User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
Powered by CHEST Physician, Clinician Reviews, MDedge Family Medicine, Internal Medicine News, and The Journal of Clinical Outcomes Management.
CDC director cites rise in hospitalizations in urging teen vaccinations
“I am deeply concerned by the numbers of hospitalized adolescents and saddened to see the number of adolescents who required treatment in intensive care units or mechanical ventilation,” CDC Director Rochelle Walensky, MD, said in a statement.
While urging teenagers to wear masks and take precautions around others, she asked “parents, relatives, and close friends to join me and talk with teens about the importance of these prevention strategies and to encourage them to get vaccinated.”
Dr. Walensky referred to the CDC’s Morbidity and Mortality Weekly Report that showed adolescent hospitalizations peaked at 2.1 per 100,000 in early January 2021, then dropped to 0.6 per 100,000 in mid-March.
Alarmingly, hospitalizations rose to 1.3 per 100,000 in April, and a number of teens required serious interventions.
“Among hospitalized adolescents, nearly one-third required intensive care unit admission, and 5% required invasive mechanical ventilation,” the report said. No deaths occurred.
The study looked at 376 adolescents aged 12-17 who were hospitalized and tested positive for coronavirus. Of that group, 204 were hospitalized for COVID-19 and the other 172 were hospitalized for reasons not directly related to COVID-19.
Of the 204 hospitalized for COVID-19, 70.6% had an underlying medical condition such as obesity or chronic lung disease.
The study noted that children and teenagers have lower hospitalization rates and generally show less severe symptoms than do older people.
Possible causes for the rise in adolescent COVID-19 hospitalizations include the arrival of variants, the growing number of children returning to in-person education, and the changes in mask-wearing and other safety precautions, the study said.
The American Academy of Pediatrics said that as of May 27, 4 million children have tested positive for COVID-19 since the pandemic began, with about 34,500 new child cases reported for the week ending May 27.
The AAP said children have represented 14.1% of total cases since the pandemic began, but for the week ending May 27, children represented 24.3% of new reported weekly COVID-19 cases.
On May 10, the FDA granted emergency use authorization for the Pfizer coronavirus vaccine to be given to children aged 12-15 years. Previously, the FDA had authorized the Pfizer vaccine for people aged 16 years and up, whereas the Moderna and Johnson & Johnson vaccines are authorized for people aged 18 years and up.
“Vaccination is our way out of this pandemic,” Dr. Walensky said in her statement. “I continue to see promising signs in CDC data that we are nearing the end of this pandemic in this country; however, we all have to do our part and get vaccinated to cross the finish line.”
A version of this article was first published on WebMD.com.
“I am deeply concerned by the numbers of hospitalized adolescents and saddened to see the number of adolescents who required treatment in intensive care units or mechanical ventilation,” CDC Director Rochelle Walensky, MD, said in a statement.
While urging teenagers to wear masks and take precautions around others, she asked “parents, relatives, and close friends to join me and talk with teens about the importance of these prevention strategies and to encourage them to get vaccinated.”
Dr. Walensky referred to the CDC’s Morbidity and Mortality Weekly Report that showed adolescent hospitalizations peaked at 2.1 per 100,000 in early January 2021, then dropped to 0.6 per 100,000 in mid-March.
Alarmingly, hospitalizations rose to 1.3 per 100,000 in April, and a number of teens required serious interventions.
“Among hospitalized adolescents, nearly one-third required intensive care unit admission, and 5% required invasive mechanical ventilation,” the report said. No deaths occurred.
The study looked at 376 adolescents aged 12-17 who were hospitalized and tested positive for coronavirus. Of that group, 204 were hospitalized for COVID-19 and the other 172 were hospitalized for reasons not directly related to COVID-19.
Of the 204 hospitalized for COVID-19, 70.6% had an underlying medical condition such as obesity or chronic lung disease.
The study noted that children and teenagers have lower hospitalization rates and generally show less severe symptoms than do older people.
Possible causes for the rise in adolescent COVID-19 hospitalizations include the arrival of variants, the growing number of children returning to in-person education, and the changes in mask-wearing and other safety precautions, the study said.
The American Academy of Pediatrics said that as of May 27, 4 million children have tested positive for COVID-19 since the pandemic began, with about 34,500 new child cases reported for the week ending May 27.
The AAP said children have represented 14.1% of total cases since the pandemic began, but for the week ending May 27, children represented 24.3% of new reported weekly COVID-19 cases.
On May 10, the FDA granted emergency use authorization for the Pfizer coronavirus vaccine to be given to children aged 12-15 years. Previously, the FDA had authorized the Pfizer vaccine for people aged 16 years and up, whereas the Moderna and Johnson & Johnson vaccines are authorized for people aged 18 years and up.
“Vaccination is our way out of this pandemic,” Dr. Walensky said in her statement. “I continue to see promising signs in CDC data that we are nearing the end of this pandemic in this country; however, we all have to do our part and get vaccinated to cross the finish line.”
A version of this article was first published on WebMD.com.
“I am deeply concerned by the numbers of hospitalized adolescents and saddened to see the number of adolescents who required treatment in intensive care units or mechanical ventilation,” CDC Director Rochelle Walensky, MD, said in a statement.
While urging teenagers to wear masks and take precautions around others, she asked “parents, relatives, and close friends to join me and talk with teens about the importance of these prevention strategies and to encourage them to get vaccinated.”
Dr. Walensky referred to the CDC’s Morbidity and Mortality Weekly Report that showed adolescent hospitalizations peaked at 2.1 per 100,000 in early January 2021, then dropped to 0.6 per 100,000 in mid-March.
Alarmingly, hospitalizations rose to 1.3 per 100,000 in April, and a number of teens required serious interventions.
“Among hospitalized adolescents, nearly one-third required intensive care unit admission, and 5% required invasive mechanical ventilation,” the report said. No deaths occurred.
The study looked at 376 adolescents aged 12-17 who were hospitalized and tested positive for coronavirus. Of that group, 204 were hospitalized for COVID-19 and the other 172 were hospitalized for reasons not directly related to COVID-19.
Of the 204 hospitalized for COVID-19, 70.6% had an underlying medical condition such as obesity or chronic lung disease.
The study noted that children and teenagers have lower hospitalization rates and generally show less severe symptoms than do older people.
Possible causes for the rise in adolescent COVID-19 hospitalizations include the arrival of variants, the growing number of children returning to in-person education, and the changes in mask-wearing and other safety precautions, the study said.
The American Academy of Pediatrics said that as of May 27, 4 million children have tested positive for COVID-19 since the pandemic began, with about 34,500 new child cases reported for the week ending May 27.
The AAP said children have represented 14.1% of total cases since the pandemic began, but for the week ending May 27, children represented 24.3% of new reported weekly COVID-19 cases.
On May 10, the FDA granted emergency use authorization for the Pfizer coronavirus vaccine to be given to children aged 12-15 years. Previously, the FDA had authorized the Pfizer vaccine for people aged 16 years and up, whereas the Moderna and Johnson & Johnson vaccines are authorized for people aged 18 years and up.
“Vaccination is our way out of this pandemic,” Dr. Walensky said in her statement. “I continue to see promising signs in CDC data that we are nearing the end of this pandemic in this country; however, we all have to do our part and get vaccinated to cross the finish line.”
A version of this article was first published on WebMD.com.
Collaborative effort reduces COPD readmissions, costs
Medicare exacts a penalty whenever it deems that hospitals have too many patients with chronic obstructive pulmonary disease who have been re-admitted within 30 days of discharge for care related to the disease. For acute-care hospitals the solution to reducing chronic obstructive pulmonary disease (COPD) re-admissions has been elusive, but members of a COPD chronic care management collaborative think they have found at least a partial solution.
Among 33 centers participating in the performance improvement program, the aggregated cost avoidance for emergency department (ED) visits was estimated at $351,000, and the savings for hospital re-visits avoided was an estimated $2.6 million, reported Valerie Press, MD, MPH, from the University of Chicago, and co-authors from the health care performance-improvement company Vizient.
The investigators described their chronic care management collaborative in a thematic poster presented during the American Thoracic Society’s virtual international conference (Abstract A1688).
“I’ve been working in the space of COPD re-admissions pretty much since Medicare started its penalty program,” Dr. Press said in an interview.
“At both my own institution and nationally, we’ve been trying to understand the policy that went into place to reduce what was considered to be excessive readmissions after a COPD admission, but there really wasn’t a lot of evidence to suggest how to do this at the time the policy went into place,” she said.
The Centers for Medicare & Medicaid Services (CMS) initiated its Hospital Readmission Reduction Program for COPD in 2014.
“The challenge with COPD is that we have not found really successful interventions to decrease readmissions,” commented Laura C. Myers, MD, MPH, in an interview. Dr. Myers, who studies optimal care delivery models for patients with COPD at Kaiser Permanente Northern California in Oakland, was not involved in the study.
She said that although the aggregate cost savings in the study by Dr. Press and colleagues are relatively modest, “if you extrapolate across the country, then those numbers could potentially be impressive.”
Collaboration details
Dr. Press was a subject matter expert for the collaborative, which included 47 Vizient member sites in the Southeast, Southwest, Midwest, and Northeast and Northwest coasts. Of these centers, 33 completed both parts of the collaboration.
The program included bi-monthly didactic sessions and site report and discussion sessions with peer-to-peer networking for a total of 6 months. During the sessions, meeting participants discussed best practices, received expert coaching, and provided progress updates on performance improvement projects.
“The goal was for them to identify the gaps or needs they had at their hospitals or practices, and then to try to put in place one or more interventions,” Dr. Press said. “This wasn’t a research program. It wasn’t standardized, and not all hospitals had to do the same program.”
The participants submitted reports for baseline and post-collaboration periods on both an intervention’s “reach,” defined as the percentage of patients who received a specified intervention, and on two outcome measures.
The interventions measured included spirometry, follow-up visits scheduled within 7 to 14 days of discharge, patients receiving COPD education, pulmonary referrals, and adherence to the COPD clinical pathway.
The outcome measures were the rate of COPD-related ED visits and hospital readmissions.
Revisits reduced
At the end of the program, 83% of participating sites had reductions in either ED visits or readmissions, and of this group, five sites had decreases in both measures.
Among all sites with improved metrics, the average rate of COPD-related ED revisits declined from 12.7% to 9%, and average inpatient readmissions declined from 20.1% to 15.6%.
As noted, the estimated cost savings in ED revisits avoided was $351,00, and the estimated savings in hospital readmission costs was $2.6 million.
“Although the centers didn’t have to participate in both parts, we did see in our results that the programs that participated fully had better results,” Dr. Press said.
“Historically, we’ve had such difficulty in decreasing COPD readmissions, and it’s nice to see something that actually works, both for patients and for conserving health resources,” Dr. Myers commented.
The study was supported by Vizient. Dr. Press disclosed honoraria from the company in her role as subject matter expert. Dr. Myers reported no conflicts of interest.
Medicare exacts a penalty whenever it deems that hospitals have too many patients with chronic obstructive pulmonary disease who have been re-admitted within 30 days of discharge for care related to the disease. For acute-care hospitals the solution to reducing chronic obstructive pulmonary disease (COPD) re-admissions has been elusive, but members of a COPD chronic care management collaborative think they have found at least a partial solution.
Among 33 centers participating in the performance improvement program, the aggregated cost avoidance for emergency department (ED) visits was estimated at $351,000, and the savings for hospital re-visits avoided was an estimated $2.6 million, reported Valerie Press, MD, MPH, from the University of Chicago, and co-authors from the health care performance-improvement company Vizient.
The investigators described their chronic care management collaborative in a thematic poster presented during the American Thoracic Society’s virtual international conference (Abstract A1688).
“I’ve been working in the space of COPD re-admissions pretty much since Medicare started its penalty program,” Dr. Press said in an interview.
“At both my own institution and nationally, we’ve been trying to understand the policy that went into place to reduce what was considered to be excessive readmissions after a COPD admission, but there really wasn’t a lot of evidence to suggest how to do this at the time the policy went into place,” she said.
The Centers for Medicare & Medicaid Services (CMS) initiated its Hospital Readmission Reduction Program for COPD in 2014.
“The challenge with COPD is that we have not found really successful interventions to decrease readmissions,” commented Laura C. Myers, MD, MPH, in an interview. Dr. Myers, who studies optimal care delivery models for patients with COPD at Kaiser Permanente Northern California in Oakland, was not involved in the study.
She said that although the aggregate cost savings in the study by Dr. Press and colleagues are relatively modest, “if you extrapolate across the country, then those numbers could potentially be impressive.”
Collaboration details
Dr. Press was a subject matter expert for the collaborative, which included 47 Vizient member sites in the Southeast, Southwest, Midwest, and Northeast and Northwest coasts. Of these centers, 33 completed both parts of the collaboration.
The program included bi-monthly didactic sessions and site report and discussion sessions with peer-to-peer networking for a total of 6 months. During the sessions, meeting participants discussed best practices, received expert coaching, and provided progress updates on performance improvement projects.
“The goal was for them to identify the gaps or needs they had at their hospitals or practices, and then to try to put in place one or more interventions,” Dr. Press said. “This wasn’t a research program. It wasn’t standardized, and not all hospitals had to do the same program.”
The participants submitted reports for baseline and post-collaboration periods on both an intervention’s “reach,” defined as the percentage of patients who received a specified intervention, and on two outcome measures.
The interventions measured included spirometry, follow-up visits scheduled within 7 to 14 days of discharge, patients receiving COPD education, pulmonary referrals, and adherence to the COPD clinical pathway.
The outcome measures were the rate of COPD-related ED visits and hospital readmissions.
Revisits reduced
At the end of the program, 83% of participating sites had reductions in either ED visits or readmissions, and of this group, five sites had decreases in both measures.
Among all sites with improved metrics, the average rate of COPD-related ED revisits declined from 12.7% to 9%, and average inpatient readmissions declined from 20.1% to 15.6%.
As noted, the estimated cost savings in ED revisits avoided was $351,00, and the estimated savings in hospital readmission costs was $2.6 million.
“Although the centers didn’t have to participate in both parts, we did see in our results that the programs that participated fully had better results,” Dr. Press said.
“Historically, we’ve had such difficulty in decreasing COPD readmissions, and it’s nice to see something that actually works, both for patients and for conserving health resources,” Dr. Myers commented.
The study was supported by Vizient. Dr. Press disclosed honoraria from the company in her role as subject matter expert. Dr. Myers reported no conflicts of interest.
Medicare exacts a penalty whenever it deems that hospitals have too many patients with chronic obstructive pulmonary disease who have been re-admitted within 30 days of discharge for care related to the disease. For acute-care hospitals the solution to reducing chronic obstructive pulmonary disease (COPD) re-admissions has been elusive, but members of a COPD chronic care management collaborative think they have found at least a partial solution.
Among 33 centers participating in the performance improvement program, the aggregated cost avoidance for emergency department (ED) visits was estimated at $351,000, and the savings for hospital re-visits avoided was an estimated $2.6 million, reported Valerie Press, MD, MPH, from the University of Chicago, and co-authors from the health care performance-improvement company Vizient.
The investigators described their chronic care management collaborative in a thematic poster presented during the American Thoracic Society’s virtual international conference (Abstract A1688).
“I’ve been working in the space of COPD re-admissions pretty much since Medicare started its penalty program,” Dr. Press said in an interview.
“At both my own institution and nationally, we’ve been trying to understand the policy that went into place to reduce what was considered to be excessive readmissions after a COPD admission, but there really wasn’t a lot of evidence to suggest how to do this at the time the policy went into place,” she said.
The Centers for Medicare & Medicaid Services (CMS) initiated its Hospital Readmission Reduction Program for COPD in 2014.
“The challenge with COPD is that we have not found really successful interventions to decrease readmissions,” commented Laura C. Myers, MD, MPH, in an interview. Dr. Myers, who studies optimal care delivery models for patients with COPD at Kaiser Permanente Northern California in Oakland, was not involved in the study.
She said that although the aggregate cost savings in the study by Dr. Press and colleagues are relatively modest, “if you extrapolate across the country, then those numbers could potentially be impressive.”
Collaboration details
Dr. Press was a subject matter expert for the collaborative, which included 47 Vizient member sites in the Southeast, Southwest, Midwest, and Northeast and Northwest coasts. Of these centers, 33 completed both parts of the collaboration.
The program included bi-monthly didactic sessions and site report and discussion sessions with peer-to-peer networking for a total of 6 months. During the sessions, meeting participants discussed best practices, received expert coaching, and provided progress updates on performance improvement projects.
“The goal was for them to identify the gaps or needs they had at their hospitals or practices, and then to try to put in place one or more interventions,” Dr. Press said. “This wasn’t a research program. It wasn’t standardized, and not all hospitals had to do the same program.”
The participants submitted reports for baseline and post-collaboration periods on both an intervention’s “reach,” defined as the percentage of patients who received a specified intervention, and on two outcome measures.
The interventions measured included spirometry, follow-up visits scheduled within 7 to 14 days of discharge, patients receiving COPD education, pulmonary referrals, and adherence to the COPD clinical pathway.
The outcome measures were the rate of COPD-related ED visits and hospital readmissions.
Revisits reduced
At the end of the program, 83% of participating sites had reductions in either ED visits or readmissions, and of this group, five sites had decreases in both measures.
Among all sites with improved metrics, the average rate of COPD-related ED revisits declined from 12.7% to 9%, and average inpatient readmissions declined from 20.1% to 15.6%.
As noted, the estimated cost savings in ED revisits avoided was $351,00, and the estimated savings in hospital readmission costs was $2.6 million.
“Although the centers didn’t have to participate in both parts, we did see in our results that the programs that participated fully had better results,” Dr. Press said.
“Historically, we’ve had such difficulty in decreasing COPD readmissions, and it’s nice to see something that actually works, both for patients and for conserving health resources,” Dr. Myers commented.
The study was supported by Vizient. Dr. Press disclosed honoraria from the company in her role as subject matter expert. Dr. Myers reported no conflicts of interest.
FROM ATS 2021
Secondhand smoke in childhood and adulthood linked to increased risk of rheumatoid arthritis
Secondhand smoke exposure in both childhood and adulthood is associated with an increased risk of rheumatoid arthritis in women, according to a study presented at the annual European Congress of Rheumatology.
“These results suggest that smoking by-products, whether actively or passively inhaled or absorbed, could generate autoimmunity, at least towards antigens involved in rheumatoid arthritis pathogenesis,” said Yann Nguyen, MD, MPH, of the center for research in epidemiology and population health at the University of Paris-Saclay in Villejuif and of Beaujon Hospital at the University of Paris in Clichy, France.
Previous research has already repeatedly implicated smoking as a risk factor for rheumatoid arthritis positive for anticitrullinated protein antibodies (ACPA), especially in those who have the HLA-DRB1-shared epitope (SE) alleles, Dr. Nguyen explained to attendees. This study looked at whether exposure to others’ smoke had any similar associations.
The researchers relied on the French prospective cohort study known as E3N-EPIC (Etude Epidémiologique auprès de femmes de la Mutuelle Générale de l’Education Nationale), which is designed to examine potential associations between environmental factors and chronic disease. Of the 98,995 healthy French women the longitudinal study has tracked since 1990, this study included 79,806 participants with an average age of 49 years. A total of 698 women developed rheumatoid arthritis during the study an average of 11.7 years after baseline.
Exposure to secondhand smoke, or passive smoking, in childhood was defined as spending several hours a day in a smoky room as a child, based on participants’ self-report. Adult exposure to passive smoking referred to women’s self-report of spending at least 1 hour a day around actively smoking adults. Researchers further stratified participants according to whether they currently smoke, have never smoked, or used to smoke. Additional covariates in the fully adjusted models included body mass index and educational level.
About one in seven of the women (13.5%) reported exposure to childhood passive smoking, and just over half (53.6%) reported passive smoking exposure as adults. Overall, 58.9% of participants had secondhand exposure in adulthood or childhood, and 8.25% had both.
A positive association existed between childhood exposure and rheumatoid arthritis in the unadjusted and adjusted models. In the fully adjusted model, the risk of rheumatoid arthritis was 1.24 times greater overall for those exposed to secondhand smoke in childhood compared with those who had no exposure. The risk was even greater, however, among women who had never smoked (hazard ratio, 1.42), and the association was not statistically significant in women who had ever smoked.
Similarly, risk of rheumatoid arthritis was greater among those women reporting exposure to passive smoking in adulthood in the unadjusted and adjusted models (HR, 1.19 after adjustment). Once again, women who had never smoked had a modestly higher increased risk (HR, 1.27) if they had secondhand smoke exposure in adulthood, but no statistically significant association existed for women who were current or former smokers.
Although research had previously shown the association between active smoking and rheumatoid arthritis, these new findings suggest clinicians need to emphasize to their patients this additional negative effect from smoking.
Dr. Nguyen, Dr. Carmona, and Dr. Schulze-Koops have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Secondhand smoke exposure in both childhood and adulthood is associated with an increased risk of rheumatoid arthritis in women, according to a study presented at the annual European Congress of Rheumatology.
“These results suggest that smoking by-products, whether actively or passively inhaled or absorbed, could generate autoimmunity, at least towards antigens involved in rheumatoid arthritis pathogenesis,” said Yann Nguyen, MD, MPH, of the center for research in epidemiology and population health at the University of Paris-Saclay in Villejuif and of Beaujon Hospital at the University of Paris in Clichy, France.
Previous research has already repeatedly implicated smoking as a risk factor for rheumatoid arthritis positive for anticitrullinated protein antibodies (ACPA), especially in those who have the HLA-DRB1-shared epitope (SE) alleles, Dr. Nguyen explained to attendees. This study looked at whether exposure to others’ smoke had any similar associations.
The researchers relied on the French prospective cohort study known as E3N-EPIC (Etude Epidémiologique auprès de femmes de la Mutuelle Générale de l’Education Nationale), which is designed to examine potential associations between environmental factors and chronic disease. Of the 98,995 healthy French women the longitudinal study has tracked since 1990, this study included 79,806 participants with an average age of 49 years. A total of 698 women developed rheumatoid arthritis during the study an average of 11.7 years after baseline.
Exposure to secondhand smoke, or passive smoking, in childhood was defined as spending several hours a day in a smoky room as a child, based on participants’ self-report. Adult exposure to passive smoking referred to women’s self-report of spending at least 1 hour a day around actively smoking adults. Researchers further stratified participants according to whether they currently smoke, have never smoked, or used to smoke. Additional covariates in the fully adjusted models included body mass index and educational level.
About one in seven of the women (13.5%) reported exposure to childhood passive smoking, and just over half (53.6%) reported passive smoking exposure as adults. Overall, 58.9% of participants had secondhand exposure in adulthood or childhood, and 8.25% had both.
A positive association existed between childhood exposure and rheumatoid arthritis in the unadjusted and adjusted models. In the fully adjusted model, the risk of rheumatoid arthritis was 1.24 times greater overall for those exposed to secondhand smoke in childhood compared with those who had no exposure. The risk was even greater, however, among women who had never smoked (hazard ratio, 1.42), and the association was not statistically significant in women who had ever smoked.
Similarly, risk of rheumatoid arthritis was greater among those women reporting exposure to passive smoking in adulthood in the unadjusted and adjusted models (HR, 1.19 after adjustment). Once again, women who had never smoked had a modestly higher increased risk (HR, 1.27) if they had secondhand smoke exposure in adulthood, but no statistically significant association existed for women who were current or former smokers.
Although research had previously shown the association between active smoking and rheumatoid arthritis, these new findings suggest clinicians need to emphasize to their patients this additional negative effect from smoking.
Dr. Nguyen, Dr. Carmona, and Dr. Schulze-Koops have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Secondhand smoke exposure in both childhood and adulthood is associated with an increased risk of rheumatoid arthritis in women, according to a study presented at the annual European Congress of Rheumatology.
“These results suggest that smoking by-products, whether actively or passively inhaled or absorbed, could generate autoimmunity, at least towards antigens involved in rheumatoid arthritis pathogenesis,” said Yann Nguyen, MD, MPH, of the center for research in epidemiology and population health at the University of Paris-Saclay in Villejuif and of Beaujon Hospital at the University of Paris in Clichy, France.
Previous research has already repeatedly implicated smoking as a risk factor for rheumatoid arthritis positive for anticitrullinated protein antibodies (ACPA), especially in those who have the HLA-DRB1-shared epitope (SE) alleles, Dr. Nguyen explained to attendees. This study looked at whether exposure to others’ smoke had any similar associations.
The researchers relied on the French prospective cohort study known as E3N-EPIC (Etude Epidémiologique auprès de femmes de la Mutuelle Générale de l’Education Nationale), which is designed to examine potential associations between environmental factors and chronic disease. Of the 98,995 healthy French women the longitudinal study has tracked since 1990, this study included 79,806 participants with an average age of 49 years. A total of 698 women developed rheumatoid arthritis during the study an average of 11.7 years after baseline.
Exposure to secondhand smoke, or passive smoking, in childhood was defined as spending several hours a day in a smoky room as a child, based on participants’ self-report. Adult exposure to passive smoking referred to women’s self-report of spending at least 1 hour a day around actively smoking adults. Researchers further stratified participants according to whether they currently smoke, have never smoked, or used to smoke. Additional covariates in the fully adjusted models included body mass index and educational level.
About one in seven of the women (13.5%) reported exposure to childhood passive smoking, and just over half (53.6%) reported passive smoking exposure as adults. Overall, 58.9% of participants had secondhand exposure in adulthood or childhood, and 8.25% had both.
A positive association existed between childhood exposure and rheumatoid arthritis in the unadjusted and adjusted models. In the fully adjusted model, the risk of rheumatoid arthritis was 1.24 times greater overall for those exposed to secondhand smoke in childhood compared with those who had no exposure. The risk was even greater, however, among women who had never smoked (hazard ratio, 1.42), and the association was not statistically significant in women who had ever smoked.
Similarly, risk of rheumatoid arthritis was greater among those women reporting exposure to passive smoking in adulthood in the unadjusted and adjusted models (HR, 1.19 after adjustment). Once again, women who had never smoked had a modestly higher increased risk (HR, 1.27) if they had secondhand smoke exposure in adulthood, but no statistically significant association existed for women who were current or former smokers.
Although research had previously shown the association between active smoking and rheumatoid arthritis, these new findings suggest clinicians need to emphasize to their patients this additional negative effect from smoking.
Dr. Nguyen, Dr. Carmona, and Dr. Schulze-Koops have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM THE EULAR 2021 CONGRESS
Gene variant confirmed as strong predictor of lung disease in RA
Carriers have more than twofold greater risk
Patients with rheumatoid arthritis who carry a specific allele of the gene MUC5B have about double the risk of developing interstitial lung disease when compared with noncarriers, according to a large Finnish biobank study presented at the annual European Congress of Rheumatology.
“The risk difference [or carriers relative to noncarriers] started at about age 65, with a bigger difference [for] men than women,” reported Antti Palomäki, MD, PhD, of the center for rheumatology and clinical immunology at Turku (Finland) University.
The gain-of-function MUC5B variant, which encodes mucin 5B, was first linked to RA-associated interstitial lung disease (ILD) more than 3 years ago. At that time, it was already a known genetic risk factor for idiopathic pulmonary fibrosis in the general population. The new data confirm the association in a longitudinal analysis of a large biobank and suggest the association might have clinical utility.
“This is not ready for clinical practice at the moment. We do not yet know whether we can change therapy to reduce risk,” Dr. Palomäki said, adding “in the future we can look.”
One question that might be asked in clinical studies using MUC5B as a tool to assess and modify risk of ILD in patients with RA is whether one therapy is better than another in avoiding or delaying development of lung fibrosis. Dr. Palomäki noted that biologics, for example, might be a more favorable choice in patients with RA who are at high risk of developing ILD.
The association of the MUC5B variant with increased ILD incidence in patients with RA was drawn from a data set known as FinnGen, a biobank collection of epidemiologic cohorts and hospital samples with genotypes of about 10% of the Finnish population. Follow-up extends to 46 years in some of these individuals.
When 248,4000 individuals in this data set were evaluated, 5,534 had a diagnosis of RA. Of these, 178 (3.2%) developed ILD. About 20% of both those with and without RA were MUC5B variant carriers, meaning the remainder were not.
Sex and age factor into lifetime risk
In patients with RA, the lifetime rate of ILD among MUC5B variant carriers was 16.8% versus only 6.1% among noncarriers. This finding translated into a hazard ratio for ILD of 2.27 (95% confidence interval, 1.75–2.96) for variant carriers versus noncarriers.
The lifetime rate of ILD in patients with RA was greater in men versus women regardless of carrier status (18.5% vs. 8.5%). For women, the lifetime rate was lower for carriers, although the difference relative to female noncarriers was greater (14.5% vs. 4.7%).
ILD, whether in the general population or in patients with RA, is a disease of advancing age. When Dr. Palomäki showed a graph, the rise in ILD incidence did not start in any population, whether those with or without RA and regardless of carrier status, until about age 55. In those without RA and in noncarriers of the variant, ILD incidence remained low and began a discernible climb at around age 70.
In those who did not have RA but were positive for the variant, the rates rose more than twice as fast, particularly after age 70. In people who had RA but not the variant, the rate of ILD was greater than in patients who carried the variant without RA, starting the climb earlier and rising more steeply with age. In those with RA and the variant, the climb in ILD incidence rose rapidly after age 65 years even though the incidence remained fairly similar between all of these groups at age 60.
Putting the findings into context
The need to develop ways to prevent ILD in RA is urgent. ILD is one of the most common extraarticular manifestations of RA, developing in up to 60% of patients with RA in older age groups when evaluated with imaging, according to Dr. Palomäki. Although it develops into a clinically significant complication in only about 10% of these patients, ILD still is a significant cause of illness and death in elderly patients with RA.
In the 2018 study that first linked the MUC5B variant to RA-ILD, the investigators also found that the variant was associated with an increased likelihood of developing the usual interstitial pneumonia type of ILD on imaging. David Schwartz, MD, professor of medicine, pulmonary sciences, and critical care and chair of the department of medicine at the University of Colorado at Denver, Aurora, was a senior author of that study. He said these findings build on the 2018 study.
“While the gain-of-function MUC5B promoter variant is important in predicting who will develop RA-ILD, these findings also suggest that MUC5B may be involved in the etiology of RA-ILD, at least for those with the MUC5B variant,” he said.
“The study also raises the possibility that there are several subtypes of RA-ILD, and the subtype that is driven by MUC5B may respond differently to RA biologics or therapeutic agents to treat ILD,” he added.
In the discussion following the presentation by Dr. Palomäki, others agreed, with that statement including Dr. Palomäki. He expressed interest in clinical studies comparing different classes of RA therapies for their relative impact on the risk of developing ILD.Dr. Palomäki reported financial relationships with AbbVie, Merck, Pfizer, and Sanofi. Dr. Schwartz is the founder of Eleven P15, which is developing methods for early diagnosis and treatment of pulmonary fibrosis.
Carriers have more than twofold greater risk
Carriers have more than twofold greater risk
Patients with rheumatoid arthritis who carry a specific allele of the gene MUC5B have about double the risk of developing interstitial lung disease when compared with noncarriers, according to a large Finnish biobank study presented at the annual European Congress of Rheumatology.
“The risk difference [or carriers relative to noncarriers] started at about age 65, with a bigger difference [for] men than women,” reported Antti Palomäki, MD, PhD, of the center for rheumatology and clinical immunology at Turku (Finland) University.
The gain-of-function MUC5B variant, which encodes mucin 5B, was first linked to RA-associated interstitial lung disease (ILD) more than 3 years ago. At that time, it was already a known genetic risk factor for idiopathic pulmonary fibrosis in the general population. The new data confirm the association in a longitudinal analysis of a large biobank and suggest the association might have clinical utility.
“This is not ready for clinical practice at the moment. We do not yet know whether we can change therapy to reduce risk,” Dr. Palomäki said, adding “in the future we can look.”
One question that might be asked in clinical studies using MUC5B as a tool to assess and modify risk of ILD in patients with RA is whether one therapy is better than another in avoiding or delaying development of lung fibrosis. Dr. Palomäki noted that biologics, for example, might be a more favorable choice in patients with RA who are at high risk of developing ILD.
The association of the MUC5B variant with increased ILD incidence in patients with RA was drawn from a data set known as FinnGen, a biobank collection of epidemiologic cohorts and hospital samples with genotypes of about 10% of the Finnish population. Follow-up extends to 46 years in some of these individuals.
When 248,4000 individuals in this data set were evaluated, 5,534 had a diagnosis of RA. Of these, 178 (3.2%) developed ILD. About 20% of both those with and without RA were MUC5B variant carriers, meaning the remainder were not.
Sex and age factor into lifetime risk
In patients with RA, the lifetime rate of ILD among MUC5B variant carriers was 16.8% versus only 6.1% among noncarriers. This finding translated into a hazard ratio for ILD of 2.27 (95% confidence interval, 1.75–2.96) for variant carriers versus noncarriers.
The lifetime rate of ILD in patients with RA was greater in men versus women regardless of carrier status (18.5% vs. 8.5%). For women, the lifetime rate was lower for carriers, although the difference relative to female noncarriers was greater (14.5% vs. 4.7%).
ILD, whether in the general population or in patients with RA, is a disease of advancing age. When Dr. Palomäki showed a graph, the rise in ILD incidence did not start in any population, whether those with or without RA and regardless of carrier status, until about age 55. In those without RA and in noncarriers of the variant, ILD incidence remained low and began a discernible climb at around age 70.
In those who did not have RA but were positive for the variant, the rates rose more than twice as fast, particularly after age 70. In people who had RA but not the variant, the rate of ILD was greater than in patients who carried the variant without RA, starting the climb earlier and rising more steeply with age. In those with RA and the variant, the climb in ILD incidence rose rapidly after age 65 years even though the incidence remained fairly similar between all of these groups at age 60.
Putting the findings into context
The need to develop ways to prevent ILD in RA is urgent. ILD is one of the most common extraarticular manifestations of RA, developing in up to 60% of patients with RA in older age groups when evaluated with imaging, according to Dr. Palomäki. Although it develops into a clinically significant complication in only about 10% of these patients, ILD still is a significant cause of illness and death in elderly patients with RA.
In the 2018 study that first linked the MUC5B variant to RA-ILD, the investigators also found that the variant was associated with an increased likelihood of developing the usual interstitial pneumonia type of ILD on imaging. David Schwartz, MD, professor of medicine, pulmonary sciences, and critical care and chair of the department of medicine at the University of Colorado at Denver, Aurora, was a senior author of that study. He said these findings build on the 2018 study.
“While the gain-of-function MUC5B promoter variant is important in predicting who will develop RA-ILD, these findings also suggest that MUC5B may be involved in the etiology of RA-ILD, at least for those with the MUC5B variant,” he said.
“The study also raises the possibility that there are several subtypes of RA-ILD, and the subtype that is driven by MUC5B may respond differently to RA biologics or therapeutic agents to treat ILD,” he added.
In the discussion following the presentation by Dr. Palomäki, others agreed, with that statement including Dr. Palomäki. He expressed interest in clinical studies comparing different classes of RA therapies for their relative impact on the risk of developing ILD.Dr. Palomäki reported financial relationships with AbbVie, Merck, Pfizer, and Sanofi. Dr. Schwartz is the founder of Eleven P15, which is developing methods for early diagnosis and treatment of pulmonary fibrosis.
Patients with rheumatoid arthritis who carry a specific allele of the gene MUC5B have about double the risk of developing interstitial lung disease when compared with noncarriers, according to a large Finnish biobank study presented at the annual European Congress of Rheumatology.
“The risk difference [or carriers relative to noncarriers] started at about age 65, with a bigger difference [for] men than women,” reported Antti Palomäki, MD, PhD, of the center for rheumatology and clinical immunology at Turku (Finland) University.
The gain-of-function MUC5B variant, which encodes mucin 5B, was first linked to RA-associated interstitial lung disease (ILD) more than 3 years ago. At that time, it was already a known genetic risk factor for idiopathic pulmonary fibrosis in the general population. The new data confirm the association in a longitudinal analysis of a large biobank and suggest the association might have clinical utility.
“This is not ready for clinical practice at the moment. We do not yet know whether we can change therapy to reduce risk,” Dr. Palomäki said, adding “in the future we can look.”
One question that might be asked in clinical studies using MUC5B as a tool to assess and modify risk of ILD in patients with RA is whether one therapy is better than another in avoiding or delaying development of lung fibrosis. Dr. Palomäki noted that biologics, for example, might be a more favorable choice in patients with RA who are at high risk of developing ILD.
The association of the MUC5B variant with increased ILD incidence in patients with RA was drawn from a data set known as FinnGen, a biobank collection of epidemiologic cohorts and hospital samples with genotypes of about 10% of the Finnish population. Follow-up extends to 46 years in some of these individuals.
When 248,4000 individuals in this data set were evaluated, 5,534 had a diagnosis of RA. Of these, 178 (3.2%) developed ILD. About 20% of both those with and without RA were MUC5B variant carriers, meaning the remainder were not.
Sex and age factor into lifetime risk
In patients with RA, the lifetime rate of ILD among MUC5B variant carriers was 16.8% versus only 6.1% among noncarriers. This finding translated into a hazard ratio for ILD of 2.27 (95% confidence interval, 1.75–2.96) for variant carriers versus noncarriers.
The lifetime rate of ILD in patients with RA was greater in men versus women regardless of carrier status (18.5% vs. 8.5%). For women, the lifetime rate was lower for carriers, although the difference relative to female noncarriers was greater (14.5% vs. 4.7%).
ILD, whether in the general population or in patients with RA, is a disease of advancing age. When Dr. Palomäki showed a graph, the rise in ILD incidence did not start in any population, whether those with or without RA and regardless of carrier status, until about age 55. In those without RA and in noncarriers of the variant, ILD incidence remained low and began a discernible climb at around age 70.
In those who did not have RA but were positive for the variant, the rates rose more than twice as fast, particularly after age 70. In people who had RA but not the variant, the rate of ILD was greater than in patients who carried the variant without RA, starting the climb earlier and rising more steeply with age. In those with RA and the variant, the climb in ILD incidence rose rapidly after age 65 years even though the incidence remained fairly similar between all of these groups at age 60.
Putting the findings into context
The need to develop ways to prevent ILD in RA is urgent. ILD is one of the most common extraarticular manifestations of RA, developing in up to 60% of patients with RA in older age groups when evaluated with imaging, according to Dr. Palomäki. Although it develops into a clinically significant complication in only about 10% of these patients, ILD still is a significant cause of illness and death in elderly patients with RA.
In the 2018 study that first linked the MUC5B variant to RA-ILD, the investigators also found that the variant was associated with an increased likelihood of developing the usual interstitial pneumonia type of ILD on imaging. David Schwartz, MD, professor of medicine, pulmonary sciences, and critical care and chair of the department of medicine at the University of Colorado at Denver, Aurora, was a senior author of that study. He said these findings build on the 2018 study.
“While the gain-of-function MUC5B promoter variant is important in predicting who will develop RA-ILD, these findings also suggest that MUC5B may be involved in the etiology of RA-ILD, at least for those with the MUC5B variant,” he said.
“The study also raises the possibility that there are several subtypes of RA-ILD, and the subtype that is driven by MUC5B may respond differently to RA biologics or therapeutic agents to treat ILD,” he added.
In the discussion following the presentation by Dr. Palomäki, others agreed, with that statement including Dr. Palomäki. He expressed interest in clinical studies comparing different classes of RA therapies for their relative impact on the risk of developing ILD.Dr. Palomäki reported financial relationships with AbbVie, Merck, Pfizer, and Sanofi. Dr. Schwartz is the founder of Eleven P15, which is developing methods for early diagnosis and treatment of pulmonary fibrosis.
FROM THE EULAR 2021 CONGRESS
Nintedanib slows interstitial lung disease in RA patients
Subgroup analysis from INBUILD trial finds results similar to overall study cohort
In a new subgroup analysis of a previously published multinational trial, the preservation of lung function with nintedanib (Ofev) was about the same in patients with interstitial lung disease related to rheumatoid arthritis (RA-ILD) as it was in patients with other etiologies, according to data presented at the annual European Congress of Rheumatology.
“There was no significant heterogeneity across any of several characteristics we evaluated,” reported Clive Kelly, MBBS, of the Institute of Cellular Medicine at Newcastle University (England).
The INBUILD trial, which enrolled more than 600 patients in 15 countries with a range of fibrosing lung diseases, was published almost 2 years ago. On the primary endpoint of rate of decline in forced vital capacity (FVC), the medians were –80.8 mL per year among those randomized to nintedanib and –187.8 mL per year (P < .001) on placebo.
The INBUILD study provided evidence that fibrosing lung diseases have a common pathobiologic mechanism that can be slowed by targeting intracellular kinases. Nintedanib inhibits several growth factor receptors as well as nonreceptor tyrosine kinases, but its exact mechanism for slowing fibrosing lung diseases remains unclear. Initially approved for, nintedanib received approvals from the FDA for systemic sclerosis–associated ILD in 2019 and for chronic fibrosing ILD with progressive phenotypes in 2020 after being initially approved for the treatment of idiopathic pulmonary fibrosis in 2014.
When asked for comment, Paul F. Dellaripa, MD, an associate professor of medicine in the division of rheumatology, immunology, and allergy at Harvard Medical School, Boston, indicated these data are helpful in considering strategies for RA patients with ILD, but he encouraged collaboration between joint and lung specialists.
“Antifibrotic agents for patients with progressive ILD in autoimmune diseases like RA is a welcome addition to our care of this challenging complication,” said Dr. Dellaripa, who has published frequently on the diagnosis and treatment of lung diseases associated with RA. Yet, treatment must be individualized, he added.
“It will be incumbent for rheumatologists to incorporate lung health as a critical part of patient care and work closely with pulmonologists to consider when to institute antifibrotic therapy in patients with ILD,” he said.
Details of subanalysis
In the RA-ILD subpopulation of 89 patients, there was no further decline in FVC from 24 weeks after randomization to the end of 52 weeks for those on nintedanib, but the decline remained steady over the full course of follow-up among those in the placebo group. At 52 weeks, the decline in the placebo group reached –200 mL at the end of 52 weeks. As a result, the between-group relative reduction in FVC at 52 weeks of 116.7 mL favoring nintedanib over placebo (P < .037) slightly exceeded the 107-mL reduction (P < .001) observed in the overall INBUILD study population.
Among other subgroups the investigators evaluated, outcomes with nintedanib did not differ when patients were split into groups with higher or lower baseline levels of high-sensitivity C-reactive protein, regardless of whether the groups were defined by levels above and below 1 mg/L or 3 mg/L. The same was true for those who were taking nonbiologic disease-modifying antirheumatic drugs or glucocorticoids.
However, for these latter analyses, Dr. Kelly conceded that the differences were based on small numbers of patients and so cannot be considered conclusive.
The adverse event most closely associated with nintedanib in the RA-ILD population was diarrhea, just as in the overall study, and it was more than twice as frequent in the RA-ILD patients receiving the active therapy, compared with placebo (54.8% vs. 25.5%). Nausea was also more common (21.4% vs. 10.6%), and so was decreased appetite (11.9% vs. 2.1%) and weight reduction (9.5% vs. 2.1%).
Lung-related adverse events, such as bronchiolitis (21.4% vs. 17.0%) and dyspnea (11.9% vs. 10.6%), were only slightly more frequent in the nintedanib group. Nasopharyngitis (7.1% vs. 12.8%) was less common. Side effects leading to treatment discontinuation were higher on nintedanib (19.0% vs. 12.8%)
The RA-ILD subgroup represented 13.4% of those randomized in INBUILD. The mean time since diagnosis of RA was about 10 years. More than 60% were smokers or former smokers. At baseline, the mean FVC of predicted was 71%. More than 85% had a usual interstitial pneumonia (UIP) radiologic pattern.
Acute exacerbations and death were not evaluated in the RA-ILD subpopulation, but these were secondary endpoints in the published INBUILD study according to the presence or absence of a UIP-like fibrotic pattern. For the combined endpoint of acute exacerbation of ILD or death, the protection associated with nintedanib approached statistical significance for the population overall (odds ratio, 0.68; 95% confidence interval, 0.46-1.01) and reached significance for those with a UIP pattern (OR, 0.61; 95% CI, 0.38-0.98).
Nintedanib led to lower death rates at 52 weeks in the overall population (8.1% vs. 11.5% with placebo) and in the group with a UIP pattern (9.7% vs. 15.0% with placebo).
Dr. Kelly has financial relationships with multiple pharmaceutical companies, including Boehringer Ingelheim, which provided funding for INBUILD and this subpopulation analysis. Dr. Dellaripa reported financial relationships with Bristol-Myers Squibb and Genentech.
Subgroup analysis from INBUILD trial finds results similar to overall study cohort
Subgroup analysis from INBUILD trial finds results similar to overall study cohort
In a new subgroup analysis of a previously published multinational trial, the preservation of lung function with nintedanib (Ofev) was about the same in patients with interstitial lung disease related to rheumatoid arthritis (RA-ILD) as it was in patients with other etiologies, according to data presented at the annual European Congress of Rheumatology.
“There was no significant heterogeneity across any of several characteristics we evaluated,” reported Clive Kelly, MBBS, of the Institute of Cellular Medicine at Newcastle University (England).
The INBUILD trial, which enrolled more than 600 patients in 15 countries with a range of fibrosing lung diseases, was published almost 2 years ago. On the primary endpoint of rate of decline in forced vital capacity (FVC), the medians were –80.8 mL per year among those randomized to nintedanib and –187.8 mL per year (P < .001) on placebo.
The INBUILD study provided evidence that fibrosing lung diseases have a common pathobiologic mechanism that can be slowed by targeting intracellular kinases. Nintedanib inhibits several growth factor receptors as well as nonreceptor tyrosine kinases, but its exact mechanism for slowing fibrosing lung diseases remains unclear. Initially approved for, nintedanib received approvals from the FDA for systemic sclerosis–associated ILD in 2019 and for chronic fibrosing ILD with progressive phenotypes in 2020 after being initially approved for the treatment of idiopathic pulmonary fibrosis in 2014.
When asked for comment, Paul F. Dellaripa, MD, an associate professor of medicine in the division of rheumatology, immunology, and allergy at Harvard Medical School, Boston, indicated these data are helpful in considering strategies for RA patients with ILD, but he encouraged collaboration between joint and lung specialists.
“Antifibrotic agents for patients with progressive ILD in autoimmune diseases like RA is a welcome addition to our care of this challenging complication,” said Dr. Dellaripa, who has published frequently on the diagnosis and treatment of lung diseases associated with RA. Yet, treatment must be individualized, he added.
“It will be incumbent for rheumatologists to incorporate lung health as a critical part of patient care and work closely with pulmonologists to consider when to institute antifibrotic therapy in patients with ILD,” he said.
Details of subanalysis
In the RA-ILD subpopulation of 89 patients, there was no further decline in FVC from 24 weeks after randomization to the end of 52 weeks for those on nintedanib, but the decline remained steady over the full course of follow-up among those in the placebo group. At 52 weeks, the decline in the placebo group reached –200 mL at the end of 52 weeks. As a result, the between-group relative reduction in FVC at 52 weeks of 116.7 mL favoring nintedanib over placebo (P < .037) slightly exceeded the 107-mL reduction (P < .001) observed in the overall INBUILD study population.
Among other subgroups the investigators evaluated, outcomes with nintedanib did not differ when patients were split into groups with higher or lower baseline levels of high-sensitivity C-reactive protein, regardless of whether the groups were defined by levels above and below 1 mg/L or 3 mg/L. The same was true for those who were taking nonbiologic disease-modifying antirheumatic drugs or glucocorticoids.
However, for these latter analyses, Dr. Kelly conceded that the differences were based on small numbers of patients and so cannot be considered conclusive.
The adverse event most closely associated with nintedanib in the RA-ILD population was diarrhea, just as in the overall study, and it was more than twice as frequent in the RA-ILD patients receiving the active therapy, compared with placebo (54.8% vs. 25.5%). Nausea was also more common (21.4% vs. 10.6%), and so was decreased appetite (11.9% vs. 2.1%) and weight reduction (9.5% vs. 2.1%).
Lung-related adverse events, such as bronchiolitis (21.4% vs. 17.0%) and dyspnea (11.9% vs. 10.6%), were only slightly more frequent in the nintedanib group. Nasopharyngitis (7.1% vs. 12.8%) was less common. Side effects leading to treatment discontinuation were higher on nintedanib (19.0% vs. 12.8%)
The RA-ILD subgroup represented 13.4% of those randomized in INBUILD. The mean time since diagnosis of RA was about 10 years. More than 60% were smokers or former smokers. At baseline, the mean FVC of predicted was 71%. More than 85% had a usual interstitial pneumonia (UIP) radiologic pattern.
Acute exacerbations and death were not evaluated in the RA-ILD subpopulation, but these were secondary endpoints in the published INBUILD study according to the presence or absence of a UIP-like fibrotic pattern. For the combined endpoint of acute exacerbation of ILD or death, the protection associated with nintedanib approached statistical significance for the population overall (odds ratio, 0.68; 95% confidence interval, 0.46-1.01) and reached significance for those with a UIP pattern (OR, 0.61; 95% CI, 0.38-0.98).
Nintedanib led to lower death rates at 52 weeks in the overall population (8.1% vs. 11.5% with placebo) and in the group with a UIP pattern (9.7% vs. 15.0% with placebo).
Dr. Kelly has financial relationships with multiple pharmaceutical companies, including Boehringer Ingelheim, which provided funding for INBUILD and this subpopulation analysis. Dr. Dellaripa reported financial relationships with Bristol-Myers Squibb and Genentech.
In a new subgroup analysis of a previously published multinational trial, the preservation of lung function with nintedanib (Ofev) was about the same in patients with interstitial lung disease related to rheumatoid arthritis (RA-ILD) as it was in patients with other etiologies, according to data presented at the annual European Congress of Rheumatology.
“There was no significant heterogeneity across any of several characteristics we evaluated,” reported Clive Kelly, MBBS, of the Institute of Cellular Medicine at Newcastle University (England).
The INBUILD trial, which enrolled more than 600 patients in 15 countries with a range of fibrosing lung diseases, was published almost 2 years ago. On the primary endpoint of rate of decline in forced vital capacity (FVC), the medians were –80.8 mL per year among those randomized to nintedanib and –187.8 mL per year (P < .001) on placebo.
The INBUILD study provided evidence that fibrosing lung diseases have a common pathobiologic mechanism that can be slowed by targeting intracellular kinases. Nintedanib inhibits several growth factor receptors as well as nonreceptor tyrosine kinases, but its exact mechanism for slowing fibrosing lung diseases remains unclear. Initially approved for, nintedanib received approvals from the FDA for systemic sclerosis–associated ILD in 2019 and for chronic fibrosing ILD with progressive phenotypes in 2020 after being initially approved for the treatment of idiopathic pulmonary fibrosis in 2014.
When asked for comment, Paul F. Dellaripa, MD, an associate professor of medicine in the division of rheumatology, immunology, and allergy at Harvard Medical School, Boston, indicated these data are helpful in considering strategies for RA patients with ILD, but he encouraged collaboration between joint and lung specialists.
“Antifibrotic agents for patients with progressive ILD in autoimmune diseases like RA is a welcome addition to our care of this challenging complication,” said Dr. Dellaripa, who has published frequently on the diagnosis and treatment of lung diseases associated with RA. Yet, treatment must be individualized, he added.
“It will be incumbent for rheumatologists to incorporate lung health as a critical part of patient care and work closely with pulmonologists to consider when to institute antifibrotic therapy in patients with ILD,” he said.
Details of subanalysis
In the RA-ILD subpopulation of 89 patients, there was no further decline in FVC from 24 weeks after randomization to the end of 52 weeks for those on nintedanib, but the decline remained steady over the full course of follow-up among those in the placebo group. At 52 weeks, the decline in the placebo group reached –200 mL at the end of 52 weeks. As a result, the between-group relative reduction in FVC at 52 weeks of 116.7 mL favoring nintedanib over placebo (P < .037) slightly exceeded the 107-mL reduction (P < .001) observed in the overall INBUILD study population.
Among other subgroups the investigators evaluated, outcomes with nintedanib did not differ when patients were split into groups with higher or lower baseline levels of high-sensitivity C-reactive protein, regardless of whether the groups were defined by levels above and below 1 mg/L or 3 mg/L. The same was true for those who were taking nonbiologic disease-modifying antirheumatic drugs or glucocorticoids.
However, for these latter analyses, Dr. Kelly conceded that the differences were based on small numbers of patients and so cannot be considered conclusive.
The adverse event most closely associated with nintedanib in the RA-ILD population was diarrhea, just as in the overall study, and it was more than twice as frequent in the RA-ILD patients receiving the active therapy, compared with placebo (54.8% vs. 25.5%). Nausea was also more common (21.4% vs. 10.6%), and so was decreased appetite (11.9% vs. 2.1%) and weight reduction (9.5% vs. 2.1%).
Lung-related adverse events, such as bronchiolitis (21.4% vs. 17.0%) and dyspnea (11.9% vs. 10.6%), were only slightly more frequent in the nintedanib group. Nasopharyngitis (7.1% vs. 12.8%) was less common. Side effects leading to treatment discontinuation were higher on nintedanib (19.0% vs. 12.8%)
The RA-ILD subgroup represented 13.4% of those randomized in INBUILD. The mean time since diagnosis of RA was about 10 years. More than 60% were smokers or former smokers. At baseline, the mean FVC of predicted was 71%. More than 85% had a usual interstitial pneumonia (UIP) radiologic pattern.
Acute exacerbations and death were not evaluated in the RA-ILD subpopulation, but these were secondary endpoints in the published INBUILD study according to the presence or absence of a UIP-like fibrotic pattern. For the combined endpoint of acute exacerbation of ILD or death, the protection associated with nintedanib approached statistical significance for the population overall (odds ratio, 0.68; 95% confidence interval, 0.46-1.01) and reached significance for those with a UIP pattern (OR, 0.61; 95% CI, 0.38-0.98).
Nintedanib led to lower death rates at 52 weeks in the overall population (8.1% vs. 11.5% with placebo) and in the group with a UIP pattern (9.7% vs. 15.0% with placebo).
Dr. Kelly has financial relationships with multiple pharmaceutical companies, including Boehringer Ingelheim, which provided funding for INBUILD and this subpopulation analysis. Dr. Dellaripa reported financial relationships with Bristol-Myers Squibb and Genentech.
FROM THE EULAR 2021 CONGRESS
Subclinical myocarditis found in some athletes post COVID
Myocarditis is present in a small percentage of competitive athletes after COVID-19 infection, even in those without symptoms, new research suggests.
In a cohort study of 1,597 competitive collegiate athletes undergoing comprehensive cardiovascular testing in the United States, the prevalence of clinical myocarditis based on a symptom-based screening strategy was only 0.31%.
But screening with cardiac MRI increased the prevalence of clinical and subclinical myocarditis by a factor of 7.4, to 2.3%, the authors reported.
The findings are published online May 27, 2021, in JAMA Cardiology.
“It was the largest study to evaluate college athletes who have had COVID with extensive cardiac testing, including MRI, and this gave us a very objective look at the cardiac findings, as they were not purely based upon a subjective evaluation of symptoms,” lead investigator Curt J. Daniels, MD, professor at Ohio State University Wexner Medical Center, Columbus, said in an interview.
“Unfortunately, our study showed that athletes can be asymptomatic, or at least not report symptoms. This is a very subjective feature, and we don’t know if they don’t report symptoms because they didn’t want to get tested. That is why we took a very objective approach,” Dr. Daniels said.
The finding that more than half of the asymptomatic athletes had myocarditis, or as the investigators called it, “subclinical myocarditis,” was a surprise, he acknowledged.
“More than half of the athletes found to have myocarditis reported no symptoms, and yes, that was a surprise, because prior to this study, the protocols that had been published stated that you had to have symptoms to even enter into the protocol for cardiac MRI. But, as our ... paper shows, if we had followed that protocol, we only would have found about 5 cases of myocarditis, as opposed to the total of 37 we found with cardiac MRI,” Dr. Daniels said.
In October 2020, the American College of Cardiology’s Sports and Exercise Council recommended that cardiac MRI be limited to athletes who exhibited symptoms as part of their guide to ensuring a safe return to play.
As reported by this news organization the council recommended a tiered approach to screening based on the presence of symptoms, followed by electrocardiography, injury biomarkers, and echocardiography. Any abnormalities detected were to be further characterized by the selective use of cardiac MRI.
At the time, there were relatively few data to support the recommendations, and all stakeholders called for larger datasets to better drive informed recommendations in the future.
In the current study, Dr. Daniels and associates conducted comprehensive cardiac screening – including ECG, troponin testing, echocardiography, and cardiac MRI – of 1,597 college athlete survivors of COVID-19.
The athletes were part of the Big Ten athletic conference, which consists of 13 major American universities.
Cardiac MRI revealed that 37 (2.3%) of these athletes demonstrated diagnostic criteria for COVID-19 myocarditis; of these, 20 had no cardiovascular symptoms and had normal ECGs, echocardiography, and troponin test results.
“These patients would not have been identified without CMR imaging. If we were going according to the older protocol, we would not have made this discovery. Cardiac MRI is the most sensitive and specific test for myocardial inflammation, there is no argument about that,” Dr. Daniels said.
The catch is, cardiac MRI is expensive and often difficult to access, especially in remote, rural, or other underserviced areas.
“You can’t get an MRI for every person who has had COVID, it’s just not feasible,” Dr. Daniels said. “We are not advocating that everybody get an MRI. But we do hope that our study creates awareness among clinicians and athletes themselves that if you’ve had COVID, even if you’re asymptomatic, there may be some heart changes. So be aware when you start to exercise again, if you have any symptoms, pause and seek medical care.”
Kudos to the sports cardiology community
In an accompanying editorial, James E. Udelson, MD, Ethan J. Rowin, MD, and Barry J. Maron, MD, from the CardioVascular Center at Tufts Medical Center, Boston, applauded the sports cardiology community for its diligence in acquiring and publishing data about the post–COVID-19 prevalence of cardiac abnormalities in competitive athletes.
“It is a real tribute to the sports cardiology community. There has been an amazing growth of information, and they not only gathered this information, they analyzed and published it, starting out with a study of 29 or 30 athletes, and now thousands,” Dr. Udelson said in an interview.
At the start of the pandemic, it appeared that 15%-20% of athletes had myocarditis, and athletic conferences were discussing canceling sports events.
However, with greater numbers comes a more accurate picture of the extent of the problem.
“Once you get thousands of subjects in these studies, you can hone in on what the real number is, so now we understand that if you screen everybody with a cardiac MRI, 1%, 2%, or 3% will have some evidence of what looks like myocarditis,” he said.
Dr. Udelson agreed that doing cardiac imaging in everyone is not feasible.
“This study looked at a very large number of people who all had an MRI, but that doesn’t mean everyone should have them. If you just do an echo, an EKG, and a troponin test, and if everything is normal, which is kind of what current recommendations are, this paper tells us that we are going to miss one or two people out of a hundred, and that might be okay,” he said. “So, if you are at a huge university that has a large medical center and you want to screen all your athletes with MRI, great. But if you’re at a high school in a remote area, you know that the alternative, not having an MRI, isn’t so bad, either.”
A version of this article first appeared on Medscape.com.
Myocarditis is present in a small percentage of competitive athletes after COVID-19 infection, even in those without symptoms, new research suggests.
In a cohort study of 1,597 competitive collegiate athletes undergoing comprehensive cardiovascular testing in the United States, the prevalence of clinical myocarditis based on a symptom-based screening strategy was only 0.31%.
But screening with cardiac MRI increased the prevalence of clinical and subclinical myocarditis by a factor of 7.4, to 2.3%, the authors reported.
The findings are published online May 27, 2021, in JAMA Cardiology.
“It was the largest study to evaluate college athletes who have had COVID with extensive cardiac testing, including MRI, and this gave us a very objective look at the cardiac findings, as they were not purely based upon a subjective evaluation of symptoms,” lead investigator Curt J. Daniels, MD, professor at Ohio State University Wexner Medical Center, Columbus, said in an interview.
“Unfortunately, our study showed that athletes can be asymptomatic, or at least not report symptoms. This is a very subjective feature, and we don’t know if they don’t report symptoms because they didn’t want to get tested. That is why we took a very objective approach,” Dr. Daniels said.
The finding that more than half of the asymptomatic athletes had myocarditis, or as the investigators called it, “subclinical myocarditis,” was a surprise, he acknowledged.
“More than half of the athletes found to have myocarditis reported no symptoms, and yes, that was a surprise, because prior to this study, the protocols that had been published stated that you had to have symptoms to even enter into the protocol for cardiac MRI. But, as our ... paper shows, if we had followed that protocol, we only would have found about 5 cases of myocarditis, as opposed to the total of 37 we found with cardiac MRI,” Dr. Daniels said.
In October 2020, the American College of Cardiology’s Sports and Exercise Council recommended that cardiac MRI be limited to athletes who exhibited symptoms as part of their guide to ensuring a safe return to play.
As reported by this news organization the council recommended a tiered approach to screening based on the presence of symptoms, followed by electrocardiography, injury biomarkers, and echocardiography. Any abnormalities detected were to be further characterized by the selective use of cardiac MRI.
At the time, there were relatively few data to support the recommendations, and all stakeholders called for larger datasets to better drive informed recommendations in the future.
In the current study, Dr. Daniels and associates conducted comprehensive cardiac screening – including ECG, troponin testing, echocardiography, and cardiac MRI – of 1,597 college athlete survivors of COVID-19.
The athletes were part of the Big Ten athletic conference, which consists of 13 major American universities.
Cardiac MRI revealed that 37 (2.3%) of these athletes demonstrated diagnostic criteria for COVID-19 myocarditis; of these, 20 had no cardiovascular symptoms and had normal ECGs, echocardiography, and troponin test results.
“These patients would not have been identified without CMR imaging. If we were going according to the older protocol, we would not have made this discovery. Cardiac MRI is the most sensitive and specific test for myocardial inflammation, there is no argument about that,” Dr. Daniels said.
The catch is, cardiac MRI is expensive and often difficult to access, especially in remote, rural, or other underserviced areas.
“You can’t get an MRI for every person who has had COVID, it’s just not feasible,” Dr. Daniels said. “We are not advocating that everybody get an MRI. But we do hope that our study creates awareness among clinicians and athletes themselves that if you’ve had COVID, even if you’re asymptomatic, there may be some heart changes. So be aware when you start to exercise again, if you have any symptoms, pause and seek medical care.”
Kudos to the sports cardiology community
In an accompanying editorial, James E. Udelson, MD, Ethan J. Rowin, MD, and Barry J. Maron, MD, from the CardioVascular Center at Tufts Medical Center, Boston, applauded the sports cardiology community for its diligence in acquiring and publishing data about the post–COVID-19 prevalence of cardiac abnormalities in competitive athletes.
“It is a real tribute to the sports cardiology community. There has been an amazing growth of information, and they not only gathered this information, they analyzed and published it, starting out with a study of 29 or 30 athletes, and now thousands,” Dr. Udelson said in an interview.
At the start of the pandemic, it appeared that 15%-20% of athletes had myocarditis, and athletic conferences were discussing canceling sports events.
However, with greater numbers comes a more accurate picture of the extent of the problem.
“Once you get thousands of subjects in these studies, you can hone in on what the real number is, so now we understand that if you screen everybody with a cardiac MRI, 1%, 2%, or 3% will have some evidence of what looks like myocarditis,” he said.
Dr. Udelson agreed that doing cardiac imaging in everyone is not feasible.
“This study looked at a very large number of people who all had an MRI, but that doesn’t mean everyone should have them. If you just do an echo, an EKG, and a troponin test, and if everything is normal, which is kind of what current recommendations are, this paper tells us that we are going to miss one or two people out of a hundred, and that might be okay,” he said. “So, if you are at a huge university that has a large medical center and you want to screen all your athletes with MRI, great. But if you’re at a high school in a remote area, you know that the alternative, not having an MRI, isn’t so bad, either.”
A version of this article first appeared on Medscape.com.
Myocarditis is present in a small percentage of competitive athletes after COVID-19 infection, even in those without symptoms, new research suggests.
In a cohort study of 1,597 competitive collegiate athletes undergoing comprehensive cardiovascular testing in the United States, the prevalence of clinical myocarditis based on a symptom-based screening strategy was only 0.31%.
But screening with cardiac MRI increased the prevalence of clinical and subclinical myocarditis by a factor of 7.4, to 2.3%, the authors reported.
The findings are published online May 27, 2021, in JAMA Cardiology.
“It was the largest study to evaluate college athletes who have had COVID with extensive cardiac testing, including MRI, and this gave us a very objective look at the cardiac findings, as they were not purely based upon a subjective evaluation of symptoms,” lead investigator Curt J. Daniels, MD, professor at Ohio State University Wexner Medical Center, Columbus, said in an interview.
“Unfortunately, our study showed that athletes can be asymptomatic, or at least not report symptoms. This is a very subjective feature, and we don’t know if they don’t report symptoms because they didn’t want to get tested. That is why we took a very objective approach,” Dr. Daniels said.
The finding that more than half of the asymptomatic athletes had myocarditis, or as the investigators called it, “subclinical myocarditis,” was a surprise, he acknowledged.
“More than half of the athletes found to have myocarditis reported no symptoms, and yes, that was a surprise, because prior to this study, the protocols that had been published stated that you had to have symptoms to even enter into the protocol for cardiac MRI. But, as our ... paper shows, if we had followed that protocol, we only would have found about 5 cases of myocarditis, as opposed to the total of 37 we found with cardiac MRI,” Dr. Daniels said.
In October 2020, the American College of Cardiology’s Sports and Exercise Council recommended that cardiac MRI be limited to athletes who exhibited symptoms as part of their guide to ensuring a safe return to play.
As reported by this news organization the council recommended a tiered approach to screening based on the presence of symptoms, followed by electrocardiography, injury biomarkers, and echocardiography. Any abnormalities detected were to be further characterized by the selective use of cardiac MRI.
At the time, there were relatively few data to support the recommendations, and all stakeholders called for larger datasets to better drive informed recommendations in the future.
In the current study, Dr. Daniels and associates conducted comprehensive cardiac screening – including ECG, troponin testing, echocardiography, and cardiac MRI – of 1,597 college athlete survivors of COVID-19.
The athletes were part of the Big Ten athletic conference, which consists of 13 major American universities.
Cardiac MRI revealed that 37 (2.3%) of these athletes demonstrated diagnostic criteria for COVID-19 myocarditis; of these, 20 had no cardiovascular symptoms and had normal ECGs, echocardiography, and troponin test results.
“These patients would not have been identified without CMR imaging. If we were going according to the older protocol, we would not have made this discovery. Cardiac MRI is the most sensitive and specific test for myocardial inflammation, there is no argument about that,” Dr. Daniels said.
The catch is, cardiac MRI is expensive and often difficult to access, especially in remote, rural, or other underserviced areas.
“You can’t get an MRI for every person who has had COVID, it’s just not feasible,” Dr. Daniels said. “We are not advocating that everybody get an MRI. But we do hope that our study creates awareness among clinicians and athletes themselves that if you’ve had COVID, even if you’re asymptomatic, there may be some heart changes. So be aware when you start to exercise again, if you have any symptoms, pause and seek medical care.”
Kudos to the sports cardiology community
In an accompanying editorial, James E. Udelson, MD, Ethan J. Rowin, MD, and Barry J. Maron, MD, from the CardioVascular Center at Tufts Medical Center, Boston, applauded the sports cardiology community for its diligence in acquiring and publishing data about the post–COVID-19 prevalence of cardiac abnormalities in competitive athletes.
“It is a real tribute to the sports cardiology community. There has been an amazing growth of information, and they not only gathered this information, they analyzed and published it, starting out with a study of 29 or 30 athletes, and now thousands,” Dr. Udelson said in an interview.
At the start of the pandemic, it appeared that 15%-20% of athletes had myocarditis, and athletic conferences were discussing canceling sports events.
However, with greater numbers comes a more accurate picture of the extent of the problem.
“Once you get thousands of subjects in these studies, you can hone in on what the real number is, so now we understand that if you screen everybody with a cardiac MRI, 1%, 2%, or 3% will have some evidence of what looks like myocarditis,” he said.
Dr. Udelson agreed that doing cardiac imaging in everyone is not feasible.
“This study looked at a very large number of people who all had an MRI, but that doesn’t mean everyone should have them. If you just do an echo, an EKG, and a troponin test, and if everything is normal, which is kind of what current recommendations are, this paper tells us that we are going to miss one or two people out of a hundred, and that might be okay,” he said. “So, if you are at a huge university that has a large medical center and you want to screen all your athletes with MRI, great. But if you’re at a high school in a remote area, you know that the alternative, not having an MRI, isn’t so bad, either.”
A version of this article first appeared on Medscape.com.
DOJ charges 14 with COVID-19–related fraud nearing $150M
The U.S. Department of Justice (DOJ) on May 26 announced charges against 14 defendants across the country who allegedly engaged in health care fraud schemes that exploited the COVID-19 pandemic and resulted in over $143 million in false billings to Medicare.
Among the defendants, a DOJ news release said, were a telemedicine company executive, a physician, marketers, and medical business owners.
In addition, the Centers for Medicare and Medicaid Services separately announced that it had taken “adverse administrative actions” against more than 50 providers for their involvement in fraud schemes related to COVID-19 or the abuse of CMS programs that were designed to encourage access to medical care during the pandemic.
Several of the defendants allegedly offered COVID-19 tests to Medicare beneficiaries in senior living facilities, drive-through COVID-19 testing sites, and medical offices to induce the beneficiaries to provide their personal identifying information and a saliva or a blood sample.
The DOJ charges claim the defendants then misused the information and the samples to submit claims to Medicare for unrelated, medically unnecessary, and far more expensive lab tests, including cancer genetic testing, allergy testing, and respiratory pathogen panel tests.
In some cases, it’s alleged, the lab results were not provided to the individuals in a timely fashion or were not reliable.
Other defendants are charged with exploiting temporary changes in CMS telehealth regulations that were designed to increase access to health care during the pandemic. In these cases, which the DOJ said were the first charges related to the expansion of telehealth under the COVID-19 emergency declaration, the defendants allegedly submitted false and fraudulent claims to Medicare for sham telemedicine encounters that did not occur.
“As part of these cases, medical professionals are alleged to have [been] offered and paid bribes in exchange for the medical professionals’ referral of unnecessary testing,” the DOJ news release said. However, no physicians were identified by the department.
Commenting on this aspect of the law enforcement action, FBI Director Christopher Wray said in the release: “Medical providers have been the unsung heroes for the American public throughout the pandemic. It’s disheartening that some have abused their authorities and committed COVID-19–related fraud against trusting citizens. The FBI, along with our federal law enforcement and private sector partners, are committed to continuing to combat health care fraud and protect the American people.”
The law enforcement action includes the third set of criminal charges related to the misuse of Provider Relief Fund monies, according to the release.
More than 340 individuals were charged in September 2020 with submitting $6 billion in fraudulent claims to federal health care programs and private insurers for telehealth consultations and substance abuse treatment. About $4.5 billion of that was related to telehealth, as reported by this news organization.
The new criminal charges were brought in federal district courts in Arkansas, California, Louisiana, Florida, New Jersey, and New York.
Case summaries
The DOJ provided several case summaries. One defendant, lab owner Billy Joe Taylor of Lavaca, Ark., was charged with participating in a scheme to defraud the government of over $42 million by filing false claims that were billed in combination with COVID-19 testing claims. He also allegedly billed for tests that were not performed.
Petros Hannesyan of Burbank, Calif., the owner of a home health agency, was charged with obtaining over $229,000 from COVID-19 relief programs under false pretenses. His firm allegedly misappropriated funds from the CARES Act Provider Relief Fund and submitted false loan applications and a false loan agreement to the Economic Injury Disaster Loan Program.
Michael Stein and Leonel Palatnik of Palm Beach County, Fla., were charged in a connection with an alleged $73 million conspiracy to defraud the government and to pay and receive health care kickbacks during the pandemic.
Mr. Stein, who owned a “purported” consulting company, and Mr. Palatnik, who owned testing labs in Texas, allegedly exploited Medicare’s waiver of telehealth restrictions “by offering telehealth providers access to Medicare beneficiaries for whom they could bill consultations. In exchange, these providers agreed to refer beneficiaries to [Mr. Palatnik’s] laboratories for expensive and medically unnecessary cancer and cardiovascular genetic testing.”
A version of this article first appeared on Medscape.com.
The U.S. Department of Justice (DOJ) on May 26 announced charges against 14 defendants across the country who allegedly engaged in health care fraud schemes that exploited the COVID-19 pandemic and resulted in over $143 million in false billings to Medicare.
Among the defendants, a DOJ news release said, were a telemedicine company executive, a physician, marketers, and medical business owners.
In addition, the Centers for Medicare and Medicaid Services separately announced that it had taken “adverse administrative actions” against more than 50 providers for their involvement in fraud schemes related to COVID-19 or the abuse of CMS programs that were designed to encourage access to medical care during the pandemic.
Several of the defendants allegedly offered COVID-19 tests to Medicare beneficiaries in senior living facilities, drive-through COVID-19 testing sites, and medical offices to induce the beneficiaries to provide their personal identifying information and a saliva or a blood sample.
The DOJ charges claim the defendants then misused the information and the samples to submit claims to Medicare for unrelated, medically unnecessary, and far more expensive lab tests, including cancer genetic testing, allergy testing, and respiratory pathogen panel tests.
In some cases, it’s alleged, the lab results were not provided to the individuals in a timely fashion or were not reliable.
Other defendants are charged with exploiting temporary changes in CMS telehealth regulations that were designed to increase access to health care during the pandemic. In these cases, which the DOJ said were the first charges related to the expansion of telehealth under the COVID-19 emergency declaration, the defendants allegedly submitted false and fraudulent claims to Medicare for sham telemedicine encounters that did not occur.
“As part of these cases, medical professionals are alleged to have [been] offered and paid bribes in exchange for the medical professionals’ referral of unnecessary testing,” the DOJ news release said. However, no physicians were identified by the department.
Commenting on this aspect of the law enforcement action, FBI Director Christopher Wray said in the release: “Medical providers have been the unsung heroes for the American public throughout the pandemic. It’s disheartening that some have abused their authorities and committed COVID-19–related fraud against trusting citizens. The FBI, along with our federal law enforcement and private sector partners, are committed to continuing to combat health care fraud and protect the American people.”
The law enforcement action includes the third set of criminal charges related to the misuse of Provider Relief Fund monies, according to the release.
More than 340 individuals were charged in September 2020 with submitting $6 billion in fraudulent claims to federal health care programs and private insurers for telehealth consultations and substance abuse treatment. About $4.5 billion of that was related to telehealth, as reported by this news organization.
The new criminal charges were brought in federal district courts in Arkansas, California, Louisiana, Florida, New Jersey, and New York.
Case summaries
The DOJ provided several case summaries. One defendant, lab owner Billy Joe Taylor of Lavaca, Ark., was charged with participating in a scheme to defraud the government of over $42 million by filing false claims that were billed in combination with COVID-19 testing claims. He also allegedly billed for tests that were not performed.
Petros Hannesyan of Burbank, Calif., the owner of a home health agency, was charged with obtaining over $229,000 from COVID-19 relief programs under false pretenses. His firm allegedly misappropriated funds from the CARES Act Provider Relief Fund and submitted false loan applications and a false loan agreement to the Economic Injury Disaster Loan Program.
Michael Stein and Leonel Palatnik of Palm Beach County, Fla., were charged in a connection with an alleged $73 million conspiracy to defraud the government and to pay and receive health care kickbacks during the pandemic.
Mr. Stein, who owned a “purported” consulting company, and Mr. Palatnik, who owned testing labs in Texas, allegedly exploited Medicare’s waiver of telehealth restrictions “by offering telehealth providers access to Medicare beneficiaries for whom they could bill consultations. In exchange, these providers agreed to refer beneficiaries to [Mr. Palatnik’s] laboratories for expensive and medically unnecessary cancer and cardiovascular genetic testing.”
A version of this article first appeared on Medscape.com.
The U.S. Department of Justice (DOJ) on May 26 announced charges against 14 defendants across the country who allegedly engaged in health care fraud schemes that exploited the COVID-19 pandemic and resulted in over $143 million in false billings to Medicare.
Among the defendants, a DOJ news release said, were a telemedicine company executive, a physician, marketers, and medical business owners.
In addition, the Centers for Medicare and Medicaid Services separately announced that it had taken “adverse administrative actions” against more than 50 providers for their involvement in fraud schemes related to COVID-19 or the abuse of CMS programs that were designed to encourage access to medical care during the pandemic.
Several of the defendants allegedly offered COVID-19 tests to Medicare beneficiaries in senior living facilities, drive-through COVID-19 testing sites, and medical offices to induce the beneficiaries to provide their personal identifying information and a saliva or a blood sample.
The DOJ charges claim the defendants then misused the information and the samples to submit claims to Medicare for unrelated, medically unnecessary, and far more expensive lab tests, including cancer genetic testing, allergy testing, and respiratory pathogen panel tests.
In some cases, it’s alleged, the lab results were not provided to the individuals in a timely fashion or were not reliable.
Other defendants are charged with exploiting temporary changes in CMS telehealth regulations that were designed to increase access to health care during the pandemic. In these cases, which the DOJ said were the first charges related to the expansion of telehealth under the COVID-19 emergency declaration, the defendants allegedly submitted false and fraudulent claims to Medicare for sham telemedicine encounters that did not occur.
“As part of these cases, medical professionals are alleged to have [been] offered and paid bribes in exchange for the medical professionals’ referral of unnecessary testing,” the DOJ news release said. However, no physicians were identified by the department.
Commenting on this aspect of the law enforcement action, FBI Director Christopher Wray said in the release: “Medical providers have been the unsung heroes for the American public throughout the pandemic. It’s disheartening that some have abused their authorities and committed COVID-19–related fraud against trusting citizens. The FBI, along with our federal law enforcement and private sector partners, are committed to continuing to combat health care fraud and protect the American people.”
The law enforcement action includes the third set of criminal charges related to the misuse of Provider Relief Fund monies, according to the release.
More than 340 individuals were charged in September 2020 with submitting $6 billion in fraudulent claims to federal health care programs and private insurers for telehealth consultations and substance abuse treatment. About $4.5 billion of that was related to telehealth, as reported by this news organization.
The new criminal charges were brought in federal district courts in Arkansas, California, Louisiana, Florida, New Jersey, and New York.
Case summaries
The DOJ provided several case summaries. One defendant, lab owner Billy Joe Taylor of Lavaca, Ark., was charged with participating in a scheme to defraud the government of over $42 million by filing false claims that were billed in combination with COVID-19 testing claims. He also allegedly billed for tests that were not performed.
Petros Hannesyan of Burbank, Calif., the owner of a home health agency, was charged with obtaining over $229,000 from COVID-19 relief programs under false pretenses. His firm allegedly misappropriated funds from the CARES Act Provider Relief Fund and submitted false loan applications and a false loan agreement to the Economic Injury Disaster Loan Program.
Michael Stein and Leonel Palatnik of Palm Beach County, Fla., were charged in a connection with an alleged $73 million conspiracy to defraud the government and to pay and receive health care kickbacks during the pandemic.
Mr. Stein, who owned a “purported” consulting company, and Mr. Palatnik, who owned testing labs in Texas, allegedly exploited Medicare’s waiver of telehealth restrictions “by offering telehealth providers access to Medicare beneficiaries for whom they could bill consultations. In exchange, these providers agreed to refer beneficiaries to [Mr. Palatnik’s] laboratories for expensive and medically unnecessary cancer and cardiovascular genetic testing.”
A version of this article first appeared on Medscape.com.
Children aged 12-15 years continue to close COVID-19 vaccination gap
More children aged 12-15 years already have received at least one dose of a COVID-19 vaccine than have 16- and 17-year-olds, based on data from the Centers for Disease Control and Prevention.
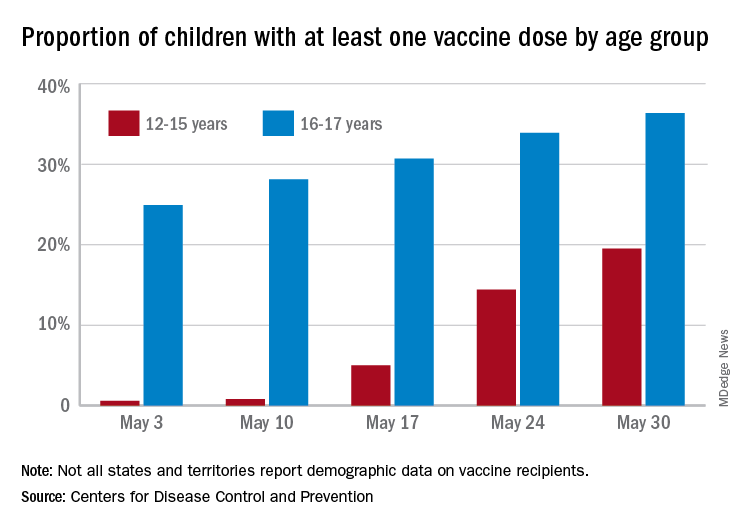
with those figures representing increases of 31.6% and 6.6% in the past week, respectively. Since the overall size of the 12-15 population is much larger, however, the proportion vaccinated is still smaller: 19.5% to 36.4%, according to the CDC’s COVID Data Tracker.
A look at full vaccination status shows that only 0.7% of those aged 12-15 years have received both doses of a two-dose vaccine or one dose of the single-shot variety, compared with 24% of those aged 16-17. For the country as a whole, 50.5% of all ages have received at least one dose and 40.7% are fully vaccinated, the CDC said.
Children aged 12-15 represent the largest share of the U.S. population (23.4%) initiating vaccination in the 14 days ending May 30, while children aged 16-17 made up just 4.5% of those getting their first dose. The younger group’s later entry into the vaccination pool shows up again when looking at completion rates, though, representing just 0.4% of all Americans who reached full vaccination during that same 14-day period, compared with 4.6% of the older children, the CDC data show.
Not all states are reporting data such as age for vaccine recipients, the CDC noted, and there are other variables that affect data collection. “Demographic data ... might differ by populations prioritized within each state or jurisdiction’s vaccination phase. Every geographic area has a different racial and ethnic composition, and not all are in the same vaccination phase,” the CDC said.
More children aged 12-15 years already have received at least one dose of a COVID-19 vaccine than have 16- and 17-year-olds, based on data from the Centers for Disease Control and Prevention.

with those figures representing increases of 31.6% and 6.6% in the past week, respectively. Since the overall size of the 12-15 population is much larger, however, the proportion vaccinated is still smaller: 19.5% to 36.4%, according to the CDC’s COVID Data Tracker.
A look at full vaccination status shows that only 0.7% of those aged 12-15 years have received both doses of a two-dose vaccine or one dose of the single-shot variety, compared with 24% of those aged 16-17. For the country as a whole, 50.5% of all ages have received at least one dose and 40.7% are fully vaccinated, the CDC said.
Children aged 12-15 represent the largest share of the U.S. population (23.4%) initiating vaccination in the 14 days ending May 30, while children aged 16-17 made up just 4.5% of those getting their first dose. The younger group’s later entry into the vaccination pool shows up again when looking at completion rates, though, representing just 0.4% of all Americans who reached full vaccination during that same 14-day period, compared with 4.6% of the older children, the CDC data show.
Not all states are reporting data such as age for vaccine recipients, the CDC noted, and there are other variables that affect data collection. “Demographic data ... might differ by populations prioritized within each state or jurisdiction’s vaccination phase. Every geographic area has a different racial and ethnic composition, and not all are in the same vaccination phase,” the CDC said.
More children aged 12-15 years already have received at least one dose of a COVID-19 vaccine than have 16- and 17-year-olds, based on data from the Centers for Disease Control and Prevention.

with those figures representing increases of 31.6% and 6.6% in the past week, respectively. Since the overall size of the 12-15 population is much larger, however, the proportion vaccinated is still smaller: 19.5% to 36.4%, according to the CDC’s COVID Data Tracker.
A look at full vaccination status shows that only 0.7% of those aged 12-15 years have received both doses of a two-dose vaccine or one dose of the single-shot variety, compared with 24% of those aged 16-17. For the country as a whole, 50.5% of all ages have received at least one dose and 40.7% are fully vaccinated, the CDC said.
Children aged 12-15 represent the largest share of the U.S. population (23.4%) initiating vaccination in the 14 days ending May 30, while children aged 16-17 made up just 4.5% of those getting their first dose. The younger group’s later entry into the vaccination pool shows up again when looking at completion rates, though, representing just 0.4% of all Americans who reached full vaccination during that same 14-day period, compared with 4.6% of the older children, the CDC data show.
Not all states are reporting data such as age for vaccine recipients, the CDC noted, and there are other variables that affect data collection. “Demographic data ... might differ by populations prioritized within each state or jurisdiction’s vaccination phase. Every geographic area has a different racial and ethnic composition, and not all are in the same vaccination phase,” the CDC said.
Clean indoor air is vital for infection control
Health workers already know that indoor air quality can be as important to human health as clean water and uncontaminated food. But before the COVID-19 pandemic, its importance in the prevention of respiratory illnesses outside of health circles was only whispered about.
Now, a team of nearly 40 scientists from 14 countries is calling for “a paradigm shift,” so that improvements in indoor air quality are viewed as essential to curb respiratory infections.
Most countries do not have indoor air-quality standards, the scientists point out in their recent report, and those that do often fall short in scope and enforcement.
“We expect everywhere in the world to have clean water flowing from our taps. In most parts of the developed world, it is happening and we take it completely for granted,” said lead investigator Lidia Morawska, PhD, of the International Laboratory for Air Quality and Health at the Queensland University of Technology in Brisbane, Australia.
But bacteria and viruses can circulate freely in the air, and “no one thinks about this, whatsoever, apart from health care facilities,” she said.
A first step is to recognize the risk posed by airborne pathogens, something not yet universally acknowledged. The investigators also want the World Health Organization to extend its guidelines to cover airborne pathogens, and for ventilation standards to include higher airflow and filtration rates.
Germany has been at the forefront of air-quality measures, Dr. Morawska said. Years ago, she observed a monitor showing the carbon dioxide level and relative humidity in the room where she was attending a meeting. The screen was accompanied by red, yellow, and green signals to communicate risk. Such indicators are also commonly displayed in German schools so teachers know when to open the windows or adjust the ventilation.
Monitors show carbon dioxide levels
But this is not yet being done in most other countries, Dr. Morawska said. Levels of carbon dioxide are one measure of indoor air quality, but they serve as a proxy for ventilation, she pointed out. Although the technology is available, sensors that can test a variety of components in a building in real time are not yet affordable.
Dr. Morawska envisions a future where the air quality numbers of the places people frequent are displayed so they know the risk for airborne transmission of respiratory illnesses. And people can begin to expect clean indoor air when they enter a business, office, or entertainment space and request changes when the air quality dips and improvement is needed, she said.
It is a daunting challenge to clean indoor air for several reasons. Air is not containable in the same way water is, which makes it difficult to trace contaminants. And infections transmitted through dirty water and food are usually evident immediately, whereas infections transmitted through airborne pathogens can take days to develop. Plus, the necessary infrastructure changes will be expensive.
However, the initial cost required to change the flow and quality of indoor air might be less than the cost of infections, the scientists pointed out. It is estimated that the global harm caused by COVID-19 alone costs $1 trillion each month.
“In the United States, the yearly cost – direct and indirect – of influenza has been calculated at $11.2 billion. For respiratory infections other than influenza, the yearly cost stood at $40 billion,” the team noted.
“If even half of this was caused by inhalation, we are still talking about massive costs,” said Dr. Morawska.
Bigger is not always better
It is tempting to see the solution as increased ventilation, said Ehsan Mousavi, PhD, assistant professor of construction science and management at Clemson (S.C.) University, who studies indoor air quality and ventilation in hospitals.
“We are ventilating the heck out of hospitals,” he said in an interview. But there is much debate about how much ventilation is the right amount. Too much and “you can blow pathogens into an open wound,” he explained. “Bigger is not always better.”
And there is still debate about the best mix of outside and recirculated air. An increase in the intake of outdoor air can refresh indoor air if it is clean, but that depends on where you live, he pointed out.
The mix used in most standard office buildings is 15% outside air and 85% recirculated air, Dr. Mousavi said. Boosting the percentage of outside air increases costs and energy use.
In fact, it can take five times more energy to ventilate hospital spaces than office spaces, he reported.
Engineers searching for clean-air solutions need to know what particulates are in the air and whether they are harmful to humans, but the sensors currently available can’t identify whether a virus is present in real time.
Samples have to be taken to a lab and, “by the time you know a virus was in the space, the moment is gone,” Dr. Mousavi explained.
More research is needed. “We need a reasonable answer that looks at the problem holistically, not just from the infectious disease perspective,” he said.
Hydrating indoor air
Research is making it clear that health care environments can play a significant role in patient recovery, according to Stephanie Taylor, MD. Dr. Taylor is president of Building4Health, which she founded to help businesses assess the quality of air in their buildings and find solutions. The company uses an algorithm to arrive at a health assessment score.
Air hydration is the most important aspect to target, she said.
Since the 1980s, research has shown that a relative humidity of 40%-60% is healthy for humans, she said. Currently, in an office building in a winter climate, the humidity level is more like 20%.
Canada is the first country to officially recommend the 40%-60% range for senior citizen centers and residential homes.
“Properly hydrated air supports our immune system and prevents skin problems and respiratory problems. It also inactivates many bacteria and viruses,” Dr. Taylor explained. Inhaling dry air compromises the ability of the body to restrict influenza virus infection, researchers showed in a 2019 study.
In the case of COVID-19, as virus particles attach to water molecules, they get bigger and heavier and eventually drop out of the breathing zone and onto surfaces where they can be wiped away, she explained.
But when the particles “are very small – like 5 microns in diameter – and you inhale them, they can lodge deep in the lungs,” she said.
In properly hydrated air, particles will be larger – about 10-20 microns when they attach to the water vapor – so they will get stuck in the nose or the back of the throat, where they can be washed away by mucous and not travel to the lungs.
“Indoor air metrics” can support our health or contribute to disease, “not just over time, but quickly, within minutes or hours,” she said.
No one expects the world’s building stock to suddenly upgrade to the ideal air quality. “But that doesn’t mean we shouldn’t move in that direction,” Dr. Taylor said. Changes can start small and gradually increase.
New research targets indoor air
Humidity is one of the key areas for current research, said Karl Rockne, PhD, director of the environmental engineering program at the National Science Foundation.
“When a virus comes out, it’s not just a naked virus, which is exceptionally small. It’s a virus encapsulated in liquid. And that’s why the humidity is so key. The degree of humidity can determine how fast the water evaporates from the particle,” he said in an interview.
In the wake of COVID-19, his institution is funding more cross-disciplinary research in biology, building science, architecture, and physics, he pointed out.
One such effort involved the development of a sensor that can capture live COVID-19 virus. This so-called “smoking gun,” which proved that the virus can spread through the air, took the combined expertise of professionals in medicine, engineering, and several other disciplines.
Currently, investigators are examining indoor air quality and water supplies in offices that have been left empty during the pandemic, and the effect they will have on human health. And others are looking at the way outside air quality affects indoor air quality, particularly where outdoor air quality is poor, such as in areas experiencing wildfires.
So will COVID-19 be the catalyst that finally drives changes to building design, regulation, and public expectations of air quality in the spaces where we spend close to 90% of our time?
“If not COVID, what else? It affected every country, every sector,” Dr. Morawska said. “There’s enough momentum now to do something about this. And enough realization there is a problem.”
A version of this article first appeared on Medscape.com.
Health workers already know that indoor air quality can be as important to human health as clean water and uncontaminated food. But before the COVID-19 pandemic, its importance in the prevention of respiratory illnesses outside of health circles was only whispered about.
Now, a team of nearly 40 scientists from 14 countries is calling for “a paradigm shift,” so that improvements in indoor air quality are viewed as essential to curb respiratory infections.
Most countries do not have indoor air-quality standards, the scientists point out in their recent report, and those that do often fall short in scope and enforcement.
“We expect everywhere in the world to have clean water flowing from our taps. In most parts of the developed world, it is happening and we take it completely for granted,” said lead investigator Lidia Morawska, PhD, of the International Laboratory for Air Quality and Health at the Queensland University of Technology in Brisbane, Australia.
But bacteria and viruses can circulate freely in the air, and “no one thinks about this, whatsoever, apart from health care facilities,” she said.
A first step is to recognize the risk posed by airborne pathogens, something not yet universally acknowledged. The investigators also want the World Health Organization to extend its guidelines to cover airborne pathogens, and for ventilation standards to include higher airflow and filtration rates.
Germany has been at the forefront of air-quality measures, Dr. Morawska said. Years ago, she observed a monitor showing the carbon dioxide level and relative humidity in the room where she was attending a meeting. The screen was accompanied by red, yellow, and green signals to communicate risk. Such indicators are also commonly displayed in German schools so teachers know when to open the windows or adjust the ventilation.
Monitors show carbon dioxide levels
But this is not yet being done in most other countries, Dr. Morawska said. Levels of carbon dioxide are one measure of indoor air quality, but they serve as a proxy for ventilation, she pointed out. Although the technology is available, sensors that can test a variety of components in a building in real time are not yet affordable.
Dr. Morawska envisions a future where the air quality numbers of the places people frequent are displayed so they know the risk for airborne transmission of respiratory illnesses. And people can begin to expect clean indoor air when they enter a business, office, or entertainment space and request changes when the air quality dips and improvement is needed, she said.
It is a daunting challenge to clean indoor air for several reasons. Air is not containable in the same way water is, which makes it difficult to trace contaminants. And infections transmitted through dirty water and food are usually evident immediately, whereas infections transmitted through airborne pathogens can take days to develop. Plus, the necessary infrastructure changes will be expensive.
However, the initial cost required to change the flow and quality of indoor air might be less than the cost of infections, the scientists pointed out. It is estimated that the global harm caused by COVID-19 alone costs $1 trillion each month.
“In the United States, the yearly cost – direct and indirect – of influenza has been calculated at $11.2 billion. For respiratory infections other than influenza, the yearly cost stood at $40 billion,” the team noted.
“If even half of this was caused by inhalation, we are still talking about massive costs,” said Dr. Morawska.
Bigger is not always better
It is tempting to see the solution as increased ventilation, said Ehsan Mousavi, PhD, assistant professor of construction science and management at Clemson (S.C.) University, who studies indoor air quality and ventilation in hospitals.
“We are ventilating the heck out of hospitals,” he said in an interview. But there is much debate about how much ventilation is the right amount. Too much and “you can blow pathogens into an open wound,” he explained. “Bigger is not always better.”
And there is still debate about the best mix of outside and recirculated air. An increase in the intake of outdoor air can refresh indoor air if it is clean, but that depends on where you live, he pointed out.
The mix used in most standard office buildings is 15% outside air and 85% recirculated air, Dr. Mousavi said. Boosting the percentage of outside air increases costs and energy use.
In fact, it can take five times more energy to ventilate hospital spaces than office spaces, he reported.
Engineers searching for clean-air solutions need to know what particulates are in the air and whether they are harmful to humans, but the sensors currently available can’t identify whether a virus is present in real time.
Samples have to be taken to a lab and, “by the time you know a virus was in the space, the moment is gone,” Dr. Mousavi explained.
More research is needed. “We need a reasonable answer that looks at the problem holistically, not just from the infectious disease perspective,” he said.
Hydrating indoor air
Research is making it clear that health care environments can play a significant role in patient recovery, according to Stephanie Taylor, MD. Dr. Taylor is president of Building4Health, which she founded to help businesses assess the quality of air in their buildings and find solutions. The company uses an algorithm to arrive at a health assessment score.
Air hydration is the most important aspect to target, she said.
Since the 1980s, research has shown that a relative humidity of 40%-60% is healthy for humans, she said. Currently, in an office building in a winter climate, the humidity level is more like 20%.
Canada is the first country to officially recommend the 40%-60% range for senior citizen centers and residential homes.
“Properly hydrated air supports our immune system and prevents skin problems and respiratory problems. It also inactivates many bacteria and viruses,” Dr. Taylor explained. Inhaling dry air compromises the ability of the body to restrict influenza virus infection, researchers showed in a 2019 study.
In the case of COVID-19, as virus particles attach to water molecules, they get bigger and heavier and eventually drop out of the breathing zone and onto surfaces where they can be wiped away, she explained.
But when the particles “are very small – like 5 microns in diameter – and you inhale them, they can lodge deep in the lungs,” she said.
In properly hydrated air, particles will be larger – about 10-20 microns when they attach to the water vapor – so they will get stuck in the nose or the back of the throat, where they can be washed away by mucous and not travel to the lungs.
“Indoor air metrics” can support our health or contribute to disease, “not just over time, but quickly, within minutes or hours,” she said.
No one expects the world’s building stock to suddenly upgrade to the ideal air quality. “But that doesn’t mean we shouldn’t move in that direction,” Dr. Taylor said. Changes can start small and gradually increase.
New research targets indoor air
Humidity is one of the key areas for current research, said Karl Rockne, PhD, director of the environmental engineering program at the National Science Foundation.
“When a virus comes out, it’s not just a naked virus, which is exceptionally small. It’s a virus encapsulated in liquid. And that’s why the humidity is so key. The degree of humidity can determine how fast the water evaporates from the particle,” he said in an interview.
In the wake of COVID-19, his institution is funding more cross-disciplinary research in biology, building science, architecture, and physics, he pointed out.
One such effort involved the development of a sensor that can capture live COVID-19 virus. This so-called “smoking gun,” which proved that the virus can spread through the air, took the combined expertise of professionals in medicine, engineering, and several other disciplines.
Currently, investigators are examining indoor air quality and water supplies in offices that have been left empty during the pandemic, and the effect they will have on human health. And others are looking at the way outside air quality affects indoor air quality, particularly where outdoor air quality is poor, such as in areas experiencing wildfires.
So will COVID-19 be the catalyst that finally drives changes to building design, regulation, and public expectations of air quality in the spaces where we spend close to 90% of our time?
“If not COVID, what else? It affected every country, every sector,” Dr. Morawska said. “There’s enough momentum now to do something about this. And enough realization there is a problem.”
A version of this article first appeared on Medscape.com.
Health workers already know that indoor air quality can be as important to human health as clean water and uncontaminated food. But before the COVID-19 pandemic, its importance in the prevention of respiratory illnesses outside of health circles was only whispered about.
Now, a team of nearly 40 scientists from 14 countries is calling for “a paradigm shift,” so that improvements in indoor air quality are viewed as essential to curb respiratory infections.
Most countries do not have indoor air-quality standards, the scientists point out in their recent report, and those that do often fall short in scope and enforcement.
“We expect everywhere in the world to have clean water flowing from our taps. In most parts of the developed world, it is happening and we take it completely for granted,” said lead investigator Lidia Morawska, PhD, of the International Laboratory for Air Quality and Health at the Queensland University of Technology in Brisbane, Australia.
But bacteria and viruses can circulate freely in the air, and “no one thinks about this, whatsoever, apart from health care facilities,” she said.
A first step is to recognize the risk posed by airborne pathogens, something not yet universally acknowledged. The investigators also want the World Health Organization to extend its guidelines to cover airborne pathogens, and for ventilation standards to include higher airflow and filtration rates.
Germany has been at the forefront of air-quality measures, Dr. Morawska said. Years ago, she observed a monitor showing the carbon dioxide level and relative humidity in the room where she was attending a meeting. The screen was accompanied by red, yellow, and green signals to communicate risk. Such indicators are also commonly displayed in German schools so teachers know when to open the windows or adjust the ventilation.
Monitors show carbon dioxide levels
But this is not yet being done in most other countries, Dr. Morawska said. Levels of carbon dioxide are one measure of indoor air quality, but they serve as a proxy for ventilation, she pointed out. Although the technology is available, sensors that can test a variety of components in a building in real time are not yet affordable.
Dr. Morawska envisions a future where the air quality numbers of the places people frequent are displayed so they know the risk for airborne transmission of respiratory illnesses. And people can begin to expect clean indoor air when they enter a business, office, or entertainment space and request changes when the air quality dips and improvement is needed, she said.
It is a daunting challenge to clean indoor air for several reasons. Air is not containable in the same way water is, which makes it difficult to trace contaminants. And infections transmitted through dirty water and food are usually evident immediately, whereas infections transmitted through airborne pathogens can take days to develop. Plus, the necessary infrastructure changes will be expensive.
However, the initial cost required to change the flow and quality of indoor air might be less than the cost of infections, the scientists pointed out. It is estimated that the global harm caused by COVID-19 alone costs $1 trillion each month.
“In the United States, the yearly cost – direct and indirect – of influenza has been calculated at $11.2 billion. For respiratory infections other than influenza, the yearly cost stood at $40 billion,” the team noted.
“If even half of this was caused by inhalation, we are still talking about massive costs,” said Dr. Morawska.
Bigger is not always better
It is tempting to see the solution as increased ventilation, said Ehsan Mousavi, PhD, assistant professor of construction science and management at Clemson (S.C.) University, who studies indoor air quality and ventilation in hospitals.
“We are ventilating the heck out of hospitals,” he said in an interview. But there is much debate about how much ventilation is the right amount. Too much and “you can blow pathogens into an open wound,” he explained. “Bigger is not always better.”
And there is still debate about the best mix of outside and recirculated air. An increase in the intake of outdoor air can refresh indoor air if it is clean, but that depends on where you live, he pointed out.
The mix used in most standard office buildings is 15% outside air and 85% recirculated air, Dr. Mousavi said. Boosting the percentage of outside air increases costs and energy use.
In fact, it can take five times more energy to ventilate hospital spaces than office spaces, he reported.
Engineers searching for clean-air solutions need to know what particulates are in the air and whether they are harmful to humans, but the sensors currently available can’t identify whether a virus is present in real time.
Samples have to be taken to a lab and, “by the time you know a virus was in the space, the moment is gone,” Dr. Mousavi explained.
More research is needed. “We need a reasonable answer that looks at the problem holistically, not just from the infectious disease perspective,” he said.
Hydrating indoor air
Research is making it clear that health care environments can play a significant role in patient recovery, according to Stephanie Taylor, MD. Dr. Taylor is president of Building4Health, which she founded to help businesses assess the quality of air in their buildings and find solutions. The company uses an algorithm to arrive at a health assessment score.
Air hydration is the most important aspect to target, she said.
Since the 1980s, research has shown that a relative humidity of 40%-60% is healthy for humans, she said. Currently, in an office building in a winter climate, the humidity level is more like 20%.
Canada is the first country to officially recommend the 40%-60% range for senior citizen centers and residential homes.
“Properly hydrated air supports our immune system and prevents skin problems and respiratory problems. It also inactivates many bacteria and viruses,” Dr. Taylor explained. Inhaling dry air compromises the ability of the body to restrict influenza virus infection, researchers showed in a 2019 study.
In the case of COVID-19, as virus particles attach to water molecules, they get bigger and heavier and eventually drop out of the breathing zone and onto surfaces where they can be wiped away, she explained.
But when the particles “are very small – like 5 microns in diameter – and you inhale them, they can lodge deep in the lungs,” she said.
In properly hydrated air, particles will be larger – about 10-20 microns when they attach to the water vapor – so they will get stuck in the nose or the back of the throat, where they can be washed away by mucous and not travel to the lungs.
“Indoor air metrics” can support our health or contribute to disease, “not just over time, but quickly, within minutes or hours,” she said.
No one expects the world’s building stock to suddenly upgrade to the ideal air quality. “But that doesn’t mean we shouldn’t move in that direction,” Dr. Taylor said. Changes can start small and gradually increase.
New research targets indoor air
Humidity is one of the key areas for current research, said Karl Rockne, PhD, director of the environmental engineering program at the National Science Foundation.
“When a virus comes out, it’s not just a naked virus, which is exceptionally small. It’s a virus encapsulated in liquid. And that’s why the humidity is so key. The degree of humidity can determine how fast the water evaporates from the particle,” he said in an interview.
In the wake of COVID-19, his institution is funding more cross-disciplinary research in biology, building science, architecture, and physics, he pointed out.
One such effort involved the development of a sensor that can capture live COVID-19 virus. This so-called “smoking gun,” which proved that the virus can spread through the air, took the combined expertise of professionals in medicine, engineering, and several other disciplines.
Currently, investigators are examining indoor air quality and water supplies in offices that have been left empty during the pandemic, and the effect they will have on human health. And others are looking at the way outside air quality affects indoor air quality, particularly where outdoor air quality is poor, such as in areas experiencing wildfires.
So will COVID-19 be the catalyst that finally drives changes to building design, regulation, and public expectations of air quality in the spaces where we spend close to 90% of our time?
“If not COVID, what else? It affected every country, every sector,” Dr. Morawska said. “There’s enough momentum now to do something about this. And enough realization there is a problem.”
A version of this article first appeared on Medscape.com.
Obstructive sleep apnea linked to COVID-19 risk
Greater severity of obstructive sleep apnea (OSA) is associated with a higher risk of contracting COVID-19, and positive airway pressure (PAP) treatment may counter that risk, according to a retrospective analysis from the records of Kaiser Permanente Southern California.
OSA patients often worry that PAP therapy might increase risk of severe COVID-19, said Dennis Hwang, MD, who presented the study at the American Thoracic Society’s virtual international conference (Abstract A1108). But the findings should be reassuring. “If you have obstructive sleep apnea, and you’re supposed to be using PAP, we recommend that you continue using PAP. It’s good for your overall wellness and reducing the risk of cardiovascular disease, but as it relates to COVID-19, it’s possible that it could protect. And there doesn’t appear to be any risk of increased severity of illness (with use of PAP),” Dr. Hwang said in an interview. He is medical director of sleep medicine for Kaiser Permanente San Bernardino County and cochair of sleep medicine for Kaiser Southern California.
He noted that the retrospective nature of the study makes it difficult to pin down whether PAP therapy is truly protective, “but I think there’s enough that we’ve been able conceptually to understand, to suggest that a direct causative relationship is possible,” said Dr. Hwang.
The results may imply that OSA patients should pay special attention to their OSA when there’s concern about exposure to an infectious agent like SARS-CoV-2. “The intermittent hypoxia at night, which can linger over to the day as increased sympathetic activity, increased heart rate. All of these are stresses to the body. So if you’re going to get infected, you want to start at a healthier level. You want to eliminate your sleep apnea to help reduce your risk of morbidity,” said Esra Tasali, MD, who was asked to comment on the study. Dr. Tasali is associate professor of medicine at the University of Chicago, and director of the Sleep Research Center there.
During the Q&A session after the talk, audience members asked about the timing of PAP use during COVID-19 infection, for example how often it was used during the asymptomatic phase of infection and if PAP has a positive effect. The data were not available, but “I think that the way to go is to understand this chronology,” said Dr. Tasali.
The researchers examined records between 2015 and 2020, using sleep study data, remotely collected daily PAP data, and electronic health records, all from Kaiser Permanente Southern California. Included subjects were adults who had enrolled before Feb. 1, 2020, and had sleep diagnostic or PAP data on record by March 1, 2020. The researchers analyzed PAP adherence between March 1, 2020, and the time of COVID-19 diagnosis, or until the study ended on July 31, 2020.
Patients were defined as being untreated (< 2 hours/night PAP), moderately treated (2-3.9 hours/night), or well treated (4 or more hours/night). Apnea hypopnea index (AHI) was used to determine severity. The analysis included 81,932 patients (39.8% were women, mean age was 54.0 years, 9.9% were Black, and 34.5% were Hispanic). A total of 1.7% of subjects without OSA experienced COVID-19 infection, compared to 1.8% with OSA; 0.3% with OSA were hospitalized and 0.07% underwent intensive care or died.
There were some differences between the two groups. The non-USA population was younger (mean age 47.0 vs. 54.5 years), was less likely to be men (44% vs. 60.3%), had a lower mean body mass index (30.4 vs. 34.3), had fewer comorbidities according to the Charleston Comorbidity Index (1.3 vs. 2.0), and were less likely to have hypertension (5.6% vs. 12.4%; P < .0001 for all).
Infection rates were higher in patients with more severe OSA. The rates in untreated mild, moderate, and severe OSA were 2%, 2%, and 2.4% respectively. The rate among all treated patients was 1.4% (P < .0001). Infection rates also dropped among patients with better treatment: untreated, 2.1%; moderately treated, 1.7%; and well treated, 1.3% (P < .0001).
Not having OSA was associated with a lower infection risk than was having OSA (odds ratio [OR], 0.82; 95% confidence interval, 0.70-0.96). Compared to untreated patients, there was lower infection risk in the moderately treated (OR, 0.82; 95% CI, 0.65-1.03) and well treated (OR, 0.68; 95% CI, 0.59-0.79) groups. Higher infection rates were associated with obesity, higher Charlson Comorbidity score (> 2; OR, 1.29; 95% CI, 1.09-1.53), Black (OR, 1.51; 95% CI, 1.24-1.84) and Hispanic ethnicities (OR, 2.23; 95% CI, 1.96-2.54), and Medicaid enrollment. Increasing age was associated with lower risk of infection, with each 5-year increment linked to reduced risk (OR, 0.88; 95% CI, 0.86-0.90). Dr. Hwang suggested that the age association may be because older individuals were more likely to follow social distancing and other precautions.
A multivariate analysis found that OSA was associated with infection risk according to OSA severity, including mild (OR, 1.21; 95% CI, 1.01-1.44), and moderate to severe (OR, 1.27; 95% CI, 1.07-1.51). There was no association between hospitalization rate or ICU admission/death and presence of OSA or PAP adherence in the data presented, but Dr. Hwang said that an updated analysis suggests that OSA may be associated with a risk of greater COVID-19 severity.
The control group was composed of individuals who had undergone sleep testing, but found to not have OSA. Still, they aren’t necessarily representative of the general population, since symptoms likely drove them to testing. A high percentage were also obese, and the average BMI was 30. “It’s certainly not a ‘normal population,’ but the advantage of what we did in terms of using this control group is that they underwent sleep testing, so they were proven to have no obstructive sleep apnea, whereas if we used a general population, we just don’t know,” said Dr. Hwang.
The study received technical and data support from Somnoware, and was funded by Kaiser Permanente. Dr. Tasali has no relevant financial disclosures.
Greater severity of obstructive sleep apnea (OSA) is associated with a higher risk of contracting COVID-19, and positive airway pressure (PAP) treatment may counter that risk, according to a retrospective analysis from the records of Kaiser Permanente Southern California.
OSA patients often worry that PAP therapy might increase risk of severe COVID-19, said Dennis Hwang, MD, who presented the study at the American Thoracic Society’s virtual international conference (Abstract A1108). But the findings should be reassuring. “If you have obstructive sleep apnea, and you’re supposed to be using PAP, we recommend that you continue using PAP. It’s good for your overall wellness and reducing the risk of cardiovascular disease, but as it relates to COVID-19, it’s possible that it could protect. And there doesn’t appear to be any risk of increased severity of illness (with use of PAP),” Dr. Hwang said in an interview. He is medical director of sleep medicine for Kaiser Permanente San Bernardino County and cochair of sleep medicine for Kaiser Southern California.
He noted that the retrospective nature of the study makes it difficult to pin down whether PAP therapy is truly protective, “but I think there’s enough that we’ve been able conceptually to understand, to suggest that a direct causative relationship is possible,” said Dr. Hwang.
The results may imply that OSA patients should pay special attention to their OSA when there’s concern about exposure to an infectious agent like SARS-CoV-2. “The intermittent hypoxia at night, which can linger over to the day as increased sympathetic activity, increased heart rate. All of these are stresses to the body. So if you’re going to get infected, you want to start at a healthier level. You want to eliminate your sleep apnea to help reduce your risk of morbidity,” said Esra Tasali, MD, who was asked to comment on the study. Dr. Tasali is associate professor of medicine at the University of Chicago, and director of the Sleep Research Center there.
During the Q&A session after the talk, audience members asked about the timing of PAP use during COVID-19 infection, for example how often it was used during the asymptomatic phase of infection and if PAP has a positive effect. The data were not available, but “I think that the way to go is to understand this chronology,” said Dr. Tasali.
The researchers examined records between 2015 and 2020, using sleep study data, remotely collected daily PAP data, and electronic health records, all from Kaiser Permanente Southern California. Included subjects were adults who had enrolled before Feb. 1, 2020, and had sleep diagnostic or PAP data on record by March 1, 2020. The researchers analyzed PAP adherence between March 1, 2020, and the time of COVID-19 diagnosis, or until the study ended on July 31, 2020.
Patients were defined as being untreated (< 2 hours/night PAP), moderately treated (2-3.9 hours/night), or well treated (4 or more hours/night). Apnea hypopnea index (AHI) was used to determine severity. The analysis included 81,932 patients (39.8% were women, mean age was 54.0 years, 9.9% were Black, and 34.5% were Hispanic). A total of 1.7% of subjects without OSA experienced COVID-19 infection, compared to 1.8% with OSA; 0.3% with OSA were hospitalized and 0.07% underwent intensive care or died.
There were some differences between the two groups. The non-USA population was younger (mean age 47.0 vs. 54.5 years), was less likely to be men (44% vs. 60.3%), had a lower mean body mass index (30.4 vs. 34.3), had fewer comorbidities according to the Charleston Comorbidity Index (1.3 vs. 2.0), and were less likely to have hypertension (5.6% vs. 12.4%; P < .0001 for all).
Infection rates were higher in patients with more severe OSA. The rates in untreated mild, moderate, and severe OSA were 2%, 2%, and 2.4% respectively. The rate among all treated patients was 1.4% (P < .0001). Infection rates also dropped among patients with better treatment: untreated, 2.1%; moderately treated, 1.7%; and well treated, 1.3% (P < .0001).
Not having OSA was associated with a lower infection risk than was having OSA (odds ratio [OR], 0.82; 95% confidence interval, 0.70-0.96). Compared to untreated patients, there was lower infection risk in the moderately treated (OR, 0.82; 95% CI, 0.65-1.03) and well treated (OR, 0.68; 95% CI, 0.59-0.79) groups. Higher infection rates were associated with obesity, higher Charlson Comorbidity score (> 2; OR, 1.29; 95% CI, 1.09-1.53), Black (OR, 1.51; 95% CI, 1.24-1.84) and Hispanic ethnicities (OR, 2.23; 95% CI, 1.96-2.54), and Medicaid enrollment. Increasing age was associated with lower risk of infection, with each 5-year increment linked to reduced risk (OR, 0.88; 95% CI, 0.86-0.90). Dr. Hwang suggested that the age association may be because older individuals were more likely to follow social distancing and other precautions.
A multivariate analysis found that OSA was associated with infection risk according to OSA severity, including mild (OR, 1.21; 95% CI, 1.01-1.44), and moderate to severe (OR, 1.27; 95% CI, 1.07-1.51). There was no association between hospitalization rate or ICU admission/death and presence of OSA or PAP adherence in the data presented, but Dr. Hwang said that an updated analysis suggests that OSA may be associated with a risk of greater COVID-19 severity.
The control group was composed of individuals who had undergone sleep testing, but found to not have OSA. Still, they aren’t necessarily representative of the general population, since symptoms likely drove them to testing. A high percentage were also obese, and the average BMI was 30. “It’s certainly not a ‘normal population,’ but the advantage of what we did in terms of using this control group is that they underwent sleep testing, so they were proven to have no obstructive sleep apnea, whereas if we used a general population, we just don’t know,” said Dr. Hwang.
The study received technical and data support from Somnoware, and was funded by Kaiser Permanente. Dr. Tasali has no relevant financial disclosures.
Greater severity of obstructive sleep apnea (OSA) is associated with a higher risk of contracting COVID-19, and positive airway pressure (PAP) treatment may counter that risk, according to a retrospective analysis from the records of Kaiser Permanente Southern California.
OSA patients often worry that PAP therapy might increase risk of severe COVID-19, said Dennis Hwang, MD, who presented the study at the American Thoracic Society’s virtual international conference (Abstract A1108). But the findings should be reassuring. “If you have obstructive sleep apnea, and you’re supposed to be using PAP, we recommend that you continue using PAP. It’s good for your overall wellness and reducing the risk of cardiovascular disease, but as it relates to COVID-19, it’s possible that it could protect. And there doesn’t appear to be any risk of increased severity of illness (with use of PAP),” Dr. Hwang said in an interview. He is medical director of sleep medicine for Kaiser Permanente San Bernardino County and cochair of sleep medicine for Kaiser Southern California.
He noted that the retrospective nature of the study makes it difficult to pin down whether PAP therapy is truly protective, “but I think there’s enough that we’ve been able conceptually to understand, to suggest that a direct causative relationship is possible,” said Dr. Hwang.
The results may imply that OSA patients should pay special attention to their OSA when there’s concern about exposure to an infectious agent like SARS-CoV-2. “The intermittent hypoxia at night, which can linger over to the day as increased sympathetic activity, increased heart rate. All of these are stresses to the body. So if you’re going to get infected, you want to start at a healthier level. You want to eliminate your sleep apnea to help reduce your risk of morbidity,” said Esra Tasali, MD, who was asked to comment on the study. Dr. Tasali is associate professor of medicine at the University of Chicago, and director of the Sleep Research Center there.
During the Q&A session after the talk, audience members asked about the timing of PAP use during COVID-19 infection, for example how often it was used during the asymptomatic phase of infection and if PAP has a positive effect. The data were not available, but “I think that the way to go is to understand this chronology,” said Dr. Tasali.
The researchers examined records between 2015 and 2020, using sleep study data, remotely collected daily PAP data, and electronic health records, all from Kaiser Permanente Southern California. Included subjects were adults who had enrolled before Feb. 1, 2020, and had sleep diagnostic or PAP data on record by March 1, 2020. The researchers analyzed PAP adherence between March 1, 2020, and the time of COVID-19 diagnosis, or until the study ended on July 31, 2020.
Patients were defined as being untreated (< 2 hours/night PAP), moderately treated (2-3.9 hours/night), or well treated (4 or more hours/night). Apnea hypopnea index (AHI) was used to determine severity. The analysis included 81,932 patients (39.8% were women, mean age was 54.0 years, 9.9% were Black, and 34.5% were Hispanic). A total of 1.7% of subjects without OSA experienced COVID-19 infection, compared to 1.8% with OSA; 0.3% with OSA were hospitalized and 0.07% underwent intensive care or died.
There were some differences between the two groups. The non-USA population was younger (mean age 47.0 vs. 54.5 years), was less likely to be men (44% vs. 60.3%), had a lower mean body mass index (30.4 vs. 34.3), had fewer comorbidities according to the Charleston Comorbidity Index (1.3 vs. 2.0), and were less likely to have hypertension (5.6% vs. 12.4%; P < .0001 for all).
Infection rates were higher in patients with more severe OSA. The rates in untreated mild, moderate, and severe OSA were 2%, 2%, and 2.4% respectively. The rate among all treated patients was 1.4% (P < .0001). Infection rates also dropped among patients with better treatment: untreated, 2.1%; moderately treated, 1.7%; and well treated, 1.3% (P < .0001).
Not having OSA was associated with a lower infection risk than was having OSA (odds ratio [OR], 0.82; 95% confidence interval, 0.70-0.96). Compared to untreated patients, there was lower infection risk in the moderately treated (OR, 0.82; 95% CI, 0.65-1.03) and well treated (OR, 0.68; 95% CI, 0.59-0.79) groups. Higher infection rates were associated with obesity, higher Charlson Comorbidity score (> 2; OR, 1.29; 95% CI, 1.09-1.53), Black (OR, 1.51; 95% CI, 1.24-1.84) and Hispanic ethnicities (OR, 2.23; 95% CI, 1.96-2.54), and Medicaid enrollment. Increasing age was associated with lower risk of infection, with each 5-year increment linked to reduced risk (OR, 0.88; 95% CI, 0.86-0.90). Dr. Hwang suggested that the age association may be because older individuals were more likely to follow social distancing and other precautions.
A multivariate analysis found that OSA was associated with infection risk according to OSA severity, including mild (OR, 1.21; 95% CI, 1.01-1.44), and moderate to severe (OR, 1.27; 95% CI, 1.07-1.51). There was no association between hospitalization rate or ICU admission/death and presence of OSA or PAP adherence in the data presented, but Dr. Hwang said that an updated analysis suggests that OSA may be associated with a risk of greater COVID-19 severity.
The control group was composed of individuals who had undergone sleep testing, but found to not have OSA. Still, they aren’t necessarily representative of the general population, since symptoms likely drove them to testing. A high percentage were also obese, and the average BMI was 30. “It’s certainly not a ‘normal population,’ but the advantage of what we did in terms of using this control group is that they underwent sleep testing, so they were proven to have no obstructive sleep apnea, whereas if we used a general population, we just don’t know,” said Dr. Hwang.
The study received technical and data support from Somnoware, and was funded by Kaiser Permanente. Dr. Tasali has no relevant financial disclosures.
FROM ATS 2021