User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
Powered by CHEST Physician, Clinician Reviews, MDedge Family Medicine, Internal Medicine News, and The Journal of Clinical Outcomes Management.
‘Profound human toll’ in excess deaths from COVID-19 calculated in two studies
However, additional deaths could be indirectly related because people avoided emergency care during the pandemic, new research shows.
Deaths linked to COVID-19 varied by state and phase of the pandemic, as reported in a study from researchers at Virginia Commonwealth University and Yale University that was published online October 12 in JAMA.
Another study published online simultaneously in JAMA took more of an international perspective. Investigators from the University of Pennsylvania and Harvard University found that in America there were more excess deaths and there was higher all-cause mortality during the pandemic than in 18 other countries.
Although the ongoing number of deaths attributable to COVID-19 continues to garner attention, there can be a lag of weeks or months in how long it takes some public health agencies to update their figures.
“For the public at large, the take-home message is twofold: that the number of deaths caused by the pandemic exceeds publicly reported COVID-19 death counts by 20% and that states that reopened or lifted restrictions early suffered a protracted surge in excess deaths that extended into the summer,” lead author of the US-focused study, Steven H. Woolf, MD, MPH, told Medscape Medical News.
The take-away for physicians is in the bigger picture – it is likely that the COVID-19 pandemic is responsible for deaths from other conditions as well. “Surges in COVID-19 were accompanied by an increase in deaths attributed to other causes, such as heart disease and Alzheimer’s disease and dementia,” said Woolf, director emeritus and senior adviser at the Center on Society and Health and professor in the Department of Family Medicine and Population Health at the Virginia Commonwealth University School of Medicine in Richmond, Virginia.
The investigators identified 225,530 excess US deaths in the 5 months from March to July. They report that 67% were directly attributable to COVID-19.
Deaths linked to COVID-19 included those in which the disease was listed as an underlying or contributing cause. US total death rates are “remarkably consistent” year after year, and the investigators calculated a 20% overall jump in mortality.
The study included data from the National Center for Health Statistics and the US Census Bureau for 48 states and the District of Columbia. Connecticut and North Carolina were excluded because of missing data.
Woolf and colleagues also found statistically higher rates of deaths from two other causes, heart disease and Alzheimer’s disease/dementia.
Altered states
New York, New Jersey, Massachusetts, Louisiana, Arizona, Mississippi, Maryland, Delaware, Rhode Island, and Michigan had the highest per capita excess death rates. Three states experienced the shortest epidemics during the study period: New York, New Jersey, and Massachusetts.
Some lessons could be learned by looking at how individual states managed large numbers of people with COVID-19. “Although we suspected that states that reopened early might have put themselves at risk of a pandemic surge, the consistency with which that occurred and the devastating numbers of deaths they suffered was a surprise,” Woolf said.
“The goal of our study is not to look in the rearview mirror and lament what happened months ago but to learn the lesson going forward: Our country will be unable to take control of this pandemic without more robust efforts to control community spread,” Woolf said. “Our study found that states that did this well, such as New York and New Jersey, experienced large surges but bent the curve and were back to baseline in less than 10 weeks.
“If we could do this as a country, countless lives could be saved.”
A global perspective
The United States experienced high mortality linked to COVID-19, as well as high all-cause mortality, compared with 18 other countries, as reported in the study by University of Pennsylvania and Harvard University researchers.
The United States ranked third, with 72 deaths per 100,000 people, among countries with moderate or high mortality. Although perhaps not surprising given the state of SARS-CoV-2 infection across the United States, a question remains as to what extent the relatively high mortality rate is linked to early outbreaks vs “poor long-term response,” the researchers note.
Alyssa Bilinski, MSc, and lead author Ezekiel J. Emanuel, MD, PhD, chair of the Department of Medical Ethics and Health Policy at the University of Pennsylvania Perelman School of Medicine in Philadelphia, calculated the difference in COVID-19 deaths among countries through Sept. 19, 2020. On this date, the United States reported a total 198,589 COVID-19 deaths.
They calculated that, if the US death rates were similar to those in Australia, the United States would have experienced 187,661 fewer COVID-19 deaths. If similar to those of Canada, there would have been 117,622 fewer deaths in the United States.
The US death rate was lower than six other countries with high COVID-19 mortality in the early spring, including Belgium, Spain, and the United Kingdom. However, after May 10, the per capita mortality rate in the United States exceeded the others.
Between May 10 and Sept. 19, the death rate in Italy was 9.1 per 100,000, vs 36.9 per 100,000.
“After the first peak in early spring, US death rates from COVID-19 and from all causes remained higher than even countries with high COVID-19 mortality,” the researchers note. “This may have been a result of several factors, including weak public health infrastructure and a decentralized, inconsistent US response to the pandemic.”
“Mortifying and motivating”
Woolf and colleagues estimate that more than 225,000 excess deaths occurred in recent months; this represents a 20% increase over expected deaths, note Harvey V. Fineberg, MD, PhD, of the Gordon and Betty Moore Foundation, in an accompanying editorial in JAMA.
“Importantly, a condition such as COVID-19 can contribute both directly and indirectly to excess mortality,” he writes.
Although the direct contribution to the mortality rates by those infected is straightforward, “the indirect contribution may relate to circumstances or choices due to the COVID-19 pandemic: for example, a patient who develops symptoms of a stroke is too concerned about COVID-19 to go to the emergency department, and a potentially reversible condition becomes fatal.”
Fineberg notes that “a general indication of the death toll from COVID-19 and the excess deaths related to the pandemic, as presented by Woolf et al, are sufficiently mortifying and motivating.”
“Profound human toll”
“The importance of the estimate by Woolf et al – which suggests that for the entirety of 2020, more than 400,000 excess deaths will occur – cannot be overstated, because it accounts for what could be declines in some causes of death, like motor vehicle crashes, but increases in others, like myocardial infarction,” write Howard Bauchner, MD, editor in chief of JAMA, and Phil B. Fontanarosa, MD, MBA, executive editor of JAMA, in another accompanying editorial.
“These deaths reflect a true measure of the human cost of the Great Pandemic of 2020,” they add.
The study from Emanuel and Bilinski was notable for calculating the excess COVID-19 and all-cause mortality to Sept. 2020, they note. “After the initial peak in early spring, US death rates from COVID-19 and from all causes remained higher than rates in countries with high COVID-19 mortality.”
“Few people will forget the Great Pandemic of 2020, where and how they lived, how it substantially changed their lives, and for many, the profound human toll it has taken,” Bauchner and Fontanarosa write.
The study by Woolf and colleagues was supported by National Center for Advancing Translational Sciences, the National Institute on Aging, and the National Institute of Allergy and Infectious Diseases. The study by Bilinski and Emanuel was partially funded by the Colton Foundation. Woolf, Emanuel, Fineberg, Bauchner, and Fontanarosa have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
However, additional deaths could be indirectly related because people avoided emergency care during the pandemic, new research shows.
Deaths linked to COVID-19 varied by state and phase of the pandemic, as reported in a study from researchers at Virginia Commonwealth University and Yale University that was published online October 12 in JAMA.
Another study published online simultaneously in JAMA took more of an international perspective. Investigators from the University of Pennsylvania and Harvard University found that in America there were more excess deaths and there was higher all-cause mortality during the pandemic than in 18 other countries.
Although the ongoing number of deaths attributable to COVID-19 continues to garner attention, there can be a lag of weeks or months in how long it takes some public health agencies to update their figures.
“For the public at large, the take-home message is twofold: that the number of deaths caused by the pandemic exceeds publicly reported COVID-19 death counts by 20% and that states that reopened or lifted restrictions early suffered a protracted surge in excess deaths that extended into the summer,” lead author of the US-focused study, Steven H. Woolf, MD, MPH, told Medscape Medical News.
The take-away for physicians is in the bigger picture – it is likely that the COVID-19 pandemic is responsible for deaths from other conditions as well. “Surges in COVID-19 were accompanied by an increase in deaths attributed to other causes, such as heart disease and Alzheimer’s disease and dementia,” said Woolf, director emeritus and senior adviser at the Center on Society and Health and professor in the Department of Family Medicine and Population Health at the Virginia Commonwealth University School of Medicine in Richmond, Virginia.
The investigators identified 225,530 excess US deaths in the 5 months from March to July. They report that 67% were directly attributable to COVID-19.
Deaths linked to COVID-19 included those in which the disease was listed as an underlying or contributing cause. US total death rates are “remarkably consistent” year after year, and the investigators calculated a 20% overall jump in mortality.
The study included data from the National Center for Health Statistics and the US Census Bureau for 48 states and the District of Columbia. Connecticut and North Carolina were excluded because of missing data.
Woolf and colleagues also found statistically higher rates of deaths from two other causes, heart disease and Alzheimer’s disease/dementia.
Altered states
New York, New Jersey, Massachusetts, Louisiana, Arizona, Mississippi, Maryland, Delaware, Rhode Island, and Michigan had the highest per capita excess death rates. Three states experienced the shortest epidemics during the study period: New York, New Jersey, and Massachusetts.
Some lessons could be learned by looking at how individual states managed large numbers of people with COVID-19. “Although we suspected that states that reopened early might have put themselves at risk of a pandemic surge, the consistency with which that occurred and the devastating numbers of deaths they suffered was a surprise,” Woolf said.
“The goal of our study is not to look in the rearview mirror and lament what happened months ago but to learn the lesson going forward: Our country will be unable to take control of this pandemic without more robust efforts to control community spread,” Woolf said. “Our study found that states that did this well, such as New York and New Jersey, experienced large surges but bent the curve and were back to baseline in less than 10 weeks.
“If we could do this as a country, countless lives could be saved.”
A global perspective
The United States experienced high mortality linked to COVID-19, as well as high all-cause mortality, compared with 18 other countries, as reported in the study by University of Pennsylvania and Harvard University researchers.
The United States ranked third, with 72 deaths per 100,000 people, among countries with moderate or high mortality. Although perhaps not surprising given the state of SARS-CoV-2 infection across the United States, a question remains as to what extent the relatively high mortality rate is linked to early outbreaks vs “poor long-term response,” the researchers note.
Alyssa Bilinski, MSc, and lead author Ezekiel J. Emanuel, MD, PhD, chair of the Department of Medical Ethics and Health Policy at the University of Pennsylvania Perelman School of Medicine in Philadelphia, calculated the difference in COVID-19 deaths among countries through Sept. 19, 2020. On this date, the United States reported a total 198,589 COVID-19 deaths.
They calculated that, if the US death rates were similar to those in Australia, the United States would have experienced 187,661 fewer COVID-19 deaths. If similar to those of Canada, there would have been 117,622 fewer deaths in the United States.
The US death rate was lower than six other countries with high COVID-19 mortality in the early spring, including Belgium, Spain, and the United Kingdom. However, after May 10, the per capita mortality rate in the United States exceeded the others.
Between May 10 and Sept. 19, the death rate in Italy was 9.1 per 100,000, vs 36.9 per 100,000.
“After the first peak in early spring, US death rates from COVID-19 and from all causes remained higher than even countries with high COVID-19 mortality,” the researchers note. “This may have been a result of several factors, including weak public health infrastructure and a decentralized, inconsistent US response to the pandemic.”
“Mortifying and motivating”
Woolf and colleagues estimate that more than 225,000 excess deaths occurred in recent months; this represents a 20% increase over expected deaths, note Harvey V. Fineberg, MD, PhD, of the Gordon and Betty Moore Foundation, in an accompanying editorial in JAMA.
“Importantly, a condition such as COVID-19 can contribute both directly and indirectly to excess mortality,” he writes.
Although the direct contribution to the mortality rates by those infected is straightforward, “the indirect contribution may relate to circumstances or choices due to the COVID-19 pandemic: for example, a patient who develops symptoms of a stroke is too concerned about COVID-19 to go to the emergency department, and a potentially reversible condition becomes fatal.”
Fineberg notes that “a general indication of the death toll from COVID-19 and the excess deaths related to the pandemic, as presented by Woolf et al, are sufficiently mortifying and motivating.”
“Profound human toll”
“The importance of the estimate by Woolf et al – which suggests that for the entirety of 2020, more than 400,000 excess deaths will occur – cannot be overstated, because it accounts for what could be declines in some causes of death, like motor vehicle crashes, but increases in others, like myocardial infarction,” write Howard Bauchner, MD, editor in chief of JAMA, and Phil B. Fontanarosa, MD, MBA, executive editor of JAMA, in another accompanying editorial.
“These deaths reflect a true measure of the human cost of the Great Pandemic of 2020,” they add.
The study from Emanuel and Bilinski was notable for calculating the excess COVID-19 and all-cause mortality to Sept. 2020, they note. “After the initial peak in early spring, US death rates from COVID-19 and from all causes remained higher than rates in countries with high COVID-19 mortality.”
“Few people will forget the Great Pandemic of 2020, where and how they lived, how it substantially changed their lives, and for many, the profound human toll it has taken,” Bauchner and Fontanarosa write.
The study by Woolf and colleagues was supported by National Center for Advancing Translational Sciences, the National Institute on Aging, and the National Institute of Allergy and Infectious Diseases. The study by Bilinski and Emanuel was partially funded by the Colton Foundation. Woolf, Emanuel, Fineberg, Bauchner, and Fontanarosa have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
However, additional deaths could be indirectly related because people avoided emergency care during the pandemic, new research shows.
Deaths linked to COVID-19 varied by state and phase of the pandemic, as reported in a study from researchers at Virginia Commonwealth University and Yale University that was published online October 12 in JAMA.
Another study published online simultaneously in JAMA took more of an international perspective. Investigators from the University of Pennsylvania and Harvard University found that in America there were more excess deaths and there was higher all-cause mortality during the pandemic than in 18 other countries.
Although the ongoing number of deaths attributable to COVID-19 continues to garner attention, there can be a lag of weeks or months in how long it takes some public health agencies to update their figures.
“For the public at large, the take-home message is twofold: that the number of deaths caused by the pandemic exceeds publicly reported COVID-19 death counts by 20% and that states that reopened or lifted restrictions early suffered a protracted surge in excess deaths that extended into the summer,” lead author of the US-focused study, Steven H. Woolf, MD, MPH, told Medscape Medical News.
The take-away for physicians is in the bigger picture – it is likely that the COVID-19 pandemic is responsible for deaths from other conditions as well. “Surges in COVID-19 were accompanied by an increase in deaths attributed to other causes, such as heart disease and Alzheimer’s disease and dementia,” said Woolf, director emeritus and senior adviser at the Center on Society and Health and professor in the Department of Family Medicine and Population Health at the Virginia Commonwealth University School of Medicine in Richmond, Virginia.
The investigators identified 225,530 excess US deaths in the 5 months from March to July. They report that 67% were directly attributable to COVID-19.
Deaths linked to COVID-19 included those in which the disease was listed as an underlying or contributing cause. US total death rates are “remarkably consistent” year after year, and the investigators calculated a 20% overall jump in mortality.
The study included data from the National Center for Health Statistics and the US Census Bureau for 48 states and the District of Columbia. Connecticut and North Carolina were excluded because of missing data.
Woolf and colleagues also found statistically higher rates of deaths from two other causes, heart disease and Alzheimer’s disease/dementia.
Altered states
New York, New Jersey, Massachusetts, Louisiana, Arizona, Mississippi, Maryland, Delaware, Rhode Island, and Michigan had the highest per capita excess death rates. Three states experienced the shortest epidemics during the study period: New York, New Jersey, and Massachusetts.
Some lessons could be learned by looking at how individual states managed large numbers of people with COVID-19. “Although we suspected that states that reopened early might have put themselves at risk of a pandemic surge, the consistency with which that occurred and the devastating numbers of deaths they suffered was a surprise,” Woolf said.
“The goal of our study is not to look in the rearview mirror and lament what happened months ago but to learn the lesson going forward: Our country will be unable to take control of this pandemic without more robust efforts to control community spread,” Woolf said. “Our study found that states that did this well, such as New York and New Jersey, experienced large surges but bent the curve and were back to baseline in less than 10 weeks.
“If we could do this as a country, countless lives could be saved.”
A global perspective
The United States experienced high mortality linked to COVID-19, as well as high all-cause mortality, compared with 18 other countries, as reported in the study by University of Pennsylvania and Harvard University researchers.
The United States ranked third, with 72 deaths per 100,000 people, among countries with moderate or high mortality. Although perhaps not surprising given the state of SARS-CoV-2 infection across the United States, a question remains as to what extent the relatively high mortality rate is linked to early outbreaks vs “poor long-term response,” the researchers note.
Alyssa Bilinski, MSc, and lead author Ezekiel J. Emanuel, MD, PhD, chair of the Department of Medical Ethics and Health Policy at the University of Pennsylvania Perelman School of Medicine in Philadelphia, calculated the difference in COVID-19 deaths among countries through Sept. 19, 2020. On this date, the United States reported a total 198,589 COVID-19 deaths.
They calculated that, if the US death rates were similar to those in Australia, the United States would have experienced 187,661 fewer COVID-19 deaths. If similar to those of Canada, there would have been 117,622 fewer deaths in the United States.
The US death rate was lower than six other countries with high COVID-19 mortality in the early spring, including Belgium, Spain, and the United Kingdom. However, after May 10, the per capita mortality rate in the United States exceeded the others.
Between May 10 and Sept. 19, the death rate in Italy was 9.1 per 100,000, vs 36.9 per 100,000.
“After the first peak in early spring, US death rates from COVID-19 and from all causes remained higher than even countries with high COVID-19 mortality,” the researchers note. “This may have been a result of several factors, including weak public health infrastructure and a decentralized, inconsistent US response to the pandemic.”
“Mortifying and motivating”
Woolf and colleagues estimate that more than 225,000 excess deaths occurred in recent months; this represents a 20% increase over expected deaths, note Harvey V. Fineberg, MD, PhD, of the Gordon and Betty Moore Foundation, in an accompanying editorial in JAMA.
“Importantly, a condition such as COVID-19 can contribute both directly and indirectly to excess mortality,” he writes.
Although the direct contribution to the mortality rates by those infected is straightforward, “the indirect contribution may relate to circumstances or choices due to the COVID-19 pandemic: for example, a patient who develops symptoms of a stroke is too concerned about COVID-19 to go to the emergency department, and a potentially reversible condition becomes fatal.”
Fineberg notes that “a general indication of the death toll from COVID-19 and the excess deaths related to the pandemic, as presented by Woolf et al, are sufficiently mortifying and motivating.”
“Profound human toll”
“The importance of the estimate by Woolf et al – which suggests that for the entirety of 2020, more than 400,000 excess deaths will occur – cannot be overstated, because it accounts for what could be declines in some causes of death, like motor vehicle crashes, but increases in others, like myocardial infarction,” write Howard Bauchner, MD, editor in chief of JAMA, and Phil B. Fontanarosa, MD, MBA, executive editor of JAMA, in another accompanying editorial.
“These deaths reflect a true measure of the human cost of the Great Pandemic of 2020,” they add.
The study from Emanuel and Bilinski was notable for calculating the excess COVID-19 and all-cause mortality to Sept. 2020, they note. “After the initial peak in early spring, US death rates from COVID-19 and from all causes remained higher than rates in countries with high COVID-19 mortality.”
“Few people will forget the Great Pandemic of 2020, where and how they lived, how it substantially changed their lives, and for many, the profound human toll it has taken,” Bauchner and Fontanarosa write.
The study by Woolf and colleagues was supported by National Center for Advancing Translational Sciences, the National Institute on Aging, and the National Institute of Allergy and Infectious Diseases. The study by Bilinski and Emanuel was partially funded by the Colton Foundation. Woolf, Emanuel, Fineberg, Bauchner, and Fontanarosa have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
Ruling out PE in pregnancy
ILLUSTRATIVE CASE
A 28-year-old G2P1001 at 28 weeks’ gestation presents to your clinic with 1 day of dyspnea and palpitations. Her pregnancy has been otherwise uncomplicated. She reports worsening dyspnea with mild exertion but denies other symptoms, including leg swelling.
The current incidence of venous thromboembolism (VTE) in pregnant women is estimated to be a relatively low 5 to 12 events per 10,000 pregnancies, yet the condition is the leading cause of maternal mortality in developed countries.2,3,4 Currently, there are conflicting recommendations among relevant organization guidelines regarding the use of D-dimer testing to aid in the diagnosis of pulmonary embolism (PE) during pregnancy. Both the Working Group in Women’s Health of the Society of Thrombosis and Haemostasis (GTH) and the European Society of Cardiology (ESC) recommend using D-dimer testing to rule out PE in pregnant women (ESC Class IIa, level of evidence B based on small studies, retrospective studies, and observational studies; GTH provides no grade).5,6
Conversely, the Royal College of Obstetricians and Gynaecologists (RCOG), the Society of Obstetricians and Gynaecologists of Canada (SOGC), and the American Thoracic Society (ATS)/Society of Thoracic Radiology recommend against the use of D-dimer testing in pregnant women because pregnant women were excluded from D-dimer validation studies (RCOG and SOGC Grade D; ATS weak recommendation).4,7,8 The American College of Obstetricians and Gynecologists does not have specific recommendations regarding the use of D-dimer testing during pregnancy, but has endorsed the ATS guidelines.4,9 In addition, SOGC recommends against the use of clinical prediction scores (Grade D), and RCOG states that there is no evidence to support their use (Grade C).7,8 The remaining societies do not make a recommendation for or against the use of clinical prediction scores because of the absence of high-quality evidence regarding their use in the pregnant patient population.4,5,6
STUDY SUMMARY
Prospective validation of a strategy to diagnose PE in pregnant women
This multicenter, multinational, prospective diagnostic study involving 395 pregnant women evaluated the accuracy of PE diagnosis across 11 centers in France and Switzerland from August 2008 through July 2016.1 Patients with clinically suspected PE were evaluated in emergency departments. Patients were tested according to a diagnostic algorithm that included pretest clinical probability using the revised Geneva Score for Pulmonary Embolism (www.mdcalc.com/geneva-score-revised-pulmonary-embolism), a clinical prediction tool that uses patient history, presenting symptoms, and clinical signs to classify patients as being at low (0-3/25), intermediate (4-10/25), or high (≥ 11/25) risk;10 high-sensitivity D-dimer testing; bilateral lower limb compression ultrasonography (CUS); computed tomography pulmonary angiography (CTPA); and a ventilation-perfusion (V/Q) scan.
PE was excluded in patients who had a low or intermediate pretest clinical probability score and a negative D-dimer test result (< 500 mcg/L). Patients with a high pretest probability score or positive D-dimer test result underwent CUS, and, if negative, subsequent CTPA. A V/Q scan was performed if the CTPA was inconclusive. If the work-up was negative, PE was excluded.
Untreated pregnant women had clinical follow-up at 3 months. Any cases of suspected VTE were evaluated by a 3-member independent adjudication committee blinded to the initial diagnostic work-up. The primary outcome was the rate of adjudicated VTE events during the 3-month follow-up period. PE was diagnosed in 28 patients (7.1%) and excluded in 367 (clinical probability score and negative D-dimer test result [n = 46], negative CTPA result [n = 290], normal or low-probability V/Q scan [n = 17], and other reason [n = 14]). Twenty-two women received anticoagulation during the follow-up period for other reasons (mainly history of previous VTE disease). No symptomatic VTE events occurred in any of the women after the diagnostic work-up was negative, including among those patients who were ruled out with only the clinical prediction tool and a negative D-dimer test result (rate 0.0%; 95% confidence interval [CI], 0.0%-1%).
WHAT’S NEW
Clinical probability and D-dimer rule out PE in pregnant women
This study ruled out PE in patients with low/intermediate risk as determined by the revised Geneva score and a D-dimer test, enabling patients to avoid further diagnostic testing. This low-cost strategy can be applied easily to the pregnant population.
CAVEATS
Additional research is still needed
From the results of this study, 11.6% of patients (n = 46) had a PE ruled out utilizing the revised Geneva score in conjunction with a D-dimer test result, with avoidance of chest imaging. However, this study was powered for the entire treatment algorithm and was not specifically powered for patients with low- or intermediate-risk pretest probability scores. Since this is the first published prospective diagnostic study of VTE in pregnancy, further research is needed to confirm the findings that a clinical prediction tool and a negative D-dimer test result can safely rule out PE in pregnant women.
In addition, further research is needed to determine pregnancy-adapted D-dimer cut-off values, as the researchers of this study noted that < 500 mcg/L was useful in the first and second trimester, but that levels increased as gestational age increased.
CHALLENGES TO IMPLEMENTATION
None to speak of
Implementing a diagnostic algorithm that incorporates sequential assessment of pretest clinical probability based on the revised Geneva score and a D-dimer measurement should be relatively easy to implement, as both methods are readily available and relatively inexpensive.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
1. Righini M, Robert-Ebadi H, Elias A, et al. Diagnosis of pulmonary embolism during pregnancy. A multicenter prospective management outcome study. Ann Intern Med. 2018;169:766-773.
2. Knight M, Kenyon S, Brocklehurst P, et al. Saving lives, improving mothers’ care: lessons learned to inform future maternity care from the UK and Ireland confidential enquiries into maternal deaths and morbidity 2009-2012. Oxford: National Perinatal Epidemiology Unit, University of Oxford; 2014.
3. Bourjeily G, Paidas M, Khalil H, et al. Pulmonary embolism in pregnancy. Lancet. 2010;375:500-512.
4. Leung AN, Bull TM, Jaeschke R, et al. An official American Thoracic Society/Society of Thoracic Radiology clinical practice guideline: evaluation of suspected pulmonary embolism in pregnancy. Am J Resp Crit Care Med. 2011;184:1200-1208.
5. Konstantinides SV, Meyer G, Becattini C, et al. 2019 ESC Guidelines for the diagnosis and management of acute pulmonary embolism developed in collaboration with the European Respiratory Society (ERS). Eur Heart J. 2020;41:543-603.
6. Linnemann B, Bauersachs R, Rott H, et al. Working Group in Women’s Health of the Society of Thrombosis and Haemostasis. Diagnosis of pregnancy-associated venous thromboembolism-position paper of the Working Group in Women’s Health of the Society of Thrombosis and Haemostasis (GTH). Vasa. 2016;45:87-101.
7. Royal College of Obstetricians & Gynaecologists. Thromboembolic disease in pregnancy and the puerperium: acute management. Green‐top Guideline No. 37b. April 2015.
8. Chan WS, Rey E, Kent NE, et al. Venous thromboembolism and antithrombotic therapy in pregnancy. J Obstet Gynaecol Can. 2014;36:527-553.
9. James A, Birsner M, Kaimal A, American College of Obstetricians and Gynecologists’ Committee on Practice Bulletins‐Obstetrics. ACOG Practice Bulletin No. 196: thromboembolism in pregnancy. Obstet Gynecol. 2018;132:e1-e17.
10. Le Gal G, Righini M, Roy PM, et al. Prediction of pulmonary embolism in the emergency department: the revised Geneva score. Ann Intern Med. 2006;144:165-171.
ILLUSTRATIVE CASE
A 28-year-old G2P1001 at 28 weeks’ gestation presents to your clinic with 1 day of dyspnea and palpitations. Her pregnancy has been otherwise uncomplicated. She reports worsening dyspnea with mild exertion but denies other symptoms, including leg swelling.
The current incidence of venous thromboembolism (VTE) in pregnant women is estimated to be a relatively low 5 to 12 events per 10,000 pregnancies, yet the condition is the leading cause of maternal mortality in developed countries.2,3,4 Currently, there are conflicting recommendations among relevant organization guidelines regarding the use of D-dimer testing to aid in the diagnosis of pulmonary embolism (PE) during pregnancy. Both the Working Group in Women’s Health of the Society of Thrombosis and Haemostasis (GTH) and the European Society of Cardiology (ESC) recommend using D-dimer testing to rule out PE in pregnant women (ESC Class IIa, level of evidence B based on small studies, retrospective studies, and observational studies; GTH provides no grade).5,6
Conversely, the Royal College of Obstetricians and Gynaecologists (RCOG), the Society of Obstetricians and Gynaecologists of Canada (SOGC), and the American Thoracic Society (ATS)/Society of Thoracic Radiology recommend against the use of D-dimer testing in pregnant women because pregnant women were excluded from D-dimer validation studies (RCOG and SOGC Grade D; ATS weak recommendation).4,7,8 The American College of Obstetricians and Gynecologists does not have specific recommendations regarding the use of D-dimer testing during pregnancy, but has endorsed the ATS guidelines.4,9 In addition, SOGC recommends against the use of clinical prediction scores (Grade D), and RCOG states that there is no evidence to support their use (Grade C).7,8 The remaining societies do not make a recommendation for or against the use of clinical prediction scores because of the absence of high-quality evidence regarding their use in the pregnant patient population.4,5,6
STUDY SUMMARY
Prospective validation of a strategy to diagnose PE in pregnant women
This multicenter, multinational, prospective diagnostic study involving 395 pregnant women evaluated the accuracy of PE diagnosis across 11 centers in France and Switzerland from August 2008 through July 2016.1 Patients with clinically suspected PE were evaluated in emergency departments. Patients were tested according to a diagnostic algorithm that included pretest clinical probability using the revised Geneva Score for Pulmonary Embolism (www.mdcalc.com/geneva-score-revised-pulmonary-embolism), a clinical prediction tool that uses patient history, presenting symptoms, and clinical signs to classify patients as being at low (0-3/25), intermediate (4-10/25), or high (≥ 11/25) risk;10 high-sensitivity D-dimer testing; bilateral lower limb compression ultrasonography (CUS); computed tomography pulmonary angiography (CTPA); and a ventilation-perfusion (V/Q) scan.
PE was excluded in patients who had a low or intermediate pretest clinical probability score and a negative D-dimer test result (< 500 mcg/L). Patients with a high pretest probability score or positive D-dimer test result underwent CUS, and, if negative, subsequent CTPA. A V/Q scan was performed if the CTPA was inconclusive. If the work-up was negative, PE was excluded.
Untreated pregnant women had clinical follow-up at 3 months. Any cases of suspected VTE were evaluated by a 3-member independent adjudication committee blinded to the initial diagnostic work-up. The primary outcome was the rate of adjudicated VTE events during the 3-month follow-up period. PE was diagnosed in 28 patients (7.1%) and excluded in 367 (clinical probability score and negative D-dimer test result [n = 46], negative CTPA result [n = 290], normal or low-probability V/Q scan [n = 17], and other reason [n = 14]). Twenty-two women received anticoagulation during the follow-up period for other reasons (mainly history of previous VTE disease). No symptomatic VTE events occurred in any of the women after the diagnostic work-up was negative, including among those patients who were ruled out with only the clinical prediction tool and a negative D-dimer test result (rate 0.0%; 95% confidence interval [CI], 0.0%-1%).
WHAT’S NEW
Clinical probability and D-dimer rule out PE in pregnant women
This study ruled out PE in patients with low/intermediate risk as determined by the revised Geneva score and a D-dimer test, enabling patients to avoid further diagnostic testing. This low-cost strategy can be applied easily to the pregnant population.
CAVEATS
Additional research is still needed
From the results of this study, 11.6% of patients (n = 46) had a PE ruled out utilizing the revised Geneva score in conjunction with a D-dimer test result, with avoidance of chest imaging. However, this study was powered for the entire treatment algorithm and was not specifically powered for patients with low- or intermediate-risk pretest probability scores. Since this is the first published prospective diagnostic study of VTE in pregnancy, further research is needed to confirm the findings that a clinical prediction tool and a negative D-dimer test result can safely rule out PE in pregnant women.
In addition, further research is needed to determine pregnancy-adapted D-dimer cut-off values, as the researchers of this study noted that < 500 mcg/L was useful in the first and second trimester, but that levels increased as gestational age increased.
CHALLENGES TO IMPLEMENTATION
None to speak of
Implementing a diagnostic algorithm that incorporates sequential assessment of pretest clinical probability based on the revised Geneva score and a D-dimer measurement should be relatively easy to implement, as both methods are readily available and relatively inexpensive.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
ILLUSTRATIVE CASE
A 28-year-old G2P1001 at 28 weeks’ gestation presents to your clinic with 1 day of dyspnea and palpitations. Her pregnancy has been otherwise uncomplicated. She reports worsening dyspnea with mild exertion but denies other symptoms, including leg swelling.
The current incidence of venous thromboembolism (VTE) in pregnant women is estimated to be a relatively low 5 to 12 events per 10,000 pregnancies, yet the condition is the leading cause of maternal mortality in developed countries.2,3,4 Currently, there are conflicting recommendations among relevant organization guidelines regarding the use of D-dimer testing to aid in the diagnosis of pulmonary embolism (PE) during pregnancy. Both the Working Group in Women’s Health of the Society of Thrombosis and Haemostasis (GTH) and the European Society of Cardiology (ESC) recommend using D-dimer testing to rule out PE in pregnant women (ESC Class IIa, level of evidence B based on small studies, retrospective studies, and observational studies; GTH provides no grade).5,6
Conversely, the Royal College of Obstetricians and Gynaecologists (RCOG), the Society of Obstetricians and Gynaecologists of Canada (SOGC), and the American Thoracic Society (ATS)/Society of Thoracic Radiology recommend against the use of D-dimer testing in pregnant women because pregnant women were excluded from D-dimer validation studies (RCOG and SOGC Grade D; ATS weak recommendation).4,7,8 The American College of Obstetricians and Gynecologists does not have specific recommendations regarding the use of D-dimer testing during pregnancy, but has endorsed the ATS guidelines.4,9 In addition, SOGC recommends against the use of clinical prediction scores (Grade D), and RCOG states that there is no evidence to support their use (Grade C).7,8 The remaining societies do not make a recommendation for or against the use of clinical prediction scores because of the absence of high-quality evidence regarding their use in the pregnant patient population.4,5,6
STUDY SUMMARY
Prospective validation of a strategy to diagnose PE in pregnant women
This multicenter, multinational, prospective diagnostic study involving 395 pregnant women evaluated the accuracy of PE diagnosis across 11 centers in France and Switzerland from August 2008 through July 2016.1 Patients with clinically suspected PE were evaluated in emergency departments. Patients were tested according to a diagnostic algorithm that included pretest clinical probability using the revised Geneva Score for Pulmonary Embolism (www.mdcalc.com/geneva-score-revised-pulmonary-embolism), a clinical prediction tool that uses patient history, presenting symptoms, and clinical signs to classify patients as being at low (0-3/25), intermediate (4-10/25), or high (≥ 11/25) risk;10 high-sensitivity D-dimer testing; bilateral lower limb compression ultrasonography (CUS); computed tomography pulmonary angiography (CTPA); and a ventilation-perfusion (V/Q) scan.
PE was excluded in patients who had a low or intermediate pretest clinical probability score and a negative D-dimer test result (< 500 mcg/L). Patients with a high pretest probability score or positive D-dimer test result underwent CUS, and, if negative, subsequent CTPA. A V/Q scan was performed if the CTPA was inconclusive. If the work-up was negative, PE was excluded.
Untreated pregnant women had clinical follow-up at 3 months. Any cases of suspected VTE were evaluated by a 3-member independent adjudication committee blinded to the initial diagnostic work-up. The primary outcome was the rate of adjudicated VTE events during the 3-month follow-up period. PE was diagnosed in 28 patients (7.1%) and excluded in 367 (clinical probability score and negative D-dimer test result [n = 46], negative CTPA result [n = 290], normal or low-probability V/Q scan [n = 17], and other reason [n = 14]). Twenty-two women received anticoagulation during the follow-up period for other reasons (mainly history of previous VTE disease). No symptomatic VTE events occurred in any of the women after the diagnostic work-up was negative, including among those patients who were ruled out with only the clinical prediction tool and a negative D-dimer test result (rate 0.0%; 95% confidence interval [CI], 0.0%-1%).
WHAT’S NEW
Clinical probability and D-dimer rule out PE in pregnant women
This study ruled out PE in patients with low/intermediate risk as determined by the revised Geneva score and a D-dimer test, enabling patients to avoid further diagnostic testing. This low-cost strategy can be applied easily to the pregnant population.
CAVEATS
Additional research is still needed
From the results of this study, 11.6% of patients (n = 46) had a PE ruled out utilizing the revised Geneva score in conjunction with a D-dimer test result, with avoidance of chest imaging. However, this study was powered for the entire treatment algorithm and was not specifically powered for patients with low- or intermediate-risk pretest probability scores. Since this is the first published prospective diagnostic study of VTE in pregnancy, further research is needed to confirm the findings that a clinical prediction tool and a negative D-dimer test result can safely rule out PE in pregnant women.
In addition, further research is needed to determine pregnancy-adapted D-dimer cut-off values, as the researchers of this study noted that < 500 mcg/L was useful in the first and second trimester, but that levels increased as gestational age increased.
CHALLENGES TO IMPLEMENTATION
None to speak of
Implementing a diagnostic algorithm that incorporates sequential assessment of pretest clinical probability based on the revised Geneva score and a D-dimer measurement should be relatively easy to implement, as both methods are readily available and relatively inexpensive.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
1. Righini M, Robert-Ebadi H, Elias A, et al. Diagnosis of pulmonary embolism during pregnancy. A multicenter prospective management outcome study. Ann Intern Med. 2018;169:766-773.
2. Knight M, Kenyon S, Brocklehurst P, et al. Saving lives, improving mothers’ care: lessons learned to inform future maternity care from the UK and Ireland confidential enquiries into maternal deaths and morbidity 2009-2012. Oxford: National Perinatal Epidemiology Unit, University of Oxford; 2014.
3. Bourjeily G, Paidas M, Khalil H, et al. Pulmonary embolism in pregnancy. Lancet. 2010;375:500-512.
4. Leung AN, Bull TM, Jaeschke R, et al. An official American Thoracic Society/Society of Thoracic Radiology clinical practice guideline: evaluation of suspected pulmonary embolism in pregnancy. Am J Resp Crit Care Med. 2011;184:1200-1208.
5. Konstantinides SV, Meyer G, Becattini C, et al. 2019 ESC Guidelines for the diagnosis and management of acute pulmonary embolism developed in collaboration with the European Respiratory Society (ERS). Eur Heart J. 2020;41:543-603.
6. Linnemann B, Bauersachs R, Rott H, et al. Working Group in Women’s Health of the Society of Thrombosis and Haemostasis. Diagnosis of pregnancy-associated venous thromboembolism-position paper of the Working Group in Women’s Health of the Society of Thrombosis and Haemostasis (GTH). Vasa. 2016;45:87-101.
7. Royal College of Obstetricians & Gynaecologists. Thromboembolic disease in pregnancy and the puerperium: acute management. Green‐top Guideline No. 37b. April 2015.
8. Chan WS, Rey E, Kent NE, et al. Venous thromboembolism and antithrombotic therapy in pregnancy. J Obstet Gynaecol Can. 2014;36:527-553.
9. James A, Birsner M, Kaimal A, American College of Obstetricians and Gynecologists’ Committee on Practice Bulletins‐Obstetrics. ACOG Practice Bulletin No. 196: thromboembolism in pregnancy. Obstet Gynecol. 2018;132:e1-e17.
10. Le Gal G, Righini M, Roy PM, et al. Prediction of pulmonary embolism in the emergency department: the revised Geneva score. Ann Intern Med. 2006;144:165-171.
1. Righini M, Robert-Ebadi H, Elias A, et al. Diagnosis of pulmonary embolism during pregnancy. A multicenter prospective management outcome study. Ann Intern Med. 2018;169:766-773.
2. Knight M, Kenyon S, Brocklehurst P, et al. Saving lives, improving mothers’ care: lessons learned to inform future maternity care from the UK and Ireland confidential enquiries into maternal deaths and morbidity 2009-2012. Oxford: National Perinatal Epidemiology Unit, University of Oxford; 2014.
3. Bourjeily G, Paidas M, Khalil H, et al. Pulmonary embolism in pregnancy. Lancet. 2010;375:500-512.
4. Leung AN, Bull TM, Jaeschke R, et al. An official American Thoracic Society/Society of Thoracic Radiology clinical practice guideline: evaluation of suspected pulmonary embolism in pregnancy. Am J Resp Crit Care Med. 2011;184:1200-1208.
5. Konstantinides SV, Meyer G, Becattini C, et al. 2019 ESC Guidelines for the diagnosis and management of acute pulmonary embolism developed in collaboration with the European Respiratory Society (ERS). Eur Heart J. 2020;41:543-603.
6. Linnemann B, Bauersachs R, Rott H, et al. Working Group in Women’s Health of the Society of Thrombosis and Haemostasis. Diagnosis of pregnancy-associated venous thromboembolism-position paper of the Working Group in Women’s Health of the Society of Thrombosis and Haemostasis (GTH). Vasa. 2016;45:87-101.
7. Royal College of Obstetricians & Gynaecologists. Thromboembolic disease in pregnancy and the puerperium: acute management. Green‐top Guideline No. 37b. April 2015.
8. Chan WS, Rey E, Kent NE, et al. Venous thromboembolism and antithrombotic therapy in pregnancy. J Obstet Gynaecol Can. 2014;36:527-553.
9. James A, Birsner M, Kaimal A, American College of Obstetricians and Gynecologists’ Committee on Practice Bulletins‐Obstetrics. ACOG Practice Bulletin No. 196: thromboembolism in pregnancy. Obstet Gynecol. 2018;132:e1-e17.
10. Le Gal G, Righini M, Roy PM, et al. Prediction of pulmonary embolism in the emergency department: the revised Geneva score. Ann Intern Med. 2006;144:165-171.
PRACTICE CHANGER
Use a clinical probability score to identify patients at low or intermediate risk for pulmonary embolism (PE) and combine that with a high-sensitivity D-dimer test to rule out PE in pregnant women.
STRENGTH OF RECOMMENDATION
B: Prospective diagnostic management outcome study.1
Righini M, Robert-Ebadi H, Elias A, et al. Diagnosis of pulmonary embolism during pregnancy: a multicenter prospective management outcome study. Ann Intern Med. 2018;169:766-773.1
Fourteen-day sports hiatus recommended for children after COVID-19
Children should not return to sports for 14 days after exposure to COVID-19, and those with moderate symptoms should undergo an electrocardiogram before returning, according to the American Academy of Pediatrics.
said Susannah Briskin, MD, a pediatric sports medicine specialist at Rainbow Babies and Children’s Hospital in Cleveland.
“There has been emerging evidence about cases of myocarditis occurring in athletes, including athletes who are asymptomatic with COVID-19,” she said in an interview.
The update aligns the AAP recommendations with those from the American College of Cardiologists, she added.
Recent imaging studies have turned up signs of myocarditis in athletes recovering from mild or asymptomatic cases of COVID-19 and have prompted calls for clearer guidelines about imaging studies and return to play.
Viral myocarditis poses a risk to athletes because it can lead to potentially fatal arrhythmias, Dr. Briskin said.
Although children benefit from participating in sports, these activities also put them at risk of contracting COVID-19 and spreading it to others, the guidance noted.
To balance the risks and benefits, the academy proposed guidelines that vary depending on the severity of the presentation.
In the first category are patients with a severe presentation (hypotension, arrhythmias, need for intubation or extracorporeal membrane oxygenation support, kidney or cardiac failure) or with multisystem inflammatory syndrome. Clinicians should treat these patients as though they have myocarditis. Patients should be restricted from engaging in sports and other exercise for 3-6 months, the guidance stated.
The primary care physician and “appropriate pediatric medical subspecialist, preferably in consultation with a pediatric cardiologist,” should clear them before they return to activities. In examining patients for return to play, clinicians should focus on cardiac symptoms, including chest pain, shortness of breath, fatigue, palpitations, or syncope, the guidance said.
In another category are patients with cardiac symptoms, those with concerning findings on examination, and those with moderate symptoms of COVID-19, including prolonged fever. These patients should undergo an ECG and possibly be referred to a pediatric cardiologist, the guidelines said. These symptoms must be absent for at least 14 days before these patients can return to sports, and the athletes should obtain clearance from their primary care physicians before they resume.
In a third category are patients who have been infected with SARS-CoV-2 or who have had close contact with someone who was infected but who have not themselves experienced symptoms. These athletes should refrain from sports for at least 14 days, the guidelines said.
Children who don’t fall into any of these categories should not be tested for the virus or antibodies to it before participation in sports, the academy said.
The guidelines don’t vary depending on the sport. But the academy has issued separate guidance for parents and guardians to help them evaluate the risk for COVID-19 transmission by sport.
Athletes participating in “sports that have greater amount of contact time or proximity to people would be at higher risk for contracting COVID-19,” Dr. Briskin said. “But I think that’s all fairly common sense, given the recommendations for non–sport-related activity just in terms of social distancing and masking.”
The new guidance called on sports organizers to minimize contact by, for example, modifying drills and conditioning. It recommended that athletes wear masks except during vigorous exercise or when participating in water sports, as well as in other circumstances in which the mask could become a safety hazard.
They also recommended using handwashing stations or hand sanitizer, avoiding contact with shared surfaces, and avoiding small rooms and areas with poor ventilation.
Dr. Briskin disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Children should not return to sports for 14 days after exposure to COVID-19, and those with moderate symptoms should undergo an electrocardiogram before returning, according to the American Academy of Pediatrics.
said Susannah Briskin, MD, a pediatric sports medicine specialist at Rainbow Babies and Children’s Hospital in Cleveland.
“There has been emerging evidence about cases of myocarditis occurring in athletes, including athletes who are asymptomatic with COVID-19,” she said in an interview.
The update aligns the AAP recommendations with those from the American College of Cardiologists, she added.
Recent imaging studies have turned up signs of myocarditis in athletes recovering from mild or asymptomatic cases of COVID-19 and have prompted calls for clearer guidelines about imaging studies and return to play.
Viral myocarditis poses a risk to athletes because it can lead to potentially fatal arrhythmias, Dr. Briskin said.
Although children benefit from participating in sports, these activities also put them at risk of contracting COVID-19 and spreading it to others, the guidance noted.
To balance the risks and benefits, the academy proposed guidelines that vary depending on the severity of the presentation.
In the first category are patients with a severe presentation (hypotension, arrhythmias, need for intubation or extracorporeal membrane oxygenation support, kidney or cardiac failure) or with multisystem inflammatory syndrome. Clinicians should treat these patients as though they have myocarditis. Patients should be restricted from engaging in sports and other exercise for 3-6 months, the guidance stated.
The primary care physician and “appropriate pediatric medical subspecialist, preferably in consultation with a pediatric cardiologist,” should clear them before they return to activities. In examining patients for return to play, clinicians should focus on cardiac symptoms, including chest pain, shortness of breath, fatigue, palpitations, or syncope, the guidance said.
In another category are patients with cardiac symptoms, those with concerning findings on examination, and those with moderate symptoms of COVID-19, including prolonged fever. These patients should undergo an ECG and possibly be referred to a pediatric cardiologist, the guidelines said. These symptoms must be absent for at least 14 days before these patients can return to sports, and the athletes should obtain clearance from their primary care physicians before they resume.
In a third category are patients who have been infected with SARS-CoV-2 or who have had close contact with someone who was infected but who have not themselves experienced symptoms. These athletes should refrain from sports for at least 14 days, the guidelines said.
Children who don’t fall into any of these categories should not be tested for the virus or antibodies to it before participation in sports, the academy said.
The guidelines don’t vary depending on the sport. But the academy has issued separate guidance for parents and guardians to help them evaluate the risk for COVID-19 transmission by sport.
Athletes participating in “sports that have greater amount of contact time or proximity to people would be at higher risk for contracting COVID-19,” Dr. Briskin said. “But I think that’s all fairly common sense, given the recommendations for non–sport-related activity just in terms of social distancing and masking.”
The new guidance called on sports organizers to minimize contact by, for example, modifying drills and conditioning. It recommended that athletes wear masks except during vigorous exercise or when participating in water sports, as well as in other circumstances in which the mask could become a safety hazard.
They also recommended using handwashing stations or hand sanitizer, avoiding contact with shared surfaces, and avoiding small rooms and areas with poor ventilation.
Dr. Briskin disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Children should not return to sports for 14 days after exposure to COVID-19, and those with moderate symptoms should undergo an electrocardiogram before returning, according to the American Academy of Pediatrics.
said Susannah Briskin, MD, a pediatric sports medicine specialist at Rainbow Babies and Children’s Hospital in Cleveland.
“There has been emerging evidence about cases of myocarditis occurring in athletes, including athletes who are asymptomatic with COVID-19,” she said in an interview.
The update aligns the AAP recommendations with those from the American College of Cardiologists, she added.
Recent imaging studies have turned up signs of myocarditis in athletes recovering from mild or asymptomatic cases of COVID-19 and have prompted calls for clearer guidelines about imaging studies and return to play.
Viral myocarditis poses a risk to athletes because it can lead to potentially fatal arrhythmias, Dr. Briskin said.
Although children benefit from participating in sports, these activities also put them at risk of contracting COVID-19 and spreading it to others, the guidance noted.
To balance the risks and benefits, the academy proposed guidelines that vary depending on the severity of the presentation.
In the first category are patients with a severe presentation (hypotension, arrhythmias, need for intubation or extracorporeal membrane oxygenation support, kidney or cardiac failure) or with multisystem inflammatory syndrome. Clinicians should treat these patients as though they have myocarditis. Patients should be restricted from engaging in sports and other exercise for 3-6 months, the guidance stated.
The primary care physician and “appropriate pediatric medical subspecialist, preferably in consultation with a pediatric cardiologist,” should clear them before they return to activities. In examining patients for return to play, clinicians should focus on cardiac symptoms, including chest pain, shortness of breath, fatigue, palpitations, or syncope, the guidance said.
In another category are patients with cardiac symptoms, those with concerning findings on examination, and those with moderate symptoms of COVID-19, including prolonged fever. These patients should undergo an ECG and possibly be referred to a pediatric cardiologist, the guidelines said. These symptoms must be absent for at least 14 days before these patients can return to sports, and the athletes should obtain clearance from their primary care physicians before they resume.
In a third category are patients who have been infected with SARS-CoV-2 or who have had close contact with someone who was infected but who have not themselves experienced symptoms. These athletes should refrain from sports for at least 14 days, the guidelines said.
Children who don’t fall into any of these categories should not be tested for the virus or antibodies to it before participation in sports, the academy said.
The guidelines don’t vary depending on the sport. But the academy has issued separate guidance for parents and guardians to help them evaluate the risk for COVID-19 transmission by sport.
Athletes participating in “sports that have greater amount of contact time or proximity to people would be at higher risk for contracting COVID-19,” Dr. Briskin said. “But I think that’s all fairly common sense, given the recommendations for non–sport-related activity just in terms of social distancing and masking.”
The new guidance called on sports organizers to minimize contact by, for example, modifying drills and conditioning. It recommended that athletes wear masks except during vigorous exercise or when participating in water sports, as well as in other circumstances in which the mask could become a safety hazard.
They also recommended using handwashing stations or hand sanitizer, avoiding contact with shared surfaces, and avoiding small rooms and areas with poor ventilation.
Dr. Briskin disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Remdesivir effective, well-tolerated in final trial report
Drug beats placebo across multiple endpoints in COVID-19 patients
In May 2020, remdesivir received Food and Drug Administration approval for emergency treatment of severe COVID-19 on the basis of a preliminary report on this trial. In August 2020, the FDA expanded the indication to include all hospitalized adult and pediatric patients with suspected or laboratory-confirmed COVID-19 infection irrespective of severity.
“Our findings were consistent with the findings of the preliminary report: a 10-day course of remdesivir was superior to placebo in the treatment of hospitalized patients with COVID-19,” reported a team of investigators led by John H. Beigel, MD, of the Division of Microbiology and Infectious Diseases at the National Institute of Allergy and Infectious Diseases, in the New England Journal of Medicine.
The drug’s broadened indication was not based on the ACTT-1 trial, according to Dr. Beigel. “Other data have demonstrated that remdesivir shortens recovery in patients with lower acuity. In our study, evidence of pneumonia was an enrollment requirement,” he explained in an interview.
In the newly published final ACTT-1 data, the median time to recovery was 10 days for those on active therapy versus 15 days for those randomized to placebo. With a rate ratio of 1.29 (P less than .001), this translated to a recovery that was about one third faster.
In this final report, remdesivir’s significant advantage over placebo regarding the trial’s primary endpoint was reinforced by efficacy on multiple secondary endpoints.
This benefits on multiple secondary endpoints included a 50% greater odds ratio (OR, 1.5; 95% CI, 1.2-1.9) of significant clinical improvement by day 15 after adjustment for baseline severity, a shorter initial length of hospital stay (12 vs. 17 days) and fewer days on oxygen supplementation (13 vs. 21 days) for the subgroup of patients on oxygen at enrollment.
Although the numerically lower mortality in the remdesivir arm (6.75 vs. 11.9%) did not reach statistical significance, Dr. Beigel said, “mortality was moving in the same direction as the other key endpoints.”
According to the study investigators, the types of rates of adverse events on remdesivir, which inhibits viral replication, “were generally similar in the remdesivir and placebo groups.”
In ACTT-1, 1,062 patients were randomized to remdesivir (200 mg loading dose followed by 100 mg daily for up to 9 days) or placebo. Patients were enrolled at study sites in North America, Europe, and Asia.
The data of ACTT-1 confirm a benefit from remdesivir in hospitalized COVID-19 patients with severe disease, but Dr. Beigel said he agrees with the current FDA indication that supports treatment in any hospitalized COVID-19 patient.
“We saw bigger benefits in patients with more severe infections. The benefits are not as large in patients with mild disease, but I think remdesivir should be considered in any hospitalized patient,” Dr. Beigel said.
This point of view is shared.
“I would give this drug to anyone in the hospital infected with COVID-19 assuming there was an ample supply and no need for rationing,” said Donna E. Sweet, MD, professor of internal medicine, University of Kansas, Wichita. She noted that this study has implications for hospital and hospital staff, as well as for patients.
“This type of reduction in recovery time means a reduction in potential exposures to hospital staff, a reduced need for PPE [personal protective equipment], and it will free up beds in the ICU [intensive care unit],” said Dr. Sweet, who also serves as an editorial advisory board member for Internal Medicine News.
An infectious disease specialist at the University of Minnesota also considers remdesivir to have an important role for conserving resources that deserves emphasis.
The reduction in time to recovery “is of benefit to the health system by maintaining hospital bed capacity,” said David R. Boulware, MD, professor of medicine at the University of Minnesota, Minneapolis.
According to his reading of the available data, including those from ACTT-1, the benefit appears to be greatest in those with a moderate degree of illness, which he defined as “sick enough to be hospitalized and require oxygen, yet not severely sick [and] requiring a ventilator or [extracorporeal membrane oxygenation].”
This does not preclude a benefit in those with more severe or milder disease, but patients with mild disease “are likely to recover regardless – or despite – whatever therapy they receive,” he said.
Dr. Beigel, the principal investigator of this trial, reports no potential conflicts of interest.
SOURCE: Beigel JH et al. N Engl J Med. 2020 Oct 8. doi: 10.1056/NEJMoa2007764.
Drug beats placebo across multiple endpoints in COVID-19 patients
Drug beats placebo across multiple endpoints in COVID-19 patients
In May 2020, remdesivir received Food and Drug Administration approval for emergency treatment of severe COVID-19 on the basis of a preliminary report on this trial. In August 2020, the FDA expanded the indication to include all hospitalized adult and pediatric patients with suspected or laboratory-confirmed COVID-19 infection irrespective of severity.
“Our findings were consistent with the findings of the preliminary report: a 10-day course of remdesivir was superior to placebo in the treatment of hospitalized patients with COVID-19,” reported a team of investigators led by John H. Beigel, MD, of the Division of Microbiology and Infectious Diseases at the National Institute of Allergy and Infectious Diseases, in the New England Journal of Medicine.
The drug’s broadened indication was not based on the ACTT-1 trial, according to Dr. Beigel. “Other data have demonstrated that remdesivir shortens recovery in patients with lower acuity. In our study, evidence of pneumonia was an enrollment requirement,” he explained in an interview.
In the newly published final ACTT-1 data, the median time to recovery was 10 days for those on active therapy versus 15 days for those randomized to placebo. With a rate ratio of 1.29 (P less than .001), this translated to a recovery that was about one third faster.
In this final report, remdesivir’s significant advantage over placebo regarding the trial’s primary endpoint was reinforced by efficacy on multiple secondary endpoints.
This benefits on multiple secondary endpoints included a 50% greater odds ratio (OR, 1.5; 95% CI, 1.2-1.9) of significant clinical improvement by day 15 after adjustment for baseline severity, a shorter initial length of hospital stay (12 vs. 17 days) and fewer days on oxygen supplementation (13 vs. 21 days) for the subgroup of patients on oxygen at enrollment.
Although the numerically lower mortality in the remdesivir arm (6.75 vs. 11.9%) did not reach statistical significance, Dr. Beigel said, “mortality was moving in the same direction as the other key endpoints.”
According to the study investigators, the types of rates of adverse events on remdesivir, which inhibits viral replication, “were generally similar in the remdesivir and placebo groups.”
In ACTT-1, 1,062 patients were randomized to remdesivir (200 mg loading dose followed by 100 mg daily for up to 9 days) or placebo. Patients were enrolled at study sites in North America, Europe, and Asia.
The data of ACTT-1 confirm a benefit from remdesivir in hospitalized COVID-19 patients with severe disease, but Dr. Beigel said he agrees with the current FDA indication that supports treatment in any hospitalized COVID-19 patient.
“We saw bigger benefits in patients with more severe infections. The benefits are not as large in patients with mild disease, but I think remdesivir should be considered in any hospitalized patient,” Dr. Beigel said.
This point of view is shared.
“I would give this drug to anyone in the hospital infected with COVID-19 assuming there was an ample supply and no need for rationing,” said Donna E. Sweet, MD, professor of internal medicine, University of Kansas, Wichita. She noted that this study has implications for hospital and hospital staff, as well as for patients.
“This type of reduction in recovery time means a reduction in potential exposures to hospital staff, a reduced need for PPE [personal protective equipment], and it will free up beds in the ICU [intensive care unit],” said Dr. Sweet, who also serves as an editorial advisory board member for Internal Medicine News.
An infectious disease specialist at the University of Minnesota also considers remdesivir to have an important role for conserving resources that deserves emphasis.
The reduction in time to recovery “is of benefit to the health system by maintaining hospital bed capacity,” said David R. Boulware, MD, professor of medicine at the University of Minnesota, Minneapolis.
According to his reading of the available data, including those from ACTT-1, the benefit appears to be greatest in those with a moderate degree of illness, which he defined as “sick enough to be hospitalized and require oxygen, yet not severely sick [and] requiring a ventilator or [extracorporeal membrane oxygenation].”
This does not preclude a benefit in those with more severe or milder disease, but patients with mild disease “are likely to recover regardless – or despite – whatever therapy they receive,” he said.
Dr. Beigel, the principal investigator of this trial, reports no potential conflicts of interest.
SOURCE: Beigel JH et al. N Engl J Med. 2020 Oct 8. doi: 10.1056/NEJMoa2007764.
In May 2020, remdesivir received Food and Drug Administration approval for emergency treatment of severe COVID-19 on the basis of a preliminary report on this trial. In August 2020, the FDA expanded the indication to include all hospitalized adult and pediatric patients with suspected or laboratory-confirmed COVID-19 infection irrespective of severity.
“Our findings were consistent with the findings of the preliminary report: a 10-day course of remdesivir was superior to placebo in the treatment of hospitalized patients with COVID-19,” reported a team of investigators led by John H. Beigel, MD, of the Division of Microbiology and Infectious Diseases at the National Institute of Allergy and Infectious Diseases, in the New England Journal of Medicine.
The drug’s broadened indication was not based on the ACTT-1 trial, according to Dr. Beigel. “Other data have demonstrated that remdesivir shortens recovery in patients with lower acuity. In our study, evidence of pneumonia was an enrollment requirement,” he explained in an interview.
In the newly published final ACTT-1 data, the median time to recovery was 10 days for those on active therapy versus 15 days for those randomized to placebo. With a rate ratio of 1.29 (P less than .001), this translated to a recovery that was about one third faster.
In this final report, remdesivir’s significant advantage over placebo regarding the trial’s primary endpoint was reinforced by efficacy on multiple secondary endpoints.
This benefits on multiple secondary endpoints included a 50% greater odds ratio (OR, 1.5; 95% CI, 1.2-1.9) of significant clinical improvement by day 15 after adjustment for baseline severity, a shorter initial length of hospital stay (12 vs. 17 days) and fewer days on oxygen supplementation (13 vs. 21 days) for the subgroup of patients on oxygen at enrollment.
Although the numerically lower mortality in the remdesivir arm (6.75 vs. 11.9%) did not reach statistical significance, Dr. Beigel said, “mortality was moving in the same direction as the other key endpoints.”
According to the study investigators, the types of rates of adverse events on remdesivir, which inhibits viral replication, “were generally similar in the remdesivir and placebo groups.”
In ACTT-1, 1,062 patients were randomized to remdesivir (200 mg loading dose followed by 100 mg daily for up to 9 days) or placebo. Patients were enrolled at study sites in North America, Europe, and Asia.
The data of ACTT-1 confirm a benefit from remdesivir in hospitalized COVID-19 patients with severe disease, but Dr. Beigel said he agrees with the current FDA indication that supports treatment in any hospitalized COVID-19 patient.
“We saw bigger benefits in patients with more severe infections. The benefits are not as large in patients with mild disease, but I think remdesivir should be considered in any hospitalized patient,” Dr. Beigel said.
This point of view is shared.
“I would give this drug to anyone in the hospital infected with COVID-19 assuming there was an ample supply and no need for rationing,” said Donna E. Sweet, MD, professor of internal medicine, University of Kansas, Wichita. She noted that this study has implications for hospital and hospital staff, as well as for patients.
“This type of reduction in recovery time means a reduction in potential exposures to hospital staff, a reduced need for PPE [personal protective equipment], and it will free up beds in the ICU [intensive care unit],” said Dr. Sweet, who also serves as an editorial advisory board member for Internal Medicine News.
An infectious disease specialist at the University of Minnesota also considers remdesivir to have an important role for conserving resources that deserves emphasis.
The reduction in time to recovery “is of benefit to the health system by maintaining hospital bed capacity,” said David R. Boulware, MD, professor of medicine at the University of Minnesota, Minneapolis.
According to his reading of the available data, including those from ACTT-1, the benefit appears to be greatest in those with a moderate degree of illness, which he defined as “sick enough to be hospitalized and require oxygen, yet not severely sick [and] requiring a ventilator or [extracorporeal membrane oxygenation].”
This does not preclude a benefit in those with more severe or milder disease, but patients with mild disease “are likely to recover regardless – or despite – whatever therapy they receive,” he said.
Dr. Beigel, the principal investigator of this trial, reports no potential conflicts of interest.
SOURCE: Beigel JH et al. N Engl J Med. 2020 Oct 8. doi: 10.1056/NEJMoa2007764.
More data on impact of corticosteroids on COVID-19 mortality in patients with COPD
, a study of almost 1 million individuals in the United Kingdom has shown.
Patients with chronic obstructive pulmonary disease or asthma who used ICS on a regular basis were more likely to die from COVID-19 than COPD or asthma patients who were prescribed non-ICS therapies, reported co-lead author Anna Schultze, PhD, of London School of Hygiene & Tropical Medicine and colleagues.
Of note, the increased risk of death among ICS users likely stemmed from greater severity of preexisting chronic respiratory conditions, instead of directly from ICS usage, which has little apparent impact on COVID-19 mortality, the investigators wrote in Lancet Respiratory Medicine.
These findings conflict with a hypothesis proposed early in the pandemic: that ICS may protect individuals from SARS-CoV-2 infection and poor outcomes with COVID-19.
According to Megan Conroy, MD, of the department of internal medicine at the Ohio State University Wexner Medical Center, Columbus, this hypothesis was based on some unexpected epidemiological findings.
“In general, we tend to think people with underlying lung disease – like COPD or asthma – to be at higher risk for severe forms of lower respiratory tract infections,” Dr. Conroy said. “Somewhat surprisingly, early data in the pandemic showed patients with COPD and asthma [were] underrepresented [among patients with COVID] when compared to the prevalence of these diseases in the population.”
This raised the possibility of an incidental protective effect from regular ICS therapy, which “had some strong theoretic pathophysiologic basis,” Dr. Conroy said, referring to research that demonstrated ICS-mediated downregulation of SARS-CoV-2 entry receptors ACE2 and TMPRSS2.
Dr. Schultze and colleagues noted that investigators for two ongoing randomized controlled trials (NCT04331054, NCT04330586) are studying ICS as an intervention for COVID-19; but neither trial includes individuals already taking ICS for chronic respiratory disease.
The present observational study therefore aimed to assess mortality risk within this population. Data were drawn from electronic health records and a U.K. national mortality database, with follow-up ranging from March 1 to May 6, 2020. Eligibility required a relevant prescription within 4 months of first follow-up. In the COPD group, patients were prescribed a long-acting beta agonist plus a long-acting muscarinic antagonist (LABA–LAMA), LABA alone, LABA plus ICS, LABA–LAMA plus ICS, or ICS alone (if prescribed LABA within 4 months).
In the asthma group, patients received low/medium-dose ICS, high-dose ICS, or a short-acting beta agonist (SABA) alone. Patients with COPD were at least 35 years of age, while those with asthma were 18 years or older. Hazard ratios were adjusted for a variety of covariates, including respiratory disease–exacerbation history, age, sex, body mass index, hypertension, diabetes, and others.
These eligibility criteria returned 148,557 patients with COPD and 818,490 with asthma.
Patients with COPD who were prescribed ICS plus LABA-LAMA or ICS plus LABA had an increased risk of COVID-19-related death, compared with those who did not receive ICS (adjusted hazard ratio, 1.39; 95% confidence interval, 1.10-1.76). Separate analyses of patients who received a triple combination (LABA–LAMA plus ICS) versus those who took a dual combination (LABA plus ICS) showed that triple-combination therapy was significantly associated with increased COVID-19-related mortality (aHR, 1.43; 95% CI, 1.12-1.83), while dual-combination therapy was less so (aHR, 1.29; 95% CI, 0.96-1.74). Non–COVID-19–related mortality was significantly increased for all COPD patients who were prescribed ICS, with or without adjustment for covariates.
Asthma patients prescribed high-dose ICS instead of SABA alone had a slightly greater risk of COVID-19–related death, based on an adjusted hazard ratio of 1.55 (95% CI, 1.10-2.18). Those with asthma who received low/medium–dose ICS demonstrated a slight trend toward increased mortality risk, but this was not significant (aHR, 1.14; 95% CI, 0.85-1.54). ICS usage in the asthma group was not linked with a significant increase in non–COVID-19–related death.
“In summary, we found no evidence of a beneficial effect of regular ICS use among people with COPD and asthma on COVID-19–related mortality,” the investigators concluded.
In agreement with the investigators, Dr. Conroy said that the increased mortality rate among ICS users should not be misconstrued as a medication-related risk.
“While the study found that those with COPD or asthma taking ICS and high-dose ICS were at an increased risk of death, this could easily be explained by the likelihood that those are the patients who are more likely to have more severe underlying lung disease,” Dr. Conroy said. “While this observational study did attempt to control for exacerbation history, the ability to do so by electronic health records data is certainly imperfect.”
With this in mind, patients with chronic respiratory disease should be encouraged to adhere to their usual treatment regimen, Dr. Conroy added.
“There isn’t evidence to increase or decrease medications just because of the pandemic,” she said. “A patient with asthma or COPD should continue to take the medications that are needed to achieve good control of their lung disease.”
The study was funded by the U.K. Medical Research Council. The investigators reported additional relationships with the Wellcome Trust, the Good Thinking Foundation, the Laura and John Arnold Foundation, and others. Dr. Conroy reported no conflicts of interest.
SOURCE: Schultze A et al. Lancet Respir Med. 2020 Sep 24. doi: 10.1016/ S2213-2600(20)30415-X.
, a study of almost 1 million individuals in the United Kingdom has shown.
Patients with chronic obstructive pulmonary disease or asthma who used ICS on a regular basis were more likely to die from COVID-19 than COPD or asthma patients who were prescribed non-ICS therapies, reported co-lead author Anna Schultze, PhD, of London School of Hygiene & Tropical Medicine and colleagues.
Of note, the increased risk of death among ICS users likely stemmed from greater severity of preexisting chronic respiratory conditions, instead of directly from ICS usage, which has little apparent impact on COVID-19 mortality, the investigators wrote in Lancet Respiratory Medicine.
These findings conflict with a hypothesis proposed early in the pandemic: that ICS may protect individuals from SARS-CoV-2 infection and poor outcomes with COVID-19.
According to Megan Conroy, MD, of the department of internal medicine at the Ohio State University Wexner Medical Center, Columbus, this hypothesis was based on some unexpected epidemiological findings.
“In general, we tend to think people with underlying lung disease – like COPD or asthma – to be at higher risk for severe forms of lower respiratory tract infections,” Dr. Conroy said. “Somewhat surprisingly, early data in the pandemic showed patients with COPD and asthma [were] underrepresented [among patients with COVID] when compared to the prevalence of these diseases in the population.”
This raised the possibility of an incidental protective effect from regular ICS therapy, which “had some strong theoretic pathophysiologic basis,” Dr. Conroy said, referring to research that demonstrated ICS-mediated downregulation of SARS-CoV-2 entry receptors ACE2 and TMPRSS2.
Dr. Schultze and colleagues noted that investigators for two ongoing randomized controlled trials (NCT04331054, NCT04330586) are studying ICS as an intervention for COVID-19; but neither trial includes individuals already taking ICS for chronic respiratory disease.
The present observational study therefore aimed to assess mortality risk within this population. Data were drawn from electronic health records and a U.K. national mortality database, with follow-up ranging from March 1 to May 6, 2020. Eligibility required a relevant prescription within 4 months of first follow-up. In the COPD group, patients were prescribed a long-acting beta agonist plus a long-acting muscarinic antagonist (LABA–LAMA), LABA alone, LABA plus ICS, LABA–LAMA plus ICS, or ICS alone (if prescribed LABA within 4 months).
In the asthma group, patients received low/medium-dose ICS, high-dose ICS, or a short-acting beta agonist (SABA) alone. Patients with COPD were at least 35 years of age, while those with asthma were 18 years or older. Hazard ratios were adjusted for a variety of covariates, including respiratory disease–exacerbation history, age, sex, body mass index, hypertension, diabetes, and others.
These eligibility criteria returned 148,557 patients with COPD and 818,490 with asthma.
Patients with COPD who were prescribed ICS plus LABA-LAMA or ICS plus LABA had an increased risk of COVID-19-related death, compared with those who did not receive ICS (adjusted hazard ratio, 1.39; 95% confidence interval, 1.10-1.76). Separate analyses of patients who received a triple combination (LABA–LAMA plus ICS) versus those who took a dual combination (LABA plus ICS) showed that triple-combination therapy was significantly associated with increased COVID-19-related mortality (aHR, 1.43; 95% CI, 1.12-1.83), while dual-combination therapy was less so (aHR, 1.29; 95% CI, 0.96-1.74). Non–COVID-19–related mortality was significantly increased for all COPD patients who were prescribed ICS, with or without adjustment for covariates.
Asthma patients prescribed high-dose ICS instead of SABA alone had a slightly greater risk of COVID-19–related death, based on an adjusted hazard ratio of 1.55 (95% CI, 1.10-2.18). Those with asthma who received low/medium–dose ICS demonstrated a slight trend toward increased mortality risk, but this was not significant (aHR, 1.14; 95% CI, 0.85-1.54). ICS usage in the asthma group was not linked with a significant increase in non–COVID-19–related death.
“In summary, we found no evidence of a beneficial effect of regular ICS use among people with COPD and asthma on COVID-19–related mortality,” the investigators concluded.
In agreement with the investigators, Dr. Conroy said that the increased mortality rate among ICS users should not be misconstrued as a medication-related risk.
“While the study found that those with COPD or asthma taking ICS and high-dose ICS were at an increased risk of death, this could easily be explained by the likelihood that those are the patients who are more likely to have more severe underlying lung disease,” Dr. Conroy said. “While this observational study did attempt to control for exacerbation history, the ability to do so by electronic health records data is certainly imperfect.”
With this in mind, patients with chronic respiratory disease should be encouraged to adhere to their usual treatment regimen, Dr. Conroy added.
“There isn’t evidence to increase or decrease medications just because of the pandemic,” she said. “A patient with asthma or COPD should continue to take the medications that are needed to achieve good control of their lung disease.”
The study was funded by the U.K. Medical Research Council. The investigators reported additional relationships with the Wellcome Trust, the Good Thinking Foundation, the Laura and John Arnold Foundation, and others. Dr. Conroy reported no conflicts of interest.
SOURCE: Schultze A et al. Lancet Respir Med. 2020 Sep 24. doi: 10.1016/ S2213-2600(20)30415-X.
, a study of almost 1 million individuals in the United Kingdom has shown.
Patients with chronic obstructive pulmonary disease or asthma who used ICS on a regular basis were more likely to die from COVID-19 than COPD or asthma patients who were prescribed non-ICS therapies, reported co-lead author Anna Schultze, PhD, of London School of Hygiene & Tropical Medicine and colleagues.
Of note, the increased risk of death among ICS users likely stemmed from greater severity of preexisting chronic respiratory conditions, instead of directly from ICS usage, which has little apparent impact on COVID-19 mortality, the investigators wrote in Lancet Respiratory Medicine.
These findings conflict with a hypothesis proposed early in the pandemic: that ICS may protect individuals from SARS-CoV-2 infection and poor outcomes with COVID-19.
According to Megan Conroy, MD, of the department of internal medicine at the Ohio State University Wexner Medical Center, Columbus, this hypothesis was based on some unexpected epidemiological findings.
“In general, we tend to think people with underlying lung disease – like COPD or asthma – to be at higher risk for severe forms of lower respiratory tract infections,” Dr. Conroy said. “Somewhat surprisingly, early data in the pandemic showed patients with COPD and asthma [were] underrepresented [among patients with COVID] when compared to the prevalence of these diseases in the population.”
This raised the possibility of an incidental protective effect from regular ICS therapy, which “had some strong theoretic pathophysiologic basis,” Dr. Conroy said, referring to research that demonstrated ICS-mediated downregulation of SARS-CoV-2 entry receptors ACE2 and TMPRSS2.
Dr. Schultze and colleagues noted that investigators for two ongoing randomized controlled trials (NCT04331054, NCT04330586) are studying ICS as an intervention for COVID-19; but neither trial includes individuals already taking ICS for chronic respiratory disease.
The present observational study therefore aimed to assess mortality risk within this population. Data were drawn from electronic health records and a U.K. national mortality database, with follow-up ranging from March 1 to May 6, 2020. Eligibility required a relevant prescription within 4 months of first follow-up. In the COPD group, patients were prescribed a long-acting beta agonist plus a long-acting muscarinic antagonist (LABA–LAMA), LABA alone, LABA plus ICS, LABA–LAMA plus ICS, or ICS alone (if prescribed LABA within 4 months).
In the asthma group, patients received low/medium-dose ICS, high-dose ICS, or a short-acting beta agonist (SABA) alone. Patients with COPD were at least 35 years of age, while those with asthma were 18 years or older. Hazard ratios were adjusted for a variety of covariates, including respiratory disease–exacerbation history, age, sex, body mass index, hypertension, diabetes, and others.
These eligibility criteria returned 148,557 patients with COPD and 818,490 with asthma.
Patients with COPD who were prescribed ICS plus LABA-LAMA or ICS plus LABA had an increased risk of COVID-19-related death, compared with those who did not receive ICS (adjusted hazard ratio, 1.39; 95% confidence interval, 1.10-1.76). Separate analyses of patients who received a triple combination (LABA–LAMA plus ICS) versus those who took a dual combination (LABA plus ICS) showed that triple-combination therapy was significantly associated with increased COVID-19-related mortality (aHR, 1.43; 95% CI, 1.12-1.83), while dual-combination therapy was less so (aHR, 1.29; 95% CI, 0.96-1.74). Non–COVID-19–related mortality was significantly increased for all COPD patients who were prescribed ICS, with or without adjustment for covariates.
Asthma patients prescribed high-dose ICS instead of SABA alone had a slightly greater risk of COVID-19–related death, based on an adjusted hazard ratio of 1.55 (95% CI, 1.10-2.18). Those with asthma who received low/medium–dose ICS demonstrated a slight trend toward increased mortality risk, but this was not significant (aHR, 1.14; 95% CI, 0.85-1.54). ICS usage in the asthma group was not linked with a significant increase in non–COVID-19–related death.
“In summary, we found no evidence of a beneficial effect of regular ICS use among people with COPD and asthma on COVID-19–related mortality,” the investigators concluded.
In agreement with the investigators, Dr. Conroy said that the increased mortality rate among ICS users should not be misconstrued as a medication-related risk.
“While the study found that those with COPD or asthma taking ICS and high-dose ICS were at an increased risk of death, this could easily be explained by the likelihood that those are the patients who are more likely to have more severe underlying lung disease,” Dr. Conroy said. “While this observational study did attempt to control for exacerbation history, the ability to do so by electronic health records data is certainly imperfect.”
With this in mind, patients with chronic respiratory disease should be encouraged to adhere to their usual treatment regimen, Dr. Conroy added.
“There isn’t evidence to increase or decrease medications just because of the pandemic,” she said. “A patient with asthma or COPD should continue to take the medications that are needed to achieve good control of their lung disease.”
The study was funded by the U.K. Medical Research Council. The investigators reported additional relationships with the Wellcome Trust, the Good Thinking Foundation, the Laura and John Arnold Foundation, and others. Dr. Conroy reported no conflicts of interest.
SOURCE: Schultze A et al. Lancet Respir Med. 2020 Sep 24. doi: 10.1016/ S2213-2600(20)30415-X.
FROM LANCET RESPIRATORY MEDICINE
One measure of child COVID-19 may be trending downward
After increasing for several weeks, the proportion of new COVID-19 cases occurring in children has dropped for the second week in a row, according to data in a new report from the American Academy of Pediatrics and the Children’s Hospital Association.
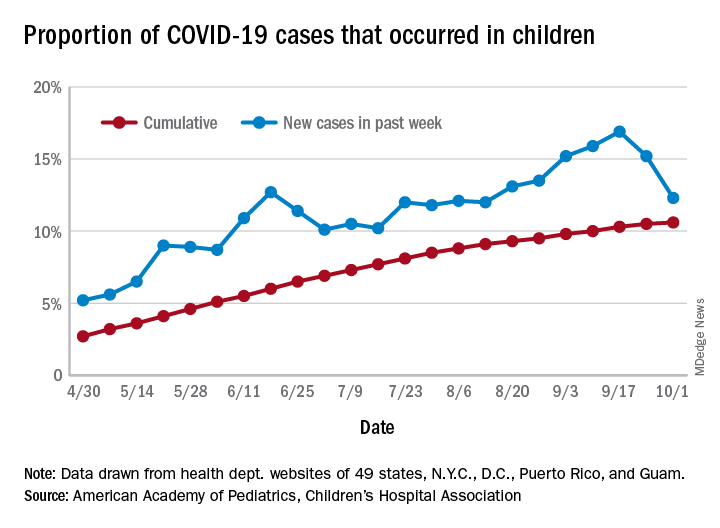
COVID-19 cases in children accounted for 12.3% of all new cases in the United States for the week ending Oct. 1, down from 15.2% the previous week. That measure had reached its highest point, 16.9%, just one week earlier (Sept. 17), the AAP and the CHA said in their weekly COVID-19 report.
based on data from the health departments of 49 states (New York does not provide ages on its website), as well as the District of Columbia, New York City, Puerto Rico, and Guam.
The child COVID-19 rate for the United States was 874 per 100,000 children as of Oct. 1, and that figure has doubled since the end of July. At the state level, the highest rates can be found in Tennessee (2,031.4 per 100,000), North Dakota (2,029.6), and South Carolina (2,002.6), with the lowest rates in Vermont (168.9), Maine (229.1), and New Hampshire (268.3), the AAP/CHA report shows.
The children of Wyoming make up the largest share, 22.4%, of any state’s COVID-19 cases, followed by North Dakota and Tennessee, both at 18.3%. New Jersey is lower than any other state at 3.9%, although New York City is a slightly lower 3.6%, the AAP and CHA said.
“The data are limited because the states differ in how they report the data, and it is unknown how many children have been infected but not tested. It is unclear how much of the increase in child cases is due to increased testing capacity,” the AAP said in an earlier statement.
After increasing for several weeks, the proportion of new COVID-19 cases occurring in children has dropped for the second week in a row, according to data in a new report from the American Academy of Pediatrics and the Children’s Hospital Association.

COVID-19 cases in children accounted for 12.3% of all new cases in the United States for the week ending Oct. 1, down from 15.2% the previous week. That measure had reached its highest point, 16.9%, just one week earlier (Sept. 17), the AAP and the CHA said in their weekly COVID-19 report.
based on data from the health departments of 49 states (New York does not provide ages on its website), as well as the District of Columbia, New York City, Puerto Rico, and Guam.
The child COVID-19 rate for the United States was 874 per 100,000 children as of Oct. 1, and that figure has doubled since the end of July. At the state level, the highest rates can be found in Tennessee (2,031.4 per 100,000), North Dakota (2,029.6), and South Carolina (2,002.6), with the lowest rates in Vermont (168.9), Maine (229.1), and New Hampshire (268.3), the AAP/CHA report shows.
The children of Wyoming make up the largest share, 22.4%, of any state’s COVID-19 cases, followed by North Dakota and Tennessee, both at 18.3%. New Jersey is lower than any other state at 3.9%, although New York City is a slightly lower 3.6%, the AAP and CHA said.
“The data are limited because the states differ in how they report the data, and it is unknown how many children have been infected but not tested. It is unclear how much of the increase in child cases is due to increased testing capacity,” the AAP said in an earlier statement.
After increasing for several weeks, the proportion of new COVID-19 cases occurring in children has dropped for the second week in a row, according to data in a new report from the American Academy of Pediatrics and the Children’s Hospital Association.

COVID-19 cases in children accounted for 12.3% of all new cases in the United States for the week ending Oct. 1, down from 15.2% the previous week. That measure had reached its highest point, 16.9%, just one week earlier (Sept. 17), the AAP and the CHA said in their weekly COVID-19 report.
based on data from the health departments of 49 states (New York does not provide ages on its website), as well as the District of Columbia, New York City, Puerto Rico, and Guam.
The child COVID-19 rate for the United States was 874 per 100,000 children as of Oct. 1, and that figure has doubled since the end of July. At the state level, the highest rates can be found in Tennessee (2,031.4 per 100,000), North Dakota (2,029.6), and South Carolina (2,002.6), with the lowest rates in Vermont (168.9), Maine (229.1), and New Hampshire (268.3), the AAP/CHA report shows.
The children of Wyoming make up the largest share, 22.4%, of any state’s COVID-19 cases, followed by North Dakota and Tennessee, both at 18.3%. New Jersey is lower than any other state at 3.9%, although New York City is a slightly lower 3.6%, the AAP and CHA said.
“The data are limited because the states differ in how they report the data, and it is unknown how many children have been infected but not tested. It is unclear how much of the increase in child cases is due to increased testing capacity,” the AAP said in an earlier statement.
CMS gives hospitals 14 weeks to start daily COVID, flu reports
The federal government is giving hospitals 14 weeks to comply with daily reporting requirements for COVID-19.
The Centers for Medicare & Medicaid Services will send letters on October 7 to all 6,200 hospitals that receive reimbursement from the two federal health programs informing them of how well they are doing now, said CMS Administrator Seema Verma on a press call.
Verma would not give an estimate on how many hospitals are currently not compliant. But Deborah Birx, MD, a member of the White House Coronavirus Task Force, said on the call that 86% of hospitals are currently reporting daily.
Federal officials on the call also announced that hospitals would have the option to begin reporting certain data on influenza starting October 19, but that it would become mandatory a few weeks later.
The reporting is important “to really ensure that we’re triangulating all data to understand where this epidemic is, how it’s moving through different populations, and ensuring that we’re meeting the needs of specific hospitals and communities,” Birx said.
The federal government began a new hospital reporting system in April but did not require hospitals to participate until it quietly issued guidance in mid-July informing facilities that they should no longer report to the Centers for Disease Control and Prevention (CDC).
The move perplexed many public health experts and epidemiologists, who expressed concern that asking hospitals to use a new data system during a pandemic could result in delays and lost information. The new HHS data collection site, HHS Protect, is being managed by a private contractor, not the CDC, which also raised alarms.
The final CMS rule issued in August went into effect immediately, without any chance for comment or revision. CMS said at the time that the pandemic was reason enough to skip over the normal bureaucratic process.
Hospitals were not pleased. But Verma claimed that since then CMS had been working with hospital organizations on enforcement.
“We’re going to do everything we can to facilitate reporting, including an enforcement timeline that will provide hospitals ample opportunity to come into compliance,” she said.
Hospitals that do not comply will get a notice every 3 weeks. Three weeks after the second notice, they’ll get weekly notices for a month, and a final termination notice at 14 weeks.
The Federation of American Hospitals (FAH), however, said their members were still not happy. “It is both inappropriate and frankly overkill for CMS to tie compliance with reporting to Medicare conditions of participation,” said FAH President and CEO Chip Kahn in a statement. He called the CMS proposal “sledgehammer enforcement,” and said that the continuing data request might weaken hospitals’ response to the pandemic because it would divert time and money away from patient care.
Rick Pollack, president and CEO of the American Hospital Association called the CMS rule an “overly heavy-handed approach that could jeopardize access to hospital care for all Americans.” He noted in a statement that barring hospitals from Medicare and Medicaid could harm beneficiaries and the effort to provide COVID care.
Pollack also noted that AHA has “observed errors in data processing and confusion about exactly what was being requested at the hospital, state, contractor, and federal level, and has worked diligently with the federal agencies to identify and correct those problems.”
The document that lays out U.S. Department of Health and Human Services (HHS) Protect reporting requirements were updated again on October 6 to add influenza data. The hospitals must report on total patients with laboratory-confirmed flu; previous day’s flu admissions; total ICU patients with lab-confirmed flu; total inpatients with either flu or COVID-19; and the previous day’s deaths for flu and COVID.
CDC Director Robert Redfield, MD, said on the press call that the new data will give the agency crucial hospital-level information and perhaps better estimates of the flu burden. Flu trends have been tracked using the CDC’s Influenza Hospitalization Surveillance Network (FluSurv-NET), which will not be replaced, Redfield said. But that network only tracks hospitalizations in 14 states and does not provide information in “nearly real-time,” he said.
Having the new data “will give us a true situational awareness of severe respiratory illness, provide local hospitalization trends, and help direct resources such as antiretrovirals to address potential increased impact of flu and COVID cocirculation,” Redfield said.
This article first appeared on Medscape.com.
The federal government is giving hospitals 14 weeks to comply with daily reporting requirements for COVID-19.
The Centers for Medicare & Medicaid Services will send letters on October 7 to all 6,200 hospitals that receive reimbursement from the two federal health programs informing them of how well they are doing now, said CMS Administrator Seema Verma on a press call.
Verma would not give an estimate on how many hospitals are currently not compliant. But Deborah Birx, MD, a member of the White House Coronavirus Task Force, said on the call that 86% of hospitals are currently reporting daily.
Federal officials on the call also announced that hospitals would have the option to begin reporting certain data on influenza starting October 19, but that it would become mandatory a few weeks later.
The reporting is important “to really ensure that we’re triangulating all data to understand where this epidemic is, how it’s moving through different populations, and ensuring that we’re meeting the needs of specific hospitals and communities,” Birx said.
The federal government began a new hospital reporting system in April but did not require hospitals to participate until it quietly issued guidance in mid-July informing facilities that they should no longer report to the Centers for Disease Control and Prevention (CDC).
The move perplexed many public health experts and epidemiologists, who expressed concern that asking hospitals to use a new data system during a pandemic could result in delays and lost information. The new HHS data collection site, HHS Protect, is being managed by a private contractor, not the CDC, which also raised alarms.
The final CMS rule issued in August went into effect immediately, without any chance for comment or revision. CMS said at the time that the pandemic was reason enough to skip over the normal bureaucratic process.
Hospitals were not pleased. But Verma claimed that since then CMS had been working with hospital organizations on enforcement.
“We’re going to do everything we can to facilitate reporting, including an enforcement timeline that will provide hospitals ample opportunity to come into compliance,” she said.
Hospitals that do not comply will get a notice every 3 weeks. Three weeks after the second notice, they’ll get weekly notices for a month, and a final termination notice at 14 weeks.
The Federation of American Hospitals (FAH), however, said their members were still not happy. “It is both inappropriate and frankly overkill for CMS to tie compliance with reporting to Medicare conditions of participation,” said FAH President and CEO Chip Kahn in a statement. He called the CMS proposal “sledgehammer enforcement,” and said that the continuing data request might weaken hospitals’ response to the pandemic because it would divert time and money away from patient care.
Rick Pollack, president and CEO of the American Hospital Association called the CMS rule an “overly heavy-handed approach that could jeopardize access to hospital care for all Americans.” He noted in a statement that barring hospitals from Medicare and Medicaid could harm beneficiaries and the effort to provide COVID care.
Pollack also noted that AHA has “observed errors in data processing and confusion about exactly what was being requested at the hospital, state, contractor, and federal level, and has worked diligently with the federal agencies to identify and correct those problems.”
The document that lays out U.S. Department of Health and Human Services (HHS) Protect reporting requirements were updated again on October 6 to add influenza data. The hospitals must report on total patients with laboratory-confirmed flu; previous day’s flu admissions; total ICU patients with lab-confirmed flu; total inpatients with either flu or COVID-19; and the previous day’s deaths for flu and COVID.
CDC Director Robert Redfield, MD, said on the press call that the new data will give the agency crucial hospital-level information and perhaps better estimates of the flu burden. Flu trends have been tracked using the CDC’s Influenza Hospitalization Surveillance Network (FluSurv-NET), which will not be replaced, Redfield said. But that network only tracks hospitalizations in 14 states and does not provide information in “nearly real-time,” he said.
Having the new data “will give us a true situational awareness of severe respiratory illness, provide local hospitalization trends, and help direct resources such as antiretrovirals to address potential increased impact of flu and COVID cocirculation,” Redfield said.
This article first appeared on Medscape.com.
The federal government is giving hospitals 14 weeks to comply with daily reporting requirements for COVID-19.
The Centers for Medicare & Medicaid Services will send letters on October 7 to all 6,200 hospitals that receive reimbursement from the two federal health programs informing them of how well they are doing now, said CMS Administrator Seema Verma on a press call.
Verma would not give an estimate on how many hospitals are currently not compliant. But Deborah Birx, MD, a member of the White House Coronavirus Task Force, said on the call that 86% of hospitals are currently reporting daily.
Federal officials on the call also announced that hospitals would have the option to begin reporting certain data on influenza starting October 19, but that it would become mandatory a few weeks later.
The reporting is important “to really ensure that we’re triangulating all data to understand where this epidemic is, how it’s moving through different populations, and ensuring that we’re meeting the needs of specific hospitals and communities,” Birx said.
The federal government began a new hospital reporting system in April but did not require hospitals to participate until it quietly issued guidance in mid-July informing facilities that they should no longer report to the Centers for Disease Control and Prevention (CDC).
The move perplexed many public health experts and epidemiologists, who expressed concern that asking hospitals to use a new data system during a pandemic could result in delays and lost information. The new HHS data collection site, HHS Protect, is being managed by a private contractor, not the CDC, which also raised alarms.
The final CMS rule issued in August went into effect immediately, without any chance for comment or revision. CMS said at the time that the pandemic was reason enough to skip over the normal bureaucratic process.
Hospitals were not pleased. But Verma claimed that since then CMS had been working with hospital organizations on enforcement.
“We’re going to do everything we can to facilitate reporting, including an enforcement timeline that will provide hospitals ample opportunity to come into compliance,” she said.
Hospitals that do not comply will get a notice every 3 weeks. Three weeks after the second notice, they’ll get weekly notices for a month, and a final termination notice at 14 weeks.
The Federation of American Hospitals (FAH), however, said their members were still not happy. “It is both inappropriate and frankly overkill for CMS to tie compliance with reporting to Medicare conditions of participation,” said FAH President and CEO Chip Kahn in a statement. He called the CMS proposal “sledgehammer enforcement,” and said that the continuing data request might weaken hospitals’ response to the pandemic because it would divert time and money away from patient care.
Rick Pollack, president and CEO of the American Hospital Association called the CMS rule an “overly heavy-handed approach that could jeopardize access to hospital care for all Americans.” He noted in a statement that barring hospitals from Medicare and Medicaid could harm beneficiaries and the effort to provide COVID care.
Pollack also noted that AHA has “observed errors in data processing and confusion about exactly what was being requested at the hospital, state, contractor, and federal level, and has worked diligently with the federal agencies to identify and correct those problems.”
The document that lays out U.S. Department of Health and Human Services (HHS) Protect reporting requirements were updated again on October 6 to add influenza data. The hospitals must report on total patients with laboratory-confirmed flu; previous day’s flu admissions; total ICU patients with lab-confirmed flu; total inpatients with either flu or COVID-19; and the previous day’s deaths for flu and COVID.
CDC Director Robert Redfield, MD, said on the press call that the new data will give the agency crucial hospital-level information and perhaps better estimates of the flu burden. Flu trends have been tracked using the CDC’s Influenza Hospitalization Surveillance Network (FluSurv-NET), which will not be replaced, Redfield said. But that network only tracks hospitalizations in 14 states and does not provide information in “nearly real-time,” he said.
Having the new data “will give us a true situational awareness of severe respiratory illness, provide local hospitalization trends, and help direct resources such as antiretrovirals to address potential increased impact of flu and COVID cocirculation,” Redfield said.
This article first appeared on Medscape.com.
FDA posts COVID vaccine guidance amid White House pushback
while medical and trade associations called for a thorough review of any such product before approval.
The FDA took the unusual step of posting background materials much earlier than usual for its planned Oct. 22 advisory committee meeting on potential vaccines for COVID-19. The FDA also on Tuesday afternoon released a new guidance document, expanding on a previous set of recommendations the agency released in June.
In the new guidance document, FDA officials outline what will be required for even a limited clearance, known as an emergency use authorization (EUA), for a COVID-19 vaccine.
“Data from phase 3 studies should include a median follow-up duration of at least 2 months after completion of the full vaccination regimen to help provide adequate information to assess a vaccine’s benefit-risk profile,” the FDA said in the document.
FDA staff have emphasized the higher bar that drugmakers and regulators face in considering approval of a COVID-19 vaccine.
“Vaccines are complex biological products, and an EUA for a COVID-19 vaccine may allow for rapid and widespread deployment for administration of the vaccine to millions of individuals, including healthy people,” the agency staff said in the briefing documents.
The FDA’s briefing document for the Oct. 22 meeting appears to be markedly at odds with the claim Trump made in a video Monday night, in which he told the American public that “vaccines are coming momentarily.”
Trump, who is in a tightly contested presidential race against Democratic candidate Joe Biden, has repeatedly made claims of the potential arrival of COVID vaccines that are at odds with timelines offered with guarded optimism by experts in infectious diseases.
But based on these new guidelines from the FDA, it appears that the White House may now endorse the FDA’s stance, according to a Wall Street Journal report based on “people familiar with the matter.”
The publication reports that the White House, which has yet to officially comment, “endorsed the U.S. Food and Drug Administration’s plans for assessing whether a Covid-19 vaccine should be given widely, casting aside objections to requirements that would likely mean a shot won’t be cleared until after Election Day, people familiar with the matter said.”
Anthony S. Fauci, MD, director of the National Institute of Allergy and Infectious Diseases, on Monday night said during a virtual appearance at the twenty-first annual New Yorker Festival that there could be evidence as early as November or December about whether one of the vaccines now in testing will work out. He declared himself to have “cautious optimism” about potential rollout of vaccines as early as late 2020 or early 2021.
Peter Lurie, MD, MPH, who earlier served as the FDA’s associate commissioner for public health strategy and analysis, described the agency’s release of the briefing document as being a positive development.
News organizations, including the New York Times, have reported that the White House had sought to block the FDA from releasing further instructions for companies developing COVID-19 vaccines. The Associated Press on Tuesday said that a senior Trump administration official confirmed that the White House had blocked earlier FDA plans to formally publish the safety guidelines based on the 2-month data requirement, arguing that there was “no clinical or medical reason” for it.
“It is an encouraging sign that, despite opposition from the White House, the Food and Drug Administration has effectively published guidelines for emergency release of a vaccine for COVID-19 by disclosing the advice it has been providing to individual sponsors,” said Dr. Lurie, who is now executive director and president of the Center for Science in the Public Interest.
In a news release, he said the White House had sought to keep the FDA guidance under wraps “so it could maintain the public fiction that a safe and effective vaccine could be available before Election Day or even so that it could force emergency authorization of a vaccine with more limited follow-up.”
“Even the pharmaceutical industry has been clamoring for the release of these guidelines. We all want a safe and effective vaccine to end the pandemic, and we want it sooner rather than later,” Dr. Lurie said. “But we can’t afford for the Trump administration to bungle vaccine review the way they’ve bungled nearly every other aspect of its pandemic response.”
Tuesday also saw a flood of statements in support of FDA officials, including tweets from the chief executive of Pfizer, which is among the leaders in the race to develop a COVID-19 vaccine. Pfizer’s Albert Bourla, DVM, PhD, said that the FDA’s “public servants are known for their high integrity and scientific expertise and we have full faith in their ability to set appropriate standards for the approval of a COVID vaccine or treatment.”
The American Medical Association on Tuesday announced a public webinar on Wednesday where its president, Susan R. Bailey, MD, will discuss the COVID-19 vaccine review process with Peter Marks, MD, PhD, director of the Center for Biologics Evaluation and Research at the FDA. The AMA described this webinar as part of work “to restore trust in science and science-based decision-making among policymakers and the public.”
“To ensure media and the physician community are continuously informed about the federal review process for COVID-19 vaccine candidates, the AMA will host a webinar series to gain fact-based insights from the nation’s highest-ranking subject matter experts working to protect the health of the public,” the organization said in announcing the webinar.
In a statement, leaders of the Association of American Medical Colleges said that the FDA’s Vaccines and Related Biological Products Advisory Committee should evaluate any COVID-19 candidate vaccines prior to the FDA issuing an EUA.
“Full approval of a new vaccine or biologic requires demonstration of safety and effectiveness through a process that includes evaluation by the VRBPAC. Their recommendations are considered by FDA staff who ultimately have the authority to approve the new product,” said AAMC chief scientific officer Ross McKinney Jr, MD, and AAMC CEO David J. Skorton, MD, in the statement.
Thomas M. File Jr., MD, president of the Infectious Diseases Society of America, said in a statement that his association again asked the White House to “follow medical and scientific expertise in efforts to combat COVID-19.”
“It is imperative that a vaccine be approved on the basis of FDA’s quality standards and that its safety and efficacy are established before it is authorized,” Dr. File said. “A vaccine that has been approved with speed, rather than safety and efficacy, at the forefront will compound the challenges posed by this pandemic. FDA guidelines for approval that set standards the American people can trust are essential to the success of a vaccine.”
Stephen J. Ubl, chief executive of the Pharmaceutical Research and Manufacturers of America, said in a statement that his association “supports any efforts by FDA to provide clarifying guidance and we have engaged with the agency to support bringing greater transparency to the review process for COVID-19 vaccines.”
“To help address this public health crisis, our companies have also taken unprecedented steps to share vaccine clinical trial protocols and data in real time,” Mr. Ubl said. “We welcome the agency’s efforts to instill confidence in the rigorous safety of these potential vaccines.”
On Oct. 1, Michelle McMurry-Heath, MD, PhD, president and chief executive of the Biotechnology Innovation Organization, released publicly her letter urging Department of Health & Human Services Secretary Alex Azar to “publicly release all new guidance” related to a COVID-19 vaccine. Such a move would bolster public confidence in the vaccine, she said.
“We cannot allow a lack of transparency to undermine confidence in the vaccine development process. The public must have full faith in the scientific process and the rigor of FDA’s regulatory oversight if we are to end the pandemic,” she wrote in the Oct. 1 letter to Azar. “Releasing any additional guidance on granting emergency use authorization for a vaccine will go a long way in accomplishing this critical goal.”
This article first appeared on Medscape.com.
while medical and trade associations called for a thorough review of any such product before approval.
The FDA took the unusual step of posting background materials much earlier than usual for its planned Oct. 22 advisory committee meeting on potential vaccines for COVID-19. The FDA also on Tuesday afternoon released a new guidance document, expanding on a previous set of recommendations the agency released in June.
In the new guidance document, FDA officials outline what will be required for even a limited clearance, known as an emergency use authorization (EUA), for a COVID-19 vaccine.
“Data from phase 3 studies should include a median follow-up duration of at least 2 months after completion of the full vaccination regimen to help provide adequate information to assess a vaccine’s benefit-risk profile,” the FDA said in the document.
FDA staff have emphasized the higher bar that drugmakers and regulators face in considering approval of a COVID-19 vaccine.
“Vaccines are complex biological products, and an EUA for a COVID-19 vaccine may allow for rapid and widespread deployment for administration of the vaccine to millions of individuals, including healthy people,” the agency staff said in the briefing documents.
The FDA’s briefing document for the Oct. 22 meeting appears to be markedly at odds with the claim Trump made in a video Monday night, in which he told the American public that “vaccines are coming momentarily.”
Trump, who is in a tightly contested presidential race against Democratic candidate Joe Biden, has repeatedly made claims of the potential arrival of COVID vaccines that are at odds with timelines offered with guarded optimism by experts in infectious diseases.
But based on these new guidelines from the FDA, it appears that the White House may now endorse the FDA’s stance, according to a Wall Street Journal report based on “people familiar with the matter.”
The publication reports that the White House, which has yet to officially comment, “endorsed the U.S. Food and Drug Administration’s plans for assessing whether a Covid-19 vaccine should be given widely, casting aside objections to requirements that would likely mean a shot won’t be cleared until after Election Day, people familiar with the matter said.”
Anthony S. Fauci, MD, director of the National Institute of Allergy and Infectious Diseases, on Monday night said during a virtual appearance at the twenty-first annual New Yorker Festival that there could be evidence as early as November or December about whether one of the vaccines now in testing will work out. He declared himself to have “cautious optimism” about potential rollout of vaccines as early as late 2020 or early 2021.
Peter Lurie, MD, MPH, who earlier served as the FDA’s associate commissioner for public health strategy and analysis, described the agency’s release of the briefing document as being a positive development.
News organizations, including the New York Times, have reported that the White House had sought to block the FDA from releasing further instructions for companies developing COVID-19 vaccines. The Associated Press on Tuesday said that a senior Trump administration official confirmed that the White House had blocked earlier FDA plans to formally publish the safety guidelines based on the 2-month data requirement, arguing that there was “no clinical or medical reason” for it.
“It is an encouraging sign that, despite opposition from the White House, the Food and Drug Administration has effectively published guidelines for emergency release of a vaccine for COVID-19 by disclosing the advice it has been providing to individual sponsors,” said Dr. Lurie, who is now executive director and president of the Center for Science in the Public Interest.
In a news release, he said the White House had sought to keep the FDA guidance under wraps “so it could maintain the public fiction that a safe and effective vaccine could be available before Election Day or even so that it could force emergency authorization of a vaccine with more limited follow-up.”
“Even the pharmaceutical industry has been clamoring for the release of these guidelines. We all want a safe and effective vaccine to end the pandemic, and we want it sooner rather than later,” Dr. Lurie said. “But we can’t afford for the Trump administration to bungle vaccine review the way they’ve bungled nearly every other aspect of its pandemic response.”
Tuesday also saw a flood of statements in support of FDA officials, including tweets from the chief executive of Pfizer, which is among the leaders in the race to develop a COVID-19 vaccine. Pfizer’s Albert Bourla, DVM, PhD, said that the FDA’s “public servants are known for their high integrity and scientific expertise and we have full faith in their ability to set appropriate standards for the approval of a COVID vaccine or treatment.”
The American Medical Association on Tuesday announced a public webinar on Wednesday where its president, Susan R. Bailey, MD, will discuss the COVID-19 vaccine review process with Peter Marks, MD, PhD, director of the Center for Biologics Evaluation and Research at the FDA. The AMA described this webinar as part of work “to restore trust in science and science-based decision-making among policymakers and the public.”
“To ensure media and the physician community are continuously informed about the federal review process for COVID-19 vaccine candidates, the AMA will host a webinar series to gain fact-based insights from the nation’s highest-ranking subject matter experts working to protect the health of the public,” the organization said in announcing the webinar.
In a statement, leaders of the Association of American Medical Colleges said that the FDA’s Vaccines and Related Biological Products Advisory Committee should evaluate any COVID-19 candidate vaccines prior to the FDA issuing an EUA.
“Full approval of a new vaccine or biologic requires demonstration of safety and effectiveness through a process that includes evaluation by the VRBPAC. Their recommendations are considered by FDA staff who ultimately have the authority to approve the new product,” said AAMC chief scientific officer Ross McKinney Jr, MD, and AAMC CEO David J. Skorton, MD, in the statement.
Thomas M. File Jr., MD, president of the Infectious Diseases Society of America, said in a statement that his association again asked the White House to “follow medical and scientific expertise in efforts to combat COVID-19.”
“It is imperative that a vaccine be approved on the basis of FDA’s quality standards and that its safety and efficacy are established before it is authorized,” Dr. File said. “A vaccine that has been approved with speed, rather than safety and efficacy, at the forefront will compound the challenges posed by this pandemic. FDA guidelines for approval that set standards the American people can trust are essential to the success of a vaccine.”
Stephen J. Ubl, chief executive of the Pharmaceutical Research and Manufacturers of America, said in a statement that his association “supports any efforts by FDA to provide clarifying guidance and we have engaged with the agency to support bringing greater transparency to the review process for COVID-19 vaccines.”
“To help address this public health crisis, our companies have also taken unprecedented steps to share vaccine clinical trial protocols and data in real time,” Mr. Ubl said. “We welcome the agency’s efforts to instill confidence in the rigorous safety of these potential vaccines.”
On Oct. 1, Michelle McMurry-Heath, MD, PhD, president and chief executive of the Biotechnology Innovation Organization, released publicly her letter urging Department of Health & Human Services Secretary Alex Azar to “publicly release all new guidance” related to a COVID-19 vaccine. Such a move would bolster public confidence in the vaccine, she said.
“We cannot allow a lack of transparency to undermine confidence in the vaccine development process. The public must have full faith in the scientific process and the rigor of FDA’s regulatory oversight if we are to end the pandemic,” she wrote in the Oct. 1 letter to Azar. “Releasing any additional guidance on granting emergency use authorization for a vaccine will go a long way in accomplishing this critical goal.”
This article first appeared on Medscape.com.
while medical and trade associations called for a thorough review of any such product before approval.
The FDA took the unusual step of posting background materials much earlier than usual for its planned Oct. 22 advisory committee meeting on potential vaccines for COVID-19. The FDA also on Tuesday afternoon released a new guidance document, expanding on a previous set of recommendations the agency released in June.
In the new guidance document, FDA officials outline what will be required for even a limited clearance, known as an emergency use authorization (EUA), for a COVID-19 vaccine.
“Data from phase 3 studies should include a median follow-up duration of at least 2 months after completion of the full vaccination regimen to help provide adequate information to assess a vaccine’s benefit-risk profile,” the FDA said in the document.
FDA staff have emphasized the higher bar that drugmakers and regulators face in considering approval of a COVID-19 vaccine.
“Vaccines are complex biological products, and an EUA for a COVID-19 vaccine may allow for rapid and widespread deployment for administration of the vaccine to millions of individuals, including healthy people,” the agency staff said in the briefing documents.
The FDA’s briefing document for the Oct. 22 meeting appears to be markedly at odds with the claim Trump made in a video Monday night, in which he told the American public that “vaccines are coming momentarily.”
Trump, who is in a tightly contested presidential race against Democratic candidate Joe Biden, has repeatedly made claims of the potential arrival of COVID vaccines that are at odds with timelines offered with guarded optimism by experts in infectious diseases.
But based on these new guidelines from the FDA, it appears that the White House may now endorse the FDA’s stance, according to a Wall Street Journal report based on “people familiar with the matter.”
The publication reports that the White House, which has yet to officially comment, “endorsed the U.S. Food and Drug Administration’s plans for assessing whether a Covid-19 vaccine should be given widely, casting aside objections to requirements that would likely mean a shot won’t be cleared until after Election Day, people familiar with the matter said.”
Anthony S. Fauci, MD, director of the National Institute of Allergy and Infectious Diseases, on Monday night said during a virtual appearance at the twenty-first annual New Yorker Festival that there could be evidence as early as November or December about whether one of the vaccines now in testing will work out. He declared himself to have “cautious optimism” about potential rollout of vaccines as early as late 2020 or early 2021.
Peter Lurie, MD, MPH, who earlier served as the FDA’s associate commissioner for public health strategy and analysis, described the agency’s release of the briefing document as being a positive development.
News organizations, including the New York Times, have reported that the White House had sought to block the FDA from releasing further instructions for companies developing COVID-19 vaccines. The Associated Press on Tuesday said that a senior Trump administration official confirmed that the White House had blocked earlier FDA plans to formally publish the safety guidelines based on the 2-month data requirement, arguing that there was “no clinical or medical reason” for it.
“It is an encouraging sign that, despite opposition from the White House, the Food and Drug Administration has effectively published guidelines for emergency release of a vaccine for COVID-19 by disclosing the advice it has been providing to individual sponsors,” said Dr. Lurie, who is now executive director and president of the Center for Science in the Public Interest.
In a news release, he said the White House had sought to keep the FDA guidance under wraps “so it could maintain the public fiction that a safe and effective vaccine could be available before Election Day or even so that it could force emergency authorization of a vaccine with more limited follow-up.”
“Even the pharmaceutical industry has been clamoring for the release of these guidelines. We all want a safe and effective vaccine to end the pandemic, and we want it sooner rather than later,” Dr. Lurie said. “But we can’t afford for the Trump administration to bungle vaccine review the way they’ve bungled nearly every other aspect of its pandemic response.”
Tuesday also saw a flood of statements in support of FDA officials, including tweets from the chief executive of Pfizer, which is among the leaders in the race to develop a COVID-19 vaccine. Pfizer’s Albert Bourla, DVM, PhD, said that the FDA’s “public servants are known for their high integrity and scientific expertise and we have full faith in their ability to set appropriate standards for the approval of a COVID vaccine or treatment.”
The American Medical Association on Tuesday announced a public webinar on Wednesday where its president, Susan R. Bailey, MD, will discuss the COVID-19 vaccine review process with Peter Marks, MD, PhD, director of the Center for Biologics Evaluation and Research at the FDA. The AMA described this webinar as part of work “to restore trust in science and science-based decision-making among policymakers and the public.”
“To ensure media and the physician community are continuously informed about the federal review process for COVID-19 vaccine candidates, the AMA will host a webinar series to gain fact-based insights from the nation’s highest-ranking subject matter experts working to protect the health of the public,” the organization said in announcing the webinar.
In a statement, leaders of the Association of American Medical Colleges said that the FDA’s Vaccines and Related Biological Products Advisory Committee should evaluate any COVID-19 candidate vaccines prior to the FDA issuing an EUA.
“Full approval of a new vaccine or biologic requires demonstration of safety and effectiveness through a process that includes evaluation by the VRBPAC. Their recommendations are considered by FDA staff who ultimately have the authority to approve the new product,” said AAMC chief scientific officer Ross McKinney Jr, MD, and AAMC CEO David J. Skorton, MD, in the statement.
Thomas M. File Jr., MD, president of the Infectious Diseases Society of America, said in a statement that his association again asked the White House to “follow medical and scientific expertise in efforts to combat COVID-19.”
“It is imperative that a vaccine be approved on the basis of FDA’s quality standards and that its safety and efficacy are established before it is authorized,” Dr. File said. “A vaccine that has been approved with speed, rather than safety and efficacy, at the forefront will compound the challenges posed by this pandemic. FDA guidelines for approval that set standards the American people can trust are essential to the success of a vaccine.”
Stephen J. Ubl, chief executive of the Pharmaceutical Research and Manufacturers of America, said in a statement that his association “supports any efforts by FDA to provide clarifying guidance and we have engaged with the agency to support bringing greater transparency to the review process for COVID-19 vaccines.”
“To help address this public health crisis, our companies have also taken unprecedented steps to share vaccine clinical trial protocols and data in real time,” Mr. Ubl said. “We welcome the agency’s efforts to instill confidence in the rigorous safety of these potential vaccines.”
On Oct. 1, Michelle McMurry-Heath, MD, PhD, president and chief executive of the Biotechnology Innovation Organization, released publicly her letter urging Department of Health & Human Services Secretary Alex Azar to “publicly release all new guidance” related to a COVID-19 vaccine. Such a move would bolster public confidence in the vaccine, she said.
“We cannot allow a lack of transparency to undermine confidence in the vaccine development process. The public must have full faith in the scientific process and the rigor of FDA’s regulatory oversight if we are to end the pandemic,” she wrote in the Oct. 1 letter to Azar. “Releasing any additional guidance on granting emergency use authorization for a vaccine will go a long way in accomplishing this critical goal.”
This article first appeared on Medscape.com.
Stroke may be the first symptom of COVID-19 in younger patients
new research suggests. Investigators carried out a meta-analysis of data, including 160 patients with COVID-19 and stroke, and found that nearly half of patients under the age of 50 were asymptomatic at the time of stroke onset.
Although younger patients had the highest risk of stroke, the highest risk of death was in patients who were older, had other chronic conditions, and had more severe COVID-19–associated respiratory symptoms.
“One of the most eye-opening findings of this study is that, for patients under 50 years old, many were totally asymptomatic when they had a stroke related to COVID-19, [which] means that, for these patients, the stroke was their first symptom of the disease,” lead author Luciano Sposato, MD, MBA, associate professor and chair in stroke research at Western University, London, Ont.
The study was published online Sept. 15 in Neurology.
Anecdotal reports
“In early April of 2020, we realized that COVID-19 was a highly thrombogenic disease,” said Dr. Sposato. “Almost in parallel, I started to see anecdotal reports in social media of strokes occurring in patients with COVID-19, and there were also very few case reports.”
The investigators “thought it would be a good idea to put all the data together in one paper,” he said, and began by conducting a systematic review of 10 published studies of COVID-19 and stroke (n = 125 patients), which were then pooled with 35 unpublished cases from Canada, the United States, and Iran for a total of 160 cases.
The analysis examined in-hospital mortality rates of patients with stroke and COVID-19.
In addition, the researchers conducted a second review of 150 papers, encompassing a final cohort of 3,306 COVID-19 patients with stroke of any type and 5,322 with ischemic stroke.
“Some studies reported data for only ischemic stroke, and some reported data for all strokes considered together, which resulted in a different number of patients on each meta-analysis, with a lower number of ‘any stroke’ cases,” Dr. Sposato explained. “This review looked at the number of patients who developed a stroke during admission and included thousands of patients.”
Dr. Sposato noted that the first review was conducted on single case reports and small case series “to understand the clinical characteristics of strokes in patients with COVID-19 on an individual patient level,” since “large studies, including hundreds of thousands of patients, usually do not provide the level of detail for a descriptive analysis of the clinical characteristics of a disease.”
Cluster analyses were used to “identify specific clinical phenotypes and their relationship with death.” Patients were stratified into three age groups: <50, 50-70, and >70 years (“young,” “middle aged,” and “older,” respectively). The median age was 65 years and 43% were female.
Mortality ‘remarkably high’
The review showed that 1.8% (95% confidence interval, 0.9%-3.7%) of patients experienced a new stroke, while 1.5% (95% CI, 0.8%-2.8%) of these experienced an ischemic stroke. “These numbers are higher than historical data for other infectious diseases – for example, 0.75% in SARS-CoV-1, 0.78% in sepsis, and 0.2% in influenza,” Dr. Sposato commented.
Moreover, “this number may be an underestimate, given that many patients die without a confirmed diagnosis and that some patients did not come to the emergency department when experiencing mild symptoms during the first months of the pandemic,” he added.
Focusing on the review of 160 patients, the researchers described in-hospital mortality for strokes of all types and for ischemic strokes alone as “remarkably high” (34.4% [95% CI, 27.2%-42.4%] and 35.7% [95% CI, 27.5%-44.8%], respectively), with most deaths occurring among ischemic stroke patients.
“This high mortality rate is higher than the [roughly] 15% to 30% reported for stroke patients without COVID-19 admitted to intensive care units,” Dr. Sposato said.
High-risk phenotype
Many “young” COVID-19 patients (under age 50) who had a stroke (42.9%) had no previous risk factors or comorbidities. Moreover, in almost half of these patients (48.3%), stroke was more likely to occur before the onset of any COVID-19 respiratory symptoms.
Additionally, younger patients showed the highest frequency of elevated cardiac troponin compared with middle-aged and older patients (71.4% vs. 48.4% and 27.8%, respectively). On the other hand, mortality was 67% lower in younger versus older patients (odds ratio, 0.33; 95% CI, 0.12-0.94; P = .039).
Dr. Sposato noted that the proportion of ischemic stroke patients with large-vessel occlusion was “higher than previously reported” for patients with stroke without COVID-19 (47% compared with 29%, respectively).
“We should consider COVID-19 as a new cause or risk factor for stroke. At least, patients with stroke should probably be tested for SARS-CoV-2 infection if they are young and present with a large-vessel occlusion, even in the absence of typical COVID-19 respiratory symptoms,” he suggested.
The researchers identified a “high-risk phenotype” for death for all types of stroke considered together: older age, a higher burden of comorbidities, and severe COVID-19 respiratory symptoms. Patients with all three characteristics had the highest in-hospital mortality rate (58.6%) and a threefold risk of death, compared with the rest of the cohort (OR, 3.52; 95% CI, 1.53-8.09; P = .003).
“Several potential mechanisms can explain the increased risk of stroke among COVID-19 patients, but perhaps the most important one is increased thrombogenesis secondary to an exaggerated inflammatory response,” Dr. Sposato said.
Not just elders
Commenting on the study, Jodi Edwards, PhD, director of the Brain and Heart Nexus Research Program at the University of Ottawa Heart Institute, said the findings are “consistent with and underscore public health messaging emphasizing that COVID-19 does not only affect the elderly and those with underlying health conditions, but can have serious and even fatal consequences at any age.”
Dr. Edwards, who was not involved with the study, emphasized that “adherence to public health recommendations is critical to begin to reduce the rising incidence in younger adults.”
Dr. Sposato acknowledged that the study was small and that there “can be problems associated with a systematic review of case reports, such as publication bias, lack of completeness of data, etc, so more research is needed.”
Dr. Sposato is supported by the Kathleen & Dr. Henry Barnett Research Chair in Stroke Research at Western University, the Edward and Alma Saraydar Neurosciences Fund of the London Health Sciences Foundation, and the Opportunities Fund of the Academic Health Sciences Centre Alternative Funding Plan of the Academic Medical Organization of Southwestern Ontario. Dr. Sposato reported speaker honoraria from Boehringer Ingelheim, Pfizer, Gore, and Bayer and research/quality improvement grants from Boehringer Ingelheim and Bayer. The other authors’ disclosures are listed on the original article. Dr. Edwards has disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
new research suggests. Investigators carried out a meta-analysis of data, including 160 patients with COVID-19 and stroke, and found that nearly half of patients under the age of 50 were asymptomatic at the time of stroke onset.
Although younger patients had the highest risk of stroke, the highest risk of death was in patients who were older, had other chronic conditions, and had more severe COVID-19–associated respiratory symptoms.
“One of the most eye-opening findings of this study is that, for patients under 50 years old, many were totally asymptomatic when they had a stroke related to COVID-19, [which] means that, for these patients, the stroke was their first symptom of the disease,” lead author Luciano Sposato, MD, MBA, associate professor and chair in stroke research at Western University, London, Ont.
The study was published online Sept. 15 in Neurology.
Anecdotal reports
“In early April of 2020, we realized that COVID-19 was a highly thrombogenic disease,” said Dr. Sposato. “Almost in parallel, I started to see anecdotal reports in social media of strokes occurring in patients with COVID-19, and there were also very few case reports.”
The investigators “thought it would be a good idea to put all the data together in one paper,” he said, and began by conducting a systematic review of 10 published studies of COVID-19 and stroke (n = 125 patients), which were then pooled with 35 unpublished cases from Canada, the United States, and Iran for a total of 160 cases.
The analysis examined in-hospital mortality rates of patients with stroke and COVID-19.
In addition, the researchers conducted a second review of 150 papers, encompassing a final cohort of 3,306 COVID-19 patients with stroke of any type and 5,322 with ischemic stroke.
“Some studies reported data for only ischemic stroke, and some reported data for all strokes considered together, which resulted in a different number of patients on each meta-analysis, with a lower number of ‘any stroke’ cases,” Dr. Sposato explained. “This review looked at the number of patients who developed a stroke during admission and included thousands of patients.”
Dr. Sposato noted that the first review was conducted on single case reports and small case series “to understand the clinical characteristics of strokes in patients with COVID-19 on an individual patient level,” since “large studies, including hundreds of thousands of patients, usually do not provide the level of detail for a descriptive analysis of the clinical characteristics of a disease.”
Cluster analyses were used to “identify specific clinical phenotypes and their relationship with death.” Patients were stratified into three age groups: <50, 50-70, and >70 years (“young,” “middle aged,” and “older,” respectively). The median age was 65 years and 43% were female.
Mortality ‘remarkably high’
The review showed that 1.8% (95% confidence interval, 0.9%-3.7%) of patients experienced a new stroke, while 1.5% (95% CI, 0.8%-2.8%) of these experienced an ischemic stroke. “These numbers are higher than historical data for other infectious diseases – for example, 0.75% in SARS-CoV-1, 0.78% in sepsis, and 0.2% in influenza,” Dr. Sposato commented.
Moreover, “this number may be an underestimate, given that many patients die without a confirmed diagnosis and that some patients did not come to the emergency department when experiencing mild symptoms during the first months of the pandemic,” he added.
Focusing on the review of 160 patients, the researchers described in-hospital mortality for strokes of all types and for ischemic strokes alone as “remarkably high” (34.4% [95% CI, 27.2%-42.4%] and 35.7% [95% CI, 27.5%-44.8%], respectively), with most deaths occurring among ischemic stroke patients.
“This high mortality rate is higher than the [roughly] 15% to 30% reported for stroke patients without COVID-19 admitted to intensive care units,” Dr. Sposato said.
High-risk phenotype
Many “young” COVID-19 patients (under age 50) who had a stroke (42.9%) had no previous risk factors or comorbidities. Moreover, in almost half of these patients (48.3%), stroke was more likely to occur before the onset of any COVID-19 respiratory symptoms.
Additionally, younger patients showed the highest frequency of elevated cardiac troponin compared with middle-aged and older patients (71.4% vs. 48.4% and 27.8%, respectively). On the other hand, mortality was 67% lower in younger versus older patients (odds ratio, 0.33; 95% CI, 0.12-0.94; P = .039).
Dr. Sposato noted that the proportion of ischemic stroke patients with large-vessel occlusion was “higher than previously reported” for patients with stroke without COVID-19 (47% compared with 29%, respectively).
“We should consider COVID-19 as a new cause or risk factor for stroke. At least, patients with stroke should probably be tested for SARS-CoV-2 infection if they are young and present with a large-vessel occlusion, even in the absence of typical COVID-19 respiratory symptoms,” he suggested.
The researchers identified a “high-risk phenotype” for death for all types of stroke considered together: older age, a higher burden of comorbidities, and severe COVID-19 respiratory symptoms. Patients with all three characteristics had the highest in-hospital mortality rate (58.6%) and a threefold risk of death, compared with the rest of the cohort (OR, 3.52; 95% CI, 1.53-8.09; P = .003).
“Several potential mechanisms can explain the increased risk of stroke among COVID-19 patients, but perhaps the most important one is increased thrombogenesis secondary to an exaggerated inflammatory response,” Dr. Sposato said.
Not just elders
Commenting on the study, Jodi Edwards, PhD, director of the Brain and Heart Nexus Research Program at the University of Ottawa Heart Institute, said the findings are “consistent with and underscore public health messaging emphasizing that COVID-19 does not only affect the elderly and those with underlying health conditions, but can have serious and even fatal consequences at any age.”
Dr. Edwards, who was not involved with the study, emphasized that “adherence to public health recommendations is critical to begin to reduce the rising incidence in younger adults.”
Dr. Sposato acknowledged that the study was small and that there “can be problems associated with a systematic review of case reports, such as publication bias, lack of completeness of data, etc, so more research is needed.”
Dr. Sposato is supported by the Kathleen & Dr. Henry Barnett Research Chair in Stroke Research at Western University, the Edward and Alma Saraydar Neurosciences Fund of the London Health Sciences Foundation, and the Opportunities Fund of the Academic Health Sciences Centre Alternative Funding Plan of the Academic Medical Organization of Southwestern Ontario. Dr. Sposato reported speaker honoraria from Boehringer Ingelheim, Pfizer, Gore, and Bayer and research/quality improvement grants from Boehringer Ingelheim and Bayer. The other authors’ disclosures are listed on the original article. Dr. Edwards has disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
new research suggests. Investigators carried out a meta-analysis of data, including 160 patients with COVID-19 and stroke, and found that nearly half of patients under the age of 50 were asymptomatic at the time of stroke onset.
Although younger patients had the highest risk of stroke, the highest risk of death was in patients who were older, had other chronic conditions, and had more severe COVID-19–associated respiratory symptoms.
“One of the most eye-opening findings of this study is that, for patients under 50 years old, many were totally asymptomatic when they had a stroke related to COVID-19, [which] means that, for these patients, the stroke was their first symptom of the disease,” lead author Luciano Sposato, MD, MBA, associate professor and chair in stroke research at Western University, London, Ont.
The study was published online Sept. 15 in Neurology.
Anecdotal reports
“In early April of 2020, we realized that COVID-19 was a highly thrombogenic disease,” said Dr. Sposato. “Almost in parallel, I started to see anecdotal reports in social media of strokes occurring in patients with COVID-19, and there were also very few case reports.”
The investigators “thought it would be a good idea to put all the data together in one paper,” he said, and began by conducting a systematic review of 10 published studies of COVID-19 and stroke (n = 125 patients), which were then pooled with 35 unpublished cases from Canada, the United States, and Iran for a total of 160 cases.
The analysis examined in-hospital mortality rates of patients with stroke and COVID-19.
In addition, the researchers conducted a second review of 150 papers, encompassing a final cohort of 3,306 COVID-19 patients with stroke of any type and 5,322 with ischemic stroke.
“Some studies reported data for only ischemic stroke, and some reported data for all strokes considered together, which resulted in a different number of patients on each meta-analysis, with a lower number of ‘any stroke’ cases,” Dr. Sposato explained. “This review looked at the number of patients who developed a stroke during admission and included thousands of patients.”
Dr. Sposato noted that the first review was conducted on single case reports and small case series “to understand the clinical characteristics of strokes in patients with COVID-19 on an individual patient level,” since “large studies, including hundreds of thousands of patients, usually do not provide the level of detail for a descriptive analysis of the clinical characteristics of a disease.”
Cluster analyses were used to “identify specific clinical phenotypes and their relationship with death.” Patients were stratified into three age groups: <50, 50-70, and >70 years (“young,” “middle aged,” and “older,” respectively). The median age was 65 years and 43% were female.
Mortality ‘remarkably high’
The review showed that 1.8% (95% confidence interval, 0.9%-3.7%) of patients experienced a new stroke, while 1.5% (95% CI, 0.8%-2.8%) of these experienced an ischemic stroke. “These numbers are higher than historical data for other infectious diseases – for example, 0.75% in SARS-CoV-1, 0.78% in sepsis, and 0.2% in influenza,” Dr. Sposato commented.
Moreover, “this number may be an underestimate, given that many patients die without a confirmed diagnosis and that some patients did not come to the emergency department when experiencing mild symptoms during the first months of the pandemic,” he added.
Focusing on the review of 160 patients, the researchers described in-hospital mortality for strokes of all types and for ischemic strokes alone as “remarkably high” (34.4% [95% CI, 27.2%-42.4%] and 35.7% [95% CI, 27.5%-44.8%], respectively), with most deaths occurring among ischemic stroke patients.
“This high mortality rate is higher than the [roughly] 15% to 30% reported for stroke patients without COVID-19 admitted to intensive care units,” Dr. Sposato said.
High-risk phenotype
Many “young” COVID-19 patients (under age 50) who had a stroke (42.9%) had no previous risk factors or comorbidities. Moreover, in almost half of these patients (48.3%), stroke was more likely to occur before the onset of any COVID-19 respiratory symptoms.
Additionally, younger patients showed the highest frequency of elevated cardiac troponin compared with middle-aged and older patients (71.4% vs. 48.4% and 27.8%, respectively). On the other hand, mortality was 67% lower in younger versus older patients (odds ratio, 0.33; 95% CI, 0.12-0.94; P = .039).
Dr. Sposato noted that the proportion of ischemic stroke patients with large-vessel occlusion was “higher than previously reported” for patients with stroke without COVID-19 (47% compared with 29%, respectively).
“We should consider COVID-19 as a new cause or risk factor for stroke. At least, patients with stroke should probably be tested for SARS-CoV-2 infection if they are young and present with a large-vessel occlusion, even in the absence of typical COVID-19 respiratory symptoms,” he suggested.
The researchers identified a “high-risk phenotype” for death for all types of stroke considered together: older age, a higher burden of comorbidities, and severe COVID-19 respiratory symptoms. Patients with all three characteristics had the highest in-hospital mortality rate (58.6%) and a threefold risk of death, compared with the rest of the cohort (OR, 3.52; 95% CI, 1.53-8.09; P = .003).
“Several potential mechanisms can explain the increased risk of stroke among COVID-19 patients, but perhaps the most important one is increased thrombogenesis secondary to an exaggerated inflammatory response,” Dr. Sposato said.
Not just elders
Commenting on the study, Jodi Edwards, PhD, director of the Brain and Heart Nexus Research Program at the University of Ottawa Heart Institute, said the findings are “consistent with and underscore public health messaging emphasizing that COVID-19 does not only affect the elderly and those with underlying health conditions, but can have serious and even fatal consequences at any age.”
Dr. Edwards, who was not involved with the study, emphasized that “adherence to public health recommendations is critical to begin to reduce the rising incidence in younger adults.”
Dr. Sposato acknowledged that the study was small and that there “can be problems associated with a systematic review of case reports, such as publication bias, lack of completeness of data, etc, so more research is needed.”
Dr. Sposato is supported by the Kathleen & Dr. Henry Barnett Research Chair in Stroke Research at Western University, the Edward and Alma Saraydar Neurosciences Fund of the London Health Sciences Foundation, and the Opportunities Fund of the Academic Health Sciences Centre Alternative Funding Plan of the Academic Medical Organization of Southwestern Ontario. Dr. Sposato reported speaker honoraria from Boehringer Ingelheim, Pfizer, Gore, and Bayer and research/quality improvement grants from Boehringer Ingelheim and Bayer. The other authors’ disclosures are listed on the original article. Dr. Edwards has disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
From Neurology
CDC flips, acknowledges aerosol spread of COVID-19
The information reiterates, however, that “COVID-19 is thought to spread mainly through close contact from person to person, including between people who are physically near each other (within about 6 feet). People who are infected but do not show symptoms can also spread the virus to others.”
In a statement to the media, the CDC said, “Today’s update acknowledges the existence of some published reports showing limited, uncommon circumstances where people with COVID-19 infected others who were more than 6 feet away or shortly after the COVID-19–positive person left an area. In these instances, transmission occurred in poorly ventilated and enclosed spaces that often involved activities that caused heavier breathing, like singing or exercise. Such environments and activities may contribute to the buildup of virus-carrying particles.”
“This is HUGE and been long delayed. But glad it’s now CDC official,” tweeted Eric Feigl-Ding, MD, an epidemiologist and health economist at Harvard University, Boston on Oct. 5.
The CDC announcement follows an abrupt flip-flop on information last month surrounding the aerosol spread of the virus.
Information deleted from website last month
On September 18, the CDC had added to its existing guidance that the virus is spread “through respiratory droplets or small particles, such as those in aerosols, produced when an infected person coughs, sneezes, sings, talks, or breathes. These particles can be inhaled into the nose, mouth, airways, and lungs and cause infection.”
The CDC then deleted that guidance on Sept. 21, saying it was a draft update released in error.
A key element of the now-deleted guidance said, “this is thought to be the main way the virus spreads.”
The information updated today reverses the now-deleted guidance and says aerosol transmission is not the main way the virus spreads.
It states that people who are within 6 feet of a person with COVID-19 or have direct contact with that person have the greatest risk of infection.
The CDC reiterated in the statement to the media today, “People can protect themselves from the virus that causes COVID-19 by staying at least 6 feet away from others, wearing a mask that covers their nose and mouth, washing their hands frequently, cleaning touched surfaces often, and staying home when sick.”
Among the journals that have published evidence on aerosol spread is Clinical Infectious Diseases, which, on July 6, published the paper, “It Is Time to Address Airborne Transmission of Coronavirus Disease 2019,” which was supported by 239 scientists.
The authors wrote, “there is significant potential for inhalation exposure to viruses in microscopic respiratory droplets (microdroplets) at short to medium distances (up to several meters, or room scale).”
Aerosols and airborne transmission “are the only way to explain super-spreader events we are seeing,” said Kimberly Prather, PhD, an atmospheric chemist at the University of California at San Diego, in an interview Oct. 5 with the Washington Post.
Dr. Prather added that, once aerosolization is acknowledged, this becomes a “fixable” problem through proper ventilation.
“Wear masks at all times indoors when others are present,” Dr. Prather said. But when inside, she said, there’s no such thing as a completely safe social distance.
This article first appeared on Medscape.com.
The information reiterates, however, that “COVID-19 is thought to spread mainly through close contact from person to person, including between people who are physically near each other (within about 6 feet). People who are infected but do not show symptoms can also spread the virus to others.”
In a statement to the media, the CDC said, “Today’s update acknowledges the existence of some published reports showing limited, uncommon circumstances where people with COVID-19 infected others who were more than 6 feet away or shortly after the COVID-19–positive person left an area. In these instances, transmission occurred in poorly ventilated and enclosed spaces that often involved activities that caused heavier breathing, like singing or exercise. Such environments and activities may contribute to the buildup of virus-carrying particles.”
“This is HUGE and been long delayed. But glad it’s now CDC official,” tweeted Eric Feigl-Ding, MD, an epidemiologist and health economist at Harvard University, Boston on Oct. 5.
The CDC announcement follows an abrupt flip-flop on information last month surrounding the aerosol spread of the virus.
Information deleted from website last month
On September 18, the CDC had added to its existing guidance that the virus is spread “through respiratory droplets or small particles, such as those in aerosols, produced when an infected person coughs, sneezes, sings, talks, or breathes. These particles can be inhaled into the nose, mouth, airways, and lungs and cause infection.”
The CDC then deleted that guidance on Sept. 21, saying it was a draft update released in error.
A key element of the now-deleted guidance said, “this is thought to be the main way the virus spreads.”
The information updated today reverses the now-deleted guidance and says aerosol transmission is not the main way the virus spreads.
It states that people who are within 6 feet of a person with COVID-19 or have direct contact with that person have the greatest risk of infection.
The CDC reiterated in the statement to the media today, “People can protect themselves from the virus that causes COVID-19 by staying at least 6 feet away from others, wearing a mask that covers their nose and mouth, washing their hands frequently, cleaning touched surfaces often, and staying home when sick.”
Among the journals that have published evidence on aerosol spread is Clinical Infectious Diseases, which, on July 6, published the paper, “It Is Time to Address Airborne Transmission of Coronavirus Disease 2019,” which was supported by 239 scientists.
The authors wrote, “there is significant potential for inhalation exposure to viruses in microscopic respiratory droplets (microdroplets) at short to medium distances (up to several meters, or room scale).”
Aerosols and airborne transmission “are the only way to explain super-spreader events we are seeing,” said Kimberly Prather, PhD, an atmospheric chemist at the University of California at San Diego, in an interview Oct. 5 with the Washington Post.
Dr. Prather added that, once aerosolization is acknowledged, this becomes a “fixable” problem through proper ventilation.
“Wear masks at all times indoors when others are present,” Dr. Prather said. But when inside, she said, there’s no such thing as a completely safe social distance.
This article first appeared on Medscape.com.
The information reiterates, however, that “COVID-19 is thought to spread mainly through close contact from person to person, including between people who are physically near each other (within about 6 feet). People who are infected but do not show symptoms can also spread the virus to others.”
In a statement to the media, the CDC said, “Today’s update acknowledges the existence of some published reports showing limited, uncommon circumstances where people with COVID-19 infected others who were more than 6 feet away or shortly after the COVID-19–positive person left an area. In these instances, transmission occurred in poorly ventilated and enclosed spaces that often involved activities that caused heavier breathing, like singing or exercise. Such environments and activities may contribute to the buildup of virus-carrying particles.”
“This is HUGE and been long delayed. But glad it’s now CDC official,” tweeted Eric Feigl-Ding, MD, an epidemiologist and health economist at Harvard University, Boston on Oct. 5.
The CDC announcement follows an abrupt flip-flop on information last month surrounding the aerosol spread of the virus.
Information deleted from website last month
On September 18, the CDC had added to its existing guidance that the virus is spread “through respiratory droplets or small particles, such as those in aerosols, produced when an infected person coughs, sneezes, sings, talks, or breathes. These particles can be inhaled into the nose, mouth, airways, and lungs and cause infection.”
The CDC then deleted that guidance on Sept. 21, saying it was a draft update released in error.
A key element of the now-deleted guidance said, “this is thought to be the main way the virus spreads.”
The information updated today reverses the now-deleted guidance and says aerosol transmission is not the main way the virus spreads.
It states that people who are within 6 feet of a person with COVID-19 or have direct contact with that person have the greatest risk of infection.
The CDC reiterated in the statement to the media today, “People can protect themselves from the virus that causes COVID-19 by staying at least 6 feet away from others, wearing a mask that covers their nose and mouth, washing their hands frequently, cleaning touched surfaces often, and staying home when sick.”
Among the journals that have published evidence on aerosol spread is Clinical Infectious Diseases, which, on July 6, published the paper, “It Is Time to Address Airborne Transmission of Coronavirus Disease 2019,” which was supported by 239 scientists.
The authors wrote, “there is significant potential for inhalation exposure to viruses in microscopic respiratory droplets (microdroplets) at short to medium distances (up to several meters, or room scale).”
Aerosols and airborne transmission “are the only way to explain super-spreader events we are seeing,” said Kimberly Prather, PhD, an atmospheric chemist at the University of California at San Diego, in an interview Oct. 5 with the Washington Post.
Dr. Prather added that, once aerosolization is acknowledged, this becomes a “fixable” problem through proper ventilation.
“Wear masks at all times indoors when others are present,” Dr. Prather said. But when inside, she said, there’s no such thing as a completely safe social distance.
This article first appeared on Medscape.com.