User login
Breakthrough COVID studies lend support to use of new boosters in immunosuppressed patients
People with immune-mediated inflammatory diseases who are taking immunosuppressants don’t mount as strong of an immune defense against the Omicron variant as they did against the original SARS-CoV-2 wild-type virus, according to two studies published in Annals of the Rheumatic Diseases. One of the studies further showed that vaccinated individuals taking immunosuppressants have poorer cross-neutralizing responses to Omicron than do healthy vaccinated individuals, even after three doses of the COVID-19 mRNA vaccines.
“We carefully suggest that if Omicron-specific vaccination can be administered, it may be an effective way to reduce the risk of breakthrough infections in patients with autoimmune rheumatic disease,” Sang Tae Choi, MD, PhD, of the University College of Medicine, Seoul, Korea, and one of the authors of the study on cross-neutralizing protection, told this news organization. “However, further research is needed on Omicron-specific vaccine effectiveness in patients with immune dysfunctions. We believe that these study results can be of great benefit in determining the strategy of vaccination in the future.”
The earlier study, published in July, examined the ability of COVID-19 vaccines to induce cross-reactive antibody responses against Omicron infections in patients with autoimmune rheumatic diseases (ARDs). The observational study involved 149 patients with ARDs and 94 health care workers as controls, all of whom provided blood samples a median 15 weeks after their second COVID vaccine dose or a median 8 weeks after their third dose. A little more than two-thirds of the patients (68.5%) had received a third mRNA vaccine dose. None of the participants previously had COVID-19.
The researchers compared the rate of breakthrough infections with the Omicron variant to the neutralizing responses in patients’ blood, specifically the cross-neutralizing antibody responses because the original mRNA vaccines targeted a different variant than Omicron. Breakthrough infections were assessed by survey questions.
“Our findings suggested that neither primary series vaccinations nor booster doses are sufficient to induce Omicron-neutralizing responses above the threshold in patients with ARDs, although responses were noticeably increased following the third dose of an mRNA vaccine,” write Woo-Joong Kim, of the Chung-Ang University College of Medicine, Seoul, Korea, and his colleagues. “This impairment of cross-neutralization responses across most of our patients contrasts starkly with a potent elicitation of the Omicron-neutralizing responses after the third vaccination in healthy recipients.”
The average neutralizing responses against the original SARS-CoV-2 strain were similar in both groups: 76% in patients with ARDs and 72% in health care workers after the second dose. The mean response after a third dose was 97% in health care workers and 88% in patients.
The average cross-neutralizing response against the Omicron variant was far lower, particularly in those with rheumatic disease: only 11.5%, which rose to 27% after the third dose. Only 39% of the patient sera showed neutralization of Omicron, even after the third dose. Meanwhile, the mean cross-neutralizing response in health care workers was 18% after the second dose and 50% after the third.
When the researchers compared seropositivity rates against the original virus to neutralizing responses against Omicron, the association between these was stronger in health care workers than in those with ARDs. In fact, among patients with ARDs who seroconverted, only 41% showed any response against Omicron. Among all the patients, most of those who didn’t respond to Omicron (93.5%) had initially seroconverted.
The researchers also looked at the ability to neutralize Omicron on the basis of disease in those who received three doses of the vaccine. About half of those with lupus (52%) showed any neutralization against Omicron, compared with 25% of those with rheumatoid arthritis, 37.5% of those with ankylosing spondylitis, 33% of those with Behçet snydrome, and all of those with adult-onset Still’s disease.
The rate of breakthrough infections was lower in patients (19%) than in health care workers (33%). A similar pattern was seen in the more recent study published Sept. 5. Researchers used data from a prospective cohort study in the Netherlands to examine incidence and severity of Omicron breakthrough infections in patients with immune-mediated inflammatory diseases. The researchers compared infection rates and severity among 1,593 vaccinated patients with inflammatory disease who were taking immunosuppressants and 579 vaccinated controls (418 patients with inflammatory disease not on immunosuppressants and 161 healthy controls).
One in five patients with inflammatory disease (21%) were taking immunosuppressants that substantially impair antibodies, such as anti-CD20 therapy, S1P modulators, or mycophenolate mofetil combination therapy, and 48% of these patients seroconverted after primary vaccination, compared with 96% of patients taking other immunosuppressants and 98% of controls.
Breakthrough infection rates were similar between the control group (31%) and those taking immunosuppressants (30%). Only three participants had severe disease requiring hospitalization: one control and two patients taking immunosuppressants.
“In both studies, the controls had similar or higher rates of breakthrough infections, compared with the immunosuppressed,” noted Alfred Kim, MD, an assistant professor of medicine at Washington University, St Louis, but he added, “one has to consider differences in mitigation strategies, such as masking, that may explain these findings.” That is, patients taking immunosuppressants may be taking fewer risks in the community or have fewer potential exposures, especially in the Korean study, wherein the controls were health care workers.
A greater disparity in infections occurred when considering seroconversion rates. Breakthrough incidence was 38% among those taking immunosuppressants who did not seroconvert, compared with 29% among those who did. A similar trend was seen in breakthrough incidence between those taking strongly antibody-impairing immunosuppressants (36% breakthrough rate) and those taking other immunosuppressants (28%).
Among those taking immunosuppressants who seroconverted, a primary series of vaccination reduced the risk of a breakthrough infection by 29%. Protection became more robust with a booster or prior infection, both of which reduced breakthrough infection risk by 39% in those taking immunosuppressants who seroconverted.
“We demonstrate in patients with immune-mediated inflammatory diseases on immunosuppressants that additional vaccinations are associated with decreased risk of SARS-CoV-2 Omicron breakthrough infections,” wrote Eileen W. Stalman, MD, PhD, of Amsterdam UMC in the Netherlands, and her colleagues.
Though neither study broke down immune response or breakthrough infection based on individual medications, Kim said that previous research allows one to extrapolate “that prior culprits of poor vaccine responses [such as B-cell depleting drugs, mycophenolate, and TNF [tumor necrosis factor] inhibitors will continue to bear the greatest burden in breakthrough infection, including Omicron.”
Overall, he found the data from both studies relatively consistent with one another.
“Those on immunosuppression, particularly mechanisms that have been established as risk factors for poor vaccine responses, are at risk of breakthrough infection during the era of Omicron,” Dr. Kim said.
The earlier study from Korea also found that “the median time between the third-dose vaccination and the date of confirmed breakthrough infection in patients with ARDs was significantly shorter, compared with that in health care workers” at just 93 days in patients versus 122 days in health care workers. They postulated that this population’s limited neutralization of Omicron explained this short-lived protection.
Most of the patients with breakthrough infections (74%) in that study showed no neutralization against Omicron, including the only two hospitalized patients, both of whom had strong responses against the original SARS-CoV-2 strain. The significant decline over time of neutralization against Omicron suggested “the potential for a substantial loss of the protection from breakthrough infection,” the authors write.
“The third dose of an mRNA vaccine could improve the cross-neutralization of the SARS-CoV-2 Omicron variant in patients with autoimmune rheumatic disease [although] more than half of the patients failed to generate Omicron-neutralizing antibodies,” Tae Choi said in an interview. “Our study sheds light on the relative deficiency of the Omicron-specific neutralizing responses in patients with autoimmune rheumatic disease and their anticipated vulnerability to breakthrough infection.”
The message for clinicians, Dr. Kim said, is to “continue to urge our patients to maintain additional and boosting doses per guidance, use pre-exposure prophylaxis such as Evusheld, and continue other mitigation strategies as they have done.”
The Dutch study was funded by The Netherlands Organization for Health Research and Development; the Korean study used no external funding.
The authors of the Korean study had no disclosures. The Dutch study’s authors reported a wide range of disclosures involving more than a dozen pharmaceutical companies but not including Pfizer or Moderna. Dr. Kim’s industry disclosures include Alexion, ANI, AstraZeneca, Aurinia, Exagen, Foghorn Therapeutics, GlaxoSmithKline, Kypha, and Pfizer.
A version of this article first appeared on Medscape.com.
People with immune-mediated inflammatory diseases who are taking immunosuppressants don’t mount as strong of an immune defense against the Omicron variant as they did against the original SARS-CoV-2 wild-type virus, according to two studies published in Annals of the Rheumatic Diseases. One of the studies further showed that vaccinated individuals taking immunosuppressants have poorer cross-neutralizing responses to Omicron than do healthy vaccinated individuals, even after three doses of the COVID-19 mRNA vaccines.
“We carefully suggest that if Omicron-specific vaccination can be administered, it may be an effective way to reduce the risk of breakthrough infections in patients with autoimmune rheumatic disease,” Sang Tae Choi, MD, PhD, of the University College of Medicine, Seoul, Korea, and one of the authors of the study on cross-neutralizing protection, told this news organization. “However, further research is needed on Omicron-specific vaccine effectiveness in patients with immune dysfunctions. We believe that these study results can be of great benefit in determining the strategy of vaccination in the future.”
The earlier study, published in July, examined the ability of COVID-19 vaccines to induce cross-reactive antibody responses against Omicron infections in patients with autoimmune rheumatic diseases (ARDs). The observational study involved 149 patients with ARDs and 94 health care workers as controls, all of whom provided blood samples a median 15 weeks after their second COVID vaccine dose or a median 8 weeks after their third dose. A little more than two-thirds of the patients (68.5%) had received a third mRNA vaccine dose. None of the participants previously had COVID-19.
The researchers compared the rate of breakthrough infections with the Omicron variant to the neutralizing responses in patients’ blood, specifically the cross-neutralizing antibody responses because the original mRNA vaccines targeted a different variant than Omicron. Breakthrough infections were assessed by survey questions.
“Our findings suggested that neither primary series vaccinations nor booster doses are sufficient to induce Omicron-neutralizing responses above the threshold in patients with ARDs, although responses were noticeably increased following the third dose of an mRNA vaccine,” write Woo-Joong Kim, of the Chung-Ang University College of Medicine, Seoul, Korea, and his colleagues. “This impairment of cross-neutralization responses across most of our patients contrasts starkly with a potent elicitation of the Omicron-neutralizing responses after the third vaccination in healthy recipients.”
The average neutralizing responses against the original SARS-CoV-2 strain were similar in both groups: 76% in patients with ARDs and 72% in health care workers after the second dose. The mean response after a third dose was 97% in health care workers and 88% in patients.
The average cross-neutralizing response against the Omicron variant was far lower, particularly in those with rheumatic disease: only 11.5%, which rose to 27% after the third dose. Only 39% of the patient sera showed neutralization of Omicron, even after the third dose. Meanwhile, the mean cross-neutralizing response in health care workers was 18% after the second dose and 50% after the third.
When the researchers compared seropositivity rates against the original virus to neutralizing responses against Omicron, the association between these was stronger in health care workers than in those with ARDs. In fact, among patients with ARDs who seroconverted, only 41% showed any response against Omicron. Among all the patients, most of those who didn’t respond to Omicron (93.5%) had initially seroconverted.
The researchers also looked at the ability to neutralize Omicron on the basis of disease in those who received three doses of the vaccine. About half of those with lupus (52%) showed any neutralization against Omicron, compared with 25% of those with rheumatoid arthritis, 37.5% of those with ankylosing spondylitis, 33% of those with Behçet snydrome, and all of those with adult-onset Still’s disease.
The rate of breakthrough infections was lower in patients (19%) than in health care workers (33%). A similar pattern was seen in the more recent study published Sept. 5. Researchers used data from a prospective cohort study in the Netherlands to examine incidence and severity of Omicron breakthrough infections in patients with immune-mediated inflammatory diseases. The researchers compared infection rates and severity among 1,593 vaccinated patients with inflammatory disease who were taking immunosuppressants and 579 vaccinated controls (418 patients with inflammatory disease not on immunosuppressants and 161 healthy controls).
One in five patients with inflammatory disease (21%) were taking immunosuppressants that substantially impair antibodies, such as anti-CD20 therapy, S1P modulators, or mycophenolate mofetil combination therapy, and 48% of these patients seroconverted after primary vaccination, compared with 96% of patients taking other immunosuppressants and 98% of controls.
Breakthrough infection rates were similar between the control group (31%) and those taking immunosuppressants (30%). Only three participants had severe disease requiring hospitalization: one control and two patients taking immunosuppressants.
“In both studies, the controls had similar or higher rates of breakthrough infections, compared with the immunosuppressed,” noted Alfred Kim, MD, an assistant professor of medicine at Washington University, St Louis, but he added, “one has to consider differences in mitigation strategies, such as masking, that may explain these findings.” That is, patients taking immunosuppressants may be taking fewer risks in the community or have fewer potential exposures, especially in the Korean study, wherein the controls were health care workers.
A greater disparity in infections occurred when considering seroconversion rates. Breakthrough incidence was 38% among those taking immunosuppressants who did not seroconvert, compared with 29% among those who did. A similar trend was seen in breakthrough incidence between those taking strongly antibody-impairing immunosuppressants (36% breakthrough rate) and those taking other immunosuppressants (28%).
Among those taking immunosuppressants who seroconverted, a primary series of vaccination reduced the risk of a breakthrough infection by 29%. Protection became more robust with a booster or prior infection, both of which reduced breakthrough infection risk by 39% in those taking immunosuppressants who seroconverted.
“We demonstrate in patients with immune-mediated inflammatory diseases on immunosuppressants that additional vaccinations are associated with decreased risk of SARS-CoV-2 Omicron breakthrough infections,” wrote Eileen W. Stalman, MD, PhD, of Amsterdam UMC in the Netherlands, and her colleagues.
Though neither study broke down immune response or breakthrough infection based on individual medications, Kim said that previous research allows one to extrapolate “that prior culprits of poor vaccine responses [such as B-cell depleting drugs, mycophenolate, and TNF [tumor necrosis factor] inhibitors will continue to bear the greatest burden in breakthrough infection, including Omicron.”
Overall, he found the data from both studies relatively consistent with one another.
“Those on immunosuppression, particularly mechanisms that have been established as risk factors for poor vaccine responses, are at risk of breakthrough infection during the era of Omicron,” Dr. Kim said.
The earlier study from Korea also found that “the median time between the third-dose vaccination and the date of confirmed breakthrough infection in patients with ARDs was significantly shorter, compared with that in health care workers” at just 93 days in patients versus 122 days in health care workers. They postulated that this population’s limited neutralization of Omicron explained this short-lived protection.
Most of the patients with breakthrough infections (74%) in that study showed no neutralization against Omicron, including the only two hospitalized patients, both of whom had strong responses against the original SARS-CoV-2 strain. The significant decline over time of neutralization against Omicron suggested “the potential for a substantial loss of the protection from breakthrough infection,” the authors write.
“The third dose of an mRNA vaccine could improve the cross-neutralization of the SARS-CoV-2 Omicron variant in patients with autoimmune rheumatic disease [although] more than half of the patients failed to generate Omicron-neutralizing antibodies,” Tae Choi said in an interview. “Our study sheds light on the relative deficiency of the Omicron-specific neutralizing responses in patients with autoimmune rheumatic disease and their anticipated vulnerability to breakthrough infection.”
The message for clinicians, Dr. Kim said, is to “continue to urge our patients to maintain additional and boosting doses per guidance, use pre-exposure prophylaxis such as Evusheld, and continue other mitigation strategies as they have done.”
The Dutch study was funded by The Netherlands Organization for Health Research and Development; the Korean study used no external funding.
The authors of the Korean study had no disclosures. The Dutch study’s authors reported a wide range of disclosures involving more than a dozen pharmaceutical companies but not including Pfizer or Moderna. Dr. Kim’s industry disclosures include Alexion, ANI, AstraZeneca, Aurinia, Exagen, Foghorn Therapeutics, GlaxoSmithKline, Kypha, and Pfizer.
A version of this article first appeared on Medscape.com.
People with immune-mediated inflammatory diseases who are taking immunosuppressants don’t mount as strong of an immune defense against the Omicron variant as they did against the original SARS-CoV-2 wild-type virus, according to two studies published in Annals of the Rheumatic Diseases. One of the studies further showed that vaccinated individuals taking immunosuppressants have poorer cross-neutralizing responses to Omicron than do healthy vaccinated individuals, even after three doses of the COVID-19 mRNA vaccines.
“We carefully suggest that if Omicron-specific vaccination can be administered, it may be an effective way to reduce the risk of breakthrough infections in patients with autoimmune rheumatic disease,” Sang Tae Choi, MD, PhD, of the University College of Medicine, Seoul, Korea, and one of the authors of the study on cross-neutralizing protection, told this news organization. “However, further research is needed on Omicron-specific vaccine effectiveness in patients with immune dysfunctions. We believe that these study results can be of great benefit in determining the strategy of vaccination in the future.”
The earlier study, published in July, examined the ability of COVID-19 vaccines to induce cross-reactive antibody responses against Omicron infections in patients with autoimmune rheumatic diseases (ARDs). The observational study involved 149 patients with ARDs and 94 health care workers as controls, all of whom provided blood samples a median 15 weeks after their second COVID vaccine dose or a median 8 weeks after their third dose. A little more than two-thirds of the patients (68.5%) had received a third mRNA vaccine dose. None of the participants previously had COVID-19.
The researchers compared the rate of breakthrough infections with the Omicron variant to the neutralizing responses in patients’ blood, specifically the cross-neutralizing antibody responses because the original mRNA vaccines targeted a different variant than Omicron. Breakthrough infections were assessed by survey questions.
“Our findings suggested that neither primary series vaccinations nor booster doses are sufficient to induce Omicron-neutralizing responses above the threshold in patients with ARDs, although responses were noticeably increased following the third dose of an mRNA vaccine,” write Woo-Joong Kim, of the Chung-Ang University College of Medicine, Seoul, Korea, and his colleagues. “This impairment of cross-neutralization responses across most of our patients contrasts starkly with a potent elicitation of the Omicron-neutralizing responses after the third vaccination in healthy recipients.”
The average neutralizing responses against the original SARS-CoV-2 strain were similar in both groups: 76% in patients with ARDs and 72% in health care workers after the second dose. The mean response after a third dose was 97% in health care workers and 88% in patients.
The average cross-neutralizing response against the Omicron variant was far lower, particularly in those with rheumatic disease: only 11.5%, which rose to 27% after the third dose. Only 39% of the patient sera showed neutralization of Omicron, even after the third dose. Meanwhile, the mean cross-neutralizing response in health care workers was 18% after the second dose and 50% after the third.
When the researchers compared seropositivity rates against the original virus to neutralizing responses against Omicron, the association between these was stronger in health care workers than in those with ARDs. In fact, among patients with ARDs who seroconverted, only 41% showed any response against Omicron. Among all the patients, most of those who didn’t respond to Omicron (93.5%) had initially seroconverted.
The researchers also looked at the ability to neutralize Omicron on the basis of disease in those who received three doses of the vaccine. About half of those with lupus (52%) showed any neutralization against Omicron, compared with 25% of those with rheumatoid arthritis, 37.5% of those with ankylosing spondylitis, 33% of those with Behçet snydrome, and all of those with adult-onset Still’s disease.
The rate of breakthrough infections was lower in patients (19%) than in health care workers (33%). A similar pattern was seen in the more recent study published Sept. 5. Researchers used data from a prospective cohort study in the Netherlands to examine incidence and severity of Omicron breakthrough infections in patients with immune-mediated inflammatory diseases. The researchers compared infection rates and severity among 1,593 vaccinated patients with inflammatory disease who were taking immunosuppressants and 579 vaccinated controls (418 patients with inflammatory disease not on immunosuppressants and 161 healthy controls).
One in five patients with inflammatory disease (21%) were taking immunosuppressants that substantially impair antibodies, such as anti-CD20 therapy, S1P modulators, or mycophenolate mofetil combination therapy, and 48% of these patients seroconverted after primary vaccination, compared with 96% of patients taking other immunosuppressants and 98% of controls.
Breakthrough infection rates were similar between the control group (31%) and those taking immunosuppressants (30%). Only three participants had severe disease requiring hospitalization: one control and two patients taking immunosuppressants.
“In both studies, the controls had similar or higher rates of breakthrough infections, compared with the immunosuppressed,” noted Alfred Kim, MD, an assistant professor of medicine at Washington University, St Louis, but he added, “one has to consider differences in mitigation strategies, such as masking, that may explain these findings.” That is, patients taking immunosuppressants may be taking fewer risks in the community or have fewer potential exposures, especially in the Korean study, wherein the controls were health care workers.
A greater disparity in infections occurred when considering seroconversion rates. Breakthrough incidence was 38% among those taking immunosuppressants who did not seroconvert, compared with 29% among those who did. A similar trend was seen in breakthrough incidence between those taking strongly antibody-impairing immunosuppressants (36% breakthrough rate) and those taking other immunosuppressants (28%).
Among those taking immunosuppressants who seroconverted, a primary series of vaccination reduced the risk of a breakthrough infection by 29%. Protection became more robust with a booster or prior infection, both of which reduced breakthrough infection risk by 39% in those taking immunosuppressants who seroconverted.
“We demonstrate in patients with immune-mediated inflammatory diseases on immunosuppressants that additional vaccinations are associated with decreased risk of SARS-CoV-2 Omicron breakthrough infections,” wrote Eileen W. Stalman, MD, PhD, of Amsterdam UMC in the Netherlands, and her colleagues.
Though neither study broke down immune response or breakthrough infection based on individual medications, Kim said that previous research allows one to extrapolate “that prior culprits of poor vaccine responses [such as B-cell depleting drugs, mycophenolate, and TNF [tumor necrosis factor] inhibitors will continue to bear the greatest burden in breakthrough infection, including Omicron.”
Overall, he found the data from both studies relatively consistent with one another.
“Those on immunosuppression, particularly mechanisms that have been established as risk factors for poor vaccine responses, are at risk of breakthrough infection during the era of Omicron,” Dr. Kim said.
The earlier study from Korea also found that “the median time between the third-dose vaccination and the date of confirmed breakthrough infection in patients with ARDs was significantly shorter, compared with that in health care workers” at just 93 days in patients versus 122 days in health care workers. They postulated that this population’s limited neutralization of Omicron explained this short-lived protection.
Most of the patients with breakthrough infections (74%) in that study showed no neutralization against Omicron, including the only two hospitalized patients, both of whom had strong responses against the original SARS-CoV-2 strain. The significant decline over time of neutralization against Omicron suggested “the potential for a substantial loss of the protection from breakthrough infection,” the authors write.
“The third dose of an mRNA vaccine could improve the cross-neutralization of the SARS-CoV-2 Omicron variant in patients with autoimmune rheumatic disease [although] more than half of the patients failed to generate Omicron-neutralizing antibodies,” Tae Choi said in an interview. “Our study sheds light on the relative deficiency of the Omicron-specific neutralizing responses in patients with autoimmune rheumatic disease and their anticipated vulnerability to breakthrough infection.”
The message for clinicians, Dr. Kim said, is to “continue to urge our patients to maintain additional and boosting doses per guidance, use pre-exposure prophylaxis such as Evusheld, and continue other mitigation strategies as they have done.”
The Dutch study was funded by The Netherlands Organization for Health Research and Development; the Korean study used no external funding.
The authors of the Korean study had no disclosures. The Dutch study’s authors reported a wide range of disclosures involving more than a dozen pharmaceutical companies but not including Pfizer or Moderna. Dr. Kim’s industry disclosures include Alexion, ANI, AstraZeneca, Aurinia, Exagen, Foghorn Therapeutics, GlaxoSmithKline, Kypha, and Pfizer.
A version of this article first appeared on Medscape.com.
COVID attacks DNA in heart, unlike flu, study says
, according to a study published in Immunology.
The study looked at the hearts of patients who died from COVID-19, the flu, and other causes. The findings could provide clues about why coronavirus has led to complications such as ongoing heart issues.
“We found a lot of DNA damage that was unique to the COVID-19 patients, which wasn’t present in the flu patients,” Arutha Kulasinghe, one of the lead study authors and a research fellow at the University of Queensland, Brisbane, Australia, told the Brisbane Times.
“So in this study, COVID-19 and flu look very different in the way they affect the heart,” he said.
Dr. Kulasinghe and colleagues analyzed the hearts of seven COVID-19 patients, two flu patients, and six patients who died from other causes. They used transcriptomic profiling, which looks at the DNA landscape of an organ, to investigate heart tissue from the patients.
Because of previous studies about heart problems associated with COVID-19, he and colleagues expected to find extreme inflammation in the heart. Instead, they found that inflammation signals had been suppressed in the heart, and markers for DNA damage and repair were much higher. They’re still unsure of the underlying cause.
“The indications here are that there’s DNA damage here, it’s not inflammation,” Dr. Kulasinghe said. “There’s something else going on that we need to figure out.”
The damage was similar to the way chronic diseases such as diabetes and cancer appear in the heart, he said, with heart tissue showing DNA damage signals.
Dr. Kulasinghe said he hopes other studies can build on the findings to develop risk models to understand which patients may face a higher risk of serious COVID-19 complications. In turn, this could help doctors provide early treatment. For instance, all seven COVID-19 patients had other chronic diseases, such as diabetes, hypertension, and heart disease.
“Ideally in the future, if you have cardiovascular disease, if you’re obese or have other complications, and you’ve got a signature in your blood that indicates you are at risk of severe disease, then we can risk-stratify patients when they are diagnosed,” he said.
The research is a preliminary step, Dr. Kulasinghe said, because of the small sample size. This type of study is often difficult to conduct because researchers have to wait for the availability of organs, as well as request permission from families for postmortem autopsies and biopsies, to be able to look at the effects on dead tissues.
“Our challenge now is to draw a clinical finding from this, which we can’t at this stage,” he added. “But it’s a really fundamental biological difference we’re observing [between COVID-19 and flu], which we need to validate with larger studies.”
A version of this article first appeared on WebMD.com.
, according to a study published in Immunology.
The study looked at the hearts of patients who died from COVID-19, the flu, and other causes. The findings could provide clues about why coronavirus has led to complications such as ongoing heart issues.
“We found a lot of DNA damage that was unique to the COVID-19 patients, which wasn’t present in the flu patients,” Arutha Kulasinghe, one of the lead study authors and a research fellow at the University of Queensland, Brisbane, Australia, told the Brisbane Times.
“So in this study, COVID-19 and flu look very different in the way they affect the heart,” he said.
Dr. Kulasinghe and colleagues analyzed the hearts of seven COVID-19 patients, two flu patients, and six patients who died from other causes. They used transcriptomic profiling, which looks at the DNA landscape of an organ, to investigate heart tissue from the patients.
Because of previous studies about heart problems associated with COVID-19, he and colleagues expected to find extreme inflammation in the heart. Instead, they found that inflammation signals had been suppressed in the heart, and markers for DNA damage and repair were much higher. They’re still unsure of the underlying cause.
“The indications here are that there’s DNA damage here, it’s not inflammation,” Dr. Kulasinghe said. “There’s something else going on that we need to figure out.”
The damage was similar to the way chronic diseases such as diabetes and cancer appear in the heart, he said, with heart tissue showing DNA damage signals.
Dr. Kulasinghe said he hopes other studies can build on the findings to develop risk models to understand which patients may face a higher risk of serious COVID-19 complications. In turn, this could help doctors provide early treatment. For instance, all seven COVID-19 patients had other chronic diseases, such as diabetes, hypertension, and heart disease.
“Ideally in the future, if you have cardiovascular disease, if you’re obese or have other complications, and you’ve got a signature in your blood that indicates you are at risk of severe disease, then we can risk-stratify patients when they are diagnosed,” he said.
The research is a preliminary step, Dr. Kulasinghe said, because of the small sample size. This type of study is often difficult to conduct because researchers have to wait for the availability of organs, as well as request permission from families for postmortem autopsies and biopsies, to be able to look at the effects on dead tissues.
“Our challenge now is to draw a clinical finding from this, which we can’t at this stage,” he added. “But it’s a really fundamental biological difference we’re observing [between COVID-19 and flu], which we need to validate with larger studies.”
A version of this article first appeared on WebMD.com.
, according to a study published in Immunology.
The study looked at the hearts of patients who died from COVID-19, the flu, and other causes. The findings could provide clues about why coronavirus has led to complications such as ongoing heart issues.
“We found a lot of DNA damage that was unique to the COVID-19 patients, which wasn’t present in the flu patients,” Arutha Kulasinghe, one of the lead study authors and a research fellow at the University of Queensland, Brisbane, Australia, told the Brisbane Times.
“So in this study, COVID-19 and flu look very different in the way they affect the heart,” he said.
Dr. Kulasinghe and colleagues analyzed the hearts of seven COVID-19 patients, two flu patients, and six patients who died from other causes. They used transcriptomic profiling, which looks at the DNA landscape of an organ, to investigate heart tissue from the patients.
Because of previous studies about heart problems associated with COVID-19, he and colleagues expected to find extreme inflammation in the heart. Instead, they found that inflammation signals had been suppressed in the heart, and markers for DNA damage and repair were much higher. They’re still unsure of the underlying cause.
“The indications here are that there’s DNA damage here, it’s not inflammation,” Dr. Kulasinghe said. “There’s something else going on that we need to figure out.”
The damage was similar to the way chronic diseases such as diabetes and cancer appear in the heart, he said, with heart tissue showing DNA damage signals.
Dr. Kulasinghe said he hopes other studies can build on the findings to develop risk models to understand which patients may face a higher risk of serious COVID-19 complications. In turn, this could help doctors provide early treatment. For instance, all seven COVID-19 patients had other chronic diseases, such as diabetes, hypertension, and heart disease.
“Ideally in the future, if you have cardiovascular disease, if you’re obese or have other complications, and you’ve got a signature in your blood that indicates you are at risk of severe disease, then we can risk-stratify patients when they are diagnosed,” he said.
The research is a preliminary step, Dr. Kulasinghe said, because of the small sample size. This type of study is often difficult to conduct because researchers have to wait for the availability of organs, as well as request permission from families for postmortem autopsies and biopsies, to be able to look at the effects on dead tissues.
“Our challenge now is to draw a clinical finding from this, which we can’t at this stage,” he added. “But it’s a really fundamental biological difference we’re observing [between COVID-19 and flu], which we need to validate with larger studies.”
A version of this article first appeared on WebMD.com.
FROM IMMUNOLOGY
Why can’t U.K. immunocompromised patients get Evusheld?
This transcript has been edited for clarity.
I’m David Kerr, professor of cancer medicine at Oxford. As I’m gearing up to have my autumnal COVID-19 booster vaccine,
This was developed by AstraZeneca. It’s a combination of two relatively long-acting antibodies (tixagevimab and cilgavimab) that bind to the spike protein on the outside of the SARS-CoV-2 virus, the virus that causes COVID-19. The antibody binds to the spike protein and prevents it from binding to and infecting or damaging cells, so it’s what’s called preexposure prophylaxis.
Although vaccination is still the best approach to protecting against and, one would hope, conferring a degree of herd protection to our population as a whole, there are some people who cannot mount an appropriate immune response and we have to take care of these folks. Because the vaccines don’t work very well for them, the vaccine itself is not sufficient to protect them.
Evusheld, in trials that have been done hitherto, can protect people who can’t mount an immune response from being infected. Between 75% and 80% of patients treated with Evusheld didn’t get COVID-19. The duration of effect seemed to be at least for 6 months, possibly longer, so it’s a really good result. This caused our medicines regulatory authority in the United Kingdom to approve the drug in March of this year. Although the drug has been approved, it’s not yet funded and not yet available for vulnerable patients.
These are patients who, for reasons of inborn genetic diseases, cannot mount an immune response; patients who are pharmacologically immune depleted; patients who have had transplants and are on immunosuppressive drugs; and some of our cancer patients, particularly those with blood or hematologic malignancies, who can receive very heavy treatment that can pound the immune system to bits.
These are, in the population as a whole, relatively small numbers, but an important number of people who are still vulnerable to developing COVID-19 despite vaccination.
Why isn’t the drug available? We have a two-stage process in the United Kingdom. We have the scientists and regulatory authorities looking at the evidence and data and saying, “Yes, it stacks up. This drug is effective and safe to some extent.”
The second phase is a health technology assessment undertaken by NICE, our National Institute for Health and Care Excellence – something that I’ve talked about a number of times before and the sometimes seemingly arbitrary decisions that they make. NICE hasn’t evaluated the drug yet, and the British government has held out because they are arguing that we don’t have enough data.
The trials with Evusheld were done before the Omicron variant dominated, as it does now; therefore, they are looking to try to work with AstraZeneca to generate more real-world data to show that Evusheld would prevent infection from the Omicron variant of the virus. Equally as important, how long does that protection last? Is it as protective against Omicron, and what’s the duration of that protection? Those bits of work are going on now.
Some real-world data are starting to emerge, showing that Evusheld will offer some degree of protection against Omicron, but there are still question marks about duration and the proportion of the population that would benefit.
NICE aren’t due to report on this – although the drug was approved in March of this year – until next year some time. That’s what’s caused a degree of consternation in the community of patients that we serve. Some of my clinical colleagues are beating the drum, saying, “We must have this drug now.” We’re still waiting on NICE to announce.
One obvious way to go around this is the government, which has bent over backwards in the United Kingdom to do as much as it can to protect the population from COVID-19. There was fantastic vaccine rollout and an extraordinary economic package to support individuals during lockdown to maintain the workforce, to support families and people at home. They’ve done a fantastic job.
Wanting to damp down this controversy, perhaps the sensible thing would be to ask NICE to evaluate the data that they have just now, to allow AstraZeneca to present whatever real-world evidence they have, and although it may not be perfect, it may be sufficient – we don’t know – to pass the NICE health technology assessment.
Watch this space. Let’s see what happens. If I were government, that’s what I would do. I would ask NICE to bring their appraisal forward. I would ask them to work with AstraZeneca to go over every ounce and iota of data that they have to see if this drug will be sufficiently effective and sufficiently cost-effective to be used before winter comes. I think the whole world is holding its breath, expecting another COVID-19 winter surge. Now would be the time to act.
What do you think? Here we are in the United Kingdom discussing yet another quasi–”health-rationing” problem. It’s not. This is about collecting more data and being as rational as possible. Can we accelerate that process? Perhaps.
Thanks for listening. I’d be very grateful for any comments that you might choose to make.
David J. Kerr, MD, DSc, is a professor of cancer medicine at the University of Oxford (England). He disclosed financial relationships with Oxford Cancer Biomarkers, Afrox, GlaxoSmithKline, Bayer HealthCare Pharmaceuticals, Genomic Health, Merck Serono, Roche, and Celleron Therapeutics.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
I’m David Kerr, professor of cancer medicine at Oxford. As I’m gearing up to have my autumnal COVID-19 booster vaccine,
This was developed by AstraZeneca. It’s a combination of two relatively long-acting antibodies (tixagevimab and cilgavimab) that bind to the spike protein on the outside of the SARS-CoV-2 virus, the virus that causes COVID-19. The antibody binds to the spike protein and prevents it from binding to and infecting or damaging cells, so it’s what’s called preexposure prophylaxis.
Although vaccination is still the best approach to protecting against and, one would hope, conferring a degree of herd protection to our population as a whole, there are some people who cannot mount an appropriate immune response and we have to take care of these folks. Because the vaccines don’t work very well for them, the vaccine itself is not sufficient to protect them.
Evusheld, in trials that have been done hitherto, can protect people who can’t mount an immune response from being infected. Between 75% and 80% of patients treated with Evusheld didn’t get COVID-19. The duration of effect seemed to be at least for 6 months, possibly longer, so it’s a really good result. This caused our medicines regulatory authority in the United Kingdom to approve the drug in March of this year. Although the drug has been approved, it’s not yet funded and not yet available for vulnerable patients.
These are patients who, for reasons of inborn genetic diseases, cannot mount an immune response; patients who are pharmacologically immune depleted; patients who have had transplants and are on immunosuppressive drugs; and some of our cancer patients, particularly those with blood or hematologic malignancies, who can receive very heavy treatment that can pound the immune system to bits.
These are, in the population as a whole, relatively small numbers, but an important number of people who are still vulnerable to developing COVID-19 despite vaccination.
Why isn’t the drug available? We have a two-stage process in the United Kingdom. We have the scientists and regulatory authorities looking at the evidence and data and saying, “Yes, it stacks up. This drug is effective and safe to some extent.”
The second phase is a health technology assessment undertaken by NICE, our National Institute for Health and Care Excellence – something that I’ve talked about a number of times before and the sometimes seemingly arbitrary decisions that they make. NICE hasn’t evaluated the drug yet, and the British government has held out because they are arguing that we don’t have enough data.
The trials with Evusheld were done before the Omicron variant dominated, as it does now; therefore, they are looking to try to work with AstraZeneca to generate more real-world data to show that Evusheld would prevent infection from the Omicron variant of the virus. Equally as important, how long does that protection last? Is it as protective against Omicron, and what’s the duration of that protection? Those bits of work are going on now.
Some real-world data are starting to emerge, showing that Evusheld will offer some degree of protection against Omicron, but there are still question marks about duration and the proportion of the population that would benefit.
NICE aren’t due to report on this – although the drug was approved in March of this year – until next year some time. That’s what’s caused a degree of consternation in the community of patients that we serve. Some of my clinical colleagues are beating the drum, saying, “We must have this drug now.” We’re still waiting on NICE to announce.
One obvious way to go around this is the government, which has bent over backwards in the United Kingdom to do as much as it can to protect the population from COVID-19. There was fantastic vaccine rollout and an extraordinary economic package to support individuals during lockdown to maintain the workforce, to support families and people at home. They’ve done a fantastic job.
Wanting to damp down this controversy, perhaps the sensible thing would be to ask NICE to evaluate the data that they have just now, to allow AstraZeneca to present whatever real-world evidence they have, and although it may not be perfect, it may be sufficient – we don’t know – to pass the NICE health technology assessment.
Watch this space. Let’s see what happens. If I were government, that’s what I would do. I would ask NICE to bring their appraisal forward. I would ask them to work with AstraZeneca to go over every ounce and iota of data that they have to see if this drug will be sufficiently effective and sufficiently cost-effective to be used before winter comes. I think the whole world is holding its breath, expecting another COVID-19 winter surge. Now would be the time to act.
What do you think? Here we are in the United Kingdom discussing yet another quasi–”health-rationing” problem. It’s not. This is about collecting more data and being as rational as possible. Can we accelerate that process? Perhaps.
Thanks for listening. I’d be very grateful for any comments that you might choose to make.
David J. Kerr, MD, DSc, is a professor of cancer medicine at the University of Oxford (England). He disclosed financial relationships with Oxford Cancer Biomarkers, Afrox, GlaxoSmithKline, Bayer HealthCare Pharmaceuticals, Genomic Health, Merck Serono, Roche, and Celleron Therapeutics.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
I’m David Kerr, professor of cancer medicine at Oxford. As I’m gearing up to have my autumnal COVID-19 booster vaccine,
This was developed by AstraZeneca. It’s a combination of two relatively long-acting antibodies (tixagevimab and cilgavimab) that bind to the spike protein on the outside of the SARS-CoV-2 virus, the virus that causes COVID-19. The antibody binds to the spike protein and prevents it from binding to and infecting or damaging cells, so it’s what’s called preexposure prophylaxis.
Although vaccination is still the best approach to protecting against and, one would hope, conferring a degree of herd protection to our population as a whole, there are some people who cannot mount an appropriate immune response and we have to take care of these folks. Because the vaccines don’t work very well for them, the vaccine itself is not sufficient to protect them.
Evusheld, in trials that have been done hitherto, can protect people who can’t mount an immune response from being infected. Between 75% and 80% of patients treated with Evusheld didn’t get COVID-19. The duration of effect seemed to be at least for 6 months, possibly longer, so it’s a really good result. This caused our medicines regulatory authority in the United Kingdom to approve the drug in March of this year. Although the drug has been approved, it’s not yet funded and not yet available for vulnerable patients.
These are patients who, for reasons of inborn genetic diseases, cannot mount an immune response; patients who are pharmacologically immune depleted; patients who have had transplants and are on immunosuppressive drugs; and some of our cancer patients, particularly those with blood or hematologic malignancies, who can receive very heavy treatment that can pound the immune system to bits.
These are, in the population as a whole, relatively small numbers, but an important number of people who are still vulnerable to developing COVID-19 despite vaccination.
Why isn’t the drug available? We have a two-stage process in the United Kingdom. We have the scientists and regulatory authorities looking at the evidence and data and saying, “Yes, it stacks up. This drug is effective and safe to some extent.”
The second phase is a health technology assessment undertaken by NICE, our National Institute for Health and Care Excellence – something that I’ve talked about a number of times before and the sometimes seemingly arbitrary decisions that they make. NICE hasn’t evaluated the drug yet, and the British government has held out because they are arguing that we don’t have enough data.
The trials with Evusheld were done before the Omicron variant dominated, as it does now; therefore, they are looking to try to work with AstraZeneca to generate more real-world data to show that Evusheld would prevent infection from the Omicron variant of the virus. Equally as important, how long does that protection last? Is it as protective against Omicron, and what’s the duration of that protection? Those bits of work are going on now.
Some real-world data are starting to emerge, showing that Evusheld will offer some degree of protection against Omicron, but there are still question marks about duration and the proportion of the population that would benefit.
NICE aren’t due to report on this – although the drug was approved in March of this year – until next year some time. That’s what’s caused a degree of consternation in the community of patients that we serve. Some of my clinical colleagues are beating the drum, saying, “We must have this drug now.” We’re still waiting on NICE to announce.
One obvious way to go around this is the government, which has bent over backwards in the United Kingdom to do as much as it can to protect the population from COVID-19. There was fantastic vaccine rollout and an extraordinary economic package to support individuals during lockdown to maintain the workforce, to support families and people at home. They’ve done a fantastic job.
Wanting to damp down this controversy, perhaps the sensible thing would be to ask NICE to evaluate the data that they have just now, to allow AstraZeneca to present whatever real-world evidence they have, and although it may not be perfect, it may be sufficient – we don’t know – to pass the NICE health technology assessment.
Watch this space. Let’s see what happens. If I were government, that’s what I would do. I would ask NICE to bring their appraisal forward. I would ask them to work with AstraZeneca to go over every ounce and iota of data that they have to see if this drug will be sufficiently effective and sufficiently cost-effective to be used before winter comes. I think the whole world is holding its breath, expecting another COVID-19 winter surge. Now would be the time to act.
What do you think? Here we are in the United Kingdom discussing yet another quasi–”health-rationing” problem. It’s not. This is about collecting more data and being as rational as possible. Can we accelerate that process? Perhaps.
Thanks for listening. I’d be very grateful for any comments that you might choose to make.
David J. Kerr, MD, DSc, is a professor of cancer medicine at the University of Oxford (England). He disclosed financial relationships with Oxford Cancer Biomarkers, Afrox, GlaxoSmithKline, Bayer HealthCare Pharmaceuticals, Genomic Health, Merck Serono, Roche, and Celleron Therapeutics.
A version of this article first appeared on Medscape.com.
Severe COVID-19–related outcomes found worse in men with RA
A retrospective study that analyzed sex disparities in patients with COVID-19 and rheumatoid arthritis found that men had more baseline comorbidities and increased risk of COVID-19–related outcomes, compared with women.
“Differences in genetics between sex and sex steroid hormones may play a role in predisposition to COVID-19 infection as well as modulating the disease progression,” according to Xiaofeng Zhou, PhD, senior director at Pfizer, New York, and the study’s lead author.
Dr. Zhou presented her findings at The Lancet Summit on Sex and Gender in Rheumatology.
Patients with chronic rheumatic diseases treated with immunomodulatory therapies may be at higher risk for more severe COVID-19 outcomes, including hospitalization, complications, and death. Research on sex-based disparities in RA patients with COVID-19 in the United States is limited, said Dr. Zhou, who embarked on a retrospective cohort study to examine the demographic and clinical characteristics of RA patients with COVID-19 and estimate the risk of possible COVID-19 outcomes by sex.
Dr. Zhou and colleagues used U.S. COVID-19 data collected through electronic health records by Optum during 2020 to June 2021. The study included adult patients with RA and a COVID-19 diagnosis (≥ 1 diagnosis code or positive SARS-CoV-2 laboratory test) and greater than or equal to 183 days of database enrollment who received treatment with immunomodulatory therapies prior to the diagnosis date. They were stratified by sex.
Investigators used logistic regression to estimate the risk of 11 possible COVID-19–related outcomes within 30 days of the COVID-19 diagnosis (hospitalization, ICU admission, pneumonia, kidney failure, thrombotic event, heart failure, acute respiratory distress syndrome [ARDS], sepsis/septic shock, mechanical ventilation/extracorporeal membrane oxygenation [ECMO], in-hospital death, and all-cause mortality), adjusting for demographics and baseline clinical covariates.
A total of 4,476 COVID-19 patients with RA (78% female) took part in the study. Male patients trended older (64 vs. 60 years) and had lower African American representation and Medicaid enrollment than female patients, but they had more baseline comorbidities such as hypertension (55% vs. 45%), hyperlipidemia (45% vs. 33%), diabetes (25% vs. 20%), coronary artery disease (28% vs. 12%), and chronic kidney disease (20% vs. 15%).
Eight of the eleven COVID-19 outcomes were significantly more likely to occur in men than women (hospitalization: odds ratio, 1.32 [95% confidence interval (CI), 1.11-1.56]; ICU admission: OR, 1.80 [95% CI, 1.36-2.40]; mechanical ventilation/ECMO: OR, 1.48 [95% CI, 1.04-2.11]; in-hospital death: OR, 1.53 [95% CI, 1.13-2.07]; all-cause mortality: OR, 1.42 [95% CI, 1.09-1.86]; sepsis: OR, 1.55 [95% CI, 1.20-2.02]; kidney failure: OR, 1.46 [95% CI, 1.15-1.85]; ARDS: OR, 1.39 [95% CI, 1.15-1.69]).
Sex hormones factor into risk
The data illustrated that men with RA had more baseline comorbidities and increased risk of COVID-19 outcomes than women.
Sex hormones regulate virus entry into host cells, respiratory function, immune response, the cardiovascular system, and coagulation, explained Dr. Zhou.
Estrogen and progesterone in women could help develop stronger and efficient immune responses to viruses and reduce virus entry into the host cells. Also, “[the] larger number of copies of ACE2 genes in women, [which] is linked with protection in the lungs against edema, permeability, and pulmonary damage, could be associated with lower incidence of severe COVID-19 outcomes, such as respiratory-related mortality and mortality,” Dr. Zhou said.
By comparison, androgens in men may increase virus entry into the host cells and promote unfavorable immune response through the induction of cytokine production and reducing the antibody response to the virus. This could lead to severe infection, Dr. Zhou said.
Sex-based differences in steroid hormones may also explain the higher incidence of morbidity and fatality that’s been observed in other studies of male patients with other infectious diseases, such as severe acute respiratory syndrome and Middle East respiratory syndrome.
Study bolsters evidence on sex disparities
The results add real-world evidence to the limited literature on sex disparities in COVID-19 outcomes among patients with RA in the United States, Dr. Zhou said. “The differential role in sex steroid hormones among women and men may shed light on clinical management of COVID-19 patients and the need to consider sex-specific approaches in clinical trials in preventing and treating COVID-19 patients,” she said.
Considering that all patients are recommended to get COVID-19 vaccinations, “it is difficult to say how this impacts clinical practice,” said Janet Pope, MD, MPH, professor of medicine in the division of rheumatology at the University of Western Ontario, London, who was not involved with the study.
Sharing results with some patients may help to encourage vaccination, thus reducing risk of poor COVID-19 outcomes, Dr. Pope said.
In future studies, Dr. Zhou suggests using multiple databases and considering other geographies beyond the United States to further understand the etiology of sexual dimorphism in COVID-19 and expand generalizability. “In addition, future research will seek to provide insights into health equity gaps in the management of COVID-19. This may inform development of precision medicines and vaccines, especially among patients on immunosuppressive treatments,” she said.
The study was sponsored by Pfizer. Dr. Zhou and other study authors are Pfizer employees and hold Pfizer stock.
A version of this article first appeared on Medscape.com.
A retrospective study that analyzed sex disparities in patients with COVID-19 and rheumatoid arthritis found that men had more baseline comorbidities and increased risk of COVID-19–related outcomes, compared with women.
“Differences in genetics between sex and sex steroid hormones may play a role in predisposition to COVID-19 infection as well as modulating the disease progression,” according to Xiaofeng Zhou, PhD, senior director at Pfizer, New York, and the study’s lead author.
Dr. Zhou presented her findings at The Lancet Summit on Sex and Gender in Rheumatology.
Patients with chronic rheumatic diseases treated with immunomodulatory therapies may be at higher risk for more severe COVID-19 outcomes, including hospitalization, complications, and death. Research on sex-based disparities in RA patients with COVID-19 in the United States is limited, said Dr. Zhou, who embarked on a retrospective cohort study to examine the demographic and clinical characteristics of RA patients with COVID-19 and estimate the risk of possible COVID-19 outcomes by sex.
Dr. Zhou and colleagues used U.S. COVID-19 data collected through electronic health records by Optum during 2020 to June 2021. The study included adult patients with RA and a COVID-19 diagnosis (≥ 1 diagnosis code or positive SARS-CoV-2 laboratory test) and greater than or equal to 183 days of database enrollment who received treatment with immunomodulatory therapies prior to the diagnosis date. They were stratified by sex.
Investigators used logistic regression to estimate the risk of 11 possible COVID-19–related outcomes within 30 days of the COVID-19 diagnosis (hospitalization, ICU admission, pneumonia, kidney failure, thrombotic event, heart failure, acute respiratory distress syndrome [ARDS], sepsis/septic shock, mechanical ventilation/extracorporeal membrane oxygenation [ECMO], in-hospital death, and all-cause mortality), adjusting for demographics and baseline clinical covariates.
A total of 4,476 COVID-19 patients with RA (78% female) took part in the study. Male patients trended older (64 vs. 60 years) and had lower African American representation and Medicaid enrollment than female patients, but they had more baseline comorbidities such as hypertension (55% vs. 45%), hyperlipidemia (45% vs. 33%), diabetes (25% vs. 20%), coronary artery disease (28% vs. 12%), and chronic kidney disease (20% vs. 15%).
Eight of the eleven COVID-19 outcomes were significantly more likely to occur in men than women (hospitalization: odds ratio, 1.32 [95% confidence interval (CI), 1.11-1.56]; ICU admission: OR, 1.80 [95% CI, 1.36-2.40]; mechanical ventilation/ECMO: OR, 1.48 [95% CI, 1.04-2.11]; in-hospital death: OR, 1.53 [95% CI, 1.13-2.07]; all-cause mortality: OR, 1.42 [95% CI, 1.09-1.86]; sepsis: OR, 1.55 [95% CI, 1.20-2.02]; kidney failure: OR, 1.46 [95% CI, 1.15-1.85]; ARDS: OR, 1.39 [95% CI, 1.15-1.69]).
Sex hormones factor into risk
The data illustrated that men with RA had more baseline comorbidities and increased risk of COVID-19 outcomes than women.
Sex hormones regulate virus entry into host cells, respiratory function, immune response, the cardiovascular system, and coagulation, explained Dr. Zhou.
Estrogen and progesterone in women could help develop stronger and efficient immune responses to viruses and reduce virus entry into the host cells. Also, “[the] larger number of copies of ACE2 genes in women, [which] is linked with protection in the lungs against edema, permeability, and pulmonary damage, could be associated with lower incidence of severe COVID-19 outcomes, such as respiratory-related mortality and mortality,” Dr. Zhou said.
By comparison, androgens in men may increase virus entry into the host cells and promote unfavorable immune response through the induction of cytokine production and reducing the antibody response to the virus. This could lead to severe infection, Dr. Zhou said.
Sex-based differences in steroid hormones may also explain the higher incidence of morbidity and fatality that’s been observed in other studies of male patients with other infectious diseases, such as severe acute respiratory syndrome and Middle East respiratory syndrome.
Study bolsters evidence on sex disparities
The results add real-world evidence to the limited literature on sex disparities in COVID-19 outcomes among patients with RA in the United States, Dr. Zhou said. “The differential role in sex steroid hormones among women and men may shed light on clinical management of COVID-19 patients and the need to consider sex-specific approaches in clinical trials in preventing and treating COVID-19 patients,” she said.
Considering that all patients are recommended to get COVID-19 vaccinations, “it is difficult to say how this impacts clinical practice,” said Janet Pope, MD, MPH, professor of medicine in the division of rheumatology at the University of Western Ontario, London, who was not involved with the study.
Sharing results with some patients may help to encourage vaccination, thus reducing risk of poor COVID-19 outcomes, Dr. Pope said.
In future studies, Dr. Zhou suggests using multiple databases and considering other geographies beyond the United States to further understand the etiology of sexual dimorphism in COVID-19 and expand generalizability. “In addition, future research will seek to provide insights into health equity gaps in the management of COVID-19. This may inform development of precision medicines and vaccines, especially among patients on immunosuppressive treatments,” she said.
The study was sponsored by Pfizer. Dr. Zhou and other study authors are Pfizer employees and hold Pfizer stock.
A version of this article first appeared on Medscape.com.
A retrospective study that analyzed sex disparities in patients with COVID-19 and rheumatoid arthritis found that men had more baseline comorbidities and increased risk of COVID-19–related outcomes, compared with women.
“Differences in genetics between sex and sex steroid hormones may play a role in predisposition to COVID-19 infection as well as modulating the disease progression,” according to Xiaofeng Zhou, PhD, senior director at Pfizer, New York, and the study’s lead author.
Dr. Zhou presented her findings at The Lancet Summit on Sex and Gender in Rheumatology.
Patients with chronic rheumatic diseases treated with immunomodulatory therapies may be at higher risk for more severe COVID-19 outcomes, including hospitalization, complications, and death. Research on sex-based disparities in RA patients with COVID-19 in the United States is limited, said Dr. Zhou, who embarked on a retrospective cohort study to examine the demographic and clinical characteristics of RA patients with COVID-19 and estimate the risk of possible COVID-19 outcomes by sex.
Dr. Zhou and colleagues used U.S. COVID-19 data collected through electronic health records by Optum during 2020 to June 2021. The study included adult patients with RA and a COVID-19 diagnosis (≥ 1 diagnosis code or positive SARS-CoV-2 laboratory test) and greater than or equal to 183 days of database enrollment who received treatment with immunomodulatory therapies prior to the diagnosis date. They were stratified by sex.
Investigators used logistic regression to estimate the risk of 11 possible COVID-19–related outcomes within 30 days of the COVID-19 diagnosis (hospitalization, ICU admission, pneumonia, kidney failure, thrombotic event, heart failure, acute respiratory distress syndrome [ARDS], sepsis/septic shock, mechanical ventilation/extracorporeal membrane oxygenation [ECMO], in-hospital death, and all-cause mortality), adjusting for demographics and baseline clinical covariates.
A total of 4,476 COVID-19 patients with RA (78% female) took part in the study. Male patients trended older (64 vs. 60 years) and had lower African American representation and Medicaid enrollment than female patients, but they had more baseline comorbidities such as hypertension (55% vs. 45%), hyperlipidemia (45% vs. 33%), diabetes (25% vs. 20%), coronary artery disease (28% vs. 12%), and chronic kidney disease (20% vs. 15%).
Eight of the eleven COVID-19 outcomes were significantly more likely to occur in men than women (hospitalization: odds ratio, 1.32 [95% confidence interval (CI), 1.11-1.56]; ICU admission: OR, 1.80 [95% CI, 1.36-2.40]; mechanical ventilation/ECMO: OR, 1.48 [95% CI, 1.04-2.11]; in-hospital death: OR, 1.53 [95% CI, 1.13-2.07]; all-cause mortality: OR, 1.42 [95% CI, 1.09-1.86]; sepsis: OR, 1.55 [95% CI, 1.20-2.02]; kidney failure: OR, 1.46 [95% CI, 1.15-1.85]; ARDS: OR, 1.39 [95% CI, 1.15-1.69]).
Sex hormones factor into risk
The data illustrated that men with RA had more baseline comorbidities and increased risk of COVID-19 outcomes than women.
Sex hormones regulate virus entry into host cells, respiratory function, immune response, the cardiovascular system, and coagulation, explained Dr. Zhou.
Estrogen and progesterone in women could help develop stronger and efficient immune responses to viruses and reduce virus entry into the host cells. Also, “[the] larger number of copies of ACE2 genes in women, [which] is linked with protection in the lungs against edema, permeability, and pulmonary damage, could be associated with lower incidence of severe COVID-19 outcomes, such as respiratory-related mortality and mortality,” Dr. Zhou said.
By comparison, androgens in men may increase virus entry into the host cells and promote unfavorable immune response through the induction of cytokine production and reducing the antibody response to the virus. This could lead to severe infection, Dr. Zhou said.
Sex-based differences in steroid hormones may also explain the higher incidence of morbidity and fatality that’s been observed in other studies of male patients with other infectious diseases, such as severe acute respiratory syndrome and Middle East respiratory syndrome.
Study bolsters evidence on sex disparities
The results add real-world evidence to the limited literature on sex disparities in COVID-19 outcomes among patients with RA in the United States, Dr. Zhou said. “The differential role in sex steroid hormones among women and men may shed light on clinical management of COVID-19 patients and the need to consider sex-specific approaches in clinical trials in preventing and treating COVID-19 patients,” she said.
Considering that all patients are recommended to get COVID-19 vaccinations, “it is difficult to say how this impacts clinical practice,” said Janet Pope, MD, MPH, professor of medicine in the division of rheumatology at the University of Western Ontario, London, who was not involved with the study.
Sharing results with some patients may help to encourage vaccination, thus reducing risk of poor COVID-19 outcomes, Dr. Pope said.
In future studies, Dr. Zhou suggests using multiple databases and considering other geographies beyond the United States to further understand the etiology of sexual dimorphism in COVID-19 and expand generalizability. “In addition, future research will seek to provide insights into health equity gaps in the management of COVID-19. This may inform development of precision medicines and vaccines, especially among patients on immunosuppressive treatments,” she said.
The study was sponsored by Pfizer. Dr. Zhou and other study authors are Pfizer employees and hold Pfizer stock.
A version of this article first appeared on Medscape.com.
FROM THE LANCET SUMMIT ON SEX AND GENDER IN RHEUMATOLOGY
COVID pandemic associated with anorexia in Canadian youth
, data suggest.
Preliminary results of the Canadian Paediatric Surveillance Program (CPSP) indicate that the pandemic has been a precipitating factor in the development of anorexia nervosa in almost half of children and adolescents studied. The pandemic also has precipitated hospitalizations for anorexia in more than one-third of cases.
“Data globally, and certainly our data here in Canada, have shown a real increase in health care utilization with the onset of the COVID-19 pandemic,” study author Debra Katzman, MD, professor of pediatrics at the Hospital for Sick Children in Toronto and the University of Toronto, said in an interview. “And when I talk about health care utilization, I’m talking about hospitalizations for eating disorders.”
The data were included in the 2021 results of the CPSP.
Focus on appearance
CPSP is a collaboration between the Public Health Agency of Canada and the Canadian Pediatric Society that consists of a network of 2,800 pediatricians and pediatric subspecialists across Canada. The latest results include surveillance studies on 14 diseases and conditions, with data collected during various periods.
From April 2020 to May 2021, researchers identified 1,800 COVID-19 cases in children and collected detailed information on 1,456 of them, including 405 cases hospitalized with pediatric inflammatory multisystem syndrome (PIMS). The median age of hospitalized cases was 3.2 years for SARS-CoV-2 infection and 5.4 years for PIMS.
Dr. Katzman and colleagues observed 118 first-time hospitalizations for anorexia nervosa between Sept. 1 and Dec. 31, 2021. More than 90% of reported cases were female, with 66% of verified cases in teens aged 14-17 years and the remainder in adolescents aged 11-13 years.
In 49% of cases, the reporting physician identified the COVID-19 pandemic as a precipitating factor in the development of anorexia nervosa. In 37% of cases, the reporting physician identified the pandemic as having precipitated the anorexia-related hospitalization.
Last year, a cross-sectional analysis of children in Canada reported that monthly hospitalizations for anorexia nervosa increased from 7.5 to 20 from March through November 2020. The monthly rate in the CPSP study was closer to 30 for first-time hospitalizations.
Dr. Katzman said that the findings about anorexia nervosa didn’t surprise her. “There was so much disruption and [so many] restrictions to young peoples’ daily routines – closures of schools and recreational activities – they lost regular connection with their peers, and they lost extracurricular and social activities,” she said. “That led to heightened anxiety and depression and really a lack of control.”
Adolescents and teens were also spending more time on social media than they were before the pandemic, she noted. “They were looking at themselves all the time, so they were getting preoccupied with their body image. There was a heightened focus on appearance, and I think that things like public-health mitigation strategies – things like hand washing, social distancing, mask wearing – may have impacted the psychological well-being of young people.”
The closure of outpatient facilities, long waiting lists to get into facilities that were opened, and “coronaphobia” about going to physicians’ offices and emergency departments compounded the problem, Dr. Katzman added.
The long-term effects of COVID and eating disorders in children are unknown, Dr. Katzman said. “This is sort of a wake-up call for the health care system that during times of stress or pandemics or crises, these kinds of things can happen, and we need to be prepared to provide the resources for vulnerable populations moving forward,” she said.
Heightened anxiety
Commenting on the data, Margaret Thew, APNP, director of the eating disorders program at Children’s Wisconsin in Milwaukee, said that isolation due to school closures and negative social media messages created the “perfect storm” for eating disorders in adolescents and teenagers because of higher rates of anxiety and depression. Ms. Thew was not involved in the research.
The storm is not over yet, she said. “What everyone needs to keep in mind is that we still have this very heightened state of anxiety and depression ... for adolescents, teenagers, and preteens alike,” Ms. Thew said in an interview, “and we know that many of them are not coping with their anxiety very well.”
In her experience, since the start of the pandemic, the average age of pediatric patients with eating disorders declined from 16 to 15 years, and the youngest age declined from 12 to 11 years.
Overall, the CPSP results show that children are affected by mental health issues at an earlier age than before the pandemic, said Ms. Thew. “Years ago, we wouldn’t have thought that an 8-year-old needed to be screened for some of these risk factors, but now we’re definitely getting more younger children who are struggling, and I think it’s taking too long for them to get the care they need because it’s being overlooked,” she said.
The report was funded by the Public Health Agency of Canada, Health Canada, Alberta Children’s Hospital Research Institute, Bethanys Hope Foundation, CHEO Research Institute, and Children’s Hospital Research Institute of Manitoba. Dr. Katzman and Ms. Thew have no relevant disclosures.
A version of this article first appeared on Medscape.com.
, data suggest.
Preliminary results of the Canadian Paediatric Surveillance Program (CPSP) indicate that the pandemic has been a precipitating factor in the development of anorexia nervosa in almost half of children and adolescents studied. The pandemic also has precipitated hospitalizations for anorexia in more than one-third of cases.
“Data globally, and certainly our data here in Canada, have shown a real increase in health care utilization with the onset of the COVID-19 pandemic,” study author Debra Katzman, MD, professor of pediatrics at the Hospital for Sick Children in Toronto and the University of Toronto, said in an interview. “And when I talk about health care utilization, I’m talking about hospitalizations for eating disorders.”
The data were included in the 2021 results of the CPSP.
Focus on appearance
CPSP is a collaboration between the Public Health Agency of Canada and the Canadian Pediatric Society that consists of a network of 2,800 pediatricians and pediatric subspecialists across Canada. The latest results include surveillance studies on 14 diseases and conditions, with data collected during various periods.
From April 2020 to May 2021, researchers identified 1,800 COVID-19 cases in children and collected detailed information on 1,456 of them, including 405 cases hospitalized with pediatric inflammatory multisystem syndrome (PIMS). The median age of hospitalized cases was 3.2 years for SARS-CoV-2 infection and 5.4 years for PIMS.
Dr. Katzman and colleagues observed 118 first-time hospitalizations for anorexia nervosa between Sept. 1 and Dec. 31, 2021. More than 90% of reported cases were female, with 66% of verified cases in teens aged 14-17 years and the remainder in adolescents aged 11-13 years.
In 49% of cases, the reporting physician identified the COVID-19 pandemic as a precipitating factor in the development of anorexia nervosa. In 37% of cases, the reporting physician identified the pandemic as having precipitated the anorexia-related hospitalization.
Last year, a cross-sectional analysis of children in Canada reported that monthly hospitalizations for anorexia nervosa increased from 7.5 to 20 from March through November 2020. The monthly rate in the CPSP study was closer to 30 for first-time hospitalizations.
Dr. Katzman said that the findings about anorexia nervosa didn’t surprise her. “There was so much disruption and [so many] restrictions to young peoples’ daily routines – closures of schools and recreational activities – they lost regular connection with their peers, and they lost extracurricular and social activities,” she said. “That led to heightened anxiety and depression and really a lack of control.”
Adolescents and teens were also spending more time on social media than they were before the pandemic, she noted. “They were looking at themselves all the time, so they were getting preoccupied with their body image. There was a heightened focus on appearance, and I think that things like public-health mitigation strategies – things like hand washing, social distancing, mask wearing – may have impacted the psychological well-being of young people.”
The closure of outpatient facilities, long waiting lists to get into facilities that were opened, and “coronaphobia” about going to physicians’ offices and emergency departments compounded the problem, Dr. Katzman added.
The long-term effects of COVID and eating disorders in children are unknown, Dr. Katzman said. “This is sort of a wake-up call for the health care system that during times of stress or pandemics or crises, these kinds of things can happen, and we need to be prepared to provide the resources for vulnerable populations moving forward,” she said.
Heightened anxiety
Commenting on the data, Margaret Thew, APNP, director of the eating disorders program at Children’s Wisconsin in Milwaukee, said that isolation due to school closures and negative social media messages created the “perfect storm” for eating disorders in adolescents and teenagers because of higher rates of anxiety and depression. Ms. Thew was not involved in the research.
The storm is not over yet, she said. “What everyone needs to keep in mind is that we still have this very heightened state of anxiety and depression ... for adolescents, teenagers, and preteens alike,” Ms. Thew said in an interview, “and we know that many of them are not coping with their anxiety very well.”
In her experience, since the start of the pandemic, the average age of pediatric patients with eating disorders declined from 16 to 15 years, and the youngest age declined from 12 to 11 years.
Overall, the CPSP results show that children are affected by mental health issues at an earlier age than before the pandemic, said Ms. Thew. “Years ago, we wouldn’t have thought that an 8-year-old needed to be screened for some of these risk factors, but now we’re definitely getting more younger children who are struggling, and I think it’s taking too long for them to get the care they need because it’s being overlooked,” she said.
The report was funded by the Public Health Agency of Canada, Health Canada, Alberta Children’s Hospital Research Institute, Bethanys Hope Foundation, CHEO Research Institute, and Children’s Hospital Research Institute of Manitoba. Dr. Katzman and Ms. Thew have no relevant disclosures.
A version of this article first appeared on Medscape.com.
, data suggest.
Preliminary results of the Canadian Paediatric Surveillance Program (CPSP) indicate that the pandemic has been a precipitating factor in the development of anorexia nervosa in almost half of children and adolescents studied. The pandemic also has precipitated hospitalizations for anorexia in more than one-third of cases.
“Data globally, and certainly our data here in Canada, have shown a real increase in health care utilization with the onset of the COVID-19 pandemic,” study author Debra Katzman, MD, professor of pediatrics at the Hospital for Sick Children in Toronto and the University of Toronto, said in an interview. “And when I talk about health care utilization, I’m talking about hospitalizations for eating disorders.”
The data were included in the 2021 results of the CPSP.
Focus on appearance
CPSP is a collaboration between the Public Health Agency of Canada and the Canadian Pediatric Society that consists of a network of 2,800 pediatricians and pediatric subspecialists across Canada. The latest results include surveillance studies on 14 diseases and conditions, with data collected during various periods.
From April 2020 to May 2021, researchers identified 1,800 COVID-19 cases in children and collected detailed information on 1,456 of them, including 405 cases hospitalized with pediatric inflammatory multisystem syndrome (PIMS). The median age of hospitalized cases was 3.2 years for SARS-CoV-2 infection and 5.4 years for PIMS.
Dr. Katzman and colleagues observed 118 first-time hospitalizations for anorexia nervosa between Sept. 1 and Dec. 31, 2021. More than 90% of reported cases were female, with 66% of verified cases in teens aged 14-17 years and the remainder in adolescents aged 11-13 years.
In 49% of cases, the reporting physician identified the COVID-19 pandemic as a precipitating factor in the development of anorexia nervosa. In 37% of cases, the reporting physician identified the pandemic as having precipitated the anorexia-related hospitalization.
Last year, a cross-sectional analysis of children in Canada reported that monthly hospitalizations for anorexia nervosa increased from 7.5 to 20 from March through November 2020. The monthly rate in the CPSP study was closer to 30 for first-time hospitalizations.
Dr. Katzman said that the findings about anorexia nervosa didn’t surprise her. “There was so much disruption and [so many] restrictions to young peoples’ daily routines – closures of schools and recreational activities – they lost regular connection with their peers, and they lost extracurricular and social activities,” she said. “That led to heightened anxiety and depression and really a lack of control.”
Adolescents and teens were also spending more time on social media than they were before the pandemic, she noted. “They were looking at themselves all the time, so they were getting preoccupied with their body image. There was a heightened focus on appearance, and I think that things like public-health mitigation strategies – things like hand washing, social distancing, mask wearing – may have impacted the psychological well-being of young people.”
The closure of outpatient facilities, long waiting lists to get into facilities that were opened, and “coronaphobia” about going to physicians’ offices and emergency departments compounded the problem, Dr. Katzman added.
The long-term effects of COVID and eating disorders in children are unknown, Dr. Katzman said. “This is sort of a wake-up call for the health care system that during times of stress or pandemics or crises, these kinds of things can happen, and we need to be prepared to provide the resources for vulnerable populations moving forward,” she said.
Heightened anxiety
Commenting on the data, Margaret Thew, APNP, director of the eating disorders program at Children’s Wisconsin in Milwaukee, said that isolation due to school closures and negative social media messages created the “perfect storm” for eating disorders in adolescents and teenagers because of higher rates of anxiety and depression. Ms. Thew was not involved in the research.
The storm is not over yet, she said. “What everyone needs to keep in mind is that we still have this very heightened state of anxiety and depression ... for adolescents, teenagers, and preteens alike,” Ms. Thew said in an interview, “and we know that many of them are not coping with their anxiety very well.”
In her experience, since the start of the pandemic, the average age of pediatric patients with eating disorders declined from 16 to 15 years, and the youngest age declined from 12 to 11 years.
Overall, the CPSP results show that children are affected by mental health issues at an earlier age than before the pandemic, said Ms. Thew. “Years ago, we wouldn’t have thought that an 8-year-old needed to be screened for some of these risk factors, but now we’re definitely getting more younger children who are struggling, and I think it’s taking too long for them to get the care they need because it’s being overlooked,” she said.
The report was funded by the Public Health Agency of Canada, Health Canada, Alberta Children’s Hospital Research Institute, Bethanys Hope Foundation, CHEO Research Institute, and Children’s Hospital Research Institute of Manitoba. Dr. Katzman and Ms. Thew have no relevant disclosures.
A version of this article first appeared on Medscape.com.
Long COVID could cost the economy trillions, experts predict
from restaurants struggling to replace low-wage workers, to airlines scrambling to replace crew, to overwhelmed hospitals, experts are predicting.
“There’s a lot we need to do to understand what it takes to enable disabled people to participate more in the economy,” said Katie Bach, a senior fellow with Brookings Institution and the author of a study looking into long COVID’s impact on the labor market.
Data from June 2022 from the Centers for Disease Control and Prevention shows that, of the 40% of American adults who contracted COVID-19, nearly one in five still have long COVID symptoms. That works out to 1 in 13, or 7.5%, of the overall U.S. adult population.
Drawing from the CDC data, Ms. Bach estimates in her August 2022 report that as many as 4 million working-age Americans are too sick with long COVID to perform their jobs. That works out to as much as $230 billion in lost wages, or almost 1% of the U.S. GDP.
“This is a big deal,” she said. “We’re talking potentially hundreds of billions of dollars a year and that this is big enough to have a measurable impact on the labor market.”
Other sources have suggested lower figures, but the conclusions are the same: Long COVID is an urgent issue that will cost tens of billions of dollars a year in lost wages alone, Ms. Bach said. But it’s not just lost income for workers. There is a cost for businesses and the public.
Throughout the pandemic, COVID-19’s crippling force could be felt across multiple industries. While business has picked up again, staffing shortages remain a challenge. At some airports this summer, air passengers spent hours in security lines; were stranded for days as flights were canceled, rebooked, and canceled again on short notice; and waited weeks for lost luggage. Restaurants have had to cut back their hours. Those seeking medical care had longer than usual wait times in EDs and urgent care clinics. Some EDs temporarily closed.
These challenges have been attributed in part to the “great resignation” and in part because so many infected workers were out, especially during the Omicron waves. But increasingly, economists and health care professionals alike worry about long COVID’s impact on employers and the broader economy.
David Cutler, PhD, a professor of economics at Harvard University, Cambridge, Mass., believes the total economic loss could be as high as $3.7 trillion, when factoring in the lost quality of life, the cost in lost earnings, and the cost of higher spending on medical care. His estimate is more than a trillion dollars higher than a previous projection he and fellow economist Lawrence Summers, PhD, made in 2020. The reason? Long COVID.
“The higher estimate is largely a result of the greater prevalence of long COVID than we had guessed at the time,” Dr. Cutler wrote in a paper released in July.
“There are about 10 times the number of people with long COVID as have died of COVID. Because long COVID is so new, there is uncertainty about all of the numbers involved in the calculations. Still, the costs here are conservative, based on only cases to date.”
In Ms. Bach’s Brookings report, she projected that, if recovery from long COVID does not pick up and the population of Americans with long COVID were to grow by 10% a year, the annual cost of lost wages alone could reach half a trillion dollars in a decade.
Meanwhile, a working paper by the National Bureau of Economic Research found that workers who missed an entire week of work because of probable COVID-19 illnesses were roughly 7 percentage points less likely to be working a year later, compared with those who did not miss work for health reasons.
“It’s not just individuals with long COVID who are suffering from this. It impacts their families, their livelihoods, and the economy on a global scale. So, we have to raise awareness about those ripple effects,” said Linda Geng, MD, a clinical assistant professor of medicine with Stanford (Calif.) University’s Primary Care and Population Health.
“I think it’s hard for the public to grasp ... and understand the scale of this public health crisis.”
Debilitating fatigue
Long COVID is roughly defined; the CDC defines it as symptoms that linger 3 or more months after a patient first catches the virus.
The symptoms vary and include profound fatigue and brain issues.
“It’s a new degree of extreme and debilitating fatigue and exhaustion, to the point where you can’t do your daily tasks,” said Dr. Geng, who is also the codirector of Stanford’s Post-Acute COVID-19 Syndrome Clinic.
“People can be so debilitated, they can’t even do basic things, like the activities of daily living, let alone do their job, particularly if it’s physically or mentally demanding.”
Patients can also have postexertional malaise, where they feel especially bad and symptoms worsen when they exert themselves physically or mentally, Dr. Geng said. Compounding the issue for many long COVID patients is their trouble getting restful sleep. Those with brain fog have issues with memory, processing information, focused concentration, confusion, making mistakes, and multitasking. Pain is another debilitating symptom that can disrupt daily life and ability to work.
Even people with relatively mild infections can end up with long COVID, Dr. Geng said, noting that many of the patients at the Stanford clinic were never hospitalized with their initial infections. While existing research and Dr. Geng’s clinical experience show that long COVID can hit any age, she most commonly sees patients from ages 20 to their 60s, with an average age in the 40s – people in their prime working ages.
Jason Furman, PhD, a former White House economic adviser who is now a professor at Harvard University, noted in August that the labor force participation rate was far below what could be explained by standard demographic changes like an aging population, with the decline evident across all age groups. Dr. Furman does not speculate about why, but others have.
“We are pessimistic: Both the aging of the population and the impact of long COVID imply that the participation rate will be slow to return to its prepandemic level,” Anna Wong, Yelena Shulyatyeva, Andrew Husby, and Eliza Winger, economists with Bloomberg Economics, wrote in a research note.
Supportive policies
There is some evidence that vaccination reduces the risk of long COVID, but not completely, and it is too early to know if repeat infections increase long COVID risks. There is also no definitive data on how fast or how many people are recovering. Economists often assume that those with long COVID will recover at some point, Ms. Bach noted, but she is careful not to make assumptions.
“If people aren’t recovering, then this group keeps getting bigger,” she said. “We’re still adding, and if people aren’t coming out of that group, this becomes a bigger and bigger problem.”
For now, the number of new people being diagnosed with long COVID appears to have slowed, Ms. Bach said, but it remains to be seen whether the trend can be sustained.
“If people are impaired longer than we think and if the impairment turns out to be severe, then we can have a lot of people who need services like disability insurance,” Dr. Cutler said.
“That could put a really big strain on public sector programs and our ability to meet those needs.”
Policies that support the research and clinical work necessary to prevent and treat long COVID are essential, experts say.
“To me, that is the biggest economic imperative, to say nothing of human suffering,” said Ms. Bach.
Employers also have a role, and experts say there are a number of accommodations businesses should consider. What happens when an employee has long COVID? Can accommodations be made that allow them to continue working productively? If they spend a great deal of time commuting, can they work from home? What can employers do so that family members do not have to drop out of the workforce to take care of loved ones with long COVID?
Disability insurance
To be sure, there is one piece of the puzzle that does not quite fit, according to Dr. Cutler and Ms. Bach. There is no sign yet of a large increase in federal disability insurance applications, and no one quite knows why. Publicly available government data shows that online applications actually dipped by about 4% each year between 2019 and 2021. Applications in 2022 appear on track to remain slightly below prepandemic levels.
To qualify for Social Security Disability Insurance (SSDI), people need to have a disability that lasts at least a year.
“If you’re disabled with long COVID, who knows, right? You don’t know,” said Ms. Bach. “Two of the most dominant symptoms of long COVID are fatigue and brain fog. So, I’ve heard from people that the process of going through an SSDI application is really hard.”
Some long COVID patients told Ms. Bach they simply assumed they would not get SSDI and did not even bother applying. She stressed that working Americans with debilitating long COVID should be aware that their condition is protected by the Americans with Disabilities Act. But the challenge, based on guidance issued by the government, is that not all cases of long COVID qualify as a disability and that individual assessments are necessary.
While more long COVID data are needed, Ms. Bach believes there is enough information for decisionmakers to go after the issue more aggressively. She pointed to the $1.15 billion in funding that Congress earmarked for the National Institutes of Health over the course of 4 years in support of research into the long-term health effects of COVID-19.
“Now, $250 million a year sounds like a lot of money until you start talking about the cost of lost wages – just lost wages,” Ms. Bach said. “That’s not lost productivity. That’s not the cost of people whose family members are sick. Who have to reduce their own labor force participation. That’s not medical costs. Suddenly, $250 million doesn’t really sound like that much.”
A version of this article first appeared on WebMD.com.
from restaurants struggling to replace low-wage workers, to airlines scrambling to replace crew, to overwhelmed hospitals, experts are predicting.
“There’s a lot we need to do to understand what it takes to enable disabled people to participate more in the economy,” said Katie Bach, a senior fellow with Brookings Institution and the author of a study looking into long COVID’s impact on the labor market.
Data from June 2022 from the Centers for Disease Control and Prevention shows that, of the 40% of American adults who contracted COVID-19, nearly one in five still have long COVID symptoms. That works out to 1 in 13, or 7.5%, of the overall U.S. adult population.
Drawing from the CDC data, Ms. Bach estimates in her August 2022 report that as many as 4 million working-age Americans are too sick with long COVID to perform their jobs. That works out to as much as $230 billion in lost wages, or almost 1% of the U.S. GDP.
“This is a big deal,” she said. “We’re talking potentially hundreds of billions of dollars a year and that this is big enough to have a measurable impact on the labor market.”
Other sources have suggested lower figures, but the conclusions are the same: Long COVID is an urgent issue that will cost tens of billions of dollars a year in lost wages alone, Ms. Bach said. But it’s not just lost income for workers. There is a cost for businesses and the public.
Throughout the pandemic, COVID-19’s crippling force could be felt across multiple industries. While business has picked up again, staffing shortages remain a challenge. At some airports this summer, air passengers spent hours in security lines; were stranded for days as flights were canceled, rebooked, and canceled again on short notice; and waited weeks for lost luggage. Restaurants have had to cut back their hours. Those seeking medical care had longer than usual wait times in EDs and urgent care clinics. Some EDs temporarily closed.
These challenges have been attributed in part to the “great resignation” and in part because so many infected workers were out, especially during the Omicron waves. But increasingly, economists and health care professionals alike worry about long COVID’s impact on employers and the broader economy.
David Cutler, PhD, a professor of economics at Harvard University, Cambridge, Mass., believes the total economic loss could be as high as $3.7 trillion, when factoring in the lost quality of life, the cost in lost earnings, and the cost of higher spending on medical care. His estimate is more than a trillion dollars higher than a previous projection he and fellow economist Lawrence Summers, PhD, made in 2020. The reason? Long COVID.
“The higher estimate is largely a result of the greater prevalence of long COVID than we had guessed at the time,” Dr. Cutler wrote in a paper released in July.
“There are about 10 times the number of people with long COVID as have died of COVID. Because long COVID is so new, there is uncertainty about all of the numbers involved in the calculations. Still, the costs here are conservative, based on only cases to date.”
In Ms. Bach’s Brookings report, she projected that, if recovery from long COVID does not pick up and the population of Americans with long COVID were to grow by 10% a year, the annual cost of lost wages alone could reach half a trillion dollars in a decade.
Meanwhile, a working paper by the National Bureau of Economic Research found that workers who missed an entire week of work because of probable COVID-19 illnesses were roughly 7 percentage points less likely to be working a year later, compared with those who did not miss work for health reasons.
“It’s not just individuals with long COVID who are suffering from this. It impacts their families, their livelihoods, and the economy on a global scale. So, we have to raise awareness about those ripple effects,” said Linda Geng, MD, a clinical assistant professor of medicine with Stanford (Calif.) University’s Primary Care and Population Health.
“I think it’s hard for the public to grasp ... and understand the scale of this public health crisis.”
Debilitating fatigue
Long COVID is roughly defined; the CDC defines it as symptoms that linger 3 or more months after a patient first catches the virus.
The symptoms vary and include profound fatigue and brain issues.
“It’s a new degree of extreme and debilitating fatigue and exhaustion, to the point where you can’t do your daily tasks,” said Dr. Geng, who is also the codirector of Stanford’s Post-Acute COVID-19 Syndrome Clinic.
“People can be so debilitated, they can’t even do basic things, like the activities of daily living, let alone do their job, particularly if it’s physically or mentally demanding.”
Patients can also have postexertional malaise, where they feel especially bad and symptoms worsen when they exert themselves physically or mentally, Dr. Geng said. Compounding the issue for many long COVID patients is their trouble getting restful sleep. Those with brain fog have issues with memory, processing information, focused concentration, confusion, making mistakes, and multitasking. Pain is another debilitating symptom that can disrupt daily life and ability to work.
Even people with relatively mild infections can end up with long COVID, Dr. Geng said, noting that many of the patients at the Stanford clinic were never hospitalized with their initial infections. While existing research and Dr. Geng’s clinical experience show that long COVID can hit any age, she most commonly sees patients from ages 20 to their 60s, with an average age in the 40s – people in their prime working ages.
Jason Furman, PhD, a former White House economic adviser who is now a professor at Harvard University, noted in August that the labor force participation rate was far below what could be explained by standard demographic changes like an aging population, with the decline evident across all age groups. Dr. Furman does not speculate about why, but others have.
“We are pessimistic: Both the aging of the population and the impact of long COVID imply that the participation rate will be slow to return to its prepandemic level,” Anna Wong, Yelena Shulyatyeva, Andrew Husby, and Eliza Winger, economists with Bloomberg Economics, wrote in a research note.
Supportive policies
There is some evidence that vaccination reduces the risk of long COVID, but not completely, and it is too early to know if repeat infections increase long COVID risks. There is also no definitive data on how fast or how many people are recovering. Economists often assume that those with long COVID will recover at some point, Ms. Bach noted, but she is careful not to make assumptions.
“If people aren’t recovering, then this group keeps getting bigger,” she said. “We’re still adding, and if people aren’t coming out of that group, this becomes a bigger and bigger problem.”
For now, the number of new people being diagnosed with long COVID appears to have slowed, Ms. Bach said, but it remains to be seen whether the trend can be sustained.
“If people are impaired longer than we think and if the impairment turns out to be severe, then we can have a lot of people who need services like disability insurance,” Dr. Cutler said.
“That could put a really big strain on public sector programs and our ability to meet those needs.”
Policies that support the research and clinical work necessary to prevent and treat long COVID are essential, experts say.
“To me, that is the biggest economic imperative, to say nothing of human suffering,” said Ms. Bach.
Employers also have a role, and experts say there are a number of accommodations businesses should consider. What happens when an employee has long COVID? Can accommodations be made that allow them to continue working productively? If they spend a great deal of time commuting, can they work from home? What can employers do so that family members do not have to drop out of the workforce to take care of loved ones with long COVID?
Disability insurance
To be sure, there is one piece of the puzzle that does not quite fit, according to Dr. Cutler and Ms. Bach. There is no sign yet of a large increase in federal disability insurance applications, and no one quite knows why. Publicly available government data shows that online applications actually dipped by about 4% each year between 2019 and 2021. Applications in 2022 appear on track to remain slightly below prepandemic levels.
To qualify for Social Security Disability Insurance (SSDI), people need to have a disability that lasts at least a year.
“If you’re disabled with long COVID, who knows, right? You don’t know,” said Ms. Bach. “Two of the most dominant symptoms of long COVID are fatigue and brain fog. So, I’ve heard from people that the process of going through an SSDI application is really hard.”
Some long COVID patients told Ms. Bach they simply assumed they would not get SSDI and did not even bother applying. She stressed that working Americans with debilitating long COVID should be aware that their condition is protected by the Americans with Disabilities Act. But the challenge, based on guidance issued by the government, is that not all cases of long COVID qualify as a disability and that individual assessments are necessary.
While more long COVID data are needed, Ms. Bach believes there is enough information for decisionmakers to go after the issue more aggressively. She pointed to the $1.15 billion in funding that Congress earmarked for the National Institutes of Health over the course of 4 years in support of research into the long-term health effects of COVID-19.
“Now, $250 million a year sounds like a lot of money until you start talking about the cost of lost wages – just lost wages,” Ms. Bach said. “That’s not lost productivity. That’s not the cost of people whose family members are sick. Who have to reduce their own labor force participation. That’s not medical costs. Suddenly, $250 million doesn’t really sound like that much.”
A version of this article first appeared on WebMD.com.
from restaurants struggling to replace low-wage workers, to airlines scrambling to replace crew, to overwhelmed hospitals, experts are predicting.
“There’s a lot we need to do to understand what it takes to enable disabled people to participate more in the economy,” said Katie Bach, a senior fellow with Brookings Institution and the author of a study looking into long COVID’s impact on the labor market.
Data from June 2022 from the Centers for Disease Control and Prevention shows that, of the 40% of American adults who contracted COVID-19, nearly one in five still have long COVID symptoms. That works out to 1 in 13, or 7.5%, of the overall U.S. adult population.
Drawing from the CDC data, Ms. Bach estimates in her August 2022 report that as many as 4 million working-age Americans are too sick with long COVID to perform their jobs. That works out to as much as $230 billion in lost wages, or almost 1% of the U.S. GDP.
“This is a big deal,” she said. “We’re talking potentially hundreds of billions of dollars a year and that this is big enough to have a measurable impact on the labor market.”
Other sources have suggested lower figures, but the conclusions are the same: Long COVID is an urgent issue that will cost tens of billions of dollars a year in lost wages alone, Ms. Bach said. But it’s not just lost income for workers. There is a cost for businesses and the public.
Throughout the pandemic, COVID-19’s crippling force could be felt across multiple industries. While business has picked up again, staffing shortages remain a challenge. At some airports this summer, air passengers spent hours in security lines; were stranded for days as flights were canceled, rebooked, and canceled again on short notice; and waited weeks for lost luggage. Restaurants have had to cut back their hours. Those seeking medical care had longer than usual wait times in EDs and urgent care clinics. Some EDs temporarily closed.
These challenges have been attributed in part to the “great resignation” and in part because so many infected workers were out, especially during the Omicron waves. But increasingly, economists and health care professionals alike worry about long COVID’s impact on employers and the broader economy.
David Cutler, PhD, a professor of economics at Harvard University, Cambridge, Mass., believes the total economic loss could be as high as $3.7 trillion, when factoring in the lost quality of life, the cost in lost earnings, and the cost of higher spending on medical care. His estimate is more than a trillion dollars higher than a previous projection he and fellow economist Lawrence Summers, PhD, made in 2020. The reason? Long COVID.
“The higher estimate is largely a result of the greater prevalence of long COVID than we had guessed at the time,” Dr. Cutler wrote in a paper released in July.
“There are about 10 times the number of people with long COVID as have died of COVID. Because long COVID is so new, there is uncertainty about all of the numbers involved in the calculations. Still, the costs here are conservative, based on only cases to date.”
In Ms. Bach’s Brookings report, she projected that, if recovery from long COVID does not pick up and the population of Americans with long COVID were to grow by 10% a year, the annual cost of lost wages alone could reach half a trillion dollars in a decade.
Meanwhile, a working paper by the National Bureau of Economic Research found that workers who missed an entire week of work because of probable COVID-19 illnesses were roughly 7 percentage points less likely to be working a year later, compared with those who did not miss work for health reasons.
“It’s not just individuals with long COVID who are suffering from this. It impacts their families, their livelihoods, and the economy on a global scale. So, we have to raise awareness about those ripple effects,” said Linda Geng, MD, a clinical assistant professor of medicine with Stanford (Calif.) University’s Primary Care and Population Health.
“I think it’s hard for the public to grasp ... and understand the scale of this public health crisis.”
Debilitating fatigue
Long COVID is roughly defined; the CDC defines it as symptoms that linger 3 or more months after a patient first catches the virus.
The symptoms vary and include profound fatigue and brain issues.
“It’s a new degree of extreme and debilitating fatigue and exhaustion, to the point where you can’t do your daily tasks,” said Dr. Geng, who is also the codirector of Stanford’s Post-Acute COVID-19 Syndrome Clinic.
“People can be so debilitated, they can’t even do basic things, like the activities of daily living, let alone do their job, particularly if it’s physically or mentally demanding.”
Patients can also have postexertional malaise, where they feel especially bad and symptoms worsen when they exert themselves physically or mentally, Dr. Geng said. Compounding the issue for many long COVID patients is their trouble getting restful sleep. Those with brain fog have issues with memory, processing information, focused concentration, confusion, making mistakes, and multitasking. Pain is another debilitating symptom that can disrupt daily life and ability to work.
Even people with relatively mild infections can end up with long COVID, Dr. Geng said, noting that many of the patients at the Stanford clinic were never hospitalized with their initial infections. While existing research and Dr. Geng’s clinical experience show that long COVID can hit any age, she most commonly sees patients from ages 20 to their 60s, with an average age in the 40s – people in their prime working ages.
Jason Furman, PhD, a former White House economic adviser who is now a professor at Harvard University, noted in August that the labor force participation rate was far below what could be explained by standard demographic changes like an aging population, with the decline evident across all age groups. Dr. Furman does not speculate about why, but others have.
“We are pessimistic: Both the aging of the population and the impact of long COVID imply that the participation rate will be slow to return to its prepandemic level,” Anna Wong, Yelena Shulyatyeva, Andrew Husby, and Eliza Winger, economists with Bloomberg Economics, wrote in a research note.
Supportive policies
There is some evidence that vaccination reduces the risk of long COVID, but not completely, and it is too early to know if repeat infections increase long COVID risks. There is also no definitive data on how fast or how many people are recovering. Economists often assume that those with long COVID will recover at some point, Ms. Bach noted, but she is careful not to make assumptions.
“If people aren’t recovering, then this group keeps getting bigger,” she said. “We’re still adding, and if people aren’t coming out of that group, this becomes a bigger and bigger problem.”
For now, the number of new people being diagnosed with long COVID appears to have slowed, Ms. Bach said, but it remains to be seen whether the trend can be sustained.
“If people are impaired longer than we think and if the impairment turns out to be severe, then we can have a lot of people who need services like disability insurance,” Dr. Cutler said.
“That could put a really big strain on public sector programs and our ability to meet those needs.”
Policies that support the research and clinical work necessary to prevent and treat long COVID are essential, experts say.
“To me, that is the biggest economic imperative, to say nothing of human suffering,” said Ms. Bach.
Employers also have a role, and experts say there are a number of accommodations businesses should consider. What happens when an employee has long COVID? Can accommodations be made that allow them to continue working productively? If they spend a great deal of time commuting, can they work from home? What can employers do so that family members do not have to drop out of the workforce to take care of loved ones with long COVID?
Disability insurance
To be sure, there is one piece of the puzzle that does not quite fit, according to Dr. Cutler and Ms. Bach. There is no sign yet of a large increase in federal disability insurance applications, and no one quite knows why. Publicly available government data shows that online applications actually dipped by about 4% each year between 2019 and 2021. Applications in 2022 appear on track to remain slightly below prepandemic levels.
To qualify for Social Security Disability Insurance (SSDI), people need to have a disability that lasts at least a year.
“If you’re disabled with long COVID, who knows, right? You don’t know,” said Ms. Bach. “Two of the most dominant symptoms of long COVID are fatigue and brain fog. So, I’ve heard from people that the process of going through an SSDI application is really hard.”
Some long COVID patients told Ms. Bach they simply assumed they would not get SSDI and did not even bother applying. She stressed that working Americans with debilitating long COVID should be aware that their condition is protected by the Americans with Disabilities Act. But the challenge, based on guidance issued by the government, is that not all cases of long COVID qualify as a disability and that individual assessments are necessary.
While more long COVID data are needed, Ms. Bach believes there is enough information for decisionmakers to go after the issue more aggressively. She pointed to the $1.15 billion in funding that Congress earmarked for the National Institutes of Health over the course of 4 years in support of research into the long-term health effects of COVID-19.
“Now, $250 million a year sounds like a lot of money until you start talking about the cost of lost wages – just lost wages,” Ms. Bach said. “That’s not lost productivity. That’s not the cost of people whose family members are sick. Who have to reduce their own labor force participation. That’s not medical costs. Suddenly, $250 million doesn’t really sound like that much.”
A version of this article first appeared on WebMD.com.
What we know about long COVID so far
Long COVID: The name says it all. It’s an illness that, for many people, has not yet stopped.
Eric Roach became ill with COVID-19 in November 2020, and he’s still sick. “I have brain fog, memory loss,” says the 67-year-old Navy veteran from Spearfish, S.D. “The fatigue has just been insane.”
Long COVID, more formally known as post-acute sequelae of COVID (PASC), is the lay term to describe when people start to recover, or seem to recover, from a bout of COVID-19 but then continue to suffer from symptoms. For some, it’s gone on for 2 years or longer. While the governments of the United Statesand several other countries formally recognize the existence of long COVID, the National Institutes of Health (NIH) has yet to formally define it. There’s no approved treatment, and the causes are not understood.
Here’s what is known: and it is affecting enough people to cause concern for employers, health insurers, and governments.
First, the many symptoms
According to the Centers for Disease Control and Prvention, long COVID symptoms may include:
- Tiredness or fatigue that interferes with daily life.
- Symptoms that get worse after physical or mental effort.
- Fever.
- Difficulty breathing or shortness of breath.
- Cough.
- Chest pain.
- Heart palpitations.
- Difficulty thinking or concentrating (sometimes referred to as “brain fog”).
- Headache.
- Sleep problems.
- Dizziness when standing.
- Pins-and-needles feelings.
- Change in smell or taste.
- Depression or anxiety.
- Diarrhea.
- Stomach pain.
- Joint or muscle pain.
- Rash.
- Changes in menstrual cycles.
“People with post-COVID conditions may develop or continue to have symptoms that are hard to explain and manage,” the CDC says on its website. “Clinical evaluations and results of routine blood tests, chest x-rays, and electrocardiograms may be normal. The symptoms are similar to those reported by people with ME/CFS (myalgic encephalomyelitis/chronic fatigue syndrome) and other poorly understood chronic illnesses that may occur after other infections.”
Doctors may not fully appreciate the subtle nature of some of the symptoms.
“People with these unexplained symptoms may be misunderstood by their health care providers, which can result in a long time for them to get a diagnosis and receive appropriate care or treatment,” the CDC says.
Health professionals should recognize that long COVID can be disabling, the U.S. Department of Health and Human Services says. “Long COVID can substantially limit a major life activity,” HHS says in civil rights guidance. One possible example: “A person with long COVID who has lung damage that causes shortness of breath, fatigue, and related effects is substantially limited in respiratory function, among other major life activities,” the HHS notes.
How many people are affected?
This has been difficult to judge because not everyone who has had COVID-19 gets tested for it and there are no formal diagnostic criteria yet for long COVID. The CDC estimates that 19% of patients in the United States who have ever had COVID-19 have long COVID symptoms.
Some estimates go higher. A University of Oxford study in September 2021 found more than a third of patients had symptoms of long COVID between 3 months and 6 months after a COVID-19 diagnosis. As many as 55% of COVID-19 patients in one Chinese study had one or more lingering symptoms 2 years later, Lixue Huang, MD, of the China-Japan Friendship Hospital in Beijing, and colleagues reported in the journal Lancet Respiratory Medicine in May.
According to the CDC, age is a factor. “Older adults are less likely to have long COVID than younger adults. Nearly three times as many adults ages 50-59 currently have long COVID than those age 80 and older,” the CDC says. Women and racial and ethnic minorities are more likely to be affected.
Many people are experiencing neurological effects, such as the so-called brain fog, according to Ziyad Al-Aly, MD, of Washington University and the VA St. Louis Health Care System, and colleagues, whose report was published in Nature Medicine in September. They estimated that 6.6 million Americans have brain impairments associated with COVID infection.
“Some of the neurologic disorders reported here are serious chronic conditions that will impact some people for a lifetime,” they wrote. “Given the colossal scale of the pandemic, and even though the absolute numbers reported in this work are small, these may translate into a large number of affected individuals around the world – and this will likely contribute to a rise in the burden of neurologic diseases.”
Causes
It’s not clear what the underlying causes are, but most research points to a combination of factors. Suspects include ongoing inflammation, tiny blood clots, and reactivation of latent viruses. In May, Brent Palmer, PhD, of the University of Colorado, Denver, and colleagues found people with long COVID had persistent activation of T-cells that were specific for SARS-CoV-2.
COVID-19 itself can damage organs, and long COVID might be caused by ongoing damage. In August, Alexandros Rovas, MD, of University Hospital Munster in Germany, and colleagues found patients with long COVID had evidence of damage to their capillaries. “Whether, to what extent, and when the observed damage might be reversible remains unclear,” they wrote in the journal Angiogenesis.
People with long COVID have immune responses to other viruses, such as Epstein-Barr – evidence that COVID-19 might reactivate latent viruses. “Our data suggest the involvement of persistent antigen, reactivation of latent herpesviruses, and chronic inflammation,” immunobiologist Akiko Iwasaki, PhD, of Yale University, New Haven, Conn., and colleagues wrote in a study posted in August that had not yet been peer-reviewed for publication.
This might be causing an autoimmune response. “The infection may cause the immune system to start making autoantibodies that attack a person’s own organs and tissues,” the NIH says.
There could be other factors. A study by Harvard researchers found that people who felt stressed, depressed, or lonely before catching COVID-19 were more likely to develop long COVID afterward. “Distress was more strongly associated with developing long COVID than physical health risk factors such as obesity, asthma, and hypertension,” Siwen Wang, MD, a research fellow with Harvard University’s T.H. Chan School of Public Health, Boston, said in a statement. Plus, nearly 44% of those in the study developed COVID-19 infections after having been assessed for stress, Dr. Wang and colleagues reported in the journal JAMA Psychiatry.
Vaccine protection
There’s evidence that vaccination protects against long COVID, both by preventing infection in the first place, but also even for people who have breakthrough infections.
A meta-analysis covering studies involving 17 million people found evidence vaccination might reduce the severity of COVID-19 or might help the body clear any lingering virus after an infection.
“Overall, vaccination was associated with reduced risks or odds of long COVID, with preliminary evidence suggesting that two doses are more effective than one dose,” wrote Cesar Fernandez de las Penas, PhD, of King Juan Carlos University in Madrid, and colleagues. Their report is in The Lancet’s eClinicalMedicine.
A team in Milan found that unvaccinated people in their study were nearly three times as likely to have serious symptoms for longer than 4 weeks compared to vaccinated volunteers. According to their report in JAMA, Elena Azzolini, MD, PhD, assistant professor at Humanitas Research Hospital, and colleagues found two or three doses of vaccine reduced the risk of hospitalization from COVID to 16% or 17% compared to 42% for the unvaccinated.
Treatments
With no diagnostic criteria and no understanding of the causes, it’s hard for doctors to determine treatments.
Most experts dealing with long COVID, even those at the specialty centers that have been set up at hospitals and health systems in the United States, recommend that patients start with their primary care doctors before moving on to specialists.
“The mainstay of management is supportive, holistic care, symptom control, and detection of treatable complications,” Trish Greenhalgh, MD, professor of primary care health sciences at the University of Oxford, England, and colleagues wrote in the journal The BMJ in September. “Patients with long COVID greatly value input from their primary care clinician. Generalist clinicians can help patients considerably by hearing the patient’s story and validating their experience … (and) making the diagnosis of long COVID (which does not have to be by exclusion) and excluding alternative diagnoses.”
Evidence is building that long COVID closely resembles other postviral conditions – something that can provide clues for treatment. For example, several studies indicate that exercise doesn’t help most patients.
But there are approaches that can work. Treatments may include pulmonary rehabilitation; autonomic conditioning therapy, which includes breathing therapy; and cognitive rehabilitation to relieve brain fog. Doctors are also trying the antidepressant amitriptyline to help with sleep disturbances and headaches; the antiseizure medication gabapentin to help with pain, numbness, and other neurological symptoms; and drugs to relieve low blood pressure in patients experiencing postural orthostatic tachycardia syndrome (POTS).
The NIH is sponsoring studies that have recruited just over 8,200 adults. And more than two dozen researchers from Harvard; Stanford; the University of California, San Francisco; the J. Craig Venter Institute; Johns Hopkins University; the University of Pennsylvania; Mount Sinai Hospitals; Cardiff University; and Yale announced in September they were forming the Long COVID Research Initiative to speed up studies.
The group, with funding from private enterprise, plans to conduct tissue biopsy, imaging studies, and autopsies and will search for potential biomarkers in the blood of patients.
A version of this article first appeared on WebMD.com.
Long COVID: The name says it all. It’s an illness that, for many people, has not yet stopped.
Eric Roach became ill with COVID-19 in November 2020, and he’s still sick. “I have brain fog, memory loss,” says the 67-year-old Navy veteran from Spearfish, S.D. “The fatigue has just been insane.”
Long COVID, more formally known as post-acute sequelae of COVID (PASC), is the lay term to describe when people start to recover, or seem to recover, from a bout of COVID-19 but then continue to suffer from symptoms. For some, it’s gone on for 2 years or longer. While the governments of the United Statesand several other countries formally recognize the existence of long COVID, the National Institutes of Health (NIH) has yet to formally define it. There’s no approved treatment, and the causes are not understood.
Here’s what is known: and it is affecting enough people to cause concern for employers, health insurers, and governments.
First, the many symptoms
According to the Centers for Disease Control and Prvention, long COVID symptoms may include:
- Tiredness or fatigue that interferes with daily life.
- Symptoms that get worse after physical or mental effort.
- Fever.
- Difficulty breathing or shortness of breath.
- Cough.
- Chest pain.
- Heart palpitations.
- Difficulty thinking or concentrating (sometimes referred to as “brain fog”).
- Headache.
- Sleep problems.
- Dizziness when standing.
- Pins-and-needles feelings.
- Change in smell or taste.
- Depression or anxiety.
- Diarrhea.
- Stomach pain.
- Joint or muscle pain.
- Rash.
- Changes in menstrual cycles.
“People with post-COVID conditions may develop or continue to have symptoms that are hard to explain and manage,” the CDC says on its website. “Clinical evaluations and results of routine blood tests, chest x-rays, and electrocardiograms may be normal. The symptoms are similar to those reported by people with ME/CFS (myalgic encephalomyelitis/chronic fatigue syndrome) and other poorly understood chronic illnesses that may occur after other infections.”
Doctors may not fully appreciate the subtle nature of some of the symptoms.
“People with these unexplained symptoms may be misunderstood by their health care providers, which can result in a long time for them to get a diagnosis and receive appropriate care or treatment,” the CDC says.
Health professionals should recognize that long COVID can be disabling, the U.S. Department of Health and Human Services says. “Long COVID can substantially limit a major life activity,” HHS says in civil rights guidance. One possible example: “A person with long COVID who has lung damage that causes shortness of breath, fatigue, and related effects is substantially limited in respiratory function, among other major life activities,” the HHS notes.
How many people are affected?
This has been difficult to judge because not everyone who has had COVID-19 gets tested for it and there are no formal diagnostic criteria yet for long COVID. The CDC estimates that 19% of patients in the United States who have ever had COVID-19 have long COVID symptoms.
Some estimates go higher. A University of Oxford study in September 2021 found more than a third of patients had symptoms of long COVID between 3 months and 6 months after a COVID-19 diagnosis. As many as 55% of COVID-19 patients in one Chinese study had one or more lingering symptoms 2 years later, Lixue Huang, MD, of the China-Japan Friendship Hospital in Beijing, and colleagues reported in the journal Lancet Respiratory Medicine in May.
According to the CDC, age is a factor. “Older adults are less likely to have long COVID than younger adults. Nearly three times as many adults ages 50-59 currently have long COVID than those age 80 and older,” the CDC says. Women and racial and ethnic minorities are more likely to be affected.
Many people are experiencing neurological effects, such as the so-called brain fog, according to Ziyad Al-Aly, MD, of Washington University and the VA St. Louis Health Care System, and colleagues, whose report was published in Nature Medicine in September. They estimated that 6.6 million Americans have brain impairments associated with COVID infection.
“Some of the neurologic disorders reported here are serious chronic conditions that will impact some people for a lifetime,” they wrote. “Given the colossal scale of the pandemic, and even though the absolute numbers reported in this work are small, these may translate into a large number of affected individuals around the world – and this will likely contribute to a rise in the burden of neurologic diseases.”
Causes
It’s not clear what the underlying causes are, but most research points to a combination of factors. Suspects include ongoing inflammation, tiny blood clots, and reactivation of latent viruses. In May, Brent Palmer, PhD, of the University of Colorado, Denver, and colleagues found people with long COVID had persistent activation of T-cells that were specific for SARS-CoV-2.
COVID-19 itself can damage organs, and long COVID might be caused by ongoing damage. In August, Alexandros Rovas, MD, of University Hospital Munster in Germany, and colleagues found patients with long COVID had evidence of damage to their capillaries. “Whether, to what extent, and when the observed damage might be reversible remains unclear,” they wrote in the journal Angiogenesis.
People with long COVID have immune responses to other viruses, such as Epstein-Barr – evidence that COVID-19 might reactivate latent viruses. “Our data suggest the involvement of persistent antigen, reactivation of latent herpesviruses, and chronic inflammation,” immunobiologist Akiko Iwasaki, PhD, of Yale University, New Haven, Conn., and colleagues wrote in a study posted in August that had not yet been peer-reviewed for publication.
This might be causing an autoimmune response. “The infection may cause the immune system to start making autoantibodies that attack a person’s own organs and tissues,” the NIH says.
There could be other factors. A study by Harvard researchers found that people who felt stressed, depressed, or lonely before catching COVID-19 were more likely to develop long COVID afterward. “Distress was more strongly associated with developing long COVID than physical health risk factors such as obesity, asthma, and hypertension,” Siwen Wang, MD, a research fellow with Harvard University’s T.H. Chan School of Public Health, Boston, said in a statement. Plus, nearly 44% of those in the study developed COVID-19 infections after having been assessed for stress, Dr. Wang and colleagues reported in the journal JAMA Psychiatry.
Vaccine protection
There’s evidence that vaccination protects against long COVID, both by preventing infection in the first place, but also even for people who have breakthrough infections.
A meta-analysis covering studies involving 17 million people found evidence vaccination might reduce the severity of COVID-19 or might help the body clear any lingering virus after an infection.
“Overall, vaccination was associated with reduced risks or odds of long COVID, with preliminary evidence suggesting that two doses are more effective than one dose,” wrote Cesar Fernandez de las Penas, PhD, of King Juan Carlos University in Madrid, and colleagues. Their report is in The Lancet’s eClinicalMedicine.
A team in Milan found that unvaccinated people in their study were nearly three times as likely to have serious symptoms for longer than 4 weeks compared to vaccinated volunteers. According to their report in JAMA, Elena Azzolini, MD, PhD, assistant professor at Humanitas Research Hospital, and colleagues found two or three doses of vaccine reduced the risk of hospitalization from COVID to 16% or 17% compared to 42% for the unvaccinated.
Treatments
With no diagnostic criteria and no understanding of the causes, it’s hard for doctors to determine treatments.
Most experts dealing with long COVID, even those at the specialty centers that have been set up at hospitals and health systems in the United States, recommend that patients start with their primary care doctors before moving on to specialists.
“The mainstay of management is supportive, holistic care, symptom control, and detection of treatable complications,” Trish Greenhalgh, MD, professor of primary care health sciences at the University of Oxford, England, and colleagues wrote in the journal The BMJ in September. “Patients with long COVID greatly value input from their primary care clinician. Generalist clinicians can help patients considerably by hearing the patient’s story and validating their experience … (and) making the diagnosis of long COVID (which does not have to be by exclusion) and excluding alternative diagnoses.”
Evidence is building that long COVID closely resembles other postviral conditions – something that can provide clues for treatment. For example, several studies indicate that exercise doesn’t help most patients.
But there are approaches that can work. Treatments may include pulmonary rehabilitation; autonomic conditioning therapy, which includes breathing therapy; and cognitive rehabilitation to relieve brain fog. Doctors are also trying the antidepressant amitriptyline to help with sleep disturbances and headaches; the antiseizure medication gabapentin to help with pain, numbness, and other neurological symptoms; and drugs to relieve low blood pressure in patients experiencing postural orthostatic tachycardia syndrome (POTS).
The NIH is sponsoring studies that have recruited just over 8,200 adults. And more than two dozen researchers from Harvard; Stanford; the University of California, San Francisco; the J. Craig Venter Institute; Johns Hopkins University; the University of Pennsylvania; Mount Sinai Hospitals; Cardiff University; and Yale announced in September they were forming the Long COVID Research Initiative to speed up studies.
The group, with funding from private enterprise, plans to conduct tissue biopsy, imaging studies, and autopsies and will search for potential biomarkers in the blood of patients.
A version of this article first appeared on WebMD.com.
Long COVID: The name says it all. It’s an illness that, for many people, has not yet stopped.
Eric Roach became ill with COVID-19 in November 2020, and he’s still sick. “I have brain fog, memory loss,” says the 67-year-old Navy veteran from Spearfish, S.D. “The fatigue has just been insane.”
Long COVID, more formally known as post-acute sequelae of COVID (PASC), is the lay term to describe when people start to recover, or seem to recover, from a bout of COVID-19 but then continue to suffer from symptoms. For some, it’s gone on for 2 years or longer. While the governments of the United Statesand several other countries formally recognize the existence of long COVID, the National Institutes of Health (NIH) has yet to formally define it. There’s no approved treatment, and the causes are not understood.
Here’s what is known: and it is affecting enough people to cause concern for employers, health insurers, and governments.
First, the many symptoms
According to the Centers for Disease Control and Prvention, long COVID symptoms may include:
- Tiredness or fatigue that interferes with daily life.
- Symptoms that get worse after physical or mental effort.
- Fever.
- Difficulty breathing or shortness of breath.
- Cough.
- Chest pain.
- Heart palpitations.
- Difficulty thinking or concentrating (sometimes referred to as “brain fog”).
- Headache.
- Sleep problems.
- Dizziness when standing.
- Pins-and-needles feelings.
- Change in smell or taste.
- Depression or anxiety.
- Diarrhea.
- Stomach pain.
- Joint or muscle pain.
- Rash.
- Changes in menstrual cycles.
“People with post-COVID conditions may develop or continue to have symptoms that are hard to explain and manage,” the CDC says on its website. “Clinical evaluations and results of routine blood tests, chest x-rays, and electrocardiograms may be normal. The symptoms are similar to those reported by people with ME/CFS (myalgic encephalomyelitis/chronic fatigue syndrome) and other poorly understood chronic illnesses that may occur after other infections.”
Doctors may not fully appreciate the subtle nature of some of the symptoms.
“People with these unexplained symptoms may be misunderstood by their health care providers, which can result in a long time for them to get a diagnosis and receive appropriate care or treatment,” the CDC says.
Health professionals should recognize that long COVID can be disabling, the U.S. Department of Health and Human Services says. “Long COVID can substantially limit a major life activity,” HHS says in civil rights guidance. One possible example: “A person with long COVID who has lung damage that causes shortness of breath, fatigue, and related effects is substantially limited in respiratory function, among other major life activities,” the HHS notes.
How many people are affected?
This has been difficult to judge because not everyone who has had COVID-19 gets tested for it and there are no formal diagnostic criteria yet for long COVID. The CDC estimates that 19% of patients in the United States who have ever had COVID-19 have long COVID symptoms.
Some estimates go higher. A University of Oxford study in September 2021 found more than a third of patients had symptoms of long COVID between 3 months and 6 months after a COVID-19 diagnosis. As many as 55% of COVID-19 patients in one Chinese study had one or more lingering symptoms 2 years later, Lixue Huang, MD, of the China-Japan Friendship Hospital in Beijing, and colleagues reported in the journal Lancet Respiratory Medicine in May.
According to the CDC, age is a factor. “Older adults are less likely to have long COVID than younger adults. Nearly three times as many adults ages 50-59 currently have long COVID than those age 80 and older,” the CDC says. Women and racial and ethnic minorities are more likely to be affected.
Many people are experiencing neurological effects, such as the so-called brain fog, according to Ziyad Al-Aly, MD, of Washington University and the VA St. Louis Health Care System, and colleagues, whose report was published in Nature Medicine in September. They estimated that 6.6 million Americans have brain impairments associated with COVID infection.
“Some of the neurologic disorders reported here are serious chronic conditions that will impact some people for a lifetime,” they wrote. “Given the colossal scale of the pandemic, and even though the absolute numbers reported in this work are small, these may translate into a large number of affected individuals around the world – and this will likely contribute to a rise in the burden of neurologic diseases.”
Causes
It’s not clear what the underlying causes are, but most research points to a combination of factors. Suspects include ongoing inflammation, tiny blood clots, and reactivation of latent viruses. In May, Brent Palmer, PhD, of the University of Colorado, Denver, and colleagues found people with long COVID had persistent activation of T-cells that were specific for SARS-CoV-2.
COVID-19 itself can damage organs, and long COVID might be caused by ongoing damage. In August, Alexandros Rovas, MD, of University Hospital Munster in Germany, and colleagues found patients with long COVID had evidence of damage to their capillaries. “Whether, to what extent, and when the observed damage might be reversible remains unclear,” they wrote in the journal Angiogenesis.
People with long COVID have immune responses to other viruses, such as Epstein-Barr – evidence that COVID-19 might reactivate latent viruses. “Our data suggest the involvement of persistent antigen, reactivation of latent herpesviruses, and chronic inflammation,” immunobiologist Akiko Iwasaki, PhD, of Yale University, New Haven, Conn., and colleagues wrote in a study posted in August that had not yet been peer-reviewed for publication.
This might be causing an autoimmune response. “The infection may cause the immune system to start making autoantibodies that attack a person’s own organs and tissues,” the NIH says.
There could be other factors. A study by Harvard researchers found that people who felt stressed, depressed, or lonely before catching COVID-19 were more likely to develop long COVID afterward. “Distress was more strongly associated with developing long COVID than physical health risk factors such as obesity, asthma, and hypertension,” Siwen Wang, MD, a research fellow with Harvard University’s T.H. Chan School of Public Health, Boston, said in a statement. Plus, nearly 44% of those in the study developed COVID-19 infections after having been assessed for stress, Dr. Wang and colleagues reported in the journal JAMA Psychiatry.
Vaccine protection
There’s evidence that vaccination protects against long COVID, both by preventing infection in the first place, but also even for people who have breakthrough infections.
A meta-analysis covering studies involving 17 million people found evidence vaccination might reduce the severity of COVID-19 or might help the body clear any lingering virus after an infection.
“Overall, vaccination was associated with reduced risks or odds of long COVID, with preliminary evidence suggesting that two doses are more effective than one dose,” wrote Cesar Fernandez de las Penas, PhD, of King Juan Carlos University in Madrid, and colleagues. Their report is in The Lancet’s eClinicalMedicine.
A team in Milan found that unvaccinated people in their study were nearly three times as likely to have serious symptoms for longer than 4 weeks compared to vaccinated volunteers. According to their report in JAMA, Elena Azzolini, MD, PhD, assistant professor at Humanitas Research Hospital, and colleagues found two or three doses of vaccine reduced the risk of hospitalization from COVID to 16% or 17% compared to 42% for the unvaccinated.
Treatments
With no diagnostic criteria and no understanding of the causes, it’s hard for doctors to determine treatments.
Most experts dealing with long COVID, even those at the specialty centers that have been set up at hospitals and health systems in the United States, recommend that patients start with their primary care doctors before moving on to specialists.
“The mainstay of management is supportive, holistic care, symptom control, and detection of treatable complications,” Trish Greenhalgh, MD, professor of primary care health sciences at the University of Oxford, England, and colleagues wrote in the journal The BMJ in September. “Patients with long COVID greatly value input from their primary care clinician. Generalist clinicians can help patients considerably by hearing the patient’s story and validating their experience … (and) making the diagnosis of long COVID (which does not have to be by exclusion) and excluding alternative diagnoses.”
Evidence is building that long COVID closely resembles other postviral conditions – something that can provide clues for treatment. For example, several studies indicate that exercise doesn’t help most patients.
But there are approaches that can work. Treatments may include pulmonary rehabilitation; autonomic conditioning therapy, which includes breathing therapy; and cognitive rehabilitation to relieve brain fog. Doctors are also trying the antidepressant amitriptyline to help with sleep disturbances and headaches; the antiseizure medication gabapentin to help with pain, numbness, and other neurological symptoms; and drugs to relieve low blood pressure in patients experiencing postural orthostatic tachycardia syndrome (POTS).
The NIH is sponsoring studies that have recruited just over 8,200 adults. And more than two dozen researchers from Harvard; Stanford; the University of California, San Francisco; the J. Craig Venter Institute; Johns Hopkins University; the University of Pennsylvania; Mount Sinai Hospitals; Cardiff University; and Yale announced in September they were forming the Long COVID Research Initiative to speed up studies.
The group, with funding from private enterprise, plans to conduct tissue biopsy, imaging studies, and autopsies and will search for potential biomarkers in the blood of patients.
A version of this article first appeared on WebMD.com.
Children and COVID: September slowdown continues
New COVID-19 cases and hospital admissions in children continue to decline, while the slow pace of vaccinations has not deterred manufacturers from seeking new emergency authorizations.
Since reaching a post-Omicron peak of 112,000 in late May, the number of weekly cases has fluctuated, with no stretch of increases or decreases lasting more than 4 weeks or the weekly count rising above 97,000 or falling lower than the current 55,000, according to state-level data collected by the American Academy of Pediatrics and the Children’s Hospital Association.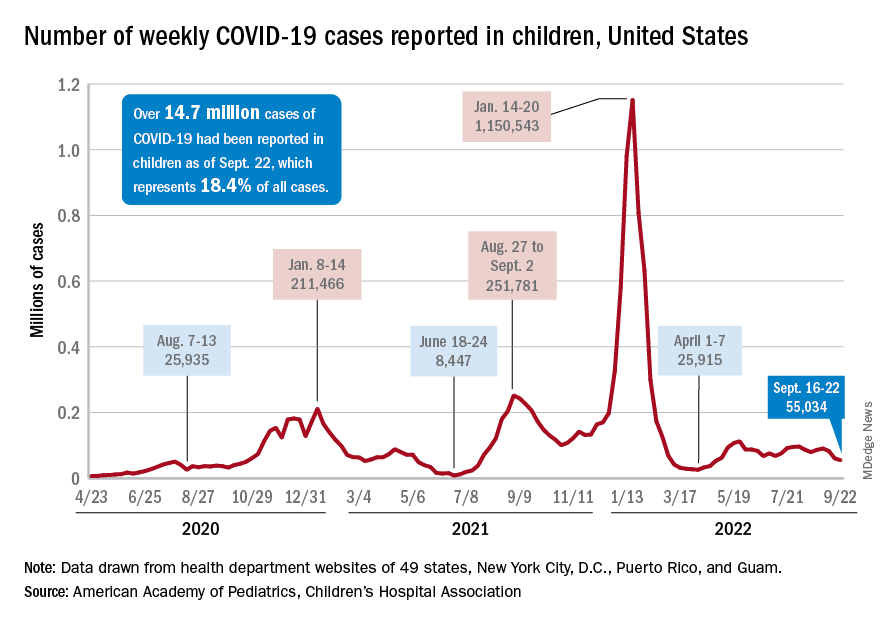
New admissions with confirmed COVID for children aged 0-17 years, which did not follow that pattern and instead continued to rise through the spring and early summer, have been largely decreasing in recent weeks and had fallen to 0.27 per 100,000 population as of Sept. 21 after peaking at 0.46 per 100,000 in late July, the Centers for Disease Control and Prevention reported. A similar decline has been seen for emergency department visits since late August.
The biggest vaccination news of the week came from Moderna and Pfizer and BioNTech, which are each seeking emergency authorization from the Food and Drug Administration for bivalent vaccine boosters that target both the original COVID strain and the BA.4 and BA.5 strains of Omicron.
“Pfizer’s booster would be for children 5 to 11 who have completed a primary vaccination series [and] Moderna’s updated boosters would be for children ages 6 to 17 who have completed a primary vaccination series,” WebMD said.
Although almost 61% of children aged 12-17 years are already fully vaccinated, that is not the case among those aged 5-11, of whom only 31.4% have completed the initial vaccine regimen. Since becoming eligible in June, just 1.9% of children under 5 years of age have been fully vaccinated and 6.3% have received at least one dose, the CDC said on its COVID Data Tracker. The latest data put the already boosted child populations at 28.8% for 12- to 17-year-olds and 14.8% in those aged 5-11.
About 51,000 children under age 5 years received their initial COVID vaccination during the week of Sept. 15-21, and the trend for that measure is one of gradual decline since July. Among the older children that same week, there were 28,000 initial vaccinations in the 5- to 11-year-olds and 18,000 for those aged 12-17, and activity in both age groups has largely stagnated since the spring, according to a separate AAP report based on CDC data.
New COVID-19 cases and hospital admissions in children continue to decline, while the slow pace of vaccinations has not deterred manufacturers from seeking new emergency authorizations.
Since reaching a post-Omicron peak of 112,000 in late May, the number of weekly cases has fluctuated, with no stretch of increases or decreases lasting more than 4 weeks or the weekly count rising above 97,000 or falling lower than the current 55,000, according to state-level data collected by the American Academy of Pediatrics and the Children’s Hospital Association.
New admissions with confirmed COVID for children aged 0-17 years, which did not follow that pattern and instead continued to rise through the spring and early summer, have been largely decreasing in recent weeks and had fallen to 0.27 per 100,000 population as of Sept. 21 after peaking at 0.46 per 100,000 in late July, the Centers for Disease Control and Prevention reported. A similar decline has been seen for emergency department visits since late August.
The biggest vaccination news of the week came from Moderna and Pfizer and BioNTech, which are each seeking emergency authorization from the Food and Drug Administration for bivalent vaccine boosters that target both the original COVID strain and the BA.4 and BA.5 strains of Omicron.
“Pfizer’s booster would be for children 5 to 11 who have completed a primary vaccination series [and] Moderna’s updated boosters would be for children ages 6 to 17 who have completed a primary vaccination series,” WebMD said.
Although almost 61% of children aged 12-17 years are already fully vaccinated, that is not the case among those aged 5-11, of whom only 31.4% have completed the initial vaccine regimen. Since becoming eligible in June, just 1.9% of children under 5 years of age have been fully vaccinated and 6.3% have received at least one dose, the CDC said on its COVID Data Tracker. The latest data put the already boosted child populations at 28.8% for 12- to 17-year-olds and 14.8% in those aged 5-11.
About 51,000 children under age 5 years received their initial COVID vaccination during the week of Sept. 15-21, and the trend for that measure is one of gradual decline since July. Among the older children that same week, there were 28,000 initial vaccinations in the 5- to 11-year-olds and 18,000 for those aged 12-17, and activity in both age groups has largely stagnated since the spring, according to a separate AAP report based on CDC data.
New COVID-19 cases and hospital admissions in children continue to decline, while the slow pace of vaccinations has not deterred manufacturers from seeking new emergency authorizations.
Since reaching a post-Omicron peak of 112,000 in late May, the number of weekly cases has fluctuated, with no stretch of increases or decreases lasting more than 4 weeks or the weekly count rising above 97,000 or falling lower than the current 55,000, according to state-level data collected by the American Academy of Pediatrics and the Children’s Hospital Association.
New admissions with confirmed COVID for children aged 0-17 years, which did not follow that pattern and instead continued to rise through the spring and early summer, have been largely decreasing in recent weeks and had fallen to 0.27 per 100,000 population as of Sept. 21 after peaking at 0.46 per 100,000 in late July, the Centers for Disease Control and Prevention reported. A similar decline has been seen for emergency department visits since late August.
The biggest vaccination news of the week came from Moderna and Pfizer and BioNTech, which are each seeking emergency authorization from the Food and Drug Administration for bivalent vaccine boosters that target both the original COVID strain and the BA.4 and BA.5 strains of Omicron.
“Pfizer’s booster would be for children 5 to 11 who have completed a primary vaccination series [and] Moderna’s updated boosters would be for children ages 6 to 17 who have completed a primary vaccination series,” WebMD said.
Although almost 61% of children aged 12-17 years are already fully vaccinated, that is not the case among those aged 5-11, of whom only 31.4% have completed the initial vaccine regimen. Since becoming eligible in June, just 1.9% of children under 5 years of age have been fully vaccinated and 6.3% have received at least one dose, the CDC said on its COVID Data Tracker. The latest data put the already boosted child populations at 28.8% for 12- to 17-year-olds and 14.8% in those aged 5-11.
About 51,000 children under age 5 years received their initial COVID vaccination during the week of Sept. 15-21, and the trend for that measure is one of gradual decline since July. Among the older children that same week, there were 28,000 initial vaccinations in the 5- to 11-year-olds and 18,000 for those aged 12-17, and activity in both age groups has largely stagnated since the spring, according to a separate AAP report based on CDC data.
Desperate long COVID patients turn to unproven alternative therapies
Entrepreneur Maya McNulty, 49, was one of the first victims of the COVID-19 pandemic. The Schenectady, N.Y., businesswoman spent 2 months in the hospital after catching the disease in March 2020. That September, she was diagnosed with long COVID.
“Even a simple task such as unloading the dishwasher became a major challenge,” she says.
Over the next several months, Ms. McNulty saw a range of specialists, including neurologists, pulmonologists, and cardiologists. She had months of physical therapy and respiratory therapy to help regain strength and lung function. While many of the doctors she saw were sympathetic to what she was going through, not all were.
“I saw one neurologist who told me to my face that she didn’t believe in long COVID,” she recalls. “It was particularly astonishing since the hospital they were affiliated with had a long COVID clinic.”
Ms. McNulty began to connect with other patients with long COVID through a support group she created at the end of 2020 on the social media app Clubhouse. They exchanged ideas and stories about what had helped one another, which led her to try, over the next year, a plant-based diet, Chinese medicine, and vitamin C supplements, among other treatments.
She also acted on unscientific reports she found online and did her own research, which led her to discover claims that some asthma patients with chronic coughing responded well to halotherapy, or dry salt therapy, during which patients inhale micro-particles of salt into their lungs to reduce inflammation, widen airways, and thin mucus. She’s been doing this procedure at a clinic near her home for over a year and credits it with helping with her chronic cough, especially as she recovers from her second bout of COVID-19.
It’s not cheap – a single half-hour session can cost up to $50 and isn’t covered by insurance. There’s also no good research to suggest that it can help with long COVID, according to the Cleveland Clinic.
Ms. McNulty understands that but says many people who live with long COVID turn to these treatments out of a sense of desperation.
“When it comes to this condition, we kind of have to be our own advocates. People are so desperate and feel so gaslit by doctors who don’t believe in their symptoms that they play Russian roulette with their body,” she says. “Most just want some hope and a way to relieve pain.”
Across the country, 16 million Americans have long COVID, according to the Brookings Institution’s analysis of a 2022 Census Bureau report. The report also estimated that up to a quarter of them have such debilitating symptoms that they are no longer able to work. While long COVID centers may offer therapies to help relieve symptoms, “there are no evidence-based established treatments for long COVID at this point,” says Andrew Schamess, MD, a professor of internal medicine at Ohio State Wexner Medical Center, who runs its Post-COVID Recovery Program. “You can’t blame patients for looking for alternative remedies to help them. Unfortunately, there are also a lot of people out to make a buck who are selling unproven and disproven therapies.”
Sniffing out the snake oil
With few evidence-based treatments for long COVID, patients with debilitating symptoms can be tempted by unproven options. One that has gotten a lot of attention is hyperbaric oxygen. This therapy has traditionally been used to treat divers who have decompression sickness, or “the bends.” It’s also being touted by some clinics as an effective treatment for long COVID.
A very small trial of 73 patients with long COVID, published in the journal Scientific Reports, found that those treated in a high-pressure oxygen system 5 days a week for 2 months showed improvements in brain fog, pain, energy, sleep, anxiety, and depression, compared with similar patients who got sham treatments. But larger studies are needed to show how well it works, notes Dr. Schamess.
“It’s very expensive – roughly $120 per session – and there just isn’t the evidence there to support its use,” he says.
In addition, the therapy itself carries risks, such as ear and sinus pain, middle ear injury, temporary vision changes, and, very rarely, lung collapse, according to the U.S. Food and Drug Administration.
One “particularly troubling” treatment being offered, says Kathleen Bell, MD, chair of the department of physical medicine and rehabilitation at the University of Texas Southwestern Medical Center, is stem cell therapy. This therapy is still in its infancy, but it’s being marketed by some clinics as a way to prevent COVID-19 and also treat long-haul symptoms.
The FDA has issued advisories that there are no products approved to treat long COVID and recommends against their use, except in a clinical trial.
“There’s absolutely no regulation – you don’t know what you’re getting, and there’s no research to suggest this therapy even works,” says Dr. Bell. It’s also prohibitively expensive – one Cayman Islands–based company advertises its treatment for as much as $25,000.
Patients with long COVID are even traveling as far as Cyprus, Germany, and Switzerland for a procedure known as blood washing, in which large needles are inserted into veins to filter blood and remove lipids and inflammatory proteins, the British Medical Journal reported in July. Some patients are also prescribed blood thinners to remove microscopic blood clots that may contribute to long COVID. But this treatment is also expensive, with many people paying $10,000-$15,000 out of pocket, and there’s no published evidence to suggest it works, according to the BMJ.
It can be particularly hard to discern what may work and what’s unproven, since many primary care providers are themselves unfamiliar with even traditional long COVID treatments, Dr. Bell says.
Sorting through supplements
Yufang Lin, MD, an integrative specialist at the Cleveland Clinic, says many patients with long COVID enter her office with bags of supplements.
“There’s no data on them, and in large quantities, they may even be harmful,” she says.
Instead, she works closely with the Cleveland Clinic’s long COVID center to do a thorough workup of each patient, which often includes screening for certain nutritional deficiencies.
“Anecdotally, we do see many patients with long COVID who are deficient in these vitamins and minerals,” says Dr. Lin. “If someone is low, we will suggest the appropriate supplement. Otherwise, we work with them to institute some dietary changes.”
This usually involves a plant-based, anti-inflammatory eating pattern such as the Mediterranean diet, which is rich in fruits, vegetables, whole grains, nuts, fatty fish, and healthy fats such as olive oil and avocados.
Other supplements some doctors recommend for patients with long COVID are meant to treat inflammation, Dr. Bell says, although there’s not good evidence they work. One is the antioxidant coenzyme Q10.
But a small preprint study published in The Lancet, of 121 patients with long COVID who took 500 milligrams a day of coenzyme Q10 for 6 weeks saw no differences in recovery, compared with those who took a placebo. Because the study is still a preprint, it has not been peer-reviewed.
Another is probiotics. A small study, published in the journal Infectious Diseases Diagnosis & Treatment, found that a blend of five lactobacillus probiotics, along with a prebiotic called inulin, taken for 30 days, helped with long-term COVID symptoms such as coughing and fatigue. But larger studies need to be done to support their use.
One that may have more promise is omega-3 fatty acids. Like many other supplements, these may help with long COVID by easing inflammation, says Steven Flanagan, MD, a rehabilitation medicine specialist at NYU Langone who works with long COVID patients. Researchers at the Mount Sinai School of Medicine, New York, are studying whether a supplement can help patients who have lost their sense of taste or smell after an infection, but results aren’t yet available.
Among the few alternatives that have been shown to help patients are mindfulness-based therapies – in particular, mindfulness-based forms of exercise such as tai chi and qi gong may be helpful, as they combine a gentle workout with stress reduction.
“Both incorporate meditation, which helps not only to relieve some of the anxiety associated with long COVID but allows patients to redirect their thought process so that they can cope with symptoms better,” says Dr. Flanagan.
A 2022 study, published in BMJ Open, found that these two activities reduced inflammatory markers and improved respiratory muscle strength and function in patients recovering from COVID-19.
“I recommend these activities to all my long COVID patients, as it’s inexpensive and easy to find classes to do either at home or in their community,” he says. “Even if it doesn’t improve their long COVID symptoms, it has other benefits such as increased strength and flexibility that can boost their overall health.”
A version of this article first appeared on WebMD.com.
Entrepreneur Maya McNulty, 49, was one of the first victims of the COVID-19 pandemic. The Schenectady, N.Y., businesswoman spent 2 months in the hospital after catching the disease in March 2020. That September, she was diagnosed with long COVID.
“Even a simple task such as unloading the dishwasher became a major challenge,” she says.
Over the next several months, Ms. McNulty saw a range of specialists, including neurologists, pulmonologists, and cardiologists. She had months of physical therapy and respiratory therapy to help regain strength and lung function. While many of the doctors she saw were sympathetic to what she was going through, not all were.
“I saw one neurologist who told me to my face that she didn’t believe in long COVID,” she recalls. “It was particularly astonishing since the hospital they were affiliated with had a long COVID clinic.”
Ms. McNulty began to connect with other patients with long COVID through a support group she created at the end of 2020 on the social media app Clubhouse. They exchanged ideas and stories about what had helped one another, which led her to try, over the next year, a plant-based diet, Chinese medicine, and vitamin C supplements, among other treatments.
She also acted on unscientific reports she found online and did her own research, which led her to discover claims that some asthma patients with chronic coughing responded well to halotherapy, or dry salt therapy, during which patients inhale micro-particles of salt into their lungs to reduce inflammation, widen airways, and thin mucus. She’s been doing this procedure at a clinic near her home for over a year and credits it with helping with her chronic cough, especially as she recovers from her second bout of COVID-19.
It’s not cheap – a single half-hour session can cost up to $50 and isn’t covered by insurance. There’s also no good research to suggest that it can help with long COVID, according to the Cleveland Clinic.
Ms. McNulty understands that but says many people who live with long COVID turn to these treatments out of a sense of desperation.
“When it comes to this condition, we kind of have to be our own advocates. People are so desperate and feel so gaslit by doctors who don’t believe in their symptoms that they play Russian roulette with their body,” she says. “Most just want some hope and a way to relieve pain.”
Across the country, 16 million Americans have long COVID, according to the Brookings Institution’s analysis of a 2022 Census Bureau report. The report also estimated that up to a quarter of them have such debilitating symptoms that they are no longer able to work. While long COVID centers may offer therapies to help relieve symptoms, “there are no evidence-based established treatments for long COVID at this point,” says Andrew Schamess, MD, a professor of internal medicine at Ohio State Wexner Medical Center, who runs its Post-COVID Recovery Program. “You can’t blame patients for looking for alternative remedies to help them. Unfortunately, there are also a lot of people out to make a buck who are selling unproven and disproven therapies.”
Sniffing out the snake oil
With few evidence-based treatments for long COVID, patients with debilitating symptoms can be tempted by unproven options. One that has gotten a lot of attention is hyperbaric oxygen. This therapy has traditionally been used to treat divers who have decompression sickness, or “the bends.” It’s also being touted by some clinics as an effective treatment for long COVID.
A very small trial of 73 patients with long COVID, published in the journal Scientific Reports, found that those treated in a high-pressure oxygen system 5 days a week for 2 months showed improvements in brain fog, pain, energy, sleep, anxiety, and depression, compared with similar patients who got sham treatments. But larger studies are needed to show how well it works, notes Dr. Schamess.
“It’s very expensive – roughly $120 per session – and there just isn’t the evidence there to support its use,” he says.
In addition, the therapy itself carries risks, such as ear and sinus pain, middle ear injury, temporary vision changes, and, very rarely, lung collapse, according to the U.S. Food and Drug Administration.
One “particularly troubling” treatment being offered, says Kathleen Bell, MD, chair of the department of physical medicine and rehabilitation at the University of Texas Southwestern Medical Center, is stem cell therapy. This therapy is still in its infancy, but it’s being marketed by some clinics as a way to prevent COVID-19 and also treat long-haul symptoms.
The FDA has issued advisories that there are no products approved to treat long COVID and recommends against their use, except in a clinical trial.
“There’s absolutely no regulation – you don’t know what you’re getting, and there’s no research to suggest this therapy even works,” says Dr. Bell. It’s also prohibitively expensive – one Cayman Islands–based company advertises its treatment for as much as $25,000.
Patients with long COVID are even traveling as far as Cyprus, Germany, and Switzerland for a procedure known as blood washing, in which large needles are inserted into veins to filter blood and remove lipids and inflammatory proteins, the British Medical Journal reported in July. Some patients are also prescribed blood thinners to remove microscopic blood clots that may contribute to long COVID. But this treatment is also expensive, with many people paying $10,000-$15,000 out of pocket, and there’s no published evidence to suggest it works, according to the BMJ.
It can be particularly hard to discern what may work and what’s unproven, since many primary care providers are themselves unfamiliar with even traditional long COVID treatments, Dr. Bell says.
Sorting through supplements
Yufang Lin, MD, an integrative specialist at the Cleveland Clinic, says many patients with long COVID enter her office with bags of supplements.
“There’s no data on them, and in large quantities, they may even be harmful,” she says.
Instead, she works closely with the Cleveland Clinic’s long COVID center to do a thorough workup of each patient, which often includes screening for certain nutritional deficiencies.
“Anecdotally, we do see many patients with long COVID who are deficient in these vitamins and minerals,” says Dr. Lin. “If someone is low, we will suggest the appropriate supplement. Otherwise, we work with them to institute some dietary changes.”
This usually involves a plant-based, anti-inflammatory eating pattern such as the Mediterranean diet, which is rich in fruits, vegetables, whole grains, nuts, fatty fish, and healthy fats such as olive oil and avocados.
Other supplements some doctors recommend for patients with long COVID are meant to treat inflammation, Dr. Bell says, although there’s not good evidence they work. One is the antioxidant coenzyme Q10.
But a small preprint study published in The Lancet, of 121 patients with long COVID who took 500 milligrams a day of coenzyme Q10 for 6 weeks saw no differences in recovery, compared with those who took a placebo. Because the study is still a preprint, it has not been peer-reviewed.
Another is probiotics. A small study, published in the journal Infectious Diseases Diagnosis & Treatment, found that a blend of five lactobacillus probiotics, along with a prebiotic called inulin, taken for 30 days, helped with long-term COVID symptoms such as coughing and fatigue. But larger studies need to be done to support their use.
One that may have more promise is omega-3 fatty acids. Like many other supplements, these may help with long COVID by easing inflammation, says Steven Flanagan, MD, a rehabilitation medicine specialist at NYU Langone who works with long COVID patients. Researchers at the Mount Sinai School of Medicine, New York, are studying whether a supplement can help patients who have lost their sense of taste or smell after an infection, but results aren’t yet available.
Among the few alternatives that have been shown to help patients are mindfulness-based therapies – in particular, mindfulness-based forms of exercise such as tai chi and qi gong may be helpful, as they combine a gentle workout with stress reduction.
“Both incorporate meditation, which helps not only to relieve some of the anxiety associated with long COVID but allows patients to redirect their thought process so that they can cope with symptoms better,” says Dr. Flanagan.
A 2022 study, published in BMJ Open, found that these two activities reduced inflammatory markers and improved respiratory muscle strength and function in patients recovering from COVID-19.
“I recommend these activities to all my long COVID patients, as it’s inexpensive and easy to find classes to do either at home or in their community,” he says. “Even if it doesn’t improve their long COVID symptoms, it has other benefits such as increased strength and flexibility that can boost their overall health.”
A version of this article first appeared on WebMD.com.
Entrepreneur Maya McNulty, 49, was one of the first victims of the COVID-19 pandemic. The Schenectady, N.Y., businesswoman spent 2 months in the hospital after catching the disease in March 2020. That September, she was diagnosed with long COVID.
“Even a simple task such as unloading the dishwasher became a major challenge,” she says.
Over the next several months, Ms. McNulty saw a range of specialists, including neurologists, pulmonologists, and cardiologists. She had months of physical therapy and respiratory therapy to help regain strength and lung function. While many of the doctors she saw were sympathetic to what she was going through, not all were.
“I saw one neurologist who told me to my face that she didn’t believe in long COVID,” she recalls. “It was particularly astonishing since the hospital they were affiliated with had a long COVID clinic.”
Ms. McNulty began to connect with other patients with long COVID through a support group she created at the end of 2020 on the social media app Clubhouse. They exchanged ideas and stories about what had helped one another, which led her to try, over the next year, a plant-based diet, Chinese medicine, and vitamin C supplements, among other treatments.
She also acted on unscientific reports she found online and did her own research, which led her to discover claims that some asthma patients with chronic coughing responded well to halotherapy, or dry salt therapy, during which patients inhale micro-particles of salt into their lungs to reduce inflammation, widen airways, and thin mucus. She’s been doing this procedure at a clinic near her home for over a year and credits it with helping with her chronic cough, especially as she recovers from her second bout of COVID-19.
It’s not cheap – a single half-hour session can cost up to $50 and isn’t covered by insurance. There’s also no good research to suggest that it can help with long COVID, according to the Cleveland Clinic.
Ms. McNulty understands that but says many people who live with long COVID turn to these treatments out of a sense of desperation.
“When it comes to this condition, we kind of have to be our own advocates. People are so desperate and feel so gaslit by doctors who don’t believe in their symptoms that they play Russian roulette with their body,” she says. “Most just want some hope and a way to relieve pain.”
Across the country, 16 million Americans have long COVID, according to the Brookings Institution’s analysis of a 2022 Census Bureau report. The report also estimated that up to a quarter of them have such debilitating symptoms that they are no longer able to work. While long COVID centers may offer therapies to help relieve symptoms, “there are no evidence-based established treatments for long COVID at this point,” says Andrew Schamess, MD, a professor of internal medicine at Ohio State Wexner Medical Center, who runs its Post-COVID Recovery Program. “You can’t blame patients for looking for alternative remedies to help them. Unfortunately, there are also a lot of people out to make a buck who are selling unproven and disproven therapies.”
Sniffing out the snake oil
With few evidence-based treatments for long COVID, patients with debilitating symptoms can be tempted by unproven options. One that has gotten a lot of attention is hyperbaric oxygen. This therapy has traditionally been used to treat divers who have decompression sickness, or “the bends.” It’s also being touted by some clinics as an effective treatment for long COVID.
A very small trial of 73 patients with long COVID, published in the journal Scientific Reports, found that those treated in a high-pressure oxygen system 5 days a week for 2 months showed improvements in brain fog, pain, energy, sleep, anxiety, and depression, compared with similar patients who got sham treatments. But larger studies are needed to show how well it works, notes Dr. Schamess.
“It’s very expensive – roughly $120 per session – and there just isn’t the evidence there to support its use,” he says.
In addition, the therapy itself carries risks, such as ear and sinus pain, middle ear injury, temporary vision changes, and, very rarely, lung collapse, according to the U.S. Food and Drug Administration.
One “particularly troubling” treatment being offered, says Kathleen Bell, MD, chair of the department of physical medicine and rehabilitation at the University of Texas Southwestern Medical Center, is stem cell therapy. This therapy is still in its infancy, but it’s being marketed by some clinics as a way to prevent COVID-19 and also treat long-haul symptoms.
The FDA has issued advisories that there are no products approved to treat long COVID and recommends against their use, except in a clinical trial.
“There’s absolutely no regulation – you don’t know what you’re getting, and there’s no research to suggest this therapy even works,” says Dr. Bell. It’s also prohibitively expensive – one Cayman Islands–based company advertises its treatment for as much as $25,000.
Patients with long COVID are even traveling as far as Cyprus, Germany, and Switzerland for a procedure known as blood washing, in which large needles are inserted into veins to filter blood and remove lipids and inflammatory proteins, the British Medical Journal reported in July. Some patients are also prescribed blood thinners to remove microscopic blood clots that may contribute to long COVID. But this treatment is also expensive, with many people paying $10,000-$15,000 out of pocket, and there’s no published evidence to suggest it works, according to the BMJ.
It can be particularly hard to discern what may work and what’s unproven, since many primary care providers are themselves unfamiliar with even traditional long COVID treatments, Dr. Bell says.
Sorting through supplements
Yufang Lin, MD, an integrative specialist at the Cleveland Clinic, says many patients with long COVID enter her office with bags of supplements.
“There’s no data on them, and in large quantities, they may even be harmful,” she says.
Instead, she works closely with the Cleveland Clinic’s long COVID center to do a thorough workup of each patient, which often includes screening for certain nutritional deficiencies.
“Anecdotally, we do see many patients with long COVID who are deficient in these vitamins and minerals,” says Dr. Lin. “If someone is low, we will suggest the appropriate supplement. Otherwise, we work with them to institute some dietary changes.”
This usually involves a plant-based, anti-inflammatory eating pattern such as the Mediterranean diet, which is rich in fruits, vegetables, whole grains, nuts, fatty fish, and healthy fats such as olive oil and avocados.
Other supplements some doctors recommend for patients with long COVID are meant to treat inflammation, Dr. Bell says, although there’s not good evidence they work. One is the antioxidant coenzyme Q10.
But a small preprint study published in The Lancet, of 121 patients with long COVID who took 500 milligrams a day of coenzyme Q10 for 6 weeks saw no differences in recovery, compared with those who took a placebo. Because the study is still a preprint, it has not been peer-reviewed.
Another is probiotics. A small study, published in the journal Infectious Diseases Diagnosis & Treatment, found that a blend of five lactobacillus probiotics, along with a prebiotic called inulin, taken for 30 days, helped with long-term COVID symptoms such as coughing and fatigue. But larger studies need to be done to support their use.
One that may have more promise is omega-3 fatty acids. Like many other supplements, these may help with long COVID by easing inflammation, says Steven Flanagan, MD, a rehabilitation medicine specialist at NYU Langone who works with long COVID patients. Researchers at the Mount Sinai School of Medicine, New York, are studying whether a supplement can help patients who have lost their sense of taste or smell after an infection, but results aren’t yet available.
Among the few alternatives that have been shown to help patients are mindfulness-based therapies – in particular, mindfulness-based forms of exercise such as tai chi and qi gong may be helpful, as they combine a gentle workout with stress reduction.
“Both incorporate meditation, which helps not only to relieve some of the anxiety associated with long COVID but allows patients to redirect their thought process so that they can cope with symptoms better,” says Dr. Flanagan.
A 2022 study, published in BMJ Open, found that these two activities reduced inflammatory markers and improved respiratory muscle strength and function in patients recovering from COVID-19.
“I recommend these activities to all my long COVID patients, as it’s inexpensive and easy to find classes to do either at home or in their community,” he says. “Even if it doesn’t improve their long COVID symptoms, it has other benefits such as increased strength and flexibility that can boost their overall health.”
A version of this article first appeared on WebMD.com.
Limiting antibiotic overprescription in pandemics: New guidelines
A statement by the Society for Healthcare Epidemiology of America, published online in Infection Control & Hospital Epidemiology, offers health care providers guidelines on how to prevent inappropriate antibiotic use in future pandemics and to avoid some of the negative scenarios that have been seen with COVID-19.
According to the U.S. Centers of Disease Control and Prevention,
The culprit might be the widespread antibiotic overprescription during the current pandemic. A 2022 meta-analysis revealed that in high-income countries, 58% of patients with COVID-19 were given antibiotics, whereas in lower- and middle-income countries, 89% of patients were put on such drugs. Some hospitals in Europe and the United States reported similarly elevated numbers, sometimes approaching 100%.
“We’ve lost control,” Natasha Pettit, PharmD, pharmacy director at University of Chicago Medicine, told this news organization. Dr. Pettit was not involved in the SHEA study. “Even if CDC didn’t come out with that data, I can tell you right now more of my time is spent trying to figure out how to manage these multi-drug–resistant infections, and we are running out of options for these patients,”
“Dealing with uncertainty, exhaustion, [and] critical illness in often young, otherwise healthy patients meant doctors wanted to do something for their patients,” said Tamar Barlam, MD, an infectious diseases expert at the Boston Medical Center who led the development of the SHEA white paper, in an interview.
That something often was a prescription for antibiotics, even without a clear indication that they were actually needed. A British study revealed that in times of pandemic uncertainty, clinicians often reached for antibiotics “just in case” and referred to conservative prescribing as “bravery.”
Studies have shown, however, that bacterial co-infections in COVID-19 are rare. A 2020 meta-analysis of 24 studies concluded that only 3.5% of patients had a bacterial co-infection on presentation, and 14.3% had a secondary infection. Similar patterns had previously been observed in other viral outbreaks. Research on MERS-CoV, for example, documented only 1% of patients with a bacterial co-infection on admission. During the 2009 H1N1 influenza pandemic, that number was 12% of non–ICU hospitalized patients.
Yet, according to Dr. Pettit, even when such data became available, it didn’t necessarily change prescribing patterns. “Information was coming at us so quickly, I think the providers didn’t have a moment to see the data, to understand what it meant for their prescribing. Having external guidance earlier on would have been hugely helpful,” she told this news organization.
That’s where the newly published SHEA statement comes in: It outlines recommendations on when to prescribe antibiotics during a respiratory viral pandemic, what tests to order, and when to de-escalate or discontinue the treatment. These recommendations include, for instance, advice to not trust inflammatory markers as reliable indicators of bacterial or fungal infection and to not use procalcitonin routinely to aid in the decision to initiate antibiotics.
According to Dr. Barlam, one of the crucial lessons here is that if clinicians see patients with symptoms that are consistent with the current pandemic, they should trust their own impressions and avoid reaching for antimicrobials “just in case.”
Another important lesson is that antibiotic stewardship programs have a huge role to play during pandemics. They should not only monitor prescribing but also compile new information on bacterial co-infections as it gets released and make sure it reaches the clinicians in a clear form.
Evidence suggests that such programs and guidelines do work to limit unnecessary antibiotic use. In one medical center in Chicago, for example, before recommendations on when to initiate and discontinue antimicrobials were released, over 74% of COVID-19 patients received antibiotics. After guidelines were put in place, the use of such drugs fell to 42%.
Dr. Pettit believes, however, that it’s important not to leave each medical center to its own devices. “Hindsight is always twenty-twenty,” she said, “but I think it would be great that, if we start hearing about a pathogen that might lead to another pandemic, we should have a mechanism in place to call together an expert body to get guidance for how antimicrobial stewardship programs should get involved.”
One of the authors of the SHEA statement, Susan Seo, reports an investigator-initiated Merck grant on cost-effectiveness of letermovir in hematopoietic stem cell transplant patients. Another author, Graeme Forrest, reports a clinical study grant from Regeneron for inpatient monoclonals against SARS-CoV-2. All other authors report no conflicts of interest. The study was independently supported.
A version of this article first appeared on Medscape.com.
A statement by the Society for Healthcare Epidemiology of America, published online in Infection Control & Hospital Epidemiology, offers health care providers guidelines on how to prevent inappropriate antibiotic use in future pandemics and to avoid some of the negative scenarios that have been seen with COVID-19.
According to the U.S. Centers of Disease Control and Prevention,
The culprit might be the widespread antibiotic overprescription during the current pandemic. A 2022 meta-analysis revealed that in high-income countries, 58% of patients with COVID-19 were given antibiotics, whereas in lower- and middle-income countries, 89% of patients were put on such drugs. Some hospitals in Europe and the United States reported similarly elevated numbers, sometimes approaching 100%.
“We’ve lost control,” Natasha Pettit, PharmD, pharmacy director at University of Chicago Medicine, told this news organization. Dr. Pettit was not involved in the SHEA study. “Even if CDC didn’t come out with that data, I can tell you right now more of my time is spent trying to figure out how to manage these multi-drug–resistant infections, and we are running out of options for these patients,”
“Dealing with uncertainty, exhaustion, [and] critical illness in often young, otherwise healthy patients meant doctors wanted to do something for their patients,” said Tamar Barlam, MD, an infectious diseases expert at the Boston Medical Center who led the development of the SHEA white paper, in an interview.
That something often was a prescription for antibiotics, even without a clear indication that they were actually needed. A British study revealed that in times of pandemic uncertainty, clinicians often reached for antibiotics “just in case” and referred to conservative prescribing as “bravery.”
Studies have shown, however, that bacterial co-infections in COVID-19 are rare. A 2020 meta-analysis of 24 studies concluded that only 3.5% of patients had a bacterial co-infection on presentation, and 14.3% had a secondary infection. Similar patterns had previously been observed in other viral outbreaks. Research on MERS-CoV, for example, documented only 1% of patients with a bacterial co-infection on admission. During the 2009 H1N1 influenza pandemic, that number was 12% of non–ICU hospitalized patients.
Yet, according to Dr. Pettit, even when such data became available, it didn’t necessarily change prescribing patterns. “Information was coming at us so quickly, I think the providers didn’t have a moment to see the data, to understand what it meant for their prescribing. Having external guidance earlier on would have been hugely helpful,” she told this news organization.
That’s where the newly published SHEA statement comes in: It outlines recommendations on when to prescribe antibiotics during a respiratory viral pandemic, what tests to order, and when to de-escalate or discontinue the treatment. These recommendations include, for instance, advice to not trust inflammatory markers as reliable indicators of bacterial or fungal infection and to not use procalcitonin routinely to aid in the decision to initiate antibiotics.
According to Dr. Barlam, one of the crucial lessons here is that if clinicians see patients with symptoms that are consistent with the current pandemic, they should trust their own impressions and avoid reaching for antimicrobials “just in case.”
Another important lesson is that antibiotic stewardship programs have a huge role to play during pandemics. They should not only monitor prescribing but also compile new information on bacterial co-infections as it gets released and make sure it reaches the clinicians in a clear form.
Evidence suggests that such programs and guidelines do work to limit unnecessary antibiotic use. In one medical center in Chicago, for example, before recommendations on when to initiate and discontinue antimicrobials were released, over 74% of COVID-19 patients received antibiotics. After guidelines were put in place, the use of such drugs fell to 42%.
Dr. Pettit believes, however, that it’s important not to leave each medical center to its own devices. “Hindsight is always twenty-twenty,” she said, “but I think it would be great that, if we start hearing about a pathogen that might lead to another pandemic, we should have a mechanism in place to call together an expert body to get guidance for how antimicrobial stewardship programs should get involved.”
One of the authors of the SHEA statement, Susan Seo, reports an investigator-initiated Merck grant on cost-effectiveness of letermovir in hematopoietic stem cell transplant patients. Another author, Graeme Forrest, reports a clinical study grant from Regeneron for inpatient monoclonals against SARS-CoV-2. All other authors report no conflicts of interest. The study was independently supported.
A version of this article first appeared on Medscape.com.
A statement by the Society for Healthcare Epidemiology of America, published online in Infection Control & Hospital Epidemiology, offers health care providers guidelines on how to prevent inappropriate antibiotic use in future pandemics and to avoid some of the negative scenarios that have been seen with COVID-19.
According to the U.S. Centers of Disease Control and Prevention,
The culprit might be the widespread antibiotic overprescription during the current pandemic. A 2022 meta-analysis revealed that in high-income countries, 58% of patients with COVID-19 were given antibiotics, whereas in lower- and middle-income countries, 89% of patients were put on such drugs. Some hospitals in Europe and the United States reported similarly elevated numbers, sometimes approaching 100%.
“We’ve lost control,” Natasha Pettit, PharmD, pharmacy director at University of Chicago Medicine, told this news organization. Dr. Pettit was not involved in the SHEA study. “Even if CDC didn’t come out with that data, I can tell you right now more of my time is spent trying to figure out how to manage these multi-drug–resistant infections, and we are running out of options for these patients,”
“Dealing with uncertainty, exhaustion, [and] critical illness in often young, otherwise healthy patients meant doctors wanted to do something for their patients,” said Tamar Barlam, MD, an infectious diseases expert at the Boston Medical Center who led the development of the SHEA white paper, in an interview.
That something often was a prescription for antibiotics, even without a clear indication that they were actually needed. A British study revealed that in times of pandemic uncertainty, clinicians often reached for antibiotics “just in case” and referred to conservative prescribing as “bravery.”
Studies have shown, however, that bacterial co-infections in COVID-19 are rare. A 2020 meta-analysis of 24 studies concluded that only 3.5% of patients had a bacterial co-infection on presentation, and 14.3% had a secondary infection. Similar patterns had previously been observed in other viral outbreaks. Research on MERS-CoV, for example, documented only 1% of patients with a bacterial co-infection on admission. During the 2009 H1N1 influenza pandemic, that number was 12% of non–ICU hospitalized patients.
Yet, according to Dr. Pettit, even when such data became available, it didn’t necessarily change prescribing patterns. “Information was coming at us so quickly, I think the providers didn’t have a moment to see the data, to understand what it meant for their prescribing. Having external guidance earlier on would have been hugely helpful,” she told this news organization.
That’s where the newly published SHEA statement comes in: It outlines recommendations on when to prescribe antibiotics during a respiratory viral pandemic, what tests to order, and when to de-escalate or discontinue the treatment. These recommendations include, for instance, advice to not trust inflammatory markers as reliable indicators of bacterial or fungal infection and to not use procalcitonin routinely to aid in the decision to initiate antibiotics.
According to Dr. Barlam, one of the crucial lessons here is that if clinicians see patients with symptoms that are consistent with the current pandemic, they should trust their own impressions and avoid reaching for antimicrobials “just in case.”
Another important lesson is that antibiotic stewardship programs have a huge role to play during pandemics. They should not only monitor prescribing but also compile new information on bacterial co-infections as it gets released and make sure it reaches the clinicians in a clear form.
Evidence suggests that such programs and guidelines do work to limit unnecessary antibiotic use. In one medical center in Chicago, for example, before recommendations on when to initiate and discontinue antimicrobials were released, over 74% of COVID-19 patients received antibiotics. After guidelines were put in place, the use of such drugs fell to 42%.
Dr. Pettit believes, however, that it’s important not to leave each medical center to its own devices. “Hindsight is always twenty-twenty,” she said, “but I think it would be great that, if we start hearing about a pathogen that might lead to another pandemic, we should have a mechanism in place to call together an expert body to get guidance for how antimicrobial stewardship programs should get involved.”
One of the authors of the SHEA statement, Susan Seo, reports an investigator-initiated Merck grant on cost-effectiveness of letermovir in hematopoietic stem cell transplant patients. Another author, Graeme Forrest, reports a clinical study grant from Regeneron for inpatient monoclonals against SARS-CoV-2. All other authors report no conflicts of interest. The study was independently supported.
A version of this article first appeared on Medscape.com.
FROM INFECTION CONTROL & HOSPITAL EPIDEMIOLOGY