User login
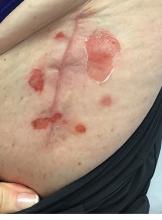
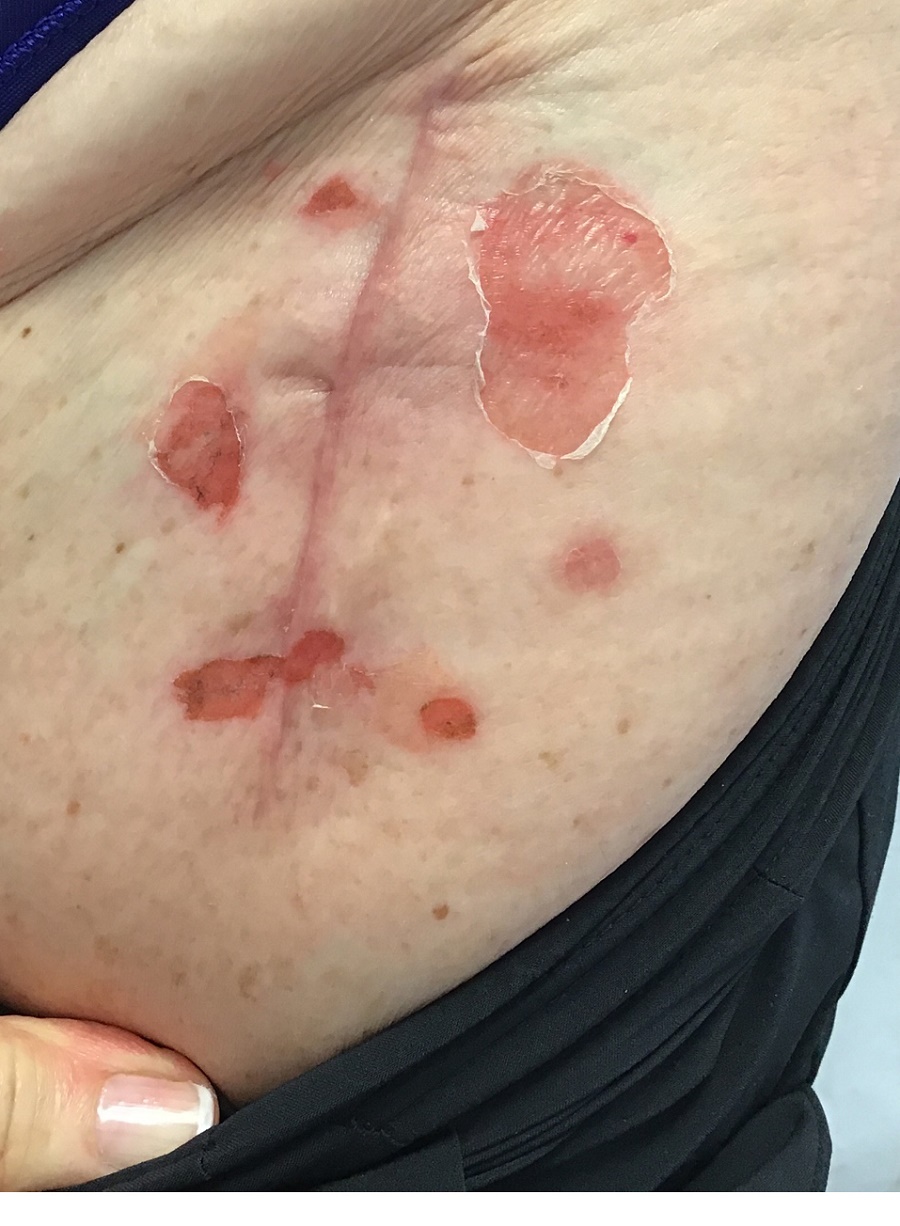
A 71-year-old White female developed erosions after hip replacement surgery 2 months prior to presentation
The patient had been diagnosed with pemphigus vulgaris (PV) 1 year prior to presentation with erosions on the axilla. Biopsy at that time revealed intraepithelial acantholytic blistering with areas of suprabasilar and subcorneal clefting. Direct immunofluorescence was positive for linear/granular IgG deposition throughout the epithelial cell surfaces, as well as linear/granular C3 deposits of the lower two thirds of the epithelial strata, consistent for pemphigus vulgaris.
There is likely a genetic predisposition. Medications that may induce pemphigus include penicillamine, nifedipine, or captopril.
Clinically, PV presents with flaccid blistering lesions that may be cutaneous and/or mucosal. Bullae can progress to erosions and crusting, which then heal with pigment alteration but not scarring. The most commonly affected sites are the mouth, intertriginous areas, face, and neck. Mucosal lesions can involve the lips, esophagus, conjunctiva, and genitals.
Biopsy for histology and direct immunofluorescence is important in distinguishing between PV and other blistering disorders. Up to 75% of patients with active disease also have a positive indirect immunofluorescence with circulating IgG.
There are numerous reports in the literature of PV occurring in previous surgical scars, and areas of friction or trauma. This so-called Koebner’s phenomenon is seen more commonly in several dermatologic conditions, such as psoriasis, lichen planus, verruca vulgaris, and vitiligo.
Treatment for PV is generally immunosuppressive. Systemic therapy usually begins with prednisone and then is transitioned to a steroid sparing agent such as mycophenolate mofetil. Other steroid sparing agents include azathioprine, methotrexate, cyclophosphamide, and intravenous immunoglobulin. Secondary infections are possible and should be treated. Topical therapies aimed at reducing pain, especially in mucosal lesions, can be beneficial.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Florida. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to [email protected].
References
Cerottini JP et al. Eur J Dermatol. 2000 Oct-Nov;10(7):546-7.
Reichert-Penetrat S et al. Eur J Dermatol. 1998 Jan-Feb;8(1):60-2.
Saini P et al. Skinmed. 2020 Aug 1;18(4):252-253.
The patient had been diagnosed with pemphigus vulgaris (PV) 1 year prior to presentation with erosions on the axilla. Biopsy at that time revealed intraepithelial acantholytic blistering with areas of suprabasilar and subcorneal clefting. Direct immunofluorescence was positive for linear/granular IgG deposition throughout the epithelial cell surfaces, as well as linear/granular C3 deposits of the lower two thirds of the epithelial strata, consistent for pemphigus vulgaris.
There is likely a genetic predisposition. Medications that may induce pemphigus include penicillamine, nifedipine, or captopril.
Clinically, PV presents with flaccid blistering lesions that may be cutaneous and/or mucosal. Bullae can progress to erosions and crusting, which then heal with pigment alteration but not scarring. The most commonly affected sites are the mouth, intertriginous areas, face, and neck. Mucosal lesions can involve the lips, esophagus, conjunctiva, and genitals.
Biopsy for histology and direct immunofluorescence is important in distinguishing between PV and other blistering disorders. Up to 75% of patients with active disease also have a positive indirect immunofluorescence with circulating IgG.
There are numerous reports in the literature of PV occurring in previous surgical scars, and areas of friction or trauma. This so-called Koebner’s phenomenon is seen more commonly in several dermatologic conditions, such as psoriasis, lichen planus, verruca vulgaris, and vitiligo.
Treatment for PV is generally immunosuppressive. Systemic therapy usually begins with prednisone and then is transitioned to a steroid sparing agent such as mycophenolate mofetil. Other steroid sparing agents include azathioprine, methotrexate, cyclophosphamide, and intravenous immunoglobulin. Secondary infections are possible and should be treated. Topical therapies aimed at reducing pain, especially in mucosal lesions, can be beneficial.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Florida. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to [email protected].
References
Cerottini JP et al. Eur J Dermatol. 2000 Oct-Nov;10(7):546-7.
Reichert-Penetrat S et al. Eur J Dermatol. 1998 Jan-Feb;8(1):60-2.
Saini P et al. Skinmed. 2020 Aug 1;18(4):252-253.
The patient had been diagnosed with pemphigus vulgaris (PV) 1 year prior to presentation with erosions on the axilla. Biopsy at that time revealed intraepithelial acantholytic blistering with areas of suprabasilar and subcorneal clefting. Direct immunofluorescence was positive for linear/granular IgG deposition throughout the epithelial cell surfaces, as well as linear/granular C3 deposits of the lower two thirds of the epithelial strata, consistent for pemphigus vulgaris.
There is likely a genetic predisposition. Medications that may induce pemphigus include penicillamine, nifedipine, or captopril.
Clinically, PV presents with flaccid blistering lesions that may be cutaneous and/or mucosal. Bullae can progress to erosions and crusting, which then heal with pigment alteration but not scarring. The most commonly affected sites are the mouth, intertriginous areas, face, and neck. Mucosal lesions can involve the lips, esophagus, conjunctiva, and genitals.
Biopsy for histology and direct immunofluorescence is important in distinguishing between PV and other blistering disorders. Up to 75% of patients with active disease also have a positive indirect immunofluorescence with circulating IgG.
There are numerous reports in the literature of PV occurring in previous surgical scars, and areas of friction or trauma. This so-called Koebner’s phenomenon is seen more commonly in several dermatologic conditions, such as psoriasis, lichen planus, verruca vulgaris, and vitiligo.
Treatment for PV is generally immunosuppressive. Systemic therapy usually begins with prednisone and then is transitioned to a steroid sparing agent such as mycophenolate mofetil. Other steroid sparing agents include azathioprine, methotrexate, cyclophosphamide, and intravenous immunoglobulin. Secondary infections are possible and should be treated. Topical therapies aimed at reducing pain, especially in mucosal lesions, can be beneficial.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Florida. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to [email protected].
References
Cerottini JP et al. Eur J Dermatol. 2000 Oct-Nov;10(7):546-7.
Reichert-Penetrat S et al. Eur J Dermatol. 1998 Jan-Feb;8(1):60-2.
Saini P et al. Skinmed. 2020 Aug 1;18(4):252-253.
Myth of the Month: Vitamin C vs the Common Cold
Case: A 38-year-old presents for acute onset runny nose, cough, and fever for the last 3 days. Her children at home have a similar presentation. She believes that she has been managing her symptoms well with Tylenol and rest. The patient is up to date on her COVID and flu shots and was wondering if there was anything else she could have done to prevent her symptoms. She saw a commercial about vitamin C supplements boosting the immune system and was wondering about their efficacy. How would you respond?
Studies of Vitamin C
Linus Pauling, FRS, did a summary of four relatively small published studies of vitamin C and concluded that vitamin C supplementation helped prevent and lessen colds.1 He mentioned a placebo-controlled study of vitamin C with viral inoculation which did not show any effect. His overall conclusion of efficacy for vitamin C led to the widespread belief that vitamin C was a proven effective therapy to prevent and treat the common cold. Since then, multiple trials and studies have examined the effect of vitamin C on the prevention and treatment of colds.
The Cochrane Review conducted a meta-analysis comparing 29 placebo-controlled trials involving 11,306 participants.2 Criteria included vitamin C supplementation of 0.2 g-1 g/day to study its efficacy in preventing the common cold. The analysis showed that supplemental vitamin C did not significantly reduce the incidence of colds. However, there was a statistically significant 8% reduction in adults and 14% in children in the duration of colds. In terms of treatment, there was no evidence of vitamin C’s efficacy.
A 2001 study conducted a small double-blind, randomized control trial to evaluate large doses of vitamin C as treatment for the common cold.3 Volunteers were divided and instructed to take varying doses ranging from 1 to 3 g of vitamin C vs a placebo at the onset of cold-like symptoms. Subjects were expected to assess the duration and severity of their cold. The data showed no significant difference in the severity or duration of cold symptoms between small or large vitamin C doses or placebo.
A more recent meta-analysis by Hemilä and Chalker looked at 10 placebo-controlled trials of vitamin C for the prevention and treatment of colds.4 The analysis showed a small 15% reduction in more severe cold symptoms.
Summary
While vitamin C is safe, there is no evidence for its ability to prevent the common cold. Although the Cochrane review and more a recent meta-analysis by Hemilä and Chalker demonstrated statistical significance in shortening the duration of symptoms, it was a minimal reduction with little clinical significance. .
Ms. Ibabao is a fourth year medical student at the University of Washington School of Medicine; Dr. Paauw is Professor of Medicine, Rathmann Family Foundation Endowed Chair Patient-centered Clinical Education, at the University of Washington School of Medicine, Seattle. They have no conflicts of interest.
References
1. Pauling L. The significance of the evidence about ascorbic acid and the common cold. Proc Natl Acad Sci USA. 1971;68:2678-2671.
2. Hemilä H, Chalker E. Vitamin C for preventing and treating the common cold. Cochrane Database of Systematic Reviews. 2013;1(1).
3. Audera C et al. Mega‐dose vitamin C in treatment of the common cold: a randomised controlled trial. Med J Australia. 2001;175(7):359-362.
4. Hemilä H, Chalker E. Vitamin C reduces the severity of common colds: a meta-analysis. BMC Public Health. 2023;23:2468.
Case: A 38-year-old presents for acute onset runny nose, cough, and fever for the last 3 days. Her children at home have a similar presentation. She believes that she has been managing her symptoms well with Tylenol and rest. The patient is up to date on her COVID and flu shots and was wondering if there was anything else she could have done to prevent her symptoms. She saw a commercial about vitamin C supplements boosting the immune system and was wondering about their efficacy. How would you respond?
Studies of Vitamin C
Linus Pauling, FRS, did a summary of four relatively small published studies of vitamin C and concluded that vitamin C supplementation helped prevent and lessen colds.1 He mentioned a placebo-controlled study of vitamin C with viral inoculation which did not show any effect. His overall conclusion of efficacy for vitamin C led to the widespread belief that vitamin C was a proven effective therapy to prevent and treat the common cold. Since then, multiple trials and studies have examined the effect of vitamin C on the prevention and treatment of colds.
The Cochrane Review conducted a meta-analysis comparing 29 placebo-controlled trials involving 11,306 participants.2 Criteria included vitamin C supplementation of 0.2 g-1 g/day to study its efficacy in preventing the common cold. The analysis showed that supplemental vitamin C did not significantly reduce the incidence of colds. However, there was a statistically significant 8% reduction in adults and 14% in children in the duration of colds. In terms of treatment, there was no evidence of vitamin C’s efficacy.
A 2001 study conducted a small double-blind, randomized control trial to evaluate large doses of vitamin C as treatment for the common cold.3 Volunteers were divided and instructed to take varying doses ranging from 1 to 3 g of vitamin C vs a placebo at the onset of cold-like symptoms. Subjects were expected to assess the duration and severity of their cold. The data showed no significant difference in the severity or duration of cold symptoms between small or large vitamin C doses or placebo.
A more recent meta-analysis by Hemilä and Chalker looked at 10 placebo-controlled trials of vitamin C for the prevention and treatment of colds.4 The analysis showed a small 15% reduction in more severe cold symptoms.
Summary
While vitamin C is safe, there is no evidence for its ability to prevent the common cold. Although the Cochrane review and more a recent meta-analysis by Hemilä and Chalker demonstrated statistical significance in shortening the duration of symptoms, it was a minimal reduction with little clinical significance. .
Ms. Ibabao is a fourth year medical student at the University of Washington School of Medicine; Dr. Paauw is Professor of Medicine, Rathmann Family Foundation Endowed Chair Patient-centered Clinical Education, at the University of Washington School of Medicine, Seattle. They have no conflicts of interest.
References
1. Pauling L. The significance of the evidence about ascorbic acid and the common cold. Proc Natl Acad Sci USA. 1971;68:2678-2671.
2. Hemilä H, Chalker E. Vitamin C for preventing and treating the common cold. Cochrane Database of Systematic Reviews. 2013;1(1).
3. Audera C et al. Mega‐dose vitamin C in treatment of the common cold: a randomised controlled trial. Med J Australia. 2001;175(7):359-362.
4. Hemilä H, Chalker E. Vitamin C reduces the severity of common colds: a meta-analysis. BMC Public Health. 2023;23:2468.
Case: A 38-year-old presents for acute onset runny nose, cough, and fever for the last 3 days. Her children at home have a similar presentation. She believes that she has been managing her symptoms well with Tylenol and rest. The patient is up to date on her COVID and flu shots and was wondering if there was anything else she could have done to prevent her symptoms. She saw a commercial about vitamin C supplements boosting the immune system and was wondering about their efficacy. How would you respond?
Studies of Vitamin C
Linus Pauling, FRS, did a summary of four relatively small published studies of vitamin C and concluded that vitamin C supplementation helped prevent and lessen colds.1 He mentioned a placebo-controlled study of vitamin C with viral inoculation which did not show any effect. His overall conclusion of efficacy for vitamin C led to the widespread belief that vitamin C was a proven effective therapy to prevent and treat the common cold. Since then, multiple trials and studies have examined the effect of vitamin C on the prevention and treatment of colds.
The Cochrane Review conducted a meta-analysis comparing 29 placebo-controlled trials involving 11,306 participants.2 Criteria included vitamin C supplementation of 0.2 g-1 g/day to study its efficacy in preventing the common cold. The analysis showed that supplemental vitamin C did not significantly reduce the incidence of colds. However, there was a statistically significant 8% reduction in adults and 14% in children in the duration of colds. In terms of treatment, there was no evidence of vitamin C’s efficacy.
A 2001 study conducted a small double-blind, randomized control trial to evaluate large doses of vitamin C as treatment for the common cold.3 Volunteers were divided and instructed to take varying doses ranging from 1 to 3 g of vitamin C vs a placebo at the onset of cold-like symptoms. Subjects were expected to assess the duration and severity of their cold. The data showed no significant difference in the severity or duration of cold symptoms between small or large vitamin C doses or placebo.
A more recent meta-analysis by Hemilä and Chalker looked at 10 placebo-controlled trials of vitamin C for the prevention and treatment of colds.4 The analysis showed a small 15% reduction in more severe cold symptoms.
Summary
While vitamin C is safe, there is no evidence for its ability to prevent the common cold. Although the Cochrane review and more a recent meta-analysis by Hemilä and Chalker demonstrated statistical significance in shortening the duration of symptoms, it was a minimal reduction with little clinical significance. .
Ms. Ibabao is a fourth year medical student at the University of Washington School of Medicine; Dr. Paauw is Professor of Medicine, Rathmann Family Foundation Endowed Chair Patient-centered Clinical Education, at the University of Washington School of Medicine, Seattle. They have no conflicts of interest.
References
1. Pauling L. The significance of the evidence about ascorbic acid and the common cold. Proc Natl Acad Sci USA. 1971;68:2678-2671.
2. Hemilä H, Chalker E. Vitamin C for preventing and treating the common cold. Cochrane Database of Systematic Reviews. 2013;1(1).
3. Audera C et al. Mega‐dose vitamin C in treatment of the common cold: a randomised controlled trial. Med J Australia. 2001;175(7):359-362.
4. Hemilä H, Chalker E. Vitamin C reduces the severity of common colds: a meta-analysis. BMC Public Health. 2023;23:2468.
Bimekizumab Gains FDA Approval for Psoriatic Arthritis, Axial Spondyloarthritis
The Food and Drug Administration has approved bimekizumab-bkzx (Bimzelx; UCB) for adult patients with active psoriatic arthritis (PsA), active nonradiographic axial spondyloarthritis (nr-axSpA) with objective signs of inflammation, and active ankylosing spondylitis (AS).
The drug, an interleukin (IL)–17A and IL-17F inhibitor, was first approved in October 2023 for treatment of moderate to severe plaque psoriasis in adults who are candidates for systemic therapy or phototherapy.
“In psoriatic arthritis and across the spectrum of axSpA, clinical study results and real-world experience outside the US have highlighted that Bimzelx can help patients achieve high thresholds of clinical response that are rapid in onset and sustained up to 2 years,” said Emmanuel Caeymaex, executive vice president, head of patient impact, and chief commercial officer of UCB in a press release.
The recommended dosage of bimekizumab for adult patients with active PsA, nr-axSpA, or AS is 160 mg by subcutaneous injection every 4 weeks. For patients with PsA and coexistent moderate to severe plaque psoriasis, the dosage is the same as for patients with plaque psoriasis. The dosing for plaque psoriasis is to administer 320 mg (two 160-mg injections) by subcutaneous injection at weeks 0, 4, 8, 12, and 16, then every 8 weeks thereafter. For patients weighing ≥ 120 kg, consider a dose of 320 mg every 4 weeks after week 16.
PsA Clinical Trials
The approval for PsA was based on data from two phase 3 clinical trials, including 852 participants naive to biologics (BE OPTIMAL) and 400 participants with inadequate response to treatment with one or two tumor necrosis factor (TNF) inhibitors (BE COMPLETE). Both studies met their primary endpoint, 50% improvement in American College of Rheumatology response criteria (ACR50) at 16 weeks, as well as ranked secondary endpoints. Secondary endpoints included minimal disease activity (MDA) and Psoriasis Area and Severity Index 100 (complete skin clearance) at week 16.
At 16 weeks:
- About 44% of both the biologic-naive (189 of 431) and TNF inhibitor–resistant (116 of 267) groups receiving bimekizumab achieved ACR50 response, compared with 10% (28 of 281) and 7% (9 of 133) receiving placebo, respectively.
- About 45% of all patients treated with bimekizumab achieved MDA.
- Nearly 60% of TNF inhibitor–resistant patients had complete skin clearance.
These responses generally were sustained for 1 year. The most common adverse reactions are upper respiratory tract infections, oral candidiasis, headache, diarrhea, and urinary tract infection.
NR-axSpA and AS Clinical Trials
The approval for active nr-axSpA and active AS was based on data from two clinical studies, BE MOBILE 1 (nr-axSpA) and BE MOBILE 2 (AS). Both studies met their primary endpoint, 40% improvement in Assessment of Spondyloarthritis International Society response criteria (ASAS40) at 16 weeks.
Key findings included:
- In nr-axSpA patients, 47.7% (61 of 128) receiving bimekizumab achieved ASAS40 at week 16, compared with 21.4% (27 of 126) receiving placebo.
- In AS patients, 44.8% (99 of 221) in the bimekizumab group achieved ASAS40 response at week 16 vs 22.5% (25 of 111) receiving placebo.
- At 1 year in both groups, 60% treated with bimekizumab achieved an Ankylosing Spondylitis Disease Activity Score < 2.1.
In nr-axSpA, the most common adverse reactions are upper respiratory tract infections, oral candidiasis, headache, diarrhea, cough, fatigue, musculoskeletal pain, myalgia, tonsillitis, increase in transaminase, and urinary tract infection. In AS, the most common adverse reactions are upper respiratory tract infections, oral candidiasis, headache, diarrhea, injection-site pain, rash, and vulvovaginal mycotic infection.
Bimekizumab was approved by the European Commission for the same rheumatologic indications in June 2023.
Bimekizumab is currently available to eligible patients in the United States, according to the press release.
A version of this article first appeared on Medscape.com.
The Food and Drug Administration has approved bimekizumab-bkzx (Bimzelx; UCB) for adult patients with active psoriatic arthritis (PsA), active nonradiographic axial spondyloarthritis (nr-axSpA) with objective signs of inflammation, and active ankylosing spondylitis (AS).
The drug, an interleukin (IL)–17A and IL-17F inhibitor, was first approved in October 2023 for treatment of moderate to severe plaque psoriasis in adults who are candidates for systemic therapy or phototherapy.
“In psoriatic arthritis and across the spectrum of axSpA, clinical study results and real-world experience outside the US have highlighted that Bimzelx can help patients achieve high thresholds of clinical response that are rapid in onset and sustained up to 2 years,” said Emmanuel Caeymaex, executive vice president, head of patient impact, and chief commercial officer of UCB in a press release.
The recommended dosage of bimekizumab for adult patients with active PsA, nr-axSpA, or AS is 160 mg by subcutaneous injection every 4 weeks. For patients with PsA and coexistent moderate to severe plaque psoriasis, the dosage is the same as for patients with plaque psoriasis. The dosing for plaque psoriasis is to administer 320 mg (two 160-mg injections) by subcutaneous injection at weeks 0, 4, 8, 12, and 16, then every 8 weeks thereafter. For patients weighing ≥ 120 kg, consider a dose of 320 mg every 4 weeks after week 16.
PsA Clinical Trials
The approval for PsA was based on data from two phase 3 clinical trials, including 852 participants naive to biologics (BE OPTIMAL) and 400 participants with inadequate response to treatment with one or two tumor necrosis factor (TNF) inhibitors (BE COMPLETE). Both studies met their primary endpoint, 50% improvement in American College of Rheumatology response criteria (ACR50) at 16 weeks, as well as ranked secondary endpoints. Secondary endpoints included minimal disease activity (MDA) and Psoriasis Area and Severity Index 100 (complete skin clearance) at week 16.
At 16 weeks:
- About 44% of both the biologic-naive (189 of 431) and TNF inhibitor–resistant (116 of 267) groups receiving bimekizumab achieved ACR50 response, compared with 10% (28 of 281) and 7% (9 of 133) receiving placebo, respectively.
- About 45% of all patients treated with bimekizumab achieved MDA.
- Nearly 60% of TNF inhibitor–resistant patients had complete skin clearance.
These responses generally were sustained for 1 year. The most common adverse reactions are upper respiratory tract infections, oral candidiasis, headache, diarrhea, and urinary tract infection.
NR-axSpA and AS Clinical Trials
The approval for active nr-axSpA and active AS was based on data from two clinical studies, BE MOBILE 1 (nr-axSpA) and BE MOBILE 2 (AS). Both studies met their primary endpoint, 40% improvement in Assessment of Spondyloarthritis International Society response criteria (ASAS40) at 16 weeks.
Key findings included:
- In nr-axSpA patients, 47.7% (61 of 128) receiving bimekizumab achieved ASAS40 at week 16, compared with 21.4% (27 of 126) receiving placebo.
- In AS patients, 44.8% (99 of 221) in the bimekizumab group achieved ASAS40 response at week 16 vs 22.5% (25 of 111) receiving placebo.
- At 1 year in both groups, 60% treated with bimekizumab achieved an Ankylosing Spondylitis Disease Activity Score < 2.1.
In nr-axSpA, the most common adverse reactions are upper respiratory tract infections, oral candidiasis, headache, diarrhea, cough, fatigue, musculoskeletal pain, myalgia, tonsillitis, increase in transaminase, and urinary tract infection. In AS, the most common adverse reactions are upper respiratory tract infections, oral candidiasis, headache, diarrhea, injection-site pain, rash, and vulvovaginal mycotic infection.
Bimekizumab was approved by the European Commission for the same rheumatologic indications in June 2023.
Bimekizumab is currently available to eligible patients in the United States, according to the press release.
A version of this article first appeared on Medscape.com.
The Food and Drug Administration has approved bimekizumab-bkzx (Bimzelx; UCB) for adult patients with active psoriatic arthritis (PsA), active nonradiographic axial spondyloarthritis (nr-axSpA) with objective signs of inflammation, and active ankylosing spondylitis (AS).
The drug, an interleukin (IL)–17A and IL-17F inhibitor, was first approved in October 2023 for treatment of moderate to severe plaque psoriasis in adults who are candidates for systemic therapy or phototherapy.
“In psoriatic arthritis and across the spectrum of axSpA, clinical study results and real-world experience outside the US have highlighted that Bimzelx can help patients achieve high thresholds of clinical response that are rapid in onset and sustained up to 2 years,” said Emmanuel Caeymaex, executive vice president, head of patient impact, and chief commercial officer of UCB in a press release.
The recommended dosage of bimekizumab for adult patients with active PsA, nr-axSpA, or AS is 160 mg by subcutaneous injection every 4 weeks. For patients with PsA and coexistent moderate to severe plaque psoriasis, the dosage is the same as for patients with plaque psoriasis. The dosing for plaque psoriasis is to administer 320 mg (two 160-mg injections) by subcutaneous injection at weeks 0, 4, 8, 12, and 16, then every 8 weeks thereafter. For patients weighing ≥ 120 kg, consider a dose of 320 mg every 4 weeks after week 16.
PsA Clinical Trials
The approval for PsA was based on data from two phase 3 clinical trials, including 852 participants naive to biologics (BE OPTIMAL) and 400 participants with inadequate response to treatment with one or two tumor necrosis factor (TNF) inhibitors (BE COMPLETE). Both studies met their primary endpoint, 50% improvement in American College of Rheumatology response criteria (ACR50) at 16 weeks, as well as ranked secondary endpoints. Secondary endpoints included minimal disease activity (MDA) and Psoriasis Area and Severity Index 100 (complete skin clearance) at week 16.
At 16 weeks:
- About 44% of both the biologic-naive (189 of 431) and TNF inhibitor–resistant (116 of 267) groups receiving bimekizumab achieved ACR50 response, compared with 10% (28 of 281) and 7% (9 of 133) receiving placebo, respectively.
- About 45% of all patients treated with bimekizumab achieved MDA.
- Nearly 60% of TNF inhibitor–resistant patients had complete skin clearance.
These responses generally were sustained for 1 year. The most common adverse reactions are upper respiratory tract infections, oral candidiasis, headache, diarrhea, and urinary tract infection.
NR-axSpA and AS Clinical Trials
The approval for active nr-axSpA and active AS was based on data from two clinical studies, BE MOBILE 1 (nr-axSpA) and BE MOBILE 2 (AS). Both studies met their primary endpoint, 40% improvement in Assessment of Spondyloarthritis International Society response criteria (ASAS40) at 16 weeks.
Key findings included:
- In nr-axSpA patients, 47.7% (61 of 128) receiving bimekizumab achieved ASAS40 at week 16, compared with 21.4% (27 of 126) receiving placebo.
- In AS patients, 44.8% (99 of 221) in the bimekizumab group achieved ASAS40 response at week 16 vs 22.5% (25 of 111) receiving placebo.
- At 1 year in both groups, 60% treated with bimekizumab achieved an Ankylosing Spondylitis Disease Activity Score < 2.1.
In nr-axSpA, the most common adverse reactions are upper respiratory tract infections, oral candidiasis, headache, diarrhea, cough, fatigue, musculoskeletal pain, myalgia, tonsillitis, increase in transaminase, and urinary tract infection. In AS, the most common adverse reactions are upper respiratory tract infections, oral candidiasis, headache, diarrhea, injection-site pain, rash, and vulvovaginal mycotic infection.
Bimekizumab was approved by the European Commission for the same rheumatologic indications in June 2023.
Bimekizumab is currently available to eligible patients in the United States, according to the press release.
A version of this article first appeared on Medscape.com.
Dealing with Hot Flashes? Try Hypnosis
There’s an unexpected treatment for hot flashes and other menopause symptoms that’s getting more popular: clinical hypnosis.
Hypnosis is a state of highly focused attention that works through disassociating, or putting aside your conscious awareness of things that would ordinarily be in your consciousness, said David Spiegel, MD, a psychiatrist with Stanford Medical School in Califonrnia.
“It increases your cognitive flexibility – a way to approach an old problem from a new point of view and just let go of your older ways of thinking about it,” he said.
Usually around age 50, women have menopause, which is the end of their menstrual cycles. Estrogen levels drop, and hot flashes can happen 12-15 times per day, said Gary Elkins, PhD, a psychology and neuroscience professor at Baylor University in Waco, Texas.
Both clinical hypnosis and cognitive-behavioral therapy, a common form of talk therapy, have been shown to work as non-hormonal treatments for hot flashes, particularly for women who are unable to take hormones for health reasons, such as having a history with an estrogen-sensitive cancer (like breast cancer), according to research published by the Menopause Society in 2023.
A new review presented at the 2024 annual meeting of the Menopause Society in Chicago analyzed 23 studies from 1996 to 2022 and compared how well clinical hypnosis and cognitive behavioral therapy worked as treatments for hot flashes and other menopause symptoms. Researchers found that clinical hypnosis is better at helping make hot flashes less frequent and less intense, even reducing symptoms by 60%. Findings on cognitive-behavioral therapy, on the other hand, showed only slight hot flash reduction, though it helped reduce daily stress linked with hot flashes.
Hypnosis can address the “perfect storm” of mental and physical issues that come with menopause symptoms, explained Dr. Spiegel, who created a popular self-hypnosis app called Reveri. “You’re having a reduction in your levels of estrogen and progesterone, but it’s also a reminder that you’re going into a different stage of life where you’re no longer fertile, you’re getting older,” he said. “[With hypnosis], you can disassociate pain and your awareness of things that ordinarily would impede your consciousness and make you miserable.”
A hypnosis session can help you separate psychological discomfort from physical discomfort, Dr. Spiegel said. “Typically, people in hypnosis dealing with menopause will imagine they’re floating in a lake, feeling cool, tingling, numbness. They can literally change how hot they feel. They can change the hot flash and imagine themselves cool, comfortable. If they’re worried about something, picture it on an imaginary screen. Just picture it, but not feel it.”
Hypnosis for Sleep
Hot flashes that happen at night are called night sweats and can hinder your sleep. Hypnotherapy can help reduce both hot flashes and night sweats, to the point where sleep is not interrupted, Dr. Elkins said. “While sleep improves with the hypnotherapy intervention, it also involves general relaxation,” said Dr. Elkins, who is the director of the Mind-Body Medicine Research Laboratory at Baylor University. “As women practice self-hypnosis at night, they’re entering a more calm and relaxed state, which also may facilitate good sleep or improve sleep duration and sleep quality.”
Our subconscious mind influences our sleep patterns largely through experiences vs. words or thoughts, according to Emilie Leyes, a certified hypnotherapist based in Philadelphia. This explains why simply reciting the words “I’m relaxed,” when you’re stressed, is often less effective than a few deep breaths or a warm hug from a family member or friend, said Ms. Leyes, who hosts a brain-training podcast for mindset transformation called How to Like Your Life.
“In a similar way, hypnosis, which directly accesses the subconscious, allows us to offer our minds new, powerful experiences to reduce our stress, improve our mood, and increase our access to positive emotions,” she said. “Repeatedly exposing ourselves to these positive experiences in our minds can increase our capacity to feel good, and impact how we feel in our everyday lives.”
Your First Hypnosis Session
A hypnosis session always begins with deep relaxation, which can help your mind and body grow accustomed to what it’s like to feel calm, said Ms. Leyes. “By giving the brain and body experiences of safety, relaxation, and inner peace, we can more easily let go of our stressful thoughts of the day and drift off to sleep with ease at night.”
You will often start by sitting or lying in a comfortable position, and then the hypnotic induction begins with a focus of attention, according to Dr. Elkins. The person concentrates, with their eyelids closed, and then are given suggestions for deepening their relaxed state. “Usually that’s a safe, pleasant place, such as walking through the mountains or being near a beach,” he said. “And within that, suggestions are given that target the mechanism that underlies the symptoms [such as hot flashes].”
Dr. Spiegel usually starts off with a neutral test that can help measure how hypnotizable a person is on a 0-to-10 scale. For example, instructing the client to imagine that their hand is floating in the air. If they pull their hand down and it floats back up, the client finds they can “actually dissociate the psychological from the physiological aspects of their experience – their left hand feels different from their right hand,” Dr. Spiegel said. “I use that as an example for them to say, ‘look how you can change how your body feels. Now, let’s use it to help you with your anxiety with your menopausal symptoms.’ ”
A version of this article appeared on WebMD.com.
There’s an unexpected treatment for hot flashes and other menopause symptoms that’s getting more popular: clinical hypnosis.
Hypnosis is a state of highly focused attention that works through disassociating, or putting aside your conscious awareness of things that would ordinarily be in your consciousness, said David Spiegel, MD, a psychiatrist with Stanford Medical School in Califonrnia.
“It increases your cognitive flexibility – a way to approach an old problem from a new point of view and just let go of your older ways of thinking about it,” he said.
Usually around age 50, women have menopause, which is the end of their menstrual cycles. Estrogen levels drop, and hot flashes can happen 12-15 times per day, said Gary Elkins, PhD, a psychology and neuroscience professor at Baylor University in Waco, Texas.
Both clinical hypnosis and cognitive-behavioral therapy, a common form of talk therapy, have been shown to work as non-hormonal treatments for hot flashes, particularly for women who are unable to take hormones for health reasons, such as having a history with an estrogen-sensitive cancer (like breast cancer), according to research published by the Menopause Society in 2023.
A new review presented at the 2024 annual meeting of the Menopause Society in Chicago analyzed 23 studies from 1996 to 2022 and compared how well clinical hypnosis and cognitive behavioral therapy worked as treatments for hot flashes and other menopause symptoms. Researchers found that clinical hypnosis is better at helping make hot flashes less frequent and less intense, even reducing symptoms by 60%. Findings on cognitive-behavioral therapy, on the other hand, showed only slight hot flash reduction, though it helped reduce daily stress linked with hot flashes.
Hypnosis can address the “perfect storm” of mental and physical issues that come with menopause symptoms, explained Dr. Spiegel, who created a popular self-hypnosis app called Reveri. “You’re having a reduction in your levels of estrogen and progesterone, but it’s also a reminder that you’re going into a different stage of life where you’re no longer fertile, you’re getting older,” he said. “[With hypnosis], you can disassociate pain and your awareness of things that ordinarily would impede your consciousness and make you miserable.”
A hypnosis session can help you separate psychological discomfort from physical discomfort, Dr. Spiegel said. “Typically, people in hypnosis dealing with menopause will imagine they’re floating in a lake, feeling cool, tingling, numbness. They can literally change how hot they feel. They can change the hot flash and imagine themselves cool, comfortable. If they’re worried about something, picture it on an imaginary screen. Just picture it, but not feel it.”
Hypnosis for Sleep
Hot flashes that happen at night are called night sweats and can hinder your sleep. Hypnotherapy can help reduce both hot flashes and night sweats, to the point where sleep is not interrupted, Dr. Elkins said. “While sleep improves with the hypnotherapy intervention, it also involves general relaxation,” said Dr. Elkins, who is the director of the Mind-Body Medicine Research Laboratory at Baylor University. “As women practice self-hypnosis at night, they’re entering a more calm and relaxed state, which also may facilitate good sleep or improve sleep duration and sleep quality.”
Our subconscious mind influences our sleep patterns largely through experiences vs. words or thoughts, according to Emilie Leyes, a certified hypnotherapist based in Philadelphia. This explains why simply reciting the words “I’m relaxed,” when you’re stressed, is often less effective than a few deep breaths or a warm hug from a family member or friend, said Ms. Leyes, who hosts a brain-training podcast for mindset transformation called How to Like Your Life.
“In a similar way, hypnosis, which directly accesses the subconscious, allows us to offer our minds new, powerful experiences to reduce our stress, improve our mood, and increase our access to positive emotions,” she said. “Repeatedly exposing ourselves to these positive experiences in our minds can increase our capacity to feel good, and impact how we feel in our everyday lives.”
Your First Hypnosis Session
A hypnosis session always begins with deep relaxation, which can help your mind and body grow accustomed to what it’s like to feel calm, said Ms. Leyes. “By giving the brain and body experiences of safety, relaxation, and inner peace, we can more easily let go of our stressful thoughts of the day and drift off to sleep with ease at night.”
You will often start by sitting or lying in a comfortable position, and then the hypnotic induction begins with a focus of attention, according to Dr. Elkins. The person concentrates, with their eyelids closed, and then are given suggestions for deepening their relaxed state. “Usually that’s a safe, pleasant place, such as walking through the mountains or being near a beach,” he said. “And within that, suggestions are given that target the mechanism that underlies the symptoms [such as hot flashes].”
Dr. Spiegel usually starts off with a neutral test that can help measure how hypnotizable a person is on a 0-to-10 scale. For example, instructing the client to imagine that their hand is floating in the air. If they pull their hand down and it floats back up, the client finds they can “actually dissociate the psychological from the physiological aspects of their experience – their left hand feels different from their right hand,” Dr. Spiegel said. “I use that as an example for them to say, ‘look how you can change how your body feels. Now, let’s use it to help you with your anxiety with your menopausal symptoms.’ ”
A version of this article appeared on WebMD.com.
There’s an unexpected treatment for hot flashes and other menopause symptoms that’s getting more popular: clinical hypnosis.
Hypnosis is a state of highly focused attention that works through disassociating, or putting aside your conscious awareness of things that would ordinarily be in your consciousness, said David Spiegel, MD, a psychiatrist with Stanford Medical School in Califonrnia.
“It increases your cognitive flexibility – a way to approach an old problem from a new point of view and just let go of your older ways of thinking about it,” he said.
Usually around age 50, women have menopause, which is the end of their menstrual cycles. Estrogen levels drop, and hot flashes can happen 12-15 times per day, said Gary Elkins, PhD, a psychology and neuroscience professor at Baylor University in Waco, Texas.
Both clinical hypnosis and cognitive-behavioral therapy, a common form of talk therapy, have been shown to work as non-hormonal treatments for hot flashes, particularly for women who are unable to take hormones for health reasons, such as having a history with an estrogen-sensitive cancer (like breast cancer), according to research published by the Menopause Society in 2023.
A new review presented at the 2024 annual meeting of the Menopause Society in Chicago analyzed 23 studies from 1996 to 2022 and compared how well clinical hypnosis and cognitive behavioral therapy worked as treatments for hot flashes and other menopause symptoms. Researchers found that clinical hypnosis is better at helping make hot flashes less frequent and less intense, even reducing symptoms by 60%. Findings on cognitive-behavioral therapy, on the other hand, showed only slight hot flash reduction, though it helped reduce daily stress linked with hot flashes.
Hypnosis can address the “perfect storm” of mental and physical issues that come with menopause symptoms, explained Dr. Spiegel, who created a popular self-hypnosis app called Reveri. “You’re having a reduction in your levels of estrogen and progesterone, but it’s also a reminder that you’re going into a different stage of life where you’re no longer fertile, you’re getting older,” he said. “[With hypnosis], you can disassociate pain and your awareness of things that ordinarily would impede your consciousness and make you miserable.”
A hypnosis session can help you separate psychological discomfort from physical discomfort, Dr. Spiegel said. “Typically, people in hypnosis dealing with menopause will imagine they’re floating in a lake, feeling cool, tingling, numbness. They can literally change how hot they feel. They can change the hot flash and imagine themselves cool, comfortable. If they’re worried about something, picture it on an imaginary screen. Just picture it, but not feel it.”
Hypnosis for Sleep
Hot flashes that happen at night are called night sweats and can hinder your sleep. Hypnotherapy can help reduce both hot flashes and night sweats, to the point where sleep is not interrupted, Dr. Elkins said. “While sleep improves with the hypnotherapy intervention, it also involves general relaxation,” said Dr. Elkins, who is the director of the Mind-Body Medicine Research Laboratory at Baylor University. “As women practice self-hypnosis at night, they’re entering a more calm and relaxed state, which also may facilitate good sleep or improve sleep duration and sleep quality.”
Our subconscious mind influences our sleep patterns largely through experiences vs. words or thoughts, according to Emilie Leyes, a certified hypnotherapist based in Philadelphia. This explains why simply reciting the words “I’m relaxed,” when you’re stressed, is often less effective than a few deep breaths or a warm hug from a family member or friend, said Ms. Leyes, who hosts a brain-training podcast for mindset transformation called How to Like Your Life.
“In a similar way, hypnosis, which directly accesses the subconscious, allows us to offer our minds new, powerful experiences to reduce our stress, improve our mood, and increase our access to positive emotions,” she said. “Repeatedly exposing ourselves to these positive experiences in our minds can increase our capacity to feel good, and impact how we feel in our everyday lives.”
Your First Hypnosis Session
A hypnosis session always begins with deep relaxation, which can help your mind and body grow accustomed to what it’s like to feel calm, said Ms. Leyes. “By giving the brain and body experiences of safety, relaxation, and inner peace, we can more easily let go of our stressful thoughts of the day and drift off to sleep with ease at night.”
You will often start by sitting or lying in a comfortable position, and then the hypnotic induction begins with a focus of attention, according to Dr. Elkins. The person concentrates, with their eyelids closed, and then are given suggestions for deepening their relaxed state. “Usually that’s a safe, pleasant place, such as walking through the mountains or being near a beach,” he said. “And within that, suggestions are given that target the mechanism that underlies the symptoms [such as hot flashes].”
Dr. Spiegel usually starts off with a neutral test that can help measure how hypnotizable a person is on a 0-to-10 scale. For example, instructing the client to imagine that their hand is floating in the air. If they pull their hand down and it floats back up, the client finds they can “actually dissociate the psychological from the physiological aspects of their experience – their left hand feels different from their right hand,” Dr. Spiegel said. “I use that as an example for them to say, ‘look how you can change how your body feels. Now, let’s use it to help you with your anxiety with your menopausal symptoms.’ ”
A version of this article appeared on WebMD.com.
Does Bariatric Surgery Also Improve Thyroid Function?
TOPLINE:
Metabolic/bariatric surgery (MBS) reduces thyroid-stimulating hormone (TSH), free triiodothyronine (fT3) levels, and thyroid hormone resistance indices in patients with obesity, changes strongly correlated with improvement in body composition.
METHODOLOGY:
- Recent studies have linked obesity with increased levels of TSH and thyroid hormones; however, the role that body fat distribution plays in this association remains unclear.
- This retrospective observational study evaluated the effects of MBS on thyroid hormone levels and thyroid hormone resistance in euthyroid individuals with obesity, focusing on the correlation with changes in body composition.
- Researchers included 470 patients with obesity (mean age, 33.4 years; mean body mass index [BMI], 37.9; 63.2% women) and 118 control individuals without obesity (mean BMI, 21.8), who had had normal levels of TSH, fT3, and free thyroxine.
- Among the patients with obesity, 125 underwent MBS and had thyroid tests both before and ≥ 3 months after surgery.
- Data on body composition and thyroid function were collected, and correlations between baseline and changes in thyroid function and body composition were assessed.
TAKEAWAY:
- Individuals with obesity had higher baseline TSH and fT3 levels (P < .001) and thyroid feedback quantile-based index (TFQI; P = .047) than those without obesity, with the values decreasing after MBS (all P < .001).
- Among individuals with obesity, preoperative TSH was positively correlated with the visceral fat area (VFA; P = .019) and body fat percentage (P = .013) and negatively correlated with skeletal muscle mass percentage (P = .024)
- The decrease in TSH post-surgery positively correlated with decreased VFA (P = .021) and decreased body fat percentage (P = .031).
- Decrease in VFA and body fat percentage after MBS was also associated with improved central thyroid hormone resistance indicated by TFQI.
IN PRACTICE:
“The relationship between obesity and [thyroid hormone] is bidirectional, indicating that addressing underlying thyroid disturbance could potentially benefit weight loss and metabolism,” the authors wrote.
SOURCE:
This study was led by Yu Yan, MD, Department of Pancreatic and Metabolic Surgery, Medical School of Southeast University, Nanjing Drum Tower Hospital, Nanjing, China, and published online in The Journal of Clinical Endocrinology & Metabolism.
LIMITATIONS:
The retrospective nature of this study limited the ability to definitively attribute changes in thyroid function and thyroid hormone resistance to changes in body composition. The relatively short duration of the study and the exclusion of individuals taking medications affecting thyroid function may also limit the generalizability of the findings.
DISCLOSURES:
This study was supported by the Fundings for Clinical Trials from the Affiliated Drum Tower Hospital, Nanjing University Medical School, Nanjing, China. The authors declared no potential conflicts of interest.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
Metabolic/bariatric surgery (MBS) reduces thyroid-stimulating hormone (TSH), free triiodothyronine (fT3) levels, and thyroid hormone resistance indices in patients with obesity, changes strongly correlated with improvement in body composition.
METHODOLOGY:
- Recent studies have linked obesity with increased levels of TSH and thyroid hormones; however, the role that body fat distribution plays in this association remains unclear.
- This retrospective observational study evaluated the effects of MBS on thyroid hormone levels and thyroid hormone resistance in euthyroid individuals with obesity, focusing on the correlation with changes in body composition.
- Researchers included 470 patients with obesity (mean age, 33.4 years; mean body mass index [BMI], 37.9; 63.2% women) and 118 control individuals without obesity (mean BMI, 21.8), who had had normal levels of TSH, fT3, and free thyroxine.
- Among the patients with obesity, 125 underwent MBS and had thyroid tests both before and ≥ 3 months after surgery.
- Data on body composition and thyroid function were collected, and correlations between baseline and changes in thyroid function and body composition were assessed.
TAKEAWAY:
- Individuals with obesity had higher baseline TSH and fT3 levels (P < .001) and thyroid feedback quantile-based index (TFQI; P = .047) than those without obesity, with the values decreasing after MBS (all P < .001).
- Among individuals with obesity, preoperative TSH was positively correlated with the visceral fat area (VFA; P = .019) and body fat percentage (P = .013) and negatively correlated with skeletal muscle mass percentage (P = .024)
- The decrease in TSH post-surgery positively correlated with decreased VFA (P = .021) and decreased body fat percentage (P = .031).
- Decrease in VFA and body fat percentage after MBS was also associated with improved central thyroid hormone resistance indicated by TFQI.
IN PRACTICE:
“The relationship between obesity and [thyroid hormone] is bidirectional, indicating that addressing underlying thyroid disturbance could potentially benefit weight loss and metabolism,” the authors wrote.
SOURCE:
This study was led by Yu Yan, MD, Department of Pancreatic and Metabolic Surgery, Medical School of Southeast University, Nanjing Drum Tower Hospital, Nanjing, China, and published online in The Journal of Clinical Endocrinology & Metabolism.
LIMITATIONS:
The retrospective nature of this study limited the ability to definitively attribute changes in thyroid function and thyroid hormone resistance to changes in body composition. The relatively short duration of the study and the exclusion of individuals taking medications affecting thyroid function may also limit the generalizability of the findings.
DISCLOSURES:
This study was supported by the Fundings for Clinical Trials from the Affiliated Drum Tower Hospital, Nanjing University Medical School, Nanjing, China. The authors declared no potential conflicts of interest.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
Metabolic/bariatric surgery (MBS) reduces thyroid-stimulating hormone (TSH), free triiodothyronine (fT3) levels, and thyroid hormone resistance indices in patients with obesity, changes strongly correlated with improvement in body composition.
METHODOLOGY:
- Recent studies have linked obesity with increased levels of TSH and thyroid hormones; however, the role that body fat distribution plays in this association remains unclear.
- This retrospective observational study evaluated the effects of MBS on thyroid hormone levels and thyroid hormone resistance in euthyroid individuals with obesity, focusing on the correlation with changes in body composition.
- Researchers included 470 patients with obesity (mean age, 33.4 years; mean body mass index [BMI], 37.9; 63.2% women) and 118 control individuals without obesity (mean BMI, 21.8), who had had normal levels of TSH, fT3, and free thyroxine.
- Among the patients with obesity, 125 underwent MBS and had thyroid tests both before and ≥ 3 months after surgery.
- Data on body composition and thyroid function were collected, and correlations between baseline and changes in thyroid function and body composition were assessed.
TAKEAWAY:
- Individuals with obesity had higher baseline TSH and fT3 levels (P < .001) and thyroid feedback quantile-based index (TFQI; P = .047) than those without obesity, with the values decreasing after MBS (all P < .001).
- Among individuals with obesity, preoperative TSH was positively correlated with the visceral fat area (VFA; P = .019) and body fat percentage (P = .013) and negatively correlated with skeletal muscle mass percentage (P = .024)
- The decrease in TSH post-surgery positively correlated with decreased VFA (P = .021) and decreased body fat percentage (P = .031).
- Decrease in VFA and body fat percentage after MBS was also associated with improved central thyroid hormone resistance indicated by TFQI.
IN PRACTICE:
“The relationship between obesity and [thyroid hormone] is bidirectional, indicating that addressing underlying thyroid disturbance could potentially benefit weight loss and metabolism,” the authors wrote.
SOURCE:
This study was led by Yu Yan, MD, Department of Pancreatic and Metabolic Surgery, Medical School of Southeast University, Nanjing Drum Tower Hospital, Nanjing, China, and published online in The Journal of Clinical Endocrinology & Metabolism.
LIMITATIONS:
The retrospective nature of this study limited the ability to definitively attribute changes in thyroid function and thyroid hormone resistance to changes in body composition. The relatively short duration of the study and the exclusion of individuals taking medications affecting thyroid function may also limit the generalizability of the findings.
DISCLOSURES:
This study was supported by the Fundings for Clinical Trials from the Affiliated Drum Tower Hospital, Nanjing University Medical School, Nanjing, China. The authors declared no potential conflicts of interest.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
Psilocybin Bests SSRI for Major Depression in First Long-Term Comparison
MILAN — Psilocybin leads to a better overall outcome in the treatment of moderate to severe major depressive disorder (MDD) than the selective serotonin reuptake inhibitor (SSRI) escitalopram, results of the first long-term comparison of the two treatments suggest.
“This is the first work to compare the long-term effects of these two drugs in the context of overall well-being, not just freedom from depression,” study investigator Tommaso Barba, PhD candidate at Imperial College London in England, said in a press release. “Psilocybin outperformed escitalopram in several measures of well-being, meaning in life, work, and social functioning.”
Findings from the 6-month follow-up study of a phase 2 double-blind, randomized, controlled trial were presented at the 37th European College of Neuropsychopharmacology (ECNP) Congress and published simultaneously in The Lancet eClinicalMedicine
Addressing a Treatment ‘Mismatch’
The findings are important because they address “a mismatch” between what psychiatrists and what patients think is important, Mr. Barba said in an interview.
“Psychiatrists really focus on negative symptoms of depression. So, if you are not sad anymore, if your sleep or appetite is not impaired, they think you’re better. But if you look at what patients define as important, they say it’s the degree in which their life is meaningful, in which they can connect with people around them, in which they can function in everyday life,” Mr. Barba said.
“The study suggests that psilocybin therapy might be a more holistic treatment option for depression,” added co–first author David Erritzoe, MD, PhD, clinical director and deputy head of the Centre for Psychedelic Research, Imperial College London. “This could make a substantial difference in the overall happiness and daily activities of those suffering from depression, providing a more joined-up approach to mental health treatment.”
The initial single-center study included 59 adults with MDD (mean age, 41 years) who were randomized to receive either psilocybin or escitalopram over a 6-week period. The psilocybin arm (n = 30) received two 25-mg oral doses of psilocybin therapy (PT), and the escitalopram arm (n = 29) received 10-20 mg of daily escitalopram plus two (placebo-like) 1-mg doses of psilocybin (ET). Both groups received psychological support.
Based on change in depression scores on the 16-item Quick Inventory of Depressive Symptomatology–Self-Report (QIDS-SR-16) at week 6, the initial study results suggested noninferiority between the two treatments in terms of depressive symptoms (primary outcome), but superiority of PT for secondary outcomes including “well-being, anhedonia, social functioning, sexual functioning, and related variables, with fewer side effects compared to ET,” the researchers noted.
The new 6-month follow-up findings, with monthly questionnaires and no additional study treatment or psychiatric treatment restrictions, measured the QIDS-SR-16, plus Work and Social Adjustment Scale (WSAS), Meaning in Life Questionnaire, Flourishing Scale (FS), and Watts Connectedness Scale (WCS).
Again, both groups maintained similar results on the QIDS-SR-16, with slightly greater reductions in depressive symptoms for PT in the first month (positive false discovery rate [pFDR] = 0.021), but not thereafter.
At both 3 and 6 months, there were greater improvements in WSAS scores for the PT group (pFDR < 0.001 and pFDR = 0.01, respectively), and also greater improvements in meaning in life across all follow-up timepoints (pFDR < 0.001).
There was also greater improvement in the PT group regarding WCS at both 3 and 6 months (pFDR = 0.02, and pFDR = 0.04) and comparable FS improvements for both groups across all timepoints.
Confounding follow-up interventions may have muddied the results, with 30.7% of PT participants and 43.5% of ET participants receiving an additional intervention during this period.
The researchers conclude that while a short course of SSRIs combined with intensive therapeutic support (around 20 hours) “might be enough to induce sustained antidepressant effects,” patients treated with psilocybin showed greater improvements in general functioning, connectedness, and meaning in life.
Although not reassessed in the follow-up, the initial study showed that adverse events, particularly sexual functioning, favored psilocybin, said Mr. Barba. “The two treatments seemed to go in opposite directions with psilocybin seeming to improve it and the antidepressant to suppress it. Other side effects associated with psilocybin were less diverse — mainly headaches at the end of the day — but with escitalopram they were way more diverse and more impairing.”
Although many therapists may be unfamiliar with psilocybin-assisted psychotherapy, “it’s not a difficult skill to master. It might require some specialization, but I think if you’re a good psychotherapist, you can learn how to implement psilocybin into your practice,” he said.
“Normally the journey is quite inward, so patients do not require active support during the psychedelic experience [around 6 hours]. Sometimes they do require some hand-holding, or helping them to ‘let go’, or breathing exercises. The important part is the integration work that comes afterwards,” Mr. Barba added.
He said he envisions a therapy program that involves “psychiatrists working together with psychotherapists. The psychotherapists would be more in charge of the active guiding, and the psychiatrist would do the prescribing, with the follow-up psychological support on Zoom.”
He added a word of caution for therapists that “psilocybin requires active confrontation of painful, negative emotions and people who take this drug need to be open and prepared for the idea that they are going into a state where they may probably end up crying and confronting whatever they are maybe running away from in their lives. Not everyone may want to do this.”
A New Treatment Paradigm?
In a comment, Johan Lundberg, MD, PhD, adjunct professor of psychiatry at the Department of Clinical Neuroscience, Karolinska Institute, Stockholm, Sweden, said the study addresses a key outstanding question about the long-term effects of one or two doses of psilocybin.
“It’s a 6-month follow-up of a short treatment intervention, so in that sense, it’s of high interest. It has been talked about that psilocybin might have a long-term effect, but this is the first study that has followed this for a longer term.”
But Dr. Lundberg also pointed out that one shortcoming of the study is the diversity of treatments following the intervention.
“They didn’t have control over whether patients received other treatments or when they started. So, that is a key concern. But they transparently reported that, and we do know there was a difference in reported ability to perform activities of daily life, and that is important.”
He added that if psilocybin is eventually approved, it would likely come with an education package for providers — “which is already the case with other treatments like ECT [electroconvulsive therapy] or TMS [transcranial magnetic stimulation] — you have to learn how to do it.”
James Rucker, MD, PhD, psychiatrist and senior clinical lecturer at King’s College London, who was not involved in the research, also noted that they have tended to attribute differences observed in this study to comparative differences between the drugs themselves.
However, he noted, it is also possible that the results reflect biased reporting between groups. This is more likely here because studies involving psilocybin tend to attract those with positive preconceptions about psilocybin and negative preconceptions about conventional antidepressants, and study participants were unblinded during the long-term follow-up phase, so knew which condition they were allocated to.
“This said, the nature of depression varies hugely between individuals, and this calls for the development of a similarly varied suite of treatment paradigms. Psilocybin therapy is certainly a different paradigm of treatment to escitalopram. The observation of similar levels of effectiveness to antidepressants here is encouraging to see alongside the much larger trials of psilocybin currently underway here in the UK, Europe, and the US,” Dr. Rucker added.
This work was supported by The Alexander Mosley Charitable Trust and by the founding partners of Imperial College London’s Centre for Psychedelic Research.
Mr. Barba reported having received consulting fees from Adamo Bioscience. Both Dr. Lundberg and Dr. Rucker are involved in psilocybin research, but neither reported financial links.
A version of this article first appeared on Medscape.com.
MILAN — Psilocybin leads to a better overall outcome in the treatment of moderate to severe major depressive disorder (MDD) than the selective serotonin reuptake inhibitor (SSRI) escitalopram, results of the first long-term comparison of the two treatments suggest.
“This is the first work to compare the long-term effects of these two drugs in the context of overall well-being, not just freedom from depression,” study investigator Tommaso Barba, PhD candidate at Imperial College London in England, said in a press release. “Psilocybin outperformed escitalopram in several measures of well-being, meaning in life, work, and social functioning.”
Findings from the 6-month follow-up study of a phase 2 double-blind, randomized, controlled trial were presented at the 37th European College of Neuropsychopharmacology (ECNP) Congress and published simultaneously in The Lancet eClinicalMedicine
Addressing a Treatment ‘Mismatch’
The findings are important because they address “a mismatch” between what psychiatrists and what patients think is important, Mr. Barba said in an interview.
“Psychiatrists really focus on negative symptoms of depression. So, if you are not sad anymore, if your sleep or appetite is not impaired, they think you’re better. But if you look at what patients define as important, they say it’s the degree in which their life is meaningful, in which they can connect with people around them, in which they can function in everyday life,” Mr. Barba said.
“The study suggests that psilocybin therapy might be a more holistic treatment option for depression,” added co–first author David Erritzoe, MD, PhD, clinical director and deputy head of the Centre for Psychedelic Research, Imperial College London. “This could make a substantial difference in the overall happiness and daily activities of those suffering from depression, providing a more joined-up approach to mental health treatment.”
The initial single-center study included 59 adults with MDD (mean age, 41 years) who were randomized to receive either psilocybin or escitalopram over a 6-week period. The psilocybin arm (n = 30) received two 25-mg oral doses of psilocybin therapy (PT), and the escitalopram arm (n = 29) received 10-20 mg of daily escitalopram plus two (placebo-like) 1-mg doses of psilocybin (ET). Both groups received psychological support.
Based on change in depression scores on the 16-item Quick Inventory of Depressive Symptomatology–Self-Report (QIDS-SR-16) at week 6, the initial study results suggested noninferiority between the two treatments in terms of depressive symptoms (primary outcome), but superiority of PT for secondary outcomes including “well-being, anhedonia, social functioning, sexual functioning, and related variables, with fewer side effects compared to ET,” the researchers noted.
The new 6-month follow-up findings, with monthly questionnaires and no additional study treatment or psychiatric treatment restrictions, measured the QIDS-SR-16, plus Work and Social Adjustment Scale (WSAS), Meaning in Life Questionnaire, Flourishing Scale (FS), and Watts Connectedness Scale (WCS).
Again, both groups maintained similar results on the QIDS-SR-16, with slightly greater reductions in depressive symptoms for PT in the first month (positive false discovery rate [pFDR] = 0.021), but not thereafter.
At both 3 and 6 months, there were greater improvements in WSAS scores for the PT group (pFDR < 0.001 and pFDR = 0.01, respectively), and also greater improvements in meaning in life across all follow-up timepoints (pFDR < 0.001).
There was also greater improvement in the PT group regarding WCS at both 3 and 6 months (pFDR = 0.02, and pFDR = 0.04) and comparable FS improvements for both groups across all timepoints.
Confounding follow-up interventions may have muddied the results, with 30.7% of PT participants and 43.5% of ET participants receiving an additional intervention during this period.
The researchers conclude that while a short course of SSRIs combined with intensive therapeutic support (around 20 hours) “might be enough to induce sustained antidepressant effects,” patients treated with psilocybin showed greater improvements in general functioning, connectedness, and meaning in life.
Although not reassessed in the follow-up, the initial study showed that adverse events, particularly sexual functioning, favored psilocybin, said Mr. Barba. “The two treatments seemed to go in opposite directions with psilocybin seeming to improve it and the antidepressant to suppress it. Other side effects associated with psilocybin were less diverse — mainly headaches at the end of the day — but with escitalopram they were way more diverse and more impairing.”
Although many therapists may be unfamiliar with psilocybin-assisted psychotherapy, “it’s not a difficult skill to master. It might require some specialization, but I think if you’re a good psychotherapist, you can learn how to implement psilocybin into your practice,” he said.
“Normally the journey is quite inward, so patients do not require active support during the psychedelic experience [around 6 hours]. Sometimes they do require some hand-holding, or helping them to ‘let go’, or breathing exercises. The important part is the integration work that comes afterwards,” Mr. Barba added.
He said he envisions a therapy program that involves “psychiatrists working together with psychotherapists. The psychotherapists would be more in charge of the active guiding, and the psychiatrist would do the prescribing, with the follow-up psychological support on Zoom.”
He added a word of caution for therapists that “psilocybin requires active confrontation of painful, negative emotions and people who take this drug need to be open and prepared for the idea that they are going into a state where they may probably end up crying and confronting whatever they are maybe running away from in their lives. Not everyone may want to do this.”
A New Treatment Paradigm?
In a comment, Johan Lundberg, MD, PhD, adjunct professor of psychiatry at the Department of Clinical Neuroscience, Karolinska Institute, Stockholm, Sweden, said the study addresses a key outstanding question about the long-term effects of one or two doses of psilocybin.
“It’s a 6-month follow-up of a short treatment intervention, so in that sense, it’s of high interest. It has been talked about that psilocybin might have a long-term effect, but this is the first study that has followed this for a longer term.”
But Dr. Lundberg also pointed out that one shortcoming of the study is the diversity of treatments following the intervention.
“They didn’t have control over whether patients received other treatments or when they started. So, that is a key concern. But they transparently reported that, and we do know there was a difference in reported ability to perform activities of daily life, and that is important.”
He added that if psilocybin is eventually approved, it would likely come with an education package for providers — “which is already the case with other treatments like ECT [electroconvulsive therapy] or TMS [transcranial magnetic stimulation] — you have to learn how to do it.”
James Rucker, MD, PhD, psychiatrist and senior clinical lecturer at King’s College London, who was not involved in the research, also noted that they have tended to attribute differences observed in this study to comparative differences between the drugs themselves.
However, he noted, it is also possible that the results reflect biased reporting between groups. This is more likely here because studies involving psilocybin tend to attract those with positive preconceptions about psilocybin and negative preconceptions about conventional antidepressants, and study participants were unblinded during the long-term follow-up phase, so knew which condition they were allocated to.
“This said, the nature of depression varies hugely between individuals, and this calls for the development of a similarly varied suite of treatment paradigms. Psilocybin therapy is certainly a different paradigm of treatment to escitalopram. The observation of similar levels of effectiveness to antidepressants here is encouraging to see alongside the much larger trials of psilocybin currently underway here in the UK, Europe, and the US,” Dr. Rucker added.
This work was supported by The Alexander Mosley Charitable Trust and by the founding partners of Imperial College London’s Centre for Psychedelic Research.
Mr. Barba reported having received consulting fees from Adamo Bioscience. Both Dr. Lundberg and Dr. Rucker are involved in psilocybin research, but neither reported financial links.
A version of this article first appeared on Medscape.com.
MILAN — Psilocybin leads to a better overall outcome in the treatment of moderate to severe major depressive disorder (MDD) than the selective serotonin reuptake inhibitor (SSRI) escitalopram, results of the first long-term comparison of the two treatments suggest.
“This is the first work to compare the long-term effects of these two drugs in the context of overall well-being, not just freedom from depression,” study investigator Tommaso Barba, PhD candidate at Imperial College London in England, said in a press release. “Psilocybin outperformed escitalopram in several measures of well-being, meaning in life, work, and social functioning.”
Findings from the 6-month follow-up study of a phase 2 double-blind, randomized, controlled trial were presented at the 37th European College of Neuropsychopharmacology (ECNP) Congress and published simultaneously in The Lancet eClinicalMedicine
Addressing a Treatment ‘Mismatch’
The findings are important because they address “a mismatch” between what psychiatrists and what patients think is important, Mr. Barba said in an interview.
“Psychiatrists really focus on negative symptoms of depression. So, if you are not sad anymore, if your sleep or appetite is not impaired, they think you’re better. But if you look at what patients define as important, they say it’s the degree in which their life is meaningful, in which they can connect with people around them, in which they can function in everyday life,” Mr. Barba said.
“The study suggests that psilocybin therapy might be a more holistic treatment option for depression,” added co–first author David Erritzoe, MD, PhD, clinical director and deputy head of the Centre for Psychedelic Research, Imperial College London. “This could make a substantial difference in the overall happiness and daily activities of those suffering from depression, providing a more joined-up approach to mental health treatment.”
The initial single-center study included 59 adults with MDD (mean age, 41 years) who were randomized to receive either psilocybin or escitalopram over a 6-week period. The psilocybin arm (n = 30) received two 25-mg oral doses of psilocybin therapy (PT), and the escitalopram arm (n = 29) received 10-20 mg of daily escitalopram plus two (placebo-like) 1-mg doses of psilocybin (ET). Both groups received psychological support.
Based on change in depression scores on the 16-item Quick Inventory of Depressive Symptomatology–Self-Report (QIDS-SR-16) at week 6, the initial study results suggested noninferiority between the two treatments in terms of depressive symptoms (primary outcome), but superiority of PT for secondary outcomes including “well-being, anhedonia, social functioning, sexual functioning, and related variables, with fewer side effects compared to ET,” the researchers noted.
The new 6-month follow-up findings, with monthly questionnaires and no additional study treatment or psychiatric treatment restrictions, measured the QIDS-SR-16, plus Work and Social Adjustment Scale (WSAS), Meaning in Life Questionnaire, Flourishing Scale (FS), and Watts Connectedness Scale (WCS).
Again, both groups maintained similar results on the QIDS-SR-16, with slightly greater reductions in depressive symptoms for PT in the first month (positive false discovery rate [pFDR] = 0.021), but not thereafter.
At both 3 and 6 months, there were greater improvements in WSAS scores for the PT group (pFDR < 0.001 and pFDR = 0.01, respectively), and also greater improvements in meaning in life across all follow-up timepoints (pFDR < 0.001).
There was also greater improvement in the PT group regarding WCS at both 3 and 6 months (pFDR = 0.02, and pFDR = 0.04) and comparable FS improvements for both groups across all timepoints.
Confounding follow-up interventions may have muddied the results, with 30.7% of PT participants and 43.5% of ET participants receiving an additional intervention during this period.
The researchers conclude that while a short course of SSRIs combined with intensive therapeutic support (around 20 hours) “might be enough to induce sustained antidepressant effects,” patients treated with psilocybin showed greater improvements in general functioning, connectedness, and meaning in life.
Although not reassessed in the follow-up, the initial study showed that adverse events, particularly sexual functioning, favored psilocybin, said Mr. Barba. “The two treatments seemed to go in opposite directions with psilocybin seeming to improve it and the antidepressant to suppress it. Other side effects associated with psilocybin were less diverse — mainly headaches at the end of the day — but with escitalopram they were way more diverse and more impairing.”
Although many therapists may be unfamiliar with psilocybin-assisted psychotherapy, “it’s not a difficult skill to master. It might require some specialization, but I think if you’re a good psychotherapist, you can learn how to implement psilocybin into your practice,” he said.
“Normally the journey is quite inward, so patients do not require active support during the psychedelic experience [around 6 hours]. Sometimes they do require some hand-holding, or helping them to ‘let go’, or breathing exercises. The important part is the integration work that comes afterwards,” Mr. Barba added.
He said he envisions a therapy program that involves “psychiatrists working together with psychotherapists. The psychotherapists would be more in charge of the active guiding, and the psychiatrist would do the prescribing, with the follow-up psychological support on Zoom.”
He added a word of caution for therapists that “psilocybin requires active confrontation of painful, negative emotions and people who take this drug need to be open and prepared for the idea that they are going into a state where they may probably end up crying and confronting whatever they are maybe running away from in their lives. Not everyone may want to do this.”
A New Treatment Paradigm?
In a comment, Johan Lundberg, MD, PhD, adjunct professor of psychiatry at the Department of Clinical Neuroscience, Karolinska Institute, Stockholm, Sweden, said the study addresses a key outstanding question about the long-term effects of one or two doses of psilocybin.
“It’s a 6-month follow-up of a short treatment intervention, so in that sense, it’s of high interest. It has been talked about that psilocybin might have a long-term effect, but this is the first study that has followed this for a longer term.”
But Dr. Lundberg also pointed out that one shortcoming of the study is the diversity of treatments following the intervention.
“They didn’t have control over whether patients received other treatments or when they started. So, that is a key concern. But they transparently reported that, and we do know there was a difference in reported ability to perform activities of daily life, and that is important.”
He added that if psilocybin is eventually approved, it would likely come with an education package for providers — “which is already the case with other treatments like ECT [electroconvulsive therapy] or TMS [transcranial magnetic stimulation] — you have to learn how to do it.”
James Rucker, MD, PhD, psychiatrist and senior clinical lecturer at King’s College London, who was not involved in the research, also noted that they have tended to attribute differences observed in this study to comparative differences between the drugs themselves.
However, he noted, it is also possible that the results reflect biased reporting between groups. This is more likely here because studies involving psilocybin tend to attract those with positive preconceptions about psilocybin and negative preconceptions about conventional antidepressants, and study participants were unblinded during the long-term follow-up phase, so knew which condition they were allocated to.
“This said, the nature of depression varies hugely between individuals, and this calls for the development of a similarly varied suite of treatment paradigms. Psilocybin therapy is certainly a different paradigm of treatment to escitalopram. The observation of similar levels of effectiveness to antidepressants here is encouraging to see alongside the much larger trials of psilocybin currently underway here in the UK, Europe, and the US,” Dr. Rucker added.
This work was supported by The Alexander Mosley Charitable Trust and by the founding partners of Imperial College London’s Centre for Psychedelic Research.
Mr. Barba reported having received consulting fees from Adamo Bioscience. Both Dr. Lundberg and Dr. Rucker are involved in psilocybin research, but neither reported financial links.
A version of this article first appeared on Medscape.com.
FROM ECNP 2024
Novel Agent First to Slow Disability in Nonrelapsing Secondary MS
COPENHAGEN — A new investigational drug has become the first agent to slow disability in patients with nonrelapsing secondary progressive multiple sclerosis (nrSPMS).
In addition, tolebrutinib almost doubled the number of patients who experienced confirmed disability improvement from 5% to 10%.
However, these benefits come with the potential safety issue of liver toxicity, with raised liver enzymes reported in 4% of patients and very severe liver enzyme rises occurring in 0.5% of patients, one of whom died after undergoing a liver transplant.
The results were presented by Robert Fox, MD, vice chair of research at the Cleveland Clinic’s Neurological Institute in Ohio, at the 2024 ECTRIMS annual meeting.
“We have finally found a therapy that can alter the compartmentalized inflammation that is driving progressive MS,” he said.
Dr. Fox pointed out that the population enrolled in the HERCULES trial had stopped having clinical relapses. “These are the patients for whom current immunomodulator therapies really don’t work at all — they don’t slow disability. This trial suggests that tolebrutinib can fill that void and now we have something to offer this patient group,” he said.
He estimated that up to 30% of patients with MS at his clinic may fall into this category.
A typical patient with nrSPMS who was included in this trial may have experienced a gradual decline in the distance they can walk or the ease with which they could climb stairs, he explained.
“I would project that this therapy will slow down that gradual decline, and, in some patients, it may actually stop the decline,” he added.
Dr. Fox said that BTK inhibitors are believed to have two main mechanisms of action relevant to MS — down-regulating B cells, probably mostly in the periphery, and, as these agents can cross the blood-brain barrier, they also appear to reduce the inflammatory activity of microglia and macrophages in the brain.
He noted that the disability progression in nrSPMS patients is thought to be caused by compartmentalized inflammation in the brain, which is what tolebrutinib may be targeting.
He noted that siponimod has also shown benefit in secondary progressive MS in the EXPAND trial, but the benefit was almost entirely restricted to patients who had experienced recent relapses.
Ocrelizumab has been shown to be beneficial in a trial in primary progressive MS, but again, a large proportion of patients in that study had active focal inflammation at baselineEngl J Med. 2017;376:209-220).
Trial Results
The HERCULES trial included 1131 patients with nrSPMS, defined as having an Expanded Disability Status Scale score (EDSS) between 3.0 and 6.5, no clinical relapses in the previous 24 months, and documented evidence of disability accumulation in the previous 12 months.
They were randomly assigned (2:1) to receive 60 mg tolebrutinib as an oral daily dose or placebo for up to approximately 48 months. This was an event-driven trial, with 288 6-month confirmed disability progression events required.
About 23% of patients in each group discontinued treatment and 12%-17% who had confirmed disability progression elected to crossover to open-label tolebrutinib.
The study population had an average age of 49 years, had a median EDSS score of 6, and a mean time since last clinical relapse of over 7 years.
“So, this was a really very quiescent patient population in terms of focal inflammation,” Dr. Fox noted.
Results showed that the primary endpoint showed a 31% reduction in the risk of 6-month confirmed disability progression (26.9% tolebrutinib vs. 37.2% placebo; hazard ratio [HR], 0.69; 95% CI, 0.55-0.88).
Rates of 3-month confirmed disability progression were 32.6% in the tolebrutinib group versus 41.5% with placebo — a 24% risk reduction.
In addition, 6-month confirmed disability improvement was achieved by 10% of tolebrutinib patients versus 5% in the placebo group (HR, 1.88; 95% CI, 1.10-3.21).
A ‘Head-scratcher’ Finding
Surprisingly, he noted, tolebrutinib did not appear to slow brain atrophy.
“Despite seeing a benefit on disability progression, we saw no significant slowing of brain atrophy or brain volume loss over the course of the study,” Dr. Fox reported.
He described this discordance between disability rates and brain volume loss rates as “a bit of a head-scratcher.”
In terms of safety, the main concern is liver enzyme elevations, which occurred at greater than three times the upper limit of normal (ULN) in 4.1% of the tolebrutinib group vs 1.6% in the placebo group.
A small (0.5%) proportion of patients treated with tolebrutinib experienced very severe elevations (> 20 x ULN) in liver enzymes, and one of these patients had to have a liver transplant and died because of postoperative complications, “a reminder that this can be a very serious complication of this drug,” said Dr. Fox.
However, he noted that all the very severe liver enzyme rises occurred in the first 3 months and it is now recommended that patients undergo weekly liver enzyme monitoring for the first 12 weeks of treatment.
Other adverse effects that were increased slightly in tolebrutinib group were upper respiratory infections and possibly hypertension.
Weekly Liver Enzyme Testing
Dr. Fox cautioned that patients starting tolebrutinib would need to undergo weekly liver enzyme testing in the first few months of treatment. “They would need to be very attentive to this monitoring, but if they are willing to do that, then I think many of these patients will be very eager to take this drug that may slow down their disability progression.”
The drug’s manufacturer, Sanofi, said the trial results will form the basis for applications to global regulatory authorities with submissions starting later in 2024.
Commenting on the trial, Ludwig Kappos, MD, professor of neurology at University Hospital and University of Basel, Switzerland, said the trial was important as it had shown “a robust effect on confirmed disability progression in a population of secondary progressive MS with no or very low signs of focal inflammation.”
“The effect is similar and probably more pronounced than that seen in the siponimod trial also in advanced secondary progressive MS,” he added.
Dr. Kappos believes more work will be needed to make sure the liver toxicity can be prevented, “but if that can be resolved then patients could have a significant delay in accumulating disability.”
GEMINI Trials Also Show Slowed Disability
Two other phase 3 trials of tolebrutinib were presented during the same ECTRIMS session — GEMINI 1 and 2 — which compared the new drug with teriflunomide, a standard of care treatment, in participants with relapsing MS. Neither study met the primary endpoint of an improvement in annualized relapse rates, compared with teriflunomide.
However, with respect to the key secondary endpoint, in a pooled analysis of data from GEMINI 1 and 2, tolebrutinib delayed the time to onset of 6-month confirmed disability worsening by 29%, a finding in line with the main results of the HERCULES trial.
“The significant impact of tolebrutinib on disability accumulation versus teriflunomide, in the absence of a statistically superior impact on relapses, also suggests that tolebrutinib may address smoldering neuroinflammation, which manifests as progression independent of relapses” Dr. Fox said.
The HERCULES trial was sponsored by Sanofi. Dr. Fox is a paid adviser to Sanofi. Dr. Kappos led the EXPAND trial of siponimod in SPMS.
A version of this article first appeared on Medscape.com.
COPENHAGEN — A new investigational drug has become the first agent to slow disability in patients with nonrelapsing secondary progressive multiple sclerosis (nrSPMS).
In addition, tolebrutinib almost doubled the number of patients who experienced confirmed disability improvement from 5% to 10%.
However, these benefits come with the potential safety issue of liver toxicity, with raised liver enzymes reported in 4% of patients and very severe liver enzyme rises occurring in 0.5% of patients, one of whom died after undergoing a liver transplant.
The results were presented by Robert Fox, MD, vice chair of research at the Cleveland Clinic’s Neurological Institute in Ohio, at the 2024 ECTRIMS annual meeting.
“We have finally found a therapy that can alter the compartmentalized inflammation that is driving progressive MS,” he said.
Dr. Fox pointed out that the population enrolled in the HERCULES trial had stopped having clinical relapses. “These are the patients for whom current immunomodulator therapies really don’t work at all — they don’t slow disability. This trial suggests that tolebrutinib can fill that void and now we have something to offer this patient group,” he said.
He estimated that up to 30% of patients with MS at his clinic may fall into this category.
A typical patient with nrSPMS who was included in this trial may have experienced a gradual decline in the distance they can walk or the ease with which they could climb stairs, he explained.
“I would project that this therapy will slow down that gradual decline, and, in some patients, it may actually stop the decline,” he added.
Dr. Fox said that BTK inhibitors are believed to have two main mechanisms of action relevant to MS — down-regulating B cells, probably mostly in the periphery, and, as these agents can cross the blood-brain barrier, they also appear to reduce the inflammatory activity of microglia and macrophages in the brain.
He noted that the disability progression in nrSPMS patients is thought to be caused by compartmentalized inflammation in the brain, which is what tolebrutinib may be targeting.
He noted that siponimod has also shown benefit in secondary progressive MS in the EXPAND trial, but the benefit was almost entirely restricted to patients who had experienced recent relapses.
Ocrelizumab has been shown to be beneficial in a trial in primary progressive MS, but again, a large proportion of patients in that study had active focal inflammation at baselineEngl J Med. 2017;376:209-220).
Trial Results
The HERCULES trial included 1131 patients with nrSPMS, defined as having an Expanded Disability Status Scale score (EDSS) between 3.0 and 6.5, no clinical relapses in the previous 24 months, and documented evidence of disability accumulation in the previous 12 months.
They were randomly assigned (2:1) to receive 60 mg tolebrutinib as an oral daily dose or placebo for up to approximately 48 months. This was an event-driven trial, with 288 6-month confirmed disability progression events required.
About 23% of patients in each group discontinued treatment and 12%-17% who had confirmed disability progression elected to crossover to open-label tolebrutinib.
The study population had an average age of 49 years, had a median EDSS score of 6, and a mean time since last clinical relapse of over 7 years.
“So, this was a really very quiescent patient population in terms of focal inflammation,” Dr. Fox noted.
Results showed that the primary endpoint showed a 31% reduction in the risk of 6-month confirmed disability progression (26.9% tolebrutinib vs. 37.2% placebo; hazard ratio [HR], 0.69; 95% CI, 0.55-0.88).
Rates of 3-month confirmed disability progression were 32.6% in the tolebrutinib group versus 41.5% with placebo — a 24% risk reduction.
In addition, 6-month confirmed disability improvement was achieved by 10% of tolebrutinib patients versus 5% in the placebo group (HR, 1.88; 95% CI, 1.10-3.21).
A ‘Head-scratcher’ Finding
Surprisingly, he noted, tolebrutinib did not appear to slow brain atrophy.
“Despite seeing a benefit on disability progression, we saw no significant slowing of brain atrophy or brain volume loss over the course of the study,” Dr. Fox reported.
He described this discordance between disability rates and brain volume loss rates as “a bit of a head-scratcher.”
In terms of safety, the main concern is liver enzyme elevations, which occurred at greater than three times the upper limit of normal (ULN) in 4.1% of the tolebrutinib group vs 1.6% in the placebo group.
A small (0.5%) proportion of patients treated with tolebrutinib experienced very severe elevations (> 20 x ULN) in liver enzymes, and one of these patients had to have a liver transplant and died because of postoperative complications, “a reminder that this can be a very serious complication of this drug,” said Dr. Fox.
However, he noted that all the very severe liver enzyme rises occurred in the first 3 months and it is now recommended that patients undergo weekly liver enzyme monitoring for the first 12 weeks of treatment.
Other adverse effects that were increased slightly in tolebrutinib group were upper respiratory infections and possibly hypertension.
Weekly Liver Enzyme Testing
Dr. Fox cautioned that patients starting tolebrutinib would need to undergo weekly liver enzyme testing in the first few months of treatment. “They would need to be very attentive to this monitoring, but if they are willing to do that, then I think many of these patients will be very eager to take this drug that may slow down their disability progression.”
The drug’s manufacturer, Sanofi, said the trial results will form the basis for applications to global regulatory authorities with submissions starting later in 2024.
Commenting on the trial, Ludwig Kappos, MD, professor of neurology at University Hospital and University of Basel, Switzerland, said the trial was important as it had shown “a robust effect on confirmed disability progression in a population of secondary progressive MS with no or very low signs of focal inflammation.”
“The effect is similar and probably more pronounced than that seen in the siponimod trial also in advanced secondary progressive MS,” he added.
Dr. Kappos believes more work will be needed to make sure the liver toxicity can be prevented, “but if that can be resolved then patients could have a significant delay in accumulating disability.”
GEMINI Trials Also Show Slowed Disability
Two other phase 3 trials of tolebrutinib were presented during the same ECTRIMS session — GEMINI 1 and 2 — which compared the new drug with teriflunomide, a standard of care treatment, in participants with relapsing MS. Neither study met the primary endpoint of an improvement in annualized relapse rates, compared with teriflunomide.
However, with respect to the key secondary endpoint, in a pooled analysis of data from GEMINI 1 and 2, tolebrutinib delayed the time to onset of 6-month confirmed disability worsening by 29%, a finding in line with the main results of the HERCULES trial.
“The significant impact of tolebrutinib on disability accumulation versus teriflunomide, in the absence of a statistically superior impact on relapses, also suggests that tolebrutinib may address smoldering neuroinflammation, which manifests as progression independent of relapses” Dr. Fox said.
The HERCULES trial was sponsored by Sanofi. Dr. Fox is a paid adviser to Sanofi. Dr. Kappos led the EXPAND trial of siponimod in SPMS.
A version of this article first appeared on Medscape.com.
COPENHAGEN — A new investigational drug has become the first agent to slow disability in patients with nonrelapsing secondary progressive multiple sclerosis (nrSPMS).
In addition, tolebrutinib almost doubled the number of patients who experienced confirmed disability improvement from 5% to 10%.
However, these benefits come with the potential safety issue of liver toxicity, with raised liver enzymes reported in 4% of patients and very severe liver enzyme rises occurring in 0.5% of patients, one of whom died after undergoing a liver transplant.
The results were presented by Robert Fox, MD, vice chair of research at the Cleveland Clinic’s Neurological Institute in Ohio, at the 2024 ECTRIMS annual meeting.
“We have finally found a therapy that can alter the compartmentalized inflammation that is driving progressive MS,” he said.
Dr. Fox pointed out that the population enrolled in the HERCULES trial had stopped having clinical relapses. “These are the patients for whom current immunomodulator therapies really don’t work at all — they don’t slow disability. This trial suggests that tolebrutinib can fill that void and now we have something to offer this patient group,” he said.
He estimated that up to 30% of patients with MS at his clinic may fall into this category.
A typical patient with nrSPMS who was included in this trial may have experienced a gradual decline in the distance they can walk or the ease with which they could climb stairs, he explained.
“I would project that this therapy will slow down that gradual decline, and, in some patients, it may actually stop the decline,” he added.
Dr. Fox said that BTK inhibitors are believed to have two main mechanisms of action relevant to MS — down-regulating B cells, probably mostly in the periphery, and, as these agents can cross the blood-brain barrier, they also appear to reduce the inflammatory activity of microglia and macrophages in the brain.
He noted that the disability progression in nrSPMS patients is thought to be caused by compartmentalized inflammation in the brain, which is what tolebrutinib may be targeting.
He noted that siponimod has also shown benefit in secondary progressive MS in the EXPAND trial, but the benefit was almost entirely restricted to patients who had experienced recent relapses.
Ocrelizumab has been shown to be beneficial in a trial in primary progressive MS, but again, a large proportion of patients in that study had active focal inflammation at baselineEngl J Med. 2017;376:209-220).
Trial Results
The HERCULES trial included 1131 patients with nrSPMS, defined as having an Expanded Disability Status Scale score (EDSS) between 3.0 and 6.5, no clinical relapses in the previous 24 months, and documented evidence of disability accumulation in the previous 12 months.
They were randomly assigned (2:1) to receive 60 mg tolebrutinib as an oral daily dose or placebo for up to approximately 48 months. This was an event-driven trial, with 288 6-month confirmed disability progression events required.
About 23% of patients in each group discontinued treatment and 12%-17% who had confirmed disability progression elected to crossover to open-label tolebrutinib.
The study population had an average age of 49 years, had a median EDSS score of 6, and a mean time since last clinical relapse of over 7 years.
“So, this was a really very quiescent patient population in terms of focal inflammation,” Dr. Fox noted.
Results showed that the primary endpoint showed a 31% reduction in the risk of 6-month confirmed disability progression (26.9% tolebrutinib vs. 37.2% placebo; hazard ratio [HR], 0.69; 95% CI, 0.55-0.88).
Rates of 3-month confirmed disability progression were 32.6% in the tolebrutinib group versus 41.5% with placebo — a 24% risk reduction.
In addition, 6-month confirmed disability improvement was achieved by 10% of tolebrutinib patients versus 5% in the placebo group (HR, 1.88; 95% CI, 1.10-3.21).
A ‘Head-scratcher’ Finding
Surprisingly, he noted, tolebrutinib did not appear to slow brain atrophy.
“Despite seeing a benefit on disability progression, we saw no significant slowing of brain atrophy or brain volume loss over the course of the study,” Dr. Fox reported.
He described this discordance between disability rates and brain volume loss rates as “a bit of a head-scratcher.”
In terms of safety, the main concern is liver enzyme elevations, which occurred at greater than three times the upper limit of normal (ULN) in 4.1% of the tolebrutinib group vs 1.6% in the placebo group.
A small (0.5%) proportion of patients treated with tolebrutinib experienced very severe elevations (> 20 x ULN) in liver enzymes, and one of these patients had to have a liver transplant and died because of postoperative complications, “a reminder that this can be a very serious complication of this drug,” said Dr. Fox.
However, he noted that all the very severe liver enzyme rises occurred in the first 3 months and it is now recommended that patients undergo weekly liver enzyme monitoring for the first 12 weeks of treatment.
Other adverse effects that were increased slightly in tolebrutinib group were upper respiratory infections and possibly hypertension.
Weekly Liver Enzyme Testing
Dr. Fox cautioned that patients starting tolebrutinib would need to undergo weekly liver enzyme testing in the first few months of treatment. “They would need to be very attentive to this monitoring, but if they are willing to do that, then I think many of these patients will be very eager to take this drug that may slow down their disability progression.”
The drug’s manufacturer, Sanofi, said the trial results will form the basis for applications to global regulatory authorities with submissions starting later in 2024.
Commenting on the trial, Ludwig Kappos, MD, professor of neurology at University Hospital and University of Basel, Switzerland, said the trial was important as it had shown “a robust effect on confirmed disability progression in a population of secondary progressive MS with no or very low signs of focal inflammation.”
“The effect is similar and probably more pronounced than that seen in the siponimod trial also in advanced secondary progressive MS,” he added.
Dr. Kappos believes more work will be needed to make sure the liver toxicity can be prevented, “but if that can be resolved then patients could have a significant delay in accumulating disability.”
GEMINI Trials Also Show Slowed Disability
Two other phase 3 trials of tolebrutinib were presented during the same ECTRIMS session — GEMINI 1 and 2 — which compared the new drug with teriflunomide, a standard of care treatment, in participants with relapsing MS. Neither study met the primary endpoint of an improvement in annualized relapse rates, compared with teriflunomide.
However, with respect to the key secondary endpoint, in a pooled analysis of data from GEMINI 1 and 2, tolebrutinib delayed the time to onset of 6-month confirmed disability worsening by 29%, a finding in line with the main results of the HERCULES trial.
“The significant impact of tolebrutinib on disability accumulation versus teriflunomide, in the absence of a statistically superior impact on relapses, also suggests that tolebrutinib may address smoldering neuroinflammation, which manifests as progression independent of relapses” Dr. Fox said.
The HERCULES trial was sponsored by Sanofi. Dr. Fox is a paid adviser to Sanofi. Dr. Kappos led the EXPAND trial of siponimod in SPMS.
A version of this article first appeared on Medscape.com.
FROM ECTRIMS 2024
Muscle Relaxants for Chronic Pain: Where Is the Greatest Evidence?
TOPLINE:
The long-term use of muscle relaxants may benefit patients with painful spasms or cramps and neck pain, according to a systematic review of clinical studies, but they do not appear to be beneficial for low back pain, fibromyalgia, or headaches and can have adverse effects such as sedation and dry mouth.
METHODOLOGY:
- Researchers conducted a systematic review to evaluate the effectiveness of long-term use (≥ 4 weeks) of muscle relaxants for chronic pain lasting ≥ 3 months.
- They identified 30 randomized clinical trials involving 1314 patients and 14 cohort studies involving 1168 patients, grouped according to the categories of low back pain, fibromyalgia, painful cramps or spasticity, headaches, and other syndromes.
- Baclofen, tizanidine, cyclobenzaprine, eperisone, quinine, carisoprodol, orphenadrine, chlormezanone, and methocarbamol were the muscle relaxants assessed in comparison with placebo, other treatments, or untreated individuals.
TAKEAWAY:
- The long-term use of muscle relaxants reduced pain intensity in those with painful spasms or cramps and neck pain. Baclofen, orphenadrine, carisoprodol, and methocarbamol improved cramp frequency, while the use of eperisone and chlormezanone improved neck pain and enhanced the quality of sleep, respectively, in those with neck osteoarthritis.
- While some studies suggested that muscle relaxants reduced pain intensity in those with back pain and fibromyalgia, between-group differences were not observed. The benefits seen with some medications diminished after their discontinuation.
- Despite tizanidine improving pain severity in headaches, 25% participants dropped out owing to adverse effects. Although certain muscle relaxants demonstrated pain relief, others did not.
- The most common adverse effects of muscle relaxants were somnolence and dry mouth. Other adverse events included vomiting, diarrhea, nausea, weakness, and constipation.
IN PRACTICE:
“For patients already prescribed long-term SMRs [skeletal muscle relaxants], interventions are needed to assist clinicians to engage in shared decision-making with patients about deprescribing SMRs. This may be particularly true for older patients for whom risks of adverse events may be greater,” the authors wrote. “Clinicians should be vigilant for adverse effects and consider deprescribing if pain-related goals are not met.”
SOURCE:
The study, led by Benjamin J. Oldfield, MD, MHS, Yale School of Medicine, New Haven, Connecticut, was published online on September 19, 2024, in JAMA Network Open
LIMITATIONS:
This systematic review was limited to publications written in English, Spanish, and Italian language, potentially excluding studies from other regions. Variations in clinical sites, definitions of pain syndromes, medications, and durations of therapy prevented the possibility of conducting meta-analyses. Only quantitative studies were included, excluding valuable insights into patient experiences offered by qualitative studies.
DISCLOSURES:
The study was supported by the National Institute on Drug Abuse. The authors declared no conflicts of interest.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
The long-term use of muscle relaxants may benefit patients with painful spasms or cramps and neck pain, according to a systematic review of clinical studies, but they do not appear to be beneficial for low back pain, fibromyalgia, or headaches and can have adverse effects such as sedation and dry mouth.
METHODOLOGY:
- Researchers conducted a systematic review to evaluate the effectiveness of long-term use (≥ 4 weeks) of muscle relaxants for chronic pain lasting ≥ 3 months.
- They identified 30 randomized clinical trials involving 1314 patients and 14 cohort studies involving 1168 patients, grouped according to the categories of low back pain, fibromyalgia, painful cramps or spasticity, headaches, and other syndromes.
- Baclofen, tizanidine, cyclobenzaprine, eperisone, quinine, carisoprodol, orphenadrine, chlormezanone, and methocarbamol were the muscle relaxants assessed in comparison with placebo, other treatments, or untreated individuals.
TAKEAWAY:
- The long-term use of muscle relaxants reduced pain intensity in those with painful spasms or cramps and neck pain. Baclofen, orphenadrine, carisoprodol, and methocarbamol improved cramp frequency, while the use of eperisone and chlormezanone improved neck pain and enhanced the quality of sleep, respectively, in those with neck osteoarthritis.
- While some studies suggested that muscle relaxants reduced pain intensity in those with back pain and fibromyalgia, between-group differences were not observed. The benefits seen with some medications diminished after their discontinuation.
- Despite tizanidine improving pain severity in headaches, 25% participants dropped out owing to adverse effects. Although certain muscle relaxants demonstrated pain relief, others did not.
- The most common adverse effects of muscle relaxants were somnolence and dry mouth. Other adverse events included vomiting, diarrhea, nausea, weakness, and constipation.
IN PRACTICE:
“For patients already prescribed long-term SMRs [skeletal muscle relaxants], interventions are needed to assist clinicians to engage in shared decision-making with patients about deprescribing SMRs. This may be particularly true for older patients for whom risks of adverse events may be greater,” the authors wrote. “Clinicians should be vigilant for adverse effects and consider deprescribing if pain-related goals are not met.”
SOURCE:
The study, led by Benjamin J. Oldfield, MD, MHS, Yale School of Medicine, New Haven, Connecticut, was published online on September 19, 2024, in JAMA Network Open
LIMITATIONS:
This systematic review was limited to publications written in English, Spanish, and Italian language, potentially excluding studies from other regions. Variations in clinical sites, definitions of pain syndromes, medications, and durations of therapy prevented the possibility of conducting meta-analyses. Only quantitative studies were included, excluding valuable insights into patient experiences offered by qualitative studies.
DISCLOSURES:
The study was supported by the National Institute on Drug Abuse. The authors declared no conflicts of interest.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
The long-term use of muscle relaxants may benefit patients with painful spasms or cramps and neck pain, according to a systematic review of clinical studies, but they do not appear to be beneficial for low back pain, fibromyalgia, or headaches and can have adverse effects such as sedation and dry mouth.
METHODOLOGY:
- Researchers conducted a systematic review to evaluate the effectiveness of long-term use (≥ 4 weeks) of muscle relaxants for chronic pain lasting ≥ 3 months.
- They identified 30 randomized clinical trials involving 1314 patients and 14 cohort studies involving 1168 patients, grouped according to the categories of low back pain, fibromyalgia, painful cramps or spasticity, headaches, and other syndromes.
- Baclofen, tizanidine, cyclobenzaprine, eperisone, quinine, carisoprodol, orphenadrine, chlormezanone, and methocarbamol were the muscle relaxants assessed in comparison with placebo, other treatments, or untreated individuals.
TAKEAWAY:
- The long-term use of muscle relaxants reduced pain intensity in those with painful spasms or cramps and neck pain. Baclofen, orphenadrine, carisoprodol, and methocarbamol improved cramp frequency, while the use of eperisone and chlormezanone improved neck pain and enhanced the quality of sleep, respectively, in those with neck osteoarthritis.
- While some studies suggested that muscle relaxants reduced pain intensity in those with back pain and fibromyalgia, between-group differences were not observed. The benefits seen with some medications diminished after their discontinuation.
- Despite tizanidine improving pain severity in headaches, 25% participants dropped out owing to adverse effects. Although certain muscle relaxants demonstrated pain relief, others did not.
- The most common adverse effects of muscle relaxants were somnolence and dry mouth. Other adverse events included vomiting, diarrhea, nausea, weakness, and constipation.
IN PRACTICE:
“For patients already prescribed long-term SMRs [skeletal muscle relaxants], interventions are needed to assist clinicians to engage in shared decision-making with patients about deprescribing SMRs. This may be particularly true for older patients for whom risks of adverse events may be greater,” the authors wrote. “Clinicians should be vigilant for adverse effects and consider deprescribing if pain-related goals are not met.”
SOURCE:
The study, led by Benjamin J. Oldfield, MD, MHS, Yale School of Medicine, New Haven, Connecticut, was published online on September 19, 2024, in JAMA Network Open
LIMITATIONS:
This systematic review was limited to publications written in English, Spanish, and Italian language, potentially excluding studies from other regions. Variations in clinical sites, definitions of pain syndromes, medications, and durations of therapy prevented the possibility of conducting meta-analyses. Only quantitative studies were included, excluding valuable insights into patient experiences offered by qualitative studies.
DISCLOSURES:
The study was supported by the National Institute on Drug Abuse. The authors declared no conflicts of interest.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
Patient Navigators in Rheumatology Set to Expand in Importance, Scope With New Medicare Codes
When a large rheumatology clinic in Richmond, Virginia, heard that Medicare would be reimbursing patient navigators, they decided to launch their own virtual navigator program.
“We read about it and felt like it was the perfect representation of what we were already trying to do,” said Blake Wehman, founder and CEO of Remission Medical, which offers virtual diagnosis and longitudinal care in rheumatology.
Mr. Wehman has plans to start submitting for these principal illness navigation (PIN) codes in 2025.
The Centers for Medicare & Medicaid Services (CMS) in 2024 began paying navigators who assist Medicare patients with high-risk conditions, which could include rheumatologic diseases. “The codes are not limited to a specific set of diagnoses; rather, the definition of a serious, high-risk condition is dependent on clinical judgment,” the agency clarified.
CMS established this provision in the CY 2024 Physician Fee Schedule final rule.
Reimbursing patient navigators is long overdue, noted Edith Williams, PhD, MS, director of the Center for Community Health and Prevention and founding director of the Office of Health Equity Research at the University of Rochester in New York. “It’s something our patients need. It’s something that the science is telling us can impact outcomes as an adjunct to clinical care,” she said.
Dr. Williams said the new CMS codes “got our departments talking about what this policy is and how it would translate into patient care.”
The codes apply when navigators are assigned to support patients with high-risk conditions who need assistance connecting with clinical and other resources, including any unmet social determinants of health needs, or in diagnosis or treatment of their medical problems.
“Having a navigator by their side to help get through all the clinical and administrative challenges gives people an advocate and a partner who is with them and their families every step of the way to help make the journey easier,” said a CMS spokesperson.
Not all navigator programs may qualify for the new codes. Some are supported by grants and don’t bill patient insurance. However, they all share a common goal: to guide patients through the healthcare continuum and assist with appointments and medication adherence.
Identifying ‘Root Causes’ of Barriers
Navigators represent a wide variety of backgrounds, ranging from healthcare professionals to students or even patients themselves. They generally don’t provide medical advice. “However, we are responsible for making sure our patients and their families are educated and aware, then assist with guidance on their path,” said Katie Costillo, BSW, CPPN, patient navigator and program manager with the Lupus Foundation of America, Heartland Region.
“Training and experience in engaging and building rapport is essential to assisting patients overcome obstacles that limit their access to healthcare,” she said. Narrowing down with patients the root causes of their barriers and then identifying appropriate and available community resources is key.
Studies have demonstrated the effectiveness of adding a navigator to a rheumatology patient’s care plan. In one study, a group of Boston researchers determined that navigators played a useful role in reducing adherence barriers to oral disease-modifying antirheumatic drugs. The navigators uncovered several concerns among 107 rheumatology patients, including fear of adverse events and medication effectiveness.
They also helped to facilitate patient-physician communication, developed strategies to improve medication adherence, and provided medication and diagnosis education. Patients reported satisfaction with the navigator experience.
A study Dr. Williams coauthored that examined behavioral interventions to support African American women with systemic lupus erythematosus found that patient navigator participants had superior coping scores, compared with those engaged in peer-to-peer methodology and patient support groups.
“We had a lot of success with the mentorship program, too,” Dr. Williams said. Navigator services, however, offer more one-on-one attention, “and it’s more tailored to what the person needs rather than the set curriculum that the mentors delivered to their mentees.”
Supporting Patients With Lupus
Ideally, navigators should be able to relate to patients and know what they’re going through, Dr. Williams said. This is someone whom the patient can trust and depend on. “That’s where the benefit of having someone who is also a patient lies because they’re ultimately relatable to other patients. But different institutions have taken different approaches to this.”
Some programs focus on specific rheumatologic conditions. The Lupus Foundation of America, for example, established patient navigator programs to assist patients with lupus in four markets across the country.
The Heartland patient navigator program is available for all patients with lupus within its region, which includes Kansas, Missouri, and central and southern Illinois. As a navigator, Ms. Costillo has been assisting patients since 2022. In 2023, she began meeting with patients at the Washington University Lupus Clinic (WULC) in St. Louis, Missouri.
Navigators work directly with patients before and after their appointment to ensure follow-up and reduce missed appointments. “They help lupus patients connect with community services and overcoming barriers to access and care. The goal of this position is to improve overall disease management, which results in better health outcomes,” Ms. Costillo said.
Since its inception, the patient navigator program at WULC has shown a decrease in patient no-call no-shows and an increase in requests to reschedule as opposed to not showing up for their scheduled appointment, based on history.
Patients have reported fewer barriers to transportation and improvement in access to resources, support, and disease education. “Our patients have also stated [that] meeting with the navigator during their appointments has helped them to feel heard, understood, and supported,” Ms. Costillo said.
Navigator Work Is Not Without Challenges
A total of 90% of patients with lupus are women, and women of color are two to three times more likely to develop lupus in their lifetime.
“Based on socioeconomic statistics, lupus patients are in a demographic that is commonly underserved, underfunded, and often overlooked. Finding appropriate local community resources for a patient who must choose between feeding her family or paying for transportation to multiple physician appointments is a common problem,” Ms. Costillo said.
Much of the assistance that became available during the COVID pandemic is starting to disappear. “With the rising costs of daily living, we are having to find creative and alternative ways to break down barriers and find support to fill those gaps,” she continued.
Getting insurance coverage of patients is another challenge. Many patients with lupus will be prescribed a treatment that insurance refuses to cover even after the physician disputes it.
Additionally, many patients with lupus are unable to work to support their family. A majority who apply for Social Security Disability Insurance are denied on their first and second attempts, “requiring multiple hearings and pages of documentation from their physicians,” Ms. Costillo said.
Students Serve as Navigators
One inner-city program is seeking to increase access to healthcare services to patients with lupus and lupus nephritis in underserved communities. In 2021, SUNY Downstate Health Sciences University in New York City, in partnership with the Brooklyn Free Clinic and Brooklyn Health Disparities Center, launched a program to teach navigator skills to second-year medical students.
The students assist patients at the Arthritis Clinic at University Hospital at Downstate. “Many of our patients have either low medical literacy or difficulty with English. Many of them are immigrants,” said Ellen M. Ginzler, MD, MPH, SUNY Downstate’s professor emerita and former vice-chair for research and rheumatology division chief.
Dr. Ginzler sought out navigator candidates who showed a strong interest in working with underserved patients with complicated, severe disease who struggled with keeping appointments or adhering to medication regimens. The program also gave preference to students fluent in other languages such as Spanish.
All these efforts have generated improvements in care.
Assessing the program’s effectiveness in a cross-sectional study, Dr. Ginzler and colleagues reported that 94% of navigators were able to schedule appointments and 87% assisted with prescriptions. Navigators also had high success rates in answering medical questions, getting in touch with a patient’s doctor, and reminding patients of medical appointments.
Medical student Jeremy Wilson, a coauthor of the study, served as a navigator for a woman with lupus and scleroderma for many years, along with other comorbidities.
Mr. Wilson went above and beyond for this patient, helping to secure social services supports that included accompanying her to clinic visits and serving as her advocate. “She found an enormous difference in how she was treated when she went to these clinics because the doctors in those clinics took her much more seriously,” Dr. Ginzler said. Mr. Wilson ran interference to secure clinic appointments and worked with the patient’s rheumatology fellow in the clinic to get approval for medications.
Mr. Wilson and the patient formed a great bond. “It not only helped the patient, but it helped Jeremy tremendously in terms of how he felt about his medical career,” Dr. Ginzler said.
The program has since expanded to include patients with other rheumatic diseases, such as rheumatoid arthritis and psoriatic arthritis, and also offers navigator services in dermatology.
A total of 21 students to date have completed the second year of the program. “We’ve just selected eight more,” Dr. Ginzler said. Some of the students continue to do the program in their third or even fourth year as they’re applying for residencies.
A student-run, unpublished survey of nine students in the SUNY program found that all nine reported high confidence in identifying social factors that impact patient health and well-being, compared with four who reported high confidence prior to starting the program. “Additionally, students reported increased confidence in providing comprehensive care in rheumatology and dermatology, and interdisciplinary collaboration,” study author Alejandra K. Moncayo, MPH, and colleagues wrote.
When Navigators Go Virtual
Remission Medical offers its navigator service through its own standalone virtual clinic.
Pain associated with rheumatologic conditions increases the urgency to see a doctor. The goal of the virtual RemissionNavigator program is to meet rheumatology patients where they live, to bridge care gaps and reduce wait times, said Mr. Wehman.
RemissionNavigator accomplishes this through video visits and unlimited texting to its network of board-certified rheumatologists or rheumatology-focused advanced practice providers. Experts can answer questions about why labs are ordered, why a patient may have received a certain diagnosis, or provide detailed explanations of a rheumatic condition.
“There are instances where improvement for the patient means waiting a couple days for us versus 45 days for their brick-and-mortar choice,” Mr. Wehman said.
The program currently has 36 subscribers to Remission’s services, which include navigation. “We have 15 providers in a blend of employed and contracted relationships with Remission,” Mr. Wehman said.
Even in its infancy, the navigator program has produced some success stories. “We had a patient tell us that thanks to us, he was seen faster, found relief immediately through our diagnosis and prescription of methotrexate, felt better at work, lost weight, and was happier in general,” Mr. Wehman said.
Another patient was making monthly, 90-minute trips to Richmond for infusion services. Through the virtual program’s assistance, she is now receiving care from home and can get her monthly infusions at a local clinic.
Ultimately, the goal is to help rheumatology move into an era of value-based care where the transition from fee-for-service to per patient will enable optimized care models and better accessibility, Mr. Wehman said. “It will not happen overnight, but every day we work toward this future.”
VA Targets Rheumatology Care
The Department of Veterans Affairs (VA) has also explored the use of navigator services in rheumatology, including virtual services.
VA uses an integrated, interdisciplinary model that manages each veteran’s individual healthcare needs through a coordinated effort among providers, nurses, social workers, pharmacists, and other health professionals, according to VA press secretary Terrence Hayes.
Care coordination may include supporting scheduling appointments, managing chronic conditions, and coordinating care across different medical departments. “This coordination is particularly important in managing complex rheumatologic conditions, where multiple providers may be involved,” Mr. Hayes said.
Additionally, VA has launched a national telerheumatology initiative to improve access to rheumatology providers in rural areas. The initiative will assist veterans in understanding the telehealth system, navigating appointments, and ensuring they have the necessary technology for virtual consultations.
“It will also facilitate communication between rheumatologists, primary care providers, and other specialists, ensuring that all team members are aligned in their approach to the veteran’s care,” Mr. Hayes said.
Who Will Take Advantage of New Codes?
Currently, Remission Medical operates on a cash-pay model, but the company intends to transition to insurance-based coverage in 2025.
Remission Medical also partners directly with preexisting healthcare systems and clinics such as Sentara Health and OrthoVirginia, where a PIN program, powered by Remission Medical’s virtual rheumatology network, may be explored as well.
The company offers its partners synchronous virtual visits and e-consults. It’s likely that these larger organizations will explore coverage for navigator services for Medicare and private insurance. “We can be there to support them as they decide to implement this,” Mr. Wehman said.
Taking advantage of CMS’s navigator PIN codes is an eventual goal. Remission Medical has not submitted the codes yet, “but we do intend to as we continue to grow our membership count,” Mr. Wehman said. “We hope to provide coverage for most of the US and submit the codes to reimbursement by early to mid-2025.”
In terms of reimbursement, the VA operates under a different payment model than Medicare or private insurance, focusing on providing integrated care within the VA system rather than reimbursing for specific services such as patient navigation.
While the SUNY clinic takes care of Medicare patients, it’s unlikely that the new CMS codes for navigators would apply to medical students. Students get paid a monthly stipend for doing navigator work. “There’s a policy about what students can get paid, and how many hours they can work,” Dr. Ginzler clarified.
The SUNY Downstate and Lupus Foundation navigator programs rely on grants to sustain their services. Aurinia Pharmaceuticals has funded both programs, and the SUNY program received an additional grant from Janssen to expand its offerings.
Because it’s grant funded, the navigator position at the Lupus Foundation does not bill patient insurance, Ms. Costillo explained.
Navigator Work Requires Training
Before they start working with patients, navigators often go through a vetting or training process. At Remission Medical, a clinical leadership team does a synchronous interview, background check, and CV review of its potential navigators.
Even before she became a navigator, Ms. Costillo had a strong baseline education in this work. She has a bachelor’s degree in social work and 15 years of experience in social services working with disabled, vulnerable, and underserved populations. Some of her fellow navigators at the Lupus Foundation of America also have degrees in social work.
Ms. Costillo underwent training with the Patient-Centered Education & Research Institute to become a certified professional patient navigator. Her name is on the national registry. The curriculum covered various aspects of medical care such as patient and care team interactions and communications, health and clinical knowledge, patient care coordination and resources, and using evidence-based approaches.
“For our lupus patients, it is essential that navigators understand the disease and the impact on patients and families, treatments available and those in the pipelines, and also the ins and outs of various insurance options,” Ms. Costillo said.
Mr. Wehman, Dr. Williams, and Ms. Costillo reported no disclosures. Dr. Ginzler has been a consultant for Aurinia Pharmaceuticals.
A version of this article first appeared on Medscape.com.
When a large rheumatology clinic in Richmond, Virginia, heard that Medicare would be reimbursing patient navigators, they decided to launch their own virtual navigator program.
“We read about it and felt like it was the perfect representation of what we were already trying to do,” said Blake Wehman, founder and CEO of Remission Medical, which offers virtual diagnosis and longitudinal care in rheumatology.
Mr. Wehman has plans to start submitting for these principal illness navigation (PIN) codes in 2025.
The Centers for Medicare & Medicaid Services (CMS) in 2024 began paying navigators who assist Medicare patients with high-risk conditions, which could include rheumatologic diseases. “The codes are not limited to a specific set of diagnoses; rather, the definition of a serious, high-risk condition is dependent on clinical judgment,” the agency clarified.
CMS established this provision in the CY 2024 Physician Fee Schedule final rule.
Reimbursing patient navigators is long overdue, noted Edith Williams, PhD, MS, director of the Center for Community Health and Prevention and founding director of the Office of Health Equity Research at the University of Rochester in New York. “It’s something our patients need. It’s something that the science is telling us can impact outcomes as an adjunct to clinical care,” she said.
Dr. Williams said the new CMS codes “got our departments talking about what this policy is and how it would translate into patient care.”
The codes apply when navigators are assigned to support patients with high-risk conditions who need assistance connecting with clinical and other resources, including any unmet social determinants of health needs, or in diagnosis or treatment of their medical problems.
“Having a navigator by their side to help get through all the clinical and administrative challenges gives people an advocate and a partner who is with them and their families every step of the way to help make the journey easier,” said a CMS spokesperson.
Not all navigator programs may qualify for the new codes. Some are supported by grants and don’t bill patient insurance. However, they all share a common goal: to guide patients through the healthcare continuum and assist with appointments and medication adherence.
Identifying ‘Root Causes’ of Barriers
Navigators represent a wide variety of backgrounds, ranging from healthcare professionals to students or even patients themselves. They generally don’t provide medical advice. “However, we are responsible for making sure our patients and their families are educated and aware, then assist with guidance on their path,” said Katie Costillo, BSW, CPPN, patient navigator and program manager with the Lupus Foundation of America, Heartland Region.
“Training and experience in engaging and building rapport is essential to assisting patients overcome obstacles that limit their access to healthcare,” she said. Narrowing down with patients the root causes of their barriers and then identifying appropriate and available community resources is key.
Studies have demonstrated the effectiveness of adding a navigator to a rheumatology patient’s care plan. In one study, a group of Boston researchers determined that navigators played a useful role in reducing adherence barriers to oral disease-modifying antirheumatic drugs. The navigators uncovered several concerns among 107 rheumatology patients, including fear of adverse events and medication effectiveness.
They also helped to facilitate patient-physician communication, developed strategies to improve medication adherence, and provided medication and diagnosis education. Patients reported satisfaction with the navigator experience.
A study Dr. Williams coauthored that examined behavioral interventions to support African American women with systemic lupus erythematosus found that patient navigator participants had superior coping scores, compared with those engaged in peer-to-peer methodology and patient support groups.
“We had a lot of success with the mentorship program, too,” Dr. Williams said. Navigator services, however, offer more one-on-one attention, “and it’s more tailored to what the person needs rather than the set curriculum that the mentors delivered to their mentees.”
Supporting Patients With Lupus
Ideally, navigators should be able to relate to patients and know what they’re going through, Dr. Williams said. This is someone whom the patient can trust and depend on. “That’s where the benefit of having someone who is also a patient lies because they’re ultimately relatable to other patients. But different institutions have taken different approaches to this.”
Some programs focus on specific rheumatologic conditions. The Lupus Foundation of America, for example, established patient navigator programs to assist patients with lupus in four markets across the country.
The Heartland patient navigator program is available for all patients with lupus within its region, which includes Kansas, Missouri, and central and southern Illinois. As a navigator, Ms. Costillo has been assisting patients since 2022. In 2023, she began meeting with patients at the Washington University Lupus Clinic (WULC) in St. Louis, Missouri.
Navigators work directly with patients before and after their appointment to ensure follow-up and reduce missed appointments. “They help lupus patients connect with community services and overcoming barriers to access and care. The goal of this position is to improve overall disease management, which results in better health outcomes,” Ms. Costillo said.
Since its inception, the patient navigator program at WULC has shown a decrease in patient no-call no-shows and an increase in requests to reschedule as opposed to not showing up for their scheduled appointment, based on history.
Patients have reported fewer barriers to transportation and improvement in access to resources, support, and disease education. “Our patients have also stated [that] meeting with the navigator during their appointments has helped them to feel heard, understood, and supported,” Ms. Costillo said.
Navigator Work Is Not Without Challenges
A total of 90% of patients with lupus are women, and women of color are two to three times more likely to develop lupus in their lifetime.
“Based on socioeconomic statistics, lupus patients are in a demographic that is commonly underserved, underfunded, and often overlooked. Finding appropriate local community resources for a patient who must choose between feeding her family or paying for transportation to multiple physician appointments is a common problem,” Ms. Costillo said.
Much of the assistance that became available during the COVID pandemic is starting to disappear. “With the rising costs of daily living, we are having to find creative and alternative ways to break down barriers and find support to fill those gaps,” she continued.
Getting insurance coverage of patients is another challenge. Many patients with lupus will be prescribed a treatment that insurance refuses to cover even after the physician disputes it.
Additionally, many patients with lupus are unable to work to support their family. A majority who apply for Social Security Disability Insurance are denied on their first and second attempts, “requiring multiple hearings and pages of documentation from their physicians,” Ms. Costillo said.
Students Serve as Navigators
One inner-city program is seeking to increase access to healthcare services to patients with lupus and lupus nephritis in underserved communities. In 2021, SUNY Downstate Health Sciences University in New York City, in partnership with the Brooklyn Free Clinic and Brooklyn Health Disparities Center, launched a program to teach navigator skills to second-year medical students.
The students assist patients at the Arthritis Clinic at University Hospital at Downstate. “Many of our patients have either low medical literacy or difficulty with English. Many of them are immigrants,” said Ellen M. Ginzler, MD, MPH, SUNY Downstate’s professor emerita and former vice-chair for research and rheumatology division chief.
Dr. Ginzler sought out navigator candidates who showed a strong interest in working with underserved patients with complicated, severe disease who struggled with keeping appointments or adhering to medication regimens. The program also gave preference to students fluent in other languages such as Spanish.
All these efforts have generated improvements in care.
Assessing the program’s effectiveness in a cross-sectional study, Dr. Ginzler and colleagues reported that 94% of navigators were able to schedule appointments and 87% assisted with prescriptions. Navigators also had high success rates in answering medical questions, getting in touch with a patient’s doctor, and reminding patients of medical appointments.
Medical student Jeremy Wilson, a coauthor of the study, served as a navigator for a woman with lupus and scleroderma for many years, along with other comorbidities.
Mr. Wilson went above and beyond for this patient, helping to secure social services supports that included accompanying her to clinic visits and serving as her advocate. “She found an enormous difference in how she was treated when she went to these clinics because the doctors in those clinics took her much more seriously,” Dr. Ginzler said. Mr. Wilson ran interference to secure clinic appointments and worked with the patient’s rheumatology fellow in the clinic to get approval for medications.
Mr. Wilson and the patient formed a great bond. “It not only helped the patient, but it helped Jeremy tremendously in terms of how he felt about his medical career,” Dr. Ginzler said.
The program has since expanded to include patients with other rheumatic diseases, such as rheumatoid arthritis and psoriatic arthritis, and also offers navigator services in dermatology.
A total of 21 students to date have completed the second year of the program. “We’ve just selected eight more,” Dr. Ginzler said. Some of the students continue to do the program in their third or even fourth year as they’re applying for residencies.
A student-run, unpublished survey of nine students in the SUNY program found that all nine reported high confidence in identifying social factors that impact patient health and well-being, compared with four who reported high confidence prior to starting the program. “Additionally, students reported increased confidence in providing comprehensive care in rheumatology and dermatology, and interdisciplinary collaboration,” study author Alejandra K. Moncayo, MPH, and colleagues wrote.
When Navigators Go Virtual
Remission Medical offers its navigator service through its own standalone virtual clinic.
Pain associated with rheumatologic conditions increases the urgency to see a doctor. The goal of the virtual RemissionNavigator program is to meet rheumatology patients where they live, to bridge care gaps and reduce wait times, said Mr. Wehman.
RemissionNavigator accomplishes this through video visits and unlimited texting to its network of board-certified rheumatologists or rheumatology-focused advanced practice providers. Experts can answer questions about why labs are ordered, why a patient may have received a certain diagnosis, or provide detailed explanations of a rheumatic condition.
“There are instances where improvement for the patient means waiting a couple days for us versus 45 days for their brick-and-mortar choice,” Mr. Wehman said.
The program currently has 36 subscribers to Remission’s services, which include navigation. “We have 15 providers in a blend of employed and contracted relationships with Remission,” Mr. Wehman said.
Even in its infancy, the navigator program has produced some success stories. “We had a patient tell us that thanks to us, he was seen faster, found relief immediately through our diagnosis and prescription of methotrexate, felt better at work, lost weight, and was happier in general,” Mr. Wehman said.
Another patient was making monthly, 90-minute trips to Richmond for infusion services. Through the virtual program’s assistance, she is now receiving care from home and can get her monthly infusions at a local clinic.
Ultimately, the goal is to help rheumatology move into an era of value-based care where the transition from fee-for-service to per patient will enable optimized care models and better accessibility, Mr. Wehman said. “It will not happen overnight, but every day we work toward this future.”
VA Targets Rheumatology Care
The Department of Veterans Affairs (VA) has also explored the use of navigator services in rheumatology, including virtual services.
VA uses an integrated, interdisciplinary model that manages each veteran’s individual healthcare needs through a coordinated effort among providers, nurses, social workers, pharmacists, and other health professionals, according to VA press secretary Terrence Hayes.
Care coordination may include supporting scheduling appointments, managing chronic conditions, and coordinating care across different medical departments. “This coordination is particularly important in managing complex rheumatologic conditions, where multiple providers may be involved,” Mr. Hayes said.
Additionally, VA has launched a national telerheumatology initiative to improve access to rheumatology providers in rural areas. The initiative will assist veterans in understanding the telehealth system, navigating appointments, and ensuring they have the necessary technology for virtual consultations.
“It will also facilitate communication between rheumatologists, primary care providers, and other specialists, ensuring that all team members are aligned in their approach to the veteran’s care,” Mr. Hayes said.
Who Will Take Advantage of New Codes?
Currently, Remission Medical operates on a cash-pay model, but the company intends to transition to insurance-based coverage in 2025.
Remission Medical also partners directly with preexisting healthcare systems and clinics such as Sentara Health and OrthoVirginia, where a PIN program, powered by Remission Medical’s virtual rheumatology network, may be explored as well.
The company offers its partners synchronous virtual visits and e-consults. It’s likely that these larger organizations will explore coverage for navigator services for Medicare and private insurance. “We can be there to support them as they decide to implement this,” Mr. Wehman said.
Taking advantage of CMS’s navigator PIN codes is an eventual goal. Remission Medical has not submitted the codes yet, “but we do intend to as we continue to grow our membership count,” Mr. Wehman said. “We hope to provide coverage for most of the US and submit the codes to reimbursement by early to mid-2025.”
In terms of reimbursement, the VA operates under a different payment model than Medicare or private insurance, focusing on providing integrated care within the VA system rather than reimbursing for specific services such as patient navigation.
While the SUNY clinic takes care of Medicare patients, it’s unlikely that the new CMS codes for navigators would apply to medical students. Students get paid a monthly stipend for doing navigator work. “There’s a policy about what students can get paid, and how many hours they can work,” Dr. Ginzler clarified.
The SUNY Downstate and Lupus Foundation navigator programs rely on grants to sustain their services. Aurinia Pharmaceuticals has funded both programs, and the SUNY program received an additional grant from Janssen to expand its offerings.
Because it’s grant funded, the navigator position at the Lupus Foundation does not bill patient insurance, Ms. Costillo explained.
Navigator Work Requires Training
Before they start working with patients, navigators often go through a vetting or training process. At Remission Medical, a clinical leadership team does a synchronous interview, background check, and CV review of its potential navigators.
Even before she became a navigator, Ms. Costillo had a strong baseline education in this work. She has a bachelor’s degree in social work and 15 years of experience in social services working with disabled, vulnerable, and underserved populations. Some of her fellow navigators at the Lupus Foundation of America also have degrees in social work.
Ms. Costillo underwent training with the Patient-Centered Education & Research Institute to become a certified professional patient navigator. Her name is on the national registry. The curriculum covered various aspects of medical care such as patient and care team interactions and communications, health and clinical knowledge, patient care coordination and resources, and using evidence-based approaches.
“For our lupus patients, it is essential that navigators understand the disease and the impact on patients and families, treatments available and those in the pipelines, and also the ins and outs of various insurance options,” Ms. Costillo said.
Mr. Wehman, Dr. Williams, and Ms. Costillo reported no disclosures. Dr. Ginzler has been a consultant for Aurinia Pharmaceuticals.
A version of this article first appeared on Medscape.com.
When a large rheumatology clinic in Richmond, Virginia, heard that Medicare would be reimbursing patient navigators, they decided to launch their own virtual navigator program.
“We read about it and felt like it was the perfect representation of what we were already trying to do,” said Blake Wehman, founder and CEO of Remission Medical, which offers virtual diagnosis and longitudinal care in rheumatology.
Mr. Wehman has plans to start submitting for these principal illness navigation (PIN) codes in 2025.
The Centers for Medicare & Medicaid Services (CMS) in 2024 began paying navigators who assist Medicare patients with high-risk conditions, which could include rheumatologic diseases. “The codes are not limited to a specific set of diagnoses; rather, the definition of a serious, high-risk condition is dependent on clinical judgment,” the agency clarified.
CMS established this provision in the CY 2024 Physician Fee Schedule final rule.
Reimbursing patient navigators is long overdue, noted Edith Williams, PhD, MS, director of the Center for Community Health and Prevention and founding director of the Office of Health Equity Research at the University of Rochester in New York. “It’s something our patients need. It’s something that the science is telling us can impact outcomes as an adjunct to clinical care,” she said.
Dr. Williams said the new CMS codes “got our departments talking about what this policy is and how it would translate into patient care.”
The codes apply when navigators are assigned to support patients with high-risk conditions who need assistance connecting with clinical and other resources, including any unmet social determinants of health needs, or in diagnosis or treatment of their medical problems.
“Having a navigator by their side to help get through all the clinical and administrative challenges gives people an advocate and a partner who is with them and their families every step of the way to help make the journey easier,” said a CMS spokesperson.
Not all navigator programs may qualify for the new codes. Some are supported by grants and don’t bill patient insurance. However, they all share a common goal: to guide patients through the healthcare continuum and assist with appointments and medication adherence.
Identifying ‘Root Causes’ of Barriers
Navigators represent a wide variety of backgrounds, ranging from healthcare professionals to students or even patients themselves. They generally don’t provide medical advice. “However, we are responsible for making sure our patients and their families are educated and aware, then assist with guidance on their path,” said Katie Costillo, BSW, CPPN, patient navigator and program manager with the Lupus Foundation of America, Heartland Region.
“Training and experience in engaging and building rapport is essential to assisting patients overcome obstacles that limit their access to healthcare,” she said. Narrowing down with patients the root causes of their barriers and then identifying appropriate and available community resources is key.
Studies have demonstrated the effectiveness of adding a navigator to a rheumatology patient’s care plan. In one study, a group of Boston researchers determined that navigators played a useful role in reducing adherence barriers to oral disease-modifying antirheumatic drugs. The navigators uncovered several concerns among 107 rheumatology patients, including fear of adverse events and medication effectiveness.
They also helped to facilitate patient-physician communication, developed strategies to improve medication adherence, and provided medication and diagnosis education. Patients reported satisfaction with the navigator experience.
A study Dr. Williams coauthored that examined behavioral interventions to support African American women with systemic lupus erythematosus found that patient navigator participants had superior coping scores, compared with those engaged in peer-to-peer methodology and patient support groups.
“We had a lot of success with the mentorship program, too,” Dr. Williams said. Navigator services, however, offer more one-on-one attention, “and it’s more tailored to what the person needs rather than the set curriculum that the mentors delivered to their mentees.”
Supporting Patients With Lupus
Ideally, navigators should be able to relate to patients and know what they’re going through, Dr. Williams said. This is someone whom the patient can trust and depend on. “That’s where the benefit of having someone who is also a patient lies because they’re ultimately relatable to other patients. But different institutions have taken different approaches to this.”
Some programs focus on specific rheumatologic conditions. The Lupus Foundation of America, for example, established patient navigator programs to assist patients with lupus in four markets across the country.
The Heartland patient navigator program is available for all patients with lupus within its region, which includes Kansas, Missouri, and central and southern Illinois. As a navigator, Ms. Costillo has been assisting patients since 2022. In 2023, she began meeting with patients at the Washington University Lupus Clinic (WULC) in St. Louis, Missouri.
Navigators work directly with patients before and after their appointment to ensure follow-up and reduce missed appointments. “They help lupus patients connect with community services and overcoming barriers to access and care. The goal of this position is to improve overall disease management, which results in better health outcomes,” Ms. Costillo said.
Since its inception, the patient navigator program at WULC has shown a decrease in patient no-call no-shows and an increase in requests to reschedule as opposed to not showing up for their scheduled appointment, based on history.
Patients have reported fewer barriers to transportation and improvement in access to resources, support, and disease education. “Our patients have also stated [that] meeting with the navigator during their appointments has helped them to feel heard, understood, and supported,” Ms. Costillo said.
Navigator Work Is Not Without Challenges
A total of 90% of patients with lupus are women, and women of color are two to three times more likely to develop lupus in their lifetime.
“Based on socioeconomic statistics, lupus patients are in a demographic that is commonly underserved, underfunded, and often overlooked. Finding appropriate local community resources for a patient who must choose between feeding her family or paying for transportation to multiple physician appointments is a common problem,” Ms. Costillo said.
Much of the assistance that became available during the COVID pandemic is starting to disappear. “With the rising costs of daily living, we are having to find creative and alternative ways to break down barriers and find support to fill those gaps,” she continued.
Getting insurance coverage of patients is another challenge. Many patients with lupus will be prescribed a treatment that insurance refuses to cover even after the physician disputes it.
Additionally, many patients with lupus are unable to work to support their family. A majority who apply for Social Security Disability Insurance are denied on their first and second attempts, “requiring multiple hearings and pages of documentation from their physicians,” Ms. Costillo said.
Students Serve as Navigators
One inner-city program is seeking to increase access to healthcare services to patients with lupus and lupus nephritis in underserved communities. In 2021, SUNY Downstate Health Sciences University in New York City, in partnership with the Brooklyn Free Clinic and Brooklyn Health Disparities Center, launched a program to teach navigator skills to second-year medical students.
The students assist patients at the Arthritis Clinic at University Hospital at Downstate. “Many of our patients have either low medical literacy or difficulty with English. Many of them are immigrants,” said Ellen M. Ginzler, MD, MPH, SUNY Downstate’s professor emerita and former vice-chair for research and rheumatology division chief.
Dr. Ginzler sought out navigator candidates who showed a strong interest in working with underserved patients with complicated, severe disease who struggled with keeping appointments or adhering to medication regimens. The program also gave preference to students fluent in other languages such as Spanish.
All these efforts have generated improvements in care.
Assessing the program’s effectiveness in a cross-sectional study, Dr. Ginzler and colleagues reported that 94% of navigators were able to schedule appointments and 87% assisted with prescriptions. Navigators also had high success rates in answering medical questions, getting in touch with a patient’s doctor, and reminding patients of medical appointments.
Medical student Jeremy Wilson, a coauthor of the study, served as a navigator for a woman with lupus and scleroderma for many years, along with other comorbidities.
Mr. Wilson went above and beyond for this patient, helping to secure social services supports that included accompanying her to clinic visits and serving as her advocate. “She found an enormous difference in how she was treated when she went to these clinics because the doctors in those clinics took her much more seriously,” Dr. Ginzler said. Mr. Wilson ran interference to secure clinic appointments and worked with the patient’s rheumatology fellow in the clinic to get approval for medications.
Mr. Wilson and the patient formed a great bond. “It not only helped the patient, but it helped Jeremy tremendously in terms of how he felt about his medical career,” Dr. Ginzler said.
The program has since expanded to include patients with other rheumatic diseases, such as rheumatoid arthritis and psoriatic arthritis, and also offers navigator services in dermatology.
A total of 21 students to date have completed the second year of the program. “We’ve just selected eight more,” Dr. Ginzler said. Some of the students continue to do the program in their third or even fourth year as they’re applying for residencies.
A student-run, unpublished survey of nine students in the SUNY program found that all nine reported high confidence in identifying social factors that impact patient health and well-being, compared with four who reported high confidence prior to starting the program. “Additionally, students reported increased confidence in providing comprehensive care in rheumatology and dermatology, and interdisciplinary collaboration,” study author Alejandra K. Moncayo, MPH, and colleagues wrote.
When Navigators Go Virtual
Remission Medical offers its navigator service through its own standalone virtual clinic.
Pain associated with rheumatologic conditions increases the urgency to see a doctor. The goal of the virtual RemissionNavigator program is to meet rheumatology patients where they live, to bridge care gaps and reduce wait times, said Mr. Wehman.
RemissionNavigator accomplishes this through video visits and unlimited texting to its network of board-certified rheumatologists or rheumatology-focused advanced practice providers. Experts can answer questions about why labs are ordered, why a patient may have received a certain diagnosis, or provide detailed explanations of a rheumatic condition.
“There are instances where improvement for the patient means waiting a couple days for us versus 45 days for their brick-and-mortar choice,” Mr. Wehman said.
The program currently has 36 subscribers to Remission’s services, which include navigation. “We have 15 providers in a blend of employed and contracted relationships with Remission,” Mr. Wehman said.
Even in its infancy, the navigator program has produced some success stories. “We had a patient tell us that thanks to us, he was seen faster, found relief immediately through our diagnosis and prescription of methotrexate, felt better at work, lost weight, and was happier in general,” Mr. Wehman said.
Another patient was making monthly, 90-minute trips to Richmond for infusion services. Through the virtual program’s assistance, she is now receiving care from home and can get her monthly infusions at a local clinic.
Ultimately, the goal is to help rheumatology move into an era of value-based care where the transition from fee-for-service to per patient will enable optimized care models and better accessibility, Mr. Wehman said. “It will not happen overnight, but every day we work toward this future.”
VA Targets Rheumatology Care
The Department of Veterans Affairs (VA) has also explored the use of navigator services in rheumatology, including virtual services.
VA uses an integrated, interdisciplinary model that manages each veteran’s individual healthcare needs through a coordinated effort among providers, nurses, social workers, pharmacists, and other health professionals, according to VA press secretary Terrence Hayes.
Care coordination may include supporting scheduling appointments, managing chronic conditions, and coordinating care across different medical departments. “This coordination is particularly important in managing complex rheumatologic conditions, where multiple providers may be involved,” Mr. Hayes said.
Additionally, VA has launched a national telerheumatology initiative to improve access to rheumatology providers in rural areas. The initiative will assist veterans in understanding the telehealth system, navigating appointments, and ensuring they have the necessary technology for virtual consultations.
“It will also facilitate communication between rheumatologists, primary care providers, and other specialists, ensuring that all team members are aligned in their approach to the veteran’s care,” Mr. Hayes said.
Who Will Take Advantage of New Codes?
Currently, Remission Medical operates on a cash-pay model, but the company intends to transition to insurance-based coverage in 2025.
Remission Medical also partners directly with preexisting healthcare systems and clinics such as Sentara Health and OrthoVirginia, where a PIN program, powered by Remission Medical’s virtual rheumatology network, may be explored as well.
The company offers its partners synchronous virtual visits and e-consults. It’s likely that these larger organizations will explore coverage for navigator services for Medicare and private insurance. “We can be there to support them as they decide to implement this,” Mr. Wehman said.
Taking advantage of CMS’s navigator PIN codes is an eventual goal. Remission Medical has not submitted the codes yet, “but we do intend to as we continue to grow our membership count,” Mr. Wehman said. “We hope to provide coverage for most of the US and submit the codes to reimbursement by early to mid-2025.”
In terms of reimbursement, the VA operates under a different payment model than Medicare or private insurance, focusing on providing integrated care within the VA system rather than reimbursing for specific services such as patient navigation.
While the SUNY clinic takes care of Medicare patients, it’s unlikely that the new CMS codes for navigators would apply to medical students. Students get paid a monthly stipend for doing navigator work. “There’s a policy about what students can get paid, and how many hours they can work,” Dr. Ginzler clarified.
The SUNY Downstate and Lupus Foundation navigator programs rely on grants to sustain their services. Aurinia Pharmaceuticals has funded both programs, and the SUNY program received an additional grant from Janssen to expand its offerings.
Because it’s grant funded, the navigator position at the Lupus Foundation does not bill patient insurance, Ms. Costillo explained.
Navigator Work Requires Training
Before they start working with patients, navigators often go through a vetting or training process. At Remission Medical, a clinical leadership team does a synchronous interview, background check, and CV review of its potential navigators.
Even before she became a navigator, Ms. Costillo had a strong baseline education in this work. She has a bachelor’s degree in social work and 15 years of experience in social services working with disabled, vulnerable, and underserved populations. Some of her fellow navigators at the Lupus Foundation of America also have degrees in social work.
Ms. Costillo underwent training with the Patient-Centered Education & Research Institute to become a certified professional patient navigator. Her name is on the national registry. The curriculum covered various aspects of medical care such as patient and care team interactions and communications, health and clinical knowledge, patient care coordination and resources, and using evidence-based approaches.
“For our lupus patients, it is essential that navigators understand the disease and the impact on patients and families, treatments available and those in the pipelines, and also the ins and outs of various insurance options,” Ms. Costillo said.
Mr. Wehman, Dr. Williams, and Ms. Costillo reported no disclosures. Dr. Ginzler has been a consultant for Aurinia Pharmaceuticals.
A version of this article first appeared on Medscape.com.
FDA Expands Indication for Amivantamab in Lung Cancer
Amivantamab with carboplatin and pemetrexed is now indicated for adults with locally advanced or metastatic NSCLC with EGFR exon 19 deletions or exon 21 L858R substitution mutations whose disease has progressed on or after treatment with an EGFR tyrosine kinase inhibitor (TKI).
The FDA has already approved first-line use of amivantamab in combination with carboplatin and pemetrexed in patients with locally advanced or metastatic NSCLC with EGFR exon 20 insertion mutations, as reported by Medscape Medical News.
The second-line approval for amivantamab plus chemotherapy “may address the most common mechanisms of treatment resistance to third-generation EGFR TKIs, such as osimertinib, in the first line,” Martin Dietrich, MD, PhD, oncologist, Cancer Care Centers of Brevard in Florida, said in a company news release.
“This multitargeted combination extended progression-free survival (PFS) and improved overall response compared to chemotherapy alone, offering an important and effective new second-line option for patients,” Dr. Dietrich added.
The second-line indication is supported by the phase 3 MARIPOSA-2 study, which included 657 patients with locally advanced or metastatic NSCLC with EGFR exon 19 deletions or exon 21 L858R substitution mutations and disease progression on or after receiving osimertinib.
The study demonstrated a 52% reduced risk of disease progression or death when amivantamab was added to carboplatin and pemetrexed (hazard ratio, 0.48).
Median PFS was 6.3 months with amivantamab vs 4.2 months with chemotherapy alone. The confirmed objective response rate was 53% in the amivantamab plus chemotherapy group vs 29% in the chemotherapy only group.
The most common adverse reactions, occurring in at least 20% of patients, were rash, infusion-related reactions, fatigue, nail toxicity, nausea, constipation, edema, stomatitis, decreased appetite, musculoskeletal pain, vomiting, and COVID-19 infection.
The company noted that amivantamab in combination with chemotherapy is the only category 1 treatment option in National Comprehensive Cancer Network clinical practice guidelines for patients with EGFR-mutated NSCLC who have progressed on osimertinib and who are symptomatic with multiple lesions.
A version of this article appeared on Medscape.com.
Amivantamab with carboplatin and pemetrexed is now indicated for adults with locally advanced or metastatic NSCLC with EGFR exon 19 deletions or exon 21 L858R substitution mutations whose disease has progressed on or after treatment with an EGFR tyrosine kinase inhibitor (TKI).
The FDA has already approved first-line use of amivantamab in combination with carboplatin and pemetrexed in patients with locally advanced or metastatic NSCLC with EGFR exon 20 insertion mutations, as reported by Medscape Medical News.
The second-line approval for amivantamab plus chemotherapy “may address the most common mechanisms of treatment resistance to third-generation EGFR TKIs, such as osimertinib, in the first line,” Martin Dietrich, MD, PhD, oncologist, Cancer Care Centers of Brevard in Florida, said in a company news release.
“This multitargeted combination extended progression-free survival (PFS) and improved overall response compared to chemotherapy alone, offering an important and effective new second-line option for patients,” Dr. Dietrich added.
The second-line indication is supported by the phase 3 MARIPOSA-2 study, which included 657 patients with locally advanced or metastatic NSCLC with EGFR exon 19 deletions or exon 21 L858R substitution mutations and disease progression on or after receiving osimertinib.
The study demonstrated a 52% reduced risk of disease progression or death when amivantamab was added to carboplatin and pemetrexed (hazard ratio, 0.48).
Median PFS was 6.3 months with amivantamab vs 4.2 months with chemotherapy alone. The confirmed objective response rate was 53% in the amivantamab plus chemotherapy group vs 29% in the chemotherapy only group.
The most common adverse reactions, occurring in at least 20% of patients, were rash, infusion-related reactions, fatigue, nail toxicity, nausea, constipation, edema, stomatitis, decreased appetite, musculoskeletal pain, vomiting, and COVID-19 infection.
The company noted that amivantamab in combination with chemotherapy is the only category 1 treatment option in National Comprehensive Cancer Network clinical practice guidelines for patients with EGFR-mutated NSCLC who have progressed on osimertinib and who are symptomatic with multiple lesions.
A version of this article appeared on Medscape.com.
Amivantamab with carboplatin and pemetrexed is now indicated for adults with locally advanced or metastatic NSCLC with EGFR exon 19 deletions or exon 21 L858R substitution mutations whose disease has progressed on or after treatment with an EGFR tyrosine kinase inhibitor (TKI).
The FDA has already approved first-line use of amivantamab in combination with carboplatin and pemetrexed in patients with locally advanced or metastatic NSCLC with EGFR exon 20 insertion mutations, as reported by Medscape Medical News.
The second-line approval for amivantamab plus chemotherapy “may address the most common mechanisms of treatment resistance to third-generation EGFR TKIs, such as osimertinib, in the first line,” Martin Dietrich, MD, PhD, oncologist, Cancer Care Centers of Brevard in Florida, said in a company news release.
“This multitargeted combination extended progression-free survival (PFS) and improved overall response compared to chemotherapy alone, offering an important and effective new second-line option for patients,” Dr. Dietrich added.
The second-line indication is supported by the phase 3 MARIPOSA-2 study, which included 657 patients with locally advanced or metastatic NSCLC with EGFR exon 19 deletions or exon 21 L858R substitution mutations and disease progression on or after receiving osimertinib.
The study demonstrated a 52% reduced risk of disease progression or death when amivantamab was added to carboplatin and pemetrexed (hazard ratio, 0.48).
Median PFS was 6.3 months with amivantamab vs 4.2 months with chemotherapy alone. The confirmed objective response rate was 53% in the amivantamab plus chemotherapy group vs 29% in the chemotherapy only group.
The most common adverse reactions, occurring in at least 20% of patients, were rash, infusion-related reactions, fatigue, nail toxicity, nausea, constipation, edema, stomatitis, decreased appetite, musculoskeletal pain, vomiting, and COVID-19 infection.
The company noted that amivantamab in combination with chemotherapy is the only category 1 treatment option in National Comprehensive Cancer Network clinical practice guidelines for patients with EGFR-mutated NSCLC who have progressed on osimertinib and who are symptomatic with multiple lesions.
A version of this article appeared on Medscape.com.