User login
Revolutionizing Headache Medicine: The Role of Artificial Intelligence
As we move further into the 21st century, technology continues to revolutionize various facets of our lives. Healthcare is a prime example. Advances in technology have dramatically reshaped the way we develop medications, diagnose diseases, and enhance patient care. The rise of artificial intelligence (AI) and the widespread adoption of digital health technologies have marked a significant milestone in improving the quality of care. AI, with its ability to leverage algorithms, deep learning, and machine learning to process data, make decisions, and perform tasks autonomously, is becoming an integral part of modern society. It is embedded in various technologies that we rely on daily, from smartphones and smart home devices to content recommendations on streaming services and social media platforms.
In healthcare, AI has applications in numerous fields, such as radiology. AI streamlines processes such as organizing patient appointments, optimizing radiation protocols for safety and efficiency, and enhancing the documentation process through advanced image analysis. AI technology plays an integral role in imaging tasks like image enhancement, lesion detection, and precise measurement. In difficult-to-interpret radiologic studies, such as some mammography images, it can be a crucial aid to the radiologist. Additionally, the use of AI has significantly improved remote patient monitoring that enables healthcare professionals to monitor and assess patient conditions without needing in-person visits. Remote patient monitoring gained prominence during the COVID-19 pandemic and continues to be a valuable tool in post pandemic care. Study results have highlighted that AI-driven ambient dictation tools have increased provider engagement with patients during consultations while reducing the time spent documenting in electronic health records.
Like many other medical specialties, headache medicine also uses AI. Most prominently, AI has been used in models and engines in assisting with headache diagnoses. A noteworthy example of AI in headache medicine is the development of an online, computer-based diagnostic engine (CDE) by Rapoport et al, called BonTriage. This tool is designed to diagnose headaches by employing a rule set based on the International Classification of Headache Disorders-3 (ICHD-3) criteria for primary headache disorders while also evaluating secondary headaches and medication overuse headaches. By leveraging machine learning, the CDE has the potential to streamline the diagnostic process, reducing the number of questions needed to reach a diagnosis and making the experience more efficient. This information can then be printed as a PDF file and taken by the patient to a healthcare professional for further discussion, fostering a more accurate, fluid, and conversational consultation.
A study was conducted to evaluate the accuracy of the CDE. Participants were randomly assigned to 1 of 2 sequences: (1) using the CDE followed by a structured standard interview with a headache specialist using the same ICHD-3 criteria or (2) starting with the structured standard interview followed by the CDE. The results demonstrated nearly perfect agreement in diagnosing migraine and probable migraine between the CDE and structured standard interview (κ = 0.82, 95% CI: 0.74, 0.90). The CDE demonstrated a diagnostic accuracy of 91.6% (95% CI: 86.9%, 95.0%), a sensitivity rate of 89.0% (95% CI: 82.5%, 93.7%), and a specificity rate of 97.0% (95% CI: 89.5%, 99.6%).
A diagnostic engine such as this can save time that clinicians spend on documentation and allow more time for discussion with the patient. For instance, a patient can take the printout received from the CDE to an appointment; the printout gives a detailed history plus information about social and psychological issues, a list of medications taken, and results of previous testing. The CDE system was originally designed to help patients see a specialist in the environment of a nationwide lack of headache specialists. There are currently 45 million patients with headaches who are seeking treatment with only around 550 certified headache specialists in the United States. The CDE printed information can help a patient obtain a consultation from a clinician quickly and start evaluation and treatment earlier. This expert online consultation is currently free of charge.
Kwon et al developed a machine learning–based model designed to automatically classify headache disorders using data from a questionnaire. Their model was able to predict diagnoses for conditions such as migraine, tension-type headaches, trigeminal autonomic cephalalgia, epicranial headache, and thunderclap headaches. The model was trained on data from 2162 patients, all diagnosed by headache specialists, and achieved an overall accuracy of 81%, with a sensitivity of 88% and a specificity of 95% for diagnosing migraines. However, the model’s performance was less robust when applied to other headache disorders.
Katsuki et al developed an AI model to help non specialists accurately diagnose headaches. This model analyzed 17 variables and was trained on data from 2800 patients, with additional testing and refinement using data from another 200 patients. To evaluate its effectiveness, 2 groups of non-headache specialists each assessed 50 patients: 1 group relied solely on their expertise, while the other used the AI model. The group without AI assistance achieved an overall accuracy of 46% (κ = 0.21), while the group using the AI model significantly improved, reaching an overall accuracy of 83.2% (κ = 0.68).
Building on their work with AI for diagnosing headaches, Katsuki et al conducted a study using a smartphone application that tracked user-reported headache events alongside local weather data. The AI model revealed that lower barometric pressure, higher humidity, and increased rainfall were linked to the onset of headache attacks. The application also identified triggers for headaches in specific weather patterns, such as a drop in barometric pressure noted 6 hours before headache onset. The application of AI in monitoring weather changes could be crucial, especially given concerns that the rising frequency of severe weather events due to climate change may be exacerbating the severity and burden of migraine. Additionally, recent post hoc analyses of fremanezumab clinical trials have provided further evidence that weather changes can trigger headaches.
Rapoport and colleagues have also developed an application called Migraine Mentor, which accurately tracks headaches, triggers, health data, and response to medication on a smartphone. The patient spends 3 minutes a day answering a few questions about their day and whether they had a headache or took any medication. At 1 or 2 months, Migraine Mentor can generate a detailed report with data and current trends that is sent to the patient, which the patient can then share with the clinician. The application also reminds patients when to document data and take medication.
However, although the use of AI in headache medicine appears promising, caution must be exercised to ensure proper results and information are disseminated. One rapidly expanding application of AI is the widely popular ChatGPT. ChatGPT, which stands for generative pretraining transformer, is a type of large language model (LLM). An LLM is a deep learning algorithm designed to recognize, translate, predict, summarize, and generate text responses based on a given prompt. This model is trained on an extensive dataset that includes a diverse array of books, articles, and websites, exposing it to various language structures and styles. This training enables ChatGPT to generate responses that closely mimic human communication. LLMs are being used more and more in medicine to assist with generating patient documentation and educational materials.
However, Dr Fred Cohen published a perspective piece detailing how LLMs (such as ChatGPT) can produce misleading and inaccurate answers. In his example, he tasked ChatGPT to describe the epidemiology of migraines in penguins; the AI model generated a well-written and highly believable manuscript titled, “Migraine Under the Ice: Understanding Headaches in Antarctica's Feathered Friends.” The manuscript highlights that migraines are more prevalent in male penguins compared to females, with the peak age of onset occurring between 4 and 5 years. Additionally, emperor and king penguins are identified as being more susceptible to developing migraines compared to other penguin species. The paper was fictitious (as no studies on migraine in penguins have been written to date), exemplifying that these models can produce nonfactual materials.
For years, technological advancements have been reshaping many aspects of life, and medicine is no exception. AI has been successfully applied to streamline medical documentation, develop new drug targets, and deepen our understanding of various diseases. The field of headache medicine now also uses AI. Recent developments show significant promise, with AI aiding in the diagnosis of migraine and other headache disorders. AI models have even been used in the identification of potential drug targets for migraine treatment. Although there are still limitations to overcome, the future of AI in headache medicine appears bright.
If you would like to read more about Dr. Cohen’s work on AI and migraine, please visit fredcohenmd.com or TikTok @fredcohenmd.
As we move further into the 21st century, technology continues to revolutionize various facets of our lives. Healthcare is a prime example. Advances in technology have dramatically reshaped the way we develop medications, diagnose diseases, and enhance patient care. The rise of artificial intelligence (AI) and the widespread adoption of digital health technologies have marked a significant milestone in improving the quality of care. AI, with its ability to leverage algorithms, deep learning, and machine learning to process data, make decisions, and perform tasks autonomously, is becoming an integral part of modern society. It is embedded in various technologies that we rely on daily, from smartphones and smart home devices to content recommendations on streaming services and social media platforms.
In healthcare, AI has applications in numerous fields, such as radiology. AI streamlines processes such as organizing patient appointments, optimizing radiation protocols for safety and efficiency, and enhancing the documentation process through advanced image analysis. AI technology plays an integral role in imaging tasks like image enhancement, lesion detection, and precise measurement. In difficult-to-interpret radiologic studies, such as some mammography images, it can be a crucial aid to the radiologist. Additionally, the use of AI has significantly improved remote patient monitoring that enables healthcare professionals to monitor and assess patient conditions without needing in-person visits. Remote patient monitoring gained prominence during the COVID-19 pandemic and continues to be a valuable tool in post pandemic care. Study results have highlighted that AI-driven ambient dictation tools have increased provider engagement with patients during consultations while reducing the time spent documenting in electronic health records.
Like many other medical specialties, headache medicine also uses AI. Most prominently, AI has been used in models and engines in assisting with headache diagnoses. A noteworthy example of AI in headache medicine is the development of an online, computer-based diagnostic engine (CDE) by Rapoport et al, called BonTriage. This tool is designed to diagnose headaches by employing a rule set based on the International Classification of Headache Disorders-3 (ICHD-3) criteria for primary headache disorders while also evaluating secondary headaches and medication overuse headaches. By leveraging machine learning, the CDE has the potential to streamline the diagnostic process, reducing the number of questions needed to reach a diagnosis and making the experience more efficient. This information can then be printed as a PDF file and taken by the patient to a healthcare professional for further discussion, fostering a more accurate, fluid, and conversational consultation.
A study was conducted to evaluate the accuracy of the CDE. Participants were randomly assigned to 1 of 2 sequences: (1) using the CDE followed by a structured standard interview with a headache specialist using the same ICHD-3 criteria or (2) starting with the structured standard interview followed by the CDE. The results demonstrated nearly perfect agreement in diagnosing migraine and probable migraine between the CDE and structured standard interview (κ = 0.82, 95% CI: 0.74, 0.90). The CDE demonstrated a diagnostic accuracy of 91.6% (95% CI: 86.9%, 95.0%), a sensitivity rate of 89.0% (95% CI: 82.5%, 93.7%), and a specificity rate of 97.0% (95% CI: 89.5%, 99.6%).
A diagnostic engine such as this can save time that clinicians spend on documentation and allow more time for discussion with the patient. For instance, a patient can take the printout received from the CDE to an appointment; the printout gives a detailed history plus information about social and psychological issues, a list of medications taken, and results of previous testing. The CDE system was originally designed to help patients see a specialist in the environment of a nationwide lack of headache specialists. There are currently 45 million patients with headaches who are seeking treatment with only around 550 certified headache specialists in the United States. The CDE printed information can help a patient obtain a consultation from a clinician quickly and start evaluation and treatment earlier. This expert online consultation is currently free of charge.
Kwon et al developed a machine learning–based model designed to automatically classify headache disorders using data from a questionnaire. Their model was able to predict diagnoses for conditions such as migraine, tension-type headaches, trigeminal autonomic cephalalgia, epicranial headache, and thunderclap headaches. The model was trained on data from 2162 patients, all diagnosed by headache specialists, and achieved an overall accuracy of 81%, with a sensitivity of 88% and a specificity of 95% for diagnosing migraines. However, the model’s performance was less robust when applied to other headache disorders.
Katsuki et al developed an AI model to help non specialists accurately diagnose headaches. This model analyzed 17 variables and was trained on data from 2800 patients, with additional testing and refinement using data from another 200 patients. To evaluate its effectiveness, 2 groups of non-headache specialists each assessed 50 patients: 1 group relied solely on their expertise, while the other used the AI model. The group without AI assistance achieved an overall accuracy of 46% (κ = 0.21), while the group using the AI model significantly improved, reaching an overall accuracy of 83.2% (κ = 0.68).
Building on their work with AI for diagnosing headaches, Katsuki et al conducted a study using a smartphone application that tracked user-reported headache events alongside local weather data. The AI model revealed that lower barometric pressure, higher humidity, and increased rainfall were linked to the onset of headache attacks. The application also identified triggers for headaches in specific weather patterns, such as a drop in barometric pressure noted 6 hours before headache onset. The application of AI in monitoring weather changes could be crucial, especially given concerns that the rising frequency of severe weather events due to climate change may be exacerbating the severity and burden of migraine. Additionally, recent post hoc analyses of fremanezumab clinical trials have provided further evidence that weather changes can trigger headaches.
Rapoport and colleagues have also developed an application called Migraine Mentor, which accurately tracks headaches, triggers, health data, and response to medication on a smartphone. The patient spends 3 minutes a day answering a few questions about their day and whether they had a headache or took any medication. At 1 or 2 months, Migraine Mentor can generate a detailed report with data and current trends that is sent to the patient, which the patient can then share with the clinician. The application also reminds patients when to document data and take medication.
However, although the use of AI in headache medicine appears promising, caution must be exercised to ensure proper results and information are disseminated. One rapidly expanding application of AI is the widely popular ChatGPT. ChatGPT, which stands for generative pretraining transformer, is a type of large language model (LLM). An LLM is a deep learning algorithm designed to recognize, translate, predict, summarize, and generate text responses based on a given prompt. This model is trained on an extensive dataset that includes a diverse array of books, articles, and websites, exposing it to various language structures and styles. This training enables ChatGPT to generate responses that closely mimic human communication. LLMs are being used more and more in medicine to assist with generating patient documentation and educational materials.
However, Dr Fred Cohen published a perspective piece detailing how LLMs (such as ChatGPT) can produce misleading and inaccurate answers. In his example, he tasked ChatGPT to describe the epidemiology of migraines in penguins; the AI model generated a well-written and highly believable manuscript titled, “Migraine Under the Ice: Understanding Headaches in Antarctica's Feathered Friends.” The manuscript highlights that migraines are more prevalent in male penguins compared to females, with the peak age of onset occurring between 4 and 5 years. Additionally, emperor and king penguins are identified as being more susceptible to developing migraines compared to other penguin species. The paper was fictitious (as no studies on migraine in penguins have been written to date), exemplifying that these models can produce nonfactual materials.
For years, technological advancements have been reshaping many aspects of life, and medicine is no exception. AI has been successfully applied to streamline medical documentation, develop new drug targets, and deepen our understanding of various diseases. The field of headache medicine now also uses AI. Recent developments show significant promise, with AI aiding in the diagnosis of migraine and other headache disorders. AI models have even been used in the identification of potential drug targets for migraine treatment. Although there are still limitations to overcome, the future of AI in headache medicine appears bright.
If you would like to read more about Dr. Cohen’s work on AI and migraine, please visit fredcohenmd.com or TikTok @fredcohenmd.
As we move further into the 21st century, technology continues to revolutionize various facets of our lives. Healthcare is a prime example. Advances in technology have dramatically reshaped the way we develop medications, diagnose diseases, and enhance patient care. The rise of artificial intelligence (AI) and the widespread adoption of digital health technologies have marked a significant milestone in improving the quality of care. AI, with its ability to leverage algorithms, deep learning, and machine learning to process data, make decisions, and perform tasks autonomously, is becoming an integral part of modern society. It is embedded in various technologies that we rely on daily, from smartphones and smart home devices to content recommendations on streaming services and social media platforms.
In healthcare, AI has applications in numerous fields, such as radiology. AI streamlines processes such as organizing patient appointments, optimizing radiation protocols for safety and efficiency, and enhancing the documentation process through advanced image analysis. AI technology plays an integral role in imaging tasks like image enhancement, lesion detection, and precise measurement. In difficult-to-interpret radiologic studies, such as some mammography images, it can be a crucial aid to the radiologist. Additionally, the use of AI has significantly improved remote patient monitoring that enables healthcare professionals to monitor and assess patient conditions without needing in-person visits. Remote patient monitoring gained prominence during the COVID-19 pandemic and continues to be a valuable tool in post pandemic care. Study results have highlighted that AI-driven ambient dictation tools have increased provider engagement with patients during consultations while reducing the time spent documenting in electronic health records.
Like many other medical specialties, headache medicine also uses AI. Most prominently, AI has been used in models and engines in assisting with headache diagnoses. A noteworthy example of AI in headache medicine is the development of an online, computer-based diagnostic engine (CDE) by Rapoport et al, called BonTriage. This tool is designed to diagnose headaches by employing a rule set based on the International Classification of Headache Disorders-3 (ICHD-3) criteria for primary headache disorders while also evaluating secondary headaches and medication overuse headaches. By leveraging machine learning, the CDE has the potential to streamline the diagnostic process, reducing the number of questions needed to reach a diagnosis and making the experience more efficient. This information can then be printed as a PDF file and taken by the patient to a healthcare professional for further discussion, fostering a more accurate, fluid, and conversational consultation.
A study was conducted to evaluate the accuracy of the CDE. Participants were randomly assigned to 1 of 2 sequences: (1) using the CDE followed by a structured standard interview with a headache specialist using the same ICHD-3 criteria or (2) starting with the structured standard interview followed by the CDE. The results demonstrated nearly perfect agreement in diagnosing migraine and probable migraine between the CDE and structured standard interview (κ = 0.82, 95% CI: 0.74, 0.90). The CDE demonstrated a diagnostic accuracy of 91.6% (95% CI: 86.9%, 95.0%), a sensitivity rate of 89.0% (95% CI: 82.5%, 93.7%), and a specificity rate of 97.0% (95% CI: 89.5%, 99.6%).
A diagnostic engine such as this can save time that clinicians spend on documentation and allow more time for discussion with the patient. For instance, a patient can take the printout received from the CDE to an appointment; the printout gives a detailed history plus information about social and psychological issues, a list of medications taken, and results of previous testing. The CDE system was originally designed to help patients see a specialist in the environment of a nationwide lack of headache specialists. There are currently 45 million patients with headaches who are seeking treatment with only around 550 certified headache specialists in the United States. The CDE printed information can help a patient obtain a consultation from a clinician quickly and start evaluation and treatment earlier. This expert online consultation is currently free of charge.
Kwon et al developed a machine learning–based model designed to automatically classify headache disorders using data from a questionnaire. Their model was able to predict diagnoses for conditions such as migraine, tension-type headaches, trigeminal autonomic cephalalgia, epicranial headache, and thunderclap headaches. The model was trained on data from 2162 patients, all diagnosed by headache specialists, and achieved an overall accuracy of 81%, with a sensitivity of 88% and a specificity of 95% for diagnosing migraines. However, the model’s performance was less robust when applied to other headache disorders.
Katsuki et al developed an AI model to help non specialists accurately diagnose headaches. This model analyzed 17 variables and was trained on data from 2800 patients, with additional testing and refinement using data from another 200 patients. To evaluate its effectiveness, 2 groups of non-headache specialists each assessed 50 patients: 1 group relied solely on their expertise, while the other used the AI model. The group without AI assistance achieved an overall accuracy of 46% (κ = 0.21), while the group using the AI model significantly improved, reaching an overall accuracy of 83.2% (κ = 0.68).
Building on their work with AI for diagnosing headaches, Katsuki et al conducted a study using a smartphone application that tracked user-reported headache events alongside local weather data. The AI model revealed that lower barometric pressure, higher humidity, and increased rainfall were linked to the onset of headache attacks. The application also identified triggers for headaches in specific weather patterns, such as a drop in barometric pressure noted 6 hours before headache onset. The application of AI in monitoring weather changes could be crucial, especially given concerns that the rising frequency of severe weather events due to climate change may be exacerbating the severity and burden of migraine. Additionally, recent post hoc analyses of fremanezumab clinical trials have provided further evidence that weather changes can trigger headaches.
Rapoport and colleagues have also developed an application called Migraine Mentor, which accurately tracks headaches, triggers, health data, and response to medication on a smartphone. The patient spends 3 minutes a day answering a few questions about their day and whether they had a headache or took any medication. At 1 or 2 months, Migraine Mentor can generate a detailed report with data and current trends that is sent to the patient, which the patient can then share with the clinician. The application also reminds patients when to document data and take medication.
However, although the use of AI in headache medicine appears promising, caution must be exercised to ensure proper results and information are disseminated. One rapidly expanding application of AI is the widely popular ChatGPT. ChatGPT, which stands for generative pretraining transformer, is a type of large language model (LLM). An LLM is a deep learning algorithm designed to recognize, translate, predict, summarize, and generate text responses based on a given prompt. This model is trained on an extensive dataset that includes a diverse array of books, articles, and websites, exposing it to various language structures and styles. This training enables ChatGPT to generate responses that closely mimic human communication. LLMs are being used more and more in medicine to assist with generating patient documentation and educational materials.
However, Dr Fred Cohen published a perspective piece detailing how LLMs (such as ChatGPT) can produce misleading and inaccurate answers. In his example, he tasked ChatGPT to describe the epidemiology of migraines in penguins; the AI model generated a well-written and highly believable manuscript titled, “Migraine Under the Ice: Understanding Headaches in Antarctica's Feathered Friends.” The manuscript highlights that migraines are more prevalent in male penguins compared to females, with the peak age of onset occurring between 4 and 5 years. Additionally, emperor and king penguins are identified as being more susceptible to developing migraines compared to other penguin species. The paper was fictitious (as no studies on migraine in penguins have been written to date), exemplifying that these models can produce nonfactual materials.
For years, technological advancements have been reshaping many aspects of life, and medicine is no exception. AI has been successfully applied to streamline medical documentation, develop new drug targets, and deepen our understanding of various diseases. The field of headache medicine now also uses AI. Recent developments show significant promise, with AI aiding in the diagnosis of migraine and other headache disorders. AI models have even been used in the identification of potential drug targets for migraine treatment. Although there are still limitations to overcome, the future of AI in headache medicine appears bright.
If you would like to read more about Dr. Cohen’s work on AI and migraine, please visit fredcohenmd.com or TikTok @fredcohenmd.
Guidance Will Aid Pediatric to Adult Diabetes Care Transfer
MADRID — A new consensus statement in development will aim to advise on best practices for navigating the transition of youth with diabetes from pediatric to adult diabetes care, despite limited data.
Expected to be released in early 2025, the statement will be a joint effort of the International Society for Pediatric and Adolescent Diabetes (ISPAD), the American Diabetes Association (ADA), and the European Association for the Study of Diabetes (EASD). It will provide guidance on advance transition planning, the care transfer itself, and follow-up. Writing panel members presented an update on the statement’s development on September 13, 2024, at EASD’s annual meeting.
The care transition period is critical because “adolescents and young adults are the least likely of all age groups to achieve glycemic targets for a variety of physiological and psychosocial reasons ... Up to 60% of these individuals don’t transfer successfully from pediatric to adult care, with declines in attendance, adverse medical outcomes, and mental health challenges,” Frank J. Snoek, PhD, emeritus professor of medical psychology at Amsterdam University Medical College, Amsterdam, the Netherlands, said in introductory remarks at the EASD session.
Session chair Carine De Beaufort, MD, a pediatric endocrinologist in Luxembourg City, Luxembourg, told this news organization, “We know it’s a continuing process, which is extremely important for young people to move into the world. The last formal recommendations were published in 2011, so we thought it was time for an update. What we realized in doing a systematic review and scoping review is that there are a lot of suggestions and ideas not really associated with robust data, and it’s not so easy to get good outcome indicators.”
The final statement will provide clinical guidance but, at the same time, “will be very transparent where more work is needed,” she said.
Sarah Lyons, MD, associate professor of pediatrics at Baylor College of Medicine, Houston, broadly outlined the document. Pre-transition planning will include readiness assessments for transfer from pediatric to adult care. The transfer phase will include measures to prevent gaps in care. And the post-transition phase will cover incorporation into adult care, with follow-up of the individual’s progress for a period.
Across the three stages, the document is expected to recommend a multidisciplinary team approach including psychological support, education and assessment, family and peer support, and care coordination. It will also address practical considerations for patients and professionals including costs and insurance.
It will build upon previous guidelines, including those of ADA and general guidance on transition from pediatric to adult healthcare from the American Academy of Pediatrics. “Ideally, this process will be continuous, comprehensive, coordinated, individualized, and developmentally appropriate,” Dr. Lyons said.
‘It Shouldn’t Be Just One Conversation ... It Needs to Be a Process’
Asked to comment, ISPAD president David Maahs, MD, the Lucile Salter Packard Professor of Pediatrics and Division Chief of Pediatric Endocrinology at Stanford University, Palo Alto, California, told this news organization, “It shouldn’t be just one conversation and one visit. It needs to be a process where you talk about the need to transition to adult endocrine care and prepare the person with diabetes and their family for that transition. One of the challenges is if they don’t make it to that first appointment and you assume that they did, and then that’s one place where there can be a gap that people fall through the two systems.”
Dr. Maahs added, “Another issue that’s a big problem in the United States is that children lose their parents’ insurance at 26 ... Some become uninsured after that, or their insurance plan isn’t accepted by the adult provider.”
‘There Does Not Appear to Be Sufficient Data’
Steven James, PhD, RN, of the University of the Sunshine Coast, Brisbane, Australia, presented the limited data upon which the statement will be based. A systematic literature review yielded just 26 intervention trials looking at care transition for youth with type 1 or type 2 diabetes, including seven clinical trials with only one randomized.
In that trial, in which 205 youth aged 17-20 years were randomized to a structured 18-month transition program with a transition coordinator, the intervention was associated with increased clinic attendance, improved satisfaction with care, and decreased diabetes-related distress, but the benefits weren’t maintained 12 months after completion of the intervention.
The other trials produced mixed results in terms of metabolic outcomes, with improvements in A1c and reductions in diabetic ketoacidosis and hospitalizations seen in some but not others. Healthcare outcomes and utilization, psychosocial outcomes, transition-related knowledge, self-care, and care satisfaction were only occasionally assessed, Dr. James noted.
“The field is lacking empirically supported interventions that can improve patient physiologic and psychologic outcomes, prevent poor clinic attendance, and improve patient satisfaction in medical care ... There still does not appear to be sufficient data related to the impact of transition readiness or transfer-to-adult care programs.”
‘Quite a Lot of Variation in Practices Worldwide’
Dr. James also presented results from two online surveys undertaken by the document writing panel. One recently published survey in Diabetes Research and Clinical Practice examined healthcare professionals’ experiences and perceptions around diabetes care transitions. Of 372 respondents (75% physicians) from around the world — including a third in low-middle-income countries — fewer than half reported using transition readiness checklists (32.8%), provided written transition information (29.6%), or had a dedicated staff member to aid in the process (23.7%).
Similarly, few involved a psychologist (25.3%) or had a structured transition education program (22.6%). Even in high-income countries, fewer than half reported using these measures. Overall, a majority (91.9%) reported barriers to offering patients a positive transition experience.
“This shows to me that there is quite a lot of variation in practices worldwide ... There is a pressing need for an international consensus transition guideline,” Dr. James said.
Among the respondents’ beliefs, 53.8% thought that discussions about transitioning should be initiated at ages 15-17 years, while 27.8% thought 12-14 years was more appropriate. Large majorities favored use of a transition readiness checklist (93.6%), combined transition clinics (80.6%), having a dedicated transition coordinator/staff member available (85.8%), and involving a psychologist in the transition process (80.6%).
A similar survey of patients and carers will be published soon and will be included in the new statement’s evidence base, Dr. James said.
Dr. Maahs said that endorsement of the upcoming guidance from three different medical societies should help raise the profile of the issue. “Hopefully three professional organizations are able to speak with a united and louder voice than if it was just one group or one set of authors. I think this consensus statement can raise awareness, improve care, and help advocate for better care.”
Dr. De Beaufort, Dr. James, and Dr. Lyons had no disclosures. Dr. Snoek is an adviser/speaker for Abbott, Lilly, Novo Nordisk, and Sanofi and receives funding from Breakthrough T1D, Sanofi, and Novo Nordisk. Dr. Maahs has had research support from the National Institutes of Health, Breakthrough T1D, National Science Foundation, and the Helmsley Charitable Trust, and his institution has had research support from Medtronic, Dexcom, Insulet, Bigfoot Biomedical, Tandem, and Roche. He has consulted for Abbott, Aditxt, the Helmsley Charitable Trust, LifeScan, MannKind, Sanofi, Novo Nordisk, Eli Lilly, Medtronic, Insulet, Dompe, BioSpex, Provention Bio, Kriya, Enable Biosciences, and Bayer.
A version of this article first appeared on Medscape.com.
MADRID — A new consensus statement in development will aim to advise on best practices for navigating the transition of youth with diabetes from pediatric to adult diabetes care, despite limited data.
Expected to be released in early 2025, the statement will be a joint effort of the International Society for Pediatric and Adolescent Diabetes (ISPAD), the American Diabetes Association (ADA), and the European Association for the Study of Diabetes (EASD). It will provide guidance on advance transition planning, the care transfer itself, and follow-up. Writing panel members presented an update on the statement’s development on September 13, 2024, at EASD’s annual meeting.
The care transition period is critical because “adolescents and young adults are the least likely of all age groups to achieve glycemic targets for a variety of physiological and psychosocial reasons ... Up to 60% of these individuals don’t transfer successfully from pediatric to adult care, with declines in attendance, adverse medical outcomes, and mental health challenges,” Frank J. Snoek, PhD, emeritus professor of medical psychology at Amsterdam University Medical College, Amsterdam, the Netherlands, said in introductory remarks at the EASD session.
Session chair Carine De Beaufort, MD, a pediatric endocrinologist in Luxembourg City, Luxembourg, told this news organization, “We know it’s a continuing process, which is extremely important for young people to move into the world. The last formal recommendations were published in 2011, so we thought it was time for an update. What we realized in doing a systematic review and scoping review is that there are a lot of suggestions and ideas not really associated with robust data, and it’s not so easy to get good outcome indicators.”
The final statement will provide clinical guidance but, at the same time, “will be very transparent where more work is needed,” she said.
Sarah Lyons, MD, associate professor of pediatrics at Baylor College of Medicine, Houston, broadly outlined the document. Pre-transition planning will include readiness assessments for transfer from pediatric to adult care. The transfer phase will include measures to prevent gaps in care. And the post-transition phase will cover incorporation into adult care, with follow-up of the individual’s progress for a period.
Across the three stages, the document is expected to recommend a multidisciplinary team approach including psychological support, education and assessment, family and peer support, and care coordination. It will also address practical considerations for patients and professionals including costs and insurance.
It will build upon previous guidelines, including those of ADA and general guidance on transition from pediatric to adult healthcare from the American Academy of Pediatrics. “Ideally, this process will be continuous, comprehensive, coordinated, individualized, and developmentally appropriate,” Dr. Lyons said.
‘It Shouldn’t Be Just One Conversation ... It Needs to Be a Process’
Asked to comment, ISPAD president David Maahs, MD, the Lucile Salter Packard Professor of Pediatrics and Division Chief of Pediatric Endocrinology at Stanford University, Palo Alto, California, told this news organization, “It shouldn’t be just one conversation and one visit. It needs to be a process where you talk about the need to transition to adult endocrine care and prepare the person with diabetes and their family for that transition. One of the challenges is if they don’t make it to that first appointment and you assume that they did, and then that’s one place where there can be a gap that people fall through the two systems.”
Dr. Maahs added, “Another issue that’s a big problem in the United States is that children lose their parents’ insurance at 26 ... Some become uninsured after that, or their insurance plan isn’t accepted by the adult provider.”
‘There Does Not Appear to Be Sufficient Data’
Steven James, PhD, RN, of the University of the Sunshine Coast, Brisbane, Australia, presented the limited data upon which the statement will be based. A systematic literature review yielded just 26 intervention trials looking at care transition for youth with type 1 or type 2 diabetes, including seven clinical trials with only one randomized.
In that trial, in which 205 youth aged 17-20 years were randomized to a structured 18-month transition program with a transition coordinator, the intervention was associated with increased clinic attendance, improved satisfaction with care, and decreased diabetes-related distress, but the benefits weren’t maintained 12 months after completion of the intervention.
The other trials produced mixed results in terms of metabolic outcomes, with improvements in A1c and reductions in diabetic ketoacidosis and hospitalizations seen in some but not others. Healthcare outcomes and utilization, psychosocial outcomes, transition-related knowledge, self-care, and care satisfaction were only occasionally assessed, Dr. James noted.
“The field is lacking empirically supported interventions that can improve patient physiologic and psychologic outcomes, prevent poor clinic attendance, and improve patient satisfaction in medical care ... There still does not appear to be sufficient data related to the impact of transition readiness or transfer-to-adult care programs.”
‘Quite a Lot of Variation in Practices Worldwide’
Dr. James also presented results from two online surveys undertaken by the document writing panel. One recently published survey in Diabetes Research and Clinical Practice examined healthcare professionals’ experiences and perceptions around diabetes care transitions. Of 372 respondents (75% physicians) from around the world — including a third in low-middle-income countries — fewer than half reported using transition readiness checklists (32.8%), provided written transition information (29.6%), or had a dedicated staff member to aid in the process (23.7%).
Similarly, few involved a psychologist (25.3%) or had a structured transition education program (22.6%). Even in high-income countries, fewer than half reported using these measures. Overall, a majority (91.9%) reported barriers to offering patients a positive transition experience.
“This shows to me that there is quite a lot of variation in practices worldwide ... There is a pressing need for an international consensus transition guideline,” Dr. James said.
Among the respondents’ beliefs, 53.8% thought that discussions about transitioning should be initiated at ages 15-17 years, while 27.8% thought 12-14 years was more appropriate. Large majorities favored use of a transition readiness checklist (93.6%), combined transition clinics (80.6%), having a dedicated transition coordinator/staff member available (85.8%), and involving a psychologist in the transition process (80.6%).
A similar survey of patients and carers will be published soon and will be included in the new statement’s evidence base, Dr. James said.
Dr. Maahs said that endorsement of the upcoming guidance from three different medical societies should help raise the profile of the issue. “Hopefully three professional organizations are able to speak with a united and louder voice than if it was just one group or one set of authors. I think this consensus statement can raise awareness, improve care, and help advocate for better care.”
Dr. De Beaufort, Dr. James, and Dr. Lyons had no disclosures. Dr. Snoek is an adviser/speaker for Abbott, Lilly, Novo Nordisk, and Sanofi and receives funding from Breakthrough T1D, Sanofi, and Novo Nordisk. Dr. Maahs has had research support from the National Institutes of Health, Breakthrough T1D, National Science Foundation, and the Helmsley Charitable Trust, and his institution has had research support from Medtronic, Dexcom, Insulet, Bigfoot Biomedical, Tandem, and Roche. He has consulted for Abbott, Aditxt, the Helmsley Charitable Trust, LifeScan, MannKind, Sanofi, Novo Nordisk, Eli Lilly, Medtronic, Insulet, Dompe, BioSpex, Provention Bio, Kriya, Enable Biosciences, and Bayer.
A version of this article first appeared on Medscape.com.
MADRID — A new consensus statement in development will aim to advise on best practices for navigating the transition of youth with diabetes from pediatric to adult diabetes care, despite limited data.
Expected to be released in early 2025, the statement will be a joint effort of the International Society for Pediatric and Adolescent Diabetes (ISPAD), the American Diabetes Association (ADA), and the European Association for the Study of Diabetes (EASD). It will provide guidance on advance transition planning, the care transfer itself, and follow-up. Writing panel members presented an update on the statement’s development on September 13, 2024, at EASD’s annual meeting.
The care transition period is critical because “adolescents and young adults are the least likely of all age groups to achieve glycemic targets for a variety of physiological and psychosocial reasons ... Up to 60% of these individuals don’t transfer successfully from pediatric to adult care, with declines in attendance, adverse medical outcomes, and mental health challenges,” Frank J. Snoek, PhD, emeritus professor of medical psychology at Amsterdam University Medical College, Amsterdam, the Netherlands, said in introductory remarks at the EASD session.
Session chair Carine De Beaufort, MD, a pediatric endocrinologist in Luxembourg City, Luxembourg, told this news organization, “We know it’s a continuing process, which is extremely important for young people to move into the world. The last formal recommendations were published in 2011, so we thought it was time for an update. What we realized in doing a systematic review and scoping review is that there are a lot of suggestions and ideas not really associated with robust data, and it’s not so easy to get good outcome indicators.”
The final statement will provide clinical guidance but, at the same time, “will be very transparent where more work is needed,” she said.
Sarah Lyons, MD, associate professor of pediatrics at Baylor College of Medicine, Houston, broadly outlined the document. Pre-transition planning will include readiness assessments for transfer from pediatric to adult care. The transfer phase will include measures to prevent gaps in care. And the post-transition phase will cover incorporation into adult care, with follow-up of the individual’s progress for a period.
Across the three stages, the document is expected to recommend a multidisciplinary team approach including psychological support, education and assessment, family and peer support, and care coordination. It will also address practical considerations for patients and professionals including costs and insurance.
It will build upon previous guidelines, including those of ADA and general guidance on transition from pediatric to adult healthcare from the American Academy of Pediatrics. “Ideally, this process will be continuous, comprehensive, coordinated, individualized, and developmentally appropriate,” Dr. Lyons said.
‘It Shouldn’t Be Just One Conversation ... It Needs to Be a Process’
Asked to comment, ISPAD president David Maahs, MD, the Lucile Salter Packard Professor of Pediatrics and Division Chief of Pediatric Endocrinology at Stanford University, Palo Alto, California, told this news organization, “It shouldn’t be just one conversation and one visit. It needs to be a process where you talk about the need to transition to adult endocrine care and prepare the person with diabetes and their family for that transition. One of the challenges is if they don’t make it to that first appointment and you assume that they did, and then that’s one place where there can be a gap that people fall through the two systems.”
Dr. Maahs added, “Another issue that’s a big problem in the United States is that children lose their parents’ insurance at 26 ... Some become uninsured after that, or their insurance plan isn’t accepted by the adult provider.”
‘There Does Not Appear to Be Sufficient Data’
Steven James, PhD, RN, of the University of the Sunshine Coast, Brisbane, Australia, presented the limited data upon which the statement will be based. A systematic literature review yielded just 26 intervention trials looking at care transition for youth with type 1 or type 2 diabetes, including seven clinical trials with only one randomized.
In that trial, in which 205 youth aged 17-20 years were randomized to a structured 18-month transition program with a transition coordinator, the intervention was associated with increased clinic attendance, improved satisfaction with care, and decreased diabetes-related distress, but the benefits weren’t maintained 12 months after completion of the intervention.
The other trials produced mixed results in terms of metabolic outcomes, with improvements in A1c and reductions in diabetic ketoacidosis and hospitalizations seen in some but not others. Healthcare outcomes and utilization, psychosocial outcomes, transition-related knowledge, self-care, and care satisfaction were only occasionally assessed, Dr. James noted.
“The field is lacking empirically supported interventions that can improve patient physiologic and psychologic outcomes, prevent poor clinic attendance, and improve patient satisfaction in medical care ... There still does not appear to be sufficient data related to the impact of transition readiness or transfer-to-adult care programs.”
‘Quite a Lot of Variation in Practices Worldwide’
Dr. James also presented results from two online surveys undertaken by the document writing panel. One recently published survey in Diabetes Research and Clinical Practice examined healthcare professionals’ experiences and perceptions around diabetes care transitions. Of 372 respondents (75% physicians) from around the world — including a third in low-middle-income countries — fewer than half reported using transition readiness checklists (32.8%), provided written transition information (29.6%), or had a dedicated staff member to aid in the process (23.7%).
Similarly, few involved a psychologist (25.3%) or had a structured transition education program (22.6%). Even in high-income countries, fewer than half reported using these measures. Overall, a majority (91.9%) reported barriers to offering patients a positive transition experience.
“This shows to me that there is quite a lot of variation in practices worldwide ... There is a pressing need for an international consensus transition guideline,” Dr. James said.
Among the respondents’ beliefs, 53.8% thought that discussions about transitioning should be initiated at ages 15-17 years, while 27.8% thought 12-14 years was more appropriate. Large majorities favored use of a transition readiness checklist (93.6%), combined transition clinics (80.6%), having a dedicated transition coordinator/staff member available (85.8%), and involving a psychologist in the transition process (80.6%).
A similar survey of patients and carers will be published soon and will be included in the new statement’s evidence base, Dr. James said.
Dr. Maahs said that endorsement of the upcoming guidance from three different medical societies should help raise the profile of the issue. “Hopefully three professional organizations are able to speak with a united and louder voice than if it was just one group or one set of authors. I think this consensus statement can raise awareness, improve care, and help advocate for better care.”
Dr. De Beaufort, Dr. James, and Dr. Lyons had no disclosures. Dr. Snoek is an adviser/speaker for Abbott, Lilly, Novo Nordisk, and Sanofi and receives funding from Breakthrough T1D, Sanofi, and Novo Nordisk. Dr. Maahs has had research support from the National Institutes of Health, Breakthrough T1D, National Science Foundation, and the Helmsley Charitable Trust, and his institution has had research support from Medtronic, Dexcom, Insulet, Bigfoot Biomedical, Tandem, and Roche. He has consulted for Abbott, Aditxt, the Helmsley Charitable Trust, LifeScan, MannKind, Sanofi, Novo Nordisk, Eli Lilly, Medtronic, Insulet, Dompe, BioSpex, Provention Bio, Kriya, Enable Biosciences, and Bayer.
A version of this article first appeared on Medscape.com.
FROM EASD 2024
Triptans Trump Newer, More Expensive Meds for Acute Migraine
new research suggested.
Results of a large systematic review and meta-analysis showed that eletriptan, rizatriptan, sumatriptan, and zolmitriptan were more effective than lasmiditan, rimegepant, and ubrogepant, which investigators found were as effective as nonsteroidal anti-inflammatory drugs (NSAIDs).
International guidelines generally endorse NSAIDs as the first-line treatment for migraine and recommend triptans for moderate to severe episodes or when the response to NSAIDs is insufficient.
However, based on the study’s findings, these four triptans should be considered the treatment of choice for migraine, study investigator Andrea Cipriani, MD, PhD, professor of psychiatry at the University of Oxford in England and director of the Oxford Health Clinical Research Facility, told a press briefing.
The investigators added that these particular triptans should be “included in the WHO [World Health Organization] List of Essential Medicines to promote global accessibility and uniform standards of care.”
The study was published online in The BMJ.
Filling the Knowledge Gap
To date, almost all migraine studies have compared migraine drugs with placebo, so there’s a knowledge gap, said Dr. Cipriani. As a result, “there’s no clear consensus among experts and guidelines about which specific drug classes should be prescribed initially, and this is a clinical problem.”
The researchers pointed out that, in recent years, lasmiditan and gepants have been introduced as further treatment options, especially for patients in whom triptans are contraindicated because of their potential vasoconstrictive effects and higher risk for ischemic events.
The analysis included 137 double-blind, randomized, controlled trials that were primarily sponsored by the pharmaceutical industry. It included 89,445 adult outpatients with migraine (mean age, 40.3 years; 85.6% women).
Only drugs licensed for migraine or headache that are recommended in at least one country were included. Researchers divided these 17 drugs into five categories: Antipyretics (paracetamol), ditans (lasmiditan), gepants (rimegepant and ubrogepant), NSAIDs (acetylsalicylic acid, celecoxib, diclofenac potassium, ibuprofen, naproxen sodium, and phenazone), and triptans (almotriptan, eletriptan, frovatriptan, naratriptan, rizatriptan, sumatriptan, and zolmitriptan).
The study’s primary outcomes were freedom from pain at 2 hours and at 2-24 hours, without the use of rescue drugs.
Investigators used sumatriptan as the reference intervention because it is the most commonly prescribed migraine drug and is included in the WHO Model Lists of Essential Medicines.
The study showed all active interventions were better than placebo for pain freedom at 2 hours; with the exception of paracetamol and naratriptan, all were better for sustained pain freedom from 2 to 24 hours.
When the active interventions were compared with each other, eletriptan outperformed other drugs for achieving pain freedom at 2 hours. It was followed by rizatriptan, sumatriptan, and zolmitriptan (odds ratio [OR], 1.35-3.01). For sustained pain freedom up to 24 hours, the most efficacious interventions were eletriptan (OR, 1.41-2.73) and ibuprofen (OR, 3.16-4.82).
As for secondary efficacy outcomes, in head-to-head comparisons, eletriptan was superior to nearly all other active interventions for pain relief at 2 hours and for the use of rescue drugs.
As for adverse events, dizziness was more commonly associated with lasmiditan, eletriptan, sumatriptan, and zolmitriptan, while fatigue and sedation occurred more frequently with eletriptan and lasmiditan. Nausea was more often associated with lasmiditan, sumatriptan, zolmitriptan, and ubrogepant. Eletriptan was the only intervention most frequently associated with chest pain or discomfort.
Need to Update Guidelines?
Considering the new results, Dr. Cipriani and study coauthor Messoud Ashina, MD, PhD, professor of neurology, University of Copenhagen in Denmark, said clinical guidelines for acute migraine management should be updated.
While triptans are contraindicated in patients with vascular disease, the researchers noted that “cerebrovascular events may present primarily as migraine-like headaches, and misdiagnosis of transient ischemic attack and minor stroke as migraine is not rare.”
Recently, lasmiditan, rimegepant, and ubrogepant — which are not associated with vasoconstrictive effects — have been promoted as alternatives in patients for whom triptans are contraindicated or not tolerated. But the high costs of these drugs put them out of reach for some patients, the investigators noted.
Triptans are widely underutilized, Dr. Ashina noted during the press briefing. Current use ranges from 17% to 22% in the United States and from 3% to 22.5% in Europe.
The investigators said that triptans have been shown to be superior and should be promoted globally, adding that the limited access and substantial underutilization of these medications are “missed opportunities to offer more effective treatments.”
The new results underscore the importance of head-to-head trials, which is the gold standard, said Dr. Cipriani.
The investigators noted that the study’s main limitation was the quality of the data, which was deemed to be low, or very low, for most comparisons. Other potential limitations included lack of individual patient data; exclusion of combination drugs; inclusion of only oral treatments; and not considering type of oral formulation, consistency in response across migraine episodes, or cost-effectiveness.
The study also did not cover important clinical issues that might inform treatment decision-making, including drug overuse headache or potential withdrawal symptoms. And the authors were unable to quantify some outcomes, such as global functioning.
‘Best Profile’?
Reached for comment, Neurologist Nina Riggins, MD, PhD, Headache Center of Excellence, Palo Alto VA Medical Center in California, praised the authors for a “great job” of bringing attention to the topic.
However, she noted that the investigators’ characterization of the four triptans as having the “best profile” for acute migraine gave her pause.
“Calling triptans the medication with the ‘best profile’ might be not applicable in many cases,” she said. For example, those who need acute medication more than two to three times a week in addition to those with cardiovascular contraindications to triptans may fall outside of that category.
Dr. Riggins said that “it makes sense” that longer-acting triptans like frovatriptan and naratriptan may not rank highly for efficacy within the first 2 hours. However, these agents likely offer a superior therapeutic profile in specific contexts, such as menstrual-related migraine.
In addition, while triptans are known to cause medication overuse headache, this may not be the case with gepants, she noted.
In a release from the Science Media Center, a nonprofit organization promoting voices and views of the scientific community, Eloísa Rubio-Beltrán, PhD, research associate with The Migraine Trust at the Wolfson Sensory, Pain and Regeneration Centre, King’s College London in England, said the findings should affect migraine treatment guidelines.
“As the study highlights, due to their high efficacy and low cost, triptans should be the first-line treatment option for the acute treatment of migraine. These results should inform treatment guidelines and support the inclusion of the best performing triptans into the List of Essential Medicines, to optimize treatment, allowing patients to access more efficacious options,” said Dr. Rubio-Beltrán.
It is also important to note that gepants and ditans were developed to offer alternatives for patients who show no improvement from triptans, she added.
She pointed out that these medications were not developed as a substitute for triptans, but rather to expand the number of treatment options for migraine.
“Nonetheless,” she added, “this study highlights the need for further research on the pathophysiology of migraine, which will allow the discovery of novel targets, and thus, novel treatments options that will benefit all patient populations.”
The study was funded by the NIHR Oxford Health Biomedical Research Centre and the Lundbeck Foundation. Dr. Cipriani reported receiving research, educational, and consultancy fees from Italian Network for Pediatric Clinical Trials, Fondazione Cariplo, Lundbeck, and Angelini Pharma. Dr. Ashina is a consultant, speaker, or scientific adviser for AbbVie, Amgen, AstraZeneca, Eli Lilly, GSK, Lundbeck, Novartis, Pfizer, and Teva; is the past president of the International Headache Society; and an associate editor of The Journal of Headache and Pain and Brain. Dr. Riggins has consulted for Gerson Lehrman Group; participated in compensated work with AcademicCME and Association of Migraine Disorders; was a principal investigator on research with electroCore, Theranica, and Eli Lilly; serves on advisory boards for Theranica, Teva, Lundbeck, Amneal Pharmaceuticals, NeurologyLive, and Miles for Migraine; and is a project advisor for Clinical Awareness Initiative with Clinical Neurological Society of America. Dr. Rubio-Beltrán reported serving as a junior editorial board member of The Journal of Headache and Pain and a junior representative of the International Headache Society; receiving research support from The Migraine Trust, Eli Lilly, CoLucid Pharmaceuticals, Amgen, Novartis, and Kallyope; and receiving travel support from CoLucid Pharmaceuticals, Allergan, and Novartis.
A version of this article first appeared on Medscape.com.
new research suggested.
Results of a large systematic review and meta-analysis showed that eletriptan, rizatriptan, sumatriptan, and zolmitriptan were more effective than lasmiditan, rimegepant, and ubrogepant, which investigators found were as effective as nonsteroidal anti-inflammatory drugs (NSAIDs).
International guidelines generally endorse NSAIDs as the first-line treatment for migraine and recommend triptans for moderate to severe episodes or when the response to NSAIDs is insufficient.
However, based on the study’s findings, these four triptans should be considered the treatment of choice for migraine, study investigator Andrea Cipriani, MD, PhD, professor of psychiatry at the University of Oxford in England and director of the Oxford Health Clinical Research Facility, told a press briefing.
The investigators added that these particular triptans should be “included in the WHO [World Health Organization] List of Essential Medicines to promote global accessibility and uniform standards of care.”
The study was published online in The BMJ.
Filling the Knowledge Gap
To date, almost all migraine studies have compared migraine drugs with placebo, so there’s a knowledge gap, said Dr. Cipriani. As a result, “there’s no clear consensus among experts and guidelines about which specific drug classes should be prescribed initially, and this is a clinical problem.”
The researchers pointed out that, in recent years, lasmiditan and gepants have been introduced as further treatment options, especially for patients in whom triptans are contraindicated because of their potential vasoconstrictive effects and higher risk for ischemic events.
The analysis included 137 double-blind, randomized, controlled trials that were primarily sponsored by the pharmaceutical industry. It included 89,445 adult outpatients with migraine (mean age, 40.3 years; 85.6% women).
Only drugs licensed for migraine or headache that are recommended in at least one country were included. Researchers divided these 17 drugs into five categories: Antipyretics (paracetamol), ditans (lasmiditan), gepants (rimegepant and ubrogepant), NSAIDs (acetylsalicylic acid, celecoxib, diclofenac potassium, ibuprofen, naproxen sodium, and phenazone), and triptans (almotriptan, eletriptan, frovatriptan, naratriptan, rizatriptan, sumatriptan, and zolmitriptan).
The study’s primary outcomes were freedom from pain at 2 hours and at 2-24 hours, without the use of rescue drugs.
Investigators used sumatriptan as the reference intervention because it is the most commonly prescribed migraine drug and is included in the WHO Model Lists of Essential Medicines.
The study showed all active interventions were better than placebo for pain freedom at 2 hours; with the exception of paracetamol and naratriptan, all were better for sustained pain freedom from 2 to 24 hours.
When the active interventions were compared with each other, eletriptan outperformed other drugs for achieving pain freedom at 2 hours. It was followed by rizatriptan, sumatriptan, and zolmitriptan (odds ratio [OR], 1.35-3.01). For sustained pain freedom up to 24 hours, the most efficacious interventions were eletriptan (OR, 1.41-2.73) and ibuprofen (OR, 3.16-4.82).
As for secondary efficacy outcomes, in head-to-head comparisons, eletriptan was superior to nearly all other active interventions for pain relief at 2 hours and for the use of rescue drugs.
As for adverse events, dizziness was more commonly associated with lasmiditan, eletriptan, sumatriptan, and zolmitriptan, while fatigue and sedation occurred more frequently with eletriptan and lasmiditan. Nausea was more often associated with lasmiditan, sumatriptan, zolmitriptan, and ubrogepant. Eletriptan was the only intervention most frequently associated with chest pain or discomfort.
Need to Update Guidelines?
Considering the new results, Dr. Cipriani and study coauthor Messoud Ashina, MD, PhD, professor of neurology, University of Copenhagen in Denmark, said clinical guidelines for acute migraine management should be updated.
While triptans are contraindicated in patients with vascular disease, the researchers noted that “cerebrovascular events may present primarily as migraine-like headaches, and misdiagnosis of transient ischemic attack and minor stroke as migraine is not rare.”
Recently, lasmiditan, rimegepant, and ubrogepant — which are not associated with vasoconstrictive effects — have been promoted as alternatives in patients for whom triptans are contraindicated or not tolerated. But the high costs of these drugs put them out of reach for some patients, the investigators noted.
Triptans are widely underutilized, Dr. Ashina noted during the press briefing. Current use ranges from 17% to 22% in the United States and from 3% to 22.5% in Europe.
The investigators said that triptans have been shown to be superior and should be promoted globally, adding that the limited access and substantial underutilization of these medications are “missed opportunities to offer more effective treatments.”
The new results underscore the importance of head-to-head trials, which is the gold standard, said Dr. Cipriani.
The investigators noted that the study’s main limitation was the quality of the data, which was deemed to be low, or very low, for most comparisons. Other potential limitations included lack of individual patient data; exclusion of combination drugs; inclusion of only oral treatments; and not considering type of oral formulation, consistency in response across migraine episodes, or cost-effectiveness.
The study also did not cover important clinical issues that might inform treatment decision-making, including drug overuse headache or potential withdrawal symptoms. And the authors were unable to quantify some outcomes, such as global functioning.
‘Best Profile’?
Reached for comment, Neurologist Nina Riggins, MD, PhD, Headache Center of Excellence, Palo Alto VA Medical Center in California, praised the authors for a “great job” of bringing attention to the topic.
However, she noted that the investigators’ characterization of the four triptans as having the “best profile” for acute migraine gave her pause.
“Calling triptans the medication with the ‘best profile’ might be not applicable in many cases,” she said. For example, those who need acute medication more than two to three times a week in addition to those with cardiovascular contraindications to triptans may fall outside of that category.
Dr. Riggins said that “it makes sense” that longer-acting triptans like frovatriptan and naratriptan may not rank highly for efficacy within the first 2 hours. However, these agents likely offer a superior therapeutic profile in specific contexts, such as menstrual-related migraine.
In addition, while triptans are known to cause medication overuse headache, this may not be the case with gepants, she noted.
In a release from the Science Media Center, a nonprofit organization promoting voices and views of the scientific community, Eloísa Rubio-Beltrán, PhD, research associate with The Migraine Trust at the Wolfson Sensory, Pain and Regeneration Centre, King’s College London in England, said the findings should affect migraine treatment guidelines.
“As the study highlights, due to their high efficacy and low cost, triptans should be the first-line treatment option for the acute treatment of migraine. These results should inform treatment guidelines and support the inclusion of the best performing triptans into the List of Essential Medicines, to optimize treatment, allowing patients to access more efficacious options,” said Dr. Rubio-Beltrán.
It is also important to note that gepants and ditans were developed to offer alternatives for patients who show no improvement from triptans, she added.
She pointed out that these medications were not developed as a substitute for triptans, but rather to expand the number of treatment options for migraine.
“Nonetheless,” she added, “this study highlights the need for further research on the pathophysiology of migraine, which will allow the discovery of novel targets, and thus, novel treatments options that will benefit all patient populations.”
The study was funded by the NIHR Oxford Health Biomedical Research Centre and the Lundbeck Foundation. Dr. Cipriani reported receiving research, educational, and consultancy fees from Italian Network for Pediatric Clinical Trials, Fondazione Cariplo, Lundbeck, and Angelini Pharma. Dr. Ashina is a consultant, speaker, or scientific adviser for AbbVie, Amgen, AstraZeneca, Eli Lilly, GSK, Lundbeck, Novartis, Pfizer, and Teva; is the past president of the International Headache Society; and an associate editor of The Journal of Headache and Pain and Brain. Dr. Riggins has consulted for Gerson Lehrman Group; participated in compensated work with AcademicCME and Association of Migraine Disorders; was a principal investigator on research with electroCore, Theranica, and Eli Lilly; serves on advisory boards for Theranica, Teva, Lundbeck, Amneal Pharmaceuticals, NeurologyLive, and Miles for Migraine; and is a project advisor for Clinical Awareness Initiative with Clinical Neurological Society of America. Dr. Rubio-Beltrán reported serving as a junior editorial board member of The Journal of Headache and Pain and a junior representative of the International Headache Society; receiving research support from The Migraine Trust, Eli Lilly, CoLucid Pharmaceuticals, Amgen, Novartis, and Kallyope; and receiving travel support from CoLucid Pharmaceuticals, Allergan, and Novartis.
A version of this article first appeared on Medscape.com.
new research suggested.
Results of a large systematic review and meta-analysis showed that eletriptan, rizatriptan, sumatriptan, and zolmitriptan were more effective than lasmiditan, rimegepant, and ubrogepant, which investigators found were as effective as nonsteroidal anti-inflammatory drugs (NSAIDs).
International guidelines generally endorse NSAIDs as the first-line treatment for migraine and recommend triptans for moderate to severe episodes or when the response to NSAIDs is insufficient.
However, based on the study’s findings, these four triptans should be considered the treatment of choice for migraine, study investigator Andrea Cipriani, MD, PhD, professor of psychiatry at the University of Oxford in England and director of the Oxford Health Clinical Research Facility, told a press briefing.
The investigators added that these particular triptans should be “included in the WHO [World Health Organization] List of Essential Medicines to promote global accessibility and uniform standards of care.”
The study was published online in The BMJ.
Filling the Knowledge Gap
To date, almost all migraine studies have compared migraine drugs with placebo, so there’s a knowledge gap, said Dr. Cipriani. As a result, “there’s no clear consensus among experts and guidelines about which specific drug classes should be prescribed initially, and this is a clinical problem.”
The researchers pointed out that, in recent years, lasmiditan and gepants have been introduced as further treatment options, especially for patients in whom triptans are contraindicated because of their potential vasoconstrictive effects and higher risk for ischemic events.
The analysis included 137 double-blind, randomized, controlled trials that were primarily sponsored by the pharmaceutical industry. It included 89,445 adult outpatients with migraine (mean age, 40.3 years; 85.6% women).
Only drugs licensed for migraine or headache that are recommended in at least one country were included. Researchers divided these 17 drugs into five categories: Antipyretics (paracetamol), ditans (lasmiditan), gepants (rimegepant and ubrogepant), NSAIDs (acetylsalicylic acid, celecoxib, diclofenac potassium, ibuprofen, naproxen sodium, and phenazone), and triptans (almotriptan, eletriptan, frovatriptan, naratriptan, rizatriptan, sumatriptan, and zolmitriptan).
The study’s primary outcomes were freedom from pain at 2 hours and at 2-24 hours, without the use of rescue drugs.
Investigators used sumatriptan as the reference intervention because it is the most commonly prescribed migraine drug and is included in the WHO Model Lists of Essential Medicines.
The study showed all active interventions were better than placebo for pain freedom at 2 hours; with the exception of paracetamol and naratriptan, all were better for sustained pain freedom from 2 to 24 hours.
When the active interventions were compared with each other, eletriptan outperformed other drugs for achieving pain freedom at 2 hours. It was followed by rizatriptan, sumatriptan, and zolmitriptan (odds ratio [OR], 1.35-3.01). For sustained pain freedom up to 24 hours, the most efficacious interventions were eletriptan (OR, 1.41-2.73) and ibuprofen (OR, 3.16-4.82).
As for secondary efficacy outcomes, in head-to-head comparisons, eletriptan was superior to nearly all other active interventions for pain relief at 2 hours and for the use of rescue drugs.
As for adverse events, dizziness was more commonly associated with lasmiditan, eletriptan, sumatriptan, and zolmitriptan, while fatigue and sedation occurred more frequently with eletriptan and lasmiditan. Nausea was more often associated with lasmiditan, sumatriptan, zolmitriptan, and ubrogepant. Eletriptan was the only intervention most frequently associated with chest pain or discomfort.
Need to Update Guidelines?
Considering the new results, Dr. Cipriani and study coauthor Messoud Ashina, MD, PhD, professor of neurology, University of Copenhagen in Denmark, said clinical guidelines for acute migraine management should be updated.
While triptans are contraindicated in patients with vascular disease, the researchers noted that “cerebrovascular events may present primarily as migraine-like headaches, and misdiagnosis of transient ischemic attack and minor stroke as migraine is not rare.”
Recently, lasmiditan, rimegepant, and ubrogepant — which are not associated with vasoconstrictive effects — have been promoted as alternatives in patients for whom triptans are contraindicated or not tolerated. But the high costs of these drugs put them out of reach for some patients, the investigators noted.
Triptans are widely underutilized, Dr. Ashina noted during the press briefing. Current use ranges from 17% to 22% in the United States and from 3% to 22.5% in Europe.
The investigators said that triptans have been shown to be superior and should be promoted globally, adding that the limited access and substantial underutilization of these medications are “missed opportunities to offer more effective treatments.”
The new results underscore the importance of head-to-head trials, which is the gold standard, said Dr. Cipriani.
The investigators noted that the study’s main limitation was the quality of the data, which was deemed to be low, or very low, for most comparisons. Other potential limitations included lack of individual patient data; exclusion of combination drugs; inclusion of only oral treatments; and not considering type of oral formulation, consistency in response across migraine episodes, or cost-effectiveness.
The study also did not cover important clinical issues that might inform treatment decision-making, including drug overuse headache or potential withdrawal symptoms. And the authors were unable to quantify some outcomes, such as global functioning.
‘Best Profile’?
Reached for comment, Neurologist Nina Riggins, MD, PhD, Headache Center of Excellence, Palo Alto VA Medical Center in California, praised the authors for a “great job” of bringing attention to the topic.
However, she noted that the investigators’ characterization of the four triptans as having the “best profile” for acute migraine gave her pause.
“Calling triptans the medication with the ‘best profile’ might be not applicable in many cases,” she said. For example, those who need acute medication more than two to three times a week in addition to those with cardiovascular contraindications to triptans may fall outside of that category.
Dr. Riggins said that “it makes sense” that longer-acting triptans like frovatriptan and naratriptan may not rank highly for efficacy within the first 2 hours. However, these agents likely offer a superior therapeutic profile in specific contexts, such as menstrual-related migraine.
In addition, while triptans are known to cause medication overuse headache, this may not be the case with gepants, she noted.
In a release from the Science Media Center, a nonprofit organization promoting voices and views of the scientific community, Eloísa Rubio-Beltrán, PhD, research associate with The Migraine Trust at the Wolfson Sensory, Pain and Regeneration Centre, King’s College London in England, said the findings should affect migraine treatment guidelines.
“As the study highlights, due to their high efficacy and low cost, triptans should be the first-line treatment option for the acute treatment of migraine. These results should inform treatment guidelines and support the inclusion of the best performing triptans into the List of Essential Medicines, to optimize treatment, allowing patients to access more efficacious options,” said Dr. Rubio-Beltrán.
It is also important to note that gepants and ditans were developed to offer alternatives for patients who show no improvement from triptans, she added.
She pointed out that these medications were not developed as a substitute for triptans, but rather to expand the number of treatment options for migraine.
“Nonetheless,” she added, “this study highlights the need for further research on the pathophysiology of migraine, which will allow the discovery of novel targets, and thus, novel treatments options that will benefit all patient populations.”
The study was funded by the NIHR Oxford Health Biomedical Research Centre and the Lundbeck Foundation. Dr. Cipriani reported receiving research, educational, and consultancy fees from Italian Network for Pediatric Clinical Trials, Fondazione Cariplo, Lundbeck, and Angelini Pharma. Dr. Ashina is a consultant, speaker, or scientific adviser for AbbVie, Amgen, AstraZeneca, Eli Lilly, GSK, Lundbeck, Novartis, Pfizer, and Teva; is the past president of the International Headache Society; and an associate editor of The Journal of Headache and Pain and Brain. Dr. Riggins has consulted for Gerson Lehrman Group; participated in compensated work with AcademicCME and Association of Migraine Disorders; was a principal investigator on research with electroCore, Theranica, and Eli Lilly; serves on advisory boards for Theranica, Teva, Lundbeck, Amneal Pharmaceuticals, NeurologyLive, and Miles for Migraine; and is a project advisor for Clinical Awareness Initiative with Clinical Neurological Society of America. Dr. Rubio-Beltrán reported serving as a junior editorial board member of The Journal of Headache and Pain and a junior representative of the International Headache Society; receiving research support from The Migraine Trust, Eli Lilly, CoLucid Pharmaceuticals, Amgen, Novartis, and Kallyope; and receiving travel support from CoLucid Pharmaceuticals, Allergan, and Novartis.
A version of this article first appeared on Medscape.com.
FROM THE BMJ
Positive Stem Cell Transplant Data Is Increasing Its Use
COPENHAGEN —
With only one completed randomized trial available, HSCT remains experimental but the number of patients treated with this approach has now reached substantial numbers over 20 years of experience at multiple centers, according to two representative real-world studies presented at the 2024 ECTRIMS annual meeting.
The latest data are wholly consistent with a 2019 multinational randomized trial that found HSCT, which is a one-time intervention, to be relatively well tolerated and more effective than disease-modifying therapy (DMT) for median time to progression in patients refractory to DMT.
Relative to 24 months in the DMT group, median time to relapse was not reached among those randomized to HSCT because there were too few events. The hazard ratio (HR) reduction for progression was greater than 90% (HR, 0.07; P < 0.001). Other endpoints, such as EDSS, which improved in the group receiving HSCT but declined on DMT, also favored the single-treatment therapy.
Two Real-World Experiences With HSCT Reported
Of the two multicenter real world studies presented at ECTRIMS, one included 363 patients treated at one of 14 participating public hospitals in the United Kingdom since 2002. This analysis was uncontrolled. The other, with 97 patients treated at one of 20 participating centers in Italy since 1999, compared HSCT to alemtuzumab retrospectively.
In the UK data, presented by Paolo Muraro, MD, PhD, Senior Consultant, Division of Brain Sciences, Imperial College, London, England, 94.6% were in relapse-free survival (RFS) at 2 years and 88.6% at 5 years after undergoing HSCT. He called these numbers “impressive.”
In addition, MRI-free activity survival (MFS) was 88.2% and 78.8% at 2 and 5 years, respectively, Dr. Muraro said.
On the Expanded Disability Status Scale (EDSS), the cumulative incidence of improvement was 24.6% at 2 years and 28.6% at 5 years. There was no evidence of disease activity on the endpoints of symptoms, relapse, and MRI (NEDA-3) in 72% of patients at 2 years and 48.5% at 5 years.
Relative to historical response rates in a refractory population, Dr. Muraro considered these results favorable. Although there were four deaths, producing a treatment-related mortality of 1.1%, all occurred at an early stage of the HSCT program when there was limited experience in the management of cytopenias and other acute complications of HSCT.
“In this real-world cohort, stem cell transplant led to a durable remission of inflammatory activity and to clinical stability even though this included patients with a high EDSS at baseline [median 6.0] and a substantial proportion [39%) with progressive disease,” Dr. Muraro said.
In the Italian data, presented by Alessio Signori, MD, PhD, associate professor, Department of Health Sciences, University of Genoa, Italy, the 97 HSCT patients were matched with 314 treated with alemtuzumab over the same period and compared with propensity score overlap weighting. Baseline features were comparable.
HSCT Outperforms Alemtuzumab on All Measures
After a median follow-up of 62 months in the HSCT group and 30 months in the alemtuzumab group, HSCT outperformed drug therapy on all efficacy measures. When translated into HR, HSCT relative to alemtuzumab was associated with a 50% reduction in the probability of disability progression (HR, 0.50; P = 0.025), a 66% reduction in probability of relapse (HR, 0.34; P < 0.001), and a 62% reduction in the probability of MRI activity (HR, 0.38; P < 0.001).
“At 5 years, 58.3% of patients in the HSCT group versus 22.3% of the patients in the alemtuzumab group maintained NEDA-3,” said Dr. Signori, who reported that this difference represented a greater than 50% reduction (HR, 0.48; P < 0.001).
In this study there were two treatment-related deaths. Both occurred within 30 days of HSCT and, again, were confined to the early experience with HSCT.
There are questions that remain unanswered, such as whether there are predictors of response to HSCT. Although sustained responses have been greater on HSCT than drug therapy on average, poor responses appeared to be more common among those with a progressive phenotype in the UK experience.
Although patients with progressive disease were excluded from the Italian study, a real-world experience published 2 years ago also indicated that patients with progressive forms of MS respond less well to HSCT. Conducted in the United States at a single center (Northwestern University, Chicago) with 414 patients of whom 93 had progressive disease, EDSS progressively declined over the 5 years of follow-up among those with secondary progressive MS even as it improved in those with relapsing-remitting multiple sclerosis.
HSCT Considered on Compassionate Basis
Overall, the HSCT experience as a rescue therapy for MS patients with an inadequate response to DMT has led a growing number of centers active in this area to offer this option on a compassionate basis, according to Dr. Muraro and Dr. Signori. Joachim Burman, MD, PhD, who was the moderator of the scientific session at ECTRIMS and the leader of a research program into HSCT for MS at Uppsala University, Uppsala, Sweden, agreed.
The increasing number of centers offering HSCT to MS patients does not preclude the need or the value of the ongoing phase 3 trials, according to Dr. Burman, but he suggested that there is a growing focus of how it should be used, not whether it will be used.
Ultimately, he speculated that HSCT, once accepted as a mainstream option for MS, will probably be confined to the 5%-10% of patients with very aggressive disease. This is not for lack of safety or efficacy, but he sees several barriers to using this approach first-line, outside of special situations.
“You need a team of several different specialists to offer HSCT,” he said, suggesting that this approach to MS is much more complicated than a visit to a neurologist’s office for a DMT prescription. However, he thinks other barriers, such as concern about safety, are dissipating.
HSCT “is still being characterized as a high-risk procedure, but I would object to that,” he said. “The deaths associated with HSCT largely occurred 20 years ago when the field was new.”
Dr. Muraro reported a financial relationship with Cellerys AG that is unrelated to this study. Dr. Signori reported financial relationships with Chiesi, Horizon, and Novartis. Dr. Burman reports no potential conflicts of interest.
COPENHAGEN —
With only one completed randomized trial available, HSCT remains experimental but the number of patients treated with this approach has now reached substantial numbers over 20 years of experience at multiple centers, according to two representative real-world studies presented at the 2024 ECTRIMS annual meeting.
The latest data are wholly consistent with a 2019 multinational randomized trial that found HSCT, which is a one-time intervention, to be relatively well tolerated and more effective than disease-modifying therapy (DMT) for median time to progression in patients refractory to DMT.
Relative to 24 months in the DMT group, median time to relapse was not reached among those randomized to HSCT because there were too few events. The hazard ratio (HR) reduction for progression was greater than 90% (HR, 0.07; P < 0.001). Other endpoints, such as EDSS, which improved in the group receiving HSCT but declined on DMT, also favored the single-treatment therapy.
Two Real-World Experiences With HSCT Reported
Of the two multicenter real world studies presented at ECTRIMS, one included 363 patients treated at one of 14 participating public hospitals in the United Kingdom since 2002. This analysis was uncontrolled. The other, with 97 patients treated at one of 20 participating centers in Italy since 1999, compared HSCT to alemtuzumab retrospectively.
In the UK data, presented by Paolo Muraro, MD, PhD, Senior Consultant, Division of Brain Sciences, Imperial College, London, England, 94.6% were in relapse-free survival (RFS) at 2 years and 88.6% at 5 years after undergoing HSCT. He called these numbers “impressive.”
In addition, MRI-free activity survival (MFS) was 88.2% and 78.8% at 2 and 5 years, respectively, Dr. Muraro said.
On the Expanded Disability Status Scale (EDSS), the cumulative incidence of improvement was 24.6% at 2 years and 28.6% at 5 years. There was no evidence of disease activity on the endpoints of symptoms, relapse, and MRI (NEDA-3) in 72% of patients at 2 years and 48.5% at 5 years.
Relative to historical response rates in a refractory population, Dr. Muraro considered these results favorable. Although there were four deaths, producing a treatment-related mortality of 1.1%, all occurred at an early stage of the HSCT program when there was limited experience in the management of cytopenias and other acute complications of HSCT.
“In this real-world cohort, stem cell transplant led to a durable remission of inflammatory activity and to clinical stability even though this included patients with a high EDSS at baseline [median 6.0] and a substantial proportion [39%) with progressive disease,” Dr. Muraro said.
In the Italian data, presented by Alessio Signori, MD, PhD, associate professor, Department of Health Sciences, University of Genoa, Italy, the 97 HSCT patients were matched with 314 treated with alemtuzumab over the same period and compared with propensity score overlap weighting. Baseline features were comparable.
HSCT Outperforms Alemtuzumab on All Measures
After a median follow-up of 62 months in the HSCT group and 30 months in the alemtuzumab group, HSCT outperformed drug therapy on all efficacy measures. When translated into HR, HSCT relative to alemtuzumab was associated with a 50% reduction in the probability of disability progression (HR, 0.50; P = 0.025), a 66% reduction in probability of relapse (HR, 0.34; P < 0.001), and a 62% reduction in the probability of MRI activity (HR, 0.38; P < 0.001).
“At 5 years, 58.3% of patients in the HSCT group versus 22.3% of the patients in the alemtuzumab group maintained NEDA-3,” said Dr. Signori, who reported that this difference represented a greater than 50% reduction (HR, 0.48; P < 0.001).
In this study there were two treatment-related deaths. Both occurred within 30 days of HSCT and, again, were confined to the early experience with HSCT.
There are questions that remain unanswered, such as whether there are predictors of response to HSCT. Although sustained responses have been greater on HSCT than drug therapy on average, poor responses appeared to be more common among those with a progressive phenotype in the UK experience.
Although patients with progressive disease were excluded from the Italian study, a real-world experience published 2 years ago also indicated that patients with progressive forms of MS respond less well to HSCT. Conducted in the United States at a single center (Northwestern University, Chicago) with 414 patients of whom 93 had progressive disease, EDSS progressively declined over the 5 years of follow-up among those with secondary progressive MS even as it improved in those with relapsing-remitting multiple sclerosis.
HSCT Considered on Compassionate Basis
Overall, the HSCT experience as a rescue therapy for MS patients with an inadequate response to DMT has led a growing number of centers active in this area to offer this option on a compassionate basis, according to Dr. Muraro and Dr. Signori. Joachim Burman, MD, PhD, who was the moderator of the scientific session at ECTRIMS and the leader of a research program into HSCT for MS at Uppsala University, Uppsala, Sweden, agreed.
The increasing number of centers offering HSCT to MS patients does not preclude the need or the value of the ongoing phase 3 trials, according to Dr. Burman, but he suggested that there is a growing focus of how it should be used, not whether it will be used.
Ultimately, he speculated that HSCT, once accepted as a mainstream option for MS, will probably be confined to the 5%-10% of patients with very aggressive disease. This is not for lack of safety or efficacy, but he sees several barriers to using this approach first-line, outside of special situations.
“You need a team of several different specialists to offer HSCT,” he said, suggesting that this approach to MS is much more complicated than a visit to a neurologist’s office for a DMT prescription. However, he thinks other barriers, such as concern about safety, are dissipating.
HSCT “is still being characterized as a high-risk procedure, but I would object to that,” he said. “The deaths associated with HSCT largely occurred 20 years ago when the field was new.”
Dr. Muraro reported a financial relationship with Cellerys AG that is unrelated to this study. Dr. Signori reported financial relationships with Chiesi, Horizon, and Novartis. Dr. Burman reports no potential conflicts of interest.
COPENHAGEN —
With only one completed randomized trial available, HSCT remains experimental but the number of patients treated with this approach has now reached substantial numbers over 20 years of experience at multiple centers, according to two representative real-world studies presented at the 2024 ECTRIMS annual meeting.
The latest data are wholly consistent with a 2019 multinational randomized trial that found HSCT, which is a one-time intervention, to be relatively well tolerated and more effective than disease-modifying therapy (DMT) for median time to progression in patients refractory to DMT.
Relative to 24 months in the DMT group, median time to relapse was not reached among those randomized to HSCT because there were too few events. The hazard ratio (HR) reduction for progression was greater than 90% (HR, 0.07; P < 0.001). Other endpoints, such as EDSS, which improved in the group receiving HSCT but declined on DMT, also favored the single-treatment therapy.
Two Real-World Experiences With HSCT Reported
Of the two multicenter real world studies presented at ECTRIMS, one included 363 patients treated at one of 14 participating public hospitals in the United Kingdom since 2002. This analysis was uncontrolled. The other, with 97 patients treated at one of 20 participating centers in Italy since 1999, compared HSCT to alemtuzumab retrospectively.
In the UK data, presented by Paolo Muraro, MD, PhD, Senior Consultant, Division of Brain Sciences, Imperial College, London, England, 94.6% were in relapse-free survival (RFS) at 2 years and 88.6% at 5 years after undergoing HSCT. He called these numbers “impressive.”
In addition, MRI-free activity survival (MFS) was 88.2% and 78.8% at 2 and 5 years, respectively, Dr. Muraro said.
On the Expanded Disability Status Scale (EDSS), the cumulative incidence of improvement was 24.6% at 2 years and 28.6% at 5 years. There was no evidence of disease activity on the endpoints of symptoms, relapse, and MRI (NEDA-3) in 72% of patients at 2 years and 48.5% at 5 years.
Relative to historical response rates in a refractory population, Dr. Muraro considered these results favorable. Although there were four deaths, producing a treatment-related mortality of 1.1%, all occurred at an early stage of the HSCT program when there was limited experience in the management of cytopenias and other acute complications of HSCT.
“In this real-world cohort, stem cell transplant led to a durable remission of inflammatory activity and to clinical stability even though this included patients with a high EDSS at baseline [median 6.0] and a substantial proportion [39%) with progressive disease,” Dr. Muraro said.
In the Italian data, presented by Alessio Signori, MD, PhD, associate professor, Department of Health Sciences, University of Genoa, Italy, the 97 HSCT patients were matched with 314 treated with alemtuzumab over the same period and compared with propensity score overlap weighting. Baseline features were comparable.
HSCT Outperforms Alemtuzumab on All Measures
After a median follow-up of 62 months in the HSCT group and 30 months in the alemtuzumab group, HSCT outperformed drug therapy on all efficacy measures. When translated into HR, HSCT relative to alemtuzumab was associated with a 50% reduction in the probability of disability progression (HR, 0.50; P = 0.025), a 66% reduction in probability of relapse (HR, 0.34; P < 0.001), and a 62% reduction in the probability of MRI activity (HR, 0.38; P < 0.001).
“At 5 years, 58.3% of patients in the HSCT group versus 22.3% of the patients in the alemtuzumab group maintained NEDA-3,” said Dr. Signori, who reported that this difference represented a greater than 50% reduction (HR, 0.48; P < 0.001).
In this study there were two treatment-related deaths. Both occurred within 30 days of HSCT and, again, were confined to the early experience with HSCT.
There are questions that remain unanswered, such as whether there are predictors of response to HSCT. Although sustained responses have been greater on HSCT than drug therapy on average, poor responses appeared to be more common among those with a progressive phenotype in the UK experience.
Although patients with progressive disease were excluded from the Italian study, a real-world experience published 2 years ago also indicated that patients with progressive forms of MS respond less well to HSCT. Conducted in the United States at a single center (Northwestern University, Chicago) with 414 patients of whom 93 had progressive disease, EDSS progressively declined over the 5 years of follow-up among those with secondary progressive MS even as it improved in those with relapsing-remitting multiple sclerosis.
HSCT Considered on Compassionate Basis
Overall, the HSCT experience as a rescue therapy for MS patients with an inadequate response to DMT has led a growing number of centers active in this area to offer this option on a compassionate basis, according to Dr. Muraro and Dr. Signori. Joachim Burman, MD, PhD, who was the moderator of the scientific session at ECTRIMS and the leader of a research program into HSCT for MS at Uppsala University, Uppsala, Sweden, agreed.
The increasing number of centers offering HSCT to MS patients does not preclude the need or the value of the ongoing phase 3 trials, according to Dr. Burman, but he suggested that there is a growing focus of how it should be used, not whether it will be used.
Ultimately, he speculated that HSCT, once accepted as a mainstream option for MS, will probably be confined to the 5%-10% of patients with very aggressive disease. This is not for lack of safety or efficacy, but he sees several barriers to using this approach first-line, outside of special situations.
“You need a team of several different specialists to offer HSCT,” he said, suggesting that this approach to MS is much more complicated than a visit to a neurologist’s office for a DMT prescription. However, he thinks other barriers, such as concern about safety, are dissipating.
HSCT “is still being characterized as a high-risk procedure, but I would object to that,” he said. “The deaths associated with HSCT largely occurred 20 years ago when the field was new.”
Dr. Muraro reported a financial relationship with Cellerys AG that is unrelated to this study. Dr. Signori reported financial relationships with Chiesi, Horizon, and Novartis. Dr. Burman reports no potential conflicts of interest.
FROM ECTRIMS 2024
Over One Third of Patients Develop Exocrine Pancreatic Insufficiency After Acute Pancreatitis
TOPLINE:
Over one third of patients with acute pancreatitis develop exocrine pancreatic insufficiency (EPI) at 12 months, with the key predictors being idiopathic etiology, moderately severe or severe disease, and preexisting diabetes.
METHODOLOGY:
- EPI has traditionally been associated with chronic pancreatitis, but its prevalence and natural history following acute pancreatitis are less well defined.
- Researchers conducted a prospective cohort study including 85 hospital inpatients (mean age, 54.7 years; 48.2% women) diagnosed with acute pancreatitis from three tertiary institutions in the United States.
- Severity of acute pancreatitis was classified according to the Revised Atlanta Criteria.
- EPI was assessed by measuring fecal elastase 1 (FE-1) levels from stool samples at baseline and at 3 and 12 months after enrollment. EPI was defined by FE-1 levels ≤ 200 μg/g stool, with mild and severe EPI categorized by FE-1 levels of 101-200 μg/g stool and ≤ 100 μg/g stool, respectively.
- The prevalence of EPI was assessed at 3 and 12 months after acute pancreatitis. The study also identified the predictors of EPI, including the role of etiology and severity of acute pancreatitis and preexisting diabetes.
TAKEAWAY:
- EPI was present in 34.1% participants at 12 months after an acute pancreatitis attack, with 22.4% having severe EPI.
- Even 12.8% of those with an index mild attack of acute pancreatitis had severe EPI at 12 months.
- The odds of developing EPI at 12 months increased fourfold with idiopathic etiology of acute pancreatitis (P = .0094).
- The odds of developing EPI increased over threefold with moderately severe or severe acute pancreatitis (P = .025) and preexisting diabetes (P = .031).
- The prevalence of severe EPI after acute pancreatitis decreased from 29% at baseline to 26% at 3 months and 22% at 12 months.
IN PRACTICE:
“While specific subpopulations may have identified clinical risk factors, it will remain important to have a low threshold for testing and treatment as there remains much to learn about mechanisms leading to EPI after [acute pancreatitis],” the authors wrote.
SOURCE:
This study, led by Anna Evans Phillips, MD, MS, University of Pittsburgh School of Medicine in Pennsylvania, was published online in eClinicalMedicine.
LIMITATIONS:
Participants were often transferred from other hospitals with differing management techniques, which may have introduced selection bias. The use of FE-1 levels may have had diagnostic limitations. The study did not assess the impact of pancreatic enzyme replacement therapy on recovery from EPI. Some patients with early chronic pancreatitis may have been included owing to the lack of diagnostic clarity.
DISCLOSURES:
The study was supported by an investigator-initiated research grant from AbbVie. Some authors received funding for research from AbbVie. One of the authors declared serving as a consultant and scientific advisory board member and being an equity holder in biotechnology, biopharmaceutical, and diagnostics companies. Another author declared support from the Cystic Fibrosis Foundation and the American Society for Parenteral and Enteral Nutrition.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
Over one third of patients with acute pancreatitis develop exocrine pancreatic insufficiency (EPI) at 12 months, with the key predictors being idiopathic etiology, moderately severe or severe disease, and preexisting diabetes.
METHODOLOGY:
- EPI has traditionally been associated with chronic pancreatitis, but its prevalence and natural history following acute pancreatitis are less well defined.
- Researchers conducted a prospective cohort study including 85 hospital inpatients (mean age, 54.7 years; 48.2% women) diagnosed with acute pancreatitis from three tertiary institutions in the United States.
- Severity of acute pancreatitis was classified according to the Revised Atlanta Criteria.
- EPI was assessed by measuring fecal elastase 1 (FE-1) levels from stool samples at baseline and at 3 and 12 months after enrollment. EPI was defined by FE-1 levels ≤ 200 μg/g stool, with mild and severe EPI categorized by FE-1 levels of 101-200 μg/g stool and ≤ 100 μg/g stool, respectively.
- The prevalence of EPI was assessed at 3 and 12 months after acute pancreatitis. The study also identified the predictors of EPI, including the role of etiology and severity of acute pancreatitis and preexisting diabetes.
TAKEAWAY:
- EPI was present in 34.1% participants at 12 months after an acute pancreatitis attack, with 22.4% having severe EPI.
- Even 12.8% of those with an index mild attack of acute pancreatitis had severe EPI at 12 months.
- The odds of developing EPI at 12 months increased fourfold with idiopathic etiology of acute pancreatitis (P = .0094).
- The odds of developing EPI increased over threefold with moderately severe or severe acute pancreatitis (P = .025) and preexisting diabetes (P = .031).
- The prevalence of severe EPI after acute pancreatitis decreased from 29% at baseline to 26% at 3 months and 22% at 12 months.
IN PRACTICE:
“While specific subpopulations may have identified clinical risk factors, it will remain important to have a low threshold for testing and treatment as there remains much to learn about mechanisms leading to EPI after [acute pancreatitis],” the authors wrote.
SOURCE:
This study, led by Anna Evans Phillips, MD, MS, University of Pittsburgh School of Medicine in Pennsylvania, was published online in eClinicalMedicine.
LIMITATIONS:
Participants were often transferred from other hospitals with differing management techniques, which may have introduced selection bias. The use of FE-1 levels may have had diagnostic limitations. The study did not assess the impact of pancreatic enzyme replacement therapy on recovery from EPI. Some patients with early chronic pancreatitis may have been included owing to the lack of diagnostic clarity.
DISCLOSURES:
The study was supported by an investigator-initiated research grant from AbbVie. Some authors received funding for research from AbbVie. One of the authors declared serving as a consultant and scientific advisory board member and being an equity holder in biotechnology, biopharmaceutical, and diagnostics companies. Another author declared support from the Cystic Fibrosis Foundation and the American Society for Parenteral and Enteral Nutrition.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
Over one third of patients with acute pancreatitis develop exocrine pancreatic insufficiency (EPI) at 12 months, with the key predictors being idiopathic etiology, moderately severe or severe disease, and preexisting diabetes.
METHODOLOGY:
- EPI has traditionally been associated with chronic pancreatitis, but its prevalence and natural history following acute pancreatitis are less well defined.
- Researchers conducted a prospective cohort study including 85 hospital inpatients (mean age, 54.7 years; 48.2% women) diagnosed with acute pancreatitis from three tertiary institutions in the United States.
- Severity of acute pancreatitis was classified according to the Revised Atlanta Criteria.
- EPI was assessed by measuring fecal elastase 1 (FE-1) levels from stool samples at baseline and at 3 and 12 months after enrollment. EPI was defined by FE-1 levels ≤ 200 μg/g stool, with mild and severe EPI categorized by FE-1 levels of 101-200 μg/g stool and ≤ 100 μg/g stool, respectively.
- The prevalence of EPI was assessed at 3 and 12 months after acute pancreatitis. The study also identified the predictors of EPI, including the role of etiology and severity of acute pancreatitis and preexisting diabetes.
TAKEAWAY:
- EPI was present in 34.1% participants at 12 months after an acute pancreatitis attack, with 22.4% having severe EPI.
- Even 12.8% of those with an index mild attack of acute pancreatitis had severe EPI at 12 months.
- The odds of developing EPI at 12 months increased fourfold with idiopathic etiology of acute pancreatitis (P = .0094).
- The odds of developing EPI increased over threefold with moderately severe or severe acute pancreatitis (P = .025) and preexisting diabetes (P = .031).
- The prevalence of severe EPI after acute pancreatitis decreased from 29% at baseline to 26% at 3 months and 22% at 12 months.
IN PRACTICE:
“While specific subpopulations may have identified clinical risk factors, it will remain important to have a low threshold for testing and treatment as there remains much to learn about mechanisms leading to EPI after [acute pancreatitis],” the authors wrote.
SOURCE:
This study, led by Anna Evans Phillips, MD, MS, University of Pittsburgh School of Medicine in Pennsylvania, was published online in eClinicalMedicine.
LIMITATIONS:
Participants were often transferred from other hospitals with differing management techniques, which may have introduced selection bias. The use of FE-1 levels may have had diagnostic limitations. The study did not assess the impact of pancreatic enzyme replacement therapy on recovery from EPI. Some patients with early chronic pancreatitis may have been included owing to the lack of diagnostic clarity.
DISCLOSURES:
The study was supported by an investigator-initiated research grant from AbbVie. Some authors received funding for research from AbbVie. One of the authors declared serving as a consultant and scientific advisory board member and being an equity holder in biotechnology, biopharmaceutical, and diagnostics companies. Another author declared support from the Cystic Fibrosis Foundation and the American Society for Parenteral and Enteral Nutrition.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
Benralizumab Now FDA Approved to Treat EGPA Vasculitis
The Food and Drug Administration (FDA) has approved benralizumab (Fasenra) for the treatment of adults with eosinophilic granulomatosis with polyangiitis (EGPA), formerly known as Churg-Strauss syndrome.
The drug is the second approved biologic for the treatment of EGPA. The first, mepolizumab (Nucala), was approved in 2017.
“This disease has a devastating impact on patients and the quality of their life, and they need more treatment options. The approval of another treatment in EGPA is welcome news to the approximately 15,000 patients living in the US with this difficult-to-treat rare disease,” said Joyce Kullman, executive director of the Vasculitis Foundation, in a press release on September 18.
Benralizumab, developed by AstraZeneca, is a monoclonal antibody against the interleukin-5 alpha receptor expressed on eosinophils. The drug was first approved in 2017 as an add-on treatment for patients 12 years and older with severe eosinophilic asthma, and is now approved for use in children aged 6 years and older.
The new indication was based on positive results from a noninferiority trial comparing benralizumab and mepolizumab. For the trial, published in the New England Journal of Medicine earlier in 2024, 140 adults with relapsing or refractory EGPA were randomized to a 30-mg subcutaneous injection of benralizumab or three separate 100-mg mepolizumab injections every 4 weeks for 1 year. At weeks 36 and 48, 59% of patients in the benralizumab group and 56% of patients in the mepolizumab group achieved remission (95% CI, –13 to 18; P = .73 for superiority). From week 42 to 52, 41% of patients who received benralizumab completely stopped taking oral glucocorticoids, compared with 26% of those who received mepolizumab.
“Patients often rely on long-term oral corticosteroids, which can cause serious and lasting side effects. Benralizumab is a much-needed treatment option, with data showing that not only is remission an achievable goal for EGPA patients, but benralizumab can also help patients taper off steroid therapy,” Michael Wechsler, MD, director of The Asthma Institute at National Jewish Health in Denver, Colorado, and the international coordinating investigator for the clinical trial, said in the press release.
Benralizumab is administered via subcutaneous injection. In adults with EGPA, the recommended dosage is 30 mg every 4 weeks for the first three doses, then once every 8 weeks.
The most common adverse reactions include headache and pharyngitis, according to the prescribing information.
Benralizumab is also in development for the treatment of chronic obstructive pulmonary disease, chronic rhinosinusitis with nasal polyps, and hypereosinophilic syndrome.
A version of this article first appeared on Medscape.com.
The Food and Drug Administration (FDA) has approved benralizumab (Fasenra) for the treatment of adults with eosinophilic granulomatosis with polyangiitis (EGPA), formerly known as Churg-Strauss syndrome.
The drug is the second approved biologic for the treatment of EGPA. The first, mepolizumab (Nucala), was approved in 2017.
“This disease has a devastating impact on patients and the quality of their life, and they need more treatment options. The approval of another treatment in EGPA is welcome news to the approximately 15,000 patients living in the US with this difficult-to-treat rare disease,” said Joyce Kullman, executive director of the Vasculitis Foundation, in a press release on September 18.
Benralizumab, developed by AstraZeneca, is a monoclonal antibody against the interleukin-5 alpha receptor expressed on eosinophils. The drug was first approved in 2017 as an add-on treatment for patients 12 years and older with severe eosinophilic asthma, and is now approved for use in children aged 6 years and older.
The new indication was based on positive results from a noninferiority trial comparing benralizumab and mepolizumab. For the trial, published in the New England Journal of Medicine earlier in 2024, 140 adults with relapsing or refractory EGPA were randomized to a 30-mg subcutaneous injection of benralizumab or three separate 100-mg mepolizumab injections every 4 weeks for 1 year. At weeks 36 and 48, 59% of patients in the benralizumab group and 56% of patients in the mepolizumab group achieved remission (95% CI, –13 to 18; P = .73 for superiority). From week 42 to 52, 41% of patients who received benralizumab completely stopped taking oral glucocorticoids, compared with 26% of those who received mepolizumab.
“Patients often rely on long-term oral corticosteroids, which can cause serious and lasting side effects. Benralizumab is a much-needed treatment option, with data showing that not only is remission an achievable goal for EGPA patients, but benralizumab can also help patients taper off steroid therapy,” Michael Wechsler, MD, director of The Asthma Institute at National Jewish Health in Denver, Colorado, and the international coordinating investigator for the clinical trial, said in the press release.
Benralizumab is administered via subcutaneous injection. In adults with EGPA, the recommended dosage is 30 mg every 4 weeks for the first three doses, then once every 8 weeks.
The most common adverse reactions include headache and pharyngitis, according to the prescribing information.
Benralizumab is also in development for the treatment of chronic obstructive pulmonary disease, chronic rhinosinusitis with nasal polyps, and hypereosinophilic syndrome.
A version of this article first appeared on Medscape.com.
The Food and Drug Administration (FDA) has approved benralizumab (Fasenra) for the treatment of adults with eosinophilic granulomatosis with polyangiitis (EGPA), formerly known as Churg-Strauss syndrome.
The drug is the second approved biologic for the treatment of EGPA. The first, mepolizumab (Nucala), was approved in 2017.
“This disease has a devastating impact on patients and the quality of their life, and they need more treatment options. The approval of another treatment in EGPA is welcome news to the approximately 15,000 patients living in the US with this difficult-to-treat rare disease,” said Joyce Kullman, executive director of the Vasculitis Foundation, in a press release on September 18.
Benralizumab, developed by AstraZeneca, is a monoclonal antibody against the interleukin-5 alpha receptor expressed on eosinophils. The drug was first approved in 2017 as an add-on treatment for patients 12 years and older with severe eosinophilic asthma, and is now approved for use in children aged 6 years and older.
The new indication was based on positive results from a noninferiority trial comparing benralizumab and mepolizumab. For the trial, published in the New England Journal of Medicine earlier in 2024, 140 adults with relapsing or refractory EGPA were randomized to a 30-mg subcutaneous injection of benralizumab or three separate 100-mg mepolizumab injections every 4 weeks for 1 year. At weeks 36 and 48, 59% of patients in the benralizumab group and 56% of patients in the mepolizumab group achieved remission (95% CI, –13 to 18; P = .73 for superiority). From week 42 to 52, 41% of patients who received benralizumab completely stopped taking oral glucocorticoids, compared with 26% of those who received mepolizumab.
“Patients often rely on long-term oral corticosteroids, which can cause serious and lasting side effects. Benralizumab is a much-needed treatment option, with data showing that not only is remission an achievable goal for EGPA patients, but benralizumab can also help patients taper off steroid therapy,” Michael Wechsler, MD, director of The Asthma Institute at National Jewish Health in Denver, Colorado, and the international coordinating investigator for the clinical trial, said in the press release.
Benralizumab is administered via subcutaneous injection. In adults with EGPA, the recommended dosage is 30 mg every 4 weeks for the first three doses, then once every 8 weeks.
The most common adverse reactions include headache and pharyngitis, according to the prescribing information.
Benralizumab is also in development for the treatment of chronic obstructive pulmonary disease, chronic rhinosinusitis with nasal polyps, and hypereosinophilic syndrome.
A version of this article first appeared on Medscape.com.
Comorbidity Control Might Slow MS Activity
COPENHAGEN — The largest and perhaps most rigorous study to demonstrate an association between the presence of comorbidities and accelerated progression of multiple sclerosis (MS) is sufficiently compelling that both the study author and an independent expert maintained clinical practice should be adjusted.
Even while acknowledging that “it is hard to make causative statements” on the basis of these types of data, the findings are sufficiently compelling to suggest that comorbidities “should be a pressing concern” in MS management, according to Amber Salter, PhD, an associate professor of biostatistics at the University of Texas Southwestern Medical School, Dallas.
The strong association in this meta-analysis, presented at the 2024 ECTRIMS annual meeting, were drawn from 15 multicenter phase 3 treatment trials with 16,794 participants followed for at least 2 years, Dr. Salter reported. Her data were published simultaneously in JAMA Neurology.
“One of the strengths of this study is that the data on comorbidities were collected prospectively as part of these trials,” explained Mark S. Freedman, MD, director of the Multiple Sclerosis Research Center at Ottawa Hospital in Canada. He agreed with Dr. Salter that it is reasonable to apply these findings to clinical practice given their consistency with numerous other studies and the value of what he termed as “a holistic approach” to improving outcomes in MS.
Meta-Analysis Avoids Weaknesses of Previous Data
There are many potential weaknesses of past observational studies that the authors of this meta-analysis hoped to avoid. These include the possibility that MS patients with comorbidities might be less likely to take or adhere to disease-modifying therapies (DMT) or that comorbidity burden might masquerade or be misinterpreted as MS progression. By employing data from phase 3 DMT trials, Dr. Salter maintained that prospectively collected data monitored carefully over an extended follow-up allows the impact of comorbidities on outcome to be evaluated in a more controlled fashion.
Dr. Freedman liked the design of this study, but he admitted that he was surprised by the result.
“Phase 3 trials typically include exclusion criteria for significant comorbidities, so I did not think they would be able to show any meaningful differences,” Dr. Freedman said in an interview.
For the main outcome of evidence of disease activity (EDA), defined as confirmed disability worsening measured with the Expanded Disability Status Scale (EDSS), relapse activity, or any new or enlarging lesions on MRI, the differences reached significance even after adjustments for multiple potentially confounding factors.
MS Activity Increases Significantly with More Comorbidities
Compared with no comorbidity, the presence of three or more comorbidities were associated with a significant 14% increase in the adjusted hazard ratio (aHR) of EDA (aHR, 1.14; 95% CI, 1.02-1.28), Dr. Salter reported. If there were two or more cardiometabolic comorbidities, the risk of EDA was increased 21% (aHR, 1.21; 95% CI 1.08-1.37).
The list of comorbidities considered in this study was drawn from the International Advisory Committee on Clinical Trials in MS. It included numerous cardiometabolic comorbidities, such as hypertension, hyperlipidemia, diabetes, ischemic heart disease, cerebrovascular disease, and peripheral vascular disease. It also included chronic lung diseases, such as asthma and chronic obstructive pulmonary disease; psychiatric diseases, such as depression and anxiety; and miscellaneous autoimmune conditions.
The number of comorbidities was categorized for analysis as zero, one, two, or three or more. However, Dr. Salter acknowledged that these phase 3 trials did include comorbidity exclusion criteria. In fact, severe forms of most of these comorbidities were exclusion criteria in at least some studies. Yet, the prevalence of one or more comorbidities was still 45.4% in the total population from this meta-analysis.
By themselves alone, ischemic heart disease (aHR, 1.63), cerebrovascular disease (aHR, 1.70) and at least one psychiatric disorder (aHR, 1.14) were all significant for increased MS activity at the end of 2 years by a 95% confidence interval that did not cross the line of unity.
When the EDA endpoints were evaluated individually, not even three or more comorbidities was associated with an increased rate of active lesions on MRI at the end of follow-up, but two or more and three or more comorbidities were associated with a significantly increased risk of disability worsening (aHR, 1.16 and aHR, 1.31, respectively) and relapse (aHR, 1.16 for both).
An Underestimation of Associations?
Prospective trials are still needed to show that treating comorbidities improves outcome in MS, but randomization will be problematic if it means withholding treatment for conditions with risks independent of MS, Dr. Salter said. Although the data from this analysis did not permit an analysis of how relative severity of comorbidities affected MS outcome, she reiterated that most patients with severe comorbidities were likely excluded from inclusion in the studies anyway.
“We think that we are probably seeing an underestimation of an associations between comorbidity and increased MS activity,” Dr. Salter said. While she reported that confounding cannot be ruled out, the robust associations identified in a meta-analysis “limit the possibility of bias or chance findings.”
Asked if the message that clinicians should treat comorbidities to reduce MS activity is a reasonable conclusion in the absence of proof that treatment is beneficial, Dr. Freedman looked both to the body of evidence and to the common sense behind the recommendation.
Basically, Dr. Freedman believes that comorbidities should be addressed routinely and rigorously even if there was no evidence that they improve MS outcome. These data provide just one other source of support for a practice that should be conducted anyway.
Dr. Salter reported financial relationships with Abata Therapeutics, Gryphon Bio, and Owl Therapeutics. Dr. Freedman reported financial relationships with more than 10 pharmaceutical companies.
COPENHAGEN — The largest and perhaps most rigorous study to demonstrate an association between the presence of comorbidities and accelerated progression of multiple sclerosis (MS) is sufficiently compelling that both the study author and an independent expert maintained clinical practice should be adjusted.
Even while acknowledging that “it is hard to make causative statements” on the basis of these types of data, the findings are sufficiently compelling to suggest that comorbidities “should be a pressing concern” in MS management, according to Amber Salter, PhD, an associate professor of biostatistics at the University of Texas Southwestern Medical School, Dallas.
The strong association in this meta-analysis, presented at the 2024 ECTRIMS annual meeting, were drawn from 15 multicenter phase 3 treatment trials with 16,794 participants followed for at least 2 years, Dr. Salter reported. Her data were published simultaneously in JAMA Neurology.
“One of the strengths of this study is that the data on comorbidities were collected prospectively as part of these trials,” explained Mark S. Freedman, MD, director of the Multiple Sclerosis Research Center at Ottawa Hospital in Canada. He agreed with Dr. Salter that it is reasonable to apply these findings to clinical practice given their consistency with numerous other studies and the value of what he termed as “a holistic approach” to improving outcomes in MS.
Meta-Analysis Avoids Weaknesses of Previous Data
There are many potential weaknesses of past observational studies that the authors of this meta-analysis hoped to avoid. These include the possibility that MS patients with comorbidities might be less likely to take or adhere to disease-modifying therapies (DMT) or that comorbidity burden might masquerade or be misinterpreted as MS progression. By employing data from phase 3 DMT trials, Dr. Salter maintained that prospectively collected data monitored carefully over an extended follow-up allows the impact of comorbidities on outcome to be evaluated in a more controlled fashion.
Dr. Freedman liked the design of this study, but he admitted that he was surprised by the result.
“Phase 3 trials typically include exclusion criteria for significant comorbidities, so I did not think they would be able to show any meaningful differences,” Dr. Freedman said in an interview.
For the main outcome of evidence of disease activity (EDA), defined as confirmed disability worsening measured with the Expanded Disability Status Scale (EDSS), relapse activity, or any new or enlarging lesions on MRI, the differences reached significance even after adjustments for multiple potentially confounding factors.
MS Activity Increases Significantly with More Comorbidities
Compared with no comorbidity, the presence of three or more comorbidities were associated with a significant 14% increase in the adjusted hazard ratio (aHR) of EDA (aHR, 1.14; 95% CI, 1.02-1.28), Dr. Salter reported. If there were two or more cardiometabolic comorbidities, the risk of EDA was increased 21% (aHR, 1.21; 95% CI 1.08-1.37).
The list of comorbidities considered in this study was drawn from the International Advisory Committee on Clinical Trials in MS. It included numerous cardiometabolic comorbidities, such as hypertension, hyperlipidemia, diabetes, ischemic heart disease, cerebrovascular disease, and peripheral vascular disease. It also included chronic lung diseases, such as asthma and chronic obstructive pulmonary disease; psychiatric diseases, such as depression and anxiety; and miscellaneous autoimmune conditions.
The number of comorbidities was categorized for analysis as zero, one, two, or three or more. However, Dr. Salter acknowledged that these phase 3 trials did include comorbidity exclusion criteria. In fact, severe forms of most of these comorbidities were exclusion criteria in at least some studies. Yet, the prevalence of one or more comorbidities was still 45.4% in the total population from this meta-analysis.
By themselves alone, ischemic heart disease (aHR, 1.63), cerebrovascular disease (aHR, 1.70) and at least one psychiatric disorder (aHR, 1.14) were all significant for increased MS activity at the end of 2 years by a 95% confidence interval that did not cross the line of unity.
When the EDA endpoints were evaluated individually, not even three or more comorbidities was associated with an increased rate of active lesions on MRI at the end of follow-up, but two or more and three or more comorbidities were associated with a significantly increased risk of disability worsening (aHR, 1.16 and aHR, 1.31, respectively) and relapse (aHR, 1.16 for both).
An Underestimation of Associations?
Prospective trials are still needed to show that treating comorbidities improves outcome in MS, but randomization will be problematic if it means withholding treatment for conditions with risks independent of MS, Dr. Salter said. Although the data from this analysis did not permit an analysis of how relative severity of comorbidities affected MS outcome, she reiterated that most patients with severe comorbidities were likely excluded from inclusion in the studies anyway.
“We think that we are probably seeing an underestimation of an associations between comorbidity and increased MS activity,” Dr. Salter said. While she reported that confounding cannot be ruled out, the robust associations identified in a meta-analysis “limit the possibility of bias or chance findings.”
Asked if the message that clinicians should treat comorbidities to reduce MS activity is a reasonable conclusion in the absence of proof that treatment is beneficial, Dr. Freedman looked both to the body of evidence and to the common sense behind the recommendation.
Basically, Dr. Freedman believes that comorbidities should be addressed routinely and rigorously even if there was no evidence that they improve MS outcome. These data provide just one other source of support for a practice that should be conducted anyway.
Dr. Salter reported financial relationships with Abata Therapeutics, Gryphon Bio, and Owl Therapeutics. Dr. Freedman reported financial relationships with more than 10 pharmaceutical companies.
COPENHAGEN — The largest and perhaps most rigorous study to demonstrate an association between the presence of comorbidities and accelerated progression of multiple sclerosis (MS) is sufficiently compelling that both the study author and an independent expert maintained clinical practice should be adjusted.
Even while acknowledging that “it is hard to make causative statements” on the basis of these types of data, the findings are sufficiently compelling to suggest that comorbidities “should be a pressing concern” in MS management, according to Amber Salter, PhD, an associate professor of biostatistics at the University of Texas Southwestern Medical School, Dallas.
The strong association in this meta-analysis, presented at the 2024 ECTRIMS annual meeting, were drawn from 15 multicenter phase 3 treatment trials with 16,794 participants followed for at least 2 years, Dr. Salter reported. Her data were published simultaneously in JAMA Neurology.
“One of the strengths of this study is that the data on comorbidities were collected prospectively as part of these trials,” explained Mark S. Freedman, MD, director of the Multiple Sclerosis Research Center at Ottawa Hospital in Canada. He agreed with Dr. Salter that it is reasonable to apply these findings to clinical practice given their consistency with numerous other studies and the value of what he termed as “a holistic approach” to improving outcomes in MS.
Meta-Analysis Avoids Weaknesses of Previous Data
There are many potential weaknesses of past observational studies that the authors of this meta-analysis hoped to avoid. These include the possibility that MS patients with comorbidities might be less likely to take or adhere to disease-modifying therapies (DMT) or that comorbidity burden might masquerade or be misinterpreted as MS progression. By employing data from phase 3 DMT trials, Dr. Salter maintained that prospectively collected data monitored carefully over an extended follow-up allows the impact of comorbidities on outcome to be evaluated in a more controlled fashion.
Dr. Freedman liked the design of this study, but he admitted that he was surprised by the result.
“Phase 3 trials typically include exclusion criteria for significant comorbidities, so I did not think they would be able to show any meaningful differences,” Dr. Freedman said in an interview.
For the main outcome of evidence of disease activity (EDA), defined as confirmed disability worsening measured with the Expanded Disability Status Scale (EDSS), relapse activity, or any new or enlarging lesions on MRI, the differences reached significance even after adjustments for multiple potentially confounding factors.
MS Activity Increases Significantly with More Comorbidities
Compared with no comorbidity, the presence of three or more comorbidities were associated with a significant 14% increase in the adjusted hazard ratio (aHR) of EDA (aHR, 1.14; 95% CI, 1.02-1.28), Dr. Salter reported. If there were two or more cardiometabolic comorbidities, the risk of EDA was increased 21% (aHR, 1.21; 95% CI 1.08-1.37).
The list of comorbidities considered in this study was drawn from the International Advisory Committee on Clinical Trials in MS. It included numerous cardiometabolic comorbidities, such as hypertension, hyperlipidemia, diabetes, ischemic heart disease, cerebrovascular disease, and peripheral vascular disease. It also included chronic lung diseases, such as asthma and chronic obstructive pulmonary disease; psychiatric diseases, such as depression and anxiety; and miscellaneous autoimmune conditions.
The number of comorbidities was categorized for analysis as zero, one, two, or three or more. However, Dr. Salter acknowledged that these phase 3 trials did include comorbidity exclusion criteria. In fact, severe forms of most of these comorbidities were exclusion criteria in at least some studies. Yet, the prevalence of one or more comorbidities was still 45.4% in the total population from this meta-analysis.
By themselves alone, ischemic heart disease (aHR, 1.63), cerebrovascular disease (aHR, 1.70) and at least one psychiatric disorder (aHR, 1.14) were all significant for increased MS activity at the end of 2 years by a 95% confidence interval that did not cross the line of unity.
When the EDA endpoints were evaluated individually, not even three or more comorbidities was associated with an increased rate of active lesions on MRI at the end of follow-up, but two or more and three or more comorbidities were associated with a significantly increased risk of disability worsening (aHR, 1.16 and aHR, 1.31, respectively) and relapse (aHR, 1.16 for both).
An Underestimation of Associations?
Prospective trials are still needed to show that treating comorbidities improves outcome in MS, but randomization will be problematic if it means withholding treatment for conditions with risks independent of MS, Dr. Salter said. Although the data from this analysis did not permit an analysis of how relative severity of comorbidities affected MS outcome, she reiterated that most patients with severe comorbidities were likely excluded from inclusion in the studies anyway.
“We think that we are probably seeing an underestimation of an associations between comorbidity and increased MS activity,” Dr. Salter said. While she reported that confounding cannot be ruled out, the robust associations identified in a meta-analysis “limit the possibility of bias or chance findings.”
Asked if the message that clinicians should treat comorbidities to reduce MS activity is a reasonable conclusion in the absence of proof that treatment is beneficial, Dr. Freedman looked both to the body of evidence and to the common sense behind the recommendation.
Basically, Dr. Freedman believes that comorbidities should be addressed routinely and rigorously even if there was no evidence that they improve MS outcome. These data provide just one other source of support for a practice that should be conducted anyway.
Dr. Salter reported financial relationships with Abata Therapeutics, Gryphon Bio, and Owl Therapeutics. Dr. Freedman reported financial relationships with more than 10 pharmaceutical companies.
FROM ECTRIMS 2024
Rheumatology RCTs Have Lower Representation of Women as Authors
TOPLINE:
Women are underrepresented as authors in randomized controlled trials (RCTs) published in rheumatology from 2009 to 2023. RCTs from Africa had higher women representation as authors, while RCTs from Asia and Europe and industry-funded RCTs had lower representation of women.
METHODOLOGY:
- Researchers analyzed 1092 RCTs published in rheumatology from 2009 to 2023 involving 10,794 authors to evaluate the temporal trends and the factors influencing women’s authorship.
- The gender of authors was determined on the basis of their first names and countries of affiliation using a gender application programming interface service.
- The study assessed the association of women’s authorship with various factors using generalized estimating equations by considering women’s gender as the main binary outcome.
- Various covariates influencing women’s authorship such as geographic location, sponsorship type, intervention type, and journal impact factor were also evaluated.
TAKEAWAY:
- Overall, women accounted for 34.1% of authors in RCTs published in rheumatology from 2009 to 2023. They had less representation as first and last authors than men (36.8% vs 50.0% and 26.1% vs 61.2%, respectively).
- RCTs from Africa had higher odds of being authored by women than those from North America (odds ratio [OR], 2.34; 95% CI, 1.02-5.38). Women were also less represented as authors in RCTs from Asia and Europe.
- Their representation as authors was lower in industry-funded RCTs as well (OR, 0.64; 95% CI, 0.56-0.73).
- Women were less likely to be in senior author positions such as last (OR, 0.72) or penultimate (OR, 0.70; P < .001 for both) authors than in middle author positions.
IN PRACTICE:
“Implementing structured policies and supporting women through mentorship and leadership opportunities are crucial steps toward a more inclusive and dynamic research environment,” the authors wrote.
SOURCE:
This study was led by Kim Lauper, MD, Geneva University Hospitals, Division of Rheumatology and Faculty of Medicine, University of Geneva, Switzerland, and was published online on August 26, 2024, in medRxiv.
LIMITATIONS:
This study relied on binary gender data, which did not encompass nonbinary or other gender identities. Moreover, the accuracy of gender determination from names, although robust, had inherent limitations that could have affected the interpretation of results.
DISCLOSURES:
This study did not receive any funding. The authors declared no competing interests.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
TOPLINE:
Women are underrepresented as authors in randomized controlled trials (RCTs) published in rheumatology from 2009 to 2023. RCTs from Africa had higher women representation as authors, while RCTs from Asia and Europe and industry-funded RCTs had lower representation of women.
METHODOLOGY:
- Researchers analyzed 1092 RCTs published in rheumatology from 2009 to 2023 involving 10,794 authors to evaluate the temporal trends and the factors influencing women’s authorship.
- The gender of authors was determined on the basis of their first names and countries of affiliation using a gender application programming interface service.
- The study assessed the association of women’s authorship with various factors using generalized estimating equations by considering women’s gender as the main binary outcome.
- Various covariates influencing women’s authorship such as geographic location, sponsorship type, intervention type, and journal impact factor were also evaluated.
TAKEAWAY:
- Overall, women accounted for 34.1% of authors in RCTs published in rheumatology from 2009 to 2023. They had less representation as first and last authors than men (36.8% vs 50.0% and 26.1% vs 61.2%, respectively).
- RCTs from Africa had higher odds of being authored by women than those from North America (odds ratio [OR], 2.34; 95% CI, 1.02-5.38). Women were also less represented as authors in RCTs from Asia and Europe.
- Their representation as authors was lower in industry-funded RCTs as well (OR, 0.64; 95% CI, 0.56-0.73).
- Women were less likely to be in senior author positions such as last (OR, 0.72) or penultimate (OR, 0.70; P < .001 for both) authors than in middle author positions.
IN PRACTICE:
“Implementing structured policies and supporting women through mentorship and leadership opportunities are crucial steps toward a more inclusive and dynamic research environment,” the authors wrote.
SOURCE:
This study was led by Kim Lauper, MD, Geneva University Hospitals, Division of Rheumatology and Faculty of Medicine, University of Geneva, Switzerland, and was published online on August 26, 2024, in medRxiv.
LIMITATIONS:
This study relied on binary gender data, which did not encompass nonbinary or other gender identities. Moreover, the accuracy of gender determination from names, although robust, had inherent limitations that could have affected the interpretation of results.
DISCLOSURES:
This study did not receive any funding. The authors declared no competing interests.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
TOPLINE:
Women are underrepresented as authors in randomized controlled trials (RCTs) published in rheumatology from 2009 to 2023. RCTs from Africa had higher women representation as authors, while RCTs from Asia and Europe and industry-funded RCTs had lower representation of women.
METHODOLOGY:
- Researchers analyzed 1092 RCTs published in rheumatology from 2009 to 2023 involving 10,794 authors to evaluate the temporal trends and the factors influencing women’s authorship.
- The gender of authors was determined on the basis of their first names and countries of affiliation using a gender application programming interface service.
- The study assessed the association of women’s authorship with various factors using generalized estimating equations by considering women’s gender as the main binary outcome.
- Various covariates influencing women’s authorship such as geographic location, sponsorship type, intervention type, and journal impact factor were also evaluated.
TAKEAWAY:
- Overall, women accounted for 34.1% of authors in RCTs published in rheumatology from 2009 to 2023. They had less representation as first and last authors than men (36.8% vs 50.0% and 26.1% vs 61.2%, respectively).
- RCTs from Africa had higher odds of being authored by women than those from North America (odds ratio [OR], 2.34; 95% CI, 1.02-5.38). Women were also less represented as authors in RCTs from Asia and Europe.
- Their representation as authors was lower in industry-funded RCTs as well (OR, 0.64; 95% CI, 0.56-0.73).
- Women were less likely to be in senior author positions such as last (OR, 0.72) or penultimate (OR, 0.70; P < .001 for both) authors than in middle author positions.
IN PRACTICE:
“Implementing structured policies and supporting women through mentorship and leadership opportunities are crucial steps toward a more inclusive and dynamic research environment,” the authors wrote.
SOURCE:
This study was led by Kim Lauper, MD, Geneva University Hospitals, Division of Rheumatology and Faculty of Medicine, University of Geneva, Switzerland, and was published online on August 26, 2024, in medRxiv.
LIMITATIONS:
This study relied on binary gender data, which did not encompass nonbinary or other gender identities. Moreover, the accuracy of gender determination from names, although robust, had inherent limitations that could have affected the interpretation of results.
DISCLOSURES:
This study did not receive any funding. The authors declared no competing interests.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
Rheumatology Clinic Interventions for Smoking, Blood Pressure ‘Make a Big Difference’
Two relatively simple interventions — addressing high blood pressure (BP) and smoking cessation — could make a huge difference for patients with rheumatic disease. Patients with autoimmune disease are up to three times more likely to develop cardiovascular disease (CVD) than the general population. In addition to compounding CVD, smoking is tied to the development of certain autoimmune conditions, as well as worse outcomes. Christie Bartels, MD, chief of the Division of Rheumatology at the University of Wisconsin School of Medicine and Public Health, Madison, has focused her research on improving cardiac health in inflammatory diseases. This news organization spoke with Bartels about two short interventions she developed that tackle hypertension and smoking cessation during regular visits, each taking less than 3 minutes.
How Do These Programs Address Cardiac Disease Prevention?
The BP and Quit Connect programs help clinics systematically address the two most modifiable risk factors for CVD: high BP and smoking. There’s also evidence that addressing these two risk factors improves outcomes in rheumatic diseases. Hypertension predicts an increase in lupus damage. Particularly in lupus nephritis, hypertension will increase the risk for CVD and kidney failure. People who use tobacco have worse outcomes in diseases like rheumatoid arthritis, psoriatic arthritis, and lupus, as well as more CVD, and antirheumatic drugs may not work as well.
In 90 seconds to 3 minutes, staff can do protocol-based care, which we’ve done across 20,000-plus visits. We showed we can improve population level rates of high BP and BP control, as well as increase smoking quitting rates across different patient settings.
What Is the Quit Connect Program?
The Quit Connect program is a 10- to 90-second point of care intervention. During rooming, staff (medical assistants and nurses) ask patients: “A) Do you smoke? and B) Have you thought about cutting back or quitting in the next 30 days?”
It turns out, when you ask the question that way, between a third and a half of people say that they’ve thought about cutting back or quitting. Then, we can get patients connected directly to Quitline, a free public service across all 50 states that smokers can use to get cessation support.
If patients are ready, we ask if we can arrange for them to receive a call from a Quitline coach about setting a quit date or receiving free nicotine replacement therapy. The beautiful thing is when that all happens, A) it’s free to the patient, and B) the results from the Quitline can be recorded right back to the electronic health record.
In our most recent publication in Arthritis Care & Research, we documented bringing Quit Connect to Grady Hospital in downtown Atlanta. It’s a safety net hospital, where 80% patients are Black and 70%-80% patients are on public insurance or uninsured. Using this protocol, we improved Quitline referrals 20-fold.
What Is the BP Connect Program?
At least half of the encounters in United States happen in specialty clinics. Unfortunately, when patients get their BP measured in a specialty clinic that’s not a cardiology or a vascular clinic, often, even if the pressure is high, the clinic doesn’t give patients feedback on that. The problem is because we haven’t said anything, that gives people the false reassurance that their BP is okay.
We’ve developed a 3-minute protocol to ask, advise, and connect. The idea is that if we measure a high BP, then we remeasure and confirm that it’s high. Then, we advise why it matters in rheumatic disease: Patients with rheumatic diseases are already at an increased risk for heart disease, and controlling BP can make a big difference. Then, we connect patients with high BP back to primary care.
Specifically, a SmartSet — an electronic medical record feature — prompts different actions based on confirmed high BP readings:
- If systolic BP ≥ 140-159, the SmartSet directs scheduling a visit to a nurse or primary care provider.
- If systolic BP ≥ 160-179, the next primary care visit anticipates the need to see a prescriber.
- If systolic BP ≥ 180, then the medical assistant or nurse at the visit is instructed to notify the provider who can arrange a provider-to-provider handoff for safety to exclude a hypertensive emergency.
That order goes to the scheduler to call primary care to coordinate follow-up. BP Connect doubled the likelihood of a guideline-recommended follow-up in primary care within 30 days. All patients benefited, and disparities decreased. BP Connect has had 1100 downloads, and both BP and Quit Connect programs are endorsed by the Centers for Disease Control and Prevention and Million Hearts.
How Do These Programs Affect Clinical Practice?
We developed these interventions with a health system engineer, and we time stamped everything. Part of the sustainability of this model is that it fits within a regular workflow. As a practicing rheumatologist, I understand that time is a precious commodity.
The interventions are in partnership with frontline staff. We’ve received feedback that they feel pride participating in these initiatives. They can say, because of me, 30 patients followed up last month for high BP, or 10 patients took a referral to the Quitline last year. We celebrate these accomplishments with the staff.
What Are the Next Steps for These Programs?
Public-facing toolkits for both BP and Quit Connect programs are available online. We have implemented [these programs] in a rural setting, in an urban setting, in Milwaukee and in Atlanta, and we are looking in the future to do a larger, multistate implementation study. If folks are interested, we’d love to partner with them to look at disseminating this further.
A version of this article appeared on Medscape.com.
Two relatively simple interventions — addressing high blood pressure (BP) and smoking cessation — could make a huge difference for patients with rheumatic disease. Patients with autoimmune disease are up to three times more likely to develop cardiovascular disease (CVD) than the general population. In addition to compounding CVD, smoking is tied to the development of certain autoimmune conditions, as well as worse outcomes. Christie Bartels, MD, chief of the Division of Rheumatology at the University of Wisconsin School of Medicine and Public Health, Madison, has focused her research on improving cardiac health in inflammatory diseases. This news organization spoke with Bartels about two short interventions she developed that tackle hypertension and smoking cessation during regular visits, each taking less than 3 minutes.
How Do These Programs Address Cardiac Disease Prevention?
The BP and Quit Connect programs help clinics systematically address the two most modifiable risk factors for CVD: high BP and smoking. There’s also evidence that addressing these two risk factors improves outcomes in rheumatic diseases. Hypertension predicts an increase in lupus damage. Particularly in lupus nephritis, hypertension will increase the risk for CVD and kidney failure. People who use tobacco have worse outcomes in diseases like rheumatoid arthritis, psoriatic arthritis, and lupus, as well as more CVD, and antirheumatic drugs may not work as well.
In 90 seconds to 3 minutes, staff can do protocol-based care, which we’ve done across 20,000-plus visits. We showed we can improve population level rates of high BP and BP control, as well as increase smoking quitting rates across different patient settings.
What Is the Quit Connect Program?
The Quit Connect program is a 10- to 90-second point of care intervention. During rooming, staff (medical assistants and nurses) ask patients: “A) Do you smoke? and B) Have you thought about cutting back or quitting in the next 30 days?”
It turns out, when you ask the question that way, between a third and a half of people say that they’ve thought about cutting back or quitting. Then, we can get patients connected directly to Quitline, a free public service across all 50 states that smokers can use to get cessation support.
If patients are ready, we ask if we can arrange for them to receive a call from a Quitline coach about setting a quit date or receiving free nicotine replacement therapy. The beautiful thing is when that all happens, A) it’s free to the patient, and B) the results from the Quitline can be recorded right back to the electronic health record.
In our most recent publication in Arthritis Care & Research, we documented bringing Quit Connect to Grady Hospital in downtown Atlanta. It’s a safety net hospital, where 80% patients are Black and 70%-80% patients are on public insurance or uninsured. Using this protocol, we improved Quitline referrals 20-fold.
What Is the BP Connect Program?
At least half of the encounters in United States happen in specialty clinics. Unfortunately, when patients get their BP measured in a specialty clinic that’s not a cardiology or a vascular clinic, often, even if the pressure is high, the clinic doesn’t give patients feedback on that. The problem is because we haven’t said anything, that gives people the false reassurance that their BP is okay.
We’ve developed a 3-minute protocol to ask, advise, and connect. The idea is that if we measure a high BP, then we remeasure and confirm that it’s high. Then, we advise why it matters in rheumatic disease: Patients with rheumatic diseases are already at an increased risk for heart disease, and controlling BP can make a big difference. Then, we connect patients with high BP back to primary care.
Specifically, a SmartSet — an electronic medical record feature — prompts different actions based on confirmed high BP readings:
- If systolic BP ≥ 140-159, the SmartSet directs scheduling a visit to a nurse or primary care provider.
- If systolic BP ≥ 160-179, the next primary care visit anticipates the need to see a prescriber.
- If systolic BP ≥ 180, then the medical assistant or nurse at the visit is instructed to notify the provider who can arrange a provider-to-provider handoff for safety to exclude a hypertensive emergency.
That order goes to the scheduler to call primary care to coordinate follow-up. BP Connect doubled the likelihood of a guideline-recommended follow-up in primary care within 30 days. All patients benefited, and disparities decreased. BP Connect has had 1100 downloads, and both BP and Quit Connect programs are endorsed by the Centers for Disease Control and Prevention and Million Hearts.
How Do These Programs Affect Clinical Practice?
We developed these interventions with a health system engineer, and we time stamped everything. Part of the sustainability of this model is that it fits within a regular workflow. As a practicing rheumatologist, I understand that time is a precious commodity.
The interventions are in partnership with frontline staff. We’ve received feedback that they feel pride participating in these initiatives. They can say, because of me, 30 patients followed up last month for high BP, or 10 patients took a referral to the Quitline last year. We celebrate these accomplishments with the staff.
What Are the Next Steps for These Programs?
Public-facing toolkits for both BP and Quit Connect programs are available online. We have implemented [these programs] in a rural setting, in an urban setting, in Milwaukee and in Atlanta, and we are looking in the future to do a larger, multistate implementation study. If folks are interested, we’d love to partner with them to look at disseminating this further.
A version of this article appeared on Medscape.com.
Two relatively simple interventions — addressing high blood pressure (BP) and smoking cessation — could make a huge difference for patients with rheumatic disease. Patients with autoimmune disease are up to three times more likely to develop cardiovascular disease (CVD) than the general population. In addition to compounding CVD, smoking is tied to the development of certain autoimmune conditions, as well as worse outcomes. Christie Bartels, MD, chief of the Division of Rheumatology at the University of Wisconsin School of Medicine and Public Health, Madison, has focused her research on improving cardiac health in inflammatory diseases. This news organization spoke with Bartels about two short interventions she developed that tackle hypertension and smoking cessation during regular visits, each taking less than 3 minutes.
How Do These Programs Address Cardiac Disease Prevention?
The BP and Quit Connect programs help clinics systematically address the two most modifiable risk factors for CVD: high BP and smoking. There’s also evidence that addressing these two risk factors improves outcomes in rheumatic diseases. Hypertension predicts an increase in lupus damage. Particularly in lupus nephritis, hypertension will increase the risk for CVD and kidney failure. People who use tobacco have worse outcomes in diseases like rheumatoid arthritis, psoriatic arthritis, and lupus, as well as more CVD, and antirheumatic drugs may not work as well.
In 90 seconds to 3 minutes, staff can do protocol-based care, which we’ve done across 20,000-plus visits. We showed we can improve population level rates of high BP and BP control, as well as increase smoking quitting rates across different patient settings.
What Is the Quit Connect Program?
The Quit Connect program is a 10- to 90-second point of care intervention. During rooming, staff (medical assistants and nurses) ask patients: “A) Do you smoke? and B) Have you thought about cutting back or quitting in the next 30 days?”
It turns out, when you ask the question that way, between a third and a half of people say that they’ve thought about cutting back or quitting. Then, we can get patients connected directly to Quitline, a free public service across all 50 states that smokers can use to get cessation support.
If patients are ready, we ask if we can arrange for them to receive a call from a Quitline coach about setting a quit date or receiving free nicotine replacement therapy. The beautiful thing is when that all happens, A) it’s free to the patient, and B) the results from the Quitline can be recorded right back to the electronic health record.
In our most recent publication in Arthritis Care & Research, we documented bringing Quit Connect to Grady Hospital in downtown Atlanta. It’s a safety net hospital, where 80% patients are Black and 70%-80% patients are on public insurance or uninsured. Using this protocol, we improved Quitline referrals 20-fold.
What Is the BP Connect Program?
At least half of the encounters in United States happen in specialty clinics. Unfortunately, when patients get their BP measured in a specialty clinic that’s not a cardiology or a vascular clinic, often, even if the pressure is high, the clinic doesn’t give patients feedback on that. The problem is because we haven’t said anything, that gives people the false reassurance that their BP is okay.
We’ve developed a 3-minute protocol to ask, advise, and connect. The idea is that if we measure a high BP, then we remeasure and confirm that it’s high. Then, we advise why it matters in rheumatic disease: Patients with rheumatic diseases are already at an increased risk for heart disease, and controlling BP can make a big difference. Then, we connect patients with high BP back to primary care.
Specifically, a SmartSet — an electronic medical record feature — prompts different actions based on confirmed high BP readings:
- If systolic BP ≥ 140-159, the SmartSet directs scheduling a visit to a nurse or primary care provider.
- If systolic BP ≥ 160-179, the next primary care visit anticipates the need to see a prescriber.
- If systolic BP ≥ 180, then the medical assistant or nurse at the visit is instructed to notify the provider who can arrange a provider-to-provider handoff for safety to exclude a hypertensive emergency.
That order goes to the scheduler to call primary care to coordinate follow-up. BP Connect doubled the likelihood of a guideline-recommended follow-up in primary care within 30 days. All patients benefited, and disparities decreased. BP Connect has had 1100 downloads, and both BP and Quit Connect programs are endorsed by the Centers for Disease Control and Prevention and Million Hearts.
How Do These Programs Affect Clinical Practice?
We developed these interventions with a health system engineer, and we time stamped everything. Part of the sustainability of this model is that it fits within a regular workflow. As a practicing rheumatologist, I understand that time is a precious commodity.
The interventions are in partnership with frontline staff. We’ve received feedback that they feel pride participating in these initiatives. They can say, because of me, 30 patients followed up last month for high BP, or 10 patients took a referral to the Quitline last year. We celebrate these accomplishments with the staff.
What Are the Next Steps for These Programs?
Public-facing toolkits for both BP and Quit Connect programs are available online. We have implemented [these programs] in a rural setting, in an urban setting, in Milwaukee and in Atlanta, and we are looking in the future to do a larger, multistate implementation study. If folks are interested, we’d love to partner with them to look at disseminating this further.
A version of this article appeared on Medscape.com.
Short Interval Repeat Colonoscopy After Inadequate Bowel Preparation Is Low Among Veterans
Colorectal cancer (CRC) is the third-most diagnosed cancer after breast and lung cancer, and is the second leading cause of global cancer related deaths.1 In 2023 in the United States, > 150,000 individuals were diagnosed with CRC and 52,000 died.2
Colonoscopy is an effective CRC screening method and the lone method recommended for polyp surveillance. Inadequate bowel preparation (IBP) has been estimated to occur in about 6% to 26% of colonoscopies. 3,4 The prevalence varies based on a variety of comorbidities, including immobility, diabetes mellitus, neurologic disorders, and use of opioids, with more occurrences of IBP noted in older adult, non-English speaking, and male individuals.4-6
The quality of bowel preparation is integral to the effectiveness of screening and surveillance colonoscopies. IBP has been associated with missed adenomas and significantly lower adenoma detection rates.7-9 In particular, IBP is independently associated with an increased risk of CRC in the future.3 Accordingly, the US Multisociety Task Force recommends repeat colonoscopies for individuals with IBP within 1 year.10 Ensuring that these individuals receive repeat colonoscopies is an essential part of CRC prevention. The benefit of repeat colonoscopy after IBP is highlighted by a retrospective analysis from Fung and colleagues that showed 81% of repeat colonoscopies had adequate bowel preparation, with higher numbers of adenomas detected on repeat compared to initial colonoscopies.11
Given the impact of bowel preparation quality on the diagnostic capability of the colonoscopy, adherence to guidelines for repeat colonoscopies in cases of IBP is paramount for effective CRC prevention. This study aims to measure the frequency of repeat colonoscopy after IBP and the factors associated with adherence to recommendations.
METHODS
Individuals who underwent colonoscopy at the Minneapolis Veterans Affairs Medical Center (MVAMC) from January 1, 2016, to October 19, 2021, were identified to allow for 400 days of follow-up from the index colonoscopy to the data collection date. During the COVID-19 pandemic, the colonoscopy procedure capacity was reduced by 50% from June 1, 2020, to December 1, 2020, delaying nonurgent procedures, including screening and surveillance colonoscopies.
Individuals who underwent colonoscopy for CRC screening or polyp surveillance, or following a positive fecal immunohistochemistry test (FIT) or virtual computed tomography colonoscopy were included. Patients with colonoscopy indications for iron deficiency anemia, gastrointestinal bleeding, disease activity assessment of inflammatory bowel disease, abdominal pain, or changes in bowel movement pattern were excluded. IBP was defined as recording a Boston Bowel Preparation Scale (BBPS) score of < 6, or < 2 in any segment, or described as poor or inadequate using the Aronchick scale.
Age, sex, race, marital status, distance to MVAMC, smoking status, comorbidities, and concurrent medication use, including antiplatelet, anticoagulation, and prescription opiates at the time of index colonoscopy were obtained from the Veterans Health Administration (VHA) Corporate Data Warehouse (CDW) using structured query language processing of colonoscopy procedure notes to extract preparation scores and other procedure information. The CDW contains extracts from VHA clinical and administrative systems that contain complete clinical data from October 1999.12 Current smoking status was defined as any smoking activity at the time the questionnaire was administered during a routine clinic visit within 400 days from the index colonoscopy.
Only individuals who were recommended to have repeat colonoscopy within 1 year were included. The intervals of 365 days and 400 days (1 year + about 1 additional month) were used in the event that the individual had a delay in scheduling their 1-year repeat colonoscopy. For individuals who did not undergo a colonoscopy at MVAMC within 400 days, a manual chart review of all available records was performed to determine whether a colonoscopy was performed at a non-VA facility.
Patients received written instructions for bowel preparation 2 weeks prior to the procedure. The preparation included magnesium citrate and a split dose of 4 liters of polyethylene glycol. Patients were also advised to start a low-fiber diet 3 days prior to the procedure and a clear liquid diet the day before the procedure. Patients with a history of IBP or those undergoing procedures with anesthesia received an additional 2 liters for a total of 6 liters of polyethylene glycol.
Statistical analysis
Baseline characteristics were reported as mean (SD) or median and IQR for continuous variables and percentage for categorical variables. Individuals who returned for colonoscopy within 400 days were compared to those who did not identify factors associated with adherence to recommendations. The data on individuals who returned for colonoscopy within 400 days were also analyzed for additional minor delays in the timing of the repeat colonoscopy. Continuous data were compared using Mann-Whitney U tests. Categorical data were compared using X2 or Fisher exact tests. Missing data were imputed from the analyses. All analyses were performed using SAS JMP Pro version 16. P < .05 was considered statistically significant.
RESULTS
There were 18,241 total colonoscopies performed between January 1, 2016, to October 19, 2021, and 13,818 colonoscopies had indications for screening for colon cancer, positive FIT, virtual colonoscopy, or surveillance. Of the 10,466 unique patients there were 5369 patients for polyp surveillance, 4054 patients for CRC screening, and 1043 patients for positive FIT or virtual colonoscopy. Of these, 571 individuals (5.5%) had IBP. Repeat colonoscopy within 1 year was recommended for 485 individuals (84.9%) who were included in this study (153 CRC screenings and 46 positive FITs) but not for 86 individuals (15.1%) (Figure 1). Among included patients, the mean (SD) age was 66.6 (7.2) years, and the majority were male (460 [94.8%]) and White (435 [89.7%]) (Table). Two hundred and forty-three (50.1%) were married.
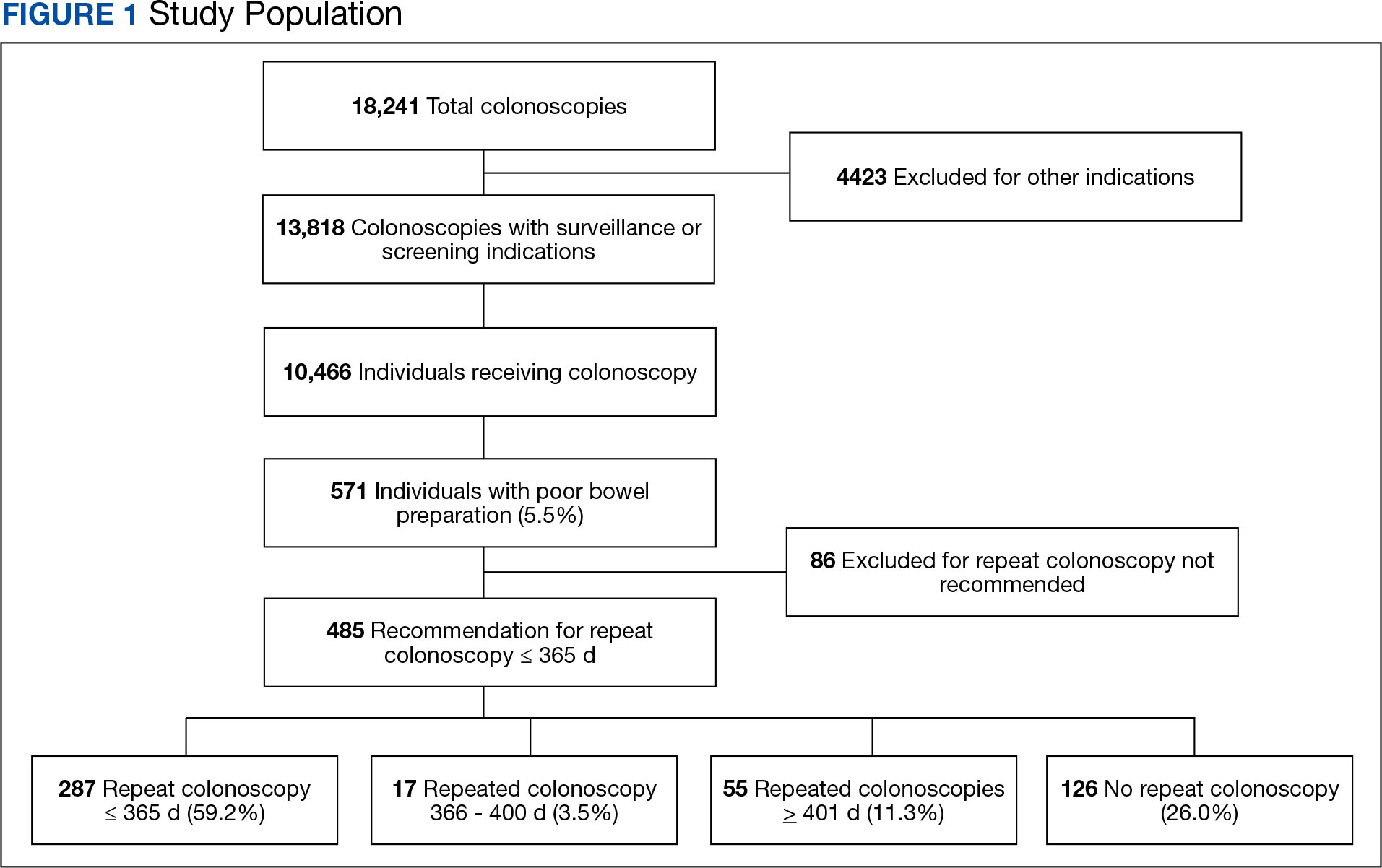
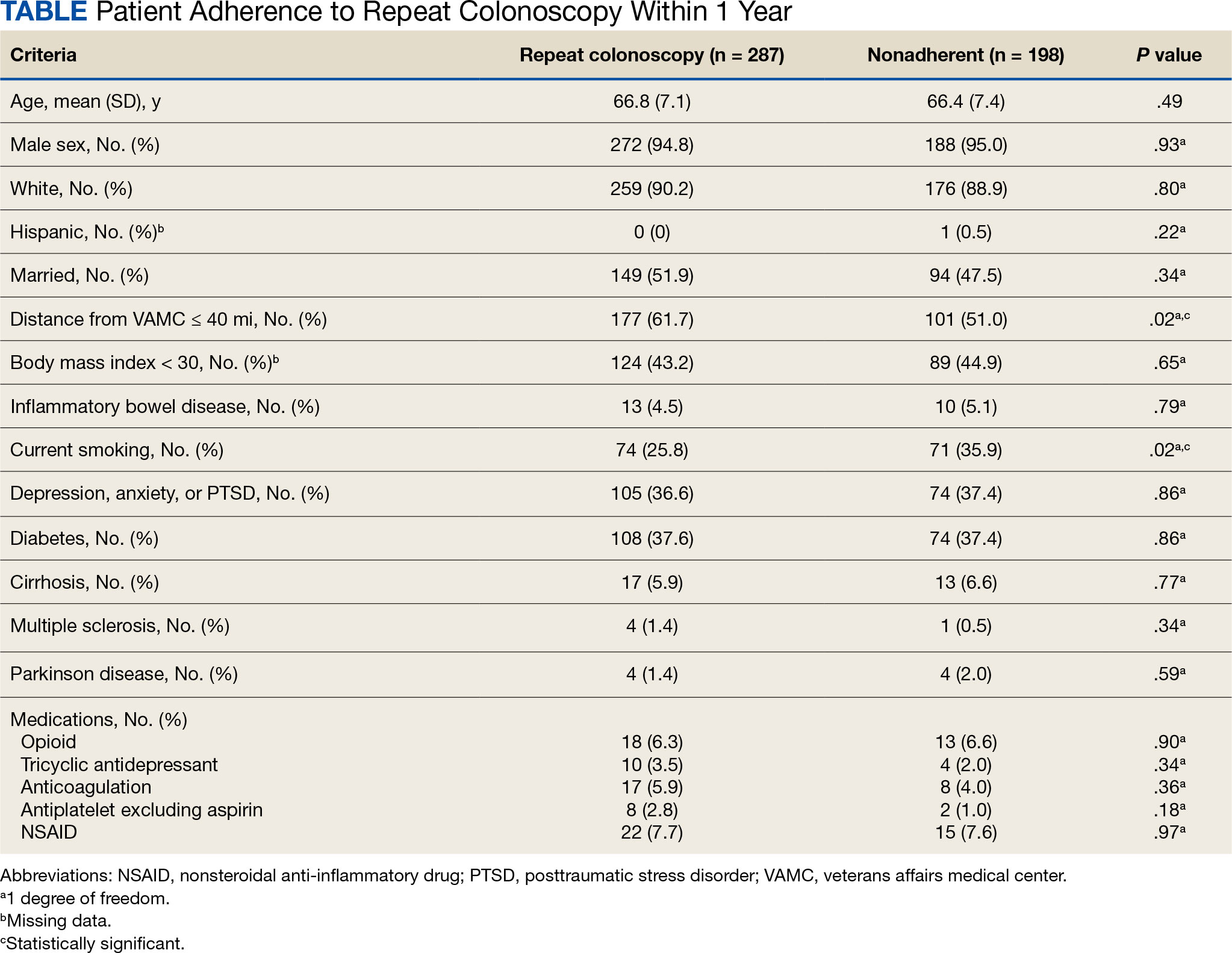
Adherence to Recommended Interval Colonoscopy
Of the 485 patients with IBP who were recommended for follow-up colonoscopy, 287 (59.2%) had a colonoscopy within 1 year, and 198 (40.8%) did not; 17 patients (13.5%) had repeat colonoscopy within 366 to 400 days. Five (1.0%) individuals had a repeat colonoscopy the next day, and 77 (15.9%) had a repeat colonoscopy within 7 days. One hundred and twentysix (26.0%) individuals underwent no repeat colonoscopy during the study period (Figure 2).
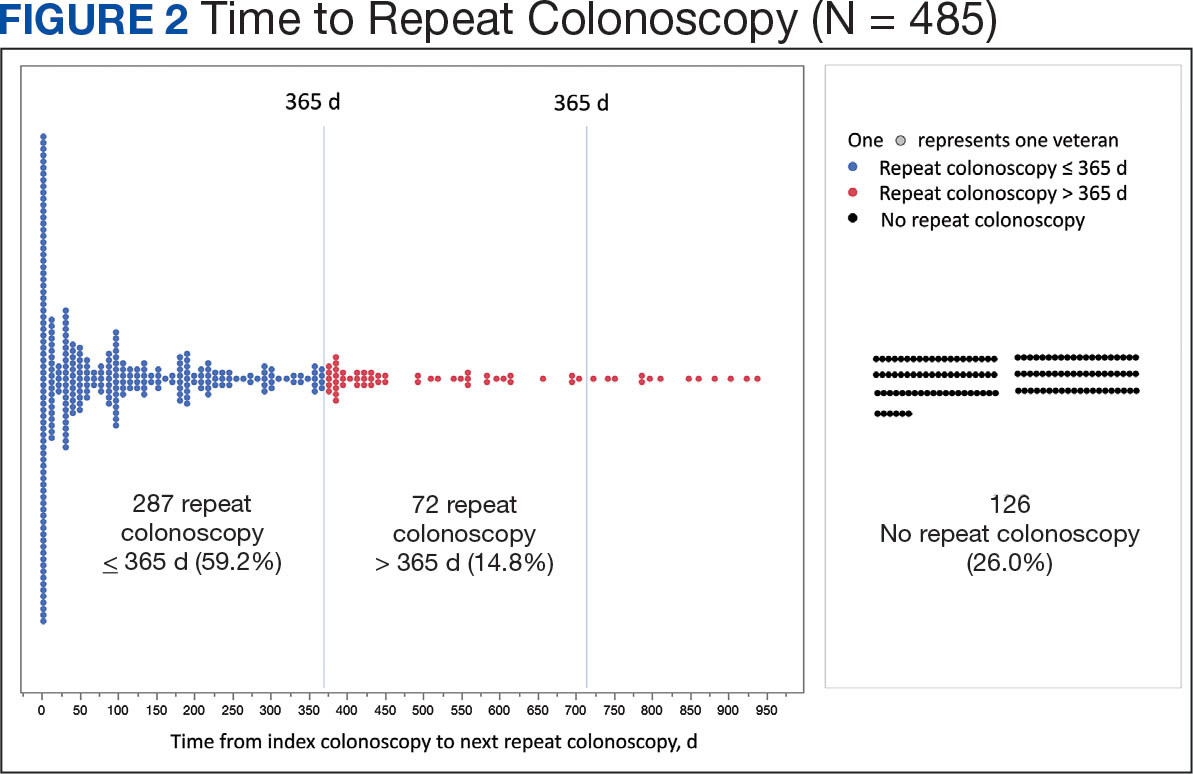
To account for the COVID-19 pandemic, the adherence rate of repeat colonoscopy within 1 year prepandemic (January 1, 2016, to December 1, 2018) was calculated along with the adherence rate postpandemic (January 1, 2019 to the end of the study). The rates were similar: 199 of 330 (60.3%) individuals prepandemic vs 88 of 155 (56.8%) individuals postpandemic (Figure 3).
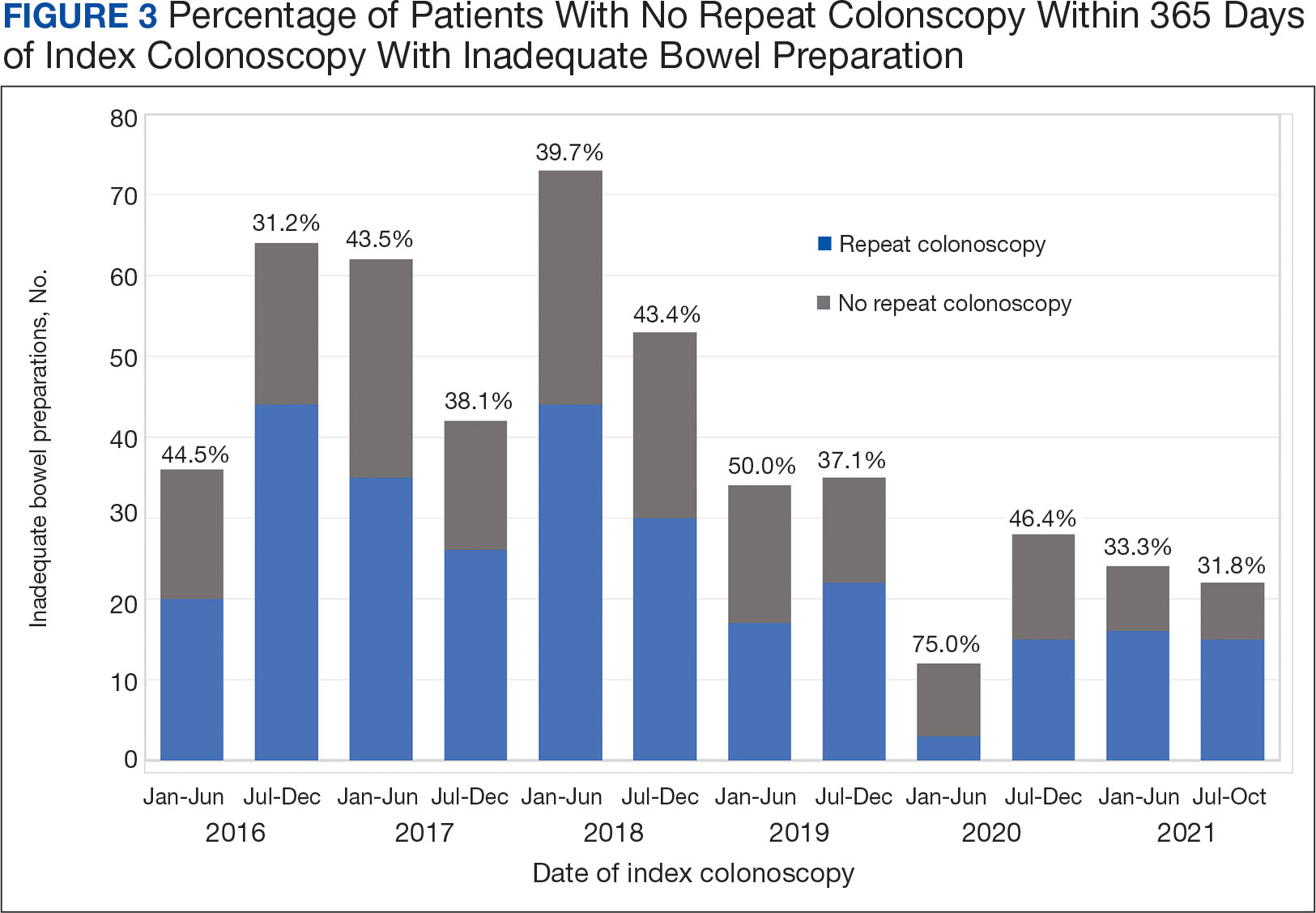
Significant Associations
Age, sex, and race were not associated with adherence to repeat colonoscopy within 1 year. Individuals living ≤ 40 miles from the endoscopy center were more likely to undergo a repeat colonoscopy within 1 year compared with those who lived > 40 miles away (61.7% vs 51.0%, P = .02). Current smoking status was associated with a lower rate of repeat colonoscopy within 1 year (25.8% vs 35.9%; P = .02). There were no differences with respect to inflammatory bowel disease diagnosis, mental health diagnosis, diabetes mellitus, cirrhosis, or medications used, including opioids, anticoagulation, and antiplatelet therapy.
Outcomes
Among individuals who had a repeat colonoscopy the day after the index colonoscopy, 53 of 56 individuals (94.6%) had adequate bowel preparation. Among individuals who had a repeat colonoscopy within 7 days, 70 of 77 (90.9%) had adequate bowel preparation. Of 287 individuals with a repeat colonoscopy within 1 year, 251 (87.5%) had adequate bowel preparation on the repeat colonoscopy. By 400 days after the index colonoscopy, 268 of 304 individuals (88.2%) had adequate bowel preparation.
In this study conducted at a large VA medical center, we found that 5.6% of individuals undergoing colonoscopies had IBP, a rate comparable to prior studies (6% to 26%).3,4 Only 59.2% of individuals underwent repeat colonoscopies within 1 year, as recommended after an index colonoscopy with IBP. Smoking and living longer distances (> 40 miles) from the endoscopy center were associated with a decreased adherence to the repeat colonoscopy recommendation.
Current guidelines recommend repeat colonoscopy for individuals with IBP within 1 year.10 In cases of IBP, the advanced adenoma miss rate is 36% upon repeat colonoscopy within 1 year.13 Despite the importance of a follow-up colonoscopy, clinician adherence with this recommendation remains low.10,14,15 However, in this study cohort, 485 of 571 individuals with IBP (84.9%) received recommendations for a repeat colonoscopy within 1 year. In the US, only 31.9% of 260,314 colonoscopies with IBP included recommendations for a follow-up colonoscopy within 1 year.14 This could be related to variations in endoscopist practice as well as patient risk factors for developing polyps, including family history of cancer and personal history of prior polyps. The findings of multiple polyps, high-risk adenomas, and cancer on the index colonoscopy also influences the endoscopist for repeat colonoscopy within 1 year.14
The timing for repeat colonoscopies within 1 year will be determined by the patients, clinicians, and available scheduling. In this study, the earlier repeat colonoscopies, especially those occurring the day after the index colonoscopy, had the highest success rate of adequate bowel preparation. In a prior study, repeating colonoscopies within the same day or the next day was also found to have a higher rate of adequate bowel preparation than repeat colonoscopies within 1 year (88.9% vs 83.5%).16
Ensuring the return of individuals with IBP for repeat colonoscopy is a challenging task. We identified that individuals who live further away from MVAMC and current smokers had a decreased probability of returning for a repeat colonoscopy. Toro and colleagues found a 68.7% return rate for a repeat colonoscopy within 1 year with individuals age ≥ 60 years, and patients who were White were less likely to proceed with a repeat colonoscopy within 1 year.17 The study did not provide data regarding smoking status or distance to the endoscopy center.17 In a prior study of veterans, the dual diagnosis of psychiatric disorders and substance abuse was associated with missed and canceled colonoscopy appointments.18 The distance to the endoscopy center has also been previously identified as a barrier to a colonoscopy following an abnormal FIT.19 Although not identified in this study due to the homogenous demographic profile, social determinants of health such as socioeconomic status, education, and insurance coverage are known barriers to cancer screening but were not evaluated in this study.20
Based on the identified risk factors, we have created a model for utilizing those risk factors to identify individuals at higher risk for noncompliance (ie, those who live further away from the endoscopy center or currently smoke). These individuals are proactively offered to use an intraprocedural bowel cleansing device to achieve adequate bowel preparation or priority rescheduling for a next-day colonoscopy.
Limitations
This study was a single-center study of the veteran population, which is predominantly White and male, thus limiting generalizability. The study is also limited by minimal available data on adenoma detection and colon cancer incidence on subsequent colonoscopies.
CONCLUSIONS
The rate of IBP was 5.5% in individuals undergoing colonoscopy for colon cancer screening, surveillance, positive FIT, or computed tomography colonography. Only 59.2% of those with IBP underwent the recommended repeat colonoscopy within 1 year. Smoking and distance to the endoscopy center were associated with a decreased adherence to the repeat colonoscopy recommendation. Additional efforts are needed to ensure that individuals with IBP return for timely repeat colonoscopy.
- Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71(3):209-249. doi:10.3322/caac.21660
- Siegel RL, Wagle NS, Cercek A, Smith RA, Jemal A. Colorectal cancer statistics, 2023. CA Cancer J Clin. 2023;73(3):233-254. doi:10.3322/caac.21772
- Atkin W, Wooldrage K, Brenner A, et al. Adenoma surveillance and colorectal cancer incidence: a retrospective, multicentre, cohort study. Lancet Oncol. 2017;18(6):823- 834. doi:10.1016/S1470-2045(17)30187-0
- Froehlich F, Wietlisbach V, Gonvers JJ, Burnand B, Vader JP. Impact of colonic cleansing on quality and diagnostic yield of colonoscopy: the European Panel of Appropriateness of Gastrointestinal Endoscopy European multicenter study. Gastrointest Endosc. 2005;61(3):378- 384. doi:10.1016/s0016-5107(04)02776-2
- Mahmood S, Farooqui SM, Madhoun MF. Predictors of inadequate bowel preparation for colonoscopy: a systematic review and meta-analysis. Eur J Gastroenterol Hepatol. 2018;30(8):819-826. doi:10.1097/MEG.0000000000001175
- ASGE Standards of Practice Committee, Saltzman JR, Cash BD, et al. Bowel preparation before colonoscopy. Gastrointest Endosc. 2015;81(4):781-794. doi:10.1016/j.gie.2014.09.048
- Clark BT, Protiva P, Nagar A, et al. Quantification of Adequate Bowel Preparation for Screening or Surveillance Colonoscopy in Men. Gastroenterology. 2016;150(2):396- e15. doi:10.1053/j.gastro.2015.09.041
- Sulz MC, Kröger A, Prakash M, Manser CN, Heinrich H, Misselwitz B. Meta-Analysis of the Effect of Bowel Preparation on Adenoma Detection: Early Adenomas Affected Stronger than Advanced Adenomas. PLoS One. 2016;11(6):e0154149. Published 2016 Jun 3. doi:10.1371/journal.pone.0154149
- Chokshi RV, Hovis CE, Hollander T, Early DS, Wang JS. Prevalence of missed adenomas in patients with inadequate bowel preparation on screening colonoscopy. Gastrointest Endosc. 2012;75(6):1197-1203. doi:10.1016/j.gie.2012.01.005
- Lieberman DA, Rex DK, Winawer SJ, Giardiello FM, Johnson DA, Levin TR. Guidelines for colonoscopy surveillance after screening and polypectomy: a consensus update by the US Multi-Society Task Force on Colorectal Cancer. Gastroenterology. 2012;143(3):844-857. doi:10.1053/j.gastro.2012.06.001
- Fung P, Syed A, Cole R, Farah K. Poor bowel prep: are you really going to come back within a year? Abstract presented at American Gastroenterological Association DDW 2021, May 21-23, 2021. doi:10.1016/S0016-5085(21)01204-X
- US Department of Veterans Affairs, VA Health Systems Research. Corporate data warehouse (CDW). Updated January 11, 2023. Accessed August 6, 2024. https://www.hsrd.research.va.gov/for_researchers/cdw.cfm
- Lebwohl B, Kastrinos F, Glick M, Rosenbaum AJ, Wang T, Neugut AI. The impact of suboptimal bowel preparation on adenoma miss rates and the factors associated with early repeat colonoscopy. Gastrointest Endosc. 2011;73(6):1207-1214. doi:10.1016/j.gie.2011.01.051
- Calderwood AH, Holub JL, Greenwald DA. Recommendations for follow-up interval after colonoscopy with inadequate bowel preparation in a national colonoscopy quality registry. Gastrointest Endosc. 2022;95(2):360-367. e2. doi:10.1016/j.gie.2021.09.027
- Latorre M, Roy A, Spyrou E, Garcia-Carrasquillo R, Rosenberg R, Lebwohl B. Adherence to guidelines after poor colonoscopy preparation: experience from a patient navigator program. Gastroenterology. 2016;151(1):P196. doi:10.1053/j.gastro.2016.05.027
- Bouquet E, Tomal J, Choksi Y. Next-day screening colonoscopy following inadequate bowel preparation may improve quality of preparation and adenoma detection in a veteran population. Am J Gastroenterol. 2020;115:S259. doi:10.14309/ajg.0000000000000853
- Toro B, Dawkins G, Friedenberg FK, Ehrlich AC. Risk factors for failure to return after a poor preparation colonoscopy: experience in a safety-net hospital, 255. Abstract presented at ACG October 2016. https://journals.lww.com/ajg/fulltext/2016/10001/risk_factors_for_failure_to_return_after_a_poor.255.aspx
- Partin MR, Gravely A, Gellad ZF, et al. Factors Associated With Missed and Cancelled Colonoscopy Appointments at Veterans Health Administration Facilities. Clin Gastroenterol Hepatol. 2016;14(2):259-267. doi:10.1016/j.cgh.2015.07.051
- Idos GE, Bonner JD, Haghighat S, et al. Bridging the Gap: Patient Navigation Increases Colonoscopy Follow-up After Abnormal FIT. Clin Transl Gastroenterol. 2021;12(2):e00307. doi:10.14309/ctg.0000000000000307
- Islami F, Baeker Bispo J, Lee H, et al. American Cancer Society’s report on the status of cancer disparities in the United States, 2023. CA Cancer J Clin. 2024;74(2):136- 166. doi:10.3322/caac.21812
Colorectal cancer (CRC) is the third-most diagnosed cancer after breast and lung cancer, and is the second leading cause of global cancer related deaths.1 In 2023 in the United States, > 150,000 individuals were diagnosed with CRC and 52,000 died.2
Colonoscopy is an effective CRC screening method and the lone method recommended for polyp surveillance. Inadequate bowel preparation (IBP) has been estimated to occur in about 6% to 26% of colonoscopies. 3,4 The prevalence varies based on a variety of comorbidities, including immobility, diabetes mellitus, neurologic disorders, and use of opioids, with more occurrences of IBP noted in older adult, non-English speaking, and male individuals.4-6
The quality of bowel preparation is integral to the effectiveness of screening and surveillance colonoscopies. IBP has been associated with missed adenomas and significantly lower adenoma detection rates.7-9 In particular, IBP is independently associated with an increased risk of CRC in the future.3 Accordingly, the US Multisociety Task Force recommends repeat colonoscopies for individuals with IBP within 1 year.10 Ensuring that these individuals receive repeat colonoscopies is an essential part of CRC prevention. The benefit of repeat colonoscopy after IBP is highlighted by a retrospective analysis from Fung and colleagues that showed 81% of repeat colonoscopies had adequate bowel preparation, with higher numbers of adenomas detected on repeat compared to initial colonoscopies.11
Given the impact of bowel preparation quality on the diagnostic capability of the colonoscopy, adherence to guidelines for repeat colonoscopies in cases of IBP is paramount for effective CRC prevention. This study aims to measure the frequency of repeat colonoscopy after IBP and the factors associated with adherence to recommendations.
METHODS
Individuals who underwent colonoscopy at the Minneapolis Veterans Affairs Medical Center (MVAMC) from January 1, 2016, to October 19, 2021, were identified to allow for 400 days of follow-up from the index colonoscopy to the data collection date. During the COVID-19 pandemic, the colonoscopy procedure capacity was reduced by 50% from June 1, 2020, to December 1, 2020, delaying nonurgent procedures, including screening and surveillance colonoscopies.
Individuals who underwent colonoscopy for CRC screening or polyp surveillance, or following a positive fecal immunohistochemistry test (FIT) or virtual computed tomography colonoscopy were included. Patients with colonoscopy indications for iron deficiency anemia, gastrointestinal bleeding, disease activity assessment of inflammatory bowel disease, abdominal pain, or changes in bowel movement pattern were excluded. IBP was defined as recording a Boston Bowel Preparation Scale (BBPS) score of < 6, or < 2 in any segment, or described as poor or inadequate using the Aronchick scale.
Age, sex, race, marital status, distance to MVAMC, smoking status, comorbidities, and concurrent medication use, including antiplatelet, anticoagulation, and prescription opiates at the time of index colonoscopy were obtained from the Veterans Health Administration (VHA) Corporate Data Warehouse (CDW) using structured query language processing of colonoscopy procedure notes to extract preparation scores and other procedure information. The CDW contains extracts from VHA clinical and administrative systems that contain complete clinical data from October 1999.12 Current smoking status was defined as any smoking activity at the time the questionnaire was administered during a routine clinic visit within 400 days from the index colonoscopy.
Only individuals who were recommended to have repeat colonoscopy within 1 year were included. The intervals of 365 days and 400 days (1 year + about 1 additional month) were used in the event that the individual had a delay in scheduling their 1-year repeat colonoscopy. For individuals who did not undergo a colonoscopy at MVAMC within 400 days, a manual chart review of all available records was performed to determine whether a colonoscopy was performed at a non-VA facility.
Patients received written instructions for bowel preparation 2 weeks prior to the procedure. The preparation included magnesium citrate and a split dose of 4 liters of polyethylene glycol. Patients were also advised to start a low-fiber diet 3 days prior to the procedure and a clear liquid diet the day before the procedure. Patients with a history of IBP or those undergoing procedures with anesthesia received an additional 2 liters for a total of 6 liters of polyethylene glycol.
Statistical analysis
Baseline characteristics were reported as mean (SD) or median and IQR for continuous variables and percentage for categorical variables. Individuals who returned for colonoscopy within 400 days were compared to those who did not identify factors associated with adherence to recommendations. The data on individuals who returned for colonoscopy within 400 days were also analyzed for additional minor delays in the timing of the repeat colonoscopy. Continuous data were compared using Mann-Whitney U tests. Categorical data were compared using X2 or Fisher exact tests. Missing data were imputed from the analyses. All analyses were performed using SAS JMP Pro version 16. P < .05 was considered statistically significant.
RESULTS
There were 18,241 total colonoscopies performed between January 1, 2016, to October 19, 2021, and 13,818 colonoscopies had indications for screening for colon cancer, positive FIT, virtual colonoscopy, or surveillance. Of the 10,466 unique patients there were 5369 patients for polyp surveillance, 4054 patients for CRC screening, and 1043 patients for positive FIT or virtual colonoscopy. Of these, 571 individuals (5.5%) had IBP. Repeat colonoscopy within 1 year was recommended for 485 individuals (84.9%) who were included in this study (153 CRC screenings and 46 positive FITs) but not for 86 individuals (15.1%) (Figure 1). Among included patients, the mean (SD) age was 66.6 (7.2) years, and the majority were male (460 [94.8%]) and White (435 [89.7%]) (Table). Two hundred and forty-three (50.1%) were married.


Adherence to Recommended Interval Colonoscopy
Of the 485 patients with IBP who were recommended for follow-up colonoscopy, 287 (59.2%) had a colonoscopy within 1 year, and 198 (40.8%) did not; 17 patients (13.5%) had repeat colonoscopy within 366 to 400 days. Five (1.0%) individuals had a repeat colonoscopy the next day, and 77 (15.9%) had a repeat colonoscopy within 7 days. One hundred and twentysix (26.0%) individuals underwent no repeat colonoscopy during the study period (Figure 2).

To account for the COVID-19 pandemic, the adherence rate of repeat colonoscopy within 1 year prepandemic (January 1, 2016, to December 1, 2018) was calculated along with the adherence rate postpandemic (January 1, 2019 to the end of the study). The rates were similar: 199 of 330 (60.3%) individuals prepandemic vs 88 of 155 (56.8%) individuals postpandemic (Figure 3).

Significant Associations
Age, sex, and race were not associated with adherence to repeat colonoscopy within 1 year. Individuals living ≤ 40 miles from the endoscopy center were more likely to undergo a repeat colonoscopy within 1 year compared with those who lived > 40 miles away (61.7% vs 51.0%, P = .02). Current smoking status was associated with a lower rate of repeat colonoscopy within 1 year (25.8% vs 35.9%; P = .02). There were no differences with respect to inflammatory bowel disease diagnosis, mental health diagnosis, diabetes mellitus, cirrhosis, or medications used, including opioids, anticoagulation, and antiplatelet therapy.
Outcomes
Among individuals who had a repeat colonoscopy the day after the index colonoscopy, 53 of 56 individuals (94.6%) had adequate bowel preparation. Among individuals who had a repeat colonoscopy within 7 days, 70 of 77 (90.9%) had adequate bowel preparation. Of 287 individuals with a repeat colonoscopy within 1 year, 251 (87.5%) had adequate bowel preparation on the repeat colonoscopy. By 400 days after the index colonoscopy, 268 of 304 individuals (88.2%) had adequate bowel preparation.
In this study conducted at a large VA medical center, we found that 5.6% of individuals undergoing colonoscopies had IBP, a rate comparable to prior studies (6% to 26%).3,4 Only 59.2% of individuals underwent repeat colonoscopies within 1 year, as recommended after an index colonoscopy with IBP. Smoking and living longer distances (> 40 miles) from the endoscopy center were associated with a decreased adherence to the repeat colonoscopy recommendation.
Current guidelines recommend repeat colonoscopy for individuals with IBP within 1 year.10 In cases of IBP, the advanced adenoma miss rate is 36% upon repeat colonoscopy within 1 year.13 Despite the importance of a follow-up colonoscopy, clinician adherence with this recommendation remains low.10,14,15 However, in this study cohort, 485 of 571 individuals with IBP (84.9%) received recommendations for a repeat colonoscopy within 1 year. In the US, only 31.9% of 260,314 colonoscopies with IBP included recommendations for a follow-up colonoscopy within 1 year.14 This could be related to variations in endoscopist practice as well as patient risk factors for developing polyps, including family history of cancer and personal history of prior polyps. The findings of multiple polyps, high-risk adenomas, and cancer on the index colonoscopy also influences the endoscopist for repeat colonoscopy within 1 year.14
The timing for repeat colonoscopies within 1 year will be determined by the patients, clinicians, and available scheduling. In this study, the earlier repeat colonoscopies, especially those occurring the day after the index colonoscopy, had the highest success rate of adequate bowel preparation. In a prior study, repeating colonoscopies within the same day or the next day was also found to have a higher rate of adequate bowel preparation than repeat colonoscopies within 1 year (88.9% vs 83.5%).16
Ensuring the return of individuals with IBP for repeat colonoscopy is a challenging task. We identified that individuals who live further away from MVAMC and current smokers had a decreased probability of returning for a repeat colonoscopy. Toro and colleagues found a 68.7% return rate for a repeat colonoscopy within 1 year with individuals age ≥ 60 years, and patients who were White were less likely to proceed with a repeat colonoscopy within 1 year.17 The study did not provide data regarding smoking status or distance to the endoscopy center.17 In a prior study of veterans, the dual diagnosis of psychiatric disorders and substance abuse was associated with missed and canceled colonoscopy appointments.18 The distance to the endoscopy center has also been previously identified as a barrier to a colonoscopy following an abnormal FIT.19 Although not identified in this study due to the homogenous demographic profile, social determinants of health such as socioeconomic status, education, and insurance coverage are known barriers to cancer screening but were not evaluated in this study.20
Based on the identified risk factors, we have created a model for utilizing those risk factors to identify individuals at higher risk for noncompliance (ie, those who live further away from the endoscopy center or currently smoke). These individuals are proactively offered to use an intraprocedural bowel cleansing device to achieve adequate bowel preparation or priority rescheduling for a next-day colonoscopy.
Limitations
This study was a single-center study of the veteran population, which is predominantly White and male, thus limiting generalizability. The study is also limited by minimal available data on adenoma detection and colon cancer incidence on subsequent colonoscopies.
CONCLUSIONS
The rate of IBP was 5.5% in individuals undergoing colonoscopy for colon cancer screening, surveillance, positive FIT, or computed tomography colonography. Only 59.2% of those with IBP underwent the recommended repeat colonoscopy within 1 year. Smoking and distance to the endoscopy center were associated with a decreased adherence to the repeat colonoscopy recommendation. Additional efforts are needed to ensure that individuals with IBP return for timely repeat colonoscopy.
Colorectal cancer (CRC) is the third-most diagnosed cancer after breast and lung cancer, and is the second leading cause of global cancer related deaths.1 In 2023 in the United States, > 150,000 individuals were diagnosed with CRC and 52,000 died.2
Colonoscopy is an effective CRC screening method and the lone method recommended for polyp surveillance. Inadequate bowel preparation (IBP) has been estimated to occur in about 6% to 26% of colonoscopies. 3,4 The prevalence varies based on a variety of comorbidities, including immobility, diabetes mellitus, neurologic disorders, and use of opioids, with more occurrences of IBP noted in older adult, non-English speaking, and male individuals.4-6
The quality of bowel preparation is integral to the effectiveness of screening and surveillance colonoscopies. IBP has been associated with missed adenomas and significantly lower adenoma detection rates.7-9 In particular, IBP is independently associated with an increased risk of CRC in the future.3 Accordingly, the US Multisociety Task Force recommends repeat colonoscopies for individuals with IBP within 1 year.10 Ensuring that these individuals receive repeat colonoscopies is an essential part of CRC prevention. The benefit of repeat colonoscopy after IBP is highlighted by a retrospective analysis from Fung and colleagues that showed 81% of repeat colonoscopies had adequate bowel preparation, with higher numbers of adenomas detected on repeat compared to initial colonoscopies.11
Given the impact of bowel preparation quality on the diagnostic capability of the colonoscopy, adherence to guidelines for repeat colonoscopies in cases of IBP is paramount for effective CRC prevention. This study aims to measure the frequency of repeat colonoscopy after IBP and the factors associated with adherence to recommendations.
METHODS
Individuals who underwent colonoscopy at the Minneapolis Veterans Affairs Medical Center (MVAMC) from January 1, 2016, to October 19, 2021, were identified to allow for 400 days of follow-up from the index colonoscopy to the data collection date. During the COVID-19 pandemic, the colonoscopy procedure capacity was reduced by 50% from June 1, 2020, to December 1, 2020, delaying nonurgent procedures, including screening and surveillance colonoscopies.
Individuals who underwent colonoscopy for CRC screening or polyp surveillance, or following a positive fecal immunohistochemistry test (FIT) or virtual computed tomography colonoscopy were included. Patients with colonoscopy indications for iron deficiency anemia, gastrointestinal bleeding, disease activity assessment of inflammatory bowel disease, abdominal pain, or changes in bowel movement pattern were excluded. IBP was defined as recording a Boston Bowel Preparation Scale (BBPS) score of < 6, or < 2 in any segment, or described as poor or inadequate using the Aronchick scale.
Age, sex, race, marital status, distance to MVAMC, smoking status, comorbidities, and concurrent medication use, including antiplatelet, anticoagulation, and prescription opiates at the time of index colonoscopy were obtained from the Veterans Health Administration (VHA) Corporate Data Warehouse (CDW) using structured query language processing of colonoscopy procedure notes to extract preparation scores and other procedure information. The CDW contains extracts from VHA clinical and administrative systems that contain complete clinical data from October 1999.12 Current smoking status was defined as any smoking activity at the time the questionnaire was administered during a routine clinic visit within 400 days from the index colonoscopy.
Only individuals who were recommended to have repeat colonoscopy within 1 year were included. The intervals of 365 days and 400 days (1 year + about 1 additional month) were used in the event that the individual had a delay in scheduling their 1-year repeat colonoscopy. For individuals who did not undergo a colonoscopy at MVAMC within 400 days, a manual chart review of all available records was performed to determine whether a colonoscopy was performed at a non-VA facility.
Patients received written instructions for bowel preparation 2 weeks prior to the procedure. The preparation included magnesium citrate and a split dose of 4 liters of polyethylene glycol. Patients were also advised to start a low-fiber diet 3 days prior to the procedure and a clear liquid diet the day before the procedure. Patients with a history of IBP or those undergoing procedures with anesthesia received an additional 2 liters for a total of 6 liters of polyethylene glycol.
Statistical analysis
Baseline characteristics were reported as mean (SD) or median and IQR for continuous variables and percentage for categorical variables. Individuals who returned for colonoscopy within 400 days were compared to those who did not identify factors associated with adherence to recommendations. The data on individuals who returned for colonoscopy within 400 days were also analyzed for additional minor delays in the timing of the repeat colonoscopy. Continuous data were compared using Mann-Whitney U tests. Categorical data were compared using X2 or Fisher exact tests. Missing data were imputed from the analyses. All analyses were performed using SAS JMP Pro version 16. P < .05 was considered statistically significant.
RESULTS
There were 18,241 total colonoscopies performed between January 1, 2016, to October 19, 2021, and 13,818 colonoscopies had indications for screening for colon cancer, positive FIT, virtual colonoscopy, or surveillance. Of the 10,466 unique patients there were 5369 patients for polyp surveillance, 4054 patients for CRC screening, and 1043 patients for positive FIT or virtual colonoscopy. Of these, 571 individuals (5.5%) had IBP. Repeat colonoscopy within 1 year was recommended for 485 individuals (84.9%) who were included in this study (153 CRC screenings and 46 positive FITs) but not for 86 individuals (15.1%) (Figure 1). Among included patients, the mean (SD) age was 66.6 (7.2) years, and the majority were male (460 [94.8%]) and White (435 [89.7%]) (Table). Two hundred and forty-three (50.1%) were married.


Adherence to Recommended Interval Colonoscopy
Of the 485 patients with IBP who were recommended for follow-up colonoscopy, 287 (59.2%) had a colonoscopy within 1 year, and 198 (40.8%) did not; 17 patients (13.5%) had repeat colonoscopy within 366 to 400 days. Five (1.0%) individuals had a repeat colonoscopy the next day, and 77 (15.9%) had a repeat colonoscopy within 7 days. One hundred and twentysix (26.0%) individuals underwent no repeat colonoscopy during the study period (Figure 2).

To account for the COVID-19 pandemic, the adherence rate of repeat colonoscopy within 1 year prepandemic (January 1, 2016, to December 1, 2018) was calculated along with the adherence rate postpandemic (January 1, 2019 to the end of the study). The rates were similar: 199 of 330 (60.3%) individuals prepandemic vs 88 of 155 (56.8%) individuals postpandemic (Figure 3).

Significant Associations
Age, sex, and race were not associated with adherence to repeat colonoscopy within 1 year. Individuals living ≤ 40 miles from the endoscopy center were more likely to undergo a repeat colonoscopy within 1 year compared with those who lived > 40 miles away (61.7% vs 51.0%, P = .02). Current smoking status was associated with a lower rate of repeat colonoscopy within 1 year (25.8% vs 35.9%; P = .02). There were no differences with respect to inflammatory bowel disease diagnosis, mental health diagnosis, diabetes mellitus, cirrhosis, or medications used, including opioids, anticoagulation, and antiplatelet therapy.
Outcomes
Among individuals who had a repeat colonoscopy the day after the index colonoscopy, 53 of 56 individuals (94.6%) had adequate bowel preparation. Among individuals who had a repeat colonoscopy within 7 days, 70 of 77 (90.9%) had adequate bowel preparation. Of 287 individuals with a repeat colonoscopy within 1 year, 251 (87.5%) had adequate bowel preparation on the repeat colonoscopy. By 400 days after the index colonoscopy, 268 of 304 individuals (88.2%) had adequate bowel preparation.
In this study conducted at a large VA medical center, we found that 5.6% of individuals undergoing colonoscopies had IBP, a rate comparable to prior studies (6% to 26%).3,4 Only 59.2% of individuals underwent repeat colonoscopies within 1 year, as recommended after an index colonoscopy with IBP. Smoking and living longer distances (> 40 miles) from the endoscopy center were associated with a decreased adherence to the repeat colonoscopy recommendation.
Current guidelines recommend repeat colonoscopy for individuals with IBP within 1 year.10 In cases of IBP, the advanced adenoma miss rate is 36% upon repeat colonoscopy within 1 year.13 Despite the importance of a follow-up colonoscopy, clinician adherence with this recommendation remains low.10,14,15 However, in this study cohort, 485 of 571 individuals with IBP (84.9%) received recommendations for a repeat colonoscopy within 1 year. In the US, only 31.9% of 260,314 colonoscopies with IBP included recommendations for a follow-up colonoscopy within 1 year.14 This could be related to variations in endoscopist practice as well as patient risk factors for developing polyps, including family history of cancer and personal history of prior polyps. The findings of multiple polyps, high-risk adenomas, and cancer on the index colonoscopy also influences the endoscopist for repeat colonoscopy within 1 year.14
The timing for repeat colonoscopies within 1 year will be determined by the patients, clinicians, and available scheduling. In this study, the earlier repeat colonoscopies, especially those occurring the day after the index colonoscopy, had the highest success rate of adequate bowel preparation. In a prior study, repeating colonoscopies within the same day or the next day was also found to have a higher rate of adequate bowel preparation than repeat colonoscopies within 1 year (88.9% vs 83.5%).16
Ensuring the return of individuals with IBP for repeat colonoscopy is a challenging task. We identified that individuals who live further away from MVAMC and current smokers had a decreased probability of returning for a repeat colonoscopy. Toro and colleagues found a 68.7% return rate for a repeat colonoscopy within 1 year with individuals age ≥ 60 years, and patients who were White were less likely to proceed with a repeat colonoscopy within 1 year.17 The study did not provide data regarding smoking status or distance to the endoscopy center.17 In a prior study of veterans, the dual diagnosis of psychiatric disorders and substance abuse was associated with missed and canceled colonoscopy appointments.18 The distance to the endoscopy center has also been previously identified as a barrier to a colonoscopy following an abnormal FIT.19 Although not identified in this study due to the homogenous demographic profile, social determinants of health such as socioeconomic status, education, and insurance coverage are known barriers to cancer screening but were not evaluated in this study.20
Based on the identified risk factors, we have created a model for utilizing those risk factors to identify individuals at higher risk for noncompliance (ie, those who live further away from the endoscopy center or currently smoke). These individuals are proactively offered to use an intraprocedural bowel cleansing device to achieve adequate bowel preparation or priority rescheduling for a next-day colonoscopy.
Limitations
This study was a single-center study of the veteran population, which is predominantly White and male, thus limiting generalizability. The study is also limited by minimal available data on adenoma detection and colon cancer incidence on subsequent colonoscopies.
CONCLUSIONS
The rate of IBP was 5.5% in individuals undergoing colonoscopy for colon cancer screening, surveillance, positive FIT, or computed tomography colonography. Only 59.2% of those with IBP underwent the recommended repeat colonoscopy within 1 year. Smoking and distance to the endoscopy center were associated with a decreased adherence to the repeat colonoscopy recommendation. Additional efforts are needed to ensure that individuals with IBP return for timely repeat colonoscopy.
- Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71(3):209-249. doi:10.3322/caac.21660
- Siegel RL, Wagle NS, Cercek A, Smith RA, Jemal A. Colorectal cancer statistics, 2023. CA Cancer J Clin. 2023;73(3):233-254. doi:10.3322/caac.21772
- Atkin W, Wooldrage K, Brenner A, et al. Adenoma surveillance and colorectal cancer incidence: a retrospective, multicentre, cohort study. Lancet Oncol. 2017;18(6):823- 834. doi:10.1016/S1470-2045(17)30187-0
- Froehlich F, Wietlisbach V, Gonvers JJ, Burnand B, Vader JP. Impact of colonic cleansing on quality and diagnostic yield of colonoscopy: the European Panel of Appropriateness of Gastrointestinal Endoscopy European multicenter study. Gastrointest Endosc. 2005;61(3):378- 384. doi:10.1016/s0016-5107(04)02776-2
- Mahmood S, Farooqui SM, Madhoun MF. Predictors of inadequate bowel preparation for colonoscopy: a systematic review and meta-analysis. Eur J Gastroenterol Hepatol. 2018;30(8):819-826. doi:10.1097/MEG.0000000000001175
- ASGE Standards of Practice Committee, Saltzman JR, Cash BD, et al. Bowel preparation before colonoscopy. Gastrointest Endosc. 2015;81(4):781-794. doi:10.1016/j.gie.2014.09.048
- Clark BT, Protiva P, Nagar A, et al. Quantification of Adequate Bowel Preparation for Screening or Surveillance Colonoscopy in Men. Gastroenterology. 2016;150(2):396- e15. doi:10.1053/j.gastro.2015.09.041
- Sulz MC, Kröger A, Prakash M, Manser CN, Heinrich H, Misselwitz B. Meta-Analysis of the Effect of Bowel Preparation on Adenoma Detection: Early Adenomas Affected Stronger than Advanced Adenomas. PLoS One. 2016;11(6):e0154149. Published 2016 Jun 3. doi:10.1371/journal.pone.0154149
- Chokshi RV, Hovis CE, Hollander T, Early DS, Wang JS. Prevalence of missed adenomas in patients with inadequate bowel preparation on screening colonoscopy. Gastrointest Endosc. 2012;75(6):1197-1203. doi:10.1016/j.gie.2012.01.005
- Lieberman DA, Rex DK, Winawer SJ, Giardiello FM, Johnson DA, Levin TR. Guidelines for colonoscopy surveillance after screening and polypectomy: a consensus update by the US Multi-Society Task Force on Colorectal Cancer. Gastroenterology. 2012;143(3):844-857. doi:10.1053/j.gastro.2012.06.001
- Fung P, Syed A, Cole R, Farah K. Poor bowel prep: are you really going to come back within a year? Abstract presented at American Gastroenterological Association DDW 2021, May 21-23, 2021. doi:10.1016/S0016-5085(21)01204-X
- US Department of Veterans Affairs, VA Health Systems Research. Corporate data warehouse (CDW). Updated January 11, 2023. Accessed August 6, 2024. https://www.hsrd.research.va.gov/for_researchers/cdw.cfm
- Lebwohl B, Kastrinos F, Glick M, Rosenbaum AJ, Wang T, Neugut AI. The impact of suboptimal bowel preparation on adenoma miss rates and the factors associated with early repeat colonoscopy. Gastrointest Endosc. 2011;73(6):1207-1214. doi:10.1016/j.gie.2011.01.051
- Calderwood AH, Holub JL, Greenwald DA. Recommendations for follow-up interval after colonoscopy with inadequate bowel preparation in a national colonoscopy quality registry. Gastrointest Endosc. 2022;95(2):360-367. e2. doi:10.1016/j.gie.2021.09.027
- Latorre M, Roy A, Spyrou E, Garcia-Carrasquillo R, Rosenberg R, Lebwohl B. Adherence to guidelines after poor colonoscopy preparation: experience from a patient navigator program. Gastroenterology. 2016;151(1):P196. doi:10.1053/j.gastro.2016.05.027
- Bouquet E, Tomal J, Choksi Y. Next-day screening colonoscopy following inadequate bowel preparation may improve quality of preparation and adenoma detection in a veteran population. Am J Gastroenterol. 2020;115:S259. doi:10.14309/ajg.0000000000000853
- Toro B, Dawkins G, Friedenberg FK, Ehrlich AC. Risk factors for failure to return after a poor preparation colonoscopy: experience in a safety-net hospital, 255. Abstract presented at ACG October 2016. https://journals.lww.com/ajg/fulltext/2016/10001/risk_factors_for_failure_to_return_after_a_poor.255.aspx
- Partin MR, Gravely A, Gellad ZF, et al. Factors Associated With Missed and Cancelled Colonoscopy Appointments at Veterans Health Administration Facilities. Clin Gastroenterol Hepatol. 2016;14(2):259-267. doi:10.1016/j.cgh.2015.07.051
- Idos GE, Bonner JD, Haghighat S, et al. Bridging the Gap: Patient Navigation Increases Colonoscopy Follow-up After Abnormal FIT. Clin Transl Gastroenterol. 2021;12(2):e00307. doi:10.14309/ctg.0000000000000307
- Islami F, Baeker Bispo J, Lee H, et al. American Cancer Society’s report on the status of cancer disparities in the United States, 2023. CA Cancer J Clin. 2024;74(2):136- 166. doi:10.3322/caac.21812
- Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71(3):209-249. doi:10.3322/caac.21660
- Siegel RL, Wagle NS, Cercek A, Smith RA, Jemal A. Colorectal cancer statistics, 2023. CA Cancer J Clin. 2023;73(3):233-254. doi:10.3322/caac.21772
- Atkin W, Wooldrage K, Brenner A, et al. Adenoma surveillance and colorectal cancer incidence: a retrospective, multicentre, cohort study. Lancet Oncol. 2017;18(6):823- 834. doi:10.1016/S1470-2045(17)30187-0
- Froehlich F, Wietlisbach V, Gonvers JJ, Burnand B, Vader JP. Impact of colonic cleansing on quality and diagnostic yield of colonoscopy: the European Panel of Appropriateness of Gastrointestinal Endoscopy European multicenter study. Gastrointest Endosc. 2005;61(3):378- 384. doi:10.1016/s0016-5107(04)02776-2
- Mahmood S, Farooqui SM, Madhoun MF. Predictors of inadequate bowel preparation for colonoscopy: a systematic review and meta-analysis. Eur J Gastroenterol Hepatol. 2018;30(8):819-826. doi:10.1097/MEG.0000000000001175
- ASGE Standards of Practice Committee, Saltzman JR, Cash BD, et al. Bowel preparation before colonoscopy. Gastrointest Endosc. 2015;81(4):781-794. doi:10.1016/j.gie.2014.09.048
- Clark BT, Protiva P, Nagar A, et al. Quantification of Adequate Bowel Preparation for Screening or Surveillance Colonoscopy in Men. Gastroenterology. 2016;150(2):396- e15. doi:10.1053/j.gastro.2015.09.041
- Sulz MC, Kröger A, Prakash M, Manser CN, Heinrich H, Misselwitz B. Meta-Analysis of the Effect of Bowel Preparation on Adenoma Detection: Early Adenomas Affected Stronger than Advanced Adenomas. PLoS One. 2016;11(6):e0154149. Published 2016 Jun 3. doi:10.1371/journal.pone.0154149
- Chokshi RV, Hovis CE, Hollander T, Early DS, Wang JS. Prevalence of missed adenomas in patients with inadequate bowel preparation on screening colonoscopy. Gastrointest Endosc. 2012;75(6):1197-1203. doi:10.1016/j.gie.2012.01.005
- Lieberman DA, Rex DK, Winawer SJ, Giardiello FM, Johnson DA, Levin TR. Guidelines for colonoscopy surveillance after screening and polypectomy: a consensus update by the US Multi-Society Task Force on Colorectal Cancer. Gastroenterology. 2012;143(3):844-857. doi:10.1053/j.gastro.2012.06.001
- Fung P, Syed A, Cole R, Farah K. Poor bowel prep: are you really going to come back within a year? Abstract presented at American Gastroenterological Association DDW 2021, May 21-23, 2021. doi:10.1016/S0016-5085(21)01204-X
- US Department of Veterans Affairs, VA Health Systems Research. Corporate data warehouse (CDW). Updated January 11, 2023. Accessed August 6, 2024. https://www.hsrd.research.va.gov/for_researchers/cdw.cfm
- Lebwohl B, Kastrinos F, Glick M, Rosenbaum AJ, Wang T, Neugut AI. The impact of suboptimal bowel preparation on adenoma miss rates and the factors associated with early repeat colonoscopy. Gastrointest Endosc. 2011;73(6):1207-1214. doi:10.1016/j.gie.2011.01.051
- Calderwood AH, Holub JL, Greenwald DA. Recommendations for follow-up interval after colonoscopy with inadequate bowel preparation in a national colonoscopy quality registry. Gastrointest Endosc. 2022;95(2):360-367. e2. doi:10.1016/j.gie.2021.09.027
- Latorre M, Roy A, Spyrou E, Garcia-Carrasquillo R, Rosenberg R, Lebwohl B. Adherence to guidelines after poor colonoscopy preparation: experience from a patient navigator program. Gastroenterology. 2016;151(1):P196. doi:10.1053/j.gastro.2016.05.027
- Bouquet E, Tomal J, Choksi Y. Next-day screening colonoscopy following inadequate bowel preparation may improve quality of preparation and adenoma detection in a veteran population. Am J Gastroenterol. 2020;115:S259. doi:10.14309/ajg.0000000000000853
- Toro B, Dawkins G, Friedenberg FK, Ehrlich AC. Risk factors for failure to return after a poor preparation colonoscopy: experience in a safety-net hospital, 255. Abstract presented at ACG October 2016. https://journals.lww.com/ajg/fulltext/2016/10001/risk_factors_for_failure_to_return_after_a_poor.255.aspx
- Partin MR, Gravely A, Gellad ZF, et al. Factors Associated With Missed and Cancelled Colonoscopy Appointments at Veterans Health Administration Facilities. Clin Gastroenterol Hepatol. 2016;14(2):259-267. doi:10.1016/j.cgh.2015.07.051
- Idos GE, Bonner JD, Haghighat S, et al. Bridging the Gap: Patient Navigation Increases Colonoscopy Follow-up After Abnormal FIT. Clin Transl Gastroenterol. 2021;12(2):e00307. doi:10.14309/ctg.0000000000000307
- Islami F, Baeker Bispo J, Lee H, et al. American Cancer Society’s report on the status of cancer disparities in the United States, 2023. CA Cancer J Clin. 2024;74(2):136- 166. doi:10.3322/caac.21812






