User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
nav[contains(@class, 'nav-ce-stack nav-ce-stack__large-screen')]
header[@id='header']
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'main-prefix')]
footer[@id='footer']
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
div[contains(@class, 'view-medstat-quiz-listing-panes')]
Survey: 2020 will see more attacks on ACA
When physicians gaze into their crystal balls to predict what’s coming in 2020, they see continued efforts to defund the Affordable Care Act – meaning the ACA will still be around to be defunded – but they don’t see a lot of support for universal health care, according to health care market research company InCrowd.

Expectations for universal health care came in at 18% of the 100 generalists and 101 specialists who responded to InCrowd’s fifth annual health care predictions survey, which left 82% who thought that “election outcomes will result in universal healthcare support” was somewhat or very unlikely in 2020.
One respondent, a specialist from California, commented that “the global data on universal healthcare for all shows that it results in overall improved population health. Unfortunately, we are so polarized in the US against universal healthcare driven by bias from health insurance companies and decision makers that are quick to ignore scientific data.”
This was the first time InCrowd asked physicians about universal health care, but ACA-related predictions have been included before, and all three scenarios presented were deemed to be increasingly likely, compared with 2019.
Respondents thought that federal government defunding was more likely to occur in 2020 (80%) than in 2019 (73%), but increased majorities also said that preexisting conditions coverage would continue (78% in 2020 vs. 70% in 2019) and that the ACA would remain in place (74% in 2020 vs. 60% in 2019), InCrowd reported after the survey, which was conducted from Dec. 30, 2019, to Jan. 2, 2020.
A respondent who thought the ACA will be eliminated said, “I have as many uninsured today as before the ACA. They are just different. Mainly younger patients who spend less in a year on healthcare than one month’s premium.” Another suggested that eliminateing it “will limit access to care and overload [emergency departments]. More people will die.”
Cost was addressed in a separate survey question that asked how physicians could help to reduce health care spending in 2020.
The leading answer, given by 37% of respondents, was for physicians to “inform themselves of costs and adapt cost-saving prescription practices.” Next came “limit use of expensive tests and scans” with 21%, followed by “prescribe generics when possible” at 20%, which was a substantial drop from the 38% it garnered in 2019, InCrowd noted.
“Participation in [shared savings] programs and risk-based incentive programs and pay-for-performance programs” would provide “better stewardship of resources,” a primary care physician from Michigan wrote.
When the survey turned to pharmaceutical industry predictions for 2020, cost was the major issue.
“What’s interesting about this year’s data is that we’re seeing less emphasis on the importance of bringing innovative, new therapies to market faster … versus expanding affordability, which was nearly a unanimous top priority for respondents,” Daniel S. Fitzgerald, InCrowd’s CEO and president, said in a separate statement.
When physicians gaze into their crystal balls to predict what’s coming in 2020, they see continued efforts to defund the Affordable Care Act – meaning the ACA will still be around to be defunded – but they don’t see a lot of support for universal health care, according to health care market research company InCrowd.

Expectations for universal health care came in at 18% of the 100 generalists and 101 specialists who responded to InCrowd’s fifth annual health care predictions survey, which left 82% who thought that “election outcomes will result in universal healthcare support” was somewhat or very unlikely in 2020.
One respondent, a specialist from California, commented that “the global data on universal healthcare for all shows that it results in overall improved population health. Unfortunately, we are so polarized in the US against universal healthcare driven by bias from health insurance companies and decision makers that are quick to ignore scientific data.”
This was the first time InCrowd asked physicians about universal health care, but ACA-related predictions have been included before, and all three scenarios presented were deemed to be increasingly likely, compared with 2019.
Respondents thought that federal government defunding was more likely to occur in 2020 (80%) than in 2019 (73%), but increased majorities also said that preexisting conditions coverage would continue (78% in 2020 vs. 70% in 2019) and that the ACA would remain in place (74% in 2020 vs. 60% in 2019), InCrowd reported after the survey, which was conducted from Dec. 30, 2019, to Jan. 2, 2020.
A respondent who thought the ACA will be eliminated said, “I have as many uninsured today as before the ACA. They are just different. Mainly younger patients who spend less in a year on healthcare than one month’s premium.” Another suggested that eliminateing it “will limit access to care and overload [emergency departments]. More people will die.”
Cost was addressed in a separate survey question that asked how physicians could help to reduce health care spending in 2020.
The leading answer, given by 37% of respondents, was for physicians to “inform themselves of costs and adapt cost-saving prescription practices.” Next came “limit use of expensive tests and scans” with 21%, followed by “prescribe generics when possible” at 20%, which was a substantial drop from the 38% it garnered in 2019, InCrowd noted.
“Participation in [shared savings] programs and risk-based incentive programs and pay-for-performance programs” would provide “better stewardship of resources,” a primary care physician from Michigan wrote.
When the survey turned to pharmaceutical industry predictions for 2020, cost was the major issue.
“What’s interesting about this year’s data is that we’re seeing less emphasis on the importance of bringing innovative, new therapies to market faster … versus expanding affordability, which was nearly a unanimous top priority for respondents,” Daniel S. Fitzgerald, InCrowd’s CEO and president, said in a separate statement.
When physicians gaze into their crystal balls to predict what’s coming in 2020, they see continued efforts to defund the Affordable Care Act – meaning the ACA will still be around to be defunded – but they don’t see a lot of support for universal health care, according to health care market research company InCrowd.

Expectations for universal health care came in at 18% of the 100 generalists and 101 specialists who responded to InCrowd’s fifth annual health care predictions survey, which left 82% who thought that “election outcomes will result in universal healthcare support” was somewhat or very unlikely in 2020.
One respondent, a specialist from California, commented that “the global data on universal healthcare for all shows that it results in overall improved population health. Unfortunately, we are so polarized in the US against universal healthcare driven by bias from health insurance companies and decision makers that are quick to ignore scientific data.”
This was the first time InCrowd asked physicians about universal health care, but ACA-related predictions have been included before, and all three scenarios presented were deemed to be increasingly likely, compared with 2019.
Respondents thought that federal government defunding was more likely to occur in 2020 (80%) than in 2019 (73%), but increased majorities also said that preexisting conditions coverage would continue (78% in 2020 vs. 70% in 2019) and that the ACA would remain in place (74% in 2020 vs. 60% in 2019), InCrowd reported after the survey, which was conducted from Dec. 30, 2019, to Jan. 2, 2020.
A respondent who thought the ACA will be eliminated said, “I have as many uninsured today as before the ACA. They are just different. Mainly younger patients who spend less in a year on healthcare than one month’s premium.” Another suggested that eliminateing it “will limit access to care and overload [emergency departments]. More people will die.”
Cost was addressed in a separate survey question that asked how physicians could help to reduce health care spending in 2020.
The leading answer, given by 37% of respondents, was for physicians to “inform themselves of costs and adapt cost-saving prescription practices.” Next came “limit use of expensive tests and scans” with 21%, followed by “prescribe generics when possible” at 20%, which was a substantial drop from the 38% it garnered in 2019, InCrowd noted.
“Participation in [shared savings] programs and risk-based incentive programs and pay-for-performance programs” would provide “better stewardship of resources,” a primary care physician from Michigan wrote.
When the survey turned to pharmaceutical industry predictions for 2020, cost was the major issue.
“What’s interesting about this year’s data is that we’re seeing less emphasis on the importance of bringing innovative, new therapies to market faster … versus expanding affordability, which was nearly a unanimous top priority for respondents,” Daniel S. Fitzgerald, InCrowd’s CEO and president, said in a separate statement.
FDA rules to ban ESDs for self-injurious, aggressive behavior
The Food and Drug Administration has banned all electrical stimulation devices used for self-injurious or aggressive behavior because of an unreasonable risk of illness or injury. This marks only the third time the FDA has banned a medical device since it gained the authority to do so.
Electrical stimulation devices (ESDs) administer electric shocks through electrodes attached to the skin during self-injurious or aggressive behavior in an attempt to condition the patient to stop engaging in that behavior, according to the FDA press release. Current evidence indicates that use of these devices can lead to worsening of underlying symptoms, depression, anxiety, PTSD, pain, burns, and tissue damage; in contrast, evidence supporting their use is weak. In addition, many patients exposed to ESDs have intellectual or developmental disabilities and might not be able to adequately communicate their level of pain.
“Since ESDs were first marketed more than 20 years ago, we have gained a better understanding of the danger these devices present to public health. Through advancements in medical science, there are now more treatment options available to reduce or stop self-injurious or aggressive behavior, thus avoiding the substantial risk ESDs present,” William H. Maisel, MD, MPH, director of the Office of Product Evaluation and Quality in the FDA’s Center for Devices and Radiological Health, said in the release.
The ruling follows a 2016 proposal to ban ESDs from the marketplace; the proposed rule received more than 1,500 comments from stakeholders, such as parents of people with intellectual and developmental disabilities, state agencies and their sister public-private organizations, the affected manufacturer and residential facility, some of the facility’s employees, and parents of individual residents, as well as from state and federal legislators and advocacy groups. Nearly all supported the ban.
The rule will go into effect 30 days after publication of the rule in the Federal Register, and compliance is required within 180 days.
The Food and Drug Administration has banned all electrical stimulation devices used for self-injurious or aggressive behavior because of an unreasonable risk of illness or injury. This marks only the third time the FDA has banned a medical device since it gained the authority to do so.
Electrical stimulation devices (ESDs) administer electric shocks through electrodes attached to the skin during self-injurious or aggressive behavior in an attempt to condition the patient to stop engaging in that behavior, according to the FDA press release. Current evidence indicates that use of these devices can lead to worsening of underlying symptoms, depression, anxiety, PTSD, pain, burns, and tissue damage; in contrast, evidence supporting their use is weak. In addition, many patients exposed to ESDs have intellectual or developmental disabilities and might not be able to adequately communicate their level of pain.
“Since ESDs were first marketed more than 20 years ago, we have gained a better understanding of the danger these devices present to public health. Through advancements in medical science, there are now more treatment options available to reduce or stop self-injurious or aggressive behavior, thus avoiding the substantial risk ESDs present,” William H. Maisel, MD, MPH, director of the Office of Product Evaluation and Quality in the FDA’s Center for Devices and Radiological Health, said in the release.
The ruling follows a 2016 proposal to ban ESDs from the marketplace; the proposed rule received more than 1,500 comments from stakeholders, such as parents of people with intellectual and developmental disabilities, state agencies and their sister public-private organizations, the affected manufacturer and residential facility, some of the facility’s employees, and parents of individual residents, as well as from state and federal legislators and advocacy groups. Nearly all supported the ban.
The rule will go into effect 30 days after publication of the rule in the Federal Register, and compliance is required within 180 days.
The Food and Drug Administration has banned all electrical stimulation devices used for self-injurious or aggressive behavior because of an unreasonable risk of illness or injury. This marks only the third time the FDA has banned a medical device since it gained the authority to do so.
Electrical stimulation devices (ESDs) administer electric shocks through electrodes attached to the skin during self-injurious or aggressive behavior in an attempt to condition the patient to stop engaging in that behavior, according to the FDA press release. Current evidence indicates that use of these devices can lead to worsening of underlying symptoms, depression, anxiety, PTSD, pain, burns, and tissue damage; in contrast, evidence supporting their use is weak. In addition, many patients exposed to ESDs have intellectual or developmental disabilities and might not be able to adequately communicate their level of pain.
“Since ESDs were first marketed more than 20 years ago, we have gained a better understanding of the danger these devices present to public health. Through advancements in medical science, there are now more treatment options available to reduce or stop self-injurious or aggressive behavior, thus avoiding the substantial risk ESDs present,” William H. Maisel, MD, MPH, director of the Office of Product Evaluation and Quality in the FDA’s Center for Devices and Radiological Health, said in the release.
The ruling follows a 2016 proposal to ban ESDs from the marketplace; the proposed rule received more than 1,500 comments from stakeholders, such as parents of people with intellectual and developmental disabilities, state agencies and their sister public-private organizations, the affected manufacturer and residential facility, some of the facility’s employees, and parents of individual residents, as well as from state and federal legislators and advocacy groups. Nearly all supported the ban.
The rule will go into effect 30 days after publication of the rule in the Federal Register, and compliance is required within 180 days.
FDA moves to expand coronavirus testing capacity; CDC clarifies testing criteria
The White House Coronavirus Task Force appeared at a press briefing March 2 to provide updates about testing strategies and public health coordination to address the current outbreak of the coronavirus COVID-19. Speaking at the briefing, led by Vice President Mike Pence, Centers for Disease Control and Prevention (CDC) director Robert Redfield, MD, said, “Working with our public health partners we continue to be able to identify new community cases and use our public health efforts to aggressively confirm, isolate, and do contact tracking.” Calling state, local, tribal, and territorial public health departments “the backbone of the public health system in our country,” Dr. Redfield noted that he expected many more confirmed COVID-19 cases to emerge.

At least some of the expected increase in confirmed cases of COVID-19 will occur because of expanded testing capacity, noted several of the task force members. On Feb. 29, the Food and Drug Administration issued a the virus that is causing the current outbreak of COVID-19.
Highly qualified laboratories, including both those run by public agencies and private labs, are now authorized to begin using their own validated test for the virus as long as they submit an Emergency Use Authorization (EUA) to the Food and Drug Administration within 15 days of notifying the agency of validation.
“To effectively respond to the COVID-19 outbreak, rapid detection of cases and contacts, appropriate clinical management and infection control, and implementation of community mitigation efforts are critical. This can best be achieved with wide availability of testing capabilities in health care settings, reference and commercial laboratories, and at the point of care,” the agency wrote in a press announcement of the expedited test expansion.
On Feb. 4, the Secretary of the Department of Health & Human Services declared a coronavirus public health emergency. The FDA was then authorized to allow individual laboratories with validated coronavirus tests to begin testing samples immediately. The goal is a more rapid and expanded testing capacity in the United States.
“The global emergence of COVID-19 is concerning, and we appreciate the efforts of the FDA to help bring more testing capability to the U.S.,” Nancy Messonnier, MD, director of the CDC’s National Center for Immunization and Respiratory Diseases (NCIRD), said in the press release.
The new guidance that permits the immediate use of clinical tests after individual development and validation, said the FDA, only applies to labs already certified to perform high complexity testing under Clinical Laboratory Improvement Amendments. Many governmental, academic, and private laboratories fall into this category, however.
“Under this policy, we expect certain laboratories who develop validated tests for coronavirus would begin using them right away prior to FDA review,” said Jeffrey Shuren, MD, JD, director of the FDA’s Center for Devices and Radiological Health. “We believe this action will support laboratories across the country working on this urgent public health situation,” he added in the press release.
“By the end of this week, close to a million tests will be available,” FDA Commissioner Stephen M. Hahn, MD, said during the March 2 briefing.*
Updated criteria
The CDC is maintaining updated criteria for the virus testing on its website. Testing criteria are based both on clinical features and epidemiologic risk.
Individuals with less severe clinical features – those who have either fever or signs and symptoms of lower respiratory disease such as cough or shortness of breath, but who don’t require hospitalization – should be tested if they have high epidemiologic risk. “High risk” is defined by the CDC as any individual, including health care workers, who has had close contact with a person with confirmed COVID-19 within the past 2 weeks. For health care workers, testing can be considered even if they have relatively mild respiratory symptoms or have had contact with a person who is suspected, but not yet confirmed, to have coronavirus.
In its testing guidance, the CDC recognizes that defining close contact is difficult. General guidelines are that individuals are considered to have been in close contact with a person who has COVID-19 if they were within about six feet of the person for a prolonged period, or cared for or have spent a prolonged amount of time in the same room or house as a person with confirmed COVID-19.
Individuals who have both fever and signs or symptoms of lower respiratory illness who require hospitalization should be tested if they have a history of travel from any affected geographic area within 14 days of the onset of their symptoms. The CDC now defines “affected geographic area” as any country or region that has at least a CDC Level 2 Travel Health Notice for COVID-19, so that the testing criteria themselves don’t need to be updated when new geographic areas are included in these alerts. As of March 3, China, Iran, Italy, Japan, and South Korea all have Level 2 or 3 travel alerts.
The CDC now recommends that any patient who has severe acute lower respiratory illness that requires hospitalization and doesn’t have an alternative diagnosis should be tested, even without any identified source of exposure.
“Despite seeing these new cases, the risk to the American people is low,” said the CDC’s Dr. Redfield. In response to a question from the press about how fast the coronavirus will spread across the United States, Dr. Redfield said, “From the beginning we’ve anticipated seeing community cases pop up.” He added that as these cases arise, testing and public health strategies will focus on unearthing linkages and contacts to learn how the virus is spreading. “We’ll use the public health strategies that we can to limit that transmission,” he said.
*An earlier version of this article misattributed this quote.
The White House Coronavirus Task Force appeared at a press briefing March 2 to provide updates about testing strategies and public health coordination to address the current outbreak of the coronavirus COVID-19. Speaking at the briefing, led by Vice President Mike Pence, Centers for Disease Control and Prevention (CDC) director Robert Redfield, MD, said, “Working with our public health partners we continue to be able to identify new community cases and use our public health efforts to aggressively confirm, isolate, and do contact tracking.” Calling state, local, tribal, and territorial public health departments “the backbone of the public health system in our country,” Dr. Redfield noted that he expected many more confirmed COVID-19 cases to emerge.

At least some of the expected increase in confirmed cases of COVID-19 will occur because of expanded testing capacity, noted several of the task force members. On Feb. 29, the Food and Drug Administration issued a the virus that is causing the current outbreak of COVID-19.
Highly qualified laboratories, including both those run by public agencies and private labs, are now authorized to begin using their own validated test for the virus as long as they submit an Emergency Use Authorization (EUA) to the Food and Drug Administration within 15 days of notifying the agency of validation.
“To effectively respond to the COVID-19 outbreak, rapid detection of cases and contacts, appropriate clinical management and infection control, and implementation of community mitigation efforts are critical. This can best be achieved with wide availability of testing capabilities in health care settings, reference and commercial laboratories, and at the point of care,” the agency wrote in a press announcement of the expedited test expansion.
On Feb. 4, the Secretary of the Department of Health & Human Services declared a coronavirus public health emergency. The FDA was then authorized to allow individual laboratories with validated coronavirus tests to begin testing samples immediately. The goal is a more rapid and expanded testing capacity in the United States.
“The global emergence of COVID-19 is concerning, and we appreciate the efforts of the FDA to help bring more testing capability to the U.S.,” Nancy Messonnier, MD, director of the CDC’s National Center for Immunization and Respiratory Diseases (NCIRD), said in the press release.
The new guidance that permits the immediate use of clinical tests after individual development and validation, said the FDA, only applies to labs already certified to perform high complexity testing under Clinical Laboratory Improvement Amendments. Many governmental, academic, and private laboratories fall into this category, however.
“Under this policy, we expect certain laboratories who develop validated tests for coronavirus would begin using them right away prior to FDA review,” said Jeffrey Shuren, MD, JD, director of the FDA’s Center for Devices and Radiological Health. “We believe this action will support laboratories across the country working on this urgent public health situation,” he added in the press release.
“By the end of this week, close to a million tests will be available,” FDA Commissioner Stephen M. Hahn, MD, said during the March 2 briefing.*
Updated criteria
The CDC is maintaining updated criteria for the virus testing on its website. Testing criteria are based both on clinical features and epidemiologic risk.
Individuals with less severe clinical features – those who have either fever or signs and symptoms of lower respiratory disease such as cough or shortness of breath, but who don’t require hospitalization – should be tested if they have high epidemiologic risk. “High risk” is defined by the CDC as any individual, including health care workers, who has had close contact with a person with confirmed COVID-19 within the past 2 weeks. For health care workers, testing can be considered even if they have relatively mild respiratory symptoms or have had contact with a person who is suspected, but not yet confirmed, to have coronavirus.
In its testing guidance, the CDC recognizes that defining close contact is difficult. General guidelines are that individuals are considered to have been in close contact with a person who has COVID-19 if they were within about six feet of the person for a prolonged period, or cared for or have spent a prolonged amount of time in the same room or house as a person with confirmed COVID-19.
Individuals who have both fever and signs or symptoms of lower respiratory illness who require hospitalization should be tested if they have a history of travel from any affected geographic area within 14 days of the onset of their symptoms. The CDC now defines “affected geographic area” as any country or region that has at least a CDC Level 2 Travel Health Notice for COVID-19, so that the testing criteria themselves don’t need to be updated when new geographic areas are included in these alerts. As of March 3, China, Iran, Italy, Japan, and South Korea all have Level 2 or 3 travel alerts.
The CDC now recommends that any patient who has severe acute lower respiratory illness that requires hospitalization and doesn’t have an alternative diagnosis should be tested, even without any identified source of exposure.
“Despite seeing these new cases, the risk to the American people is low,” said the CDC’s Dr. Redfield. In response to a question from the press about how fast the coronavirus will spread across the United States, Dr. Redfield said, “From the beginning we’ve anticipated seeing community cases pop up.” He added that as these cases arise, testing and public health strategies will focus on unearthing linkages and contacts to learn how the virus is spreading. “We’ll use the public health strategies that we can to limit that transmission,” he said.
*An earlier version of this article misattributed this quote.
The White House Coronavirus Task Force appeared at a press briefing March 2 to provide updates about testing strategies and public health coordination to address the current outbreak of the coronavirus COVID-19. Speaking at the briefing, led by Vice President Mike Pence, Centers for Disease Control and Prevention (CDC) director Robert Redfield, MD, said, “Working with our public health partners we continue to be able to identify new community cases and use our public health efforts to aggressively confirm, isolate, and do contact tracking.” Calling state, local, tribal, and territorial public health departments “the backbone of the public health system in our country,” Dr. Redfield noted that he expected many more confirmed COVID-19 cases to emerge.

At least some of the expected increase in confirmed cases of COVID-19 will occur because of expanded testing capacity, noted several of the task force members. On Feb. 29, the Food and Drug Administration issued a the virus that is causing the current outbreak of COVID-19.
Highly qualified laboratories, including both those run by public agencies and private labs, are now authorized to begin using their own validated test for the virus as long as they submit an Emergency Use Authorization (EUA) to the Food and Drug Administration within 15 days of notifying the agency of validation.
“To effectively respond to the COVID-19 outbreak, rapid detection of cases and contacts, appropriate clinical management and infection control, and implementation of community mitigation efforts are critical. This can best be achieved with wide availability of testing capabilities in health care settings, reference and commercial laboratories, and at the point of care,” the agency wrote in a press announcement of the expedited test expansion.
On Feb. 4, the Secretary of the Department of Health & Human Services declared a coronavirus public health emergency. The FDA was then authorized to allow individual laboratories with validated coronavirus tests to begin testing samples immediately. The goal is a more rapid and expanded testing capacity in the United States.
“The global emergence of COVID-19 is concerning, and we appreciate the efforts of the FDA to help bring more testing capability to the U.S.,” Nancy Messonnier, MD, director of the CDC’s National Center for Immunization and Respiratory Diseases (NCIRD), said in the press release.
The new guidance that permits the immediate use of clinical tests after individual development and validation, said the FDA, only applies to labs already certified to perform high complexity testing under Clinical Laboratory Improvement Amendments. Many governmental, academic, and private laboratories fall into this category, however.
“Under this policy, we expect certain laboratories who develop validated tests for coronavirus would begin using them right away prior to FDA review,” said Jeffrey Shuren, MD, JD, director of the FDA’s Center for Devices and Radiological Health. “We believe this action will support laboratories across the country working on this urgent public health situation,” he added in the press release.
“By the end of this week, close to a million tests will be available,” FDA Commissioner Stephen M. Hahn, MD, said during the March 2 briefing.*
Updated criteria
The CDC is maintaining updated criteria for the virus testing on its website. Testing criteria are based both on clinical features and epidemiologic risk.
Individuals with less severe clinical features – those who have either fever or signs and symptoms of lower respiratory disease such as cough or shortness of breath, but who don’t require hospitalization – should be tested if they have high epidemiologic risk. “High risk” is defined by the CDC as any individual, including health care workers, who has had close contact with a person with confirmed COVID-19 within the past 2 weeks. For health care workers, testing can be considered even if they have relatively mild respiratory symptoms or have had contact with a person who is suspected, but not yet confirmed, to have coronavirus.
In its testing guidance, the CDC recognizes that defining close contact is difficult. General guidelines are that individuals are considered to have been in close contact with a person who has COVID-19 if they were within about six feet of the person for a prolonged period, or cared for or have spent a prolonged amount of time in the same room or house as a person with confirmed COVID-19.
Individuals who have both fever and signs or symptoms of lower respiratory illness who require hospitalization should be tested if they have a history of travel from any affected geographic area within 14 days of the onset of their symptoms. The CDC now defines “affected geographic area” as any country or region that has at least a CDC Level 2 Travel Health Notice for COVID-19, so that the testing criteria themselves don’t need to be updated when new geographic areas are included in these alerts. As of March 3, China, Iran, Italy, Japan, and South Korea all have Level 2 or 3 travel alerts.
The CDC now recommends that any patient who has severe acute lower respiratory illness that requires hospitalization and doesn’t have an alternative diagnosis should be tested, even without any identified source of exposure.
“Despite seeing these new cases, the risk to the American people is low,” said the CDC’s Dr. Redfield. In response to a question from the press about how fast the coronavirus will spread across the United States, Dr. Redfield said, “From the beginning we’ve anticipated seeing community cases pop up.” He added that as these cases arise, testing and public health strategies will focus on unearthing linkages and contacts to learn how the virus is spreading. “We’ll use the public health strategies that we can to limit that transmission,” he said.
*An earlier version of this article misattributed this quote.
FROM A PRESS BRIEFING BY THE WHITE HOUSE CORONAVIRUS TASK FORCE
What medical conferences are being canceled by coronavirus?
In a typical year, March marks the start of conference season, made all the more attractive by collegial gatherings and travel to warmer climes. But 2020 has already proven anything but typical as the number of novel coronavirus cases continues to increase around the globe. As a potential pandemic looms, these meetings – full of handshakes and crowded lecture halls – are also nirvana for opportunistic viruses. As are the airports, airplanes, and cabs required to get there.
So, as COVID-19 continues to spread, medical and scientific societies must make some difficult decisions. In Europe, at least a few societies have already suspended their upcoming meetings, while France has temporarily banned all gatherings over 5000 people.
In the United States, however, most medical conferences are moving forward as planned – at least for now. But one conference of 10,000 attendees, the American Physical Society annual meeting, which was scheduled for March 2-6 in Denver, was canceled the day before the meeting started. Although it’s not a medical conference, it speaks to the “rapidly escalating health concerns” that all conference organizers must grapple with.
APS Physics Meetings
@APSMeetings
Due to rapidly escalating health concerns relating to the spread of the coronavirus disease (COVID-19), the 2020 APS March Meeting in Denver, CO, has been canceled. Please do not travel to Denver to attend the March Meeting. More information will follow shortly. #apsmarch
734 9:59 PM - Feb 29, 2020
Just one smaller medical meeting, the Ataxia Conference, which was scheduled for March 6-7 in Denver, has been canceled.
Most societies hosting these meetings have put out statements to their attendees saying that they’re monitoring the situation and will adapt as necessary. The United States and Canadian Academy of Pathology, which is holding its annual meeting in Los Angeles this week, sent out an email beforehand asking international travelers to consider staying home. The Healthcare Information and Management Systems Society (HIMSS) Global Health Conference, which is slated to have about 50,000 attendees from around the world, has declared itself a “handshake-free” conference but otherwise intends to move ahead as planned.
All of these conferences will be pushing forward without at least one prominent group of attendees. New York University’s Langone Health has removed its employees from the decision-making process and instead is taking a proactive stance: The health system just declared a 60-day (minimum) ban preventing employees from attending any meetings or conferences and from all domestic and international work-related travel.
Here’s what some of the societies have said to attendees about their intent to proceed or modify their plans:
- Conference on Retroviruses and Opportunistic Infections (CROI), Boston, 3/8/20 - 3/11/20: Monitoring the situation and seeking input from local, state, and federal infectious-disease and public-health experts. Final decision expected by the evening of March 3.
- American Academy of Allergy, Asthma & Immunology (AAAAI), Philadelphia, 3/13/20 - 3/16/20: Monitoring developments but no plans to cancel or postpone at this time.
- American Academy of Orthopedic Surgeons (AAOS), Orlando, 3/24/20 - 3/28/20: Proceeding as planned.
- American Academy of Dermatology (AAD), Denver, 3/20/20 - 3/24/20: The AAD’s 2020 Annual Meeting is scheduled to take place as planned. The organization will increase the number of hand-sanitizing stations throughout the convention center, and it is adding a nursing station specifically designated for anyone with flu-like symptoms.
- American College of Cardiology (ACC), Chicago, 3/28/20 - 3/30/20: The organization is working with attendees, faculty, exhibitors, and other stakeholders in affected countries to ensure access to research and education from the meeting, but is otherwise proceeding as planned.
- Endocrine Society (ENDO), San Francisco, 3/28/20 - 3/31/20: ENDO 2020 will take place as scheduled, but this is an evolving situation worldwide. The society will continue to monitor and provide updates on its FAQ page.
- American College of Physicians Internal Medicine (ACP IM), Los Angeles, 4/23/20 - 4/25/20: ACP leadership is closely monitoring the COVID-19 situation and is actively working with the Centers for Disease Control and Prevention (CDC) to ensure authoritative communication of safety updates and recommendations as the situation evolves.
- American Association for Cancer Research (AACR), San Diego, 4/24/20 - 4/29/20: At this time, there is no plan to cancel or postpone any scheduled AACR meetings. The organization is tracking all travel restrictions as well as information and guidance from the CDC and World Health Organization.
- American Academy of Neurology (AAN), Toronto, 4/25/20 - 5/1/20: The group is continuing to closely monitor the situation in Toronto and will provide updates as the situation warrants.
This article originally appeared on Medscape.com.
In a typical year, March marks the start of conference season, made all the more attractive by collegial gatherings and travel to warmer climes. But 2020 has already proven anything but typical as the number of novel coronavirus cases continues to increase around the globe. As a potential pandemic looms, these meetings – full of handshakes and crowded lecture halls – are also nirvana for opportunistic viruses. As are the airports, airplanes, and cabs required to get there.
So, as COVID-19 continues to spread, medical and scientific societies must make some difficult decisions. In Europe, at least a few societies have already suspended their upcoming meetings, while France has temporarily banned all gatherings over 5000 people.
In the United States, however, most medical conferences are moving forward as planned – at least for now. But one conference of 10,000 attendees, the American Physical Society annual meeting, which was scheduled for March 2-6 in Denver, was canceled the day before the meeting started. Although it’s not a medical conference, it speaks to the “rapidly escalating health concerns” that all conference organizers must grapple with.
APS Physics Meetings
@APSMeetings
Due to rapidly escalating health concerns relating to the spread of the coronavirus disease (COVID-19), the 2020 APS March Meeting in Denver, CO, has been canceled. Please do not travel to Denver to attend the March Meeting. More information will follow shortly. #apsmarch
734 9:59 PM - Feb 29, 2020
Just one smaller medical meeting, the Ataxia Conference, which was scheduled for March 6-7 in Denver, has been canceled.
Most societies hosting these meetings have put out statements to their attendees saying that they’re monitoring the situation and will adapt as necessary. The United States and Canadian Academy of Pathology, which is holding its annual meeting in Los Angeles this week, sent out an email beforehand asking international travelers to consider staying home. The Healthcare Information and Management Systems Society (HIMSS) Global Health Conference, which is slated to have about 50,000 attendees from around the world, has declared itself a “handshake-free” conference but otherwise intends to move ahead as planned.
All of these conferences will be pushing forward without at least one prominent group of attendees. New York University’s Langone Health has removed its employees from the decision-making process and instead is taking a proactive stance: The health system just declared a 60-day (minimum) ban preventing employees from attending any meetings or conferences and from all domestic and international work-related travel.
Here’s what some of the societies have said to attendees about their intent to proceed or modify their plans:
- Conference on Retroviruses and Opportunistic Infections (CROI), Boston, 3/8/20 - 3/11/20: Monitoring the situation and seeking input from local, state, and federal infectious-disease and public-health experts. Final decision expected by the evening of March 3.
- American Academy of Allergy, Asthma & Immunology (AAAAI), Philadelphia, 3/13/20 - 3/16/20: Monitoring developments but no plans to cancel or postpone at this time.
- American Academy of Orthopedic Surgeons (AAOS), Orlando, 3/24/20 - 3/28/20: Proceeding as planned.
- American Academy of Dermatology (AAD), Denver, 3/20/20 - 3/24/20: The AAD’s 2020 Annual Meeting is scheduled to take place as planned. The organization will increase the number of hand-sanitizing stations throughout the convention center, and it is adding a nursing station specifically designated for anyone with flu-like symptoms.
- American College of Cardiology (ACC), Chicago, 3/28/20 - 3/30/20: The organization is working with attendees, faculty, exhibitors, and other stakeholders in affected countries to ensure access to research and education from the meeting, but is otherwise proceeding as planned.
- Endocrine Society (ENDO), San Francisco, 3/28/20 - 3/31/20: ENDO 2020 will take place as scheduled, but this is an evolving situation worldwide. The society will continue to monitor and provide updates on its FAQ page.
- American College of Physicians Internal Medicine (ACP IM), Los Angeles, 4/23/20 - 4/25/20: ACP leadership is closely monitoring the COVID-19 situation and is actively working with the Centers for Disease Control and Prevention (CDC) to ensure authoritative communication of safety updates and recommendations as the situation evolves.
- American Association for Cancer Research (AACR), San Diego, 4/24/20 - 4/29/20: At this time, there is no plan to cancel or postpone any scheduled AACR meetings. The organization is tracking all travel restrictions as well as information and guidance from the CDC and World Health Organization.
- American Academy of Neurology (AAN), Toronto, 4/25/20 - 5/1/20: The group is continuing to closely monitor the situation in Toronto and will provide updates as the situation warrants.
This article originally appeared on Medscape.com.
In a typical year, March marks the start of conference season, made all the more attractive by collegial gatherings and travel to warmer climes. But 2020 has already proven anything but typical as the number of novel coronavirus cases continues to increase around the globe. As a potential pandemic looms, these meetings – full of handshakes and crowded lecture halls – are also nirvana for opportunistic viruses. As are the airports, airplanes, and cabs required to get there.
So, as COVID-19 continues to spread, medical and scientific societies must make some difficult decisions. In Europe, at least a few societies have already suspended their upcoming meetings, while France has temporarily banned all gatherings over 5000 people.
In the United States, however, most medical conferences are moving forward as planned – at least for now. But one conference of 10,000 attendees, the American Physical Society annual meeting, which was scheduled for March 2-6 in Denver, was canceled the day before the meeting started. Although it’s not a medical conference, it speaks to the “rapidly escalating health concerns” that all conference organizers must grapple with.
APS Physics Meetings
@APSMeetings
Due to rapidly escalating health concerns relating to the spread of the coronavirus disease (COVID-19), the 2020 APS March Meeting in Denver, CO, has been canceled. Please do not travel to Denver to attend the March Meeting. More information will follow shortly. #apsmarch
734 9:59 PM - Feb 29, 2020
Just one smaller medical meeting, the Ataxia Conference, which was scheduled for March 6-7 in Denver, has been canceled.
Most societies hosting these meetings have put out statements to their attendees saying that they’re monitoring the situation and will adapt as necessary. The United States and Canadian Academy of Pathology, which is holding its annual meeting in Los Angeles this week, sent out an email beforehand asking international travelers to consider staying home. The Healthcare Information and Management Systems Society (HIMSS) Global Health Conference, which is slated to have about 50,000 attendees from around the world, has declared itself a “handshake-free” conference but otherwise intends to move ahead as planned.
All of these conferences will be pushing forward without at least one prominent group of attendees. New York University’s Langone Health has removed its employees from the decision-making process and instead is taking a proactive stance: The health system just declared a 60-day (minimum) ban preventing employees from attending any meetings or conferences and from all domestic and international work-related travel.
Here’s what some of the societies have said to attendees about their intent to proceed or modify their plans:
- Conference on Retroviruses and Opportunistic Infections (CROI), Boston, 3/8/20 - 3/11/20: Monitoring the situation and seeking input from local, state, and federal infectious-disease and public-health experts. Final decision expected by the evening of March 3.
- American Academy of Allergy, Asthma & Immunology (AAAAI), Philadelphia, 3/13/20 - 3/16/20: Monitoring developments but no plans to cancel or postpone at this time.
- American Academy of Orthopedic Surgeons (AAOS), Orlando, 3/24/20 - 3/28/20: Proceeding as planned.
- American Academy of Dermatology (AAD), Denver, 3/20/20 - 3/24/20: The AAD’s 2020 Annual Meeting is scheduled to take place as planned. The organization will increase the number of hand-sanitizing stations throughout the convention center, and it is adding a nursing station specifically designated for anyone with flu-like symptoms.
- American College of Cardiology (ACC), Chicago, 3/28/20 - 3/30/20: The organization is working with attendees, faculty, exhibitors, and other stakeholders in affected countries to ensure access to research and education from the meeting, but is otherwise proceeding as planned.
- Endocrine Society (ENDO), San Francisco, 3/28/20 - 3/31/20: ENDO 2020 will take place as scheduled, but this is an evolving situation worldwide. The society will continue to monitor and provide updates on its FAQ page.
- American College of Physicians Internal Medicine (ACP IM), Los Angeles, 4/23/20 - 4/25/20: ACP leadership is closely monitoring the COVID-19 situation and is actively working with the Centers for Disease Control and Prevention (CDC) to ensure authoritative communication of safety updates and recommendations as the situation evolves.
- American Association for Cancer Research (AACR), San Diego, 4/24/20 - 4/29/20: At this time, there is no plan to cancel or postpone any scheduled AACR meetings. The organization is tracking all travel restrictions as well as information and guidance from the CDC and World Health Organization.
- American Academy of Neurology (AAN), Toronto, 4/25/20 - 5/1/20: The group is continuing to closely monitor the situation in Toronto and will provide updates as the situation warrants.
This article originally appeared on Medscape.com.
rTMS for depression continues to evolve
LAS VEGAS – Repetitive transcranial magnetic stimulation methods for treatment-resistant depression continue to be refined.
“Original studies have relatively low response rates, but we’re seeing better response rates as we figure out the localization, the parameters, the wave form, and how frequently you can give it,” Alan F. Schatzberg, MD, said at an annual psychopharmacology update held by the Nevada Psychiatric Association.
Repetitive transcranial magnetic stimulation (rTMS) involves the application of a magnetic field to a particular area of the brain, typically the dorsal lateral aspect of the prefrontal cortex. “It’s a weaker stimulant than electroconvulsive therapy, but it’s more focused and a lot safer,” said Dr. Schatzberg, professor of psychiatry and behavioral sciences at Stanford (Calif.) University. “It does not require anesthesia. In fact, it does seem to have some antidepressant effects.”
The original trial that applied this technology was conducted in 301 medication-free patients with major depression who had not benefited from prior treatment (Biol Psychiatry. 2007;62[11]:1208-16). Of the 301 patients, 155 received active rTMS, while 146 received sham rTMS. Treatment sessions were conducted five times per week for 4-6 weeks. The primary outcome was the symptom score change as assessed at week 4 with the Montgomery-Åsberg Depression Rating Scale (MADRS). Secondary outcomes included changes on the 17- and 24-item Hamilton Depression Rating Scale (HAMD), and response and remission rates with the MADRS and HAMD.
Response rates were significantly higher with active TMS on all three scales at weeks 4 and 6. Remission rates were approximately twofold higher with active TMS at week 6 and significant on the MADRS and HAMD24 scales (but not the HAMD17 scale). “The response rate for patients receiving active treatment was about 20%, and the remission at 6 weeks was about 18%,” said Dr. Schatzberg, who was an adviser to the study. “It was about twofold higher than in the sham group. It’s not dramatically effective, but it certainly is better than the sham control.” The MADRS score dropped about 6 points in the rTMS group, compared with about 2 points in the sham group, while the HAMD 24 score dropped about 7 points in the rTMS group, compared with about 3.5 points in the sham group.
In a separate, multisite, sham-controlled trial supported by the National Institutes of Health, researchers enrolled 199 antidepressant drug-free patients to determine whether daily left prefrontal rTMS safely and effectively treats major depressive disorder (Arch Gen Psychiatry. 2010;67[5]:507-16). Over the course of 3 weeks, the researchers delivered rTMS to the left prefrontal cortex for 37.5 minutes (3,000 pulses per session) using a figure-eight solid-core coil. Sham rTMS used a similar coil with a metal insert blocking the magnetic field and scalp electrodes that delivered matched somatosensory sensations. The retention rate was 88%, and no device-related serious adverse events were reported. A significantly greater proportion of patients treated with rTMS achieved remission, compared with those in the sham group (15% vs. 5%, respectively; P = .02). The odds of attaining remission were 4.2 times greater with active rTMS than with the sham treatment.
“These are not huge remission and response rates,” Dr. Schatzberg said of the results from this and other studies. “What can we do to start increasing efficacy? One thing you can do is design a better coil. You can alter the site of application, and you can change the pulse frequency and the pulse number. You can also change the brain wave focus. Theta seems to be mostly associated with hippocampal function around memory. Because of that, a number of groups starting giving theta waves.”
In one such study, researchers used accelerated, high-dose intermittent theta burst stimulation (iTBS) to treat highly treatment-resistant depression patients (Brain. 2018;141[3]:e18). The treatment lasted 5 days and consisted of 10 sessions per day, with 50 minutes between each session. “It’s a much more intensive system that delivers about 90,000 pulses,” said Dr. Schatzberg, who directs the Stanford Mood Disorders Center. Most patients remitted, but the durability of therapeutic response was weak, and all patients relapsed within 2 weeks post treatment.
“There’s more work to be done, but rTMS is really a good technology,” he concluded. “I think we will achieve much higher rates of success with this treatment once we push the envelope a little bit.”
Dr. Schatzberg disclosed that he has served a consultant to Alkermes, Avanir, Bracket, Compass, Delpor, Epiodyne, Janssen, Jazz, Lundbeck, McKinsey, Merck, Myriad Genetics, Owl, Neuronetics, Pfizer, Sage, and Sunovion. He has received research funding from Janssen and also holds an ownership interest in Corcept, Dermira, Delpor, Epiodyne, Incyte Genetics, Madrigal, Merck, Owl Analytics, Seattle Genetics, Titan, and Xhale.
LAS VEGAS – Repetitive transcranial magnetic stimulation methods for treatment-resistant depression continue to be refined.
“Original studies have relatively low response rates, but we’re seeing better response rates as we figure out the localization, the parameters, the wave form, and how frequently you can give it,” Alan F. Schatzberg, MD, said at an annual psychopharmacology update held by the Nevada Psychiatric Association.
Repetitive transcranial magnetic stimulation (rTMS) involves the application of a magnetic field to a particular area of the brain, typically the dorsal lateral aspect of the prefrontal cortex. “It’s a weaker stimulant than electroconvulsive therapy, but it’s more focused and a lot safer,” said Dr. Schatzberg, professor of psychiatry and behavioral sciences at Stanford (Calif.) University. “It does not require anesthesia. In fact, it does seem to have some antidepressant effects.”
The original trial that applied this technology was conducted in 301 medication-free patients with major depression who had not benefited from prior treatment (Biol Psychiatry. 2007;62[11]:1208-16). Of the 301 patients, 155 received active rTMS, while 146 received sham rTMS. Treatment sessions were conducted five times per week for 4-6 weeks. The primary outcome was the symptom score change as assessed at week 4 with the Montgomery-Åsberg Depression Rating Scale (MADRS). Secondary outcomes included changes on the 17- and 24-item Hamilton Depression Rating Scale (HAMD), and response and remission rates with the MADRS and HAMD.
Response rates were significantly higher with active TMS on all three scales at weeks 4 and 6. Remission rates were approximately twofold higher with active TMS at week 6 and significant on the MADRS and HAMD24 scales (but not the HAMD17 scale). “The response rate for patients receiving active treatment was about 20%, and the remission at 6 weeks was about 18%,” said Dr. Schatzberg, who was an adviser to the study. “It was about twofold higher than in the sham group. It’s not dramatically effective, but it certainly is better than the sham control.” The MADRS score dropped about 6 points in the rTMS group, compared with about 2 points in the sham group, while the HAMD 24 score dropped about 7 points in the rTMS group, compared with about 3.5 points in the sham group.
In a separate, multisite, sham-controlled trial supported by the National Institutes of Health, researchers enrolled 199 antidepressant drug-free patients to determine whether daily left prefrontal rTMS safely and effectively treats major depressive disorder (Arch Gen Psychiatry. 2010;67[5]:507-16). Over the course of 3 weeks, the researchers delivered rTMS to the left prefrontal cortex for 37.5 minutes (3,000 pulses per session) using a figure-eight solid-core coil. Sham rTMS used a similar coil with a metal insert blocking the magnetic field and scalp electrodes that delivered matched somatosensory sensations. The retention rate was 88%, and no device-related serious adverse events were reported. A significantly greater proportion of patients treated with rTMS achieved remission, compared with those in the sham group (15% vs. 5%, respectively; P = .02). The odds of attaining remission were 4.2 times greater with active rTMS than with the sham treatment.
“These are not huge remission and response rates,” Dr. Schatzberg said of the results from this and other studies. “What can we do to start increasing efficacy? One thing you can do is design a better coil. You can alter the site of application, and you can change the pulse frequency and the pulse number. You can also change the brain wave focus. Theta seems to be mostly associated with hippocampal function around memory. Because of that, a number of groups starting giving theta waves.”
In one such study, researchers used accelerated, high-dose intermittent theta burst stimulation (iTBS) to treat highly treatment-resistant depression patients (Brain. 2018;141[3]:e18). The treatment lasted 5 days and consisted of 10 sessions per day, with 50 minutes between each session. “It’s a much more intensive system that delivers about 90,000 pulses,” said Dr. Schatzberg, who directs the Stanford Mood Disorders Center. Most patients remitted, but the durability of therapeutic response was weak, and all patients relapsed within 2 weeks post treatment.
“There’s more work to be done, but rTMS is really a good technology,” he concluded. “I think we will achieve much higher rates of success with this treatment once we push the envelope a little bit.”
Dr. Schatzberg disclosed that he has served a consultant to Alkermes, Avanir, Bracket, Compass, Delpor, Epiodyne, Janssen, Jazz, Lundbeck, McKinsey, Merck, Myriad Genetics, Owl, Neuronetics, Pfizer, Sage, and Sunovion. He has received research funding from Janssen and also holds an ownership interest in Corcept, Dermira, Delpor, Epiodyne, Incyte Genetics, Madrigal, Merck, Owl Analytics, Seattle Genetics, Titan, and Xhale.
LAS VEGAS – Repetitive transcranial magnetic stimulation methods for treatment-resistant depression continue to be refined.
“Original studies have relatively low response rates, but we’re seeing better response rates as we figure out the localization, the parameters, the wave form, and how frequently you can give it,” Alan F. Schatzberg, MD, said at an annual psychopharmacology update held by the Nevada Psychiatric Association.
Repetitive transcranial magnetic stimulation (rTMS) involves the application of a magnetic field to a particular area of the brain, typically the dorsal lateral aspect of the prefrontal cortex. “It’s a weaker stimulant than electroconvulsive therapy, but it’s more focused and a lot safer,” said Dr. Schatzberg, professor of psychiatry and behavioral sciences at Stanford (Calif.) University. “It does not require anesthesia. In fact, it does seem to have some antidepressant effects.”
The original trial that applied this technology was conducted in 301 medication-free patients with major depression who had not benefited from prior treatment (Biol Psychiatry. 2007;62[11]:1208-16). Of the 301 patients, 155 received active rTMS, while 146 received sham rTMS. Treatment sessions were conducted five times per week for 4-6 weeks. The primary outcome was the symptom score change as assessed at week 4 with the Montgomery-Åsberg Depression Rating Scale (MADRS). Secondary outcomes included changes on the 17- and 24-item Hamilton Depression Rating Scale (HAMD), and response and remission rates with the MADRS and HAMD.
Response rates were significantly higher with active TMS on all three scales at weeks 4 and 6. Remission rates were approximately twofold higher with active TMS at week 6 and significant on the MADRS and HAMD24 scales (but not the HAMD17 scale). “The response rate for patients receiving active treatment was about 20%, and the remission at 6 weeks was about 18%,” said Dr. Schatzberg, who was an adviser to the study. “It was about twofold higher than in the sham group. It’s not dramatically effective, but it certainly is better than the sham control.” The MADRS score dropped about 6 points in the rTMS group, compared with about 2 points in the sham group, while the HAMD 24 score dropped about 7 points in the rTMS group, compared with about 3.5 points in the sham group.
In a separate, multisite, sham-controlled trial supported by the National Institutes of Health, researchers enrolled 199 antidepressant drug-free patients to determine whether daily left prefrontal rTMS safely and effectively treats major depressive disorder (Arch Gen Psychiatry. 2010;67[5]:507-16). Over the course of 3 weeks, the researchers delivered rTMS to the left prefrontal cortex for 37.5 minutes (3,000 pulses per session) using a figure-eight solid-core coil. Sham rTMS used a similar coil with a metal insert blocking the magnetic field and scalp electrodes that delivered matched somatosensory sensations. The retention rate was 88%, and no device-related serious adverse events were reported. A significantly greater proportion of patients treated with rTMS achieved remission, compared with those in the sham group (15% vs. 5%, respectively; P = .02). The odds of attaining remission were 4.2 times greater with active rTMS than with the sham treatment.
“These are not huge remission and response rates,” Dr. Schatzberg said of the results from this and other studies. “What can we do to start increasing efficacy? One thing you can do is design a better coil. You can alter the site of application, and you can change the pulse frequency and the pulse number. You can also change the brain wave focus. Theta seems to be mostly associated with hippocampal function around memory. Because of that, a number of groups starting giving theta waves.”
In one such study, researchers used accelerated, high-dose intermittent theta burst stimulation (iTBS) to treat highly treatment-resistant depression patients (Brain. 2018;141[3]:e18). The treatment lasted 5 days and consisted of 10 sessions per day, with 50 minutes between each session. “It’s a much more intensive system that delivers about 90,000 pulses,” said Dr. Schatzberg, who directs the Stanford Mood Disorders Center. Most patients remitted, but the durability of therapeutic response was weak, and all patients relapsed within 2 weeks post treatment.
“There’s more work to be done, but rTMS is really a good technology,” he concluded. “I think we will achieve much higher rates of success with this treatment once we push the envelope a little bit.”
Dr. Schatzberg disclosed that he has served a consultant to Alkermes, Avanir, Bracket, Compass, Delpor, Epiodyne, Janssen, Jazz, Lundbeck, McKinsey, Merck, Myriad Genetics, Owl, Neuronetics, Pfizer, Sage, and Sunovion. He has received research funding from Janssen and also holds an ownership interest in Corcept, Dermira, Delpor, Epiodyne, Incyte Genetics, Madrigal, Merck, Owl Analytics, Seattle Genetics, Titan, and Xhale.
REPORTING FROM NPA 2020
Borderline personality disorder common in chronic pain patients
NATIONAL HARBOR, MD. – A significant proportion of patients who suffer from chronic pain also have features of borderline personality disorder (BPD), new research shows.
Results of a systematic literature review showed 23% of patients with chronic noncancer pain (CNCP) had some features of BPD, including difficulty maintaining relationships, as well as affect and mood instability.
“The fact that one-fourth of individuals with CNCP could have co-occurring BPD underscores the need for improved access to good psychological care,” lead investigator Fei Cao, MD, PhD, University of Missouri at Kansas City, said in an interview.
“If we treat the borderline personality disorder and address the psychiatric needs as well as the pain needs of the patient, then we will be able to treat their pain more successfully,” Cao said.
The findings were presented at the American Academy of Pain Medicine (AAPM) 2020 Annual Meeting.
Treatment resistance
Cao noted that a “significant number” of CNCP patients have at least some resistance to any type of pain treatment and speculated that BPD may increase treatment-resistant chronic pain.
Initially an anesthesiologist and pain medicine specialist, Cao later became a psychiatrist after recognizing the importance of addressing the underlying psychological needs of patients with chronic pain.
He noted that there is a strong psychological component to chronic pain and that many patients with chronic pain have suffered psychological trauma.
“You have to think about what may have happened to these patients. That is most important. I would not say these are difficult patients. I would say we just don’t know what happened to them,” he said.
To gain a better understanding of the prevalence of BPD in patients suffering from chronic pain and potentially provide some unexploited targets for chronic pain management, the investigators analyzed data from 11 studies published between 1994 and 2019. They found the prevalence of BPD among CNCP patients was 23.3%. Pain types included chronic headache (11.3%), arthritis (27.5%), and chronic spinal cord pain (24.3%).
We also have to treat their BPD. This can then make pain easier to control. Chronic pain management is often long-term and requires good compliance. A diagnosis of BPD might suggest poor compliance,” said Cao.
Screen for BPD
The study findings, he added, indicate a need to screen for BPD in patients with chronic pain. Interventions that are effective in the treatment of BPD and CNCP include cognitive-behavioral therapy, dialectical behavior therapy, antidepressants, and anticonvulsants.
“These should be considered as the first-line treatment in persons with comorbid pain and BPD,” Cao said.
Commenting on the findings, Ann E. Hansen, DVM, MD, Chronic Pain Wellness Center, Phoenix VA Health Care System, Arizona, said the study illustrates the multifactorial nature of chronic pain syndromes, and underscores the importance of a multidisciplinary approach to evaluation and treatment.
“The authors present data showing that BPD is a common diagnosis in patients with chronic pain, thus raising provider awareness to consider BPD and to involve behavioral health colleagues in comanaging these complex patients to achieve optimal outcomes,” Hansen said.
Cao and Hansen have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
SOURCE: Cao F et al. American Academy of Pain Medicine (AAPM) 2020 Annual Meeting, Abstract 505.
NATIONAL HARBOR, MD. – A significant proportion of patients who suffer from chronic pain also have features of borderline personality disorder (BPD), new research shows.
Results of a systematic literature review showed 23% of patients with chronic noncancer pain (CNCP) had some features of BPD, including difficulty maintaining relationships, as well as affect and mood instability.
“The fact that one-fourth of individuals with CNCP could have co-occurring BPD underscores the need for improved access to good psychological care,” lead investigator Fei Cao, MD, PhD, University of Missouri at Kansas City, said in an interview.
“If we treat the borderline personality disorder and address the psychiatric needs as well as the pain needs of the patient, then we will be able to treat their pain more successfully,” Cao said.
The findings were presented at the American Academy of Pain Medicine (AAPM) 2020 Annual Meeting.
Treatment resistance
Cao noted that a “significant number” of CNCP patients have at least some resistance to any type of pain treatment and speculated that BPD may increase treatment-resistant chronic pain.
Initially an anesthesiologist and pain medicine specialist, Cao later became a psychiatrist after recognizing the importance of addressing the underlying psychological needs of patients with chronic pain.
He noted that there is a strong psychological component to chronic pain and that many patients with chronic pain have suffered psychological trauma.
“You have to think about what may have happened to these patients. That is most important. I would not say these are difficult patients. I would say we just don’t know what happened to them,” he said.
To gain a better understanding of the prevalence of BPD in patients suffering from chronic pain and potentially provide some unexploited targets for chronic pain management, the investigators analyzed data from 11 studies published between 1994 and 2019. They found the prevalence of BPD among CNCP patients was 23.3%. Pain types included chronic headache (11.3%), arthritis (27.5%), and chronic spinal cord pain (24.3%).
We also have to treat their BPD. This can then make pain easier to control. Chronic pain management is often long-term and requires good compliance. A diagnosis of BPD might suggest poor compliance,” said Cao.
Screen for BPD
The study findings, he added, indicate a need to screen for BPD in patients with chronic pain. Interventions that are effective in the treatment of BPD and CNCP include cognitive-behavioral therapy, dialectical behavior therapy, antidepressants, and anticonvulsants.
“These should be considered as the first-line treatment in persons with comorbid pain and BPD,” Cao said.
Commenting on the findings, Ann E. Hansen, DVM, MD, Chronic Pain Wellness Center, Phoenix VA Health Care System, Arizona, said the study illustrates the multifactorial nature of chronic pain syndromes, and underscores the importance of a multidisciplinary approach to evaluation and treatment.
“The authors present data showing that BPD is a common diagnosis in patients with chronic pain, thus raising provider awareness to consider BPD and to involve behavioral health colleagues in comanaging these complex patients to achieve optimal outcomes,” Hansen said.
Cao and Hansen have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
SOURCE: Cao F et al. American Academy of Pain Medicine (AAPM) 2020 Annual Meeting, Abstract 505.
NATIONAL HARBOR, MD. – A significant proportion of patients who suffer from chronic pain also have features of borderline personality disorder (BPD), new research shows.
Results of a systematic literature review showed 23% of patients with chronic noncancer pain (CNCP) had some features of BPD, including difficulty maintaining relationships, as well as affect and mood instability.
“The fact that one-fourth of individuals with CNCP could have co-occurring BPD underscores the need for improved access to good psychological care,” lead investigator Fei Cao, MD, PhD, University of Missouri at Kansas City, said in an interview.
“If we treat the borderline personality disorder and address the psychiatric needs as well as the pain needs of the patient, then we will be able to treat their pain more successfully,” Cao said.
The findings were presented at the American Academy of Pain Medicine (AAPM) 2020 Annual Meeting.
Treatment resistance
Cao noted that a “significant number” of CNCP patients have at least some resistance to any type of pain treatment and speculated that BPD may increase treatment-resistant chronic pain.
Initially an anesthesiologist and pain medicine specialist, Cao later became a psychiatrist after recognizing the importance of addressing the underlying psychological needs of patients with chronic pain.
He noted that there is a strong psychological component to chronic pain and that many patients with chronic pain have suffered psychological trauma.
“You have to think about what may have happened to these patients. That is most important. I would not say these are difficult patients. I would say we just don’t know what happened to them,” he said.
To gain a better understanding of the prevalence of BPD in patients suffering from chronic pain and potentially provide some unexploited targets for chronic pain management, the investigators analyzed data from 11 studies published between 1994 and 2019. They found the prevalence of BPD among CNCP patients was 23.3%. Pain types included chronic headache (11.3%), arthritis (27.5%), and chronic spinal cord pain (24.3%).
We also have to treat their BPD. This can then make pain easier to control. Chronic pain management is often long-term and requires good compliance. A diagnosis of BPD might suggest poor compliance,” said Cao.
Screen for BPD
The study findings, he added, indicate a need to screen for BPD in patients with chronic pain. Interventions that are effective in the treatment of BPD and CNCP include cognitive-behavioral therapy, dialectical behavior therapy, antidepressants, and anticonvulsants.
“These should be considered as the first-line treatment in persons with comorbid pain and BPD,” Cao said.
Commenting on the findings, Ann E. Hansen, DVM, MD, Chronic Pain Wellness Center, Phoenix VA Health Care System, Arizona, said the study illustrates the multifactorial nature of chronic pain syndromes, and underscores the importance of a multidisciplinary approach to evaluation and treatment.
“The authors present data showing that BPD is a common diagnosis in patients with chronic pain, thus raising provider awareness to consider BPD and to involve behavioral health colleagues in comanaging these complex patients to achieve optimal outcomes,” Hansen said.
Cao and Hansen have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
SOURCE: Cao F et al. American Academy of Pain Medicine (AAPM) 2020 Annual Meeting, Abstract 505.
REPORTING FROM THE AAPM 2020 ANNUAL MEETING
Washington State grapples with coronavirus outbreak
As the first COVID-19 outbreak in the United States emerges in Washington State, the city of Seattle, King County, and Washington State health officials provided the beginnings of a roadmap for how the region will address the rapidly evolving health crisis.
Health officials announced that four new cases were reported over the weekend in King County, Wash. There have now been 10 hospitalizations and 6 COVID-19 deaths at Evergreen Health, Kirkland, Wash. Of the deaths, five were King County residents and one was a resident of Snohomish County. Three patients died on March 1; all were in their 70s or 80s with comorbidities. Two had been residents of the Life Care senior residential facility that is at the center of the Kirkland outbreak. The number of cases in Washington now totals 18, with four cases in Snohomish County and the balance in neighboring King County.
Approximately 29 cases are under investigation with test results pending; a Centers for Disease Control and Prevention (CDC) team is on-site.
Speaking at a news conference March 2, officials sought to strike a balance between giving the community a realistic appraisal of the likely scope of the COVID-19 outbreak and avoiding sparking a panic.
“This is a complex and unprecedented challenge nationally, globally, and locally. The vast majority of the infected have mild or moderate disease and do not need hospitalization,” said Jeffrey Duchin, MD, health officer and chief, Communicable Disease EPI/Immunization Section, Public Health, Seattle and King County, and a professor of infectious diseases at the University of Washington, Seattle. “On the other hand, it’s obvious that this infection can cause very serious disease in people who are older and have underlying health conditions. We expect cases to continue to increase. We are taking the situation extremely seriously; the risk for all of us becoming infected is increasing. ...There is the potential for many to become ill at the same time.”
Among the measures being taken immediately are the purchase by King County of a hotel to house individuals who require isolation and those who are convalescing from the virus. Officials are also placing a number of prefabricated stand-alone housing units on public grounds in Seattle, with the recognition that the area has a large transient and homeless community. The stand-alone units will house homeless individuals who need isolation, treatment, or recuperation but who aren’t ill enough to be hospitalized.
Dr. Duchin said that testing capacity is ramping up rapidly in Washington State: The state lab can now accommodate up to about 200 tests daily, and expects to be able to do up to 1,000 daily soon. The University of Washington’s testing capacity will come online March 2 or 3 as a testing facility with similar initial and future peak testing capacities.
The testing strategy will continue to include very ill individuals with pneumonia or other respiratory illness of unknown etiology, but will also expand to include less ill people. This shift is being made in accordance with a shift in CDC guidelines, because of increased testing capacity, and to provide a better picture of the severity, scope, geography, and timing of the current COVID-19 outbreak in the greater Seattle area.
No school closures or cancellation of gatherings are currently recommended by public health authorities. There are currently no COVID-19 cases in Washington schools. The expectation is that any recommendations regarding closures will be re-evaluated as the outbreak progresses.
Repeatedly, officials asked the general public to employ basic measures such as handwashing and avoidance of touching the face, and to spare masks for the ill and for those who care for them. “The vast majority of people will not have serious illness. In turn we need to do everything we can to help those health care workers. I’m asking the public to do things like save the masks for our health care workers. …We need assets for our front-line health care workers and also for those who may be needing them,” said King County Health Department director Patty Hayes, RN, MN.
Now is also the time for households to initiate basic emergency preparedness measures, such as having adequate food and medication, and to make arrangements for childcare in the event of school closures, said several officials.
“We can decrease the impact on our health care system by reducing our individual risk. We are making individual- and community-level recommendations to limit the spread of disease. These are very similar to what we recommend for influenza,” said Dr. Duchin.
Ettore Palazzo, MD, chief medical and quality officer at EvergreenHealth, gave a sense of how the hospital is coping with being Ground Zero for COVID-19 in the United States. “We have made adjustments for airborne precautions,” he said, including transforming the entire critical care unit to a negative pressure unit. “We have these capabilities in other parts of the hospital as well.” Staff are working hard, but thus far staffing has kept pace with demand, he said, but all are feeling the strain already.
Dr. Duchin made the point that Washington is relatively well equipped to handle the increasingly likely scenario of a large spike in coronavirus cases, since it’s part of the Northwest Healthcare Response Network. The network is planning for sharing resources such as staff, respirators, and intensive care unit beds as circumstances warrant.
“What you just heard illustrates the challenge of this disease,” said Dr. Duchin, summing up. “The public health service and clinical health care delivery systems don’t have the capacity to track down every case in the community. I’m guessing we will see more cases of coronavirus than we see of influenza. At some point we will be shifting from counting every case” to focusing on outbreaks and the critically ill in hospitals, he said.
“We are still trying to contain the outbreak, but we are at the same time pivoting to a more community-based approach,” similar to the approach with influenza, said Dr. Duchin.
A summary of deaths and ongoing cases, drawn from the press release, is below:
The four new cases are:
• A male in his 50s, hospitalized at Highline Hospital. He has no known exposures. He is in stable but critical condition. He had no underlying health conditions.
• A male in his 70s, a resident of Life Care, hospitalized at EvergreenHealth in Kirkland. The man had underlying health conditions, and died March 1.
• A female in her 70s, a resident of Life Care, hospitalized at EvergreenHealth in Kirkland. The woman had underlying health conditions, and died March 1.
• A female in her 80s, a resident of Life Care, was hospitalized at EvergreenHealth. She is in critical condition.
In addition, a woman in her 80s, who was already reported as in critical condition at Evergreen, has died. She died on March 1.
Ten other cases, already reported earlier by Public Health, include:
• A female in her 80s, hospitalized at EvergreenHealth in Kirkland. This person has now died, and is reported as such above.
• A female in her 90s, hospitalized at EvergreenHealth in Kirkland. The woman has underlying health conditions, and is in critical condition.
• A male in his 70s, hospitalized at EvergreenHealth in Kirkland. The man has underlying health conditions, and is in critical condition.
• A male in his 70s was hospitalized at EvergreenHealth. He had underlying health conditions and died on Feb. 29.
• A man in his 60s, hospitalized at Valley Medical Center in Renton.
• A man in 60s, hospitalized at Virginia Mason Medical Center.
• A woman in her 50s, who had traveled to South Korea; recovering at home.
• A woman in her 70s, who was a resident of Life Care in Kirkland, hospitalized at EvergreenHealth.
• A woman in her 40s, employed by Life Care, who is hospitalized at Overlake Medical Center.
• A man in his 50s, who was hospitalized and died at EvergreenHealth.
As the first COVID-19 outbreak in the United States emerges in Washington State, the city of Seattle, King County, and Washington State health officials provided the beginnings of a roadmap for how the region will address the rapidly evolving health crisis.
Health officials announced that four new cases were reported over the weekend in King County, Wash. There have now been 10 hospitalizations and 6 COVID-19 deaths at Evergreen Health, Kirkland, Wash. Of the deaths, five were King County residents and one was a resident of Snohomish County. Three patients died on March 1; all were in their 70s or 80s with comorbidities. Two had been residents of the Life Care senior residential facility that is at the center of the Kirkland outbreak. The number of cases in Washington now totals 18, with four cases in Snohomish County and the balance in neighboring King County.
Approximately 29 cases are under investigation with test results pending; a Centers for Disease Control and Prevention (CDC) team is on-site.
Speaking at a news conference March 2, officials sought to strike a balance between giving the community a realistic appraisal of the likely scope of the COVID-19 outbreak and avoiding sparking a panic.
“This is a complex and unprecedented challenge nationally, globally, and locally. The vast majority of the infected have mild or moderate disease and do not need hospitalization,” said Jeffrey Duchin, MD, health officer and chief, Communicable Disease EPI/Immunization Section, Public Health, Seattle and King County, and a professor of infectious diseases at the University of Washington, Seattle. “On the other hand, it’s obvious that this infection can cause very serious disease in people who are older and have underlying health conditions. We expect cases to continue to increase. We are taking the situation extremely seriously; the risk for all of us becoming infected is increasing. ...There is the potential for many to become ill at the same time.”
Among the measures being taken immediately are the purchase by King County of a hotel to house individuals who require isolation and those who are convalescing from the virus. Officials are also placing a number of prefabricated stand-alone housing units on public grounds in Seattle, with the recognition that the area has a large transient and homeless community. The stand-alone units will house homeless individuals who need isolation, treatment, or recuperation but who aren’t ill enough to be hospitalized.
Dr. Duchin said that testing capacity is ramping up rapidly in Washington State: The state lab can now accommodate up to about 200 tests daily, and expects to be able to do up to 1,000 daily soon. The University of Washington’s testing capacity will come online March 2 or 3 as a testing facility with similar initial and future peak testing capacities.
The testing strategy will continue to include very ill individuals with pneumonia or other respiratory illness of unknown etiology, but will also expand to include less ill people. This shift is being made in accordance with a shift in CDC guidelines, because of increased testing capacity, and to provide a better picture of the severity, scope, geography, and timing of the current COVID-19 outbreak in the greater Seattle area.
No school closures or cancellation of gatherings are currently recommended by public health authorities. There are currently no COVID-19 cases in Washington schools. The expectation is that any recommendations regarding closures will be re-evaluated as the outbreak progresses.
Repeatedly, officials asked the general public to employ basic measures such as handwashing and avoidance of touching the face, and to spare masks for the ill and for those who care for them. “The vast majority of people will not have serious illness. In turn we need to do everything we can to help those health care workers. I’m asking the public to do things like save the masks for our health care workers. …We need assets for our front-line health care workers and also for those who may be needing them,” said King County Health Department director Patty Hayes, RN, MN.
Now is also the time for households to initiate basic emergency preparedness measures, such as having adequate food and medication, and to make arrangements for childcare in the event of school closures, said several officials.
“We can decrease the impact on our health care system by reducing our individual risk. We are making individual- and community-level recommendations to limit the spread of disease. These are very similar to what we recommend for influenza,” said Dr. Duchin.
Ettore Palazzo, MD, chief medical and quality officer at EvergreenHealth, gave a sense of how the hospital is coping with being Ground Zero for COVID-19 in the United States. “We have made adjustments for airborne precautions,” he said, including transforming the entire critical care unit to a negative pressure unit. “We have these capabilities in other parts of the hospital as well.” Staff are working hard, but thus far staffing has kept pace with demand, he said, but all are feeling the strain already.
Dr. Duchin made the point that Washington is relatively well equipped to handle the increasingly likely scenario of a large spike in coronavirus cases, since it’s part of the Northwest Healthcare Response Network. The network is planning for sharing resources such as staff, respirators, and intensive care unit beds as circumstances warrant.
“What you just heard illustrates the challenge of this disease,” said Dr. Duchin, summing up. “The public health service and clinical health care delivery systems don’t have the capacity to track down every case in the community. I’m guessing we will see more cases of coronavirus than we see of influenza. At some point we will be shifting from counting every case” to focusing on outbreaks and the critically ill in hospitals, he said.
“We are still trying to contain the outbreak, but we are at the same time pivoting to a more community-based approach,” similar to the approach with influenza, said Dr. Duchin.
A summary of deaths and ongoing cases, drawn from the press release, is below:
The four new cases are:
• A male in his 50s, hospitalized at Highline Hospital. He has no known exposures. He is in stable but critical condition. He had no underlying health conditions.
• A male in his 70s, a resident of Life Care, hospitalized at EvergreenHealth in Kirkland. The man had underlying health conditions, and died March 1.
• A female in her 70s, a resident of Life Care, hospitalized at EvergreenHealth in Kirkland. The woman had underlying health conditions, and died March 1.
• A female in her 80s, a resident of Life Care, was hospitalized at EvergreenHealth. She is in critical condition.
In addition, a woman in her 80s, who was already reported as in critical condition at Evergreen, has died. She died on March 1.
Ten other cases, already reported earlier by Public Health, include:
• A female in her 80s, hospitalized at EvergreenHealth in Kirkland. This person has now died, and is reported as such above.
• A female in her 90s, hospitalized at EvergreenHealth in Kirkland. The woman has underlying health conditions, and is in critical condition.
• A male in his 70s, hospitalized at EvergreenHealth in Kirkland. The man has underlying health conditions, and is in critical condition.
• A male in his 70s was hospitalized at EvergreenHealth. He had underlying health conditions and died on Feb. 29.
• A man in his 60s, hospitalized at Valley Medical Center in Renton.
• A man in 60s, hospitalized at Virginia Mason Medical Center.
• A woman in her 50s, who had traveled to South Korea; recovering at home.
• A woman in her 70s, who was a resident of Life Care in Kirkland, hospitalized at EvergreenHealth.
• A woman in her 40s, employed by Life Care, who is hospitalized at Overlake Medical Center.
• A man in his 50s, who was hospitalized and died at EvergreenHealth.
As the first COVID-19 outbreak in the United States emerges in Washington State, the city of Seattle, King County, and Washington State health officials provided the beginnings of a roadmap for how the region will address the rapidly evolving health crisis.
Health officials announced that four new cases were reported over the weekend in King County, Wash. There have now been 10 hospitalizations and 6 COVID-19 deaths at Evergreen Health, Kirkland, Wash. Of the deaths, five were King County residents and one was a resident of Snohomish County. Three patients died on March 1; all were in their 70s or 80s with comorbidities. Two had been residents of the Life Care senior residential facility that is at the center of the Kirkland outbreak. The number of cases in Washington now totals 18, with four cases in Snohomish County and the balance in neighboring King County.
Approximately 29 cases are under investigation with test results pending; a Centers for Disease Control and Prevention (CDC) team is on-site.
Speaking at a news conference March 2, officials sought to strike a balance between giving the community a realistic appraisal of the likely scope of the COVID-19 outbreak and avoiding sparking a panic.
“This is a complex and unprecedented challenge nationally, globally, and locally. The vast majority of the infected have mild or moderate disease and do not need hospitalization,” said Jeffrey Duchin, MD, health officer and chief, Communicable Disease EPI/Immunization Section, Public Health, Seattle and King County, and a professor of infectious diseases at the University of Washington, Seattle. “On the other hand, it’s obvious that this infection can cause very serious disease in people who are older and have underlying health conditions. We expect cases to continue to increase. We are taking the situation extremely seriously; the risk for all of us becoming infected is increasing. ...There is the potential for many to become ill at the same time.”
Among the measures being taken immediately are the purchase by King County of a hotel to house individuals who require isolation and those who are convalescing from the virus. Officials are also placing a number of prefabricated stand-alone housing units on public grounds in Seattle, with the recognition that the area has a large transient and homeless community. The stand-alone units will house homeless individuals who need isolation, treatment, or recuperation but who aren’t ill enough to be hospitalized.
Dr. Duchin said that testing capacity is ramping up rapidly in Washington State: The state lab can now accommodate up to about 200 tests daily, and expects to be able to do up to 1,000 daily soon. The University of Washington’s testing capacity will come online March 2 or 3 as a testing facility with similar initial and future peak testing capacities.
The testing strategy will continue to include very ill individuals with pneumonia or other respiratory illness of unknown etiology, but will also expand to include less ill people. This shift is being made in accordance with a shift in CDC guidelines, because of increased testing capacity, and to provide a better picture of the severity, scope, geography, and timing of the current COVID-19 outbreak in the greater Seattle area.
No school closures or cancellation of gatherings are currently recommended by public health authorities. There are currently no COVID-19 cases in Washington schools. The expectation is that any recommendations regarding closures will be re-evaluated as the outbreak progresses.
Repeatedly, officials asked the general public to employ basic measures such as handwashing and avoidance of touching the face, and to spare masks for the ill and for those who care for them. “The vast majority of people will not have serious illness. In turn we need to do everything we can to help those health care workers. I’m asking the public to do things like save the masks for our health care workers. …We need assets for our front-line health care workers and also for those who may be needing them,” said King County Health Department director Patty Hayes, RN, MN.
Now is also the time for households to initiate basic emergency preparedness measures, such as having adequate food and medication, and to make arrangements for childcare in the event of school closures, said several officials.
“We can decrease the impact on our health care system by reducing our individual risk. We are making individual- and community-level recommendations to limit the spread of disease. These are very similar to what we recommend for influenza,” said Dr. Duchin.
Ettore Palazzo, MD, chief medical and quality officer at EvergreenHealth, gave a sense of how the hospital is coping with being Ground Zero for COVID-19 in the United States. “We have made adjustments for airborne precautions,” he said, including transforming the entire critical care unit to a negative pressure unit. “We have these capabilities in other parts of the hospital as well.” Staff are working hard, but thus far staffing has kept pace with demand, he said, but all are feeling the strain already.
Dr. Duchin made the point that Washington is relatively well equipped to handle the increasingly likely scenario of a large spike in coronavirus cases, since it’s part of the Northwest Healthcare Response Network. The network is planning for sharing resources such as staff, respirators, and intensive care unit beds as circumstances warrant.
“What you just heard illustrates the challenge of this disease,” said Dr. Duchin, summing up. “The public health service and clinical health care delivery systems don’t have the capacity to track down every case in the community. I’m guessing we will see more cases of coronavirus than we see of influenza. At some point we will be shifting from counting every case” to focusing on outbreaks and the critically ill in hospitals, he said.
“We are still trying to contain the outbreak, but we are at the same time pivoting to a more community-based approach,” similar to the approach with influenza, said Dr. Duchin.
A summary of deaths and ongoing cases, drawn from the press release, is below:
The four new cases are:
• A male in his 50s, hospitalized at Highline Hospital. He has no known exposures. He is in stable but critical condition. He had no underlying health conditions.
• A male in his 70s, a resident of Life Care, hospitalized at EvergreenHealth in Kirkland. The man had underlying health conditions, and died March 1.
• A female in her 70s, a resident of Life Care, hospitalized at EvergreenHealth in Kirkland. The woman had underlying health conditions, and died March 1.
• A female in her 80s, a resident of Life Care, was hospitalized at EvergreenHealth. She is in critical condition.
In addition, a woman in her 80s, who was already reported as in critical condition at Evergreen, has died. She died on March 1.
Ten other cases, already reported earlier by Public Health, include:
• A female in her 80s, hospitalized at EvergreenHealth in Kirkland. This person has now died, and is reported as such above.
• A female in her 90s, hospitalized at EvergreenHealth in Kirkland. The woman has underlying health conditions, and is in critical condition.
• A male in his 70s, hospitalized at EvergreenHealth in Kirkland. The man has underlying health conditions, and is in critical condition.
• A male in his 70s was hospitalized at EvergreenHealth. He had underlying health conditions and died on Feb. 29.
• A man in his 60s, hospitalized at Valley Medical Center in Renton.
• A man in 60s, hospitalized at Virginia Mason Medical Center.
• A woman in her 50s, who had traveled to South Korea; recovering at home.
• A woman in her 70s, who was a resident of Life Care in Kirkland, hospitalized at EvergreenHealth.
• A woman in her 40s, employed by Life Care, who is hospitalized at Overlake Medical Center.
• A man in his 50s, who was hospitalized and died at EvergreenHealth.
FROM A KING COUNTY, WASH. NEWS BRIEFING
Bad behavior by medical trainees target of new proposal
Some instances of unprofessional behavior by medical trainees are universally deemed egregious and worthy of discipline — for example, looking up a friend’s medical data after HIPAA training.
Conversely, some professionalism lapses may be widely thought of as a teaching and consoling moment, such as the human error involved in forgetting a scheduled repositioning of a patient.
But between the extremes is a vast gray area. To deal with those cases appropriately, Jason Wasserman, PhD, and colleagues propose a new framework by which to judge each infraction.
The framework draws from “just culture” concepts used to evaluate medical errors, Wasserman, associate professor of biomedical science at Oakland University William Beaumont School of Medicine in Rochester, Michigan, told Medscape Medical News. Such an approach takes into account the environment in which the error was made, the knowledge and intent of the person making the error, and the severity and consequences of the infraction so that trainees and institutions can learn from mistakes.
“Trainees by definition are not going to fully get it,” he explained. “By definition they’re not going to fully achieve professional expectations. So how can we respond to the things we need to respond to, but do it in a way that’s educational?”
Wasserman and coauthors’ framework for remediation, which they published February 20 in The New England Journal of Medicine, takes into account several questions: Was the expectation clear? Were there factors beyond the trainees› control? What were the trainees› intentions and did they understand the consequences? Did the person genuinely believe the action was inconsequential?
An example requiring discipline, the authors say, would be using a crib sheet during an exam. In that case the intent is clear, there is no defensible belief that the action is inconsequential, and there is a clear understanding the action is wrong.
But a response of “affirm, support, and advise” is more appropriate, for example, when a student’s alarm doesn’t go off after a power outage and they miss a mandatory meeting.
Wasserman points out that this framework won’t cover all situations.
“This is not an algorithm for answering your questions about what to do,” he said. “It’s an architecture for clarifying the discussion about that. It can really tease out all the threads that need to be considered to best respond to and correct the professionalism lapse, but do it in a way that is developmentally appropriate.”
A Core Competency
For two decades, professionalism has been considered a core competency of medical education. In 1999, the Accreditation Council for Graduate Medical Education and the American Board of Medical Specialties formalized it as such. In 2013, the Association of American Medical Colleges formally required related professionalism competencies.
However, identifying lapses has operated largely on an “I-know-it-when-I-see-it” basis, leading to widely varying remediation practices judged by a small number of faculty members or administrators.
The ideas outlined by Wasserman and colleagues are “a terrific application of the ‘just-culture’ framework,” according to Nicole Treadway, MD, a first-year primary care resident at Emory School of Medicine in Atlanta, Georgia.
At Emory, discussions of professionalism start from day 1 of medical school and the subject is revisited throughout training in small groups, Treadway told Medscape Medical News.
But, she said, as the authors point out, definitions of unprofessionalism are not always clear and the examples the authors put forward help put lapses in context.
The framework also allows for looking at mistakes in light of the stress trainees encounter and the greater chance of making a professionalism error in those situations, she noted.
In her own work, she says, because she is juggling both inpatient and outpatient care, she is finding it is easy to get behind on correspondence or communicating lab results or having follow-up conversations.
Those delays could be seen as lapses in professionalism, but under this framework, there may be system solutions or training opportunities to consider.
“We do need this organizational architecture, and I think it could serve us well in really helping us identify and appropriately respond to what we see regarding professionalism,” she said.
Framework Helps Standardize Thinking
She said having a universal framework also helps because while standards of professionalism are easier to monitor in a single medical school, when students scatter to other hospitals for clinical training, those hospitals may have different professionalism standards.
Wasserman agrees, saying, “This could be easily adopted in any environment where people deal with professionalism lapses. I don’t even think it’s necessarily relegated to trainees. It’s a great way to think about any kind of lapses, just as hospitals think about medical errors.”
He said the next step is presenting the framework at various medical schools for feedback and research to see whether the framework improves processes.
Potential criticism, he said, might come from those who say such a construct avoids punishing students who make errors.
“There will always be people who say we’re pandering to medical students whenever we worry about the learning environment,” he said. “There are old-school purists who say when people screw up you should punish them.”
But he adds healthcare broadly has moved past that thinking.
“People recognized 20 years ago or more from the standpoint of improving healthcare systems and safety that is a bad strategy. You’ll never get error-free humans working in your system, and what you have to do is consider how the system is functioning and think about ways to optimize the system so people can be their best within it.”
Wasserman and Treadway have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
Some instances of unprofessional behavior by medical trainees are universally deemed egregious and worthy of discipline — for example, looking up a friend’s medical data after HIPAA training.
Conversely, some professionalism lapses may be widely thought of as a teaching and consoling moment, such as the human error involved in forgetting a scheduled repositioning of a patient.
But between the extremes is a vast gray area. To deal with those cases appropriately, Jason Wasserman, PhD, and colleagues propose a new framework by which to judge each infraction.
The framework draws from “just culture” concepts used to evaluate medical errors, Wasserman, associate professor of biomedical science at Oakland University William Beaumont School of Medicine in Rochester, Michigan, told Medscape Medical News. Such an approach takes into account the environment in which the error was made, the knowledge and intent of the person making the error, and the severity and consequences of the infraction so that trainees and institutions can learn from mistakes.
“Trainees by definition are not going to fully get it,” he explained. “By definition they’re not going to fully achieve professional expectations. So how can we respond to the things we need to respond to, but do it in a way that’s educational?”
Wasserman and coauthors’ framework for remediation, which they published February 20 in The New England Journal of Medicine, takes into account several questions: Was the expectation clear? Were there factors beyond the trainees› control? What were the trainees› intentions and did they understand the consequences? Did the person genuinely believe the action was inconsequential?
An example requiring discipline, the authors say, would be using a crib sheet during an exam. In that case the intent is clear, there is no defensible belief that the action is inconsequential, and there is a clear understanding the action is wrong.
But a response of “affirm, support, and advise” is more appropriate, for example, when a student’s alarm doesn’t go off after a power outage and they miss a mandatory meeting.
Wasserman points out that this framework won’t cover all situations.
“This is not an algorithm for answering your questions about what to do,” he said. “It’s an architecture for clarifying the discussion about that. It can really tease out all the threads that need to be considered to best respond to and correct the professionalism lapse, but do it in a way that is developmentally appropriate.”
A Core Competency
For two decades, professionalism has been considered a core competency of medical education. In 1999, the Accreditation Council for Graduate Medical Education and the American Board of Medical Specialties formalized it as such. In 2013, the Association of American Medical Colleges formally required related professionalism competencies.
However, identifying lapses has operated largely on an “I-know-it-when-I-see-it” basis, leading to widely varying remediation practices judged by a small number of faculty members or administrators.
The ideas outlined by Wasserman and colleagues are “a terrific application of the ‘just-culture’ framework,” according to Nicole Treadway, MD, a first-year primary care resident at Emory School of Medicine in Atlanta, Georgia.
At Emory, discussions of professionalism start from day 1 of medical school and the subject is revisited throughout training in small groups, Treadway told Medscape Medical News.
But, she said, as the authors point out, definitions of unprofessionalism are not always clear and the examples the authors put forward help put lapses in context.
The framework also allows for looking at mistakes in light of the stress trainees encounter and the greater chance of making a professionalism error in those situations, she noted.
In her own work, she says, because she is juggling both inpatient and outpatient care, she is finding it is easy to get behind on correspondence or communicating lab results or having follow-up conversations.
Those delays could be seen as lapses in professionalism, but under this framework, there may be system solutions or training opportunities to consider.
“We do need this organizational architecture, and I think it could serve us well in really helping us identify and appropriately respond to what we see regarding professionalism,” she said.
Framework Helps Standardize Thinking
She said having a universal framework also helps because while standards of professionalism are easier to monitor in a single medical school, when students scatter to other hospitals for clinical training, those hospitals may have different professionalism standards.
Wasserman agrees, saying, “This could be easily adopted in any environment where people deal with professionalism lapses. I don’t even think it’s necessarily relegated to trainees. It’s a great way to think about any kind of lapses, just as hospitals think about medical errors.”
He said the next step is presenting the framework at various medical schools for feedback and research to see whether the framework improves processes.
Potential criticism, he said, might come from those who say such a construct avoids punishing students who make errors.
“There will always be people who say we’re pandering to medical students whenever we worry about the learning environment,” he said. “There are old-school purists who say when people screw up you should punish them.”
But he adds healthcare broadly has moved past that thinking.
“People recognized 20 years ago or more from the standpoint of improving healthcare systems and safety that is a bad strategy. You’ll never get error-free humans working in your system, and what you have to do is consider how the system is functioning and think about ways to optimize the system so people can be their best within it.”
Wasserman and Treadway have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
Some instances of unprofessional behavior by medical trainees are universally deemed egregious and worthy of discipline — for example, looking up a friend’s medical data after HIPAA training.
Conversely, some professionalism lapses may be widely thought of as a teaching and consoling moment, such as the human error involved in forgetting a scheduled repositioning of a patient.
But between the extremes is a vast gray area. To deal with those cases appropriately, Jason Wasserman, PhD, and colleagues propose a new framework by which to judge each infraction.
The framework draws from “just culture” concepts used to evaluate medical errors, Wasserman, associate professor of biomedical science at Oakland University William Beaumont School of Medicine in Rochester, Michigan, told Medscape Medical News. Such an approach takes into account the environment in which the error was made, the knowledge and intent of the person making the error, and the severity and consequences of the infraction so that trainees and institutions can learn from mistakes.
“Trainees by definition are not going to fully get it,” he explained. “By definition they’re not going to fully achieve professional expectations. So how can we respond to the things we need to respond to, but do it in a way that’s educational?”
Wasserman and coauthors’ framework for remediation, which they published February 20 in The New England Journal of Medicine, takes into account several questions: Was the expectation clear? Were there factors beyond the trainees› control? What were the trainees› intentions and did they understand the consequences? Did the person genuinely believe the action was inconsequential?
An example requiring discipline, the authors say, would be using a crib sheet during an exam. In that case the intent is clear, there is no defensible belief that the action is inconsequential, and there is a clear understanding the action is wrong.
But a response of “affirm, support, and advise” is more appropriate, for example, when a student’s alarm doesn’t go off after a power outage and they miss a mandatory meeting.
Wasserman points out that this framework won’t cover all situations.
“This is not an algorithm for answering your questions about what to do,” he said. “It’s an architecture for clarifying the discussion about that. It can really tease out all the threads that need to be considered to best respond to and correct the professionalism lapse, but do it in a way that is developmentally appropriate.”
A Core Competency
For two decades, professionalism has been considered a core competency of medical education. In 1999, the Accreditation Council for Graduate Medical Education and the American Board of Medical Specialties formalized it as such. In 2013, the Association of American Medical Colleges formally required related professionalism competencies.
However, identifying lapses has operated largely on an “I-know-it-when-I-see-it” basis, leading to widely varying remediation practices judged by a small number of faculty members or administrators.
The ideas outlined by Wasserman and colleagues are “a terrific application of the ‘just-culture’ framework,” according to Nicole Treadway, MD, a first-year primary care resident at Emory School of Medicine in Atlanta, Georgia.
At Emory, discussions of professionalism start from day 1 of medical school and the subject is revisited throughout training in small groups, Treadway told Medscape Medical News.
But, she said, as the authors point out, definitions of unprofessionalism are not always clear and the examples the authors put forward help put lapses in context.
The framework also allows for looking at mistakes in light of the stress trainees encounter and the greater chance of making a professionalism error in those situations, she noted.
In her own work, she says, because she is juggling both inpatient and outpatient care, she is finding it is easy to get behind on correspondence or communicating lab results or having follow-up conversations.
Those delays could be seen as lapses in professionalism, but under this framework, there may be system solutions or training opportunities to consider.
“We do need this organizational architecture, and I think it could serve us well in really helping us identify and appropriately respond to what we see regarding professionalism,” she said.
Framework Helps Standardize Thinking
She said having a universal framework also helps because while standards of professionalism are easier to monitor in a single medical school, when students scatter to other hospitals for clinical training, those hospitals may have different professionalism standards.
Wasserman agrees, saying, “This could be easily adopted in any environment where people deal with professionalism lapses. I don’t even think it’s necessarily relegated to trainees. It’s a great way to think about any kind of lapses, just as hospitals think about medical errors.”
He said the next step is presenting the framework at various medical schools for feedback and research to see whether the framework improves processes.
Potential criticism, he said, might come from those who say such a construct avoids punishing students who make errors.
“There will always be people who say we’re pandering to medical students whenever we worry about the learning environment,” he said. “There are old-school purists who say when people screw up you should punish them.”
But he adds healthcare broadly has moved past that thinking.
“People recognized 20 years ago or more from the standpoint of improving healthcare systems and safety that is a bad strategy. You’ll never get error-free humans working in your system, and what you have to do is consider how the system is functioning and think about ways to optimize the system so people can be their best within it.”
Wasserman and Treadway have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
The fate of the ACA now rests with the U.S. Supreme Court
The U.S. Supreme Court has agreed to hear Texas v. California, a closely watched case that could upend the Affordable Care Act.
The justices will hear oral arguments in the case in fall 2020, with a ruling likely in 2021.
The Texas case, consolidated with a similar challenge, stems from a lawsuit by 20 Republican state attorneys general and governors that was filed after Congress zeroed out the ACA’s individual mandate penalty in 2017. The plaintiffs contend the now-valueless mandate is no longer constitutional and thus, the entire ACA should be struck down. Since the Trump administration declined to defend the ACA, a coalition of Democratic attorneys general and governors intervened in the case as defendants.
In 2018, a Texas district court ruled in favor of the plaintiffs and declared the entire health care law invalid. The 5th U.S. Circuit Court of Appeals partially affirmed the district court’s decision, ruling that the mandate was unconstitutional, but sending the case back to the lower court for more analysis on severability. On March 2, the U.S. Supreme Court granted two petitions by the defendants requesting that the high court review the appeals court decision.
The review follows a previous look at the ACA’s mandate by the Supreme Court in 2012. In National Federation of Independent Business v. Sebelius, justices upheld the ACA’s insurance mandate as constitutional, ruling the requirement was authorized by Congress’ power to levy taxes. The vote was 5-4, with Chief Justice John G. Roberts Jr. in agreement with the court’s four more liberal members.
The U.S. Supreme Court has agreed to hear Texas v. California, a closely watched case that could upend the Affordable Care Act.
The justices will hear oral arguments in the case in fall 2020, with a ruling likely in 2021.
The Texas case, consolidated with a similar challenge, stems from a lawsuit by 20 Republican state attorneys general and governors that was filed after Congress zeroed out the ACA’s individual mandate penalty in 2017. The plaintiffs contend the now-valueless mandate is no longer constitutional and thus, the entire ACA should be struck down. Since the Trump administration declined to defend the ACA, a coalition of Democratic attorneys general and governors intervened in the case as defendants.
In 2018, a Texas district court ruled in favor of the plaintiffs and declared the entire health care law invalid. The 5th U.S. Circuit Court of Appeals partially affirmed the district court’s decision, ruling that the mandate was unconstitutional, but sending the case back to the lower court for more analysis on severability. On March 2, the U.S. Supreme Court granted two petitions by the defendants requesting that the high court review the appeals court decision.
The review follows a previous look at the ACA’s mandate by the Supreme Court in 2012. In National Federation of Independent Business v. Sebelius, justices upheld the ACA’s insurance mandate as constitutional, ruling the requirement was authorized by Congress’ power to levy taxes. The vote was 5-4, with Chief Justice John G. Roberts Jr. in agreement with the court’s four more liberal members.
The U.S. Supreme Court has agreed to hear Texas v. California, a closely watched case that could upend the Affordable Care Act.
The justices will hear oral arguments in the case in fall 2020, with a ruling likely in 2021.
The Texas case, consolidated with a similar challenge, stems from a lawsuit by 20 Republican state attorneys general and governors that was filed after Congress zeroed out the ACA’s individual mandate penalty in 2017. The plaintiffs contend the now-valueless mandate is no longer constitutional and thus, the entire ACA should be struck down. Since the Trump administration declined to defend the ACA, a coalition of Democratic attorneys general and governors intervened in the case as defendants.
In 2018, a Texas district court ruled in favor of the plaintiffs and declared the entire health care law invalid. The 5th U.S. Circuit Court of Appeals partially affirmed the district court’s decision, ruling that the mandate was unconstitutional, but sending the case back to the lower court for more analysis on severability. On March 2, the U.S. Supreme Court granted two petitions by the defendants requesting that the high court review the appeals court decision.
The review follows a previous look at the ACA’s mandate by the Supreme Court in 2012. In National Federation of Independent Business v. Sebelius, justices upheld the ACA’s insurance mandate as constitutional, ruling the requirement was authorized by Congress’ power to levy taxes. The vote was 5-4, with Chief Justice John G. Roberts Jr. in agreement with the court’s four more liberal members.
Second-generation long-acting injectable antipsychotics: A practical guide
There are currently 7 FDA-approved second-generation long-acting injectable antipsychotics (LAIAs).1-7 These LAIAs provide a unique dosage form that allows patients to receive an antipsychotic without taking oral medications every day, or multiple times per day. This may be an appealing option for patients and clinicians, but because there are several types of LAIAs available, it may be difficult to determine which LAIA characteristics are best for a given patient.
Since the FDA approved the first second-generation LAIA, risperidone long-acting injectable (LAI),1 in 2003, 6 additional second-generation LAIAs have been approved:
- aripiprazole LAI
- aripiprazole lauroxil LAI
- olanzapine pamoate LAI
- paliperidone palmitate monthly injection
- paliperidone palmitate 3-month LAI
- risperidone LAI for subcutaneous (SQ) injection.
When discussing medication options with patients, clinicians need to consider factors that are unique to each LAIA. In this article, I describe the similarities and differences among the second-generation LAIAs, and address common questions about these medications.
A major potential benefit: Increased adherence
One potential benefit of all LAIAs is increased medication adherence compared with oral antipsychotics. One meta-analysis of 21 randomized controlled trials (RCTs) that compared LAIAs with oral antipsychotics and included 5,176 patients found that LAIAs had a similar efficacy to oral antipsychotics in preventing relapse.8 However, a meta-analysis of 25 mirror-image studies comparing LAIAs with oral antipsychotics that included 5,940 patients found that LAIAs were superior in preventing hospitalization.9 In these mirror-image studies, participants received oral antipsychotics first and then switched to LAIAs, and the 2 study periods were compared. Because mirror-image studies are observational, participants do not engage with research teams to the extent that they do in RCTs.9 Although mirror-image studies have limitations, participants in these studies may be a better representation of patients encountered in clinical practice due to the extensive monitoring and follow-up RCT participants typically receive.9
Differences in FDA-approved indications
The 7 currently available LAIAs vary in terms of FDA-approved indications, dose options, frequency, need for oral antipsychotic overlap, route of administration, and other factors. Table 11-7 summarizes some of these differences. Although all second-generation LAIAs are approved for schizophrenia,1-7 risperidone LAI and aripiprazole LAI are also approved for bipolar I disorder.1,4 Paliperidone palmitate monthly injection is the only LAIA approved for treating patients with schizoaffective disorder.2
Starting doses
For most LAIAs, the starting dose is the same as the maintenance dose (Table 11-7). One exception is paliperidone palmitate monthly injection, which requires a 234-mg dose on Day 1 followed by a 156-mg dose on Day 8 for all patients, regardless of the maintenance dose required.2 The 156-mg dose may be given 4 days before or after Day 8.2 The first maintenance dose of paliperidone palmitate monthly injection should be administered 5 weeks after the 234-mg dose on Day 1.2 Before starting paliperidone palmitate 3-month injection, patients should be stable on paliperidone palmitate monthly injection for 4 months, and the 2 most recent doses of paliperidone palmitate monthly injection should be the same.3
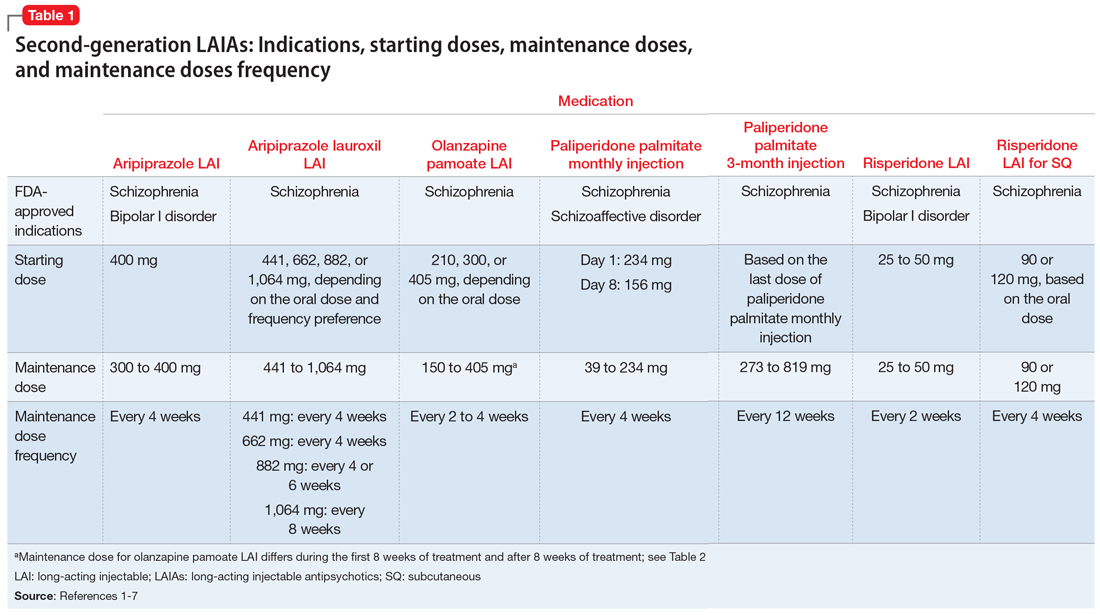
Maintenance doses
Dosing frequency may be an important factor for some patients when deciding to receive a LAIA. The frequency of the maintenance doses for all second-generation LAIAs varies from every 2 weeks to 12 weeks (Table 11-7). Paliperidone palmitate 3-month LAI is the only LAIA that is administered every 12 weeks.3 Some dosages of aripiprazole lauroxil LAI are administered every 6 or 8 weeks.6 All other second-generation LAIAs are given every 2 to 4 weeks.
Continue to: Start with an oral antipsychotic
Start with an oral antipsychotic
Before starting any LAIA, patients should receive the oral formulation of that antipsychotic to establish tolerability.1-7 Four of the 7 available LAIAs have an oral-to-LAI dose equivalency recommendation in their prescribing information (Table 22,5-7). This can help clinicians estimate the LAIA maintenance dose required to control a patient’s symptoms. If a dose adjustment is needed once a patient starts an LAIA, the dose adjustment can be made when the next injection is due.2
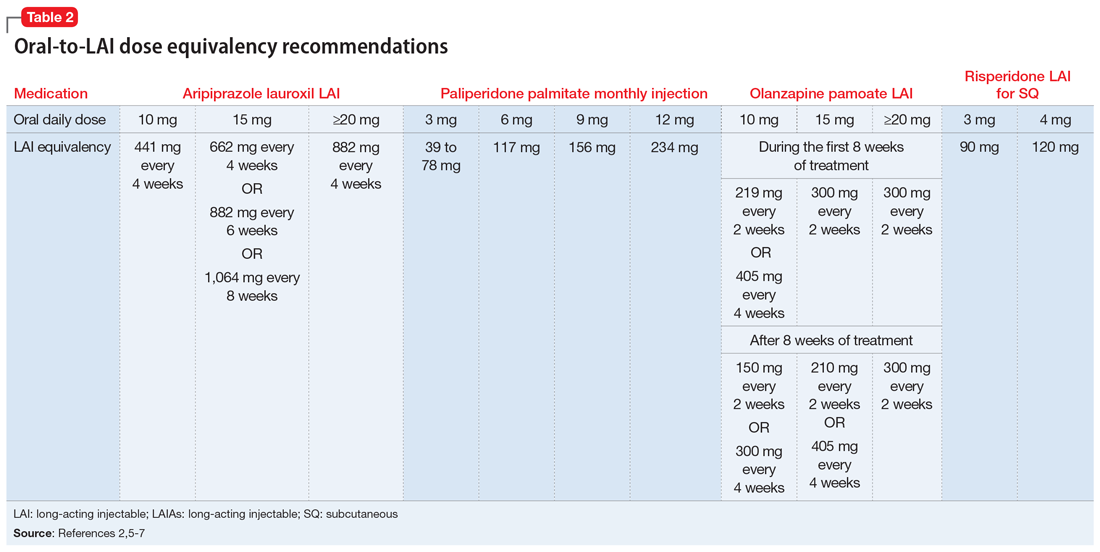
There are 2 important considerations when prescribing olanzapine pamoate LAI. First, the recommended dose for olanzapine pamoate LAI based on oral olanzapine doses differs during the first 8 weeks of treatment compared with after 8 weeks of treatment (Table 22,5-7). Additionally, because there are both short-acting and long-acting injections of olanzapine, it is essential to choose the correct formulation when prescribing this medication.5
Overlap with an oral antipsychotic might be necessary
Administration of several of the LAIAs may require overlap with an oral antipsychotic (Table 31,2,4-7). Patients who refuse to take oral medications may benefit from one of the LAIAs that does not require oral overlap—paliperidone palmitate monthly injection, olanzapine pamoate LAI, and risperidone LAI for SQ.2,5,7 Risperidone LAI requires overlap with oral risperidone for 3 weeks.1
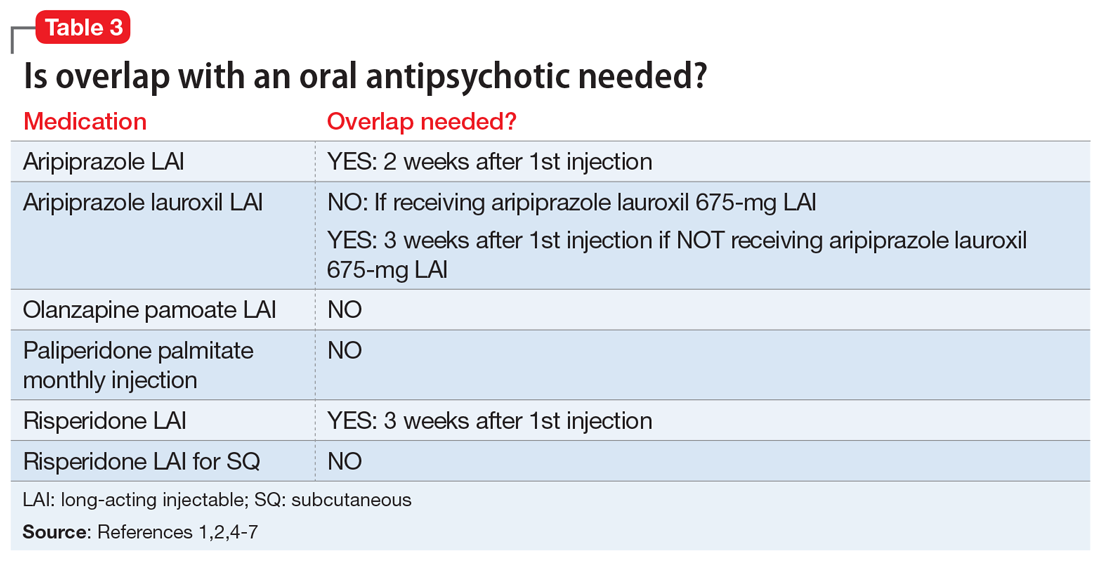
Aripiprazole is available in 2 LAI formulations: aripiprazole LAI and aripiprazole lauroxil LAI. Aripiprazole lauroxil is a prodrug of aripiprazole, and these 2 LAI medications differ in available dose options and dosing frequency.4,6 Aripiprazole LAI requires an oral overlap for 2 weeks after the first injection, whereas aripiprazole lauroxil LAI requires 3 weeks of oral overlap unless aripiprazole lauroxil 675-mg LAI is administered (Figure6).4,6,10
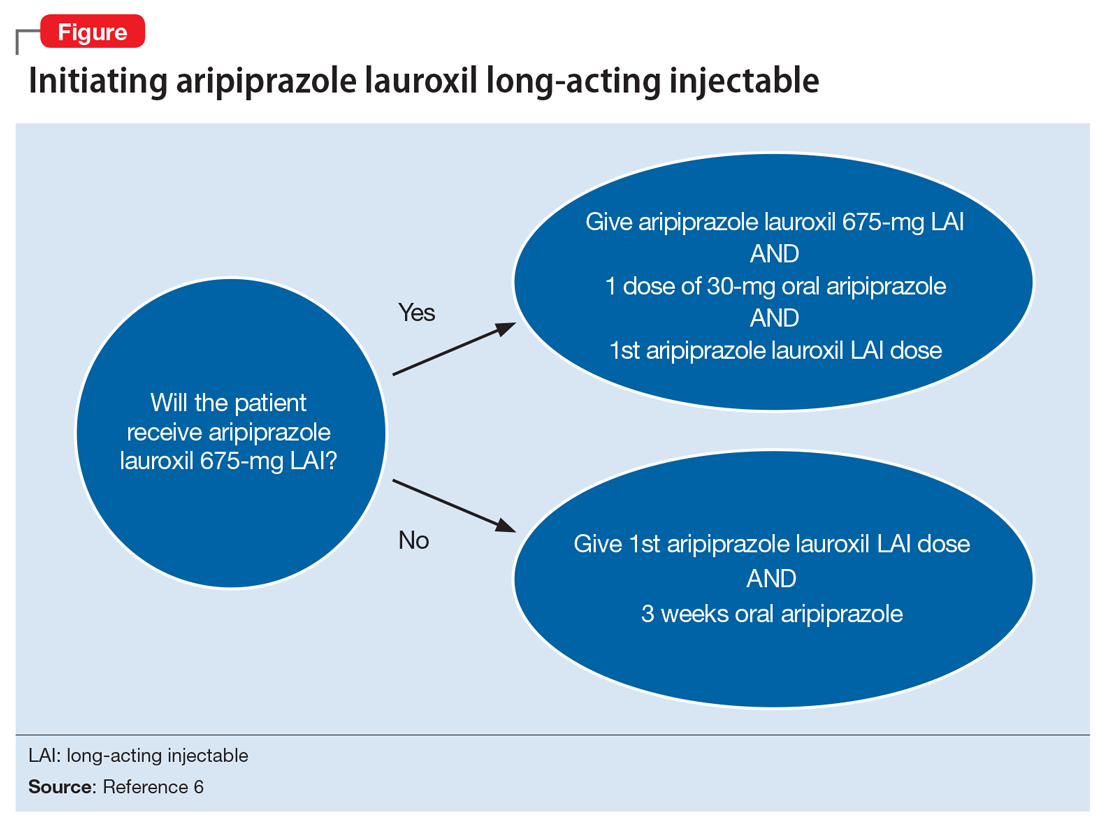
Aripiprazole lauroxil 675-mg LAI is formulated with drug particles that are smaller than those in aripiprazole lauroxil LAI.11 The smaller particle size results in faster dissolution and a more rapid increase in plasma aripiprazole levels. Aripiprazole lauroxil 675-mg LAI is a single injection that should be given with one 30-mg dose of oral aripiprazole.10 This combination results in aripiprazole concentrations that are comparable to aripiprazole lauroxil LAI and oral aripiprazole overlap for 3 weeks after the first injection.10
Continue to: The starting dose of aripiprazole lauroxil LAI...
The starting dose of aripiprazole lauroxil LAI may be administered on the same day as aripiprazole lauroxil 675-mg LAI and the 30-mg oral aripiprazole dose, or it may be administered up to 10 days after.10 Aripiprazole lauroxil LAI and aripiprazole lauroxil 675-mg LAI are not interchangeable due to differing pharmacokinetic profiles.6,10 Aripiprazole lauroxil 675-mg LAI may be used to re-initiate treatment in a patient who missed doses of aripiprazole lauroxil LAI.10 Aripiprazole lauroxil LAI and aripiprazole lauroxil 675 mg should not be injected together into the same deltoid or gluteal muscle.
Be mindful of differences in dosing windows
Each LAIA has a specific frequency recommendation, but due to scheduling or other factors, it may not be possible for patients to receive their injection on the specified day. The prescribing information for some LAIAs provides a dosing window (Table 41-7). The prescribing information for risperidone LAI, olanzapine pamoate LAI, and risperidone LAI for SQ does not specify how many days the injection can be administered before or after the due date; however, the prescribing information for risperidone LAI for SQ indicates that if the injection is not given on the due date, it should be administered as soon as possible after that.1,5,7
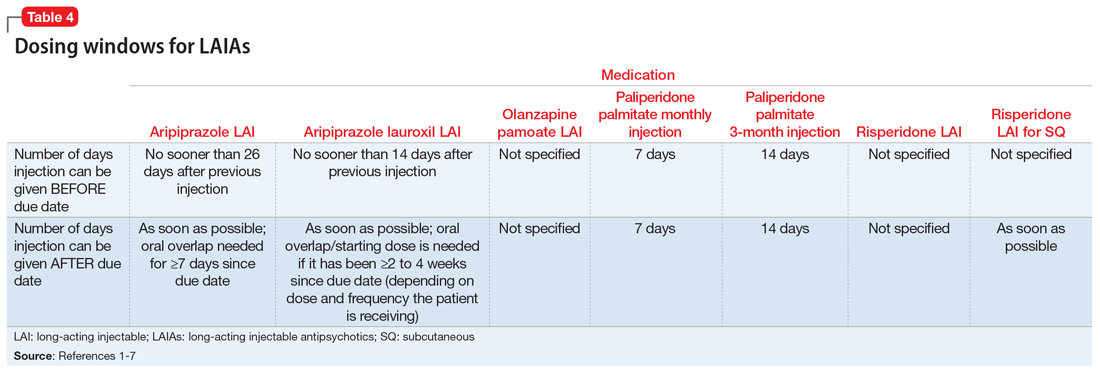
Paliperidone palmitate monthly injection and paliperidone palmitate 3-month LAI have the clearest recommendations for a dosing window. Paliperidone palmitate monthly injection may be administered 7 days before or after the 4-week due date, and paliperidone palmitate 3-month LAI can be administered 14 days before or after the 12-week due date.2,3
Aripiprazole LAI should not be administered sooner than 26 days after the previous injection, which means that it can be administered up to 2 days before the 4-week due date.4 If administered after the due date, it should be given as soon as possible, although oral overlap is not needed until ≥7 days past the due date.4
Aripiprazole lauroxil LAI has similar recommendations to aripiprazole LAI in that it should not be administered sooner than 14 days after the previous injection.6 If it is given after the due date, it should be administered as soon as possible; oral overlap/starting dose is needed if it has been ≥2 to 4 weeks since the due date, depending on which dose and frequency the patient is receiving.6
Continue to: Recommendations for missed doses
Recommendations for missed doses
Each LAIA has specific recommendations for missed dosing. Carpenter and Wong12 reviewed the recommendations for managing missed LAIA doses in
Consider patient preference
Patient preference for the type and location of the injection may factor into a clinician’s choice of LAIA (Table 51-7,10). Risperidone LAI for SQ is the only LAIA that is administered as an SQ abdominal injection.7 All other LAIAs are IM injections in the deltoid or gluteal muscle.1-6 All doses of risperidone LAI, paliperidone palmitate 3-month LAI, aripiprazole LAI, and aripiprazole lauroxil 675-mg LAI can be administered in the deltoid or gluteal muscle.1,3,4,10 Deltoid administration is required for the 2 starting doses of paliperidone palmitate monthly injection, but maintenance doses can be administered in the deltoid or gluteal muscle. Because administration into the deltoid results in a higher concentration of the drug compared with gluteal administration, administering the 2 starting doses of paliperidone palmitate monthly injection into the deltoid helps to rapidly attain therapeutic concentrations.2 Olanzapine pamoate LAI should be administered only in the gluteal muscle.5 The 441-mg dose of aripiprazole lauroxil LAI may be administered in the deltoid or gluteal muscle, but all other doses of aripiprazole lauroxil LAI should be administered only in the gluteal muscle.6
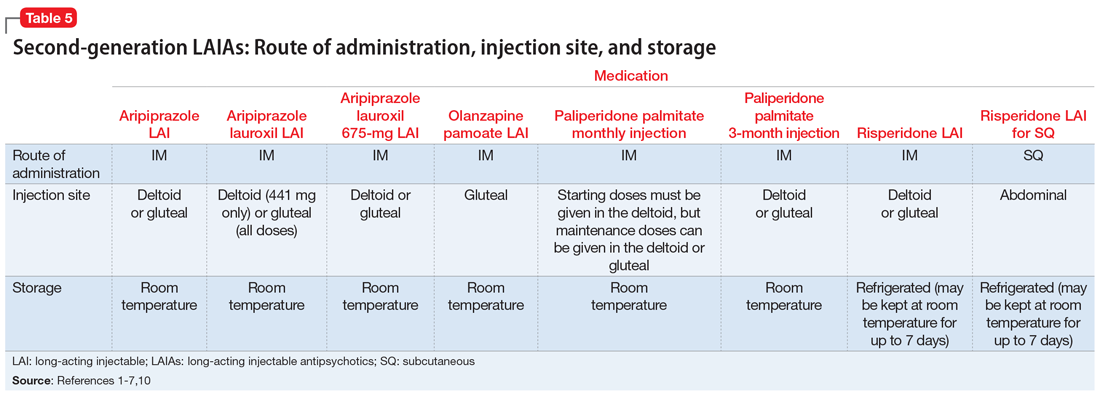
Storage
Most LAIAs can be stored at room temperature2-6; however, risperidone LAI and risperidone LAI for SQ need to be stored in the refrigerator. Both risperidone LAI and risperidone LAI for SQ may be kept at room temperature for up to 7 days. If they are not used within 7 days at room temperature, they should be discarded.1,7
Clinical pearls for specific LAIAs
Aripiprazole LAI. The recommended starting and maintenance dose for aripiprazole LAI is 400 mg monthly, unless the patient has drug interactions or other factors that require dose adjustment. If patients experience adverse reactions to the 400-mg dose, a reduction to 300 mg monthly could be considered.4
Olanzapine pamoate LAI has a Risk Evaluation and Mitigation Strategy (REMS) due to the potential for post-injection delirium/sedation syndrome (PDSS). Prescribing clinicians, dispensing pharmacies, and administering health care facilities must all be certified to prescribe, dispense, or administer olanzapine pamoate LAI. The patient must also be enrolled in the REMS program.13 Patients must be observed by health care staff for 3 hours after receiving a dose of olanzapine pamoate LAI to monitor for signs and symptoms of PDSS.5
Continue to: Risperidone LAI
Risperidone LAI. When increasing the dose of risperidone LAI, do not expect to see the clinical effects of the new dose earlier than 3 weeks after initiating the higher dose, because the main release of the medication starts at 3 weeks after the injection.1
Risperidone LAI for SQ has specific recommendations for the LAI dose based on whether the patient was stable when receiving 3 or 4 mg/d of oral risperidone. If patients are stable on <3 or >4 mg/d, they may not be candidates for risperidone LAI for SQ.7
Table 61-7,10 lists additional factors to consider when prescribing a specific LAIA.
Bottom Line
Second-generation long-acting injectable antipsychotics (LAIAs) have the potential to increase medication adherence. There are important differences among the 7 currently available LAIAs. For effective prescribing, clinicians need to understand each medication’s unique aspects, including dosing options, frequency, need for oral antipsychotic overlap, and route of administration.
Related Resources
- Correll CU, Citrome L, Haddad PM, et al. The use of long-acting injectable antipsychotics in schizophrenia: evaluating the evidence. J Clin Psychiatry. 2016;77(suppl 3):1-24.
- Peters L, Krogmann A, von Hardenberg L, et al. Long-acting injections in schizophrenia: a 3-year update on randomized controlled trials published January 2016-March 2019. Curr Psychiatry Rep. 2019;21(12):124.
Drug Brand Names
Aripiprazole • Abilify
Aripiprazole long-acting injectable • Abilify Maintena
Aripiprazole lauroxil extended-release injectable suspension • Aristada
Aripiprazole lauroxil 675 mg • Aristada Initio
Olanzapine pamoate long-acting injection • Zyprexa Relprevv
Paliperidone palmitate monthly long-acting injection • Invega Sustenna
Paliperidone palmitate 3-month injection • Invega Trinza
Risperidone • Risperdal
Risperidone long-acting injection • Risperdal Consta
Risperidone long-acting injection for SQ • Perseris
1. Risperdal Consta [package insert]. Titusville, NJ: Janssen Pharmaceuticals, Inc.; 2019.
2. Invega Sustenna [package insert]. Titusville, NJ: Janssen Pharmaceuticals, Inc.; 2019.
3. Invega Trinza [package insert]. Titusville, NJ: Janssen Pharmaceuticals, Inc.; 2019.
4. Abilify Maintena [package insert]. Rockville, MD: Otsuka America Pharmaceutical, Inc.; 2019.
5. Zyprexa Relprevv [package insert]. Indianapolis; IN: Eli Lilly and Co.; 2019.
6. Aristada [package insert]. Waltham, MA: Alkermes, Inc.; 2019.
7. Perseris [package insert]. North Chesterfield, VA: Indivior, Inc.; 2018.
8. Kishimoto T, Robenzadeh A, Leucht C, et al. Long-acting injectable vs oral antipsychotics for relapse prevention in schizophrenia: a meta-analysis of randomized trials. Schizophr Bull. 2014;40(1):192-213.
9. Kishimoto T, Nitta M, Borenstein M, et al. Long-acting injectable versus oral antipsychotics in schizophrenia: a systematic review and meta-analysis of mirror-image studies. J Clin Psychiatry. 2013;74(10):957-965.
10. Aristada Initio [package insert]. Waltham, MA: Alkermes, Inc.; 2019.
11. Jain R, Meyer J, Wehr A, et al. Size matters: the importance of particle size in a newly developed injectable formulation for the treatment of schizophrenia. CNS Spectr. 2019:1-8.
12. Carpenter J, Wong KK. Long-acting injectable antipsychotics: what to do about missed doses. Current Psychiatry. 2018;17(7):10-12,14-19,56.
13. US Food and Drug Administration. Approved Risk Evaluation and Mitigation Strategies (REMS) zyprexa relprevv (olanzapine). https://www.accessdata.fda.gov/scripts/cder/rems/index.cfm?event=IndvRemsDetails.page&REMS=74. Updated April 11, 2019. Accessed January 27, 2020.
There are currently 7 FDA-approved second-generation long-acting injectable antipsychotics (LAIAs).1-7 These LAIAs provide a unique dosage form that allows patients to receive an antipsychotic without taking oral medications every day, or multiple times per day. This may be an appealing option for patients and clinicians, but because there are several types of LAIAs available, it may be difficult to determine which LAIA characteristics are best for a given patient.
Since the FDA approved the first second-generation LAIA, risperidone long-acting injectable (LAI),1 in 2003, 6 additional second-generation LAIAs have been approved:
- aripiprazole LAI
- aripiprazole lauroxil LAI
- olanzapine pamoate LAI
- paliperidone palmitate monthly injection
- paliperidone palmitate 3-month LAI
- risperidone LAI for subcutaneous (SQ) injection.
When discussing medication options with patients, clinicians need to consider factors that are unique to each LAIA. In this article, I describe the similarities and differences among the second-generation LAIAs, and address common questions about these medications.
A major potential benefit: Increased adherence
One potential benefit of all LAIAs is increased medication adherence compared with oral antipsychotics. One meta-analysis of 21 randomized controlled trials (RCTs) that compared LAIAs with oral antipsychotics and included 5,176 patients found that LAIAs had a similar efficacy to oral antipsychotics in preventing relapse.8 However, a meta-analysis of 25 mirror-image studies comparing LAIAs with oral antipsychotics that included 5,940 patients found that LAIAs were superior in preventing hospitalization.9 In these mirror-image studies, participants received oral antipsychotics first and then switched to LAIAs, and the 2 study periods were compared. Because mirror-image studies are observational, participants do not engage with research teams to the extent that they do in RCTs.9 Although mirror-image studies have limitations, participants in these studies may be a better representation of patients encountered in clinical practice due to the extensive monitoring and follow-up RCT participants typically receive.9
Differences in FDA-approved indications
The 7 currently available LAIAs vary in terms of FDA-approved indications, dose options, frequency, need for oral antipsychotic overlap, route of administration, and other factors. Table 11-7 summarizes some of these differences. Although all second-generation LAIAs are approved for schizophrenia,1-7 risperidone LAI and aripiprazole LAI are also approved for bipolar I disorder.1,4 Paliperidone palmitate monthly injection is the only LAIA approved for treating patients with schizoaffective disorder.2
Starting doses
For most LAIAs, the starting dose is the same as the maintenance dose (Table 11-7). One exception is paliperidone palmitate monthly injection, which requires a 234-mg dose on Day 1 followed by a 156-mg dose on Day 8 for all patients, regardless of the maintenance dose required.2 The 156-mg dose may be given 4 days before or after Day 8.2 The first maintenance dose of paliperidone palmitate monthly injection should be administered 5 weeks after the 234-mg dose on Day 1.2 Before starting paliperidone palmitate 3-month injection, patients should be stable on paliperidone palmitate monthly injection for 4 months, and the 2 most recent doses of paliperidone palmitate monthly injection should be the same.3

Maintenance doses
Dosing frequency may be an important factor for some patients when deciding to receive a LAIA. The frequency of the maintenance doses for all second-generation LAIAs varies from every 2 weeks to 12 weeks (Table 11-7). Paliperidone palmitate 3-month LAI is the only LAIA that is administered every 12 weeks.3 Some dosages of aripiprazole lauroxil LAI are administered every 6 or 8 weeks.6 All other second-generation LAIAs are given every 2 to 4 weeks.
Continue to: Start with an oral antipsychotic
Start with an oral antipsychotic
Before starting any LAIA, patients should receive the oral formulation of that antipsychotic to establish tolerability.1-7 Four of the 7 available LAIAs have an oral-to-LAI dose equivalency recommendation in their prescribing information (Table 22,5-7). This can help clinicians estimate the LAIA maintenance dose required to control a patient’s symptoms. If a dose adjustment is needed once a patient starts an LAIA, the dose adjustment can be made when the next injection is due.2

There are 2 important considerations when prescribing olanzapine pamoate LAI. First, the recommended dose for olanzapine pamoate LAI based on oral olanzapine doses differs during the first 8 weeks of treatment compared with after 8 weeks of treatment (Table 22,5-7). Additionally, because there are both short-acting and long-acting injections of olanzapine, it is essential to choose the correct formulation when prescribing this medication.5
Overlap with an oral antipsychotic might be necessary
Administration of several of the LAIAs may require overlap with an oral antipsychotic (Table 31,2,4-7). Patients who refuse to take oral medications may benefit from one of the LAIAs that does not require oral overlap—paliperidone palmitate monthly injection, olanzapine pamoate LAI, and risperidone LAI for SQ.2,5,7 Risperidone LAI requires overlap with oral risperidone for 3 weeks.1

Aripiprazole is available in 2 LAI formulations: aripiprazole LAI and aripiprazole lauroxil LAI. Aripiprazole lauroxil is a prodrug of aripiprazole, and these 2 LAI medications differ in available dose options and dosing frequency.4,6 Aripiprazole LAI requires an oral overlap for 2 weeks after the first injection, whereas aripiprazole lauroxil LAI requires 3 weeks of oral overlap unless aripiprazole lauroxil 675-mg LAI is administered (Figure6).4,6,10

Aripiprazole lauroxil 675-mg LAI is formulated with drug particles that are smaller than those in aripiprazole lauroxil LAI.11 The smaller particle size results in faster dissolution and a more rapid increase in plasma aripiprazole levels. Aripiprazole lauroxil 675-mg LAI is a single injection that should be given with one 30-mg dose of oral aripiprazole.10 This combination results in aripiprazole concentrations that are comparable to aripiprazole lauroxil LAI and oral aripiprazole overlap for 3 weeks after the first injection.10
Continue to: The starting dose of aripiprazole lauroxil LAI...
The starting dose of aripiprazole lauroxil LAI may be administered on the same day as aripiprazole lauroxil 675-mg LAI and the 30-mg oral aripiprazole dose, or it may be administered up to 10 days after.10 Aripiprazole lauroxil LAI and aripiprazole lauroxil 675-mg LAI are not interchangeable due to differing pharmacokinetic profiles.6,10 Aripiprazole lauroxil 675-mg LAI may be used to re-initiate treatment in a patient who missed doses of aripiprazole lauroxil LAI.10 Aripiprazole lauroxil LAI and aripiprazole lauroxil 675 mg should not be injected together into the same deltoid or gluteal muscle.
Be mindful of differences in dosing windows
Each LAIA has a specific frequency recommendation, but due to scheduling or other factors, it may not be possible for patients to receive their injection on the specified day. The prescribing information for some LAIAs provides a dosing window (Table 41-7). The prescribing information for risperidone LAI, olanzapine pamoate LAI, and risperidone LAI for SQ does not specify how many days the injection can be administered before or after the due date; however, the prescribing information for risperidone LAI for SQ indicates that if the injection is not given on the due date, it should be administered as soon as possible after that.1,5,7

Paliperidone palmitate monthly injection and paliperidone palmitate 3-month LAI have the clearest recommendations for a dosing window. Paliperidone palmitate monthly injection may be administered 7 days before or after the 4-week due date, and paliperidone palmitate 3-month LAI can be administered 14 days before or after the 12-week due date.2,3
Aripiprazole LAI should not be administered sooner than 26 days after the previous injection, which means that it can be administered up to 2 days before the 4-week due date.4 If administered after the due date, it should be given as soon as possible, although oral overlap is not needed until ≥7 days past the due date.4
Aripiprazole lauroxil LAI has similar recommendations to aripiprazole LAI in that it should not be administered sooner than 14 days after the previous injection.6 If it is given after the due date, it should be administered as soon as possible; oral overlap/starting dose is needed if it has been ≥2 to 4 weeks since the due date, depending on which dose and frequency the patient is receiving.6
Continue to: Recommendations for missed doses
Recommendations for missed doses
Each LAIA has specific recommendations for missed dosing. Carpenter and Wong12 reviewed the recommendations for managing missed LAIA doses in
Consider patient preference
Patient preference for the type and location of the injection may factor into a clinician’s choice of LAIA (Table 51-7,10). Risperidone LAI for SQ is the only LAIA that is administered as an SQ abdominal injection.7 All other LAIAs are IM injections in the deltoid or gluteal muscle.1-6 All doses of risperidone LAI, paliperidone palmitate 3-month LAI, aripiprazole LAI, and aripiprazole lauroxil 675-mg LAI can be administered in the deltoid or gluteal muscle.1,3,4,10 Deltoid administration is required for the 2 starting doses of paliperidone palmitate monthly injection, but maintenance doses can be administered in the deltoid or gluteal muscle. Because administration into the deltoid results in a higher concentration of the drug compared with gluteal administration, administering the 2 starting doses of paliperidone palmitate monthly injection into the deltoid helps to rapidly attain therapeutic concentrations.2 Olanzapine pamoate LAI should be administered only in the gluteal muscle.5 The 441-mg dose of aripiprazole lauroxil LAI may be administered in the deltoid or gluteal muscle, but all other doses of aripiprazole lauroxil LAI should be administered only in the gluteal muscle.6

Storage
Most LAIAs can be stored at room temperature2-6; however, risperidone LAI and risperidone LAI for SQ need to be stored in the refrigerator. Both risperidone LAI and risperidone LAI for SQ may be kept at room temperature for up to 7 days. If they are not used within 7 days at room temperature, they should be discarded.1,7
Clinical pearls for specific LAIAs
Aripiprazole LAI. The recommended starting and maintenance dose for aripiprazole LAI is 400 mg monthly, unless the patient has drug interactions or other factors that require dose adjustment. If patients experience adverse reactions to the 400-mg dose, a reduction to 300 mg monthly could be considered.4
Olanzapine pamoate LAI has a Risk Evaluation and Mitigation Strategy (REMS) due to the potential for post-injection delirium/sedation syndrome (PDSS). Prescribing clinicians, dispensing pharmacies, and administering health care facilities must all be certified to prescribe, dispense, or administer olanzapine pamoate LAI. The patient must also be enrolled in the REMS program.13 Patients must be observed by health care staff for 3 hours after receiving a dose of olanzapine pamoate LAI to monitor for signs and symptoms of PDSS.5
Continue to: Risperidone LAI
Risperidone LAI. When increasing the dose of risperidone LAI, do not expect to see the clinical effects of the new dose earlier than 3 weeks after initiating the higher dose, because the main release of the medication starts at 3 weeks after the injection.1
Risperidone LAI for SQ has specific recommendations for the LAI dose based on whether the patient was stable when receiving 3 or 4 mg/d of oral risperidone. If patients are stable on <3 or >4 mg/d, they may not be candidates for risperidone LAI for SQ.7
Table 61-7,10 lists additional factors to consider when prescribing a specific LAIA.
Bottom Line
Second-generation long-acting injectable antipsychotics (LAIAs) have the potential to increase medication adherence. There are important differences among the 7 currently available LAIAs. For effective prescribing, clinicians need to understand each medication’s unique aspects, including dosing options, frequency, need for oral antipsychotic overlap, and route of administration.
Related Resources
- Correll CU, Citrome L, Haddad PM, et al. The use of long-acting injectable antipsychotics in schizophrenia: evaluating the evidence. J Clin Psychiatry. 2016;77(suppl 3):1-24.
- Peters L, Krogmann A, von Hardenberg L, et al. Long-acting injections in schizophrenia: a 3-year update on randomized controlled trials published January 2016-March 2019. Curr Psychiatry Rep. 2019;21(12):124.
Drug Brand Names
Aripiprazole • Abilify
Aripiprazole long-acting injectable • Abilify Maintena
Aripiprazole lauroxil extended-release injectable suspension • Aristada
Aripiprazole lauroxil 675 mg • Aristada Initio
Olanzapine pamoate long-acting injection • Zyprexa Relprevv
Paliperidone palmitate monthly long-acting injection • Invega Sustenna
Paliperidone palmitate 3-month injection • Invega Trinza
Risperidone • Risperdal
Risperidone long-acting injection • Risperdal Consta
Risperidone long-acting injection for SQ • Perseris
There are currently 7 FDA-approved second-generation long-acting injectable antipsychotics (LAIAs).1-7 These LAIAs provide a unique dosage form that allows patients to receive an antipsychotic without taking oral medications every day, or multiple times per day. This may be an appealing option for patients and clinicians, but because there are several types of LAIAs available, it may be difficult to determine which LAIA characteristics are best for a given patient.
Since the FDA approved the first second-generation LAIA, risperidone long-acting injectable (LAI),1 in 2003, 6 additional second-generation LAIAs have been approved:
- aripiprazole LAI
- aripiprazole lauroxil LAI
- olanzapine pamoate LAI
- paliperidone palmitate monthly injection
- paliperidone palmitate 3-month LAI
- risperidone LAI for subcutaneous (SQ) injection.
When discussing medication options with patients, clinicians need to consider factors that are unique to each LAIA. In this article, I describe the similarities and differences among the second-generation LAIAs, and address common questions about these medications.
A major potential benefit: Increased adherence
One potential benefit of all LAIAs is increased medication adherence compared with oral antipsychotics. One meta-analysis of 21 randomized controlled trials (RCTs) that compared LAIAs with oral antipsychotics and included 5,176 patients found that LAIAs had a similar efficacy to oral antipsychotics in preventing relapse.8 However, a meta-analysis of 25 mirror-image studies comparing LAIAs with oral antipsychotics that included 5,940 patients found that LAIAs were superior in preventing hospitalization.9 In these mirror-image studies, participants received oral antipsychotics first and then switched to LAIAs, and the 2 study periods were compared. Because mirror-image studies are observational, participants do not engage with research teams to the extent that they do in RCTs.9 Although mirror-image studies have limitations, participants in these studies may be a better representation of patients encountered in clinical practice due to the extensive monitoring and follow-up RCT participants typically receive.9
Differences in FDA-approved indications
The 7 currently available LAIAs vary in terms of FDA-approved indications, dose options, frequency, need for oral antipsychotic overlap, route of administration, and other factors. Table 11-7 summarizes some of these differences. Although all second-generation LAIAs are approved for schizophrenia,1-7 risperidone LAI and aripiprazole LAI are also approved for bipolar I disorder.1,4 Paliperidone palmitate monthly injection is the only LAIA approved for treating patients with schizoaffective disorder.2
Starting doses
For most LAIAs, the starting dose is the same as the maintenance dose (Table 11-7). One exception is paliperidone palmitate monthly injection, which requires a 234-mg dose on Day 1 followed by a 156-mg dose on Day 8 for all patients, regardless of the maintenance dose required.2 The 156-mg dose may be given 4 days before or after Day 8.2 The first maintenance dose of paliperidone palmitate monthly injection should be administered 5 weeks after the 234-mg dose on Day 1.2 Before starting paliperidone palmitate 3-month injection, patients should be stable on paliperidone palmitate monthly injection for 4 months, and the 2 most recent doses of paliperidone palmitate monthly injection should be the same.3

Maintenance doses
Dosing frequency may be an important factor for some patients when deciding to receive a LAIA. The frequency of the maintenance doses for all second-generation LAIAs varies from every 2 weeks to 12 weeks (Table 11-7). Paliperidone palmitate 3-month LAI is the only LAIA that is administered every 12 weeks.3 Some dosages of aripiprazole lauroxil LAI are administered every 6 or 8 weeks.6 All other second-generation LAIAs are given every 2 to 4 weeks.
Continue to: Start with an oral antipsychotic
Start with an oral antipsychotic
Before starting any LAIA, patients should receive the oral formulation of that antipsychotic to establish tolerability.1-7 Four of the 7 available LAIAs have an oral-to-LAI dose equivalency recommendation in their prescribing information (Table 22,5-7). This can help clinicians estimate the LAIA maintenance dose required to control a patient’s symptoms. If a dose adjustment is needed once a patient starts an LAIA, the dose adjustment can be made when the next injection is due.2

There are 2 important considerations when prescribing olanzapine pamoate LAI. First, the recommended dose for olanzapine pamoate LAI based on oral olanzapine doses differs during the first 8 weeks of treatment compared with after 8 weeks of treatment (Table 22,5-7). Additionally, because there are both short-acting and long-acting injections of olanzapine, it is essential to choose the correct formulation when prescribing this medication.5
Overlap with an oral antipsychotic might be necessary
Administration of several of the LAIAs may require overlap with an oral antipsychotic (Table 31,2,4-7). Patients who refuse to take oral medications may benefit from one of the LAIAs that does not require oral overlap—paliperidone palmitate monthly injection, olanzapine pamoate LAI, and risperidone LAI for SQ.2,5,7 Risperidone LAI requires overlap with oral risperidone for 3 weeks.1

Aripiprazole is available in 2 LAI formulations: aripiprazole LAI and aripiprazole lauroxil LAI. Aripiprazole lauroxil is a prodrug of aripiprazole, and these 2 LAI medications differ in available dose options and dosing frequency.4,6 Aripiprazole LAI requires an oral overlap for 2 weeks after the first injection, whereas aripiprazole lauroxil LAI requires 3 weeks of oral overlap unless aripiprazole lauroxil 675-mg LAI is administered (Figure6).4,6,10

Aripiprazole lauroxil 675-mg LAI is formulated with drug particles that are smaller than those in aripiprazole lauroxil LAI.11 The smaller particle size results in faster dissolution and a more rapid increase in plasma aripiprazole levels. Aripiprazole lauroxil 675-mg LAI is a single injection that should be given with one 30-mg dose of oral aripiprazole.10 This combination results in aripiprazole concentrations that are comparable to aripiprazole lauroxil LAI and oral aripiprazole overlap for 3 weeks after the first injection.10
Continue to: The starting dose of aripiprazole lauroxil LAI...
The starting dose of aripiprazole lauroxil LAI may be administered on the same day as aripiprazole lauroxil 675-mg LAI and the 30-mg oral aripiprazole dose, or it may be administered up to 10 days after.10 Aripiprazole lauroxil LAI and aripiprazole lauroxil 675-mg LAI are not interchangeable due to differing pharmacokinetic profiles.6,10 Aripiprazole lauroxil 675-mg LAI may be used to re-initiate treatment in a patient who missed doses of aripiprazole lauroxil LAI.10 Aripiprazole lauroxil LAI and aripiprazole lauroxil 675 mg should not be injected together into the same deltoid or gluteal muscle.
Be mindful of differences in dosing windows
Each LAIA has a specific frequency recommendation, but due to scheduling or other factors, it may not be possible for patients to receive their injection on the specified day. The prescribing information for some LAIAs provides a dosing window (Table 41-7). The prescribing information for risperidone LAI, olanzapine pamoate LAI, and risperidone LAI for SQ does not specify how many days the injection can be administered before or after the due date; however, the prescribing information for risperidone LAI for SQ indicates that if the injection is not given on the due date, it should be administered as soon as possible after that.1,5,7

Paliperidone palmitate monthly injection and paliperidone palmitate 3-month LAI have the clearest recommendations for a dosing window. Paliperidone palmitate monthly injection may be administered 7 days before or after the 4-week due date, and paliperidone palmitate 3-month LAI can be administered 14 days before or after the 12-week due date.2,3
Aripiprazole LAI should not be administered sooner than 26 days after the previous injection, which means that it can be administered up to 2 days before the 4-week due date.4 If administered after the due date, it should be given as soon as possible, although oral overlap is not needed until ≥7 days past the due date.4
Aripiprazole lauroxil LAI has similar recommendations to aripiprazole LAI in that it should not be administered sooner than 14 days after the previous injection.6 If it is given after the due date, it should be administered as soon as possible; oral overlap/starting dose is needed if it has been ≥2 to 4 weeks since the due date, depending on which dose and frequency the patient is receiving.6
Continue to: Recommendations for missed doses
Recommendations for missed doses
Each LAIA has specific recommendations for missed dosing. Carpenter and Wong12 reviewed the recommendations for managing missed LAIA doses in
Consider patient preference
Patient preference for the type and location of the injection may factor into a clinician’s choice of LAIA (Table 51-7,10). Risperidone LAI for SQ is the only LAIA that is administered as an SQ abdominal injection.7 All other LAIAs are IM injections in the deltoid or gluteal muscle.1-6 All doses of risperidone LAI, paliperidone palmitate 3-month LAI, aripiprazole LAI, and aripiprazole lauroxil 675-mg LAI can be administered in the deltoid or gluteal muscle.1,3,4,10 Deltoid administration is required for the 2 starting doses of paliperidone palmitate monthly injection, but maintenance doses can be administered in the deltoid or gluteal muscle. Because administration into the deltoid results in a higher concentration of the drug compared with gluteal administration, administering the 2 starting doses of paliperidone palmitate monthly injection into the deltoid helps to rapidly attain therapeutic concentrations.2 Olanzapine pamoate LAI should be administered only in the gluteal muscle.5 The 441-mg dose of aripiprazole lauroxil LAI may be administered in the deltoid or gluteal muscle, but all other doses of aripiprazole lauroxil LAI should be administered only in the gluteal muscle.6

Storage
Most LAIAs can be stored at room temperature2-6; however, risperidone LAI and risperidone LAI for SQ need to be stored in the refrigerator. Both risperidone LAI and risperidone LAI for SQ may be kept at room temperature for up to 7 days. If they are not used within 7 days at room temperature, they should be discarded.1,7
Clinical pearls for specific LAIAs
Aripiprazole LAI. The recommended starting and maintenance dose for aripiprazole LAI is 400 mg monthly, unless the patient has drug interactions or other factors that require dose adjustment. If patients experience adverse reactions to the 400-mg dose, a reduction to 300 mg monthly could be considered.4
Olanzapine pamoate LAI has a Risk Evaluation and Mitigation Strategy (REMS) due to the potential for post-injection delirium/sedation syndrome (PDSS). Prescribing clinicians, dispensing pharmacies, and administering health care facilities must all be certified to prescribe, dispense, or administer olanzapine pamoate LAI. The patient must also be enrolled in the REMS program.13 Patients must be observed by health care staff for 3 hours after receiving a dose of olanzapine pamoate LAI to monitor for signs and symptoms of PDSS.5
Continue to: Risperidone LAI
Risperidone LAI. When increasing the dose of risperidone LAI, do not expect to see the clinical effects of the new dose earlier than 3 weeks after initiating the higher dose, because the main release of the medication starts at 3 weeks after the injection.1
Risperidone LAI for SQ has specific recommendations for the LAI dose based on whether the patient was stable when receiving 3 or 4 mg/d of oral risperidone. If patients are stable on <3 or >4 mg/d, they may not be candidates for risperidone LAI for SQ.7
Table 61-7,10 lists additional factors to consider when prescribing a specific LAIA.
Bottom Line
Second-generation long-acting injectable antipsychotics (LAIAs) have the potential to increase medication adherence. There are important differences among the 7 currently available LAIAs. For effective prescribing, clinicians need to understand each medication’s unique aspects, including dosing options, frequency, need for oral antipsychotic overlap, and route of administration.
Related Resources
- Correll CU, Citrome L, Haddad PM, et al. The use of long-acting injectable antipsychotics in schizophrenia: evaluating the evidence. J Clin Psychiatry. 2016;77(suppl 3):1-24.
- Peters L, Krogmann A, von Hardenberg L, et al. Long-acting injections in schizophrenia: a 3-year update on randomized controlled trials published January 2016-March 2019. Curr Psychiatry Rep. 2019;21(12):124.
Drug Brand Names
Aripiprazole • Abilify
Aripiprazole long-acting injectable • Abilify Maintena
Aripiprazole lauroxil extended-release injectable suspension • Aristada
Aripiprazole lauroxil 675 mg • Aristada Initio
Olanzapine pamoate long-acting injection • Zyprexa Relprevv
Paliperidone palmitate monthly long-acting injection • Invega Sustenna
Paliperidone palmitate 3-month injection • Invega Trinza
Risperidone • Risperdal
Risperidone long-acting injection • Risperdal Consta
Risperidone long-acting injection for SQ • Perseris
1. Risperdal Consta [package insert]. Titusville, NJ: Janssen Pharmaceuticals, Inc.; 2019.
2. Invega Sustenna [package insert]. Titusville, NJ: Janssen Pharmaceuticals, Inc.; 2019.
3. Invega Trinza [package insert]. Titusville, NJ: Janssen Pharmaceuticals, Inc.; 2019.
4. Abilify Maintena [package insert]. Rockville, MD: Otsuka America Pharmaceutical, Inc.; 2019.
5. Zyprexa Relprevv [package insert]. Indianapolis; IN: Eli Lilly and Co.; 2019.
6. Aristada [package insert]. Waltham, MA: Alkermes, Inc.; 2019.
7. Perseris [package insert]. North Chesterfield, VA: Indivior, Inc.; 2018.
8. Kishimoto T, Robenzadeh A, Leucht C, et al. Long-acting injectable vs oral antipsychotics for relapse prevention in schizophrenia: a meta-analysis of randomized trials. Schizophr Bull. 2014;40(1):192-213.
9. Kishimoto T, Nitta M, Borenstein M, et al. Long-acting injectable versus oral antipsychotics in schizophrenia: a systematic review and meta-analysis of mirror-image studies. J Clin Psychiatry. 2013;74(10):957-965.
10. Aristada Initio [package insert]. Waltham, MA: Alkermes, Inc.; 2019.
11. Jain R, Meyer J, Wehr A, et al. Size matters: the importance of particle size in a newly developed injectable formulation for the treatment of schizophrenia. CNS Spectr. 2019:1-8.
12. Carpenter J, Wong KK. Long-acting injectable antipsychotics: what to do about missed doses. Current Psychiatry. 2018;17(7):10-12,14-19,56.
13. US Food and Drug Administration. Approved Risk Evaluation and Mitigation Strategies (REMS) zyprexa relprevv (olanzapine). https://www.accessdata.fda.gov/scripts/cder/rems/index.cfm?event=IndvRemsDetails.page&REMS=74. Updated April 11, 2019. Accessed January 27, 2020.
1. Risperdal Consta [package insert]. Titusville, NJ: Janssen Pharmaceuticals, Inc.; 2019.
2. Invega Sustenna [package insert]. Titusville, NJ: Janssen Pharmaceuticals, Inc.; 2019.
3. Invega Trinza [package insert]. Titusville, NJ: Janssen Pharmaceuticals, Inc.; 2019.
4. Abilify Maintena [package insert]. Rockville, MD: Otsuka America Pharmaceutical, Inc.; 2019.
5. Zyprexa Relprevv [package insert]. Indianapolis; IN: Eli Lilly and Co.; 2019.
6. Aristada [package insert]. Waltham, MA: Alkermes, Inc.; 2019.
7. Perseris [package insert]. North Chesterfield, VA: Indivior, Inc.; 2018.
8. Kishimoto T, Robenzadeh A, Leucht C, et al. Long-acting injectable vs oral antipsychotics for relapse prevention in schizophrenia: a meta-analysis of randomized trials. Schizophr Bull. 2014;40(1):192-213.
9. Kishimoto T, Nitta M, Borenstein M, et al. Long-acting injectable versus oral antipsychotics in schizophrenia: a systematic review and meta-analysis of mirror-image studies. J Clin Psychiatry. 2013;74(10):957-965.
10. Aristada Initio [package insert]. Waltham, MA: Alkermes, Inc.; 2019.
11. Jain R, Meyer J, Wehr A, et al. Size matters: the importance of particle size in a newly developed injectable formulation for the treatment of schizophrenia. CNS Spectr. 2019:1-8.
12. Carpenter J, Wong KK. Long-acting injectable antipsychotics: what to do about missed doses. Current Psychiatry. 2018;17(7):10-12,14-19,56.
13. US Food and Drug Administration. Approved Risk Evaluation and Mitigation Strategies (REMS) zyprexa relprevv (olanzapine). https://www.accessdata.fda.gov/scripts/cder/rems/index.cfm?event=IndvRemsDetails.page&REMS=74. Updated April 11, 2019. Accessed January 27, 2020.








