User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
Powered by CHEST Physician, Clinician Reviews, MDedge Family Medicine, Internal Medicine News, and The Journal of Clinical Outcomes Management.
New COVID-19 vaccinations decline again in 12- to 15-year-olds
Even though less than 21% of all children aged 12-15 years are fully vaccinated against COVID-19, the number seeking first vaccinations continues to decline, according to data from the Centers for Disease Control and Prevention.

and 462,000 during the week ending June 14. Collectively, 30.2% of 12- to 15-year-olds have gotten at least one dose of vaccine so far and 20.7% are now fully vaccinated, the CDC said on its COVID Data Tracker site.
Among children aged 16-17 years, who were able to start the vaccination process earlier, 42.9% have received at least one dose and 34.0% have completed the COVID-19 vaccine regimen. Vaccine initiation – measured as the proportion of all individuals getting a first shot over the previous 2 weeks – has been consistently around 4.8% during the month of June for this age group but has dropped from 17.9% on June 7 to 14.3% on June 28 for those aged 12-15, the CDC data show.
Looking at the same measure for vaccine completion, 16.7% of all those who reached full vaccination status in the 14 days ending June 28 were 12- to 15-years-olds, down from 21.5% on June 21 and 19.6% on June 14. The numbers for those aged 15-16 were, respectively, 4.6%, 4.5%, and 4.2%, the CDC reported.
Fortunately, in the wake of recent vaccination trends, new cases of COVID-19 in children were down to their lowest level – just 8,447 for the week ending June 24 – since May of 2020, according to a new report from the American Academy of Pediatrics and the Children’s Hospital Association.
New cases had been well over 15,000 the previous week (June 17), following weeks of 14,000 (June 10) and 16,000 (June 3) new cases, so the latest drop down to just four digits represents a 1-week decline of over 46% in the 49 states (excluding New York) that are reporting age distribution, along with the District of Columbia, New York City, Puerto Rico, and Guam.
The cumulative number of child COVID-19 cases in those jurisdictions is about 4.03 million since the beginning of the pandemic, which represents 14.2% of all cases in the United States. At the state level, the cumulative rate of cases in children is highest in Vermont (22.7%) and lowest in Florida (8.9%), which uses an age range of 0-14 years for children, compared with 0-17 or 0-19 for most states, the AAP and CHA said.
Severe illness has been rare in children, which is reflected in the proportion of children among all hospitalizations, 2.2% in 24 jurisdictions, and the proportion of deaths, 0.06% in 46 jurisdictions, since the start of the pandemic, the AAP and CHA said, with a total of 336 COVID-19–related deaths reported.
Even though less than 21% of all children aged 12-15 years are fully vaccinated against COVID-19, the number seeking first vaccinations continues to decline, according to data from the Centers for Disease Control and Prevention.

and 462,000 during the week ending June 14. Collectively, 30.2% of 12- to 15-year-olds have gotten at least one dose of vaccine so far and 20.7% are now fully vaccinated, the CDC said on its COVID Data Tracker site.
Among children aged 16-17 years, who were able to start the vaccination process earlier, 42.9% have received at least one dose and 34.0% have completed the COVID-19 vaccine regimen. Vaccine initiation – measured as the proportion of all individuals getting a first shot over the previous 2 weeks – has been consistently around 4.8% during the month of June for this age group but has dropped from 17.9% on June 7 to 14.3% on June 28 for those aged 12-15, the CDC data show.
Looking at the same measure for vaccine completion, 16.7% of all those who reached full vaccination status in the 14 days ending June 28 were 12- to 15-years-olds, down from 21.5% on June 21 and 19.6% on June 14. The numbers for those aged 15-16 were, respectively, 4.6%, 4.5%, and 4.2%, the CDC reported.
Fortunately, in the wake of recent vaccination trends, new cases of COVID-19 in children were down to their lowest level – just 8,447 for the week ending June 24 – since May of 2020, according to a new report from the American Academy of Pediatrics and the Children’s Hospital Association.
New cases had been well over 15,000 the previous week (June 17), following weeks of 14,000 (June 10) and 16,000 (June 3) new cases, so the latest drop down to just four digits represents a 1-week decline of over 46% in the 49 states (excluding New York) that are reporting age distribution, along with the District of Columbia, New York City, Puerto Rico, and Guam.
The cumulative number of child COVID-19 cases in those jurisdictions is about 4.03 million since the beginning of the pandemic, which represents 14.2% of all cases in the United States. At the state level, the cumulative rate of cases in children is highest in Vermont (22.7%) and lowest in Florida (8.9%), which uses an age range of 0-14 years for children, compared with 0-17 or 0-19 for most states, the AAP and CHA said.
Severe illness has been rare in children, which is reflected in the proportion of children among all hospitalizations, 2.2% in 24 jurisdictions, and the proportion of deaths, 0.06% in 46 jurisdictions, since the start of the pandemic, the AAP and CHA said, with a total of 336 COVID-19–related deaths reported.
Even though less than 21% of all children aged 12-15 years are fully vaccinated against COVID-19, the number seeking first vaccinations continues to decline, according to data from the Centers for Disease Control and Prevention.

and 462,000 during the week ending June 14. Collectively, 30.2% of 12- to 15-year-olds have gotten at least one dose of vaccine so far and 20.7% are now fully vaccinated, the CDC said on its COVID Data Tracker site.
Among children aged 16-17 years, who were able to start the vaccination process earlier, 42.9% have received at least one dose and 34.0% have completed the COVID-19 vaccine regimen. Vaccine initiation – measured as the proportion of all individuals getting a first shot over the previous 2 weeks – has been consistently around 4.8% during the month of June for this age group but has dropped from 17.9% on June 7 to 14.3% on June 28 for those aged 12-15, the CDC data show.
Looking at the same measure for vaccine completion, 16.7% of all those who reached full vaccination status in the 14 days ending June 28 were 12- to 15-years-olds, down from 21.5% on June 21 and 19.6% on June 14. The numbers for those aged 15-16 were, respectively, 4.6%, 4.5%, and 4.2%, the CDC reported.
Fortunately, in the wake of recent vaccination trends, new cases of COVID-19 in children were down to their lowest level – just 8,447 for the week ending June 24 – since May of 2020, according to a new report from the American Academy of Pediatrics and the Children’s Hospital Association.
New cases had been well over 15,000 the previous week (June 17), following weeks of 14,000 (June 10) and 16,000 (June 3) new cases, so the latest drop down to just four digits represents a 1-week decline of over 46% in the 49 states (excluding New York) that are reporting age distribution, along with the District of Columbia, New York City, Puerto Rico, and Guam.
The cumulative number of child COVID-19 cases in those jurisdictions is about 4.03 million since the beginning of the pandemic, which represents 14.2% of all cases in the United States. At the state level, the cumulative rate of cases in children is highest in Vermont (22.7%) and lowest in Florida (8.9%), which uses an age range of 0-14 years for children, compared with 0-17 or 0-19 for most states, the AAP and CHA said.
Severe illness has been rare in children, which is reflected in the proportion of children among all hospitalizations, 2.2% in 24 jurisdictions, and the proportion of deaths, 0.06% in 46 jurisdictions, since the start of the pandemic, the AAP and CHA said, with a total of 336 COVID-19–related deaths reported.
Pfizer halts distribution of stop-smoking pill Chantix
The pharmaceutical company is also recalling some lots of Chantix that may have high levels of NDMA, Reuters reported.
Pfizer told Reuters the distribution pause was ordered out of abundance of caution while further testing is conducted. The FDA approved varenicline, which is marketed as Chantix, in 2006.
“The benefits of Chantix outweigh the very low potential risks, if any, posed by nitrosamine exposure from varenicline on top of other common sources over a lifetime,” Pfizer spokesperson Steven Danehy said in an email, according to Reuters.
The FDA has not issued a recall on Chantix. In Canada, however, health authorities on June 8 instituted a recall for Champix, the name under which the drug is sold in that nation.
The Chantix website says it’s a 3- to 6-month treatment that helps people overcome the need to smoke tobacco. The website says more than 13 million people have been prescribed Chantix.
Other health concerns have been raised about Chantix, such as mental health side effects.
In 2016, however, researchers concluded Chantix did not appear to raise the risk of serious health disorders such as depression, anxiety, and suicidal thoughts.
A version of this article first appeared on WebMD.com.
The pharmaceutical company is also recalling some lots of Chantix that may have high levels of NDMA, Reuters reported.
Pfizer told Reuters the distribution pause was ordered out of abundance of caution while further testing is conducted. The FDA approved varenicline, which is marketed as Chantix, in 2006.
“The benefits of Chantix outweigh the very low potential risks, if any, posed by nitrosamine exposure from varenicline on top of other common sources over a lifetime,” Pfizer spokesperson Steven Danehy said in an email, according to Reuters.
The FDA has not issued a recall on Chantix. In Canada, however, health authorities on June 8 instituted a recall for Champix, the name under which the drug is sold in that nation.
The Chantix website says it’s a 3- to 6-month treatment that helps people overcome the need to smoke tobacco. The website says more than 13 million people have been prescribed Chantix.
Other health concerns have been raised about Chantix, such as mental health side effects.
In 2016, however, researchers concluded Chantix did not appear to raise the risk of serious health disorders such as depression, anxiety, and suicidal thoughts.
A version of this article first appeared on WebMD.com.
The pharmaceutical company is also recalling some lots of Chantix that may have high levels of NDMA, Reuters reported.
Pfizer told Reuters the distribution pause was ordered out of abundance of caution while further testing is conducted. The FDA approved varenicline, which is marketed as Chantix, in 2006.
“The benefits of Chantix outweigh the very low potential risks, if any, posed by nitrosamine exposure from varenicline on top of other common sources over a lifetime,” Pfizer spokesperson Steven Danehy said in an email, according to Reuters.
The FDA has not issued a recall on Chantix. In Canada, however, health authorities on June 8 instituted a recall for Champix, the name under which the drug is sold in that nation.
The Chantix website says it’s a 3- to 6-month treatment that helps people overcome the need to smoke tobacco. The website says more than 13 million people have been prescribed Chantix.
Other health concerns have been raised about Chantix, such as mental health side effects.
In 2016, however, researchers concluded Chantix did not appear to raise the risk of serious health disorders such as depression, anxiety, and suicidal thoughts.
A version of this article first appeared on WebMD.com.
FDA to add myocarditis warning to mRNA COVID-19 vaccines
The Food and Drug Administration is adding a warning to mRNA COVID-19 vaccines’ fact sheets as medical experts continue to investigate cases of heart inflammation, which are rare but are more likely to occur in young men and teen boys.
Doran Fink, MD, PhD, deputy director of the FDA’s division of vaccines and related products applications, told a Centers for Disease Control and Prevention expert panel on June 23 that the FDA is finalizing language on a warning statement for health care providers, vaccine recipients, and parents or caregivers of teens.
The incidents are more likely to follow the second dose of the Pfizer or Moderna vaccine, with chest pain and other symptoms occurring within several days to a week, the warning will note.
“Based on limited follow-up, most cases appear to have been associated with resolution of symptoms, but limited information is available about potential long-term sequelae,” Dr. Fink said, describing the statement to the Advisory Committee on Immunization Practices, independent experts who advise the CDC.
“Symptoms suggestive of myocarditis or pericarditis should result in vaccine recipients seeking medical attention,” he said.
Benefits outweigh risks
Although no formal vote occurred after the meeting, the ACIP members delivered a strong endorsement for continuing to vaccinate 12- to 29-year-olds with the Pfizer and Moderna vaccines despite the warning.
“To me it’s clear, based on current information, that the benefits of vaccine clearly outweigh the risks,” said ACIP member Veronica McNally, president and CEO of the Franny Strong Foundation in Bloomfield, Mich., a sentiment echoed by other members.
As ACIP was meeting, leaders of the nation’s major physician, nurse, and public health associations issued a statement supporting continued vaccination: “The facts are clear: this is an extremely rare side effect, and only an exceedingly small number of people will experience it after vaccination.
“Importantly, for the young people who do, most cases are mild, and individuals recover often on their own or with minimal treatment. In addition, we know that myocarditis and pericarditis are much more common if you get COVID-19, and the risks to the heart from COVID-19 infection can be more severe.”
ACIP heard the evidence behind that claim. According to the Vaccine Safety Datalink, which contains data from more than 12 million medical records, myocarditis or pericarditis occurs in 12- to 39-year-olds at a rate of 8 per 1 million after the second Pfizer dose and 19.8 per 1 million after the second Moderna dose.
The CDC continues to investigate the link between the mRNA vaccines and heart inflammation, including any differences between the vaccines.
Most of the symptoms resolved quickly, said Tom Shimabukuro, deputy director of CDC’s Immunization Safety Office. Of 323 cases analyzed by the CDC, 309 were hospitalized, 295 were discharged, and 218, or 79%, had recovered from symptoms.
“Most postvaccine myocarditis has been responding to minimal treatment,” pediatric cardiologist Matthew Oster, MD, MPH, from Children’s Healthcare of Atlanta, told the panel.
COVID ‘risks are higher’
Overall, the CDC has reported 2,767 COVID-19 deaths among people aged 12-29 years, and there have been 4,018 reported cases of the COVID-linked inflammatory disorder MIS-C since the beginning of the pandemic.
That amounts to 1 MIS-C case in every 3,200 COVID infections – 36% of them among teens aged 12-20 years and 62% among children who are Hispanic or Black and non-Hispanic, according to a CDC presentation.
The CDC estimated that every 1 million second-dose COVID vaccines administered to 12- to 17-year-old boys could prevent 5,700 cases of COVID-19, 215 hospitalizations, 71 ICU admissions, and 2 deaths. There could also be 56-69 myocarditis cases.
The emergence of new variants in the United States and the skewed pattern of vaccination around the country also may increase the risk to unvaccinated young people, noted Grace Lee, MD, MPH, chair of the ACIP’s COVID-19 Vaccine Safety Technical Subgroup and a pediatric infectious disease physician at Stanford (Calif.) Children’s Health.
“If you’re in an area with low vaccination, the risks are higher,” she said. “The benefits [of the vaccine] are going to be far, far greater than any risk.”
Individuals, parents, and their clinicians should consider the full scope of risk when making decisions about vaccination, she said.
As the pandemic evolves, medical experts have to balance the known risks and benefits while they gather more information, said William Schaffner, MD, an infectious disease physician at Vanderbilt University, Nashville, Tenn., and medical director of the National Foundation for Infectious Diseases.
“The story is not over,” Dr. Schaffner said in an interview. “Clearly, we are still working in the face of a pandemic, so there’s urgency to continue vaccinating. But they would like to know more about the long-term consequences of the myocarditis.”
Booster possibilities
Meanwhile, ACIP began conversations on the parameters for a possible vaccine booster. For now, there are simply questions: Would a third vaccine help the immunocompromised gain protection? Should people get a different type of vaccine – mRNA versus adenovirus vector – for their booster? Most important, how long do antibodies last?
“Prior to going around giving everyone boosters, we really need to improve the overall vaccination coverage,” said Helen Keipp Talbot, MD, associate professor of medicine at Vanderbilt University. “That will protect everyone.”
A version of this article first appeared on Medscape.com.
The Food and Drug Administration is adding a warning to mRNA COVID-19 vaccines’ fact sheets as medical experts continue to investigate cases of heart inflammation, which are rare but are more likely to occur in young men and teen boys.
Doran Fink, MD, PhD, deputy director of the FDA’s division of vaccines and related products applications, told a Centers for Disease Control and Prevention expert panel on June 23 that the FDA is finalizing language on a warning statement for health care providers, vaccine recipients, and parents or caregivers of teens.
The incidents are more likely to follow the second dose of the Pfizer or Moderna vaccine, with chest pain and other symptoms occurring within several days to a week, the warning will note.
“Based on limited follow-up, most cases appear to have been associated with resolution of symptoms, but limited information is available about potential long-term sequelae,” Dr. Fink said, describing the statement to the Advisory Committee on Immunization Practices, independent experts who advise the CDC.
“Symptoms suggestive of myocarditis or pericarditis should result in vaccine recipients seeking medical attention,” he said.
Benefits outweigh risks
Although no formal vote occurred after the meeting, the ACIP members delivered a strong endorsement for continuing to vaccinate 12- to 29-year-olds with the Pfizer and Moderna vaccines despite the warning.
“To me it’s clear, based on current information, that the benefits of vaccine clearly outweigh the risks,” said ACIP member Veronica McNally, president and CEO of the Franny Strong Foundation in Bloomfield, Mich., a sentiment echoed by other members.
As ACIP was meeting, leaders of the nation’s major physician, nurse, and public health associations issued a statement supporting continued vaccination: “The facts are clear: this is an extremely rare side effect, and only an exceedingly small number of people will experience it after vaccination.
“Importantly, for the young people who do, most cases are mild, and individuals recover often on their own or with minimal treatment. In addition, we know that myocarditis and pericarditis are much more common if you get COVID-19, and the risks to the heart from COVID-19 infection can be more severe.”
ACIP heard the evidence behind that claim. According to the Vaccine Safety Datalink, which contains data from more than 12 million medical records, myocarditis or pericarditis occurs in 12- to 39-year-olds at a rate of 8 per 1 million after the second Pfizer dose and 19.8 per 1 million after the second Moderna dose.
The CDC continues to investigate the link between the mRNA vaccines and heart inflammation, including any differences between the vaccines.
Most of the symptoms resolved quickly, said Tom Shimabukuro, deputy director of CDC’s Immunization Safety Office. Of 323 cases analyzed by the CDC, 309 were hospitalized, 295 were discharged, and 218, or 79%, had recovered from symptoms.
“Most postvaccine myocarditis has been responding to minimal treatment,” pediatric cardiologist Matthew Oster, MD, MPH, from Children’s Healthcare of Atlanta, told the panel.
COVID ‘risks are higher’
Overall, the CDC has reported 2,767 COVID-19 deaths among people aged 12-29 years, and there have been 4,018 reported cases of the COVID-linked inflammatory disorder MIS-C since the beginning of the pandemic.
That amounts to 1 MIS-C case in every 3,200 COVID infections – 36% of them among teens aged 12-20 years and 62% among children who are Hispanic or Black and non-Hispanic, according to a CDC presentation.
The CDC estimated that every 1 million second-dose COVID vaccines administered to 12- to 17-year-old boys could prevent 5,700 cases of COVID-19, 215 hospitalizations, 71 ICU admissions, and 2 deaths. There could also be 56-69 myocarditis cases.
The emergence of new variants in the United States and the skewed pattern of vaccination around the country also may increase the risk to unvaccinated young people, noted Grace Lee, MD, MPH, chair of the ACIP’s COVID-19 Vaccine Safety Technical Subgroup and a pediatric infectious disease physician at Stanford (Calif.) Children’s Health.
“If you’re in an area with low vaccination, the risks are higher,” she said. “The benefits [of the vaccine] are going to be far, far greater than any risk.”
Individuals, parents, and their clinicians should consider the full scope of risk when making decisions about vaccination, she said.
As the pandemic evolves, medical experts have to balance the known risks and benefits while they gather more information, said William Schaffner, MD, an infectious disease physician at Vanderbilt University, Nashville, Tenn., and medical director of the National Foundation for Infectious Diseases.
“The story is not over,” Dr. Schaffner said in an interview. “Clearly, we are still working in the face of a pandemic, so there’s urgency to continue vaccinating. But they would like to know more about the long-term consequences of the myocarditis.”
Booster possibilities
Meanwhile, ACIP began conversations on the parameters for a possible vaccine booster. For now, there are simply questions: Would a third vaccine help the immunocompromised gain protection? Should people get a different type of vaccine – mRNA versus adenovirus vector – for their booster? Most important, how long do antibodies last?
“Prior to going around giving everyone boosters, we really need to improve the overall vaccination coverage,” said Helen Keipp Talbot, MD, associate professor of medicine at Vanderbilt University. “That will protect everyone.”
A version of this article first appeared on Medscape.com.
The Food and Drug Administration is adding a warning to mRNA COVID-19 vaccines’ fact sheets as medical experts continue to investigate cases of heart inflammation, which are rare but are more likely to occur in young men and teen boys.
Doran Fink, MD, PhD, deputy director of the FDA’s division of vaccines and related products applications, told a Centers for Disease Control and Prevention expert panel on June 23 that the FDA is finalizing language on a warning statement for health care providers, vaccine recipients, and parents or caregivers of teens.
The incidents are more likely to follow the second dose of the Pfizer or Moderna vaccine, with chest pain and other symptoms occurring within several days to a week, the warning will note.
“Based on limited follow-up, most cases appear to have been associated with resolution of symptoms, but limited information is available about potential long-term sequelae,” Dr. Fink said, describing the statement to the Advisory Committee on Immunization Practices, independent experts who advise the CDC.
“Symptoms suggestive of myocarditis or pericarditis should result in vaccine recipients seeking medical attention,” he said.
Benefits outweigh risks
Although no formal vote occurred after the meeting, the ACIP members delivered a strong endorsement for continuing to vaccinate 12- to 29-year-olds with the Pfizer and Moderna vaccines despite the warning.
“To me it’s clear, based on current information, that the benefits of vaccine clearly outweigh the risks,” said ACIP member Veronica McNally, president and CEO of the Franny Strong Foundation in Bloomfield, Mich., a sentiment echoed by other members.
As ACIP was meeting, leaders of the nation’s major physician, nurse, and public health associations issued a statement supporting continued vaccination: “The facts are clear: this is an extremely rare side effect, and only an exceedingly small number of people will experience it after vaccination.
“Importantly, for the young people who do, most cases are mild, and individuals recover often on their own or with minimal treatment. In addition, we know that myocarditis and pericarditis are much more common if you get COVID-19, and the risks to the heart from COVID-19 infection can be more severe.”
ACIP heard the evidence behind that claim. According to the Vaccine Safety Datalink, which contains data from more than 12 million medical records, myocarditis or pericarditis occurs in 12- to 39-year-olds at a rate of 8 per 1 million after the second Pfizer dose and 19.8 per 1 million after the second Moderna dose.
The CDC continues to investigate the link between the mRNA vaccines and heart inflammation, including any differences between the vaccines.
Most of the symptoms resolved quickly, said Tom Shimabukuro, deputy director of CDC’s Immunization Safety Office. Of 323 cases analyzed by the CDC, 309 were hospitalized, 295 were discharged, and 218, or 79%, had recovered from symptoms.
“Most postvaccine myocarditis has been responding to minimal treatment,” pediatric cardiologist Matthew Oster, MD, MPH, from Children’s Healthcare of Atlanta, told the panel.
COVID ‘risks are higher’
Overall, the CDC has reported 2,767 COVID-19 deaths among people aged 12-29 years, and there have been 4,018 reported cases of the COVID-linked inflammatory disorder MIS-C since the beginning of the pandemic.
That amounts to 1 MIS-C case in every 3,200 COVID infections – 36% of them among teens aged 12-20 years and 62% among children who are Hispanic or Black and non-Hispanic, according to a CDC presentation.
The CDC estimated that every 1 million second-dose COVID vaccines administered to 12- to 17-year-old boys could prevent 5,700 cases of COVID-19, 215 hospitalizations, 71 ICU admissions, and 2 deaths. There could also be 56-69 myocarditis cases.
The emergence of new variants in the United States and the skewed pattern of vaccination around the country also may increase the risk to unvaccinated young people, noted Grace Lee, MD, MPH, chair of the ACIP’s COVID-19 Vaccine Safety Technical Subgroup and a pediatric infectious disease physician at Stanford (Calif.) Children’s Health.
“If you’re in an area with low vaccination, the risks are higher,” she said. “The benefits [of the vaccine] are going to be far, far greater than any risk.”
Individuals, parents, and their clinicians should consider the full scope of risk when making decisions about vaccination, she said.
As the pandemic evolves, medical experts have to balance the known risks and benefits while they gather more information, said William Schaffner, MD, an infectious disease physician at Vanderbilt University, Nashville, Tenn., and medical director of the National Foundation for Infectious Diseases.
“The story is not over,” Dr. Schaffner said in an interview. “Clearly, we are still working in the face of a pandemic, so there’s urgency to continue vaccinating. But they would like to know more about the long-term consequences of the myocarditis.”
Booster possibilities
Meanwhile, ACIP began conversations on the parameters for a possible vaccine booster. For now, there are simply questions: Would a third vaccine help the immunocompromised gain protection? Should people get a different type of vaccine – mRNA versus adenovirus vector – for their booster? Most important, how long do antibodies last?
“Prior to going around giving everyone boosters, we really need to improve the overall vaccination coverage,” said Helen Keipp Talbot, MD, associate professor of medicine at Vanderbilt University. “That will protect everyone.”
A version of this article first appeared on Medscape.com.
‘Dreck’ to drama: How the media handled, and got handled by, COVID
For well over a year, the COVID-19 pandemic has been the biggest story in the world, costing millions of lives, impacting a presidential election, and quaking economies around the world.
But as vaccination rates increase and restrictions relax across the United States, relief is beginning to mix with reflection. Part of that contemplation means grappling with how the media depicted the crisis – in ways that were helpful, harmful, and somewhere in between.
“This story was so overwhelming, and the amount of journalism done about it was also overwhelming, and it’s going to be a while before we can do any kind of comprehensive overview of how journalism really performed,” said Maryn McKenna, an independent journalist and journalism professor at Emory University, Atlanta, who specializes in public and global health.
Some ‘heroically good’ reporting
The pandemic hit at a time when journalism was under a lot of pressure from external forces – undermined by politics, swimming through a sea of misinformation, and pressed by financial pressure to produce more stories more quickly, said Emily Bell, founding director of the Tow Center for Digital Journalism at Columbia University, New York.
The pandemic drove enormous audiences to news outlets, as people searched for reliable information, and increased the appreciation many people felt for the work of journalists, she said.
“I think there’s been some heroically good reporting and some really empathetic reporting as well,” said Ms. Bell. She cites The New York Times stories honoring the nearly 100,000 people lost to COVID-19 in May 2020 and The Atlantic’s COVID Tracking Project as exceptionally good examples.
Journalism is part of a complex, and evolving, information ecosystem characterized by “traditional” television, radio, and newspapers but also social media, search engine results, niche online news outlets, and clickbait sites.
On the one hand, social media provided a way for physicians, nurses, and scientists to speak directly to the world about their experiences and research. On the other hand, it’s challenging to elevate the really good work of traditional media over all of the bad or unhelpful signals, said Ms. Bell.
But, at the end of the day, much of journalism is a business. There are incentives in the market for tabloids to do sensational coverage and for outlets to push misleading, clickbait headlines, Ms. Bell said.
“Sometimes we’ll criticize journalists for ‘getting it wrong,’ but they might be getting it right in their business model but getting it wrong in terms of what it’s doing for society,” she said.
“We need to do a self-examination, when or if the dust from this ever settles, [on] how much of the past year was viewed as a business opportunity and did that get in the way of informing the public adequately,” Ms. McKenna said.
Digital platforms and journalists also need to reflect on how narratives build on one another, particularly online, said Ms. Bell. If you search for side effects of the Johnson & Johnson vaccine, for example, you will see a list of dozens of headlines that might give you the impression this is a major problem without the context that these effects are exceedingly rare, she notes.
There was also a personnel problem. Shrinking newsrooms over the last decade meant many outlets didn’t have dedicated science and health reporting, or very few staffers, if any. During the pandemic, suddenly general assignment and politics reporters had to be science and health reporters, too.
“You have a hard enough time with these issues if you’re a fairly seasoned science journalist,” said Gary Schwitzer, a former head of the health care news unit for CNN, journalism professor at the University of Minnesota, and founder of the watchdog site HealthNewsReview.org.
And outlets that had the staffing didn’t always put science reporters to full use, Ms. McKenna said. In March and April of 2020, major media outlets should have sent science reporters, not politics reporters, to President Donald Trump’s White House press briefings, which often included incorrect statements about COVID-19 science.
“I just don’t feel that the big outlets understood that that expertise would have made a difference,” she said.
New challenges, old problems
Some of the science journalism done during the pandemic has been some of the best ever seen in this country, said Mr. Schwitzer. But between the peaks of excellence, there is “the daily drumbeat coverage of dreck,” he added.
Many of the issues with this dreck coverage aren’t new or unique to the pandemic. For example, over the last year there have been far too many news stories based solely on weak information sources, like a drug company press release or a not-yet-peer-reviewed preprint article that hasn’t been put into proper context, said Mr. Schwitzer.
A quality science story should always include an independent perspective, he said, but many COVID-19 stories missed that perspective. This isn’t a new issue for science coverage – at Health News Review, Mr. Schwitzer and his colleagues saw stories without appropriate independent sources every day for 15 years.
It’s also challenging to write about uncertainty without over- or underselling what scientists know about a particular phenomenon. “We know that the media in general tends to portray science as more certain than it is,” said Dominique Brossard, PhD, professor and department chair at the University of Wisconsin–Madison and an expert on the intersection between science, media, and policy. This can lead to confusion when the science, and the advice based on that science, changes.
“The public has a really difficult time understanding what uncertainty means within science,” said Todd P. Newman, PhD, assistant professor at the University of Wisconsin–Madison who studies strategic communication within the context of science, technology, and the environment.
“I think the media generally has been good on the subject,” said Paul Offit, MD, director of the Vaccine Education Center, attending physician in the Division of Infectious Diseases at the Children’s Hospital of Philadelphia, and a prominent expert voice throughout the pandemic. “I think where they’ve been imperfect is they tend to be a little more dramatic in terms of how we’re doing.”
Dr. Offit isn’t the only expert to point to the drama of COVID-19 coverage. A study published in March 2021 by the National Bureau of Economic Research found 87% of stories by major U.S. media outlets leaned negative in the tone of their COVID-19 reporting, compared with 50% of stories from non-U.S. major outlets and 64% of articles in scientific journals. The negative emphasis persists even around positive developments, like vaccine trials and school re-openings.
John Whyte, MD, chief medical officer for WebMD, said he is very proud of the way WebMD and Medscape ramped up production of video series and other content to give health care providers the most up-to-date guidance on a rapidly evolving medical situation.
“But I think as [we] started to make progress – especially in the last 6 months – the coverage was never balanced enough; any positive news was immediately proceeded by negative,” he said.
“You want to be honest, but you also don’t want to be alarmist – and that’s where I think the challenge is at times in the media,” said Dr. Whyte. “We didn’t put enough optimism in at times, especially in recent months.”
“Any good coverage on vaccines immediately [was] covered by ‘[we] might need boosters in the fall.’ Why can’t [we] have an opportunity to breathe for a little while and see the good news?” he asked.
Variants or scariants?
Negativity and fear shaped much of the coverage around variants and vaccines earlier this year. In February 2021, Zeynep Tufekci, PhD, a sociologist at the University of North Carolina at Chapel Hill school of information and library science, wrote in The Atlantic about how much reporting has not reflected “the truly amazing reality of these vaccines,” and has instead highlighted “a chorus of relentless pessimism.”
This felt especially true earlier in 2021, when lots of coverage repeatedly emphasized what vaccinated people still could not do.
Eric Topol, MD, editor-in-chief of Medscape and executive vice president of Scripps Research in La Jolla, California, said New York Times editors told him earlier in the pandemic that he couldn’t use the word “scariant” in an opinion piece about the media’s overly fearful and sometimes inaccurate reporting around COVID-19 variants because they worried it would seem like the Times was coming after other media outlets.
“A variant is innocent until proven guilty,” said Dr. Topol. Had journalists approached the subject from that point of view, he said we would have seen “much more faithful reporting.”
Dr. Brossard and Dr. Newman worry that focusing on uncommon negative behavior, like people who break social distancing and mask rules by gathering at the beach or the bar, makes those actions seem more common than they actually are.
The evidence suggests that “if you show these kinds of things to people, you encourage them to do the same behavior,” said Dr. Brossard.
There have been other mistakes along the way, too. Early in the pandemic, many outlets pointed viewers to official government sources of information, some of which, like the White House press briefings in March and April of 2020, ended up being some of the most virulent spreaders of misinformation, said Ms. Bell.
Before that, a handful of journalists like Roxanne Khamsi were the few pushing back against the dominant media narrative in early 2020 that the novel coronavirus was less concerning than the seasonal flu.
“Science journalists have always been writing about studies that sometimes contradict each other, and what’s happened is that has only been condensed in time,” said Ms. Khamsi, a health care reporter for outlets like WIRED magazine and The New York Times and a former chief news editor for Nature Medicine.
Politics and misinformation
It’s impossible to talk about media coverage of COVID-19 without touching on politics and misinformation.
Coverage of the pandemic was politicized and polarized from the very beginning, said Sedona Chinn, PhD, an assistant professor at the University of Wisconsin–Madison who researches the prevalence and effects of scientific disagreements in media.
By looking at network news transcripts and articles from national outlets like the Washington Post and The New York Times, Dr. Chinn and her colleagues were able to determine politicization of coverage by counting the mentions of politicians versus scientists in COVID-19 coverage and polarization by looking at how different or similar the language was surrounding mentions of Republicans and Democrats.
If the two parties were working together or on the same page, they reasoned, the language would be similar.
From mid-March through May 2020, Dr. Chinn and fellow researchers found politicians were featured more often than scientists in newspaper coverage and as frequently as scientists in network news coverage. They also found polarized language around Republicans and Democrats, particularly in stories describing duels between the (at the time) Republican national government and Democratic state and local leaders.
It’s possible that polarization in news coverage helped contribute to polarized attitudes around the virus, the authors write in the study, which was published in August 2020 in the journal Science Communication.
The politicization and polarization of the issue is mirrored in our fractured media environment, where people tend to read, listen, and watch outlets that align with their political leanings. If that trusted outlet features misinformation, the people who follow it are more likely to accept that false information as truth, said Matt Motta, PhD, a political scientist at Oklahoma State University whose research includes public opinion and science communication.
This is true across the political spectrum, he said. When it comes to COVID-19, however, right-wing media outlets like Fox News and Breitbart are more likely to promote conspiratorial tropes and misinformation about the pandemic, according to Dr. Motta and his collaborator Dominik Stecula, PhD, a political scientist at Colorado State University who studies the news media environment and its effects on society.
Across the media ecosystem, reporting on the “infodemic” accompanying the pandemic – the rapid spread of misinformation and disinformation about the virus – has been a major challenge. Outlets may not be creating the misinformation, but they are the ones choosing to give it a platform, said Dr. Motta.
By repeating a false idea, even with the goal of debunking it, you can unintentionally cause the information to stick in people’s minds, said Dr. Brossard.
“Just because something is controversial doesn’t mean it’s worth covering,” said Dr. Motta. Using vaccines as an example, he said many reporters and scientists alike assume that if people have all the facts, they’ll land on the side of science.
“That is just fundamentally not how people think about the decision to get vaccinated,” he said. Instead, the choice is wrapped up with cultural factors, religious beliefs, political identity, and more.
The factors and challenges that shaped the media’s coverage of the pandemic aren’t going anywhere. Improving science and medical coverage in the future is a collective project for journalists, scientists, and everyone in between, said Dr. Newman.
“I call on scientists, too, to think really deeply about how they’re communicating – and especially how they’re communicating what they know and don’t know,” he said.
A version of this article first appeared on Medscape.com.
For well over a year, the COVID-19 pandemic has been the biggest story in the world, costing millions of lives, impacting a presidential election, and quaking economies around the world.
But as vaccination rates increase and restrictions relax across the United States, relief is beginning to mix with reflection. Part of that contemplation means grappling with how the media depicted the crisis – in ways that were helpful, harmful, and somewhere in between.
“This story was so overwhelming, and the amount of journalism done about it was also overwhelming, and it’s going to be a while before we can do any kind of comprehensive overview of how journalism really performed,” said Maryn McKenna, an independent journalist and journalism professor at Emory University, Atlanta, who specializes in public and global health.
Some ‘heroically good’ reporting
The pandemic hit at a time when journalism was under a lot of pressure from external forces – undermined by politics, swimming through a sea of misinformation, and pressed by financial pressure to produce more stories more quickly, said Emily Bell, founding director of the Tow Center for Digital Journalism at Columbia University, New York.
The pandemic drove enormous audiences to news outlets, as people searched for reliable information, and increased the appreciation many people felt for the work of journalists, she said.
“I think there’s been some heroically good reporting and some really empathetic reporting as well,” said Ms. Bell. She cites The New York Times stories honoring the nearly 100,000 people lost to COVID-19 in May 2020 and The Atlantic’s COVID Tracking Project as exceptionally good examples.
Journalism is part of a complex, and evolving, information ecosystem characterized by “traditional” television, radio, and newspapers but also social media, search engine results, niche online news outlets, and clickbait sites.
On the one hand, social media provided a way for physicians, nurses, and scientists to speak directly to the world about their experiences and research. On the other hand, it’s challenging to elevate the really good work of traditional media over all of the bad or unhelpful signals, said Ms. Bell.
But, at the end of the day, much of journalism is a business. There are incentives in the market for tabloids to do sensational coverage and for outlets to push misleading, clickbait headlines, Ms. Bell said.
“Sometimes we’ll criticize journalists for ‘getting it wrong,’ but they might be getting it right in their business model but getting it wrong in terms of what it’s doing for society,” she said.
“We need to do a self-examination, when or if the dust from this ever settles, [on] how much of the past year was viewed as a business opportunity and did that get in the way of informing the public adequately,” Ms. McKenna said.
Digital platforms and journalists also need to reflect on how narratives build on one another, particularly online, said Ms. Bell. If you search for side effects of the Johnson & Johnson vaccine, for example, you will see a list of dozens of headlines that might give you the impression this is a major problem without the context that these effects are exceedingly rare, she notes.
There was also a personnel problem. Shrinking newsrooms over the last decade meant many outlets didn’t have dedicated science and health reporting, or very few staffers, if any. During the pandemic, suddenly general assignment and politics reporters had to be science and health reporters, too.
“You have a hard enough time with these issues if you’re a fairly seasoned science journalist,” said Gary Schwitzer, a former head of the health care news unit for CNN, journalism professor at the University of Minnesota, and founder of the watchdog site HealthNewsReview.org.
And outlets that had the staffing didn’t always put science reporters to full use, Ms. McKenna said. In March and April of 2020, major media outlets should have sent science reporters, not politics reporters, to President Donald Trump’s White House press briefings, which often included incorrect statements about COVID-19 science.
“I just don’t feel that the big outlets understood that that expertise would have made a difference,” she said.
New challenges, old problems
Some of the science journalism done during the pandemic has been some of the best ever seen in this country, said Mr. Schwitzer. But between the peaks of excellence, there is “the daily drumbeat coverage of dreck,” he added.
Many of the issues with this dreck coverage aren’t new or unique to the pandemic. For example, over the last year there have been far too many news stories based solely on weak information sources, like a drug company press release or a not-yet-peer-reviewed preprint article that hasn’t been put into proper context, said Mr. Schwitzer.
A quality science story should always include an independent perspective, he said, but many COVID-19 stories missed that perspective. This isn’t a new issue for science coverage – at Health News Review, Mr. Schwitzer and his colleagues saw stories without appropriate independent sources every day for 15 years.
It’s also challenging to write about uncertainty without over- or underselling what scientists know about a particular phenomenon. “We know that the media in general tends to portray science as more certain than it is,” said Dominique Brossard, PhD, professor and department chair at the University of Wisconsin–Madison and an expert on the intersection between science, media, and policy. This can lead to confusion when the science, and the advice based on that science, changes.
“The public has a really difficult time understanding what uncertainty means within science,” said Todd P. Newman, PhD, assistant professor at the University of Wisconsin–Madison who studies strategic communication within the context of science, technology, and the environment.
“I think the media generally has been good on the subject,” said Paul Offit, MD, director of the Vaccine Education Center, attending physician in the Division of Infectious Diseases at the Children’s Hospital of Philadelphia, and a prominent expert voice throughout the pandemic. “I think where they’ve been imperfect is they tend to be a little more dramatic in terms of how we’re doing.”
Dr. Offit isn’t the only expert to point to the drama of COVID-19 coverage. A study published in March 2021 by the National Bureau of Economic Research found 87% of stories by major U.S. media outlets leaned negative in the tone of their COVID-19 reporting, compared with 50% of stories from non-U.S. major outlets and 64% of articles in scientific journals. The negative emphasis persists even around positive developments, like vaccine trials and school re-openings.
John Whyte, MD, chief medical officer for WebMD, said he is very proud of the way WebMD and Medscape ramped up production of video series and other content to give health care providers the most up-to-date guidance on a rapidly evolving medical situation.
“But I think as [we] started to make progress – especially in the last 6 months – the coverage was never balanced enough; any positive news was immediately proceeded by negative,” he said.
“You want to be honest, but you also don’t want to be alarmist – and that’s where I think the challenge is at times in the media,” said Dr. Whyte. “We didn’t put enough optimism in at times, especially in recent months.”
“Any good coverage on vaccines immediately [was] covered by ‘[we] might need boosters in the fall.’ Why can’t [we] have an opportunity to breathe for a little while and see the good news?” he asked.
Variants or scariants?
Negativity and fear shaped much of the coverage around variants and vaccines earlier this year. In February 2021, Zeynep Tufekci, PhD, a sociologist at the University of North Carolina at Chapel Hill school of information and library science, wrote in The Atlantic about how much reporting has not reflected “the truly amazing reality of these vaccines,” and has instead highlighted “a chorus of relentless pessimism.”
This felt especially true earlier in 2021, when lots of coverage repeatedly emphasized what vaccinated people still could not do.
Eric Topol, MD, editor-in-chief of Medscape and executive vice president of Scripps Research in La Jolla, California, said New York Times editors told him earlier in the pandemic that he couldn’t use the word “scariant” in an opinion piece about the media’s overly fearful and sometimes inaccurate reporting around COVID-19 variants because they worried it would seem like the Times was coming after other media outlets.
“A variant is innocent until proven guilty,” said Dr. Topol. Had journalists approached the subject from that point of view, he said we would have seen “much more faithful reporting.”
Dr. Brossard and Dr. Newman worry that focusing on uncommon negative behavior, like people who break social distancing and mask rules by gathering at the beach or the bar, makes those actions seem more common than they actually are.
The evidence suggests that “if you show these kinds of things to people, you encourage them to do the same behavior,” said Dr. Brossard.
There have been other mistakes along the way, too. Early in the pandemic, many outlets pointed viewers to official government sources of information, some of which, like the White House press briefings in March and April of 2020, ended up being some of the most virulent spreaders of misinformation, said Ms. Bell.
Before that, a handful of journalists like Roxanne Khamsi were the few pushing back against the dominant media narrative in early 2020 that the novel coronavirus was less concerning than the seasonal flu.
“Science journalists have always been writing about studies that sometimes contradict each other, and what’s happened is that has only been condensed in time,” said Ms. Khamsi, a health care reporter for outlets like WIRED magazine and The New York Times and a former chief news editor for Nature Medicine.
Politics and misinformation
It’s impossible to talk about media coverage of COVID-19 without touching on politics and misinformation.
Coverage of the pandemic was politicized and polarized from the very beginning, said Sedona Chinn, PhD, an assistant professor at the University of Wisconsin–Madison who researches the prevalence and effects of scientific disagreements in media.
By looking at network news transcripts and articles from national outlets like the Washington Post and The New York Times, Dr. Chinn and her colleagues were able to determine politicization of coverage by counting the mentions of politicians versus scientists in COVID-19 coverage and polarization by looking at how different or similar the language was surrounding mentions of Republicans and Democrats.
If the two parties were working together or on the same page, they reasoned, the language would be similar.
From mid-March through May 2020, Dr. Chinn and fellow researchers found politicians were featured more often than scientists in newspaper coverage and as frequently as scientists in network news coverage. They also found polarized language around Republicans and Democrats, particularly in stories describing duels between the (at the time) Republican national government and Democratic state and local leaders.
It’s possible that polarization in news coverage helped contribute to polarized attitudes around the virus, the authors write in the study, which was published in August 2020 in the journal Science Communication.
The politicization and polarization of the issue is mirrored in our fractured media environment, where people tend to read, listen, and watch outlets that align with their political leanings. If that trusted outlet features misinformation, the people who follow it are more likely to accept that false information as truth, said Matt Motta, PhD, a political scientist at Oklahoma State University whose research includes public opinion and science communication.
This is true across the political spectrum, he said. When it comes to COVID-19, however, right-wing media outlets like Fox News and Breitbart are more likely to promote conspiratorial tropes and misinformation about the pandemic, according to Dr. Motta and his collaborator Dominik Stecula, PhD, a political scientist at Colorado State University who studies the news media environment and its effects on society.
Across the media ecosystem, reporting on the “infodemic” accompanying the pandemic – the rapid spread of misinformation and disinformation about the virus – has been a major challenge. Outlets may not be creating the misinformation, but they are the ones choosing to give it a platform, said Dr. Motta.
By repeating a false idea, even with the goal of debunking it, you can unintentionally cause the information to stick in people’s minds, said Dr. Brossard.
“Just because something is controversial doesn’t mean it’s worth covering,” said Dr. Motta. Using vaccines as an example, he said many reporters and scientists alike assume that if people have all the facts, they’ll land on the side of science.
“That is just fundamentally not how people think about the decision to get vaccinated,” he said. Instead, the choice is wrapped up with cultural factors, religious beliefs, political identity, and more.
The factors and challenges that shaped the media’s coverage of the pandemic aren’t going anywhere. Improving science and medical coverage in the future is a collective project for journalists, scientists, and everyone in between, said Dr. Newman.
“I call on scientists, too, to think really deeply about how they’re communicating – and especially how they’re communicating what they know and don’t know,” he said.
A version of this article first appeared on Medscape.com.
For well over a year, the COVID-19 pandemic has been the biggest story in the world, costing millions of lives, impacting a presidential election, and quaking economies around the world.
But as vaccination rates increase and restrictions relax across the United States, relief is beginning to mix with reflection. Part of that contemplation means grappling with how the media depicted the crisis – in ways that were helpful, harmful, and somewhere in between.
“This story was so overwhelming, and the amount of journalism done about it was also overwhelming, and it’s going to be a while before we can do any kind of comprehensive overview of how journalism really performed,” said Maryn McKenna, an independent journalist and journalism professor at Emory University, Atlanta, who specializes in public and global health.
Some ‘heroically good’ reporting
The pandemic hit at a time when journalism was under a lot of pressure from external forces – undermined by politics, swimming through a sea of misinformation, and pressed by financial pressure to produce more stories more quickly, said Emily Bell, founding director of the Tow Center for Digital Journalism at Columbia University, New York.
The pandemic drove enormous audiences to news outlets, as people searched for reliable information, and increased the appreciation many people felt for the work of journalists, she said.
“I think there’s been some heroically good reporting and some really empathetic reporting as well,” said Ms. Bell. She cites The New York Times stories honoring the nearly 100,000 people lost to COVID-19 in May 2020 and The Atlantic’s COVID Tracking Project as exceptionally good examples.
Journalism is part of a complex, and evolving, information ecosystem characterized by “traditional” television, radio, and newspapers but also social media, search engine results, niche online news outlets, and clickbait sites.
On the one hand, social media provided a way for physicians, nurses, and scientists to speak directly to the world about their experiences and research. On the other hand, it’s challenging to elevate the really good work of traditional media over all of the bad or unhelpful signals, said Ms. Bell.
But, at the end of the day, much of journalism is a business. There are incentives in the market for tabloids to do sensational coverage and for outlets to push misleading, clickbait headlines, Ms. Bell said.
“Sometimes we’ll criticize journalists for ‘getting it wrong,’ but they might be getting it right in their business model but getting it wrong in terms of what it’s doing for society,” she said.
“We need to do a self-examination, when or if the dust from this ever settles, [on] how much of the past year was viewed as a business opportunity and did that get in the way of informing the public adequately,” Ms. McKenna said.
Digital platforms and journalists also need to reflect on how narratives build on one another, particularly online, said Ms. Bell. If you search for side effects of the Johnson & Johnson vaccine, for example, you will see a list of dozens of headlines that might give you the impression this is a major problem without the context that these effects are exceedingly rare, she notes.
There was also a personnel problem. Shrinking newsrooms over the last decade meant many outlets didn’t have dedicated science and health reporting, or very few staffers, if any. During the pandemic, suddenly general assignment and politics reporters had to be science and health reporters, too.
“You have a hard enough time with these issues if you’re a fairly seasoned science journalist,” said Gary Schwitzer, a former head of the health care news unit for CNN, journalism professor at the University of Minnesota, and founder of the watchdog site HealthNewsReview.org.
And outlets that had the staffing didn’t always put science reporters to full use, Ms. McKenna said. In March and April of 2020, major media outlets should have sent science reporters, not politics reporters, to President Donald Trump’s White House press briefings, which often included incorrect statements about COVID-19 science.
“I just don’t feel that the big outlets understood that that expertise would have made a difference,” she said.
New challenges, old problems
Some of the science journalism done during the pandemic has been some of the best ever seen in this country, said Mr. Schwitzer. But between the peaks of excellence, there is “the daily drumbeat coverage of dreck,” he added.
Many of the issues with this dreck coverage aren’t new or unique to the pandemic. For example, over the last year there have been far too many news stories based solely on weak information sources, like a drug company press release or a not-yet-peer-reviewed preprint article that hasn’t been put into proper context, said Mr. Schwitzer.
A quality science story should always include an independent perspective, he said, but many COVID-19 stories missed that perspective. This isn’t a new issue for science coverage – at Health News Review, Mr. Schwitzer and his colleagues saw stories without appropriate independent sources every day for 15 years.
It’s also challenging to write about uncertainty without over- or underselling what scientists know about a particular phenomenon. “We know that the media in general tends to portray science as more certain than it is,” said Dominique Brossard, PhD, professor and department chair at the University of Wisconsin–Madison and an expert on the intersection between science, media, and policy. This can lead to confusion when the science, and the advice based on that science, changes.
“The public has a really difficult time understanding what uncertainty means within science,” said Todd P. Newman, PhD, assistant professor at the University of Wisconsin–Madison who studies strategic communication within the context of science, technology, and the environment.
“I think the media generally has been good on the subject,” said Paul Offit, MD, director of the Vaccine Education Center, attending physician in the Division of Infectious Diseases at the Children’s Hospital of Philadelphia, and a prominent expert voice throughout the pandemic. “I think where they’ve been imperfect is they tend to be a little more dramatic in terms of how we’re doing.”
Dr. Offit isn’t the only expert to point to the drama of COVID-19 coverage. A study published in March 2021 by the National Bureau of Economic Research found 87% of stories by major U.S. media outlets leaned negative in the tone of their COVID-19 reporting, compared with 50% of stories from non-U.S. major outlets and 64% of articles in scientific journals. The negative emphasis persists even around positive developments, like vaccine trials and school re-openings.
John Whyte, MD, chief medical officer for WebMD, said he is very proud of the way WebMD and Medscape ramped up production of video series and other content to give health care providers the most up-to-date guidance on a rapidly evolving medical situation.
“But I think as [we] started to make progress – especially in the last 6 months – the coverage was never balanced enough; any positive news was immediately proceeded by negative,” he said.
“You want to be honest, but you also don’t want to be alarmist – and that’s where I think the challenge is at times in the media,” said Dr. Whyte. “We didn’t put enough optimism in at times, especially in recent months.”
“Any good coverage on vaccines immediately [was] covered by ‘[we] might need boosters in the fall.’ Why can’t [we] have an opportunity to breathe for a little while and see the good news?” he asked.
Variants or scariants?
Negativity and fear shaped much of the coverage around variants and vaccines earlier this year. In February 2021, Zeynep Tufekci, PhD, a sociologist at the University of North Carolina at Chapel Hill school of information and library science, wrote in The Atlantic about how much reporting has not reflected “the truly amazing reality of these vaccines,” and has instead highlighted “a chorus of relentless pessimism.”
This felt especially true earlier in 2021, when lots of coverage repeatedly emphasized what vaccinated people still could not do.
Eric Topol, MD, editor-in-chief of Medscape and executive vice president of Scripps Research in La Jolla, California, said New York Times editors told him earlier in the pandemic that he couldn’t use the word “scariant” in an opinion piece about the media’s overly fearful and sometimes inaccurate reporting around COVID-19 variants because they worried it would seem like the Times was coming after other media outlets.
“A variant is innocent until proven guilty,” said Dr. Topol. Had journalists approached the subject from that point of view, he said we would have seen “much more faithful reporting.”
Dr. Brossard and Dr. Newman worry that focusing on uncommon negative behavior, like people who break social distancing and mask rules by gathering at the beach or the bar, makes those actions seem more common than they actually are.
The evidence suggests that “if you show these kinds of things to people, you encourage them to do the same behavior,” said Dr. Brossard.
There have been other mistakes along the way, too. Early in the pandemic, many outlets pointed viewers to official government sources of information, some of which, like the White House press briefings in March and April of 2020, ended up being some of the most virulent spreaders of misinformation, said Ms. Bell.
Before that, a handful of journalists like Roxanne Khamsi were the few pushing back against the dominant media narrative in early 2020 that the novel coronavirus was less concerning than the seasonal flu.
“Science journalists have always been writing about studies that sometimes contradict each other, and what’s happened is that has only been condensed in time,” said Ms. Khamsi, a health care reporter for outlets like WIRED magazine and The New York Times and a former chief news editor for Nature Medicine.
Politics and misinformation
It’s impossible to talk about media coverage of COVID-19 without touching on politics and misinformation.
Coverage of the pandemic was politicized and polarized from the very beginning, said Sedona Chinn, PhD, an assistant professor at the University of Wisconsin–Madison who researches the prevalence and effects of scientific disagreements in media.
By looking at network news transcripts and articles from national outlets like the Washington Post and The New York Times, Dr. Chinn and her colleagues were able to determine politicization of coverage by counting the mentions of politicians versus scientists in COVID-19 coverage and polarization by looking at how different or similar the language was surrounding mentions of Republicans and Democrats.
If the two parties were working together or on the same page, they reasoned, the language would be similar.
From mid-March through May 2020, Dr. Chinn and fellow researchers found politicians were featured more often than scientists in newspaper coverage and as frequently as scientists in network news coverage. They also found polarized language around Republicans and Democrats, particularly in stories describing duels between the (at the time) Republican national government and Democratic state and local leaders.
It’s possible that polarization in news coverage helped contribute to polarized attitudes around the virus, the authors write in the study, which was published in August 2020 in the journal Science Communication.
The politicization and polarization of the issue is mirrored in our fractured media environment, where people tend to read, listen, and watch outlets that align with their political leanings. If that trusted outlet features misinformation, the people who follow it are more likely to accept that false information as truth, said Matt Motta, PhD, a political scientist at Oklahoma State University whose research includes public opinion and science communication.
This is true across the political spectrum, he said. When it comes to COVID-19, however, right-wing media outlets like Fox News and Breitbart are more likely to promote conspiratorial tropes and misinformation about the pandemic, according to Dr. Motta and his collaborator Dominik Stecula, PhD, a political scientist at Colorado State University who studies the news media environment and its effects on society.
Across the media ecosystem, reporting on the “infodemic” accompanying the pandemic – the rapid spread of misinformation and disinformation about the virus – has been a major challenge. Outlets may not be creating the misinformation, but they are the ones choosing to give it a platform, said Dr. Motta.
By repeating a false idea, even with the goal of debunking it, you can unintentionally cause the information to stick in people’s minds, said Dr. Brossard.
“Just because something is controversial doesn’t mean it’s worth covering,” said Dr. Motta. Using vaccines as an example, he said many reporters and scientists alike assume that if people have all the facts, they’ll land on the side of science.
“That is just fundamentally not how people think about the decision to get vaccinated,” he said. Instead, the choice is wrapped up with cultural factors, religious beliefs, political identity, and more.
The factors and challenges that shaped the media’s coverage of the pandemic aren’t going anywhere. Improving science and medical coverage in the future is a collective project for journalists, scientists, and everyone in between, said Dr. Newman.
“I call on scientists, too, to think really deeply about how they’re communicating – and especially how they’re communicating what they know and don’t know,” he said.
A version of this article first appeared on Medscape.com.
Children and COVID: Vaccination trends beginning to diverge
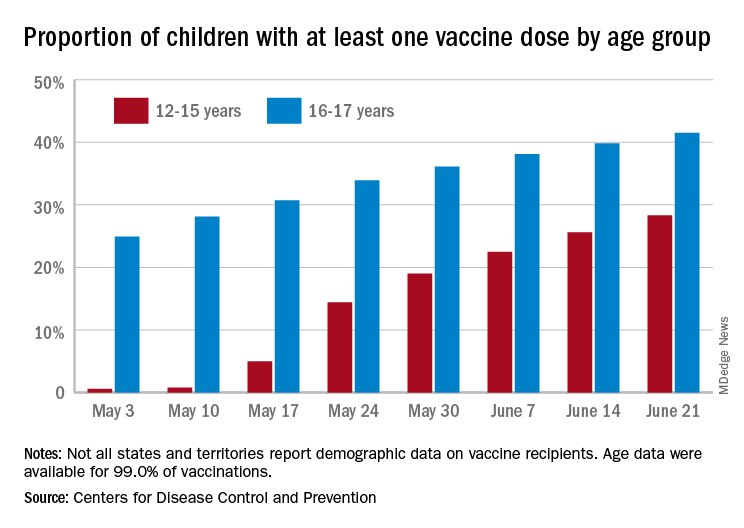
As more adolescents became eligible for a second dose of the Pfizer vaccine since it received approval from the Food and Drug Administration in mid-May, the share of 12- to 15-year-olds considered fully vaccinated rose from 11.4% on June 14 to 17.8% on June 28, an increase of 56%, the CDC’s COVID Data Tracker indicated June 22.
For children aged 16-17 years, who have been receiving the vaccine since early April, full vaccination rose by 9.6% in that same week, going from 29.1% on June 14 to 31.9% on June 21. The cumulative numbers for first vaccinations are higher, of course, but are rising more slowly in both age groups: 41.5% of those aged 16-17 had received at least one dose by June 21 (up by 4.3%), with the 12- to 15-year-olds at 28.3% (up by 10.5%), based on the CDC data.
Limiting the time frame to just the last 2 weeks, however, shows the opposite of rising among the younger children. During the 2 weeks ending June 7, 17.9% of those initiating a first dose were 12-15 years old, but that 2-week figure slipped to 17.1% as of June 14 and was down to 16.0% on June 21. The older group was slow but steady over that time: 4.8%, 4.7%, and 4.8%, the CDC said. To give those figures some context, those aged 25-39 years represented 23.7% of past-2-week initiations on June 7 and 24.3% on June 21.
Although no COVID-19 vaccine has been approved for children under 12 years, about 0.4% of that age group – just over 167,000 children – have received a first dose and almost 91,000 are fully vaccinated, according to CDC data.

As more adolescents became eligible for a second dose of the Pfizer vaccine since it received approval from the Food and Drug Administration in mid-May, the share of 12- to 15-year-olds considered fully vaccinated rose from 11.4% on June 14 to 17.8% on June 28, an increase of 56%, the CDC’s COVID Data Tracker indicated June 22.
For children aged 16-17 years, who have been receiving the vaccine since early April, full vaccination rose by 9.6% in that same week, going from 29.1% on June 14 to 31.9% on June 21. The cumulative numbers for first vaccinations are higher, of course, but are rising more slowly in both age groups: 41.5% of those aged 16-17 had received at least one dose by June 21 (up by 4.3%), with the 12- to 15-year-olds at 28.3% (up by 10.5%), based on the CDC data.
Limiting the time frame to just the last 2 weeks, however, shows the opposite of rising among the younger children. During the 2 weeks ending June 7, 17.9% of those initiating a first dose were 12-15 years old, but that 2-week figure slipped to 17.1% as of June 14 and was down to 16.0% on June 21. The older group was slow but steady over that time: 4.8%, 4.7%, and 4.8%, the CDC said. To give those figures some context, those aged 25-39 years represented 23.7% of past-2-week initiations on June 7 and 24.3% on June 21.
Although no COVID-19 vaccine has been approved for children under 12 years, about 0.4% of that age group – just over 167,000 children – have received a first dose and almost 91,000 are fully vaccinated, according to CDC data.

As more adolescents became eligible for a second dose of the Pfizer vaccine since it received approval from the Food and Drug Administration in mid-May, the share of 12- to 15-year-olds considered fully vaccinated rose from 11.4% on June 14 to 17.8% on June 28, an increase of 56%, the CDC’s COVID Data Tracker indicated June 22.
For children aged 16-17 years, who have been receiving the vaccine since early April, full vaccination rose by 9.6% in that same week, going from 29.1% on June 14 to 31.9% on June 21. The cumulative numbers for first vaccinations are higher, of course, but are rising more slowly in both age groups: 41.5% of those aged 16-17 had received at least one dose by June 21 (up by 4.3%), with the 12- to 15-year-olds at 28.3% (up by 10.5%), based on the CDC data.
Limiting the time frame to just the last 2 weeks, however, shows the opposite of rising among the younger children. During the 2 weeks ending June 7, 17.9% of those initiating a first dose were 12-15 years old, but that 2-week figure slipped to 17.1% as of June 14 and was down to 16.0% on June 21. The older group was slow but steady over that time: 4.8%, 4.7%, and 4.8%, the CDC said. To give those figures some context, those aged 25-39 years represented 23.7% of past-2-week initiations on June 7 and 24.3% on June 21.
Although no COVID-19 vaccine has been approved for children under 12 years, about 0.4% of that age group – just over 167,000 children – have received a first dose and almost 91,000 are fully vaccinated, according to CDC data.
FDA approves OTC antihistamine nasal spray
, making it the first nasal antihistamine available over the counter in the United States.
The 0.15% strength of azelastine hydrochloride nasal spray is now approved for nonprescription treatment of seasonal and perennial allergic rhinitis in adults and children 6 years of age or older, the agency said. The 0.1% strength remains a prescription product that is indicated in younger children.
The “approval provides individuals an option for a safe and effective nasal antihistamine without requiring the assistance of a health care provider,” Theresa M. Michele, MD, director of the office of nonprescription drugs in the FDA’s Center for Drug Evaluation and Research, said in a prepared statement.
The FDA granted the nonprescription approval to Bayer Healthcare LLC, which said in a press release that the nasal spray would be available in national mass retail locations starting in the first quarter of 2022.
Oral antihistamines such as cetirizine (Zyrtec), loratadine (Claritin), and fexofenadine (Allegra) have been on store shelves for years. Azelastine 0.15% will be the first and only over-the-counter antihistamine for indoor and outdoor allergy relief in a nasal formulation, Bayer said.
An over-the-counter nasal antihistamine could be a better option for some allergy sufferers when compared with what is already over the counter, said Tracy Prematta, MD, a private practice allergist in Havertown, Pa.
“In general, I like the nasal antihistamines,” Dr. Prematta said in an interview. “They work quickly, whereas the nasal steroids don’t, and I think a lot of people who go to the drugstore looking for allergy relief are actually looking for something quick-acting.”
However, the cost of the over-the-counter azelastine may play a big role in whether patients go with the prescription or nonprescription option, according to Dr. Prematta.
Bayer has not yet set the price for nonprescription azelastine, a company spokesperson told this news organization.
The change in azelastine approval status happened through a regulatory process called an Rx-to-OTC switch. According to the FDA, products switched to nonprescription status need to have data demonstrating that they are safe and effective as self-medication when used as directed.
The product manufacturer has to show that consumers know how to use the drug safely and effectively without a health care professional supervising them, the FDA said.
The FDA considers the change in status for azelastine a partial Rx-to-OTC switch, since the 0.15% strength is now over the counter and the 0.1% strength remains a prescription product.
The 0.1% strength is indicated for perennial allergies in children 6 months to 6 years old, and seasonal allergies for children 2-6 years old, according to the FDA.
Drowsiness is a side effect of azelastine, the FDA said. According to prescribing information, consumers using the nasal spray need to be careful when driving or operating machinery, and should avoid alcohol.
Using the product with alcohol, sedatives, or tranquilizers may increase drowsiness, the agency added.
Sedation is also common with the oral antihistamines people take to treat their allergies, said Dr. Prematta, who added that patients may also complain of dry mouth, nose, or throat.
Although some allergy sufferers dislike the taste of antihistamine nasal spray, they can try to overcome that issue by tilting the head forward, pointing the tip of the nozzle toward the outside of the nose, and sniffing gently, Dr. Prematta said.
“That really minimizes what gets in the back of your throat, so taste becomes less of a problem,” she explained.
Dr. Prematta has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, making it the first nasal antihistamine available over the counter in the United States.
The 0.15% strength of azelastine hydrochloride nasal spray is now approved for nonprescription treatment of seasonal and perennial allergic rhinitis in adults and children 6 years of age or older, the agency said. The 0.1% strength remains a prescription product that is indicated in younger children.
The “approval provides individuals an option for a safe and effective nasal antihistamine without requiring the assistance of a health care provider,” Theresa M. Michele, MD, director of the office of nonprescription drugs in the FDA’s Center for Drug Evaluation and Research, said in a prepared statement.
The FDA granted the nonprescription approval to Bayer Healthcare LLC, which said in a press release that the nasal spray would be available in national mass retail locations starting in the first quarter of 2022.
Oral antihistamines such as cetirizine (Zyrtec), loratadine (Claritin), and fexofenadine (Allegra) have been on store shelves for years. Azelastine 0.15% will be the first and only over-the-counter antihistamine for indoor and outdoor allergy relief in a nasal formulation, Bayer said.
An over-the-counter nasal antihistamine could be a better option for some allergy sufferers when compared with what is already over the counter, said Tracy Prematta, MD, a private practice allergist in Havertown, Pa.
“In general, I like the nasal antihistamines,” Dr. Prematta said in an interview. “They work quickly, whereas the nasal steroids don’t, and I think a lot of people who go to the drugstore looking for allergy relief are actually looking for something quick-acting.”
However, the cost of the over-the-counter azelastine may play a big role in whether patients go with the prescription or nonprescription option, according to Dr. Prematta.
Bayer has not yet set the price for nonprescription azelastine, a company spokesperson told this news organization.
The change in azelastine approval status happened through a regulatory process called an Rx-to-OTC switch. According to the FDA, products switched to nonprescription status need to have data demonstrating that they are safe and effective as self-medication when used as directed.
The product manufacturer has to show that consumers know how to use the drug safely and effectively without a health care professional supervising them, the FDA said.
The FDA considers the change in status for azelastine a partial Rx-to-OTC switch, since the 0.15% strength is now over the counter and the 0.1% strength remains a prescription product.
The 0.1% strength is indicated for perennial allergies in children 6 months to 6 years old, and seasonal allergies for children 2-6 years old, according to the FDA.
Drowsiness is a side effect of azelastine, the FDA said. According to prescribing information, consumers using the nasal spray need to be careful when driving or operating machinery, and should avoid alcohol.
Using the product with alcohol, sedatives, or tranquilizers may increase drowsiness, the agency added.
Sedation is also common with the oral antihistamines people take to treat their allergies, said Dr. Prematta, who added that patients may also complain of dry mouth, nose, or throat.
Although some allergy sufferers dislike the taste of antihistamine nasal spray, they can try to overcome that issue by tilting the head forward, pointing the tip of the nozzle toward the outside of the nose, and sniffing gently, Dr. Prematta said.
“That really minimizes what gets in the back of your throat, so taste becomes less of a problem,” she explained.
Dr. Prematta has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, making it the first nasal antihistamine available over the counter in the United States.
The 0.15% strength of azelastine hydrochloride nasal spray is now approved for nonprescription treatment of seasonal and perennial allergic rhinitis in adults and children 6 years of age or older, the agency said. The 0.1% strength remains a prescription product that is indicated in younger children.
The “approval provides individuals an option for a safe and effective nasal antihistamine without requiring the assistance of a health care provider,” Theresa M. Michele, MD, director of the office of nonprescription drugs in the FDA’s Center for Drug Evaluation and Research, said in a prepared statement.
The FDA granted the nonprescription approval to Bayer Healthcare LLC, which said in a press release that the nasal spray would be available in national mass retail locations starting in the first quarter of 2022.
Oral antihistamines such as cetirizine (Zyrtec), loratadine (Claritin), and fexofenadine (Allegra) have been on store shelves for years. Azelastine 0.15% will be the first and only over-the-counter antihistamine for indoor and outdoor allergy relief in a nasal formulation, Bayer said.
An over-the-counter nasal antihistamine could be a better option for some allergy sufferers when compared with what is already over the counter, said Tracy Prematta, MD, a private practice allergist in Havertown, Pa.
“In general, I like the nasal antihistamines,” Dr. Prematta said in an interview. “They work quickly, whereas the nasal steroids don’t, and I think a lot of people who go to the drugstore looking for allergy relief are actually looking for something quick-acting.”
However, the cost of the over-the-counter azelastine may play a big role in whether patients go with the prescription or nonprescription option, according to Dr. Prematta.
Bayer has not yet set the price for nonprescription azelastine, a company spokesperson told this news organization.
The change in azelastine approval status happened through a regulatory process called an Rx-to-OTC switch. According to the FDA, products switched to nonprescription status need to have data demonstrating that they are safe and effective as self-medication when used as directed.
The product manufacturer has to show that consumers know how to use the drug safely and effectively without a health care professional supervising them, the FDA said.
The FDA considers the change in status for azelastine a partial Rx-to-OTC switch, since the 0.15% strength is now over the counter and the 0.1% strength remains a prescription product.
The 0.1% strength is indicated for perennial allergies in children 6 months to 6 years old, and seasonal allergies for children 2-6 years old, according to the FDA.
Drowsiness is a side effect of azelastine, the FDA said. According to prescribing information, consumers using the nasal spray need to be careful when driving or operating machinery, and should avoid alcohol.
Using the product with alcohol, sedatives, or tranquilizers may increase drowsiness, the agency added.
Sedation is also common with the oral antihistamines people take to treat their allergies, said Dr. Prematta, who added that patients may also complain of dry mouth, nose, or throat.
Although some allergy sufferers dislike the taste of antihistamine nasal spray, they can try to overcome that issue by tilting the head forward, pointing the tip of the nozzle toward the outside of the nose, and sniffing gently, Dr. Prematta said.
“That really minimizes what gets in the back of your throat, so taste becomes less of a problem,” she explained.
Dr. Prematta has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Diaphragmatic endometriosis diagnosed many years after symptom onset
Diaphragmatic endometriosis is often diagnosed several years after the start of symptoms – mainly moderate to severe pain – and this is potentially because of general lack of awareness of diaphragmatic endometriosis among the general population and medical professionals.
Findings of the international survey that explored the diagnosis and treatment of diaphragmatic endometriosis were presented at this year’s Royal College of Obstetricians and Gynecologists 2021 Virtual World Congress by medical student Rachel Piccus, MSc, based at the University of Birmingham (England). Robert Sutcliffe, MD, consultant in hepatobiliary and pancreatic surgery, at Queen Elizabeth Hospital Birmingham was senior author. Results were also published in the May 2021 issue of the European Journal of Obstetrics and Gynaecology and Reproductive Biology.
The study found that it took an average of five visits to a primary physician before referral to a gynecologist.
“Late diagnosis could also be due to the idea that diaphragmatic endometriosis symptoms often present before pelvic symptoms and therefore the site of pain is considered atypical for pelvic endometriosis,” Ms. Piccus said, adding that “clinicians are screening for cyclical pain, which is typical of endometriosis, but our study has shown that pain can in fact be more frequent – daily and weekly.”
These significant diagnostic delays, seen from the time of the initial primary care and gynecology consultation has the potential to significantly affect quality of life as seen in pelvic endometriosis, said Ms. Piccus. “These delays are partly due to a lack of awareness among gynecologists, but could also be due to pelvic laparoscopy being insufficient to examine the diaphragm behind the liver.”
Justin Clark, MD, consultant gynaecologist, Birmingham (England) Women’s and Children Hospital, moderated the session and agreed that the study highlights the need for greater awareness of this variant of endometriosis. “Whilst endometriosis affecting the diaphragm, subdiaphragm, and thorax is rare, the condition causes substantial morbidity.”
“Greater knowledge of thoracic endometriosis amongst clinicians in both primary and secondary care is needed to ensure accurate and timely diagnosis,” he added.
Diaphragmatic endometriosis is estimated to affect 1%-1.5% of all endometriosis patients and presents as cyclical pain in the chest, abdomen, and shoulder tip, as well as other respiratory symptoms such as catamenial pneumothorax and difficulty breathing.
“Cross-sectional imaging has shown low sensitivity so upper abdominal laparoscopy is the gold standard; however, this has implications for diagnostic delay because a strong clinical suspicion is required to refer for this invasive procedure,” explained Ms. Piccus referring to one of the reasons underpinning the need for the study.
When successfully diagnosed, treatment requires excision or ablation surgery and studies show symptomatic relief in 75%-100% of cases.
To gauge the extent of delayed diagnosis as well as treatment outcomes from a patient perspective, Ms. Piccus circulated an anonymous online survey among women with a previous history of surgery for diaphragmatic endometriosis.
Diaphragmatic endometriosis pain – daily and weekly as well as cyclical
A total of 137 participants responded to the survey, with a median age of 34 years (range, 19-53). Median age of diaphragmatic endometriosis onset was 27 years (range, 11-50), and importantly, diaphragmatic endometriosis symptoms started before pelvic symptoms in 90 respondents (66%).
The dominant symptom was pain. A total of 38% reported cyclical pain (related to endometrial shedding during menstruation), 15% weekly pain, and 47% daily pain, both of which were worse during the menstrual cycle. Furthermore, 14% reported other symptoms including catamenial pneumothorax, difficulty breathing, and hemoptysis.
“Whilst this cyclical pain is typical of endometriosis, we see that diagnostic delays may be due to misdiagnosis because clinicians are screening for this cyclical pain whilst our study has shown that pain can in fact be more frequent, being daily and weekly,” noted Ms. Piccus. Moderate to severe pain was reported in 67% of respondents and moderate in 31%, only 2% reported pain as mild.
Location of pain comprised moderate to severe pain in the upper abdomen (68%), chest (64%) and shoulder (54%). Pain was right-sided in 54%, left-sided in 11% and bilateral in 35%. Upper back and neck were also reported as sites of pain.
Indirectly providing a measure of the lack of awareness of diaphragmatic endometriosis on behalf of primary care, 122 participants reported initially visiting their primary care physician for help and 65 were given a diagnosis – in only 14 cases was that diaphragmatic endometriosis. There were a range of other gynecologic (e.g. ovarian cyst, two), respiratory (spontaneous pneumothorax, seven), gastrointestinal (gastritis/reflux, eight), musculoskeletal (six), and psychological (anxiety/stress, four) diagnoses.
A median of 5 primary care consultations (range, 1-100) were required before referral to a gynecologist, with 30% seeing a primary care physician over 10 times. A further 14 patients self-referred to gynecologist.
“These findings have implications for diagnostic delay, added Ms. Piccus. “While the majority of respondents were diagnosed less than a year from the first GP visit, the median delay was 2 years, with 31% diagnosed after 5 or more years. One took 23 years for an initial diagnosis.”
Most cases were diagnosed at the time of surgery – 93%, with 52% at pelvic laparoscopy, 35% upper abdominal laparoscopy, with 30% requiring two or more laparoscopies before they were diagnosed with diaphragmatic endometriosis. A total of 7% were diagnosed via cross-sectional imaging prior to surgery.
Treatment outcomes for diaphragmatic endometriosis
Reflecting the literature, surgery to remove the endometriosis lesions was mainly laparoscopic with 47% abdominal excisions, and 29% abdominal ablations; 6% received open abdominal procedures, and 18% received open thoracotomy or video-assisted thoracoscopic surgery.
The survey asked about postoperative symptoms 6 months after surgery and at the time of survey. Symptoms at 6 months post surgery had completely resolved in 18%, shown significant improvement in 48%, and no improvement in 20%. Worsening of symptoms was seen in 14%. Long-term pain was reported by 21% as severe, 27% as moderate, 35% as mild, and 17% had no symptoms.
Further findings included that 23% underwent additional procedures to treat their diaphragmatic endometriosis, and that there was no significant difference between excision and ablation, nor between age of onset of symptoms or length of diagnostic delay.
“Surgical treatment to remove these extra pelvic deposits of endometriosis will depend upon the type and distribution of thoracic endometriosis and a variety of surgical specialties may need to be involved including gynecologists, cardiothoracic, and upper gastrointestinal/liver surgeons,” Dr. Clark said.
He added that familiar hormonal medical treatments for more typical pelvic endometriosis should also be considered for primary and maintenance treatment. “These data suggest a high symptomatic recurrence rate after surgical treatment and so medical treatments should be considered to try and minimize the risks of endometriosis symptoms returning.”
Dr. Clark also pointed out that multidisciplinary clinical teams should be established in specialized centers to plan surgical and medical management to enhance clinical outcomes and collect data to better understand this enigmatic condition.
Ms. Piccus and Dr. Clark have no relevant conflicts of interest.
Diaphragmatic endometriosis is often diagnosed several years after the start of symptoms – mainly moderate to severe pain – and this is potentially because of general lack of awareness of diaphragmatic endometriosis among the general population and medical professionals.
Findings of the international survey that explored the diagnosis and treatment of diaphragmatic endometriosis were presented at this year’s Royal College of Obstetricians and Gynecologists 2021 Virtual World Congress by medical student Rachel Piccus, MSc, based at the University of Birmingham (England). Robert Sutcliffe, MD, consultant in hepatobiliary and pancreatic surgery, at Queen Elizabeth Hospital Birmingham was senior author. Results were also published in the May 2021 issue of the European Journal of Obstetrics and Gynaecology and Reproductive Biology.
The study found that it took an average of five visits to a primary physician before referral to a gynecologist.
“Late diagnosis could also be due to the idea that diaphragmatic endometriosis symptoms often present before pelvic symptoms and therefore the site of pain is considered atypical for pelvic endometriosis,” Ms. Piccus said, adding that “clinicians are screening for cyclical pain, which is typical of endometriosis, but our study has shown that pain can in fact be more frequent – daily and weekly.”
These significant diagnostic delays, seen from the time of the initial primary care and gynecology consultation has the potential to significantly affect quality of life as seen in pelvic endometriosis, said Ms. Piccus. “These delays are partly due to a lack of awareness among gynecologists, but could also be due to pelvic laparoscopy being insufficient to examine the diaphragm behind the liver.”
Justin Clark, MD, consultant gynaecologist, Birmingham (England) Women’s and Children Hospital, moderated the session and agreed that the study highlights the need for greater awareness of this variant of endometriosis. “Whilst endometriosis affecting the diaphragm, subdiaphragm, and thorax is rare, the condition causes substantial morbidity.”
“Greater knowledge of thoracic endometriosis amongst clinicians in both primary and secondary care is needed to ensure accurate and timely diagnosis,” he added.
Diaphragmatic endometriosis is estimated to affect 1%-1.5% of all endometriosis patients and presents as cyclical pain in the chest, abdomen, and shoulder tip, as well as other respiratory symptoms such as catamenial pneumothorax and difficulty breathing.
“Cross-sectional imaging has shown low sensitivity so upper abdominal laparoscopy is the gold standard; however, this has implications for diagnostic delay because a strong clinical suspicion is required to refer for this invasive procedure,” explained Ms. Piccus referring to one of the reasons underpinning the need for the study.
When successfully diagnosed, treatment requires excision or ablation surgery and studies show symptomatic relief in 75%-100% of cases.
To gauge the extent of delayed diagnosis as well as treatment outcomes from a patient perspective, Ms. Piccus circulated an anonymous online survey among women with a previous history of surgery for diaphragmatic endometriosis.
Diaphragmatic endometriosis pain – daily and weekly as well as cyclical
A total of 137 participants responded to the survey, with a median age of 34 years (range, 19-53). Median age of diaphragmatic endometriosis onset was 27 years (range, 11-50), and importantly, diaphragmatic endometriosis symptoms started before pelvic symptoms in 90 respondents (66%).
The dominant symptom was pain. A total of 38% reported cyclical pain (related to endometrial shedding during menstruation), 15% weekly pain, and 47% daily pain, both of which were worse during the menstrual cycle. Furthermore, 14% reported other symptoms including catamenial pneumothorax, difficulty breathing, and hemoptysis.
“Whilst this cyclical pain is typical of endometriosis, we see that diagnostic delays may be due to misdiagnosis because clinicians are screening for this cyclical pain whilst our study has shown that pain can in fact be more frequent, being daily and weekly,” noted Ms. Piccus. Moderate to severe pain was reported in 67% of respondents and moderate in 31%, only 2% reported pain as mild.
Location of pain comprised moderate to severe pain in the upper abdomen (68%), chest (64%) and shoulder (54%). Pain was right-sided in 54%, left-sided in 11% and bilateral in 35%. Upper back and neck were also reported as sites of pain.
Indirectly providing a measure of the lack of awareness of diaphragmatic endometriosis on behalf of primary care, 122 participants reported initially visiting their primary care physician for help and 65 were given a diagnosis – in only 14 cases was that diaphragmatic endometriosis. There were a range of other gynecologic (e.g. ovarian cyst, two), respiratory (spontaneous pneumothorax, seven), gastrointestinal (gastritis/reflux, eight), musculoskeletal (six), and psychological (anxiety/stress, four) diagnoses.
A median of 5 primary care consultations (range, 1-100) were required before referral to a gynecologist, with 30% seeing a primary care physician over 10 times. A further 14 patients self-referred to gynecologist.
“These findings have implications for diagnostic delay, added Ms. Piccus. “While the majority of respondents were diagnosed less than a year from the first GP visit, the median delay was 2 years, with 31% diagnosed after 5 or more years. One took 23 years for an initial diagnosis.”
Most cases were diagnosed at the time of surgery – 93%, with 52% at pelvic laparoscopy, 35% upper abdominal laparoscopy, with 30% requiring two or more laparoscopies before they were diagnosed with diaphragmatic endometriosis. A total of 7% were diagnosed via cross-sectional imaging prior to surgery.
Treatment outcomes for diaphragmatic endometriosis
Reflecting the literature, surgery to remove the endometriosis lesions was mainly laparoscopic with 47% abdominal excisions, and 29% abdominal ablations; 6% received open abdominal procedures, and 18% received open thoracotomy or video-assisted thoracoscopic surgery.
The survey asked about postoperative symptoms 6 months after surgery and at the time of survey. Symptoms at 6 months post surgery had completely resolved in 18%, shown significant improvement in 48%, and no improvement in 20%. Worsening of symptoms was seen in 14%. Long-term pain was reported by 21% as severe, 27% as moderate, 35% as mild, and 17% had no symptoms.
Further findings included that 23% underwent additional procedures to treat their diaphragmatic endometriosis, and that there was no significant difference between excision and ablation, nor between age of onset of symptoms or length of diagnostic delay.
“Surgical treatment to remove these extra pelvic deposits of endometriosis will depend upon the type and distribution of thoracic endometriosis and a variety of surgical specialties may need to be involved including gynecologists, cardiothoracic, and upper gastrointestinal/liver surgeons,” Dr. Clark said.
He added that familiar hormonal medical treatments for more typical pelvic endometriosis should also be considered for primary and maintenance treatment. “These data suggest a high symptomatic recurrence rate after surgical treatment and so medical treatments should be considered to try and minimize the risks of endometriosis symptoms returning.”
Dr. Clark also pointed out that multidisciplinary clinical teams should be established in specialized centers to plan surgical and medical management to enhance clinical outcomes and collect data to better understand this enigmatic condition.
Ms. Piccus and Dr. Clark have no relevant conflicts of interest.
Diaphragmatic endometriosis is often diagnosed several years after the start of symptoms – mainly moderate to severe pain – and this is potentially because of general lack of awareness of diaphragmatic endometriosis among the general population and medical professionals.
Findings of the international survey that explored the diagnosis and treatment of diaphragmatic endometriosis were presented at this year’s Royal College of Obstetricians and Gynecologists 2021 Virtual World Congress by medical student Rachel Piccus, MSc, based at the University of Birmingham (England). Robert Sutcliffe, MD, consultant in hepatobiliary and pancreatic surgery, at Queen Elizabeth Hospital Birmingham was senior author. Results were also published in the May 2021 issue of the European Journal of Obstetrics and Gynaecology and Reproductive Biology.
The study found that it took an average of five visits to a primary physician before referral to a gynecologist.
“Late diagnosis could also be due to the idea that diaphragmatic endometriosis symptoms often present before pelvic symptoms and therefore the site of pain is considered atypical for pelvic endometriosis,” Ms. Piccus said, adding that “clinicians are screening for cyclical pain, which is typical of endometriosis, but our study has shown that pain can in fact be more frequent – daily and weekly.”
These significant diagnostic delays, seen from the time of the initial primary care and gynecology consultation has the potential to significantly affect quality of life as seen in pelvic endometriosis, said Ms. Piccus. “These delays are partly due to a lack of awareness among gynecologists, but could also be due to pelvic laparoscopy being insufficient to examine the diaphragm behind the liver.”
Justin Clark, MD, consultant gynaecologist, Birmingham (England) Women’s and Children Hospital, moderated the session and agreed that the study highlights the need for greater awareness of this variant of endometriosis. “Whilst endometriosis affecting the diaphragm, subdiaphragm, and thorax is rare, the condition causes substantial morbidity.”
“Greater knowledge of thoracic endometriosis amongst clinicians in both primary and secondary care is needed to ensure accurate and timely diagnosis,” he added.
Diaphragmatic endometriosis is estimated to affect 1%-1.5% of all endometriosis patients and presents as cyclical pain in the chest, abdomen, and shoulder tip, as well as other respiratory symptoms such as catamenial pneumothorax and difficulty breathing.
“Cross-sectional imaging has shown low sensitivity so upper abdominal laparoscopy is the gold standard; however, this has implications for diagnostic delay because a strong clinical suspicion is required to refer for this invasive procedure,” explained Ms. Piccus referring to one of the reasons underpinning the need for the study.
When successfully diagnosed, treatment requires excision or ablation surgery and studies show symptomatic relief in 75%-100% of cases.
To gauge the extent of delayed diagnosis as well as treatment outcomes from a patient perspective, Ms. Piccus circulated an anonymous online survey among women with a previous history of surgery for diaphragmatic endometriosis.
Diaphragmatic endometriosis pain – daily and weekly as well as cyclical
A total of 137 participants responded to the survey, with a median age of 34 years (range, 19-53). Median age of diaphragmatic endometriosis onset was 27 years (range, 11-50), and importantly, diaphragmatic endometriosis symptoms started before pelvic symptoms in 90 respondents (66%).
The dominant symptom was pain. A total of 38% reported cyclical pain (related to endometrial shedding during menstruation), 15% weekly pain, and 47% daily pain, both of which were worse during the menstrual cycle. Furthermore, 14% reported other symptoms including catamenial pneumothorax, difficulty breathing, and hemoptysis.
“Whilst this cyclical pain is typical of endometriosis, we see that diagnostic delays may be due to misdiagnosis because clinicians are screening for this cyclical pain whilst our study has shown that pain can in fact be more frequent, being daily and weekly,” noted Ms. Piccus. Moderate to severe pain was reported in 67% of respondents and moderate in 31%, only 2% reported pain as mild.
Location of pain comprised moderate to severe pain in the upper abdomen (68%), chest (64%) and shoulder (54%). Pain was right-sided in 54%, left-sided in 11% and bilateral in 35%. Upper back and neck were also reported as sites of pain.
Indirectly providing a measure of the lack of awareness of diaphragmatic endometriosis on behalf of primary care, 122 participants reported initially visiting their primary care physician for help and 65 were given a diagnosis – in only 14 cases was that diaphragmatic endometriosis. There were a range of other gynecologic (e.g. ovarian cyst, two), respiratory (spontaneous pneumothorax, seven), gastrointestinal (gastritis/reflux, eight), musculoskeletal (six), and psychological (anxiety/stress, four) diagnoses.
A median of 5 primary care consultations (range, 1-100) were required before referral to a gynecologist, with 30% seeing a primary care physician over 10 times. A further 14 patients self-referred to gynecologist.
“These findings have implications for diagnostic delay, added Ms. Piccus. “While the majority of respondents were diagnosed less than a year from the first GP visit, the median delay was 2 years, with 31% diagnosed after 5 or more years. One took 23 years for an initial diagnosis.”
Most cases were diagnosed at the time of surgery – 93%, with 52% at pelvic laparoscopy, 35% upper abdominal laparoscopy, with 30% requiring two or more laparoscopies before they were diagnosed with diaphragmatic endometriosis. A total of 7% were diagnosed via cross-sectional imaging prior to surgery.
Treatment outcomes for diaphragmatic endometriosis
Reflecting the literature, surgery to remove the endometriosis lesions was mainly laparoscopic with 47% abdominal excisions, and 29% abdominal ablations; 6% received open abdominal procedures, and 18% received open thoracotomy or video-assisted thoracoscopic surgery.
The survey asked about postoperative symptoms 6 months after surgery and at the time of survey. Symptoms at 6 months post surgery had completely resolved in 18%, shown significant improvement in 48%, and no improvement in 20%. Worsening of symptoms was seen in 14%. Long-term pain was reported by 21% as severe, 27% as moderate, 35% as mild, and 17% had no symptoms.
Further findings included that 23% underwent additional procedures to treat their diaphragmatic endometriosis, and that there was no significant difference between excision and ablation, nor between age of onset of symptoms or length of diagnostic delay.
“Surgical treatment to remove these extra pelvic deposits of endometriosis will depend upon the type and distribution of thoracic endometriosis and a variety of surgical specialties may need to be involved including gynecologists, cardiothoracic, and upper gastrointestinal/liver surgeons,” Dr. Clark said.
He added that familiar hormonal medical treatments for more typical pelvic endometriosis should also be considered for primary and maintenance treatment. “These data suggest a high symptomatic recurrence rate after surgical treatment and so medical treatments should be considered to try and minimize the risks of endometriosis symptoms returning.”
Dr. Clark also pointed out that multidisciplinary clinical teams should be established in specialized centers to plan surgical and medical management to enhance clinical outcomes and collect data to better understand this enigmatic condition.
Ms. Piccus and Dr. Clark have no relevant conflicts of interest.
Prophylactic anticoagulation tied to lower death rate in COVID
Prophylactic anticoagulation to prevent venous thromboembolism (VTE) was associated with reduced 60-day mortality in patients with COVID-19 who were ill enough to require hospitalization, a new report shows.
In a cohort study of more than 1,300 hospitalized patients with COVID-19 infection across 30 hospitals in Michigan, both prophylactic- and therapeutic-dose anticoagulation were associated with reduced in-hospital mortality; however, at 60 days, only prophylactic-dose anticoagulation remained associated with lower mortality.
And adherence was key; nonadherence, or missing 2 days or more of anticoagulation, was linked to more deaths at 60 days.
The findings, which were published online June 11 in JAMA Network Open, are final proof that a prophylactic anticoagulation strategy for the hospitalized COVID population is, indeed, the right one, Valerie M. Vaughn, MD, director of hospital medicine research at the University of Utah, Salt Lake City, said in an interview.
“We’ve probably always known that patients with COVID need prophylaxis for VTE, but we found that early on, unfortunately, that wasn’t being done,” Dr. Vaughn said.
“Now, we see that prophylactic rates have increased. We always knew to use anticoagulation prophylactically in patients who were hospitalized with infection because of their risk for VTE, so this study just drives home that proper adherence to an anticoagulation protocol improves mortality,” she said.
Dr. Vaughn was on the front lines when COVID-19 came to Michigan, where the research was conducted.
“We probably should have been anticoagulating from the get-go, but you have to remember that in the early days of COVID, the hospitals in Michigan were being overwhelmed. They didn’t have PPE. They were taking care of patients outside of their typical hospital beds or setting up field hospitals,” she said. “It was not quite as bad as New York, but at the University of Michigan, we set up four or five ICUs outside of our normal care.”
They also converted the top floor of their pediatric hospital into an ICU to take care of patients with COVID during the first surge, she added. “We didn’t know much about this disease, but faced with this influx of patients, many of whom were dying with blood clots, we had to do something.”
Some hospitals began prophylactically anticoagulating their patients, but others hesitated before adopting the strategy. “But now we feel confident that prophylactic anticoagulation, done according to the right protocol, with no interruptions in the treatment, is beneficial,” Dr. Vaughn said.
The best medication choice is enoxaparin (Lovenox), which can be given once a day, as opposed to heparin, which needs to be given via injection three times a day, she said.
“Prophylactic dose anticoagulation is typically given by an injection under the skin, but a lot of times, I’ve had patients tell me they feel like a human pin cushion and have all these bruises from being stuck with needles every day, which I can totally relate to,” she said.
“It is important for us as clinicians to explain that we’re having to poke our patients because it is good for them and will help them fight COVID,” she added. “Also having the once-a-day option is going to be a lot better for adherence, and adherence to the protocol, not missing any days, is key to the better outcome.”
Dr. Vaughn and her team reviewed the charts of 1,351 patients (48% women, 49% Black, median age 64 [range 52-75]) who were hospitalized throughout Michigan during the first several months of the COVID-19 pandemic, from March to June 2020.
Only 18 patients (1.3%) had a confirmed VTE and 219 patients (16.2%) received treatment-dose anticoagulation.
The researchers noted that use of treatment-dose anticoagulation without imaging ranged from 0% to 29% across hospitals and increased significantly over time.
Of the 1,127 patients who received anticoagulation, 392 (34.8%) missed 2 days or more of prophylaxis.
In addition, there were varying rates of missed prophylaxis among the hospitals, from 11% to 61%, but these rates decreased markedly over time.
Missed doses were associated with a higher 60-day mortality (adjusted hazard ratio, 1.31; 95% confidence interval, 1.03-1.67), but not in-hospital mortality (aHR, 0.97; 95% CI, 0.91-1.03).
Compared with no anticoagulation, receiving any dose of anticoagulation was associated with lower in-hospital mortality.
However, only prophylactic-dose anticoagulation remained associated with lower mortality at 60 days. The adjusted hazard ratio for prophylactic-dose anticoagulation was 0.71 (95% CI, 0.51-0.90), compared with 0.92 (95% CI, 0.63-1.35) for treatment-dose anticoagulation.
Study boosts confidence
Despite its limitations, the study should make clinicians more confident that the use of prophylactic anticoagulation is warranted for hospitalized patients with COVID-19, write Andrew B. Dicks, MD, and Ido Weinberg, MD, from Massachusetts General Hospital, Boston, in an invited commentary.
“Practically, we still lack the granular data we need to help guide us in patient-by-patient decision-making – such as anticoagulation agent choice, dosage, and duration of therapy – especially as dictated by acuity of patient illness,” Dr. Dicks and Dr. Weinberg note.
“While we still await the data from randomized controlled trials to guide the optimal anticoagulation dose and duration, this study adds significant merit to the previously published recommendations from several different medical organizations regarding the use of prophylactic anticoagulation in hospitalized patients with COVID-19,” Dr. Dicks told this news organization.
The study was supported by Blue Cross and Blue Shield of Michigan and Blue Care Network as part of their Value Partnerships program. Dr. Vaughn has reported receiving speaking fees from Thermo Fisher Scientific. Dr. Dicks and Dr. Weinberg have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Prophylactic anticoagulation to prevent venous thromboembolism (VTE) was associated with reduced 60-day mortality in patients with COVID-19 who were ill enough to require hospitalization, a new report shows.
In a cohort study of more than 1,300 hospitalized patients with COVID-19 infection across 30 hospitals in Michigan, both prophylactic- and therapeutic-dose anticoagulation were associated with reduced in-hospital mortality; however, at 60 days, only prophylactic-dose anticoagulation remained associated with lower mortality.
And adherence was key; nonadherence, or missing 2 days or more of anticoagulation, was linked to more deaths at 60 days.
The findings, which were published online June 11 in JAMA Network Open, are final proof that a prophylactic anticoagulation strategy for the hospitalized COVID population is, indeed, the right one, Valerie M. Vaughn, MD, director of hospital medicine research at the University of Utah, Salt Lake City, said in an interview.
“We’ve probably always known that patients with COVID need prophylaxis for VTE, but we found that early on, unfortunately, that wasn’t being done,” Dr. Vaughn said.
“Now, we see that prophylactic rates have increased. We always knew to use anticoagulation prophylactically in patients who were hospitalized with infection because of their risk for VTE, so this study just drives home that proper adherence to an anticoagulation protocol improves mortality,” she said.
Dr. Vaughn was on the front lines when COVID-19 came to Michigan, where the research was conducted.
“We probably should have been anticoagulating from the get-go, but you have to remember that in the early days of COVID, the hospitals in Michigan were being overwhelmed. They didn’t have PPE. They were taking care of patients outside of their typical hospital beds or setting up field hospitals,” she said. “It was not quite as bad as New York, but at the University of Michigan, we set up four or five ICUs outside of our normal care.”
They also converted the top floor of their pediatric hospital into an ICU to take care of patients with COVID during the first surge, she added. “We didn’t know much about this disease, but faced with this influx of patients, many of whom were dying with blood clots, we had to do something.”
Some hospitals began prophylactically anticoagulating their patients, but others hesitated before adopting the strategy. “But now we feel confident that prophylactic anticoagulation, done according to the right protocol, with no interruptions in the treatment, is beneficial,” Dr. Vaughn said.
The best medication choice is enoxaparin (Lovenox), which can be given once a day, as opposed to heparin, which needs to be given via injection three times a day, she said.
“Prophylactic dose anticoagulation is typically given by an injection under the skin, but a lot of times, I’ve had patients tell me they feel like a human pin cushion and have all these bruises from being stuck with needles every day, which I can totally relate to,” she said.
“It is important for us as clinicians to explain that we’re having to poke our patients because it is good for them and will help them fight COVID,” she added. “Also having the once-a-day option is going to be a lot better for adherence, and adherence to the protocol, not missing any days, is key to the better outcome.”
Dr. Vaughn and her team reviewed the charts of 1,351 patients (48% women, 49% Black, median age 64 [range 52-75]) who were hospitalized throughout Michigan during the first several months of the COVID-19 pandemic, from March to June 2020.
Only 18 patients (1.3%) had a confirmed VTE and 219 patients (16.2%) received treatment-dose anticoagulation.
The researchers noted that use of treatment-dose anticoagulation without imaging ranged from 0% to 29% across hospitals and increased significantly over time.
Of the 1,127 patients who received anticoagulation, 392 (34.8%) missed 2 days or more of prophylaxis.
In addition, there were varying rates of missed prophylaxis among the hospitals, from 11% to 61%, but these rates decreased markedly over time.
Missed doses were associated with a higher 60-day mortality (adjusted hazard ratio, 1.31; 95% confidence interval, 1.03-1.67), but not in-hospital mortality (aHR, 0.97; 95% CI, 0.91-1.03).
Compared with no anticoagulation, receiving any dose of anticoagulation was associated with lower in-hospital mortality.
However, only prophylactic-dose anticoagulation remained associated with lower mortality at 60 days. The adjusted hazard ratio for prophylactic-dose anticoagulation was 0.71 (95% CI, 0.51-0.90), compared with 0.92 (95% CI, 0.63-1.35) for treatment-dose anticoagulation.
Study boosts confidence
Despite its limitations, the study should make clinicians more confident that the use of prophylactic anticoagulation is warranted for hospitalized patients with COVID-19, write Andrew B. Dicks, MD, and Ido Weinberg, MD, from Massachusetts General Hospital, Boston, in an invited commentary.
“Practically, we still lack the granular data we need to help guide us in patient-by-patient decision-making – such as anticoagulation agent choice, dosage, and duration of therapy – especially as dictated by acuity of patient illness,” Dr. Dicks and Dr. Weinberg note.
“While we still await the data from randomized controlled trials to guide the optimal anticoagulation dose and duration, this study adds significant merit to the previously published recommendations from several different medical organizations regarding the use of prophylactic anticoagulation in hospitalized patients with COVID-19,” Dr. Dicks told this news organization.
The study was supported by Blue Cross and Blue Shield of Michigan and Blue Care Network as part of their Value Partnerships program. Dr. Vaughn has reported receiving speaking fees from Thermo Fisher Scientific. Dr. Dicks and Dr. Weinberg have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Prophylactic anticoagulation to prevent venous thromboembolism (VTE) was associated with reduced 60-day mortality in patients with COVID-19 who were ill enough to require hospitalization, a new report shows.
In a cohort study of more than 1,300 hospitalized patients with COVID-19 infection across 30 hospitals in Michigan, both prophylactic- and therapeutic-dose anticoagulation were associated with reduced in-hospital mortality; however, at 60 days, only prophylactic-dose anticoagulation remained associated with lower mortality.
And adherence was key; nonadherence, or missing 2 days or more of anticoagulation, was linked to more deaths at 60 days.
The findings, which were published online June 11 in JAMA Network Open, are final proof that a prophylactic anticoagulation strategy for the hospitalized COVID population is, indeed, the right one, Valerie M. Vaughn, MD, director of hospital medicine research at the University of Utah, Salt Lake City, said in an interview.
“We’ve probably always known that patients with COVID need prophylaxis for VTE, but we found that early on, unfortunately, that wasn’t being done,” Dr. Vaughn said.
“Now, we see that prophylactic rates have increased. We always knew to use anticoagulation prophylactically in patients who were hospitalized with infection because of their risk for VTE, so this study just drives home that proper adherence to an anticoagulation protocol improves mortality,” she said.
Dr. Vaughn was on the front lines when COVID-19 came to Michigan, where the research was conducted.
“We probably should have been anticoagulating from the get-go, but you have to remember that in the early days of COVID, the hospitals in Michigan were being overwhelmed. They didn’t have PPE. They were taking care of patients outside of their typical hospital beds or setting up field hospitals,” she said. “It was not quite as bad as New York, but at the University of Michigan, we set up four or five ICUs outside of our normal care.”
They also converted the top floor of their pediatric hospital into an ICU to take care of patients with COVID during the first surge, she added. “We didn’t know much about this disease, but faced with this influx of patients, many of whom were dying with blood clots, we had to do something.”
Some hospitals began prophylactically anticoagulating their patients, but others hesitated before adopting the strategy. “But now we feel confident that prophylactic anticoagulation, done according to the right protocol, with no interruptions in the treatment, is beneficial,” Dr. Vaughn said.
The best medication choice is enoxaparin (Lovenox), which can be given once a day, as opposed to heparin, which needs to be given via injection three times a day, she said.
“Prophylactic dose anticoagulation is typically given by an injection under the skin, but a lot of times, I’ve had patients tell me they feel like a human pin cushion and have all these bruises from being stuck with needles every day, which I can totally relate to,” she said.
“It is important for us as clinicians to explain that we’re having to poke our patients because it is good for them and will help them fight COVID,” she added. “Also having the once-a-day option is going to be a lot better for adherence, and adherence to the protocol, not missing any days, is key to the better outcome.”
Dr. Vaughn and her team reviewed the charts of 1,351 patients (48% women, 49% Black, median age 64 [range 52-75]) who were hospitalized throughout Michigan during the first several months of the COVID-19 pandemic, from March to June 2020.
Only 18 patients (1.3%) had a confirmed VTE and 219 patients (16.2%) received treatment-dose anticoagulation.
The researchers noted that use of treatment-dose anticoagulation without imaging ranged from 0% to 29% across hospitals and increased significantly over time.
Of the 1,127 patients who received anticoagulation, 392 (34.8%) missed 2 days or more of prophylaxis.
In addition, there were varying rates of missed prophylaxis among the hospitals, from 11% to 61%, but these rates decreased markedly over time.
Missed doses were associated with a higher 60-day mortality (adjusted hazard ratio, 1.31; 95% confidence interval, 1.03-1.67), but not in-hospital mortality (aHR, 0.97; 95% CI, 0.91-1.03).
Compared with no anticoagulation, receiving any dose of anticoagulation was associated with lower in-hospital mortality.
However, only prophylactic-dose anticoagulation remained associated with lower mortality at 60 days. The adjusted hazard ratio for prophylactic-dose anticoagulation was 0.71 (95% CI, 0.51-0.90), compared with 0.92 (95% CI, 0.63-1.35) for treatment-dose anticoagulation.
Study boosts confidence
Despite its limitations, the study should make clinicians more confident that the use of prophylactic anticoagulation is warranted for hospitalized patients with COVID-19, write Andrew B. Dicks, MD, and Ido Weinberg, MD, from Massachusetts General Hospital, Boston, in an invited commentary.
“Practically, we still lack the granular data we need to help guide us in patient-by-patient decision-making – such as anticoagulation agent choice, dosage, and duration of therapy – especially as dictated by acuity of patient illness,” Dr. Dicks and Dr. Weinberg note.
“While we still await the data from randomized controlled trials to guide the optimal anticoagulation dose and duration, this study adds significant merit to the previously published recommendations from several different medical organizations regarding the use of prophylactic anticoagulation in hospitalized patients with COVID-19,” Dr. Dicks told this news organization.
The study was supported by Blue Cross and Blue Shield of Michigan and Blue Care Network as part of their Value Partnerships program. Dr. Vaughn has reported receiving speaking fees from Thermo Fisher Scientific. Dr. Dicks and Dr. Weinberg have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Giving flu and COVID-19 shots at same time appears safe, effective: Study
Overall, the NVX-CoV2373 vaccine (Novavax) is showing 89.8% efficacy in an ongoing, placebo-controlled phase 3 study. When the researchers gave a smaller group of 431 volunteers from the same study an influenza shot at the same time, efficacy dropped slightly to 87.5%.
“These results demonstrate the promising opportunity for concomitant vaccination, which may lead to higher vaccination rates and further protection against both viruses,” said study coauthor Raja Rajaram, MD, medical affairs lead, Europe, Middle East, and Africa at Seqirus, the company that supplied the influenza vaccines for the research.
The research was published online June 13 as a medRxiv preprint.
“With these COVID-19 vaccines, there are essentially no concurrent use studies,” Paul A. Offit, MD, told this news organization when asked to comment.
Traditionally, how a new vaccine might interact with existing vaccines is studied before the product is cleared for use. That was not the case, however, with the COVID-19 vaccines made available through expedited emergency use authorization.
The researchers found no major safety concerns associated with concomitant vaccination, Dr. Rajaram said. In addition to safety, the aim of the current study was to determine whether either vaccine changes the immunogenicity or effectiveness of the other.
“It’s a small study, but it’s certainly encouraging to know that there didn’t seem to be a big decrease in immunogenicity either way and the safety profile was similar. Not identical, but similar,” added Dr. Offit, director of the Vaccine Education Center at Children’s Hospital of Philadelphia.
Some adverse events were more common in the co-administration group. For example, injection-site tenderness was reported by 70%, versus 58% for those who got the COVID-19 shot alone. The same was true for pain at the injection site, 40% versus 29%; fatigue, 28% versus 19%; and muscle pain, 28% versus 21%.
Rates of unsolicited adverse events, adverse events that required medical attention, and serious adverse events were low and well balanced between groups.
Fewer antibodies important?
Although co-administering the two vaccines did not change the immune response for the influenza vaccine, the spike protein antibody response to the COVID-19 vaccine was less robust.
Antibody titer levels at day 35 were 46,678 among people in the Novavax vaccine alone group, compared with 31,236 titers in the participants who received both vaccines.
“This impact did not seem to be clinically meaningful as vaccine efficacy appeared to be preserved,” the researchers noted.
Gregory A. Poland, MD, an internist and part of the Vaccine Research Group at Mayo Clinic in Rochester, Minn., agreed. “I highly doubt that is significant,” he said in an interview.
Dr. Rajaram said the antibody findings are “slightly surprising but not completely unexpected” because the same observation has been made in other combination vaccine studies. He added that the antibody levels “remain very high, although we do not yet know what antibody levels are required to achieve protection against COVID-19.”
The decrease could become more concerning if people start with fewer antibodies and they drop over time with normal waning of protection, Dr. Poland said. This group could include people over age 65 or people who are immunocompromised. More data would be needed to confirm this, he added.
A boost for booster vaccines?
The research could carry implications for future COVID-19 booster shots, Dr. Poland said.
“Overall, the study results are reassuring and of potential practical importance if we have to give booster doses. It will make it easier to give them both in one visit,” said Dr. Poland, who was not affiliated with the research.
Although Novavax could be positioning itself as a logical choice for a COVID-19 booster based on the findings, Dr. Offit believes it is more important to focus on having more COVID-19 vaccine options available.
“There may be, as we say at the track, ‘courses for horses,’ ” he said, meaning that different vaccines may be better suited for different situations.
“It’s likely we’re going to find these vaccines have different safety profiles, they may have different populations for whom they work best, and they may have differences in terms of their long-term durability,” he added. Also, some may prove more effective against certain variants of concern.
The Novavax vaccine would add a new class of COVID-19 vaccine to the mRNA and adenovirus vaccines. NVX-CoV2373 is a recombinant spike protein vaccine.
“I think the more vaccines that are available here, the better,” Dr. Offit said.
Study limitations
Dr. Poland shared some caveats. The study was primarily conducted in adults aged 18-64 years, so there is less certainty on what could happen in people over 65. Furthermore, co-administration was evaluated after the first dose of the Novavax vaccine. “The reason I bring that up is most of the COVID-19 vaccine reactogenicity occurs with dose two, not dose one.
“All in all, it’s an important first step – but it’s only a first step,” Dr. Poland said. “We need more data, including in elderly people who are primarily at risk for morbidity and mortality from the flu.”
He suggested expanding the research to study co-administration of COVID-19 vaccines with different formulations of influenza vaccines.
The study was supported by Novavax. Dr. Offit had no relevant financial disclosures. Dr. Poland serves as a consultant to all of the COVID-19 vaccine companies.
A version of this article first appeared on Medscape.com.
Overall, the NVX-CoV2373 vaccine (Novavax) is showing 89.8% efficacy in an ongoing, placebo-controlled phase 3 study. When the researchers gave a smaller group of 431 volunteers from the same study an influenza shot at the same time, efficacy dropped slightly to 87.5%.
“These results demonstrate the promising opportunity for concomitant vaccination, which may lead to higher vaccination rates and further protection against both viruses,” said study coauthor Raja Rajaram, MD, medical affairs lead, Europe, Middle East, and Africa at Seqirus, the company that supplied the influenza vaccines for the research.
The research was published online June 13 as a medRxiv preprint.
“With these COVID-19 vaccines, there are essentially no concurrent use studies,” Paul A. Offit, MD, told this news organization when asked to comment.
Traditionally, how a new vaccine might interact with existing vaccines is studied before the product is cleared for use. That was not the case, however, with the COVID-19 vaccines made available through expedited emergency use authorization.
The researchers found no major safety concerns associated with concomitant vaccination, Dr. Rajaram said. In addition to safety, the aim of the current study was to determine whether either vaccine changes the immunogenicity or effectiveness of the other.
“It’s a small study, but it’s certainly encouraging to know that there didn’t seem to be a big decrease in immunogenicity either way and the safety profile was similar. Not identical, but similar,” added Dr. Offit, director of the Vaccine Education Center at Children’s Hospital of Philadelphia.
Some adverse events were more common in the co-administration group. For example, injection-site tenderness was reported by 70%, versus 58% for those who got the COVID-19 shot alone. The same was true for pain at the injection site, 40% versus 29%; fatigue, 28% versus 19%; and muscle pain, 28% versus 21%.
Rates of unsolicited adverse events, adverse events that required medical attention, and serious adverse events were low and well balanced between groups.
Fewer antibodies important?
Although co-administering the two vaccines did not change the immune response for the influenza vaccine, the spike protein antibody response to the COVID-19 vaccine was less robust.
Antibody titer levels at day 35 were 46,678 among people in the Novavax vaccine alone group, compared with 31,236 titers in the participants who received both vaccines.
“This impact did not seem to be clinically meaningful as vaccine efficacy appeared to be preserved,” the researchers noted.
Gregory A. Poland, MD, an internist and part of the Vaccine Research Group at Mayo Clinic in Rochester, Minn., agreed. “I highly doubt that is significant,” he said in an interview.
Dr. Rajaram said the antibody findings are “slightly surprising but not completely unexpected” because the same observation has been made in other combination vaccine studies. He added that the antibody levels “remain very high, although we do not yet know what antibody levels are required to achieve protection against COVID-19.”
The decrease could become more concerning if people start with fewer antibodies and they drop over time with normal waning of protection, Dr. Poland said. This group could include people over age 65 or people who are immunocompromised. More data would be needed to confirm this, he added.
A boost for booster vaccines?
The research could carry implications for future COVID-19 booster shots, Dr. Poland said.
“Overall, the study results are reassuring and of potential practical importance if we have to give booster doses. It will make it easier to give them both in one visit,” said Dr. Poland, who was not affiliated with the research.
Although Novavax could be positioning itself as a logical choice for a COVID-19 booster based on the findings, Dr. Offit believes it is more important to focus on having more COVID-19 vaccine options available.
“There may be, as we say at the track, ‘courses for horses,’ ” he said, meaning that different vaccines may be better suited for different situations.
“It’s likely we’re going to find these vaccines have different safety profiles, they may have different populations for whom they work best, and they may have differences in terms of their long-term durability,” he added. Also, some may prove more effective against certain variants of concern.
The Novavax vaccine would add a new class of COVID-19 vaccine to the mRNA and adenovirus vaccines. NVX-CoV2373 is a recombinant spike protein vaccine.
“I think the more vaccines that are available here, the better,” Dr. Offit said.
Study limitations
Dr. Poland shared some caveats. The study was primarily conducted in adults aged 18-64 years, so there is less certainty on what could happen in people over 65. Furthermore, co-administration was evaluated after the first dose of the Novavax vaccine. “The reason I bring that up is most of the COVID-19 vaccine reactogenicity occurs with dose two, not dose one.
“All in all, it’s an important first step – but it’s only a first step,” Dr. Poland said. “We need more data, including in elderly people who are primarily at risk for morbidity and mortality from the flu.”
He suggested expanding the research to study co-administration of COVID-19 vaccines with different formulations of influenza vaccines.
The study was supported by Novavax. Dr. Offit had no relevant financial disclosures. Dr. Poland serves as a consultant to all of the COVID-19 vaccine companies.
A version of this article first appeared on Medscape.com.
Overall, the NVX-CoV2373 vaccine (Novavax) is showing 89.8% efficacy in an ongoing, placebo-controlled phase 3 study. When the researchers gave a smaller group of 431 volunteers from the same study an influenza shot at the same time, efficacy dropped slightly to 87.5%.
“These results demonstrate the promising opportunity for concomitant vaccination, which may lead to higher vaccination rates and further protection against both viruses,” said study coauthor Raja Rajaram, MD, medical affairs lead, Europe, Middle East, and Africa at Seqirus, the company that supplied the influenza vaccines for the research.
The research was published online June 13 as a medRxiv preprint.
“With these COVID-19 vaccines, there are essentially no concurrent use studies,” Paul A. Offit, MD, told this news organization when asked to comment.
Traditionally, how a new vaccine might interact with existing vaccines is studied before the product is cleared for use. That was not the case, however, with the COVID-19 vaccines made available through expedited emergency use authorization.
The researchers found no major safety concerns associated with concomitant vaccination, Dr. Rajaram said. In addition to safety, the aim of the current study was to determine whether either vaccine changes the immunogenicity or effectiveness of the other.
“It’s a small study, but it’s certainly encouraging to know that there didn’t seem to be a big decrease in immunogenicity either way and the safety profile was similar. Not identical, but similar,” added Dr. Offit, director of the Vaccine Education Center at Children’s Hospital of Philadelphia.
Some adverse events were more common in the co-administration group. For example, injection-site tenderness was reported by 70%, versus 58% for those who got the COVID-19 shot alone. The same was true for pain at the injection site, 40% versus 29%; fatigue, 28% versus 19%; and muscle pain, 28% versus 21%.
Rates of unsolicited adverse events, adverse events that required medical attention, and serious adverse events were low and well balanced between groups.
Fewer antibodies important?
Although co-administering the two vaccines did not change the immune response for the influenza vaccine, the spike protein antibody response to the COVID-19 vaccine was less robust.
Antibody titer levels at day 35 were 46,678 among people in the Novavax vaccine alone group, compared with 31,236 titers in the participants who received both vaccines.
“This impact did not seem to be clinically meaningful as vaccine efficacy appeared to be preserved,” the researchers noted.
Gregory A. Poland, MD, an internist and part of the Vaccine Research Group at Mayo Clinic in Rochester, Minn., agreed. “I highly doubt that is significant,” he said in an interview.
Dr. Rajaram said the antibody findings are “slightly surprising but not completely unexpected” because the same observation has been made in other combination vaccine studies. He added that the antibody levels “remain very high, although we do not yet know what antibody levels are required to achieve protection against COVID-19.”
The decrease could become more concerning if people start with fewer antibodies and they drop over time with normal waning of protection, Dr. Poland said. This group could include people over age 65 or people who are immunocompromised. More data would be needed to confirm this, he added.
A boost for booster vaccines?
The research could carry implications for future COVID-19 booster shots, Dr. Poland said.
“Overall, the study results are reassuring and of potential practical importance if we have to give booster doses. It will make it easier to give them both in one visit,” said Dr. Poland, who was not affiliated with the research.
Although Novavax could be positioning itself as a logical choice for a COVID-19 booster based on the findings, Dr. Offit believes it is more important to focus on having more COVID-19 vaccine options available.
“There may be, as we say at the track, ‘courses for horses,’ ” he said, meaning that different vaccines may be better suited for different situations.
“It’s likely we’re going to find these vaccines have different safety profiles, they may have different populations for whom they work best, and they may have differences in terms of their long-term durability,” he added. Also, some may prove more effective against certain variants of concern.
The Novavax vaccine would add a new class of COVID-19 vaccine to the mRNA and adenovirus vaccines. NVX-CoV2373 is a recombinant spike protein vaccine.
“I think the more vaccines that are available here, the better,” Dr. Offit said.
Study limitations
Dr. Poland shared some caveats. The study was primarily conducted in adults aged 18-64 years, so there is less certainty on what could happen in people over 65. Furthermore, co-administration was evaluated after the first dose of the Novavax vaccine. “The reason I bring that up is most of the COVID-19 vaccine reactogenicity occurs with dose two, not dose one.
“All in all, it’s an important first step – but it’s only a first step,” Dr. Poland said. “We need more data, including in elderly people who are primarily at risk for morbidity and mortality from the flu.”
He suggested expanding the research to study co-administration of COVID-19 vaccines with different formulations of influenza vaccines.
The study was supported by Novavax. Dr. Offit had no relevant financial disclosures. Dr. Poland serves as a consultant to all of the COVID-19 vaccine companies.
A version of this article first appeared on Medscape.com.
AHA: Don’t delay COVID shot while CDC reviews myocarditis cases
While the investigation into cases of myocarditis possibly associated with COVID vaccines proceeds, the American Heart Association/American Stroke Association (ASA) continue to urge everyone who is eligible for the vaccine to get it without delay.
“We remain confident that the benefits of vaccination far exceed the very unusual risks,” the leadership of the AHA/ASA said in a statement issued June 12.
“The risks of COVID-19 infection include its potentially fatal consequences and the potential long-term health effects that are still revealing themselves, including lingering consequences affecting the heart, brain, vascular system, and other organs after infection,” they point out.
Late last week, the Centers for Disease Control and Prevention alerted health care providers that the COVID-19 Vaccine Safety Technical Work Group (VaST) of the Advisory Committee on Immunization Practices (ACIP) will meet June 18 to review cases of myocarditis reported in adolescents and young adults after they received a COVID-19 vaccine manufactured by Pfizer-BioNTech or Moderna.
The CDC is monitoring the Vaccine Adverse Events Reporting System (VAERS) and the Vaccine Safety Datalink (VSD) for cases of myocarditis that have been associated with the mRNA vaccines against SARS-CoV-2 from Pfizer and Moderna.
These cases may occur more often in males than females and more frequently after the second dose than the first dose of either mRNA vaccine. Symptoms typically occur in the 3 days after administration.
“The CDC’s ongoing investigation into cases of suspected myocarditis reflects a strong and steadfast commitment to transparency and the importance of scientific rigor on all fronts. We applaud the CDC’s unwavering efforts to lead our nation’s scientific and public health efforts, including ensuring the continued safety of the COVID-19 vaccines,” the AHA/ASA states.
They emphasize that vaccinations should continue, and say it’s important to consider the details of the suspected myocarditis cases being investigated by the CDC.
As of June 11, more than 306 million doses of COVID-19 vaccines have been administered in the United States (since Dec. 14, 2020) and nearly 43% of Americans – more than 142 million people – are now fully vaccinated.
According to the June 10 CDC VAERS report detailing adverse events through May 31:
- 789 cases of suspected myocarditis have been reported, with 475 involving people younger than 30 years; 79 cases reported were in patients 16 or 17 years old.
- The vast majority (81%) of the 270 patients younger than 30 years who were discharged from care after suspected myocarditis related to COVID-19 vaccination have recovered fully; the remaining 19% of patients report ongoing symptoms or complete data are missing.
- 196 cases of suspected myocarditis after a COVID-19 vaccine were reported in young adults 18 to 24 years of age, which is higher than expected for this age group.
As of May 31, only about 9% of the COVID-19 vaccine doses administered were to people 16 to 24 years of age, which is why this “higher-than-normal rate of possible myocarditis cases” warrants investigation, the AHA/ASA says.
They note that these suspected myocarditis cases were reported to VAERS because of their proximity to COVID-19 vaccine administration.
It remains to be determined which cases meet the clinical criteria for a diagnosis of myocarditis and whether they have any direct connection to the COVID-19 vaccine, the AHA/ASA says.
They urge all health care professionals to be aware of “very rare” adverse events that could be related to a COVID-19 vaccine, including myocarditis, blood clots, low platelets, and symptoms of severe inflammation.
They advise asking patients who present with symptoms related to these conditions about the timing of recent COVID vaccinations, as needed, to confirm the diagnosis and provide appropriate treatment quickly.
The AHA will be at the CDC’s June 18 meeting to review the latest evidence on cases of suspected myocarditis after the COVID-19 vaccine, the statement adds.
The statement notes that it reflects the views of the AHA/ASA and its scientific leadership, including current president Mitchel S.V. Elkind, MD, PhD; immediate past-president Robert A. Harrington, MD; president-elect Donald M. Lloyd-Jones, MD; AHA/ASA chief science and medical officer Mariell Jessup, MD; and chief medical officer for prevention Eduardo Sanchez, MD, MPH.
A version of this article first appeared on Medscape.com.
While the investigation into cases of myocarditis possibly associated with COVID vaccines proceeds, the American Heart Association/American Stroke Association (ASA) continue to urge everyone who is eligible for the vaccine to get it without delay.
“We remain confident that the benefits of vaccination far exceed the very unusual risks,” the leadership of the AHA/ASA said in a statement issued June 12.
“The risks of COVID-19 infection include its potentially fatal consequences and the potential long-term health effects that are still revealing themselves, including lingering consequences affecting the heart, brain, vascular system, and other organs after infection,” they point out.
Late last week, the Centers for Disease Control and Prevention alerted health care providers that the COVID-19 Vaccine Safety Technical Work Group (VaST) of the Advisory Committee on Immunization Practices (ACIP) will meet June 18 to review cases of myocarditis reported in adolescents and young adults after they received a COVID-19 vaccine manufactured by Pfizer-BioNTech or Moderna.
The CDC is monitoring the Vaccine Adverse Events Reporting System (VAERS) and the Vaccine Safety Datalink (VSD) for cases of myocarditis that have been associated with the mRNA vaccines against SARS-CoV-2 from Pfizer and Moderna.
These cases may occur more often in males than females and more frequently after the second dose than the first dose of either mRNA vaccine. Symptoms typically occur in the 3 days after administration.
“The CDC’s ongoing investigation into cases of suspected myocarditis reflects a strong and steadfast commitment to transparency and the importance of scientific rigor on all fronts. We applaud the CDC’s unwavering efforts to lead our nation’s scientific and public health efforts, including ensuring the continued safety of the COVID-19 vaccines,” the AHA/ASA states.
They emphasize that vaccinations should continue, and say it’s important to consider the details of the suspected myocarditis cases being investigated by the CDC.
As of June 11, more than 306 million doses of COVID-19 vaccines have been administered in the United States (since Dec. 14, 2020) and nearly 43% of Americans – more than 142 million people – are now fully vaccinated.
According to the June 10 CDC VAERS report detailing adverse events through May 31:
- 789 cases of suspected myocarditis have been reported, with 475 involving people younger than 30 years; 79 cases reported were in patients 16 or 17 years old.
- The vast majority (81%) of the 270 patients younger than 30 years who were discharged from care after suspected myocarditis related to COVID-19 vaccination have recovered fully; the remaining 19% of patients report ongoing symptoms or complete data are missing.
- 196 cases of suspected myocarditis after a COVID-19 vaccine were reported in young adults 18 to 24 years of age, which is higher than expected for this age group.
As of May 31, only about 9% of the COVID-19 vaccine doses administered were to people 16 to 24 years of age, which is why this “higher-than-normal rate of possible myocarditis cases” warrants investigation, the AHA/ASA says.
They note that these suspected myocarditis cases were reported to VAERS because of their proximity to COVID-19 vaccine administration.
It remains to be determined which cases meet the clinical criteria for a diagnosis of myocarditis and whether they have any direct connection to the COVID-19 vaccine, the AHA/ASA says.
They urge all health care professionals to be aware of “very rare” adverse events that could be related to a COVID-19 vaccine, including myocarditis, blood clots, low platelets, and symptoms of severe inflammation.
They advise asking patients who present with symptoms related to these conditions about the timing of recent COVID vaccinations, as needed, to confirm the diagnosis and provide appropriate treatment quickly.
The AHA will be at the CDC’s June 18 meeting to review the latest evidence on cases of suspected myocarditis after the COVID-19 vaccine, the statement adds.
The statement notes that it reflects the views of the AHA/ASA and its scientific leadership, including current president Mitchel S.V. Elkind, MD, PhD; immediate past-president Robert A. Harrington, MD; president-elect Donald M. Lloyd-Jones, MD; AHA/ASA chief science and medical officer Mariell Jessup, MD; and chief medical officer for prevention Eduardo Sanchez, MD, MPH.
A version of this article first appeared on Medscape.com.
While the investigation into cases of myocarditis possibly associated with COVID vaccines proceeds, the American Heart Association/American Stroke Association (ASA) continue to urge everyone who is eligible for the vaccine to get it without delay.
“We remain confident that the benefits of vaccination far exceed the very unusual risks,” the leadership of the AHA/ASA said in a statement issued June 12.
“The risks of COVID-19 infection include its potentially fatal consequences and the potential long-term health effects that are still revealing themselves, including lingering consequences affecting the heart, brain, vascular system, and other organs after infection,” they point out.
Late last week, the Centers for Disease Control and Prevention alerted health care providers that the COVID-19 Vaccine Safety Technical Work Group (VaST) of the Advisory Committee on Immunization Practices (ACIP) will meet June 18 to review cases of myocarditis reported in adolescents and young adults after they received a COVID-19 vaccine manufactured by Pfizer-BioNTech or Moderna.
The CDC is monitoring the Vaccine Adverse Events Reporting System (VAERS) and the Vaccine Safety Datalink (VSD) for cases of myocarditis that have been associated with the mRNA vaccines against SARS-CoV-2 from Pfizer and Moderna.
These cases may occur more often in males than females and more frequently after the second dose than the first dose of either mRNA vaccine. Symptoms typically occur in the 3 days after administration.
“The CDC’s ongoing investigation into cases of suspected myocarditis reflects a strong and steadfast commitment to transparency and the importance of scientific rigor on all fronts. We applaud the CDC’s unwavering efforts to lead our nation’s scientific and public health efforts, including ensuring the continued safety of the COVID-19 vaccines,” the AHA/ASA states.
They emphasize that vaccinations should continue, and say it’s important to consider the details of the suspected myocarditis cases being investigated by the CDC.
As of June 11, more than 306 million doses of COVID-19 vaccines have been administered in the United States (since Dec. 14, 2020) and nearly 43% of Americans – more than 142 million people – are now fully vaccinated.
According to the June 10 CDC VAERS report detailing adverse events through May 31:
- 789 cases of suspected myocarditis have been reported, with 475 involving people younger than 30 years; 79 cases reported were in patients 16 or 17 years old.
- The vast majority (81%) of the 270 patients younger than 30 years who were discharged from care after suspected myocarditis related to COVID-19 vaccination have recovered fully; the remaining 19% of patients report ongoing symptoms or complete data are missing.
- 196 cases of suspected myocarditis after a COVID-19 vaccine were reported in young adults 18 to 24 years of age, which is higher than expected for this age group.
As of May 31, only about 9% of the COVID-19 vaccine doses administered were to people 16 to 24 years of age, which is why this “higher-than-normal rate of possible myocarditis cases” warrants investigation, the AHA/ASA says.
They note that these suspected myocarditis cases were reported to VAERS because of their proximity to COVID-19 vaccine administration.
It remains to be determined which cases meet the clinical criteria for a diagnosis of myocarditis and whether they have any direct connection to the COVID-19 vaccine, the AHA/ASA says.
They urge all health care professionals to be aware of “very rare” adverse events that could be related to a COVID-19 vaccine, including myocarditis, blood clots, low platelets, and symptoms of severe inflammation.
They advise asking patients who present with symptoms related to these conditions about the timing of recent COVID vaccinations, as needed, to confirm the diagnosis and provide appropriate treatment quickly.
The AHA will be at the CDC’s June 18 meeting to review the latest evidence on cases of suspected myocarditis after the COVID-19 vaccine, the statement adds.
The statement notes that it reflects the views of the AHA/ASA and its scientific leadership, including current president Mitchel S.V. Elkind, MD, PhD; immediate past-president Robert A. Harrington, MD; president-elect Donald M. Lloyd-Jones, MD; AHA/ASA chief science and medical officer Mariell Jessup, MD; and chief medical officer for prevention Eduardo Sanchez, MD, MPH.
A version of this article first appeared on Medscape.com.

