User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
nav[contains(@class, 'nav-ce-stack nav-ce-stack__large-screen')]
header[@id='header']
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'main-prefix')]
footer[@id='footer']
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
div[contains(@class, 'view-medstat-quiz-listing-panes')]
Pharmacotherapy for alcohol use disorder in patients with hepatic impairment
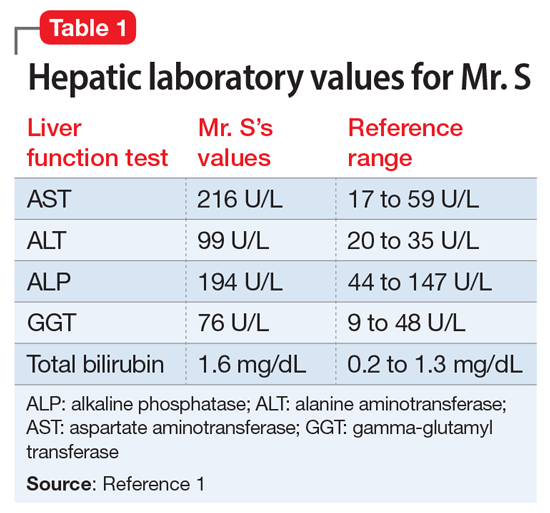
Mr. S, age 64, presents for an outpatient follow-up after a recent hospital discharge for alcohol detoxification. He reports a long history of alcohol use, which has resulted in numerous hospital admissions. He has recently been receiving care from a gastroenterologist because the results of laboratory testing suggested hepatic impairment (Table 1). Mr. S says that a friend of his was able to stop drinking by taking a medication, and he wonders if he can be prescribed a medication to help him as well.
A chart review shows that Mr. S recently underwent paracentesis, during which 6 liters of fluid were removed. Additionally, an abdominal ultrasound confirmed hepatic cirrhosis.
According to the World Health Organization, alcohol consumption contributes to 3 million deaths annually.2 The highest proportion of these deaths (21.3%) is due to alcohol-associated gastrointestinal complications, including alcoholic and infectious hepatitis, pancreatitis, and cirrhosis. Because the liver is the primary site of ethanol metabolism, it sustains the greatest degree of tissue injury with heavy alcohol consumption. Additionally, the association of harmful use of alcohol with risky sexual behavior may partially explain the higher prevalence of viral hepatitis among persons with alcohol use disorder (AUD) compared with the general population. Alcoholic liver disease (ALD) progresses through several stages, beginning with hepatic steatosis and progressing through alcohol-related hepatitis, fibrosis, cirrhosis, and potentially hepatocellular carcinoma.3
Liver markers of alcohol use
Although biological markers can be used in clinical practice to screen and monitor for alcohol abuse, making a diagnosis of ALD can be challenging. Typically, a history of heavy alcohol consumption in addition to certain physical signs and laboratory tests for liver disease are the best indicators of ALD. However, the clinical assessment can be confounded by patients who deny or minimize how much alcohol they have consumed. Furthermore, physical and laboratory findings may not be specific to ALD.
Liver enzymes, including aspartate aminotransferase (AST), alanine aminotransferase (ALT), and gamma-glutamyltransferase (GGT), have historically been used as the basis of diagnosing ALD. In addition to elevated bilirubin and evidence of macrocytic anemia, elevations in these enzymes may suggest heavy alcohol use, but these values alone are inadequate to establish ALD. Gamma-glutamyltransferase is found in cell membranes of several body tissues, including the liver and spleen, and therefore is not specific to liver damage. However, elevated GGT is the best indicator of excessive alcohol consumption because it has greater sensitivity than AST and ALT.1,3,4
Although these biomarkers are helpful in diagnosing ALD, they lose some of their utility in patients with advanced liver disease. Patients with severe liver dysfunction may not have elevated serum aminotransferase levels because the degree of liver enzyme elevation does not correlate well with the severity of ALD. For example, patients with advanced cirrhosis may have liver enzyme levels that appear normal. However, the pattern of elevation in transaminases can be helpful in making a diagnosis of liver dysfunction; using the ratio of AST to ALT may aid in diagnosing ALD, because AST is elevated more than twice that of ALT in >80% of patients with ALD.1,3,4
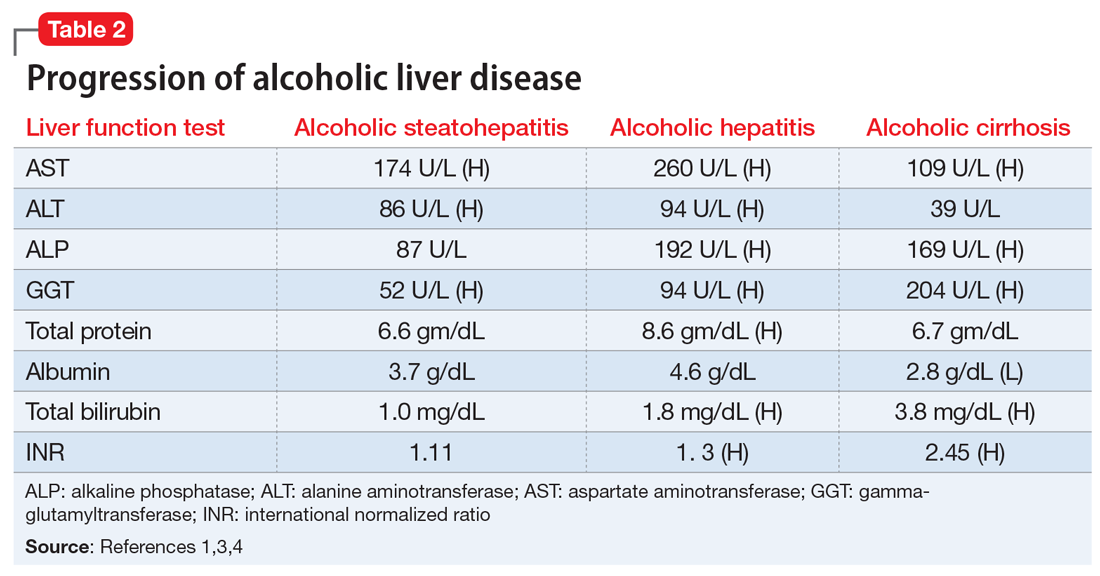
Table 21,3,4 shows the progression of ALD from steatohepatitis to alcoholic hepatitis to cirrhosis. In steatohepatitis, transaminitis is present but all other biomarkers normal. In alcoholic hepatitis, transaminitis is present along with elevated alkaline phosphatase, elevated bilirubin, and elevated international normalized ratio (INR). In alcoholic cirrhosis, the AST-to-ALT ratio is >2, and hypoalbuminemia, hyperbilirubinemia, and coagulopathy (evidenced by elevated INR) are present, consistent with long-term liver damage.1,3,4
Continue to: FDA-approved medications
FDA-approved medications
Three medications—acamprosate, naltrexone, and disulfiram—currently are FDA-approved for treating AUD.5,6 Additionally, several other medications have shown varying levels of efficacy in treating patients with AUD but are not FDA-approved for this indication (Table 3).5-8
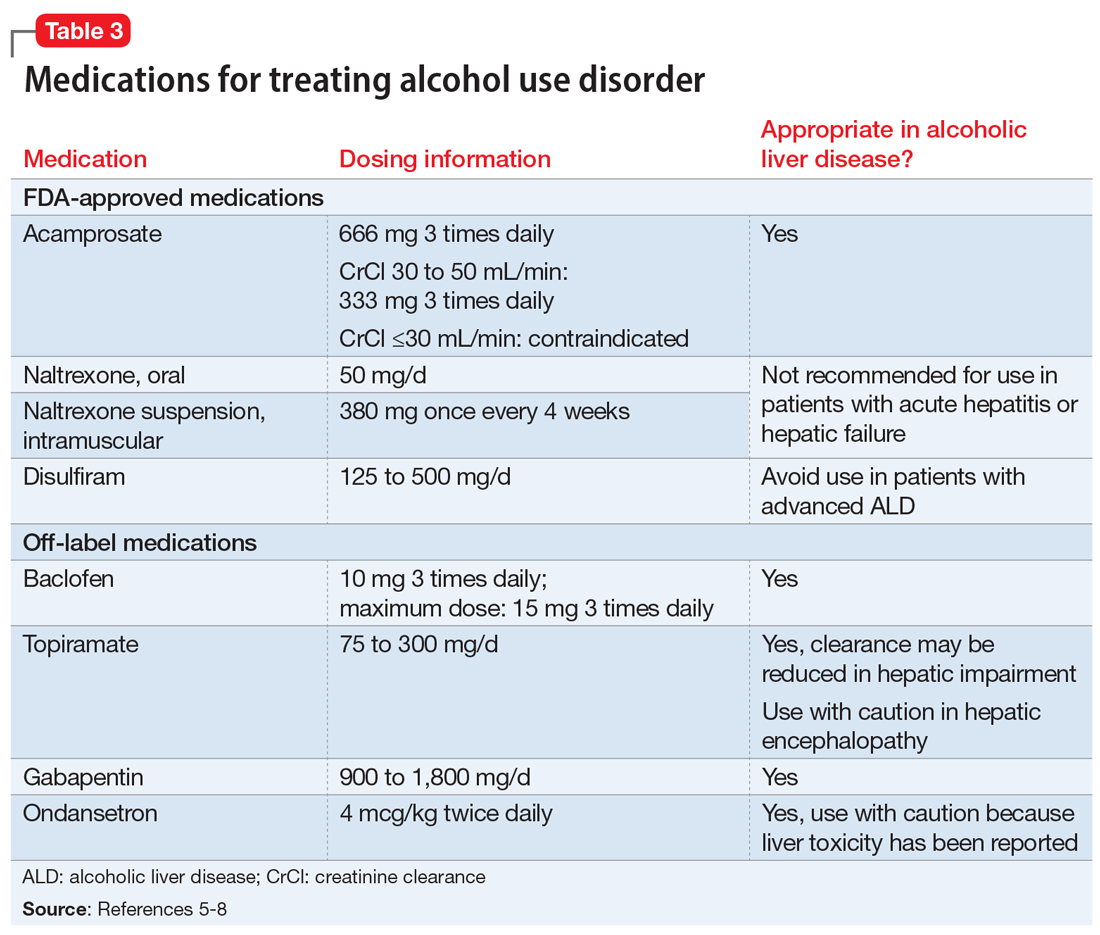
Acamprosate is thought to create a balance of inhibitor and excitatory neurotransmitters by functioning as a glutamate antagonist and gamma-aminobutyric acid (GABA) agonist. This is speculated to aid in abstinence from alcohol. Data suggests that acamprosate may be more effective for maintaining abstinence than for inducing remission in individuals who have not yet detoxified from alcohol. Because of its renal excretion, acamprosate is the only FDA-approved medication for AUD that is not associated with liver toxicity. The most commonly reported adverse effect with acamprosate use is diarrhea.
Naltrexone, a mu-opioid receptor antagonist, is available in both tablet and long-acting IM injection formulations. Naltrexone blocks the binding of endorphins created by alcohol consumption to opioid receptors. This results in diminished dopamine release and is speculated to decrease reward and positive reinforcement with alcohol consumption, leading to fewer heavy drinking days. Due to hepatic metabolism, naltrexone use carries a risk of liver injury. Cases of hepatitis and clinically significant liver dysfunction as well as transient, asymptomatic, hepatic transaminase elevations have been observed in patients who receive naltrexone. Because of the absence of first-pass metabolism, long-acting IM naltrexone may produce less hepatotoxicity than the oral formulation. When the FDA approved both formulations of naltrexone, a “black-box” warning was issued concerning the risk of liver damage; however, these warnings have since been removed from their respective prescribing information.
Disulfiram inhibits acetaldehyde dehydrogenase, resulting in elevated acetaldehyde concentrations after consuming alcohol. In theory, this medication reduces a person’s desire to drink due to the negative physiological and physical effects associated with increased acetaldehyde, including hypotension, flushing, nausea, and vomiting. Although most of these reactions are short-lived, disulfiram can induce hepatotoxicity and liver failure that may prove fatal. Disulfiram should be avoided in patients with advanced ALD.
Off-label medications for AUD
Additional pharmacotherapeutic agents have been evaluated in patients with AUD. Baclofen, topiramate, gabapentin, and ondansetron have shown varying levels of efficacy and pose minimal concern in patients with ALD.
Continue to: Baclofen
Baclofen. Although findings are conflicting, baclofen is the only agent that has been specifically studied for treating AUD in patients with ALD. A GABA B receptor antagonist, baclofen is currently FDA-approved for treating spasticity. In a series of open-label and double-blind studies, baclofen has been shown to effectively reduce alcohol intake, promote abstinence, and prevent relapse.5,6 Further studies identified a possible dose-related response, noting that 20 mg taken 3 times daily may confer additional response over 10 mg taken 3 times daily.5,6 Conversely, the ALPADIR study failed to demonstrate superiority of baclofen vs placebo in the maintenance of abstinence from alcohol despite dosing at 180 mg/d.9 This study did, however, find a significant reduction in alcohol craving in favor of baclofen.9 Further, in a randomized controlled trial (RCT) conducted in veterans with chronic hepatitis C, baclofen 30 mg/d failed to show superiority over placebo with regard to increasing abstinence or reducing alcohol use
Topiramate. A recent meta-analysis found that topiramate use may result in fewer drinking days, heavy drinking days, and number of drinks per drinking day.7 Additionally, topiramate has demonstrated a statistically significant reduction in alcohol craving as well as the ability to decrease all liver function test values.5 This agent should be used with caution in patients with hepatic encephalopathy because the adverse cognitive effects associated with topiramate may confound the clinical course and treatment of such.
Gabapentin. The use of gabapentin to treat patients with AUD is supported by multiple RCTs. In studies that evaluated dose-related response, higher doses of gabapentin (up to 1,800 mg/d) showed greater efficacy than lower doses (ie, 900 mg/d).8 Because gabapentin does not undergo hepatic metabolism, its use in patients with ALD is considered safe. Although the abuse potential of gabapentin is less defined in patients with AUD, there have been reports of abuse in other high-risk populations (ie, those with opioid use disorder, incarcerated persons, and those who misuse prescriptions recreationally).8
Ondansetron is speculated to decrease the reward from alcohol via the down-regulation of dopaminergic neurons. Studies examining ondansetron for patients with AUD have found that it decreases alcohol cravings in those with early-onset alcoholism (initial onset at age ≤25), but not in late-onset alcoholism (initial onset at age >25).5 However, the ondansetron doses used in these trials were very low (4 mcg/kg), and those doses are not available commercially.5
CASE CONTINUED
Following a discussion of available pharmacotherapeutic options for AUD, Mr. S is started on baclofen, 10 mg 3 times daily, with plans for dose titration. At a 2-week follow-up appointment, Mr. S reports that he had not been taking baclofen as often as instructed; however, he denies further alcohol consumption and re-commits to baclofen treatment. Unfortunately, Mr. S is soon admitted to hospice care due to continued decompensation and is unable to attend any additional outpatient follow-up appointments. Three months after his initial outpatient contact, Mr. S dies due to alcoholic cirrhosis.
Related Resources
• Crabb DW, Im GY, Szabo G, et al. Diagnosis and treatment of alcohol-related liver diseases: 2019 practice guidance from the American Association for the Study of Liver Diseases. Hepatology. 2020;71(1):306-333.
• Murail AR, Carey WD. Disease management. Liver test interpretation - approach to the patient with liver disease: a guide to commonly used liver tests. Cleveland Clinic Center for Continuing Education. Updated August 2017. www.clevelandclinicmeded. com/medicalpubs/diseasemanagement/hepatology/ guide-to-common-liver-tests/
Drug Brand Names
Acamprosate • Campral
Baclofen • Lioresal
Disulfiram • Antabuse
Gabapentin • Neurontin
Naltrexone • Revia, Vivitrol
Ondansetron • Zofran
Topiramate • Topamax
1. Agrawal S, Dhiman RK, Limdi JK. Evaluation of abnormal liver function tests. Postgrad Med J. 2016;92(1086):223-234.
2. World Health Organization. Global status report on alcohol and health 2018. Published 2018. Accessed November 5, 2020. https://www.who.int/substance_abuse/publications/global_alcohol_report/gsr_2018/en/
3. Osna NA, Donohue TM, Kharbanda KK. Alcoholic liver disease: pathogenesis and current management. Alcohol Res. 2017;38(2):147-161.
4. Leggio L, Lee MR. Treatment of alcohol use disorder in patients with alcoholic liver disease. Am J Med. 2017;130(2):124-134.
5. Addolorato G, Mirijello A, Leggio L, et al. Management of alcohol dependence in patients with liver disease. CNS Drugs. 2013;27(4):287-299.
6. Vuittonet CL, Halse M, Leggio L, et al. Pharmacotherapy for alcoholic patients with alcoholic liver disease. Am J Health Syst Pharm. 2014;71(15):1265-1276.
7. Jonas DE, Amick HR, Feltner C, et al. Pharmacotherapy for adults with alcohol use disorders in outpatient settings. JAMA. 2014;311(18):1889-1900.
8. Mason BJ, Quello S, Shadan F. Gabapentin for the treatment of alcohol use disorder. Expert Opin Investig Drugs. 2018;27(1):113-124.
9. Reynaud M, Aubin HJ, Trinquet F, et al. A randomized, placebo-controlled study of high-dose baclofen in alcohol-dependent patients-the ALPADIR study. Alcohol Alcohol. 2017;52(4):439-446.
10. Hauser P, Fuller B, Ho S, et al. The safety and efficacy of baclofen to reduce alcohol use in veterans with chronic hepatitis C: a randomized controlled trial. Addiction. 2017;112(7):1173-1183.
Mr. S, age 64, presents for an outpatient follow-up after a recent hospital discharge for alcohol detoxification. He reports a long history of alcohol use, which has resulted in numerous hospital admissions. He has recently been receiving care from a gastroenterologist because the results of laboratory testing suggested hepatic impairment (Table 1). Mr. S says that a friend of his was able to stop drinking by taking a medication, and he wonders if he can be prescribed a medication to help him as well.

A chart review shows that Mr. S recently underwent paracentesis, during which 6 liters of fluid were removed. Additionally, an abdominal ultrasound confirmed hepatic cirrhosis.
According to the World Health Organization, alcohol consumption contributes to 3 million deaths annually.2 The highest proportion of these deaths (21.3%) is due to alcohol-associated gastrointestinal complications, including alcoholic and infectious hepatitis, pancreatitis, and cirrhosis. Because the liver is the primary site of ethanol metabolism, it sustains the greatest degree of tissue injury with heavy alcohol consumption. Additionally, the association of harmful use of alcohol with risky sexual behavior may partially explain the higher prevalence of viral hepatitis among persons with alcohol use disorder (AUD) compared with the general population. Alcoholic liver disease (ALD) progresses through several stages, beginning with hepatic steatosis and progressing through alcohol-related hepatitis, fibrosis, cirrhosis, and potentially hepatocellular carcinoma.3
Liver markers of alcohol use
Although biological markers can be used in clinical practice to screen and monitor for alcohol abuse, making a diagnosis of ALD can be challenging. Typically, a history of heavy alcohol consumption in addition to certain physical signs and laboratory tests for liver disease are the best indicators of ALD. However, the clinical assessment can be confounded by patients who deny or minimize how much alcohol they have consumed. Furthermore, physical and laboratory findings may not be specific to ALD.
Liver enzymes, including aspartate aminotransferase (AST), alanine aminotransferase (ALT), and gamma-glutamyltransferase (GGT), have historically been used as the basis of diagnosing ALD. In addition to elevated bilirubin and evidence of macrocytic anemia, elevations in these enzymes may suggest heavy alcohol use, but these values alone are inadequate to establish ALD. Gamma-glutamyltransferase is found in cell membranes of several body tissues, including the liver and spleen, and therefore is not specific to liver damage. However, elevated GGT is the best indicator of excessive alcohol consumption because it has greater sensitivity than AST and ALT.1,3,4
Although these biomarkers are helpful in diagnosing ALD, they lose some of their utility in patients with advanced liver disease. Patients with severe liver dysfunction may not have elevated serum aminotransferase levels because the degree of liver enzyme elevation does not correlate well with the severity of ALD. For example, patients with advanced cirrhosis may have liver enzyme levels that appear normal. However, the pattern of elevation in transaminases can be helpful in making a diagnosis of liver dysfunction; using the ratio of AST to ALT may aid in diagnosing ALD, because AST is elevated more than twice that of ALT in >80% of patients with ALD.1,3,4
Table 21,3,4 shows the progression of ALD from steatohepatitis to alcoholic hepatitis to cirrhosis. In steatohepatitis, transaminitis is present but all other biomarkers normal. In alcoholic hepatitis, transaminitis is present along with elevated alkaline phosphatase, elevated bilirubin, and elevated international normalized ratio (INR). In alcoholic cirrhosis, the AST-to-ALT ratio is >2, and hypoalbuminemia, hyperbilirubinemia, and coagulopathy (evidenced by elevated INR) are present, consistent with long-term liver damage.1,3,4

Continue to: FDA-approved medications
FDA-approved medications
Three medications—acamprosate, naltrexone, and disulfiram—currently are FDA-approved for treating AUD.5,6 Additionally, several other medications have shown varying levels of efficacy in treating patients with AUD but are not FDA-approved for this indication (Table 3).5-8

Acamprosate is thought to create a balance of inhibitor and excitatory neurotransmitters by functioning as a glutamate antagonist and gamma-aminobutyric acid (GABA) agonist. This is speculated to aid in abstinence from alcohol. Data suggests that acamprosate may be more effective for maintaining abstinence than for inducing remission in individuals who have not yet detoxified from alcohol. Because of its renal excretion, acamprosate is the only FDA-approved medication for AUD that is not associated with liver toxicity. The most commonly reported adverse effect with acamprosate use is diarrhea.
Naltrexone, a mu-opioid receptor antagonist, is available in both tablet and long-acting IM injection formulations. Naltrexone blocks the binding of endorphins created by alcohol consumption to opioid receptors. This results in diminished dopamine release and is speculated to decrease reward and positive reinforcement with alcohol consumption, leading to fewer heavy drinking days. Due to hepatic metabolism, naltrexone use carries a risk of liver injury. Cases of hepatitis and clinically significant liver dysfunction as well as transient, asymptomatic, hepatic transaminase elevations have been observed in patients who receive naltrexone. Because of the absence of first-pass metabolism, long-acting IM naltrexone may produce less hepatotoxicity than the oral formulation. When the FDA approved both formulations of naltrexone, a “black-box” warning was issued concerning the risk of liver damage; however, these warnings have since been removed from their respective prescribing information.
Disulfiram inhibits acetaldehyde dehydrogenase, resulting in elevated acetaldehyde concentrations after consuming alcohol. In theory, this medication reduces a person’s desire to drink due to the negative physiological and physical effects associated with increased acetaldehyde, including hypotension, flushing, nausea, and vomiting. Although most of these reactions are short-lived, disulfiram can induce hepatotoxicity and liver failure that may prove fatal. Disulfiram should be avoided in patients with advanced ALD.
Off-label medications for AUD
Additional pharmacotherapeutic agents have been evaluated in patients with AUD. Baclofen, topiramate, gabapentin, and ondansetron have shown varying levels of efficacy and pose minimal concern in patients with ALD.
Continue to: Baclofen
Baclofen. Although findings are conflicting, baclofen is the only agent that has been specifically studied for treating AUD in patients with ALD. A GABA B receptor antagonist, baclofen is currently FDA-approved for treating spasticity. In a series of open-label and double-blind studies, baclofen has been shown to effectively reduce alcohol intake, promote abstinence, and prevent relapse.5,6 Further studies identified a possible dose-related response, noting that 20 mg taken 3 times daily may confer additional response over 10 mg taken 3 times daily.5,6 Conversely, the ALPADIR study failed to demonstrate superiority of baclofen vs placebo in the maintenance of abstinence from alcohol despite dosing at 180 mg/d.9 This study did, however, find a significant reduction in alcohol craving in favor of baclofen.9 Further, in a randomized controlled trial (RCT) conducted in veterans with chronic hepatitis C, baclofen 30 mg/d failed to show superiority over placebo with regard to increasing abstinence or reducing alcohol use
Topiramate. A recent meta-analysis found that topiramate use may result in fewer drinking days, heavy drinking days, and number of drinks per drinking day.7 Additionally, topiramate has demonstrated a statistically significant reduction in alcohol craving as well as the ability to decrease all liver function test values.5 This agent should be used with caution in patients with hepatic encephalopathy because the adverse cognitive effects associated with topiramate may confound the clinical course and treatment of such.
Gabapentin. The use of gabapentin to treat patients with AUD is supported by multiple RCTs. In studies that evaluated dose-related response, higher doses of gabapentin (up to 1,800 mg/d) showed greater efficacy than lower doses (ie, 900 mg/d).8 Because gabapentin does not undergo hepatic metabolism, its use in patients with ALD is considered safe. Although the abuse potential of gabapentin is less defined in patients with AUD, there have been reports of abuse in other high-risk populations (ie, those with opioid use disorder, incarcerated persons, and those who misuse prescriptions recreationally).8
Ondansetron is speculated to decrease the reward from alcohol via the down-regulation of dopaminergic neurons. Studies examining ondansetron for patients with AUD have found that it decreases alcohol cravings in those with early-onset alcoholism (initial onset at age ≤25), but not in late-onset alcoholism (initial onset at age >25).5 However, the ondansetron doses used in these trials were very low (4 mcg/kg), and those doses are not available commercially.5
CASE CONTINUED
Following a discussion of available pharmacotherapeutic options for AUD, Mr. S is started on baclofen, 10 mg 3 times daily, with plans for dose titration. At a 2-week follow-up appointment, Mr. S reports that he had not been taking baclofen as often as instructed; however, he denies further alcohol consumption and re-commits to baclofen treatment. Unfortunately, Mr. S is soon admitted to hospice care due to continued decompensation and is unable to attend any additional outpatient follow-up appointments. Three months after his initial outpatient contact, Mr. S dies due to alcoholic cirrhosis.
Related Resources
• Crabb DW, Im GY, Szabo G, et al. Diagnosis and treatment of alcohol-related liver diseases: 2019 practice guidance from the American Association for the Study of Liver Diseases. Hepatology. 2020;71(1):306-333.
• Murail AR, Carey WD. Disease management. Liver test interpretation - approach to the patient with liver disease: a guide to commonly used liver tests. Cleveland Clinic Center for Continuing Education. Updated August 2017. www.clevelandclinicmeded. com/medicalpubs/diseasemanagement/hepatology/ guide-to-common-liver-tests/
Drug Brand Names
Acamprosate • Campral
Baclofen • Lioresal
Disulfiram • Antabuse
Gabapentin • Neurontin
Naltrexone • Revia, Vivitrol
Ondansetron • Zofran
Topiramate • Topamax
Mr. S, age 64, presents for an outpatient follow-up after a recent hospital discharge for alcohol detoxification. He reports a long history of alcohol use, which has resulted in numerous hospital admissions. He has recently been receiving care from a gastroenterologist because the results of laboratory testing suggested hepatic impairment (Table 1). Mr. S says that a friend of his was able to stop drinking by taking a medication, and he wonders if he can be prescribed a medication to help him as well.

A chart review shows that Mr. S recently underwent paracentesis, during which 6 liters of fluid were removed. Additionally, an abdominal ultrasound confirmed hepatic cirrhosis.
According to the World Health Organization, alcohol consumption contributes to 3 million deaths annually.2 The highest proportion of these deaths (21.3%) is due to alcohol-associated gastrointestinal complications, including alcoholic and infectious hepatitis, pancreatitis, and cirrhosis. Because the liver is the primary site of ethanol metabolism, it sustains the greatest degree of tissue injury with heavy alcohol consumption. Additionally, the association of harmful use of alcohol with risky sexual behavior may partially explain the higher prevalence of viral hepatitis among persons with alcohol use disorder (AUD) compared with the general population. Alcoholic liver disease (ALD) progresses through several stages, beginning with hepatic steatosis and progressing through alcohol-related hepatitis, fibrosis, cirrhosis, and potentially hepatocellular carcinoma.3
Liver markers of alcohol use
Although biological markers can be used in clinical practice to screen and monitor for alcohol abuse, making a diagnosis of ALD can be challenging. Typically, a history of heavy alcohol consumption in addition to certain physical signs and laboratory tests for liver disease are the best indicators of ALD. However, the clinical assessment can be confounded by patients who deny or minimize how much alcohol they have consumed. Furthermore, physical and laboratory findings may not be specific to ALD.
Liver enzymes, including aspartate aminotransferase (AST), alanine aminotransferase (ALT), and gamma-glutamyltransferase (GGT), have historically been used as the basis of diagnosing ALD. In addition to elevated bilirubin and evidence of macrocytic anemia, elevations in these enzymes may suggest heavy alcohol use, but these values alone are inadequate to establish ALD. Gamma-glutamyltransferase is found in cell membranes of several body tissues, including the liver and spleen, and therefore is not specific to liver damage. However, elevated GGT is the best indicator of excessive alcohol consumption because it has greater sensitivity than AST and ALT.1,3,4
Although these biomarkers are helpful in diagnosing ALD, they lose some of their utility in patients with advanced liver disease. Patients with severe liver dysfunction may not have elevated serum aminotransferase levels because the degree of liver enzyme elevation does not correlate well with the severity of ALD. For example, patients with advanced cirrhosis may have liver enzyme levels that appear normal. However, the pattern of elevation in transaminases can be helpful in making a diagnosis of liver dysfunction; using the ratio of AST to ALT may aid in diagnosing ALD, because AST is elevated more than twice that of ALT in >80% of patients with ALD.1,3,4
Table 21,3,4 shows the progression of ALD from steatohepatitis to alcoholic hepatitis to cirrhosis. In steatohepatitis, transaminitis is present but all other biomarkers normal. In alcoholic hepatitis, transaminitis is present along with elevated alkaline phosphatase, elevated bilirubin, and elevated international normalized ratio (INR). In alcoholic cirrhosis, the AST-to-ALT ratio is >2, and hypoalbuminemia, hyperbilirubinemia, and coagulopathy (evidenced by elevated INR) are present, consistent with long-term liver damage.1,3,4

Continue to: FDA-approved medications
FDA-approved medications
Three medications—acamprosate, naltrexone, and disulfiram—currently are FDA-approved for treating AUD.5,6 Additionally, several other medications have shown varying levels of efficacy in treating patients with AUD but are not FDA-approved for this indication (Table 3).5-8

Acamprosate is thought to create a balance of inhibitor and excitatory neurotransmitters by functioning as a glutamate antagonist and gamma-aminobutyric acid (GABA) agonist. This is speculated to aid in abstinence from alcohol. Data suggests that acamprosate may be more effective for maintaining abstinence than for inducing remission in individuals who have not yet detoxified from alcohol. Because of its renal excretion, acamprosate is the only FDA-approved medication for AUD that is not associated with liver toxicity. The most commonly reported adverse effect with acamprosate use is diarrhea.
Naltrexone, a mu-opioid receptor antagonist, is available in both tablet and long-acting IM injection formulations. Naltrexone blocks the binding of endorphins created by alcohol consumption to opioid receptors. This results in diminished dopamine release and is speculated to decrease reward and positive reinforcement with alcohol consumption, leading to fewer heavy drinking days. Due to hepatic metabolism, naltrexone use carries a risk of liver injury. Cases of hepatitis and clinically significant liver dysfunction as well as transient, asymptomatic, hepatic transaminase elevations have been observed in patients who receive naltrexone. Because of the absence of first-pass metabolism, long-acting IM naltrexone may produce less hepatotoxicity than the oral formulation. When the FDA approved both formulations of naltrexone, a “black-box” warning was issued concerning the risk of liver damage; however, these warnings have since been removed from their respective prescribing information.
Disulfiram inhibits acetaldehyde dehydrogenase, resulting in elevated acetaldehyde concentrations after consuming alcohol. In theory, this medication reduces a person’s desire to drink due to the negative physiological and physical effects associated with increased acetaldehyde, including hypotension, flushing, nausea, and vomiting. Although most of these reactions are short-lived, disulfiram can induce hepatotoxicity and liver failure that may prove fatal. Disulfiram should be avoided in patients with advanced ALD.
Off-label medications for AUD
Additional pharmacotherapeutic agents have been evaluated in patients with AUD. Baclofen, topiramate, gabapentin, and ondansetron have shown varying levels of efficacy and pose minimal concern in patients with ALD.
Continue to: Baclofen
Baclofen. Although findings are conflicting, baclofen is the only agent that has been specifically studied for treating AUD in patients with ALD. A GABA B receptor antagonist, baclofen is currently FDA-approved for treating spasticity. In a series of open-label and double-blind studies, baclofen has been shown to effectively reduce alcohol intake, promote abstinence, and prevent relapse.5,6 Further studies identified a possible dose-related response, noting that 20 mg taken 3 times daily may confer additional response over 10 mg taken 3 times daily.5,6 Conversely, the ALPADIR study failed to demonstrate superiority of baclofen vs placebo in the maintenance of abstinence from alcohol despite dosing at 180 mg/d.9 This study did, however, find a significant reduction in alcohol craving in favor of baclofen.9 Further, in a randomized controlled trial (RCT) conducted in veterans with chronic hepatitis C, baclofen 30 mg/d failed to show superiority over placebo with regard to increasing abstinence or reducing alcohol use
Topiramate. A recent meta-analysis found that topiramate use may result in fewer drinking days, heavy drinking days, and number of drinks per drinking day.7 Additionally, topiramate has demonstrated a statistically significant reduction in alcohol craving as well as the ability to decrease all liver function test values.5 This agent should be used with caution in patients with hepatic encephalopathy because the adverse cognitive effects associated with topiramate may confound the clinical course and treatment of such.
Gabapentin. The use of gabapentin to treat patients with AUD is supported by multiple RCTs. In studies that evaluated dose-related response, higher doses of gabapentin (up to 1,800 mg/d) showed greater efficacy than lower doses (ie, 900 mg/d).8 Because gabapentin does not undergo hepatic metabolism, its use in patients with ALD is considered safe. Although the abuse potential of gabapentin is less defined in patients with AUD, there have been reports of abuse in other high-risk populations (ie, those with opioid use disorder, incarcerated persons, and those who misuse prescriptions recreationally).8
Ondansetron is speculated to decrease the reward from alcohol via the down-regulation of dopaminergic neurons. Studies examining ondansetron for patients with AUD have found that it decreases alcohol cravings in those with early-onset alcoholism (initial onset at age ≤25), but not in late-onset alcoholism (initial onset at age >25).5 However, the ondansetron doses used in these trials were very low (4 mcg/kg), and those doses are not available commercially.5
CASE CONTINUED
Following a discussion of available pharmacotherapeutic options for AUD, Mr. S is started on baclofen, 10 mg 3 times daily, with plans for dose titration. At a 2-week follow-up appointment, Mr. S reports that he had not been taking baclofen as often as instructed; however, he denies further alcohol consumption and re-commits to baclofen treatment. Unfortunately, Mr. S is soon admitted to hospice care due to continued decompensation and is unable to attend any additional outpatient follow-up appointments. Three months after his initial outpatient contact, Mr. S dies due to alcoholic cirrhosis.
Related Resources
• Crabb DW, Im GY, Szabo G, et al. Diagnosis and treatment of alcohol-related liver diseases: 2019 practice guidance from the American Association for the Study of Liver Diseases. Hepatology. 2020;71(1):306-333.
• Murail AR, Carey WD. Disease management. Liver test interpretation - approach to the patient with liver disease: a guide to commonly used liver tests. Cleveland Clinic Center for Continuing Education. Updated August 2017. www.clevelandclinicmeded. com/medicalpubs/diseasemanagement/hepatology/ guide-to-common-liver-tests/
Drug Brand Names
Acamprosate • Campral
Baclofen • Lioresal
Disulfiram • Antabuse
Gabapentin • Neurontin
Naltrexone • Revia, Vivitrol
Ondansetron • Zofran
Topiramate • Topamax
1. Agrawal S, Dhiman RK, Limdi JK. Evaluation of abnormal liver function tests. Postgrad Med J. 2016;92(1086):223-234.
2. World Health Organization. Global status report on alcohol and health 2018. Published 2018. Accessed November 5, 2020. https://www.who.int/substance_abuse/publications/global_alcohol_report/gsr_2018/en/
3. Osna NA, Donohue TM, Kharbanda KK. Alcoholic liver disease: pathogenesis and current management. Alcohol Res. 2017;38(2):147-161.
4. Leggio L, Lee MR. Treatment of alcohol use disorder in patients with alcoholic liver disease. Am J Med. 2017;130(2):124-134.
5. Addolorato G, Mirijello A, Leggio L, et al. Management of alcohol dependence in patients with liver disease. CNS Drugs. 2013;27(4):287-299.
6. Vuittonet CL, Halse M, Leggio L, et al. Pharmacotherapy for alcoholic patients with alcoholic liver disease. Am J Health Syst Pharm. 2014;71(15):1265-1276.
7. Jonas DE, Amick HR, Feltner C, et al. Pharmacotherapy for adults with alcohol use disorders in outpatient settings. JAMA. 2014;311(18):1889-1900.
8. Mason BJ, Quello S, Shadan F. Gabapentin for the treatment of alcohol use disorder. Expert Opin Investig Drugs. 2018;27(1):113-124.
9. Reynaud M, Aubin HJ, Trinquet F, et al. A randomized, placebo-controlled study of high-dose baclofen in alcohol-dependent patients-the ALPADIR study. Alcohol Alcohol. 2017;52(4):439-446.
10. Hauser P, Fuller B, Ho S, et al. The safety and efficacy of baclofen to reduce alcohol use in veterans with chronic hepatitis C: a randomized controlled trial. Addiction. 2017;112(7):1173-1183.
1. Agrawal S, Dhiman RK, Limdi JK. Evaluation of abnormal liver function tests. Postgrad Med J. 2016;92(1086):223-234.
2. World Health Organization. Global status report on alcohol and health 2018. Published 2018. Accessed November 5, 2020. https://www.who.int/substance_abuse/publications/global_alcohol_report/gsr_2018/en/
3. Osna NA, Donohue TM, Kharbanda KK. Alcoholic liver disease: pathogenesis and current management. Alcohol Res. 2017;38(2):147-161.
4. Leggio L, Lee MR. Treatment of alcohol use disorder in patients with alcoholic liver disease. Am J Med. 2017;130(2):124-134.
5. Addolorato G, Mirijello A, Leggio L, et al. Management of alcohol dependence in patients with liver disease. CNS Drugs. 2013;27(4):287-299.
6. Vuittonet CL, Halse M, Leggio L, et al. Pharmacotherapy for alcoholic patients with alcoholic liver disease. Am J Health Syst Pharm. 2014;71(15):1265-1276.
7. Jonas DE, Amick HR, Feltner C, et al. Pharmacotherapy for adults with alcohol use disorders in outpatient settings. JAMA. 2014;311(18):1889-1900.
8. Mason BJ, Quello S, Shadan F. Gabapentin for the treatment of alcohol use disorder. Expert Opin Investig Drugs. 2018;27(1):113-124.
9. Reynaud M, Aubin HJ, Trinquet F, et al. A randomized, placebo-controlled study of high-dose baclofen in alcohol-dependent patients-the ALPADIR study. Alcohol Alcohol. 2017;52(4):439-446.
10. Hauser P, Fuller B, Ho S, et al. The safety and efficacy of baclofen to reduce alcohol use in veterans with chronic hepatitis C: a randomized controlled trial. Addiction. 2017;112(7):1173-1183.
Scant risk for SARS-CoV-2 from hospital air
Everywhere they look within hospitals, researchers find RNA from SARS-CoV-2 in the air. But viable viruses typically are found only close to patients, according to a review of published studies.
The finding supports recommendations to use surgical masks in most parts of the hospital, reserving respirators (such as N95 or FFP2) for aerosol-generating procedures on patients’ respiratory tracts, said Gabriel Birgand, PhD, an infectious disease researcher at Imperial College London.
“When the virus is spreading a lot in the community, it’s probably more likely for you to be contaminated in your friends’ areas or in your building than in your work area, where you are well equipped and compliant with all the measures,” he said in an interview. “So it’s pretty good news.”
The systematic review by Dr. Birgand and colleagues was published in JAMA Network Open.
Recommended precautions to protect health care workers from SARS-CoV-2 infections remain controversial. Most authorities believe droplets are the primary route of transmission, which would mean surgical masks may be sufficient protection. But some research has suggested transmission by aerosols as well, making N95 respirators seem necessary. There is even disagreement about the definitions of the words “aerosol” and “droplet.”
To better understand where traces of the virus can be found in the air in hospitals, Dr. Birgand and colleagues analyzed all the studies they could find on the subject in English.
They identified 24 articles with original data. All of the studies used reverse transcription–polymerase chain reaction (PCR) tests to identify SARS-CoV-2 RNA. In five studies, attempts were also made to culture viable viruses. Three studies assessed the particle size relative to RNA concentration or viral titer.
Of 893 air samples across the 24 studies, 52.7% were taken from areas close to patients, 26.5% were taken in clinical areas, 13.7% in staff areas, 4.7% in public areas, and 2.4% in toilets or bathrooms.
Among those studies that quantified RNA, the median interquartile range of concentrations varied from 1.0 x 103 copies/m3 in clinical areas to 9.7 x 103 copies/m3 in toilets or bathrooms.
One study found an RNA concentration of 2.0 x 103 copies for particle sizes >4 mcm and 1.3 x 103 copies/m3 for particle sizes ≤4 mcm, both in patients’ rooms.
Three studies included viral cultures; of those, two resulted in positive cultures, both in a non-ICU setting. In one study, 3 of 39 samples were positive, and in the other, 4 of 4 were positive. Viral cultures in toilets, clinical areas, staff areas, and public areas were negative.
One of these studies assessed viral concentration and found that the median interquartile range was 4.8 tissue culture infectious dose (TCID50)/m3 for particles <1 mcm, 4.27 TCID50/m3 for particles 1-4 mcm, and 1.82 TCID50/m3 for particles >4 mcm.
Although viable viruses weren’t found in staff areas, the presence of viral RNA in places such as dining rooms and meeting rooms raises a concern, Dr. Birgand said.
“All of these staff areas are probably playing an important role in contamination,” he said. “It’s pretty easy to see when you are dining, you are not wearing a face mask, and it’s associated with a strong risk when there is a strong dissemination of the virus in the community.”
Studies on contact tracing among health care workers have also identified meeting rooms and dining rooms as the second most common source of infection after community contact, he said.
In general, the findings of the review correspond to epidemiologic studies, said Angela Rasmussen, PhD, a virologist with the Georgetown University Center for Global Health Science and Security, Washington, who was not involved in the review. “Absent aerosol-generating procedures, health care workers are largely not getting infected when they take droplet precautions.”
One reason may be that patients shed the most infectious viruses a couple of days before and after symptoms begin. By the time they’re hospitalized, they’re less likely to be contagious but may continue to shed viral RNA.
“We don’t really know the basis for the persistence of RNA being produced long after people have been infected and have recovered from the acute infection,” she said, “but it has been observed quite frequently.”
Although the virus cannot remain viable for very long in the air, remnants may still be detected in the form of RNA, Dr. Rasmussen said. In addition, hospitals often do a good job of ventilation.
She pointed out that it can be difficult to cultivate viruses in air samples because of contaminants such as bacteria and fungi. “That’s one of the limitations of a study like this. You’re not really sure if it’s because there’s no viable virus there or because you just aren’t able to collect samples that would allow you to determine that.”
Dr. Birgand and colleagues acknowledged other limitations. The studies they reviewed used different approaches to sampling. Different procedures may have been underway in the rooms being sampled, and factors such as temperature and humidity could have affected the results. In addition, the studies used different cycle thresholds for PCR positivity.
A version of this article first appeared on Medscape.com.
Everywhere they look within hospitals, researchers find RNA from SARS-CoV-2 in the air. But viable viruses typically are found only close to patients, according to a review of published studies.
The finding supports recommendations to use surgical masks in most parts of the hospital, reserving respirators (such as N95 or FFP2) for aerosol-generating procedures on patients’ respiratory tracts, said Gabriel Birgand, PhD, an infectious disease researcher at Imperial College London.
“When the virus is spreading a lot in the community, it’s probably more likely for you to be contaminated in your friends’ areas or in your building than in your work area, where you are well equipped and compliant with all the measures,” he said in an interview. “So it’s pretty good news.”
The systematic review by Dr. Birgand and colleagues was published in JAMA Network Open.
Recommended precautions to protect health care workers from SARS-CoV-2 infections remain controversial. Most authorities believe droplets are the primary route of transmission, which would mean surgical masks may be sufficient protection. But some research has suggested transmission by aerosols as well, making N95 respirators seem necessary. There is even disagreement about the definitions of the words “aerosol” and “droplet.”
To better understand where traces of the virus can be found in the air in hospitals, Dr. Birgand and colleagues analyzed all the studies they could find on the subject in English.
They identified 24 articles with original data. All of the studies used reverse transcription–polymerase chain reaction (PCR) tests to identify SARS-CoV-2 RNA. In five studies, attempts were also made to culture viable viruses. Three studies assessed the particle size relative to RNA concentration or viral titer.
Of 893 air samples across the 24 studies, 52.7% were taken from areas close to patients, 26.5% were taken in clinical areas, 13.7% in staff areas, 4.7% in public areas, and 2.4% in toilets or bathrooms.
Among those studies that quantified RNA, the median interquartile range of concentrations varied from 1.0 x 103 copies/m3 in clinical areas to 9.7 x 103 copies/m3 in toilets or bathrooms.
One study found an RNA concentration of 2.0 x 103 copies for particle sizes >4 mcm and 1.3 x 103 copies/m3 for particle sizes ≤4 mcm, both in patients’ rooms.
Three studies included viral cultures; of those, two resulted in positive cultures, both in a non-ICU setting. In one study, 3 of 39 samples were positive, and in the other, 4 of 4 were positive. Viral cultures in toilets, clinical areas, staff areas, and public areas were negative.
One of these studies assessed viral concentration and found that the median interquartile range was 4.8 tissue culture infectious dose (TCID50)/m3 for particles <1 mcm, 4.27 TCID50/m3 for particles 1-4 mcm, and 1.82 TCID50/m3 for particles >4 mcm.
Although viable viruses weren’t found in staff areas, the presence of viral RNA in places such as dining rooms and meeting rooms raises a concern, Dr. Birgand said.
“All of these staff areas are probably playing an important role in contamination,” he said. “It’s pretty easy to see when you are dining, you are not wearing a face mask, and it’s associated with a strong risk when there is a strong dissemination of the virus in the community.”
Studies on contact tracing among health care workers have also identified meeting rooms and dining rooms as the second most common source of infection after community contact, he said.
In general, the findings of the review correspond to epidemiologic studies, said Angela Rasmussen, PhD, a virologist with the Georgetown University Center for Global Health Science and Security, Washington, who was not involved in the review. “Absent aerosol-generating procedures, health care workers are largely not getting infected when they take droplet precautions.”
One reason may be that patients shed the most infectious viruses a couple of days before and after symptoms begin. By the time they’re hospitalized, they’re less likely to be contagious but may continue to shed viral RNA.
“We don’t really know the basis for the persistence of RNA being produced long after people have been infected and have recovered from the acute infection,” she said, “but it has been observed quite frequently.”
Although the virus cannot remain viable for very long in the air, remnants may still be detected in the form of RNA, Dr. Rasmussen said. In addition, hospitals often do a good job of ventilation.
She pointed out that it can be difficult to cultivate viruses in air samples because of contaminants such as bacteria and fungi. “That’s one of the limitations of a study like this. You’re not really sure if it’s because there’s no viable virus there or because you just aren’t able to collect samples that would allow you to determine that.”
Dr. Birgand and colleagues acknowledged other limitations. The studies they reviewed used different approaches to sampling. Different procedures may have been underway in the rooms being sampled, and factors such as temperature and humidity could have affected the results. In addition, the studies used different cycle thresholds for PCR positivity.
A version of this article first appeared on Medscape.com.
Everywhere they look within hospitals, researchers find RNA from SARS-CoV-2 in the air. But viable viruses typically are found only close to patients, according to a review of published studies.
The finding supports recommendations to use surgical masks in most parts of the hospital, reserving respirators (such as N95 or FFP2) for aerosol-generating procedures on patients’ respiratory tracts, said Gabriel Birgand, PhD, an infectious disease researcher at Imperial College London.
“When the virus is spreading a lot in the community, it’s probably more likely for you to be contaminated in your friends’ areas or in your building than in your work area, where you are well equipped and compliant with all the measures,” he said in an interview. “So it’s pretty good news.”
The systematic review by Dr. Birgand and colleagues was published in JAMA Network Open.
Recommended precautions to protect health care workers from SARS-CoV-2 infections remain controversial. Most authorities believe droplets are the primary route of transmission, which would mean surgical masks may be sufficient protection. But some research has suggested transmission by aerosols as well, making N95 respirators seem necessary. There is even disagreement about the definitions of the words “aerosol” and “droplet.”
To better understand where traces of the virus can be found in the air in hospitals, Dr. Birgand and colleagues analyzed all the studies they could find on the subject in English.
They identified 24 articles with original data. All of the studies used reverse transcription–polymerase chain reaction (PCR) tests to identify SARS-CoV-2 RNA. In five studies, attempts were also made to culture viable viruses. Three studies assessed the particle size relative to RNA concentration or viral titer.
Of 893 air samples across the 24 studies, 52.7% were taken from areas close to patients, 26.5% were taken in clinical areas, 13.7% in staff areas, 4.7% in public areas, and 2.4% in toilets or bathrooms.
Among those studies that quantified RNA, the median interquartile range of concentrations varied from 1.0 x 103 copies/m3 in clinical areas to 9.7 x 103 copies/m3 in toilets or bathrooms.
One study found an RNA concentration of 2.0 x 103 copies for particle sizes >4 mcm and 1.3 x 103 copies/m3 for particle sizes ≤4 mcm, both in patients’ rooms.
Three studies included viral cultures; of those, two resulted in positive cultures, both in a non-ICU setting. In one study, 3 of 39 samples were positive, and in the other, 4 of 4 were positive. Viral cultures in toilets, clinical areas, staff areas, and public areas were negative.
One of these studies assessed viral concentration and found that the median interquartile range was 4.8 tissue culture infectious dose (TCID50)/m3 for particles <1 mcm, 4.27 TCID50/m3 for particles 1-4 mcm, and 1.82 TCID50/m3 for particles >4 mcm.
Although viable viruses weren’t found in staff areas, the presence of viral RNA in places such as dining rooms and meeting rooms raises a concern, Dr. Birgand said.
“All of these staff areas are probably playing an important role in contamination,” he said. “It’s pretty easy to see when you are dining, you are not wearing a face mask, and it’s associated with a strong risk when there is a strong dissemination of the virus in the community.”
Studies on contact tracing among health care workers have also identified meeting rooms and dining rooms as the second most common source of infection after community contact, he said.
In general, the findings of the review correspond to epidemiologic studies, said Angela Rasmussen, PhD, a virologist with the Georgetown University Center for Global Health Science and Security, Washington, who was not involved in the review. “Absent aerosol-generating procedures, health care workers are largely not getting infected when they take droplet precautions.”
One reason may be that patients shed the most infectious viruses a couple of days before and after symptoms begin. By the time they’re hospitalized, they’re less likely to be contagious but may continue to shed viral RNA.
“We don’t really know the basis for the persistence of RNA being produced long after people have been infected and have recovered from the acute infection,” she said, “but it has been observed quite frequently.”
Although the virus cannot remain viable for very long in the air, remnants may still be detected in the form of RNA, Dr. Rasmussen said. In addition, hospitals often do a good job of ventilation.
She pointed out that it can be difficult to cultivate viruses in air samples because of contaminants such as bacteria and fungi. “That’s one of the limitations of a study like this. You’re not really sure if it’s because there’s no viable virus there or because you just aren’t able to collect samples that would allow you to determine that.”
Dr. Birgand and colleagues acknowledged other limitations. The studies they reviewed used different approaches to sampling. Different procedures may have been underway in the rooms being sampled, and factors such as temperature and humidity could have affected the results. In addition, the studies used different cycle thresholds for PCR positivity.
A version of this article first appeared on Medscape.com.
New resilience center targets traumatized health care workers
A physician assistant participating in a virtual workshop began to cry, confessing that she felt overwhelmed with guilt because New Yorkers were hailing her as a frontline hero in the pandemic. That was when Joe Ciavarro knew he was in the right place.
“She was saying all the things I could not verbalize because I, too, didn’t feel like I deserved all this praise and thousands of people cheering for us every evening when people were losing jobs, didn’t have money for food, and their loved ones were dying without family at their side,” says Mr. Ciavarro, a PA at Mount Sinai Medical Center in New York.
Mr. Ciavarro, who also manages 170 other PAs on two of Mount Sinai’s campuses in Manhattan, has been on the front lines since COVID-19 first hit; he lost a colleague and friend to suicide in September.
The mental anguish from his job prompted him to sign up for the resilience workshop offered by Mount Sinai’s Center for Stress, Resilience, and Personal Growth. The center – the first of its kind in North America – was launched in June to help health care workers like him cope with the intense psychological pressures they were facing. The weekly workshops became a safe place where Mr. Ciavarro and other staff members could share their darkest fears and learn ways to help them deal with their situation.
“It’s been grueling but we learned how to take care of ourselves so we can take care of our patients,” said Mr. Ciavarro. “This has become like a guided group therapy session on ways to manage and develop resilience. And I feel like my emotions are validated, knowing that others feel the same way.”
Caring for their own
Medical professionals treating patients with COVID-19 are in similar predicaments, and the psychological fallout is enormous: They’re exhausted by the seemingly never-ending patient load and staffing shortages, and haunted by fears for their own safety and that of their families. Studies in China, Canada, and Italy have revealed that a significant number of doctors and nurses in the early days of the pandemic experienced high levels of distress, depression, anxiety, nightmares, and insomnia.
after witnessing the deaths of so many patients who were alone, without family.
But the resilience workshop that Mr. Ciavarro attended offers some hope and is part of a multifaceted program that aims to be a model for other institutions and communities. The Mount Sinai health system already had some programs in place, including centers for 9/11 responders, for spirituality and health, and a wellness program to aid burned-out doctors. But the leadership at Mount Sinai, which includes psychiatrist Dennis Charney, MD, dean of the medical school and a leading expert on PTSD, knew early in the pandemic that emotional and psychological distress would plague health care workers, according to Deborah Marin, MD, director of the new center.
“We decided to quickly put in place a program that we could do virtually, with workshops and apps, that would give access to several services above and beyond what was already going on,” says Dr. Marin, a professor of psychiatry at the Icahn School of Medicine at Mount Sinai, New York, who also directs their center for spirituality and health.
The key components include a comprehensive screening tool that helps doctors at the center identify which potential participants are most at risk. Participants build personal inventories that detail the intensity of work-related exposures, personal or family stressors that have arisen because of the pandemic, or any mental health conditions or substance abuse problems that may make staff members more vulnerable.
The weekly workshops led by trained staff are designed to give participants the tools to foster resilience and process their experiences. Online apps provide feedback on their progress and engage them with video and other resources around meditation, relaxation, and resilience techniques.
In addition, all 40,000 members of the Mount Sinai staff are eligible for up to 14 one-on-one sessions with psychologists and psychiatrists who specialize in treating trauma.
“That’s highly unusual – to offer this at no cost to everyone,” said Dr. Marin. “We also have a treatment service that is specifically focused on behavioral health care, so people can learn better coping strategies, and we also have social workers to provide coaching.”
While the center doesn’t have specific numbers on how many nurses, physicians, and other staff have participated in treatment, they have trained over 70 peer leaders for their five workshops that home in on the most important factors of resilience.
“We’ve gotten enthusiastic responses from PAs and nurses,” said Craig Katz, MD, an expert in disaster psychiatry at Mount Sinai and a workshop moderator. Physicians have been slower to get on board. “Doctors are a tough nut to crack – it’s largely a culture where they may burn out but don’t want to talk about it. And asking for help is a hard transition for physicians to make.”
How to protect in midst of trauma
In formulating the program’s platform, Mount Sinai experts drew upon their extensive experience aiding 9/11 responders at the World Trade Center (WTC), as well as their system-wide wellness program that aids demoralized and burned-out physicians. While the reach of the pandemic is much broader than 9/11, experts see some commonalities in conditions that emerge after traumatic events, and they also discovered what can help.
“We learned from our WTC experience about what are protective factors – what are the social supports that buffer against depression, anxiety, and PTSD,” said Jonathan DePierro, PhD, clinical director of CSRPG and a psychologist at the Mount Sinai WTC Mental Health Program. “We also learned that people who have more prolonged exposures are at greater risk of developing mental health difficulties.”
The program itself reflects these lessons – and that’s why it’s open to all employees, not just medical professionals. Housekeepers, security staffers, even construction workers are also dealing with their lives being in danger. “That wasn’t in their job description,” said Dr. DePierro. “These people tend to have fewer social and economic resources, make less money and have fewer structural supports, which makes them even more vulnerable.”
Dr. Charney’s strategies on building resilience became a bible of sorts for the workshops, according to Dr. Katz, who authored the training curriculum. Sessions deal with how to build up reservoirs of realistic optimism, keep gratitude journals, find spiritual meaning in their lives, maintain physical wellness and create networks of social support. The workshops are meant to help participants create action plans, to reach out for support in their social networks, and keep the focus on the positives.
The goal is to give demoralized health care workers a renewed sense of competence. “The resilience workshop is a launching point to get people to show up and talk,” said Dr. Katz. “And if we do that, we’ve accomplished a lot just getting people in the door.”
The center will also have a research component to identify what works and what doesn’t so their platform can provide a template for other institutions; Dr. Marin said they’ve gotten inquiries about the program from major hospital systems in Michigan and California. They’ll also conduct longitudinal research to determine what lingering problems persist among healthcare workers over time.
Since the center opened its virtual doors, the curriculum has also been altered in response to feedback from the support staff, many of whom live in the community that surrounds Mount Sinai in northern Manhattan, which is largely lower-income Latinx and Black individuals. Workshop materials have been translated into Spanish and now feature people who reflect a more diverse set of experiences.
“Many of our employees and the population we serve identify as non-White so we’ve been doing outreach with a lot of the local unions,” said Dr. Marin. “Our next step is to take what we’re doing and work with local community organizations.”
A version of this article first appeared on Medscape.com.
A physician assistant participating in a virtual workshop began to cry, confessing that she felt overwhelmed with guilt because New Yorkers were hailing her as a frontline hero in the pandemic. That was when Joe Ciavarro knew he was in the right place.
“She was saying all the things I could not verbalize because I, too, didn’t feel like I deserved all this praise and thousands of people cheering for us every evening when people were losing jobs, didn’t have money for food, and their loved ones were dying without family at their side,” says Mr. Ciavarro, a PA at Mount Sinai Medical Center in New York.
Mr. Ciavarro, who also manages 170 other PAs on two of Mount Sinai’s campuses in Manhattan, has been on the front lines since COVID-19 first hit; he lost a colleague and friend to suicide in September.
The mental anguish from his job prompted him to sign up for the resilience workshop offered by Mount Sinai’s Center for Stress, Resilience, and Personal Growth. The center – the first of its kind in North America – was launched in June to help health care workers like him cope with the intense psychological pressures they were facing. The weekly workshops became a safe place where Mr. Ciavarro and other staff members could share their darkest fears and learn ways to help them deal with their situation.
“It’s been grueling but we learned how to take care of ourselves so we can take care of our patients,” said Mr. Ciavarro. “This has become like a guided group therapy session on ways to manage and develop resilience. And I feel like my emotions are validated, knowing that others feel the same way.”
Caring for their own
Medical professionals treating patients with COVID-19 are in similar predicaments, and the psychological fallout is enormous: They’re exhausted by the seemingly never-ending patient load and staffing shortages, and haunted by fears for their own safety and that of their families. Studies in China, Canada, and Italy have revealed that a significant number of doctors and nurses in the early days of the pandemic experienced high levels of distress, depression, anxiety, nightmares, and insomnia.
after witnessing the deaths of so many patients who were alone, without family.
But the resilience workshop that Mr. Ciavarro attended offers some hope and is part of a multifaceted program that aims to be a model for other institutions and communities. The Mount Sinai health system already had some programs in place, including centers for 9/11 responders, for spirituality and health, and a wellness program to aid burned-out doctors. But the leadership at Mount Sinai, which includes psychiatrist Dennis Charney, MD, dean of the medical school and a leading expert on PTSD, knew early in the pandemic that emotional and psychological distress would plague health care workers, according to Deborah Marin, MD, director of the new center.
“We decided to quickly put in place a program that we could do virtually, with workshops and apps, that would give access to several services above and beyond what was already going on,” says Dr. Marin, a professor of psychiatry at the Icahn School of Medicine at Mount Sinai, New York, who also directs their center for spirituality and health.
The key components include a comprehensive screening tool that helps doctors at the center identify which potential participants are most at risk. Participants build personal inventories that detail the intensity of work-related exposures, personal or family stressors that have arisen because of the pandemic, or any mental health conditions or substance abuse problems that may make staff members more vulnerable.
The weekly workshops led by trained staff are designed to give participants the tools to foster resilience and process their experiences. Online apps provide feedback on their progress and engage them with video and other resources around meditation, relaxation, and resilience techniques.
In addition, all 40,000 members of the Mount Sinai staff are eligible for up to 14 one-on-one sessions with psychologists and psychiatrists who specialize in treating trauma.
“That’s highly unusual – to offer this at no cost to everyone,” said Dr. Marin. “We also have a treatment service that is specifically focused on behavioral health care, so people can learn better coping strategies, and we also have social workers to provide coaching.”
While the center doesn’t have specific numbers on how many nurses, physicians, and other staff have participated in treatment, they have trained over 70 peer leaders for their five workshops that home in on the most important factors of resilience.
“We’ve gotten enthusiastic responses from PAs and nurses,” said Craig Katz, MD, an expert in disaster psychiatry at Mount Sinai and a workshop moderator. Physicians have been slower to get on board. “Doctors are a tough nut to crack – it’s largely a culture where they may burn out but don’t want to talk about it. And asking for help is a hard transition for physicians to make.”
How to protect in midst of trauma
In formulating the program’s platform, Mount Sinai experts drew upon their extensive experience aiding 9/11 responders at the World Trade Center (WTC), as well as their system-wide wellness program that aids demoralized and burned-out physicians. While the reach of the pandemic is much broader than 9/11, experts see some commonalities in conditions that emerge after traumatic events, and they also discovered what can help.
“We learned from our WTC experience about what are protective factors – what are the social supports that buffer against depression, anxiety, and PTSD,” said Jonathan DePierro, PhD, clinical director of CSRPG and a psychologist at the Mount Sinai WTC Mental Health Program. “We also learned that people who have more prolonged exposures are at greater risk of developing mental health difficulties.”
The program itself reflects these lessons – and that’s why it’s open to all employees, not just medical professionals. Housekeepers, security staffers, even construction workers are also dealing with their lives being in danger. “That wasn’t in their job description,” said Dr. DePierro. “These people tend to have fewer social and economic resources, make less money and have fewer structural supports, which makes them even more vulnerable.”
Dr. Charney’s strategies on building resilience became a bible of sorts for the workshops, according to Dr. Katz, who authored the training curriculum. Sessions deal with how to build up reservoirs of realistic optimism, keep gratitude journals, find spiritual meaning in their lives, maintain physical wellness and create networks of social support. The workshops are meant to help participants create action plans, to reach out for support in their social networks, and keep the focus on the positives.
The goal is to give demoralized health care workers a renewed sense of competence. “The resilience workshop is a launching point to get people to show up and talk,” said Dr. Katz. “And if we do that, we’ve accomplished a lot just getting people in the door.”
The center will also have a research component to identify what works and what doesn’t so their platform can provide a template for other institutions; Dr. Marin said they’ve gotten inquiries about the program from major hospital systems in Michigan and California. They’ll also conduct longitudinal research to determine what lingering problems persist among healthcare workers over time.
Since the center opened its virtual doors, the curriculum has also been altered in response to feedback from the support staff, many of whom live in the community that surrounds Mount Sinai in northern Manhattan, which is largely lower-income Latinx and Black individuals. Workshop materials have been translated into Spanish and now feature people who reflect a more diverse set of experiences.
“Many of our employees and the population we serve identify as non-White so we’ve been doing outreach with a lot of the local unions,” said Dr. Marin. “Our next step is to take what we’re doing and work with local community organizations.”
A version of this article first appeared on Medscape.com.
A physician assistant participating in a virtual workshop began to cry, confessing that she felt overwhelmed with guilt because New Yorkers were hailing her as a frontline hero in the pandemic. That was when Joe Ciavarro knew he was in the right place.
“She was saying all the things I could not verbalize because I, too, didn’t feel like I deserved all this praise and thousands of people cheering for us every evening when people were losing jobs, didn’t have money for food, and their loved ones were dying without family at their side,” says Mr. Ciavarro, a PA at Mount Sinai Medical Center in New York.
Mr. Ciavarro, who also manages 170 other PAs on two of Mount Sinai’s campuses in Manhattan, has been on the front lines since COVID-19 first hit; he lost a colleague and friend to suicide in September.
The mental anguish from his job prompted him to sign up for the resilience workshop offered by Mount Sinai’s Center for Stress, Resilience, and Personal Growth. The center – the first of its kind in North America – was launched in June to help health care workers like him cope with the intense psychological pressures they were facing. The weekly workshops became a safe place where Mr. Ciavarro and other staff members could share their darkest fears and learn ways to help them deal with their situation.
“It’s been grueling but we learned how to take care of ourselves so we can take care of our patients,” said Mr. Ciavarro. “This has become like a guided group therapy session on ways to manage and develop resilience. And I feel like my emotions are validated, knowing that others feel the same way.”
Caring for their own
Medical professionals treating patients with COVID-19 are in similar predicaments, and the psychological fallout is enormous: They’re exhausted by the seemingly never-ending patient load and staffing shortages, and haunted by fears for their own safety and that of their families. Studies in China, Canada, and Italy have revealed that a significant number of doctors and nurses in the early days of the pandemic experienced high levels of distress, depression, anxiety, nightmares, and insomnia.
after witnessing the deaths of so many patients who were alone, without family.
But the resilience workshop that Mr. Ciavarro attended offers some hope and is part of a multifaceted program that aims to be a model for other institutions and communities. The Mount Sinai health system already had some programs in place, including centers for 9/11 responders, for spirituality and health, and a wellness program to aid burned-out doctors. But the leadership at Mount Sinai, which includes psychiatrist Dennis Charney, MD, dean of the medical school and a leading expert on PTSD, knew early in the pandemic that emotional and psychological distress would plague health care workers, according to Deborah Marin, MD, director of the new center.
“We decided to quickly put in place a program that we could do virtually, with workshops and apps, that would give access to several services above and beyond what was already going on,” says Dr. Marin, a professor of psychiatry at the Icahn School of Medicine at Mount Sinai, New York, who also directs their center for spirituality and health.
The key components include a comprehensive screening tool that helps doctors at the center identify which potential participants are most at risk. Participants build personal inventories that detail the intensity of work-related exposures, personal or family stressors that have arisen because of the pandemic, or any mental health conditions or substance abuse problems that may make staff members more vulnerable.
The weekly workshops led by trained staff are designed to give participants the tools to foster resilience and process their experiences. Online apps provide feedback on their progress and engage them with video and other resources around meditation, relaxation, and resilience techniques.
In addition, all 40,000 members of the Mount Sinai staff are eligible for up to 14 one-on-one sessions with psychologists and psychiatrists who specialize in treating trauma.
“That’s highly unusual – to offer this at no cost to everyone,” said Dr. Marin. “We also have a treatment service that is specifically focused on behavioral health care, so people can learn better coping strategies, and we also have social workers to provide coaching.”
While the center doesn’t have specific numbers on how many nurses, physicians, and other staff have participated in treatment, they have trained over 70 peer leaders for their five workshops that home in on the most important factors of resilience.
“We’ve gotten enthusiastic responses from PAs and nurses,” said Craig Katz, MD, an expert in disaster psychiatry at Mount Sinai and a workshop moderator. Physicians have been slower to get on board. “Doctors are a tough nut to crack – it’s largely a culture where they may burn out but don’t want to talk about it. And asking for help is a hard transition for physicians to make.”
How to protect in midst of trauma
In formulating the program’s platform, Mount Sinai experts drew upon their extensive experience aiding 9/11 responders at the World Trade Center (WTC), as well as their system-wide wellness program that aids demoralized and burned-out physicians. While the reach of the pandemic is much broader than 9/11, experts see some commonalities in conditions that emerge after traumatic events, and they also discovered what can help.
“We learned from our WTC experience about what are protective factors – what are the social supports that buffer against depression, anxiety, and PTSD,” said Jonathan DePierro, PhD, clinical director of CSRPG and a psychologist at the Mount Sinai WTC Mental Health Program. “We also learned that people who have more prolonged exposures are at greater risk of developing mental health difficulties.”
The program itself reflects these lessons – and that’s why it’s open to all employees, not just medical professionals. Housekeepers, security staffers, even construction workers are also dealing with their lives being in danger. “That wasn’t in their job description,” said Dr. DePierro. “These people tend to have fewer social and economic resources, make less money and have fewer structural supports, which makes them even more vulnerable.”
Dr. Charney’s strategies on building resilience became a bible of sorts for the workshops, according to Dr. Katz, who authored the training curriculum. Sessions deal with how to build up reservoirs of realistic optimism, keep gratitude journals, find spiritual meaning in their lives, maintain physical wellness and create networks of social support. The workshops are meant to help participants create action plans, to reach out for support in their social networks, and keep the focus on the positives.
The goal is to give demoralized health care workers a renewed sense of competence. “The resilience workshop is a launching point to get people to show up and talk,” said Dr. Katz. “And if we do that, we’ve accomplished a lot just getting people in the door.”
The center will also have a research component to identify what works and what doesn’t so their platform can provide a template for other institutions; Dr. Marin said they’ve gotten inquiries about the program from major hospital systems in Michigan and California. They’ll also conduct longitudinal research to determine what lingering problems persist among healthcare workers over time.
Since the center opened its virtual doors, the curriculum has also been altered in response to feedback from the support staff, many of whom live in the community that surrounds Mount Sinai in northern Manhattan, which is largely lower-income Latinx and Black individuals. Workshop materials have been translated into Spanish and now feature people who reflect a more diverse set of experiences.
“Many of our employees and the population we serve identify as non-White so we’ve been doing outreach with a lot of the local unions,” said Dr. Marin. “Our next step is to take what we’re doing and work with local community organizations.”
A version of this article first appeared on Medscape.com.
Intense intervention may boost addiction program retention
An intense and assertive “won’t take no for an answer” approach is effective for engaging in treatment young adults with substance abuse who have been in and out of various recovery programs for years, new research suggests.
The Youth Opioid Recovery Support (YORS) program is a team effort that includes home delivery of the prescribed medication, family engagement, assertive outreach, and contingency management.
In a new study of 42 patients in recovery for substance use disorder (SUD), those who were treated with extended-release naltrexone or extended-release buprenorphine plus YORS received more outpatient doses of their medication, and rates of opioid relapse at 12 and 24 weeks were lower compared with their peers who received only treatment as usual.
“ coinvestigator Marc Fishman, MD, director of the Maryland Treatment Centers, Johns Hopkins University, Baltimore, said in an interview.
The findings were presented at the virtual American Academy of Addiction Psychiatry 31st Annual Meeting.
Treatment barriers
Young adults with SUD are difficult to reach, which leads to decreased addiction program retention, decreased medication adherence, early drop out, waxing and waning motivation, and worse outcomes, compared with older adults with SUD, Dr. Fishman said.
In July, positive results from a pilot trial conducted by the investigators of YORS were published online in Addiction.
In that study, 41 young adults aged 18-26 years who intended to undergo treatment for SUD with extended-release naltrexone were randomly assigned to also undergo YORS or treatment as usual, which consisted of a standard referral to outpatient care following an inpatient stay.
The primary outcomes were number of medication doses received over 24 weeks and relapse to opioid use, which was defined as 10 or more days of use within 28 days at 24 weeks.
Participants in the YORS group received more doses of extended-release naltrexone (mean, 4.28; standard deviation, 2.3) than participants in the treatment-as-usual group (mean, 0.70; SD, 1.2; P < .01).
In the YORS group, rates of relapse at both 12 and 24 weeks were lower, and there were fewer overall days of opioid use.
For the current study, the investigators wanted to test whether there was a possible effect when patients were given a choice of medication. In the earlier trial, patients did not have a choice – they had to take extended-release naltrexone. In this study, they could opt for it or extended-release buprenorphine.
The researchers recruited 22 young adults (aged 18-26 years) from their inpatient clinic to participate. Half the patients chose to take extended-release naltrexone, and the other half chose extended-release buprenorphine.
The groups were then compared to a historical group of 20 patients who received treatment as usual and served as the control group.
Positive outcomes
As in the first study, outcomes in the new study were better with YORS.
All participants who underwent YORS received more outpatient medication doses at 12 weeks and 24 weeks than those who received treatment as usual (1.91 vs. 0.40 and 3.76 vs. 0.70, respectively; P < .001).
For the YORS group, rates of opioid relapse were lower at 12 weeks (27.3% vs. 75.0%) and at 24 weeks (52.9% vs. 95.0%; P < .01.)
All components of YORS work together to improve retention, Dr. Fishman noted. Patients do much better if a relative such as a mother, father, or grandmother is closely involved, he added.
Also important is drug delivery.
“In some ways, this is similar to the assertive community treatment, or ACT, for schizophrenia. Like substance use disorder, schizophrenia requires long-acting injectable antipsychotics. When that is delivered to the patient through an organized delivery service like YORS, it improves outcomes,” said Dr. Fishman.
SUD is a chronic, relapsing illness in which an individual’s judgment is impaired, he added.
“ACT has become a relatively standard feature of treatment in most communities in this country and internationally and is sustainable under public sector funding, so it’s not an impossible leap to say it could be done. But it will not be cheap,” Dr. Fishman said.
Removing barriers
In a comment, Serra Akyar, MD, a psychiatry resident at Northwell Health’s Staten Island University Hospital, New York, said that the YORS program may appear to be labor intensive.
“However, the combination of medication-assisted treatment and support are essential to the treatment of opioid use disorder, especially for young adults. Developing effective interventions for young adults is particularly important, given the plasticity of their brains,” said Dr. Akyar, who was not involved with the research.
Inability to access medication and a lack of a supportive environment, both in everyday life and in regards to therapy, are barriers to successful treatment, she noted.
“The YORS intervention aims to remove these barriers to further enhance engagement to care through a combination of medication delivery and family engagement and assertive outreach via text messaging, a modality presumed to be well received by youth,” Dr. Akyar said.
Despite having a limited sample size, the study shows how a comprehensive intervention can have a large impact on the maintenance of medication adherence and reduction of relapse in young adults, she added.
“Its early success is encouraging and warrants further study on a larger scale to determine long-term effectiveness, overall costs and feasibility, generalizability, and whether certain independent factors exist that may predict medication adherence and reduction of relapse,” she said.
Wraparound support
The study is also a significant reminder that the opioid crisis has affected the young adult population, who are very vulnerable to OUD, said Jose Vito, MD, child, adolescent, and addiction psychiatrist at New York University.
“The study made me realize the importance of the four components of YORS, which were the outreach, family involvement, home delivery, and monetary incentives,” Dr. Vito said in an interview.
All of these components, in addition to extended-release naltrexone or extended-release buprenorphine, “have contributed to lower rates of opioid relapse, and the relapses are much later in the course of treatment if they do occur,” he said.
Overall, the findings demonstrate the importance of not giving up on these youths, he noted.
“Programs like YORS that provide wraparound support can help alleviate the opioid health care crisis by keeping these young adults in treatment,” Dr. Vito concluded.
The study was funded by the University of Maryland Center for Addiction Research, Education, and Service. Dr. Fishman has a financial relationship with Alkermes.
A version of this article first appeared on Medscape.com.
An intense and assertive “won’t take no for an answer” approach is effective for engaging in treatment young adults with substance abuse who have been in and out of various recovery programs for years, new research suggests.
The Youth Opioid Recovery Support (YORS) program is a team effort that includes home delivery of the prescribed medication, family engagement, assertive outreach, and contingency management.
In a new study of 42 patients in recovery for substance use disorder (SUD), those who were treated with extended-release naltrexone or extended-release buprenorphine plus YORS received more outpatient doses of their medication, and rates of opioid relapse at 12 and 24 weeks were lower compared with their peers who received only treatment as usual.
“ coinvestigator Marc Fishman, MD, director of the Maryland Treatment Centers, Johns Hopkins University, Baltimore, said in an interview.
The findings were presented at the virtual American Academy of Addiction Psychiatry 31st Annual Meeting.
Treatment barriers
Young adults with SUD are difficult to reach, which leads to decreased addiction program retention, decreased medication adherence, early drop out, waxing and waning motivation, and worse outcomes, compared with older adults with SUD, Dr. Fishman said.
In July, positive results from a pilot trial conducted by the investigators of YORS were published online in Addiction.
In that study, 41 young adults aged 18-26 years who intended to undergo treatment for SUD with extended-release naltrexone were randomly assigned to also undergo YORS or treatment as usual, which consisted of a standard referral to outpatient care following an inpatient stay.
The primary outcomes were number of medication doses received over 24 weeks and relapse to opioid use, which was defined as 10 or more days of use within 28 days at 24 weeks.
Participants in the YORS group received more doses of extended-release naltrexone (mean, 4.28; standard deviation, 2.3) than participants in the treatment-as-usual group (mean, 0.70; SD, 1.2; P < .01).
In the YORS group, rates of relapse at both 12 and 24 weeks were lower, and there were fewer overall days of opioid use.
For the current study, the investigators wanted to test whether there was a possible effect when patients were given a choice of medication. In the earlier trial, patients did not have a choice – they had to take extended-release naltrexone. In this study, they could opt for it or extended-release buprenorphine.
The researchers recruited 22 young adults (aged 18-26 years) from their inpatient clinic to participate. Half the patients chose to take extended-release naltrexone, and the other half chose extended-release buprenorphine.
The groups were then compared to a historical group of 20 patients who received treatment as usual and served as the control group.
Positive outcomes
As in the first study, outcomes in the new study were better with YORS.
All participants who underwent YORS received more outpatient medication doses at 12 weeks and 24 weeks than those who received treatment as usual (1.91 vs. 0.40 and 3.76 vs. 0.70, respectively; P < .001).
For the YORS group, rates of opioid relapse were lower at 12 weeks (27.3% vs. 75.0%) and at 24 weeks (52.9% vs. 95.0%; P < .01.)
All components of YORS work together to improve retention, Dr. Fishman noted. Patients do much better if a relative such as a mother, father, or grandmother is closely involved, he added.
Also important is drug delivery.
“In some ways, this is similar to the assertive community treatment, or ACT, for schizophrenia. Like substance use disorder, schizophrenia requires long-acting injectable antipsychotics. When that is delivered to the patient through an organized delivery service like YORS, it improves outcomes,” said Dr. Fishman.
SUD is a chronic, relapsing illness in which an individual’s judgment is impaired, he added.
“ACT has become a relatively standard feature of treatment in most communities in this country and internationally and is sustainable under public sector funding, so it’s not an impossible leap to say it could be done. But it will not be cheap,” Dr. Fishman said.
Removing barriers
In a comment, Serra Akyar, MD, a psychiatry resident at Northwell Health’s Staten Island University Hospital, New York, said that the YORS program may appear to be labor intensive.
“However, the combination of medication-assisted treatment and support are essential to the treatment of opioid use disorder, especially for young adults. Developing effective interventions for young adults is particularly important, given the plasticity of their brains,” said Dr. Akyar, who was not involved with the research.
Inability to access medication and a lack of a supportive environment, both in everyday life and in regards to therapy, are barriers to successful treatment, she noted.
“The YORS intervention aims to remove these barriers to further enhance engagement to care through a combination of medication delivery and family engagement and assertive outreach via text messaging, a modality presumed to be well received by youth,” Dr. Akyar said.
Despite having a limited sample size, the study shows how a comprehensive intervention can have a large impact on the maintenance of medication adherence and reduction of relapse in young adults, she added.
“Its early success is encouraging and warrants further study on a larger scale to determine long-term effectiveness, overall costs and feasibility, generalizability, and whether certain independent factors exist that may predict medication adherence and reduction of relapse,” she said.
Wraparound support
The study is also a significant reminder that the opioid crisis has affected the young adult population, who are very vulnerable to OUD, said Jose Vito, MD, child, adolescent, and addiction psychiatrist at New York University.
“The study made me realize the importance of the four components of YORS, which were the outreach, family involvement, home delivery, and monetary incentives,” Dr. Vito said in an interview.
All of these components, in addition to extended-release naltrexone or extended-release buprenorphine, “have contributed to lower rates of opioid relapse, and the relapses are much later in the course of treatment if they do occur,” he said.
Overall, the findings demonstrate the importance of not giving up on these youths, he noted.
“Programs like YORS that provide wraparound support can help alleviate the opioid health care crisis by keeping these young adults in treatment,” Dr. Vito concluded.
The study was funded by the University of Maryland Center for Addiction Research, Education, and Service. Dr. Fishman has a financial relationship with Alkermes.
A version of this article first appeared on Medscape.com.
An intense and assertive “won’t take no for an answer” approach is effective for engaging in treatment young adults with substance abuse who have been in and out of various recovery programs for years, new research suggests.
The Youth Opioid Recovery Support (YORS) program is a team effort that includes home delivery of the prescribed medication, family engagement, assertive outreach, and contingency management.
In a new study of 42 patients in recovery for substance use disorder (SUD), those who were treated with extended-release naltrexone or extended-release buprenorphine plus YORS received more outpatient doses of their medication, and rates of opioid relapse at 12 and 24 weeks were lower compared with their peers who received only treatment as usual.
“ coinvestigator Marc Fishman, MD, director of the Maryland Treatment Centers, Johns Hopkins University, Baltimore, said in an interview.
The findings were presented at the virtual American Academy of Addiction Psychiatry 31st Annual Meeting.
Treatment barriers
Young adults with SUD are difficult to reach, which leads to decreased addiction program retention, decreased medication adherence, early drop out, waxing and waning motivation, and worse outcomes, compared with older adults with SUD, Dr. Fishman said.
In July, positive results from a pilot trial conducted by the investigators of YORS were published online in Addiction.
In that study, 41 young adults aged 18-26 years who intended to undergo treatment for SUD with extended-release naltrexone were randomly assigned to also undergo YORS or treatment as usual, which consisted of a standard referral to outpatient care following an inpatient stay.
The primary outcomes were number of medication doses received over 24 weeks and relapse to opioid use, which was defined as 10 or more days of use within 28 days at 24 weeks.
Participants in the YORS group received more doses of extended-release naltrexone (mean, 4.28; standard deviation, 2.3) than participants in the treatment-as-usual group (mean, 0.70; SD, 1.2; P < .01).
In the YORS group, rates of relapse at both 12 and 24 weeks were lower, and there were fewer overall days of opioid use.
For the current study, the investigators wanted to test whether there was a possible effect when patients were given a choice of medication. In the earlier trial, patients did not have a choice – they had to take extended-release naltrexone. In this study, they could opt for it or extended-release buprenorphine.
The researchers recruited 22 young adults (aged 18-26 years) from their inpatient clinic to participate. Half the patients chose to take extended-release naltrexone, and the other half chose extended-release buprenorphine.
The groups were then compared to a historical group of 20 patients who received treatment as usual and served as the control group.
Positive outcomes
As in the first study, outcomes in the new study were better with YORS.
All participants who underwent YORS received more outpatient medication doses at 12 weeks and 24 weeks than those who received treatment as usual (1.91 vs. 0.40 and 3.76 vs. 0.70, respectively; P < .001).
For the YORS group, rates of opioid relapse were lower at 12 weeks (27.3% vs. 75.0%) and at 24 weeks (52.9% vs. 95.0%; P < .01.)
All components of YORS work together to improve retention, Dr. Fishman noted. Patients do much better if a relative such as a mother, father, or grandmother is closely involved, he added.
Also important is drug delivery.
“In some ways, this is similar to the assertive community treatment, or ACT, for schizophrenia. Like substance use disorder, schizophrenia requires long-acting injectable antipsychotics. When that is delivered to the patient through an organized delivery service like YORS, it improves outcomes,” said Dr. Fishman.
SUD is a chronic, relapsing illness in which an individual’s judgment is impaired, he added.
“ACT has become a relatively standard feature of treatment in most communities in this country and internationally and is sustainable under public sector funding, so it’s not an impossible leap to say it could be done. But it will not be cheap,” Dr. Fishman said.
Removing barriers
In a comment, Serra Akyar, MD, a psychiatry resident at Northwell Health’s Staten Island University Hospital, New York, said that the YORS program may appear to be labor intensive.
“However, the combination of medication-assisted treatment and support are essential to the treatment of opioid use disorder, especially for young adults. Developing effective interventions for young adults is particularly important, given the plasticity of their brains,” said Dr. Akyar, who was not involved with the research.
Inability to access medication and a lack of a supportive environment, both in everyday life and in regards to therapy, are barriers to successful treatment, she noted.
“The YORS intervention aims to remove these barriers to further enhance engagement to care through a combination of medication delivery and family engagement and assertive outreach via text messaging, a modality presumed to be well received by youth,” Dr. Akyar said.
Despite having a limited sample size, the study shows how a comprehensive intervention can have a large impact on the maintenance of medication adherence and reduction of relapse in young adults, she added.
“Its early success is encouraging and warrants further study on a larger scale to determine long-term effectiveness, overall costs and feasibility, generalizability, and whether certain independent factors exist that may predict medication adherence and reduction of relapse,” she said.
Wraparound support
The study is also a significant reminder that the opioid crisis has affected the young adult population, who are very vulnerable to OUD, said Jose Vito, MD, child, adolescent, and addiction psychiatrist at New York University.
“The study made me realize the importance of the four components of YORS, which were the outreach, family involvement, home delivery, and monetary incentives,” Dr. Vito said in an interview.
All of these components, in addition to extended-release naltrexone or extended-release buprenorphine, “have contributed to lower rates of opioid relapse, and the relapses are much later in the course of treatment if they do occur,” he said.
Overall, the findings demonstrate the importance of not giving up on these youths, he noted.
“Programs like YORS that provide wraparound support can help alleviate the opioid health care crisis by keeping these young adults in treatment,” Dr. Vito concluded.
The study was funded by the University of Maryland Center for Addiction Research, Education, and Service. Dr. Fishman has a financial relationship with Alkermes.
A version of this article first appeared on Medscape.com.
COVID-19 mortality rates declined, but vary by hospital
Mortality rates for inpatients with COVID-19 dropped significantly during the first 6 months of the pandemic, but outcomes depend on the hospital where patients receive care, new data show.
“[T]he characteristic that is most associated with poor or worsening hospital outcomes is high or increasing community case rates,” write David A. Asch, MD, MBA, executive director of the Center for Health Care Innovation at the University of Pennsylvania in Philadelphia, and colleagues.
The relationship between COVID-19 mortality rates and local disease prevalence suggests that “hospitals do worse when they are burdened with cases and is consistent with imperatives to flatten the curve,” the authors continue. “As case rates of COVID-19 increase across the nation, hospital mortality outcomes may worsen.”
The researchers published their study online December 22 in JAMA Internal Medicine.
The quick and substantial improvement in survival “is a tribute in part to new science — for example, the science that revealed the benefits of dexamethasone,” Asch told Medscape Medical News. “But it’s also a tribute to the doctors and nurses in the hospitals who developed experience. It’s a cliché to refer to them as heroes, but that is what they are. The science and the heroic experience continues on, and so I’m optimistic that we’ll see even more improvement over time.”
However, the data also indicate that “with lots of disease in the community, hospitals may have a harder time keeping patients alive,” Asch said. “And of course the reason this is bad news is that community level case rates are rising all over, and in some cases at rapid rates. With that rise, we might be giving back some of our past gains in survival — just as the vaccine is beginning to be distributed.”
Examining mortality trends
The researchers analyzed administrative claims data from a large national health insurer. They included data from 38,517 adults who were admitted with COVID-19 to 955 US hospitals between January 1 and June 30 of this year. The investigators estimated hospitals’ risk-standardized rate of 30-day in-hospital mortality or referral to hospice, adjusted for patient-level characteristics.
Overall, 3179 patients (8.25%) died, and 1433 patients (3.7%) were referred to hospice. Risk-standardized mortality or hospice referral rates for individual hospitals ranged from 5.7% to 24.7%. The average rate was 9.1% in the best-performing quintile, compared with 15.7% in the worst-performing quintile.
In a subset of 398 hospitals that had at least 10 patients admitted for COVID-19 during early (January 1 through April 30) and later periods (between May 1 and June 30), rates in all but one hospital improved, and 94% improved by at least 25%. The average risk-standardized event rate declined from 16.6% to 9.3%.
“That rate of relative improvement is striking and encouraging, but perhaps not surprising,” Asch and coauthors write. “Early efforts at treating patients with COVID-19 were based on experience with previously known causes of severe respiratory illness. Later efforts could draw on experiences specific to SARS-CoV-2 infection.”
For instance, doctors tried different inpatient management approaches, such as early vs late assisted ventilation, differences in oxygen flow, prone or supine positioning, and anticoagulation. “Those efforts varied in how systematically they were evaluated, but our results suggest that valuable experience was gained,” the authors note.
In addition, variation between hospitals could reflect differences in quality or different admission thresholds, they continue.
The study provides “a reason for optimism that our healthcare system has improved in our ability to care for persons with COVID-19,” write Leon Boudourakis, MD, MHS, and Amit Uppal, MD, in a related commentary. Boudourakis and Uppal are both affiliated with NYC Health + Hospitals in New York City and with SUNY Downstate and New York University School of Medicine, respectively.
Similar improvements in mortality rates have been reported in the United Kingdom and in a New York City hospital system, the editorialists note. The lower mortality rates may represent clinical, healthcare system, and epidemiologic trends.
“Since the first wave of serious COVID-19 cases, physicians have learned a great deal about the best ways to treat this serious infection,” they say. “Steroids may decrease mortality in patients with respiratory failure. Remdesivir may shorten hospitalizations of patients with serious illness. Anticoagulation and prone positioning may help certain patients. Using noninvasive ventilation and high-flow oxygen therapy may spare subsets of patients from the harms of intubation, such as ventilator-induced lung injury.»
Overwhelmed hospitals
“Hospitals do not perform as well when they are overwhelmed,” which may be a reason for the correlation between community prevalence and mortality rates, Boudourakis and Uppal suggested. “In particular, patients with a precarious respiratory status require expert, meticulous therapy to avoid intubation; those who undergo intubation or have kidney failure require nuanced and timely expert care with ventilatory adjustments and kidney replacement therapy, which are difficult to perform optimally when hospital capacity is strained.”
Although the death rate has fallen to about 9% for hospitalized patients, “9% is still high,” Asch said.
“Our results show that hospitals can’t do it on their own,” Asch said. “They need all of us to keep the community spread of the disease down. The right answer now is the right answer since the beginning of the pandemic: Keep your distance, wash your hands, and wear a mask.”
Asch, Boudourakis, and Uppal have disclosed no relevant financial relationships. A study coauthor reported personal fees and grants from pharmaceutical companies outside the submitted work.
A version of this article first appeared on Medscape.com.
Mortality rates for inpatients with COVID-19 dropped significantly during the first 6 months of the pandemic, but outcomes depend on the hospital where patients receive care, new data show.
“[T]he characteristic that is most associated with poor or worsening hospital outcomes is high or increasing community case rates,” write David A. Asch, MD, MBA, executive director of the Center for Health Care Innovation at the University of Pennsylvania in Philadelphia, and colleagues.
The relationship between COVID-19 mortality rates and local disease prevalence suggests that “hospitals do worse when they are burdened with cases and is consistent with imperatives to flatten the curve,” the authors continue. “As case rates of COVID-19 increase across the nation, hospital mortality outcomes may worsen.”
The researchers published their study online December 22 in JAMA Internal Medicine.
The quick and substantial improvement in survival “is a tribute in part to new science — for example, the science that revealed the benefits of dexamethasone,” Asch told Medscape Medical News. “But it’s also a tribute to the doctors and nurses in the hospitals who developed experience. It’s a cliché to refer to them as heroes, but that is what they are. The science and the heroic experience continues on, and so I’m optimistic that we’ll see even more improvement over time.”
However, the data also indicate that “with lots of disease in the community, hospitals may have a harder time keeping patients alive,” Asch said. “And of course the reason this is bad news is that community level case rates are rising all over, and in some cases at rapid rates. With that rise, we might be giving back some of our past gains in survival — just as the vaccine is beginning to be distributed.”
Examining mortality trends
The researchers analyzed administrative claims data from a large national health insurer. They included data from 38,517 adults who were admitted with COVID-19 to 955 US hospitals between January 1 and June 30 of this year. The investigators estimated hospitals’ risk-standardized rate of 30-day in-hospital mortality or referral to hospice, adjusted for patient-level characteristics.
Overall, 3179 patients (8.25%) died, and 1433 patients (3.7%) were referred to hospice. Risk-standardized mortality or hospice referral rates for individual hospitals ranged from 5.7% to 24.7%. The average rate was 9.1% in the best-performing quintile, compared with 15.7% in the worst-performing quintile.
In a subset of 398 hospitals that had at least 10 patients admitted for COVID-19 during early (January 1 through April 30) and later periods (between May 1 and June 30), rates in all but one hospital improved, and 94% improved by at least 25%. The average risk-standardized event rate declined from 16.6% to 9.3%.
“That rate of relative improvement is striking and encouraging, but perhaps not surprising,” Asch and coauthors write. “Early efforts at treating patients with COVID-19 were based on experience with previously known causes of severe respiratory illness. Later efforts could draw on experiences specific to SARS-CoV-2 infection.”
For instance, doctors tried different inpatient management approaches, such as early vs late assisted ventilation, differences in oxygen flow, prone or supine positioning, and anticoagulation. “Those efforts varied in how systematically they were evaluated, but our results suggest that valuable experience was gained,” the authors note.
In addition, variation between hospitals could reflect differences in quality or different admission thresholds, they continue.
The study provides “a reason for optimism that our healthcare system has improved in our ability to care for persons with COVID-19,” write Leon Boudourakis, MD, MHS, and Amit Uppal, MD, in a related commentary. Boudourakis and Uppal are both affiliated with NYC Health + Hospitals in New York City and with SUNY Downstate and New York University School of Medicine, respectively.
Similar improvements in mortality rates have been reported in the United Kingdom and in a New York City hospital system, the editorialists note. The lower mortality rates may represent clinical, healthcare system, and epidemiologic trends.
“Since the first wave of serious COVID-19 cases, physicians have learned a great deal about the best ways to treat this serious infection,” they say. “Steroids may decrease mortality in patients with respiratory failure. Remdesivir may shorten hospitalizations of patients with serious illness. Anticoagulation and prone positioning may help certain patients. Using noninvasive ventilation and high-flow oxygen therapy may spare subsets of patients from the harms of intubation, such as ventilator-induced lung injury.»
Overwhelmed hospitals
“Hospitals do not perform as well when they are overwhelmed,” which may be a reason for the correlation between community prevalence and mortality rates, Boudourakis and Uppal suggested. “In particular, patients with a precarious respiratory status require expert, meticulous therapy to avoid intubation; those who undergo intubation or have kidney failure require nuanced and timely expert care with ventilatory adjustments and kidney replacement therapy, which are difficult to perform optimally when hospital capacity is strained.”
Although the death rate has fallen to about 9% for hospitalized patients, “9% is still high,” Asch said.
“Our results show that hospitals can’t do it on their own,” Asch said. “They need all of us to keep the community spread of the disease down. The right answer now is the right answer since the beginning of the pandemic: Keep your distance, wash your hands, and wear a mask.”
Asch, Boudourakis, and Uppal have disclosed no relevant financial relationships. A study coauthor reported personal fees and grants from pharmaceutical companies outside the submitted work.
A version of this article first appeared on Medscape.com.
Mortality rates for inpatients with COVID-19 dropped significantly during the first 6 months of the pandemic, but outcomes depend on the hospital where patients receive care, new data show.
“[T]he characteristic that is most associated with poor or worsening hospital outcomes is high or increasing community case rates,” write David A. Asch, MD, MBA, executive director of the Center for Health Care Innovation at the University of Pennsylvania in Philadelphia, and colleagues.
The relationship between COVID-19 mortality rates and local disease prevalence suggests that “hospitals do worse when they are burdened with cases and is consistent with imperatives to flatten the curve,” the authors continue. “As case rates of COVID-19 increase across the nation, hospital mortality outcomes may worsen.”
The researchers published their study online December 22 in JAMA Internal Medicine.
The quick and substantial improvement in survival “is a tribute in part to new science — for example, the science that revealed the benefits of dexamethasone,” Asch told Medscape Medical News. “But it’s also a tribute to the doctors and nurses in the hospitals who developed experience. It’s a cliché to refer to them as heroes, but that is what they are. The science and the heroic experience continues on, and so I’m optimistic that we’ll see even more improvement over time.”
However, the data also indicate that “with lots of disease in the community, hospitals may have a harder time keeping patients alive,” Asch said. “And of course the reason this is bad news is that community level case rates are rising all over, and in some cases at rapid rates. With that rise, we might be giving back some of our past gains in survival — just as the vaccine is beginning to be distributed.”
Examining mortality trends
The researchers analyzed administrative claims data from a large national health insurer. They included data from 38,517 adults who were admitted with COVID-19 to 955 US hospitals between January 1 and June 30 of this year. The investigators estimated hospitals’ risk-standardized rate of 30-day in-hospital mortality or referral to hospice, adjusted for patient-level characteristics.
Overall, 3179 patients (8.25%) died, and 1433 patients (3.7%) were referred to hospice. Risk-standardized mortality or hospice referral rates for individual hospitals ranged from 5.7% to 24.7%. The average rate was 9.1% in the best-performing quintile, compared with 15.7% in the worst-performing quintile.
In a subset of 398 hospitals that had at least 10 patients admitted for COVID-19 during early (January 1 through April 30) and later periods (between May 1 and June 30), rates in all but one hospital improved, and 94% improved by at least 25%. The average risk-standardized event rate declined from 16.6% to 9.3%.
“That rate of relative improvement is striking and encouraging, but perhaps not surprising,” Asch and coauthors write. “Early efforts at treating patients with COVID-19 were based on experience with previously known causes of severe respiratory illness. Later efforts could draw on experiences specific to SARS-CoV-2 infection.”
For instance, doctors tried different inpatient management approaches, such as early vs late assisted ventilation, differences in oxygen flow, prone or supine positioning, and anticoagulation. “Those efforts varied in how systematically they were evaluated, but our results suggest that valuable experience was gained,” the authors note.
In addition, variation between hospitals could reflect differences in quality or different admission thresholds, they continue.
The study provides “a reason for optimism that our healthcare system has improved in our ability to care for persons with COVID-19,” write Leon Boudourakis, MD, MHS, and Amit Uppal, MD, in a related commentary. Boudourakis and Uppal are both affiliated with NYC Health + Hospitals in New York City and with SUNY Downstate and New York University School of Medicine, respectively.
Similar improvements in mortality rates have been reported in the United Kingdom and in a New York City hospital system, the editorialists note. The lower mortality rates may represent clinical, healthcare system, and epidemiologic trends.
“Since the first wave of serious COVID-19 cases, physicians have learned a great deal about the best ways to treat this serious infection,” they say. “Steroids may decrease mortality in patients with respiratory failure. Remdesivir may shorten hospitalizations of patients with serious illness. Anticoagulation and prone positioning may help certain patients. Using noninvasive ventilation and high-flow oxygen therapy may spare subsets of patients from the harms of intubation, such as ventilator-induced lung injury.»
Overwhelmed hospitals
“Hospitals do not perform as well when they are overwhelmed,” which may be a reason for the correlation between community prevalence and mortality rates, Boudourakis and Uppal suggested. “In particular, patients with a precarious respiratory status require expert, meticulous therapy to avoid intubation; those who undergo intubation or have kidney failure require nuanced and timely expert care with ventilatory adjustments and kidney replacement therapy, which are difficult to perform optimally when hospital capacity is strained.”
Although the death rate has fallen to about 9% for hospitalized patients, “9% is still high,” Asch said.
“Our results show that hospitals can’t do it on their own,” Asch said. “They need all of us to keep the community spread of the disease down. The right answer now is the right answer since the beginning of the pandemic: Keep your distance, wash your hands, and wear a mask.”
Asch, Boudourakis, and Uppal have disclosed no relevant financial relationships. A study coauthor reported personal fees and grants from pharmaceutical companies outside the submitted work.
A version of this article first appeared on Medscape.com.
Shared medical appointments may bridge the opioid treatment gap
Shared medical appointments (SMAs) are an acceptable way to receive treatment for opioid use disorder (OUD), new research suggests.
In a survey study, participants attending an urban outpatient buprenorphine clinic reported a high degree of satisfaction with SMAs. However, the majority also reported they preferred individual appointments.
Still, SMAs may serve a role in providing comprehensive care for certain subpopulations with OUD who are prone to isolation and may also increase capacity to treat more patients with a substance use disorder (SUD), said coinvestigator Serra Akyar, MD, Northwell Health Staten Island University Hospital, New York.
“By providing education and a forum for sharing, SMAs can lead to changes in behavior and enhance and reinforce coping and problem-solving skills,” Dr. Akyar said in an interview.
The findings were presented at the virtual American Academy of Addiction Psychiatry 31st Annual Meeting.
SMA vs. group therapy
SMA is not a form of group therapy, Dr. Akyar noted. Group therapy has a psychotherapy component and is led by a therapist. SMAs do not have a psychotherapeutic or a behavioral therapy component but provide education and an opportunity for sharing personal experiences of recovery.
“For example, the doctor participating in the group describes what happens in the brain to drive addiction and fellow participants share their personal anecdotes of recovery, including their struggles and successes,” Dr. Akyar said.
“ given the differences in the type of care each group provides,” she added.
Recent research on SMAs for OUD is limited. Although previous studies have shown that the practice is highly acceptable and has comparable or better retention in care rates with buprenorphine versus individual appointments, these studies have been conducted in predominantly White populations and in suburban settings.
For the new study, the investigators wanted to examine how acceptable SMAs for OUD would be in an urban setting involving predominantly racial and ethnic minorities.
They administered a 15-minute survey to patients with OUD who were attending the Comprehensive Addiction Resources and Education Center, an outpatient psychiatry clinic located at New Jersey Medical School, from December 2019 to February 2020.
Of the 42 participants who initially consented, 39 completed the survey. The majority of the responders were Black (64.1%), had an annual income that was less than $20,000 (61.5%), and/or were unemployed or disabled (69.3%).
Most of the participants agreed or strongly agreed with the following statements:
- Scheduling appointments for SMAs is easy.
- I gain valuable information from the responses to other patients’ questions in SMAs.
- There is enough time for questions during SMAs.
- I gain valuable information from the doctor and social worker in SMAs.
- My medical needs are met during SMAs.
- I would recommend an SMA to other patients.
- Since starting SMAs, I find it easier to stick to my treatment plan.
- I have a lot of support outside of SMAs.
- People in SMAs give me the support I need to stick to my treatment plan.
Interestingly, despite the overall high satisfaction with SMAs, just 33% of participants said they preferred them to one-on-one visits, Dr. Akyar noted.
Further analyses showed that total satisfaction scores were positively associated with older age, being on disability, or being in retirement.
Bridging the gap
In a comment, Philip Wong, MD, New Jersey Medical School, Newark, noted that a more widespread use of SMAs could potentially bridge the treatment gap that currently exists in the United States.
“For providers, SMAs help reduce costs, improve productivity, prevent repeating of common advice, and increase outreach. These are all important at a time when the need for OUD treatment is increasing. This is especially true for places like Newark, which is one of the prime epicenters of the opioid epidemic,” said Dr. Wong.
Although he was not involved with this research, he and his colleagues recently conducted a literature review of publications relating to SMAs and found seven peer-reviewed articles. However, none was appropriately designed to compare SMAs with traditional one-on-one recovery treatment.
“We definitely need more clinical studies to further our understanding of SMAs as a tool for the medication-assisted treatment of opioid use disorder,” Dr. Wong said.
“There are currently a very limited number of physicians who can prescribe medication-assisted treatment in the first place. So, if that one provider can reach a larger community by doing these SMAs, then the potential is very great in terms of addressing the opioid epidemic,” he said.
David Kan, MD, chief medical officer of Bright Heart Health, San Ramon, Calif., agreed.
“SMAs are promising because they are efficient and allow more people to access treatment,” Dr. Kan said in an interview.
“Although the mechanism of SMA satisfaction is unclear, other research shows peer support and groups helpful for SUD treatment as a whole. SMA takes the best of many worlds and increases the potential number of patients treated for SUD,” he said.
Also asked to comment, Lewei (Allison) Lin, MD, University of Michigan, Ann Arbor, said SMAs “are one of a number of important interventions that should be considered” in order to increase availability and access to medication providers for OUD.
However, more research is needed “to examine the impact on treatment uptake and patient and provider experiences,” said Dr. Lin.
Dr. Akyar, Dr. Wong, Dr. Kan, and Dr. Lin disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Shared medical appointments (SMAs) are an acceptable way to receive treatment for opioid use disorder (OUD), new research suggests.
In a survey study, participants attending an urban outpatient buprenorphine clinic reported a high degree of satisfaction with SMAs. However, the majority also reported they preferred individual appointments.
Still, SMAs may serve a role in providing comprehensive care for certain subpopulations with OUD who are prone to isolation and may also increase capacity to treat more patients with a substance use disorder (SUD), said coinvestigator Serra Akyar, MD, Northwell Health Staten Island University Hospital, New York.
“By providing education and a forum for sharing, SMAs can lead to changes in behavior and enhance and reinforce coping and problem-solving skills,” Dr. Akyar said in an interview.
The findings were presented at the virtual American Academy of Addiction Psychiatry 31st Annual Meeting.
SMA vs. group therapy
SMA is not a form of group therapy, Dr. Akyar noted. Group therapy has a psychotherapy component and is led by a therapist. SMAs do not have a psychotherapeutic or a behavioral therapy component but provide education and an opportunity for sharing personal experiences of recovery.
“For example, the doctor participating in the group describes what happens in the brain to drive addiction and fellow participants share their personal anecdotes of recovery, including their struggles and successes,” Dr. Akyar said.
“ given the differences in the type of care each group provides,” she added.
Recent research on SMAs for OUD is limited. Although previous studies have shown that the practice is highly acceptable and has comparable or better retention in care rates with buprenorphine versus individual appointments, these studies have been conducted in predominantly White populations and in suburban settings.
For the new study, the investigators wanted to examine how acceptable SMAs for OUD would be in an urban setting involving predominantly racial and ethnic minorities.
They administered a 15-minute survey to patients with OUD who were attending the Comprehensive Addiction Resources and Education Center, an outpatient psychiatry clinic located at New Jersey Medical School, from December 2019 to February 2020.
Of the 42 participants who initially consented, 39 completed the survey. The majority of the responders were Black (64.1%), had an annual income that was less than $20,000 (61.5%), and/or were unemployed or disabled (69.3%).
Most of the participants agreed or strongly agreed with the following statements:
- Scheduling appointments for SMAs is easy.
- I gain valuable information from the responses to other patients’ questions in SMAs.
- There is enough time for questions during SMAs.
- I gain valuable information from the doctor and social worker in SMAs.
- My medical needs are met during SMAs.
- I would recommend an SMA to other patients.
- Since starting SMAs, I find it easier to stick to my treatment plan.
- I have a lot of support outside of SMAs.
- People in SMAs give me the support I need to stick to my treatment plan.
Interestingly, despite the overall high satisfaction with SMAs, just 33% of participants said they preferred them to one-on-one visits, Dr. Akyar noted.
Further analyses showed that total satisfaction scores were positively associated with older age, being on disability, or being in retirement.
Bridging the gap
In a comment, Philip Wong, MD, New Jersey Medical School, Newark, noted that a more widespread use of SMAs could potentially bridge the treatment gap that currently exists in the United States.
“For providers, SMAs help reduce costs, improve productivity, prevent repeating of common advice, and increase outreach. These are all important at a time when the need for OUD treatment is increasing. This is especially true for places like Newark, which is one of the prime epicenters of the opioid epidemic,” said Dr. Wong.
Although he was not involved with this research, he and his colleagues recently conducted a literature review of publications relating to SMAs and found seven peer-reviewed articles. However, none was appropriately designed to compare SMAs with traditional one-on-one recovery treatment.
“We definitely need more clinical studies to further our understanding of SMAs as a tool for the medication-assisted treatment of opioid use disorder,” Dr. Wong said.
“There are currently a very limited number of physicians who can prescribe medication-assisted treatment in the first place. So, if that one provider can reach a larger community by doing these SMAs, then the potential is very great in terms of addressing the opioid epidemic,” he said.
David Kan, MD, chief medical officer of Bright Heart Health, San Ramon, Calif., agreed.
“SMAs are promising because they are efficient and allow more people to access treatment,” Dr. Kan said in an interview.
“Although the mechanism of SMA satisfaction is unclear, other research shows peer support and groups helpful for SUD treatment as a whole. SMA takes the best of many worlds and increases the potential number of patients treated for SUD,” he said.
Also asked to comment, Lewei (Allison) Lin, MD, University of Michigan, Ann Arbor, said SMAs “are one of a number of important interventions that should be considered” in order to increase availability and access to medication providers for OUD.
However, more research is needed “to examine the impact on treatment uptake and patient and provider experiences,” said Dr. Lin.
Dr. Akyar, Dr. Wong, Dr. Kan, and Dr. Lin disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Shared medical appointments (SMAs) are an acceptable way to receive treatment for opioid use disorder (OUD), new research suggests.
In a survey study, participants attending an urban outpatient buprenorphine clinic reported a high degree of satisfaction with SMAs. However, the majority also reported they preferred individual appointments.
Still, SMAs may serve a role in providing comprehensive care for certain subpopulations with OUD who are prone to isolation and may also increase capacity to treat more patients with a substance use disorder (SUD), said coinvestigator Serra Akyar, MD, Northwell Health Staten Island University Hospital, New York.
“By providing education and a forum for sharing, SMAs can lead to changes in behavior and enhance and reinforce coping and problem-solving skills,” Dr. Akyar said in an interview.
The findings were presented at the virtual American Academy of Addiction Psychiatry 31st Annual Meeting.
SMA vs. group therapy
SMA is not a form of group therapy, Dr. Akyar noted. Group therapy has a psychotherapy component and is led by a therapist. SMAs do not have a psychotherapeutic or a behavioral therapy component but provide education and an opportunity for sharing personal experiences of recovery.
“For example, the doctor participating in the group describes what happens in the brain to drive addiction and fellow participants share their personal anecdotes of recovery, including their struggles and successes,” Dr. Akyar said.
“ given the differences in the type of care each group provides,” she added.
Recent research on SMAs for OUD is limited. Although previous studies have shown that the practice is highly acceptable and has comparable or better retention in care rates with buprenorphine versus individual appointments, these studies have been conducted in predominantly White populations and in suburban settings.
For the new study, the investigators wanted to examine how acceptable SMAs for OUD would be in an urban setting involving predominantly racial and ethnic minorities.
They administered a 15-minute survey to patients with OUD who were attending the Comprehensive Addiction Resources and Education Center, an outpatient psychiatry clinic located at New Jersey Medical School, from December 2019 to February 2020.
Of the 42 participants who initially consented, 39 completed the survey. The majority of the responders were Black (64.1%), had an annual income that was less than $20,000 (61.5%), and/or were unemployed or disabled (69.3%).
Most of the participants agreed or strongly agreed with the following statements:
- Scheduling appointments for SMAs is easy.
- I gain valuable information from the responses to other patients’ questions in SMAs.
- There is enough time for questions during SMAs.
- I gain valuable information from the doctor and social worker in SMAs.
- My medical needs are met during SMAs.
- I would recommend an SMA to other patients.
- Since starting SMAs, I find it easier to stick to my treatment plan.
- I have a lot of support outside of SMAs.
- People in SMAs give me the support I need to stick to my treatment plan.
Interestingly, despite the overall high satisfaction with SMAs, just 33% of participants said they preferred them to one-on-one visits, Dr. Akyar noted.
Further analyses showed that total satisfaction scores were positively associated with older age, being on disability, or being in retirement.
Bridging the gap
In a comment, Philip Wong, MD, New Jersey Medical School, Newark, noted that a more widespread use of SMAs could potentially bridge the treatment gap that currently exists in the United States.
“For providers, SMAs help reduce costs, improve productivity, prevent repeating of common advice, and increase outreach. These are all important at a time when the need for OUD treatment is increasing. This is especially true for places like Newark, which is one of the prime epicenters of the opioid epidemic,” said Dr. Wong.
Although he was not involved with this research, he and his colleagues recently conducted a literature review of publications relating to SMAs and found seven peer-reviewed articles. However, none was appropriately designed to compare SMAs with traditional one-on-one recovery treatment.
“We definitely need more clinical studies to further our understanding of SMAs as a tool for the medication-assisted treatment of opioid use disorder,” Dr. Wong said.
“There are currently a very limited number of physicians who can prescribe medication-assisted treatment in the first place. So, if that one provider can reach a larger community by doing these SMAs, then the potential is very great in terms of addressing the opioid epidemic,” he said.
David Kan, MD, chief medical officer of Bright Heart Health, San Ramon, Calif., agreed.
“SMAs are promising because they are efficient and allow more people to access treatment,” Dr. Kan said in an interview.
“Although the mechanism of SMA satisfaction is unclear, other research shows peer support and groups helpful for SUD treatment as a whole. SMA takes the best of many worlds and increases the potential number of patients treated for SUD,” he said.
Also asked to comment, Lewei (Allison) Lin, MD, University of Michigan, Ann Arbor, said SMAs “are one of a number of important interventions that should be considered” in order to increase availability and access to medication providers for OUD.
However, more research is needed “to examine the impact on treatment uptake and patient and provider experiences,” said Dr. Lin.
Dr. Akyar, Dr. Wong, Dr. Kan, and Dr. Lin disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
‘She’s not a real doctor, she’s a psych doctor’
During a particularly hectic day, Janeni Nayagan, MD, a first-year resident in the department of psychiatry and behavioral health at Cooper University Hospital, Camden, N.J., was taken aback – but not surprised – by a patient’s comment.
In the middle of an emergent situation, she overheard a patient say that Dr. Nayagan wasn’t a “real doctor, she’s a psych doctor.”
“When it happened, I wasn’t particularly angry. It was something I knew I would hear eventually because I’d heard of others experiencing it,” Dr. Nayagan said in an interview. “Psychiatry is one of the fields of medicine that is often questioned in terms of legitimacy, and I knew that when I applied for psych residency,” she said.
Nevertheless, she wrote a post about the incident on Twitter, and she discovered she was far from alone. She posted her original tweet at the start of a night shift and was surprised by the number of responses when she opened her account the next day.
So far, Dr. Nayagan’s initial tweet has garnered 86 replies, 960 likes, and 35 retweets. Some clinicians reported similar experiences from both patients and colleagues, and others offered advice on how to handle such slights.
“There were a lot from people within mental health, but I also received responses from pathologists, radiologists, and others. I didn’t realize how much this experience pervaded through medicine, where a certain specialty would be told: You’re not a real doctor. So it was nice having that support,” Dr. Nayagan said.
Dr. Nayagan noted that psychiatrists encounter “specialty bias” to a greater extent than other medical professionals, and it can be a deterrent for medical students when considering psychiatry as a career path.
“It is something that has been around for a while, but it’s surprising that it’s still prevalent in 2020,” Dr. Nayagan said.
‘Busting the myth’
This type of bias is real, agreed Kaz Nelson, MD, associate professor of psychiatry and behavioral sciences and vice chair for education at the University of Minnesota, Minneapolis, and former director of the psychiatry residency program at the school for 8 years.
She said there is “discrimination” toward psychiatrists, but noted that the problem is improving.
“I think we’re better now than 2 years or 5 years or 10 years ago, so we’re heading in the right direction. But ,” Dr. Nelson said in an interview.
“Psychiatrists have the same robust biomedical training as all specialties. They completed medical school and have this additional specialty and in some cases subspecialty training that is comprehensive of biology, psychology, and social components. We call that using a biopsychosocial model,” she said.
However, she noted that, when talking with students about the field of psychiatry, there’s an awareness “that perhaps we aren’t wearing a white coat” or a stethoscope around the neck.
“That gets translated as, You are giving up medicine – you got this medical training, and you’re not using it. And I have to bust that myth and convey that it really is a privilege to be able to integrate these aspects of knowledge and expertise. It’s in no way giving up medicine – we’re practicing medicine every single day,” said Dr. Nelson.
Remnants of stigma remain
Tristan Gorrindo, MD, deputy medical director and chief of the division of education at the American Psychiatric Association, noted “remnants of stigma” still occur.
“In my mind, it’s really a misunderstanding of the relationship between mental health and physical health,” Dr. Gorrindo said in an interview.
“There’s still this notion that holds over from an old belief that the mind and the body are separate. However, the contemporary thinking in most of modern medicine is that mental illness and physical illness are really one and the same, and they influence each other in a very dynamic way all the time,” he said.
“Psychiatrists stand in both worlds. They’re really the bridge to both the psychiatric and physical aspects,” he added.
Dr. Gorrindo agreed with Dr. Nelson that this understanding has become more prevalent during past few decades.
“Within society, it’s become much more acceptable for people to talk about their mental illness and seek treatment. In a way, shedding daylight on this issue has allowed psychiatry to step forward and demonstrate its value,” he said.
“I think over time we’re going to see that stigma or specialty bias become an anachronism that will fall by the wayside as we see psychiatry more broadly integrated and accepted within the entire house of medicine,” said Dr. Gorrindo.
Taking a toll
Although some responders on Twitter advised Dr. Nayagan and other psychiatrists to “educate with a smile” when faced with specialty discrimination, Dr. Nelson noted that it’s important to recognize that experiencing “microaggressions” takes a toll.
“Anytime you’re given a signal that you aren’t really a physician or you’re not doing a real job, whether it’s based on race, gender, ethnicity, or being a psychiatrist, there is a cost. I’d say, know what you’re doing and hold your head up high, but recognize that there’s a cost for which you may need community and support from colleagues,” she said.
“Together, our culture is changing, and the future is bright. But it’s a little bit of an oversimplification to say, ‘Just brush it off.’ We must recognize that there’s a burden that comes from those forms of exclusion,” Dr. Nelson concluded.
A version of this article first appeared on Medscape.com.
During a particularly hectic day, Janeni Nayagan, MD, a first-year resident in the department of psychiatry and behavioral health at Cooper University Hospital, Camden, N.J., was taken aback – but not surprised – by a patient’s comment.
In the middle of an emergent situation, she overheard a patient say that Dr. Nayagan wasn’t a “real doctor, she’s a psych doctor.”
“When it happened, I wasn’t particularly angry. It was something I knew I would hear eventually because I’d heard of others experiencing it,” Dr. Nayagan said in an interview. “Psychiatry is one of the fields of medicine that is often questioned in terms of legitimacy, and I knew that when I applied for psych residency,” she said.
Nevertheless, she wrote a post about the incident on Twitter, and she discovered she was far from alone. She posted her original tweet at the start of a night shift and was surprised by the number of responses when she opened her account the next day.
So far, Dr. Nayagan’s initial tweet has garnered 86 replies, 960 likes, and 35 retweets. Some clinicians reported similar experiences from both patients and colleagues, and others offered advice on how to handle such slights.
“There were a lot from people within mental health, but I also received responses from pathologists, radiologists, and others. I didn’t realize how much this experience pervaded through medicine, where a certain specialty would be told: You’re not a real doctor. So it was nice having that support,” Dr. Nayagan said.
Dr. Nayagan noted that psychiatrists encounter “specialty bias” to a greater extent than other medical professionals, and it can be a deterrent for medical students when considering psychiatry as a career path.
“It is something that has been around for a while, but it’s surprising that it’s still prevalent in 2020,” Dr. Nayagan said.
‘Busting the myth’
This type of bias is real, agreed Kaz Nelson, MD, associate professor of psychiatry and behavioral sciences and vice chair for education at the University of Minnesota, Minneapolis, and former director of the psychiatry residency program at the school for 8 years.
She said there is “discrimination” toward psychiatrists, but noted that the problem is improving.
“I think we’re better now than 2 years or 5 years or 10 years ago, so we’re heading in the right direction. But ,” Dr. Nelson said in an interview.
“Psychiatrists have the same robust biomedical training as all specialties. They completed medical school and have this additional specialty and in some cases subspecialty training that is comprehensive of biology, psychology, and social components. We call that using a biopsychosocial model,” she said.
However, she noted that, when talking with students about the field of psychiatry, there’s an awareness “that perhaps we aren’t wearing a white coat” or a stethoscope around the neck.
“That gets translated as, You are giving up medicine – you got this medical training, and you’re not using it. And I have to bust that myth and convey that it really is a privilege to be able to integrate these aspects of knowledge and expertise. It’s in no way giving up medicine – we’re practicing medicine every single day,” said Dr. Nelson.
Remnants of stigma remain
Tristan Gorrindo, MD, deputy medical director and chief of the division of education at the American Psychiatric Association, noted “remnants of stigma” still occur.
“In my mind, it’s really a misunderstanding of the relationship between mental health and physical health,” Dr. Gorrindo said in an interview.
“There’s still this notion that holds over from an old belief that the mind and the body are separate. However, the contemporary thinking in most of modern medicine is that mental illness and physical illness are really one and the same, and they influence each other in a very dynamic way all the time,” he said.
“Psychiatrists stand in both worlds. They’re really the bridge to both the psychiatric and physical aspects,” he added.
Dr. Gorrindo agreed with Dr. Nelson that this understanding has become more prevalent during past few decades.
“Within society, it’s become much more acceptable for people to talk about their mental illness and seek treatment. In a way, shedding daylight on this issue has allowed psychiatry to step forward and demonstrate its value,” he said.
“I think over time we’re going to see that stigma or specialty bias become an anachronism that will fall by the wayside as we see psychiatry more broadly integrated and accepted within the entire house of medicine,” said Dr. Gorrindo.
Taking a toll
Although some responders on Twitter advised Dr. Nayagan and other psychiatrists to “educate with a smile” when faced with specialty discrimination, Dr. Nelson noted that it’s important to recognize that experiencing “microaggressions” takes a toll.
“Anytime you’re given a signal that you aren’t really a physician or you’re not doing a real job, whether it’s based on race, gender, ethnicity, or being a psychiatrist, there is a cost. I’d say, know what you’re doing and hold your head up high, but recognize that there’s a cost for which you may need community and support from colleagues,” she said.
“Together, our culture is changing, and the future is bright. But it’s a little bit of an oversimplification to say, ‘Just brush it off.’ We must recognize that there’s a burden that comes from those forms of exclusion,” Dr. Nelson concluded.
A version of this article first appeared on Medscape.com.
During a particularly hectic day, Janeni Nayagan, MD, a first-year resident in the department of psychiatry and behavioral health at Cooper University Hospital, Camden, N.J., was taken aback – but not surprised – by a patient’s comment.
In the middle of an emergent situation, she overheard a patient say that Dr. Nayagan wasn’t a “real doctor, she’s a psych doctor.”
“When it happened, I wasn’t particularly angry. It was something I knew I would hear eventually because I’d heard of others experiencing it,” Dr. Nayagan said in an interview. “Psychiatry is one of the fields of medicine that is often questioned in terms of legitimacy, and I knew that when I applied for psych residency,” she said.
Nevertheless, she wrote a post about the incident on Twitter, and she discovered she was far from alone. She posted her original tweet at the start of a night shift and was surprised by the number of responses when she opened her account the next day.
So far, Dr. Nayagan’s initial tweet has garnered 86 replies, 960 likes, and 35 retweets. Some clinicians reported similar experiences from both patients and colleagues, and others offered advice on how to handle such slights.
“There were a lot from people within mental health, but I also received responses from pathologists, radiologists, and others. I didn’t realize how much this experience pervaded through medicine, where a certain specialty would be told: You’re not a real doctor. So it was nice having that support,” Dr. Nayagan said.
Dr. Nayagan noted that psychiatrists encounter “specialty bias” to a greater extent than other medical professionals, and it can be a deterrent for medical students when considering psychiatry as a career path.
“It is something that has been around for a while, but it’s surprising that it’s still prevalent in 2020,” Dr. Nayagan said.
‘Busting the myth’
This type of bias is real, agreed Kaz Nelson, MD, associate professor of psychiatry and behavioral sciences and vice chair for education at the University of Minnesota, Minneapolis, and former director of the psychiatry residency program at the school for 8 years.
She said there is “discrimination” toward psychiatrists, but noted that the problem is improving.
“I think we’re better now than 2 years or 5 years or 10 years ago, so we’re heading in the right direction. But ,” Dr. Nelson said in an interview.
“Psychiatrists have the same robust biomedical training as all specialties. They completed medical school and have this additional specialty and in some cases subspecialty training that is comprehensive of biology, psychology, and social components. We call that using a biopsychosocial model,” she said.
However, she noted that, when talking with students about the field of psychiatry, there’s an awareness “that perhaps we aren’t wearing a white coat” or a stethoscope around the neck.
“That gets translated as, You are giving up medicine – you got this medical training, and you’re not using it. And I have to bust that myth and convey that it really is a privilege to be able to integrate these aspects of knowledge and expertise. It’s in no way giving up medicine – we’re practicing medicine every single day,” said Dr. Nelson.
Remnants of stigma remain
Tristan Gorrindo, MD, deputy medical director and chief of the division of education at the American Psychiatric Association, noted “remnants of stigma” still occur.
“In my mind, it’s really a misunderstanding of the relationship between mental health and physical health,” Dr. Gorrindo said in an interview.
“There’s still this notion that holds over from an old belief that the mind and the body are separate. However, the contemporary thinking in most of modern medicine is that mental illness and physical illness are really one and the same, and they influence each other in a very dynamic way all the time,” he said.
“Psychiatrists stand in both worlds. They’re really the bridge to both the psychiatric and physical aspects,” he added.
Dr. Gorrindo agreed with Dr. Nelson that this understanding has become more prevalent during past few decades.
“Within society, it’s become much more acceptable for people to talk about their mental illness and seek treatment. In a way, shedding daylight on this issue has allowed psychiatry to step forward and demonstrate its value,” he said.
“I think over time we’re going to see that stigma or specialty bias become an anachronism that will fall by the wayside as we see psychiatry more broadly integrated and accepted within the entire house of medicine,” said Dr. Gorrindo.
Taking a toll
Although some responders on Twitter advised Dr. Nayagan and other psychiatrists to “educate with a smile” when faced with specialty discrimination, Dr. Nelson noted that it’s important to recognize that experiencing “microaggressions” takes a toll.
“Anytime you’re given a signal that you aren’t really a physician or you’re not doing a real job, whether it’s based on race, gender, ethnicity, or being a psychiatrist, there is a cost. I’d say, know what you’re doing and hold your head up high, but recognize that there’s a cost for which you may need community and support from colleagues,” she said.
“Together, our culture is changing, and the future is bright. But it’s a little bit of an oversimplification to say, ‘Just brush it off.’ We must recognize that there’s a burden that comes from those forms of exclusion,” Dr. Nelson concluded.
A version of this article first appeared on Medscape.com.
Moderna’s COVID-19 vaccine deemed ‘highly effective,’ but further studies needed
The Food and Drug Administration’s Vaccines and Related Biological Products Advisory Committee (VRBPAC) evaluated
The panel acknowledged that further studies will be required post issuance of an Emergency Use Authorization (EUA) to collect additional data on the safety and effectiveness of the vaccine. A briefing document released by the FDA on Dec. 17, 2020, summarized interim results and included recommendations from VRBPAC on use of Moderna’s mRNA-1273 COVID-19 vaccine.
“On November 30, 2020, ModernaTX (the Sponsor) submitted an EUA request to FDA for an investigational COVID-19 vaccine (mRNA-1273) intended to prevent COVID-19,” the committee wrote.
The mRNA-1273 vaccine trial
Among 30,351 individuals aged 18 years and older, the efficacy, safety, and immunogenicity of the mRNA-1273 vaccine candidate was evaluated in a randomized, stratified, observer-blind, placebo-controlled phase 3 study. Participants were randomly assigned (1:1) to receive two injections of either 100 mcg of mRNA-1273 (n = 15,181) or saline placebo (n = 15,170) administered intramuscularly on day 1 and day 29.
The primary efficacy endpoint was efficacy of mRNA-1273 against PCR-confirmed COVID-19 with onset at least 14 days following the second dose. The primary safety endpoint was to characterize the safety of the vaccine following one or two doses.
Efficacy
Among 27,817 subjects included in the first interim analysis (data cutoff: Nov. 7, 2020), 5 cases of COVID-19 with onset at least 14 days after the second dose occurred among vaccine recipients and 90 case occurred among placebo recipients, corresponding to 94.5% vaccine efficacy (95% confidence interval, 86.5%-97.8%).
“Subgroup analyses of the primary efficacy endpoint showed similar efficacy point estimates across age groups, genders, racial and ethnic groups, and participants with medical comorbidities associated with high risk of severe COVID-19,” they reported.
Data from the final scheduled analysis of the primary efficacy endpoint (data cutoff: Nov. 21, 2020; median follow-up of >2 months after dose 2), demonstrated 94.1% vaccine efficacy (95% confidence interval, 89.3%-96.8%), corresponding to 11 cases of COVID-19 in the vaccine group and 185 cases in the placebo group.
When stratified by age, the vaccine efficacy was 95.6% (95% CI, 90.6%-97.9%) for individuals 18-64 years of age and 86.4% (95% CI, 61.4%-95.5%) for those 65 years of age or older.
In addition, results from secondary analyses indicated benefit for mRNA-1273 in preventing severe COVID-19 cases, COVID-19 in those with prior SARS-CoV-2 infection, and infection after the first dose, but these data were not conclusive.
Safety
Among 30,350 subjects included in the first interim analysis (data cutoff: Nov. 11, 2020; median follow-up of 7 weeks post second dose), no specific safety concerns were observed that would prevent issuance of an EUA.
Additional safety data (data cutoff: Nov. 25, 2020; median follow-up of 9 weeks post second dose) were provided on Dec. 7, 2020, but did not change the conclusions from the first interim analysis.
The most common vaccine-related adverse reactions were injection site pain (91.6%), fatigue (68.5%), headache (63.0%), muscle pain (59.6%), joint pain (44.8%), and chills (43.4%).
“The frequency of serious adverse events (SAEs) was low (1.0% in the mRNA-1273 arm and 1.0% in the placebo arm), without meaningful imbalances between study arms,” they reported.
Myocardial infarction (0.03%), nephrolithiasis (0.02%), and cholecystitis (0.02%) were the most common SAEs that were numerically greater in the vaccine arm than the placebo arm; however, the small number of cases does not infer a casual relationship.
“The 2-dose vaccination regimen was highly effective in preventing PCR-confirmed COVID-19 occurring at least 14 days after receipt of the second dose,” the committee wrote. “[However], it is critical to continue to gather data about the vaccine even after it is made available under EUA.”
The associated phase 3 study was sponsored by ModernaTX.
SOURCE: FDA Briefing Document: Moderna COVID-19 Vaccine. FDA Vaccines and Related Biological Products Advisory Committee. Published Dec. 17, 2020.
The Food and Drug Administration’s Vaccines and Related Biological Products Advisory Committee (VRBPAC) evaluated
The panel acknowledged that further studies will be required post issuance of an Emergency Use Authorization (EUA) to collect additional data on the safety and effectiveness of the vaccine. A briefing document released by the FDA on Dec. 17, 2020, summarized interim results and included recommendations from VRBPAC on use of Moderna’s mRNA-1273 COVID-19 vaccine.
“On November 30, 2020, ModernaTX (the Sponsor) submitted an EUA request to FDA for an investigational COVID-19 vaccine (mRNA-1273) intended to prevent COVID-19,” the committee wrote.
The mRNA-1273 vaccine trial
Among 30,351 individuals aged 18 years and older, the efficacy, safety, and immunogenicity of the mRNA-1273 vaccine candidate was evaluated in a randomized, stratified, observer-blind, placebo-controlled phase 3 study. Participants were randomly assigned (1:1) to receive two injections of either 100 mcg of mRNA-1273 (n = 15,181) or saline placebo (n = 15,170) administered intramuscularly on day 1 and day 29.
The primary efficacy endpoint was efficacy of mRNA-1273 against PCR-confirmed COVID-19 with onset at least 14 days following the second dose. The primary safety endpoint was to characterize the safety of the vaccine following one or two doses.
Efficacy
Among 27,817 subjects included in the first interim analysis (data cutoff: Nov. 7, 2020), 5 cases of COVID-19 with onset at least 14 days after the second dose occurred among vaccine recipients and 90 case occurred among placebo recipients, corresponding to 94.5% vaccine efficacy (95% confidence interval, 86.5%-97.8%).
“Subgroup analyses of the primary efficacy endpoint showed similar efficacy point estimates across age groups, genders, racial and ethnic groups, and participants with medical comorbidities associated with high risk of severe COVID-19,” they reported.
Data from the final scheduled analysis of the primary efficacy endpoint (data cutoff: Nov. 21, 2020; median follow-up of >2 months after dose 2), demonstrated 94.1% vaccine efficacy (95% confidence interval, 89.3%-96.8%), corresponding to 11 cases of COVID-19 in the vaccine group and 185 cases in the placebo group.
When stratified by age, the vaccine efficacy was 95.6% (95% CI, 90.6%-97.9%) for individuals 18-64 years of age and 86.4% (95% CI, 61.4%-95.5%) for those 65 years of age or older.
In addition, results from secondary analyses indicated benefit for mRNA-1273 in preventing severe COVID-19 cases, COVID-19 in those with prior SARS-CoV-2 infection, and infection after the first dose, but these data were not conclusive.
Safety
Among 30,350 subjects included in the first interim analysis (data cutoff: Nov. 11, 2020; median follow-up of 7 weeks post second dose), no specific safety concerns were observed that would prevent issuance of an EUA.
Additional safety data (data cutoff: Nov. 25, 2020; median follow-up of 9 weeks post second dose) were provided on Dec. 7, 2020, but did not change the conclusions from the first interim analysis.
The most common vaccine-related adverse reactions were injection site pain (91.6%), fatigue (68.5%), headache (63.0%), muscle pain (59.6%), joint pain (44.8%), and chills (43.4%).
“The frequency of serious adverse events (SAEs) was low (1.0% in the mRNA-1273 arm and 1.0% in the placebo arm), without meaningful imbalances between study arms,” they reported.
Myocardial infarction (0.03%), nephrolithiasis (0.02%), and cholecystitis (0.02%) were the most common SAEs that were numerically greater in the vaccine arm than the placebo arm; however, the small number of cases does not infer a casual relationship.
“The 2-dose vaccination regimen was highly effective in preventing PCR-confirmed COVID-19 occurring at least 14 days after receipt of the second dose,” the committee wrote. “[However], it is critical to continue to gather data about the vaccine even after it is made available under EUA.”
The associated phase 3 study was sponsored by ModernaTX.
SOURCE: FDA Briefing Document: Moderna COVID-19 Vaccine. FDA Vaccines and Related Biological Products Advisory Committee. Published Dec. 17, 2020.
The Food and Drug Administration’s Vaccines and Related Biological Products Advisory Committee (VRBPAC) evaluated
The panel acknowledged that further studies will be required post issuance of an Emergency Use Authorization (EUA) to collect additional data on the safety and effectiveness of the vaccine. A briefing document released by the FDA on Dec. 17, 2020, summarized interim results and included recommendations from VRBPAC on use of Moderna’s mRNA-1273 COVID-19 vaccine.
“On November 30, 2020, ModernaTX (the Sponsor) submitted an EUA request to FDA for an investigational COVID-19 vaccine (mRNA-1273) intended to prevent COVID-19,” the committee wrote.
The mRNA-1273 vaccine trial
Among 30,351 individuals aged 18 years and older, the efficacy, safety, and immunogenicity of the mRNA-1273 vaccine candidate was evaluated in a randomized, stratified, observer-blind, placebo-controlled phase 3 study. Participants were randomly assigned (1:1) to receive two injections of either 100 mcg of mRNA-1273 (n = 15,181) or saline placebo (n = 15,170) administered intramuscularly on day 1 and day 29.
The primary efficacy endpoint was efficacy of mRNA-1273 against PCR-confirmed COVID-19 with onset at least 14 days following the second dose. The primary safety endpoint was to characterize the safety of the vaccine following one or two doses.
Efficacy
Among 27,817 subjects included in the first interim analysis (data cutoff: Nov. 7, 2020), 5 cases of COVID-19 with onset at least 14 days after the second dose occurred among vaccine recipients and 90 case occurred among placebo recipients, corresponding to 94.5% vaccine efficacy (95% confidence interval, 86.5%-97.8%).
“Subgroup analyses of the primary efficacy endpoint showed similar efficacy point estimates across age groups, genders, racial and ethnic groups, and participants with medical comorbidities associated with high risk of severe COVID-19,” they reported.
Data from the final scheduled analysis of the primary efficacy endpoint (data cutoff: Nov. 21, 2020; median follow-up of >2 months after dose 2), demonstrated 94.1% vaccine efficacy (95% confidence interval, 89.3%-96.8%), corresponding to 11 cases of COVID-19 in the vaccine group and 185 cases in the placebo group.
When stratified by age, the vaccine efficacy was 95.6% (95% CI, 90.6%-97.9%) for individuals 18-64 years of age and 86.4% (95% CI, 61.4%-95.5%) for those 65 years of age or older.
In addition, results from secondary analyses indicated benefit for mRNA-1273 in preventing severe COVID-19 cases, COVID-19 in those with prior SARS-CoV-2 infection, and infection after the first dose, but these data were not conclusive.
Safety
Among 30,350 subjects included in the first interim analysis (data cutoff: Nov. 11, 2020; median follow-up of 7 weeks post second dose), no specific safety concerns were observed that would prevent issuance of an EUA.
Additional safety data (data cutoff: Nov. 25, 2020; median follow-up of 9 weeks post second dose) were provided on Dec. 7, 2020, but did not change the conclusions from the first interim analysis.
The most common vaccine-related adverse reactions were injection site pain (91.6%), fatigue (68.5%), headache (63.0%), muscle pain (59.6%), joint pain (44.8%), and chills (43.4%).
“The frequency of serious adverse events (SAEs) was low (1.0% in the mRNA-1273 arm and 1.0% in the placebo arm), without meaningful imbalances between study arms,” they reported.
Myocardial infarction (0.03%), nephrolithiasis (0.02%), and cholecystitis (0.02%) were the most common SAEs that were numerically greater in the vaccine arm than the placebo arm; however, the small number of cases does not infer a casual relationship.
“The 2-dose vaccination regimen was highly effective in preventing PCR-confirmed COVID-19 occurring at least 14 days after receipt of the second dose,” the committee wrote. “[However], it is critical to continue to gather data about the vaccine even after it is made available under EUA.”
The associated phase 3 study was sponsored by ModernaTX.
SOURCE: FDA Briefing Document: Moderna COVID-19 Vaccine. FDA Vaccines and Related Biological Products Advisory Committee. Published Dec. 17, 2020.
Key clinical point: The FDA’s Vaccines and Related Biological Products Advisory Committee regarded Moderna’s COVID-19 vaccine as highly effective with a favorable safety profile, based on interim phase 3 results.
Major finding: The two-dose vaccine regimen had a low frequency of serious adverse events (1.0% each in the mRNA-1273 and placebo arms, respectively) and demonstrated 94.1% (95% CI, 89.3%-96.8%) vaccine efficacy.
Study details: A briefing document summarized interim data and recommendations from the FDA’s VRBPAC on Moderna’s mRNA-1273 COVID-19 vaccine.
Disclosures: The associated phase 3 study was sponsored by ModernaTX.
Source: FDA Briefing Document: Moderna COVID-19 Vaccine. FDA Vaccines and Related Biological Products Advisory Committee. Published Dec. 17, 2020.
Threatening to burn the house down
CASE Agitated and aggressive
Mr. X, age 61, who has Alzheimer’s disease, is brought to the emergency department (ED) by his family after he is found to be confused, becomes physically aggressive with family members, and threatens to burn the house down. His family reports that earlier that day, he was paranoid that somebody was trying to kill him and he tried to leave the house. Mr. X has been experiencing visual hallucinations and delusional thoughts that made him aggressive towards his son. After an initial laboratory workup in the ED, Mr. X’s bloodwork comes back positive for mild leukocytosis, indicating the possibility of an infectious etiology. Mr. X is admitted to the hospital for further evaluation of his altered mental status.
HISTORY Decline over 2 years
This is Mr. X’s third inpatient admission for agitation and psychosis. His current medications—twice daily divalproex sodium extended release (ER), 250 mg every morning and 500 mg at every bedtime, and prazosin, 2 mg/d at bedtime—have been only partially effective. His medical history includes osteoarthritis, back pain, and heterozygous factor V Leiden (not on anticoagulation). He quit smoking tobacco several years ago and has no history of substance use. He has no family history of dementia. Previous trials of cholinesterase inhibitors, antipsychotics, and antidepressants resulted in only minimal improvement in his agitation and psychosis.
A chart review shows that 2 years before his current hospital admission, Mr. X had presented to his primary care physician with slurred speech, forgetfulness, missing words, and transient reading difficulties. His initial laboratory workup and MRI came back normal. He was placed on short-term disability due to work-related errors. He was referred to the hospital’s Memory Clinic 2 years ago, where his Mini-Mental State Exam score was 20/30, indicating mild cognitive impairment. Stroke workup was negative. Due to significant language deficits, a differential diagnosis for Alzheimer’s disease vs primary progressive aphasia vs frontotemporal dementia was made. He screened positive for amyloid PET scan, which confirmed the diagnosis of Alzheimer’s disease.
Neuropsychological testing showed similarities with logopenic variant of primary progressive aphasia, which in many cases is present in Alzheimer’s disease. Mr. X was prescribed anticholinesterase inhibitors, including donepezil, 10 mg/d, and rivastigmine patch, 9.5 mg/d; and memantine, 10 mg/d, which he could not tolerate because of adverse effects. During the next year, Mr. X deteriorated and presented to the ED a few times with significant psychotic symptoms and aggression. He had a poor response to various pharmacologic and nonpharmacologic interventions during this time.
EVALUATION Continued problematic behaviors
During his hospitalization, Mr. X continues to be agitated and paranoid and is placed in restraints. He is unable to respond to his name and cannot follow simple verbal commands. Results of his laboratory workup are within normal limits. His mild leukocytosis resolves with no active signs of infection. Psychiatry is consulted for management of his behavioral and psychological symptoms of dementia (BPSD).
Continue to: Mr. X is started on olanzapine...
Mr. X is started on olanzapine and lorazepam as needed for agitation, and his twice daily divalproex sodium ER is increased to 250 every morning and 750 mg at every bedtime. However, Mr. X remains agitated and requires restraints. Olanzapine is switched from an as-needed dose to scheduled doses of 10 mg every morning and 15 mg at every bedtime, to address his psychosis and agitation.
On Day 24 of hospitalization, Mr. X’s ammonia levels are checked and are found to be 69 µ/dL, which is high (normal range: 15 to 45 µ/dL). Divalproex sodium ER is eventually tapered and discontinued. Mr. X is started on carbamazepine, which is titrated to 400 mg twice daily and results in some improvement in his behavior. He continues to receive carbamazepine and is started on dextromethorphan-quinidine, 10 mg/d, and increased to 10 mg twice daily; however, Mr. X continues to be verbally aggressive with staff, throws food, wanders around, and tries to leave the hospital unit, so he is placed in restraints and continues to require a sitter.
[polldaddy:10698428]
The authors' observations
Dementia typically affects older adults, but its onset can occur before age 60. It is a syndrome rather than a specific illness; the most common types are Alzheimer’s disease, vascular dementia, dementia with Lewy bodies, and frontotemporal dementia. Diagnostic clarity and an evidence-based treatment plan are crucial for improving the quality of life for both the patient and their caregivers. The Table outlines the differential diagnosis of cognitive deficits. New-onset cognitive deficits warrant neuroimaging, and other testing may also be needed.
Behavioral and psychological symptoms of dementia
Noncognitive symptoms occur in 98% of individuals with dementia at some point in their disease and are often the most distressing to both caregivers and patients.1 Behavioral and psychological symptoms of dementia, including apathy, depression, sleep disorders, hallucinations, delusions, psychosis, agitation, and aggression, are exceedingly prevalent.2 Although these symptoms pose a significant burden, there are no clear published treatment guidelines; however, the American Psychiatric Association and the American Geriatric Society recommend using nonpharmacologic approaches as the first-line of treatment for patients with BPSD.3,4
Nonpharmacologic treatments
Due to the unfavorable adverse effects profiles of medications commonly used to treat dementia, nonpharmacologic treatment approaches have always played a crucial role for managing BPSD. Interventions such as music therapy, aromatherapy, art therapy, behavioral therapy, reality orientation, tailored activities, and physical exercises, have shown promising results for alleviating BPSD.5-7
Continue to: Pharmacologic therapies should be used...
Pharmacologic treatments
Pharmacologic therapies should be used when nonpharmacologic approaches are unsuccessful, or when a patient is at imminent risk to harm themselves or others.
Antipsychotics. Although there is conflicting data regarding the use of antipsychotics in older adults, these agents are the most common pharmacologic treatment for patients with BPSD. Several studies examining the efficacy of antipsychotics for treating BPSD have demonstrated an increased risk of cerebrovascular events, including stroke and death due to any cause.8 While the use of antipsychotics increases the risk of mortality in older adults, the absolute risk is still low.9
Antipsychotics used to treat BPSD include:
- Risperidone is well studied in older adults and has shown benefit for treating aggression, agitation, and psychosis.10
- Quetiapine has a favorable adverse effects profile and may help improve sleep and reduce anxiety.10
- Olanzapine. Low-dose olanzapine has been modestly effective in decreasing agitation and aggression in patients with Alzheimer’s and vascular dementias.11
- Aripiprazole has shown modest benefit in treating psychosis and agitation in patients with dementia but may be associated with insomnia or activation symptoms at lower doses.10
- Ziprasidone. Case reports have found benefit with oral and injectable forms.12
Antidepressants. In the CitAD study, which was a placebo-controlled randomized trial, citalopram titrated to a target of 30 mg/d was found to be effective in reducing BPSD.13 However, QTc prolongation limits the use of citalopram. Sertraline was studied in 1 small, randomized trial against haloperidol but showed no additional benefit.14
Mood stabilizers. In a small, randomized trial, carbamazepine was helpful for patients with BPSD who were resistant to treatment with antipsychotics, with efficacy demonstrated over 6 weeks.15 No other mood stabilizers have had significant positive results in treating BPSD.16
Anxiolytic medications. Some research suggests that the occasional use of lorazepam, as necessary, is acceptable for patients with extreme agitation or aggression when behavioral interventions or sleep aids are ineffective.17 Various case reports and case series have suggested gabapentin may be effective for BPSD.18
Prazosin. In a small randomized placebo-controlled trial, the commonly used antihypertensive agent prazosin reduced agitation and aggression in patients with Alzheimer’s dementia, at doses from 1 to 6 mg/d.19 Postural hypotension, the main adverse effect associated with prazosin, can limit its use.
Trazodone. Some research suggests trazodone can reduce irritability and aggression in patients with Alzheimer’s disease.20
Dextromethorphan/quinidine. In a 10-week phase 2 randomized clinical trial of patients with probable Alzheimer’s disease dementia, combination dextromethorphan/quinidine reduced agitation and was generally well tolerated.21
For patients such as Mr. X who do not respond to multiple pharmacologic treatments, electroconvulsive therapy (ECT) may be an option.
Continue to: Because Mr. X does not respond...
TREATMENT A trial of ECT
Because Mr. X does not respond to the standard treatment protocols, the treatment team and Mr. X’s family discuss the use of ECT to control his agitation. Consent is obtained from his legal guardian and Mr. X is medically cleared to receive ECT. Mr. X receives 3 ECT treatments per week. During the first week, Mr. X experiences post-treatment agitation and confusion. The frequency of ECT treatments is reduced to 2 treatments per week, and then 1 session per week. Mr. X starts to show improvement in his agitation and ECT is continued at 1 session per week for 7 weeks.
The authors’ observations
Electroconvulsive therapy has been an effective treatment for patients with treatment-resistant depression and has shown benefit in treating other psychiatric conditions such as acute mania, catatonia, psychotic disorders, and Parkinson’s disease.22 Its use as an off-label treatment for chronic neuropathic pain has also been well documented.23 Although ECT is not indicated for treating agitation and aggression in patients with dementia, its effectiveness for these symptoms has been discussed extensively in the literature.22,24-26
Electroconvulsive therapy treatment can be divided into 2 phases: an acute phase during which ECT is administered 2 to 3 times a week for 4 to 5 weeks, and a maintenance phase of weekly treatments for 4 weeks and then biweekly treatments for 8 weeks.26 Although extensive research supports the safe use of ECT in older adults, concerns for worsening cognitive impairment can deter patients and families from agreeing to this treatment.
Adverse effects of ECT such as headaches and postictal confusion are generally mild and transient. Severe adverse effects such as seizures, severe confusion, and delirium are uncommon.25 The number of ECT treatments required for a good effect ranges from 2 to 18, and the most common position for electrodes placement is bilateral. Outcomes can be measured by using rating scales such as the Cohen-Mansfield Agitation Inventory, Neuropsychiatric Inventory, Social Dysfunction and Aggression Scale, Clinical Global Impression scale, and Pittsford Agitation Scale.25 Obtaining consent from patients with dementia is generally not possible because these patients generally lack the capacity to make medical decisions. Clinicians should refer to their state laws regarding medical-decision making in such cases. The patient’s next of kin or medical power of attorney should be contacted, and the risks and benefits should be discussed before starting ECT.
OUTCOME Lasting improvement
Due to Mr. X’s improvement after ECT, on hospital Day 124, the restraints are removed and he no longer requires a sitter. He starts responding to his name and following simple verbal commands. Electroconvulsive therapy is tapered to every other week, and eventually stopped as his status improves. Mr. X continues to do well and is maintained on the same dosages of olanzapine, carbamazepine, and dextromethorphan-quinidine he had been receiving prior to discharge.
Related Resources
• Van den Berg JF, Kruithof HC, Kok RM, et al. Electroconvulsive therapy for agitation and aggression in dementia: a systematic review. Am J Geriatr Psychiatry. 2018;26(4):419-434.
• Kales HC, Mulsant BH, Sajatovic M. Prescribing antipsychotics in geriatric patients: Focus on dementia. Current Psychiatry. 2017;16(12):24-30.
Drug Brand Names
Aripiprazole • Abilify
Carbamazepine • Tegretol
Citalopram • Celexa
Dextromethorphan- quinidine • Nuedexta
Divalproex sodium ER • Depakote
Donepezil • Aricept
Gabapentin • Neurontin
Haloperidol • Haldol
Lorazepam • Ativan
Memantine • Namenda
Olanzapine • Zyprexa
Prazosin • Minipress
Quetiapine • Seroquel
Risperidone • Risperdal
Rivastigmine • Exelon
Sertraline • Zoloft
Trazodone • Desyrel, Oleptro
Ziprasidone • Geodon
1. Kales HC, Gitlin LN, Lyketsos CG. Management of neuropsychiatric symptoms of dementia in clinical settings: recommendations from a multidisciplinary expert panel. J Am Geriatr Soc. 2014;62(4):762-769.
2. Scarmeas N, Brandt J, Albert M, et al. Delusions and hallucinations are associated with worse outcome in Alzheimer disease. Arch Neurol. 2005;62(10):1601-1608.
3. Reus VI, Fochtmann LJ, Eyler AE, et al. The American Psychiatric Association Practice Guideline on the use of antipsychotics to treat agitation or psychosis in patients with dementia. Am J Psychiatry. 2016;173(5):543-546.
4. AGS Executive Committee. A guide to the management of psychotic disorders and neuropsychiatric symptoms of dementia in older adults. The American Geriatrics Society. Published April 2011. Accessed September 24, 2020. https://qioprogram.org/sites/default/files/AGS_Guidelines_for_Telligen.pdf
5. Yang MH, Lin LC, Wu SC, et al. Comparison of the efficacy of aroma-acupressure and aromatherapy for the treatment of dementia-associated agitation. BMC Complement Altern Med. 2015;15:93.
6. Cerga-Pashoja A, Lowery D, Bhattacharya R, et al. Evaluation of exercise on individuals with dementia and their carers: a randomised controlled trial. Trials. 2010;11:53.
7. Chen RC, Liu CL, Lin MH, et al. Non-pharmacological treatment reducing not only behavioral symptoms, but also psychotic symptoms of older adults with dementia: a prospective cohort study in Taiwan. Geriatr Gerontol Int. 2014;14(2):440-446.
8. Schneider LS, Dagerman KS, Insel P. Risk of death with atypical antipsychotic drug treatment for dementia: meta-analysis of randomized placebo-controlled trials. JAMA. 2005;294(15):1934-1943.
9. Lenzer J. FDA warns about using antipsychotic drugs for dementia. BMJ. 2005;330(7497):922.
10. Burke AD, Tariot PN. Atypical antipsychotics in the elderly: a review of therapeutic trends and clinical outcomes. Expert Opin Pharmacother. 2009;10(15):2407-2414.
11. Moretti R, Torre R, Antonello T, et al. Olanzapine as a possible treatment of behavioral symptoms in vascular dementia: risks of cerebrovascular events. J Neurol. 2005;252(10):1186-1193.
12. Cole SA, Saleem R, Shea WP, et al. Ziprasidone for agitation or psychosis in dementia: four cases. Int J Psychiatry Med. 2005;35(1):91-98.
13. Porsteinsson AP, Drye LT, Pollock BG, et al. Effect of citalopram on agitation in Alzheimer disease: the CitAD randomized clinical trial. JAMA. 2014;311(7):682-691.
14. Gaber S, Ronzoli S, Bruno A, et al. Sertraline versus small doses of haloperidol in the treatment of agitated behavior in patients with dementia. Arch Gerontol Geriatr Suppl. 2001; 7:159-162.
15. Olin JT, Fox LS, Pawluczyk S, et al. A pilot randomized trial of carbamazepine for behavioral symptoms in treatment-resistant outpatients with Alzheimer disease. Am J Geriatr Psychiatry. 2001;9(4):400-405.
16. Konovalov S, Muralee S, Tampi RR. Anticonvulsants for the treatment of behavioral and psychological symptoms of dementia: a literature review. Int Psychogeriatr. 2008;20(2):293-308.
17. Davies SJC, Burhan AM, Kim D. Sequential drug treatment algorithm for agitation and aggression in Alzheimer’s and mixed dementia. J Psychopharmacol. 2018;32(5):509-523.
18. Kim Y, Wilkins KM, Tampi RR. Use of gabapentin in the treatment of behavioural and psychological symptoms of dementia: a review of the evidence. Drugs Aging. 2008;25(3):187-196.
19. Wang LY, Shofer JB, Rohde K, et al. Prazosin for the treatment of behavioral symptoms in patients with Alzheimer disease with agitation and aggression. Am J Geriatr Psychiatry. 2009; 17(9):744-751.
20. López-Pousa S, Garre-Olmo J, Vilalta-Franch J, et al. Trazodone for Alzheimer’s disease: a naturalistic follow-up study. Arch Gerontol Geriatr. 2008;47(2):207-215.
21. Cummings JL, Lyketsos CG, Peskind ER. Effect of dextromethorphan-quinidine on agitation in patients with Alzheimer disease dementia: a randomized clinical trial. JAMA. 2015;314(12):1242-1254.
22. Ujkaj M, Davidoff DA, Seiner SJ, et al. Safety and efficacy of electroconvulsive therapy for the treatment of agitation and aggression in patients with dementia. Am J Geriatr Psychiatry. 2012;20(1):61-72.
23. McDaniel WW. Electroconvulsive therapy in complex regional pain syndromes. J ECT. 2003;19(4):226-229.
24. Glass OM, Forester BP, Hermida AP. Electroconvulsive therapy (ECT) for treating agitation in dementia (major neurocognitive disorder)–a promising option. Int Psychogeriatr. 2017;29(5):717-726.
25. Van den Berg JF, Kruithof HC, Kok RM, et al. Electroconvulsive therapy for agitation and aggression in dementia: a systematic review. Am J Geriatr Psychiatry. 2018;26(4):419-434.
26. Isserles M, Daskalakis ZJ, Kumar S, et al. Clinical effectiveness and tolerability of electroconvulsive therapy in patients with neuropsychiatric symptoms of dementia. J Alzheimers Dis. 2017;57(1):45-51.
CASE Agitated and aggressive
Mr. X, age 61, who has Alzheimer’s disease, is brought to the emergency department (ED) by his family after he is found to be confused, becomes physically aggressive with family members, and threatens to burn the house down. His family reports that earlier that day, he was paranoid that somebody was trying to kill him and he tried to leave the house. Mr. X has been experiencing visual hallucinations and delusional thoughts that made him aggressive towards his son. After an initial laboratory workup in the ED, Mr. X’s bloodwork comes back positive for mild leukocytosis, indicating the possibility of an infectious etiology. Mr. X is admitted to the hospital for further evaluation of his altered mental status.
HISTORY Decline over 2 years
This is Mr. X’s third inpatient admission for agitation and psychosis. His current medications—twice daily divalproex sodium extended release (ER), 250 mg every morning and 500 mg at every bedtime, and prazosin, 2 mg/d at bedtime—have been only partially effective. His medical history includes osteoarthritis, back pain, and heterozygous factor V Leiden (not on anticoagulation). He quit smoking tobacco several years ago and has no history of substance use. He has no family history of dementia. Previous trials of cholinesterase inhibitors, antipsychotics, and antidepressants resulted in only minimal improvement in his agitation and psychosis.
A chart review shows that 2 years before his current hospital admission, Mr. X had presented to his primary care physician with slurred speech, forgetfulness, missing words, and transient reading difficulties. His initial laboratory workup and MRI came back normal. He was placed on short-term disability due to work-related errors. He was referred to the hospital’s Memory Clinic 2 years ago, where his Mini-Mental State Exam score was 20/30, indicating mild cognitive impairment. Stroke workup was negative. Due to significant language deficits, a differential diagnosis for Alzheimer’s disease vs primary progressive aphasia vs frontotemporal dementia was made. He screened positive for amyloid PET scan, which confirmed the diagnosis of Alzheimer’s disease.
Neuropsychological testing showed similarities with logopenic variant of primary progressive aphasia, which in many cases is present in Alzheimer’s disease. Mr. X was prescribed anticholinesterase inhibitors, including donepezil, 10 mg/d, and rivastigmine patch, 9.5 mg/d; and memantine, 10 mg/d, which he could not tolerate because of adverse effects. During the next year, Mr. X deteriorated and presented to the ED a few times with significant psychotic symptoms and aggression. He had a poor response to various pharmacologic and nonpharmacologic interventions during this time.
EVALUATION Continued problematic behaviors
During his hospitalization, Mr. X continues to be agitated and paranoid and is placed in restraints. He is unable to respond to his name and cannot follow simple verbal commands. Results of his laboratory workup are within normal limits. His mild leukocytosis resolves with no active signs of infection. Psychiatry is consulted for management of his behavioral and psychological symptoms of dementia (BPSD).
Continue to: Mr. X is started on olanzapine...
Mr. X is started on olanzapine and lorazepam as needed for agitation, and his twice daily divalproex sodium ER is increased to 250 every morning and 750 mg at every bedtime. However, Mr. X remains agitated and requires restraints. Olanzapine is switched from an as-needed dose to scheduled doses of 10 mg every morning and 15 mg at every bedtime, to address his psychosis and agitation.
On Day 24 of hospitalization, Mr. X’s ammonia levels are checked and are found to be 69 µ/dL, which is high (normal range: 15 to 45 µ/dL). Divalproex sodium ER is eventually tapered and discontinued. Mr. X is started on carbamazepine, which is titrated to 400 mg twice daily and results in some improvement in his behavior. He continues to receive carbamazepine and is started on dextromethorphan-quinidine, 10 mg/d, and increased to 10 mg twice daily; however, Mr. X continues to be verbally aggressive with staff, throws food, wanders around, and tries to leave the hospital unit, so he is placed in restraints and continues to require a sitter.
[polldaddy:10698428]
The authors' observations
Dementia typically affects older adults, but its onset can occur before age 60. It is a syndrome rather than a specific illness; the most common types are Alzheimer’s disease, vascular dementia, dementia with Lewy bodies, and frontotemporal dementia. Diagnostic clarity and an evidence-based treatment plan are crucial for improving the quality of life for both the patient and their caregivers. The Table outlines the differential diagnosis of cognitive deficits. New-onset cognitive deficits warrant neuroimaging, and other testing may also be needed.

Behavioral and psychological symptoms of dementia
Noncognitive symptoms occur in 98% of individuals with dementia at some point in their disease and are often the most distressing to both caregivers and patients.1 Behavioral and psychological symptoms of dementia, including apathy, depression, sleep disorders, hallucinations, delusions, psychosis, agitation, and aggression, are exceedingly prevalent.2 Although these symptoms pose a significant burden, there are no clear published treatment guidelines; however, the American Psychiatric Association and the American Geriatric Society recommend using nonpharmacologic approaches as the first-line of treatment for patients with BPSD.3,4
Nonpharmacologic treatments
Due to the unfavorable adverse effects profiles of medications commonly used to treat dementia, nonpharmacologic treatment approaches have always played a crucial role for managing BPSD. Interventions such as music therapy, aromatherapy, art therapy, behavioral therapy, reality orientation, tailored activities, and physical exercises, have shown promising results for alleviating BPSD.5-7
Continue to: Pharmacologic therapies should be used...
Pharmacologic treatments
Pharmacologic therapies should be used when nonpharmacologic approaches are unsuccessful, or when a patient is at imminent risk to harm themselves or others.
Antipsychotics. Although there is conflicting data regarding the use of antipsychotics in older adults, these agents are the most common pharmacologic treatment for patients with BPSD. Several studies examining the efficacy of antipsychotics for treating BPSD have demonstrated an increased risk of cerebrovascular events, including stroke and death due to any cause.8 While the use of antipsychotics increases the risk of mortality in older adults, the absolute risk is still low.9
Antipsychotics used to treat BPSD include:
- Risperidone is well studied in older adults and has shown benefit for treating aggression, agitation, and psychosis.10
- Quetiapine has a favorable adverse effects profile and may help improve sleep and reduce anxiety.10
- Olanzapine. Low-dose olanzapine has been modestly effective in decreasing agitation and aggression in patients with Alzheimer’s and vascular dementias.11
- Aripiprazole has shown modest benefit in treating psychosis and agitation in patients with dementia but may be associated with insomnia or activation symptoms at lower doses.10
- Ziprasidone. Case reports have found benefit with oral and injectable forms.12
Antidepressants. In the CitAD study, which was a placebo-controlled randomized trial, citalopram titrated to a target of 30 mg/d was found to be effective in reducing BPSD.13 However, QTc prolongation limits the use of citalopram. Sertraline was studied in 1 small, randomized trial against haloperidol but showed no additional benefit.14
Mood stabilizers. In a small, randomized trial, carbamazepine was helpful for patients with BPSD who were resistant to treatment with antipsychotics, with efficacy demonstrated over 6 weeks.15 No other mood stabilizers have had significant positive results in treating BPSD.16
Anxiolytic medications. Some research suggests that the occasional use of lorazepam, as necessary, is acceptable for patients with extreme agitation or aggression when behavioral interventions or sleep aids are ineffective.17 Various case reports and case series have suggested gabapentin may be effective for BPSD.18
Prazosin. In a small randomized placebo-controlled trial, the commonly used antihypertensive agent prazosin reduced agitation and aggression in patients with Alzheimer’s dementia, at doses from 1 to 6 mg/d.19 Postural hypotension, the main adverse effect associated with prazosin, can limit its use.
Trazodone. Some research suggests trazodone can reduce irritability and aggression in patients with Alzheimer’s disease.20
Dextromethorphan/quinidine. In a 10-week phase 2 randomized clinical trial of patients with probable Alzheimer’s disease dementia, combination dextromethorphan/quinidine reduced agitation and was generally well tolerated.21
For patients such as Mr. X who do not respond to multiple pharmacologic treatments, electroconvulsive therapy (ECT) may be an option.
Continue to: Because Mr. X does not respond...
TREATMENT A trial of ECT
Because Mr. X does not respond to the standard treatment protocols, the treatment team and Mr. X’s family discuss the use of ECT to control his agitation. Consent is obtained from his legal guardian and Mr. X is medically cleared to receive ECT. Mr. X receives 3 ECT treatments per week. During the first week, Mr. X experiences post-treatment agitation and confusion. The frequency of ECT treatments is reduced to 2 treatments per week, and then 1 session per week. Mr. X starts to show improvement in his agitation and ECT is continued at 1 session per week for 7 weeks.
The authors’ observations
Electroconvulsive therapy has been an effective treatment for patients with treatment-resistant depression and has shown benefit in treating other psychiatric conditions such as acute mania, catatonia, psychotic disorders, and Parkinson’s disease.22 Its use as an off-label treatment for chronic neuropathic pain has also been well documented.23 Although ECT is not indicated for treating agitation and aggression in patients with dementia, its effectiveness for these symptoms has been discussed extensively in the literature.22,24-26
Electroconvulsive therapy treatment can be divided into 2 phases: an acute phase during which ECT is administered 2 to 3 times a week for 4 to 5 weeks, and a maintenance phase of weekly treatments for 4 weeks and then biweekly treatments for 8 weeks.26 Although extensive research supports the safe use of ECT in older adults, concerns for worsening cognitive impairment can deter patients and families from agreeing to this treatment.
Adverse effects of ECT such as headaches and postictal confusion are generally mild and transient. Severe adverse effects such as seizures, severe confusion, and delirium are uncommon.25 The number of ECT treatments required for a good effect ranges from 2 to 18, and the most common position for electrodes placement is bilateral. Outcomes can be measured by using rating scales such as the Cohen-Mansfield Agitation Inventory, Neuropsychiatric Inventory, Social Dysfunction and Aggression Scale, Clinical Global Impression scale, and Pittsford Agitation Scale.25 Obtaining consent from patients with dementia is generally not possible because these patients generally lack the capacity to make medical decisions. Clinicians should refer to their state laws regarding medical-decision making in such cases. The patient’s next of kin or medical power of attorney should be contacted, and the risks and benefits should be discussed before starting ECT.
OUTCOME Lasting improvement
Due to Mr. X’s improvement after ECT, on hospital Day 124, the restraints are removed and he no longer requires a sitter. He starts responding to his name and following simple verbal commands. Electroconvulsive therapy is tapered to every other week, and eventually stopped as his status improves. Mr. X continues to do well and is maintained on the same dosages of olanzapine, carbamazepine, and dextromethorphan-quinidine he had been receiving prior to discharge.
Related Resources
• Van den Berg JF, Kruithof HC, Kok RM, et al. Electroconvulsive therapy for agitation and aggression in dementia: a systematic review. Am J Geriatr Psychiatry. 2018;26(4):419-434.
• Kales HC, Mulsant BH, Sajatovic M. Prescribing antipsychotics in geriatric patients: Focus on dementia. Current Psychiatry. 2017;16(12):24-30.
Drug Brand Names
Aripiprazole • Abilify
Carbamazepine • Tegretol
Citalopram • Celexa
Dextromethorphan- quinidine • Nuedexta
Divalproex sodium ER • Depakote
Donepezil • Aricept
Gabapentin • Neurontin
Haloperidol • Haldol
Lorazepam • Ativan
Memantine • Namenda
Olanzapine • Zyprexa
Prazosin • Minipress
Quetiapine • Seroquel
Risperidone • Risperdal
Rivastigmine • Exelon
Sertraline • Zoloft
Trazodone • Desyrel, Oleptro
Ziprasidone • Geodon
CASE Agitated and aggressive
Mr. X, age 61, who has Alzheimer’s disease, is brought to the emergency department (ED) by his family after he is found to be confused, becomes physically aggressive with family members, and threatens to burn the house down. His family reports that earlier that day, he was paranoid that somebody was trying to kill him and he tried to leave the house. Mr. X has been experiencing visual hallucinations and delusional thoughts that made him aggressive towards his son. After an initial laboratory workup in the ED, Mr. X’s bloodwork comes back positive for mild leukocytosis, indicating the possibility of an infectious etiology. Mr. X is admitted to the hospital for further evaluation of his altered mental status.
HISTORY Decline over 2 years
This is Mr. X’s third inpatient admission for agitation and psychosis. His current medications—twice daily divalproex sodium extended release (ER), 250 mg every morning and 500 mg at every bedtime, and prazosin, 2 mg/d at bedtime—have been only partially effective. His medical history includes osteoarthritis, back pain, and heterozygous factor V Leiden (not on anticoagulation). He quit smoking tobacco several years ago and has no history of substance use. He has no family history of dementia. Previous trials of cholinesterase inhibitors, antipsychotics, and antidepressants resulted in only minimal improvement in his agitation and psychosis.
A chart review shows that 2 years before his current hospital admission, Mr. X had presented to his primary care physician with slurred speech, forgetfulness, missing words, and transient reading difficulties. His initial laboratory workup and MRI came back normal. He was placed on short-term disability due to work-related errors. He was referred to the hospital’s Memory Clinic 2 years ago, where his Mini-Mental State Exam score was 20/30, indicating mild cognitive impairment. Stroke workup was negative. Due to significant language deficits, a differential diagnosis for Alzheimer’s disease vs primary progressive aphasia vs frontotemporal dementia was made. He screened positive for amyloid PET scan, which confirmed the diagnosis of Alzheimer’s disease.
Neuropsychological testing showed similarities with logopenic variant of primary progressive aphasia, which in many cases is present in Alzheimer’s disease. Mr. X was prescribed anticholinesterase inhibitors, including donepezil, 10 mg/d, and rivastigmine patch, 9.5 mg/d; and memantine, 10 mg/d, which he could not tolerate because of adverse effects. During the next year, Mr. X deteriorated and presented to the ED a few times with significant psychotic symptoms and aggression. He had a poor response to various pharmacologic and nonpharmacologic interventions during this time.
EVALUATION Continued problematic behaviors
During his hospitalization, Mr. X continues to be agitated and paranoid and is placed in restraints. He is unable to respond to his name and cannot follow simple verbal commands. Results of his laboratory workup are within normal limits. His mild leukocytosis resolves with no active signs of infection. Psychiatry is consulted for management of his behavioral and psychological symptoms of dementia (BPSD).
Continue to: Mr. X is started on olanzapine...
Mr. X is started on olanzapine and lorazepam as needed for agitation, and his twice daily divalproex sodium ER is increased to 250 every morning and 750 mg at every bedtime. However, Mr. X remains agitated and requires restraints. Olanzapine is switched from an as-needed dose to scheduled doses of 10 mg every morning and 15 mg at every bedtime, to address his psychosis and agitation.
On Day 24 of hospitalization, Mr. X’s ammonia levels are checked and are found to be 69 µ/dL, which is high (normal range: 15 to 45 µ/dL). Divalproex sodium ER is eventually tapered and discontinued. Mr. X is started on carbamazepine, which is titrated to 400 mg twice daily and results in some improvement in his behavior. He continues to receive carbamazepine and is started on dextromethorphan-quinidine, 10 mg/d, and increased to 10 mg twice daily; however, Mr. X continues to be verbally aggressive with staff, throws food, wanders around, and tries to leave the hospital unit, so he is placed in restraints and continues to require a sitter.
[polldaddy:10698428]
The authors' observations
Dementia typically affects older adults, but its onset can occur before age 60. It is a syndrome rather than a specific illness; the most common types are Alzheimer’s disease, vascular dementia, dementia with Lewy bodies, and frontotemporal dementia. Diagnostic clarity and an evidence-based treatment plan are crucial for improving the quality of life for both the patient and their caregivers. The Table outlines the differential diagnosis of cognitive deficits. New-onset cognitive deficits warrant neuroimaging, and other testing may also be needed.

Behavioral and psychological symptoms of dementia
Noncognitive symptoms occur in 98% of individuals with dementia at some point in their disease and are often the most distressing to both caregivers and patients.1 Behavioral and psychological symptoms of dementia, including apathy, depression, sleep disorders, hallucinations, delusions, psychosis, agitation, and aggression, are exceedingly prevalent.2 Although these symptoms pose a significant burden, there are no clear published treatment guidelines; however, the American Psychiatric Association and the American Geriatric Society recommend using nonpharmacologic approaches as the first-line of treatment for patients with BPSD.3,4
Nonpharmacologic treatments
Due to the unfavorable adverse effects profiles of medications commonly used to treat dementia, nonpharmacologic treatment approaches have always played a crucial role for managing BPSD. Interventions such as music therapy, aromatherapy, art therapy, behavioral therapy, reality orientation, tailored activities, and physical exercises, have shown promising results for alleviating BPSD.5-7
Continue to: Pharmacologic therapies should be used...
Pharmacologic treatments
Pharmacologic therapies should be used when nonpharmacologic approaches are unsuccessful, or when a patient is at imminent risk to harm themselves or others.
Antipsychotics. Although there is conflicting data regarding the use of antipsychotics in older adults, these agents are the most common pharmacologic treatment for patients with BPSD. Several studies examining the efficacy of antipsychotics for treating BPSD have demonstrated an increased risk of cerebrovascular events, including stroke and death due to any cause.8 While the use of antipsychotics increases the risk of mortality in older adults, the absolute risk is still low.9
Antipsychotics used to treat BPSD include:
- Risperidone is well studied in older adults and has shown benefit for treating aggression, agitation, and psychosis.10
- Quetiapine has a favorable adverse effects profile and may help improve sleep and reduce anxiety.10
- Olanzapine. Low-dose olanzapine has been modestly effective in decreasing agitation and aggression in patients with Alzheimer’s and vascular dementias.11
- Aripiprazole has shown modest benefit in treating psychosis and agitation in patients with dementia but may be associated with insomnia or activation symptoms at lower doses.10
- Ziprasidone. Case reports have found benefit with oral and injectable forms.12
Antidepressants. In the CitAD study, which was a placebo-controlled randomized trial, citalopram titrated to a target of 30 mg/d was found to be effective in reducing BPSD.13 However, QTc prolongation limits the use of citalopram. Sertraline was studied in 1 small, randomized trial against haloperidol but showed no additional benefit.14
Mood stabilizers. In a small, randomized trial, carbamazepine was helpful for patients with BPSD who were resistant to treatment with antipsychotics, with efficacy demonstrated over 6 weeks.15 No other mood stabilizers have had significant positive results in treating BPSD.16
Anxiolytic medications. Some research suggests that the occasional use of lorazepam, as necessary, is acceptable for patients with extreme agitation or aggression when behavioral interventions or sleep aids are ineffective.17 Various case reports and case series have suggested gabapentin may be effective for BPSD.18
Prazosin. In a small randomized placebo-controlled trial, the commonly used antihypertensive agent prazosin reduced agitation and aggression in patients with Alzheimer’s dementia, at doses from 1 to 6 mg/d.19 Postural hypotension, the main adverse effect associated with prazosin, can limit its use.
Trazodone. Some research suggests trazodone can reduce irritability and aggression in patients with Alzheimer’s disease.20
Dextromethorphan/quinidine. In a 10-week phase 2 randomized clinical trial of patients with probable Alzheimer’s disease dementia, combination dextromethorphan/quinidine reduced agitation and was generally well tolerated.21
For patients such as Mr. X who do not respond to multiple pharmacologic treatments, electroconvulsive therapy (ECT) may be an option.
Continue to: Because Mr. X does not respond...
TREATMENT A trial of ECT
Because Mr. X does not respond to the standard treatment protocols, the treatment team and Mr. X’s family discuss the use of ECT to control his agitation. Consent is obtained from his legal guardian and Mr. X is medically cleared to receive ECT. Mr. X receives 3 ECT treatments per week. During the first week, Mr. X experiences post-treatment agitation and confusion. The frequency of ECT treatments is reduced to 2 treatments per week, and then 1 session per week. Mr. X starts to show improvement in his agitation and ECT is continued at 1 session per week for 7 weeks.
The authors’ observations
Electroconvulsive therapy has been an effective treatment for patients with treatment-resistant depression and has shown benefit in treating other psychiatric conditions such as acute mania, catatonia, psychotic disorders, and Parkinson’s disease.22 Its use as an off-label treatment for chronic neuropathic pain has also been well documented.23 Although ECT is not indicated for treating agitation and aggression in patients with dementia, its effectiveness for these symptoms has been discussed extensively in the literature.22,24-26
Electroconvulsive therapy treatment can be divided into 2 phases: an acute phase during which ECT is administered 2 to 3 times a week for 4 to 5 weeks, and a maintenance phase of weekly treatments for 4 weeks and then biweekly treatments for 8 weeks.26 Although extensive research supports the safe use of ECT in older adults, concerns for worsening cognitive impairment can deter patients and families from agreeing to this treatment.
Adverse effects of ECT such as headaches and postictal confusion are generally mild and transient. Severe adverse effects such as seizures, severe confusion, and delirium are uncommon.25 The number of ECT treatments required for a good effect ranges from 2 to 18, and the most common position for electrodes placement is bilateral. Outcomes can be measured by using rating scales such as the Cohen-Mansfield Agitation Inventory, Neuropsychiatric Inventory, Social Dysfunction and Aggression Scale, Clinical Global Impression scale, and Pittsford Agitation Scale.25 Obtaining consent from patients with dementia is generally not possible because these patients generally lack the capacity to make medical decisions. Clinicians should refer to their state laws regarding medical-decision making in such cases. The patient’s next of kin or medical power of attorney should be contacted, and the risks and benefits should be discussed before starting ECT.
OUTCOME Lasting improvement
Due to Mr. X’s improvement after ECT, on hospital Day 124, the restraints are removed and he no longer requires a sitter. He starts responding to his name and following simple verbal commands. Electroconvulsive therapy is tapered to every other week, and eventually stopped as his status improves. Mr. X continues to do well and is maintained on the same dosages of olanzapine, carbamazepine, and dextromethorphan-quinidine he had been receiving prior to discharge.
Related Resources
• Van den Berg JF, Kruithof HC, Kok RM, et al. Electroconvulsive therapy for agitation and aggression in dementia: a systematic review. Am J Geriatr Psychiatry. 2018;26(4):419-434.
• Kales HC, Mulsant BH, Sajatovic M. Prescribing antipsychotics in geriatric patients: Focus on dementia. Current Psychiatry. 2017;16(12):24-30.
Drug Brand Names
Aripiprazole • Abilify
Carbamazepine • Tegretol
Citalopram • Celexa
Dextromethorphan- quinidine • Nuedexta
Divalproex sodium ER • Depakote
Donepezil • Aricept
Gabapentin • Neurontin
Haloperidol • Haldol
Lorazepam • Ativan
Memantine • Namenda
Olanzapine • Zyprexa
Prazosin • Minipress
Quetiapine • Seroquel
Risperidone • Risperdal
Rivastigmine • Exelon
Sertraline • Zoloft
Trazodone • Desyrel, Oleptro
Ziprasidone • Geodon
1. Kales HC, Gitlin LN, Lyketsos CG. Management of neuropsychiatric symptoms of dementia in clinical settings: recommendations from a multidisciplinary expert panel. J Am Geriatr Soc. 2014;62(4):762-769.
2. Scarmeas N, Brandt J, Albert M, et al. Delusions and hallucinations are associated with worse outcome in Alzheimer disease. Arch Neurol. 2005;62(10):1601-1608.
3. Reus VI, Fochtmann LJ, Eyler AE, et al. The American Psychiatric Association Practice Guideline on the use of antipsychotics to treat agitation or psychosis in patients with dementia. Am J Psychiatry. 2016;173(5):543-546.
4. AGS Executive Committee. A guide to the management of psychotic disorders and neuropsychiatric symptoms of dementia in older adults. The American Geriatrics Society. Published April 2011. Accessed September 24, 2020. https://qioprogram.org/sites/default/files/AGS_Guidelines_for_Telligen.pdf
5. Yang MH, Lin LC, Wu SC, et al. Comparison of the efficacy of aroma-acupressure and aromatherapy for the treatment of dementia-associated agitation. BMC Complement Altern Med. 2015;15:93.
6. Cerga-Pashoja A, Lowery D, Bhattacharya R, et al. Evaluation of exercise on individuals with dementia and their carers: a randomised controlled trial. Trials. 2010;11:53.
7. Chen RC, Liu CL, Lin MH, et al. Non-pharmacological treatment reducing not only behavioral symptoms, but also psychotic symptoms of older adults with dementia: a prospective cohort study in Taiwan. Geriatr Gerontol Int. 2014;14(2):440-446.
8. Schneider LS, Dagerman KS, Insel P. Risk of death with atypical antipsychotic drug treatment for dementia: meta-analysis of randomized placebo-controlled trials. JAMA. 2005;294(15):1934-1943.
9. Lenzer J. FDA warns about using antipsychotic drugs for dementia. BMJ. 2005;330(7497):922.
10. Burke AD, Tariot PN. Atypical antipsychotics in the elderly: a review of therapeutic trends and clinical outcomes. Expert Opin Pharmacother. 2009;10(15):2407-2414.
11. Moretti R, Torre R, Antonello T, et al. Olanzapine as a possible treatment of behavioral symptoms in vascular dementia: risks of cerebrovascular events. J Neurol. 2005;252(10):1186-1193.
12. Cole SA, Saleem R, Shea WP, et al. Ziprasidone for agitation or psychosis in dementia: four cases. Int J Psychiatry Med. 2005;35(1):91-98.
13. Porsteinsson AP, Drye LT, Pollock BG, et al. Effect of citalopram on agitation in Alzheimer disease: the CitAD randomized clinical trial. JAMA. 2014;311(7):682-691.
14. Gaber S, Ronzoli S, Bruno A, et al. Sertraline versus small doses of haloperidol in the treatment of agitated behavior in patients with dementia. Arch Gerontol Geriatr Suppl. 2001; 7:159-162.
15. Olin JT, Fox LS, Pawluczyk S, et al. A pilot randomized trial of carbamazepine for behavioral symptoms in treatment-resistant outpatients with Alzheimer disease. Am J Geriatr Psychiatry. 2001;9(4):400-405.
16. Konovalov S, Muralee S, Tampi RR. Anticonvulsants for the treatment of behavioral and psychological symptoms of dementia: a literature review. Int Psychogeriatr. 2008;20(2):293-308.
17. Davies SJC, Burhan AM, Kim D. Sequential drug treatment algorithm for agitation and aggression in Alzheimer’s and mixed dementia. J Psychopharmacol. 2018;32(5):509-523.
18. Kim Y, Wilkins KM, Tampi RR. Use of gabapentin in the treatment of behavioural and psychological symptoms of dementia: a review of the evidence. Drugs Aging. 2008;25(3):187-196.
19. Wang LY, Shofer JB, Rohde K, et al. Prazosin for the treatment of behavioral symptoms in patients with Alzheimer disease with agitation and aggression. Am J Geriatr Psychiatry. 2009; 17(9):744-751.
20. López-Pousa S, Garre-Olmo J, Vilalta-Franch J, et al. Trazodone for Alzheimer’s disease: a naturalistic follow-up study. Arch Gerontol Geriatr. 2008;47(2):207-215.
21. Cummings JL, Lyketsos CG, Peskind ER. Effect of dextromethorphan-quinidine on agitation in patients with Alzheimer disease dementia: a randomized clinical trial. JAMA. 2015;314(12):1242-1254.
22. Ujkaj M, Davidoff DA, Seiner SJ, et al. Safety and efficacy of electroconvulsive therapy for the treatment of agitation and aggression in patients with dementia. Am J Geriatr Psychiatry. 2012;20(1):61-72.
23. McDaniel WW. Electroconvulsive therapy in complex regional pain syndromes. J ECT. 2003;19(4):226-229.
24. Glass OM, Forester BP, Hermida AP. Electroconvulsive therapy (ECT) for treating agitation in dementia (major neurocognitive disorder)–a promising option. Int Psychogeriatr. 2017;29(5):717-726.
25. Van den Berg JF, Kruithof HC, Kok RM, et al. Electroconvulsive therapy for agitation and aggression in dementia: a systematic review. Am J Geriatr Psychiatry. 2018;26(4):419-434.
26. Isserles M, Daskalakis ZJ, Kumar S, et al. Clinical effectiveness and tolerability of electroconvulsive therapy in patients with neuropsychiatric symptoms of dementia. J Alzheimers Dis. 2017;57(1):45-51.
1. Kales HC, Gitlin LN, Lyketsos CG. Management of neuropsychiatric symptoms of dementia in clinical settings: recommendations from a multidisciplinary expert panel. J Am Geriatr Soc. 2014;62(4):762-769.
2. Scarmeas N, Brandt J, Albert M, et al. Delusions and hallucinations are associated with worse outcome in Alzheimer disease. Arch Neurol. 2005;62(10):1601-1608.
3. Reus VI, Fochtmann LJ, Eyler AE, et al. The American Psychiatric Association Practice Guideline on the use of antipsychotics to treat agitation or psychosis in patients with dementia. Am J Psychiatry. 2016;173(5):543-546.
4. AGS Executive Committee. A guide to the management of psychotic disorders and neuropsychiatric symptoms of dementia in older adults. The American Geriatrics Society. Published April 2011. Accessed September 24, 2020. https://qioprogram.org/sites/default/files/AGS_Guidelines_for_Telligen.pdf
5. Yang MH, Lin LC, Wu SC, et al. Comparison of the efficacy of aroma-acupressure and aromatherapy for the treatment of dementia-associated agitation. BMC Complement Altern Med. 2015;15:93.
6. Cerga-Pashoja A, Lowery D, Bhattacharya R, et al. Evaluation of exercise on individuals with dementia and their carers: a randomised controlled trial. Trials. 2010;11:53.
7. Chen RC, Liu CL, Lin MH, et al. Non-pharmacological treatment reducing not only behavioral symptoms, but also psychotic symptoms of older adults with dementia: a prospective cohort study in Taiwan. Geriatr Gerontol Int. 2014;14(2):440-446.
8. Schneider LS, Dagerman KS, Insel P. Risk of death with atypical antipsychotic drug treatment for dementia: meta-analysis of randomized placebo-controlled trials. JAMA. 2005;294(15):1934-1943.
9. Lenzer J. FDA warns about using antipsychotic drugs for dementia. BMJ. 2005;330(7497):922.
10. Burke AD, Tariot PN. Atypical antipsychotics in the elderly: a review of therapeutic trends and clinical outcomes. Expert Opin Pharmacother. 2009;10(15):2407-2414.
11. Moretti R, Torre R, Antonello T, et al. Olanzapine as a possible treatment of behavioral symptoms in vascular dementia: risks of cerebrovascular events. J Neurol. 2005;252(10):1186-1193.
12. Cole SA, Saleem R, Shea WP, et al. Ziprasidone for agitation or psychosis in dementia: four cases. Int J Psychiatry Med. 2005;35(1):91-98.
13. Porsteinsson AP, Drye LT, Pollock BG, et al. Effect of citalopram on agitation in Alzheimer disease: the CitAD randomized clinical trial. JAMA. 2014;311(7):682-691.
14. Gaber S, Ronzoli S, Bruno A, et al. Sertraline versus small doses of haloperidol in the treatment of agitated behavior in patients with dementia. Arch Gerontol Geriatr Suppl. 2001; 7:159-162.
15. Olin JT, Fox LS, Pawluczyk S, et al. A pilot randomized trial of carbamazepine for behavioral symptoms in treatment-resistant outpatients with Alzheimer disease. Am J Geriatr Psychiatry. 2001;9(4):400-405.
16. Konovalov S, Muralee S, Tampi RR. Anticonvulsants for the treatment of behavioral and psychological symptoms of dementia: a literature review. Int Psychogeriatr. 2008;20(2):293-308.
17. Davies SJC, Burhan AM, Kim D. Sequential drug treatment algorithm for agitation and aggression in Alzheimer’s and mixed dementia. J Psychopharmacol. 2018;32(5):509-523.
18. Kim Y, Wilkins KM, Tampi RR. Use of gabapentin in the treatment of behavioural and psychological symptoms of dementia: a review of the evidence. Drugs Aging. 2008;25(3):187-196.
19. Wang LY, Shofer JB, Rohde K, et al. Prazosin for the treatment of behavioral symptoms in patients with Alzheimer disease with agitation and aggression. Am J Geriatr Psychiatry. 2009; 17(9):744-751.
20. López-Pousa S, Garre-Olmo J, Vilalta-Franch J, et al. Trazodone for Alzheimer’s disease: a naturalistic follow-up study. Arch Gerontol Geriatr. 2008;47(2):207-215.
21. Cummings JL, Lyketsos CG, Peskind ER. Effect of dextromethorphan-quinidine on agitation in patients with Alzheimer disease dementia: a randomized clinical trial. JAMA. 2015;314(12):1242-1254.
22. Ujkaj M, Davidoff DA, Seiner SJ, et al. Safety and efficacy of electroconvulsive therapy for the treatment of agitation and aggression in patients with dementia. Am J Geriatr Psychiatry. 2012;20(1):61-72.
23. McDaniel WW. Electroconvulsive therapy in complex regional pain syndromes. J ECT. 2003;19(4):226-229.
24. Glass OM, Forester BP, Hermida AP. Electroconvulsive therapy (ECT) for treating agitation in dementia (major neurocognitive disorder)–a promising option. Int Psychogeriatr. 2017;29(5):717-726.
25. Van den Berg JF, Kruithof HC, Kok RM, et al. Electroconvulsive therapy for agitation and aggression in dementia: a systematic review. Am J Geriatr Psychiatry. 2018;26(4):419-434.
26. Isserles M, Daskalakis ZJ, Kumar S, et al. Clinical effectiveness and tolerability of electroconvulsive therapy in patients with neuropsychiatric symptoms of dementia. J Alzheimers Dis. 2017;57(1):45-51.
Child abuse visits to EDs declined in 2020, but not admissions
but the visits in 2020 were significantly more likely to result in hospitalization, based on analysis of a national ED database.
The number of ED visits involving child abuse and neglect was down by 53% during the 4-week period from March 29 to April 25, 2020, compared with the 4 weeks from March 31 to April 27, 2019. The proportion of those ED visits that ended in hospitalizations, however, increased from 2.1% in 2019 to 3.2% in 2020, Elizabeth Swedo, MD, and associates at the Centers for Disease Control and Prevention said in the Morbidity and Mortality Weekly Report.
“ED visits related to suspected or confirmed child abuse and neglect decreased beginning the week of March 15, 2020, coinciding with the declaration of a national emergency related to COVID-19 and implementation of community mitigation measures,” they wrote.
An earlier study involving the same database (the National Syndromic Surveillance Program) showed that, over the two same 4-week periods, the volume of all ED visits in 2020 was down 72% for children aged 10 years and younger and 71% for those aged 11-14 years.
In the current study, however, all age subgroups had significant increases in hospital admissions. The proportion of ED visits related to child abuse and neglect that resulted in hospitalization rose from 3.5% in 2019 to 5.3% in 2020 among ages 0-4 years, 0.7% to 1.3% for ages 5-11 years, and 1.6% to 2.2% for adolescents aged 12-17, Dr. Swedo and associates reported.
The absence of a corresponding drop in hospitalizations may be tied to risk factors related to the pandemic, “such as loss of income, increased stress related to parental child care and schooling responsibilities, and increased substance use and mental health conditions among adults,” the investigators added.
The National Syndromic Surveillance Program receives daily data from 3,310 EDs in 47 states, but the number of facilities meeting the investigators’ criteria averaged 2,970 a week for the 8 weeks of the study period.
SOURCE: Swedo E et al. MMWR. 2020 Dec. 11;69(49):1841-7.
but the visits in 2020 were significantly more likely to result in hospitalization, based on analysis of a national ED database.

The number of ED visits involving child abuse and neglect was down by 53% during the 4-week period from March 29 to April 25, 2020, compared with the 4 weeks from March 31 to April 27, 2019. The proportion of those ED visits that ended in hospitalizations, however, increased from 2.1% in 2019 to 3.2% in 2020, Elizabeth Swedo, MD, and associates at the Centers for Disease Control and Prevention said in the Morbidity and Mortality Weekly Report.
“ED visits related to suspected or confirmed child abuse and neglect decreased beginning the week of March 15, 2020, coinciding with the declaration of a national emergency related to COVID-19 and implementation of community mitigation measures,” they wrote.
An earlier study involving the same database (the National Syndromic Surveillance Program) showed that, over the two same 4-week periods, the volume of all ED visits in 2020 was down 72% for children aged 10 years and younger and 71% for those aged 11-14 years.
In the current study, however, all age subgroups had significant increases in hospital admissions. The proportion of ED visits related to child abuse and neglect that resulted in hospitalization rose from 3.5% in 2019 to 5.3% in 2020 among ages 0-4 years, 0.7% to 1.3% for ages 5-11 years, and 1.6% to 2.2% for adolescents aged 12-17, Dr. Swedo and associates reported.
The absence of a corresponding drop in hospitalizations may be tied to risk factors related to the pandemic, “such as loss of income, increased stress related to parental child care and schooling responsibilities, and increased substance use and mental health conditions among adults,” the investigators added.
The National Syndromic Surveillance Program receives daily data from 3,310 EDs in 47 states, but the number of facilities meeting the investigators’ criteria averaged 2,970 a week for the 8 weeks of the study period.
SOURCE: Swedo E et al. MMWR. 2020 Dec. 11;69(49):1841-7.
but the visits in 2020 were significantly more likely to result in hospitalization, based on analysis of a national ED database.

The number of ED visits involving child abuse and neglect was down by 53% during the 4-week period from March 29 to April 25, 2020, compared with the 4 weeks from March 31 to April 27, 2019. The proportion of those ED visits that ended in hospitalizations, however, increased from 2.1% in 2019 to 3.2% in 2020, Elizabeth Swedo, MD, and associates at the Centers for Disease Control and Prevention said in the Morbidity and Mortality Weekly Report.
“ED visits related to suspected or confirmed child abuse and neglect decreased beginning the week of March 15, 2020, coinciding with the declaration of a national emergency related to COVID-19 and implementation of community mitigation measures,” they wrote.
An earlier study involving the same database (the National Syndromic Surveillance Program) showed that, over the two same 4-week periods, the volume of all ED visits in 2020 was down 72% for children aged 10 years and younger and 71% for those aged 11-14 years.
In the current study, however, all age subgroups had significant increases in hospital admissions. The proportion of ED visits related to child abuse and neglect that resulted in hospitalization rose from 3.5% in 2019 to 5.3% in 2020 among ages 0-4 years, 0.7% to 1.3% for ages 5-11 years, and 1.6% to 2.2% for adolescents aged 12-17, Dr. Swedo and associates reported.
The absence of a corresponding drop in hospitalizations may be tied to risk factors related to the pandemic, “such as loss of income, increased stress related to parental child care and schooling responsibilities, and increased substance use and mental health conditions among adults,” the investigators added.
The National Syndromic Surveillance Program receives daily data from 3,310 EDs in 47 states, but the number of facilities meeting the investigators’ criteria averaged 2,970 a week for the 8 weeks of the study period.
SOURCE: Swedo E et al. MMWR. 2020 Dec. 11;69(49):1841-7.
FROM MMWR