysis of the Cholesterol and Recurrent Events (CARE) trial, a secondary prevention trial of pravastatin (Pravachol) vs placebo, showed a similar effect for patients with a glomerular filtration rate (GFR) less than 60 mL/min/1.73 m2 at baseline.9
A meta-analysis of 27 randomized trials (39,704 participants) concluded that, compared with no treatment, statins slowed the loss of GFR by a mean of 1.22 mL/min/year (95% confidence interval 0.44–2.0).10
Statins may confer this benefit independently of lipid-lowering. These drugs seem to decrease proteinuria, possibly by improving endothelial function or decreasing inflammation.11 A meta-analysis (1,384 patients) noted that 13 of 15 published studies found an antiproteinuric effect, with a greater effect in those with greater baseline proteinuria.12
The Prospective Evaluation of Proteinuria and Renal Function in Diabetic Patients With Progressive Renal Disease Trial (PLANET) will enroll 345 diabetic patients with protein-uria and hypercholesterolemia and examine the effects of rosuvastatin (Crestor) and atorvastatin on proteinuria and GFR.13
Cardioprotective effects in stages 1–4
Since patients with chronic kidney disease were excluded from most of the major statin trials, the best evidence in those with non-dialysis-dependent disease comes from post hoc analysis of data from the CARE study.14 While this trial excluded patients with more than 2+ proteinuria on dipstick analysis and those with creatinine values greater than 1.5 times the upper limit of normal, 1,711 of the initial 4,159 patients had a creatinine clearance of less than 75 mL/min; the mean creatinine clearance in this subgroup was 61. In this subgroup, pravastatin therapy was associated with a significantly lower risk of cardiovascular death or recurrent nonfatal myocardial infarction (MI) (hazard ratio 0.72, P < 0.05).
Similarly, in the 4,491 patients with chronic kidney disease (mean GFR 55 mL/min/1.73 m2) in the Pravastatin Pooling Project, the hazard of new MI, cardiovascular death, or cardiac intervention was nearly 25% lower in the pravastatin group.15
The ongoing Study of Heart and Renal Protection (SHARP),16 a randomized trial of ezetimibe/simvastatin (Vytorin) that enrolled 6,000 people with stages 3 to 4 kidney disease and 3,000 dialysis patients, will help in determining whether statin therapy prevents new vascular events. The study was launched in 2003 and has now completed enrollment. The primary outcome measure will be the time to first vascular event; secondary analyses will address whether statins decrease proteinuria or slow the progression of kidney disease.
Cardioprotective effects in dialysis patients
The only major randomized trial of statins ever conducted in dialysis patients with diabetes, the German Diabetes and Dialysis Study (4D), did not find atorvastatin 20 mg to have any benefit compared with placebo in reducing a composite end point of death from cardiac causes, stroke, and nonfatal MI over a median of 4 years of follow-up, despite a decrease in LDL-C of over 40% in the treatment group.17 Adverse events were similar in the two groups. The lack of a detectable benefit may be due to differences in the cardiovascular milieu in dialysis patients, who may have more advanced disease, with preexisting cardiac remodeling and congestive heart failure, which may not be modified to the same extent by statin therapy. Alternatively, the dose of atorvastatin may have been too low, or 4 years of treatment may not be sufficient to detect a benefit in these patients.
An ongoing prospective, randomized, placebo-controlled trial in 3,000 hemodialysis patients, called A Study to Evaluate the Use of Rosuvastatin in Subjects on Regular Haemodialysis: an Assessment of Survival and Cardiovascular Events (AURORA),18 will help to clarify the role of statins in this population.
CONCLUSION
The National Kidney Foundation guidelines1,19 note that people with chronic kidney disease are at high risk of cardiovascular disease and therefore should be treated according to guidelines for treating traditional risk factors in high-risk groups. We believe that those with dyslipidemia who are in stages 1 through 4, particularly those with other risk factors for coronary heart disease, should receive a statin, with an LDL-C target of less than 100 mg/dL, even though we have few data from large trials focused on this population and even though LDL-C may not be the only reason to consider statin use. The pleiotropic effects of statins on proteinuria and progression of kidney function loss may be of benefit in this population as well, although we would not recommend starting a statin solely for these effects until more data are available.
Despite the negative results of the 4D trial, given the relative safety of statins and the lack of any trial data suggesting harm in patients with chronic kidney disease, in our practice we treat dialysis patients with known cardiovascular disease with a statin, with a target LDL-C level less than 100 mg/dL. In dialysis patients without known cardiovascular disease, the use of a statin is even more controversial, and decisions should be made on an individual basis.
Results from the SHARP, AURORA, and PLANET trials, each of which is focused on patients with chronic kidney disease, will help determine whether statins benefit patients at this stage of disease.
- National Kidney Foundation. K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis 2002; 39 suppl 1:S1–S266.
- Shlipak MG, Sarnak MJ, Katz R, et al. Cystatin C and the risk of death and cardiovascular events among elderly persons. N Engl J Med 2005; 352:2049–2060.
- Foley RN, Parfrey PS, Sarnak MJ. Clinical epidemiology of cardiovascular disease in chronic renal disease. Am J Kidney Dis 1998; 32 suppl 3:S112–S119.
- Manjunath G, Tighiouart H, Coresh J, et al. Level of kidney function as a risk factor for cardiovascular outcomes in the elderly. Kidney Int 2003; 63:1121–1129.
- Baigent C, Keech A, Kearney PM, et al Cholesterol Treatment Trialists’ (CTT) Collaborators. Efficacy and safety of cholesterol-lowering treatment: prospective meta-analysis of data from 90,056 participants in 14 randomised trials of statins. Lancet 2005; 366:1267–1278.
- Hyre AD, Fox CS, Astor BC, Cohen AJ, Muntner P. The impact of reclassifying moderate CKD as a coronary heart disease risk equivalent on the number of US adults recommended lipid-lowering treatment. Am J Kidney Dis 2007; 49:37–45.
- Coca SG, Krumholz HM, Garg AX, Parikh CR. Underrepresentation of renal disease in randomized controlled trials of cardiovascular disease. JAMA 2006; 296:1377–1384.
- Athyros VG, Mikhailidis DP, Papageorgiou AA, et al. The effect of statins versus untreated dyslipidaemia on renal function in patients with coronary heart disease. A subgroup analysis of the Greek atorvastatin and coronary heart disease evaluation (GREACE) study. J Clin Pathol 2004; 57:728–734.
- Tonelli M, Moye L, Sacks FM, Cole T, Curhan GC Cholesterol and Recurrent Events Trial Investigators. Effect of pravastatin on loss of renal function in people with moderate chronic renal insufficiency and cardiovascular disease. J Am Soc Nephrol 2003; 14:1605–1613.
- Sandhu S, Wiebe N, Fried LF, Tonelli M. Statins for improving renal outcomes: a meta-analysis. J Am Soc Nephrol 2006; 17:2006–2016.
- Balk EM, Lau J, Goudas LC, et al. Effects of statins on nonlipid serum markers associated with cardiovascular disease: a systematic review. Ann Intern Med 2003; 139:670–682.
- Douglas K, O’Malley PG, Jackson JL. Meta-analysis: the effect of statins on albuminuria. Ann Intern Med 2006; 145:117–124.
- US National Institutes of Health. Prospective Evaluation of Proteinuria and Renal Function in Diabetic Patients with Progressive Renal Disease (PLANET 1). Accessed December 6, 2007. www.clinicaltrials.gov/ct/show/NCT00296374?order=1.
- Tonelli M, Moye L, Sacks FM, Kiberd B, Curhan G Cholesterol and Recurrent Events (CARE) Trial Investigators. Pravastatin for secondary prevention of cardiovascular events in persons with mild chronic renal insufficiency. Ann Intern Med 2003; 138:98–104.
- Tonelli M, Isles C, Curhan GC, et al. Effect of pravastatin on cardiovascular events in people with chronic kidney disease. Circulation 2004; 110:1557–1563.
- Baigent C, Landry M. Study of Heart and Renal Protection (SHARP). Kidney Int Suppl 2003; 84:S207–S210.
- Wanner C, Krane V, Marz W, et al German Diabetes and Dialysis Study Investigators. Atorvastatin in patients with type 2 diabetes mellitus undergoing hemodialysis. N Engl J Med 2005; 353:238–248.
- Fellstrom BC, Holdaas H, Jardine AG. Why do we need a statin trial in hemodialysis patients? Kidney Int 2003; 84 suppl:S204–S206.
- KDOQI Clinical Practice Guidelines and Clinical Practice Recommendations for Diabetes and Chronic Kidney Disease. Am J Kidney Dis 2007; 49 suppl 2:S12–154.
- National Kidney Foundation. K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis 2002; 39 suppl 1:S1–S266.
- Shlipak MG, Sarnak MJ, Katz R, et al. Cystatin C and the risk of death and cardiovascular events among elderly persons. N Engl J Med 2005; 352:2049–2060.
- Foley RN, Parfrey PS, Sarnak MJ. Clinical epidemiology of cardiovascular disease in chronic renal disease. Am J Kidney Dis 1998; 32 suppl 3:S112–S119.
- Manjunath G, Tighiouart H, Coresh J, et al. Level of kidney function as a risk factor for cardiovascular outcomes in the elderly. Kidney Int 2003; 63:1121–1129.
- Baigent C, Keech A, Kearney PM, et al Cholesterol Treatment Trialists’ (CTT) Collaborators. Efficacy and safety of cholesterol-lowering treatment: prospective meta-analysis of data from 90,056 participants in 14 randomised trials of statins. Lancet 2005; 366:1267–1278.
- Hyre AD, Fox CS, Astor BC, Cohen AJ, Muntner P. The impact of reclassifying moderate CKD as a coronary heart disease risk equivalent on the number of US adults recommended lipid-lowering treatment. Am J Kidney Dis 2007; 49:37–45.
- Coca SG, Krumholz HM, Garg AX, Parikh CR. Underrepresentation of renal disease in randomized controlled trials of cardiovascular disease. JAMA 2006; 296:1377–1384.
- Athyros VG, Mikhailidis DP, Papageorgiou AA, et al. The effect of statins versus untreated dyslipidaemia on renal function in patients with coronary heart disease. A subgroup analysis of the Greek atorvastatin and coronary heart disease evaluation (GREACE) study. J Clin Pathol 2004; 57:728–734.
- Tonelli M, Moye L, Sacks FM, Cole T, Curhan GC Cholesterol and Recurrent Events Trial Investigators. Effect of pravastatin on loss of renal function in people with moderate chronic renal insufficiency and cardiovascular disease. J Am Soc Nephrol 2003; 14:1605–1613.
- Sandhu S, Wiebe N, Fried LF, Tonelli M. Statins for improving renal outcomes: a meta-analysis. J Am Soc Nephrol 2006; 17:2006–2016.
- Balk EM, Lau J, Goudas LC, et al. Effects of statins on nonlipid serum markers associated with cardiovascular disease: a systematic review. Ann Intern Med 2003; 139:670–682.
- Douglas K, O’Malley PG, Jackson JL. Meta-analysis: the effect of statins on albuminuria. Ann Intern Med 2006; 145:117–124.
- US National Institutes of Health. Prospective Evaluation of Proteinuria and Renal Function in Diabetic Patients with Progressive Renal Disease (PLANET 1). Accessed December 6, 2007. www.clinicaltrials.gov/ct/show/NCT00296374?order=1.
- Tonelli M, Moye L, Sacks FM, Kiberd B, Curhan G Cholesterol and Recurrent Events (CARE) Trial Investigators. Pravastatin for secondary prevention of cardiovascular events in persons with mild chronic renal insufficiency. Ann Intern Med 2003; 138:98–104.
- Tonelli M, Isles C, Curhan GC, et al. Effect of pravastatin on cardiovascular events in people with chronic kidney disease. Circulation 2004; 110:1557–1563.
- Baigent C, Landry M. Study of Heart and Renal Protection (SHARP). Kidney Int Suppl 2003; 84:S207–S210.
- Wanner C, Krane V, Marz W, et al German Diabetes and Dialysis Study Investigators. Atorvastatin in patients with type 2 diabetes mellitus undergoing hemodialysis. N Engl J Med 2005; 353:238–248.
- Fellstrom BC, Holdaas H, Jardine AG. Why do we need a statin trial in hemodialysis patients? Kidney Int 2003; 84 suppl:S204–S206.
- KDOQI Clinical Practice Guidelines and Clinical Practice Recommendations for Diabetes and Chronic Kidney Disease. Am J Kidney Dis 2007; 49 suppl 2:S12–154.
Protecting Patients with HCV from Lymphoma
Erratum (2007;80:284-288)
What's Eating You? Pubic Lice (Pthirus pubis)
A shift in referral patterns for HIV/AIDS patients
- Funding for specific training programs on HIV/AIDS care should be targeted to community health centers, where there is sufficient volume of HIV patients and an already demonstrated expertise amongst clinical faculty.
Purpose With the rapid development (and complex prescribing patterns) of drugs for HIV/AIDS care, it is challenging for physicians to keep current. We conducted a follow-up study to a 1994 cohort study to see how care and referral patterns have changed over the last decade. In this study, we examined how family physicians in Massachusetts were caring for their HIV-infected patients, and explored whether FPs were referring more patients to specialists for care compared with a decade ago.
Methods We designed a cross-sectional survey as an 11-year follow-up to a previous study. It was mailed in 2005 to the active membership of the Massachusetts academy of Family Physicians.
Results Compared with the cohort of 1994, the number of HIV+ patients in individual practices remained about the same, but the number of practices with no AIDS patients was significantly higher. 85.3% of FPs noted that they were more likely to refer HIV/AIDS patients immediately compared with their own practice patterns a decade ago. In this study, 39.0% of current respondents referred HIV+ patients immediately, 57.0% co-managed patients, and 4.1% managed these patients alone (the data for the 1994 cohort was 7.0%, 45.8%, and 47.2%, respectively; P<.0001).
Similar changes were seen in regard to care patterns for AIDS patients. among the current cohort, 61.7% reported that they referred patients immediately, compared with only 18.3% in 1994; 36.8% noted that they co-managed these patients (vs 74.3% in 1994); and only 1.5% reported that they managed these patients alone (vs 7.4% in 1994; P<.0001).
Conclusions A significant shift amongst FPs with regard to their referral patterns for patients with HIV/AIDS has occurred over the last decade. The community health center has emerged as a resource for patients with HIV/AIDS. Funding for specific training programs on HIV/AIDS care should be targeted to community health centers.
In June 2008, it will be 27 years since the first reported clusters of Pneumocystis pneumonia cases, which were the earliest published reports of the HIV epidemic.1 There are now more than a million individuals infected with HIV in the United States.2
Since the first antiretroviral drug, azidothymidine (AZT), was approved in 1987, more than a dozen medications have been introduced to treat this illness.2 Care for patients with HIV/AIDS is rapidly evolving—so much so that the International AIDS Society publishes revised treatment guidelines every 2 years.3 The US Department of Health and Human Services also issues frequent guidelines for HIV care.4
Methods
Asking FPs about their HIV/AIDS management practices
This cross-sectional survey was designed as an 11-year follow-up to a previous research study, described in greater detail elsewhere.12
In June 2005, we obtained a membership listing from the Massachusetts Academy of Family Physicians (MAFP). Using the total design methodology described by Dillman,13 a cover letter and survey instrument were mailed to current MAFP members (N=777). Nonresponders were sent a reminder postcard 2 weeks later, and a second survey 2 weeks after that. A final reminder and survey were mailed to the remaining nonrespondents a month later. No incentives were offered for the completion of the survey.
The survey instrument was developed using the original data collection tool from 1994, supplemented by questions assessing any changes in patient management over the previous 11 years. The 31-item survey included questions about the sociodemographic and practice characteristics of the respondents, their patient mix, their education, and their management of patients with HIV/AIDS, as well as a series of Likert-type attitudinal questions assessing their knowledge, access to specialists, and safety concerns regarding HIV.
Outcomes measured: Changes in care and referral patterns
The main outcomes of the survey were changes in the management of patients with HIV/AIDS, particularly regarding referral patterns compared with the previous decade. Univariate and bivariate statistics, frequency and percentile distributions, as well as means and medians, were used to characterize the physician cohort, their practice characteristics, referral patterns, and attitudes. We also examined a variety of relationships (eg, differences by gender, years of practice, and involvement in teaching), including changes from the data we collected in 1994 (using a repeated cross-sectional design approach).
Data were analyzed using SPSS for Windows, version 14 (SPSS, Inc, Chicago, Ill). Depending on the categorical or continuous nature of the data, chi-square and t-tests were used to assess significance at the .05 level. These bivariate analyses were subsequently used to select which independent variables we would include in the stepwise logistic regression analyses.
This study was reviewed by the University of Massachusetts’ Institutional Review Board for the conduct of human subject research and received an exemption from formal review.
Results
Who were the respondents?
In all, 369 completed surveys were returned, along with 44 uncompleted surveys (returned as undeliverable or with an indication that the respondent was no longer practicing), for a response rate of 53.2% (413/777) and a completion rate of 47.4% (369/777).
The respondents included an equal representation of male and female physicians. The majority practiced in non- urban settings (62.6%), and nearly one half (44.8%) had finished their residency since the first survey was sent. Half (54.9%) participated in a solo or single-specialty practice. FPs more often reported teaching medical students regularly (52.9%) than being involved in residency education (30.9%). More than a third (34.2%) reported that ≥15% of their patients participated in “high-risk” behaviors for HIV. (The definition of high-risk was left to the individual respondent.)
Compared with our earlier survey, respondents are now more likely to be female, more likely to be practicing in an urban setting, and slightly more likely to teach medical students. Current respondents also reported that they had fewer patients involved in high-risk behaviors.
The majority (63.8%) indicated that they had at least one HIV+ patient in their practice, with 39.0% also reporting at least one AIDS patient. Compared with the previous cohort studied, HIV+ patient numbers remained about the same; however, the number of practices caring for at least 1 AIDS patient was significantly lower (TABLE 1).
TABLE 1
Demographics of FPs responding (1994 vs 2005)
| SOCIODEMOGRAPHIC AND PRACTICE FACTORS | 1994 SURVEY (N=281)* N(%) | 2005 SURVEY (N=389)* N(%) | P VALUE |
|---|---|---|---|
| Gender | |||
| Male | 205 (74.0%) | 188 (50.9%) | <.0001 |
| Female | 72 (26.0%) | 181 (49.1%) | |
| Year of residency completion | |||
| After 1994 | — | 163 (44.8%) | not tested |
| 1986—1994 | 117 (44.5%) | 107 (29.4%) | |
| Before 1986 | 146 (55.5%) | 94 (25.8%) | |
| Practice location | |||
| Urban | 81 (29.0%) | 135 (37.4%) | |
| Suburban/rural | 198 (71.0%) | 226 (62.6%) | .026 |
| Practice type | |||
| Solo/single specialty | 169 (61.0%) | 200 (54.9%) | |
| Multispecialty/HMO/CHC | 90 (32.5%) | 134 (36.8%) | |
| Hospital-based clinic | 18 (6.5%) | 30 (8.2%) | .289 |
| Teach medical students regularly | |||
| Yes | 127 (45.7%) | 193 (52.9%) | |
| No | 151 (54.3%) | 172 (47.1%) | .071 |
| Teach residents regularly | |||
| Yes | 72 (25.9%) | 112 (30.9%) | |
| No | 206 (74.1%) | 251 (69.1%) | .169 |
| % of patients who engage in high-risk behavior | |||
| ≥15% | 74 (29.4%) | 123 (34.2%) | |
| 6%–14% | 75 (29.8%) | 140 (38.9%) | |
| ≤5% | 103 (40.9%) | 97 (26.9%) | .0012 |
| Number of HIV+ patients in practice | |||
| ≥3 patients | 88 (31.4%) | 89 (24.5%) | |
| 1–2 patients | 103 (36.8%) | 143 (39.3%) | |
| None | 89 (31.8%) | 132 (36.3%) | .136 |
| Number of AIDS patients in practice | |||
| ≥3 patients | 43 (15.5%) | 47 (12.9%) | |
| 1–2 patients | 95 (34.2%) | 95 (26.1%) | |
| None | 140 (50.3%) | 222 (61.0%) | .025 |
| HMO, health maintenance organization; CHC, community health center. | |||
| * Some numbers may not total to the individual cohort N because of sporadic missing data. The response rate for the 1994 survey was 60% and for the 2005 survey it was 50%. Percentages are based on the number of responders to each question, not the total number of respondents. | |||
HIV/AIDS care and changing referral patterns
When asked how referral patterns had changed over the past decade, the overwhelming majority (94.4%) reported being far less likely to manage HIV/AIDS patients alone. Just over half (56.2%) of the physicians in the current survey indicated being more likely to co-manage patients, and an impressive 85.3% noted that they were more likely to refer patients immediately compared with their own practice patterns a decade ago.
Changing care patterns for asymptomatic HIV+ patients. A total of 39.0% of current respondents referred patients immediately, 57.0% co-managed patients, while only 4.1% managed these patients alone (the data for the 1994 cohort was 7.0%, 45.8%, and 47.2%, respectively; P<.0001).
AIDS patients have similar patterns. Similar changes were seen in regard to care patterns for AIDS patients. Among the current cohort, 61.7% reported that they referred these patients immediately, compared with only 18.3% in 1994; 36.8% noted that they co-managed these patients (vs 74.3% in 1994); and only 1.5% reported that they managed these patients alone (vs 7.4% in 1994; P<.0001).
Use of resources. When they were asked which resources they used to help provide care for HIV patients, 39.9% indicated an HIV clinic at the closest teaching hospital (vs 28.4% in 1994); 41.4% noted a specialist at the community hospital where they practiced (vs 52.6% in 1994); and the remainder were fairly equally distributed (in both cohort years) between a local community health center that treats HIV+ patients, a consultant requested by the patient, or an FP colleague (P=.0003).
Which FPs are likely to refer? Given the small number of respondents who manage asymptomatic HIV+ and AIDS patients alone, we focused our analyses between those who refer immediately and those who co-manage care.
Those who reported referring asymptomatic HIV+ patients immediately were less likely to have ≥3 HIV+ or ≥3 AIDS patients in their current practice. They were significantly more likely to be in a group practice and significantly less likely to work at a community health center. They also reported being less likely to teach medical students and residents (TABLE 2).
Similar findings in referral patterns were also seen in the management of symptomatic HIV+/AIDS patients (TABLE 3). In addition, those physicians who immediately refer AIDS patients were also less likely to report that >15% of their patients were involved in high-risk behaviors.
TABLE 2
Care of asymptomatic HIV+ patients—which FPs refer, which co-manage?
| REFER IMMEDIATELY (N=134)* | CO-MANAGE (N=196*) | P VALUE | |
|---|---|---|---|
| Practice type | |||
| Solo practice | 24 (17.9%) | 30 (15.6%) | .001 |
| Single specialty | 58 (43.3%) | 70 (36.5%) | |
| HMO | 3 (2.2%) | 0 (0.0%) | |
| Multispecialty | 22 (16.4%) | 20 (10.4%) | |
| CHC | 14 (10.4%) | 57 (29.7%) | |
| Hospital-based clinic | 13 (9.7%) | 15 (7.8%) | |
| Teach medical students regularly | |||
| Yes | 51 (38.1%) | 118 (61.5%) | <.001 |
| No | 83 (61.9%) | 74 (38.5%) | |
| Teach residents regularly | |||
| Yes | 20 (15.0%) | 78 (40.8%) | <.001 |
| No | 113 (85.0%) | 113 (59.2%) | |
| Number of HIV+ patients in practice | |||
| <3 patients | 121 (91.0%) | 132 (68.0%) | <.001 |
| ≥3 patients | 12 (9.0%) | 62 (32.0%) | |
| Number of AIDS patients in practice | |||
| <3 patients | 128 (96.2%) | 165 (85.1%) | .001 |
| ≥3 patients | 5 (3.8%) | 29 (14.9%) | |
| % of patients who engage in high-risk behavior | |||
| 0%—5% | 44 (33.1%) | 44 (23.0%) | .133 |
| 6%—15% | 46 (34.6%) | 78 (40.8%) | |
| >15% | 43 (32.3%) | 69 (36.1%) | |
| HMO, health maintenance organization; CHC, community health center. | |||
| * Total N=369. We removed those physicians who noted “manage alone” because of the small sample size. Each item’s total may not be the total number of respondents due to unanswered questions. Percentages are based on the total number of responders to each question, not the total number of respondents. | |||
Who is caring for HIV/AIDS patients? Those FPs who reported an increase over 11 years in the number of patients with HIV/AIDS in their practices were more likely to practice in a community health center (P<.001), and were more likely to teach medical students (P=.002) and residents (P<.001). Additionally, these FPs reported a higher percentage of patients with high-risk behaviors (P=.008). These FPs were less likely to report that they didn’t have time to care for HIV/AIDS patients (P=.037). They felt more knowledgeable about HIV (P=.005) and AIDS care (P<.001), and were more likely to learn about HIV/AIDS care through formal CME (P=.001).
In contrast, those FPs with <10 HIV/AIDS patients in their practice were more likely to be in rural practices (P=.006), to have been in practice longer (mean, 14.75 vs 12.35 years; P=.042), and to teach medical students (P=.045). There were no differences noted between gender, practice arrangement, or residency education.
TABLE 3
Care of symptomatic HIV+/AIDS patients—which FPs refer, which co-manage?
| Refer IMMEDIATELY (N=134)* | CO-MANAGE (n=196)* | P VALUE | |
|---|---|---|---|
| Practice type | |||
| Solo practice | 37 (17.7%) | 17 (13.7%) | <.001 |
| Single specialty | 91 (43.5%) | 42 (33.9%) | |
| HMO | 3 (1.4%) | 0 (0.0%) | |
| Multispecialty | 34 (16.3%) | 10 (8.1%) | |
| CHC | 25 (12.0%) | 46 (37.1%) | |
| Hospital-based clinic | 19 (9.1%) | 9 (7.2%) | |
| Teach medical students regularly | |||
| Yes | 92 (43.6%) | 82 (67.2%) | |
| No | 119 (56.4%) | 40 (32.8%) | <.001 |
| Teach residents regularly | |||
| Yes | 40 (19.1%) | 60 (49.2%) | |
| No | 169 (80.9%) | 62 (50.8%) | <.001 |
| Number of HIV+ patients in practice | |||
| <3 patients | 180 (86.1%) | 77 (61.6%) | |
| ≥3 patients | 29 (13.9%) | 48 (38.4%) | <.001 |
| Number of AIDS patients in practice | |||
| <3 patients | 203 (97.1%) | 93 (74.4%) | |
| ≥3 patients | 6 (2.9%) | 32 (25.6%) | <.001 |
| % of patients who engage in high-risk behavior | |||
| 0%—5% | 67 (32.1%) | 21 (17.2%) | |
| 6%—15% | 83 (39.7%) | 49 (40.2%) | |
| >15% | 59 (28.2%) | 52 (42.6%) | .004 |
| HMO, health maintenance organization; CHC, community health center. | |||
| * Total N=369. We removed those physicians who noted “manage alone” because of small sample size. Each item’s total may not be the total number of respondents due to unanswered questions. Percentages are based on the total number of responders to each question, not the total number of respondents. | |||
Multivariate analyses
To identify factors that contributed the most to the immediate referral of asymptomatic HIV+ patients, we employed a stepwise logistic regression analysis based on the results of our bivariate analyses.
FPs were less likely to refer immediately if they were female, practiced in a community health center, had a higher number of HIV patients in their practice, learned about HIV/AIDS care during residency as well as through formal CME programs, taught medical students regularly, and felt more knowledgeable about HIV/AIDS care. They were more likely to refer these patients if they reported having no time to care for HIV/AIDS patients. A similar model was observed for the referral of symptomatic AIDS patients (TABLE 4).
TABLE 4
Multivariate analyses of factors related to referring patients immediately vs co-managing
| INDEPENDENT FACTORS | IMEDIATE REFERRALS FOR ASYMPT OMATIC HIV+ PATIENTS (N=297) OR * (95% CI ) | IMEDIATE REFERRALS FOR SYMPT OMATIC HIV+/AIDS PATIENTS (N=302) OR* (95% CI ) |
|---|---|---|
| Gender | ||
| Male | 1.0 | 1.0 |
| Female | 0.505 (0.283–0.901) | 0.311 (0.168–0.576) |
| Practice location: Community health center | ||
| No | 1.0 | ns† |
| Yes | 0.402 (0.178–0.910) | |
| Number of HIV+ patients in practice | ||
| 4-level ordinal variable: | ||
| 1=None; 2=1-2; 3=3-10; 4≥10 | 0.637 (0.440–0.923) | not in model |
| Number of AIDS patients in practice | ||
| 4-level ordinal variable: | ns† | 0.514 (0.343–0.769) |
| 1=None; 2=1-2; 3=3-10; 4≥10 | ||
| Learned about HIV/AIDS care during residency | ||
| No | 1.0 | ns† |
| Yes | 0.476 (0.243–0.930) | |
| Learned about HIV/AIDS care from formal CME programs | ||
| No | 1.0 | 1.0 |
| Yes | 0.468 (0.257–0.851) | 0.375 (0.204–0.691) |
| Participate in teaching medical students regularly | ||
| No | 1.0 | 1.0 |
| Yes | 0.417 (0.238–0.732) | 0.531 (0.281–1.003) |
| Participate in teaching residents regularly | ||
| No | ns† | 1.0 |
| Yes | 0.537 (0.279–1.035) | |
| Feel knowledgeable about HIV care | ||
| No | 1.0 | 1.0 |
| Yes | 0.345 (0.187–0.638) | 0.357 (0.176–0.728) |
| Have no time in my practice to care for HIV/AIDS patients | ||
| No | 1.0 | 1.0 |
| Yes | 2.076 (1.155–3.729) | 4.306 (2.098–8.838) |
| * An OR of 1.0 reflects the referent category within each of the independent factors. | ||
| † ns = not significant in the stepwise regression model. | ||
| OR, odds ratio; CI, confidence interval; CME, continuing medical education. | ||
Discussion
Referral patterns change with demographics and new treatments
In comparing data with our survey from 1994, we found significant differences in care and referral patterns for HIV/AIDS patients.
FPs are more likely to refer, and right away. FPs are more likely to refer HIV patients immediately, compared with a decade ago; this likely results from many factors. The complexity of this disease and the rapid rate of change in management have been well documented.3
Keeping up-to-date with current practice guidelines and managing complications of treatment protocols can be time-consuming. Also, more physicians in our current survey reported having no AIDS patients in their practices compared with 1994.
The emergence of community health centers. Another interesting finding, based on the results of both our bivariate and multivariate analyses, was to see the community health center emerge as a resource for patients with HIV/AIDS. This may reflect the more urban location of community health centers and the higher prevalence of HIV/AIDS patients in those locales, an increased involvement with teaching, and an increased volume of patients with HIV/AIDS—all resulting in an increased knowledge of HIV/AIDS care. Additionally, community health centers are capable of providing a more comprehensive range of services than a traditional practice, through the support of the Federal Ryan White CARE Act. This likely plays a role in the increasing numbers of such patients being cared for in this setting.
Implications for future training of FPs. Optimal care of HIV patients requires a combination of disease-specific expertise and primary care skills and organization.14 Recent literature demonstrates that generalist physicians are able to develop condition-specific knowledge similar to those with specialty training—if they have a substantial caseload, and if they make an effort to stay current in a particular area.15 Residency training sites, particularly community health centers, will likely emerge as leaders in the training of primary care physicians to care for this disease. The ongoing expertise of faculty in these sites will be a vital aspect of this training.
Limitations of this study Our survey was limited to members of the MAFP, and may not be generalizable to other primary care providers. It also may not be generalizable to other states, given the demographics of Massachusetts and the availability of health care in a more urban environment. The availability of HIV resources and referral centers may vary from state to state.
The survey relied upon self-report and may be prone to either over- or under-reporting of current practice and recall of changes over the past decade.
Also, a higher response rate among male FPs, with females being less likely to refer patients, may have understated the relationship between gender and referral patterns for these patients.
Quality of care? it’s still a question. The quality of care provided by the subset of family physicians that are caring for their HIV/AIDS patients was not studied in this article. As this group continues to train new physicians and provide ongoing care for these patients, it will be important to measure the quality of care being provided.9,16
Will the role of the FP in HIV/AIDS care expand?
Our study demonstrates a significant shift amongst FPs with regard to their referral patterns for patients with HIV/AIDS over the last decade. This overall shift likely reflects the complexity of caring for these patients.
However, as these patients have longer survival rates, primary care offices will likely be seeing more individuals with HIV disease. While these patients may be followed by specialists, the role of the primary care physician in providing care may well expand. Funding for specific training programs on HIV/AIDS care should be targeted to community health centers where there is sufficient volume of HIV patients and an already demonstrated expertise amongst clinical faculty.
Acknowledgments
We gratefully acknowledge the survey implementation and data entry efforts of Denise West and the expert review by Jeff Baxter, MD.
Correspondence
Philip O. Fournier, MD, University of Massachusetts Medical School, 55 Lake Avenue North, Worcester, MA 01655; [email protected]
1. Centers for Disease Control and Prevention. Pneumocystis pneumonia—Los Angeles. MMWR Morb Mortal Wkly Rep 1981;30:250-252.
2. Frame PT. HIV disease in primary care. Prim Care 2004;30:205-237.
3. Hammer SM, Saag MS, Schechter M, et al. Treatment for adult HIV infection 2006. Recommendations of the International AIDS Society—USA Panel. JAMA 2006;296:827-843.
4. US Department of Health and Human Services. Guidelines and Standards. Available at: www.aids.gov/treatment/guidelines/index.html. Accessed January 17, 2008.
5. Sherer RI, Stieglitz K, Narra J, et al. HIV multidisciplinary teams work: Support services improve access to and retention in HIV primary care. AIDS Care 2002;14(Suppl 1):S31-S44.
6. Kitahata MM, Van Rompaey SE, Dillingham PW, et al. Primary care delivery is associated with greater physician experience and improved survival among persons with AIDS. J Gen Intern Med 2003;18:95-103.
7. Sackoff JE, Hanna DB, Pfeiffer MR, Torian L. Causes of death among persons with AIDS in the era of highly active antiretroviral therapy: New york City. Ann Intern Med 2006;145:397-406.
8. Kirchner J. Who should care for patients with HIV/AIDS? Am Fam Physician 2006;73:290-291.
9. Landon BE, Wilson IB, McInnes K, et al. Physician specialization and the quality of care for human immunodeficiency virus infection. Arch Intern Med 2005;165:1133-1139.
10. Gemson DH, Colombotos J, Elinson J, et al. Acquired immunodeficiency syndrome prevention: Knowledge, attitudes, and practices of primary care physicians. Arch Intern Med 1991;151:1102-1108.
11. Skiest DJ, Keiser P. Human immunodeficiency virus infection in patients older than 50 years: A survey of primary care physicians’ beliefs, practices, and knowledge. Arch Intern Med 1991;151:1102-1108.
12. Fournier PO, Baldor RA, Warfield ME, Frazier B. Patients with HIV/AIDS: Physicians’ knowledge, attitudes, and referral practices. J Fam Pract 1997;44:85-89.
13. Dillman DA. Mail and Internet Surveys: The Tailored Design Method. Hoboken, NJ: John Wiley & Sons; 2000.
14. Hecht FM, Wilson IB, Wu AW, et al. Optimizing care for persons with HIV infection. Ann Intern Med 1999;131:136-143.
15. Wilson IB, Landon BE, Ding L, et al. A national study of the relationship of care site HIV specialization to early adoption of highly active antiretroviral therapy. Med Care 2005;43:12-20.
16. Wilson IB, Landon BE, Hirschhorn LR, et al. Quality of HIV care provided by nurse practitioners, physicians assistants, and physicians. Ann Intern Med 2005;143:729-736.
- Funding for specific training programs on HIV/AIDS care should be targeted to community health centers, where there is sufficient volume of HIV patients and an already demonstrated expertise amongst clinical faculty.
Purpose With the rapid development (and complex prescribing patterns) of drugs for HIV/AIDS care, it is challenging for physicians to keep current. We conducted a follow-up study to a 1994 cohort study to see how care and referral patterns have changed over the last decade. In this study, we examined how family physicians in Massachusetts were caring for their HIV-infected patients, and explored whether FPs were referring more patients to specialists for care compared with a decade ago.
Methods We designed a cross-sectional survey as an 11-year follow-up to a previous study. It was mailed in 2005 to the active membership of the Massachusetts academy of Family Physicians.
Results Compared with the cohort of 1994, the number of HIV+ patients in individual practices remained about the same, but the number of practices with no AIDS patients was significantly higher. 85.3% of FPs noted that they were more likely to refer HIV/AIDS patients immediately compared with their own practice patterns a decade ago. In this study, 39.0% of current respondents referred HIV+ patients immediately, 57.0% co-managed patients, and 4.1% managed these patients alone (the data for the 1994 cohort was 7.0%, 45.8%, and 47.2%, respectively; P<.0001).
Similar changes were seen in regard to care patterns for AIDS patients. among the current cohort, 61.7% reported that they referred patients immediately, compared with only 18.3% in 1994; 36.8% noted that they co-managed these patients (vs 74.3% in 1994); and only 1.5% reported that they managed these patients alone (vs 7.4% in 1994; P<.0001).
Conclusions A significant shift amongst FPs with regard to their referral patterns for patients with HIV/AIDS has occurred over the last decade. The community health center has emerged as a resource for patients with HIV/AIDS. Funding for specific training programs on HIV/AIDS care should be targeted to community health centers.
In June 2008, it will be 27 years since the first reported clusters of Pneumocystis pneumonia cases, which were the earliest published reports of the HIV epidemic.1 There are now more than a million individuals infected with HIV in the United States.2
Since the first antiretroviral drug, azidothymidine (AZT), was approved in 1987, more than a dozen medications have been introduced to treat this illness.2 Care for patients with HIV/AIDS is rapidly evolving—so much so that the International AIDS Society publishes revised treatment guidelines every 2 years.3 The US Department of Health and Human Services also issues frequent guidelines for HIV care.4
Methods
Asking FPs about their HIV/AIDS management practices
This cross-sectional survey was designed as an 11-year follow-up to a previous research study, described in greater detail elsewhere.12
In June 2005, we obtained a membership listing from the Massachusetts Academy of Family Physicians (MAFP). Using the total design methodology described by Dillman,13 a cover letter and survey instrument were mailed to current MAFP members (N=777). Nonresponders were sent a reminder postcard 2 weeks later, and a second survey 2 weeks after that. A final reminder and survey were mailed to the remaining nonrespondents a month later. No incentives were offered for the completion of the survey.
The survey instrument was developed using the original data collection tool from 1994, supplemented by questions assessing any changes in patient management over the previous 11 years. The 31-item survey included questions about the sociodemographic and practice characteristics of the respondents, their patient mix, their education, and their management of patients with HIV/AIDS, as well as a series of Likert-type attitudinal questions assessing their knowledge, access to specialists, and safety concerns regarding HIV.
Outcomes measured: Changes in care and referral patterns
The main outcomes of the survey were changes in the management of patients with HIV/AIDS, particularly regarding referral patterns compared with the previous decade. Univariate and bivariate statistics, frequency and percentile distributions, as well as means and medians, were used to characterize the physician cohort, their practice characteristics, referral patterns, and attitudes. We also examined a variety of relationships (eg, differences by gender, years of practice, and involvement in teaching), including changes from the data we collected in 1994 (using a repeated cross-sectional design approach).
Data were analyzed using SPSS for Windows, version 14 (SPSS, Inc, Chicago, Ill). Depending on the categorical or continuous nature of the data, chi-square and t-tests were used to assess significance at the .05 level. These bivariate analyses were subsequently used to select which independent variables we would include in the stepwise logistic regression analyses.
This study was reviewed by the University of Massachusetts’ Institutional Review Board for the conduct of human subject research and received an exemption from formal review.
Results
Who were the respondents?
In all, 369 completed surveys were returned, along with 44 uncompleted surveys (returned as undeliverable or with an indication that the respondent was no longer practicing), for a response rate of 53.2% (413/777) and a completion rate of 47.4% (369/777).
The respondents included an equal representation of male and female physicians. The majority practiced in non- urban settings (62.6%), and nearly one half (44.8%) had finished their residency since the first survey was sent. Half (54.9%) participated in a solo or single-specialty practice. FPs more often reported teaching medical students regularly (52.9%) than being involved in residency education (30.9%). More than a third (34.2%) reported that ≥15% of their patients participated in “high-risk” behaviors for HIV. (The definition of high-risk was left to the individual respondent.)
Compared with our earlier survey, respondents are now more likely to be female, more likely to be practicing in an urban setting, and slightly more likely to teach medical students. Current respondents also reported that they had fewer patients involved in high-risk behaviors.
The majority (63.8%) indicated that they had at least one HIV+ patient in their practice, with 39.0% also reporting at least one AIDS patient. Compared with the previous cohort studied, HIV+ patient numbers remained about the same; however, the number of practices caring for at least 1 AIDS patient was significantly lower (TABLE 1).
TABLE 1
Demographics of FPs responding (1994 vs 2005)
| SOCIODEMOGRAPHIC AND PRACTICE FACTORS | 1994 SURVEY (N=281)* N(%) | 2005 SURVEY (N=389)* N(%) | P VALUE |
|---|---|---|---|
| Gender | |||
| Male | 205 (74.0%) | 188 (50.9%) | <.0001 |
| Female | 72 (26.0%) | 181 (49.1%) | |
| Year of residency completion | |||
| After 1994 | — | 163 (44.8%) | not tested |
| 1986—1994 | 117 (44.5%) | 107 (29.4%) | |
| Before 1986 | 146 (55.5%) | 94 (25.8%) | |
| Practice location | |||
| Urban | 81 (29.0%) | 135 (37.4%) | |
| Suburban/rural | 198 (71.0%) | 226 (62.6%) | .026 |
| Practice type | |||
| Solo/single specialty | 169 (61.0%) | 200 (54.9%) | |
| Multispecialty/HMO/CHC | 90 (32.5%) | 134 (36.8%) | |
| Hospital-based clinic | 18 (6.5%) | 30 (8.2%) | .289 |
| Teach medical students regularly | |||
| Yes | 127 (45.7%) | 193 (52.9%) | |
| No | 151 (54.3%) | 172 (47.1%) | .071 |
| Teach residents regularly | |||
| Yes | 72 (25.9%) | 112 (30.9%) | |
| No | 206 (74.1%) | 251 (69.1%) | .169 |
| % of patients who engage in high-risk behavior | |||
| ≥15% | 74 (29.4%) | 123 (34.2%) | |
| 6%–14% | 75 (29.8%) | 140 (38.9%) | |
| ≤5% | 103 (40.9%) | 97 (26.9%) | .0012 |
| Number of HIV+ patients in practice | |||
| ≥3 patients | 88 (31.4%) | 89 (24.5%) | |
| 1–2 patients | 103 (36.8%) | 143 (39.3%) | |
| None | 89 (31.8%) | 132 (36.3%) | .136 |
| Number of AIDS patients in practice | |||
| ≥3 patients | 43 (15.5%) | 47 (12.9%) | |
| 1–2 patients | 95 (34.2%) | 95 (26.1%) | |
| None | 140 (50.3%) | 222 (61.0%) | .025 |
| HMO, health maintenance organization; CHC, community health center. | |||
| * Some numbers may not total to the individual cohort N because of sporadic missing data. The response rate for the 1994 survey was 60% and for the 2005 survey it was 50%. Percentages are based on the number of responders to each question, not the total number of respondents. | |||
HIV/AIDS care and changing referral patterns
When asked how referral patterns had changed over the past decade, the overwhelming majority (94.4%) reported being far less likely to manage HIV/AIDS patients alone. Just over half (56.2%) of the physicians in the current survey indicated being more likely to co-manage patients, and an impressive 85.3% noted that they were more likely to refer patients immediately compared with their own practice patterns a decade ago.
Changing care patterns for asymptomatic HIV+ patients. A total of 39.0% of current respondents referred patients immediately, 57.0% co-managed patients, while only 4.1% managed these patients alone (the data for the 1994 cohort was 7.0%, 45.8%, and 47.2%, respectively; P<.0001).
AIDS patients have similar patterns. Similar changes were seen in regard to care patterns for AIDS patients. Among the current cohort, 61.7% reported that they referred these patients immediately, compared with only 18.3% in 1994; 36.8% noted that they co-managed these patients (vs 74.3% in 1994); and only 1.5% reported that they managed these patients alone (vs 7.4% in 1994; P<.0001).
Use of resources. When they were asked which resources they used to help provide care for HIV patients, 39.9% indicated an HIV clinic at the closest teaching hospital (vs 28.4% in 1994); 41.4% noted a specialist at the community hospital where they practiced (vs 52.6% in 1994); and the remainder were fairly equally distributed (in both cohort years) between a local community health center that treats HIV+ patients, a consultant requested by the patient, or an FP colleague (P=.0003).
Which FPs are likely to refer? Given the small number of respondents who manage asymptomatic HIV+ and AIDS patients alone, we focused our analyses between those who refer immediately and those who co-manage care.
Those who reported referring asymptomatic HIV+ patients immediately were less likely to have ≥3 HIV+ or ≥3 AIDS patients in their current practice. They were significantly more likely to be in a group practice and significantly less likely to work at a community health center. They also reported being less likely to teach medical students and residents (TABLE 2).
Similar findings in referral patterns were also seen in the management of symptomatic HIV+/AIDS patients (TABLE 3). In addition, those physicians who immediately refer AIDS patients were also less likely to report that >15% of their patients were involved in high-risk behaviors.
TABLE 2
Care of asymptomatic HIV+ patients—which FPs refer, which co-manage?
| REFER IMMEDIATELY (N=134)* | CO-MANAGE (N=196*) | P VALUE | |
|---|---|---|---|
| Practice type | |||
| Solo practice | 24 (17.9%) | 30 (15.6%) | .001 |
| Single specialty | 58 (43.3%) | 70 (36.5%) | |
| HMO | 3 (2.2%) | 0 (0.0%) | |
| Multispecialty | 22 (16.4%) | 20 (10.4%) | |
| CHC | 14 (10.4%) | 57 (29.7%) | |
| Hospital-based clinic | 13 (9.7%) | 15 (7.8%) | |
| Teach medical students regularly | |||
| Yes | 51 (38.1%) | 118 (61.5%) | <.001 |
| No | 83 (61.9%) | 74 (38.5%) | |
| Teach residents regularly | |||
| Yes | 20 (15.0%) | 78 (40.8%) | <.001 |
| No | 113 (85.0%) | 113 (59.2%) | |
| Number of HIV+ patients in practice | |||
| <3 patients | 121 (91.0%) | 132 (68.0%) | <.001 |
| ≥3 patients | 12 (9.0%) | 62 (32.0%) | |
| Number of AIDS patients in practice | |||
| <3 patients | 128 (96.2%) | 165 (85.1%) | .001 |
| ≥3 patients | 5 (3.8%) | 29 (14.9%) | |
| % of patients who engage in high-risk behavior | |||
| 0%—5% | 44 (33.1%) | 44 (23.0%) | .133 |
| 6%—15% | 46 (34.6%) | 78 (40.8%) | |
| >15% | 43 (32.3%) | 69 (36.1%) | |
| HMO, health maintenance organization; CHC, community health center. | |||
| * Total N=369. We removed those physicians who noted “manage alone” because of the small sample size. Each item’s total may not be the total number of respondents due to unanswered questions. Percentages are based on the total number of responders to each question, not the total number of respondents. | |||
Who is caring for HIV/AIDS patients? Those FPs who reported an increase over 11 years in the number of patients with HIV/AIDS in their practices were more likely to practice in a community health center (P<.001), and were more likely to teach medical students (P=.002) and residents (P<.001). Additionally, these FPs reported a higher percentage of patients with high-risk behaviors (P=.008). These FPs were less likely to report that they didn’t have time to care for HIV/AIDS patients (P=.037). They felt more knowledgeable about HIV (P=.005) and AIDS care (P<.001), and were more likely to learn about HIV/AIDS care through formal CME (P=.001).
In contrast, those FPs with <10 HIV/AIDS patients in their practice were more likely to be in rural practices (P=.006), to have been in practice longer (mean, 14.75 vs 12.35 years; P=.042), and to teach medical students (P=.045). There were no differences noted between gender, practice arrangement, or residency education.
TABLE 3
Care of symptomatic HIV+/AIDS patients—which FPs refer, which co-manage?
| Refer IMMEDIATELY (N=134)* | CO-MANAGE (n=196)* | P VALUE | |
|---|---|---|---|
| Practice type | |||
| Solo practice | 37 (17.7%) | 17 (13.7%) | <.001 |
| Single specialty | 91 (43.5%) | 42 (33.9%) | |
| HMO | 3 (1.4%) | 0 (0.0%) | |
| Multispecialty | 34 (16.3%) | 10 (8.1%) | |
| CHC | 25 (12.0%) | 46 (37.1%) | |
| Hospital-based clinic | 19 (9.1%) | 9 (7.2%) | |
| Teach medical students regularly | |||
| Yes | 92 (43.6%) | 82 (67.2%) | |
| No | 119 (56.4%) | 40 (32.8%) | <.001 |
| Teach residents regularly | |||
| Yes | 40 (19.1%) | 60 (49.2%) | |
| No | 169 (80.9%) | 62 (50.8%) | <.001 |
| Number of HIV+ patients in practice | |||
| <3 patients | 180 (86.1%) | 77 (61.6%) | |
| ≥3 patients | 29 (13.9%) | 48 (38.4%) | <.001 |
| Number of AIDS patients in practice | |||
| <3 patients | 203 (97.1%) | 93 (74.4%) | |
| ≥3 patients | 6 (2.9%) | 32 (25.6%) | <.001 |
| % of patients who engage in high-risk behavior | |||
| 0%—5% | 67 (32.1%) | 21 (17.2%) | |
| 6%—15% | 83 (39.7%) | 49 (40.2%) | |
| >15% | 59 (28.2%) | 52 (42.6%) | .004 |
| HMO, health maintenance organization; CHC, community health center. | |||
| * Total N=369. We removed those physicians who noted “manage alone” because of small sample size. Each item’s total may not be the total number of respondents due to unanswered questions. Percentages are based on the total number of responders to each question, not the total number of respondents. | |||
Multivariate analyses
To identify factors that contributed the most to the immediate referral of asymptomatic HIV+ patients, we employed a stepwise logistic regression analysis based on the results of our bivariate analyses.
FPs were less likely to refer immediately if they were female, practiced in a community health center, had a higher number of HIV patients in their practice, learned about HIV/AIDS care during residency as well as through formal CME programs, taught medical students regularly, and felt more knowledgeable about HIV/AIDS care. They were more likely to refer these patients if they reported having no time to care for HIV/AIDS patients. A similar model was observed for the referral of symptomatic AIDS patients (TABLE 4).
TABLE 4
Multivariate analyses of factors related to referring patients immediately vs co-managing
| INDEPENDENT FACTORS | IMEDIATE REFERRALS FOR ASYMPT OMATIC HIV+ PATIENTS (N=297) OR * (95% CI ) | IMEDIATE REFERRALS FOR SYMPT OMATIC HIV+/AIDS PATIENTS (N=302) OR* (95% CI ) |
|---|---|---|
| Gender | ||
| Male | 1.0 | 1.0 |
| Female | 0.505 (0.283–0.901) | 0.311 (0.168–0.576) |
| Practice location: Community health center | ||
| No | 1.0 | ns† |
| Yes | 0.402 (0.178–0.910) | |
| Number of HIV+ patients in practice | ||
| 4-level ordinal variable: | ||
| 1=None; 2=1-2; 3=3-10; 4≥10 | 0.637 (0.440–0.923) | not in model |
| Number of AIDS patients in practice | ||
| 4-level ordinal variable: | ns† | 0.514 (0.343–0.769) |
| 1=None; 2=1-2; 3=3-10; 4≥10 | ||
| Learned about HIV/AIDS care during residency | ||
| No | 1.0 | ns† |
| Yes | 0.476 (0.243–0.930) | |
| Learned about HIV/AIDS care from formal CME programs | ||
| No | 1.0 | 1.0 |
| Yes | 0.468 (0.257–0.851) | 0.375 (0.204–0.691) |
| Participate in teaching medical students regularly | ||
| No | 1.0 | 1.0 |
| Yes | 0.417 (0.238–0.732) | 0.531 (0.281–1.003) |
| Participate in teaching residents regularly | ||
| No | ns† | 1.0 |
| Yes | 0.537 (0.279–1.035) | |
| Feel knowledgeable about HIV care | ||
| No | 1.0 | 1.0 |
| Yes | 0.345 (0.187–0.638) | 0.357 (0.176–0.728) |
| Have no time in my practice to care for HIV/AIDS patients | ||
| No | 1.0 | 1.0 |
| Yes | 2.076 (1.155–3.729) | 4.306 (2.098–8.838) |
| * An OR of 1.0 reflects the referent category within each of the independent factors. | ||
| † ns = not significant in the stepwise regression model. | ||
| OR, odds ratio; CI, confidence interval; CME, continuing medical education. | ||
Discussion
Referral patterns change with demographics and new treatments
In comparing data with our survey from 1994, we found significant differences in care and referral patterns for HIV/AIDS patients.
FPs are more likely to refer, and right away. FPs are more likely to refer HIV patients immediately, compared with a decade ago; this likely results from many factors. The complexity of this disease and the rapid rate of change in management have been well documented.3
Keeping up-to-date with current practice guidelines and managing complications of treatment protocols can be time-consuming. Also, more physicians in our current survey reported having no AIDS patients in their practices compared with 1994.
The emergence of community health centers. Another interesting finding, based on the results of both our bivariate and multivariate analyses, was to see the community health center emerge as a resource for patients with HIV/AIDS. This may reflect the more urban location of community health centers and the higher prevalence of HIV/AIDS patients in those locales, an increased involvement with teaching, and an increased volume of patients with HIV/AIDS—all resulting in an increased knowledge of HIV/AIDS care. Additionally, community health centers are capable of providing a more comprehensive range of services than a traditional practice, through the support of the Federal Ryan White CARE Act. This likely plays a role in the increasing numbers of such patients being cared for in this setting.
Implications for future training of FPs. Optimal care of HIV patients requires a combination of disease-specific expertise and primary care skills and organization.14 Recent literature demonstrates that generalist physicians are able to develop condition-specific knowledge similar to those with specialty training—if they have a substantial caseload, and if they make an effort to stay current in a particular area.15 Residency training sites, particularly community health centers, will likely emerge as leaders in the training of primary care physicians to care for this disease. The ongoing expertise of faculty in these sites will be a vital aspect of this training.
Limitations of this study Our survey was limited to members of the MAFP, and may not be generalizable to other primary care providers. It also may not be generalizable to other states, given the demographics of Massachusetts and the availability of health care in a more urban environment. The availability of HIV resources and referral centers may vary from state to state.
The survey relied upon self-report and may be prone to either over- or under-reporting of current practice and recall of changes over the past decade.
Also, a higher response rate among male FPs, with females being less likely to refer patients, may have understated the relationship between gender and referral patterns for these patients.
Quality of care? it’s still a question. The quality of care provided by the subset of family physicians that are caring for their HIV/AIDS patients was not studied in this article. As this group continues to train new physicians and provide ongoing care for these patients, it will be important to measure the quality of care being provided.9,16
Will the role of the FP in HIV/AIDS care expand?
Our study demonstrates a significant shift amongst FPs with regard to their referral patterns for patients with HIV/AIDS over the last decade. This overall shift likely reflects the complexity of caring for these patients.
However, as these patients have longer survival rates, primary care offices will likely be seeing more individuals with HIV disease. While these patients may be followed by specialists, the role of the primary care physician in providing care may well expand. Funding for specific training programs on HIV/AIDS care should be targeted to community health centers where there is sufficient volume of HIV patients and an already demonstrated expertise amongst clinical faculty.
Acknowledgments
We gratefully acknowledge the survey implementation and data entry efforts of Denise West and the expert review by Jeff Baxter, MD.
Correspondence
Philip O. Fournier, MD, University of Massachusetts Medical School, 55 Lake Avenue North, Worcester, MA 01655; [email protected]
- Funding for specific training programs on HIV/AIDS care should be targeted to community health centers, where there is sufficient volume of HIV patients and an already demonstrated expertise amongst clinical faculty.
Purpose With the rapid development (and complex prescribing patterns) of drugs for HIV/AIDS care, it is challenging for physicians to keep current. We conducted a follow-up study to a 1994 cohort study to see how care and referral patterns have changed over the last decade. In this study, we examined how family physicians in Massachusetts were caring for their HIV-infected patients, and explored whether FPs were referring more patients to specialists for care compared with a decade ago.
Methods We designed a cross-sectional survey as an 11-year follow-up to a previous study. It was mailed in 2005 to the active membership of the Massachusetts academy of Family Physicians.
Results Compared with the cohort of 1994, the number of HIV+ patients in individual practices remained about the same, but the number of practices with no AIDS patients was significantly higher. 85.3% of FPs noted that they were more likely to refer HIV/AIDS patients immediately compared with their own practice patterns a decade ago. In this study, 39.0% of current respondents referred HIV+ patients immediately, 57.0% co-managed patients, and 4.1% managed these patients alone (the data for the 1994 cohort was 7.0%, 45.8%, and 47.2%, respectively; P<.0001).
Similar changes were seen in regard to care patterns for AIDS patients. among the current cohort, 61.7% reported that they referred patients immediately, compared with only 18.3% in 1994; 36.8% noted that they co-managed these patients (vs 74.3% in 1994); and only 1.5% reported that they managed these patients alone (vs 7.4% in 1994; P<.0001).
Conclusions A significant shift amongst FPs with regard to their referral patterns for patients with HIV/AIDS has occurred over the last decade. The community health center has emerged as a resource for patients with HIV/AIDS. Funding for specific training programs on HIV/AIDS care should be targeted to community health centers.
In June 2008, it will be 27 years since the first reported clusters of Pneumocystis pneumonia cases, which were the earliest published reports of the HIV epidemic.1 There are now more than a million individuals infected with HIV in the United States.2
Since the first antiretroviral drug, azidothymidine (AZT), was approved in 1987, more than a dozen medications have been introduced to treat this illness.2 Care for patients with HIV/AIDS is rapidly evolving—so much so that the International AIDS Society publishes revised treatment guidelines every 2 years.3 The US Department of Health and Human Services also issues frequent guidelines for HIV care.4
Methods
Asking FPs about their HIV/AIDS management practices
This cross-sectional survey was designed as an 11-year follow-up to a previous research study, described in greater detail elsewhere.12
In June 2005, we obtained a membership listing from the Massachusetts Academy of Family Physicians (MAFP). Using the total design methodology described by Dillman,13 a cover letter and survey instrument were mailed to current MAFP members (N=777). Nonresponders were sent a reminder postcard 2 weeks later, and a second survey 2 weeks after that. A final reminder and survey were mailed to the remaining nonrespondents a month later. No incentives were offered for the completion of the survey.
The survey instrument was developed using the original data collection tool from 1994, supplemented by questions assessing any changes in patient management over the previous 11 years. The 31-item survey included questions about the sociodemographic and practice characteristics of the respondents, their patient mix, their education, and their management of patients with HIV/AIDS, as well as a series of Likert-type attitudinal questions assessing their knowledge, access to specialists, and safety concerns regarding HIV.
Outcomes measured: Changes in care and referral patterns
The main outcomes of the survey were changes in the management of patients with HIV/AIDS, particularly regarding referral patterns compared with the previous decade. Univariate and bivariate statistics, frequency and percentile distributions, as well as means and medians, were used to characterize the physician cohort, their practice characteristics, referral patterns, and attitudes. We also examined a variety of relationships (eg, differences by gender, years of practice, and involvement in teaching), including changes from the data we collected in 1994 (using a repeated cross-sectional design approach).
Data were analyzed using SPSS for Windows, version 14 (SPSS, Inc, Chicago, Ill). Depending on the categorical or continuous nature of the data, chi-square and t-tests were used to assess significance at the .05 level. These bivariate analyses were subsequently used to select which independent variables we would include in the stepwise logistic regression analyses.
This study was reviewed by the University of Massachusetts’ Institutional Review Board for the conduct of human subject research and received an exemption from formal review.
Results
Who were the respondents?
In all, 369 completed surveys were returned, along with 44 uncompleted surveys (returned as undeliverable or with an indication that the respondent was no longer practicing), for a response rate of 53.2% (413/777) and a completion rate of 47.4% (369/777).
The respondents included an equal representation of male and female physicians. The majority practiced in non- urban settings (62.6%), and nearly one half (44.8%) had finished their residency since the first survey was sent. Half (54.9%) participated in a solo or single-specialty practice. FPs more often reported teaching medical students regularly (52.9%) than being involved in residency education (30.9%). More than a third (34.2%) reported that ≥15% of their patients participated in “high-risk” behaviors for HIV. (The definition of high-risk was left to the individual respondent.)
Compared with our earlier survey, respondents are now more likely to be female, more likely to be practicing in an urban setting, and slightly more likely to teach medical students. Current respondents also reported that they had fewer patients involved in high-risk behaviors.
The majority (63.8%) indicated that they had at least one HIV+ patient in their practice, with 39.0% also reporting at least one AIDS patient. Compared with the previous cohort studied, HIV+ patient numbers remained about the same; however, the number of practices caring for at least 1 AIDS patient was significantly lower (TABLE 1).
TABLE 1
Demographics of FPs responding (1994 vs 2005)
| SOCIODEMOGRAPHIC AND PRACTICE FACTORS | 1994 SURVEY (N=281)* N(%) | 2005 SURVEY (N=389)* N(%) | P VALUE |
|---|---|---|---|
| Gender | |||
| Male | 205 (74.0%) | 188 (50.9%) | <.0001 |
| Female | 72 (26.0%) | 181 (49.1%) | |
| Year of residency completion | |||
| After 1994 | — | 163 (44.8%) | not tested |
| 1986—1994 | 117 (44.5%) | 107 (29.4%) | |
| Before 1986 | 146 (55.5%) | 94 (25.8%) | |
| Practice location | |||
| Urban | 81 (29.0%) | 135 (37.4%) | |
| Suburban/rural | 198 (71.0%) | 226 (62.6%) | .026 |
| Practice type | |||
| Solo/single specialty | 169 (61.0%) | 200 (54.9%) | |
| Multispecialty/HMO/CHC | 90 (32.5%) | 134 (36.8%) | |
| Hospital-based clinic | 18 (6.5%) | 30 (8.2%) | .289 |
| Teach medical students regularly | |||
| Yes | 127 (45.7%) | 193 (52.9%) | |
| No | 151 (54.3%) | 172 (47.1%) | .071 |
| Teach residents regularly | |||
| Yes | 72 (25.9%) | 112 (30.9%) | |
| No | 206 (74.1%) | 251 (69.1%) | .169 |
| % of patients who engage in high-risk behavior | |||
| ≥15% | 74 (29.4%) | 123 (34.2%) | |
| 6%–14% | 75 (29.8%) | 140 (38.9%) | |
| ≤5% | 103 (40.9%) | 97 (26.9%) | .0012 |
| Number of HIV+ patients in practice | |||
| ≥3 patients | 88 (31.4%) | 89 (24.5%) | |
| 1–2 patients | 103 (36.8%) | 143 (39.3%) | |
| None | 89 (31.8%) | 132 (36.3%) | .136 |
| Number of AIDS patients in practice | |||
| ≥3 patients | 43 (15.5%) | 47 (12.9%) | |
| 1–2 patients | 95 (34.2%) | 95 (26.1%) | |
| None | 140 (50.3%) | 222 (61.0%) | .025 |
| HMO, health maintenance organization; CHC, community health center. | |||
| * Some numbers may not total to the individual cohort N because of sporadic missing data. The response rate for the 1994 survey was 60% and for the 2005 survey it was 50%. Percentages are based on the number of responders to each question, not the total number of respondents. | |||
HIV/AIDS care and changing referral patterns
When asked how referral patterns had changed over the past decade, the overwhelming majority (94.4%) reported being far less likely to manage HIV/AIDS patients alone. Just over half (56.2%) of the physicians in the current survey indicated being more likely to co-manage patients, and an impressive 85.3% noted that they were more likely to refer patients immediately compared with their own practice patterns a decade ago.
Changing care patterns for asymptomatic HIV+ patients. A total of 39.0% of current respondents referred patients immediately, 57.0% co-managed patients, while only 4.1% managed these patients alone (the data for the 1994 cohort was 7.0%, 45.8%, and 47.2%, respectively; P<.0001).
AIDS patients have similar patterns. Similar changes were seen in regard to care patterns for AIDS patients. Among the current cohort, 61.7% reported that they referred these patients immediately, compared with only 18.3% in 1994; 36.8% noted that they co-managed these patients (vs 74.3% in 1994); and only 1.5% reported that they managed these patients alone (vs 7.4% in 1994; P<.0001).
Use of resources. When they were asked which resources they used to help provide care for HIV patients, 39.9% indicated an HIV clinic at the closest teaching hospital (vs 28.4% in 1994); 41.4% noted a specialist at the community hospital where they practiced (vs 52.6% in 1994); and the remainder were fairly equally distributed (in both cohort years) between a local community health center that treats HIV+ patients, a consultant requested by the patient, or an FP colleague (P=.0003).
Which FPs are likely to refer? Given the small number of respondents who manage asymptomatic HIV+ and AIDS patients alone, we focused our analyses between those who refer immediately and those who co-manage care.
Those who reported referring asymptomatic HIV+ patients immediately were less likely to have ≥3 HIV+ or ≥3 AIDS patients in their current practice. They were significantly more likely to be in a group practice and significantly less likely to work at a community health center. They also reported being less likely to teach medical students and residents (TABLE 2).
Similar findings in referral patterns were also seen in the management of symptomatic HIV+/AIDS patients (TABLE 3). In addition, those physicians who immediately refer AIDS patients were also less likely to report that >15% of their patients were involved in high-risk behaviors.
TABLE 2
Care of asymptomatic HIV+ patients—which FPs refer, which co-manage?
| REFER IMMEDIATELY (N=134)* | CO-MANAGE (N=196*) | P VALUE | |
|---|---|---|---|
| Practice type | |||
| Solo practice | 24 (17.9%) | 30 (15.6%) | .001 |
| Single specialty | 58 (43.3%) | 70 (36.5%) | |
| HMO | 3 (2.2%) | 0 (0.0%) | |
| Multispecialty | 22 (16.4%) | 20 (10.4%) | |
| CHC | 14 (10.4%) | 57 (29.7%) | |
| Hospital-based clinic | 13 (9.7%) | 15 (7.8%) | |
| Teach medical students regularly | |||
| Yes | 51 (38.1%) | 118 (61.5%) | <.001 |
| No | 83 (61.9%) | 74 (38.5%) | |
| Teach residents regularly | |||
| Yes | 20 (15.0%) | 78 (40.8%) | <.001 |
| No | 113 (85.0%) | 113 (59.2%) | |
| Number of HIV+ patients in practice | |||
| <3 patients | 121 (91.0%) | 132 (68.0%) | <.001 |
| ≥3 patients | 12 (9.0%) | 62 (32.0%) | |
| Number of AIDS patients in practice | |||
| <3 patients | 128 (96.2%) | 165 (85.1%) | .001 |
| ≥3 patients | 5 (3.8%) | 29 (14.9%) | |
| % of patients who engage in high-risk behavior | |||
| 0%—5% | 44 (33.1%) | 44 (23.0%) | .133 |
| 6%—15% | 46 (34.6%) | 78 (40.8%) | |
| >15% | 43 (32.3%) | 69 (36.1%) | |
| HMO, health maintenance organization; CHC, community health center. | |||
| * Total N=369. We removed those physicians who noted “manage alone” because of the small sample size. Each item’s total may not be the total number of respondents due to unanswered questions. Percentages are based on the total number of responders to each question, not the total number of respondents. | |||
Who is caring for HIV/AIDS patients? Those FPs who reported an increase over 11 years in the number of patients with HIV/AIDS in their practices were more likely to practice in a community health center (P<.001), and were more likely to teach medical students (P=.002) and residents (P<.001). Additionally, these FPs reported a higher percentage of patients with high-risk behaviors (P=.008). These FPs were less likely to report that they didn’t have time to care for HIV/AIDS patients (P=.037). They felt more knowledgeable about HIV (P=.005) and AIDS care (P<.001), and were more likely to learn about HIV/AIDS care through formal CME (P=.001).
In contrast, those FPs with <10 HIV/AIDS patients in their practice were more likely to be in rural practices (P=.006), to have been in practice longer (mean, 14.75 vs 12.35 years; P=.042), and to teach medical students (P=.045). There were no differences noted between gender, practice arrangement, or residency education.
TABLE 3
Care of symptomatic HIV+/AIDS patients—which FPs refer, which co-manage?
| Refer IMMEDIATELY (N=134)* | CO-MANAGE (n=196)* | P VALUE | |
|---|---|---|---|
| Practice type | |||
| Solo practice | 37 (17.7%) | 17 (13.7%) | <.001 |
| Single specialty | 91 (43.5%) | 42 (33.9%) | |
| HMO | 3 (1.4%) | 0 (0.0%) | |
| Multispecialty | 34 (16.3%) | 10 (8.1%) | |
| CHC | 25 (12.0%) | 46 (37.1%) | |
| Hospital-based clinic | 19 (9.1%) | 9 (7.2%) | |
| Teach medical students regularly | |||
| Yes | 92 (43.6%) | 82 (67.2%) | |
| No | 119 (56.4%) | 40 (32.8%) | <.001 |
| Teach residents regularly | |||
| Yes | 40 (19.1%) | 60 (49.2%) | |
| No | 169 (80.9%) | 62 (50.8%) | <.001 |
| Number of HIV+ patients in practice | |||
| <3 patients | 180 (86.1%) | 77 (61.6%) | |
| ≥3 patients | 29 (13.9%) | 48 (38.4%) | <.001 |
| Number of AIDS patients in practice | |||
| <3 patients | 203 (97.1%) | 93 (74.4%) | |
| ≥3 patients | 6 (2.9%) | 32 (25.6%) | <.001 |
| % of patients who engage in high-risk behavior | |||
| 0%—5% | 67 (32.1%) | 21 (17.2%) | |
| 6%—15% | 83 (39.7%) | 49 (40.2%) | |
| >15% | 59 (28.2%) | 52 (42.6%) | .004 |
| HMO, health maintenance organization; CHC, community health center. | |||
| * Total N=369. We removed those physicians who noted “manage alone” because of small sample size. Each item’s total may not be the total number of respondents due to unanswered questions. Percentages are based on the total number of responders to each question, not the total number of respondents. | |||
Multivariate analyses
To identify factors that contributed the most to the immediate referral of asymptomatic HIV+ patients, we employed a stepwise logistic regression analysis based on the results of our bivariate analyses.
FPs were less likely to refer immediately if they were female, practiced in a community health center, had a higher number of HIV patients in their practice, learned about HIV/AIDS care during residency as well as through formal CME programs, taught medical students regularly, and felt more knowledgeable about HIV/AIDS care. They were more likely to refer these patients if they reported having no time to care for HIV/AIDS patients. A similar model was observed for the referral of symptomatic AIDS patients (TABLE 4).
TABLE 4
Multivariate analyses of factors related to referring patients immediately vs co-managing
| INDEPENDENT FACTORS | IMEDIATE REFERRALS FOR ASYMPT OMATIC HIV+ PATIENTS (N=297) OR * (95% CI ) | IMEDIATE REFERRALS FOR SYMPT OMATIC HIV+/AIDS PATIENTS (N=302) OR* (95% CI ) |
|---|---|---|
| Gender | ||
| Male | 1.0 | 1.0 |
| Female | 0.505 (0.283–0.901) | 0.311 (0.168–0.576) |
| Practice location: Community health center | ||
| No | 1.0 | ns† |
| Yes | 0.402 (0.178–0.910) | |
| Number of HIV+ patients in practice | ||
| 4-level ordinal variable: | ||
| 1=None; 2=1-2; 3=3-10; 4≥10 | 0.637 (0.440–0.923) | not in model |
| Number of AIDS patients in practice | ||
| 4-level ordinal variable: | ns† | 0.514 (0.343–0.769) |
| 1=None; 2=1-2; 3=3-10; 4≥10 | ||
| Learned about HIV/AIDS care during residency | ||
| No | 1.0 | ns† |
| Yes | 0.476 (0.243–0.930) | |
| Learned about HIV/AIDS care from formal CME programs | ||
| No | 1.0 | 1.0 |
| Yes | 0.468 (0.257–0.851) | 0.375 (0.204–0.691) |
| Participate in teaching medical students regularly | ||
| No | 1.0 | 1.0 |
| Yes | 0.417 (0.238–0.732) | 0.531 (0.281–1.003) |
| Participate in teaching residents regularly | ||
| No | ns† | 1.0 |
| Yes | 0.537 (0.279–1.035) | |
| Feel knowledgeable about HIV care | ||
| No | 1.0 | 1.0 |
| Yes | 0.345 (0.187–0.638) | 0.357 (0.176–0.728) |
| Have no time in my practice to care for HIV/AIDS patients | ||
| No | 1.0 | 1.0 |
| Yes | 2.076 (1.155–3.729) | 4.306 (2.098–8.838) |
| * An OR of 1.0 reflects the referent category within each of the independent factors. | ||
| † ns = not significant in the stepwise regression model. | ||
| OR, odds ratio; CI, confidence interval; CME, continuing medical education. | ||
Discussion
Referral patterns change with demographics and new treatments
In comparing data with our survey from 1994, we found significant differences in care and referral patterns for HIV/AIDS patients.
FPs are more likely to refer, and right away. FPs are more likely to refer HIV patients immediately, compared with a decade ago; this likely results from many factors. The complexity of this disease and the rapid rate of change in management have been well documented.3
Keeping up-to-date with current practice guidelines and managing complications of treatment protocols can be time-consuming. Also, more physicians in our current survey reported having no AIDS patients in their practices compared with 1994.
The emergence of community health centers. Another interesting finding, based on the results of both our bivariate and multivariate analyses, was to see the community health center emerge as a resource for patients with HIV/AIDS. This may reflect the more urban location of community health centers and the higher prevalence of HIV/AIDS patients in those locales, an increased involvement with teaching, and an increased volume of patients with HIV/AIDS—all resulting in an increased knowledge of HIV/AIDS care. Additionally, community health centers are capable of providing a more comprehensive range of services than a traditional practice, through the support of the Federal Ryan White CARE Act. This likely plays a role in the increasing numbers of such patients being cared for in this setting.
Implications for future training of FPs. Optimal care of HIV patients requires a combination of disease-specific expertise and primary care skills and organization.14 Recent literature demonstrates that generalist physicians are able to develop condition-specific knowledge similar to those with specialty training—if they have a substantial caseload, and if they make an effort to stay current in a particular area.15 Residency training sites, particularly community health centers, will likely emerge as leaders in the training of primary care physicians to care for this disease. The ongoing expertise of faculty in these sites will be a vital aspect of this training.
Limitations of this study Our survey was limited to members of the MAFP, and may not be generalizable to other primary care providers. It also may not be generalizable to other states, given the demographics of Massachusetts and the availability of health care in a more urban environment. The availability of HIV resources and referral centers may vary from state to state.
The survey relied upon self-report and may be prone to either over- or under-reporting of current practice and recall of changes over the past decade.
Also, a higher response rate among male FPs, with females being less likely to refer patients, may have understated the relationship between gender and referral patterns for these patients.
Quality of care? it’s still a question. The quality of care provided by the subset of family physicians that are caring for their HIV/AIDS patients was not studied in this article. As this group continues to train new physicians and provide ongoing care for these patients, it will be important to measure the quality of care being provided.9,16
Will the role of the FP in HIV/AIDS care expand?
Our study demonstrates a significant shift amongst FPs with regard to their referral patterns for patients with HIV/AIDS over the last decade. This overall shift likely reflects the complexity of caring for these patients.
However, as these patients have longer survival rates, primary care offices will likely be seeing more individuals with HIV disease. While these patients may be followed by specialists, the role of the primary care physician in providing care may well expand. Funding for specific training programs on HIV/AIDS care should be targeted to community health centers where there is sufficient volume of HIV patients and an already demonstrated expertise amongst clinical faculty.
Acknowledgments
We gratefully acknowledge the survey implementation and data entry efforts of Denise West and the expert review by Jeff Baxter, MD.
Correspondence
Philip O. Fournier, MD, University of Massachusetts Medical School, 55 Lake Avenue North, Worcester, MA 01655; [email protected]
1. Centers for Disease Control and Prevention. Pneumocystis pneumonia—Los Angeles. MMWR Morb Mortal Wkly Rep 1981;30:250-252.
2. Frame PT. HIV disease in primary care. Prim Care 2004;30:205-237.
3. Hammer SM, Saag MS, Schechter M, et al. Treatment for adult HIV infection 2006. Recommendations of the International AIDS Society—USA Panel. JAMA 2006;296:827-843.
4. US Department of Health and Human Services. Guidelines and Standards. Available at: www.aids.gov/treatment/guidelines/index.html. Accessed January 17, 2008.
5. Sherer RI, Stieglitz K, Narra J, et al. HIV multidisciplinary teams work: Support services improve access to and retention in HIV primary care. AIDS Care 2002;14(Suppl 1):S31-S44.
6. Kitahata MM, Van Rompaey SE, Dillingham PW, et al. Primary care delivery is associated with greater physician experience and improved survival among persons with AIDS. J Gen Intern Med 2003;18:95-103.
7. Sackoff JE, Hanna DB, Pfeiffer MR, Torian L. Causes of death among persons with AIDS in the era of highly active antiretroviral therapy: New york City. Ann Intern Med 2006;145:397-406.
8. Kirchner J. Who should care for patients with HIV/AIDS? Am Fam Physician 2006;73:290-291.
9. Landon BE, Wilson IB, McInnes K, et al. Physician specialization and the quality of care for human immunodeficiency virus infection. Arch Intern Med 2005;165:1133-1139.
10. Gemson DH, Colombotos J, Elinson J, et al. Acquired immunodeficiency syndrome prevention: Knowledge, attitudes, and practices of primary care physicians. Arch Intern Med 1991;151:1102-1108.
11. Skiest DJ, Keiser P. Human immunodeficiency virus infection in patients older than 50 years: A survey of primary care physicians’ beliefs, practices, and knowledge. Arch Intern Med 1991;151:1102-1108.
12. Fournier PO, Baldor RA, Warfield ME, Frazier B. Patients with HIV/AIDS: Physicians’ knowledge, attitudes, and referral practices. J Fam Pract 1997;44:85-89.
13. Dillman DA. Mail and Internet Surveys: The Tailored Design Method. Hoboken, NJ: John Wiley & Sons; 2000.
14. Hecht FM, Wilson IB, Wu AW, et al. Optimizing care for persons with HIV infection. Ann Intern Med 1999;131:136-143.
15. Wilson IB, Landon BE, Ding L, et al. A national study of the relationship of care site HIV specialization to early adoption of highly active antiretroviral therapy. Med Care 2005;43:12-20.
16. Wilson IB, Landon BE, Hirschhorn LR, et al. Quality of HIV care provided by nurse practitioners, physicians assistants, and physicians. Ann Intern Med 2005;143:729-736.
1. Centers for Disease Control and Prevention. Pneumocystis pneumonia—Los Angeles. MMWR Morb Mortal Wkly Rep 1981;30:250-252.
2. Frame PT. HIV disease in primary care. Prim Care 2004;30:205-237.
3. Hammer SM, Saag MS, Schechter M, et al. Treatment for adult HIV infection 2006. Recommendations of the International AIDS Society—USA Panel. JAMA 2006;296:827-843.
4. US Department of Health and Human Services. Guidelines and Standards. Available at: www.aids.gov/treatment/guidelines/index.html. Accessed January 17, 2008.
5. Sherer RI, Stieglitz K, Narra J, et al. HIV multidisciplinary teams work: Support services improve access to and retention in HIV primary care. AIDS Care 2002;14(Suppl 1):S31-S44.
6. Kitahata MM, Van Rompaey SE, Dillingham PW, et al. Primary care delivery is associated with greater physician experience and improved survival among persons with AIDS. J Gen Intern Med 2003;18:95-103.
7. Sackoff JE, Hanna DB, Pfeiffer MR, Torian L. Causes of death among persons with AIDS in the era of highly active antiretroviral therapy: New york City. Ann Intern Med 2006;145:397-406.
8. Kirchner J. Who should care for patients with HIV/AIDS? Am Fam Physician 2006;73:290-291.
9. Landon BE, Wilson IB, McInnes K, et al. Physician specialization and the quality of care for human immunodeficiency virus infection. Arch Intern Med 2005;165:1133-1139.
10. Gemson DH, Colombotos J, Elinson J, et al. Acquired immunodeficiency syndrome prevention: Knowledge, attitudes, and practices of primary care physicians. Arch Intern Med 1991;151:1102-1108.
11. Skiest DJ, Keiser P. Human immunodeficiency virus infection in patients older than 50 years: A survey of primary care physicians’ beliefs, practices, and knowledge. Arch Intern Med 1991;151:1102-1108.
12. Fournier PO, Baldor RA, Warfield ME, Frazier B. Patients with HIV/AIDS: Physicians’ knowledge, attitudes, and referral practices. J Fam Pract 1997;44:85-89.
13. Dillman DA. Mail and Internet Surveys: The Tailored Design Method. Hoboken, NJ: John Wiley & Sons; 2000.
14. Hecht FM, Wilson IB, Wu AW, et al. Optimizing care for persons with HIV infection. Ann Intern Med 1999;131:136-143.
15. Wilson IB, Landon BE, Ding L, et al. A national study of the relationship of care site HIV specialization to early adoption of highly active antiretroviral therapy. Med Care 2005;43:12-20.
16. Wilson IB, Landon BE, Hirschhorn LR, et al. Quality of HIV care provided by nurse practitioners, physicians assistants, and physicians. Ann Intern Med 2005;143:729-736.
REIMBURSEMENT ADVISER
In the July 2007 issue of OBG Management, I wrote an article about the Medicare Physician Quality Reporting Initiative (PQRI) program, which could have earned you as much as a 1.5% bonus at the end of that year (read this article). For 2008, there are many more quality measures for which you can qualify.
For example, there are now measures for screening, such as colon cancer screening and mammography. And more:
- New measure 113 allows you to note that you documented the result of a fecal occult blood test
- If you document, at the time of a problem visit, the result of a recent mammogram, you can report measure 112
- Measures 114 and 115 relate to inquiring about a patient’s tobacco use and then advising her to quit—activities customarily performed by ObGyns.
For details on how to participate in this program (and to see how easy it is to report measures), visit the Centers for Medicare & Medicaid Services (CMS) at www.cms.hhs.gov/PQRI/35_2008PQRI-Information.asp. Download “2008 PRQI Quality Measure Specifications.”
For coding tips on managing obstetric anal sphincter injury, see this issue’s cover article
Reimbursement for repair of your surgical injury?
When the injury occurs during the surgery and is repaired at that time, Medicare does not allow the surgeon who caused the injury to bill separately for repairing it. If another physician is called in to make the repair, however, he (she) is reimbursed for the work. According to Medicare’s General Correct Coding Policies for National Correct Coding Initiative Policy Manual for Medicare Services, “When a complication described by codes defining complications arises during an operative session…a separate service for treating the complication is not to be reported.”
A return to the operating room for a complication would be reimbursed, however; report this by adding a modifier -78 to the surgical code for the complication repair (for example, 49002 [re-opening of a recent laparotomy for hemorrhage exploration]).
Most private payers allow separate billing for repair of iatrogenic injury.
In the July 2007 issue of OBG Management, I wrote an article about the Medicare Physician Quality Reporting Initiative (PQRI) program, which could have earned you as much as a 1.5% bonus at the end of that year (read this article). For 2008, there are many more quality measures for which you can qualify.
For example, there are now measures for screening, such as colon cancer screening and mammography. And more:
- New measure 113 allows you to note that you documented the result of a fecal occult blood test
- If you document, at the time of a problem visit, the result of a recent mammogram, you can report measure 112
- Measures 114 and 115 relate to inquiring about a patient’s tobacco use and then advising her to quit—activities customarily performed by ObGyns.
For details on how to participate in this program (and to see how easy it is to report measures), visit the Centers for Medicare & Medicaid Services (CMS) at www.cms.hhs.gov/PQRI/35_2008PQRI-Information.asp. Download “2008 PRQI Quality Measure Specifications.”
For coding tips on managing obstetric anal sphincter injury, see this issue’s cover article
Reimbursement for repair of your surgical injury?
When the injury occurs during the surgery and is repaired at that time, Medicare does not allow the surgeon who caused the injury to bill separately for repairing it. If another physician is called in to make the repair, however, he (she) is reimbursed for the work. According to Medicare’s General Correct Coding Policies for National Correct Coding Initiative Policy Manual for Medicare Services, “When a complication described by codes defining complications arises during an operative session…a separate service for treating the complication is not to be reported.”
A return to the operating room for a complication would be reimbursed, however; report this by adding a modifier -78 to the surgical code for the complication repair (for example, 49002 [re-opening of a recent laparotomy for hemorrhage exploration]).
Most private payers allow separate billing for repair of iatrogenic injury.
In the July 2007 issue of OBG Management, I wrote an article about the Medicare Physician Quality Reporting Initiative (PQRI) program, which could have earned you as much as a 1.5% bonus at the end of that year (read this article). For 2008, there are many more quality measures for which you can qualify.
For example, there are now measures for screening, such as colon cancer screening and mammography. And more:
- New measure 113 allows you to note that you documented the result of a fecal occult blood test
- If you document, at the time of a problem visit, the result of a recent mammogram, you can report measure 112
- Measures 114 and 115 relate to inquiring about a patient’s tobacco use and then advising her to quit—activities customarily performed by ObGyns.
For details on how to participate in this program (and to see how easy it is to report measures), visit the Centers for Medicare & Medicaid Services (CMS) at www.cms.hhs.gov/PQRI/35_2008PQRI-Information.asp. Download “2008 PRQI Quality Measure Specifications.”
For coding tips on managing obstetric anal sphincter injury, see this issue’s cover article
Reimbursement for repair of your surgical injury?
When the injury occurs during the surgery and is repaired at that time, Medicare does not allow the surgeon who caused the injury to bill separately for repairing it. If another physician is called in to make the repair, however, he (she) is reimbursed for the work. According to Medicare’s General Correct Coding Policies for National Correct Coding Initiative Policy Manual for Medicare Services, “When a complication described by codes defining complications arises during an operative session…a separate service for treating the complication is not to be reported.”
A return to the operating room for a complication would be reimbursed, however; report this by adding a modifier -78 to the surgical code for the complication repair (for example, 49002 [re-opening of a recent laparotomy for hemorrhage exploration]).
Most private payers allow separate billing for repair of iatrogenic injury.
Was the patient still suicidal?
THE PATIENT. A 30-year-old police officer reports thoughts of suicide. He was under investigation for illegal work-related activities and feared he would have to report his coworkers’ involvement in these activities and lose his job.
CASE FACTS. The patient was voluntarily hospitalized for 4 days and received medication and inpatient psychotherapy. When he was discharged, a psychiatrist prescribed follow-up outpatient psychotherapy and antidepressant and antipsychotic medications. The next day, the officer fatally shot himself.
THE PATIENT’S FAMILY’S CLAIM. The psychiatrist did not adequately weigh the patient’s depression and stressors, including possibly losing his job, and did not properly assess suicidal ideation. Also, the patient’s mother claims she attended the discharge meeting with the psychiatrist and that her son expressed suicidal intentions at that time.
THE DOCTOR’S DEFENSE. The patient believed he could get another job if necessary and was no longer contemplating suicide. Also, he was a voluntary patient and could not be hospitalized any longer without consent.
Submit your verdict and find out how the court ruled at CurrentPsychiatry.com. Click on “Have more to say about this topic?” to comment.
Cases are selected by Current Psychiatry from Medical Malpractice Verdicts, Settlements & Experts, with permission of its editor, Lewis Laska of Nashville, TN (www.verdictslaska.com). Information may be incomplete in some instances, but these cases represent clinical situations that typically result in litigation.
THE PATIENT. A 30-year-old police officer reports thoughts of suicide. He was under investigation for illegal work-related activities and feared he would have to report his coworkers’ involvement in these activities and lose his job.
CASE FACTS. The patient was voluntarily hospitalized for 4 days and received medication and inpatient psychotherapy. When he was discharged, a psychiatrist prescribed follow-up outpatient psychotherapy and antidepressant and antipsychotic medications. The next day, the officer fatally shot himself.
THE PATIENT’S FAMILY’S CLAIM. The psychiatrist did not adequately weigh the patient’s depression and stressors, including possibly losing his job, and did not properly assess suicidal ideation. Also, the patient’s mother claims she attended the discharge meeting with the psychiatrist and that her son expressed suicidal intentions at that time.
THE DOCTOR’S DEFENSE. The patient believed he could get another job if necessary and was no longer contemplating suicide. Also, he was a voluntary patient and could not be hospitalized any longer without consent.
Submit your verdict and find out how the court ruled at CurrentPsychiatry.com. Click on “Have more to say about this topic?” to comment.
THE PATIENT. A 30-year-old police officer reports thoughts of suicide. He was under investigation for illegal work-related activities and feared he would have to report his coworkers’ involvement in these activities and lose his job.
CASE FACTS. The patient was voluntarily hospitalized for 4 days and received medication and inpatient psychotherapy. When he was discharged, a psychiatrist prescribed follow-up outpatient psychotherapy and antidepressant and antipsychotic medications. The next day, the officer fatally shot himself.
THE PATIENT’S FAMILY’S CLAIM. The psychiatrist did not adequately weigh the patient’s depression and stressors, including possibly losing his job, and did not properly assess suicidal ideation. Also, the patient’s mother claims she attended the discharge meeting with the psychiatrist and that her son expressed suicidal intentions at that time.
THE DOCTOR’S DEFENSE. The patient believed he could get another job if necessary and was no longer contemplating suicide. Also, he was a voluntary patient and could not be hospitalized any longer without consent.
Submit your verdict and find out how the court ruled at CurrentPsychiatry.com. Click on “Have more to say about this topic?” to comment.
Cases are selected by Current Psychiatry from Medical Malpractice Verdicts, Settlements & Experts, with permission of its editor, Lewis Laska of Nashville, TN (www.verdictslaska.com). Information may be incomplete in some instances, but these cases represent clinical situations that typically result in litigation.
Cases are selected by Current Psychiatry from Medical Malpractice Verdicts, Settlements & Experts, with permission of its editor, Lewis Laska of Nashville, TN (www.verdictslaska.com). Information may be incomplete in some instances, but these cases represent clinical situations that typically result in litigation.
Tips to make documentation easier, faster, and more satisfying
Current Psychiatry’s malpractice column is evolving. Previously, “Malpractice Verdicts,” used case decisions to initiate discussions of clinical situations that can generate lawsuits. The verdicts remain as “Malpractice Minute”, but Current Psychiatry has invited me to contribute a new column, “Malpractice Rx,” that will solicit questions and address practicing clinicians’ concerns about malpractice risk.
To start this dialogue, I’ll begin with a question that often comes up in discussions with colleagues, and especially when I teach psychiatry residents: “What should I document?” In this article, we will review why proper documentation is essential. We’ll also look at some ideas that might make documentation easier, more efficient, and more satisfying.
- If so, please submit your malpractice-related questions to Dr. Mossman at [email protected].
- Include your name, address, and practice location. If your question is chosen for publication, your name can be withheld by request.
- All readers who submit questions will be included in quarterly drawings for a $50 gift certificate for Professional Risk Management Services, Inc’s online marketplace of risk management publications and resources (www.prms.com).
When I was in medical school, my professors said the primary reason for accurate charting was to communicate with the rest of the treatment team. This is still true. But in these sadder-but-wiser days, when I ask psychiatry residents “What is the purpose of documentation?” they always answer, “to create a legal record.”
Documentation plays many roles (Table 1). From the standpoint of preventing a malpractice judgment, the clinical record can accomplish 3 important things:
Lawsuit deterrence. Records are a key source—and often the only source—of information an attorney uses when deciding whether to file a lawsuit. An attorney won’t risk time and money on a malpractice case if the clinical record suggests that a psychiatrist was conscientious and met the standard of care.1
Impression management. The patient’s chart is what plaintiffs’ and defendants’ experts use when forming their initial opinions about the quality of care delivered.
Credibility. Clinical records are the most believable source of information about what you observed, what you thought, what you did, why you did it, and when you did it. The adage “if it wasn’t written, it didn’t happen” is not always applicable,2 but if an adverse event occurs, a defendant doctor’s verbal testimony about delivering good care will be more convincing when backed up by documentation created before the event.
Table 1
Purposes of medical record documentation
|
Improving documentation
Because it is impossible to describe everything you see, hear, say, do, and think during clinical encounters with patients, you must make choices about what to include in the record. The components of good documentation depend on the clinical context, but the following general principles may avert some malpractice actions.
1 More is better. Psychiatric practice often requires you to be discreet about patients’ personal information. Within appropriate bounds, however, the more information the record contains about objective findings, patients’ statements, clinical judgments, and your decision making, the better the portrayal of competent care.
2 Record the time and date. When attorneys and experts try to reconstruct what happened before an adverse occurrence, knowing the exact time you saw the patient, recorded findings, wrote orders, followed up on lab tests, or discussed problems with others—including family and treatment team members—can make a big difference.
3 Sooner is better. The most credible charting is done during or just after a service is rendered. Charting completed after an adverse event is vulnerable to accusations of fabrication.
4 Describe your thinking. Most aspects of clinical medicine are far from certain. Documenting the reasoning behind your diagnosis and treatment selection—what you’ve ruled out, what still seems tentative, and what risks and benefits you’ve weighed—helps emphasize this reality.3 After something bad happens, people retrospectively regard the event as more probable than it really was.4 Documenting your uncertainty and ways of addressing it may help counter this “hindsight bias.” It also shows that you were thoughtful and took therapeutic steps prudently.
5
6 Clarify capacity. Jurors may believe that all psychiatric patients are incompetent, and plaintiff’s attorneys sometimes try to create the impression that patients are completely controlled by weird whims and aberrant thoughts. To counter this, when appropriate indicate in the chart that the patient can handle responsibilities such as reporting side effects, seeking emergency attention, or notifying you about changes in thought or mood.3,5
7 Manage appearance and content. Under Health Insurance Portability and Accountability Act (HIPAA) regulations, patients have the right to review their medical records.6 If a lawsuit occurs, the records might be read out loud in court. Documentation will make a better impression if it is clear, legible, and free of gratuitous comments.
8 Include quotations. Documenting verbatim statements from a patient, such as “I’ve never considered suicide,” can quickly convey key information that you considered when making a therapeutic decision.
Technical approaches
Table 27,8 lists several techniques and technologies that might improve documentation. For example, computer users can create templates or customize software to quickly produce thorough documentation for frequently encountered procedures or clinical events. Whether these approaches are useful and appropriate will depend on your work setting, but all aim to improve the speed and quality of clinical documentation.
Think creatively about improving documentation. Even if you’re never sued, better documentation helps you and your patients. For example, several years ago a colleague9 designed an emergency room form that allowed clinicians to complete in a few seconds a Brief Psychiatric Rating Scale on every patient we evaluated. This innovation shortened the time needed to document a systematic, comprehensive assessment and increased the quantity, quality, and reliability of information in patients’ records.
Table 2
Purposes of medical record documentation
| Idea | Comment |
|---|---|
| Use speech recognition | You speak faster than you write. Transcription software |
| Software | accuracy has improved in the last few years. |
| Use handouts and | Patients often do not remember or understand much of what |
| medication instructions | doctors tell them,7,8 so handouts may be more useful than verbal instructions. Good handouts about medications are available on the Internet. Note in the chart that you gave the patient the document. |
| Seek anonymous | Documenting consultations shows you are prudent and |
| consultations with colleagues | a colleague agreed with your treatment. |
| Ask patients to rate their | This practice may improve your information gathering |
| own symptoms and progress | and help document what the patient told you. |
| Use standard rating scales | Rating scales can help you record more information in a scientifically validated format. |
| Use macros and templates | Macros can reduce time needed for documentation. Your memory isn’t perfect, but templates can help you include everything you need to cover. |
1. Simpson S, Stacy M. Avoiding the malpractice snare: documenting suicide risk assessment. J Psychiatr Pract 2004;10:185-9.
2. Zurad EG. Don’t be the target of a malpractice suit. Fam Pract Manag 2006;13(6):57-64.
3. Gutheil TG. Fundamentals of medical record documentation. Psychiatry 2004;1:26-8.
4. Fischhoff B, Beyth R. “I knew it would happen” remembered probabilities of once-future things. Organ Behav Hum Perform 1975;13:1-16.
5. Appelbaum PS, Gutheil TG. Clinical handbook of psychiatry and the law 4th ed. Philadelphia, PA: Lippincott Williams and Wilkins; 2007.
6. 45 CFR § 164.524(a)(1).
7. Rogers AE, Addington-Hall JM, Abery AJ, et al. Knowledge and communication difficulties for patients with chronic heart failure: qualitative study. BMJ 2000;321:605-7.
8. Chesanow N. Are you getting through? Med Econ 2006;83(13):41,45-6.
9. Somoza E, Somoza JR. A neural-network approach to predicting admission decisions in a psychiatric emergency room. Med Decis Making 1993;13:273-80.
Current Psychiatry’s malpractice column is evolving. Previously, “Malpractice Verdicts,” used case decisions to initiate discussions of clinical situations that can generate lawsuits. The verdicts remain as “Malpractice Minute”, but Current Psychiatry has invited me to contribute a new column, “Malpractice Rx,” that will solicit questions and address practicing clinicians’ concerns about malpractice risk.
To start this dialogue, I’ll begin with a question that often comes up in discussions with colleagues, and especially when I teach psychiatry residents: “What should I document?” In this article, we will review why proper documentation is essential. We’ll also look at some ideas that might make documentation easier, more efficient, and more satisfying.
- If so, please submit your malpractice-related questions to Dr. Mossman at [email protected].
- Include your name, address, and practice location. If your question is chosen for publication, your name can be withheld by request.
- All readers who submit questions will be included in quarterly drawings for a $50 gift certificate for Professional Risk Management Services, Inc’s online marketplace of risk management publications and resources (www.prms.com).
When I was in medical school, my professors said the primary reason for accurate charting was to communicate with the rest of the treatment team. This is still true. But in these sadder-but-wiser days, when I ask psychiatry residents “What is the purpose of documentation?” they always answer, “to create a legal record.”
Documentation plays many roles (Table 1). From the standpoint of preventing a malpractice judgment, the clinical record can accomplish 3 important things:
Lawsuit deterrence. Records are a key source—and often the only source—of information an attorney uses when deciding whether to file a lawsuit. An attorney won’t risk time and money on a malpractice case if the clinical record suggests that a psychiatrist was conscientious and met the standard of care.1
Impression management. The patient’s chart is what plaintiffs’ and defendants’ experts use when forming their initial opinions about the quality of care delivered.
Credibility. Clinical records are the most believable source of information about what you observed, what you thought, what you did, why you did it, and when you did it. The adage “if it wasn’t written, it didn’t happen” is not always applicable,2 but if an adverse event occurs, a defendant doctor’s verbal testimony about delivering good care will be more convincing when backed up by documentation created before the event.
Table 1
Purposes of medical record documentation
|
Improving documentation
Because it is impossible to describe everything you see, hear, say, do, and think during clinical encounters with patients, you must make choices about what to include in the record. The components of good documentation depend on the clinical context, but the following general principles may avert some malpractice actions.
1 More is better. Psychiatric practice often requires you to be discreet about patients’ personal information. Within appropriate bounds, however, the more information the record contains about objective findings, patients’ statements, clinical judgments, and your decision making, the better the portrayal of competent care.
2 Record the time and date. When attorneys and experts try to reconstruct what happened before an adverse occurrence, knowing the exact time you saw the patient, recorded findings, wrote orders, followed up on lab tests, or discussed problems with others—including family and treatment team members—can make a big difference.
3 Sooner is better. The most credible charting is done during or just after a service is rendered. Charting completed after an adverse event is vulnerable to accusations of fabrication.
4 Describe your thinking. Most aspects of clinical medicine are far from certain. Documenting the reasoning behind your diagnosis and treatment selection—what you’ve ruled out, what still seems tentative, and what risks and benefits you’ve weighed—helps emphasize this reality.3 After something bad happens, people retrospectively regard the event as more probable than it really was.4 Documenting your uncertainty and ways of addressing it may help counter this “hindsight bias.” It also shows that you were thoughtful and took therapeutic steps prudently.
5
6 Clarify capacity. Jurors may believe that all psychiatric patients are incompetent, and plaintiff’s attorneys sometimes try to create the impression that patients are completely controlled by weird whims and aberrant thoughts. To counter this, when appropriate indicate in the chart that the patient can handle responsibilities such as reporting side effects, seeking emergency attention, or notifying you about changes in thought or mood.3,5
7 Manage appearance and content. Under Health Insurance Portability and Accountability Act (HIPAA) regulations, patients have the right to review their medical records.6 If a lawsuit occurs, the records might be read out loud in court. Documentation will make a better impression if it is clear, legible, and free of gratuitous comments.
8 Include quotations. Documenting verbatim statements from a patient, such as “I’ve never considered suicide,” can quickly convey key information that you considered when making a therapeutic decision.
Technical approaches
Table 27,8 lists several techniques and technologies that might improve documentation. For example, computer users can create templates or customize software to quickly produce thorough documentation for frequently encountered procedures or clinical events. Whether these approaches are useful and appropriate will depend on your work setting, but all aim to improve the speed and quality of clinical documentation.
Think creatively about improving documentation. Even if you’re never sued, better documentation helps you and your patients. For example, several years ago a colleague9 designed an emergency room form that allowed clinicians to complete in a few seconds a Brief Psychiatric Rating Scale on every patient we evaluated. This innovation shortened the time needed to document a systematic, comprehensive assessment and increased the quantity, quality, and reliability of information in patients’ records.
Table 2
Purposes of medical record documentation
| Idea | Comment |
|---|---|
| Use speech recognition | You speak faster than you write. Transcription software |
| Software | accuracy has improved in the last few years. |
| Use handouts and | Patients often do not remember or understand much of what |
| medication instructions | doctors tell them,7,8 so handouts may be more useful than verbal instructions. Good handouts about medications are available on the Internet. Note in the chart that you gave the patient the document. |
| Seek anonymous | Documenting consultations shows you are prudent and |
| consultations with colleagues | a colleague agreed with your treatment. |
| Ask patients to rate their | This practice may improve your information gathering |
| own symptoms and progress | and help document what the patient told you. |
| Use standard rating scales | Rating scales can help you record more information in a scientifically validated format. |
| Use macros and templates | Macros can reduce time needed for documentation. Your memory isn’t perfect, but templates can help you include everything you need to cover. |
Current Psychiatry’s malpractice column is evolving. Previously, “Malpractice Verdicts,” used case decisions to initiate discussions of clinical situations that can generate lawsuits. The verdicts remain as “Malpractice Minute”, but Current Psychiatry has invited me to contribute a new column, “Malpractice Rx,” that will solicit questions and address practicing clinicians’ concerns about malpractice risk.
To start this dialogue, I’ll begin with a question that often comes up in discussions with colleagues, and especially when I teach psychiatry residents: “What should I document?” In this article, we will review why proper documentation is essential. We’ll also look at some ideas that might make documentation easier, more efficient, and more satisfying.
- If so, please submit your malpractice-related questions to Dr. Mossman at [email protected].
- Include your name, address, and practice location. If your question is chosen for publication, your name can be withheld by request.
- All readers who submit questions will be included in quarterly drawings for a $50 gift certificate for Professional Risk Management Services, Inc’s online marketplace of risk management publications and resources (www.prms.com).
When I was in medical school, my professors said the primary reason for accurate charting was to communicate with the rest of the treatment team. This is still true. But in these sadder-but-wiser days, when I ask psychiatry residents “What is the purpose of documentation?” they always answer, “to create a legal record.”
Documentation plays many roles (Table 1). From the standpoint of preventing a malpractice judgment, the clinical record can accomplish 3 important things:
Lawsuit deterrence. Records are a key source—and often the only source—of information an attorney uses when deciding whether to file a lawsuit. An attorney won’t risk time and money on a malpractice case if the clinical record suggests that a psychiatrist was conscientious and met the standard of care.1
Impression management. The patient’s chart is what plaintiffs’ and defendants’ experts use when forming their initial opinions about the quality of care delivered.
Credibility. Clinical records are the most believable source of information about what you observed, what you thought, what you did, why you did it, and when you did it. The adage “if it wasn’t written, it didn’t happen” is not always applicable,2 but if an adverse event occurs, a defendant doctor’s verbal testimony about delivering good care will be more convincing when backed up by documentation created before the event.
Table 1
Purposes of medical record documentation
|
Improving documentation
Because it is impossible to describe everything you see, hear, say, do, and think during clinical encounters with patients, you must make choices about what to include in the record. The components of good documentation depend on the clinical context, but the following general principles may avert some malpractice actions.
1 More is better. Psychiatric practice often requires you to be discreet about patients’ personal information. Within appropriate bounds, however, the more information the record contains about objective findings, patients’ statements, clinical judgments, and your decision making, the better the portrayal of competent care.
2 Record the time and date. When attorneys and experts try to reconstruct what happened before an adverse occurrence, knowing the exact time you saw the patient, recorded findings, wrote orders, followed up on lab tests, or discussed problems with others—including family and treatment team members—can make a big difference.
3 Sooner is better. The most credible charting is done during or just after a service is rendered. Charting completed after an adverse event is vulnerable to accusations of fabrication.
4 Describe your thinking. Most aspects of clinical medicine are far from certain. Documenting the reasoning behind your diagnosis and treatment selection—what you’ve ruled out, what still seems tentative, and what risks and benefits you’ve weighed—helps emphasize this reality.3 After something bad happens, people retrospectively regard the event as more probable than it really was.4 Documenting your uncertainty and ways of addressing it may help counter this “hindsight bias.” It also shows that you were thoughtful and took therapeutic steps prudently.
5
6 Clarify capacity. Jurors may believe that all psychiatric patients are incompetent, and plaintiff’s attorneys sometimes try to create the impression that patients are completely controlled by weird whims and aberrant thoughts. To counter this, when appropriate indicate in the chart that the patient can handle responsibilities such as reporting side effects, seeking emergency attention, or notifying you about changes in thought or mood.3,5
7 Manage appearance and content. Under Health Insurance Portability and Accountability Act (HIPAA) regulations, patients have the right to review their medical records.6 If a lawsuit occurs, the records might be read out loud in court. Documentation will make a better impression if it is clear, legible, and free of gratuitous comments.
8 Include quotations. Documenting verbatim statements from a patient, such as “I’ve never considered suicide,” can quickly convey key information that you considered when making a therapeutic decision.
Technical approaches
Table 27,8 lists several techniques and technologies that might improve documentation. For example, computer users can create templates or customize software to quickly produce thorough documentation for frequently encountered procedures or clinical events. Whether these approaches are useful and appropriate will depend on your work setting, but all aim to improve the speed and quality of clinical documentation.
Think creatively about improving documentation. Even if you’re never sued, better documentation helps you and your patients. For example, several years ago a colleague9 designed an emergency room form that allowed clinicians to complete in a few seconds a Brief Psychiatric Rating Scale on every patient we evaluated. This innovation shortened the time needed to document a systematic, comprehensive assessment and increased the quantity, quality, and reliability of information in patients’ records.
Table 2
Purposes of medical record documentation
| Idea | Comment |
|---|---|
| Use speech recognition | You speak faster than you write. Transcription software |
| Software | accuracy has improved in the last few years. |
| Use handouts and | Patients often do not remember or understand much of what |
| medication instructions | doctors tell them,7,8 so handouts may be more useful than verbal instructions. Good handouts about medications are available on the Internet. Note in the chart that you gave the patient the document. |
| Seek anonymous | Documenting consultations shows you are prudent and |
| consultations with colleagues | a colleague agreed with your treatment. |
| Ask patients to rate their | This practice may improve your information gathering |
| own symptoms and progress | and help document what the patient told you. |
| Use standard rating scales | Rating scales can help you record more information in a scientifically validated format. |
| Use macros and templates | Macros can reduce time needed for documentation. Your memory isn’t perfect, but templates can help you include everything you need to cover. |
1. Simpson S, Stacy M. Avoiding the malpractice snare: documenting suicide risk assessment. J Psychiatr Pract 2004;10:185-9.
2. Zurad EG. Don’t be the target of a malpractice suit. Fam Pract Manag 2006;13(6):57-64.
3. Gutheil TG. Fundamentals of medical record documentation. Psychiatry 2004;1:26-8.
4. Fischhoff B, Beyth R. “I knew it would happen” remembered probabilities of once-future things. Organ Behav Hum Perform 1975;13:1-16.
5. Appelbaum PS, Gutheil TG. Clinical handbook of psychiatry and the law 4th ed. Philadelphia, PA: Lippincott Williams and Wilkins; 2007.
6. 45 CFR § 164.524(a)(1).
7. Rogers AE, Addington-Hall JM, Abery AJ, et al. Knowledge and communication difficulties for patients with chronic heart failure: qualitative study. BMJ 2000;321:605-7.
8. Chesanow N. Are you getting through? Med Econ 2006;83(13):41,45-6.
9. Somoza E, Somoza JR. A neural-network approach to predicting admission decisions in a psychiatric emergency room. Med Decis Making 1993;13:273-80.
1. Simpson S, Stacy M. Avoiding the malpractice snare: documenting suicide risk assessment. J Psychiatr Pract 2004;10:185-9.
2. Zurad EG. Don’t be the target of a malpractice suit. Fam Pract Manag 2006;13(6):57-64.
3. Gutheil TG. Fundamentals of medical record documentation. Psychiatry 2004;1:26-8.
4. Fischhoff B, Beyth R. “I knew it would happen” remembered probabilities of once-future things. Organ Behav Hum Perform 1975;13:1-16.
5. Appelbaum PS, Gutheil TG. Clinical handbook of psychiatry and the law 4th ed. Philadelphia, PA: Lippincott Williams and Wilkins; 2007.
6. 45 CFR § 164.524(a)(1).
7. Rogers AE, Addington-Hall JM, Abery AJ, et al. Knowledge and communication difficulties for patients with chronic heart failure: qualitative study. BMJ 2000;321:605-7.
8. Chesanow N. Are you getting through? Med Econ 2006;83(13):41,45-6.
9. Somoza E, Somoza JR. A neural-network approach to predicting admission decisions in a psychiatric emergency room. Med Decis Making 1993;13:273-80.