User login
Whooping Cough Rising Fast, Especially Among Teens
Whooping cough is surging in the United States, with four times as many cases reported so far this year, compared to all of 2023.
The CDC said 14,569 cases had been reported as of Sept. 14, compared to 3475 in all of 2023.
Whooping cough, also called pertussis, is a respiratory illness spread through coughing, sneezing, or breathing very close to another person. Babies are given the DTaP vaccine to protect against whooping cough, diphtheria, and tetanus. Because the vaccine effectiveness wanes faster for whooping cough than the two other illnesses, boosters are recommended every decade or so.
Why the Whooping Cough Vaccine Is Important
Whooping cough is a very contagious bacteria, so vaccination is an important step to avoid it.
But many children in their tweens aren’t getting boosters, and that age group is driving the whooping cough outbreak.
“With the increase in vaccine hesitancy that has been going on since the COVID-19 pandemic, we’re seeing outbreaks occurring in kids who are not vaccinated,” Tina Tan, MD, president-elect of the Infectious Diseases Society of America, told NBC News.
Also, people are not social distancing the way they did during the height of the COVID pandemic, when whooping cough numbers went down.
“Levels of pertussis dropped dramatically when we were all masking, and now this huge increase is getting us back to pre-pandemic levels, and probably a little above that,” Thomas Murray, MD, a Yale Medicine pediatric infectious diseases specialist, said in a news release from the school. “It’s a contagious respiratory virus that can spread fairly quickly through the population.”
FDA advisers were scheduled to meet Sept. 20 to discuss developing more effective boosters for whooping cough.
A version of this article appeared on WebMD.com.
Whooping cough is surging in the United States, with four times as many cases reported so far this year, compared to all of 2023.
The CDC said 14,569 cases had been reported as of Sept. 14, compared to 3475 in all of 2023.
Whooping cough, also called pertussis, is a respiratory illness spread through coughing, sneezing, or breathing very close to another person. Babies are given the DTaP vaccine to protect against whooping cough, diphtheria, and tetanus. Because the vaccine effectiveness wanes faster for whooping cough than the two other illnesses, boosters are recommended every decade or so.
Why the Whooping Cough Vaccine Is Important
Whooping cough is a very contagious bacteria, so vaccination is an important step to avoid it.
But many children in their tweens aren’t getting boosters, and that age group is driving the whooping cough outbreak.
“With the increase in vaccine hesitancy that has been going on since the COVID-19 pandemic, we’re seeing outbreaks occurring in kids who are not vaccinated,” Tina Tan, MD, president-elect of the Infectious Diseases Society of America, told NBC News.
Also, people are not social distancing the way they did during the height of the COVID pandemic, when whooping cough numbers went down.
“Levels of pertussis dropped dramatically when we were all masking, and now this huge increase is getting us back to pre-pandemic levels, and probably a little above that,” Thomas Murray, MD, a Yale Medicine pediatric infectious diseases specialist, said in a news release from the school. “It’s a contagious respiratory virus that can spread fairly quickly through the population.”
FDA advisers were scheduled to meet Sept. 20 to discuss developing more effective boosters for whooping cough.
A version of this article appeared on WebMD.com.
Whooping cough is surging in the United States, with four times as many cases reported so far this year, compared to all of 2023.
The CDC said 14,569 cases had been reported as of Sept. 14, compared to 3475 in all of 2023.
Whooping cough, also called pertussis, is a respiratory illness spread through coughing, sneezing, or breathing very close to another person. Babies are given the DTaP vaccine to protect against whooping cough, diphtheria, and tetanus. Because the vaccine effectiveness wanes faster for whooping cough than the two other illnesses, boosters are recommended every decade or so.
Why the Whooping Cough Vaccine Is Important
Whooping cough is a very contagious bacteria, so vaccination is an important step to avoid it.
But many children in their tweens aren’t getting boosters, and that age group is driving the whooping cough outbreak.
“With the increase in vaccine hesitancy that has been going on since the COVID-19 pandemic, we’re seeing outbreaks occurring in kids who are not vaccinated,” Tina Tan, MD, president-elect of the Infectious Diseases Society of America, told NBC News.
Also, people are not social distancing the way they did during the height of the COVID pandemic, when whooping cough numbers went down.
“Levels of pertussis dropped dramatically when we were all masking, and now this huge increase is getting us back to pre-pandemic levels, and probably a little above that,” Thomas Murray, MD, a Yale Medicine pediatric infectious diseases specialist, said in a news release from the school. “It’s a contagious respiratory virus that can spread fairly quickly through the population.”
FDA advisers were scheduled to meet Sept. 20 to discuss developing more effective boosters for whooping cough.
A version of this article appeared on WebMD.com.
Feds Sue Three Biggest Pharmacy Benefit Managers Over Insulin Costs
The US Federal Trade Commission (FTC) has sued the nation’s three largest pharmacy benefit managers (PBMs), alleging that they have steered patients to buying higher-priced insulins so that they can reap more profits.
The three PBMs administer 80% of prescriptions in the United States, the FTC said in a statement announcing the action.
The agency filed an administrative complaint, which means its allegations will be tried in a formal hearing before an administrative law judge. It will not be heard in a criminal court.
The three PBMs “have extracted millions of dollars off the backs of patients who need life-saving medications,” Rahul Rao, deputy director of the FTC’s Bureau of Competition, said in the statement.
The FTC action is not the first taken by a government agency against PBMs. Ohio Attorney General Dave Yost sued Express Scripts and Prime Therapeutics in March 2023, alleging antitrust violations.
The FTC’s complaint, which is not yet public, alleges that PBMs excluded lower-priced insulins from their formularies “in favor of high list price, highly rebated insulin products.”
The FTC describes a market in which PBMs, as they consolidated market power, began to extract higher rebates from drug makers. In turn, insulin manufacturers started raising their prices. That allowed PBMs to collect larger rebates, even as drug makers profited, according to the FTC.
The PBMs “engaged in unfair methods of competition and unfair acts or practices under Section 5 of the FTC Act by incentivizing manufacturers to inflate insulin list prices, restricting patients’ access to more affordable insulins on drug formularies and shifting the cost of high list price insulins to vulnerable patient populations,” said the FTC, in its statement.
Andrea Nelson, chief legal officer for The Cigna Group, said in a statement that the lawsuit “continues a troubling pattern from the FTC of unsubstantiated and ideologically-driven attacks on pharmacy benefit managers, following the FTC’s biased and misleading July 2024 report, which Express Scripts demanded the Commission retract earlier this week.”
Conduct ‘Raises Serious Concerns’
Drug makers are not off the hook, said the FTC. Mr. Rao said in a separate statement that “all drug manufacturers should be on notice that their participation in the type of conduct challenged here raises serious concerns and that the Bureau of Competition may recommend suing drug manufacturers in any future enforcement actions.”
The lawsuit comes on the heels of a report issued by the FTC in July, in which it accused the industry of driving small pharmacies out of business and of having extraordinary control over where Americans access prescription drugs and how much they pay.
The agency also noted in that report that some PBMs had still not responded to its requests for information, some 2 years after first asking.
Cigna’s Express Scripts sued the FTC on September 17, 2024, claiming that the report hurt the company’s reputation.
The report is “74 pages of unsupported innuendo leveled against Express Scripts and other PBMs under a false and defamatory headline and accompanied by a false and defamatory press release,” said the Cigna suit.
Cigna is seeking to have the report scrubbed from the FTC website and an injunction that would bar FTC Chairwoman Lina Khan from participating in any FTC business relating to Express Scripts.
Cigna’s Ms. Nelson accused the FTC of trying to “score political points” and said that forcing PBMs to include some drugs on its formularies “will drive drug prices higher in this country.”
CVS Health’s Caremark and UnitedHealth’s Optum also pushed back, as did the industry trade group, the Pharmaceutical Care Management Association.
“This action not only fails to accurately consider the role of the entire prescription drug supply chain, but disregards positive progress, supported by PBMs, in making insulin more affordable for patients,” the association said in a statement. “In contrast to the rhetoric, the current insulin market is actually working, with PBMs effectively leveraging greater competition to drive down insulin prices and doing their part to make insulin affordable for patients through innovative programs,” said the group.
“The FTC has missed the mark entirely,” David Whitrap, vice president for external affairs at CVS Health, said in a statement emailed to this news organization.
CVS Health members “on average pay less than $25, far below list prices and far below the Biden Administration’s $35 cap,” said Mr. Whitrap, who added that the PBM had protected customers from “pharma price-gouging.”
UnitedHealth’s Optum also said that it had reduced insulin prices for members to an average of less than $18 per month. “This baseless action demonstrates a profound misunderstanding of how drug pricing works,” wrote Elizabeth Hoff, a spokesperson for UnitedHealth’s Optum Rx, in an email to this news organization.
A version of this article appeared on Medscape.com.
The US Federal Trade Commission (FTC) has sued the nation’s three largest pharmacy benefit managers (PBMs), alleging that they have steered patients to buying higher-priced insulins so that they can reap more profits.
The three PBMs administer 80% of prescriptions in the United States, the FTC said in a statement announcing the action.
The agency filed an administrative complaint, which means its allegations will be tried in a formal hearing before an administrative law judge. It will not be heard in a criminal court.
The three PBMs “have extracted millions of dollars off the backs of patients who need life-saving medications,” Rahul Rao, deputy director of the FTC’s Bureau of Competition, said in the statement.
The FTC action is not the first taken by a government agency against PBMs. Ohio Attorney General Dave Yost sued Express Scripts and Prime Therapeutics in March 2023, alleging antitrust violations.
The FTC’s complaint, which is not yet public, alleges that PBMs excluded lower-priced insulins from their formularies “in favor of high list price, highly rebated insulin products.”
The FTC describes a market in which PBMs, as they consolidated market power, began to extract higher rebates from drug makers. In turn, insulin manufacturers started raising their prices. That allowed PBMs to collect larger rebates, even as drug makers profited, according to the FTC.
The PBMs “engaged in unfair methods of competition and unfair acts or practices under Section 5 of the FTC Act by incentivizing manufacturers to inflate insulin list prices, restricting patients’ access to more affordable insulins on drug formularies and shifting the cost of high list price insulins to vulnerable patient populations,” said the FTC, in its statement.
Andrea Nelson, chief legal officer for The Cigna Group, said in a statement that the lawsuit “continues a troubling pattern from the FTC of unsubstantiated and ideologically-driven attacks on pharmacy benefit managers, following the FTC’s biased and misleading July 2024 report, which Express Scripts demanded the Commission retract earlier this week.”
Conduct ‘Raises Serious Concerns’
Drug makers are not off the hook, said the FTC. Mr. Rao said in a separate statement that “all drug manufacturers should be on notice that their participation in the type of conduct challenged here raises serious concerns and that the Bureau of Competition may recommend suing drug manufacturers in any future enforcement actions.”
The lawsuit comes on the heels of a report issued by the FTC in July, in which it accused the industry of driving small pharmacies out of business and of having extraordinary control over where Americans access prescription drugs and how much they pay.
The agency also noted in that report that some PBMs had still not responded to its requests for information, some 2 years after first asking.
Cigna’s Express Scripts sued the FTC on September 17, 2024, claiming that the report hurt the company’s reputation.
The report is “74 pages of unsupported innuendo leveled against Express Scripts and other PBMs under a false and defamatory headline and accompanied by a false and defamatory press release,” said the Cigna suit.
Cigna is seeking to have the report scrubbed from the FTC website and an injunction that would bar FTC Chairwoman Lina Khan from participating in any FTC business relating to Express Scripts.
Cigna’s Ms. Nelson accused the FTC of trying to “score political points” and said that forcing PBMs to include some drugs on its formularies “will drive drug prices higher in this country.”
CVS Health’s Caremark and UnitedHealth’s Optum also pushed back, as did the industry trade group, the Pharmaceutical Care Management Association.
“This action not only fails to accurately consider the role of the entire prescription drug supply chain, but disregards positive progress, supported by PBMs, in making insulin more affordable for patients,” the association said in a statement. “In contrast to the rhetoric, the current insulin market is actually working, with PBMs effectively leveraging greater competition to drive down insulin prices and doing their part to make insulin affordable for patients through innovative programs,” said the group.
“The FTC has missed the mark entirely,” David Whitrap, vice president for external affairs at CVS Health, said in a statement emailed to this news organization.
CVS Health members “on average pay less than $25, far below list prices and far below the Biden Administration’s $35 cap,” said Mr. Whitrap, who added that the PBM had protected customers from “pharma price-gouging.”
UnitedHealth’s Optum also said that it had reduced insulin prices for members to an average of less than $18 per month. “This baseless action demonstrates a profound misunderstanding of how drug pricing works,” wrote Elizabeth Hoff, a spokesperson for UnitedHealth’s Optum Rx, in an email to this news organization.
A version of this article appeared on Medscape.com.
The US Federal Trade Commission (FTC) has sued the nation’s three largest pharmacy benefit managers (PBMs), alleging that they have steered patients to buying higher-priced insulins so that they can reap more profits.
The three PBMs administer 80% of prescriptions in the United States, the FTC said in a statement announcing the action.
The agency filed an administrative complaint, which means its allegations will be tried in a formal hearing before an administrative law judge. It will not be heard in a criminal court.
The three PBMs “have extracted millions of dollars off the backs of patients who need life-saving medications,” Rahul Rao, deputy director of the FTC’s Bureau of Competition, said in the statement.
The FTC action is not the first taken by a government agency against PBMs. Ohio Attorney General Dave Yost sued Express Scripts and Prime Therapeutics in March 2023, alleging antitrust violations.
The FTC’s complaint, which is not yet public, alleges that PBMs excluded lower-priced insulins from their formularies “in favor of high list price, highly rebated insulin products.”
The FTC describes a market in which PBMs, as they consolidated market power, began to extract higher rebates from drug makers. In turn, insulin manufacturers started raising their prices. That allowed PBMs to collect larger rebates, even as drug makers profited, according to the FTC.
The PBMs “engaged in unfair methods of competition and unfair acts or practices under Section 5 of the FTC Act by incentivizing manufacturers to inflate insulin list prices, restricting patients’ access to more affordable insulins on drug formularies and shifting the cost of high list price insulins to vulnerable patient populations,” said the FTC, in its statement.
Andrea Nelson, chief legal officer for The Cigna Group, said in a statement that the lawsuit “continues a troubling pattern from the FTC of unsubstantiated and ideologically-driven attacks on pharmacy benefit managers, following the FTC’s biased and misleading July 2024 report, which Express Scripts demanded the Commission retract earlier this week.”
Conduct ‘Raises Serious Concerns’
Drug makers are not off the hook, said the FTC. Mr. Rao said in a separate statement that “all drug manufacturers should be on notice that their participation in the type of conduct challenged here raises serious concerns and that the Bureau of Competition may recommend suing drug manufacturers in any future enforcement actions.”
The lawsuit comes on the heels of a report issued by the FTC in July, in which it accused the industry of driving small pharmacies out of business and of having extraordinary control over where Americans access prescription drugs and how much they pay.
The agency also noted in that report that some PBMs had still not responded to its requests for information, some 2 years after first asking.
Cigna’s Express Scripts sued the FTC on September 17, 2024, claiming that the report hurt the company’s reputation.
The report is “74 pages of unsupported innuendo leveled against Express Scripts and other PBMs under a false and defamatory headline and accompanied by a false and defamatory press release,” said the Cigna suit.
Cigna is seeking to have the report scrubbed from the FTC website and an injunction that would bar FTC Chairwoman Lina Khan from participating in any FTC business relating to Express Scripts.
Cigna’s Ms. Nelson accused the FTC of trying to “score political points” and said that forcing PBMs to include some drugs on its formularies “will drive drug prices higher in this country.”
CVS Health’s Caremark and UnitedHealth’s Optum also pushed back, as did the industry trade group, the Pharmaceutical Care Management Association.
“This action not only fails to accurately consider the role of the entire prescription drug supply chain, but disregards positive progress, supported by PBMs, in making insulin more affordable for patients,” the association said in a statement. “In contrast to the rhetoric, the current insulin market is actually working, with PBMs effectively leveraging greater competition to drive down insulin prices and doing their part to make insulin affordable for patients through innovative programs,” said the group.
“The FTC has missed the mark entirely,” David Whitrap, vice president for external affairs at CVS Health, said in a statement emailed to this news organization.
CVS Health members “on average pay less than $25, far below list prices and far below the Biden Administration’s $35 cap,” said Mr. Whitrap, who added that the PBM had protected customers from “pharma price-gouging.”
UnitedHealth’s Optum also said that it had reduced insulin prices for members to an average of less than $18 per month. “This baseless action demonstrates a profound misunderstanding of how drug pricing works,” wrote Elizabeth Hoff, a spokesperson for UnitedHealth’s Optum Rx, in an email to this news organization.
A version of this article appeared on Medscape.com.
Isatuximab Approved First-Line for Transplant-Ineligible MM
The new first-line indication joins two previous approvals of the CD38 antibody for later-line indications, one for relapsed disease with carfilzomib and dexamethasone, the other with pomalidomide and dexamethasone after at least two prior regimens that include lenalidomide and a proteasome inhibitor.
In addition to other MM indications, isatuximab’s anti-CD38 competitor on the US market, daratumumab (Darzalex — Johnson & Johnson), also carries a first-line indication for transplant-ineligible MM in combination with either lenalidomide and dexamethasone or bortezomib, melphalan, and prednisone.
Isatuximab’s new approval is based on the open-label IMROZ trial in 446 patients randomized 3:2 to either isatuximab or placebo on a background of bortezomib, lenalidomide, and dexamethasone.
At a median follow-up of 59.7 months, estimated progression-free survival (PFS) was 63.2% with isatuximab add-on vs 45.2% in the placebo arm. Median PFS was not reached in the isatuximab group but 54.3 months with placebo (hazard ratio, 0.60; 98.5% CI, 0.41-0.88; P < .001).
In a press release announcing the results, Sanofi said “IMROZ is the first global phase 3 study of an anti-CD38 monoclonal antibody” to show benefit in combination with bortezomib, lenalidomide, and dexamethasone, the current standard of care for transplant-ineligible MM.
Upper respiratory tract infections, diarrhea, fatigue, peripheral sensory neuropathy, pneumonia, musculoskeletal pain, cataract, constipation, peripheral edema, rash, infusion-related reaction, insomnia, and COVID-19 were the most common adverse events in the isatuximab arm of IMROZ, occurring in 20% or more of subjects.
Eleven percent of isatuximab patients died during treatment vs 5.5% in the placebo group, driven primarily by infections.
The recommended dose of isatuximab is 10 mg/kg every week for 4 weeks followed by every 2 weeks until disease progression or unacceptable toxicity.
The cost is approximately $843 for 5 mL of the 20 mg/mL intravenous solution, according to Drugs.com.
A version of this article appeared on Medscape.com.
The new first-line indication joins two previous approvals of the CD38 antibody for later-line indications, one for relapsed disease with carfilzomib and dexamethasone, the other with pomalidomide and dexamethasone after at least two prior regimens that include lenalidomide and a proteasome inhibitor.
In addition to other MM indications, isatuximab’s anti-CD38 competitor on the US market, daratumumab (Darzalex — Johnson & Johnson), also carries a first-line indication for transplant-ineligible MM in combination with either lenalidomide and dexamethasone or bortezomib, melphalan, and prednisone.
Isatuximab’s new approval is based on the open-label IMROZ trial in 446 patients randomized 3:2 to either isatuximab or placebo on a background of bortezomib, lenalidomide, and dexamethasone.
At a median follow-up of 59.7 months, estimated progression-free survival (PFS) was 63.2% with isatuximab add-on vs 45.2% in the placebo arm. Median PFS was not reached in the isatuximab group but 54.3 months with placebo (hazard ratio, 0.60; 98.5% CI, 0.41-0.88; P < .001).
In a press release announcing the results, Sanofi said “IMROZ is the first global phase 3 study of an anti-CD38 monoclonal antibody” to show benefit in combination with bortezomib, lenalidomide, and dexamethasone, the current standard of care for transplant-ineligible MM.
Upper respiratory tract infections, diarrhea, fatigue, peripheral sensory neuropathy, pneumonia, musculoskeletal pain, cataract, constipation, peripheral edema, rash, infusion-related reaction, insomnia, and COVID-19 were the most common adverse events in the isatuximab arm of IMROZ, occurring in 20% or more of subjects.
Eleven percent of isatuximab patients died during treatment vs 5.5% in the placebo group, driven primarily by infections.
The recommended dose of isatuximab is 10 mg/kg every week for 4 weeks followed by every 2 weeks until disease progression or unacceptable toxicity.
The cost is approximately $843 for 5 mL of the 20 mg/mL intravenous solution, according to Drugs.com.
A version of this article appeared on Medscape.com.
The new first-line indication joins two previous approvals of the CD38 antibody for later-line indications, one for relapsed disease with carfilzomib and dexamethasone, the other with pomalidomide and dexamethasone after at least two prior regimens that include lenalidomide and a proteasome inhibitor.
In addition to other MM indications, isatuximab’s anti-CD38 competitor on the US market, daratumumab (Darzalex — Johnson & Johnson), also carries a first-line indication for transplant-ineligible MM in combination with either lenalidomide and dexamethasone or bortezomib, melphalan, and prednisone.
Isatuximab’s new approval is based on the open-label IMROZ trial in 446 patients randomized 3:2 to either isatuximab or placebo on a background of bortezomib, lenalidomide, and dexamethasone.
At a median follow-up of 59.7 months, estimated progression-free survival (PFS) was 63.2% with isatuximab add-on vs 45.2% in the placebo arm. Median PFS was not reached in the isatuximab group but 54.3 months with placebo (hazard ratio, 0.60; 98.5% CI, 0.41-0.88; P < .001).
In a press release announcing the results, Sanofi said “IMROZ is the first global phase 3 study of an anti-CD38 monoclonal antibody” to show benefit in combination with bortezomib, lenalidomide, and dexamethasone, the current standard of care for transplant-ineligible MM.
Upper respiratory tract infections, diarrhea, fatigue, peripheral sensory neuropathy, pneumonia, musculoskeletal pain, cataract, constipation, peripheral edema, rash, infusion-related reaction, insomnia, and COVID-19 were the most common adverse events in the isatuximab arm of IMROZ, occurring in 20% or more of subjects.
Eleven percent of isatuximab patients died during treatment vs 5.5% in the placebo group, driven primarily by infections.
The recommended dose of isatuximab is 10 mg/kg every week for 4 weeks followed by every 2 weeks until disease progression or unacceptable toxicity.
The cost is approximately $843 for 5 mL of the 20 mg/mL intravenous solution, according to Drugs.com.
A version of this article appeared on Medscape.com.
Hypnosis May Offer Relief During Sharp Debridement of Skin Ulcers
TOPLINE:
Hypnosis reduces pain during sharp debridement of skin ulcers in patients with immune-mediated inflammatory diseases, with most patients reporting decreased pain awareness and lasting pain relief for 2-3 days after the procedure.
METHODOLOGY:
- Researchers reported their experience with the anecdotal use of hypnosis for pain management in debridement of skin ulcers in immune-mediated inflammatory diseases.
- They studied 16 participants (14 women; mean age, 56 years; 14 with systemic sclerosis or morphea) with recurrent skin ulcerations requiring sharp debridement, who presented to a wound care clinic at the Leeds Teaching Hospitals NHS Trust, Leeds, United Kingdom. The participants had negative experiences with pharmacologic pain management.
- Participants consented to hypnosis during debridement as the only mode of analgesia, conducted by the same hypnosis-trained, experienced healthcare professional in charge of their ulcer care.
- Ulcer pain scores were recorded using a numerical rating pain scale before and immediately after debridement, with a score of 0 indicating no pain and 10 indicating worst pain.
TAKEAWAY:
- Hypnosis reduced the median pre-debridement ulcer pain score from 8 (interquartile range [IQR], 7-10) to 0.5 (IQR, 0-2) immediately after the procedure.
- Of 16 participants, 14 reported being aware of the procedure but not feeling the pain, with only two participants experiencing a brief spike in pain.
- The other two participants reported experiencing reduced awareness and being pain-free during the procedure.
- Five participants reported a lasting decrease in pain perception for 2-3 days after the procedure.
IN PRACTICE:
“These preliminary data underscore the potential for the integration of hypnosis into the management of intervention-related pain in clinical care,” the authors wrote.
SOURCE:
The study was led by Begonya Alcacer-Pitarch, PhD, Leeds Institute of Rheumatic and Musculoskeletal Medicine, the University of Leeds, and Chapel Allerton Hospital in Leeds, United Kingdom. It was published as a correspondence on September 10, 2024, in The Lancet Rheumatology.
LIMITATIONS:
The small sample size may limit the generalizability of the findings. The methods used for data collection were not standardized, and the individuals included in the study may have introduced selection bias.
DISCLOSURES:
The study did not have a funding source. The authors declared no relevant conflicts of interest.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
TOPLINE:
Hypnosis reduces pain during sharp debridement of skin ulcers in patients with immune-mediated inflammatory diseases, with most patients reporting decreased pain awareness and lasting pain relief for 2-3 days after the procedure.
METHODOLOGY:
- Researchers reported their experience with the anecdotal use of hypnosis for pain management in debridement of skin ulcers in immune-mediated inflammatory diseases.
- They studied 16 participants (14 women; mean age, 56 years; 14 with systemic sclerosis or morphea) with recurrent skin ulcerations requiring sharp debridement, who presented to a wound care clinic at the Leeds Teaching Hospitals NHS Trust, Leeds, United Kingdom. The participants had negative experiences with pharmacologic pain management.
- Participants consented to hypnosis during debridement as the only mode of analgesia, conducted by the same hypnosis-trained, experienced healthcare professional in charge of their ulcer care.
- Ulcer pain scores were recorded using a numerical rating pain scale before and immediately after debridement, with a score of 0 indicating no pain and 10 indicating worst pain.
TAKEAWAY:
- Hypnosis reduced the median pre-debridement ulcer pain score from 8 (interquartile range [IQR], 7-10) to 0.5 (IQR, 0-2) immediately after the procedure.
- Of 16 participants, 14 reported being aware of the procedure but not feeling the pain, with only two participants experiencing a brief spike in pain.
- The other two participants reported experiencing reduced awareness and being pain-free during the procedure.
- Five participants reported a lasting decrease in pain perception for 2-3 days after the procedure.
IN PRACTICE:
“These preliminary data underscore the potential for the integration of hypnosis into the management of intervention-related pain in clinical care,” the authors wrote.
SOURCE:
The study was led by Begonya Alcacer-Pitarch, PhD, Leeds Institute of Rheumatic and Musculoskeletal Medicine, the University of Leeds, and Chapel Allerton Hospital in Leeds, United Kingdom. It was published as a correspondence on September 10, 2024, in The Lancet Rheumatology.
LIMITATIONS:
The small sample size may limit the generalizability of the findings. The methods used for data collection were not standardized, and the individuals included in the study may have introduced selection bias.
DISCLOSURES:
The study did not have a funding source. The authors declared no relevant conflicts of interest.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
TOPLINE:
Hypnosis reduces pain during sharp debridement of skin ulcers in patients with immune-mediated inflammatory diseases, with most patients reporting decreased pain awareness and lasting pain relief for 2-3 days after the procedure.
METHODOLOGY:
- Researchers reported their experience with the anecdotal use of hypnosis for pain management in debridement of skin ulcers in immune-mediated inflammatory diseases.
- They studied 16 participants (14 women; mean age, 56 years; 14 with systemic sclerosis or morphea) with recurrent skin ulcerations requiring sharp debridement, who presented to a wound care clinic at the Leeds Teaching Hospitals NHS Trust, Leeds, United Kingdom. The participants had negative experiences with pharmacologic pain management.
- Participants consented to hypnosis during debridement as the only mode of analgesia, conducted by the same hypnosis-trained, experienced healthcare professional in charge of their ulcer care.
- Ulcer pain scores were recorded using a numerical rating pain scale before and immediately after debridement, with a score of 0 indicating no pain and 10 indicating worst pain.
TAKEAWAY:
- Hypnosis reduced the median pre-debridement ulcer pain score from 8 (interquartile range [IQR], 7-10) to 0.5 (IQR, 0-2) immediately after the procedure.
- Of 16 participants, 14 reported being aware of the procedure but not feeling the pain, with only two participants experiencing a brief spike in pain.
- The other two participants reported experiencing reduced awareness and being pain-free during the procedure.
- Five participants reported a lasting decrease in pain perception for 2-3 days after the procedure.
IN PRACTICE:
“These preliminary data underscore the potential for the integration of hypnosis into the management of intervention-related pain in clinical care,” the authors wrote.
SOURCE:
The study was led by Begonya Alcacer-Pitarch, PhD, Leeds Institute of Rheumatic and Musculoskeletal Medicine, the University of Leeds, and Chapel Allerton Hospital in Leeds, United Kingdom. It was published as a correspondence on September 10, 2024, in The Lancet Rheumatology.
LIMITATIONS:
The small sample size may limit the generalizability of the findings. The methods used for data collection were not standardized, and the individuals included in the study may have introduced selection bias.
DISCLOSURES:
The study did not have a funding source. The authors declared no relevant conflicts of interest.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
Treating Family: Ethicist Discusses Whether It’s Appropriate
This transcript has been edited for clarity.
There’s a very interesting story in the medical press. A few years ago, a plastic surgeon named Edmond Cabbabe was preparing to do a follow-up cosmetic procedure on his wife at Mercy Hospital South, which is a big hospital in the St. Louis, Missouri, area.
He put her on the operating schedule, and he had done that when he had performed the original operation on her. On the day of the surgery, he got a call from the hospital saying the procedure was canceled. They said that the hospital’s policy, maybe a new one, would not allow doctors to operate on family members.
This physician was a past president of the Missouri State Medical Association. I think he was also on the board or president of the American Medical Association (AMA) Foundation. This was a physician not only in a skilled area where he felt confident he could take care of his wife, but also someone who was prominent in medical politics and medical policy.
The AMA forever has had a policy that says don’t treat relatives. This physician basically said, I think that policy is too restrictive, too cautious, and it doesn’t make much sense to continue to say that you can’t treat family and friends.
By implication, he was saying, I know exactly what I’m doing in my field and I know exactly what I’m doing with her procedure. I should have a right to perform it. I think I do a great job and I’d be best for her.
If you look at medical boards, every once in a while in some state, someone is brought up on a charge of doing different things with family members and saying that they’re going to get censured. They don’t usually lose their license, but they get a reprimand or get told that is just not ethical to do.
I think, in the long run, the policy about not treating your family and friends makes sense. The problem is, as is well known from the social sciences and psychology, people get biased when they deal with those they care about, love, and hold close to them.
It’s hard for the doctor to be objective when dealing with people that they really like or love. It’s also difficult for patients because they may not want to bring up something or they are uncomfortable talking with a doctor who’s a family member or close friend. They may not want to complain. They may be a little bit embarrassed about things. It just adds an emotional edge, I think, that’s difficult.
All that said, do I know doctors who regularly prescribe, say, an ointment for something that’s itchy or some kind of a pill when allergy season breaks out? I do. Do I think they’re acting in a horribly unethical manner? I don’t.
You need some judgment here. There are absolutely minor things where objectivity, fear, and anxiety are not in play. You’re going to be able to prescribe the routine thing for the routine itch without worrying too much about whether it’s a stranger, a friend, or your daughter.
What sorts of things am I really talking about when I say that minor variability ought to be allowed? It’s one thing when someone has poison ivy and they’re going to need some kind of standard medicine to treat it. A very different area that’s much more dangerous, and one I would avoid, is in the mental health field, and for that matter, the pain field.
It’s tempting to say: “Oh, my relative is just having a bad time. I’ll give her a little bit of antidepressant medicine,” or “They seem to be having pain after an operation or something, and I’m going to give them a little bit of pain meds just to get them through.”
Those areas are flying red flags. It’s easy to abuse and easy for someone to become a user and manipulate a friend or a doctor who’s a relative into getting things that another doctor wouldn’t be giving. I think that’s the space where you’ve got to exercise extreme caution.
Time and again, when those people get called up in front of the boards for treating relatives, it’s in those spaces of mental health, anxiety, and pain control. Again, when you know that there’s a likelihood of abuse, I think that’s the place where the line has to hold. Don’t treat the relative. Don’t treat the friend.
At the end of the day, I wouldn’t change the AMA policy. I think we should keep it in place and morally try to discourage doctors from caring for those they’re close to or they have emotional ties to.
At the same time, as with all ethical situations, there has to be a little bit of wiggle room for those super-minor cases where it just makes sense to say: “You don’t have to go find somebody else to do this. I can prescribe this ointment or this minor thing for you. No one’s objectivity is going to be soured, and you’re not going to feel in any way at risk because I’m going to prescribe this for you.”
Common sense ought to prevail. The default position is don’t do it; however, maybe with a tiny bit of space for what’s minor, what’s routine, and what really does just save people some inconvenience, there I might just give a little.
Dr. Caplan, Director, Division of Medical Ethics, New York University Langone Medical Center, New York City, has disclosed relationships with Johnson & Johnson’s Panel for Compassionate Drug Use and Medscape.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
There’s a very interesting story in the medical press. A few years ago, a plastic surgeon named Edmond Cabbabe was preparing to do a follow-up cosmetic procedure on his wife at Mercy Hospital South, which is a big hospital in the St. Louis, Missouri, area.
He put her on the operating schedule, and he had done that when he had performed the original operation on her. On the day of the surgery, he got a call from the hospital saying the procedure was canceled. They said that the hospital’s policy, maybe a new one, would not allow doctors to operate on family members.
This physician was a past president of the Missouri State Medical Association. I think he was also on the board or president of the American Medical Association (AMA) Foundation. This was a physician not only in a skilled area where he felt confident he could take care of his wife, but also someone who was prominent in medical politics and medical policy.
The AMA forever has had a policy that says don’t treat relatives. This physician basically said, I think that policy is too restrictive, too cautious, and it doesn’t make much sense to continue to say that you can’t treat family and friends.
By implication, he was saying, I know exactly what I’m doing in my field and I know exactly what I’m doing with her procedure. I should have a right to perform it. I think I do a great job and I’d be best for her.
If you look at medical boards, every once in a while in some state, someone is brought up on a charge of doing different things with family members and saying that they’re going to get censured. They don’t usually lose their license, but they get a reprimand or get told that is just not ethical to do.
I think, in the long run, the policy about not treating your family and friends makes sense. The problem is, as is well known from the social sciences and psychology, people get biased when they deal with those they care about, love, and hold close to them.
It’s hard for the doctor to be objective when dealing with people that they really like or love. It’s also difficult for patients because they may not want to bring up something or they are uncomfortable talking with a doctor who’s a family member or close friend. They may not want to complain. They may be a little bit embarrassed about things. It just adds an emotional edge, I think, that’s difficult.
All that said, do I know doctors who regularly prescribe, say, an ointment for something that’s itchy or some kind of a pill when allergy season breaks out? I do. Do I think they’re acting in a horribly unethical manner? I don’t.
You need some judgment here. There are absolutely minor things where objectivity, fear, and anxiety are not in play. You’re going to be able to prescribe the routine thing for the routine itch without worrying too much about whether it’s a stranger, a friend, or your daughter.
What sorts of things am I really talking about when I say that minor variability ought to be allowed? It’s one thing when someone has poison ivy and they’re going to need some kind of standard medicine to treat it. A very different area that’s much more dangerous, and one I would avoid, is in the mental health field, and for that matter, the pain field.
It’s tempting to say: “Oh, my relative is just having a bad time. I’ll give her a little bit of antidepressant medicine,” or “They seem to be having pain after an operation or something, and I’m going to give them a little bit of pain meds just to get them through.”
Those areas are flying red flags. It’s easy to abuse and easy for someone to become a user and manipulate a friend or a doctor who’s a relative into getting things that another doctor wouldn’t be giving. I think that’s the space where you’ve got to exercise extreme caution.
Time and again, when those people get called up in front of the boards for treating relatives, it’s in those spaces of mental health, anxiety, and pain control. Again, when you know that there’s a likelihood of abuse, I think that’s the place where the line has to hold. Don’t treat the relative. Don’t treat the friend.
At the end of the day, I wouldn’t change the AMA policy. I think we should keep it in place and morally try to discourage doctors from caring for those they’re close to or they have emotional ties to.
At the same time, as with all ethical situations, there has to be a little bit of wiggle room for those super-minor cases where it just makes sense to say: “You don’t have to go find somebody else to do this. I can prescribe this ointment or this minor thing for you. No one’s objectivity is going to be soured, and you’re not going to feel in any way at risk because I’m going to prescribe this for you.”
Common sense ought to prevail. The default position is don’t do it; however, maybe with a tiny bit of space for what’s minor, what’s routine, and what really does just save people some inconvenience, there I might just give a little.
Dr. Caplan, Director, Division of Medical Ethics, New York University Langone Medical Center, New York City, has disclosed relationships with Johnson & Johnson’s Panel for Compassionate Drug Use and Medscape.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
There’s a very interesting story in the medical press. A few years ago, a plastic surgeon named Edmond Cabbabe was preparing to do a follow-up cosmetic procedure on his wife at Mercy Hospital South, which is a big hospital in the St. Louis, Missouri, area.
He put her on the operating schedule, and he had done that when he had performed the original operation on her. On the day of the surgery, he got a call from the hospital saying the procedure was canceled. They said that the hospital’s policy, maybe a new one, would not allow doctors to operate on family members.
This physician was a past president of the Missouri State Medical Association. I think he was also on the board or president of the American Medical Association (AMA) Foundation. This was a physician not only in a skilled area where he felt confident he could take care of his wife, but also someone who was prominent in medical politics and medical policy.
The AMA forever has had a policy that says don’t treat relatives. This physician basically said, I think that policy is too restrictive, too cautious, and it doesn’t make much sense to continue to say that you can’t treat family and friends.
By implication, he was saying, I know exactly what I’m doing in my field and I know exactly what I’m doing with her procedure. I should have a right to perform it. I think I do a great job and I’d be best for her.
If you look at medical boards, every once in a while in some state, someone is brought up on a charge of doing different things with family members and saying that they’re going to get censured. They don’t usually lose their license, but they get a reprimand or get told that is just not ethical to do.
I think, in the long run, the policy about not treating your family and friends makes sense. The problem is, as is well known from the social sciences and psychology, people get biased when they deal with those they care about, love, and hold close to them.
It’s hard for the doctor to be objective when dealing with people that they really like or love. It’s also difficult for patients because they may not want to bring up something or they are uncomfortable talking with a doctor who’s a family member or close friend. They may not want to complain. They may be a little bit embarrassed about things. It just adds an emotional edge, I think, that’s difficult.
All that said, do I know doctors who regularly prescribe, say, an ointment for something that’s itchy or some kind of a pill when allergy season breaks out? I do. Do I think they’re acting in a horribly unethical manner? I don’t.
You need some judgment here. There are absolutely minor things where objectivity, fear, and anxiety are not in play. You’re going to be able to prescribe the routine thing for the routine itch without worrying too much about whether it’s a stranger, a friend, or your daughter.
What sorts of things am I really talking about when I say that minor variability ought to be allowed? It’s one thing when someone has poison ivy and they’re going to need some kind of standard medicine to treat it. A very different area that’s much more dangerous, and one I would avoid, is in the mental health field, and for that matter, the pain field.
It’s tempting to say: “Oh, my relative is just having a bad time. I’ll give her a little bit of antidepressant medicine,” or “They seem to be having pain after an operation or something, and I’m going to give them a little bit of pain meds just to get them through.”
Those areas are flying red flags. It’s easy to abuse and easy for someone to become a user and manipulate a friend or a doctor who’s a relative into getting things that another doctor wouldn’t be giving. I think that’s the space where you’ve got to exercise extreme caution.
Time and again, when those people get called up in front of the boards for treating relatives, it’s in those spaces of mental health, anxiety, and pain control. Again, when you know that there’s a likelihood of abuse, I think that’s the place where the line has to hold. Don’t treat the relative. Don’t treat the friend.
At the end of the day, I wouldn’t change the AMA policy. I think we should keep it in place and morally try to discourage doctors from caring for those they’re close to or they have emotional ties to.
At the same time, as with all ethical situations, there has to be a little bit of wiggle room for those super-minor cases where it just makes sense to say: “You don’t have to go find somebody else to do this. I can prescribe this ointment or this minor thing for you. No one’s objectivity is going to be soured, and you’re not going to feel in any way at risk because I’m going to prescribe this for you.”
Common sense ought to prevail. The default position is don’t do it; however, maybe with a tiny bit of space for what’s minor, what’s routine, and what really does just save people some inconvenience, there I might just give a little.
Dr. Caplan, Director, Division of Medical Ethics, New York University Langone Medical Center, New York City, has disclosed relationships with Johnson & Johnson’s Panel for Compassionate Drug Use and Medscape.
A version of this article first appeared on Medscape.com.
Reflectance Confocal Microscopy as a Diagnostic Aid in Allergic Contact Dermatitis to Mango Sap
The mango tree (Mangifera indica) produces nutrient-dense fruit—known colloquially as the “king of fruits”—that is widely consumed across the world. Native to southern Asia, the mango tree is a member of the Anacardiaceae family, a large family of flowering, fruit-bearing plants.1 Many members of the Anacardiaceae family, which includes poison ivy and poison oak, are known to produce urushiol, a skin irritant associated with allergic contact dermatitis (ACD).2 Interestingly, despite its widespread consumption and categorization in the Anacardiaceae family, allergic reactions to mango are comparatively rare; they occur as either immediate type I hypersensitivity reactions manifesting with rapid-onset symptoms such as urticaria, wheezing, and angioedema, or delayed type IV hypersensitivity reactions manifesting as ACD.3 Although exposure to components of the mango tree has been most characteristically linked to type IV hypersensitivity reactions, there remain fewer than 40 reported cases of mango-induced ACD since it was first described in 1939.4
Evaluation of ACD most commonly includes a thorough clinical assessment with diagnostic support from patch testing and histopathologic review following skin biopsy. In recent years, reflectance confocal microscopy (RCM) has shown promising potential to join the repertoire of diagnostic tools for ACD by enabling dynamic and high-resolution imaging of contact dermatitis in vivo.5-10 Reflectance confocal microscopy is a noninvasive optical imaging technique that uses a low-energy diode laser to penetrate the layers of the skin. The resulting reflected light generates images that facilitate visualization of cutaneous structures to the depth of the papillary dermis.11 While it is most commonly used in skin cancer diagnostics, preliminary studies also have shown an emerging role for RCM in the evaluation of eczematous and inflammatory skin disease, including contact dermatitis.5-10 Herein, we present a unique case of mango sap–induced ACD imaged and diagnosed in real time via RCM.
Case Report
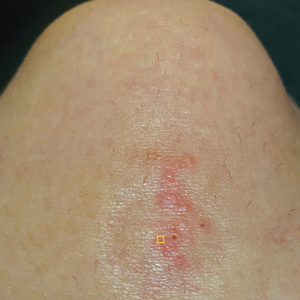
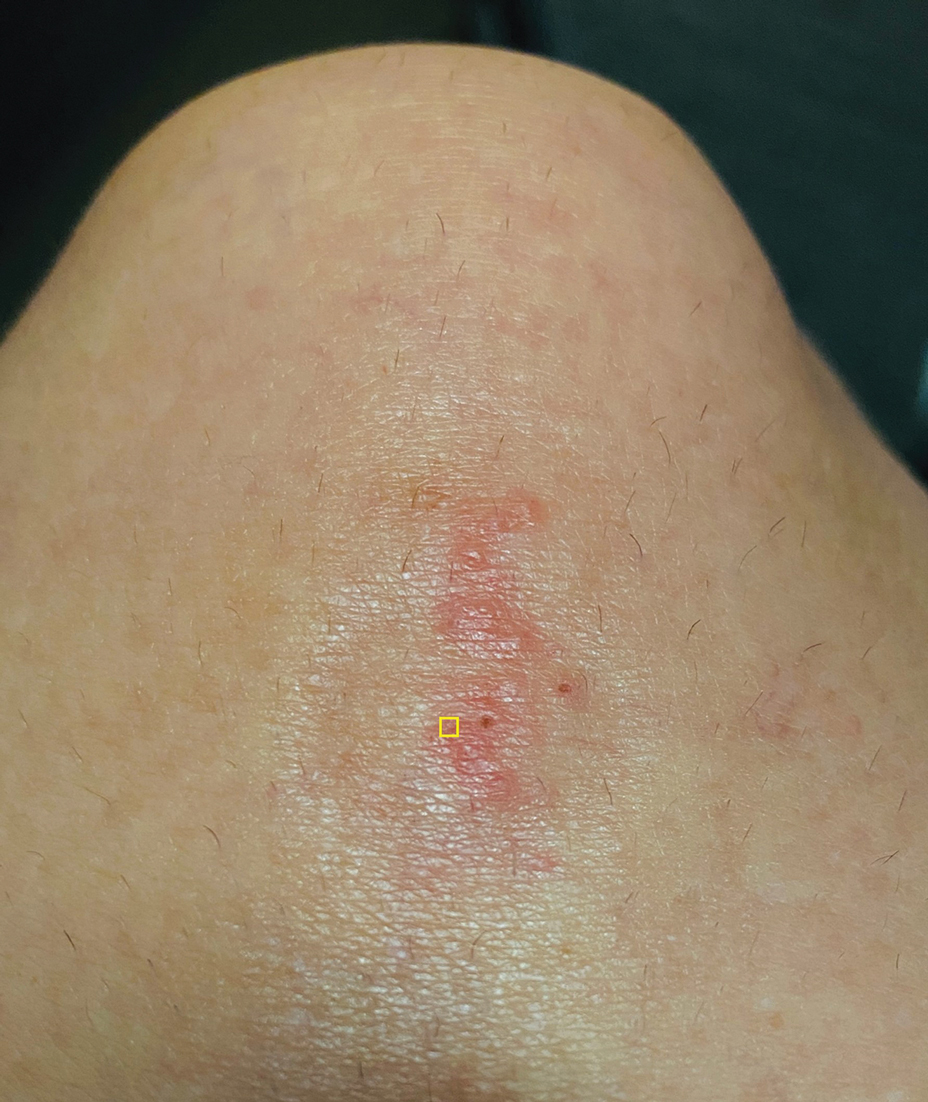
A 39-year-old woman presented to our clinic with a pruritic vesicular eruption on the right leg of 2 weeks’ duration that initially had developed within 7 days of exposure to mango tree sap (Figure 1). The patient reported having experienced similar pruritic eruptions in the past following contact with mango sap while eating mangos but denied any history of reactions from ingestion of the fruit. She also reported a history of robust reactions to poison ivy; however, a timeline specifying the order of first exposure to these irritants was unknown. She denied any personal or family history of atopic conditions.
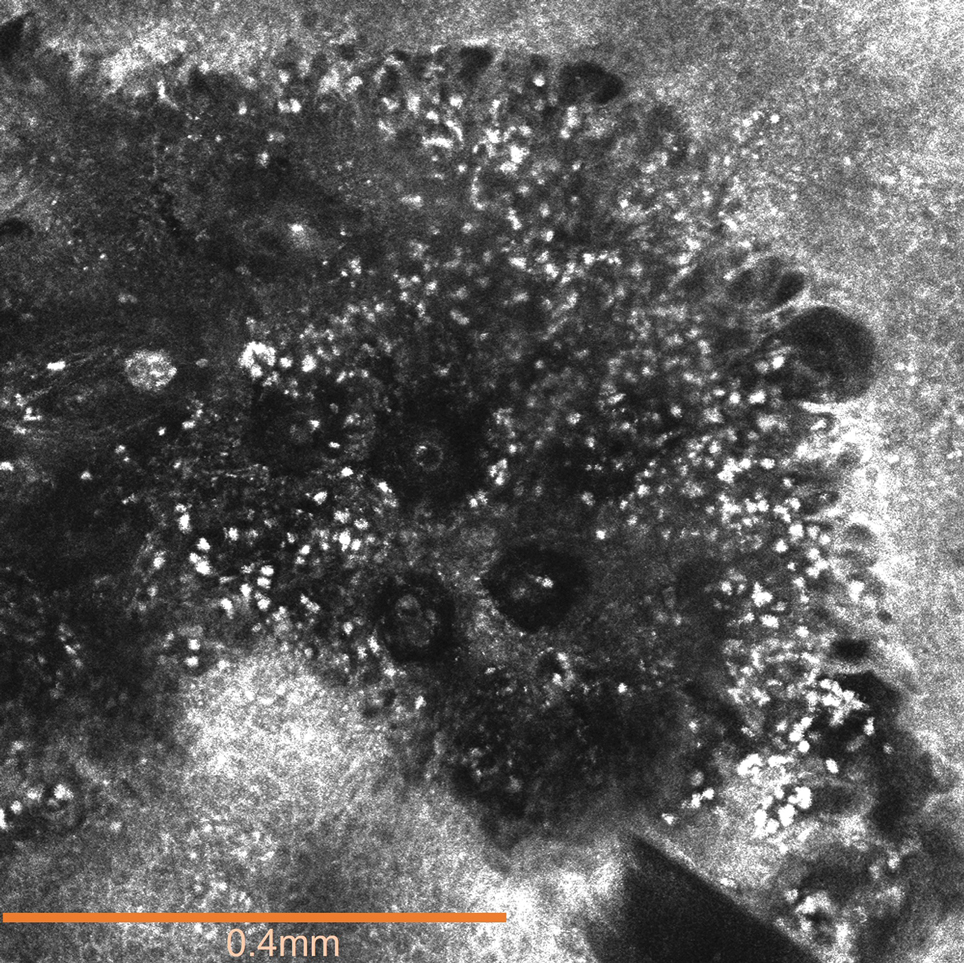
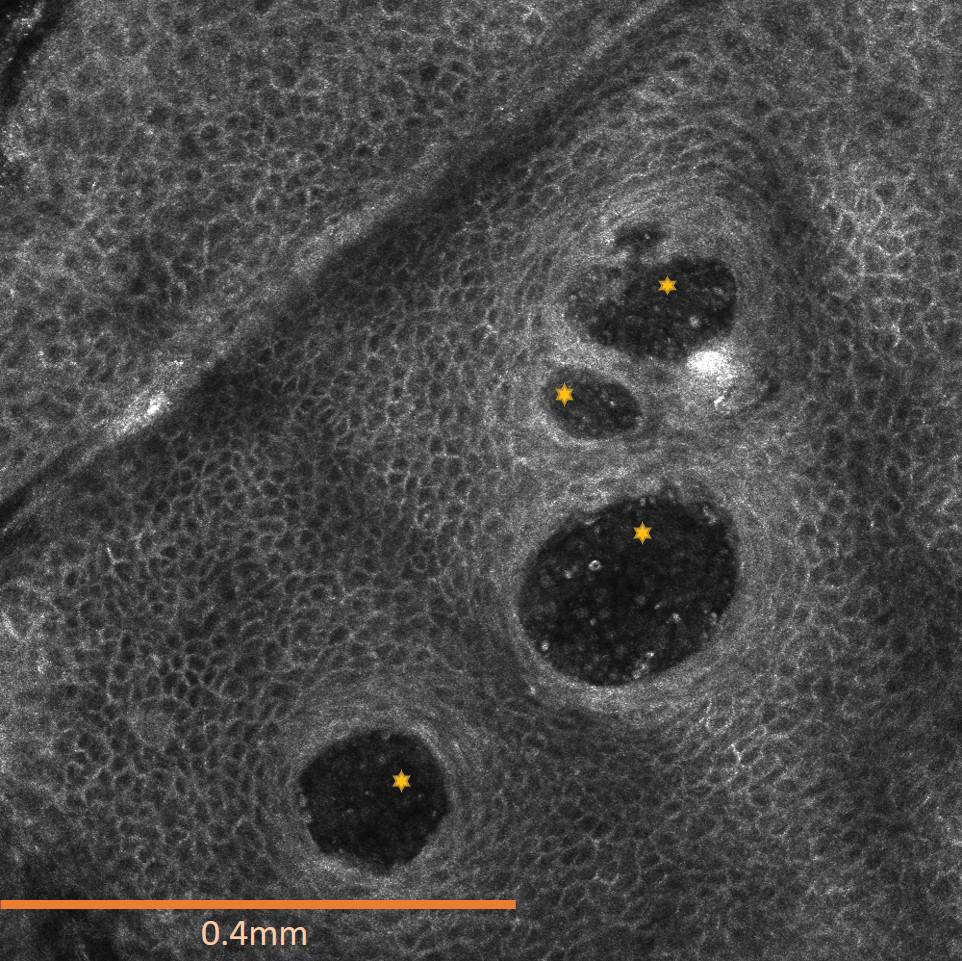
The affected skin was imaged in real time during clinic using RCM, which showed an inflammatory infiltrate represented by dark spongiotic vesicles containing bright cells (Figure 2). Additional RCM imaging at the level of the stratum spinosum showed dark spongiotic areas with bright inflammatory cells infiltrating the vesicles, which were surrounded by normal skin showing a typical epidermal honeycomb pattern (Figure 3). These findings were diagnostic of ACD secondary to exposure to mango sap. The patient was advised to apply clobetasol cream 0.05% to the affected area. Notable improvement of the rash was noted within 10 days of treatment.
Comment
Exposure to the mango tree and its fruit is a rare cause of ACD, with few reported cases in the literature. The majority of known instances have occurred in non–mango-cultivating countries, largely the United States, although cases also have been reported in Canada, Australia, France, Japan, and Thailand.3,12 Mango-induced contact allergy follows a roughly equal distribution between males and females and most often occurs in young adults during the third and fourth decades of life.4,12-21 Importantly, delayed-type hypersensitivity reactions to mango can manifest as either localized or systemic ACD. Localized ACD can be induced via direct contact with the mango tree and its components or ingestion of the fruit.3,12,22 Conversely, systemic ACD is primarily stimulated by ingestion of the fruit. In our case, the patient had no history of allergy following mango ingestion, and her ACD was prompted by isolated contact with mango sap. The time from exposure to symptom onset of known instances of mango ACD varies widely, ranging from less than 24 hours to as long as 9 days.3,12 Diagnosis of mango-induced ACD largely is guided by clinical findings. Presenting symptoms often include an eczematous, vesicular, pruritic rash on affected areas of the skin, frequently the head, neck, and extremities. Patients also commonly present with linear papulovesicular lesions and periorbital or perioral edema.
The suspected allergens responsible for mango-induced ACD are derived from resorcinol—specifically heptadecadienyl resorcinol, heptadecenyl resorcinol, and pentadecyl resorcinol, which are collectively known as mango allergens.23 These allergens can be found within the pulp and skin of the mango fruit as well as in the bark and leaves of the mango tree, which may explain observed allergic reactions to components of both the mango fruit and tree.12 Similar to these resorcinol derivatives, the urushiol resin found in poison ivy and poison oak is a catechol derivative.2 Importantly, both resorcinols and catechols are isomers of the same aromatic phenol—dihydroxybenzene. Because of these similarities, it is thought that the allergens in mangos may cross-react with urushiol in poison ivy or poison oak.23 Alongside their shared categorization in the Anacardiaceae family, it is hypothesized that this cross-reactivity underlies the sensitization that has been noted between mango and poison ivy or poison oak exposure.12,23,24 Thus, ACD often can occur on initial contact with the mango tree or its components, as a prior exposure to poison ivy or poison oak may serve as the inciting factor for hypersensitization. The majority of reported cases in the literature also occurred in countries where exposure to poison ivy and poison oak are common, further supporting the notion that these compounds may provide a sensitizing trigger for a future mango contact allergy.12
A detailed clinical history combined with adjunctive diagnostic support from patch testing and histopathology of biopsied skin lesions classically are used in the diagnosis of mango-induced ACD. Due to its ability to provide quick and noninvasive in vivo imaging of cutaneous lesions, RCM's applications have expanded to include evaluation of inflammatory skin diseases such as contact dermatitis. Many features of contact dermatitis identified via RCM are common between ACD and irritant contact dermatitis (ICD) and include disruption of the stratum corneum, parakeratosis, vesiculation, spongiosis, and exocytosis.6,10,25 Studies also have described features shown via RCM that are unique to ACD, including vasodilation and intercellular edema, compared to more distinct targetoid keratinocytes and detached corneocytes seen in ICD.6,10,25 Studies by Astner et al5,6 demonstrated a wide range of sensitivity from 52% to 96% and a high specificity of RCM greater than 95% for many of the aforementioned features of contact dermatitis, including disruption of the stratum corneum, parakeratosis, spongiosis, and exocytosis. Additional studies have further strengthened these findings, demonstrating sensitivity and specificity values of 83% and 92% for contact dermatitis under RCM, respectively.26 Importantly, given the similarities and potentially large overlap of features between ACD and ICD identified via RCM as well as findings seen on physical examination and histopathology, an emphasis on clinical correlation is essential when differentiating between these 2 variants of contact dermatitis. Thus, taken in consideration with clinical contexts, RCM has shown potent diagnostic accuracy and great potential to support the evaluation of ACD alongside patch testing and histopathology.
Final Thoughts
Contact allergy to the mango tree and its components is uncommon. We report a unique case of mango sap–induced ACD evaluated and diagnosed via dynamic visualization under RCM. As a noninvasive and reproducible imaging technique with resolutions comparable to histopathologic analysis, RCM is a promising tool that can be used to support the diagnostic evaluation of ACD.
- Shah KA, Patel MB, Patel RJ, et al. Mangifera indica (mango). Pharmacogn Rev. 2010;4:42-48.
- Lofgran T, Mahabal GD. Toxicodendron toxicity. StatPearls [Internet]. Updated May 16, 2023. Accessed September 19, 2024. https://www.ncbi.nlm.nih.gov/books/NBK557866
- Sareen R, Shah A. Hypersensitivity manifestations to the fruit mango. Asia Pac Allergy. 2011;1:43-49.
- Zakon SJ. Contact dermatitis due to mango. JAMA. 1939;113:1808.
- Astner S, Gonzalez E, Cheung A, et al. Pilot study on the sensitivity and specificity of in vivo reflectance confocal microscopy in the diagnosis of allergic contact dermatitis. J Am Acad Dermatol. 2005;53:986-992.
- Astner S, Gonzalez S, Gonzalez E. Noninvasive evaluation of allergic and irritant contact dermatitis by in vivo reflectance confocal microscopy. Dermatitis. 2006;17:182-191.
- Csuka EA, Ward SC, Ekelem C, et al. Reflectance confocal microscopy, optical coherence tomography, and multiphoton microscopy in inflammatory skin disease diagnosis. Lasers Surg Med. 2021;53:776-797.
- Guichard A, Fanian F, Girardin P, et al. Allergic patch test and contact dermatitis by in vivo reflectance confocal microscopy [in French]. Ann Dermatol Venereol. 2014;141:805-807.
- Sakanashi EN, Matsumura M, Kikuchi K, et al. A comparative study of allergic contact dermatitis by patch test versus reflectance confocal laser microscopy, with nickel and cobalt. Eur J Dermatol. 2010;20:705-711.
- Swindells K, Burnett N, Rius-Diaz F, et al. Reflectance confocal microscopy may differentiate acute allergic and irritant contact dermatitis in vivo. J Am Acad Dermatol. 2004;50:220-228.
- Shahriari N, Grant-Kels JM, Rabinovitz H, et al. Reflectance confocal microscopy: principles, basic terminology, clinical indications, limitations, and practical considerations. J Am Acad Dermatol. 2021;84:1-14.
- Berghea EC, Craiu M, Ali S, et al. Contact allergy induced by mango (Mangifera indica): a relevant topic? Medicina (Kaunas). 2021;57:1240.
- O’Hern K, Zhang F, Zug KA, et al. “Mango slice” dermatitis: pediatric allergic contact dermatitis to mango pulp and skin. Dermatitis. 2022;33:E46-E47.
- Raison-Peyron N, Aljaber F, Al Ali OA, et al. Mango dermatitis: an unusual cause of eyelid dermatitis in France. Contact Dermatitis. 2021;85:599-600.
- Alipour Tehrany Y, Coulombe J. Mango allergic contact dermatitis. Contact Dermatitis. 2021;85:241-242.
- Yoo MJ, Carius BM. Mango dermatitis after urushiol sensitization. Clin Pract Cases Emerg Med. 2019;3:361-363.
- Miyazawa H, Nishie W, Hata H, et al. A severe case of mango dermatitis. J Eur Acad Dermatol Venereol. 2018;32:E160-E161.
- Trehan I, Meuli GJ. Mango contact allergy. J Travel Med. 2010;17:284.
- Wiwanitkit V. Mango dermatitis. Indian J Dermatol. 2008;53:158.
- Weinstein S, Bassiri-Tehrani S, Cohen DE. Allergic contact dermatitis to mango flesh. Int J Dermatol. 2004;43:195-196.
- Calvert ML, Robertson I, Samaratunga H. Mango dermatitis: allergic contact dermatitis to Mangifera indica. Australas J Dermatol. 1996;37:59-60.
- Thoo CH, Freeman S. Hypersensitivity reaction to the ingestion of mango flesh. Australas J Dermatol. 2008;49:116-119.
- Oka K, Saito F, Yasuhara T, et al. A study of cross-reactions between mango contact allergens and urushiol. Contact Dermatitis. 2004;51:292-296.
- Keil H, Wasserman D, Dawson CR. Mango dermatitis and its relationship to poison ivy hypersensitivity. Ann Allergy. 1946;4: 268-281.
- Maarouf M, Costello CM, Gonzalez S, et al. In vivo reflectance confocal microscopy: emerging role in noninvasive diagnosis and monitoring of eczematous dermatoses. Actas Dermosifiliogr (Engl Ed). 2019;110:626-636.
- Koller S, Gerger A, Ahlgrimm-Siess V, et al. In vivo reflectance confocal microscopy of erythematosquamous skin diseases. Exp Dermatol. 2009;18:536-540.
The mango tree (Mangifera indica) produces nutrient-dense fruit—known colloquially as the “king of fruits”—that is widely consumed across the world. Native to southern Asia, the mango tree is a member of the Anacardiaceae family, a large family of flowering, fruit-bearing plants.1 Many members of the Anacardiaceae family, which includes poison ivy and poison oak, are known to produce urushiol, a skin irritant associated with allergic contact dermatitis (ACD).2 Interestingly, despite its widespread consumption and categorization in the Anacardiaceae family, allergic reactions to mango are comparatively rare; they occur as either immediate type I hypersensitivity reactions manifesting with rapid-onset symptoms such as urticaria, wheezing, and angioedema, or delayed type IV hypersensitivity reactions manifesting as ACD.3 Although exposure to components of the mango tree has been most characteristically linked to type IV hypersensitivity reactions, there remain fewer than 40 reported cases of mango-induced ACD since it was first described in 1939.4
Evaluation of ACD most commonly includes a thorough clinical assessment with diagnostic support from patch testing and histopathologic review following skin biopsy. In recent years, reflectance confocal microscopy (RCM) has shown promising potential to join the repertoire of diagnostic tools for ACD by enabling dynamic and high-resolution imaging of contact dermatitis in vivo.5-10 Reflectance confocal microscopy is a noninvasive optical imaging technique that uses a low-energy diode laser to penetrate the layers of the skin. The resulting reflected light generates images that facilitate visualization of cutaneous structures to the depth of the papillary dermis.11 While it is most commonly used in skin cancer diagnostics, preliminary studies also have shown an emerging role for RCM in the evaluation of eczematous and inflammatory skin disease, including contact dermatitis.5-10 Herein, we present a unique case of mango sap–induced ACD imaged and diagnosed in real time via RCM.
Case Report
A 39-year-old woman presented to our clinic with a pruritic vesicular eruption on the right leg of 2 weeks’ duration that initially had developed within 7 days of exposure to mango tree sap (Figure 1). The patient reported having experienced similar pruritic eruptions in the past following contact with mango sap while eating mangos but denied any history of reactions from ingestion of the fruit. She also reported a history of robust reactions to poison ivy; however, a timeline specifying the order of first exposure to these irritants was unknown. She denied any personal or family history of atopic conditions.

The affected skin was imaged in real time during clinic using RCM, which showed an inflammatory infiltrate represented by dark spongiotic vesicles containing bright cells (Figure 2). Additional RCM imaging at the level of the stratum spinosum showed dark spongiotic areas with bright inflammatory cells infiltrating the vesicles, which were surrounded by normal skin showing a typical epidermal honeycomb pattern (Figure 3). These findings were diagnostic of ACD secondary to exposure to mango sap. The patient was advised to apply clobetasol cream 0.05% to the affected area. Notable improvement of the rash was noted within 10 days of treatment.


Comment
Exposure to the mango tree and its fruit is a rare cause of ACD, with few reported cases in the literature. The majority of known instances have occurred in non–mango-cultivating countries, largely the United States, although cases also have been reported in Canada, Australia, France, Japan, and Thailand.3,12 Mango-induced contact allergy follows a roughly equal distribution between males and females and most often occurs in young adults during the third and fourth decades of life.4,12-21 Importantly, delayed-type hypersensitivity reactions to mango can manifest as either localized or systemic ACD. Localized ACD can be induced via direct contact with the mango tree and its components or ingestion of the fruit.3,12,22 Conversely, systemic ACD is primarily stimulated by ingestion of the fruit. In our case, the patient had no history of allergy following mango ingestion, and her ACD was prompted by isolated contact with mango sap. The time from exposure to symptom onset of known instances of mango ACD varies widely, ranging from less than 24 hours to as long as 9 days.3,12 Diagnosis of mango-induced ACD largely is guided by clinical findings. Presenting symptoms often include an eczematous, vesicular, pruritic rash on affected areas of the skin, frequently the head, neck, and extremities. Patients also commonly present with linear papulovesicular lesions and periorbital or perioral edema.
The suspected allergens responsible for mango-induced ACD are derived from resorcinol—specifically heptadecadienyl resorcinol, heptadecenyl resorcinol, and pentadecyl resorcinol, which are collectively known as mango allergens.23 These allergens can be found within the pulp and skin of the mango fruit as well as in the bark and leaves of the mango tree, which may explain observed allergic reactions to components of both the mango fruit and tree.12 Similar to these resorcinol derivatives, the urushiol resin found in poison ivy and poison oak is a catechol derivative.2 Importantly, both resorcinols and catechols are isomers of the same aromatic phenol—dihydroxybenzene. Because of these similarities, it is thought that the allergens in mangos may cross-react with urushiol in poison ivy or poison oak.23 Alongside their shared categorization in the Anacardiaceae family, it is hypothesized that this cross-reactivity underlies the sensitization that has been noted between mango and poison ivy or poison oak exposure.12,23,24 Thus, ACD often can occur on initial contact with the mango tree or its components, as a prior exposure to poison ivy or poison oak may serve as the inciting factor for hypersensitization. The majority of reported cases in the literature also occurred in countries where exposure to poison ivy and poison oak are common, further supporting the notion that these compounds may provide a sensitizing trigger for a future mango contact allergy.12
A detailed clinical history combined with adjunctive diagnostic support from patch testing and histopathology of biopsied skin lesions classically are used in the diagnosis of mango-induced ACD. Due to its ability to provide quick and noninvasive in vivo imaging of cutaneous lesions, RCM's applications have expanded to include evaluation of inflammatory skin diseases such as contact dermatitis. Many features of contact dermatitis identified via RCM are common between ACD and irritant contact dermatitis (ICD) and include disruption of the stratum corneum, parakeratosis, vesiculation, spongiosis, and exocytosis.6,10,25 Studies also have described features shown via RCM that are unique to ACD, including vasodilation and intercellular edema, compared to more distinct targetoid keratinocytes and detached corneocytes seen in ICD.6,10,25 Studies by Astner et al5,6 demonstrated a wide range of sensitivity from 52% to 96% and a high specificity of RCM greater than 95% for many of the aforementioned features of contact dermatitis, including disruption of the stratum corneum, parakeratosis, spongiosis, and exocytosis. Additional studies have further strengthened these findings, demonstrating sensitivity and specificity values of 83% and 92% for contact dermatitis under RCM, respectively.26 Importantly, given the similarities and potentially large overlap of features between ACD and ICD identified via RCM as well as findings seen on physical examination and histopathology, an emphasis on clinical correlation is essential when differentiating between these 2 variants of contact dermatitis. Thus, taken in consideration with clinical contexts, RCM has shown potent diagnostic accuracy and great potential to support the evaluation of ACD alongside patch testing and histopathology.
Final Thoughts
Contact allergy to the mango tree and its components is uncommon. We report a unique case of mango sap–induced ACD evaluated and diagnosed via dynamic visualization under RCM. As a noninvasive and reproducible imaging technique with resolutions comparable to histopathologic analysis, RCM is a promising tool that can be used to support the diagnostic evaluation of ACD.
The mango tree (Mangifera indica) produces nutrient-dense fruit—known colloquially as the “king of fruits”—that is widely consumed across the world. Native to southern Asia, the mango tree is a member of the Anacardiaceae family, a large family of flowering, fruit-bearing plants.1 Many members of the Anacardiaceae family, which includes poison ivy and poison oak, are known to produce urushiol, a skin irritant associated with allergic contact dermatitis (ACD).2 Interestingly, despite its widespread consumption and categorization in the Anacardiaceae family, allergic reactions to mango are comparatively rare; they occur as either immediate type I hypersensitivity reactions manifesting with rapid-onset symptoms such as urticaria, wheezing, and angioedema, or delayed type IV hypersensitivity reactions manifesting as ACD.3 Although exposure to components of the mango tree has been most characteristically linked to type IV hypersensitivity reactions, there remain fewer than 40 reported cases of mango-induced ACD since it was first described in 1939.4
Evaluation of ACD most commonly includes a thorough clinical assessment with diagnostic support from patch testing and histopathologic review following skin biopsy. In recent years, reflectance confocal microscopy (RCM) has shown promising potential to join the repertoire of diagnostic tools for ACD by enabling dynamic and high-resolution imaging of contact dermatitis in vivo.5-10 Reflectance confocal microscopy is a noninvasive optical imaging technique that uses a low-energy diode laser to penetrate the layers of the skin. The resulting reflected light generates images that facilitate visualization of cutaneous structures to the depth of the papillary dermis.11 While it is most commonly used in skin cancer diagnostics, preliminary studies also have shown an emerging role for RCM in the evaluation of eczematous and inflammatory skin disease, including contact dermatitis.5-10 Herein, we present a unique case of mango sap–induced ACD imaged and diagnosed in real time via RCM.
Case Report
A 39-year-old woman presented to our clinic with a pruritic vesicular eruption on the right leg of 2 weeks’ duration that initially had developed within 7 days of exposure to mango tree sap (Figure 1). The patient reported having experienced similar pruritic eruptions in the past following contact with mango sap while eating mangos but denied any history of reactions from ingestion of the fruit. She also reported a history of robust reactions to poison ivy; however, a timeline specifying the order of first exposure to these irritants was unknown. She denied any personal or family history of atopic conditions.

The affected skin was imaged in real time during clinic using RCM, which showed an inflammatory infiltrate represented by dark spongiotic vesicles containing bright cells (Figure 2). Additional RCM imaging at the level of the stratum spinosum showed dark spongiotic areas with bright inflammatory cells infiltrating the vesicles, which were surrounded by normal skin showing a typical epidermal honeycomb pattern (Figure 3). These findings were diagnostic of ACD secondary to exposure to mango sap. The patient was advised to apply clobetasol cream 0.05% to the affected area. Notable improvement of the rash was noted within 10 days of treatment.


Comment
Exposure to the mango tree and its fruit is a rare cause of ACD, with few reported cases in the literature. The majority of known instances have occurred in non–mango-cultivating countries, largely the United States, although cases also have been reported in Canada, Australia, France, Japan, and Thailand.3,12 Mango-induced contact allergy follows a roughly equal distribution between males and females and most often occurs in young adults during the third and fourth decades of life.4,12-21 Importantly, delayed-type hypersensitivity reactions to mango can manifest as either localized or systemic ACD. Localized ACD can be induced via direct contact with the mango tree and its components or ingestion of the fruit.3,12,22 Conversely, systemic ACD is primarily stimulated by ingestion of the fruit. In our case, the patient had no history of allergy following mango ingestion, and her ACD was prompted by isolated contact with mango sap. The time from exposure to symptom onset of known instances of mango ACD varies widely, ranging from less than 24 hours to as long as 9 days.3,12 Diagnosis of mango-induced ACD largely is guided by clinical findings. Presenting symptoms often include an eczematous, vesicular, pruritic rash on affected areas of the skin, frequently the head, neck, and extremities. Patients also commonly present with linear papulovesicular lesions and periorbital or perioral edema.
The suspected allergens responsible for mango-induced ACD are derived from resorcinol—specifically heptadecadienyl resorcinol, heptadecenyl resorcinol, and pentadecyl resorcinol, which are collectively known as mango allergens.23 These allergens can be found within the pulp and skin of the mango fruit as well as in the bark and leaves of the mango tree, which may explain observed allergic reactions to components of both the mango fruit and tree.12 Similar to these resorcinol derivatives, the urushiol resin found in poison ivy and poison oak is a catechol derivative.2 Importantly, both resorcinols and catechols are isomers of the same aromatic phenol—dihydroxybenzene. Because of these similarities, it is thought that the allergens in mangos may cross-react with urushiol in poison ivy or poison oak.23 Alongside their shared categorization in the Anacardiaceae family, it is hypothesized that this cross-reactivity underlies the sensitization that has been noted between mango and poison ivy or poison oak exposure.12,23,24 Thus, ACD often can occur on initial contact with the mango tree or its components, as a prior exposure to poison ivy or poison oak may serve as the inciting factor for hypersensitization. The majority of reported cases in the literature also occurred in countries where exposure to poison ivy and poison oak are common, further supporting the notion that these compounds may provide a sensitizing trigger for a future mango contact allergy.12
A detailed clinical history combined with adjunctive diagnostic support from patch testing and histopathology of biopsied skin lesions classically are used in the diagnosis of mango-induced ACD. Due to its ability to provide quick and noninvasive in vivo imaging of cutaneous lesions, RCM's applications have expanded to include evaluation of inflammatory skin diseases such as contact dermatitis. Many features of contact dermatitis identified via RCM are common between ACD and irritant contact dermatitis (ICD) and include disruption of the stratum corneum, parakeratosis, vesiculation, spongiosis, and exocytosis.6,10,25 Studies also have described features shown via RCM that are unique to ACD, including vasodilation and intercellular edema, compared to more distinct targetoid keratinocytes and detached corneocytes seen in ICD.6,10,25 Studies by Astner et al5,6 demonstrated a wide range of sensitivity from 52% to 96% and a high specificity of RCM greater than 95% for many of the aforementioned features of contact dermatitis, including disruption of the stratum corneum, parakeratosis, spongiosis, and exocytosis. Additional studies have further strengthened these findings, demonstrating sensitivity and specificity values of 83% and 92% for contact dermatitis under RCM, respectively.26 Importantly, given the similarities and potentially large overlap of features between ACD and ICD identified via RCM as well as findings seen on physical examination and histopathology, an emphasis on clinical correlation is essential when differentiating between these 2 variants of contact dermatitis. Thus, taken in consideration with clinical contexts, RCM has shown potent diagnostic accuracy and great potential to support the evaluation of ACD alongside patch testing and histopathology.
Final Thoughts
Contact allergy to the mango tree and its components is uncommon. We report a unique case of mango sap–induced ACD evaluated and diagnosed via dynamic visualization under RCM. As a noninvasive and reproducible imaging technique with resolutions comparable to histopathologic analysis, RCM is a promising tool that can be used to support the diagnostic evaluation of ACD.
- Shah KA, Patel MB, Patel RJ, et al. Mangifera indica (mango). Pharmacogn Rev. 2010;4:42-48.
- Lofgran T, Mahabal GD. Toxicodendron toxicity. StatPearls [Internet]. Updated May 16, 2023. Accessed September 19, 2024. https://www.ncbi.nlm.nih.gov/books/NBK557866
- Sareen R, Shah A. Hypersensitivity manifestations to the fruit mango. Asia Pac Allergy. 2011;1:43-49.
- Zakon SJ. Contact dermatitis due to mango. JAMA. 1939;113:1808.
- Astner S, Gonzalez E, Cheung A, et al. Pilot study on the sensitivity and specificity of in vivo reflectance confocal microscopy in the diagnosis of allergic contact dermatitis. J Am Acad Dermatol. 2005;53:986-992.
- Astner S, Gonzalez S, Gonzalez E. Noninvasive evaluation of allergic and irritant contact dermatitis by in vivo reflectance confocal microscopy. Dermatitis. 2006;17:182-191.
- Csuka EA, Ward SC, Ekelem C, et al. Reflectance confocal microscopy, optical coherence tomography, and multiphoton microscopy in inflammatory skin disease diagnosis. Lasers Surg Med. 2021;53:776-797.
- Guichard A, Fanian F, Girardin P, et al. Allergic patch test and contact dermatitis by in vivo reflectance confocal microscopy [in French]. Ann Dermatol Venereol. 2014;141:805-807.
- Sakanashi EN, Matsumura M, Kikuchi K, et al. A comparative study of allergic contact dermatitis by patch test versus reflectance confocal laser microscopy, with nickel and cobalt. Eur J Dermatol. 2010;20:705-711.
- Swindells K, Burnett N, Rius-Diaz F, et al. Reflectance confocal microscopy may differentiate acute allergic and irritant contact dermatitis in vivo. J Am Acad Dermatol. 2004;50:220-228.
- Shahriari N, Grant-Kels JM, Rabinovitz H, et al. Reflectance confocal microscopy: principles, basic terminology, clinical indications, limitations, and practical considerations. J Am Acad Dermatol. 2021;84:1-14.
- Berghea EC, Craiu M, Ali S, et al. Contact allergy induced by mango (Mangifera indica): a relevant topic? Medicina (Kaunas). 2021;57:1240.
- O’Hern K, Zhang F, Zug KA, et al. “Mango slice” dermatitis: pediatric allergic contact dermatitis to mango pulp and skin. Dermatitis. 2022;33:E46-E47.
- Raison-Peyron N, Aljaber F, Al Ali OA, et al. Mango dermatitis: an unusual cause of eyelid dermatitis in France. Contact Dermatitis. 2021;85:599-600.
- Alipour Tehrany Y, Coulombe J. Mango allergic contact dermatitis. Contact Dermatitis. 2021;85:241-242.
- Yoo MJ, Carius BM. Mango dermatitis after urushiol sensitization. Clin Pract Cases Emerg Med. 2019;3:361-363.
- Miyazawa H, Nishie W, Hata H, et al. A severe case of mango dermatitis. J Eur Acad Dermatol Venereol. 2018;32:E160-E161.
- Trehan I, Meuli GJ. Mango contact allergy. J Travel Med. 2010;17:284.
- Wiwanitkit V. Mango dermatitis. Indian J Dermatol. 2008;53:158.
- Weinstein S, Bassiri-Tehrani S, Cohen DE. Allergic contact dermatitis to mango flesh. Int J Dermatol. 2004;43:195-196.
- Calvert ML, Robertson I, Samaratunga H. Mango dermatitis: allergic contact dermatitis to Mangifera indica. Australas J Dermatol. 1996;37:59-60.
- Thoo CH, Freeman S. Hypersensitivity reaction to the ingestion of mango flesh. Australas J Dermatol. 2008;49:116-119.
- Oka K, Saito F, Yasuhara T, et al. A study of cross-reactions between mango contact allergens and urushiol. Contact Dermatitis. 2004;51:292-296.
- Keil H, Wasserman D, Dawson CR. Mango dermatitis and its relationship to poison ivy hypersensitivity. Ann Allergy. 1946;4: 268-281.
- Maarouf M, Costello CM, Gonzalez S, et al. In vivo reflectance confocal microscopy: emerging role in noninvasive diagnosis and monitoring of eczematous dermatoses. Actas Dermosifiliogr (Engl Ed). 2019;110:626-636.
- Koller S, Gerger A, Ahlgrimm-Siess V, et al. In vivo reflectance confocal microscopy of erythematosquamous skin diseases. Exp Dermatol. 2009;18:536-540.
- Shah KA, Patel MB, Patel RJ, et al. Mangifera indica (mango). Pharmacogn Rev. 2010;4:42-48.
- Lofgran T, Mahabal GD. Toxicodendron toxicity. StatPearls [Internet]. Updated May 16, 2023. Accessed September 19, 2024. https://www.ncbi.nlm.nih.gov/books/NBK557866
- Sareen R, Shah A. Hypersensitivity manifestations to the fruit mango. Asia Pac Allergy. 2011;1:43-49.
- Zakon SJ. Contact dermatitis due to mango. JAMA. 1939;113:1808.
- Astner S, Gonzalez E, Cheung A, et al. Pilot study on the sensitivity and specificity of in vivo reflectance confocal microscopy in the diagnosis of allergic contact dermatitis. J Am Acad Dermatol. 2005;53:986-992.
- Astner S, Gonzalez S, Gonzalez E. Noninvasive evaluation of allergic and irritant contact dermatitis by in vivo reflectance confocal microscopy. Dermatitis. 2006;17:182-191.
- Csuka EA, Ward SC, Ekelem C, et al. Reflectance confocal microscopy, optical coherence tomography, and multiphoton microscopy in inflammatory skin disease diagnosis. Lasers Surg Med. 2021;53:776-797.
- Guichard A, Fanian F, Girardin P, et al. Allergic patch test and contact dermatitis by in vivo reflectance confocal microscopy [in French]. Ann Dermatol Venereol. 2014;141:805-807.
- Sakanashi EN, Matsumura M, Kikuchi K, et al. A comparative study of allergic contact dermatitis by patch test versus reflectance confocal laser microscopy, with nickel and cobalt. Eur J Dermatol. 2010;20:705-711.
- Swindells K, Burnett N, Rius-Diaz F, et al. Reflectance confocal microscopy may differentiate acute allergic and irritant contact dermatitis in vivo. J Am Acad Dermatol. 2004;50:220-228.
- Shahriari N, Grant-Kels JM, Rabinovitz H, et al. Reflectance confocal microscopy: principles, basic terminology, clinical indications, limitations, and practical considerations. J Am Acad Dermatol. 2021;84:1-14.
- Berghea EC, Craiu M, Ali S, et al. Contact allergy induced by mango (Mangifera indica): a relevant topic? Medicina (Kaunas). 2021;57:1240.
- O’Hern K, Zhang F, Zug KA, et al. “Mango slice” dermatitis: pediatric allergic contact dermatitis to mango pulp and skin. Dermatitis. 2022;33:E46-E47.
- Raison-Peyron N, Aljaber F, Al Ali OA, et al. Mango dermatitis: an unusual cause of eyelid dermatitis in France. Contact Dermatitis. 2021;85:599-600.
- Alipour Tehrany Y, Coulombe J. Mango allergic contact dermatitis. Contact Dermatitis. 2021;85:241-242.
- Yoo MJ, Carius BM. Mango dermatitis after urushiol sensitization. Clin Pract Cases Emerg Med. 2019;3:361-363.
- Miyazawa H, Nishie W, Hata H, et al. A severe case of mango dermatitis. J Eur Acad Dermatol Venereol. 2018;32:E160-E161.
- Trehan I, Meuli GJ. Mango contact allergy. J Travel Med. 2010;17:284.
- Wiwanitkit V. Mango dermatitis. Indian J Dermatol. 2008;53:158.
- Weinstein S, Bassiri-Tehrani S, Cohen DE. Allergic contact dermatitis to mango flesh. Int J Dermatol. 2004;43:195-196.
- Calvert ML, Robertson I, Samaratunga H. Mango dermatitis: allergic contact dermatitis to Mangifera indica. Australas J Dermatol. 1996;37:59-60.
- Thoo CH, Freeman S. Hypersensitivity reaction to the ingestion of mango flesh. Australas J Dermatol. 2008;49:116-119.
- Oka K, Saito F, Yasuhara T, et al. A study of cross-reactions between mango contact allergens and urushiol. Contact Dermatitis. 2004;51:292-296.
- Keil H, Wasserman D, Dawson CR. Mango dermatitis and its relationship to poison ivy hypersensitivity. Ann Allergy. 1946;4: 268-281.
- Maarouf M, Costello CM, Gonzalez S, et al. In vivo reflectance confocal microscopy: emerging role in noninvasive diagnosis and monitoring of eczematous dermatoses. Actas Dermosifiliogr (Engl Ed). 2019;110:626-636.
- Koller S, Gerger A, Ahlgrimm-Siess V, et al. In vivo reflectance confocal microscopy of erythematosquamous skin diseases. Exp Dermatol. 2009;18:536-540.
Practice Points
- Contact with mango tree sap can induce allergic contact dermatitis.
- Reflectance confocal microscopy (RCM) is a noninvasive imaging technique that can provide real-time in vivo visualization of affected skin in contact dermatitis.
- Predominant findings of contact dermatitis under RCM include disruption of the stratum corneum; parakeratosis; vesiculation; spongiosis; and exocytosis, vasodilation, and intercellular edema more specific to the allergic subtype.
Disability Reduction Is a Twist in Negative BTKi RRMS Trial
COPENHAGEN — In two phase 3 head-to-head comparing the Bruton tyrosine kinase inhibitor (BTKi) tolebrutinib to the immunomodulatory teriflunomide for relapsing-remitting multiple sclerosis (RRMS), there was no advantage on the primary endpoint of relapse, but the greater protection against disability, a secondary endpoint, might change thinking about BTKis as a potential MS therapy.
For annualized relapse rate (ARR), which is the basis on which these two drugs were compared, “there was no difference between tolebrutinib and teriflunomide,” reported Jiwon Oh, MD, Medical Director, Barlo Multiple Sclerosis Program, St. Michael’s Hospital, University of Toronto, Canada.
In the similar GEMINI 1 and 2 trials, the ARRs were nearly identical in the first, (0.13 and 0.12), and completely identical in the second (0.11) for tolebrutinib and teriflunomide, respectively.
Although Negative, GEMINI Trials Offer Intriguing Data
These data rule out the study hypothesis that a BTKi offers greater protection against relapse than a commonly used immunomodulator, but Dr. Oh suggested the study is still potentially relevant to MS research.
“There is hope,” Dr. Oh said, when reporting the findings of the GEMINI I and II trials during the latebreaker session at the 2024 ECTRIMS annual meeting. Ultimately, a substantial part of this hope was derived from the consistency of the GEMINI data with the placebo-controlled HERCULES trial of tolebrutinib presented immediately afterwards, but the disparity between the primary and secondary outcomes of GEMINI are, by themselves, relevant, suggesting that targets of treatment change as MS progresses from an acute to a chronic inflammatory process.
BTKi Associated With Reduced Disability
At 3 months, the rate of confirmed disability worsening (CDW) in the pooled GEMINI trials was 18.5% and 14.7% for tolebrutinib and teriflunomide, respectively, producing at 27% reduction in hazard ratio (HR) for this outcome (HR 0.73; P = .0018). At 6 months, the protection against disability (13.2% vs. 9.9%) persisted for tolebrutinib relative to teriflunomide (HR 0.71; P = .023).*
For the outcome of a confirmed disability improvement at 6 months, the higher rate in the tolebrutinib arm did not reach statistical significance (12.8% vs. 12.0%), but it did suggest a favorable trend (HR 1.22; P = .17).
While Dr. Oh acknowledged that secondary outcomes can only be considered hypothesis generating when the primary outcome is negative, she said these outcomes provide intriguing support for the potential of this BTKi drug to inhibit “smoldering inflammation.” Even if tolebrutinib was no more effective than teriflunomide against the acute inflammation that drives relapse, the GEMINI trials data support greater inhibition of the chronic inflammation implicated in progression in the absence of flares.
On MRI, the annualized rate of new and enlarging T2 lesions, although numerically higher in the tolebrutinib group, did not differ significantly in either GEMINI 1 (5.6 vs. 5.2; P = .46) or GEMINI 2 (5.1 vs. 4.4; P = .24). The least mean square difference in brain volume at end of study relative to 6 months into the study was 0.2% less in the tolebrutinib arm than the teriflunomide arm (P = .0002) in GEMINI 1, but the 0.04 numerical advantage for tolebrutinib did not reach statistical significance in GEMINI 2 (P = .43).
Of the 974 patients randomized in GEMINI 1 and 899 randomized in GEMINI 2, about 85% completed the 3-year trial. Almost all had RRMS (99%) rather than progressing MS. The median age was approximately 36 years, the baseline EDSS score was approximately 1.2, and the median time since diagnosis was about 6.5 years. The mean number of relapses in the prior year was approximately 0.6.
In GEMINI, the secondary outcomes foreshadowed the positive findings in the phase 3 HERCULES trial that came immediately after Dr. Oh’s GEMINI trials presentation. The HERCULES trial associated tolebrutinib with a 31% reduction in the risk of confirmed disability progression (CDW) relative to placebo in patients with non-relapsing secondary progressive MS (nrSPMS).
In HERCULES, 1172 patients with nrSPMS were randomized in a 2:1 fashion to tolebrutinib or placebo. For the primary endpoint of CDW at 6 months, tolebrutinib demonstrated a major and highly significant reduction in this primary endpoint (HR 0.69; P = .00026).
BTKi Disability Protection Supported By Progressive MS Trial
“This is the first trial to show significant slowing of disability in people with nrSPMS,” reported the principal investigator Robert J. Fox, MD, Vice Chair of the Neurological Institute at Cleveland Clinic, Cleveland, Ohio.
For disability improvement at 6 months, tolebrutinib was associated with a nearly 2-fold improvement (HR 1.88; P = .021). According to both Dr. Oh and Dr. Fox the results of these two major phase 3 tolebrutinib studies support the principle that the BTKi, which was shown to offer inhibition of relapse comparable to teriflunomide in the GEMINI trials, offers a greater inhibition of chronic inflammation.
“These results are consistent with the hypothesis that acute focal inflammation and smoldering neuroinflammation are two distinct biological processes,” Dr. Oh said.
Dr. Fox said that the HERCULES results will be submitted to regulatory authorities with the goal of securing an indication for tolebrutinib for nrSPMS.
Both Dr. Oh and Dr. Fox suggested these results are likely to reorient thinking about the pathophysiology of MS progression and how different processes can be targeted in the future. Other experts agreed.
“I think we are starting to look at different endpoints than ARR, particularly at those that might better reflect progression in later stages of MS and that are independent of ARR,” said Dalia Rotstein, MD, MS researcher and an assistant professor of neurology, University of Toronto, Canada.
A moderator of the ECTRIMS latebreaker session, she suggested that the differences between outcomes of the GEMINI trials and HERCULES trials might have relevance to each other even if the GEMINI trials did not meet their primary endpoint.
Dr. Oh reported financial relationships with Amgen, Biogen, Eli Lilly, EMD Serono, Novartis, Roche, and Sanofi, which provided funding for the GEMINI trials. Dr. Fox reported financial relationships with more than 15 pharmaceutical companies, including Sanofi, which also provided funding for the HERCULES trial. Dr. Rotstein reported financial relationships with Alexion, Biogen, EMD Serono, Horizon, Novartis, Roche, Sanofi, and Touch IME.
*Correction, 9/26/24: A previous version of this article contained an incorrect P value.
COPENHAGEN — In two phase 3 head-to-head comparing the Bruton tyrosine kinase inhibitor (BTKi) tolebrutinib to the immunomodulatory teriflunomide for relapsing-remitting multiple sclerosis (RRMS), there was no advantage on the primary endpoint of relapse, but the greater protection against disability, a secondary endpoint, might change thinking about BTKis as a potential MS therapy.
For annualized relapse rate (ARR), which is the basis on which these two drugs were compared, “there was no difference between tolebrutinib and teriflunomide,” reported Jiwon Oh, MD, Medical Director, Barlo Multiple Sclerosis Program, St. Michael’s Hospital, University of Toronto, Canada.
In the similar GEMINI 1 and 2 trials, the ARRs were nearly identical in the first, (0.13 and 0.12), and completely identical in the second (0.11) for tolebrutinib and teriflunomide, respectively.
Although Negative, GEMINI Trials Offer Intriguing Data
These data rule out the study hypothesis that a BTKi offers greater protection against relapse than a commonly used immunomodulator, but Dr. Oh suggested the study is still potentially relevant to MS research.
“There is hope,” Dr. Oh said, when reporting the findings of the GEMINI I and II trials during the latebreaker session at the 2024 ECTRIMS annual meeting. Ultimately, a substantial part of this hope was derived from the consistency of the GEMINI data with the placebo-controlled HERCULES trial of tolebrutinib presented immediately afterwards, but the disparity between the primary and secondary outcomes of GEMINI are, by themselves, relevant, suggesting that targets of treatment change as MS progresses from an acute to a chronic inflammatory process.
BTKi Associated With Reduced Disability
At 3 months, the rate of confirmed disability worsening (CDW) in the pooled GEMINI trials was 18.5% and 14.7% for tolebrutinib and teriflunomide, respectively, producing at 27% reduction in hazard ratio (HR) for this outcome (HR 0.73; P = .0018). At 6 months, the protection against disability (13.2% vs. 9.9%) persisted for tolebrutinib relative to teriflunomide (HR 0.71; P = .023).*
For the outcome of a confirmed disability improvement at 6 months, the higher rate in the tolebrutinib arm did not reach statistical significance (12.8% vs. 12.0%), but it did suggest a favorable trend (HR 1.22; P = .17).
While Dr. Oh acknowledged that secondary outcomes can only be considered hypothesis generating when the primary outcome is negative, she said these outcomes provide intriguing support for the potential of this BTKi drug to inhibit “smoldering inflammation.” Even if tolebrutinib was no more effective than teriflunomide against the acute inflammation that drives relapse, the GEMINI trials data support greater inhibition of the chronic inflammation implicated in progression in the absence of flares.
On MRI, the annualized rate of new and enlarging T2 lesions, although numerically higher in the tolebrutinib group, did not differ significantly in either GEMINI 1 (5.6 vs. 5.2; P = .46) or GEMINI 2 (5.1 vs. 4.4; P = .24). The least mean square difference in brain volume at end of study relative to 6 months into the study was 0.2% less in the tolebrutinib arm than the teriflunomide arm (P = .0002) in GEMINI 1, but the 0.04 numerical advantage for tolebrutinib did not reach statistical significance in GEMINI 2 (P = .43).
Of the 974 patients randomized in GEMINI 1 and 899 randomized in GEMINI 2, about 85% completed the 3-year trial. Almost all had RRMS (99%) rather than progressing MS. The median age was approximately 36 years, the baseline EDSS score was approximately 1.2, and the median time since diagnosis was about 6.5 years. The mean number of relapses in the prior year was approximately 0.6.
In GEMINI, the secondary outcomes foreshadowed the positive findings in the phase 3 HERCULES trial that came immediately after Dr. Oh’s GEMINI trials presentation. The HERCULES trial associated tolebrutinib with a 31% reduction in the risk of confirmed disability progression (CDW) relative to placebo in patients with non-relapsing secondary progressive MS (nrSPMS).
In HERCULES, 1172 patients with nrSPMS were randomized in a 2:1 fashion to tolebrutinib or placebo. For the primary endpoint of CDW at 6 months, tolebrutinib demonstrated a major and highly significant reduction in this primary endpoint (HR 0.69; P = .00026).
BTKi Disability Protection Supported By Progressive MS Trial
“This is the first trial to show significant slowing of disability in people with nrSPMS,” reported the principal investigator Robert J. Fox, MD, Vice Chair of the Neurological Institute at Cleveland Clinic, Cleveland, Ohio.
For disability improvement at 6 months, tolebrutinib was associated with a nearly 2-fold improvement (HR 1.88; P = .021). According to both Dr. Oh and Dr. Fox the results of these two major phase 3 tolebrutinib studies support the principle that the BTKi, which was shown to offer inhibition of relapse comparable to teriflunomide in the GEMINI trials, offers a greater inhibition of chronic inflammation.
“These results are consistent with the hypothesis that acute focal inflammation and smoldering neuroinflammation are two distinct biological processes,” Dr. Oh said.
Dr. Fox said that the HERCULES results will be submitted to regulatory authorities with the goal of securing an indication for tolebrutinib for nrSPMS.
Both Dr. Oh and Dr. Fox suggested these results are likely to reorient thinking about the pathophysiology of MS progression and how different processes can be targeted in the future. Other experts agreed.
“I think we are starting to look at different endpoints than ARR, particularly at those that might better reflect progression in later stages of MS and that are independent of ARR,” said Dalia Rotstein, MD, MS researcher and an assistant professor of neurology, University of Toronto, Canada.
A moderator of the ECTRIMS latebreaker session, she suggested that the differences between outcomes of the GEMINI trials and HERCULES trials might have relevance to each other even if the GEMINI trials did not meet their primary endpoint.
Dr. Oh reported financial relationships with Amgen, Biogen, Eli Lilly, EMD Serono, Novartis, Roche, and Sanofi, which provided funding for the GEMINI trials. Dr. Fox reported financial relationships with more than 15 pharmaceutical companies, including Sanofi, which also provided funding for the HERCULES trial. Dr. Rotstein reported financial relationships with Alexion, Biogen, EMD Serono, Horizon, Novartis, Roche, Sanofi, and Touch IME.
*Correction, 9/26/24: A previous version of this article contained an incorrect P value.
COPENHAGEN — In two phase 3 head-to-head comparing the Bruton tyrosine kinase inhibitor (BTKi) tolebrutinib to the immunomodulatory teriflunomide for relapsing-remitting multiple sclerosis (RRMS), there was no advantage on the primary endpoint of relapse, but the greater protection against disability, a secondary endpoint, might change thinking about BTKis as a potential MS therapy.
For annualized relapse rate (ARR), which is the basis on which these two drugs were compared, “there was no difference between tolebrutinib and teriflunomide,” reported Jiwon Oh, MD, Medical Director, Barlo Multiple Sclerosis Program, St. Michael’s Hospital, University of Toronto, Canada.
In the similar GEMINI 1 and 2 trials, the ARRs were nearly identical in the first, (0.13 and 0.12), and completely identical in the second (0.11) for tolebrutinib and teriflunomide, respectively.
Although Negative, GEMINI Trials Offer Intriguing Data
These data rule out the study hypothesis that a BTKi offers greater protection against relapse than a commonly used immunomodulator, but Dr. Oh suggested the study is still potentially relevant to MS research.
“There is hope,” Dr. Oh said, when reporting the findings of the GEMINI I and II trials during the latebreaker session at the 2024 ECTRIMS annual meeting. Ultimately, a substantial part of this hope was derived from the consistency of the GEMINI data with the placebo-controlled HERCULES trial of tolebrutinib presented immediately afterwards, but the disparity between the primary and secondary outcomes of GEMINI are, by themselves, relevant, suggesting that targets of treatment change as MS progresses from an acute to a chronic inflammatory process.
BTKi Associated With Reduced Disability
At 3 months, the rate of confirmed disability worsening (CDW) in the pooled GEMINI trials was 18.5% and 14.7% for tolebrutinib and teriflunomide, respectively, producing at 27% reduction in hazard ratio (HR) for this outcome (HR 0.73; P = .0018). At 6 months, the protection against disability (13.2% vs. 9.9%) persisted for tolebrutinib relative to teriflunomide (HR 0.71; P = .023).*
For the outcome of a confirmed disability improvement at 6 months, the higher rate in the tolebrutinib arm did not reach statistical significance (12.8% vs. 12.0%), but it did suggest a favorable trend (HR 1.22; P = .17).
While Dr. Oh acknowledged that secondary outcomes can only be considered hypothesis generating when the primary outcome is negative, she said these outcomes provide intriguing support for the potential of this BTKi drug to inhibit “smoldering inflammation.” Even if tolebrutinib was no more effective than teriflunomide against the acute inflammation that drives relapse, the GEMINI trials data support greater inhibition of the chronic inflammation implicated in progression in the absence of flares.
On MRI, the annualized rate of new and enlarging T2 lesions, although numerically higher in the tolebrutinib group, did not differ significantly in either GEMINI 1 (5.6 vs. 5.2; P = .46) or GEMINI 2 (5.1 vs. 4.4; P = .24). The least mean square difference in brain volume at end of study relative to 6 months into the study was 0.2% less in the tolebrutinib arm than the teriflunomide arm (P = .0002) in GEMINI 1, but the 0.04 numerical advantage for tolebrutinib did not reach statistical significance in GEMINI 2 (P = .43).
Of the 974 patients randomized in GEMINI 1 and 899 randomized in GEMINI 2, about 85% completed the 3-year trial. Almost all had RRMS (99%) rather than progressing MS. The median age was approximately 36 years, the baseline EDSS score was approximately 1.2, and the median time since diagnosis was about 6.5 years. The mean number of relapses in the prior year was approximately 0.6.
In GEMINI, the secondary outcomes foreshadowed the positive findings in the phase 3 HERCULES trial that came immediately after Dr. Oh’s GEMINI trials presentation. The HERCULES trial associated tolebrutinib with a 31% reduction in the risk of confirmed disability progression (CDW) relative to placebo in patients with non-relapsing secondary progressive MS (nrSPMS).
In HERCULES, 1172 patients with nrSPMS were randomized in a 2:1 fashion to tolebrutinib or placebo. For the primary endpoint of CDW at 6 months, tolebrutinib demonstrated a major and highly significant reduction in this primary endpoint (HR 0.69; P = .00026).
BTKi Disability Protection Supported By Progressive MS Trial
“This is the first trial to show significant slowing of disability in people with nrSPMS,” reported the principal investigator Robert J. Fox, MD, Vice Chair of the Neurological Institute at Cleveland Clinic, Cleveland, Ohio.
For disability improvement at 6 months, tolebrutinib was associated with a nearly 2-fold improvement (HR 1.88; P = .021). According to both Dr. Oh and Dr. Fox the results of these two major phase 3 tolebrutinib studies support the principle that the BTKi, which was shown to offer inhibition of relapse comparable to teriflunomide in the GEMINI trials, offers a greater inhibition of chronic inflammation.
“These results are consistent with the hypothesis that acute focal inflammation and smoldering neuroinflammation are two distinct biological processes,” Dr. Oh said.
Dr. Fox said that the HERCULES results will be submitted to regulatory authorities with the goal of securing an indication for tolebrutinib for nrSPMS.
Both Dr. Oh and Dr. Fox suggested these results are likely to reorient thinking about the pathophysiology of MS progression and how different processes can be targeted in the future. Other experts agreed.
“I think we are starting to look at different endpoints than ARR, particularly at those that might better reflect progression in later stages of MS and that are independent of ARR,” said Dalia Rotstein, MD, MS researcher and an assistant professor of neurology, University of Toronto, Canada.
A moderator of the ECTRIMS latebreaker session, she suggested that the differences between outcomes of the GEMINI trials and HERCULES trials might have relevance to each other even if the GEMINI trials did not meet their primary endpoint.
Dr. Oh reported financial relationships with Amgen, Biogen, Eli Lilly, EMD Serono, Novartis, Roche, and Sanofi, which provided funding for the GEMINI trials. Dr. Fox reported financial relationships with more than 15 pharmaceutical companies, including Sanofi, which also provided funding for the HERCULES trial. Dr. Rotstein reported financial relationships with Alexion, Biogen, EMD Serono, Horizon, Novartis, Roche, Sanofi, and Touch IME.
*Correction, 9/26/24: A previous version of this article contained an incorrect P value.
FROM ECTRIMS 2024
Transient Eruption of Verrucous Keratoses During Encorafenib Therapy: Adverse Event or Paraneoplastic Phenomenon?
To the Editor:
Mutations of the BRAF protein kinase gene are implicated in a variety of malignancies.1 BRAF mutations in malignancies cause the mitogen-activated protein kinase (MAPK) pathway to become constitutively active, which results in unchecked cellular proliferation,2,3 making the BRAF mutation an attractive target for inhibition with pharmacologic agents to potentially halt cancer growth.4 Vemurafenib—the first selective BRAF inhibitor used in clinical practice—initially was approved by the US Food and Drug Administration in 2011. The approval of dabrafenib followed in 2013 and most recently encorafenib in 2018.5
Although targeted treatment of BRAF-mutated malignancies with BRAF inhibitors has become common, it often is associated with cutaneous adverse events (AEs), such as rash, pruritus, photosensitivity, actinic keratosis, and verrucous keratosis. Some reports demonstrate these events in up to 95% of patients undergoing BRAF inhibitor treatment.6 In several cases the eruption of verrucous keratoses is among the most common cutaneous AEs seen among patients receiving BRAF inhibitor treatment.5-7
In general, lesions can appear days to months after therapy is initiated and may resolve after switching to dual therapy with a MEK inhibitor or with complete cessation of BRAF inhibitor therapy.5,7,8 One case of spontaneous resolution of vemurafenib-associated panniculitis during ongoing BRAF inhibitor therapy has been reported9; however, spontaneous resolution of cutaneous AEs is uncommon. Herein, we describe verrucous keratoses in a patient undergoing treatment with encorafenib that resolved spontaneously despite ongoing BRAF inhibitor therapy.
A 61-year-old woman presented to the emergency department with pain in the right lower quadrant. Computed tomography (CT) of the abdomen and pelvis revealed a large ovarian mass. Subsequent bloodwork revealed elevated carcinoembryonic antigen levels. The patient underwent a hysterectomy, bilateral salpingo-oophorectomy, omentectomy, right hemicolectomy with ileotransverse side-to-side anastomosis, right pelvic lymph node reduction, and complete cytoreduction. Histopathology revealed an adenocarcinoma of the cecum with tumor invasion into the visceral peritoneum and metastases to the left ovary, fallopian tube, and omentum. A BRAF V600E mutation was detected.
Two months after the initial presentation, the patient started her first cycle of chemotherapy with a combination of folinic acid, fluorouracil, and oxaliplatin. She completed 11 cycles of this regimen, then was switched to capecitabine and oxaliplatin for an additional 2 cycles due to insurance concerns. At the end of treatment, there was no evidence of disease on CT, thus the patient was followed with observation. However, she presented 10 months later to the emergency department with abdominal pain, and CT revealed new lesions in the liver that were concerning for potential metastases. She started oral encorafenib 300 mg/d and intravenous cetuximab 500 mg weekly; after 1 week, encorafenib was reduced to 150 mg/d due to nausea and loss of appetite. Within 2 weeks of starting treatment, the patient reported the relatively abrupt appearance of more than 50 small papules across the shoulders and back (Figure 1A). She was referred to dermatology, and shave biopsies of 2 lesions—one from the left anterior thigh, the other from the right posterior shoulder—revealed verrucous keratosis pathology (Figure 2). At this time, encorafenib was increased again to 300 mg/d as the patient had been tolerating the reduced dose. She continued to report the appearance of new lesions for the next 3 months, after which the lesions were stable for approximately 2 months. By 2.5 months after initiation of therapy, the patient had undergone CT demonstrating resolution of the liver lesions. At 5 months of therapy, the patient reported a stable to slightly reduced number of skin lesions but had begun to experience worsening joint pain, and the dosage of encorafenib was reduced to 225 mg/d. At 7 months of therapy, the dosage was further reduced to 150 mg/d due to persistent arthralgia. A follow-up examination at 10 months of therapy showed improvement in the number and size of the verrucous keratoses, and near resolution was seen by 14 months after the initial onset of the lesions (Figure 1B). At 20 months after initial onset, only 1 remaining verrucous keratosis was identified on physical examination and biopsy. The patient had continued a regimen of encorafenib 150 mg/d and weekly intravenous 500 mg cetuximab up to this point. Over the entire time period that the patient was seen, up to 12 lesions located in high-friction areas had become irritated and were treated with cryotherapy, but this contributed only minorly to the patient’s overall presentation.
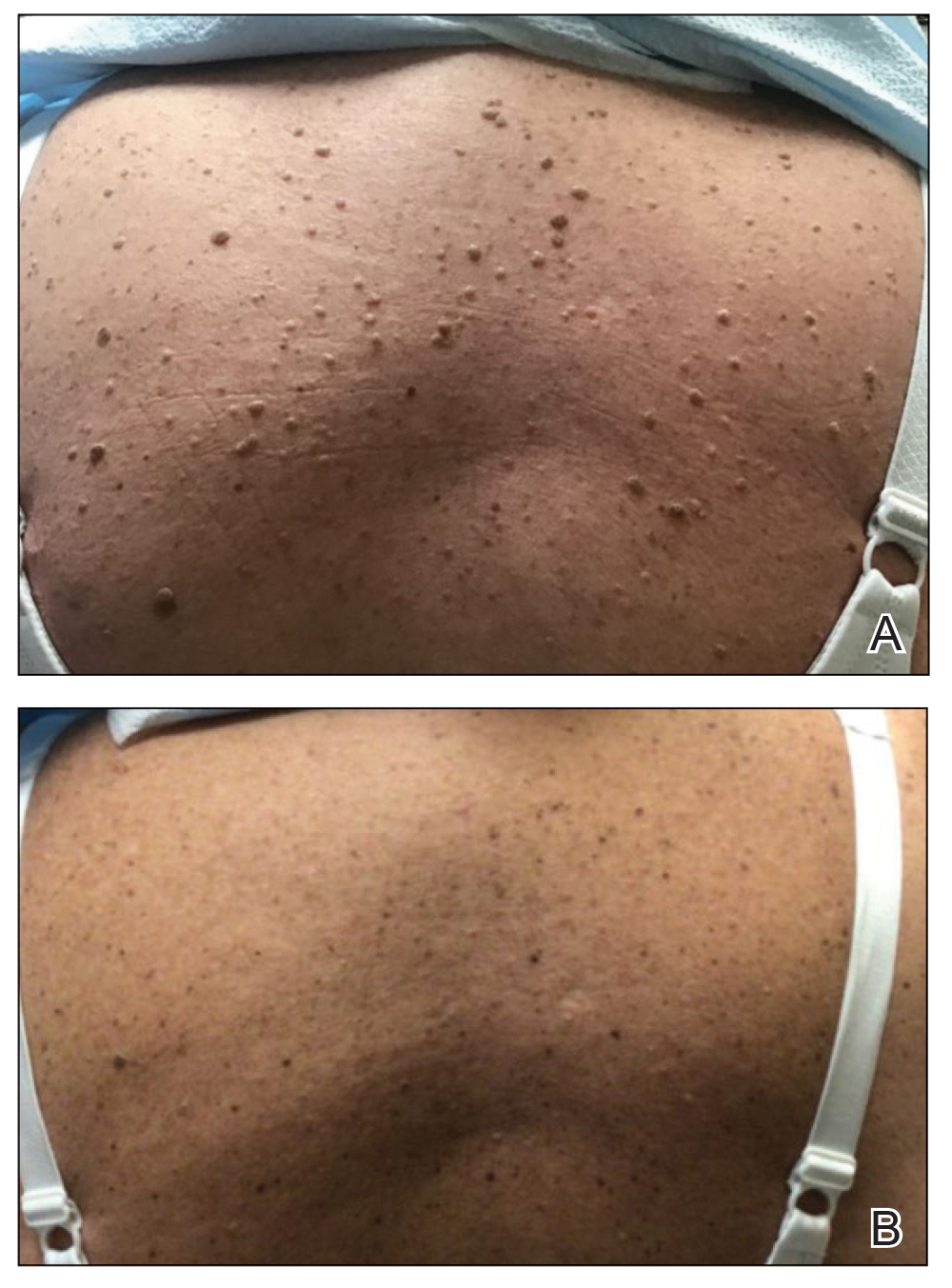
Verrucous keratosis is a known cutaneous AE of BRAF inhibitor treatment with vemurafenib and dabrafenib, with fewer cases attributed to encorafenib.5,6 Within the oncologic setting, the eruption of verrucous papules as a paraneoplastic phenomenon is heavily debated in the literature and is known as the Leser-Trélat sign. This phenomenon is commonly associated with adenocarcinomas of the gastrointestinal tract, as seen in our patient.10 Based on Curth’s postulates—the criteria used to evaluate the relationship between an internal malignancy and a cutaneous disorder—this was unlikely in our patient. The criteria, which do not all need to be met to suggest a paraneoplastic phenomenon, include concurrent onset of the malignancy and the dermatosis, parallel course, association of a specific dermatosis with a specific malignancy, statistical significance of the association, and the presence of a genetic basis for the association.11 Several features favored a drug-related cutaneous eruption vs a paraneoplastic phenomenon: (1) the malignancy was identified months before the cutaneous eruptions manifested; (2) the cutaneous lesions appeared once treatment had already been initiated; and (3) the cutaneous lesions persisted long after the malignancy was no longer identifiable on CT. Indeed, eruption of the papules temporally coincided closely with the initiation of BRAF inhibitor therapy, arguing for correlation.
As a suspected BRAF inhibitor–associated cutaneous AE, the eruption of verrucous keratoses in our patient is remarkable for its spontaneous resolution despite ongoing therapy. It is speculated that keratinocytic proliferation while on BRAF inhibitor therapy may be caused by a paradoxical increase in signaling through CRAF, another Raf isoform that plays a role in the induction of terminal differentiation of keratinocytes, with a subsequent increase in MAPK signaling.12-14 Self-resolution of this cycle despite continuing BRAF inhibitor therapy suggests the possible involvement of balancing and/or alternative mechanistic pathways that may be related to the immune system. Although verrucous keratoses are considered benign proliferations and do not necessarily require any specific treatment or reduction in BRAF inhibitor dosage, they may be treated with cryotherapy, electrocautery, shave removal, or excision,15 which often is done if the lesions become inflamed and cause pain. Additionally, some patients may feel distress from the appearance of the lesions and desire treatment for this reason. Understanding that verrucous keratoses can be a transient cutaneous AE rather than a persistent one may be useful to clinicians as they manage AEs during BRAF inhibitor therapy.
- Pakneshan S, Salajegheh A, Smith RA, Lam AK. Clinicopathological relevance of BRAF mutations in human cancer. Pathology. 2013;45:346-356. doi:10.1097/PAT.0b013e328360b61d
- Dhomen N, Marais R. BRAF signaling and targeted therapies in melanoma. Hematol Oncol Clin North Am. 2009;23:529-545. doi:10.1016/j.hoc.2009.04.001
- Long GV, Menzies AM, Nagrial AM, et al. Prognostic and clinicopathologic associations of oncogenic BRAF in metastatic melanoma. J Clin Oncol. 2011;29:1239-1246. doi:10.1200/JCO.2010.32.4327
- Ji Z, Flaherty KT, Tsao H. Targeting the RAS pathway in melanoma. Trends Mol Med. 2012;18:27-35. doi:10.1016/j.molmed.2011.08.001
- Gouda MA, Subbiah V. Precision oncology for BRAF-mutant cancers with BRAF and MEK inhibitors: from melanoma to tissue-agnostic therapy. ESMO Open. 2023;8:100788. doi:10.1016/j.esmoop.2023.100788
- Gençler B, Gönül M. Cutaneous side effects of BRAF inhibitors in advanced melanoma: review of the literature. Dermatol Res Pract. 2016;2016:5361569. doi:10.1155/2016/5361569.
- Chu EY, Wanat KA, Miller CJ, et al. Diverse cutaneous side effects associated with BRAF inhibitor therapy: a clinicopathologic study. J Am Acad Dermatol. 2012;67:1265-1272. doi:10.1016/j.jaad.2012.04.008
- Naqash AR, File DM, Ziemer CM, et al. Cutaneous adverse reactions in B-RAF positive metastatic melanoma following sequential treatment with B-RAF/MEK inhibitors and immune checkpoint blockade or vice versa. a single-institutional case-series. J Immunother Cancer. 2019;7:4. doi:10.1186/s40425-018-0475-y
- Maldonado-Seral C, Berros-Fombella JP, Vivanco-Allende B, et al. Vemurafenib-associated neutrophilic panniculitis: an emergent adverse effect of variable severity. Dermatol Online J. 2013;19:16. doi:10.5070/d370x41670
- Mirali S, Mufti A, Lansang RP, et al. Eruptive seborrheic keratoses are associated with a co-occurring malignancy in the majority of reported cases: a systematic review. J Cutan Med Surg. 2022;26:57-62. doi:10.1177/12034754211035124
- Thiers BH, Sahn RE, Callen JP. Cutaneous manifestations of internal malignancy. CA Cancer J Clin. 2009;59:73-98. doi:10.3322/caac.20005
- Hatzivassiliou G, Song K, Yen I, et al. RAF inhibitors prime wild-type RAF to activate the MAPK pathway and enhance growth. Nature. 2010;464:431-435. doi:10.1038/nature08833
- Heidorn SJ, Milagre C, Whittaker S, et al. Kinase-dead BRAF and oncogenic RAS cooperate to drive tumor progression through CRAF. Cell. 2010;140:209-221. doi:10.1016/j.cell.2009.12.040
- Poulikakos PI, Zhang C, Bollag G, et al. RAF inhibitors transactivate RAF dimers and ERK signaling in cells with wild-type BRAF. Nature. 2010;464:427-430. doi:10.1038/nature08902
- Hayat MA. Brain Metastases from Primary Tumors, Volume 3: Epidemiology, Biology, and Therapy of Melanoma and Other Cancers. Academic Press; 2016.
To the Editor:
Mutations of the BRAF protein kinase gene are implicated in a variety of malignancies.1 BRAF mutations in malignancies cause the mitogen-activated protein kinase (MAPK) pathway to become constitutively active, which results in unchecked cellular proliferation,2,3 making the BRAF mutation an attractive target for inhibition with pharmacologic agents to potentially halt cancer growth.4 Vemurafenib—the first selective BRAF inhibitor used in clinical practice—initially was approved by the US Food and Drug Administration in 2011. The approval of dabrafenib followed in 2013 and most recently encorafenib in 2018.5
Although targeted treatment of BRAF-mutated malignancies with BRAF inhibitors has become common, it often is associated with cutaneous adverse events (AEs), such as rash, pruritus, photosensitivity, actinic keratosis, and verrucous keratosis. Some reports demonstrate these events in up to 95% of patients undergoing BRAF inhibitor treatment.6 In several cases the eruption of verrucous keratoses is among the most common cutaneous AEs seen among patients receiving BRAF inhibitor treatment.5-7
In general, lesions can appear days to months after therapy is initiated and may resolve after switching to dual therapy with a MEK inhibitor or with complete cessation of BRAF inhibitor therapy.5,7,8 One case of spontaneous resolution of vemurafenib-associated panniculitis during ongoing BRAF inhibitor therapy has been reported9; however, spontaneous resolution of cutaneous AEs is uncommon. Herein, we describe verrucous keratoses in a patient undergoing treatment with encorafenib that resolved spontaneously despite ongoing BRAF inhibitor therapy.
A 61-year-old woman presented to the emergency department with pain in the right lower quadrant. Computed tomography (CT) of the abdomen and pelvis revealed a large ovarian mass. Subsequent bloodwork revealed elevated carcinoembryonic antigen levels. The patient underwent a hysterectomy, bilateral salpingo-oophorectomy, omentectomy, right hemicolectomy with ileotransverse side-to-side anastomosis, right pelvic lymph node reduction, and complete cytoreduction. Histopathology revealed an adenocarcinoma of the cecum with tumor invasion into the visceral peritoneum and metastases to the left ovary, fallopian tube, and omentum. A BRAF V600E mutation was detected.
Two months after the initial presentation, the patient started her first cycle of chemotherapy with a combination of folinic acid, fluorouracil, and oxaliplatin. She completed 11 cycles of this regimen, then was switched to capecitabine and oxaliplatin for an additional 2 cycles due to insurance concerns. At the end of treatment, there was no evidence of disease on CT, thus the patient was followed with observation. However, she presented 10 months later to the emergency department with abdominal pain, and CT revealed new lesions in the liver that were concerning for potential metastases. She started oral encorafenib 300 mg/d and intravenous cetuximab 500 mg weekly; after 1 week, encorafenib was reduced to 150 mg/d due to nausea and loss of appetite. Within 2 weeks of starting treatment, the patient reported the relatively abrupt appearance of more than 50 small papules across the shoulders and back (Figure 1A). She was referred to dermatology, and shave biopsies of 2 lesions—one from the left anterior thigh, the other from the right posterior shoulder—revealed verrucous keratosis pathology (Figure 2). At this time, encorafenib was increased again to 300 mg/d as the patient had been tolerating the reduced dose. She continued to report the appearance of new lesions for the next 3 months, after which the lesions were stable for approximately 2 months. By 2.5 months after initiation of therapy, the patient had undergone CT demonstrating resolution of the liver lesions. At 5 months of therapy, the patient reported a stable to slightly reduced number of skin lesions but had begun to experience worsening joint pain, and the dosage of encorafenib was reduced to 225 mg/d. At 7 months of therapy, the dosage was further reduced to 150 mg/d due to persistent arthralgia. A follow-up examination at 10 months of therapy showed improvement in the number and size of the verrucous keratoses, and near resolution was seen by 14 months after the initial onset of the lesions (Figure 1B). At 20 months after initial onset, only 1 remaining verrucous keratosis was identified on physical examination and biopsy. The patient had continued a regimen of encorafenib 150 mg/d and weekly intravenous 500 mg cetuximab up to this point. Over the entire time period that the patient was seen, up to 12 lesions located in high-friction areas had become irritated and were treated with cryotherapy, but this contributed only minorly to the patient’s overall presentation.


Verrucous keratosis is a known cutaneous AE of BRAF inhibitor treatment with vemurafenib and dabrafenib, with fewer cases attributed to encorafenib.5,6 Within the oncologic setting, the eruption of verrucous papules as a paraneoplastic phenomenon is heavily debated in the literature and is known as the Leser-Trélat sign. This phenomenon is commonly associated with adenocarcinomas of the gastrointestinal tract, as seen in our patient.10 Based on Curth’s postulates—the criteria used to evaluate the relationship between an internal malignancy and a cutaneous disorder—this was unlikely in our patient. The criteria, which do not all need to be met to suggest a paraneoplastic phenomenon, include concurrent onset of the malignancy and the dermatosis, parallel course, association of a specific dermatosis with a specific malignancy, statistical significance of the association, and the presence of a genetic basis for the association.11 Several features favored a drug-related cutaneous eruption vs a paraneoplastic phenomenon: (1) the malignancy was identified months before the cutaneous eruptions manifested; (2) the cutaneous lesions appeared once treatment had already been initiated; and (3) the cutaneous lesions persisted long after the malignancy was no longer identifiable on CT. Indeed, eruption of the papules temporally coincided closely with the initiation of BRAF inhibitor therapy, arguing for correlation.
As a suspected BRAF inhibitor–associated cutaneous AE, the eruption of verrucous keratoses in our patient is remarkable for its spontaneous resolution despite ongoing therapy. It is speculated that keratinocytic proliferation while on BRAF inhibitor therapy may be caused by a paradoxical increase in signaling through CRAF, another Raf isoform that plays a role in the induction of terminal differentiation of keratinocytes, with a subsequent increase in MAPK signaling.12-14 Self-resolution of this cycle despite continuing BRAF inhibitor therapy suggests the possible involvement of balancing and/or alternative mechanistic pathways that may be related to the immune system. Although verrucous keratoses are considered benign proliferations and do not necessarily require any specific treatment or reduction in BRAF inhibitor dosage, they may be treated with cryotherapy, electrocautery, shave removal, or excision,15 which often is done if the lesions become inflamed and cause pain. Additionally, some patients may feel distress from the appearance of the lesions and desire treatment for this reason. Understanding that verrucous keratoses can be a transient cutaneous AE rather than a persistent one may be useful to clinicians as they manage AEs during BRAF inhibitor therapy.
To the Editor:
Mutations of the BRAF protein kinase gene are implicated in a variety of malignancies.1 BRAF mutations in malignancies cause the mitogen-activated protein kinase (MAPK) pathway to become constitutively active, which results in unchecked cellular proliferation,2,3 making the BRAF mutation an attractive target for inhibition with pharmacologic agents to potentially halt cancer growth.4 Vemurafenib—the first selective BRAF inhibitor used in clinical practice—initially was approved by the US Food and Drug Administration in 2011. The approval of dabrafenib followed in 2013 and most recently encorafenib in 2018.5
Although targeted treatment of BRAF-mutated malignancies with BRAF inhibitors has become common, it often is associated with cutaneous adverse events (AEs), such as rash, pruritus, photosensitivity, actinic keratosis, and verrucous keratosis. Some reports demonstrate these events in up to 95% of patients undergoing BRAF inhibitor treatment.6 In several cases the eruption of verrucous keratoses is among the most common cutaneous AEs seen among patients receiving BRAF inhibitor treatment.5-7
In general, lesions can appear days to months after therapy is initiated and may resolve after switching to dual therapy with a MEK inhibitor or with complete cessation of BRAF inhibitor therapy.5,7,8 One case of spontaneous resolution of vemurafenib-associated panniculitis during ongoing BRAF inhibitor therapy has been reported9; however, spontaneous resolution of cutaneous AEs is uncommon. Herein, we describe verrucous keratoses in a patient undergoing treatment with encorafenib that resolved spontaneously despite ongoing BRAF inhibitor therapy.
A 61-year-old woman presented to the emergency department with pain in the right lower quadrant. Computed tomography (CT) of the abdomen and pelvis revealed a large ovarian mass. Subsequent bloodwork revealed elevated carcinoembryonic antigen levels. The patient underwent a hysterectomy, bilateral salpingo-oophorectomy, omentectomy, right hemicolectomy with ileotransverse side-to-side anastomosis, right pelvic lymph node reduction, and complete cytoreduction. Histopathology revealed an adenocarcinoma of the cecum with tumor invasion into the visceral peritoneum and metastases to the left ovary, fallopian tube, and omentum. A BRAF V600E mutation was detected.
Two months after the initial presentation, the patient started her first cycle of chemotherapy with a combination of folinic acid, fluorouracil, and oxaliplatin. She completed 11 cycles of this regimen, then was switched to capecitabine and oxaliplatin for an additional 2 cycles due to insurance concerns. At the end of treatment, there was no evidence of disease on CT, thus the patient was followed with observation. However, she presented 10 months later to the emergency department with abdominal pain, and CT revealed new lesions in the liver that were concerning for potential metastases. She started oral encorafenib 300 mg/d and intravenous cetuximab 500 mg weekly; after 1 week, encorafenib was reduced to 150 mg/d due to nausea and loss of appetite. Within 2 weeks of starting treatment, the patient reported the relatively abrupt appearance of more than 50 small papules across the shoulders and back (Figure 1A). She was referred to dermatology, and shave biopsies of 2 lesions—one from the left anterior thigh, the other from the right posterior shoulder—revealed verrucous keratosis pathology (Figure 2). At this time, encorafenib was increased again to 300 mg/d as the patient had been tolerating the reduced dose. She continued to report the appearance of new lesions for the next 3 months, after which the lesions were stable for approximately 2 months. By 2.5 months after initiation of therapy, the patient had undergone CT demonstrating resolution of the liver lesions. At 5 months of therapy, the patient reported a stable to slightly reduced number of skin lesions but had begun to experience worsening joint pain, and the dosage of encorafenib was reduced to 225 mg/d. At 7 months of therapy, the dosage was further reduced to 150 mg/d due to persistent arthralgia. A follow-up examination at 10 months of therapy showed improvement in the number and size of the verrucous keratoses, and near resolution was seen by 14 months after the initial onset of the lesions (Figure 1B). At 20 months after initial onset, only 1 remaining verrucous keratosis was identified on physical examination and biopsy. The patient had continued a regimen of encorafenib 150 mg/d and weekly intravenous 500 mg cetuximab up to this point. Over the entire time period that the patient was seen, up to 12 lesions located in high-friction areas had become irritated and were treated with cryotherapy, but this contributed only minorly to the patient’s overall presentation.


Verrucous keratosis is a known cutaneous AE of BRAF inhibitor treatment with vemurafenib and dabrafenib, with fewer cases attributed to encorafenib.5,6 Within the oncologic setting, the eruption of verrucous papules as a paraneoplastic phenomenon is heavily debated in the literature and is known as the Leser-Trélat sign. This phenomenon is commonly associated with adenocarcinomas of the gastrointestinal tract, as seen in our patient.10 Based on Curth’s postulates—the criteria used to evaluate the relationship between an internal malignancy and a cutaneous disorder—this was unlikely in our patient. The criteria, which do not all need to be met to suggest a paraneoplastic phenomenon, include concurrent onset of the malignancy and the dermatosis, parallel course, association of a specific dermatosis with a specific malignancy, statistical significance of the association, and the presence of a genetic basis for the association.11 Several features favored a drug-related cutaneous eruption vs a paraneoplastic phenomenon: (1) the malignancy was identified months before the cutaneous eruptions manifested; (2) the cutaneous lesions appeared once treatment had already been initiated; and (3) the cutaneous lesions persisted long after the malignancy was no longer identifiable on CT. Indeed, eruption of the papules temporally coincided closely with the initiation of BRAF inhibitor therapy, arguing for correlation.
As a suspected BRAF inhibitor–associated cutaneous AE, the eruption of verrucous keratoses in our patient is remarkable for its spontaneous resolution despite ongoing therapy. It is speculated that keratinocytic proliferation while on BRAF inhibitor therapy may be caused by a paradoxical increase in signaling through CRAF, another Raf isoform that plays a role in the induction of terminal differentiation of keratinocytes, with a subsequent increase in MAPK signaling.12-14 Self-resolution of this cycle despite continuing BRAF inhibitor therapy suggests the possible involvement of balancing and/or alternative mechanistic pathways that may be related to the immune system. Although verrucous keratoses are considered benign proliferations and do not necessarily require any specific treatment or reduction in BRAF inhibitor dosage, they may be treated with cryotherapy, electrocautery, shave removal, or excision,15 which often is done if the lesions become inflamed and cause pain. Additionally, some patients may feel distress from the appearance of the lesions and desire treatment for this reason. Understanding that verrucous keratoses can be a transient cutaneous AE rather than a persistent one may be useful to clinicians as they manage AEs during BRAF inhibitor therapy.
- Pakneshan S, Salajegheh A, Smith RA, Lam AK. Clinicopathological relevance of BRAF mutations in human cancer. Pathology. 2013;45:346-356. doi:10.1097/PAT.0b013e328360b61d
- Dhomen N, Marais R. BRAF signaling and targeted therapies in melanoma. Hematol Oncol Clin North Am. 2009;23:529-545. doi:10.1016/j.hoc.2009.04.001
- Long GV, Menzies AM, Nagrial AM, et al. Prognostic and clinicopathologic associations of oncogenic BRAF in metastatic melanoma. J Clin Oncol. 2011;29:1239-1246. doi:10.1200/JCO.2010.32.4327
- Ji Z, Flaherty KT, Tsao H. Targeting the RAS pathway in melanoma. Trends Mol Med. 2012;18:27-35. doi:10.1016/j.molmed.2011.08.001
- Gouda MA, Subbiah V. Precision oncology for BRAF-mutant cancers with BRAF and MEK inhibitors: from melanoma to tissue-agnostic therapy. ESMO Open. 2023;8:100788. doi:10.1016/j.esmoop.2023.100788
- Gençler B, Gönül M. Cutaneous side effects of BRAF inhibitors in advanced melanoma: review of the literature. Dermatol Res Pract. 2016;2016:5361569. doi:10.1155/2016/5361569.
- Chu EY, Wanat KA, Miller CJ, et al. Diverse cutaneous side effects associated with BRAF inhibitor therapy: a clinicopathologic study. J Am Acad Dermatol. 2012;67:1265-1272. doi:10.1016/j.jaad.2012.04.008
- Naqash AR, File DM, Ziemer CM, et al. Cutaneous adverse reactions in B-RAF positive metastatic melanoma following sequential treatment with B-RAF/MEK inhibitors and immune checkpoint blockade or vice versa. a single-institutional case-series. J Immunother Cancer. 2019;7:4. doi:10.1186/s40425-018-0475-y
- Maldonado-Seral C, Berros-Fombella JP, Vivanco-Allende B, et al. Vemurafenib-associated neutrophilic panniculitis: an emergent adverse effect of variable severity. Dermatol Online J. 2013;19:16. doi:10.5070/d370x41670
- Mirali S, Mufti A, Lansang RP, et al. Eruptive seborrheic keratoses are associated with a co-occurring malignancy in the majority of reported cases: a systematic review. J Cutan Med Surg. 2022;26:57-62. doi:10.1177/12034754211035124
- Thiers BH, Sahn RE, Callen JP. Cutaneous manifestations of internal malignancy. CA Cancer J Clin. 2009;59:73-98. doi:10.3322/caac.20005
- Hatzivassiliou G, Song K, Yen I, et al. RAF inhibitors prime wild-type RAF to activate the MAPK pathway and enhance growth. Nature. 2010;464:431-435. doi:10.1038/nature08833
- Heidorn SJ, Milagre C, Whittaker S, et al. Kinase-dead BRAF and oncogenic RAS cooperate to drive tumor progression through CRAF. Cell. 2010;140:209-221. doi:10.1016/j.cell.2009.12.040
- Poulikakos PI, Zhang C, Bollag G, et al. RAF inhibitors transactivate RAF dimers and ERK signaling in cells with wild-type BRAF. Nature. 2010;464:427-430. doi:10.1038/nature08902
- Hayat MA. Brain Metastases from Primary Tumors, Volume 3: Epidemiology, Biology, and Therapy of Melanoma and Other Cancers. Academic Press; 2016.
- Pakneshan S, Salajegheh A, Smith RA, Lam AK. Clinicopathological relevance of BRAF mutations in human cancer. Pathology. 2013;45:346-356. doi:10.1097/PAT.0b013e328360b61d
- Dhomen N, Marais R. BRAF signaling and targeted therapies in melanoma. Hematol Oncol Clin North Am. 2009;23:529-545. doi:10.1016/j.hoc.2009.04.001
- Long GV, Menzies AM, Nagrial AM, et al. Prognostic and clinicopathologic associations of oncogenic BRAF in metastatic melanoma. J Clin Oncol. 2011;29:1239-1246. doi:10.1200/JCO.2010.32.4327
- Ji Z, Flaherty KT, Tsao H. Targeting the RAS pathway in melanoma. Trends Mol Med. 2012;18:27-35. doi:10.1016/j.molmed.2011.08.001
- Gouda MA, Subbiah V. Precision oncology for BRAF-mutant cancers with BRAF and MEK inhibitors: from melanoma to tissue-agnostic therapy. ESMO Open. 2023;8:100788. doi:10.1016/j.esmoop.2023.100788
- Gençler B, Gönül M. Cutaneous side effects of BRAF inhibitors in advanced melanoma: review of the literature. Dermatol Res Pract. 2016;2016:5361569. doi:10.1155/2016/5361569.
- Chu EY, Wanat KA, Miller CJ, et al. Diverse cutaneous side effects associated with BRAF inhibitor therapy: a clinicopathologic study. J Am Acad Dermatol. 2012;67:1265-1272. doi:10.1016/j.jaad.2012.04.008
- Naqash AR, File DM, Ziemer CM, et al. Cutaneous adverse reactions in B-RAF positive metastatic melanoma following sequential treatment with B-RAF/MEK inhibitors and immune checkpoint blockade or vice versa. a single-institutional case-series. J Immunother Cancer. 2019;7:4. doi:10.1186/s40425-018-0475-y
- Maldonado-Seral C, Berros-Fombella JP, Vivanco-Allende B, et al. Vemurafenib-associated neutrophilic panniculitis: an emergent adverse effect of variable severity. Dermatol Online J. 2013;19:16. doi:10.5070/d370x41670
- Mirali S, Mufti A, Lansang RP, et al. Eruptive seborrheic keratoses are associated with a co-occurring malignancy in the majority of reported cases: a systematic review. J Cutan Med Surg. 2022;26:57-62. doi:10.1177/12034754211035124
- Thiers BH, Sahn RE, Callen JP. Cutaneous manifestations of internal malignancy. CA Cancer J Clin. 2009;59:73-98. doi:10.3322/caac.20005
- Hatzivassiliou G, Song K, Yen I, et al. RAF inhibitors prime wild-type RAF to activate the MAPK pathway and enhance growth. Nature. 2010;464:431-435. doi:10.1038/nature08833
- Heidorn SJ, Milagre C, Whittaker S, et al. Kinase-dead BRAF and oncogenic RAS cooperate to drive tumor progression through CRAF. Cell. 2010;140:209-221. doi:10.1016/j.cell.2009.12.040
- Poulikakos PI, Zhang C, Bollag G, et al. RAF inhibitors transactivate RAF dimers and ERK signaling in cells with wild-type BRAF. Nature. 2010;464:427-430. doi:10.1038/nature08902
- Hayat MA. Brain Metastases from Primary Tumors, Volume 3: Epidemiology, Biology, and Therapy of Melanoma and Other Cancers. Academic Press; 2016.
Practice Points
- Verrucous keratoses are common cutaneous adverse events (AEs) associated with BRAF inhibitor therapy.
- Verrucous papules may be a paraneoplastic phenomenon and can be differentiated from a treatment-related AE based on the timing and progression in relation to tumor burden.
- Although treatment of particularly bothersome lesions with cryotherapy may be warranted, verrucous papules secondary to BRAF inhibitor therapy may resolve spontaneously.
Is Intravenous Iron More Effective Than Oral Iron for Anemia During Pregnancy?
TOPLINE:
Intravenous iron reduced iron deficiency more effectively than oral iron, which is often distasteful, among pregnant women in Nigeria. However, no significant difference was found in the prevalence of anemia or preterm birth between the two groups.
METHODOLOGY:
- A total of 1056 pregnant women aged 15-49 years with hemoglobin concentrations 10 g/dL at 20-32 weeks’ gestation were included in the trial.
- Participants were randomly assigned to receive either a single dose of intravenous ferric carboxymaltose (20 mg/kg to a maximum of 1000 mg) or oral ferrous sulphate (200 mg; 65 mg elemental iron) three times daily until 6 weeks postpartum.
- Primary outcomes were maternal anemia (hemoglobin, < 11 g/dL) at 36 weeks’ gestation and preterm birth before 37 weeks’ gestation.
- Secondary outcomes were iron deficiency, iron deficiency anemia, maternal depression, infections, immunization, and breastfeeding practices.
- The trial was conducted in 11 health facilities in Lagos and Kano, Nigeria, with follow-up visits at 2 weeks and 6 weeks postpartum.
TAKEAWAY:
- No significant difference was found in the prevalence of anemia at 36 weeks’ gestation between the intravenous and oral iron groups (58% vs 61%; P = .36).
- Intravenous iron was more effective at reducing iron deficiency (5% vs 16%; P = .0001) and iron deficiency anemia (2% vs 10%; P = .0001) at 36 weeks’ gestation.
- The incidence of preterm birth did not significantly differ between the intravenous and oral iron groups (14% vs 15%; P = .66).
- Intravenous iron led to a higher mean hemoglobin concentration from baseline to 4 weeks in both iron-deficient and non–iron-deficient subgroups.
IN PRACTICE:
“Although the effect on overall anaemia did not differ, intravenous iron reduced the prevalence of iron deficiency to a greater extent than oral iron and was considered to be safe. We recommend that intravenous iron be considered for anaemic pregnant women in Nigeria and similar settings,” wrote the authors of the study.
SOURCE:
This study was led by Bosede B. Afolabi, Department of Obstetrics and Gynaecology, Faculty of Clinical Sciences, College of Medicine, University of Lagos, Nigeria. It was published online in The Lancet Global Health.
LIMITATIONS:
The study’s sample size estimation assumed a 25% rate of preterm births, but the actual rate was only 14.5%, which potentially underpowered the study to measure this outcome. Most participants were enrolled after 20 weeks’ gestation, which limited the ability to explore the effect of treatment duration. The interpretation of postpartum hemorrhage was limited by the use of visual assessment to determine blood loss, which is subjective.
DISCLOSURES:
A coathor, Kristi S. Annerstedt, PhD, reported participation on the ALERT project Data Safety Monitoring Board. Additional disclosures are noted in the original article. The study was supported by grants from the Bill & Melinda Gates Foundation.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
Intravenous iron reduced iron deficiency more effectively than oral iron, which is often distasteful, among pregnant women in Nigeria. However, no significant difference was found in the prevalence of anemia or preterm birth between the two groups.
METHODOLOGY:
- A total of 1056 pregnant women aged 15-49 years with hemoglobin concentrations 10 g/dL at 20-32 weeks’ gestation were included in the trial.
- Participants were randomly assigned to receive either a single dose of intravenous ferric carboxymaltose (20 mg/kg to a maximum of 1000 mg) or oral ferrous sulphate (200 mg; 65 mg elemental iron) three times daily until 6 weeks postpartum.
- Primary outcomes were maternal anemia (hemoglobin, < 11 g/dL) at 36 weeks’ gestation and preterm birth before 37 weeks’ gestation.
- Secondary outcomes were iron deficiency, iron deficiency anemia, maternal depression, infections, immunization, and breastfeeding practices.
- The trial was conducted in 11 health facilities in Lagos and Kano, Nigeria, with follow-up visits at 2 weeks and 6 weeks postpartum.
TAKEAWAY:
- No significant difference was found in the prevalence of anemia at 36 weeks’ gestation between the intravenous and oral iron groups (58% vs 61%; P = .36).
- Intravenous iron was more effective at reducing iron deficiency (5% vs 16%; P = .0001) and iron deficiency anemia (2% vs 10%; P = .0001) at 36 weeks’ gestation.
- The incidence of preterm birth did not significantly differ between the intravenous and oral iron groups (14% vs 15%; P = .66).
- Intravenous iron led to a higher mean hemoglobin concentration from baseline to 4 weeks in both iron-deficient and non–iron-deficient subgroups.
IN PRACTICE:
“Although the effect on overall anaemia did not differ, intravenous iron reduced the prevalence of iron deficiency to a greater extent than oral iron and was considered to be safe. We recommend that intravenous iron be considered for anaemic pregnant women in Nigeria and similar settings,” wrote the authors of the study.
SOURCE:
This study was led by Bosede B. Afolabi, Department of Obstetrics and Gynaecology, Faculty of Clinical Sciences, College of Medicine, University of Lagos, Nigeria. It was published online in The Lancet Global Health.
LIMITATIONS:
The study’s sample size estimation assumed a 25% rate of preterm births, but the actual rate was only 14.5%, which potentially underpowered the study to measure this outcome. Most participants were enrolled after 20 weeks’ gestation, which limited the ability to explore the effect of treatment duration. The interpretation of postpartum hemorrhage was limited by the use of visual assessment to determine blood loss, which is subjective.
DISCLOSURES:
A coathor, Kristi S. Annerstedt, PhD, reported participation on the ALERT project Data Safety Monitoring Board. Additional disclosures are noted in the original article. The study was supported by grants from the Bill & Melinda Gates Foundation.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
Intravenous iron reduced iron deficiency more effectively than oral iron, which is often distasteful, among pregnant women in Nigeria. However, no significant difference was found in the prevalence of anemia or preterm birth between the two groups.
METHODOLOGY:
- A total of 1056 pregnant women aged 15-49 years with hemoglobin concentrations 10 g/dL at 20-32 weeks’ gestation were included in the trial.
- Participants were randomly assigned to receive either a single dose of intravenous ferric carboxymaltose (20 mg/kg to a maximum of 1000 mg) or oral ferrous sulphate (200 mg; 65 mg elemental iron) three times daily until 6 weeks postpartum.
- Primary outcomes were maternal anemia (hemoglobin, < 11 g/dL) at 36 weeks’ gestation and preterm birth before 37 weeks’ gestation.
- Secondary outcomes were iron deficiency, iron deficiency anemia, maternal depression, infections, immunization, and breastfeeding practices.
- The trial was conducted in 11 health facilities in Lagos and Kano, Nigeria, with follow-up visits at 2 weeks and 6 weeks postpartum.
TAKEAWAY:
- No significant difference was found in the prevalence of anemia at 36 weeks’ gestation between the intravenous and oral iron groups (58% vs 61%; P = .36).
- Intravenous iron was more effective at reducing iron deficiency (5% vs 16%; P = .0001) and iron deficiency anemia (2% vs 10%; P = .0001) at 36 weeks’ gestation.
- The incidence of preterm birth did not significantly differ between the intravenous and oral iron groups (14% vs 15%; P = .66).
- Intravenous iron led to a higher mean hemoglobin concentration from baseline to 4 weeks in both iron-deficient and non–iron-deficient subgroups.
IN PRACTICE:
“Although the effect on overall anaemia did not differ, intravenous iron reduced the prevalence of iron deficiency to a greater extent than oral iron and was considered to be safe. We recommend that intravenous iron be considered for anaemic pregnant women in Nigeria and similar settings,” wrote the authors of the study.
SOURCE:
This study was led by Bosede B. Afolabi, Department of Obstetrics and Gynaecology, Faculty of Clinical Sciences, College of Medicine, University of Lagos, Nigeria. It was published online in The Lancet Global Health.
LIMITATIONS:
The study’s sample size estimation assumed a 25% rate of preterm births, but the actual rate was only 14.5%, which potentially underpowered the study to measure this outcome. Most participants were enrolled after 20 weeks’ gestation, which limited the ability to explore the effect of treatment duration. The interpretation of postpartum hemorrhage was limited by the use of visual assessment to determine blood loss, which is subjective.
DISCLOSURES:
A coathor, Kristi S. Annerstedt, PhD, reported participation on the ALERT project Data Safety Monitoring Board. Additional disclosures are noted in the original article. The study was supported by grants from the Bill & Melinda Gates Foundation.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
Seborrheic Dermatitis in Black Patients: New Therapies Offer Hope
NEW YORK — not only in this group but also overall, now that there is an approved therapy with an array of alternatives and adjunctive medications, according to Shawn Kwatra, MD.
The list of therapies effective against SD, often employed in combination, is lengthy, but topical 0.3% roflumilast foam (Zoryve), approved by the Food and Drug Administration (FDA) late last year for treating SD, has a high rate of efficacy and should now be considered a first-line treatment option, according to Dr. Kwatra, professor and chair of the Department of Dermatology, University of Maryland School of Medicine, Baltimore.
New Approved Therapy Draws Attention to SD
Emphasizing that topical roflumilast does not necessarily replace the use of over-the-counter shampoos and emollients or a list of prescription drugs used off-label to control this condition, he said it is also important for another reason.
“It shines a light on this disease,” said Dr. Kwatra, speaking at the 2024 Skin of Color Update. While his comments were focused primarily on individuals with darker skin, his major take home messages were broadly relevant across skin types.
He acknowledged that for years he “had not given seborrheic dermatitis the respect that it deserves” even though this condition comes after only acne and eczema as chief complaints among Black individuals seeing a dermatologist. The estimated global incidence is 5%, according to Dr. Kwatra, but he considers this estimate of an often “forgotten disease” too low.
One reason is that many individuals self-treat with over-the-counter solutions and never bring the complaint to a clinician. Dr. Kwatra said that he now looks for it routinely and points it out to patients who have come to him for another reason.
In patients with darker skin, the signs of SD can differ. While scalp involvement is generally easy to identify across skin types, the inflammation and erythema, sebum production, scaling and itch, and Malassezia that accompanies and drives SD might be missed in a patient with darker skin without specifically looking for these signs.
Skin and Gut Microbiome Involvement Suspected
The underlying causes of SD are understood as an inflammatory process involving keratinocyte disruption and proliferation that ultimately impairs skin barrier function, causes water loss, and produces scale stemming from stratum corneum, but Dr. Kwatra said that there is increasing evidence of a major role for both the skin and gut microbiome.
In regard to the skin microbiome, Malassezia has long been recognized as linked to SD and is a target of treatment, but evidence that the gut microbiome might be participating is relatively new. One clue comes from the fact that oral antifungal therapies, such as itraconazole, are known to reduce risk for SD relapse, an effect that might be a function of their ability to modulate the gut microbiome, according to Dr. Kwatra.
Topical roflumilast, a phosphodiesterase-4 inhibitor, was effective for SD in a vehicle-controlled phase 3 trial published in 2023. He characterized the adverse event profile as “pretty clean,” but he emphasized that a role for many other strategies remains. This is particularly true for challenging forms of SD. For example, topical tacrolimus provided meaningful protection against relapse over a period of more than 6 months in a 2021 trial that enrolled patients with severe facial SD.
The topical Janus kinase inhibitor ruxolitinib, 1.5%, (approved for atopic dermatitis and vitiligo) has also been reported to be effective for refractory facial SD. It is being evaluated in a phase 2 study of SD, according to Dr. Kwatra. A topical PDE4 inhibitor is also being evaluated for SD in a phase 2 study, he said.
Given the heterogeneity of the presentation of SD and the value of combining different mechanisms of action, Dr. Kwatra does not think any drug by itself will be a cure for SD. However, the chances of success with current drug combinations are high.
It is for this reason that Dr. Kwatra encourages clinicians to look for this disease routinely, including among patients who have a different presenting complaint. “Patients do not always bring it up, so bring it up,” he said.
This is good advice, according to Andrew F. Alexis, MD, MPH, professor of clinical dermatology and Vice-chair for Diversity and Inclusion of the Department of Dermatology, Weill Cornell Medicine, New York City. He agreed that the recent introduction of a therapy approved by the FDA is an impetus to look for SD and to talk with patients about treatment options.
In addition, while he also considers roflumilast foam to be a first-line drug, he agreed that combination therapies might be needed to increase the likely of rapid control of scalp and skin involvement. “SD is probably underestimated as a clinical problem, and we do have good treatments to offer for the patients who are affected,” he said at the meeting.
Dr. Kwatra reported no relevant disclosures. Dr. Alexis reported financial relationships with more than 25 pharmaceutical companies.
A version of this article appeared on Medscape.com.
NEW YORK — not only in this group but also overall, now that there is an approved therapy with an array of alternatives and adjunctive medications, according to Shawn Kwatra, MD.
The list of therapies effective against SD, often employed in combination, is lengthy, but topical 0.3% roflumilast foam (Zoryve), approved by the Food and Drug Administration (FDA) late last year for treating SD, has a high rate of efficacy and should now be considered a first-line treatment option, according to Dr. Kwatra, professor and chair of the Department of Dermatology, University of Maryland School of Medicine, Baltimore.
New Approved Therapy Draws Attention to SD
Emphasizing that topical roflumilast does not necessarily replace the use of over-the-counter shampoos and emollients or a list of prescription drugs used off-label to control this condition, he said it is also important for another reason.
“It shines a light on this disease,” said Dr. Kwatra, speaking at the 2024 Skin of Color Update. While his comments were focused primarily on individuals with darker skin, his major take home messages were broadly relevant across skin types.
He acknowledged that for years he “had not given seborrheic dermatitis the respect that it deserves” even though this condition comes after only acne and eczema as chief complaints among Black individuals seeing a dermatologist. The estimated global incidence is 5%, according to Dr. Kwatra, but he considers this estimate of an often “forgotten disease” too low.
One reason is that many individuals self-treat with over-the-counter solutions and never bring the complaint to a clinician. Dr. Kwatra said that he now looks for it routinely and points it out to patients who have come to him for another reason.
In patients with darker skin, the signs of SD can differ. While scalp involvement is generally easy to identify across skin types, the inflammation and erythema, sebum production, scaling and itch, and Malassezia that accompanies and drives SD might be missed in a patient with darker skin without specifically looking for these signs.
Skin and Gut Microbiome Involvement Suspected
The underlying causes of SD are understood as an inflammatory process involving keratinocyte disruption and proliferation that ultimately impairs skin barrier function, causes water loss, and produces scale stemming from stratum corneum, but Dr. Kwatra said that there is increasing evidence of a major role for both the skin and gut microbiome.
In regard to the skin microbiome, Malassezia has long been recognized as linked to SD and is a target of treatment, but evidence that the gut microbiome might be participating is relatively new. One clue comes from the fact that oral antifungal therapies, such as itraconazole, are known to reduce risk for SD relapse, an effect that might be a function of their ability to modulate the gut microbiome, according to Dr. Kwatra.
Topical roflumilast, a phosphodiesterase-4 inhibitor, was effective for SD in a vehicle-controlled phase 3 trial published in 2023. He characterized the adverse event profile as “pretty clean,” but he emphasized that a role for many other strategies remains. This is particularly true for challenging forms of SD. For example, topical tacrolimus provided meaningful protection against relapse over a period of more than 6 months in a 2021 trial that enrolled patients with severe facial SD.
The topical Janus kinase inhibitor ruxolitinib, 1.5%, (approved for atopic dermatitis and vitiligo) has also been reported to be effective for refractory facial SD. It is being evaluated in a phase 2 study of SD, according to Dr. Kwatra. A topical PDE4 inhibitor is also being evaluated for SD in a phase 2 study, he said.
Given the heterogeneity of the presentation of SD and the value of combining different mechanisms of action, Dr. Kwatra does not think any drug by itself will be a cure for SD. However, the chances of success with current drug combinations are high.
It is for this reason that Dr. Kwatra encourages clinicians to look for this disease routinely, including among patients who have a different presenting complaint. “Patients do not always bring it up, so bring it up,” he said.
This is good advice, according to Andrew F. Alexis, MD, MPH, professor of clinical dermatology and Vice-chair for Diversity and Inclusion of the Department of Dermatology, Weill Cornell Medicine, New York City. He agreed that the recent introduction of a therapy approved by the FDA is an impetus to look for SD and to talk with patients about treatment options.
In addition, while he also considers roflumilast foam to be a first-line drug, he agreed that combination therapies might be needed to increase the likely of rapid control of scalp and skin involvement. “SD is probably underestimated as a clinical problem, and we do have good treatments to offer for the patients who are affected,” he said at the meeting.
Dr. Kwatra reported no relevant disclosures. Dr. Alexis reported financial relationships with more than 25 pharmaceutical companies.
A version of this article appeared on Medscape.com.
NEW YORK — not only in this group but also overall, now that there is an approved therapy with an array of alternatives and adjunctive medications, according to Shawn Kwatra, MD.
The list of therapies effective against SD, often employed in combination, is lengthy, but topical 0.3% roflumilast foam (Zoryve), approved by the Food and Drug Administration (FDA) late last year for treating SD, has a high rate of efficacy and should now be considered a first-line treatment option, according to Dr. Kwatra, professor and chair of the Department of Dermatology, University of Maryland School of Medicine, Baltimore.
New Approved Therapy Draws Attention to SD
Emphasizing that topical roflumilast does not necessarily replace the use of over-the-counter shampoos and emollients or a list of prescription drugs used off-label to control this condition, he said it is also important for another reason.
“It shines a light on this disease,” said Dr. Kwatra, speaking at the 2024 Skin of Color Update. While his comments were focused primarily on individuals with darker skin, his major take home messages were broadly relevant across skin types.
He acknowledged that for years he “had not given seborrheic dermatitis the respect that it deserves” even though this condition comes after only acne and eczema as chief complaints among Black individuals seeing a dermatologist. The estimated global incidence is 5%, according to Dr. Kwatra, but he considers this estimate of an often “forgotten disease” too low.
One reason is that many individuals self-treat with over-the-counter solutions and never bring the complaint to a clinician. Dr. Kwatra said that he now looks for it routinely and points it out to patients who have come to him for another reason.
In patients with darker skin, the signs of SD can differ. While scalp involvement is generally easy to identify across skin types, the inflammation and erythema, sebum production, scaling and itch, and Malassezia that accompanies and drives SD might be missed in a patient with darker skin without specifically looking for these signs.
Skin and Gut Microbiome Involvement Suspected
The underlying causes of SD are understood as an inflammatory process involving keratinocyte disruption and proliferation that ultimately impairs skin barrier function, causes water loss, and produces scale stemming from stratum corneum, but Dr. Kwatra said that there is increasing evidence of a major role for both the skin and gut microbiome.
In regard to the skin microbiome, Malassezia has long been recognized as linked to SD and is a target of treatment, but evidence that the gut microbiome might be participating is relatively new. One clue comes from the fact that oral antifungal therapies, such as itraconazole, are known to reduce risk for SD relapse, an effect that might be a function of their ability to modulate the gut microbiome, according to Dr. Kwatra.
Topical roflumilast, a phosphodiesterase-4 inhibitor, was effective for SD in a vehicle-controlled phase 3 trial published in 2023. He characterized the adverse event profile as “pretty clean,” but he emphasized that a role for many other strategies remains. This is particularly true for challenging forms of SD. For example, topical tacrolimus provided meaningful protection against relapse over a period of more than 6 months in a 2021 trial that enrolled patients with severe facial SD.
The topical Janus kinase inhibitor ruxolitinib, 1.5%, (approved for atopic dermatitis and vitiligo) has also been reported to be effective for refractory facial SD. It is being evaluated in a phase 2 study of SD, according to Dr. Kwatra. A topical PDE4 inhibitor is also being evaluated for SD in a phase 2 study, he said.
Given the heterogeneity of the presentation of SD and the value of combining different mechanisms of action, Dr. Kwatra does not think any drug by itself will be a cure for SD. However, the chances of success with current drug combinations are high.
It is for this reason that Dr. Kwatra encourages clinicians to look for this disease routinely, including among patients who have a different presenting complaint. “Patients do not always bring it up, so bring it up,” he said.
This is good advice, according to Andrew F. Alexis, MD, MPH, professor of clinical dermatology and Vice-chair for Diversity and Inclusion of the Department of Dermatology, Weill Cornell Medicine, New York City. He agreed that the recent introduction of a therapy approved by the FDA is an impetus to look for SD and to talk with patients about treatment options.
In addition, while he also considers roflumilast foam to be a first-line drug, he agreed that combination therapies might be needed to increase the likely of rapid control of scalp and skin involvement. “SD is probably underestimated as a clinical problem, and we do have good treatments to offer for the patients who are affected,” he said at the meeting.
Dr. Kwatra reported no relevant disclosures. Dr. Alexis reported financial relationships with more than 25 pharmaceutical companies.
A version of this article appeared on Medscape.com.
FROM SOC 2024