User login
Melanoma: Neoadjuvant Immunotherapy Provides Optimal Survival Results
BARCELONA, SPAIN — with immunotherapy or a targeted agent or targeted therapy plus immunotherapy, according to a large-scale pooled analysis from the International Neoadjuvant Melanoma Consortium.
Importantly, the analysis — presented at the annual meeting of the European Society for Medical Oncology — showed that achieving a major pathological response to neoadjuvant therapy is a key indicator of survival outcomes.
After 3 years of follow-up, the results showed that neoadjuvant therapy is not delaying melanoma recurrence, “it’s actually preventing it,” coinvestigator Hussein A. Tawbi, MD, PhD, Department of Melanoma Medical Oncology, University of Texas MD Anderson Cancer Center, Houston, said in an interview. That’s “a big deal.”
Since 2010, the introduction of novel adjuvant and neoadjuvant therapies for high-risk stage III resectable melanoma has led to incremental gains for patients, said Georgina V. Long, MD, PhD, BSc, chair of Melanoma Medical Oncology and Translational Research at the University of Sydney in Australia, who presented the results.
The first pooled analysis of neoadjuvant therapy in 189 patients, published in 2021, indicated that those who achieved a major pathological response — defined as either a pathological complete response (with no remaining vital tumor) or a near-complete pathological response (with vital tumor ≤ 10%) — had the best recurrence-free survival rates.
In the current study, the researchers expanded their cohort to include 818 patients from 18 centers. Patients received at least one dose of neoadjuvant therapy — either combination immunotherapy, combination of targeted and immunotherapy agents, or monotherapy with either an immune checkpoint inhibitor or a targeted agent.
The median age was 59 years, and 38% of patients were women. The median follow-up so far is 38.8 months.
Overall, the 3-year event-free survival was 74% in patients who received any immunotherapy, 72% in those who received immunotherapy plus a targeted BRAF/MEK therapy, and just 37% in those who received targeted therapy alone. Similarly, 3-year recurrence-free survival rates were highest in patients who received immunotherapy at 77% vs 73% in those who received immunotherapy plus a targeted BRAF/MEK therapy and just 37% in those who received targeted therapy alone.
Looking specifically at progressive death 1 (PD-1)–based immunotherapy regimens, combination therapy led to a 3-year event-free survival rate between 77% and 95%, depending on the specific combinations, vs 64% with PD-1 monotherapy and 37% with combination targeted therapy.
Overall, patients who had a major pathological response were more likely to be recurrence free at 3 years. The 3-year recurrence-free survival was 88% in patients with a complete response, 68% in those with a partial pathological response, and 40% in those without a response.
Patients who received immunotherapy were more likely to have major pathological response. The 3-year recurrence-free survival was about 94% in patients who received combination or monotherapy with immune checkpoint inhibition, and about 87% in those who received immunotherapy plus targeted therapy. The recurrence-free survival rate was much lower in patients given only BRAF/MEK inhibitors.
The current overall survival data, which are still immature, suggested a few differences when stratifying the patients by treatment. Almost all patients with a major pathological response were alive at 3 years, compared with 86% of those with a partial pathological response and 70% of those without a pathological response.
Overall, the results showed that immunotherapy — as either combination or monotherapy — is “quite a bit” better than targeted therapy with BRAF/MEK agents, which offers no substantial benefit, said Dr. Twabi.
“When you see the same pattern happening in study after study, in a very clear, robust way, it actually becomes very powerful,” he explained.
Rebecca A. Dent, MD, MSc, chair of the ESMO Scientific Committee who was not involved in the study, told a press conference that the introduction of immunotherapy and combination immunotherapy has dramatically changed outcomes in melanoma.
Commenting on the current study results, Dr. Dent said that “combination immunotherapy is clearly showing exceptional stability in terms of long-term benefits.”
The question now is what are the toxicities and costs that come with combination immunotherapy, said Dr. Dent, from National Cancer Centre Singapore and Duke-NUS Medical School, Singapore.
No funding source was declared. Dr. Long declared relationships with a variety of companies, including AstraZeneca UK Limited, Bayer Healthcare Pharmaceuticals, Boehringer Ingelheim, Merck Sharp & Dohme, Novartis, and Regeneron. Dr. Twabi declared relationships with Bristol-Myers Squibb, Novartis, Merck, Genentech, GlaxoSmithKline, Eisai, and others. Dr. Dent declared relationships with AstraZeneca, Roche, Eisai, Gilead Sciences, Eli Lilly, Merck, and Pfizer.
A version of this article appeared on Medscape.com.
BARCELONA, SPAIN — with immunotherapy or a targeted agent or targeted therapy plus immunotherapy, according to a large-scale pooled analysis from the International Neoadjuvant Melanoma Consortium.
Importantly, the analysis — presented at the annual meeting of the European Society for Medical Oncology — showed that achieving a major pathological response to neoadjuvant therapy is a key indicator of survival outcomes.
After 3 years of follow-up, the results showed that neoadjuvant therapy is not delaying melanoma recurrence, “it’s actually preventing it,” coinvestigator Hussein A. Tawbi, MD, PhD, Department of Melanoma Medical Oncology, University of Texas MD Anderson Cancer Center, Houston, said in an interview. That’s “a big deal.”
Since 2010, the introduction of novel adjuvant and neoadjuvant therapies for high-risk stage III resectable melanoma has led to incremental gains for patients, said Georgina V. Long, MD, PhD, BSc, chair of Melanoma Medical Oncology and Translational Research at the University of Sydney in Australia, who presented the results.
The first pooled analysis of neoadjuvant therapy in 189 patients, published in 2021, indicated that those who achieved a major pathological response — defined as either a pathological complete response (with no remaining vital tumor) or a near-complete pathological response (with vital tumor ≤ 10%) — had the best recurrence-free survival rates.
In the current study, the researchers expanded their cohort to include 818 patients from 18 centers. Patients received at least one dose of neoadjuvant therapy — either combination immunotherapy, combination of targeted and immunotherapy agents, or monotherapy with either an immune checkpoint inhibitor or a targeted agent.
The median age was 59 years, and 38% of patients were women. The median follow-up so far is 38.8 months.
Overall, the 3-year event-free survival was 74% in patients who received any immunotherapy, 72% in those who received immunotherapy plus a targeted BRAF/MEK therapy, and just 37% in those who received targeted therapy alone. Similarly, 3-year recurrence-free survival rates were highest in patients who received immunotherapy at 77% vs 73% in those who received immunotherapy plus a targeted BRAF/MEK therapy and just 37% in those who received targeted therapy alone.
Looking specifically at progressive death 1 (PD-1)–based immunotherapy regimens, combination therapy led to a 3-year event-free survival rate between 77% and 95%, depending on the specific combinations, vs 64% with PD-1 monotherapy and 37% with combination targeted therapy.
Overall, patients who had a major pathological response were more likely to be recurrence free at 3 years. The 3-year recurrence-free survival was 88% in patients with a complete response, 68% in those with a partial pathological response, and 40% in those without a response.
Patients who received immunotherapy were more likely to have major pathological response. The 3-year recurrence-free survival was about 94% in patients who received combination or monotherapy with immune checkpoint inhibition, and about 87% in those who received immunotherapy plus targeted therapy. The recurrence-free survival rate was much lower in patients given only BRAF/MEK inhibitors.
The current overall survival data, which are still immature, suggested a few differences when stratifying the patients by treatment. Almost all patients with a major pathological response were alive at 3 years, compared with 86% of those with a partial pathological response and 70% of those without a pathological response.
Overall, the results showed that immunotherapy — as either combination or monotherapy — is “quite a bit” better than targeted therapy with BRAF/MEK agents, which offers no substantial benefit, said Dr. Twabi.
“When you see the same pattern happening in study after study, in a very clear, robust way, it actually becomes very powerful,” he explained.
Rebecca A. Dent, MD, MSc, chair of the ESMO Scientific Committee who was not involved in the study, told a press conference that the introduction of immunotherapy and combination immunotherapy has dramatically changed outcomes in melanoma.
Commenting on the current study results, Dr. Dent said that “combination immunotherapy is clearly showing exceptional stability in terms of long-term benefits.”
The question now is what are the toxicities and costs that come with combination immunotherapy, said Dr. Dent, from National Cancer Centre Singapore and Duke-NUS Medical School, Singapore.
No funding source was declared. Dr. Long declared relationships with a variety of companies, including AstraZeneca UK Limited, Bayer Healthcare Pharmaceuticals, Boehringer Ingelheim, Merck Sharp & Dohme, Novartis, and Regeneron. Dr. Twabi declared relationships with Bristol-Myers Squibb, Novartis, Merck, Genentech, GlaxoSmithKline, Eisai, and others. Dr. Dent declared relationships with AstraZeneca, Roche, Eisai, Gilead Sciences, Eli Lilly, Merck, and Pfizer.
A version of this article appeared on Medscape.com.
BARCELONA, SPAIN — with immunotherapy or a targeted agent or targeted therapy plus immunotherapy, according to a large-scale pooled analysis from the International Neoadjuvant Melanoma Consortium.
Importantly, the analysis — presented at the annual meeting of the European Society for Medical Oncology — showed that achieving a major pathological response to neoadjuvant therapy is a key indicator of survival outcomes.
After 3 years of follow-up, the results showed that neoadjuvant therapy is not delaying melanoma recurrence, “it’s actually preventing it,” coinvestigator Hussein A. Tawbi, MD, PhD, Department of Melanoma Medical Oncology, University of Texas MD Anderson Cancer Center, Houston, said in an interview. That’s “a big deal.”
Since 2010, the introduction of novel adjuvant and neoadjuvant therapies for high-risk stage III resectable melanoma has led to incremental gains for patients, said Georgina V. Long, MD, PhD, BSc, chair of Melanoma Medical Oncology and Translational Research at the University of Sydney in Australia, who presented the results.
The first pooled analysis of neoadjuvant therapy in 189 patients, published in 2021, indicated that those who achieved a major pathological response — defined as either a pathological complete response (with no remaining vital tumor) or a near-complete pathological response (with vital tumor ≤ 10%) — had the best recurrence-free survival rates.
In the current study, the researchers expanded their cohort to include 818 patients from 18 centers. Patients received at least one dose of neoadjuvant therapy — either combination immunotherapy, combination of targeted and immunotherapy agents, or monotherapy with either an immune checkpoint inhibitor or a targeted agent.
The median age was 59 years, and 38% of patients were women. The median follow-up so far is 38.8 months.
Overall, the 3-year event-free survival was 74% in patients who received any immunotherapy, 72% in those who received immunotherapy plus a targeted BRAF/MEK therapy, and just 37% in those who received targeted therapy alone. Similarly, 3-year recurrence-free survival rates were highest in patients who received immunotherapy at 77% vs 73% in those who received immunotherapy plus a targeted BRAF/MEK therapy and just 37% in those who received targeted therapy alone.
Looking specifically at progressive death 1 (PD-1)–based immunotherapy regimens, combination therapy led to a 3-year event-free survival rate between 77% and 95%, depending on the specific combinations, vs 64% with PD-1 monotherapy and 37% with combination targeted therapy.
Overall, patients who had a major pathological response were more likely to be recurrence free at 3 years. The 3-year recurrence-free survival was 88% in patients with a complete response, 68% in those with a partial pathological response, and 40% in those without a response.
Patients who received immunotherapy were more likely to have major pathological response. The 3-year recurrence-free survival was about 94% in patients who received combination or monotherapy with immune checkpoint inhibition, and about 87% in those who received immunotherapy plus targeted therapy. The recurrence-free survival rate was much lower in patients given only BRAF/MEK inhibitors.
The current overall survival data, which are still immature, suggested a few differences when stratifying the patients by treatment. Almost all patients with a major pathological response were alive at 3 years, compared with 86% of those with a partial pathological response and 70% of those without a pathological response.
Overall, the results showed that immunotherapy — as either combination or monotherapy — is “quite a bit” better than targeted therapy with BRAF/MEK agents, which offers no substantial benefit, said Dr. Twabi.
“When you see the same pattern happening in study after study, in a very clear, robust way, it actually becomes very powerful,” he explained.
Rebecca A. Dent, MD, MSc, chair of the ESMO Scientific Committee who was not involved in the study, told a press conference that the introduction of immunotherapy and combination immunotherapy has dramatically changed outcomes in melanoma.
Commenting on the current study results, Dr. Dent said that “combination immunotherapy is clearly showing exceptional stability in terms of long-term benefits.”
The question now is what are the toxicities and costs that come with combination immunotherapy, said Dr. Dent, from National Cancer Centre Singapore and Duke-NUS Medical School, Singapore.
No funding source was declared. Dr. Long declared relationships with a variety of companies, including AstraZeneca UK Limited, Bayer Healthcare Pharmaceuticals, Boehringer Ingelheim, Merck Sharp & Dohme, Novartis, and Regeneron. Dr. Twabi declared relationships with Bristol-Myers Squibb, Novartis, Merck, Genentech, GlaxoSmithKline, Eisai, and others. Dr. Dent declared relationships with AstraZeneca, Roche, Eisai, Gilead Sciences, Eli Lilly, Merck, and Pfizer.
A version of this article appeared on Medscape.com.
FROM ESMO 2024
Identifying Drug-Induced Rashes in Skin of Color: Heightened Awareness Can Accelerate Diagnosis
NEW YORK — Because of their heterogeneity in appearance, to speed the diagnosis.
This risk for a delayed or missed diagnosis in patients with darker skin is shared across skin rashes, but drug-induced hypersensitivity syndrome (DIHS) is a telling example, according to Joanna Harp, MD, director of the Inpatient Dermatology Consult Service, NewYork–Presbyterian Hospital, New York City.
DIHS, also known as a drug reaction with eosinophilia and systemic symptoms, is a type IV hypersensitivity reaction, Dr. Harp explained. While the fact that this disorder does not always include eosinophilia prompted the DIHS acronym, the maculopapular rash often serves as a critical clue of the underlying etiology.
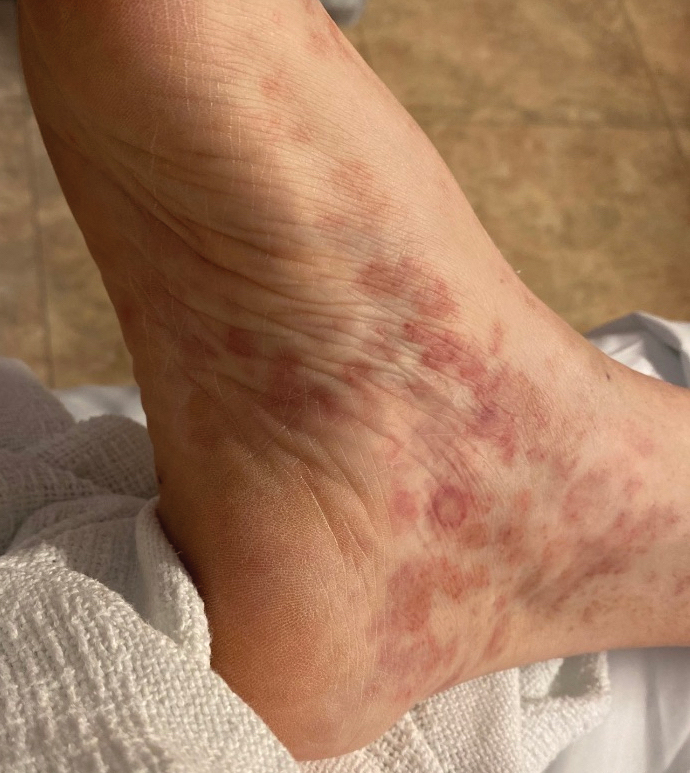
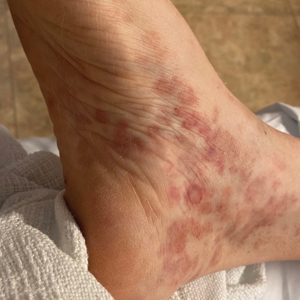
In patients with darker skin, DIHS skin manifestations “can look different, can be more severe, and can have worse outcomes,” Dr. Harp said. As with other skin rashes that are primarily erythematous, the DIHS rash is often more subtle in Black-skinned patients, typically appearing gray or violaceous rather than red.
“The high amount of scale can be a clue,” said Dr. Harp, speaking at the 2024 Skin of Color Update. Scale is particularly prominent among Black patients, she said, because of the greater relative transepidermal water loss than lighter skin, increasing dryness and susceptibility to scale.
The maculopapular rash is “similar to a simple drug eruption, although it is usually more impressive,” she said. Emphasizing that DIHS is a systemic disease, she noted that the characteristic rash is typically accompanied by inflammation in multiple organs that not only includes the mucous membranes but can include major organs such as the lungs, kidneys, and heart.
In patients with DIHS and many of the even more serious types of rashes traced to drug exposures, such as Stevens-Johnson syndrome (SJS) or erythema multiforme, the delay to appearance of the rash from the time of exposure can be the most confusing element.
“It can be months for some drugs such as allopurinol,” said Dr. Harp, pointing out that Black and Asian patients are more likely to carry the HLA-B*5801 genotype, a known risk factor for allopurinol hypersensitivity.
Signs of AGEP Can Be Subtle in Black Patients
Some of the same principles for diagnosing drug-induced rash in darker skin can also be applied to acute generalized exanthematous pustulosis (AGEP), another type IV hypersensitivity reaction. Like all drug-induced rashes, the earlier AGEP is recognized and treated, the better the outcome, but in Black patients, the signs can be subtle.
“The onset is usually fast and occurs in 1-2 days after [the causative drug] exposure,” said Dr. Harp, adding that antibiotics, such as cephalosporins or penicillin, and calcium channel blockers are among the prominent causes of AGEP.
One of the hallmark signs of early-onset AGEP are tiny erythematous pustules in flexural areas, such as the neck or the armpits. The issue of detecting erythema in darker skin is also relevant to this area, but there is an additional problem, according to Dr. Harp. The pustules often dry up quickly, leaving a neutrophilic scale that further complicates the effort to see the characteristic erythema.
“If you see a lot of scale, look for erythema underneath. Think of inflammation,” Dr. Harp said, explaining that the clinical appearance evolves quickly. “If you do not see the pustules, it does not mean they were not there; you just missed them.”
In addition to the flexural areas, “AGEP loves the ears, the face, and the geographic tongue,” she said, offering several pearls to help with the diagnosis. These include side lighting to make papules easier to see, pressing on the skin to highlight the difference between erythematous skin and blanched skin, and checking less pigmented skin, such as on the hands and feet, which makes erythema easier to see.
Steroids are often the first-line treatment for drug-induced skin rashes, but Dr. Harp moves to etanercept or cyclosporine for the most serious drug reactions, such as SJS and toxic epidermal necrolysis.
Etanercept is typically her first choice because patients with systemic hypersensitivity reactions with major organ involvement are often quite ill, making cyclosporine harder to use. In her experience, etanercept has been well tolerated.
Conversely, she cautioned against the use of intravenous immunoglobulin (IVIG). Although this has been used traditionally for severe drug hypersensitivity reactions, “the data are not there,” she said. The data are stronger for a combination of high-dose steroids and IVIG, but she thinks even these data are inconsistent and not as strong as the data supporting etanercept or cyclosporine. She encouraged centers still using IVIG to consider alternatives.
After drug sensitivity reactions are controlled, follow-up care is particularly important for Black patients who face greater risks for sequelae, such as hypopigmentation, hyperpigmentation, or keloids. She recommended aggressive use of emollients and sunscreens for an extended period after lesions resolve to lessen these risks.
Differences in the manifestations of drug-induced skin rashes by race and ethnicity are important and perhaps underappreciated, agreed Shawn Kwatra, MD, professor and chairman of the Department of Dermatology, University of Maryland, Baltimore.
Asked to comment at the meeting, Dr. Kwatra said that he appreciated Dr. Harp’s effort to translate published data and her experience into an overview that increases awareness of the risk for missed or delayed diagnoses of drug-induced rashes in skin of color. He noted that the strategies to identify erythema and pustules, such as increased suspicion in skin of color and the extra steps to rule them out, such as the use of side lighting in the case of pustules for AGEP, are simple and practical.
Dr. Harp and Dr. Kwatra had no relevant disclosures.
A version of this article appeared on Medscape.com.
NEW YORK — Because of their heterogeneity in appearance, to speed the diagnosis.
This risk for a delayed or missed diagnosis in patients with darker skin is shared across skin rashes, but drug-induced hypersensitivity syndrome (DIHS) is a telling example, according to Joanna Harp, MD, director of the Inpatient Dermatology Consult Service, NewYork–Presbyterian Hospital, New York City.
DIHS, also known as a drug reaction with eosinophilia and systemic symptoms, is a type IV hypersensitivity reaction, Dr. Harp explained. While the fact that this disorder does not always include eosinophilia prompted the DIHS acronym, the maculopapular rash often serves as a critical clue of the underlying etiology.
In patients with darker skin, DIHS skin manifestations “can look different, can be more severe, and can have worse outcomes,” Dr. Harp said. As with other skin rashes that are primarily erythematous, the DIHS rash is often more subtle in Black-skinned patients, typically appearing gray or violaceous rather than red.
“The high amount of scale can be a clue,” said Dr. Harp, speaking at the 2024 Skin of Color Update. Scale is particularly prominent among Black patients, she said, because of the greater relative transepidermal water loss than lighter skin, increasing dryness and susceptibility to scale.
The maculopapular rash is “similar to a simple drug eruption, although it is usually more impressive,” she said. Emphasizing that DIHS is a systemic disease, she noted that the characteristic rash is typically accompanied by inflammation in multiple organs that not only includes the mucous membranes but can include major organs such as the lungs, kidneys, and heart.
In patients with DIHS and many of the even more serious types of rashes traced to drug exposures, such as Stevens-Johnson syndrome (SJS) or erythema multiforme, the delay to appearance of the rash from the time of exposure can be the most confusing element.
“It can be months for some drugs such as allopurinol,” said Dr. Harp, pointing out that Black and Asian patients are more likely to carry the HLA-B*5801 genotype, a known risk factor for allopurinol hypersensitivity.
Signs of AGEP Can Be Subtle in Black Patients
Some of the same principles for diagnosing drug-induced rash in darker skin can also be applied to acute generalized exanthematous pustulosis (AGEP), another type IV hypersensitivity reaction. Like all drug-induced rashes, the earlier AGEP is recognized and treated, the better the outcome, but in Black patients, the signs can be subtle.
“The onset is usually fast and occurs in 1-2 days after [the causative drug] exposure,” said Dr. Harp, adding that antibiotics, such as cephalosporins or penicillin, and calcium channel blockers are among the prominent causes of AGEP.
One of the hallmark signs of early-onset AGEP are tiny erythematous pustules in flexural areas, such as the neck or the armpits. The issue of detecting erythema in darker skin is also relevant to this area, but there is an additional problem, according to Dr. Harp. The pustules often dry up quickly, leaving a neutrophilic scale that further complicates the effort to see the characteristic erythema.
“If you see a lot of scale, look for erythema underneath. Think of inflammation,” Dr. Harp said, explaining that the clinical appearance evolves quickly. “If you do not see the pustules, it does not mean they were not there; you just missed them.”
In addition to the flexural areas, “AGEP loves the ears, the face, and the geographic tongue,” she said, offering several pearls to help with the diagnosis. These include side lighting to make papules easier to see, pressing on the skin to highlight the difference between erythematous skin and blanched skin, and checking less pigmented skin, such as on the hands and feet, which makes erythema easier to see.
Steroids are often the first-line treatment for drug-induced skin rashes, but Dr. Harp moves to etanercept or cyclosporine for the most serious drug reactions, such as SJS and toxic epidermal necrolysis.
Etanercept is typically her first choice because patients with systemic hypersensitivity reactions with major organ involvement are often quite ill, making cyclosporine harder to use. In her experience, etanercept has been well tolerated.
Conversely, she cautioned against the use of intravenous immunoglobulin (IVIG). Although this has been used traditionally for severe drug hypersensitivity reactions, “the data are not there,” she said. The data are stronger for a combination of high-dose steroids and IVIG, but she thinks even these data are inconsistent and not as strong as the data supporting etanercept or cyclosporine. She encouraged centers still using IVIG to consider alternatives.
After drug sensitivity reactions are controlled, follow-up care is particularly important for Black patients who face greater risks for sequelae, such as hypopigmentation, hyperpigmentation, or keloids. She recommended aggressive use of emollients and sunscreens for an extended period after lesions resolve to lessen these risks.
Differences in the manifestations of drug-induced skin rashes by race and ethnicity are important and perhaps underappreciated, agreed Shawn Kwatra, MD, professor and chairman of the Department of Dermatology, University of Maryland, Baltimore.
Asked to comment at the meeting, Dr. Kwatra said that he appreciated Dr. Harp’s effort to translate published data and her experience into an overview that increases awareness of the risk for missed or delayed diagnoses of drug-induced rashes in skin of color. He noted that the strategies to identify erythema and pustules, such as increased suspicion in skin of color and the extra steps to rule them out, such as the use of side lighting in the case of pustules for AGEP, are simple and practical.
Dr. Harp and Dr. Kwatra had no relevant disclosures.
A version of this article appeared on Medscape.com.
NEW YORK — Because of their heterogeneity in appearance, to speed the diagnosis.
This risk for a delayed or missed diagnosis in patients with darker skin is shared across skin rashes, but drug-induced hypersensitivity syndrome (DIHS) is a telling example, according to Joanna Harp, MD, director of the Inpatient Dermatology Consult Service, NewYork–Presbyterian Hospital, New York City.
DIHS, also known as a drug reaction with eosinophilia and systemic symptoms, is a type IV hypersensitivity reaction, Dr. Harp explained. While the fact that this disorder does not always include eosinophilia prompted the DIHS acronym, the maculopapular rash often serves as a critical clue of the underlying etiology.
In patients with darker skin, DIHS skin manifestations “can look different, can be more severe, and can have worse outcomes,” Dr. Harp said. As with other skin rashes that are primarily erythematous, the DIHS rash is often more subtle in Black-skinned patients, typically appearing gray or violaceous rather than red.
“The high amount of scale can be a clue,” said Dr. Harp, speaking at the 2024 Skin of Color Update. Scale is particularly prominent among Black patients, she said, because of the greater relative transepidermal water loss than lighter skin, increasing dryness and susceptibility to scale.
The maculopapular rash is “similar to a simple drug eruption, although it is usually more impressive,” she said. Emphasizing that DIHS is a systemic disease, she noted that the characteristic rash is typically accompanied by inflammation in multiple organs that not only includes the mucous membranes but can include major organs such as the lungs, kidneys, and heart.
In patients with DIHS and many of the even more serious types of rashes traced to drug exposures, such as Stevens-Johnson syndrome (SJS) or erythema multiforme, the delay to appearance of the rash from the time of exposure can be the most confusing element.
“It can be months for some drugs such as allopurinol,” said Dr. Harp, pointing out that Black and Asian patients are more likely to carry the HLA-B*5801 genotype, a known risk factor for allopurinol hypersensitivity.
Signs of AGEP Can Be Subtle in Black Patients
Some of the same principles for diagnosing drug-induced rash in darker skin can also be applied to acute generalized exanthematous pustulosis (AGEP), another type IV hypersensitivity reaction. Like all drug-induced rashes, the earlier AGEP is recognized and treated, the better the outcome, but in Black patients, the signs can be subtle.
“The onset is usually fast and occurs in 1-2 days after [the causative drug] exposure,” said Dr. Harp, adding that antibiotics, such as cephalosporins or penicillin, and calcium channel blockers are among the prominent causes of AGEP.
One of the hallmark signs of early-onset AGEP are tiny erythematous pustules in flexural areas, such as the neck or the armpits. The issue of detecting erythema in darker skin is also relevant to this area, but there is an additional problem, according to Dr. Harp. The pustules often dry up quickly, leaving a neutrophilic scale that further complicates the effort to see the characteristic erythema.
“If you see a lot of scale, look for erythema underneath. Think of inflammation,” Dr. Harp said, explaining that the clinical appearance evolves quickly. “If you do not see the pustules, it does not mean they were not there; you just missed them.”
In addition to the flexural areas, “AGEP loves the ears, the face, and the geographic tongue,” she said, offering several pearls to help with the diagnosis. These include side lighting to make papules easier to see, pressing on the skin to highlight the difference between erythematous skin and blanched skin, and checking less pigmented skin, such as on the hands and feet, which makes erythema easier to see.
Steroids are often the first-line treatment for drug-induced skin rashes, but Dr. Harp moves to etanercept or cyclosporine for the most serious drug reactions, such as SJS and toxic epidermal necrolysis.
Etanercept is typically her first choice because patients with systemic hypersensitivity reactions with major organ involvement are often quite ill, making cyclosporine harder to use. In her experience, etanercept has been well tolerated.
Conversely, she cautioned against the use of intravenous immunoglobulin (IVIG). Although this has been used traditionally for severe drug hypersensitivity reactions, “the data are not there,” she said. The data are stronger for a combination of high-dose steroids and IVIG, but she thinks even these data are inconsistent and not as strong as the data supporting etanercept or cyclosporine. She encouraged centers still using IVIG to consider alternatives.
After drug sensitivity reactions are controlled, follow-up care is particularly important for Black patients who face greater risks for sequelae, such as hypopigmentation, hyperpigmentation, or keloids. She recommended aggressive use of emollients and sunscreens for an extended period after lesions resolve to lessen these risks.
Differences in the manifestations of drug-induced skin rashes by race and ethnicity are important and perhaps underappreciated, agreed Shawn Kwatra, MD, professor and chairman of the Department of Dermatology, University of Maryland, Baltimore.
Asked to comment at the meeting, Dr. Kwatra said that he appreciated Dr. Harp’s effort to translate published data and her experience into an overview that increases awareness of the risk for missed or delayed diagnoses of drug-induced rashes in skin of color. He noted that the strategies to identify erythema and pustules, such as increased suspicion in skin of color and the extra steps to rule them out, such as the use of side lighting in the case of pustules for AGEP, are simple and practical.
Dr. Harp and Dr. Kwatra had no relevant disclosures.
A version of this article appeared on Medscape.com.
FROM SOC 2024
FDA Initiative Aims to Improve Diversity in Clinical Trials
NEW YORK — Underrepresentation by gender and race in major clinical trials has been a cause for complaint for decades, but the Food and Drug Administration (FDA) has drafted a regulatory solution to this issue expected to be implemented sometime in 2025.
This initiative, known as the according to Valerie M. Harvey, MD, MPH, associate clinical professor, Edward Via College of Osteopathic Medicine, Blacksburg, Virginia. These rules will be codified, she said at the 2024 Skin of Color Update.
Once the DAP is enacted, “the sponsor must specify the rationale and goals for study enrollment by age, ethnicity, sex, and race,” she said. Furthermore, the submission to the FDA must “describe the methods to meet the diversity benchmarks.”
Lack of Trial Diversity Is Common Across Medicine
Although she focused on the relevance of this initiative to dermatology, Dr. Harvey said the lack of diversity in clinical trials is pervasive throughout medicine. In one survey of randomized controlled trials, less than 60% of trials even specified the race and ethnicity of the participants. In recent psoriasis trials, only 30% met a diversity definition of ≥ 20% of patients identifying as minority (Black, Hispanic, Asian, or other non-White group), said Dr. Harvey, who practices dermatology in Newport News, Virginia.
The FDA draft guidance for the DAP was released in June 2024 and is now available for submitting comments (until September 26). The plan is expected to be published in June 2025, according to Dr. Harvey. It will pertain to all pivotal and phase 3 trials enrolling 180 days after the publication date and will be relevant to all drugs and biologics as well as certain devices.
This initiative could be a critical step toward ensuring diversity in major clinical trials after years of stagnation, Dr. Harvey said, noting that despite repeated calls for more diversity in clinical trials, the literature suggests “little progress.”
However, she said that increasing diversity in clinical trials is just one step toward gathering data about the generalizability of efficacy and safety across racial and ethnic groups. A much more complex issue involves how race and ethnicity are defined in order to understand differences, if any, for efficacy and risk.
“Race is a dynamic social construct and a poor measure for biologic variation and skin color,” Dr. Harvey said. This means that work is needed to address the more complex issue of race and ethnicity stratification that will help clinicians understand the relative benefits and risks for the drugs in these trials.
Rather than differences based on genetic or other sources of biologic differences, she said, outcomes by race alone are often suspected of reflecting disparities in access to healthcare rather than a difference in therapeutic response.
Skin Color Is Inadequate to Define Race
When stratifying patients by race or ethnicity, Dr. Harvey said that “we have to be very, very careful in considering the study purpose and what the study question is.” A study attempting to compare benefits and risks among subgroups by race or ethnicity will require descriptors beyond skin color.
The recognized limitations of measuring skin tone as a surrogate of race are one reason for widespread interest in moving away from the Fitzpatrick skin type (FST) rating that has been widely considered a standard, according to Dr. Harvey. Several alternatives have been proposed, including the Monk Skin Tone Scale, the Individual Typology Angle, and the Eumelanin Human Skin Color Scale, but she cautioned that these are less well validated and generally have the limitations of the FST.
If skin color was ever useful for grouping individuals on the basis of shared physiology, growing rates of intermarriage and immigration have made skin color increasingly irrelevant to racial identity. If the goal is to evaluate the safety and efficacy of drugs across racial groups and ethnicities, the characterization of populations will almost certainly require multiple descriptors and biomarkers, she said.
“It is very important to have many tools for characterizing patients by skin type,” Susan Taylor, MD, professor of dermatology and vice chair for diversity, equity, and inclusion for the Department of Dermatology, University of Pennsylvania, Philadelphia, said in an interview at the meeting.
The reason is “there are limitations to all of them,” she said, noting also that the questions being asked about how and if skin color and race are relevant to therapeutic options differ by the question, such as innate response or access to care.
Dr. Taylor is part of a workshop that she said is evaluating a combination of instruments for characterizing skin color and race in ways relevant to the specific question being asked.
The solutions might differ. While simple clinical assessments involving skin color might be made with methods captured on a smartphone app, Dr. Taylor acknowledged that far more complex tools might be required to document the effect of racial or ethnic differences in drug efficacy and safety in a research setting.
Outside of a research setting, any tools that might be useful for assessing race as a variable must be practical, according to Dr. Harvey. She suggested that these must be time efficient, of reasonable cost, and most importantly, reliable.
Tools meeting these criteria do not currently exist, but Dr. Harvey said the work is underway. She expects a “top-down” collaborative approach to validate alternatives to the FST. If such tools can be developed with buy-in from the FDA, they might be particularly useful for translating trial data to patient care, she added.
Dr. Harvey reported financial relationships with AbbVie, Bristol-Myers Squibb, Janssen, Johnson & Johnson, L’Oréal, and SkinCeuticals. Dr. Taylor, president-elect of the American Academy of Dermatology, reported financial relationships with more than 25 pharmaceutical and cosmetic companies.
A version of this article appeared on Medscape.com.
NEW YORK — Underrepresentation by gender and race in major clinical trials has been a cause for complaint for decades, but the Food and Drug Administration (FDA) has drafted a regulatory solution to this issue expected to be implemented sometime in 2025.
This initiative, known as the according to Valerie M. Harvey, MD, MPH, associate clinical professor, Edward Via College of Osteopathic Medicine, Blacksburg, Virginia. These rules will be codified, she said at the 2024 Skin of Color Update.
Once the DAP is enacted, “the sponsor must specify the rationale and goals for study enrollment by age, ethnicity, sex, and race,” she said. Furthermore, the submission to the FDA must “describe the methods to meet the diversity benchmarks.”
Lack of Trial Diversity Is Common Across Medicine
Although she focused on the relevance of this initiative to dermatology, Dr. Harvey said the lack of diversity in clinical trials is pervasive throughout medicine. In one survey of randomized controlled trials, less than 60% of trials even specified the race and ethnicity of the participants. In recent psoriasis trials, only 30% met a diversity definition of ≥ 20% of patients identifying as minority (Black, Hispanic, Asian, or other non-White group), said Dr. Harvey, who practices dermatology in Newport News, Virginia.
The FDA draft guidance for the DAP was released in June 2024 and is now available for submitting comments (until September 26). The plan is expected to be published in June 2025, according to Dr. Harvey. It will pertain to all pivotal and phase 3 trials enrolling 180 days after the publication date and will be relevant to all drugs and biologics as well as certain devices.
This initiative could be a critical step toward ensuring diversity in major clinical trials after years of stagnation, Dr. Harvey said, noting that despite repeated calls for more diversity in clinical trials, the literature suggests “little progress.”
However, she said that increasing diversity in clinical trials is just one step toward gathering data about the generalizability of efficacy and safety across racial and ethnic groups. A much more complex issue involves how race and ethnicity are defined in order to understand differences, if any, for efficacy and risk.
“Race is a dynamic social construct and a poor measure for biologic variation and skin color,” Dr. Harvey said. This means that work is needed to address the more complex issue of race and ethnicity stratification that will help clinicians understand the relative benefits and risks for the drugs in these trials.
Rather than differences based on genetic or other sources of biologic differences, she said, outcomes by race alone are often suspected of reflecting disparities in access to healthcare rather than a difference in therapeutic response.
Skin Color Is Inadequate to Define Race
When stratifying patients by race or ethnicity, Dr. Harvey said that “we have to be very, very careful in considering the study purpose and what the study question is.” A study attempting to compare benefits and risks among subgroups by race or ethnicity will require descriptors beyond skin color.
The recognized limitations of measuring skin tone as a surrogate of race are one reason for widespread interest in moving away from the Fitzpatrick skin type (FST) rating that has been widely considered a standard, according to Dr. Harvey. Several alternatives have been proposed, including the Monk Skin Tone Scale, the Individual Typology Angle, and the Eumelanin Human Skin Color Scale, but she cautioned that these are less well validated and generally have the limitations of the FST.
If skin color was ever useful for grouping individuals on the basis of shared physiology, growing rates of intermarriage and immigration have made skin color increasingly irrelevant to racial identity. If the goal is to evaluate the safety and efficacy of drugs across racial groups and ethnicities, the characterization of populations will almost certainly require multiple descriptors and biomarkers, she said.
“It is very important to have many tools for characterizing patients by skin type,” Susan Taylor, MD, professor of dermatology and vice chair for diversity, equity, and inclusion for the Department of Dermatology, University of Pennsylvania, Philadelphia, said in an interview at the meeting.
The reason is “there are limitations to all of them,” she said, noting also that the questions being asked about how and if skin color and race are relevant to therapeutic options differ by the question, such as innate response or access to care.
Dr. Taylor is part of a workshop that she said is evaluating a combination of instruments for characterizing skin color and race in ways relevant to the specific question being asked.
The solutions might differ. While simple clinical assessments involving skin color might be made with methods captured on a smartphone app, Dr. Taylor acknowledged that far more complex tools might be required to document the effect of racial or ethnic differences in drug efficacy and safety in a research setting.
Outside of a research setting, any tools that might be useful for assessing race as a variable must be practical, according to Dr. Harvey. She suggested that these must be time efficient, of reasonable cost, and most importantly, reliable.
Tools meeting these criteria do not currently exist, but Dr. Harvey said the work is underway. She expects a “top-down” collaborative approach to validate alternatives to the FST. If such tools can be developed with buy-in from the FDA, they might be particularly useful for translating trial data to patient care, she added.
Dr. Harvey reported financial relationships with AbbVie, Bristol-Myers Squibb, Janssen, Johnson & Johnson, L’Oréal, and SkinCeuticals. Dr. Taylor, president-elect of the American Academy of Dermatology, reported financial relationships with more than 25 pharmaceutical and cosmetic companies.
A version of this article appeared on Medscape.com.
NEW YORK — Underrepresentation by gender and race in major clinical trials has been a cause for complaint for decades, but the Food and Drug Administration (FDA) has drafted a regulatory solution to this issue expected to be implemented sometime in 2025.
This initiative, known as the according to Valerie M. Harvey, MD, MPH, associate clinical professor, Edward Via College of Osteopathic Medicine, Blacksburg, Virginia. These rules will be codified, she said at the 2024 Skin of Color Update.
Once the DAP is enacted, “the sponsor must specify the rationale and goals for study enrollment by age, ethnicity, sex, and race,” she said. Furthermore, the submission to the FDA must “describe the methods to meet the diversity benchmarks.”
Lack of Trial Diversity Is Common Across Medicine
Although she focused on the relevance of this initiative to dermatology, Dr. Harvey said the lack of diversity in clinical trials is pervasive throughout medicine. In one survey of randomized controlled trials, less than 60% of trials even specified the race and ethnicity of the participants. In recent psoriasis trials, only 30% met a diversity definition of ≥ 20% of patients identifying as minority (Black, Hispanic, Asian, or other non-White group), said Dr. Harvey, who practices dermatology in Newport News, Virginia.
The FDA draft guidance for the DAP was released in June 2024 and is now available for submitting comments (until September 26). The plan is expected to be published in June 2025, according to Dr. Harvey. It will pertain to all pivotal and phase 3 trials enrolling 180 days after the publication date and will be relevant to all drugs and biologics as well as certain devices.
This initiative could be a critical step toward ensuring diversity in major clinical trials after years of stagnation, Dr. Harvey said, noting that despite repeated calls for more diversity in clinical trials, the literature suggests “little progress.”
However, she said that increasing diversity in clinical trials is just one step toward gathering data about the generalizability of efficacy and safety across racial and ethnic groups. A much more complex issue involves how race and ethnicity are defined in order to understand differences, if any, for efficacy and risk.
“Race is a dynamic social construct and a poor measure for biologic variation and skin color,” Dr. Harvey said. This means that work is needed to address the more complex issue of race and ethnicity stratification that will help clinicians understand the relative benefits and risks for the drugs in these trials.
Rather than differences based on genetic or other sources of biologic differences, she said, outcomes by race alone are often suspected of reflecting disparities in access to healthcare rather than a difference in therapeutic response.
Skin Color Is Inadequate to Define Race
When stratifying patients by race or ethnicity, Dr. Harvey said that “we have to be very, very careful in considering the study purpose and what the study question is.” A study attempting to compare benefits and risks among subgroups by race or ethnicity will require descriptors beyond skin color.
The recognized limitations of measuring skin tone as a surrogate of race are one reason for widespread interest in moving away from the Fitzpatrick skin type (FST) rating that has been widely considered a standard, according to Dr. Harvey. Several alternatives have been proposed, including the Monk Skin Tone Scale, the Individual Typology Angle, and the Eumelanin Human Skin Color Scale, but she cautioned that these are less well validated and generally have the limitations of the FST.
If skin color was ever useful for grouping individuals on the basis of shared physiology, growing rates of intermarriage and immigration have made skin color increasingly irrelevant to racial identity. If the goal is to evaluate the safety and efficacy of drugs across racial groups and ethnicities, the characterization of populations will almost certainly require multiple descriptors and biomarkers, she said.
“It is very important to have many tools for characterizing patients by skin type,” Susan Taylor, MD, professor of dermatology and vice chair for diversity, equity, and inclusion for the Department of Dermatology, University of Pennsylvania, Philadelphia, said in an interview at the meeting.
The reason is “there are limitations to all of them,” she said, noting also that the questions being asked about how and if skin color and race are relevant to therapeutic options differ by the question, such as innate response or access to care.
Dr. Taylor is part of a workshop that she said is evaluating a combination of instruments for characterizing skin color and race in ways relevant to the specific question being asked.
The solutions might differ. While simple clinical assessments involving skin color might be made with methods captured on a smartphone app, Dr. Taylor acknowledged that far more complex tools might be required to document the effect of racial or ethnic differences in drug efficacy and safety in a research setting.
Outside of a research setting, any tools that might be useful for assessing race as a variable must be practical, according to Dr. Harvey. She suggested that these must be time efficient, of reasonable cost, and most importantly, reliable.
Tools meeting these criteria do not currently exist, but Dr. Harvey said the work is underway. She expects a “top-down” collaborative approach to validate alternatives to the FST. If such tools can be developed with buy-in from the FDA, they might be particularly useful for translating trial data to patient care, she added.
Dr. Harvey reported financial relationships with AbbVie, Bristol-Myers Squibb, Janssen, Johnson & Johnson, L’Oréal, and SkinCeuticals. Dr. Taylor, president-elect of the American Academy of Dermatology, reported financial relationships with more than 25 pharmaceutical and cosmetic companies.
A version of this article appeared on Medscape.com.
FROM SOC 2024
Most Women With Genitourinary Syndrome of Menopause Do Not Receive Effective Treatment
CHICAGO — The vast majority of women experiencing genitourinary syndrome of menopause (GSM) symptoms did not receive a prescription for hormonal vaginal therapies prior to seeking care at a specialized menopause clinic, according to research presented at the annual meeting of The Menopause Society.
“GSM symptoms are very common and affect women’s health and quality of life, often worsening without effective therapy,” Leticia Hernández Galán, PhD, of the Department of Obstetrics & Gynecology, McMaster University, Hamilton, Ontario, Canada, and colleagues reported. “We have demonstrated that most women seeking specialty care in an urban center with GSM symptoms have not been given a trial of local vaginal therapies by referring providers despite guidelines about safety and lack of contraindications. Given very long wait times for menopause providers in Canada, improved education for both women and their providers is needed to reduce needless suffering and improve care.”
Stephanie Faubion, MD, MBA, director of the Mayo Clinic Women’s Health in Jacksonville, Florida, and medical director of The Menopause Society, was not involved with the study but agreed with the authors’ assessment of the findings.
“This study highlights the treatment gap for women with genitourinary syndrome of menopause,” Dr. Faubion told this news organization. “Clearly, there is underutilization of low-dose vaginal hormonal therapies, which are known to be safe and effective. We still have work to do in terms of educating both women and providers on established treatment options for this common concern in menopausal women.”
The findings match previous ones that found a majority of women with GSM do not receive treatment. A 2017 study, which was cited in the 2020 Menopause Society position statement on the condition, found that half of women with GSM had never used any treatment.
GSM is the current term that replaces previously used “vulvovaginal atrophy” and “atrophic vaginitis” because it encompasses all the menopause symptoms and signs associated with menopause that affect the vagina, vulva, and urinary tract. Anywhere from 50% to 84% of postmenopausal women experience GSM, the authors noted, with symptoms that include “burning, itching, or irritation of the vulva” and “lack of lubrication and discomfort or pain with sexual activity as well as dysuria, increased frequency or urgency of urination, and increased risk for urinary tract infections.”
First-line treatment of mild GSM often includes nonhormonal vaginal lubricants and moisturizers, but vaginal estrogen is considered the most effective treatment for more severe or bothersome cases. Other treatments include systematic hormone therapy and ospemifene or other selective estrogen receptor modulators.
Increased Risk for Urinary Tract Infections (UTIs)
Untreated GSM is not simply a quality of life issue; it increases the risk of developing serious UTIs, explained JoAnn Pinkerton, MD, a professor of obstetrics and gynecology at the University of Virginia, Charlottesville, who was not involved in the study.
“Estrogen depletion alters the vaginal epithelium, with distinct impairments in lubrication, elasticity, pH, and blood flow,” Dr. Pinkerton said. “The vaginal microbiome changes, with increasing pH following menopause and loss of lactobacillus predominance. These alterations allow a more hospitable environment for bacterial growth and increase the risk of UTI.”
Vaginal estrogen, meanwhile, reduces UTI risk because it “increases the presence of lactobacillus in the vagina due to improvements in vaginal pH, rebuilding superficial cells, elasticity, and connectivity,” she said.
The study assessed the incidence of GSM among patients at a single specialized Canadian institution, St. Joseph’s Healthcare Menopause Clinic in Hamilton, Ontario, between January 2021 and August 2024. Patients completed a Menopause Rating Scale that quantified two sets of GSM symptoms relating to “dryness of the vagina” and “bladder problems.” Patients also answered questions about the provider they had seen before coming to the specialized clinic and whether they had been prescribed local vaginal products before their visit.
Among 529 patients, the average age was 51, and the vast majority (88%) had some amount of tertiary education beyond high school. Only 21.5% were still menstruating, whereas the other respondents had stopped menstruating. The patient population was mostly White (85.6%), with Black, Hispanic, Asian, Middle Eastern, and Indigenous patients making up most of the other patient groups.
Among the 521 patients who answered the question on vaginal dryness, answers were similarly split between none (26%), mild (23%), moderate (21%), severe (15%), and very severe (15%). One third of the 526 women (34%) who answered the question on bladder problems said they had none, whereas the remainder reported their problems as mild (24%), moderate (24%), severe (11%), or very severe (7%).
Despite about half the participants reporting moderate to very severe vaginal dryness, 85% of them had not been prescribed local vaginal hormone therapies before their visit to the menopause clinic. Women were more likely to have been prescribed a localized therapy if they were older, were postmenopausal instead of perimenopausal, or had a female healthcare provider prior to this visit.
The survey also asked about the specialty and years in practice for the providers women had seen before visiting the clinic, but neither of these were predictors for receiving a hormone prescription. The patient’s education, partner status, and ethnicity were also not associated with the likelihood of a prescription.
Among 62 women who had been prescribed a vaginal hormone treatment, most were prescribed Vagifem (29%) or Premarin Vaginal cream (26%), followed by Intrarosa (19%), Estragyn cream (16%), Estring (3%), or something else (18%).
Serious Complications of GSM
Dr. Pinkerton described how GSM, particularly in older women, can run the risk of becoming life-threatening if untreated and unrecognized.
“For some women, UTIs can lead to urosepsis, as both the vaginal tissues and bladder tissues are thin with blood vessels close to the surface,” Dr. Pinkerton said. “What may have started as a UTI, can ascend to the kidneys or get into the bloodstream, which, in some, can develop into urosepsis, which can be life-threatening. The bacterial pathogen initiates the disease process, but host immune responses drive whether sepsis develops and its severity.”
The research by Dr. Hernández Galán was funded by the Canadian Institutes of Health Research, the Canadian Menopause Society, and Pfizer. Dr. Faubion had no disclosures, and Dr. Pinkerton has run a trial funded by Bayer and is a consultant for Bayer and Pfizer.
A version of this article first appeared on Medscape.com.
CHICAGO — The vast majority of women experiencing genitourinary syndrome of menopause (GSM) symptoms did not receive a prescription for hormonal vaginal therapies prior to seeking care at a specialized menopause clinic, according to research presented at the annual meeting of The Menopause Society.
“GSM symptoms are very common and affect women’s health and quality of life, often worsening without effective therapy,” Leticia Hernández Galán, PhD, of the Department of Obstetrics & Gynecology, McMaster University, Hamilton, Ontario, Canada, and colleagues reported. “We have demonstrated that most women seeking specialty care in an urban center with GSM symptoms have not been given a trial of local vaginal therapies by referring providers despite guidelines about safety and lack of contraindications. Given very long wait times for menopause providers in Canada, improved education for both women and their providers is needed to reduce needless suffering and improve care.”
Stephanie Faubion, MD, MBA, director of the Mayo Clinic Women’s Health in Jacksonville, Florida, and medical director of The Menopause Society, was not involved with the study but agreed with the authors’ assessment of the findings.
“This study highlights the treatment gap for women with genitourinary syndrome of menopause,” Dr. Faubion told this news organization. “Clearly, there is underutilization of low-dose vaginal hormonal therapies, which are known to be safe and effective. We still have work to do in terms of educating both women and providers on established treatment options for this common concern in menopausal women.”
The findings match previous ones that found a majority of women with GSM do not receive treatment. A 2017 study, which was cited in the 2020 Menopause Society position statement on the condition, found that half of women with GSM had never used any treatment.
GSM is the current term that replaces previously used “vulvovaginal atrophy” and “atrophic vaginitis” because it encompasses all the menopause symptoms and signs associated with menopause that affect the vagina, vulva, and urinary tract. Anywhere from 50% to 84% of postmenopausal women experience GSM, the authors noted, with symptoms that include “burning, itching, or irritation of the vulva” and “lack of lubrication and discomfort or pain with sexual activity as well as dysuria, increased frequency or urgency of urination, and increased risk for urinary tract infections.”
First-line treatment of mild GSM often includes nonhormonal vaginal lubricants and moisturizers, but vaginal estrogen is considered the most effective treatment for more severe or bothersome cases. Other treatments include systematic hormone therapy and ospemifene or other selective estrogen receptor modulators.
Increased Risk for Urinary Tract Infections (UTIs)
Untreated GSM is not simply a quality of life issue; it increases the risk of developing serious UTIs, explained JoAnn Pinkerton, MD, a professor of obstetrics and gynecology at the University of Virginia, Charlottesville, who was not involved in the study.
“Estrogen depletion alters the vaginal epithelium, with distinct impairments in lubrication, elasticity, pH, and blood flow,” Dr. Pinkerton said. “The vaginal microbiome changes, with increasing pH following menopause and loss of lactobacillus predominance. These alterations allow a more hospitable environment for bacterial growth and increase the risk of UTI.”
Vaginal estrogen, meanwhile, reduces UTI risk because it “increases the presence of lactobacillus in the vagina due to improvements in vaginal pH, rebuilding superficial cells, elasticity, and connectivity,” she said.
The study assessed the incidence of GSM among patients at a single specialized Canadian institution, St. Joseph’s Healthcare Menopause Clinic in Hamilton, Ontario, between January 2021 and August 2024. Patients completed a Menopause Rating Scale that quantified two sets of GSM symptoms relating to “dryness of the vagina” and “bladder problems.” Patients also answered questions about the provider they had seen before coming to the specialized clinic and whether they had been prescribed local vaginal products before their visit.
Among 529 patients, the average age was 51, and the vast majority (88%) had some amount of tertiary education beyond high school. Only 21.5% were still menstruating, whereas the other respondents had stopped menstruating. The patient population was mostly White (85.6%), with Black, Hispanic, Asian, Middle Eastern, and Indigenous patients making up most of the other patient groups.
Among the 521 patients who answered the question on vaginal dryness, answers were similarly split between none (26%), mild (23%), moderate (21%), severe (15%), and very severe (15%). One third of the 526 women (34%) who answered the question on bladder problems said they had none, whereas the remainder reported their problems as mild (24%), moderate (24%), severe (11%), or very severe (7%).
Despite about half the participants reporting moderate to very severe vaginal dryness, 85% of them had not been prescribed local vaginal hormone therapies before their visit to the menopause clinic. Women were more likely to have been prescribed a localized therapy if they were older, were postmenopausal instead of perimenopausal, or had a female healthcare provider prior to this visit.
The survey also asked about the specialty and years in practice for the providers women had seen before visiting the clinic, but neither of these were predictors for receiving a hormone prescription. The patient’s education, partner status, and ethnicity were also not associated with the likelihood of a prescription.
Among 62 women who had been prescribed a vaginal hormone treatment, most were prescribed Vagifem (29%) or Premarin Vaginal cream (26%), followed by Intrarosa (19%), Estragyn cream (16%), Estring (3%), or something else (18%).
Serious Complications of GSM
Dr. Pinkerton described how GSM, particularly in older women, can run the risk of becoming life-threatening if untreated and unrecognized.
“For some women, UTIs can lead to urosepsis, as both the vaginal tissues and bladder tissues are thin with blood vessels close to the surface,” Dr. Pinkerton said. “What may have started as a UTI, can ascend to the kidneys or get into the bloodstream, which, in some, can develop into urosepsis, which can be life-threatening. The bacterial pathogen initiates the disease process, but host immune responses drive whether sepsis develops and its severity.”
The research by Dr. Hernández Galán was funded by the Canadian Institutes of Health Research, the Canadian Menopause Society, and Pfizer. Dr. Faubion had no disclosures, and Dr. Pinkerton has run a trial funded by Bayer and is a consultant for Bayer and Pfizer.
A version of this article first appeared on Medscape.com.
CHICAGO — The vast majority of women experiencing genitourinary syndrome of menopause (GSM) symptoms did not receive a prescription for hormonal vaginal therapies prior to seeking care at a specialized menopause clinic, according to research presented at the annual meeting of The Menopause Society.
“GSM symptoms are very common and affect women’s health and quality of life, often worsening without effective therapy,” Leticia Hernández Galán, PhD, of the Department of Obstetrics & Gynecology, McMaster University, Hamilton, Ontario, Canada, and colleagues reported. “We have demonstrated that most women seeking specialty care in an urban center with GSM symptoms have not been given a trial of local vaginal therapies by referring providers despite guidelines about safety and lack of contraindications. Given very long wait times for menopause providers in Canada, improved education for both women and their providers is needed to reduce needless suffering and improve care.”
Stephanie Faubion, MD, MBA, director of the Mayo Clinic Women’s Health in Jacksonville, Florida, and medical director of The Menopause Society, was not involved with the study but agreed with the authors’ assessment of the findings.
“This study highlights the treatment gap for women with genitourinary syndrome of menopause,” Dr. Faubion told this news organization. “Clearly, there is underutilization of low-dose vaginal hormonal therapies, which are known to be safe and effective. We still have work to do in terms of educating both women and providers on established treatment options for this common concern in menopausal women.”
The findings match previous ones that found a majority of women with GSM do not receive treatment. A 2017 study, which was cited in the 2020 Menopause Society position statement on the condition, found that half of women with GSM had never used any treatment.
GSM is the current term that replaces previously used “vulvovaginal atrophy” and “atrophic vaginitis” because it encompasses all the menopause symptoms and signs associated with menopause that affect the vagina, vulva, and urinary tract. Anywhere from 50% to 84% of postmenopausal women experience GSM, the authors noted, with symptoms that include “burning, itching, or irritation of the vulva” and “lack of lubrication and discomfort or pain with sexual activity as well as dysuria, increased frequency or urgency of urination, and increased risk for urinary tract infections.”
First-line treatment of mild GSM often includes nonhormonal vaginal lubricants and moisturizers, but vaginal estrogen is considered the most effective treatment for more severe or bothersome cases. Other treatments include systematic hormone therapy and ospemifene or other selective estrogen receptor modulators.
Increased Risk for Urinary Tract Infections (UTIs)
Untreated GSM is not simply a quality of life issue; it increases the risk of developing serious UTIs, explained JoAnn Pinkerton, MD, a professor of obstetrics and gynecology at the University of Virginia, Charlottesville, who was not involved in the study.
“Estrogen depletion alters the vaginal epithelium, with distinct impairments in lubrication, elasticity, pH, and blood flow,” Dr. Pinkerton said. “The vaginal microbiome changes, with increasing pH following menopause and loss of lactobacillus predominance. These alterations allow a more hospitable environment for bacterial growth and increase the risk of UTI.”
Vaginal estrogen, meanwhile, reduces UTI risk because it “increases the presence of lactobacillus in the vagina due to improvements in vaginal pH, rebuilding superficial cells, elasticity, and connectivity,” she said.
The study assessed the incidence of GSM among patients at a single specialized Canadian institution, St. Joseph’s Healthcare Menopause Clinic in Hamilton, Ontario, between January 2021 and August 2024. Patients completed a Menopause Rating Scale that quantified two sets of GSM symptoms relating to “dryness of the vagina” and “bladder problems.” Patients also answered questions about the provider they had seen before coming to the specialized clinic and whether they had been prescribed local vaginal products before their visit.
Among 529 patients, the average age was 51, and the vast majority (88%) had some amount of tertiary education beyond high school. Only 21.5% were still menstruating, whereas the other respondents had stopped menstruating. The patient population was mostly White (85.6%), with Black, Hispanic, Asian, Middle Eastern, and Indigenous patients making up most of the other patient groups.
Among the 521 patients who answered the question on vaginal dryness, answers were similarly split between none (26%), mild (23%), moderate (21%), severe (15%), and very severe (15%). One third of the 526 women (34%) who answered the question on bladder problems said they had none, whereas the remainder reported their problems as mild (24%), moderate (24%), severe (11%), or very severe (7%).
Despite about half the participants reporting moderate to very severe vaginal dryness, 85% of them had not been prescribed local vaginal hormone therapies before their visit to the menopause clinic. Women were more likely to have been prescribed a localized therapy if they were older, were postmenopausal instead of perimenopausal, or had a female healthcare provider prior to this visit.
The survey also asked about the specialty and years in practice for the providers women had seen before visiting the clinic, but neither of these were predictors for receiving a hormone prescription. The patient’s education, partner status, and ethnicity were also not associated with the likelihood of a prescription.
Among 62 women who had been prescribed a vaginal hormone treatment, most were prescribed Vagifem (29%) or Premarin Vaginal cream (26%), followed by Intrarosa (19%), Estragyn cream (16%), Estring (3%), or something else (18%).
Serious Complications of GSM
Dr. Pinkerton described how GSM, particularly in older women, can run the risk of becoming life-threatening if untreated and unrecognized.
“For some women, UTIs can lead to urosepsis, as both the vaginal tissues and bladder tissues are thin with blood vessels close to the surface,” Dr. Pinkerton said. “What may have started as a UTI, can ascend to the kidneys or get into the bloodstream, which, in some, can develop into urosepsis, which can be life-threatening. The bacterial pathogen initiates the disease process, but host immune responses drive whether sepsis develops and its severity.”
The research by Dr. Hernández Galán was funded by the Canadian Institutes of Health Research, the Canadian Menopause Society, and Pfizer. Dr. Faubion had no disclosures, and Dr. Pinkerton has run a trial funded by Bayer and is a consultant for Bayer and Pfizer.
A version of this article first appeared on Medscape.com.
FROM THE MENOPAUSE SOCIETY 2024
Hormone Therapy for Menopause Remains at Historic Lows Despite Effectiveness and Safety Profile
CHICAGO — before the publication of the 2002 Women’s Health Initiative (WHI) study that misguidedly cast doubt on the safety of HT. Though subsequent research has addressed the flaws of the WHI study and supports the use of HT in most menopausal women younger than 60 years, use of this therapy has never recovered, according to research presented at the annual meeting of The Menopause Society (formerly The North American Menopause Society).
“Despite evidence supporting the efficacy and safety of HT, usage rates of US Food and Drug Administration–approved HT remain low,” Stephanie Faubion, MD, MBA, director of the Mayo Clinic Women’s Health in Jacksonville, Florida, and medical director of The Menopause Society, told attendees. “Improved education of clinicians and patients is critically needed.”
Today, “there is more clarity on the risk/benefit ratio of HT use with the benefits typically outweighing the risks in women who initiate therapy under the age of 60 years and within 10 years of menopause onset.”
Using medical and pharmacy claims data from OptumLabs, Dr. Faubion and her colleagues examined utilization rates from 2007 to 2023 of transdermal vs oral estrogen and of conjugated estrogen vs estradiol in women aged 40 years or older. The data included more than 200 million people throughout the United States covered by commercial insurance or Medicare Advantage. The researchers defined annual rate of HT use as the proportion of women who had at least 180 days of a filled prescription for a systemic HT preparation with estrogen.
The study population increased from an estimated 2 million women in 2007 to 4.5 million women in 2023, and the average age of enrollees increased from 53 in 2007 to 66 in 2023. Starting at 4.6% in 2007, HT use steadily declined to a low of 1.8% in 2023 for the whole cohort of women aged 40 years or older.
Though rates remained highest in women aged 50-64 years, it still declined within each age group: From 6% in 2007 to 3.6% in 2023 among women aged 50-54 years, from 7.3% to 3.8% among women aged 55-59 years, and from 7.5% to 2.9% among women aged 60-64 years. It also declined in younger women, from 3.2% in 2007 to 1.5% in 2023 in those aged 45-50 years. Estradiol was the most common formulation used, and oral administration was the most common route.
The researchers also saw a gradual decline during the study period in the use of high-dose oral HT and an increase in the use of low-dose oral HT, whereas standard dosages remained fairly consistent as the most common dose prescribed. Similarly, the use of high transdermal doses declined, whereas low transdermal doses increased and surpassed the use of standard doses. Conjugated estrogen use plummeted during the study period across all age groups, from 2%-5% in most age groups to < 1% in all age groups by 2023.
One limitation of the study was that it could not examine rates of compounded HT use because those would not be reflected in insurance claims, pointed out JoAnn Pinkerton, MD, a professor of ob.gyn. at the University of Virginia in Charlottesville, Virginia, who was not involved in the study. Dr. Pinkerton found it surprising that the numbers were so low, despite the fact that research estimates suggest less than 15% of menopausal women are receiving adequate treatment, she told this news organization. “You can see there’s a large unmet need to get treatment,” she said. “All major medical societies say the same thing: For healthy, symptomatic menopausal women, you can use hormone therapy safely and effectively.”
The lack of education among providers is likely the biggest reason for the decline, Dr. Pinkerton says. “I think it’s because there’s a whole group of providers that did not receive any training, and that’s OB/GYNs, internal medicine, family practice, endocrinologists,” she said. “Now that people are starting to feel more confident that we can use it safely, we’re trying to get that training out to people about vasomotor symptoms, about hormone therapy, and now about new nonhormone therapies.”
Dr. Pinkerton noted that The Menopause Society has begun a new teaching program, Menopause Step-by-Step, aimed at providing short articles on the basics of menopause, HT, non-HT, and vaginal issues.
A separate poster presented at the conference provides insight into another potential factor contributing to low HT rates. A survey of 1050 American and Canadian women found that 90% discussed their symptoms with their healthcare providers, yet only 25% said their doctor identified the symptoms as likely due to perimenopause or menopause on their first visit — and only 10% of respondents said their doctor was the one to bring up perimenopause/menopause.
The respondents comprised a convenience sample of those who saw the survey on social media, in an email, or on the website of Morphus, a Toronto-based company aimed at providing support, information, and products related to menopause. Though the survey is ongoing, the analyzed responses are from March to May 2024.
Though 40% of the women said their provider attributed their symptoms to perimenopause or menopause on the second or third visit, 18% saw a provider four to five times, and 17% saw a provider more than five times before the provider considered menopause as a cause. About a third of the women (35%) brought it up to their doctor themselves and found their provider receptive, but 40% said the response was dismissive when they brought it up, and 15% said the topic was never broached at all.
Andrea Donsky, RHN, founder of Morphus who conducted the study, found these numbers surprising because she would have hoped that more doctors would have brought up perimenopause/menopause sooner. “We still have a lot of work to do to help educate women and healthcare providers,” Ms. Donsky told this news organization. “A lot of women spend years not knowing they’re in this phase of life, so they visit their doctors/HCPs [healthcare providers] many times because the connection isn’t made on the first visit.”
Danielle Meitiv, MS, a study co-author and health coach based in Silver Spring, Maryland, added, “Everyone wonders why we end up with Dr. Google; that’s the only doctor who’s talking to us about menopause.”
Dr. Pinkerton was less surprised by these survey findings. “As a menopause specialist, my most common new patient is a perimenopausal woman who feels like she hasn’t been listened to,” whether it’s her primary care doctor, her ob.gyn., or another clinician. “If the provider doesn’t ask or if the women doesn’t tell, then you don’t have the conversation,” Dr. Pinkerton said. “So many women in perimenopause are busy with work, families, partnerships, aging parents — all of the issues that they’re dealing with — that when they start to have sleep issues or mood issues or easy crying, they relate it to their life stressors, instead of recognizing that it’s fluctuating hormones.”
When Ms. Donsky examined the 1223 responses they had received through August 2024, the most common treatments advised for symptoms were antidepressants and HT, both recommended by 38% of providers. Other common recommendations were to “lose weight,” “eat less and exercise more,” supplements, or birth control pills.
Dr. Faubion had no disclosures, and her study used no external funding. Dr. Pinkerton has run a trial funded by Bayer and is a consultant for Bayer and Pfizer. Ms. Donsky is the owner of Morphus. Ms. Meitiv had no disclosures. The poster on women’s experiences with providers was funded by Morphus Inc.
A version of this article first appeared on Medscape.com.
CHICAGO — before the publication of the 2002 Women’s Health Initiative (WHI) study that misguidedly cast doubt on the safety of HT. Though subsequent research has addressed the flaws of the WHI study and supports the use of HT in most menopausal women younger than 60 years, use of this therapy has never recovered, according to research presented at the annual meeting of The Menopause Society (formerly The North American Menopause Society).
“Despite evidence supporting the efficacy and safety of HT, usage rates of US Food and Drug Administration–approved HT remain low,” Stephanie Faubion, MD, MBA, director of the Mayo Clinic Women’s Health in Jacksonville, Florida, and medical director of The Menopause Society, told attendees. “Improved education of clinicians and patients is critically needed.”
Today, “there is more clarity on the risk/benefit ratio of HT use with the benefits typically outweighing the risks in women who initiate therapy under the age of 60 years and within 10 years of menopause onset.”
Using medical and pharmacy claims data from OptumLabs, Dr. Faubion and her colleagues examined utilization rates from 2007 to 2023 of transdermal vs oral estrogen and of conjugated estrogen vs estradiol in women aged 40 years or older. The data included more than 200 million people throughout the United States covered by commercial insurance or Medicare Advantage. The researchers defined annual rate of HT use as the proportion of women who had at least 180 days of a filled prescription for a systemic HT preparation with estrogen.
The study population increased from an estimated 2 million women in 2007 to 4.5 million women in 2023, and the average age of enrollees increased from 53 in 2007 to 66 in 2023. Starting at 4.6% in 2007, HT use steadily declined to a low of 1.8% in 2023 for the whole cohort of women aged 40 years or older.
Though rates remained highest in women aged 50-64 years, it still declined within each age group: From 6% in 2007 to 3.6% in 2023 among women aged 50-54 years, from 7.3% to 3.8% among women aged 55-59 years, and from 7.5% to 2.9% among women aged 60-64 years. It also declined in younger women, from 3.2% in 2007 to 1.5% in 2023 in those aged 45-50 years. Estradiol was the most common formulation used, and oral administration was the most common route.
The researchers also saw a gradual decline during the study period in the use of high-dose oral HT and an increase in the use of low-dose oral HT, whereas standard dosages remained fairly consistent as the most common dose prescribed. Similarly, the use of high transdermal doses declined, whereas low transdermal doses increased and surpassed the use of standard doses. Conjugated estrogen use plummeted during the study period across all age groups, from 2%-5% in most age groups to < 1% in all age groups by 2023.
One limitation of the study was that it could not examine rates of compounded HT use because those would not be reflected in insurance claims, pointed out JoAnn Pinkerton, MD, a professor of ob.gyn. at the University of Virginia in Charlottesville, Virginia, who was not involved in the study. Dr. Pinkerton found it surprising that the numbers were so low, despite the fact that research estimates suggest less than 15% of menopausal women are receiving adequate treatment, she told this news organization. “You can see there’s a large unmet need to get treatment,” she said. “All major medical societies say the same thing: For healthy, symptomatic menopausal women, you can use hormone therapy safely and effectively.”
The lack of education among providers is likely the biggest reason for the decline, Dr. Pinkerton says. “I think it’s because there’s a whole group of providers that did not receive any training, and that’s OB/GYNs, internal medicine, family practice, endocrinologists,” she said. “Now that people are starting to feel more confident that we can use it safely, we’re trying to get that training out to people about vasomotor symptoms, about hormone therapy, and now about new nonhormone therapies.”
Dr. Pinkerton noted that The Menopause Society has begun a new teaching program, Menopause Step-by-Step, aimed at providing short articles on the basics of menopause, HT, non-HT, and vaginal issues.
A separate poster presented at the conference provides insight into another potential factor contributing to low HT rates. A survey of 1050 American and Canadian women found that 90% discussed their symptoms with their healthcare providers, yet only 25% said their doctor identified the symptoms as likely due to perimenopause or menopause on their first visit — and only 10% of respondents said their doctor was the one to bring up perimenopause/menopause.
The respondents comprised a convenience sample of those who saw the survey on social media, in an email, or on the website of Morphus, a Toronto-based company aimed at providing support, information, and products related to menopause. Though the survey is ongoing, the analyzed responses are from March to May 2024.
Though 40% of the women said their provider attributed their symptoms to perimenopause or menopause on the second or third visit, 18% saw a provider four to five times, and 17% saw a provider more than five times before the provider considered menopause as a cause. About a third of the women (35%) brought it up to their doctor themselves and found their provider receptive, but 40% said the response was dismissive when they brought it up, and 15% said the topic was never broached at all.
Andrea Donsky, RHN, founder of Morphus who conducted the study, found these numbers surprising because she would have hoped that more doctors would have brought up perimenopause/menopause sooner. “We still have a lot of work to do to help educate women and healthcare providers,” Ms. Donsky told this news organization. “A lot of women spend years not knowing they’re in this phase of life, so they visit their doctors/HCPs [healthcare providers] many times because the connection isn’t made on the first visit.”
Danielle Meitiv, MS, a study co-author and health coach based in Silver Spring, Maryland, added, “Everyone wonders why we end up with Dr. Google; that’s the only doctor who’s talking to us about menopause.”
Dr. Pinkerton was less surprised by these survey findings. “As a menopause specialist, my most common new patient is a perimenopausal woman who feels like she hasn’t been listened to,” whether it’s her primary care doctor, her ob.gyn., or another clinician. “If the provider doesn’t ask or if the women doesn’t tell, then you don’t have the conversation,” Dr. Pinkerton said. “So many women in perimenopause are busy with work, families, partnerships, aging parents — all of the issues that they’re dealing with — that when they start to have sleep issues or mood issues or easy crying, they relate it to their life stressors, instead of recognizing that it’s fluctuating hormones.”
When Ms. Donsky examined the 1223 responses they had received through August 2024, the most common treatments advised for symptoms were antidepressants and HT, both recommended by 38% of providers. Other common recommendations were to “lose weight,” “eat less and exercise more,” supplements, or birth control pills.
Dr. Faubion had no disclosures, and her study used no external funding. Dr. Pinkerton has run a trial funded by Bayer and is a consultant for Bayer and Pfizer. Ms. Donsky is the owner of Morphus. Ms. Meitiv had no disclosures. The poster on women’s experiences with providers was funded by Morphus Inc.
A version of this article first appeared on Medscape.com.
CHICAGO — before the publication of the 2002 Women’s Health Initiative (WHI) study that misguidedly cast doubt on the safety of HT. Though subsequent research has addressed the flaws of the WHI study and supports the use of HT in most menopausal women younger than 60 years, use of this therapy has never recovered, according to research presented at the annual meeting of The Menopause Society (formerly The North American Menopause Society).
“Despite evidence supporting the efficacy and safety of HT, usage rates of US Food and Drug Administration–approved HT remain low,” Stephanie Faubion, MD, MBA, director of the Mayo Clinic Women’s Health in Jacksonville, Florida, and medical director of The Menopause Society, told attendees. “Improved education of clinicians and patients is critically needed.”
Today, “there is more clarity on the risk/benefit ratio of HT use with the benefits typically outweighing the risks in women who initiate therapy under the age of 60 years and within 10 years of menopause onset.”
Using medical and pharmacy claims data from OptumLabs, Dr. Faubion and her colleagues examined utilization rates from 2007 to 2023 of transdermal vs oral estrogen and of conjugated estrogen vs estradiol in women aged 40 years or older. The data included more than 200 million people throughout the United States covered by commercial insurance or Medicare Advantage. The researchers defined annual rate of HT use as the proportion of women who had at least 180 days of a filled prescription for a systemic HT preparation with estrogen.
The study population increased from an estimated 2 million women in 2007 to 4.5 million women in 2023, and the average age of enrollees increased from 53 in 2007 to 66 in 2023. Starting at 4.6% in 2007, HT use steadily declined to a low of 1.8% in 2023 for the whole cohort of women aged 40 years or older.
Though rates remained highest in women aged 50-64 years, it still declined within each age group: From 6% in 2007 to 3.6% in 2023 among women aged 50-54 years, from 7.3% to 3.8% among women aged 55-59 years, and from 7.5% to 2.9% among women aged 60-64 years. It also declined in younger women, from 3.2% in 2007 to 1.5% in 2023 in those aged 45-50 years. Estradiol was the most common formulation used, and oral administration was the most common route.
The researchers also saw a gradual decline during the study period in the use of high-dose oral HT and an increase in the use of low-dose oral HT, whereas standard dosages remained fairly consistent as the most common dose prescribed. Similarly, the use of high transdermal doses declined, whereas low transdermal doses increased and surpassed the use of standard doses. Conjugated estrogen use plummeted during the study period across all age groups, from 2%-5% in most age groups to < 1% in all age groups by 2023.
One limitation of the study was that it could not examine rates of compounded HT use because those would not be reflected in insurance claims, pointed out JoAnn Pinkerton, MD, a professor of ob.gyn. at the University of Virginia in Charlottesville, Virginia, who was not involved in the study. Dr. Pinkerton found it surprising that the numbers were so low, despite the fact that research estimates suggest less than 15% of menopausal women are receiving adequate treatment, she told this news organization. “You can see there’s a large unmet need to get treatment,” she said. “All major medical societies say the same thing: For healthy, symptomatic menopausal women, you can use hormone therapy safely and effectively.”
The lack of education among providers is likely the biggest reason for the decline, Dr. Pinkerton says. “I think it’s because there’s a whole group of providers that did not receive any training, and that’s OB/GYNs, internal medicine, family practice, endocrinologists,” she said. “Now that people are starting to feel more confident that we can use it safely, we’re trying to get that training out to people about vasomotor symptoms, about hormone therapy, and now about new nonhormone therapies.”
Dr. Pinkerton noted that The Menopause Society has begun a new teaching program, Menopause Step-by-Step, aimed at providing short articles on the basics of menopause, HT, non-HT, and vaginal issues.
A separate poster presented at the conference provides insight into another potential factor contributing to low HT rates. A survey of 1050 American and Canadian women found that 90% discussed their symptoms with their healthcare providers, yet only 25% said their doctor identified the symptoms as likely due to perimenopause or menopause on their first visit — and only 10% of respondents said their doctor was the one to bring up perimenopause/menopause.
The respondents comprised a convenience sample of those who saw the survey on social media, in an email, or on the website of Morphus, a Toronto-based company aimed at providing support, information, and products related to menopause. Though the survey is ongoing, the analyzed responses are from March to May 2024.
Though 40% of the women said their provider attributed their symptoms to perimenopause or menopause on the second or third visit, 18% saw a provider four to five times, and 17% saw a provider more than five times before the provider considered menopause as a cause. About a third of the women (35%) brought it up to their doctor themselves and found their provider receptive, but 40% said the response was dismissive when they brought it up, and 15% said the topic was never broached at all.
Andrea Donsky, RHN, founder of Morphus who conducted the study, found these numbers surprising because she would have hoped that more doctors would have brought up perimenopause/menopause sooner. “We still have a lot of work to do to help educate women and healthcare providers,” Ms. Donsky told this news organization. “A lot of women spend years not knowing they’re in this phase of life, so they visit their doctors/HCPs [healthcare providers] many times because the connection isn’t made on the first visit.”
Danielle Meitiv, MS, a study co-author and health coach based in Silver Spring, Maryland, added, “Everyone wonders why we end up with Dr. Google; that’s the only doctor who’s talking to us about menopause.”
Dr. Pinkerton was less surprised by these survey findings. “As a menopause specialist, my most common new patient is a perimenopausal woman who feels like she hasn’t been listened to,” whether it’s her primary care doctor, her ob.gyn., or another clinician. “If the provider doesn’t ask or if the women doesn’t tell, then you don’t have the conversation,” Dr. Pinkerton said. “So many women in perimenopause are busy with work, families, partnerships, aging parents — all of the issues that they’re dealing with — that when they start to have sleep issues or mood issues or easy crying, they relate it to their life stressors, instead of recognizing that it’s fluctuating hormones.”
When Ms. Donsky examined the 1223 responses they had received through August 2024, the most common treatments advised for symptoms were antidepressants and HT, both recommended by 38% of providers. Other common recommendations were to “lose weight,” “eat less and exercise more,” supplements, or birth control pills.
Dr. Faubion had no disclosures, and her study used no external funding. Dr. Pinkerton has run a trial funded by Bayer and is a consultant for Bayer and Pfizer. Ms. Donsky is the owner of Morphus. Ms. Meitiv had no disclosures. The poster on women’s experiences with providers was funded by Morphus Inc.
A version of this article first appeared on Medscape.com.
FROM THE MENOPAUSE SOCIETY 2024
Transgender Women and Prostate Cancer: It’s Complicated
The Veterans Health Administration (VHA) provides care for about 10,000 transgender women, and clinicians must understand their distinctive needs for prostate cancer screening, a urologist told cancer specialists during a presentation at the 2024 annual meeting of the Association of VA Hematology/Oncology in Atlanta.
Even if they’ve undergone gender reassignment surgery, “all transgender women still have a prostate, so therefore they remain at risk of prostate cancer and could still be considered for prostate cancer screening,” said Farnoosh Nik-Ahd, MD, a resident physician at the University of California San Francisco. However, “clinicians and patients may not be aware of prostate cancer risk, so that they may not think [of screening] transgender women.”
Nik-Ahd also noted another complication: The results of prostate screening tests may be misleading in this population.
Transgender women were born biologically male but now identify as female. These individuals may have undergone gender reassignment surgery to remove male genitalia, but the procedures do not remove the prostate. They also might be taking estrogen therapy. “Prostate cancer is a hormonally driven cancer, and the exact impact of gender-affirming hormones on prostate cancer risk and development is unknown,” Nik-Ahd said.
In a 2023 study in JAMA, Nik-Ahd and colleagues identified 155 cases of prostate cancer in transgender women within the VHA (about 14 cases per year) from 2000 to 2022. Of these patients, 116 had never used estrogen, while 17 had used it previously and 22 used it at diagnosis.
The median age of patients was 61 years, 88% identified as White, and the median prostate-specific antigen (PSA) was 6.8 ng/mL. “Given estimates of 10,000 transgender women in the US Department of Veterans Affairs, 33 cases per year would be expected. Instead, only about 14 per year were observed,” the researchers wrote. “Lower rates may stem from less PSA screening owing to barriers including lack of prostate cancer risk awareness or stigma, the suppressive effects of estrogen on prostate cancer development, or prostate cancers being missed in transgender women because of misinterpretation of ‘normal’ PSA levels among those receiving gender-affirming hormone therapies.”
In the presentation, Nik-Ahd said, “PSA density, which is a marker of prostate cancer aggressiveness, was highest in transgender women who were actively on estrogen.”
She noted, “the existing thyrotropin reference ranges, which is what we use to interpret PSA values, are all based on data from cisgender men.” The ranges would be expected to be far lower in transgender women who are taking estrogen, potentially throwing off screening tests, she said, and “ultimately missing clinically significant prostate cancer.”
In the larger picture, there are no specific guidelines about PSA screening in transgender women, she said.
A recent study published in JAMA by Nik-Ahd and colleagues examined PSA levels in 210 transgender women (mean age 60 years) treated within the VHA from 2000 to 2023. All were aged 40 to 80 years, had received estrogen for at least 6 months (mean duration 4.7 years), and didn’t have prostate cancer diagnoses.
“Median (IQR) PSA was 0.02 (0-0.2) ng/mL and the 95th percentile value was 0.6 ng/mL,” the report found. “PSAs were undetectable in 36% of patients (23% and 49% of PSAs in patients without and with orchiectomy, respectively).”
The researchers write that “the historic cut point of 4 ng/mL, often used as a threshold for further evaluation, is likely far too high a threshold for this population.”
Nik-Ahd noted, “clinicians should interpret PSA values in transgender women on estrogen with extreme caution. In this population, normal might actually not be normal, and a value that is considered normal might be very abnormal for somebody who is on estrogen. If you're unsure of whether a PSA value is appropriate for a transgender woman on estrogen, refer that patient to a urologist so they can undergo further evaluation.”
Farnoosh Nik-Ahd discloses consulting for Janssen.
The Veterans Health Administration (VHA) provides care for about 10,000 transgender women, and clinicians must understand their distinctive needs for prostate cancer screening, a urologist told cancer specialists during a presentation at the 2024 annual meeting of the Association of VA Hematology/Oncology in Atlanta.
Even if they’ve undergone gender reassignment surgery, “all transgender women still have a prostate, so therefore they remain at risk of prostate cancer and could still be considered for prostate cancer screening,” said Farnoosh Nik-Ahd, MD, a resident physician at the University of California San Francisco. However, “clinicians and patients may not be aware of prostate cancer risk, so that they may not think [of screening] transgender women.”
Nik-Ahd also noted another complication: The results of prostate screening tests may be misleading in this population.
Transgender women were born biologically male but now identify as female. These individuals may have undergone gender reassignment surgery to remove male genitalia, but the procedures do not remove the prostate. They also might be taking estrogen therapy. “Prostate cancer is a hormonally driven cancer, and the exact impact of gender-affirming hormones on prostate cancer risk and development is unknown,” Nik-Ahd said.
In a 2023 study in JAMA, Nik-Ahd and colleagues identified 155 cases of prostate cancer in transgender women within the VHA (about 14 cases per year) from 2000 to 2022. Of these patients, 116 had never used estrogen, while 17 had used it previously and 22 used it at diagnosis.
The median age of patients was 61 years, 88% identified as White, and the median prostate-specific antigen (PSA) was 6.8 ng/mL. “Given estimates of 10,000 transgender women in the US Department of Veterans Affairs, 33 cases per year would be expected. Instead, only about 14 per year were observed,” the researchers wrote. “Lower rates may stem from less PSA screening owing to barriers including lack of prostate cancer risk awareness or stigma, the suppressive effects of estrogen on prostate cancer development, or prostate cancers being missed in transgender women because of misinterpretation of ‘normal’ PSA levels among those receiving gender-affirming hormone therapies.”
In the presentation, Nik-Ahd said, “PSA density, which is a marker of prostate cancer aggressiveness, was highest in transgender women who were actively on estrogen.”
She noted, “the existing thyrotropin reference ranges, which is what we use to interpret PSA values, are all based on data from cisgender men.” The ranges would be expected to be far lower in transgender women who are taking estrogen, potentially throwing off screening tests, she said, and “ultimately missing clinically significant prostate cancer.”
In the larger picture, there are no specific guidelines about PSA screening in transgender women, she said.
A recent study published in JAMA by Nik-Ahd and colleagues examined PSA levels in 210 transgender women (mean age 60 years) treated within the VHA from 2000 to 2023. All were aged 40 to 80 years, had received estrogen for at least 6 months (mean duration 4.7 years), and didn’t have prostate cancer diagnoses.
“Median (IQR) PSA was 0.02 (0-0.2) ng/mL and the 95th percentile value was 0.6 ng/mL,” the report found. “PSAs were undetectable in 36% of patients (23% and 49% of PSAs in patients without and with orchiectomy, respectively).”
The researchers write that “the historic cut point of 4 ng/mL, often used as a threshold for further evaluation, is likely far too high a threshold for this population.”
Nik-Ahd noted, “clinicians should interpret PSA values in transgender women on estrogen with extreme caution. In this population, normal might actually not be normal, and a value that is considered normal might be very abnormal for somebody who is on estrogen. If you're unsure of whether a PSA value is appropriate for a transgender woman on estrogen, refer that patient to a urologist so they can undergo further evaluation.”
Farnoosh Nik-Ahd discloses consulting for Janssen.
The Veterans Health Administration (VHA) provides care for about 10,000 transgender women, and clinicians must understand their distinctive needs for prostate cancer screening, a urologist told cancer specialists during a presentation at the 2024 annual meeting of the Association of VA Hematology/Oncology in Atlanta.
Even if they’ve undergone gender reassignment surgery, “all transgender women still have a prostate, so therefore they remain at risk of prostate cancer and could still be considered for prostate cancer screening,” said Farnoosh Nik-Ahd, MD, a resident physician at the University of California San Francisco. However, “clinicians and patients may not be aware of prostate cancer risk, so that they may not think [of screening] transgender women.”
Nik-Ahd also noted another complication: The results of prostate screening tests may be misleading in this population.
Transgender women were born biologically male but now identify as female. These individuals may have undergone gender reassignment surgery to remove male genitalia, but the procedures do not remove the prostate. They also might be taking estrogen therapy. “Prostate cancer is a hormonally driven cancer, and the exact impact of gender-affirming hormones on prostate cancer risk and development is unknown,” Nik-Ahd said.
In a 2023 study in JAMA, Nik-Ahd and colleagues identified 155 cases of prostate cancer in transgender women within the VHA (about 14 cases per year) from 2000 to 2022. Of these patients, 116 had never used estrogen, while 17 had used it previously and 22 used it at diagnosis.
The median age of patients was 61 years, 88% identified as White, and the median prostate-specific antigen (PSA) was 6.8 ng/mL. “Given estimates of 10,000 transgender women in the US Department of Veterans Affairs, 33 cases per year would be expected. Instead, only about 14 per year were observed,” the researchers wrote. “Lower rates may stem from less PSA screening owing to barriers including lack of prostate cancer risk awareness or stigma, the suppressive effects of estrogen on prostate cancer development, or prostate cancers being missed in transgender women because of misinterpretation of ‘normal’ PSA levels among those receiving gender-affirming hormone therapies.”
In the presentation, Nik-Ahd said, “PSA density, which is a marker of prostate cancer aggressiveness, was highest in transgender women who were actively on estrogen.”
She noted, “the existing thyrotropin reference ranges, which is what we use to interpret PSA values, are all based on data from cisgender men.” The ranges would be expected to be far lower in transgender women who are taking estrogen, potentially throwing off screening tests, she said, and “ultimately missing clinically significant prostate cancer.”
In the larger picture, there are no specific guidelines about PSA screening in transgender women, she said.
A recent study published in JAMA by Nik-Ahd and colleagues examined PSA levels in 210 transgender women (mean age 60 years) treated within the VHA from 2000 to 2023. All were aged 40 to 80 years, had received estrogen for at least 6 months (mean duration 4.7 years), and didn’t have prostate cancer diagnoses.
“Median (IQR) PSA was 0.02 (0-0.2) ng/mL and the 95th percentile value was 0.6 ng/mL,” the report found. “PSAs were undetectable in 36% of patients (23% and 49% of PSAs in patients without and with orchiectomy, respectively).”
The researchers write that “the historic cut point of 4 ng/mL, often used as a threshold for further evaluation, is likely far too high a threshold for this population.”
Nik-Ahd noted, “clinicians should interpret PSA values in transgender women on estrogen with extreme caution. In this population, normal might actually not be normal, and a value that is considered normal might be very abnormal for somebody who is on estrogen. If you're unsure of whether a PSA value is appropriate for a transgender woman on estrogen, refer that patient to a urologist so they can undergo further evaluation.”
Farnoosh Nik-Ahd discloses consulting for Janssen.
Are You Using the Correct Medication or a Look-Alike?
Five years have passed since the member states of the World Health Organization (WHO) gathered at the 72nd World Health Assembly and decided that September 17 should be recognized as World Patient Safety Day, acknowledging it as a global health priority.
WHO data indicate the following findings related to medical safety:
- One in 10 patients is harmed while receiving healthcare, and 3 million die as a result.
- More than half of these incidents could be prevented.
- Indirect costs could amount to several billion US dollars annually.
Given the magnitude of preventable harm related to medication use, in 2017, the WHO launched the third Global Patient Safety Challenge: Medication Without Harm with the goal of reducing serious and preventable harm related to medication by 50%. In addition, considering the volume of medication packages prescribed in 2023 by physicians in Spain’s National Health System, it is necessary to understand the most common types of medication errors to provide an effective and efficient response.
According to Spain’s Institute for Safe Medication Practices (ISMP), the 10 types of medication errors detected in 2020 with the most serious consequences were the following:
- Errors due to omission or delay in medication.
- Administration of medication to the wrong patient.
- Errors related to allergies or known adverse effects of medications.
- Dosing errors in pediatric patients.
- Errors due to similarities in the labeling or packaging of marketed medications.
- Errors associated with the lack of use of smart infusion pumps.
- Errors due to accidental administration of neuromuscular blocking agents.
- Incorrect intravenous administration of oral liquid medications.
- Errors in medication reconciliation upon hospital admission and discharge.
- Errors due to patient misunderstandings regarding medication use.
I would like to focus on the fifth item, errors due to similarities in the labeling or packaging of marketed medications.
Medications with similar names or with similar labeling or packaging are known as “look alike–sound alike” medications. They are estimated to account for between 6.2% and 14.7% of all medication errors. Confusion can arise due to spelling and phonetic similarities.
As shown in bulletin no. 50 of the ISMP, difficulties in distinguishing different medications or different presentations of the same medication due to similar packaging and labeling have frequently been associated with reported incidents.
Most cases involve either medications marketed by the same laboratory with a design based on brand image or different medications marketed by different laboratories in screen-printed ampoules used in the same settings.
In 2020, the ISMP published 11 new cases of labeling or packaging that may promote errors on its website. It reported 49 incidents to the Spanish Agency for Medicines and Medical Devices.
Shortages caused by the COVID-19 pandemic have further contributed to these incidents, as healthcare facilities sometimes had to change the medications they usually acquired and purchase whatever was available, without being able to select products that would not be confused with existing medications in the facility.
The ISMP recommends the following general practices for healthcare institutions, professionals, and patients to prevent these errors:
- Develop short lists of easily confused medication names and distribute them among all healthcare professionals.
- Prioritize medication names by active ingredient instead of brand name.
- For similar names, highlight the differences in capital letters, eg, DOBUTamine, DOPamine.
- For similar active ingredients, use brand names.
- Avoid placing similar medications near each other.
- Prescribe all medications electronically to minimize the risk of selecting the wrong medication.
- Make manual prescriptions legible, with clearly written dosages and pharmaceutical forms.
- Encourage patients to actively participate in their treatment and consult a clinician if they have any questions about the medications they are receiving.
- Raise awareness among patients, family members, and caregivers about the issues caused by medication name confusion and inform them about how to avoid these errors.
- Instruct patients to focus on and always use the active ingredient name as an identifying element for the medications they are taking.
- Review treatments with patients to ensure they know the medications they are taking.
Julia María Ruiz Redondo is the regional nursing advisor inspector of Spanish Society of General and Family Physicians of Castilla-La Mancha (SEMG-CLM), coordinator of the National Working Group on Public Health in the SEMG, and director of the international public health master’s degree at TECH Technological University. This article is the result of an editorial collaboration between the SEMG and Univadis, which you can access here.
This story was translated from Univadis Spain, which is part of the Medscape professional network, using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
Five years have passed since the member states of the World Health Organization (WHO) gathered at the 72nd World Health Assembly and decided that September 17 should be recognized as World Patient Safety Day, acknowledging it as a global health priority.
WHO data indicate the following findings related to medical safety:
- One in 10 patients is harmed while receiving healthcare, and 3 million die as a result.
- More than half of these incidents could be prevented.
- Indirect costs could amount to several billion US dollars annually.
Given the magnitude of preventable harm related to medication use, in 2017, the WHO launched the third Global Patient Safety Challenge: Medication Without Harm with the goal of reducing serious and preventable harm related to medication by 50%. In addition, considering the volume of medication packages prescribed in 2023 by physicians in Spain’s National Health System, it is necessary to understand the most common types of medication errors to provide an effective and efficient response.
According to Spain’s Institute for Safe Medication Practices (ISMP), the 10 types of medication errors detected in 2020 with the most serious consequences were the following:
- Errors due to omission or delay in medication.
- Administration of medication to the wrong patient.
- Errors related to allergies or known adverse effects of medications.
- Dosing errors in pediatric patients.
- Errors due to similarities in the labeling or packaging of marketed medications.
- Errors associated with the lack of use of smart infusion pumps.
- Errors due to accidental administration of neuromuscular blocking agents.
- Incorrect intravenous administration of oral liquid medications.
- Errors in medication reconciliation upon hospital admission and discharge.
- Errors due to patient misunderstandings regarding medication use.
I would like to focus on the fifth item, errors due to similarities in the labeling or packaging of marketed medications.
Medications with similar names or with similar labeling or packaging are known as “look alike–sound alike” medications. They are estimated to account for between 6.2% and 14.7% of all medication errors. Confusion can arise due to spelling and phonetic similarities.
As shown in bulletin no. 50 of the ISMP, difficulties in distinguishing different medications or different presentations of the same medication due to similar packaging and labeling have frequently been associated with reported incidents.
Most cases involve either medications marketed by the same laboratory with a design based on brand image or different medications marketed by different laboratories in screen-printed ampoules used in the same settings.
In 2020, the ISMP published 11 new cases of labeling or packaging that may promote errors on its website. It reported 49 incidents to the Spanish Agency for Medicines and Medical Devices.
Shortages caused by the COVID-19 pandemic have further contributed to these incidents, as healthcare facilities sometimes had to change the medications they usually acquired and purchase whatever was available, without being able to select products that would not be confused with existing medications in the facility.
The ISMP recommends the following general practices for healthcare institutions, professionals, and patients to prevent these errors:
- Develop short lists of easily confused medication names and distribute them among all healthcare professionals.
- Prioritize medication names by active ingredient instead of brand name.
- For similar names, highlight the differences in capital letters, eg, DOBUTamine, DOPamine.
- For similar active ingredients, use brand names.
- Avoid placing similar medications near each other.
- Prescribe all medications electronically to minimize the risk of selecting the wrong medication.
- Make manual prescriptions legible, with clearly written dosages and pharmaceutical forms.
- Encourage patients to actively participate in their treatment and consult a clinician if they have any questions about the medications they are receiving.
- Raise awareness among patients, family members, and caregivers about the issues caused by medication name confusion and inform them about how to avoid these errors.
- Instruct patients to focus on and always use the active ingredient name as an identifying element for the medications they are taking.
- Review treatments with patients to ensure they know the medications they are taking.
Julia María Ruiz Redondo is the regional nursing advisor inspector of Spanish Society of General and Family Physicians of Castilla-La Mancha (SEMG-CLM), coordinator of the National Working Group on Public Health in the SEMG, and director of the international public health master’s degree at TECH Technological University. This article is the result of an editorial collaboration between the SEMG and Univadis, which you can access here.
This story was translated from Univadis Spain, which is part of the Medscape professional network, using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
Five years have passed since the member states of the World Health Organization (WHO) gathered at the 72nd World Health Assembly and decided that September 17 should be recognized as World Patient Safety Day, acknowledging it as a global health priority.
WHO data indicate the following findings related to medical safety:
- One in 10 patients is harmed while receiving healthcare, and 3 million die as a result.
- More than half of these incidents could be prevented.
- Indirect costs could amount to several billion US dollars annually.
Given the magnitude of preventable harm related to medication use, in 2017, the WHO launched the third Global Patient Safety Challenge: Medication Without Harm with the goal of reducing serious and preventable harm related to medication by 50%. In addition, considering the volume of medication packages prescribed in 2023 by physicians in Spain’s National Health System, it is necessary to understand the most common types of medication errors to provide an effective and efficient response.
According to Spain’s Institute for Safe Medication Practices (ISMP), the 10 types of medication errors detected in 2020 with the most serious consequences were the following:
- Errors due to omission or delay in medication.
- Administration of medication to the wrong patient.
- Errors related to allergies or known adverse effects of medications.
- Dosing errors in pediatric patients.
- Errors due to similarities in the labeling or packaging of marketed medications.
- Errors associated with the lack of use of smart infusion pumps.
- Errors due to accidental administration of neuromuscular blocking agents.
- Incorrect intravenous administration of oral liquid medications.
- Errors in medication reconciliation upon hospital admission and discharge.
- Errors due to patient misunderstandings regarding medication use.
I would like to focus on the fifth item, errors due to similarities in the labeling or packaging of marketed medications.
Medications with similar names or with similar labeling or packaging are known as “look alike–sound alike” medications. They are estimated to account for between 6.2% and 14.7% of all medication errors. Confusion can arise due to spelling and phonetic similarities.
As shown in bulletin no. 50 of the ISMP, difficulties in distinguishing different medications or different presentations of the same medication due to similar packaging and labeling have frequently been associated with reported incidents.
Most cases involve either medications marketed by the same laboratory with a design based on brand image or different medications marketed by different laboratories in screen-printed ampoules used in the same settings.
In 2020, the ISMP published 11 new cases of labeling or packaging that may promote errors on its website. It reported 49 incidents to the Spanish Agency for Medicines and Medical Devices.
Shortages caused by the COVID-19 pandemic have further contributed to these incidents, as healthcare facilities sometimes had to change the medications they usually acquired and purchase whatever was available, without being able to select products that would not be confused with existing medications in the facility.
The ISMP recommends the following general practices for healthcare institutions, professionals, and patients to prevent these errors:
- Develop short lists of easily confused medication names and distribute them among all healthcare professionals.
- Prioritize medication names by active ingredient instead of brand name.
- For similar names, highlight the differences in capital letters, eg, DOBUTamine, DOPamine.
- For similar active ingredients, use brand names.
- Avoid placing similar medications near each other.
- Prescribe all medications electronically to minimize the risk of selecting the wrong medication.
- Make manual prescriptions legible, with clearly written dosages and pharmaceutical forms.
- Encourage patients to actively participate in their treatment and consult a clinician if they have any questions about the medications they are receiving.
- Raise awareness among patients, family members, and caregivers about the issues caused by medication name confusion and inform them about how to avoid these errors.
- Instruct patients to focus on and always use the active ingredient name as an identifying element for the medications they are taking.
- Review treatments with patients to ensure they know the medications they are taking.
Julia María Ruiz Redondo is the regional nursing advisor inspector of Spanish Society of General and Family Physicians of Castilla-La Mancha (SEMG-CLM), coordinator of the National Working Group on Public Health in the SEMG, and director of the international public health master’s degree at TECH Technological University. This article is the result of an editorial collaboration between the SEMG and Univadis, which you can access here.
This story was translated from Univadis Spain, which is part of the Medscape professional network, using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
Cancer Risk: Are Pesticides the New Smoking?
Pesticides have transformed modern agriculture by boosting production yields and helping alleviate food insecurity amid rapid global population growth. However, from a public health perspective, exposure to pesticides has been linked to numerous harmful effects, including neurologic disorders like Parkinson’s disease, weakened immune function, and an increased risk for cancer.
thus offering a limited perspective.
A comprehensive assessment of how pesticide use affects cancer risk across a broader population has yet to be conducted.
A recent population-level study aimed to address this gap by evaluating cancer risks in the US population using a model that accounts for pesticide use and adjusts for various factors. The goal was to identify regional disparities in exposure and contribute to the development of public health policies that protect populations from potential harm.
Calculating Cancer Risk
Researchers developed a model using several data sources to estimate the additional cancer risk from agricultural pesticide use. Key data included:
- Pesticide use data from the US Geological Survey in 2019, which covered 69 agricultural pesticides across 3143 counties
- Cancer incidence rates per 100,000 people, which were collected between 2015 and 2019 by the National Institutes of Health and the Centers for Disease Control and Prevention; these data covered various cancers, including bladder, colorectal, leukemia, lung, non-Hodgkin lymphoma, and pancreatic cancers
- Covariates, including smoking prevalence, the Social Vulnerability Index, agricultural land use, and total US population in 2019
Pesticide use profile patterns were developed using latent class analysis, a statistical method used to identify homogeneous subgroups within a heterogeneous population. A generalized linear model then estimated how these pesticide use patterns and the covariates affected cancer incidence.
The model highlighted regions with the highest and lowest “additional” cancer risks linked to pesticide exposure, calculating the estimated increase in cancer cases per year that resulted from variations in agricultural pesticide use.
Midwest Most Affected
While this model doesn’t establish causality or assess individual risk, it reveals regional trends in the association between pesticide use patterns and cancer incidence from a population-based perspective.
The Midwest, known for its high corn production, emerged as the region most affected by pesticide use. Compared with regions with the lowest risk, the Midwest faced an additional 154,541 cancer cases annually across all types. For colorectal and pancreatic cancers, the yearly increases were 20,927 and 3835 cases, respectively. Similar trends were observed for leukemia and non-Hodgkin lymphoma.
Pesticides vs Smoking
The researchers also estimated the additional cancer risk related to smoking, using the same model. They found that pesticides contributed to a higher risk for cancer than smoking in several cases.
The most significant difference was observed with non-Hodgkin lymphoma, where pesticides were linked to 154.1% more cases than smoking. For all cancers combined, as well as bladder cancer and leukemia, the increases were moderate: 18.7%, 19.3%, and 21.0%, respectively.
This result highlights the importance of considering pesticide exposure alongside smoking when studying cancer risks.
Expanding Scope of Research
Some limitations of this study should be noted. Certain counties lacked complete data, and there was heterogeneity in the size and population of the counties studied. The research also did not account for seasonal and migrant workers, who are likely to be heavily exposed. In addition, the data used in the study were not independently validated, and they could not be used to assess individual risk.
The effect of pesticides on human health is a vast and critical field of research, often focusing on a limited range of pesticides or specific cancers. This study stands out by taking a broader, more holistic approach, aiming to highlight regional inequalities and identify less-studied pesticides that could be future research priorities.
Given the significant public health impact, the authors encouraged the authorities to share these findings with the most vulnerable communities to raise awareness.
This story was translated from JIM using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
Pesticides have transformed modern agriculture by boosting production yields and helping alleviate food insecurity amid rapid global population growth. However, from a public health perspective, exposure to pesticides has been linked to numerous harmful effects, including neurologic disorders like Parkinson’s disease, weakened immune function, and an increased risk for cancer.
thus offering a limited perspective.
A comprehensive assessment of how pesticide use affects cancer risk across a broader population has yet to be conducted.
A recent population-level study aimed to address this gap by evaluating cancer risks in the US population using a model that accounts for pesticide use and adjusts for various factors. The goal was to identify regional disparities in exposure and contribute to the development of public health policies that protect populations from potential harm.
Calculating Cancer Risk
Researchers developed a model using several data sources to estimate the additional cancer risk from agricultural pesticide use. Key data included:
- Pesticide use data from the US Geological Survey in 2019, which covered 69 agricultural pesticides across 3143 counties
- Cancer incidence rates per 100,000 people, which were collected between 2015 and 2019 by the National Institutes of Health and the Centers for Disease Control and Prevention; these data covered various cancers, including bladder, colorectal, leukemia, lung, non-Hodgkin lymphoma, and pancreatic cancers
- Covariates, including smoking prevalence, the Social Vulnerability Index, agricultural land use, and total US population in 2019
Pesticide use profile patterns were developed using latent class analysis, a statistical method used to identify homogeneous subgroups within a heterogeneous population. A generalized linear model then estimated how these pesticide use patterns and the covariates affected cancer incidence.
The model highlighted regions with the highest and lowest “additional” cancer risks linked to pesticide exposure, calculating the estimated increase in cancer cases per year that resulted from variations in agricultural pesticide use.
Midwest Most Affected
While this model doesn’t establish causality or assess individual risk, it reveals regional trends in the association between pesticide use patterns and cancer incidence from a population-based perspective.
The Midwest, known for its high corn production, emerged as the region most affected by pesticide use. Compared with regions with the lowest risk, the Midwest faced an additional 154,541 cancer cases annually across all types. For colorectal and pancreatic cancers, the yearly increases were 20,927 and 3835 cases, respectively. Similar trends were observed for leukemia and non-Hodgkin lymphoma.
Pesticides vs Smoking
The researchers also estimated the additional cancer risk related to smoking, using the same model. They found that pesticides contributed to a higher risk for cancer than smoking in several cases.
The most significant difference was observed with non-Hodgkin lymphoma, where pesticides were linked to 154.1% more cases than smoking. For all cancers combined, as well as bladder cancer and leukemia, the increases were moderate: 18.7%, 19.3%, and 21.0%, respectively.
This result highlights the importance of considering pesticide exposure alongside smoking when studying cancer risks.
Expanding Scope of Research
Some limitations of this study should be noted. Certain counties lacked complete data, and there was heterogeneity in the size and population of the counties studied. The research also did not account for seasonal and migrant workers, who are likely to be heavily exposed. In addition, the data used in the study were not independently validated, and they could not be used to assess individual risk.
The effect of pesticides on human health is a vast and critical field of research, often focusing on a limited range of pesticides or specific cancers. This study stands out by taking a broader, more holistic approach, aiming to highlight regional inequalities and identify less-studied pesticides that could be future research priorities.
Given the significant public health impact, the authors encouraged the authorities to share these findings with the most vulnerable communities to raise awareness.
This story was translated from JIM using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
Pesticides have transformed modern agriculture by boosting production yields and helping alleviate food insecurity amid rapid global population growth. However, from a public health perspective, exposure to pesticides has been linked to numerous harmful effects, including neurologic disorders like Parkinson’s disease, weakened immune function, and an increased risk for cancer.
thus offering a limited perspective.
A comprehensive assessment of how pesticide use affects cancer risk across a broader population has yet to be conducted.
A recent population-level study aimed to address this gap by evaluating cancer risks in the US population using a model that accounts for pesticide use and adjusts for various factors. The goal was to identify regional disparities in exposure and contribute to the development of public health policies that protect populations from potential harm.
Calculating Cancer Risk
Researchers developed a model using several data sources to estimate the additional cancer risk from agricultural pesticide use. Key data included:
- Pesticide use data from the US Geological Survey in 2019, which covered 69 agricultural pesticides across 3143 counties
- Cancer incidence rates per 100,000 people, which were collected between 2015 and 2019 by the National Institutes of Health and the Centers for Disease Control and Prevention; these data covered various cancers, including bladder, colorectal, leukemia, lung, non-Hodgkin lymphoma, and pancreatic cancers
- Covariates, including smoking prevalence, the Social Vulnerability Index, agricultural land use, and total US population in 2019
Pesticide use profile patterns were developed using latent class analysis, a statistical method used to identify homogeneous subgroups within a heterogeneous population. A generalized linear model then estimated how these pesticide use patterns and the covariates affected cancer incidence.
The model highlighted regions with the highest and lowest “additional” cancer risks linked to pesticide exposure, calculating the estimated increase in cancer cases per year that resulted from variations in agricultural pesticide use.
Midwest Most Affected
While this model doesn’t establish causality or assess individual risk, it reveals regional trends in the association between pesticide use patterns and cancer incidence from a population-based perspective.
The Midwest, known for its high corn production, emerged as the region most affected by pesticide use. Compared with regions with the lowest risk, the Midwest faced an additional 154,541 cancer cases annually across all types. For colorectal and pancreatic cancers, the yearly increases were 20,927 and 3835 cases, respectively. Similar trends were observed for leukemia and non-Hodgkin lymphoma.
Pesticides vs Smoking
The researchers also estimated the additional cancer risk related to smoking, using the same model. They found that pesticides contributed to a higher risk for cancer than smoking in several cases.
The most significant difference was observed with non-Hodgkin lymphoma, where pesticides were linked to 154.1% more cases than smoking. For all cancers combined, as well as bladder cancer and leukemia, the increases were moderate: 18.7%, 19.3%, and 21.0%, respectively.
This result highlights the importance of considering pesticide exposure alongside smoking when studying cancer risks.
Expanding Scope of Research
Some limitations of this study should be noted. Certain counties lacked complete data, and there was heterogeneity in the size and population of the counties studied. The research also did not account for seasonal and migrant workers, who are likely to be heavily exposed. In addition, the data used in the study were not independently validated, and they could not be used to assess individual risk.
The effect of pesticides on human health is a vast and critical field of research, often focusing on a limited range of pesticides or specific cancers. This study stands out by taking a broader, more holistic approach, aiming to highlight regional inequalities and identify less-studied pesticides that could be future research priorities.
Given the significant public health impact, the authors encouraged the authorities to share these findings with the most vulnerable communities to raise awareness.
This story was translated from JIM using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
Semaglutide Bests Liraglutide in Long-Term Weight Loss
Patients with obesity or type 2 diabetes (T2D) who stuck with their medication for a year lost more weight with semaglutide than with liraglutide, a new study reported.
Researchers at the Cleveland Clinic reviewed records for 3389 adult patients with obesity who were prescribed one of the glucagon-like peptide 1 (GLP-1) medications for either T2D or obesity between 2015 and 2022. They found that patients who took either semaglutide or liraglutide for obesity were more likely to lose weight than those prescribed the medications for T2D and that semaglutide was associated with greater weight loss.
The study, published in JAMA Network Open, identified “key characteristics that could inform the probability of achieving sustained weight loss of a magnitude large enough to provide clinically significant health benefits,” said lead author Hamlet Gasoyan, PhD, a staff investigator at the Center for Value-Based Care Research in the Department of Internal Medicine of Primary Care Institute, Cleveland Clinic, Cleveland.
Only about 40% of patients continued to take the medications at 1 year. Those who did not continue did not achieve the same level of weight loss, Dr. Gasoyan told this news organization. He and his colleagues will study the factors that lead patients to stop taking the medications in a future paper.
The results from the current paper give patients and clinicians reasonable expectations on the trajectory of weight loss when the drugs are prescribed for diabetes vs obesity, said Dr. Gasoyan, assistant professor of medicine at Cleveland Clinic Lerner College of Medicine of Case Western Reserve University, Cleveland.
Semaglutide Superior
Because of the study’s timeframe, the majority of GLP-1s were prescribed for T2D. Liraglutide was approved (as Saxenda) for obesity in December 2020 and semaglutide (as Wegovy) for obesity in June 2021.
The authors were able to capture fills under the brand names and doses approved by the US Food and Drug Administration (FDA) for obesity (Wegovy, 1.7 or 2.4 mg; Saxenda, 3.0 mg), as well as those approved for T2D (Ozempic, 0.5, 1.0, or 2.0 mg; Victoza, 1.2 or 1.8 mg).
The researchers reported that among the 3389 patients, 1341 (39.6%) were prescribed semaglutide and 1444 (42.6%) were prescribed liraglutide for T2D. For obesity, 227 (6.7%) were prescribed liraglutide, and 377 (11.1%) were prescribed semaglutide.
Overall, those with diabetes had a −3.2% mean weight change compared with those with obesity who had a −5.9% mean weight change.
Semaglutide consistently outperformed liraglutide, particularly in obesity.
Overall, at 1 year, the mean percentage weight change among those with obesity was −5.1% with semaglutide compared with −2.2% with liraglutide (P < .001).
At 1 year, among those with obesity who were persistent in semaglutide use (defined as 90-275 medication days) had a mean body weight of −12.9% vs −5.6% in those taking liraglutide.
Overall, about 40% of patients were persistent at 1 year. But the figure was higher for semaglutide (45.8%) and lower for liraglutide (35.6%).
Liraglutide requires daily injections compared with semaglutide that requires weekly injections. The authors did not study the reasons for medication adherence or discontinuation.
Key factors for achieving a greater than 10% weight loss — considered clinically meaningful — included taking semaglutide, receiving a GLP-1 for obesity, persistent medication use, high dosage, and being female.
Real-World Data Welcomed
Michael Weintraub, MD, an obesity medicine specialist and clinical assistant professor at NYU Langone Health, New York City, said that having real-world data on GLP-1 effectiveness has been much needed.
The researchers “did a really good job at stratifying these patients,” he told this news organization, saying that the study “adds to the literature in terms of what we might expect and what things we should look out for when we want to obtain the maximum degree of weight loss and attain overall better metabolic health for our patients.”
One strength: The researchers were able to capture when someone actually filled a prescription, he said. Clinicians don’t always know whether a prescription for a GLP-1 has been filled because patients might go without the drug because of insurance hurdles or supply issues, he said.
Dr. Weintraub was not surprised that the study showed that both GLP-1s produced more weight loss in those with obesity than in those with T2D, as that has become a common finding. No one has been able to explain why there is such a difference, said Dr. Weintraub. “As a field, we actually don’t know the reason behind that yet,” he said.
Given the small number of patients prescribed semaglutide for obesity, that “limits the generalizability,” he said.
Even so, semaglutide is increasingly proving superior, Dr. Weintraub said. “I would reach towards semaglutide every time either for individuals with type 2 diabetes or individuals with obesity,” he said. “The major limitation, though, is insurance coverage rather than, unfortunately, my clinical decision-making.”
He also still sees a role for liraglutide. It will go off patent soon and that could “lead to a lower price point and hopefully greater access for patients,” he said.
Dr. Gasoyan and Dr. Weintraub reported no relevant financial relationships. One coauthor reported receiving advisory board fees from Novo Nordisk and research funding from Eli Lilly during the conduct of the study.
A version of this article first appeared on Medscape.com.
Patients with obesity or type 2 diabetes (T2D) who stuck with their medication for a year lost more weight with semaglutide than with liraglutide, a new study reported.
Researchers at the Cleveland Clinic reviewed records for 3389 adult patients with obesity who were prescribed one of the glucagon-like peptide 1 (GLP-1) medications for either T2D or obesity between 2015 and 2022. They found that patients who took either semaglutide or liraglutide for obesity were more likely to lose weight than those prescribed the medications for T2D and that semaglutide was associated with greater weight loss.
The study, published in JAMA Network Open, identified “key characteristics that could inform the probability of achieving sustained weight loss of a magnitude large enough to provide clinically significant health benefits,” said lead author Hamlet Gasoyan, PhD, a staff investigator at the Center for Value-Based Care Research in the Department of Internal Medicine of Primary Care Institute, Cleveland Clinic, Cleveland.
Only about 40% of patients continued to take the medications at 1 year. Those who did not continue did not achieve the same level of weight loss, Dr. Gasoyan told this news organization. He and his colleagues will study the factors that lead patients to stop taking the medications in a future paper.
The results from the current paper give patients and clinicians reasonable expectations on the trajectory of weight loss when the drugs are prescribed for diabetes vs obesity, said Dr. Gasoyan, assistant professor of medicine at Cleveland Clinic Lerner College of Medicine of Case Western Reserve University, Cleveland.
Semaglutide Superior
Because of the study’s timeframe, the majority of GLP-1s were prescribed for T2D. Liraglutide was approved (as Saxenda) for obesity in December 2020 and semaglutide (as Wegovy) for obesity in June 2021.
The authors were able to capture fills under the brand names and doses approved by the US Food and Drug Administration (FDA) for obesity (Wegovy, 1.7 or 2.4 mg; Saxenda, 3.0 mg), as well as those approved for T2D (Ozempic, 0.5, 1.0, or 2.0 mg; Victoza, 1.2 or 1.8 mg).
The researchers reported that among the 3389 patients, 1341 (39.6%) were prescribed semaglutide and 1444 (42.6%) were prescribed liraglutide for T2D. For obesity, 227 (6.7%) were prescribed liraglutide, and 377 (11.1%) were prescribed semaglutide.
Overall, those with diabetes had a −3.2% mean weight change compared with those with obesity who had a −5.9% mean weight change.
Semaglutide consistently outperformed liraglutide, particularly in obesity.
Overall, at 1 year, the mean percentage weight change among those with obesity was −5.1% with semaglutide compared with −2.2% with liraglutide (P < .001).
At 1 year, among those with obesity who were persistent in semaglutide use (defined as 90-275 medication days) had a mean body weight of −12.9% vs −5.6% in those taking liraglutide.
Overall, about 40% of patients were persistent at 1 year. But the figure was higher for semaglutide (45.8%) and lower for liraglutide (35.6%).
Liraglutide requires daily injections compared with semaglutide that requires weekly injections. The authors did not study the reasons for medication adherence or discontinuation.
Key factors for achieving a greater than 10% weight loss — considered clinically meaningful — included taking semaglutide, receiving a GLP-1 for obesity, persistent medication use, high dosage, and being female.
Real-World Data Welcomed
Michael Weintraub, MD, an obesity medicine specialist and clinical assistant professor at NYU Langone Health, New York City, said that having real-world data on GLP-1 effectiveness has been much needed.
The researchers “did a really good job at stratifying these patients,” he told this news organization, saying that the study “adds to the literature in terms of what we might expect and what things we should look out for when we want to obtain the maximum degree of weight loss and attain overall better metabolic health for our patients.”
One strength: The researchers were able to capture when someone actually filled a prescription, he said. Clinicians don’t always know whether a prescription for a GLP-1 has been filled because patients might go without the drug because of insurance hurdles or supply issues, he said.
Dr. Weintraub was not surprised that the study showed that both GLP-1s produced more weight loss in those with obesity than in those with T2D, as that has become a common finding. No one has been able to explain why there is such a difference, said Dr. Weintraub. “As a field, we actually don’t know the reason behind that yet,” he said.
Given the small number of patients prescribed semaglutide for obesity, that “limits the generalizability,” he said.
Even so, semaglutide is increasingly proving superior, Dr. Weintraub said. “I would reach towards semaglutide every time either for individuals with type 2 diabetes or individuals with obesity,” he said. “The major limitation, though, is insurance coverage rather than, unfortunately, my clinical decision-making.”
He also still sees a role for liraglutide. It will go off patent soon and that could “lead to a lower price point and hopefully greater access for patients,” he said.
Dr. Gasoyan and Dr. Weintraub reported no relevant financial relationships. One coauthor reported receiving advisory board fees from Novo Nordisk and research funding from Eli Lilly during the conduct of the study.
A version of this article first appeared on Medscape.com.
Patients with obesity or type 2 diabetes (T2D) who stuck with their medication for a year lost more weight with semaglutide than with liraglutide, a new study reported.
Researchers at the Cleveland Clinic reviewed records for 3389 adult patients with obesity who were prescribed one of the glucagon-like peptide 1 (GLP-1) medications for either T2D or obesity between 2015 and 2022. They found that patients who took either semaglutide or liraglutide for obesity were more likely to lose weight than those prescribed the medications for T2D and that semaglutide was associated with greater weight loss.
The study, published in JAMA Network Open, identified “key characteristics that could inform the probability of achieving sustained weight loss of a magnitude large enough to provide clinically significant health benefits,” said lead author Hamlet Gasoyan, PhD, a staff investigator at the Center for Value-Based Care Research in the Department of Internal Medicine of Primary Care Institute, Cleveland Clinic, Cleveland.
Only about 40% of patients continued to take the medications at 1 year. Those who did not continue did not achieve the same level of weight loss, Dr. Gasoyan told this news organization. He and his colleagues will study the factors that lead patients to stop taking the medications in a future paper.
The results from the current paper give patients and clinicians reasonable expectations on the trajectory of weight loss when the drugs are prescribed for diabetes vs obesity, said Dr. Gasoyan, assistant professor of medicine at Cleveland Clinic Lerner College of Medicine of Case Western Reserve University, Cleveland.
Semaglutide Superior
Because of the study’s timeframe, the majority of GLP-1s were prescribed for T2D. Liraglutide was approved (as Saxenda) for obesity in December 2020 and semaglutide (as Wegovy) for obesity in June 2021.
The authors were able to capture fills under the brand names and doses approved by the US Food and Drug Administration (FDA) for obesity (Wegovy, 1.7 or 2.4 mg; Saxenda, 3.0 mg), as well as those approved for T2D (Ozempic, 0.5, 1.0, or 2.0 mg; Victoza, 1.2 or 1.8 mg).
The researchers reported that among the 3389 patients, 1341 (39.6%) were prescribed semaglutide and 1444 (42.6%) were prescribed liraglutide for T2D. For obesity, 227 (6.7%) were prescribed liraglutide, and 377 (11.1%) were prescribed semaglutide.
Overall, those with diabetes had a −3.2% mean weight change compared with those with obesity who had a −5.9% mean weight change.
Semaglutide consistently outperformed liraglutide, particularly in obesity.
Overall, at 1 year, the mean percentage weight change among those with obesity was −5.1% with semaglutide compared with −2.2% with liraglutide (P < .001).
At 1 year, among those with obesity who were persistent in semaglutide use (defined as 90-275 medication days) had a mean body weight of −12.9% vs −5.6% in those taking liraglutide.
Overall, about 40% of patients were persistent at 1 year. But the figure was higher for semaglutide (45.8%) and lower for liraglutide (35.6%).
Liraglutide requires daily injections compared with semaglutide that requires weekly injections. The authors did not study the reasons for medication adherence or discontinuation.
Key factors for achieving a greater than 10% weight loss — considered clinically meaningful — included taking semaglutide, receiving a GLP-1 for obesity, persistent medication use, high dosage, and being female.
Real-World Data Welcomed
Michael Weintraub, MD, an obesity medicine specialist and clinical assistant professor at NYU Langone Health, New York City, said that having real-world data on GLP-1 effectiveness has been much needed.
The researchers “did a really good job at stratifying these patients,” he told this news organization, saying that the study “adds to the literature in terms of what we might expect and what things we should look out for when we want to obtain the maximum degree of weight loss and attain overall better metabolic health for our patients.”
One strength: The researchers were able to capture when someone actually filled a prescription, he said. Clinicians don’t always know whether a prescription for a GLP-1 has been filled because patients might go without the drug because of insurance hurdles or supply issues, he said.
Dr. Weintraub was not surprised that the study showed that both GLP-1s produced more weight loss in those with obesity than in those with T2D, as that has become a common finding. No one has been able to explain why there is such a difference, said Dr. Weintraub. “As a field, we actually don’t know the reason behind that yet,” he said.
Given the small number of patients prescribed semaglutide for obesity, that “limits the generalizability,” he said.
Even so, semaglutide is increasingly proving superior, Dr. Weintraub said. “I would reach towards semaglutide every time either for individuals with type 2 diabetes or individuals with obesity,” he said. “The major limitation, though, is insurance coverage rather than, unfortunately, my clinical decision-making.”
He also still sees a role for liraglutide. It will go off patent soon and that could “lead to a lower price point and hopefully greater access for patients,” he said.
Dr. Gasoyan and Dr. Weintraub reported no relevant financial relationships. One coauthor reported receiving advisory board fees from Novo Nordisk and research funding from Eli Lilly during the conduct of the study.
A version of this article first appeared on Medscape.com.
FROM JAMA NETWORK OPEN
Nonscaly Red-Brown Macules on the Feet and Ankles
THE DIAGNOSIS: Secondary Syphilis
Histopathology demonstrated a mild superficial perivascular and interstitial infiltrate composed of lymphocytes, histiocytes, and rare plasma cells with a background of extravasated erythrocytes (Figure, A). Treponema pallidum staining highlighted multiple spirochetes along the dermoepidermal junction and in the superficial dermis (Figure, B). Direct immunofluorescence was negative. Laboratory workup revealed a reactive rapid plasma reagin screen with a titer of 1:16 and positive IgG and IgM treponemal antibodies. The patient was diagnosed with secondary syphilis and was treated with a single dose of 2.4 million U of intramuscular benzathine penicillin G, with notable improvement of the rash and arthritis symptoms at 2-week follow-up.
Syphilis is a sexually transmitted infection caused by the spirochete T pallidum that progresses through active and latent stages. The incidence of both the primary and secondary stages of syphilis was at a historic low in the year 2000 and has increased annually since then.1 Syphilis is more common in men, and men who have sex with men (MSM) are disproportionately affected. Although the incidence of syphilis in MSM has increased since 2000, rates have slowed, with slight decreases in this population between 2019 and 2020.1 Conversely, rates among women have increased substantially in recent years, suggesting a more recent epidemic affecting heterosexual men and women.2
Classically, the primary stage of syphilis manifests as an asymptomatic papule followed by a painless ulcer (chancre) that heals spontaneously. The secondary stage of syphilis results from dissemination of T pallidum and is characterized by a wide range of mucocutaneous manifestations and prodromal symptoms. The most common cutaneous manifestation is a diffuse, nonpruritic, papulosquamous rash with red-brown scaly macules or papules on the trunk and extremities.3 The palms and soles commonly are involved. Mucosal patches, “snail-track” ulcers in the mouth, and condylomata lata are the characteristic mucosal lesions of secondary syphilis. Mucocutaneous findings typically are preceded by systemic signs including fever, malaise, myalgia, and generalized lymphadenopathy. However, syphilis is considered “the great mimicker,” with new reports of unusual presentations of the disease. In addition to papulosquamous morphologies, pustular, targetoid, psoriasiform, and noduloulcerative (also known as lues maligna) forms of syphilis have been reported.3-5
The histopathologic features of secondary syphilis also are variable. Classically, secondary syphilis demonstrates vacuolar interface dermatitis and acanthosis with slender elongated rete ridges. Other well-known features include endothelial swelling and the presence of plasma cells in most cases.6 However, the histopathologic features of secondary syphilis may vary depending on the morphology of the skin eruption and when the biopsy is taken. Our patient lacked the classic histopathologic features of secondary syphilis. However, because syphilis was in the clinical differential diagnosis, a treponemal stain was ordered and confirmed the diagnosis. Immunohistochemical stains using antibodies to treponemal antigens have a reported sensitivity of 71% to 100% and are highly specific.7 Although the combination of endothelial swelling, interstitial inflammation, irregular acanthosis, and elongated rete ridges should raise the possibility of syphilis, a treponemal stain may be useful to identify spirochetes if clinical suspicion exists.8
Given our patient’s known history of GPA, leukocytoclastic vasculitis was high on the list of differential diagnoses. However, leukocytoclastic vasculitis most classically manifests as petechiae and palpable purpura, and unlike in secondary syphilis, the palms and soles are less commonly involved. Because our patient’s rash was mainly localized to the lower limbs, the differential also included 2 pigmented purpuric dermatoses (PPDs): progressive pigmentary purpura (Schamberg disease) and purpura annularis telangiectodes (Majocchi disease). Progressive pigmentary purpura is the most common manifestation of PPD and appears as cayenne pepper–colored macules that coalesce into golden brown–pigmented patches on the legs.9 Purpura annularis telangiectodes is another variant of PPD that manifests as pinpoint telangiectatic macules that progress to annular hyperpigmented patches with central clearing. Although PPDs frequently occur on the lower extremities, reports of plantar involvement are rare.10 Annular lichen planus manifests as violaceous papules with a clear center; however, it would be atypical for these lesions to be restricted to the feet and ankles. Palmoplantar lichen planus can mimic secondary syphilis clinically, but these cases manifest as hyperkeratotic pruritic papules on the palms and soles in contrast to the faint brown asymptomatic macules noted in our case.11
Our case highlights an unusual presentation of secondary syphilis and demonstrates the challenge of diagnosing this entity on clinical presentation alone. Because this patient lacked the classic clinical and histopathologic features of secondary syphilis, a skin biopsy with positive immunohistochemical staining for treponemal antigens was necessary to make the diagnosis. Given the variability in presentation of secondary syphilis, a biopsy or serologic testing may be necessary to make a proper diagnosis.
- Centers for Disease Control and Prevention. Sexually transmitted disease surveillance 2020. Accessed September 4, 2024. https://www.cdc.gov/std/statistics/2020/2020-SR-4-10-2023.pdf
- Ghanem KG, Ram S, Rice PA. The modern epidemic of syphilis. N Engl J Med. 2020;382:845-854. doi:10.1056/NEJMra1901593
- Forrestel AK, Kovarik CL, Katz KA. Sexually acquired syphilis: historical aspects, microbiology, epidemiology, and clinical manifestations. J Am Acad Dermatol. 2020;82:1-14. doi:10.1016/j.jaad.2019.02.073
- Wu MC, Hsu CK, Lee JY, et al. Erythema multiforme-like secondary syphilis in a HIV-positive bisexual man. Acta Derm Venereol. 2010;90:647-648. doi:10.2340/00015555-0920
- Kopelman H, Lin A, Jorizzo JL. A pemphigus-like presentation of secondary syphilis. JAAD Case Rep. 2019;5:861-864. doi:10.1016/j.jdcr.2019.07.021
- Liu XK, Li J. Histologic features of secondary syphilis. Dermatology. 2020;236:145-150. doi:10.1159/000502641
- Forrestel AK, Kovarik CL, Katz KA. Sexually acquired syphilis: laboratory diagnosis, management, and prevention. J Am Acad Dermatol. 2020;82:17-28. doi:10.1016/j.jaad.2019.02.074
- Flamm A, Parikh K, Xie Q, et al. Histologic features of secondary syphilis: a multicenter retrospective review. J Am Acad Dermatol. 2015;73:1025-1030. doi:10.1016/j.jaad.2015.08.062
- Kim DH, Seo SH, Ahn HH, et al. Characteristics and clinical manifestations of pigmented purpuric dermatosis. Ann Dermatol. 2015;27:404-410. doi:10.5021/ad.2015.27.4.404
- Sivendran M, Mowad C. Hyperpigmented patches on shins, palms, and soles. JAMA Dermatol. 2013;149:223. doi:10.1001/2013.jamadermatol.652a
- Kim YS, Kim MH, Kim CW, et al. A case of palmoplantar lichen planus mimicking secondary syphilis. Ann Dermatol. 2009;21:429-431.doi:10.5021/ad.2009.21.4.429
THE DIAGNOSIS: Secondary Syphilis
Histopathology demonstrated a mild superficial perivascular and interstitial infiltrate composed of lymphocytes, histiocytes, and rare plasma cells with a background of extravasated erythrocytes (Figure, A). Treponema pallidum staining highlighted multiple spirochetes along the dermoepidermal junction and in the superficial dermis (Figure, B). Direct immunofluorescence was negative. Laboratory workup revealed a reactive rapid plasma reagin screen with a titer of 1:16 and positive IgG and IgM treponemal antibodies. The patient was diagnosed with secondary syphilis and was treated with a single dose of 2.4 million U of intramuscular benzathine penicillin G, with notable improvement of the rash and arthritis symptoms at 2-week follow-up.

Syphilis is a sexually transmitted infection caused by the spirochete T pallidum that progresses through active and latent stages. The incidence of both the primary and secondary stages of syphilis was at a historic low in the year 2000 and has increased annually since then.1 Syphilis is more common in men, and men who have sex with men (MSM) are disproportionately affected. Although the incidence of syphilis in MSM has increased since 2000, rates have slowed, with slight decreases in this population between 2019 and 2020.1 Conversely, rates among women have increased substantially in recent years, suggesting a more recent epidemic affecting heterosexual men and women.2
Classically, the primary stage of syphilis manifests as an asymptomatic papule followed by a painless ulcer (chancre) that heals spontaneously. The secondary stage of syphilis results from dissemination of T pallidum and is characterized by a wide range of mucocutaneous manifestations and prodromal symptoms. The most common cutaneous manifestation is a diffuse, nonpruritic, papulosquamous rash with red-brown scaly macules or papules on the trunk and extremities.3 The palms and soles commonly are involved. Mucosal patches, “snail-track” ulcers in the mouth, and condylomata lata are the characteristic mucosal lesions of secondary syphilis. Mucocutaneous findings typically are preceded by systemic signs including fever, malaise, myalgia, and generalized lymphadenopathy. However, syphilis is considered “the great mimicker,” with new reports of unusual presentations of the disease. In addition to papulosquamous morphologies, pustular, targetoid, psoriasiform, and noduloulcerative (also known as lues maligna) forms of syphilis have been reported.3-5
The histopathologic features of secondary syphilis also are variable. Classically, secondary syphilis demonstrates vacuolar interface dermatitis and acanthosis with slender elongated rete ridges. Other well-known features include endothelial swelling and the presence of plasma cells in most cases.6 However, the histopathologic features of secondary syphilis may vary depending on the morphology of the skin eruption and when the biopsy is taken. Our patient lacked the classic histopathologic features of secondary syphilis. However, because syphilis was in the clinical differential diagnosis, a treponemal stain was ordered and confirmed the diagnosis. Immunohistochemical stains using antibodies to treponemal antigens have a reported sensitivity of 71% to 100% and are highly specific.7 Although the combination of endothelial swelling, interstitial inflammation, irregular acanthosis, and elongated rete ridges should raise the possibility of syphilis, a treponemal stain may be useful to identify spirochetes if clinical suspicion exists.8
Given our patient’s known history of GPA, leukocytoclastic vasculitis was high on the list of differential diagnoses. However, leukocytoclastic vasculitis most classically manifests as petechiae and palpable purpura, and unlike in secondary syphilis, the palms and soles are less commonly involved. Because our patient’s rash was mainly localized to the lower limbs, the differential also included 2 pigmented purpuric dermatoses (PPDs): progressive pigmentary purpura (Schamberg disease) and purpura annularis telangiectodes (Majocchi disease). Progressive pigmentary purpura is the most common manifestation of PPD and appears as cayenne pepper–colored macules that coalesce into golden brown–pigmented patches on the legs.9 Purpura annularis telangiectodes is another variant of PPD that manifests as pinpoint telangiectatic macules that progress to annular hyperpigmented patches with central clearing. Although PPDs frequently occur on the lower extremities, reports of plantar involvement are rare.10 Annular lichen planus manifests as violaceous papules with a clear center; however, it would be atypical for these lesions to be restricted to the feet and ankles. Palmoplantar lichen planus can mimic secondary syphilis clinically, but these cases manifest as hyperkeratotic pruritic papules on the palms and soles in contrast to the faint brown asymptomatic macules noted in our case.11
Our case highlights an unusual presentation of secondary syphilis and demonstrates the challenge of diagnosing this entity on clinical presentation alone. Because this patient lacked the classic clinical and histopathologic features of secondary syphilis, a skin biopsy with positive immunohistochemical staining for treponemal antigens was necessary to make the diagnosis. Given the variability in presentation of secondary syphilis, a biopsy or serologic testing may be necessary to make a proper diagnosis.
THE DIAGNOSIS: Secondary Syphilis
Histopathology demonstrated a mild superficial perivascular and interstitial infiltrate composed of lymphocytes, histiocytes, and rare plasma cells with a background of extravasated erythrocytes (Figure, A). Treponema pallidum staining highlighted multiple spirochetes along the dermoepidermal junction and in the superficial dermis (Figure, B). Direct immunofluorescence was negative. Laboratory workup revealed a reactive rapid plasma reagin screen with a titer of 1:16 and positive IgG and IgM treponemal antibodies. The patient was diagnosed with secondary syphilis and was treated with a single dose of 2.4 million U of intramuscular benzathine penicillin G, with notable improvement of the rash and arthritis symptoms at 2-week follow-up.

Syphilis is a sexually transmitted infection caused by the spirochete T pallidum that progresses through active and latent stages. The incidence of both the primary and secondary stages of syphilis was at a historic low in the year 2000 and has increased annually since then.1 Syphilis is more common in men, and men who have sex with men (MSM) are disproportionately affected. Although the incidence of syphilis in MSM has increased since 2000, rates have slowed, with slight decreases in this population between 2019 and 2020.1 Conversely, rates among women have increased substantially in recent years, suggesting a more recent epidemic affecting heterosexual men and women.2
Classically, the primary stage of syphilis manifests as an asymptomatic papule followed by a painless ulcer (chancre) that heals spontaneously. The secondary stage of syphilis results from dissemination of T pallidum and is characterized by a wide range of mucocutaneous manifestations and prodromal symptoms. The most common cutaneous manifestation is a diffuse, nonpruritic, papulosquamous rash with red-brown scaly macules or papules on the trunk and extremities.3 The palms and soles commonly are involved. Mucosal patches, “snail-track” ulcers in the mouth, and condylomata lata are the characteristic mucosal lesions of secondary syphilis. Mucocutaneous findings typically are preceded by systemic signs including fever, malaise, myalgia, and generalized lymphadenopathy. However, syphilis is considered “the great mimicker,” with new reports of unusual presentations of the disease. In addition to papulosquamous morphologies, pustular, targetoid, psoriasiform, and noduloulcerative (also known as lues maligna) forms of syphilis have been reported.3-5
The histopathologic features of secondary syphilis also are variable. Classically, secondary syphilis demonstrates vacuolar interface dermatitis and acanthosis with slender elongated rete ridges. Other well-known features include endothelial swelling and the presence of plasma cells in most cases.6 However, the histopathologic features of secondary syphilis may vary depending on the morphology of the skin eruption and when the biopsy is taken. Our patient lacked the classic histopathologic features of secondary syphilis. However, because syphilis was in the clinical differential diagnosis, a treponemal stain was ordered and confirmed the diagnosis. Immunohistochemical stains using antibodies to treponemal antigens have a reported sensitivity of 71% to 100% and are highly specific.7 Although the combination of endothelial swelling, interstitial inflammation, irregular acanthosis, and elongated rete ridges should raise the possibility of syphilis, a treponemal stain may be useful to identify spirochetes if clinical suspicion exists.8
Given our patient’s known history of GPA, leukocytoclastic vasculitis was high on the list of differential diagnoses. However, leukocytoclastic vasculitis most classically manifests as petechiae and palpable purpura, and unlike in secondary syphilis, the palms and soles are less commonly involved. Because our patient’s rash was mainly localized to the lower limbs, the differential also included 2 pigmented purpuric dermatoses (PPDs): progressive pigmentary purpura (Schamberg disease) and purpura annularis telangiectodes (Majocchi disease). Progressive pigmentary purpura is the most common manifestation of PPD and appears as cayenne pepper–colored macules that coalesce into golden brown–pigmented patches on the legs.9 Purpura annularis telangiectodes is another variant of PPD that manifests as pinpoint telangiectatic macules that progress to annular hyperpigmented patches with central clearing. Although PPDs frequently occur on the lower extremities, reports of plantar involvement are rare.10 Annular lichen planus manifests as violaceous papules with a clear center; however, it would be atypical for these lesions to be restricted to the feet and ankles. Palmoplantar lichen planus can mimic secondary syphilis clinically, but these cases manifest as hyperkeratotic pruritic papules on the palms and soles in contrast to the faint brown asymptomatic macules noted in our case.11
Our case highlights an unusual presentation of secondary syphilis and demonstrates the challenge of diagnosing this entity on clinical presentation alone. Because this patient lacked the classic clinical and histopathologic features of secondary syphilis, a skin biopsy with positive immunohistochemical staining for treponemal antigens was necessary to make the diagnosis. Given the variability in presentation of secondary syphilis, a biopsy or serologic testing may be necessary to make a proper diagnosis.
- Centers for Disease Control and Prevention. Sexually transmitted disease surveillance 2020. Accessed September 4, 2024. https://www.cdc.gov/std/statistics/2020/2020-SR-4-10-2023.pdf
- Ghanem KG, Ram S, Rice PA. The modern epidemic of syphilis. N Engl J Med. 2020;382:845-854. doi:10.1056/NEJMra1901593
- Forrestel AK, Kovarik CL, Katz KA. Sexually acquired syphilis: historical aspects, microbiology, epidemiology, and clinical manifestations. J Am Acad Dermatol. 2020;82:1-14. doi:10.1016/j.jaad.2019.02.073
- Wu MC, Hsu CK, Lee JY, et al. Erythema multiforme-like secondary syphilis in a HIV-positive bisexual man. Acta Derm Venereol. 2010;90:647-648. doi:10.2340/00015555-0920
- Kopelman H, Lin A, Jorizzo JL. A pemphigus-like presentation of secondary syphilis. JAAD Case Rep. 2019;5:861-864. doi:10.1016/j.jdcr.2019.07.021
- Liu XK, Li J. Histologic features of secondary syphilis. Dermatology. 2020;236:145-150. doi:10.1159/000502641
- Forrestel AK, Kovarik CL, Katz KA. Sexually acquired syphilis: laboratory diagnosis, management, and prevention. J Am Acad Dermatol. 2020;82:17-28. doi:10.1016/j.jaad.2019.02.074
- Flamm A, Parikh K, Xie Q, et al. Histologic features of secondary syphilis: a multicenter retrospective review. J Am Acad Dermatol. 2015;73:1025-1030. doi:10.1016/j.jaad.2015.08.062
- Kim DH, Seo SH, Ahn HH, et al. Characteristics and clinical manifestations of pigmented purpuric dermatosis. Ann Dermatol. 2015;27:404-410. doi:10.5021/ad.2015.27.4.404
- Sivendran M, Mowad C. Hyperpigmented patches on shins, palms, and soles. JAMA Dermatol. 2013;149:223. doi:10.1001/2013.jamadermatol.652a
- Kim YS, Kim MH, Kim CW, et al. A case of palmoplantar lichen planus mimicking secondary syphilis. Ann Dermatol. 2009;21:429-431.doi:10.5021/ad.2009.21.4.429
- Centers for Disease Control and Prevention. Sexually transmitted disease surveillance 2020. Accessed September 4, 2024. https://www.cdc.gov/std/statistics/2020/2020-SR-4-10-2023.pdf
- Ghanem KG, Ram S, Rice PA. The modern epidemic of syphilis. N Engl J Med. 2020;382:845-854. doi:10.1056/NEJMra1901593
- Forrestel AK, Kovarik CL, Katz KA. Sexually acquired syphilis: historical aspects, microbiology, epidemiology, and clinical manifestations. J Am Acad Dermatol. 2020;82:1-14. doi:10.1016/j.jaad.2019.02.073
- Wu MC, Hsu CK, Lee JY, et al. Erythema multiforme-like secondary syphilis in a HIV-positive bisexual man. Acta Derm Venereol. 2010;90:647-648. doi:10.2340/00015555-0920
- Kopelman H, Lin A, Jorizzo JL. A pemphigus-like presentation of secondary syphilis. JAAD Case Rep. 2019;5:861-864. doi:10.1016/j.jdcr.2019.07.021
- Liu XK, Li J. Histologic features of secondary syphilis. Dermatology. 2020;236:145-150. doi:10.1159/000502641
- Forrestel AK, Kovarik CL, Katz KA. Sexually acquired syphilis: laboratory diagnosis, management, and prevention. J Am Acad Dermatol. 2020;82:17-28. doi:10.1016/j.jaad.2019.02.074
- Flamm A, Parikh K, Xie Q, et al. Histologic features of secondary syphilis: a multicenter retrospective review. J Am Acad Dermatol. 2015;73:1025-1030. doi:10.1016/j.jaad.2015.08.062
- Kim DH, Seo SH, Ahn HH, et al. Characteristics and clinical manifestations of pigmented purpuric dermatosis. Ann Dermatol. 2015;27:404-410. doi:10.5021/ad.2015.27.4.404
- Sivendran M, Mowad C. Hyperpigmented patches on shins, palms, and soles. JAMA Dermatol. 2013;149:223. doi:10.1001/2013.jamadermatol.652a
- Kim YS, Kim MH, Kim CW, et al. A case of palmoplantar lichen planus mimicking secondary syphilis. Ann Dermatol. 2009;21:429-431.doi:10.5021/ad.2009.21.4.429
A 59-year-old man presented with a nontender nonpruritic rash on the feet of 2 days’ duration. The patient had a several-year history of granulomatosis with polyangiitis (GPA) and was taking methotrexate and prednisone. The rash appeared suddenly—first on the right foot and then on the left foot—and was preceded by 1 week of worsening polyarthralgia, most notably in the ankles. He denied any fever, chills, sore throat, or weight loss. His typical GPA symptoms included inflammatory arthritis, scleritis, leukocytoclastic vasculitis, and sinonasal and renal involvement. He recently experienced exacerbation of inflammatory arthritis that required an increase in the prednisone dosage (from 40 mg to 60 mg daily), but there were no other GPA symptoms. He had a history of multiple female sexual partners but no known history of HIV and no recent testing for sexually transmitted infections. Hepatitis C antibody testing performed 5 years earlier was nonreactive. He denied any illicit drug use, recent travel, sick contacts, or new medications.
Dermatologic examination revealed nonscaly, clustered, red-brown macules, some with central clearing, on the medial and lateral aspects of the feet and ankles with a few faint copper-colored macules on the palms and soles. The ankles had full range of motion with no edema or effusion. There were no oral or genital lesions. The remainder of the skin examination was normal. Punch biopsies of skin on the left foot were obtained for histopathology and direct immunofluorescence.