User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
nav[contains(@class, 'nav-ce-stack nav-ce-stack__large-screen')]
header[@id='header']
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'main-prefix')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
div[contains(@class, 'view-medstat-quiz-listing-panes')]
div[contains(@class, 'pane-article-sidebar-latest-news')]
2022 Update on female sexual health
Many authors have commented on the lack of research into female sexual dysfunction, especially when compared with the hundreds of research publications related to male sexual health and dysfunction. Not surprisingly, very little has been published in the past year on the subject of female sexual health.
Recently, the International Society for the Study of Women’s Sexual Health (ISSWSH) published 2 important papers: a guideline on the use of testosterone for hypoactive sexual desire disorder (HSDD) in women and a consensus document on the management of persistent genital arousal disorder (PGAD). The lack of funding and support for female sexual health leaves women’s health professionals with little education or guidance on how to identify and treat conditions that are likely as common in women as erectile dysfunction is in men. While we would like to rely on randomized trials to inform our clinical care, the very limited literature on female sexual health makes this difficult. Bringing together experienced clinicians who focus their practices on sexual health, ISSWSH has provided some much-needed recommendations for the management of difficult conditions.
ISSWSH provides clinical guidance on testosterone therapy for women with HSDD
Parish S, Simon J, Davis S, et al. International Society for the Study of Women’s Sexual Health clinical practice guideline for the use of systemic testosterone for hypoactive sexual desire disorder in women. J Sex Med. 2021;18:849-867.
For development of the ISSWSH clinical practice guideline on testosterone therapy for women with HSDD, 16 international researchers and clinicians were convened. A modified Delphi method was used to establish consensus at the meeting on the recommended indications for testosterone treatment, formulations, and when measurement of testosterone levels is appropriate.
An extensive evidence-based literature review was performed, which included original research, meta-analyses, reviews, and clinical practice guidelines, to address the use of testosterone in women for management of HSDD. Notably, in 2019, representatives of 10 medical societies published a Global Consensus Position Statement on the Use of Testosterone Therapy for Women that reviewed the existing literature on testosterone’s effects on sexual dysfunction, mood, cognition, musculoskeletal, cardiovascular, and breast health as well as androgenic side effects and adverse events.1 Based on their review, the only evidence-based indication for testosterone use is for the treatment of HSDD.
Testosterone formulations, HSDD diagnosis, and sex steroid physiology
More than 10 years ago, the US Food and Drug Administration (FDA) reviewed an application for the use of a transdermal testosterone patch (Intrinsa) in women for the treatment of HSDD. Efficacy of treatment was clearly demonstrated, and no safety signals were found in the placebo-controlled trial. Based, however, on the opinions of regulators who were “concerned” about the potential for cardiovascular adverse outcomes and worry that the peripheral conversion of testosterone to estradiol might lead to an increase in breast cancer—worry generated from the findings of the Women’s Health Initiative (which did not demonstrate an increase in breast cancer risk with estrogen alone but only when estrogen was combined with medroxyprogesterone acetate)—the FDA declined to approve the testosterone patch for women.
The Diagnostic and Statistical Manual of Mental Disorders, 4th Edition (DSM-IV) defined HSDD as “persistent or recurrent deficiency or absence of sexual fantasies and desire for sexual activity with marked distress or interpersonal difficulty.” The guideline authors noted that although the DSM-5 edition merged female arousal disorder with desire disorder into a single diagnosis, they used the DSM-IV definition as it had been the basis for the studies and literature reviewed. HSDD is a prevalent condition worldwide that affects between 12% and 53% of peri- and postmenopausal women.
The consensus guideline authors extensively reviewed the physiology and mechanism of action of sex steroids in women, particularly their impact on sexual function and the biologic alterations that occur during peri- and postmenopause.
Continue to: Consensus position and recommendations...
Consensus position and recommendations
The ISSWSH consensus guideline concluded that there is a moderate therapeutic benefit in adding testosterone therapy to achieve up to premenopausal levels in postmenopausal women with self-reported reduction in sexual desire that is causing distress as determined by a validated instrument.
The authors advise baseline hormone testing to rule out androgen excess and baseline renal, lipid, liver, and metabolic testing, even though transdermal testosterone therapy was not shown to alter these parameters in randomized trials of more than 3,000 women. Laboratory assays for both total and free testosterone are “highly unreliable” in the female range as they have been calibrated for male levels of hormone.
FDA-approved testosterone treatments for men with hypogonadism include transdermal gels, patches, intramuscular injection, and an oral formulation. Dosing for women is approximately one-tenth the dosage for treatment of men. Patients should be informed that this treatment is off-label and that long-term studies to establish safety are not available. The authors advised against the use of compounded formulations based on the National Academies of Science, Engineering, and Medicine guidelines, but they went on to say that if compounded products are used, the pharmacy should adhere to Good Manufacturing Practice and Active Pharmaceutical Ingredients standards.
Transdermal testosterone is beneficial for the treatment of HSDD in postmenopausal women after other causes of decreased desire, such as dyspareunia, relationship issues, and other general medical conditions, have been ruled out. There is no diagnostic laboratory test to confirm HSDD or to use as a therapeutic target in treatment (for total or free testosterone, as these are highly unreliable laboratory values). Although large trials have identified no safety signals, they were generally limited to 6 months in duration. Prescribing one-tenth the dose indicated for male hypogonadism results in premenopausal testosterone levels for most women. If there is no benefit after 6 months of treatment, testosterone should be discontinued.
Rare, complex sexual function disorder requires integrated biopsychosocial approach, says ISSWSH
Goldstein I, Komisaruk BR, Pukall CF, et al. International Society for the Study of Women’s Sexual Health (ISSWSH) review of epidemiology and pathophysiology, and a consensus nomenclature and process of care for the management of persistent genital arousal disorder/genito-pelvic dyesthesia (PGAD/GPD). J Sex Med. 2021;18:665-697.
Persistent genital arousal disorder is a poorly understood and relatively rare sexual dysfunction in women. The American College of Obstetricians and Gynecologists (ACOG) Practice Bulletin on Female Sexual Dysfunction does not mention this condition, leaving women’s health practitioners with little guidance as to diagnosis or management.2 Prevalence for the condition is estimated at 1% to 3%. The symptoms may be intermittent or continuous.
In a recent ISSWSH review, a consensus panel defined 5 criteria for this disorder: the perception of genital arousal that is involuntary, unrelated to sexual desire, without any identified cause, not relieved with orgasm, and distressing to the patient. The panel made a clear distinction between PGAD/ genito-pelvic dysesthesia (GPD) and Compulsive Sexual Behavior Disorder (defined by the International Classification of Diseases revision 11 as “a persistent pattern of failure to control intense, repetitive sexual impulses or urges). Because there is considerable overlap with syndromes of genital dysesthesia—itching, burning, tingling, or pain— the consensus panel elected to expand the nomenclature to describe both persistent genital arousal and genito-pelvic dysesthesia as a single syndrome, namely, PGAD/GPD.
Continue to: Negative impact of PGAD/GPD...
Negative impact of PGAD/GPD
The consensus panel identified several contributors to the overall morbidity of this complex disorder, including end organ pathology, peripheral nerve, spinal cord and central sensory processing malfunction, and significant psychological issues. PGAD/GPD also may be associated with spinal cysts, cauda equina pathology, and withdrawal from selective serotonin reuptake inhibitors (SSRIs). Functional magnetic resonance imaging has identified specific brain regions (for example, the paracentral lobule) that are active during clitoral stimulation and that also activate during patients’ experience of persistent genital arousal.
PGAD/GPD negatively impacts sexual function, mental health, and ability to function in daily life. Of major importance is that a large proportion of people with this disorder have significant mental health disorders; in a survey, 54% of patients with PGAD reported suicidal ideation, compared with 25% of participants in a control group.
Evaluation and management recommendations
Diagnosis and management of PGAD/GPD are directed at the 5 areas of evaluation:
- end organ
- pelvis and perineum (assess for pelvic floor tension myalgia, pudendal neuropathy, pelvic congestion syndrome, or pelvic arteriovenous malformation)
- cauda equina (evaluate for neurologic deficits related to cysts compressing S2-S3 nerve roots)
- spinal cord (serotonin and norepinephrine pathways modulate nociceptive sensory activity; either SSRI/serotonin and norepinephrine reuptake inhibitor (SNRI) withdrawal or treatment could impact PGAD/ GPD based on their actions in the spinal cord)
- brain.
The consensus panel recommends an integrated biopsychosocial model for evaluation and treatment of PGAD/GPD. Comorbid mental health conditions, such as depression and anxiety, are common. Small studies suggest that a history of sexual trauma may contribute to catastrophizing and the experience of distressing persistent genital sensations, either arousal or dyesthesia, with 46.7% to 52.6% of patients reporting childhood sexual abuse.3
PGAD/GPD is a poorly recognized source of major distress to a small but significant group of patients. Diagnosis and management require a multidisciplinary team to identify end organ, pharmacologic, neurologic, vascular, and emotional components that contribute to the syndrome. Treatment requires a biopsychosocial approach that addresses the various sources of aberrant sensory processing, including end organ disease, neuropathic signaling, spinal cord pathways, and brain signal processing. Recognizing the existence of, and approaches to, this disorder will help gynecologists understand the considerable distress and potential life-threatening consequences our patients with PGAD/GPD experience.
Future possibilities and current actualities for patient care
Research dollars and investment in female sexual dysfunction remain inadequate to address the considerable gaps that exist in evidence-based clinical guidelines. ISSWSH is working to help clinicians approach these evidence gaps with guidelines and consensus statements to help women’s health professionals identify and manage our patients with sexual concerns and symptoms. An expert consensus guideline on the assessment and management of female orgasmic disorder is currently under development (personal communication, Dr. Sheryl Kingsberg). In addition, a phase 2b trial is underway to assess the impact of topical sildenafil cream for the treatment of female arousal disorder. Stay tuned for the results of these studies.
For now, women’s health professionals have 2 FDA-approved treatment options for premenopausal women with arousal disorder, flibanserin (a daily oral medication that requires abstinence from alcohol) and bremelanotide (an injectable medication that can be used just prior to a sexual encounter). For postmenopausal women, there are no FDA-approved therapies; however, based on the ISSWSH guideline summarized above, transdermal testosterone may be offered to postmenopausal women with distressing loss of sexual desire in doses approximately one-tenth those used to treat men with androgen deficiency. These small doses are challenging to achieve consistently with the delivery systems available for FDA-approved products sold for men.
The National Academies of Science, Engineering, and Medicine advise against the use of compounded hormonal products due to the potential for inconsistency and lack of FDA oversight in the manufacturing/compounding process. I have found and used some compounding pharmacies that are dedicated to safety, quality control, and compliance; test their products; and provide consistent, reliable compounded drugs for my patients. Consideration of compounded testosterone should be discussed with patients, and they should be informed of the current professional association guidelines. Testosterone creams may be compounded to a 1% product—20 mg/mL. Researchers in Australia have demonstrated that 5 mg of transdermal testosterone cream (one-quarter of a mL) results in typical premenopausal testosterone levels.4 When prescribing testosterone for postmenopausal women, check in with them after 6 weeks of treatment to assess impact and check blood levels to ensure that levels are not too high.
Testosterone pellets and intramuscular testosterone are not recommended and in fact should be actively avoided. These methods of administration are associated with extreme variation in hormone levels over time. There are typically supraphysiologic and quite high levels immediately after implantation or injection, followed by fairly significant drop-offs and rapid return of symptoms over time. This may lead to more and more frequent dosing and markedly elevated serum levels.
Management of PGAD/GPD is difficult, but knowing it exists as a valid syndrome will help clinicians validate patients’ symptoms and begin to approach appropriate evaluation and workup targeted to the 5 domains suggested by the ISSWSH expert panel. It is useful to understand the possible relationship to initiation or withdrawal from SSRIs or SNRIs and how aberrant norepinephrine signaling along the sensory pathways may contribute to genital dysesthesia or chronic sensations of arousal. Nonpharmacologic therapies, such as cognitive-behavioral therapy and others, are essential components of the multifaceted approach to treatment. Finally, many complex problems, such as chronic pelvic pain, vestibulodynia, vulvodynia, and chronic fatigue syndrome, are associated with childhood adverse experiences and sexual trauma. Approaching these patients with trauma-informed care is important to create the trust and therapeutic environment they need for successful multidisciplinary care. ●
- Davis SR, Baber R, Panay N, et al. Global consensus position statement on the use of testosterone therapy for women. J Sex Med. 2019;16:1331-1337.
- American College of Obstetricians and Gynecologists’ Committee on Practice Bulletins—Gynecology. ACOG practice bulletin no. 213: Female sexual dysfunction: clinical management guidelines for obstetrician-gynecologists. Obstet Gynecol. 2019;134:e1-e18.
- Leiblum S, Seehuus M, Goldmeier D, et al. Psychological, medical, and pharmacological correlates of persistent genital arousal disorder. J Sex Med. 2007;4:1358-1366.
- Fooladi E, Reuter SE, Bell RJ, et al. Pharmacokinetics of a transdermal testosterone cream in healthy postmenopausal women. Menopause. 2015;22:44-49.
Many authors have commented on the lack of research into female sexual dysfunction, especially when compared with the hundreds of research publications related to male sexual health and dysfunction. Not surprisingly, very little has been published in the past year on the subject of female sexual health.
Recently, the International Society for the Study of Women’s Sexual Health (ISSWSH) published 2 important papers: a guideline on the use of testosterone for hypoactive sexual desire disorder (HSDD) in women and a consensus document on the management of persistent genital arousal disorder (PGAD). The lack of funding and support for female sexual health leaves women’s health professionals with little education or guidance on how to identify and treat conditions that are likely as common in women as erectile dysfunction is in men. While we would like to rely on randomized trials to inform our clinical care, the very limited literature on female sexual health makes this difficult. Bringing together experienced clinicians who focus their practices on sexual health, ISSWSH has provided some much-needed recommendations for the management of difficult conditions.
ISSWSH provides clinical guidance on testosterone therapy for women with HSDD
Parish S, Simon J, Davis S, et al. International Society for the Study of Women’s Sexual Health clinical practice guideline for the use of systemic testosterone for hypoactive sexual desire disorder in women. J Sex Med. 2021;18:849-867.
For development of the ISSWSH clinical practice guideline on testosterone therapy for women with HSDD, 16 international researchers and clinicians were convened. A modified Delphi method was used to establish consensus at the meeting on the recommended indications for testosterone treatment, formulations, and when measurement of testosterone levels is appropriate.
An extensive evidence-based literature review was performed, which included original research, meta-analyses, reviews, and clinical practice guidelines, to address the use of testosterone in women for management of HSDD. Notably, in 2019, representatives of 10 medical societies published a Global Consensus Position Statement on the Use of Testosterone Therapy for Women that reviewed the existing literature on testosterone’s effects on sexual dysfunction, mood, cognition, musculoskeletal, cardiovascular, and breast health as well as androgenic side effects and adverse events.1 Based on their review, the only evidence-based indication for testosterone use is for the treatment of HSDD.
Testosterone formulations, HSDD diagnosis, and sex steroid physiology
More than 10 years ago, the US Food and Drug Administration (FDA) reviewed an application for the use of a transdermal testosterone patch (Intrinsa) in women for the treatment of HSDD. Efficacy of treatment was clearly demonstrated, and no safety signals were found in the placebo-controlled trial. Based, however, on the opinions of regulators who were “concerned” about the potential for cardiovascular adverse outcomes and worry that the peripheral conversion of testosterone to estradiol might lead to an increase in breast cancer—worry generated from the findings of the Women’s Health Initiative (which did not demonstrate an increase in breast cancer risk with estrogen alone but only when estrogen was combined with medroxyprogesterone acetate)—the FDA declined to approve the testosterone patch for women.
The Diagnostic and Statistical Manual of Mental Disorders, 4th Edition (DSM-IV) defined HSDD as “persistent or recurrent deficiency or absence of sexual fantasies and desire for sexual activity with marked distress or interpersonal difficulty.” The guideline authors noted that although the DSM-5 edition merged female arousal disorder with desire disorder into a single diagnosis, they used the DSM-IV definition as it had been the basis for the studies and literature reviewed. HSDD is a prevalent condition worldwide that affects between 12% and 53% of peri- and postmenopausal women.
The consensus guideline authors extensively reviewed the physiology and mechanism of action of sex steroids in women, particularly their impact on sexual function and the biologic alterations that occur during peri- and postmenopause.
Continue to: Consensus position and recommendations...
Consensus position and recommendations
The ISSWSH consensus guideline concluded that there is a moderate therapeutic benefit in adding testosterone therapy to achieve up to premenopausal levels in postmenopausal women with self-reported reduction in sexual desire that is causing distress as determined by a validated instrument.
The authors advise baseline hormone testing to rule out androgen excess and baseline renal, lipid, liver, and metabolic testing, even though transdermal testosterone therapy was not shown to alter these parameters in randomized trials of more than 3,000 women. Laboratory assays for both total and free testosterone are “highly unreliable” in the female range as they have been calibrated for male levels of hormone.
FDA-approved testosterone treatments for men with hypogonadism include transdermal gels, patches, intramuscular injection, and an oral formulation. Dosing for women is approximately one-tenth the dosage for treatment of men. Patients should be informed that this treatment is off-label and that long-term studies to establish safety are not available. The authors advised against the use of compounded formulations based on the National Academies of Science, Engineering, and Medicine guidelines, but they went on to say that if compounded products are used, the pharmacy should adhere to Good Manufacturing Practice and Active Pharmaceutical Ingredients standards.
Transdermal testosterone is beneficial for the treatment of HSDD in postmenopausal women after other causes of decreased desire, such as dyspareunia, relationship issues, and other general medical conditions, have been ruled out. There is no diagnostic laboratory test to confirm HSDD or to use as a therapeutic target in treatment (for total or free testosterone, as these are highly unreliable laboratory values). Although large trials have identified no safety signals, they were generally limited to 6 months in duration. Prescribing one-tenth the dose indicated for male hypogonadism results in premenopausal testosterone levels for most women. If there is no benefit after 6 months of treatment, testosterone should be discontinued.
Rare, complex sexual function disorder requires integrated biopsychosocial approach, says ISSWSH
Goldstein I, Komisaruk BR, Pukall CF, et al. International Society for the Study of Women’s Sexual Health (ISSWSH) review of epidemiology and pathophysiology, and a consensus nomenclature and process of care for the management of persistent genital arousal disorder/genito-pelvic dyesthesia (PGAD/GPD). J Sex Med. 2021;18:665-697.
Persistent genital arousal disorder is a poorly understood and relatively rare sexual dysfunction in women. The American College of Obstetricians and Gynecologists (ACOG) Practice Bulletin on Female Sexual Dysfunction does not mention this condition, leaving women’s health practitioners with little guidance as to diagnosis or management.2 Prevalence for the condition is estimated at 1% to 3%. The symptoms may be intermittent or continuous.
In a recent ISSWSH review, a consensus panel defined 5 criteria for this disorder: the perception of genital arousal that is involuntary, unrelated to sexual desire, without any identified cause, not relieved with orgasm, and distressing to the patient. The panel made a clear distinction between PGAD/ genito-pelvic dysesthesia (GPD) and Compulsive Sexual Behavior Disorder (defined by the International Classification of Diseases revision 11 as “a persistent pattern of failure to control intense, repetitive sexual impulses or urges). Because there is considerable overlap with syndromes of genital dysesthesia—itching, burning, tingling, or pain— the consensus panel elected to expand the nomenclature to describe both persistent genital arousal and genito-pelvic dysesthesia as a single syndrome, namely, PGAD/GPD.
Continue to: Negative impact of PGAD/GPD...
Negative impact of PGAD/GPD
The consensus panel identified several contributors to the overall morbidity of this complex disorder, including end organ pathology, peripheral nerve, spinal cord and central sensory processing malfunction, and significant psychological issues. PGAD/GPD also may be associated with spinal cysts, cauda equina pathology, and withdrawal from selective serotonin reuptake inhibitors (SSRIs). Functional magnetic resonance imaging has identified specific brain regions (for example, the paracentral lobule) that are active during clitoral stimulation and that also activate during patients’ experience of persistent genital arousal.
PGAD/GPD negatively impacts sexual function, mental health, and ability to function in daily life. Of major importance is that a large proportion of people with this disorder have significant mental health disorders; in a survey, 54% of patients with PGAD reported suicidal ideation, compared with 25% of participants in a control group.
Evaluation and management recommendations
Diagnosis and management of PGAD/GPD are directed at the 5 areas of evaluation:
- end organ
- pelvis and perineum (assess for pelvic floor tension myalgia, pudendal neuropathy, pelvic congestion syndrome, or pelvic arteriovenous malformation)
- cauda equina (evaluate for neurologic deficits related to cysts compressing S2-S3 nerve roots)
- spinal cord (serotonin and norepinephrine pathways modulate nociceptive sensory activity; either SSRI/serotonin and norepinephrine reuptake inhibitor (SNRI) withdrawal or treatment could impact PGAD/ GPD based on their actions in the spinal cord)
- brain.
The consensus panel recommends an integrated biopsychosocial model for evaluation and treatment of PGAD/GPD. Comorbid mental health conditions, such as depression and anxiety, are common. Small studies suggest that a history of sexual trauma may contribute to catastrophizing and the experience of distressing persistent genital sensations, either arousal or dyesthesia, with 46.7% to 52.6% of patients reporting childhood sexual abuse.3
PGAD/GPD is a poorly recognized source of major distress to a small but significant group of patients. Diagnosis and management require a multidisciplinary team to identify end organ, pharmacologic, neurologic, vascular, and emotional components that contribute to the syndrome. Treatment requires a biopsychosocial approach that addresses the various sources of aberrant sensory processing, including end organ disease, neuropathic signaling, spinal cord pathways, and brain signal processing. Recognizing the existence of, and approaches to, this disorder will help gynecologists understand the considerable distress and potential life-threatening consequences our patients with PGAD/GPD experience.
Future possibilities and current actualities for patient care
Research dollars and investment in female sexual dysfunction remain inadequate to address the considerable gaps that exist in evidence-based clinical guidelines. ISSWSH is working to help clinicians approach these evidence gaps with guidelines and consensus statements to help women’s health professionals identify and manage our patients with sexual concerns and symptoms. An expert consensus guideline on the assessment and management of female orgasmic disorder is currently under development (personal communication, Dr. Sheryl Kingsberg). In addition, a phase 2b trial is underway to assess the impact of topical sildenafil cream for the treatment of female arousal disorder. Stay tuned for the results of these studies.
For now, women’s health professionals have 2 FDA-approved treatment options for premenopausal women with arousal disorder, flibanserin (a daily oral medication that requires abstinence from alcohol) and bremelanotide (an injectable medication that can be used just prior to a sexual encounter). For postmenopausal women, there are no FDA-approved therapies; however, based on the ISSWSH guideline summarized above, transdermal testosterone may be offered to postmenopausal women with distressing loss of sexual desire in doses approximately one-tenth those used to treat men with androgen deficiency. These small doses are challenging to achieve consistently with the delivery systems available for FDA-approved products sold for men.
The National Academies of Science, Engineering, and Medicine advise against the use of compounded hormonal products due to the potential for inconsistency and lack of FDA oversight in the manufacturing/compounding process. I have found and used some compounding pharmacies that are dedicated to safety, quality control, and compliance; test their products; and provide consistent, reliable compounded drugs for my patients. Consideration of compounded testosterone should be discussed with patients, and they should be informed of the current professional association guidelines. Testosterone creams may be compounded to a 1% product—20 mg/mL. Researchers in Australia have demonstrated that 5 mg of transdermal testosterone cream (one-quarter of a mL) results in typical premenopausal testosterone levels.4 When prescribing testosterone for postmenopausal women, check in with them after 6 weeks of treatment to assess impact and check blood levels to ensure that levels are not too high.
Testosterone pellets and intramuscular testosterone are not recommended and in fact should be actively avoided. These methods of administration are associated with extreme variation in hormone levels over time. There are typically supraphysiologic and quite high levels immediately after implantation or injection, followed by fairly significant drop-offs and rapid return of symptoms over time. This may lead to more and more frequent dosing and markedly elevated serum levels.
Management of PGAD/GPD is difficult, but knowing it exists as a valid syndrome will help clinicians validate patients’ symptoms and begin to approach appropriate evaluation and workup targeted to the 5 domains suggested by the ISSWSH expert panel. It is useful to understand the possible relationship to initiation or withdrawal from SSRIs or SNRIs and how aberrant norepinephrine signaling along the sensory pathways may contribute to genital dysesthesia or chronic sensations of arousal. Nonpharmacologic therapies, such as cognitive-behavioral therapy and others, are essential components of the multifaceted approach to treatment. Finally, many complex problems, such as chronic pelvic pain, vestibulodynia, vulvodynia, and chronic fatigue syndrome, are associated with childhood adverse experiences and sexual trauma. Approaching these patients with trauma-informed care is important to create the trust and therapeutic environment they need for successful multidisciplinary care. ●
Many authors have commented on the lack of research into female sexual dysfunction, especially when compared with the hundreds of research publications related to male sexual health and dysfunction. Not surprisingly, very little has been published in the past year on the subject of female sexual health.
Recently, the International Society for the Study of Women’s Sexual Health (ISSWSH) published 2 important papers: a guideline on the use of testosterone for hypoactive sexual desire disorder (HSDD) in women and a consensus document on the management of persistent genital arousal disorder (PGAD). The lack of funding and support for female sexual health leaves women’s health professionals with little education or guidance on how to identify and treat conditions that are likely as common in women as erectile dysfunction is in men. While we would like to rely on randomized trials to inform our clinical care, the very limited literature on female sexual health makes this difficult. Bringing together experienced clinicians who focus their practices on sexual health, ISSWSH has provided some much-needed recommendations for the management of difficult conditions.
ISSWSH provides clinical guidance on testosterone therapy for women with HSDD
Parish S, Simon J, Davis S, et al. International Society for the Study of Women’s Sexual Health clinical practice guideline for the use of systemic testosterone for hypoactive sexual desire disorder in women. J Sex Med. 2021;18:849-867.
For development of the ISSWSH clinical practice guideline on testosterone therapy for women with HSDD, 16 international researchers and clinicians were convened. A modified Delphi method was used to establish consensus at the meeting on the recommended indications for testosterone treatment, formulations, and when measurement of testosterone levels is appropriate.
An extensive evidence-based literature review was performed, which included original research, meta-analyses, reviews, and clinical practice guidelines, to address the use of testosterone in women for management of HSDD. Notably, in 2019, representatives of 10 medical societies published a Global Consensus Position Statement on the Use of Testosterone Therapy for Women that reviewed the existing literature on testosterone’s effects on sexual dysfunction, mood, cognition, musculoskeletal, cardiovascular, and breast health as well as androgenic side effects and adverse events.1 Based on their review, the only evidence-based indication for testosterone use is for the treatment of HSDD.
Testosterone formulations, HSDD diagnosis, and sex steroid physiology
More than 10 years ago, the US Food and Drug Administration (FDA) reviewed an application for the use of a transdermal testosterone patch (Intrinsa) in women for the treatment of HSDD. Efficacy of treatment was clearly demonstrated, and no safety signals were found in the placebo-controlled trial. Based, however, on the opinions of regulators who were “concerned” about the potential for cardiovascular adverse outcomes and worry that the peripheral conversion of testosterone to estradiol might lead to an increase in breast cancer—worry generated from the findings of the Women’s Health Initiative (which did not demonstrate an increase in breast cancer risk with estrogen alone but only when estrogen was combined with medroxyprogesterone acetate)—the FDA declined to approve the testosterone patch for women.
The Diagnostic and Statistical Manual of Mental Disorders, 4th Edition (DSM-IV) defined HSDD as “persistent or recurrent deficiency or absence of sexual fantasies and desire for sexual activity with marked distress or interpersonal difficulty.” The guideline authors noted that although the DSM-5 edition merged female arousal disorder with desire disorder into a single diagnosis, they used the DSM-IV definition as it had been the basis for the studies and literature reviewed. HSDD is a prevalent condition worldwide that affects between 12% and 53% of peri- and postmenopausal women.
The consensus guideline authors extensively reviewed the physiology and mechanism of action of sex steroids in women, particularly their impact on sexual function and the biologic alterations that occur during peri- and postmenopause.
Continue to: Consensus position and recommendations...
Consensus position and recommendations
The ISSWSH consensus guideline concluded that there is a moderate therapeutic benefit in adding testosterone therapy to achieve up to premenopausal levels in postmenopausal women with self-reported reduction in sexual desire that is causing distress as determined by a validated instrument.
The authors advise baseline hormone testing to rule out androgen excess and baseline renal, lipid, liver, and metabolic testing, even though transdermal testosterone therapy was not shown to alter these parameters in randomized trials of more than 3,000 women. Laboratory assays for both total and free testosterone are “highly unreliable” in the female range as they have been calibrated for male levels of hormone.
FDA-approved testosterone treatments for men with hypogonadism include transdermal gels, patches, intramuscular injection, and an oral formulation. Dosing for women is approximately one-tenth the dosage for treatment of men. Patients should be informed that this treatment is off-label and that long-term studies to establish safety are not available. The authors advised against the use of compounded formulations based on the National Academies of Science, Engineering, and Medicine guidelines, but they went on to say that if compounded products are used, the pharmacy should adhere to Good Manufacturing Practice and Active Pharmaceutical Ingredients standards.
Transdermal testosterone is beneficial for the treatment of HSDD in postmenopausal women after other causes of decreased desire, such as dyspareunia, relationship issues, and other general medical conditions, have been ruled out. There is no diagnostic laboratory test to confirm HSDD or to use as a therapeutic target in treatment (for total or free testosterone, as these are highly unreliable laboratory values). Although large trials have identified no safety signals, they were generally limited to 6 months in duration. Prescribing one-tenth the dose indicated for male hypogonadism results in premenopausal testosterone levels for most women. If there is no benefit after 6 months of treatment, testosterone should be discontinued.
Rare, complex sexual function disorder requires integrated biopsychosocial approach, says ISSWSH
Goldstein I, Komisaruk BR, Pukall CF, et al. International Society for the Study of Women’s Sexual Health (ISSWSH) review of epidemiology and pathophysiology, and a consensus nomenclature and process of care for the management of persistent genital arousal disorder/genito-pelvic dyesthesia (PGAD/GPD). J Sex Med. 2021;18:665-697.
Persistent genital arousal disorder is a poorly understood and relatively rare sexual dysfunction in women. The American College of Obstetricians and Gynecologists (ACOG) Practice Bulletin on Female Sexual Dysfunction does not mention this condition, leaving women’s health practitioners with little guidance as to diagnosis or management.2 Prevalence for the condition is estimated at 1% to 3%. The symptoms may be intermittent or continuous.
In a recent ISSWSH review, a consensus panel defined 5 criteria for this disorder: the perception of genital arousal that is involuntary, unrelated to sexual desire, without any identified cause, not relieved with orgasm, and distressing to the patient. The panel made a clear distinction between PGAD/ genito-pelvic dysesthesia (GPD) and Compulsive Sexual Behavior Disorder (defined by the International Classification of Diseases revision 11 as “a persistent pattern of failure to control intense, repetitive sexual impulses or urges). Because there is considerable overlap with syndromes of genital dysesthesia—itching, burning, tingling, or pain— the consensus panel elected to expand the nomenclature to describe both persistent genital arousal and genito-pelvic dysesthesia as a single syndrome, namely, PGAD/GPD.
Continue to: Negative impact of PGAD/GPD...
Negative impact of PGAD/GPD
The consensus panel identified several contributors to the overall morbidity of this complex disorder, including end organ pathology, peripheral nerve, spinal cord and central sensory processing malfunction, and significant psychological issues. PGAD/GPD also may be associated with spinal cysts, cauda equina pathology, and withdrawal from selective serotonin reuptake inhibitors (SSRIs). Functional magnetic resonance imaging has identified specific brain regions (for example, the paracentral lobule) that are active during clitoral stimulation and that also activate during patients’ experience of persistent genital arousal.
PGAD/GPD negatively impacts sexual function, mental health, and ability to function in daily life. Of major importance is that a large proportion of people with this disorder have significant mental health disorders; in a survey, 54% of patients with PGAD reported suicidal ideation, compared with 25% of participants in a control group.
Evaluation and management recommendations
Diagnosis and management of PGAD/GPD are directed at the 5 areas of evaluation:
- end organ
- pelvis and perineum (assess for pelvic floor tension myalgia, pudendal neuropathy, pelvic congestion syndrome, or pelvic arteriovenous malformation)
- cauda equina (evaluate for neurologic deficits related to cysts compressing S2-S3 nerve roots)
- spinal cord (serotonin and norepinephrine pathways modulate nociceptive sensory activity; either SSRI/serotonin and norepinephrine reuptake inhibitor (SNRI) withdrawal or treatment could impact PGAD/ GPD based on their actions in the spinal cord)
- brain.
The consensus panel recommends an integrated biopsychosocial model for evaluation and treatment of PGAD/GPD. Comorbid mental health conditions, such as depression and anxiety, are common. Small studies suggest that a history of sexual trauma may contribute to catastrophizing and the experience of distressing persistent genital sensations, either arousal or dyesthesia, with 46.7% to 52.6% of patients reporting childhood sexual abuse.3
PGAD/GPD is a poorly recognized source of major distress to a small but significant group of patients. Diagnosis and management require a multidisciplinary team to identify end organ, pharmacologic, neurologic, vascular, and emotional components that contribute to the syndrome. Treatment requires a biopsychosocial approach that addresses the various sources of aberrant sensory processing, including end organ disease, neuropathic signaling, spinal cord pathways, and brain signal processing. Recognizing the existence of, and approaches to, this disorder will help gynecologists understand the considerable distress and potential life-threatening consequences our patients with PGAD/GPD experience.
Future possibilities and current actualities for patient care
Research dollars and investment in female sexual dysfunction remain inadequate to address the considerable gaps that exist in evidence-based clinical guidelines. ISSWSH is working to help clinicians approach these evidence gaps with guidelines and consensus statements to help women’s health professionals identify and manage our patients with sexual concerns and symptoms. An expert consensus guideline on the assessment and management of female orgasmic disorder is currently under development (personal communication, Dr. Sheryl Kingsberg). In addition, a phase 2b trial is underway to assess the impact of topical sildenafil cream for the treatment of female arousal disorder. Stay tuned for the results of these studies.
For now, women’s health professionals have 2 FDA-approved treatment options for premenopausal women with arousal disorder, flibanserin (a daily oral medication that requires abstinence from alcohol) and bremelanotide (an injectable medication that can be used just prior to a sexual encounter). For postmenopausal women, there are no FDA-approved therapies; however, based on the ISSWSH guideline summarized above, transdermal testosterone may be offered to postmenopausal women with distressing loss of sexual desire in doses approximately one-tenth those used to treat men with androgen deficiency. These small doses are challenging to achieve consistently with the delivery systems available for FDA-approved products sold for men.
The National Academies of Science, Engineering, and Medicine advise against the use of compounded hormonal products due to the potential for inconsistency and lack of FDA oversight in the manufacturing/compounding process. I have found and used some compounding pharmacies that are dedicated to safety, quality control, and compliance; test their products; and provide consistent, reliable compounded drugs for my patients. Consideration of compounded testosterone should be discussed with patients, and they should be informed of the current professional association guidelines. Testosterone creams may be compounded to a 1% product—20 mg/mL. Researchers in Australia have demonstrated that 5 mg of transdermal testosterone cream (one-quarter of a mL) results in typical premenopausal testosterone levels.4 When prescribing testosterone for postmenopausal women, check in with them after 6 weeks of treatment to assess impact and check blood levels to ensure that levels are not too high.
Testosterone pellets and intramuscular testosterone are not recommended and in fact should be actively avoided. These methods of administration are associated with extreme variation in hormone levels over time. There are typically supraphysiologic and quite high levels immediately after implantation or injection, followed by fairly significant drop-offs and rapid return of symptoms over time. This may lead to more and more frequent dosing and markedly elevated serum levels.
Management of PGAD/GPD is difficult, but knowing it exists as a valid syndrome will help clinicians validate patients’ symptoms and begin to approach appropriate evaluation and workup targeted to the 5 domains suggested by the ISSWSH expert panel. It is useful to understand the possible relationship to initiation or withdrawal from SSRIs or SNRIs and how aberrant norepinephrine signaling along the sensory pathways may contribute to genital dysesthesia or chronic sensations of arousal. Nonpharmacologic therapies, such as cognitive-behavioral therapy and others, are essential components of the multifaceted approach to treatment. Finally, many complex problems, such as chronic pelvic pain, vestibulodynia, vulvodynia, and chronic fatigue syndrome, are associated with childhood adverse experiences and sexual trauma. Approaching these patients with trauma-informed care is important to create the trust and therapeutic environment they need for successful multidisciplinary care. ●
- Davis SR, Baber R, Panay N, et al. Global consensus position statement on the use of testosterone therapy for women. J Sex Med. 2019;16:1331-1337.
- American College of Obstetricians and Gynecologists’ Committee on Practice Bulletins—Gynecology. ACOG practice bulletin no. 213: Female sexual dysfunction: clinical management guidelines for obstetrician-gynecologists. Obstet Gynecol. 2019;134:e1-e18.
- Leiblum S, Seehuus M, Goldmeier D, et al. Psychological, medical, and pharmacological correlates of persistent genital arousal disorder. J Sex Med. 2007;4:1358-1366.
- Fooladi E, Reuter SE, Bell RJ, et al. Pharmacokinetics of a transdermal testosterone cream in healthy postmenopausal women. Menopause. 2015;22:44-49.
- Davis SR, Baber R, Panay N, et al. Global consensus position statement on the use of testosterone therapy for women. J Sex Med. 2019;16:1331-1337.
- American College of Obstetricians and Gynecologists’ Committee on Practice Bulletins—Gynecology. ACOG practice bulletin no. 213: Female sexual dysfunction: clinical management guidelines for obstetrician-gynecologists. Obstet Gynecol. 2019;134:e1-e18.
- Leiblum S, Seehuus M, Goldmeier D, et al. Psychological, medical, and pharmacological correlates of persistent genital arousal disorder. J Sex Med. 2007;4:1358-1366.
- Fooladi E, Reuter SE, Bell RJ, et al. Pharmacokinetics of a transdermal testosterone cream in healthy postmenopausal women. Menopause. 2015;22:44-49.
Should we rethink maternal monitoring of fetal movement through “kick counts”?

It is time to reconsider the recommendation for practicing fetal kick counts. A meta-analysis demonstrated no decrease in the outcome of stillbirth, but instead an increased risk of iatrogenic delivery.1
CASE 1 8 vs 10 fetal movements in 2 hours
Ms. M is 38 weeks pregnant with an uncomplicated pregnancy. She calls your practice with concerns about fetal kick counts. During her prenatal care, she was counseled to ensure that the baby moved 10 times over a period of 2 hours. This morning, however, she only perceived 8 movements in 2 hours. She is scheduled for evaluation with a nonstress test (NST) on the labor and delivery unit. The NST reveals a reassuring, reactive tracing. Ultrasonography evaluation demonstrates a normal amniotic fluid index and normal fetal growth. The patient is reassured, returns home, and goes on to deliver a healthy baby at 39 weeks and 5 days.
Perception of decreased movement triggers evaluation and monitoring
Maternal perception of normal fetal movement has conceivably been used throughout history as a means of reassurance of fetal well-being; it is highly predictive of fetal viability.2,3 When fetal movement is lacking or decreased, it can be an alarm sign and may result in concerns by the mother that her baby is unwell. Maternal perception of decreased fetal movements affects 5% to 15% of all pregnancies.2,4 While decreased fetal movement can be associated with poor perinatal outcomes such as fetal growth restriction, oligohydramnios, and neuro-developmental disability, it also can be reflective of more benign issues such as anterior placenta, maternal activity, maternal caffeine or sugar consumption, or maternal position.4,5
However, the definition of decreased fetal movement is subject to significant variation, from a total absence of movement over an entire day or what has commonly become accepted as the definition of fetal kick counts with Pearson’s Cardiff chart (which was defined in the 1970s as 10 movements within 12 hours).6,7 Today, women in the United States are commonly recommended to monitor their baby over a 2-hour period and to look for 10 movements during that time.8 Anything less is considered reduced fetal movement and results in recommendations to undergo assessment of previously known high-risk conditions or any possible underlying conditions, such as hypertension, gestational diabetes, or fetal growth restriction. Further evaluation with more objective measures such as electronic fetal monitoring or ultrasonography with biophysical profile are often recommended concurrently.9
It is estimated that up to 15% of women present reporting decreased fetal movement in the third trimester and, as such, require additional monitoring and evaluation. This is not without cost of time and money to the health care system and pregnant patients.
It is uncertain that fetal kick counting prevents stillbirth
Intrauterine fetal demise is neither an uncommon nor completely preventable outcome, despite advances in antenatal care. Many cases occur without evidence of fetal abnormality or other risk factors, and 30% to 55% of women who experience intrauterine fetal demise experience decreased fetal movement in the preceding week.10 It makes physiologic sense that a fetus’ adaptive response to decreased oxygenation is reduced fetal movement, resulting from the prioritization of blood to the fetal brain and other organs over skeletal muscle.4,9,11 Results of a 1976 small study of 61 low-risk pregnancies seemed to confirm that a decrease in fetal movement preceded intrauterine death by 3 to 4 days. Conversely, they found that a normal fetal movement count was generally associated with a good neonatal outcome.6 Thus, experts have long extrapolated that decreased fetal movement can be an indicator for utero-placental insufficiency and, in turn, chronic or acute hypoxia.
However, in larger studies, the ability of fetal movement counting to predict fetal death and fetal compromise appears limited.8,10,11 A meta-analysis of studies, including 5 randomized controlled trials and 468,000 fetuses, compared the incidence of stillbirth in women receiving instructions for fetal movement counting versus women who did not. Rates of stillbirth were the same for each group, demonstrating no advantage to fetal kick counts to prevent a poor perinatal outcome, including stillbirth.1
CASE 2 Reported reduced fetal movement over 4 weeks
Ms. E is a 20-year-old nullipara at 36 weeks’ and 6 days gestation who has come in to triage weekly for the last 4 weeks with concerns about decreased fetal movement. She states that she goes for several hours each day without feeling 10 movements in 2 hours. Recent fetal growth recorded 3 weeks ago was in the 45th percentile, and the amniotic fluid index has been above 10 cm on each weekly ultrasound. Her weekly NSTs have been reactive, and she has been normotensive. However, because she has had several weeks of persistent decreased fetal movement, the labor and delivery team opts to keep her for induction as she is “close to term.”
Decreased kick count frequency may increase unnecessary interventions
Women with fewer kick counts are more likely to present with concerns about the well-being of their baby. In a survey of obstetricians and midwives, a large proportion of providers were more apt to recommend delivery or admission to the hospital for women presenting with decreased fetal movements.2 It stands to reason that recommendations for delivery or admission can lead to outcomes like preterm delivery or recommendations for cesarean delivery (CD). However, using fetal kick counts to portend stillbirth or other poor fetal and neonatal outcomes has been shown to be limited in its value with the AFFIRM trial.10 The results of this large study, which included more than 400,000 pregnancies from 37 hospitals, show the challenges of any study to address the use of management strategies for recent change in the frequency of fetal movements in the reduction of and cause of stillbirth. Additionally, the relatively low risk of stillbirth overall (4.06 stillbirths per 1,000 livebirths during the intervention period and 4.40 per 1,000 livebirths during the control period) but higher incidence of other outcomes, such as prolonged (>48 hours) antepartum admission (6.7% in the intervention period and 6.2% in the control period), induction of labor (40.7% in the intervention period and 35.9% in the control period), and CD (28.4% and 25.5%, respectively) may result in increased harm for many women rather than the intended benefit of preventing stillbirth.10,12
Mindfetalness may be a viable and valuable alternative to kick counts
Alternatives have been proposed as a measure of fetal movement without using kick counts specifically. Mindfetalness has been a method studied in Sweden; its purpose is to strengthen the mother’s awareness of her baby through developing an understanding of the fetal-movement pattern. It is practiced starting at 28 weeks’ gestation for 15 minutes a day, with the woman instructed to lie on her left side and discern the intensity and character of the movements, as well as frequency, without overtly counting the movements.12 In one small study, women felt more connected to their babies and felt less worried.12 In a much larger study of 13,000 women, the authors found no evidence of harm from generalized awareness of fetal movements in a population of pregnant women at or beyond 32 weeks; in fact, they did see significant reductions in iatrogenic outcomes such as CDs and labor inductions
The case for movement awareness over kick counts
Stillbirth risk does not appear to be modified by the use of methods to detect fetal movement.10,12 However, a perceived decrease in fetal kick counts has been shown to result in increased interventions and preterm deliveries. A more prudent approach appears to be educating mothers about general fetal movement, which appears to reduce potentially unnecessary visits and interventions without sacrificing the ability to reassure mothers about the well-being of their babies in utero. ●
- Haezell AEP, Green M, Wright C, et al. Midwives’ and obstetricians’ knowledge and management of women presenting with decreased fetal movements. Acta Obstetricia et Gynecologica. 2008:87;331-339. doi: 10.1080/00016340801902034.
- Froen JF. A kick from within – fetal movement counting and the cancelled progress in antenatal care. J Perinat Med. 2004;32:13-24. doi: 10.1515/JPM.2004.003.
- Heazell AEP, Froen JF. Methods of fetal movement counting and the detection of fetal compromise. J Obstet Gynaecol. 2008;28:147-154. doi: 10.1080/01443610801912618.
- Froen JF, Heazell AEP, Holm Tveit JV, et al. Fetal movement assessment. Semin Perinatal. 2008;32:243-246. doi: 10.1053/j.semperi.2008.04.004
- Pearson JF, Weaver JB. Fetal activity and fetal wellbeing: an evaluation. British Med J. 1976;1:1305-1307. doi: 10.1136/bmj.1.6021.1305.
- Pearson JF. Fetal movements – a new approach to antenatal care. Nursing Mirror Midwives J. 1977;144:49-51.
- American College of Obstetricians and Gynecologists’ Committee on Obstetric Practice, Society for Maternal-Fetal Medicine. Indications for outpatient antenatal fetal surveillance: ACOG committee opinion no. 828. Obstet Gynecol. 2021;137:e177-197. doi: 10.1097/AOG.0000000000004407.
- Christensen FC, Rayburn WF. Fetal movement counts. Obstet Gynecol Clin North Am. 1999;26:4(607-621). doi: 10.1016/s0889-8545(05)70102-9.
- Norman JE, Heazell AEP, Rodriguez A, et al. Awareness of fetal movements and care package to reduce fetal mortality (AFFIRM): a stepped wedge cluster-randomized trial. Lancet. 2018;392:1629-1638. doi: 10.1016/S0140-6736(18)31543-5.
- Warrender LK, Batra G, Bernatavicius G, et al. Maternal perception of reduced fetal movement is associated with altered placental structure and function. PLoS One. 2012;7:4. doi: 10.1371/journal.pone.0034851.
- Bellussi F, Po’ G, Livi A, et al. Fetal movement counting and perinatal mortality. A systematic review and meta-analysis. Obstet Gynecol. 2020;135:453-462. doi: 10.1097/AOG.0000000000003645.
- Akselsson A, Georgsson S, Lindgren H, et al. Women’s attitudes, experiences and compliance concerning the use of mindfetalness – a method for systematic observation of fetal movements in late pregnancy. BMC Pregnancy Childbirth. 2017;17:1-7. doi: 10.1186/s12884-017-1548-5.
- Akselsson A, Lindgren H, Skokic V, et al. A decrease in cesarean sections and labor inductions among Swedish women by awareness of fetal movements with the Mindfetalness method. BMC Pregnancy Childbirth. 2020;20;577:1-10.

It is time to reconsider the recommendation for practicing fetal kick counts. A meta-analysis demonstrated no decrease in the outcome of stillbirth, but instead an increased risk of iatrogenic delivery.1
CASE 1 8 vs 10 fetal movements in 2 hours
Ms. M is 38 weeks pregnant with an uncomplicated pregnancy. She calls your practice with concerns about fetal kick counts. During her prenatal care, she was counseled to ensure that the baby moved 10 times over a period of 2 hours. This morning, however, she only perceived 8 movements in 2 hours. She is scheduled for evaluation with a nonstress test (NST) on the labor and delivery unit. The NST reveals a reassuring, reactive tracing. Ultrasonography evaluation demonstrates a normal amniotic fluid index and normal fetal growth. The patient is reassured, returns home, and goes on to deliver a healthy baby at 39 weeks and 5 days.
Perception of decreased movement triggers evaluation and monitoring
Maternal perception of normal fetal movement has conceivably been used throughout history as a means of reassurance of fetal well-being; it is highly predictive of fetal viability.2,3 When fetal movement is lacking or decreased, it can be an alarm sign and may result in concerns by the mother that her baby is unwell. Maternal perception of decreased fetal movements affects 5% to 15% of all pregnancies.2,4 While decreased fetal movement can be associated with poor perinatal outcomes such as fetal growth restriction, oligohydramnios, and neuro-developmental disability, it also can be reflective of more benign issues such as anterior placenta, maternal activity, maternal caffeine or sugar consumption, or maternal position.4,5
However, the definition of decreased fetal movement is subject to significant variation, from a total absence of movement over an entire day or what has commonly become accepted as the definition of fetal kick counts with Pearson’s Cardiff chart (which was defined in the 1970s as 10 movements within 12 hours).6,7 Today, women in the United States are commonly recommended to monitor their baby over a 2-hour period and to look for 10 movements during that time.8 Anything less is considered reduced fetal movement and results in recommendations to undergo assessment of previously known high-risk conditions or any possible underlying conditions, such as hypertension, gestational diabetes, or fetal growth restriction. Further evaluation with more objective measures such as electronic fetal monitoring or ultrasonography with biophysical profile are often recommended concurrently.9
It is estimated that up to 15% of women present reporting decreased fetal movement in the third trimester and, as such, require additional monitoring and evaluation. This is not without cost of time and money to the health care system and pregnant patients.
It is uncertain that fetal kick counting prevents stillbirth
Intrauterine fetal demise is neither an uncommon nor completely preventable outcome, despite advances in antenatal care. Many cases occur without evidence of fetal abnormality or other risk factors, and 30% to 55% of women who experience intrauterine fetal demise experience decreased fetal movement in the preceding week.10 It makes physiologic sense that a fetus’ adaptive response to decreased oxygenation is reduced fetal movement, resulting from the prioritization of blood to the fetal brain and other organs over skeletal muscle.4,9,11 Results of a 1976 small study of 61 low-risk pregnancies seemed to confirm that a decrease in fetal movement preceded intrauterine death by 3 to 4 days. Conversely, they found that a normal fetal movement count was generally associated with a good neonatal outcome.6 Thus, experts have long extrapolated that decreased fetal movement can be an indicator for utero-placental insufficiency and, in turn, chronic or acute hypoxia.
However, in larger studies, the ability of fetal movement counting to predict fetal death and fetal compromise appears limited.8,10,11 A meta-analysis of studies, including 5 randomized controlled trials and 468,000 fetuses, compared the incidence of stillbirth in women receiving instructions for fetal movement counting versus women who did not. Rates of stillbirth were the same for each group, demonstrating no advantage to fetal kick counts to prevent a poor perinatal outcome, including stillbirth.1
CASE 2 Reported reduced fetal movement over 4 weeks
Ms. E is a 20-year-old nullipara at 36 weeks’ and 6 days gestation who has come in to triage weekly for the last 4 weeks with concerns about decreased fetal movement. She states that she goes for several hours each day without feeling 10 movements in 2 hours. Recent fetal growth recorded 3 weeks ago was in the 45th percentile, and the amniotic fluid index has been above 10 cm on each weekly ultrasound. Her weekly NSTs have been reactive, and she has been normotensive. However, because she has had several weeks of persistent decreased fetal movement, the labor and delivery team opts to keep her for induction as she is “close to term.”
Decreased kick count frequency may increase unnecessary interventions
Women with fewer kick counts are more likely to present with concerns about the well-being of their baby. In a survey of obstetricians and midwives, a large proportion of providers were more apt to recommend delivery or admission to the hospital for women presenting with decreased fetal movements.2 It stands to reason that recommendations for delivery or admission can lead to outcomes like preterm delivery or recommendations for cesarean delivery (CD). However, using fetal kick counts to portend stillbirth or other poor fetal and neonatal outcomes has been shown to be limited in its value with the AFFIRM trial.10 The results of this large study, which included more than 400,000 pregnancies from 37 hospitals, show the challenges of any study to address the use of management strategies for recent change in the frequency of fetal movements in the reduction of and cause of stillbirth. Additionally, the relatively low risk of stillbirth overall (4.06 stillbirths per 1,000 livebirths during the intervention period and 4.40 per 1,000 livebirths during the control period) but higher incidence of other outcomes, such as prolonged (>48 hours) antepartum admission (6.7% in the intervention period and 6.2% in the control period), induction of labor (40.7% in the intervention period and 35.9% in the control period), and CD (28.4% and 25.5%, respectively) may result in increased harm for many women rather than the intended benefit of preventing stillbirth.10,12
Mindfetalness may be a viable and valuable alternative to kick counts
Alternatives have been proposed as a measure of fetal movement without using kick counts specifically. Mindfetalness has been a method studied in Sweden; its purpose is to strengthen the mother’s awareness of her baby through developing an understanding of the fetal-movement pattern. It is practiced starting at 28 weeks’ gestation for 15 minutes a day, with the woman instructed to lie on her left side and discern the intensity and character of the movements, as well as frequency, without overtly counting the movements.12 In one small study, women felt more connected to their babies and felt less worried.12 In a much larger study of 13,000 women, the authors found no evidence of harm from generalized awareness of fetal movements in a population of pregnant women at or beyond 32 weeks; in fact, they did see significant reductions in iatrogenic outcomes such as CDs and labor inductions
The case for movement awareness over kick counts
Stillbirth risk does not appear to be modified by the use of methods to detect fetal movement.10,12 However, a perceived decrease in fetal kick counts has been shown to result in increased interventions and preterm deliveries. A more prudent approach appears to be educating mothers about general fetal movement, which appears to reduce potentially unnecessary visits and interventions without sacrificing the ability to reassure mothers about the well-being of their babies in utero. ●

It is time to reconsider the recommendation for practicing fetal kick counts. A meta-analysis demonstrated no decrease in the outcome of stillbirth, but instead an increased risk of iatrogenic delivery.1
CASE 1 8 vs 10 fetal movements in 2 hours
Ms. M is 38 weeks pregnant with an uncomplicated pregnancy. She calls your practice with concerns about fetal kick counts. During her prenatal care, she was counseled to ensure that the baby moved 10 times over a period of 2 hours. This morning, however, she only perceived 8 movements in 2 hours. She is scheduled for evaluation with a nonstress test (NST) on the labor and delivery unit. The NST reveals a reassuring, reactive tracing. Ultrasonography evaluation demonstrates a normal amniotic fluid index and normal fetal growth. The patient is reassured, returns home, and goes on to deliver a healthy baby at 39 weeks and 5 days.
Perception of decreased movement triggers evaluation and monitoring
Maternal perception of normal fetal movement has conceivably been used throughout history as a means of reassurance of fetal well-being; it is highly predictive of fetal viability.2,3 When fetal movement is lacking or decreased, it can be an alarm sign and may result in concerns by the mother that her baby is unwell. Maternal perception of decreased fetal movements affects 5% to 15% of all pregnancies.2,4 While decreased fetal movement can be associated with poor perinatal outcomes such as fetal growth restriction, oligohydramnios, and neuro-developmental disability, it also can be reflective of more benign issues such as anterior placenta, maternal activity, maternal caffeine or sugar consumption, or maternal position.4,5
However, the definition of decreased fetal movement is subject to significant variation, from a total absence of movement over an entire day or what has commonly become accepted as the definition of fetal kick counts with Pearson’s Cardiff chart (which was defined in the 1970s as 10 movements within 12 hours).6,7 Today, women in the United States are commonly recommended to monitor their baby over a 2-hour period and to look for 10 movements during that time.8 Anything less is considered reduced fetal movement and results in recommendations to undergo assessment of previously known high-risk conditions or any possible underlying conditions, such as hypertension, gestational diabetes, or fetal growth restriction. Further evaluation with more objective measures such as electronic fetal monitoring or ultrasonography with biophysical profile are often recommended concurrently.9
It is estimated that up to 15% of women present reporting decreased fetal movement in the third trimester and, as such, require additional monitoring and evaluation. This is not without cost of time and money to the health care system and pregnant patients.
It is uncertain that fetal kick counting prevents stillbirth
Intrauterine fetal demise is neither an uncommon nor completely preventable outcome, despite advances in antenatal care. Many cases occur without evidence of fetal abnormality or other risk factors, and 30% to 55% of women who experience intrauterine fetal demise experience decreased fetal movement in the preceding week.10 It makes physiologic sense that a fetus’ adaptive response to decreased oxygenation is reduced fetal movement, resulting from the prioritization of blood to the fetal brain and other organs over skeletal muscle.4,9,11 Results of a 1976 small study of 61 low-risk pregnancies seemed to confirm that a decrease in fetal movement preceded intrauterine death by 3 to 4 days. Conversely, they found that a normal fetal movement count was generally associated with a good neonatal outcome.6 Thus, experts have long extrapolated that decreased fetal movement can be an indicator for utero-placental insufficiency and, in turn, chronic or acute hypoxia.
However, in larger studies, the ability of fetal movement counting to predict fetal death and fetal compromise appears limited.8,10,11 A meta-analysis of studies, including 5 randomized controlled trials and 468,000 fetuses, compared the incidence of stillbirth in women receiving instructions for fetal movement counting versus women who did not. Rates of stillbirth were the same for each group, demonstrating no advantage to fetal kick counts to prevent a poor perinatal outcome, including stillbirth.1
CASE 2 Reported reduced fetal movement over 4 weeks
Ms. E is a 20-year-old nullipara at 36 weeks’ and 6 days gestation who has come in to triage weekly for the last 4 weeks with concerns about decreased fetal movement. She states that she goes for several hours each day without feeling 10 movements in 2 hours. Recent fetal growth recorded 3 weeks ago was in the 45th percentile, and the amniotic fluid index has been above 10 cm on each weekly ultrasound. Her weekly NSTs have been reactive, and she has been normotensive. However, because she has had several weeks of persistent decreased fetal movement, the labor and delivery team opts to keep her for induction as she is “close to term.”
Decreased kick count frequency may increase unnecessary interventions
Women with fewer kick counts are more likely to present with concerns about the well-being of their baby. In a survey of obstetricians and midwives, a large proportion of providers were more apt to recommend delivery or admission to the hospital for women presenting with decreased fetal movements.2 It stands to reason that recommendations for delivery or admission can lead to outcomes like preterm delivery or recommendations for cesarean delivery (CD). However, using fetal kick counts to portend stillbirth or other poor fetal and neonatal outcomes has been shown to be limited in its value with the AFFIRM trial.10 The results of this large study, which included more than 400,000 pregnancies from 37 hospitals, show the challenges of any study to address the use of management strategies for recent change in the frequency of fetal movements in the reduction of and cause of stillbirth. Additionally, the relatively low risk of stillbirth overall (4.06 stillbirths per 1,000 livebirths during the intervention period and 4.40 per 1,000 livebirths during the control period) but higher incidence of other outcomes, such as prolonged (>48 hours) antepartum admission (6.7% in the intervention period and 6.2% in the control period), induction of labor (40.7% in the intervention period and 35.9% in the control period), and CD (28.4% and 25.5%, respectively) may result in increased harm for many women rather than the intended benefit of preventing stillbirth.10,12
Mindfetalness may be a viable and valuable alternative to kick counts
Alternatives have been proposed as a measure of fetal movement without using kick counts specifically. Mindfetalness has been a method studied in Sweden; its purpose is to strengthen the mother’s awareness of her baby through developing an understanding of the fetal-movement pattern. It is practiced starting at 28 weeks’ gestation for 15 minutes a day, with the woman instructed to lie on her left side and discern the intensity and character of the movements, as well as frequency, without overtly counting the movements.12 In one small study, women felt more connected to their babies and felt less worried.12 In a much larger study of 13,000 women, the authors found no evidence of harm from generalized awareness of fetal movements in a population of pregnant women at or beyond 32 weeks; in fact, they did see significant reductions in iatrogenic outcomes such as CDs and labor inductions
The case for movement awareness over kick counts
Stillbirth risk does not appear to be modified by the use of methods to detect fetal movement.10,12 However, a perceived decrease in fetal kick counts has been shown to result in increased interventions and preterm deliveries. A more prudent approach appears to be educating mothers about general fetal movement, which appears to reduce potentially unnecessary visits and interventions without sacrificing the ability to reassure mothers about the well-being of their babies in utero. ●
- Haezell AEP, Green M, Wright C, et al. Midwives’ and obstetricians’ knowledge and management of women presenting with decreased fetal movements. Acta Obstetricia et Gynecologica. 2008:87;331-339. doi: 10.1080/00016340801902034.
- Froen JF. A kick from within – fetal movement counting and the cancelled progress in antenatal care. J Perinat Med. 2004;32:13-24. doi: 10.1515/JPM.2004.003.
- Heazell AEP, Froen JF. Methods of fetal movement counting and the detection of fetal compromise. J Obstet Gynaecol. 2008;28:147-154. doi: 10.1080/01443610801912618.
- Froen JF, Heazell AEP, Holm Tveit JV, et al. Fetal movement assessment. Semin Perinatal. 2008;32:243-246. doi: 10.1053/j.semperi.2008.04.004
- Pearson JF, Weaver JB. Fetal activity and fetal wellbeing: an evaluation. British Med J. 1976;1:1305-1307. doi: 10.1136/bmj.1.6021.1305.
- Pearson JF. Fetal movements – a new approach to antenatal care. Nursing Mirror Midwives J. 1977;144:49-51.
- American College of Obstetricians and Gynecologists’ Committee on Obstetric Practice, Society for Maternal-Fetal Medicine. Indications for outpatient antenatal fetal surveillance: ACOG committee opinion no. 828. Obstet Gynecol. 2021;137:e177-197. doi: 10.1097/AOG.0000000000004407.
- Christensen FC, Rayburn WF. Fetal movement counts. Obstet Gynecol Clin North Am. 1999;26:4(607-621). doi: 10.1016/s0889-8545(05)70102-9.
- Norman JE, Heazell AEP, Rodriguez A, et al. Awareness of fetal movements and care package to reduce fetal mortality (AFFIRM): a stepped wedge cluster-randomized trial. Lancet. 2018;392:1629-1638. doi: 10.1016/S0140-6736(18)31543-5.
- Warrender LK, Batra G, Bernatavicius G, et al. Maternal perception of reduced fetal movement is associated with altered placental structure and function. PLoS One. 2012;7:4. doi: 10.1371/journal.pone.0034851.
- Bellussi F, Po’ G, Livi A, et al. Fetal movement counting and perinatal mortality. A systematic review and meta-analysis. Obstet Gynecol. 2020;135:453-462. doi: 10.1097/AOG.0000000000003645.
- Akselsson A, Georgsson S, Lindgren H, et al. Women’s attitudes, experiences and compliance concerning the use of mindfetalness – a method for systematic observation of fetal movements in late pregnancy. BMC Pregnancy Childbirth. 2017;17:1-7. doi: 10.1186/s12884-017-1548-5.
- Akselsson A, Lindgren H, Skokic V, et al. A decrease in cesarean sections and labor inductions among Swedish women by awareness of fetal movements with the Mindfetalness method. BMC Pregnancy Childbirth. 2020;20;577:1-10.
- Haezell AEP, Green M, Wright C, et al. Midwives’ and obstetricians’ knowledge and management of women presenting with decreased fetal movements. Acta Obstetricia et Gynecologica. 2008:87;331-339. doi: 10.1080/00016340801902034.
- Froen JF. A kick from within – fetal movement counting and the cancelled progress in antenatal care. J Perinat Med. 2004;32:13-24. doi: 10.1515/JPM.2004.003.
- Heazell AEP, Froen JF. Methods of fetal movement counting and the detection of fetal compromise. J Obstet Gynaecol. 2008;28:147-154. doi: 10.1080/01443610801912618.
- Froen JF, Heazell AEP, Holm Tveit JV, et al. Fetal movement assessment. Semin Perinatal. 2008;32:243-246. doi: 10.1053/j.semperi.2008.04.004
- Pearson JF, Weaver JB. Fetal activity and fetal wellbeing: an evaluation. British Med J. 1976;1:1305-1307. doi: 10.1136/bmj.1.6021.1305.
- Pearson JF. Fetal movements – a new approach to antenatal care. Nursing Mirror Midwives J. 1977;144:49-51.
- American College of Obstetricians and Gynecologists’ Committee on Obstetric Practice, Society for Maternal-Fetal Medicine. Indications for outpatient antenatal fetal surveillance: ACOG committee opinion no. 828. Obstet Gynecol. 2021;137:e177-197. doi: 10.1097/AOG.0000000000004407.
- Christensen FC, Rayburn WF. Fetal movement counts. Obstet Gynecol Clin North Am. 1999;26:4(607-621). doi: 10.1016/s0889-8545(05)70102-9.
- Norman JE, Heazell AEP, Rodriguez A, et al. Awareness of fetal movements and care package to reduce fetal mortality (AFFIRM): a stepped wedge cluster-randomized trial. Lancet. 2018;392:1629-1638. doi: 10.1016/S0140-6736(18)31543-5.
- Warrender LK, Batra G, Bernatavicius G, et al. Maternal perception of reduced fetal movement is associated with altered placental structure and function. PLoS One. 2012;7:4. doi: 10.1371/journal.pone.0034851.
- Bellussi F, Po’ G, Livi A, et al. Fetal movement counting and perinatal mortality. A systematic review and meta-analysis. Obstet Gynecol. 2020;135:453-462. doi: 10.1097/AOG.0000000000003645.
- Akselsson A, Georgsson S, Lindgren H, et al. Women’s attitudes, experiences and compliance concerning the use of mindfetalness – a method for systematic observation of fetal movements in late pregnancy. BMC Pregnancy Childbirth. 2017;17:1-7. doi: 10.1186/s12884-017-1548-5.
- Akselsson A, Lindgren H, Skokic V, et al. A decrease in cesarean sections and labor inductions among Swedish women by awareness of fetal movements with the Mindfetalness method. BMC Pregnancy Childbirth. 2020;20;577:1-10.
How should you advise your 54-year-old patient about the use of HT?
CASE Healthy woman with hot flashes inquires about HT
A 54-year-old healthy woman with a history of hypothyroidism taking thyroid replacement medication comes in for her annual visit. Her last menstrual period was over 2 years ago and she reports severe hot flashes. They have greatly affected her quality of life and she must take frequent breaks at work. She wakes up frequently at night due to night sweats, which is impacting her sleep and, subsequently, her energy level. She has noted increased vaginal dryness so has been abstaining from sexual intercourse due to the discomfort. She has an intact uterus. Her family history is significant for heart disease, diagnosed in her mother at age 75.
On physical examination, she is normotensive and well-appearing. Her body mass index (BMI) is 21 kg/m2. Labs obtained prior to her visit show normal renal and liver function. Her high-density lipid (HDL) level is 55 mg/dL, her low-density lipid (LDL) level is 80 mg/dL, and her triglyceride level is 100 mg/dL; HbA1c is 5.5 mmol/mol.
She is interested in learning more about menopausal hormone therapy (HT) and whether or not she would be a candidate.
What information do you need to know to counsel and manage this patient?
Menopausal HT prescribing practices have changed over the last few decades as a better understanding of the risks and benefits of treatment have emerged. Prior to 2002, HT was commonly used for treatment of symptoms associated with menopause and was thought to have beneficial effects for chronic disease prevention.1-4 After data from the Women’s Health Initiative (WHI) was released, concerns arose around the effect of HT on cardiovascular health and risk of breast cancer. As a result, HT prescriptions fell precipitously after around 2002.5 Since then, postintervention analysis and cumulative 18-year follow-up of WHI data, along with results from subsequent randomized controlled trials, including the Kronos Early Estrogen Prevention Study (KEEPS) and the Early Versus Late Intervention Trial with Estradiol (ELITE), have demonstrated a favorable safety profile for healthy women starting HT early in menopause (less than age 60, or within 10 years from their final menstrual period).5-11
There are many types, formulations, and routes of HT, and the effects and risks differ for each (TABLE). For example, oral estrogen therapy, such as conjugated equine estrogens, portend a higher risk of adverse effects compared with transdermal formulations. Topical and transdermal estrogens bypass first-pass hepatic metabolism and thus are associated with a lower risk of venous thromboembolism (VTE) compared with oral formulations.12-14 A progestogen such as micronized progesterone is used in postmenopausal women with a uterus to protect the endometrium from unopposed estrogen therapy (ET). While it comes in oral and transdermal forms, the oral formulation is most widely used and studied in the United States; transdermal forms do not provide adequate endometrial protection and should not be used in combination therapy.15,16
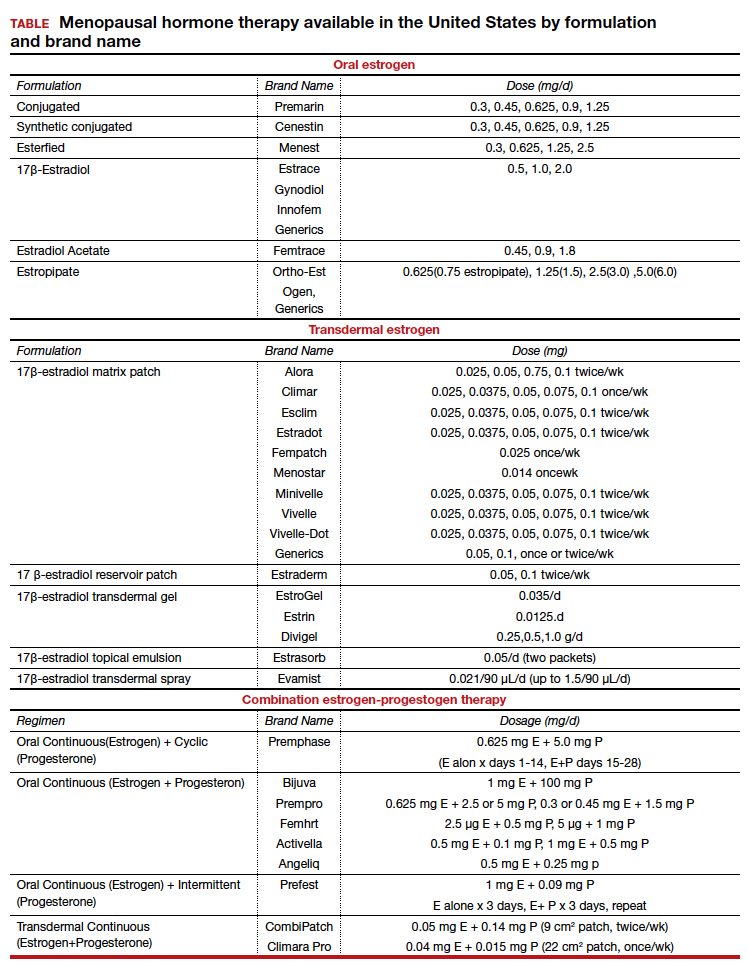
Risks and benefits
Cardiovascular risk
Over time, the benefits and risks of HT use in menopausal patients have been further elucidated and defined, although they remain complex and dependent on patient clinical characteristics. HT remains the most effective treatment for vasomotor symptoms (VMS) and the genitourinary syndrome of menopause.17,18 In 2002, concerns for increased cardiovascular disease (CVD) and breast cancer risk resulted in early cessation of the WHI trial. Since that time the risk of CVD in postmenopausal women taking HT has been found to be more nuanced. In fact, updates in the literature have shown that HT results in a reduction of coronary heart disease if started in healthy women younger than age 60 years within 10 years of menopause.7,9-11 With this updated information, the North American Menopause society (NAMS), American College of Obstetricians and Gynecologists and the Endocrine Society have published guidelines supporting the initiation of HT for symptomatic healthy women: under the age of 60, within 10 years of menopause, and without contraindications. After age 60 years and further from menopause, the benefits and risks become less known.18-20
Risk stratification allows for more comprehensive counseling in use of HT for treatment of bothersome VMS. From a cardiovascular health standpoint, calculating an atherosclerotic CVD (ASCVD) risk score helps to evaluate appropriateness of HT prescribing:
- For those with low 10-year CVD risk (<5%), either oral or transdermal HT is appropriate.
- For those with moderate 10-year CVD risk (5%-10%), transdermal HT is recommended over oral HT.
- For those with high 10-year CVD risk (>10%), HT is not recommended.19,21
Breast cancer risk
Follow up since the initial WHI publication have shown that breast cancer risk is largely dependent on the formulation and route of HT used. Oral estrogen combined with a progestogen has been shown to increase the risk of invasive breast cancer, though very rarely.22 To put it into context, the absolute risk of breast cancer based on follow-up studies from WHI showed less than 1 additional case per 1,000 person years of use; less risk than associated with drinking 2 glasses of wine per day and similar to that of obesity and/or sedentary lifestyle.23,24 Studies have shown estrogen treatment alone for postmenopausal women does not appear to increase the risk of breast cancer. In fact, follow-up data from WHI showed a nonsignificant reduction in breast cancer risk for those taking ET alone.25
Breast cancer risk stratification is helpful when determining appropriateness of HT in postmenopausal women. Generally, if using risk stratification models for breast cancer (ie, Gail Risk model or international breast cancer intervention study [IBIS] tool), a patient who is average to moderate risk, HT can be offered with appropriate counseling. By contrast, a patient who is high risk should have a more detailed discussion about their risk (surveillance and risk-reducing treatments), and they may consider nonhormonal options for treatment of VMS. Women with a history of breast cancer should not be prescribed systemic HT.
Continue to: Additional HT benefits...
Additional HT benefits
The benefits of HT in postmenopausal women include improved bone health and reduction of fractures; reduction of risk for type 2 diabetes mellitus (T2DM); improvement of insulin sensitivity; improvement of lipid profiles with increased HDL and decreased LDL levels; and reduction of colon cancer risk.25 For women aged younger than 60 years who start HT within 10 years of their last menstrual period, HT has been shown to cause a reduction in all-cause mortality. Important risks to counsel patients on when starting HT include the low risk of stroke and venous thromboembolism (VTE) when using oral formulations.26
CASE Resolved
Her ASCVD risk score, based on her history, estimates her 10-year CVD risk to be low (<5%). Thus, from a cardiovascular standpoint, either oral or transdermal HT would be an appropriate option. Her IBIS 10-year score is 1.5%, placing her in a low-risk category for breast cancer based on her personal and family history. Given that she is less than 60 years of age and within 10 years of menopause, along with her low-risk stratification for CVD and breast cancer, she would be an appropriate patient to begin combined HT with an estrogen plus an oral progesterone, such as an estradiol patch 0.0375 mg twice weekly, along with oral micronized progesterone 100 mg nightly. The dose could be increased over time based on symptoms and tolerability of the treatment.
ALTERNATE CASE 1 The patient has additional risk factors
Consider the patient case with the following additions to her history: the patient has a BMI of 34 kg/m2, a history of well-controlled hypertension while taking amlodipine 5 mg, and an ASCVD risk score of 7.5%. She reports severe VMS that are greatly impacting her quality of life. How would your recommendations or counseling change?
Focus on healthy lifestyle
Obesity and hypertension, both common chronic conditions, pose additional risks to be accounted for when counseling on and approaching HT prescribing. Her alternate ASCVD risk score places her at moderate risk for CVD within 10 years, based on guidelines as discussed above. It would still be appropriate to offer her combined HT after a shared decision-making discussion that includes a focus on healthy lifestyle habits.
Consider transdermal HT in obese women
Longitudinal studies have found that weight gain is more a consequence of aging, regardless of menopausal status. Fat distribution and body composition changes are a menopause-related phenomenon driven by estrogen deficiency. HT has been shown to preserve lean body mass and reduce visceral adiposity, resulting in favorable effects of body composition. Still, obesity results in increased risk of CVD, VTE, and certain hormone-sensitive cancers.27 When considering HT in obese patients, a transdermal estrogen route is preferred to reduce risks.
For women with hypertension, prescribe transdermal HT
Overall, studies have found that HT has a neutral effect on blood pressure.25 When considering formulation of HT, micronized progesterone, dydrogesterone, and drospirenone seem to be most neutral and possibly even beneficial on blood pressure compared with synthetic progestins.26 Oral estrogen is associated with increased vasoconstriction and/or increased sodium retention with resultant worsened regulation of blood pressure in women with hypertension, so transdermal estrogen is preferred for women with hypertension.26 Hypertension is a component of the ASCVD risk score; factoring this into a patient’s clinical picture is important when discussing appropriateness of HT prescribing. To minimize risks, the transdermal route of estrogen is preferred for those with hypertension.
Continue to: ALTERNATE CASE 1 Resolved...
ALTERNATE CASE 1 Resolved
She has a moderate ASCVD risk score, is obese, and has a history of hypertension. Through shared decision making, you ultimately start her on transdermal estrogen and micronized progesterone to treat her quality-of-life-impacting VMS, a formulation that is most likely to mitigate the possible risks in her clinical case. You see her back in the clinic every 3-6 months to monitor her blood pressure.
ALTERNATE CASE 2 The patient has a high risk for breast cancer
The patient reveals further her significant family history of breast cancer in her maternal grandmother and mother, both diagnosed in their 50s. You calculate her risk of breast cancer with a model that incorporates family history. Her Tyrer Cuzick-IBIS 10-year risk score is >5% and lifetime risk is >20%, putting her at high risk for breast cancer. Since she has a uterus and would need concomitant progesterone therapy, her risk for breast cancer is higher than if she was taking ET alone. Ultimately, together you and the patient decide to trial nonhormonal options for her VMS.
What are nonhormonal options for treatment of VMS?
While HT remains the most effective treatment for VMS, there are multiple nonhormonal treatments for women who are either at too high a risk for HT or who favor other options, which are outlined in the NAMS 2015 nonhormonal management position statement.27 Cognitive behavioral therapy (CBT) has been shown to decrease bother related to VMS but not frequency. Clinical hypnosis has been shown to reduce hot flash frequency and improve sleep. Paroxetine salt (7.5 mg/day) remains the only FDA nonhormonal-approved medication for treatment of moderate to severe vasomotor symptoms. Off label use of other selective serotonin reuptake inhibitors (SSRIs) and selective norepinephrine reuptake inhibitors have been shown in studies to reduce VMS including paroxetine at slightly higher doses (10 mg/day–20 mg/day), citalopram (10 mg/day–20 mg/day), escitalopram (10 mg/day–20 mg/day), venlafaxine (37.5 mg/day–150 mg/day), and desvenlafaxine (50 mg/day–100 mg/day). Other treatments that could be considered include off-label use of gabapentin (900 mg/day–2,400 mg/day), oxybutynin (2.5–5 mg twice daily) or clonidine (0.1 mg/day–1 mg/day divided in doses) since they all have data demonstrating they are beneficial at reducing VMS.
Nonhormonal options that may be helpful but are recommended with caution due to lack of data include weight loss, mindfulness-based stress reduction, s-equol derivatives of soy isoflavones and a stellate ganglion block. Further evidence and studies are needed for the aforementioned options.27
ALTERNATE CASE 2 Resolved
She may consider any of the nonhormonal options discussed. If she meets with a medical breast specialist to discuss her elevated risk of breast cancer and considers starting risk-reducing medications, particularly tamoxifen, you will want to avoid medications that have significant CPY 2D6 inhibition, such as paroxetine and fluoxetine. Safer choices would include venlafaxine, escitalopram, or citalopram.
The bottom line
In summary, the benefits and risks of HT in the treatment of VMS remain nuanced. For healthy women younger than 60 years of age and within 10 years from their last menstrual period, the benefits of HT largely outweigh the risks. Shared decision making, along with individualized and appropriate risk stratification specific for women, can guide appropriateness of HT prescribing. For those women who cannot take HT or choose not to, there are many nonhormonal options that will help manage their bothersome VMS. ●
- Carr BR, Wilson JD. Disorders of the ovary and female reproductive tract. In: Isselbacher KJ, Braunwald E, Wilson JD, eds. Harrisons’ Principles of Internal Medicine, 13th ed. New York, NY: McGraw-Hill; 1994:2016-2017.
- Davidson MH, Maki KC, Marx P, et al. Effects of continuous estrogen and estrogen-progestin replacement regimens on cardiovascular risk markers in postmenopausel women. Arch Intern Med. 2000;160:3315-3325. doi: 10.1001/archinte.160.21.3315.
- Grodstein F, Manson JE, Colditz GA, et al. A prospective, observational study of postmenopausal hormone therapy and primary prevention of cardiovascular disease. Ann Intern Med. 2000;133:933-941. doi: 10.7326/0003-4819-133-12-200012190-00008.
- Grady D, Rubin SM, Petitti DB, et al. Hormone therapy to prevent disease and prolong life in postmenopausal women. Ann Intern Med. 1992;117:1016-1037. doi: 10.7326/0003-4819-117-12-1016.
- Rossouw JE, Manson JE, Kaunitz AM, et al. Lessons learned from the Women’s Health Initiative trials of menopausal hormone therapy. Obstet Gynecol. 2013;121:172-176. doi: 10.1097/aog.0b013e31827a08c8.
- Manson JE, Hsia J, Johnson KC, et al. Estrogen plus progestin and the risk of coronary heart disease. NEJM. 2003;349:523-534. doi: 10.1056/NEJMoa030808.
- Manson JE, Chlebowski RT, Stefanick ML, et al. Menopausal hormone therapy and health outcomes during the intervention and extended poststopping phases of the Women’s Health Initiative randomized trials. JAMA. 2013;310:1353-1368. doi: 10.1001/jama.2013.278040.
- Santen RJ, Allred DC, Ardoin SP, et al. Postmenopausal hormone therapy: an Endocrine Society scientific statement. J Clin Endocrinol Metab. 2010;95:S1-S66. doi: 10.1210/jc.2009-2509.
- Manson JE, Aragaki AK, Rossouw JE, et al. Menopausal hormone therapy and long-term all-cause and cause-specific mortality: the Women’s Health Initiative randomized trials. JAMA. 2017;318:927-938. doi: 10.1001/jama.2017.11217.
- Hodis HN, Mack WJ, Henderson VW, et al. Vacular effects of early versus late postmenopausal treatment with estradiol. NEJM. 2016;374:1221-1231. doi: 10.1056/NEJMoa1505241.
- Taylor HS, Tal A, Pal L, et al. Effects of oral vs transdermal estrogen therapy on sexual function in early postmenopause: ancillary study of the Kronos Early Estrogen Prevention Study (KEEPS). JAMA Intern Med. 2017;177:1471-1479. doi: 10.1001/jamainternmed.2017.3877.
- Liu JH, Pinkerton JV. Prescription therapies. In: CJ Crandall, ed. Menopause Practice: A Clinician’s Guide, 6th ed. Pepper Pike, OH: The North American Menopause Society; 2019:277-309.
- Files J, Kling JM. Transdermal delivery of bioidentical estrogen in menopausal hormone therapy: a clinical review. Expert Opin Drug Deliv. 2020;17:543-549. doi: 10.1080/17425247.2020.1700949.
- Canonico M, Carcaillon L, Plu-Bureau G, et al. Postmenopausal hormone therapy and risk of stroke: impact of the route of estrogen administration and type of progestogen. Stroke. 2016;47:1734-1741. doi: 10.1161/STROKEAHA.116.013052.
- Hitchcok CL, Prior JC. Oral micronized progesterone for vasomotor symptoms—a placebo-controlled randomized trial in healthy post-menopausal women. Menopause. 2001;8:10-16.
- Effects of hormone replacement therapy on endometrial histology in postmenopausal women. The Postmenopausal Estrogen/Progestin Interventions (PEPI) Trial. The writing Group for the PEPI Trial. JAMA. 1996;275:370-375. doi: 10.1001/jama.1996.03530290040035.
- Pinkerton JV. Hormone therapy for postmenopausal women. N Engl J Med. 2020;382:446-55. doi:10.1056/NEJMcp1714787.
- The 2022 hormone therapy position statement of The North American Menopause Society. Menopause. 2022;29:767-794. doi:10.1097/GME.00000000000000002028.
- Stuenkel CA, Davis SR, Gompel A, et al. Treatment of symptoms of the menopause: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2015;100:3975-4011. doi: 10.1210/jc.2015-2236.
- American College of Obstetricians and Gynecologists. Practice Bulletin No. 141: Management of Menopausal Symptoms. Obstet Gynecol. 2014;123:202-216. doi: 10.1097/01.AOG.0000441353.20693.78.
- Manson JE. Current recommendations: what is the clinician to do? Fertil Steril. 2014;101:916. World Cancer Research Fund/American Institute for Cancer Research. Continuous Update Project Expert Report 2018. Alcoholic drinks and the risk of cancer. https://www.wcrf.org/sites/default/files/Alcoholic-Drinks.pdf. 2018.
- Anderson GL, Chlebowski RT, Aragaki AK, et al. Conjugated equine oestrogen and breast cancer incidence and mortality in postmenopausal women with hysterectomy: Extended follow-up of Women’s Health Initiative randomized placebo-controlled trial. Lancet Oncol. 2012;5:476-486. doi: 10.1016/S1470-2045(12)70075-X.
- World Cancer Research Fund/American Institute for Cancer Research. Continuous Update Project Expert Report 2018. Diet, nutrition, physical activity and breast cancer. www.aicr.org/continuous-update-project/breast-cancer.html. 2018.
- Anderson GL, Chlebowski RT, Aragaki AK, et al. Conjugated equine oestrogen and breast cancer incidence and mortality in postmenopausal women with hysterectomy: Extended follow-up of the Women’s Health Initiative randomized placebo-controlled trial. Lancet Oncol. 2012;5:476-486. doi: 10.1016/S1470-2045(12)70075-X.
- Mehta J, Kling JM, Manson JE. Risks, benefits and treatment modalities of menopausal hormone therapy: current concepts. Front Endocrinol (Laussane). 2021;12:564781. doi: 10.3389/fendo.2021.564781.
- Kapoor E, Kling JM, Lobo AS, et al. Menopausal hormone therapy in women with chronic medical conditions. Best Pract Res Clin Endocrinol Metab. 2021:35;101578. doi: 10.1016/j.beem.2021.101578.
- NAMS position statement advisory panel. Nonhormonal management of menopause-associated vasomotor symptoms: 2015 position statement of The North American Menopause Society. Menopause. 2015:22:1155-72. doi: 10.1097/GME.0000000000000546.
CASE Healthy woman with hot flashes inquires about HT
A 54-year-old healthy woman with a history of hypothyroidism taking thyroid replacement medication comes in for her annual visit. Her last menstrual period was over 2 years ago and she reports severe hot flashes. They have greatly affected her quality of life and she must take frequent breaks at work. She wakes up frequently at night due to night sweats, which is impacting her sleep and, subsequently, her energy level. She has noted increased vaginal dryness so has been abstaining from sexual intercourse due to the discomfort. She has an intact uterus. Her family history is significant for heart disease, diagnosed in her mother at age 75.
On physical examination, she is normotensive and well-appearing. Her body mass index (BMI) is 21 kg/m2. Labs obtained prior to her visit show normal renal and liver function. Her high-density lipid (HDL) level is 55 mg/dL, her low-density lipid (LDL) level is 80 mg/dL, and her triglyceride level is 100 mg/dL; HbA1c is 5.5 mmol/mol.
She is interested in learning more about menopausal hormone therapy (HT) and whether or not she would be a candidate.
What information do you need to know to counsel and manage this patient?
Menopausal HT prescribing practices have changed over the last few decades as a better understanding of the risks and benefits of treatment have emerged. Prior to 2002, HT was commonly used for treatment of symptoms associated with menopause and was thought to have beneficial effects for chronic disease prevention.1-4 After data from the Women’s Health Initiative (WHI) was released, concerns arose around the effect of HT on cardiovascular health and risk of breast cancer. As a result, HT prescriptions fell precipitously after around 2002.5 Since then, postintervention analysis and cumulative 18-year follow-up of WHI data, along with results from subsequent randomized controlled trials, including the Kronos Early Estrogen Prevention Study (KEEPS) and the Early Versus Late Intervention Trial with Estradiol (ELITE), have demonstrated a favorable safety profile for healthy women starting HT early in menopause (less than age 60, or within 10 years from their final menstrual period).5-11
There are many types, formulations, and routes of HT, and the effects and risks differ for each (TABLE). For example, oral estrogen therapy, such as conjugated equine estrogens, portend a higher risk of adverse effects compared with transdermal formulations. Topical and transdermal estrogens bypass first-pass hepatic metabolism and thus are associated with a lower risk of venous thromboembolism (VTE) compared with oral formulations.12-14 A progestogen such as micronized progesterone is used in postmenopausal women with a uterus to protect the endometrium from unopposed estrogen therapy (ET). While it comes in oral and transdermal forms, the oral formulation is most widely used and studied in the United States; transdermal forms do not provide adequate endometrial protection and should not be used in combination therapy.15,16

Risks and benefits
Cardiovascular risk
Over time, the benefits and risks of HT use in menopausal patients have been further elucidated and defined, although they remain complex and dependent on patient clinical characteristics. HT remains the most effective treatment for vasomotor symptoms (VMS) and the genitourinary syndrome of menopause.17,18 In 2002, concerns for increased cardiovascular disease (CVD) and breast cancer risk resulted in early cessation of the WHI trial. Since that time the risk of CVD in postmenopausal women taking HT has been found to be more nuanced. In fact, updates in the literature have shown that HT results in a reduction of coronary heart disease if started in healthy women younger than age 60 years within 10 years of menopause.7,9-11 With this updated information, the North American Menopause society (NAMS), American College of Obstetricians and Gynecologists and the Endocrine Society have published guidelines supporting the initiation of HT for symptomatic healthy women: under the age of 60, within 10 years of menopause, and without contraindications. After age 60 years and further from menopause, the benefits and risks become less known.18-20
Risk stratification allows for more comprehensive counseling in use of HT for treatment of bothersome VMS. From a cardiovascular health standpoint, calculating an atherosclerotic CVD (ASCVD) risk score helps to evaluate appropriateness of HT prescribing:
- For those with low 10-year CVD risk (<5%), either oral or transdermal HT is appropriate.
- For those with moderate 10-year CVD risk (5%-10%), transdermal HT is recommended over oral HT.
- For those with high 10-year CVD risk (>10%), HT is not recommended.19,21
Breast cancer risk
Follow up since the initial WHI publication have shown that breast cancer risk is largely dependent on the formulation and route of HT used. Oral estrogen combined with a progestogen has been shown to increase the risk of invasive breast cancer, though very rarely.22 To put it into context, the absolute risk of breast cancer based on follow-up studies from WHI showed less than 1 additional case per 1,000 person years of use; less risk than associated with drinking 2 glasses of wine per day and similar to that of obesity and/or sedentary lifestyle.23,24 Studies have shown estrogen treatment alone for postmenopausal women does not appear to increase the risk of breast cancer. In fact, follow-up data from WHI showed a nonsignificant reduction in breast cancer risk for those taking ET alone.25
Breast cancer risk stratification is helpful when determining appropriateness of HT in postmenopausal women. Generally, if using risk stratification models for breast cancer (ie, Gail Risk model or international breast cancer intervention study [IBIS] tool), a patient who is average to moderate risk, HT can be offered with appropriate counseling. By contrast, a patient who is high risk should have a more detailed discussion about their risk (surveillance and risk-reducing treatments), and they may consider nonhormonal options for treatment of VMS. Women with a history of breast cancer should not be prescribed systemic HT.
Continue to: Additional HT benefits...
Additional HT benefits
The benefits of HT in postmenopausal women include improved bone health and reduction of fractures; reduction of risk for type 2 diabetes mellitus (T2DM); improvement of insulin sensitivity; improvement of lipid profiles with increased HDL and decreased LDL levels; and reduction of colon cancer risk.25 For women aged younger than 60 years who start HT within 10 years of their last menstrual period, HT has been shown to cause a reduction in all-cause mortality. Important risks to counsel patients on when starting HT include the low risk of stroke and venous thromboembolism (VTE) when using oral formulations.26
CASE Resolved
Her ASCVD risk score, based on her history, estimates her 10-year CVD risk to be low (<5%). Thus, from a cardiovascular standpoint, either oral or transdermal HT would be an appropriate option. Her IBIS 10-year score is 1.5%, placing her in a low-risk category for breast cancer based on her personal and family history. Given that she is less than 60 years of age and within 10 years of menopause, along with her low-risk stratification for CVD and breast cancer, she would be an appropriate patient to begin combined HT with an estrogen plus an oral progesterone, such as an estradiol patch 0.0375 mg twice weekly, along with oral micronized progesterone 100 mg nightly. The dose could be increased over time based on symptoms and tolerability of the treatment.
ALTERNATE CASE 1 The patient has additional risk factors
Consider the patient case with the following additions to her history: the patient has a BMI of 34 kg/m2, a history of well-controlled hypertension while taking amlodipine 5 mg, and an ASCVD risk score of 7.5%. She reports severe VMS that are greatly impacting her quality of life. How would your recommendations or counseling change?
Focus on healthy lifestyle
Obesity and hypertension, both common chronic conditions, pose additional risks to be accounted for when counseling on and approaching HT prescribing. Her alternate ASCVD risk score places her at moderate risk for CVD within 10 years, based on guidelines as discussed above. It would still be appropriate to offer her combined HT after a shared decision-making discussion that includes a focus on healthy lifestyle habits.
Consider transdermal HT in obese women
Longitudinal studies have found that weight gain is more a consequence of aging, regardless of menopausal status. Fat distribution and body composition changes are a menopause-related phenomenon driven by estrogen deficiency. HT has been shown to preserve lean body mass and reduce visceral adiposity, resulting in favorable effects of body composition. Still, obesity results in increased risk of CVD, VTE, and certain hormone-sensitive cancers.27 When considering HT in obese patients, a transdermal estrogen route is preferred to reduce risks.
For women with hypertension, prescribe transdermal HT
Overall, studies have found that HT has a neutral effect on blood pressure.25 When considering formulation of HT, micronized progesterone, dydrogesterone, and drospirenone seem to be most neutral and possibly even beneficial on blood pressure compared with synthetic progestins.26 Oral estrogen is associated with increased vasoconstriction and/or increased sodium retention with resultant worsened regulation of blood pressure in women with hypertension, so transdermal estrogen is preferred for women with hypertension.26 Hypertension is a component of the ASCVD risk score; factoring this into a patient’s clinical picture is important when discussing appropriateness of HT prescribing. To minimize risks, the transdermal route of estrogen is preferred for those with hypertension.
Continue to: ALTERNATE CASE 1 Resolved...
ALTERNATE CASE 1 Resolved
She has a moderate ASCVD risk score, is obese, and has a history of hypertension. Through shared decision making, you ultimately start her on transdermal estrogen and micronized progesterone to treat her quality-of-life-impacting VMS, a formulation that is most likely to mitigate the possible risks in her clinical case. You see her back in the clinic every 3-6 months to monitor her blood pressure.
ALTERNATE CASE 2 The patient has a high risk for breast cancer
The patient reveals further her significant family history of breast cancer in her maternal grandmother and mother, both diagnosed in their 50s. You calculate her risk of breast cancer with a model that incorporates family history. Her Tyrer Cuzick-IBIS 10-year risk score is >5% and lifetime risk is >20%, putting her at high risk for breast cancer. Since she has a uterus and would need concomitant progesterone therapy, her risk for breast cancer is higher than if she was taking ET alone. Ultimately, together you and the patient decide to trial nonhormonal options for her VMS.
What are nonhormonal options for treatment of VMS?
While HT remains the most effective treatment for VMS, there are multiple nonhormonal treatments for women who are either at too high a risk for HT or who favor other options, which are outlined in the NAMS 2015 nonhormonal management position statement.27 Cognitive behavioral therapy (CBT) has been shown to decrease bother related to VMS but not frequency. Clinical hypnosis has been shown to reduce hot flash frequency and improve sleep. Paroxetine salt (7.5 mg/day) remains the only FDA nonhormonal-approved medication for treatment of moderate to severe vasomotor symptoms. Off label use of other selective serotonin reuptake inhibitors (SSRIs) and selective norepinephrine reuptake inhibitors have been shown in studies to reduce VMS including paroxetine at slightly higher doses (10 mg/day–20 mg/day), citalopram (10 mg/day–20 mg/day), escitalopram (10 mg/day–20 mg/day), venlafaxine (37.5 mg/day–150 mg/day), and desvenlafaxine (50 mg/day–100 mg/day). Other treatments that could be considered include off-label use of gabapentin (900 mg/day–2,400 mg/day), oxybutynin (2.5–5 mg twice daily) or clonidine (0.1 mg/day–1 mg/day divided in doses) since they all have data demonstrating they are beneficial at reducing VMS.
Nonhormonal options that may be helpful but are recommended with caution due to lack of data include weight loss, mindfulness-based stress reduction, s-equol derivatives of soy isoflavones and a stellate ganglion block. Further evidence and studies are needed for the aforementioned options.27
ALTERNATE CASE 2 Resolved
She may consider any of the nonhormonal options discussed. If she meets with a medical breast specialist to discuss her elevated risk of breast cancer and considers starting risk-reducing medications, particularly tamoxifen, you will want to avoid medications that have significant CPY 2D6 inhibition, such as paroxetine and fluoxetine. Safer choices would include venlafaxine, escitalopram, or citalopram.
The bottom line
In summary, the benefits and risks of HT in the treatment of VMS remain nuanced. For healthy women younger than 60 years of age and within 10 years from their last menstrual period, the benefits of HT largely outweigh the risks. Shared decision making, along with individualized and appropriate risk stratification specific for women, can guide appropriateness of HT prescribing. For those women who cannot take HT or choose not to, there are many nonhormonal options that will help manage their bothersome VMS. ●
CASE Healthy woman with hot flashes inquires about HT
A 54-year-old healthy woman with a history of hypothyroidism taking thyroid replacement medication comes in for her annual visit. Her last menstrual period was over 2 years ago and she reports severe hot flashes. They have greatly affected her quality of life and she must take frequent breaks at work. She wakes up frequently at night due to night sweats, which is impacting her sleep and, subsequently, her energy level. She has noted increased vaginal dryness so has been abstaining from sexual intercourse due to the discomfort. She has an intact uterus. Her family history is significant for heart disease, diagnosed in her mother at age 75.
On physical examination, she is normotensive and well-appearing. Her body mass index (BMI) is 21 kg/m2. Labs obtained prior to her visit show normal renal and liver function. Her high-density lipid (HDL) level is 55 mg/dL, her low-density lipid (LDL) level is 80 mg/dL, and her triglyceride level is 100 mg/dL; HbA1c is 5.5 mmol/mol.
She is interested in learning more about menopausal hormone therapy (HT) and whether or not she would be a candidate.
What information do you need to know to counsel and manage this patient?
Menopausal HT prescribing practices have changed over the last few decades as a better understanding of the risks and benefits of treatment have emerged. Prior to 2002, HT was commonly used for treatment of symptoms associated with menopause and was thought to have beneficial effects for chronic disease prevention.1-4 After data from the Women’s Health Initiative (WHI) was released, concerns arose around the effect of HT on cardiovascular health and risk of breast cancer. As a result, HT prescriptions fell precipitously after around 2002.5 Since then, postintervention analysis and cumulative 18-year follow-up of WHI data, along with results from subsequent randomized controlled trials, including the Kronos Early Estrogen Prevention Study (KEEPS) and the Early Versus Late Intervention Trial with Estradiol (ELITE), have demonstrated a favorable safety profile for healthy women starting HT early in menopause (less than age 60, or within 10 years from their final menstrual period).5-11
There are many types, formulations, and routes of HT, and the effects and risks differ for each (TABLE). For example, oral estrogen therapy, such as conjugated equine estrogens, portend a higher risk of adverse effects compared with transdermal formulations. Topical and transdermal estrogens bypass first-pass hepatic metabolism and thus are associated with a lower risk of venous thromboembolism (VTE) compared with oral formulations.12-14 A progestogen such as micronized progesterone is used in postmenopausal women with a uterus to protect the endometrium from unopposed estrogen therapy (ET). While it comes in oral and transdermal forms, the oral formulation is most widely used and studied in the United States; transdermal forms do not provide adequate endometrial protection and should not be used in combination therapy.15,16

Risks and benefits
Cardiovascular risk
Over time, the benefits and risks of HT use in menopausal patients have been further elucidated and defined, although they remain complex and dependent on patient clinical characteristics. HT remains the most effective treatment for vasomotor symptoms (VMS) and the genitourinary syndrome of menopause.17,18 In 2002, concerns for increased cardiovascular disease (CVD) and breast cancer risk resulted in early cessation of the WHI trial. Since that time the risk of CVD in postmenopausal women taking HT has been found to be more nuanced. In fact, updates in the literature have shown that HT results in a reduction of coronary heart disease if started in healthy women younger than age 60 years within 10 years of menopause.7,9-11 With this updated information, the North American Menopause society (NAMS), American College of Obstetricians and Gynecologists and the Endocrine Society have published guidelines supporting the initiation of HT for symptomatic healthy women: under the age of 60, within 10 years of menopause, and without contraindications. After age 60 years and further from menopause, the benefits and risks become less known.18-20
Risk stratification allows for more comprehensive counseling in use of HT for treatment of bothersome VMS. From a cardiovascular health standpoint, calculating an atherosclerotic CVD (ASCVD) risk score helps to evaluate appropriateness of HT prescribing:
- For those with low 10-year CVD risk (<5%), either oral or transdermal HT is appropriate.
- For those with moderate 10-year CVD risk (5%-10%), transdermal HT is recommended over oral HT.
- For those with high 10-year CVD risk (>10%), HT is not recommended.19,21
Breast cancer risk
Follow up since the initial WHI publication have shown that breast cancer risk is largely dependent on the formulation and route of HT used. Oral estrogen combined with a progestogen has been shown to increase the risk of invasive breast cancer, though very rarely.22 To put it into context, the absolute risk of breast cancer based on follow-up studies from WHI showed less than 1 additional case per 1,000 person years of use; less risk than associated with drinking 2 glasses of wine per day and similar to that of obesity and/or sedentary lifestyle.23,24 Studies have shown estrogen treatment alone for postmenopausal women does not appear to increase the risk of breast cancer. In fact, follow-up data from WHI showed a nonsignificant reduction in breast cancer risk for those taking ET alone.25
Breast cancer risk stratification is helpful when determining appropriateness of HT in postmenopausal women. Generally, if using risk stratification models for breast cancer (ie, Gail Risk model or international breast cancer intervention study [IBIS] tool), a patient who is average to moderate risk, HT can be offered with appropriate counseling. By contrast, a patient who is high risk should have a more detailed discussion about their risk (surveillance and risk-reducing treatments), and they may consider nonhormonal options for treatment of VMS. Women with a history of breast cancer should not be prescribed systemic HT.
Continue to: Additional HT benefits...
Additional HT benefits
The benefits of HT in postmenopausal women include improved bone health and reduction of fractures; reduction of risk for type 2 diabetes mellitus (T2DM); improvement of insulin sensitivity; improvement of lipid profiles with increased HDL and decreased LDL levels; and reduction of colon cancer risk.25 For women aged younger than 60 years who start HT within 10 years of their last menstrual period, HT has been shown to cause a reduction in all-cause mortality. Important risks to counsel patients on when starting HT include the low risk of stroke and venous thromboembolism (VTE) when using oral formulations.26
CASE Resolved
Her ASCVD risk score, based on her history, estimates her 10-year CVD risk to be low (<5%). Thus, from a cardiovascular standpoint, either oral or transdermal HT would be an appropriate option. Her IBIS 10-year score is 1.5%, placing her in a low-risk category for breast cancer based on her personal and family history. Given that she is less than 60 years of age and within 10 years of menopause, along with her low-risk stratification for CVD and breast cancer, she would be an appropriate patient to begin combined HT with an estrogen plus an oral progesterone, such as an estradiol patch 0.0375 mg twice weekly, along with oral micronized progesterone 100 mg nightly. The dose could be increased over time based on symptoms and tolerability of the treatment.
ALTERNATE CASE 1 The patient has additional risk factors
Consider the patient case with the following additions to her history: the patient has a BMI of 34 kg/m2, a history of well-controlled hypertension while taking amlodipine 5 mg, and an ASCVD risk score of 7.5%. She reports severe VMS that are greatly impacting her quality of life. How would your recommendations or counseling change?
Focus on healthy lifestyle
Obesity and hypertension, both common chronic conditions, pose additional risks to be accounted for when counseling on and approaching HT prescribing. Her alternate ASCVD risk score places her at moderate risk for CVD within 10 years, based on guidelines as discussed above. It would still be appropriate to offer her combined HT after a shared decision-making discussion that includes a focus on healthy lifestyle habits.
Consider transdermal HT in obese women
Longitudinal studies have found that weight gain is more a consequence of aging, regardless of menopausal status. Fat distribution and body composition changes are a menopause-related phenomenon driven by estrogen deficiency. HT has been shown to preserve lean body mass and reduce visceral adiposity, resulting in favorable effects of body composition. Still, obesity results in increased risk of CVD, VTE, and certain hormone-sensitive cancers.27 When considering HT in obese patients, a transdermal estrogen route is preferred to reduce risks.
For women with hypertension, prescribe transdermal HT
Overall, studies have found that HT has a neutral effect on blood pressure.25 When considering formulation of HT, micronized progesterone, dydrogesterone, and drospirenone seem to be most neutral and possibly even beneficial on blood pressure compared with synthetic progestins.26 Oral estrogen is associated with increased vasoconstriction and/or increased sodium retention with resultant worsened regulation of blood pressure in women with hypertension, so transdermal estrogen is preferred for women with hypertension.26 Hypertension is a component of the ASCVD risk score; factoring this into a patient’s clinical picture is important when discussing appropriateness of HT prescribing. To minimize risks, the transdermal route of estrogen is preferred for those with hypertension.
Continue to: ALTERNATE CASE 1 Resolved...
ALTERNATE CASE 1 Resolved
She has a moderate ASCVD risk score, is obese, and has a history of hypertension. Through shared decision making, you ultimately start her on transdermal estrogen and micronized progesterone to treat her quality-of-life-impacting VMS, a formulation that is most likely to mitigate the possible risks in her clinical case. You see her back in the clinic every 3-6 months to monitor her blood pressure.
ALTERNATE CASE 2 The patient has a high risk for breast cancer
The patient reveals further her significant family history of breast cancer in her maternal grandmother and mother, both diagnosed in their 50s. You calculate her risk of breast cancer with a model that incorporates family history. Her Tyrer Cuzick-IBIS 10-year risk score is >5% and lifetime risk is >20%, putting her at high risk for breast cancer. Since she has a uterus and would need concomitant progesterone therapy, her risk for breast cancer is higher than if she was taking ET alone. Ultimately, together you and the patient decide to trial nonhormonal options for her VMS.
What are nonhormonal options for treatment of VMS?
While HT remains the most effective treatment for VMS, there are multiple nonhormonal treatments for women who are either at too high a risk for HT or who favor other options, which are outlined in the NAMS 2015 nonhormonal management position statement.27 Cognitive behavioral therapy (CBT) has been shown to decrease bother related to VMS but not frequency. Clinical hypnosis has been shown to reduce hot flash frequency and improve sleep. Paroxetine salt (7.5 mg/day) remains the only FDA nonhormonal-approved medication for treatment of moderate to severe vasomotor symptoms. Off label use of other selective serotonin reuptake inhibitors (SSRIs) and selective norepinephrine reuptake inhibitors have been shown in studies to reduce VMS including paroxetine at slightly higher doses (10 mg/day–20 mg/day), citalopram (10 mg/day–20 mg/day), escitalopram (10 mg/day–20 mg/day), venlafaxine (37.5 mg/day–150 mg/day), and desvenlafaxine (50 mg/day–100 mg/day). Other treatments that could be considered include off-label use of gabapentin (900 mg/day–2,400 mg/day), oxybutynin (2.5–5 mg twice daily) or clonidine (0.1 mg/day–1 mg/day divided in doses) since they all have data demonstrating they are beneficial at reducing VMS.
Nonhormonal options that may be helpful but are recommended with caution due to lack of data include weight loss, mindfulness-based stress reduction, s-equol derivatives of soy isoflavones and a stellate ganglion block. Further evidence and studies are needed for the aforementioned options.27
ALTERNATE CASE 2 Resolved
She may consider any of the nonhormonal options discussed. If she meets with a medical breast specialist to discuss her elevated risk of breast cancer and considers starting risk-reducing medications, particularly tamoxifen, you will want to avoid medications that have significant CPY 2D6 inhibition, such as paroxetine and fluoxetine. Safer choices would include venlafaxine, escitalopram, or citalopram.
The bottom line
In summary, the benefits and risks of HT in the treatment of VMS remain nuanced. For healthy women younger than 60 years of age and within 10 years from their last menstrual period, the benefits of HT largely outweigh the risks. Shared decision making, along with individualized and appropriate risk stratification specific for women, can guide appropriateness of HT prescribing. For those women who cannot take HT or choose not to, there are many nonhormonal options that will help manage their bothersome VMS. ●
- Carr BR, Wilson JD. Disorders of the ovary and female reproductive tract. In: Isselbacher KJ, Braunwald E, Wilson JD, eds. Harrisons’ Principles of Internal Medicine, 13th ed. New York, NY: McGraw-Hill; 1994:2016-2017.
- Davidson MH, Maki KC, Marx P, et al. Effects of continuous estrogen and estrogen-progestin replacement regimens on cardiovascular risk markers in postmenopausel women. Arch Intern Med. 2000;160:3315-3325. doi: 10.1001/archinte.160.21.3315.
- Grodstein F, Manson JE, Colditz GA, et al. A prospective, observational study of postmenopausal hormone therapy and primary prevention of cardiovascular disease. Ann Intern Med. 2000;133:933-941. doi: 10.7326/0003-4819-133-12-200012190-00008.
- Grady D, Rubin SM, Petitti DB, et al. Hormone therapy to prevent disease and prolong life in postmenopausal women. Ann Intern Med. 1992;117:1016-1037. doi: 10.7326/0003-4819-117-12-1016.
- Rossouw JE, Manson JE, Kaunitz AM, et al. Lessons learned from the Women’s Health Initiative trials of menopausal hormone therapy. Obstet Gynecol. 2013;121:172-176. doi: 10.1097/aog.0b013e31827a08c8.
- Manson JE, Hsia J, Johnson KC, et al. Estrogen plus progestin and the risk of coronary heart disease. NEJM. 2003;349:523-534. doi: 10.1056/NEJMoa030808.
- Manson JE, Chlebowski RT, Stefanick ML, et al. Menopausal hormone therapy and health outcomes during the intervention and extended poststopping phases of the Women’s Health Initiative randomized trials. JAMA. 2013;310:1353-1368. doi: 10.1001/jama.2013.278040.
- Santen RJ, Allred DC, Ardoin SP, et al. Postmenopausal hormone therapy: an Endocrine Society scientific statement. J Clin Endocrinol Metab. 2010;95:S1-S66. doi: 10.1210/jc.2009-2509.
- Manson JE, Aragaki AK, Rossouw JE, et al. Menopausal hormone therapy and long-term all-cause and cause-specific mortality: the Women’s Health Initiative randomized trials. JAMA. 2017;318:927-938. doi: 10.1001/jama.2017.11217.
- Hodis HN, Mack WJ, Henderson VW, et al. Vacular effects of early versus late postmenopausal treatment with estradiol. NEJM. 2016;374:1221-1231. doi: 10.1056/NEJMoa1505241.
- Taylor HS, Tal A, Pal L, et al. Effects of oral vs transdermal estrogen therapy on sexual function in early postmenopause: ancillary study of the Kronos Early Estrogen Prevention Study (KEEPS). JAMA Intern Med. 2017;177:1471-1479. doi: 10.1001/jamainternmed.2017.3877.
- Liu JH, Pinkerton JV. Prescription therapies. In: CJ Crandall, ed. Menopause Practice: A Clinician’s Guide, 6th ed. Pepper Pike, OH: The North American Menopause Society; 2019:277-309.
- Files J, Kling JM. Transdermal delivery of bioidentical estrogen in menopausal hormone therapy: a clinical review. Expert Opin Drug Deliv. 2020;17:543-549. doi: 10.1080/17425247.2020.1700949.
- Canonico M, Carcaillon L, Plu-Bureau G, et al. Postmenopausal hormone therapy and risk of stroke: impact of the route of estrogen administration and type of progestogen. Stroke. 2016;47:1734-1741. doi: 10.1161/STROKEAHA.116.013052.
- Hitchcok CL, Prior JC. Oral micronized progesterone for vasomotor symptoms—a placebo-controlled randomized trial in healthy post-menopausal women. Menopause. 2001;8:10-16.
- Effects of hormone replacement therapy on endometrial histology in postmenopausal women. The Postmenopausal Estrogen/Progestin Interventions (PEPI) Trial. The writing Group for the PEPI Trial. JAMA. 1996;275:370-375. doi: 10.1001/jama.1996.03530290040035.
- Pinkerton JV. Hormone therapy for postmenopausal women. N Engl J Med. 2020;382:446-55. doi:10.1056/NEJMcp1714787.
- The 2022 hormone therapy position statement of The North American Menopause Society. Menopause. 2022;29:767-794. doi:10.1097/GME.00000000000000002028.
- Stuenkel CA, Davis SR, Gompel A, et al. Treatment of symptoms of the menopause: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2015;100:3975-4011. doi: 10.1210/jc.2015-2236.
- American College of Obstetricians and Gynecologists. Practice Bulletin No. 141: Management of Menopausal Symptoms. Obstet Gynecol. 2014;123:202-216. doi: 10.1097/01.AOG.0000441353.20693.78.
- Manson JE. Current recommendations: what is the clinician to do? Fertil Steril. 2014;101:916. World Cancer Research Fund/American Institute for Cancer Research. Continuous Update Project Expert Report 2018. Alcoholic drinks and the risk of cancer. https://www.wcrf.org/sites/default/files/Alcoholic-Drinks.pdf. 2018.
- Anderson GL, Chlebowski RT, Aragaki AK, et al. Conjugated equine oestrogen and breast cancer incidence and mortality in postmenopausal women with hysterectomy: Extended follow-up of Women’s Health Initiative randomized placebo-controlled trial. Lancet Oncol. 2012;5:476-486. doi: 10.1016/S1470-2045(12)70075-X.
- World Cancer Research Fund/American Institute for Cancer Research. Continuous Update Project Expert Report 2018. Diet, nutrition, physical activity and breast cancer. www.aicr.org/continuous-update-project/breast-cancer.html. 2018.
- Anderson GL, Chlebowski RT, Aragaki AK, et al. Conjugated equine oestrogen and breast cancer incidence and mortality in postmenopausal women with hysterectomy: Extended follow-up of the Women’s Health Initiative randomized placebo-controlled trial. Lancet Oncol. 2012;5:476-486. doi: 10.1016/S1470-2045(12)70075-X.
- Mehta J, Kling JM, Manson JE. Risks, benefits and treatment modalities of menopausal hormone therapy: current concepts. Front Endocrinol (Laussane). 2021;12:564781. doi: 10.3389/fendo.2021.564781.
- Kapoor E, Kling JM, Lobo AS, et al. Menopausal hormone therapy in women with chronic medical conditions. Best Pract Res Clin Endocrinol Metab. 2021:35;101578. doi: 10.1016/j.beem.2021.101578.
- NAMS position statement advisory panel. Nonhormonal management of menopause-associated vasomotor symptoms: 2015 position statement of The North American Menopause Society. Menopause. 2015:22:1155-72. doi: 10.1097/GME.0000000000000546.
- Carr BR, Wilson JD. Disorders of the ovary and female reproductive tract. In: Isselbacher KJ, Braunwald E, Wilson JD, eds. Harrisons’ Principles of Internal Medicine, 13th ed. New York, NY: McGraw-Hill; 1994:2016-2017.
- Davidson MH, Maki KC, Marx P, et al. Effects of continuous estrogen and estrogen-progestin replacement regimens on cardiovascular risk markers in postmenopausel women. Arch Intern Med. 2000;160:3315-3325. doi: 10.1001/archinte.160.21.3315.
- Grodstein F, Manson JE, Colditz GA, et al. A prospective, observational study of postmenopausal hormone therapy and primary prevention of cardiovascular disease. Ann Intern Med. 2000;133:933-941. doi: 10.7326/0003-4819-133-12-200012190-00008.
- Grady D, Rubin SM, Petitti DB, et al. Hormone therapy to prevent disease and prolong life in postmenopausal women. Ann Intern Med. 1992;117:1016-1037. doi: 10.7326/0003-4819-117-12-1016.
- Rossouw JE, Manson JE, Kaunitz AM, et al. Lessons learned from the Women’s Health Initiative trials of menopausal hormone therapy. Obstet Gynecol. 2013;121:172-176. doi: 10.1097/aog.0b013e31827a08c8.
- Manson JE, Hsia J, Johnson KC, et al. Estrogen plus progestin and the risk of coronary heart disease. NEJM. 2003;349:523-534. doi: 10.1056/NEJMoa030808.
- Manson JE, Chlebowski RT, Stefanick ML, et al. Menopausal hormone therapy and health outcomes during the intervention and extended poststopping phases of the Women’s Health Initiative randomized trials. JAMA. 2013;310:1353-1368. doi: 10.1001/jama.2013.278040.
- Santen RJ, Allred DC, Ardoin SP, et al. Postmenopausal hormone therapy: an Endocrine Society scientific statement. J Clin Endocrinol Metab. 2010;95:S1-S66. doi: 10.1210/jc.2009-2509.
- Manson JE, Aragaki AK, Rossouw JE, et al. Menopausal hormone therapy and long-term all-cause and cause-specific mortality: the Women’s Health Initiative randomized trials. JAMA. 2017;318:927-938. doi: 10.1001/jama.2017.11217.
- Hodis HN, Mack WJ, Henderson VW, et al. Vacular effects of early versus late postmenopausal treatment with estradiol. NEJM. 2016;374:1221-1231. doi: 10.1056/NEJMoa1505241.
- Taylor HS, Tal A, Pal L, et al. Effects of oral vs transdermal estrogen therapy on sexual function in early postmenopause: ancillary study of the Kronos Early Estrogen Prevention Study (KEEPS). JAMA Intern Med. 2017;177:1471-1479. doi: 10.1001/jamainternmed.2017.3877.
- Liu JH, Pinkerton JV. Prescription therapies. In: CJ Crandall, ed. Menopause Practice: A Clinician’s Guide, 6th ed. Pepper Pike, OH: The North American Menopause Society; 2019:277-309.
- Files J, Kling JM. Transdermal delivery of bioidentical estrogen in menopausal hormone therapy: a clinical review. Expert Opin Drug Deliv. 2020;17:543-549. doi: 10.1080/17425247.2020.1700949.
- Canonico M, Carcaillon L, Plu-Bureau G, et al. Postmenopausal hormone therapy and risk of stroke: impact of the route of estrogen administration and type of progestogen. Stroke. 2016;47:1734-1741. doi: 10.1161/STROKEAHA.116.013052.
- Hitchcok CL, Prior JC. Oral micronized progesterone for vasomotor symptoms—a placebo-controlled randomized trial in healthy post-menopausal women. Menopause. 2001;8:10-16.
- Effects of hormone replacement therapy on endometrial histology in postmenopausal women. The Postmenopausal Estrogen/Progestin Interventions (PEPI) Trial. The writing Group for the PEPI Trial. JAMA. 1996;275:370-375. doi: 10.1001/jama.1996.03530290040035.
- Pinkerton JV. Hormone therapy for postmenopausal women. N Engl J Med. 2020;382:446-55. doi:10.1056/NEJMcp1714787.
- The 2022 hormone therapy position statement of The North American Menopause Society. Menopause. 2022;29:767-794. doi:10.1097/GME.00000000000000002028.
- Stuenkel CA, Davis SR, Gompel A, et al. Treatment of symptoms of the menopause: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2015;100:3975-4011. doi: 10.1210/jc.2015-2236.
- American College of Obstetricians and Gynecologists. Practice Bulletin No. 141: Management of Menopausal Symptoms. Obstet Gynecol. 2014;123:202-216. doi: 10.1097/01.AOG.0000441353.20693.78.
- Manson JE. Current recommendations: what is the clinician to do? Fertil Steril. 2014;101:916. World Cancer Research Fund/American Institute for Cancer Research. Continuous Update Project Expert Report 2018. Alcoholic drinks and the risk of cancer. https://www.wcrf.org/sites/default/files/Alcoholic-Drinks.pdf. 2018.
- Anderson GL, Chlebowski RT, Aragaki AK, et al. Conjugated equine oestrogen and breast cancer incidence and mortality in postmenopausal women with hysterectomy: Extended follow-up of Women’s Health Initiative randomized placebo-controlled trial. Lancet Oncol. 2012;5:476-486. doi: 10.1016/S1470-2045(12)70075-X.
- World Cancer Research Fund/American Institute for Cancer Research. Continuous Update Project Expert Report 2018. Diet, nutrition, physical activity and breast cancer. www.aicr.org/continuous-update-project/breast-cancer.html. 2018.
- Anderson GL, Chlebowski RT, Aragaki AK, et al. Conjugated equine oestrogen and breast cancer incidence and mortality in postmenopausal women with hysterectomy: Extended follow-up of the Women’s Health Initiative randomized placebo-controlled trial. Lancet Oncol. 2012;5:476-486. doi: 10.1016/S1470-2045(12)70075-X.
- Mehta J, Kling JM, Manson JE. Risks, benefits and treatment modalities of menopausal hormone therapy: current concepts. Front Endocrinol (Laussane). 2021;12:564781. doi: 10.3389/fendo.2021.564781.
- Kapoor E, Kling JM, Lobo AS, et al. Menopausal hormone therapy in women with chronic medical conditions. Best Pract Res Clin Endocrinol Metab. 2021:35;101578. doi: 10.1016/j.beem.2021.101578.
- NAMS position statement advisory panel. Nonhormonal management of menopause-associated vasomotor symptoms: 2015 position statement of The North American Menopause Society. Menopause. 2015:22:1155-72. doi: 10.1097/GME.0000000000000546.
Management considerations for women with von Willebrand disease
Von Willebrand disease (VWD) represents the most common inherited bleeding disorder, with a prevalence of approximately 1 in 1,000 people.
Bruising and mucocutaneous bleeding (epistaxis, gingival bleeding, and bleeding after dental extraction) are the most common presenting symptoms of VWD. Because VWD substantially increases the risk of heavy menstrual bleeding (HMB) and, to some extent, intrapartum bleeding complications, and postpartum hemorrhage, women experience a disproportionate burden from VWD. Thus, ObGyns are likely to be called on to make treatment recommendations in VWD patients with these concerns.1
In 2017, the American Society of Hematology, the International Society on Thrombosis and Haemostasis, the National Hemophilia Foundation, and the World Federation of Hemophilia determined that among clinical issues related to VWD, updating guidelines for women with VWD represented the highest priority.2 Accordingly, an international group of hematologists/coagulation specialists performed systematic literature reviews to address 3 questions faced by women with VWD and their clinicians:
- What are the most effective treatments for HMB?
- What is the safest approach for women desiring neuraxial analgesia for intrapartum pain?
- What is the impact of postpartum administration of tranexamic acid (TxA) on postpartum hemorrhage (PPH)?3
Evidence on management strategies for HMB in women with VWD
The prevalence of HMB in women with VWD ranges from 50% to 92%. Reports suggest that between 5% and 24% of women presenting with this symptom have VWD.3 However, the prevalence of VWD among women seeking care for HMB relates to referral patterns, with the prevalence of VWD substantially higher in patient populations who are referred to clinicians or centers that focus on care of patients with bleeding disorders.
The systematic review authors3 identified 2 comparative studies that assessed the treatment of HMB in women with VWD. One was a crossover trial that enrolled 116 VWD patients with HMB with a mean age of 36 years.4 All participants in this trial chose not to use combination oral contraceptives (COCs) as they had not experienced good results with prior COC use. Trial participants were randomly assigned to receive either intranasal desmopressin (DDAVP; a synthetic analog of the antidiuretic agent vasopressin, which stimulates the release of VWF from endothelial cells) or oral TxA therapy for 2 menstrual cycles. Participants then crossed over to the other drug for 2 additional cycles. Although both agents significantly reduced estimated menstrual blood loss, TxA was more effective in decreasing bleeding than intranasal DDAVP.4
In a retrospective cohort study, investigators compared COC use with intranasal DDAVP in 36 adolescents who had VWD and HMB.5 Participant follow-up ranged from 6 months to 4 years. The estimated efficacy of COCs and intranasal DDAVP was 86% and 77%, respectively, a difference that did not achieve statistical significance. Some of the adolescents who used intranasal DDAVP reported severe headaches and flushing.5
In addition, the systematic review authors3 identified 5 case series that described the use of the levonorgestrel (52 mg)-releasing intrauterine device (LNG 52 IUD) in women with VWD and HMB; 4 of these addressed the efficacy of progestin-releasing IUDs in reducing HMB in this patient population.6-9 Using different approaches to define HMB, the authors of these reports followed between 7 and 26 patients with bleeding disorders (most with confirmed VWD) and HMB for variable amounts of time after placement of an LNG 52 IUD. Many of the women described in these case series had tried other HMB treatments, including COCs, without success. Although these 4 reports assessed different outcomes, all reported that placement of the LNG 52 IUD substantially reduced menstrual blood loss, often resulting in amenorrhea. Several of these reports also noted important improvements in quality of life following LNG 52 IUD placement. One case series reported LNG 52 IUD placement in 13 adolescents with VWD and HMB. The mean time to achieve amenorrhea or occasional spotting was 94 days.6
The fifth report, which followed 20 women (median age, 31 years) with HMB associated with VWD or other bleeding disorders who underwent LNG 52 IUD placement, aimed to describe IUD expulsions and malpositioned IUDs in this population. In this small group of patients, 3 IUD expulsions and 2 malpositioned IUDs were observed. Furthermore, an additional 5 women had their device removed prematurely due to patient dissatisfaction. Accordingly, the IUD continuation rate in this case series was only 50%.10
Evidence on management of pregnancy, delivery, and the postpartum period
Heavy menstrual bleeding is not the only challenge for women with VWD. While pregnancy is accompanied by higher levels of VWF, potentially offsetting the risk of bleeding at the time of delivery, the levels do not achieve the same magnitude as they would in unaffected women.11 Women are at an increased risk of primary PPH12,13 and, importantly, since VWF levels fall exponentially after delivery when women are still experiencing lochia,11 they are at increased risk of secondary or delayed PPH.
Two questions arise frequently in the care of women with VWD at the time of delivery and during the postpartum period:
- What is the safest approach for women who desire neuraxial analgesia for intrapartum pain?
- What is the impact of postpartum administration of TxA on PPH?
The second systematic review the authors performed3 focused on VWF levels in women receiving neuraxial anesthesia during labor. After screening 27 studies, the authors included 5 case series, which did not describe outcomes based on VWF levels but rather described the outcomes of women with VWF levels of greater than 0.50 IU/mL (> 50% of normal compared with a normal standard).
Meta-analysis showed that the proportion of anesthesia complications was 6%, which sounds high, but the range of complications was what would be expected in any population (hypotension, accidental dural puncture, inadequate anesthesia, and bloody tap with no further complications). No spinal, subdural, or epidural hematomas were noted.3 Such hematomas are an extremely rare complication of neuraxial anesthesia, occurring in only 1 in 200,000 or 1 in 250,000 obstetric patients14,15; accordingly, an increase in the rate of hematomas among women with VWD could go undetected. The absence of hematomas among women with VWD as reported in the systematic review does not mean there is not an increase in the rate of hematomas in women with VWD. The relative risk is unknown and caution would be advised.
The third systematic review that the authors performed3 was on TxA treatment in the postpartum period. After screening 41 studies, the authors included 2 retrospective cohort studies.16,17 The majority of the participants had VWD. With very-low-certainty evidence, the authors found that TxA reduces the risk of:
- severe primary PPH (risk ratio [RR], 0.36; 95% confidence interval [CI], 0.05–2.59)
- primary PPH (RR, 0.25; 95% CI, 0.04–1.75)
- secondary PPH (RR, 0.42; 95% CI, 0.02–0.91—does not cross 1.0).
Note that the 95% confidence intervals for severe as well as primary PPH crossed 1.0 and therefore these reductions in risk did not achieve statistical significance. Additionally, there was very-low-certainty evidence on the effect of TxA on blood transfusions, vaginal hematomas, blood loss, and thrombotic complications.3
Continue to: Our recommendations for HMB management...
Our recommendations for HMB management
When first evaluating any woman with HMB, it is important to check a blood count and ferritin level, if not already done. If there is any suggestion of iron deficiency (with or without anemia), we recommend oral iron supplementation. This is best accomplished with slow-release iron supplement formulations (or less expensive generic or house brands that contain less than 65 mg of elemental iron per tablet) taken every other day. Such preparations may cause fewer gastrointestinal adverse effects than other oral iron formulations.18 Although it may appear counterintuitive, oral iron is better absorbed (and also may cause fewer gastrointestinal adverse effects) when taken every other day.19
Initial management of HMB, whether or not a bleeding disorder is present, often consists or oral hormonal management. If no contraindications are present, we recommend initiation of a COC with a short hormone-free interval (for example, a 24/4 formulation). If contraindications to contraceptive doses of estrogen are present, continuous use of norethindrone acetate 5-mg tablets or off-label use of combination tablets with 5 µg of ethinyl estradiol and 1 mg of norethindrone acetate (a formulation approved for the treatment of menopausal symptoms) is appropriate.20
Once a patient is established on oral hormonal management, placement of a levonorgestrel-releasing IUD should be considered. Given that expulsion rates may be higher in women with HMB, if feasible, consider using abdominal ultrasound guidance for IUD placement.
For women with VWD who fail first-line therapy (hormonal management) or are trying to become pregnant, TxA (two 650-mg tablets 3 times daily for up to 5 days during episodes of heavy flow) can reduce HMB.20,21
Our recommendations for management of pregnancy and delivery
The second and third systematic reviews discussed above provide very limited guidance on comprehensive management. The care of the pregnant patient with VWD starts with assessment of VWF levels and making an accurate diagnosis. This usually requires the input of a hematologist or other expert in hemostasis. If no recent VWF levels are available, the ObGyn can obtain a von Willebrand panel that includes VWF antigen, VWF activity (most commonly ristocetin cofactor), and factor VIII.
Levels should be reassessed around 36 weeks’ gestation in anticipation of delivery. VWF levels increase during pregnancy; accordingly, in mild, type 1 VWD, half the time treatment is not necessary.11 If VWF activity is less than 50 IU/dL (less than 50% of normal) at 36 weeks’ gestation, the patient should receive VWF concentrate (dosed in VWF units). This requires consultation with hematology and specialized pharmacy support.
For these reasons, the patient with a VWF level less than 50% should be delivered in a referral center with the necessary resources. Anesthesia should be aware of the patient. Unless
Due to the quantity of fluids administered during labor or at the time of delivery and the coexistent administration of oxytocin, desmopressin (synthetic vasopressin) should not be used without monitoring sodium levels, should not be dosed more than once, or should be avoided altogether due to the risk of water intoxication.
If the patient has sustained VWF and factor VIII levels greater than 50 IU/dL, she would be a candidate to deliver in her local hospital and receive neuraxial anesthesia.
Based on the best data we have for women with VWD, a patient with a VWF greater than 50 IU/dL is no more likely to experience PPH than other women.11 Intravenous TxA can be used for prevention or treatment of immediate postpartum bleeding per protocol (1 g after cord clamp and 1 g 30 minutes or more later).22 Oral TxA can be used for prevention or treatment of delayed postpartum bleeding as per HMB. Regardless of the outcome of any testing during pregnancy, nonsteroidal anti-inflammatory drugs should be avoided postpartum and the patient should be monitored closely for bleeding.
Neonatal care
As for the fetus/neonate, the parents should be aware that the infant has a 50% chance of inheriting VWD. If the baby’s father has no history of bleeding, it is unlikely that the infant would be any more affected than the patient herself. Nonetheless, cord blood (in one or more light blue top tubes) should be obtained at the time of delivery and sent for a von Willebrand panel. If the infant is male, a circumcision should be postponed until VWD is ruled out. In addition, fetal invasive procedures should be avoided during labor. Fetal scalp electrode placement should be avoided. Operative vaginal delivery also should be avoided. Cesarean delivery would be preferred to operative vaginal delivery, but if operative vaginal delivery is unavoidable, use of forceps is preferred to vacuum extraction. ●
- ACOG committee opinion no. 451: Von Willebrand disease in women. Obstet Gynecol. 2009;114:1439-1443. doi: 10.1097 /AOG.0b013e3181c6f975.
- Kalot MA, Al-Khatib M, Connell NT, et al; VWD Working Group. An international survey to inform priorities for new guidance on von Willebrand disease. Hemophilia. 2020;26:106-116. doi: 10.1111/hae.13881.
- Brignardello-Petersen R, El Alayli A, Husainat N, et al. Gynecologic and obstetric management of women with von Willebrand disease: summary of 3 systematic reviews of the literature. Blood Adv. 2022;6:228-237. doi: 10.1182 /bloodadvances.2021005589.
- Kouides PA, Byams VR, Philipp CS, et al. Multisite management study of menorrhagia with abnormal laboratory haemostasis: a prospective crossover study of intranasal desmopressin and oral tranexamic acid. Br J Haematol. 2009;145:212-220. doi: 10.1111/j.1365-2141.2009.07610.x.
- Amesse LS, Pfaff-Amesse T, Leonardi R, et al. Oral contraceptives and DDAVP nasal spray: patterns of use in managing vWD-associated menorrhagia: a single-institution study. J Pediatr Hematol Oncol. 2005;27:357-363. doi: 10.1097/01.mph.0000173175.95152.95.
- Adeyemi-Fowode OA, Santos XM, Dietrich JE, et al. Levonorgestrel-releasing intrauterine device use in female adolescents with heavy menstrual bleeding and bleeding disorders: single institution review. J Pediatr Adolesc Gynecol. 2017;30:479-483. doi: 10.1016/j.jpag.2016.04.001.
- Chi C, Huq FY, Kadir RA. Levonorgestrel-releasing intrauterine system for the management of heavy menstrual bleeding in women with inherited bleeding disorders: long-term follow-up. Contraception. 2011;83:242-247. doi: 10.1016/j.contraception.2010.07.010.
- Kingman CE, Kadir RA, Lee CA, et al. The use of levonorgestrel-releasing intrauterine system for treatment of menorrhagia in women with inherited bleeding disorders. BJOG. 2004;111:1425-1428. doi: 10.1111/j.1471-0528.2004.00305.x.
- Lukes AS, Reardon B, Arepally G. Use of the levonorgestrel-releasing intrauterine system in women with hemostatic disorders. Fertil Steril. 2008;90:673-677. doi: 10.1016 /j.fertnstert.2007.07.1315.
- Rimmer E, Jamieson MA, James P. Malposition and expulsion of the levonorgestrel intrauterine system among women with inherited bleeding disorders. Haemophilia. 2013;19:933-938. doi: 10.1111/hae.12184.
- James AH, Konkle BA, Kouides P, et al. Postpartum von Willebrand factor levels in women with and without von Willebrand disease and implications for prophylaxis. Haemophilia. 2015;21:81-87. doi: 10.1111/hae.12568.
- James AH, Jamison MG. Bleeding events and other complications during pregnancy and childbirth in women with von Willebrand disease. J Thromb Haemost. 2007;5: 1165-1169. doi: 10.1111/j.1538-7836.2007.02563.x.
- Al-Zirqi I, Vangen S, Forsen L, et al. Prevalence and risk factors of severe obstetric haemorrhage. BJOG. 2008;115:1265-1272. doi: 10.1111/j.1471-0528.2008.01859.x.
- Moen V, Dahlgren N, Irestedt L. Severe neurological complications after central neuraxial blockades in Sweden 1990-1999. Anesthesiology. 2004;101:950-959. doi: 10.1097/00000542-200410000-00021.
- D’Angelo R, Smiley RM, Riley ET, et al. Serious complications related to obstetric anesthesia: the serious complication repository project of the Society for Obstetric Anesthesia and Perinatology. Anesthesiology. 2014;120:1505-1512. doi: 10.1097/ALN.000000000000253.
- Govorov I, Lofgren S, Chaireti R, et al. Postpartum hemorrhage in women with von Willebrand disease—a retrospective observational study. PLos One. 2016;11:e0164683. doi: 10.1371/journal.pone.0164683.
- Hawke L, Grabell J, Sim W, et al. Obstetric bleeding among women with inherited bleeding disorders: a retrospective study. Haemophilia. 2016;22:906-911. doi: 10.1111/hae.13067.
- James AH. Iron deficiency anemia in pregnancy. Obstet Gynecol. 2021;138:663-674. doi:10.1097/AOG .000000000000.4559.
- Stoffel NU, Cercamondi CI, Brittenham G, et al. Iron absorption from oral iron supplements given on consecutive versus alternate days and as single morning doses versus twice-daily split dosing in iron-depleted women: two open-label, randomised controlled trials. Lancet Haematol. 2017;4:e524-e533. doi: 10.1016/S2352-3026(17)30182-5.
- Kaunitz AM. Abnormal uterine bleeding in reproductiveage women. JAMA. 2019;321:2126-2127. doi: 10.1001 /jama.2019.5248.
- James AH, Kouides PA, Abdul-Kadir R, et al. Von Willebrand disease and other bleeding disorders in women: consensus on diagnosis and management from an international expert panel. Am J Obstet Gynecol. 2009;201:12.e1-8. doi: 10.1016 /j.ajog.2009.04.024.
- WOMAN Trial Collaborators. Effect of early tranexamic acid administration on mortality, hysterectomy, and other morbidities in women with post-partum haemorrhage (WOMAN): an international, randomised, double-blind, placebo-controlled trial. Lancet. 2017;389:2105-2116. doi: 10.1016/S0140-6736(17)30638-4.
Von Willebrand disease (VWD) represents the most common inherited bleeding disorder, with a prevalence of approximately 1 in 1,000 people.
Bruising and mucocutaneous bleeding (epistaxis, gingival bleeding, and bleeding after dental extraction) are the most common presenting symptoms of VWD. Because VWD substantially increases the risk of heavy menstrual bleeding (HMB) and, to some extent, intrapartum bleeding complications, and postpartum hemorrhage, women experience a disproportionate burden from VWD. Thus, ObGyns are likely to be called on to make treatment recommendations in VWD patients with these concerns.1
In 2017, the American Society of Hematology, the International Society on Thrombosis and Haemostasis, the National Hemophilia Foundation, and the World Federation of Hemophilia determined that among clinical issues related to VWD, updating guidelines for women with VWD represented the highest priority.2 Accordingly, an international group of hematologists/coagulation specialists performed systematic literature reviews to address 3 questions faced by women with VWD and their clinicians:
- What are the most effective treatments for HMB?
- What is the safest approach for women desiring neuraxial analgesia for intrapartum pain?
- What is the impact of postpartum administration of tranexamic acid (TxA) on postpartum hemorrhage (PPH)?3
Evidence on management strategies for HMB in women with VWD
The prevalence of HMB in women with VWD ranges from 50% to 92%. Reports suggest that between 5% and 24% of women presenting with this symptom have VWD.3 However, the prevalence of VWD among women seeking care for HMB relates to referral patterns, with the prevalence of VWD substantially higher in patient populations who are referred to clinicians or centers that focus on care of patients with bleeding disorders.
The systematic review authors3 identified 2 comparative studies that assessed the treatment of HMB in women with VWD. One was a crossover trial that enrolled 116 VWD patients with HMB with a mean age of 36 years.4 All participants in this trial chose not to use combination oral contraceptives (COCs) as they had not experienced good results with prior COC use. Trial participants were randomly assigned to receive either intranasal desmopressin (DDAVP; a synthetic analog of the antidiuretic agent vasopressin, which stimulates the release of VWF from endothelial cells) or oral TxA therapy for 2 menstrual cycles. Participants then crossed over to the other drug for 2 additional cycles. Although both agents significantly reduced estimated menstrual blood loss, TxA was more effective in decreasing bleeding than intranasal DDAVP.4
In a retrospective cohort study, investigators compared COC use with intranasal DDAVP in 36 adolescents who had VWD and HMB.5 Participant follow-up ranged from 6 months to 4 years. The estimated efficacy of COCs and intranasal DDAVP was 86% and 77%, respectively, a difference that did not achieve statistical significance. Some of the adolescents who used intranasal DDAVP reported severe headaches and flushing.5
In addition, the systematic review authors3 identified 5 case series that described the use of the levonorgestrel (52 mg)-releasing intrauterine device (LNG 52 IUD) in women with VWD and HMB; 4 of these addressed the efficacy of progestin-releasing IUDs in reducing HMB in this patient population.6-9 Using different approaches to define HMB, the authors of these reports followed between 7 and 26 patients with bleeding disorders (most with confirmed VWD) and HMB for variable amounts of time after placement of an LNG 52 IUD. Many of the women described in these case series had tried other HMB treatments, including COCs, without success. Although these 4 reports assessed different outcomes, all reported that placement of the LNG 52 IUD substantially reduced menstrual blood loss, often resulting in amenorrhea. Several of these reports also noted important improvements in quality of life following LNG 52 IUD placement. One case series reported LNG 52 IUD placement in 13 adolescents with VWD and HMB. The mean time to achieve amenorrhea or occasional spotting was 94 days.6
The fifth report, which followed 20 women (median age, 31 years) with HMB associated with VWD or other bleeding disorders who underwent LNG 52 IUD placement, aimed to describe IUD expulsions and malpositioned IUDs in this population. In this small group of patients, 3 IUD expulsions and 2 malpositioned IUDs were observed. Furthermore, an additional 5 women had their device removed prematurely due to patient dissatisfaction. Accordingly, the IUD continuation rate in this case series was only 50%.10
Evidence on management of pregnancy, delivery, and the postpartum period
Heavy menstrual bleeding is not the only challenge for women with VWD. While pregnancy is accompanied by higher levels of VWF, potentially offsetting the risk of bleeding at the time of delivery, the levels do not achieve the same magnitude as they would in unaffected women.11 Women are at an increased risk of primary PPH12,13 and, importantly, since VWF levels fall exponentially after delivery when women are still experiencing lochia,11 they are at increased risk of secondary or delayed PPH.
Two questions arise frequently in the care of women with VWD at the time of delivery and during the postpartum period:
- What is the safest approach for women who desire neuraxial analgesia for intrapartum pain?
- What is the impact of postpartum administration of TxA on PPH?
The second systematic review the authors performed3 focused on VWF levels in women receiving neuraxial anesthesia during labor. After screening 27 studies, the authors included 5 case series, which did not describe outcomes based on VWF levels but rather described the outcomes of women with VWF levels of greater than 0.50 IU/mL (> 50% of normal compared with a normal standard).
Meta-analysis showed that the proportion of anesthesia complications was 6%, which sounds high, but the range of complications was what would be expected in any population (hypotension, accidental dural puncture, inadequate anesthesia, and bloody tap with no further complications). No spinal, subdural, or epidural hematomas were noted.3 Such hematomas are an extremely rare complication of neuraxial anesthesia, occurring in only 1 in 200,000 or 1 in 250,000 obstetric patients14,15; accordingly, an increase in the rate of hematomas among women with VWD could go undetected. The absence of hematomas among women with VWD as reported in the systematic review does not mean there is not an increase in the rate of hematomas in women with VWD. The relative risk is unknown and caution would be advised.
The third systematic review that the authors performed3 was on TxA treatment in the postpartum period. After screening 41 studies, the authors included 2 retrospective cohort studies.16,17 The majority of the participants had VWD. With very-low-certainty evidence, the authors found that TxA reduces the risk of:
- severe primary PPH (risk ratio [RR], 0.36; 95% confidence interval [CI], 0.05–2.59)
- primary PPH (RR, 0.25; 95% CI, 0.04–1.75)
- secondary PPH (RR, 0.42; 95% CI, 0.02–0.91—does not cross 1.0).
Note that the 95% confidence intervals for severe as well as primary PPH crossed 1.0 and therefore these reductions in risk did not achieve statistical significance. Additionally, there was very-low-certainty evidence on the effect of TxA on blood transfusions, vaginal hematomas, blood loss, and thrombotic complications.3
Continue to: Our recommendations for HMB management...
Our recommendations for HMB management
When first evaluating any woman with HMB, it is important to check a blood count and ferritin level, if not already done. If there is any suggestion of iron deficiency (with or without anemia), we recommend oral iron supplementation. This is best accomplished with slow-release iron supplement formulations (or less expensive generic or house brands that contain less than 65 mg of elemental iron per tablet) taken every other day. Such preparations may cause fewer gastrointestinal adverse effects than other oral iron formulations.18 Although it may appear counterintuitive, oral iron is better absorbed (and also may cause fewer gastrointestinal adverse effects) when taken every other day.19
Initial management of HMB, whether or not a bleeding disorder is present, often consists or oral hormonal management. If no contraindications are present, we recommend initiation of a COC with a short hormone-free interval (for example, a 24/4 formulation). If contraindications to contraceptive doses of estrogen are present, continuous use of norethindrone acetate 5-mg tablets or off-label use of combination tablets with 5 µg of ethinyl estradiol and 1 mg of norethindrone acetate (a formulation approved for the treatment of menopausal symptoms) is appropriate.20
Once a patient is established on oral hormonal management, placement of a levonorgestrel-releasing IUD should be considered. Given that expulsion rates may be higher in women with HMB, if feasible, consider using abdominal ultrasound guidance for IUD placement.
For women with VWD who fail first-line therapy (hormonal management) or are trying to become pregnant, TxA (two 650-mg tablets 3 times daily for up to 5 days during episodes of heavy flow) can reduce HMB.20,21
Our recommendations for management of pregnancy and delivery
The second and third systematic reviews discussed above provide very limited guidance on comprehensive management. The care of the pregnant patient with VWD starts with assessment of VWF levels and making an accurate diagnosis. This usually requires the input of a hematologist or other expert in hemostasis. If no recent VWF levels are available, the ObGyn can obtain a von Willebrand panel that includes VWF antigen, VWF activity (most commonly ristocetin cofactor), and factor VIII.
Levels should be reassessed around 36 weeks’ gestation in anticipation of delivery. VWF levels increase during pregnancy; accordingly, in mild, type 1 VWD, half the time treatment is not necessary.11 If VWF activity is less than 50 IU/dL (less than 50% of normal) at 36 weeks’ gestation, the patient should receive VWF concentrate (dosed in VWF units). This requires consultation with hematology and specialized pharmacy support.
For these reasons, the patient with a VWF level less than 50% should be delivered in a referral center with the necessary resources. Anesthesia should be aware of the patient. Unless
Due to the quantity of fluids administered during labor or at the time of delivery and the coexistent administration of oxytocin, desmopressin (synthetic vasopressin) should not be used without monitoring sodium levels, should not be dosed more than once, or should be avoided altogether due to the risk of water intoxication.
If the patient has sustained VWF and factor VIII levels greater than 50 IU/dL, she would be a candidate to deliver in her local hospital and receive neuraxial anesthesia.
Based on the best data we have for women with VWD, a patient with a VWF greater than 50 IU/dL is no more likely to experience PPH than other women.11 Intravenous TxA can be used for prevention or treatment of immediate postpartum bleeding per protocol (1 g after cord clamp and 1 g 30 minutes or more later).22 Oral TxA can be used for prevention or treatment of delayed postpartum bleeding as per HMB. Regardless of the outcome of any testing during pregnancy, nonsteroidal anti-inflammatory drugs should be avoided postpartum and the patient should be monitored closely for bleeding.
Neonatal care
As for the fetus/neonate, the parents should be aware that the infant has a 50% chance of inheriting VWD. If the baby’s father has no history of bleeding, it is unlikely that the infant would be any more affected than the patient herself. Nonetheless, cord blood (in one or more light blue top tubes) should be obtained at the time of delivery and sent for a von Willebrand panel. If the infant is male, a circumcision should be postponed until VWD is ruled out. In addition, fetal invasive procedures should be avoided during labor. Fetal scalp electrode placement should be avoided. Operative vaginal delivery also should be avoided. Cesarean delivery would be preferred to operative vaginal delivery, but if operative vaginal delivery is unavoidable, use of forceps is preferred to vacuum extraction. ●
Von Willebrand disease (VWD) represents the most common inherited bleeding disorder, with a prevalence of approximately 1 in 1,000 people.
Bruising and mucocutaneous bleeding (epistaxis, gingival bleeding, and bleeding after dental extraction) are the most common presenting symptoms of VWD. Because VWD substantially increases the risk of heavy menstrual bleeding (HMB) and, to some extent, intrapartum bleeding complications, and postpartum hemorrhage, women experience a disproportionate burden from VWD. Thus, ObGyns are likely to be called on to make treatment recommendations in VWD patients with these concerns.1
In 2017, the American Society of Hematology, the International Society on Thrombosis and Haemostasis, the National Hemophilia Foundation, and the World Federation of Hemophilia determined that among clinical issues related to VWD, updating guidelines for women with VWD represented the highest priority.2 Accordingly, an international group of hematologists/coagulation specialists performed systematic literature reviews to address 3 questions faced by women with VWD and their clinicians:
- What are the most effective treatments for HMB?
- What is the safest approach for women desiring neuraxial analgesia for intrapartum pain?
- What is the impact of postpartum administration of tranexamic acid (TxA) on postpartum hemorrhage (PPH)?3
Evidence on management strategies for HMB in women with VWD
The prevalence of HMB in women with VWD ranges from 50% to 92%. Reports suggest that between 5% and 24% of women presenting with this symptom have VWD.3 However, the prevalence of VWD among women seeking care for HMB relates to referral patterns, with the prevalence of VWD substantially higher in patient populations who are referred to clinicians or centers that focus on care of patients with bleeding disorders.
The systematic review authors3 identified 2 comparative studies that assessed the treatment of HMB in women with VWD. One was a crossover trial that enrolled 116 VWD patients with HMB with a mean age of 36 years.4 All participants in this trial chose not to use combination oral contraceptives (COCs) as they had not experienced good results with prior COC use. Trial participants were randomly assigned to receive either intranasal desmopressin (DDAVP; a synthetic analog of the antidiuretic agent vasopressin, which stimulates the release of VWF from endothelial cells) or oral TxA therapy for 2 menstrual cycles. Participants then crossed over to the other drug for 2 additional cycles. Although both agents significantly reduced estimated menstrual blood loss, TxA was more effective in decreasing bleeding than intranasal DDAVP.4
In a retrospective cohort study, investigators compared COC use with intranasal DDAVP in 36 adolescents who had VWD and HMB.5 Participant follow-up ranged from 6 months to 4 years. The estimated efficacy of COCs and intranasal DDAVP was 86% and 77%, respectively, a difference that did not achieve statistical significance. Some of the adolescents who used intranasal DDAVP reported severe headaches and flushing.5
In addition, the systematic review authors3 identified 5 case series that described the use of the levonorgestrel (52 mg)-releasing intrauterine device (LNG 52 IUD) in women with VWD and HMB; 4 of these addressed the efficacy of progestin-releasing IUDs in reducing HMB in this patient population.6-9 Using different approaches to define HMB, the authors of these reports followed between 7 and 26 patients with bleeding disorders (most with confirmed VWD) and HMB for variable amounts of time after placement of an LNG 52 IUD. Many of the women described in these case series had tried other HMB treatments, including COCs, without success. Although these 4 reports assessed different outcomes, all reported that placement of the LNG 52 IUD substantially reduced menstrual blood loss, often resulting in amenorrhea. Several of these reports also noted important improvements in quality of life following LNG 52 IUD placement. One case series reported LNG 52 IUD placement in 13 adolescents with VWD and HMB. The mean time to achieve amenorrhea or occasional spotting was 94 days.6
The fifth report, which followed 20 women (median age, 31 years) with HMB associated with VWD or other bleeding disorders who underwent LNG 52 IUD placement, aimed to describe IUD expulsions and malpositioned IUDs in this population. In this small group of patients, 3 IUD expulsions and 2 malpositioned IUDs were observed. Furthermore, an additional 5 women had their device removed prematurely due to patient dissatisfaction. Accordingly, the IUD continuation rate in this case series was only 50%.10
Evidence on management of pregnancy, delivery, and the postpartum period
Heavy menstrual bleeding is not the only challenge for women with VWD. While pregnancy is accompanied by higher levels of VWF, potentially offsetting the risk of bleeding at the time of delivery, the levels do not achieve the same magnitude as they would in unaffected women.11 Women are at an increased risk of primary PPH12,13 and, importantly, since VWF levels fall exponentially after delivery when women are still experiencing lochia,11 they are at increased risk of secondary or delayed PPH.
Two questions arise frequently in the care of women with VWD at the time of delivery and during the postpartum period:
- What is the safest approach for women who desire neuraxial analgesia for intrapartum pain?
- What is the impact of postpartum administration of TxA on PPH?
The second systematic review the authors performed3 focused on VWF levels in women receiving neuraxial anesthesia during labor. After screening 27 studies, the authors included 5 case series, which did not describe outcomes based on VWF levels but rather described the outcomes of women with VWF levels of greater than 0.50 IU/mL (> 50% of normal compared with a normal standard).
Meta-analysis showed that the proportion of anesthesia complications was 6%, which sounds high, but the range of complications was what would be expected in any population (hypotension, accidental dural puncture, inadequate anesthesia, and bloody tap with no further complications). No spinal, subdural, or epidural hematomas were noted.3 Such hematomas are an extremely rare complication of neuraxial anesthesia, occurring in only 1 in 200,000 or 1 in 250,000 obstetric patients14,15; accordingly, an increase in the rate of hematomas among women with VWD could go undetected. The absence of hematomas among women with VWD as reported in the systematic review does not mean there is not an increase in the rate of hematomas in women with VWD. The relative risk is unknown and caution would be advised.
The third systematic review that the authors performed3 was on TxA treatment in the postpartum period. After screening 41 studies, the authors included 2 retrospective cohort studies.16,17 The majority of the participants had VWD. With very-low-certainty evidence, the authors found that TxA reduces the risk of:
- severe primary PPH (risk ratio [RR], 0.36; 95% confidence interval [CI], 0.05–2.59)
- primary PPH (RR, 0.25; 95% CI, 0.04–1.75)
- secondary PPH (RR, 0.42; 95% CI, 0.02–0.91—does not cross 1.0).
Note that the 95% confidence intervals for severe as well as primary PPH crossed 1.0 and therefore these reductions in risk did not achieve statistical significance. Additionally, there was very-low-certainty evidence on the effect of TxA on blood transfusions, vaginal hematomas, blood loss, and thrombotic complications.3
Continue to: Our recommendations for HMB management...
Our recommendations for HMB management
When first evaluating any woman with HMB, it is important to check a blood count and ferritin level, if not already done. If there is any suggestion of iron deficiency (with or without anemia), we recommend oral iron supplementation. This is best accomplished with slow-release iron supplement formulations (or less expensive generic or house brands that contain less than 65 mg of elemental iron per tablet) taken every other day. Such preparations may cause fewer gastrointestinal adverse effects than other oral iron formulations.18 Although it may appear counterintuitive, oral iron is better absorbed (and also may cause fewer gastrointestinal adverse effects) when taken every other day.19
Initial management of HMB, whether or not a bleeding disorder is present, often consists or oral hormonal management. If no contraindications are present, we recommend initiation of a COC with a short hormone-free interval (for example, a 24/4 formulation). If contraindications to contraceptive doses of estrogen are present, continuous use of norethindrone acetate 5-mg tablets or off-label use of combination tablets with 5 µg of ethinyl estradiol and 1 mg of norethindrone acetate (a formulation approved for the treatment of menopausal symptoms) is appropriate.20
Once a patient is established on oral hormonal management, placement of a levonorgestrel-releasing IUD should be considered. Given that expulsion rates may be higher in women with HMB, if feasible, consider using abdominal ultrasound guidance for IUD placement.
For women with VWD who fail first-line therapy (hormonal management) or are trying to become pregnant, TxA (two 650-mg tablets 3 times daily for up to 5 days during episodes of heavy flow) can reduce HMB.20,21
Our recommendations for management of pregnancy and delivery
The second and third systematic reviews discussed above provide very limited guidance on comprehensive management. The care of the pregnant patient with VWD starts with assessment of VWF levels and making an accurate diagnosis. This usually requires the input of a hematologist or other expert in hemostasis. If no recent VWF levels are available, the ObGyn can obtain a von Willebrand panel that includes VWF antigen, VWF activity (most commonly ristocetin cofactor), and factor VIII.
Levels should be reassessed around 36 weeks’ gestation in anticipation of delivery. VWF levels increase during pregnancy; accordingly, in mild, type 1 VWD, half the time treatment is not necessary.11 If VWF activity is less than 50 IU/dL (less than 50% of normal) at 36 weeks’ gestation, the patient should receive VWF concentrate (dosed in VWF units). This requires consultation with hematology and specialized pharmacy support.
For these reasons, the patient with a VWF level less than 50% should be delivered in a referral center with the necessary resources. Anesthesia should be aware of the patient. Unless
Due to the quantity of fluids administered during labor or at the time of delivery and the coexistent administration of oxytocin, desmopressin (synthetic vasopressin) should not be used without monitoring sodium levels, should not be dosed more than once, or should be avoided altogether due to the risk of water intoxication.
If the patient has sustained VWF and factor VIII levels greater than 50 IU/dL, she would be a candidate to deliver in her local hospital and receive neuraxial anesthesia.
Based on the best data we have for women with VWD, a patient with a VWF greater than 50 IU/dL is no more likely to experience PPH than other women.11 Intravenous TxA can be used for prevention or treatment of immediate postpartum bleeding per protocol (1 g after cord clamp and 1 g 30 minutes or more later).22 Oral TxA can be used for prevention or treatment of delayed postpartum bleeding as per HMB. Regardless of the outcome of any testing during pregnancy, nonsteroidal anti-inflammatory drugs should be avoided postpartum and the patient should be monitored closely for bleeding.
Neonatal care
As for the fetus/neonate, the parents should be aware that the infant has a 50% chance of inheriting VWD. If the baby’s father has no history of bleeding, it is unlikely that the infant would be any more affected than the patient herself. Nonetheless, cord blood (in one or more light blue top tubes) should be obtained at the time of delivery and sent for a von Willebrand panel. If the infant is male, a circumcision should be postponed until VWD is ruled out. In addition, fetal invasive procedures should be avoided during labor. Fetal scalp electrode placement should be avoided. Operative vaginal delivery also should be avoided. Cesarean delivery would be preferred to operative vaginal delivery, but if operative vaginal delivery is unavoidable, use of forceps is preferred to vacuum extraction. ●
- ACOG committee opinion no. 451: Von Willebrand disease in women. Obstet Gynecol. 2009;114:1439-1443. doi: 10.1097 /AOG.0b013e3181c6f975.
- Kalot MA, Al-Khatib M, Connell NT, et al; VWD Working Group. An international survey to inform priorities for new guidance on von Willebrand disease. Hemophilia. 2020;26:106-116. doi: 10.1111/hae.13881.
- Brignardello-Petersen R, El Alayli A, Husainat N, et al. Gynecologic and obstetric management of women with von Willebrand disease: summary of 3 systematic reviews of the literature. Blood Adv. 2022;6:228-237. doi: 10.1182 /bloodadvances.2021005589.
- Kouides PA, Byams VR, Philipp CS, et al. Multisite management study of menorrhagia with abnormal laboratory haemostasis: a prospective crossover study of intranasal desmopressin and oral tranexamic acid. Br J Haematol. 2009;145:212-220. doi: 10.1111/j.1365-2141.2009.07610.x.
- Amesse LS, Pfaff-Amesse T, Leonardi R, et al. Oral contraceptives and DDAVP nasal spray: patterns of use in managing vWD-associated menorrhagia: a single-institution study. J Pediatr Hematol Oncol. 2005;27:357-363. doi: 10.1097/01.mph.0000173175.95152.95.
- Adeyemi-Fowode OA, Santos XM, Dietrich JE, et al. Levonorgestrel-releasing intrauterine device use in female adolescents with heavy menstrual bleeding and bleeding disorders: single institution review. J Pediatr Adolesc Gynecol. 2017;30:479-483. doi: 10.1016/j.jpag.2016.04.001.
- Chi C, Huq FY, Kadir RA. Levonorgestrel-releasing intrauterine system for the management of heavy menstrual bleeding in women with inherited bleeding disorders: long-term follow-up. Contraception. 2011;83:242-247. doi: 10.1016/j.contraception.2010.07.010.
- Kingman CE, Kadir RA, Lee CA, et al. The use of levonorgestrel-releasing intrauterine system for treatment of menorrhagia in women with inherited bleeding disorders. BJOG. 2004;111:1425-1428. doi: 10.1111/j.1471-0528.2004.00305.x.
- Lukes AS, Reardon B, Arepally G. Use of the levonorgestrel-releasing intrauterine system in women with hemostatic disorders. Fertil Steril. 2008;90:673-677. doi: 10.1016 /j.fertnstert.2007.07.1315.
- Rimmer E, Jamieson MA, James P. Malposition and expulsion of the levonorgestrel intrauterine system among women with inherited bleeding disorders. Haemophilia. 2013;19:933-938. doi: 10.1111/hae.12184.
- James AH, Konkle BA, Kouides P, et al. Postpartum von Willebrand factor levels in women with and without von Willebrand disease and implications for prophylaxis. Haemophilia. 2015;21:81-87. doi: 10.1111/hae.12568.
- James AH, Jamison MG. Bleeding events and other complications during pregnancy and childbirth in women with von Willebrand disease. J Thromb Haemost. 2007;5: 1165-1169. doi: 10.1111/j.1538-7836.2007.02563.x.
- Al-Zirqi I, Vangen S, Forsen L, et al. Prevalence and risk factors of severe obstetric haemorrhage. BJOG. 2008;115:1265-1272. doi: 10.1111/j.1471-0528.2008.01859.x.
- Moen V, Dahlgren N, Irestedt L. Severe neurological complications after central neuraxial blockades in Sweden 1990-1999. Anesthesiology. 2004;101:950-959. doi: 10.1097/00000542-200410000-00021.
- D’Angelo R, Smiley RM, Riley ET, et al. Serious complications related to obstetric anesthesia: the serious complication repository project of the Society for Obstetric Anesthesia and Perinatology. Anesthesiology. 2014;120:1505-1512. doi: 10.1097/ALN.000000000000253.
- Govorov I, Lofgren S, Chaireti R, et al. Postpartum hemorrhage in women with von Willebrand disease—a retrospective observational study. PLos One. 2016;11:e0164683. doi: 10.1371/journal.pone.0164683.
- Hawke L, Grabell J, Sim W, et al. Obstetric bleeding among women with inherited bleeding disorders: a retrospective study. Haemophilia. 2016;22:906-911. doi: 10.1111/hae.13067.
- James AH. Iron deficiency anemia in pregnancy. Obstet Gynecol. 2021;138:663-674. doi:10.1097/AOG .000000000000.4559.
- Stoffel NU, Cercamondi CI, Brittenham G, et al. Iron absorption from oral iron supplements given on consecutive versus alternate days and as single morning doses versus twice-daily split dosing in iron-depleted women: two open-label, randomised controlled trials. Lancet Haematol. 2017;4:e524-e533. doi: 10.1016/S2352-3026(17)30182-5.
- Kaunitz AM. Abnormal uterine bleeding in reproductiveage women. JAMA. 2019;321:2126-2127. doi: 10.1001 /jama.2019.5248.
- James AH, Kouides PA, Abdul-Kadir R, et al. Von Willebrand disease and other bleeding disorders in women: consensus on diagnosis and management from an international expert panel. Am J Obstet Gynecol. 2009;201:12.e1-8. doi: 10.1016 /j.ajog.2009.04.024.
- WOMAN Trial Collaborators. Effect of early tranexamic acid administration on mortality, hysterectomy, and other morbidities in women with post-partum haemorrhage (WOMAN): an international, randomised, double-blind, placebo-controlled trial. Lancet. 2017;389:2105-2116. doi: 10.1016/S0140-6736(17)30638-4.
- ACOG committee opinion no. 451: Von Willebrand disease in women. Obstet Gynecol. 2009;114:1439-1443. doi: 10.1097 /AOG.0b013e3181c6f975.
- Kalot MA, Al-Khatib M, Connell NT, et al; VWD Working Group. An international survey to inform priorities for new guidance on von Willebrand disease. Hemophilia. 2020;26:106-116. doi: 10.1111/hae.13881.
- Brignardello-Petersen R, El Alayli A, Husainat N, et al. Gynecologic and obstetric management of women with von Willebrand disease: summary of 3 systematic reviews of the literature. Blood Adv. 2022;6:228-237. doi: 10.1182 /bloodadvances.2021005589.
- Kouides PA, Byams VR, Philipp CS, et al. Multisite management study of menorrhagia with abnormal laboratory haemostasis: a prospective crossover study of intranasal desmopressin and oral tranexamic acid. Br J Haematol. 2009;145:212-220. doi: 10.1111/j.1365-2141.2009.07610.x.
- Amesse LS, Pfaff-Amesse T, Leonardi R, et al. Oral contraceptives and DDAVP nasal spray: patterns of use in managing vWD-associated menorrhagia: a single-institution study. J Pediatr Hematol Oncol. 2005;27:357-363. doi: 10.1097/01.mph.0000173175.95152.95.
- Adeyemi-Fowode OA, Santos XM, Dietrich JE, et al. Levonorgestrel-releasing intrauterine device use in female adolescents with heavy menstrual bleeding and bleeding disorders: single institution review. J Pediatr Adolesc Gynecol. 2017;30:479-483. doi: 10.1016/j.jpag.2016.04.001.
- Chi C, Huq FY, Kadir RA. Levonorgestrel-releasing intrauterine system for the management of heavy menstrual bleeding in women with inherited bleeding disorders: long-term follow-up. Contraception. 2011;83:242-247. doi: 10.1016/j.contraception.2010.07.010.
- Kingman CE, Kadir RA, Lee CA, et al. The use of levonorgestrel-releasing intrauterine system for treatment of menorrhagia in women with inherited bleeding disorders. BJOG. 2004;111:1425-1428. doi: 10.1111/j.1471-0528.2004.00305.x.
- Lukes AS, Reardon B, Arepally G. Use of the levonorgestrel-releasing intrauterine system in women with hemostatic disorders. Fertil Steril. 2008;90:673-677. doi: 10.1016 /j.fertnstert.2007.07.1315.
- Rimmer E, Jamieson MA, James P. Malposition and expulsion of the levonorgestrel intrauterine system among women with inherited bleeding disorders. Haemophilia. 2013;19:933-938. doi: 10.1111/hae.12184.
- James AH, Konkle BA, Kouides P, et al. Postpartum von Willebrand factor levels in women with and without von Willebrand disease and implications for prophylaxis. Haemophilia. 2015;21:81-87. doi: 10.1111/hae.12568.
- James AH, Jamison MG. Bleeding events and other complications during pregnancy and childbirth in women with von Willebrand disease. J Thromb Haemost. 2007;5: 1165-1169. doi: 10.1111/j.1538-7836.2007.02563.x.
- Al-Zirqi I, Vangen S, Forsen L, et al. Prevalence and risk factors of severe obstetric haemorrhage. BJOG. 2008;115:1265-1272. doi: 10.1111/j.1471-0528.2008.01859.x.
- Moen V, Dahlgren N, Irestedt L. Severe neurological complications after central neuraxial blockades in Sweden 1990-1999. Anesthesiology. 2004;101:950-959. doi: 10.1097/00000542-200410000-00021.
- D’Angelo R, Smiley RM, Riley ET, et al. Serious complications related to obstetric anesthesia: the serious complication repository project of the Society for Obstetric Anesthesia and Perinatology. Anesthesiology. 2014;120:1505-1512. doi: 10.1097/ALN.000000000000253.
- Govorov I, Lofgren S, Chaireti R, et al. Postpartum hemorrhage in women with von Willebrand disease—a retrospective observational study. PLos One. 2016;11:e0164683. doi: 10.1371/journal.pone.0164683.
- Hawke L, Grabell J, Sim W, et al. Obstetric bleeding among women with inherited bleeding disorders: a retrospective study. Haemophilia. 2016;22:906-911. doi: 10.1111/hae.13067.
- James AH. Iron deficiency anemia in pregnancy. Obstet Gynecol. 2021;138:663-674. doi:10.1097/AOG .000000000000.4559.
- Stoffel NU, Cercamondi CI, Brittenham G, et al. Iron absorption from oral iron supplements given on consecutive versus alternate days and as single morning doses versus twice-daily split dosing in iron-depleted women: two open-label, randomised controlled trials. Lancet Haematol. 2017;4:e524-e533. doi: 10.1016/S2352-3026(17)30182-5.
- Kaunitz AM. Abnormal uterine bleeding in reproductiveage women. JAMA. 2019;321:2126-2127. doi: 10.1001 /jama.2019.5248.
- James AH, Kouides PA, Abdul-Kadir R, et al. Von Willebrand disease and other bleeding disorders in women: consensus on diagnosis and management from an international expert panel. Am J Obstet Gynecol. 2009;201:12.e1-8. doi: 10.1016 /j.ajog.2009.04.024.
- WOMAN Trial Collaborators. Effect of early tranexamic acid administration on mortality, hysterectomy, and other morbidities in women with post-partum haemorrhage (WOMAN): an international, randomised, double-blind, placebo-controlled trial. Lancet. 2017;389:2105-2116. doi: 10.1016/S0140-6736(17)30638-4.
HIV management in pregnancy
Human immunodeficiency virus (HIV) is a single-stranded enveloped RNA retrovirus that was first described in the 1980s and is known for its severity of systemic immune dysregulation and associated opportunistic infections. It is transmitted through contact with blood or bodily fluids, and it can be transmitted vertically, most often at the time of delivery. Since the advent of antiretroviral therapy, the average life expectancy and natural course of HIV infection has improved notably.1
In 2019, just over 1 million adults and adolescents in the United States were living with the diagnosis of HIV.2 In the same year, the rate of new HIV diagnoses in the United States had stabilized at a rate of 13.2 new cases per 100,000 individuals.2 Among this cohort, individuals identifying as females at birth accounted for 19% of the total population living with HIV.2 Sexual contact was the most common route of transmission, followed by injection drug use—77% and 20%, respectively.2
It is important to note that the incidence and prevalence of HIV does not reflect the individuals who unknowingly are living with the disease. The disease burden associated with HIV infection and the availability of effective treatment modalities has led to the recommendation that all individuals undergo HIV screening at least once in their lifetime.3 Early identification of HIV infection is important to optimize the health of all individuals and future generations.
The interplay between high-risk sexual practices and the risk for HIV exposure and unintended pregnancy places the ObGyn at the forefront of HIV prevention and identification. Early diagnosis and standardized treatment with antiretroviral therapies have led to both a dramatic improvement in adult disease burden and a dramatic decrease in perinatal transmission.4,5 In 2019, perinatal transmission accounted for less than 1% of HIV transmission in the United States.2 This is a decrease of greater than 54% from 2014, which, again, emphasizes the role of the ObGyn in HIV management.6
Preconception care: Gynecologic screening, diagnosis, and management
The Centers for Disease Control and Prevention (CDC) recommends that an individual undergo HIV screening at least once in their lifetime.3 HIV screening algorithms have changed over the last 20 years to reduce the number of false-positive and/or false-negative results obtained through HIV antibody testing alone.7 HIV-1/2 antibody/antigen immunoassay is recommended as the initial screening test. If reactive, this should be followed by an HIV p24-specific antigen test. Reactivity for both the HIV-1/2 immunoassay and the HIV p24-specific antigen test confirms the diagnosis of HIV infection. However, if HIV p24-specific antigen testing is indeterminate or an acute HIV infection is suspected, an HIV nucleic acid test (NAT) should be performed.7,8
Upon a positive diagnosis, a multidisciplinary team approach is recommended to address the mental, social, and physical care of the patient. Team members should include an adult medicine clinician, an infectious disease clinician, an ObGyn, social services staff, and behavioral health support to achieve the goal of obtaining and maintaining the patient’s optimal health status.
TABLE 1 lists the recommended initial laboratory assessments that should follow a new diagnosis of HIV infection. Based on the laboratory results, the indicated vaccinations, antibiotic prophylaxis for opportunistic infections, and optimal combined antiretroviral therapy (cART) can be determined.9 The vaccinations listed in TABLE 2 should be up to date.10,11 Additionally, cervical cancer screening with cytology and human papillomavirus (HPV) testing and treatment should be performed in accordance with the 2019 American Society for Cervical Cancer Prevention (ASCCP) guidelines.12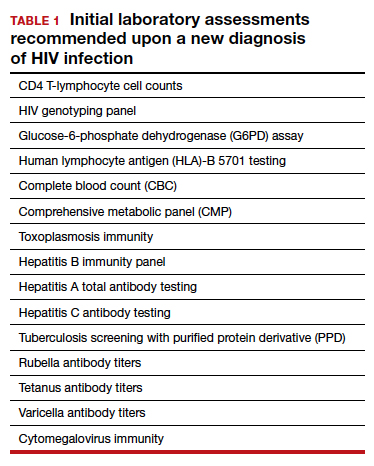
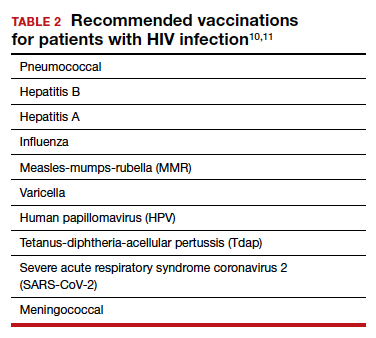
Promptly initiating cART is of utmost importance; this decreases the rate of HIV transmission via sexual contact and decreases the rate of perinatal transmission.5,13 Results of the initial laboratory assessment, hepatitis B status, and desire for pregnancy/contraception should be considered when initiating cART.3,14,15
It is imperative to discuss sharing the positive diagnostic results with the patient’s partner. The CDC provides guidance for these discussions,16 which should address the use of preexposure prophylaxis (PrEP) if partner screening establishes partner serodiscordance (that is, HIV positivity in one partner and HIV negativity in the other partner). PrEP is a single pill approved by the US Food and Drug Administration (FDA) that combines tenofovir 300 mg and emtricitabine 200 mg daily17 and has been recommended since 2012.18-20 PrEP also should be considered in sexually active individuals who have higher-risk behaviors within an area with high HIV prevalence.18-21 Despite the CDC’s strong recommendations for PrEP use, lack of insurance coverage and high cost are barriers to universal use. The National Alliance of State and Territorial AIDS Directors (NASTAD) provides a list of patient and copayment assistance programs that can be found at the NASTAD website: https://nastad.org/prepcost-resources/prep-assitance-programs.
Continue to: Preconception considerations...
Preconception considerations
In individuals with known HIV infection, preconception consultation with an ObGyn or maternal-fetal medicine (MFM) specialist should be recommended prior to conception.22 Preconception recommendations include addressing optimization of maternal medical comorbidities, addressing routine health screening and vaccinations, performing sexually transmitted infection screening, and optimizing HIV disease status.3,22,23
With the assistance of adult medicine and infectious disease clinicians, a cART regimen that is sufficient to reliably maintain viral suppression (that is, viral load < 50 copies/mL on 2 separate occasions at least 3 months apart) and is safe for use in pregnancy should be established.3 In serodiscordant couples, recommended mechanisms to prevent HIV transmission during conception include sustained viral suppression in the HIV-positive partner, PrEP use in the HIV-negative partner, and timing of unprotected intercourse during peak fertility only.3
Antepartum care
The initial prenatal visit
Women who have no prior screening for HIV or prior negative HIV results should undergo HIV screening at the first prenatal visit.3 Screening should be performed in accordance with the “opt out method.”6 Using this method, a woman without a known diagnosis of HIV infection is told that she will undergo HIV screening as a component of routine prenatal care unless she decides that she does not want this test performed.6,24,25 At the time of screening, all pregnant women should be provided with comprehensive information regarding HIV screening, HIV screening results, and the implications of HIV infection on pregnancy.26
In the pregnant patient with confirmed HIV infection, all preconception considerations should be addressed. If not already in place, referrals to appropriate providers (infectious disease specialist, ObGyn, MFM specialist) and ancillary support staff (social services, behavioral health support) should be arranged. All efforts should be implemented to optimize additional medical comorbidities. TABLE 3 lists additional prenatal testing requirements.22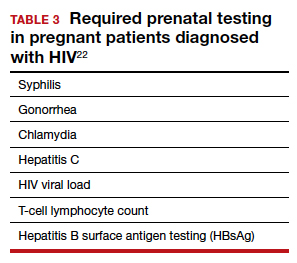
Antiretroviral therapy should be assessed for safety and efficacy in pregnancy and should comply with the CDC recommendations for cART in pregnancy.3 Patients with a T-lymphocyte cell count of less than 200 cells/mm3 and/or a viral load greater than 50 copies/mL despite adherent cART use should be referred to an infectious disease specialist to determine the need for alternative cART and/or the need for chemoprophylaxis against opportunistic infections.23
First and second trimester
Antiretroviral adherence and barriers to adherence should be addressed at every prenatal visit. If the patient is started on antiretroviral therapy in pregnancy or is switched to an alternative cART regimen, viral load assessment should be performed 2 to 4 weeks after the start or change in cART and then repeated monthly until undetectable levels are achieved.3,26 If an undetectable viral load cannot be obtained, cART adherence should be thoroughly evaluated, and the patient should be referred to an infectious disease or HIV treatment specialist.26
If the initial prenatal testing indicates an undetectable viral load, repeat viral load assessment can be performed every 3 months throughout the pregnancy.3 If initial prenatal testing indicates an undetectable HIV viral load and the T-lymphocyte count is greater than 200 cells/mm3, repeat viral load testing can be performed every 6 months to ensure stability.3
Early screening for gestational diabetes should be performed in patients receiving protease inhibitors because these agents may interfere with carbohydrate tolerance.22,26
Continue to: Third trimester...
Third trimester
Women with negative HIV screening at the initial prenatal evaluation should undergo repeat HIV screening in the third trimester if they are at high risk for HIV exposure.25 Factors that determine high-risk status are listed in TABLE 4.27 Sexually transmitted infection screening should be repeated in the third trimester.26
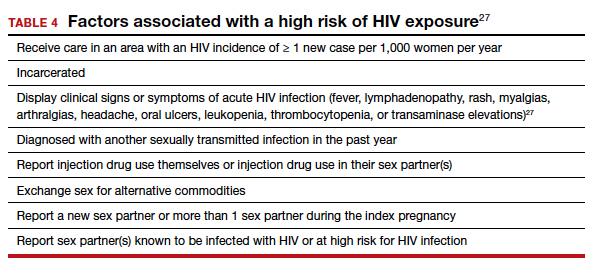
Repeat assessment of the viral load should be completed between 34 and 36 weeks’ gestation or sooner if additional indications for early term or late preterm delivery arise.3 Viral load assessments aid in determining delivery timing and route and the need for zidovudine (ZDV) treatment (FIGURE).
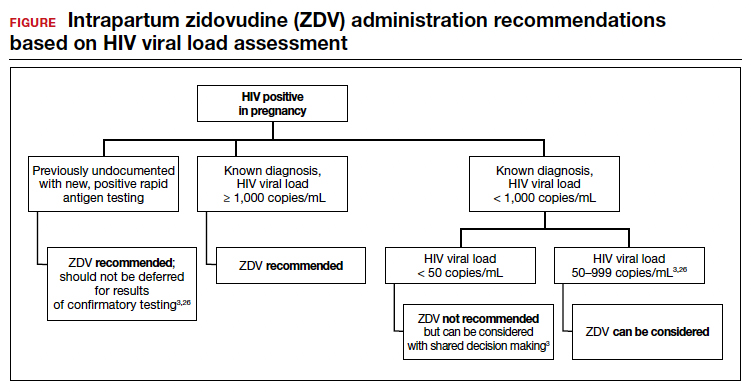
Studies that were performed prior to standardized cART use found higher rates of perinatal transmission associated with vaginal delivery when compared with cesarean delivery (CD).28-30 However, these studies did not account for measures of viral load within their study populations.28-30
In more recent studies performed in the era of standardized cART and viral load monitoring, CD does not provide protection from perinatal transmission when the maternal viral load is less than 1,000 copies/mL at the time of delivery.31 Similarly, delivery prior to 40 weeks’ gestation does not confer protection from perinatal transmission.32
Alternatively, if the maternal viral load is 1,000 copies/mL or greater, CD should be considered to reduce the risk of perinatal transmission. A scheduled, elective CD at 38 weeks’ gestation is recommended in those with a maternal viral load of 1,000 copies/mL or greater and no medical indication for earlier delivery in order to decrease the likelihood of labor onset and/or rupture of membranes prior to delivery.3,33
Intrapartum care
Rapid antigen testing (with follow-up confirmatory testing as indicated) is recommended in patients presenting to labor and delivery with no prior documentation of HIV status.3,8,26
Despite a significant decrease in perinatal transmission rates over the last 30 years, a large proportion of perinatal transmission cases are thought to result from intrapartum fetal exposure. While the mechanism of transmission is not known, a correlation between maternal viral load and risk for perinatal transmission has been shown. A maternal viral load of less than 1,000 copies/mL has been associated with a perinatal transmission risk of less than 2%.34,35 A maternal viral load between 50 and 999 copies/mL has been associated with a perinatal transmission rate of 1% to 2% compared with less than 1% for a maternal viral load of less than 50 copies/mL or undetectable measures.5,36,37
These differences in perinatal transmission rates have prompted the recommendation for administration of ZDV for a minimum of 3 hours prior to delivery in mothers with a viral load of 1,000 copies/mL or greater.4,38 The recommended ZDV dosing is: a 1-hour intravenous loading dose of 2 mg/kg followed by continuous infusion of 1 mg/kg per hour until delivery.39,40 Patients who opt for vaginal delivery despite nonsuppressed viral loads (≥1,000 copies/mL) after thorough perinatal counseling should receive ZDV at the start of labor through delivery.3 All patients should be continued on cART throughout their intrapartum and postpartum course.
The duration of membrane rupture and the use of invasive fetal monitoring (that is, fetal scalp electrodes) have been assessed as mechanisms of perinatal transmission. Although they were performed prior to the standardized use of cART, several studies demonstrated that increased perinatal transmission rates were associated with invasive fetal monitoring.34,41,42 While limited data have refuted this finding in women with suppressed viral loads (< 50 copies/mL), the American College of Obstetricians and Gynecologists recommends avoiding the use of invasive fetal monitoring in labor.26
Pre-cART studies demonstrated increased rates of perinatal transmission with longer durations of membrane rupture prior to delivery.43,44 More recent studies have reevaluated this association and determined that the increased perinatal transmission rates are more likely associated with higher maternal viral loads at the time of delivery rather than duration of membrane rupture.45-47 No clear evidence describes when or if CD after the onset of labor or rupture of membranes provides protection from perinatal HIV transmission in pregnant women with HIV receiving no antiretroviral drugs or only ZDV during labor.43,48 CD can be considered for patients in whom scheduled, pre-labor CD was planned who present in labor or with rupture of membranes prior to scheduled CD.26 These, and additional intrapartum considerations, are listed in TABLE 5.49,50
Appropriate personal protective equipment should be available and donned for all providers present throughout intrapartum management and at delivery.23,26 Should any provider injury occur, immediate cleansing of the injury site should be performed, followed by referral to proper workplace supervisors for additional laboratory testing and antiretroviral prophylaxis.
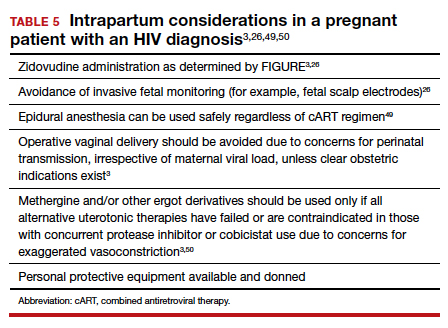
Continue to: Postpartum care...
Postpartum care
Postpartum contraception should be offered and provided in accordance with patient request. Regardless of other birth control methods, strict condom use should be advised. PrEP should be discussed and offered for all partners of serodiscordant couples.
Upon outpatient follow-up, assessment and provision of routine health maintenance should be performed. Any abnormal cervical pathology encountered during prenatal care should be managed in accordance with ASCCP guidelines.12 Follow-up care should be established with adult medicine, infectious disease, and ObGyn clinicians.26
Neonatal considerations
Neonates born to mothers with positive or unknown HIV status should undergo expedited HIV testing.51,52 Consultation should be conducted with pediatric or neonatology colleagues to determine the antiretroviral regimen and duration of therapy based on presumed HIV status of the neonate. Ideally, antiretroviral therapy should be initiated within 6 hours of delivery.3,53
Formula feeding should be implemented as maternal HIV infection is one of the few contraindications to breastfeeding.54,55 The risk of late breast milk transmission, defined as postnatal transmission that occurs after 1 month of age, may vary based on maternal viral load, but it has been reported as high as 8.9 transmissions per 100 person-years of breastfeeding.56
Resources available
Care of the pregnant patient with HIV and the reduction of perinatal transmission both depend on early diagnosis of HIV and effective treatment with cART. Such patients benefit from a team-based care model that includes the ObGyn and/or MFM specialist, infectious disease clinician, pediatrician, and social worker. As guidelines evolve for care of these patients, a reference checklist, such as the examples provided at the Society for Maternal-Fetal Medicine website (smfm.org) or at HIV.gov, provide an outline for:
- management before, during, and after pregnancy
- suggestions for management teams of interest to successfully carry out the checklist requirements
- proposals for measurements of quality performance with the use of checklists in the management of HIV in pregnancy.
In addition, assistance with clinical decision making for patients with HIV in pregnancy can be obtained via telephone consultation with the National Clinician Consultation Center–Perinatal HIV/AIDS (888-448-8765), which is available 24 hours a day, 7 days a week. ●
- Samji H, Cescon A, Hogg RS, et al; North American AIDS Cohort Collaboration on Research and Design (NA-ACCORD) of IeDEA. Closing the gap: increases in life expectancy among treated HIV-positive individuals in the United States and Canada. PLoS One. 2013;8:e81355. doi: 10.1371/journal. pone.0081355.
- Centers for Disease Control and Prevention. May 1, 2021. HIV Surveillance Report, 2019, vol. 32: Diagnosis of HIV infection in the United States and dependent areas, 2019. Accessed February 15, 2022. http://www.cdc.gov/hiv/library/reports /hiv-surveillance.html
- Panel on Antiretroviral Therapy and Medical Management of Children Living with HIV. Guidelines for the use of antiretroviral agents in pediatric HIV infection. https: //clinicalinfo.hiv.gov/en/guidelines/pediatric-arv. Accessed February 15, 2022.
- Connor EM, Sperling RS, Gelber R, et al. Reduction of maternal-infant transmission of human immunodeficiency virus type 1 with zidovudine treatment. Pediatric AIDS Clinical Trials Group Protocol 076 Study Group. N Engl J Med. 1994;331:1173-1180.
- Townsend CL, Byrne L, Cortina-Borja M, et al. Earlier initiation of ART and further decline in mother-to-child HIV transmission rates, 2000-2011. AIDS. 2014;28:1049-1057.
- Centers for Disease Control and Prevention. January 26, 2022. HIV and pregnant women, infants, and children. Accessed February 15, 2022. https://www.cdc.gov/hiv/group/gender /pregnantwomen/index.html
- Centers for Disease Control and Prevention. 2018 Quick reference guide: Recommended laboratory HIV testing algorithm for serum or plasma specimens. National Center for HIV/AIDS, Viral Hepatitis, and TB Prevention (US); Division of HIV/AIDS Prevention; Association of Public Health Laboratories. Updated January 2018. https://stacks. cdc.gov/view/cdc/50872
- Centers for Disease Control and Prevention, Association of Public Health Laboratories. June 27, 2014. Laboratory testing for the diagnosis of HIV infection: updated recommendations. Accessed February 15, 2022. http://stacks.cdc.gov/view /cdc/23447
- Guidelines for the prevention and treatment of opportunistic infections in adults and adolescents with HIV. Updated April 12, 2022. Accessed July 6, 2022. https://clinicalinfo.hiv .gov/en/guidelines/adult-and-adolescent-opportunistic -infection/whats-new-guidelines
- Rubin LG, Levin MJ, Ljungman P, et al. 2013 IDSA clinical practice guideline for vaccination of the immunocompromised host. Clin Infect Dis. 2014;58: e44–e100. doi: 10.1093/cid/cit684.
- Centers for Disease Control and Prevention. ACIP: Guidance for vaccine recommendations for pregnant and breastfeeding women. Accessed July 5, 2022. https://www.cdc.gov /vaccines/hcp/acip-recs/rec-vac-preg.html?CDC_AA _refVal=https%3A%2F%2Fwww.cdc.gov%2Fvaccines%2Facip %2Fcommittee%2Fguidance%2Frec-vac-preg.html
- Perkins RB, Guido RS, Castle PE, et al; for the 2019 ASCCP Risk-Based Management Consensus Guidelines Committee. 2019 ASCCP risk-based management consensus guidelines for abnormal cervical cancer screening tests and cancer precursors. J Low Genit Tract Dis. 2020;24:102-131. doi: 10.1097/LGT.0000000000000525. Erratum in: J Low Genit Tract Dis. 2020;24:427.
- Cohen MS, Chen YQ, McCauley M, et al; HPTN 052 Study Team. Prevention of HIV-1 infection with early antiretroviral therapy. N Engl J Med. 2011;365:493-505.
- Drug interactions between antiretroviral agents and hormonal contraceptives. Accessed July 6, 2022. https://clinicalinfo .hiv.gov/en/table/table-3-drug-interactions-between -antiretroviral-agents-and-hormonal-contraceptives
- Panel on Treatment of HIV During Pregnancy and Prevention of Perinatal Transmission. Recommendations for use of antiretroviral drugs in pregnancy and interventions to reduce perinatal HIV transmission in the United States. Accessed July 7, 2022. https://clinicalinfo.hiv.gov/en/guidelines/perinatal /whats-new-guidelines
- Centers for Disease Control and Prevention. Recommendations for partner services programs for HIV infection, syphilis, gonorrhea, and chlamydial infection. MMWR Recomm Rep. 2008;57(RR-9):1–83.
- Gilead Sciences, Inc. Truvada (emtricitabine 200 mg/ tenofovir disoproxil fumarate 300 mg tablets). Accessed July 6, 2022. https://truvada.com
- Centers for Disease Control and Prevention. Interim guidance for clinicians considering the use of preexposure prophylaxis for the prevention of HIV infection in heterosexually active adults. MMWR Morb Mortal Wkly Rep. 2012;61:586-589.
- Baeten JM, Donnell D, Ndase P, et al; Partners PrEP Study Team. Antiretroviral prophylaxis for HIV prevention in heterosexual men and women. N Engl J Med. 2012;367: 399-410.
- Celum C, Baeten JM. Antiretroviral-based HIV-1 prevention: antiretroviral treatment and pre-exposure prophylaxis. Antivir Ther. 2012;17:1483-1493.
- Thigpen MC, Kebaabetswe PM, Paxton LA, et al; TDF2 Study Group. Antiretroviral preexposure prophylaxis for heterosexual HIV transmission in Botswana. N Engl J Med. 2012;367:423-434.
- Society for Maternal-Fetal Medicine. Special statement: updated checklists for pregnancy management in persons with HIV. Accessed July 5, 2022. https://www.smfm.org /publications/334-smfm-special-statement-updated -checklists-for-pregnancy-management-in-persons-with-hiv
- American College of Obstetricians and Gynecologists. ACOG committee opinion no. 752. Prenatal and perinatal human immunodeficiency virus testing. Obstet Gynecol. 2018;132:e138-e142.
- Human immunodeficiency virus screening. Joint statement of the American Academy of Pediatrics and the American College of Obstetricians and Gynecologists. Pediatrics. 1999;104(1 pt 1):128.
- Branson BM, Handsfield HH, Lampe MA, et al; Centers for Disease Control and Prevention. Revised recommendations for HIV testing of adults, adolescents, and pregnant women in health care settings. MMWR Recomm Rep. 2006; 55(RR-14):1-17.
- American College of Obstetricians and Gynecologists. ACOG committee opinion no. 751. Labor and delivery management of women with human immunodeficiency virus infection. Obstet Gynecol. 2018;132:e131-e137.
- Centers for Disease Control and Prevention. Factors increasing the risk of acquiring or transmitting HIV. November 12, 2019. Accessed July 29, 2022. https://www.cdc .gov/hiv/risk/estimates/riskfactors.html
- Mandelbrot L, Le Chenadec J, Berrebi A, et al. Perinatal HIV1 transmission: interaction between zidovudine prophylaxis and mode of delivery in the French Perinatal Cohort. JAMA. 1998;280:55-60.
- European Mode of Delivery Collaboration. Elective caesarean-section versus vaginal delivery in prevention of vertical HIV-1 transmission: a randomised clinical trial. Lancet. 1999;353:1035-1039.
- International Perinatal HIV Group; Andiman W, Bryson Y, de Martino M, et al. The mode of delivery and the risk of vertical transmission of human immunodeficiency virus type 1—a meta-analysis of 15 prospective cohort studies. N Engl J Med. 1999;340:977-987.
- Briand N, Jasseron C, Sibiude J, et al. Cesarean section for HIV-infected women in the combination antiretroviral therapies era, 2000–2010. Am J Obstet Gynecol. 2013;209: 335.e1-335.e12.
- Scott RK, Chakhtoura N, Burke MM, et al. Delivery after 40 weeks of gestation in pregnant women with well-controlled human immunodeficiency virus. Obstet Gynecol. 2017;130:502-510.
- American College of Obstetricians and Gynecologists. Committee opinion no. 560. Medically indicated late-preterm and early-term deliveries. Obstet Gynecol. 2013;121:908-910.
- Mofenson LM, Lambert JS, Stiehm ER, et al. Risk factors for perinatal transmission of human immunodeficiency virus type 1 in women treated with zidovudine. Pediatric AIDS Clinical Trials Group Study 185 Team. N Engl J Med. 1999;341:385-393.
- Garcia PM, Kalish LA, Pitt J, et al. Maternal levels of plasma human immunodeficiency virus type 1 RNA and the risk of perinatal transmission. Women and Infants Transmission Study Group. N Engl J Med. 1999;341:394-402.
- Briand N, Warszawski J, Mandelbrot L, et al; ANRS-EPF CO1CO11 Study Group. Is intrapartum intravenous zidovudine for prevention of mother-to-child HIV-1 transmission still useful in the combination antiretroviral therapy era? Clin Infect Dis. 2013;57:903-914.
- Myer L, Phillips TK, McIntyre JA, et al. HIV viraemia and mother-to-child transmission risk after antiretroviral therapy initiation in pregnancy in Cape Town, South Africa. HIV Med. 2017;18:80-88.
- Rodman JH, Flynn PM, Robbins B, et al. Systemic pharmacokinetics and cellular pharmacology of zidovudine in human immunodeficiency virus type 1-infected women and newborn infants. J Infect Dis. 1999;180:1844-1850.
- Wade NA, Birkhead GS, Warren BL, et al. Abbreviated regimens of zidovudine prophylaxis and perinatal transmission of the human immunodeficiency virus. N Engl J Med. 1998;339:1409-1414.
- Nielsen-Saines K, Watts HD, Veloso VS, et al; NICHD HPTN 040/PACTG 1043 Protocol Team. Three postpartum antiretroviral regimens to prevent intrapartum HIV infection. N Engl J Med. 2012;366:2368-2379.
- Mandelbrot L, Mayaux MJ, Bongain A, et al. Obstetric factors and mother-to-child transmission of human immunodeficiency virus type 1: the French perinatal cohorts. SEROGEST French Pediatric HIV Infection Study Group. Am J Obstet Gynecol. 1996;175(3 pt 1):661-667.
- Shapiro DE, Sperling RS, Mandelbrot L, et al. Risk factors for perinatal human immunodeficiency virus transmission in patients receiving zidovudine prophylaxis. Pediatric AIDS Clinical Trials Group protocol 076 Study Group. Obstet Gynecol. 1999;94:897-908.
- International Perinatal HIV Group. Duration of ruptured membranes and vertical transmission of HIV-1: a meta-analysis from 15 prospective cohort studies. AIDS. 2001;15:357-368.
- Nielsen TF, Hokegard KH. Postoperative cesarean section morbidity: a prospective study. Am J Obstet Gynecol. 1983;146:911-916.
- Mark S, Murphy KE, Read S, et al. HIV mother-to-child transmission, mode of delivery, and duration of rupture of membranes: experience in the current era. Infect Dis Obstet Gynecol. 2012;2012:267969.
- Cotter AM, Brookfield KF, Duthely LM, et al. Duration of membrane rupture and risk of perinatal transmission of HIV1 in the era of combination antiretroviral therapy. Am J Obstet Gynecol. 2012;207:482.e1-482.e5.
- Peters H, Byrne L, De Ruiter A, et al. Duration of ruptured membranes and mother-to-child HIV transmission: a prospective population-based surveillance study. BJOG. 2016;123:975-981.
- Jamieson DJ, Read JS, Kourtis AP, et al. Cesarean delivery for HIV-infected women: recommendations and controversies. Am J Obstet Gynecol. 2007;197(3 suppl):S96-S100.
- Cambic CR, Avram MJ, Gupta DK, et al. Effect of ritonavir-induced cytochrome P450 3A4 inhibition on plasma fentanyl concentrations during patient-controlled epidural labor analgesia: a pharmacokinetic simulation. Int J Obstet Anesth. 2014;23:45-51.
- Navarro J, Curran A, Burgos J, et al. Acute leg ischaemia in an HIV-infected patient receiving antiretroviral treatment. Antivir Ther. 2017;22:89-90.
- American Academy of Pediatrics, American College of Obstetricians and Gynecologists. Guidelines for Perinatal Care. 8th ed. American Academy of Pediatrics, American College of Obstetricians and Gynecologists; 2017.
- Siberry GK, Abzug MJ, Nachman S, et al; Panel on Opportunistic Infections in HIV-Exposed and HIV-Infected Children. Guidelines for the prevention and treatment of opportunistic infections in HIV-exposed and HIV-infected children: recommendations from the National Institutes of Health, Centers for Disease Control and Prevention, the HIV Medicine Association of the Infectious Diseases Society of America, the Pediatric Infectious Diseases Society, and the American Academy of Pediatrics. Pediatr Infect Dis J. 32(suppl 2[0 2]):i–KK4.
- Panel on Antiretroviral Therapy and Medical Management of Children Living with HIV. Guidelines for the use of antiretroviral agents in pediatric HIV infection. Accessed February 15, 2022. https://clinicalinfo.hiv.gov/en/guidelines /pediatric-arv
- Committee on Health Care for Underserved Women, American College of Obstetricians and Gynecologists. ACOG committee opinion no. 361. Breastfeeding: maternal and infant aspects. Obstet Gynecol. 2007;109(2 pt 1):479-480.
- Committee on Pediatric AIDS; Mofenson LM, Flynn PM, Aldrovandi GM, et al. Infant feeding and transmission of human immunodeficiency virus in the United States. Pediatrics. 2013;131:391-396.
- Breastfeeding and HIV International Transmission Study Group; Coutsoudis A, Dabis F, Fawzi W, et al. Late postnatal transmission of HIV-1 in breast-fed children: an individual patient data meta-analysis. J Infect Dis. 2004;189:2154-2166.
Human immunodeficiency virus (HIV) is a single-stranded enveloped RNA retrovirus that was first described in the 1980s and is known for its severity of systemic immune dysregulation and associated opportunistic infections. It is transmitted through contact with blood or bodily fluids, and it can be transmitted vertically, most often at the time of delivery. Since the advent of antiretroviral therapy, the average life expectancy and natural course of HIV infection has improved notably.1
In 2019, just over 1 million adults and adolescents in the United States were living with the diagnosis of HIV.2 In the same year, the rate of new HIV diagnoses in the United States had stabilized at a rate of 13.2 new cases per 100,000 individuals.2 Among this cohort, individuals identifying as females at birth accounted for 19% of the total population living with HIV.2 Sexual contact was the most common route of transmission, followed by injection drug use—77% and 20%, respectively.2
It is important to note that the incidence and prevalence of HIV does not reflect the individuals who unknowingly are living with the disease. The disease burden associated with HIV infection and the availability of effective treatment modalities has led to the recommendation that all individuals undergo HIV screening at least once in their lifetime.3 Early identification of HIV infection is important to optimize the health of all individuals and future generations.
The interplay between high-risk sexual practices and the risk for HIV exposure and unintended pregnancy places the ObGyn at the forefront of HIV prevention and identification. Early diagnosis and standardized treatment with antiretroviral therapies have led to both a dramatic improvement in adult disease burden and a dramatic decrease in perinatal transmission.4,5 In 2019, perinatal transmission accounted for less than 1% of HIV transmission in the United States.2 This is a decrease of greater than 54% from 2014, which, again, emphasizes the role of the ObGyn in HIV management.6
Preconception care: Gynecologic screening, diagnosis, and management
The Centers for Disease Control and Prevention (CDC) recommends that an individual undergo HIV screening at least once in their lifetime.3 HIV screening algorithms have changed over the last 20 years to reduce the number of false-positive and/or false-negative results obtained through HIV antibody testing alone.7 HIV-1/2 antibody/antigen immunoassay is recommended as the initial screening test. If reactive, this should be followed by an HIV p24-specific antigen test. Reactivity for both the HIV-1/2 immunoassay and the HIV p24-specific antigen test confirms the diagnosis of HIV infection. However, if HIV p24-specific antigen testing is indeterminate or an acute HIV infection is suspected, an HIV nucleic acid test (NAT) should be performed.7,8
Upon a positive diagnosis, a multidisciplinary team approach is recommended to address the mental, social, and physical care of the patient. Team members should include an adult medicine clinician, an infectious disease clinician, an ObGyn, social services staff, and behavioral health support to achieve the goal of obtaining and maintaining the patient’s optimal health status.
TABLE 1 lists the recommended initial laboratory assessments that should follow a new diagnosis of HIV infection. Based on the laboratory results, the indicated vaccinations, antibiotic prophylaxis for opportunistic infections, and optimal combined antiretroviral therapy (cART) can be determined.9 The vaccinations listed in TABLE 2 should be up to date.10,11 Additionally, cervical cancer screening with cytology and human papillomavirus (HPV) testing and treatment should be performed in accordance with the 2019 American Society for Cervical Cancer Prevention (ASCCP) guidelines.12

Promptly initiating cART is of utmost importance; this decreases the rate of HIV transmission via sexual contact and decreases the rate of perinatal transmission.5,13 Results of the initial laboratory assessment, hepatitis B status, and desire for pregnancy/contraception should be considered when initiating cART.3,14,15
It is imperative to discuss sharing the positive diagnostic results with the patient’s partner. The CDC provides guidance for these discussions,16 which should address the use of preexposure prophylaxis (PrEP) if partner screening establishes partner serodiscordance (that is, HIV positivity in one partner and HIV negativity in the other partner). PrEP is a single pill approved by the US Food and Drug Administration (FDA) that combines tenofovir 300 mg and emtricitabine 200 mg daily17 and has been recommended since 2012.18-20 PrEP also should be considered in sexually active individuals who have higher-risk behaviors within an area with high HIV prevalence.18-21 Despite the CDC’s strong recommendations for PrEP use, lack of insurance coverage and high cost are barriers to universal use. The National Alliance of State and Territorial AIDS Directors (NASTAD) provides a list of patient and copayment assistance programs that can be found at the NASTAD website: https://nastad.org/prepcost-resources/prep-assitance-programs.
Continue to: Preconception considerations...
Preconception considerations
In individuals with known HIV infection, preconception consultation with an ObGyn or maternal-fetal medicine (MFM) specialist should be recommended prior to conception.22 Preconception recommendations include addressing optimization of maternal medical comorbidities, addressing routine health screening and vaccinations, performing sexually transmitted infection screening, and optimizing HIV disease status.3,22,23
With the assistance of adult medicine and infectious disease clinicians, a cART regimen that is sufficient to reliably maintain viral suppression (that is, viral load < 50 copies/mL on 2 separate occasions at least 3 months apart) and is safe for use in pregnancy should be established.3 In serodiscordant couples, recommended mechanisms to prevent HIV transmission during conception include sustained viral suppression in the HIV-positive partner, PrEP use in the HIV-negative partner, and timing of unprotected intercourse during peak fertility only.3
Antepartum care
The initial prenatal visit
Women who have no prior screening for HIV or prior negative HIV results should undergo HIV screening at the first prenatal visit.3 Screening should be performed in accordance with the “opt out method.”6 Using this method, a woman without a known diagnosis of HIV infection is told that she will undergo HIV screening as a component of routine prenatal care unless she decides that she does not want this test performed.6,24,25 At the time of screening, all pregnant women should be provided with comprehensive information regarding HIV screening, HIV screening results, and the implications of HIV infection on pregnancy.26
In the pregnant patient with confirmed HIV infection, all preconception considerations should be addressed. If not already in place, referrals to appropriate providers (infectious disease specialist, ObGyn, MFM specialist) and ancillary support staff (social services, behavioral health support) should be arranged. All efforts should be implemented to optimize additional medical comorbidities. TABLE 3 lists additional prenatal testing requirements.22
Antiretroviral therapy should be assessed for safety and efficacy in pregnancy and should comply with the CDC recommendations for cART in pregnancy.3 Patients with a T-lymphocyte cell count of less than 200 cells/mm3 and/or a viral load greater than 50 copies/mL despite adherent cART use should be referred to an infectious disease specialist to determine the need for alternative cART and/or the need for chemoprophylaxis against opportunistic infections.23
First and second trimester
Antiretroviral adherence and barriers to adherence should be addressed at every prenatal visit. If the patient is started on antiretroviral therapy in pregnancy or is switched to an alternative cART regimen, viral load assessment should be performed 2 to 4 weeks after the start or change in cART and then repeated monthly until undetectable levels are achieved.3,26 If an undetectable viral load cannot be obtained, cART adherence should be thoroughly evaluated, and the patient should be referred to an infectious disease or HIV treatment specialist.26
If the initial prenatal testing indicates an undetectable viral load, repeat viral load assessment can be performed every 3 months throughout the pregnancy.3 If initial prenatal testing indicates an undetectable HIV viral load and the T-lymphocyte count is greater than 200 cells/mm3, repeat viral load testing can be performed every 6 months to ensure stability.3
Early screening for gestational diabetes should be performed in patients receiving protease inhibitors because these agents may interfere with carbohydrate tolerance.22,26
Continue to: Third trimester...
Third trimester
Women with negative HIV screening at the initial prenatal evaluation should undergo repeat HIV screening in the third trimester if they are at high risk for HIV exposure.25 Factors that determine high-risk status are listed in TABLE 4.27 Sexually transmitted infection screening should be repeated in the third trimester.26

Repeat assessment of the viral load should be completed between 34 and 36 weeks’ gestation or sooner if additional indications for early term or late preterm delivery arise.3 Viral load assessments aid in determining delivery timing and route and the need for zidovudine (ZDV) treatment (FIGURE).

Studies that were performed prior to standardized cART use found higher rates of perinatal transmission associated with vaginal delivery when compared with cesarean delivery (CD).28-30 However, these studies did not account for measures of viral load within their study populations.28-30
In more recent studies performed in the era of standardized cART and viral load monitoring, CD does not provide protection from perinatal transmission when the maternal viral load is less than 1,000 copies/mL at the time of delivery.31 Similarly, delivery prior to 40 weeks’ gestation does not confer protection from perinatal transmission.32
Alternatively, if the maternal viral load is 1,000 copies/mL or greater, CD should be considered to reduce the risk of perinatal transmission. A scheduled, elective CD at 38 weeks’ gestation is recommended in those with a maternal viral load of 1,000 copies/mL or greater and no medical indication for earlier delivery in order to decrease the likelihood of labor onset and/or rupture of membranes prior to delivery.3,33
Intrapartum care
Rapid antigen testing (with follow-up confirmatory testing as indicated) is recommended in patients presenting to labor and delivery with no prior documentation of HIV status.3,8,26
Despite a significant decrease in perinatal transmission rates over the last 30 years, a large proportion of perinatal transmission cases are thought to result from intrapartum fetal exposure. While the mechanism of transmission is not known, a correlation between maternal viral load and risk for perinatal transmission has been shown. A maternal viral load of less than 1,000 copies/mL has been associated with a perinatal transmission risk of less than 2%.34,35 A maternal viral load between 50 and 999 copies/mL has been associated with a perinatal transmission rate of 1% to 2% compared with less than 1% for a maternal viral load of less than 50 copies/mL or undetectable measures.5,36,37
These differences in perinatal transmission rates have prompted the recommendation for administration of ZDV for a minimum of 3 hours prior to delivery in mothers with a viral load of 1,000 copies/mL or greater.4,38 The recommended ZDV dosing is: a 1-hour intravenous loading dose of 2 mg/kg followed by continuous infusion of 1 mg/kg per hour until delivery.39,40 Patients who opt for vaginal delivery despite nonsuppressed viral loads (≥1,000 copies/mL) after thorough perinatal counseling should receive ZDV at the start of labor through delivery.3 All patients should be continued on cART throughout their intrapartum and postpartum course.
The duration of membrane rupture and the use of invasive fetal monitoring (that is, fetal scalp electrodes) have been assessed as mechanisms of perinatal transmission. Although they were performed prior to the standardized use of cART, several studies demonstrated that increased perinatal transmission rates were associated with invasive fetal monitoring.34,41,42 While limited data have refuted this finding in women with suppressed viral loads (< 50 copies/mL), the American College of Obstetricians and Gynecologists recommends avoiding the use of invasive fetal monitoring in labor.26
Pre-cART studies demonstrated increased rates of perinatal transmission with longer durations of membrane rupture prior to delivery.43,44 More recent studies have reevaluated this association and determined that the increased perinatal transmission rates are more likely associated with higher maternal viral loads at the time of delivery rather than duration of membrane rupture.45-47 No clear evidence describes when or if CD after the onset of labor or rupture of membranes provides protection from perinatal HIV transmission in pregnant women with HIV receiving no antiretroviral drugs or only ZDV during labor.43,48 CD can be considered for patients in whom scheduled, pre-labor CD was planned who present in labor or with rupture of membranes prior to scheduled CD.26 These, and additional intrapartum considerations, are listed in TABLE 5.49,50
Appropriate personal protective equipment should be available and donned for all providers present throughout intrapartum management and at delivery.23,26 Should any provider injury occur, immediate cleansing of the injury site should be performed, followed by referral to proper workplace supervisors for additional laboratory testing and antiretroviral prophylaxis.

Continue to: Postpartum care...
Postpartum care
Postpartum contraception should be offered and provided in accordance with patient request. Regardless of other birth control methods, strict condom use should be advised. PrEP should be discussed and offered for all partners of serodiscordant couples.
Upon outpatient follow-up, assessment and provision of routine health maintenance should be performed. Any abnormal cervical pathology encountered during prenatal care should be managed in accordance with ASCCP guidelines.12 Follow-up care should be established with adult medicine, infectious disease, and ObGyn clinicians.26
Neonatal considerations
Neonates born to mothers with positive or unknown HIV status should undergo expedited HIV testing.51,52 Consultation should be conducted with pediatric or neonatology colleagues to determine the antiretroviral regimen and duration of therapy based on presumed HIV status of the neonate. Ideally, antiretroviral therapy should be initiated within 6 hours of delivery.3,53
Formula feeding should be implemented as maternal HIV infection is one of the few contraindications to breastfeeding.54,55 The risk of late breast milk transmission, defined as postnatal transmission that occurs after 1 month of age, may vary based on maternal viral load, but it has been reported as high as 8.9 transmissions per 100 person-years of breastfeeding.56
Resources available
Care of the pregnant patient with HIV and the reduction of perinatal transmission both depend on early diagnosis of HIV and effective treatment with cART. Such patients benefit from a team-based care model that includes the ObGyn and/or MFM specialist, infectious disease clinician, pediatrician, and social worker. As guidelines evolve for care of these patients, a reference checklist, such as the examples provided at the Society for Maternal-Fetal Medicine website (smfm.org) or at HIV.gov, provide an outline for:
- management before, during, and after pregnancy
- suggestions for management teams of interest to successfully carry out the checklist requirements
- proposals for measurements of quality performance with the use of checklists in the management of HIV in pregnancy.
In addition, assistance with clinical decision making for patients with HIV in pregnancy can be obtained via telephone consultation with the National Clinician Consultation Center–Perinatal HIV/AIDS (888-448-8765), which is available 24 hours a day, 7 days a week. ●
Human immunodeficiency virus (HIV) is a single-stranded enveloped RNA retrovirus that was first described in the 1980s and is known for its severity of systemic immune dysregulation and associated opportunistic infections. It is transmitted through contact with blood or bodily fluids, and it can be transmitted vertically, most often at the time of delivery. Since the advent of antiretroviral therapy, the average life expectancy and natural course of HIV infection has improved notably.1
In 2019, just over 1 million adults and adolescents in the United States were living with the diagnosis of HIV.2 In the same year, the rate of new HIV diagnoses in the United States had stabilized at a rate of 13.2 new cases per 100,000 individuals.2 Among this cohort, individuals identifying as females at birth accounted for 19% of the total population living with HIV.2 Sexual contact was the most common route of transmission, followed by injection drug use—77% and 20%, respectively.2
It is important to note that the incidence and prevalence of HIV does not reflect the individuals who unknowingly are living with the disease. The disease burden associated with HIV infection and the availability of effective treatment modalities has led to the recommendation that all individuals undergo HIV screening at least once in their lifetime.3 Early identification of HIV infection is important to optimize the health of all individuals and future generations.
The interplay between high-risk sexual practices and the risk for HIV exposure and unintended pregnancy places the ObGyn at the forefront of HIV prevention and identification. Early diagnosis and standardized treatment with antiretroviral therapies have led to both a dramatic improvement in adult disease burden and a dramatic decrease in perinatal transmission.4,5 In 2019, perinatal transmission accounted for less than 1% of HIV transmission in the United States.2 This is a decrease of greater than 54% from 2014, which, again, emphasizes the role of the ObGyn in HIV management.6
Preconception care: Gynecologic screening, diagnosis, and management
The Centers for Disease Control and Prevention (CDC) recommends that an individual undergo HIV screening at least once in their lifetime.3 HIV screening algorithms have changed over the last 20 years to reduce the number of false-positive and/or false-negative results obtained through HIV antibody testing alone.7 HIV-1/2 antibody/antigen immunoassay is recommended as the initial screening test. If reactive, this should be followed by an HIV p24-specific antigen test. Reactivity for both the HIV-1/2 immunoassay and the HIV p24-specific antigen test confirms the diagnosis of HIV infection. However, if HIV p24-specific antigen testing is indeterminate or an acute HIV infection is suspected, an HIV nucleic acid test (NAT) should be performed.7,8
Upon a positive diagnosis, a multidisciplinary team approach is recommended to address the mental, social, and physical care of the patient. Team members should include an adult medicine clinician, an infectious disease clinician, an ObGyn, social services staff, and behavioral health support to achieve the goal of obtaining and maintaining the patient’s optimal health status.
TABLE 1 lists the recommended initial laboratory assessments that should follow a new diagnosis of HIV infection. Based on the laboratory results, the indicated vaccinations, antibiotic prophylaxis for opportunistic infections, and optimal combined antiretroviral therapy (cART) can be determined.9 The vaccinations listed in TABLE 2 should be up to date.10,11 Additionally, cervical cancer screening with cytology and human papillomavirus (HPV) testing and treatment should be performed in accordance with the 2019 American Society for Cervical Cancer Prevention (ASCCP) guidelines.12

Promptly initiating cART is of utmost importance; this decreases the rate of HIV transmission via sexual contact and decreases the rate of perinatal transmission.5,13 Results of the initial laboratory assessment, hepatitis B status, and desire for pregnancy/contraception should be considered when initiating cART.3,14,15
It is imperative to discuss sharing the positive diagnostic results with the patient’s partner. The CDC provides guidance for these discussions,16 which should address the use of preexposure prophylaxis (PrEP) if partner screening establishes partner serodiscordance (that is, HIV positivity in one partner and HIV negativity in the other partner). PrEP is a single pill approved by the US Food and Drug Administration (FDA) that combines tenofovir 300 mg and emtricitabine 200 mg daily17 and has been recommended since 2012.18-20 PrEP also should be considered in sexually active individuals who have higher-risk behaviors within an area with high HIV prevalence.18-21 Despite the CDC’s strong recommendations for PrEP use, lack of insurance coverage and high cost are barriers to universal use. The National Alliance of State and Territorial AIDS Directors (NASTAD) provides a list of patient and copayment assistance programs that can be found at the NASTAD website: https://nastad.org/prepcost-resources/prep-assitance-programs.
Continue to: Preconception considerations...
Preconception considerations
In individuals with known HIV infection, preconception consultation with an ObGyn or maternal-fetal medicine (MFM) specialist should be recommended prior to conception.22 Preconception recommendations include addressing optimization of maternal medical comorbidities, addressing routine health screening and vaccinations, performing sexually transmitted infection screening, and optimizing HIV disease status.3,22,23
With the assistance of adult medicine and infectious disease clinicians, a cART regimen that is sufficient to reliably maintain viral suppression (that is, viral load < 50 copies/mL on 2 separate occasions at least 3 months apart) and is safe for use in pregnancy should be established.3 In serodiscordant couples, recommended mechanisms to prevent HIV transmission during conception include sustained viral suppression in the HIV-positive partner, PrEP use in the HIV-negative partner, and timing of unprotected intercourse during peak fertility only.3
Antepartum care
The initial prenatal visit
Women who have no prior screening for HIV or prior negative HIV results should undergo HIV screening at the first prenatal visit.3 Screening should be performed in accordance with the “opt out method.”6 Using this method, a woman without a known diagnosis of HIV infection is told that she will undergo HIV screening as a component of routine prenatal care unless she decides that she does not want this test performed.6,24,25 At the time of screening, all pregnant women should be provided with comprehensive information regarding HIV screening, HIV screening results, and the implications of HIV infection on pregnancy.26
In the pregnant patient with confirmed HIV infection, all preconception considerations should be addressed. If not already in place, referrals to appropriate providers (infectious disease specialist, ObGyn, MFM specialist) and ancillary support staff (social services, behavioral health support) should be arranged. All efforts should be implemented to optimize additional medical comorbidities. TABLE 3 lists additional prenatal testing requirements.22
Antiretroviral therapy should be assessed for safety and efficacy in pregnancy and should comply with the CDC recommendations for cART in pregnancy.3 Patients with a T-lymphocyte cell count of less than 200 cells/mm3 and/or a viral load greater than 50 copies/mL despite adherent cART use should be referred to an infectious disease specialist to determine the need for alternative cART and/or the need for chemoprophylaxis against opportunistic infections.23
First and second trimester
Antiretroviral adherence and barriers to adherence should be addressed at every prenatal visit. If the patient is started on antiretroviral therapy in pregnancy or is switched to an alternative cART regimen, viral load assessment should be performed 2 to 4 weeks after the start or change in cART and then repeated monthly until undetectable levels are achieved.3,26 If an undetectable viral load cannot be obtained, cART adherence should be thoroughly evaluated, and the patient should be referred to an infectious disease or HIV treatment specialist.26
If the initial prenatal testing indicates an undetectable viral load, repeat viral load assessment can be performed every 3 months throughout the pregnancy.3 If initial prenatal testing indicates an undetectable HIV viral load and the T-lymphocyte count is greater than 200 cells/mm3, repeat viral load testing can be performed every 6 months to ensure stability.3
Early screening for gestational diabetes should be performed in patients receiving protease inhibitors because these agents may interfere with carbohydrate tolerance.22,26
Continue to: Third trimester...
Third trimester
Women with negative HIV screening at the initial prenatal evaluation should undergo repeat HIV screening in the third trimester if they are at high risk for HIV exposure.25 Factors that determine high-risk status are listed in TABLE 4.27 Sexually transmitted infection screening should be repeated in the third trimester.26

Repeat assessment of the viral load should be completed between 34 and 36 weeks’ gestation or sooner if additional indications for early term or late preterm delivery arise.3 Viral load assessments aid in determining delivery timing and route and the need for zidovudine (ZDV) treatment (FIGURE).

Studies that were performed prior to standardized cART use found higher rates of perinatal transmission associated with vaginal delivery when compared with cesarean delivery (CD).28-30 However, these studies did not account for measures of viral load within their study populations.28-30
In more recent studies performed in the era of standardized cART and viral load monitoring, CD does not provide protection from perinatal transmission when the maternal viral load is less than 1,000 copies/mL at the time of delivery.31 Similarly, delivery prior to 40 weeks’ gestation does not confer protection from perinatal transmission.32
Alternatively, if the maternal viral load is 1,000 copies/mL or greater, CD should be considered to reduce the risk of perinatal transmission. A scheduled, elective CD at 38 weeks’ gestation is recommended in those with a maternal viral load of 1,000 copies/mL or greater and no medical indication for earlier delivery in order to decrease the likelihood of labor onset and/or rupture of membranes prior to delivery.3,33
Intrapartum care
Rapid antigen testing (with follow-up confirmatory testing as indicated) is recommended in patients presenting to labor and delivery with no prior documentation of HIV status.3,8,26
Despite a significant decrease in perinatal transmission rates over the last 30 years, a large proportion of perinatal transmission cases are thought to result from intrapartum fetal exposure. While the mechanism of transmission is not known, a correlation between maternal viral load and risk for perinatal transmission has been shown. A maternal viral load of less than 1,000 copies/mL has been associated with a perinatal transmission risk of less than 2%.34,35 A maternal viral load between 50 and 999 copies/mL has been associated with a perinatal transmission rate of 1% to 2% compared with less than 1% for a maternal viral load of less than 50 copies/mL or undetectable measures.5,36,37
These differences in perinatal transmission rates have prompted the recommendation for administration of ZDV for a minimum of 3 hours prior to delivery in mothers with a viral load of 1,000 copies/mL or greater.4,38 The recommended ZDV dosing is: a 1-hour intravenous loading dose of 2 mg/kg followed by continuous infusion of 1 mg/kg per hour until delivery.39,40 Patients who opt for vaginal delivery despite nonsuppressed viral loads (≥1,000 copies/mL) after thorough perinatal counseling should receive ZDV at the start of labor through delivery.3 All patients should be continued on cART throughout their intrapartum and postpartum course.
The duration of membrane rupture and the use of invasive fetal monitoring (that is, fetal scalp electrodes) have been assessed as mechanisms of perinatal transmission. Although they were performed prior to the standardized use of cART, several studies demonstrated that increased perinatal transmission rates were associated with invasive fetal monitoring.34,41,42 While limited data have refuted this finding in women with suppressed viral loads (< 50 copies/mL), the American College of Obstetricians and Gynecologists recommends avoiding the use of invasive fetal monitoring in labor.26
Pre-cART studies demonstrated increased rates of perinatal transmission with longer durations of membrane rupture prior to delivery.43,44 More recent studies have reevaluated this association and determined that the increased perinatal transmission rates are more likely associated with higher maternal viral loads at the time of delivery rather than duration of membrane rupture.45-47 No clear evidence describes when or if CD after the onset of labor or rupture of membranes provides protection from perinatal HIV transmission in pregnant women with HIV receiving no antiretroviral drugs or only ZDV during labor.43,48 CD can be considered for patients in whom scheduled, pre-labor CD was planned who present in labor or with rupture of membranes prior to scheduled CD.26 These, and additional intrapartum considerations, are listed in TABLE 5.49,50
Appropriate personal protective equipment should be available and donned for all providers present throughout intrapartum management and at delivery.23,26 Should any provider injury occur, immediate cleansing of the injury site should be performed, followed by referral to proper workplace supervisors for additional laboratory testing and antiretroviral prophylaxis.

Continue to: Postpartum care...
Postpartum care
Postpartum contraception should be offered and provided in accordance with patient request. Regardless of other birth control methods, strict condom use should be advised. PrEP should be discussed and offered for all partners of serodiscordant couples.
Upon outpatient follow-up, assessment and provision of routine health maintenance should be performed. Any abnormal cervical pathology encountered during prenatal care should be managed in accordance with ASCCP guidelines.12 Follow-up care should be established with adult medicine, infectious disease, and ObGyn clinicians.26
Neonatal considerations
Neonates born to mothers with positive or unknown HIV status should undergo expedited HIV testing.51,52 Consultation should be conducted with pediatric or neonatology colleagues to determine the antiretroviral regimen and duration of therapy based on presumed HIV status of the neonate. Ideally, antiretroviral therapy should be initiated within 6 hours of delivery.3,53
Formula feeding should be implemented as maternal HIV infection is one of the few contraindications to breastfeeding.54,55 The risk of late breast milk transmission, defined as postnatal transmission that occurs after 1 month of age, may vary based on maternal viral load, but it has been reported as high as 8.9 transmissions per 100 person-years of breastfeeding.56
Resources available
Care of the pregnant patient with HIV and the reduction of perinatal transmission both depend on early diagnosis of HIV and effective treatment with cART. Such patients benefit from a team-based care model that includes the ObGyn and/or MFM specialist, infectious disease clinician, pediatrician, and social worker. As guidelines evolve for care of these patients, a reference checklist, such as the examples provided at the Society for Maternal-Fetal Medicine website (smfm.org) or at HIV.gov, provide an outline for:
- management before, during, and after pregnancy
- suggestions for management teams of interest to successfully carry out the checklist requirements
- proposals for measurements of quality performance with the use of checklists in the management of HIV in pregnancy.
In addition, assistance with clinical decision making for patients with HIV in pregnancy can be obtained via telephone consultation with the National Clinician Consultation Center–Perinatal HIV/AIDS (888-448-8765), which is available 24 hours a day, 7 days a week. ●
- Samji H, Cescon A, Hogg RS, et al; North American AIDS Cohort Collaboration on Research and Design (NA-ACCORD) of IeDEA. Closing the gap: increases in life expectancy among treated HIV-positive individuals in the United States and Canada. PLoS One. 2013;8:e81355. doi: 10.1371/journal. pone.0081355.
- Centers for Disease Control and Prevention. May 1, 2021. HIV Surveillance Report, 2019, vol. 32: Diagnosis of HIV infection in the United States and dependent areas, 2019. Accessed February 15, 2022. http://www.cdc.gov/hiv/library/reports /hiv-surveillance.html
- Panel on Antiretroviral Therapy and Medical Management of Children Living with HIV. Guidelines for the use of antiretroviral agents in pediatric HIV infection. https: //clinicalinfo.hiv.gov/en/guidelines/pediatric-arv. Accessed February 15, 2022.
- Connor EM, Sperling RS, Gelber R, et al. Reduction of maternal-infant transmission of human immunodeficiency virus type 1 with zidovudine treatment. Pediatric AIDS Clinical Trials Group Protocol 076 Study Group. N Engl J Med. 1994;331:1173-1180.
- Townsend CL, Byrne L, Cortina-Borja M, et al. Earlier initiation of ART and further decline in mother-to-child HIV transmission rates, 2000-2011. AIDS. 2014;28:1049-1057.
- Centers for Disease Control and Prevention. January 26, 2022. HIV and pregnant women, infants, and children. Accessed February 15, 2022. https://www.cdc.gov/hiv/group/gender /pregnantwomen/index.html
- Centers for Disease Control and Prevention. 2018 Quick reference guide: Recommended laboratory HIV testing algorithm for serum or plasma specimens. National Center for HIV/AIDS, Viral Hepatitis, and TB Prevention (US); Division of HIV/AIDS Prevention; Association of Public Health Laboratories. Updated January 2018. https://stacks. cdc.gov/view/cdc/50872
- Centers for Disease Control and Prevention, Association of Public Health Laboratories. June 27, 2014. Laboratory testing for the diagnosis of HIV infection: updated recommendations. Accessed February 15, 2022. http://stacks.cdc.gov/view /cdc/23447
- Guidelines for the prevention and treatment of opportunistic infections in adults and adolescents with HIV. Updated April 12, 2022. Accessed July 6, 2022. https://clinicalinfo.hiv .gov/en/guidelines/adult-and-adolescent-opportunistic -infection/whats-new-guidelines
- Rubin LG, Levin MJ, Ljungman P, et al. 2013 IDSA clinical practice guideline for vaccination of the immunocompromised host. Clin Infect Dis. 2014;58: e44–e100. doi: 10.1093/cid/cit684.
- Centers for Disease Control and Prevention. ACIP: Guidance for vaccine recommendations for pregnant and breastfeeding women. Accessed July 5, 2022. https://www.cdc.gov /vaccines/hcp/acip-recs/rec-vac-preg.html?CDC_AA _refVal=https%3A%2F%2Fwww.cdc.gov%2Fvaccines%2Facip %2Fcommittee%2Fguidance%2Frec-vac-preg.html
- Perkins RB, Guido RS, Castle PE, et al; for the 2019 ASCCP Risk-Based Management Consensus Guidelines Committee. 2019 ASCCP risk-based management consensus guidelines for abnormal cervical cancer screening tests and cancer precursors. J Low Genit Tract Dis. 2020;24:102-131. doi: 10.1097/LGT.0000000000000525. Erratum in: J Low Genit Tract Dis. 2020;24:427.
- Cohen MS, Chen YQ, McCauley M, et al; HPTN 052 Study Team. Prevention of HIV-1 infection with early antiretroviral therapy. N Engl J Med. 2011;365:493-505.
- Drug interactions between antiretroviral agents and hormonal contraceptives. Accessed July 6, 2022. https://clinicalinfo .hiv.gov/en/table/table-3-drug-interactions-between -antiretroviral-agents-and-hormonal-contraceptives
- Panel on Treatment of HIV During Pregnancy and Prevention of Perinatal Transmission. Recommendations for use of antiretroviral drugs in pregnancy and interventions to reduce perinatal HIV transmission in the United States. Accessed July 7, 2022. https://clinicalinfo.hiv.gov/en/guidelines/perinatal /whats-new-guidelines
- Centers for Disease Control and Prevention. Recommendations for partner services programs for HIV infection, syphilis, gonorrhea, and chlamydial infection. MMWR Recomm Rep. 2008;57(RR-9):1–83.
- Gilead Sciences, Inc. Truvada (emtricitabine 200 mg/ tenofovir disoproxil fumarate 300 mg tablets). Accessed July 6, 2022. https://truvada.com
- Centers for Disease Control and Prevention. Interim guidance for clinicians considering the use of preexposure prophylaxis for the prevention of HIV infection in heterosexually active adults. MMWR Morb Mortal Wkly Rep. 2012;61:586-589.
- Baeten JM, Donnell D, Ndase P, et al; Partners PrEP Study Team. Antiretroviral prophylaxis for HIV prevention in heterosexual men and women. N Engl J Med. 2012;367: 399-410.
- Celum C, Baeten JM. Antiretroviral-based HIV-1 prevention: antiretroviral treatment and pre-exposure prophylaxis. Antivir Ther. 2012;17:1483-1493.
- Thigpen MC, Kebaabetswe PM, Paxton LA, et al; TDF2 Study Group. Antiretroviral preexposure prophylaxis for heterosexual HIV transmission in Botswana. N Engl J Med. 2012;367:423-434.
- Society for Maternal-Fetal Medicine. Special statement: updated checklists for pregnancy management in persons with HIV. Accessed July 5, 2022. https://www.smfm.org /publications/334-smfm-special-statement-updated -checklists-for-pregnancy-management-in-persons-with-hiv
- American College of Obstetricians and Gynecologists. ACOG committee opinion no. 752. Prenatal and perinatal human immunodeficiency virus testing. Obstet Gynecol. 2018;132:e138-e142.
- Human immunodeficiency virus screening. Joint statement of the American Academy of Pediatrics and the American College of Obstetricians and Gynecologists. Pediatrics. 1999;104(1 pt 1):128.
- Branson BM, Handsfield HH, Lampe MA, et al; Centers for Disease Control and Prevention. Revised recommendations for HIV testing of adults, adolescents, and pregnant women in health care settings. MMWR Recomm Rep. 2006; 55(RR-14):1-17.
- American College of Obstetricians and Gynecologists. ACOG committee opinion no. 751. Labor and delivery management of women with human immunodeficiency virus infection. Obstet Gynecol. 2018;132:e131-e137.
- Centers for Disease Control and Prevention. Factors increasing the risk of acquiring or transmitting HIV. November 12, 2019. Accessed July 29, 2022. https://www.cdc .gov/hiv/risk/estimates/riskfactors.html
- Mandelbrot L, Le Chenadec J, Berrebi A, et al. Perinatal HIV1 transmission: interaction between zidovudine prophylaxis and mode of delivery in the French Perinatal Cohort. JAMA. 1998;280:55-60.
- European Mode of Delivery Collaboration. Elective caesarean-section versus vaginal delivery in prevention of vertical HIV-1 transmission: a randomised clinical trial. Lancet. 1999;353:1035-1039.
- International Perinatal HIV Group; Andiman W, Bryson Y, de Martino M, et al. The mode of delivery and the risk of vertical transmission of human immunodeficiency virus type 1—a meta-analysis of 15 prospective cohort studies. N Engl J Med. 1999;340:977-987.
- Briand N, Jasseron C, Sibiude J, et al. Cesarean section for HIV-infected women in the combination antiretroviral therapies era, 2000–2010. Am J Obstet Gynecol. 2013;209: 335.e1-335.e12.
- Scott RK, Chakhtoura N, Burke MM, et al. Delivery after 40 weeks of gestation in pregnant women with well-controlled human immunodeficiency virus. Obstet Gynecol. 2017;130:502-510.
- American College of Obstetricians and Gynecologists. Committee opinion no. 560. Medically indicated late-preterm and early-term deliveries. Obstet Gynecol. 2013;121:908-910.
- Mofenson LM, Lambert JS, Stiehm ER, et al. Risk factors for perinatal transmission of human immunodeficiency virus type 1 in women treated with zidovudine. Pediatric AIDS Clinical Trials Group Study 185 Team. N Engl J Med. 1999;341:385-393.
- Garcia PM, Kalish LA, Pitt J, et al. Maternal levels of plasma human immunodeficiency virus type 1 RNA and the risk of perinatal transmission. Women and Infants Transmission Study Group. N Engl J Med. 1999;341:394-402.
- Briand N, Warszawski J, Mandelbrot L, et al; ANRS-EPF CO1CO11 Study Group. Is intrapartum intravenous zidovudine for prevention of mother-to-child HIV-1 transmission still useful in the combination antiretroviral therapy era? Clin Infect Dis. 2013;57:903-914.
- Myer L, Phillips TK, McIntyre JA, et al. HIV viraemia and mother-to-child transmission risk after antiretroviral therapy initiation in pregnancy in Cape Town, South Africa. HIV Med. 2017;18:80-88.
- Rodman JH, Flynn PM, Robbins B, et al. Systemic pharmacokinetics and cellular pharmacology of zidovudine in human immunodeficiency virus type 1-infected women and newborn infants. J Infect Dis. 1999;180:1844-1850.
- Wade NA, Birkhead GS, Warren BL, et al. Abbreviated regimens of zidovudine prophylaxis and perinatal transmission of the human immunodeficiency virus. N Engl J Med. 1998;339:1409-1414.
- Nielsen-Saines K, Watts HD, Veloso VS, et al; NICHD HPTN 040/PACTG 1043 Protocol Team. Three postpartum antiretroviral regimens to prevent intrapartum HIV infection. N Engl J Med. 2012;366:2368-2379.
- Mandelbrot L, Mayaux MJ, Bongain A, et al. Obstetric factors and mother-to-child transmission of human immunodeficiency virus type 1: the French perinatal cohorts. SEROGEST French Pediatric HIV Infection Study Group. Am J Obstet Gynecol. 1996;175(3 pt 1):661-667.
- Shapiro DE, Sperling RS, Mandelbrot L, et al. Risk factors for perinatal human immunodeficiency virus transmission in patients receiving zidovudine prophylaxis. Pediatric AIDS Clinical Trials Group protocol 076 Study Group. Obstet Gynecol. 1999;94:897-908.
- International Perinatal HIV Group. Duration of ruptured membranes and vertical transmission of HIV-1: a meta-analysis from 15 prospective cohort studies. AIDS. 2001;15:357-368.
- Nielsen TF, Hokegard KH. Postoperative cesarean section morbidity: a prospective study. Am J Obstet Gynecol. 1983;146:911-916.
- Mark S, Murphy KE, Read S, et al. HIV mother-to-child transmission, mode of delivery, and duration of rupture of membranes: experience in the current era. Infect Dis Obstet Gynecol. 2012;2012:267969.
- Cotter AM, Brookfield KF, Duthely LM, et al. Duration of membrane rupture and risk of perinatal transmission of HIV1 in the era of combination antiretroviral therapy. Am J Obstet Gynecol. 2012;207:482.e1-482.e5.
- Peters H, Byrne L, De Ruiter A, et al. Duration of ruptured membranes and mother-to-child HIV transmission: a prospective population-based surveillance study. BJOG. 2016;123:975-981.
- Jamieson DJ, Read JS, Kourtis AP, et al. Cesarean delivery for HIV-infected women: recommendations and controversies. Am J Obstet Gynecol. 2007;197(3 suppl):S96-S100.
- Cambic CR, Avram MJ, Gupta DK, et al. Effect of ritonavir-induced cytochrome P450 3A4 inhibition on plasma fentanyl concentrations during patient-controlled epidural labor analgesia: a pharmacokinetic simulation. Int J Obstet Anesth. 2014;23:45-51.
- Navarro J, Curran A, Burgos J, et al. Acute leg ischaemia in an HIV-infected patient receiving antiretroviral treatment. Antivir Ther. 2017;22:89-90.
- American Academy of Pediatrics, American College of Obstetricians and Gynecologists. Guidelines for Perinatal Care. 8th ed. American Academy of Pediatrics, American College of Obstetricians and Gynecologists; 2017.
- Siberry GK, Abzug MJ, Nachman S, et al; Panel on Opportunistic Infections in HIV-Exposed and HIV-Infected Children. Guidelines for the prevention and treatment of opportunistic infections in HIV-exposed and HIV-infected children: recommendations from the National Institutes of Health, Centers for Disease Control and Prevention, the HIV Medicine Association of the Infectious Diseases Society of America, the Pediatric Infectious Diseases Society, and the American Academy of Pediatrics. Pediatr Infect Dis J. 32(suppl 2[0 2]):i–KK4.
- Panel on Antiretroviral Therapy and Medical Management of Children Living with HIV. Guidelines for the use of antiretroviral agents in pediatric HIV infection. Accessed February 15, 2022. https://clinicalinfo.hiv.gov/en/guidelines /pediatric-arv
- Committee on Health Care for Underserved Women, American College of Obstetricians and Gynecologists. ACOG committee opinion no. 361. Breastfeeding: maternal and infant aspects. Obstet Gynecol. 2007;109(2 pt 1):479-480.
- Committee on Pediatric AIDS; Mofenson LM, Flynn PM, Aldrovandi GM, et al. Infant feeding and transmission of human immunodeficiency virus in the United States. Pediatrics. 2013;131:391-396.
- Breastfeeding and HIV International Transmission Study Group; Coutsoudis A, Dabis F, Fawzi W, et al. Late postnatal transmission of HIV-1 in breast-fed children: an individual patient data meta-analysis. J Infect Dis. 2004;189:2154-2166.
- Samji H, Cescon A, Hogg RS, et al; North American AIDS Cohort Collaboration on Research and Design (NA-ACCORD) of IeDEA. Closing the gap: increases in life expectancy among treated HIV-positive individuals in the United States and Canada. PLoS One. 2013;8:e81355. doi: 10.1371/journal. pone.0081355.
- Centers for Disease Control and Prevention. May 1, 2021. HIV Surveillance Report, 2019, vol. 32: Diagnosis of HIV infection in the United States and dependent areas, 2019. Accessed February 15, 2022. http://www.cdc.gov/hiv/library/reports /hiv-surveillance.html
- Panel on Antiretroviral Therapy and Medical Management of Children Living with HIV. Guidelines for the use of antiretroviral agents in pediatric HIV infection. https: //clinicalinfo.hiv.gov/en/guidelines/pediatric-arv. Accessed February 15, 2022.
- Connor EM, Sperling RS, Gelber R, et al. Reduction of maternal-infant transmission of human immunodeficiency virus type 1 with zidovudine treatment. Pediatric AIDS Clinical Trials Group Protocol 076 Study Group. N Engl J Med. 1994;331:1173-1180.
- Townsend CL, Byrne L, Cortina-Borja M, et al. Earlier initiation of ART and further decline in mother-to-child HIV transmission rates, 2000-2011. AIDS. 2014;28:1049-1057.
- Centers for Disease Control and Prevention. January 26, 2022. HIV and pregnant women, infants, and children. Accessed February 15, 2022. https://www.cdc.gov/hiv/group/gender /pregnantwomen/index.html
- Centers for Disease Control and Prevention. 2018 Quick reference guide: Recommended laboratory HIV testing algorithm for serum or plasma specimens. National Center for HIV/AIDS, Viral Hepatitis, and TB Prevention (US); Division of HIV/AIDS Prevention; Association of Public Health Laboratories. Updated January 2018. https://stacks. cdc.gov/view/cdc/50872
- Centers for Disease Control and Prevention, Association of Public Health Laboratories. June 27, 2014. Laboratory testing for the diagnosis of HIV infection: updated recommendations. Accessed February 15, 2022. http://stacks.cdc.gov/view /cdc/23447
- Guidelines for the prevention and treatment of opportunistic infections in adults and adolescents with HIV. Updated April 12, 2022. Accessed July 6, 2022. https://clinicalinfo.hiv .gov/en/guidelines/adult-and-adolescent-opportunistic -infection/whats-new-guidelines
- Rubin LG, Levin MJ, Ljungman P, et al. 2013 IDSA clinical practice guideline for vaccination of the immunocompromised host. Clin Infect Dis. 2014;58: e44–e100. doi: 10.1093/cid/cit684.
- Centers for Disease Control and Prevention. ACIP: Guidance for vaccine recommendations for pregnant and breastfeeding women. Accessed July 5, 2022. https://www.cdc.gov /vaccines/hcp/acip-recs/rec-vac-preg.html?CDC_AA _refVal=https%3A%2F%2Fwww.cdc.gov%2Fvaccines%2Facip %2Fcommittee%2Fguidance%2Frec-vac-preg.html
- Perkins RB, Guido RS, Castle PE, et al; for the 2019 ASCCP Risk-Based Management Consensus Guidelines Committee. 2019 ASCCP risk-based management consensus guidelines for abnormal cervical cancer screening tests and cancer precursors. J Low Genit Tract Dis. 2020;24:102-131. doi: 10.1097/LGT.0000000000000525. Erratum in: J Low Genit Tract Dis. 2020;24:427.
- Cohen MS, Chen YQ, McCauley M, et al; HPTN 052 Study Team. Prevention of HIV-1 infection with early antiretroviral therapy. N Engl J Med. 2011;365:493-505.
- Drug interactions between antiretroviral agents and hormonal contraceptives. Accessed July 6, 2022. https://clinicalinfo .hiv.gov/en/table/table-3-drug-interactions-between -antiretroviral-agents-and-hormonal-contraceptives
- Panel on Treatment of HIV During Pregnancy and Prevention of Perinatal Transmission. Recommendations for use of antiretroviral drugs in pregnancy and interventions to reduce perinatal HIV transmission in the United States. Accessed July 7, 2022. https://clinicalinfo.hiv.gov/en/guidelines/perinatal /whats-new-guidelines
- Centers for Disease Control and Prevention. Recommendations for partner services programs for HIV infection, syphilis, gonorrhea, and chlamydial infection. MMWR Recomm Rep. 2008;57(RR-9):1–83.
- Gilead Sciences, Inc. Truvada (emtricitabine 200 mg/ tenofovir disoproxil fumarate 300 mg tablets). Accessed July 6, 2022. https://truvada.com
- Centers for Disease Control and Prevention. Interim guidance for clinicians considering the use of preexposure prophylaxis for the prevention of HIV infection in heterosexually active adults. MMWR Morb Mortal Wkly Rep. 2012;61:586-589.
- Baeten JM, Donnell D, Ndase P, et al; Partners PrEP Study Team. Antiretroviral prophylaxis for HIV prevention in heterosexual men and women. N Engl J Med. 2012;367: 399-410.
- Celum C, Baeten JM. Antiretroviral-based HIV-1 prevention: antiretroviral treatment and pre-exposure prophylaxis. Antivir Ther. 2012;17:1483-1493.
- Thigpen MC, Kebaabetswe PM, Paxton LA, et al; TDF2 Study Group. Antiretroviral preexposure prophylaxis for heterosexual HIV transmission in Botswana. N Engl J Med. 2012;367:423-434.
- Society for Maternal-Fetal Medicine. Special statement: updated checklists for pregnancy management in persons with HIV. Accessed July 5, 2022. https://www.smfm.org /publications/334-smfm-special-statement-updated -checklists-for-pregnancy-management-in-persons-with-hiv
- American College of Obstetricians and Gynecologists. ACOG committee opinion no. 752. Prenatal and perinatal human immunodeficiency virus testing. Obstet Gynecol. 2018;132:e138-e142.
- Human immunodeficiency virus screening. Joint statement of the American Academy of Pediatrics and the American College of Obstetricians and Gynecologists. Pediatrics. 1999;104(1 pt 1):128.
- Branson BM, Handsfield HH, Lampe MA, et al; Centers for Disease Control and Prevention. Revised recommendations for HIV testing of adults, adolescents, and pregnant women in health care settings. MMWR Recomm Rep. 2006; 55(RR-14):1-17.
- American College of Obstetricians and Gynecologists. ACOG committee opinion no. 751. Labor and delivery management of women with human immunodeficiency virus infection. Obstet Gynecol. 2018;132:e131-e137.
- Centers for Disease Control and Prevention. Factors increasing the risk of acquiring or transmitting HIV. November 12, 2019. Accessed July 29, 2022. https://www.cdc .gov/hiv/risk/estimates/riskfactors.html
- Mandelbrot L, Le Chenadec J, Berrebi A, et al. Perinatal HIV1 transmission: interaction between zidovudine prophylaxis and mode of delivery in the French Perinatal Cohort. JAMA. 1998;280:55-60.
- European Mode of Delivery Collaboration. Elective caesarean-section versus vaginal delivery in prevention of vertical HIV-1 transmission: a randomised clinical trial. Lancet. 1999;353:1035-1039.
- International Perinatal HIV Group; Andiman W, Bryson Y, de Martino M, et al. The mode of delivery and the risk of vertical transmission of human immunodeficiency virus type 1—a meta-analysis of 15 prospective cohort studies. N Engl J Med. 1999;340:977-987.
- Briand N, Jasseron C, Sibiude J, et al. Cesarean section for HIV-infected women in the combination antiretroviral therapies era, 2000–2010. Am J Obstet Gynecol. 2013;209: 335.e1-335.e12.
- Scott RK, Chakhtoura N, Burke MM, et al. Delivery after 40 weeks of gestation in pregnant women with well-controlled human immunodeficiency virus. Obstet Gynecol. 2017;130:502-510.
- American College of Obstetricians and Gynecologists. Committee opinion no. 560. Medically indicated late-preterm and early-term deliveries. Obstet Gynecol. 2013;121:908-910.
- Mofenson LM, Lambert JS, Stiehm ER, et al. Risk factors for perinatal transmission of human immunodeficiency virus type 1 in women treated with zidovudine. Pediatric AIDS Clinical Trials Group Study 185 Team. N Engl J Med. 1999;341:385-393.
- Garcia PM, Kalish LA, Pitt J, et al. Maternal levels of plasma human immunodeficiency virus type 1 RNA and the risk of perinatal transmission. Women and Infants Transmission Study Group. N Engl J Med. 1999;341:394-402.
- Briand N, Warszawski J, Mandelbrot L, et al; ANRS-EPF CO1CO11 Study Group. Is intrapartum intravenous zidovudine for prevention of mother-to-child HIV-1 transmission still useful in the combination antiretroviral therapy era? Clin Infect Dis. 2013;57:903-914.
- Myer L, Phillips TK, McIntyre JA, et al. HIV viraemia and mother-to-child transmission risk after antiretroviral therapy initiation in pregnancy in Cape Town, South Africa. HIV Med. 2017;18:80-88.
- Rodman JH, Flynn PM, Robbins B, et al. Systemic pharmacokinetics and cellular pharmacology of zidovudine in human immunodeficiency virus type 1-infected women and newborn infants. J Infect Dis. 1999;180:1844-1850.
- Wade NA, Birkhead GS, Warren BL, et al. Abbreviated regimens of zidovudine prophylaxis and perinatal transmission of the human immunodeficiency virus. N Engl J Med. 1998;339:1409-1414.
- Nielsen-Saines K, Watts HD, Veloso VS, et al; NICHD HPTN 040/PACTG 1043 Protocol Team. Three postpartum antiretroviral regimens to prevent intrapartum HIV infection. N Engl J Med. 2012;366:2368-2379.
- Mandelbrot L, Mayaux MJ, Bongain A, et al. Obstetric factors and mother-to-child transmission of human immunodeficiency virus type 1: the French perinatal cohorts. SEROGEST French Pediatric HIV Infection Study Group. Am J Obstet Gynecol. 1996;175(3 pt 1):661-667.
- Shapiro DE, Sperling RS, Mandelbrot L, et al. Risk factors for perinatal human immunodeficiency virus transmission in patients receiving zidovudine prophylaxis. Pediatric AIDS Clinical Trials Group protocol 076 Study Group. Obstet Gynecol. 1999;94:897-908.
- International Perinatal HIV Group. Duration of ruptured membranes and vertical transmission of HIV-1: a meta-analysis from 15 prospective cohort studies. AIDS. 2001;15:357-368.
- Nielsen TF, Hokegard KH. Postoperative cesarean section morbidity: a prospective study. Am J Obstet Gynecol. 1983;146:911-916.
- Mark S, Murphy KE, Read S, et al. HIV mother-to-child transmission, mode of delivery, and duration of rupture of membranes: experience in the current era. Infect Dis Obstet Gynecol. 2012;2012:267969.
- Cotter AM, Brookfield KF, Duthely LM, et al. Duration of membrane rupture and risk of perinatal transmission of HIV1 in the era of combination antiretroviral therapy. Am J Obstet Gynecol. 2012;207:482.e1-482.e5.
- Peters H, Byrne L, De Ruiter A, et al. Duration of ruptured membranes and mother-to-child HIV transmission: a prospective population-based surveillance study. BJOG. 2016;123:975-981.
- Jamieson DJ, Read JS, Kourtis AP, et al. Cesarean delivery for HIV-infected women: recommendations and controversies. Am J Obstet Gynecol. 2007;197(3 suppl):S96-S100.
- Cambic CR, Avram MJ, Gupta DK, et al. Effect of ritonavir-induced cytochrome P450 3A4 inhibition on plasma fentanyl concentrations during patient-controlled epidural labor analgesia: a pharmacokinetic simulation. Int J Obstet Anesth. 2014;23:45-51.
- Navarro J, Curran A, Burgos J, et al. Acute leg ischaemia in an HIV-infected patient receiving antiretroviral treatment. Antivir Ther. 2017;22:89-90.
- American Academy of Pediatrics, American College of Obstetricians and Gynecologists. Guidelines for Perinatal Care. 8th ed. American Academy of Pediatrics, American College of Obstetricians and Gynecologists; 2017.
- Siberry GK, Abzug MJ, Nachman S, et al; Panel on Opportunistic Infections in HIV-Exposed and HIV-Infected Children. Guidelines for the prevention and treatment of opportunistic infections in HIV-exposed and HIV-infected children: recommendations from the National Institutes of Health, Centers for Disease Control and Prevention, the HIV Medicine Association of the Infectious Diseases Society of America, the Pediatric Infectious Diseases Society, and the American Academy of Pediatrics. Pediatr Infect Dis J. 32(suppl 2[0 2]):i–KK4.
- Panel on Antiretroviral Therapy and Medical Management of Children Living with HIV. Guidelines for the use of antiretroviral agents in pediatric HIV infection. Accessed February 15, 2022. https://clinicalinfo.hiv.gov/en/guidelines /pediatric-arv
- Committee on Health Care for Underserved Women, American College of Obstetricians and Gynecologists. ACOG committee opinion no. 361. Breastfeeding: maternal and infant aspects. Obstet Gynecol. 2007;109(2 pt 1):479-480.
- Committee on Pediatric AIDS; Mofenson LM, Flynn PM, Aldrovandi GM, et al. Infant feeding and transmission of human immunodeficiency virus in the United States. Pediatrics. 2013;131:391-396.
- Breastfeeding and HIV International Transmission Study Group; Coutsoudis A, Dabis F, Fawzi W, et al. Late postnatal transmission of HIV-1 in breast-fed children: an individual patient data meta-analysis. J Infect Dis. 2004;189:2154-2166.
Are single-incision mini-slings the new gold standard for stress urinary incontinence?
Abdel-Fattah M, Cooper D, Davidson T, et al. Single-incision mini-slings for stress urinary incontinence in women. N Engl J Med. 2022;386:1230-1243.
EXPERT COMMENTARY
A joint society position statement by the American Urogynecologic Society and the Society of Urodynamics, Female Pelvic Medicine and Urogenital Reconstruction published in December 2021 declared synthetic midurethral slings, first cleared for use in the United States in the early 1990s, the most extensively studied anti-incontinence operation and the standard of care for the treatment of female stress urinary incontinence.1 Full-length retropubic and transobturator (out-in and in-out) slings have been extensively evaluated for safety and efficacy in well-conducted randomized trials.2 Single-incision mini-slings (SIMS) were first cleared for use in 2006, but they lack the long-term safety and comparative effectiveness data of full-length standard midurethral slings (SMUS).3 Furthermore, several iterations of the mini-slings have come to market but have been withdrawn or modified to allow for adjustability.
The SIMS trial by Abdel-Fattah and colleagues, published recently in the New England Journal of Medicine, is one of the few randomized trials with long-term (3 year) subjective and objective outcome data based on comparison of adjustable single-incision mini-slings versus standard full-length midurethral slings.
Details of the study
The SIMS trial is a noninferiority multicenter randomized controlled trial funded by the National Institute for Health Research at 21 hospitals in the United Kingdom that compared adjustable mini-sling procedures performed under local anesthesia with full-length retrotropubic and transobturator sling procedures performed under general anesthesia. Patients and surgeons were not masked to study group assignment because of the differences in anesthesia, and patients with greater than stage 2 prolapse were excluded from the trial.
The primary outcome was Patient Global Impression of Improvement (PGI-I) based on a 7-point Likert scale, with success defined as very much improved or much improved at 15 months and failure defined as all other responses (improved, same, worse, much worse, and very much worse). A noninferiority margin was set at 10 percentage points at 15 months.
Secondary outcomes and adverse events at 36 months included postoperative pain, return to normal activities, objective success based on a 24-hour pad test weight of less than 8 g, and tape exposure, organ injury, new or worsening urinary urgency, dyspareunia, and need for prolonged catheterization.
A total of 596 women were enrolled in the study, 298 in the mini-sling arm and 298 in the standard midurethral sling arm. Baseline characteristics were similar in both groups with most sling procedures being performed by general consultant gynecologists (>60%) versus subspecialist urogynecologists.
Results. Success at 15 months, based on the PGI-I responses of very much better or much better, was noted in 79.1% of patients in the mini-sling group (212/268) versus 75.6% in the full-length sling group (189/250). The authors deemed mini-slings noninferior to standard full-length slings (adjusted risk difference, 4.6 percentage points; 95% confidence interval [CI], -2.7 to 11.8; P<.001 for noninferiority). Success rates declined but remained similar in both groups at 36 months: 72% in the mini-sling group (177/246) and 66.8% (157/235) in the full-length sling group.
More than 70% of mini-incision slings were Altis (Coloplast) and 22% were Ajust (CR Bard; since withdrawn from the market). The majority of standard midurethral full-length slings were transobturator slings (52.9%) versus retropubic slings (35.6%).
While blood loss, organ injury, and 36-month objective 24-hour pad test did not differ between groups, there were significant differences in other secondary outcomes. Dyspareunia and coital incontinence were more common with mini-slings at 15 and 36 months, reported in 11.7% of the mini-sling group and 4.8% of the full-length group (P<.01). Groin or thigh pain did not differ significantly between groups at 36 months (14.1% in mini-sling and 14.9% in full-length sling group, P = .61). Mesh exposure was noted in 3.3% of those with mini-slings and 1.9% of those with standard midurethral slings. The need for surgical intervention to treat recurrent stress incontinence or mesh removal for voiding dysfunction, pain, or mesh exposure also did not differ between groups (8.7% of the mini-sling group and 4.6% of the midurethral sling group; P = .12).
Study strengths and limitations
The strengths of this pragmatic randomized trial are in the use of clinically important and validated patient-reported subjective and objective outcomes in an adequately powered multisite trial of long duration (36 months). This study is important in demonstrating noninferiority of the mini-sling procedure compared with full-length slings, especially given this trial’s timing when there was a pause or suspension of sling mesh use in the United Kingdom beginning in 2018.
Study limitations include the loss to follow-up with diminished response rate of 87.1% at 15 months and 81.4% at 36 months and the inability to adequately assess for the uncommon outcomes, such as mesh-related complications and groin pain.
Further analysis needed
The high rate of dyspareunia (11.7%) with mini-slings deserves further analysis and consideration of whether or not to implant them in patients who are sexually active. Groin or thigh pain did not differ at 36 months but reported pain coincided with the higher percentage of transobturator slings placed over retropubic slings. Prior randomized trials of transobturator versus retropubic midurethral slings have demonstrated this same phenomenon of increased groin pain with the transobturator approach.2 Furthermore, this study by Abdel-Fattah and colleagues excluded patients with advanced anterior or apical prolapse, but one trial is currently underway in the United States.4
In conclusion, this trial suggests some advantages of single-incision mini-slings—ability to perform the procedure under local anesthesia, less synthetic mesh implantation with theoretically decreased risk of bladder perforation or bowel injury, and potential for easier removal compared with full-length slings. Disadvantages include dyspareunia and mesh exposure, which could be significant trade-offs for patients. ●
In the IDEAL framework for evaluating new surgical innovations, the recommended process begins with an idea, followed by development by a few surgeons in a few patients, then exploration in a feasibility randomized controlled trial, an assessment in larger trials by many surgeons, and long-term follow-up.5 The SIMS trial falls under the assessment tab of the IDEAL framework and represents a much-needed study prior to widespread adoption of single-incision mini-slings. The higher dyspareunia rate in women undergoing single-incision mini-slings deserves further evaluation.
CHERYL B. IGLESIA, MD
- Joint position statement on midurethral slings for stress urinary incontinence. Female Pelvic Med Reconstr Surg. 2021;27:707-710. doi: 10.1097/SPV.0000000000001096.
- Richter HE, Albo ME, Zyczynski HM, et al; Urinary Incontinence Treatment Network. Retropubic versus transobturator midurethral slings for stress incontinence. N Engl J Med. 2010;362:2066-2076.
- Nambiar A, Cody JD, Jeffery ST. Single-incision sling operations for urinary incontinence in women. Cochrane Database Syst Rev. 2014;6:CD008709.
- National Institutes of Health. Retropubic vs single-incision mid-urethral sling for stress urinary incontinence. ClinicalTrials.gov identifier NCT03520114. Accessed July16, 2022. https://www.clinicaltrials.gov/ct2/show/NCT0352011 4?cond=altis+sling&draw=2&rank=6
- McCulloch P, Altman DG, Campbell WB, et al. No surgical innovation without evaluation: the IDEAL recommendations. Lancet. 2009;374:1105-1111.
Abdel-Fattah M, Cooper D, Davidson T, et al. Single-incision mini-slings for stress urinary incontinence in women. N Engl J Med. 2022;386:1230-1243.
EXPERT COMMENTARY
A joint society position statement by the American Urogynecologic Society and the Society of Urodynamics, Female Pelvic Medicine and Urogenital Reconstruction published in December 2021 declared synthetic midurethral slings, first cleared for use in the United States in the early 1990s, the most extensively studied anti-incontinence operation and the standard of care for the treatment of female stress urinary incontinence.1 Full-length retropubic and transobturator (out-in and in-out) slings have been extensively evaluated for safety and efficacy in well-conducted randomized trials.2 Single-incision mini-slings (SIMS) were first cleared for use in 2006, but they lack the long-term safety and comparative effectiveness data of full-length standard midurethral slings (SMUS).3 Furthermore, several iterations of the mini-slings have come to market but have been withdrawn or modified to allow for adjustability.
The SIMS trial by Abdel-Fattah and colleagues, published recently in the New England Journal of Medicine, is one of the few randomized trials with long-term (3 year) subjective and objective outcome data based on comparison of adjustable single-incision mini-slings versus standard full-length midurethral slings.
Details of the study
The SIMS trial is a noninferiority multicenter randomized controlled trial funded by the National Institute for Health Research at 21 hospitals in the United Kingdom that compared adjustable mini-sling procedures performed under local anesthesia with full-length retrotropubic and transobturator sling procedures performed under general anesthesia. Patients and surgeons were not masked to study group assignment because of the differences in anesthesia, and patients with greater than stage 2 prolapse were excluded from the trial.
The primary outcome was Patient Global Impression of Improvement (PGI-I) based on a 7-point Likert scale, with success defined as very much improved or much improved at 15 months and failure defined as all other responses (improved, same, worse, much worse, and very much worse). A noninferiority margin was set at 10 percentage points at 15 months.
Secondary outcomes and adverse events at 36 months included postoperative pain, return to normal activities, objective success based on a 24-hour pad test weight of less than 8 g, and tape exposure, organ injury, new or worsening urinary urgency, dyspareunia, and need for prolonged catheterization.
A total of 596 women were enrolled in the study, 298 in the mini-sling arm and 298 in the standard midurethral sling arm. Baseline characteristics were similar in both groups with most sling procedures being performed by general consultant gynecologists (>60%) versus subspecialist urogynecologists.
Results. Success at 15 months, based on the PGI-I responses of very much better or much better, was noted in 79.1% of patients in the mini-sling group (212/268) versus 75.6% in the full-length sling group (189/250). The authors deemed mini-slings noninferior to standard full-length slings (adjusted risk difference, 4.6 percentage points; 95% confidence interval [CI], -2.7 to 11.8; P<.001 for noninferiority). Success rates declined but remained similar in both groups at 36 months: 72% in the mini-sling group (177/246) and 66.8% (157/235) in the full-length sling group.
More than 70% of mini-incision slings were Altis (Coloplast) and 22% were Ajust (CR Bard; since withdrawn from the market). The majority of standard midurethral full-length slings were transobturator slings (52.9%) versus retropubic slings (35.6%).
While blood loss, organ injury, and 36-month objective 24-hour pad test did not differ between groups, there were significant differences in other secondary outcomes. Dyspareunia and coital incontinence were more common with mini-slings at 15 and 36 months, reported in 11.7% of the mini-sling group and 4.8% of the full-length group (P<.01). Groin or thigh pain did not differ significantly between groups at 36 months (14.1% in mini-sling and 14.9% in full-length sling group, P = .61). Mesh exposure was noted in 3.3% of those with mini-slings and 1.9% of those with standard midurethral slings. The need for surgical intervention to treat recurrent stress incontinence or mesh removal for voiding dysfunction, pain, or mesh exposure also did not differ between groups (8.7% of the mini-sling group and 4.6% of the midurethral sling group; P = .12).
Study strengths and limitations
The strengths of this pragmatic randomized trial are in the use of clinically important and validated patient-reported subjective and objective outcomes in an adequately powered multisite trial of long duration (36 months). This study is important in demonstrating noninferiority of the mini-sling procedure compared with full-length slings, especially given this trial’s timing when there was a pause or suspension of sling mesh use in the United Kingdom beginning in 2018.
Study limitations include the loss to follow-up with diminished response rate of 87.1% at 15 months and 81.4% at 36 months and the inability to adequately assess for the uncommon outcomes, such as mesh-related complications and groin pain.
Further analysis needed
The high rate of dyspareunia (11.7%) with mini-slings deserves further analysis and consideration of whether or not to implant them in patients who are sexually active. Groin or thigh pain did not differ at 36 months but reported pain coincided with the higher percentage of transobturator slings placed over retropubic slings. Prior randomized trials of transobturator versus retropubic midurethral slings have demonstrated this same phenomenon of increased groin pain with the transobturator approach.2 Furthermore, this study by Abdel-Fattah and colleagues excluded patients with advanced anterior or apical prolapse, but one trial is currently underway in the United States.4
In conclusion, this trial suggests some advantages of single-incision mini-slings—ability to perform the procedure under local anesthesia, less synthetic mesh implantation with theoretically decreased risk of bladder perforation or bowel injury, and potential for easier removal compared with full-length slings. Disadvantages include dyspareunia and mesh exposure, which could be significant trade-offs for patients. ●
In the IDEAL framework for evaluating new surgical innovations, the recommended process begins with an idea, followed by development by a few surgeons in a few patients, then exploration in a feasibility randomized controlled trial, an assessment in larger trials by many surgeons, and long-term follow-up.5 The SIMS trial falls under the assessment tab of the IDEAL framework and represents a much-needed study prior to widespread adoption of single-incision mini-slings. The higher dyspareunia rate in women undergoing single-incision mini-slings deserves further evaluation.
CHERYL B. IGLESIA, MD
Abdel-Fattah M, Cooper D, Davidson T, et al. Single-incision mini-slings for stress urinary incontinence in women. N Engl J Med. 2022;386:1230-1243.
EXPERT COMMENTARY
A joint society position statement by the American Urogynecologic Society and the Society of Urodynamics, Female Pelvic Medicine and Urogenital Reconstruction published in December 2021 declared synthetic midurethral slings, first cleared for use in the United States in the early 1990s, the most extensively studied anti-incontinence operation and the standard of care for the treatment of female stress urinary incontinence.1 Full-length retropubic and transobturator (out-in and in-out) slings have been extensively evaluated for safety and efficacy in well-conducted randomized trials.2 Single-incision mini-slings (SIMS) were first cleared for use in 2006, but they lack the long-term safety and comparative effectiveness data of full-length standard midurethral slings (SMUS).3 Furthermore, several iterations of the mini-slings have come to market but have been withdrawn or modified to allow for adjustability.
The SIMS trial by Abdel-Fattah and colleagues, published recently in the New England Journal of Medicine, is one of the few randomized trials with long-term (3 year) subjective and objective outcome data based on comparison of adjustable single-incision mini-slings versus standard full-length midurethral slings.
Details of the study
The SIMS trial is a noninferiority multicenter randomized controlled trial funded by the National Institute for Health Research at 21 hospitals in the United Kingdom that compared adjustable mini-sling procedures performed under local anesthesia with full-length retrotropubic and transobturator sling procedures performed under general anesthesia. Patients and surgeons were not masked to study group assignment because of the differences in anesthesia, and patients with greater than stage 2 prolapse were excluded from the trial.
The primary outcome was Patient Global Impression of Improvement (PGI-I) based on a 7-point Likert scale, with success defined as very much improved or much improved at 15 months and failure defined as all other responses (improved, same, worse, much worse, and very much worse). A noninferiority margin was set at 10 percentage points at 15 months.
Secondary outcomes and adverse events at 36 months included postoperative pain, return to normal activities, objective success based on a 24-hour pad test weight of less than 8 g, and tape exposure, organ injury, new or worsening urinary urgency, dyspareunia, and need for prolonged catheterization.
A total of 596 women were enrolled in the study, 298 in the mini-sling arm and 298 in the standard midurethral sling arm. Baseline characteristics were similar in both groups with most sling procedures being performed by general consultant gynecologists (>60%) versus subspecialist urogynecologists.
Results. Success at 15 months, based on the PGI-I responses of very much better or much better, was noted in 79.1% of patients in the mini-sling group (212/268) versus 75.6% in the full-length sling group (189/250). The authors deemed mini-slings noninferior to standard full-length slings (adjusted risk difference, 4.6 percentage points; 95% confidence interval [CI], -2.7 to 11.8; P<.001 for noninferiority). Success rates declined but remained similar in both groups at 36 months: 72% in the mini-sling group (177/246) and 66.8% (157/235) in the full-length sling group.
More than 70% of mini-incision slings were Altis (Coloplast) and 22% were Ajust (CR Bard; since withdrawn from the market). The majority of standard midurethral full-length slings were transobturator slings (52.9%) versus retropubic slings (35.6%).
While blood loss, organ injury, and 36-month objective 24-hour pad test did not differ between groups, there were significant differences in other secondary outcomes. Dyspareunia and coital incontinence were more common with mini-slings at 15 and 36 months, reported in 11.7% of the mini-sling group and 4.8% of the full-length group (P<.01). Groin or thigh pain did not differ significantly between groups at 36 months (14.1% in mini-sling and 14.9% in full-length sling group, P = .61). Mesh exposure was noted in 3.3% of those with mini-slings and 1.9% of those with standard midurethral slings. The need for surgical intervention to treat recurrent stress incontinence or mesh removal for voiding dysfunction, pain, or mesh exposure also did not differ between groups (8.7% of the mini-sling group and 4.6% of the midurethral sling group; P = .12).
Study strengths and limitations
The strengths of this pragmatic randomized trial are in the use of clinically important and validated patient-reported subjective and objective outcomes in an adequately powered multisite trial of long duration (36 months). This study is important in demonstrating noninferiority of the mini-sling procedure compared with full-length slings, especially given this trial’s timing when there was a pause or suspension of sling mesh use in the United Kingdom beginning in 2018.
Study limitations include the loss to follow-up with diminished response rate of 87.1% at 15 months and 81.4% at 36 months and the inability to adequately assess for the uncommon outcomes, such as mesh-related complications and groin pain.
Further analysis needed
The high rate of dyspareunia (11.7%) with mini-slings deserves further analysis and consideration of whether or not to implant them in patients who are sexually active. Groin or thigh pain did not differ at 36 months but reported pain coincided with the higher percentage of transobturator slings placed over retropubic slings. Prior randomized trials of transobturator versus retropubic midurethral slings have demonstrated this same phenomenon of increased groin pain with the transobturator approach.2 Furthermore, this study by Abdel-Fattah and colleagues excluded patients with advanced anterior or apical prolapse, but one trial is currently underway in the United States.4
In conclusion, this trial suggests some advantages of single-incision mini-slings—ability to perform the procedure under local anesthesia, less synthetic mesh implantation with theoretically decreased risk of bladder perforation or bowel injury, and potential for easier removal compared with full-length slings. Disadvantages include dyspareunia and mesh exposure, which could be significant trade-offs for patients. ●
In the IDEAL framework for evaluating new surgical innovations, the recommended process begins with an idea, followed by development by a few surgeons in a few patients, then exploration in a feasibility randomized controlled trial, an assessment in larger trials by many surgeons, and long-term follow-up.5 The SIMS trial falls under the assessment tab of the IDEAL framework and represents a much-needed study prior to widespread adoption of single-incision mini-slings. The higher dyspareunia rate in women undergoing single-incision mini-slings deserves further evaluation.
CHERYL B. IGLESIA, MD
- Joint position statement on midurethral slings for stress urinary incontinence. Female Pelvic Med Reconstr Surg. 2021;27:707-710. doi: 10.1097/SPV.0000000000001096.
- Richter HE, Albo ME, Zyczynski HM, et al; Urinary Incontinence Treatment Network. Retropubic versus transobturator midurethral slings for stress incontinence. N Engl J Med. 2010;362:2066-2076.
- Nambiar A, Cody JD, Jeffery ST. Single-incision sling operations for urinary incontinence in women. Cochrane Database Syst Rev. 2014;6:CD008709.
- National Institutes of Health. Retropubic vs single-incision mid-urethral sling for stress urinary incontinence. ClinicalTrials.gov identifier NCT03520114. Accessed July16, 2022. https://www.clinicaltrials.gov/ct2/show/NCT0352011 4?cond=altis+sling&draw=2&rank=6
- McCulloch P, Altman DG, Campbell WB, et al. No surgical innovation without evaluation: the IDEAL recommendations. Lancet. 2009;374:1105-1111.
- Joint position statement on midurethral slings for stress urinary incontinence. Female Pelvic Med Reconstr Surg. 2021;27:707-710. doi: 10.1097/SPV.0000000000001096.
- Richter HE, Albo ME, Zyczynski HM, et al; Urinary Incontinence Treatment Network. Retropubic versus transobturator midurethral slings for stress incontinence. N Engl J Med. 2010;362:2066-2076.
- Nambiar A, Cody JD, Jeffery ST. Single-incision sling operations for urinary incontinence in women. Cochrane Database Syst Rev. 2014;6:CD008709.
- National Institutes of Health. Retropubic vs single-incision mid-urethral sling for stress urinary incontinence. ClinicalTrials.gov identifier NCT03520114. Accessed July16, 2022. https://www.clinicaltrials.gov/ct2/show/NCT0352011 4?cond=altis+sling&draw=2&rank=6
- McCulloch P, Altman DG, Campbell WB, et al. No surgical innovation without evaluation: the IDEAL recommendations. Lancet. 2009;374:1105-1111.
Mifepristone for the treatment of miscarriage and fetal demise
In the uterus, coordinated myometrial cell contraction is not triggered by neural activation; instead, myometrial cells work together as a contractile syncytium through cell-to-cell gap junction connections permitting the intercellular sharing of small molecules, which in turn facilitates activation of the actin-myosin contractile apparatus and coordinated uterine contraction. In myometrial cells, connexin 43 (Cx43) is the main gap junction protein. Cx43 permits the passage of small hydrophilic molecules (ATP) and ions (calcium) cell to cell. Estradiol increases Cx43 synthesis in human myometrial cells.1 Progesterone decreases Cx43 synthesis effectively isolating myometrial cells, reducing cell-to-cell sharing of chemicals that stimulate contraction, blocking coordinated uterine contraction.2 Progesterone suppression of Cx43 synthesis helps to prevent premature uterine contraction during pregnancy. At term, decreases in progesterone levels result in an increase in Cx43 synthesis, facilitating the onset of effective labor. In myometrial cells, antiprogestins, including mifepristone, increase the number of gap junction connections, facilitating a coordinated contractile signal in response to misoprostol or oxytocin.3,4
It takes time for antiprogestins to stimulate myometrial cell production of Cx43. In the rat myometrium the administration of mifepristone results in a 2.5-fold increase of Cx43 mRNA transcripts within 9 hours and a 5.6-fold increase in 24 hours.3 Hence, most mifepristone treatment protocols involve administering mifepristone and waiting 24 to 48 hours before administering an agent that stimulates myometrial contraction, such as misoprostol. Antiprogestins also increase the sensitivity of myometrial cells to oxytocin stimulation of uterine contractions by increasing Cx43 concentration.4
Progesterone also regulates other important biological processes in the cervix, decidua, placenta, and cervix. Antiprogestins can facilitate cervical ripening and disrupt decidual function, interfering with the attachment of pregnancy tissue.5 In the cervix, antiprogestins increase matrix metalloproteinase expression, disrupting collagen organization, decreasing cervical tensile strength and leading to cervical ripening.6
Pharmacology of mifepristone
Mifepristone is an antiprogestin and antiglucocorticoid with high-affinity binding to both the progesterone and glucocorticoid receptors (FIGURE 1). The phenylaminodimethyl group at C-11 of mifepristone changes the positional equilibrium of helix 12 of the progesterone receptor, reducing the ability of the receptor to bind required co-activators, limiting receptor binding to DNA, resulting in an antiprogesterone effect.7 At the low, single-dose used for treatment of miscarriage and fetal demise (200 mg one dose), mifepristone is an antiprogestin. At the high, daily dose used for the treatment of hyperglycemia caused by Cushing disease (≥ 300 mg daily), mifepristone is also an antiglucocorticoid.
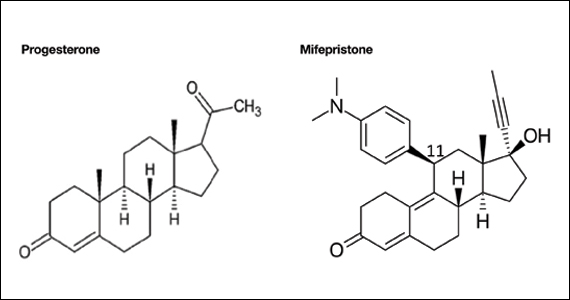
Although mifepristone is a powerful antiglucocorticoid, in patients with an intact hypothalamic-pituitary-adrenal axis, mifepristone does not cause adrenal insufficiency. In people with an intact hypothalamic-pituitary-adrenal axis, daily administration of mifepristone (≥ 200 mg) for 7 days or longer results in an increase in pituitary secretion of ACTH and adrenal secretion of cortisol, largely overcoming the antiglucocorticoid action of mifepristone.8-10 This compensatory increase in ACTH and cortisol is not possible in patients who have had a hypophysectomy or bilateral adrenalectomy or have adrenal suppression due to long-term treatment with high doses of glucocorticoids. Mifepristone is contraindicated for patients with these conditions because it may cause glucocorticoid insufficiency by blocking glucocorticoid receptors.
The terminal half-life of mifepristone is 18 hours.11 Following oral administration of a single dose of mifepristone 200 mg the peak circulating concentration is reached in 90 minutes. Mifepristone is metabolized by CYP3A4 and is also a strong inhibitor of CYP3A4. Contraindications to the use of mifepristone include adrenal failure, porphyria, hemorrhagic diseases, anticoagulation, an IUD in the uterus, ectopic pregnancy, long-term glucocorticoid administration, and an undiagnosed adnexal mass.
Continue to: Mifepristone-misoprostol for the treatment of early missed miscarriage with a gestational sac...
Mifepristone-misoprostol for the treatment of early missed miscarriage with a gestational sac
For patients with a miscarriage, the treatment options to resolve the pregnancy loss are expectant management, medication, or surgery.12 Joint decision-making is recommended to establish a management plan that supports the patient’s values. Expectant management is most likely to result in a multi-week process to achieve completion of the miscarriage. A surgical procedure is most likely to result in rapid resolution of the miscarriage with the greatest rate of success. Surgical evacuation of the uterus may be the preferred option for patients who have excessive uterine bleeding or concerning vital signs. Both medical and surgical management are more likely than expectant management to successfully resolve the miscarriage.13
In the past, the standard approach to medication management of a miscarriage was the administration of one or more doses of misoprostol, a synthetic prostaglandin E1. However, two large trials have reported that the dual-medication sequence of mifepristone followed 24 to 48 hours later by misoprostol is more effective than misoprostol alone for resolving a miscarriage.14,15 This is probably due to mifepristone making the uterus more responsive to the effects of misoprostol.
Schreiber and colleagues14 reported a study of 300 patients with an anembryonic gestation or embryonic demise, between 5 and 12 completed weeks of gestation, who were randomly assigned to treatment with mifepristone (200 mg) followed in 24 to 48 hours with vaginal misoprostol (800 µg) or vaginal misoprostol (800 µg) alone. Ultrasonography was performed 1 to 4 days after misoprostol administration. Successful treatment was defined as expulsion of the gestational sac plus no additional surgical or medical intervention within 30 days after treatment. In this study, the dual-medication regimen of mifepristone-misoprostol was more successful than misoprostol alone in resolving the miscarriage, 84% and 67%, respectively (relative risk [RR], 1.25; 95% confidence interval [CI], 1.09–1.43).
Surgical evacuation of the uterus occurred less often with mifepristone-misoprostol treatment than with misoprostol monotherapy—9% and 24%, respectively (RR, 0.37; 95% CI, 0.21–0.68). Pelvic infection occurred in 2 patients (1.3%) in each group. Uterine bleeding managed with blood transfusion occurred in 3 patients who received mifepristone-misoprostol and 1 patient who received misoprostol alone. In this study, clinical factors including active bleeding, parity, and gestational age did not influence treatment success with the mifepristone-misoprostol regimen.16 The mifepristone-misoprostol regimen was reported to be more cost-effective than misoprostol alone.17
Chu and colleagues15 reported a study of medication treatment of missed miscarriage that included more than 700 patients randomly assigned to treatment with mifepristone-misoprostol or placebo-misoprostol. Missed miscarriage was diagnosed by an ultrasound demonstrating a gestational sac and a nonviable pregnancy. The doses of mifepristone and misoprostol were 200 mg and 800 µg, respectively. In this study the misoprostol was administered 48 hours following mifepristone or placebo using a vaginal, oral, or buccal route, but 90% of patients used the vaginal route. Treatment was considered successful if the patient passed the gestational sac as determined by an ultrasound performed 7 days after entry into the study. If the gestational sac was passed, the patients were asked to do a urine pregnancy test 3 weeks after entering the study to conclude their care episode. If patients did not pass the gestational sac, they were offered a second dose of misoprostol or surgical evacuation. In this study, mifepristone-misoprostol resulted in fewer patients who did not pass the gestational sac within 7 days after entry into the study than placebo (mifepristone-misoprostol, 17% vs placebo-misoprostol, 24% (P=.043). Surgical intervention was performed in 25% of patients treated with placebo-misoprostol and 17% of patients treated with mifepristone-misoprostol (RR, 0.73; 95% CI, 0.53–0.95; P=.021). A cost-effectiveness analysis of the trial results reported that the combination of mifepristone-misoprostol was less costly than misoprostol alone for the management of missed miscarriages.18
Misoprostol can be administered by an oral, buccal, rectal, or vaginal route.19,20 Vaginal administration results in higher circulating concentrations of misoprostol than buccal administration, but both routes of administration produce similar mean uterine tone and mean uterine activity as measured by an intrauterine pressure transducer over 5 hours.21 Hence, at our institution, we most often use buccal administration of misoprostol. To assess effectiveness of mifepristone-misoprostol treatment, 1 week after treatment with a pelvic ultrasound to detect expulsion of the gestational sac. Alternatively, a urine pregnancy test can be performed 3 weeks following medication treatment. The mifepristone-misoprostol regimen is not approved by the US Food and Drug Administration for the treatment of miscarriage.
Continue to: Mifepristone-misoprostol for the treatment of fetal demise...
Mifepristone-misoprostol for the treatment of fetal demise
Fetal loss in the second or third trimesters is a devastating experience for most patients, painfully echoing in the heart and mind for years. Empathic and effective treatment of fetal loss may reduce the adverse impact of the event. Multiple studies have reported that combinations of mifepristone and misoprostol reduced the time from initiation of labor contractions to birth compared with misoprostol alone.22-28 In addition, the combination of mifepristone-misoprostol reduced the amount of misoprostol needed to achieve delivery.22,23
In one clinical trial, 66 patients with fetal demise between 14 and 28 weeks’ gestation were randomized to receive mifepristone 200 mg or placebo.22 Twenty-four to 48 hours later, misoprostol for induction of labor was initiated. Among the patients from 14 to 23 completed weeks of gestation, the misoprostol dose was 400 µg vaginally every 6 hours. For patients from 24 to 28 weeks gestation, the misoprostol dose was 200 µg vaginally every 4 hours. The median times from initiation of misoprostol to birth for the patients in the mifepristone and placebo groups were 6.8 hours and 10.5 hours (P=.002).
Compared with the patients in the placebo-misoprostol group, the patients in the mifepristone-misoprostol group required fewer doses of misoprostol (2.1 vs 3.4; P=.002) and a lower total dose of misoprostol (768 µg vs 1,182 µg; P=.003). All patients in the mifepristone group delivered within 24 hours. By contrast, 13% of the patients in the placebo group delivered more than 24 hours after the initiation of misoprostol treatment. Five patients were readmitted with retained products of conception needing suction curettage—4 in the placebo group and 1 in the mifepristone group.22
In a second clinical trial, 110 patients with fetal demise after 20 weeks of gestation were randomized to receive mifepristone 200 mg or placebo.23 Thirty-six to 48 hours later, misoprostol for induction of labor was initiated. Among the patients from 20 to 25 completed weeks of gestation, the misoprostol dose was 100 µg vaginally every 6 hours for a maximum of 4 doses. For patients ≥26 weeks gestation, the misoprostol dose was 50 µg vaginally every 4 hours for a maximum of 6 doses. The median times from initiation of misoprostol to birth for the patients in the mifepristone and placebo groups were 9.8 hours and 16.3 hours. (P=.001).
Compared with the patients in the placebo-misoprostol group, the patients in the mifepristone-misoprostol group required a lower total dose of misoprostol (110 µg vs 198 µg, P<.001).
Delivery within 24 hours following initiation of misoprostol occurred in 93% and 73% of the patients in the mifepristone and placebo groups, respectively (P<.001). Compared with patients in the mifepristone group, shivering occurred more frequently among the patients in the placebo group (7.5% vs 19.2%; P=.09), likely because they received greater doses of misoprostol.23
Miscarriage and fetal demise frequently cause patients to experience a range of emotions including denial, numbness, grief, anger, guilt, and depression. It may take months or years for people to progress to a tentative acceptance of the loss, refocusing on future aspirations. Empathic care and timely and effective medical intervention to resolve the pregnancy loss optimize outcomes. For medication treatment of miscarriage and fetal demise, mifepristone is an important agent because it improves the success rate for resolution of miscarriage without surgery and it shortens the time of labor for inductions for fetal demise. Obstetrician-gynecologists are the specialists leading advances in treatment of miscarriage and fetal demise. I encourage you to use mifepristone in your care of appropriate patients with miscarriage and fetal demise. ●
- Andersen J, Grine E, Eng L, et al. Expression of connexin-43 in human myometrium and leiomyoma. Am J Obstet Gynecol. 1993;169:1266-1276. doi: 10.1016/0002-9378(93)90293-r.
- Ou CW, Orsino A, Lye SJ. Expression of connexin-43 and connexin-26 in the rat myometrium during pregnancy and labor is differentially regulated by mechanical and hormonal signals. Endocrinology. 1997;138:5398-5407. doi: 10.1210 /endo.138.12.5624.
- Petrocelli T, Lye SJ. Regulation of transcripts encoding the myometrial gap junction protein, connexin-43, by estrogen and progesterone. Endocrinology. 1993;133:284-290. doi: 10.1210 /endo.133.1.8391423.
- Chwalisz K, Fahrenholz F, Hackenberg M, et al. The progesterone antagonist onapristone increases the effectiveness of oxytocin to produce delivery without changing the myometrial oxytocin receptor concentration. Am J Obstet Gynecol. 1991;165:1760-1770. doi: 10.1016/0002 -9378(91)90030-u.
- Large MJ, DeMayo FJ. The regulation of embryo implantation and endometrial decidualization by progesterone receptor signaling. Mol Cell Endocrinol. 2012;358:155-165. doi: 10.1016 /j.mce.2011.07.027.
- Clark K, Ji H, Feltovich H, et al. Mifepristone-induced cervical ripening: structural, biomechanical and molecular events. Am J Obstet Gynecol. 2006;194:1391-1398. doi: 10.1016 /j.ajog.2005.11.026.
- Raaijmakers HCA, Versteegh JE, Uitdehaag JCM. T he x-ray structure of RU486 bound to the progesterone receptor in a destabilized agonist conformation. J Biol Chem. 2009;284:19572-19579. doi: 10.1074/jbc.M109.007872.
- Yuen KCJ, Moraitis A, Nguyen D. Evaluation of evidence of adrenal insufficiency in trials of normocortisolemic patients treated with mifepristone. J Endocr Soc. 2017;1:237-246. doi: 10.1210 /js.2016-1097.
- Spitz IM, Grunberg SM, Chabbert-Buffet N, et al. Management of patients receiving long-term treatment with mifepristone. Fertil Steril. 2005;84:1719-1726. doi: 10.1016 /j.fertnstert.2005.05.056.
- Bertagna X, Escourolle H, Pinquier JL, et al. Administration of RU 486 for 8 days in normal volunteers: antiglucocorticoid effect with no evidence of peripheral cortisol deprivation. J Clin Endocrinol Metab. 1994;78:375-380. doi: 10.1210 /jcem.78.2.8106625.
- Mifeprex [package insert]. New York, NY: Danco Laboratories; March 2016.
- Early pregnancy loss. ACOG Practice Bulletin No 200. American College of Obstetricians and Gynecologists. Obstet Gynecol. 2018;132:e197-e207. doi: /AOG.0000000000002899. 10.1097
- Chu J, Devall AJ, Hardy P, et al. What is the best method for managing early miscarriage? BMJ. 2020;368:l6483. doi: 10.1136/bmj.l6438.
- Schreiber C, Creinin MD, Atrio J, et al. Mifepristone pretreatment for the medical management of early pregnancy loss. N Engl J Med. 2018;378:2161-2170. doi: 10.1056 /NEJMoa1715726.
- Chu JJ, Devall AJ, Beeson LE, et al. Mifepristone and misoprostol versus misoprostol alone for the management of missed miscarriage (MifeMiso): a randomised, double-blind, placebo-controlled trial. Lancet. 2020;396:770-778. doi: 10.1016 /S0140-6736(20)31788-8.
- Sonalkar S, Koelper N, Creinin MD, et al. Management of early pregnancy loss with mifepristone and misoprostol: clinical predictors of treatment success from a randomized trial. Am J Obstet Gynecol. 2020;223:551.e1-e7. doi: 10.1016/j. ajog.2020.04.006. 17.
- Nagendra D, Koelper N, Loza-Avalos SE, et al. Cost-effectiveness of mifepristone pretreatment for the medical management of nonviable early pregnancy: secondary analysis of a randomized clinical trial. JAMA Netw Open. 2020;3:e201594. doi: 10.1001/jamanetworkopen.2020.1594.
- Okeke-Ogwulu CB, Williams EV, Chu JJ, et al. Cost-effectiveness of mifepristone and misoprostol versus misoprostol alone for the management of missed miscarriage: an economic evaluation based on the MifeMiso trial. BJOG. 2021;128: 1534-1545. doi: 10.1111/1471-0528.16737.
- Tang OS, Schweer H, Seyberth HW, et al. Pharmacokinetics of different routes of administration of misoprostol. Hum Reprod. 2002;17:332336. doi: 10.1093/humrep/17.2.332.
- Schaff EA, DiCenzo R, Fielding SL. Comparison of misoprostol plasma concentrations following buccal and sublingual administration. Contraception. 2005;71:22-25. doi: 10.1016 /j.contraception.2004.06.014.
- Meckstroth KR, Whitaker AK, Bertisch S, et al. Misoprostol administered by epithelial routes: drug absorption and uterine response. Obstet Gynecol. 2006;108:582-590. doi: 10.1097/01 .AOG.0000230398.32794.9d.
- Allanson ER, Copson S, Spilsbury K, et al. Pretreatment with mifepristone compared with misoprostol alone for delivery after fetal death between 14 and 28 weeks of gestation. Obstet Gynecol. 2021;137:801-809. doi: 10.1097 /AOG.0000000000004344.
- Chaudhuri P, Datta S. Mifepristone and misoprostol compared with misoprostol alone for induction of labor in intrauterine fetal death: a randomized trial. J Obstet Gynaecol Res. 2015;41:1884-1890. doi: 10.1111/jog.12815.
- Fyfe R, Murray H. Comparison of induction of labour regimens for termination of pregnancy with and without mifepristone, from 20 to 41 weeks gestation. Aust N Z J Obstet Gynaecol. 2017;57:604-608. doi: 10.1111 /ajo.12648.
- Panda S, Jha V, Singh S. Role of combination of mifepristone and misoprostol verses misoprostol alone in induction of labour in late intrauterine fetal death: a prospective study. J Family Reprod Health. 2013;7:177-179.
- Vayrynen W, Heikinheimo O, Nuutila M. Misoprostol-only versus mifepristone plus misoprostol in induction of labor following intrauterine fetal death. Acta Obstet Gynecol Scand. 2007;86: 701-705. doi: 10.1080/00016340701379853.
- Sharma D, Singhal SR, Poonam AP. Comparison of mifepristone combination with misoprostol and misoprostol alone in the management of intrauterine death. Taiwan J Obstet Gynecol. 2011;50:322-325. doi: 10.1016/j.tjog.2011.07.007.
- Stibbe KJM, de Weerd S. Induction of delivery by mifepristone and misoprostol in termination of pregnancy and intrauterine fetal death: 2nd and 3rd trimester induction of labour. Arch Gynecol Obstet. 2012;286:795-796. doi: 10.1007 /s00404-012-2289-3.
In the uterus, coordinated myometrial cell contraction is not triggered by neural activation; instead, myometrial cells work together as a contractile syncytium through cell-to-cell gap junction connections permitting the intercellular sharing of small molecules, which in turn facilitates activation of the actin-myosin contractile apparatus and coordinated uterine contraction. In myometrial cells, connexin 43 (Cx43) is the main gap junction protein. Cx43 permits the passage of small hydrophilic molecules (ATP) and ions (calcium) cell to cell. Estradiol increases Cx43 synthesis in human myometrial cells.1 Progesterone decreases Cx43 synthesis effectively isolating myometrial cells, reducing cell-to-cell sharing of chemicals that stimulate contraction, blocking coordinated uterine contraction.2 Progesterone suppression of Cx43 synthesis helps to prevent premature uterine contraction during pregnancy. At term, decreases in progesterone levels result in an increase in Cx43 synthesis, facilitating the onset of effective labor. In myometrial cells, antiprogestins, including mifepristone, increase the number of gap junction connections, facilitating a coordinated contractile signal in response to misoprostol or oxytocin.3,4
It takes time for antiprogestins to stimulate myometrial cell production of Cx43. In the rat myometrium the administration of mifepristone results in a 2.5-fold increase of Cx43 mRNA transcripts within 9 hours and a 5.6-fold increase in 24 hours.3 Hence, most mifepristone treatment protocols involve administering mifepristone and waiting 24 to 48 hours before administering an agent that stimulates myometrial contraction, such as misoprostol. Antiprogestins also increase the sensitivity of myometrial cells to oxytocin stimulation of uterine contractions by increasing Cx43 concentration.4
Progesterone also regulates other important biological processes in the cervix, decidua, placenta, and cervix. Antiprogestins can facilitate cervical ripening and disrupt decidual function, interfering with the attachment of pregnancy tissue.5 In the cervix, antiprogestins increase matrix metalloproteinase expression, disrupting collagen organization, decreasing cervical tensile strength and leading to cervical ripening.6
Pharmacology of mifepristone
Mifepristone is an antiprogestin and antiglucocorticoid with high-affinity binding to both the progesterone and glucocorticoid receptors (FIGURE 1). The phenylaminodimethyl group at C-11 of mifepristone changes the positional equilibrium of helix 12 of the progesterone receptor, reducing the ability of the receptor to bind required co-activators, limiting receptor binding to DNA, resulting in an antiprogesterone effect.7 At the low, single-dose used for treatment of miscarriage and fetal demise (200 mg one dose), mifepristone is an antiprogestin. At the high, daily dose used for the treatment of hyperglycemia caused by Cushing disease (≥ 300 mg daily), mifepristone is also an antiglucocorticoid.

Although mifepristone is a powerful antiglucocorticoid, in patients with an intact hypothalamic-pituitary-adrenal axis, mifepristone does not cause adrenal insufficiency. In people with an intact hypothalamic-pituitary-adrenal axis, daily administration of mifepristone (≥ 200 mg) for 7 days or longer results in an increase in pituitary secretion of ACTH and adrenal secretion of cortisol, largely overcoming the antiglucocorticoid action of mifepristone.8-10 This compensatory increase in ACTH and cortisol is not possible in patients who have had a hypophysectomy or bilateral adrenalectomy or have adrenal suppression due to long-term treatment with high doses of glucocorticoids. Mifepristone is contraindicated for patients with these conditions because it may cause glucocorticoid insufficiency by blocking glucocorticoid receptors.
The terminal half-life of mifepristone is 18 hours.11 Following oral administration of a single dose of mifepristone 200 mg the peak circulating concentration is reached in 90 minutes. Mifepristone is metabolized by CYP3A4 and is also a strong inhibitor of CYP3A4. Contraindications to the use of mifepristone include adrenal failure, porphyria, hemorrhagic diseases, anticoagulation, an IUD in the uterus, ectopic pregnancy, long-term glucocorticoid administration, and an undiagnosed adnexal mass.
Continue to: Mifepristone-misoprostol for the treatment of early missed miscarriage with a gestational sac...
Mifepristone-misoprostol for the treatment of early missed miscarriage with a gestational sac
For patients with a miscarriage, the treatment options to resolve the pregnancy loss are expectant management, medication, or surgery.12 Joint decision-making is recommended to establish a management plan that supports the patient’s values. Expectant management is most likely to result in a multi-week process to achieve completion of the miscarriage. A surgical procedure is most likely to result in rapid resolution of the miscarriage with the greatest rate of success. Surgical evacuation of the uterus may be the preferred option for patients who have excessive uterine bleeding or concerning vital signs. Both medical and surgical management are more likely than expectant management to successfully resolve the miscarriage.13
In the past, the standard approach to medication management of a miscarriage was the administration of one or more doses of misoprostol, a synthetic prostaglandin E1. However, two large trials have reported that the dual-medication sequence of mifepristone followed 24 to 48 hours later by misoprostol is more effective than misoprostol alone for resolving a miscarriage.14,15 This is probably due to mifepristone making the uterus more responsive to the effects of misoprostol.
Schreiber and colleagues14 reported a study of 300 patients with an anembryonic gestation or embryonic demise, between 5 and 12 completed weeks of gestation, who were randomly assigned to treatment with mifepristone (200 mg) followed in 24 to 48 hours with vaginal misoprostol (800 µg) or vaginal misoprostol (800 µg) alone. Ultrasonography was performed 1 to 4 days after misoprostol administration. Successful treatment was defined as expulsion of the gestational sac plus no additional surgical or medical intervention within 30 days after treatment. In this study, the dual-medication regimen of mifepristone-misoprostol was more successful than misoprostol alone in resolving the miscarriage, 84% and 67%, respectively (relative risk [RR], 1.25; 95% confidence interval [CI], 1.09–1.43).
Surgical evacuation of the uterus occurred less often with mifepristone-misoprostol treatment than with misoprostol monotherapy—9% and 24%, respectively (RR, 0.37; 95% CI, 0.21–0.68). Pelvic infection occurred in 2 patients (1.3%) in each group. Uterine bleeding managed with blood transfusion occurred in 3 patients who received mifepristone-misoprostol and 1 patient who received misoprostol alone. In this study, clinical factors including active bleeding, parity, and gestational age did not influence treatment success with the mifepristone-misoprostol regimen.16 The mifepristone-misoprostol regimen was reported to be more cost-effective than misoprostol alone.17
Chu and colleagues15 reported a study of medication treatment of missed miscarriage that included more than 700 patients randomly assigned to treatment with mifepristone-misoprostol or placebo-misoprostol. Missed miscarriage was diagnosed by an ultrasound demonstrating a gestational sac and a nonviable pregnancy. The doses of mifepristone and misoprostol were 200 mg and 800 µg, respectively. In this study the misoprostol was administered 48 hours following mifepristone or placebo using a vaginal, oral, or buccal route, but 90% of patients used the vaginal route. Treatment was considered successful if the patient passed the gestational sac as determined by an ultrasound performed 7 days after entry into the study. If the gestational sac was passed, the patients were asked to do a urine pregnancy test 3 weeks after entering the study to conclude their care episode. If patients did not pass the gestational sac, they were offered a second dose of misoprostol or surgical evacuation. In this study, mifepristone-misoprostol resulted in fewer patients who did not pass the gestational sac within 7 days after entry into the study than placebo (mifepristone-misoprostol, 17% vs placebo-misoprostol, 24% (P=.043). Surgical intervention was performed in 25% of patients treated with placebo-misoprostol and 17% of patients treated with mifepristone-misoprostol (RR, 0.73; 95% CI, 0.53–0.95; P=.021). A cost-effectiveness analysis of the trial results reported that the combination of mifepristone-misoprostol was less costly than misoprostol alone for the management of missed miscarriages.18
Misoprostol can be administered by an oral, buccal, rectal, or vaginal route.19,20 Vaginal administration results in higher circulating concentrations of misoprostol than buccal administration, but both routes of administration produce similar mean uterine tone and mean uterine activity as measured by an intrauterine pressure transducer over 5 hours.21 Hence, at our institution, we most often use buccal administration of misoprostol. To assess effectiveness of mifepristone-misoprostol treatment, 1 week after treatment with a pelvic ultrasound to detect expulsion of the gestational sac. Alternatively, a urine pregnancy test can be performed 3 weeks following medication treatment. The mifepristone-misoprostol regimen is not approved by the US Food and Drug Administration for the treatment of miscarriage.
Continue to: Mifepristone-misoprostol for the treatment of fetal demise...
Mifepristone-misoprostol for the treatment of fetal demise
Fetal loss in the second or third trimesters is a devastating experience for most patients, painfully echoing in the heart and mind for years. Empathic and effective treatment of fetal loss may reduce the adverse impact of the event. Multiple studies have reported that combinations of mifepristone and misoprostol reduced the time from initiation of labor contractions to birth compared with misoprostol alone.22-28 In addition, the combination of mifepristone-misoprostol reduced the amount of misoprostol needed to achieve delivery.22,23
In one clinical trial, 66 patients with fetal demise between 14 and 28 weeks’ gestation were randomized to receive mifepristone 200 mg or placebo.22 Twenty-four to 48 hours later, misoprostol for induction of labor was initiated. Among the patients from 14 to 23 completed weeks of gestation, the misoprostol dose was 400 µg vaginally every 6 hours. For patients from 24 to 28 weeks gestation, the misoprostol dose was 200 µg vaginally every 4 hours. The median times from initiation of misoprostol to birth for the patients in the mifepristone and placebo groups were 6.8 hours and 10.5 hours (P=.002).
Compared with the patients in the placebo-misoprostol group, the patients in the mifepristone-misoprostol group required fewer doses of misoprostol (2.1 vs 3.4; P=.002) and a lower total dose of misoprostol (768 µg vs 1,182 µg; P=.003). All patients in the mifepristone group delivered within 24 hours. By contrast, 13% of the patients in the placebo group delivered more than 24 hours after the initiation of misoprostol treatment. Five patients were readmitted with retained products of conception needing suction curettage—4 in the placebo group and 1 in the mifepristone group.22
In a second clinical trial, 110 patients with fetal demise after 20 weeks of gestation were randomized to receive mifepristone 200 mg or placebo.23 Thirty-six to 48 hours later, misoprostol for induction of labor was initiated. Among the patients from 20 to 25 completed weeks of gestation, the misoprostol dose was 100 µg vaginally every 6 hours for a maximum of 4 doses. For patients ≥26 weeks gestation, the misoprostol dose was 50 µg vaginally every 4 hours for a maximum of 6 doses. The median times from initiation of misoprostol to birth for the patients in the mifepristone and placebo groups were 9.8 hours and 16.3 hours. (P=.001).
Compared with the patients in the placebo-misoprostol group, the patients in the mifepristone-misoprostol group required a lower total dose of misoprostol (110 µg vs 198 µg, P<.001).
Delivery within 24 hours following initiation of misoprostol occurred in 93% and 73% of the patients in the mifepristone and placebo groups, respectively (P<.001). Compared with patients in the mifepristone group, shivering occurred more frequently among the patients in the placebo group (7.5% vs 19.2%; P=.09), likely because they received greater doses of misoprostol.23
Miscarriage and fetal demise frequently cause patients to experience a range of emotions including denial, numbness, grief, anger, guilt, and depression. It may take months or years for people to progress to a tentative acceptance of the loss, refocusing on future aspirations. Empathic care and timely and effective medical intervention to resolve the pregnancy loss optimize outcomes. For medication treatment of miscarriage and fetal demise, mifepristone is an important agent because it improves the success rate for resolution of miscarriage without surgery and it shortens the time of labor for inductions for fetal demise. Obstetrician-gynecologists are the specialists leading advances in treatment of miscarriage and fetal demise. I encourage you to use mifepristone in your care of appropriate patients with miscarriage and fetal demise. ●
In the uterus, coordinated myometrial cell contraction is not triggered by neural activation; instead, myometrial cells work together as a contractile syncytium through cell-to-cell gap junction connections permitting the intercellular sharing of small molecules, which in turn facilitates activation of the actin-myosin contractile apparatus and coordinated uterine contraction. In myometrial cells, connexin 43 (Cx43) is the main gap junction protein. Cx43 permits the passage of small hydrophilic molecules (ATP) and ions (calcium) cell to cell. Estradiol increases Cx43 synthesis in human myometrial cells.1 Progesterone decreases Cx43 synthesis effectively isolating myometrial cells, reducing cell-to-cell sharing of chemicals that stimulate contraction, blocking coordinated uterine contraction.2 Progesterone suppression of Cx43 synthesis helps to prevent premature uterine contraction during pregnancy. At term, decreases in progesterone levels result in an increase in Cx43 synthesis, facilitating the onset of effective labor. In myometrial cells, antiprogestins, including mifepristone, increase the number of gap junction connections, facilitating a coordinated contractile signal in response to misoprostol or oxytocin.3,4
It takes time for antiprogestins to stimulate myometrial cell production of Cx43. In the rat myometrium the administration of mifepristone results in a 2.5-fold increase of Cx43 mRNA transcripts within 9 hours and a 5.6-fold increase in 24 hours.3 Hence, most mifepristone treatment protocols involve administering mifepristone and waiting 24 to 48 hours before administering an agent that stimulates myometrial contraction, such as misoprostol. Antiprogestins also increase the sensitivity of myometrial cells to oxytocin stimulation of uterine contractions by increasing Cx43 concentration.4
Progesterone also regulates other important biological processes in the cervix, decidua, placenta, and cervix. Antiprogestins can facilitate cervical ripening and disrupt decidual function, interfering with the attachment of pregnancy tissue.5 In the cervix, antiprogestins increase matrix metalloproteinase expression, disrupting collagen organization, decreasing cervical tensile strength and leading to cervical ripening.6
Pharmacology of mifepristone
Mifepristone is an antiprogestin and antiglucocorticoid with high-affinity binding to both the progesterone and glucocorticoid receptors (FIGURE 1). The phenylaminodimethyl group at C-11 of mifepristone changes the positional equilibrium of helix 12 of the progesterone receptor, reducing the ability of the receptor to bind required co-activators, limiting receptor binding to DNA, resulting in an antiprogesterone effect.7 At the low, single-dose used for treatment of miscarriage and fetal demise (200 mg one dose), mifepristone is an antiprogestin. At the high, daily dose used for the treatment of hyperglycemia caused by Cushing disease (≥ 300 mg daily), mifepristone is also an antiglucocorticoid.

Although mifepristone is a powerful antiglucocorticoid, in patients with an intact hypothalamic-pituitary-adrenal axis, mifepristone does not cause adrenal insufficiency. In people with an intact hypothalamic-pituitary-adrenal axis, daily administration of mifepristone (≥ 200 mg) for 7 days or longer results in an increase in pituitary secretion of ACTH and adrenal secretion of cortisol, largely overcoming the antiglucocorticoid action of mifepristone.8-10 This compensatory increase in ACTH and cortisol is not possible in patients who have had a hypophysectomy or bilateral adrenalectomy or have adrenal suppression due to long-term treatment with high doses of glucocorticoids. Mifepristone is contraindicated for patients with these conditions because it may cause glucocorticoid insufficiency by blocking glucocorticoid receptors.
The terminal half-life of mifepristone is 18 hours.11 Following oral administration of a single dose of mifepristone 200 mg the peak circulating concentration is reached in 90 minutes. Mifepristone is metabolized by CYP3A4 and is also a strong inhibitor of CYP3A4. Contraindications to the use of mifepristone include adrenal failure, porphyria, hemorrhagic diseases, anticoagulation, an IUD in the uterus, ectopic pregnancy, long-term glucocorticoid administration, and an undiagnosed adnexal mass.
Continue to: Mifepristone-misoprostol for the treatment of early missed miscarriage with a gestational sac...
Mifepristone-misoprostol for the treatment of early missed miscarriage with a gestational sac
For patients with a miscarriage, the treatment options to resolve the pregnancy loss are expectant management, medication, or surgery.12 Joint decision-making is recommended to establish a management plan that supports the patient’s values. Expectant management is most likely to result in a multi-week process to achieve completion of the miscarriage. A surgical procedure is most likely to result in rapid resolution of the miscarriage with the greatest rate of success. Surgical evacuation of the uterus may be the preferred option for patients who have excessive uterine bleeding or concerning vital signs. Both medical and surgical management are more likely than expectant management to successfully resolve the miscarriage.13
In the past, the standard approach to medication management of a miscarriage was the administration of one or more doses of misoprostol, a synthetic prostaglandin E1. However, two large trials have reported that the dual-medication sequence of mifepristone followed 24 to 48 hours later by misoprostol is more effective than misoprostol alone for resolving a miscarriage.14,15 This is probably due to mifepristone making the uterus more responsive to the effects of misoprostol.
Schreiber and colleagues14 reported a study of 300 patients with an anembryonic gestation or embryonic demise, between 5 and 12 completed weeks of gestation, who were randomly assigned to treatment with mifepristone (200 mg) followed in 24 to 48 hours with vaginal misoprostol (800 µg) or vaginal misoprostol (800 µg) alone. Ultrasonography was performed 1 to 4 days after misoprostol administration. Successful treatment was defined as expulsion of the gestational sac plus no additional surgical or medical intervention within 30 days after treatment. In this study, the dual-medication regimen of mifepristone-misoprostol was more successful than misoprostol alone in resolving the miscarriage, 84% and 67%, respectively (relative risk [RR], 1.25; 95% confidence interval [CI], 1.09–1.43).
Surgical evacuation of the uterus occurred less often with mifepristone-misoprostol treatment than with misoprostol monotherapy—9% and 24%, respectively (RR, 0.37; 95% CI, 0.21–0.68). Pelvic infection occurred in 2 patients (1.3%) in each group. Uterine bleeding managed with blood transfusion occurred in 3 patients who received mifepristone-misoprostol and 1 patient who received misoprostol alone. In this study, clinical factors including active bleeding, parity, and gestational age did not influence treatment success with the mifepristone-misoprostol regimen.16 The mifepristone-misoprostol regimen was reported to be more cost-effective than misoprostol alone.17
Chu and colleagues15 reported a study of medication treatment of missed miscarriage that included more than 700 patients randomly assigned to treatment with mifepristone-misoprostol or placebo-misoprostol. Missed miscarriage was diagnosed by an ultrasound demonstrating a gestational sac and a nonviable pregnancy. The doses of mifepristone and misoprostol were 200 mg and 800 µg, respectively. In this study the misoprostol was administered 48 hours following mifepristone or placebo using a vaginal, oral, or buccal route, but 90% of patients used the vaginal route. Treatment was considered successful if the patient passed the gestational sac as determined by an ultrasound performed 7 days after entry into the study. If the gestational sac was passed, the patients were asked to do a urine pregnancy test 3 weeks after entering the study to conclude their care episode. If patients did not pass the gestational sac, they were offered a second dose of misoprostol or surgical evacuation. In this study, mifepristone-misoprostol resulted in fewer patients who did not pass the gestational sac within 7 days after entry into the study than placebo (mifepristone-misoprostol, 17% vs placebo-misoprostol, 24% (P=.043). Surgical intervention was performed in 25% of patients treated with placebo-misoprostol and 17% of patients treated with mifepristone-misoprostol (RR, 0.73; 95% CI, 0.53–0.95; P=.021). A cost-effectiveness analysis of the trial results reported that the combination of mifepristone-misoprostol was less costly than misoprostol alone for the management of missed miscarriages.18
Misoprostol can be administered by an oral, buccal, rectal, or vaginal route.19,20 Vaginal administration results in higher circulating concentrations of misoprostol than buccal administration, but both routes of administration produce similar mean uterine tone and mean uterine activity as measured by an intrauterine pressure transducer over 5 hours.21 Hence, at our institution, we most often use buccal administration of misoprostol. To assess effectiveness of mifepristone-misoprostol treatment, 1 week after treatment with a pelvic ultrasound to detect expulsion of the gestational sac. Alternatively, a urine pregnancy test can be performed 3 weeks following medication treatment. The mifepristone-misoprostol regimen is not approved by the US Food and Drug Administration for the treatment of miscarriage.
Continue to: Mifepristone-misoprostol for the treatment of fetal demise...
Mifepristone-misoprostol for the treatment of fetal demise
Fetal loss in the second or third trimesters is a devastating experience for most patients, painfully echoing in the heart and mind for years. Empathic and effective treatment of fetal loss may reduce the adverse impact of the event. Multiple studies have reported that combinations of mifepristone and misoprostol reduced the time from initiation of labor contractions to birth compared with misoprostol alone.22-28 In addition, the combination of mifepristone-misoprostol reduced the amount of misoprostol needed to achieve delivery.22,23
In one clinical trial, 66 patients with fetal demise between 14 and 28 weeks’ gestation were randomized to receive mifepristone 200 mg or placebo.22 Twenty-four to 48 hours later, misoprostol for induction of labor was initiated. Among the patients from 14 to 23 completed weeks of gestation, the misoprostol dose was 400 µg vaginally every 6 hours. For patients from 24 to 28 weeks gestation, the misoprostol dose was 200 µg vaginally every 4 hours. The median times from initiation of misoprostol to birth for the patients in the mifepristone and placebo groups were 6.8 hours and 10.5 hours (P=.002).
Compared with the patients in the placebo-misoprostol group, the patients in the mifepristone-misoprostol group required fewer doses of misoprostol (2.1 vs 3.4; P=.002) and a lower total dose of misoprostol (768 µg vs 1,182 µg; P=.003). All patients in the mifepristone group delivered within 24 hours. By contrast, 13% of the patients in the placebo group delivered more than 24 hours after the initiation of misoprostol treatment. Five patients were readmitted with retained products of conception needing suction curettage—4 in the placebo group and 1 in the mifepristone group.22
In a second clinical trial, 110 patients with fetal demise after 20 weeks of gestation were randomized to receive mifepristone 200 mg or placebo.23 Thirty-six to 48 hours later, misoprostol for induction of labor was initiated. Among the patients from 20 to 25 completed weeks of gestation, the misoprostol dose was 100 µg vaginally every 6 hours for a maximum of 4 doses. For patients ≥26 weeks gestation, the misoprostol dose was 50 µg vaginally every 4 hours for a maximum of 6 doses. The median times from initiation of misoprostol to birth for the patients in the mifepristone and placebo groups were 9.8 hours and 16.3 hours. (P=.001).
Compared with the patients in the placebo-misoprostol group, the patients in the mifepristone-misoprostol group required a lower total dose of misoprostol (110 µg vs 198 µg, P<.001).
Delivery within 24 hours following initiation of misoprostol occurred in 93% and 73% of the patients in the mifepristone and placebo groups, respectively (P<.001). Compared with patients in the mifepristone group, shivering occurred more frequently among the patients in the placebo group (7.5% vs 19.2%; P=.09), likely because they received greater doses of misoprostol.23
Miscarriage and fetal demise frequently cause patients to experience a range of emotions including denial, numbness, grief, anger, guilt, and depression. It may take months or years for people to progress to a tentative acceptance of the loss, refocusing on future aspirations. Empathic care and timely and effective medical intervention to resolve the pregnancy loss optimize outcomes. For medication treatment of miscarriage and fetal demise, mifepristone is an important agent because it improves the success rate for resolution of miscarriage without surgery and it shortens the time of labor for inductions for fetal demise. Obstetrician-gynecologists are the specialists leading advances in treatment of miscarriage and fetal demise. I encourage you to use mifepristone in your care of appropriate patients with miscarriage and fetal demise. ●
- Andersen J, Grine E, Eng L, et al. Expression of connexin-43 in human myometrium and leiomyoma. Am J Obstet Gynecol. 1993;169:1266-1276. doi: 10.1016/0002-9378(93)90293-r.
- Ou CW, Orsino A, Lye SJ. Expression of connexin-43 and connexin-26 in the rat myometrium during pregnancy and labor is differentially regulated by mechanical and hormonal signals. Endocrinology. 1997;138:5398-5407. doi: 10.1210 /endo.138.12.5624.
- Petrocelli T, Lye SJ. Regulation of transcripts encoding the myometrial gap junction protein, connexin-43, by estrogen and progesterone. Endocrinology. 1993;133:284-290. doi: 10.1210 /endo.133.1.8391423.
- Chwalisz K, Fahrenholz F, Hackenberg M, et al. The progesterone antagonist onapristone increases the effectiveness of oxytocin to produce delivery without changing the myometrial oxytocin receptor concentration. Am J Obstet Gynecol. 1991;165:1760-1770. doi: 10.1016/0002 -9378(91)90030-u.
- Large MJ, DeMayo FJ. The regulation of embryo implantation and endometrial decidualization by progesterone receptor signaling. Mol Cell Endocrinol. 2012;358:155-165. doi: 10.1016 /j.mce.2011.07.027.
- Clark K, Ji H, Feltovich H, et al. Mifepristone-induced cervical ripening: structural, biomechanical and molecular events. Am J Obstet Gynecol. 2006;194:1391-1398. doi: 10.1016 /j.ajog.2005.11.026.
- Raaijmakers HCA, Versteegh JE, Uitdehaag JCM. T he x-ray structure of RU486 bound to the progesterone receptor in a destabilized agonist conformation. J Biol Chem. 2009;284:19572-19579. doi: 10.1074/jbc.M109.007872.
- Yuen KCJ, Moraitis A, Nguyen D. Evaluation of evidence of adrenal insufficiency in trials of normocortisolemic patients treated with mifepristone. J Endocr Soc. 2017;1:237-246. doi: 10.1210 /js.2016-1097.
- Spitz IM, Grunberg SM, Chabbert-Buffet N, et al. Management of patients receiving long-term treatment with mifepristone. Fertil Steril. 2005;84:1719-1726. doi: 10.1016 /j.fertnstert.2005.05.056.
- Bertagna X, Escourolle H, Pinquier JL, et al. Administration of RU 486 for 8 days in normal volunteers: antiglucocorticoid effect with no evidence of peripheral cortisol deprivation. J Clin Endocrinol Metab. 1994;78:375-380. doi: 10.1210 /jcem.78.2.8106625.
- Mifeprex [package insert]. New York, NY: Danco Laboratories; March 2016.
- Early pregnancy loss. ACOG Practice Bulletin No 200. American College of Obstetricians and Gynecologists. Obstet Gynecol. 2018;132:e197-e207. doi: /AOG.0000000000002899. 10.1097
- Chu J, Devall AJ, Hardy P, et al. What is the best method for managing early miscarriage? BMJ. 2020;368:l6483. doi: 10.1136/bmj.l6438.
- Schreiber C, Creinin MD, Atrio J, et al. Mifepristone pretreatment for the medical management of early pregnancy loss. N Engl J Med. 2018;378:2161-2170. doi: 10.1056 /NEJMoa1715726.
- Chu JJ, Devall AJ, Beeson LE, et al. Mifepristone and misoprostol versus misoprostol alone for the management of missed miscarriage (MifeMiso): a randomised, double-blind, placebo-controlled trial. Lancet. 2020;396:770-778. doi: 10.1016 /S0140-6736(20)31788-8.
- Sonalkar S, Koelper N, Creinin MD, et al. Management of early pregnancy loss with mifepristone and misoprostol: clinical predictors of treatment success from a randomized trial. Am J Obstet Gynecol. 2020;223:551.e1-e7. doi: 10.1016/j. ajog.2020.04.006. 17.
- Nagendra D, Koelper N, Loza-Avalos SE, et al. Cost-effectiveness of mifepristone pretreatment for the medical management of nonviable early pregnancy: secondary analysis of a randomized clinical trial. JAMA Netw Open. 2020;3:e201594. doi: 10.1001/jamanetworkopen.2020.1594.
- Okeke-Ogwulu CB, Williams EV, Chu JJ, et al. Cost-effectiveness of mifepristone and misoprostol versus misoprostol alone for the management of missed miscarriage: an economic evaluation based on the MifeMiso trial. BJOG. 2021;128: 1534-1545. doi: 10.1111/1471-0528.16737.
- Tang OS, Schweer H, Seyberth HW, et al. Pharmacokinetics of different routes of administration of misoprostol. Hum Reprod. 2002;17:332336. doi: 10.1093/humrep/17.2.332.
- Schaff EA, DiCenzo R, Fielding SL. Comparison of misoprostol plasma concentrations following buccal and sublingual administration. Contraception. 2005;71:22-25. doi: 10.1016 /j.contraception.2004.06.014.
- Meckstroth KR, Whitaker AK, Bertisch S, et al. Misoprostol administered by epithelial routes: drug absorption and uterine response. Obstet Gynecol. 2006;108:582-590. doi: 10.1097/01 .AOG.0000230398.32794.9d.
- Allanson ER, Copson S, Spilsbury K, et al. Pretreatment with mifepristone compared with misoprostol alone for delivery after fetal death between 14 and 28 weeks of gestation. Obstet Gynecol. 2021;137:801-809. doi: 10.1097 /AOG.0000000000004344.
- Chaudhuri P, Datta S. Mifepristone and misoprostol compared with misoprostol alone for induction of labor in intrauterine fetal death: a randomized trial. J Obstet Gynaecol Res. 2015;41:1884-1890. doi: 10.1111/jog.12815.
- Fyfe R, Murray H. Comparison of induction of labour regimens for termination of pregnancy with and without mifepristone, from 20 to 41 weeks gestation. Aust N Z J Obstet Gynaecol. 2017;57:604-608. doi: 10.1111 /ajo.12648.
- Panda S, Jha V, Singh S. Role of combination of mifepristone and misoprostol verses misoprostol alone in induction of labour in late intrauterine fetal death: a prospective study. J Family Reprod Health. 2013;7:177-179.
- Vayrynen W, Heikinheimo O, Nuutila M. Misoprostol-only versus mifepristone plus misoprostol in induction of labor following intrauterine fetal death. Acta Obstet Gynecol Scand. 2007;86: 701-705. doi: 10.1080/00016340701379853.
- Sharma D, Singhal SR, Poonam AP. Comparison of mifepristone combination with misoprostol and misoprostol alone in the management of intrauterine death. Taiwan J Obstet Gynecol. 2011;50:322-325. doi: 10.1016/j.tjog.2011.07.007.
- Stibbe KJM, de Weerd S. Induction of delivery by mifepristone and misoprostol in termination of pregnancy and intrauterine fetal death: 2nd and 3rd trimester induction of labour. Arch Gynecol Obstet. 2012;286:795-796. doi: 10.1007 /s00404-012-2289-3.
- Andersen J, Grine E, Eng L, et al. Expression of connexin-43 in human myometrium and leiomyoma. Am J Obstet Gynecol. 1993;169:1266-1276. doi: 10.1016/0002-9378(93)90293-r.
- Ou CW, Orsino A, Lye SJ. Expression of connexin-43 and connexin-26 in the rat myometrium during pregnancy and labor is differentially regulated by mechanical and hormonal signals. Endocrinology. 1997;138:5398-5407. doi: 10.1210 /endo.138.12.5624.
- Petrocelli T, Lye SJ. Regulation of transcripts encoding the myometrial gap junction protein, connexin-43, by estrogen and progesterone. Endocrinology. 1993;133:284-290. doi: 10.1210 /endo.133.1.8391423.
- Chwalisz K, Fahrenholz F, Hackenberg M, et al. The progesterone antagonist onapristone increases the effectiveness of oxytocin to produce delivery without changing the myometrial oxytocin receptor concentration. Am J Obstet Gynecol. 1991;165:1760-1770. doi: 10.1016/0002 -9378(91)90030-u.
- Large MJ, DeMayo FJ. The regulation of embryo implantation and endometrial decidualization by progesterone receptor signaling. Mol Cell Endocrinol. 2012;358:155-165. doi: 10.1016 /j.mce.2011.07.027.
- Clark K, Ji H, Feltovich H, et al. Mifepristone-induced cervical ripening: structural, biomechanical and molecular events. Am J Obstet Gynecol. 2006;194:1391-1398. doi: 10.1016 /j.ajog.2005.11.026.
- Raaijmakers HCA, Versteegh JE, Uitdehaag JCM. T he x-ray structure of RU486 bound to the progesterone receptor in a destabilized agonist conformation. J Biol Chem. 2009;284:19572-19579. doi: 10.1074/jbc.M109.007872.
- Yuen KCJ, Moraitis A, Nguyen D. Evaluation of evidence of adrenal insufficiency in trials of normocortisolemic patients treated with mifepristone. J Endocr Soc. 2017;1:237-246. doi: 10.1210 /js.2016-1097.
- Spitz IM, Grunberg SM, Chabbert-Buffet N, et al. Management of patients receiving long-term treatment with mifepristone. Fertil Steril. 2005;84:1719-1726. doi: 10.1016 /j.fertnstert.2005.05.056.
- Bertagna X, Escourolle H, Pinquier JL, et al. Administration of RU 486 for 8 days in normal volunteers: antiglucocorticoid effect with no evidence of peripheral cortisol deprivation. J Clin Endocrinol Metab. 1994;78:375-380. doi: 10.1210 /jcem.78.2.8106625.
- Mifeprex [package insert]. New York, NY: Danco Laboratories; March 2016.
- Early pregnancy loss. ACOG Practice Bulletin No 200. American College of Obstetricians and Gynecologists. Obstet Gynecol. 2018;132:e197-e207. doi: /AOG.0000000000002899. 10.1097
- Chu J, Devall AJ, Hardy P, et al. What is the best method for managing early miscarriage? BMJ. 2020;368:l6483. doi: 10.1136/bmj.l6438.
- Schreiber C, Creinin MD, Atrio J, et al. Mifepristone pretreatment for the medical management of early pregnancy loss. N Engl J Med. 2018;378:2161-2170. doi: 10.1056 /NEJMoa1715726.
- Chu JJ, Devall AJ, Beeson LE, et al. Mifepristone and misoprostol versus misoprostol alone for the management of missed miscarriage (MifeMiso): a randomised, double-blind, placebo-controlled trial. Lancet. 2020;396:770-778. doi: 10.1016 /S0140-6736(20)31788-8.
- Sonalkar S, Koelper N, Creinin MD, et al. Management of early pregnancy loss with mifepristone and misoprostol: clinical predictors of treatment success from a randomized trial. Am J Obstet Gynecol. 2020;223:551.e1-e7. doi: 10.1016/j. ajog.2020.04.006. 17.
- Nagendra D, Koelper N, Loza-Avalos SE, et al. Cost-effectiveness of mifepristone pretreatment for the medical management of nonviable early pregnancy: secondary analysis of a randomized clinical trial. JAMA Netw Open. 2020;3:e201594. doi: 10.1001/jamanetworkopen.2020.1594.
- Okeke-Ogwulu CB, Williams EV, Chu JJ, et al. Cost-effectiveness of mifepristone and misoprostol versus misoprostol alone for the management of missed miscarriage: an economic evaluation based on the MifeMiso trial. BJOG. 2021;128: 1534-1545. doi: 10.1111/1471-0528.16737.
- Tang OS, Schweer H, Seyberth HW, et al. Pharmacokinetics of different routes of administration of misoprostol. Hum Reprod. 2002;17:332336. doi: 10.1093/humrep/17.2.332.
- Schaff EA, DiCenzo R, Fielding SL. Comparison of misoprostol plasma concentrations following buccal and sublingual administration. Contraception. 2005;71:22-25. doi: 10.1016 /j.contraception.2004.06.014.
- Meckstroth KR, Whitaker AK, Bertisch S, et al. Misoprostol administered by epithelial routes: drug absorption and uterine response. Obstet Gynecol. 2006;108:582-590. doi: 10.1097/01 .AOG.0000230398.32794.9d.
- Allanson ER, Copson S, Spilsbury K, et al. Pretreatment with mifepristone compared with misoprostol alone for delivery after fetal death between 14 and 28 weeks of gestation. Obstet Gynecol. 2021;137:801-809. doi: 10.1097 /AOG.0000000000004344.
- Chaudhuri P, Datta S. Mifepristone and misoprostol compared with misoprostol alone for induction of labor in intrauterine fetal death: a randomized trial. J Obstet Gynaecol Res. 2015;41:1884-1890. doi: 10.1111/jog.12815.
- Fyfe R, Murray H. Comparison of induction of labour regimens for termination of pregnancy with and without mifepristone, from 20 to 41 weeks gestation. Aust N Z J Obstet Gynaecol. 2017;57:604-608. doi: 10.1111 /ajo.12648.
- Panda S, Jha V, Singh S. Role of combination of mifepristone and misoprostol verses misoprostol alone in induction of labour in late intrauterine fetal death: a prospective study. J Family Reprod Health. 2013;7:177-179.
- Vayrynen W, Heikinheimo O, Nuutila M. Misoprostol-only versus mifepristone plus misoprostol in induction of labor following intrauterine fetal death. Acta Obstet Gynecol Scand. 2007;86: 701-705. doi: 10.1080/00016340701379853.
- Sharma D, Singhal SR, Poonam AP. Comparison of mifepristone combination with misoprostol and misoprostol alone in the management of intrauterine death. Taiwan J Obstet Gynecol. 2011;50:322-325. doi: 10.1016/j.tjog.2011.07.007.
- Stibbe KJM, de Weerd S. Induction of delivery by mifepristone and misoprostol in termination of pregnancy and intrauterine fetal death: 2nd and 3rd trimester induction of labour. Arch Gynecol Obstet. 2012;286:795-796. doi: 10.1007 /s00404-012-2289-3.
Monkeypox: Another emerging threat?
CASE Pregnant woman’s husband is ill after traveling
A 29-year-old primigravid woman at 18 weeks’ gestation just returned from a 10-day trip to Nigeria with her husband. While in Nigeria, the couple went on safari. On several occasions during the safari, they consumed bushmeat prepared by their guides. Her husband now has severe malaise, fever, chills, myalgias, cough, and prominent submandibular, cervical, and inguinal adenopathy. In addition, he has developed a diffuse papular-vesicular rash on his trunk and extremities.
- What is the most likely diagnosis?
- Does this condition pose a danger to his wife?
- What treatment is indicated for his wife?
What we know
In recent weeks, the specter of another poorly understood biological threat has emerged in the medical literature and lay press: monkeypox. This article will first review the epidemiology, clinical manifestations, and diagnosis of this infection, followed by a discussion of how to prevent and treat the condition, with special emphasis on the risks that this infection poses in pregnant women.
Virology
The monkeypox virus is a member of the orthopoxvirus genus. The variola (smallpox) virus and vaccinia virus are included in this genus. It is one of the largest of all viruses, measuring 200-250 nm. It is enveloped and contains double-stranded DNA. Its natural reservoir is probably African rodents. Two distinct strains of monkeypox exist in different geographical regions of Africa: the Central African clade and the West African clade. The Central African clade is significantly more virulent than the latter, with a mortality rate approaching 10%, versus 1% in the West African clade. The incubation period of the virus ranges from 4-20 days and averages 12 days.1,2
Epidemiology
Monkeypox was first discovered in 1958 by Preben von Magnus in a colony of research monkeys in Copenhagen, Denmark. The first case of monkeypox in humans occurred in the Democratic Republic of Congo in 1970 in a 9-year-old boy. Subsequently, cases were reported in the Ivory Coast, Liberia, Nigeria, and Sierra Leone. The infection was limited to the rain forests of central and western Africa until 2003. At that time, the first cases in the United States were reported. The US cases occurred in the Midwest and were traced to exposure to pet prairie dogs. These animals all came from a single distributor, and they apparently were infected when they were housed in the same space with Gambian rats, which are well recognized reservoirs of monkeypox in their native habitat in Africa.1-3
A limited outbreak of monkeypox occurred in the United Kingdom in 2018. Seventy-one cases, with no fatalities, were reported. In 2021 another US case of monkeypox was reported in Dallas, Texas, in an individual who had recently traveled to the United States from Nigeria. A second US case was reported in November 2021 from a patient in Maryland who had returned from a visit to Nigeria. Those were the only 2 reported cases of monkeypox in the United States in 2021.1-3
Then in early May 2022, the United Kingdom reported 9 cases of monkeypox. The first infected patient had recently traveled to Nigeria and, subsequently, infected 2 members of his family.4 On May 18, the Massachusetts Department of Public Health confirmed a case of monkeypox in an adult man who had recently traveled to Canada. As of July 7, 6,027 cases have been reported from at least 39 countries.
The current outbreak is unusual in that, previously, almost all cases occurred in western and central Africa in remote tropical rain forests. Infection usually resulted from close exposure to rats, rabbits, squirrels, monkeys, porcupines, and gazelles. Exposure occurred when persons captured, slaughtered, prepared, and then ate these animals for food without properly cooking the flesh.
The leading theory is that the present outbreak originated among men who had sex with men at 2 raves held in Spain and Belgium. The virus appears to have been spread by skin-to-skin contact, by respiratory droplets, by contact with contaminated bedding, and probably by sperm.2,4,6
Continue to: Clinical manifestations...
Clinical manifestations
Monkeypox evolves through 2 stages: a pre-eruptive stage and an eruptive stage. Prodromal symptoms include malaise, severe headache, myalgias, fever, drenching sweats, backache, fatigue, sore throat, dyspnea, and cough. Within 2-3 days, the characteristic skin eruption develops. The lesions usually begin on the face and then spread in a centrifugal manner to the trunk and extremities, including the palms of the hands and soles of the feet. The lesions typically progress from macules to papules to vesicles to pustules. They then crust and scab over. An interesting additional finding is the presence of prominent lymphadenopathy behind the ear, beneath the mandible, in the neck, and in the groin.1
Several different illnesses must be considered in the differential diagnosis of monkeypox infection. They include measles, scabies, secondary syphilis, and medication-associated allergic reactions. However, the 2 conditions most likely to be confused with monkeypox are chickenpox (varicella) and smallpox. Lymphadenopathy is much more prominent in monkeypox compared with chickenpox. Moreover, with monkeypox, all lesions tend to be at the same stage of evolution as opposed to appearing in crops as they do in chickenpox. Smallpox would be extremely unlikely in the absence of a recognized laboratory accident or a bioterrorism incident.7
Diagnosis
The presumptive diagnosis of monkeypox infection is made primarily based on clinical examination. However, laboratory testing is indicated to definitively differentiate monkeypox from other orthopoxvirus infections such as varicella and smallpox.
In specialized laboratories that employ highly trained personnel and maintain strict safety precautions, the virus can be isolated in mammalian cell cultures. Electron microscopy is a valuable tool for identifying the characteristic brick-shaped poxvirus virions. Routine histologic examination of a lesion will show ballooning degeneration of keratinocytes, prominent spongiosis, dermal edema, and acute inflammation, although these findings are not unique to monkeypox.1
The Centers for Disease Control and Prevention (CDC) has developed serologic tests that detect immunoglobulin (Ig) M- and IgG-specific antibody. However, the most useful and practical diagnostic test is assessment of a skin scraping by polymerase chain reaction (PCR). This test is more sensitive than assessment of serum PCR.1
When the diagnosis of monkeypox is being considered, the clinician should coordinate testing through the local and state public health departments and through the CDC. Effective communication with all agencies will ensure that laboratory specimens are processed in a timely and efficient manner. The CDC website presents information on specimen collection.8
How do we manage monkeypox?
Prevention
The first step in prevention of infection is to isolate infected individuals until all lesions have dried and crusted over. Susceptible people should avoid close contact with skin lesions, respiratory and genital secretions, and bedding of patients who are infected.
The ultimate preventive measure, however, is vaccination of susceptible people either immediately before exposure (eg, military personnel, first responders, infection control investigators, health care workers) or immediately after exposure (general population). Older individuals who received the original smallpox vaccine likely have immunity to monkeypox infection. Unfortunately, very few women who currently are of reproductive age received this vaccine because its use was discontinued in the United States in the early 1970s. Therefore, the vast majority of our patients are uniquely susceptible to this infection and should be vaccinated if there is an outbreak of monkeypox in their locality.7,9
The current preferred vaccine for prevention of both smallpox and monkeypox is the Jynneos (Bavarian Nordic A/S) vaccine.10 This agent incorporates a replication-deficient live virus and does not pose the same risk for adverse events as the original versions of the smallpox vaccine. Jynneos is administered subcutaneously rather than by scarification. Two 0.5-mL doses, delivered 28 days apart, are required for optimal effect. The vaccine must be obtained from local and state health departments, in consultation with the CDC.7,9
There is very little published information on the safety of the Jynneos vaccine in pregnant or lactating women, although animal data are reassuring. Moreover, the dangers of monkeypox infection are significant, and in the event of an outbreak, vaccination of susceptible individuals, including pregnant women, is indicated.
- Monkeypox is a member of the orthopoxvirus genus and is closely related to the smallpox virus. It is a large, double-stranded, enveloped DNA virus.
- The virus is transmitted primarily by close contact with infected animals or other humans or by consumption of contaminated bushmeat.
- The infection evolves in 2 phases. The pre-eruptive phase is characterized by severe flu-like symptoms and signs. The eruptive phase is distinguished by a diffuse papular-vesicular rash.
- The most valuable test for confirming the diagnosis is a polymerase chain reaction test of a fresh skin lesion.
- In women who are pregnant, monkeypox has been associated with spontaneous abortion and fetal death.
- Three antiviral agents may be of value in treating infected patients: cidofovir, brincidofovir, and tecovirimat. Only the latter has an acceptable safety profile for women who are pregnant or lactating.
- The new nonreplicating smallpox vaccine Jynneos (Bavarian Nordic A/S) is of great value for pre- and post-exposure prophylaxis.
Continue to: Treatment...
Treatment
Infected pregnant women should receive acetaminophen 1,000 mg orally every 8 hours, to control fever and provide analgesia. An antihistamine such as diphenhydramine 25 mg orally every 6-8 hours, may be used to control pruritus and provide mild sedation. Adequate fluid intake and optimal nutrition should be encouraged. Skin lesions should be inspected regularly to detect signs of superimposed bacterial infections. Small, localized bacterial skin infections can be treated with topical application of mupirocin ointment 2%, 3 times daily for 7-14 days. For diffuse and more severe bacterial skin infections, a systemic antibiotic may be necessary. Reasonable choices include amoxicillin-clavulanate 875 mg/125 mg orally every 12 hours, or trimethoprim-sulfamethoxazole double strength 800 mg/160 mg orally every 12 hours.11 The latter agent should be avoided in the first trimester of pregnancy because of potential teratogenic effects.
Several specific agents are available through the CDC for treatment of orthopoxvirus infections such as smallpox and monkeypox. Information about these agents is summarized in the TABLE.12-16
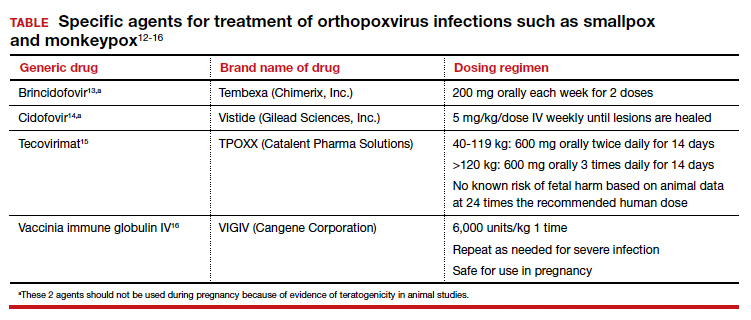
Unique considerations in pregnancy
Because monkeypox is so rare, there is very little information about the effects of this infection in pregnant women. The report most commonly cited in the literature is that by Mbala et al, which was published in 2017.17 These authors described 4 pregnant patients in the Democratic Republic of Congo who contracted monkeypox infection over a 4-year period. All 4 women were hospitalized and treated with systemic antibiotics, antiparasitic medications, and analgesics. One patient delivered a healthy infant. Two women had spontaneous abortions in the first trimester. The fourth patient experienced a stillbirth at 22 weeks’ gestation. At postmortem examination, the fetus had diffuse cutaneous lesions, prominent hepatomegaly, and hydrops. No structural malformations were noted. The placenta demonstrated numerous punctate hemorrhages, and high concentrations of virus were recovered from the placenta and from fetal tissue.
Although the information on pregnancy outcome is quite limited, it seems clear that the virus can cross the placenta and cause adverse effects such as spontaneous abortion and fetal death. Accordingly, I think the following guidelines are a reasonable approach to a pregnant patient who has been exposed to monkeypox or who has developed manifestations of infection.3,7,9
- In the event of a community outbreak, bioterrorism event, or exposure to a person with suspected or confirmed monkeypox infection, the pregnant patient should receive the Jynneos vaccine.
- The pregnant patient should be isolated from any individual with suspected or confirmed monkeypox.
- If infection develops despite these measures, the patient should be treated with either tecovirimat or vaccinia immune globulin IV. Hospitalization may be necessary for seriously ill individuals.
- Within 2 weeks of infection, a comprehensive ultrasound examination should be performed to assess for structural abnormalities in the fetus.
- Subsequently, serial ultrasound examinations should be performed at intervals of 4-6 weeks to assess fetal growth and re-evaluate fetal anatomy.
- Following delivery, a detailed neonatal examination should be performed to assess for evidence of viral injury. Neonatal skin lesions and neonatal serum can be assessed by PCR for monkeypox virus. The newborn should be isolated from the mother until all the mother’s lesions have dried and crusted over.
CASE Resolved
Given the husband’s recent travel to Nigeria and consumption of bushmeat, he most likely has monkeypox. The infection can be spread from person to person by close contact; thus, his wife is at risk. The couple should isolate until all of his lesions have dried and crusted over. The woman also should receive the Jynneos vaccine. If she becomes symptomatic, she should be treated with tecovirimat or vaccinia immune globulin IV. ●
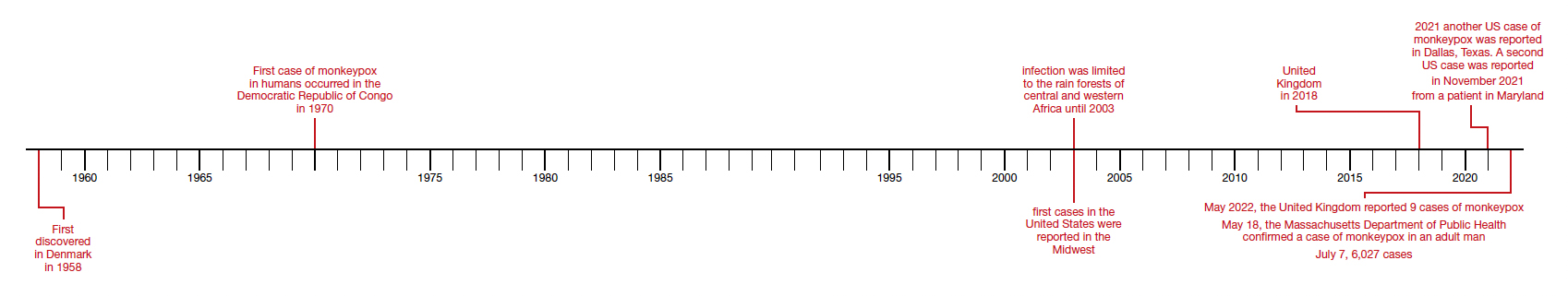
- Isaacs SN, Shenoy ES. Monkeypox. UpToDate. Updated June 28,2022. Accessed July 1, 2022. https://www.uptodate.com /contents/monkeypox?topicRef=8349&source=see_link
- Graham MB. Monkeypox. Medscape. Updated June 29, 2022. Accessed July 1, 2022. https://emedicine.medscape.com /article/1134714-overview.
- Khalil A, Samara A, O’Brien P, et al. Monkeypox and pregnancy: what do obstetricians need to know? Ultrasound Obstet Gynecol. 2022;60:22-27. doi:10.1002/uog.24968.
- World Health Organization. Monkeypox-United Kingdom of Great Britain and Northern Ireland. May 18, 2022. Accessed July 1, 2022. https://www.who.int/emergencies/diseaseoutbreak-news/item/2022-DON383.
- WHO reports two new monkeypox deaths, cases in new areas. Reuters. July 7, 2022. https://www.reuters.com/world /who-reports-two-new-monkeypox-deaths-2022-07-07/. Accessed July 19, 2022.
- World Health Organization. Multi-country monkeypox outbreak in non-endemic countries: update. May 29, 2022. Accessed July 1, 2022. https://www.who.int /emergencies/disease-outbreak-news/item/2022 -DON388#:~:text=Multi%2Dcountry%20monkeypox%20 outbreak%20in%20non%2Dendemic%20countries%3A%20 Update,-29%20May%202022&text=Since%2013%20 May%202022%2C%20monkeypox,Epidemiological%20 investigations%20are%20ongoing.
- Cono J, Cragan JD, Jamieson DJ, Rasmussen SA. Prophylaxis and treatment of pregnant women for emerging infections andbioterrorism emergencies. Emerg Infect Dis. 2006;12:16311637. doi:10.3201/eid1211.060618.
- Centers for Disease Control and Prevention. Preparation and collection of specimens. Reviewed June 29, 2022. Accessed July 6, 2022. https://www.cdc.gov/poxvirus /monkeypox/clinicians/prep-collection-specimens.html.
- Rao AK, Petersen BW, Whitehill F, et al. Monkeypox vaccination. MMWR Morb Mortal Wkly Rep. 2022;71:734-742. doi:10.15585/mmwr.mm7122e1.
- Smallpox and monkeypox vaccine, live, nonreplicating. Package insert. Bavarian Nordic A/S; 2021. Accessed July 1, 2022. https://www.fda.gov/media/131078/download.
- Duff P. Commonly used antibiotics in ObGyn practice. OBG Manag. 2022;34:29, 36-40. doi:10.12788/obgm.0191.
- Centers for Disease Control and Prevention. Treatment information for healthcare professionals: interim clinical guidance for the treatment of monkeypox. Reviewed June 17, 2022. Accessed July 1, 2022. https://www.cdc.gov/poxvirus /monkeypox/clinicians/treatment.html.
- Brincidofovir. Prescribing information. Chimerix, Inc.; 2021. Accessed July 1, 2022. https://www.accessdata.fda.gov /drugsatfda_docs/label/2021/214460s000,214461s000lbl.pdf.
- Cidofovir. Package insert. Gilead Sciences, Inc.; 2010. Accessed July 1, 2022. https://www.gilead.com/~/media /Files/pdfs/medicines/other/vistide/vistide.pdf.
- Tecovirimat. Prescribing information. Catalent Pharma Solutions; 2022. Accessed July 1, 2022. https://www.accessdata.fda.gov/drugsatfda_docs /label/2022/214518s000lbl.pdf.
- Vaccinia immune globulin IV. Prescribing information. Cangene Corporation; 2010. Accessed July 1, 2022. https: //www.fda.gov/media/77004/download.
- Mbala PK, Huggins JW, Riu-Rovira T, et al. Maternal and fetal outcomes among pregnant women with human monkeypox infection in the Democratic Republic of Congo. J Infect Dis. 2017;216:824-828. doi:10.1093/infdis/jix260.
CASE Pregnant woman’s husband is ill after traveling
A 29-year-old primigravid woman at 18 weeks’ gestation just returned from a 10-day trip to Nigeria with her husband. While in Nigeria, the couple went on safari. On several occasions during the safari, they consumed bushmeat prepared by their guides. Her husband now has severe malaise, fever, chills, myalgias, cough, and prominent submandibular, cervical, and inguinal adenopathy. In addition, he has developed a diffuse papular-vesicular rash on his trunk and extremities.
- What is the most likely diagnosis?
- Does this condition pose a danger to his wife?
- What treatment is indicated for his wife?
What we know
In recent weeks, the specter of another poorly understood biological threat has emerged in the medical literature and lay press: monkeypox. This article will first review the epidemiology, clinical manifestations, and diagnosis of this infection, followed by a discussion of how to prevent and treat the condition, with special emphasis on the risks that this infection poses in pregnant women.
Virology
The monkeypox virus is a member of the orthopoxvirus genus. The variola (smallpox) virus and vaccinia virus are included in this genus. It is one of the largest of all viruses, measuring 200-250 nm. It is enveloped and contains double-stranded DNA. Its natural reservoir is probably African rodents. Two distinct strains of monkeypox exist in different geographical regions of Africa: the Central African clade and the West African clade. The Central African clade is significantly more virulent than the latter, with a mortality rate approaching 10%, versus 1% in the West African clade. The incubation period of the virus ranges from 4-20 days and averages 12 days.1,2
Epidemiology
Monkeypox was first discovered in 1958 by Preben von Magnus in a colony of research monkeys in Copenhagen, Denmark. The first case of monkeypox in humans occurred in the Democratic Republic of Congo in 1970 in a 9-year-old boy. Subsequently, cases were reported in the Ivory Coast, Liberia, Nigeria, and Sierra Leone. The infection was limited to the rain forests of central and western Africa until 2003. At that time, the first cases in the United States were reported. The US cases occurred in the Midwest and were traced to exposure to pet prairie dogs. These animals all came from a single distributor, and they apparently were infected when they were housed in the same space with Gambian rats, which are well recognized reservoirs of monkeypox in their native habitat in Africa.1-3
A limited outbreak of monkeypox occurred in the United Kingdom in 2018. Seventy-one cases, with no fatalities, were reported. In 2021 another US case of monkeypox was reported in Dallas, Texas, in an individual who had recently traveled to the United States from Nigeria. A second US case was reported in November 2021 from a patient in Maryland who had returned from a visit to Nigeria. Those were the only 2 reported cases of monkeypox in the United States in 2021.1-3
Then in early May 2022, the United Kingdom reported 9 cases of monkeypox. The first infected patient had recently traveled to Nigeria and, subsequently, infected 2 members of his family.4 On May 18, the Massachusetts Department of Public Health confirmed a case of monkeypox in an adult man who had recently traveled to Canada. As of July 7, 6,027 cases have been reported from at least 39 countries.
The current outbreak is unusual in that, previously, almost all cases occurred in western and central Africa in remote tropical rain forests. Infection usually resulted from close exposure to rats, rabbits, squirrels, monkeys, porcupines, and gazelles. Exposure occurred when persons captured, slaughtered, prepared, and then ate these animals for food without properly cooking the flesh.
The leading theory is that the present outbreak originated among men who had sex with men at 2 raves held in Spain and Belgium. The virus appears to have been spread by skin-to-skin contact, by respiratory droplets, by contact with contaminated bedding, and probably by sperm.2,4,6
Continue to: Clinical manifestations...
Clinical manifestations
Monkeypox evolves through 2 stages: a pre-eruptive stage and an eruptive stage. Prodromal symptoms include malaise, severe headache, myalgias, fever, drenching sweats, backache, fatigue, sore throat, dyspnea, and cough. Within 2-3 days, the characteristic skin eruption develops. The lesions usually begin on the face and then spread in a centrifugal manner to the trunk and extremities, including the palms of the hands and soles of the feet. The lesions typically progress from macules to papules to vesicles to pustules. They then crust and scab over. An interesting additional finding is the presence of prominent lymphadenopathy behind the ear, beneath the mandible, in the neck, and in the groin.1
Several different illnesses must be considered in the differential diagnosis of monkeypox infection. They include measles, scabies, secondary syphilis, and medication-associated allergic reactions. However, the 2 conditions most likely to be confused with monkeypox are chickenpox (varicella) and smallpox. Lymphadenopathy is much more prominent in monkeypox compared with chickenpox. Moreover, with monkeypox, all lesions tend to be at the same stage of evolution as opposed to appearing in crops as they do in chickenpox. Smallpox would be extremely unlikely in the absence of a recognized laboratory accident or a bioterrorism incident.7
Diagnosis
The presumptive diagnosis of monkeypox infection is made primarily based on clinical examination. However, laboratory testing is indicated to definitively differentiate monkeypox from other orthopoxvirus infections such as varicella and smallpox.
In specialized laboratories that employ highly trained personnel and maintain strict safety precautions, the virus can be isolated in mammalian cell cultures. Electron microscopy is a valuable tool for identifying the characteristic brick-shaped poxvirus virions. Routine histologic examination of a lesion will show ballooning degeneration of keratinocytes, prominent spongiosis, dermal edema, and acute inflammation, although these findings are not unique to monkeypox.1
The Centers for Disease Control and Prevention (CDC) has developed serologic tests that detect immunoglobulin (Ig) M- and IgG-specific antibody. However, the most useful and practical diagnostic test is assessment of a skin scraping by polymerase chain reaction (PCR). This test is more sensitive than assessment of serum PCR.1
When the diagnosis of monkeypox is being considered, the clinician should coordinate testing through the local and state public health departments and through the CDC. Effective communication with all agencies will ensure that laboratory specimens are processed in a timely and efficient manner. The CDC website presents information on specimen collection.8
How do we manage monkeypox?
Prevention
The first step in prevention of infection is to isolate infected individuals until all lesions have dried and crusted over. Susceptible people should avoid close contact with skin lesions, respiratory and genital secretions, and bedding of patients who are infected.
The ultimate preventive measure, however, is vaccination of susceptible people either immediately before exposure (eg, military personnel, first responders, infection control investigators, health care workers) or immediately after exposure (general population). Older individuals who received the original smallpox vaccine likely have immunity to monkeypox infection. Unfortunately, very few women who currently are of reproductive age received this vaccine because its use was discontinued in the United States in the early 1970s. Therefore, the vast majority of our patients are uniquely susceptible to this infection and should be vaccinated if there is an outbreak of monkeypox in their locality.7,9
The current preferred vaccine for prevention of both smallpox and monkeypox is the Jynneos (Bavarian Nordic A/S) vaccine.10 This agent incorporates a replication-deficient live virus and does not pose the same risk for adverse events as the original versions of the smallpox vaccine. Jynneos is administered subcutaneously rather than by scarification. Two 0.5-mL doses, delivered 28 days apart, are required for optimal effect. The vaccine must be obtained from local and state health departments, in consultation with the CDC.7,9
There is very little published information on the safety of the Jynneos vaccine in pregnant or lactating women, although animal data are reassuring. Moreover, the dangers of monkeypox infection are significant, and in the event of an outbreak, vaccination of susceptible individuals, including pregnant women, is indicated.
- Monkeypox is a member of the orthopoxvirus genus and is closely related to the smallpox virus. It is a large, double-stranded, enveloped DNA virus.
- The virus is transmitted primarily by close contact with infected animals or other humans or by consumption of contaminated bushmeat.
- The infection evolves in 2 phases. The pre-eruptive phase is characterized by severe flu-like symptoms and signs. The eruptive phase is distinguished by a diffuse papular-vesicular rash.
- The most valuable test for confirming the diagnosis is a polymerase chain reaction test of a fresh skin lesion.
- In women who are pregnant, monkeypox has been associated with spontaneous abortion and fetal death.
- Three antiviral agents may be of value in treating infected patients: cidofovir, brincidofovir, and tecovirimat. Only the latter has an acceptable safety profile for women who are pregnant or lactating.
- The new nonreplicating smallpox vaccine Jynneos (Bavarian Nordic A/S) is of great value for pre- and post-exposure prophylaxis.
Continue to: Treatment...
Treatment
Infected pregnant women should receive acetaminophen 1,000 mg orally every 8 hours, to control fever and provide analgesia. An antihistamine such as diphenhydramine 25 mg orally every 6-8 hours, may be used to control pruritus and provide mild sedation. Adequate fluid intake and optimal nutrition should be encouraged. Skin lesions should be inspected regularly to detect signs of superimposed bacterial infections. Small, localized bacterial skin infections can be treated with topical application of mupirocin ointment 2%, 3 times daily for 7-14 days. For diffuse and more severe bacterial skin infections, a systemic antibiotic may be necessary. Reasonable choices include amoxicillin-clavulanate 875 mg/125 mg orally every 12 hours, or trimethoprim-sulfamethoxazole double strength 800 mg/160 mg orally every 12 hours.11 The latter agent should be avoided in the first trimester of pregnancy because of potential teratogenic effects.
Several specific agents are available through the CDC for treatment of orthopoxvirus infections such as smallpox and monkeypox. Information about these agents is summarized in the TABLE.12-16

Unique considerations in pregnancy
Because monkeypox is so rare, there is very little information about the effects of this infection in pregnant women. The report most commonly cited in the literature is that by Mbala et al, which was published in 2017.17 These authors described 4 pregnant patients in the Democratic Republic of Congo who contracted monkeypox infection over a 4-year period. All 4 women were hospitalized and treated with systemic antibiotics, antiparasitic medications, and analgesics. One patient delivered a healthy infant. Two women had spontaneous abortions in the first trimester. The fourth patient experienced a stillbirth at 22 weeks’ gestation. At postmortem examination, the fetus had diffuse cutaneous lesions, prominent hepatomegaly, and hydrops. No structural malformations were noted. The placenta demonstrated numerous punctate hemorrhages, and high concentrations of virus were recovered from the placenta and from fetal tissue.
Although the information on pregnancy outcome is quite limited, it seems clear that the virus can cross the placenta and cause adverse effects such as spontaneous abortion and fetal death. Accordingly, I think the following guidelines are a reasonable approach to a pregnant patient who has been exposed to monkeypox or who has developed manifestations of infection.3,7,9
- In the event of a community outbreak, bioterrorism event, or exposure to a person with suspected or confirmed monkeypox infection, the pregnant patient should receive the Jynneos vaccine.
- The pregnant patient should be isolated from any individual with suspected or confirmed monkeypox.
- If infection develops despite these measures, the patient should be treated with either tecovirimat or vaccinia immune globulin IV. Hospitalization may be necessary for seriously ill individuals.
- Within 2 weeks of infection, a comprehensive ultrasound examination should be performed to assess for structural abnormalities in the fetus.
- Subsequently, serial ultrasound examinations should be performed at intervals of 4-6 weeks to assess fetal growth and re-evaluate fetal anatomy.
- Following delivery, a detailed neonatal examination should be performed to assess for evidence of viral injury. Neonatal skin lesions and neonatal serum can be assessed by PCR for monkeypox virus. The newborn should be isolated from the mother until all the mother’s lesions have dried and crusted over.
CASE Resolved
Given the husband’s recent travel to Nigeria and consumption of bushmeat, he most likely has monkeypox. The infection can be spread from person to person by close contact; thus, his wife is at risk. The couple should isolate until all of his lesions have dried and crusted over. The woman also should receive the Jynneos vaccine. If she becomes symptomatic, she should be treated with tecovirimat or vaccinia immune globulin IV. ●

CASE Pregnant woman’s husband is ill after traveling
A 29-year-old primigravid woman at 18 weeks’ gestation just returned from a 10-day trip to Nigeria with her husband. While in Nigeria, the couple went on safari. On several occasions during the safari, they consumed bushmeat prepared by their guides. Her husband now has severe malaise, fever, chills, myalgias, cough, and prominent submandibular, cervical, and inguinal adenopathy. In addition, he has developed a diffuse papular-vesicular rash on his trunk and extremities.
- What is the most likely diagnosis?
- Does this condition pose a danger to his wife?
- What treatment is indicated for his wife?
What we know
In recent weeks, the specter of another poorly understood biological threat has emerged in the medical literature and lay press: monkeypox. This article will first review the epidemiology, clinical manifestations, and diagnosis of this infection, followed by a discussion of how to prevent and treat the condition, with special emphasis on the risks that this infection poses in pregnant women.
Virology
The monkeypox virus is a member of the orthopoxvirus genus. The variola (smallpox) virus and vaccinia virus are included in this genus. It is one of the largest of all viruses, measuring 200-250 nm. It is enveloped and contains double-stranded DNA. Its natural reservoir is probably African rodents. Two distinct strains of monkeypox exist in different geographical regions of Africa: the Central African clade and the West African clade. The Central African clade is significantly more virulent than the latter, with a mortality rate approaching 10%, versus 1% in the West African clade. The incubation period of the virus ranges from 4-20 days and averages 12 days.1,2
Epidemiology
Monkeypox was first discovered in 1958 by Preben von Magnus in a colony of research monkeys in Copenhagen, Denmark. The first case of monkeypox in humans occurred in the Democratic Republic of Congo in 1970 in a 9-year-old boy. Subsequently, cases were reported in the Ivory Coast, Liberia, Nigeria, and Sierra Leone. The infection was limited to the rain forests of central and western Africa until 2003. At that time, the first cases in the United States were reported. The US cases occurred in the Midwest and were traced to exposure to pet prairie dogs. These animals all came from a single distributor, and they apparently were infected when they were housed in the same space with Gambian rats, which are well recognized reservoirs of monkeypox in their native habitat in Africa.1-3
A limited outbreak of monkeypox occurred in the United Kingdom in 2018. Seventy-one cases, with no fatalities, were reported. In 2021 another US case of monkeypox was reported in Dallas, Texas, in an individual who had recently traveled to the United States from Nigeria. A second US case was reported in November 2021 from a patient in Maryland who had returned from a visit to Nigeria. Those were the only 2 reported cases of monkeypox in the United States in 2021.1-3
Then in early May 2022, the United Kingdom reported 9 cases of monkeypox. The first infected patient had recently traveled to Nigeria and, subsequently, infected 2 members of his family.4 On May 18, the Massachusetts Department of Public Health confirmed a case of monkeypox in an adult man who had recently traveled to Canada. As of July 7, 6,027 cases have been reported from at least 39 countries.
The current outbreak is unusual in that, previously, almost all cases occurred in western and central Africa in remote tropical rain forests. Infection usually resulted from close exposure to rats, rabbits, squirrels, monkeys, porcupines, and gazelles. Exposure occurred when persons captured, slaughtered, prepared, and then ate these animals for food without properly cooking the flesh.
The leading theory is that the present outbreak originated among men who had sex with men at 2 raves held in Spain and Belgium. The virus appears to have been spread by skin-to-skin contact, by respiratory droplets, by contact with contaminated bedding, and probably by sperm.2,4,6
Continue to: Clinical manifestations...
Clinical manifestations
Monkeypox evolves through 2 stages: a pre-eruptive stage and an eruptive stage. Prodromal symptoms include malaise, severe headache, myalgias, fever, drenching sweats, backache, fatigue, sore throat, dyspnea, and cough. Within 2-3 days, the characteristic skin eruption develops. The lesions usually begin on the face and then spread in a centrifugal manner to the trunk and extremities, including the palms of the hands and soles of the feet. The lesions typically progress from macules to papules to vesicles to pustules. They then crust and scab over. An interesting additional finding is the presence of prominent lymphadenopathy behind the ear, beneath the mandible, in the neck, and in the groin.1
Several different illnesses must be considered in the differential diagnosis of monkeypox infection. They include measles, scabies, secondary syphilis, and medication-associated allergic reactions. However, the 2 conditions most likely to be confused with monkeypox are chickenpox (varicella) and smallpox. Lymphadenopathy is much more prominent in monkeypox compared with chickenpox. Moreover, with monkeypox, all lesions tend to be at the same stage of evolution as opposed to appearing in crops as they do in chickenpox. Smallpox would be extremely unlikely in the absence of a recognized laboratory accident or a bioterrorism incident.7
Diagnosis
The presumptive diagnosis of monkeypox infection is made primarily based on clinical examination. However, laboratory testing is indicated to definitively differentiate monkeypox from other orthopoxvirus infections such as varicella and smallpox.
In specialized laboratories that employ highly trained personnel and maintain strict safety precautions, the virus can be isolated in mammalian cell cultures. Electron microscopy is a valuable tool for identifying the characteristic brick-shaped poxvirus virions. Routine histologic examination of a lesion will show ballooning degeneration of keratinocytes, prominent spongiosis, dermal edema, and acute inflammation, although these findings are not unique to monkeypox.1
The Centers for Disease Control and Prevention (CDC) has developed serologic tests that detect immunoglobulin (Ig) M- and IgG-specific antibody. However, the most useful and practical diagnostic test is assessment of a skin scraping by polymerase chain reaction (PCR). This test is more sensitive than assessment of serum PCR.1
When the diagnosis of monkeypox is being considered, the clinician should coordinate testing through the local and state public health departments and through the CDC. Effective communication with all agencies will ensure that laboratory specimens are processed in a timely and efficient manner. The CDC website presents information on specimen collection.8
How do we manage monkeypox?
Prevention
The first step in prevention of infection is to isolate infected individuals until all lesions have dried and crusted over. Susceptible people should avoid close contact with skin lesions, respiratory and genital secretions, and bedding of patients who are infected.
The ultimate preventive measure, however, is vaccination of susceptible people either immediately before exposure (eg, military personnel, first responders, infection control investigators, health care workers) or immediately after exposure (general population). Older individuals who received the original smallpox vaccine likely have immunity to monkeypox infection. Unfortunately, very few women who currently are of reproductive age received this vaccine because its use was discontinued in the United States in the early 1970s. Therefore, the vast majority of our patients are uniquely susceptible to this infection and should be vaccinated if there is an outbreak of monkeypox in their locality.7,9
The current preferred vaccine for prevention of both smallpox and monkeypox is the Jynneos (Bavarian Nordic A/S) vaccine.10 This agent incorporates a replication-deficient live virus and does not pose the same risk for adverse events as the original versions of the smallpox vaccine. Jynneos is administered subcutaneously rather than by scarification. Two 0.5-mL doses, delivered 28 days apart, are required for optimal effect. The vaccine must be obtained from local and state health departments, in consultation with the CDC.7,9
There is very little published information on the safety of the Jynneos vaccine in pregnant or lactating women, although animal data are reassuring. Moreover, the dangers of monkeypox infection are significant, and in the event of an outbreak, vaccination of susceptible individuals, including pregnant women, is indicated.
- Monkeypox is a member of the orthopoxvirus genus and is closely related to the smallpox virus. It is a large, double-stranded, enveloped DNA virus.
- The virus is transmitted primarily by close contact with infected animals or other humans or by consumption of contaminated bushmeat.
- The infection evolves in 2 phases. The pre-eruptive phase is characterized by severe flu-like symptoms and signs. The eruptive phase is distinguished by a diffuse papular-vesicular rash.
- The most valuable test for confirming the diagnosis is a polymerase chain reaction test of a fresh skin lesion.
- In women who are pregnant, monkeypox has been associated with spontaneous abortion and fetal death.
- Three antiviral agents may be of value in treating infected patients: cidofovir, brincidofovir, and tecovirimat. Only the latter has an acceptable safety profile for women who are pregnant or lactating.
- The new nonreplicating smallpox vaccine Jynneos (Bavarian Nordic A/S) is of great value for pre- and post-exposure prophylaxis.
Continue to: Treatment...
Treatment
Infected pregnant women should receive acetaminophen 1,000 mg orally every 8 hours, to control fever and provide analgesia. An antihistamine such as diphenhydramine 25 mg orally every 6-8 hours, may be used to control pruritus and provide mild sedation. Adequate fluid intake and optimal nutrition should be encouraged. Skin lesions should be inspected regularly to detect signs of superimposed bacterial infections. Small, localized bacterial skin infections can be treated with topical application of mupirocin ointment 2%, 3 times daily for 7-14 days. For diffuse and more severe bacterial skin infections, a systemic antibiotic may be necessary. Reasonable choices include amoxicillin-clavulanate 875 mg/125 mg orally every 12 hours, or trimethoprim-sulfamethoxazole double strength 800 mg/160 mg orally every 12 hours.11 The latter agent should be avoided in the first trimester of pregnancy because of potential teratogenic effects.
Several specific agents are available through the CDC for treatment of orthopoxvirus infections such as smallpox and monkeypox. Information about these agents is summarized in the TABLE.12-16

Unique considerations in pregnancy
Because monkeypox is so rare, there is very little information about the effects of this infection in pregnant women. The report most commonly cited in the literature is that by Mbala et al, which was published in 2017.17 These authors described 4 pregnant patients in the Democratic Republic of Congo who contracted monkeypox infection over a 4-year period. All 4 women were hospitalized and treated with systemic antibiotics, antiparasitic medications, and analgesics. One patient delivered a healthy infant. Two women had spontaneous abortions in the first trimester. The fourth patient experienced a stillbirth at 22 weeks’ gestation. At postmortem examination, the fetus had diffuse cutaneous lesions, prominent hepatomegaly, and hydrops. No structural malformations were noted. The placenta demonstrated numerous punctate hemorrhages, and high concentrations of virus were recovered from the placenta and from fetal tissue.
Although the information on pregnancy outcome is quite limited, it seems clear that the virus can cross the placenta and cause adverse effects such as spontaneous abortion and fetal death. Accordingly, I think the following guidelines are a reasonable approach to a pregnant patient who has been exposed to monkeypox or who has developed manifestations of infection.3,7,9
- In the event of a community outbreak, bioterrorism event, or exposure to a person with suspected or confirmed monkeypox infection, the pregnant patient should receive the Jynneos vaccine.
- The pregnant patient should be isolated from any individual with suspected or confirmed monkeypox.
- If infection develops despite these measures, the patient should be treated with either tecovirimat or vaccinia immune globulin IV. Hospitalization may be necessary for seriously ill individuals.
- Within 2 weeks of infection, a comprehensive ultrasound examination should be performed to assess for structural abnormalities in the fetus.
- Subsequently, serial ultrasound examinations should be performed at intervals of 4-6 weeks to assess fetal growth and re-evaluate fetal anatomy.
- Following delivery, a detailed neonatal examination should be performed to assess for evidence of viral injury. Neonatal skin lesions and neonatal serum can be assessed by PCR for monkeypox virus. The newborn should be isolated from the mother until all the mother’s lesions have dried and crusted over.
CASE Resolved
Given the husband’s recent travel to Nigeria and consumption of bushmeat, he most likely has monkeypox. The infection can be spread from person to person by close contact; thus, his wife is at risk. The couple should isolate until all of his lesions have dried and crusted over. The woman also should receive the Jynneos vaccine. If she becomes symptomatic, she should be treated with tecovirimat or vaccinia immune globulin IV. ●

- Isaacs SN, Shenoy ES. Monkeypox. UpToDate. Updated June 28,2022. Accessed July 1, 2022. https://www.uptodate.com /contents/monkeypox?topicRef=8349&source=see_link
- Graham MB. Monkeypox. Medscape. Updated June 29, 2022. Accessed July 1, 2022. https://emedicine.medscape.com /article/1134714-overview.
- Khalil A, Samara A, O’Brien P, et al. Monkeypox and pregnancy: what do obstetricians need to know? Ultrasound Obstet Gynecol. 2022;60:22-27. doi:10.1002/uog.24968.
- World Health Organization. Monkeypox-United Kingdom of Great Britain and Northern Ireland. May 18, 2022. Accessed July 1, 2022. https://www.who.int/emergencies/diseaseoutbreak-news/item/2022-DON383.
- WHO reports two new monkeypox deaths, cases in new areas. Reuters. July 7, 2022. https://www.reuters.com/world /who-reports-two-new-monkeypox-deaths-2022-07-07/. Accessed July 19, 2022.
- World Health Organization. Multi-country monkeypox outbreak in non-endemic countries: update. May 29, 2022. Accessed July 1, 2022. https://www.who.int /emergencies/disease-outbreak-news/item/2022 -DON388#:~:text=Multi%2Dcountry%20monkeypox%20 outbreak%20in%20non%2Dendemic%20countries%3A%20 Update,-29%20May%202022&text=Since%2013%20 May%202022%2C%20monkeypox,Epidemiological%20 investigations%20are%20ongoing.
- Cono J, Cragan JD, Jamieson DJ, Rasmussen SA. Prophylaxis and treatment of pregnant women for emerging infections andbioterrorism emergencies. Emerg Infect Dis. 2006;12:16311637. doi:10.3201/eid1211.060618.
- Centers for Disease Control and Prevention. Preparation and collection of specimens. Reviewed June 29, 2022. Accessed July 6, 2022. https://www.cdc.gov/poxvirus /monkeypox/clinicians/prep-collection-specimens.html.
- Rao AK, Petersen BW, Whitehill F, et al. Monkeypox vaccination. MMWR Morb Mortal Wkly Rep. 2022;71:734-742. doi:10.15585/mmwr.mm7122e1.
- Smallpox and monkeypox vaccine, live, nonreplicating. Package insert. Bavarian Nordic A/S; 2021. Accessed July 1, 2022. https://www.fda.gov/media/131078/download.
- Duff P. Commonly used antibiotics in ObGyn practice. OBG Manag. 2022;34:29, 36-40. doi:10.12788/obgm.0191.
- Centers for Disease Control and Prevention. Treatment information for healthcare professionals: interim clinical guidance for the treatment of monkeypox. Reviewed June 17, 2022. Accessed July 1, 2022. https://www.cdc.gov/poxvirus /monkeypox/clinicians/treatment.html.
- Brincidofovir. Prescribing information. Chimerix, Inc.; 2021. Accessed July 1, 2022. https://www.accessdata.fda.gov /drugsatfda_docs/label/2021/214460s000,214461s000lbl.pdf.
- Cidofovir. Package insert. Gilead Sciences, Inc.; 2010. Accessed July 1, 2022. https://www.gilead.com/~/media /Files/pdfs/medicines/other/vistide/vistide.pdf.
- Tecovirimat. Prescribing information. Catalent Pharma Solutions; 2022. Accessed July 1, 2022. https://www.accessdata.fda.gov/drugsatfda_docs /label/2022/214518s000lbl.pdf.
- Vaccinia immune globulin IV. Prescribing information. Cangene Corporation; 2010. Accessed July 1, 2022. https: //www.fda.gov/media/77004/download.
- Mbala PK, Huggins JW, Riu-Rovira T, et al. Maternal and fetal outcomes among pregnant women with human monkeypox infection in the Democratic Republic of Congo. J Infect Dis. 2017;216:824-828. doi:10.1093/infdis/jix260.
- Isaacs SN, Shenoy ES. Monkeypox. UpToDate. Updated June 28,2022. Accessed July 1, 2022. https://www.uptodate.com /contents/monkeypox?topicRef=8349&source=see_link
- Graham MB. Monkeypox. Medscape. Updated June 29, 2022. Accessed July 1, 2022. https://emedicine.medscape.com /article/1134714-overview.
- Khalil A, Samara A, O’Brien P, et al. Monkeypox and pregnancy: what do obstetricians need to know? Ultrasound Obstet Gynecol. 2022;60:22-27. doi:10.1002/uog.24968.
- World Health Organization. Monkeypox-United Kingdom of Great Britain and Northern Ireland. May 18, 2022. Accessed July 1, 2022. https://www.who.int/emergencies/diseaseoutbreak-news/item/2022-DON383.
- WHO reports two new monkeypox deaths, cases in new areas. Reuters. July 7, 2022. https://www.reuters.com/world /who-reports-two-new-monkeypox-deaths-2022-07-07/. Accessed July 19, 2022.
- World Health Organization. Multi-country monkeypox outbreak in non-endemic countries: update. May 29, 2022. Accessed July 1, 2022. https://www.who.int /emergencies/disease-outbreak-news/item/2022 -DON388#:~:text=Multi%2Dcountry%20monkeypox%20 outbreak%20in%20non%2Dendemic%20countries%3A%20 Update,-29%20May%202022&text=Since%2013%20 May%202022%2C%20monkeypox,Epidemiological%20 investigations%20are%20ongoing.
- Cono J, Cragan JD, Jamieson DJ, Rasmussen SA. Prophylaxis and treatment of pregnant women for emerging infections andbioterrorism emergencies. Emerg Infect Dis. 2006;12:16311637. doi:10.3201/eid1211.060618.
- Centers for Disease Control and Prevention. Preparation and collection of specimens. Reviewed June 29, 2022. Accessed July 6, 2022. https://www.cdc.gov/poxvirus /monkeypox/clinicians/prep-collection-specimens.html.
- Rao AK, Petersen BW, Whitehill F, et al. Monkeypox vaccination. MMWR Morb Mortal Wkly Rep. 2022;71:734-742. doi:10.15585/mmwr.mm7122e1.
- Smallpox and monkeypox vaccine, live, nonreplicating. Package insert. Bavarian Nordic A/S; 2021. Accessed July 1, 2022. https://www.fda.gov/media/131078/download.
- Duff P. Commonly used antibiotics in ObGyn practice. OBG Manag. 2022;34:29, 36-40. doi:10.12788/obgm.0191.
- Centers for Disease Control and Prevention. Treatment information for healthcare professionals: interim clinical guidance for the treatment of monkeypox. Reviewed June 17, 2022. Accessed July 1, 2022. https://www.cdc.gov/poxvirus /monkeypox/clinicians/treatment.html.
- Brincidofovir. Prescribing information. Chimerix, Inc.; 2021. Accessed July 1, 2022. https://www.accessdata.fda.gov /drugsatfda_docs/label/2021/214460s000,214461s000lbl.pdf.
- Cidofovir. Package insert. Gilead Sciences, Inc.; 2010. Accessed July 1, 2022. https://www.gilead.com/~/media /Files/pdfs/medicines/other/vistide/vistide.pdf.
- Tecovirimat. Prescribing information. Catalent Pharma Solutions; 2022. Accessed July 1, 2022. https://www.accessdata.fda.gov/drugsatfda_docs /label/2022/214518s000lbl.pdf.
- Vaccinia immune globulin IV. Prescribing information. Cangene Corporation; 2010. Accessed July 1, 2022. https: //www.fda.gov/media/77004/download.
- Mbala PK, Huggins JW, Riu-Rovira T, et al. Maternal and fetal outcomes among pregnant women with human monkeypox infection in the Democratic Republic of Congo. J Infect Dis. 2017;216:824-828. doi:10.1093/infdis/jix260.
Power-morcellation hysterectomies declined and most performed with no containment bag
The use of laparoscopic power morcellators for minimally invasive hysterectomy has significantly decreased, and while the use of containment bags increased after the U.S. Food and Drug Administration’s 2014 safety warning about power morcellators, most procedures employing them are still performed without bags, according to a large database study in Obstetrics & Gynecology.
Containment bags are thought to limit the dissemination of potentially pathologic tissue, including unsuspected cancerous cells.
Rates of uterine cancer in women having morcellation were similar before and after the 2014 FDA guidance, and containment bags were used in only a small proportion of women with uterine cancer, according to findings from a research group led by Jason D. Wright, MD, of the division of gynecologic oncology at Columbia University, New York.
“Despite warnings from professional societies and regulatory agencies, as well as intense public scrutiny after the FDA warnings, the majority of morcellated uterine cancers occurred with uncontained laparoscopic power morcellation,” Dr. Wright and associates wrote, adding that the findings have important policy implications. First, efforts are needed to ensure morcellation is avoided in women with pathologic abnormalities. Second, despite regulatory approval, the safety and efficacy of containment bags remain uncertain, and the use and outcomes of these devices should be monitored closely.
The authors noted that laparoscopic power morcellation with a containment bag actually carries a small but significant increase in the risk of complications, compared with uncontained morcellation.
The study
Drawing on the Premier Healthcare Database, the researchers looked at deidentified patients aged 18 years or older who underwent laparoscopic supracervical hysterectomy from 2010 to 2018. The largest age group having the procedure consisted of women aged 40-49.
Patients were stratified based on use of laparoscopic power morcellators.
The cohort was further stratified as either pre–FDA guidance (2010 quarter 1 to 2014 quarter 1) or post–FDA guidance (2014 quarter 2 to 2018 quarter 2).
In the final cohort of 67,115 patients, laparoscopic power morcellator use decreased from 66.7% in 2013 quarter 4 to 13.3% by 2018 quarter 2. The likelihood of using this device decreased by 9.5% for each quarter elapsed in the post–FDA warning period (risk ratio, 0.91; 95% confidence interval, 0.90-0.91).
In other findings, containment bag use rose from 5.2% in 2013 quarter 4 to 15.2% by 2018 quarter 2. The likelihood of containment bag use rose by 3% for each quarter elapsed in the post–FDA warning period (RR, 1.03; 95% CI, 1.02-1.05).
Among women who underwent surgery with laparoscopic power morcellator use, uterine cancers or sarcomas were identified in 54 (0.17%) before the FDA guidance, compared with 7 (0.12%) after the guidance (P = .45).
Containment bags were used in 11.1% of women with uterine cancers or sarcomas before the FDA guidance, compared with 14.3% after the guidance (P = .12). The perioperative complication rate was 3.3% among women who had laparoscopic power morcellator use without a containment bag, compared with 4.5% (P = .001) in those with a containment bag (adjusted RR, 1.35; 95% CI, 1.12-1.64).
A related editorial argued that the backlash against power morcellation was unwarranted and an example of “reactionary medicine.”
Ben A. Abdu, MD, and Cameron Lowry, MD, of the department of obstetrics and gynecology at the University of Tennessee Health Science Center in Memphis, noted that with the known advantages of laparoscopy over laparotomy – decreased blood loss, decreased pain, and fewer wound complications and infections – it is of paramount importance to continue to offer minimally invasive surgery whenever possible. After the FDA raised safety concerns, there was a rise in the rate of open abdominal hysterectomy, which was accompanied by an increase in surgical morbidity. “Perhaps for now we should avoid throwing the baby out with the bath water,” they wrote.
The editorialists pointed out that any surgery may entail unintended complications. “It is also important to remember that there is a risk of dissemination of malignant tissue whether or not power morcellation is used, and it has even been observed in laparotomy,” they stated, noting that bag rupture and tissue spillage can occur even when the containment bag remains intact.
The downward trend in the use of power morcellators observed by Dr. Wright’s group is of serious concern, the commentators added, especially because the FDA communication was made in response to a rare occurrence and possibly resting on an overestimation of risk. “Based on their review of the medical literature at the time, the FDA cited prevalence estimates of 1 in 352 for any uterine sarcoma and 1 in 498 for leiomyosarcoma,” they wrote. “Many authors have expressed concern that the FDA data review was overestimated.” For example, they cite a meta-analysis using prospective data in which the prevalence of occult leiomyosarcoma was estimated at 1 in 8,300. Despite this extremely low prevalence, there has been an almost total nationwide hospital moratorium on the use of power morcellation, which will likely continue. Some manufacturers have ceased or limited production, distribution, and sales of these devices, they noted.
According to Dr. Michael L. Nimaroff, MD, however, chief of minimally invasive gynecologic surgery at Northwell Health in New Hyde Park, N.Y., the general post–FDA-guidance backlash did not have much effect on expert practitioners in this surgical field. “Those of us who specialize in minimally invasive gynecologic surgery, which has many benefits for the patients, never pivoted,” he told this news organization. “We continued to perform it but more conscientiously and with more concern for safety.”
As for morcellator use, added Dr. Nimaroff, specialists were so accustomed to doing these surgeries before the containment systems were made available that they don’t miss the power morcellator. “We actually retrieve tissue manually, and most of our morcellations, if they’re not contained manually, are retrieved vaginally or through a slightly bigger incision. So patients still benefit from minimally invasive surgery, and in some cases these techniques actually shorten the operation.”
This study received no external funding. Dr. Wright is editor in chief of Obstetrics & Gynecology. He reported royalties from UpToDate and has received research support from Merck. Coauthor Dr. Hou has served as a consultant for Foundation Medicine and Natera. Dr. Abdu and Dr. Lowry disclosed no competing interests, as did Dr. Nimaroff.
The use of laparoscopic power morcellators for minimally invasive hysterectomy has significantly decreased, and while the use of containment bags increased after the U.S. Food and Drug Administration’s 2014 safety warning about power morcellators, most procedures employing them are still performed without bags, according to a large database study in Obstetrics & Gynecology.
Containment bags are thought to limit the dissemination of potentially pathologic tissue, including unsuspected cancerous cells.
Rates of uterine cancer in women having morcellation were similar before and after the 2014 FDA guidance, and containment bags were used in only a small proportion of women with uterine cancer, according to findings from a research group led by Jason D. Wright, MD, of the division of gynecologic oncology at Columbia University, New York.
“Despite warnings from professional societies and regulatory agencies, as well as intense public scrutiny after the FDA warnings, the majority of morcellated uterine cancers occurred with uncontained laparoscopic power morcellation,” Dr. Wright and associates wrote, adding that the findings have important policy implications. First, efforts are needed to ensure morcellation is avoided in women with pathologic abnormalities. Second, despite regulatory approval, the safety and efficacy of containment bags remain uncertain, and the use and outcomes of these devices should be monitored closely.
The authors noted that laparoscopic power morcellation with a containment bag actually carries a small but significant increase in the risk of complications, compared with uncontained morcellation.
The study
Drawing on the Premier Healthcare Database, the researchers looked at deidentified patients aged 18 years or older who underwent laparoscopic supracervical hysterectomy from 2010 to 2018. The largest age group having the procedure consisted of women aged 40-49.
Patients were stratified based on use of laparoscopic power morcellators.
The cohort was further stratified as either pre–FDA guidance (2010 quarter 1 to 2014 quarter 1) or post–FDA guidance (2014 quarter 2 to 2018 quarter 2).
In the final cohort of 67,115 patients, laparoscopic power morcellator use decreased from 66.7% in 2013 quarter 4 to 13.3% by 2018 quarter 2. The likelihood of using this device decreased by 9.5% for each quarter elapsed in the post–FDA warning period (risk ratio, 0.91; 95% confidence interval, 0.90-0.91).
In other findings, containment bag use rose from 5.2% in 2013 quarter 4 to 15.2% by 2018 quarter 2. The likelihood of containment bag use rose by 3% for each quarter elapsed in the post–FDA warning period (RR, 1.03; 95% CI, 1.02-1.05).
Among women who underwent surgery with laparoscopic power morcellator use, uterine cancers or sarcomas were identified in 54 (0.17%) before the FDA guidance, compared with 7 (0.12%) after the guidance (P = .45).
Containment bags were used in 11.1% of women with uterine cancers or sarcomas before the FDA guidance, compared with 14.3% after the guidance (P = .12). The perioperative complication rate was 3.3% among women who had laparoscopic power morcellator use without a containment bag, compared with 4.5% (P = .001) in those with a containment bag (adjusted RR, 1.35; 95% CI, 1.12-1.64).
A related editorial argued that the backlash against power morcellation was unwarranted and an example of “reactionary medicine.”
Ben A. Abdu, MD, and Cameron Lowry, MD, of the department of obstetrics and gynecology at the University of Tennessee Health Science Center in Memphis, noted that with the known advantages of laparoscopy over laparotomy – decreased blood loss, decreased pain, and fewer wound complications and infections – it is of paramount importance to continue to offer minimally invasive surgery whenever possible. After the FDA raised safety concerns, there was a rise in the rate of open abdominal hysterectomy, which was accompanied by an increase in surgical morbidity. “Perhaps for now we should avoid throwing the baby out with the bath water,” they wrote.
The editorialists pointed out that any surgery may entail unintended complications. “It is also important to remember that there is a risk of dissemination of malignant tissue whether or not power morcellation is used, and it has even been observed in laparotomy,” they stated, noting that bag rupture and tissue spillage can occur even when the containment bag remains intact.
The downward trend in the use of power morcellators observed by Dr. Wright’s group is of serious concern, the commentators added, especially because the FDA communication was made in response to a rare occurrence and possibly resting on an overestimation of risk. “Based on their review of the medical literature at the time, the FDA cited prevalence estimates of 1 in 352 for any uterine sarcoma and 1 in 498 for leiomyosarcoma,” they wrote. “Many authors have expressed concern that the FDA data review was overestimated.” For example, they cite a meta-analysis using prospective data in which the prevalence of occult leiomyosarcoma was estimated at 1 in 8,300. Despite this extremely low prevalence, there has been an almost total nationwide hospital moratorium on the use of power morcellation, which will likely continue. Some manufacturers have ceased or limited production, distribution, and sales of these devices, they noted.
According to Dr. Michael L. Nimaroff, MD, however, chief of minimally invasive gynecologic surgery at Northwell Health in New Hyde Park, N.Y., the general post–FDA-guidance backlash did not have much effect on expert practitioners in this surgical field. “Those of us who specialize in minimally invasive gynecologic surgery, which has many benefits for the patients, never pivoted,” he told this news organization. “We continued to perform it but more conscientiously and with more concern for safety.”
As for morcellator use, added Dr. Nimaroff, specialists were so accustomed to doing these surgeries before the containment systems were made available that they don’t miss the power morcellator. “We actually retrieve tissue manually, and most of our morcellations, if they’re not contained manually, are retrieved vaginally or through a slightly bigger incision. So patients still benefit from minimally invasive surgery, and in some cases these techniques actually shorten the operation.”
This study received no external funding. Dr. Wright is editor in chief of Obstetrics & Gynecology. He reported royalties from UpToDate and has received research support from Merck. Coauthor Dr. Hou has served as a consultant for Foundation Medicine and Natera. Dr. Abdu and Dr. Lowry disclosed no competing interests, as did Dr. Nimaroff.
The use of laparoscopic power morcellators for minimally invasive hysterectomy has significantly decreased, and while the use of containment bags increased after the U.S. Food and Drug Administration’s 2014 safety warning about power morcellators, most procedures employing them are still performed without bags, according to a large database study in Obstetrics & Gynecology.
Containment bags are thought to limit the dissemination of potentially pathologic tissue, including unsuspected cancerous cells.
Rates of uterine cancer in women having morcellation were similar before and after the 2014 FDA guidance, and containment bags were used in only a small proportion of women with uterine cancer, according to findings from a research group led by Jason D. Wright, MD, of the division of gynecologic oncology at Columbia University, New York.
“Despite warnings from professional societies and regulatory agencies, as well as intense public scrutiny after the FDA warnings, the majority of morcellated uterine cancers occurred with uncontained laparoscopic power morcellation,” Dr. Wright and associates wrote, adding that the findings have important policy implications. First, efforts are needed to ensure morcellation is avoided in women with pathologic abnormalities. Second, despite regulatory approval, the safety and efficacy of containment bags remain uncertain, and the use and outcomes of these devices should be monitored closely.
The authors noted that laparoscopic power morcellation with a containment bag actually carries a small but significant increase in the risk of complications, compared with uncontained morcellation.
The study
Drawing on the Premier Healthcare Database, the researchers looked at deidentified patients aged 18 years or older who underwent laparoscopic supracervical hysterectomy from 2010 to 2018. The largest age group having the procedure consisted of women aged 40-49.
Patients were stratified based on use of laparoscopic power morcellators.
The cohort was further stratified as either pre–FDA guidance (2010 quarter 1 to 2014 quarter 1) or post–FDA guidance (2014 quarter 2 to 2018 quarter 2).
In the final cohort of 67,115 patients, laparoscopic power morcellator use decreased from 66.7% in 2013 quarter 4 to 13.3% by 2018 quarter 2. The likelihood of using this device decreased by 9.5% for each quarter elapsed in the post–FDA warning period (risk ratio, 0.91; 95% confidence interval, 0.90-0.91).
In other findings, containment bag use rose from 5.2% in 2013 quarter 4 to 15.2% by 2018 quarter 2. The likelihood of containment bag use rose by 3% for each quarter elapsed in the post–FDA warning period (RR, 1.03; 95% CI, 1.02-1.05).
Among women who underwent surgery with laparoscopic power morcellator use, uterine cancers or sarcomas were identified in 54 (0.17%) before the FDA guidance, compared with 7 (0.12%) after the guidance (P = .45).
Containment bags were used in 11.1% of women with uterine cancers or sarcomas before the FDA guidance, compared with 14.3% after the guidance (P = .12). The perioperative complication rate was 3.3% among women who had laparoscopic power morcellator use without a containment bag, compared with 4.5% (P = .001) in those with a containment bag (adjusted RR, 1.35; 95% CI, 1.12-1.64).
A related editorial argued that the backlash against power morcellation was unwarranted and an example of “reactionary medicine.”
Ben A. Abdu, MD, and Cameron Lowry, MD, of the department of obstetrics and gynecology at the University of Tennessee Health Science Center in Memphis, noted that with the known advantages of laparoscopy over laparotomy – decreased blood loss, decreased pain, and fewer wound complications and infections – it is of paramount importance to continue to offer minimally invasive surgery whenever possible. After the FDA raised safety concerns, there was a rise in the rate of open abdominal hysterectomy, which was accompanied by an increase in surgical morbidity. “Perhaps for now we should avoid throwing the baby out with the bath water,” they wrote.
The editorialists pointed out that any surgery may entail unintended complications. “It is also important to remember that there is a risk of dissemination of malignant tissue whether or not power morcellation is used, and it has even been observed in laparotomy,” they stated, noting that bag rupture and tissue spillage can occur even when the containment bag remains intact.
The downward trend in the use of power morcellators observed by Dr. Wright’s group is of serious concern, the commentators added, especially because the FDA communication was made in response to a rare occurrence and possibly resting on an overestimation of risk. “Based on their review of the medical literature at the time, the FDA cited prevalence estimates of 1 in 352 for any uterine sarcoma and 1 in 498 for leiomyosarcoma,” they wrote. “Many authors have expressed concern that the FDA data review was overestimated.” For example, they cite a meta-analysis using prospective data in which the prevalence of occult leiomyosarcoma was estimated at 1 in 8,300. Despite this extremely low prevalence, there has been an almost total nationwide hospital moratorium on the use of power morcellation, which will likely continue. Some manufacturers have ceased or limited production, distribution, and sales of these devices, they noted.
According to Dr. Michael L. Nimaroff, MD, however, chief of minimally invasive gynecologic surgery at Northwell Health in New Hyde Park, N.Y., the general post–FDA-guidance backlash did not have much effect on expert practitioners in this surgical field. “Those of us who specialize in minimally invasive gynecologic surgery, which has many benefits for the patients, never pivoted,” he told this news organization. “We continued to perform it but more conscientiously and with more concern for safety.”
As for morcellator use, added Dr. Nimaroff, specialists were so accustomed to doing these surgeries before the containment systems were made available that they don’t miss the power morcellator. “We actually retrieve tissue manually, and most of our morcellations, if they’re not contained manually, are retrieved vaginally or through a slightly bigger incision. So patients still benefit from minimally invasive surgery, and in some cases these techniques actually shorten the operation.”
This study received no external funding. Dr. Wright is editor in chief of Obstetrics & Gynecology. He reported royalties from UpToDate and has received research support from Merck. Coauthor Dr. Hou has served as a consultant for Foundation Medicine and Natera. Dr. Abdu and Dr. Lowry disclosed no competing interests, as did Dr. Nimaroff.
FROM OBSTETRICS & GYNECOLOGY
Does cannabis help with menopause symptoms?
Many women with symptoms of menopause are turning to cannabis for help, researchers have found, despite a lack of evidence that the drug works for these issues.
In a survey of perimenopausal and menopausal women who said they’ve used cannabis, nearly 80% said they use medical marijuana to alleviate symptoms such as sleep disturbances, hot flashes, and mood swings.
“Increasingly, we see greater numbers of individuals exploiting the use of cannabis and cannabinoids for lots of conditions. We realized there was no long-term data on how women were treating themselves for conditions like menopause,” said Staci Gruber, PhD, director of the Marijuana Investigations for Neuroscientific Discovery (MIND) program at McLean Hospital, an affiliate of Harvard Medical School, Boston, who led the study.
Dr. Gruber and her colleagues surveyed 131 perimenopausal and 127 postmenopausal women about their use of cannabis, identifying them through targeted advertising and social media platforms such as Twitter, Facebook, and Reddit.
The survey, published in Menopause, found 83.5% reported habitual cannabis use and 86% said they were current users. Around half of the women reported mixed medical/recreational use; 30.8% reported recreational use only and 17.7% said they only used medical forms of the drug.
The three most common modes of cannabis use were smoking a joint, bowl, or bong (84.3%); using edibles (78.3%);, and vaping oils (52.6%).
The researchers found that women in perimenopause reported markedly worse symptoms than did those in menopause, and these women tended to use a wider variety of cannabis products.
Dr. Gruber said clinicians should be asking their menopausal patients if they use cannabis to alleviate their symptoms.
Stephanie Faubion, MD, MBA, a women’s health expert at Mayo Clinic in Rochester, Minn., and Jacksonville, Fla., said the looming question is whether cannabis in fact works in these patients.
“What we need is to figure out whether it works for women, and that hasn’t been studied yet,” she said.
Dr. Faubion, medical director for the North American Menopause Society, said the society is now conducting a review of worldwide data on nonhormonal treatments for symptoms of menopause. The report, which will examine the most current research on the effects of cannabis, hypnosis, diet, exercise, acupuncture, yoga, and meditation, will be released in 2023, she said.
Dr. Gruber said she hopes her group’s research will open the doors to more detailed explorations of how strains of cannabis and their levels of cannabidiol, a chemical compound in cannabis plants, and tetrahydrocannabinol, the main psychoactive component in cannabis, affect the symptoms women experience from menopause. Clinical trials for products aimed at specific symptoms also will be important, she added.
“We have a paucity of data from primary care clinicians,” Dr. Gruber said. “We, as researchers and clinicians, should be providing women with the research to make informed choices.”
The study was supported by private donations to the MIND program at McLean Hospital. No funding sources were involved in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Dr. Gruber reported grants from the National Institute on Drug Abuse, Foria/Praxis Ventures, and Charlotte’s Web. She reported personal fees from the Coalition for Cannabis Policy, Education and Regulation; Beth Israel Deaconess; Fenway Health; Greenwich Biosciences Cannabis Education Working Group; and National Academy of Neuropsychology outside the submitted work. Dr. Faubion reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Many women with symptoms of menopause are turning to cannabis for help, researchers have found, despite a lack of evidence that the drug works for these issues.
In a survey of perimenopausal and menopausal women who said they’ve used cannabis, nearly 80% said they use medical marijuana to alleviate symptoms such as sleep disturbances, hot flashes, and mood swings.
“Increasingly, we see greater numbers of individuals exploiting the use of cannabis and cannabinoids for lots of conditions. We realized there was no long-term data on how women were treating themselves for conditions like menopause,” said Staci Gruber, PhD, director of the Marijuana Investigations for Neuroscientific Discovery (MIND) program at McLean Hospital, an affiliate of Harvard Medical School, Boston, who led the study.
Dr. Gruber and her colleagues surveyed 131 perimenopausal and 127 postmenopausal women about their use of cannabis, identifying them through targeted advertising and social media platforms such as Twitter, Facebook, and Reddit.
The survey, published in Menopause, found 83.5% reported habitual cannabis use and 86% said they were current users. Around half of the women reported mixed medical/recreational use; 30.8% reported recreational use only and 17.7% said they only used medical forms of the drug.
The three most common modes of cannabis use were smoking a joint, bowl, or bong (84.3%); using edibles (78.3%);, and vaping oils (52.6%).
The researchers found that women in perimenopause reported markedly worse symptoms than did those in menopause, and these women tended to use a wider variety of cannabis products.
Dr. Gruber said clinicians should be asking their menopausal patients if they use cannabis to alleviate their symptoms.
Stephanie Faubion, MD, MBA, a women’s health expert at Mayo Clinic in Rochester, Minn., and Jacksonville, Fla., said the looming question is whether cannabis in fact works in these patients.
“What we need is to figure out whether it works for women, and that hasn’t been studied yet,” she said.
Dr. Faubion, medical director for the North American Menopause Society, said the society is now conducting a review of worldwide data on nonhormonal treatments for symptoms of menopause. The report, which will examine the most current research on the effects of cannabis, hypnosis, diet, exercise, acupuncture, yoga, and meditation, will be released in 2023, she said.
Dr. Gruber said she hopes her group’s research will open the doors to more detailed explorations of how strains of cannabis and their levels of cannabidiol, a chemical compound in cannabis plants, and tetrahydrocannabinol, the main psychoactive component in cannabis, affect the symptoms women experience from menopause. Clinical trials for products aimed at specific symptoms also will be important, she added.
“We have a paucity of data from primary care clinicians,” Dr. Gruber said. “We, as researchers and clinicians, should be providing women with the research to make informed choices.”
The study was supported by private donations to the MIND program at McLean Hospital. No funding sources were involved in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Dr. Gruber reported grants from the National Institute on Drug Abuse, Foria/Praxis Ventures, and Charlotte’s Web. She reported personal fees from the Coalition for Cannabis Policy, Education and Regulation; Beth Israel Deaconess; Fenway Health; Greenwich Biosciences Cannabis Education Working Group; and National Academy of Neuropsychology outside the submitted work. Dr. Faubion reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Many women with symptoms of menopause are turning to cannabis for help, researchers have found, despite a lack of evidence that the drug works for these issues.
In a survey of perimenopausal and menopausal women who said they’ve used cannabis, nearly 80% said they use medical marijuana to alleviate symptoms such as sleep disturbances, hot flashes, and mood swings.
“Increasingly, we see greater numbers of individuals exploiting the use of cannabis and cannabinoids for lots of conditions. We realized there was no long-term data on how women were treating themselves for conditions like menopause,” said Staci Gruber, PhD, director of the Marijuana Investigations for Neuroscientific Discovery (MIND) program at McLean Hospital, an affiliate of Harvard Medical School, Boston, who led the study.
Dr. Gruber and her colleagues surveyed 131 perimenopausal and 127 postmenopausal women about their use of cannabis, identifying them through targeted advertising and social media platforms such as Twitter, Facebook, and Reddit.
The survey, published in Menopause, found 83.5% reported habitual cannabis use and 86% said they were current users. Around half of the women reported mixed medical/recreational use; 30.8% reported recreational use only and 17.7% said they only used medical forms of the drug.
The three most common modes of cannabis use were smoking a joint, bowl, or bong (84.3%); using edibles (78.3%);, and vaping oils (52.6%).
The researchers found that women in perimenopause reported markedly worse symptoms than did those in menopause, and these women tended to use a wider variety of cannabis products.
Dr. Gruber said clinicians should be asking their menopausal patients if they use cannabis to alleviate their symptoms.
Stephanie Faubion, MD, MBA, a women’s health expert at Mayo Clinic in Rochester, Minn., and Jacksonville, Fla., said the looming question is whether cannabis in fact works in these patients.
“What we need is to figure out whether it works for women, and that hasn’t been studied yet,” she said.
Dr. Faubion, medical director for the North American Menopause Society, said the society is now conducting a review of worldwide data on nonhormonal treatments for symptoms of menopause. The report, which will examine the most current research on the effects of cannabis, hypnosis, diet, exercise, acupuncture, yoga, and meditation, will be released in 2023, she said.
Dr. Gruber said she hopes her group’s research will open the doors to more detailed explorations of how strains of cannabis and their levels of cannabidiol, a chemical compound in cannabis plants, and tetrahydrocannabinol, the main psychoactive component in cannabis, affect the symptoms women experience from menopause. Clinical trials for products aimed at specific symptoms also will be important, she added.
“We have a paucity of data from primary care clinicians,” Dr. Gruber said. “We, as researchers and clinicians, should be providing women with the research to make informed choices.”
The study was supported by private donations to the MIND program at McLean Hospital. No funding sources were involved in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Dr. Gruber reported grants from the National Institute on Drug Abuse, Foria/Praxis Ventures, and Charlotte’s Web. She reported personal fees from the Coalition for Cannabis Policy, Education and Regulation; Beth Israel Deaconess; Fenway Health; Greenwich Biosciences Cannabis Education Working Group; and National Academy of Neuropsychology outside the submitted work. Dr. Faubion reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.