User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
Powered by CHEST Physician, Clinician Reviews, MDedge Family Medicine, Internal Medicine News, and The Journal of Clinical Outcomes Management.
New biomarkers may predict interstitial lung disease progression in patients with systemic sclerosis
Quantitative assessment of the extent of interstitial lung disease in patients with systemic sclerosis and levels of certain proteins in bronchoalveolar lavage samples have potential for predicting mortality and disease progression, according to two analyses of data from the Scleroderma Lung Study I and II.
The analyses, presented at the annual European Congress of Rheumatology, aim to improve current prognostic abilities in patients with systemic sclerosis–interstitial lung disease (SSc-ILD). Although forced vital capacity is commonly used as a biomarker for survival in many SSc-ILD trials, other factors can affect FVC, such as respiratory muscle weakness and skin fibrosis. Further, FVC correlates poorly with patient-reported outcomes, explained first author Elizabeth Volkmann, MD, director of the scleroderma program at the University of California, Los Angeles, and the founder and codirector of the UCLA connective tissue disease–related interstitial lung disease program.
Dr. Volkmann presented two studies that investigated the potential of radiographic and protein biomarkers for predicting mortality and identifying patients at risk for ILD progression. The biomarkers may also help to identify patients who would benefit most from immunosuppressive therapy.
The first study found that tracking the quantitative extent of ILD (QILD) over time with high-resolution CT (HRCT) predicted poorer outcomes and could therefore act as a surrogate endpoint for mortality among patients with SSc-ILD. The other study identified associations between specific proteins from bronchoalveolar lavage (BAL) and the likelihood of ILD progression, although some associations were treatment dependent.
Jacob M. van Laar, MD, PhD, professor of rheumatology at the University Medical Center Utrecht (the Netherlands), who was not involved in the study, found the results intriguing and noted the importance of further validation in research before these biomarkers are considered for clinical use.
“It would be wonderful if we can tailor therapy based on BAL biomarkers in the future, as clinicians often struggle to decide on selection, timing, and duration of immunosuppressive treatment,” Dr. van Laar told this news organization. “This has become even more relevant with the introduction of new drugs such as nintedanib.”
Extent of ILD progression as a surrogate for mortality
Scleroderma Lung Study I involved 158 patients with SSc-ILD who were randomly assigned to receive either cyclophosphamide or placebo for 12 months. Scleroderma Lung Study II included 142 patients with SSc-ILD who were randomly assigned to receive either mycophenolate for 24 months or cyclophosphamide for 12 months followed by placebo for 12 months.
The researchers calculated QILD in the whole lung at baseline, at 12 months in the first trial, and at 24 months in the second trial. However, only 82 participants from the first trial and 90 participants from the second trial underwent HRCT. Demographic and disease characteristics were similar between the two groups on follow-up scans.
Follow-up continued for 12 years for patients in the first trial and 8 years in the second. The researchers compared survival rates between the 41% of participants from the first study and 31% of participants from the second study who had poorer QILD scores (at least a 2% increase) with the participants who had stable or improved scores (less than 2% increase).
Participants from both trials had significantly poorer long-term survival if their QILD scores had increased by at least 2% at follow-up (P = .01 for I; P = .019 for II). The association was no longer significant after adjustment for baseline FVC, age, and modified Rodnan skin score in the first trial (hazard ratio, 1.98; P = .089), but it remained significant for participants of the second trial (HR, 3.86; P = .014).
“Data from two independent trial cohorts demonstrated that radiographic progression of SSc-ILD at 1 and 2 years is associated with worse long-term survival,” Dr. Volkmann told attendees.
However, FVC did not significantly predict risk of mortality in either trial.
“To me, the most striking finding from the first study was that change in QILD performed better as a predictor of survival than change in FVC,” Dr. van Laar said in an interview. “This indicates QILD is fit for purpose and worth including in future clinical trials.”
Limitations of the study included lack of HRCT for all participants in the trials and the difference in timing (1 year and 2 years) of HRCT assessment between the two trials. The greater hazard ratio for worsened QILD in the second trial may suggest that assessment at 2 years provides more reliable data as a biomarker, Dr. Volkmann said.
“QILD may represent a better proxy for how a patient feels, functions, and survives than FVC,” she said.
Treatment-dependent biomarkers for worsening lung fibrosis
In the second study, the researchers looked for any associations between changes in the radiographic extent of SSc-ILD and 68 proteins from BAL.
“Being able to risk-stratify patients with interstitial lung disease at the time of diagnosis and predict which patients are likely to have a stable versus progressive disease course is critical for making important treatment decisions for these patients,” Dr. Volkmann told attendees.
The second study she presented involved Scleroderma Lung Study I. Of the 158 participants, 144 underwent a bronchoscopy, yielding BAL protein samples from 103 participants. The researchers determined the extent of radiographic fibrosis in the whole lung with quantitative imaging analysis of HRCT of the chest at baseline and 12 months.
Although the researchers identified several statistically significant associations between certain proteins and changes in radiographic fibrosis, “baseline protein levels were differentially associated with the course of ILD based on treatment status,” she told attendees.
For example, increased levels of the following proteins were linked to poor radiographic fibrosis scores for patients who received placebo:
- Granulocyte-macrophage colony-stimulating factor
- Interleukin-1
- Monocyte chemoattractant protein–3
- Chemokine ligand–5
- Transforming growth factor–beta
- Hepatocyte growth factor
- Stem cell factor
- IL-4
- TGF-alpha
Yet increases in these proteins predicted improvement in radiographic fibrosis in patients who had taken cyclophosphamide.
Independently of treatment, the researchers also identified an association between higher levels of fractalkine and poorer radiographic fibrosis scores and between higher IL-7 levels and improved radiographic fibrosis scores.
After adjusting for treatment arm and baseline severity of ILD, significant associations remained between change in radiographic fibrosis score and IL-1, MCP-3, surfactant protein C, IL-7 and CCL-5 levels.
“Biomarker discovery is really central to our ability to risk stratify patients with SSc-ILD,” Dr. Volkmann told attendees. “Understanding how biomarkers predict outcomes in treated and untreated patients may improve personalized medicine to patients with SSc-ILD and could also reveal novel treatment targets.”
Dr. van Laar said in an interview that this study’s biggest strength lay in its large sample size and in the comprehensiveness of the biomarkers studied.
“The findings are interesting from a research perspective and potentially relevant for clinical practice, but the utility of measuring biomarkers in BAL should be further studied for predictive value on clinical endpoints,” Dr. van Laar said. “BAL is an invasive procedure [that] is not routinely done.”
The research was funded by the National Institutes of Health. Dr. Volkmann has consulted for Boehringer Ingelheim and received grant funding from Corbus, Forbius, and Kadmon. Dr. van Laar has received grant funding or personal fees from Arthrogen, Arxx Therapeutics, AstraZeneca, Bristol-Myers Squibb, Eli Lilly, Gesynta, Leadiant, Merck Sharp & Dohme, Roche, Sanofi, and Thermofisher.
A version of this article first appeared on Medscape.com.
Quantitative assessment of the extent of interstitial lung disease in patients with systemic sclerosis and levels of certain proteins in bronchoalveolar lavage samples have potential for predicting mortality and disease progression, according to two analyses of data from the Scleroderma Lung Study I and II.
The analyses, presented at the annual European Congress of Rheumatology, aim to improve current prognostic abilities in patients with systemic sclerosis–interstitial lung disease (SSc-ILD). Although forced vital capacity is commonly used as a biomarker for survival in many SSc-ILD trials, other factors can affect FVC, such as respiratory muscle weakness and skin fibrosis. Further, FVC correlates poorly with patient-reported outcomes, explained first author Elizabeth Volkmann, MD, director of the scleroderma program at the University of California, Los Angeles, and the founder and codirector of the UCLA connective tissue disease–related interstitial lung disease program.
Dr. Volkmann presented two studies that investigated the potential of radiographic and protein biomarkers for predicting mortality and identifying patients at risk for ILD progression. The biomarkers may also help to identify patients who would benefit most from immunosuppressive therapy.
The first study found that tracking the quantitative extent of ILD (QILD) over time with high-resolution CT (HRCT) predicted poorer outcomes and could therefore act as a surrogate endpoint for mortality among patients with SSc-ILD. The other study identified associations between specific proteins from bronchoalveolar lavage (BAL) and the likelihood of ILD progression, although some associations were treatment dependent.
Jacob M. van Laar, MD, PhD, professor of rheumatology at the University Medical Center Utrecht (the Netherlands), who was not involved in the study, found the results intriguing and noted the importance of further validation in research before these biomarkers are considered for clinical use.
“It would be wonderful if we can tailor therapy based on BAL biomarkers in the future, as clinicians often struggle to decide on selection, timing, and duration of immunosuppressive treatment,” Dr. van Laar told this news organization. “This has become even more relevant with the introduction of new drugs such as nintedanib.”
Extent of ILD progression as a surrogate for mortality
Scleroderma Lung Study I involved 158 patients with SSc-ILD who were randomly assigned to receive either cyclophosphamide or placebo for 12 months. Scleroderma Lung Study II included 142 patients with SSc-ILD who were randomly assigned to receive either mycophenolate for 24 months or cyclophosphamide for 12 months followed by placebo for 12 months.
The researchers calculated QILD in the whole lung at baseline, at 12 months in the first trial, and at 24 months in the second trial. However, only 82 participants from the first trial and 90 participants from the second trial underwent HRCT. Demographic and disease characteristics were similar between the two groups on follow-up scans.
Follow-up continued for 12 years for patients in the first trial and 8 years in the second. The researchers compared survival rates between the 41% of participants from the first study and 31% of participants from the second study who had poorer QILD scores (at least a 2% increase) with the participants who had stable or improved scores (less than 2% increase).
Participants from both trials had significantly poorer long-term survival if their QILD scores had increased by at least 2% at follow-up (P = .01 for I; P = .019 for II). The association was no longer significant after adjustment for baseline FVC, age, and modified Rodnan skin score in the first trial (hazard ratio, 1.98; P = .089), but it remained significant for participants of the second trial (HR, 3.86; P = .014).
“Data from two independent trial cohorts demonstrated that radiographic progression of SSc-ILD at 1 and 2 years is associated with worse long-term survival,” Dr. Volkmann told attendees.
However, FVC did not significantly predict risk of mortality in either trial.
“To me, the most striking finding from the first study was that change in QILD performed better as a predictor of survival than change in FVC,” Dr. van Laar said in an interview. “This indicates QILD is fit for purpose and worth including in future clinical trials.”
Limitations of the study included lack of HRCT for all participants in the trials and the difference in timing (1 year and 2 years) of HRCT assessment between the two trials. The greater hazard ratio for worsened QILD in the second trial may suggest that assessment at 2 years provides more reliable data as a biomarker, Dr. Volkmann said.
“QILD may represent a better proxy for how a patient feels, functions, and survives than FVC,” she said.
Treatment-dependent biomarkers for worsening lung fibrosis
In the second study, the researchers looked for any associations between changes in the radiographic extent of SSc-ILD and 68 proteins from BAL.
“Being able to risk-stratify patients with interstitial lung disease at the time of diagnosis and predict which patients are likely to have a stable versus progressive disease course is critical for making important treatment decisions for these patients,” Dr. Volkmann told attendees.
The second study she presented involved Scleroderma Lung Study I. Of the 158 participants, 144 underwent a bronchoscopy, yielding BAL protein samples from 103 participants. The researchers determined the extent of radiographic fibrosis in the whole lung with quantitative imaging analysis of HRCT of the chest at baseline and 12 months.
Although the researchers identified several statistically significant associations between certain proteins and changes in radiographic fibrosis, “baseline protein levels were differentially associated with the course of ILD based on treatment status,” she told attendees.
For example, increased levels of the following proteins were linked to poor radiographic fibrosis scores for patients who received placebo:
- Granulocyte-macrophage colony-stimulating factor
- Interleukin-1
- Monocyte chemoattractant protein–3
- Chemokine ligand–5
- Transforming growth factor–beta
- Hepatocyte growth factor
- Stem cell factor
- IL-4
- TGF-alpha
Yet increases in these proteins predicted improvement in radiographic fibrosis in patients who had taken cyclophosphamide.
Independently of treatment, the researchers also identified an association between higher levels of fractalkine and poorer radiographic fibrosis scores and between higher IL-7 levels and improved radiographic fibrosis scores.
After adjusting for treatment arm and baseline severity of ILD, significant associations remained between change in radiographic fibrosis score and IL-1, MCP-3, surfactant protein C, IL-7 and CCL-5 levels.
“Biomarker discovery is really central to our ability to risk stratify patients with SSc-ILD,” Dr. Volkmann told attendees. “Understanding how biomarkers predict outcomes in treated and untreated patients may improve personalized medicine to patients with SSc-ILD and could also reveal novel treatment targets.”
Dr. van Laar said in an interview that this study’s biggest strength lay in its large sample size and in the comprehensiveness of the biomarkers studied.
“The findings are interesting from a research perspective and potentially relevant for clinical practice, but the utility of measuring biomarkers in BAL should be further studied for predictive value on clinical endpoints,” Dr. van Laar said. “BAL is an invasive procedure [that] is not routinely done.”
The research was funded by the National Institutes of Health. Dr. Volkmann has consulted for Boehringer Ingelheim and received grant funding from Corbus, Forbius, and Kadmon. Dr. van Laar has received grant funding or personal fees from Arthrogen, Arxx Therapeutics, AstraZeneca, Bristol-Myers Squibb, Eli Lilly, Gesynta, Leadiant, Merck Sharp & Dohme, Roche, Sanofi, and Thermofisher.
A version of this article first appeared on Medscape.com.
Quantitative assessment of the extent of interstitial lung disease in patients with systemic sclerosis and levels of certain proteins in bronchoalveolar lavage samples have potential for predicting mortality and disease progression, according to two analyses of data from the Scleroderma Lung Study I and II.
The analyses, presented at the annual European Congress of Rheumatology, aim to improve current prognostic abilities in patients with systemic sclerosis–interstitial lung disease (SSc-ILD). Although forced vital capacity is commonly used as a biomarker for survival in many SSc-ILD trials, other factors can affect FVC, such as respiratory muscle weakness and skin fibrosis. Further, FVC correlates poorly with patient-reported outcomes, explained first author Elizabeth Volkmann, MD, director of the scleroderma program at the University of California, Los Angeles, and the founder and codirector of the UCLA connective tissue disease–related interstitial lung disease program.
Dr. Volkmann presented two studies that investigated the potential of radiographic and protein biomarkers for predicting mortality and identifying patients at risk for ILD progression. The biomarkers may also help to identify patients who would benefit most from immunosuppressive therapy.
The first study found that tracking the quantitative extent of ILD (QILD) over time with high-resolution CT (HRCT) predicted poorer outcomes and could therefore act as a surrogate endpoint for mortality among patients with SSc-ILD. The other study identified associations between specific proteins from bronchoalveolar lavage (BAL) and the likelihood of ILD progression, although some associations were treatment dependent.
Jacob M. van Laar, MD, PhD, professor of rheumatology at the University Medical Center Utrecht (the Netherlands), who was not involved in the study, found the results intriguing and noted the importance of further validation in research before these biomarkers are considered for clinical use.
“It would be wonderful if we can tailor therapy based on BAL biomarkers in the future, as clinicians often struggle to decide on selection, timing, and duration of immunosuppressive treatment,” Dr. van Laar told this news organization. “This has become even more relevant with the introduction of new drugs such as nintedanib.”
Extent of ILD progression as a surrogate for mortality
Scleroderma Lung Study I involved 158 patients with SSc-ILD who were randomly assigned to receive either cyclophosphamide or placebo for 12 months. Scleroderma Lung Study II included 142 patients with SSc-ILD who were randomly assigned to receive either mycophenolate for 24 months or cyclophosphamide for 12 months followed by placebo for 12 months.
The researchers calculated QILD in the whole lung at baseline, at 12 months in the first trial, and at 24 months in the second trial. However, only 82 participants from the first trial and 90 participants from the second trial underwent HRCT. Demographic and disease characteristics were similar between the two groups on follow-up scans.
Follow-up continued for 12 years for patients in the first trial and 8 years in the second. The researchers compared survival rates between the 41% of participants from the first study and 31% of participants from the second study who had poorer QILD scores (at least a 2% increase) with the participants who had stable or improved scores (less than 2% increase).
Participants from both trials had significantly poorer long-term survival if their QILD scores had increased by at least 2% at follow-up (P = .01 for I; P = .019 for II). The association was no longer significant after adjustment for baseline FVC, age, and modified Rodnan skin score in the first trial (hazard ratio, 1.98; P = .089), but it remained significant for participants of the second trial (HR, 3.86; P = .014).
“Data from two independent trial cohorts demonstrated that radiographic progression of SSc-ILD at 1 and 2 years is associated with worse long-term survival,” Dr. Volkmann told attendees.
However, FVC did not significantly predict risk of mortality in either trial.
“To me, the most striking finding from the first study was that change in QILD performed better as a predictor of survival than change in FVC,” Dr. van Laar said in an interview. “This indicates QILD is fit for purpose and worth including in future clinical trials.”
Limitations of the study included lack of HRCT for all participants in the trials and the difference in timing (1 year and 2 years) of HRCT assessment between the two trials. The greater hazard ratio for worsened QILD in the second trial may suggest that assessment at 2 years provides more reliable data as a biomarker, Dr. Volkmann said.
“QILD may represent a better proxy for how a patient feels, functions, and survives than FVC,” she said.
Treatment-dependent biomarkers for worsening lung fibrosis
In the second study, the researchers looked for any associations between changes in the radiographic extent of SSc-ILD and 68 proteins from BAL.
“Being able to risk-stratify patients with interstitial lung disease at the time of diagnosis and predict which patients are likely to have a stable versus progressive disease course is critical for making important treatment decisions for these patients,” Dr. Volkmann told attendees.
The second study she presented involved Scleroderma Lung Study I. Of the 158 participants, 144 underwent a bronchoscopy, yielding BAL protein samples from 103 participants. The researchers determined the extent of radiographic fibrosis in the whole lung with quantitative imaging analysis of HRCT of the chest at baseline and 12 months.
Although the researchers identified several statistically significant associations between certain proteins and changes in radiographic fibrosis, “baseline protein levels were differentially associated with the course of ILD based on treatment status,” she told attendees.
For example, increased levels of the following proteins were linked to poor radiographic fibrosis scores for patients who received placebo:
- Granulocyte-macrophage colony-stimulating factor
- Interleukin-1
- Monocyte chemoattractant protein–3
- Chemokine ligand–5
- Transforming growth factor–beta
- Hepatocyte growth factor
- Stem cell factor
- IL-4
- TGF-alpha
Yet increases in these proteins predicted improvement in radiographic fibrosis in patients who had taken cyclophosphamide.
Independently of treatment, the researchers also identified an association between higher levels of fractalkine and poorer radiographic fibrosis scores and between higher IL-7 levels and improved radiographic fibrosis scores.
After adjusting for treatment arm and baseline severity of ILD, significant associations remained between change in radiographic fibrosis score and IL-1, MCP-3, surfactant protein C, IL-7 and CCL-5 levels.
“Biomarker discovery is really central to our ability to risk stratify patients with SSc-ILD,” Dr. Volkmann told attendees. “Understanding how biomarkers predict outcomes in treated and untreated patients may improve personalized medicine to patients with SSc-ILD and could also reveal novel treatment targets.”
Dr. van Laar said in an interview that this study’s biggest strength lay in its large sample size and in the comprehensiveness of the biomarkers studied.
“The findings are interesting from a research perspective and potentially relevant for clinical practice, but the utility of measuring biomarkers in BAL should be further studied for predictive value on clinical endpoints,” Dr. van Laar said. “BAL is an invasive procedure [that] is not routinely done.”
The research was funded by the National Institutes of Health. Dr. Volkmann has consulted for Boehringer Ingelheim and received grant funding from Corbus, Forbius, and Kadmon. Dr. van Laar has received grant funding or personal fees from Arthrogen, Arxx Therapeutics, AstraZeneca, Bristol-Myers Squibb, Eli Lilly, Gesynta, Leadiant, Merck Sharp & Dohme, Roche, Sanofi, and Thermofisher.
A version of this article first appeared on Medscape.com.
As new cases fall, U.S. passes 4 million children with COVID-19
Even as the number of new COVID-19 cases continues to drop, the United States reached the 4-million mark for infected children, according to a report from the American Academy of Pediatrics and the Children’s Hospital Association.
. That weekly total, the lowest since June of 2020, comes from 49 states (excluding N.Y.), the District of Columbia, New York City, Puerto Rico, and Guam, the AAP and CHA said in their weekly COVID-19 report.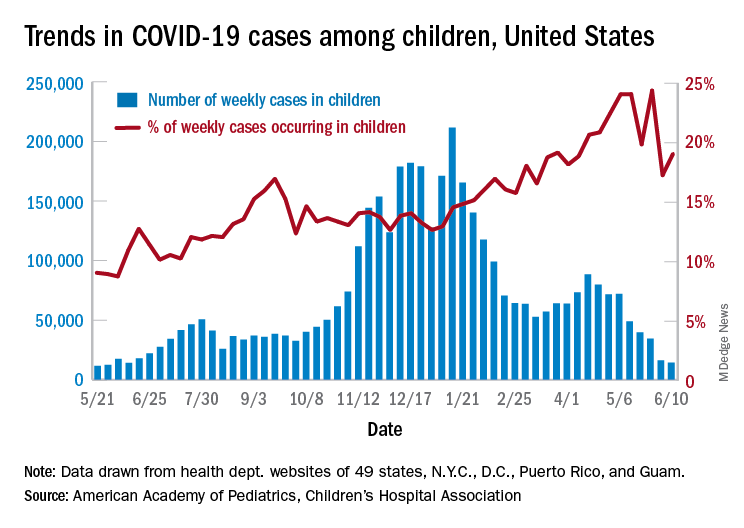
Children represent 14.1% of all COVID-19 cases since the beginning of the pandemic, while the corresponding figure for the week ending June 10 was 19.0%. That weekly proportion of cases among children had been rising pretty steadily through the winter and early spring, but the situation has become much more volatile over the last month, the AAP/CHA data show.
Use of the Pfizer-BioNTech vaccine in children aged 16-17 years, of course, didn’t begin until April, and the vaccine wasn’t authorized for children aged 12-15 years until mid-May. The Moderna and Johnson & Johnson vaccines have not received such authorization yet, but Moderna is in the process of seeking an emergency-use recommendation from the Food and Drug Administration.
In the younger group of children who are currently eligible, completion of the vaccine regimen took a big jump in the week ending June 14, according to the Centers for Disease Control and Prevention. The cumulative share of those aged 12-15 years who had received a second dose jumped from 4.1% on June 7 to 11.4% on June 14, with comparable numbers for 16- and 17-year-olds coming in at 26.4% and 29.1%.
Activity over just the last 14 days, however, shows a slight decrease in children aged 12-15 getting a first dose: For just the 2 weeks ending June 7, 17.9% of all children in the age group initiated a first dose, but for the 14 days ending June 14, only 17.1% of the age group did so, the CDC said on its COVID Data Tracker site.
For children aged 16-17 years – of whom less than 30% have reached full vaccination – activity seems to have stagnated: 4.8% of all 16- to 17-year-olds initiated a first vaccination during the 14 days ending June 7, compared with 4.7% who did so during the 14 days ending June 14, the CDC reported.
Older age groups with higher completion rates are still producing greater vaccine initiation. As of June 14, those aged 25-39 years had a completion rate of 41.9% and 24.0% of the age group had received a first dose in the previous 2 weeks, while 61.4% of those aged 50-64 were fully vaccinated, and 18.0% had gotten their first dose, the CDC data indicate.
Even as the number of new COVID-19 cases continues to drop, the United States reached the 4-million mark for infected children, according to a report from the American Academy of Pediatrics and the Children’s Hospital Association.
. That weekly total, the lowest since June of 2020, comes from 49 states (excluding N.Y.), the District of Columbia, New York City, Puerto Rico, and Guam, the AAP and CHA said in their weekly COVID-19 report.
Children represent 14.1% of all COVID-19 cases since the beginning of the pandemic, while the corresponding figure for the week ending June 10 was 19.0%. That weekly proportion of cases among children had been rising pretty steadily through the winter and early spring, but the situation has become much more volatile over the last month, the AAP/CHA data show.
Use of the Pfizer-BioNTech vaccine in children aged 16-17 years, of course, didn’t begin until April, and the vaccine wasn’t authorized for children aged 12-15 years until mid-May. The Moderna and Johnson & Johnson vaccines have not received such authorization yet, but Moderna is in the process of seeking an emergency-use recommendation from the Food and Drug Administration.
In the younger group of children who are currently eligible, completion of the vaccine regimen took a big jump in the week ending June 14, according to the Centers for Disease Control and Prevention. The cumulative share of those aged 12-15 years who had received a second dose jumped from 4.1% on June 7 to 11.4% on June 14, with comparable numbers for 16- and 17-year-olds coming in at 26.4% and 29.1%.
Activity over just the last 14 days, however, shows a slight decrease in children aged 12-15 getting a first dose: For just the 2 weeks ending June 7, 17.9% of all children in the age group initiated a first dose, but for the 14 days ending June 14, only 17.1% of the age group did so, the CDC said on its COVID Data Tracker site.
For children aged 16-17 years – of whom less than 30% have reached full vaccination – activity seems to have stagnated: 4.8% of all 16- to 17-year-olds initiated a first vaccination during the 14 days ending June 7, compared with 4.7% who did so during the 14 days ending June 14, the CDC reported.
Older age groups with higher completion rates are still producing greater vaccine initiation. As of June 14, those aged 25-39 years had a completion rate of 41.9% and 24.0% of the age group had received a first dose in the previous 2 weeks, while 61.4% of those aged 50-64 were fully vaccinated, and 18.0% had gotten their first dose, the CDC data indicate.
Even as the number of new COVID-19 cases continues to drop, the United States reached the 4-million mark for infected children, according to a report from the American Academy of Pediatrics and the Children’s Hospital Association.
. That weekly total, the lowest since June of 2020, comes from 49 states (excluding N.Y.), the District of Columbia, New York City, Puerto Rico, and Guam, the AAP and CHA said in their weekly COVID-19 report.
Children represent 14.1% of all COVID-19 cases since the beginning of the pandemic, while the corresponding figure for the week ending June 10 was 19.0%. That weekly proportion of cases among children had been rising pretty steadily through the winter and early spring, but the situation has become much more volatile over the last month, the AAP/CHA data show.
Use of the Pfizer-BioNTech vaccine in children aged 16-17 years, of course, didn’t begin until April, and the vaccine wasn’t authorized for children aged 12-15 years until mid-May. The Moderna and Johnson & Johnson vaccines have not received such authorization yet, but Moderna is in the process of seeking an emergency-use recommendation from the Food and Drug Administration.
In the younger group of children who are currently eligible, completion of the vaccine regimen took a big jump in the week ending June 14, according to the Centers for Disease Control and Prevention. The cumulative share of those aged 12-15 years who had received a second dose jumped from 4.1% on June 7 to 11.4% on June 14, with comparable numbers for 16- and 17-year-olds coming in at 26.4% and 29.1%.
Activity over just the last 14 days, however, shows a slight decrease in children aged 12-15 getting a first dose: For just the 2 weeks ending June 7, 17.9% of all children in the age group initiated a first dose, but for the 14 days ending June 14, only 17.1% of the age group did so, the CDC said on its COVID Data Tracker site.
For children aged 16-17 years – of whom less than 30% have reached full vaccination – activity seems to have stagnated: 4.8% of all 16- to 17-year-olds initiated a first vaccination during the 14 days ending June 7, compared with 4.7% who did so during the 14 days ending June 14, the CDC reported.
Older age groups with higher completion rates are still producing greater vaccine initiation. As of June 14, those aged 25-39 years had a completion rate of 41.9% and 24.0% of the age group had received a first dose in the previous 2 weeks, while 61.4% of those aged 50-64 were fully vaccinated, and 18.0% had gotten their first dose, the CDC data indicate.
Judge tosses hospital staff suit over vaccine mandate
A federal judge in Texas has dismissed a lawsuit from 117 Houston Methodist Hospital workers who refused to get a COVID-19 vaccine and said it was illegal to require them to do so.
In the ruling issued June 12, U.S. District Judge Lynn Hughes upheld the hospital’s policy and said the vaccination requirement didn’t break any federal laws.
“This is not coercion,” Judge Hughes wrote in the ruling.
“Methodist is trying to do their business of saving lives without giving them the COVID-19 virus,” he wrote. “It is a choice made to keep staff, patients, and their families safer.”
In April, the Houston Methodist Hospital system announced a policy that required employees to be vaccinated by June 7 or request an exemption. After the deadline, 178 of 26,000 employees refused to get inoculated and were placed on suspension without pay. The employees said the vaccine was unsafe and “experimental.” In his ruling, Judge Hughes said their claim was false and irrelevant.
“Texas law only protects employees from being terminated for refusing to commit an act carrying criminal penalties to the worker,” he wrote. “Receiving a COVID-19 vaccination is not an illegal act, and it carries no criminal penalties.”
He denounced the “press-release style of the complaint” and the comparison of the hospital’s vaccine policy to forced experimentation by the Nazis against Jewish people during the Holocaust.
“Equating the injection requirement to medical experimentation in concentration camps is reprehensible,” he wrote. “Nazi doctors conducted medical experiments on victims that caused pain, mutilation, permanent disability, and in many cases, death.”
Judge Hughes also said that employees can “freely choose” to accept or refuse a COVID-19 vaccine. If they refuse, they “simply need to work somewhere else,” he wrote.
“If a worker refuses an assignment, changed office, earlier start time, or other directive, he may be properly fired,” Judge Hughes said. “Every employment includes limits on the worker’s behavior in exchange for his remuneration. This is all part of the bargain.”
The ruling could set a precedent for similar COVID-19 vaccine lawsuits across the country, NPR reported. Houston Methodist was one of the first hospitals to require staff to be vaccinated. After the ruling on June 12, the hospital system wrote in a statement that it was “pleased and reassured” that Judge Hughes dismissed a “frivolous lawsuit.”
The hospital system will begin to terminate the 178 employees who were suspended if they don’t get a vaccine by June 21.
Jennifer Bridges, a nurse who has led the campaign against the vaccine policy, said she and the other plaintiffs will appeal the decision, according to KHOU.
“We’re OK with this decision. We are appealing. This will be taken all the way to the Supreme Court,” she told the news station. “This is far from over. This is literally only the beginning.”
A version of this article first appeared on WebMD.com.
A federal judge in Texas has dismissed a lawsuit from 117 Houston Methodist Hospital workers who refused to get a COVID-19 vaccine and said it was illegal to require them to do so.
In the ruling issued June 12, U.S. District Judge Lynn Hughes upheld the hospital’s policy and said the vaccination requirement didn’t break any federal laws.
“This is not coercion,” Judge Hughes wrote in the ruling.
“Methodist is trying to do their business of saving lives without giving them the COVID-19 virus,” he wrote. “It is a choice made to keep staff, patients, and their families safer.”
In April, the Houston Methodist Hospital system announced a policy that required employees to be vaccinated by June 7 or request an exemption. After the deadline, 178 of 26,000 employees refused to get inoculated and were placed on suspension without pay. The employees said the vaccine was unsafe and “experimental.” In his ruling, Judge Hughes said their claim was false and irrelevant.
“Texas law only protects employees from being terminated for refusing to commit an act carrying criminal penalties to the worker,” he wrote. “Receiving a COVID-19 vaccination is not an illegal act, and it carries no criminal penalties.”
He denounced the “press-release style of the complaint” and the comparison of the hospital’s vaccine policy to forced experimentation by the Nazis against Jewish people during the Holocaust.
“Equating the injection requirement to medical experimentation in concentration camps is reprehensible,” he wrote. “Nazi doctors conducted medical experiments on victims that caused pain, mutilation, permanent disability, and in many cases, death.”
Judge Hughes also said that employees can “freely choose” to accept or refuse a COVID-19 vaccine. If they refuse, they “simply need to work somewhere else,” he wrote.
“If a worker refuses an assignment, changed office, earlier start time, or other directive, he may be properly fired,” Judge Hughes said. “Every employment includes limits on the worker’s behavior in exchange for his remuneration. This is all part of the bargain.”
The ruling could set a precedent for similar COVID-19 vaccine lawsuits across the country, NPR reported. Houston Methodist was one of the first hospitals to require staff to be vaccinated. After the ruling on June 12, the hospital system wrote in a statement that it was “pleased and reassured” that Judge Hughes dismissed a “frivolous lawsuit.”
The hospital system will begin to terminate the 178 employees who were suspended if they don’t get a vaccine by June 21.
Jennifer Bridges, a nurse who has led the campaign against the vaccine policy, said she and the other plaintiffs will appeal the decision, according to KHOU.
“We’re OK with this decision. We are appealing. This will be taken all the way to the Supreme Court,” she told the news station. “This is far from over. This is literally only the beginning.”
A version of this article first appeared on WebMD.com.
A federal judge in Texas has dismissed a lawsuit from 117 Houston Methodist Hospital workers who refused to get a COVID-19 vaccine and said it was illegal to require them to do so.
In the ruling issued June 12, U.S. District Judge Lynn Hughes upheld the hospital’s policy and said the vaccination requirement didn’t break any federal laws.
“This is not coercion,” Judge Hughes wrote in the ruling.
“Methodist is trying to do their business of saving lives without giving them the COVID-19 virus,” he wrote. “It is a choice made to keep staff, patients, and their families safer.”
In April, the Houston Methodist Hospital system announced a policy that required employees to be vaccinated by June 7 or request an exemption. After the deadline, 178 of 26,000 employees refused to get inoculated and were placed on suspension without pay. The employees said the vaccine was unsafe and “experimental.” In his ruling, Judge Hughes said their claim was false and irrelevant.
“Texas law only protects employees from being terminated for refusing to commit an act carrying criminal penalties to the worker,” he wrote. “Receiving a COVID-19 vaccination is not an illegal act, and it carries no criminal penalties.”
He denounced the “press-release style of the complaint” and the comparison of the hospital’s vaccine policy to forced experimentation by the Nazis against Jewish people during the Holocaust.
“Equating the injection requirement to medical experimentation in concentration camps is reprehensible,” he wrote. “Nazi doctors conducted medical experiments on victims that caused pain, mutilation, permanent disability, and in many cases, death.”
Judge Hughes also said that employees can “freely choose” to accept or refuse a COVID-19 vaccine. If they refuse, they “simply need to work somewhere else,” he wrote.
“If a worker refuses an assignment, changed office, earlier start time, or other directive, he may be properly fired,” Judge Hughes said. “Every employment includes limits on the worker’s behavior in exchange for his remuneration. This is all part of the bargain.”
The ruling could set a precedent for similar COVID-19 vaccine lawsuits across the country, NPR reported. Houston Methodist was one of the first hospitals to require staff to be vaccinated. After the ruling on June 12, the hospital system wrote in a statement that it was “pleased and reassured” that Judge Hughes dismissed a “frivolous lawsuit.”
The hospital system will begin to terminate the 178 employees who were suspended if they don’t get a vaccine by June 21.
Jennifer Bridges, a nurse who has led the campaign against the vaccine policy, said she and the other plaintiffs will appeal the decision, according to KHOU.
“We’re OK with this decision. We are appealing. This will be taken all the way to the Supreme Court,” she told the news station. “This is far from over. This is literally only the beginning.”
A version of this article first appeared on WebMD.com.
Conflicting medical opinions: Black lungs, Big Coal, and bias
In 2008, the U.S. Department of Labor (DOL) paid for Tony Adams, a 48-year-old coal miner, to have a chest x-ray. His doctor found stage I black lung disease. Yet Mr. Adams’ claim for medical benefits was denied. This was because the insurance group that represented his employer hired a different – more credentialed – doctor as its medical expert. That doctor said he saw no such evidence. The judge ruled in favor of the mining company on the basis of the latter’s “expertise.”
Before he died 5 years later, at age 53, Mr. Adams went through this process again. In fact, he did it four more times. Each time, his doctor found evidence of black lung, but the company’s medical expert did not. He died without receiving benefits. Among the causes of death listed on his autopsy were cardiopulmonary arrest and coal worker’s pneumoconiosis (CWP): black lung.
Since his death in 2013, two judges have awarded Mr. Adams’ benefits to his widow, Linda. Both times, the mining company appealed the decision, most recently in December 2020. She’s not giving up. “Two weeks before he died, he told me, ‘I’m going to die of black lung,’ ” Linda recalled. “‘But I don’t want you to give up on black lung. There are too many people screwing these miners out of what they deserve.’”
There has long been suspicion among miners and their advocates that doctors used by coal companies to fight claims like Mr. Adams’ are in the pocket of “Big Coal.” At the very least, some say these physicians are swayed by their client’s preference when reading a coal miner’s chest x-ray. A recent study published in Annals of the American Thoracic Society provides empirical evidence that these doctors’ conflict of interest – namely, that parties representing coal companies hired them – appears to influence their medical opinion.
Proof of a ‘broken system’
The Annals study examined 63,780 radiograph classifications made by 264 physicians – all certified as B-readers, a certification by the National Institute for Occupational Safety and Health (NIOSH) for physicians who demonstrate proficiency in classifying radiographs of pneumoconiosis. The results showed that doctors hired by miners identified black lung 49% of the time; those hired by coal companies identified black lung only 15% of the time.
The study also found that B-readers contracted by employers read results differently for different clients. The same doctors were significantly less likely to say a miner’s lungs were negative for CWP when they were hired by the DOL (77.2%) than when they were hired by a coal company or its insurers (90.2%).
The bias does appear to work both ways: B-readers hired by miners and miners’ attorneys were more likely to find evidence of black lung when they worked with plaintiffs. However, a much higher number of doctors appeared to be biased in favor of the companies. “There were 3X more B-readers providing 8X more classifications among those affiliated with employers compared to those affiliated with miners,” the study concluded.
The authors suggest that one reason for this was the difference in pay. Some company-hired doctors made as much as $750 per reading, about 10 times what miner-hired doctors were paid.
“We knew [about the potential bias] from our work over the decades taking care of these guys,” said Robert A. Cohen, MD, a pulmonologist and the study’s senior author. “But then you see it with P values that are incredibly statistically significant ...”
The study finally put numbers to a problem that many working with black lung claims had always assumed. Those within the system are accustomed to seeing names of the same doctors on documents and reports, with little to no overlap between those hired by the defense and the plaintiffs.
“The vast majority of the time, we know what a report will say based on the doctor’s name,” said Evan Smith, JD, advocacy director at AppalReD Legal Aid, in Prestonsburg, Ky.. It is far more surprising, he said, when a defense-hired doctor agrees with a miner-hired doctor.
Over the years, Katherine DePonte, MD, a radiologist and B-reader in West Virginia, has often seen an “almost textbook appearance” of CWP, only to later learn that “another radiologist read it as negative.” She explained, “They would use some other term, like ‘old granulomatous disease.’”
Employer-hired doctors often do acknowledge the same lung damage on the radiograph as miner-hired docs; they simply don’t attribute it to coal dust. Common “alternative diagnoses” include chronic obstructive pulmonary disease or histoplasmosis. “I know a number don’t believe this disease of coal worker pneumoconiosis exists [at all],” Dr. DePonte said.
What’s inarguable is that, even as coal mining in Appalachia is on the decline, black lung disease is on the rise. NIOSH now estimates that it affects over 20% of long-term (25+ years) coal workers in central Appalachia. That’s the highest prevalence in a quarter of a century.
Mr. Smith said that at its most basic level, these doctors’ conflicts of interest “lead to people who have the disease that these benefits are for, having them denied.” People like Tony Adams. Whether the doctors involved are complicit or just conservative, critics say they have become a fixture of a broken system.
Financial bias or difference of opinion?
Broken system or not, evidence suggests that the problem can’t be blamed solely on medical experts. Dr. DePonte primarily reads for the DOL and miners. “Not that I necessarily chose that,” she said. “You get pigeonholed.”
Some say that the bias demonstrated by the Annals study is at least partially driven by the litigation process itself. It is an adversarial system. As such, attorneys on both sides are naturally inclined to seek out doctors who will best support their clients’ cases. Doctors with a legitimately conservative perspective on what constitutes black lung are more sought after by the coal companies’ attorneys.
“It can often be impossible to tell whether the money is driving a change in the behavior or if the behavior is causing them to be sought out,” said Matt McCoy, PhD, a medical ethicist who specializes in conflicts of interest at the University of Pennsylvania, Philadelphia.
Although some believe that certain doctors are driven purely by financial incentive and offer a specific reading to secure repeat business, B-readers can end up working exclusively for companies because of other reasons. Wes Addington, JD, an attorney at the Appalachian Citizens’ Law Center, Whitesburg, Ky., said some doctors appear to have an authentically different – often antiquated – view of the disease.
Perhaps the most extreme example is Paul Wheeler, MD, a highly credentialed Johns Hopkins radiologist who was exposed for false medical testimony in Chris Hamby’s 2013 Pulitzer Prize reporting. In 1,500 readings, Dr. Wheeler never diagnosed a single case of severe black lung. And yet, Dr. Cohen, Mr. Addington, Mr. Smith, and other experts all agree that Dr. Wheeler appeared to wholeheartedly believe that his view of black lung was accurate. That made him a valuable asset to mining companies.
Since Dr. Wheeler’s exposure, there has been a greater sense of accountability among B-readers, said John Cline, JD, a West Virginia–based attorney who represents miners with federal black lung claims. “Radiologists were thinking, ‘Somebody could be watching me.’ Even if they thought they were doing this in the shadows, it made people more cautious,” he said.
The data used in the Annals study predate Mr. Hamby’s investigation, going back to 2000. Thus, it is possible that, as Mr. Cline argues, things may be different now. However, Lee S. Friedman, PhD, associate professor at the University of Illinois at Chicago, who is the lead author of the study, remains skeptical.
“While the Wheeler case might have dampened some physicians [who were] completely skewing their readings always negative, I think it’s premature or incorrect” to say it resolved the issue, he said. “Did they all change their behavior the morning after? It doesn’t seem likely, given the evidence of financial conflicts of interest and behavior that’s been demonstrated.”
Skewing the evidence?
Mr. Hamby’s 2013 reporting also revealed that even when company-hired doctors did diagnose CWP, law firms were burying those readings. In 2016, the DOL attempted to stop this practice. The agency made suppression of written evidence illegal – emphasis on written.
Law firms can’t hide positive reports, but they can prevent them. Dr. Cohen explained that now, “a doctor on the phone says, ‘I will read this as positive.’ Then the company says, ‘No, thank you,’ we will send you a check.”
This practice was confirmed by Kim Adcock, MD, a retired radiologist and B-reader in Littleton, Colo., who primarily reads for 26 law firms. Some of his clients want a report no matter how he reads the radiograph. However, some want him to call them first if he’s going to read the radiograph as positive. Dr. Adcock said this practice skews the dataset to make company-hired docs appear to read more negatively than they actually do.
Because the dataset used in the study is from the Federal Black Lung Program (FBLP), it includes only readings that made it to court. Dr. Adcock said he reads approximately 2,000 radiographs a year, although only a few of his readings appeared in the study’s dataset, according to a search by Dr. Friedman. This difference is likely because the study evaluated only readings between 2000 and 2013, the year Dr. Adcock started B-reading.
“I think it’s important to get a message that, to a certain extent, contravenes this paper. Yes, we should have some reservations about the conclusions,” Dr. Adcock explained. “There are people out there attempting to do the best job they could do.”
Law firms shopping for the reading they want and censoring the ones they don’t might alter the FBLP data, but experts say that doesn’t change the underlying problem. “In any case like this, where you’re looking at individuals going up against corporations,” Dr. McCoy said, “[corporations] are able to marshal their resources and hire more officials in a way claimants can’t, and that’s a baseline concern here.”
Battling bias
Admitting bias is notoriously difficult; thus, it isn’t surprising that many doctors involved refuse to believe they are influenced by money, incentives, or other biases. Dr. DePonte said she’s not swayed by money, nor does she actively take a pro-miner stance. She views herself as more of an advocate for accuracy. However, she did say that it has traditionally been far more difficult for miners to prove their cases, a problem that has improved with new regulations in recent years.
In Colorado, Dr. Adcock’s approach is to stay as far removed from the litigation process as possible. He said he has limited understanding of how his reports are used or how claims are filed and awarded. He leans heavily on his initial – almost instantaneous – impression of a chest x-ray.
Dr. DePonte and Dr. Adcock were both hired as experts on Tony Adams’ case. In 2008, Dr. DePonte read his chest x-ray as positive for early-stage black lung (1/0). Dr. Adcock also read two of Adams’ four chest x-rays, one in 2009 and the other in 2013. He read them as negative. When asked about the case, which autopsy confirmed as black lung, Dr. Adcock explained that positive histopathology doesn’t mean the radiograph reading was wrong, only that the disease didn’t show on that radiograph. He said his “highest ambition” is to be “an objective finder of fact” and that he trusts the process to work out the truth.
That process didn’t work in time for Tony Adams. Dr. Friedman argues that people who provide expert testimony have an ethical responsibility to know how their testimony is being used; to do otherwise, he says, is “willful ignorance.” Still, the Annals study authors, along with Dr. DePonte, Mr. Cline, and West Virginia attorney Sam Petsonk, say that the process is getting fairer, thanks to new policies developed over the past 5 years by the DOL.
“The DOL has worked very hard to reconcile the final award rate (around 30%) with the incidence of disease in the population (between 20% and 25%),” Mr. Petsonk said. Although the study calls into question the integrity of the system and the doctors within it, it’s critical for miners to know that the system is working and that they can get benefits, he explained. Many fear that cynicism about the system drives miners away and causes them to resort to Social Security or long-term disability.
Fixing what’s broken
The Annals study’s authors propose some solutions to the problems they quantified. The first is a sort of “super panel” that collectively evaluates readings. Although a completely unbiased panel would be nice, such impartiality is likely unsustainable, Mr. Smith said. He believes that over time, the panel would become vulnerable to politics and would work in favor of the companies.
Even without a panel, a method to provide greater transparency could be a great start, some suggest. The DOL could make the entire FBLP database public and analyze it annually. The authors also propose a flat fee for readings. Even now, Dr. Adcock said he doesn’t make anywhere close to the upper limit of $750 per readings. “My understanding is around $125 is a pretty characteristic fee [for reading a chest x-ray],” he elaborated. “Everyone I’ve had a conversation with is within 25 bucks [of that].”
That said, Dr. Adcock is not currently listed among the heavy readers who appear in the data used for the study; it’s possible that his experience is not representative. Some readers who were included in that dataset read more than 10 times the average number of classifications per reader – the average was 242 classifications – and read 95% of chest x-rays as negative, according to Dr. Friedman. This news organization obtained the names of two doctors whose readings were 95% negative on a high volume of cases. Neither agreed to an interview.
It’s possible that if the dataset had included readings from more recent years, Dr. Adcock would have appeared more frequently, given his personal estimates. That’s why the study authors recommend that the DOL conduct this kind of analysis annually in order to get an accurate picture of who is contributing to these cases, in what way, and how often. By doing so, readers who appear biased could be identified and addressed with more regularity, Dr. Friedman said.
Even if the rate were more consistent and the data were more frequently analyzed, the very nature of the adversarial system will put any potential solution at risk. “I’m not sure there’s a foolproof system that can be devised that can’t be corrupted in time,” Mr. Cline said.
A version of this article first appeared on Medscape.com.
In 2008, the U.S. Department of Labor (DOL) paid for Tony Adams, a 48-year-old coal miner, to have a chest x-ray. His doctor found stage I black lung disease. Yet Mr. Adams’ claim for medical benefits was denied. This was because the insurance group that represented his employer hired a different – more credentialed – doctor as its medical expert. That doctor said he saw no such evidence. The judge ruled in favor of the mining company on the basis of the latter’s “expertise.”
Before he died 5 years later, at age 53, Mr. Adams went through this process again. In fact, he did it four more times. Each time, his doctor found evidence of black lung, but the company’s medical expert did not. He died without receiving benefits. Among the causes of death listed on his autopsy were cardiopulmonary arrest and coal worker’s pneumoconiosis (CWP): black lung.
Since his death in 2013, two judges have awarded Mr. Adams’ benefits to his widow, Linda. Both times, the mining company appealed the decision, most recently in December 2020. She’s not giving up. “Two weeks before he died, he told me, ‘I’m going to die of black lung,’ ” Linda recalled. “‘But I don’t want you to give up on black lung. There are too many people screwing these miners out of what they deserve.’”
There has long been suspicion among miners and their advocates that doctors used by coal companies to fight claims like Mr. Adams’ are in the pocket of “Big Coal.” At the very least, some say these physicians are swayed by their client’s preference when reading a coal miner’s chest x-ray. A recent study published in Annals of the American Thoracic Society provides empirical evidence that these doctors’ conflict of interest – namely, that parties representing coal companies hired them – appears to influence their medical opinion.
Proof of a ‘broken system’
The Annals study examined 63,780 radiograph classifications made by 264 physicians – all certified as B-readers, a certification by the National Institute for Occupational Safety and Health (NIOSH) for physicians who demonstrate proficiency in classifying radiographs of pneumoconiosis. The results showed that doctors hired by miners identified black lung 49% of the time; those hired by coal companies identified black lung only 15% of the time.
The study also found that B-readers contracted by employers read results differently for different clients. The same doctors were significantly less likely to say a miner’s lungs were negative for CWP when they were hired by the DOL (77.2%) than when they were hired by a coal company or its insurers (90.2%).
The bias does appear to work both ways: B-readers hired by miners and miners’ attorneys were more likely to find evidence of black lung when they worked with plaintiffs. However, a much higher number of doctors appeared to be biased in favor of the companies. “There were 3X more B-readers providing 8X more classifications among those affiliated with employers compared to those affiliated with miners,” the study concluded.
The authors suggest that one reason for this was the difference in pay. Some company-hired doctors made as much as $750 per reading, about 10 times what miner-hired doctors were paid.
“We knew [about the potential bias] from our work over the decades taking care of these guys,” said Robert A. Cohen, MD, a pulmonologist and the study’s senior author. “But then you see it with P values that are incredibly statistically significant ...”
The study finally put numbers to a problem that many working with black lung claims had always assumed. Those within the system are accustomed to seeing names of the same doctors on documents and reports, with little to no overlap between those hired by the defense and the plaintiffs.
“The vast majority of the time, we know what a report will say based on the doctor’s name,” said Evan Smith, JD, advocacy director at AppalReD Legal Aid, in Prestonsburg, Ky.. It is far more surprising, he said, when a defense-hired doctor agrees with a miner-hired doctor.
Over the years, Katherine DePonte, MD, a radiologist and B-reader in West Virginia, has often seen an “almost textbook appearance” of CWP, only to later learn that “another radiologist read it as negative.” She explained, “They would use some other term, like ‘old granulomatous disease.’”
Employer-hired doctors often do acknowledge the same lung damage on the radiograph as miner-hired docs; they simply don’t attribute it to coal dust. Common “alternative diagnoses” include chronic obstructive pulmonary disease or histoplasmosis. “I know a number don’t believe this disease of coal worker pneumoconiosis exists [at all],” Dr. DePonte said.
What’s inarguable is that, even as coal mining in Appalachia is on the decline, black lung disease is on the rise. NIOSH now estimates that it affects over 20% of long-term (25+ years) coal workers in central Appalachia. That’s the highest prevalence in a quarter of a century.
Mr. Smith said that at its most basic level, these doctors’ conflicts of interest “lead to people who have the disease that these benefits are for, having them denied.” People like Tony Adams. Whether the doctors involved are complicit or just conservative, critics say they have become a fixture of a broken system.
Financial bias or difference of opinion?
Broken system or not, evidence suggests that the problem can’t be blamed solely on medical experts. Dr. DePonte primarily reads for the DOL and miners. “Not that I necessarily chose that,” she said. “You get pigeonholed.”
Some say that the bias demonstrated by the Annals study is at least partially driven by the litigation process itself. It is an adversarial system. As such, attorneys on both sides are naturally inclined to seek out doctors who will best support their clients’ cases. Doctors with a legitimately conservative perspective on what constitutes black lung are more sought after by the coal companies’ attorneys.
“It can often be impossible to tell whether the money is driving a change in the behavior or if the behavior is causing them to be sought out,” said Matt McCoy, PhD, a medical ethicist who specializes in conflicts of interest at the University of Pennsylvania, Philadelphia.
Although some believe that certain doctors are driven purely by financial incentive and offer a specific reading to secure repeat business, B-readers can end up working exclusively for companies because of other reasons. Wes Addington, JD, an attorney at the Appalachian Citizens’ Law Center, Whitesburg, Ky., said some doctors appear to have an authentically different – often antiquated – view of the disease.
Perhaps the most extreme example is Paul Wheeler, MD, a highly credentialed Johns Hopkins radiologist who was exposed for false medical testimony in Chris Hamby’s 2013 Pulitzer Prize reporting. In 1,500 readings, Dr. Wheeler never diagnosed a single case of severe black lung. And yet, Dr. Cohen, Mr. Addington, Mr. Smith, and other experts all agree that Dr. Wheeler appeared to wholeheartedly believe that his view of black lung was accurate. That made him a valuable asset to mining companies.
Since Dr. Wheeler’s exposure, there has been a greater sense of accountability among B-readers, said John Cline, JD, a West Virginia–based attorney who represents miners with federal black lung claims. “Radiologists were thinking, ‘Somebody could be watching me.’ Even if they thought they were doing this in the shadows, it made people more cautious,” he said.
The data used in the Annals study predate Mr. Hamby’s investigation, going back to 2000. Thus, it is possible that, as Mr. Cline argues, things may be different now. However, Lee S. Friedman, PhD, associate professor at the University of Illinois at Chicago, who is the lead author of the study, remains skeptical.
“While the Wheeler case might have dampened some physicians [who were] completely skewing their readings always negative, I think it’s premature or incorrect” to say it resolved the issue, he said. “Did they all change their behavior the morning after? It doesn’t seem likely, given the evidence of financial conflicts of interest and behavior that’s been demonstrated.”
Skewing the evidence?
Mr. Hamby’s 2013 reporting also revealed that even when company-hired doctors did diagnose CWP, law firms were burying those readings. In 2016, the DOL attempted to stop this practice. The agency made suppression of written evidence illegal – emphasis on written.
Law firms can’t hide positive reports, but they can prevent them. Dr. Cohen explained that now, “a doctor on the phone says, ‘I will read this as positive.’ Then the company says, ‘No, thank you,’ we will send you a check.”
This practice was confirmed by Kim Adcock, MD, a retired radiologist and B-reader in Littleton, Colo., who primarily reads for 26 law firms. Some of his clients want a report no matter how he reads the radiograph. However, some want him to call them first if he’s going to read the radiograph as positive. Dr. Adcock said this practice skews the dataset to make company-hired docs appear to read more negatively than they actually do.
Because the dataset used in the study is from the Federal Black Lung Program (FBLP), it includes only readings that made it to court. Dr. Adcock said he reads approximately 2,000 radiographs a year, although only a few of his readings appeared in the study’s dataset, according to a search by Dr. Friedman. This difference is likely because the study evaluated only readings between 2000 and 2013, the year Dr. Adcock started B-reading.
“I think it’s important to get a message that, to a certain extent, contravenes this paper. Yes, we should have some reservations about the conclusions,” Dr. Adcock explained. “There are people out there attempting to do the best job they could do.”
Law firms shopping for the reading they want and censoring the ones they don’t might alter the FBLP data, but experts say that doesn’t change the underlying problem. “In any case like this, where you’re looking at individuals going up against corporations,” Dr. McCoy said, “[corporations] are able to marshal their resources and hire more officials in a way claimants can’t, and that’s a baseline concern here.”
Battling bias
Admitting bias is notoriously difficult; thus, it isn’t surprising that many doctors involved refuse to believe they are influenced by money, incentives, or other biases. Dr. DePonte said she’s not swayed by money, nor does she actively take a pro-miner stance. She views herself as more of an advocate for accuracy. However, she did say that it has traditionally been far more difficult for miners to prove their cases, a problem that has improved with new regulations in recent years.
In Colorado, Dr. Adcock’s approach is to stay as far removed from the litigation process as possible. He said he has limited understanding of how his reports are used or how claims are filed and awarded. He leans heavily on his initial – almost instantaneous – impression of a chest x-ray.
Dr. DePonte and Dr. Adcock were both hired as experts on Tony Adams’ case. In 2008, Dr. DePonte read his chest x-ray as positive for early-stage black lung (1/0). Dr. Adcock also read two of Adams’ four chest x-rays, one in 2009 and the other in 2013. He read them as negative. When asked about the case, which autopsy confirmed as black lung, Dr. Adcock explained that positive histopathology doesn’t mean the radiograph reading was wrong, only that the disease didn’t show on that radiograph. He said his “highest ambition” is to be “an objective finder of fact” and that he trusts the process to work out the truth.
That process didn’t work in time for Tony Adams. Dr. Friedman argues that people who provide expert testimony have an ethical responsibility to know how their testimony is being used; to do otherwise, he says, is “willful ignorance.” Still, the Annals study authors, along with Dr. DePonte, Mr. Cline, and West Virginia attorney Sam Petsonk, say that the process is getting fairer, thanks to new policies developed over the past 5 years by the DOL.
“The DOL has worked very hard to reconcile the final award rate (around 30%) with the incidence of disease in the population (between 20% and 25%),” Mr. Petsonk said. Although the study calls into question the integrity of the system and the doctors within it, it’s critical for miners to know that the system is working and that they can get benefits, he explained. Many fear that cynicism about the system drives miners away and causes them to resort to Social Security or long-term disability.
Fixing what’s broken
The Annals study’s authors propose some solutions to the problems they quantified. The first is a sort of “super panel” that collectively evaluates readings. Although a completely unbiased panel would be nice, such impartiality is likely unsustainable, Mr. Smith said. He believes that over time, the panel would become vulnerable to politics and would work in favor of the companies.
Even without a panel, a method to provide greater transparency could be a great start, some suggest. The DOL could make the entire FBLP database public and analyze it annually. The authors also propose a flat fee for readings. Even now, Dr. Adcock said he doesn’t make anywhere close to the upper limit of $750 per readings. “My understanding is around $125 is a pretty characteristic fee [for reading a chest x-ray],” he elaborated. “Everyone I’ve had a conversation with is within 25 bucks [of that].”
That said, Dr. Adcock is not currently listed among the heavy readers who appear in the data used for the study; it’s possible that his experience is not representative. Some readers who were included in that dataset read more than 10 times the average number of classifications per reader – the average was 242 classifications – and read 95% of chest x-rays as negative, according to Dr. Friedman. This news organization obtained the names of two doctors whose readings were 95% negative on a high volume of cases. Neither agreed to an interview.
It’s possible that if the dataset had included readings from more recent years, Dr. Adcock would have appeared more frequently, given his personal estimates. That’s why the study authors recommend that the DOL conduct this kind of analysis annually in order to get an accurate picture of who is contributing to these cases, in what way, and how often. By doing so, readers who appear biased could be identified and addressed with more regularity, Dr. Friedman said.
Even if the rate were more consistent and the data were more frequently analyzed, the very nature of the adversarial system will put any potential solution at risk. “I’m not sure there’s a foolproof system that can be devised that can’t be corrupted in time,” Mr. Cline said.
A version of this article first appeared on Medscape.com.
In 2008, the U.S. Department of Labor (DOL) paid for Tony Adams, a 48-year-old coal miner, to have a chest x-ray. His doctor found stage I black lung disease. Yet Mr. Adams’ claim for medical benefits was denied. This was because the insurance group that represented his employer hired a different – more credentialed – doctor as its medical expert. That doctor said he saw no such evidence. The judge ruled in favor of the mining company on the basis of the latter’s “expertise.”
Before he died 5 years later, at age 53, Mr. Adams went through this process again. In fact, he did it four more times. Each time, his doctor found evidence of black lung, but the company’s medical expert did not. He died without receiving benefits. Among the causes of death listed on his autopsy were cardiopulmonary arrest and coal worker’s pneumoconiosis (CWP): black lung.
Since his death in 2013, two judges have awarded Mr. Adams’ benefits to his widow, Linda. Both times, the mining company appealed the decision, most recently in December 2020. She’s not giving up. “Two weeks before he died, he told me, ‘I’m going to die of black lung,’ ” Linda recalled. “‘But I don’t want you to give up on black lung. There are too many people screwing these miners out of what they deserve.’”
There has long been suspicion among miners and their advocates that doctors used by coal companies to fight claims like Mr. Adams’ are in the pocket of “Big Coal.” At the very least, some say these physicians are swayed by their client’s preference when reading a coal miner’s chest x-ray. A recent study published in Annals of the American Thoracic Society provides empirical evidence that these doctors’ conflict of interest – namely, that parties representing coal companies hired them – appears to influence their medical opinion.
Proof of a ‘broken system’
The Annals study examined 63,780 radiograph classifications made by 264 physicians – all certified as B-readers, a certification by the National Institute for Occupational Safety and Health (NIOSH) for physicians who demonstrate proficiency in classifying radiographs of pneumoconiosis. The results showed that doctors hired by miners identified black lung 49% of the time; those hired by coal companies identified black lung only 15% of the time.
The study also found that B-readers contracted by employers read results differently for different clients. The same doctors were significantly less likely to say a miner’s lungs were negative for CWP when they were hired by the DOL (77.2%) than when they were hired by a coal company or its insurers (90.2%).
The bias does appear to work both ways: B-readers hired by miners and miners’ attorneys were more likely to find evidence of black lung when they worked with plaintiffs. However, a much higher number of doctors appeared to be biased in favor of the companies. “There were 3X more B-readers providing 8X more classifications among those affiliated with employers compared to those affiliated with miners,” the study concluded.
The authors suggest that one reason for this was the difference in pay. Some company-hired doctors made as much as $750 per reading, about 10 times what miner-hired doctors were paid.
“We knew [about the potential bias] from our work over the decades taking care of these guys,” said Robert A. Cohen, MD, a pulmonologist and the study’s senior author. “But then you see it with P values that are incredibly statistically significant ...”
The study finally put numbers to a problem that many working with black lung claims had always assumed. Those within the system are accustomed to seeing names of the same doctors on documents and reports, with little to no overlap between those hired by the defense and the plaintiffs.
“The vast majority of the time, we know what a report will say based on the doctor’s name,” said Evan Smith, JD, advocacy director at AppalReD Legal Aid, in Prestonsburg, Ky.. It is far more surprising, he said, when a defense-hired doctor agrees with a miner-hired doctor.
Over the years, Katherine DePonte, MD, a radiologist and B-reader in West Virginia, has often seen an “almost textbook appearance” of CWP, only to later learn that “another radiologist read it as negative.” She explained, “They would use some other term, like ‘old granulomatous disease.’”
Employer-hired doctors often do acknowledge the same lung damage on the radiograph as miner-hired docs; they simply don’t attribute it to coal dust. Common “alternative diagnoses” include chronic obstructive pulmonary disease or histoplasmosis. “I know a number don’t believe this disease of coal worker pneumoconiosis exists [at all],” Dr. DePonte said.
What’s inarguable is that, even as coal mining in Appalachia is on the decline, black lung disease is on the rise. NIOSH now estimates that it affects over 20% of long-term (25+ years) coal workers in central Appalachia. That’s the highest prevalence in a quarter of a century.
Mr. Smith said that at its most basic level, these doctors’ conflicts of interest “lead to people who have the disease that these benefits are for, having them denied.” People like Tony Adams. Whether the doctors involved are complicit or just conservative, critics say they have become a fixture of a broken system.
Financial bias or difference of opinion?
Broken system or not, evidence suggests that the problem can’t be blamed solely on medical experts. Dr. DePonte primarily reads for the DOL and miners. “Not that I necessarily chose that,” she said. “You get pigeonholed.”
Some say that the bias demonstrated by the Annals study is at least partially driven by the litigation process itself. It is an adversarial system. As such, attorneys on both sides are naturally inclined to seek out doctors who will best support their clients’ cases. Doctors with a legitimately conservative perspective on what constitutes black lung are more sought after by the coal companies’ attorneys.
“It can often be impossible to tell whether the money is driving a change in the behavior or if the behavior is causing them to be sought out,” said Matt McCoy, PhD, a medical ethicist who specializes in conflicts of interest at the University of Pennsylvania, Philadelphia.
Although some believe that certain doctors are driven purely by financial incentive and offer a specific reading to secure repeat business, B-readers can end up working exclusively for companies because of other reasons. Wes Addington, JD, an attorney at the Appalachian Citizens’ Law Center, Whitesburg, Ky., said some doctors appear to have an authentically different – often antiquated – view of the disease.
Perhaps the most extreme example is Paul Wheeler, MD, a highly credentialed Johns Hopkins radiologist who was exposed for false medical testimony in Chris Hamby’s 2013 Pulitzer Prize reporting. In 1,500 readings, Dr. Wheeler never diagnosed a single case of severe black lung. And yet, Dr. Cohen, Mr. Addington, Mr. Smith, and other experts all agree that Dr. Wheeler appeared to wholeheartedly believe that his view of black lung was accurate. That made him a valuable asset to mining companies.
Since Dr. Wheeler’s exposure, there has been a greater sense of accountability among B-readers, said John Cline, JD, a West Virginia–based attorney who represents miners with federal black lung claims. “Radiologists were thinking, ‘Somebody could be watching me.’ Even if they thought they were doing this in the shadows, it made people more cautious,” he said.
The data used in the Annals study predate Mr. Hamby’s investigation, going back to 2000. Thus, it is possible that, as Mr. Cline argues, things may be different now. However, Lee S. Friedman, PhD, associate professor at the University of Illinois at Chicago, who is the lead author of the study, remains skeptical.
“While the Wheeler case might have dampened some physicians [who were] completely skewing their readings always negative, I think it’s premature or incorrect” to say it resolved the issue, he said. “Did they all change their behavior the morning after? It doesn’t seem likely, given the evidence of financial conflicts of interest and behavior that’s been demonstrated.”
Skewing the evidence?
Mr. Hamby’s 2013 reporting also revealed that even when company-hired doctors did diagnose CWP, law firms were burying those readings. In 2016, the DOL attempted to stop this practice. The agency made suppression of written evidence illegal – emphasis on written.
Law firms can’t hide positive reports, but they can prevent them. Dr. Cohen explained that now, “a doctor on the phone says, ‘I will read this as positive.’ Then the company says, ‘No, thank you,’ we will send you a check.”
This practice was confirmed by Kim Adcock, MD, a retired radiologist and B-reader in Littleton, Colo., who primarily reads for 26 law firms. Some of his clients want a report no matter how he reads the radiograph. However, some want him to call them first if he’s going to read the radiograph as positive. Dr. Adcock said this practice skews the dataset to make company-hired docs appear to read more negatively than they actually do.
Because the dataset used in the study is from the Federal Black Lung Program (FBLP), it includes only readings that made it to court. Dr. Adcock said he reads approximately 2,000 radiographs a year, although only a few of his readings appeared in the study’s dataset, according to a search by Dr. Friedman. This difference is likely because the study evaluated only readings between 2000 and 2013, the year Dr. Adcock started B-reading.
“I think it’s important to get a message that, to a certain extent, contravenes this paper. Yes, we should have some reservations about the conclusions,” Dr. Adcock explained. “There are people out there attempting to do the best job they could do.”
Law firms shopping for the reading they want and censoring the ones they don’t might alter the FBLP data, but experts say that doesn’t change the underlying problem. “In any case like this, where you’re looking at individuals going up against corporations,” Dr. McCoy said, “[corporations] are able to marshal their resources and hire more officials in a way claimants can’t, and that’s a baseline concern here.”
Battling bias
Admitting bias is notoriously difficult; thus, it isn’t surprising that many doctors involved refuse to believe they are influenced by money, incentives, or other biases. Dr. DePonte said she’s not swayed by money, nor does she actively take a pro-miner stance. She views herself as more of an advocate for accuracy. However, she did say that it has traditionally been far more difficult for miners to prove their cases, a problem that has improved with new regulations in recent years.
In Colorado, Dr. Adcock’s approach is to stay as far removed from the litigation process as possible. He said he has limited understanding of how his reports are used or how claims are filed and awarded. He leans heavily on his initial – almost instantaneous – impression of a chest x-ray.
Dr. DePonte and Dr. Adcock were both hired as experts on Tony Adams’ case. In 2008, Dr. DePonte read his chest x-ray as positive for early-stage black lung (1/0). Dr. Adcock also read two of Adams’ four chest x-rays, one in 2009 and the other in 2013. He read them as negative. When asked about the case, which autopsy confirmed as black lung, Dr. Adcock explained that positive histopathology doesn’t mean the radiograph reading was wrong, only that the disease didn’t show on that radiograph. He said his “highest ambition” is to be “an objective finder of fact” and that he trusts the process to work out the truth.
That process didn’t work in time for Tony Adams. Dr. Friedman argues that people who provide expert testimony have an ethical responsibility to know how their testimony is being used; to do otherwise, he says, is “willful ignorance.” Still, the Annals study authors, along with Dr. DePonte, Mr. Cline, and West Virginia attorney Sam Petsonk, say that the process is getting fairer, thanks to new policies developed over the past 5 years by the DOL.
“The DOL has worked very hard to reconcile the final award rate (around 30%) with the incidence of disease in the population (between 20% and 25%),” Mr. Petsonk said. Although the study calls into question the integrity of the system and the doctors within it, it’s critical for miners to know that the system is working and that they can get benefits, he explained. Many fear that cynicism about the system drives miners away and causes them to resort to Social Security or long-term disability.
Fixing what’s broken
The Annals study’s authors propose some solutions to the problems they quantified. The first is a sort of “super panel” that collectively evaluates readings. Although a completely unbiased panel would be nice, such impartiality is likely unsustainable, Mr. Smith said. He believes that over time, the panel would become vulnerable to politics and would work in favor of the companies.
Even without a panel, a method to provide greater transparency could be a great start, some suggest. The DOL could make the entire FBLP database public and analyze it annually. The authors also propose a flat fee for readings. Even now, Dr. Adcock said he doesn’t make anywhere close to the upper limit of $750 per readings. “My understanding is around $125 is a pretty characteristic fee [for reading a chest x-ray],” he elaborated. “Everyone I’ve had a conversation with is within 25 bucks [of that].”
That said, Dr. Adcock is not currently listed among the heavy readers who appear in the data used for the study; it’s possible that his experience is not representative. Some readers who were included in that dataset read more than 10 times the average number of classifications per reader – the average was 242 classifications – and read 95% of chest x-rays as negative, according to Dr. Friedman. This news organization obtained the names of two doctors whose readings were 95% negative on a high volume of cases. Neither agreed to an interview.
It’s possible that if the dataset had included readings from more recent years, Dr. Adcock would have appeared more frequently, given his personal estimates. That’s why the study authors recommend that the DOL conduct this kind of analysis annually in order to get an accurate picture of who is contributing to these cases, in what way, and how often. By doing so, readers who appear biased could be identified and addressed with more regularity, Dr. Friedman said.
Even if the rate were more consistent and the data were more frequently analyzed, the very nature of the adversarial system will put any potential solution at risk. “I’m not sure there’s a foolproof system that can be devised that can’t be corrupted in time,” Mr. Cline said.
A version of this article first appeared on Medscape.com.
OSHA issues new rules on COVID-19 safety for health care workers
The U.S. Occupational Safety and Health Administration issued its long-awaited Emergency Temporary Standard (ETS) for COVID-19 June 10, surprising many by including only health care workers in the new emergency workplace safety rules.
“The ETS is an overdue step toward protecting health care workers, especially those working in long-term care facilities and home health care who are at greatly increased risk of infection,” said George Washington University, Washington, professor and former Obama administration Assistant Secretary of Labor David Michaels, PhD, MPH. “OSHA’s failure to issue a COVID-specific standard in other high-risk industries, like meat and poultry processing, corrections, homeless shelters, and retail establishments is disappointing. If exposure is not controlled in these workplaces, they will continue to be important drivers of infections.”
With the new regulations in place, about 10.3 million health care workers at hospitals, nursing homes, and assisted living facilities, as well as emergency responders and home health care workers, should be guaranteed protection standards that replace former guidance.
The new protections include supplying personal protective equipment and ensuring proper usage (for example, mandatory seal checks on respirators); screening everyone who enters the facility for COVID-19; ensuring proper ventilation; and establishing physical distancing requirements (6 feet) for unvaccinated workers. It also requires employers to give workers time off for vaccination. An antiretaliation clause could shield workers who complain about unsafe conditions.
“The science tells us that health care workers, particularly those who come into regular contact with the virus, are most at risk at this point in the pandemic,” Labor Secretary Marty Walsh said on a press call. “So following an extensive review of the science and data, OSHA determined that a health care–specific safety requirement will make the biggest impact.”
But questions remain, said James Brudney, JD, a professor at Fordham Law School in New York and former chief counsel of the U.S. Senate Subcommittee on Labor. The standard doesn’t amplify or address existing rules regarding a right to refuse unsafe work, for example, so employees may still feel they are risking their jobs to complain, despite the antiretaliation clause.
And although vaccinated employees don’t have to adhere to the same distancing and masking standards in many instances, the standard doesn’t spell out how employers should determine their workers’ vaccination status – instead leaving that determination to employers through their own policies and procedures. (California’s state OSHA office rules specify the mechanism for documentation of vaccination.)
The Trump administration did not issue an ETS, saying OSHA’s general duty clause sufficed. President Joe Biden took the opposite approach, calling for an investigation into an ETS on his first day in office. But the process took months longer than promised.
“I know it’s been a long time coming,” Mr. Walsh acknowledged. “Our health care workers from the very beginning have been put at risk.
While health care unions had asked for mandated safety standards sooner, National Nurses United, the country’s largest labor union for registered nurses, still welcomed the rules.
“An ETS is a major step toward requiring accountability for hospitals who consistently put their budget goals and profits over our health and safety,” Zenei Triunfo-Cortez, RN, one of NNU’s three presidents, said in a statement June 9 anticipating the publication of the rules.
The rules do not apply to retail pharmacies, ambulatory care settings that screen nonemployees for COVID-19, or certain other settings in which all employees are vaccinated and people with suspected or confirmed COVID-19 cannot enter.
The agency said it will work with states that have already issued local regulations, including two states that issued temporary standards of their own, Virginia and California.
Employers will have 2 weeks to comply with most of the regulations after they’re published in the Federal Register. The standards will expire in 6 months but could then become permanent, as Virginia’s did in January.
A version of this article first appeared on Medscape.com.
The U.S. Occupational Safety and Health Administration issued its long-awaited Emergency Temporary Standard (ETS) for COVID-19 June 10, surprising many by including only health care workers in the new emergency workplace safety rules.
“The ETS is an overdue step toward protecting health care workers, especially those working in long-term care facilities and home health care who are at greatly increased risk of infection,” said George Washington University, Washington, professor and former Obama administration Assistant Secretary of Labor David Michaels, PhD, MPH. “OSHA’s failure to issue a COVID-specific standard in other high-risk industries, like meat and poultry processing, corrections, homeless shelters, and retail establishments is disappointing. If exposure is not controlled in these workplaces, they will continue to be important drivers of infections.”
With the new regulations in place, about 10.3 million health care workers at hospitals, nursing homes, and assisted living facilities, as well as emergency responders and home health care workers, should be guaranteed protection standards that replace former guidance.
The new protections include supplying personal protective equipment and ensuring proper usage (for example, mandatory seal checks on respirators); screening everyone who enters the facility for COVID-19; ensuring proper ventilation; and establishing physical distancing requirements (6 feet) for unvaccinated workers. It also requires employers to give workers time off for vaccination. An antiretaliation clause could shield workers who complain about unsafe conditions.
“The science tells us that health care workers, particularly those who come into regular contact with the virus, are most at risk at this point in the pandemic,” Labor Secretary Marty Walsh said on a press call. “So following an extensive review of the science and data, OSHA determined that a health care–specific safety requirement will make the biggest impact.”
But questions remain, said James Brudney, JD, a professor at Fordham Law School in New York and former chief counsel of the U.S. Senate Subcommittee on Labor. The standard doesn’t amplify or address existing rules regarding a right to refuse unsafe work, for example, so employees may still feel they are risking their jobs to complain, despite the antiretaliation clause.
And although vaccinated employees don’t have to adhere to the same distancing and masking standards in many instances, the standard doesn’t spell out how employers should determine their workers’ vaccination status – instead leaving that determination to employers through their own policies and procedures. (California’s state OSHA office rules specify the mechanism for documentation of vaccination.)
The Trump administration did not issue an ETS, saying OSHA’s general duty clause sufficed. President Joe Biden took the opposite approach, calling for an investigation into an ETS on his first day in office. But the process took months longer than promised.
“I know it’s been a long time coming,” Mr. Walsh acknowledged. “Our health care workers from the very beginning have been put at risk.
While health care unions had asked for mandated safety standards sooner, National Nurses United, the country’s largest labor union for registered nurses, still welcomed the rules.
“An ETS is a major step toward requiring accountability for hospitals who consistently put their budget goals and profits over our health and safety,” Zenei Triunfo-Cortez, RN, one of NNU’s three presidents, said in a statement June 9 anticipating the publication of the rules.
The rules do not apply to retail pharmacies, ambulatory care settings that screen nonemployees for COVID-19, or certain other settings in which all employees are vaccinated and people with suspected or confirmed COVID-19 cannot enter.
The agency said it will work with states that have already issued local regulations, including two states that issued temporary standards of their own, Virginia and California.
Employers will have 2 weeks to comply with most of the regulations after they’re published in the Federal Register. The standards will expire in 6 months but could then become permanent, as Virginia’s did in January.
A version of this article first appeared on Medscape.com.
The U.S. Occupational Safety and Health Administration issued its long-awaited Emergency Temporary Standard (ETS) for COVID-19 June 10, surprising many by including only health care workers in the new emergency workplace safety rules.
“The ETS is an overdue step toward protecting health care workers, especially those working in long-term care facilities and home health care who are at greatly increased risk of infection,” said George Washington University, Washington, professor and former Obama administration Assistant Secretary of Labor David Michaels, PhD, MPH. “OSHA’s failure to issue a COVID-specific standard in other high-risk industries, like meat and poultry processing, corrections, homeless shelters, and retail establishments is disappointing. If exposure is not controlled in these workplaces, they will continue to be important drivers of infections.”
With the new regulations in place, about 10.3 million health care workers at hospitals, nursing homes, and assisted living facilities, as well as emergency responders and home health care workers, should be guaranteed protection standards that replace former guidance.
The new protections include supplying personal protective equipment and ensuring proper usage (for example, mandatory seal checks on respirators); screening everyone who enters the facility for COVID-19; ensuring proper ventilation; and establishing physical distancing requirements (6 feet) for unvaccinated workers. It also requires employers to give workers time off for vaccination. An antiretaliation clause could shield workers who complain about unsafe conditions.
“The science tells us that health care workers, particularly those who come into regular contact with the virus, are most at risk at this point in the pandemic,” Labor Secretary Marty Walsh said on a press call. “So following an extensive review of the science and data, OSHA determined that a health care–specific safety requirement will make the biggest impact.”
But questions remain, said James Brudney, JD, a professor at Fordham Law School in New York and former chief counsel of the U.S. Senate Subcommittee on Labor. The standard doesn’t amplify or address existing rules regarding a right to refuse unsafe work, for example, so employees may still feel they are risking their jobs to complain, despite the antiretaliation clause.
And although vaccinated employees don’t have to adhere to the same distancing and masking standards in many instances, the standard doesn’t spell out how employers should determine their workers’ vaccination status – instead leaving that determination to employers through their own policies and procedures. (California’s state OSHA office rules specify the mechanism for documentation of vaccination.)
The Trump administration did not issue an ETS, saying OSHA’s general duty clause sufficed. President Joe Biden took the opposite approach, calling for an investigation into an ETS on his first day in office. But the process took months longer than promised.
“I know it’s been a long time coming,” Mr. Walsh acknowledged. “Our health care workers from the very beginning have been put at risk.
While health care unions had asked for mandated safety standards sooner, National Nurses United, the country’s largest labor union for registered nurses, still welcomed the rules.
“An ETS is a major step toward requiring accountability for hospitals who consistently put their budget goals and profits over our health and safety,” Zenei Triunfo-Cortez, RN, one of NNU’s three presidents, said in a statement June 9 anticipating the publication of the rules.
The rules do not apply to retail pharmacies, ambulatory care settings that screen nonemployees for COVID-19, or certain other settings in which all employees are vaccinated and people with suspected or confirmed COVID-19 cannot enter.
The agency said it will work with states that have already issued local regulations, including two states that issued temporary standards of their own, Virginia and California.
Employers will have 2 weeks to comply with most of the regulations after they’re published in the Federal Register. The standards will expire in 6 months but could then become permanent, as Virginia’s did in January.
A version of this article first appeared on Medscape.com.
Mavrilimumab may aid severe COVID-19 recovery
Inhibiting granulocyte/macrophage–colony stimulating factor (GM-CSF) with mavrilimumab prevented some patients with severe COVID-19 pneumonia and hyperinflammation from needing mechanical ventilation and reduced their risk of dying versus placebo in a phase 2 study.
There was no difference in outcomes between the two doses of mavrilimumab used in the trial (6 mg/kg or 10 mg/kg) and combined data showed a higher percentage of patients achieving the primary endpoint of being alive and free of mechanical ventilation at 29 days, at 87%, versus placebo, at 74%.
The P value was 0.12, “which achieved the prespecified evidentiary standard of 0.2,” according to Lara Pupim, MD, vice president of clinical research and development at Kiniksa Pharmaceuticals in Lexington, Mass.
Importantly, there was a 61% reduction in the risk of dying if patients had received mavrilimumab rather than placebo, she reported at the annual European Congress of Rheumatology. Mortality at day 29 was 21% in the placebo arm but just 8% in the combined mavrilimumab arms (P = .07).
Hendrik Schulze-Koops, MD, called it a “surprising study” and that “the outcome is very spectacular” in his short appraisal of the study during the Clinical Highlights session on the final day of the congress.
Mavrilimumab was “a compound that we would not have thought that would have such an impact on the outcome of COVID-19 infected patients,” Dr. Schulze-Koops of Ludwig Maximilian University of Munich added.
In this small study, “there was a consistent suggestion of a biological effect across key endpoints,” Richard Conway, MBChB, PhD, a consultant rheumatologist at St. James’s Hospital in Dublin, pointed out in an interview.
“Similar to tocilizumab, the benefits with mavrilimumab appear to be in addition to those seen with glucocorticoids, as 96% of patients received dexamethasone,” Dr. Conway observed. Furthermore, nearly one-third received antiviral or remdesivir treatment.
“This study was likely underpowered to assess a clinically meaningful benefit,” he said, adding that “there is insufficient evidence at present to begin using mavrilimumab as an alternative to currently available agents.” That said, “these results are promising for future studies.”
Rationale for GM-CSF inhibition with mavrilimumab in COVID-19 pneumonia
“The cytokine GM-CSF is vital to both lung homeostasis and regulation of inflammation in autoimmunity,” Dr. Pupim explained.
She added that “GM-CSF is implicated in the mechanism of aberrant immune cell infiltration and activation in the lungs, and it may contribute to respiratory failure and death in patients with severe COVID-19 pneumonia and systemic hyperinflammation.”
The efficacy and safety of blocking GM-CSF with mavrilimumab have been shown previously in phase 2 studies in other diseases, Dr. Pupim noted. This includes patients with rheumatoid arthritis and those with giant cell arteritis.
“It was hypothesized that GM-CSF receptor–alpha blockade may reduce infiltration of pathogenic cells into the lung and may suppress inflammation in COVID-19 pneumonia in hyperinflammation,” she explained.
Study details and other outcome results
The study presented by Dr. Pupim was a phase 2/3 double-blind, placebo-controlled trial predominantly conducted in Brazil, the United States, and South Africa, with some participation in Peru and Chile.
Patients were eligible for inclusion if they had had a positive COVID-19 test within 14 days of randomization and had been hospitalized but not ventilated. Evidence of bilateral pneumonia on chest x-ray or CT scan and clinical laboratory evidence indicative of hyperinflammation were also prerequisites for study enrollment.
The ongoing study comprised two cohorts, Dr. Pupim explained: patients who have not been ventilated and those who have recently been ventilated. Dr. Pupim presented the data on the nonventilated cohort, noting that there was a total of 116 patients aged a mean of 57 years.
Patients were randomized to one of three treatment arms: two groups received a single intravenous infusion of mavrilimumab, either 6 mg/kg or 10 mg/kg, and the third group got a placebo.
“Using a time-to-event approach, looking at mechanical ventilation-free survival, mavrilimumab recipients experienced a 65% reduction in the risk of mechanical ventilation or death,” Dr. Pupim said (P = .0175).
“Separation in the Kaplan-Meier curves was evident very early after study drug administration,” she added.
There were trends toward a faster benefit with mavrilimumab than placebo in two other key secondary endpoints: the median time to achieving a two-point clinical improvement (7 vs. 11 days) and the median time to room air (7 vs. 9 days).
Timing of mavrilimumab administration and safety
Study coauthor and chief clinical development officer at Kiniksa, Arian Pano, MD, answered questions on the presentation. When asked about the timing of giving mavrilimumab, he said: “Based on these data it is before they go to ventilation, as soon as you have symptoms of hyperinflammation and a need for oxygen.”
Mavrilimumab is given as a single infusion “and has been well tolerated; virtually no interruptions occurred in this study.”
No serious adverse events related to mavrilimumab were seen, and adverse events, including secondary infections, which are known complications of COVID-19, occurred less frequently in mavrilimumab recipients, compared with placebo.
Dr. Pupim reported that there was a case of tuberculosis in one patient treated with mavrilimumab (10 mg/kg). That case had occurred in an “endemic area for tuberculosis,” and the patient had been screened before entry but only via a sputum sample.
“Prior to these events, the patient received high-dose corticosteroids, a known risk factor for reactivation of TB, and thus the potential additive contribution of mavrilimumab, if any, is uncertain.” Dr. Pupim said.
“Thrombotic events, another known complication of COVID-19, occurred in the placebo arm only,” she added.
Dr. Pano commented that the study has now “seamlessly continued to phase 3. So, basically, we did not stop the study. At the end of phase 2, we just locked the database and collected the data.” Both the 6 mg/kg and 10 mg/kg are being studied, but it’s “very likely [that] 6 mg/kg could be the dose that we may bring forward to the clinic in terms of registration, but that’s at this point in time. We will need to wait for the phase 3 data,” he observed. Those findings will hopefully be available later this year.
Kiniksa funded the study. Dr. Pupim, Dr. Pano, and multiple study coinvestigators are employees of the company.
Dr. Schulze-Koops was not involved in the study and had no specific disclosures. Dr. Conway had no financial disclosures to make in relation to his comments.
Inhibiting granulocyte/macrophage–colony stimulating factor (GM-CSF) with mavrilimumab prevented some patients with severe COVID-19 pneumonia and hyperinflammation from needing mechanical ventilation and reduced their risk of dying versus placebo in a phase 2 study.
There was no difference in outcomes between the two doses of mavrilimumab used in the trial (6 mg/kg or 10 mg/kg) and combined data showed a higher percentage of patients achieving the primary endpoint of being alive and free of mechanical ventilation at 29 days, at 87%, versus placebo, at 74%.
The P value was 0.12, “which achieved the prespecified evidentiary standard of 0.2,” according to Lara Pupim, MD, vice president of clinical research and development at Kiniksa Pharmaceuticals in Lexington, Mass.
Importantly, there was a 61% reduction in the risk of dying if patients had received mavrilimumab rather than placebo, she reported at the annual European Congress of Rheumatology. Mortality at day 29 was 21% in the placebo arm but just 8% in the combined mavrilimumab arms (P = .07).
Hendrik Schulze-Koops, MD, called it a “surprising study” and that “the outcome is very spectacular” in his short appraisal of the study during the Clinical Highlights session on the final day of the congress.
Mavrilimumab was “a compound that we would not have thought that would have such an impact on the outcome of COVID-19 infected patients,” Dr. Schulze-Koops of Ludwig Maximilian University of Munich added.
In this small study, “there was a consistent suggestion of a biological effect across key endpoints,” Richard Conway, MBChB, PhD, a consultant rheumatologist at St. James’s Hospital in Dublin, pointed out in an interview.
“Similar to tocilizumab, the benefits with mavrilimumab appear to be in addition to those seen with glucocorticoids, as 96% of patients received dexamethasone,” Dr. Conway observed. Furthermore, nearly one-third received antiviral or remdesivir treatment.
“This study was likely underpowered to assess a clinically meaningful benefit,” he said, adding that “there is insufficient evidence at present to begin using mavrilimumab as an alternative to currently available agents.” That said, “these results are promising for future studies.”
Rationale for GM-CSF inhibition with mavrilimumab in COVID-19 pneumonia
“The cytokine GM-CSF is vital to both lung homeostasis and regulation of inflammation in autoimmunity,” Dr. Pupim explained.
She added that “GM-CSF is implicated in the mechanism of aberrant immune cell infiltration and activation in the lungs, and it may contribute to respiratory failure and death in patients with severe COVID-19 pneumonia and systemic hyperinflammation.”
The efficacy and safety of blocking GM-CSF with mavrilimumab have been shown previously in phase 2 studies in other diseases, Dr. Pupim noted. This includes patients with rheumatoid arthritis and those with giant cell arteritis.
“It was hypothesized that GM-CSF receptor–alpha blockade may reduce infiltration of pathogenic cells into the lung and may suppress inflammation in COVID-19 pneumonia in hyperinflammation,” she explained.
Study details and other outcome results
The study presented by Dr. Pupim was a phase 2/3 double-blind, placebo-controlled trial predominantly conducted in Brazil, the United States, and South Africa, with some participation in Peru and Chile.
Patients were eligible for inclusion if they had had a positive COVID-19 test within 14 days of randomization and had been hospitalized but not ventilated. Evidence of bilateral pneumonia on chest x-ray or CT scan and clinical laboratory evidence indicative of hyperinflammation were also prerequisites for study enrollment.
The ongoing study comprised two cohorts, Dr. Pupim explained: patients who have not been ventilated and those who have recently been ventilated. Dr. Pupim presented the data on the nonventilated cohort, noting that there was a total of 116 patients aged a mean of 57 years.
Patients were randomized to one of three treatment arms: two groups received a single intravenous infusion of mavrilimumab, either 6 mg/kg or 10 mg/kg, and the third group got a placebo.
“Using a time-to-event approach, looking at mechanical ventilation-free survival, mavrilimumab recipients experienced a 65% reduction in the risk of mechanical ventilation or death,” Dr. Pupim said (P = .0175).
“Separation in the Kaplan-Meier curves was evident very early after study drug administration,” she added.
There were trends toward a faster benefit with mavrilimumab than placebo in two other key secondary endpoints: the median time to achieving a two-point clinical improvement (7 vs. 11 days) and the median time to room air (7 vs. 9 days).
Timing of mavrilimumab administration and safety
Study coauthor and chief clinical development officer at Kiniksa, Arian Pano, MD, answered questions on the presentation. When asked about the timing of giving mavrilimumab, he said: “Based on these data it is before they go to ventilation, as soon as you have symptoms of hyperinflammation and a need for oxygen.”
Mavrilimumab is given as a single infusion “and has been well tolerated; virtually no interruptions occurred in this study.”
No serious adverse events related to mavrilimumab were seen, and adverse events, including secondary infections, which are known complications of COVID-19, occurred less frequently in mavrilimumab recipients, compared with placebo.
Dr. Pupim reported that there was a case of tuberculosis in one patient treated with mavrilimumab (10 mg/kg). That case had occurred in an “endemic area for tuberculosis,” and the patient had been screened before entry but only via a sputum sample.
“Prior to these events, the patient received high-dose corticosteroids, a known risk factor for reactivation of TB, and thus the potential additive contribution of mavrilimumab, if any, is uncertain.” Dr. Pupim said.
“Thrombotic events, another known complication of COVID-19, occurred in the placebo arm only,” she added.
Dr. Pano commented that the study has now “seamlessly continued to phase 3. So, basically, we did not stop the study. At the end of phase 2, we just locked the database and collected the data.” Both the 6 mg/kg and 10 mg/kg are being studied, but it’s “very likely [that] 6 mg/kg could be the dose that we may bring forward to the clinic in terms of registration, but that’s at this point in time. We will need to wait for the phase 3 data,” he observed. Those findings will hopefully be available later this year.
Kiniksa funded the study. Dr. Pupim, Dr. Pano, and multiple study coinvestigators are employees of the company.
Dr. Schulze-Koops was not involved in the study and had no specific disclosures. Dr. Conway had no financial disclosures to make in relation to his comments.
Inhibiting granulocyte/macrophage–colony stimulating factor (GM-CSF) with mavrilimumab prevented some patients with severe COVID-19 pneumonia and hyperinflammation from needing mechanical ventilation and reduced their risk of dying versus placebo in a phase 2 study.
There was no difference in outcomes between the two doses of mavrilimumab used in the trial (6 mg/kg or 10 mg/kg) and combined data showed a higher percentage of patients achieving the primary endpoint of being alive and free of mechanical ventilation at 29 days, at 87%, versus placebo, at 74%.
The P value was 0.12, “which achieved the prespecified evidentiary standard of 0.2,” according to Lara Pupim, MD, vice president of clinical research and development at Kiniksa Pharmaceuticals in Lexington, Mass.
Importantly, there was a 61% reduction in the risk of dying if patients had received mavrilimumab rather than placebo, she reported at the annual European Congress of Rheumatology. Mortality at day 29 was 21% in the placebo arm but just 8% in the combined mavrilimumab arms (P = .07).
Hendrik Schulze-Koops, MD, called it a “surprising study” and that “the outcome is very spectacular” in his short appraisal of the study during the Clinical Highlights session on the final day of the congress.
Mavrilimumab was “a compound that we would not have thought that would have such an impact on the outcome of COVID-19 infected patients,” Dr. Schulze-Koops of Ludwig Maximilian University of Munich added.
In this small study, “there was a consistent suggestion of a biological effect across key endpoints,” Richard Conway, MBChB, PhD, a consultant rheumatologist at St. James’s Hospital in Dublin, pointed out in an interview.
“Similar to tocilizumab, the benefits with mavrilimumab appear to be in addition to those seen with glucocorticoids, as 96% of patients received dexamethasone,” Dr. Conway observed. Furthermore, nearly one-third received antiviral or remdesivir treatment.
“This study was likely underpowered to assess a clinically meaningful benefit,” he said, adding that “there is insufficient evidence at present to begin using mavrilimumab as an alternative to currently available agents.” That said, “these results are promising for future studies.”
Rationale for GM-CSF inhibition with mavrilimumab in COVID-19 pneumonia
“The cytokine GM-CSF is vital to both lung homeostasis and regulation of inflammation in autoimmunity,” Dr. Pupim explained.
She added that “GM-CSF is implicated in the mechanism of aberrant immune cell infiltration and activation in the lungs, and it may contribute to respiratory failure and death in patients with severe COVID-19 pneumonia and systemic hyperinflammation.”
The efficacy and safety of blocking GM-CSF with mavrilimumab have been shown previously in phase 2 studies in other diseases, Dr. Pupim noted. This includes patients with rheumatoid arthritis and those with giant cell arteritis.
“It was hypothesized that GM-CSF receptor–alpha blockade may reduce infiltration of pathogenic cells into the lung and may suppress inflammation in COVID-19 pneumonia in hyperinflammation,” she explained.
Study details and other outcome results
The study presented by Dr. Pupim was a phase 2/3 double-blind, placebo-controlled trial predominantly conducted in Brazil, the United States, and South Africa, with some participation in Peru and Chile.
Patients were eligible for inclusion if they had had a positive COVID-19 test within 14 days of randomization and had been hospitalized but not ventilated. Evidence of bilateral pneumonia on chest x-ray or CT scan and clinical laboratory evidence indicative of hyperinflammation were also prerequisites for study enrollment.
The ongoing study comprised two cohorts, Dr. Pupim explained: patients who have not been ventilated and those who have recently been ventilated. Dr. Pupim presented the data on the nonventilated cohort, noting that there was a total of 116 patients aged a mean of 57 years.
Patients were randomized to one of three treatment arms: two groups received a single intravenous infusion of mavrilimumab, either 6 mg/kg or 10 mg/kg, and the third group got a placebo.
“Using a time-to-event approach, looking at mechanical ventilation-free survival, mavrilimumab recipients experienced a 65% reduction in the risk of mechanical ventilation or death,” Dr. Pupim said (P = .0175).
“Separation in the Kaplan-Meier curves was evident very early after study drug administration,” she added.
There were trends toward a faster benefit with mavrilimumab than placebo in two other key secondary endpoints: the median time to achieving a two-point clinical improvement (7 vs. 11 days) and the median time to room air (7 vs. 9 days).
Timing of mavrilimumab administration and safety
Study coauthor and chief clinical development officer at Kiniksa, Arian Pano, MD, answered questions on the presentation. When asked about the timing of giving mavrilimumab, he said: “Based on these data it is before they go to ventilation, as soon as you have symptoms of hyperinflammation and a need for oxygen.”
Mavrilimumab is given as a single infusion “and has been well tolerated; virtually no interruptions occurred in this study.”
No serious adverse events related to mavrilimumab were seen, and adverse events, including secondary infections, which are known complications of COVID-19, occurred less frequently in mavrilimumab recipients, compared with placebo.
Dr. Pupim reported that there was a case of tuberculosis in one patient treated with mavrilimumab (10 mg/kg). That case had occurred in an “endemic area for tuberculosis,” and the patient had been screened before entry but only via a sputum sample.
“Prior to these events, the patient received high-dose corticosteroids, a known risk factor for reactivation of TB, and thus the potential additive contribution of mavrilimumab, if any, is uncertain.” Dr. Pupim said.
“Thrombotic events, another known complication of COVID-19, occurred in the placebo arm only,” she added.
Dr. Pano commented that the study has now “seamlessly continued to phase 3. So, basically, we did not stop the study. At the end of phase 2, we just locked the database and collected the data.” Both the 6 mg/kg and 10 mg/kg are being studied, but it’s “very likely [that] 6 mg/kg could be the dose that we may bring forward to the clinic in terms of registration, but that’s at this point in time. We will need to wait for the phase 3 data,” he observed. Those findings will hopefully be available later this year.
Kiniksa funded the study. Dr. Pupim, Dr. Pano, and multiple study coinvestigators are employees of the company.
Dr. Schulze-Koops was not involved in the study and had no specific disclosures. Dr. Conway had no financial disclosures to make in relation to his comments.
FROM EULAR 2021 CONGRESS
Vinorelbine survival benefit in mesothelioma overshadowed by advances in immuno-oncology
After decades of off-label use, vinorelbine finally has a randomized clinical trial supporting its efficacy as a second-line treatment of mesothelioma, an investigator reported at the annual meeting of the American Society of Clinical Oncology.
However, the development of other treatment regimens, and notably immuno-oncology approaches, are pushing this classic chemotherapy option to later lines of therapy in patients with malignant pleural mesothelioma (MPM), a speaker said at the meeting.
Adding vinorelbine to active symptom control statistically improved progression-free survival (PFS), among patients with MPM who had prior platinum-based therapy, according to results of the randomized Vinorelbine in Mesothelioma (VIM) trial.
Median PFS reached 4.2 months in the vinorelbine arm, versus 2.8 months for active symptom control alone, study results show.
That finding , coupled with safety results, supports the off-label use of vinorelbine as a treatment option for patients with relapsed MPM, according to investigator Dean Anthony Fennell, FRCP, PhD, of the University of Leicester (England).
“Vinorelbine appears to be a safe and effective treatment and could be considered as a treatment option for patients with relapsed mesothelioma,” Prof. Fennell said in his presentation at ASCO (Abstract 8507).
Changing status
While that welcome pronouncement was a long time coming, there are now other promising treatment approaches that relegate vinorelbine to a “lower priority” in the treatment algorithm, said discussant Anna K. Nowak, MBBS, FRACP, PhD, of the University of Western Australia, Nedlands.
In October 2020, the U.S. Food and Drug Administration approved the combination of ipilimumab and nivolumab for the first-line treatment of unresectable malignant pleural mesothelioma, on the basis of results from CHECKMATE-743, a randomized, open-label trial.
“Certainly, we know now that first-line ipilimumab and nivolumab is of very substantial benefit to these patients, and we still have clinical trials open in this space as well,” Prof. Nowak said in her discussion at ASCO.
There is “no doubt” that many patients with mesothelioma should at some point receive an IO agent, particularly now with recently reported randomized clinical trial evidence of an overall survival benefit, she added.
“This really pushes vinorelbine out to be a third- or fourth-line treatment,” she added, “and we know that there are usually diminishing returns from using chemotherapies further down the treatment algorithm.”
Trial details
The VIM trial described at ASCO by Prof. Fennell was a randomized, controlled phase 2 including 154 patients with MPM that had progressed after first-line chemotherapy.
“Vinorelbine has shown useful clinical activity in single-arm phase two studies, however, the specific efficacy of vinorelbine has not been evaluated in an appropriately controlled randomized trial,” Prof. Fennell said in this presentation.
Patients in the trial were randomized 2:1 to either vinorelbine plus active supportive care or active supportive care alone. Vinorelbine was given initially at 60 mg/m2 weekly every 21 days, escalated to 80 mg/m2 from cycle 2.
The median age of patients in VIM was approximately 71 years, and about 80% were male.
More partial responses were seen in the vinorelbine arm, at 3.1% of patients, compared with 1.8% for active supportive care, according to Prof. Fennell. Likewise, the rate of stable disease was higher in the vinorelbine arm, at 62.2%, versus 46.4% in the control arm.
The primary outcome of the study, PFS, was significantly improved in the vinorelbine arm, according to Dr. Fennell. The median PFS was 4.2 months in the vinorelbine arm and 2.8 months in the supportive care arm (P = .002), translating into a hazard ratio of 0.60 (95% confidence interval, 0.41-0.86), Prof. Fennell reported.
The most common grade 3-4 adverse event was neutropenia, occurring in 12.5% of the vinorelbine-treated patients and no patients managed with supportive care alone, according to the report. Other grade 3-4 adverse events occurred in fewer than 10% of patients and included dyspnea, lower respiratory infection, lymphopenia, and fatigue, among others.
Overall survival (OS) was not statistically different between vinorelbine and supportive care arms, with median OS of 9.3 months and 9.1 months, respectively.
However, a number of patients in the control arm went on to receive subsequent therapy, including 15 (or about 27%) who went into CONFIRM, a randomized phase 3 trial that, as recently reported, met its coprimary endpoints of improve OS and PFS with nivolumab vs. placebo in relapsed malignant mesothelioma.
Investigators also sought to test the hypothesis that BRCA1-negative patients might be chemoresistant, based in part on preclinical models demonstrating that BRCA1 predicted sensitivity to vinorelbine. However, there was no difference in PFS by BRCA1 expression in the VIM study, according to Prof. Fennell.
Taken together, findings of the VIM trial suggest vinorelbine is a “modestly active” agent with low cost and acceptable toxicity, according to Prof. Nowak.
“It is incumbent on us to have clear discussions with our patients on the risks and benefits of trying this as a subsequent-line therapy, in the context of this evidence that was generated as a second-line therapy,” she said in her discussant remarks on the study.
“I would say that it is a lower priority in our algorithm than cisplatin and pemetrexed, or of course, immuno-oncology agents,” she added.
Dr. Fennell reported disclosures related to AstraZeneca, Astex Therapeutics, Bayer, and multiple other pharmaceutical companies. Dr. Nowak reported disclosures with AstraZeneca, Atara Biotherapeutics, Boehringer Ingelheim, and multiple other pharmaceutical companies.
After decades of off-label use, vinorelbine finally has a randomized clinical trial supporting its efficacy as a second-line treatment of mesothelioma, an investigator reported at the annual meeting of the American Society of Clinical Oncology.
However, the development of other treatment regimens, and notably immuno-oncology approaches, are pushing this classic chemotherapy option to later lines of therapy in patients with malignant pleural mesothelioma (MPM), a speaker said at the meeting.
Adding vinorelbine to active symptom control statistically improved progression-free survival (PFS), among patients with MPM who had prior platinum-based therapy, according to results of the randomized Vinorelbine in Mesothelioma (VIM) trial.
Median PFS reached 4.2 months in the vinorelbine arm, versus 2.8 months for active symptom control alone, study results show.
That finding , coupled with safety results, supports the off-label use of vinorelbine as a treatment option for patients with relapsed MPM, according to investigator Dean Anthony Fennell, FRCP, PhD, of the University of Leicester (England).
“Vinorelbine appears to be a safe and effective treatment and could be considered as a treatment option for patients with relapsed mesothelioma,” Prof. Fennell said in his presentation at ASCO (Abstract 8507).
Changing status
While that welcome pronouncement was a long time coming, there are now other promising treatment approaches that relegate vinorelbine to a “lower priority” in the treatment algorithm, said discussant Anna K. Nowak, MBBS, FRACP, PhD, of the University of Western Australia, Nedlands.
In October 2020, the U.S. Food and Drug Administration approved the combination of ipilimumab and nivolumab for the first-line treatment of unresectable malignant pleural mesothelioma, on the basis of results from CHECKMATE-743, a randomized, open-label trial.
“Certainly, we know now that first-line ipilimumab and nivolumab is of very substantial benefit to these patients, and we still have clinical trials open in this space as well,” Prof. Nowak said in her discussion at ASCO.
There is “no doubt” that many patients with mesothelioma should at some point receive an IO agent, particularly now with recently reported randomized clinical trial evidence of an overall survival benefit, she added.
“This really pushes vinorelbine out to be a third- or fourth-line treatment,” she added, “and we know that there are usually diminishing returns from using chemotherapies further down the treatment algorithm.”
Trial details
The VIM trial described at ASCO by Prof. Fennell was a randomized, controlled phase 2 including 154 patients with MPM that had progressed after first-line chemotherapy.
“Vinorelbine has shown useful clinical activity in single-arm phase two studies, however, the specific efficacy of vinorelbine has not been evaluated in an appropriately controlled randomized trial,” Prof. Fennell said in this presentation.
Patients in the trial were randomized 2:1 to either vinorelbine plus active supportive care or active supportive care alone. Vinorelbine was given initially at 60 mg/m2 weekly every 21 days, escalated to 80 mg/m2 from cycle 2.
The median age of patients in VIM was approximately 71 years, and about 80% were male.
More partial responses were seen in the vinorelbine arm, at 3.1% of patients, compared with 1.8% for active supportive care, according to Prof. Fennell. Likewise, the rate of stable disease was higher in the vinorelbine arm, at 62.2%, versus 46.4% in the control arm.
The primary outcome of the study, PFS, was significantly improved in the vinorelbine arm, according to Dr. Fennell. The median PFS was 4.2 months in the vinorelbine arm and 2.8 months in the supportive care arm (P = .002), translating into a hazard ratio of 0.60 (95% confidence interval, 0.41-0.86), Prof. Fennell reported.
The most common grade 3-4 adverse event was neutropenia, occurring in 12.5% of the vinorelbine-treated patients and no patients managed with supportive care alone, according to the report. Other grade 3-4 adverse events occurred in fewer than 10% of patients and included dyspnea, lower respiratory infection, lymphopenia, and fatigue, among others.
Overall survival (OS) was not statistically different between vinorelbine and supportive care arms, with median OS of 9.3 months and 9.1 months, respectively.
However, a number of patients in the control arm went on to receive subsequent therapy, including 15 (or about 27%) who went into CONFIRM, a randomized phase 3 trial that, as recently reported, met its coprimary endpoints of improve OS and PFS with nivolumab vs. placebo in relapsed malignant mesothelioma.
Investigators also sought to test the hypothesis that BRCA1-negative patients might be chemoresistant, based in part on preclinical models demonstrating that BRCA1 predicted sensitivity to vinorelbine. However, there was no difference in PFS by BRCA1 expression in the VIM study, according to Prof. Fennell.
Taken together, findings of the VIM trial suggest vinorelbine is a “modestly active” agent with low cost and acceptable toxicity, according to Prof. Nowak.
“It is incumbent on us to have clear discussions with our patients on the risks and benefits of trying this as a subsequent-line therapy, in the context of this evidence that was generated as a second-line therapy,” she said in her discussant remarks on the study.
“I would say that it is a lower priority in our algorithm than cisplatin and pemetrexed, or of course, immuno-oncology agents,” she added.
Dr. Fennell reported disclosures related to AstraZeneca, Astex Therapeutics, Bayer, and multiple other pharmaceutical companies. Dr. Nowak reported disclosures with AstraZeneca, Atara Biotherapeutics, Boehringer Ingelheim, and multiple other pharmaceutical companies.
After decades of off-label use, vinorelbine finally has a randomized clinical trial supporting its efficacy as a second-line treatment of mesothelioma, an investigator reported at the annual meeting of the American Society of Clinical Oncology.
However, the development of other treatment regimens, and notably immuno-oncology approaches, are pushing this classic chemotherapy option to later lines of therapy in patients with malignant pleural mesothelioma (MPM), a speaker said at the meeting.
Adding vinorelbine to active symptom control statistically improved progression-free survival (PFS), among patients with MPM who had prior platinum-based therapy, according to results of the randomized Vinorelbine in Mesothelioma (VIM) trial.
Median PFS reached 4.2 months in the vinorelbine arm, versus 2.8 months for active symptom control alone, study results show.
That finding , coupled with safety results, supports the off-label use of vinorelbine as a treatment option for patients with relapsed MPM, according to investigator Dean Anthony Fennell, FRCP, PhD, of the University of Leicester (England).
“Vinorelbine appears to be a safe and effective treatment and could be considered as a treatment option for patients with relapsed mesothelioma,” Prof. Fennell said in his presentation at ASCO (Abstract 8507).
Changing status
While that welcome pronouncement was a long time coming, there are now other promising treatment approaches that relegate vinorelbine to a “lower priority” in the treatment algorithm, said discussant Anna K. Nowak, MBBS, FRACP, PhD, of the University of Western Australia, Nedlands.
In October 2020, the U.S. Food and Drug Administration approved the combination of ipilimumab and nivolumab for the first-line treatment of unresectable malignant pleural mesothelioma, on the basis of results from CHECKMATE-743, a randomized, open-label trial.
“Certainly, we know now that first-line ipilimumab and nivolumab is of very substantial benefit to these patients, and we still have clinical trials open in this space as well,” Prof. Nowak said in her discussion at ASCO.
There is “no doubt” that many patients with mesothelioma should at some point receive an IO agent, particularly now with recently reported randomized clinical trial evidence of an overall survival benefit, she added.
“This really pushes vinorelbine out to be a third- or fourth-line treatment,” she added, “and we know that there are usually diminishing returns from using chemotherapies further down the treatment algorithm.”
Trial details
The VIM trial described at ASCO by Prof. Fennell was a randomized, controlled phase 2 including 154 patients with MPM that had progressed after first-line chemotherapy.
“Vinorelbine has shown useful clinical activity in single-arm phase two studies, however, the specific efficacy of vinorelbine has not been evaluated in an appropriately controlled randomized trial,” Prof. Fennell said in this presentation.
Patients in the trial were randomized 2:1 to either vinorelbine plus active supportive care or active supportive care alone. Vinorelbine was given initially at 60 mg/m2 weekly every 21 days, escalated to 80 mg/m2 from cycle 2.
The median age of patients in VIM was approximately 71 years, and about 80% were male.
More partial responses were seen in the vinorelbine arm, at 3.1% of patients, compared with 1.8% for active supportive care, according to Prof. Fennell. Likewise, the rate of stable disease was higher in the vinorelbine arm, at 62.2%, versus 46.4% in the control arm.
The primary outcome of the study, PFS, was significantly improved in the vinorelbine arm, according to Dr. Fennell. The median PFS was 4.2 months in the vinorelbine arm and 2.8 months in the supportive care arm (P = .002), translating into a hazard ratio of 0.60 (95% confidence interval, 0.41-0.86), Prof. Fennell reported.
The most common grade 3-4 adverse event was neutropenia, occurring in 12.5% of the vinorelbine-treated patients and no patients managed with supportive care alone, according to the report. Other grade 3-4 adverse events occurred in fewer than 10% of patients and included dyspnea, lower respiratory infection, lymphopenia, and fatigue, among others.
Overall survival (OS) was not statistically different between vinorelbine and supportive care arms, with median OS of 9.3 months and 9.1 months, respectively.
However, a number of patients in the control arm went on to receive subsequent therapy, including 15 (or about 27%) who went into CONFIRM, a randomized phase 3 trial that, as recently reported, met its coprimary endpoints of improve OS and PFS with nivolumab vs. placebo in relapsed malignant mesothelioma.
Investigators also sought to test the hypothesis that BRCA1-negative patients might be chemoresistant, based in part on preclinical models demonstrating that BRCA1 predicted sensitivity to vinorelbine. However, there was no difference in PFS by BRCA1 expression in the VIM study, according to Prof. Fennell.
Taken together, findings of the VIM trial suggest vinorelbine is a “modestly active” agent with low cost and acceptable toxicity, according to Prof. Nowak.
“It is incumbent on us to have clear discussions with our patients on the risks and benefits of trying this as a subsequent-line therapy, in the context of this evidence that was generated as a second-line therapy,” she said in her discussant remarks on the study.
“I would say that it is a lower priority in our algorithm than cisplatin and pemetrexed, or of course, immuno-oncology agents,” she added.
Dr. Fennell reported disclosures related to AstraZeneca, Astex Therapeutics, Bayer, and multiple other pharmaceutical companies. Dr. Nowak reported disclosures with AstraZeneca, Atara Biotherapeutics, Boehringer Ingelheim, and multiple other pharmaceutical companies.
REPORTING FROM ASCO 2021
Free U.K. tool could help guide COVID-19 care for cancer patients
An online support tool for health care professionals that recommends whether to admit or discharge a cancer patient with COVID-19, based on their risk of a severe complication, has been developed by researchers from Manchester.
The team used machine learning on data from more than 900 cancer patients with COVID-19, conducting multiple analyses to arrive at a set of features that could accurately predict the need for admission or oxygen therapy, as well as the risk of death.
Dr. Rebecca Lee, The Christie NHS Foundation Trust, Manchester, and colleagues then developed thresholds to derive a score that recommended admission in 95% of patients who went on to need oxygen and an even greater proportion of those who later died.
The research was presented at the 2021 American Society of Clinical Oncology (ASCO) Annual Meeting on June 4.
CORONET
The resulting COVID-19 Risk in Oncology Evaluation Tool (CORONET) model “performs very well at predicting admission and severity of COVID-19 in patients with cancer,” Dr. Lee said. “We have set pragmatic and clinically relevant thresholds that focus on the safety regarding an admission versus discharge decision.”
To help health care professionals, the researchers have built a free online support tool that allows them to enter data and receive a recommendation “as to whether their patient should be considered for discharge, considered for admission, or is at high risk of having a severe outcome of coronavirus,” Dr. Lee explained.
“The health care professional can then explore the recommendation by seeing how their patient … compares with the rest of the cohort.”
The tool also includes a “diagram showing which features are most important to recommend a discharge decision versus an admission decision for each individual patient.”
Clinically intuitive
Dr. Alexi Wright, associate professor, Dana-Faber Cancer Institute, Boston, who was not involved in the study, commented that there were many things that were “really nice about the study.”
“First and foremost that they were establishing a tool to efficiently triage [patients] presenting with COVID,” she said, adding that it was “clinically intuitive” that the team made “pragmatic choices,” and the use of a random forest algorithm means the results are “very interpretable.”
However, Dr. Wright wondered whether the results can be replicated.
Alongside a lack of information on the deaths in the cohort, she pointed out that “ideally you have three data sets, with a training set, a testing set, and a validation set.”
The CORONET model was, however, trained and evaluated on the same dataset, “so it really needs external validation before it would be ready for direct clinical application.”
She continued that there is a “critical need to establish that studies can both be reproduced and replicated,” noting that a recent review showed that 85% of machine-learning studies that were used to detect COVID-19 using chest radiographs “failed fundamental reproducibility and quality checks.”
Risk factors
Dr. Lee began her presentation by reminding the audience that cancer patients are at increased risk of severe COVID-19 and death, with older age, male sex, nosocomial infection, higher ECOG performance status, and active cancer among the risk factors for mortality.
“However, outcomes are very heterogeneous, ranging from patients without symptoms at all to cases with multi-organ failure and death,” she said.
It is consequently “very important for the treating clinician to determine which patients could be safely discharged to the community versus those who need additional support in being admitted to hospital.”
To develop a tool that could distinguish between those two groups of patients, the researchers collected data on 1,743 cancer patients, which was reduced down to 920 patients after excluding those without laboratory confirmed COVID-19 and those with missing data.
Using recursive feature elimination, they selected 10 key patient features associated with prognosis, then compared a lasso regression model with a random forest model, with the latter performing the best.
The team then divided their patients into four cohorts, with the model trained on three cohorts and tested on the fourth. This resulted in the CORONET score, with the final model determined by testing it against the entire patient population.
Next, thresholds were determined for assessing patients for admission versus discharge, as well as for severity of illness, giving the final CORONET model, from which the online tool was developed.
Checking performance
The results showed that the model was able to predict admission with an area under the receiver operating characteristics curve (AUROC) of 0.82 for admission, 0.85 for oxygen requirement, and 0.79 for death.
Further analysis revealed that the most important feature at the time of presentation for determining outcome was the National Early Warning Score 2 (NEWS2), “which is a composite score of heart rate, respiratory rate, saturations and confusion level,” Dr. Lee said.
In addition, C-reactive protein levels, albumin, age, and platelet counts “were also very important features,” she continued, “and these have also been shown in a number of different studies to be important at determining the outcome from coronavirus.”
To examine the performance of the CORONET score further, they applied it to a European hospital dataset, ESMO-CoCARE registry data, and a U.S. cohort, the COVID-19 and Cancer Consortium Registry (CCC19). They found that the score discriminated between patients, but it did so with some degree of heterogeneity.
This was largely driven by higher patient age among the U.S. patients, a higher NEWS2 score, and lower albumin levels, Dr. Lee said.
To ensure the score’s applicability to clinical practice, the team set pragmatic thresholds to determine whether or not a patient required admission or whether they were at risk of dying.
For admission, they set a sensitivity of 85% and a specificity of 56%, while for mortality they set a sensitivity of 43% and a specificity of 92%.
When this was converted into a decision support tool, the model recommended hospital admission for 95% of patients who eventually required oxygen and 97% of patients who died.
The study was funded by The Christie Charitable Foundation. Dr. Lee declares relationships with AstraZeneca and Bristol-Myers Squibb (Inst). Dr. Wright declares relationships with NCCN/AstraZeneca (Inst).
A version of this article first appeared on Medscape.com.
An online support tool for health care professionals that recommends whether to admit or discharge a cancer patient with COVID-19, based on their risk of a severe complication, has been developed by researchers from Manchester.
The team used machine learning on data from more than 900 cancer patients with COVID-19, conducting multiple analyses to arrive at a set of features that could accurately predict the need for admission or oxygen therapy, as well as the risk of death.
Dr. Rebecca Lee, The Christie NHS Foundation Trust, Manchester, and colleagues then developed thresholds to derive a score that recommended admission in 95% of patients who went on to need oxygen and an even greater proportion of those who later died.
The research was presented at the 2021 American Society of Clinical Oncology (ASCO) Annual Meeting on June 4.
CORONET
The resulting COVID-19 Risk in Oncology Evaluation Tool (CORONET) model “performs very well at predicting admission and severity of COVID-19 in patients with cancer,” Dr. Lee said. “We have set pragmatic and clinically relevant thresholds that focus on the safety regarding an admission versus discharge decision.”
To help health care professionals, the researchers have built a free online support tool that allows them to enter data and receive a recommendation “as to whether their patient should be considered for discharge, considered for admission, or is at high risk of having a severe outcome of coronavirus,” Dr. Lee explained.
“The health care professional can then explore the recommendation by seeing how their patient … compares with the rest of the cohort.”
The tool also includes a “diagram showing which features are most important to recommend a discharge decision versus an admission decision for each individual patient.”
Clinically intuitive
Dr. Alexi Wright, associate professor, Dana-Faber Cancer Institute, Boston, who was not involved in the study, commented that there were many things that were “really nice about the study.”
“First and foremost that they were establishing a tool to efficiently triage [patients] presenting with COVID,” she said, adding that it was “clinically intuitive” that the team made “pragmatic choices,” and the use of a random forest algorithm means the results are “very interpretable.”
However, Dr. Wright wondered whether the results can be replicated.
Alongside a lack of information on the deaths in the cohort, she pointed out that “ideally you have three data sets, with a training set, a testing set, and a validation set.”
The CORONET model was, however, trained and evaluated on the same dataset, “so it really needs external validation before it would be ready for direct clinical application.”
She continued that there is a “critical need to establish that studies can both be reproduced and replicated,” noting that a recent review showed that 85% of machine-learning studies that were used to detect COVID-19 using chest radiographs “failed fundamental reproducibility and quality checks.”
Risk factors
Dr. Lee began her presentation by reminding the audience that cancer patients are at increased risk of severe COVID-19 and death, with older age, male sex, nosocomial infection, higher ECOG performance status, and active cancer among the risk factors for mortality.
“However, outcomes are very heterogeneous, ranging from patients without symptoms at all to cases with multi-organ failure and death,” she said.
It is consequently “very important for the treating clinician to determine which patients could be safely discharged to the community versus those who need additional support in being admitted to hospital.”
To develop a tool that could distinguish between those two groups of patients, the researchers collected data on 1,743 cancer patients, which was reduced down to 920 patients after excluding those without laboratory confirmed COVID-19 and those with missing data.
Using recursive feature elimination, they selected 10 key patient features associated with prognosis, then compared a lasso regression model with a random forest model, with the latter performing the best.
The team then divided their patients into four cohorts, with the model trained on three cohorts and tested on the fourth. This resulted in the CORONET score, with the final model determined by testing it against the entire patient population.
Next, thresholds were determined for assessing patients for admission versus discharge, as well as for severity of illness, giving the final CORONET model, from which the online tool was developed.
Checking performance
The results showed that the model was able to predict admission with an area under the receiver operating characteristics curve (AUROC) of 0.82 for admission, 0.85 for oxygen requirement, and 0.79 for death.
Further analysis revealed that the most important feature at the time of presentation for determining outcome was the National Early Warning Score 2 (NEWS2), “which is a composite score of heart rate, respiratory rate, saturations and confusion level,” Dr. Lee said.
In addition, C-reactive protein levels, albumin, age, and platelet counts “were also very important features,” she continued, “and these have also been shown in a number of different studies to be important at determining the outcome from coronavirus.”
To examine the performance of the CORONET score further, they applied it to a European hospital dataset, ESMO-CoCARE registry data, and a U.S. cohort, the COVID-19 and Cancer Consortium Registry (CCC19). They found that the score discriminated between patients, but it did so with some degree of heterogeneity.
This was largely driven by higher patient age among the U.S. patients, a higher NEWS2 score, and lower albumin levels, Dr. Lee said.
To ensure the score’s applicability to clinical practice, the team set pragmatic thresholds to determine whether or not a patient required admission or whether they were at risk of dying.
For admission, they set a sensitivity of 85% and a specificity of 56%, while for mortality they set a sensitivity of 43% and a specificity of 92%.
When this was converted into a decision support tool, the model recommended hospital admission for 95% of patients who eventually required oxygen and 97% of patients who died.
The study was funded by The Christie Charitable Foundation. Dr. Lee declares relationships with AstraZeneca and Bristol-Myers Squibb (Inst). Dr. Wright declares relationships with NCCN/AstraZeneca (Inst).
A version of this article first appeared on Medscape.com.
An online support tool for health care professionals that recommends whether to admit or discharge a cancer patient with COVID-19, based on their risk of a severe complication, has been developed by researchers from Manchester.
The team used machine learning on data from more than 900 cancer patients with COVID-19, conducting multiple analyses to arrive at a set of features that could accurately predict the need for admission or oxygen therapy, as well as the risk of death.
Dr. Rebecca Lee, The Christie NHS Foundation Trust, Manchester, and colleagues then developed thresholds to derive a score that recommended admission in 95% of patients who went on to need oxygen and an even greater proportion of those who later died.
The research was presented at the 2021 American Society of Clinical Oncology (ASCO) Annual Meeting on June 4.
CORONET
The resulting COVID-19 Risk in Oncology Evaluation Tool (CORONET) model “performs very well at predicting admission and severity of COVID-19 in patients with cancer,” Dr. Lee said. “We have set pragmatic and clinically relevant thresholds that focus on the safety regarding an admission versus discharge decision.”
To help health care professionals, the researchers have built a free online support tool that allows them to enter data and receive a recommendation “as to whether their patient should be considered for discharge, considered for admission, or is at high risk of having a severe outcome of coronavirus,” Dr. Lee explained.
“The health care professional can then explore the recommendation by seeing how their patient … compares with the rest of the cohort.”
The tool also includes a “diagram showing which features are most important to recommend a discharge decision versus an admission decision for each individual patient.”
Clinically intuitive
Dr. Alexi Wright, associate professor, Dana-Faber Cancer Institute, Boston, who was not involved in the study, commented that there were many things that were “really nice about the study.”
“First and foremost that they were establishing a tool to efficiently triage [patients] presenting with COVID,” she said, adding that it was “clinically intuitive” that the team made “pragmatic choices,” and the use of a random forest algorithm means the results are “very interpretable.”
However, Dr. Wright wondered whether the results can be replicated.
Alongside a lack of information on the deaths in the cohort, she pointed out that “ideally you have three data sets, with a training set, a testing set, and a validation set.”
The CORONET model was, however, trained and evaluated on the same dataset, “so it really needs external validation before it would be ready for direct clinical application.”
She continued that there is a “critical need to establish that studies can both be reproduced and replicated,” noting that a recent review showed that 85% of machine-learning studies that were used to detect COVID-19 using chest radiographs “failed fundamental reproducibility and quality checks.”
Risk factors
Dr. Lee began her presentation by reminding the audience that cancer patients are at increased risk of severe COVID-19 and death, with older age, male sex, nosocomial infection, higher ECOG performance status, and active cancer among the risk factors for mortality.
“However, outcomes are very heterogeneous, ranging from patients without symptoms at all to cases with multi-organ failure and death,” she said.
It is consequently “very important for the treating clinician to determine which patients could be safely discharged to the community versus those who need additional support in being admitted to hospital.”
To develop a tool that could distinguish between those two groups of patients, the researchers collected data on 1,743 cancer patients, which was reduced down to 920 patients after excluding those without laboratory confirmed COVID-19 and those with missing data.
Using recursive feature elimination, they selected 10 key patient features associated with prognosis, then compared a lasso regression model with a random forest model, with the latter performing the best.
The team then divided their patients into four cohorts, with the model trained on three cohorts and tested on the fourth. This resulted in the CORONET score, with the final model determined by testing it against the entire patient population.
Next, thresholds were determined for assessing patients for admission versus discharge, as well as for severity of illness, giving the final CORONET model, from which the online tool was developed.
Checking performance
The results showed that the model was able to predict admission with an area under the receiver operating characteristics curve (AUROC) of 0.82 for admission, 0.85 for oxygen requirement, and 0.79 for death.
Further analysis revealed that the most important feature at the time of presentation for determining outcome was the National Early Warning Score 2 (NEWS2), “which is a composite score of heart rate, respiratory rate, saturations and confusion level,” Dr. Lee said.
In addition, C-reactive protein levels, albumin, age, and platelet counts “were also very important features,” she continued, “and these have also been shown in a number of different studies to be important at determining the outcome from coronavirus.”
To examine the performance of the CORONET score further, they applied it to a European hospital dataset, ESMO-CoCARE registry data, and a U.S. cohort, the COVID-19 and Cancer Consortium Registry (CCC19). They found that the score discriminated between patients, but it did so with some degree of heterogeneity.
This was largely driven by higher patient age among the U.S. patients, a higher NEWS2 score, and lower albumin levels, Dr. Lee said.
To ensure the score’s applicability to clinical practice, the team set pragmatic thresholds to determine whether or not a patient required admission or whether they were at risk of dying.
For admission, they set a sensitivity of 85% and a specificity of 56%, while for mortality they set a sensitivity of 43% and a specificity of 92%.
When this was converted into a decision support tool, the model recommended hospital admission for 95% of patients who eventually required oxygen and 97% of patients who died.
The study was funded by The Christie Charitable Foundation. Dr. Lee declares relationships with AstraZeneca and Bristol-Myers Squibb (Inst). Dr. Wright declares relationships with NCCN/AstraZeneca (Inst).
A version of this article first appeared on Medscape.com.
By the numbers: Children and COVID-19 prevention
Over 6.3 million doses of COVID-19 vaccine have been administered to children aged 12-17 years as of June 7, according to data from the Centers for Disease Control and Prevention.
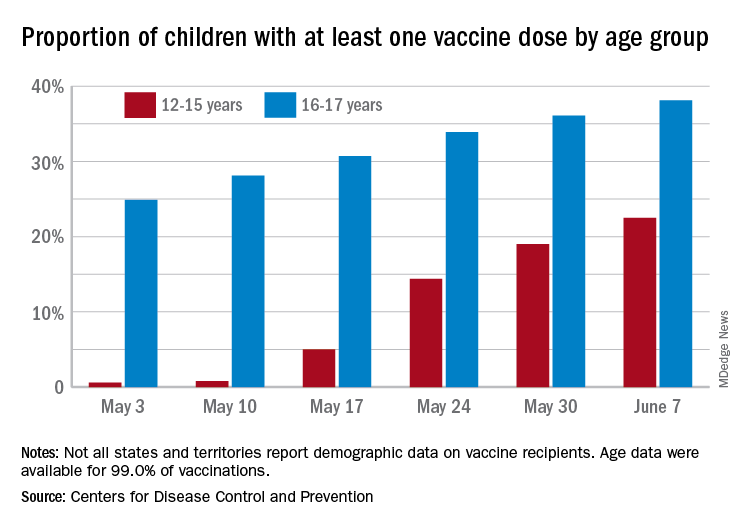
The latest results from the CDC’s COVID Data Tracker show that , with the corresponding figures for vaccine completion coming in at 4.1% and 26.4%. Compared with a week earlier, those numbers are up by 15.4% (one dose) and 486% (completion) for the younger group and by 4.7% and 8.6%, respectively, for the older children.
Children aged 12-15 represented 17.9% of all persons who initiated vaccination in the last 14 days up to June 7, while children aged 16-17 made up 4.8% of vaccine initiation over that period. The 25- to 39-year-olds, at 23.7% of all vaccine initiators, were the only group ahead of those aged 12-15, and the 50- to 64-year-olds were just behind at 17.7%, the CDC data show.
Both groups of children were on the low side, however, when it came to vaccine completion in the last 14 days, with those aged 12-15 at 6.7% of the total and those aged 16-17 years at 4.3%. The only age groups lower than that were ≥75 at 3.5% and <12 at 0.2%, and the highest share of vaccine completion was 26.0% for those aged 25-39, which also happens to be the group with the largest share of the U.S. population (20.5%), the CDC said.
People considered fully vaccinated are those who have received the second dose of a two-dose series or one dose of a single-shot vaccine, but children under age 18 years are eligible only for the Pfizer-BioNTech version, the CDC noted.
Meanwhile, back on the incidence side of the COVID-19 pandemic, the number of new cases in U.S. children for the week ending June 3 was at its lowest point (16,281) since mid-June of 2020, according to a report from the American Academy of Pediatrics and the Children’s Hospital Association.
Cases among children now total 3.99 million, which represents 14.1% of cases among all ages, a proportion that hasn’t increased since mid-May, which hasn’t happened since the two groups started keeping track in mid-April of 2020 in the 49 states (excluding New York), the District of Columbia, New York City, Puerto Rico, and Guam that report such data by age.
Less encouraging was the CDC’s report that “COVID-19-associated hospitalization rates among adolescents ages 12-17 years increased during March and April, following declines in January and February 2021.”
Children have been experiencing much lower rates of severe disease than those of adults throughout the pandemic, the CDC pointed out, but “recent increases in COVID-19-associated hospitalization rates and the potential for severe disease in adolescents reinforce the importance of continued prevention strategies, including vaccination and the correct and consistent use of masks in those who are not yet fully vaccinated.”
Over 6.3 million doses of COVID-19 vaccine have been administered to children aged 12-17 years as of June 7, according to data from the Centers for Disease Control and Prevention.

The latest results from the CDC’s COVID Data Tracker show that , with the corresponding figures for vaccine completion coming in at 4.1% and 26.4%. Compared with a week earlier, those numbers are up by 15.4% (one dose) and 486% (completion) for the younger group and by 4.7% and 8.6%, respectively, for the older children.
Children aged 12-15 represented 17.9% of all persons who initiated vaccination in the last 14 days up to June 7, while children aged 16-17 made up 4.8% of vaccine initiation over that period. The 25- to 39-year-olds, at 23.7% of all vaccine initiators, were the only group ahead of those aged 12-15, and the 50- to 64-year-olds were just behind at 17.7%, the CDC data show.
Both groups of children were on the low side, however, when it came to vaccine completion in the last 14 days, with those aged 12-15 at 6.7% of the total and those aged 16-17 years at 4.3%. The only age groups lower than that were ≥75 at 3.5% and <12 at 0.2%, and the highest share of vaccine completion was 26.0% for those aged 25-39, which also happens to be the group with the largest share of the U.S. population (20.5%), the CDC said.
People considered fully vaccinated are those who have received the second dose of a two-dose series or one dose of a single-shot vaccine, but children under age 18 years are eligible only for the Pfizer-BioNTech version, the CDC noted.
Meanwhile, back on the incidence side of the COVID-19 pandemic, the number of new cases in U.S. children for the week ending June 3 was at its lowest point (16,281) since mid-June of 2020, according to a report from the American Academy of Pediatrics and the Children’s Hospital Association.
Cases among children now total 3.99 million, which represents 14.1% of cases among all ages, a proportion that hasn’t increased since mid-May, which hasn’t happened since the two groups started keeping track in mid-April of 2020 in the 49 states (excluding New York), the District of Columbia, New York City, Puerto Rico, and Guam that report such data by age.
Less encouraging was the CDC’s report that “COVID-19-associated hospitalization rates among adolescents ages 12-17 years increased during March and April, following declines in January and February 2021.”
Children have been experiencing much lower rates of severe disease than those of adults throughout the pandemic, the CDC pointed out, but “recent increases in COVID-19-associated hospitalization rates and the potential for severe disease in adolescents reinforce the importance of continued prevention strategies, including vaccination and the correct and consistent use of masks in those who are not yet fully vaccinated.”
Over 6.3 million doses of COVID-19 vaccine have been administered to children aged 12-17 years as of June 7, according to data from the Centers for Disease Control and Prevention.

The latest results from the CDC’s COVID Data Tracker show that , with the corresponding figures for vaccine completion coming in at 4.1% and 26.4%. Compared with a week earlier, those numbers are up by 15.4% (one dose) and 486% (completion) for the younger group and by 4.7% and 8.6%, respectively, for the older children.
Children aged 12-15 represented 17.9% of all persons who initiated vaccination in the last 14 days up to June 7, while children aged 16-17 made up 4.8% of vaccine initiation over that period. The 25- to 39-year-olds, at 23.7% of all vaccine initiators, were the only group ahead of those aged 12-15, and the 50- to 64-year-olds were just behind at 17.7%, the CDC data show.
Both groups of children were on the low side, however, when it came to vaccine completion in the last 14 days, with those aged 12-15 at 6.7% of the total and those aged 16-17 years at 4.3%. The only age groups lower than that were ≥75 at 3.5% and <12 at 0.2%, and the highest share of vaccine completion was 26.0% for those aged 25-39, which also happens to be the group with the largest share of the U.S. population (20.5%), the CDC said.
People considered fully vaccinated are those who have received the second dose of a two-dose series or one dose of a single-shot vaccine, but children under age 18 years are eligible only for the Pfizer-BioNTech version, the CDC noted.
Meanwhile, back on the incidence side of the COVID-19 pandemic, the number of new cases in U.S. children for the week ending June 3 was at its lowest point (16,281) since mid-June of 2020, according to a report from the American Academy of Pediatrics and the Children’s Hospital Association.
Cases among children now total 3.99 million, which represents 14.1% of cases among all ages, a proportion that hasn’t increased since mid-May, which hasn’t happened since the two groups started keeping track in mid-April of 2020 in the 49 states (excluding New York), the District of Columbia, New York City, Puerto Rico, and Guam that report such data by age.
Less encouraging was the CDC’s report that “COVID-19-associated hospitalization rates among adolescents ages 12-17 years increased during March and April, following declines in January and February 2021.”
Children have been experiencing much lower rates of severe disease than those of adults throughout the pandemic, the CDC pointed out, but “recent increases in COVID-19-associated hospitalization rates and the potential for severe disease in adolescents reinforce the importance of continued prevention strategies, including vaccination and the correct and consistent use of masks in those who are not yet fully vaccinated.”
NIAID advances universal flu vaccine candidate into phase 1 trial
Last month, U.S. government researchers began a test of an experimental influenza vaccine that they hope will provide long-lasting immunity against multiple strains of the virus. Their project adds to the many approaches that have been tried in the decades-long quest for a universal flu shot.
For the first time, the National Institute of Allergy and Infectious Diseases (NIAID) is testing an investigational flu vaccine, known as FluMos-v1, on people. Researchers in recent years have targeted the stalk or stem of an influenza surface protein called hemagglutinin (HA) in trying to develop better flu vaccines. NIAID said FluMos-v1 is designed to spark production of antibodies against the HA protein from different virus strains, which could make it superior to vaccines now available, NIAID said.
“It could be longer lasting than the traditional flu vaccine and give us what we call super seasonal protection that might go beyond just one flu season to next year’s or the year after, or offer additional protection in a pandemic setting,” Alicia T. Widge, MD, of NIAID’s Vaccine Research Center, who is the principal investigator of the trial, said in an interview.
The phase 1 study (NCT04896086) aims to enroll 35 participants, 15 of whom will receive a single intramuscular injection of a comparator treatment, Flucelvax, which has already been approved by the U.S. Food and Drug Administration. The FluMos-v1 group will start with five participants who will receive one 20-μg dose. If no safety problems emerge at that dosage, another 15 volunteers will receive one 60-μg dose of the investigational vaccine.
The incorporation of a comparator group in the phase 1 study may help investigators get an early idea of how well FluMos-v1 compares to a marketed product, Dr. Widge said. The test will be carried out through the National Institutes of Health Clinical Center.
‘Renaissance’ of flu-vaccine research?
Currently, flu vaccines are reformulated each year in an attempt to match the dominant strain for the upcoming season, an effort that often falls notably short. The estimated vaccine effectiveness rate in the United States has ranged from a low of 19% to a high of 60% in recent years, according to the Centers for Disease Control and Prevention.
Scientists have been working for decades on a universal flu vaccine that would offer better results but haven’t yet identified the right strategy to outwit mutations in the virus. Recent setbacks include BiondVax Pharmaceuticals’ October 2020 announcement of a failed phase 3 trial of its experimental M-001 universal flu vaccine candidate.
But advances in understanding the immune system may set the stage for a “renaissance” in efforts to develop a universal flu vaccine, Michael Osterholm, PhD, MPH, director of the Center for Infectious Disease Research and Policy at the University of Minnesota, said in an interview.
The COVID-19 pandemic has spurred greater interest in the need to develop a universal flu vaccine, he said. Dr. Osterholm said he is “more optimistic now than ever” about the chances for developing vaccines that can fend off multiple strains over longer periods, although the goal of a shot that can ward off influenza in all cases may remain elusive.
“How good can we make them? Will they ever be really universal? Will they have long periods of protection? I don’t think any of us know that yet,” Dr. Osterholm said. “But this is not the influenza vaccine world of 5 or 7 years ago.”
The mRNA technology used to develop the world’s first approved COVID-19 vaccines, for example, may be applied against influenza, Dr. Osterholm said.
In January 2021, Moderna announced plans to test three development candidates for a seasonal influenza vaccine and aims to start a phase 1 study this year. In an April interview on CNBC’s Squawk Box program, Moderna’s chief executive, Stephané Bancel, spoke about the company’s plans to eventually create a combination vaccine for SARS-Cov-2 and flu viruses.
SARS-CoV-2 “is not going away.” Like flu, this virus will persist and change forms, Ms. Bancel said. Creating a flu shot that outperforms the existing ones would boost confidence in influenza vaccines, which many people now skip, Ms. Bancel said. People might someday be able to get a combination of this more effective flu shot with a COVID-19 vaccine booster in their local pharmacies.
“You can take one dose and then have a nice winter,” Ms. Bancel said of Moderna’s goal for a combination vaccine.
A version of this article first appeared on Medscape.com.
Last month, U.S. government researchers began a test of an experimental influenza vaccine that they hope will provide long-lasting immunity against multiple strains of the virus. Their project adds to the many approaches that have been tried in the decades-long quest for a universal flu shot.
For the first time, the National Institute of Allergy and Infectious Diseases (NIAID) is testing an investigational flu vaccine, known as FluMos-v1, on people. Researchers in recent years have targeted the stalk or stem of an influenza surface protein called hemagglutinin (HA) in trying to develop better flu vaccines. NIAID said FluMos-v1 is designed to spark production of antibodies against the HA protein from different virus strains, which could make it superior to vaccines now available, NIAID said.
“It could be longer lasting than the traditional flu vaccine and give us what we call super seasonal protection that might go beyond just one flu season to next year’s or the year after, or offer additional protection in a pandemic setting,” Alicia T. Widge, MD, of NIAID’s Vaccine Research Center, who is the principal investigator of the trial, said in an interview.
The phase 1 study (NCT04896086) aims to enroll 35 participants, 15 of whom will receive a single intramuscular injection of a comparator treatment, Flucelvax, which has already been approved by the U.S. Food and Drug Administration. The FluMos-v1 group will start with five participants who will receive one 20-μg dose. If no safety problems emerge at that dosage, another 15 volunteers will receive one 60-μg dose of the investigational vaccine.
The incorporation of a comparator group in the phase 1 study may help investigators get an early idea of how well FluMos-v1 compares to a marketed product, Dr. Widge said. The test will be carried out through the National Institutes of Health Clinical Center.
‘Renaissance’ of flu-vaccine research?
Currently, flu vaccines are reformulated each year in an attempt to match the dominant strain for the upcoming season, an effort that often falls notably short. The estimated vaccine effectiveness rate in the United States has ranged from a low of 19% to a high of 60% in recent years, according to the Centers for Disease Control and Prevention.
Scientists have been working for decades on a universal flu vaccine that would offer better results but haven’t yet identified the right strategy to outwit mutations in the virus. Recent setbacks include BiondVax Pharmaceuticals’ October 2020 announcement of a failed phase 3 trial of its experimental M-001 universal flu vaccine candidate.
But advances in understanding the immune system may set the stage for a “renaissance” in efforts to develop a universal flu vaccine, Michael Osterholm, PhD, MPH, director of the Center for Infectious Disease Research and Policy at the University of Minnesota, said in an interview.
The COVID-19 pandemic has spurred greater interest in the need to develop a universal flu vaccine, he said. Dr. Osterholm said he is “more optimistic now than ever” about the chances for developing vaccines that can fend off multiple strains over longer periods, although the goal of a shot that can ward off influenza in all cases may remain elusive.
“How good can we make them? Will they ever be really universal? Will they have long periods of protection? I don’t think any of us know that yet,” Dr. Osterholm said. “But this is not the influenza vaccine world of 5 or 7 years ago.”
The mRNA technology used to develop the world’s first approved COVID-19 vaccines, for example, may be applied against influenza, Dr. Osterholm said.
In January 2021, Moderna announced plans to test three development candidates for a seasonal influenza vaccine and aims to start a phase 1 study this year. In an April interview on CNBC’s Squawk Box program, Moderna’s chief executive, Stephané Bancel, spoke about the company’s plans to eventually create a combination vaccine for SARS-Cov-2 and flu viruses.
SARS-CoV-2 “is not going away.” Like flu, this virus will persist and change forms, Ms. Bancel said. Creating a flu shot that outperforms the existing ones would boost confidence in influenza vaccines, which many people now skip, Ms. Bancel said. People might someday be able to get a combination of this more effective flu shot with a COVID-19 vaccine booster in their local pharmacies.
“You can take one dose and then have a nice winter,” Ms. Bancel said of Moderna’s goal for a combination vaccine.
A version of this article first appeared on Medscape.com.
Last month, U.S. government researchers began a test of an experimental influenza vaccine that they hope will provide long-lasting immunity against multiple strains of the virus. Their project adds to the many approaches that have been tried in the decades-long quest for a universal flu shot.
For the first time, the National Institute of Allergy and Infectious Diseases (NIAID) is testing an investigational flu vaccine, known as FluMos-v1, on people. Researchers in recent years have targeted the stalk or stem of an influenza surface protein called hemagglutinin (HA) in trying to develop better flu vaccines. NIAID said FluMos-v1 is designed to spark production of antibodies against the HA protein from different virus strains, which could make it superior to vaccines now available, NIAID said.
“It could be longer lasting than the traditional flu vaccine and give us what we call super seasonal protection that might go beyond just one flu season to next year’s or the year after, or offer additional protection in a pandemic setting,” Alicia T. Widge, MD, of NIAID’s Vaccine Research Center, who is the principal investigator of the trial, said in an interview.
The phase 1 study (NCT04896086) aims to enroll 35 participants, 15 of whom will receive a single intramuscular injection of a comparator treatment, Flucelvax, which has already been approved by the U.S. Food and Drug Administration. The FluMos-v1 group will start with five participants who will receive one 20-μg dose. If no safety problems emerge at that dosage, another 15 volunteers will receive one 60-μg dose of the investigational vaccine.
The incorporation of a comparator group in the phase 1 study may help investigators get an early idea of how well FluMos-v1 compares to a marketed product, Dr. Widge said. The test will be carried out through the National Institutes of Health Clinical Center.
‘Renaissance’ of flu-vaccine research?
Currently, flu vaccines are reformulated each year in an attempt to match the dominant strain for the upcoming season, an effort that often falls notably short. The estimated vaccine effectiveness rate in the United States has ranged from a low of 19% to a high of 60% in recent years, according to the Centers for Disease Control and Prevention.
Scientists have been working for decades on a universal flu vaccine that would offer better results but haven’t yet identified the right strategy to outwit mutations in the virus. Recent setbacks include BiondVax Pharmaceuticals’ October 2020 announcement of a failed phase 3 trial of its experimental M-001 universal flu vaccine candidate.
But advances in understanding the immune system may set the stage for a “renaissance” in efforts to develop a universal flu vaccine, Michael Osterholm, PhD, MPH, director of the Center for Infectious Disease Research and Policy at the University of Minnesota, said in an interview.
The COVID-19 pandemic has spurred greater interest in the need to develop a universal flu vaccine, he said. Dr. Osterholm said he is “more optimistic now than ever” about the chances for developing vaccines that can fend off multiple strains over longer periods, although the goal of a shot that can ward off influenza in all cases may remain elusive.
“How good can we make them? Will they ever be really universal? Will they have long periods of protection? I don’t think any of us know that yet,” Dr. Osterholm said. “But this is not the influenza vaccine world of 5 or 7 years ago.”
The mRNA technology used to develop the world’s first approved COVID-19 vaccines, for example, may be applied against influenza, Dr. Osterholm said.
In January 2021, Moderna announced plans to test three development candidates for a seasonal influenza vaccine and aims to start a phase 1 study this year. In an April interview on CNBC’s Squawk Box program, Moderna’s chief executive, Stephané Bancel, spoke about the company’s plans to eventually create a combination vaccine for SARS-Cov-2 and flu viruses.
SARS-CoV-2 “is not going away.” Like flu, this virus will persist and change forms, Ms. Bancel said. Creating a flu shot that outperforms the existing ones would boost confidence in influenza vaccines, which many people now skip, Ms. Bancel said. People might someday be able to get a combination of this more effective flu shot with a COVID-19 vaccine booster in their local pharmacies.
“You can take one dose and then have a nice winter,” Ms. Bancel said of Moderna’s goal for a combination vaccine.
A version of this article first appeared on Medscape.com.





