User login
Identifying serious causes of back pain: Cancer, infection, fracture
Back pain is one of the most common complaints that internists and primary care physicians encounter.1 Although back pain is nonspecific, some hallmark signs and symptoms indicate that a patient is more likely to have a serious disorder. This article contrasts the presentation of cancer, infections, and fractures with the more common and benign conditions that cause back pain and provides guidance for diagnosis.
UNCOMMON, BUT MUST BE CONSIDERED
Although a variety of tissues can contribute to pain—intervertebral disks, vertebrae, ligaments, neural structures, muscles, and fascia—and many disorders can damage these tissues, most patients with back or neck pain have a benign condition. Back pain is typically caused by age-related degenerative changes or by minor repetitive trauma; with supportive care and physical therapy, up to 90% of patients with back pain of this nature improve substantially within 4 weeks.2
Serious, destructive diseases are uncommon causes of back pain: malignancy, infection, ankylosing spondylitis, and epidural abscess together account for fewer than 1% of cases of back pain in a typical primary care practice. But their clinical impact is out of proportion to their prevalence. The fear of overlooking a serious condition influences any practitioner’s approach to back pain and is a common reason for ordering multiple imaging studies and consultations.3 Therefore, the time, effort, and resources invested in ruling out these disorders is considerable.
Whether a patient with back pain has an ominous disease can usually be determined with a careful history, physical examination, and appropriate diagnostic studies. Once a serious diagnosis is ruled out, attention can be focused on rehabilitation and back care.
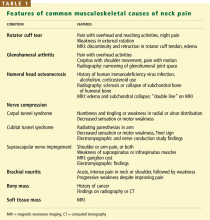
Back pain can also be due to musculoskeletal disorders, peptic ulcers, pancreatitis, pyelonephritis, aortic aneurysms, and other serious conditions, which we have discussed in other articles in this journal.4–6
SPINAL CANCER AND METASTASES
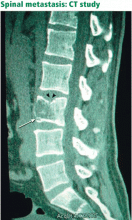
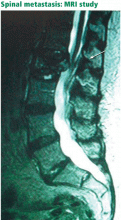
Since back pain is the presenting symptom in 90% of patients with spinal tumors,7 neoplasia belongs in the differential diagnosis of any patient with persistent, unremitting back pain. However, it is also important to recognize atypical presentations of neoplasia, such as a painless neurologic deficit, which should prompt an urgent workup.
The spine is one of the most common sites of metastasis: about 20,000 cases arise each year.8 Brihaye et al9 reviewed 1,477 cases of spinal metastases with epidural involvement and found that 16.5% arose from primary tumors in the breast, 15.6% from the lung, 9.2% from the prostate, and 6.5% from the kidney.
Cancer pain is persistent and progressive
Benign back pain often arises from a known injury, is relieved by rest, and increases with activities that load the disk (eg, sitting, getting up from bed or a chair), lumbar flexion with or without rotation, lifting, vibration (eg, riding in a car), coughing, sneezing, laughing, and the Valsalva maneuver. It is most commonly focal to the lumbosacral junction, the lumbar muscles, and the buttocks. Pain due to injury or a flare-up of degenerative disease typically begins to subside after 4 to 6 weeks and responds to nonsteroidal anti-inflammatory drugs and physical therapy.10
In contrast, pain caused by spinal neoplasia is typically persistent and progressive and is not alleviated by rest. Often the pain is worse at night, waking the patient from sleep. Back pain is typically focal to the level of the lesion and may be associated with belt-like thoracic pain or radicular symptoms of pain or weakness in the legs. A spinal mass can cause neurologic signs or symptoms by directly compressing the spinal cord or nerve roots, mimicking disk herniation or stenosis.11,12
Pathologic fractures resulting from vertebral destruction may be the first—and unfortunately a late—presentation of a tumor.
Ask about, look for, signs and symptoms of cancer
In taking the history, one should ask about possible signs and symptoms of systemic disease such as fatigue, weight loss, and changes in bowel habits. Hemoptysis, lymphadenopathy, subcutaneous or breast masses, nipple discharge, atypical vaginal bleeding, or blood in the stool suggest malignancy and should direct the specific diagnostic approach.13 A history of cancer, even if remote, should raise suspicion, as should major risk factors such as smoking.
Because most spinal tumors are metastases, a clinical examination of the breast, lungs, abdomen, thyroid, and prostate are appropriate starting points.14 The spine should be examined to identify sites of focal pain. A neurologic examination should be done to evaluate any signs of neurologic compromise or abnormal reflexes. Signs or symptoms of spinal cord compression should be investigated immediately.
Cancer usually elevates the ESR, CRP
If cancer is suspected, initial tests should include a complete blood cell count, erythrocyte sedimentation rate, C-reactive protein level, urinalysis, prostate-specific antigen level, and fecal occult blood testing. Normal results can considerably relieve suspicion of cancer: the erythrocyte sedimentation rate and C-reactive protein level are almost always elevated with systemic neoplasia.
Imaging tests
Unfortunately, spinal tumors cannot be well visualized on radiographs until significant destruction has occurred.15
A bone scan can usually detect tumors other than the purely lytic ones such as myeloma and has a sensitivity of 74%, a specificity of 81%, and a positive predictive value of 64% for vertebral metastasis in patients with back pain.16
INFECTION CAN BE INDOLENT OR ACUTE
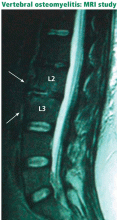
Spinal infection is a serious condition that can take an indolent, smoldering course or, alternatively, can erupt into sepsis or rapidly progressive vertebral destruction. Although the latter conditions are hard to miss, early diskitis and osteomyelitis can be difficult to differentiate from idiopathic back pain. In a series of 101 patients with vertebral osteomyelitis, misdiagnosis occurred in 33.7%, and the average delay from the onset of clinical manifestations to diagnosis was 2.6 months.20 Tuberculosis can be even more elusive: in a series of 78 patients diagnosed with definite or probable tuberculous vertebral osteomyelitis, the mean delay to diagnosis was about 6 months.21
Acute spinal infections are most often pyogenic; chronic infections may be pyogenic, fungal, or granulomatous.
Vertebral osteomyelitis accounts for 2% to 7% of all cases of osteomyelitis and is an uncommon cause of back pain.22 Any source of infection (eg, dental abscess, pneumonia) can seed the spine; urinary tract infection is the most common. Patients with immunocompromise or diabetes are most at risk.23 The onset is usually insidious with focal back pain at the level of involvement.
History and physical examination reveal localized pain
Spinal infections typically cause pain that is worsened with weight-bearing and activity and is relieved only when lying down. Chronic infection is usually associated with weight loss, fatigue, fevers, and night sweats.
Pain is usually well localized and reproduced by palpation or percussion over the involved level. Severe pain can sometimes be elicited by sitting the patient up or by changing the patient’s position. Focal kyphosis may be detectable if the vertebra has collapsed.
In a series of 41 patients with pyogenic infectious spondylitis, 90% had localized back pain aggravated by percussion, 59% had radicular signs and symptoms, and 29% had neurologic signs of spinal cord compression, including hyperreflexia, clonus, the Babinski sign (extension of the toes upward when the sole of the foot is stroked upwards), or the Hoffmann sign (flexion of the thumb elicited by flicking the end of a middle finger).24
LABORATORY RESULTS TYPICALLY INDICATE INFECTION
The erythrocyte sedimentation rate is the most sensitive test for infection, and an elevated rate may be the only abnormal laboratory finding: Digby and Kersley25 found that the rate was increased in all of 30 patients with nontuberculous pyogenic osteomyelitis of the spine. The C-reactive protein level is also usually elevated, but 40% of patients have a normal white blood cell count.25 Results of other laboratory tests are typically in the normal range. Tuberculin skin testing should be done for patients at high risk of the disease (eg, immigrants from areas of endemic disease, non-Hispanic blacks, immunocompromised patients, and those with known exposure to tuberculosis). Patients with high fever, chills, or rigors should have cultures taken of blood, urine, and sputum and from any intravenous lines.
Imaging changes may not appear for months
CT, on the other hand, may be better for showing the extent of bone involvement. In cases of vertebral osteomyelitis and intervertebral disk space infection, simultaneous involvement of the adjacent vertebral end plates and the intervertebral disk are the major findings.30
Signs of infection using T1-weighted MRI include low-signal marrow or disk spaces within the vertebral body, loss of definition of end plates (which appear hypointense compared with the bone marrow), and destruction of the cortical margins of the involved vertebral bodies. T2-weighted MRI typically discloses high signals of the affected areas of the vertebral body and disk. Contrast should be used to increase specificity; enhancement may be the first sign of an acute inflammatory process.31
CT and MRI can help identify sequestra, perilesional sclerosis, and epidural or soft tissue abscesses. Guided biopsy may be needed to differentiate between abscess, hematoma, tumor, and inflammation.
MRI findings: Pyogenic vs tuberculous spondylitis
MRI can help differentiate pyogenic vertebral osteomyelitis from tubercular disease, although findings may be similar (eg, both conditions have a high signal on T2-weighted images).32 Jung et al,33 in a retrospective study of 52 patients with spondylitis, found that compared with patients with pyogenic infections, patients with tuberculous spondylitis had a significantly higher incidence of a well-defined paraspinal abnormal signal on MRI, a thin and smooth abscess wall, a paraspinal or intraosseous abscess, subligamentous spread to three or more vertebral levels, involvement of multiple vertebral bodies, thoracic spine involvement, and a hyperintense signal on T2-weighted images. Other MRI features characteristically seen in patients with tuberculous spinal disease are anterior corner destruction, a relative preservation of the intervertebral disk, and large soft-tissue abscesses with calcifications.34
Prompt diagnosis and aggressive treatment needed
Pigrau et al35 found that spinal osteomyelitis is highly associated with endocarditis: among 606 patients with infectious endocarditis, 28 (4.6%) had pyogenic vertebral osteomyelitis, and among 91 patients with pyogenic vertebral osteomyelitis, 28 (30.8%) had infectious endocarditis.
McHenry et al36 retrospectively studied outcomes of 253 patients with vertebral osteomyelitis after a median of 6.5 years (range 2 days to 38 years): 11% died, more than one-third of survivors had residual disability, and 14% had a relapse. Surgery resulted in recovery or improvement in 86 (79%) of 109 patients. Independent risk factors for adverse outcome (death or incomplete recovery) were neurologic compromise, increased time to diagnosis, and having a hospital-acquired infection (P = .004). Relapse commonly developed in patients with severe vertebral destruction and abscesses, which appeared some time after surgical drainage or debridement. Recurrent bacteremia, paravertebral abscesses, and chronically draining sinuses were independently associated with relapse (P = .001). MRI, done in 110 patients, was often performed late in the course of infection and did not significantly affect outcome. The authors stressed that an optimal outcome of vertebral osteomyelitis requires heightened awareness, early diagnosis, prompt identification of pathogens, reversal of complications, and prolonged antimicrobial therapy.
Epidural abscess may also be present
Epidural abscess occurs in 10% of spine infections. About half of patients with an epidural abscess are misdiagnosed on their initial evaluation.37,38 Patients initially complain of local spine pain, followed by radicular pain, weakness, and finally paralysis. Between 12% and 30% of patients report a history of trauma, even as minor as a fall, preceding the infection.38,39
Radiologic findings are frequently equivocal, and MRI is preferred; gadolinium enhancement further increases sensitivity.39,40 Spinal canal abscesses usually appear hypointense on T1-weighted images and hyperintense on T2-weighted images, with ring enhancement surrounding the abscess area in contrast studies.41 MRI may give negative findings in the early stages of a spinal canal infection and so may need to be repeated.41 MRI may not help distinguish an epidural from a subdural abscess. However, primary spinal epidural abscesses without concomitant vertebral osteomyelitis are rare; therefore, the finding of associated vertebral osteomyelitis makes a spinal epidural abscess more likely.
FRACTURES
Fractures of the spine can be asymptomatic and may have no preceding trauma. They can be due to osteoporosis, malignancy, infection, or metabolic disorders such as renal osteodystrophy or hyperparathyroidism. Fractures in normal bone are almost always associated with trauma. Any suspicion of infection or malignancy should be investigated.
Corticosteroids increase risk
Any patient with back pain who is receiving corticosteroid therapy should be considered as having a compression fracture until proven otherwise.3 De Vries et al42 found that in a database of nearly 200,000 patients receiving glucocorticoids, risk increased substantially with increasing cumulative exposure. Those who intermittently received high doses (= 15 mg/day) and those who had no or little previous exposure to corticosteroids (cumulative exposure = 1 g) had only a slightly increased risk of osteoporotic fracture, and their risk of fracture of the hip and femur was not increased. In contrast, patients who received a daily dose of at least 30 mg and whose cumulative exposure was more than 5 g had a relative risk of osteoporotic vertebral fracture of 14.42 (95% confidence interval 8.29–25.08).
Osteoporotic compression fractures are common in the elderly
Osteoporosis involves reduced bone density, disrupted trabecular architecture, and increased susceptibility to fractures. About 700,000 vertebral body compression fractures occur in the United States each year43: about 10% result in hospitalization, involving an average stay of 8 days.44 Osteoporotic compression fractures are highly associated with age older than 65, female sex, and European descent.45,46 The estimated lifetime risk of a clinically evident vertebral fracture after age 50 years is 16% among postmenopausal white women and 5% among white men.47
A single osteoporotic vertebral compression fracture increases the risk of subsequent fractures by a factor of five, and up to 20% of patients with a vertebral compression fracture are likely to have another one within the same year if osteoporosis remains untreated.48 Population studies suggest that the death rate among patients who have osteoporotic vertebral compression fractures increases with the number of involved vertebrae.43
Unfortunately, osteoporotic vertebral compression fractures are not always easily amenable to treatment: up to 30% of patients who are symptomatic and seek treatment do not respond adequately to nonsurgical methods.49,50 However, new minimally invasive interventions such as vertebral augmentation make timely evaluation clinically relevant.
History, physical examination
Patients may present with a history of trauma with associated back pain or a neurologic deficit. In osteoporotic patients, the trauma may have been minimal, eg, a sneeze, a fall from a chair, or a slip and fall in the home. Pain tends to be worse when standing erect and occasionally when lying flat.
The patient is commonly visibly uncomfortable and may be limited to a wheelchair or stoop forward when standing. The spine may show an absence of the midline crease or an exaggerated thoracic kyphosis. Pain is typically reproduced by deep pressure over the spinous process at the involved level. Compression fractures rarely cause neurologic deficits but should always be considered.
Fractures commonly occur in the thoracolumbar region but may be anywhere in the spine. Fractures in the upper thoracic spine may indicate an underlying malignant tumor, and a thorough search for a possible primary lesion should always be carried out for fractures in this location.
Laboratory testing
Routine laboratory evaluation and thyroid function tests should be done, as well as a 24-hour urine specimen for collagen breakdown products, calcium, phosphate, and creatinine levels. Serum and urine protein electrophoresis should be performed if myeloma is suspected. A white blood-cell count, erythrocyte sedimentation rate, and C-reactive protein level help determine if an underlying infection caused the fracture.
MRI needed if plain films reveal fracture or are equivocal
Anteroposterior and lateral roentgenograms should be taken first; they typically show osteopenia. A fracture in the vertebral body is characterized by loss of height and by wedging. Osseous fragments can occasionally be seen in the spinal canal.
Sagittal short tau inversion recovery sequences, which use specifically timed pulse sequences to suppress fat signals, show high-intensity signal changes in areas of edema from acute or healing fractures. They provide a sensitive but nonspecific marker of abnormality.
Dual energy x-ray absorptiometry helps determine the extent of osteoporosis.
Bone scans should only be used for patients with suspected metastatic disease.
Patients with ankylosing spondylitis need thorough workup
Ankylosing spondylitis predisposes to serious spinal injury. Even after only minor trauma, patients with ankylosing spondylitis and acute, severe back pain should be thoroughly evaluated for fracture with CT and MRI of the entire spine. Plain radiography should not be relied on for these patients because of the risk of misinterpretation, delayed diagnosis, and poorer outcomes.52,53
NEUROLOGIC COMPROMISE—A RED FLAG
Cauda equina compression classically presents with back pain, bilateral sciatica, saddle anesthesia, and lower extremity weakness progressing to paraplegia, but in practice these symptoms are variably present and diagnosing the condition often requires a high degree of suspicion. Hyporeflexia is typically a sign of cauda equina compression, while hyperreflexia, clonus, and the Babinski sign suggest spinal cord compression, requiring an evaluation of the cervical and thoracic spine. Cauda equina compression typically involves urinary retention; in contrast, cord compression typically causes incontinence.55
If either cauda equina or spinal cord compression is detected during an initial examination, an immediate more extensive evaluation is warranted. MRI is the study of choice.
Spinal epidural hematoma
Spinal epidural hematoma is a rare but dramatic cause of paralysis in elderly patients. In most cases, there is no antecedent trauma. Lawton et al,56 in a series of 30 patients treated surgically for spinal epidural hematoma, found that 73% resulted from spine surgery, epidural catheterization, or anticoagulation therapy. Other possible causes of epidural hematoma include vascular malformations, angiomas, aneurysms, hypertension, and aspirin therapy.57
The same study56 found that the time from the first symptom to maximal neurologic deficit ranged from a few minutes to 4 days, with the average interval being nearly 13 hours.
Although painless onset has been reported,58 spinal epidural hematoma typically presents with acute pain at the level of the lesion, which is often rapidly followed by paraplegia or quadriplegia, depending on the location of the hemorrhage. Sometimes the onset of pain is preceded by a sudden increase of venous pressure from coughing, sneezing, or straining at stool. Urinary retention often develops at an early stage.
Most lesions occur in the thoracic region and extend into the cervicothoracic or the thoracolumbar area. The pain distribution may be radicular, mimicking a ruptured intervertebral disk.
Evaluation should be with MRI. Acute hemorrhage is characterized by a marked decrease in signal intensity on T2-weighted images. Subacute hematoma has increased signal intensity on both T1- and T2-weighted images.56
Early recognition, MRI confirmation, and treatment should be accomplished as soon as possible.56 Recovery depends on the severity of the neurologic deficit and the duration of symptoms before treatment. Lawton et al56 found that patients taken to surgery within 12 hours had better neurologic outcomes than patients with identical preoperative neurologic status whose surgery was delayed beyond 12 hours. Surgery should not be withheld because of advanced age or poor health: in 10 reported cases in which surgery was delayed, all patients died.59
- Deyo RA, Tsui-Wu YJ. Descriptive epidemiology of low-back pain and its related medical care in the United States. Spine 1987; 12:264–268.
- Pengel LH, Herbert RD, Maher CG, Refshauge KM. Acute back pain: systematic review of its prognosis. BMJ 2003; 327:323–325.
- Deyo RA, Rainville J, Kent DL. What can the history and physical examination tell us about back pain? JAMA 1992; 268:760–765.
- Pateder DB, Brems J, Lieberman I, Bell GR, McLain RF. Masquerade: nonspinal musculoskeletal disorders that mimic spinal conditions. Cleve Clin J Med 2008; 75:50–56.
- Klineberg E, Mazanec D, Orr D, Demicco R, Bell G, McLain R. Masquerade: medical causes of back pain. Cleve Clin J Med 2007; 74:905–913.
- McLain RF, Bell G, Montgomery W. Masquerade: systemic causes of back pain. Cleve Clin J Med In press.
- Gilbert RW, Kim JH, Posner JB. Epidural spinal cord compression from metastatic tumor: diagnosis and treatment. Ann Neurol 1978; 3:40–51.
- Black P. Spinal metastasis: current status and recommended guidelines for management. Neurosurgery 1979; 5:726–746.
- Brihaye J, Ectors P, Lemort M, Van Houtte P. The management of spinal epidural metastases. Adv Tech Stand Neurosurg 1988; 16:121–176.
- Patel RK, Slipman CW. Lumbar degenerative disk disease. Emedicine. Accessed March 25, 2008. http://www.emedicine.com/pmr/topic67.htm.
- Deyo RA, Diehl AK. Cancer as a cause of back pain: frequency, clinical presentation, and diagnostic strategies. J Gen Intern Med 1988; 3:230–238.
- Rosen P, Barkin RM, Danzl DF, et al. Emergency Medicine: Concepts and Clinical Practice. 4th ed. St Louis, MO: Mosby; 1998:2100–2102.
- Abbruzzese JL, Abbruzzese MC, Lenzi R, Hess KR, Raber MN. Analysis of a diagnostic strategy for patients with suspected tumors of unknown origin. J Clin Oncol 1995; 13:2094–2103.
- McCarthy EF, Frassica FJ. Pathology of Bone and Joint Disorders: With Clinical and Radiographic Correlation. Philadelphia: WB Saunders; 1998.
- Edelstyn GA, Gillespie PJ, Grebbell FS. The radiological demonstration of osseous metastases. Experimental observations. Clin Radiol 1967; 18:158–162.
- Han LJ, Au-Yong TK, Tong WC, Chu KS, Szeto LT, Wong CP. Comparison of bone single-photon emission tomography and planar imaging in the detection of vertebral metastases in patients with back pain. Eur J Nucl Med 1998; 25:635–638.
- Feun LG, Savaraj N. Detection of occult bone metastasis by MRI scan. J Fla Med Assoc 1990; 77:881–883.
- Citrin DL, Bessent RG, Greig WR. A comparison of the sensitivity and accuracy of the 99TCm-phosphate bone scan and skeletal radiograph in the diagnosis of bone metastases. Clin Radiol 1977; 28:107–117.
- Runge VM, Lee C, Iten AL, Williams NM. Contrast-enhanced magnetic resonance imaging in a spinal epidural tumor model. Invest Radiol 1997; 32:589–595.
- Buranapanitkit B, Lim A, Geater A. Misdiagnosis in vertebral osteomyelitis: problems and factors. J Med Assoc Thai 2001; 84:1743–1750.
- Colmenero JD, Jiménez-Mejías ME, Reguera JM, et al. Tuberculous vertebral osteomyelitis in the new millennium: still a diagnostic and therapeutic challenge. Eur J Clin Microbiol Infect Dis 2004; 23:477–483.
- Waldvogel FA, Medoff G, Swartz MN. Osteomyelitis: a review of clinical features, therapeutic considerations and unusual aspects. 3. Osteomyelitis associated with vascular insufficiency. N Engl J Med 1970; 282:316–322.
- Carragee EJ. Pyogenic vertebral osteomyelitis. J Bone Joint Surg Am 1997; 79:874–880.
- Kapeller P, Fazekas F, Krametter D, et al. Pyogenic infectious spondylitis: clinical, laboratory and MRI features. Eur Neurol 1997; 38:94–98.
- Digby JM, Kersley JB. Pyogenic non-tuberculous spinal infection: an analysis of thirty cases. J Bone Joint Surg Br 1979; 61:47–55.
- Modic MT, Feiglin DH, Piraino DW, et al. Vertebral osteomyelitis: assessment using MR. Radiology 1985; 157:157–166.
- Szypryt EP, Hardy JG, Hinton CE, Worthington BS, Mulholland RC. A comparison between magnetic resonance imaging and scintigraphic bone imaging in the diagnosis of disk space infection in an animal model. Spine 1988; 13:1042–1048.
- Küker W, Mull M, Mayfrank L, Töpper R, Thron A. Epidural spinal infection. Variability of clinical and magnetic resonance imaging findings. Spine 1997; 22:544–551.
- Tung GA, Yim JW, Mermel LA, Philip L, Rogg JM. Spinal epidural abscess: correlation between MRI findings and outcome. Neuroradiology 1999; 41:904–909.
- Sapico FL, Montgomerie JZ. Vertebral osteomyelitis. Infect Dis Clin North Am 1990; 4:539–550.
- Tali ET. Spinal infections. Eur J Radiol 2004; 50:120–133.
- Smith AS, Weinstein MA, Mizushima A, et al. MR imaging characteristics of tuberculous spondylitis vs vertebral osteomyelitis. AJR Am J Roentgenol 1989; 153:399–405.
- Jung NY, Jee WH, Ha KY, Park CK, Byun JY. Discrimination of tuberculous spondylitis from pyogenic spondylitis on MRI. AJR Am J Roentgenol 2004; 182:1405–1410.
- Joseffer SS, Cooper PR. Modern imaging of spinal tuberculosis. J Neurosurg Spine 2005; 2:145–150.
- Pigrau C, Almirante B, Flores X, et al. Spontaneous pyogenic vertebral osteomyelitis and endocarditis: incidence, risk factors, and outcome. Am J Med 2005; 118:1287.
- McHenry MC, Easley KA, Locker GA. Vertebral osteomyelitis: long-term outcome for 253 patients from 7 Cleveland-area hospitals. Clin Infect Dis 2002; 34:1342–1350.
- Danner RL, Hartman BJ. Update on spinal epidural abscess: 35 cases and review of the literature. Rev Infect Dis 1987; 9:265–274.
- Kaufman DM, Kaplan JG, Litman N. Infectious agents in spinal epidural abscesses. Neurology 1980; 30:844–850.
- Rezai AR, Woo HH, Errico TJ, Cooper PR. Contemporary management of spinal osteomyelitis. Neurosurgery 1999; 44:1018–1026.
- Bertino RE, Porter BA, Stimac GK, Tepper SJ. Imaging spinal osteomyelitis and epidural abscess with short TI inversion recovery (STIR). AJNR Am J Neuroradiol 1988; 9:563–564.
- Rigamonti D, Liem L, Sampath P, et al. Spinal epidural abscess: contemporary trends in etiology, evaluation, and management. Surg Neurol 1999; 52:189–197.
- De Vries F, Bracke M, Leufkens HG, Lammers JW, Cooper C, Van Staa TP. Fracture risk with intermittent high-dose oral glucocorticoid therapy. Arthritis Rheum 2007; 56:208–214.
- Cooper C, Atkinson EJ, O’Fallon WM, Melton LJ. Incidence of clinically diagnosed vertebral fractures: a population-based study in Rochester, Minnesota, 1985–1989. J Bone Miner Res 1992; 7:221–227.
- Kim DH, Vaccaro AR. Osteoporotic compression fractures of the spine; current options and considerations for treatment. Spine J 2006; 6:479–487.
- Cohn SH, Abesamis C, Yasumura S, Aloia JF, Zanzi I, Ellis KJ. Comparative skeletal mass and radial bone mineral content in black and white women. Metabolism 1977; 26:171–178.
- Tobias JH, Hutchinson AP, Hunt LP, et al. Use of clinical risk factors to identify postmenopausal women with vertebral fractures. Osteoporos Int 2007; 18:35–43.
- Melton LJ, Kallmes DF. Epidemiology of vertebral fractures: implications for vertebral augmentation. Acad Radiol 2006; 13:538–545.
- Silverman SL. The clinical consequences of vertebral compression fracture. Bone 1992; 13:S27–S31.
- Melton LJ, Kan SH, Frye MA, Wahner HW, O’Fallon WM, Riggs BL. Epidemiology of vertebral fractures in women. Am J Epidemiol 1989; 129:1000–1011.
- Wasnich RD. Vertebral fracture epidemiology. Bone 1996; 18:179S–183S.
- Yamato M, Nishimura G, Kuramochi E, Saiki N, Fujioka M. MR appearance at different ages of osteoporotic compression fractures of the vertebrae. Radiat Med 1998; 16:329–334.
- Einsiedel T, Schmelz A, Arand M, et al. Injuries of the cervical spine in patients with ankylosing spondylitis: experience at two trauma centers. J Neurosurg Spine 2006; 5:33–45.
- Olerud C, Frost A, Bring J. Spinal fractures in patients with ankylosing spondylitis. Eur Spine J 1996; 5:51–55.
- Spangfort EV. The lumbar disk herniation. A computer-aided analysis of 2,504 operations. Acta Orthop Scand Suppl 1972; 142:1–95.
- Kostuik JP, Harrington I, Alexander D, Rand W, Evans D. Cauda equina syndrome and lumbar disk herniation. J Bone Joint Surg Am 1986; 68:386–391.
- Lawton MT, Porter RW, Heiserman JE, Jacobowitz R, Sonntag VK, Dickman CA. Surgical management of spinal epidural hematoma: relationship between surgical timing and neurological outcome. J Neurosurg 1995; 83:1–7.
- Simmons EH, Grobler LJ. Acute spinal epidural hematoma. J Bone Joint Surg Am 1978; 60:395–396.
- Senelick RC, Norwood CW, Cohen GH. “Painless” spinal epidural hematoma during anticoagulant therapy”. Neurology 1976; 26:213–225.
- Watts C, Porto L. Recognizing spontaneous spinal epidural hematoma. Geriatrics 1976; 31:97–99.
Back pain is one of the most common complaints that internists and primary care physicians encounter.1 Although back pain is nonspecific, some hallmark signs and symptoms indicate that a patient is more likely to have a serious disorder. This article contrasts the presentation of cancer, infections, and fractures with the more common and benign conditions that cause back pain and provides guidance for diagnosis.
UNCOMMON, BUT MUST BE CONSIDERED
Although a variety of tissues can contribute to pain—intervertebral disks, vertebrae, ligaments, neural structures, muscles, and fascia—and many disorders can damage these tissues, most patients with back or neck pain have a benign condition. Back pain is typically caused by age-related degenerative changes or by minor repetitive trauma; with supportive care and physical therapy, up to 90% of patients with back pain of this nature improve substantially within 4 weeks.2
Serious, destructive diseases are uncommon causes of back pain: malignancy, infection, ankylosing spondylitis, and epidural abscess together account for fewer than 1% of cases of back pain in a typical primary care practice. But their clinical impact is out of proportion to their prevalence. The fear of overlooking a serious condition influences any practitioner’s approach to back pain and is a common reason for ordering multiple imaging studies and consultations.3 Therefore, the time, effort, and resources invested in ruling out these disorders is considerable.
Whether a patient with back pain has an ominous disease can usually be determined with a careful history, physical examination, and appropriate diagnostic studies. Once a serious diagnosis is ruled out, attention can be focused on rehabilitation and back care.
Back pain can also be due to musculoskeletal disorders, peptic ulcers, pancreatitis, pyelonephritis, aortic aneurysms, and other serious conditions, which we have discussed in other articles in this journal.4–6
SPINAL CANCER AND METASTASES
Since back pain is the presenting symptom in 90% of patients with spinal tumors,7 neoplasia belongs in the differential diagnosis of any patient with persistent, unremitting back pain. However, it is also important to recognize atypical presentations of neoplasia, such as a painless neurologic deficit, which should prompt an urgent workup.
The spine is one of the most common sites of metastasis: about 20,000 cases arise each year.8 Brihaye et al9 reviewed 1,477 cases of spinal metastases with epidural involvement and found that 16.5% arose from primary tumors in the breast, 15.6% from the lung, 9.2% from the prostate, and 6.5% from the kidney.
Cancer pain is persistent and progressive
Benign back pain often arises from a known injury, is relieved by rest, and increases with activities that load the disk (eg, sitting, getting up from bed or a chair), lumbar flexion with or without rotation, lifting, vibration (eg, riding in a car), coughing, sneezing, laughing, and the Valsalva maneuver. It is most commonly focal to the lumbosacral junction, the lumbar muscles, and the buttocks. Pain due to injury or a flare-up of degenerative disease typically begins to subside after 4 to 6 weeks and responds to nonsteroidal anti-inflammatory drugs and physical therapy.10
In contrast, pain caused by spinal neoplasia is typically persistent and progressive and is not alleviated by rest. Often the pain is worse at night, waking the patient from sleep. Back pain is typically focal to the level of the lesion and may be associated with belt-like thoracic pain or radicular symptoms of pain or weakness in the legs. A spinal mass can cause neurologic signs or symptoms by directly compressing the spinal cord or nerve roots, mimicking disk herniation or stenosis.11,12
Pathologic fractures resulting from vertebral destruction may be the first—and unfortunately a late—presentation of a tumor.
Ask about, look for, signs and symptoms of cancer
In taking the history, one should ask about possible signs and symptoms of systemic disease such as fatigue, weight loss, and changes in bowel habits. Hemoptysis, lymphadenopathy, subcutaneous or breast masses, nipple discharge, atypical vaginal bleeding, or blood in the stool suggest malignancy and should direct the specific diagnostic approach.13 A history of cancer, even if remote, should raise suspicion, as should major risk factors such as smoking.
Because most spinal tumors are metastases, a clinical examination of the breast, lungs, abdomen, thyroid, and prostate are appropriate starting points.14 The spine should be examined to identify sites of focal pain. A neurologic examination should be done to evaluate any signs of neurologic compromise or abnormal reflexes. Signs or symptoms of spinal cord compression should be investigated immediately.
Cancer usually elevates the ESR, CRP
If cancer is suspected, initial tests should include a complete blood cell count, erythrocyte sedimentation rate, C-reactive protein level, urinalysis, prostate-specific antigen level, and fecal occult blood testing. Normal results can considerably relieve suspicion of cancer: the erythrocyte sedimentation rate and C-reactive protein level are almost always elevated with systemic neoplasia.
Imaging tests
Unfortunately, spinal tumors cannot be well visualized on radiographs until significant destruction has occurred.15
A bone scan can usually detect tumors other than the purely lytic ones such as myeloma and has a sensitivity of 74%, a specificity of 81%, and a positive predictive value of 64% for vertebral metastasis in patients with back pain.16
INFECTION CAN BE INDOLENT OR ACUTE
Spinal infection is a serious condition that can take an indolent, smoldering course or, alternatively, can erupt into sepsis or rapidly progressive vertebral destruction. Although the latter conditions are hard to miss, early diskitis and osteomyelitis can be difficult to differentiate from idiopathic back pain. In a series of 101 patients with vertebral osteomyelitis, misdiagnosis occurred in 33.7%, and the average delay from the onset of clinical manifestations to diagnosis was 2.6 months.20 Tuberculosis can be even more elusive: in a series of 78 patients diagnosed with definite or probable tuberculous vertebral osteomyelitis, the mean delay to diagnosis was about 6 months.21
Acute spinal infections are most often pyogenic; chronic infections may be pyogenic, fungal, or granulomatous.
Vertebral osteomyelitis accounts for 2% to 7% of all cases of osteomyelitis and is an uncommon cause of back pain.22 Any source of infection (eg, dental abscess, pneumonia) can seed the spine; urinary tract infection is the most common. Patients with immunocompromise or diabetes are most at risk.23 The onset is usually insidious with focal back pain at the level of involvement.
History and physical examination reveal localized pain
Spinal infections typically cause pain that is worsened with weight-bearing and activity and is relieved only when lying down. Chronic infection is usually associated with weight loss, fatigue, fevers, and night sweats.
Pain is usually well localized and reproduced by palpation or percussion over the involved level. Severe pain can sometimes be elicited by sitting the patient up or by changing the patient’s position. Focal kyphosis may be detectable if the vertebra has collapsed.
In a series of 41 patients with pyogenic infectious spondylitis, 90% had localized back pain aggravated by percussion, 59% had radicular signs and symptoms, and 29% had neurologic signs of spinal cord compression, including hyperreflexia, clonus, the Babinski sign (extension of the toes upward when the sole of the foot is stroked upwards), or the Hoffmann sign (flexion of the thumb elicited by flicking the end of a middle finger).24
LABORATORY RESULTS TYPICALLY INDICATE INFECTION
The erythrocyte sedimentation rate is the most sensitive test for infection, and an elevated rate may be the only abnormal laboratory finding: Digby and Kersley25 found that the rate was increased in all of 30 patients with nontuberculous pyogenic osteomyelitis of the spine. The C-reactive protein level is also usually elevated, but 40% of patients have a normal white blood cell count.25 Results of other laboratory tests are typically in the normal range. Tuberculin skin testing should be done for patients at high risk of the disease (eg, immigrants from areas of endemic disease, non-Hispanic blacks, immunocompromised patients, and those with known exposure to tuberculosis). Patients with high fever, chills, or rigors should have cultures taken of blood, urine, and sputum and from any intravenous lines.
Imaging changes may not appear for months
CT, on the other hand, may be better for showing the extent of bone involvement. In cases of vertebral osteomyelitis and intervertebral disk space infection, simultaneous involvement of the adjacent vertebral end plates and the intervertebral disk are the major findings.30
Signs of infection using T1-weighted MRI include low-signal marrow or disk spaces within the vertebral body, loss of definition of end plates (which appear hypointense compared with the bone marrow), and destruction of the cortical margins of the involved vertebral bodies. T2-weighted MRI typically discloses high signals of the affected areas of the vertebral body and disk. Contrast should be used to increase specificity; enhancement may be the first sign of an acute inflammatory process.31
CT and MRI can help identify sequestra, perilesional sclerosis, and epidural or soft tissue abscesses. Guided biopsy may be needed to differentiate between abscess, hematoma, tumor, and inflammation.
MRI findings: Pyogenic vs tuberculous spondylitis
MRI can help differentiate pyogenic vertebral osteomyelitis from tubercular disease, although findings may be similar (eg, both conditions have a high signal on T2-weighted images).32 Jung et al,33 in a retrospective study of 52 patients with spondylitis, found that compared with patients with pyogenic infections, patients with tuberculous spondylitis had a significantly higher incidence of a well-defined paraspinal abnormal signal on MRI, a thin and smooth abscess wall, a paraspinal or intraosseous abscess, subligamentous spread to three or more vertebral levels, involvement of multiple vertebral bodies, thoracic spine involvement, and a hyperintense signal on T2-weighted images. Other MRI features characteristically seen in patients with tuberculous spinal disease are anterior corner destruction, a relative preservation of the intervertebral disk, and large soft-tissue abscesses with calcifications.34
Prompt diagnosis and aggressive treatment needed
Pigrau et al35 found that spinal osteomyelitis is highly associated with endocarditis: among 606 patients with infectious endocarditis, 28 (4.6%) had pyogenic vertebral osteomyelitis, and among 91 patients with pyogenic vertebral osteomyelitis, 28 (30.8%) had infectious endocarditis.
McHenry et al36 retrospectively studied outcomes of 253 patients with vertebral osteomyelitis after a median of 6.5 years (range 2 days to 38 years): 11% died, more than one-third of survivors had residual disability, and 14% had a relapse. Surgery resulted in recovery or improvement in 86 (79%) of 109 patients. Independent risk factors for adverse outcome (death or incomplete recovery) were neurologic compromise, increased time to diagnosis, and having a hospital-acquired infection (P = .004). Relapse commonly developed in patients with severe vertebral destruction and abscesses, which appeared some time after surgical drainage or debridement. Recurrent bacteremia, paravertebral abscesses, and chronically draining sinuses were independently associated with relapse (P = .001). MRI, done in 110 patients, was often performed late in the course of infection and did not significantly affect outcome. The authors stressed that an optimal outcome of vertebral osteomyelitis requires heightened awareness, early diagnosis, prompt identification of pathogens, reversal of complications, and prolonged antimicrobial therapy.
Epidural abscess may also be present
Epidural abscess occurs in 10% of spine infections. About half of patients with an epidural abscess are misdiagnosed on their initial evaluation.37,38 Patients initially complain of local spine pain, followed by radicular pain, weakness, and finally paralysis. Between 12% and 30% of patients report a history of trauma, even as minor as a fall, preceding the infection.38,39
Radiologic findings are frequently equivocal, and MRI is preferred; gadolinium enhancement further increases sensitivity.39,40 Spinal canal abscesses usually appear hypointense on T1-weighted images and hyperintense on T2-weighted images, with ring enhancement surrounding the abscess area in contrast studies.41 MRI may give negative findings in the early stages of a spinal canal infection and so may need to be repeated.41 MRI may not help distinguish an epidural from a subdural abscess. However, primary spinal epidural abscesses without concomitant vertebral osteomyelitis are rare; therefore, the finding of associated vertebral osteomyelitis makes a spinal epidural abscess more likely.
FRACTURES
Fractures of the spine can be asymptomatic and may have no preceding trauma. They can be due to osteoporosis, malignancy, infection, or metabolic disorders such as renal osteodystrophy or hyperparathyroidism. Fractures in normal bone are almost always associated with trauma. Any suspicion of infection or malignancy should be investigated.
Corticosteroids increase risk
Any patient with back pain who is receiving corticosteroid therapy should be considered as having a compression fracture until proven otherwise.3 De Vries et al42 found that in a database of nearly 200,000 patients receiving glucocorticoids, risk increased substantially with increasing cumulative exposure. Those who intermittently received high doses (= 15 mg/day) and those who had no or little previous exposure to corticosteroids (cumulative exposure = 1 g) had only a slightly increased risk of osteoporotic fracture, and their risk of fracture of the hip and femur was not increased. In contrast, patients who received a daily dose of at least 30 mg and whose cumulative exposure was more than 5 g had a relative risk of osteoporotic vertebral fracture of 14.42 (95% confidence interval 8.29–25.08).
Osteoporotic compression fractures are common in the elderly
Osteoporosis involves reduced bone density, disrupted trabecular architecture, and increased susceptibility to fractures. About 700,000 vertebral body compression fractures occur in the United States each year43: about 10% result in hospitalization, involving an average stay of 8 days.44 Osteoporotic compression fractures are highly associated with age older than 65, female sex, and European descent.45,46 The estimated lifetime risk of a clinically evident vertebral fracture after age 50 years is 16% among postmenopausal white women and 5% among white men.47
A single osteoporotic vertebral compression fracture increases the risk of subsequent fractures by a factor of five, and up to 20% of patients with a vertebral compression fracture are likely to have another one within the same year if osteoporosis remains untreated.48 Population studies suggest that the death rate among patients who have osteoporotic vertebral compression fractures increases with the number of involved vertebrae.43
Unfortunately, osteoporotic vertebral compression fractures are not always easily amenable to treatment: up to 30% of patients who are symptomatic and seek treatment do not respond adequately to nonsurgical methods.49,50 However, new minimally invasive interventions such as vertebral augmentation make timely evaluation clinically relevant.
History, physical examination
Patients may present with a history of trauma with associated back pain or a neurologic deficit. In osteoporotic patients, the trauma may have been minimal, eg, a sneeze, a fall from a chair, or a slip and fall in the home. Pain tends to be worse when standing erect and occasionally when lying flat.
The patient is commonly visibly uncomfortable and may be limited to a wheelchair or stoop forward when standing. The spine may show an absence of the midline crease or an exaggerated thoracic kyphosis. Pain is typically reproduced by deep pressure over the spinous process at the involved level. Compression fractures rarely cause neurologic deficits but should always be considered.
Fractures commonly occur in the thoracolumbar region but may be anywhere in the spine. Fractures in the upper thoracic spine may indicate an underlying malignant tumor, and a thorough search for a possible primary lesion should always be carried out for fractures in this location.
Laboratory testing
Routine laboratory evaluation and thyroid function tests should be done, as well as a 24-hour urine specimen for collagen breakdown products, calcium, phosphate, and creatinine levels. Serum and urine protein electrophoresis should be performed if myeloma is suspected. A white blood-cell count, erythrocyte sedimentation rate, and C-reactive protein level help determine if an underlying infection caused the fracture.
MRI needed if plain films reveal fracture or are equivocal
Anteroposterior and lateral roentgenograms should be taken first; they typically show osteopenia. A fracture in the vertebral body is characterized by loss of height and by wedging. Osseous fragments can occasionally be seen in the spinal canal.
Sagittal short tau inversion recovery sequences, which use specifically timed pulse sequences to suppress fat signals, show high-intensity signal changes in areas of edema from acute or healing fractures. They provide a sensitive but nonspecific marker of abnormality.
Dual energy x-ray absorptiometry helps determine the extent of osteoporosis.
Bone scans should only be used for patients with suspected metastatic disease.
Patients with ankylosing spondylitis need thorough workup
Ankylosing spondylitis predisposes to serious spinal injury. Even after only minor trauma, patients with ankylosing spondylitis and acute, severe back pain should be thoroughly evaluated for fracture with CT and MRI of the entire spine. Plain radiography should not be relied on for these patients because of the risk of misinterpretation, delayed diagnosis, and poorer outcomes.52,53
NEUROLOGIC COMPROMISE—A RED FLAG
Cauda equina compression classically presents with back pain, bilateral sciatica, saddle anesthesia, and lower extremity weakness progressing to paraplegia, but in practice these symptoms are variably present and diagnosing the condition often requires a high degree of suspicion. Hyporeflexia is typically a sign of cauda equina compression, while hyperreflexia, clonus, and the Babinski sign suggest spinal cord compression, requiring an evaluation of the cervical and thoracic spine. Cauda equina compression typically involves urinary retention; in contrast, cord compression typically causes incontinence.55
If either cauda equina or spinal cord compression is detected during an initial examination, an immediate more extensive evaluation is warranted. MRI is the study of choice.
Spinal epidural hematoma
Spinal epidural hematoma is a rare but dramatic cause of paralysis in elderly patients. In most cases, there is no antecedent trauma. Lawton et al,56 in a series of 30 patients treated surgically for spinal epidural hematoma, found that 73% resulted from spine surgery, epidural catheterization, or anticoagulation therapy. Other possible causes of epidural hematoma include vascular malformations, angiomas, aneurysms, hypertension, and aspirin therapy.57
The same study56 found that the time from the first symptom to maximal neurologic deficit ranged from a few minutes to 4 days, with the average interval being nearly 13 hours.
Although painless onset has been reported,58 spinal epidural hematoma typically presents with acute pain at the level of the lesion, which is often rapidly followed by paraplegia or quadriplegia, depending on the location of the hemorrhage. Sometimes the onset of pain is preceded by a sudden increase of venous pressure from coughing, sneezing, or straining at stool. Urinary retention often develops at an early stage.
Most lesions occur in the thoracic region and extend into the cervicothoracic or the thoracolumbar area. The pain distribution may be radicular, mimicking a ruptured intervertebral disk.
Evaluation should be with MRI. Acute hemorrhage is characterized by a marked decrease in signal intensity on T2-weighted images. Subacute hematoma has increased signal intensity on both T1- and T2-weighted images.56
Early recognition, MRI confirmation, and treatment should be accomplished as soon as possible.56 Recovery depends on the severity of the neurologic deficit and the duration of symptoms before treatment. Lawton et al56 found that patients taken to surgery within 12 hours had better neurologic outcomes than patients with identical preoperative neurologic status whose surgery was delayed beyond 12 hours. Surgery should not be withheld because of advanced age or poor health: in 10 reported cases in which surgery was delayed, all patients died.59
Back pain is one of the most common complaints that internists and primary care physicians encounter.1 Although back pain is nonspecific, some hallmark signs and symptoms indicate that a patient is more likely to have a serious disorder. This article contrasts the presentation of cancer, infections, and fractures with the more common and benign conditions that cause back pain and provides guidance for diagnosis.
UNCOMMON, BUT MUST BE CONSIDERED
Although a variety of tissues can contribute to pain—intervertebral disks, vertebrae, ligaments, neural structures, muscles, and fascia—and many disorders can damage these tissues, most patients with back or neck pain have a benign condition. Back pain is typically caused by age-related degenerative changes or by minor repetitive trauma; with supportive care and physical therapy, up to 90% of patients with back pain of this nature improve substantially within 4 weeks.2
Serious, destructive diseases are uncommon causes of back pain: malignancy, infection, ankylosing spondylitis, and epidural abscess together account for fewer than 1% of cases of back pain in a typical primary care practice. But their clinical impact is out of proportion to their prevalence. The fear of overlooking a serious condition influences any practitioner’s approach to back pain and is a common reason for ordering multiple imaging studies and consultations.3 Therefore, the time, effort, and resources invested in ruling out these disorders is considerable.
Whether a patient with back pain has an ominous disease can usually be determined with a careful history, physical examination, and appropriate diagnostic studies. Once a serious diagnosis is ruled out, attention can be focused on rehabilitation and back care.
Back pain can also be due to musculoskeletal disorders, peptic ulcers, pancreatitis, pyelonephritis, aortic aneurysms, and other serious conditions, which we have discussed in other articles in this journal.4–6
SPINAL CANCER AND METASTASES
Since back pain is the presenting symptom in 90% of patients with spinal tumors,7 neoplasia belongs in the differential diagnosis of any patient with persistent, unremitting back pain. However, it is also important to recognize atypical presentations of neoplasia, such as a painless neurologic deficit, which should prompt an urgent workup.
The spine is one of the most common sites of metastasis: about 20,000 cases arise each year.8 Brihaye et al9 reviewed 1,477 cases of spinal metastases with epidural involvement and found that 16.5% arose from primary tumors in the breast, 15.6% from the lung, 9.2% from the prostate, and 6.5% from the kidney.
Cancer pain is persistent and progressive
Benign back pain often arises from a known injury, is relieved by rest, and increases with activities that load the disk (eg, sitting, getting up from bed or a chair), lumbar flexion with or without rotation, lifting, vibration (eg, riding in a car), coughing, sneezing, laughing, and the Valsalva maneuver. It is most commonly focal to the lumbosacral junction, the lumbar muscles, and the buttocks. Pain due to injury or a flare-up of degenerative disease typically begins to subside after 4 to 6 weeks and responds to nonsteroidal anti-inflammatory drugs and physical therapy.10
In contrast, pain caused by spinal neoplasia is typically persistent and progressive and is not alleviated by rest. Often the pain is worse at night, waking the patient from sleep. Back pain is typically focal to the level of the lesion and may be associated with belt-like thoracic pain or radicular symptoms of pain or weakness in the legs. A spinal mass can cause neurologic signs or symptoms by directly compressing the spinal cord or nerve roots, mimicking disk herniation or stenosis.11,12
Pathologic fractures resulting from vertebral destruction may be the first—and unfortunately a late—presentation of a tumor.
Ask about, look for, signs and symptoms of cancer
In taking the history, one should ask about possible signs and symptoms of systemic disease such as fatigue, weight loss, and changes in bowel habits. Hemoptysis, lymphadenopathy, subcutaneous or breast masses, nipple discharge, atypical vaginal bleeding, or blood in the stool suggest malignancy and should direct the specific diagnostic approach.13 A history of cancer, even if remote, should raise suspicion, as should major risk factors such as smoking.
Because most spinal tumors are metastases, a clinical examination of the breast, lungs, abdomen, thyroid, and prostate are appropriate starting points.14 The spine should be examined to identify sites of focal pain. A neurologic examination should be done to evaluate any signs of neurologic compromise or abnormal reflexes. Signs or symptoms of spinal cord compression should be investigated immediately.
Cancer usually elevates the ESR, CRP
If cancer is suspected, initial tests should include a complete blood cell count, erythrocyte sedimentation rate, C-reactive protein level, urinalysis, prostate-specific antigen level, and fecal occult blood testing. Normal results can considerably relieve suspicion of cancer: the erythrocyte sedimentation rate and C-reactive protein level are almost always elevated with systemic neoplasia.
Imaging tests
Unfortunately, spinal tumors cannot be well visualized on radiographs until significant destruction has occurred.15
A bone scan can usually detect tumors other than the purely lytic ones such as myeloma and has a sensitivity of 74%, a specificity of 81%, and a positive predictive value of 64% for vertebral metastasis in patients with back pain.16
INFECTION CAN BE INDOLENT OR ACUTE
Spinal infection is a serious condition that can take an indolent, smoldering course or, alternatively, can erupt into sepsis or rapidly progressive vertebral destruction. Although the latter conditions are hard to miss, early diskitis and osteomyelitis can be difficult to differentiate from idiopathic back pain. In a series of 101 patients with vertebral osteomyelitis, misdiagnosis occurred in 33.7%, and the average delay from the onset of clinical manifestations to diagnosis was 2.6 months.20 Tuberculosis can be even more elusive: in a series of 78 patients diagnosed with definite or probable tuberculous vertebral osteomyelitis, the mean delay to diagnosis was about 6 months.21
Acute spinal infections are most often pyogenic; chronic infections may be pyogenic, fungal, or granulomatous.
Vertebral osteomyelitis accounts for 2% to 7% of all cases of osteomyelitis and is an uncommon cause of back pain.22 Any source of infection (eg, dental abscess, pneumonia) can seed the spine; urinary tract infection is the most common. Patients with immunocompromise or diabetes are most at risk.23 The onset is usually insidious with focal back pain at the level of involvement.
History and physical examination reveal localized pain
Spinal infections typically cause pain that is worsened with weight-bearing and activity and is relieved only when lying down. Chronic infection is usually associated with weight loss, fatigue, fevers, and night sweats.
Pain is usually well localized and reproduced by palpation or percussion over the involved level. Severe pain can sometimes be elicited by sitting the patient up or by changing the patient’s position. Focal kyphosis may be detectable if the vertebra has collapsed.
In a series of 41 patients with pyogenic infectious spondylitis, 90% had localized back pain aggravated by percussion, 59% had radicular signs and symptoms, and 29% had neurologic signs of spinal cord compression, including hyperreflexia, clonus, the Babinski sign (extension of the toes upward when the sole of the foot is stroked upwards), or the Hoffmann sign (flexion of the thumb elicited by flicking the end of a middle finger).24
LABORATORY RESULTS TYPICALLY INDICATE INFECTION
The erythrocyte sedimentation rate is the most sensitive test for infection, and an elevated rate may be the only abnormal laboratory finding: Digby and Kersley25 found that the rate was increased in all of 30 patients with nontuberculous pyogenic osteomyelitis of the spine. The C-reactive protein level is also usually elevated, but 40% of patients have a normal white blood cell count.25 Results of other laboratory tests are typically in the normal range. Tuberculin skin testing should be done for patients at high risk of the disease (eg, immigrants from areas of endemic disease, non-Hispanic blacks, immunocompromised patients, and those with known exposure to tuberculosis). Patients with high fever, chills, or rigors should have cultures taken of blood, urine, and sputum and from any intravenous lines.
Imaging changes may not appear for months
CT, on the other hand, may be better for showing the extent of bone involvement. In cases of vertebral osteomyelitis and intervertebral disk space infection, simultaneous involvement of the adjacent vertebral end plates and the intervertebral disk are the major findings.30
Signs of infection using T1-weighted MRI include low-signal marrow or disk spaces within the vertebral body, loss of definition of end plates (which appear hypointense compared with the bone marrow), and destruction of the cortical margins of the involved vertebral bodies. T2-weighted MRI typically discloses high signals of the affected areas of the vertebral body and disk. Contrast should be used to increase specificity; enhancement may be the first sign of an acute inflammatory process.31
CT and MRI can help identify sequestra, perilesional sclerosis, and epidural or soft tissue abscesses. Guided biopsy may be needed to differentiate between abscess, hematoma, tumor, and inflammation.
MRI findings: Pyogenic vs tuberculous spondylitis
MRI can help differentiate pyogenic vertebral osteomyelitis from tubercular disease, although findings may be similar (eg, both conditions have a high signal on T2-weighted images).32 Jung et al,33 in a retrospective study of 52 patients with spondylitis, found that compared with patients with pyogenic infections, patients with tuberculous spondylitis had a significantly higher incidence of a well-defined paraspinal abnormal signal on MRI, a thin and smooth abscess wall, a paraspinal or intraosseous abscess, subligamentous spread to three or more vertebral levels, involvement of multiple vertebral bodies, thoracic spine involvement, and a hyperintense signal on T2-weighted images. Other MRI features characteristically seen in patients with tuberculous spinal disease are anterior corner destruction, a relative preservation of the intervertebral disk, and large soft-tissue abscesses with calcifications.34
Prompt diagnosis and aggressive treatment needed
Pigrau et al35 found that spinal osteomyelitis is highly associated with endocarditis: among 606 patients with infectious endocarditis, 28 (4.6%) had pyogenic vertebral osteomyelitis, and among 91 patients with pyogenic vertebral osteomyelitis, 28 (30.8%) had infectious endocarditis.
McHenry et al36 retrospectively studied outcomes of 253 patients with vertebral osteomyelitis after a median of 6.5 years (range 2 days to 38 years): 11% died, more than one-third of survivors had residual disability, and 14% had a relapse. Surgery resulted in recovery or improvement in 86 (79%) of 109 patients. Independent risk factors for adverse outcome (death or incomplete recovery) were neurologic compromise, increased time to diagnosis, and having a hospital-acquired infection (P = .004). Relapse commonly developed in patients with severe vertebral destruction and abscesses, which appeared some time after surgical drainage or debridement. Recurrent bacteremia, paravertebral abscesses, and chronically draining sinuses were independently associated with relapse (P = .001). MRI, done in 110 patients, was often performed late in the course of infection and did not significantly affect outcome. The authors stressed that an optimal outcome of vertebral osteomyelitis requires heightened awareness, early diagnosis, prompt identification of pathogens, reversal of complications, and prolonged antimicrobial therapy.
Epidural abscess may also be present
Epidural abscess occurs in 10% of spine infections. About half of patients with an epidural abscess are misdiagnosed on their initial evaluation.37,38 Patients initially complain of local spine pain, followed by radicular pain, weakness, and finally paralysis. Between 12% and 30% of patients report a history of trauma, even as minor as a fall, preceding the infection.38,39
Radiologic findings are frequently equivocal, and MRI is preferred; gadolinium enhancement further increases sensitivity.39,40 Spinal canal abscesses usually appear hypointense on T1-weighted images and hyperintense on T2-weighted images, with ring enhancement surrounding the abscess area in contrast studies.41 MRI may give negative findings in the early stages of a spinal canal infection and so may need to be repeated.41 MRI may not help distinguish an epidural from a subdural abscess. However, primary spinal epidural abscesses without concomitant vertebral osteomyelitis are rare; therefore, the finding of associated vertebral osteomyelitis makes a spinal epidural abscess more likely.
FRACTURES
Fractures of the spine can be asymptomatic and may have no preceding trauma. They can be due to osteoporosis, malignancy, infection, or metabolic disorders such as renal osteodystrophy or hyperparathyroidism. Fractures in normal bone are almost always associated with trauma. Any suspicion of infection or malignancy should be investigated.
Corticosteroids increase risk
Any patient with back pain who is receiving corticosteroid therapy should be considered as having a compression fracture until proven otherwise.3 De Vries et al42 found that in a database of nearly 200,000 patients receiving glucocorticoids, risk increased substantially with increasing cumulative exposure. Those who intermittently received high doses (= 15 mg/day) and those who had no or little previous exposure to corticosteroids (cumulative exposure = 1 g) had only a slightly increased risk of osteoporotic fracture, and their risk of fracture of the hip and femur was not increased. In contrast, patients who received a daily dose of at least 30 mg and whose cumulative exposure was more than 5 g had a relative risk of osteoporotic vertebral fracture of 14.42 (95% confidence interval 8.29–25.08).
Osteoporotic compression fractures are common in the elderly
Osteoporosis involves reduced bone density, disrupted trabecular architecture, and increased susceptibility to fractures. About 700,000 vertebral body compression fractures occur in the United States each year43: about 10% result in hospitalization, involving an average stay of 8 days.44 Osteoporotic compression fractures are highly associated with age older than 65, female sex, and European descent.45,46 The estimated lifetime risk of a clinically evident vertebral fracture after age 50 years is 16% among postmenopausal white women and 5% among white men.47
A single osteoporotic vertebral compression fracture increases the risk of subsequent fractures by a factor of five, and up to 20% of patients with a vertebral compression fracture are likely to have another one within the same year if osteoporosis remains untreated.48 Population studies suggest that the death rate among patients who have osteoporotic vertebral compression fractures increases with the number of involved vertebrae.43
Unfortunately, osteoporotic vertebral compression fractures are not always easily amenable to treatment: up to 30% of patients who are symptomatic and seek treatment do not respond adequately to nonsurgical methods.49,50 However, new minimally invasive interventions such as vertebral augmentation make timely evaluation clinically relevant.
History, physical examination
Patients may present with a history of trauma with associated back pain or a neurologic deficit. In osteoporotic patients, the trauma may have been minimal, eg, a sneeze, a fall from a chair, or a slip and fall in the home. Pain tends to be worse when standing erect and occasionally when lying flat.
The patient is commonly visibly uncomfortable and may be limited to a wheelchair or stoop forward when standing. The spine may show an absence of the midline crease or an exaggerated thoracic kyphosis. Pain is typically reproduced by deep pressure over the spinous process at the involved level. Compression fractures rarely cause neurologic deficits but should always be considered.
Fractures commonly occur in the thoracolumbar region but may be anywhere in the spine. Fractures in the upper thoracic spine may indicate an underlying malignant tumor, and a thorough search for a possible primary lesion should always be carried out for fractures in this location.
Laboratory testing
Routine laboratory evaluation and thyroid function tests should be done, as well as a 24-hour urine specimen for collagen breakdown products, calcium, phosphate, and creatinine levels. Serum and urine protein electrophoresis should be performed if myeloma is suspected. A white blood-cell count, erythrocyte sedimentation rate, and C-reactive protein level help determine if an underlying infection caused the fracture.
MRI needed if plain films reveal fracture or are equivocal
Anteroposterior and lateral roentgenograms should be taken first; they typically show osteopenia. A fracture in the vertebral body is characterized by loss of height and by wedging. Osseous fragments can occasionally be seen in the spinal canal.
Sagittal short tau inversion recovery sequences, which use specifically timed pulse sequences to suppress fat signals, show high-intensity signal changes in areas of edema from acute or healing fractures. They provide a sensitive but nonspecific marker of abnormality.
Dual energy x-ray absorptiometry helps determine the extent of osteoporosis.
Bone scans should only be used for patients with suspected metastatic disease.
Patients with ankylosing spondylitis need thorough workup
Ankylosing spondylitis predisposes to serious spinal injury. Even after only minor trauma, patients with ankylosing spondylitis and acute, severe back pain should be thoroughly evaluated for fracture with CT and MRI of the entire spine. Plain radiography should not be relied on for these patients because of the risk of misinterpretation, delayed diagnosis, and poorer outcomes.52,53
NEUROLOGIC COMPROMISE—A RED FLAG
Cauda equina compression classically presents with back pain, bilateral sciatica, saddle anesthesia, and lower extremity weakness progressing to paraplegia, but in practice these symptoms are variably present and diagnosing the condition often requires a high degree of suspicion. Hyporeflexia is typically a sign of cauda equina compression, while hyperreflexia, clonus, and the Babinski sign suggest spinal cord compression, requiring an evaluation of the cervical and thoracic spine. Cauda equina compression typically involves urinary retention; in contrast, cord compression typically causes incontinence.55
If either cauda equina or spinal cord compression is detected during an initial examination, an immediate more extensive evaluation is warranted. MRI is the study of choice.
Spinal epidural hematoma
Spinal epidural hematoma is a rare but dramatic cause of paralysis in elderly patients. In most cases, there is no antecedent trauma. Lawton et al,56 in a series of 30 patients treated surgically for spinal epidural hematoma, found that 73% resulted from spine surgery, epidural catheterization, or anticoagulation therapy. Other possible causes of epidural hematoma include vascular malformations, angiomas, aneurysms, hypertension, and aspirin therapy.57
The same study56 found that the time from the first symptom to maximal neurologic deficit ranged from a few minutes to 4 days, with the average interval being nearly 13 hours.
Although painless onset has been reported,58 spinal epidural hematoma typically presents with acute pain at the level of the lesion, which is often rapidly followed by paraplegia or quadriplegia, depending on the location of the hemorrhage. Sometimes the onset of pain is preceded by a sudden increase of venous pressure from coughing, sneezing, or straining at stool. Urinary retention often develops at an early stage.
Most lesions occur in the thoracic region and extend into the cervicothoracic or the thoracolumbar area. The pain distribution may be radicular, mimicking a ruptured intervertebral disk.
Evaluation should be with MRI. Acute hemorrhage is characterized by a marked decrease in signal intensity on T2-weighted images. Subacute hematoma has increased signal intensity on both T1- and T2-weighted images.56
Early recognition, MRI confirmation, and treatment should be accomplished as soon as possible.56 Recovery depends on the severity of the neurologic deficit and the duration of symptoms before treatment. Lawton et al56 found that patients taken to surgery within 12 hours had better neurologic outcomes than patients with identical preoperative neurologic status whose surgery was delayed beyond 12 hours. Surgery should not be withheld because of advanced age or poor health: in 10 reported cases in which surgery was delayed, all patients died.59
- Deyo RA, Tsui-Wu YJ. Descriptive epidemiology of low-back pain and its related medical care in the United States. Spine 1987; 12:264–268.
- Pengel LH, Herbert RD, Maher CG, Refshauge KM. Acute back pain: systematic review of its prognosis. BMJ 2003; 327:323–325.
- Deyo RA, Rainville J, Kent DL. What can the history and physical examination tell us about back pain? JAMA 1992; 268:760–765.
- Pateder DB, Brems J, Lieberman I, Bell GR, McLain RF. Masquerade: nonspinal musculoskeletal disorders that mimic spinal conditions. Cleve Clin J Med 2008; 75:50–56.
- Klineberg E, Mazanec D, Orr D, Demicco R, Bell G, McLain R. Masquerade: medical causes of back pain. Cleve Clin J Med 2007; 74:905–913.
- McLain RF, Bell G, Montgomery W. Masquerade: systemic causes of back pain. Cleve Clin J Med In press.
- Gilbert RW, Kim JH, Posner JB. Epidural spinal cord compression from metastatic tumor: diagnosis and treatment. Ann Neurol 1978; 3:40–51.
- Black P. Spinal metastasis: current status and recommended guidelines for management. Neurosurgery 1979; 5:726–746.
- Brihaye J, Ectors P, Lemort M, Van Houtte P. The management of spinal epidural metastases. Adv Tech Stand Neurosurg 1988; 16:121–176.
- Patel RK, Slipman CW. Lumbar degenerative disk disease. Emedicine. Accessed March 25, 2008. http://www.emedicine.com/pmr/topic67.htm.
- Deyo RA, Diehl AK. Cancer as a cause of back pain: frequency, clinical presentation, and diagnostic strategies. J Gen Intern Med 1988; 3:230–238.
- Rosen P, Barkin RM, Danzl DF, et al. Emergency Medicine: Concepts and Clinical Practice. 4th ed. St Louis, MO: Mosby; 1998:2100–2102.
- Abbruzzese JL, Abbruzzese MC, Lenzi R, Hess KR, Raber MN. Analysis of a diagnostic strategy for patients with suspected tumors of unknown origin. J Clin Oncol 1995; 13:2094–2103.
- McCarthy EF, Frassica FJ. Pathology of Bone and Joint Disorders: With Clinical and Radiographic Correlation. Philadelphia: WB Saunders; 1998.
- Edelstyn GA, Gillespie PJ, Grebbell FS. The radiological demonstration of osseous metastases. Experimental observations. Clin Radiol 1967; 18:158–162.
- Han LJ, Au-Yong TK, Tong WC, Chu KS, Szeto LT, Wong CP. Comparison of bone single-photon emission tomography and planar imaging in the detection of vertebral metastases in patients with back pain. Eur J Nucl Med 1998; 25:635–638.
- Feun LG, Savaraj N. Detection of occult bone metastasis by MRI scan. J Fla Med Assoc 1990; 77:881–883.
- Citrin DL, Bessent RG, Greig WR. A comparison of the sensitivity and accuracy of the 99TCm-phosphate bone scan and skeletal radiograph in the diagnosis of bone metastases. Clin Radiol 1977; 28:107–117.
- Runge VM, Lee C, Iten AL, Williams NM. Contrast-enhanced magnetic resonance imaging in a spinal epidural tumor model. Invest Radiol 1997; 32:589–595.
- Buranapanitkit B, Lim A, Geater A. Misdiagnosis in vertebral osteomyelitis: problems and factors. J Med Assoc Thai 2001; 84:1743–1750.
- Colmenero JD, Jiménez-Mejías ME, Reguera JM, et al. Tuberculous vertebral osteomyelitis in the new millennium: still a diagnostic and therapeutic challenge. Eur J Clin Microbiol Infect Dis 2004; 23:477–483.
- Waldvogel FA, Medoff G, Swartz MN. Osteomyelitis: a review of clinical features, therapeutic considerations and unusual aspects. 3. Osteomyelitis associated with vascular insufficiency. N Engl J Med 1970; 282:316–322.
- Carragee EJ. Pyogenic vertebral osteomyelitis. J Bone Joint Surg Am 1997; 79:874–880.
- Kapeller P, Fazekas F, Krametter D, et al. Pyogenic infectious spondylitis: clinical, laboratory and MRI features. Eur Neurol 1997; 38:94–98.
- Digby JM, Kersley JB. Pyogenic non-tuberculous spinal infection: an analysis of thirty cases. J Bone Joint Surg Br 1979; 61:47–55.
- Modic MT, Feiglin DH, Piraino DW, et al. Vertebral osteomyelitis: assessment using MR. Radiology 1985; 157:157–166.
- Szypryt EP, Hardy JG, Hinton CE, Worthington BS, Mulholland RC. A comparison between magnetic resonance imaging and scintigraphic bone imaging in the diagnosis of disk space infection in an animal model. Spine 1988; 13:1042–1048.
- Küker W, Mull M, Mayfrank L, Töpper R, Thron A. Epidural spinal infection. Variability of clinical and magnetic resonance imaging findings. Spine 1997; 22:544–551.
- Tung GA, Yim JW, Mermel LA, Philip L, Rogg JM. Spinal epidural abscess: correlation between MRI findings and outcome. Neuroradiology 1999; 41:904–909.
- Sapico FL, Montgomerie JZ. Vertebral osteomyelitis. Infect Dis Clin North Am 1990; 4:539–550.
- Tali ET. Spinal infections. Eur J Radiol 2004; 50:120–133.
- Smith AS, Weinstein MA, Mizushima A, et al. MR imaging characteristics of tuberculous spondylitis vs vertebral osteomyelitis. AJR Am J Roentgenol 1989; 153:399–405.
- Jung NY, Jee WH, Ha KY, Park CK, Byun JY. Discrimination of tuberculous spondylitis from pyogenic spondylitis on MRI. AJR Am J Roentgenol 2004; 182:1405–1410.
- Joseffer SS, Cooper PR. Modern imaging of spinal tuberculosis. J Neurosurg Spine 2005; 2:145–150.
- Pigrau C, Almirante B, Flores X, et al. Spontaneous pyogenic vertebral osteomyelitis and endocarditis: incidence, risk factors, and outcome. Am J Med 2005; 118:1287.
- McHenry MC, Easley KA, Locker GA. Vertebral osteomyelitis: long-term outcome for 253 patients from 7 Cleveland-area hospitals. Clin Infect Dis 2002; 34:1342–1350.
- Danner RL, Hartman BJ. Update on spinal epidural abscess: 35 cases and review of the literature. Rev Infect Dis 1987; 9:265–274.
- Kaufman DM, Kaplan JG, Litman N. Infectious agents in spinal epidural abscesses. Neurology 1980; 30:844–850.
- Rezai AR, Woo HH, Errico TJ, Cooper PR. Contemporary management of spinal osteomyelitis. Neurosurgery 1999; 44:1018–1026.
- Bertino RE, Porter BA, Stimac GK, Tepper SJ. Imaging spinal osteomyelitis and epidural abscess with short TI inversion recovery (STIR). AJNR Am J Neuroradiol 1988; 9:563–564.
- Rigamonti D, Liem L, Sampath P, et al. Spinal epidural abscess: contemporary trends in etiology, evaluation, and management. Surg Neurol 1999; 52:189–197.
- De Vries F, Bracke M, Leufkens HG, Lammers JW, Cooper C, Van Staa TP. Fracture risk with intermittent high-dose oral glucocorticoid therapy. Arthritis Rheum 2007; 56:208–214.
- Cooper C, Atkinson EJ, O’Fallon WM, Melton LJ. Incidence of clinically diagnosed vertebral fractures: a population-based study in Rochester, Minnesota, 1985–1989. J Bone Miner Res 1992; 7:221–227.
- Kim DH, Vaccaro AR. Osteoporotic compression fractures of the spine; current options and considerations for treatment. Spine J 2006; 6:479–487.
- Cohn SH, Abesamis C, Yasumura S, Aloia JF, Zanzi I, Ellis KJ. Comparative skeletal mass and radial bone mineral content in black and white women. Metabolism 1977; 26:171–178.
- Tobias JH, Hutchinson AP, Hunt LP, et al. Use of clinical risk factors to identify postmenopausal women with vertebral fractures. Osteoporos Int 2007; 18:35–43.
- Melton LJ, Kallmes DF. Epidemiology of vertebral fractures: implications for vertebral augmentation. Acad Radiol 2006; 13:538–545.
- Silverman SL. The clinical consequences of vertebral compression fracture. Bone 1992; 13:S27–S31.
- Melton LJ, Kan SH, Frye MA, Wahner HW, O’Fallon WM, Riggs BL. Epidemiology of vertebral fractures in women. Am J Epidemiol 1989; 129:1000–1011.
- Wasnich RD. Vertebral fracture epidemiology. Bone 1996; 18:179S–183S.
- Yamato M, Nishimura G, Kuramochi E, Saiki N, Fujioka M. MR appearance at different ages of osteoporotic compression fractures of the vertebrae. Radiat Med 1998; 16:329–334.
- Einsiedel T, Schmelz A, Arand M, et al. Injuries of the cervical spine in patients with ankylosing spondylitis: experience at two trauma centers. J Neurosurg Spine 2006; 5:33–45.
- Olerud C, Frost A, Bring J. Spinal fractures in patients with ankylosing spondylitis. Eur Spine J 1996; 5:51–55.
- Spangfort EV. The lumbar disk herniation. A computer-aided analysis of 2,504 operations. Acta Orthop Scand Suppl 1972; 142:1–95.
- Kostuik JP, Harrington I, Alexander D, Rand W, Evans D. Cauda equina syndrome and lumbar disk herniation. J Bone Joint Surg Am 1986; 68:386–391.
- Lawton MT, Porter RW, Heiserman JE, Jacobowitz R, Sonntag VK, Dickman CA. Surgical management of spinal epidural hematoma: relationship between surgical timing and neurological outcome. J Neurosurg 1995; 83:1–7.
- Simmons EH, Grobler LJ. Acute spinal epidural hematoma. J Bone Joint Surg Am 1978; 60:395–396.
- Senelick RC, Norwood CW, Cohen GH. “Painless” spinal epidural hematoma during anticoagulant therapy”. Neurology 1976; 26:213–225.
- Watts C, Porto L. Recognizing spontaneous spinal epidural hematoma. Geriatrics 1976; 31:97–99.
- Deyo RA, Tsui-Wu YJ. Descriptive epidemiology of low-back pain and its related medical care in the United States. Spine 1987; 12:264–268.
- Pengel LH, Herbert RD, Maher CG, Refshauge KM. Acute back pain: systematic review of its prognosis. BMJ 2003; 327:323–325.
- Deyo RA, Rainville J, Kent DL. What can the history and physical examination tell us about back pain? JAMA 1992; 268:760–765.
- Pateder DB, Brems J, Lieberman I, Bell GR, McLain RF. Masquerade: nonspinal musculoskeletal disorders that mimic spinal conditions. Cleve Clin J Med 2008; 75:50–56.
- Klineberg E, Mazanec D, Orr D, Demicco R, Bell G, McLain R. Masquerade: medical causes of back pain. Cleve Clin J Med 2007; 74:905–913.
- McLain RF, Bell G, Montgomery W. Masquerade: systemic causes of back pain. Cleve Clin J Med In press.
- Gilbert RW, Kim JH, Posner JB. Epidural spinal cord compression from metastatic tumor: diagnosis and treatment. Ann Neurol 1978; 3:40–51.
- Black P. Spinal metastasis: current status and recommended guidelines for management. Neurosurgery 1979; 5:726–746.
- Brihaye J, Ectors P, Lemort M, Van Houtte P. The management of spinal epidural metastases. Adv Tech Stand Neurosurg 1988; 16:121–176.
- Patel RK, Slipman CW. Lumbar degenerative disk disease. Emedicine. Accessed March 25, 2008. http://www.emedicine.com/pmr/topic67.htm.
- Deyo RA, Diehl AK. Cancer as a cause of back pain: frequency, clinical presentation, and diagnostic strategies. J Gen Intern Med 1988; 3:230–238.
- Rosen P, Barkin RM, Danzl DF, et al. Emergency Medicine: Concepts and Clinical Practice. 4th ed. St Louis, MO: Mosby; 1998:2100–2102.
- Abbruzzese JL, Abbruzzese MC, Lenzi R, Hess KR, Raber MN. Analysis of a diagnostic strategy for patients with suspected tumors of unknown origin. J Clin Oncol 1995; 13:2094–2103.
- McCarthy EF, Frassica FJ. Pathology of Bone and Joint Disorders: With Clinical and Radiographic Correlation. Philadelphia: WB Saunders; 1998.
- Edelstyn GA, Gillespie PJ, Grebbell FS. The radiological demonstration of osseous metastases. Experimental observations. Clin Radiol 1967; 18:158–162.
- Han LJ, Au-Yong TK, Tong WC, Chu KS, Szeto LT, Wong CP. Comparison of bone single-photon emission tomography and planar imaging in the detection of vertebral metastases in patients with back pain. Eur J Nucl Med 1998; 25:635–638.
- Feun LG, Savaraj N. Detection of occult bone metastasis by MRI scan. J Fla Med Assoc 1990; 77:881–883.
- Citrin DL, Bessent RG, Greig WR. A comparison of the sensitivity and accuracy of the 99TCm-phosphate bone scan and skeletal radiograph in the diagnosis of bone metastases. Clin Radiol 1977; 28:107–117.
- Runge VM, Lee C, Iten AL, Williams NM. Contrast-enhanced magnetic resonance imaging in a spinal epidural tumor model. Invest Radiol 1997; 32:589–595.
- Buranapanitkit B, Lim A, Geater A. Misdiagnosis in vertebral osteomyelitis: problems and factors. J Med Assoc Thai 2001; 84:1743–1750.
- Colmenero JD, Jiménez-Mejías ME, Reguera JM, et al. Tuberculous vertebral osteomyelitis in the new millennium: still a diagnostic and therapeutic challenge. Eur J Clin Microbiol Infect Dis 2004; 23:477–483.
- Waldvogel FA, Medoff G, Swartz MN. Osteomyelitis: a review of clinical features, therapeutic considerations and unusual aspects. 3. Osteomyelitis associated with vascular insufficiency. N Engl J Med 1970; 282:316–322.
- Carragee EJ. Pyogenic vertebral osteomyelitis. J Bone Joint Surg Am 1997; 79:874–880.
- Kapeller P, Fazekas F, Krametter D, et al. Pyogenic infectious spondylitis: clinical, laboratory and MRI features. Eur Neurol 1997; 38:94–98.
- Digby JM, Kersley JB. Pyogenic non-tuberculous spinal infection: an analysis of thirty cases. J Bone Joint Surg Br 1979; 61:47–55.
- Modic MT, Feiglin DH, Piraino DW, et al. Vertebral osteomyelitis: assessment using MR. Radiology 1985; 157:157–166.
- Szypryt EP, Hardy JG, Hinton CE, Worthington BS, Mulholland RC. A comparison between magnetic resonance imaging and scintigraphic bone imaging in the diagnosis of disk space infection in an animal model. Spine 1988; 13:1042–1048.
- Küker W, Mull M, Mayfrank L, Töpper R, Thron A. Epidural spinal infection. Variability of clinical and magnetic resonance imaging findings. Spine 1997; 22:544–551.
- Tung GA, Yim JW, Mermel LA, Philip L, Rogg JM. Spinal epidural abscess: correlation between MRI findings and outcome. Neuroradiology 1999; 41:904–909.
- Sapico FL, Montgomerie JZ. Vertebral osteomyelitis. Infect Dis Clin North Am 1990; 4:539–550.
- Tali ET. Spinal infections. Eur J Radiol 2004; 50:120–133.
- Smith AS, Weinstein MA, Mizushima A, et al. MR imaging characteristics of tuberculous spondylitis vs vertebral osteomyelitis. AJR Am J Roentgenol 1989; 153:399–405.
- Jung NY, Jee WH, Ha KY, Park CK, Byun JY. Discrimination of tuberculous spondylitis from pyogenic spondylitis on MRI. AJR Am J Roentgenol 2004; 182:1405–1410.
- Joseffer SS, Cooper PR. Modern imaging of spinal tuberculosis. J Neurosurg Spine 2005; 2:145–150.
- Pigrau C, Almirante B, Flores X, et al. Spontaneous pyogenic vertebral osteomyelitis and endocarditis: incidence, risk factors, and outcome. Am J Med 2005; 118:1287.
- McHenry MC, Easley KA, Locker GA. Vertebral osteomyelitis: long-term outcome for 253 patients from 7 Cleveland-area hospitals. Clin Infect Dis 2002; 34:1342–1350.
- Danner RL, Hartman BJ. Update on spinal epidural abscess: 35 cases and review of the literature. Rev Infect Dis 1987; 9:265–274.
- Kaufman DM, Kaplan JG, Litman N. Infectious agents in spinal epidural abscesses. Neurology 1980; 30:844–850.
- Rezai AR, Woo HH, Errico TJ, Cooper PR. Contemporary management of spinal osteomyelitis. Neurosurgery 1999; 44:1018–1026.
- Bertino RE, Porter BA, Stimac GK, Tepper SJ. Imaging spinal osteomyelitis and epidural abscess with short TI inversion recovery (STIR). AJNR Am J Neuroradiol 1988; 9:563–564.
- Rigamonti D, Liem L, Sampath P, et al. Spinal epidural abscess: contemporary trends in etiology, evaluation, and management. Surg Neurol 1999; 52:189–197.
- De Vries F, Bracke M, Leufkens HG, Lammers JW, Cooper C, Van Staa TP. Fracture risk with intermittent high-dose oral glucocorticoid therapy. Arthritis Rheum 2007; 56:208–214.
- Cooper C, Atkinson EJ, O’Fallon WM, Melton LJ. Incidence of clinically diagnosed vertebral fractures: a population-based study in Rochester, Minnesota, 1985–1989. J Bone Miner Res 1992; 7:221–227.
- Kim DH, Vaccaro AR. Osteoporotic compression fractures of the spine; current options and considerations for treatment. Spine J 2006; 6:479–487.
- Cohn SH, Abesamis C, Yasumura S, Aloia JF, Zanzi I, Ellis KJ. Comparative skeletal mass and radial bone mineral content in black and white women. Metabolism 1977; 26:171–178.
- Tobias JH, Hutchinson AP, Hunt LP, et al. Use of clinical risk factors to identify postmenopausal women with vertebral fractures. Osteoporos Int 2007; 18:35–43.
- Melton LJ, Kallmes DF. Epidemiology of vertebral fractures: implications for vertebral augmentation. Acad Radiol 2006; 13:538–545.
- Silverman SL. The clinical consequences of vertebral compression fracture. Bone 1992; 13:S27–S31.
- Melton LJ, Kan SH, Frye MA, Wahner HW, O’Fallon WM, Riggs BL. Epidemiology of vertebral fractures in women. Am J Epidemiol 1989; 129:1000–1011.
- Wasnich RD. Vertebral fracture epidemiology. Bone 1996; 18:179S–183S.
- Yamato M, Nishimura G, Kuramochi E, Saiki N, Fujioka M. MR appearance at different ages of osteoporotic compression fractures of the vertebrae. Radiat Med 1998; 16:329–334.
- Einsiedel T, Schmelz A, Arand M, et al. Injuries of the cervical spine in patients with ankylosing spondylitis: experience at two trauma centers. J Neurosurg Spine 2006; 5:33–45.
- Olerud C, Frost A, Bring J. Spinal fractures in patients with ankylosing spondylitis. Eur Spine J 1996; 5:51–55.
- Spangfort EV. The lumbar disk herniation. A computer-aided analysis of 2,504 operations. Acta Orthop Scand Suppl 1972; 142:1–95.
- Kostuik JP, Harrington I, Alexander D, Rand W, Evans D. Cauda equina syndrome and lumbar disk herniation. J Bone Joint Surg Am 1986; 68:386–391.
- Lawton MT, Porter RW, Heiserman JE, Jacobowitz R, Sonntag VK, Dickman CA. Surgical management of spinal epidural hematoma: relationship between surgical timing and neurological outcome. J Neurosurg 1995; 83:1–7.
- Simmons EH, Grobler LJ. Acute spinal epidural hematoma. J Bone Joint Surg Am 1978; 60:395–396.
- Senelick RC, Norwood CW, Cohen GH. “Painless” spinal epidural hematoma during anticoagulant therapy”. Neurology 1976; 26:213–225.
- Watts C, Porto L. Recognizing spontaneous spinal epidural hematoma. Geriatrics 1976; 31:97–99.
KEY POINTS
- A primary tumor or metastasis to the spine tends to cause unremitting back pain that worsens at night and is accompanied by systemic disease and abnormal laboratory findings.
- Infection typically causes focal pain, an elevated erythrocyte sedimentation rate (the most sensitive laboratory test) and C-reactive protein level, and sometimes neurologic signs and symptoms.
- Fractures cause focal pain and should be suspected especially in older white women and patients who take corticosteroids or who have ankylosing spondylitis.
- Plain radiography can help detect fractures, but magnetic resonance imaging is needed to evaluate spinal tumors, soft tissue infections, and epidural abscesses, and to further evaluate neural compression due to fractures.
In reply: Medical causes of back pain
In Reply: We appreciate Dr. Hirsch’s comments and are pleased to expand the discussion of this important point.
He is correct in his assertion that dissection and aneurysm are distinct processes. But the goal of this review was to remind practitioners to consider the aorta as a possible source of pain when it occurs acutely or in an atypical manner.
A number of aortic processes can cause back pain, and aneurysm and dissection are two of them, aneurysm being more common than aortic dissection. But the pain can also be from aortic ulceration, aortitis, contained rupture of an aneurysm, and other more esoteric problems.
Aortic dissection often presents as a tearing, severe, thoracic back pain. Pain from a progressive abdominal aneurysm is more commonly referred to the lower back or flank and can be severe and unrelenting. It is rarely described as a tearing pain like that of dissection.
It is difficult on initial physical examination to distinguish aneurysm from dissection. The key to diagnosing aneurysm is to detect the pulsatile abdominal mass. A pulsatile, tender abdominal mass with hypotension and back pain is classically associated with rupture of an abdominal aortic aneurysm. The combination of back pain, a deficit in peripheral pulses, and hypertension is more often associated with dissection.
Without imaging and appropriate consultation, it is difficult for even an experienced provider to definitively diagnose these disorders. Without a bit of suspicion, even with a careful physical examination either disorder might be overlooked entirely, with disastrous effect. The purpose of our review was to remind the reader that these conditions, while uncommon or even rare, do occur and should be sought out in patients presenting with acute, atypical lumbar and thoracic back pain. As with each of the conditions discussed in this review, the decision to linger a bit over the patient’s history and then perform a basic, focused physical examination can be life-saving.
In Reply: We appreciate Dr. Hirsch’s comments and are pleased to expand the discussion of this important point.
He is correct in his assertion that dissection and aneurysm are distinct processes. But the goal of this review was to remind practitioners to consider the aorta as a possible source of pain when it occurs acutely or in an atypical manner.
A number of aortic processes can cause back pain, and aneurysm and dissection are two of them, aneurysm being more common than aortic dissection. But the pain can also be from aortic ulceration, aortitis, contained rupture of an aneurysm, and other more esoteric problems.
Aortic dissection often presents as a tearing, severe, thoracic back pain. Pain from a progressive abdominal aneurysm is more commonly referred to the lower back or flank and can be severe and unrelenting. It is rarely described as a tearing pain like that of dissection.
It is difficult on initial physical examination to distinguish aneurysm from dissection. The key to diagnosing aneurysm is to detect the pulsatile abdominal mass. A pulsatile, tender abdominal mass with hypotension and back pain is classically associated with rupture of an abdominal aortic aneurysm. The combination of back pain, a deficit in peripheral pulses, and hypertension is more often associated with dissection.
Without imaging and appropriate consultation, it is difficult for even an experienced provider to definitively diagnose these disorders. Without a bit of suspicion, even with a careful physical examination either disorder might be overlooked entirely, with disastrous effect. The purpose of our review was to remind the reader that these conditions, while uncommon or even rare, do occur and should be sought out in patients presenting with acute, atypical lumbar and thoracic back pain. As with each of the conditions discussed in this review, the decision to linger a bit over the patient’s history and then perform a basic, focused physical examination can be life-saving.
In Reply: We appreciate Dr. Hirsch’s comments and are pleased to expand the discussion of this important point.
He is correct in his assertion that dissection and aneurysm are distinct processes. But the goal of this review was to remind practitioners to consider the aorta as a possible source of pain when it occurs acutely or in an atypical manner.
A number of aortic processes can cause back pain, and aneurysm and dissection are two of them, aneurysm being more common than aortic dissection. But the pain can also be from aortic ulceration, aortitis, contained rupture of an aneurysm, and other more esoteric problems.
Aortic dissection often presents as a tearing, severe, thoracic back pain. Pain from a progressive abdominal aneurysm is more commonly referred to the lower back or flank and can be severe and unrelenting. It is rarely described as a tearing pain like that of dissection.
It is difficult on initial physical examination to distinguish aneurysm from dissection. The key to diagnosing aneurysm is to detect the pulsatile abdominal mass. A pulsatile, tender abdominal mass with hypotension and back pain is classically associated with rupture of an abdominal aortic aneurysm. The combination of back pain, a deficit in peripheral pulses, and hypertension is more often associated with dissection.
Without imaging and appropriate consultation, it is difficult for even an experienced provider to definitively diagnose these disorders. Without a bit of suspicion, even with a careful physical examination either disorder might be overlooked entirely, with disastrous effect. The purpose of our review was to remind the reader that these conditions, while uncommon or even rare, do occur and should be sought out in patients presenting with acute, atypical lumbar and thoracic back pain. As with each of the conditions discussed in this review, the decision to linger a bit over the patient’s history and then perform a basic, focused physical examination can be life-saving.
Masquerade: Nonspinal musculoskeletal disorders that mimic spinal conditions
Not all pain in the neck or back actually originates from the spine. Sometimes pain in the neck or back is caused by a problem in the shoulder or hip or from peripheral nerve compression in the arms or legs.
This article focuses on the diagnostic features of common—and uncommon—nonspinal musculoskeletal problems that can masquerade as disorders of the spine. A myriad of nonmusculoskeletal disorders can also cause neck or back pain, but they are beyond the scope of this article. Medical disorders that can present as possible spinal problems have been reviewed in the December 2007 issue of the Cleveland Clinic Journal of Medicine.
CAUSE OF NECK OR BACK PAIN IS NOT ALWAYS OBVIOUS
Pain in the neck or back is one of the most common reasons for visits to primary care physicians.
Usually the diagnosis is straightforward, but atypical pain patterns frequently make the cause of the problem difficult to decipher.1 Axial neck or back pain is in many cases caused by problems in the joints, muscles, tendons, or ligaments of the arms or legs because the nerves in these structures arise from the spinal cord.2 Because these structures can move relative to one another, pain often varies with position, further confusing the picture.2 Despite these challenges, a correct diagnosis can usually be made on the basis of the history, physical examination, and ancillary testing.
NONSPINAL MUSCULOSKELETAL CAUSES OF NECK PAIN
Many shoulder problems present as neck pain
Shoulder problems frequently cause neck pain4 because the shoulder and neck are near the brachial plexus, which connects them. The shoulder joint is a complex of several structures; problems in any of them can present with specific features that can be distinguished from neck problems.5
In general, shoulder problems in older people are due to degenerative conditions, whereas younger people generally have problems arising from trauma, inflammation, or instability.1
Rotator cuff disease is one of the most common shoulder problems that can present with neck pain. The rotator cuff consists of four muscles—the supraspinatus, infraspinatus, subscapularis, and teres minor—which form a common tendon that attaches to the proximal humeral tuberosities and allows rotation of the arm at the glenohumeral joint.1 The rotator cuff probably undergoes both mechanical and biologic degeneration over time, making it prone to painful tears.
Rotator cuff tears can cause pain in the anterolateral or medial aspect of the shoulder or in the trapezius and neck area.1,6 Many older patients present with pain in the trapezius and paraspinal muscles.2,5,7 Many patients report pain when they raise the arms over their head or when they reach and hold the arm away from the body (eg, holding the steering wheel while driving), and at night while lying on the affected side.1
On physical examination, weakness of the rotator cuff muscles can be detected by externally rotating the shoulder or applying a downward force to the arm with the shoulder abducted 90 degrees, forward flexed 30 degrees, and internally rotated with the thumbs pointing to the ground.1
Magnetic resonance imaging (MRI) can very accurately diagnose a rotator cuff tear: diagnostic findings include a discontinuity and retraction in the rotator cuff tendon and edema.
Not all rotator cuff tears are symptomatic.6 If a rotator cuff tear is evident on MRI but the patient does not have pain at night or during overhead activity, then neck pain is more likely due to spinal disease.
Glenohumeral arthritis is another common shoulder problem that can cause axial neck pain.1 Most cases are idiopathic, although many patients have a history of rheumatoid arthritis, prior shoulder trauma, or glenohumeral instability for which they may have had surgery. Patients with shoulder arthritis usually also have arthritis in the cervical spine.
Patients report pain in the trapezius muscle and possibly a sensation of swelling around the shoulder joint, as well as difficulty with overhead activities such as combing hair or applying makeup.1
The most significant clinical finding is eliciting the shoulder pain with motion. Patients may also have limited range of motion accompanied by pain and crepitation.1
Humeral head osteonecrosis is a less common intra-articular problem that can cause neck pain. It occurs most frequently with human immunodeficiency virus infection, alcoholism, or corticosteroid use.8 Radiography shows sclerosis or collapse of the subchondral bone of the humeral head. MRI is best for detecting early changes of osteonecrosis.
Peripheral nerve compression may mimic cervical radiculopathy
Peripheral nerve compression is common and may present with paresthesias mimicking a cervical radiculopathy.9
Carpal tunnel syndrome usually presents with hand numbness and tingling or decreased sensation in the median nerve distribution (the radial three digits). Thenar atrophy is present in advanced cases.1,9 Carpal tunnel syndrome may also present with nonspecific hand pain or other symptoms. Chowet al9 found that 84% of patients with carpal tunnel syndrome had nocturnal hand paresthesias, 82% had paresthesias that were aggravated by hand activity, and 64% had hand pain. However, some patients with cervical spondylosis also had these symptoms: 10% had hand pain, 7% had nocturnal hand paresthesias, and 10% had paresthesias that were aggravated by hand activity.
Cubital tunnel syndrome can also present with radiating arm symptoms and is usually associated with pain at the elbow and a positive Tinel sign (ie, tapping over the cubital tunnel—at the elbow between the olecranon process and the medial epicondyle—elicits pain and tingling in the small and ring fingers).1 Electromyography and a nerve conduction study can help determine the diagnosis.
Suprascapular nerve impingement is another peripheral nerve problem that can mimic a cervical spine problem.1,10 The supraspinatus and infraspinatus muscles and can become entrapped by a ganglion cyst at the suprascapular notch of the scapula. The condition is more commonly seen in young, active patients who participate in overhead activities (eg, volleyball or tennis).
Chronic suprascapular nerve impingement can cause weakness and atrophy of the supraspinatus or infraspinatus muscles or both and can be detected on physical examination and confirmed by electromyography.1,10 Electromyography is best for diagnosing peripheral nerve compression: a decreased amplitude and increased latency indicates severe nerve compression. MRI can reveal a ganglion cyst if it is the source of nerve compression at the notch.
Brachial neuritis: Acute, severe neck or shoulder pain, followed by weakness
Brachial neuritis (Parsonage-Turner syndrome) presents with abrupt onset of intense pain in the neck or shoulder, mimicking a cervical spine radiculopathy. The pain typically improves over several days to weeks,11 but may be followed by weakness of the arm muscles. The cause of this condition is unclear.
Brachial neuritis characteristically involves multiple nerve roots and the rapid onset of severe pain.11 Cervical radiculopathy, on the other hand, usually starts insidiously and has a single dermatomal distribution. Another distinguishing feature is that neck movement typically exacerbates the symptoms of cervical radiculopathy but not of brachial neuritis.12 Brachial neuritis should be suspected in patients who have these features and who do not respond to conventional therapy.11
A mass can be detected with imaging studies
A mass in or around the shoulder can present as neck or arm pain by compressing or stretching nervous structures or connective tissues in the shoulder.13
Bony masses. Although most bony lesions in the shoulder are benign (osteochondromaor bone cysts), malignant osseous lesions such as metastatic disease and primary bone sarcomas also occur. Metastatic disease should be suspected in older patients with a history of malignancy, even if the presentation is atypical.13 Most bony lesions can be diagnosed by radiography or CT.
Soft tissue masses (eg, lipomas, elastofibromas, and sarcomas) can also cause a confusing pain pattern when they arise in the shoulder. They can be diagnosed with MRI.13
NONSPINAL MUSCULOSKELETAL CAUSES OF BACK PAIN
Hip and spine arthritis are commonly found together
Several studies found that if a patient has problems in both the spine and the hip, treating only one of the conditions may not relieve the pain.11,16,17 Birrell et al15 evaluated patients with concomitant hip and spinal disease and found that most patients who underwent total hip arthroplasty followed by spinal decompression had excellent results.
Other studies suggested that it is better to treat spinal stenosis first, because neurologic sequelae could result if it is left untreated.16 On the other hand, several other studies found that patients with symptoms and spinal stenosis seen by radiography can function for years without neurologic compromise.14,15,18 Conflicting data such as these make it difficult to determine whether hip disease or spinal disease should be treated first in patients with both conditions. Generally, the more symptomatic condition is treated first, unless a neurologic problem is progressing.
Recent studies examined clinical features that help distinguish symptomatic hip disease from spine disease in patients with concomitant radiographic hip and spine arthritis.15,18 Limping, groin pain, and limited and painful internal rotation of the hip strongly implicate the hip as the source of pain. Brown et al18 found that patients with a limp were seven times more likely to have pain from the hip alone or from the spine and hip combined than from the spine alone. Patients with groin pain or painful and limited internal rotation of the hip were 14 times more likely to have either the hip or the hip and spine as the source of pain. A positive straight-leg-raising sign or a contralateral straight-leg-raising sign strongly suggests the spine as the source of pain.12 (Straight-leg tests are performed with the patient lying on a table and the examiner lifting the leg while the knee is straight. The test is positive if pain is elicited between 30 and 70 degrees.)
Femoral necrosis or fractures are detectable by imaging
Femoral head osteonecrosis is another intra-articular hip process that can cause backpain.13 As is also true of osteonecrosis of the shoulder, patients who abuse alcohol or take corticosteroids are at increased risk. Recently, human immunodeficiency virus has also been associated with this condition.
Femoral head osteonecrosis typically presents with insidiously worsening reduction of hip rotation and pain in the buttock, thigh, and groin. The pain is not in a dermatomal pattern and is usually unilateral but can be bilateral.18
Radiographs can be diagnostic for femoral head collapse in late disease. MRI is best for diagnosing early disease before collapse occurs.
Occult or impending femoral neck fracture (ie, in metastatic or metabolic bone disease) usually presents with groin pain, similar to hip osteoarthritis and osteonecrosis,13 but it can also present with vague back pain with or without groin pain. The pain is produced by weight-bearing on the affected leg. Young patients with femoral neck stress fractures or primary benign bone lesions of the hip can also present with buttock pain that can be misinterpreted as coming from the back.
MRI of the pelvis and proximal femur is best for diagnosing a stress fracture and some bone lesions, because they are often not visible on radiographs.
Because the rate of osteonecrosis is very high in displaced femoral neck fractures, it is important that an impending fracture be detected and treated before a complete fracture occurs.
Hip dysplasia requires early treatment
Hip dysplasia, in which the hip joint does not develop normally, can present as back, buttock, and groin pain in young patients. Back pain may be caused by asymmetric spinal loading and abnormal muscular tension in the lumbar spine.13 Early diagnosis is important so that it can be surgically treated (with osteotomies of the proximal femur or pelvis, or both) to preserve hip function.
Piriformis syndrome occurs in athletic patients
Piriformis syndrome, which mimics sciatica from a spinal cause, is controversial because the diagnosis must be based on history and clinical findings without any objective imaging or electrodiagnostic testing. The condition is thought to be caused by sciatic nerve entrapment and compression under the piriformis muscle, which externally rotates the hip and may become swollen and inflamed inactive, athletic people.13,16
The diagnosis is confirmed on physical examination if the pain is replicated when the piriformis muscle is stretched by externally rotating the hip (ie, with the patient supine, flexing the affected hip and knee and pulling the ipsilateral knee toward the contralateral shoulder).13,16
Imaging studies of the spine or hip are notd iagnostic but should be done to look for other possible causes of the pain.
Some patients with this condition are helped by exercises to stretch the hip muscles, particularly the external rotators.
Bursitis causes localized tenderness
Trochanteric bursitis is a fairly common soft-tissue problem that can cause pain along the lateral aspect of the hip and proximal thigh. Unlike radiculopathy, the condition causes localized tenderness over the greater trochanter.
Ischial bursitis can cause back pain and can be differentiated from spinal pathology by localized tenderness over the ischial tuberosity.
Peripheral nerve compression can cause radicular pain
Peripheral nerve compression in and around the leg can cause radicular pain that mimics lumbar spine pathology.
The lateral femoral cutaneous nerve, if compressed and irritated as it exits the pelvis, can cause meralgia paresthetica, which is characterized by pain, numbness, and tinglingin the anterolateral proximal thigh, mimicking an L1 or L2 radiculopathy. Many patients report that the pain worsens when they wear a belt or tight pants and improves when they remove or loosen them.
The saphenous and peroneal nerves can be compressed around the knee, causing paresthesias in the medial and lateral aspect of the knee and leg, respectively, mimicking a radiculopathy of the nerve roots at L3-L4 (saphenous nerve) and L5 (peroneal nerve).
The tibial nerve can be compressed in the tarsal tunnel on the medial aspect of the ankle, causing distal paresthesias in the medial aspect of the foot, mimicking radiculopathy at L4-L5.
Stimulating the area of nerve compression by external compression or tapping with the examiner’s fingers generally causes paresthesias and aggravates the symptoms. Electromyography can also help with diagnosis.
- McFarland EG. Examination of the Shoulder: The Complete Guide. New York: Thieme; 2006.
- Macnab I, McCulloch J. Neck Ache and Shoulder Pain. Baltimore: Williams & Wilkins; 1994.
- Tong HC, Haig AJ, Yamakawa K. The Spurling test and cervical radiculopathy. Spine 2002; 27:156–159.
- Gorski JM, Schwartz LH. Shoulder impingement presenting as neck pain. J Bone Joint Surg Am 2003; 85-A:635–638.
- Borenstein DG, Wiesel SW, Boden SD. Neck Pain: Medical Diagnosis and Comprehensive Management. Philadelphia: WB Saunders; 1996.
- Spindler KP, Dovan TT, McCarty EC. Assessment and management of the painful shoulder. Clin Cornerstone 2001; 3:26–37.
- Margoles MS. The pain chart: spatial properties of pain. In: Melzack R,editor. Pain Measurement and Assessment. New York: Raven Press; 1983:215–225.
- Pateder DB, Park HB, Chronopoulos E, Fayad LM, McFarland EG.Humeral head osteonecrosis after anterior shoulder stabilization in an adolescent. A case report. J Bone Joint Surg Am 2004; 86-A:2290–2293.
- Chow CS, Hung LK, Chiu CP, et al. Is symptomatology useful in distinguishing between carpal tunnel syndrome and cervical spondylosis? Hand Surg 2005; 10:1–5.
- Johnson TR. Shoulder. In: Snider RK, editor. Essentials of Musculoskeletal Care. 1st ed. Rosemont, Ill.: American Academy of Orthopaedic Surgeons; 1997.
- Mamula CJ, Erhard RE, Piva SR. Cervical radiculopathy or Parsonage-Turner syndrome: differential diagnosis of a patient with neck and upper extremity symptoms. J Orthop Sports Phys Ther 2005; 35:659–664.
- Hoppenfeld S. Physical Examination of the Spine and Extremities. New York: Appleton-Century-Crofts; 1976.
- McCarthy EF, Frassica FJ. Pathology of Bone and Joint Disorders: With Clinical and Radiographic Correlation. Philadelphia: WB Saunders; 1998.
- Borenstein D. Does osteoarthritis of the lumbar spine cause chronic low back pain? Curr Pain Headache Rep 2004; 8:512–517.
- Birrell F, Lunt M, Macfarlane G, Silman A. Association between pain in the hip region and radiographic changes of osteoarthritis: results from a population-based study. Rheumatology (Oxford) 2005; 44:337–341. Erratum in: Rheumatology (Oxford) 2005; 44:569.
- Papadopoulos EC, Khan SN. Piriformis syndrome and low back pain: a new classification and review of the literature. Orthop Clin North Am 2004; 35:65–71.
- Offierski CM, MacNab I. Hip-spine syndrome. Spine 1983; 8:316–321.
- Brown MD, Gomez-Marin O, Brookfield KF, Li PS. Differential diagnosis of hip disease versus spine disease. Clin Orthop Relat Res 2004; 419:280–284.
Not all pain in the neck or back actually originates from the spine. Sometimes pain in the neck or back is caused by a problem in the shoulder or hip or from peripheral nerve compression in the arms or legs.
This article focuses on the diagnostic features of common—and uncommon—nonspinal musculoskeletal problems that can masquerade as disorders of the spine. A myriad of nonmusculoskeletal disorders can also cause neck or back pain, but they are beyond the scope of this article. Medical disorders that can present as possible spinal problems have been reviewed in the December 2007 issue of the Cleveland Clinic Journal of Medicine.
CAUSE OF NECK OR BACK PAIN IS NOT ALWAYS OBVIOUS
Pain in the neck or back is one of the most common reasons for visits to primary care physicians.
Usually the diagnosis is straightforward, but atypical pain patterns frequently make the cause of the problem difficult to decipher.1 Axial neck or back pain is in many cases caused by problems in the joints, muscles, tendons, or ligaments of the arms or legs because the nerves in these structures arise from the spinal cord.2 Because these structures can move relative to one another, pain often varies with position, further confusing the picture.2 Despite these challenges, a correct diagnosis can usually be made on the basis of the history, physical examination, and ancillary testing.
NONSPINAL MUSCULOSKELETAL CAUSES OF NECK PAIN
Many shoulder problems present as neck pain
Shoulder problems frequently cause neck pain4 because the shoulder and neck are near the brachial plexus, which connects them. The shoulder joint is a complex of several structures; problems in any of them can present with specific features that can be distinguished from neck problems.5
In general, shoulder problems in older people are due to degenerative conditions, whereas younger people generally have problems arising from trauma, inflammation, or instability.1
Rotator cuff disease is one of the most common shoulder problems that can present with neck pain. The rotator cuff consists of four muscles—the supraspinatus, infraspinatus, subscapularis, and teres minor—which form a common tendon that attaches to the proximal humeral tuberosities and allows rotation of the arm at the glenohumeral joint.1 The rotator cuff probably undergoes both mechanical and biologic degeneration over time, making it prone to painful tears.
Rotator cuff tears can cause pain in the anterolateral or medial aspect of the shoulder or in the trapezius and neck area.1,6 Many older patients present with pain in the trapezius and paraspinal muscles.2,5,7 Many patients report pain when they raise the arms over their head or when they reach and hold the arm away from the body (eg, holding the steering wheel while driving), and at night while lying on the affected side.1
On physical examination, weakness of the rotator cuff muscles can be detected by externally rotating the shoulder or applying a downward force to the arm with the shoulder abducted 90 degrees, forward flexed 30 degrees, and internally rotated with the thumbs pointing to the ground.1
Magnetic resonance imaging (MRI) can very accurately diagnose a rotator cuff tear: diagnostic findings include a discontinuity and retraction in the rotator cuff tendon and edema.
Not all rotator cuff tears are symptomatic.6 If a rotator cuff tear is evident on MRI but the patient does not have pain at night or during overhead activity, then neck pain is more likely due to spinal disease.
Glenohumeral arthritis is another common shoulder problem that can cause axial neck pain.1 Most cases are idiopathic, although many patients have a history of rheumatoid arthritis, prior shoulder trauma, or glenohumeral instability for which they may have had surgery. Patients with shoulder arthritis usually also have arthritis in the cervical spine.
Patients report pain in the trapezius muscle and possibly a sensation of swelling around the shoulder joint, as well as difficulty with overhead activities such as combing hair or applying makeup.1
The most significant clinical finding is eliciting the shoulder pain with motion. Patients may also have limited range of motion accompanied by pain and crepitation.1
Humeral head osteonecrosis is a less common intra-articular problem that can cause neck pain. It occurs most frequently with human immunodeficiency virus infection, alcoholism, or corticosteroid use.8 Radiography shows sclerosis or collapse of the subchondral bone of the humeral head. MRI is best for detecting early changes of osteonecrosis.
Peripheral nerve compression may mimic cervical radiculopathy
Peripheral nerve compression is common and may present with paresthesias mimicking a cervical radiculopathy.9
Carpal tunnel syndrome usually presents with hand numbness and tingling or decreased sensation in the median nerve distribution (the radial three digits). Thenar atrophy is present in advanced cases.1,9 Carpal tunnel syndrome may also present with nonspecific hand pain or other symptoms. Chowet al9 found that 84% of patients with carpal tunnel syndrome had nocturnal hand paresthesias, 82% had paresthesias that were aggravated by hand activity, and 64% had hand pain. However, some patients with cervical spondylosis also had these symptoms: 10% had hand pain, 7% had nocturnal hand paresthesias, and 10% had paresthesias that were aggravated by hand activity.
Cubital tunnel syndrome can also present with radiating arm symptoms and is usually associated with pain at the elbow and a positive Tinel sign (ie, tapping over the cubital tunnel—at the elbow between the olecranon process and the medial epicondyle—elicits pain and tingling in the small and ring fingers).1 Electromyography and a nerve conduction study can help determine the diagnosis.
Suprascapular nerve impingement is another peripheral nerve problem that can mimic a cervical spine problem.1,10 The supraspinatus and infraspinatus muscles and can become entrapped by a ganglion cyst at the suprascapular notch of the scapula. The condition is more commonly seen in young, active patients who participate in overhead activities (eg, volleyball or tennis).
Chronic suprascapular nerve impingement can cause weakness and atrophy of the supraspinatus or infraspinatus muscles or both and can be detected on physical examination and confirmed by electromyography.1,10 Electromyography is best for diagnosing peripheral nerve compression: a decreased amplitude and increased latency indicates severe nerve compression. MRI can reveal a ganglion cyst if it is the source of nerve compression at the notch.
Brachial neuritis: Acute, severe neck or shoulder pain, followed by weakness
Brachial neuritis (Parsonage-Turner syndrome) presents with abrupt onset of intense pain in the neck or shoulder, mimicking a cervical spine radiculopathy. The pain typically improves over several days to weeks,11 but may be followed by weakness of the arm muscles. The cause of this condition is unclear.
Brachial neuritis characteristically involves multiple nerve roots and the rapid onset of severe pain.11 Cervical radiculopathy, on the other hand, usually starts insidiously and has a single dermatomal distribution. Another distinguishing feature is that neck movement typically exacerbates the symptoms of cervical radiculopathy but not of brachial neuritis.12 Brachial neuritis should be suspected in patients who have these features and who do not respond to conventional therapy.11
A mass can be detected with imaging studies
A mass in or around the shoulder can present as neck or arm pain by compressing or stretching nervous structures or connective tissues in the shoulder.13
Bony masses. Although most bony lesions in the shoulder are benign (osteochondromaor bone cysts), malignant osseous lesions such as metastatic disease and primary bone sarcomas also occur. Metastatic disease should be suspected in older patients with a history of malignancy, even if the presentation is atypical.13 Most bony lesions can be diagnosed by radiography or CT.
Soft tissue masses (eg, lipomas, elastofibromas, and sarcomas) can also cause a confusing pain pattern when they arise in the shoulder. They can be diagnosed with MRI.13
NONSPINAL MUSCULOSKELETAL CAUSES OF BACK PAIN
Hip and spine arthritis are commonly found together
Several studies found that if a patient has problems in both the spine and the hip, treating only one of the conditions may not relieve the pain.11,16,17 Birrell et al15 evaluated patients with concomitant hip and spinal disease and found that most patients who underwent total hip arthroplasty followed by spinal decompression had excellent results.
Other studies suggested that it is better to treat spinal stenosis first, because neurologic sequelae could result if it is left untreated.16 On the other hand, several other studies found that patients with symptoms and spinal stenosis seen by radiography can function for years without neurologic compromise.14,15,18 Conflicting data such as these make it difficult to determine whether hip disease or spinal disease should be treated first in patients with both conditions. Generally, the more symptomatic condition is treated first, unless a neurologic problem is progressing.
Recent studies examined clinical features that help distinguish symptomatic hip disease from spine disease in patients with concomitant radiographic hip and spine arthritis.15,18 Limping, groin pain, and limited and painful internal rotation of the hip strongly implicate the hip as the source of pain. Brown et al18 found that patients with a limp were seven times more likely to have pain from the hip alone or from the spine and hip combined than from the spine alone. Patients with groin pain or painful and limited internal rotation of the hip were 14 times more likely to have either the hip or the hip and spine as the source of pain. A positive straight-leg-raising sign or a contralateral straight-leg-raising sign strongly suggests the spine as the source of pain.12 (Straight-leg tests are performed with the patient lying on a table and the examiner lifting the leg while the knee is straight. The test is positive if pain is elicited between 30 and 70 degrees.)
Femoral necrosis or fractures are detectable by imaging
Femoral head osteonecrosis is another intra-articular hip process that can cause backpain.13 As is also true of osteonecrosis of the shoulder, patients who abuse alcohol or take corticosteroids are at increased risk. Recently, human immunodeficiency virus has also been associated with this condition.
Femoral head osteonecrosis typically presents with insidiously worsening reduction of hip rotation and pain in the buttock, thigh, and groin. The pain is not in a dermatomal pattern and is usually unilateral but can be bilateral.18
Radiographs can be diagnostic for femoral head collapse in late disease. MRI is best for diagnosing early disease before collapse occurs.
Occult or impending femoral neck fracture (ie, in metastatic or metabolic bone disease) usually presents with groin pain, similar to hip osteoarthritis and osteonecrosis,13 but it can also present with vague back pain with or without groin pain. The pain is produced by weight-bearing on the affected leg. Young patients with femoral neck stress fractures or primary benign bone lesions of the hip can also present with buttock pain that can be misinterpreted as coming from the back.
MRI of the pelvis and proximal femur is best for diagnosing a stress fracture and some bone lesions, because they are often not visible on radiographs.
Because the rate of osteonecrosis is very high in displaced femoral neck fractures, it is important that an impending fracture be detected and treated before a complete fracture occurs.
Hip dysplasia requires early treatment
Hip dysplasia, in which the hip joint does not develop normally, can present as back, buttock, and groin pain in young patients. Back pain may be caused by asymmetric spinal loading and abnormal muscular tension in the lumbar spine.13 Early diagnosis is important so that it can be surgically treated (with osteotomies of the proximal femur or pelvis, or both) to preserve hip function.
Piriformis syndrome occurs in athletic patients
Piriformis syndrome, which mimics sciatica from a spinal cause, is controversial because the diagnosis must be based on history and clinical findings without any objective imaging or electrodiagnostic testing. The condition is thought to be caused by sciatic nerve entrapment and compression under the piriformis muscle, which externally rotates the hip and may become swollen and inflamed inactive, athletic people.13,16
The diagnosis is confirmed on physical examination if the pain is replicated when the piriformis muscle is stretched by externally rotating the hip (ie, with the patient supine, flexing the affected hip and knee and pulling the ipsilateral knee toward the contralateral shoulder).13,16
Imaging studies of the spine or hip are notd iagnostic but should be done to look for other possible causes of the pain.
Some patients with this condition are helped by exercises to stretch the hip muscles, particularly the external rotators.
Bursitis causes localized tenderness
Trochanteric bursitis is a fairly common soft-tissue problem that can cause pain along the lateral aspect of the hip and proximal thigh. Unlike radiculopathy, the condition causes localized tenderness over the greater trochanter.
Ischial bursitis can cause back pain and can be differentiated from spinal pathology by localized tenderness over the ischial tuberosity.
Peripheral nerve compression can cause radicular pain
Peripheral nerve compression in and around the leg can cause radicular pain that mimics lumbar spine pathology.
The lateral femoral cutaneous nerve, if compressed and irritated as it exits the pelvis, can cause meralgia paresthetica, which is characterized by pain, numbness, and tinglingin the anterolateral proximal thigh, mimicking an L1 or L2 radiculopathy. Many patients report that the pain worsens when they wear a belt or tight pants and improves when they remove or loosen them.
The saphenous and peroneal nerves can be compressed around the knee, causing paresthesias in the medial and lateral aspect of the knee and leg, respectively, mimicking a radiculopathy of the nerve roots at L3-L4 (saphenous nerve) and L5 (peroneal nerve).
The tibial nerve can be compressed in the tarsal tunnel on the medial aspect of the ankle, causing distal paresthesias in the medial aspect of the foot, mimicking radiculopathy at L4-L5.
Stimulating the area of nerve compression by external compression or tapping with the examiner’s fingers generally causes paresthesias and aggravates the symptoms. Electromyography can also help with diagnosis.
Not all pain in the neck or back actually originates from the spine. Sometimes pain in the neck or back is caused by a problem in the shoulder or hip or from peripheral nerve compression in the arms or legs.
This article focuses on the diagnostic features of common—and uncommon—nonspinal musculoskeletal problems that can masquerade as disorders of the spine. A myriad of nonmusculoskeletal disorders can also cause neck or back pain, but they are beyond the scope of this article. Medical disorders that can present as possible spinal problems have been reviewed in the December 2007 issue of the Cleveland Clinic Journal of Medicine.
CAUSE OF NECK OR BACK PAIN IS NOT ALWAYS OBVIOUS
Pain in the neck or back is one of the most common reasons for visits to primary care physicians.
Usually the diagnosis is straightforward, but atypical pain patterns frequently make the cause of the problem difficult to decipher.1 Axial neck or back pain is in many cases caused by problems in the joints, muscles, tendons, or ligaments of the arms or legs because the nerves in these structures arise from the spinal cord.2 Because these structures can move relative to one another, pain often varies with position, further confusing the picture.2 Despite these challenges, a correct diagnosis can usually be made on the basis of the history, physical examination, and ancillary testing.
NONSPINAL MUSCULOSKELETAL CAUSES OF NECK PAIN
Many shoulder problems present as neck pain
Shoulder problems frequently cause neck pain4 because the shoulder and neck are near the brachial plexus, which connects them. The shoulder joint is a complex of several structures; problems in any of them can present with specific features that can be distinguished from neck problems.5
In general, shoulder problems in older people are due to degenerative conditions, whereas younger people generally have problems arising from trauma, inflammation, or instability.1
Rotator cuff disease is one of the most common shoulder problems that can present with neck pain. The rotator cuff consists of four muscles—the supraspinatus, infraspinatus, subscapularis, and teres minor—which form a common tendon that attaches to the proximal humeral tuberosities and allows rotation of the arm at the glenohumeral joint.1 The rotator cuff probably undergoes both mechanical and biologic degeneration over time, making it prone to painful tears.
Rotator cuff tears can cause pain in the anterolateral or medial aspect of the shoulder or in the trapezius and neck area.1,6 Many older patients present with pain in the trapezius and paraspinal muscles.2,5,7 Many patients report pain when they raise the arms over their head or when they reach and hold the arm away from the body (eg, holding the steering wheel while driving), and at night while lying on the affected side.1
On physical examination, weakness of the rotator cuff muscles can be detected by externally rotating the shoulder or applying a downward force to the arm with the shoulder abducted 90 degrees, forward flexed 30 degrees, and internally rotated with the thumbs pointing to the ground.1
Magnetic resonance imaging (MRI) can very accurately diagnose a rotator cuff tear: diagnostic findings include a discontinuity and retraction in the rotator cuff tendon and edema.
Not all rotator cuff tears are symptomatic.6 If a rotator cuff tear is evident on MRI but the patient does not have pain at night or during overhead activity, then neck pain is more likely due to spinal disease.
Glenohumeral arthritis is another common shoulder problem that can cause axial neck pain.1 Most cases are idiopathic, although many patients have a history of rheumatoid arthritis, prior shoulder trauma, or glenohumeral instability for which they may have had surgery. Patients with shoulder arthritis usually also have arthritis in the cervical spine.
Patients report pain in the trapezius muscle and possibly a sensation of swelling around the shoulder joint, as well as difficulty with overhead activities such as combing hair or applying makeup.1
The most significant clinical finding is eliciting the shoulder pain with motion. Patients may also have limited range of motion accompanied by pain and crepitation.1
Humeral head osteonecrosis is a less common intra-articular problem that can cause neck pain. It occurs most frequently with human immunodeficiency virus infection, alcoholism, or corticosteroid use.8 Radiography shows sclerosis or collapse of the subchondral bone of the humeral head. MRI is best for detecting early changes of osteonecrosis.
Peripheral nerve compression may mimic cervical radiculopathy
Peripheral nerve compression is common and may present with paresthesias mimicking a cervical radiculopathy.9
Carpal tunnel syndrome usually presents with hand numbness and tingling or decreased sensation in the median nerve distribution (the radial three digits). Thenar atrophy is present in advanced cases.1,9 Carpal tunnel syndrome may also present with nonspecific hand pain or other symptoms. Chowet al9 found that 84% of patients with carpal tunnel syndrome had nocturnal hand paresthesias, 82% had paresthesias that were aggravated by hand activity, and 64% had hand pain. However, some patients with cervical spondylosis also had these symptoms: 10% had hand pain, 7% had nocturnal hand paresthesias, and 10% had paresthesias that were aggravated by hand activity.
Cubital tunnel syndrome can also present with radiating arm symptoms and is usually associated with pain at the elbow and a positive Tinel sign (ie, tapping over the cubital tunnel—at the elbow between the olecranon process and the medial epicondyle—elicits pain and tingling in the small and ring fingers).1 Electromyography and a nerve conduction study can help determine the diagnosis.
Suprascapular nerve impingement is another peripheral nerve problem that can mimic a cervical spine problem.1,10 The supraspinatus and infraspinatus muscles and can become entrapped by a ganglion cyst at the suprascapular notch of the scapula. The condition is more commonly seen in young, active patients who participate in overhead activities (eg, volleyball or tennis).
Chronic suprascapular nerve impingement can cause weakness and atrophy of the supraspinatus or infraspinatus muscles or both and can be detected on physical examination and confirmed by electromyography.1,10 Electromyography is best for diagnosing peripheral nerve compression: a decreased amplitude and increased latency indicates severe nerve compression. MRI can reveal a ganglion cyst if it is the source of nerve compression at the notch.
Brachial neuritis: Acute, severe neck or shoulder pain, followed by weakness
Brachial neuritis (Parsonage-Turner syndrome) presents with abrupt onset of intense pain in the neck or shoulder, mimicking a cervical spine radiculopathy. The pain typically improves over several days to weeks,11 but may be followed by weakness of the arm muscles. The cause of this condition is unclear.
Brachial neuritis characteristically involves multiple nerve roots and the rapid onset of severe pain.11 Cervical radiculopathy, on the other hand, usually starts insidiously and has a single dermatomal distribution. Another distinguishing feature is that neck movement typically exacerbates the symptoms of cervical radiculopathy but not of brachial neuritis.12 Brachial neuritis should be suspected in patients who have these features and who do not respond to conventional therapy.11
A mass can be detected with imaging studies
A mass in or around the shoulder can present as neck or arm pain by compressing or stretching nervous structures or connective tissues in the shoulder.13
Bony masses. Although most bony lesions in the shoulder are benign (osteochondromaor bone cysts), malignant osseous lesions such as metastatic disease and primary bone sarcomas also occur. Metastatic disease should be suspected in older patients with a history of malignancy, even if the presentation is atypical.13 Most bony lesions can be diagnosed by radiography or CT.
Soft tissue masses (eg, lipomas, elastofibromas, and sarcomas) can also cause a confusing pain pattern when they arise in the shoulder. They can be diagnosed with MRI.13
NONSPINAL MUSCULOSKELETAL CAUSES OF BACK PAIN
Hip and spine arthritis are commonly found together
Several studies found that if a patient has problems in both the spine and the hip, treating only one of the conditions may not relieve the pain.11,16,17 Birrell et al15 evaluated patients with concomitant hip and spinal disease and found that most patients who underwent total hip arthroplasty followed by spinal decompression had excellent results.
Other studies suggested that it is better to treat spinal stenosis first, because neurologic sequelae could result if it is left untreated.16 On the other hand, several other studies found that patients with symptoms and spinal stenosis seen by radiography can function for years without neurologic compromise.14,15,18 Conflicting data such as these make it difficult to determine whether hip disease or spinal disease should be treated first in patients with both conditions. Generally, the more symptomatic condition is treated first, unless a neurologic problem is progressing.
Recent studies examined clinical features that help distinguish symptomatic hip disease from spine disease in patients with concomitant radiographic hip and spine arthritis.15,18 Limping, groin pain, and limited and painful internal rotation of the hip strongly implicate the hip as the source of pain. Brown et al18 found that patients with a limp were seven times more likely to have pain from the hip alone or from the spine and hip combined than from the spine alone. Patients with groin pain or painful and limited internal rotation of the hip were 14 times more likely to have either the hip or the hip and spine as the source of pain. A positive straight-leg-raising sign or a contralateral straight-leg-raising sign strongly suggests the spine as the source of pain.12 (Straight-leg tests are performed with the patient lying on a table and the examiner lifting the leg while the knee is straight. The test is positive if pain is elicited between 30 and 70 degrees.)
Femoral necrosis or fractures are detectable by imaging
Femoral head osteonecrosis is another intra-articular hip process that can cause backpain.13 As is also true of osteonecrosis of the shoulder, patients who abuse alcohol or take corticosteroids are at increased risk. Recently, human immunodeficiency virus has also been associated with this condition.
Femoral head osteonecrosis typically presents with insidiously worsening reduction of hip rotation and pain in the buttock, thigh, and groin. The pain is not in a dermatomal pattern and is usually unilateral but can be bilateral.18
Radiographs can be diagnostic for femoral head collapse in late disease. MRI is best for diagnosing early disease before collapse occurs.
Occult or impending femoral neck fracture (ie, in metastatic or metabolic bone disease) usually presents with groin pain, similar to hip osteoarthritis and osteonecrosis,13 but it can also present with vague back pain with or without groin pain. The pain is produced by weight-bearing on the affected leg. Young patients with femoral neck stress fractures or primary benign bone lesions of the hip can also present with buttock pain that can be misinterpreted as coming from the back.
MRI of the pelvis and proximal femur is best for diagnosing a stress fracture and some bone lesions, because they are often not visible on radiographs.
Because the rate of osteonecrosis is very high in displaced femoral neck fractures, it is important that an impending fracture be detected and treated before a complete fracture occurs.
Hip dysplasia requires early treatment
Hip dysplasia, in which the hip joint does not develop normally, can present as back, buttock, and groin pain in young patients. Back pain may be caused by asymmetric spinal loading and abnormal muscular tension in the lumbar spine.13 Early diagnosis is important so that it can be surgically treated (with osteotomies of the proximal femur or pelvis, or both) to preserve hip function.
Piriformis syndrome occurs in athletic patients
Piriformis syndrome, which mimics sciatica from a spinal cause, is controversial because the diagnosis must be based on history and clinical findings without any objective imaging or electrodiagnostic testing. The condition is thought to be caused by sciatic nerve entrapment and compression under the piriformis muscle, which externally rotates the hip and may become swollen and inflamed inactive, athletic people.13,16
The diagnosis is confirmed on physical examination if the pain is replicated when the piriformis muscle is stretched by externally rotating the hip (ie, with the patient supine, flexing the affected hip and knee and pulling the ipsilateral knee toward the contralateral shoulder).13,16
Imaging studies of the spine or hip are notd iagnostic but should be done to look for other possible causes of the pain.
Some patients with this condition are helped by exercises to stretch the hip muscles, particularly the external rotators.
Bursitis causes localized tenderness
Trochanteric bursitis is a fairly common soft-tissue problem that can cause pain along the lateral aspect of the hip and proximal thigh. Unlike radiculopathy, the condition causes localized tenderness over the greater trochanter.
Ischial bursitis can cause back pain and can be differentiated from spinal pathology by localized tenderness over the ischial tuberosity.
Peripheral nerve compression can cause radicular pain
Peripheral nerve compression in and around the leg can cause radicular pain that mimics lumbar spine pathology.
The lateral femoral cutaneous nerve, if compressed and irritated as it exits the pelvis, can cause meralgia paresthetica, which is characterized by pain, numbness, and tinglingin the anterolateral proximal thigh, mimicking an L1 or L2 radiculopathy. Many patients report that the pain worsens when they wear a belt or tight pants and improves when they remove or loosen them.
The saphenous and peroneal nerves can be compressed around the knee, causing paresthesias in the medial and lateral aspect of the knee and leg, respectively, mimicking a radiculopathy of the nerve roots at L3-L4 (saphenous nerve) and L5 (peroneal nerve).
The tibial nerve can be compressed in the tarsal tunnel on the medial aspect of the ankle, causing distal paresthesias in the medial aspect of the foot, mimicking radiculopathy at L4-L5.
Stimulating the area of nerve compression by external compression or tapping with the examiner’s fingers generally causes paresthesias and aggravates the symptoms. Electromyography can also help with diagnosis.
- McFarland EG. Examination of the Shoulder: The Complete Guide. New York: Thieme; 2006.
- Macnab I, McCulloch J. Neck Ache and Shoulder Pain. Baltimore: Williams & Wilkins; 1994.
- Tong HC, Haig AJ, Yamakawa K. The Spurling test and cervical radiculopathy. Spine 2002; 27:156–159.
- Gorski JM, Schwartz LH. Shoulder impingement presenting as neck pain. J Bone Joint Surg Am 2003; 85-A:635–638.
- Borenstein DG, Wiesel SW, Boden SD. Neck Pain: Medical Diagnosis and Comprehensive Management. Philadelphia: WB Saunders; 1996.
- Spindler KP, Dovan TT, McCarty EC. Assessment and management of the painful shoulder. Clin Cornerstone 2001; 3:26–37.
- Margoles MS. The pain chart: spatial properties of pain. In: Melzack R,editor. Pain Measurement and Assessment. New York: Raven Press; 1983:215–225.
- Pateder DB, Park HB, Chronopoulos E, Fayad LM, McFarland EG.Humeral head osteonecrosis after anterior shoulder stabilization in an adolescent. A case report. J Bone Joint Surg Am 2004; 86-A:2290–2293.
- Chow CS, Hung LK, Chiu CP, et al. Is symptomatology useful in distinguishing between carpal tunnel syndrome and cervical spondylosis? Hand Surg 2005; 10:1–5.
- Johnson TR. Shoulder. In: Snider RK, editor. Essentials of Musculoskeletal Care. 1st ed. Rosemont, Ill.: American Academy of Orthopaedic Surgeons; 1997.
- Mamula CJ, Erhard RE, Piva SR. Cervical radiculopathy or Parsonage-Turner syndrome: differential diagnosis of a patient with neck and upper extremity symptoms. J Orthop Sports Phys Ther 2005; 35:659–664.
- Hoppenfeld S. Physical Examination of the Spine and Extremities. New York: Appleton-Century-Crofts; 1976.
- McCarthy EF, Frassica FJ. Pathology of Bone and Joint Disorders: With Clinical and Radiographic Correlation. Philadelphia: WB Saunders; 1998.
- Borenstein D. Does osteoarthritis of the lumbar spine cause chronic low back pain? Curr Pain Headache Rep 2004; 8:512–517.
- Birrell F, Lunt M, Macfarlane G, Silman A. Association between pain in the hip region and radiographic changes of osteoarthritis: results from a population-based study. Rheumatology (Oxford) 2005; 44:337–341. Erratum in: Rheumatology (Oxford) 2005; 44:569.
- Papadopoulos EC, Khan SN. Piriformis syndrome and low back pain: a new classification and review of the literature. Orthop Clin North Am 2004; 35:65–71.
- Offierski CM, MacNab I. Hip-spine syndrome. Spine 1983; 8:316–321.
- Brown MD, Gomez-Marin O, Brookfield KF, Li PS. Differential diagnosis of hip disease versus spine disease. Clin Orthop Relat Res 2004; 419:280–284.
- McFarland EG. Examination of the Shoulder: The Complete Guide. New York: Thieme; 2006.
- Macnab I, McCulloch J. Neck Ache and Shoulder Pain. Baltimore: Williams & Wilkins; 1994.
- Tong HC, Haig AJ, Yamakawa K. The Spurling test and cervical radiculopathy. Spine 2002; 27:156–159.
- Gorski JM, Schwartz LH. Shoulder impingement presenting as neck pain. J Bone Joint Surg Am 2003; 85-A:635–638.
- Borenstein DG, Wiesel SW, Boden SD. Neck Pain: Medical Diagnosis and Comprehensive Management. Philadelphia: WB Saunders; 1996.
- Spindler KP, Dovan TT, McCarty EC. Assessment and management of the painful shoulder. Clin Cornerstone 2001; 3:26–37.
- Margoles MS. The pain chart: spatial properties of pain. In: Melzack R,editor. Pain Measurement and Assessment. New York: Raven Press; 1983:215–225.
- Pateder DB, Park HB, Chronopoulos E, Fayad LM, McFarland EG.Humeral head osteonecrosis after anterior shoulder stabilization in an adolescent. A case report. J Bone Joint Surg Am 2004; 86-A:2290–2293.
- Chow CS, Hung LK, Chiu CP, et al. Is symptomatology useful in distinguishing between carpal tunnel syndrome and cervical spondylosis? Hand Surg 2005; 10:1–5.
- Johnson TR. Shoulder. In: Snider RK, editor. Essentials of Musculoskeletal Care. 1st ed. Rosemont, Ill.: American Academy of Orthopaedic Surgeons; 1997.
- Mamula CJ, Erhard RE, Piva SR. Cervical radiculopathy or Parsonage-Turner syndrome: differential diagnosis of a patient with neck and upper extremity symptoms. J Orthop Sports Phys Ther 2005; 35:659–664.
- Hoppenfeld S. Physical Examination of the Spine and Extremities. New York: Appleton-Century-Crofts; 1976.
- McCarthy EF, Frassica FJ. Pathology of Bone and Joint Disorders: With Clinical and Radiographic Correlation. Philadelphia: WB Saunders; 1998.
- Borenstein D. Does osteoarthritis of the lumbar spine cause chronic low back pain? Curr Pain Headache Rep 2004; 8:512–517.
- Birrell F, Lunt M, Macfarlane G, Silman A. Association between pain in the hip region and radiographic changes of osteoarthritis: results from a population-based study. Rheumatology (Oxford) 2005; 44:337–341. Erratum in: Rheumatology (Oxford) 2005; 44:569.
- Papadopoulos EC, Khan SN. Piriformis syndrome and low back pain: a new classification and review of the literature. Orthop Clin North Am 2004; 35:65–71.
- Offierski CM, MacNab I. Hip-spine syndrome. Spine 1983; 8:316–321.
- Brown MD, Gomez-Marin O, Brookfield KF, Li PS. Differential diagnosis of hip disease versus spine disease. Clin Orthop Relat Res 2004; 419:280–284.
KEY POINTS
- Neck pain is commonly caused by shoulder problems such as rotator cuff disease, glenohumeral arthritis, and humeral head osteonecrosis.
- Brachial neuritis involves acute, severe neck or shoulder pain, followed by weakness as pain resolves.
- Low back pain can be caused by hip or spine arthritis, femoral head osteonecrosis, an occult or impending femoral neck fracture, hip dysplasia, piriformis syndrome, and bursitis.
- Bony and soft tissue masses can be detected with imaging studies.
- Peripheral nerve compression can mimic cervical or lumbar spine radiculopathy. Electromyography and eliciting symptoms by tapping over the compressed nerve aid in making a diagnosis.
- Patients with human immunodeficiency virus infection, alcoholism, or corticosteroid use are at increased risk of developing osteonecrosis of the humeral or femoral head.