User login
COVID vaccination does not appear to worsen symptoms of Parkinson’s disease
Nonmotor symptoms seemed to improve after SARS-CoV-2 vaccination, although the investigators could not verify a causal relationship.
Vaccination programs should continue for patients with Parkinson’s disease, they said, reporting their clinical results at the International Congress of Parkinson’s Disease and Movement Disorders.
The International Parkinson and Movement Disorder Society has recommended vaccining patients with Parkinson’s disease. “All approved mRNA-based and viral vector vaccines are not expected to interact with Parkinson’s disease, but patients [still] report concern with regard to the benefits, risks, and safeness in Parkinson’s disease,” Mayela Rodríguez-Violante, MD, MSc, and colleagues wrote in an abstract of their findings.
Social isolation may be contributing to these beliefs and concerns, though this is inconclusive.
Investigators from Mexico City conducted a retrospective study of patients with Parkinson’s disease to see how COVID-19 vaccination affected motor and nonmotor symptoms. They enlisted 60 patients (66.7% were male; aged 65.7 ± 11.35 years) who received either a vector-viral vaccine (Vaxzevria Coronavirus) or an mRNA vaccine (BNT162b2).
A Wilcoxon signed-rank test assessed scale differences before and after vaccination, measuring motor involvement (Unified Parkinson’s Disease Rating Scale), nonmotor involvement (Non-Motor Rating Scale [NMSS]), cognitive impairment (Montreal Cognitive Assessment), and quality of life (8-item Parkinson’s Disease Questionnaire index).
Investigators found no significant difference between scales, although they did notice a marked improvement in non-motor symptoms.
“The main takeaway is that vaccination against COVID-19 does not appear to worsen motor or nonmotor symptoms in persons with Parkinson’s disease. The benefits outweigh the risks,” said Dr. Rodríguez-Violante, the study’s lead author and a movement disorder specialist at the National Institute of Neurology and Neurosurgery, Mexico City.
Next steps are to increase the sample size to see if it’s possible to have a similar number in terms of type of vaccine, said Dr. Rodríguez-Violante. “Also, the data presented refers to primary series doses so booster effects will also be studied.”
Few studies have looked at vaccines and their possible effects on this patient population. However, a 2021 study of 181 patients with Parkinson’s disease reported that 2 (1.1%) had adverse effects after receiving the BNT162b2 mRNA vaccine. One of the patients, a 61-year-old woman with a decade-long history of Parkinson’s disease, developed severe, continuous, generalized dyskinesia 6 hours after a first dose of vaccine. The second patient was 79 years old and had Parkinson’s disease for 5 years. She developed fever, confusion, delusions, and continuous severe dyskinesia for 3 days following her vaccination.
“This highlights that there is a variability in the response triggered by the vaccine that might likely depend on individual immunological profiles … clinicians should be aware of this possibility and monitor their patients after they receive their vaccination,” Roberto Erro, MD, PhD and colleagues wrote in the Movement Disorders journal.
Nonmotor symptoms seemed to improve after SARS-CoV-2 vaccination, although the investigators could not verify a causal relationship.
Vaccination programs should continue for patients with Parkinson’s disease, they said, reporting their clinical results at the International Congress of Parkinson’s Disease and Movement Disorders.
The International Parkinson and Movement Disorder Society has recommended vaccining patients with Parkinson’s disease. “All approved mRNA-based and viral vector vaccines are not expected to interact with Parkinson’s disease, but patients [still] report concern with regard to the benefits, risks, and safeness in Parkinson’s disease,” Mayela Rodríguez-Violante, MD, MSc, and colleagues wrote in an abstract of their findings.
Social isolation may be contributing to these beliefs and concerns, though this is inconclusive.
Investigators from Mexico City conducted a retrospective study of patients with Parkinson’s disease to see how COVID-19 vaccination affected motor and nonmotor symptoms. They enlisted 60 patients (66.7% were male; aged 65.7 ± 11.35 years) who received either a vector-viral vaccine (Vaxzevria Coronavirus) or an mRNA vaccine (BNT162b2).
A Wilcoxon signed-rank test assessed scale differences before and after vaccination, measuring motor involvement (Unified Parkinson’s Disease Rating Scale), nonmotor involvement (Non-Motor Rating Scale [NMSS]), cognitive impairment (Montreal Cognitive Assessment), and quality of life (8-item Parkinson’s Disease Questionnaire index).
Investigators found no significant difference between scales, although they did notice a marked improvement in non-motor symptoms.
“The main takeaway is that vaccination against COVID-19 does not appear to worsen motor or nonmotor symptoms in persons with Parkinson’s disease. The benefits outweigh the risks,” said Dr. Rodríguez-Violante, the study’s lead author and a movement disorder specialist at the National Institute of Neurology and Neurosurgery, Mexico City.
Next steps are to increase the sample size to see if it’s possible to have a similar number in terms of type of vaccine, said Dr. Rodríguez-Violante. “Also, the data presented refers to primary series doses so booster effects will also be studied.”
Few studies have looked at vaccines and their possible effects on this patient population. However, a 2021 study of 181 patients with Parkinson’s disease reported that 2 (1.1%) had adverse effects after receiving the BNT162b2 mRNA vaccine. One of the patients, a 61-year-old woman with a decade-long history of Parkinson’s disease, developed severe, continuous, generalized dyskinesia 6 hours after a first dose of vaccine. The second patient was 79 years old and had Parkinson’s disease for 5 years. She developed fever, confusion, delusions, and continuous severe dyskinesia for 3 days following her vaccination.
“This highlights that there is a variability in the response triggered by the vaccine that might likely depend on individual immunological profiles … clinicians should be aware of this possibility and monitor their patients after they receive their vaccination,” Roberto Erro, MD, PhD and colleagues wrote in the Movement Disorders journal.
Nonmotor symptoms seemed to improve after SARS-CoV-2 vaccination, although the investigators could not verify a causal relationship.
Vaccination programs should continue for patients with Parkinson’s disease, they said, reporting their clinical results at the International Congress of Parkinson’s Disease and Movement Disorders.
The International Parkinson and Movement Disorder Society has recommended vaccining patients with Parkinson’s disease. “All approved mRNA-based and viral vector vaccines are not expected to interact with Parkinson’s disease, but patients [still] report concern with regard to the benefits, risks, and safeness in Parkinson’s disease,” Mayela Rodríguez-Violante, MD, MSc, and colleagues wrote in an abstract of their findings.
Social isolation may be contributing to these beliefs and concerns, though this is inconclusive.
Investigators from Mexico City conducted a retrospective study of patients with Parkinson’s disease to see how COVID-19 vaccination affected motor and nonmotor symptoms. They enlisted 60 patients (66.7% were male; aged 65.7 ± 11.35 years) who received either a vector-viral vaccine (Vaxzevria Coronavirus) or an mRNA vaccine (BNT162b2).
A Wilcoxon signed-rank test assessed scale differences before and after vaccination, measuring motor involvement (Unified Parkinson’s Disease Rating Scale), nonmotor involvement (Non-Motor Rating Scale [NMSS]), cognitive impairment (Montreal Cognitive Assessment), and quality of life (8-item Parkinson’s Disease Questionnaire index).
Investigators found no significant difference between scales, although they did notice a marked improvement in non-motor symptoms.
“The main takeaway is that vaccination against COVID-19 does not appear to worsen motor or nonmotor symptoms in persons with Parkinson’s disease. The benefits outweigh the risks,” said Dr. Rodríguez-Violante, the study’s lead author and a movement disorder specialist at the National Institute of Neurology and Neurosurgery, Mexico City.
Next steps are to increase the sample size to see if it’s possible to have a similar number in terms of type of vaccine, said Dr. Rodríguez-Violante. “Also, the data presented refers to primary series doses so booster effects will also be studied.”
Few studies have looked at vaccines and their possible effects on this patient population. However, a 2021 study of 181 patients with Parkinson’s disease reported that 2 (1.1%) had adverse effects after receiving the BNT162b2 mRNA vaccine. One of the patients, a 61-year-old woman with a decade-long history of Parkinson’s disease, developed severe, continuous, generalized dyskinesia 6 hours after a first dose of vaccine. The second patient was 79 years old and had Parkinson’s disease for 5 years. She developed fever, confusion, delusions, and continuous severe dyskinesia for 3 days following her vaccination.
“This highlights that there is a variability in the response triggered by the vaccine that might likely depend on individual immunological profiles … clinicians should be aware of this possibility and monitor their patients after they receive their vaccination,” Roberto Erro, MD, PhD and colleagues wrote in the Movement Disorders journal.
FROM MDS 2022
Children and COVID: Weekly cases drop to lowest level since April
A hefty decline in new COVID-19 cases among children resulted in the lowest weekly total since late April, according to a report from the American Academy of Pediatrics and the Children’s Hospital Association.
, making for 2 consecutive weeks of declines after almost 91,000 cases were recorded for the week ending Sept. 1, the AAP and CHA said in their latest COVID report of state-level data.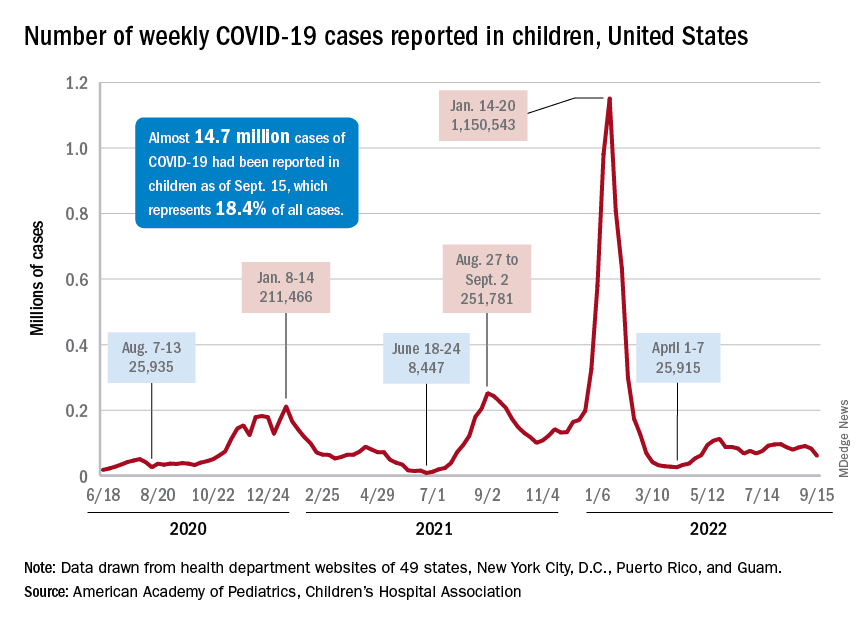
The last time the weekly count was under 60,000 came during the week of April 22-28, when 53,000 were reported by state and territorial health departments in the midst of a 7-week stretch of rising cases. Since that streak ended in mid-May, however, “reported weekly cases have plateaued, fluctuating between a low, now of 60,300 cases and a high of about 112,000,” the AAP noted.
Emergency department visits and hospital admissions, which showed less fluctuation over the summer and more steady rise and fall, have both dropped in recent weeks and are now approaching late May/early June rates, according to data from the Centers for Disease Control and Prevention.
On Sept. 15, for example, ED visits for children under 12 years with diagnosed COVID were just 2.2% of all visits, lower than at any time since May 19 and down from a summer high of 6.8% in late July. Hospital admissions for children aged 0-17 years also rose steadily through June and July, reaching 0.46 per 100,000 population on July 30, but have since slipped to 0.29 per 100,000 as of Sept. 17, the CDC said on its COVID Data Tracker.
Vaccination continues to be a tough sell
Vaccination activity among the most recently eligible age group, in the meantime, remains tepid. Just 6.0% of children under age 5 had received at least one dose of COVID-19 vaccine as of Sept. 13, about 3 months since its final approval in June, and 1.6% were fully vaccinated. For the two older groups of children with separate vaccine approvals, 31.5% of those aged 5-11 years and 43.3% of those aged 12-15 had received at least one dose 3 months after their vaccinations began, the CDC data show.
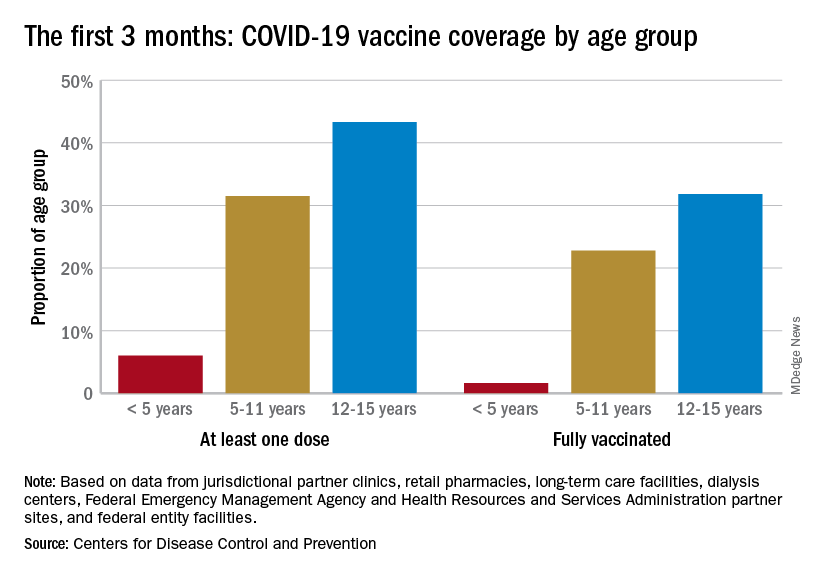
In the 2 weeks ending Sept. 14, almost 59,000 children under age 5 received their initial COVID-19 vaccine dose, as did 28,000 5- to 11-year-olds and 14,000 children aged 12-17. Children under age 5 years represented almost 20% of all Americans getting a first dose during Sept. 1-14, compared with 9.7% for those aged 5-11 and 4.8% for the 12- to 17-year-olds, the CDC said.
At the state level, children under age 5 years in the District of Columbia, where 28% have received at least one dose, and Vermont, at 24%, are the most likely to be vaccinated. The states with the lowest rates in this age group are Alabama, Louisiana, and Mississippi, all of which are at 2%. Vermont and D.C. have the highest rates for ages 5-11 at 70% each, and Alabama (17%) is the lowest, while D.C. (100%), Rhode Island (99%), and Massachusetts (99%) are highest for children aged 12-17 years and Wyoming (41%) is the lowest, the AAP said in a separate report.
A hefty decline in new COVID-19 cases among children resulted in the lowest weekly total since late April, according to a report from the American Academy of Pediatrics and the Children’s Hospital Association.
, making for 2 consecutive weeks of declines after almost 91,000 cases were recorded for the week ending Sept. 1, the AAP and CHA said in their latest COVID report of state-level data.
The last time the weekly count was under 60,000 came during the week of April 22-28, when 53,000 were reported by state and territorial health departments in the midst of a 7-week stretch of rising cases. Since that streak ended in mid-May, however, “reported weekly cases have plateaued, fluctuating between a low, now of 60,300 cases and a high of about 112,000,” the AAP noted.
Emergency department visits and hospital admissions, which showed less fluctuation over the summer and more steady rise and fall, have both dropped in recent weeks and are now approaching late May/early June rates, according to data from the Centers for Disease Control and Prevention.
On Sept. 15, for example, ED visits for children under 12 years with diagnosed COVID were just 2.2% of all visits, lower than at any time since May 19 and down from a summer high of 6.8% in late July. Hospital admissions for children aged 0-17 years also rose steadily through June and July, reaching 0.46 per 100,000 population on July 30, but have since slipped to 0.29 per 100,000 as of Sept. 17, the CDC said on its COVID Data Tracker.
Vaccination continues to be a tough sell
Vaccination activity among the most recently eligible age group, in the meantime, remains tepid. Just 6.0% of children under age 5 had received at least one dose of COVID-19 vaccine as of Sept. 13, about 3 months since its final approval in June, and 1.6% were fully vaccinated. For the two older groups of children with separate vaccine approvals, 31.5% of those aged 5-11 years and 43.3% of those aged 12-15 had received at least one dose 3 months after their vaccinations began, the CDC data show.

In the 2 weeks ending Sept. 14, almost 59,000 children under age 5 received their initial COVID-19 vaccine dose, as did 28,000 5- to 11-year-olds and 14,000 children aged 12-17. Children under age 5 years represented almost 20% of all Americans getting a first dose during Sept. 1-14, compared with 9.7% for those aged 5-11 and 4.8% for the 12- to 17-year-olds, the CDC said.
At the state level, children under age 5 years in the District of Columbia, where 28% have received at least one dose, and Vermont, at 24%, are the most likely to be vaccinated. The states with the lowest rates in this age group are Alabama, Louisiana, and Mississippi, all of which are at 2%. Vermont and D.C. have the highest rates for ages 5-11 at 70% each, and Alabama (17%) is the lowest, while D.C. (100%), Rhode Island (99%), and Massachusetts (99%) are highest for children aged 12-17 years and Wyoming (41%) is the lowest, the AAP said in a separate report.
A hefty decline in new COVID-19 cases among children resulted in the lowest weekly total since late April, according to a report from the American Academy of Pediatrics and the Children’s Hospital Association.
, making for 2 consecutive weeks of declines after almost 91,000 cases were recorded for the week ending Sept. 1, the AAP and CHA said in their latest COVID report of state-level data.
The last time the weekly count was under 60,000 came during the week of April 22-28, when 53,000 were reported by state and territorial health departments in the midst of a 7-week stretch of rising cases. Since that streak ended in mid-May, however, “reported weekly cases have plateaued, fluctuating between a low, now of 60,300 cases and a high of about 112,000,” the AAP noted.
Emergency department visits and hospital admissions, which showed less fluctuation over the summer and more steady rise and fall, have both dropped in recent weeks and are now approaching late May/early June rates, according to data from the Centers for Disease Control and Prevention.
On Sept. 15, for example, ED visits for children under 12 years with diagnosed COVID were just 2.2% of all visits, lower than at any time since May 19 and down from a summer high of 6.8% in late July. Hospital admissions for children aged 0-17 years also rose steadily through June and July, reaching 0.46 per 100,000 population on July 30, but have since slipped to 0.29 per 100,000 as of Sept. 17, the CDC said on its COVID Data Tracker.
Vaccination continues to be a tough sell
Vaccination activity among the most recently eligible age group, in the meantime, remains tepid. Just 6.0% of children under age 5 had received at least one dose of COVID-19 vaccine as of Sept. 13, about 3 months since its final approval in June, and 1.6% were fully vaccinated. For the two older groups of children with separate vaccine approvals, 31.5% of those aged 5-11 years and 43.3% of those aged 12-15 had received at least one dose 3 months after their vaccinations began, the CDC data show.

In the 2 weeks ending Sept. 14, almost 59,000 children under age 5 received their initial COVID-19 vaccine dose, as did 28,000 5- to 11-year-olds and 14,000 children aged 12-17. Children under age 5 years represented almost 20% of all Americans getting a first dose during Sept. 1-14, compared with 9.7% for those aged 5-11 and 4.8% for the 12- to 17-year-olds, the CDC said.
At the state level, children under age 5 years in the District of Columbia, where 28% have received at least one dose, and Vermont, at 24%, are the most likely to be vaccinated. The states with the lowest rates in this age group are Alabama, Louisiana, and Mississippi, all of which are at 2%. Vermont and D.C. have the highest rates for ages 5-11 at 70% each, and Alabama (17%) is the lowest, while D.C. (100%), Rhode Island (99%), and Massachusetts (99%) are highest for children aged 12-17 years and Wyoming (41%) is the lowest, the AAP said in a separate report.
COVID-19 linked to increased Alzheimer’s risk
The study of more than 6 million people aged 65 years or older found a 50%-80% increased risk for AD in the year after COVID-19; the risk was especially high for women older than 85 years.
However, the investigators were quick to point out that the observational retrospective study offers no evidence that COVID-19 causes AD. There could be a viral etiology at play, or the connection could be related to inflammation in neural tissue from the SARS-CoV-2 infection. Or it could simply be that exposure to the health care system for COVID-19 increased the odds of detection of existing undiagnosed AD cases.
Whatever the case, these findings point to a potential spike in AD cases, which is a cause for concern, study investigator Pamela Davis, MD, PhD, a professor in the Center for Community Health Integration at Case Western Reserve University, Cleveland, said in an interview.
“COVID may be giving us a legacy of ongoing medical difficulties,” Dr. Davis said. “We were already concerned about having a very large care burden and cost burden from Alzheimer’s disease. If this is another burden that’s increased by COVID, this is something we’re really going to have to prepare for.”
The findings were published online in Journal of Alzheimer’s Disease.
Increased risk
Earlier research points to a potential link between COVID-19 and increased risk for AD and Parkinson’s disease.
For the current study, researchers analyzed anonymous electronic health records of 6.2 million adults aged 65 years or older who received medical treatment between February 2020 and May 2021 and had no prior diagnosis of AD. The database includes information on almost 30% of the entire U.S. population.
Overall, there were 410,748 cases of COVID-19 during the study period.
The overall risk for new diagnosis of AD in the COVID-19 cohort was close to double that of those who did not have COVID-19 (0.68% vs. 0.35%, respectively).
After propensity-score matching, those who have had COVID-19 had a significantly higher risk for an AD diagnosis compared with those who were not infected (hazard ratio [HR], 1.69; 95% confidence interval [CI],1.53-1.72).
Risk for AD was elevated in all age groups, regardless of gender or ethnicity. Researchers did not collect data on COVID-19 severity, and the medical codes for long COVID were not published until after the study had ended.
Those with the highest risk were individuals older than 85 years (HR, 1.89; 95% CI, 1.73-2.07) and women (HR, 1.82; 95% CI, 1.69-1.97).
“We expected to see some impact, but I was surprised that it was as potent as it was,” Dr. Davis said.
Association, not causation
Heather Snyder, PhD, Alzheimer’s Association vice president of medical and scientific relations, who commented on the findings for this article, called the study interesting but emphasized caution in interpreting the results.
“Because this study only showed an association through medical records, we cannot know what the underlying mechanisms driving this association are without more research,” Dr. Snyder said. “If you have had COVID-19, it doesn’t mean you’re going to get dementia. But if you have had COVID-19 and are experiencing long-term symptoms including cognitive difficulties, talk to your doctor.”
Dr. Davis agreed, noting that this type of study offers information on association, but not causation. “I do think that this makes it imperative that we continue to follow the population for what’s going on in various neurodegenerative diseases,” Dr. Davis said.
The study was funded by the National Institute of Aging, National Institute on Alcohol Abuse and Alcoholism, the Clinical and Translational Science Collaborative of Cleveland, and the National Cancer Institute. Dr. Synder reports no relevant financial conflicts.
A version of this article first appeared on Medscape.com.
The study of more than 6 million people aged 65 years or older found a 50%-80% increased risk for AD in the year after COVID-19; the risk was especially high for women older than 85 years.
However, the investigators were quick to point out that the observational retrospective study offers no evidence that COVID-19 causes AD. There could be a viral etiology at play, or the connection could be related to inflammation in neural tissue from the SARS-CoV-2 infection. Or it could simply be that exposure to the health care system for COVID-19 increased the odds of detection of existing undiagnosed AD cases.
Whatever the case, these findings point to a potential spike in AD cases, which is a cause for concern, study investigator Pamela Davis, MD, PhD, a professor in the Center for Community Health Integration at Case Western Reserve University, Cleveland, said in an interview.
“COVID may be giving us a legacy of ongoing medical difficulties,” Dr. Davis said. “We were already concerned about having a very large care burden and cost burden from Alzheimer’s disease. If this is another burden that’s increased by COVID, this is something we’re really going to have to prepare for.”
The findings were published online in Journal of Alzheimer’s Disease.
Increased risk
Earlier research points to a potential link between COVID-19 and increased risk for AD and Parkinson’s disease.
For the current study, researchers analyzed anonymous electronic health records of 6.2 million adults aged 65 years or older who received medical treatment between February 2020 and May 2021 and had no prior diagnosis of AD. The database includes information on almost 30% of the entire U.S. population.
Overall, there were 410,748 cases of COVID-19 during the study period.
The overall risk for new diagnosis of AD in the COVID-19 cohort was close to double that of those who did not have COVID-19 (0.68% vs. 0.35%, respectively).
After propensity-score matching, those who have had COVID-19 had a significantly higher risk for an AD diagnosis compared with those who were not infected (hazard ratio [HR], 1.69; 95% confidence interval [CI],1.53-1.72).
Risk for AD was elevated in all age groups, regardless of gender or ethnicity. Researchers did not collect data on COVID-19 severity, and the medical codes for long COVID were not published until after the study had ended.
Those with the highest risk were individuals older than 85 years (HR, 1.89; 95% CI, 1.73-2.07) and women (HR, 1.82; 95% CI, 1.69-1.97).
“We expected to see some impact, but I was surprised that it was as potent as it was,” Dr. Davis said.
Association, not causation
Heather Snyder, PhD, Alzheimer’s Association vice president of medical and scientific relations, who commented on the findings for this article, called the study interesting but emphasized caution in interpreting the results.
“Because this study only showed an association through medical records, we cannot know what the underlying mechanisms driving this association are without more research,” Dr. Snyder said. “If you have had COVID-19, it doesn’t mean you’re going to get dementia. But if you have had COVID-19 and are experiencing long-term symptoms including cognitive difficulties, talk to your doctor.”
Dr. Davis agreed, noting that this type of study offers information on association, but not causation. “I do think that this makes it imperative that we continue to follow the population for what’s going on in various neurodegenerative diseases,” Dr. Davis said.
The study was funded by the National Institute of Aging, National Institute on Alcohol Abuse and Alcoholism, the Clinical and Translational Science Collaborative of Cleveland, and the National Cancer Institute. Dr. Synder reports no relevant financial conflicts.
A version of this article first appeared on Medscape.com.
The study of more than 6 million people aged 65 years or older found a 50%-80% increased risk for AD in the year after COVID-19; the risk was especially high for women older than 85 years.
However, the investigators were quick to point out that the observational retrospective study offers no evidence that COVID-19 causes AD. There could be a viral etiology at play, or the connection could be related to inflammation in neural tissue from the SARS-CoV-2 infection. Or it could simply be that exposure to the health care system for COVID-19 increased the odds of detection of existing undiagnosed AD cases.
Whatever the case, these findings point to a potential spike in AD cases, which is a cause for concern, study investigator Pamela Davis, MD, PhD, a professor in the Center for Community Health Integration at Case Western Reserve University, Cleveland, said in an interview.
“COVID may be giving us a legacy of ongoing medical difficulties,” Dr. Davis said. “We were already concerned about having a very large care burden and cost burden from Alzheimer’s disease. If this is another burden that’s increased by COVID, this is something we’re really going to have to prepare for.”
The findings were published online in Journal of Alzheimer’s Disease.
Increased risk
Earlier research points to a potential link between COVID-19 and increased risk for AD and Parkinson’s disease.
For the current study, researchers analyzed anonymous electronic health records of 6.2 million adults aged 65 years or older who received medical treatment between February 2020 and May 2021 and had no prior diagnosis of AD. The database includes information on almost 30% of the entire U.S. population.
Overall, there were 410,748 cases of COVID-19 during the study period.
The overall risk for new diagnosis of AD in the COVID-19 cohort was close to double that of those who did not have COVID-19 (0.68% vs. 0.35%, respectively).
After propensity-score matching, those who have had COVID-19 had a significantly higher risk for an AD diagnosis compared with those who were not infected (hazard ratio [HR], 1.69; 95% confidence interval [CI],1.53-1.72).
Risk for AD was elevated in all age groups, regardless of gender or ethnicity. Researchers did not collect data on COVID-19 severity, and the medical codes for long COVID were not published until after the study had ended.
Those with the highest risk were individuals older than 85 years (HR, 1.89; 95% CI, 1.73-2.07) and women (HR, 1.82; 95% CI, 1.69-1.97).
“We expected to see some impact, but I was surprised that it was as potent as it was,” Dr. Davis said.
Association, not causation
Heather Snyder, PhD, Alzheimer’s Association vice president of medical and scientific relations, who commented on the findings for this article, called the study interesting but emphasized caution in interpreting the results.
“Because this study only showed an association through medical records, we cannot know what the underlying mechanisms driving this association are without more research,” Dr. Snyder said. “If you have had COVID-19, it doesn’t mean you’re going to get dementia. But if you have had COVID-19 and are experiencing long-term symptoms including cognitive difficulties, talk to your doctor.”
Dr. Davis agreed, noting that this type of study offers information on association, but not causation. “I do think that this makes it imperative that we continue to follow the population for what’s going on in various neurodegenerative diseases,” Dr. Davis said.
The study was funded by the National Institute of Aging, National Institute on Alcohol Abuse and Alcoholism, the Clinical and Translational Science Collaborative of Cleveland, and the National Cancer Institute. Dr. Synder reports no relevant financial conflicts.
A version of this article first appeared on Medscape.com.
FROM THE JOURNAL OF ALZHEIMER’S DISEASE
People of color bearing brunt of long COVID, doctors say
From the earliest days of the COVID-19 pandemic, people of color have been hardest hit by the virus. Now, many doctors and researchers are seeing big disparities come about in who gets care for long COVID.
Long COVID can affect patients from all walks of life. said Alba Miranda Azola, MD, codirector of the post–acute COVID-19 team at Johns Hopkins University, Baltimore.
Non-White patients are more apt to lack access to primary care, face insurance barriers to see specialists, struggle with time off work or transportation for appointments, and have financial barriers to care as copayments for therapy pile up.
“We are getting a very skewed population of Caucasian wealthy people who are coming to our clinic because they have the ability to access care, they have good insurance, and they are looking on the internet and find us,” Dr. Azola said.
This mix of patients at Dr. Azola’s clinic is out of step with the demographics of Baltimore, where the majority of residents are Black, half of them earn less than $52,000 a year, and one in five live in poverty. And this isn’t unique to Hopkins. Many of the dozens of specialized long COVID clinics that have cropped up around the country are also seeing an unequal share of affluent White patients, experts say.
It’s also a patient mix that very likely doesn’t reflect who is most apt to have long COVID.
During the pandemic, people who identified as Black, Hispanic, American Indian, or Alaska Native were more likely to be diagnosed with COVID than people who identified as White, according to the Centers for Disease Control and Prevention. These people of color were also at least twice as likely to be hospitalized with severe infections, and at least 70% more likely to die.
“Data repeatedly show the disproportionate impact of COVID-19 on racial and ethnic minority populations, as well as other population groups such as people living in rural or frontier areas, people experiencing homelessness, essential and frontline workers, people with disabilities, people with substance use disorders, people who are incarcerated, and non–U.S.-born persons,” John Brooks, MD, chief medical officer for COVID-19 response at the CDC, said during testimony before the U.S. House Energy and Commerce Subcommittee on Health in April 2021.
“While we do not yet have clear data on the impact of post-COVID conditions on racial and ethnic minority populations and other disadvantaged communities, we do believe that they are likely to be disproportionately impacted ... and less likely to be able to access health care services,” Dr. Brooks said at the time.
The picture that’s emerging of long COVID suggests that the condition impacts about one in five adults. It’s more common among Hispanic adults than among people who identify as Black, Asian, or White. It’s also more common among those who identify as other races or multiple races, according survey data collected by the CDC.
It’s hard to say how accurate this snapshot is because researchers need to do a better job of identifying and following people with long COVID, said Monica Verduzco-Gutierrez, MD, chair of rehabilitation medicine and director of the COVID-19 Recovery Clinic at the University of Texas Health Science Center at San Antonio. A major limitation of surveys like the ones done by the CDC to monitor long COVID is that only people who realize they have the condition can get counted.
“Some people from historically marginalized groups may have less health literacy to know about impacts of long COVID,” she said.
Lack of awareness may keep people with persistent symptoms from seeking medical attention, leaving many long COVID cases undiagnosed.
When some patients do seek help, their complaints may not be acknowledged or understood. Often, cultural bias or structural racism can get in the way of diagnosis and treatment, Dr. Azola said.
“I hate to say this, but there is probably bias among providers,” she said. “For example, I am Puerto Rican, and the way we describe symptoms as Latinos may sound exaggerated or may be brushed aside or lost in translation. I think we miss a lot of patients being diagnosed or referred to specialists because the primary care provider they see maybe leans into this cultural bias of thinking this is just a Latino being dramatic.”
There’s some evidence that treatment for long COVID may differ by race even when symptoms are similar. One study of more than 400,000 patients, for example, found no racial differences in the proportion of people who have six common long COVID symptoms: shortness of breath, fatigue, weakness, pain, trouble with thinking skills, and a hard time getting around. Despite this, Black patients were significantly less likely to receive outpatient rehabilitation services to treat these symptoms.
Benjamin Abramoff, MD, who leads the long COVID collaborative for the American Academy of Physical Medicine and Rehabilitation, draws parallels between what happens with long COVID to another common health problem often undertreated among patients of color: pain. With both long COVID and chronic pain, one major barrier to care is “just getting taken seriously by providers,” he said.
“There is significant evidence that racial bias has led to less prescription of pain medications to people of color,” Dr. Abramoff said. “Just as pain can be difficult to get objective measures of, long COVID symptoms can also be difficult to objectively measure and requires trust between the provider and patient.”
Geography can be another barrier to care, said Aaron Friedberg, MD, clinical colead of the post-COVID recovery program at Ohio State University Wexner Medical Center, Columbus. Many communities hardest hit by COVID – particularly in high-poverty urban neighborhoods – have long had limited access to care. The pandemic worsened staffing shortages at many hospitals and clinics in these communities, leaving patients even fewer options close to home.
“I often have patients driving several hours to come to our clinic, and that can create significant challenges both because of the financial burden and time required to coordinate that type of travel, but also because post-COVID symptoms can make it extremely challenging to tolerate that type of travel,” Dr. Friedberg said.
Even though the complete picture of who has long COVID – and who’s getting treated and getting good outcomes – is still emerging, it’s very clear at this point in the pandemic that access isn’t equal among everyone and that many low-income and non-White patients are missing out on needed treatments, Friedberg said.
“One thing that is clear is that there are many people suffering alone from these conditions,” he said.
A version of this article first appeared on WebMD.com.
From the earliest days of the COVID-19 pandemic, people of color have been hardest hit by the virus. Now, many doctors and researchers are seeing big disparities come about in who gets care for long COVID.
Long COVID can affect patients from all walks of life. said Alba Miranda Azola, MD, codirector of the post–acute COVID-19 team at Johns Hopkins University, Baltimore.
Non-White patients are more apt to lack access to primary care, face insurance barriers to see specialists, struggle with time off work or transportation for appointments, and have financial barriers to care as copayments for therapy pile up.
“We are getting a very skewed population of Caucasian wealthy people who are coming to our clinic because they have the ability to access care, they have good insurance, and they are looking on the internet and find us,” Dr. Azola said.
This mix of patients at Dr. Azola’s clinic is out of step with the demographics of Baltimore, where the majority of residents are Black, half of them earn less than $52,000 a year, and one in five live in poverty. And this isn’t unique to Hopkins. Many of the dozens of specialized long COVID clinics that have cropped up around the country are also seeing an unequal share of affluent White patients, experts say.
It’s also a patient mix that very likely doesn’t reflect who is most apt to have long COVID.
During the pandemic, people who identified as Black, Hispanic, American Indian, or Alaska Native were more likely to be diagnosed with COVID than people who identified as White, according to the Centers for Disease Control and Prevention. These people of color were also at least twice as likely to be hospitalized with severe infections, and at least 70% more likely to die.
“Data repeatedly show the disproportionate impact of COVID-19 on racial and ethnic minority populations, as well as other population groups such as people living in rural or frontier areas, people experiencing homelessness, essential and frontline workers, people with disabilities, people with substance use disorders, people who are incarcerated, and non–U.S.-born persons,” John Brooks, MD, chief medical officer for COVID-19 response at the CDC, said during testimony before the U.S. House Energy and Commerce Subcommittee on Health in April 2021.
“While we do not yet have clear data on the impact of post-COVID conditions on racial and ethnic minority populations and other disadvantaged communities, we do believe that they are likely to be disproportionately impacted ... and less likely to be able to access health care services,” Dr. Brooks said at the time.
The picture that’s emerging of long COVID suggests that the condition impacts about one in five adults. It’s more common among Hispanic adults than among people who identify as Black, Asian, or White. It’s also more common among those who identify as other races or multiple races, according survey data collected by the CDC.
It’s hard to say how accurate this snapshot is because researchers need to do a better job of identifying and following people with long COVID, said Monica Verduzco-Gutierrez, MD, chair of rehabilitation medicine and director of the COVID-19 Recovery Clinic at the University of Texas Health Science Center at San Antonio. A major limitation of surveys like the ones done by the CDC to monitor long COVID is that only people who realize they have the condition can get counted.
“Some people from historically marginalized groups may have less health literacy to know about impacts of long COVID,” she said.
Lack of awareness may keep people with persistent symptoms from seeking medical attention, leaving many long COVID cases undiagnosed.
When some patients do seek help, their complaints may not be acknowledged or understood. Often, cultural bias or structural racism can get in the way of diagnosis and treatment, Dr. Azola said.
“I hate to say this, but there is probably bias among providers,” she said. “For example, I am Puerto Rican, and the way we describe symptoms as Latinos may sound exaggerated or may be brushed aside or lost in translation. I think we miss a lot of patients being diagnosed or referred to specialists because the primary care provider they see maybe leans into this cultural bias of thinking this is just a Latino being dramatic.”
There’s some evidence that treatment for long COVID may differ by race even when symptoms are similar. One study of more than 400,000 patients, for example, found no racial differences in the proportion of people who have six common long COVID symptoms: shortness of breath, fatigue, weakness, pain, trouble with thinking skills, and a hard time getting around. Despite this, Black patients were significantly less likely to receive outpatient rehabilitation services to treat these symptoms.
Benjamin Abramoff, MD, who leads the long COVID collaborative for the American Academy of Physical Medicine and Rehabilitation, draws parallels between what happens with long COVID to another common health problem often undertreated among patients of color: pain. With both long COVID and chronic pain, one major barrier to care is “just getting taken seriously by providers,” he said.
“There is significant evidence that racial bias has led to less prescription of pain medications to people of color,” Dr. Abramoff said. “Just as pain can be difficult to get objective measures of, long COVID symptoms can also be difficult to objectively measure and requires trust between the provider and patient.”
Geography can be another barrier to care, said Aaron Friedberg, MD, clinical colead of the post-COVID recovery program at Ohio State University Wexner Medical Center, Columbus. Many communities hardest hit by COVID – particularly in high-poverty urban neighborhoods – have long had limited access to care. The pandemic worsened staffing shortages at many hospitals and clinics in these communities, leaving patients even fewer options close to home.
“I often have patients driving several hours to come to our clinic, and that can create significant challenges both because of the financial burden and time required to coordinate that type of travel, but also because post-COVID symptoms can make it extremely challenging to tolerate that type of travel,” Dr. Friedberg said.
Even though the complete picture of who has long COVID – and who’s getting treated and getting good outcomes – is still emerging, it’s very clear at this point in the pandemic that access isn’t equal among everyone and that many low-income and non-White patients are missing out on needed treatments, Friedberg said.
“One thing that is clear is that there are many people suffering alone from these conditions,” he said.
A version of this article first appeared on WebMD.com.
From the earliest days of the COVID-19 pandemic, people of color have been hardest hit by the virus. Now, many doctors and researchers are seeing big disparities come about in who gets care for long COVID.
Long COVID can affect patients from all walks of life. said Alba Miranda Azola, MD, codirector of the post–acute COVID-19 team at Johns Hopkins University, Baltimore.
Non-White patients are more apt to lack access to primary care, face insurance barriers to see specialists, struggle with time off work or transportation for appointments, and have financial barriers to care as copayments for therapy pile up.
“We are getting a very skewed population of Caucasian wealthy people who are coming to our clinic because they have the ability to access care, they have good insurance, and they are looking on the internet and find us,” Dr. Azola said.
This mix of patients at Dr. Azola’s clinic is out of step with the demographics of Baltimore, where the majority of residents are Black, half of them earn less than $52,000 a year, and one in five live in poverty. And this isn’t unique to Hopkins. Many of the dozens of specialized long COVID clinics that have cropped up around the country are also seeing an unequal share of affluent White patients, experts say.
It’s also a patient mix that very likely doesn’t reflect who is most apt to have long COVID.
During the pandemic, people who identified as Black, Hispanic, American Indian, or Alaska Native were more likely to be diagnosed with COVID than people who identified as White, according to the Centers for Disease Control and Prevention. These people of color were also at least twice as likely to be hospitalized with severe infections, and at least 70% more likely to die.
“Data repeatedly show the disproportionate impact of COVID-19 on racial and ethnic minority populations, as well as other population groups such as people living in rural or frontier areas, people experiencing homelessness, essential and frontline workers, people with disabilities, people with substance use disorders, people who are incarcerated, and non–U.S.-born persons,” John Brooks, MD, chief medical officer for COVID-19 response at the CDC, said during testimony before the U.S. House Energy and Commerce Subcommittee on Health in April 2021.
“While we do not yet have clear data on the impact of post-COVID conditions on racial and ethnic minority populations and other disadvantaged communities, we do believe that they are likely to be disproportionately impacted ... and less likely to be able to access health care services,” Dr. Brooks said at the time.
The picture that’s emerging of long COVID suggests that the condition impacts about one in five adults. It’s more common among Hispanic adults than among people who identify as Black, Asian, or White. It’s also more common among those who identify as other races or multiple races, according survey data collected by the CDC.
It’s hard to say how accurate this snapshot is because researchers need to do a better job of identifying and following people with long COVID, said Monica Verduzco-Gutierrez, MD, chair of rehabilitation medicine and director of the COVID-19 Recovery Clinic at the University of Texas Health Science Center at San Antonio. A major limitation of surveys like the ones done by the CDC to monitor long COVID is that only people who realize they have the condition can get counted.
“Some people from historically marginalized groups may have less health literacy to know about impacts of long COVID,” she said.
Lack of awareness may keep people with persistent symptoms from seeking medical attention, leaving many long COVID cases undiagnosed.
When some patients do seek help, their complaints may not be acknowledged or understood. Often, cultural bias or structural racism can get in the way of diagnosis and treatment, Dr. Azola said.
“I hate to say this, but there is probably bias among providers,” she said. “For example, I am Puerto Rican, and the way we describe symptoms as Latinos may sound exaggerated or may be brushed aside or lost in translation. I think we miss a lot of patients being diagnosed or referred to specialists because the primary care provider they see maybe leans into this cultural bias of thinking this is just a Latino being dramatic.”
There’s some evidence that treatment for long COVID may differ by race even when symptoms are similar. One study of more than 400,000 patients, for example, found no racial differences in the proportion of people who have six common long COVID symptoms: shortness of breath, fatigue, weakness, pain, trouble with thinking skills, and a hard time getting around. Despite this, Black patients were significantly less likely to receive outpatient rehabilitation services to treat these symptoms.
Benjamin Abramoff, MD, who leads the long COVID collaborative for the American Academy of Physical Medicine and Rehabilitation, draws parallels between what happens with long COVID to another common health problem often undertreated among patients of color: pain. With both long COVID and chronic pain, one major barrier to care is “just getting taken seriously by providers,” he said.
“There is significant evidence that racial bias has led to less prescription of pain medications to people of color,” Dr. Abramoff said. “Just as pain can be difficult to get objective measures of, long COVID symptoms can also be difficult to objectively measure and requires trust between the provider and patient.”
Geography can be another barrier to care, said Aaron Friedberg, MD, clinical colead of the post-COVID recovery program at Ohio State University Wexner Medical Center, Columbus. Many communities hardest hit by COVID – particularly in high-poverty urban neighborhoods – have long had limited access to care. The pandemic worsened staffing shortages at many hospitals and clinics in these communities, leaving patients even fewer options close to home.
“I often have patients driving several hours to come to our clinic, and that can create significant challenges both because of the financial burden and time required to coordinate that type of travel, but also because post-COVID symptoms can make it extremely challenging to tolerate that type of travel,” Dr. Friedberg said.
Even though the complete picture of who has long COVID – and who’s getting treated and getting good outcomes – is still emerging, it’s very clear at this point in the pandemic that access isn’t equal among everyone and that many low-income and non-White patients are missing out on needed treatments, Friedberg said.
“One thing that is clear is that there are many people suffering alone from these conditions,” he said.
A version of this article first appeared on WebMD.com.
Children and COVID: New cases took a downturn in September
After 2 weeks of increases in the number of new COVID-19 cases in children – a trend that just happened to coincide with the start of a new school year – there were fewer cases reported during the first full week of September, according to the American Academy of Pediatrics and the Children’s Hospital Association.
, the AAP and CHA said in their weekly COVID-19 report, noting also that seven states and the District of Columbia no longer update their online dashboards while others publish new data less often than every week.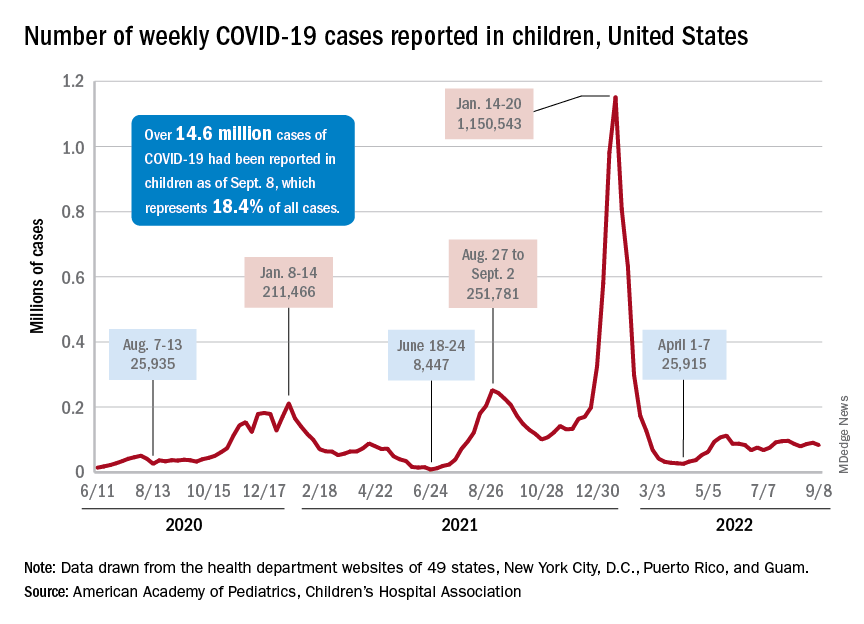
The drop in new cases was accompanied by declines in emergency department visits and hospital admissions, both of which had shown some signs of resurgence in mid- to late August. The brief rise in ED visits seemed to be age-related, occurring in those aged 12 years and older but not in younger children, whose ED visit rate fell steadily through August. Through the first week of September, however, 7-day averages were down for both those aged 12-15 and for 16- to 17-year-olds, the Centers for Disease Control and Prevention reported.
The rate of new hospital admissions of children with confirmed COVID-19, available only for ages 0-17 years, has declined every day since Aug. 28, when it reached 0.44 per 100,000 population after a week of climbing, the CDC said on its COVID Data Tracker.
Cumulatively, about 156,000 children were hospitalized with COVID from Aug. 1, 2020 to Sept. 10, 2022, according to the CDC, which puts the total number of pediatric cases at just over 15 million and deaths at 1,778. Those last two figures represent 17.4% and about 0.4% of all U.S. cases and deaths. The AAP and CHA estimate that about 14.6 million child cases have been reported so far, which is 18.4% of cases in all ages.
Vaccinations are slowly adding up
On the prevention side of the health care system’s response to COVID, the CDC’s cumulative numbers looked like this as of Sept. 6:
- 1.1 million children under age 5 (about 5.8% of the age group) had received at least one dose of vaccine, and 280,000 (1.4%) were fully vaccinated.
- Almost 11 million (38.2%) children aged 5-11 had gotten one dose, and 8.9 million (31.1%) were fully vaccinated.
- 17.9 million (70.8%) children aged 12-17 had received at least one dose, and 15.3 million (60.5%) were fully vaccinated.
Over the 14 days ending Sept. 7, children aged 2-4 years made up the largest group (21.4%) of Americans getting their first vaccine doses, while those aged 5-11 years were the third largest age group at 16.7% of all vaccinees (25- to 49-year-olds were second). The situation was reversed for vaccine completion over the last 2 weeks: Those aged 5-11 were first at 24.7%, and the 2- to 4-year-olds were third at 16.7% (those aged 25-49 were second again), according to the COVID Data Tracker.
After 2 weeks of increases in the number of new COVID-19 cases in children – a trend that just happened to coincide with the start of a new school year – there were fewer cases reported during the first full week of September, according to the American Academy of Pediatrics and the Children’s Hospital Association.
, the AAP and CHA said in their weekly COVID-19 report, noting also that seven states and the District of Columbia no longer update their online dashboards while others publish new data less often than every week.
The drop in new cases was accompanied by declines in emergency department visits and hospital admissions, both of which had shown some signs of resurgence in mid- to late August. The brief rise in ED visits seemed to be age-related, occurring in those aged 12 years and older but not in younger children, whose ED visit rate fell steadily through August. Through the first week of September, however, 7-day averages were down for both those aged 12-15 and for 16- to 17-year-olds, the Centers for Disease Control and Prevention reported.
The rate of new hospital admissions of children with confirmed COVID-19, available only for ages 0-17 years, has declined every day since Aug. 28, when it reached 0.44 per 100,000 population after a week of climbing, the CDC said on its COVID Data Tracker.
Cumulatively, about 156,000 children were hospitalized with COVID from Aug. 1, 2020 to Sept. 10, 2022, according to the CDC, which puts the total number of pediatric cases at just over 15 million and deaths at 1,778. Those last two figures represent 17.4% and about 0.4% of all U.S. cases and deaths. The AAP and CHA estimate that about 14.6 million child cases have been reported so far, which is 18.4% of cases in all ages.
Vaccinations are slowly adding up
On the prevention side of the health care system’s response to COVID, the CDC’s cumulative numbers looked like this as of Sept. 6:
- 1.1 million children under age 5 (about 5.8% of the age group) had received at least one dose of vaccine, and 280,000 (1.4%) were fully vaccinated.
- Almost 11 million (38.2%) children aged 5-11 had gotten one dose, and 8.9 million (31.1%) were fully vaccinated.
- 17.9 million (70.8%) children aged 12-17 had received at least one dose, and 15.3 million (60.5%) were fully vaccinated.
Over the 14 days ending Sept. 7, children aged 2-4 years made up the largest group (21.4%) of Americans getting their first vaccine doses, while those aged 5-11 years were the third largest age group at 16.7% of all vaccinees (25- to 49-year-olds were second). The situation was reversed for vaccine completion over the last 2 weeks: Those aged 5-11 were first at 24.7%, and the 2- to 4-year-olds were third at 16.7% (those aged 25-49 were second again), according to the COVID Data Tracker.
After 2 weeks of increases in the number of new COVID-19 cases in children – a trend that just happened to coincide with the start of a new school year – there were fewer cases reported during the first full week of September, according to the American Academy of Pediatrics and the Children’s Hospital Association.
, the AAP and CHA said in their weekly COVID-19 report, noting also that seven states and the District of Columbia no longer update their online dashboards while others publish new data less often than every week.
The drop in new cases was accompanied by declines in emergency department visits and hospital admissions, both of which had shown some signs of resurgence in mid- to late August. The brief rise in ED visits seemed to be age-related, occurring in those aged 12 years and older but not in younger children, whose ED visit rate fell steadily through August. Through the first week of September, however, 7-day averages were down for both those aged 12-15 and for 16- to 17-year-olds, the Centers for Disease Control and Prevention reported.
The rate of new hospital admissions of children with confirmed COVID-19, available only for ages 0-17 years, has declined every day since Aug. 28, when it reached 0.44 per 100,000 population after a week of climbing, the CDC said on its COVID Data Tracker.
Cumulatively, about 156,000 children were hospitalized with COVID from Aug. 1, 2020 to Sept. 10, 2022, according to the CDC, which puts the total number of pediatric cases at just over 15 million and deaths at 1,778. Those last two figures represent 17.4% and about 0.4% of all U.S. cases and deaths. The AAP and CHA estimate that about 14.6 million child cases have been reported so far, which is 18.4% of cases in all ages.
Vaccinations are slowly adding up
On the prevention side of the health care system’s response to COVID, the CDC’s cumulative numbers looked like this as of Sept. 6:
- 1.1 million children under age 5 (about 5.8% of the age group) had received at least one dose of vaccine, and 280,000 (1.4%) were fully vaccinated.
- Almost 11 million (38.2%) children aged 5-11 had gotten one dose, and 8.9 million (31.1%) were fully vaccinated.
- 17.9 million (70.8%) children aged 12-17 had received at least one dose, and 15.3 million (60.5%) were fully vaccinated.
Over the 14 days ending Sept. 7, children aged 2-4 years made up the largest group (21.4%) of Americans getting their first vaccine doses, while those aged 5-11 years were the third largest age group at 16.7% of all vaccinees (25- to 49-year-olds were second). The situation was reversed for vaccine completion over the last 2 weeks: Those aged 5-11 were first at 24.7%, and the 2- to 4-year-olds were third at 16.7% (those aged 25-49 were second again), according to the COVID Data Tracker.
FAQ: New COVID Omicron boosters
Here are answers to frequently asked questions about the shots produced by Moderna and Pfizer/BioNTech, based on information provided by the CDC and Keri Althoff, PhD, and virologist Andrew Pekosz, PhD, Johns Hopkins Bloomberg School of Public Health epidemiologists.
Question: Who is eligible for the new bivalent boosters?
Answer: The CDC greenlighted the upgraded Pfizer/BioNTech shots for Americans 12 and older and the Moderna booster for those 18 and over, if they have received a primary vaccine series or a booster at least 2 months before.
The boosters have been redesigned to protect against the predominant BA.4 and BA.5 strains of the virus. The Biden administration is making 160 million of the booster shots available free of charge through pharmacies, doctor’s offices, clinics, and state health departments.
Q: What about children under 12?
A: The new boosters are not approved for children under 12. Additional testing and trials need to be conducted for safety and effectiveness. But officials recommend that children 5 and above receive the primary vaccine series and be boosted with one shot. Children 6 months to under 5 years are not yet eligible for boosters.
Pfizer said it hopes to ask the Food and Drug Administration for authorization in 5- to 11-year-olds in October.
Q: How do the new bivalent boosters differ from previous shots?
A: The new shots use the same mRNA technology as the prior Moderna and Pfizer/BioNTech vaccines and boosters but have been upgraded to target the newer Omicron strains. The shots use mRNA created in a lab to teach our cells to produce a specific protein that triggers an immune-system response and make antibodies that help protect us from SARS-CoV-2, the virus that causes COVID.
The recipe for the new shots incorporates the so-called “spike protein” of both the original (ancestral) strain of the virus and more highly transmissible Omicron strains (BA.4, BA.5). Once your body produces these proteins, your immune system kicks into gear to mount a response.
It’s also possible – but yet to be determined – that the new bivalent boosters will offer protection against newer but less common strains known as BA.4.6 and BA.2.75.
Q: Are there any new risks or side effects associated with these boosters?
A: Health experts don’t expect to see anything beyond what has already been noted with prior mRNA vaccines, with the vast majority of recipients experiencing only mild issues such as redness from the shot, soreness, and fatigue.
Q: Do I need one of the new shots if I’ve already had past boosters or had COVID?
A: Yes. Even if you’ve been infected with COVID in the past year and/or received the prior series of primary vaccines and boosters, you should get a bivalent Omicron shot.
Doing so will give you broader immunity against COVID and also help limit the emergence of other variants. The more Americans with high immunity, the better; it makes it less likely other variants will emerge that can escape the immunity provided by vaccines and COVID infections.
Q: How long should I wait, from the time of my last shot, before getting a new booster?
A: The bivalent boosters are most effective when given after a period of time has passed between your last shot and the new one. A 2- to 3-month waiting period is the minimum, but some evidence suggests extending it out to 4-6 months might be good timing.
To determine when you should get a new booster, check out the CDC’s Stay Up to Date with COVID-19 Vaccines Including Boosters website.
Q: What if I’ve recently had COVID?
A: There are no specific rules about a waiting period after COVID infection. But if you have been infected with the virus in the last 8 weeks, you may want to wait for 8 weeks to pass before receiving the bivalent booster to allow your immune system to get greater benefit from the shot.
Q: If I never got the original vaccines, do I need to get those shots first?
A: Yes. The bivalent vaccine has a lower dose of mRNA than the vaccines used in the primary series of vaccines, rolled out in late 2020. The bivalent vaccine is authorized for use as a booster dose and not a primary vaccine series dose.
Q: Do the Omicron-specific boosters entirely replace the other boosters?
A: Yes. The new booster shots, which target the original strain and the Omicron subvariants, are now the only available boosters for people ages 12 and older. The FDA no longer authorizes the previous booster doses for people in the approved age groups.
Q: What if I received a non-mRNA vaccine produced by Novavax or Johnson & Johnson? Should I still get an mRNA booster?
A: You can mix and match COVID vaccines, and you are eligible to get the bivalent booster 8 weeks after completing the primary COVID vaccination series – whether that was two doses of mRNA or Novavax, or one shot of J&J.
Q: How effective are the new boosters?
A: Scientists don’t have complete effectiveness data from the bivalent vaccines yet. But because the new boosters contain mRNA from the Omicron and the original strains, they are believed to offer greater protection against COVID overall.
Cellular-level data support this, with studies showing the bivalent vaccines increase neutralizing antibodies to BA.4/BA.5 strains. Scientists regard these kinds of studies as surrogate stand-ins for clinical trials. But officials will be studying the effectiveness of the new boosters, examining to what degree they reduce hospitalizations and deaths.
Q: How long will the boosters’ protection last?
A: Research shows that vaccine effectiveness eventually wanes, which is why we have the boosters. Scientists will be monitoring to see how long the protection lasts from the bivalent boosters through studies of antibody levels as well as assessments of severe COVID illnesses over time, throughout the fall and winter.
Q: Is it OK to get a flu shot and a COVID booster at the same time?
A: Yes. In fact, it’s important to get a flu shot this year because some experts believe we could see overlapping COVID-influenza surges this fall – a phenomenon some have fancifully called a “twindemic.” Getting a flu shot and COVID booster – simultaneously, if possible – is particularly important if you’re in a high-risk group.
People who are susceptible to severe complications from COVID – such as older people, people with weakened immune systems, and those with chronic health conditions – are also especially vulnerable to severe influenza complications.
Q: Will a new booster mean I can stop wearing a mask, social distancing, avoiding crowded indoor spaces, and taking other precautions to avoid COVID?
A: No. It’s still a good idea to mask up, keep your distance from others, avoid indoor spaces with people whose vaccine status is unknown, and take other precautions against COVID.
Although the new boosters are front of mind, it’s a good idea to also use other tools in the toolbox, as well, particularly if you have contact with someone who is older, immune-suppressed, or has a chronic condition that puts them at higher risk from COVID.
Keep in mind: The community risk of infection nationwide is still high today, with about 67,400 new cases and nearly 320 deaths reported each day in the United States, according to the latest CDC reports.A version of this article first appeared on WebMD.
Here are answers to frequently asked questions about the shots produced by Moderna and Pfizer/BioNTech, based on information provided by the CDC and Keri Althoff, PhD, and virologist Andrew Pekosz, PhD, Johns Hopkins Bloomberg School of Public Health epidemiologists.
Question: Who is eligible for the new bivalent boosters?
Answer: The CDC greenlighted the upgraded Pfizer/BioNTech shots for Americans 12 and older and the Moderna booster for those 18 and over, if they have received a primary vaccine series or a booster at least 2 months before.
The boosters have been redesigned to protect against the predominant BA.4 and BA.5 strains of the virus. The Biden administration is making 160 million of the booster shots available free of charge through pharmacies, doctor’s offices, clinics, and state health departments.
Q: What about children under 12?
A: The new boosters are not approved for children under 12. Additional testing and trials need to be conducted for safety and effectiveness. But officials recommend that children 5 and above receive the primary vaccine series and be boosted with one shot. Children 6 months to under 5 years are not yet eligible for boosters.
Pfizer said it hopes to ask the Food and Drug Administration for authorization in 5- to 11-year-olds in October.
Q: How do the new bivalent boosters differ from previous shots?
A: The new shots use the same mRNA technology as the prior Moderna and Pfizer/BioNTech vaccines and boosters but have been upgraded to target the newer Omicron strains. The shots use mRNA created in a lab to teach our cells to produce a specific protein that triggers an immune-system response and make antibodies that help protect us from SARS-CoV-2, the virus that causes COVID.
The recipe for the new shots incorporates the so-called “spike protein” of both the original (ancestral) strain of the virus and more highly transmissible Omicron strains (BA.4, BA.5). Once your body produces these proteins, your immune system kicks into gear to mount a response.
It’s also possible – but yet to be determined – that the new bivalent boosters will offer protection against newer but less common strains known as BA.4.6 and BA.2.75.
Q: Are there any new risks or side effects associated with these boosters?
A: Health experts don’t expect to see anything beyond what has already been noted with prior mRNA vaccines, with the vast majority of recipients experiencing only mild issues such as redness from the shot, soreness, and fatigue.
Q: Do I need one of the new shots if I’ve already had past boosters or had COVID?
A: Yes. Even if you’ve been infected with COVID in the past year and/or received the prior series of primary vaccines and boosters, you should get a bivalent Omicron shot.
Doing so will give you broader immunity against COVID and also help limit the emergence of other variants. The more Americans with high immunity, the better; it makes it less likely other variants will emerge that can escape the immunity provided by vaccines and COVID infections.
Q: How long should I wait, from the time of my last shot, before getting a new booster?
A: The bivalent boosters are most effective when given after a period of time has passed between your last shot and the new one. A 2- to 3-month waiting period is the minimum, but some evidence suggests extending it out to 4-6 months might be good timing.
To determine when you should get a new booster, check out the CDC’s Stay Up to Date with COVID-19 Vaccines Including Boosters website.
Q: What if I’ve recently had COVID?
A: There are no specific rules about a waiting period after COVID infection. But if you have been infected with the virus in the last 8 weeks, you may want to wait for 8 weeks to pass before receiving the bivalent booster to allow your immune system to get greater benefit from the shot.
Q: If I never got the original vaccines, do I need to get those shots first?
A: Yes. The bivalent vaccine has a lower dose of mRNA than the vaccines used in the primary series of vaccines, rolled out in late 2020. The bivalent vaccine is authorized for use as a booster dose and not a primary vaccine series dose.
Q: Do the Omicron-specific boosters entirely replace the other boosters?
A: Yes. The new booster shots, which target the original strain and the Omicron subvariants, are now the only available boosters for people ages 12 and older. The FDA no longer authorizes the previous booster doses for people in the approved age groups.
Q: What if I received a non-mRNA vaccine produced by Novavax or Johnson & Johnson? Should I still get an mRNA booster?
A: You can mix and match COVID vaccines, and you are eligible to get the bivalent booster 8 weeks after completing the primary COVID vaccination series – whether that was two doses of mRNA or Novavax, or one shot of J&J.
Q: How effective are the new boosters?
A: Scientists don’t have complete effectiveness data from the bivalent vaccines yet. But because the new boosters contain mRNA from the Omicron and the original strains, they are believed to offer greater protection against COVID overall.
Cellular-level data support this, with studies showing the bivalent vaccines increase neutralizing antibodies to BA.4/BA.5 strains. Scientists regard these kinds of studies as surrogate stand-ins for clinical trials. But officials will be studying the effectiveness of the new boosters, examining to what degree they reduce hospitalizations and deaths.
Q: How long will the boosters’ protection last?
A: Research shows that vaccine effectiveness eventually wanes, which is why we have the boosters. Scientists will be monitoring to see how long the protection lasts from the bivalent boosters through studies of antibody levels as well as assessments of severe COVID illnesses over time, throughout the fall and winter.
Q: Is it OK to get a flu shot and a COVID booster at the same time?
A: Yes. In fact, it’s important to get a flu shot this year because some experts believe we could see overlapping COVID-influenza surges this fall – a phenomenon some have fancifully called a “twindemic.” Getting a flu shot and COVID booster – simultaneously, if possible – is particularly important if you’re in a high-risk group.
People who are susceptible to severe complications from COVID – such as older people, people with weakened immune systems, and those with chronic health conditions – are also especially vulnerable to severe influenza complications.
Q: Will a new booster mean I can stop wearing a mask, social distancing, avoiding crowded indoor spaces, and taking other precautions to avoid COVID?
A: No. It’s still a good idea to mask up, keep your distance from others, avoid indoor spaces with people whose vaccine status is unknown, and take other precautions against COVID.
Although the new boosters are front of mind, it’s a good idea to also use other tools in the toolbox, as well, particularly if you have contact with someone who is older, immune-suppressed, or has a chronic condition that puts them at higher risk from COVID.
Keep in mind: The community risk of infection nationwide is still high today, with about 67,400 new cases and nearly 320 deaths reported each day in the United States, according to the latest CDC reports.A version of this article first appeared on WebMD.
Here are answers to frequently asked questions about the shots produced by Moderna and Pfizer/BioNTech, based on information provided by the CDC and Keri Althoff, PhD, and virologist Andrew Pekosz, PhD, Johns Hopkins Bloomberg School of Public Health epidemiologists.
Question: Who is eligible for the new bivalent boosters?
Answer: The CDC greenlighted the upgraded Pfizer/BioNTech shots for Americans 12 and older and the Moderna booster for those 18 and over, if they have received a primary vaccine series or a booster at least 2 months before.
The boosters have been redesigned to protect against the predominant BA.4 and BA.5 strains of the virus. The Biden administration is making 160 million of the booster shots available free of charge through pharmacies, doctor’s offices, clinics, and state health departments.
Q: What about children under 12?
A: The new boosters are not approved for children under 12. Additional testing and trials need to be conducted for safety and effectiveness. But officials recommend that children 5 and above receive the primary vaccine series and be boosted with one shot. Children 6 months to under 5 years are not yet eligible for boosters.
Pfizer said it hopes to ask the Food and Drug Administration for authorization in 5- to 11-year-olds in October.
Q: How do the new bivalent boosters differ from previous shots?
A: The new shots use the same mRNA technology as the prior Moderna and Pfizer/BioNTech vaccines and boosters but have been upgraded to target the newer Omicron strains. The shots use mRNA created in a lab to teach our cells to produce a specific protein that triggers an immune-system response and make antibodies that help protect us from SARS-CoV-2, the virus that causes COVID.
The recipe for the new shots incorporates the so-called “spike protein” of both the original (ancestral) strain of the virus and more highly transmissible Omicron strains (BA.4, BA.5). Once your body produces these proteins, your immune system kicks into gear to mount a response.
It’s also possible – but yet to be determined – that the new bivalent boosters will offer protection against newer but less common strains known as BA.4.6 and BA.2.75.
Q: Are there any new risks or side effects associated with these boosters?
A: Health experts don’t expect to see anything beyond what has already been noted with prior mRNA vaccines, with the vast majority of recipients experiencing only mild issues such as redness from the shot, soreness, and fatigue.
Q: Do I need one of the new shots if I’ve already had past boosters or had COVID?
A: Yes. Even if you’ve been infected with COVID in the past year and/or received the prior series of primary vaccines and boosters, you should get a bivalent Omicron shot.
Doing so will give you broader immunity against COVID and also help limit the emergence of other variants. The more Americans with high immunity, the better; it makes it less likely other variants will emerge that can escape the immunity provided by vaccines and COVID infections.
Q: How long should I wait, from the time of my last shot, before getting a new booster?
A: The bivalent boosters are most effective when given after a period of time has passed between your last shot and the new one. A 2- to 3-month waiting period is the minimum, but some evidence suggests extending it out to 4-6 months might be good timing.
To determine when you should get a new booster, check out the CDC’s Stay Up to Date with COVID-19 Vaccines Including Boosters website.
Q: What if I’ve recently had COVID?
A: There are no specific rules about a waiting period after COVID infection. But if you have been infected with the virus in the last 8 weeks, you may want to wait for 8 weeks to pass before receiving the bivalent booster to allow your immune system to get greater benefit from the shot.
Q: If I never got the original vaccines, do I need to get those shots first?
A: Yes. The bivalent vaccine has a lower dose of mRNA than the vaccines used in the primary series of vaccines, rolled out in late 2020. The bivalent vaccine is authorized for use as a booster dose and not a primary vaccine series dose.
Q: Do the Omicron-specific boosters entirely replace the other boosters?
A: Yes. The new booster shots, which target the original strain and the Omicron subvariants, are now the only available boosters for people ages 12 and older. The FDA no longer authorizes the previous booster doses for people in the approved age groups.
Q: What if I received a non-mRNA vaccine produced by Novavax or Johnson & Johnson? Should I still get an mRNA booster?
A: You can mix and match COVID vaccines, and you are eligible to get the bivalent booster 8 weeks after completing the primary COVID vaccination series – whether that was two doses of mRNA or Novavax, or one shot of J&J.
Q: How effective are the new boosters?
A: Scientists don’t have complete effectiveness data from the bivalent vaccines yet. But because the new boosters contain mRNA from the Omicron and the original strains, they are believed to offer greater protection against COVID overall.
Cellular-level data support this, with studies showing the bivalent vaccines increase neutralizing antibodies to BA.4/BA.5 strains. Scientists regard these kinds of studies as surrogate stand-ins for clinical trials. But officials will be studying the effectiveness of the new boosters, examining to what degree they reduce hospitalizations and deaths.
Q: How long will the boosters’ protection last?
A: Research shows that vaccine effectiveness eventually wanes, which is why we have the boosters. Scientists will be monitoring to see how long the protection lasts from the bivalent boosters through studies of antibody levels as well as assessments of severe COVID illnesses over time, throughout the fall and winter.
Q: Is it OK to get a flu shot and a COVID booster at the same time?
A: Yes. In fact, it’s important to get a flu shot this year because some experts believe we could see overlapping COVID-influenza surges this fall – a phenomenon some have fancifully called a “twindemic.” Getting a flu shot and COVID booster – simultaneously, if possible – is particularly important if you’re in a high-risk group.
People who are susceptible to severe complications from COVID – such as older people, people with weakened immune systems, and those with chronic health conditions – are also especially vulnerable to severe influenza complications.
Q: Will a new booster mean I can stop wearing a mask, social distancing, avoiding crowded indoor spaces, and taking other precautions to avoid COVID?
A: No. It’s still a good idea to mask up, keep your distance from others, avoid indoor spaces with people whose vaccine status is unknown, and take other precautions against COVID.
Although the new boosters are front of mind, it’s a good idea to also use other tools in the toolbox, as well, particularly if you have contact with someone who is older, immune-suppressed, or has a chronic condition that puts them at higher risk from COVID.
Keep in mind: The community risk of infection nationwide is still high today, with about 67,400 new cases and nearly 320 deaths reported each day in the United States, according to the latest CDC reports.A version of this article first appeared on WebMD.
Risk factors linked to post–COVID vaccination death identified
The researchers have identified factors that put a person at greater risk of COVID-related death after they have completed both doses of the primary COVID vaccination schedule and a booster dose.
For their research, published in JAMA Network Open, researchers from the Office for National Statistics (ONS); Public Health Scotland; the University of Strathclyde, Glasgow; and the University of Edinburgh used data from the ONS Public linked data set combining the 2011 Census of England and covering 80% of the population of England. The study population included 19,473,570 individuals aged 18-100 years (mean age 60.8 years, 45.2% men, 92.0% White individuals) living in England who had completed both doses of their primary vaccination schedule and had received their mRNA booster 14 days or more prior to Dec. 31, 2021. The outcome of interest was time to death involving COVID-19 occurring between Jan. 1 and March 16, 2022.
Prioritization of booster doses and COVID-19 treatments
The authors highlighted how it had become “critical” to identify risk factors associated with COVID-19 death in those who had been vaccinated and pointed out that existing evidence was “based on people who have received one or two doses of a COVID-19 vaccine and were infected by the Alpha or Delta variant”. They emphasized that establishing which groups are at increased risk of COVID-19 death after receiving a booster is crucial for the “prioritization of further booster doses and access to COVID-19 therapeutics.”
During the study period the authors found that there were 4,781 (0.02%) deaths involving COVID-19 and 58,020 (0.3%) deaths from other causes. Of those who died of coronavirus, the mean age was 83.3 years, and the authors highlighted how “age was the most important characteristic” associated with the risk of postbooster COVID-19 death. They added that, compared with a 50-year-old, the HR for an 80-year-old individual was 31.3 (95% confidence interval, 26.1-37.6).
They found that women were at lower risk than men with an HR of 0.52 (95% CI, 0.49-0.55). An increased risk of COVID-19 death was also associated with living in a care home or in a socioeconomically deprived area.
Of note, they said that “there was no association between the risk of COVID-19 death and ethnicity, except for those of Indian background”, who they explained were at slightly elevated risk, compared with White individuals. However, they explained how the association with ethnicity was “unclear and differed from previous studies”, with their findings likely to be due “largely to the pronounced differences in vaccination uptake” between ethnic groups in previous studies.
Dementia concern
With regard to existing health conditions the authors commented that “most of the QCovid risk groups were associated with an increased HR of postbooster breakthrough death, except for of congenital heart disease, asthma, and prior fracture.”
Risk was particularly elevated, they said, for people with severe combined immunodeficiency (HR, 6.2; 95% CI, 3.3-11.5), and they also identified several conditions associated with HRs of greater than 3, including dementia.
In July, Alzheimer’s Research UK urged the Government to boost the development and deployment of new dementia treatments having found that a significant proportion of people who died of COVID-19 in 2020 and 2021 were living with the condition. At the time, data published by the ONS of deaths caused by coronavirus in England and Wales in 2021 showed dementia to be the second-most common pre-existing condition.
David Thomas, head of policy at Alzheimer’s Research UK, said: “We’ve known for some time that people with dementia have been hit disproportionately hard during the pandemic, but this new data serves as a stark reminder of the growing challenge we face in tackling the condition, and the urgent need to address it.”
The authors of the new research acknowledged the study’s limitations, notably that only data for the population living in England who were enumerated in the 2011 Census of England and Wales was included.
However, subpopulations “remain at increased risk of COVID-19 fatality” after receiving a booster vaccine during the Omicron wave, they pointed out.
“The subpopulations with the highest risk should be considered a priority for COVID-19 therapeutics and further booster doses,” they urged.
A version of this article first appeared on Medscape UK.
The researchers have identified factors that put a person at greater risk of COVID-related death after they have completed both doses of the primary COVID vaccination schedule and a booster dose.
For their research, published in JAMA Network Open, researchers from the Office for National Statistics (ONS); Public Health Scotland; the University of Strathclyde, Glasgow; and the University of Edinburgh used data from the ONS Public linked data set combining the 2011 Census of England and covering 80% of the population of England. The study population included 19,473,570 individuals aged 18-100 years (mean age 60.8 years, 45.2% men, 92.0% White individuals) living in England who had completed both doses of their primary vaccination schedule and had received their mRNA booster 14 days or more prior to Dec. 31, 2021. The outcome of interest was time to death involving COVID-19 occurring between Jan. 1 and March 16, 2022.
Prioritization of booster doses and COVID-19 treatments
The authors highlighted how it had become “critical” to identify risk factors associated with COVID-19 death in those who had been vaccinated and pointed out that existing evidence was “based on people who have received one or two doses of a COVID-19 vaccine and were infected by the Alpha or Delta variant”. They emphasized that establishing which groups are at increased risk of COVID-19 death after receiving a booster is crucial for the “prioritization of further booster doses and access to COVID-19 therapeutics.”
During the study period the authors found that there were 4,781 (0.02%) deaths involving COVID-19 and 58,020 (0.3%) deaths from other causes. Of those who died of coronavirus, the mean age was 83.3 years, and the authors highlighted how “age was the most important characteristic” associated with the risk of postbooster COVID-19 death. They added that, compared with a 50-year-old, the HR for an 80-year-old individual was 31.3 (95% confidence interval, 26.1-37.6).
They found that women were at lower risk than men with an HR of 0.52 (95% CI, 0.49-0.55). An increased risk of COVID-19 death was also associated with living in a care home or in a socioeconomically deprived area.
Of note, they said that “there was no association between the risk of COVID-19 death and ethnicity, except for those of Indian background”, who they explained were at slightly elevated risk, compared with White individuals. However, they explained how the association with ethnicity was “unclear and differed from previous studies”, with their findings likely to be due “largely to the pronounced differences in vaccination uptake” between ethnic groups in previous studies.
Dementia concern
With regard to existing health conditions the authors commented that “most of the QCovid risk groups were associated with an increased HR of postbooster breakthrough death, except for of congenital heart disease, asthma, and prior fracture.”
Risk was particularly elevated, they said, for people with severe combined immunodeficiency (HR, 6.2; 95% CI, 3.3-11.5), and they also identified several conditions associated with HRs of greater than 3, including dementia.
In July, Alzheimer’s Research UK urged the Government to boost the development and deployment of new dementia treatments having found that a significant proportion of people who died of COVID-19 in 2020 and 2021 were living with the condition. At the time, data published by the ONS of deaths caused by coronavirus in England and Wales in 2021 showed dementia to be the second-most common pre-existing condition.
David Thomas, head of policy at Alzheimer’s Research UK, said: “We’ve known for some time that people with dementia have been hit disproportionately hard during the pandemic, but this new data serves as a stark reminder of the growing challenge we face in tackling the condition, and the urgent need to address it.”
The authors of the new research acknowledged the study’s limitations, notably that only data for the population living in England who were enumerated in the 2011 Census of England and Wales was included.
However, subpopulations “remain at increased risk of COVID-19 fatality” after receiving a booster vaccine during the Omicron wave, they pointed out.
“The subpopulations with the highest risk should be considered a priority for COVID-19 therapeutics and further booster doses,” they urged.
A version of this article first appeared on Medscape UK.
The researchers have identified factors that put a person at greater risk of COVID-related death after they have completed both doses of the primary COVID vaccination schedule and a booster dose.
For their research, published in JAMA Network Open, researchers from the Office for National Statistics (ONS); Public Health Scotland; the University of Strathclyde, Glasgow; and the University of Edinburgh used data from the ONS Public linked data set combining the 2011 Census of England and covering 80% of the population of England. The study population included 19,473,570 individuals aged 18-100 years (mean age 60.8 years, 45.2% men, 92.0% White individuals) living in England who had completed both doses of their primary vaccination schedule and had received their mRNA booster 14 days or more prior to Dec. 31, 2021. The outcome of interest was time to death involving COVID-19 occurring between Jan. 1 and March 16, 2022.
Prioritization of booster doses and COVID-19 treatments
The authors highlighted how it had become “critical” to identify risk factors associated with COVID-19 death in those who had been vaccinated and pointed out that existing evidence was “based on people who have received one or two doses of a COVID-19 vaccine and were infected by the Alpha or Delta variant”. They emphasized that establishing which groups are at increased risk of COVID-19 death after receiving a booster is crucial for the “prioritization of further booster doses and access to COVID-19 therapeutics.”
During the study period the authors found that there were 4,781 (0.02%) deaths involving COVID-19 and 58,020 (0.3%) deaths from other causes. Of those who died of coronavirus, the mean age was 83.3 years, and the authors highlighted how “age was the most important characteristic” associated with the risk of postbooster COVID-19 death. They added that, compared with a 50-year-old, the HR for an 80-year-old individual was 31.3 (95% confidence interval, 26.1-37.6).
They found that women were at lower risk than men with an HR of 0.52 (95% CI, 0.49-0.55). An increased risk of COVID-19 death was also associated with living in a care home or in a socioeconomically deprived area.
Of note, they said that “there was no association between the risk of COVID-19 death and ethnicity, except for those of Indian background”, who they explained were at slightly elevated risk, compared with White individuals. However, they explained how the association with ethnicity was “unclear and differed from previous studies”, with their findings likely to be due “largely to the pronounced differences in vaccination uptake” between ethnic groups in previous studies.
Dementia concern
With regard to existing health conditions the authors commented that “most of the QCovid risk groups were associated with an increased HR of postbooster breakthrough death, except for of congenital heart disease, asthma, and prior fracture.”
Risk was particularly elevated, they said, for people with severe combined immunodeficiency (HR, 6.2; 95% CI, 3.3-11.5), and they also identified several conditions associated with HRs of greater than 3, including dementia.
In July, Alzheimer’s Research UK urged the Government to boost the development and deployment of new dementia treatments having found that a significant proportion of people who died of COVID-19 in 2020 and 2021 were living with the condition. At the time, data published by the ONS of deaths caused by coronavirus in England and Wales in 2021 showed dementia to be the second-most common pre-existing condition.
David Thomas, head of policy at Alzheimer’s Research UK, said: “We’ve known for some time that people with dementia have been hit disproportionately hard during the pandemic, but this new data serves as a stark reminder of the growing challenge we face in tackling the condition, and the urgent need to address it.”
The authors of the new research acknowledged the study’s limitations, notably that only data for the population living in England who were enumerated in the 2011 Census of England and Wales was included.
However, subpopulations “remain at increased risk of COVID-19 fatality” after receiving a booster vaccine during the Omicron wave, they pointed out.
“The subpopulations with the highest risk should be considered a priority for COVID-19 therapeutics and further booster doses,” they urged.
A version of this article first appeared on Medscape UK.
FROM JAMA NETWORK OPEN
Prior psychological distress tied to ‘long-COVID’ conditions
In an analysis of almost 55,000 adult participants in three ongoing studies, having depression, anxiety, worry, perceived stress, or loneliness early in the pandemic, before SARS-CoV-2 infection, was associated with a 50% increased risk for developing long COVID. These types of psychological distress were also associated with a 15% to 51% greater risk for impairment in daily life among individuals with long COVID.
Psychological distress was even more strongly associated with developing long COVID than were physical health risk factors, and the increased risk was not explained by health behaviors such as smoking or physical comorbidities, researchers note.
“Our findings suggest the need to consider psychological health in addition to physical health as risk factors of long COVID-19,” lead author Siwen Wang, MD, postdoctoral fellow, department of nutrition, Harvard T. H. Chan School of Public Health, Boston, said in an interview.
“We need to increase public awareness of the importance of mental health and focus on getting mental health care for people who need it, increasing the supply of mental health clinicians and improving access to care,” she said.
The findings were published online in JAMA Psychiatry.
‘Poorly understood’
Postacute sequelae of SARS-CoV-2 (“long COVID”), which are “signs and symptoms consistent with COVID-19 that extend beyond 4 weeks from onset of infection” constitute “an emerging health issue,” the investigators write.
Dr. Wang noted that it has been estimated that 8-23 million Americans have developed long COVID. However, “despite the high prevalence and daily life impairment associated with long COVID, it is still poorly understood, and few risk factors have been established,” she said.
Although psychological distress may be implicated in long COVID, only three previous studies investigated psychological factors as potential contributors, the researchers note. Also, no study has investigated the potential role of other common manifestations of distress that have increased during the pandemic, such as loneliness and perceived stress, they add.
To investigate these issues, the researchers turned to three large ongoing longitudinal studies: the Nurses’ Health Study II (NSHII), the Nurses’ Health study 3 (NHS3), and the Growing Up Today Study (GUTS).
They analyzed data on 54,960 total participants (96.6% women; mean age, 57.5 years). Of the full group, 38% were active health care workers.
Participants completed an online COVID-19 questionnaire from April 2020 to Sept. 1, 2020 (baseline), and monthly surveys thereafter. Beginning in August 2020, surveys were administered quarterly. The end of follow-up was in November 2021.
The COVID questionnaires included questions about positive SARS-CoV-2 test results, COVID symptoms and hospitalization since March 1, 2020, and the presence of long-term COVID symptoms, such as fatigue, respiratory problems, persistent cough, muscle/joint/chest pain, smell/taste problems, confusion/disorientation/brain fog, depression/anxiety/changes in mood, headache, and memory problems.
Participants who reported these post-COVID conditions were asked about the frequency of symptoms and the degree of impairment in daily life.
Inflammation, immune dysregulation implicated?
The Patient Health Questionnaire–4 (PHQ-4) was used to assess for anxiety and depressive symptoms in the past 2 weeks. It consists of a two-item depression measure (PHQ-2) and a two-item Generalized Anxiety Disorder Scale (GAD-2).
Non–health care providers completed two additional assessments of psychological distress: the four-item Perceived Stress Scale and the three-item UCLA Loneliness Scale.
The researchers included demographic factors, weight, smoking status, marital status, and medical conditions, including diabetes, hypertension, hypercholesterolemia, asthma, and cancer, and socioeconomic factors as covariates.
For each participant, the investigators calculated the number of types of distress experienced at a high level, including probable depression, probable anxiety, worry about COVID-19, being in the top quartile of perceived stress, and loneliness.
During the 19 months of follow-up (1-47 weeks after baseline), 6% of respondents reported a positive result on a SARS-CoV-2 antibody, antigen, or polymerase chain reaction test.
Of these, 43.9% reported long-COVID conditions, with most reporting that symptoms lasted 2 months or longer; 55.8% reported at least occasional daily life impairment.
The most common post-COVID conditions were fatigue (reported by 56%), loss of smell or taste problems (44.6%), shortness of breath (25.5%), confusion/disorientation/ brain fog (24.5%), and memory issues (21.8%).
Among patients who had been infected, there was a considerably higher rate of preinfection psychological distress after adjusting for sociodemographic factors, health behaviors, and comorbidities. Each type of distress was associated with post-COVID conditions.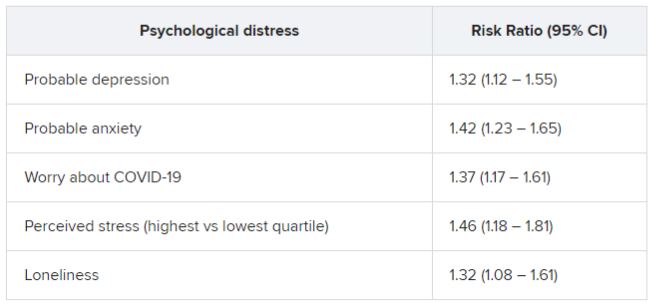
In addition, participants who had experienced at least two types of distress prior to infection were at nearly 50% increased risk for post–COVID conditions (risk ratio, 1.49; 95% confidence interval, 1.23-1.80).
Among those with post-COVID conditions, all types of distress were associated with increased risk for daily life impairment (RR range, 1.15-1.51).
Senior author Andrea Roberts, PhD, senior research scientist at the Harvard T. H. Chan School of Public Health, Boston, noted that the investigators did not examine biological mechanisms potentially underlying the association they found.
However, “based on prior research, it may be that inflammation and immune dysregulation related to psychological distress play a role in the association of distress with long COVID, but we can’t be sure,” Dr. Roberts said.
Contributes to the field
Commenting for this article, Yapeng Su, PhD, a postdoctoral researcher at the Fred Hutchinson Cancer Research Center in Seattle, called the study “great work contributing to the long-COVID research field and revealing important connections” with psychological stress prior to infection.
Dr. Su, who was not involved with the study, was previously at the Institute for Systems Biology, also in Seattle, and has written about long COVID.
He noted that the “biological mechanism of such intriguing linkage is definitely the important next step, which will likely require deep phenotyping of biological specimens from these patients longitudinally.”
Dr. Wang pointed to past research suggesting that some patients with mental illness “sometimes develop autoantibodies that have also been associated with increased risk of long COVID.” In addition, depression “affects the brain in ways that may explain certain cognitive symptoms in long COVID,” she added.
More studies are now needed to understand how psychological distress increases the risk for long COVID, said Dr. Wang.
The research was supported by grants from the Eunice Kennedy Shriver National Institute of Child Health and Human Development, the National Institutes of Health, the Dean’s Fund for Scientific Advancement Acceleration Award from the Harvard T. H. Chan School of Public Health, the Massachusetts Consortium on Pathogen Readiness Evergrande COVID-19 Response Fund Award, and the Veterans Affairs Health Services Research and Development Service funds. Dr. Wang and Dr. Roberts have reported no relevant financial relationships. The other investigators’ disclosures are listed in the original article. Dr. Su reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
In an analysis of almost 55,000 adult participants in three ongoing studies, having depression, anxiety, worry, perceived stress, or loneliness early in the pandemic, before SARS-CoV-2 infection, was associated with a 50% increased risk for developing long COVID. These types of psychological distress were also associated with a 15% to 51% greater risk for impairment in daily life among individuals with long COVID.
Psychological distress was even more strongly associated with developing long COVID than were physical health risk factors, and the increased risk was not explained by health behaviors such as smoking or physical comorbidities, researchers note.
“Our findings suggest the need to consider psychological health in addition to physical health as risk factors of long COVID-19,” lead author Siwen Wang, MD, postdoctoral fellow, department of nutrition, Harvard T. H. Chan School of Public Health, Boston, said in an interview.
“We need to increase public awareness of the importance of mental health and focus on getting mental health care for people who need it, increasing the supply of mental health clinicians and improving access to care,” she said.
The findings were published online in JAMA Psychiatry.
‘Poorly understood’
Postacute sequelae of SARS-CoV-2 (“long COVID”), which are “signs and symptoms consistent with COVID-19 that extend beyond 4 weeks from onset of infection” constitute “an emerging health issue,” the investigators write.
Dr. Wang noted that it has been estimated that 8-23 million Americans have developed long COVID. However, “despite the high prevalence and daily life impairment associated with long COVID, it is still poorly understood, and few risk factors have been established,” she said.
Although psychological distress may be implicated in long COVID, only three previous studies investigated psychological factors as potential contributors, the researchers note. Also, no study has investigated the potential role of other common manifestations of distress that have increased during the pandemic, such as loneliness and perceived stress, they add.
To investigate these issues, the researchers turned to three large ongoing longitudinal studies: the Nurses’ Health Study II (NSHII), the Nurses’ Health study 3 (NHS3), and the Growing Up Today Study (GUTS).
They analyzed data on 54,960 total participants (96.6% women; mean age, 57.5 years). Of the full group, 38% were active health care workers.
Participants completed an online COVID-19 questionnaire from April 2020 to Sept. 1, 2020 (baseline), and monthly surveys thereafter. Beginning in August 2020, surveys were administered quarterly. The end of follow-up was in November 2021.
The COVID questionnaires included questions about positive SARS-CoV-2 test results, COVID symptoms and hospitalization since March 1, 2020, and the presence of long-term COVID symptoms, such as fatigue, respiratory problems, persistent cough, muscle/joint/chest pain, smell/taste problems, confusion/disorientation/brain fog, depression/anxiety/changes in mood, headache, and memory problems.
Participants who reported these post-COVID conditions were asked about the frequency of symptoms and the degree of impairment in daily life.
Inflammation, immune dysregulation implicated?
The Patient Health Questionnaire–4 (PHQ-4) was used to assess for anxiety and depressive symptoms in the past 2 weeks. It consists of a two-item depression measure (PHQ-2) and a two-item Generalized Anxiety Disorder Scale (GAD-2).
Non–health care providers completed two additional assessments of psychological distress: the four-item Perceived Stress Scale and the three-item UCLA Loneliness Scale.
The researchers included demographic factors, weight, smoking status, marital status, and medical conditions, including diabetes, hypertension, hypercholesterolemia, asthma, and cancer, and socioeconomic factors as covariates.
For each participant, the investigators calculated the number of types of distress experienced at a high level, including probable depression, probable anxiety, worry about COVID-19, being in the top quartile of perceived stress, and loneliness.
During the 19 months of follow-up (1-47 weeks after baseline), 6% of respondents reported a positive result on a SARS-CoV-2 antibody, antigen, or polymerase chain reaction test.
Of these, 43.9% reported long-COVID conditions, with most reporting that symptoms lasted 2 months or longer; 55.8% reported at least occasional daily life impairment.
The most common post-COVID conditions were fatigue (reported by 56%), loss of smell or taste problems (44.6%), shortness of breath (25.5%), confusion/disorientation/ brain fog (24.5%), and memory issues (21.8%).
Among patients who had been infected, there was a considerably higher rate of preinfection psychological distress after adjusting for sociodemographic factors, health behaviors, and comorbidities. Each type of distress was associated with post-COVID conditions.
In addition, participants who had experienced at least two types of distress prior to infection were at nearly 50% increased risk for post–COVID conditions (risk ratio, 1.49; 95% confidence interval, 1.23-1.80).
Among those with post-COVID conditions, all types of distress were associated with increased risk for daily life impairment (RR range, 1.15-1.51).
Senior author Andrea Roberts, PhD, senior research scientist at the Harvard T. H. Chan School of Public Health, Boston, noted that the investigators did not examine biological mechanisms potentially underlying the association they found.
However, “based on prior research, it may be that inflammation and immune dysregulation related to psychological distress play a role in the association of distress with long COVID, but we can’t be sure,” Dr. Roberts said.
Contributes to the field
Commenting for this article, Yapeng Su, PhD, a postdoctoral researcher at the Fred Hutchinson Cancer Research Center in Seattle, called the study “great work contributing to the long-COVID research field and revealing important connections” with psychological stress prior to infection.
Dr. Su, who was not involved with the study, was previously at the Institute for Systems Biology, also in Seattle, and has written about long COVID.
He noted that the “biological mechanism of such intriguing linkage is definitely the important next step, which will likely require deep phenotyping of biological specimens from these patients longitudinally.”
Dr. Wang pointed to past research suggesting that some patients with mental illness “sometimes develop autoantibodies that have also been associated with increased risk of long COVID.” In addition, depression “affects the brain in ways that may explain certain cognitive symptoms in long COVID,” she added.
More studies are now needed to understand how psychological distress increases the risk for long COVID, said Dr. Wang.
The research was supported by grants from the Eunice Kennedy Shriver National Institute of Child Health and Human Development, the National Institutes of Health, the Dean’s Fund for Scientific Advancement Acceleration Award from the Harvard T. H. Chan School of Public Health, the Massachusetts Consortium on Pathogen Readiness Evergrande COVID-19 Response Fund Award, and the Veterans Affairs Health Services Research and Development Service funds. Dr. Wang and Dr. Roberts have reported no relevant financial relationships. The other investigators’ disclosures are listed in the original article. Dr. Su reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
In an analysis of almost 55,000 adult participants in three ongoing studies, having depression, anxiety, worry, perceived stress, or loneliness early in the pandemic, before SARS-CoV-2 infection, was associated with a 50% increased risk for developing long COVID. These types of psychological distress were also associated with a 15% to 51% greater risk for impairment in daily life among individuals with long COVID.
Psychological distress was even more strongly associated with developing long COVID than were physical health risk factors, and the increased risk was not explained by health behaviors such as smoking or physical comorbidities, researchers note.
“Our findings suggest the need to consider psychological health in addition to physical health as risk factors of long COVID-19,” lead author Siwen Wang, MD, postdoctoral fellow, department of nutrition, Harvard T. H. Chan School of Public Health, Boston, said in an interview.
“We need to increase public awareness of the importance of mental health and focus on getting mental health care for people who need it, increasing the supply of mental health clinicians and improving access to care,” she said.
The findings were published online in JAMA Psychiatry.
‘Poorly understood’
Postacute sequelae of SARS-CoV-2 (“long COVID”), which are “signs and symptoms consistent with COVID-19 that extend beyond 4 weeks from onset of infection” constitute “an emerging health issue,” the investigators write.
Dr. Wang noted that it has been estimated that 8-23 million Americans have developed long COVID. However, “despite the high prevalence and daily life impairment associated with long COVID, it is still poorly understood, and few risk factors have been established,” she said.
Although psychological distress may be implicated in long COVID, only three previous studies investigated psychological factors as potential contributors, the researchers note. Also, no study has investigated the potential role of other common manifestations of distress that have increased during the pandemic, such as loneliness and perceived stress, they add.
To investigate these issues, the researchers turned to three large ongoing longitudinal studies: the Nurses’ Health Study II (NSHII), the Nurses’ Health study 3 (NHS3), and the Growing Up Today Study (GUTS).
They analyzed data on 54,960 total participants (96.6% women; mean age, 57.5 years). Of the full group, 38% were active health care workers.
Participants completed an online COVID-19 questionnaire from April 2020 to Sept. 1, 2020 (baseline), and monthly surveys thereafter. Beginning in August 2020, surveys were administered quarterly. The end of follow-up was in November 2021.
The COVID questionnaires included questions about positive SARS-CoV-2 test results, COVID symptoms and hospitalization since March 1, 2020, and the presence of long-term COVID symptoms, such as fatigue, respiratory problems, persistent cough, muscle/joint/chest pain, smell/taste problems, confusion/disorientation/brain fog, depression/anxiety/changes in mood, headache, and memory problems.
Participants who reported these post-COVID conditions were asked about the frequency of symptoms and the degree of impairment in daily life.
Inflammation, immune dysregulation implicated?
The Patient Health Questionnaire–4 (PHQ-4) was used to assess for anxiety and depressive symptoms in the past 2 weeks. It consists of a two-item depression measure (PHQ-2) and a two-item Generalized Anxiety Disorder Scale (GAD-2).
Non–health care providers completed two additional assessments of psychological distress: the four-item Perceived Stress Scale and the three-item UCLA Loneliness Scale.
The researchers included demographic factors, weight, smoking status, marital status, and medical conditions, including diabetes, hypertension, hypercholesterolemia, asthma, and cancer, and socioeconomic factors as covariates.
For each participant, the investigators calculated the number of types of distress experienced at a high level, including probable depression, probable anxiety, worry about COVID-19, being in the top quartile of perceived stress, and loneliness.
During the 19 months of follow-up (1-47 weeks after baseline), 6% of respondents reported a positive result on a SARS-CoV-2 antibody, antigen, or polymerase chain reaction test.
Of these, 43.9% reported long-COVID conditions, with most reporting that symptoms lasted 2 months or longer; 55.8% reported at least occasional daily life impairment.
The most common post-COVID conditions were fatigue (reported by 56%), loss of smell or taste problems (44.6%), shortness of breath (25.5%), confusion/disorientation/ brain fog (24.5%), and memory issues (21.8%).
Among patients who had been infected, there was a considerably higher rate of preinfection psychological distress after adjusting for sociodemographic factors, health behaviors, and comorbidities. Each type of distress was associated with post-COVID conditions.
In addition, participants who had experienced at least two types of distress prior to infection were at nearly 50% increased risk for post–COVID conditions (risk ratio, 1.49; 95% confidence interval, 1.23-1.80).
Among those with post-COVID conditions, all types of distress were associated with increased risk for daily life impairment (RR range, 1.15-1.51).
Senior author Andrea Roberts, PhD, senior research scientist at the Harvard T. H. Chan School of Public Health, Boston, noted that the investigators did not examine biological mechanisms potentially underlying the association they found.
However, “based on prior research, it may be that inflammation and immune dysregulation related to psychological distress play a role in the association of distress with long COVID, but we can’t be sure,” Dr. Roberts said.
Contributes to the field
Commenting for this article, Yapeng Su, PhD, a postdoctoral researcher at the Fred Hutchinson Cancer Research Center in Seattle, called the study “great work contributing to the long-COVID research field and revealing important connections” with psychological stress prior to infection.
Dr. Su, who was not involved with the study, was previously at the Institute for Systems Biology, also in Seattle, and has written about long COVID.
He noted that the “biological mechanism of such intriguing linkage is definitely the important next step, which will likely require deep phenotyping of biological specimens from these patients longitudinally.”
Dr. Wang pointed to past research suggesting that some patients with mental illness “sometimes develop autoantibodies that have also been associated with increased risk of long COVID.” In addition, depression “affects the brain in ways that may explain certain cognitive symptoms in long COVID,” she added.
More studies are now needed to understand how psychological distress increases the risk for long COVID, said Dr. Wang.
The research was supported by grants from the Eunice Kennedy Shriver National Institute of Child Health and Human Development, the National Institutes of Health, the Dean’s Fund for Scientific Advancement Acceleration Award from the Harvard T. H. Chan School of Public Health, the Massachusetts Consortium on Pathogen Readiness Evergrande COVID-19 Response Fund Award, and the Veterans Affairs Health Services Research and Development Service funds. Dr. Wang and Dr. Roberts have reported no relevant financial relationships. The other investigators’ disclosures are listed in the original article. Dr. Su reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM JAMA PSYCHIATRY
Why some infectious disease docs are ‘encouraged’ by new bivalent COVID vaccines
A panel of infectious disease experts shared their take recently on the importance of the newly approved bivalent COVID-19 vaccines, why authorization without human data is not for them a cause for alarm, and what they are most optimistic about at this stage of the pandemic.
“I’m very encouraged by this new development,” Kathryn M. Edwards, MD, said during a media briefing sponsored by the Infectious Diseases Society of America (IDSA).
, she said. “It does seem that if you have a circulating strain BA.4 and BA.5, hitting it with the appropriate vaccine targeted for that is most immunogenic, certainly. We will hopefully see that in terms of effectiveness.”
Changing the vaccines at this point is appropriate, Walter A. Orenstein, MD, said. “One of our challenges is that this virus mutates. Our immune response is focused on an area of the virus that can change and be evaded,” said Dr. Orenstein, professor and associate director of the Emory Vaccine Center at Emory University, Atlanta.
“This is different than measles or polio,” he said. “But for influenza and now with SARS-CoV-2 ... we have to update our vaccines, because the virus changes.”
Man versus mouse
Dr. Edwards addressed the controversy over a lack of human data specific to these next-generation Pfizer/BioNTech and Moderna vaccines. “I do not want people to be unhappy or worried that the bivalent vaccine will act in a different way than the ones that we have been administering for the past 2 years.”
The Food and Drug Administration emergency use authorization may have relied primarily on animal studies, she said, but mice given a vaccine specific to BA.4 and BA.5 “have a much more robust immune response,” compared with those given a BA.1 vaccine.
Also, “over and over and over again we have seen with these SARS-CoV-2 vaccines that the mouse responses mirror the human responses,” said Dr. Edwards, scientific director of the Vanderbilt Vaccine Research Program at Vanderbilt University, Nashville, Tenn., and an IDSA fellow.
“Human data will be coming very soon to look at the immunogenicity,” she said.
A ‘glass half full’ perspective
When asked what they are most optimistic about at this point in the COVID-19 pandemic, Dr. Orenstein said, “I’m really positive in the sense that the vaccines we have are already very effective against severe disease, death, and hospitalization. I feel really good about that. And we have great tools.
“The bottom line for me is, I want to get it myself,” he said regarding the bivalent vaccine.
“There are a lot of things to be happy with,” Dr. Edwards said. “I’m kind of a glass-half-full kind of person.”
Dr. Edwards is confident that the surveillance systems now in place can accurately detect major changes in the virus, including new variants. She is also optimistic about the mRNA technology that allows rapid updates to COVID-19 vaccines.
Furthermore, “I’m happy that we’re beginning to open up – that we can go do different things that we have done in the past and feel much more comfortable,” she said.
More motivational messaging needed
Now is also a good time to renew efforts to get people vaccinated.
“We invested a lot into developing these vaccines, but I think we also need to invest in what I call ‘implementation science research,’ ” Dr. Orenstein said, the goal being to convince people to get vaccinated.
He pointed out that it’s vaccinations, not vaccines, that saves lives. “Vaccine doses that remain in the vial are 0% effective.
“When I was director of the United States’ immunization program at the CDC,” Dr. Orenstein said, “my director of communications used to say that you need the right message delivered by the right messenger through the right communications channel.”
Dr. Edwards agreed that listening to people’s concerns and respecting their questions are important. “We also need to make sure that we use the proper messenger, just as Walt said. Maybe the proper messenger isn’t an old gray-haired lady,” she said, referring to herself, “but it’s someone that lives in your community or is your primary care doctor who has taken care of you or your children for many years.”
Research on how to better motivate people to get vaccinated is warranted, Dr. Edwards said, as well as on “how to make sure that this is really a medical issue and not a political issue. That’s been a really big problem.”
A version of this article first appeared on Medscape.com.
A panel of infectious disease experts shared their take recently on the importance of the newly approved bivalent COVID-19 vaccines, why authorization without human data is not for them a cause for alarm, and what they are most optimistic about at this stage of the pandemic.
“I’m very encouraged by this new development,” Kathryn M. Edwards, MD, said during a media briefing sponsored by the Infectious Diseases Society of America (IDSA).
, she said. “It does seem that if you have a circulating strain BA.4 and BA.5, hitting it with the appropriate vaccine targeted for that is most immunogenic, certainly. We will hopefully see that in terms of effectiveness.”
Changing the vaccines at this point is appropriate, Walter A. Orenstein, MD, said. “One of our challenges is that this virus mutates. Our immune response is focused on an area of the virus that can change and be evaded,” said Dr. Orenstein, professor and associate director of the Emory Vaccine Center at Emory University, Atlanta.
“This is different than measles or polio,” he said. “But for influenza and now with SARS-CoV-2 ... we have to update our vaccines, because the virus changes.”
Man versus mouse
Dr. Edwards addressed the controversy over a lack of human data specific to these next-generation Pfizer/BioNTech and Moderna vaccines. “I do not want people to be unhappy or worried that the bivalent vaccine will act in a different way than the ones that we have been administering for the past 2 years.”
The Food and Drug Administration emergency use authorization may have relied primarily on animal studies, she said, but mice given a vaccine specific to BA.4 and BA.5 “have a much more robust immune response,” compared with those given a BA.1 vaccine.
Also, “over and over and over again we have seen with these SARS-CoV-2 vaccines that the mouse responses mirror the human responses,” said Dr. Edwards, scientific director of the Vanderbilt Vaccine Research Program at Vanderbilt University, Nashville, Tenn., and an IDSA fellow.
“Human data will be coming very soon to look at the immunogenicity,” she said.
A ‘glass half full’ perspective
When asked what they are most optimistic about at this point in the COVID-19 pandemic, Dr. Orenstein said, “I’m really positive in the sense that the vaccines we have are already very effective against severe disease, death, and hospitalization. I feel really good about that. And we have great tools.
“The bottom line for me is, I want to get it myself,” he said regarding the bivalent vaccine.
“There are a lot of things to be happy with,” Dr. Edwards said. “I’m kind of a glass-half-full kind of person.”
Dr. Edwards is confident that the surveillance systems now in place can accurately detect major changes in the virus, including new variants. She is also optimistic about the mRNA technology that allows rapid updates to COVID-19 vaccines.
Furthermore, “I’m happy that we’re beginning to open up – that we can go do different things that we have done in the past and feel much more comfortable,” she said.
More motivational messaging needed
Now is also a good time to renew efforts to get people vaccinated.
“We invested a lot into developing these vaccines, but I think we also need to invest in what I call ‘implementation science research,’ ” Dr. Orenstein said, the goal being to convince people to get vaccinated.
He pointed out that it’s vaccinations, not vaccines, that saves lives. “Vaccine doses that remain in the vial are 0% effective.
“When I was director of the United States’ immunization program at the CDC,” Dr. Orenstein said, “my director of communications used to say that you need the right message delivered by the right messenger through the right communications channel.”
Dr. Edwards agreed that listening to people’s concerns and respecting their questions are important. “We also need to make sure that we use the proper messenger, just as Walt said. Maybe the proper messenger isn’t an old gray-haired lady,” she said, referring to herself, “but it’s someone that lives in your community or is your primary care doctor who has taken care of you or your children for many years.”
Research on how to better motivate people to get vaccinated is warranted, Dr. Edwards said, as well as on “how to make sure that this is really a medical issue and not a political issue. That’s been a really big problem.”
A version of this article first appeared on Medscape.com.
A panel of infectious disease experts shared their take recently on the importance of the newly approved bivalent COVID-19 vaccines, why authorization without human data is not for them a cause for alarm, and what they are most optimistic about at this stage of the pandemic.
“I’m very encouraged by this new development,” Kathryn M. Edwards, MD, said during a media briefing sponsored by the Infectious Diseases Society of America (IDSA).
, she said. “It does seem that if you have a circulating strain BA.4 and BA.5, hitting it with the appropriate vaccine targeted for that is most immunogenic, certainly. We will hopefully see that in terms of effectiveness.”
Changing the vaccines at this point is appropriate, Walter A. Orenstein, MD, said. “One of our challenges is that this virus mutates. Our immune response is focused on an area of the virus that can change and be evaded,” said Dr. Orenstein, professor and associate director of the Emory Vaccine Center at Emory University, Atlanta.
“This is different than measles or polio,” he said. “But for influenza and now with SARS-CoV-2 ... we have to update our vaccines, because the virus changes.”
Man versus mouse
Dr. Edwards addressed the controversy over a lack of human data specific to these next-generation Pfizer/BioNTech and Moderna vaccines. “I do not want people to be unhappy or worried that the bivalent vaccine will act in a different way than the ones that we have been administering for the past 2 years.”
The Food and Drug Administration emergency use authorization may have relied primarily on animal studies, she said, but mice given a vaccine specific to BA.4 and BA.5 “have a much more robust immune response,” compared with those given a BA.1 vaccine.
Also, “over and over and over again we have seen with these SARS-CoV-2 vaccines that the mouse responses mirror the human responses,” said Dr. Edwards, scientific director of the Vanderbilt Vaccine Research Program at Vanderbilt University, Nashville, Tenn., and an IDSA fellow.
“Human data will be coming very soon to look at the immunogenicity,” she said.
A ‘glass half full’ perspective
When asked what they are most optimistic about at this point in the COVID-19 pandemic, Dr. Orenstein said, “I’m really positive in the sense that the vaccines we have are already very effective against severe disease, death, and hospitalization. I feel really good about that. And we have great tools.
“The bottom line for me is, I want to get it myself,” he said regarding the bivalent vaccine.
“There are a lot of things to be happy with,” Dr. Edwards said. “I’m kind of a glass-half-full kind of person.”
Dr. Edwards is confident that the surveillance systems now in place can accurately detect major changes in the virus, including new variants. She is also optimistic about the mRNA technology that allows rapid updates to COVID-19 vaccines.
Furthermore, “I’m happy that we’re beginning to open up – that we can go do different things that we have done in the past and feel much more comfortable,” she said.
More motivational messaging needed
Now is also a good time to renew efforts to get people vaccinated.
“We invested a lot into developing these vaccines, but I think we also need to invest in what I call ‘implementation science research,’ ” Dr. Orenstein said, the goal being to convince people to get vaccinated.
He pointed out that it’s vaccinations, not vaccines, that saves lives. “Vaccine doses that remain in the vial are 0% effective.
“When I was director of the United States’ immunization program at the CDC,” Dr. Orenstein said, “my director of communications used to say that you need the right message delivered by the right messenger through the right communications channel.”
Dr. Edwards agreed that listening to people’s concerns and respecting their questions are important. “We also need to make sure that we use the proper messenger, just as Walt said. Maybe the proper messenger isn’t an old gray-haired lady,” she said, referring to herself, “but it’s someone that lives in your community or is your primary care doctor who has taken care of you or your children for many years.”
Research on how to better motivate people to get vaccinated is warranted, Dr. Edwards said, as well as on “how to make sure that this is really a medical issue and not a political issue. That’s been a really big problem.”
A version of this article first appeared on Medscape.com.
Vitamin D supplementation shows no COVID-19 prevention
Two large studies out of the United Kingdom and Norway show vitamin D supplementation has no benefit – as low dose, high dose, or in the form of cod liver oil supplementation – in preventing COVID-19 or acute respiratory tract infections, regardless of whether individuals are deficient or not.
The studies, published in the BMJ, underscore that “vaccination is still the most effective way to protect people from COVID-19, and vitamin D and cod liver oil supplementation should not be offered to healthy people with normal vitamin D levels,” writes Peter Bergman, MD, of the Karolinska Institute, Stockholm, in an editorial published alongside the studies.
Suboptimal levels of vitamin D are known to be associated with an increased risk of acute respiratory infections, and some observational studies have linked low 25-hydroxyvitamin D (25[OH]D) with more severe COVID-19; however, data on a possible protective effect of vitamin D supplementation in preventing infection have been inconsistent.
U.K. study compares doses
To further investigate the relationship with infections, including COVID-19, in a large cohort, the authors of the first of the two BMJ studies, a phase 3 open-label trial, enrolled 6,200 people in the United Kingdom aged 16 and older between December 2020 and June 2021 who were not taking vitamin D supplements at baseline.
Half of participants were offered a finger-prick blood test, and of the 2,674 who accepted, 86.3% were found to have low concentrations of 25(OH)D (< 75 nmol/L). These participants were provided with vitamin D supplementation at a lower (800 IU/day; n = 1328) or higher dose (3,200 IU/day; n = 1,346) for 6 months. The other half of the group received no tests or supplements.
The results showed minimal differences between groups in terms of rates of developing at least one acute respiratory infection, which occurred in 5% of those in the lower-dose group, 5.7% in the higher-dose group, and 4.6% of participants not offered supplementation.
Similarly, there were no significant differences in the development of real-time PCR-confirmed COVID-19, with rates of 3.6% in the lower-dose group, 3.0% in the higher-dose group, and 2.6% in the group not offered supplementation.
The study is “the first phase 3 randomized controlled trial to evaluate the effectiveness of a test-and-treat approach for correction of suboptimal vitamin D status to prevent acute respiratory tract infections,” report the authors, led by Adrian R. Martineau, MD, PhD, of Barts and The London School of Medicine and Dentistry, Queen Mary University of London.
While uptake and supplementation in the study were favorable, “no statistically significant effect of either dose was seen on the primary outcome of swab test, doctor-confirmed acute respiratory tract infection, or on the major secondary outcome of swab test-confirmed COVID-19,” they conclude.
Traditional use of cod liver oil of benefit?
In the second study, researchers in Norway, led by Arne Soraas, MD, PhD, of the department of microbiology, Oslo University Hospital, evaluated whether that country’s long-held tradition of consuming cod liver oil during the winter to prevent vitamin D deficiency could affect the development of COVID-19 or outcomes.
For the Cod Liver Oil for COVID-19 Prevention Study (CLOC), a large cohort of 34,601 adults with a mean age of 44.9 years who were not taking daily vitamin D supplements were randomized to receive 5 mL/day of cod liver oil, representing a surrogate dose of 400 IU/day of vitamin D (n = 17,278), or placebo (n = 17,323) for up to 6 months.
In contrast with the first study, the vast majority of patients in the CLOC study (86%) had adequate vitamin D levels, defined as greater than 50 nmol/L, at baseline.
Again, however, the results showed no association between increased vitamin D supplementation with cod liver oil and PCR-confirmed COVID-19 or acute respiratory infections, with approximately 1.3% in each group testing positive for COVID-19 over a median of 164 days.
Supplementation with cod liver oil was also not associated with a reduced risk of any of the coprimary endpoints, including other acute respiratory infections.
“Daily supplementation with cod liver oil, a low-dose vitamin D, eicosapentaenoic acid, and docosahexaenoic acid supplement, for 6 months during the SARS-CoV-2pandemic among Norwegian adults did not reduce the incidence of SARS-CoV-2 infection, serious COVID-19, or other acute respiratory infections,” the authors report.
Key study limitations
In his editorial, Dr. Bergman underscores the limitations of two studies – also acknowledged by the authors – including the key confounding role of vaccines that emerged during the studies.
“The null findings of the studies should be interpreted in the context of a highly effective vaccine rolled out during both studies,” Dr. Bergman writes.
In the U.K. study, for instance, whereas only 1.2% of participants were vaccinated at baseline, the rate soared to 89.1% having received at least one dose by study end, potentially masking any effect of vitamin D, he says.
Additionally, for the Norway study, Dr. Bergman notes that cod liver oil also contains a substantial amount of vitamin A, which can be a potent immunomodulator.
“Excessive intake of vitamin A can cause adverse effects and may also interfere with vitamin D-mediated effects on the immune system,” he writes.
With two recent large meta-analyses showing benefits of vitamin D supplementation to be specifically among people who are vitamin D deficient, “a pragmatic approach for the clinician could be to focus on risk groups” for supplementation, Dr. Bergman writes.
“[These include] those who could be tested before supplementation, including people with dark skin, or skin that is rarely exposed to the sun, pregnant women, and elderly people with chronic diseases.”
The U.K. trial was supported by Barts Charity, Pharma Nord, the Fischer Family Foundation, DSM Nutritional Products, the Exilarch’s Foundation, the Karl R. Pfleger Foundation, the AIM Foundation, Synergy Biologics, Cytoplan, the Clinical Research Network of the U.K. National Institute for Health and Care Research, the HDR UK BREATHE Hub, the U.K. Research and Innovation Industrial Strategy Challenge Fund, Thornton & Ross, Warburtons, Hyphens Pharma, and philanthropist Matthew Isaacs.
The CLOC trial was funded by Orkla Health, the manufacturer of the cod liver oil used in the trial. Dr. Bergman has reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Two large studies out of the United Kingdom and Norway show vitamin D supplementation has no benefit – as low dose, high dose, or in the form of cod liver oil supplementation – in preventing COVID-19 or acute respiratory tract infections, regardless of whether individuals are deficient or not.
The studies, published in the BMJ, underscore that “vaccination is still the most effective way to protect people from COVID-19, and vitamin D and cod liver oil supplementation should not be offered to healthy people with normal vitamin D levels,” writes Peter Bergman, MD, of the Karolinska Institute, Stockholm, in an editorial published alongside the studies.
Suboptimal levels of vitamin D are known to be associated with an increased risk of acute respiratory infections, and some observational studies have linked low 25-hydroxyvitamin D (25[OH]D) with more severe COVID-19; however, data on a possible protective effect of vitamin D supplementation in preventing infection have been inconsistent.
U.K. study compares doses
To further investigate the relationship with infections, including COVID-19, in a large cohort, the authors of the first of the two BMJ studies, a phase 3 open-label trial, enrolled 6,200 people in the United Kingdom aged 16 and older between December 2020 and June 2021 who were not taking vitamin D supplements at baseline.
Half of participants were offered a finger-prick blood test, and of the 2,674 who accepted, 86.3% were found to have low concentrations of 25(OH)D (< 75 nmol/L). These participants were provided with vitamin D supplementation at a lower (800 IU/day; n = 1328) or higher dose (3,200 IU/day; n = 1,346) for 6 months. The other half of the group received no tests or supplements.
The results showed minimal differences between groups in terms of rates of developing at least one acute respiratory infection, which occurred in 5% of those in the lower-dose group, 5.7% in the higher-dose group, and 4.6% of participants not offered supplementation.
Similarly, there were no significant differences in the development of real-time PCR-confirmed COVID-19, with rates of 3.6% in the lower-dose group, 3.0% in the higher-dose group, and 2.6% in the group not offered supplementation.
The study is “the first phase 3 randomized controlled trial to evaluate the effectiveness of a test-and-treat approach for correction of suboptimal vitamin D status to prevent acute respiratory tract infections,” report the authors, led by Adrian R. Martineau, MD, PhD, of Barts and The London School of Medicine and Dentistry, Queen Mary University of London.
While uptake and supplementation in the study were favorable, “no statistically significant effect of either dose was seen on the primary outcome of swab test, doctor-confirmed acute respiratory tract infection, or on the major secondary outcome of swab test-confirmed COVID-19,” they conclude.
Traditional use of cod liver oil of benefit?
In the second study, researchers in Norway, led by Arne Soraas, MD, PhD, of the department of microbiology, Oslo University Hospital, evaluated whether that country’s long-held tradition of consuming cod liver oil during the winter to prevent vitamin D deficiency could affect the development of COVID-19 or outcomes.
For the Cod Liver Oil for COVID-19 Prevention Study (CLOC), a large cohort of 34,601 adults with a mean age of 44.9 years who were not taking daily vitamin D supplements were randomized to receive 5 mL/day of cod liver oil, representing a surrogate dose of 400 IU/day of vitamin D (n = 17,278), or placebo (n = 17,323) for up to 6 months.
In contrast with the first study, the vast majority of patients in the CLOC study (86%) had adequate vitamin D levels, defined as greater than 50 nmol/L, at baseline.
Again, however, the results showed no association between increased vitamin D supplementation with cod liver oil and PCR-confirmed COVID-19 or acute respiratory infections, with approximately 1.3% in each group testing positive for COVID-19 over a median of 164 days.
Supplementation with cod liver oil was also not associated with a reduced risk of any of the coprimary endpoints, including other acute respiratory infections.
“Daily supplementation with cod liver oil, a low-dose vitamin D, eicosapentaenoic acid, and docosahexaenoic acid supplement, for 6 months during the SARS-CoV-2pandemic among Norwegian adults did not reduce the incidence of SARS-CoV-2 infection, serious COVID-19, or other acute respiratory infections,” the authors report.
Key study limitations
In his editorial, Dr. Bergman underscores the limitations of two studies – also acknowledged by the authors – including the key confounding role of vaccines that emerged during the studies.
“The null findings of the studies should be interpreted in the context of a highly effective vaccine rolled out during both studies,” Dr. Bergman writes.
In the U.K. study, for instance, whereas only 1.2% of participants were vaccinated at baseline, the rate soared to 89.1% having received at least one dose by study end, potentially masking any effect of vitamin D, he says.
Additionally, for the Norway study, Dr. Bergman notes that cod liver oil also contains a substantial amount of vitamin A, which can be a potent immunomodulator.
“Excessive intake of vitamin A can cause adverse effects and may also interfere with vitamin D-mediated effects on the immune system,” he writes.
With two recent large meta-analyses showing benefits of vitamin D supplementation to be specifically among people who are vitamin D deficient, “a pragmatic approach for the clinician could be to focus on risk groups” for supplementation, Dr. Bergman writes.
“[These include] those who could be tested before supplementation, including people with dark skin, or skin that is rarely exposed to the sun, pregnant women, and elderly people with chronic diseases.”
The U.K. trial was supported by Barts Charity, Pharma Nord, the Fischer Family Foundation, DSM Nutritional Products, the Exilarch’s Foundation, the Karl R. Pfleger Foundation, the AIM Foundation, Synergy Biologics, Cytoplan, the Clinical Research Network of the U.K. National Institute for Health and Care Research, the HDR UK BREATHE Hub, the U.K. Research and Innovation Industrial Strategy Challenge Fund, Thornton & Ross, Warburtons, Hyphens Pharma, and philanthropist Matthew Isaacs.
The CLOC trial was funded by Orkla Health, the manufacturer of the cod liver oil used in the trial. Dr. Bergman has reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Two large studies out of the United Kingdom and Norway show vitamin D supplementation has no benefit – as low dose, high dose, or in the form of cod liver oil supplementation – in preventing COVID-19 or acute respiratory tract infections, regardless of whether individuals are deficient or not.
The studies, published in the BMJ, underscore that “vaccination is still the most effective way to protect people from COVID-19, and vitamin D and cod liver oil supplementation should not be offered to healthy people with normal vitamin D levels,” writes Peter Bergman, MD, of the Karolinska Institute, Stockholm, in an editorial published alongside the studies.
Suboptimal levels of vitamin D are known to be associated with an increased risk of acute respiratory infections, and some observational studies have linked low 25-hydroxyvitamin D (25[OH]D) with more severe COVID-19; however, data on a possible protective effect of vitamin D supplementation in preventing infection have been inconsistent.
U.K. study compares doses
To further investigate the relationship with infections, including COVID-19, in a large cohort, the authors of the first of the two BMJ studies, a phase 3 open-label trial, enrolled 6,200 people in the United Kingdom aged 16 and older between December 2020 and June 2021 who were not taking vitamin D supplements at baseline.
Half of participants were offered a finger-prick blood test, and of the 2,674 who accepted, 86.3% were found to have low concentrations of 25(OH)D (< 75 nmol/L). These participants were provided with vitamin D supplementation at a lower (800 IU/day; n = 1328) or higher dose (3,200 IU/day; n = 1,346) for 6 months. The other half of the group received no tests or supplements.
The results showed minimal differences between groups in terms of rates of developing at least one acute respiratory infection, which occurred in 5% of those in the lower-dose group, 5.7% in the higher-dose group, and 4.6% of participants not offered supplementation.
Similarly, there were no significant differences in the development of real-time PCR-confirmed COVID-19, with rates of 3.6% in the lower-dose group, 3.0% in the higher-dose group, and 2.6% in the group not offered supplementation.
The study is “the first phase 3 randomized controlled trial to evaluate the effectiveness of a test-and-treat approach for correction of suboptimal vitamin D status to prevent acute respiratory tract infections,” report the authors, led by Adrian R. Martineau, MD, PhD, of Barts and The London School of Medicine and Dentistry, Queen Mary University of London.
While uptake and supplementation in the study were favorable, “no statistically significant effect of either dose was seen on the primary outcome of swab test, doctor-confirmed acute respiratory tract infection, or on the major secondary outcome of swab test-confirmed COVID-19,” they conclude.
Traditional use of cod liver oil of benefit?
In the second study, researchers in Norway, led by Arne Soraas, MD, PhD, of the department of microbiology, Oslo University Hospital, evaluated whether that country’s long-held tradition of consuming cod liver oil during the winter to prevent vitamin D deficiency could affect the development of COVID-19 or outcomes.
For the Cod Liver Oil for COVID-19 Prevention Study (CLOC), a large cohort of 34,601 adults with a mean age of 44.9 years who were not taking daily vitamin D supplements were randomized to receive 5 mL/day of cod liver oil, representing a surrogate dose of 400 IU/day of vitamin D (n = 17,278), or placebo (n = 17,323) for up to 6 months.
In contrast with the first study, the vast majority of patients in the CLOC study (86%) had adequate vitamin D levels, defined as greater than 50 nmol/L, at baseline.
Again, however, the results showed no association between increased vitamin D supplementation with cod liver oil and PCR-confirmed COVID-19 or acute respiratory infections, with approximately 1.3% in each group testing positive for COVID-19 over a median of 164 days.
Supplementation with cod liver oil was also not associated with a reduced risk of any of the coprimary endpoints, including other acute respiratory infections.
“Daily supplementation with cod liver oil, a low-dose vitamin D, eicosapentaenoic acid, and docosahexaenoic acid supplement, for 6 months during the SARS-CoV-2pandemic among Norwegian adults did not reduce the incidence of SARS-CoV-2 infection, serious COVID-19, or other acute respiratory infections,” the authors report.
Key study limitations
In his editorial, Dr. Bergman underscores the limitations of two studies – also acknowledged by the authors – including the key confounding role of vaccines that emerged during the studies.
“The null findings of the studies should be interpreted in the context of a highly effective vaccine rolled out during both studies,” Dr. Bergman writes.
In the U.K. study, for instance, whereas only 1.2% of participants were vaccinated at baseline, the rate soared to 89.1% having received at least one dose by study end, potentially masking any effect of vitamin D, he says.
Additionally, for the Norway study, Dr. Bergman notes that cod liver oil also contains a substantial amount of vitamin A, which can be a potent immunomodulator.
“Excessive intake of vitamin A can cause adverse effects and may also interfere with vitamin D-mediated effects on the immune system,” he writes.
With two recent large meta-analyses showing benefits of vitamin D supplementation to be specifically among people who are vitamin D deficient, “a pragmatic approach for the clinician could be to focus on risk groups” for supplementation, Dr. Bergman writes.
“[These include] those who could be tested before supplementation, including people with dark skin, or skin that is rarely exposed to the sun, pregnant women, and elderly people with chronic diseases.”
The U.K. trial was supported by Barts Charity, Pharma Nord, the Fischer Family Foundation, DSM Nutritional Products, the Exilarch’s Foundation, the Karl R. Pfleger Foundation, the AIM Foundation, Synergy Biologics, Cytoplan, the Clinical Research Network of the U.K. National Institute for Health and Care Research, the HDR UK BREATHE Hub, the U.K. Research and Innovation Industrial Strategy Challenge Fund, Thornton & Ross, Warburtons, Hyphens Pharma, and philanthropist Matthew Isaacs.
The CLOC trial was funded by Orkla Health, the manufacturer of the cod liver oil used in the trial. Dr. Bergman has reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM BMJ