User login
More Than 100 GIs Strong for Advocacy Day 2025!
For our leaders, showing up on behalf of their patients is a privilege and an opportunity to represent the specialty with individuals who have a role in dictating health care policy.
In total, 124 members, patient advocates, and AGA staffers met with lawmakers and attended 130 meetings – 70 unique House districts and 60 unique Senate districts – with Republican and Democratic staff.
Our advocacy contingent represented the diversity of the country with 30 states represented from coast-to-coast. No matter the home state, everyone was united in the calls to Congress: to reform prior authorization, increase digestive disease funding, and secure a permanent solution for Medicare physician reimbursement.
As in past years, patient advocates participated alongside GI clinicians and researchers.
Their participation underscored the importance of including diverse voices. As patients with chronic health conditions, they were able to convey how their experiences navigating insurance barriers or managing delays to care as prescribed by their health care provider impacted their well-being and quality of life.
Throughout the day, patient advocates and GIs alike were encouraged by their meetings with congressional staffers. Conversations were constructive, engaging, and meaningful as everyone collaborated on common ground: seeking solutions to ensure GI patients have timely access to care that they need.
Many AGA leaders appreciated the value of being able to unite with colleagues to advocate and share their firsthand experiences in the lab or clinic in meetings with House and Senate staffers.
While Advocacy Day lasts a single day, its value hasn’t diminished. Thanks to the engagement and participation of the more than 100 AGA leaders and patient advocates, we can continue to build positive relationships with influential policymakers and make strides to improve and protect access to GI patient care.
For our leaders, showing up on behalf of their patients is a privilege and an opportunity to represent the specialty with individuals who have a role in dictating health care policy.
In total, 124 members, patient advocates, and AGA staffers met with lawmakers and attended 130 meetings – 70 unique House districts and 60 unique Senate districts – with Republican and Democratic staff.
Our advocacy contingent represented the diversity of the country with 30 states represented from coast-to-coast. No matter the home state, everyone was united in the calls to Congress: to reform prior authorization, increase digestive disease funding, and secure a permanent solution for Medicare physician reimbursement.
As in past years, patient advocates participated alongside GI clinicians and researchers.
Their participation underscored the importance of including diverse voices. As patients with chronic health conditions, they were able to convey how their experiences navigating insurance barriers or managing delays to care as prescribed by their health care provider impacted their well-being and quality of life.
Throughout the day, patient advocates and GIs alike were encouraged by their meetings with congressional staffers. Conversations were constructive, engaging, and meaningful as everyone collaborated on common ground: seeking solutions to ensure GI patients have timely access to care that they need.
Many AGA leaders appreciated the value of being able to unite with colleagues to advocate and share their firsthand experiences in the lab or clinic in meetings with House and Senate staffers.
While Advocacy Day lasts a single day, its value hasn’t diminished. Thanks to the engagement and participation of the more than 100 AGA leaders and patient advocates, we can continue to build positive relationships with influential policymakers and make strides to improve and protect access to GI patient care.
For our leaders, showing up on behalf of their patients is a privilege and an opportunity to represent the specialty with individuals who have a role in dictating health care policy.
In total, 124 members, patient advocates, and AGA staffers met with lawmakers and attended 130 meetings – 70 unique House districts and 60 unique Senate districts – with Republican and Democratic staff.
Our advocacy contingent represented the diversity of the country with 30 states represented from coast-to-coast. No matter the home state, everyone was united in the calls to Congress: to reform prior authorization, increase digestive disease funding, and secure a permanent solution for Medicare physician reimbursement.
As in past years, patient advocates participated alongside GI clinicians and researchers.
Their participation underscored the importance of including diverse voices. As patients with chronic health conditions, they were able to convey how their experiences navigating insurance barriers or managing delays to care as prescribed by their health care provider impacted their well-being and quality of life.
Throughout the day, patient advocates and GIs alike were encouraged by their meetings with congressional staffers. Conversations were constructive, engaging, and meaningful as everyone collaborated on common ground: seeking solutions to ensure GI patients have timely access to care that they need.
Many AGA leaders appreciated the value of being able to unite with colleagues to advocate and share their firsthand experiences in the lab or clinic in meetings with House and Senate staffers.
While Advocacy Day lasts a single day, its value hasn’t diminished. Thanks to the engagement and participation of the more than 100 AGA leaders and patient advocates, we can continue to build positive relationships with influential policymakers and make strides to improve and protect access to GI patient care.
Combining Upper-Lower GI Screening Feasible, Effective
BERLIN — , including malignancies and lesions requiring ongoing surveillance, according to an interim analysis from the TOGAS study.
“There was an abundance of benign but clinically relevant findings,” said lead investigator Jan Bornschein, MD, gastroenterologist at Oxford University Hospitals NHS Foundation Trust, Oxford, England, who presented the interim resuts of the study at United European Gastroenterology (UEG) Week 2025.
While the study found upper GI neoplasia in only 1.4% of participants, 17.8% of individuals were marked for upper GI endoscopic surveillance.
The results may inform how Europe develops gastric cancer prevention programs alongside those for colorectal cancer, said Bornschein. “If we can combine the upper GI endoscopy with other modalities [colonoscopy], the more likelihood there is that you can have a one-stop test package,” he said. “A combination, particularly for bowel and stomach, is more feasible and also more cost-effective. So far, the findings show that it’s definitely a strategy that, in my opinion, is worth implementing.”
Bornschein and the TOGAS study group hope that the combined approach will prove workable across diverse European settings and will help identify a spectrum of upper GI pathology, from cancers and dysplasia to atrophy and intestinal metaplasia, that can meaningfully affect follow-up surveillance.
Mixed Rates of GI Cancers Across Europe and the US
These findings come amid data showing rising rates of early-onset (younger than 50 years) GI cancers in the US, including colorectal, gastric, pancreatic, and esophageal tumors. These trends, previously reported by this news organization, point to environmental and lifestyle drivers, strengthening the case for earlier detection and risk-tailored strategies for upper GI neoplasia and preneoplastic conditions detected during existing colorectal cancer screening pathways.
However, Bornschein noted that prevalence varies considerably across Europe. “There are areas, particularly in the Eastern regions, and in some parts of the West, for example, Portugal, that have a very high incidence of GI cancers. In the UK or in Germany, we have noticed a decline over the years, so the numbers are actually much better than they used to be.”
The study is the second in a series of three TOGAS pilot studies and was conducted across eight centers (France, Germany, Ireland, Latvia, Lithuania, the Netherlands, Portugal, and Spain) in adults aged 50-74 years attending screening or polyp-surveillance colonoscopy.
A European Society of Gastrointestinal Endoscopy-aligned protocol defining image documentation, biopsy sampling, and quality parameters was followed to ensure a standardized approach. “Marked preneoplastic change” was defined as gastric glandular atrophy or intestinal metaplasia at the Operative Link on Gastritis Assessment/Operative Link on Gastric Intestinal Metaplasia Assessment stage III-IV and/or Endoscopic Grading of Gastric Intestinal Metaplasia > 5, triggering a need for endoscopic surveillance.
Data were gathered on colonoscopy findings (including polyp surveillance and family history), EGD findings plus biopsies, serum pepsinogen, and Helicobacter pylori serology. Outcome measures included the prevalence of gastric cancer and preneoplastic conditions, the diagnostic accuracy of pepsinogen testing, comparisons between national settings, the relevance of upper endoscopy in fecal immunochemical test-positive cases, and overall H pylori prevalence.
Neoplasia and Preneoplasia Found
A total of 846 participants were analyzed. At baseline, the mean age was 62 years, 52.2% were men, and 84.2% were White, despite efforts to recruit a more diverse population. Around 390 participants drank alcohol, and 190 smoked tobacco.
A total of 37.8% of participants had undergone prior EGD, of which 94.7% were performed more than 3 years before the study start. The history of GI surgery was 13.7%, and the history of cancer was 14.5%. Around 11% took aspirin, and 14% took proton pump inhibitors (PPIs). “We were surprised at the low prevalence of PPI use,” remarked Bornschein. “It was also good news that around half were never smokers.”
Key results for upper GI neoplasia included six patients (0.7%) with gastric cancers, three (0.4%) with esophageal cancers, and five (0.6%) with duodenal tumors. H pylori positivity was found in 303 patients (35.8%), with an additional 81 (9.6%) reporting a history of eradication.
Colorectal findings included 15 patients (1.8%) with cancers and colon polyps in 503 (59.5%) participants.
Regarding preneoplastic conditions, endoscopy identified intestinal metaplasia in 174 patients (20.6%), of which 65 (7.7%) were multifocal. Atrophy was observed in 220 patients (26.0%), with 59 (7.0%) showing multifocal atrophic changes. Both intestinal metaplasia and atrophy were found together in 105 (12.4%) patients. Barrett’s esophagus was detected in 31 (3.7%) patients.
“I’d really like to highlight these further benign gastric findings,” said Bornschein. These included gastric ulcers in 28 (3.3%) patients, erosive gastritis in 245 (29.0%) patients, esophageal ulcers in three (0.4%) patients, Los Angeles Community College District classification esophagitis in 13 (1.5%) patients, and duodenal ulcers in 10 (1.2%) patients. “These were asymptomatic, but we were able to identify them,” he noted.
“We’ve had a very low rate of complications (0.01%),” he added.” I don’t want to jinx that now. These were basically related to sedation.”
PROSPERO: Early Detection of Upper GI Conditions in a UK Population
Massimiliano di Pietro, MD, consultant gastroenterologist at Addenbrooke’s Hospital, Cambridge, England, and the principal investigator of the PROSPERO study, which aimed to determine the prevalence of premalignant upper GI conditions in routine endoscopy in the UK, commented on the findings. The TOGAS study focuses on asymptomatic individuals referred for colonoscopy and examines the value of performing an upper GI endoscopy at the same time, he explained. “This approach might identify upper GI conditions that require monitoring, in particular early cancer.”
“On the other hand, the PROSPERO study focuses on patients referred for upper GI symptoms and diagnosis,” he said. Preliminary data from that study, presented during the same session as the TOGAS trial, showed a 13.6% prevalence of premalignant upper GI conditions in a symptomatic UK patient population referred for endoscopy.
“In some respects, the findings were similar, particularly the rate of upper GI cancer at 1.4%, although there were differences in the prevalence of premalignant conditions,” he noted. “This may be explained by the fact that TOGAS is a European study, while PROSPERO is UK-based, where the distribution of upper GI cancers differs, with more esophageal adenocarcinoma vs gastric adenocarcinoma.”
Reflecting on both of the studies, Di Pietro said they are “really important in fulfilling an unmet need in the quality of upper GI endoscopy. Currently, there are no diagnostic quality indicators in upper GI endoscopy, so it’s difficult to rate the performance of endoscopists in the same way as we can in lower GI. It’s really important to understand the population prevalence, both in symptomatic and asymptomatic individuals, of premalignant and malignant upper GI conditions.”
TOGAS 2 is recruiting until February 2026, with 1200 of a potential 1600 participants recruited to date. The data will be used for implementation modeling and to inform quality indicators for future screening programs. Final results and plans for a follow-up study are expected in 2026.
Bornschein declared receiving advisory and speaker fees from Flynn Pharma and Juvisé Pharmaceuticals. Di Pietro reported having no disclosures relevant to the studies discussed.
A version of this article first appeared on Medscape.com.
BERLIN — , including malignancies and lesions requiring ongoing surveillance, according to an interim analysis from the TOGAS study.
“There was an abundance of benign but clinically relevant findings,” said lead investigator Jan Bornschein, MD, gastroenterologist at Oxford University Hospitals NHS Foundation Trust, Oxford, England, who presented the interim resuts of the study at United European Gastroenterology (UEG) Week 2025.
While the study found upper GI neoplasia in only 1.4% of participants, 17.8% of individuals were marked for upper GI endoscopic surveillance.
The results may inform how Europe develops gastric cancer prevention programs alongside those for colorectal cancer, said Bornschein. “If we can combine the upper GI endoscopy with other modalities [colonoscopy], the more likelihood there is that you can have a one-stop test package,” he said. “A combination, particularly for bowel and stomach, is more feasible and also more cost-effective. So far, the findings show that it’s definitely a strategy that, in my opinion, is worth implementing.”
Bornschein and the TOGAS study group hope that the combined approach will prove workable across diverse European settings and will help identify a spectrum of upper GI pathology, from cancers and dysplasia to atrophy and intestinal metaplasia, that can meaningfully affect follow-up surveillance.
Mixed Rates of GI Cancers Across Europe and the US
These findings come amid data showing rising rates of early-onset (younger than 50 years) GI cancers in the US, including colorectal, gastric, pancreatic, and esophageal tumors. These trends, previously reported by this news organization, point to environmental and lifestyle drivers, strengthening the case for earlier detection and risk-tailored strategies for upper GI neoplasia and preneoplastic conditions detected during existing colorectal cancer screening pathways.
However, Bornschein noted that prevalence varies considerably across Europe. “There are areas, particularly in the Eastern regions, and in some parts of the West, for example, Portugal, that have a very high incidence of GI cancers. In the UK or in Germany, we have noticed a decline over the years, so the numbers are actually much better than they used to be.”
The study is the second in a series of three TOGAS pilot studies and was conducted across eight centers (France, Germany, Ireland, Latvia, Lithuania, the Netherlands, Portugal, and Spain) in adults aged 50-74 years attending screening or polyp-surveillance colonoscopy.
A European Society of Gastrointestinal Endoscopy-aligned protocol defining image documentation, biopsy sampling, and quality parameters was followed to ensure a standardized approach. “Marked preneoplastic change” was defined as gastric glandular atrophy or intestinal metaplasia at the Operative Link on Gastritis Assessment/Operative Link on Gastric Intestinal Metaplasia Assessment stage III-IV and/or Endoscopic Grading of Gastric Intestinal Metaplasia > 5, triggering a need for endoscopic surveillance.
Data were gathered on colonoscopy findings (including polyp surveillance and family history), EGD findings plus biopsies, serum pepsinogen, and Helicobacter pylori serology. Outcome measures included the prevalence of gastric cancer and preneoplastic conditions, the diagnostic accuracy of pepsinogen testing, comparisons between national settings, the relevance of upper endoscopy in fecal immunochemical test-positive cases, and overall H pylori prevalence.
Neoplasia and Preneoplasia Found
A total of 846 participants were analyzed. At baseline, the mean age was 62 years, 52.2% were men, and 84.2% were White, despite efforts to recruit a more diverse population. Around 390 participants drank alcohol, and 190 smoked tobacco.
A total of 37.8% of participants had undergone prior EGD, of which 94.7% were performed more than 3 years before the study start. The history of GI surgery was 13.7%, and the history of cancer was 14.5%. Around 11% took aspirin, and 14% took proton pump inhibitors (PPIs). “We were surprised at the low prevalence of PPI use,” remarked Bornschein. “It was also good news that around half were never smokers.”
Key results for upper GI neoplasia included six patients (0.7%) with gastric cancers, three (0.4%) with esophageal cancers, and five (0.6%) with duodenal tumors. H pylori positivity was found in 303 patients (35.8%), with an additional 81 (9.6%) reporting a history of eradication.
Colorectal findings included 15 patients (1.8%) with cancers and colon polyps in 503 (59.5%) participants.
Regarding preneoplastic conditions, endoscopy identified intestinal metaplasia in 174 patients (20.6%), of which 65 (7.7%) were multifocal. Atrophy was observed in 220 patients (26.0%), with 59 (7.0%) showing multifocal atrophic changes. Both intestinal metaplasia and atrophy were found together in 105 (12.4%) patients. Barrett’s esophagus was detected in 31 (3.7%) patients.
“I’d really like to highlight these further benign gastric findings,” said Bornschein. These included gastric ulcers in 28 (3.3%) patients, erosive gastritis in 245 (29.0%) patients, esophageal ulcers in three (0.4%) patients, Los Angeles Community College District classification esophagitis in 13 (1.5%) patients, and duodenal ulcers in 10 (1.2%) patients. “These were asymptomatic, but we were able to identify them,” he noted.
“We’ve had a very low rate of complications (0.01%),” he added.” I don’t want to jinx that now. These were basically related to sedation.”
PROSPERO: Early Detection of Upper GI Conditions in a UK Population
Massimiliano di Pietro, MD, consultant gastroenterologist at Addenbrooke’s Hospital, Cambridge, England, and the principal investigator of the PROSPERO study, which aimed to determine the prevalence of premalignant upper GI conditions in routine endoscopy in the UK, commented on the findings. The TOGAS study focuses on asymptomatic individuals referred for colonoscopy and examines the value of performing an upper GI endoscopy at the same time, he explained. “This approach might identify upper GI conditions that require monitoring, in particular early cancer.”
“On the other hand, the PROSPERO study focuses on patients referred for upper GI symptoms and diagnosis,” he said. Preliminary data from that study, presented during the same session as the TOGAS trial, showed a 13.6% prevalence of premalignant upper GI conditions in a symptomatic UK patient population referred for endoscopy.
“In some respects, the findings were similar, particularly the rate of upper GI cancer at 1.4%, although there were differences in the prevalence of premalignant conditions,” he noted. “This may be explained by the fact that TOGAS is a European study, while PROSPERO is UK-based, where the distribution of upper GI cancers differs, with more esophageal adenocarcinoma vs gastric adenocarcinoma.”
Reflecting on both of the studies, Di Pietro said they are “really important in fulfilling an unmet need in the quality of upper GI endoscopy. Currently, there are no diagnostic quality indicators in upper GI endoscopy, so it’s difficult to rate the performance of endoscopists in the same way as we can in lower GI. It’s really important to understand the population prevalence, both in symptomatic and asymptomatic individuals, of premalignant and malignant upper GI conditions.”
TOGAS 2 is recruiting until February 2026, with 1200 of a potential 1600 participants recruited to date. The data will be used for implementation modeling and to inform quality indicators for future screening programs. Final results and plans for a follow-up study are expected in 2026.
Bornschein declared receiving advisory and speaker fees from Flynn Pharma and Juvisé Pharmaceuticals. Di Pietro reported having no disclosures relevant to the studies discussed.
A version of this article first appeared on Medscape.com.
BERLIN — , including malignancies and lesions requiring ongoing surveillance, according to an interim analysis from the TOGAS study.
“There was an abundance of benign but clinically relevant findings,” said lead investigator Jan Bornschein, MD, gastroenterologist at Oxford University Hospitals NHS Foundation Trust, Oxford, England, who presented the interim resuts of the study at United European Gastroenterology (UEG) Week 2025.
While the study found upper GI neoplasia in only 1.4% of participants, 17.8% of individuals were marked for upper GI endoscopic surveillance.
The results may inform how Europe develops gastric cancer prevention programs alongside those for colorectal cancer, said Bornschein. “If we can combine the upper GI endoscopy with other modalities [colonoscopy], the more likelihood there is that you can have a one-stop test package,” he said. “A combination, particularly for bowel and stomach, is more feasible and also more cost-effective. So far, the findings show that it’s definitely a strategy that, in my opinion, is worth implementing.”
Bornschein and the TOGAS study group hope that the combined approach will prove workable across diverse European settings and will help identify a spectrum of upper GI pathology, from cancers and dysplasia to atrophy and intestinal metaplasia, that can meaningfully affect follow-up surveillance.
Mixed Rates of GI Cancers Across Europe and the US
These findings come amid data showing rising rates of early-onset (younger than 50 years) GI cancers in the US, including colorectal, gastric, pancreatic, and esophageal tumors. These trends, previously reported by this news organization, point to environmental and lifestyle drivers, strengthening the case for earlier detection and risk-tailored strategies for upper GI neoplasia and preneoplastic conditions detected during existing colorectal cancer screening pathways.
However, Bornschein noted that prevalence varies considerably across Europe. “There are areas, particularly in the Eastern regions, and in some parts of the West, for example, Portugal, that have a very high incidence of GI cancers. In the UK or in Germany, we have noticed a decline over the years, so the numbers are actually much better than they used to be.”
The study is the second in a series of three TOGAS pilot studies and was conducted across eight centers (France, Germany, Ireland, Latvia, Lithuania, the Netherlands, Portugal, and Spain) in adults aged 50-74 years attending screening or polyp-surveillance colonoscopy.
A European Society of Gastrointestinal Endoscopy-aligned protocol defining image documentation, biopsy sampling, and quality parameters was followed to ensure a standardized approach. “Marked preneoplastic change” was defined as gastric glandular atrophy or intestinal metaplasia at the Operative Link on Gastritis Assessment/Operative Link on Gastric Intestinal Metaplasia Assessment stage III-IV and/or Endoscopic Grading of Gastric Intestinal Metaplasia > 5, triggering a need for endoscopic surveillance.
Data were gathered on colonoscopy findings (including polyp surveillance and family history), EGD findings plus biopsies, serum pepsinogen, and Helicobacter pylori serology. Outcome measures included the prevalence of gastric cancer and preneoplastic conditions, the diagnostic accuracy of pepsinogen testing, comparisons between national settings, the relevance of upper endoscopy in fecal immunochemical test-positive cases, and overall H pylori prevalence.
Neoplasia and Preneoplasia Found
A total of 846 participants were analyzed. At baseline, the mean age was 62 years, 52.2% were men, and 84.2% were White, despite efforts to recruit a more diverse population. Around 390 participants drank alcohol, and 190 smoked tobacco.
A total of 37.8% of participants had undergone prior EGD, of which 94.7% were performed more than 3 years before the study start. The history of GI surgery was 13.7%, and the history of cancer was 14.5%. Around 11% took aspirin, and 14% took proton pump inhibitors (PPIs). “We were surprised at the low prevalence of PPI use,” remarked Bornschein. “It was also good news that around half were never smokers.”
Key results for upper GI neoplasia included six patients (0.7%) with gastric cancers, three (0.4%) with esophageal cancers, and five (0.6%) with duodenal tumors. H pylori positivity was found in 303 patients (35.8%), with an additional 81 (9.6%) reporting a history of eradication.
Colorectal findings included 15 patients (1.8%) with cancers and colon polyps in 503 (59.5%) participants.
Regarding preneoplastic conditions, endoscopy identified intestinal metaplasia in 174 patients (20.6%), of which 65 (7.7%) were multifocal. Atrophy was observed in 220 patients (26.0%), with 59 (7.0%) showing multifocal atrophic changes. Both intestinal metaplasia and atrophy were found together in 105 (12.4%) patients. Barrett’s esophagus was detected in 31 (3.7%) patients.
“I’d really like to highlight these further benign gastric findings,” said Bornschein. These included gastric ulcers in 28 (3.3%) patients, erosive gastritis in 245 (29.0%) patients, esophageal ulcers in three (0.4%) patients, Los Angeles Community College District classification esophagitis in 13 (1.5%) patients, and duodenal ulcers in 10 (1.2%) patients. “These were asymptomatic, but we were able to identify them,” he noted.
“We’ve had a very low rate of complications (0.01%),” he added.” I don’t want to jinx that now. These were basically related to sedation.”
PROSPERO: Early Detection of Upper GI Conditions in a UK Population
Massimiliano di Pietro, MD, consultant gastroenterologist at Addenbrooke’s Hospital, Cambridge, England, and the principal investigator of the PROSPERO study, which aimed to determine the prevalence of premalignant upper GI conditions in routine endoscopy in the UK, commented on the findings. The TOGAS study focuses on asymptomatic individuals referred for colonoscopy and examines the value of performing an upper GI endoscopy at the same time, he explained. “This approach might identify upper GI conditions that require monitoring, in particular early cancer.”
“On the other hand, the PROSPERO study focuses on patients referred for upper GI symptoms and diagnosis,” he said. Preliminary data from that study, presented during the same session as the TOGAS trial, showed a 13.6% prevalence of premalignant upper GI conditions in a symptomatic UK patient population referred for endoscopy.
“In some respects, the findings were similar, particularly the rate of upper GI cancer at 1.4%, although there were differences in the prevalence of premalignant conditions,” he noted. “This may be explained by the fact that TOGAS is a European study, while PROSPERO is UK-based, where the distribution of upper GI cancers differs, with more esophageal adenocarcinoma vs gastric adenocarcinoma.”
Reflecting on both of the studies, Di Pietro said they are “really important in fulfilling an unmet need in the quality of upper GI endoscopy. Currently, there are no diagnostic quality indicators in upper GI endoscopy, so it’s difficult to rate the performance of endoscopists in the same way as we can in lower GI. It’s really important to understand the population prevalence, both in symptomatic and asymptomatic individuals, of premalignant and malignant upper GI conditions.”
TOGAS 2 is recruiting until February 2026, with 1200 of a potential 1600 participants recruited to date. The data will be used for implementation modeling and to inform quality indicators for future screening programs. Final results and plans for a follow-up study are expected in 2026.
Bornschein declared receiving advisory and speaker fees from Flynn Pharma and Juvisé Pharmaceuticals. Di Pietro reported having no disclosures relevant to the studies discussed.
A version of this article first appeared on Medscape.com.
Real-World Pros & Cons of the New Liver Disease Nomenclature
VIENNA – Maria Effenberger, MD, Medical University of Innsbruck, Berlin, Germany, told attendees at United European Gastroenterology (UEG) Week 2025 in Vienna, Austria.
In her presentation, “Sense and Nonsense of the New Nomenclature,” Effenberger highlighted the clinical implications of the new liver-disease terminology and pointed to a few factors still needing to be sorted out.
Both NAFLD and MASLD are steatotic liver diseasesand, notably, there are few differences between the two in clinical studies, which makes the terminology shift easier, said Effenberger. She cited a recent study showing demographic and clinical profiles of individuals classified as NAFLD and MASLD in the US were “strikingly similar,” as were the accuracy of the noninvasive tests and all-cause and cause-specific mortality rates for both conditions.
However, “the important thing about MASLD is that the term is really connected to metabolic dysfunction,” said Effenberger. To be diagnosed with MASLD, patients with liver disease need to have at least one of five cardiometabolic abnormalities: a high BMI — over 25 in White people and over 23 in Asian people; type 2 diabetes (T2D) or prediabetes; arterial hypertension; high levels of triglycerides; or a low level of high-density lipoprotein cholesterol.
“MASLD is a systemic disease, and that term represents it much better than only looking at it as a hepatological disease,” Effenberger said. “Many factors, especially inflammatory ones, influence steatosis, inflammation, and fibrosis.” These include influences from adipose tissue, the gut microbiome, the brain, a hypocaloric diet, and from steatosis of the liver itself. Proinflammatory cytokines induced by the disease can lead to inflammation throughout the body, with clinical outcomes such as stroke, heart failure, arrhythmias, myocardial infarction, chronic kidney disease.
MASLD, MetALD, or ALD?
“What is important now,” said Effenberger, is that “every patient who has liver disease should be asked two questions.” The first question is whether the patient has any of the cardiometabolic criteria outlined above. Second, is the patient consuming alcohol?
If the patient has one of the cardiometabolic criteria but doesn’t consume alcohol, “we are straight at the diagnosis of MASLD,” she explained. If the patient does consume alcohol, it depends on how much.
Patients who have at least one cardiometabolic risk factor and consume 140-350 g for men and 210-420 g for women are considered to have Metabolic and Alcohol-Associated Liver Disease (MetALD). And those with steatotic liver disease who drink alcohol above the MetALD thresholds are considered to have ALD.
Effenberger pointed to two “cons” of the new nomenclature that need to be clarified. Although MetALD has poorer outcomes than MASLD, “it’s really hard to differentiate between ALD and MASLD,” she said. Yet the distinction is important because risks for cirrhosis, hepatocellular carcinoma (HCC), and overall mortality increase more for patients diagnosed with ALD vs MASLD.
“Do MASLD patients drink alcohol? Yes they do,” Effenberger said. “And if you have MASLD and another trigger factor like alcohol, the rates of mortality, morbidity and cancer go up.”
Moderator Laurent Castera, MD, PhD, Université Paris-Cité, Paris, France, noted that a “pro” of the new nomenclature is that it is “shedding light on the importance of alcohol because when we discuss steatotic liver disease or MASLD, alcohol is always the elephant in the room,” he said. “We need to increase the awareness that even in the absence of alcohol, you can still develop cirrhosis if you have severe metabolic risk factors.”
On the other hand, he said, “We desperately need more statistics on the true prevalence of alcohol consumption. While studies suggest the prevalence is low, at around 4% or 5%, that does not match the reality, in my opinion.”
Effenberger agreed. There’s a problem in trying to zero in on alcohol consumption because of the stigma attached to it, she said. She pointed to an Austrian study assessing patients who are diagnosed with MASLD. The researchers asked them, “Do you drink alcohol?” and all the participants said “no.” However, after completing a questionnaire designed to identify alcohol use disorders, and undergoing glucuronide tests in the urine and hair, it became clear that 25%-30% of these patients actually drank alcohol on a regular basis.
Cancer, Cirrhosis, CVD
MASLD is a trigger for cancer, especially HCC, Effenberger said. A recent review affirmed that MASLD is strongly associated with HCC, especially in Southeast Asia and India. The same study showed that many patients with MASLD are getting HCC without cirrhosis, and their cancer is often detected at a later stage, however, it’s not yet clear why they are getting HCC, and further study is needed.
In addition, MASLD is also associated with higher rates of extrahepatic cancers, including cancers of the skin and androgenic cancers. This, too, requires further investigation.
Regarding cardiovascular disease (CVD) risk, Effenberger emphasized that cardiometabolic diseases are strongly linked to each other. “Therefore, if you have diabetes and MASLD, the rates of atherosclerosis and of heart insufficiency and arteriosclerotic events like stroke and heart attacks go up, leading to the question of whether a CVD risk assessment is necessary in patients with MASLD.”
One recent study suggests that yes, it is, she reported. “If a patient has MASLD and cardiometabolic risk factors, and a risk score that suggests the patient is at increased risk of CVD for 10 years, then a CT scan of the arteries of the heart is important. The increased risk could also lead to intensified medical therapy, including GLP-1s or SGLT2s.”
During the Q&A, one attendee asked whether all patients with noncirrhotic MASLD should be screened for HCC, given the increased risk. Effenberger agreed that would be the best way to identify those at high risk; however, she said, “I think science is not in a state where you can clearly define which patients will be at high risk, and so we don’t have any guidelines for that.”
Another attendee asked why HCC is more common in Indians and Asians. Effenberger said, “We don’t know, but it is likely that there is an HCC-driven genetic risk factor.”
Remaining Questions
And finally, there’s the question of “what do we do with burnt-out MASLD?” Effenberger asked. “We know the fat content of the liver decreases when liver severity goes up. Therefore, we have a lot of patients with cirrhosis whose disease is not defined as steatotic liver because the liver fat content is no longer more than 5%.”
The decrease in fat is an ongoing process, and therefore, these patients with MASLD and advanced hepatic disease need to be better represented in the nomenclature, she suggested.
No funding information was provided. Effenberger declared working with Ipsen as a potential conflict.
A version of this article first appeared on Medscape.com.
VIENNA – Maria Effenberger, MD, Medical University of Innsbruck, Berlin, Germany, told attendees at United European Gastroenterology (UEG) Week 2025 in Vienna, Austria.
In her presentation, “Sense and Nonsense of the New Nomenclature,” Effenberger highlighted the clinical implications of the new liver-disease terminology and pointed to a few factors still needing to be sorted out.
Both NAFLD and MASLD are steatotic liver diseasesand, notably, there are few differences between the two in clinical studies, which makes the terminology shift easier, said Effenberger. She cited a recent study showing demographic and clinical profiles of individuals classified as NAFLD and MASLD in the US were “strikingly similar,” as were the accuracy of the noninvasive tests and all-cause and cause-specific mortality rates for both conditions.
However, “the important thing about MASLD is that the term is really connected to metabolic dysfunction,” said Effenberger. To be diagnosed with MASLD, patients with liver disease need to have at least one of five cardiometabolic abnormalities: a high BMI — over 25 in White people and over 23 in Asian people; type 2 diabetes (T2D) or prediabetes; arterial hypertension; high levels of triglycerides; or a low level of high-density lipoprotein cholesterol.
“MASLD is a systemic disease, and that term represents it much better than only looking at it as a hepatological disease,” Effenberger said. “Many factors, especially inflammatory ones, influence steatosis, inflammation, and fibrosis.” These include influences from adipose tissue, the gut microbiome, the brain, a hypocaloric diet, and from steatosis of the liver itself. Proinflammatory cytokines induced by the disease can lead to inflammation throughout the body, with clinical outcomes such as stroke, heart failure, arrhythmias, myocardial infarction, chronic kidney disease.
MASLD, MetALD, or ALD?
“What is important now,” said Effenberger, is that “every patient who has liver disease should be asked two questions.” The first question is whether the patient has any of the cardiometabolic criteria outlined above. Second, is the patient consuming alcohol?
If the patient has one of the cardiometabolic criteria but doesn’t consume alcohol, “we are straight at the diagnosis of MASLD,” she explained. If the patient does consume alcohol, it depends on how much.
Patients who have at least one cardiometabolic risk factor and consume 140-350 g for men and 210-420 g for women are considered to have Metabolic and Alcohol-Associated Liver Disease (MetALD). And those with steatotic liver disease who drink alcohol above the MetALD thresholds are considered to have ALD.
Effenberger pointed to two “cons” of the new nomenclature that need to be clarified. Although MetALD has poorer outcomes than MASLD, “it’s really hard to differentiate between ALD and MASLD,” she said. Yet the distinction is important because risks for cirrhosis, hepatocellular carcinoma (HCC), and overall mortality increase more for patients diagnosed with ALD vs MASLD.
“Do MASLD patients drink alcohol? Yes they do,” Effenberger said. “And if you have MASLD and another trigger factor like alcohol, the rates of mortality, morbidity and cancer go up.”
Moderator Laurent Castera, MD, PhD, Université Paris-Cité, Paris, France, noted that a “pro” of the new nomenclature is that it is “shedding light on the importance of alcohol because when we discuss steatotic liver disease or MASLD, alcohol is always the elephant in the room,” he said. “We need to increase the awareness that even in the absence of alcohol, you can still develop cirrhosis if you have severe metabolic risk factors.”
On the other hand, he said, “We desperately need more statistics on the true prevalence of alcohol consumption. While studies suggest the prevalence is low, at around 4% or 5%, that does not match the reality, in my opinion.”
Effenberger agreed. There’s a problem in trying to zero in on alcohol consumption because of the stigma attached to it, she said. She pointed to an Austrian study assessing patients who are diagnosed with MASLD. The researchers asked them, “Do you drink alcohol?” and all the participants said “no.” However, after completing a questionnaire designed to identify alcohol use disorders, and undergoing glucuronide tests in the urine and hair, it became clear that 25%-30% of these patients actually drank alcohol on a regular basis.
Cancer, Cirrhosis, CVD
MASLD is a trigger for cancer, especially HCC, Effenberger said. A recent review affirmed that MASLD is strongly associated with HCC, especially in Southeast Asia and India. The same study showed that many patients with MASLD are getting HCC without cirrhosis, and their cancer is often detected at a later stage, however, it’s not yet clear why they are getting HCC, and further study is needed.
In addition, MASLD is also associated with higher rates of extrahepatic cancers, including cancers of the skin and androgenic cancers. This, too, requires further investigation.
Regarding cardiovascular disease (CVD) risk, Effenberger emphasized that cardiometabolic diseases are strongly linked to each other. “Therefore, if you have diabetes and MASLD, the rates of atherosclerosis and of heart insufficiency and arteriosclerotic events like stroke and heart attacks go up, leading to the question of whether a CVD risk assessment is necessary in patients with MASLD.”
One recent study suggests that yes, it is, she reported. “If a patient has MASLD and cardiometabolic risk factors, and a risk score that suggests the patient is at increased risk of CVD for 10 years, then a CT scan of the arteries of the heart is important. The increased risk could also lead to intensified medical therapy, including GLP-1s or SGLT2s.”
During the Q&A, one attendee asked whether all patients with noncirrhotic MASLD should be screened for HCC, given the increased risk. Effenberger agreed that would be the best way to identify those at high risk; however, she said, “I think science is not in a state where you can clearly define which patients will be at high risk, and so we don’t have any guidelines for that.”
Another attendee asked why HCC is more common in Indians and Asians. Effenberger said, “We don’t know, but it is likely that there is an HCC-driven genetic risk factor.”
Remaining Questions
And finally, there’s the question of “what do we do with burnt-out MASLD?” Effenberger asked. “We know the fat content of the liver decreases when liver severity goes up. Therefore, we have a lot of patients with cirrhosis whose disease is not defined as steatotic liver because the liver fat content is no longer more than 5%.”
The decrease in fat is an ongoing process, and therefore, these patients with MASLD and advanced hepatic disease need to be better represented in the nomenclature, she suggested.
No funding information was provided. Effenberger declared working with Ipsen as a potential conflict.
A version of this article first appeared on Medscape.com.
VIENNA – Maria Effenberger, MD, Medical University of Innsbruck, Berlin, Germany, told attendees at United European Gastroenterology (UEG) Week 2025 in Vienna, Austria.
In her presentation, “Sense and Nonsense of the New Nomenclature,” Effenberger highlighted the clinical implications of the new liver-disease terminology and pointed to a few factors still needing to be sorted out.
Both NAFLD and MASLD are steatotic liver diseasesand, notably, there are few differences between the two in clinical studies, which makes the terminology shift easier, said Effenberger. She cited a recent study showing demographic and clinical profiles of individuals classified as NAFLD and MASLD in the US were “strikingly similar,” as were the accuracy of the noninvasive tests and all-cause and cause-specific mortality rates for both conditions.
However, “the important thing about MASLD is that the term is really connected to metabolic dysfunction,” said Effenberger. To be diagnosed with MASLD, patients with liver disease need to have at least one of five cardiometabolic abnormalities: a high BMI — over 25 in White people and over 23 in Asian people; type 2 diabetes (T2D) or prediabetes; arterial hypertension; high levels of triglycerides; or a low level of high-density lipoprotein cholesterol.
“MASLD is a systemic disease, and that term represents it much better than only looking at it as a hepatological disease,” Effenberger said. “Many factors, especially inflammatory ones, influence steatosis, inflammation, and fibrosis.” These include influences from adipose tissue, the gut microbiome, the brain, a hypocaloric diet, and from steatosis of the liver itself. Proinflammatory cytokines induced by the disease can lead to inflammation throughout the body, with clinical outcomes such as stroke, heart failure, arrhythmias, myocardial infarction, chronic kidney disease.
MASLD, MetALD, or ALD?
“What is important now,” said Effenberger, is that “every patient who has liver disease should be asked two questions.” The first question is whether the patient has any of the cardiometabolic criteria outlined above. Second, is the patient consuming alcohol?
If the patient has one of the cardiometabolic criteria but doesn’t consume alcohol, “we are straight at the diagnosis of MASLD,” she explained. If the patient does consume alcohol, it depends on how much.
Patients who have at least one cardiometabolic risk factor and consume 140-350 g for men and 210-420 g for women are considered to have Metabolic and Alcohol-Associated Liver Disease (MetALD). And those with steatotic liver disease who drink alcohol above the MetALD thresholds are considered to have ALD.
Effenberger pointed to two “cons” of the new nomenclature that need to be clarified. Although MetALD has poorer outcomes than MASLD, “it’s really hard to differentiate between ALD and MASLD,” she said. Yet the distinction is important because risks for cirrhosis, hepatocellular carcinoma (HCC), and overall mortality increase more for patients diagnosed with ALD vs MASLD.
“Do MASLD patients drink alcohol? Yes they do,” Effenberger said. “And if you have MASLD and another trigger factor like alcohol, the rates of mortality, morbidity and cancer go up.”
Moderator Laurent Castera, MD, PhD, Université Paris-Cité, Paris, France, noted that a “pro” of the new nomenclature is that it is “shedding light on the importance of alcohol because when we discuss steatotic liver disease or MASLD, alcohol is always the elephant in the room,” he said. “We need to increase the awareness that even in the absence of alcohol, you can still develop cirrhosis if you have severe metabolic risk factors.”
On the other hand, he said, “We desperately need more statistics on the true prevalence of alcohol consumption. While studies suggest the prevalence is low, at around 4% or 5%, that does not match the reality, in my opinion.”
Effenberger agreed. There’s a problem in trying to zero in on alcohol consumption because of the stigma attached to it, she said. She pointed to an Austrian study assessing patients who are diagnosed with MASLD. The researchers asked them, “Do you drink alcohol?” and all the participants said “no.” However, after completing a questionnaire designed to identify alcohol use disorders, and undergoing glucuronide tests in the urine and hair, it became clear that 25%-30% of these patients actually drank alcohol on a regular basis.
Cancer, Cirrhosis, CVD
MASLD is a trigger for cancer, especially HCC, Effenberger said. A recent review affirmed that MASLD is strongly associated with HCC, especially in Southeast Asia and India. The same study showed that many patients with MASLD are getting HCC without cirrhosis, and their cancer is often detected at a later stage, however, it’s not yet clear why they are getting HCC, and further study is needed.
In addition, MASLD is also associated with higher rates of extrahepatic cancers, including cancers of the skin and androgenic cancers. This, too, requires further investigation.
Regarding cardiovascular disease (CVD) risk, Effenberger emphasized that cardiometabolic diseases are strongly linked to each other. “Therefore, if you have diabetes and MASLD, the rates of atherosclerosis and of heart insufficiency and arteriosclerotic events like stroke and heart attacks go up, leading to the question of whether a CVD risk assessment is necessary in patients with MASLD.”
One recent study suggests that yes, it is, she reported. “If a patient has MASLD and cardiometabolic risk factors, and a risk score that suggests the patient is at increased risk of CVD for 10 years, then a CT scan of the arteries of the heart is important. The increased risk could also lead to intensified medical therapy, including GLP-1s or SGLT2s.”
During the Q&A, one attendee asked whether all patients with noncirrhotic MASLD should be screened for HCC, given the increased risk. Effenberger agreed that would be the best way to identify those at high risk; however, she said, “I think science is not in a state where you can clearly define which patients will be at high risk, and so we don’t have any guidelines for that.”
Another attendee asked why HCC is more common in Indians and Asians. Effenberger said, “We don’t know, but it is likely that there is an HCC-driven genetic risk factor.”
Remaining Questions
And finally, there’s the question of “what do we do with burnt-out MASLD?” Effenberger asked. “We know the fat content of the liver decreases when liver severity goes up. Therefore, we have a lot of patients with cirrhosis whose disease is not defined as steatotic liver because the liver fat content is no longer more than 5%.”
The decrease in fat is an ongoing process, and therefore, these patients with MASLD and advanced hepatic disease need to be better represented in the nomenclature, she suggested.
No funding information was provided. Effenberger declared working with Ipsen as a potential conflict.
A version of this article first appeared on Medscape.com.
Anti-TNF Exposure Influences Efficacy of Subsequent Therapies in UC
, based on results of a large meta-analysis.
Patients previously treated with TNF antagonists were less likely to respond to lymphocyte trafficking inhibitors but more likely to achieve remission on Janus kinase (JAK) inhibitors, Han Hee Lee, MD, PhD, of the University of California San Diego, and colleagues reported.
“Treatment options for patients with moderate-severe ulcerative colitis have increased in the last decade with the availability of six different classes of medications,” investigators wrote in Clinical Gastroenterology and Hepatology (2024 Dec. doi:10.1016/j.cgh.2024.12.007). “There is wide interindividual variability in response to specific medications, and drivers of this heterogeneity are critical to understand to be able to choose the best therapy for each individual patient.”
To learn more about the impacts of anti-TNF exposure on subsequent advanced therapies, the investigators conducted a systematic review and meta-analysis of 17 phase 2 and 3 trials. The dataset included 8,871 adults with moderate-severe UC.
The primary outcome was induction of clinical remission at 6–14 weeks, most often defined as a Mayo Clinic score of 2 or lower with no subscore greater than 1. Endoscopic improvement, generally defined as a Mayo endoscopic subscore of 0 or 1, was evaluated as a secondary endpoint.
Advanced therapies were grouped by mechanism of action, including lymphocyte trafficking inhibitors, JAK inhibitors, and interleukin (IL)-12/23 and IL-23 antagonists. Odds ratios for treatment versus placebo were calculated separately for each subgroup, and a ratio of odds ratios was then used to assess whether prior TNF exposure modified drug effect. Analyses were conducted on an intention-to-treat basis, restricted to approved dosing when multiple regimens were tested.
Across five trials of lymphocyte trafficking inhibitors including 2,046 patients, efficacy was significantly greater in TNF-naïve patients compared with those who had prior TNF exposure. The odds of achieving clinical remission were nearly doubled in the TNF-naïve group (ratio of odds ratios [ROR], 1.88; 95% CI, 1.02–3.49).
In six trials of JAK inhibitors including 3,015 patients, remission rates were higher among TNF-exposed patients com-pared with TNF-naïve patients (ROR, 0.47; 95% CI, 0.22–1.01).
In six trials of IL-12/23 and IL-23 antagonists, including 3,810 patients, prior TNF exposure did not significantly modify treatment outcomes (ROR, 1.07; 95% CI, 0.64–1.80). Within individual trials, ustekinumab showed a trend toward great-er efficacy in TNF-exposed patients, whereas selective IL-23 antagonists performed similarly regardless of TNF exposure history.
Secondary analyses of endoscopic improvement yielded results consistent with the primary endpoint. Statistical heterogeneity across trials was minimal, and all included studies were rated at low risk of bias.
The investigators noted several limitations. For example, therapies were grouped broadly by mechanism of action, although specific biologic effects could potentially differ within groups. The analysis also could not account for patients who had failed two or more classes of advanced therapy, which may independently reduce the likelihood of response.
Still, Lee and colleagues suggested that the findings deserve a closer look.
“[T]here is significant heterogeneity of treatment efficacy for induction of remission with different advanced therapies in patients with moderate-severe UC based on prior exposure to TNF antagonists,” they concluded. “Future studies on the mechanistic insight for these intriguing observations are warranted.”
The study was supported by the Leona and Harry B. Helmsley Trust, the National Institutes of Health, and the Centers for Disease Control and Prevention. The investigators disclosed relationships with AbbVie, Ferring, Pfizer, and others.
, based on results of a large meta-analysis.
Patients previously treated with TNF antagonists were less likely to respond to lymphocyte trafficking inhibitors but more likely to achieve remission on Janus kinase (JAK) inhibitors, Han Hee Lee, MD, PhD, of the University of California San Diego, and colleagues reported.
“Treatment options for patients with moderate-severe ulcerative colitis have increased in the last decade with the availability of six different classes of medications,” investigators wrote in Clinical Gastroenterology and Hepatology (2024 Dec. doi:10.1016/j.cgh.2024.12.007). “There is wide interindividual variability in response to specific medications, and drivers of this heterogeneity are critical to understand to be able to choose the best therapy for each individual patient.”
To learn more about the impacts of anti-TNF exposure on subsequent advanced therapies, the investigators conducted a systematic review and meta-analysis of 17 phase 2 and 3 trials. The dataset included 8,871 adults with moderate-severe UC.
The primary outcome was induction of clinical remission at 6–14 weeks, most often defined as a Mayo Clinic score of 2 or lower with no subscore greater than 1. Endoscopic improvement, generally defined as a Mayo endoscopic subscore of 0 or 1, was evaluated as a secondary endpoint.
Advanced therapies were grouped by mechanism of action, including lymphocyte trafficking inhibitors, JAK inhibitors, and interleukin (IL)-12/23 and IL-23 antagonists. Odds ratios for treatment versus placebo were calculated separately for each subgroup, and a ratio of odds ratios was then used to assess whether prior TNF exposure modified drug effect. Analyses were conducted on an intention-to-treat basis, restricted to approved dosing when multiple regimens were tested.
Across five trials of lymphocyte trafficking inhibitors including 2,046 patients, efficacy was significantly greater in TNF-naïve patients compared with those who had prior TNF exposure. The odds of achieving clinical remission were nearly doubled in the TNF-naïve group (ratio of odds ratios [ROR], 1.88; 95% CI, 1.02–3.49).
In six trials of JAK inhibitors including 3,015 patients, remission rates were higher among TNF-exposed patients com-pared with TNF-naïve patients (ROR, 0.47; 95% CI, 0.22–1.01).
In six trials of IL-12/23 and IL-23 antagonists, including 3,810 patients, prior TNF exposure did not significantly modify treatment outcomes (ROR, 1.07; 95% CI, 0.64–1.80). Within individual trials, ustekinumab showed a trend toward great-er efficacy in TNF-exposed patients, whereas selective IL-23 antagonists performed similarly regardless of TNF exposure history.
Secondary analyses of endoscopic improvement yielded results consistent with the primary endpoint. Statistical heterogeneity across trials was minimal, and all included studies were rated at low risk of bias.
The investigators noted several limitations. For example, therapies were grouped broadly by mechanism of action, although specific biologic effects could potentially differ within groups. The analysis also could not account for patients who had failed two or more classes of advanced therapy, which may independently reduce the likelihood of response.
Still, Lee and colleagues suggested that the findings deserve a closer look.
“[T]here is significant heterogeneity of treatment efficacy for induction of remission with different advanced therapies in patients with moderate-severe UC based on prior exposure to TNF antagonists,” they concluded. “Future studies on the mechanistic insight for these intriguing observations are warranted.”
The study was supported by the Leona and Harry B. Helmsley Trust, the National Institutes of Health, and the Centers for Disease Control and Prevention. The investigators disclosed relationships with AbbVie, Ferring, Pfizer, and others.
, based on results of a large meta-analysis.
Patients previously treated with TNF antagonists were less likely to respond to lymphocyte trafficking inhibitors but more likely to achieve remission on Janus kinase (JAK) inhibitors, Han Hee Lee, MD, PhD, of the University of California San Diego, and colleagues reported.
“Treatment options for patients with moderate-severe ulcerative colitis have increased in the last decade with the availability of six different classes of medications,” investigators wrote in Clinical Gastroenterology and Hepatology (2024 Dec. doi:10.1016/j.cgh.2024.12.007). “There is wide interindividual variability in response to specific medications, and drivers of this heterogeneity are critical to understand to be able to choose the best therapy for each individual patient.”
To learn more about the impacts of anti-TNF exposure on subsequent advanced therapies, the investigators conducted a systematic review and meta-analysis of 17 phase 2 and 3 trials. The dataset included 8,871 adults with moderate-severe UC.
The primary outcome was induction of clinical remission at 6–14 weeks, most often defined as a Mayo Clinic score of 2 or lower with no subscore greater than 1. Endoscopic improvement, generally defined as a Mayo endoscopic subscore of 0 or 1, was evaluated as a secondary endpoint.
Advanced therapies were grouped by mechanism of action, including lymphocyte trafficking inhibitors, JAK inhibitors, and interleukin (IL)-12/23 and IL-23 antagonists. Odds ratios for treatment versus placebo were calculated separately for each subgroup, and a ratio of odds ratios was then used to assess whether prior TNF exposure modified drug effect. Analyses were conducted on an intention-to-treat basis, restricted to approved dosing when multiple regimens were tested.
Across five trials of lymphocyte trafficking inhibitors including 2,046 patients, efficacy was significantly greater in TNF-naïve patients compared with those who had prior TNF exposure. The odds of achieving clinical remission were nearly doubled in the TNF-naïve group (ratio of odds ratios [ROR], 1.88; 95% CI, 1.02–3.49).
In six trials of JAK inhibitors including 3,015 patients, remission rates were higher among TNF-exposed patients com-pared with TNF-naïve patients (ROR, 0.47; 95% CI, 0.22–1.01).
In six trials of IL-12/23 and IL-23 antagonists, including 3,810 patients, prior TNF exposure did not significantly modify treatment outcomes (ROR, 1.07; 95% CI, 0.64–1.80). Within individual trials, ustekinumab showed a trend toward great-er efficacy in TNF-exposed patients, whereas selective IL-23 antagonists performed similarly regardless of TNF exposure history.
Secondary analyses of endoscopic improvement yielded results consistent with the primary endpoint. Statistical heterogeneity across trials was minimal, and all included studies were rated at low risk of bias.
The investigators noted several limitations. For example, therapies were grouped broadly by mechanism of action, although specific biologic effects could potentially differ within groups. The analysis also could not account for patients who had failed two or more classes of advanced therapy, which may independently reduce the likelihood of response.
Still, Lee and colleagues suggested that the findings deserve a closer look.
“[T]here is significant heterogeneity of treatment efficacy for induction of remission with different advanced therapies in patients with moderate-severe UC based on prior exposure to TNF antagonists,” they concluded. “Future studies on the mechanistic insight for these intriguing observations are warranted.”
The study was supported by the Leona and Harry B. Helmsley Trust, the National Institutes of Health, and the Centers for Disease Control and Prevention. The investigators disclosed relationships with AbbVie, Ferring, Pfizer, and others.
FROM CLINICAL GASTROENTEROLOGY AND HEPATOLOGY
mRNA Cancer Vaccines: Pipeline Insights for Clinicians
Since 1965, messenger RNA (mRNA) vaccines have been studied for cancer treatment, but it was the technological advances in vaccines during the COVID pandemic that helped accelerate research. Currently, no vaccine has been approved for tumor treatment, although many clinical studies are ongoing worldwide. According to experts consulted by Medscape’s Portuguese edition, the outlook is very promising, and these studies are expected to open doors for personalized therapies.
In cancer treatment, the vaccine would function as an immunotherapy, in which the immune system can be “trained” to act against an invader. Just as with pathogens, the platform would use parts of the tumor — which have altered proteins or are expressed at abnormal levels — to teach the body to defend itself against cancer.
Vladmir Lima, MD, PhD, clinical oncologist at A.C. Camargo Cancer Center, São Paulo, Brazil, explained that with this technology it will be possible to produce personalized vaccines, which prevents, for example, large-scale manufacturing. “In theory, these vaccines can be developed for any tumor type, but this does not mean that efficacy will be the same for all,” he said. Because cancer has specific characteristics in each individual, it is difficult to envision a single vaccine that works for all cancers.
Current evidence suggests the vaccine could be administered after chemotherapy or radiotherapy, with the goal of reducing tumor mass and increasing the effectiveness of mRNA-based treatment, according to Ana Paula Lepique, professor and researcher in tumor immunology at the Institute of Biomedical Sciences, University of São Paulo, São Paulo.
“There is also a study with pancreatic cancer patients, in which the vaccine was administered after surgery,” she explained. “It would not work, for example, to give chemotherapy or radiotherapy while the immune response is being triggered by the vaccine. This would make the vaccine ineffective, since chemotherapy and radiotherapy are toxic to lymphocytes.”
Lepique also clarified that it is possible to combine the vaccine with immunotherapy targeting immune regulatory molecules. “In this case, in addition to administering the mRNA with the antigen, a strategy is used to improve the patient’s immune response.”
Challenges With mRNA Vaccines
Despite being a promising technology, there are challenges, warned Lepique. mRNA molecules degrade quickly when injected into the body, which can compromise vaccine efficacy. To overcome this, researchers have developed nanoencapsulation technologies that protect the molecules and allow safe use in vaccines. “Another alternative is transferring the mRNA into dendritic cells, known as antigen-presenting cells, and then administering these cells to the patient,” she explained.
Global Research Status
According to a study published this year in Med, over 120 clinical trials are exploring mRNA vaccines to treat lung, breast, prostate, and pancreatic tumors, as well as melanoma.
Lepique noted that the countries leading this research are the US, UK, Germany, China, and Japan. “Unfortunately, the US government recently cut funding for mRNA vaccine development and testing, which will likely have significant consequences,” she said.
Lepique reported that Brazilian researchers are collaborating with international institutions to develop these vaccines. “The Brazilian government, through the Ministry of Health and the Ministry of Science, Technology, and Innovation, recently announced investments in mRNA technologies for vaccines. While not specifically targeting cancer, these investments could also benefit this field,” she clarified.
Leading Studies
Lepique highlighted the most advanced studies to date:
- Pancreatic cancer: A study published in Nature in February demonstrated that a personalized mRNA vaccine reduced the risk for recurrence after surgery in 16 patients, with 3 years of follow-up.
- Melanoma: A study published in The Lancet reported improved survival in melanoma patients after mRNA vaccine administration combined with the checkpoint inhibitor pembrolizumab applied after surgical tumor resection.
- Universal vaccine: A study in Nature Biomedical Engineering described the creation of a “generic” vaccine capable of activating the patient’s immune system and inducing tumor regression. Lepique explained that this vaccine acts more as an immune response modulator than a classical neoantigen-specific vaccine. “Because it is not limited to a single neoantigen, it could potentially be universal, though further testing is needed to determine efficacy across all cancer types,” she added.
Lima highlighted a 2024 study being conducted by MSD and Moderna against lung cancer, with results yet to be published. “Patients first receive immunotherapy after surgery. Once the vaccine is ready, it is added to the ongoing immunotherapy,” he explained. The global phase 3 study involves 868 patients with resected lung cancer who previously underwent chemotherapy. Participants receive the vaccine (1 mg every 3 weeks, up to nine doses) alongside pembrolizumab (400 mg every 6 weeks, up to nine cycles) over approximately 1 year.
Other mRNA vaccines remain in early-stage development. For example, in May 2024, the UK National Health Service recruited participants for a personalized colorectal cancer mRNA vaccine trial.
Advantages of mRNA Technology
Experts noted that mRNA-based cancer vaccines are considered safer for patients because the tumor mRNA is synthesized in the laboratory. According to Lepique, these vaccines are more specific than many other cancer therapies, and therefore carry a lower risk for serious side effects.
“Clinical studies have shown that these vaccines can generate immunological memory, meaning lymphocytes that recognize tumor antigens remain in the body and can respond to recurrence,” she explained.
It is also possible to combine multiple mRNA molecules in a single vaccine, creating a platform that targets several tumor antigens simultaneously. “Formulations can additionally include adjuvants to further enhance immune responses against tumors,” she said. However, as a personalized therapy, costs are high, and vaccine formulation requires considerable time.
Lima emphasized the customization advantage: “We can take a portion of the patient’s tumor, sequence it to identify alterations, and develop a vaccine specifically for that tumor.” He also highlighted safety data, noting that the platform has been widely used in SARS-CoV-2 vaccine development, providing confidence in large-scale application. “The potential exists to achieve more personalized, tumor-directed immunotherapy with greater scalability,” he explained.
Outlook and Limitations
Lima noted that although the projected efficacy is promising, definitive results are still pending.
“We have very positive expectations, but we must wait for study outcomes. Efficacy may vary across scenarios and among patients. The immune system may also respond against the vaccine itself, potentially reducing effectiveness at times,” he explained.
According to Lima, mRNA vaccines are expected to complement current treatments, enhancing outcomes without replacing conventional approaches entirely.
“It will not be a panacea. These vaccines are likely to add to and improve strategies we already use, but they will not work for all patients in every scenario,” he concluded.
Lepique highlighted the promise of combination strategies. “The outlook is positive, particularly because multiple mRNA types can be combined in a single formulation and used alongside drugs that enhance immune responses,” she explained.
Although mRNA vaccine research has been ongoing for many years, prior results have brought both progress and setbacks. “This new protocol appears more effective [and] capable of generating immunological memory and is also safe,” she noted. Still, she cautioned that cancer presents unique challenges: “The disease has multiple mechanisms to evade immune responses. Additionally, some tumors are naturally unrecognized by the immune system, the so-called ‘cold tumors.’”
This story was translated from Medscape’s Portuguese edition. A version of this article appeared on Medscape.com.
Since 1965, messenger RNA (mRNA) vaccines have been studied for cancer treatment, but it was the technological advances in vaccines during the COVID pandemic that helped accelerate research. Currently, no vaccine has been approved for tumor treatment, although many clinical studies are ongoing worldwide. According to experts consulted by Medscape’s Portuguese edition, the outlook is very promising, and these studies are expected to open doors for personalized therapies.
In cancer treatment, the vaccine would function as an immunotherapy, in which the immune system can be “trained” to act against an invader. Just as with pathogens, the platform would use parts of the tumor — which have altered proteins or are expressed at abnormal levels — to teach the body to defend itself against cancer.
Vladmir Lima, MD, PhD, clinical oncologist at A.C. Camargo Cancer Center, São Paulo, Brazil, explained that with this technology it will be possible to produce personalized vaccines, which prevents, for example, large-scale manufacturing. “In theory, these vaccines can be developed for any tumor type, but this does not mean that efficacy will be the same for all,” he said. Because cancer has specific characteristics in each individual, it is difficult to envision a single vaccine that works for all cancers.
Current evidence suggests the vaccine could be administered after chemotherapy or radiotherapy, with the goal of reducing tumor mass and increasing the effectiveness of mRNA-based treatment, according to Ana Paula Lepique, professor and researcher in tumor immunology at the Institute of Biomedical Sciences, University of São Paulo, São Paulo.
“There is also a study with pancreatic cancer patients, in which the vaccine was administered after surgery,” she explained. “It would not work, for example, to give chemotherapy or radiotherapy while the immune response is being triggered by the vaccine. This would make the vaccine ineffective, since chemotherapy and radiotherapy are toxic to lymphocytes.”
Lepique also clarified that it is possible to combine the vaccine with immunotherapy targeting immune regulatory molecules. “In this case, in addition to administering the mRNA with the antigen, a strategy is used to improve the patient’s immune response.”
Challenges With mRNA Vaccines
Despite being a promising technology, there are challenges, warned Lepique. mRNA molecules degrade quickly when injected into the body, which can compromise vaccine efficacy. To overcome this, researchers have developed nanoencapsulation technologies that protect the molecules and allow safe use in vaccines. “Another alternative is transferring the mRNA into dendritic cells, known as antigen-presenting cells, and then administering these cells to the patient,” she explained.
Global Research Status
According to a study published this year in Med, over 120 clinical trials are exploring mRNA vaccines to treat lung, breast, prostate, and pancreatic tumors, as well as melanoma.
Lepique noted that the countries leading this research are the US, UK, Germany, China, and Japan. “Unfortunately, the US government recently cut funding for mRNA vaccine development and testing, which will likely have significant consequences,” she said.
Lepique reported that Brazilian researchers are collaborating with international institutions to develop these vaccines. “The Brazilian government, through the Ministry of Health and the Ministry of Science, Technology, and Innovation, recently announced investments in mRNA technologies for vaccines. While not specifically targeting cancer, these investments could also benefit this field,” she clarified.
Leading Studies
Lepique highlighted the most advanced studies to date:
- Pancreatic cancer: A study published in Nature in February demonstrated that a personalized mRNA vaccine reduced the risk for recurrence after surgery in 16 patients, with 3 years of follow-up.
- Melanoma: A study published in The Lancet reported improved survival in melanoma patients after mRNA vaccine administration combined with the checkpoint inhibitor pembrolizumab applied after surgical tumor resection.
- Universal vaccine: A study in Nature Biomedical Engineering described the creation of a “generic” vaccine capable of activating the patient’s immune system and inducing tumor regression. Lepique explained that this vaccine acts more as an immune response modulator than a classical neoantigen-specific vaccine. “Because it is not limited to a single neoantigen, it could potentially be universal, though further testing is needed to determine efficacy across all cancer types,” she added.
Lima highlighted a 2024 study being conducted by MSD and Moderna against lung cancer, with results yet to be published. “Patients first receive immunotherapy after surgery. Once the vaccine is ready, it is added to the ongoing immunotherapy,” he explained. The global phase 3 study involves 868 patients with resected lung cancer who previously underwent chemotherapy. Participants receive the vaccine (1 mg every 3 weeks, up to nine doses) alongside pembrolizumab (400 mg every 6 weeks, up to nine cycles) over approximately 1 year.
Other mRNA vaccines remain in early-stage development. For example, in May 2024, the UK National Health Service recruited participants for a personalized colorectal cancer mRNA vaccine trial.
Advantages of mRNA Technology
Experts noted that mRNA-based cancer vaccines are considered safer for patients because the tumor mRNA is synthesized in the laboratory. According to Lepique, these vaccines are more specific than many other cancer therapies, and therefore carry a lower risk for serious side effects.
“Clinical studies have shown that these vaccines can generate immunological memory, meaning lymphocytes that recognize tumor antigens remain in the body and can respond to recurrence,” she explained.
It is also possible to combine multiple mRNA molecules in a single vaccine, creating a platform that targets several tumor antigens simultaneously. “Formulations can additionally include adjuvants to further enhance immune responses against tumors,” she said. However, as a personalized therapy, costs are high, and vaccine formulation requires considerable time.
Lima emphasized the customization advantage: “We can take a portion of the patient’s tumor, sequence it to identify alterations, and develop a vaccine specifically for that tumor.” He also highlighted safety data, noting that the platform has been widely used in SARS-CoV-2 vaccine development, providing confidence in large-scale application. “The potential exists to achieve more personalized, tumor-directed immunotherapy with greater scalability,” he explained.
Outlook and Limitations
Lima noted that although the projected efficacy is promising, definitive results are still pending.
“We have very positive expectations, but we must wait for study outcomes. Efficacy may vary across scenarios and among patients. The immune system may also respond against the vaccine itself, potentially reducing effectiveness at times,” he explained.
According to Lima, mRNA vaccines are expected to complement current treatments, enhancing outcomes without replacing conventional approaches entirely.
“It will not be a panacea. These vaccines are likely to add to and improve strategies we already use, but they will not work for all patients in every scenario,” he concluded.
Lepique highlighted the promise of combination strategies. “The outlook is positive, particularly because multiple mRNA types can be combined in a single formulation and used alongside drugs that enhance immune responses,” she explained.
Although mRNA vaccine research has been ongoing for many years, prior results have brought both progress and setbacks. “This new protocol appears more effective [and] capable of generating immunological memory and is also safe,” she noted. Still, she cautioned that cancer presents unique challenges: “The disease has multiple mechanisms to evade immune responses. Additionally, some tumors are naturally unrecognized by the immune system, the so-called ‘cold tumors.’”
This story was translated from Medscape’s Portuguese edition. A version of this article appeared on Medscape.com.
Since 1965, messenger RNA (mRNA) vaccines have been studied for cancer treatment, but it was the technological advances in vaccines during the COVID pandemic that helped accelerate research. Currently, no vaccine has been approved for tumor treatment, although many clinical studies are ongoing worldwide. According to experts consulted by Medscape’s Portuguese edition, the outlook is very promising, and these studies are expected to open doors for personalized therapies.
In cancer treatment, the vaccine would function as an immunotherapy, in which the immune system can be “trained” to act against an invader. Just as with pathogens, the platform would use parts of the tumor — which have altered proteins or are expressed at abnormal levels — to teach the body to defend itself against cancer.
Vladmir Lima, MD, PhD, clinical oncologist at A.C. Camargo Cancer Center, São Paulo, Brazil, explained that with this technology it will be possible to produce personalized vaccines, which prevents, for example, large-scale manufacturing. “In theory, these vaccines can be developed for any tumor type, but this does not mean that efficacy will be the same for all,” he said. Because cancer has specific characteristics in each individual, it is difficult to envision a single vaccine that works for all cancers.
Current evidence suggests the vaccine could be administered after chemotherapy or radiotherapy, with the goal of reducing tumor mass and increasing the effectiveness of mRNA-based treatment, according to Ana Paula Lepique, professor and researcher in tumor immunology at the Institute of Biomedical Sciences, University of São Paulo, São Paulo.
“There is also a study with pancreatic cancer patients, in which the vaccine was administered after surgery,” she explained. “It would not work, for example, to give chemotherapy or radiotherapy while the immune response is being triggered by the vaccine. This would make the vaccine ineffective, since chemotherapy and radiotherapy are toxic to lymphocytes.”
Lepique also clarified that it is possible to combine the vaccine with immunotherapy targeting immune regulatory molecules. “In this case, in addition to administering the mRNA with the antigen, a strategy is used to improve the patient’s immune response.”
Challenges With mRNA Vaccines
Despite being a promising technology, there are challenges, warned Lepique. mRNA molecules degrade quickly when injected into the body, which can compromise vaccine efficacy. To overcome this, researchers have developed nanoencapsulation technologies that protect the molecules and allow safe use in vaccines. “Another alternative is transferring the mRNA into dendritic cells, known as antigen-presenting cells, and then administering these cells to the patient,” she explained.
Global Research Status
According to a study published this year in Med, over 120 clinical trials are exploring mRNA vaccines to treat lung, breast, prostate, and pancreatic tumors, as well as melanoma.
Lepique noted that the countries leading this research are the US, UK, Germany, China, and Japan. “Unfortunately, the US government recently cut funding for mRNA vaccine development and testing, which will likely have significant consequences,” she said.
Lepique reported that Brazilian researchers are collaborating with international institutions to develop these vaccines. “The Brazilian government, through the Ministry of Health and the Ministry of Science, Technology, and Innovation, recently announced investments in mRNA technologies for vaccines. While not specifically targeting cancer, these investments could also benefit this field,” she clarified.
Leading Studies
Lepique highlighted the most advanced studies to date:
- Pancreatic cancer: A study published in Nature in February demonstrated that a personalized mRNA vaccine reduced the risk for recurrence after surgery in 16 patients, with 3 years of follow-up.
- Melanoma: A study published in The Lancet reported improved survival in melanoma patients after mRNA vaccine administration combined with the checkpoint inhibitor pembrolizumab applied after surgical tumor resection.
- Universal vaccine: A study in Nature Biomedical Engineering described the creation of a “generic” vaccine capable of activating the patient’s immune system and inducing tumor regression. Lepique explained that this vaccine acts more as an immune response modulator than a classical neoantigen-specific vaccine. “Because it is not limited to a single neoantigen, it could potentially be universal, though further testing is needed to determine efficacy across all cancer types,” she added.
Lima highlighted a 2024 study being conducted by MSD and Moderna against lung cancer, with results yet to be published. “Patients first receive immunotherapy after surgery. Once the vaccine is ready, it is added to the ongoing immunotherapy,” he explained. The global phase 3 study involves 868 patients with resected lung cancer who previously underwent chemotherapy. Participants receive the vaccine (1 mg every 3 weeks, up to nine doses) alongside pembrolizumab (400 mg every 6 weeks, up to nine cycles) over approximately 1 year.
Other mRNA vaccines remain in early-stage development. For example, in May 2024, the UK National Health Service recruited participants for a personalized colorectal cancer mRNA vaccine trial.
Advantages of mRNA Technology
Experts noted that mRNA-based cancer vaccines are considered safer for patients because the tumor mRNA is synthesized in the laboratory. According to Lepique, these vaccines are more specific than many other cancer therapies, and therefore carry a lower risk for serious side effects.
“Clinical studies have shown that these vaccines can generate immunological memory, meaning lymphocytes that recognize tumor antigens remain in the body and can respond to recurrence,” she explained.
It is also possible to combine multiple mRNA molecules in a single vaccine, creating a platform that targets several tumor antigens simultaneously. “Formulations can additionally include adjuvants to further enhance immune responses against tumors,” she said. However, as a personalized therapy, costs are high, and vaccine formulation requires considerable time.
Lima emphasized the customization advantage: “We can take a portion of the patient’s tumor, sequence it to identify alterations, and develop a vaccine specifically for that tumor.” He also highlighted safety data, noting that the platform has been widely used in SARS-CoV-2 vaccine development, providing confidence in large-scale application. “The potential exists to achieve more personalized, tumor-directed immunotherapy with greater scalability,” he explained.
Outlook and Limitations
Lima noted that although the projected efficacy is promising, definitive results are still pending.
“We have very positive expectations, but we must wait for study outcomes. Efficacy may vary across scenarios and among patients. The immune system may also respond against the vaccine itself, potentially reducing effectiveness at times,” he explained.
According to Lima, mRNA vaccines are expected to complement current treatments, enhancing outcomes without replacing conventional approaches entirely.
“It will not be a panacea. These vaccines are likely to add to and improve strategies we already use, but they will not work for all patients in every scenario,” he concluded.
Lepique highlighted the promise of combination strategies. “The outlook is positive, particularly because multiple mRNA types can be combined in a single formulation and used alongside drugs that enhance immune responses,” she explained.
Although mRNA vaccine research has been ongoing for many years, prior results have brought both progress and setbacks. “This new protocol appears more effective [and] capable of generating immunological memory and is also safe,” she noted. Still, she cautioned that cancer presents unique challenges: “The disease has multiple mechanisms to evade immune responses. Additionally, some tumors are naturally unrecognized by the immune system, the so-called ‘cold tumors.’”
This story was translated from Medscape’s Portuguese edition. A version of this article appeared on Medscape.com.
Hepatitis D Virus Classified as Carcinogenic: Implications
The International Agency for Research on Cancer (IARC) of the World Health Organization has classified hepatitis D virus (HDV) as carcinogenic, citing sufficient evidence and placing it alongside hepatitis B virus (HBV) and hepatitis C virus (HCV) as a cause of hepatocellular carcinoma (HCC).
Individuals with HBV-HDV coinfection face an elevated risk for liver cancer, highlighting the need for HBV vaccination, systematic screening, and early antiviral treatment to reduce the progression to cirrhosis and HCC.
About 12 million people globally have HBV-HDV coinfection, representing 5% of all chronic HBV cases. The prevalence of this condition varies regionally, with a likely underdiagnosis. True coinfection rates may reach 13%-14%, the highest in Europe’s Mediterranean region.
Virus Biology
HDV is an incomplete virus that infects hepatocytes and requires the envelope protein of hepatitis B surface antigen (HBsAg) for cell exit. Infection occurs only with chronic HBV infection, either as a superinfection or simultaneous acquisition. Humans are the only known natural host.
HDV coinfection worsens HBV-induced hepatic inflammation and prognosis, and up to 80% of patients develop cirrhosis. Triple infection with the HBV virus, HDV, and HIV further increases this risk, and the global prevalence is likely underestimated.
Cancer Risk
HDV infection significantly increases the risk for HCC compared with HBV infection alone. Many patients die from decompensated cirrhosis or HCC, reflecting the aggressive nature of coinfection.
The molecular mechanisms underlying HDV oncogenesis remain unclear. Research conducted over the past 15 years has provided insights that could inform the development of more effective treatments.
Early vaccination prophylaxis is critical for reducing the risk for HCC, despite limited options.
Treatment Options
Randomized controlled trials have demonstrated antiviral efficacy for:
- Pegylated interferon alpha (Peg-IFN) is approved for HBV and is active against HDV.
- Bulevirtide, a synthetic myristoylated lipopeptide entry inhibitor, is used alone or in combination with Peg-IFN.
Suppression of HBV remains central. Nucleoside and nucleotide analogs, such as entecavir, tenofovir alafenamide fumarate, and tenofovir disoproxil fumarate, significantly reduce HCC progression in treated patients compared with untreated patients at risk.
Promising therapeutics include lonafarnib, a farnesyltransferase inhibitor that blocks HDV particle formation, and nucleic acid polymers targeting the host chaperone DNAJB12 to inhibit HBV and HDV replication.
Guideline Updates
The 2023 addendum to the S3 guidelines covers the prophylaxis, diagnosis, and treatment of HBV, including HDV management.
IARC experts also re-evaluated the human cytomegalovirus and Merkel cell polyomavirus. Complete assessments are expected in the next edition of IARC Monographs.
HBV Vaccination
HBV vaccination is the only effective prophylaxis against HBV and HDV. Introduced in 1982 for high-risk groups, it reduced chronic infections, with the WHO expanding its recommendations from 1992 onward.
Infants and young children are at the highest risk of developing this disease. Acute HBV infection often resolves in adults, but infants face up to a 90% risk of developing chronic infection. Newborns of mothers with chronic or undiagnosed HBV infections are particularly vulnerable.
Routine infant immunization includes three doses, with the first dose administered within 12 hours of birth. In Germany, the Standing Committee on Vaccination (STIKO) recommends the administration of combination vaccines, with the hexavalent vaccine administered at 2, 4, and 11 months in a 2 + 1 schedule.
Timely vaccination is crucial because undetected chronic infections often lead to late-stage HCC diagnosis. Adults in high-risk groups should receive HBV vaccination counseling.
STIKO recommends vaccination for close contacts of individuals who are HBsAg-positive, individuals with high-risk sexual contacts, immunocompromised persons, and those with preexisting conditions that increase the risk for severe HBV infection.
Since 2021, insured adults aged 35 years or older in Germany have undergone one-time HBV and HCV screening. HDV testing is recommended for all HBsAg-positive patients. Current frameworks may miss cases, and additional or personalized screening could improve the detection of previously unrecognized infections.
This story was translated from Univadis Germany.
A version of this article appeared on Medscape.com.
The International Agency for Research on Cancer (IARC) of the World Health Organization has classified hepatitis D virus (HDV) as carcinogenic, citing sufficient evidence and placing it alongside hepatitis B virus (HBV) and hepatitis C virus (HCV) as a cause of hepatocellular carcinoma (HCC).
Individuals with HBV-HDV coinfection face an elevated risk for liver cancer, highlighting the need for HBV vaccination, systematic screening, and early antiviral treatment to reduce the progression to cirrhosis and HCC.
About 12 million people globally have HBV-HDV coinfection, representing 5% of all chronic HBV cases. The prevalence of this condition varies regionally, with a likely underdiagnosis. True coinfection rates may reach 13%-14%, the highest in Europe’s Mediterranean region.
Virus Biology
HDV is an incomplete virus that infects hepatocytes and requires the envelope protein of hepatitis B surface antigen (HBsAg) for cell exit. Infection occurs only with chronic HBV infection, either as a superinfection or simultaneous acquisition. Humans are the only known natural host.
HDV coinfection worsens HBV-induced hepatic inflammation and prognosis, and up to 80% of patients develop cirrhosis. Triple infection with the HBV virus, HDV, and HIV further increases this risk, and the global prevalence is likely underestimated.
Cancer Risk
HDV infection significantly increases the risk for HCC compared with HBV infection alone. Many patients die from decompensated cirrhosis or HCC, reflecting the aggressive nature of coinfection.
The molecular mechanisms underlying HDV oncogenesis remain unclear. Research conducted over the past 15 years has provided insights that could inform the development of more effective treatments.
Early vaccination prophylaxis is critical for reducing the risk for HCC, despite limited options.
Treatment Options
Randomized controlled trials have demonstrated antiviral efficacy for:
- Pegylated interferon alpha (Peg-IFN) is approved for HBV and is active against HDV.
- Bulevirtide, a synthetic myristoylated lipopeptide entry inhibitor, is used alone or in combination with Peg-IFN.
Suppression of HBV remains central. Nucleoside and nucleotide analogs, such as entecavir, tenofovir alafenamide fumarate, and tenofovir disoproxil fumarate, significantly reduce HCC progression in treated patients compared with untreated patients at risk.
Promising therapeutics include lonafarnib, a farnesyltransferase inhibitor that blocks HDV particle formation, and nucleic acid polymers targeting the host chaperone DNAJB12 to inhibit HBV and HDV replication.
Guideline Updates
The 2023 addendum to the S3 guidelines covers the prophylaxis, diagnosis, and treatment of HBV, including HDV management.
IARC experts also re-evaluated the human cytomegalovirus and Merkel cell polyomavirus. Complete assessments are expected in the next edition of IARC Monographs.
HBV Vaccination
HBV vaccination is the only effective prophylaxis against HBV and HDV. Introduced in 1982 for high-risk groups, it reduced chronic infections, with the WHO expanding its recommendations from 1992 onward.
Infants and young children are at the highest risk of developing this disease. Acute HBV infection often resolves in adults, but infants face up to a 90% risk of developing chronic infection. Newborns of mothers with chronic or undiagnosed HBV infections are particularly vulnerable.
Routine infant immunization includes three doses, with the first dose administered within 12 hours of birth. In Germany, the Standing Committee on Vaccination (STIKO) recommends the administration of combination vaccines, with the hexavalent vaccine administered at 2, 4, and 11 months in a 2 + 1 schedule.
Timely vaccination is crucial because undetected chronic infections often lead to late-stage HCC diagnosis. Adults in high-risk groups should receive HBV vaccination counseling.
STIKO recommends vaccination for close contacts of individuals who are HBsAg-positive, individuals with high-risk sexual contacts, immunocompromised persons, and those with preexisting conditions that increase the risk for severe HBV infection.
Since 2021, insured adults aged 35 years or older in Germany have undergone one-time HBV and HCV screening. HDV testing is recommended for all HBsAg-positive patients. Current frameworks may miss cases, and additional or personalized screening could improve the detection of previously unrecognized infections.
This story was translated from Univadis Germany.
A version of this article appeared on Medscape.com.
The International Agency for Research on Cancer (IARC) of the World Health Organization has classified hepatitis D virus (HDV) as carcinogenic, citing sufficient evidence and placing it alongside hepatitis B virus (HBV) and hepatitis C virus (HCV) as a cause of hepatocellular carcinoma (HCC).
Individuals with HBV-HDV coinfection face an elevated risk for liver cancer, highlighting the need for HBV vaccination, systematic screening, and early antiviral treatment to reduce the progression to cirrhosis and HCC.
About 12 million people globally have HBV-HDV coinfection, representing 5% of all chronic HBV cases. The prevalence of this condition varies regionally, with a likely underdiagnosis. True coinfection rates may reach 13%-14%, the highest in Europe’s Mediterranean region.
Virus Biology
HDV is an incomplete virus that infects hepatocytes and requires the envelope protein of hepatitis B surface antigen (HBsAg) for cell exit. Infection occurs only with chronic HBV infection, either as a superinfection or simultaneous acquisition. Humans are the only known natural host.
HDV coinfection worsens HBV-induced hepatic inflammation and prognosis, and up to 80% of patients develop cirrhosis. Triple infection with the HBV virus, HDV, and HIV further increases this risk, and the global prevalence is likely underestimated.
Cancer Risk
HDV infection significantly increases the risk for HCC compared with HBV infection alone. Many patients die from decompensated cirrhosis or HCC, reflecting the aggressive nature of coinfection.
The molecular mechanisms underlying HDV oncogenesis remain unclear. Research conducted over the past 15 years has provided insights that could inform the development of more effective treatments.
Early vaccination prophylaxis is critical for reducing the risk for HCC, despite limited options.
Treatment Options
Randomized controlled trials have demonstrated antiviral efficacy for:
- Pegylated interferon alpha (Peg-IFN) is approved for HBV and is active against HDV.
- Bulevirtide, a synthetic myristoylated lipopeptide entry inhibitor, is used alone or in combination with Peg-IFN.
Suppression of HBV remains central. Nucleoside and nucleotide analogs, such as entecavir, tenofovir alafenamide fumarate, and tenofovir disoproxil fumarate, significantly reduce HCC progression in treated patients compared with untreated patients at risk.
Promising therapeutics include lonafarnib, a farnesyltransferase inhibitor that blocks HDV particle formation, and nucleic acid polymers targeting the host chaperone DNAJB12 to inhibit HBV and HDV replication.
Guideline Updates
The 2023 addendum to the S3 guidelines covers the prophylaxis, diagnosis, and treatment of HBV, including HDV management.
IARC experts also re-evaluated the human cytomegalovirus and Merkel cell polyomavirus. Complete assessments are expected in the next edition of IARC Monographs.
HBV Vaccination
HBV vaccination is the only effective prophylaxis against HBV and HDV. Introduced in 1982 for high-risk groups, it reduced chronic infections, with the WHO expanding its recommendations from 1992 onward.
Infants and young children are at the highest risk of developing this disease. Acute HBV infection often resolves in adults, but infants face up to a 90% risk of developing chronic infection. Newborns of mothers with chronic or undiagnosed HBV infections are particularly vulnerable.
Routine infant immunization includes three doses, with the first dose administered within 12 hours of birth. In Germany, the Standing Committee on Vaccination (STIKO) recommends the administration of combination vaccines, with the hexavalent vaccine administered at 2, 4, and 11 months in a 2 + 1 schedule.
Timely vaccination is crucial because undetected chronic infections often lead to late-stage HCC diagnosis. Adults in high-risk groups should receive HBV vaccination counseling.
STIKO recommends vaccination for close contacts of individuals who are HBsAg-positive, individuals with high-risk sexual contacts, immunocompromised persons, and those with preexisting conditions that increase the risk for severe HBV infection.
Since 2021, insured adults aged 35 years or older in Germany have undergone one-time HBV and HCV screening. HDV testing is recommended for all HBsAg-positive patients. Current frameworks may miss cases, and additional or personalized screening could improve the detection of previously unrecognized infections.
This story was translated from Univadis Germany.
A version of this article appeared on Medscape.com.
Physician Compensation: Gains Small, Gaps Large
Few would deny that physicians today face many challenges: a growing and aging patient population, personnel shortages, mounting paperwork, regulatory and reimbursement pressures, and personal burnout. Collectively these could work to worsen patient access to care. Yet despite these headwinds, Doximity’s survey-based Physician Compensation Report 2025 found that more than three-quarters of physicians polled would still choose to enter their profession.
“Physician burnout isn’t new. It’s been a persistent problem over the past decade,” said Amit Phull, MD, chief clinical experience officer at Doximity. “In a Doximity poll of nearly 2,000 physicians conducted in May 2025, 85% reported they feel overworked, up from 73% just four years ago. As a result, about 68% of physicians said they are looking for an employment change or considering early retirement.”
Greater awareness of contemporary trends may help physicians make more-informed career decisions and more effectively advocate for both themselves and the patients who need them, the report’s authors stated.
Compensation Lag May Impact Care
A small overall average compensation increase of 3.7% from 2023 to 2024 – a slightly lower increase than the 5.9% in the prior year – has done little to close existing pay gaps across the profession.
In 2024, average compensation for men rose 5.7% over 2023, compared with just 1.7% for women – widening the gender pay gap to 26% vs 23% in 2023 and matching the gender gap seen in 2022. And significant disparities persist between physicians caring for adults vs children. In some specialties, the pay gap between pediatric and adult specialists exceeded 80% despite practitioners’ similar levels of training and clinical complexity.
Nearly 60% of respondents said reimbursement pressures could affect their ability to serve Medicare or Medicaid patients in the next year. Additionally, 81% reported that reimbursement policies have significantly contributed to the decline of private practices, and more than a third said they could stifle practice growth with compensation concerns forcing them to delay or cancel hiring or expansion plans. Almost 90% reported an adverse impact from physician shortages, with more citing an inability or limited ability to accept new patients.
Narrowing the Gap for Primary Care?
Over the past three years, the percent pay gap between primary care and specialist medicine declined modestly, the report noted. In 2024, surgical specialists earned 87% more than primary care physicians, down from 100% in 2022. Non-surgical specialists, emergency medicine physicians, and Ob/Gyns also continued to earn significantly more than primary care physicians, though the gaps have narrowed slightly.
“These trends come at a time when primary care remains critical to meeting high patient demand, especially amid ongoing physician shortages,” the report stated. “Primary care physicians continue to earn considerably less than many of their medical colleagues despite their essential role in the healthcare system.”
Significantly, many physicians believe that current reimbursement policies have contributed to the steady decline of independent practices in their fields. According to the American Medical Association, the share of physicians working in private practices dropped by 18 percentage points from 60.1% to 42.2% from 2012 to 2024.
The Specialties
This year’s review found that among 20 specialties, the highest average compensation occurred in surgical and procedural specialties, while the lowest paid were, as mentioned, pediatric medicine and primary care. Pediatric nephrology saw the largest average compensation growth in 2024 at 15.6%, yet compensation still lagged behind adult nephrology with a 40% pay gap.
By medical discipline, gastroenterologists ranked 13th overall in average annual compensation. Gastroenterology remained in the top 20 compensated specialties, with average annual compensation of $537,870 – an increase from $514,208 in 2024, representing a 4.5% growth rate over 2023. Neurosurgeons topped the list at $749,140, followed by thoracic surgeons at $689,969 and orthopedic surgeons at $679,517.
The three lowest-paid branches were all pediatric: endocrinology at $230,426, rheumatology at $231,574, and infectious diseases at $248,322. Pediatric gastroenterology paid somewhat higher at $298,457.
The largest disparities were seen in hematology and oncology, where adult specialists earned 93% more than their pediatric peers. Pediatric gastroenterology showed an 80% pay gap. There were also substantial pay differences across cardiology, pulmonology, and rheumatology. “These gaps appear to reflect a systemic lag in pay for pediatric specialty care, even as demand for pediatric subspecialists continues to rise,” the report stated.
Practice Setting and Location
Where a doctor practices impacts the bottom line, too: in 2024 the highest compensation reported for a metro area was in Rochester, Minnesota (the Mayo Clinic effect?), at $495,532, while the lowest reported was in Durham-Chapel Hill, North Carolina, at $368,782. St. Louis, Missouri ($484,883) and Los Angeles, California ($470,198) were 2nd and 3rd at the top of the list. Rochester, Minnesota, also emerged as best for annual compensation after cost-of-living adjustment, while Boston, Massachusetts, occupied the bottom rung.
The Gender Effect
With a women’s pay increase in 2024 of just 1.7%, the gender gap returned to its 2022-level disparity of 26%, with women physicians earning an average of $120,917 less than men after adjusting for specialty, location, and years of experience.
Doximity’s analysis of data from 2014 to 2019 estimated that on average men make at least $2 million more than women over the course of a 40-year career. This gap is often attributed to the fewer hours worked by female physician with their generally heavier familial responsibilities, “but Doximity’s gender wage gap analysis controls for the number of hours worked and career stage, along with specialty, work type, employment status, region, and credentials,” Phull said.
Women physicians had lower average earnings than men physicians across all specialties, a trend consistent with prior years. As a percentage of pay, the largest gender disparity was seen in pediatric nephrology (16.5%), a specialty that in fact saw the largest annual growth in physician pay. Neurosurgery had the smallest gender gap at 11.3%, while infectious diseases came in at 11.5% and oncology at 12%.
According to Maria T. Abreu, MD, AGAF, executive director of the F. Widjaja Inflammatory Bowel Disease Institute at Cedars-Sinai Medical Center in Los Angeles and past president of AGA, the remuneration gender gap in gastroenterology is being taken seriously by AGA and several other GI societies. “The discrepancies in pay start from the beginning and therefore are magnified over time. We are helping to empower women to negotiate better as well as to gather data on the roots of inequity, she told GI & Hepatology News.
The AGA Women’s Committee has developed a project to support the advancement of women in gastroenterology, Abreu said. The initiative, which includes the AGA Gender Equity Framework and Gender Equity Road Map. focuses attention on disparities in the workplace and promotes opportunities for women’s leadership, career advancement, mentorship and physician health and wellness, she added.
Are these disparities due mainly to the “motherhood penalty,” with career interruption and time lost to maternity leave and fewer hours worked owing to the greater parenting burden of physician mothers? Or are they due to the systemic effects of gender expectations around compensation?
Hours worked appear to be a factor. A 2017 study of dual physician couples found that among childless respondents men worked an average of 57 hours and women 52 hours weekly. Compared with childless men, men with children worked similar numbers of hours weekly. However, compared with childless physicians, mothers worked significantly fewer hours – roughly 40 to 43 hours weekly – depending on the age of their youngest child.
Abreu pushed back on this stereotype. “Most women physicians, including gastroenterologists, do not take the maternity leave they are allowed because they are concerned about burdening their colleagues,” she said. “Thus, it is unlikely to explain the disparities. Many systemic issues remain challenging, but we want women to be empowered to advocate for themselves at the time of hiring and along the arc of their career paths.”
In Abreu’s view, having women assume more leadership roles in the field of gastroenterology provides an opportunity to focus on reducing the disparities in compensation.
Regardless of gender, among all physicians surveyed, autonomy and work-life balance appeared to be a high priority: 77% of doctors said they would be willing to accept or have already accepted lower pay for more autonomy or work-life balance. “Overwork appears to be especially prevalent among women physicians,” said Phull, noting that 91% of women respondents reported being overworked compared with 80% of men. “This overwork has compelled 74% of women to consider making a career change, compared with 62% of men.” Differences emerged among specialties as well: 90% of primary care physicians said they are overworked compared with 84% of surgeons and 83% of non-surgical specialists.
Looking ahead, the report raised an important question. Are we relying too heavily on physicians rather than addressing the underlying need for policies that support a healthier, more sustainable future for all? “Building that future will take more than physician dedication alone,” Phull said. “It will require meaningful collaboration across the entire health care ecosystem – including health systems, hospitals, payors, and policymakers. And physicians must not only have a voice in shaping the path forward; they must have a seat at the table.”
Abreu reported no conflicts of interest in regard to her comments.
Few would deny that physicians today face many challenges: a growing and aging patient population, personnel shortages, mounting paperwork, regulatory and reimbursement pressures, and personal burnout. Collectively these could work to worsen patient access to care. Yet despite these headwinds, Doximity’s survey-based Physician Compensation Report 2025 found that more than three-quarters of physicians polled would still choose to enter their profession.
“Physician burnout isn’t new. It’s been a persistent problem over the past decade,” said Amit Phull, MD, chief clinical experience officer at Doximity. “In a Doximity poll of nearly 2,000 physicians conducted in May 2025, 85% reported they feel overworked, up from 73% just four years ago. As a result, about 68% of physicians said they are looking for an employment change or considering early retirement.”
Greater awareness of contemporary trends may help physicians make more-informed career decisions and more effectively advocate for both themselves and the patients who need them, the report’s authors stated.
Compensation Lag May Impact Care
A small overall average compensation increase of 3.7% from 2023 to 2024 – a slightly lower increase than the 5.9% in the prior year – has done little to close existing pay gaps across the profession.
In 2024, average compensation for men rose 5.7% over 2023, compared with just 1.7% for women – widening the gender pay gap to 26% vs 23% in 2023 and matching the gender gap seen in 2022. And significant disparities persist between physicians caring for adults vs children. In some specialties, the pay gap between pediatric and adult specialists exceeded 80% despite practitioners’ similar levels of training and clinical complexity.
Nearly 60% of respondents said reimbursement pressures could affect their ability to serve Medicare or Medicaid patients in the next year. Additionally, 81% reported that reimbursement policies have significantly contributed to the decline of private practices, and more than a third said they could stifle practice growth with compensation concerns forcing them to delay or cancel hiring or expansion plans. Almost 90% reported an adverse impact from physician shortages, with more citing an inability or limited ability to accept new patients.
Narrowing the Gap for Primary Care?
Over the past three years, the percent pay gap between primary care and specialist medicine declined modestly, the report noted. In 2024, surgical specialists earned 87% more than primary care physicians, down from 100% in 2022. Non-surgical specialists, emergency medicine physicians, and Ob/Gyns also continued to earn significantly more than primary care physicians, though the gaps have narrowed slightly.
“These trends come at a time when primary care remains critical to meeting high patient demand, especially amid ongoing physician shortages,” the report stated. “Primary care physicians continue to earn considerably less than many of their medical colleagues despite their essential role in the healthcare system.”
Significantly, many physicians believe that current reimbursement policies have contributed to the steady decline of independent practices in their fields. According to the American Medical Association, the share of physicians working in private practices dropped by 18 percentage points from 60.1% to 42.2% from 2012 to 2024.
The Specialties
This year’s review found that among 20 specialties, the highest average compensation occurred in surgical and procedural specialties, while the lowest paid were, as mentioned, pediatric medicine and primary care. Pediatric nephrology saw the largest average compensation growth in 2024 at 15.6%, yet compensation still lagged behind adult nephrology with a 40% pay gap.
By medical discipline, gastroenterologists ranked 13th overall in average annual compensation. Gastroenterology remained in the top 20 compensated specialties, with average annual compensation of $537,870 – an increase from $514,208 in 2024, representing a 4.5% growth rate over 2023. Neurosurgeons topped the list at $749,140, followed by thoracic surgeons at $689,969 and orthopedic surgeons at $679,517.
The three lowest-paid branches were all pediatric: endocrinology at $230,426, rheumatology at $231,574, and infectious diseases at $248,322. Pediatric gastroenterology paid somewhat higher at $298,457.
The largest disparities were seen in hematology and oncology, where adult specialists earned 93% more than their pediatric peers. Pediatric gastroenterology showed an 80% pay gap. There were also substantial pay differences across cardiology, pulmonology, and rheumatology. “These gaps appear to reflect a systemic lag in pay for pediatric specialty care, even as demand for pediatric subspecialists continues to rise,” the report stated.
Practice Setting and Location
Where a doctor practices impacts the bottom line, too: in 2024 the highest compensation reported for a metro area was in Rochester, Minnesota (the Mayo Clinic effect?), at $495,532, while the lowest reported was in Durham-Chapel Hill, North Carolina, at $368,782. St. Louis, Missouri ($484,883) and Los Angeles, California ($470,198) were 2nd and 3rd at the top of the list. Rochester, Minnesota, also emerged as best for annual compensation after cost-of-living adjustment, while Boston, Massachusetts, occupied the bottom rung.
The Gender Effect
With a women’s pay increase in 2024 of just 1.7%, the gender gap returned to its 2022-level disparity of 26%, with women physicians earning an average of $120,917 less than men after adjusting for specialty, location, and years of experience.
Doximity’s analysis of data from 2014 to 2019 estimated that on average men make at least $2 million more than women over the course of a 40-year career. This gap is often attributed to the fewer hours worked by female physician with their generally heavier familial responsibilities, “but Doximity’s gender wage gap analysis controls for the number of hours worked and career stage, along with specialty, work type, employment status, region, and credentials,” Phull said.
Women physicians had lower average earnings than men physicians across all specialties, a trend consistent with prior years. As a percentage of pay, the largest gender disparity was seen in pediatric nephrology (16.5%), a specialty that in fact saw the largest annual growth in physician pay. Neurosurgery had the smallest gender gap at 11.3%, while infectious diseases came in at 11.5% and oncology at 12%.
According to Maria T. Abreu, MD, AGAF, executive director of the F. Widjaja Inflammatory Bowel Disease Institute at Cedars-Sinai Medical Center in Los Angeles and past president of AGA, the remuneration gender gap in gastroenterology is being taken seriously by AGA and several other GI societies. “The discrepancies in pay start from the beginning and therefore are magnified over time. We are helping to empower women to negotiate better as well as to gather data on the roots of inequity, she told GI & Hepatology News.
The AGA Women’s Committee has developed a project to support the advancement of women in gastroenterology, Abreu said. The initiative, which includes the AGA Gender Equity Framework and Gender Equity Road Map. focuses attention on disparities in the workplace and promotes opportunities for women’s leadership, career advancement, mentorship and physician health and wellness, she added.
Are these disparities due mainly to the “motherhood penalty,” with career interruption and time lost to maternity leave and fewer hours worked owing to the greater parenting burden of physician mothers? Or are they due to the systemic effects of gender expectations around compensation?
Hours worked appear to be a factor. A 2017 study of dual physician couples found that among childless respondents men worked an average of 57 hours and women 52 hours weekly. Compared with childless men, men with children worked similar numbers of hours weekly. However, compared with childless physicians, mothers worked significantly fewer hours – roughly 40 to 43 hours weekly – depending on the age of their youngest child.
Abreu pushed back on this stereotype. “Most women physicians, including gastroenterologists, do not take the maternity leave they are allowed because they are concerned about burdening their colleagues,” she said. “Thus, it is unlikely to explain the disparities. Many systemic issues remain challenging, but we want women to be empowered to advocate for themselves at the time of hiring and along the arc of their career paths.”
In Abreu’s view, having women assume more leadership roles in the field of gastroenterology provides an opportunity to focus on reducing the disparities in compensation.
Regardless of gender, among all physicians surveyed, autonomy and work-life balance appeared to be a high priority: 77% of doctors said they would be willing to accept or have already accepted lower pay for more autonomy or work-life balance. “Overwork appears to be especially prevalent among women physicians,” said Phull, noting that 91% of women respondents reported being overworked compared with 80% of men. “This overwork has compelled 74% of women to consider making a career change, compared with 62% of men.” Differences emerged among specialties as well: 90% of primary care physicians said they are overworked compared with 84% of surgeons and 83% of non-surgical specialists.
Looking ahead, the report raised an important question. Are we relying too heavily on physicians rather than addressing the underlying need for policies that support a healthier, more sustainable future for all? “Building that future will take more than physician dedication alone,” Phull said. “It will require meaningful collaboration across the entire health care ecosystem – including health systems, hospitals, payors, and policymakers. And physicians must not only have a voice in shaping the path forward; they must have a seat at the table.”
Abreu reported no conflicts of interest in regard to her comments.
Few would deny that physicians today face many challenges: a growing and aging patient population, personnel shortages, mounting paperwork, regulatory and reimbursement pressures, and personal burnout. Collectively these could work to worsen patient access to care. Yet despite these headwinds, Doximity’s survey-based Physician Compensation Report 2025 found that more than three-quarters of physicians polled would still choose to enter their profession.
“Physician burnout isn’t new. It’s been a persistent problem over the past decade,” said Amit Phull, MD, chief clinical experience officer at Doximity. “In a Doximity poll of nearly 2,000 physicians conducted in May 2025, 85% reported they feel overworked, up from 73% just four years ago. As a result, about 68% of physicians said they are looking for an employment change or considering early retirement.”
Greater awareness of contemporary trends may help physicians make more-informed career decisions and more effectively advocate for both themselves and the patients who need them, the report’s authors stated.
Compensation Lag May Impact Care
A small overall average compensation increase of 3.7% from 2023 to 2024 – a slightly lower increase than the 5.9% in the prior year – has done little to close existing pay gaps across the profession.
In 2024, average compensation for men rose 5.7% over 2023, compared with just 1.7% for women – widening the gender pay gap to 26% vs 23% in 2023 and matching the gender gap seen in 2022. And significant disparities persist between physicians caring for adults vs children. In some specialties, the pay gap between pediatric and adult specialists exceeded 80% despite practitioners’ similar levels of training and clinical complexity.
Nearly 60% of respondents said reimbursement pressures could affect their ability to serve Medicare or Medicaid patients in the next year. Additionally, 81% reported that reimbursement policies have significantly contributed to the decline of private practices, and more than a third said they could stifle practice growth with compensation concerns forcing them to delay or cancel hiring or expansion plans. Almost 90% reported an adverse impact from physician shortages, with more citing an inability or limited ability to accept new patients.
Narrowing the Gap for Primary Care?
Over the past three years, the percent pay gap between primary care and specialist medicine declined modestly, the report noted. In 2024, surgical specialists earned 87% more than primary care physicians, down from 100% in 2022. Non-surgical specialists, emergency medicine physicians, and Ob/Gyns also continued to earn significantly more than primary care physicians, though the gaps have narrowed slightly.
“These trends come at a time when primary care remains critical to meeting high patient demand, especially amid ongoing physician shortages,” the report stated. “Primary care physicians continue to earn considerably less than many of their medical colleagues despite their essential role in the healthcare system.”
Significantly, many physicians believe that current reimbursement policies have contributed to the steady decline of independent practices in their fields. According to the American Medical Association, the share of physicians working in private practices dropped by 18 percentage points from 60.1% to 42.2% from 2012 to 2024.
The Specialties
This year’s review found that among 20 specialties, the highest average compensation occurred in surgical and procedural specialties, while the lowest paid were, as mentioned, pediatric medicine and primary care. Pediatric nephrology saw the largest average compensation growth in 2024 at 15.6%, yet compensation still lagged behind adult nephrology with a 40% pay gap.
By medical discipline, gastroenterologists ranked 13th overall in average annual compensation. Gastroenterology remained in the top 20 compensated specialties, with average annual compensation of $537,870 – an increase from $514,208 in 2024, representing a 4.5% growth rate over 2023. Neurosurgeons topped the list at $749,140, followed by thoracic surgeons at $689,969 and orthopedic surgeons at $679,517.
The three lowest-paid branches were all pediatric: endocrinology at $230,426, rheumatology at $231,574, and infectious diseases at $248,322. Pediatric gastroenterology paid somewhat higher at $298,457.
The largest disparities were seen in hematology and oncology, where adult specialists earned 93% more than their pediatric peers. Pediatric gastroenterology showed an 80% pay gap. There were also substantial pay differences across cardiology, pulmonology, and rheumatology. “These gaps appear to reflect a systemic lag in pay for pediatric specialty care, even as demand for pediatric subspecialists continues to rise,” the report stated.
Practice Setting and Location
Where a doctor practices impacts the bottom line, too: in 2024 the highest compensation reported for a metro area was in Rochester, Minnesota (the Mayo Clinic effect?), at $495,532, while the lowest reported was in Durham-Chapel Hill, North Carolina, at $368,782. St. Louis, Missouri ($484,883) and Los Angeles, California ($470,198) were 2nd and 3rd at the top of the list. Rochester, Minnesota, also emerged as best for annual compensation after cost-of-living adjustment, while Boston, Massachusetts, occupied the bottom rung.
The Gender Effect
With a women’s pay increase in 2024 of just 1.7%, the gender gap returned to its 2022-level disparity of 26%, with women physicians earning an average of $120,917 less than men after adjusting for specialty, location, and years of experience.
Doximity’s analysis of data from 2014 to 2019 estimated that on average men make at least $2 million more than women over the course of a 40-year career. This gap is often attributed to the fewer hours worked by female physician with their generally heavier familial responsibilities, “but Doximity’s gender wage gap analysis controls for the number of hours worked and career stage, along with specialty, work type, employment status, region, and credentials,” Phull said.
Women physicians had lower average earnings than men physicians across all specialties, a trend consistent with prior years. As a percentage of pay, the largest gender disparity was seen in pediatric nephrology (16.5%), a specialty that in fact saw the largest annual growth in physician pay. Neurosurgery had the smallest gender gap at 11.3%, while infectious diseases came in at 11.5% and oncology at 12%.
According to Maria T. Abreu, MD, AGAF, executive director of the F. Widjaja Inflammatory Bowel Disease Institute at Cedars-Sinai Medical Center in Los Angeles and past president of AGA, the remuneration gender gap in gastroenterology is being taken seriously by AGA and several other GI societies. “The discrepancies in pay start from the beginning and therefore are magnified over time. We are helping to empower women to negotiate better as well as to gather data on the roots of inequity, she told GI & Hepatology News.
The AGA Women’s Committee has developed a project to support the advancement of women in gastroenterology, Abreu said. The initiative, which includes the AGA Gender Equity Framework and Gender Equity Road Map. focuses attention on disparities in the workplace and promotes opportunities for women’s leadership, career advancement, mentorship and physician health and wellness, she added.
Are these disparities due mainly to the “motherhood penalty,” with career interruption and time lost to maternity leave and fewer hours worked owing to the greater parenting burden of physician mothers? Or are they due to the systemic effects of gender expectations around compensation?
Hours worked appear to be a factor. A 2017 study of dual physician couples found that among childless respondents men worked an average of 57 hours and women 52 hours weekly. Compared with childless men, men with children worked similar numbers of hours weekly. However, compared with childless physicians, mothers worked significantly fewer hours – roughly 40 to 43 hours weekly – depending on the age of their youngest child.
Abreu pushed back on this stereotype. “Most women physicians, including gastroenterologists, do not take the maternity leave they are allowed because they are concerned about burdening their colleagues,” she said. “Thus, it is unlikely to explain the disparities. Many systemic issues remain challenging, but we want women to be empowered to advocate for themselves at the time of hiring and along the arc of their career paths.”
In Abreu’s view, having women assume more leadership roles in the field of gastroenterology provides an opportunity to focus on reducing the disparities in compensation.
Regardless of gender, among all physicians surveyed, autonomy and work-life balance appeared to be a high priority: 77% of doctors said they would be willing to accept or have already accepted lower pay for more autonomy or work-life balance. “Overwork appears to be especially prevalent among women physicians,” said Phull, noting that 91% of women respondents reported being overworked compared with 80% of men. “This overwork has compelled 74% of women to consider making a career change, compared with 62% of men.” Differences emerged among specialties as well: 90% of primary care physicians said they are overworked compared with 84% of surgeons and 83% of non-surgical specialists.
Looking ahead, the report raised an important question. Are we relying too heavily on physicians rather than addressing the underlying need for policies that support a healthier, more sustainable future for all? “Building that future will take more than physician dedication alone,” Phull said. “It will require meaningful collaboration across the entire health care ecosystem – including health systems, hospitals, payors, and policymakers. And physicians must not only have a voice in shaping the path forward; they must have a seat at the table.”
Abreu reported no conflicts of interest in regard to her comments.
Supporting Exceptional Researchers
Did you know that the AGA Research Foundation helped support 74 researchers this past May?
But we can’t do it without you. We depend on the generosity of our supporters to make our vision for the future a reality.
When you donate to AGA Research Foundation, you don’t just give funds – you personally give our beneficiaries grant funding that will lead to new discoveries in GI. Your support goes directly towards funding GI research, helping us address immediate needs while building the foundation for long-term solutions. Plus, you’ll become part of a community full of passionate members like you.
It’s easy to make your mark on our efforts to support investigators. Simply visit our website or learn more ways to give here: [email protected] or contact us at [email protected].
Did you know that the AGA Research Foundation helped support 74 researchers this past May?
But we can’t do it without you. We depend on the generosity of our supporters to make our vision for the future a reality.
When you donate to AGA Research Foundation, you don’t just give funds – you personally give our beneficiaries grant funding that will lead to new discoveries in GI. Your support goes directly towards funding GI research, helping us address immediate needs while building the foundation for long-term solutions. Plus, you’ll become part of a community full of passionate members like you.
It’s easy to make your mark on our efforts to support investigators. Simply visit our website or learn more ways to give here: [email protected] or contact us at [email protected].
Did you know that the AGA Research Foundation helped support 74 researchers this past May?
But we can’t do it without you. We depend on the generosity of our supporters to make our vision for the future a reality.
When you donate to AGA Research Foundation, you don’t just give funds – you personally give our beneficiaries grant funding that will lead to new discoveries in GI. Your support goes directly towards funding GI research, helping us address immediate needs while building the foundation for long-term solutions. Plus, you’ll become part of a community full of passionate members like you.
It’s easy to make your mark on our efforts to support investigators. Simply visit our website or learn more ways to give here: [email protected] or contact us at [email protected].
Streamlined Testosterone Order Template to Improve the Diagnosis and Evaluation of Hypogonadism in Veterans
Streamlined Testosterone Order Template to Improve the Diagnosis and Evaluation of Hypogonadism in Veterans
Testosterone therapy is administered following pragmatic diagnostic evaluation and workup to assess whether an adult male is hypogonadal, based on symptoms consistent with androgen deficiency and low morning serum testosterone concentrations on ≥ 2 occasions. Effects of testosterone administration include the development or maintenance of secondary sexual characteristics and increases in libido, muscle strength, fat-free mass, and bone density.
Testosterone prescriptions have markedly increased in the past 20 years, including within the US Department of Veterans Affairs (VA) health care system.1-3 This trend may be influenced by various factors, including patient perceptions of benefit, an increase in marketing, and the availability of more user-friendly formulations.
Since 2006, evidence-based clinical practice guidelines have recommended specific clinical and laboratory evaluation and counseling prior to starting testosterone replacement therapy (TRT).4-8 However, research has shown poor adherence to these recommendations, including at the VA, which raises concerns about inappropriate TRT initiation without proper diagnostic evaluation.9,10 Observational research has suggested a possible link between testosterone therapy and increased risk of cardiovascular (CV) events. The US Food and Drug Administration prescribing information includes boxed warnings about potential risks of high blood pressure, myocardial infarction, stroke, and CV-related mortality with testosterone treatment, contact transfer of transdermal testosterone, and pulmonary oil microembolism with testosterone undecanoate injections.11-15
A VA Office of Inspector General (OIG) review of VA clinician adherence to clinical and laboratory evaluation guidelines for testosterone deficiency found poor adherence among VA practitioners and made recommendations for improvement.4,15 These focused on establishing clinical signs and symptoms consistent with testosterone deficiency, confirming hypogonadism by repeated testosterone testing, determining the etiology of hypogonadism by measuring gonadotropins, initiating a discussion of risks and benefits of TRT, and assessing clinical improvement and obtaining an updated hematocrit test within 3 to 6 months of initiation.
The VA Puget Sound Health Care System (VAPSHCS) developed a local prior authorization template to assist health care practitioners (HCPs) to address the OIG recommendations. This testosterone order template (TOT) aimed to improve the diagnosis, evaluation, and monitoring of TRT in males with hypogonadism, combined with existing VA pharmacy criteria for the use of testosterone based on Endocrine Society guidelines. A version of the VAPSHCS TOT was approved as the national VA Computerized Patient Record System (CPRS) template.
Preliminary evaluation of the TOT suggested improved short-term adherence to guideline recommendations following implementation.16 This quality improvement study sought to assess the long-term effectiveness of the TOT with respect to clinical practice guideline adherence. The OIG did not address prostate-specific antigen (PSA) monitoring because understanding of the relationship between TRT and the risks of elevated PSA levels remains incomplete.6,17 This project hypothesized that implementation of a pharmacy-managed TOT incorporated into CPRS would result in higher adherence rates to guideline-recommended clinical and laboratory evaluation, in addition to counseling of men with hypogonadism prior to initiation of TRT.
Methods
Eligible participants were cisgender males who received a new testosterone prescription, had ≥ 2 clinic visits at VAPSHCS, and no previous testosterone prescription in the previous 2 years. Individuals were excluded if they had testosterone administered at VAPSHCS; were prescribed testosterone at another facility (VA or community-based); pilot tested an initial version of the TOT prior to November 30, 2019; or had an International Classification of Diseases, Tenth Revision codes for hypopituitarism, gender identity disorder, history of sexual assignment, or Klinefelter syndrome for which testosterone therapy was already approved. Patients who met the inclusion criteria were identified by an algorithm developed by the VAPSHCS pharmacoeconomist.
This quality improvement project used a retrospective, pre-post experimental design. Electronic chart review and systematic manual review of all eligible patient charts were performed for the pretemplate period (December 1, 2018, to November 30, 2019) and after the template implementation, (December 1, 2021, to November 30, 2022).
An initial version of the TOT was implemented on July 1, 2019, but was not fully integrated into CPRS until early 2020; individuals in whom the TOT was used prior to November 30, 2019, were excluded. Data from the initial period of the COVID-19 pandemic were avoided because of alterations in clinic and prescribing practices. As a quality improvement project, the TOT evaluation was exempt from formal review by the VAPSHCS Institutional Review Board, as determined by the Director of the Office of Transformation/Quality/Safety/Value.
Interventions
Testosterone is a Schedule III controlled substance with potential risks and a propensity for varied prescribing practices. It was designated as a restricted drug requiring a prior authorization drug request (PADR) for which a specific TOT was developed, approved by the VAPSHCS Pharmacy and Therapeutics Committee, and incorporated into CPRS. A team of pharmacists, primary care physicians, geriatricians, endocrinologists, and health informatics experts created and developed the TOT. Pharmacists managed and monitored its completion.
The process for prescribing testosterone via the TOT is outlined in the eAppendix. When an HCP orders testosterone in CPRS, reminders prompt them to use the TOT and indicate required laboratory measurements (an order set is provided). Completion of TOT is not necessary to order testosterone for patients with an existing diagnosis of an organic cause of hypogonadism (eg, Klinefelter syndrome or hypopituitarism) or transgender women (assigned male at birth). In the TOT, the prescriber must also indicate signs and symptoms of testosterone deficiency; required laboratory tests; and counseling regarding potential risks and benefits of TRT. A pharmacist reviews the TOT and either approves or rejects the testosterone prescription and provides follow-up guidance to the prescriber. The completed TOT serves as documentation of guideline adherence in CPRS. The TOT also includes sections for first renewal testosterone prescriptions, addressing guideline recommendations for follow-up laboratory evaluation and clinical response to TRT. Due to limited completion of this section in the posttemplate period, evaluating adherence to follow-up recommendations was not feasible.
Measures
This project assessed the percentage of patients in the posttemplate period vs pretemplate period with an approved PADR. Documentation of specific guideline-recommended measures was assessed: signs and symptoms of testosterone deficiency; ≥ 2 serum testosterone measurements (≥ 2 total, free and total, or 2 free testosterone levels, and ≥ 1 testosterone level before 10
The project also assessed the proportion of patients in the posttemplate period vs pretemplate period who had all hormone tests (≥ 2 serum testosterone and LH and FSH concentrations), all laboratory tests (hormone tests and hematocrit), and all 5 guideline-recommended measures.
Analysis
Statistical comparisons between the proportions of patients in the pretemplate and posttemplate periods for each measure were performed using a χ2 test, without correction for multiple comparisons. All analyses were conducted using Stata version 10.0. A P value < .05 was considered significant for all comparisons.
Results
Chart review identified 189 patients in the pretemplate period and 113 patients in the posttemplate period with a new testosterone prescription (Figure). After exclusions, 91 and 49 patients, respectively, met eligibility criteria (Table 1). Fifty-six patients (62%) pretemplate and 40 patients (82%) posttemplate (P = .015) had approved PADRs and comprised the groups that were analyzed (Table 2).
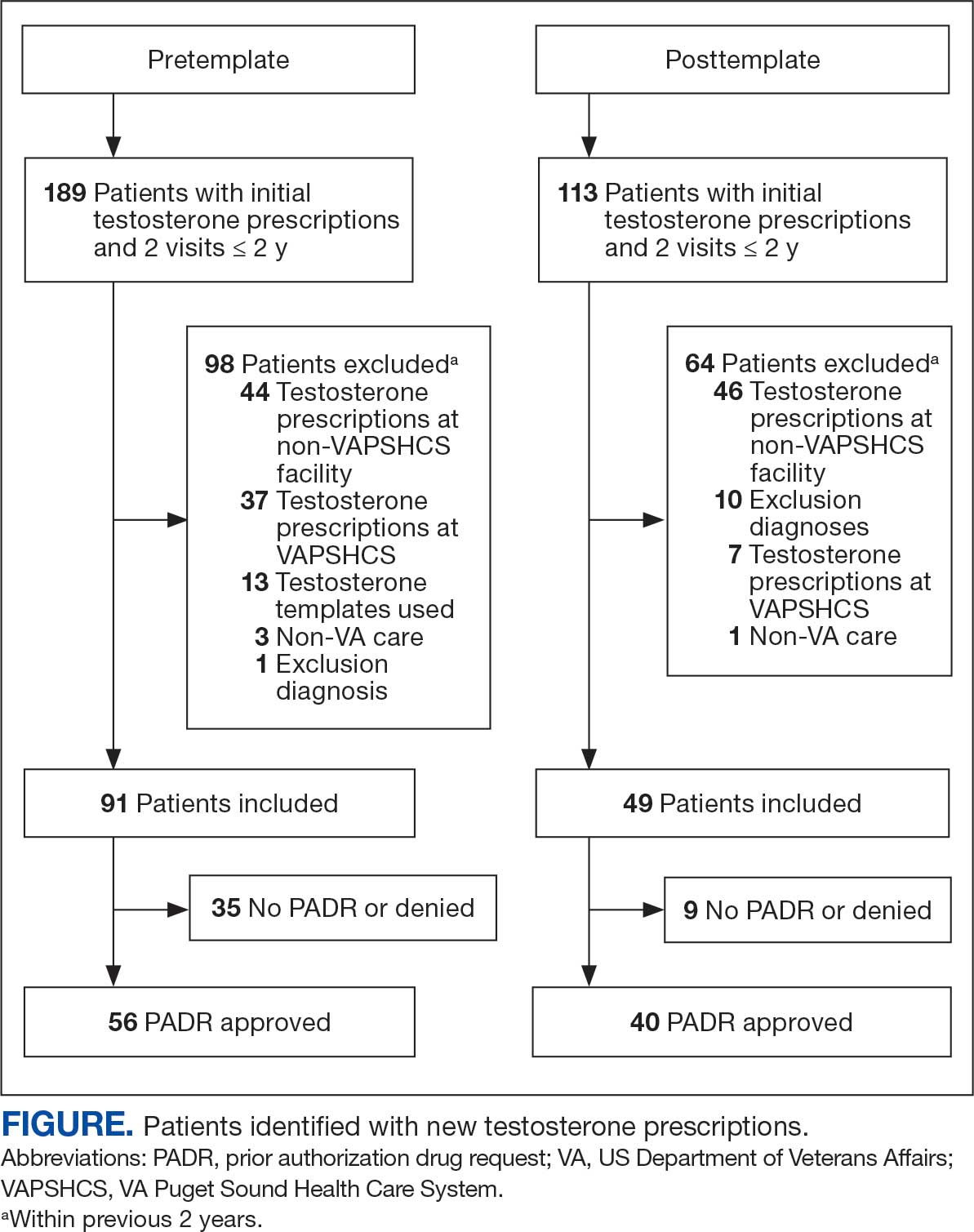
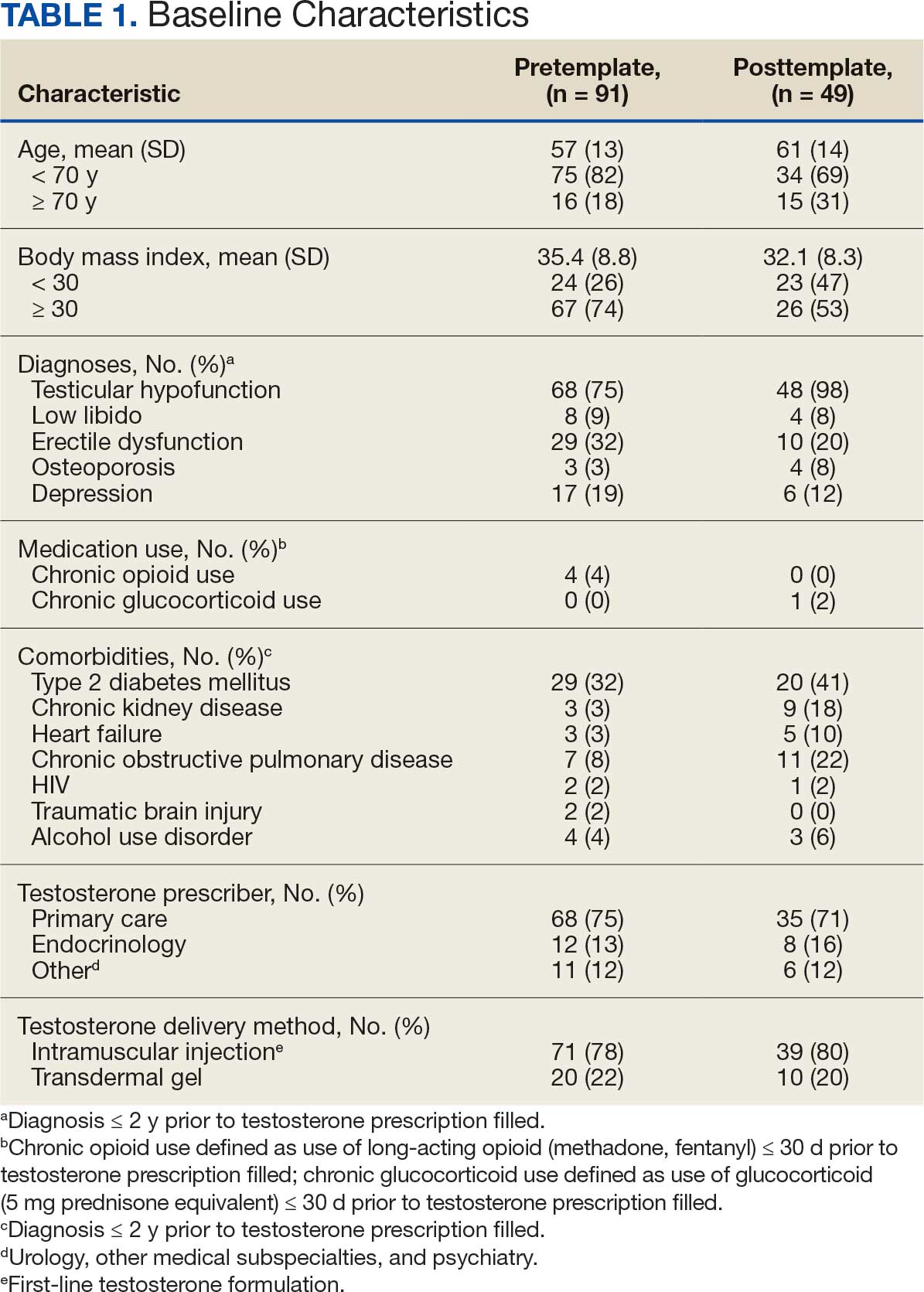

The mean age and body mass index were similar in the pretemplate and posttemplate periods, but there was variation in the proportions of patients aged < 70 years and those with a body mass index < 30 between the groups. The most common diagnosis in both groups was testicular hypofunction, and the most common comorbidity was type 2 diabetes mellitus. Concomitant use of opioids or glucocorticoids that can lower testosterone levels was rare. Most testosterone prescriptions originated from primary care clinics in both periods: 68 (75%) in the pretemplate period and 35 (71%) in the posttemplate period. Most testosterone treatment was delivered by intramuscular injection.
In the posttemplate period vs pretemplate period, the proportion of patients with an approved PADR (82% vs 62%, P = .02), and documentation of signs and symptoms of hypogonadism (93% vs 71%, P = .002) prior to starting TRT were higher, while the percentage of patients having ≥ 2 testosterone measurements (85% vs 89%, P = .53), ≥ 1 testosterone level before 10 AM (78% vs 75%, P = .70), and hematocrit measured (95% vs 91%, P = .47) were similar. Rates of LH and FSH testing were higher in the posttemplate period (80%) vs the pretemplate period (63%) but did not achieve statistical significance (P = .07), and discussion of the risks and benefits of TRT was higher in the posttemplate period (58%) vs the pretemplate period (34%) (P = .02). The percentage of patients who had all hormone measurements (total and/or free testosterone, LH, and FSH) was higher in the posttemplate period (78%) vs the pretemplate period (59%) but did not achieve statistical significance (P = .06). The rates of all guideline-recommended laboratory test orders were higher in the posttemplate period (78%) vs the pretemplate period (55%) (P = .03), and all 5 guideline-recommended clinical and laboratory measures were higher in the posttemplate period (45%) vs the pretemplate period (18%) (P = .004).
Discussion
The implementation of a pharmacy-managed TOT in CPRS demonstrated higher adherence to evidence-based guidelines for diagnosing and evaluating hypogonadism before TRT. After TOT implementation, a higher proportion of patients had documented signs and symptoms of testosterone deficiency, underwent all recommended laboratory tests, and had discussions about the risks and benefits of TRT. Adherence to 5 clinical and laboratory measures recommended by Endocrine Society guidelines was higher after TOT implementation, indicating improved prescribing practices.4
The requirement for TOT completion before testosterone prescription and its management by trained pharmacists likely contributed to higher adherence to guideline recommendations than previously reported. Integration of the TOT into CPRS with pharmacy oversight may have enhanced adherence by summarizing and codifying evidence-based guideline recommendations for clinical and biochemical evaluation prior to TRT initiation, offering relevant education to clinicians and pharmacists, automatically importing pertinent clinical information and laboratory results, and generating CPRS documentation to reduce clinician burden during patient care.
The proportion of patients with documented signs and symptoms of testosterone deficiency before TRT increased from the pretemplate period (71%) to the posttemplate period (93%), indicating that most patients receiving TRT had clinical manifestations of hypogonadism. This aligns with Endocrine Society guidelines, which define hypogonadism as a clinical disorder characterized by clinical manifestations of testosterone deficiency and persistently low serum testosterone levels on ≥ 2 separate occasions.4,6 However, recent trends in direct-to-consumer advertising for testosterone and the rise of “low T” clinics may contribute to increased testing, varied practices, and inappropriate testosterone therapy initiation (eg, in men with low testosterone levels who lack symptoms of hypogonadism).18 Improved adherence in documenting clinical hypogonadism with implementation of the TOT reinforces the value of incorporating educational material, as previously reported.11
Adherence to guideline recommendations following implementation of the TOT in this project was higher than those previously reported. In a study of 111,631 outpatient veterans prescribed testosterone from 2009 to 2012, only 18.3% had ≥ 2 testosterone prescriptions, and 3.5% had ≥ 2 testosterone, LH, and FSH levels measured prior to the initiation of a TRT.9 In a report of 63,534 insured patients who received TRT from 2010 to 2012, 40.3% had ≥ 2 testosterone prescriptions, and 12% had LH and/or FSH measured prior to the initiation.8
Low rates of guideline-recommended laboratory tests prior to initiation of testosterone treatment were reported in prior non-VA studies.19,20 Poor guideline adherence reinforces the need for clinician education or other methods to improve TRT and ensure appropriate prescribing practices across health care systems. The TOT described in this project is a sustainable clinical tool with the potential to improve testosterone prescribing practices.
The high rates of adherence to guideline recommendations at VAPSHCS likely stem from local endocrine expertise and ongoing educational initiatives, as well as the requirement for template completion before testosterone prescription. However, most testosterone prescriptions were initiated by primary care and monitored by pharmacists with varying degrees of training and clinical experience in hypogonadism and TRT.
However, adherence to guideline recommendations was modest, suggesting there is still an opportunity for improvement. The decision to initiate therapy should be made only after appropriate counseling with patients regarding its potential benefits and risks. Reports on the CV risk of TRT have been mixed. The 2023 TRAVERSE study found no increase in major adverse CV events among older men with hypogonadism and pre-existing CV risks undergoing TRT, but noted higher instances of pulmonary embolism, atrial fibrillation, and acute kidney injury.21 This highlights the need for clinicians to continue to engage in informed decision-making with patients. Effective pretreatment counseling is important but time-consuming; future TOT monitoring and modifications could consider mandatory checkboxes to document counseling on TRT risks and benefits.
The TOT described in this study could be adapted and incorporated into the prescribing process and electronic health record of larger health care systems. Use of an electronic template allows for automatic real-time dashboard monitoring of organization performance. The TOT described could be modified or simplified for specialty or primary care clinics or individual practitioners to improve adherence to evidence-based guideline recommendations and quality of care.
Strengths
A strength of this study is the multidisciplinary team (composed of stakeholders with experience in VA health care system and subject matter experts in hypogonadism) that developed and incorporated a user-friendly template for testosterone prescriptions; the use of evidence-based guideline recommendations; and the use of a structured chart review permitted accurate assessment of adherence to recommendations to document signs and symptoms of testosterone deficiency and a discussion of potential risks and benefits prior to TRT. To our knowledge, these recommendations have not been assessed in previous reports.
Limitations
The retrospective pre-post design of this study precludes a conclusion that implementation of the TOT caused the increase in adherence to guideline recommendations. Improved adherence could have resulted from the ongoing development of the preauthorization process for testosterone prescriptions or other changes over time. However, the preauthorization process had already been established for many years prior to template implementation. Forty-nine patients had new prescriptions for testosterone in the posttemplate period compared to 91 in the pretemplate period, but TRT was initiated in accordance with guideline recommendations more appropriately in the posttemplate period. The study’s sample size was small, and many eligible patients were excluded; however, exclusions were necessary to evaluate men who had new testosterone prescriptions for which the template was designed. Most men excluded were already taking testosterone.
Conclusions
The implementation of a CPRS-based TOT improved adherence to evidence-based guidelines for the diagnosis, evaluation, and counseling of patients with hypogonadism before starting TRT. While there were improvements in adherence with the TOT, the relatively low proportion of patients with documentation of TRT risks and benefits and all guideline recommendations highlights the need for additional efforts to further strengthen adherence to guideline recommendations and ensure appropriate evaluation, counseling, and prescribing practices before initiating TRT.
- Layton JB, Li D, Meier CR, et al. Testosterone lab testing and initiation in the United Kingdom and the United States, 2000 to 2011. J Clin Endocrinol Metab. 2014;99:835-842. doi:10.1210/jc.2013-3570
- Baillargeon J, Kuo YF, Westra JR, et al. Testosterone prescribing in the United States, 2002-2016. JAMA. 2018;320:200-202. doi:10.1001/jama.2018.7999
- Jasuja GK, Bhasin S, Rose AJ. Patterns of testosterone prescription overuse. Curr Opin Endocrinol Diabetes Obes. 2017;24:240-245. doi:10.1097/MED.0000000000000336
- Bhasin S, Cunningham GR, Hayes FJ, et al. Testosterone therapy in adult men with androgen deficiency syndromes: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2006;91:1995-2010. doi:10.1210/jc.2005-2847
- Bhasin S, Cunningham GR, Hayes FJ, et al. Testosterone therapy in men with androgen deficiency syndromes: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2010;95:2536-2559. doi:10.1210/jc.2009-2354
- Bhasin S, Brito JP, Cunningham GR, et al. Testosterone therapy in men with hypogonadism: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2018;103:1715-1744. doi:10.1210/jc.2018-00229
- Mulhall JP, Trost LW, Brannigan RE, et al. Evaluation and management of testosterone deficiency: AUA guideline. J Urol. 2018;200:423-432. doi:10.1016/j.juro.2018.03.115
- Muram D, Zhang X, Cui Z, et al. Use of hormone testing for the diagnosis and evaluation of male hypogonadism and monitoring of testosterone therapy: application of hormone testing guideline recommendations in clinical practice. J Sex Med. 2015;12:1886-1894. doi:10.1111/jsm.12968
- Jasuja GK, Bhasin S, Reisman JI, et al. Ascertainment of testosterone prescribing practices in the VA. Med Care. 2015;53:746-752. doi:10.1097/MLR.0000000000000398?
- Jasuja GK, Bhasin S, Reisman JI, et al. Who gets testosterone? Patient characteristics associated with testosterone prescribing in the Veteran Affairs system: a cross-sectional study. J Gen Intern Med. 2017;32:304-311. doi:10.1007/s11606-016-3940-7
- Basaria S, Coviello AD, Travison TG, et al. Adverse events associated with testosterone administration. N Engl J Med. 2010;363:109-122. doi:10.1056/NEJMoa1000485
- Vigen R, O’Donnell CI, Barón AE, et al. Association of testosterone therapy with mortality, myocardial infarction, and stroke in men with low testosterone levels. JAMA. 2013;310:1829-1836. doi:10.1001/jama.2013.280386
- Finkle WD, Greenland S, Ridgeway GK, et al. Increased risk of non-fatal myocardial infarction following testosterone therapy prescription in men. PLoS One. 2014;9:e85805. doi:10.1371/journal.pone.0085805
- US Food and Drug Administration. FDA Drug Safety Communication: FDA cautions about using testosterone products for low testosterone due to aging; requires labeling change to inform of possible increased risk of heart attack and stroke with use. FDA.gov. March 3, 2015. Updated February 28, 2025. Accessed July 8, 2025. http://www.fda.gov/Drugs/DrugSafety/ucm436259.htm
- US Dept of Veterans Affairs, Office of Inspector General. Healthcare inspection – testosterone replacement therapy initiation and follow-up evaluation in VA male patients. April 11, 2018. Accessed July 8, 2025. https://www.vaoig.gov/reports/national-healthcare-review/healthcare-inspection-testosterone-replacement-therapy
- Narla R, Mobley D, Nguyen EHK, et al. Preliminary evaluation of an order template to improve diagnosis and testosterone therapy of hypogonadism in veterans. Fed Pract. 2021;38:121-127. doi:10.12788/fp.0103
- Bhasin S, Travison TG, Pencina KM, et al. Prostate safety events during testosterone replacement therapy in men with hypogonadism: a randomized clinical trial. JAMA Netw Open. 2023;6:e2348692. doi:10.1001/jamanetworkopen.2023.48692
- Dubin JM, Jesse E, Fantus RJ, et al. Guideline-discordant care among direct-to-consumer testosterone therapy platforms. JAMA Intern Med. 2022;182:1321-1323. doi:10.1001/jamainternmed.2022.4928
- Baillargeon J, Urban RJ, Ottenbacher KJ, et al. Trends in androgen prescribing in the United States, 2001 to 2011. JAMA Intern Med. 2013;173:1465-1466. doi:10.1001/jamainternmed.2013.6895
- Locke JA, Flannigan R, Günther OP, et al. Testosterone therapy: prescribing and monitoring patterns of practice in British Columbia. Can Urol Assoc J. 2021;15:e110-e117. doi:10.5489/cuaj.6586
- Lincoff AM, Bhasin S, Flevaris P, et al. Cardiovascular safety of testosterone-replacement therapy. N Engl J Med. 2023;389:107-117. doi:10.1056/NEJMoa2215025
Testosterone therapy is administered following pragmatic diagnostic evaluation and workup to assess whether an adult male is hypogonadal, based on symptoms consistent with androgen deficiency and low morning serum testosterone concentrations on ≥ 2 occasions. Effects of testosterone administration include the development or maintenance of secondary sexual characteristics and increases in libido, muscle strength, fat-free mass, and bone density.
Testosterone prescriptions have markedly increased in the past 20 years, including within the US Department of Veterans Affairs (VA) health care system.1-3 This trend may be influenced by various factors, including patient perceptions of benefit, an increase in marketing, and the availability of more user-friendly formulations.
Since 2006, evidence-based clinical practice guidelines have recommended specific clinical and laboratory evaluation and counseling prior to starting testosterone replacement therapy (TRT).4-8 However, research has shown poor adherence to these recommendations, including at the VA, which raises concerns about inappropriate TRT initiation without proper diagnostic evaluation.9,10 Observational research has suggested a possible link between testosterone therapy and increased risk of cardiovascular (CV) events. The US Food and Drug Administration prescribing information includes boxed warnings about potential risks of high blood pressure, myocardial infarction, stroke, and CV-related mortality with testosterone treatment, contact transfer of transdermal testosterone, and pulmonary oil microembolism with testosterone undecanoate injections.11-15
A VA Office of Inspector General (OIG) review of VA clinician adherence to clinical and laboratory evaluation guidelines for testosterone deficiency found poor adherence among VA practitioners and made recommendations for improvement.4,15 These focused on establishing clinical signs and symptoms consistent with testosterone deficiency, confirming hypogonadism by repeated testosterone testing, determining the etiology of hypogonadism by measuring gonadotropins, initiating a discussion of risks and benefits of TRT, and assessing clinical improvement and obtaining an updated hematocrit test within 3 to 6 months of initiation.
The VA Puget Sound Health Care System (VAPSHCS) developed a local prior authorization template to assist health care practitioners (HCPs) to address the OIG recommendations. This testosterone order template (TOT) aimed to improve the diagnosis, evaluation, and monitoring of TRT in males with hypogonadism, combined with existing VA pharmacy criteria for the use of testosterone based on Endocrine Society guidelines. A version of the VAPSHCS TOT was approved as the national VA Computerized Patient Record System (CPRS) template.
Preliminary evaluation of the TOT suggested improved short-term adherence to guideline recommendations following implementation.16 This quality improvement study sought to assess the long-term effectiveness of the TOT with respect to clinical practice guideline adherence. The OIG did not address prostate-specific antigen (PSA) monitoring because understanding of the relationship between TRT and the risks of elevated PSA levels remains incomplete.6,17 This project hypothesized that implementation of a pharmacy-managed TOT incorporated into CPRS would result in higher adherence rates to guideline-recommended clinical and laboratory evaluation, in addition to counseling of men with hypogonadism prior to initiation of TRT.
Methods
Eligible participants were cisgender males who received a new testosterone prescription, had ≥ 2 clinic visits at VAPSHCS, and no previous testosterone prescription in the previous 2 years. Individuals were excluded if they had testosterone administered at VAPSHCS; were prescribed testosterone at another facility (VA or community-based); pilot tested an initial version of the TOT prior to November 30, 2019; or had an International Classification of Diseases, Tenth Revision codes for hypopituitarism, gender identity disorder, history of sexual assignment, or Klinefelter syndrome for which testosterone therapy was already approved. Patients who met the inclusion criteria were identified by an algorithm developed by the VAPSHCS pharmacoeconomist.
This quality improvement project used a retrospective, pre-post experimental design. Electronic chart review and systematic manual review of all eligible patient charts were performed for the pretemplate period (December 1, 2018, to November 30, 2019) and after the template implementation, (December 1, 2021, to November 30, 2022).
An initial version of the TOT was implemented on July 1, 2019, but was not fully integrated into CPRS until early 2020; individuals in whom the TOT was used prior to November 30, 2019, were excluded. Data from the initial period of the COVID-19 pandemic were avoided because of alterations in clinic and prescribing practices. As a quality improvement project, the TOT evaluation was exempt from formal review by the VAPSHCS Institutional Review Board, as determined by the Director of the Office of Transformation/Quality/Safety/Value.
Interventions
Testosterone is a Schedule III controlled substance with potential risks and a propensity for varied prescribing practices. It was designated as a restricted drug requiring a prior authorization drug request (PADR) for which a specific TOT was developed, approved by the VAPSHCS Pharmacy and Therapeutics Committee, and incorporated into CPRS. A team of pharmacists, primary care physicians, geriatricians, endocrinologists, and health informatics experts created and developed the TOT. Pharmacists managed and monitored its completion.
The process for prescribing testosterone via the TOT is outlined in the eAppendix. When an HCP orders testosterone in CPRS, reminders prompt them to use the TOT and indicate required laboratory measurements (an order set is provided). Completion of TOT is not necessary to order testosterone for patients with an existing diagnosis of an organic cause of hypogonadism (eg, Klinefelter syndrome or hypopituitarism) or transgender women (assigned male at birth). In the TOT, the prescriber must also indicate signs and symptoms of testosterone deficiency; required laboratory tests; and counseling regarding potential risks and benefits of TRT. A pharmacist reviews the TOT and either approves or rejects the testosterone prescription and provides follow-up guidance to the prescriber. The completed TOT serves as documentation of guideline adherence in CPRS. The TOT also includes sections for first renewal testosterone prescriptions, addressing guideline recommendations for follow-up laboratory evaluation and clinical response to TRT. Due to limited completion of this section in the posttemplate period, evaluating adherence to follow-up recommendations was not feasible.
Measures
This project assessed the percentage of patients in the posttemplate period vs pretemplate period with an approved PADR. Documentation of specific guideline-recommended measures was assessed: signs and symptoms of testosterone deficiency; ≥ 2 serum testosterone measurements (≥ 2 total, free and total, or 2 free testosterone levels, and ≥ 1 testosterone level before 10
The project also assessed the proportion of patients in the posttemplate period vs pretemplate period who had all hormone tests (≥ 2 serum testosterone and LH and FSH concentrations), all laboratory tests (hormone tests and hematocrit), and all 5 guideline-recommended measures.
Analysis
Statistical comparisons between the proportions of patients in the pretemplate and posttemplate periods for each measure were performed using a χ2 test, without correction for multiple comparisons. All analyses were conducted using Stata version 10.0. A P value < .05 was considered significant for all comparisons.
Results
Chart review identified 189 patients in the pretemplate period and 113 patients in the posttemplate period with a new testosterone prescription (Figure). After exclusions, 91 and 49 patients, respectively, met eligibility criteria (Table 1). Fifty-six patients (62%) pretemplate and 40 patients (82%) posttemplate (P = .015) had approved PADRs and comprised the groups that were analyzed (Table 2).



The mean age and body mass index were similar in the pretemplate and posttemplate periods, but there was variation in the proportions of patients aged < 70 years and those with a body mass index < 30 between the groups. The most common diagnosis in both groups was testicular hypofunction, and the most common comorbidity was type 2 diabetes mellitus. Concomitant use of opioids or glucocorticoids that can lower testosterone levels was rare. Most testosterone prescriptions originated from primary care clinics in both periods: 68 (75%) in the pretemplate period and 35 (71%) in the posttemplate period. Most testosterone treatment was delivered by intramuscular injection.
In the posttemplate period vs pretemplate period, the proportion of patients with an approved PADR (82% vs 62%, P = .02), and documentation of signs and symptoms of hypogonadism (93% vs 71%, P = .002) prior to starting TRT were higher, while the percentage of patients having ≥ 2 testosterone measurements (85% vs 89%, P = .53), ≥ 1 testosterone level before 10 AM (78% vs 75%, P = .70), and hematocrit measured (95% vs 91%, P = .47) were similar. Rates of LH and FSH testing were higher in the posttemplate period (80%) vs the pretemplate period (63%) but did not achieve statistical significance (P = .07), and discussion of the risks and benefits of TRT was higher in the posttemplate period (58%) vs the pretemplate period (34%) (P = .02). The percentage of patients who had all hormone measurements (total and/or free testosterone, LH, and FSH) was higher in the posttemplate period (78%) vs the pretemplate period (59%) but did not achieve statistical significance (P = .06). The rates of all guideline-recommended laboratory test orders were higher in the posttemplate period (78%) vs the pretemplate period (55%) (P = .03), and all 5 guideline-recommended clinical and laboratory measures were higher in the posttemplate period (45%) vs the pretemplate period (18%) (P = .004).
Discussion
The implementation of a pharmacy-managed TOT in CPRS demonstrated higher adherence to evidence-based guidelines for diagnosing and evaluating hypogonadism before TRT. After TOT implementation, a higher proportion of patients had documented signs and symptoms of testosterone deficiency, underwent all recommended laboratory tests, and had discussions about the risks and benefits of TRT. Adherence to 5 clinical and laboratory measures recommended by Endocrine Society guidelines was higher after TOT implementation, indicating improved prescribing practices.4
The requirement for TOT completion before testosterone prescription and its management by trained pharmacists likely contributed to higher adherence to guideline recommendations than previously reported. Integration of the TOT into CPRS with pharmacy oversight may have enhanced adherence by summarizing and codifying evidence-based guideline recommendations for clinical and biochemical evaluation prior to TRT initiation, offering relevant education to clinicians and pharmacists, automatically importing pertinent clinical information and laboratory results, and generating CPRS documentation to reduce clinician burden during patient care.
The proportion of patients with documented signs and symptoms of testosterone deficiency before TRT increased from the pretemplate period (71%) to the posttemplate period (93%), indicating that most patients receiving TRT had clinical manifestations of hypogonadism. This aligns with Endocrine Society guidelines, which define hypogonadism as a clinical disorder characterized by clinical manifestations of testosterone deficiency and persistently low serum testosterone levels on ≥ 2 separate occasions.4,6 However, recent trends in direct-to-consumer advertising for testosterone and the rise of “low T” clinics may contribute to increased testing, varied practices, and inappropriate testosterone therapy initiation (eg, in men with low testosterone levels who lack symptoms of hypogonadism).18 Improved adherence in documenting clinical hypogonadism with implementation of the TOT reinforces the value of incorporating educational material, as previously reported.11
Adherence to guideline recommendations following implementation of the TOT in this project was higher than those previously reported. In a study of 111,631 outpatient veterans prescribed testosterone from 2009 to 2012, only 18.3% had ≥ 2 testosterone prescriptions, and 3.5% had ≥ 2 testosterone, LH, and FSH levels measured prior to the initiation of a TRT.9 In a report of 63,534 insured patients who received TRT from 2010 to 2012, 40.3% had ≥ 2 testosterone prescriptions, and 12% had LH and/or FSH measured prior to the initiation.8
Low rates of guideline-recommended laboratory tests prior to initiation of testosterone treatment were reported in prior non-VA studies.19,20 Poor guideline adherence reinforces the need for clinician education or other methods to improve TRT and ensure appropriate prescribing practices across health care systems. The TOT described in this project is a sustainable clinical tool with the potential to improve testosterone prescribing practices.
The high rates of adherence to guideline recommendations at VAPSHCS likely stem from local endocrine expertise and ongoing educational initiatives, as well as the requirement for template completion before testosterone prescription. However, most testosterone prescriptions were initiated by primary care and monitored by pharmacists with varying degrees of training and clinical experience in hypogonadism and TRT.
However, adherence to guideline recommendations was modest, suggesting there is still an opportunity for improvement. The decision to initiate therapy should be made only after appropriate counseling with patients regarding its potential benefits and risks. Reports on the CV risk of TRT have been mixed. The 2023 TRAVERSE study found no increase in major adverse CV events among older men with hypogonadism and pre-existing CV risks undergoing TRT, but noted higher instances of pulmonary embolism, atrial fibrillation, and acute kidney injury.21 This highlights the need for clinicians to continue to engage in informed decision-making with patients. Effective pretreatment counseling is important but time-consuming; future TOT monitoring and modifications could consider mandatory checkboxes to document counseling on TRT risks and benefits.
The TOT described in this study could be adapted and incorporated into the prescribing process and electronic health record of larger health care systems. Use of an electronic template allows for automatic real-time dashboard monitoring of organization performance. The TOT described could be modified or simplified for specialty or primary care clinics or individual practitioners to improve adherence to evidence-based guideline recommendations and quality of care.
Strengths
A strength of this study is the multidisciplinary team (composed of stakeholders with experience in VA health care system and subject matter experts in hypogonadism) that developed and incorporated a user-friendly template for testosterone prescriptions; the use of evidence-based guideline recommendations; and the use of a structured chart review permitted accurate assessment of adherence to recommendations to document signs and symptoms of testosterone deficiency and a discussion of potential risks and benefits prior to TRT. To our knowledge, these recommendations have not been assessed in previous reports.
Limitations
The retrospective pre-post design of this study precludes a conclusion that implementation of the TOT caused the increase in adherence to guideline recommendations. Improved adherence could have resulted from the ongoing development of the preauthorization process for testosterone prescriptions or other changes over time. However, the preauthorization process had already been established for many years prior to template implementation. Forty-nine patients had new prescriptions for testosterone in the posttemplate period compared to 91 in the pretemplate period, but TRT was initiated in accordance with guideline recommendations more appropriately in the posttemplate period. The study’s sample size was small, and many eligible patients were excluded; however, exclusions were necessary to evaluate men who had new testosterone prescriptions for which the template was designed. Most men excluded were already taking testosterone.
Conclusions
The implementation of a CPRS-based TOT improved adherence to evidence-based guidelines for the diagnosis, evaluation, and counseling of patients with hypogonadism before starting TRT. While there were improvements in adherence with the TOT, the relatively low proportion of patients with documentation of TRT risks and benefits and all guideline recommendations highlights the need for additional efforts to further strengthen adherence to guideline recommendations and ensure appropriate evaluation, counseling, and prescribing practices before initiating TRT.
Testosterone therapy is administered following pragmatic diagnostic evaluation and workup to assess whether an adult male is hypogonadal, based on symptoms consistent with androgen deficiency and low morning serum testosterone concentrations on ≥ 2 occasions. Effects of testosterone administration include the development or maintenance of secondary sexual characteristics and increases in libido, muscle strength, fat-free mass, and bone density.
Testosterone prescriptions have markedly increased in the past 20 years, including within the US Department of Veterans Affairs (VA) health care system.1-3 This trend may be influenced by various factors, including patient perceptions of benefit, an increase in marketing, and the availability of more user-friendly formulations.
Since 2006, evidence-based clinical practice guidelines have recommended specific clinical and laboratory evaluation and counseling prior to starting testosterone replacement therapy (TRT).4-8 However, research has shown poor adherence to these recommendations, including at the VA, which raises concerns about inappropriate TRT initiation without proper diagnostic evaluation.9,10 Observational research has suggested a possible link between testosterone therapy and increased risk of cardiovascular (CV) events. The US Food and Drug Administration prescribing information includes boxed warnings about potential risks of high blood pressure, myocardial infarction, stroke, and CV-related mortality with testosterone treatment, contact transfer of transdermal testosterone, and pulmonary oil microembolism with testosterone undecanoate injections.11-15
A VA Office of Inspector General (OIG) review of VA clinician adherence to clinical and laboratory evaluation guidelines for testosterone deficiency found poor adherence among VA practitioners and made recommendations for improvement.4,15 These focused on establishing clinical signs and symptoms consistent with testosterone deficiency, confirming hypogonadism by repeated testosterone testing, determining the etiology of hypogonadism by measuring gonadotropins, initiating a discussion of risks and benefits of TRT, and assessing clinical improvement and obtaining an updated hematocrit test within 3 to 6 months of initiation.
The VA Puget Sound Health Care System (VAPSHCS) developed a local prior authorization template to assist health care practitioners (HCPs) to address the OIG recommendations. This testosterone order template (TOT) aimed to improve the diagnosis, evaluation, and monitoring of TRT in males with hypogonadism, combined with existing VA pharmacy criteria for the use of testosterone based on Endocrine Society guidelines. A version of the VAPSHCS TOT was approved as the national VA Computerized Patient Record System (CPRS) template.
Preliminary evaluation of the TOT suggested improved short-term adherence to guideline recommendations following implementation.16 This quality improvement study sought to assess the long-term effectiveness of the TOT with respect to clinical practice guideline adherence. The OIG did not address prostate-specific antigen (PSA) monitoring because understanding of the relationship between TRT and the risks of elevated PSA levels remains incomplete.6,17 This project hypothesized that implementation of a pharmacy-managed TOT incorporated into CPRS would result in higher adherence rates to guideline-recommended clinical and laboratory evaluation, in addition to counseling of men with hypogonadism prior to initiation of TRT.
Methods
Eligible participants were cisgender males who received a new testosterone prescription, had ≥ 2 clinic visits at VAPSHCS, and no previous testosterone prescription in the previous 2 years. Individuals were excluded if they had testosterone administered at VAPSHCS; were prescribed testosterone at another facility (VA or community-based); pilot tested an initial version of the TOT prior to November 30, 2019; or had an International Classification of Diseases, Tenth Revision codes for hypopituitarism, gender identity disorder, history of sexual assignment, or Klinefelter syndrome for which testosterone therapy was already approved. Patients who met the inclusion criteria were identified by an algorithm developed by the VAPSHCS pharmacoeconomist.
This quality improvement project used a retrospective, pre-post experimental design. Electronic chart review and systematic manual review of all eligible patient charts were performed for the pretemplate period (December 1, 2018, to November 30, 2019) and after the template implementation, (December 1, 2021, to November 30, 2022).
An initial version of the TOT was implemented on July 1, 2019, but was not fully integrated into CPRS until early 2020; individuals in whom the TOT was used prior to November 30, 2019, were excluded. Data from the initial period of the COVID-19 pandemic were avoided because of alterations in clinic and prescribing practices. As a quality improvement project, the TOT evaluation was exempt from formal review by the VAPSHCS Institutional Review Board, as determined by the Director of the Office of Transformation/Quality/Safety/Value.
Interventions
Testosterone is a Schedule III controlled substance with potential risks and a propensity for varied prescribing practices. It was designated as a restricted drug requiring a prior authorization drug request (PADR) for which a specific TOT was developed, approved by the VAPSHCS Pharmacy and Therapeutics Committee, and incorporated into CPRS. A team of pharmacists, primary care physicians, geriatricians, endocrinologists, and health informatics experts created and developed the TOT. Pharmacists managed and monitored its completion.
The process for prescribing testosterone via the TOT is outlined in the eAppendix. When an HCP orders testosterone in CPRS, reminders prompt them to use the TOT and indicate required laboratory measurements (an order set is provided). Completion of TOT is not necessary to order testosterone for patients with an existing diagnosis of an organic cause of hypogonadism (eg, Klinefelter syndrome or hypopituitarism) or transgender women (assigned male at birth). In the TOT, the prescriber must also indicate signs and symptoms of testosterone deficiency; required laboratory tests; and counseling regarding potential risks and benefits of TRT. A pharmacist reviews the TOT and either approves or rejects the testosterone prescription and provides follow-up guidance to the prescriber. The completed TOT serves as documentation of guideline adherence in CPRS. The TOT also includes sections for first renewal testosterone prescriptions, addressing guideline recommendations for follow-up laboratory evaluation and clinical response to TRT. Due to limited completion of this section in the posttemplate period, evaluating adherence to follow-up recommendations was not feasible.
Measures
This project assessed the percentage of patients in the posttemplate period vs pretemplate period with an approved PADR. Documentation of specific guideline-recommended measures was assessed: signs and symptoms of testosterone deficiency; ≥ 2 serum testosterone measurements (≥ 2 total, free and total, or 2 free testosterone levels, and ≥ 1 testosterone level before 10
The project also assessed the proportion of patients in the posttemplate period vs pretemplate period who had all hormone tests (≥ 2 serum testosterone and LH and FSH concentrations), all laboratory tests (hormone tests and hematocrit), and all 5 guideline-recommended measures.
Analysis
Statistical comparisons between the proportions of patients in the pretemplate and posttemplate periods for each measure were performed using a χ2 test, without correction for multiple comparisons. All analyses were conducted using Stata version 10.0. A P value < .05 was considered significant for all comparisons.
Results
Chart review identified 189 patients in the pretemplate period and 113 patients in the posttemplate period with a new testosterone prescription (Figure). After exclusions, 91 and 49 patients, respectively, met eligibility criteria (Table 1). Fifty-six patients (62%) pretemplate and 40 patients (82%) posttemplate (P = .015) had approved PADRs and comprised the groups that were analyzed (Table 2).



The mean age and body mass index were similar in the pretemplate and posttemplate periods, but there was variation in the proportions of patients aged < 70 years and those with a body mass index < 30 between the groups. The most common diagnosis in both groups was testicular hypofunction, and the most common comorbidity was type 2 diabetes mellitus. Concomitant use of opioids or glucocorticoids that can lower testosterone levels was rare. Most testosterone prescriptions originated from primary care clinics in both periods: 68 (75%) in the pretemplate period and 35 (71%) in the posttemplate period. Most testosterone treatment was delivered by intramuscular injection.
In the posttemplate period vs pretemplate period, the proportion of patients with an approved PADR (82% vs 62%, P = .02), and documentation of signs and symptoms of hypogonadism (93% vs 71%, P = .002) prior to starting TRT were higher, while the percentage of patients having ≥ 2 testosterone measurements (85% vs 89%, P = .53), ≥ 1 testosterone level before 10 AM (78% vs 75%, P = .70), and hematocrit measured (95% vs 91%, P = .47) were similar. Rates of LH and FSH testing were higher in the posttemplate period (80%) vs the pretemplate period (63%) but did not achieve statistical significance (P = .07), and discussion of the risks and benefits of TRT was higher in the posttemplate period (58%) vs the pretemplate period (34%) (P = .02). The percentage of patients who had all hormone measurements (total and/or free testosterone, LH, and FSH) was higher in the posttemplate period (78%) vs the pretemplate period (59%) but did not achieve statistical significance (P = .06). The rates of all guideline-recommended laboratory test orders were higher in the posttemplate period (78%) vs the pretemplate period (55%) (P = .03), and all 5 guideline-recommended clinical and laboratory measures were higher in the posttemplate period (45%) vs the pretemplate period (18%) (P = .004).
Discussion
The implementation of a pharmacy-managed TOT in CPRS demonstrated higher adherence to evidence-based guidelines for diagnosing and evaluating hypogonadism before TRT. After TOT implementation, a higher proportion of patients had documented signs and symptoms of testosterone deficiency, underwent all recommended laboratory tests, and had discussions about the risks and benefits of TRT. Adherence to 5 clinical and laboratory measures recommended by Endocrine Society guidelines was higher after TOT implementation, indicating improved prescribing practices.4
The requirement for TOT completion before testosterone prescription and its management by trained pharmacists likely contributed to higher adherence to guideline recommendations than previously reported. Integration of the TOT into CPRS with pharmacy oversight may have enhanced adherence by summarizing and codifying evidence-based guideline recommendations for clinical and biochemical evaluation prior to TRT initiation, offering relevant education to clinicians and pharmacists, automatically importing pertinent clinical information and laboratory results, and generating CPRS documentation to reduce clinician burden during patient care.
The proportion of patients with documented signs and symptoms of testosterone deficiency before TRT increased from the pretemplate period (71%) to the posttemplate period (93%), indicating that most patients receiving TRT had clinical manifestations of hypogonadism. This aligns with Endocrine Society guidelines, which define hypogonadism as a clinical disorder characterized by clinical manifestations of testosterone deficiency and persistently low serum testosterone levels on ≥ 2 separate occasions.4,6 However, recent trends in direct-to-consumer advertising for testosterone and the rise of “low T” clinics may contribute to increased testing, varied practices, and inappropriate testosterone therapy initiation (eg, in men with low testosterone levels who lack symptoms of hypogonadism).18 Improved adherence in documenting clinical hypogonadism with implementation of the TOT reinforces the value of incorporating educational material, as previously reported.11
Adherence to guideline recommendations following implementation of the TOT in this project was higher than those previously reported. In a study of 111,631 outpatient veterans prescribed testosterone from 2009 to 2012, only 18.3% had ≥ 2 testosterone prescriptions, and 3.5% had ≥ 2 testosterone, LH, and FSH levels measured prior to the initiation of a TRT.9 In a report of 63,534 insured patients who received TRT from 2010 to 2012, 40.3% had ≥ 2 testosterone prescriptions, and 12% had LH and/or FSH measured prior to the initiation.8
Low rates of guideline-recommended laboratory tests prior to initiation of testosterone treatment were reported in prior non-VA studies.19,20 Poor guideline adherence reinforces the need for clinician education or other methods to improve TRT and ensure appropriate prescribing practices across health care systems. The TOT described in this project is a sustainable clinical tool with the potential to improve testosterone prescribing practices.
The high rates of adherence to guideline recommendations at VAPSHCS likely stem from local endocrine expertise and ongoing educational initiatives, as well as the requirement for template completion before testosterone prescription. However, most testosterone prescriptions were initiated by primary care and monitored by pharmacists with varying degrees of training and clinical experience in hypogonadism and TRT.
However, adherence to guideline recommendations was modest, suggesting there is still an opportunity for improvement. The decision to initiate therapy should be made only after appropriate counseling with patients regarding its potential benefits and risks. Reports on the CV risk of TRT have been mixed. The 2023 TRAVERSE study found no increase in major adverse CV events among older men with hypogonadism and pre-existing CV risks undergoing TRT, but noted higher instances of pulmonary embolism, atrial fibrillation, and acute kidney injury.21 This highlights the need for clinicians to continue to engage in informed decision-making with patients. Effective pretreatment counseling is important but time-consuming; future TOT monitoring and modifications could consider mandatory checkboxes to document counseling on TRT risks and benefits.
The TOT described in this study could be adapted and incorporated into the prescribing process and electronic health record of larger health care systems. Use of an electronic template allows for automatic real-time dashboard monitoring of organization performance. The TOT described could be modified or simplified for specialty or primary care clinics or individual practitioners to improve adherence to evidence-based guideline recommendations and quality of care.
Strengths
A strength of this study is the multidisciplinary team (composed of stakeholders with experience in VA health care system and subject matter experts in hypogonadism) that developed and incorporated a user-friendly template for testosterone prescriptions; the use of evidence-based guideline recommendations; and the use of a structured chart review permitted accurate assessment of adherence to recommendations to document signs and symptoms of testosterone deficiency and a discussion of potential risks and benefits prior to TRT. To our knowledge, these recommendations have not been assessed in previous reports.
Limitations
The retrospective pre-post design of this study precludes a conclusion that implementation of the TOT caused the increase in adherence to guideline recommendations. Improved adherence could have resulted from the ongoing development of the preauthorization process for testosterone prescriptions or other changes over time. However, the preauthorization process had already been established for many years prior to template implementation. Forty-nine patients had new prescriptions for testosterone in the posttemplate period compared to 91 in the pretemplate period, but TRT was initiated in accordance with guideline recommendations more appropriately in the posttemplate period. The study’s sample size was small, and many eligible patients were excluded; however, exclusions were necessary to evaluate men who had new testosterone prescriptions for which the template was designed. Most men excluded were already taking testosterone.
Conclusions
The implementation of a CPRS-based TOT improved adherence to evidence-based guidelines for the diagnosis, evaluation, and counseling of patients with hypogonadism before starting TRT. While there were improvements in adherence with the TOT, the relatively low proportion of patients with documentation of TRT risks and benefits and all guideline recommendations highlights the need for additional efforts to further strengthen adherence to guideline recommendations and ensure appropriate evaluation, counseling, and prescribing practices before initiating TRT.
- Layton JB, Li D, Meier CR, et al. Testosterone lab testing and initiation in the United Kingdom and the United States, 2000 to 2011. J Clin Endocrinol Metab. 2014;99:835-842. doi:10.1210/jc.2013-3570
- Baillargeon J, Kuo YF, Westra JR, et al. Testosterone prescribing in the United States, 2002-2016. JAMA. 2018;320:200-202. doi:10.1001/jama.2018.7999
- Jasuja GK, Bhasin S, Rose AJ. Patterns of testosterone prescription overuse. Curr Opin Endocrinol Diabetes Obes. 2017;24:240-245. doi:10.1097/MED.0000000000000336
- Bhasin S, Cunningham GR, Hayes FJ, et al. Testosterone therapy in adult men with androgen deficiency syndromes: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2006;91:1995-2010. doi:10.1210/jc.2005-2847
- Bhasin S, Cunningham GR, Hayes FJ, et al. Testosterone therapy in men with androgen deficiency syndromes: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2010;95:2536-2559. doi:10.1210/jc.2009-2354
- Bhasin S, Brito JP, Cunningham GR, et al. Testosterone therapy in men with hypogonadism: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2018;103:1715-1744. doi:10.1210/jc.2018-00229
- Mulhall JP, Trost LW, Brannigan RE, et al. Evaluation and management of testosterone deficiency: AUA guideline. J Urol. 2018;200:423-432. doi:10.1016/j.juro.2018.03.115
- Muram D, Zhang X, Cui Z, et al. Use of hormone testing for the diagnosis and evaluation of male hypogonadism and monitoring of testosterone therapy: application of hormone testing guideline recommendations in clinical practice. J Sex Med. 2015;12:1886-1894. doi:10.1111/jsm.12968
- Jasuja GK, Bhasin S, Reisman JI, et al. Ascertainment of testosterone prescribing practices in the VA. Med Care. 2015;53:746-752. doi:10.1097/MLR.0000000000000398?
- Jasuja GK, Bhasin S, Reisman JI, et al. Who gets testosterone? Patient characteristics associated with testosterone prescribing in the Veteran Affairs system: a cross-sectional study. J Gen Intern Med. 2017;32:304-311. doi:10.1007/s11606-016-3940-7
- Basaria S, Coviello AD, Travison TG, et al. Adverse events associated with testosterone administration. N Engl J Med. 2010;363:109-122. doi:10.1056/NEJMoa1000485
- Vigen R, O’Donnell CI, Barón AE, et al. Association of testosterone therapy with mortality, myocardial infarction, and stroke in men with low testosterone levels. JAMA. 2013;310:1829-1836. doi:10.1001/jama.2013.280386
- Finkle WD, Greenland S, Ridgeway GK, et al. Increased risk of non-fatal myocardial infarction following testosterone therapy prescription in men. PLoS One. 2014;9:e85805. doi:10.1371/journal.pone.0085805
- US Food and Drug Administration. FDA Drug Safety Communication: FDA cautions about using testosterone products for low testosterone due to aging; requires labeling change to inform of possible increased risk of heart attack and stroke with use. FDA.gov. March 3, 2015. Updated February 28, 2025. Accessed July 8, 2025. http://www.fda.gov/Drugs/DrugSafety/ucm436259.htm
- US Dept of Veterans Affairs, Office of Inspector General. Healthcare inspection – testosterone replacement therapy initiation and follow-up evaluation in VA male patients. April 11, 2018. Accessed July 8, 2025. https://www.vaoig.gov/reports/national-healthcare-review/healthcare-inspection-testosterone-replacement-therapy
- Narla R, Mobley D, Nguyen EHK, et al. Preliminary evaluation of an order template to improve diagnosis and testosterone therapy of hypogonadism in veterans. Fed Pract. 2021;38:121-127. doi:10.12788/fp.0103
- Bhasin S, Travison TG, Pencina KM, et al. Prostate safety events during testosterone replacement therapy in men with hypogonadism: a randomized clinical trial. JAMA Netw Open. 2023;6:e2348692. doi:10.1001/jamanetworkopen.2023.48692
- Dubin JM, Jesse E, Fantus RJ, et al. Guideline-discordant care among direct-to-consumer testosterone therapy platforms. JAMA Intern Med. 2022;182:1321-1323. doi:10.1001/jamainternmed.2022.4928
- Baillargeon J, Urban RJ, Ottenbacher KJ, et al. Trends in androgen prescribing in the United States, 2001 to 2011. JAMA Intern Med. 2013;173:1465-1466. doi:10.1001/jamainternmed.2013.6895
- Locke JA, Flannigan R, Günther OP, et al. Testosterone therapy: prescribing and monitoring patterns of practice in British Columbia. Can Urol Assoc J. 2021;15:e110-e117. doi:10.5489/cuaj.6586
- Lincoff AM, Bhasin S, Flevaris P, et al. Cardiovascular safety of testosterone-replacement therapy. N Engl J Med. 2023;389:107-117. doi:10.1056/NEJMoa2215025
- Layton JB, Li D, Meier CR, et al. Testosterone lab testing and initiation in the United Kingdom and the United States, 2000 to 2011. J Clin Endocrinol Metab. 2014;99:835-842. doi:10.1210/jc.2013-3570
- Baillargeon J, Kuo YF, Westra JR, et al. Testosterone prescribing in the United States, 2002-2016. JAMA. 2018;320:200-202. doi:10.1001/jama.2018.7999
- Jasuja GK, Bhasin S, Rose AJ. Patterns of testosterone prescription overuse. Curr Opin Endocrinol Diabetes Obes. 2017;24:240-245. doi:10.1097/MED.0000000000000336
- Bhasin S, Cunningham GR, Hayes FJ, et al. Testosterone therapy in adult men with androgen deficiency syndromes: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2006;91:1995-2010. doi:10.1210/jc.2005-2847
- Bhasin S, Cunningham GR, Hayes FJ, et al. Testosterone therapy in men with androgen deficiency syndromes: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2010;95:2536-2559. doi:10.1210/jc.2009-2354
- Bhasin S, Brito JP, Cunningham GR, et al. Testosterone therapy in men with hypogonadism: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2018;103:1715-1744. doi:10.1210/jc.2018-00229
- Mulhall JP, Trost LW, Brannigan RE, et al. Evaluation and management of testosterone deficiency: AUA guideline. J Urol. 2018;200:423-432. doi:10.1016/j.juro.2018.03.115
- Muram D, Zhang X, Cui Z, et al. Use of hormone testing for the diagnosis and evaluation of male hypogonadism and monitoring of testosterone therapy: application of hormone testing guideline recommendations in clinical practice. J Sex Med. 2015;12:1886-1894. doi:10.1111/jsm.12968
- Jasuja GK, Bhasin S, Reisman JI, et al. Ascertainment of testosterone prescribing practices in the VA. Med Care. 2015;53:746-752. doi:10.1097/MLR.0000000000000398?
- Jasuja GK, Bhasin S, Reisman JI, et al. Who gets testosterone? Patient characteristics associated with testosterone prescribing in the Veteran Affairs system: a cross-sectional study. J Gen Intern Med. 2017;32:304-311. doi:10.1007/s11606-016-3940-7
- Basaria S, Coviello AD, Travison TG, et al. Adverse events associated with testosterone administration. N Engl J Med. 2010;363:109-122. doi:10.1056/NEJMoa1000485
- Vigen R, O’Donnell CI, Barón AE, et al. Association of testosterone therapy with mortality, myocardial infarction, and stroke in men with low testosterone levels. JAMA. 2013;310:1829-1836. doi:10.1001/jama.2013.280386
- Finkle WD, Greenland S, Ridgeway GK, et al. Increased risk of non-fatal myocardial infarction following testosterone therapy prescription in men. PLoS One. 2014;9:e85805. doi:10.1371/journal.pone.0085805
- US Food and Drug Administration. FDA Drug Safety Communication: FDA cautions about using testosterone products for low testosterone due to aging; requires labeling change to inform of possible increased risk of heart attack and stroke with use. FDA.gov. March 3, 2015. Updated February 28, 2025. Accessed July 8, 2025. http://www.fda.gov/Drugs/DrugSafety/ucm436259.htm
- US Dept of Veterans Affairs, Office of Inspector General. Healthcare inspection – testosterone replacement therapy initiation and follow-up evaluation in VA male patients. April 11, 2018. Accessed July 8, 2025. https://www.vaoig.gov/reports/national-healthcare-review/healthcare-inspection-testosterone-replacement-therapy
- Narla R, Mobley D, Nguyen EHK, et al. Preliminary evaluation of an order template to improve diagnosis and testosterone therapy of hypogonadism in veterans. Fed Pract. 2021;38:121-127. doi:10.12788/fp.0103
- Bhasin S, Travison TG, Pencina KM, et al. Prostate safety events during testosterone replacement therapy in men with hypogonadism: a randomized clinical trial. JAMA Netw Open. 2023;6:e2348692. doi:10.1001/jamanetworkopen.2023.48692
- Dubin JM, Jesse E, Fantus RJ, et al. Guideline-discordant care among direct-to-consumer testosterone therapy platforms. JAMA Intern Med. 2022;182:1321-1323. doi:10.1001/jamainternmed.2022.4928
- Baillargeon J, Urban RJ, Ottenbacher KJ, et al. Trends in androgen prescribing in the United States, 2001 to 2011. JAMA Intern Med. 2013;173:1465-1466. doi:10.1001/jamainternmed.2013.6895
- Locke JA, Flannigan R, Günther OP, et al. Testosterone therapy: prescribing and monitoring patterns of practice in British Columbia. Can Urol Assoc J. 2021;15:e110-e117. doi:10.5489/cuaj.6586
- Lincoff AM, Bhasin S, Flevaris P, et al. Cardiovascular safety of testosterone-replacement therapy. N Engl J Med. 2023;389:107-117. doi:10.1056/NEJMoa2215025
Streamlined Testosterone Order Template to Improve the Diagnosis and Evaluation of Hypogonadism in Veterans
Streamlined Testosterone Order Template to Improve the Diagnosis and Evaluation of Hypogonadism in Veterans