User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
nav[contains(@class, 'nav-ce-stack nav-ce-stack__large-screen')]
header[@id='header']
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'main-prefix')]
footer[@id='footer']
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
div[contains(@class, 'view-medstat-quiz-listing-panes')]
Changes in metabolism tied to risk of subsequent dementia
in new findings that may provide a prevention target.
Investigators found one of the clusters includes small high-density lipoprotein (HDL) metabolites associated with vascular dementia, while another cluster involves ketone bodies and citrate that are primarily associated with Alzheimer’s disease.
Ketone bodies, or ketones, are three related compounds – acetone, acetoacetic acid, and beta-hydroxybutyric acid (BHB) – produced by the liver during fat metabolism. Citrate is a salt or ester of citric acid.
These metabolite clusters are not only linked to the future development of dementia but also correlate with early pathology in those under age 60 years, said study investigator Cornelia M. van Duijn, PhD, professor of epidemiology at Nuffield Department of Population Health, Oxford (England) University.
“These metabolites flag early and late pathology and may be relevant as targets for prevention of dementia,” she noted.
The findings were presented at the 2021 Alzheimer’s Association International Conference.
Weight loss before dementia explained?
For the study, investigators included 125,000 patients from the UK Biobank, which includes 51,031 who were over age 60 at baseline. Of these, 1,188 developed dementia during a follow-up of about 10 years; 553 were diagnosed with Alzheimer’s disease and 298 with vascular dementia.
Researchers used a platform that covers 249 metabolic measures, including small molecules, fatty acids, and lipoprotein lipids.
They estimated risk associated with these metabolites, adjusting for age, sex, body mass index, technical variables, ethnicity, smoking, alcohol, education, metabolic and neuropsychiatric medication, and APOE4 genotypes.
Of the 249 metabolites, 47 (19%) were associated with dementia risk in those over age 60, after adjustment.
The investigators examined effect estimates for associations of metabolites with both Alzheimer’s disease and vascular dementia over age 60 versus hippocampal volume under age 60. They found a “very strong, very significant” association for Alzheimer’s disease, and a “marginally significant” association for vascular dementia, said Dr. van Duijn.
This would be expected, as there is a much stronger correlation between hippocampal and Alzheimer’s disease versus vascular dementia, she added.
“We not only see that the metabolites predict dementia, but also early pathology. This makes these findings rather interesting for targeting prevention,” she said. An analysis of total brain volume showed “very strong, very similar, very significant associations” for both Alzheimer’s disease and vascular dementia,” added Dr. van Duijn.
The researchers found a major shift in various metabolites involved in energy metabolism in the 10-year period before the diagnosis of Alzheimer’s disease. These changes include low levels of branched-chain amino acids and omega-3 fatty acids and high levels of glucose, citrate, acetone, beta-hydroxybutyrate, and acetate. “This finding is in line with that in APOE models that show reduced energy metabolism over age in the brain,” said Dr. van Duijn.
She added that high levels of some of these metabolites are associated with low body weight before dementia onset, which may explain the weight loss seen in patients before developing the disease. “Our hypothesis is that the liver is burning the fat reserves of the patients in order to provide the brain with fuel,” she explained.
Diet a prevention target?
The results also showed ketone bodies increase with age, which may represent the aging brain’s “compensation mechanism” to deal with an energy shortage, said Dr. van Duijn. “Supplementation of ketone bodies, branched-chain amino and omega-3 fatty acids may help support brain function.”
The fact that ketone bodies were positively associated with the risk of dementia is “a very important finding,” she said.
Following this and other presentations, session cochair Rima Kaddurah-Daouk, PhD, professor in psychiatry and behavioral sciences, Institute for Brain Sciences, Duke University, Durham, N.C., noted the research is “an important part of trying to decipher some of the mysteries in Alzheimer’s disease.”
The research contributes to the understanding of how nutrition and diet could influence metabolism and then the brain and is “opening the horizon” for thinking about “strategies for therapeutic interventions,” she said.
The study received funding support from the National Institute on Aging. The investigators have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
in new findings that may provide a prevention target.
Investigators found one of the clusters includes small high-density lipoprotein (HDL) metabolites associated with vascular dementia, while another cluster involves ketone bodies and citrate that are primarily associated with Alzheimer’s disease.
Ketone bodies, or ketones, are three related compounds – acetone, acetoacetic acid, and beta-hydroxybutyric acid (BHB) – produced by the liver during fat metabolism. Citrate is a salt or ester of citric acid.
These metabolite clusters are not only linked to the future development of dementia but also correlate with early pathology in those under age 60 years, said study investigator Cornelia M. van Duijn, PhD, professor of epidemiology at Nuffield Department of Population Health, Oxford (England) University.
“These metabolites flag early and late pathology and may be relevant as targets for prevention of dementia,” she noted.
The findings were presented at the 2021 Alzheimer’s Association International Conference.
Weight loss before dementia explained?
For the study, investigators included 125,000 patients from the UK Biobank, which includes 51,031 who were over age 60 at baseline. Of these, 1,188 developed dementia during a follow-up of about 10 years; 553 were diagnosed with Alzheimer’s disease and 298 with vascular dementia.
Researchers used a platform that covers 249 metabolic measures, including small molecules, fatty acids, and lipoprotein lipids.
They estimated risk associated with these metabolites, adjusting for age, sex, body mass index, technical variables, ethnicity, smoking, alcohol, education, metabolic and neuropsychiatric medication, and APOE4 genotypes.
Of the 249 metabolites, 47 (19%) were associated with dementia risk in those over age 60, after adjustment.
The investigators examined effect estimates for associations of metabolites with both Alzheimer’s disease and vascular dementia over age 60 versus hippocampal volume under age 60. They found a “very strong, very significant” association for Alzheimer’s disease, and a “marginally significant” association for vascular dementia, said Dr. van Duijn.
This would be expected, as there is a much stronger correlation between hippocampal and Alzheimer’s disease versus vascular dementia, she added.
“We not only see that the metabolites predict dementia, but also early pathology. This makes these findings rather interesting for targeting prevention,” she said. An analysis of total brain volume showed “very strong, very similar, very significant associations” for both Alzheimer’s disease and vascular dementia,” added Dr. van Duijn.
The researchers found a major shift in various metabolites involved in energy metabolism in the 10-year period before the diagnosis of Alzheimer’s disease. These changes include low levels of branched-chain amino acids and omega-3 fatty acids and high levels of glucose, citrate, acetone, beta-hydroxybutyrate, and acetate. “This finding is in line with that in APOE models that show reduced energy metabolism over age in the brain,” said Dr. van Duijn.
She added that high levels of some of these metabolites are associated with low body weight before dementia onset, which may explain the weight loss seen in patients before developing the disease. “Our hypothesis is that the liver is burning the fat reserves of the patients in order to provide the brain with fuel,” she explained.
Diet a prevention target?
The results also showed ketone bodies increase with age, which may represent the aging brain’s “compensation mechanism” to deal with an energy shortage, said Dr. van Duijn. “Supplementation of ketone bodies, branched-chain amino and omega-3 fatty acids may help support brain function.”
The fact that ketone bodies were positively associated with the risk of dementia is “a very important finding,” she said.
Following this and other presentations, session cochair Rima Kaddurah-Daouk, PhD, professor in psychiatry and behavioral sciences, Institute for Brain Sciences, Duke University, Durham, N.C., noted the research is “an important part of trying to decipher some of the mysteries in Alzheimer’s disease.”
The research contributes to the understanding of how nutrition and diet could influence metabolism and then the brain and is “opening the horizon” for thinking about “strategies for therapeutic interventions,” she said.
The study received funding support from the National Institute on Aging. The investigators have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
in new findings that may provide a prevention target.
Investigators found one of the clusters includes small high-density lipoprotein (HDL) metabolites associated with vascular dementia, while another cluster involves ketone bodies and citrate that are primarily associated with Alzheimer’s disease.
Ketone bodies, or ketones, are three related compounds – acetone, acetoacetic acid, and beta-hydroxybutyric acid (BHB) – produced by the liver during fat metabolism. Citrate is a salt or ester of citric acid.
These metabolite clusters are not only linked to the future development of dementia but also correlate with early pathology in those under age 60 years, said study investigator Cornelia M. van Duijn, PhD, professor of epidemiology at Nuffield Department of Population Health, Oxford (England) University.
“These metabolites flag early and late pathology and may be relevant as targets for prevention of dementia,” she noted.
The findings were presented at the 2021 Alzheimer’s Association International Conference.
Weight loss before dementia explained?
For the study, investigators included 125,000 patients from the UK Biobank, which includes 51,031 who were over age 60 at baseline. Of these, 1,188 developed dementia during a follow-up of about 10 years; 553 were diagnosed with Alzheimer’s disease and 298 with vascular dementia.
Researchers used a platform that covers 249 metabolic measures, including small molecules, fatty acids, and lipoprotein lipids.
They estimated risk associated with these metabolites, adjusting for age, sex, body mass index, technical variables, ethnicity, smoking, alcohol, education, metabolic and neuropsychiatric medication, and APOE4 genotypes.
Of the 249 metabolites, 47 (19%) were associated with dementia risk in those over age 60, after adjustment.
The investigators examined effect estimates for associations of metabolites with both Alzheimer’s disease and vascular dementia over age 60 versus hippocampal volume under age 60. They found a “very strong, very significant” association for Alzheimer’s disease, and a “marginally significant” association for vascular dementia, said Dr. van Duijn.
This would be expected, as there is a much stronger correlation between hippocampal and Alzheimer’s disease versus vascular dementia, she added.
“We not only see that the metabolites predict dementia, but also early pathology. This makes these findings rather interesting for targeting prevention,” she said. An analysis of total brain volume showed “very strong, very similar, very significant associations” for both Alzheimer’s disease and vascular dementia,” added Dr. van Duijn.
The researchers found a major shift in various metabolites involved in energy metabolism in the 10-year period before the diagnosis of Alzheimer’s disease. These changes include low levels of branched-chain amino acids and omega-3 fatty acids and high levels of glucose, citrate, acetone, beta-hydroxybutyrate, and acetate. “This finding is in line with that in APOE models that show reduced energy metabolism over age in the brain,” said Dr. van Duijn.
She added that high levels of some of these metabolites are associated with low body weight before dementia onset, which may explain the weight loss seen in patients before developing the disease. “Our hypothesis is that the liver is burning the fat reserves of the patients in order to provide the brain with fuel,” she explained.
Diet a prevention target?
The results also showed ketone bodies increase with age, which may represent the aging brain’s “compensation mechanism” to deal with an energy shortage, said Dr. van Duijn. “Supplementation of ketone bodies, branched-chain amino and omega-3 fatty acids may help support brain function.”
The fact that ketone bodies were positively associated with the risk of dementia is “a very important finding,” she said.
Following this and other presentations, session cochair Rima Kaddurah-Daouk, PhD, professor in psychiatry and behavioral sciences, Institute for Brain Sciences, Duke University, Durham, N.C., noted the research is “an important part of trying to decipher some of the mysteries in Alzheimer’s disease.”
The research contributes to the understanding of how nutrition and diet could influence metabolism and then the brain and is “opening the horizon” for thinking about “strategies for therapeutic interventions,” she said.
The study received funding support from the National Institute on Aging. The investigators have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM AAIC 2021
‘Staggering’ increase in global dementia cases predicted by 2050
, new global prevalence data show. “These extreme increases are due largely to demographic trends, including population growth and aging,” said study investigator Emma Nichols, MPH, a researcher at the Institute for Health Metrics and Evaluation at the University of Washington in Seattle.
“Our estimates of expected increases can and should inform policy and planning efforts that will be needed to address the needs of the growing number of individuals with dementia in the future,” Ms. Nichols said.
The latest global prevalence data were reported at the 2021 Alzheimer’s Association International Conference.
“The numbers are staggering: Nearly 153 million cases of dementia are predicted worldwide by the year 2050. To put that in context, that number is equal to approximately half of the U.S. population in 2020,” Heather M. Snyder, PhD, vice president of medical and scientific relations for the Alzheimer’s Association, said in a statement.
Prevalence by country
To more accurately forecast global dementia prevalence and produce country-level estimates, the investigators leveraged data from 1999 to 2019 from the Global Burden of Disease study, a comprehensive set of estimates of worldwide health trends.
These data suggest global dementia cases will increase from 57.4 million (50.4 to 65.1) in 2019 to 152.8 million (130.8 to 175.9) in 2050.
Regions that will experience the worst of the increase are eastern Sub-Saharan Africa, North Africa, and the Middle East.
The researchers also factored into the forecasts expected trends in obesity, diabetes, smoking, and educational attainment.
Increases in better education around the world are projected to decrease dementia prevalence by 6.2 million cases worldwide by 2050. However, anticipated trends in smoking, high body mass index, and diabetes will offset this gain, increasing global dementia cases by 6.8 million cases.
“A reversal of these expected trends in cardiovascular risks would be necessary to alter the anticipated trends,” Ms. Nichols said. “Interventions targeted at modifiable risk factors for dementia represent a viable strategy to help address the anticipated trends in dementia burden,” she added.
Need for effective prevention, treatment
Commenting on the research, Rebecca M. Edelmayer, PhD, senior director of scientific engagement at the Alzheimer’s Association, said the global increase in dementia cases is something the association has been following for many years. “We know that if we do not find effective treatments that are going to stop, slow, or prevent Alzheimer’s disease, this number will continue to grow and it will continue to impact people globally,” Dr. Edelmayer said.
She noted that although there are some positive trends, including the fact that increased education may drive down dementia risk, other factors, such as smoking, high body mass index, and high blood sugar level, are predicted to increase in prevalence.
“Some of these factors are actually counterbalancing each other, and in the end, if we don’t continue to develop culturally tailored interventions or even risk reduction strategies for individuals across the globe, we will continue to see those numbers rise overall,” Dr. Edelmayer said.
A version of this article first appeared on Medscape.com.
, new global prevalence data show. “These extreme increases are due largely to demographic trends, including population growth and aging,” said study investigator Emma Nichols, MPH, a researcher at the Institute for Health Metrics and Evaluation at the University of Washington in Seattle.
“Our estimates of expected increases can and should inform policy and planning efforts that will be needed to address the needs of the growing number of individuals with dementia in the future,” Ms. Nichols said.
The latest global prevalence data were reported at the 2021 Alzheimer’s Association International Conference.
“The numbers are staggering: Nearly 153 million cases of dementia are predicted worldwide by the year 2050. To put that in context, that number is equal to approximately half of the U.S. population in 2020,” Heather M. Snyder, PhD, vice president of medical and scientific relations for the Alzheimer’s Association, said in a statement.
Prevalence by country
To more accurately forecast global dementia prevalence and produce country-level estimates, the investigators leveraged data from 1999 to 2019 from the Global Burden of Disease study, a comprehensive set of estimates of worldwide health trends.
These data suggest global dementia cases will increase from 57.4 million (50.4 to 65.1) in 2019 to 152.8 million (130.8 to 175.9) in 2050.
Regions that will experience the worst of the increase are eastern Sub-Saharan Africa, North Africa, and the Middle East.
The researchers also factored into the forecasts expected trends in obesity, diabetes, smoking, and educational attainment.
Increases in better education around the world are projected to decrease dementia prevalence by 6.2 million cases worldwide by 2050. However, anticipated trends in smoking, high body mass index, and diabetes will offset this gain, increasing global dementia cases by 6.8 million cases.
“A reversal of these expected trends in cardiovascular risks would be necessary to alter the anticipated trends,” Ms. Nichols said. “Interventions targeted at modifiable risk factors for dementia represent a viable strategy to help address the anticipated trends in dementia burden,” she added.
Need for effective prevention, treatment
Commenting on the research, Rebecca M. Edelmayer, PhD, senior director of scientific engagement at the Alzheimer’s Association, said the global increase in dementia cases is something the association has been following for many years. “We know that if we do not find effective treatments that are going to stop, slow, or prevent Alzheimer’s disease, this number will continue to grow and it will continue to impact people globally,” Dr. Edelmayer said.
She noted that although there are some positive trends, including the fact that increased education may drive down dementia risk, other factors, such as smoking, high body mass index, and high blood sugar level, are predicted to increase in prevalence.
“Some of these factors are actually counterbalancing each other, and in the end, if we don’t continue to develop culturally tailored interventions or even risk reduction strategies for individuals across the globe, we will continue to see those numbers rise overall,” Dr. Edelmayer said.
A version of this article first appeared on Medscape.com.
, new global prevalence data show. “These extreme increases are due largely to demographic trends, including population growth and aging,” said study investigator Emma Nichols, MPH, a researcher at the Institute for Health Metrics and Evaluation at the University of Washington in Seattle.
“Our estimates of expected increases can and should inform policy and planning efforts that will be needed to address the needs of the growing number of individuals with dementia in the future,” Ms. Nichols said.
The latest global prevalence data were reported at the 2021 Alzheimer’s Association International Conference.
“The numbers are staggering: Nearly 153 million cases of dementia are predicted worldwide by the year 2050. To put that in context, that number is equal to approximately half of the U.S. population in 2020,” Heather M. Snyder, PhD, vice president of medical and scientific relations for the Alzheimer’s Association, said in a statement.
Prevalence by country
To more accurately forecast global dementia prevalence and produce country-level estimates, the investigators leveraged data from 1999 to 2019 from the Global Burden of Disease study, a comprehensive set of estimates of worldwide health trends.
These data suggest global dementia cases will increase from 57.4 million (50.4 to 65.1) in 2019 to 152.8 million (130.8 to 175.9) in 2050.
Regions that will experience the worst of the increase are eastern Sub-Saharan Africa, North Africa, and the Middle East.
The researchers also factored into the forecasts expected trends in obesity, diabetes, smoking, and educational attainment.
Increases in better education around the world are projected to decrease dementia prevalence by 6.2 million cases worldwide by 2050. However, anticipated trends in smoking, high body mass index, and diabetes will offset this gain, increasing global dementia cases by 6.8 million cases.
“A reversal of these expected trends in cardiovascular risks would be necessary to alter the anticipated trends,” Ms. Nichols said. “Interventions targeted at modifiable risk factors for dementia represent a viable strategy to help address the anticipated trends in dementia burden,” she added.
Need for effective prevention, treatment
Commenting on the research, Rebecca M. Edelmayer, PhD, senior director of scientific engagement at the Alzheimer’s Association, said the global increase in dementia cases is something the association has been following for many years. “We know that if we do not find effective treatments that are going to stop, slow, or prevent Alzheimer’s disease, this number will continue to grow and it will continue to impact people globally,” Dr. Edelmayer said.
She noted that although there are some positive trends, including the fact that increased education may drive down dementia risk, other factors, such as smoking, high body mass index, and high blood sugar level, are predicted to increase in prevalence.
“Some of these factors are actually counterbalancing each other, and in the end, if we don’t continue to develop culturally tailored interventions or even risk reduction strategies for individuals across the globe, we will continue to see those numbers rise overall,” Dr. Edelmayer said.
A version of this article first appeared on Medscape.com.
From AAIC 2021
Money buys life, and a cigarette maker wants to ‘unsmoke the world’
With COVID, the fun never ends
Welcome to America’s favorite pandemic-themed game show! Let’s play Covidiot Proof! And now, here’s your host, the lovely and talented Anthony Grouchy!
Tony: Hello everyone! Our first category today is America or [blank], and the first clue is for you, Don. This country requires “individuals to use a health pass to patronize indoor establishments such as restaurants, bars, nightclubs and cinemas.”
Don: Freedom-loving Americans would never stand for that, Tony, so I’m going to say Greece.
Tony: That’s correct, Don. One hundred points for you. Okay Joe, here’s your clue: In this country, some people wear disguises to get a COVID vaccination so their friends and families won’t find out.
Joe: Sounds like communism to me, Tony. I’ll say Cuba.
Tony: Sorry Joe, that’s incorrect. Don?
Don: The friends and families sound like freedom-loving Americans, so it must be America.
Tony: It is America. Missouri, to be exact. And now, one last question for both of you to win the game. True or false? Did the pastor of a church in Tennessee say that mask-wearers would be kicked out of the building because “I am not playing these Democrat games up in this church”?
Joe: That’s fake news, Tony. It’s gotta be false.
Tony: Incorrect! It’s absolutely true. That means today’s winner is … Joe? Yes, I’m being told that Tennessee goes to Joe.
Don: That’s bulls#&@! I won this thing! I’ll see you in court!
More money, more life
Does it seem to you that the wealthy live forever, while the less financially comfortable live shorter lives? If you answered, yes, it turns out that you’re right.
Researchers analyzed the effect of net worth at midlife with mortality. To take out genetic differences among the sample of 5,400 adults aged 46 years, the investigators also studied a subset of 2,490 twin and sibling pairs.
“The within-family association provides strong evidence that an association between wealth accumulation and life expectancy exists, because comparing siblings within the same family to each other controls for all of the life experience and biology that they share,” said coauthor Eric Finegood of Northwestern University, Chicago.
But what if one sibling has a history of cancer, heart disease, or other health conditions? The cost of treatment and employment limitations could affect someone’s ability to stack their wealth, right? Absolutely. The researchers took that into account and looked at only healthy individuals and found the same results. More money, longer life.
We have the policies and programs in place for heart health, diabetes prevention, and smoking cessation, as they are seen as major threats to public health. So why not do the same for financial security? A low bank account may just be more harmful.
Holding the ‘health care and wellness’ gun
Cigarettes are not good for us. We know this.
It’s, therefore, not surprising to learn that a business has requested for a U.K. ban on the sale of cigarettes by 2030. However, when that someone turns out to be the CEO of Philip Morris International, tobacco company and maker of Marlboro cigarettes, things get a little confusing.
Banning cigarettes, according to Jacek Olczak, would reduce confusion among consumers, many of whom feel that the alternatives are worse for their health. His company can “see the world without cigarettes ... and actually, the sooner it happens, the better it is for everyone.” A truly noble sentiment from the CEO of a large tobacco company. Nothing nefarious going on here.
And if those aren’t egregious business euphemisms, we don’t know what is.
Of course, for all the completely believable and sincere rhetoric, the fact is that Marlboros are still on the shelves. Philip Morris is still making and advertising them. If their concern was genuine, why wouldn’t they just stop manufacturing them now?
So, we ask ourselves if this a selfless act of kindness or is it an unscrupulous corporate act to get a leg up on their competitors? We’ll leave it up to the readers to decide.
Okay, we lied, it’s the second one.
Autopsy of the living dead
Imagine the absolute terror you’d feel if you opened your eyes to bright, blinding white lights only to see a bone saw 3 inches from your forehead and getting closer by the second. Horrifying for you, certainly, but think about the poor pathologist behind the saw who probably thought a zombie apocalypse was coming. This was close to being a reality for a 29-year-old prisoner at the Asturias Central Penitentiary in Spain.
Gonzalo Montoya Jiménez was discovered in his cell unresponsive. Three physicians examined him and found he was showing signs of death, such as cyanosis and rigor mortis. Mr. Jiménez was processed like any other body and was sent, in a body bag, to a hospital mortuary, where he spent time in a freezer for body preservation. Just before he was due for his autopsy, he began showing signs of life.
It’s not completely clear why this happened to poor Mr. Jiménez, but it was reported that he wasn’t feeling well the day before and that he has epilepsy. Hospital officials suggested he may have been cataleptic, possibly because he had trouble adhering to his medication schedule.
Mr. Jiménez was moved to another hospital under armed guard after coming back to life and regained consciousness after a day or so. Talk about cheating death.
With COVID, the fun never ends
Welcome to America’s favorite pandemic-themed game show! Let’s play Covidiot Proof! And now, here’s your host, the lovely and talented Anthony Grouchy!
Tony: Hello everyone! Our first category today is America or [blank], and the first clue is for you, Don. This country requires “individuals to use a health pass to patronize indoor establishments such as restaurants, bars, nightclubs and cinemas.”
Don: Freedom-loving Americans would never stand for that, Tony, so I’m going to say Greece.
Tony: That’s correct, Don. One hundred points for you. Okay Joe, here’s your clue: In this country, some people wear disguises to get a COVID vaccination so their friends and families won’t find out.
Joe: Sounds like communism to me, Tony. I’ll say Cuba.
Tony: Sorry Joe, that’s incorrect. Don?
Don: The friends and families sound like freedom-loving Americans, so it must be America.
Tony: It is America. Missouri, to be exact. And now, one last question for both of you to win the game. True or false? Did the pastor of a church in Tennessee say that mask-wearers would be kicked out of the building because “I am not playing these Democrat games up in this church”?
Joe: That’s fake news, Tony. It’s gotta be false.
Tony: Incorrect! It’s absolutely true. That means today’s winner is … Joe? Yes, I’m being told that Tennessee goes to Joe.
Don: That’s bulls#&@! I won this thing! I’ll see you in court!
More money, more life
Does it seem to you that the wealthy live forever, while the less financially comfortable live shorter lives? If you answered, yes, it turns out that you’re right.
Researchers analyzed the effect of net worth at midlife with mortality. To take out genetic differences among the sample of 5,400 adults aged 46 years, the investigators also studied a subset of 2,490 twin and sibling pairs.
“The within-family association provides strong evidence that an association between wealth accumulation and life expectancy exists, because comparing siblings within the same family to each other controls for all of the life experience and biology that they share,” said coauthor Eric Finegood of Northwestern University, Chicago.
But what if one sibling has a history of cancer, heart disease, or other health conditions? The cost of treatment and employment limitations could affect someone’s ability to stack their wealth, right? Absolutely. The researchers took that into account and looked at only healthy individuals and found the same results. More money, longer life.
We have the policies and programs in place for heart health, diabetes prevention, and smoking cessation, as they are seen as major threats to public health. So why not do the same for financial security? A low bank account may just be more harmful.
Holding the ‘health care and wellness’ gun
Cigarettes are not good for us. We know this.
It’s, therefore, not surprising to learn that a business has requested for a U.K. ban on the sale of cigarettes by 2030. However, when that someone turns out to be the CEO of Philip Morris International, tobacco company and maker of Marlboro cigarettes, things get a little confusing.
Banning cigarettes, according to Jacek Olczak, would reduce confusion among consumers, many of whom feel that the alternatives are worse for their health. His company can “see the world without cigarettes ... and actually, the sooner it happens, the better it is for everyone.” A truly noble sentiment from the CEO of a large tobacco company. Nothing nefarious going on here.
And if those aren’t egregious business euphemisms, we don’t know what is.
Of course, for all the completely believable and sincere rhetoric, the fact is that Marlboros are still on the shelves. Philip Morris is still making and advertising them. If their concern was genuine, why wouldn’t they just stop manufacturing them now?
So, we ask ourselves if this a selfless act of kindness or is it an unscrupulous corporate act to get a leg up on their competitors? We’ll leave it up to the readers to decide.
Okay, we lied, it’s the second one.
Autopsy of the living dead
Imagine the absolute terror you’d feel if you opened your eyes to bright, blinding white lights only to see a bone saw 3 inches from your forehead and getting closer by the second. Horrifying for you, certainly, but think about the poor pathologist behind the saw who probably thought a zombie apocalypse was coming. This was close to being a reality for a 29-year-old prisoner at the Asturias Central Penitentiary in Spain.
Gonzalo Montoya Jiménez was discovered in his cell unresponsive. Three physicians examined him and found he was showing signs of death, such as cyanosis and rigor mortis. Mr. Jiménez was processed like any other body and was sent, in a body bag, to a hospital mortuary, where he spent time in a freezer for body preservation. Just before he was due for his autopsy, he began showing signs of life.
It’s not completely clear why this happened to poor Mr. Jiménez, but it was reported that he wasn’t feeling well the day before and that he has epilepsy. Hospital officials suggested he may have been cataleptic, possibly because he had trouble adhering to his medication schedule.
Mr. Jiménez was moved to another hospital under armed guard after coming back to life and regained consciousness after a day or so. Talk about cheating death.
With COVID, the fun never ends
Welcome to America’s favorite pandemic-themed game show! Let’s play Covidiot Proof! And now, here’s your host, the lovely and talented Anthony Grouchy!
Tony: Hello everyone! Our first category today is America or [blank], and the first clue is for you, Don. This country requires “individuals to use a health pass to patronize indoor establishments such as restaurants, bars, nightclubs and cinemas.”
Don: Freedom-loving Americans would never stand for that, Tony, so I’m going to say Greece.
Tony: That’s correct, Don. One hundred points for you. Okay Joe, here’s your clue: In this country, some people wear disguises to get a COVID vaccination so their friends and families won’t find out.
Joe: Sounds like communism to me, Tony. I’ll say Cuba.
Tony: Sorry Joe, that’s incorrect. Don?
Don: The friends and families sound like freedom-loving Americans, so it must be America.
Tony: It is America. Missouri, to be exact. And now, one last question for both of you to win the game. True or false? Did the pastor of a church in Tennessee say that mask-wearers would be kicked out of the building because “I am not playing these Democrat games up in this church”?
Joe: That’s fake news, Tony. It’s gotta be false.
Tony: Incorrect! It’s absolutely true. That means today’s winner is … Joe? Yes, I’m being told that Tennessee goes to Joe.
Don: That’s bulls#&@! I won this thing! I’ll see you in court!
More money, more life
Does it seem to you that the wealthy live forever, while the less financially comfortable live shorter lives? If you answered, yes, it turns out that you’re right.
Researchers analyzed the effect of net worth at midlife with mortality. To take out genetic differences among the sample of 5,400 adults aged 46 years, the investigators also studied a subset of 2,490 twin and sibling pairs.
“The within-family association provides strong evidence that an association between wealth accumulation and life expectancy exists, because comparing siblings within the same family to each other controls for all of the life experience and biology that they share,” said coauthor Eric Finegood of Northwestern University, Chicago.
But what if one sibling has a history of cancer, heart disease, or other health conditions? The cost of treatment and employment limitations could affect someone’s ability to stack their wealth, right? Absolutely. The researchers took that into account and looked at only healthy individuals and found the same results. More money, longer life.
We have the policies and programs in place for heart health, diabetes prevention, and smoking cessation, as they are seen as major threats to public health. So why not do the same for financial security? A low bank account may just be more harmful.
Holding the ‘health care and wellness’ gun
Cigarettes are not good for us. We know this.
It’s, therefore, not surprising to learn that a business has requested for a U.K. ban on the sale of cigarettes by 2030. However, when that someone turns out to be the CEO of Philip Morris International, tobacco company and maker of Marlboro cigarettes, things get a little confusing.
Banning cigarettes, according to Jacek Olczak, would reduce confusion among consumers, many of whom feel that the alternatives are worse for their health. His company can “see the world without cigarettes ... and actually, the sooner it happens, the better it is for everyone.” A truly noble sentiment from the CEO of a large tobacco company. Nothing nefarious going on here.
And if those aren’t egregious business euphemisms, we don’t know what is.
Of course, for all the completely believable and sincere rhetoric, the fact is that Marlboros are still on the shelves. Philip Morris is still making and advertising them. If their concern was genuine, why wouldn’t they just stop manufacturing them now?
So, we ask ourselves if this a selfless act of kindness or is it an unscrupulous corporate act to get a leg up on their competitors? We’ll leave it up to the readers to decide.
Okay, we lied, it’s the second one.
Autopsy of the living dead
Imagine the absolute terror you’d feel if you opened your eyes to bright, blinding white lights only to see a bone saw 3 inches from your forehead and getting closer by the second. Horrifying for you, certainly, but think about the poor pathologist behind the saw who probably thought a zombie apocalypse was coming. This was close to being a reality for a 29-year-old prisoner at the Asturias Central Penitentiary in Spain.
Gonzalo Montoya Jiménez was discovered in his cell unresponsive. Three physicians examined him and found he was showing signs of death, such as cyanosis and rigor mortis. Mr. Jiménez was processed like any other body and was sent, in a body bag, to a hospital mortuary, where he spent time in a freezer for body preservation. Just before he was due for his autopsy, he began showing signs of life.
It’s not completely clear why this happened to poor Mr. Jiménez, but it was reported that he wasn’t feeling well the day before and that he has epilepsy. Hospital officials suggested he may have been cataleptic, possibly because he had trouble adhering to his medication schedule.
Mr. Jiménez was moved to another hospital under armed guard after coming back to life and regained consciousness after a day or so. Talk about cheating death.
Coffee and the brain: ‘Concerning’ new data
according to the results of a large study.
“With coffee intake, moderation is the key, and especially high levels of consumption may have adverse long-term effects on the brain,” said study investigator Elina Hypponen, PhD, professor of nutritional and genetic epidemiology and director of the Australian Center for Precision Health at the University of South Australia.
“These new data are concerning, and there is a need to conduct further carefully controlled studies to clarify the effects of coffee on the brain.”
The study was published online June 24 in Nutritional Neuroscience.
Potent stimulant
Coffee is a potent nervous system stimulant and is among the most popular nonalcoholic beverages. Some previous research suggests it benefits the brain, but the investigators noted that other research shows a negative or U-shaped relationship.
To investigate, the researchers examined data from the U.K. Biobank, a long-term prospective epidemiologic study of more than 500,000 participants aged 37-73 years who were recruited in 22 assessment centers in the United Kingdom between March 2006 and October 2010.
During the baseline assessment, information was gathered using touchscreen questionnaires, verbal interviews, and physical examinations that involved collection of blood, urine, and saliva samples. An imaging substudy was incorporated in 2014, the goal of which was to conduct brain, heart, and body MRI imaging for 100,000 participants.
The investigators conducted analyses on disease outcomes for 398,646 participants for whom information on habitual coffee consumption was available. Brain volume analyses were conducted in 17,702 participants for whom valid brain imaging data were available.
Participants reported coffee intake in cups per day. Researchers grouped coffee consumption into seven categories: nondrinkers, decaffeinated coffee drinkers, and caffeinated coffee drinkers who consumed less than 1 cup/d, 1-2 cups/d, 3-4 cups/d, 5-6 cups/d, and more than 6 cups/d.
The reference category was those who consumed 1-2 cups/d, rather than those who abstained from coffee, because persons who abstain are more likely to be at suboptimal health.
“Comparing the health of coffee drinkers to the health of those choosing to abstain from coffee will typically lead to an impression of a health benefit, even if there would not be one,” said Dr. Hypponen.
The researchers obtained total and regional brain volumes from the MRI imaging substudy starting 4-6 years after baseline assessment. They accessed information on incident dementia and stroke using primary care data, hospital admission electronic health records, national death registers, and self-reported medical conditions.
Covariates included socioeconomic, health, and other factors, such as smoking, alcohol and tea consumption, physical activity, stressful life events, and body mass index.
The investigators found that there was a linear inverse association between coffee consumption and total brain volume (fully adjusted beta per cup, –1.42; 95% confidence interval, –1.89 to –0.94), with consistent patterns for gray matter, white matter, and hippocampal volumes.
There was no evidence to support an association with white matter hyperintensity (WMH) volume (beta –0.01; 95% CI, –0.07 to 0.05).
Higher consumption, higher risk
The analysis also revealed a nonlinear association between coffee consumption and the odds of dementia (P nonlinearity = .0001), with slightly higher odds seen with non–coffee drinkers and decaffeinated-coffee drinkers and more notable increases for participants in the highest categories of coffee consumption compared with light coffee drinkers.
After adjustment for all covariates, the odds ratio of dementia among persons in the category of coffee intake was 1.53 (95% CI, 1.28-1.83). After full adjustments, the association with heavy coffee consumption and stroke was not significant, although “we can’t exclude a weak effect,” said Dr. Hypponen.
“For the highest coffee consumption group, the data support an association which may be anywhere from 0% to 37% higher odds of stroke after full adjustment,” she added.
People at risk for hypertension may develop “unpleasant sensations” and stop drinking coffee before a serious adverse event occurs, said Dr. Hypponen. In a previous study, she and her colleagues showed that those who have genetically higher blood pressure tend to drink less coffee than their counterparts without the condition.
“This type of effect might be expected to naturally limit the adverse effects of coffee on the risk of stroke,” said Dr. Hypponen.
The odds remained elevated for participants drinking more than 6 cups/d after the researchers accounted for sleep quality. There were no differences in risk between men and women or by age.
An examination of the consumption of tea, which often contains caffeine, did not show an association with brain volume or the odds of dementia or stroke.
“We don’t know whether the difference between associations seen for coffee and tea intake reflects the difference in related caffeine intake or some other explanation, such as dehydration or effects operating through blood cholesterol,” said Dr. Hypponen.
Although reverse causation is possible, there’s no reason to believe that it is relevant to the study results. Genetic evidence suggests a causal role of higher coffee intake on risk for Alzheimer’s disease. In addition, results of a clinical trial support the association between higher caffeine intake and smaller gray matter volume, said Dr. Hypponen.
The mechanisms linking coffee consumption to brain volumes and dementia are not well established. However, Dr. Hypponen noted that caffeine has been used to induce apoptosis in cancer studies using glial cells.
“Furthermore, adenosine receptors, which mediate many of the effects of caffeine in the brain, have been suggested to influence the release of growth factors, which in turn can have an influence on astrocyte proliferation and angiogenesis in the brain,” she said.
Some types of coffee contain cafestol, which increases blood cholesterol and can have adverse effects though related mechanisms, said Dr. Hypponen.
The mechanism may also involve dehydration, which may have a harmful effect on the brain. The study suggested a correlation between dehydration and high coffee intake. “Of course, if this is the case, it is good news, as then we can do something about it simply by drinking some water every time we have a cup of coffee,” she said.
Misleading conclusions
Coffee contains antioxidants, and although previous studies have suggested it might be beneficial, this hypothesis is “too simplistic,” said Dr. Hypponen. “While coffee is not going to be all ‘bad’ either, there are a lot of controversies and suggestions about beneficial effects of coffee which may not be true, or at least do not reflect the full story.”
If the drinking of coffee is at least partly determined by an individual’s health status, then that would often lead to misleading conclusions in observational studies, said Dr. Hypponen.
“When one uses as a comparison people who already have poor health and who do not drink coffee because of that, coffee intake will by default appear beneficial simply because there are more people with disease among those choosing abstinence,” she said.
Before now, there was “very little evidence about the association between coffee intake and brain morphology,” and the studies that were conducted were relatively small, said Dr. Hypponen.
One of these smaller studies included a group of women aged 13-30 years. It found that coffee consumption was not associated with total brain volumes, but the findings suggested a U-shaped association with hippocampal volume; higher values were seen both for nondrinkers and the groups with higher consumption.
A small study of elderly patients with diabetes showed no evidence of an association with white matter volume, but there was a possible age-dependent association with gray matter volume.
The largest of the earlier studies had results that were very similar to those of the current study, suggesting that increasing coffee intake is associated with smaller hippocampal volumes, said Dr. Hypponen.
One of the study’s limitations included the fact that full dietary information was available only for a subsample and that factors such as dehydration were measured at baseline rather than at the time of brain MRI.
Another possible study limitation was the use of self-reported data and the fact that lifestyle changes may have occurred between baseline and MRI or covariate measurement.
In addition, the study is subject to a healthy-volunteer bias, and its implications are restricted to White British persons. The association needs to be studied in other ethnic populations, the authors noted.
A reason to cut back?
Commenting on the findings, Walter Willett, MD, DrPH, professor of epidemiology and nutrition, Harvard T. H. Chan School of Public Health, Boston, said the study is large and quite well done.
“It does raise questions about an increase in risk of dementia with six or more cups of coffee per day,” said Dr. Willett. “At the same time, it provides reassurance about lack of adverse effects of coffee for those consuming three or four cups per day, and little increase in risk, if any, with five cups per day.”
It’s not entirely clear whether the increase in risk with six or more cups of coffee per day represents a “true effect” of coffee, inasmuch as the study did not seem to adjust fully for dietary factors, high consumption of alcohol, or past smoking, said Dr. Willett.
The findings don’t suggest that coffee lovers should give up their Java. “But six or more cups per day is a lot, and those who drink that much might consider cutting back a bit while research continues,” said Dr. Willett.
The study was supported by the National Health and Medical Research Council.
A version of this article first appeared on Medscape.com.
according to the results of a large study.
“With coffee intake, moderation is the key, and especially high levels of consumption may have adverse long-term effects on the brain,” said study investigator Elina Hypponen, PhD, professor of nutritional and genetic epidemiology and director of the Australian Center for Precision Health at the University of South Australia.
“These new data are concerning, and there is a need to conduct further carefully controlled studies to clarify the effects of coffee on the brain.”
The study was published online June 24 in Nutritional Neuroscience.
Potent stimulant
Coffee is a potent nervous system stimulant and is among the most popular nonalcoholic beverages. Some previous research suggests it benefits the brain, but the investigators noted that other research shows a negative or U-shaped relationship.
To investigate, the researchers examined data from the U.K. Biobank, a long-term prospective epidemiologic study of more than 500,000 participants aged 37-73 years who were recruited in 22 assessment centers in the United Kingdom between March 2006 and October 2010.
During the baseline assessment, information was gathered using touchscreen questionnaires, verbal interviews, and physical examinations that involved collection of blood, urine, and saliva samples. An imaging substudy was incorporated in 2014, the goal of which was to conduct brain, heart, and body MRI imaging for 100,000 participants.
The investigators conducted analyses on disease outcomes for 398,646 participants for whom information on habitual coffee consumption was available. Brain volume analyses were conducted in 17,702 participants for whom valid brain imaging data were available.
Participants reported coffee intake in cups per day. Researchers grouped coffee consumption into seven categories: nondrinkers, decaffeinated coffee drinkers, and caffeinated coffee drinkers who consumed less than 1 cup/d, 1-2 cups/d, 3-4 cups/d, 5-6 cups/d, and more than 6 cups/d.
The reference category was those who consumed 1-2 cups/d, rather than those who abstained from coffee, because persons who abstain are more likely to be at suboptimal health.
“Comparing the health of coffee drinkers to the health of those choosing to abstain from coffee will typically lead to an impression of a health benefit, even if there would not be one,” said Dr. Hypponen.
The researchers obtained total and regional brain volumes from the MRI imaging substudy starting 4-6 years after baseline assessment. They accessed information on incident dementia and stroke using primary care data, hospital admission electronic health records, national death registers, and self-reported medical conditions.
Covariates included socioeconomic, health, and other factors, such as smoking, alcohol and tea consumption, physical activity, stressful life events, and body mass index.
The investigators found that there was a linear inverse association between coffee consumption and total brain volume (fully adjusted beta per cup, –1.42; 95% confidence interval, –1.89 to –0.94), with consistent patterns for gray matter, white matter, and hippocampal volumes.
There was no evidence to support an association with white matter hyperintensity (WMH) volume (beta –0.01; 95% CI, –0.07 to 0.05).
Higher consumption, higher risk
The analysis also revealed a nonlinear association between coffee consumption and the odds of dementia (P nonlinearity = .0001), with slightly higher odds seen with non–coffee drinkers and decaffeinated-coffee drinkers and more notable increases for participants in the highest categories of coffee consumption compared with light coffee drinkers.
After adjustment for all covariates, the odds ratio of dementia among persons in the category of coffee intake was 1.53 (95% CI, 1.28-1.83). After full adjustments, the association with heavy coffee consumption and stroke was not significant, although “we can’t exclude a weak effect,” said Dr. Hypponen.
“For the highest coffee consumption group, the data support an association which may be anywhere from 0% to 37% higher odds of stroke after full adjustment,” she added.
People at risk for hypertension may develop “unpleasant sensations” and stop drinking coffee before a serious adverse event occurs, said Dr. Hypponen. In a previous study, she and her colleagues showed that those who have genetically higher blood pressure tend to drink less coffee than their counterparts without the condition.
“This type of effect might be expected to naturally limit the adverse effects of coffee on the risk of stroke,” said Dr. Hypponen.
The odds remained elevated for participants drinking more than 6 cups/d after the researchers accounted for sleep quality. There were no differences in risk between men and women or by age.
An examination of the consumption of tea, which often contains caffeine, did not show an association with brain volume or the odds of dementia or stroke.
“We don’t know whether the difference between associations seen for coffee and tea intake reflects the difference in related caffeine intake or some other explanation, such as dehydration or effects operating through blood cholesterol,” said Dr. Hypponen.
Although reverse causation is possible, there’s no reason to believe that it is relevant to the study results. Genetic evidence suggests a causal role of higher coffee intake on risk for Alzheimer’s disease. In addition, results of a clinical trial support the association between higher caffeine intake and smaller gray matter volume, said Dr. Hypponen.
The mechanisms linking coffee consumption to brain volumes and dementia are not well established. However, Dr. Hypponen noted that caffeine has been used to induce apoptosis in cancer studies using glial cells.
“Furthermore, adenosine receptors, which mediate many of the effects of caffeine in the brain, have been suggested to influence the release of growth factors, which in turn can have an influence on astrocyte proliferation and angiogenesis in the brain,” she said.
Some types of coffee contain cafestol, which increases blood cholesterol and can have adverse effects though related mechanisms, said Dr. Hypponen.
The mechanism may also involve dehydration, which may have a harmful effect on the brain. The study suggested a correlation between dehydration and high coffee intake. “Of course, if this is the case, it is good news, as then we can do something about it simply by drinking some water every time we have a cup of coffee,” she said.
Misleading conclusions
Coffee contains antioxidants, and although previous studies have suggested it might be beneficial, this hypothesis is “too simplistic,” said Dr. Hypponen. “While coffee is not going to be all ‘bad’ either, there are a lot of controversies and suggestions about beneficial effects of coffee which may not be true, or at least do not reflect the full story.”
If the drinking of coffee is at least partly determined by an individual’s health status, then that would often lead to misleading conclusions in observational studies, said Dr. Hypponen.
“When one uses as a comparison people who already have poor health and who do not drink coffee because of that, coffee intake will by default appear beneficial simply because there are more people with disease among those choosing abstinence,” she said.
Before now, there was “very little evidence about the association between coffee intake and brain morphology,” and the studies that were conducted were relatively small, said Dr. Hypponen.
One of these smaller studies included a group of women aged 13-30 years. It found that coffee consumption was not associated with total brain volumes, but the findings suggested a U-shaped association with hippocampal volume; higher values were seen both for nondrinkers and the groups with higher consumption.
A small study of elderly patients with diabetes showed no evidence of an association with white matter volume, but there was a possible age-dependent association with gray matter volume.
The largest of the earlier studies had results that were very similar to those of the current study, suggesting that increasing coffee intake is associated with smaller hippocampal volumes, said Dr. Hypponen.
One of the study’s limitations included the fact that full dietary information was available only for a subsample and that factors such as dehydration were measured at baseline rather than at the time of brain MRI.
Another possible study limitation was the use of self-reported data and the fact that lifestyle changes may have occurred between baseline and MRI or covariate measurement.
In addition, the study is subject to a healthy-volunteer bias, and its implications are restricted to White British persons. The association needs to be studied in other ethnic populations, the authors noted.
A reason to cut back?
Commenting on the findings, Walter Willett, MD, DrPH, professor of epidemiology and nutrition, Harvard T. H. Chan School of Public Health, Boston, said the study is large and quite well done.
“It does raise questions about an increase in risk of dementia with six or more cups of coffee per day,” said Dr. Willett. “At the same time, it provides reassurance about lack of adverse effects of coffee for those consuming three or four cups per day, and little increase in risk, if any, with five cups per day.”
It’s not entirely clear whether the increase in risk with six or more cups of coffee per day represents a “true effect” of coffee, inasmuch as the study did not seem to adjust fully for dietary factors, high consumption of alcohol, or past smoking, said Dr. Willett.
The findings don’t suggest that coffee lovers should give up their Java. “But six or more cups per day is a lot, and those who drink that much might consider cutting back a bit while research continues,” said Dr. Willett.
The study was supported by the National Health and Medical Research Council.
A version of this article first appeared on Medscape.com.
according to the results of a large study.
“With coffee intake, moderation is the key, and especially high levels of consumption may have adverse long-term effects on the brain,” said study investigator Elina Hypponen, PhD, professor of nutritional and genetic epidemiology and director of the Australian Center for Precision Health at the University of South Australia.
“These new data are concerning, and there is a need to conduct further carefully controlled studies to clarify the effects of coffee on the brain.”
The study was published online June 24 in Nutritional Neuroscience.
Potent stimulant
Coffee is a potent nervous system stimulant and is among the most popular nonalcoholic beverages. Some previous research suggests it benefits the brain, but the investigators noted that other research shows a negative or U-shaped relationship.
To investigate, the researchers examined data from the U.K. Biobank, a long-term prospective epidemiologic study of more than 500,000 participants aged 37-73 years who were recruited in 22 assessment centers in the United Kingdom between March 2006 and October 2010.
During the baseline assessment, information was gathered using touchscreen questionnaires, verbal interviews, and physical examinations that involved collection of blood, urine, and saliva samples. An imaging substudy was incorporated in 2014, the goal of which was to conduct brain, heart, and body MRI imaging for 100,000 participants.
The investigators conducted analyses on disease outcomes for 398,646 participants for whom information on habitual coffee consumption was available. Brain volume analyses were conducted in 17,702 participants for whom valid brain imaging data were available.
Participants reported coffee intake in cups per day. Researchers grouped coffee consumption into seven categories: nondrinkers, decaffeinated coffee drinkers, and caffeinated coffee drinkers who consumed less than 1 cup/d, 1-2 cups/d, 3-4 cups/d, 5-6 cups/d, and more than 6 cups/d.
The reference category was those who consumed 1-2 cups/d, rather than those who abstained from coffee, because persons who abstain are more likely to be at suboptimal health.
“Comparing the health of coffee drinkers to the health of those choosing to abstain from coffee will typically lead to an impression of a health benefit, even if there would not be one,” said Dr. Hypponen.
The researchers obtained total and regional brain volumes from the MRI imaging substudy starting 4-6 years after baseline assessment. They accessed information on incident dementia and stroke using primary care data, hospital admission electronic health records, national death registers, and self-reported medical conditions.
Covariates included socioeconomic, health, and other factors, such as smoking, alcohol and tea consumption, physical activity, stressful life events, and body mass index.
The investigators found that there was a linear inverse association between coffee consumption and total brain volume (fully adjusted beta per cup, –1.42; 95% confidence interval, –1.89 to –0.94), with consistent patterns for gray matter, white matter, and hippocampal volumes.
There was no evidence to support an association with white matter hyperintensity (WMH) volume (beta –0.01; 95% CI, –0.07 to 0.05).
Higher consumption, higher risk
The analysis also revealed a nonlinear association between coffee consumption and the odds of dementia (P nonlinearity = .0001), with slightly higher odds seen with non–coffee drinkers and decaffeinated-coffee drinkers and more notable increases for participants in the highest categories of coffee consumption compared with light coffee drinkers.
After adjustment for all covariates, the odds ratio of dementia among persons in the category of coffee intake was 1.53 (95% CI, 1.28-1.83). After full adjustments, the association with heavy coffee consumption and stroke was not significant, although “we can’t exclude a weak effect,” said Dr. Hypponen.
“For the highest coffee consumption group, the data support an association which may be anywhere from 0% to 37% higher odds of stroke after full adjustment,” she added.
People at risk for hypertension may develop “unpleasant sensations” and stop drinking coffee before a serious adverse event occurs, said Dr. Hypponen. In a previous study, she and her colleagues showed that those who have genetically higher blood pressure tend to drink less coffee than their counterparts without the condition.
“This type of effect might be expected to naturally limit the adverse effects of coffee on the risk of stroke,” said Dr. Hypponen.
The odds remained elevated for participants drinking more than 6 cups/d after the researchers accounted for sleep quality. There were no differences in risk between men and women or by age.
An examination of the consumption of tea, which often contains caffeine, did not show an association with brain volume or the odds of dementia or stroke.
“We don’t know whether the difference between associations seen for coffee and tea intake reflects the difference in related caffeine intake or some other explanation, such as dehydration or effects operating through blood cholesterol,” said Dr. Hypponen.
Although reverse causation is possible, there’s no reason to believe that it is relevant to the study results. Genetic evidence suggests a causal role of higher coffee intake on risk for Alzheimer’s disease. In addition, results of a clinical trial support the association between higher caffeine intake and smaller gray matter volume, said Dr. Hypponen.
The mechanisms linking coffee consumption to brain volumes and dementia are not well established. However, Dr. Hypponen noted that caffeine has been used to induce apoptosis in cancer studies using glial cells.
“Furthermore, adenosine receptors, which mediate many of the effects of caffeine in the brain, have been suggested to influence the release of growth factors, which in turn can have an influence on astrocyte proliferation and angiogenesis in the brain,” she said.
Some types of coffee contain cafestol, which increases blood cholesterol and can have adverse effects though related mechanisms, said Dr. Hypponen.
The mechanism may also involve dehydration, which may have a harmful effect on the brain. The study suggested a correlation between dehydration and high coffee intake. “Of course, if this is the case, it is good news, as then we can do something about it simply by drinking some water every time we have a cup of coffee,” she said.
Misleading conclusions
Coffee contains antioxidants, and although previous studies have suggested it might be beneficial, this hypothesis is “too simplistic,” said Dr. Hypponen. “While coffee is not going to be all ‘bad’ either, there are a lot of controversies and suggestions about beneficial effects of coffee which may not be true, or at least do not reflect the full story.”
If the drinking of coffee is at least partly determined by an individual’s health status, then that would often lead to misleading conclusions in observational studies, said Dr. Hypponen.
“When one uses as a comparison people who already have poor health and who do not drink coffee because of that, coffee intake will by default appear beneficial simply because there are more people with disease among those choosing abstinence,” she said.
Before now, there was “very little evidence about the association between coffee intake and brain morphology,” and the studies that were conducted were relatively small, said Dr. Hypponen.
One of these smaller studies included a group of women aged 13-30 years. It found that coffee consumption was not associated with total brain volumes, but the findings suggested a U-shaped association with hippocampal volume; higher values were seen both for nondrinkers and the groups with higher consumption.
A small study of elderly patients with diabetes showed no evidence of an association with white matter volume, but there was a possible age-dependent association with gray matter volume.
The largest of the earlier studies had results that were very similar to those of the current study, suggesting that increasing coffee intake is associated with smaller hippocampal volumes, said Dr. Hypponen.
One of the study’s limitations included the fact that full dietary information was available only for a subsample and that factors such as dehydration were measured at baseline rather than at the time of brain MRI.
Another possible study limitation was the use of self-reported data and the fact that lifestyle changes may have occurred between baseline and MRI or covariate measurement.
In addition, the study is subject to a healthy-volunteer bias, and its implications are restricted to White British persons. The association needs to be studied in other ethnic populations, the authors noted.
A reason to cut back?
Commenting on the findings, Walter Willett, MD, DrPH, professor of epidemiology and nutrition, Harvard T. H. Chan School of Public Health, Boston, said the study is large and quite well done.
“It does raise questions about an increase in risk of dementia with six or more cups of coffee per day,” said Dr. Willett. “At the same time, it provides reassurance about lack of adverse effects of coffee for those consuming three or four cups per day, and little increase in risk, if any, with five cups per day.”
It’s not entirely clear whether the increase in risk with six or more cups of coffee per day represents a “true effect” of coffee, inasmuch as the study did not seem to adjust fully for dietary factors, high consumption of alcohol, or past smoking, said Dr. Willett.
The findings don’t suggest that coffee lovers should give up their Java. “But six or more cups per day is a lot, and those who drink that much might consider cutting back a bit while research continues,” said Dr. Willett.
The study was supported by the National Health and Medical Research Council.
A version of this article first appeared on Medscape.com.
FROM NUTRITIONAL NEUROSCIENCE
Obesity treatment in mental illness: Is semaglutide a game changer?
It’s probably fair to say that most people would like to be thinner. More than 42% of Americans have obesity and another 30% are classified as being overweight, according to the latest statistics from the CDC.
Excess body weight is associated with many illnesses and plays a role in mental health; being heavy can take a toll on self-esteem. Many people worry that carrying excess weight makes them less attractive to potential romantic partners, and both physicians and employers treat those with obesity differently. Furthermore, in psychiatry, many of the medications we prescribe lead to weight gain.
In my clinical practice, I have listened as patients blamed themselves for their body habitus; many won’t consider biological treatments as they feel that would be “cheating” or taking an easy way out. They often point to periods in their life when they did lose weight and believe that they should be able to do it again, even if the weight loss took tremendous effort, was not sustained, and occurred decades ago.
That said, we psychiatrists often find ourselves in the position of managing obesity in our patients. I have been known to give patients who gain weight on antipsychotics either stimulants or metformin, or to add naltrexone to their Wellbutrin (bupropion) to effectively mimic a weight-loss medicine called Contrave.
Obesity a treatable medical condition
It wasn’t until 2013 that the American Medical Association recognized obesity as a medical condition.
In a New Yorker article that same year, “Diet Drugs Work: Why Won’t Doctors Prescribe Them?” Suzanne Koven wrote: “Several obesity experts told me they’ve encountered doctors who confide that they just didn’t like fat people and don’t enjoy taking care of them. Even doctors who treat obese patients feel stigmatized: ‘diet doctor’ is not a flattering term.”
Eat less, exercise more – with a blame-the-patient attitude – is still what people see as the “right” way to lose weight.
On June 4, 2021, the FDA approved semaglutide, a glucagonlike peptide–1 receptor agonist, previously used for the treatment of diabetes, for use as a weight loss agent for patients with obesity, or for those with a body mass index over 27 kg/m2 if they also have a weight-related comorbidity.
Semaglutide has three trade names, all manufactured by Novo Nordisk. The pill version is called Rybelsus and comes in 7-mg and 14-mg tablets. Ozempic is available in 0.5-mg and 1.0-mg doses and is administered weekly by subcutaneous injection for diabetes. The new, higher-dose preparation for weight loss, Wegovy, 2.4 mg, also comes as a weekly subcutaneous dose and is now available for the hefty price of $1,400 per month.
In STEP 1 trials, the higher-dose Wegovy was associated with an average 14.9% weight loss (15.3 kg) over 68 weeks, more than any other single-agent weight loss medication on the market.
GLP-1 receptor agonists work in the brain to decrease appetite, slow gastric emptying, increase insulin secretion, and stimulate brown adipose tissue thermogenesis.
Psych drugs lead to weight gain
Elaine Weiner, MD, is the medical director in the outpatient research program of the Maryland Psychiatric Research Center in Catonsville, where she treats patients with schizophrenia.
“Nearly all of our patients gain 20 pounds or more on the combinations of medications we use, mostly atypical antipsychotics,” she said. “Weight management is difficult for people who don’t have problems with motivation, but in our patients, lack of motivation is a core part of their illness, so asking them to adhere to diet and exercise regimens is of limited utility.
“Then, add to that the fact that they sometimes don’t have primary care doctors, and these issues of weight gain and metabolic syndrome come back to the psychiatrist. It is a really bad problem and we need more treatments.”
Fatima Cody Stanford, MD, MPH, MPA, is a fellowship-trained obesity medicine physician-scientist at the Massachusetts General Hospital Weight Center and Harvard Medical School, both in Boston. She has treated thousands of patients with obesity, speaks internationally on the topic of weight loss medicine, and has published over 100 peer-reviewed articles on obesity.
We spoke at length about recent changes in the field of obesity medicine and the introduction of the new GLP-1 receptor agonists.
“We as physicians have learned so little,” Dr. Stanford said. “This mantra of ‘calories in, calories out’ is not working; this is inaccurate and our focus on this has led to a rise in obesity. All calories are not created the same, and I think we are finally starting to see obesity medicine take off.”
Dr. Stanford is quick to note that obesity is a complex problem. Several different hormones are involved in regulating both appetite and satiety, processed foods promote weight gain, sleep is crucial to weight loss, and exercise helps maintain weight loss but is not usually effective in promoting it. “There are many contributors to energy storage,” she said.
The stimulant phentermine was approved in 1959. Addiction was a concern, and then in the 1990s, it was used in combination with fenfluramine to promote weight loss, a combination known as phen-fen. Fenfluramine was pulled from the market in 1997 when it was found to be associated with pulmonary hypertension and then heart valve abnormalities.
“This frightened quite a few physicians,” Dr. Stanford noted. Phentermine is still used for weight loss, either alone or together with topiramate, as a combination medication called Qsymia, nicknamed phen-top.
“Phen-top is the next best thing we have to semaglutide, and there is an average weight loss of 8%-9% of body weight. Semaglutide is going to be really significant for those people who are responders, and this has been quite well tolerated, the most common side effect being nausea,” she said.
However, she is quick to note that not everyone responds to every medication. “I use each patient’s clinical profile to determine what strategies and which medications to use.”
Cardiologists getting in the game
Michael Miller, MD, is a cardiologist at the University of Maryland, Baltimore, and author of “Heal Your Heart” (Emmaus, Pa.: Rodale, 2014). He is very enthusiastic about the approval of semaglutide.
“We are so excited because you finally can use these medicines without having to be diabetic,” Dr. Miller said. “We’re waiting on the results of the SELECT [Semaglutide Effects on Heart Disease and Stroke in Patients With Overweight or Obesity] trials looking at people who are not diabetic or who are prediabetic, to see the 5-year outcomes with regard to cardiac events.
“Usually endocrinologists prescribe these medications, but cardiologists have started to get into the game since GLP-1 receptor agonists reduce cardiovascular events.” Dr. Miller is hopeful that this medication may neutralize the weight gain caused by psychotropic medications.
Wegovy is administered via weekly injection and, like insulin, is a subcutaneous medication that patients self-administer. Will patients be amenable to injecting a medication for weight loss? Dr. Stanford said that roughly 20%-30% of her patients are hesitant when she suggests that they use liraglutide, another GLP-1 receptor agonist that is approved for weight loss, and some are very fearful of needles.
However, she also noted that during the COVID-19 pandemic, many more patients have sought treatment from obesity medicine physicians because of the association between obesity and mortality from COVID-19. Patients have been willing to consider treatments that they were not previously open to pursuing.
So if people are willing to take Wegovy and doctors are willing to prescribe it, will insurers pay for it? As of this writing, the medication is not yet available, but Ozempic, the lower-dose agent for diabetes, costs $850-$900 for a 4-week supply, according to the GoodRx website.
Liraglutide (Saxenda), the GLP-1 receptor agonist that is currently available for weight loss as a daily injectable, costs $1,300-$1,400 per month.
These medications are not covered by Medicare or Medicaid, and Dr. Stanford, who is well versed as to exactly which private insurers in Massachusetts will and will not reimburse specific medications, said her patients with insurance coverage have been known to delay retirement so that they can remain on the more expensive medications.
“For the past 8 years,” she said, “the Treat and Reduce Obesity Act has had bipartisan support in Congress but has not passed. We are still hopeful that insurers will be required to cover medical and behavioral treatments for obesity.”
As our society struggles to destigmatize so many disorders, obesity remains a highly stigmatized condition, one that our patients cannot hide and one that leads to so many other comorbid illnesses. As new treatments are approved, there will be more for physicians to offer. Semaglutide, if it becomes available to those who need it most, could be a game changer. For patients who have not had success with traditional weight-loss methods, it’s encouraging to have another option available, one that may be reasonable to try before resorting to bariatric surgery.
For decades, psychiatrists have been comfortable prescribing treatments that lead to weight gain. Now, maybe it’s time they also prescribe those that prevent it.
A version of this article first appeared on Medscape.com.
It’s probably fair to say that most people would like to be thinner. More than 42% of Americans have obesity and another 30% are classified as being overweight, according to the latest statistics from the CDC.
Excess body weight is associated with many illnesses and plays a role in mental health; being heavy can take a toll on self-esteem. Many people worry that carrying excess weight makes them less attractive to potential romantic partners, and both physicians and employers treat those with obesity differently. Furthermore, in psychiatry, many of the medications we prescribe lead to weight gain.
In my clinical practice, I have listened as patients blamed themselves for their body habitus; many won’t consider biological treatments as they feel that would be “cheating” or taking an easy way out. They often point to periods in their life when they did lose weight and believe that they should be able to do it again, even if the weight loss took tremendous effort, was not sustained, and occurred decades ago.
That said, we psychiatrists often find ourselves in the position of managing obesity in our patients. I have been known to give patients who gain weight on antipsychotics either stimulants or metformin, or to add naltrexone to their Wellbutrin (bupropion) to effectively mimic a weight-loss medicine called Contrave.
Obesity a treatable medical condition
It wasn’t until 2013 that the American Medical Association recognized obesity as a medical condition.
In a New Yorker article that same year, “Diet Drugs Work: Why Won’t Doctors Prescribe Them?” Suzanne Koven wrote: “Several obesity experts told me they’ve encountered doctors who confide that they just didn’t like fat people and don’t enjoy taking care of them. Even doctors who treat obese patients feel stigmatized: ‘diet doctor’ is not a flattering term.”
Eat less, exercise more – with a blame-the-patient attitude – is still what people see as the “right” way to lose weight.
On June 4, 2021, the FDA approved semaglutide, a glucagonlike peptide–1 receptor agonist, previously used for the treatment of diabetes, for use as a weight loss agent for patients with obesity, or for those with a body mass index over 27 kg/m2 if they also have a weight-related comorbidity.
Semaglutide has three trade names, all manufactured by Novo Nordisk. The pill version is called Rybelsus and comes in 7-mg and 14-mg tablets. Ozempic is available in 0.5-mg and 1.0-mg doses and is administered weekly by subcutaneous injection for diabetes. The new, higher-dose preparation for weight loss, Wegovy, 2.4 mg, also comes as a weekly subcutaneous dose and is now available for the hefty price of $1,400 per month.
In STEP 1 trials, the higher-dose Wegovy was associated with an average 14.9% weight loss (15.3 kg) over 68 weeks, more than any other single-agent weight loss medication on the market.
GLP-1 receptor agonists work in the brain to decrease appetite, slow gastric emptying, increase insulin secretion, and stimulate brown adipose tissue thermogenesis.
Psych drugs lead to weight gain
Elaine Weiner, MD, is the medical director in the outpatient research program of the Maryland Psychiatric Research Center in Catonsville, where she treats patients with schizophrenia.
“Nearly all of our patients gain 20 pounds or more on the combinations of medications we use, mostly atypical antipsychotics,” she said. “Weight management is difficult for people who don’t have problems with motivation, but in our patients, lack of motivation is a core part of their illness, so asking them to adhere to diet and exercise regimens is of limited utility.
“Then, add to that the fact that they sometimes don’t have primary care doctors, and these issues of weight gain and metabolic syndrome come back to the psychiatrist. It is a really bad problem and we need more treatments.”
Fatima Cody Stanford, MD, MPH, MPA, is a fellowship-trained obesity medicine physician-scientist at the Massachusetts General Hospital Weight Center and Harvard Medical School, both in Boston. She has treated thousands of patients with obesity, speaks internationally on the topic of weight loss medicine, and has published over 100 peer-reviewed articles on obesity.
We spoke at length about recent changes in the field of obesity medicine and the introduction of the new GLP-1 receptor agonists.
“We as physicians have learned so little,” Dr. Stanford said. “This mantra of ‘calories in, calories out’ is not working; this is inaccurate and our focus on this has led to a rise in obesity. All calories are not created the same, and I think we are finally starting to see obesity medicine take off.”
Dr. Stanford is quick to note that obesity is a complex problem. Several different hormones are involved in regulating both appetite and satiety, processed foods promote weight gain, sleep is crucial to weight loss, and exercise helps maintain weight loss but is not usually effective in promoting it. “There are many contributors to energy storage,” she said.
The stimulant phentermine was approved in 1959. Addiction was a concern, and then in the 1990s, it was used in combination with fenfluramine to promote weight loss, a combination known as phen-fen. Fenfluramine was pulled from the market in 1997 when it was found to be associated with pulmonary hypertension and then heart valve abnormalities.
“This frightened quite a few physicians,” Dr. Stanford noted. Phentermine is still used for weight loss, either alone or together with topiramate, as a combination medication called Qsymia, nicknamed phen-top.
“Phen-top is the next best thing we have to semaglutide, and there is an average weight loss of 8%-9% of body weight. Semaglutide is going to be really significant for those people who are responders, and this has been quite well tolerated, the most common side effect being nausea,” she said.
However, she is quick to note that not everyone responds to every medication. “I use each patient’s clinical profile to determine what strategies and which medications to use.”
Cardiologists getting in the game
Michael Miller, MD, is a cardiologist at the University of Maryland, Baltimore, and author of “Heal Your Heart” (Emmaus, Pa.: Rodale, 2014). He is very enthusiastic about the approval of semaglutide.
“We are so excited because you finally can use these medicines without having to be diabetic,” Dr. Miller said. “We’re waiting on the results of the SELECT [Semaglutide Effects on Heart Disease and Stroke in Patients With Overweight or Obesity] trials looking at people who are not diabetic or who are prediabetic, to see the 5-year outcomes with regard to cardiac events.
“Usually endocrinologists prescribe these medications, but cardiologists have started to get into the game since GLP-1 receptor agonists reduce cardiovascular events.” Dr. Miller is hopeful that this medication may neutralize the weight gain caused by psychotropic medications.
Wegovy is administered via weekly injection and, like insulin, is a subcutaneous medication that patients self-administer. Will patients be amenable to injecting a medication for weight loss? Dr. Stanford said that roughly 20%-30% of her patients are hesitant when she suggests that they use liraglutide, another GLP-1 receptor agonist that is approved for weight loss, and some are very fearful of needles.
However, she also noted that during the COVID-19 pandemic, many more patients have sought treatment from obesity medicine physicians because of the association between obesity and mortality from COVID-19. Patients have been willing to consider treatments that they were not previously open to pursuing.
So if people are willing to take Wegovy and doctors are willing to prescribe it, will insurers pay for it? As of this writing, the medication is not yet available, but Ozempic, the lower-dose agent for diabetes, costs $850-$900 for a 4-week supply, according to the GoodRx website.
Liraglutide (Saxenda), the GLP-1 receptor agonist that is currently available for weight loss as a daily injectable, costs $1,300-$1,400 per month.
These medications are not covered by Medicare or Medicaid, and Dr. Stanford, who is well versed as to exactly which private insurers in Massachusetts will and will not reimburse specific medications, said her patients with insurance coverage have been known to delay retirement so that they can remain on the more expensive medications.
“For the past 8 years,” she said, “the Treat and Reduce Obesity Act has had bipartisan support in Congress but has not passed. We are still hopeful that insurers will be required to cover medical and behavioral treatments for obesity.”
As our society struggles to destigmatize so many disorders, obesity remains a highly stigmatized condition, one that our patients cannot hide and one that leads to so many other comorbid illnesses. As new treatments are approved, there will be more for physicians to offer. Semaglutide, if it becomes available to those who need it most, could be a game changer. For patients who have not had success with traditional weight-loss methods, it’s encouraging to have another option available, one that may be reasonable to try before resorting to bariatric surgery.
For decades, psychiatrists have been comfortable prescribing treatments that lead to weight gain. Now, maybe it’s time they also prescribe those that prevent it.
A version of this article first appeared on Medscape.com.
It’s probably fair to say that most people would like to be thinner. More than 42% of Americans have obesity and another 30% are classified as being overweight, according to the latest statistics from the CDC.
Excess body weight is associated with many illnesses and plays a role in mental health; being heavy can take a toll on self-esteem. Many people worry that carrying excess weight makes them less attractive to potential romantic partners, and both physicians and employers treat those with obesity differently. Furthermore, in psychiatry, many of the medications we prescribe lead to weight gain.
In my clinical practice, I have listened as patients blamed themselves for their body habitus; many won’t consider biological treatments as they feel that would be “cheating” or taking an easy way out. They often point to periods in their life when they did lose weight and believe that they should be able to do it again, even if the weight loss took tremendous effort, was not sustained, and occurred decades ago.
That said, we psychiatrists often find ourselves in the position of managing obesity in our patients. I have been known to give patients who gain weight on antipsychotics either stimulants or metformin, or to add naltrexone to their Wellbutrin (bupropion) to effectively mimic a weight-loss medicine called Contrave.
Obesity a treatable medical condition
It wasn’t until 2013 that the American Medical Association recognized obesity as a medical condition.
In a New Yorker article that same year, “Diet Drugs Work: Why Won’t Doctors Prescribe Them?” Suzanne Koven wrote: “Several obesity experts told me they’ve encountered doctors who confide that they just didn’t like fat people and don’t enjoy taking care of them. Even doctors who treat obese patients feel stigmatized: ‘diet doctor’ is not a flattering term.”
Eat less, exercise more – with a blame-the-patient attitude – is still what people see as the “right” way to lose weight.
On June 4, 2021, the FDA approved semaglutide, a glucagonlike peptide–1 receptor agonist, previously used for the treatment of diabetes, for use as a weight loss agent for patients with obesity, or for those with a body mass index over 27 kg/m2 if they also have a weight-related comorbidity.
Semaglutide has three trade names, all manufactured by Novo Nordisk. The pill version is called Rybelsus and comes in 7-mg and 14-mg tablets. Ozempic is available in 0.5-mg and 1.0-mg doses and is administered weekly by subcutaneous injection for diabetes. The new, higher-dose preparation for weight loss, Wegovy, 2.4 mg, also comes as a weekly subcutaneous dose and is now available for the hefty price of $1,400 per month.
In STEP 1 trials, the higher-dose Wegovy was associated with an average 14.9% weight loss (15.3 kg) over 68 weeks, more than any other single-agent weight loss medication on the market.
GLP-1 receptor agonists work in the brain to decrease appetite, slow gastric emptying, increase insulin secretion, and stimulate brown adipose tissue thermogenesis.
Psych drugs lead to weight gain
Elaine Weiner, MD, is the medical director in the outpatient research program of the Maryland Psychiatric Research Center in Catonsville, where she treats patients with schizophrenia.
“Nearly all of our patients gain 20 pounds or more on the combinations of medications we use, mostly atypical antipsychotics,” she said. “Weight management is difficult for people who don’t have problems with motivation, but in our patients, lack of motivation is a core part of their illness, so asking them to adhere to diet and exercise regimens is of limited utility.
“Then, add to that the fact that they sometimes don’t have primary care doctors, and these issues of weight gain and metabolic syndrome come back to the psychiatrist. It is a really bad problem and we need more treatments.”
Fatima Cody Stanford, MD, MPH, MPA, is a fellowship-trained obesity medicine physician-scientist at the Massachusetts General Hospital Weight Center and Harvard Medical School, both in Boston. She has treated thousands of patients with obesity, speaks internationally on the topic of weight loss medicine, and has published over 100 peer-reviewed articles on obesity.
We spoke at length about recent changes in the field of obesity medicine and the introduction of the new GLP-1 receptor agonists.
“We as physicians have learned so little,” Dr. Stanford said. “This mantra of ‘calories in, calories out’ is not working; this is inaccurate and our focus on this has led to a rise in obesity. All calories are not created the same, and I think we are finally starting to see obesity medicine take off.”
Dr. Stanford is quick to note that obesity is a complex problem. Several different hormones are involved in regulating both appetite and satiety, processed foods promote weight gain, sleep is crucial to weight loss, and exercise helps maintain weight loss but is not usually effective in promoting it. “There are many contributors to energy storage,” she said.
The stimulant phentermine was approved in 1959. Addiction was a concern, and then in the 1990s, it was used in combination with fenfluramine to promote weight loss, a combination known as phen-fen. Fenfluramine was pulled from the market in 1997 when it was found to be associated with pulmonary hypertension and then heart valve abnormalities.
“This frightened quite a few physicians,” Dr. Stanford noted. Phentermine is still used for weight loss, either alone or together with topiramate, as a combination medication called Qsymia, nicknamed phen-top.
“Phen-top is the next best thing we have to semaglutide, and there is an average weight loss of 8%-9% of body weight. Semaglutide is going to be really significant for those people who are responders, and this has been quite well tolerated, the most common side effect being nausea,” she said.
However, she is quick to note that not everyone responds to every medication. “I use each patient’s clinical profile to determine what strategies and which medications to use.”
Cardiologists getting in the game
Michael Miller, MD, is a cardiologist at the University of Maryland, Baltimore, and author of “Heal Your Heart” (Emmaus, Pa.: Rodale, 2014). He is very enthusiastic about the approval of semaglutide.
“We are so excited because you finally can use these medicines without having to be diabetic,” Dr. Miller said. “We’re waiting on the results of the SELECT [Semaglutide Effects on Heart Disease and Stroke in Patients With Overweight or Obesity] trials looking at people who are not diabetic or who are prediabetic, to see the 5-year outcomes with regard to cardiac events.
“Usually endocrinologists prescribe these medications, but cardiologists have started to get into the game since GLP-1 receptor agonists reduce cardiovascular events.” Dr. Miller is hopeful that this medication may neutralize the weight gain caused by psychotropic medications.
Wegovy is administered via weekly injection and, like insulin, is a subcutaneous medication that patients self-administer. Will patients be amenable to injecting a medication for weight loss? Dr. Stanford said that roughly 20%-30% of her patients are hesitant when she suggests that they use liraglutide, another GLP-1 receptor agonist that is approved for weight loss, and some are very fearful of needles.
However, she also noted that during the COVID-19 pandemic, many more patients have sought treatment from obesity medicine physicians because of the association between obesity and mortality from COVID-19. Patients have been willing to consider treatments that they were not previously open to pursuing.
So if people are willing to take Wegovy and doctors are willing to prescribe it, will insurers pay for it? As of this writing, the medication is not yet available, but Ozempic, the lower-dose agent for diabetes, costs $850-$900 for a 4-week supply, according to the GoodRx website.
Liraglutide (Saxenda), the GLP-1 receptor agonist that is currently available for weight loss as a daily injectable, costs $1,300-$1,400 per month.
These medications are not covered by Medicare or Medicaid, and Dr. Stanford, who is well versed as to exactly which private insurers in Massachusetts will and will not reimburse specific medications, said her patients with insurance coverage have been known to delay retirement so that they can remain on the more expensive medications.
“For the past 8 years,” she said, “the Treat and Reduce Obesity Act has had bipartisan support in Congress but has not passed. We are still hopeful that insurers will be required to cover medical and behavioral treatments for obesity.”
As our society struggles to destigmatize so many disorders, obesity remains a highly stigmatized condition, one that our patients cannot hide and one that leads to so many other comorbid illnesses. As new treatments are approved, there will be more for physicians to offer. Semaglutide, if it becomes available to those who need it most, could be a game changer. For patients who have not had success with traditional weight-loss methods, it’s encouraging to have another option available, one that may be reasonable to try before resorting to bariatric surgery.
For decades, psychiatrists have been comfortable prescribing treatments that lead to weight gain. Now, maybe it’s time they also prescribe those that prevent it.
A version of this article first appeared on Medscape.com.
Remote cognitive assessments get positive mark
That is the message behind numerous publications in recent years, and the COVID-19 pandemic has accelerated that trend.
“The publications have just skyrocketed since 2018, but I think there are still some additional tests that we need to validate using this medium of assessment. Also, I think we need to kind of put on our thinking caps as a field and think outside the box. What novel tests can we develop that will capitalize upon the telehealth environment – interactive tests that are monitoring [the individuals’] performance in real time and giving the examiner feedback, things like that,” said Munro Cullum, PhD, in an interview. Dr. Cullum spoke on the topic at the 2021 Alzheimer’s Association International Conference.
Still, challenges remain, especially factors in the home environment that can adversely affect testing. “Some of our tests are a question-answer, pencil-paper sort of tests that can be well suited to a telemedicine environment, [but] other tests don’t translate as well. So we still have a ways to go to kind of get our test to the next generation when being administered during this type of assessment. But a lot of the verbal tests work extremely well,” said Dr. Cullum, who is a professor of psychiatry at the University of Texas Southwestern Medical Center, Dallas.
Preliminary evidence of equivalence
Some years ago, Dr. Cullum was interested in getting a better understanding of what existing tests could best be performed remotely, and what populations could most benefit from remote assessments. Existing studies were generally supportive of remote testing, but varied significantly in their methodology and design. He went on to publish a study in 2014 showing equivalency of existing tests in the in-person and remote environment, and that helped pave the way for a wave of more recent studies that seem to confirm equivalence of in-person methods.
“If you look at the literature overall, there is a nice, growing body of evidence suggesting support for a host of neuropsychological test instruments. For the most part, almost all have shown good reliability across test conditions,” Dr. Cullum said during the talk.
He said that he is often asked if different test norms will be required for remote tests, but that doesn’t seem to be a concern. “It looks like the regular old neuropsych test norms should serve as well in this remote assessment environment. Although as within hospital testing of patients, conservative use of norms is always an order. They are interpretive guidelines,” he added.
One concern is potential threats to validity within the home environment. He posted an image of a woman at home, taking a remote cognitive test. The desk she sat at overlooked a wooded scene, and had a sewing machine on it. A small dog lay in her lap. “So assessing the home environment, ensuring that it is as close to a clinical standard setting as possible, is certainly advised,” said Dr. Cullum.
Although much progress has been made in studying existing tests in a telemedicine environment, many commonly used tests still haven’t been studied. The risk of intrusions and distractions, and even connectivity issues, can be limiting factors. Some tests may be ineligible for remote use due to copyright issues that might prevent required materials from being displayed online. For those reasons and others, not all individuals are suited for a remote test.
Finally, remote tests should be viewed with healthy skepticism. “In doing clinical evaluations this way, we have to be extra careful to not mis- or overinterpret the findings in case there were any distractions or glitches in the examination that came up during the test,” said Dr. Cullum.
Looking toward the future
Moving forward, Dr. Cullum called for more research to design new tests to exploit the telehealth format. “I think this is a really important opportunity for new test development in neuropsychology with increasing incorporation of computerized measures and integration with more cognitive neuroscience and clinical neuropsychology principles.”
He also suggested that remote testing could be combined with neuroimaging, neuromodulation, and even portable magnetoencephalography. “These opportunities for research can enhance compliance, enhance large-scale studies to allow for the inclusion of brief cognitive outcome metrics that might not have other otherwise been [possible],” said Dr. Cullum.
During the question-and-answer session, someone asked if the momentum towards telehealth will continue once the COVID-19 pandemic recedes. “We believe telehealth is here to stay, or at least I do,” said session moderator Allison Lindauer, PhD, who was asked to comment. Dr. Lindauer is an associate professor at the Layton Aging and Alzheimer’s Disease Center in Portland, Ore.
Dr. Lindauer has also conducted studies on telehealth-delivered assessments and also found encouraging results. “Work like this says, we have confidence in our work, we can believe that what we’re assessing and what we’re doing – if we did it face to face, we would get similar results,” Dr. Lindauer said in an interview.
Plenty of challenges remain, and the most important is widely available broadband internet, said Dr. Lindauer. “We need a huge push to get broadband everywhere. Granted, you’re going to have people that don’t want to use the computer, or they’re nervous about doing it online. But in my experience, most people with enough coaching can do it and are fine with it.”
Dr. Cullum and Dr. Lindauer have no relevant financial disclosures.
That is the message behind numerous publications in recent years, and the COVID-19 pandemic has accelerated that trend.
“The publications have just skyrocketed since 2018, but I think there are still some additional tests that we need to validate using this medium of assessment. Also, I think we need to kind of put on our thinking caps as a field and think outside the box. What novel tests can we develop that will capitalize upon the telehealth environment – interactive tests that are monitoring [the individuals’] performance in real time and giving the examiner feedback, things like that,” said Munro Cullum, PhD, in an interview. Dr. Cullum spoke on the topic at the 2021 Alzheimer’s Association International Conference.
Still, challenges remain, especially factors in the home environment that can adversely affect testing. “Some of our tests are a question-answer, pencil-paper sort of tests that can be well suited to a telemedicine environment, [but] other tests don’t translate as well. So we still have a ways to go to kind of get our test to the next generation when being administered during this type of assessment. But a lot of the verbal tests work extremely well,” said Dr. Cullum, who is a professor of psychiatry at the University of Texas Southwestern Medical Center, Dallas.
Preliminary evidence of equivalence
Some years ago, Dr. Cullum was interested in getting a better understanding of what existing tests could best be performed remotely, and what populations could most benefit from remote assessments. Existing studies were generally supportive of remote testing, but varied significantly in their methodology and design. He went on to publish a study in 2014 showing equivalency of existing tests in the in-person and remote environment, and that helped pave the way for a wave of more recent studies that seem to confirm equivalence of in-person methods.
“If you look at the literature overall, there is a nice, growing body of evidence suggesting support for a host of neuropsychological test instruments. For the most part, almost all have shown good reliability across test conditions,” Dr. Cullum said during the talk.
He said that he is often asked if different test norms will be required for remote tests, but that doesn’t seem to be a concern. “It looks like the regular old neuropsych test norms should serve as well in this remote assessment environment. Although as within hospital testing of patients, conservative use of norms is always an order. They are interpretive guidelines,” he added.
One concern is potential threats to validity within the home environment. He posted an image of a woman at home, taking a remote cognitive test. The desk she sat at overlooked a wooded scene, and had a sewing machine on it. A small dog lay in her lap. “So assessing the home environment, ensuring that it is as close to a clinical standard setting as possible, is certainly advised,” said Dr. Cullum.
Although much progress has been made in studying existing tests in a telemedicine environment, many commonly used tests still haven’t been studied. The risk of intrusions and distractions, and even connectivity issues, can be limiting factors. Some tests may be ineligible for remote use due to copyright issues that might prevent required materials from being displayed online. For those reasons and others, not all individuals are suited for a remote test.
Finally, remote tests should be viewed with healthy skepticism. “In doing clinical evaluations this way, we have to be extra careful to not mis- or overinterpret the findings in case there were any distractions or glitches in the examination that came up during the test,” said Dr. Cullum.
Looking toward the future
Moving forward, Dr. Cullum called for more research to design new tests to exploit the telehealth format. “I think this is a really important opportunity for new test development in neuropsychology with increasing incorporation of computerized measures and integration with more cognitive neuroscience and clinical neuropsychology principles.”
He also suggested that remote testing could be combined with neuroimaging, neuromodulation, and even portable magnetoencephalography. “These opportunities for research can enhance compliance, enhance large-scale studies to allow for the inclusion of brief cognitive outcome metrics that might not have other otherwise been [possible],” said Dr. Cullum.
During the question-and-answer session, someone asked if the momentum towards telehealth will continue once the COVID-19 pandemic recedes. “We believe telehealth is here to stay, or at least I do,” said session moderator Allison Lindauer, PhD, who was asked to comment. Dr. Lindauer is an associate professor at the Layton Aging and Alzheimer’s Disease Center in Portland, Ore.
Dr. Lindauer has also conducted studies on telehealth-delivered assessments and also found encouraging results. “Work like this says, we have confidence in our work, we can believe that what we’re assessing and what we’re doing – if we did it face to face, we would get similar results,” Dr. Lindauer said in an interview.
Plenty of challenges remain, and the most important is widely available broadband internet, said Dr. Lindauer. “We need a huge push to get broadband everywhere. Granted, you’re going to have people that don’t want to use the computer, or they’re nervous about doing it online. But in my experience, most people with enough coaching can do it and are fine with it.”
Dr. Cullum and Dr. Lindauer have no relevant financial disclosures.
That is the message behind numerous publications in recent years, and the COVID-19 pandemic has accelerated that trend.
“The publications have just skyrocketed since 2018, but I think there are still some additional tests that we need to validate using this medium of assessment. Also, I think we need to kind of put on our thinking caps as a field and think outside the box. What novel tests can we develop that will capitalize upon the telehealth environment – interactive tests that are monitoring [the individuals’] performance in real time and giving the examiner feedback, things like that,” said Munro Cullum, PhD, in an interview. Dr. Cullum spoke on the topic at the 2021 Alzheimer’s Association International Conference.
Still, challenges remain, especially factors in the home environment that can adversely affect testing. “Some of our tests are a question-answer, pencil-paper sort of tests that can be well suited to a telemedicine environment, [but] other tests don’t translate as well. So we still have a ways to go to kind of get our test to the next generation when being administered during this type of assessment. But a lot of the verbal tests work extremely well,” said Dr. Cullum, who is a professor of psychiatry at the University of Texas Southwestern Medical Center, Dallas.
Preliminary evidence of equivalence
Some years ago, Dr. Cullum was interested in getting a better understanding of what existing tests could best be performed remotely, and what populations could most benefit from remote assessments. Existing studies were generally supportive of remote testing, but varied significantly in their methodology and design. He went on to publish a study in 2014 showing equivalency of existing tests in the in-person and remote environment, and that helped pave the way for a wave of more recent studies that seem to confirm equivalence of in-person methods.
“If you look at the literature overall, there is a nice, growing body of evidence suggesting support for a host of neuropsychological test instruments. For the most part, almost all have shown good reliability across test conditions,” Dr. Cullum said during the talk.
He said that he is often asked if different test norms will be required for remote tests, but that doesn’t seem to be a concern. “It looks like the regular old neuropsych test norms should serve as well in this remote assessment environment. Although as within hospital testing of patients, conservative use of norms is always an order. They are interpretive guidelines,” he added.
One concern is potential threats to validity within the home environment. He posted an image of a woman at home, taking a remote cognitive test. The desk she sat at overlooked a wooded scene, and had a sewing machine on it. A small dog lay in her lap. “So assessing the home environment, ensuring that it is as close to a clinical standard setting as possible, is certainly advised,” said Dr. Cullum.
Although much progress has been made in studying existing tests in a telemedicine environment, many commonly used tests still haven’t been studied. The risk of intrusions and distractions, and even connectivity issues, can be limiting factors. Some tests may be ineligible for remote use due to copyright issues that might prevent required materials from being displayed online. For those reasons and others, not all individuals are suited for a remote test.
Finally, remote tests should be viewed with healthy skepticism. “In doing clinical evaluations this way, we have to be extra careful to not mis- or overinterpret the findings in case there were any distractions or glitches in the examination that came up during the test,” said Dr. Cullum.
Looking toward the future
Moving forward, Dr. Cullum called for more research to design new tests to exploit the telehealth format. “I think this is a really important opportunity for new test development in neuropsychology with increasing incorporation of computerized measures and integration with more cognitive neuroscience and clinical neuropsychology principles.”
He also suggested that remote testing could be combined with neuroimaging, neuromodulation, and even portable magnetoencephalography. “These opportunities for research can enhance compliance, enhance large-scale studies to allow for the inclusion of brief cognitive outcome metrics that might not have other otherwise been [possible],” said Dr. Cullum.
During the question-and-answer session, someone asked if the momentum towards telehealth will continue once the COVID-19 pandemic recedes. “We believe telehealth is here to stay, or at least I do,” said session moderator Allison Lindauer, PhD, who was asked to comment. Dr. Lindauer is an associate professor at the Layton Aging and Alzheimer’s Disease Center in Portland, Ore.
Dr. Lindauer has also conducted studies on telehealth-delivered assessments and also found encouraging results. “Work like this says, we have confidence in our work, we can believe that what we’re assessing and what we’re doing – if we did it face to face, we would get similar results,” Dr. Lindauer said in an interview.
Plenty of challenges remain, and the most important is widely available broadband internet, said Dr. Lindauer. “We need a huge push to get broadband everywhere. Granted, you’re going to have people that don’t want to use the computer, or they’re nervous about doing it online. But in my experience, most people with enough coaching can do it and are fine with it.”
Dr. Cullum and Dr. Lindauer have no relevant financial disclosures.
FROM AAIC 2021
Reducing air pollution is linked to slowed brain aging and lower dementia risk
, new research reveals. The findings have implications for individual behaviors, such as avoiding areas with poor air quality, but they also have implications for public policy, said study investigator, Xinhui Wang, PhD, assistant professor of research neurology, department of neurology, University of Southern California, Los Angeles.
“Controlling air quality has great benefits not only for the short-term, for example for pulmonary function or very broadly mortality, but can impact brain function and slow memory function decline and in the long run may reduce dementia cases.”
The findings were presented at the 2021 Alzheimer’s Association International Conference.
New approach
Previous research examining the impact of reducing air pollution, which has primarily examined respiratory illnesses and mortality, showed it is beneficial. However, no previous studies have examined the impact of improved air quality on cognitive function.
The current study used a subset of participants from the Women’s Health Initiative Memory Study-Epidemiology of Cognitive Health Outcomes (WHIMS-ECHO), which evaluated whether postmenopausal women derive cognitive benefit from hormone therapy.
The analysis included 2,232 community-dwelling older women aged 74-92 (mean age, 81.5 years) who did not have dementia at study enrollment.
Researchers obtained measures of participants’ annual cognitive function from 2008 to 2018. These measures included general cognitive status assessed using the Telephone Interview for Cognitive Status-modified (TICSm) and episodic memory assessed by the telephone-based California Verbal Learning Test (CVLT).
The investigators used complex geographical covariates to estimate exposure to fine particulate matter (PM2.5) and nitrogen dioxide (NO2), in areas where individual participants lived from 1996 to 2012. The investigators averaged measures over 3-year periods immediately preceding (recent exposure) and 10 years prior to (remote exposure) enrollment, then calculated individual-level improvements in air quality as the reduction from remote to recent exposures.
The researchers examined pollution exposure and cognitive outcomes at different times to determine causation.
“Maybe the relationship isn’t causal and is just an association, so we tried to separate the timeframe for exposure and outcome and make sure the exposure was before we measured the outcome,” said Dr. Wang.
The investigators adjusted for multiple sociodemographic, lifestyle, and clinical characteristics.
Reduced dementia risk
The analysis showed air quality improved significantly for both PM2.5 and NO2 before study enrollment. “For almost 95% of the subjects in our study, air quality improved over the 10 years,” said Dr. Wang.
During a median follow-up of 6.2 years, there was a significant decline in cognitive status and episodic memory in study participants, which makes sense, said Dr. Wang, because cognitive function naturally declines with age.
However, a 10% improvement in air quality PM2.5 and NO2 resulted in a respective 14% and 26% decreased risk for dementia. This translates into a level of risk seen in women 2 to 3 years younger.
Greater air quality improvement was associated with slower decline in both general cognitive status and episodic memory.
“Participants all declined in cognitive function, but living in areas with the greatest air quality improvement slowed this decline,” said Dr. Wang.
“Whether you look at global cognitive function or memory-specific function, and whether you look at PM2.5 or NO2, slower decline was in the range of someone who is 1-2 years younger.”
The associations did not significantly differ by age, region, education, APOE ε4 genotypes, or cardiovascular risk factors.
Patients concerned about cognitive decline can take steps to avoid exposure to pollution by wearing a mask; avoiding heavy traffic, fires, and smoke; or moving to an area with better air quality, said Dr. Wang.
“But our study mainly tried to provide some evidence for policymakers and regulators,” she added.
Another study carried out by the same investigators suggests pollution may affect various cognitive functions differently. This analysis used the same cohort, timeframe, and air quality improvement indicators as the first study but examined the association with specific cognitive domains, including episodic memory, working memory, attention/executive function, and language.
The investigators found women living in locations with greater PM2.5 improvement performed better on tests of episodic memory (P = .002), working memory (P = .01) and attention/executive function (P = .01), but not language. Findings were similar for improved NO2.
When looking at air quality improvement and trajectory slopes of decline across cognitive functions, only the association between improved NO2 and slower episodic memory decline was statistically significant (P < 0.001). “The other domains were marginal or not significant,” said Dr. Wang.
“This suggests that brain regions are impacted differently,” she said, adding that various brain areas oversee different cognitive functions.
Important policy implications
Commenting on the research, Rebecca Edelmayer, PhD, senior director of scientific engagement, Alzheimer’s Association, said she welcomes new research on environmental factors that affect Alzheimer’s disease.
Whereas previous studies have linked longterm air pollution exposure to accumulation of Alzheimer’s disease-related brain plaques and increased risk of dementia, “these newer studies provide some of the first evidence to suggest that actually reducing pollution is associated with lower risk of all-cause dementia,” said Dr. Edelmayer.
Individuals can control some factors that contribute to dementia risk, such as exercise, diet, and physical activity, but it’s more difficult for them to control exposure to smog and pollution, she said.
“This is probably going to require changes to policy from federal and local governments and businesses, to start addressing the need to improve air quality to help reduce risk for dementia.”
A version of this article first appeared on Medscape.com.
, new research reveals. The findings have implications for individual behaviors, such as avoiding areas with poor air quality, but they also have implications for public policy, said study investigator, Xinhui Wang, PhD, assistant professor of research neurology, department of neurology, University of Southern California, Los Angeles.
“Controlling air quality has great benefits not only for the short-term, for example for pulmonary function or very broadly mortality, but can impact brain function and slow memory function decline and in the long run may reduce dementia cases.”
The findings were presented at the 2021 Alzheimer’s Association International Conference.
New approach
Previous research examining the impact of reducing air pollution, which has primarily examined respiratory illnesses and mortality, showed it is beneficial. However, no previous studies have examined the impact of improved air quality on cognitive function.
The current study used a subset of participants from the Women’s Health Initiative Memory Study-Epidemiology of Cognitive Health Outcomes (WHIMS-ECHO), which evaluated whether postmenopausal women derive cognitive benefit from hormone therapy.
The analysis included 2,232 community-dwelling older women aged 74-92 (mean age, 81.5 years) who did not have dementia at study enrollment.
Researchers obtained measures of participants’ annual cognitive function from 2008 to 2018. These measures included general cognitive status assessed using the Telephone Interview for Cognitive Status-modified (TICSm) and episodic memory assessed by the telephone-based California Verbal Learning Test (CVLT).
The investigators used complex geographical covariates to estimate exposure to fine particulate matter (PM2.5) and nitrogen dioxide (NO2), in areas where individual participants lived from 1996 to 2012. The investigators averaged measures over 3-year periods immediately preceding (recent exposure) and 10 years prior to (remote exposure) enrollment, then calculated individual-level improvements in air quality as the reduction from remote to recent exposures.
The researchers examined pollution exposure and cognitive outcomes at different times to determine causation.
“Maybe the relationship isn’t causal and is just an association, so we tried to separate the timeframe for exposure and outcome and make sure the exposure was before we measured the outcome,” said Dr. Wang.
The investigators adjusted for multiple sociodemographic, lifestyle, and clinical characteristics.
Reduced dementia risk
The analysis showed air quality improved significantly for both PM2.5 and NO2 before study enrollment. “For almost 95% of the subjects in our study, air quality improved over the 10 years,” said Dr. Wang.
During a median follow-up of 6.2 years, there was a significant decline in cognitive status and episodic memory in study participants, which makes sense, said Dr. Wang, because cognitive function naturally declines with age.
However, a 10% improvement in air quality PM2.5 and NO2 resulted in a respective 14% and 26% decreased risk for dementia. This translates into a level of risk seen in women 2 to 3 years younger.
Greater air quality improvement was associated with slower decline in both general cognitive status and episodic memory.
“Participants all declined in cognitive function, but living in areas with the greatest air quality improvement slowed this decline,” said Dr. Wang.
“Whether you look at global cognitive function or memory-specific function, and whether you look at PM2.5 or NO2, slower decline was in the range of someone who is 1-2 years younger.”
The associations did not significantly differ by age, region, education, APOE ε4 genotypes, or cardiovascular risk factors.
Patients concerned about cognitive decline can take steps to avoid exposure to pollution by wearing a mask; avoiding heavy traffic, fires, and smoke; or moving to an area with better air quality, said Dr. Wang.
“But our study mainly tried to provide some evidence for policymakers and regulators,” she added.
Another study carried out by the same investigators suggests pollution may affect various cognitive functions differently. This analysis used the same cohort, timeframe, and air quality improvement indicators as the first study but examined the association with specific cognitive domains, including episodic memory, working memory, attention/executive function, and language.
The investigators found women living in locations with greater PM2.5 improvement performed better on tests of episodic memory (P = .002), working memory (P = .01) and attention/executive function (P = .01), but not language. Findings were similar for improved NO2.
When looking at air quality improvement and trajectory slopes of decline across cognitive functions, only the association between improved NO2 and slower episodic memory decline was statistically significant (P < 0.001). “The other domains were marginal or not significant,” said Dr. Wang.
“This suggests that brain regions are impacted differently,” she said, adding that various brain areas oversee different cognitive functions.
Important policy implications
Commenting on the research, Rebecca Edelmayer, PhD, senior director of scientific engagement, Alzheimer’s Association, said she welcomes new research on environmental factors that affect Alzheimer’s disease.
Whereas previous studies have linked longterm air pollution exposure to accumulation of Alzheimer’s disease-related brain plaques and increased risk of dementia, “these newer studies provide some of the first evidence to suggest that actually reducing pollution is associated with lower risk of all-cause dementia,” said Dr. Edelmayer.
Individuals can control some factors that contribute to dementia risk, such as exercise, diet, and physical activity, but it’s more difficult for them to control exposure to smog and pollution, she said.
“This is probably going to require changes to policy from federal and local governments and businesses, to start addressing the need to improve air quality to help reduce risk for dementia.”
A version of this article first appeared on Medscape.com.
, new research reveals. The findings have implications for individual behaviors, such as avoiding areas with poor air quality, but they also have implications for public policy, said study investigator, Xinhui Wang, PhD, assistant professor of research neurology, department of neurology, University of Southern California, Los Angeles.
“Controlling air quality has great benefits not only for the short-term, for example for pulmonary function or very broadly mortality, but can impact brain function and slow memory function decline and in the long run may reduce dementia cases.”
The findings were presented at the 2021 Alzheimer’s Association International Conference.
New approach
Previous research examining the impact of reducing air pollution, which has primarily examined respiratory illnesses and mortality, showed it is beneficial. However, no previous studies have examined the impact of improved air quality on cognitive function.
The current study used a subset of participants from the Women’s Health Initiative Memory Study-Epidemiology of Cognitive Health Outcomes (WHIMS-ECHO), which evaluated whether postmenopausal women derive cognitive benefit from hormone therapy.
The analysis included 2,232 community-dwelling older women aged 74-92 (mean age, 81.5 years) who did not have dementia at study enrollment.
Researchers obtained measures of participants’ annual cognitive function from 2008 to 2018. These measures included general cognitive status assessed using the Telephone Interview for Cognitive Status-modified (TICSm) and episodic memory assessed by the telephone-based California Verbal Learning Test (CVLT).
The investigators used complex geographical covariates to estimate exposure to fine particulate matter (PM2.5) and nitrogen dioxide (NO2), in areas where individual participants lived from 1996 to 2012. The investigators averaged measures over 3-year periods immediately preceding (recent exposure) and 10 years prior to (remote exposure) enrollment, then calculated individual-level improvements in air quality as the reduction from remote to recent exposures.
The researchers examined pollution exposure and cognitive outcomes at different times to determine causation.
“Maybe the relationship isn’t causal and is just an association, so we tried to separate the timeframe for exposure and outcome and make sure the exposure was before we measured the outcome,” said Dr. Wang.
The investigators adjusted for multiple sociodemographic, lifestyle, and clinical characteristics.
Reduced dementia risk
The analysis showed air quality improved significantly for both PM2.5 and NO2 before study enrollment. “For almost 95% of the subjects in our study, air quality improved over the 10 years,” said Dr. Wang.
During a median follow-up of 6.2 years, there was a significant decline in cognitive status and episodic memory in study participants, which makes sense, said Dr. Wang, because cognitive function naturally declines with age.
However, a 10% improvement in air quality PM2.5 and NO2 resulted in a respective 14% and 26% decreased risk for dementia. This translates into a level of risk seen in women 2 to 3 years younger.
Greater air quality improvement was associated with slower decline in both general cognitive status and episodic memory.
“Participants all declined in cognitive function, but living in areas with the greatest air quality improvement slowed this decline,” said Dr. Wang.
“Whether you look at global cognitive function or memory-specific function, and whether you look at PM2.5 or NO2, slower decline was in the range of someone who is 1-2 years younger.”
The associations did not significantly differ by age, region, education, APOE ε4 genotypes, or cardiovascular risk factors.
Patients concerned about cognitive decline can take steps to avoid exposure to pollution by wearing a mask; avoiding heavy traffic, fires, and smoke; or moving to an area with better air quality, said Dr. Wang.
“But our study mainly tried to provide some evidence for policymakers and regulators,” she added.
Another study carried out by the same investigators suggests pollution may affect various cognitive functions differently. This analysis used the same cohort, timeframe, and air quality improvement indicators as the first study but examined the association with specific cognitive domains, including episodic memory, working memory, attention/executive function, and language.
The investigators found women living in locations with greater PM2.5 improvement performed better on tests of episodic memory (P = .002), working memory (P = .01) and attention/executive function (P = .01), but not language. Findings were similar for improved NO2.
When looking at air quality improvement and trajectory slopes of decline across cognitive functions, only the association between improved NO2 and slower episodic memory decline was statistically significant (P < 0.001). “The other domains were marginal or not significant,” said Dr. Wang.
“This suggests that brain regions are impacted differently,” she said, adding that various brain areas oversee different cognitive functions.
Important policy implications
Commenting on the research, Rebecca Edelmayer, PhD, senior director of scientific engagement, Alzheimer’s Association, said she welcomes new research on environmental factors that affect Alzheimer’s disease.
Whereas previous studies have linked longterm air pollution exposure to accumulation of Alzheimer’s disease-related brain plaques and increased risk of dementia, “these newer studies provide some of the first evidence to suggest that actually reducing pollution is associated with lower risk of all-cause dementia,” said Dr. Edelmayer.
Individuals can control some factors that contribute to dementia risk, such as exercise, diet, and physical activity, but it’s more difficult for them to control exposure to smog and pollution, she said.
“This is probably going to require changes to policy from federal and local governments and businesses, to start addressing the need to improve air quality to help reduce risk for dementia.”
A version of this article first appeared on Medscape.com.
From AAIC 2021
First guidance on appropriate use of controversial Alzheimer’s drug
, the controversial anti-amyloid drug that was approved by the U.S. Food and Drug Administration in June for adults with early Alzheimer’s disease.
“There are incredible gaps between the FDA label and what most of us in the field feel needs to happen in terms of detailed guidance on using this drug,” said panel member Alireza Atri, MD, PhD, director of the Banner Sun Health Research Institute (Banner Health) in Sun City, Arizona.
“This is a first-in-class drug where the vast majority of clinicians have no experience with it, and patients and their caregivers are already asking for it, and there are some really important conversations to be had – not only about who may qualify to begin with and also about potential effectiveness and safety,” Dr. Atri added.
The aducanumab recommendations were published online July 27 in the Journal of Prevention of Alzheimer’s Disease to coincide with their presentation at the 2021 Alzheimer’s Association International Conference.
A separate article outlining the key recommendations was published in Alzheimer’s and Dementia: Translational Research and Clinical Interventions.
Patient-centered focus
The panel recommends that aducanumab only be used for patients with clinical features similar to those of the patients who took part in the clinical trials that led to the drug’s approval – patients with mild cognitive impairment (MCI) due to Alzheimer’s disease and mild Alzheimer’s disease dementia who have brain amyloid, as confirmed on amyloid positron-emission tomography (PET) or with cerebrospinal fluid (CSF) findings consistent with Alzheimer’s disease.
“You’re giving a drug that’s been approved on accelerated status for lowering amyloid, so amyloid status needs to be verified either by an amyloid PET scan or spinal fluid,” said Dr. Atri.
The panel also recommends that patients under consideration for aducanumab treatment have no psychiatric problems; that they be medically stable with no cardiovascular or cardiopulmonary conditions; that they are not taking anticoagulants; that they have no organ failure; and that they have no active cancer except for low-grade basal and squamous cell carcinomas. Current treatment with cholinesterase inhibitors and memantine is acceptable.
Dr. Atri noted that the prescribing label for the drug provides “broad strokes about titration.” The panel recommends that the drug be titrated to the highest dose to maximize opportunity for efficacy.
Monthly infusions should begin with a dose of 1 mg/kg for the first and second infusions. They should be increased to 3 mg/kg for infusions three and four and to 6 mg/kg for the fifth and sixth infusions. The intended dose of 10 mg/kg should be administered on the seventh infusion. The target dose level of 10 mg/kg should then be continued for the foreseeable future, the panel notes.
Safety monitoring is critically important. The panel recommends structured monitoring for amyloid-related imaging abnormalities of the effusion (ARIA-E) or hemorrhagic (ARIA-H) type. Patients should undergo MRI at least 1 year before aducanumab treatment is initiated or at baseline if there are any suggestions of a focal brain event since the last MRI. MRI should again be conducted before the fifth, seventh, and 12th infusions.
The panel says the “best practice” for providing aducanumab therapy is to adopt a patient-centered focus.
‘Not a cure’
“There should be comprehensive discussions and clear communication with the patient and care partner regarding the requirements for therapy, the expected outcome of therapy, potential risks and side effects, and the required safety monitoring, as well as uncertainties regarding individual responses and benefits,” said Dr. Atri.
“Patients need to know that this is not a cure. It’s not going to actually make their cognition better, but by removing amyloid, there is a reasonable chance it’s going to slow down clinical decline,” he added.
“You could have two identical twins who would qualify, and when you have this discussion with them, based on the risk and reward calculus, one may reasonably decide, ‘this is not for me,’ and that’s really important,” Dr. Atri added.
He cautioned that these initial recommendations are “a starting point, not a finishing point,” and will be updated as needed.
“This paper takes no stance on advocating for this treatment. But now that it’s available, let’s put up some guardrails and use it appropriately and safety,” Dr. Atri said.
“Clinicians are requesting clarity and more specific information about the appropriate use of this new treatment,” Rebecca Edelmayer, PhD, senior director of scientific engagement, the Alzheimer’s Association, said in an interview.
These first appropriate-use recommendations are “a first step and will certainly evolve over time as the medication is prescribed,” Dr. Edelmayer said.
The research had no specific funding. Dr. Atri has received honoraria for consulting; participating in independent data safety monitoring boards; providing educational lectures, programs, and materials; or serving on advisory boards for AbbVie, Acadia, Allergan, the Alzheimer’s Association, Axovant, AZ Therapies, Biogen, Grifols, Harvard Medical School Graduate Continuing Education, JOMDD, Lundbeck, Merck, Roche/Genentech, Novo Nordisk, Sunovion, and Suven. Dr. Edelmayer has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, the controversial anti-amyloid drug that was approved by the U.S. Food and Drug Administration in June for adults with early Alzheimer’s disease.
“There are incredible gaps between the FDA label and what most of us in the field feel needs to happen in terms of detailed guidance on using this drug,” said panel member Alireza Atri, MD, PhD, director of the Banner Sun Health Research Institute (Banner Health) in Sun City, Arizona.
“This is a first-in-class drug where the vast majority of clinicians have no experience with it, and patients and their caregivers are already asking for it, and there are some really important conversations to be had – not only about who may qualify to begin with and also about potential effectiveness and safety,” Dr. Atri added.
The aducanumab recommendations were published online July 27 in the Journal of Prevention of Alzheimer’s Disease to coincide with their presentation at the 2021 Alzheimer’s Association International Conference.
A separate article outlining the key recommendations was published in Alzheimer’s and Dementia: Translational Research and Clinical Interventions.
Patient-centered focus
The panel recommends that aducanumab only be used for patients with clinical features similar to those of the patients who took part in the clinical trials that led to the drug’s approval – patients with mild cognitive impairment (MCI) due to Alzheimer’s disease and mild Alzheimer’s disease dementia who have brain amyloid, as confirmed on amyloid positron-emission tomography (PET) or with cerebrospinal fluid (CSF) findings consistent with Alzheimer’s disease.
“You’re giving a drug that’s been approved on accelerated status for lowering amyloid, so amyloid status needs to be verified either by an amyloid PET scan or spinal fluid,” said Dr. Atri.
The panel also recommends that patients under consideration for aducanumab treatment have no psychiatric problems; that they be medically stable with no cardiovascular or cardiopulmonary conditions; that they are not taking anticoagulants; that they have no organ failure; and that they have no active cancer except for low-grade basal and squamous cell carcinomas. Current treatment with cholinesterase inhibitors and memantine is acceptable.
Dr. Atri noted that the prescribing label for the drug provides “broad strokes about titration.” The panel recommends that the drug be titrated to the highest dose to maximize opportunity for efficacy.
Monthly infusions should begin with a dose of 1 mg/kg for the first and second infusions. They should be increased to 3 mg/kg for infusions three and four and to 6 mg/kg for the fifth and sixth infusions. The intended dose of 10 mg/kg should be administered on the seventh infusion. The target dose level of 10 mg/kg should then be continued for the foreseeable future, the panel notes.
Safety monitoring is critically important. The panel recommends structured monitoring for amyloid-related imaging abnormalities of the effusion (ARIA-E) or hemorrhagic (ARIA-H) type. Patients should undergo MRI at least 1 year before aducanumab treatment is initiated or at baseline if there are any suggestions of a focal brain event since the last MRI. MRI should again be conducted before the fifth, seventh, and 12th infusions.
The panel says the “best practice” for providing aducanumab therapy is to adopt a patient-centered focus.
‘Not a cure’
“There should be comprehensive discussions and clear communication with the patient and care partner regarding the requirements for therapy, the expected outcome of therapy, potential risks and side effects, and the required safety monitoring, as well as uncertainties regarding individual responses and benefits,” said Dr. Atri.
“Patients need to know that this is not a cure. It’s not going to actually make their cognition better, but by removing amyloid, there is a reasonable chance it’s going to slow down clinical decline,” he added.
“You could have two identical twins who would qualify, and when you have this discussion with them, based on the risk and reward calculus, one may reasonably decide, ‘this is not for me,’ and that’s really important,” Dr. Atri added.
He cautioned that these initial recommendations are “a starting point, not a finishing point,” and will be updated as needed.
“This paper takes no stance on advocating for this treatment. But now that it’s available, let’s put up some guardrails and use it appropriately and safety,” Dr. Atri said.
“Clinicians are requesting clarity and more specific information about the appropriate use of this new treatment,” Rebecca Edelmayer, PhD, senior director of scientific engagement, the Alzheimer’s Association, said in an interview.
These first appropriate-use recommendations are “a first step and will certainly evolve over time as the medication is prescribed,” Dr. Edelmayer said.
The research had no specific funding. Dr. Atri has received honoraria for consulting; participating in independent data safety monitoring boards; providing educational lectures, programs, and materials; or serving on advisory boards for AbbVie, Acadia, Allergan, the Alzheimer’s Association, Axovant, AZ Therapies, Biogen, Grifols, Harvard Medical School Graduate Continuing Education, JOMDD, Lundbeck, Merck, Roche/Genentech, Novo Nordisk, Sunovion, and Suven. Dr. Edelmayer has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, the controversial anti-amyloid drug that was approved by the U.S. Food and Drug Administration in June for adults with early Alzheimer’s disease.
“There are incredible gaps between the FDA label and what most of us in the field feel needs to happen in terms of detailed guidance on using this drug,” said panel member Alireza Atri, MD, PhD, director of the Banner Sun Health Research Institute (Banner Health) in Sun City, Arizona.
“This is a first-in-class drug where the vast majority of clinicians have no experience with it, and patients and their caregivers are already asking for it, and there are some really important conversations to be had – not only about who may qualify to begin with and also about potential effectiveness and safety,” Dr. Atri added.
The aducanumab recommendations were published online July 27 in the Journal of Prevention of Alzheimer’s Disease to coincide with their presentation at the 2021 Alzheimer’s Association International Conference.
A separate article outlining the key recommendations was published in Alzheimer’s and Dementia: Translational Research and Clinical Interventions.
Patient-centered focus
The panel recommends that aducanumab only be used for patients with clinical features similar to those of the patients who took part in the clinical trials that led to the drug’s approval – patients with mild cognitive impairment (MCI) due to Alzheimer’s disease and mild Alzheimer’s disease dementia who have brain amyloid, as confirmed on amyloid positron-emission tomography (PET) or with cerebrospinal fluid (CSF) findings consistent with Alzheimer’s disease.
“You’re giving a drug that’s been approved on accelerated status for lowering amyloid, so amyloid status needs to be verified either by an amyloid PET scan or spinal fluid,” said Dr. Atri.
The panel also recommends that patients under consideration for aducanumab treatment have no psychiatric problems; that they be medically stable with no cardiovascular or cardiopulmonary conditions; that they are not taking anticoagulants; that they have no organ failure; and that they have no active cancer except for low-grade basal and squamous cell carcinomas. Current treatment with cholinesterase inhibitors and memantine is acceptable.
Dr. Atri noted that the prescribing label for the drug provides “broad strokes about titration.” The panel recommends that the drug be titrated to the highest dose to maximize opportunity for efficacy.
Monthly infusions should begin with a dose of 1 mg/kg for the first and second infusions. They should be increased to 3 mg/kg for infusions three and four and to 6 mg/kg for the fifth and sixth infusions. The intended dose of 10 mg/kg should be administered on the seventh infusion. The target dose level of 10 mg/kg should then be continued for the foreseeable future, the panel notes.
Safety monitoring is critically important. The panel recommends structured monitoring for amyloid-related imaging abnormalities of the effusion (ARIA-E) or hemorrhagic (ARIA-H) type. Patients should undergo MRI at least 1 year before aducanumab treatment is initiated or at baseline if there are any suggestions of a focal brain event since the last MRI. MRI should again be conducted before the fifth, seventh, and 12th infusions.
The panel says the “best practice” for providing aducanumab therapy is to adopt a patient-centered focus.
‘Not a cure’
“There should be comprehensive discussions and clear communication with the patient and care partner regarding the requirements for therapy, the expected outcome of therapy, potential risks and side effects, and the required safety monitoring, as well as uncertainties regarding individual responses and benefits,” said Dr. Atri.
“Patients need to know that this is not a cure. It’s not going to actually make their cognition better, but by removing amyloid, there is a reasonable chance it’s going to slow down clinical decline,” he added.
“You could have two identical twins who would qualify, and when you have this discussion with them, based on the risk and reward calculus, one may reasonably decide, ‘this is not for me,’ and that’s really important,” Dr. Atri added.
He cautioned that these initial recommendations are “a starting point, not a finishing point,” and will be updated as needed.
“This paper takes no stance on advocating for this treatment. But now that it’s available, let’s put up some guardrails and use it appropriately and safety,” Dr. Atri said.
“Clinicians are requesting clarity and more specific information about the appropriate use of this new treatment,” Rebecca Edelmayer, PhD, senior director of scientific engagement, the Alzheimer’s Association, said in an interview.
These first appropriate-use recommendations are “a first step and will certainly evolve over time as the medication is prescribed,” Dr. Edelmayer said.
The research had no specific funding. Dr. Atri has received honoraria for consulting; participating in independent data safety monitoring boards; providing educational lectures, programs, and materials; or serving on advisory boards for AbbVie, Acadia, Allergan, the Alzheimer’s Association, Axovant, AZ Therapies, Biogen, Grifols, Harvard Medical School Graduate Continuing Education, JOMDD, Lundbeck, Merck, Roche/Genentech, Novo Nordisk, Sunovion, and Suven. Dr. Edelmayer has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
From AAIC 2021
Let’s talk about race
“I feel like my aggression is being racialized.” “Of course I wouldn’t call the cops if I felt like hurting myself. I’m Black.”
Those statements represent the heightened trauma our Black and Brown patients with mental health issues have been experiencing. In the wake of increasingly publicized police brutality against Black and Brown communities, the role race plays in mental health decompensation is evident. At this moment in time, we must continue to improve our understanding of the role race plays in psychiatric disorders. We must also ask ourselves: At times, does psychiatry worsen the traumas of the communities we serve?
Some psychiatrists are afraid to speak about race. They may believe it to be too “political.” But avoiding these necessary conversations perpetuates the trauma of those we treat. It suggests that physicians are ignorant of an issue at the forefront of patients’ mental health. Psychiatry, today, is primarily focused on the biological aspects of disease. We must not forget that psychiatry is biopsychosocial. It is imperative that psychiatrists have conversations about race – and its significance to our patients and their care.
Only 10.4% of psychiatrists in the United States comprise those considered underrepresented in medicine (URM). Yet, those very groups make up 32.6% of the U.S. population and are overrepresented in psychiatric hospitals.1 Many studies have shown that concordant racial backgrounds between patient and physician lead to a more positive patient experience2 and arguably, the subsequent potential for better health outcomes. Our efforts in addressing this disparity often fall short. URM applicants may be hesitant to join an institution where diversity is lacking or where they may be the only minority.3 While there is no simple solution, I propose that psychiatrists promote the importance of mental health to Black and Brown students of all ages by collaborating with schools and community leaders.
It is important to acknowledge that the lack of diversity within psychiatry is reflective of that among all physicians. This in part stems from the barriers to medical education that Black and Brown communities face. Those who start off with more resources or have parents who are physicians are at an advantage when trying to get into medical school. In fact, one in five medical students have a parent who is a physician4 and about three-fourths of students come from families whose income falls among the top two quintiles.5 Impoverished communities, which have a disproportionate share of Black and Brown people, cannot afford to take MCAT preparatory classes or to accept unpaid “resume building” opportunities. Many medical schools continue to place more weight on test scores and research/medical experiences, despite a shift to a more holistic review process. Institutions that have tried a different approach and accepted students from more diverse backgrounds may often overlook the challenges that URM students face while in medical school and fail to provide appropriate support resources.
The result is a failure to retain such students. A study conducted at Stony Brook (N.Y.) University showed that those underrepresented in medicine were six times more likely to get dismissed from medical school, and three times more likely to both withdraw or graduate beyond 4 years, compared with their White counterparts.6 This is a serious issue that needs to change on a structural and systemic level.
Any discussion of race and psychiatry must acknowledge psychiatry’s history of racism against Black and Brown communities to engage in racially informed discussions with our patients. Only then can we play a better role advocating against racism within the field in the future. Dating back to the 18th century, psychiatry has promoted ideologies that promote racism. Benjamin Rush, considered the “father of American Psychiatry,” believed that Black skin was a disease derived from leprosy called “negritude.” In the late 19th century, this twisted ideology continued with the invention of the term “drapetomania,” which was used to describe enslaved people who ran away as having a mental disorder.7 Black prisoners were subjected to experimental treatment with substances such as LSD and bulbocapnine to subdue them.8 This idea that minorities were dangerous and needed to be subdued translated into a higher number of schizophrenia diagnoses, particularly among Black men, as it was used as a tool to vilify them in the 1970s. Although schizophrenia is equally prevalent among Whites and non-Whites, Black people are four times more likely to be diagnosed, compared with their White counterparts, while Hispanics are three times more likely. Studies have shown that Black and Brown men are also more likely to receive higher doses of antipsychotics.9
Given this history, it is not surprising that Black and Brown representation within the field is lacking and that patients may be hesitant to share their feelings about race with us. While we can’t change history, we can take a stance condemning the harmful behavior of the past. The American Psychiatric Association issued an apology earlier this year to Black, Indigenous, and People of Color for its support in structural racism.10 This is a step in the right direction, but we need more than statements or performative actions. We need to amplify the voices of Black and Brown psychiatrists and patients, as well as highlight their current and past contributions to the field. While my educational experiences focused on the work of prominent White scholars, medical curricula should showcase the work of people like Solomon Carter Fuller, MD, a Black psychiatrist who was essential to understanding Alzheimer’s, or Joseph White, PhD, sometimes referred to as the “godfather of Black psychology.”11
At times, I have found myself witness to situations where colleagues make statements that not only do not condemn racism, but in fact encourage it. I have unfortunately heard some use the all-too-familiar rhetoric of reverse racism, such as: “They just assume I am racist because I am a White male” or “They’re being racist against me” or statements like “Don’t you think it is far-fetched to believe she was just sitting on a college campus doing nothing when the police were called?” Rhetoric such as this is problematic to the field of psychiatry and medicine as a whole – and only serves to further invalidate the feelings of our Black and Brown patients. We must increase exposure and education regarding racism to address this, especially the meaning of microaggressions, a concept many fail to understand.
Attention to the subject of racism has increased within medical schools and residency training programs in the wake of George Floyd’s death. However, most programs often make these lectures optional or only have one to two limited sessions. Furthermore, many do not make it mandatory for faculty to attend; they are arguably the most in need of this training given that they set the precedent of how to practice psychiatry. Some institutions have incorporated comprehensive antiracist curriculums into medical training. One model that has been successful is the Social Justice and Health Equity program within Yale University’s psychiatry residency. The curriculum has four tracks:
- Structural competency, which focuses on the mental health impact of extraclinical structures, for example a patient’s neighborhood and associated barriers of access.
- Human experience, which explores the interaction of patients and providers and how biases play a role.
- Advocacy, which teaches residents the written and oral skills to lobby for patient interests on a community and legislative level.
- History of psychiatry, which focuses on understanding psychiatry’s prior role in racism.
In each track, there are group discussions, cases led by faculty, and meetings with community leaders. Through this curriculum, residents learn about power, privilege, and how to interact with and advocate for patients in a way that promotes equity, rather than racial disparity.12,13 This is a model that other psychiatric residency programs can promote, emulate, and benefit from.
Educating ourselves will hopefully lead to a deeper introspection of how we interact with patients and if we are promoting antiracism through our attitude and actions. Reflecting on my own personal practice, I have noted that the interplay of race, mental health, and provider decision-making becomes particularly complex when dealing with situations in which a patient exhibits increased aggression or agitation. As a second-year psychiatric resident immersed in the inpatient world, I have become familiar with patients at higher risk and greater need. The first attempt toward de-escalation involves verbal cues without any other more intrusive measures. If that fails, intramuscular (IM) medications are typically considered. If a patient has a history of aggressive behavior, the threshold to use IM medications can decrease dramatically. This is mainly to protect ourselves and our nursing staff and to prioritize safety. While I understand this rationale, I wonder about the patient’s experience. What constitutes “aggressive” behavior? For patients who have had violence used against them because of their race or who have suffered from police brutality, having police present or threatening IM medications will increasingly trigger them and escalate the situation. The aftermath can deepen the distrust of psychiatry by Black and Brown people.
How do we then handle such situations in a way that both protects our staff from physical harm and protects our patients from racial trauma? While I don’t have a great answer, I think we can benefit from standardizing what we consider aggressive behavior and have specific criteria that patients need to exhibit before administering an IM medication. In addition, discussions with the team, including residents, nurses, and attending physicians, about how to address an emergent situation before it actually happens are essential. Specifically discussing the patient’s history and race and how it may affect the situation is not something to be shied away from. Lastly, in the event that an IM medication is administered and police are present, debriefing with the patient afterward is necessary. The patient may not be willing or able to listen to you or trust you, but taking accountability and acknowledging what happened, justified or not, is a part of the process of rebuilding rapport.
Both in the purview of the individual psychiatrist and the field of psychiatry as a whole, we need to examine our behavior and not be afraid to make changes for the betterment of our patients. We must learn to talk about race with our patients and in the process, advocate for more representation of Black and Brown psychiatrists, understanding the barriers faced by these communities when pursuing the medical field. We must educate ourselves on psychiatry’s history, and equip ourselves with knowledge and tools to promote antiracism and shape psychiatry’s future. We can then apply these very tools to challenging situations we may encounter daily with the ultimate goal of improving the mental health of our patients. This is the only way we will progress and ensure that psychiatry is an equitable, antiracist field. As Ibram X. Kendi, PhD, has written, “The heartbeat of antiracism is self-reflection, recognition, admission, and fundamentally self-critique.”
Dr. Malik is a second-year psychiatry resident at the University of California, San Diego. She has a background in policy and grassroots organizing through her time working at the National Coalition for the Homeless and the Women’s Law Project. Dr. Malik has no disclosures.
References
1. Wyse R et al. Acad Psychiatry. 2020 Oct;44(5):523-30.
2. Cooper LA et al. Ann Intern Med. 2003;139:907-15.
3. Pierre JM et al. Acad Psychiatry. 2017;41:226-32.
4. Hartocollis A. “Getting into med school without hard sciences.” New York Times. 2010 Jul 29.
5. AAMC. An updated look at the economic diversity of U.S. medical students. Analysis in Brief. 2018 Oct;18(5).
6. Rainey ML. How do we retain minority health professions students. In: Smedley BD et al. The right thing to do, the smart thing to do: Enhancing diversity in the health professions: Summary of the Symposium on Diversity in Health Professions in Honor of Herbert W. Nickens, M.D. Institute of Medicine. National Academies Press. 2001.
7. Geller J. “Structural racism in American psychiatry and APA: Part 1.” Psychiatric News. 2020 Jun 23.
8. Mohr CL and Gordon JE. Tulane: The emergence of a modern university, 1945-1980. Louisiana State University Press, Baton Rouge. 2001.
9. Metzl JM. The protest psychosis: How schizophrenia became a Black disease. Beacon Press. 2010.
10. APA’s apology to Black, indigenous and people of color for its support of structural racism in psychiatry. American Psychiatric Association. 2021 Jan 18.
11. Black pioneers in mental health. Mental Health America. 2021.
12. Belli B. For Yale’s emerging psychiatrists, confronting racism is in the curriculum. Yale News. 2020 Jul 30.
13. Jordan A and Jackson D. Social justice and health equity curriculum. Yale School of Medicine. 2019 Sep 24.
“I feel like my aggression is being racialized.” “Of course I wouldn’t call the cops if I felt like hurting myself. I’m Black.”
Those statements represent the heightened trauma our Black and Brown patients with mental health issues have been experiencing. In the wake of increasingly publicized police brutality against Black and Brown communities, the role race plays in mental health decompensation is evident. At this moment in time, we must continue to improve our understanding of the role race plays in psychiatric disorders. We must also ask ourselves: At times, does psychiatry worsen the traumas of the communities we serve?
Some psychiatrists are afraid to speak about race. They may believe it to be too “political.” But avoiding these necessary conversations perpetuates the trauma of those we treat. It suggests that physicians are ignorant of an issue at the forefront of patients’ mental health. Psychiatry, today, is primarily focused on the biological aspects of disease. We must not forget that psychiatry is biopsychosocial. It is imperative that psychiatrists have conversations about race – and its significance to our patients and their care.
Only 10.4% of psychiatrists in the United States comprise those considered underrepresented in medicine (URM). Yet, those very groups make up 32.6% of the U.S. population and are overrepresented in psychiatric hospitals.1 Many studies have shown that concordant racial backgrounds between patient and physician lead to a more positive patient experience2 and arguably, the subsequent potential for better health outcomes. Our efforts in addressing this disparity often fall short. URM applicants may be hesitant to join an institution where diversity is lacking or where they may be the only minority.3 While there is no simple solution, I propose that psychiatrists promote the importance of mental health to Black and Brown students of all ages by collaborating with schools and community leaders.
It is important to acknowledge that the lack of diversity within psychiatry is reflective of that among all physicians. This in part stems from the barriers to medical education that Black and Brown communities face. Those who start off with more resources or have parents who are physicians are at an advantage when trying to get into medical school. In fact, one in five medical students have a parent who is a physician4 and about three-fourths of students come from families whose income falls among the top two quintiles.5 Impoverished communities, which have a disproportionate share of Black and Brown people, cannot afford to take MCAT preparatory classes or to accept unpaid “resume building” opportunities. Many medical schools continue to place more weight on test scores and research/medical experiences, despite a shift to a more holistic review process. Institutions that have tried a different approach and accepted students from more diverse backgrounds may often overlook the challenges that URM students face while in medical school and fail to provide appropriate support resources.
The result is a failure to retain such students. A study conducted at Stony Brook (N.Y.) University showed that those underrepresented in medicine were six times more likely to get dismissed from medical school, and three times more likely to both withdraw or graduate beyond 4 years, compared with their White counterparts.6 This is a serious issue that needs to change on a structural and systemic level.
Any discussion of race and psychiatry must acknowledge psychiatry’s history of racism against Black and Brown communities to engage in racially informed discussions with our patients. Only then can we play a better role advocating against racism within the field in the future. Dating back to the 18th century, psychiatry has promoted ideologies that promote racism. Benjamin Rush, considered the “father of American Psychiatry,” believed that Black skin was a disease derived from leprosy called “negritude.” In the late 19th century, this twisted ideology continued with the invention of the term “drapetomania,” which was used to describe enslaved people who ran away as having a mental disorder.7 Black prisoners were subjected to experimental treatment with substances such as LSD and bulbocapnine to subdue them.8 This idea that minorities were dangerous and needed to be subdued translated into a higher number of schizophrenia diagnoses, particularly among Black men, as it was used as a tool to vilify them in the 1970s. Although schizophrenia is equally prevalent among Whites and non-Whites, Black people are four times more likely to be diagnosed, compared with their White counterparts, while Hispanics are three times more likely. Studies have shown that Black and Brown men are also more likely to receive higher doses of antipsychotics.9
Given this history, it is not surprising that Black and Brown representation within the field is lacking and that patients may be hesitant to share their feelings about race with us. While we can’t change history, we can take a stance condemning the harmful behavior of the past. The American Psychiatric Association issued an apology earlier this year to Black, Indigenous, and People of Color for its support in structural racism.10 This is a step in the right direction, but we need more than statements or performative actions. We need to amplify the voices of Black and Brown psychiatrists and patients, as well as highlight their current and past contributions to the field. While my educational experiences focused on the work of prominent White scholars, medical curricula should showcase the work of people like Solomon Carter Fuller, MD, a Black psychiatrist who was essential to understanding Alzheimer’s, or Joseph White, PhD, sometimes referred to as the “godfather of Black psychology.”11
At times, I have found myself witness to situations where colleagues make statements that not only do not condemn racism, but in fact encourage it. I have unfortunately heard some use the all-too-familiar rhetoric of reverse racism, such as: “They just assume I am racist because I am a White male” or “They’re being racist against me” or statements like “Don’t you think it is far-fetched to believe she was just sitting on a college campus doing nothing when the police were called?” Rhetoric such as this is problematic to the field of psychiatry and medicine as a whole – and only serves to further invalidate the feelings of our Black and Brown patients. We must increase exposure and education regarding racism to address this, especially the meaning of microaggressions, a concept many fail to understand.
Attention to the subject of racism has increased within medical schools and residency training programs in the wake of George Floyd’s death. However, most programs often make these lectures optional or only have one to two limited sessions. Furthermore, many do not make it mandatory for faculty to attend; they are arguably the most in need of this training given that they set the precedent of how to practice psychiatry. Some institutions have incorporated comprehensive antiracist curriculums into medical training. One model that has been successful is the Social Justice and Health Equity program within Yale University’s psychiatry residency. The curriculum has four tracks:
- Structural competency, which focuses on the mental health impact of extraclinical structures, for example a patient’s neighborhood and associated barriers of access.
- Human experience, which explores the interaction of patients and providers and how biases play a role.
- Advocacy, which teaches residents the written and oral skills to lobby for patient interests on a community and legislative level.
- History of psychiatry, which focuses on understanding psychiatry’s prior role in racism.
In each track, there are group discussions, cases led by faculty, and meetings with community leaders. Through this curriculum, residents learn about power, privilege, and how to interact with and advocate for patients in a way that promotes equity, rather than racial disparity.12,13 This is a model that other psychiatric residency programs can promote, emulate, and benefit from.
Educating ourselves will hopefully lead to a deeper introspection of how we interact with patients and if we are promoting antiracism through our attitude and actions. Reflecting on my own personal practice, I have noted that the interplay of race, mental health, and provider decision-making becomes particularly complex when dealing with situations in which a patient exhibits increased aggression or agitation. As a second-year psychiatric resident immersed in the inpatient world, I have become familiar with patients at higher risk and greater need. The first attempt toward de-escalation involves verbal cues without any other more intrusive measures. If that fails, intramuscular (IM) medications are typically considered. If a patient has a history of aggressive behavior, the threshold to use IM medications can decrease dramatically. This is mainly to protect ourselves and our nursing staff and to prioritize safety. While I understand this rationale, I wonder about the patient’s experience. What constitutes “aggressive” behavior? For patients who have had violence used against them because of their race or who have suffered from police brutality, having police present or threatening IM medications will increasingly trigger them and escalate the situation. The aftermath can deepen the distrust of psychiatry by Black and Brown people.
How do we then handle such situations in a way that both protects our staff from physical harm and protects our patients from racial trauma? While I don’t have a great answer, I think we can benefit from standardizing what we consider aggressive behavior and have specific criteria that patients need to exhibit before administering an IM medication. In addition, discussions with the team, including residents, nurses, and attending physicians, about how to address an emergent situation before it actually happens are essential. Specifically discussing the patient’s history and race and how it may affect the situation is not something to be shied away from. Lastly, in the event that an IM medication is administered and police are present, debriefing with the patient afterward is necessary. The patient may not be willing or able to listen to you or trust you, but taking accountability and acknowledging what happened, justified or not, is a part of the process of rebuilding rapport.
Both in the purview of the individual psychiatrist and the field of psychiatry as a whole, we need to examine our behavior and not be afraid to make changes for the betterment of our patients. We must learn to talk about race with our patients and in the process, advocate for more representation of Black and Brown psychiatrists, understanding the barriers faced by these communities when pursuing the medical field. We must educate ourselves on psychiatry’s history, and equip ourselves with knowledge and tools to promote antiracism and shape psychiatry’s future. We can then apply these very tools to challenging situations we may encounter daily with the ultimate goal of improving the mental health of our patients. This is the only way we will progress and ensure that psychiatry is an equitable, antiracist field. As Ibram X. Kendi, PhD, has written, “The heartbeat of antiracism is self-reflection, recognition, admission, and fundamentally self-critique.”
Dr. Malik is a second-year psychiatry resident at the University of California, San Diego. She has a background in policy and grassroots organizing through her time working at the National Coalition for the Homeless and the Women’s Law Project. Dr. Malik has no disclosures.
References
1. Wyse R et al. Acad Psychiatry. 2020 Oct;44(5):523-30.
2. Cooper LA et al. Ann Intern Med. 2003;139:907-15.
3. Pierre JM et al. Acad Psychiatry. 2017;41:226-32.
4. Hartocollis A. “Getting into med school without hard sciences.” New York Times. 2010 Jul 29.
5. AAMC. An updated look at the economic diversity of U.S. medical students. Analysis in Brief. 2018 Oct;18(5).
6. Rainey ML. How do we retain minority health professions students. In: Smedley BD et al. The right thing to do, the smart thing to do: Enhancing diversity in the health professions: Summary of the Symposium on Diversity in Health Professions in Honor of Herbert W. Nickens, M.D. Institute of Medicine. National Academies Press. 2001.
7. Geller J. “Structural racism in American psychiatry and APA: Part 1.” Psychiatric News. 2020 Jun 23.
8. Mohr CL and Gordon JE. Tulane: The emergence of a modern university, 1945-1980. Louisiana State University Press, Baton Rouge. 2001.
9. Metzl JM. The protest psychosis: How schizophrenia became a Black disease. Beacon Press. 2010.
10. APA’s apology to Black, indigenous and people of color for its support of structural racism in psychiatry. American Psychiatric Association. 2021 Jan 18.
11. Black pioneers in mental health. Mental Health America. 2021.
12. Belli B. For Yale’s emerging psychiatrists, confronting racism is in the curriculum. Yale News. 2020 Jul 30.
13. Jordan A and Jackson D. Social justice and health equity curriculum. Yale School of Medicine. 2019 Sep 24.
“I feel like my aggression is being racialized.” “Of course I wouldn’t call the cops if I felt like hurting myself. I’m Black.”
Those statements represent the heightened trauma our Black and Brown patients with mental health issues have been experiencing. In the wake of increasingly publicized police brutality against Black and Brown communities, the role race plays in mental health decompensation is evident. At this moment in time, we must continue to improve our understanding of the role race plays in psychiatric disorders. We must also ask ourselves: At times, does psychiatry worsen the traumas of the communities we serve?
Some psychiatrists are afraid to speak about race. They may believe it to be too “political.” But avoiding these necessary conversations perpetuates the trauma of those we treat. It suggests that physicians are ignorant of an issue at the forefront of patients’ mental health. Psychiatry, today, is primarily focused on the biological aspects of disease. We must not forget that psychiatry is biopsychosocial. It is imperative that psychiatrists have conversations about race – and its significance to our patients and their care.
Only 10.4% of psychiatrists in the United States comprise those considered underrepresented in medicine (URM). Yet, those very groups make up 32.6% of the U.S. population and are overrepresented in psychiatric hospitals.1 Many studies have shown that concordant racial backgrounds between patient and physician lead to a more positive patient experience2 and arguably, the subsequent potential for better health outcomes. Our efforts in addressing this disparity often fall short. URM applicants may be hesitant to join an institution where diversity is lacking or where they may be the only minority.3 While there is no simple solution, I propose that psychiatrists promote the importance of mental health to Black and Brown students of all ages by collaborating with schools and community leaders.
It is important to acknowledge that the lack of diversity within psychiatry is reflective of that among all physicians. This in part stems from the barriers to medical education that Black and Brown communities face. Those who start off with more resources or have parents who are physicians are at an advantage when trying to get into medical school. In fact, one in five medical students have a parent who is a physician4 and about three-fourths of students come from families whose income falls among the top two quintiles.5 Impoverished communities, which have a disproportionate share of Black and Brown people, cannot afford to take MCAT preparatory classes or to accept unpaid “resume building” opportunities. Many medical schools continue to place more weight on test scores and research/medical experiences, despite a shift to a more holistic review process. Institutions that have tried a different approach and accepted students from more diverse backgrounds may often overlook the challenges that URM students face while in medical school and fail to provide appropriate support resources.
The result is a failure to retain such students. A study conducted at Stony Brook (N.Y.) University showed that those underrepresented in medicine were six times more likely to get dismissed from medical school, and three times more likely to both withdraw or graduate beyond 4 years, compared with their White counterparts.6 This is a serious issue that needs to change on a structural and systemic level.
Any discussion of race and psychiatry must acknowledge psychiatry’s history of racism against Black and Brown communities to engage in racially informed discussions with our patients. Only then can we play a better role advocating against racism within the field in the future. Dating back to the 18th century, psychiatry has promoted ideologies that promote racism. Benjamin Rush, considered the “father of American Psychiatry,” believed that Black skin was a disease derived from leprosy called “negritude.” In the late 19th century, this twisted ideology continued with the invention of the term “drapetomania,” which was used to describe enslaved people who ran away as having a mental disorder.7 Black prisoners were subjected to experimental treatment with substances such as LSD and bulbocapnine to subdue them.8 This idea that minorities were dangerous and needed to be subdued translated into a higher number of schizophrenia diagnoses, particularly among Black men, as it was used as a tool to vilify them in the 1970s. Although schizophrenia is equally prevalent among Whites and non-Whites, Black people are four times more likely to be diagnosed, compared with their White counterparts, while Hispanics are three times more likely. Studies have shown that Black and Brown men are also more likely to receive higher doses of antipsychotics.9
Given this history, it is not surprising that Black and Brown representation within the field is lacking and that patients may be hesitant to share their feelings about race with us. While we can’t change history, we can take a stance condemning the harmful behavior of the past. The American Psychiatric Association issued an apology earlier this year to Black, Indigenous, and People of Color for its support in structural racism.10 This is a step in the right direction, but we need more than statements or performative actions. We need to amplify the voices of Black and Brown psychiatrists and patients, as well as highlight their current and past contributions to the field. While my educational experiences focused on the work of prominent White scholars, medical curricula should showcase the work of people like Solomon Carter Fuller, MD, a Black psychiatrist who was essential to understanding Alzheimer’s, or Joseph White, PhD, sometimes referred to as the “godfather of Black psychology.”11
At times, I have found myself witness to situations where colleagues make statements that not only do not condemn racism, but in fact encourage it. I have unfortunately heard some use the all-too-familiar rhetoric of reverse racism, such as: “They just assume I am racist because I am a White male” or “They’re being racist against me” or statements like “Don’t you think it is far-fetched to believe she was just sitting on a college campus doing nothing when the police were called?” Rhetoric such as this is problematic to the field of psychiatry and medicine as a whole – and only serves to further invalidate the feelings of our Black and Brown patients. We must increase exposure and education regarding racism to address this, especially the meaning of microaggressions, a concept many fail to understand.
Attention to the subject of racism has increased within medical schools and residency training programs in the wake of George Floyd’s death. However, most programs often make these lectures optional or only have one to two limited sessions. Furthermore, many do not make it mandatory for faculty to attend; they are arguably the most in need of this training given that they set the precedent of how to practice psychiatry. Some institutions have incorporated comprehensive antiracist curriculums into medical training. One model that has been successful is the Social Justice and Health Equity program within Yale University’s psychiatry residency. The curriculum has four tracks:
- Structural competency, which focuses on the mental health impact of extraclinical structures, for example a patient’s neighborhood and associated barriers of access.
- Human experience, which explores the interaction of patients and providers and how biases play a role.
- Advocacy, which teaches residents the written and oral skills to lobby for patient interests on a community and legislative level.
- History of psychiatry, which focuses on understanding psychiatry’s prior role in racism.
In each track, there are group discussions, cases led by faculty, and meetings with community leaders. Through this curriculum, residents learn about power, privilege, and how to interact with and advocate for patients in a way that promotes equity, rather than racial disparity.12,13 This is a model that other psychiatric residency programs can promote, emulate, and benefit from.
Educating ourselves will hopefully lead to a deeper introspection of how we interact with patients and if we are promoting antiracism through our attitude and actions. Reflecting on my own personal practice, I have noted that the interplay of race, mental health, and provider decision-making becomes particularly complex when dealing with situations in which a patient exhibits increased aggression or agitation. As a second-year psychiatric resident immersed in the inpatient world, I have become familiar with patients at higher risk and greater need. The first attempt toward de-escalation involves verbal cues without any other more intrusive measures. If that fails, intramuscular (IM) medications are typically considered. If a patient has a history of aggressive behavior, the threshold to use IM medications can decrease dramatically. This is mainly to protect ourselves and our nursing staff and to prioritize safety. While I understand this rationale, I wonder about the patient’s experience. What constitutes “aggressive” behavior? For patients who have had violence used against them because of their race or who have suffered from police brutality, having police present or threatening IM medications will increasingly trigger them and escalate the situation. The aftermath can deepen the distrust of psychiatry by Black and Brown people.
How do we then handle such situations in a way that both protects our staff from physical harm and protects our patients from racial trauma? While I don’t have a great answer, I think we can benefit from standardizing what we consider aggressive behavior and have specific criteria that patients need to exhibit before administering an IM medication. In addition, discussions with the team, including residents, nurses, and attending physicians, about how to address an emergent situation before it actually happens are essential. Specifically discussing the patient’s history and race and how it may affect the situation is not something to be shied away from. Lastly, in the event that an IM medication is administered and police are present, debriefing with the patient afterward is necessary. The patient may not be willing or able to listen to you or trust you, but taking accountability and acknowledging what happened, justified or not, is a part of the process of rebuilding rapport.
Both in the purview of the individual psychiatrist and the field of psychiatry as a whole, we need to examine our behavior and not be afraid to make changes for the betterment of our patients. We must learn to talk about race with our patients and in the process, advocate for more representation of Black and Brown psychiatrists, understanding the barriers faced by these communities when pursuing the medical field. We must educate ourselves on psychiatry’s history, and equip ourselves with knowledge and tools to promote antiracism and shape psychiatry’s future. We can then apply these very tools to challenging situations we may encounter daily with the ultimate goal of improving the mental health of our patients. This is the only way we will progress and ensure that psychiatry is an equitable, antiracist field. As Ibram X. Kendi, PhD, has written, “The heartbeat of antiracism is self-reflection, recognition, admission, and fundamentally self-critique.”
Dr. Malik is a second-year psychiatry resident at the University of California, San Diego. She has a background in policy and grassroots organizing through her time working at the National Coalition for the Homeless and the Women’s Law Project. Dr. Malik has no disclosures.
References
1. Wyse R et al. Acad Psychiatry. 2020 Oct;44(5):523-30.
2. Cooper LA et al. Ann Intern Med. 2003;139:907-15.
3. Pierre JM et al. Acad Psychiatry. 2017;41:226-32.
4. Hartocollis A. “Getting into med school without hard sciences.” New York Times. 2010 Jul 29.
5. AAMC. An updated look at the economic diversity of U.S. medical students. Analysis in Brief. 2018 Oct;18(5).
6. Rainey ML. How do we retain minority health professions students. In: Smedley BD et al. The right thing to do, the smart thing to do: Enhancing diversity in the health professions: Summary of the Symposium on Diversity in Health Professions in Honor of Herbert W. Nickens, M.D. Institute of Medicine. National Academies Press. 2001.
7. Geller J. “Structural racism in American psychiatry and APA: Part 1.” Psychiatric News. 2020 Jun 23.
8. Mohr CL and Gordon JE. Tulane: The emergence of a modern university, 1945-1980. Louisiana State University Press, Baton Rouge. 2001.
9. Metzl JM. The protest psychosis: How schizophrenia became a Black disease. Beacon Press. 2010.
10. APA’s apology to Black, indigenous and people of color for its support of structural racism in psychiatry. American Psychiatric Association. 2021 Jan 18.
11. Black pioneers in mental health. Mental Health America. 2021.
12. Belli B. For Yale’s emerging psychiatrists, confronting racism is in the curriculum. Yale News. 2020 Jul 30.
13. Jordan A and Jackson D. Social justice and health equity curriculum. Yale School of Medicine. 2019 Sep 24.
PUFAs a promising add-on for borderline personality disorder
Marine omega-3 fatty acids may be a promising add-on therapy for improving symptoms of borderline personality disorder (BPD), new research suggests.
A meta-analysis of four randomized controlled trials showed that adjunctive omega-3 fatty polyunsaturated fatty acids (PUFAs) significantly reduced overall BPD symptom severity, particularly affect dysregulation and impulsive behavior.
“Given the mechanisms of action and beneficial side effect profile, this [analysis] suggests that omega-3 fatty acids could be considered as add-on treatment” for patients with BPD, senior author Roel J. T. Mocking MD, PhD, resident in psychiatry and postdoctoral researcher at Academisch Medisch Centrum, Amsterdam, said in an interview.
The findings were published online in the Journal of Clinical Psychiatry.
Urgent need
“There are several effective treatments, but not all patients respond sufficiently,” which points to an urgent need for additional treatment options, Dr. Mocking said.
He noted that, although “several prior studies showed promising effects of omega-3 fatty acids” for patients with BPD, those studies were relatively small, which precluded more definitive overall conclusions.
The investigators wanted to combine results of the earlier studies to provide a combined estimate of overall effectiveness of the use of omega-3 fatty acids for patients with BP, with the intention of “guiding clinicians and individuals suffering from borderline personality disorder to decide on whether they should add omega-3 fatty acids to their treatment.”
The analyzed four studies that had a total of 137 patients. Three of the studies included patients diagnosed with BPD; one included individuals with recurrent self-harm, most of whom were also diagnosed with BPD.
Omega-3 fatty acids were used as monotherapy in one study. In the other studies, they were used as add-on therapy to other agents, such as antidepressants, benzodiazepines, and/or valproic acid. None of the studies included patients who were taking antipsychotics.
The type of omega-3 PUFAs were derived from marine rather than plant sources.
Three studies compared omega-3 fatty acids with placebo. One study compared valproic acid monotherapy with valproic acid plus omega-3 fatty acids and did not include a placebo group.
Significant symptom reduction
Random-effects meta-analyses showed an “overall significant decreasing effect” of omega-3 fatty acids on overall BPD symptom severity (standardized difference in means, 0.54; 95% CI, 0.91-0.17; P = .004) in the omega-3 group compared with the control group, with a medium effect size.
The investigators added that there was “no relevant heterogeneity” (P = .45).
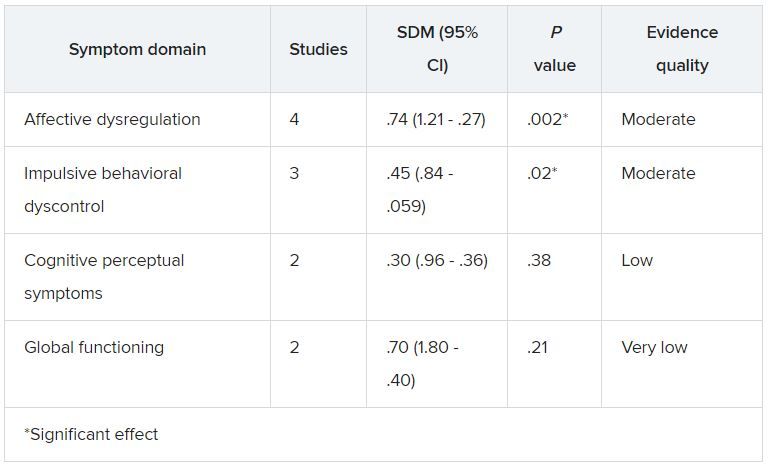
Although heterogeneity was “more pronounced” in the affective dysregulation symptom domain, it did not reach statistical significance, the researchers noted.
The impulsive behavioral dyscontrol and cognitive perceptual symptom domains had “no relevant heterogeneity.” On the other hand, there was “substantial heterogeneity” in the global functioning symptom group.
Omega-3 fatty acids “have multiple bioactive roles in the brain. For example, they form essential components of the membrane of brain cells and thereby influence the structure and functioning of the brain. They also have an effect on inflammation levels in the brain,” Dr. Mocking said.
“Because we cannot synthesize these omega-3 fatty acids ourselves, we are dependent on our diet. The main dietary source of omega-3 fatty acids is fatty fish. However, since the industrial revolution, we eat less and less fatty fish, risking deficiency of omega-3 fatty acids causing brain dysfunction,” he added.
Dr. Mocking noted that
This “suggests that they could be combined to increase overall effectiveness,” he said.
Important benefit
Commenting on the study, Roger McIntyre, MD, professor of psychiatry and pharmacology, University of Toronto, and head of the mood disorders psychopharmacology unit, said that the benefit of omega-3 “on impulsivity and mood symptoms is especially important, as these are some of the most debilitating aspects of BPD and lead to service utilization, such as ER, primary care, and specialty care.”
In addition, “impulsivity often presages suicidality,” he noted.
Dr. McIntyre, who is also chair and executive director of the Brain and Cognition Discovery Foundation in Toronto and was not involved with the study, called the effect size “quite reasonable.”
“The mechanistic story is very strong around anti-inflammatory effect, which particularly implied mood and cognition. In other words, inflammation is highly associated with mood and cognitive difficulties,” he said.
However, Dr. McIntyre also pointed to several significant challenges, including “quality assurance on the purchase of the product of fish oil, as it is not sufficiently regulated.” It is also unclear which individuals are more likely to benefit from it.
For example, major depressive disorder data have shown that “fish oils are not as effective as we hoped but are especially effective in people with baseline elevation of inflammatory markers,” Dr. McIntyre said.
“In other words, is there a way to identify a biomarkers/biosignature or phenomenology that’s more likely to identify a subgroup of people with BPD who might benefit benefiting from omega-3?” he asked.
Dr. Mocking and the other investigators reported no relevant financial relationships. Dr. McIntyre has received research grant support from CIHR/GACD/Chinese National Natural Research Foundation and speaker/consultation fees from Lundbeck, Janssen, Purdue, Pfizer, Otsuka, Allergan, Takeda, Neurocrine, Sunovion, Eisai, Minerva, Intra-Cellular, and AbbVie. Dr. McIntyre is also CEO of AltMed.
A version of this article first appeared on Medscape.com.
Marine omega-3 fatty acids may be a promising add-on therapy for improving symptoms of borderline personality disorder (BPD), new research suggests.
A meta-analysis of four randomized controlled trials showed that adjunctive omega-3 fatty polyunsaturated fatty acids (PUFAs) significantly reduced overall BPD symptom severity, particularly affect dysregulation and impulsive behavior.
“Given the mechanisms of action and beneficial side effect profile, this [analysis] suggests that omega-3 fatty acids could be considered as add-on treatment” for patients with BPD, senior author Roel J. T. Mocking MD, PhD, resident in psychiatry and postdoctoral researcher at Academisch Medisch Centrum, Amsterdam, said in an interview.
The findings were published online in the Journal of Clinical Psychiatry.
Urgent need
“There are several effective treatments, but not all patients respond sufficiently,” which points to an urgent need for additional treatment options, Dr. Mocking said.
He noted that, although “several prior studies showed promising effects of omega-3 fatty acids” for patients with BPD, those studies were relatively small, which precluded more definitive overall conclusions.
The investigators wanted to combine results of the earlier studies to provide a combined estimate of overall effectiveness of the use of omega-3 fatty acids for patients with BP, with the intention of “guiding clinicians and individuals suffering from borderline personality disorder to decide on whether they should add omega-3 fatty acids to their treatment.”
The analyzed four studies that had a total of 137 patients. Three of the studies included patients diagnosed with BPD; one included individuals with recurrent self-harm, most of whom were also diagnosed with BPD.
Omega-3 fatty acids were used as monotherapy in one study. In the other studies, they were used as add-on therapy to other agents, such as antidepressants, benzodiazepines, and/or valproic acid. None of the studies included patients who were taking antipsychotics.
The type of omega-3 PUFAs were derived from marine rather than plant sources.
Three studies compared omega-3 fatty acids with placebo. One study compared valproic acid monotherapy with valproic acid plus omega-3 fatty acids and did not include a placebo group.
Significant symptom reduction
Random-effects meta-analyses showed an “overall significant decreasing effect” of omega-3 fatty acids on overall BPD symptom severity (standardized difference in means, 0.54; 95% CI, 0.91-0.17; P = .004) in the omega-3 group compared with the control group, with a medium effect size.
The investigators added that there was “no relevant heterogeneity” (P = .45).

Although heterogeneity was “more pronounced” in the affective dysregulation symptom domain, it did not reach statistical significance, the researchers noted.
The impulsive behavioral dyscontrol and cognitive perceptual symptom domains had “no relevant heterogeneity.” On the other hand, there was “substantial heterogeneity” in the global functioning symptom group.
Omega-3 fatty acids “have multiple bioactive roles in the brain. For example, they form essential components of the membrane of brain cells and thereby influence the structure and functioning of the brain. They also have an effect on inflammation levels in the brain,” Dr. Mocking said.
“Because we cannot synthesize these omega-3 fatty acids ourselves, we are dependent on our diet. The main dietary source of omega-3 fatty acids is fatty fish. However, since the industrial revolution, we eat less and less fatty fish, risking deficiency of omega-3 fatty acids causing brain dysfunction,” he added.
Dr. Mocking noted that
This “suggests that they could be combined to increase overall effectiveness,” he said.
Important benefit
Commenting on the study, Roger McIntyre, MD, professor of psychiatry and pharmacology, University of Toronto, and head of the mood disorders psychopharmacology unit, said that the benefit of omega-3 “on impulsivity and mood symptoms is especially important, as these are some of the most debilitating aspects of BPD and lead to service utilization, such as ER, primary care, and specialty care.”
In addition, “impulsivity often presages suicidality,” he noted.
Dr. McIntyre, who is also chair and executive director of the Brain and Cognition Discovery Foundation in Toronto and was not involved with the study, called the effect size “quite reasonable.”
“The mechanistic story is very strong around anti-inflammatory effect, which particularly implied mood and cognition. In other words, inflammation is highly associated with mood and cognitive difficulties,” he said.
However, Dr. McIntyre also pointed to several significant challenges, including “quality assurance on the purchase of the product of fish oil, as it is not sufficiently regulated.” It is also unclear which individuals are more likely to benefit from it.
For example, major depressive disorder data have shown that “fish oils are not as effective as we hoped but are especially effective in people with baseline elevation of inflammatory markers,” Dr. McIntyre said.
“In other words, is there a way to identify a biomarkers/biosignature or phenomenology that’s more likely to identify a subgroup of people with BPD who might benefit benefiting from omega-3?” he asked.
Dr. Mocking and the other investigators reported no relevant financial relationships. Dr. McIntyre has received research grant support from CIHR/GACD/Chinese National Natural Research Foundation and speaker/consultation fees from Lundbeck, Janssen, Purdue, Pfizer, Otsuka, Allergan, Takeda, Neurocrine, Sunovion, Eisai, Minerva, Intra-Cellular, and AbbVie. Dr. McIntyre is also CEO of AltMed.
A version of this article first appeared on Medscape.com.
Marine omega-3 fatty acids may be a promising add-on therapy for improving symptoms of borderline personality disorder (BPD), new research suggests.
A meta-analysis of four randomized controlled trials showed that adjunctive omega-3 fatty polyunsaturated fatty acids (PUFAs) significantly reduced overall BPD symptom severity, particularly affect dysregulation and impulsive behavior.
“Given the mechanisms of action and beneficial side effect profile, this [analysis] suggests that omega-3 fatty acids could be considered as add-on treatment” for patients with BPD, senior author Roel J. T. Mocking MD, PhD, resident in psychiatry and postdoctoral researcher at Academisch Medisch Centrum, Amsterdam, said in an interview.
The findings were published online in the Journal of Clinical Psychiatry.
Urgent need
“There are several effective treatments, but not all patients respond sufficiently,” which points to an urgent need for additional treatment options, Dr. Mocking said.
He noted that, although “several prior studies showed promising effects of omega-3 fatty acids” for patients with BPD, those studies were relatively small, which precluded more definitive overall conclusions.
The investigators wanted to combine results of the earlier studies to provide a combined estimate of overall effectiveness of the use of omega-3 fatty acids for patients with BP, with the intention of “guiding clinicians and individuals suffering from borderline personality disorder to decide on whether they should add omega-3 fatty acids to their treatment.”
The analyzed four studies that had a total of 137 patients. Three of the studies included patients diagnosed with BPD; one included individuals with recurrent self-harm, most of whom were also diagnosed with BPD.
Omega-3 fatty acids were used as monotherapy in one study. In the other studies, they were used as add-on therapy to other agents, such as antidepressants, benzodiazepines, and/or valproic acid. None of the studies included patients who were taking antipsychotics.
The type of omega-3 PUFAs were derived from marine rather than plant sources.
Three studies compared omega-3 fatty acids with placebo. One study compared valproic acid monotherapy with valproic acid plus omega-3 fatty acids and did not include a placebo group.
Significant symptom reduction
Random-effects meta-analyses showed an “overall significant decreasing effect” of omega-3 fatty acids on overall BPD symptom severity (standardized difference in means, 0.54; 95% CI, 0.91-0.17; P = .004) in the omega-3 group compared with the control group, with a medium effect size.
The investigators added that there was “no relevant heterogeneity” (P = .45).

Although heterogeneity was “more pronounced” in the affective dysregulation symptom domain, it did not reach statistical significance, the researchers noted.
The impulsive behavioral dyscontrol and cognitive perceptual symptom domains had “no relevant heterogeneity.” On the other hand, there was “substantial heterogeneity” in the global functioning symptom group.
Omega-3 fatty acids “have multiple bioactive roles in the brain. For example, they form essential components of the membrane of brain cells and thereby influence the structure and functioning of the brain. They also have an effect on inflammation levels in the brain,” Dr. Mocking said.
“Because we cannot synthesize these omega-3 fatty acids ourselves, we are dependent on our diet. The main dietary source of omega-3 fatty acids is fatty fish. However, since the industrial revolution, we eat less and less fatty fish, risking deficiency of omega-3 fatty acids causing brain dysfunction,” he added.
Dr. Mocking noted that
This “suggests that they could be combined to increase overall effectiveness,” he said.
Important benefit
Commenting on the study, Roger McIntyre, MD, professor of psychiatry and pharmacology, University of Toronto, and head of the mood disorders psychopharmacology unit, said that the benefit of omega-3 “on impulsivity and mood symptoms is especially important, as these are some of the most debilitating aspects of BPD and lead to service utilization, such as ER, primary care, and specialty care.”
In addition, “impulsivity often presages suicidality,” he noted.
Dr. McIntyre, who is also chair and executive director of the Brain and Cognition Discovery Foundation in Toronto and was not involved with the study, called the effect size “quite reasonable.”
“The mechanistic story is very strong around anti-inflammatory effect, which particularly implied mood and cognition. In other words, inflammation is highly associated with mood and cognitive difficulties,” he said.
However, Dr. McIntyre also pointed to several significant challenges, including “quality assurance on the purchase of the product of fish oil, as it is not sufficiently regulated.” It is also unclear which individuals are more likely to benefit from it.
For example, major depressive disorder data have shown that “fish oils are not as effective as we hoped but are especially effective in people with baseline elevation of inflammatory markers,” Dr. McIntyre said.
“In other words, is there a way to identify a biomarkers/biosignature or phenomenology that’s more likely to identify a subgroup of people with BPD who might benefit benefiting from omega-3?” he asked.
Dr. Mocking and the other investigators reported no relevant financial relationships. Dr. McIntyre has received research grant support from CIHR/GACD/Chinese National Natural Research Foundation and speaker/consultation fees from Lundbeck, Janssen, Purdue, Pfizer, Otsuka, Allergan, Takeda, Neurocrine, Sunovion, Eisai, Minerva, Intra-Cellular, and AbbVie. Dr. McIntyre is also CEO of AltMed.
A version of this article first appeared on Medscape.com.



















