User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
nav[contains(@class, 'nav-ce-stack nav-ce-stack__large-screen')]
header[@id='header']
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'main-prefix')]
footer[@id='footer']
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
div[contains(@class, 'view-medstat-quiz-listing-panes')]
Let’s ‘cancel’ these obsolete terms in DSM
Psychiatry has made significant scientific advances over the past century. However, it is still saddled with archaic terms, with pejorative connotations, disguised as official medical diagnoses. It is time to “cancel” those terms and replace them with ones that are neutral and have not accumulated baggage.
This process of “creative destruction” of psychiatric terminology is long overdue. It is frankly disturbing that the psychiatric jargon used around the time that the American Psychiatric Association was established 175 years ago (1844) is now considered insults and epithets. We no longer work in “lunatic asylums for the insane,” and our patients with intellectual disabilities are no longer classified as “morons,” “idiots,” or “imbeciles.” Such “diagnoses” have certainly contributed to the stigma of psychiatric brain disorders. Even the noble word “asylum” has acquired a negative valence because in the past it referred to hospitals that housed persons with serious mental illness.
Thankfully, some of the outrageous terms fabricated during the condemnable and dark era of slavery 2 centuries ago were never adopted by organized psychiatry. The absurd diagnosis of “negritude,” whose tenet was that black skin is a disease curable by whitening the skin, was “invented” by none other than Benjamin Rush, the Father of Psychiatry, whose conflicted soul was depicted by concomitantly owning a slave and positioning himself as an ardent abolitionist!
Terms that need to be replaced
Fast-forward to the modern era and consider the following:
Borderline personality disorder. It is truly tragic how this confusing and non-scientific term is used as an official diagnosis for a set of seriously ill persons. It is loaded with obloquy, indignity, and derision that completely ignore the tumult, self-harm, and disability with which patients who carry this label are burdened throughout their lives, despite being intelligent. This is a serious brain disorder that has been shown to be highly genetic and is characterized by many well-established structural brain abnormalities that have been documented in neuroimaging studies.1,2 Borderline personality should not be classified as a personality disorder but as an illness with multiple signs and symptoms, including mood lability, anger, impulsivity, self-cutting, suicidal urges, feelings of abandonment, and micro-psychotic episodes. A more clinically accurate term should be coined very soon to replace borderline personality, which should be discarded to the trash heap of obsolete psychiatric terms, and no longer inflicted on patients.
Neurosis. What is the justification for continuing to use the term “neurotic” for a person who has an anxiety disorder? Is it used because Jung and Freud propagated the term “neurosis” (after it was coined by William Cullen in 1769)? Neurosis has degenerated from a psychiatric diagnosis to a scornful snub that must never be used for any patient.
Schizophrenia. This diagnosis, coined by Eugen Bleuler to replace the narrow and pessimistic “dementia praecox” proposed by Emil Kraepelin in the 1920s, initially seemed to be a neutral description of a thought disorder (split associations, not split personality). Bleuler was perceptive enough to call his book Dementia Praecox or the Group of Schizophrenias, which is consistent with the modern scientific research that confirms schizophrenia is a very heterogeneous syndrome with hundreds of genetic and environmental biotypes with a similar phenotype but a wide range of severity, treatment response, and functional outcomes. However, in subsequent decades, schizophrenia became one of the most demeaning labels in psychiatry, casting a shadow of hopelessness and disability on the people who have this serious neurologic condition with many psychiatric symptoms. The term that should replace schizophrenia should be no more degrading than stroke, multiple sclerosis, or myocardial infarction.
Continue to: Over the past 15 years...
Over the past 15 years, an expanding group of schizophrenia experts have agreed that this term must be changed to one that reflects the core features of this syndrome, and have proposed terms such as “salience syndrome,” “psychosis-spectrum,” and “reality distortion and cognitive impairment disorder.”3 In fact, several countries have already adopted a new official diagnosis for schizophrenia.4 Japan now uses the term “integration disorder,” which has significantly reduced the stigma of this brain disorder.5 South Korea changed the name to “attunement disorder.” Hong Kong and Taiwan now use “dysfunction of thought and perception.” Some researchers recommend calling schizophrenia “Bleuler’s syndrome,” a neutral eponymous designation.
One of the most irritating things about the term schizophrenia is the widespread misconception that it means “split personality.” This prompts some sports announcers to call a football team “schizophrenic” if they play well in the first half and badly in the second. The stock market is labeled “schizophrenic” if it goes up one day and way down on the next. No other medical term is misused by the media as often as the term schizophrenia.
Narcissistic personality disorder. The origin of this diagnostic category is the concept of “malignant narcissism” coined by Erich Fromm in 1964, which he designated as “the quintessence of evil.” I strongly object to implying that evil is part of any psychiatric diagnosis. Numerous studies have found structural brain abnormalities (in both gray and white matter) in patients diagnosed with psychopathic traits.6 Later, malignant narcissism was reframed as narcissistic personality disorder in 1971 by Herbert Rosenfeld. Although malignant narcissism was never accepted by either the DSM or the International Classification of Diseases, narcissistic personality disorder has been included in the DSM for the past few decades. This diagnosis reeks of disparagement and negativity. Persons with narcissistic personality disorder have been shown to have pathological brain changes in resting-state functional connectivity,7 weakened frontostriatal white matter connectivity,8,9 and a reduced frontal thickness and cortical volume.10 A distorted sense of self and others is a socially disabling disorder that should generate empathy, not disdain. Narcissistic personality disorder should be replaced by a term that accurately describes its behavioral pathology, and should not incorporate Greek mythology.
Mania. This is another unfortunate diagnosis that immediately evokes a negative image of patients who suffer from a potentially lethal brain disorder. It was fortunate that Robert Kendall coined the term “bipolar disorder” to replace “manic-depressive illness,” but mania is still being used within bipolar disorder as a prominent clinical phase. While depression accurately describes the mood in the other phase of this disorder, the term mania evokes wild, irrational behavior. Because the actual mood symptom cluster in mania is either elation/grandiosity or irritability/anger, why not replace mania with “elation/irritability phase of bipolar disorder”? It is more descriptive of the patient’s mood and is less pejorative.
Nomenclature is vital, and words do matter, especially when used as a diagnostic medical term. Psychiatry must “cancel” its archaic names, which are infused with negative connotations. Reinventing the psychiatric lexicon is a necessary act of renewal in a specialty where a poorly worded diagnostic label can morph into the equivalent of a “scarlet letter.” Think of other contemptuous terms, such as refrigerator mother, male hysteria, moral insanity, toxic parents, inadequate personality disorder, neurasthenia, or catastrophic schizophrenia.
General medicine regularly discards many of its obsolete terms.11 These include terms such as ablepsy, ague, camp fever, bloody flux, chlorosis, catarrh, consumption, dropsy, French pox, phthisis, milk sickness, and scrumpox.
Think also of how society abandoned the antediluvian names of boys and girls. Few parents these days would name their son Ackley, Allard, Arundel, Awarnach, Beldon, Durward, Grower, Kenlm, or Legolan, or name their daughter Afton, Agrona, Arantxa, Corliss, Demelza, Eartha, Maida, Obsession, Radella, or Sacrifice.In summary, a necessary part of psychiatry’s progress is shedding obsolete terminology, even if it means slaughtering some widely used “traditional” vocabulary. It is a necessary act of renewal, and the image of psychiatry will be burnished by it.
1. Nasrallah HA. Borderline personality disorder is a heritable brain disease. Current Psychiatry. 2014;13(4):19-20,32.
2. Sagarwala R, Nasrallah HA. White matter pathology in patients with borderline personality disorder: a review of controlled DTI studies. Ann Clin Psychiatry. 2020;32(4):281-286.
3. Keshavan MS, Tandon R, Nasrallah HA. Renaming schizophrenia: keeping up with the facts. Schizophr Res. 2013;148(1-3):1-2.
4. Lasalvia A, Penta E, Sartorius N, et al. Should the label “schizophrenia” be abandoned? Schizophr Res. 2015;162(1-3):276-284.
5. Takahashi H, Ideno T, Okubo S, et al. Impact of changing the Japanese term for “schizophrenia” for reasons of stereotypical beliefs of schizophrenia in Japanese youth. Schizophr Res. 2009;112(1-3):149-152.
6. Johanson M, Vaurio D, Tiihunen J, et al. A systematic literature review of neuroimaging of psychopathic traits. Front Psychiatry. 2020;10:1027.
7. Yang, W, Cun L, Du X, et al. Gender differences in brain structure and resting-state functional connectivity related to narcissistic personality. Sci Rep. 2015;5:10924.
8. Chester DS, Cynam DR, Powell DK, et al. Narcissismis associated with weakened frontostriatal connectivity: a DTI study. Soc Cogn Affect Neurosci. 2016;11(7):1036-1040.
9. Nenadic I, Gullmar D, Dietzek M, et al. Brain structure in narcissistic personality disorder: a VBM and DTI pilot study. Psychiatry Res. 2015;231(2):184-186.
10. Mao Y, Sang N, Wang Y, et al. Reduced frontal cortex thickness and cortical volume associated with pathological narcissism. Neuroscience. 2016;378:51-57.
11. Nasrallah HA. The transient truths of medical ‘progress.’ Current Psychiatry. 2014;13(6):23-24.
Psychiatry has made significant scientific advances over the past century. However, it is still saddled with archaic terms, with pejorative connotations, disguised as official medical diagnoses. It is time to “cancel” those terms and replace them with ones that are neutral and have not accumulated baggage.
This process of “creative destruction” of psychiatric terminology is long overdue. It is frankly disturbing that the psychiatric jargon used around the time that the American Psychiatric Association was established 175 years ago (1844) is now considered insults and epithets. We no longer work in “lunatic asylums for the insane,” and our patients with intellectual disabilities are no longer classified as “morons,” “idiots,” or “imbeciles.” Such “diagnoses” have certainly contributed to the stigma of psychiatric brain disorders. Even the noble word “asylum” has acquired a negative valence because in the past it referred to hospitals that housed persons with serious mental illness.
Thankfully, some of the outrageous terms fabricated during the condemnable and dark era of slavery 2 centuries ago were never adopted by organized psychiatry. The absurd diagnosis of “negritude,” whose tenet was that black skin is a disease curable by whitening the skin, was “invented” by none other than Benjamin Rush, the Father of Psychiatry, whose conflicted soul was depicted by concomitantly owning a slave and positioning himself as an ardent abolitionist!
Terms that need to be replaced
Fast-forward to the modern era and consider the following:
Borderline personality disorder. It is truly tragic how this confusing and non-scientific term is used as an official diagnosis for a set of seriously ill persons. It is loaded with obloquy, indignity, and derision that completely ignore the tumult, self-harm, and disability with which patients who carry this label are burdened throughout their lives, despite being intelligent. This is a serious brain disorder that has been shown to be highly genetic and is characterized by many well-established structural brain abnormalities that have been documented in neuroimaging studies.1,2 Borderline personality should not be classified as a personality disorder but as an illness with multiple signs and symptoms, including mood lability, anger, impulsivity, self-cutting, suicidal urges, feelings of abandonment, and micro-psychotic episodes. A more clinically accurate term should be coined very soon to replace borderline personality, which should be discarded to the trash heap of obsolete psychiatric terms, and no longer inflicted on patients.
Neurosis. What is the justification for continuing to use the term “neurotic” for a person who has an anxiety disorder? Is it used because Jung and Freud propagated the term “neurosis” (after it was coined by William Cullen in 1769)? Neurosis has degenerated from a psychiatric diagnosis to a scornful snub that must never be used for any patient.
Schizophrenia. This diagnosis, coined by Eugen Bleuler to replace the narrow and pessimistic “dementia praecox” proposed by Emil Kraepelin in the 1920s, initially seemed to be a neutral description of a thought disorder (split associations, not split personality). Bleuler was perceptive enough to call his book Dementia Praecox or the Group of Schizophrenias, which is consistent with the modern scientific research that confirms schizophrenia is a very heterogeneous syndrome with hundreds of genetic and environmental biotypes with a similar phenotype but a wide range of severity, treatment response, and functional outcomes. However, in subsequent decades, schizophrenia became one of the most demeaning labels in psychiatry, casting a shadow of hopelessness and disability on the people who have this serious neurologic condition with many psychiatric symptoms. The term that should replace schizophrenia should be no more degrading than stroke, multiple sclerosis, or myocardial infarction.
Continue to: Over the past 15 years...
Over the past 15 years, an expanding group of schizophrenia experts have agreed that this term must be changed to one that reflects the core features of this syndrome, and have proposed terms such as “salience syndrome,” “psychosis-spectrum,” and “reality distortion and cognitive impairment disorder.”3 In fact, several countries have already adopted a new official diagnosis for schizophrenia.4 Japan now uses the term “integration disorder,” which has significantly reduced the stigma of this brain disorder.5 South Korea changed the name to “attunement disorder.” Hong Kong and Taiwan now use “dysfunction of thought and perception.” Some researchers recommend calling schizophrenia “Bleuler’s syndrome,” a neutral eponymous designation.
One of the most irritating things about the term schizophrenia is the widespread misconception that it means “split personality.” This prompts some sports announcers to call a football team “schizophrenic” if they play well in the first half and badly in the second. The stock market is labeled “schizophrenic” if it goes up one day and way down on the next. No other medical term is misused by the media as often as the term schizophrenia.
Narcissistic personality disorder. The origin of this diagnostic category is the concept of “malignant narcissism” coined by Erich Fromm in 1964, which he designated as “the quintessence of evil.” I strongly object to implying that evil is part of any psychiatric diagnosis. Numerous studies have found structural brain abnormalities (in both gray and white matter) in patients diagnosed with psychopathic traits.6 Later, malignant narcissism was reframed as narcissistic personality disorder in 1971 by Herbert Rosenfeld. Although malignant narcissism was never accepted by either the DSM or the International Classification of Diseases, narcissistic personality disorder has been included in the DSM for the past few decades. This diagnosis reeks of disparagement and negativity. Persons with narcissistic personality disorder have been shown to have pathological brain changes in resting-state functional connectivity,7 weakened frontostriatal white matter connectivity,8,9 and a reduced frontal thickness and cortical volume.10 A distorted sense of self and others is a socially disabling disorder that should generate empathy, not disdain. Narcissistic personality disorder should be replaced by a term that accurately describes its behavioral pathology, and should not incorporate Greek mythology.
Mania. This is another unfortunate diagnosis that immediately evokes a negative image of patients who suffer from a potentially lethal brain disorder. It was fortunate that Robert Kendall coined the term “bipolar disorder” to replace “manic-depressive illness,” but mania is still being used within bipolar disorder as a prominent clinical phase. While depression accurately describes the mood in the other phase of this disorder, the term mania evokes wild, irrational behavior. Because the actual mood symptom cluster in mania is either elation/grandiosity or irritability/anger, why not replace mania with “elation/irritability phase of bipolar disorder”? It is more descriptive of the patient’s mood and is less pejorative.
Nomenclature is vital, and words do matter, especially when used as a diagnostic medical term. Psychiatry must “cancel” its archaic names, which are infused with negative connotations. Reinventing the psychiatric lexicon is a necessary act of renewal in a specialty where a poorly worded diagnostic label can morph into the equivalent of a “scarlet letter.” Think of other contemptuous terms, such as refrigerator mother, male hysteria, moral insanity, toxic parents, inadequate personality disorder, neurasthenia, or catastrophic schizophrenia.
General medicine regularly discards many of its obsolete terms.11 These include terms such as ablepsy, ague, camp fever, bloody flux, chlorosis, catarrh, consumption, dropsy, French pox, phthisis, milk sickness, and scrumpox.
Think also of how society abandoned the antediluvian names of boys and girls. Few parents these days would name their son Ackley, Allard, Arundel, Awarnach, Beldon, Durward, Grower, Kenlm, or Legolan, or name their daughter Afton, Agrona, Arantxa, Corliss, Demelza, Eartha, Maida, Obsession, Radella, or Sacrifice.In summary, a necessary part of psychiatry’s progress is shedding obsolete terminology, even if it means slaughtering some widely used “traditional” vocabulary. It is a necessary act of renewal, and the image of psychiatry will be burnished by it.
Psychiatry has made significant scientific advances over the past century. However, it is still saddled with archaic terms, with pejorative connotations, disguised as official medical diagnoses. It is time to “cancel” those terms and replace them with ones that are neutral and have not accumulated baggage.
This process of “creative destruction” of psychiatric terminology is long overdue. It is frankly disturbing that the psychiatric jargon used around the time that the American Psychiatric Association was established 175 years ago (1844) is now considered insults and epithets. We no longer work in “lunatic asylums for the insane,” and our patients with intellectual disabilities are no longer classified as “morons,” “idiots,” or “imbeciles.” Such “diagnoses” have certainly contributed to the stigma of psychiatric brain disorders. Even the noble word “asylum” has acquired a negative valence because in the past it referred to hospitals that housed persons with serious mental illness.
Thankfully, some of the outrageous terms fabricated during the condemnable and dark era of slavery 2 centuries ago were never adopted by organized psychiatry. The absurd diagnosis of “negritude,” whose tenet was that black skin is a disease curable by whitening the skin, was “invented” by none other than Benjamin Rush, the Father of Psychiatry, whose conflicted soul was depicted by concomitantly owning a slave and positioning himself as an ardent abolitionist!
Terms that need to be replaced
Fast-forward to the modern era and consider the following:
Borderline personality disorder. It is truly tragic how this confusing and non-scientific term is used as an official diagnosis for a set of seriously ill persons. It is loaded with obloquy, indignity, and derision that completely ignore the tumult, self-harm, and disability with which patients who carry this label are burdened throughout their lives, despite being intelligent. This is a serious brain disorder that has been shown to be highly genetic and is characterized by many well-established structural brain abnormalities that have been documented in neuroimaging studies.1,2 Borderline personality should not be classified as a personality disorder but as an illness with multiple signs and symptoms, including mood lability, anger, impulsivity, self-cutting, suicidal urges, feelings of abandonment, and micro-psychotic episodes. A more clinically accurate term should be coined very soon to replace borderline personality, which should be discarded to the trash heap of obsolete psychiatric terms, and no longer inflicted on patients.
Neurosis. What is the justification for continuing to use the term “neurotic” for a person who has an anxiety disorder? Is it used because Jung and Freud propagated the term “neurosis” (after it was coined by William Cullen in 1769)? Neurosis has degenerated from a psychiatric diagnosis to a scornful snub that must never be used for any patient.
Schizophrenia. This diagnosis, coined by Eugen Bleuler to replace the narrow and pessimistic “dementia praecox” proposed by Emil Kraepelin in the 1920s, initially seemed to be a neutral description of a thought disorder (split associations, not split personality). Bleuler was perceptive enough to call his book Dementia Praecox or the Group of Schizophrenias, which is consistent with the modern scientific research that confirms schizophrenia is a very heterogeneous syndrome with hundreds of genetic and environmental biotypes with a similar phenotype but a wide range of severity, treatment response, and functional outcomes. However, in subsequent decades, schizophrenia became one of the most demeaning labels in psychiatry, casting a shadow of hopelessness and disability on the people who have this serious neurologic condition with many psychiatric symptoms. The term that should replace schizophrenia should be no more degrading than stroke, multiple sclerosis, or myocardial infarction.
Continue to: Over the past 15 years...
Over the past 15 years, an expanding group of schizophrenia experts have agreed that this term must be changed to one that reflects the core features of this syndrome, and have proposed terms such as “salience syndrome,” “psychosis-spectrum,” and “reality distortion and cognitive impairment disorder.”3 In fact, several countries have already adopted a new official diagnosis for schizophrenia.4 Japan now uses the term “integration disorder,” which has significantly reduced the stigma of this brain disorder.5 South Korea changed the name to “attunement disorder.” Hong Kong and Taiwan now use “dysfunction of thought and perception.” Some researchers recommend calling schizophrenia “Bleuler’s syndrome,” a neutral eponymous designation.
One of the most irritating things about the term schizophrenia is the widespread misconception that it means “split personality.” This prompts some sports announcers to call a football team “schizophrenic” if they play well in the first half and badly in the second. The stock market is labeled “schizophrenic” if it goes up one day and way down on the next. No other medical term is misused by the media as often as the term schizophrenia.
Narcissistic personality disorder. The origin of this diagnostic category is the concept of “malignant narcissism” coined by Erich Fromm in 1964, which he designated as “the quintessence of evil.” I strongly object to implying that evil is part of any psychiatric diagnosis. Numerous studies have found structural brain abnormalities (in both gray and white matter) in patients diagnosed with psychopathic traits.6 Later, malignant narcissism was reframed as narcissistic personality disorder in 1971 by Herbert Rosenfeld. Although malignant narcissism was never accepted by either the DSM or the International Classification of Diseases, narcissistic personality disorder has been included in the DSM for the past few decades. This diagnosis reeks of disparagement and negativity. Persons with narcissistic personality disorder have been shown to have pathological brain changes in resting-state functional connectivity,7 weakened frontostriatal white matter connectivity,8,9 and a reduced frontal thickness and cortical volume.10 A distorted sense of self and others is a socially disabling disorder that should generate empathy, not disdain. Narcissistic personality disorder should be replaced by a term that accurately describes its behavioral pathology, and should not incorporate Greek mythology.
Mania. This is another unfortunate diagnosis that immediately evokes a negative image of patients who suffer from a potentially lethal brain disorder. It was fortunate that Robert Kendall coined the term “bipolar disorder” to replace “manic-depressive illness,” but mania is still being used within bipolar disorder as a prominent clinical phase. While depression accurately describes the mood in the other phase of this disorder, the term mania evokes wild, irrational behavior. Because the actual mood symptom cluster in mania is either elation/grandiosity or irritability/anger, why not replace mania with “elation/irritability phase of bipolar disorder”? It is more descriptive of the patient’s mood and is less pejorative.
Nomenclature is vital, and words do matter, especially when used as a diagnostic medical term. Psychiatry must “cancel” its archaic names, which are infused with negative connotations. Reinventing the psychiatric lexicon is a necessary act of renewal in a specialty where a poorly worded diagnostic label can morph into the equivalent of a “scarlet letter.” Think of other contemptuous terms, such as refrigerator mother, male hysteria, moral insanity, toxic parents, inadequate personality disorder, neurasthenia, or catastrophic schizophrenia.
General medicine regularly discards many of its obsolete terms.11 These include terms such as ablepsy, ague, camp fever, bloody flux, chlorosis, catarrh, consumption, dropsy, French pox, phthisis, milk sickness, and scrumpox.
Think also of how society abandoned the antediluvian names of boys and girls. Few parents these days would name their son Ackley, Allard, Arundel, Awarnach, Beldon, Durward, Grower, Kenlm, or Legolan, or name their daughter Afton, Agrona, Arantxa, Corliss, Demelza, Eartha, Maida, Obsession, Radella, or Sacrifice.In summary, a necessary part of psychiatry’s progress is shedding obsolete terminology, even if it means slaughtering some widely used “traditional” vocabulary. It is a necessary act of renewal, and the image of psychiatry will be burnished by it.
1. Nasrallah HA. Borderline personality disorder is a heritable brain disease. Current Psychiatry. 2014;13(4):19-20,32.
2. Sagarwala R, Nasrallah HA. White matter pathology in patients with borderline personality disorder: a review of controlled DTI studies. Ann Clin Psychiatry. 2020;32(4):281-286.
3. Keshavan MS, Tandon R, Nasrallah HA. Renaming schizophrenia: keeping up with the facts. Schizophr Res. 2013;148(1-3):1-2.
4. Lasalvia A, Penta E, Sartorius N, et al. Should the label “schizophrenia” be abandoned? Schizophr Res. 2015;162(1-3):276-284.
5. Takahashi H, Ideno T, Okubo S, et al. Impact of changing the Japanese term for “schizophrenia” for reasons of stereotypical beliefs of schizophrenia in Japanese youth. Schizophr Res. 2009;112(1-3):149-152.
6. Johanson M, Vaurio D, Tiihunen J, et al. A systematic literature review of neuroimaging of psychopathic traits. Front Psychiatry. 2020;10:1027.
7. Yang, W, Cun L, Du X, et al. Gender differences in brain structure and resting-state functional connectivity related to narcissistic personality. Sci Rep. 2015;5:10924.
8. Chester DS, Cynam DR, Powell DK, et al. Narcissismis associated with weakened frontostriatal connectivity: a DTI study. Soc Cogn Affect Neurosci. 2016;11(7):1036-1040.
9. Nenadic I, Gullmar D, Dietzek M, et al. Brain structure in narcissistic personality disorder: a VBM and DTI pilot study. Psychiatry Res. 2015;231(2):184-186.
10. Mao Y, Sang N, Wang Y, et al. Reduced frontal cortex thickness and cortical volume associated with pathological narcissism. Neuroscience. 2016;378:51-57.
11. Nasrallah HA. The transient truths of medical ‘progress.’ Current Psychiatry. 2014;13(6):23-24.
1. Nasrallah HA. Borderline personality disorder is a heritable brain disease. Current Psychiatry. 2014;13(4):19-20,32.
2. Sagarwala R, Nasrallah HA. White matter pathology in patients with borderline personality disorder: a review of controlled DTI studies. Ann Clin Psychiatry. 2020;32(4):281-286.
3. Keshavan MS, Tandon R, Nasrallah HA. Renaming schizophrenia: keeping up with the facts. Schizophr Res. 2013;148(1-3):1-2.
4. Lasalvia A, Penta E, Sartorius N, et al. Should the label “schizophrenia” be abandoned? Schizophr Res. 2015;162(1-3):276-284.
5. Takahashi H, Ideno T, Okubo S, et al. Impact of changing the Japanese term for “schizophrenia” for reasons of stereotypical beliefs of schizophrenia in Japanese youth. Schizophr Res. 2009;112(1-3):149-152.
6. Johanson M, Vaurio D, Tiihunen J, et al. A systematic literature review of neuroimaging of psychopathic traits. Front Psychiatry. 2020;10:1027.
7. Yang, W, Cun L, Du X, et al. Gender differences in brain structure and resting-state functional connectivity related to narcissistic personality. Sci Rep. 2015;5:10924.
8. Chester DS, Cynam DR, Powell DK, et al. Narcissismis associated with weakened frontostriatal connectivity: a DTI study. Soc Cogn Affect Neurosci. 2016;11(7):1036-1040.
9. Nenadic I, Gullmar D, Dietzek M, et al. Brain structure in narcissistic personality disorder: a VBM and DTI pilot study. Psychiatry Res. 2015;231(2):184-186.
10. Mao Y, Sang N, Wang Y, et al. Reduced frontal cortex thickness and cortical volume associated with pathological narcissism. Neuroscience. 2016;378:51-57.
11. Nasrallah HA. The transient truths of medical ‘progress.’ Current Psychiatry. 2014;13(6):23-24.
Caring for adults who engage in nonsuicidal self-injury
Nonsuicidal self-injury (NSSI) is the direct and deliberate destruction of body tissue without intent to die.1 Cutting is the most common form of NSSI; other methods include burning, scraping/scratching skin, interfering with wound healing, hitting, biting, self-poisoning, and purposeful non-recreational risk-taking.2,3 Although most individuals who engage in NSSI have no intention to die, suicidal ideation often precedes the initial engagement in NSSI,4 and a history of repeated NSSI is a risk factor for suicide attempts.4 In a systematic review, Cipriano et al5 found that NSSI is most common among adolescents and young adults, with onset most often occurring between age 12 and 14. Prevalence rates of NSSI are 7.5% to 46.5% in adolescents, 38.9% in university students, and 4% to 23% in adults.5
Although no medications have consistently shown efficacy for treating NSSI, research suggests cognitive-behavioral therapy and dialectical behavioral therapy may be helpful. Unfortunately, these therapies are often not available during a patient’s acute crisis.3 Because a thorough review of the treatment options for NSSI is beyond the scope of this article, here I offer tips for caring for adults who engage in NSSI. Although there are slight differences in managing NSSI in adolescents (eg, the need for parental monitoring and reducing risk of contagion), these tips also can be used with adolescents.
Explore why your patient engages in NSSI. Identifying the reasons for our patients’ NSSI makes it easier for us to empathize with them, and puts us in a better position to treat them.3 The most widely reported reasons for NSSI are to cope with distress/anguish and to exert influence on others.6 In a systematic review, self-reported reasons for NSSI also included punishing oneself for having positive feelings, punishing others, managing dissociation (ie, active pursuit of numbness), sensation-seeking (ie, to generate excitement or exhilaration), averting suicide (ie, warding off suicidal thoughts), maintaining or exploring boundaries, and expressing or coping with sexuality.6 When exploring your patient’s reasons for NSSI, determine if the behavior is based on a true suicidal desire. Because NSSI is associated with mood disorders, anxiety disorders, personality disorders, and other disorders, also assess for any underlying psychiatric conditions, and treat them accordingly because mental health treatment has been empirically proven to reduce suicide rates.2,7
Conduct a suicide risk assessment. Regardless of your patient’s reasons for NSSI, an individualized and thorough suicide risk assessment is needed to identify modifiable, non-modifiable, and protective factors that you can consider when developing a treatment plan. Key components of such assessments include (but are not limited to) current and past urges to engage in NSSI, past NSSI and suicide attempts, access to lethal means, and ability to follow a safety plan.
Avoid exaggerating the danger and importance of NSSI. Treating a patient who engages in NSSI who is motivated by a true suicidal desire and/or has underlying psychiatric conditions may prompt you to consider hospitalization and/or prescribing psychotropic medications.3 However, because most NSSI is not due to a true suicidal desire, overreacting may unwittingly communicate to the patient that self-harm is a way to sustain someone’s attention, thus reinforcing that such behaviors can help them obtain support when distressed.3 Further, overreacting will not help patients comprehend and better cope with the reasons for their self-injurious behaviors.3
Restrict your patient’s access to lethal means. Restricting access to items such as firearms, sharp objects (eg, knives and razors), medications, implements for suffocation/hanging (eg, belts), and household poisons has been empirically proven to reduce suicide rates.7 Such restrictions can also potentially reduce the likelihood of NSSI. It is important to repeatedly ask your patient if they have acquired any new means, and to listen for information that indicates they possess means that they did not previously disclose. It is also important to ask if the patient has moved existing means to an area for easier access to use them.
Create a safety plan. Written safety plans can include a list of warning signs (thoughts, images, mood, situations, behaviors) that a crisis is developing, coping strategies (eg, going for a walk, exercising, engaging in a hobby, socializing with friends or family), and contact information for 24-hour crisis hotlines, emergency rooms, and mental health clinicians.8 The Suicide Prevention Resource Center offers a safety plan template at www.sprc.org/sites/default/files/resource-program/Brown_StanleySafetyPlanTemplate.pdf.8
Offer empathy. Individuals who engage in NSSI are making a desperate call for help that requires concerned and supportive responses.3 One such response is to provide empathy. In addition to expressing concern and compassion, empathy involves recognizing and sharing your patients’ emotions. Empathy also can help you avoid any resistance during the visit by considering what is appropriate to say to patients.
Manage countertransference. You may have negative feelings toward a patient who engages in NSSI, or may even view self-harm as a willful act designed to gain attention. However, such feelings could lead you to minimize or dismiss the importance of your patient’s behaviors, which may push them to engage in more dangerous self-harm.3 Acknowledging any feelings of derision for a patient who engages in NSSI and understanding why you have these emotions will help you better understand your patient, improve rapport, and ensure that you are not impeding the delivery of appropriate clinical care.
1. Nock MK. Self-injury. Annu Rev Clin Psychol. 2010;6:339-363.
2. Klonsky ED. Non-suicidal self-injury in United States adults: prevalence, sociodemographics, topography and functions. Psychol Med. 2011;41(9):1981-1986.
3. Gunderson JG, Choi-Kain LW. Working with patients who self-injure. JAMA Psychiatry. 2019;76(9):976-977.
4. Glenn CR, Lanzillo EC, Esposito EC, et al. Examining the course of suicidal and nonsuicidal self-injurious thoughts and behaviors in outpatient and inpatient adolescents. J Abnorm Child Psychol. 2017;45(5):971-983.
5. Cipriano A, Cella S, Cotrufo P. Nonsuicidal self-injury: a systematic review. Front Psychol. 2017;8:1946. doi: 10.3389/fpsyg.2017.01946
6. Edmondson AJ, Brennan CA, House AO. Non-suicidal reasons for self-harm: a systematic review of self-reported accounts. J Affect Disord. 2016;191:109-117.
7. Mann JJ, Apter A, Bertolete J. Suicide prevention strategies: a systematic review. JAMA. 2005;294(16):2064-2074.
8. Stanley B, Brown GK. Safety planning intervention: a brief intervention to mitigate suicide risk. Cog Behav Practice. 2012;19:256-264.
Nonsuicidal self-injury (NSSI) is the direct and deliberate destruction of body tissue without intent to die.1 Cutting is the most common form of NSSI; other methods include burning, scraping/scratching skin, interfering with wound healing, hitting, biting, self-poisoning, and purposeful non-recreational risk-taking.2,3 Although most individuals who engage in NSSI have no intention to die, suicidal ideation often precedes the initial engagement in NSSI,4 and a history of repeated NSSI is a risk factor for suicide attempts.4 In a systematic review, Cipriano et al5 found that NSSI is most common among adolescents and young adults, with onset most often occurring between age 12 and 14. Prevalence rates of NSSI are 7.5% to 46.5% in adolescents, 38.9% in university students, and 4% to 23% in adults.5
Although no medications have consistently shown efficacy for treating NSSI, research suggests cognitive-behavioral therapy and dialectical behavioral therapy may be helpful. Unfortunately, these therapies are often not available during a patient’s acute crisis.3 Because a thorough review of the treatment options for NSSI is beyond the scope of this article, here I offer tips for caring for adults who engage in NSSI. Although there are slight differences in managing NSSI in adolescents (eg, the need for parental monitoring and reducing risk of contagion), these tips also can be used with adolescents.
Explore why your patient engages in NSSI. Identifying the reasons for our patients’ NSSI makes it easier for us to empathize with them, and puts us in a better position to treat them.3 The most widely reported reasons for NSSI are to cope with distress/anguish and to exert influence on others.6 In a systematic review, self-reported reasons for NSSI also included punishing oneself for having positive feelings, punishing others, managing dissociation (ie, active pursuit of numbness), sensation-seeking (ie, to generate excitement or exhilaration), averting suicide (ie, warding off suicidal thoughts), maintaining or exploring boundaries, and expressing or coping with sexuality.6 When exploring your patient’s reasons for NSSI, determine if the behavior is based on a true suicidal desire. Because NSSI is associated with mood disorders, anxiety disorders, personality disorders, and other disorders, also assess for any underlying psychiatric conditions, and treat them accordingly because mental health treatment has been empirically proven to reduce suicide rates.2,7
Conduct a suicide risk assessment. Regardless of your patient’s reasons for NSSI, an individualized and thorough suicide risk assessment is needed to identify modifiable, non-modifiable, and protective factors that you can consider when developing a treatment plan. Key components of such assessments include (but are not limited to) current and past urges to engage in NSSI, past NSSI and suicide attempts, access to lethal means, and ability to follow a safety plan.
Avoid exaggerating the danger and importance of NSSI. Treating a patient who engages in NSSI who is motivated by a true suicidal desire and/or has underlying psychiatric conditions may prompt you to consider hospitalization and/or prescribing psychotropic medications.3 However, because most NSSI is not due to a true suicidal desire, overreacting may unwittingly communicate to the patient that self-harm is a way to sustain someone’s attention, thus reinforcing that such behaviors can help them obtain support when distressed.3 Further, overreacting will not help patients comprehend and better cope with the reasons for their self-injurious behaviors.3
Restrict your patient’s access to lethal means. Restricting access to items such as firearms, sharp objects (eg, knives and razors), medications, implements for suffocation/hanging (eg, belts), and household poisons has been empirically proven to reduce suicide rates.7 Such restrictions can also potentially reduce the likelihood of NSSI. It is important to repeatedly ask your patient if they have acquired any new means, and to listen for information that indicates they possess means that they did not previously disclose. It is also important to ask if the patient has moved existing means to an area for easier access to use them.
Create a safety plan. Written safety plans can include a list of warning signs (thoughts, images, mood, situations, behaviors) that a crisis is developing, coping strategies (eg, going for a walk, exercising, engaging in a hobby, socializing with friends or family), and contact information for 24-hour crisis hotlines, emergency rooms, and mental health clinicians.8 The Suicide Prevention Resource Center offers a safety plan template at www.sprc.org/sites/default/files/resource-program/Brown_StanleySafetyPlanTemplate.pdf.8
Offer empathy. Individuals who engage in NSSI are making a desperate call for help that requires concerned and supportive responses.3 One such response is to provide empathy. In addition to expressing concern and compassion, empathy involves recognizing and sharing your patients’ emotions. Empathy also can help you avoid any resistance during the visit by considering what is appropriate to say to patients.
Manage countertransference. You may have negative feelings toward a patient who engages in NSSI, or may even view self-harm as a willful act designed to gain attention. However, such feelings could lead you to minimize or dismiss the importance of your patient’s behaviors, which may push them to engage in more dangerous self-harm.3 Acknowledging any feelings of derision for a patient who engages in NSSI and understanding why you have these emotions will help you better understand your patient, improve rapport, and ensure that you are not impeding the delivery of appropriate clinical care.
Nonsuicidal self-injury (NSSI) is the direct and deliberate destruction of body tissue without intent to die.1 Cutting is the most common form of NSSI; other methods include burning, scraping/scratching skin, interfering with wound healing, hitting, biting, self-poisoning, and purposeful non-recreational risk-taking.2,3 Although most individuals who engage in NSSI have no intention to die, suicidal ideation often precedes the initial engagement in NSSI,4 and a history of repeated NSSI is a risk factor for suicide attempts.4 In a systematic review, Cipriano et al5 found that NSSI is most common among adolescents and young adults, with onset most often occurring between age 12 and 14. Prevalence rates of NSSI are 7.5% to 46.5% in adolescents, 38.9% in university students, and 4% to 23% in adults.5
Although no medications have consistently shown efficacy for treating NSSI, research suggests cognitive-behavioral therapy and dialectical behavioral therapy may be helpful. Unfortunately, these therapies are often not available during a patient’s acute crisis.3 Because a thorough review of the treatment options for NSSI is beyond the scope of this article, here I offer tips for caring for adults who engage in NSSI. Although there are slight differences in managing NSSI in adolescents (eg, the need for parental monitoring and reducing risk of contagion), these tips also can be used with adolescents.
Explore why your patient engages in NSSI. Identifying the reasons for our patients’ NSSI makes it easier for us to empathize with them, and puts us in a better position to treat them.3 The most widely reported reasons for NSSI are to cope with distress/anguish and to exert influence on others.6 In a systematic review, self-reported reasons for NSSI also included punishing oneself for having positive feelings, punishing others, managing dissociation (ie, active pursuit of numbness), sensation-seeking (ie, to generate excitement or exhilaration), averting suicide (ie, warding off suicidal thoughts), maintaining or exploring boundaries, and expressing or coping with sexuality.6 When exploring your patient’s reasons for NSSI, determine if the behavior is based on a true suicidal desire. Because NSSI is associated with mood disorders, anxiety disorders, personality disorders, and other disorders, also assess for any underlying psychiatric conditions, and treat them accordingly because mental health treatment has been empirically proven to reduce suicide rates.2,7
Conduct a suicide risk assessment. Regardless of your patient’s reasons for NSSI, an individualized and thorough suicide risk assessment is needed to identify modifiable, non-modifiable, and protective factors that you can consider when developing a treatment plan. Key components of such assessments include (but are not limited to) current and past urges to engage in NSSI, past NSSI and suicide attempts, access to lethal means, and ability to follow a safety plan.
Avoid exaggerating the danger and importance of NSSI. Treating a patient who engages in NSSI who is motivated by a true suicidal desire and/or has underlying psychiatric conditions may prompt you to consider hospitalization and/or prescribing psychotropic medications.3 However, because most NSSI is not due to a true suicidal desire, overreacting may unwittingly communicate to the patient that self-harm is a way to sustain someone’s attention, thus reinforcing that such behaviors can help them obtain support when distressed.3 Further, overreacting will not help patients comprehend and better cope with the reasons for their self-injurious behaviors.3
Restrict your patient’s access to lethal means. Restricting access to items such as firearms, sharp objects (eg, knives and razors), medications, implements for suffocation/hanging (eg, belts), and household poisons has been empirically proven to reduce suicide rates.7 Such restrictions can also potentially reduce the likelihood of NSSI. It is important to repeatedly ask your patient if they have acquired any new means, and to listen for information that indicates they possess means that they did not previously disclose. It is also important to ask if the patient has moved existing means to an area for easier access to use them.
Create a safety plan. Written safety plans can include a list of warning signs (thoughts, images, mood, situations, behaviors) that a crisis is developing, coping strategies (eg, going for a walk, exercising, engaging in a hobby, socializing with friends or family), and contact information for 24-hour crisis hotlines, emergency rooms, and mental health clinicians.8 The Suicide Prevention Resource Center offers a safety plan template at www.sprc.org/sites/default/files/resource-program/Brown_StanleySafetyPlanTemplate.pdf.8
Offer empathy. Individuals who engage in NSSI are making a desperate call for help that requires concerned and supportive responses.3 One such response is to provide empathy. In addition to expressing concern and compassion, empathy involves recognizing and sharing your patients’ emotions. Empathy also can help you avoid any resistance during the visit by considering what is appropriate to say to patients.
Manage countertransference. You may have negative feelings toward a patient who engages in NSSI, or may even view self-harm as a willful act designed to gain attention. However, such feelings could lead you to minimize or dismiss the importance of your patient’s behaviors, which may push them to engage in more dangerous self-harm.3 Acknowledging any feelings of derision for a patient who engages in NSSI and understanding why you have these emotions will help you better understand your patient, improve rapport, and ensure that you are not impeding the delivery of appropriate clinical care.
1. Nock MK. Self-injury. Annu Rev Clin Psychol. 2010;6:339-363.
2. Klonsky ED. Non-suicidal self-injury in United States adults: prevalence, sociodemographics, topography and functions. Psychol Med. 2011;41(9):1981-1986.
3. Gunderson JG, Choi-Kain LW. Working with patients who self-injure. JAMA Psychiatry. 2019;76(9):976-977.
4. Glenn CR, Lanzillo EC, Esposito EC, et al. Examining the course of suicidal and nonsuicidal self-injurious thoughts and behaviors in outpatient and inpatient adolescents. J Abnorm Child Psychol. 2017;45(5):971-983.
5. Cipriano A, Cella S, Cotrufo P. Nonsuicidal self-injury: a systematic review. Front Psychol. 2017;8:1946. doi: 10.3389/fpsyg.2017.01946
6. Edmondson AJ, Brennan CA, House AO. Non-suicidal reasons for self-harm: a systematic review of self-reported accounts. J Affect Disord. 2016;191:109-117.
7. Mann JJ, Apter A, Bertolete J. Suicide prevention strategies: a systematic review. JAMA. 2005;294(16):2064-2074.
8. Stanley B, Brown GK. Safety planning intervention: a brief intervention to mitigate suicide risk. Cog Behav Practice. 2012;19:256-264.
1. Nock MK. Self-injury. Annu Rev Clin Psychol. 2010;6:339-363.
2. Klonsky ED. Non-suicidal self-injury in United States adults: prevalence, sociodemographics, topography and functions. Psychol Med. 2011;41(9):1981-1986.
3. Gunderson JG, Choi-Kain LW. Working with patients who self-injure. JAMA Psychiatry. 2019;76(9):976-977.
4. Glenn CR, Lanzillo EC, Esposito EC, et al. Examining the course of suicidal and nonsuicidal self-injurious thoughts and behaviors in outpatient and inpatient adolescents. J Abnorm Child Psychol. 2017;45(5):971-983.
5. Cipriano A, Cella S, Cotrufo P. Nonsuicidal self-injury: a systematic review. Front Psychol. 2017;8:1946. doi: 10.3389/fpsyg.2017.01946
6. Edmondson AJ, Brennan CA, House AO. Non-suicidal reasons for self-harm: a systematic review of self-reported accounts. J Affect Disord. 2016;191:109-117.
7. Mann JJ, Apter A, Bertolete J. Suicide prevention strategies: a systematic review. JAMA. 2005;294(16):2064-2074.
8. Stanley B, Brown GK. Safety planning intervention: a brief intervention to mitigate suicide risk. Cog Behav Practice. 2012;19:256-264.
Career Choices: Navy Psychiatry
In this Career Choices, Siddhi Bhivandkar, MD, spoke with Captain Paulette T. Cazares, MD, MPH. Dr. Cazares is Director for Mental Health at U.S. Navy Medicine Readiness and Training Command Okinawa, Japan. She also is Assistant Professor, Department of Psychiatry, Uniformed Services University, Bethesda, Maryland, and serves as Secretary of the American Medical Women’s Association, Schaumburg, Illinois.
Dr. Bhivandkar: What made you choose the Navy psychiatry track, and how did your training lead you towards this path?
Dr. Cazares: I had considered a career in the Navy early on in my education, and when I was ready to apply to medical school, I saw Uniformed Services University (USU) as one of my top choices. I wasn’t 100% sure, but after a tour and my interview, I was sold on serving those who serve.
During my clinical rotations at USU, I had great experiences in inpatient and emergency psychiatry. I became fascinated with understanding all I could about brain circuitry and chemistry, and how that interacts with the environment to create or protect individuals from disease. Once I talked with some mentors, it became clear to me that I would love a career in psychiatry, and that remains true today.
Dr. Bhivandkar: What are some of the pros and cons of working in Navy psychiatry?
Dr. Cazares: As a Navy psychiatrist, I have found great reward in caring for our nation’s volunteer force. I have had wonderful colleagues with whom I have deployed, and with whom I have served in both small military hospitals and large military training and academic centers. I have been able to work in research in military mental health, and feel I have specifically advanced the field of women’s mental health in the Navy.
I had 4 children while I have been on active duty, and had paid maternity leave for all of them, as well as practices that protected my choice to breastfeed and pump, even after returning to work. I have moved to areas of the country I didn’t expect to with the Navy, and my husband’s career took unexpected turns as a result. While this can be seen as a challenge, it can also be a surprisingly rewarding experience, seeing areas of our nation and world that I otherwise would not have seen. I have deployed and been away from family. While that was a challenge, my family came through it very strong, and I found myself a more humble human and a better clinician as a result of that time.
Dr. Bhivandkar: Based on your personal experience, what should one consider when choosing a Navy psychiatry program?
Dr. Cazares: In considering a Navy training program, one should consider that in the military, our patient population is generally young and healthy, yet also exposed to unique occupational stressors. This means that we generally see routine mental health diagnoses, and some early-break severe cases. We do not typically follow long-term patients with chronic mental illness, because those patients tend to be medically retired from active duty service.
Continue to: We see many unique populations...
We see many unique populations that have specific health care needs, including service members who work on submarines, who are pilots or military police members, and those who handle and manage weapons. We get to learn the unique balance between serving our patients, and the units they work for and in. We see the impact of occupational stress on individuals, and are part of the multidisciplinary team that helps to build resilience in our young service members.
Dr. Bhivandkar: What are some of the career options and work settings for Navy psychiatrists?
Dr. Cazares: My peers and I have worked across both operational and multiple hospital settings, with both the US Marine Corps, as well as the US Navy. Psychiatrists can apply for fellowship, as the Navy regularly trains child and adolescent psychiatrists, as well as those who want to specialize in addiction psychiatry.
We can work in large Navy medical centers on faculty, in community-style Navy hospitals both in the United States and overseas, as well as on ships, with the Marines, or in headquarters jobs, advising on policy and the future of the military health system.
Dr. Bhivandkar: What are some of the challenges of working in this field?
Dr. Cazares: Health care and the military are both demanding career fields. Like many areas of medicine, work-life harmony is an important part of a career in Navy psychiatry. I work hard to balance my own needs, and model this for those I lead.
Dr. Bhivandkar: What advice do you have for those contemplating a career in Navy psychiatry?
Dr. Cazares: Consider joining a team that offers incredible purpose. I have served wonderful patients and had incredibly impressive colleagues, and I am grateful for the choice I made to take an oath and wear the uniform.
In this Career Choices, Siddhi Bhivandkar, MD, spoke with Captain Paulette T. Cazares, MD, MPH. Dr. Cazares is Director for Mental Health at U.S. Navy Medicine Readiness and Training Command Okinawa, Japan. She also is Assistant Professor, Department of Psychiatry, Uniformed Services University, Bethesda, Maryland, and serves as Secretary of the American Medical Women’s Association, Schaumburg, Illinois.
Dr. Bhivandkar: What made you choose the Navy psychiatry track, and how did your training lead you towards this path?
Dr. Cazares: I had considered a career in the Navy early on in my education, and when I was ready to apply to medical school, I saw Uniformed Services University (USU) as one of my top choices. I wasn’t 100% sure, but after a tour and my interview, I was sold on serving those who serve.
During my clinical rotations at USU, I had great experiences in inpatient and emergency psychiatry. I became fascinated with understanding all I could about brain circuitry and chemistry, and how that interacts with the environment to create or protect individuals from disease. Once I talked with some mentors, it became clear to me that I would love a career in psychiatry, and that remains true today.
Dr. Bhivandkar: What are some of the pros and cons of working in Navy psychiatry?
Dr. Cazares: As a Navy psychiatrist, I have found great reward in caring for our nation’s volunteer force. I have had wonderful colleagues with whom I have deployed, and with whom I have served in both small military hospitals and large military training and academic centers. I have been able to work in research in military mental health, and feel I have specifically advanced the field of women’s mental health in the Navy.
I had 4 children while I have been on active duty, and had paid maternity leave for all of them, as well as practices that protected my choice to breastfeed and pump, even after returning to work. I have moved to areas of the country I didn’t expect to with the Navy, and my husband’s career took unexpected turns as a result. While this can be seen as a challenge, it can also be a surprisingly rewarding experience, seeing areas of our nation and world that I otherwise would not have seen. I have deployed and been away from family. While that was a challenge, my family came through it very strong, and I found myself a more humble human and a better clinician as a result of that time.
Dr. Bhivandkar: Based on your personal experience, what should one consider when choosing a Navy psychiatry program?
Dr. Cazares: In considering a Navy training program, one should consider that in the military, our patient population is generally young and healthy, yet also exposed to unique occupational stressors. This means that we generally see routine mental health diagnoses, and some early-break severe cases. We do not typically follow long-term patients with chronic mental illness, because those patients tend to be medically retired from active duty service.
Continue to: We see many unique populations...
We see many unique populations that have specific health care needs, including service members who work on submarines, who are pilots or military police members, and those who handle and manage weapons. We get to learn the unique balance between serving our patients, and the units they work for and in. We see the impact of occupational stress on individuals, and are part of the multidisciplinary team that helps to build resilience in our young service members.
Dr. Bhivandkar: What are some of the career options and work settings for Navy psychiatrists?
Dr. Cazares: My peers and I have worked across both operational and multiple hospital settings, with both the US Marine Corps, as well as the US Navy. Psychiatrists can apply for fellowship, as the Navy regularly trains child and adolescent psychiatrists, as well as those who want to specialize in addiction psychiatry.
We can work in large Navy medical centers on faculty, in community-style Navy hospitals both in the United States and overseas, as well as on ships, with the Marines, or in headquarters jobs, advising on policy and the future of the military health system.
Dr. Bhivandkar: What are some of the challenges of working in this field?
Dr. Cazares: Health care and the military are both demanding career fields. Like many areas of medicine, work-life harmony is an important part of a career in Navy psychiatry. I work hard to balance my own needs, and model this for those I lead.
Dr. Bhivandkar: What advice do you have for those contemplating a career in Navy psychiatry?
Dr. Cazares: Consider joining a team that offers incredible purpose. I have served wonderful patients and had incredibly impressive colleagues, and I am grateful for the choice I made to take an oath and wear the uniform.
In this Career Choices, Siddhi Bhivandkar, MD, spoke with Captain Paulette T. Cazares, MD, MPH. Dr. Cazares is Director for Mental Health at U.S. Navy Medicine Readiness and Training Command Okinawa, Japan. She also is Assistant Professor, Department of Psychiatry, Uniformed Services University, Bethesda, Maryland, and serves as Secretary of the American Medical Women’s Association, Schaumburg, Illinois.
Dr. Bhivandkar: What made you choose the Navy psychiatry track, and how did your training lead you towards this path?
Dr. Cazares: I had considered a career in the Navy early on in my education, and when I was ready to apply to medical school, I saw Uniformed Services University (USU) as one of my top choices. I wasn’t 100% sure, but after a tour and my interview, I was sold on serving those who serve.
During my clinical rotations at USU, I had great experiences in inpatient and emergency psychiatry. I became fascinated with understanding all I could about brain circuitry and chemistry, and how that interacts with the environment to create or protect individuals from disease. Once I talked with some mentors, it became clear to me that I would love a career in psychiatry, and that remains true today.
Dr. Bhivandkar: What are some of the pros and cons of working in Navy psychiatry?
Dr. Cazares: As a Navy psychiatrist, I have found great reward in caring for our nation’s volunteer force. I have had wonderful colleagues with whom I have deployed, and with whom I have served in both small military hospitals and large military training and academic centers. I have been able to work in research in military mental health, and feel I have specifically advanced the field of women’s mental health in the Navy.
I had 4 children while I have been on active duty, and had paid maternity leave for all of them, as well as practices that protected my choice to breastfeed and pump, even after returning to work. I have moved to areas of the country I didn’t expect to with the Navy, and my husband’s career took unexpected turns as a result. While this can be seen as a challenge, it can also be a surprisingly rewarding experience, seeing areas of our nation and world that I otherwise would not have seen. I have deployed and been away from family. While that was a challenge, my family came through it very strong, and I found myself a more humble human and a better clinician as a result of that time.
Dr. Bhivandkar: Based on your personal experience, what should one consider when choosing a Navy psychiatry program?
Dr. Cazares: In considering a Navy training program, one should consider that in the military, our patient population is generally young and healthy, yet also exposed to unique occupational stressors. This means that we generally see routine mental health diagnoses, and some early-break severe cases. We do not typically follow long-term patients with chronic mental illness, because those patients tend to be medically retired from active duty service.
Continue to: We see many unique populations...
We see many unique populations that have specific health care needs, including service members who work on submarines, who are pilots or military police members, and those who handle and manage weapons. We get to learn the unique balance between serving our patients, and the units they work for and in. We see the impact of occupational stress on individuals, and are part of the multidisciplinary team that helps to build resilience in our young service members.
Dr. Bhivandkar: What are some of the career options and work settings for Navy psychiatrists?
Dr. Cazares: My peers and I have worked across both operational and multiple hospital settings, with both the US Marine Corps, as well as the US Navy. Psychiatrists can apply for fellowship, as the Navy regularly trains child and adolescent psychiatrists, as well as those who want to specialize in addiction psychiatry.
We can work in large Navy medical centers on faculty, in community-style Navy hospitals both in the United States and overseas, as well as on ships, with the Marines, or in headquarters jobs, advising on policy and the future of the military health system.
Dr. Bhivandkar: What are some of the challenges of working in this field?
Dr. Cazares: Health care and the military are both demanding career fields. Like many areas of medicine, work-life harmony is an important part of a career in Navy psychiatry. I work hard to balance my own needs, and model this for those I lead.
Dr. Bhivandkar: What advice do you have for those contemplating a career in Navy psychiatry?
Dr. Cazares: Consider joining a team that offers incredible purpose. I have served wonderful patients and had incredibly impressive colleagues, and I am grateful for the choice I made to take an oath and wear the uniform.
The rebirth of psychedelic psychiatry
Mr. P, age 65, has a history of major depressive disorder (MDD), generalized anxiety disorder, and social phobia. Mr. P’s personality is high in neuroticism and he has often responded to new situations with feelings of impending doom. For him, fear, anxious rumination, helplessness, and catastrophizing are familiar mental processes.
When he was in his 30s, Mr. P had a severe major depressive episode with suicidal ideation and sought care from a psychiatrist. He began a treatment program of psychotherapy and concomitant psychopharmacotherapy with consecutive trials of fluoxetine, sertraline, and amitriptyline, each of an adequate dose and duration. With each medication, Mr. P experienced new adverse effects, including nausea, constipation, tremors, and headache. His psychiatrist transitioned him to bupropion, which helped Mr. P most. For the next several decades, Mr. P continued to experience low-grade depressive symptoms with intermittent exacerbation to mild-to-moderate major depressive episodes, but he remained adherent to his medication and continued psychotherapy.
Shortly after his 65th birthday, Mr. P experiences progressively worsening nausea and abdominal pain. Initially, he assumes the symptoms are secondary to anxiety. Taking his psychiatrist’s advice, Mr. P visits his primary care physician. A work-up reveals that Mr. P has advanced pancreatic cancer, and an oncologist estimates Mr. P has 6 months of life remaining.
Following his cancer diagnosis, Mr. P quickly develops symptoms of MDD despite continuing to take bupropion. Within a week he becomes withdrawn and hopeless, and thinks about ending his life “before God does.” His psychiatrist urges Mr. P to contact the local academic medical center because it is conducting a trial of a “new” drug, psilocybin, to treat anxiety and depression in patients with terminal illness.
Beginning in the 1940s, a growing body of scientific evidence suggested that psychedelic compounds such as lysergic acid diethylamide (LSD) could benefit individuals with various psychiatric maladies. Research interest in LSD and substances with similar effects persisted until the late 1960s. In response to the growing counterculture movement in the United States and the efforts of Harvard researchers Timothy Leary and Richard Alpert to popularize psychedelic drug use in the general population, in 1970 President Richard M. Nixon signed the Controlled Substances Act (CSA) into law. The CSA categorized LSD as a Schedule I drug, rendering its manufacture and distribution illegal. Research into the potential therapeutic benefits of LSD was effectively halted.1 In recent decades, however, there has been a quiet but growing renaissance of scientific interest in the effects of psychedelics on a variety of conditions, including terminal illness–related anxiety and depression, treatment-resistant depression, and substance use disorders (SUDs). One example is psilocybin, which is currently undergoing Phase 2 and 3 clinical trials in North America and Europe for treatment-resistant depression.
As researchers have once again picked up the torch in the pursuit of psychedelic therapeutics, jurisdictions in the United States are also relaxing their stance on these drugs. In 2019 and early 2020, Denver, Oakland, and Santa Cruz became the first 3 cities in the United States to decriminalize the possession of various psychedelic substances.2-4 With the passage of Measure 109 in November 2020, Oregon became the first state to decriminalize the use of psychedelic mushrooms in therapeutic settings.5 The combined forces of increased research and relaxed political concern related to psychedelics might make it possible for the FDA to approve their use for psychiatric conditions. Therefore, it is critical for psychiatrists to understand the psychopharmacology, range of effects, and potential risks and benefits of these agents. In this article, I describe what psychedelics are and how they work, summarize a few research findings about psilocybin, and offer a framework for psychedelic psychiatric practice in the years to come.
What are psychedelics?
Psychiatrist Humphry Osmond first coined the term “psychedelic” in 1957 at a meeting of the New York Academy of Sciences, where he was discussing his research on the effect of LSD on patients at the Weyburn Mental Hospital in Saskatchewan, Canada.6 Prior to 1957, LSD had been described as a “psychotomimetic” drug because it was believed to induce a state of psychosis similar to that experienced in schizophrenia. But LSD does not generally induce frank auditory hallucinations or clearly defined delusional beliefs. Osmond’s new term—derived from the Greek words psyche, meaning “mind,” and delos, meaning “to show”—referred to the “mind-manifesting” capacities of LSD and related drugs.6 Psychedelic drugs can cause an array of changes to an individual’s conscious experience, from relatively mild changes in visual perception to profound derangements in sense of self and reality.
Continue to: Before describing the effects...
Classic psychedelics vs other compounds
Before describing the effects of psychedelic drugs and how they may relate to their therapeutic potential, it is useful to define which compounds are considered “classic psychedelics.”
The classic psychedelics are substances that operate primarily through activation of the serotonin 5-hydroxytryptamine receptor 2A receptor (5-HT2A) (Table 17). Many psychedelic drugs are derived from natural sources, including plants, fungi, and animals. For example, N, N-dimethyltryptamine (DMT), which is one of the most potent psychedelic compounds, is found in various plant species and can be imbibed in a tea known as ayahuasca, most commonly in the context of spiritual ceremonies.
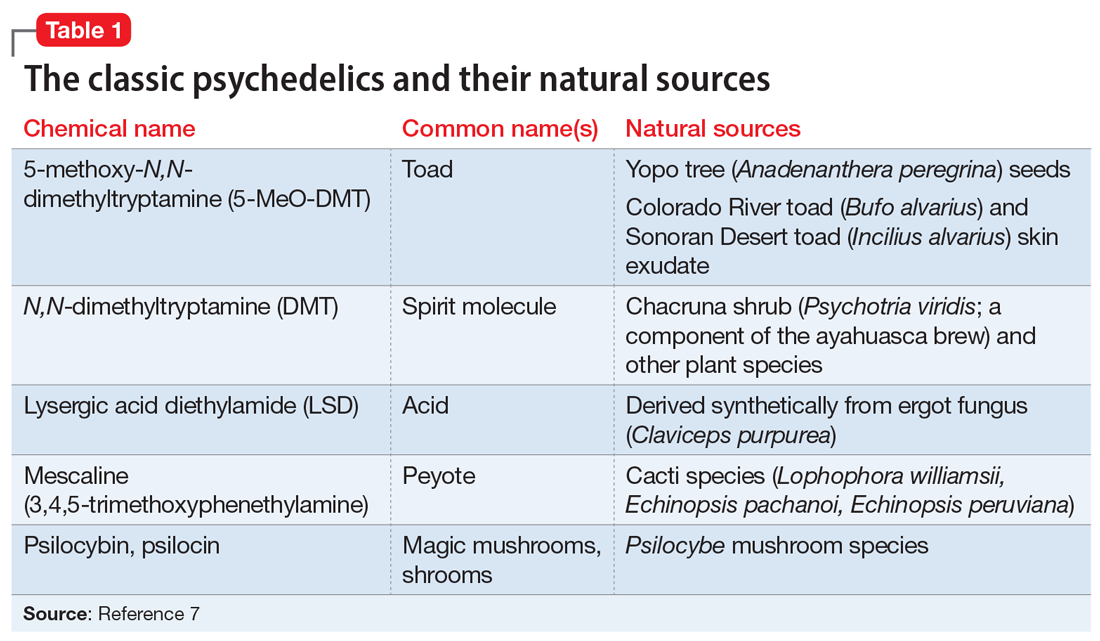
Other compounds. Some researchers continue to classify other compounds as “psychedelics,” although the mechanisms of action and effects of these compounds may vary greatly from those of the classic psychedelics. These include the dissociative anesthetics ketamine and phencyclidine (PCP), which exert their effects via N-methyl-
The DSM-58 does not differentiate between classic psychedelics and related compounds. In its chapter on Substance-Related and Addictive Disorders, the section Hallucinogen-Related Disorders provides criteria for the diagnoses of phencyclidine use disorder and other hallucinogen use disorder. Researchers generally have abandoned the term “hallucinogen” because psychedelics typically do not induce frank hallucinations. Furthermore, lumping psychedelics and compounds such as MDMA and ketamine into the category of “other hallucinogen” fails to address important distinctions between them, including diagnostically relevant issues. For example, psychedelics do not cause symptoms of physiologic dependence such as craving or a withdrawal syndrome, whereas MDMA can.9 The DSM-5 also contains a diagnosis called hallucinogen persisting perception disorder (HPPD), referring to residual distortions of visual perception that remain following psychedelic intoxication. Although the text notes the estimated prevalence of HPPD in individuals who use psychedelics is 4.2%, the condition is thought to occur infrequently in both therapeutic and recreational users.10
How psychedelics work
Psychedelics can induce a spectrum of effects that are not necessarily dose-dependent. Mild effects of intoxication include altered sensory perception in visual, auditory, proprioceptive, and somatosensory spheres, including synesthesia. Progressively more severe changes include a distorted or eliminated perception or awareness of space, time, body, and self, resulting in derealization and depersonalization. Some of the most extreme alterations of consciousness reported by users include mystical or transcendent experiences of birth, giving birth, death, exchanging bodies with a nonhuman species, and meeting otherworldly beings.11 In terms of neurophysiology, psychedelics cause altered cerebral blood flow and metabolism, increased connectivity between brain regions that do not typically communicate, and a reduction in the activity of a group of cortical structures called the default mode network (DMN).12
Continue to: Researchers hypothesize that...
Researchers hypothesize that the disruption of DMN activity may be a key mechanism accounting for psychedelics’ therapeutic effects in mental illness. The DMN is a group of structures that includes the posterior cingulate cortex, the medial prefrontal cortex, the angular gyrus, and other cortical areas that are active when an individual is not engaged in a particular mental task (for example, during mind wandering). It is thought to underlie introspection and to serve as an “orchestrator” of global brain function.13 Theoretically, then, by temporarily disrupting the neural circuits responsible for maintaining ingrained, negative thought and behavioral patterns, as observed in patients with depression or SUDs, psychedelics can help patients develop greater emotional and cognitive flexibility and identify new ways to view the world and to solve problems.
Evaluating psychedelics as therapeutic agents
The renaissance of research into psychedelics as therapeutic agents during the last 2 decades has produced some promising preliminary findings. In 2020, the American Psychiatric Association’s Work Group on Biomarkers and Novel Treatments published a review of the best evidence on the topic.14 Psilocybin is the most studied drug because compared with LSD, it carries less of a stigma and has a shorter duration of action. Psilocybin has been studied as a potential treatment for several psychiatric disorders, including terminal illness–related depression and anxiety, and SUDs.
Griffiths et al.15 In a double-blind randomized crossover study at Johns Hopkins School of Medicine, Griffiths et al15 administered a high dose (22 or 30 mg/70 kg) and a very low, placebo-like dose (1 or 3 mg/70 kg) of psilocybin at 2 separate sessions to 51 patients with terminal cancer and associated depressive and anxiety disorders. After 5 weeks, the participants assigned to one condition crossed over to the other condition. High-dose psilocybin had a significant effect on depression and anxiety symptoms within 5 weeks that persisted over 6 months of follow-up. At 6 months, 78% of participants experienced a response in depressive symptoms (≥50% decrease in GRID-Hamilton Depression Rating Scale [HAM-D-17] baseline scores) and 65% remitted (GRID-HAM-D-17 score ≤7). At 6 months, 83% of participants had a response in anxiety symptoms (≥50% decrease in Hamilton Rating Scale for Anxiety [HAM-A] baseline scores) and 57% remitted (HAM-A ≤7).
Johnson et al.16,17 In an open-label pilot study16 and ≥12-month follow-up study,17 Johnson et al administered a moderate (20 mg/70 kg) and high (30 mg/70 kg) dose of psilocybin to 15 participants enrolled in a 15-week smoking session program. The psilocybin sessions were scheduled at Weeks 5 and 7, with an optional psilocybin session at Week 13. The sessions included nondirective support from program staff, but not smoking cessation content. Relying on laboratory-verified exhaled carbon monoxide and urine cotinine measures, researchers found an 80% abstinence rate at 6 months, a 67% abstinence rate at 12 months, and a 75% abstinence rate at 2.5 years.16,17
Bogenschutz et al18 conducted a study of 10 patients who met DSM-IV criteria for alcohol dependence and had at least 2 heavy drinking days in the previous 30 days. They found that a 14-session treatment program that included 2 psilocybin-assisted psychotherapy sessions with dosages of 0.4 mg/kg resulted in a significant increase in self-reported alcohol abstinence at 4 weeks that persisted for 36 weeks.18
Although these studies were small, open-label, and had other methodologic flaws, their pilot work has led to larger-scale projects assessing psilocybin’s therapeutic potential. Psilocybin has also been studied for treatment-resistant depression and obsessive-compulsive disorder. Other clinical trials underway are investigating psilocybin for the treatment of cocaine and opioid use disorder, anorexia nervosa, and depression in Alzheimer’s disease.14 Although psilocybin is currently the best-studied psychedelic, there is some research demonstrating that LSD can also induce a persistent reduction in anxiety symptoms associated with terminal illness19 and that ayahuasca causes a rapid reduction in depressive symptoms that persists over 21 days.20
Continue to: The future of psychedelic psychiatry...
The future of psychedelic psychiatry
If psychedelic compounds become approved for the treatment of psychiatric conditions, psychiatrists will likely be responsible for prescribing them and managing patients who receive them.21Table 211,21-24 summarizes practical considerations for psychiatrists who may someday be prescribing psychedelic drugs. Areas of psychedelic treatment in which psychiatric expertise is necessary include:
- screening for patients at increased risk for a challenging or adverse experience or “bad trip”
- conducting a thorough informed consent process in which the risks are discussed and the patient’s wishes regarding potential situations are elicited
- managing acute medical and psychiatric complications, including agitation and violent behavior
- ensuring the use of trained guides during sessions.
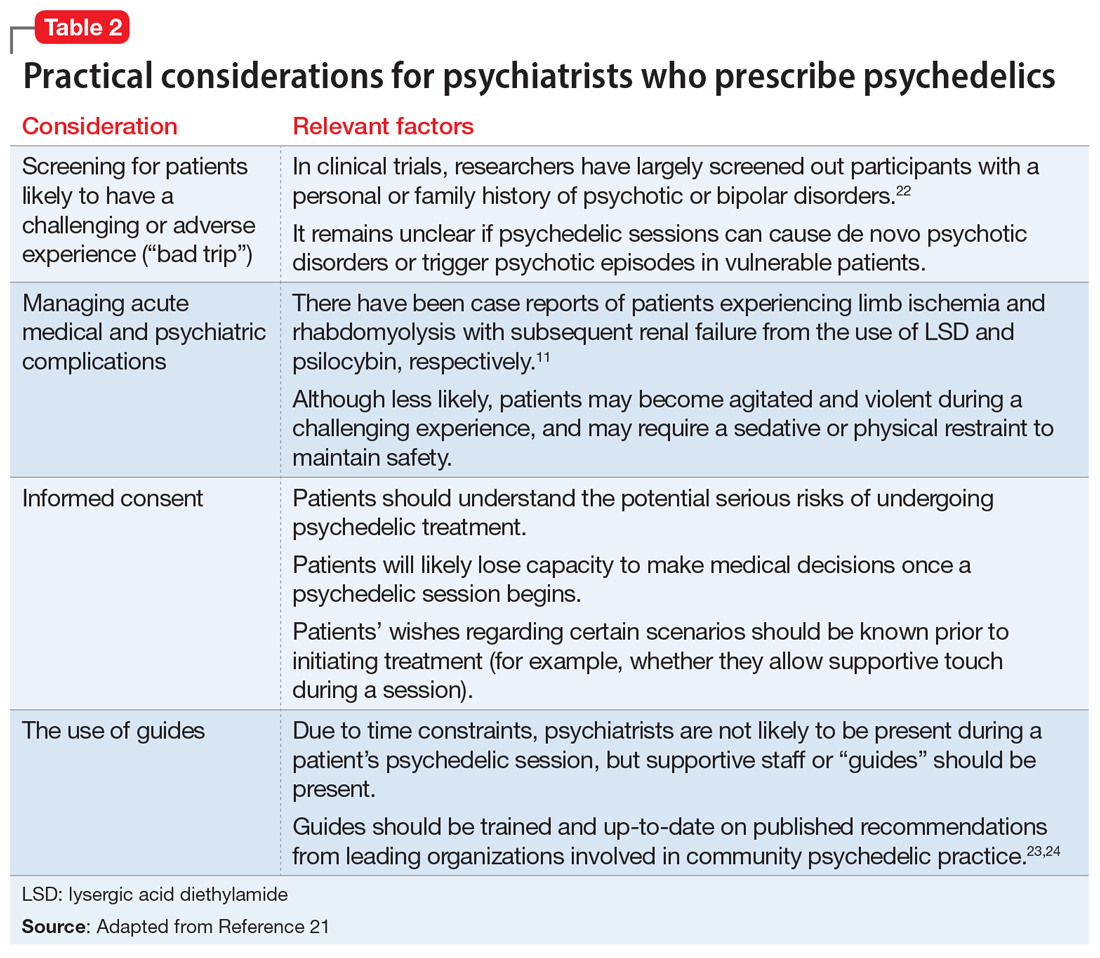
Psychiatrists who are interested in providing psychedelic-assisted therapy should understand the concept of “set and setting,” which was defined by Timothy Leary in the 1960s and is thought to play an important role in determining the types of experiences that arise during a psychedelic session.25 “Set” refers to an individual’s mindset going into a session, and “setting” refers to the environment in which the session occurs. Typical elements of each are summarized in Table 3.7 Psychiatrists will play a critical role in assessing and preparing the “set” by screening patients appropriately, assessing patient goals, and providing a thorough informed consent procedure. Psychiatrists should also be mindful of the “setting,” providing a comfortable, safe, familiar environment and access to appropriate music and eyeshades, if desired. Due to time restraints, psychiatrists are not likely to be responsible for guiding patients through sessions, and should educate themselves about ethical practices of psychedelic guides,if they are in the position to hire guides.23,24
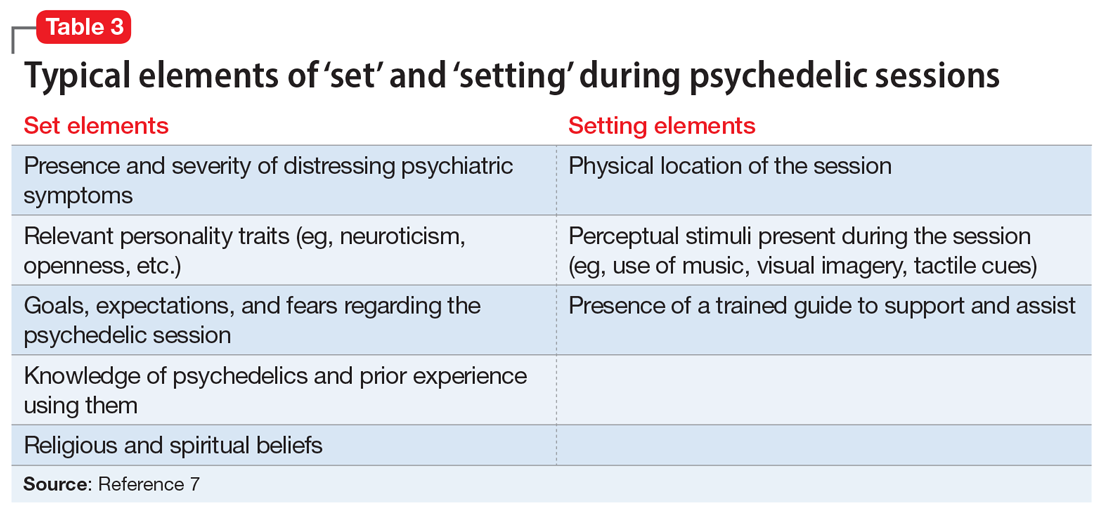
Psychiatrists may also play a role in providing psychotherapy to patients receiving treatment with psychedelics. These substances can induce both transcendent and terrifying experiences. Patients therefore require “integration” therapy sessions to assist with processing the content of their psychedelic treatment and incorporating the experiences into day-to-day life. In an online survey of nearly 2,000 individuals who used psilocybin recreationally, 7.6% reported that they had to seek treatment for enduring psychological symptoms that they attributed to their psilocybin use, including persistent anxiety, fear, paranoia, and depression.26 Integrative psychotherapy sessions may help reduce the risk of persistent negative effects from therapeutic psychedelics, as well as enhance their beneficial effects.
CASE CONTINUED
Mr. P is enrolled in the academic medical center study assessing the effect of psilocybin on terminal illness-related anxiety and depression. During a 5-hour, 30-mg psilocybin session, he initially experiences distorted visual cues, with vivid, colorful geometric patterns collapsing into each other. He then loses the concepts and experience of time, space, and his body, as his visual distortions convert to darkness. After what seems like a decade within the darkness, he sees himself lying in a hospital bed with loved ones surrounding him. He watches himself take his last breaths and his family members weep as he dies. As he regains his senses, Mr. P feels that he is being reborn.
In the therapy sessions that follow the psychedelic session, Mr. P reports feeling “finally freed” from the fear, sadness, and anger that he has felt throughout his life. He comes to accept his impending death with gratitude and peace. In his final days, he no longer experiences depression or anxiety. Mr. P’s friends and family members comment that he seems to be the best version of himself in the months that lead up to his death.
Related Resources
• Nutt D. Psychedelic drugs-a new era in psychiatry? Dialogues Clin Neurosci. 2019;21(2):139-147.
• Garcia-Romeu A, Kersgaard B, Addy PH. Clinical applications of hallucinogens: a review. Exp Clin Psychopharmacol. 2016; 24(4):229-268.
Drug Brand Names
Amitriptyline • Amitril, Elavil
Bupropion • Wellbutrin
Fluoxetine • Prozac
Sertraline • Zoloft
Bottom Line
Psychedelics are a class of consciousness-altering agents that have become a potentially promising source of new treatments for psychiatric illness. Although more evidence is needed, compounds such as psilocybin may one day become FDAapproved for conditions such as terminal illness–related depression and anxiety, and substance use disorders. When this occurs, psychiatrists should be responsible for prescribing psychedelics and managing patients who receive treatment.
1. Smith DE, Raswyck GE, Davidson LD. From Hofmann to the Haight Ashbury, and into the future: the past and potential of lysergic acid diethylamide. J Psychoactive Drugs. 2014;46(1):3-10.
2. Siegel M. Threading Denver’s magic mushrooms needle: promising as medicine, risky as recreation. USA Today. Published May 13, 2019. Accessed December 4, 2020. https://www.usatoday.com/story/opinion/2019/05/13/denver-magic-mushrooms-promising-medicine-reckless-recreation-column/1182543001
3. Epstein, K. Oakland decriminalizes ‘magic mushrooms’ and other natural psychedelics. The Washington Post. Published June 5, 2019. Accessed December 4, 2020. https://www.washingtonpost.com/nation/2019/06/05/oakland-decriminalizes-magic-mushrooms-other-natural-psychedelics
4. York JA. Santa Cruz decriminalizes natural psychedelics. Santa Cruz Sentinel. Published January 30, 2020. Accessed December 4, 2020. https://www.santacruzsentinel.com/2020/01/29/santa-cruz-decriminalizes-natural-psychedelics
5. Acker L. Oregon becomes first state to legalize psychedelic mushrooms. The Oregonian/Oregon Live. Published November 4, 2020. Accessed December 4, 2020. https://www.oregonlive.com/politics/2020/11/oregon-becomes-first-state-to-legalize-psychedelic-mushrooms.html
6. Dyck E. Flashback: psychiatric experimentation with LSD in historical perspective. Can J Psychiatry. 2005;50(7):381-388.
7. Holoyda BJ. The psychedelic renaissance and its forensic implications. J Am Acad Psychiatry Law. 2020;48(1):87-97.
8. Diagnostic and statistical manual of mental disorders, 5th ed. American Psychiatric Association; 2013.
9. Davis AK, Rosenberg H. The prevalence, intensity, and assessment of craving for MDMA/ecstasy in recreational users. J Psychoactive Drugs. 2014;46(2):154-151.
10. Halpern JH, Lerner AG, Passie T. A review of hallucinogen persisting perception disorder (HPPD) and an exploratory study of subjects claiming symptoms of HPPD. Curr Top Behav Neurosci. 2018;36:333-360.
11. Nichols DE. Psychedelics. Pharmacol Rev. 2016;68(2):264-355.
12. Nichols DE. Hallucinogens. Pharmacol Ther. 2004;101(2):131-181.
13. Carhart-Harris RL, Leech R, Hellyer PJ, et al. The entropic brain: a theory of conscious states informed by neuroimaging research with psychedelic drugs. Front Hum Neurosci. 2014;8:20.
14. Reiff CM, Richman EE, Nemeroff CB, et al. Psychedelics and psychedelic-assisted psychotherapy. Am J Psychiatry. 2020;177(5):391-410.
15. Griffiths RR, Johnson MW, Carducci MA, et al. Psilocybin produces substantial and sustained decreases in depression and anxiety in patients with life-threatening cancer: a randomized double-blind trial. J Psychopharmacol. 2016;30(12):1181-1197.
16. Johnson MW, Garcia-Romeu A, Cosimano MP, et al. Pilot study of the 5-HT2AR agonist psilocybin in the treatment of tobacco addiction. J Psychopharmacol. 2014;28(11):983-992.
17. Johnson MW, Garcia-Romeu A, Griffiths RR. Long-term follow-up of psilocybin-facilitated smoking cessation. Am J Drug Alcohol Abuse. 2017;43(1):55-60.
18. Bogenschutz MP, Forcehimes AA, Pommy JA, et al. Psilocybin-assisted treatment for alcohol dependence: a proof-of-concept study. J Psychopharmacol. 2015;29(3):1182-1190.
19. Gasser P, Holstein D, Michel Y, et al. Safety and efficacy of lysergic acid diethylamide-assisted psychotherapy for anxiety associated with life-threatening diseases. J Nerv Ment Dis. 2014;202(7):531-520.
20. Osório F de L, Sanches RF, Macedo LR, et al. Antidepressant effects of a single dose of ayahuasca in patients with recurrent depression: a preliminary report. Braz J Psychiatry. 2015;37(1):13-20.
21. Holoyda B. Psychedelic psychiatry: preparing for novel treatments involving altered states of consciousness. Psych Serv. 2020;71(12):1297-1299.
22. Johnson MW, Richards W, Griffiths RR. Human hallucinogen research: guidelines for safety. J Psychopharmacol. 2008;22(6):603-620.
23. Council on Spiritual Practices. Code of ethics for spiritual Guides. Published August 10, 2001. Accessed November 25, 2020. https://csp.org/docs/code-of-ethics-for-spiritual-guides
24. Multidisciplinary Association for Psychedelic Studies. Zendo psychedelic harm reduction training manual. Published 2017. Accessed November 25, 2020. https://zendoproject.org/wp-content/uploads/2017/06/Zendo-Manual-2017.pdf
25. Zinberg NE. Drug, set, and setting: the basis for controlled intoxicant use. Yale University Press; 1984.
26. Carbonaro TM, Bradstreet MP, Barrett FS, et al. Survey study of challenging experiences after ingesting psilocybin mushrooms: acute and enduring positive and negative consequences. J Psychopharmacol. 2016;30(12):1268-1278.
Mr. P, age 65, has a history of major depressive disorder (MDD), generalized anxiety disorder, and social phobia. Mr. P’s personality is high in neuroticism and he has often responded to new situations with feelings of impending doom. For him, fear, anxious rumination, helplessness, and catastrophizing are familiar mental processes.
When he was in his 30s, Mr. P had a severe major depressive episode with suicidal ideation and sought care from a psychiatrist. He began a treatment program of psychotherapy and concomitant psychopharmacotherapy with consecutive trials of fluoxetine, sertraline, and amitriptyline, each of an adequate dose and duration. With each medication, Mr. P experienced new adverse effects, including nausea, constipation, tremors, and headache. His psychiatrist transitioned him to bupropion, which helped Mr. P most. For the next several decades, Mr. P continued to experience low-grade depressive symptoms with intermittent exacerbation to mild-to-moderate major depressive episodes, but he remained adherent to his medication and continued psychotherapy.
Shortly after his 65th birthday, Mr. P experiences progressively worsening nausea and abdominal pain. Initially, he assumes the symptoms are secondary to anxiety. Taking his psychiatrist’s advice, Mr. P visits his primary care physician. A work-up reveals that Mr. P has advanced pancreatic cancer, and an oncologist estimates Mr. P has 6 months of life remaining.
Following his cancer diagnosis, Mr. P quickly develops symptoms of MDD despite continuing to take bupropion. Within a week he becomes withdrawn and hopeless, and thinks about ending his life “before God does.” His psychiatrist urges Mr. P to contact the local academic medical center because it is conducting a trial of a “new” drug, psilocybin, to treat anxiety and depression in patients with terminal illness.
Beginning in the 1940s, a growing body of scientific evidence suggested that psychedelic compounds such as lysergic acid diethylamide (LSD) could benefit individuals with various psychiatric maladies. Research interest in LSD and substances with similar effects persisted until the late 1960s. In response to the growing counterculture movement in the United States and the efforts of Harvard researchers Timothy Leary and Richard Alpert to popularize psychedelic drug use in the general population, in 1970 President Richard M. Nixon signed the Controlled Substances Act (CSA) into law. The CSA categorized LSD as a Schedule I drug, rendering its manufacture and distribution illegal. Research into the potential therapeutic benefits of LSD was effectively halted.1 In recent decades, however, there has been a quiet but growing renaissance of scientific interest in the effects of psychedelics on a variety of conditions, including terminal illness–related anxiety and depression, treatment-resistant depression, and substance use disorders (SUDs). One example is psilocybin, which is currently undergoing Phase 2 and 3 clinical trials in North America and Europe for treatment-resistant depression.
As researchers have once again picked up the torch in the pursuit of psychedelic therapeutics, jurisdictions in the United States are also relaxing their stance on these drugs. In 2019 and early 2020, Denver, Oakland, and Santa Cruz became the first 3 cities in the United States to decriminalize the possession of various psychedelic substances.2-4 With the passage of Measure 109 in November 2020, Oregon became the first state to decriminalize the use of psychedelic mushrooms in therapeutic settings.5 The combined forces of increased research and relaxed political concern related to psychedelics might make it possible for the FDA to approve their use for psychiatric conditions. Therefore, it is critical for psychiatrists to understand the psychopharmacology, range of effects, and potential risks and benefits of these agents. In this article, I describe what psychedelics are and how they work, summarize a few research findings about psilocybin, and offer a framework for psychedelic psychiatric practice in the years to come.
What are psychedelics?
Psychiatrist Humphry Osmond first coined the term “psychedelic” in 1957 at a meeting of the New York Academy of Sciences, where he was discussing his research on the effect of LSD on patients at the Weyburn Mental Hospital in Saskatchewan, Canada.6 Prior to 1957, LSD had been described as a “psychotomimetic” drug because it was believed to induce a state of psychosis similar to that experienced in schizophrenia. But LSD does not generally induce frank auditory hallucinations or clearly defined delusional beliefs. Osmond’s new term—derived from the Greek words psyche, meaning “mind,” and delos, meaning “to show”—referred to the “mind-manifesting” capacities of LSD and related drugs.6 Psychedelic drugs can cause an array of changes to an individual’s conscious experience, from relatively mild changes in visual perception to profound derangements in sense of self and reality.
Continue to: Before describing the effects...
Classic psychedelics vs other compounds
Before describing the effects of psychedelic drugs and how they may relate to their therapeutic potential, it is useful to define which compounds are considered “classic psychedelics.”
The classic psychedelics are substances that operate primarily through activation of the serotonin 5-hydroxytryptamine receptor 2A receptor (5-HT2A) (Table 17). Many psychedelic drugs are derived from natural sources, including plants, fungi, and animals. For example, N, N-dimethyltryptamine (DMT), which is one of the most potent psychedelic compounds, is found in various plant species and can be imbibed in a tea known as ayahuasca, most commonly in the context of spiritual ceremonies.

Other compounds. Some researchers continue to classify other compounds as “psychedelics,” although the mechanisms of action and effects of these compounds may vary greatly from those of the classic psychedelics. These include the dissociative anesthetics ketamine and phencyclidine (PCP), which exert their effects via N-methyl-
The DSM-58 does not differentiate between classic psychedelics and related compounds. In its chapter on Substance-Related and Addictive Disorders, the section Hallucinogen-Related Disorders provides criteria for the diagnoses of phencyclidine use disorder and other hallucinogen use disorder. Researchers generally have abandoned the term “hallucinogen” because psychedelics typically do not induce frank hallucinations. Furthermore, lumping psychedelics and compounds such as MDMA and ketamine into the category of “other hallucinogen” fails to address important distinctions between them, including diagnostically relevant issues. For example, psychedelics do not cause symptoms of physiologic dependence such as craving or a withdrawal syndrome, whereas MDMA can.9 The DSM-5 also contains a diagnosis called hallucinogen persisting perception disorder (HPPD), referring to residual distortions of visual perception that remain following psychedelic intoxication. Although the text notes the estimated prevalence of HPPD in individuals who use psychedelics is 4.2%, the condition is thought to occur infrequently in both therapeutic and recreational users.10
How psychedelics work
Psychedelics can induce a spectrum of effects that are not necessarily dose-dependent. Mild effects of intoxication include altered sensory perception in visual, auditory, proprioceptive, and somatosensory spheres, including synesthesia. Progressively more severe changes include a distorted or eliminated perception or awareness of space, time, body, and self, resulting in derealization and depersonalization. Some of the most extreme alterations of consciousness reported by users include mystical or transcendent experiences of birth, giving birth, death, exchanging bodies with a nonhuman species, and meeting otherworldly beings.11 In terms of neurophysiology, psychedelics cause altered cerebral blood flow and metabolism, increased connectivity between brain regions that do not typically communicate, and a reduction in the activity of a group of cortical structures called the default mode network (DMN).12
Continue to: Researchers hypothesize that...
Researchers hypothesize that the disruption of DMN activity may be a key mechanism accounting for psychedelics’ therapeutic effects in mental illness. The DMN is a group of structures that includes the posterior cingulate cortex, the medial prefrontal cortex, the angular gyrus, and other cortical areas that are active when an individual is not engaged in a particular mental task (for example, during mind wandering). It is thought to underlie introspection and to serve as an “orchestrator” of global brain function.13 Theoretically, then, by temporarily disrupting the neural circuits responsible for maintaining ingrained, negative thought and behavioral patterns, as observed in patients with depression or SUDs, psychedelics can help patients develop greater emotional and cognitive flexibility and identify new ways to view the world and to solve problems.
Evaluating psychedelics as therapeutic agents
The renaissance of research into psychedelics as therapeutic agents during the last 2 decades has produced some promising preliminary findings. In 2020, the American Psychiatric Association’s Work Group on Biomarkers and Novel Treatments published a review of the best evidence on the topic.14 Psilocybin is the most studied drug because compared with LSD, it carries less of a stigma and has a shorter duration of action. Psilocybin has been studied as a potential treatment for several psychiatric disorders, including terminal illness–related depression and anxiety, and SUDs.
Griffiths et al.15 In a double-blind randomized crossover study at Johns Hopkins School of Medicine, Griffiths et al15 administered a high dose (22 or 30 mg/70 kg) and a very low, placebo-like dose (1 or 3 mg/70 kg) of psilocybin at 2 separate sessions to 51 patients with terminal cancer and associated depressive and anxiety disorders. After 5 weeks, the participants assigned to one condition crossed over to the other condition. High-dose psilocybin had a significant effect on depression and anxiety symptoms within 5 weeks that persisted over 6 months of follow-up. At 6 months, 78% of participants experienced a response in depressive symptoms (≥50% decrease in GRID-Hamilton Depression Rating Scale [HAM-D-17] baseline scores) and 65% remitted (GRID-HAM-D-17 score ≤7). At 6 months, 83% of participants had a response in anxiety symptoms (≥50% decrease in Hamilton Rating Scale for Anxiety [HAM-A] baseline scores) and 57% remitted (HAM-A ≤7).
Johnson et al.16,17 In an open-label pilot study16 and ≥12-month follow-up study,17 Johnson et al administered a moderate (20 mg/70 kg) and high (30 mg/70 kg) dose of psilocybin to 15 participants enrolled in a 15-week smoking session program. The psilocybin sessions were scheduled at Weeks 5 and 7, with an optional psilocybin session at Week 13. The sessions included nondirective support from program staff, but not smoking cessation content. Relying on laboratory-verified exhaled carbon monoxide and urine cotinine measures, researchers found an 80% abstinence rate at 6 months, a 67% abstinence rate at 12 months, and a 75% abstinence rate at 2.5 years.16,17
Bogenschutz et al18 conducted a study of 10 patients who met DSM-IV criteria for alcohol dependence and had at least 2 heavy drinking days in the previous 30 days. They found that a 14-session treatment program that included 2 psilocybin-assisted psychotherapy sessions with dosages of 0.4 mg/kg resulted in a significant increase in self-reported alcohol abstinence at 4 weeks that persisted for 36 weeks.18
Although these studies were small, open-label, and had other methodologic flaws, their pilot work has led to larger-scale projects assessing psilocybin’s therapeutic potential. Psilocybin has also been studied for treatment-resistant depression and obsessive-compulsive disorder. Other clinical trials underway are investigating psilocybin for the treatment of cocaine and opioid use disorder, anorexia nervosa, and depression in Alzheimer’s disease.14 Although psilocybin is currently the best-studied psychedelic, there is some research demonstrating that LSD can also induce a persistent reduction in anxiety symptoms associated with terminal illness19 and that ayahuasca causes a rapid reduction in depressive symptoms that persists over 21 days.20
Continue to: The future of psychedelic psychiatry...
The future of psychedelic psychiatry
If psychedelic compounds become approved for the treatment of psychiatric conditions, psychiatrists will likely be responsible for prescribing them and managing patients who receive them.21Table 211,21-24 summarizes practical considerations for psychiatrists who may someday be prescribing psychedelic drugs. Areas of psychedelic treatment in which psychiatric expertise is necessary include:
- screening for patients at increased risk for a challenging or adverse experience or “bad trip”
- conducting a thorough informed consent process in which the risks are discussed and the patient’s wishes regarding potential situations are elicited
- managing acute medical and psychiatric complications, including agitation and violent behavior
- ensuring the use of trained guides during sessions.

Psychiatrists who are interested in providing psychedelic-assisted therapy should understand the concept of “set and setting,” which was defined by Timothy Leary in the 1960s and is thought to play an important role in determining the types of experiences that arise during a psychedelic session.25 “Set” refers to an individual’s mindset going into a session, and “setting” refers to the environment in which the session occurs. Typical elements of each are summarized in Table 3.7 Psychiatrists will play a critical role in assessing and preparing the “set” by screening patients appropriately, assessing patient goals, and providing a thorough informed consent procedure. Psychiatrists should also be mindful of the “setting,” providing a comfortable, safe, familiar environment and access to appropriate music and eyeshades, if desired. Due to time restraints, psychiatrists are not likely to be responsible for guiding patients through sessions, and should educate themselves about ethical practices of psychedelic guides,if they are in the position to hire guides.23,24

Psychiatrists may also play a role in providing psychotherapy to patients receiving treatment with psychedelics. These substances can induce both transcendent and terrifying experiences. Patients therefore require “integration” therapy sessions to assist with processing the content of their psychedelic treatment and incorporating the experiences into day-to-day life. In an online survey of nearly 2,000 individuals who used psilocybin recreationally, 7.6% reported that they had to seek treatment for enduring psychological symptoms that they attributed to their psilocybin use, including persistent anxiety, fear, paranoia, and depression.26 Integrative psychotherapy sessions may help reduce the risk of persistent negative effects from therapeutic psychedelics, as well as enhance their beneficial effects.
CASE CONTINUED
Mr. P is enrolled in the academic medical center study assessing the effect of psilocybin on terminal illness-related anxiety and depression. During a 5-hour, 30-mg psilocybin session, he initially experiences distorted visual cues, with vivid, colorful geometric patterns collapsing into each other. He then loses the concepts and experience of time, space, and his body, as his visual distortions convert to darkness. After what seems like a decade within the darkness, he sees himself lying in a hospital bed with loved ones surrounding him. He watches himself take his last breaths and his family members weep as he dies. As he regains his senses, Mr. P feels that he is being reborn.
In the therapy sessions that follow the psychedelic session, Mr. P reports feeling “finally freed” from the fear, sadness, and anger that he has felt throughout his life. He comes to accept his impending death with gratitude and peace. In his final days, he no longer experiences depression or anxiety. Mr. P’s friends and family members comment that he seems to be the best version of himself in the months that lead up to his death.
Related Resources
• Nutt D. Psychedelic drugs-a new era in psychiatry? Dialogues Clin Neurosci. 2019;21(2):139-147.
• Garcia-Romeu A, Kersgaard B, Addy PH. Clinical applications of hallucinogens: a review. Exp Clin Psychopharmacol. 2016; 24(4):229-268.
Drug Brand Names
Amitriptyline • Amitril, Elavil
Bupropion • Wellbutrin
Fluoxetine • Prozac
Sertraline • Zoloft
Bottom Line
Psychedelics are a class of consciousness-altering agents that have become a potentially promising source of new treatments for psychiatric illness. Although more evidence is needed, compounds such as psilocybin may one day become FDAapproved for conditions such as terminal illness–related depression and anxiety, and substance use disorders. When this occurs, psychiatrists should be responsible for prescribing psychedelics and managing patients who receive treatment.
Mr. P, age 65, has a history of major depressive disorder (MDD), generalized anxiety disorder, and social phobia. Mr. P’s personality is high in neuroticism and he has often responded to new situations with feelings of impending doom. For him, fear, anxious rumination, helplessness, and catastrophizing are familiar mental processes.
When he was in his 30s, Mr. P had a severe major depressive episode with suicidal ideation and sought care from a psychiatrist. He began a treatment program of psychotherapy and concomitant psychopharmacotherapy with consecutive trials of fluoxetine, sertraline, and amitriptyline, each of an adequate dose and duration. With each medication, Mr. P experienced new adverse effects, including nausea, constipation, tremors, and headache. His psychiatrist transitioned him to bupropion, which helped Mr. P most. For the next several decades, Mr. P continued to experience low-grade depressive symptoms with intermittent exacerbation to mild-to-moderate major depressive episodes, but he remained adherent to his medication and continued psychotherapy.
Shortly after his 65th birthday, Mr. P experiences progressively worsening nausea and abdominal pain. Initially, he assumes the symptoms are secondary to anxiety. Taking his psychiatrist’s advice, Mr. P visits his primary care physician. A work-up reveals that Mr. P has advanced pancreatic cancer, and an oncologist estimates Mr. P has 6 months of life remaining.
Following his cancer diagnosis, Mr. P quickly develops symptoms of MDD despite continuing to take bupropion. Within a week he becomes withdrawn and hopeless, and thinks about ending his life “before God does.” His psychiatrist urges Mr. P to contact the local academic medical center because it is conducting a trial of a “new” drug, psilocybin, to treat anxiety and depression in patients with terminal illness.
Beginning in the 1940s, a growing body of scientific evidence suggested that psychedelic compounds such as lysergic acid diethylamide (LSD) could benefit individuals with various psychiatric maladies. Research interest in LSD and substances with similar effects persisted until the late 1960s. In response to the growing counterculture movement in the United States and the efforts of Harvard researchers Timothy Leary and Richard Alpert to popularize psychedelic drug use in the general population, in 1970 President Richard M. Nixon signed the Controlled Substances Act (CSA) into law. The CSA categorized LSD as a Schedule I drug, rendering its manufacture and distribution illegal. Research into the potential therapeutic benefits of LSD was effectively halted.1 In recent decades, however, there has been a quiet but growing renaissance of scientific interest in the effects of psychedelics on a variety of conditions, including terminal illness–related anxiety and depression, treatment-resistant depression, and substance use disorders (SUDs). One example is psilocybin, which is currently undergoing Phase 2 and 3 clinical trials in North America and Europe for treatment-resistant depression.
As researchers have once again picked up the torch in the pursuit of psychedelic therapeutics, jurisdictions in the United States are also relaxing their stance on these drugs. In 2019 and early 2020, Denver, Oakland, and Santa Cruz became the first 3 cities in the United States to decriminalize the possession of various psychedelic substances.2-4 With the passage of Measure 109 in November 2020, Oregon became the first state to decriminalize the use of psychedelic mushrooms in therapeutic settings.5 The combined forces of increased research and relaxed political concern related to psychedelics might make it possible for the FDA to approve their use for psychiatric conditions. Therefore, it is critical for psychiatrists to understand the psychopharmacology, range of effects, and potential risks and benefits of these agents. In this article, I describe what psychedelics are and how they work, summarize a few research findings about psilocybin, and offer a framework for psychedelic psychiatric practice in the years to come.
What are psychedelics?
Psychiatrist Humphry Osmond first coined the term “psychedelic” in 1957 at a meeting of the New York Academy of Sciences, where he was discussing his research on the effect of LSD on patients at the Weyburn Mental Hospital in Saskatchewan, Canada.6 Prior to 1957, LSD had been described as a “psychotomimetic” drug because it was believed to induce a state of psychosis similar to that experienced in schizophrenia. But LSD does not generally induce frank auditory hallucinations or clearly defined delusional beliefs. Osmond’s new term—derived from the Greek words psyche, meaning “mind,” and delos, meaning “to show”—referred to the “mind-manifesting” capacities of LSD and related drugs.6 Psychedelic drugs can cause an array of changes to an individual’s conscious experience, from relatively mild changes in visual perception to profound derangements in sense of self and reality.
Continue to: Before describing the effects...
Classic psychedelics vs other compounds
Before describing the effects of psychedelic drugs and how they may relate to their therapeutic potential, it is useful to define which compounds are considered “classic psychedelics.”
The classic psychedelics are substances that operate primarily through activation of the serotonin 5-hydroxytryptamine receptor 2A receptor (5-HT2A) (Table 17). Many psychedelic drugs are derived from natural sources, including plants, fungi, and animals. For example, N, N-dimethyltryptamine (DMT), which is one of the most potent psychedelic compounds, is found in various plant species and can be imbibed in a tea known as ayahuasca, most commonly in the context of spiritual ceremonies.

Other compounds. Some researchers continue to classify other compounds as “psychedelics,” although the mechanisms of action and effects of these compounds may vary greatly from those of the classic psychedelics. These include the dissociative anesthetics ketamine and phencyclidine (PCP), which exert their effects via N-methyl-
The DSM-58 does not differentiate between classic psychedelics and related compounds. In its chapter on Substance-Related and Addictive Disorders, the section Hallucinogen-Related Disorders provides criteria for the diagnoses of phencyclidine use disorder and other hallucinogen use disorder. Researchers generally have abandoned the term “hallucinogen” because psychedelics typically do not induce frank hallucinations. Furthermore, lumping psychedelics and compounds such as MDMA and ketamine into the category of “other hallucinogen” fails to address important distinctions between them, including diagnostically relevant issues. For example, psychedelics do not cause symptoms of physiologic dependence such as craving or a withdrawal syndrome, whereas MDMA can.9 The DSM-5 also contains a diagnosis called hallucinogen persisting perception disorder (HPPD), referring to residual distortions of visual perception that remain following psychedelic intoxication. Although the text notes the estimated prevalence of HPPD in individuals who use psychedelics is 4.2%, the condition is thought to occur infrequently in both therapeutic and recreational users.10
How psychedelics work
Psychedelics can induce a spectrum of effects that are not necessarily dose-dependent. Mild effects of intoxication include altered sensory perception in visual, auditory, proprioceptive, and somatosensory spheres, including synesthesia. Progressively more severe changes include a distorted or eliminated perception or awareness of space, time, body, and self, resulting in derealization and depersonalization. Some of the most extreme alterations of consciousness reported by users include mystical or transcendent experiences of birth, giving birth, death, exchanging bodies with a nonhuman species, and meeting otherworldly beings.11 In terms of neurophysiology, psychedelics cause altered cerebral blood flow and metabolism, increased connectivity between brain regions that do not typically communicate, and a reduction in the activity of a group of cortical structures called the default mode network (DMN).12
Continue to: Researchers hypothesize that...
Researchers hypothesize that the disruption of DMN activity may be a key mechanism accounting for psychedelics’ therapeutic effects in mental illness. The DMN is a group of structures that includes the posterior cingulate cortex, the medial prefrontal cortex, the angular gyrus, and other cortical areas that are active when an individual is not engaged in a particular mental task (for example, during mind wandering). It is thought to underlie introspection and to serve as an “orchestrator” of global brain function.13 Theoretically, then, by temporarily disrupting the neural circuits responsible for maintaining ingrained, negative thought and behavioral patterns, as observed in patients with depression or SUDs, psychedelics can help patients develop greater emotional and cognitive flexibility and identify new ways to view the world and to solve problems.
Evaluating psychedelics as therapeutic agents
The renaissance of research into psychedelics as therapeutic agents during the last 2 decades has produced some promising preliminary findings. In 2020, the American Psychiatric Association’s Work Group on Biomarkers and Novel Treatments published a review of the best evidence on the topic.14 Psilocybin is the most studied drug because compared with LSD, it carries less of a stigma and has a shorter duration of action. Psilocybin has been studied as a potential treatment for several psychiatric disorders, including terminal illness–related depression and anxiety, and SUDs.
Griffiths et al.15 In a double-blind randomized crossover study at Johns Hopkins School of Medicine, Griffiths et al15 administered a high dose (22 or 30 mg/70 kg) and a very low, placebo-like dose (1 or 3 mg/70 kg) of psilocybin at 2 separate sessions to 51 patients with terminal cancer and associated depressive and anxiety disorders. After 5 weeks, the participants assigned to one condition crossed over to the other condition. High-dose psilocybin had a significant effect on depression and anxiety symptoms within 5 weeks that persisted over 6 months of follow-up. At 6 months, 78% of participants experienced a response in depressive symptoms (≥50% decrease in GRID-Hamilton Depression Rating Scale [HAM-D-17] baseline scores) and 65% remitted (GRID-HAM-D-17 score ≤7). At 6 months, 83% of participants had a response in anxiety symptoms (≥50% decrease in Hamilton Rating Scale for Anxiety [HAM-A] baseline scores) and 57% remitted (HAM-A ≤7).
Johnson et al.16,17 In an open-label pilot study16 and ≥12-month follow-up study,17 Johnson et al administered a moderate (20 mg/70 kg) and high (30 mg/70 kg) dose of psilocybin to 15 participants enrolled in a 15-week smoking session program. The psilocybin sessions were scheduled at Weeks 5 and 7, with an optional psilocybin session at Week 13. The sessions included nondirective support from program staff, but not smoking cessation content. Relying on laboratory-verified exhaled carbon monoxide and urine cotinine measures, researchers found an 80% abstinence rate at 6 months, a 67% abstinence rate at 12 months, and a 75% abstinence rate at 2.5 years.16,17
Bogenschutz et al18 conducted a study of 10 patients who met DSM-IV criteria for alcohol dependence and had at least 2 heavy drinking days in the previous 30 days. They found that a 14-session treatment program that included 2 psilocybin-assisted psychotherapy sessions with dosages of 0.4 mg/kg resulted in a significant increase in self-reported alcohol abstinence at 4 weeks that persisted for 36 weeks.18
Although these studies were small, open-label, and had other methodologic flaws, their pilot work has led to larger-scale projects assessing psilocybin’s therapeutic potential. Psilocybin has also been studied for treatment-resistant depression and obsessive-compulsive disorder. Other clinical trials underway are investigating psilocybin for the treatment of cocaine and opioid use disorder, anorexia nervosa, and depression in Alzheimer’s disease.14 Although psilocybin is currently the best-studied psychedelic, there is some research demonstrating that LSD can also induce a persistent reduction in anxiety symptoms associated with terminal illness19 and that ayahuasca causes a rapid reduction in depressive symptoms that persists over 21 days.20
Continue to: The future of psychedelic psychiatry...
The future of psychedelic psychiatry
If psychedelic compounds become approved for the treatment of psychiatric conditions, psychiatrists will likely be responsible for prescribing them and managing patients who receive them.21Table 211,21-24 summarizes practical considerations for psychiatrists who may someday be prescribing psychedelic drugs. Areas of psychedelic treatment in which psychiatric expertise is necessary include:
- screening for patients at increased risk for a challenging or adverse experience or “bad trip”
- conducting a thorough informed consent process in which the risks are discussed and the patient’s wishes regarding potential situations are elicited
- managing acute medical and psychiatric complications, including agitation and violent behavior
- ensuring the use of trained guides during sessions.

Psychiatrists who are interested in providing psychedelic-assisted therapy should understand the concept of “set and setting,” which was defined by Timothy Leary in the 1960s and is thought to play an important role in determining the types of experiences that arise during a psychedelic session.25 “Set” refers to an individual’s mindset going into a session, and “setting” refers to the environment in which the session occurs. Typical elements of each are summarized in Table 3.7 Psychiatrists will play a critical role in assessing and preparing the “set” by screening patients appropriately, assessing patient goals, and providing a thorough informed consent procedure. Psychiatrists should also be mindful of the “setting,” providing a comfortable, safe, familiar environment and access to appropriate music and eyeshades, if desired. Due to time restraints, psychiatrists are not likely to be responsible for guiding patients through sessions, and should educate themselves about ethical practices of psychedelic guides,if they are in the position to hire guides.23,24

Psychiatrists may also play a role in providing psychotherapy to patients receiving treatment with psychedelics. These substances can induce both transcendent and terrifying experiences. Patients therefore require “integration” therapy sessions to assist with processing the content of their psychedelic treatment and incorporating the experiences into day-to-day life. In an online survey of nearly 2,000 individuals who used psilocybin recreationally, 7.6% reported that they had to seek treatment for enduring psychological symptoms that they attributed to their psilocybin use, including persistent anxiety, fear, paranoia, and depression.26 Integrative psychotherapy sessions may help reduce the risk of persistent negative effects from therapeutic psychedelics, as well as enhance their beneficial effects.
CASE CONTINUED
Mr. P is enrolled in the academic medical center study assessing the effect of psilocybin on terminal illness-related anxiety and depression. During a 5-hour, 30-mg psilocybin session, he initially experiences distorted visual cues, with vivid, colorful geometric patterns collapsing into each other. He then loses the concepts and experience of time, space, and his body, as his visual distortions convert to darkness. After what seems like a decade within the darkness, he sees himself lying in a hospital bed with loved ones surrounding him. He watches himself take his last breaths and his family members weep as he dies. As he regains his senses, Mr. P feels that he is being reborn.
In the therapy sessions that follow the psychedelic session, Mr. P reports feeling “finally freed” from the fear, sadness, and anger that he has felt throughout his life. He comes to accept his impending death with gratitude and peace. In his final days, he no longer experiences depression or anxiety. Mr. P’s friends and family members comment that he seems to be the best version of himself in the months that lead up to his death.
Related Resources
• Nutt D. Psychedelic drugs-a new era in psychiatry? Dialogues Clin Neurosci. 2019;21(2):139-147.
• Garcia-Romeu A, Kersgaard B, Addy PH. Clinical applications of hallucinogens: a review. Exp Clin Psychopharmacol. 2016; 24(4):229-268.
Drug Brand Names
Amitriptyline • Amitril, Elavil
Bupropion • Wellbutrin
Fluoxetine • Prozac
Sertraline • Zoloft
Bottom Line
Psychedelics are a class of consciousness-altering agents that have become a potentially promising source of new treatments for psychiatric illness. Although more evidence is needed, compounds such as psilocybin may one day become FDAapproved for conditions such as terminal illness–related depression and anxiety, and substance use disorders. When this occurs, psychiatrists should be responsible for prescribing psychedelics and managing patients who receive treatment.
1. Smith DE, Raswyck GE, Davidson LD. From Hofmann to the Haight Ashbury, and into the future: the past and potential of lysergic acid diethylamide. J Psychoactive Drugs. 2014;46(1):3-10.
2. Siegel M. Threading Denver’s magic mushrooms needle: promising as medicine, risky as recreation. USA Today. Published May 13, 2019. Accessed December 4, 2020. https://www.usatoday.com/story/opinion/2019/05/13/denver-magic-mushrooms-promising-medicine-reckless-recreation-column/1182543001
3. Epstein, K. Oakland decriminalizes ‘magic mushrooms’ and other natural psychedelics. The Washington Post. Published June 5, 2019. Accessed December 4, 2020. https://www.washingtonpost.com/nation/2019/06/05/oakland-decriminalizes-magic-mushrooms-other-natural-psychedelics
4. York JA. Santa Cruz decriminalizes natural psychedelics. Santa Cruz Sentinel. Published January 30, 2020. Accessed December 4, 2020. https://www.santacruzsentinel.com/2020/01/29/santa-cruz-decriminalizes-natural-psychedelics
5. Acker L. Oregon becomes first state to legalize psychedelic mushrooms. The Oregonian/Oregon Live. Published November 4, 2020. Accessed December 4, 2020. https://www.oregonlive.com/politics/2020/11/oregon-becomes-first-state-to-legalize-psychedelic-mushrooms.html
6. Dyck E. Flashback: psychiatric experimentation with LSD in historical perspective. Can J Psychiatry. 2005;50(7):381-388.
7. Holoyda BJ. The psychedelic renaissance and its forensic implications. J Am Acad Psychiatry Law. 2020;48(1):87-97.
8. Diagnostic and statistical manual of mental disorders, 5th ed. American Psychiatric Association; 2013.
9. Davis AK, Rosenberg H. The prevalence, intensity, and assessment of craving for MDMA/ecstasy in recreational users. J Psychoactive Drugs. 2014;46(2):154-151.
10. Halpern JH, Lerner AG, Passie T. A review of hallucinogen persisting perception disorder (HPPD) and an exploratory study of subjects claiming symptoms of HPPD. Curr Top Behav Neurosci. 2018;36:333-360.
11. Nichols DE. Psychedelics. Pharmacol Rev. 2016;68(2):264-355.
12. Nichols DE. Hallucinogens. Pharmacol Ther. 2004;101(2):131-181.
13. Carhart-Harris RL, Leech R, Hellyer PJ, et al. The entropic brain: a theory of conscious states informed by neuroimaging research with psychedelic drugs. Front Hum Neurosci. 2014;8:20.
14. Reiff CM, Richman EE, Nemeroff CB, et al. Psychedelics and psychedelic-assisted psychotherapy. Am J Psychiatry. 2020;177(5):391-410.
15. Griffiths RR, Johnson MW, Carducci MA, et al. Psilocybin produces substantial and sustained decreases in depression and anxiety in patients with life-threatening cancer: a randomized double-blind trial. J Psychopharmacol. 2016;30(12):1181-1197.
16. Johnson MW, Garcia-Romeu A, Cosimano MP, et al. Pilot study of the 5-HT2AR agonist psilocybin in the treatment of tobacco addiction. J Psychopharmacol. 2014;28(11):983-992.
17. Johnson MW, Garcia-Romeu A, Griffiths RR. Long-term follow-up of psilocybin-facilitated smoking cessation. Am J Drug Alcohol Abuse. 2017;43(1):55-60.
18. Bogenschutz MP, Forcehimes AA, Pommy JA, et al. Psilocybin-assisted treatment for alcohol dependence: a proof-of-concept study. J Psychopharmacol. 2015;29(3):1182-1190.
19. Gasser P, Holstein D, Michel Y, et al. Safety and efficacy of lysergic acid diethylamide-assisted psychotherapy for anxiety associated with life-threatening diseases. J Nerv Ment Dis. 2014;202(7):531-520.
20. Osório F de L, Sanches RF, Macedo LR, et al. Antidepressant effects of a single dose of ayahuasca in patients with recurrent depression: a preliminary report. Braz J Psychiatry. 2015;37(1):13-20.
21. Holoyda B. Psychedelic psychiatry: preparing for novel treatments involving altered states of consciousness. Psych Serv. 2020;71(12):1297-1299.
22. Johnson MW, Richards W, Griffiths RR. Human hallucinogen research: guidelines for safety. J Psychopharmacol. 2008;22(6):603-620.
23. Council on Spiritual Practices. Code of ethics for spiritual Guides. Published August 10, 2001. Accessed November 25, 2020. https://csp.org/docs/code-of-ethics-for-spiritual-guides
24. Multidisciplinary Association for Psychedelic Studies. Zendo psychedelic harm reduction training manual. Published 2017. Accessed November 25, 2020. https://zendoproject.org/wp-content/uploads/2017/06/Zendo-Manual-2017.pdf
25. Zinberg NE. Drug, set, and setting: the basis for controlled intoxicant use. Yale University Press; 1984.
26. Carbonaro TM, Bradstreet MP, Barrett FS, et al. Survey study of challenging experiences after ingesting psilocybin mushrooms: acute and enduring positive and negative consequences. J Psychopharmacol. 2016;30(12):1268-1278.
1. Smith DE, Raswyck GE, Davidson LD. From Hofmann to the Haight Ashbury, and into the future: the past and potential of lysergic acid diethylamide. J Psychoactive Drugs. 2014;46(1):3-10.
2. Siegel M. Threading Denver’s magic mushrooms needle: promising as medicine, risky as recreation. USA Today. Published May 13, 2019. Accessed December 4, 2020. https://www.usatoday.com/story/opinion/2019/05/13/denver-magic-mushrooms-promising-medicine-reckless-recreation-column/1182543001
3. Epstein, K. Oakland decriminalizes ‘magic mushrooms’ and other natural psychedelics. The Washington Post. Published June 5, 2019. Accessed December 4, 2020. https://www.washingtonpost.com/nation/2019/06/05/oakland-decriminalizes-magic-mushrooms-other-natural-psychedelics
4. York JA. Santa Cruz decriminalizes natural psychedelics. Santa Cruz Sentinel. Published January 30, 2020. Accessed December 4, 2020. https://www.santacruzsentinel.com/2020/01/29/santa-cruz-decriminalizes-natural-psychedelics
5. Acker L. Oregon becomes first state to legalize psychedelic mushrooms. The Oregonian/Oregon Live. Published November 4, 2020. Accessed December 4, 2020. https://www.oregonlive.com/politics/2020/11/oregon-becomes-first-state-to-legalize-psychedelic-mushrooms.html
6. Dyck E. Flashback: psychiatric experimentation with LSD in historical perspective. Can J Psychiatry. 2005;50(7):381-388.
7. Holoyda BJ. The psychedelic renaissance and its forensic implications. J Am Acad Psychiatry Law. 2020;48(1):87-97.
8. Diagnostic and statistical manual of mental disorders, 5th ed. American Psychiatric Association; 2013.
9. Davis AK, Rosenberg H. The prevalence, intensity, and assessment of craving for MDMA/ecstasy in recreational users. J Psychoactive Drugs. 2014;46(2):154-151.
10. Halpern JH, Lerner AG, Passie T. A review of hallucinogen persisting perception disorder (HPPD) and an exploratory study of subjects claiming symptoms of HPPD. Curr Top Behav Neurosci. 2018;36:333-360.
11. Nichols DE. Psychedelics. Pharmacol Rev. 2016;68(2):264-355.
12. Nichols DE. Hallucinogens. Pharmacol Ther. 2004;101(2):131-181.
13. Carhart-Harris RL, Leech R, Hellyer PJ, et al. The entropic brain: a theory of conscious states informed by neuroimaging research with psychedelic drugs. Front Hum Neurosci. 2014;8:20.
14. Reiff CM, Richman EE, Nemeroff CB, et al. Psychedelics and psychedelic-assisted psychotherapy. Am J Psychiatry. 2020;177(5):391-410.
15. Griffiths RR, Johnson MW, Carducci MA, et al. Psilocybin produces substantial and sustained decreases in depression and anxiety in patients with life-threatening cancer: a randomized double-blind trial. J Psychopharmacol. 2016;30(12):1181-1197.
16. Johnson MW, Garcia-Romeu A, Cosimano MP, et al. Pilot study of the 5-HT2AR agonist psilocybin in the treatment of tobacco addiction. J Psychopharmacol. 2014;28(11):983-992.
17. Johnson MW, Garcia-Romeu A, Griffiths RR. Long-term follow-up of psilocybin-facilitated smoking cessation. Am J Drug Alcohol Abuse. 2017;43(1):55-60.
18. Bogenschutz MP, Forcehimes AA, Pommy JA, et al. Psilocybin-assisted treatment for alcohol dependence: a proof-of-concept study. J Psychopharmacol. 2015;29(3):1182-1190.
19. Gasser P, Holstein D, Michel Y, et al. Safety and efficacy of lysergic acid diethylamide-assisted psychotherapy for anxiety associated with life-threatening diseases. J Nerv Ment Dis. 2014;202(7):531-520.
20. Osório F de L, Sanches RF, Macedo LR, et al. Antidepressant effects of a single dose of ayahuasca in patients with recurrent depression: a preliminary report. Braz J Psychiatry. 2015;37(1):13-20.
21. Holoyda B. Psychedelic psychiatry: preparing for novel treatments involving altered states of consciousness. Psych Serv. 2020;71(12):1297-1299.
22. Johnson MW, Richards W, Griffiths RR. Human hallucinogen research: guidelines for safety. J Psychopharmacol. 2008;22(6):603-620.
23. Council on Spiritual Practices. Code of ethics for spiritual Guides. Published August 10, 2001. Accessed November 25, 2020. https://csp.org/docs/code-of-ethics-for-spiritual-guides
24. Multidisciplinary Association for Psychedelic Studies. Zendo psychedelic harm reduction training manual. Published 2017. Accessed November 25, 2020. https://zendoproject.org/wp-content/uploads/2017/06/Zendo-Manual-2017.pdf
25. Zinberg NE. Drug, set, and setting: the basis for controlled intoxicant use. Yale University Press; 1984.
26. Carbonaro TM, Bradstreet MP, Barrett FS, et al. Survey study of challenging experiences after ingesting psilocybin mushrooms: acute and enduring positive and negative consequences. J Psychopharmacol. 2016;30(12):1268-1278.
Call to arms: vaccinating the health workforce of 21 million strong
As the first American health care workers rolled up their sleeves for a COVID-19 vaccine, the images were instantly frozen in history, marking the triumph of scientific know-how and ingenuity. Cameras captured the first trucks pulling out of a warehouse in Portage, Mich., to the applause of workers and area residents. A day later, Boston Medical Center employees – some dressed in scrubs and wearing masks, face shields, and protective gowns – literally danced on the sidewalk when doses arrived. Some have photographed themselves getting the vaccine and posted it on social media, tagging it #MyCOVIDVax.
But the real story of the debut of COVID-19 vaccination is more methodical than monumental, a celebration of teamwork rather than of conquest. As hospitals waited for their first allotment, they reviewed their carefully drafted plans. They relied on each other, reaching across the usual divisions of competition and working collaboratively to share the limited supply. Their priority lists for the first vaccinations included environmental services workers who clean patient rooms and the critical care physicians who work to save lives.
“Health care workers have pulled together throughout this pandemic,” said Melanie Swift, MD, cochair of the COVID-19 Vaccine Allocation and Distribution Work Group at Mayo Clinic in Rochester, Minn. “We’ve gone through the darkest of years relying so heavily on each other,” she said. “Now we’re pulling together to get out of it.”
Still, a rollout of this magnitude has hitches. Stanford issued an apology Dec. 18 after its medical residents protested a vaccine distribution plan that left out nearly all of its residents and fellows, many of whom regularly treat patients with COVID-19.
There have already been more than 287,000 COVID-19 cases and 953 deaths among health care workers, according to the Centers for Disease Control and Prevention. In its guidance, the agency pointed out that the “continued protection of them at work, at home, and in the community remains a national priority.” That means vaccinating a workforce of about 21 million people, often the largest group of employees in a community.
“It collectively takes all of us to vaccinate our teams to maintain that stability in our health care infrastructure across the metro Atlanta area,” Christy Norman, PharmD, vice president of pharmacy services at Emory Healthcare, told reporters in a briefing as the health system awaited its first delivery.
Don’t waste a dose
One overriding imperative prevails: Hospitals don’t want to waste any doses. The storage requirements of the Pfizer vaccine make that tricky.
Once vials are removed from the pizza-box-shaped containers in ultracold storage and placed in a refrigerator, they must be used within 5 days. Thawed five-dose vials must be brought to room temperature before they are diluted, and they can remain at room temperature for no more than 2 hours. Once they are diluted with 1.8 mL of a 0.9% sodium chloride injection, the vials must be used within 6 hours.
COVID-19 precautions require employees to stay physically distant while they wait their turn for vaccination, which means the process can’t mirror typical large-scale flu immunization programs.
To prioritize groups, the vaccination planners at Mayo conducted a thorough risk stratification, considering each employee’s duties. Do they work in a dedicated COVID-19 unit? Do they handle lab tests or collect swabs? Do they work in the ICU or emergency department?
“We have applied some principles to make sure that as we roll it out, we prioritize people who are at greatest risk of ongoing exposure and who are really critical to maintaining the COVID response and other essential health services,” said Dr. Swift, associate medical director of Mayo’s occupational health service.
Mayo employees who are eligible for the first doses can sign up for appointments through the medical record system. If it seems likely that some doses will be left over at the end of the vaccination period – perhaps because of missed appointments – supervisors in high-risk areas can refer other health care workers. Mayo gave its first vaccines on Dec. 18, but the vaccination program began in earnest the following week. With the pleasant surprise that each five-dose vial actually provides six doses, 474 vials will allow for the vaccination of 2,844 employees in the top-priority group. “It’s going to expand each week or few days as we get more and more vaccine,” Dr. Swift said.
Sharing vials with small rural hospitals
Minnesota is using a hub-and-spoke system to give small rural hospitals access to the Pfizer vaccine, even though they lack ultracold storage and can’t use a minimum order of 975 doses. Large hospitals, acting as hubs, are sharing their orders. (The minimum order for Moderna is 100 doses.)
In south-central Minnesota, for example, two hub hospitals each have six spoke hospitals. Five of the 14 hospitals are independent, and the rest are part of large hospital systems, but affiliation doesn’t matter, said Eric Weller, regional health care preparedness coordinator for the South Central Healthcare Coalition. “We are all working together. It doesn’t matter what system you’re from,” he said. “We’re working for the good of the community.”
Each hospital designed a process to provide vaccine education, prioritize groups, allocate appointments, register people for vaccination, obtain signed consent forms, administer vaccines in a COVID-safe way, and provide follow-up appointments for the second dose. “We’re using some of the lessons we learned during H1N1,” said Mr. Weller, referring to immunization during the 2009 influenza pandemic. “The difference is that during H1N1, you could have lines of people.”
Coordinating the appointments will be more important than ever. “One of the vaccination strategies is to get people in groups of five, so you use one vial on those five people and don’t waste it,” he said.
Logistics are somewhat different for the Moderna vaccine, which will come in 10-dose vials that can be refrigerated for up to 30 days.
Both vaccines may produce mild flulike symptoms, such as fatigue, headache, or muscle pain, particularly after the second dose. That’s a sign that the immune system is reacting to the vaccine, but it’s also another consideration in the vaccination plans, because health care workers might take a day or two off work. “We’re not going to vaccinate a whole department at one time. It will be staggered,” said Kevin Smith, MD, medical director of the occupational medicine program at ProMedica, a health care system based in Toledo, Ohio.
Dr. Smith said he plans to encourage employees to use V-Safe, an app created by the CDC to track adverse effects in people who receive the vaccine. He pointed out that a day or two of achiness will be better than coping with the symptoms of COVID-19. Some employees who recovered from the infection still feel fatigued or haven’t regained their sense of taste and smell. “We are still monitoring quite a few employees to make sure they get back to 100%,” he said.
Hope for ending the pandemic
Public health officials have worried about vaccine hesitancy, even among health care workers, but so far, that concern seems overshadowed by enthusiasm. Dr. Smith said his department has been fielding calls from employees who want to know when they will be able to get the vaccine. “I think everyone feels relief,” he said. “We’re at the beginning of the end.”
At Mayo, Dr. Swift is surveying staff to gauge the willingness to get the vaccine, but she already senses excitement among employees. “No doubt there are still people who are hesitant, but I’m feeling a shift,” she said. “I’m feeling this momentum building of health care workers coming on board and wanting to take this vaccine, which is good, because they will set an example for their patients.”
For Colleen Kelley, MD, an infectious disease physician at Emory University in Atlanta who was principal investigator for an Emory-affiliated Moderna clinical trial site, it has been an emotional time. “Things were looking very bleak and dark for a time, and then we started to get these efficacy results that were greater than anyone imagined,” she said.
Dr. Kelley spends time talking to journalists and educating physician colleagues and hospital employees about how the vaccine was developed so quickly and how it works. “Everyone asks me, ‘Should I get it? Are you going to get it?’ My answer is ‘yes’ and ‘yes,’ “ she said. “I am 1,000% confident that the benefits of widespread vaccination outweigh the risks of continued COVID and a continued pandemic.”
A version of this article first appeared on Medscape.com.
As the first American health care workers rolled up their sleeves for a COVID-19 vaccine, the images were instantly frozen in history, marking the triumph of scientific know-how and ingenuity. Cameras captured the first trucks pulling out of a warehouse in Portage, Mich., to the applause of workers and area residents. A day later, Boston Medical Center employees – some dressed in scrubs and wearing masks, face shields, and protective gowns – literally danced on the sidewalk when doses arrived. Some have photographed themselves getting the vaccine and posted it on social media, tagging it #MyCOVIDVax.
But the real story of the debut of COVID-19 vaccination is more methodical than monumental, a celebration of teamwork rather than of conquest. As hospitals waited for their first allotment, they reviewed their carefully drafted plans. They relied on each other, reaching across the usual divisions of competition and working collaboratively to share the limited supply. Their priority lists for the first vaccinations included environmental services workers who clean patient rooms and the critical care physicians who work to save lives.
“Health care workers have pulled together throughout this pandemic,” said Melanie Swift, MD, cochair of the COVID-19 Vaccine Allocation and Distribution Work Group at Mayo Clinic in Rochester, Minn. “We’ve gone through the darkest of years relying so heavily on each other,” she said. “Now we’re pulling together to get out of it.”
Still, a rollout of this magnitude has hitches. Stanford issued an apology Dec. 18 after its medical residents protested a vaccine distribution plan that left out nearly all of its residents and fellows, many of whom regularly treat patients with COVID-19.
There have already been more than 287,000 COVID-19 cases and 953 deaths among health care workers, according to the Centers for Disease Control and Prevention. In its guidance, the agency pointed out that the “continued protection of them at work, at home, and in the community remains a national priority.” That means vaccinating a workforce of about 21 million people, often the largest group of employees in a community.
“It collectively takes all of us to vaccinate our teams to maintain that stability in our health care infrastructure across the metro Atlanta area,” Christy Norman, PharmD, vice president of pharmacy services at Emory Healthcare, told reporters in a briefing as the health system awaited its first delivery.
Don’t waste a dose
One overriding imperative prevails: Hospitals don’t want to waste any doses. The storage requirements of the Pfizer vaccine make that tricky.
Once vials are removed from the pizza-box-shaped containers in ultracold storage and placed in a refrigerator, they must be used within 5 days. Thawed five-dose vials must be brought to room temperature before they are diluted, and they can remain at room temperature for no more than 2 hours. Once they are diluted with 1.8 mL of a 0.9% sodium chloride injection, the vials must be used within 6 hours.
COVID-19 precautions require employees to stay physically distant while they wait their turn for vaccination, which means the process can’t mirror typical large-scale flu immunization programs.
To prioritize groups, the vaccination planners at Mayo conducted a thorough risk stratification, considering each employee’s duties. Do they work in a dedicated COVID-19 unit? Do they handle lab tests or collect swabs? Do they work in the ICU or emergency department?
“We have applied some principles to make sure that as we roll it out, we prioritize people who are at greatest risk of ongoing exposure and who are really critical to maintaining the COVID response and other essential health services,” said Dr. Swift, associate medical director of Mayo’s occupational health service.
Mayo employees who are eligible for the first doses can sign up for appointments through the medical record system. If it seems likely that some doses will be left over at the end of the vaccination period – perhaps because of missed appointments – supervisors in high-risk areas can refer other health care workers. Mayo gave its first vaccines on Dec. 18, but the vaccination program began in earnest the following week. With the pleasant surprise that each five-dose vial actually provides six doses, 474 vials will allow for the vaccination of 2,844 employees in the top-priority group. “It’s going to expand each week or few days as we get more and more vaccine,” Dr. Swift said.
Sharing vials with small rural hospitals
Minnesota is using a hub-and-spoke system to give small rural hospitals access to the Pfizer vaccine, even though they lack ultracold storage and can’t use a minimum order of 975 doses. Large hospitals, acting as hubs, are sharing their orders. (The minimum order for Moderna is 100 doses.)
In south-central Minnesota, for example, two hub hospitals each have six spoke hospitals. Five of the 14 hospitals are independent, and the rest are part of large hospital systems, but affiliation doesn’t matter, said Eric Weller, regional health care preparedness coordinator for the South Central Healthcare Coalition. “We are all working together. It doesn’t matter what system you’re from,” he said. “We’re working for the good of the community.”
Each hospital designed a process to provide vaccine education, prioritize groups, allocate appointments, register people for vaccination, obtain signed consent forms, administer vaccines in a COVID-safe way, and provide follow-up appointments for the second dose. “We’re using some of the lessons we learned during H1N1,” said Mr. Weller, referring to immunization during the 2009 influenza pandemic. “The difference is that during H1N1, you could have lines of people.”
Coordinating the appointments will be more important than ever. “One of the vaccination strategies is to get people in groups of five, so you use one vial on those five people and don’t waste it,” he said.
Logistics are somewhat different for the Moderna vaccine, which will come in 10-dose vials that can be refrigerated for up to 30 days.
Both vaccines may produce mild flulike symptoms, such as fatigue, headache, or muscle pain, particularly after the second dose. That’s a sign that the immune system is reacting to the vaccine, but it’s also another consideration in the vaccination plans, because health care workers might take a day or two off work. “We’re not going to vaccinate a whole department at one time. It will be staggered,” said Kevin Smith, MD, medical director of the occupational medicine program at ProMedica, a health care system based in Toledo, Ohio.
Dr. Smith said he plans to encourage employees to use V-Safe, an app created by the CDC to track adverse effects in people who receive the vaccine. He pointed out that a day or two of achiness will be better than coping with the symptoms of COVID-19. Some employees who recovered from the infection still feel fatigued or haven’t regained their sense of taste and smell. “We are still monitoring quite a few employees to make sure they get back to 100%,” he said.
Hope for ending the pandemic
Public health officials have worried about vaccine hesitancy, even among health care workers, but so far, that concern seems overshadowed by enthusiasm. Dr. Smith said his department has been fielding calls from employees who want to know when they will be able to get the vaccine. “I think everyone feels relief,” he said. “We’re at the beginning of the end.”
At Mayo, Dr. Swift is surveying staff to gauge the willingness to get the vaccine, but she already senses excitement among employees. “No doubt there are still people who are hesitant, but I’m feeling a shift,” she said. “I’m feeling this momentum building of health care workers coming on board and wanting to take this vaccine, which is good, because they will set an example for their patients.”
For Colleen Kelley, MD, an infectious disease physician at Emory University in Atlanta who was principal investigator for an Emory-affiliated Moderna clinical trial site, it has been an emotional time. “Things were looking very bleak and dark for a time, and then we started to get these efficacy results that were greater than anyone imagined,” she said.
Dr. Kelley spends time talking to journalists and educating physician colleagues and hospital employees about how the vaccine was developed so quickly and how it works. “Everyone asks me, ‘Should I get it? Are you going to get it?’ My answer is ‘yes’ and ‘yes,’ “ she said. “I am 1,000% confident that the benefits of widespread vaccination outweigh the risks of continued COVID and a continued pandemic.”
A version of this article first appeared on Medscape.com.
As the first American health care workers rolled up their sleeves for a COVID-19 vaccine, the images were instantly frozen in history, marking the triumph of scientific know-how and ingenuity. Cameras captured the first trucks pulling out of a warehouse in Portage, Mich., to the applause of workers and area residents. A day later, Boston Medical Center employees – some dressed in scrubs and wearing masks, face shields, and protective gowns – literally danced on the sidewalk when doses arrived. Some have photographed themselves getting the vaccine and posted it on social media, tagging it #MyCOVIDVax.
But the real story of the debut of COVID-19 vaccination is more methodical than monumental, a celebration of teamwork rather than of conquest. As hospitals waited for their first allotment, they reviewed their carefully drafted plans. They relied on each other, reaching across the usual divisions of competition and working collaboratively to share the limited supply. Their priority lists for the first vaccinations included environmental services workers who clean patient rooms and the critical care physicians who work to save lives.
“Health care workers have pulled together throughout this pandemic,” said Melanie Swift, MD, cochair of the COVID-19 Vaccine Allocation and Distribution Work Group at Mayo Clinic in Rochester, Minn. “We’ve gone through the darkest of years relying so heavily on each other,” she said. “Now we’re pulling together to get out of it.”
Still, a rollout of this magnitude has hitches. Stanford issued an apology Dec. 18 after its medical residents protested a vaccine distribution plan that left out nearly all of its residents and fellows, many of whom regularly treat patients with COVID-19.
There have already been more than 287,000 COVID-19 cases and 953 deaths among health care workers, according to the Centers for Disease Control and Prevention. In its guidance, the agency pointed out that the “continued protection of them at work, at home, and in the community remains a national priority.” That means vaccinating a workforce of about 21 million people, often the largest group of employees in a community.
“It collectively takes all of us to vaccinate our teams to maintain that stability in our health care infrastructure across the metro Atlanta area,” Christy Norman, PharmD, vice president of pharmacy services at Emory Healthcare, told reporters in a briefing as the health system awaited its first delivery.
Don’t waste a dose
One overriding imperative prevails: Hospitals don’t want to waste any doses. The storage requirements of the Pfizer vaccine make that tricky.
Once vials are removed from the pizza-box-shaped containers in ultracold storage and placed in a refrigerator, they must be used within 5 days. Thawed five-dose vials must be brought to room temperature before they are diluted, and they can remain at room temperature for no more than 2 hours. Once they are diluted with 1.8 mL of a 0.9% sodium chloride injection, the vials must be used within 6 hours.
COVID-19 precautions require employees to stay physically distant while they wait their turn for vaccination, which means the process can’t mirror typical large-scale flu immunization programs.
To prioritize groups, the vaccination planners at Mayo conducted a thorough risk stratification, considering each employee’s duties. Do they work in a dedicated COVID-19 unit? Do they handle lab tests or collect swabs? Do they work in the ICU or emergency department?
“We have applied some principles to make sure that as we roll it out, we prioritize people who are at greatest risk of ongoing exposure and who are really critical to maintaining the COVID response and other essential health services,” said Dr. Swift, associate medical director of Mayo’s occupational health service.
Mayo employees who are eligible for the first doses can sign up for appointments through the medical record system. If it seems likely that some doses will be left over at the end of the vaccination period – perhaps because of missed appointments – supervisors in high-risk areas can refer other health care workers. Mayo gave its first vaccines on Dec. 18, but the vaccination program began in earnest the following week. With the pleasant surprise that each five-dose vial actually provides six doses, 474 vials will allow for the vaccination of 2,844 employees in the top-priority group. “It’s going to expand each week or few days as we get more and more vaccine,” Dr. Swift said.
Sharing vials with small rural hospitals
Minnesota is using a hub-and-spoke system to give small rural hospitals access to the Pfizer vaccine, even though they lack ultracold storage and can’t use a minimum order of 975 doses. Large hospitals, acting as hubs, are sharing their orders. (The minimum order for Moderna is 100 doses.)
In south-central Minnesota, for example, two hub hospitals each have six spoke hospitals. Five of the 14 hospitals are independent, and the rest are part of large hospital systems, but affiliation doesn’t matter, said Eric Weller, regional health care preparedness coordinator for the South Central Healthcare Coalition. “We are all working together. It doesn’t matter what system you’re from,” he said. “We’re working for the good of the community.”
Each hospital designed a process to provide vaccine education, prioritize groups, allocate appointments, register people for vaccination, obtain signed consent forms, administer vaccines in a COVID-safe way, and provide follow-up appointments for the second dose. “We’re using some of the lessons we learned during H1N1,” said Mr. Weller, referring to immunization during the 2009 influenza pandemic. “The difference is that during H1N1, you could have lines of people.”
Coordinating the appointments will be more important than ever. “One of the vaccination strategies is to get people in groups of five, so you use one vial on those five people and don’t waste it,” he said.
Logistics are somewhat different for the Moderna vaccine, which will come in 10-dose vials that can be refrigerated for up to 30 days.
Both vaccines may produce mild flulike symptoms, such as fatigue, headache, or muscle pain, particularly after the second dose. That’s a sign that the immune system is reacting to the vaccine, but it’s also another consideration in the vaccination plans, because health care workers might take a day or two off work. “We’re not going to vaccinate a whole department at one time. It will be staggered,” said Kevin Smith, MD, medical director of the occupational medicine program at ProMedica, a health care system based in Toledo, Ohio.
Dr. Smith said he plans to encourage employees to use V-Safe, an app created by the CDC to track adverse effects in people who receive the vaccine. He pointed out that a day or two of achiness will be better than coping with the symptoms of COVID-19. Some employees who recovered from the infection still feel fatigued or haven’t regained their sense of taste and smell. “We are still monitoring quite a few employees to make sure they get back to 100%,” he said.
Hope for ending the pandemic
Public health officials have worried about vaccine hesitancy, even among health care workers, but so far, that concern seems overshadowed by enthusiasm. Dr. Smith said his department has been fielding calls from employees who want to know when they will be able to get the vaccine. “I think everyone feels relief,” he said. “We’re at the beginning of the end.”
At Mayo, Dr. Swift is surveying staff to gauge the willingness to get the vaccine, but she already senses excitement among employees. “No doubt there are still people who are hesitant, but I’m feeling a shift,” she said. “I’m feeling this momentum building of health care workers coming on board and wanting to take this vaccine, which is good, because they will set an example for their patients.”
For Colleen Kelley, MD, an infectious disease physician at Emory University in Atlanta who was principal investigator for an Emory-affiliated Moderna clinical trial site, it has been an emotional time. “Things were looking very bleak and dark for a time, and then we started to get these efficacy results that were greater than anyone imagined,” she said.
Dr. Kelley spends time talking to journalists and educating physician colleagues and hospital employees about how the vaccine was developed so quickly and how it works. “Everyone asks me, ‘Should I get it? Are you going to get it?’ My answer is ‘yes’ and ‘yes,’ “ she said. “I am 1,000% confident that the benefits of widespread vaccination outweigh the risks of continued COVID and a continued pandemic.”
A version of this article first appeared on Medscape.com.
Paul Summergrad, MD, on the state of psychiatry
For this Psychiatry Leaders’ Perspectives, Awais Aftab, MD, interviewed Paul Summergrad, MD. Dr. Summergrad is the Dr. Frances S. Arkin Professor and Chair of the Department of Psychiatry and Professor of Psychiatry and Medicine at Tufts University School of Medicine and Psychiatrist-in-Chief at Tufts Medical Center, Boston, Massachusetts. From 2014 to 2015, Dr. Summergrad served as the 141st president of the American Psychiatric Association, and is a past president of the American Association of Chairs of Departments of Psychiatry. Dr. Summergrad’s research focuses on mood disorders, medical/psychiatric comorbidity, and health system design. He received the Distinguished Faculty Award from Tufts University School of Medicine in 2015 and the Leadership Award of the American Association of Chairs of Departments of Psychiatry in 2018. In 2020, he was elected to the Honorary Fellowship of the Royal College of Psychiatrists, their highest honor. He is the lead editor of Textbook of Medical Psychiatry, which was published by American Psychiatric Association Publishing in 2020.
Dr. Aftab: Much of your career has been devoted to the practice of “medical psychiatry” in which you have fruitfully integrated your medical training as well as psychoanalytic training. How has this influenced your understanding of the medical model in psychiatry and psychiatry’s relationship with medicine?
Dr. Summergrad: It is a really complex and ongoing influence. I think there is a misunderstanding of what is meant by the medical model in psychiatry. It has nothing to do with the etiology of mental disorders or their treatment. At its core, the medical model is based on a syndromic view of disorders: that we attend to those symptoms of illness that occur together more frequently than they might by chance and then, based on that provisional diagnostic cluster, look for causes, risk factors, and interventions that affect the putative disorder’s course. As a consequence of that process, disorders are refined, often separated into a group of disorders, or in some cases discarded. An excellent example that we have all been living through has been our evolving understanding of COVID-19, which is now understood to be as much a clotting and inflammatory disorder as a respiratory condition.
Medical psychiatry is a different and discrete area of clinical psychiatric interest. It covers a variety of areas: the complexity of the management of patients with comorbid medical and psychiatric illness, the impact of medical illness on the course of psychiatric illness and life expectancy, and conversely the effects of psychiatric illness on the course of medical disorders—for example, the increased mortality in patients with myocardial infarction (MI) and post-MI major depression. At its core is the study of medical disorders, including neurologic conditions, that directly cause syndromes in the realm that we define as mental disorders. This was the focus of our recent Textbook of Medical Psychiatry. This has been a long-standing interest of mine since I did my medical residency at Boston City Hospital before I trained in psychiatry, and it has informed my career in many other ways.
Dr. Aftab: What do you see as some of the strengths of our profession?
Dr. Summergrad: Psychiatry has so many riches: a long clinical tradition based on close and long-term observation and interaction with patients, effective psychotherapies such as cognitive-behavioral therapy and psychodynamic therapies, and evidence-based pharmacologic and other somatic therapies.
Second, there has been substantial growth in our fundamental understanding of the neurobiology of psychiatric illness with regards to circuitry, imaging, and genetics. While many of these advances have arisen from more basic research, it is also the case that the evolution of a consistent diagnostic nomenclature in the 1970s, even with all its limitations, allowed for advances in research, diagnosis, and treatment.
Finally, our other great strengths are our roots in medicine and the importance of those skills in assessing patients and caring for active comorbidities. We are one of the last fields in clinical medicine where doctors can take the time to establish a detailed and close working relationship with our patients. We are fortunate to have this great mix of science, medicine, and interpersonal skills, which is highly rewarding.
Continue to: Dr. Aftab: Are there ways...
Dr. Aftab: Are there ways in which the status quo in psychiatry falls short of the ideal? What are our areas of relative weakness?
Dr. Summergrad: There are many, as there are in many other fields of medicine. For too many, there is a reification of a diagnostic nomenclature as being identical to detailed and thoughtful clinical evaluation. For many, the pressures of health care economics mean that they may be taking care of more inpatients than is optimal, or are under pressure to see a larger number of patients as a so-called “prescriber,” a term I think should be banished.
We have struggled significantly to have a coherent link between our clinical work, including our interventions, our emerging understanding of neuroscience and genetics, and the experiences of our patients, including the onset and timing of many of the disorders we treat. Part of this is that we lack a unified model of mental functioning that unites the onset of illness, its clinical phenomena, and any underlying pathophysiology. We operate at multiple levels of abstraction (brain-mind) compared with other medical fields. While other medical fields incorporate experience, they are more fully operating, from a pathophysiologic perspective, at a physical level alone. Even in common parlance, we can easily talk about the heart as a pump, or the kidney as a filter, but there is no corresponding way to describe what the brain-mind is and does. This could be construed as a weakness; I see it more as an intrinsic complexity of our field.
What we refer to as psychiatric disorders deal with the most intimate aspects of people’s beings: their sense of self and capacity. Many people experience our diagnostic work and nomenclature as wounding, demeaning, distancing, or defining their very essence or being as ill. There is a wonderful story that I heard from the great Elyn Saks, the constitutional law professor, regarding her own illness, about which she has been admirably open. She described a long course of significant psychotic illness, eventually diagnosed as schizophrenia, for which she received years of psychotherapy, psychopharmacology, and hospital care, both when she studied at Oxford and while she was a law student at Yale. She described that after 10 years of care, she was eventually prescribed clozapine, which made a major difference in her illness. It was about the same time that she finally accepted that she had a psychiatric illness, and it was at that very moment of acceptance that she realized that it wasn’t about her, that it didn’t define who she was in her essence. Very moving and important. In defining pathophysiology or what we call psychopathology, we need to make sure it is clear that we are not labeling or diagnosing anyone’s essential being.
I think we need to tread very carefully in these areas, including being very sensitive with our language. Much of this is in the nature of the illnesses we deal with and their profound intimacy, but again we need to be mindful of this. These areas are ones which I think contribute to a resentment of psychiatry, and are possibly related to some of the anti-psychiatry sentiments and criticisms of the so-called medical model in psychiatry, which as I noted above is, I think, not well understood.
Continue to: Dr. Aftab: What is your perception...
Dr. Aftab: What is your perception of the threats that psychiatry faces or is likely to face in the future?
Dr. Summergrad: I actually am very bullish on psychiatry’s future. While we are far from perfect, the illnesses we care for are so ubiquitous that many if not most people will experience them personally or in close family members over their lifetime. As such, there is a real and broad understanding about the need for psychiatric care; we do, however, have to always strive to do it better and with greater sensitivity to the human experiences of those who seek our care.
Dr. Aftab: What do you envision for the future of psychiatry? What opportunities lie ahead for us?
Dr. Summergrad: I think we will see an expansion of awareness of mental disorders globally. While it may seem counterintuitive to say this in the midst of a global pandemic, over the course of the last 80 years, the global burden of disease related to communicable diseases has fallen across much of the developed world and the burden of disease attributable to noncommunicable disease has increased. Psychiatric disorders are among the most frequent noncommunicable disorders and are increasing as a proportion of total illness burden. As such, the need for mental health–related care will increase dramatically over the next half century, if not longer.
Second, I think our understanding of neurobiology, the impact of medical disorders, and pathophysiology related to mechanisms such as inflammation in psychiatric disorders will increase. Likewise, our appreciation will grow for non-allelic influences on the genetics or heritability of psychiatric disorders. I don’t think we have come near to tapping the effects of epigenetics on psychiatric illnesses, and that will become increasingly important.
I also think that over time, our understanding of particular neurobiological pathways and our ability to modulate these pathways will increase. How that will eventually yield the ability to treat patients with greater precision I don’t know, but I expect that will occur. Over time, we may even learn enough to have a workable theory of mind and brain, but I am not sure that all of these mysteries will yield anytime soon, and for some of them, answers may have to come from other domains of human experience.
For this Psychiatry Leaders’ Perspectives, Awais Aftab, MD, interviewed Paul Summergrad, MD. Dr. Summergrad is the Dr. Frances S. Arkin Professor and Chair of the Department of Psychiatry and Professor of Psychiatry and Medicine at Tufts University School of Medicine and Psychiatrist-in-Chief at Tufts Medical Center, Boston, Massachusetts. From 2014 to 2015, Dr. Summergrad served as the 141st president of the American Psychiatric Association, and is a past president of the American Association of Chairs of Departments of Psychiatry. Dr. Summergrad’s research focuses on mood disorders, medical/psychiatric comorbidity, and health system design. He received the Distinguished Faculty Award from Tufts University School of Medicine in 2015 and the Leadership Award of the American Association of Chairs of Departments of Psychiatry in 2018. In 2020, he was elected to the Honorary Fellowship of the Royal College of Psychiatrists, their highest honor. He is the lead editor of Textbook of Medical Psychiatry, which was published by American Psychiatric Association Publishing in 2020.
Dr. Aftab: Much of your career has been devoted to the practice of “medical psychiatry” in which you have fruitfully integrated your medical training as well as psychoanalytic training. How has this influenced your understanding of the medical model in psychiatry and psychiatry’s relationship with medicine?
Dr. Summergrad: It is a really complex and ongoing influence. I think there is a misunderstanding of what is meant by the medical model in psychiatry. It has nothing to do with the etiology of mental disorders or their treatment. At its core, the medical model is based on a syndromic view of disorders: that we attend to those symptoms of illness that occur together more frequently than they might by chance and then, based on that provisional diagnostic cluster, look for causes, risk factors, and interventions that affect the putative disorder’s course. As a consequence of that process, disorders are refined, often separated into a group of disorders, or in some cases discarded. An excellent example that we have all been living through has been our evolving understanding of COVID-19, which is now understood to be as much a clotting and inflammatory disorder as a respiratory condition.
Medical psychiatry is a different and discrete area of clinical psychiatric interest. It covers a variety of areas: the complexity of the management of patients with comorbid medical and psychiatric illness, the impact of medical illness on the course of psychiatric illness and life expectancy, and conversely the effects of psychiatric illness on the course of medical disorders—for example, the increased mortality in patients with myocardial infarction (MI) and post-MI major depression. At its core is the study of medical disorders, including neurologic conditions, that directly cause syndromes in the realm that we define as mental disorders. This was the focus of our recent Textbook of Medical Psychiatry. This has been a long-standing interest of mine since I did my medical residency at Boston City Hospital before I trained in psychiatry, and it has informed my career in many other ways.
Dr. Aftab: What do you see as some of the strengths of our profession?
Dr. Summergrad: Psychiatry has so many riches: a long clinical tradition based on close and long-term observation and interaction with patients, effective psychotherapies such as cognitive-behavioral therapy and psychodynamic therapies, and evidence-based pharmacologic and other somatic therapies.
Second, there has been substantial growth in our fundamental understanding of the neurobiology of psychiatric illness with regards to circuitry, imaging, and genetics. While many of these advances have arisen from more basic research, it is also the case that the evolution of a consistent diagnostic nomenclature in the 1970s, even with all its limitations, allowed for advances in research, diagnosis, and treatment.
Finally, our other great strengths are our roots in medicine and the importance of those skills in assessing patients and caring for active comorbidities. We are one of the last fields in clinical medicine where doctors can take the time to establish a detailed and close working relationship with our patients. We are fortunate to have this great mix of science, medicine, and interpersonal skills, which is highly rewarding.
Continue to: Dr. Aftab: Are there ways...
Dr. Aftab: Are there ways in which the status quo in psychiatry falls short of the ideal? What are our areas of relative weakness?
Dr. Summergrad: There are many, as there are in many other fields of medicine. For too many, there is a reification of a diagnostic nomenclature as being identical to detailed and thoughtful clinical evaluation. For many, the pressures of health care economics mean that they may be taking care of more inpatients than is optimal, or are under pressure to see a larger number of patients as a so-called “prescriber,” a term I think should be banished.
We have struggled significantly to have a coherent link between our clinical work, including our interventions, our emerging understanding of neuroscience and genetics, and the experiences of our patients, including the onset and timing of many of the disorders we treat. Part of this is that we lack a unified model of mental functioning that unites the onset of illness, its clinical phenomena, and any underlying pathophysiology. We operate at multiple levels of abstraction (brain-mind) compared with other medical fields. While other medical fields incorporate experience, they are more fully operating, from a pathophysiologic perspective, at a physical level alone. Even in common parlance, we can easily talk about the heart as a pump, or the kidney as a filter, but there is no corresponding way to describe what the brain-mind is and does. This could be construed as a weakness; I see it more as an intrinsic complexity of our field.
What we refer to as psychiatric disorders deal with the most intimate aspects of people’s beings: their sense of self and capacity. Many people experience our diagnostic work and nomenclature as wounding, demeaning, distancing, or defining their very essence or being as ill. There is a wonderful story that I heard from the great Elyn Saks, the constitutional law professor, regarding her own illness, about which she has been admirably open. She described a long course of significant psychotic illness, eventually diagnosed as schizophrenia, for which she received years of psychotherapy, psychopharmacology, and hospital care, both when she studied at Oxford and while she was a law student at Yale. She described that after 10 years of care, she was eventually prescribed clozapine, which made a major difference in her illness. It was about the same time that she finally accepted that she had a psychiatric illness, and it was at that very moment of acceptance that she realized that it wasn’t about her, that it didn’t define who she was in her essence. Very moving and important. In defining pathophysiology or what we call psychopathology, we need to make sure it is clear that we are not labeling or diagnosing anyone’s essential being.
I think we need to tread very carefully in these areas, including being very sensitive with our language. Much of this is in the nature of the illnesses we deal with and their profound intimacy, but again we need to be mindful of this. These areas are ones which I think contribute to a resentment of psychiatry, and are possibly related to some of the anti-psychiatry sentiments and criticisms of the so-called medical model in psychiatry, which as I noted above is, I think, not well understood.
Continue to: Dr. Aftab: What is your perception...
Dr. Aftab: What is your perception of the threats that psychiatry faces or is likely to face in the future?
Dr. Summergrad: I actually am very bullish on psychiatry’s future. While we are far from perfect, the illnesses we care for are so ubiquitous that many if not most people will experience them personally or in close family members over their lifetime. As such, there is a real and broad understanding about the need for psychiatric care; we do, however, have to always strive to do it better and with greater sensitivity to the human experiences of those who seek our care.
Dr. Aftab: What do you envision for the future of psychiatry? What opportunities lie ahead for us?
Dr. Summergrad: I think we will see an expansion of awareness of mental disorders globally. While it may seem counterintuitive to say this in the midst of a global pandemic, over the course of the last 80 years, the global burden of disease related to communicable diseases has fallen across much of the developed world and the burden of disease attributable to noncommunicable disease has increased. Psychiatric disorders are among the most frequent noncommunicable disorders and are increasing as a proportion of total illness burden. As such, the need for mental health–related care will increase dramatically over the next half century, if not longer.
Second, I think our understanding of neurobiology, the impact of medical disorders, and pathophysiology related to mechanisms such as inflammation in psychiatric disorders will increase. Likewise, our appreciation will grow for non-allelic influences on the genetics or heritability of psychiatric disorders. I don’t think we have come near to tapping the effects of epigenetics on psychiatric illnesses, and that will become increasingly important.
I also think that over time, our understanding of particular neurobiological pathways and our ability to modulate these pathways will increase. How that will eventually yield the ability to treat patients with greater precision I don’t know, but I expect that will occur. Over time, we may even learn enough to have a workable theory of mind and brain, but I am not sure that all of these mysteries will yield anytime soon, and for some of them, answers may have to come from other domains of human experience.
For this Psychiatry Leaders’ Perspectives, Awais Aftab, MD, interviewed Paul Summergrad, MD. Dr. Summergrad is the Dr. Frances S. Arkin Professor and Chair of the Department of Psychiatry and Professor of Psychiatry and Medicine at Tufts University School of Medicine and Psychiatrist-in-Chief at Tufts Medical Center, Boston, Massachusetts. From 2014 to 2015, Dr. Summergrad served as the 141st president of the American Psychiatric Association, and is a past president of the American Association of Chairs of Departments of Psychiatry. Dr. Summergrad’s research focuses on mood disorders, medical/psychiatric comorbidity, and health system design. He received the Distinguished Faculty Award from Tufts University School of Medicine in 2015 and the Leadership Award of the American Association of Chairs of Departments of Psychiatry in 2018. In 2020, he was elected to the Honorary Fellowship of the Royal College of Psychiatrists, their highest honor. He is the lead editor of Textbook of Medical Psychiatry, which was published by American Psychiatric Association Publishing in 2020.
Dr. Aftab: Much of your career has been devoted to the practice of “medical psychiatry” in which you have fruitfully integrated your medical training as well as psychoanalytic training. How has this influenced your understanding of the medical model in psychiatry and psychiatry’s relationship with medicine?
Dr. Summergrad: It is a really complex and ongoing influence. I think there is a misunderstanding of what is meant by the medical model in psychiatry. It has nothing to do with the etiology of mental disorders or their treatment. At its core, the medical model is based on a syndromic view of disorders: that we attend to those symptoms of illness that occur together more frequently than they might by chance and then, based on that provisional diagnostic cluster, look for causes, risk factors, and interventions that affect the putative disorder’s course. As a consequence of that process, disorders are refined, often separated into a group of disorders, or in some cases discarded. An excellent example that we have all been living through has been our evolving understanding of COVID-19, which is now understood to be as much a clotting and inflammatory disorder as a respiratory condition.
Medical psychiatry is a different and discrete area of clinical psychiatric interest. It covers a variety of areas: the complexity of the management of patients with comorbid medical and psychiatric illness, the impact of medical illness on the course of psychiatric illness and life expectancy, and conversely the effects of psychiatric illness on the course of medical disorders—for example, the increased mortality in patients with myocardial infarction (MI) and post-MI major depression. At its core is the study of medical disorders, including neurologic conditions, that directly cause syndromes in the realm that we define as mental disorders. This was the focus of our recent Textbook of Medical Psychiatry. This has been a long-standing interest of mine since I did my medical residency at Boston City Hospital before I trained in psychiatry, and it has informed my career in many other ways.
Dr. Aftab: What do you see as some of the strengths of our profession?
Dr. Summergrad: Psychiatry has so many riches: a long clinical tradition based on close and long-term observation and interaction with patients, effective psychotherapies such as cognitive-behavioral therapy and psychodynamic therapies, and evidence-based pharmacologic and other somatic therapies.
Second, there has been substantial growth in our fundamental understanding of the neurobiology of psychiatric illness with regards to circuitry, imaging, and genetics. While many of these advances have arisen from more basic research, it is also the case that the evolution of a consistent diagnostic nomenclature in the 1970s, even with all its limitations, allowed for advances in research, diagnosis, and treatment.
Finally, our other great strengths are our roots in medicine and the importance of those skills in assessing patients and caring for active comorbidities. We are one of the last fields in clinical medicine where doctors can take the time to establish a detailed and close working relationship with our patients. We are fortunate to have this great mix of science, medicine, and interpersonal skills, which is highly rewarding.
Continue to: Dr. Aftab: Are there ways...
Dr. Aftab: Are there ways in which the status quo in psychiatry falls short of the ideal? What are our areas of relative weakness?
Dr. Summergrad: There are many, as there are in many other fields of medicine. For too many, there is a reification of a diagnostic nomenclature as being identical to detailed and thoughtful clinical evaluation. For many, the pressures of health care economics mean that they may be taking care of more inpatients than is optimal, or are under pressure to see a larger number of patients as a so-called “prescriber,” a term I think should be banished.
We have struggled significantly to have a coherent link between our clinical work, including our interventions, our emerging understanding of neuroscience and genetics, and the experiences of our patients, including the onset and timing of many of the disorders we treat. Part of this is that we lack a unified model of mental functioning that unites the onset of illness, its clinical phenomena, and any underlying pathophysiology. We operate at multiple levels of abstraction (brain-mind) compared with other medical fields. While other medical fields incorporate experience, they are more fully operating, from a pathophysiologic perspective, at a physical level alone. Even in common parlance, we can easily talk about the heart as a pump, or the kidney as a filter, but there is no corresponding way to describe what the brain-mind is and does. This could be construed as a weakness; I see it more as an intrinsic complexity of our field.
What we refer to as psychiatric disorders deal with the most intimate aspects of people’s beings: their sense of self and capacity. Many people experience our diagnostic work and nomenclature as wounding, demeaning, distancing, or defining their very essence or being as ill. There is a wonderful story that I heard from the great Elyn Saks, the constitutional law professor, regarding her own illness, about which she has been admirably open. She described a long course of significant psychotic illness, eventually diagnosed as schizophrenia, for which she received years of psychotherapy, psychopharmacology, and hospital care, both when she studied at Oxford and while she was a law student at Yale. She described that after 10 years of care, she was eventually prescribed clozapine, which made a major difference in her illness. It was about the same time that she finally accepted that she had a psychiatric illness, and it was at that very moment of acceptance that she realized that it wasn’t about her, that it didn’t define who she was in her essence. Very moving and important. In defining pathophysiology or what we call psychopathology, we need to make sure it is clear that we are not labeling or diagnosing anyone’s essential being.
I think we need to tread very carefully in these areas, including being very sensitive with our language. Much of this is in the nature of the illnesses we deal with and their profound intimacy, but again we need to be mindful of this. These areas are ones which I think contribute to a resentment of psychiatry, and are possibly related to some of the anti-psychiatry sentiments and criticisms of the so-called medical model in psychiatry, which as I noted above is, I think, not well understood.
Continue to: Dr. Aftab: What is your perception...
Dr. Aftab: What is your perception of the threats that psychiatry faces or is likely to face in the future?
Dr. Summergrad: I actually am very bullish on psychiatry’s future. While we are far from perfect, the illnesses we care for are so ubiquitous that many if not most people will experience them personally or in close family members over their lifetime. As such, there is a real and broad understanding about the need for psychiatric care; we do, however, have to always strive to do it better and with greater sensitivity to the human experiences of those who seek our care.
Dr. Aftab: What do you envision for the future of psychiatry? What opportunities lie ahead for us?
Dr. Summergrad: I think we will see an expansion of awareness of mental disorders globally. While it may seem counterintuitive to say this in the midst of a global pandemic, over the course of the last 80 years, the global burden of disease related to communicable diseases has fallen across much of the developed world and the burden of disease attributable to noncommunicable disease has increased. Psychiatric disorders are among the most frequent noncommunicable disorders and are increasing as a proportion of total illness burden. As such, the need for mental health–related care will increase dramatically over the next half century, if not longer.
Second, I think our understanding of neurobiology, the impact of medical disorders, and pathophysiology related to mechanisms such as inflammation in psychiatric disorders will increase. Likewise, our appreciation will grow for non-allelic influences on the genetics or heritability of psychiatric disorders. I don’t think we have come near to tapping the effects of epigenetics on psychiatric illnesses, and that will become increasingly important.
I also think that over time, our understanding of particular neurobiological pathways and our ability to modulate these pathways will increase. How that will eventually yield the ability to treat patients with greater precision I don’t know, but I expect that will occur. Over time, we may even learn enough to have a workable theory of mind and brain, but I am not sure that all of these mysteries will yield anytime soon, and for some of them, answers may have to come from other domains of human experience.
Pharmacologic management of autism spectrum disorder: A review of 7 studies
Autism spectrum disorder (ASD) is characterized by persistent deficits in social communication and social interaction, including deficits in social reciprocity, nonverbal communicative behaviors used for social interaction, and skills in developing, maintaining, and understanding relationships.1 In addition, the diagnosis of ASD requires the presence of restricted, repetitive patterns of behavior, interests, or activities.
Initially, ASD was considered a rare condition. In recent years, the reported prevalence has increased substantially. The most recent estimated prevalence is 1 in 68 children at age 8, with a male-to-female ratio of 4 to 1.2
Behavioral interventions are considered to be the most effective treatment for the core symptoms of ASD. Pharmacologic interventions are used primarily to treat associated or comorbid symptoms rather than the core symptoms. Aggression, self-injurious behavior, and irritability are common targets of pharmacotherapy in patients with ASD. Studies have provided support for the use of antipsychotic agents to treat irritability and associated aggressive behaviors in patients with autism,3 but because these agents have significant adverse effects—including extrapyramidal side effects, somnolence, and weight gain—their use requires a careful risk/benefit assessment. Stimulants have also been shown to be effective in treating comorbid attention-deficit/hyperactivity symptoms. The use of selective serotonin reuptake inhibitors (SSRIs) to manage repetitive behaviors and anxiety is also common.
Here, we review 7 recent studies of the pharmacologic management of ASD (Table).4-10 These studies examined the role of SSRIs (sertraline, fluoxetine), an acetylcholinesterase inhibitor (donepezil), atypical antipsychotics (risperidone, aripiprazole, lurasidone), natural supplements (vitamin D, omega-3), a diuretic (bumetanide), and a glutamatergic modulator (riluzole) in the treatment of ASD symptoms.
1. Potter LA, Scholze DA, Biag HMB, et al. A randomized controlled trial of sertraline in young children with autism spectrum disorder. Front Psychiatry. 2019;10:810.
Several studies have shown that SSRIs improve language development in children with Fragile X syndrome, based on the Mullen Scales of Early Learning (MSEL). A previously published trial involving children with Fragile X syndrome and comorbid ASD found that sertraline improved expressive language development. Potter et al4 examined the role of sertraline in children with ASD only.
Study Design
- In this randomized, double-blind, placebo-controlled trial, 58 children age 24 to 72 months with ASD received low-dose sertraline or placebo for 6 months.
- Of the 179 participants who were screened for eligibility, 58 were included in the study. Of these 58 participants, 32 received sertraline and 26 received placebo. The numbers of participants who discontinued from the sertraline and placebo arms were 8 and 5, respectively.
- Among those in the sertraline group, participants age <48 months received 2.5 mg/d, and those age ≥48 months received 5 mg/d.
Outcomes
- No significant differences were found on the primary outcome (MSEL expressive language raw score and age-equivalent combined score) or secondary outcomes (including Clinical Global Impressions–Improvement [CGI-I] scale at 3 months and end of treatment), as per intent-to-treat analyses.
- Sertraline was well tolerated. There was no difference in adverse effects between treatment groups and no serious adverse events.
Conclusion
- Although potentially useful for language development in patients with Fragile X syndrome with comorbid ASD, SSRIs such as sertraline have not proven efficacious for improving expressive language in patients with non-syndromic ASD.
- While 6-month treatment with low-dose sertraline in young children with ASD appears safe, the long-term effects are unknown.
Continue to: Gabis et al5 examined the safety...
2. Gabis LV, Ben-Hur R, Shefer S, et al. Improvement of language in children with autism with combined donepezil and choline treatment. J Mol Neurosci. 2019;69(2):224-234.
Gabis et al5 examined the safety and efficacy of utilizing donepezil, an acetylcholinesterase inhibitor, plus a choline supplement to treat both core features and associated symptoms in children and adolescents with ASD.
Study design
- This 9-month randomized, double-blind trial included 60 children/adolescents with ASD who were randomly assigned to receive placebo or donepezil plus a choline supplement. Participants underwent a baseline evaluation (E1), 12 weeks of treatment and re-evaluation (E2), 6 months of washout, and a final evaluation (E3).
- The baseline and final evaluations assessed changes in language performance, adaptive functioning, sleep habits, autism severity, clinical impression, and intellectual abilities. The evaluation after 12 weeks of treatment (E2) included all of these measures except intellectual abilities.
Outcomes
- Patients treated with donepezil plus a choline supplement had significant improvement in receptive language skills between E1 and E3 (P = .003).
- Patients treated with donepezil plus a choline supplement had significant worsening in scores on the Autism Treatment Evaluation Checklist (ATEC) health/physical behavior subscale between E1 and E2 (P = .012) and between E1 and E3 (P = .021).
- Improvement in receptive language skills was significant only in patients age 5 to 10 years (P = .047), whereas worsening in ATEC health/physical behavior subscale score was significant only in patients age 10 to 16 years (P = .024).
- Patients treated with donepezil plus a choline supplement reported higher percentages of gastrointestinal disturbance when compared with placebo (P = .007), and patients in the adolescent subgroup had a significant increase in irritability (P = .035).
Conclusion
- Patients age 5 to 10 years treated with donepezil plus a choline supplement exhibited improved receptive language skills. This treatment was less effective in patients age >10 years, and this group also exhibited behavioral worsening.
- Gastrointestinal disturbances were the main adverse effect of treatment with donepezil plus a choline supplement.
Continue to: The persistence of excitatory...
3. James BJ, Gales MA, Gales BJ. Bumetanide for autism spectrum disorder in children: a review of randomized controlled trials. Ann Pharmacother. 2019;53(5):537-544.
The persistence of excitatory gamma-aminobutyric acid (GABA) signaling has been found in patients with ASD. Bumetanide is a sodium-potassium-chloride cotransporter 1 (NKCC1) antagonist that not only decreases intracellular chloride, but also aberrantly decreases GABA signaling. This potent loop diuretic is a proposed treatment for symptoms of ASD. James et al6 evaluated the safety and efficacy of bumetanide use in children with ASD.
Study design
- Researchers searched the PubMed and Ovid MEDLINE databases for the terms “autism” and “bumetanide” between 1946 and 2018. A total of 26 articles were screened by title, 7 were screened by full text, and 3 articles were included in the study. The remaining articles were excluded due to study design and use of non-human subjects.
- All 3 randomized controlled trials evaluated the effects of low-dose oral bumetanide (most common dose was 0.5 mg twice daily) in a total of 208 patients age 2 to 18 years.
- Measurement scales used in the 3 studies included the Childhood Autism Rating Scale (CARS), Clinical Global Impressions Scale (CGI), Autism Behavioral Checklist (ABC), Social Responsiveness Scale (SRS), and Autism Diagnostic Observation Schedule-Generic (ADOS-G).
Outcomes
- Bumetanide improved scores on multiple autism assessment scales, including CARS, but the degree of improvement was not consistent across the 3 trials.
- There was a statistically significant improvement in ASD symptoms as measured by CGI in all 3 trials, and statistically significant improvements on the ABC and SRS in 2 trials. No improvements were noted on the ADOS-G in any of the trials.
- No dose-effect correlation was identified, but hypokalemia and polyuria were more prevalent with higher doses of bumetanide.
Conclusion
- Low-dose oral bumetanide improved social communication, social interactions, and restricted interests in patients with moderate to severe ASD. However, the 3 trials used different evaluation methods and observed varying degrees of improvement, which makes it difficult to make recommendations for or against the use of bumetanide.
- Streamlined trials with a consensus on evaluation methodology are needed to draw conclusions about the efficacy and safety of bumetanide as a treatment for ASD.
Continue to: The use of SSRIs to target...
4. Li C, Bai Y, Jin C, et al. Efficacy and safety of fluoxetine in autism spectrum disorder: a meta-analysis. Am J Ther. 2020;27(3):e312-e315.
The use of SSRIs to target symptoms of ASD has been long studied because many children with ASD have elevated serotonin levels. Several SSRIs, including fluoxetine, are FDA-approved for the treatment of obsessive-compulsive disorder, anxiety, and depression. Currently, no SSRIs are FDA-approved for treating ASD. In a meta-analysis, Li et al7 evaluated the use of fluoxetine for ASD.
Study design
- Two independent researchers searched for studies of fluoxetine treatment for ASD in Embase, Google Scholar, Ovid SP, and PubMed, with disagreement resolved by consensus.
- The researchers extracted the study design, patient demographics, and outcomes (inter-rater reliability kappa = 0.93). The primary outcomes were response rate of patients treated with fluoxetine, and change from baseline in ABC, ATEC, CARS, CGI, and Yale-Brown Obsessive Compulsive Scale (Y-BOCS) scores after fluoxetine treatment.
Outcomes
- This meta-analysis included 13 studies in which fluoxetine was used to treat a total of 303 patients with ASD. The median treatment duration was 6 months, the average age of participants was 15.23 years, and most participants (72%) were male.
- The response rate of patients treated with fluoxetine was 75%, with significant mean changes from baseline in ABC score (Helvetica Neue LT Std−3.42), ATEC score (Helvetica Neue LT Std−2.04), CGI score (Helvetica Neue LT Std−0.93), and Y-BOCS score (Helvetica Neue LT Std−1.86).
- A significantly higher incidence of hyperactivity/restlessness/agitation was noted with fluoxetine.
Conclusion
- Although 75% of participants responded to fluoxetine, the limitations of this meta-analysis included low power, inadequate quality of the included studies, and high statistical heterogeneity. In addition, the analysis found a high incidence of hyperactivity/restlessness associated with fluoxetine.
- Future randomized controlled studies may provide further clarification on managing symptoms of ASD with SSRIs.
Continue to: Irritability is a common comorbid...
5. Fallah MS, Shaikh MR, Neupane B, et al. Atypical antipsychotics for irritability in pediatric autism: a systematic review and network meta-analysis. J Child Adolesc Psychopharmacol. 2019;29(3):168-180.
Irritability is a common comorbid symptom in children with ASD. Two second-generation antipsychotics (SGAs)—risperidone and aripiprazole—are FDA-approved for irritability associated with ASD. Fallah et al8 examined the efficacy of several SGAs for treating irritability.
Study design
- This review and meta-analysis included 8 studies identified from Medline, PsycINFO, and Embase from inception to March 2018. It included double-blind, randomized controlled trials that used the Aberrant Behavior Checklist Irritability (ABC-I) to measure irritability.
- The main outcome was change in degree of irritability.
- The 8 studies compared the efficacy of risperidone, aripiprazole, lurasidone, and placebo in a total of 878 patients.
Outcomes
- Risperidone reduced ABC-I scores more than aripiprazole, lurasidone, or placebo.
- Mean differences in ABC-I scores were Helvetica Neue LT Std−6.89 for risperidone, Helvetica Neue LT Std−6.62 for aripiprazole, and Helvetica Neue LT Std−1.61 for lurasidone.
Conclusion
- Risperidone and aripiprazole were efficacious and safe for children with ASD-associated irritability.
- Lurasidone may minimally improve irritability in children with ASD.
Continue to: Irritability and hyperactivity are common...
6. Mazahery H, Conlon CA, Beck KL, et al. A randomised controlled trial of vitamin D and omega-3 long chain polyunsaturated fatty acids in the treatment of irritability and hyperactivity among children with autism spectrum disorder. J Steroid Biochem Mol Biol. 2019;187:9-16.
Irritability and hyperactivity are common comorbid symptoms in children with ASD and have been linked to lower quality of life, poor adaptive functioning, and lower responsiveness to treatments when compared to children without comorbid problem behaviors. Mazahery et al9 evaluated the efficacy of vitamin D, omega-3 long-chain polyunsaturated fatty acids (LCPUFA), or both on irritability and hyperactivity.
Study design
- In a 1-year, double-blind, placebo-controlled trial, 73 children age 2.5 to 8 years with ASD were randomly assigned to receive placebo; vitamin D, 2000 IU/d (VID); omega-3 LCPUFA, 722 mg/d (OM); or both in the aforementioned doses.
- The primary outcome was reduction in the Aberrant Behavior Checklist in the domains of irritability and hyperactivity. Caregivers also completed weekly surveys regarding adverse events, compliance, and utilization of behavioral therapies.
- Of 111 children enrolled, 73 completed the 12 months of treatment.
Outcomes
- Children who received OM and VID had a greater reduction in irritability than those who received placebo (P = .001 and P = .01, respectively).
- Children who received VID also had a reduction in irritability (P = .047).
- An explanatory analysis revealed that OM also reduced lethargy (based on the Aberrant Behavior Checklist) more significantly than placebo (P = .02 adjusted for covariates).
Conclusion
- Treatment with vitamin D, 2000 IU/d, reduced irritability and hyperactivity.
- Treatment with omega-3 LCPUFA, 722 mg/d, reduced hyperactivity and lethargy.
Continue to: Glutamatergic dysregulation has been...
7. Wink LK, Adams R, Horn PS, et al. A randomized placebo-controlled cross-over pilot study of riluzole for drug-refractory irritability in autism spectrum disorder. J Autism Dev Disord. 2018;48(9):3051-3060.
Glutamatergic dysregulation has been identified as a potential cause of ASD. Riluzole, a glutamatergic modulator that is FDA-approved for treating amyotrophic lateral sclerosis, is a drug of interest for the treatment of ASD-related irritability due to this proposed mechanism. Wink et al10 evaluated riluzole for irritability in patients with ASD.
Study design
- This randomized, double-blind, placebo-controlled, crossover pilot study evaluated the tolerability and safety of adjunctive riluzole treatment for drug-refractory irritability in 8 patients with ASD.
- Participants were age 12 to 25 years, had a diagnosis of ASD confirmed by the autism diagnostic observation schedule 2, and an ABC-I subscale score ≥18. Participants receiving ≥2 psychotropic medications or glutamatergic/GABA-modulating medications were excluded.
- Participants received either 5 weeks of riluzole followed by 5 weeks of placebo, or vice versa; both groups then had a 2-week washout period.
- Riluzole was started at 50 mg/d, and then increased in 50 mg/d–increments to a maximum of 200 mg/d by Week 4.
- Primary outcome measures were change in score on the ABC-I and CGI-I.
Outcomes
- No significant treatment effects were identified.
- All participants tolerated riluzole, 200 mg/d, but increased dosages did not result in a higher overall treatment effect.
- There were no clinically significant adverse effects or laboratory abnormalities.
Conclusion
- Riluzole, 200 mg/d, was well tolerated but had no significant effect on irritability in adolescents with ASD.
1. Diagnostic and statistical manual of mental disorders, 5th ed. American Psychiatric Association; 2013.
2. Christensen DL, Baio J, Van Naarden Braun K, et al; Centers for Disease Control and Prevention. Prevalence and characteristics of autism spectrum disorder among children aged 8 years: Autism and Developmental Disabilities Monitoring Network, 11 sites, United States, 2012. MMWR Surveill Summ. 2016;65(3):1-23.
3. Fung LK, Mahajan R, Nozzolillo A, et al. Pharmacologic treatment of severe irritability and problem behaviors in autism: a systematic review and meta-analysis. Pediatrics. 2016;137(suppl 2):S124-S135.
4. Potter LA, Scholze DA, Biag HMB, et al. A randomized controlled trial of sertraline in young children with autism spectrum disorder. Front Psychiatry. 2019;10:810.
5. Gabis LV, Ben-Hur R, Shefer S, et al. Improvement of language in children with autism with combined donepezil and choline treatment. J Mol Neurosci. 2019;69(2):224-234.
6. James BJ, Gales MA, Gales BJ. Bumetanide for autism spectrum disorder in children: a review of randomized controlled trials. Ann Pharmacother. 2019;53(5) 537-544.
7. Li C, Bai Y, Jin C, et al. Efficacy and safety of fluoxetine in autism spectrum disorder: a meta-analysis. Am J Ther. 2020;27(3):e312-e315.
8. Fallah MS, Shaikh MR, Neupane B, et al. Atypical antipsychotics for irritability in pediatric autism: a systematic review and network meta-analysis. J Child Adolesc Psychopharmacol. 2019;29(3):168-180.
9. Mazahery H, Conlon CA, Beck KL, et al. A randomised controlled trial of vitamin D and omega-3 long chain polyunsaturated fatty acids in the treatment of irritability and hyperactivity among children with autism spectrum disorder. J Steroid Biochem Mol Biol. 2019;187:9-16.
10. Wink LK, Adams R, Horn PS, et al. A randomized placebo-controlled cross-over pilot study of riluzole for drug-refractory irritability in autism spectrum disorder. J Autism Dev Disord. 2018;48(9):3051-3060.
Autism spectrum disorder (ASD) is characterized by persistent deficits in social communication and social interaction, including deficits in social reciprocity, nonverbal communicative behaviors used for social interaction, and skills in developing, maintaining, and understanding relationships.1 In addition, the diagnosis of ASD requires the presence of restricted, repetitive patterns of behavior, interests, or activities.
Initially, ASD was considered a rare condition. In recent years, the reported prevalence has increased substantially. The most recent estimated prevalence is 1 in 68 children at age 8, with a male-to-female ratio of 4 to 1.2
Behavioral interventions are considered to be the most effective treatment for the core symptoms of ASD. Pharmacologic interventions are used primarily to treat associated or comorbid symptoms rather than the core symptoms. Aggression, self-injurious behavior, and irritability are common targets of pharmacotherapy in patients with ASD. Studies have provided support for the use of antipsychotic agents to treat irritability and associated aggressive behaviors in patients with autism,3 but because these agents have significant adverse effects—including extrapyramidal side effects, somnolence, and weight gain—their use requires a careful risk/benefit assessment. Stimulants have also been shown to be effective in treating comorbid attention-deficit/hyperactivity symptoms. The use of selective serotonin reuptake inhibitors (SSRIs) to manage repetitive behaviors and anxiety is also common.
Here, we review 7 recent studies of the pharmacologic management of ASD (Table).4-10 These studies examined the role of SSRIs (sertraline, fluoxetine), an acetylcholinesterase inhibitor (donepezil), atypical antipsychotics (risperidone, aripiprazole, lurasidone), natural supplements (vitamin D, omega-3), a diuretic (bumetanide), and a glutamatergic modulator (riluzole) in the treatment of ASD symptoms.

1. Potter LA, Scholze DA, Biag HMB, et al. A randomized controlled trial of sertraline in young children with autism spectrum disorder. Front Psychiatry. 2019;10:810.
Several studies have shown that SSRIs improve language development in children with Fragile X syndrome, based on the Mullen Scales of Early Learning (MSEL). A previously published trial involving children with Fragile X syndrome and comorbid ASD found that sertraline improved expressive language development. Potter et al4 examined the role of sertraline in children with ASD only.
Study Design
- In this randomized, double-blind, placebo-controlled trial, 58 children age 24 to 72 months with ASD received low-dose sertraline or placebo for 6 months.
- Of the 179 participants who were screened for eligibility, 58 were included in the study. Of these 58 participants, 32 received sertraline and 26 received placebo. The numbers of participants who discontinued from the sertraline and placebo arms were 8 and 5, respectively.
- Among those in the sertraline group, participants age <48 months received 2.5 mg/d, and those age ≥48 months received 5 mg/d.
Outcomes
- No significant differences were found on the primary outcome (MSEL expressive language raw score and age-equivalent combined score) or secondary outcomes (including Clinical Global Impressions–Improvement [CGI-I] scale at 3 months and end of treatment), as per intent-to-treat analyses.
- Sertraline was well tolerated. There was no difference in adverse effects between treatment groups and no serious adverse events.
Conclusion
- Although potentially useful for language development in patients with Fragile X syndrome with comorbid ASD, SSRIs such as sertraline have not proven efficacious for improving expressive language in patients with non-syndromic ASD.
- While 6-month treatment with low-dose sertraline in young children with ASD appears safe, the long-term effects are unknown.
Continue to: Gabis et al5 examined the safety...
2. Gabis LV, Ben-Hur R, Shefer S, et al. Improvement of language in children with autism with combined donepezil and choline treatment. J Mol Neurosci. 2019;69(2):224-234.
Gabis et al5 examined the safety and efficacy of utilizing donepezil, an acetylcholinesterase inhibitor, plus a choline supplement to treat both core features and associated symptoms in children and adolescents with ASD.
Study design
- This 9-month randomized, double-blind trial included 60 children/adolescents with ASD who were randomly assigned to receive placebo or donepezil plus a choline supplement. Participants underwent a baseline evaluation (E1), 12 weeks of treatment and re-evaluation (E2), 6 months of washout, and a final evaluation (E3).
- The baseline and final evaluations assessed changes in language performance, adaptive functioning, sleep habits, autism severity, clinical impression, and intellectual abilities. The evaluation after 12 weeks of treatment (E2) included all of these measures except intellectual abilities.
Outcomes
- Patients treated with donepezil plus a choline supplement had significant improvement in receptive language skills between E1 and E3 (P = .003).
- Patients treated with donepezil plus a choline supplement had significant worsening in scores on the Autism Treatment Evaluation Checklist (ATEC) health/physical behavior subscale between E1 and E2 (P = .012) and between E1 and E3 (P = .021).
- Improvement in receptive language skills was significant only in patients age 5 to 10 years (P = .047), whereas worsening in ATEC health/physical behavior subscale score was significant only in patients age 10 to 16 years (P = .024).
- Patients treated with donepezil plus a choline supplement reported higher percentages of gastrointestinal disturbance when compared with placebo (P = .007), and patients in the adolescent subgroup had a significant increase in irritability (P = .035).
Conclusion
- Patients age 5 to 10 years treated with donepezil plus a choline supplement exhibited improved receptive language skills. This treatment was less effective in patients age >10 years, and this group also exhibited behavioral worsening.
- Gastrointestinal disturbances were the main adverse effect of treatment with donepezil plus a choline supplement.
Continue to: The persistence of excitatory...
3. James BJ, Gales MA, Gales BJ. Bumetanide for autism spectrum disorder in children: a review of randomized controlled trials. Ann Pharmacother. 2019;53(5):537-544.
The persistence of excitatory gamma-aminobutyric acid (GABA) signaling has been found in patients with ASD. Bumetanide is a sodium-potassium-chloride cotransporter 1 (NKCC1) antagonist that not only decreases intracellular chloride, but also aberrantly decreases GABA signaling. This potent loop diuretic is a proposed treatment for symptoms of ASD. James et al6 evaluated the safety and efficacy of bumetanide use in children with ASD.
Study design
- Researchers searched the PubMed and Ovid MEDLINE databases for the terms “autism” and “bumetanide” between 1946 and 2018. A total of 26 articles were screened by title, 7 were screened by full text, and 3 articles were included in the study. The remaining articles were excluded due to study design and use of non-human subjects.
- All 3 randomized controlled trials evaluated the effects of low-dose oral bumetanide (most common dose was 0.5 mg twice daily) in a total of 208 patients age 2 to 18 years.
- Measurement scales used in the 3 studies included the Childhood Autism Rating Scale (CARS), Clinical Global Impressions Scale (CGI), Autism Behavioral Checklist (ABC), Social Responsiveness Scale (SRS), and Autism Diagnostic Observation Schedule-Generic (ADOS-G).
Outcomes
- Bumetanide improved scores on multiple autism assessment scales, including CARS, but the degree of improvement was not consistent across the 3 trials.
- There was a statistically significant improvement in ASD symptoms as measured by CGI in all 3 trials, and statistically significant improvements on the ABC and SRS in 2 trials. No improvements were noted on the ADOS-G in any of the trials.
- No dose-effect correlation was identified, but hypokalemia and polyuria were more prevalent with higher doses of bumetanide.
Conclusion
- Low-dose oral bumetanide improved social communication, social interactions, and restricted interests in patients with moderate to severe ASD. However, the 3 trials used different evaluation methods and observed varying degrees of improvement, which makes it difficult to make recommendations for or against the use of bumetanide.
- Streamlined trials with a consensus on evaluation methodology are needed to draw conclusions about the efficacy and safety of bumetanide as a treatment for ASD.
Continue to: The use of SSRIs to target...
4. Li C, Bai Y, Jin C, et al. Efficacy and safety of fluoxetine in autism spectrum disorder: a meta-analysis. Am J Ther. 2020;27(3):e312-e315.
The use of SSRIs to target symptoms of ASD has been long studied because many children with ASD have elevated serotonin levels. Several SSRIs, including fluoxetine, are FDA-approved for the treatment of obsessive-compulsive disorder, anxiety, and depression. Currently, no SSRIs are FDA-approved for treating ASD. In a meta-analysis, Li et al7 evaluated the use of fluoxetine for ASD.
Study design
- Two independent researchers searched for studies of fluoxetine treatment for ASD in Embase, Google Scholar, Ovid SP, and PubMed, with disagreement resolved by consensus.
- The researchers extracted the study design, patient demographics, and outcomes (inter-rater reliability kappa = 0.93). The primary outcomes were response rate of patients treated with fluoxetine, and change from baseline in ABC, ATEC, CARS, CGI, and Yale-Brown Obsessive Compulsive Scale (Y-BOCS) scores after fluoxetine treatment.
Outcomes
- This meta-analysis included 13 studies in which fluoxetine was used to treat a total of 303 patients with ASD. The median treatment duration was 6 months, the average age of participants was 15.23 years, and most participants (72%) were male.
- The response rate of patients treated with fluoxetine was 75%, with significant mean changes from baseline in ABC score (Helvetica Neue LT Std−3.42), ATEC score (Helvetica Neue LT Std−2.04), CGI score (Helvetica Neue LT Std−0.93), and Y-BOCS score (Helvetica Neue LT Std−1.86).
- A significantly higher incidence of hyperactivity/restlessness/agitation was noted with fluoxetine.
Conclusion
- Although 75% of participants responded to fluoxetine, the limitations of this meta-analysis included low power, inadequate quality of the included studies, and high statistical heterogeneity. In addition, the analysis found a high incidence of hyperactivity/restlessness associated with fluoxetine.
- Future randomized controlled studies may provide further clarification on managing symptoms of ASD with SSRIs.
Continue to: Irritability is a common comorbid...
5. Fallah MS, Shaikh MR, Neupane B, et al. Atypical antipsychotics for irritability in pediatric autism: a systematic review and network meta-analysis. J Child Adolesc Psychopharmacol. 2019;29(3):168-180.
Irritability is a common comorbid symptom in children with ASD. Two second-generation antipsychotics (SGAs)—risperidone and aripiprazole—are FDA-approved for irritability associated with ASD. Fallah et al8 examined the efficacy of several SGAs for treating irritability.
Study design
- This review and meta-analysis included 8 studies identified from Medline, PsycINFO, and Embase from inception to March 2018. It included double-blind, randomized controlled trials that used the Aberrant Behavior Checklist Irritability (ABC-I) to measure irritability.
- The main outcome was change in degree of irritability.
- The 8 studies compared the efficacy of risperidone, aripiprazole, lurasidone, and placebo in a total of 878 patients.
Outcomes
- Risperidone reduced ABC-I scores more than aripiprazole, lurasidone, or placebo.
- Mean differences in ABC-I scores were Helvetica Neue LT Std−6.89 for risperidone, Helvetica Neue LT Std−6.62 for aripiprazole, and Helvetica Neue LT Std−1.61 for lurasidone.
Conclusion
- Risperidone and aripiprazole were efficacious and safe for children with ASD-associated irritability.
- Lurasidone may minimally improve irritability in children with ASD.
Continue to: Irritability and hyperactivity are common...
6. Mazahery H, Conlon CA, Beck KL, et al. A randomised controlled trial of vitamin D and omega-3 long chain polyunsaturated fatty acids in the treatment of irritability and hyperactivity among children with autism spectrum disorder. J Steroid Biochem Mol Biol. 2019;187:9-16.
Irritability and hyperactivity are common comorbid symptoms in children with ASD and have been linked to lower quality of life, poor adaptive functioning, and lower responsiveness to treatments when compared to children without comorbid problem behaviors. Mazahery et al9 evaluated the efficacy of vitamin D, omega-3 long-chain polyunsaturated fatty acids (LCPUFA), or both on irritability and hyperactivity.
Study design
- In a 1-year, double-blind, placebo-controlled trial, 73 children age 2.5 to 8 years with ASD were randomly assigned to receive placebo; vitamin D, 2000 IU/d (VID); omega-3 LCPUFA, 722 mg/d (OM); or both in the aforementioned doses.
- The primary outcome was reduction in the Aberrant Behavior Checklist in the domains of irritability and hyperactivity. Caregivers also completed weekly surveys regarding adverse events, compliance, and utilization of behavioral therapies.
- Of 111 children enrolled, 73 completed the 12 months of treatment.
Outcomes
- Children who received OM and VID had a greater reduction in irritability than those who received placebo (P = .001 and P = .01, respectively).
- Children who received VID also had a reduction in irritability (P = .047).
- An explanatory analysis revealed that OM also reduced lethargy (based on the Aberrant Behavior Checklist) more significantly than placebo (P = .02 adjusted for covariates).
Conclusion
- Treatment with vitamin D, 2000 IU/d, reduced irritability and hyperactivity.
- Treatment with omega-3 LCPUFA, 722 mg/d, reduced hyperactivity and lethargy.
Continue to: Glutamatergic dysregulation has been...
7. Wink LK, Adams R, Horn PS, et al. A randomized placebo-controlled cross-over pilot study of riluzole for drug-refractory irritability in autism spectrum disorder. J Autism Dev Disord. 2018;48(9):3051-3060.
Glutamatergic dysregulation has been identified as a potential cause of ASD. Riluzole, a glutamatergic modulator that is FDA-approved for treating amyotrophic lateral sclerosis, is a drug of interest for the treatment of ASD-related irritability due to this proposed mechanism. Wink et al10 evaluated riluzole for irritability in patients with ASD.
Study design
- This randomized, double-blind, placebo-controlled, crossover pilot study evaluated the tolerability and safety of adjunctive riluzole treatment for drug-refractory irritability in 8 patients with ASD.
- Participants were age 12 to 25 years, had a diagnosis of ASD confirmed by the autism diagnostic observation schedule 2, and an ABC-I subscale score ≥18. Participants receiving ≥2 psychotropic medications or glutamatergic/GABA-modulating medications were excluded.
- Participants received either 5 weeks of riluzole followed by 5 weeks of placebo, or vice versa; both groups then had a 2-week washout period.
- Riluzole was started at 50 mg/d, and then increased in 50 mg/d–increments to a maximum of 200 mg/d by Week 4.
- Primary outcome measures were change in score on the ABC-I and CGI-I.
Outcomes
- No significant treatment effects were identified.
- All participants tolerated riluzole, 200 mg/d, but increased dosages did not result in a higher overall treatment effect.
- There were no clinically significant adverse effects or laboratory abnormalities.
Conclusion
- Riluzole, 200 mg/d, was well tolerated but had no significant effect on irritability in adolescents with ASD.
Autism spectrum disorder (ASD) is characterized by persistent deficits in social communication and social interaction, including deficits in social reciprocity, nonverbal communicative behaviors used for social interaction, and skills in developing, maintaining, and understanding relationships.1 In addition, the diagnosis of ASD requires the presence of restricted, repetitive patterns of behavior, interests, or activities.
Initially, ASD was considered a rare condition. In recent years, the reported prevalence has increased substantially. The most recent estimated prevalence is 1 in 68 children at age 8, with a male-to-female ratio of 4 to 1.2
Behavioral interventions are considered to be the most effective treatment for the core symptoms of ASD. Pharmacologic interventions are used primarily to treat associated or comorbid symptoms rather than the core symptoms. Aggression, self-injurious behavior, and irritability are common targets of pharmacotherapy in patients with ASD. Studies have provided support for the use of antipsychotic agents to treat irritability and associated aggressive behaviors in patients with autism,3 but because these agents have significant adverse effects—including extrapyramidal side effects, somnolence, and weight gain—their use requires a careful risk/benefit assessment. Stimulants have also been shown to be effective in treating comorbid attention-deficit/hyperactivity symptoms. The use of selective serotonin reuptake inhibitors (SSRIs) to manage repetitive behaviors and anxiety is also common.
Here, we review 7 recent studies of the pharmacologic management of ASD (Table).4-10 These studies examined the role of SSRIs (sertraline, fluoxetine), an acetylcholinesterase inhibitor (donepezil), atypical antipsychotics (risperidone, aripiprazole, lurasidone), natural supplements (vitamin D, omega-3), a diuretic (bumetanide), and a glutamatergic modulator (riluzole) in the treatment of ASD symptoms.

1. Potter LA, Scholze DA, Biag HMB, et al. A randomized controlled trial of sertraline in young children with autism spectrum disorder. Front Psychiatry. 2019;10:810.
Several studies have shown that SSRIs improve language development in children with Fragile X syndrome, based on the Mullen Scales of Early Learning (MSEL). A previously published trial involving children with Fragile X syndrome and comorbid ASD found that sertraline improved expressive language development. Potter et al4 examined the role of sertraline in children with ASD only.
Study Design
- In this randomized, double-blind, placebo-controlled trial, 58 children age 24 to 72 months with ASD received low-dose sertraline or placebo for 6 months.
- Of the 179 participants who were screened for eligibility, 58 were included in the study. Of these 58 participants, 32 received sertraline and 26 received placebo. The numbers of participants who discontinued from the sertraline and placebo arms were 8 and 5, respectively.
- Among those in the sertraline group, participants age <48 months received 2.5 mg/d, and those age ≥48 months received 5 mg/d.
Outcomes
- No significant differences were found on the primary outcome (MSEL expressive language raw score and age-equivalent combined score) or secondary outcomes (including Clinical Global Impressions–Improvement [CGI-I] scale at 3 months and end of treatment), as per intent-to-treat analyses.
- Sertraline was well tolerated. There was no difference in adverse effects between treatment groups and no serious adverse events.
Conclusion
- Although potentially useful for language development in patients with Fragile X syndrome with comorbid ASD, SSRIs such as sertraline have not proven efficacious for improving expressive language in patients with non-syndromic ASD.
- While 6-month treatment with low-dose sertraline in young children with ASD appears safe, the long-term effects are unknown.
Continue to: Gabis et al5 examined the safety...
2. Gabis LV, Ben-Hur R, Shefer S, et al. Improvement of language in children with autism with combined donepezil and choline treatment. J Mol Neurosci. 2019;69(2):224-234.
Gabis et al5 examined the safety and efficacy of utilizing donepezil, an acetylcholinesterase inhibitor, plus a choline supplement to treat both core features and associated symptoms in children and adolescents with ASD.
Study design
- This 9-month randomized, double-blind trial included 60 children/adolescents with ASD who were randomly assigned to receive placebo or donepezil plus a choline supplement. Participants underwent a baseline evaluation (E1), 12 weeks of treatment and re-evaluation (E2), 6 months of washout, and a final evaluation (E3).
- The baseline and final evaluations assessed changes in language performance, adaptive functioning, sleep habits, autism severity, clinical impression, and intellectual abilities. The evaluation after 12 weeks of treatment (E2) included all of these measures except intellectual abilities.
Outcomes
- Patients treated with donepezil plus a choline supplement had significant improvement in receptive language skills between E1 and E3 (P = .003).
- Patients treated with donepezil plus a choline supplement had significant worsening in scores on the Autism Treatment Evaluation Checklist (ATEC) health/physical behavior subscale between E1 and E2 (P = .012) and between E1 and E3 (P = .021).
- Improvement in receptive language skills was significant only in patients age 5 to 10 years (P = .047), whereas worsening in ATEC health/physical behavior subscale score was significant only in patients age 10 to 16 years (P = .024).
- Patients treated with donepezil plus a choline supplement reported higher percentages of gastrointestinal disturbance when compared with placebo (P = .007), and patients in the adolescent subgroup had a significant increase in irritability (P = .035).
Conclusion
- Patients age 5 to 10 years treated with donepezil plus a choline supplement exhibited improved receptive language skills. This treatment was less effective in patients age >10 years, and this group also exhibited behavioral worsening.
- Gastrointestinal disturbances were the main adverse effect of treatment with donepezil plus a choline supplement.
Continue to: The persistence of excitatory...
3. James BJ, Gales MA, Gales BJ. Bumetanide for autism spectrum disorder in children: a review of randomized controlled trials. Ann Pharmacother. 2019;53(5):537-544.
The persistence of excitatory gamma-aminobutyric acid (GABA) signaling has been found in patients with ASD. Bumetanide is a sodium-potassium-chloride cotransporter 1 (NKCC1) antagonist that not only decreases intracellular chloride, but also aberrantly decreases GABA signaling. This potent loop diuretic is a proposed treatment for symptoms of ASD. James et al6 evaluated the safety and efficacy of bumetanide use in children with ASD.
Study design
- Researchers searched the PubMed and Ovid MEDLINE databases for the terms “autism” and “bumetanide” between 1946 and 2018. A total of 26 articles were screened by title, 7 were screened by full text, and 3 articles were included in the study. The remaining articles were excluded due to study design and use of non-human subjects.
- All 3 randomized controlled trials evaluated the effects of low-dose oral bumetanide (most common dose was 0.5 mg twice daily) in a total of 208 patients age 2 to 18 years.
- Measurement scales used in the 3 studies included the Childhood Autism Rating Scale (CARS), Clinical Global Impressions Scale (CGI), Autism Behavioral Checklist (ABC), Social Responsiveness Scale (SRS), and Autism Diagnostic Observation Schedule-Generic (ADOS-G).
Outcomes
- Bumetanide improved scores on multiple autism assessment scales, including CARS, but the degree of improvement was not consistent across the 3 trials.
- There was a statistically significant improvement in ASD symptoms as measured by CGI in all 3 trials, and statistically significant improvements on the ABC and SRS in 2 trials. No improvements were noted on the ADOS-G in any of the trials.
- No dose-effect correlation was identified, but hypokalemia and polyuria were more prevalent with higher doses of bumetanide.
Conclusion
- Low-dose oral bumetanide improved social communication, social interactions, and restricted interests in patients with moderate to severe ASD. However, the 3 trials used different evaluation methods and observed varying degrees of improvement, which makes it difficult to make recommendations for or against the use of bumetanide.
- Streamlined trials with a consensus on evaluation methodology are needed to draw conclusions about the efficacy and safety of bumetanide as a treatment for ASD.
Continue to: The use of SSRIs to target...
4. Li C, Bai Y, Jin C, et al. Efficacy and safety of fluoxetine in autism spectrum disorder: a meta-analysis. Am J Ther. 2020;27(3):e312-e315.
The use of SSRIs to target symptoms of ASD has been long studied because many children with ASD have elevated serotonin levels. Several SSRIs, including fluoxetine, are FDA-approved for the treatment of obsessive-compulsive disorder, anxiety, and depression. Currently, no SSRIs are FDA-approved for treating ASD. In a meta-analysis, Li et al7 evaluated the use of fluoxetine for ASD.
Study design
- Two independent researchers searched for studies of fluoxetine treatment for ASD in Embase, Google Scholar, Ovid SP, and PubMed, with disagreement resolved by consensus.
- The researchers extracted the study design, patient demographics, and outcomes (inter-rater reliability kappa = 0.93). The primary outcomes were response rate of patients treated with fluoxetine, and change from baseline in ABC, ATEC, CARS, CGI, and Yale-Brown Obsessive Compulsive Scale (Y-BOCS) scores after fluoxetine treatment.
Outcomes
- This meta-analysis included 13 studies in which fluoxetine was used to treat a total of 303 patients with ASD. The median treatment duration was 6 months, the average age of participants was 15.23 years, and most participants (72%) were male.
- The response rate of patients treated with fluoxetine was 75%, with significant mean changes from baseline in ABC score (Helvetica Neue LT Std−3.42), ATEC score (Helvetica Neue LT Std−2.04), CGI score (Helvetica Neue LT Std−0.93), and Y-BOCS score (Helvetica Neue LT Std−1.86).
- A significantly higher incidence of hyperactivity/restlessness/agitation was noted with fluoxetine.
Conclusion
- Although 75% of participants responded to fluoxetine, the limitations of this meta-analysis included low power, inadequate quality of the included studies, and high statistical heterogeneity. In addition, the analysis found a high incidence of hyperactivity/restlessness associated with fluoxetine.
- Future randomized controlled studies may provide further clarification on managing symptoms of ASD with SSRIs.
Continue to: Irritability is a common comorbid...
5. Fallah MS, Shaikh MR, Neupane B, et al. Atypical antipsychotics for irritability in pediatric autism: a systematic review and network meta-analysis. J Child Adolesc Psychopharmacol. 2019;29(3):168-180.
Irritability is a common comorbid symptom in children with ASD. Two second-generation antipsychotics (SGAs)—risperidone and aripiprazole—are FDA-approved for irritability associated with ASD. Fallah et al8 examined the efficacy of several SGAs for treating irritability.
Study design
- This review and meta-analysis included 8 studies identified from Medline, PsycINFO, and Embase from inception to March 2018. It included double-blind, randomized controlled trials that used the Aberrant Behavior Checklist Irritability (ABC-I) to measure irritability.
- The main outcome was change in degree of irritability.
- The 8 studies compared the efficacy of risperidone, aripiprazole, lurasidone, and placebo in a total of 878 patients.
Outcomes
- Risperidone reduced ABC-I scores more than aripiprazole, lurasidone, or placebo.
- Mean differences in ABC-I scores were Helvetica Neue LT Std−6.89 for risperidone, Helvetica Neue LT Std−6.62 for aripiprazole, and Helvetica Neue LT Std−1.61 for lurasidone.
Conclusion
- Risperidone and aripiprazole were efficacious and safe for children with ASD-associated irritability.
- Lurasidone may minimally improve irritability in children with ASD.
Continue to: Irritability and hyperactivity are common...
6. Mazahery H, Conlon CA, Beck KL, et al. A randomised controlled trial of vitamin D and omega-3 long chain polyunsaturated fatty acids in the treatment of irritability and hyperactivity among children with autism spectrum disorder. J Steroid Biochem Mol Biol. 2019;187:9-16.
Irritability and hyperactivity are common comorbid symptoms in children with ASD and have been linked to lower quality of life, poor adaptive functioning, and lower responsiveness to treatments when compared to children without comorbid problem behaviors. Mazahery et al9 evaluated the efficacy of vitamin D, omega-3 long-chain polyunsaturated fatty acids (LCPUFA), or both on irritability and hyperactivity.
Study design
- In a 1-year, double-blind, placebo-controlled trial, 73 children age 2.5 to 8 years with ASD were randomly assigned to receive placebo; vitamin D, 2000 IU/d (VID); omega-3 LCPUFA, 722 mg/d (OM); or both in the aforementioned doses.
- The primary outcome was reduction in the Aberrant Behavior Checklist in the domains of irritability and hyperactivity. Caregivers also completed weekly surveys regarding adverse events, compliance, and utilization of behavioral therapies.
- Of 111 children enrolled, 73 completed the 12 months of treatment.
Outcomes
- Children who received OM and VID had a greater reduction in irritability than those who received placebo (P = .001 and P = .01, respectively).
- Children who received VID also had a reduction in irritability (P = .047).
- An explanatory analysis revealed that OM also reduced lethargy (based on the Aberrant Behavior Checklist) more significantly than placebo (P = .02 adjusted for covariates).
Conclusion
- Treatment with vitamin D, 2000 IU/d, reduced irritability and hyperactivity.
- Treatment with omega-3 LCPUFA, 722 mg/d, reduced hyperactivity and lethargy.
Continue to: Glutamatergic dysregulation has been...
7. Wink LK, Adams R, Horn PS, et al. A randomized placebo-controlled cross-over pilot study of riluzole for drug-refractory irritability in autism spectrum disorder. J Autism Dev Disord. 2018;48(9):3051-3060.
Glutamatergic dysregulation has been identified as a potential cause of ASD. Riluzole, a glutamatergic modulator that is FDA-approved for treating amyotrophic lateral sclerosis, is a drug of interest for the treatment of ASD-related irritability due to this proposed mechanism. Wink et al10 evaluated riluzole for irritability in patients with ASD.
Study design
- This randomized, double-blind, placebo-controlled, crossover pilot study evaluated the tolerability and safety of adjunctive riluzole treatment for drug-refractory irritability in 8 patients with ASD.
- Participants were age 12 to 25 years, had a diagnosis of ASD confirmed by the autism diagnostic observation schedule 2, and an ABC-I subscale score ≥18. Participants receiving ≥2 psychotropic medications or glutamatergic/GABA-modulating medications were excluded.
- Participants received either 5 weeks of riluzole followed by 5 weeks of placebo, or vice versa; both groups then had a 2-week washout period.
- Riluzole was started at 50 mg/d, and then increased in 50 mg/d–increments to a maximum of 200 mg/d by Week 4.
- Primary outcome measures were change in score on the ABC-I and CGI-I.
Outcomes
- No significant treatment effects were identified.
- All participants tolerated riluzole, 200 mg/d, but increased dosages did not result in a higher overall treatment effect.
- There were no clinically significant adverse effects or laboratory abnormalities.
Conclusion
- Riluzole, 200 mg/d, was well tolerated but had no significant effect on irritability in adolescents with ASD.
1. Diagnostic and statistical manual of mental disorders, 5th ed. American Psychiatric Association; 2013.
2. Christensen DL, Baio J, Van Naarden Braun K, et al; Centers for Disease Control and Prevention. Prevalence and characteristics of autism spectrum disorder among children aged 8 years: Autism and Developmental Disabilities Monitoring Network, 11 sites, United States, 2012. MMWR Surveill Summ. 2016;65(3):1-23.
3. Fung LK, Mahajan R, Nozzolillo A, et al. Pharmacologic treatment of severe irritability and problem behaviors in autism: a systematic review and meta-analysis. Pediatrics. 2016;137(suppl 2):S124-S135.
4. Potter LA, Scholze DA, Biag HMB, et al. A randomized controlled trial of sertraline in young children with autism spectrum disorder. Front Psychiatry. 2019;10:810.
5. Gabis LV, Ben-Hur R, Shefer S, et al. Improvement of language in children with autism with combined donepezil and choline treatment. J Mol Neurosci. 2019;69(2):224-234.
6. James BJ, Gales MA, Gales BJ. Bumetanide for autism spectrum disorder in children: a review of randomized controlled trials. Ann Pharmacother. 2019;53(5) 537-544.
7. Li C, Bai Y, Jin C, et al. Efficacy and safety of fluoxetine in autism spectrum disorder: a meta-analysis. Am J Ther. 2020;27(3):e312-e315.
8. Fallah MS, Shaikh MR, Neupane B, et al. Atypical antipsychotics for irritability in pediatric autism: a systematic review and network meta-analysis. J Child Adolesc Psychopharmacol. 2019;29(3):168-180.
9. Mazahery H, Conlon CA, Beck KL, et al. A randomised controlled trial of vitamin D and omega-3 long chain polyunsaturated fatty acids in the treatment of irritability and hyperactivity among children with autism spectrum disorder. J Steroid Biochem Mol Biol. 2019;187:9-16.
10. Wink LK, Adams R, Horn PS, et al. A randomized placebo-controlled cross-over pilot study of riluzole for drug-refractory irritability in autism spectrum disorder. J Autism Dev Disord. 2018;48(9):3051-3060.
1. Diagnostic and statistical manual of mental disorders, 5th ed. American Psychiatric Association; 2013.
2. Christensen DL, Baio J, Van Naarden Braun K, et al; Centers for Disease Control and Prevention. Prevalence and characteristics of autism spectrum disorder among children aged 8 years: Autism and Developmental Disabilities Monitoring Network, 11 sites, United States, 2012. MMWR Surveill Summ. 2016;65(3):1-23.
3. Fung LK, Mahajan R, Nozzolillo A, et al. Pharmacologic treatment of severe irritability and problem behaviors in autism: a systematic review and meta-analysis. Pediatrics. 2016;137(suppl 2):S124-S135.
4. Potter LA, Scholze DA, Biag HMB, et al. A randomized controlled trial of sertraline in young children with autism spectrum disorder. Front Psychiatry. 2019;10:810.
5. Gabis LV, Ben-Hur R, Shefer S, et al. Improvement of language in children with autism with combined donepezil and choline treatment. J Mol Neurosci. 2019;69(2):224-234.
6. James BJ, Gales MA, Gales BJ. Bumetanide for autism spectrum disorder in children: a review of randomized controlled trials. Ann Pharmacother. 2019;53(5) 537-544.
7. Li C, Bai Y, Jin C, et al. Efficacy and safety of fluoxetine in autism spectrum disorder: a meta-analysis. Am J Ther. 2020;27(3):e312-e315.
8. Fallah MS, Shaikh MR, Neupane B, et al. Atypical antipsychotics for irritability in pediatric autism: a systematic review and network meta-analysis. J Child Adolesc Psychopharmacol. 2019;29(3):168-180.
9. Mazahery H, Conlon CA, Beck KL, et al. A randomised controlled trial of vitamin D and omega-3 long chain polyunsaturated fatty acids in the treatment of irritability and hyperactivity among children with autism spectrum disorder. J Steroid Biochem Mol Biol. 2019;187:9-16.
10. Wink LK, Adams R, Horn PS, et al. A randomized placebo-controlled cross-over pilot study of riluzole for drug-refractory irritability in autism spectrum disorder. J Autism Dev Disord. 2018;48(9):3051-3060.
COVID-19 and decision-making capacity; more
COVID-19 and decision-making capacity
Dr. Ryznar’s article “Evaluating patients’ decision-making capacity during COVID-19” (Evidence-Based Reviews,
For example, in a controversial 2007 case in Atlanta, Georgia, an attorney with active tuberculosis failed to heed medical advice to refrain from traveling.1 The patient’s uncooperativeness did not implicate concerns over his decisional capacity.1 However, his international and interstate travel triggered the Centers for Disease Control and Prevention’s legal authority under the Public Health Service Act to prevent the entry and spread of communicable disease.1-3 An authorized order from a duly constituted public health authority is issued and enforceable without regard to clinical determinations of capacity (and is generally subject to challenge via judicial or other due process mechanisms as a government-sanctioned deprivation of liberty to protect public welfare). State laws and local ordinances require physicians to notify the appropriate public health department when patients test positive for certain contagious diseases.
The difficulty with involuntarily detaining a cognitively intact patient due to concern over their contagion risk and erroneous beliefs runs considerably deeper than eliciting a “political backlash” or managing the qualms of hospital security officers. It is a fundamental matter of proper legal authority. Psychiatrists and other physicians assess patients’ decision-making capacity for specific treatment decisions on a case-by-case basis, seeking to preserve autonomy while practicing beneficence. Public health officers are agents of the state with designated authorities to control the spread of disease. A capacity determination in the absence of neurocognitive deficits implies the psychiatrist is evaluating the soundness of the patient’s ideas as opposed to their cognition, overlooking the reality that fully capable individuals can possess dubious—and even unsalutary—beliefs. While physicians educate patients about the risks of contracting and communicating infection, they are thankfully not tasked with arbitrating sociopolitical disputes at the bedside. Such controversies regarding pandemic response do not belong under the rubric of medical decision-making capacity. Conflating psychosomatic medicine consultations with public health orders risks unmooring capacity determinations from their medicolegal and bioethical foundations.
Charles G. Kels, JD
S Army Medical Center of Excellence
San Antonio, Texas
Disclaimer: The views expressed here are those of the author and do not necessarily reflect those of any government agency.
References
1. Tanne JH. Tuberculosis case exposes flaws in international public health systems. BMJ. 2007;334(7605):1187.
2. Public Health Service Act, 42 USC § 264-272 (1944).
3. Interstate and Foreign Quarantine, 42 CFR Parts 70-71 (2017).
The author responds
I appreciate Mr. Kels’s letter and explicit discussion of the limits of decision-making capacity. I agree that physicians should not overstep their legal authority and ethical mandate. The specific case discussed in my article was a patient who was symptomatic from COVID-19 who wanted to leave the hospital against medical advice. The contagious nature of this virus certainly falls under the risk/benefit analysis of the clinical situation because it is an important aspect of understanding the nature of the illness and treatment/recovery process (as a thought example, consider that such a patient lives with their elderly mother who has heart disease and chronic obstructive pulmonary disease, and the patient does not want their mother to die). From a medicolegal perspective, the risk of infection to others may not necessarily outweigh the benefit of autonomy, especially because decision-making capacity assessments are made with the purpose of balancing autonomy and beneficence of the patient, not others. I highlighted the relative importance of autonomy using the weight of the arrows in Figure 2 of my article. I did not task physicians with arbitrating sociopolitical disputes, but merely highlighted how the current climate can impact people’s personal views on COVID-19, which sometimes can run counter to scientific evidence. If a patient has an erroneous view about an illness, it is our duty to try to help them understand if it directly impacts their health or affects their decision-making process, especially in a high-stakes clinical scenario.
Elizabeth Ryznar, MD, MSc
Assistant Professor
Department of Psychiatry and Behavioral Sciences
Johns Hopkins School of Medicine
Baltimore, Maryland
Continue to: Olanzapine for treatment-resistant anxiety
Olanzapine for treatment-resistant anxiety
Ms. A, age 62, was a retired high school teacher. Her primary care physician referred her to me for persistent, disabling anxiety. Her condition was recently worsened by a trial of escitalopram, 5 mg/d, which led her to visit the emergency department (ED). There she was prescribed lorazepam, 0.5 mg as needed, which helped her somewhat. Her medical conditions included prominent gastrointestinal (GI) symptoms, with nausea and a restricted diet; tinnitus; and chronic bilateral hand tremors. Her initial Patient Health Questionnaire-9 (PHQ-9) score was 11, and her Generalized Anxiety Disorder-7 (GAD-7) score was 10.
Initially, I encouraged Ms. A to exercise regularly, and I changed her lorazepam from 0.5 mg as-needed to 0.5 mg twice a day. I also referred her to a psychologist for psychotherapy. She showed limited improvement. I increased her lorazepam to 1 mg 3 times a day and started sertraline, 12.5 mg/d, but she soon experienced chest tightness and was admitted to the ED for observation and a cardiac workup. After she visited the ED, Ms. A stopped taking sertraline.
When I next saw Ms. A, she agreed to a trial of olanzapine, 2.5 mg/d at bedtime. Three weeks later, she told me, “I feel so much better.” Her scores on the PHQ-9 and GAD-7 were 0 and 1, respectively. Her GI complaints decreased, she had gained a little weight, and her tinnitus bothered her less. Lorazepam was gradually decreased and stopped.
After approximately 2 years, Ms. A had experienced no long-term adverse effects. We agreed to gradually discontinue olanzapine. Over the next 4 months, Ms. A decreased and stopped taking olanzapine at her own discretion.Three weeks after she stopped taking olanzapine, Ms. A reported that her psychiatric and GI symptoms had returned. She still maintained weekly visits with her psychotherapist. Her GI specialist asked if I could prescribe her olanzapine again. I restarted Ms. A on olanzapine, 2.5 mg/d at bedtime. By the next month, she said she felt much better (PHQ-9: 0; GAD-7: 1). I last saw Ms. A approximately 1 year ago.
Over the years, I have usually prescribed low-dose olanzapine alone or with other medications for patients with treatment-resistance who had no overt psychotic symptoms, I have used this medication for patients with “soft” psychotic thinking marked by severe anxiety, obsessions, compulsivity, perfectionism, and/or rumination.1 Evidence suggests olanzapine also may be effective for anorexia nervosa.2 There is good evidence for its use in the DSM-5 diagnosis of avoidant/restrictive food intake disorder (“a food avoidance emotional disorder”).3,4 In retrospect, Ms. A also likely met the criteria for the diagnosis of unspecified eating disorder. Despite extensive GI workup and follow-up, physical signs of GI pathology were equivocal.
Among antipsychotics, olanzapine most closely resembles clozapine, the only antipsychotic that has been proved more efficacious than others for psychotic symptoms.5 There is also some research suggesting that olanzapine may be more efficacious.6 Obsessions and perfectionism are associated with dopamine D4 receptor activity, and D1, D2, and D3 receptors are involved in normalizing cognition and reward.7 There are appropriate concerns about adverse effects, especially metabolic syndrome and obesity, with olanzapine, but patients can have different profiles of receptor sensitivity. In my conversations with Ms. A’s primary care physician and GI specialist, metabolic syndrome was not an issue. Clearly, low-dose olanzapine was very helpful in her treatment.
Daniel Storch, MD
Key Point Health Services
Catonsville, Maryland
References
1. Goodnick PJ, Barrios CA. Use of olanzapine in non-psychotic psychiatric disorders. Expert Opin Pharmacother. 2001;2(4):667-680.
2. Brewerton TD. Psychopharmacologic management of eating disorders. Presented at: 25th Annual National Psychopharmacology Update; February 2020; Las Vegas, Nevada. Accessed December 8, 2020. https://legacy.audio-digest.org/pages/htmlos/pastissues.html?sub1=psychiatry&sub2=2020
3. Diagnostic and statistical manual of mental disorders, 5th ed. American Psychiatric Association; 2013.
4. Brewerton TD, D’Agostino M. Adjunctive use of olanzapine in the treatment of avoidant restrictive food intake disorder in children and adolescents in an eating disorders program. J Child Adolesc Psychopharmacol. 2017;27(10):920-922.
5. Lobos CA, Komossa K, Rummel-Kluge C, et al. Clozapine versus other atypical antipsychotics for schizophrenia. Cochrane Database Syst Rev. 2010;(11):CD006633.
6. Komossa K, Rummel-Kluge C, Hunger H, et al. Olanzapine versus other atypical antipsychotics for schizophrenia. Cochrane Database Syst Rev. 2010;(3):CD006654.
7. Bachner-Melman R, Lerer E, Zohar AH, et al. Anorexia nervosa, perfectionism, and dopamine D4 receptor (DRD4). Am J Med Genet B Neuropsychiatr Genet. 2007;144B(6):748-756.
Continue to: Neuro-politics and academic paralysis...
Neuro-politics and academic paralysis
I commend Dr. Nasrallah for his brief, precisely defined, scientific editorial “Neuro-politics: Will you vote with your cortex or limbic system?” (From the Editor,
I would like to see
Similar to a hurricane or tsunami that pushes water into a river, this retrograde shift of feedback pathways is demonstrated by emotional narratives that have flooded the public and drowned facts and evidence-based practice. Furthermore, the science of convenience has emerged, where facts are eligible only if they justify the narrative. Any discussion, debate, or questioning of the rationale of the approach is met with hostility, naming, shaming, and even loss of employment at universities. I have sadly learned from frightened colleagues and from reading reports by academicians whose publications have been either rejected or coerced for revision following acceptance by a peer-reviewed journal or even retracted post-publication due to complaints, harassment, and threats by the politically correct “thought police.” Diversity of thinking and freedom of speech—core values and principles in academic dialogue—have been violated. Academicians are as perplexed as laboratory rats that need to learn which lever to push in order to receive a reward and avoid punishment in an ever-shifting environment. People have been pondering, “Is it time for flight, fright, or fight?” As Buffalo Springfield’s legendary Vietnam 1960s–era song “For What it’s Worth” states: “There’s battle lines being drawn and nobody’s right if everybody’s wrong.”
What we have learned from history is that the majority of people exercise passivity and hope as bystanders in order to avoid becoming victims of “collateral damage.” Are there no modern Giordano Bruno (the martyr of science), Copernicus, or Michelangelo who would challenge the “Church of the People” that has created new language, terminology, and culture and is on the verge of creating nouveau scientific principles that could lead to a monopoly of one segment of society that threatens pluralism of thought. Do we need dystopic books such as 1984 or Fahrenheit 451, or the experience of the French and Russian revolution (epitomized by the guillotine and the gulag) to remind us that we are a step away from education and reprogramming camps that used to be called universities? The American Association of University Professors’ most recent announcement on academic freedom ominously avoids using terms such as freedom of speech, diversity of opinions, or even pluralism.
I hope that psychiatrists will lead the way back to sanity, starting with focus groups and forums. It would amount to a group cognitive-behavioral therapy of immense proportion following a paradigm of “Problem Solving,” according to Albert Bandura’s social learning model. There is simply no other constructive way to get to the cheese at the end of the maze.
Yifrah Kaminer, MD
Professor Emeritus of Psychiatry & Pediatrics
University of Connecticut School of Medicine
Farmington, Connecticut
Disclosures: The authors report no financial relationships with any companies whose products are mentioned in this article, or with manufacturers of competing products.
COVID-19 and decision-making capacity
Dr. Ryznar’s article “Evaluating patients’ decision-making capacity during COVID-19” (Evidence-Based Reviews,
For example, in a controversial 2007 case in Atlanta, Georgia, an attorney with active tuberculosis failed to heed medical advice to refrain from traveling.1 The patient’s uncooperativeness did not implicate concerns over his decisional capacity.1 However, his international and interstate travel triggered the Centers for Disease Control and Prevention’s legal authority under the Public Health Service Act to prevent the entry and spread of communicable disease.1-3 An authorized order from a duly constituted public health authority is issued and enforceable without regard to clinical determinations of capacity (and is generally subject to challenge via judicial or other due process mechanisms as a government-sanctioned deprivation of liberty to protect public welfare). State laws and local ordinances require physicians to notify the appropriate public health department when patients test positive for certain contagious diseases.
The difficulty with involuntarily detaining a cognitively intact patient due to concern over their contagion risk and erroneous beliefs runs considerably deeper than eliciting a “political backlash” or managing the qualms of hospital security officers. It is a fundamental matter of proper legal authority. Psychiatrists and other physicians assess patients’ decision-making capacity for specific treatment decisions on a case-by-case basis, seeking to preserve autonomy while practicing beneficence. Public health officers are agents of the state with designated authorities to control the spread of disease. A capacity determination in the absence of neurocognitive deficits implies the psychiatrist is evaluating the soundness of the patient’s ideas as opposed to their cognition, overlooking the reality that fully capable individuals can possess dubious—and even unsalutary—beliefs. While physicians educate patients about the risks of contracting and communicating infection, they are thankfully not tasked with arbitrating sociopolitical disputes at the bedside. Such controversies regarding pandemic response do not belong under the rubric of medical decision-making capacity. Conflating psychosomatic medicine consultations with public health orders risks unmooring capacity determinations from their medicolegal and bioethical foundations.
Charles G. Kels, JD
S Army Medical Center of Excellence
San Antonio, Texas
Disclaimer: The views expressed here are those of the author and do not necessarily reflect those of any government agency.
References
1. Tanne JH. Tuberculosis case exposes flaws in international public health systems. BMJ. 2007;334(7605):1187.
2. Public Health Service Act, 42 USC § 264-272 (1944).
3. Interstate and Foreign Quarantine, 42 CFR Parts 70-71 (2017).
The author responds
I appreciate Mr. Kels’s letter and explicit discussion of the limits of decision-making capacity. I agree that physicians should not overstep their legal authority and ethical mandate. The specific case discussed in my article was a patient who was symptomatic from COVID-19 who wanted to leave the hospital against medical advice. The contagious nature of this virus certainly falls under the risk/benefit analysis of the clinical situation because it is an important aspect of understanding the nature of the illness and treatment/recovery process (as a thought example, consider that such a patient lives with their elderly mother who has heart disease and chronic obstructive pulmonary disease, and the patient does not want their mother to die). From a medicolegal perspective, the risk of infection to others may not necessarily outweigh the benefit of autonomy, especially because decision-making capacity assessments are made with the purpose of balancing autonomy and beneficence of the patient, not others. I highlighted the relative importance of autonomy using the weight of the arrows in Figure 2 of my article. I did not task physicians with arbitrating sociopolitical disputes, but merely highlighted how the current climate can impact people’s personal views on COVID-19, which sometimes can run counter to scientific evidence. If a patient has an erroneous view about an illness, it is our duty to try to help them understand if it directly impacts their health or affects their decision-making process, especially in a high-stakes clinical scenario.
Elizabeth Ryznar, MD, MSc
Assistant Professor
Department of Psychiatry and Behavioral Sciences
Johns Hopkins School of Medicine
Baltimore, Maryland
Continue to: Olanzapine for treatment-resistant anxiety
Olanzapine for treatment-resistant anxiety
Ms. A, age 62, was a retired high school teacher. Her primary care physician referred her to me for persistent, disabling anxiety. Her condition was recently worsened by a trial of escitalopram, 5 mg/d, which led her to visit the emergency department (ED). There she was prescribed lorazepam, 0.5 mg as needed, which helped her somewhat. Her medical conditions included prominent gastrointestinal (GI) symptoms, with nausea and a restricted diet; tinnitus; and chronic bilateral hand tremors. Her initial Patient Health Questionnaire-9 (PHQ-9) score was 11, and her Generalized Anxiety Disorder-7 (GAD-7) score was 10.
Initially, I encouraged Ms. A to exercise regularly, and I changed her lorazepam from 0.5 mg as-needed to 0.5 mg twice a day. I also referred her to a psychologist for psychotherapy. She showed limited improvement. I increased her lorazepam to 1 mg 3 times a day and started sertraline, 12.5 mg/d, but she soon experienced chest tightness and was admitted to the ED for observation and a cardiac workup. After she visited the ED, Ms. A stopped taking sertraline.
When I next saw Ms. A, she agreed to a trial of olanzapine, 2.5 mg/d at bedtime. Three weeks later, she told me, “I feel so much better.” Her scores on the PHQ-9 and GAD-7 were 0 and 1, respectively. Her GI complaints decreased, she had gained a little weight, and her tinnitus bothered her less. Lorazepam was gradually decreased and stopped.
After approximately 2 years, Ms. A had experienced no long-term adverse effects. We agreed to gradually discontinue olanzapine. Over the next 4 months, Ms. A decreased and stopped taking olanzapine at her own discretion.Three weeks after she stopped taking olanzapine, Ms. A reported that her psychiatric and GI symptoms had returned. She still maintained weekly visits with her psychotherapist. Her GI specialist asked if I could prescribe her olanzapine again. I restarted Ms. A on olanzapine, 2.5 mg/d at bedtime. By the next month, she said she felt much better (PHQ-9: 0; GAD-7: 1). I last saw Ms. A approximately 1 year ago.
Over the years, I have usually prescribed low-dose olanzapine alone or with other medications for patients with treatment-resistance who had no overt psychotic symptoms, I have used this medication for patients with “soft” psychotic thinking marked by severe anxiety, obsessions, compulsivity, perfectionism, and/or rumination.1 Evidence suggests olanzapine also may be effective for anorexia nervosa.2 There is good evidence for its use in the DSM-5 diagnosis of avoidant/restrictive food intake disorder (“a food avoidance emotional disorder”).3,4 In retrospect, Ms. A also likely met the criteria for the diagnosis of unspecified eating disorder. Despite extensive GI workup and follow-up, physical signs of GI pathology were equivocal.
Among antipsychotics, olanzapine most closely resembles clozapine, the only antipsychotic that has been proved more efficacious than others for psychotic symptoms.5 There is also some research suggesting that olanzapine may be more efficacious.6 Obsessions and perfectionism are associated with dopamine D4 receptor activity, and D1, D2, and D3 receptors are involved in normalizing cognition and reward.7 There are appropriate concerns about adverse effects, especially metabolic syndrome and obesity, with olanzapine, but patients can have different profiles of receptor sensitivity. In my conversations with Ms. A’s primary care physician and GI specialist, metabolic syndrome was not an issue. Clearly, low-dose olanzapine was very helpful in her treatment.
Daniel Storch, MD
Key Point Health Services
Catonsville, Maryland
References
1. Goodnick PJ, Barrios CA. Use of olanzapine in non-psychotic psychiatric disorders. Expert Opin Pharmacother. 2001;2(4):667-680.
2. Brewerton TD. Psychopharmacologic management of eating disorders. Presented at: 25th Annual National Psychopharmacology Update; February 2020; Las Vegas, Nevada. Accessed December 8, 2020. https://legacy.audio-digest.org/pages/htmlos/pastissues.html?sub1=psychiatry&sub2=2020
3. Diagnostic and statistical manual of mental disorders, 5th ed. American Psychiatric Association; 2013.
4. Brewerton TD, D’Agostino M. Adjunctive use of olanzapine in the treatment of avoidant restrictive food intake disorder in children and adolescents in an eating disorders program. J Child Adolesc Psychopharmacol. 2017;27(10):920-922.
5. Lobos CA, Komossa K, Rummel-Kluge C, et al. Clozapine versus other atypical antipsychotics for schizophrenia. Cochrane Database Syst Rev. 2010;(11):CD006633.
6. Komossa K, Rummel-Kluge C, Hunger H, et al. Olanzapine versus other atypical antipsychotics for schizophrenia. Cochrane Database Syst Rev. 2010;(3):CD006654.
7. Bachner-Melman R, Lerer E, Zohar AH, et al. Anorexia nervosa, perfectionism, and dopamine D4 receptor (DRD4). Am J Med Genet B Neuropsychiatr Genet. 2007;144B(6):748-756.
Continue to: Neuro-politics and academic paralysis...
Neuro-politics and academic paralysis
I commend Dr. Nasrallah for his brief, precisely defined, scientific editorial “Neuro-politics: Will you vote with your cortex or limbic system?” (From the Editor,
I would like to see
Similar to a hurricane or tsunami that pushes water into a river, this retrograde shift of feedback pathways is demonstrated by emotional narratives that have flooded the public and drowned facts and evidence-based practice. Furthermore, the science of convenience has emerged, where facts are eligible only if they justify the narrative. Any discussion, debate, or questioning of the rationale of the approach is met with hostility, naming, shaming, and even loss of employment at universities. I have sadly learned from frightened colleagues and from reading reports by academicians whose publications have been either rejected or coerced for revision following acceptance by a peer-reviewed journal or even retracted post-publication due to complaints, harassment, and threats by the politically correct “thought police.” Diversity of thinking and freedom of speech—core values and principles in academic dialogue—have been violated. Academicians are as perplexed as laboratory rats that need to learn which lever to push in order to receive a reward and avoid punishment in an ever-shifting environment. People have been pondering, “Is it time for flight, fright, or fight?” As Buffalo Springfield’s legendary Vietnam 1960s–era song “For What it’s Worth” states: “There’s battle lines being drawn and nobody’s right if everybody’s wrong.”
What we have learned from history is that the majority of people exercise passivity and hope as bystanders in order to avoid becoming victims of “collateral damage.” Are there no modern Giordano Bruno (the martyr of science), Copernicus, or Michelangelo who would challenge the “Church of the People” that has created new language, terminology, and culture and is on the verge of creating nouveau scientific principles that could lead to a monopoly of one segment of society that threatens pluralism of thought. Do we need dystopic books such as 1984 or Fahrenheit 451, or the experience of the French and Russian revolution (epitomized by the guillotine and the gulag) to remind us that we are a step away from education and reprogramming camps that used to be called universities? The American Association of University Professors’ most recent announcement on academic freedom ominously avoids using terms such as freedom of speech, diversity of opinions, or even pluralism.
I hope that psychiatrists will lead the way back to sanity, starting with focus groups and forums. It would amount to a group cognitive-behavioral therapy of immense proportion following a paradigm of “Problem Solving,” according to Albert Bandura’s social learning model. There is simply no other constructive way to get to the cheese at the end of the maze.
Yifrah Kaminer, MD
Professor Emeritus of Psychiatry & Pediatrics
University of Connecticut School of Medicine
Farmington, Connecticut
Disclosures: The authors report no financial relationships with any companies whose products are mentioned in this article, or with manufacturers of competing products.
COVID-19 and decision-making capacity
Dr. Ryznar’s article “Evaluating patients’ decision-making capacity during COVID-19” (Evidence-Based Reviews,
For example, in a controversial 2007 case in Atlanta, Georgia, an attorney with active tuberculosis failed to heed medical advice to refrain from traveling.1 The patient’s uncooperativeness did not implicate concerns over his decisional capacity.1 However, his international and interstate travel triggered the Centers for Disease Control and Prevention’s legal authority under the Public Health Service Act to prevent the entry and spread of communicable disease.1-3 An authorized order from a duly constituted public health authority is issued and enforceable without regard to clinical determinations of capacity (and is generally subject to challenge via judicial or other due process mechanisms as a government-sanctioned deprivation of liberty to protect public welfare). State laws and local ordinances require physicians to notify the appropriate public health department when patients test positive for certain contagious diseases.
The difficulty with involuntarily detaining a cognitively intact patient due to concern over their contagion risk and erroneous beliefs runs considerably deeper than eliciting a “political backlash” or managing the qualms of hospital security officers. It is a fundamental matter of proper legal authority. Psychiatrists and other physicians assess patients’ decision-making capacity for specific treatment decisions on a case-by-case basis, seeking to preserve autonomy while practicing beneficence. Public health officers are agents of the state with designated authorities to control the spread of disease. A capacity determination in the absence of neurocognitive deficits implies the psychiatrist is evaluating the soundness of the patient’s ideas as opposed to their cognition, overlooking the reality that fully capable individuals can possess dubious—and even unsalutary—beliefs. While physicians educate patients about the risks of contracting and communicating infection, they are thankfully not tasked with arbitrating sociopolitical disputes at the bedside. Such controversies regarding pandemic response do not belong under the rubric of medical decision-making capacity. Conflating psychosomatic medicine consultations with public health orders risks unmooring capacity determinations from their medicolegal and bioethical foundations.
Charles G. Kels, JD
S Army Medical Center of Excellence
San Antonio, Texas
Disclaimer: The views expressed here are those of the author and do not necessarily reflect those of any government agency.
References
1. Tanne JH. Tuberculosis case exposes flaws in international public health systems. BMJ. 2007;334(7605):1187.
2. Public Health Service Act, 42 USC § 264-272 (1944).
3. Interstate and Foreign Quarantine, 42 CFR Parts 70-71 (2017).
The author responds
I appreciate Mr. Kels’s letter and explicit discussion of the limits of decision-making capacity. I agree that physicians should not overstep their legal authority and ethical mandate. The specific case discussed in my article was a patient who was symptomatic from COVID-19 who wanted to leave the hospital against medical advice. The contagious nature of this virus certainly falls under the risk/benefit analysis of the clinical situation because it is an important aspect of understanding the nature of the illness and treatment/recovery process (as a thought example, consider that such a patient lives with their elderly mother who has heart disease and chronic obstructive pulmonary disease, and the patient does not want their mother to die). From a medicolegal perspective, the risk of infection to others may not necessarily outweigh the benefit of autonomy, especially because decision-making capacity assessments are made with the purpose of balancing autonomy and beneficence of the patient, not others. I highlighted the relative importance of autonomy using the weight of the arrows in Figure 2 of my article. I did not task physicians with arbitrating sociopolitical disputes, but merely highlighted how the current climate can impact people’s personal views on COVID-19, which sometimes can run counter to scientific evidence. If a patient has an erroneous view about an illness, it is our duty to try to help them understand if it directly impacts their health or affects their decision-making process, especially in a high-stakes clinical scenario.
Elizabeth Ryznar, MD, MSc
Assistant Professor
Department of Psychiatry and Behavioral Sciences
Johns Hopkins School of Medicine
Baltimore, Maryland
Continue to: Olanzapine for treatment-resistant anxiety
Olanzapine for treatment-resistant anxiety
Ms. A, age 62, was a retired high school teacher. Her primary care physician referred her to me for persistent, disabling anxiety. Her condition was recently worsened by a trial of escitalopram, 5 mg/d, which led her to visit the emergency department (ED). There she was prescribed lorazepam, 0.5 mg as needed, which helped her somewhat. Her medical conditions included prominent gastrointestinal (GI) symptoms, with nausea and a restricted diet; tinnitus; and chronic bilateral hand tremors. Her initial Patient Health Questionnaire-9 (PHQ-9) score was 11, and her Generalized Anxiety Disorder-7 (GAD-7) score was 10.
Initially, I encouraged Ms. A to exercise regularly, and I changed her lorazepam from 0.5 mg as-needed to 0.5 mg twice a day. I also referred her to a psychologist for psychotherapy. She showed limited improvement. I increased her lorazepam to 1 mg 3 times a day and started sertraline, 12.5 mg/d, but she soon experienced chest tightness and was admitted to the ED for observation and a cardiac workup. After she visited the ED, Ms. A stopped taking sertraline.
When I next saw Ms. A, she agreed to a trial of olanzapine, 2.5 mg/d at bedtime. Three weeks later, she told me, “I feel so much better.” Her scores on the PHQ-9 and GAD-7 were 0 and 1, respectively. Her GI complaints decreased, she had gained a little weight, and her tinnitus bothered her less. Lorazepam was gradually decreased and stopped.
After approximately 2 years, Ms. A had experienced no long-term adverse effects. We agreed to gradually discontinue olanzapine. Over the next 4 months, Ms. A decreased and stopped taking olanzapine at her own discretion.Three weeks after she stopped taking olanzapine, Ms. A reported that her psychiatric and GI symptoms had returned. She still maintained weekly visits with her psychotherapist. Her GI specialist asked if I could prescribe her olanzapine again. I restarted Ms. A on olanzapine, 2.5 mg/d at bedtime. By the next month, she said she felt much better (PHQ-9: 0; GAD-7: 1). I last saw Ms. A approximately 1 year ago.
Over the years, I have usually prescribed low-dose olanzapine alone or with other medications for patients with treatment-resistance who had no overt psychotic symptoms, I have used this medication for patients with “soft” psychotic thinking marked by severe anxiety, obsessions, compulsivity, perfectionism, and/or rumination.1 Evidence suggests olanzapine also may be effective for anorexia nervosa.2 There is good evidence for its use in the DSM-5 diagnosis of avoidant/restrictive food intake disorder (“a food avoidance emotional disorder”).3,4 In retrospect, Ms. A also likely met the criteria for the diagnosis of unspecified eating disorder. Despite extensive GI workup and follow-up, physical signs of GI pathology were equivocal.
Among antipsychotics, olanzapine most closely resembles clozapine, the only antipsychotic that has been proved more efficacious than others for psychotic symptoms.5 There is also some research suggesting that olanzapine may be more efficacious.6 Obsessions and perfectionism are associated with dopamine D4 receptor activity, and D1, D2, and D3 receptors are involved in normalizing cognition and reward.7 There are appropriate concerns about adverse effects, especially metabolic syndrome and obesity, with olanzapine, but patients can have different profiles of receptor sensitivity. In my conversations with Ms. A’s primary care physician and GI specialist, metabolic syndrome was not an issue. Clearly, low-dose olanzapine was very helpful in her treatment.
Daniel Storch, MD
Key Point Health Services
Catonsville, Maryland
References
1. Goodnick PJ, Barrios CA. Use of olanzapine in non-psychotic psychiatric disorders. Expert Opin Pharmacother. 2001;2(4):667-680.
2. Brewerton TD. Psychopharmacologic management of eating disorders. Presented at: 25th Annual National Psychopharmacology Update; February 2020; Las Vegas, Nevada. Accessed December 8, 2020. https://legacy.audio-digest.org/pages/htmlos/pastissues.html?sub1=psychiatry&sub2=2020
3. Diagnostic and statistical manual of mental disorders, 5th ed. American Psychiatric Association; 2013.
4. Brewerton TD, D’Agostino M. Adjunctive use of olanzapine in the treatment of avoidant restrictive food intake disorder in children and adolescents in an eating disorders program. J Child Adolesc Psychopharmacol. 2017;27(10):920-922.
5. Lobos CA, Komossa K, Rummel-Kluge C, et al. Clozapine versus other atypical antipsychotics for schizophrenia. Cochrane Database Syst Rev. 2010;(11):CD006633.
6. Komossa K, Rummel-Kluge C, Hunger H, et al. Olanzapine versus other atypical antipsychotics for schizophrenia. Cochrane Database Syst Rev. 2010;(3):CD006654.
7. Bachner-Melman R, Lerer E, Zohar AH, et al. Anorexia nervosa, perfectionism, and dopamine D4 receptor (DRD4). Am J Med Genet B Neuropsychiatr Genet. 2007;144B(6):748-756.
Continue to: Neuro-politics and academic paralysis...
Neuro-politics and academic paralysis
I commend Dr. Nasrallah for his brief, precisely defined, scientific editorial “Neuro-politics: Will you vote with your cortex or limbic system?” (From the Editor,
I would like to see
Similar to a hurricane or tsunami that pushes water into a river, this retrograde shift of feedback pathways is demonstrated by emotional narratives that have flooded the public and drowned facts and evidence-based practice. Furthermore, the science of convenience has emerged, where facts are eligible only if they justify the narrative. Any discussion, debate, or questioning of the rationale of the approach is met with hostility, naming, shaming, and even loss of employment at universities. I have sadly learned from frightened colleagues and from reading reports by academicians whose publications have been either rejected or coerced for revision following acceptance by a peer-reviewed journal or even retracted post-publication due to complaints, harassment, and threats by the politically correct “thought police.” Diversity of thinking and freedom of speech—core values and principles in academic dialogue—have been violated. Academicians are as perplexed as laboratory rats that need to learn which lever to push in order to receive a reward and avoid punishment in an ever-shifting environment. People have been pondering, “Is it time for flight, fright, or fight?” As Buffalo Springfield’s legendary Vietnam 1960s–era song “For What it’s Worth” states: “There’s battle lines being drawn and nobody’s right if everybody’s wrong.”
What we have learned from history is that the majority of people exercise passivity and hope as bystanders in order to avoid becoming victims of “collateral damage.” Are there no modern Giordano Bruno (the martyr of science), Copernicus, or Michelangelo who would challenge the “Church of the People” that has created new language, terminology, and culture and is on the verge of creating nouveau scientific principles that could lead to a monopoly of one segment of society that threatens pluralism of thought. Do we need dystopic books such as 1984 or Fahrenheit 451, or the experience of the French and Russian revolution (epitomized by the guillotine and the gulag) to remind us that we are a step away from education and reprogramming camps that used to be called universities? The American Association of University Professors’ most recent announcement on academic freedom ominously avoids using terms such as freedom of speech, diversity of opinions, or even pluralism.
I hope that psychiatrists will lead the way back to sanity, starting with focus groups and forums. It would amount to a group cognitive-behavioral therapy of immense proportion following a paradigm of “Problem Solving,” according to Albert Bandura’s social learning model. There is simply no other constructive way to get to the cheese at the end of the maze.
Yifrah Kaminer, MD
Professor Emeritus of Psychiatry & Pediatrics
University of Connecticut School of Medicine
Farmington, Connecticut
Disclosures: The authors report no financial relationships with any companies whose products are mentioned in this article, or with manufacturers of competing products.
Constipation: A potentially serious adverse effect of clozapine that’s often overlooked
Clozapine is the most effective second-generation antipsychotic for the treatment of refractory schizophrenia. It can reduce delusions and hallucinations in patients who are unresponsive to other antipsychotic medications. Further, clozapine is the only agent known to reduce suicidal urges.1
Unfortunately, clozapine is associated with numerous adverse effects, most notably agranulocytosis, a rare but potentially fatal adverse effect that occurs in approximately 1% to 2% of patients during the first year of treatment.2 Other adverse effects associated with clozapine are weight gain, sedation, orthostatic hypotension, sialorrhea, constipation, hyperglycemia, hyperlipidemia, myocarditis, and seizures. Among these adverse effects, constipation, which can progress to life-threatening gastrointestinal (GI) hypomotility and ileus, is often overlooked. Up to 60% of patients who are administered clozapine experience constipation.3 A recent review found that potentially life-threatening clozapine-induced ileus occurred in approximately 3 per 1,000 patients, and 28 deaths have been documented.4
In this case report, I describe a patient who received clozapine and experienced constipation that led to an intestinal obstruction. I discuss the importance of prompt diagnosis and treatment approaches to prevent severe constipation in patients who are prescribed clozapine.
CASE REPORT
Mr. L, age 24, has schizophrenia, depression, mild intellectual disability, and congenital human immunodeficiency virus (HIV). He has had multiple unsuccessful antipsychotic trials but is compliant with highly active antiretroviral therapy for HIV. After experiencing worsening aggressive behavior for a third time, Mr. L was involuntarily committed to our Crises Response Center.
Mr. L was admitted to the acute inpatient psychiatry unit. He reported having auditory hallucinations, which included whispering sounds with intermittent music, mostly at night. He also reported decreased sleep, poor appetite, and low energy, but denied feelings of depression or mania.
During the mental status examination, Mr. L was calm and cooperative, but easily distracted. He said he smoked cigarettes but denied any current alcohol or illicit drug use. Mr. L’s urine drug screen was negative.
External medication records showed Mr. L had been prescribed haloperidol, risperidone, chlorpromazine, olanzapine, aripiprazole, quetiapine, bupropion, sodium valproate, and topiramate, for the treatment of schizophrenia, with no significant improvement.
Continue to: On hospital Day 3, Mr. L...
On hospital Day 3, Mr. L was started on clozapine, 12.5 mg at bedtime, and titrated to 300 mg by Day 15. The clozapine was titrated slowly; initially the dose was doubled every 2 days up to 100 mg every night at bedtime, then it was increased by 50 mg every 2 to 3 days up to 300 mg every night at bedtime. A baseline complete blood count with differential confirmed that his absolute neutrophil count (ANC) was >1,500 µL, which is above the reference range. Mr. L was closely monitored for agranulocytosis and had weekly blood work for ANC. Additionally, his information was updated regularly on the Clozapine Risk Evaluation and Mitigation Strategy website.
After Mr. L began the clozapine regimen, he had reduced mood lability, paranoia, and delusions; significantly improved auditory and visual hallucinations; and reduced distress. His sleep was improved, and he appeared pleasant with clear sensorium. During this period, Mr. L developed sialorrhea and was administered glycopyrrolate and prescribed diphenhydramine, as needed for sleep. Although he had been prescribed oral benztropine for extrapyramidal side effects prophylaxis, this medication was never administered to him during his stay in the hospital. He became stable on this regimen, and the treatment team started working on his discharge.
On hospital Day 20, Mr. L complained about abdominal pain. At first, the pain was localized to right upper quadrant; later, he had diffuse abdominal pain with distension. He reported that he had no bowel movement for 1 day. The treatment team instructed him to take nothing by mouth, and all antipsychotic and anticholinergic medications were held. Given Mr. L’s HIV status, the treatment team ordered liver function tests (LFTs) and an abdominal x-ray. Mr. L’s LFT results were normal and the x-ray findings were inconclusive. However, a CT scan of the abdomen showed an obstruction due to a 3.5-cm stoolball in the proximal transverse colon with fecal impaction. Mr. L was started on a saline enema, which resulted in him having 2 to 3 episodes of watery diarrhea, and his abdominal pain resolved.
Although Mr. L reported feeling better and started eating again, there were concerns about his watery bowel movement, so a repeat abdominal x-ray was ordered. The x-ray confirmed that Mr. L had a persistent bowel obstruction. Mr. L’s abdominal pain returned. At this time, the pain was diffuse and severe, and Mr. L was vomiting. Mr. L was started on a bisacodyl suppository immediately, and then twice daily as needed. Subsequently, Mr. L had a solid bowel movement and relief of all GI symptoms. Mr. L was administered docusate sodium twice daily. Repeated x-rays of the abdomen confirmed the obstructive changes of the small bowel had resolved.
Why constipation may be overlooked
Although constipation is a common adverse effect of many psychotropic medications, when it emerges during clozapine therapy, it can lead to ileus, which can be fatal. Mr. L’s case highlighted that clozapine use can cause intestinal obstruction, a condition that can deteriorate within a few hours to life-threatening ileus. The extent of fecal impaction can be masked by spurious diarrhea, as illustrated in Mr. L’s case.5 Clozapine has anti-serotonergic properties (5HT-2A antagonist) that may result in reduced intestinal nociception pain. This discrepancy between physical symptoms and the severity of illness may cause delays in diagnosis.4 As soon as the treatment team determined Mr. L was constipated, all medications with anticholinergic effects were held. Patients also may have difficulty reporting intestinal pain due to psychotic symptoms such as paranoia or thought disorder.6
Take steps to prevent constipation
To prevent constipation in patients receiving clozapine, minimize the use of systemic anticholinergic agents because of the adverse effects of this interaction. For example, in Mr. L’s case, he received both clozapine and glycopyrrolate. In addition, all patients who are prescribed clozapine should receive docusate sodium to prevent constipation. However, because docusate sodium alone is usually not sufficient, consider adding another agent. Osmotic laxatives, such as polyethylene glycol 3350, are suitable additional agents. If this combination does not work, then consider senna glycoside or bisacodyl, which will increase intestinal motility and help with the flow of water into the bowel, thereby improving constipation. Bulk agents should be avoided because they can make constipation worse, especially if the patient is not drinking enough water, which is often the case with patients who have psychosis.7
Ask patients about GI symptoms
Clinicians need to observe and monitor patients who receive clozapine for signs of constipation, including the frequency and difficulty of defecation during treatment.4 It is important to ask patients about bowel function. Before starting treatment with clozapine, discuss the risks of clozapine-induced intestinal obstruction with patients and caregivers, and encourage them to report any GI symptoms. Also, provide dietary advice and recommend the as-needed use of laxatives.
1. Patchan KM, Richardson C, Vyas G, et al. The risk of suicide after clozapine discontinuation: cause for concern. Ann Clinical Psychiatry. 2015;27(4):253-256.
2. Alvir JM, Lieberman JA, Safferman AZ, et al. Clozapine-induced agranulocytosis. Incidence and risk factors in the United States. N Engl J Med. 1993;329(3):162-167.
3. Hayes G, Gibler B. Clozapine-induced constipation. Am J Psychiatry. 1995;152(2):298.
4. Palmer SE, McLean RM, Ellis PM, et al. Life-threatening clozapine-induced gastrointestinal hypomotility: an analysis of 102 cases. J Clin Psychiatry. 2008;69(5):759-768.
5. Drew L, Herdson P. Clozapine and constipation: a serious issue. Aust N Z J Psychiatry. 1997; 31(1):149-150.
6. Bickerstaff LK, Harris SC, Leggett RS, et al. Pain insensitivity in schizophrenic patients: a surgical dilemma. Arch Surg. 1988;123(1):49-51.
7. Psychopharmacology Institute. How to manage adverse effects of clozapine – Part 1. Updated June 3, 2020. Accessed December 7, 2020. https://psychopharmacologyinstitute.com/publication/how-to-manage-adverse-effects-of-clozapine-part-1-2476
Clozapine is the most effective second-generation antipsychotic for the treatment of refractory schizophrenia. It can reduce delusions and hallucinations in patients who are unresponsive to other antipsychotic medications. Further, clozapine is the only agent known to reduce suicidal urges.1
Unfortunately, clozapine is associated with numerous adverse effects, most notably agranulocytosis, a rare but potentially fatal adverse effect that occurs in approximately 1% to 2% of patients during the first year of treatment.2 Other adverse effects associated with clozapine are weight gain, sedation, orthostatic hypotension, sialorrhea, constipation, hyperglycemia, hyperlipidemia, myocarditis, and seizures. Among these adverse effects, constipation, which can progress to life-threatening gastrointestinal (GI) hypomotility and ileus, is often overlooked. Up to 60% of patients who are administered clozapine experience constipation.3 A recent review found that potentially life-threatening clozapine-induced ileus occurred in approximately 3 per 1,000 patients, and 28 deaths have been documented.4
In this case report, I describe a patient who received clozapine and experienced constipation that led to an intestinal obstruction. I discuss the importance of prompt diagnosis and treatment approaches to prevent severe constipation in patients who are prescribed clozapine.
CASE REPORT
Mr. L, age 24, has schizophrenia, depression, mild intellectual disability, and congenital human immunodeficiency virus (HIV). He has had multiple unsuccessful antipsychotic trials but is compliant with highly active antiretroviral therapy for HIV. After experiencing worsening aggressive behavior for a third time, Mr. L was involuntarily committed to our Crises Response Center.
Mr. L was admitted to the acute inpatient psychiatry unit. He reported having auditory hallucinations, which included whispering sounds with intermittent music, mostly at night. He also reported decreased sleep, poor appetite, and low energy, but denied feelings of depression or mania.
During the mental status examination, Mr. L was calm and cooperative, but easily distracted. He said he smoked cigarettes but denied any current alcohol or illicit drug use. Mr. L’s urine drug screen was negative.
External medication records showed Mr. L had been prescribed haloperidol, risperidone, chlorpromazine, olanzapine, aripiprazole, quetiapine, bupropion, sodium valproate, and topiramate, for the treatment of schizophrenia, with no significant improvement.
Continue to: On hospital Day 3, Mr. L...
On hospital Day 3, Mr. L was started on clozapine, 12.5 mg at bedtime, and titrated to 300 mg by Day 15. The clozapine was titrated slowly; initially the dose was doubled every 2 days up to 100 mg every night at bedtime, then it was increased by 50 mg every 2 to 3 days up to 300 mg every night at bedtime. A baseline complete blood count with differential confirmed that his absolute neutrophil count (ANC) was >1,500 µL, which is above the reference range. Mr. L was closely monitored for agranulocytosis and had weekly blood work for ANC. Additionally, his information was updated regularly on the Clozapine Risk Evaluation and Mitigation Strategy website.
After Mr. L began the clozapine regimen, he had reduced mood lability, paranoia, and delusions; significantly improved auditory and visual hallucinations; and reduced distress. His sleep was improved, and he appeared pleasant with clear sensorium. During this period, Mr. L developed sialorrhea and was administered glycopyrrolate and prescribed diphenhydramine, as needed for sleep. Although he had been prescribed oral benztropine for extrapyramidal side effects prophylaxis, this medication was never administered to him during his stay in the hospital. He became stable on this regimen, and the treatment team started working on his discharge.
On hospital Day 20, Mr. L complained about abdominal pain. At first, the pain was localized to right upper quadrant; later, he had diffuse abdominal pain with distension. He reported that he had no bowel movement for 1 day. The treatment team instructed him to take nothing by mouth, and all antipsychotic and anticholinergic medications were held. Given Mr. L’s HIV status, the treatment team ordered liver function tests (LFTs) and an abdominal x-ray. Mr. L’s LFT results were normal and the x-ray findings were inconclusive. However, a CT scan of the abdomen showed an obstruction due to a 3.5-cm stoolball in the proximal transverse colon with fecal impaction. Mr. L was started on a saline enema, which resulted in him having 2 to 3 episodes of watery diarrhea, and his abdominal pain resolved.
Although Mr. L reported feeling better and started eating again, there were concerns about his watery bowel movement, so a repeat abdominal x-ray was ordered. The x-ray confirmed that Mr. L had a persistent bowel obstruction. Mr. L’s abdominal pain returned. At this time, the pain was diffuse and severe, and Mr. L was vomiting. Mr. L was started on a bisacodyl suppository immediately, and then twice daily as needed. Subsequently, Mr. L had a solid bowel movement and relief of all GI symptoms. Mr. L was administered docusate sodium twice daily. Repeated x-rays of the abdomen confirmed the obstructive changes of the small bowel had resolved.
Why constipation may be overlooked
Although constipation is a common adverse effect of many psychotropic medications, when it emerges during clozapine therapy, it can lead to ileus, which can be fatal. Mr. L’s case highlighted that clozapine use can cause intestinal obstruction, a condition that can deteriorate within a few hours to life-threatening ileus. The extent of fecal impaction can be masked by spurious diarrhea, as illustrated in Mr. L’s case.5 Clozapine has anti-serotonergic properties (5HT-2A antagonist) that may result in reduced intestinal nociception pain. This discrepancy between physical symptoms and the severity of illness may cause delays in diagnosis.4 As soon as the treatment team determined Mr. L was constipated, all medications with anticholinergic effects were held. Patients also may have difficulty reporting intestinal pain due to psychotic symptoms such as paranoia or thought disorder.6
Take steps to prevent constipation
To prevent constipation in patients receiving clozapine, minimize the use of systemic anticholinergic agents because of the adverse effects of this interaction. For example, in Mr. L’s case, he received both clozapine and glycopyrrolate. In addition, all patients who are prescribed clozapine should receive docusate sodium to prevent constipation. However, because docusate sodium alone is usually not sufficient, consider adding another agent. Osmotic laxatives, such as polyethylene glycol 3350, are suitable additional agents. If this combination does not work, then consider senna glycoside or bisacodyl, which will increase intestinal motility and help with the flow of water into the bowel, thereby improving constipation. Bulk agents should be avoided because they can make constipation worse, especially if the patient is not drinking enough water, which is often the case with patients who have psychosis.7
Ask patients about GI symptoms
Clinicians need to observe and monitor patients who receive clozapine for signs of constipation, including the frequency and difficulty of defecation during treatment.4 It is important to ask patients about bowel function. Before starting treatment with clozapine, discuss the risks of clozapine-induced intestinal obstruction with patients and caregivers, and encourage them to report any GI symptoms. Also, provide dietary advice and recommend the as-needed use of laxatives.
Clozapine is the most effective second-generation antipsychotic for the treatment of refractory schizophrenia. It can reduce delusions and hallucinations in patients who are unresponsive to other antipsychotic medications. Further, clozapine is the only agent known to reduce suicidal urges.1
Unfortunately, clozapine is associated with numerous adverse effects, most notably agranulocytosis, a rare but potentially fatal adverse effect that occurs in approximately 1% to 2% of patients during the first year of treatment.2 Other adverse effects associated with clozapine are weight gain, sedation, orthostatic hypotension, sialorrhea, constipation, hyperglycemia, hyperlipidemia, myocarditis, and seizures. Among these adverse effects, constipation, which can progress to life-threatening gastrointestinal (GI) hypomotility and ileus, is often overlooked. Up to 60% of patients who are administered clozapine experience constipation.3 A recent review found that potentially life-threatening clozapine-induced ileus occurred in approximately 3 per 1,000 patients, and 28 deaths have been documented.4
In this case report, I describe a patient who received clozapine and experienced constipation that led to an intestinal obstruction. I discuss the importance of prompt diagnosis and treatment approaches to prevent severe constipation in patients who are prescribed clozapine.
CASE REPORT
Mr. L, age 24, has schizophrenia, depression, mild intellectual disability, and congenital human immunodeficiency virus (HIV). He has had multiple unsuccessful antipsychotic trials but is compliant with highly active antiretroviral therapy for HIV. After experiencing worsening aggressive behavior for a third time, Mr. L was involuntarily committed to our Crises Response Center.
Mr. L was admitted to the acute inpatient psychiatry unit. He reported having auditory hallucinations, which included whispering sounds with intermittent music, mostly at night. He also reported decreased sleep, poor appetite, and low energy, but denied feelings of depression or mania.
During the mental status examination, Mr. L was calm and cooperative, but easily distracted. He said he smoked cigarettes but denied any current alcohol or illicit drug use. Mr. L’s urine drug screen was negative.
External medication records showed Mr. L had been prescribed haloperidol, risperidone, chlorpromazine, olanzapine, aripiprazole, quetiapine, bupropion, sodium valproate, and topiramate, for the treatment of schizophrenia, with no significant improvement.
Continue to: On hospital Day 3, Mr. L...
On hospital Day 3, Mr. L was started on clozapine, 12.5 mg at bedtime, and titrated to 300 mg by Day 15. The clozapine was titrated slowly; initially the dose was doubled every 2 days up to 100 mg every night at bedtime, then it was increased by 50 mg every 2 to 3 days up to 300 mg every night at bedtime. A baseline complete blood count with differential confirmed that his absolute neutrophil count (ANC) was >1,500 µL, which is above the reference range. Mr. L was closely monitored for agranulocytosis and had weekly blood work for ANC. Additionally, his information was updated regularly on the Clozapine Risk Evaluation and Mitigation Strategy website.
After Mr. L began the clozapine regimen, he had reduced mood lability, paranoia, and delusions; significantly improved auditory and visual hallucinations; and reduced distress. His sleep was improved, and he appeared pleasant with clear sensorium. During this period, Mr. L developed sialorrhea and was administered glycopyrrolate and prescribed diphenhydramine, as needed for sleep. Although he had been prescribed oral benztropine for extrapyramidal side effects prophylaxis, this medication was never administered to him during his stay in the hospital. He became stable on this regimen, and the treatment team started working on his discharge.
On hospital Day 20, Mr. L complained about abdominal pain. At first, the pain was localized to right upper quadrant; later, he had diffuse abdominal pain with distension. He reported that he had no bowel movement for 1 day. The treatment team instructed him to take nothing by mouth, and all antipsychotic and anticholinergic medications were held. Given Mr. L’s HIV status, the treatment team ordered liver function tests (LFTs) and an abdominal x-ray. Mr. L’s LFT results were normal and the x-ray findings were inconclusive. However, a CT scan of the abdomen showed an obstruction due to a 3.5-cm stoolball in the proximal transverse colon with fecal impaction. Mr. L was started on a saline enema, which resulted in him having 2 to 3 episodes of watery diarrhea, and his abdominal pain resolved.
Although Mr. L reported feeling better and started eating again, there were concerns about his watery bowel movement, so a repeat abdominal x-ray was ordered. The x-ray confirmed that Mr. L had a persistent bowel obstruction. Mr. L’s abdominal pain returned. At this time, the pain was diffuse and severe, and Mr. L was vomiting. Mr. L was started on a bisacodyl suppository immediately, and then twice daily as needed. Subsequently, Mr. L had a solid bowel movement and relief of all GI symptoms. Mr. L was administered docusate sodium twice daily. Repeated x-rays of the abdomen confirmed the obstructive changes of the small bowel had resolved.
Why constipation may be overlooked
Although constipation is a common adverse effect of many psychotropic medications, when it emerges during clozapine therapy, it can lead to ileus, which can be fatal. Mr. L’s case highlighted that clozapine use can cause intestinal obstruction, a condition that can deteriorate within a few hours to life-threatening ileus. The extent of fecal impaction can be masked by spurious diarrhea, as illustrated in Mr. L’s case.5 Clozapine has anti-serotonergic properties (5HT-2A antagonist) that may result in reduced intestinal nociception pain. This discrepancy between physical symptoms and the severity of illness may cause delays in diagnosis.4 As soon as the treatment team determined Mr. L was constipated, all medications with anticholinergic effects were held. Patients also may have difficulty reporting intestinal pain due to psychotic symptoms such as paranoia or thought disorder.6
Take steps to prevent constipation
To prevent constipation in patients receiving clozapine, minimize the use of systemic anticholinergic agents because of the adverse effects of this interaction. For example, in Mr. L’s case, he received both clozapine and glycopyrrolate. In addition, all patients who are prescribed clozapine should receive docusate sodium to prevent constipation. However, because docusate sodium alone is usually not sufficient, consider adding another agent. Osmotic laxatives, such as polyethylene glycol 3350, are suitable additional agents. If this combination does not work, then consider senna glycoside or bisacodyl, which will increase intestinal motility and help with the flow of water into the bowel, thereby improving constipation. Bulk agents should be avoided because they can make constipation worse, especially if the patient is not drinking enough water, which is often the case with patients who have psychosis.7
Ask patients about GI symptoms
Clinicians need to observe and monitor patients who receive clozapine for signs of constipation, including the frequency and difficulty of defecation during treatment.4 It is important to ask patients about bowel function. Before starting treatment with clozapine, discuss the risks of clozapine-induced intestinal obstruction with patients and caregivers, and encourage them to report any GI symptoms. Also, provide dietary advice and recommend the as-needed use of laxatives.
1. Patchan KM, Richardson C, Vyas G, et al. The risk of suicide after clozapine discontinuation: cause for concern. Ann Clinical Psychiatry. 2015;27(4):253-256.
2. Alvir JM, Lieberman JA, Safferman AZ, et al. Clozapine-induced agranulocytosis. Incidence and risk factors in the United States. N Engl J Med. 1993;329(3):162-167.
3. Hayes G, Gibler B. Clozapine-induced constipation. Am J Psychiatry. 1995;152(2):298.
4. Palmer SE, McLean RM, Ellis PM, et al. Life-threatening clozapine-induced gastrointestinal hypomotility: an analysis of 102 cases. J Clin Psychiatry. 2008;69(5):759-768.
5. Drew L, Herdson P. Clozapine and constipation: a serious issue. Aust N Z J Psychiatry. 1997; 31(1):149-150.
6. Bickerstaff LK, Harris SC, Leggett RS, et al. Pain insensitivity in schizophrenic patients: a surgical dilemma. Arch Surg. 1988;123(1):49-51.
7. Psychopharmacology Institute. How to manage adverse effects of clozapine – Part 1. Updated June 3, 2020. Accessed December 7, 2020. https://psychopharmacologyinstitute.com/publication/how-to-manage-adverse-effects-of-clozapine-part-1-2476
1. Patchan KM, Richardson C, Vyas G, et al. The risk of suicide after clozapine discontinuation: cause for concern. Ann Clinical Psychiatry. 2015;27(4):253-256.
2. Alvir JM, Lieberman JA, Safferman AZ, et al. Clozapine-induced agranulocytosis. Incidence and risk factors in the United States. N Engl J Med. 1993;329(3):162-167.
3. Hayes G, Gibler B. Clozapine-induced constipation. Am J Psychiatry. 1995;152(2):298.
4. Palmer SE, McLean RM, Ellis PM, et al. Life-threatening clozapine-induced gastrointestinal hypomotility: an analysis of 102 cases. J Clin Psychiatry. 2008;69(5):759-768.
5. Drew L, Herdson P. Clozapine and constipation: a serious issue. Aust N Z J Psychiatry. 1997; 31(1):149-150.
6. Bickerstaff LK, Harris SC, Leggett RS, et al. Pain insensitivity in schizophrenic patients: a surgical dilemma. Arch Surg. 1988;123(1):49-51.
7. Psychopharmacology Institute. How to manage adverse effects of clozapine – Part 1. Updated June 3, 2020. Accessed December 7, 2020. https://psychopharmacologyinstitute.com/publication/how-to-manage-adverse-effects-of-clozapine-part-1-2476
COVID-19 anticoagulation trials ‘paused’ for futility, safety
Parts of three linked studies investigating increased levels of anticoagulation in hospitalized COVID-19 patients have been “paused” because of futility and safety concerns, a statement from the U.S. National Heart, Lung, and Blood Institute (NHLBI) confirms.
The trials involved are the REMAP-CAP, ACTIV-4, and ATTACC studies.
The statement also says that a potential for harm in this subgroup could not be excluded, noting that increased bleeding is a known complication of full-dose anticoagulation. The trials are working urgently to undertake additional analyses, which will be made available as soon as possible.
The three clinical trial platforms are working together to test the effects of full therapeutic doses of anticoagulants vs. lower prophylactic doses in COVID-19 patients.
Informed by the deliberations of the data safety monitoring boards of these trials, all of the trial sites have paused enrollment of the most critically ill hospitalized patients with COVID-19.
Enrollment continues in the trials for moderately ill hospitalized COVID-19 patients, the statement notes.
“Whether the use of full-dose compared to low-dose anticoagulants leads to better outcomes in hospitalized patients with less COVID-19 severe disease remains a very important question,” the NHLBI statement says.
Patients who require full dose anticoagulants for another medical indication are not included in these trials.
The statement explains that COVID-19 is associated with significant inflammation and clinical and pathologic evidence of widespread blood clots. These trials were launched because clinicians have observed that many patients ill with COVID-19, including those who have died from the disease, formed blood clots throughout their bodies, even in their smallest blood vessels. This unusual clotting can cause multiple health complications, including lung failure, myocardial infarction, and stroke.
The three trials are the result of a collaboration between major international partners. The trials include: the Randomized, Embedded, Multi-factorial Adaptive Platform Trial for Community-Acquired Pneumonia (REMAP-CAP) Therapeutic Anticoagulation; Accelerating COVID-19 Therapeutic Interventions and Vaccines-4 (ACTIV-4) Antithrombotics Inpatient; and Antithrombotic Therapy to Ameliorate Complications of COVID-19 (ATTACC).
The trials, which span four continents, have the common goal of assessing the benefit of full doses of anticoagulants to treat moderately ill or critically ill adults hospitalized for COVID-19, compared with a lower dose often used to prevent blood clots in hospitalized patients.
In the United States, the ACTIV-4 trial is being led by a collaborative effort involving a number of universities, including the University of Pittsburgh and New York University.
The trials are supported by multiple international funding organizations including the National Institutes of Health, Canadian Institutes of Health Research, the National Institute for Health Research (UK), the National Health and Medical Research Council (Australia), and the PREPARE and RECOVER consortia (European Union).
A version of this story first appeared on Medscape.com.
Parts of three linked studies investigating increased levels of anticoagulation in hospitalized COVID-19 patients have been “paused” because of futility and safety concerns, a statement from the U.S. National Heart, Lung, and Blood Institute (NHLBI) confirms.
The trials involved are the REMAP-CAP, ACTIV-4, and ATTACC studies.
The statement also says that a potential for harm in this subgroup could not be excluded, noting that increased bleeding is a known complication of full-dose anticoagulation. The trials are working urgently to undertake additional analyses, which will be made available as soon as possible.
The three clinical trial platforms are working together to test the effects of full therapeutic doses of anticoagulants vs. lower prophylactic doses in COVID-19 patients.
Informed by the deliberations of the data safety monitoring boards of these trials, all of the trial sites have paused enrollment of the most critically ill hospitalized patients with COVID-19.
Enrollment continues in the trials for moderately ill hospitalized COVID-19 patients, the statement notes.
“Whether the use of full-dose compared to low-dose anticoagulants leads to better outcomes in hospitalized patients with less COVID-19 severe disease remains a very important question,” the NHLBI statement says.
Patients who require full dose anticoagulants for another medical indication are not included in these trials.
The statement explains that COVID-19 is associated with significant inflammation and clinical and pathologic evidence of widespread blood clots. These trials were launched because clinicians have observed that many patients ill with COVID-19, including those who have died from the disease, formed blood clots throughout their bodies, even in their smallest blood vessels. This unusual clotting can cause multiple health complications, including lung failure, myocardial infarction, and stroke.
The three trials are the result of a collaboration between major international partners. The trials include: the Randomized, Embedded, Multi-factorial Adaptive Platform Trial for Community-Acquired Pneumonia (REMAP-CAP) Therapeutic Anticoagulation; Accelerating COVID-19 Therapeutic Interventions and Vaccines-4 (ACTIV-4) Antithrombotics Inpatient; and Antithrombotic Therapy to Ameliorate Complications of COVID-19 (ATTACC).
The trials, which span four continents, have the common goal of assessing the benefit of full doses of anticoagulants to treat moderately ill or critically ill adults hospitalized for COVID-19, compared with a lower dose often used to prevent blood clots in hospitalized patients.
In the United States, the ACTIV-4 trial is being led by a collaborative effort involving a number of universities, including the University of Pittsburgh and New York University.
The trials are supported by multiple international funding organizations including the National Institutes of Health, Canadian Institutes of Health Research, the National Institute for Health Research (UK), the National Health and Medical Research Council (Australia), and the PREPARE and RECOVER consortia (European Union).
A version of this story first appeared on Medscape.com.
Parts of three linked studies investigating increased levels of anticoagulation in hospitalized COVID-19 patients have been “paused” because of futility and safety concerns, a statement from the U.S. National Heart, Lung, and Blood Institute (NHLBI) confirms.
The trials involved are the REMAP-CAP, ACTIV-4, and ATTACC studies.
The statement also says that a potential for harm in this subgroup could not be excluded, noting that increased bleeding is a known complication of full-dose anticoagulation. The trials are working urgently to undertake additional analyses, which will be made available as soon as possible.
The three clinical trial platforms are working together to test the effects of full therapeutic doses of anticoagulants vs. lower prophylactic doses in COVID-19 patients.
Informed by the deliberations of the data safety monitoring boards of these trials, all of the trial sites have paused enrollment of the most critically ill hospitalized patients with COVID-19.
Enrollment continues in the trials for moderately ill hospitalized COVID-19 patients, the statement notes.
“Whether the use of full-dose compared to low-dose anticoagulants leads to better outcomes in hospitalized patients with less COVID-19 severe disease remains a very important question,” the NHLBI statement says.
Patients who require full dose anticoagulants for another medical indication are not included in these trials.
The statement explains that COVID-19 is associated with significant inflammation and clinical and pathologic evidence of widespread blood clots. These trials were launched because clinicians have observed that many patients ill with COVID-19, including those who have died from the disease, formed blood clots throughout their bodies, even in their smallest blood vessels. This unusual clotting can cause multiple health complications, including lung failure, myocardial infarction, and stroke.
The three trials are the result of a collaboration between major international partners. The trials include: the Randomized, Embedded, Multi-factorial Adaptive Platform Trial for Community-Acquired Pneumonia (REMAP-CAP) Therapeutic Anticoagulation; Accelerating COVID-19 Therapeutic Interventions and Vaccines-4 (ACTIV-4) Antithrombotics Inpatient; and Antithrombotic Therapy to Ameliorate Complications of COVID-19 (ATTACC).
The trials, which span four continents, have the common goal of assessing the benefit of full doses of anticoagulants to treat moderately ill or critically ill adults hospitalized for COVID-19, compared with a lower dose often used to prevent blood clots in hospitalized patients.
In the United States, the ACTIV-4 trial is being led by a collaborative effort involving a number of universities, including the University of Pittsburgh and New York University.
The trials are supported by multiple international funding organizations including the National Institutes of Health, Canadian Institutes of Health Research, the National Institute for Health Research (UK), the National Health and Medical Research Council (Australia), and the PREPARE and RECOVER consortia (European Union).
A version of this story first appeared on Medscape.com.






