User login
Moderna reports positive COVID-19 vaccine response in kids down to 6 months
Moderna on March 23 released interim results indicating that its mRNA-1273 COVID vaccine produced “robust” neutralizing antibody titers in children aged 6 months to 6 years – levels similar to those seen in adults.
Vaccine efficacy against infection was 43.7% in children aged 6 months to 2 years and 37.5% among children aged 2-6 years, the new data from its phase 2/3 KidCOVE study show.
The company explained the lower efficacy numbers by noting that its study involving these younger children was conducted during the Omicron wave. The same decrease in efficacy against infection was reported in adults during the Omicron surge.
A majority of COVID-19 cases were mild in the approximately 6,900 children aged 6 months to 6 years in the study. No severe COVID-19 cases, hospitalizations, or deaths were reported.
The primary series of two 25-mcg doses of the vaccine given 28 days apart was generally well tolerated. Most adverse events were mild to moderate. For example, temperature greater than 38° C (>100.4° F) was reported for 17.0% of the 6-month-old to 2-year-old group and for 14.6% of the 2- to 6-year-old group. A few children, 0.2% of each group, experienced a temperature greater than 40° C (>104° F).
Moderna plans to include these response, efficacy, and safety data in an application to the Food and Drug Administration for emergency use authorization (EUA) of the vaccine in these younger children in the coming weeks.
“We now have clinical data on the performance of our vaccine from infants 6 months of age through older adults,” Moderna CEO Stephane Bancel said in a news release. He described the interim results as “good news for parents of children under 6 years of age.”
In other news
Moderna also announced that it began the FDA EUA submission process for a 50-μg two-dose primary series for children aged 6-12 years.
The company is also updating its EUA submission for a 100-mcg two-dose primary series for children and adolescents aged 12-18 years.
Similar to its booster research in adults, Moderna plans to evaluate the potential of a booster dose for all pediatric populations, including those aged 6 months to 6 years, 6-12 years, and adolescents. The company is evaluating both a booster dose of mRNA-1273 and its bivalent booster candidate (mRNA1273.214), which includes an Omicron variant booster and mRNA-1273.
A version of this article first appeared on Medscape.com.
Moderna on March 23 released interim results indicating that its mRNA-1273 COVID vaccine produced “robust” neutralizing antibody titers in children aged 6 months to 6 years – levels similar to those seen in adults.
Vaccine efficacy against infection was 43.7% in children aged 6 months to 2 years and 37.5% among children aged 2-6 years, the new data from its phase 2/3 KidCOVE study show.
The company explained the lower efficacy numbers by noting that its study involving these younger children was conducted during the Omicron wave. The same decrease in efficacy against infection was reported in adults during the Omicron surge.
A majority of COVID-19 cases were mild in the approximately 6,900 children aged 6 months to 6 years in the study. No severe COVID-19 cases, hospitalizations, or deaths were reported.
The primary series of two 25-mcg doses of the vaccine given 28 days apart was generally well tolerated. Most adverse events were mild to moderate. For example, temperature greater than 38° C (>100.4° F) was reported for 17.0% of the 6-month-old to 2-year-old group and for 14.6% of the 2- to 6-year-old group. A few children, 0.2% of each group, experienced a temperature greater than 40° C (>104° F).
Moderna plans to include these response, efficacy, and safety data in an application to the Food and Drug Administration for emergency use authorization (EUA) of the vaccine in these younger children in the coming weeks.
“We now have clinical data on the performance of our vaccine from infants 6 months of age through older adults,” Moderna CEO Stephane Bancel said in a news release. He described the interim results as “good news for parents of children under 6 years of age.”
In other news
Moderna also announced that it began the FDA EUA submission process for a 50-μg two-dose primary series for children aged 6-12 years.
The company is also updating its EUA submission for a 100-mcg two-dose primary series for children and adolescents aged 12-18 years.
Similar to its booster research in adults, Moderna plans to evaluate the potential of a booster dose for all pediatric populations, including those aged 6 months to 6 years, 6-12 years, and adolescents. The company is evaluating both a booster dose of mRNA-1273 and its bivalent booster candidate (mRNA1273.214), which includes an Omicron variant booster and mRNA-1273.
A version of this article first appeared on Medscape.com.
Moderna on March 23 released interim results indicating that its mRNA-1273 COVID vaccine produced “robust” neutralizing antibody titers in children aged 6 months to 6 years – levels similar to those seen in adults.
Vaccine efficacy against infection was 43.7% in children aged 6 months to 2 years and 37.5% among children aged 2-6 years, the new data from its phase 2/3 KidCOVE study show.
The company explained the lower efficacy numbers by noting that its study involving these younger children was conducted during the Omicron wave. The same decrease in efficacy against infection was reported in adults during the Omicron surge.
A majority of COVID-19 cases were mild in the approximately 6,900 children aged 6 months to 6 years in the study. No severe COVID-19 cases, hospitalizations, or deaths were reported.
The primary series of two 25-mcg doses of the vaccine given 28 days apart was generally well tolerated. Most adverse events were mild to moderate. For example, temperature greater than 38° C (>100.4° F) was reported for 17.0% of the 6-month-old to 2-year-old group and for 14.6% of the 2- to 6-year-old group. A few children, 0.2% of each group, experienced a temperature greater than 40° C (>104° F).
Moderna plans to include these response, efficacy, and safety data in an application to the Food and Drug Administration for emergency use authorization (EUA) of the vaccine in these younger children in the coming weeks.
“We now have clinical data on the performance of our vaccine from infants 6 months of age through older adults,” Moderna CEO Stephane Bancel said in a news release. He described the interim results as “good news for parents of children under 6 years of age.”
In other news
Moderna also announced that it began the FDA EUA submission process for a 50-μg two-dose primary series for children aged 6-12 years.
The company is also updating its EUA submission for a 100-mcg two-dose primary series for children and adolescents aged 12-18 years.
Similar to its booster research in adults, Moderna plans to evaluate the potential of a booster dose for all pediatric populations, including those aged 6 months to 6 years, 6-12 years, and adolescents. The company is evaluating both a booster dose of mRNA-1273 and its bivalent booster candidate (mRNA1273.214), which includes an Omicron variant booster and mRNA-1273.
A version of this article first appeared on Medscape.com.
Children and COVID: CDC gives perspective on hospitalizations
New COVID-19 cases in children fell by 23% as the latest weekly count dropped to its lowest level since July of 2021, based on data from the American Academy of Pediatrics and the Children’s Hospital Association.
, when the early stages of the Delta surge led to 23,551 cases, the AAP and CHA said in their weekly COVID report.
The two organizations put the total number of cases at nearly 12.8 million from the start of the pandemic to March 17, with children representing 19.0% of cases among all ages. The Centers for Disease Control and Prevention puts the cumulative number of COVID-19 cases at almost 12.0 million as of March 21, or 17.5% of the nationwide total.
COVID-related hospitalizations also continue to fall, and two new studies from the CDC put children’s experiences during the Omicron surge and the larger pandemic into perspective.
One study showed that hospitalization rates for children aged 4 years and younger during the Omicron surge were five times higher than at the peak of the Delta surge, with the highest rates occurring in infants under 6 months of age. That report was based on the CDC’s COVID-19–Associated Hospitalization Surveillance Network (COVID-NET), which covers 99 counties across 14 states (MMWR. 2022 March 18;71[11]:429-36).
The second study compared child hospitalizations during 1 year of the COVID pandemic (Oct. 1, 2020, to Sept. 30, 2021) with three influenza seasons (2017-2018 through 2019-2020). The pre-Omicron hospitalization rate for those under age 18 years, 48.2 per 100,000 children, was higher than any of the three flu seasons: 33.5 per 100,000 in 2017-2018, 33.8 in 2018-2019, and 41.7 for 2019-2020, the investigators said in a medRxiv preprint.
Most of the increased COVID burden fell on adolescents aged 12-17, they said. The COVID hospitalization rate for that age group was 59.9 per 100,000, versus 12.2-14.1 for influenza, while children aged 5-11 had a COVID-related rate of 25.0 and flu-related rates of 24.3-31.7, and those aged 0-4 had rates of 66.8 for COVID and 70.9-91.5 for the flu, Miranda J. Delahoy of the CDC’s COVID-19 Response Team and associates reported.
New COVID-19 cases in children fell by 23% as the latest weekly count dropped to its lowest level since July of 2021, based on data from the American Academy of Pediatrics and the Children’s Hospital Association.
, when the early stages of the Delta surge led to 23,551 cases, the AAP and CHA said in their weekly COVID report.
The two organizations put the total number of cases at nearly 12.8 million from the start of the pandemic to March 17, with children representing 19.0% of cases among all ages. The Centers for Disease Control and Prevention puts the cumulative number of COVID-19 cases at almost 12.0 million as of March 21, or 17.5% of the nationwide total.
COVID-related hospitalizations also continue to fall, and two new studies from the CDC put children’s experiences during the Omicron surge and the larger pandemic into perspective.
One study showed that hospitalization rates for children aged 4 years and younger during the Omicron surge were five times higher than at the peak of the Delta surge, with the highest rates occurring in infants under 6 months of age. That report was based on the CDC’s COVID-19–Associated Hospitalization Surveillance Network (COVID-NET), which covers 99 counties across 14 states (MMWR. 2022 March 18;71[11]:429-36).
The second study compared child hospitalizations during 1 year of the COVID pandemic (Oct. 1, 2020, to Sept. 30, 2021) with three influenza seasons (2017-2018 through 2019-2020). The pre-Omicron hospitalization rate for those under age 18 years, 48.2 per 100,000 children, was higher than any of the three flu seasons: 33.5 per 100,000 in 2017-2018, 33.8 in 2018-2019, and 41.7 for 2019-2020, the investigators said in a medRxiv preprint.
Most of the increased COVID burden fell on adolescents aged 12-17, they said. The COVID hospitalization rate for that age group was 59.9 per 100,000, versus 12.2-14.1 for influenza, while children aged 5-11 had a COVID-related rate of 25.0 and flu-related rates of 24.3-31.7, and those aged 0-4 had rates of 66.8 for COVID and 70.9-91.5 for the flu, Miranda J. Delahoy of the CDC’s COVID-19 Response Team and associates reported.
New COVID-19 cases in children fell by 23% as the latest weekly count dropped to its lowest level since July of 2021, based on data from the American Academy of Pediatrics and the Children’s Hospital Association.
, when the early stages of the Delta surge led to 23,551 cases, the AAP and CHA said in their weekly COVID report.
The two organizations put the total number of cases at nearly 12.8 million from the start of the pandemic to March 17, with children representing 19.0% of cases among all ages. The Centers for Disease Control and Prevention puts the cumulative number of COVID-19 cases at almost 12.0 million as of March 21, or 17.5% of the nationwide total.
COVID-related hospitalizations also continue to fall, and two new studies from the CDC put children’s experiences during the Omicron surge and the larger pandemic into perspective.
One study showed that hospitalization rates for children aged 4 years and younger during the Omicron surge were five times higher than at the peak of the Delta surge, with the highest rates occurring in infants under 6 months of age. That report was based on the CDC’s COVID-19–Associated Hospitalization Surveillance Network (COVID-NET), which covers 99 counties across 14 states (MMWR. 2022 March 18;71[11]:429-36).
The second study compared child hospitalizations during 1 year of the COVID pandemic (Oct. 1, 2020, to Sept. 30, 2021) with three influenza seasons (2017-2018 through 2019-2020). The pre-Omicron hospitalization rate for those under age 18 years, 48.2 per 100,000 children, was higher than any of the three flu seasons: 33.5 per 100,000 in 2017-2018, 33.8 in 2018-2019, and 41.7 for 2019-2020, the investigators said in a medRxiv preprint.
Most of the increased COVID burden fell on adolescents aged 12-17, they said. The COVID hospitalization rate for that age group was 59.9 per 100,000, versus 12.2-14.1 for influenza, while children aged 5-11 had a COVID-related rate of 25.0 and flu-related rates of 24.3-31.7, and those aged 0-4 had rates of 66.8 for COVID and 70.9-91.5 for the flu, Miranda J. Delahoy of the CDC’s COVID-19 Response Team and associates reported.
Mild COVID-19 infection linked to later type 2 diabetes
People who recover from a mild case of COVID-19 appear to have an increased risk for subsequent new-onset type 2 diabetes but not other types of diabetes, new data suggest.
“If confirmed, the results of the present study indicate that diabetes screening in individuals who have recovered from even mild COVID-19 should be recommended,” say Wolfgang Rathmann, MD, of the Leibniz Center for Diabetes Research at Heinrich Heine University, Düsseldorf, Germany, and colleagues.
The findings, from a nationwide primary care database in Germany, were recently published in Diabetologia.
These primary care data align with those from other studies of more seriously ill patients with COVID-19 that found increased rates of type 2 diabetes diagnoses in the subsequent months following illness, they point out.
“COVID-19 infection may lead to diabetes by upregulation of the immune system after remission, which may induce pancreatic beta-cell dysfunction and insulin resistance, or patients may have been at risk for developing diabetes due to having obesity or prediabetes, and the stress COVID-19 put on their bodies sped it up,” said Dr. Rathmann in a press release.
However, because the patients with COVID-19 in the study were only followed for about 3 months, “further follow-up is needed to understand whether type 2 diabetes after mild COVID-19 is just temporary and can be reversed after they have fully recovered or whether it leads to a chronic condition,” he noted.
Increase in type 2 diabetes 3 months after mild COVID-19
The retrospective cohort analysis was performed using data from the Disease Analyzer, a representative panel of 1,171 physician practices in Germany, from March 2020 to January 2021, with follow-up through July 2021.
Individuals with a history of COVID-19 or diabetes and those taking corticosteroids within 30 days after the index dates were excluded.
A total of 35,865 patients with confirmed SARS-CoV-2 infection were propensity score-matched on a one-to-one basis for sex, age, health insurance, and comorbidities with those who had acute respiratory tract infections (controls) but were COVID-19 negative. Median follow-up was 119 days for the COVID-19 group and 161 days for controls.
There was a 28% increased risk of type 2 diabetes for those who had COVID-19 versus controls (15.8 per 1,000 person-years vs. 12.3 per 1,000 person-years, respectively, which was significantly different, and an incidence rate ratio of 1.28).
The incidence of other types of diabetes or unspecified diabetes for the COVID-19 and control groups did not differ significantly (4.3 per 1,000 person-years vs. 3.7 per 1,000 person-years; IRR, 1.17).
Similar findings were seen in sensitivity analyses by glucose-lowering medication prescriptions and by ICD-10 codes.
Although type 2 diabetes is not likely to be a problem for the vast majority of people who have mild COVID-19, the authors recommend that anyone who has recovered from COVID-19 be aware of the warning signs and symptoms such as fatigue, frequent urination, and increased thirst, and seek treatment right away.
CoviDiab registry tracking type 1 and type 2 diabetes
Over the course of the pandemic, there have been conflicting data on whether COVID-19 induces or reveals a propensity for type 1 and type 2 diabetes.
The CoviDiab global registry is tracking this and will include diabetes type for adults and children.
The aim is to have “as many as possible cases of new-onset diabetes for which we can have also a minimum set of clinical data including type of diabetes and A1c,” coprincipal investigator Francesco Rubino, MD, of King’s College London, previously told this news organization.
“By looking at this information we can infer whether a role of COVID-19 in triggering diabetes is clinically plausible – or not – and what type of diabetes is most frequently associated with COVID-19.”
Rubino said that the CoviDiab team is approaching the data with the assumption that, at least in adults diagnosed with type 2 diabetes, the explanation might be that the person already had undiagnosed diabetes or the hyperglycemia may be stress-induced and temporary.
The German Diabetes Center is funded by the German Federal Ministry of Health and the Ministry of Culture and Science of the State of North Rhine-Westphalia. Dr. Rathmann has reported receiving consulting fees for attending educational sessions or advisory boards for AstraZeneca, Boehringer Ingelheim, and Novo Nordisk and institutional research grants from Novo Nordisk outside of the topic of the current work.
A version of this article first appeared on Medscape.com.
People who recover from a mild case of COVID-19 appear to have an increased risk for subsequent new-onset type 2 diabetes but not other types of diabetes, new data suggest.
“If confirmed, the results of the present study indicate that diabetes screening in individuals who have recovered from even mild COVID-19 should be recommended,” say Wolfgang Rathmann, MD, of the Leibniz Center for Diabetes Research at Heinrich Heine University, Düsseldorf, Germany, and colleagues.
The findings, from a nationwide primary care database in Germany, were recently published in Diabetologia.
These primary care data align with those from other studies of more seriously ill patients with COVID-19 that found increased rates of type 2 diabetes diagnoses in the subsequent months following illness, they point out.
“COVID-19 infection may lead to diabetes by upregulation of the immune system after remission, which may induce pancreatic beta-cell dysfunction and insulin resistance, or patients may have been at risk for developing diabetes due to having obesity or prediabetes, and the stress COVID-19 put on their bodies sped it up,” said Dr. Rathmann in a press release.
However, because the patients with COVID-19 in the study were only followed for about 3 months, “further follow-up is needed to understand whether type 2 diabetes after mild COVID-19 is just temporary and can be reversed after they have fully recovered or whether it leads to a chronic condition,” he noted.
Increase in type 2 diabetes 3 months after mild COVID-19
The retrospective cohort analysis was performed using data from the Disease Analyzer, a representative panel of 1,171 physician practices in Germany, from March 2020 to January 2021, with follow-up through July 2021.
Individuals with a history of COVID-19 or diabetes and those taking corticosteroids within 30 days after the index dates were excluded.
A total of 35,865 patients with confirmed SARS-CoV-2 infection were propensity score-matched on a one-to-one basis for sex, age, health insurance, and comorbidities with those who had acute respiratory tract infections (controls) but were COVID-19 negative. Median follow-up was 119 days for the COVID-19 group and 161 days for controls.
There was a 28% increased risk of type 2 diabetes for those who had COVID-19 versus controls (15.8 per 1,000 person-years vs. 12.3 per 1,000 person-years, respectively, which was significantly different, and an incidence rate ratio of 1.28).
The incidence of other types of diabetes or unspecified diabetes for the COVID-19 and control groups did not differ significantly (4.3 per 1,000 person-years vs. 3.7 per 1,000 person-years; IRR, 1.17).
Similar findings were seen in sensitivity analyses by glucose-lowering medication prescriptions and by ICD-10 codes.
Although type 2 diabetes is not likely to be a problem for the vast majority of people who have mild COVID-19, the authors recommend that anyone who has recovered from COVID-19 be aware of the warning signs and symptoms such as fatigue, frequent urination, and increased thirst, and seek treatment right away.
CoviDiab registry tracking type 1 and type 2 diabetes
Over the course of the pandemic, there have been conflicting data on whether COVID-19 induces or reveals a propensity for type 1 and type 2 diabetes.
The CoviDiab global registry is tracking this and will include diabetes type for adults and children.
The aim is to have “as many as possible cases of new-onset diabetes for which we can have also a minimum set of clinical data including type of diabetes and A1c,” coprincipal investigator Francesco Rubino, MD, of King’s College London, previously told this news organization.
“By looking at this information we can infer whether a role of COVID-19 in triggering diabetes is clinically plausible – or not – and what type of diabetes is most frequently associated with COVID-19.”
Rubino said that the CoviDiab team is approaching the data with the assumption that, at least in adults diagnosed with type 2 diabetes, the explanation might be that the person already had undiagnosed diabetes or the hyperglycemia may be stress-induced and temporary.
The German Diabetes Center is funded by the German Federal Ministry of Health and the Ministry of Culture and Science of the State of North Rhine-Westphalia. Dr. Rathmann has reported receiving consulting fees for attending educational sessions or advisory boards for AstraZeneca, Boehringer Ingelheim, and Novo Nordisk and institutional research grants from Novo Nordisk outside of the topic of the current work.
A version of this article first appeared on Medscape.com.
People who recover from a mild case of COVID-19 appear to have an increased risk for subsequent new-onset type 2 diabetes but not other types of diabetes, new data suggest.
“If confirmed, the results of the present study indicate that diabetes screening in individuals who have recovered from even mild COVID-19 should be recommended,” say Wolfgang Rathmann, MD, of the Leibniz Center for Diabetes Research at Heinrich Heine University, Düsseldorf, Germany, and colleagues.
The findings, from a nationwide primary care database in Germany, were recently published in Diabetologia.
These primary care data align with those from other studies of more seriously ill patients with COVID-19 that found increased rates of type 2 diabetes diagnoses in the subsequent months following illness, they point out.
“COVID-19 infection may lead to diabetes by upregulation of the immune system after remission, which may induce pancreatic beta-cell dysfunction and insulin resistance, or patients may have been at risk for developing diabetes due to having obesity or prediabetes, and the stress COVID-19 put on their bodies sped it up,” said Dr. Rathmann in a press release.
However, because the patients with COVID-19 in the study were only followed for about 3 months, “further follow-up is needed to understand whether type 2 diabetes after mild COVID-19 is just temporary and can be reversed after they have fully recovered or whether it leads to a chronic condition,” he noted.
Increase in type 2 diabetes 3 months after mild COVID-19
The retrospective cohort analysis was performed using data from the Disease Analyzer, a representative panel of 1,171 physician practices in Germany, from March 2020 to January 2021, with follow-up through July 2021.
Individuals with a history of COVID-19 or diabetes and those taking corticosteroids within 30 days after the index dates were excluded.
A total of 35,865 patients with confirmed SARS-CoV-2 infection were propensity score-matched on a one-to-one basis for sex, age, health insurance, and comorbidities with those who had acute respiratory tract infections (controls) but were COVID-19 negative. Median follow-up was 119 days for the COVID-19 group and 161 days for controls.
There was a 28% increased risk of type 2 diabetes for those who had COVID-19 versus controls (15.8 per 1,000 person-years vs. 12.3 per 1,000 person-years, respectively, which was significantly different, and an incidence rate ratio of 1.28).
The incidence of other types of diabetes or unspecified diabetes for the COVID-19 and control groups did not differ significantly (4.3 per 1,000 person-years vs. 3.7 per 1,000 person-years; IRR, 1.17).
Similar findings were seen in sensitivity analyses by glucose-lowering medication prescriptions and by ICD-10 codes.
Although type 2 diabetes is not likely to be a problem for the vast majority of people who have mild COVID-19, the authors recommend that anyone who has recovered from COVID-19 be aware of the warning signs and symptoms such as fatigue, frequent urination, and increased thirst, and seek treatment right away.
CoviDiab registry tracking type 1 and type 2 diabetes
Over the course of the pandemic, there have been conflicting data on whether COVID-19 induces or reveals a propensity for type 1 and type 2 diabetes.
The CoviDiab global registry is tracking this and will include diabetes type for adults and children.
The aim is to have “as many as possible cases of new-onset diabetes for which we can have also a minimum set of clinical data including type of diabetes and A1c,” coprincipal investigator Francesco Rubino, MD, of King’s College London, previously told this news organization.
“By looking at this information we can infer whether a role of COVID-19 in triggering diabetes is clinically plausible – or not – and what type of diabetes is most frequently associated with COVID-19.”
Rubino said that the CoviDiab team is approaching the data with the assumption that, at least in adults diagnosed with type 2 diabetes, the explanation might be that the person already had undiagnosed diabetes or the hyperglycemia may be stress-induced and temporary.
The German Diabetes Center is funded by the German Federal Ministry of Health and the Ministry of Culture and Science of the State of North Rhine-Westphalia. Dr. Rathmann has reported receiving consulting fees for attending educational sessions or advisory boards for AstraZeneca, Boehringer Ingelheim, and Novo Nordisk and institutional research grants from Novo Nordisk outside of the topic of the current work.
A version of this article first appeared on Medscape.com.
FROM DIABETOLOGIA
COVID-19 doesn’t spike A1c levels
Key takeaways
Results from a retrospective, observational, case-control study of more than 20,000 people from a single U.S. medical center showed a statistically significant but clinically insignificant increase in A1c in people following COVID-19 infection, in both those with and without diabetes.
After people received a diagnosis of COVID-19 infection, they were 40% more likely to also receive a diagnosis of type 2 diabetes, compared with people who tested negative for COVID-19, a difference that was significant and could be explained by the increased medical care received by people who test positive for COVID-19.
The risk of incident diabetic ketoacidosis (DKA) among people who tested positive for COVID-19 was significantly higher among those with pre-existing type 2 diabetes, those using insulin, and among Black individuals.
Why this matters
The authors said that their study is the first report of evidence that infection with COVID-19 affects A1c levels in a large, real-world clinical cohort.
Until now, the impact of COVID-19 infection on A1c remained unclear. Results from previous studies indicated that COVID-19 infection may increase A1c levels, but the studied cohorts were small and lacked uninfected controls.
The current study included 8,755 people infected with COVID-19, had data from both before and after the infection on diabetes status and A1c levels, and also included many matched, uninfected people who served as controls.
Study design
Data came from a Cleveland Clinic registry that included 81,093 people who tested positive for COVID-19 between March 2020 and May 2021 and 153,034 matched individuals who tested negative for COVID-19 during the same period.
The researchers retrospectively selected patients with an A1c recorded within 12 months before their COVID-19 test, as well as a second A1c value recorded within 12 months after COVID-19 testing. This produced a study cohort of 8,755 COVID-positive people and 11,998 matched people who tested negative for COVID-19.
To evaluate the risk of DKA onset after COVID-19 infection, the authors identified two sub-cohorts that excluded those with a history of DKA. The sub-cohorts were 701 people with type 1 diabetes and 21,830 with type 2 diabetes.
Key results
The investigators found a statistically significant but clinically insignificant A1c increase following a positive COVID-19 test, an average A1c increase of 0.06 percentage points. Those who tested negative for COVID-19 had a clinically insignificant change in their average A1c level that was of borderline statistical significance, an average increase of 0.02 percentage points (P = .05).
The statistically significant but clinically insignificant increase in A1c following infection with COVID-19 was similar in people with and without type 2 diabetes prior to infection.
In patients with type 2 diabetes who became infected with COVID-19, the researchers saw significant positive associations between higher A1c levels before infection and time to hospitalization (hazard ratio, 1.07), need for assisted breathing (HR, 1.06), and ICU admission (HR, 1.07).
Following a COVID-19 infection, people were 40% more likely to receive a diagnosis of incident type 2 diabetes, compared with matched uninfected people. The authors said a possible explanation is that after diagnosis of COVID-19, infected people in general received more intensified care that led to better identification of those with underlying type 2 diabetes.
The 701 people included with pre-existing type 1 diabetes showed no significant difference in their rate of developing DKA between those infected and not infected with COVID-19.
Among the 21,830 people with pre-existing type 2 diabetes, the DKA risk was a significant 35% greater for those who were infected with COVID-19, compared with those who were uninfected. The magnitude of this increased relative risk was even higher among the patients with type 2 diabetes who used insulin as part of their treatment.
The difference in DKA risk didn’t differ between Black and White patients who were not infected with COVID-19, but among those infected by COVID-19, Black patients were more than twice as likely to be diagnosed with DKA, compared with White patients, a significant difference.
Black patients with type 2 diabetes who became infected with COVID-19 had a significant (63%) increased rate of DKA compared with Black patients with type 2 diabetes who remained uninfected.
Limitations
The study included patients with A1c measurements made up to 12 months prior to their COVID-19 test, and hence comorbid conditions, medication changes during this period, or other factors may have affected subsequent A1c levels. To address this, the authors also assessed outcomes at 3- and 6-month intervals, which produced results consistent with the 12-month findings.
The researchers did not have A1c values for many of the more than 234,000 people in the entire registry who underwent COVID-19 testing from March 2020-May 2021 at the Cleveland Clinic, omissions that may have biased the study cohort.
This was a single-center study. Some patients may have received care outside of the center, hence records of those episodes could not be included.
Disclosures
The study received no commercial funding. Four authors received consulting and speaker honoraria and research funding from AstraZeneca, Bayer, Boehringer Ingelheim, Corcept Therapeutics, Diasome, Eli Lilly, Merck, Novo Nordisk, and Sanofi. Three authors have intellectual property related to treatment decisionmaking in the context of type 2 diabetes.
This is a summary of a preprint research study “Impacts of COVID-19 on glycemia and risk of diabetic ketoacidosis,” written by researchers at the Cleveland Clinic on medRxiv. The study has not yet been peer reviewed. The full text of the study can be found on medRxiv.org.
A version of this article first appeared on Medscape.com.
Key takeaways
Results from a retrospective, observational, case-control study of more than 20,000 people from a single U.S. medical center showed a statistically significant but clinically insignificant increase in A1c in people following COVID-19 infection, in both those with and without diabetes.
After people received a diagnosis of COVID-19 infection, they were 40% more likely to also receive a diagnosis of type 2 diabetes, compared with people who tested negative for COVID-19, a difference that was significant and could be explained by the increased medical care received by people who test positive for COVID-19.
The risk of incident diabetic ketoacidosis (DKA) among people who tested positive for COVID-19 was significantly higher among those with pre-existing type 2 diabetes, those using insulin, and among Black individuals.
Why this matters
The authors said that their study is the first report of evidence that infection with COVID-19 affects A1c levels in a large, real-world clinical cohort.
Until now, the impact of COVID-19 infection on A1c remained unclear. Results from previous studies indicated that COVID-19 infection may increase A1c levels, but the studied cohorts were small and lacked uninfected controls.
The current study included 8,755 people infected with COVID-19, had data from both before and after the infection on diabetes status and A1c levels, and also included many matched, uninfected people who served as controls.
Study design
Data came from a Cleveland Clinic registry that included 81,093 people who tested positive for COVID-19 between March 2020 and May 2021 and 153,034 matched individuals who tested negative for COVID-19 during the same period.
The researchers retrospectively selected patients with an A1c recorded within 12 months before their COVID-19 test, as well as a second A1c value recorded within 12 months after COVID-19 testing. This produced a study cohort of 8,755 COVID-positive people and 11,998 matched people who tested negative for COVID-19.
To evaluate the risk of DKA onset after COVID-19 infection, the authors identified two sub-cohorts that excluded those with a history of DKA. The sub-cohorts were 701 people with type 1 diabetes and 21,830 with type 2 diabetes.
Key results
The investigators found a statistically significant but clinically insignificant A1c increase following a positive COVID-19 test, an average A1c increase of 0.06 percentage points. Those who tested negative for COVID-19 had a clinically insignificant change in their average A1c level that was of borderline statistical significance, an average increase of 0.02 percentage points (P = .05).
The statistically significant but clinically insignificant increase in A1c following infection with COVID-19 was similar in people with and without type 2 diabetes prior to infection.
In patients with type 2 diabetes who became infected with COVID-19, the researchers saw significant positive associations between higher A1c levels before infection and time to hospitalization (hazard ratio, 1.07), need for assisted breathing (HR, 1.06), and ICU admission (HR, 1.07).
Following a COVID-19 infection, people were 40% more likely to receive a diagnosis of incident type 2 diabetes, compared with matched uninfected people. The authors said a possible explanation is that after diagnosis of COVID-19, infected people in general received more intensified care that led to better identification of those with underlying type 2 diabetes.
The 701 people included with pre-existing type 1 diabetes showed no significant difference in their rate of developing DKA between those infected and not infected with COVID-19.
Among the 21,830 people with pre-existing type 2 diabetes, the DKA risk was a significant 35% greater for those who were infected with COVID-19, compared with those who were uninfected. The magnitude of this increased relative risk was even higher among the patients with type 2 diabetes who used insulin as part of their treatment.
The difference in DKA risk didn’t differ between Black and White patients who were not infected with COVID-19, but among those infected by COVID-19, Black patients were more than twice as likely to be diagnosed with DKA, compared with White patients, a significant difference.
Black patients with type 2 diabetes who became infected with COVID-19 had a significant (63%) increased rate of DKA compared with Black patients with type 2 diabetes who remained uninfected.
Limitations
The study included patients with A1c measurements made up to 12 months prior to their COVID-19 test, and hence comorbid conditions, medication changes during this period, or other factors may have affected subsequent A1c levels. To address this, the authors also assessed outcomes at 3- and 6-month intervals, which produced results consistent with the 12-month findings.
The researchers did not have A1c values for many of the more than 234,000 people in the entire registry who underwent COVID-19 testing from March 2020-May 2021 at the Cleveland Clinic, omissions that may have biased the study cohort.
This was a single-center study. Some patients may have received care outside of the center, hence records of those episodes could not be included.
Disclosures
The study received no commercial funding. Four authors received consulting and speaker honoraria and research funding from AstraZeneca, Bayer, Boehringer Ingelheim, Corcept Therapeutics, Diasome, Eli Lilly, Merck, Novo Nordisk, and Sanofi. Three authors have intellectual property related to treatment decisionmaking in the context of type 2 diabetes.
This is a summary of a preprint research study “Impacts of COVID-19 on glycemia and risk of diabetic ketoacidosis,” written by researchers at the Cleveland Clinic on medRxiv. The study has not yet been peer reviewed. The full text of the study can be found on medRxiv.org.
A version of this article first appeared on Medscape.com.
Key takeaways
Results from a retrospective, observational, case-control study of more than 20,000 people from a single U.S. medical center showed a statistically significant but clinically insignificant increase in A1c in people following COVID-19 infection, in both those with and without diabetes.
After people received a diagnosis of COVID-19 infection, they were 40% more likely to also receive a diagnosis of type 2 diabetes, compared with people who tested negative for COVID-19, a difference that was significant and could be explained by the increased medical care received by people who test positive for COVID-19.
The risk of incident diabetic ketoacidosis (DKA) among people who tested positive for COVID-19 was significantly higher among those with pre-existing type 2 diabetes, those using insulin, and among Black individuals.
Why this matters
The authors said that their study is the first report of evidence that infection with COVID-19 affects A1c levels in a large, real-world clinical cohort.
Until now, the impact of COVID-19 infection on A1c remained unclear. Results from previous studies indicated that COVID-19 infection may increase A1c levels, but the studied cohorts were small and lacked uninfected controls.
The current study included 8,755 people infected with COVID-19, had data from both before and after the infection on diabetes status and A1c levels, and also included many matched, uninfected people who served as controls.
Study design
Data came from a Cleveland Clinic registry that included 81,093 people who tested positive for COVID-19 between March 2020 and May 2021 and 153,034 matched individuals who tested negative for COVID-19 during the same period.
The researchers retrospectively selected patients with an A1c recorded within 12 months before their COVID-19 test, as well as a second A1c value recorded within 12 months after COVID-19 testing. This produced a study cohort of 8,755 COVID-positive people and 11,998 matched people who tested negative for COVID-19.
To evaluate the risk of DKA onset after COVID-19 infection, the authors identified two sub-cohorts that excluded those with a history of DKA. The sub-cohorts were 701 people with type 1 diabetes and 21,830 with type 2 diabetes.
Key results
The investigators found a statistically significant but clinically insignificant A1c increase following a positive COVID-19 test, an average A1c increase of 0.06 percentage points. Those who tested negative for COVID-19 had a clinically insignificant change in their average A1c level that was of borderline statistical significance, an average increase of 0.02 percentage points (P = .05).
The statistically significant but clinically insignificant increase in A1c following infection with COVID-19 was similar in people with and without type 2 diabetes prior to infection.
In patients with type 2 diabetes who became infected with COVID-19, the researchers saw significant positive associations between higher A1c levels before infection and time to hospitalization (hazard ratio, 1.07), need for assisted breathing (HR, 1.06), and ICU admission (HR, 1.07).
Following a COVID-19 infection, people were 40% more likely to receive a diagnosis of incident type 2 diabetes, compared with matched uninfected people. The authors said a possible explanation is that after diagnosis of COVID-19, infected people in general received more intensified care that led to better identification of those with underlying type 2 diabetes.
The 701 people included with pre-existing type 1 diabetes showed no significant difference in their rate of developing DKA between those infected and not infected with COVID-19.
Among the 21,830 people with pre-existing type 2 diabetes, the DKA risk was a significant 35% greater for those who were infected with COVID-19, compared with those who were uninfected. The magnitude of this increased relative risk was even higher among the patients with type 2 diabetes who used insulin as part of their treatment.
The difference in DKA risk didn’t differ between Black and White patients who were not infected with COVID-19, but among those infected by COVID-19, Black patients were more than twice as likely to be diagnosed with DKA, compared with White patients, a significant difference.
Black patients with type 2 diabetes who became infected with COVID-19 had a significant (63%) increased rate of DKA compared with Black patients with type 2 diabetes who remained uninfected.
Limitations
The study included patients with A1c measurements made up to 12 months prior to their COVID-19 test, and hence comorbid conditions, medication changes during this period, or other factors may have affected subsequent A1c levels. To address this, the authors also assessed outcomes at 3- and 6-month intervals, which produced results consistent with the 12-month findings.
The researchers did not have A1c values for many of the more than 234,000 people in the entire registry who underwent COVID-19 testing from March 2020-May 2021 at the Cleveland Clinic, omissions that may have biased the study cohort.
This was a single-center study. Some patients may have received care outside of the center, hence records of those episodes could not be included.
Disclosures
The study received no commercial funding. Four authors received consulting and speaker honoraria and research funding from AstraZeneca, Bayer, Boehringer Ingelheim, Corcept Therapeutics, Diasome, Eli Lilly, Merck, Novo Nordisk, and Sanofi. Three authors have intellectual property related to treatment decisionmaking in the context of type 2 diabetes.
This is a summary of a preprint research study “Impacts of COVID-19 on glycemia and risk of diabetic ketoacidosis,” written by researchers at the Cleveland Clinic on medRxiv. The study has not yet been peer reviewed. The full text of the study can be found on medRxiv.org.
A version of this article first appeared on Medscape.com.
U.S. health officials tracking COVID-19 increase in U.K.
according to NPR.
Daily cases counts have increased 38% in the past week, according to the latest data from the U.K. Health Security Agency. Hospitalizations are up about 25% as well.
“Over the last year or so, what happens in the U.K. usually happens here a few weeks later,” Anthony S. Fauci, MD, director of the National Institute of Allergy and Infectious Diseases, told NPR.
“And right now, the U.K. is seeing somewhat of a rebound in cases,” he said.
Health officials in the United Kingdom have noted the latest increase is likely due to the contagious BA.2 Omicron subvariant, the recent loosening of coronavirus restrictions, and waning immunity from vaccinations and infections.
“All three of those factors we have here in the United States,” Dr. Fauci said. “So I would not be surprised if, in the next few weeks, we see either a plateauing … of cases or even [the curve] rebounds and slightly goes up.”
Right now, COVID-19 cases in the United Stastes have dropped to their lowest levels since July 2021, according to the latest Centers for Disease Control and Prevention data, with fewer than 30,000 daily cases. At the same time, the rate of decline in cases has slowed significantly and is beginning to plateau.
Public health experts are also pointing to wastewater surveillance data that shows an uptick in viral activity across the country. The CDC’s wastewater dashboard indicates that about 35% of sites that monitor wastewater are seeing an increase, with consistent growth in Florida, Rhode Island, and West Virginia.
“The power of wastewater surveillance is that it’s an early warning system,” Amy Kirby, the program lead for the CDC’s National Wastewater Surveillance System, told NPR.
“We are seeing evidence of increases in some communities across the country,” she said. “What looked like noise at the beginning of the week is starting to look like a true signal here at the end of the week.”
The wastewater system doesn’t distinguish between Omicron and subvariants such as BA.2. However, other CDC data has found an increase in BA.2 cases in the United States, making up about a quarter of new COVID-19 cases.
The BA.2 variant has roughly doubled each week for the last month, which means it could become the dominant coronavirus strain in the United States in coming weeks, according to USA Today. Cases appear to be spreading more quickly in the Northeast and West, making up about 39% of cases in New York and New Jersey last week.
BA.2 also accounts for nearly 39% of cases across the Northeast, including Connecticut, Maine, Massachusetts, New Hampshire, Rhode Island and Vermont, USA Today reported. In the West, which includes Arizona, California and Nevada, the subvariant makes up about 28% of new cases. In the upper West, which includes Alaska, Oregon and Washington, about 26% of cases are BA.2.
The good news is that BA.2 “doesn’t seem to evade our vaccines or immunity any more than the prior Omicron [variant]. And it doesn’t seem to lead to any more increased severity of disease,” Rochelle Walensky, MD, the CDC director, told NPR’s Morning Edition on March 18.
The effects of BA.2 will likely depend on the immunity profile in the United States, including how long it’s been since someone was vaccinated, boosted, or recovered from an infection, she said.
Health officials are watching other countries with BA.2 increases, such as Germany, Italy, and the Netherlands. Many European countries have been reporting an uptick but not implementing major restrictions or shutdowns, USA Today reported.
The BA.2 variant likely won’t lead to a major surge in severe disease or strict COVID-19 measures, Dr. Fauci told NPR, but some coronavirus protocols may need to be implemented again if cases grow dramatically.
“We must be ready to pivot and, if necessary, to go back to stricter mitigation with regard to masks,” he said.
A version of this article first appeared on WebMD.com.
according to NPR.
Daily cases counts have increased 38% in the past week, according to the latest data from the U.K. Health Security Agency. Hospitalizations are up about 25% as well.
“Over the last year or so, what happens in the U.K. usually happens here a few weeks later,” Anthony S. Fauci, MD, director of the National Institute of Allergy and Infectious Diseases, told NPR.
“And right now, the U.K. is seeing somewhat of a rebound in cases,” he said.
Health officials in the United Kingdom have noted the latest increase is likely due to the contagious BA.2 Omicron subvariant, the recent loosening of coronavirus restrictions, and waning immunity from vaccinations and infections.
“All three of those factors we have here in the United States,” Dr. Fauci said. “So I would not be surprised if, in the next few weeks, we see either a plateauing … of cases or even [the curve] rebounds and slightly goes up.”
Right now, COVID-19 cases in the United Stastes have dropped to their lowest levels since July 2021, according to the latest Centers for Disease Control and Prevention data, with fewer than 30,000 daily cases. At the same time, the rate of decline in cases has slowed significantly and is beginning to plateau.
Public health experts are also pointing to wastewater surveillance data that shows an uptick in viral activity across the country. The CDC’s wastewater dashboard indicates that about 35% of sites that monitor wastewater are seeing an increase, with consistent growth in Florida, Rhode Island, and West Virginia.
“The power of wastewater surveillance is that it’s an early warning system,” Amy Kirby, the program lead for the CDC’s National Wastewater Surveillance System, told NPR.
“We are seeing evidence of increases in some communities across the country,” she said. “What looked like noise at the beginning of the week is starting to look like a true signal here at the end of the week.”
The wastewater system doesn’t distinguish between Omicron and subvariants such as BA.2. However, other CDC data has found an increase in BA.2 cases in the United States, making up about a quarter of new COVID-19 cases.
The BA.2 variant has roughly doubled each week for the last month, which means it could become the dominant coronavirus strain in the United States in coming weeks, according to USA Today. Cases appear to be spreading more quickly in the Northeast and West, making up about 39% of cases in New York and New Jersey last week.
BA.2 also accounts for nearly 39% of cases across the Northeast, including Connecticut, Maine, Massachusetts, New Hampshire, Rhode Island and Vermont, USA Today reported. In the West, which includes Arizona, California and Nevada, the subvariant makes up about 28% of new cases. In the upper West, which includes Alaska, Oregon and Washington, about 26% of cases are BA.2.
The good news is that BA.2 “doesn’t seem to evade our vaccines or immunity any more than the prior Omicron [variant]. And it doesn’t seem to lead to any more increased severity of disease,” Rochelle Walensky, MD, the CDC director, told NPR’s Morning Edition on March 18.
The effects of BA.2 will likely depend on the immunity profile in the United States, including how long it’s been since someone was vaccinated, boosted, or recovered from an infection, she said.
Health officials are watching other countries with BA.2 increases, such as Germany, Italy, and the Netherlands. Many European countries have been reporting an uptick but not implementing major restrictions or shutdowns, USA Today reported.
The BA.2 variant likely won’t lead to a major surge in severe disease or strict COVID-19 measures, Dr. Fauci told NPR, but some coronavirus protocols may need to be implemented again if cases grow dramatically.
“We must be ready to pivot and, if necessary, to go back to stricter mitigation with regard to masks,” he said.
A version of this article first appeared on WebMD.com.
according to NPR.
Daily cases counts have increased 38% in the past week, according to the latest data from the U.K. Health Security Agency. Hospitalizations are up about 25% as well.
“Over the last year or so, what happens in the U.K. usually happens here a few weeks later,” Anthony S. Fauci, MD, director of the National Institute of Allergy and Infectious Diseases, told NPR.
“And right now, the U.K. is seeing somewhat of a rebound in cases,” he said.
Health officials in the United Kingdom have noted the latest increase is likely due to the contagious BA.2 Omicron subvariant, the recent loosening of coronavirus restrictions, and waning immunity from vaccinations and infections.
“All three of those factors we have here in the United States,” Dr. Fauci said. “So I would not be surprised if, in the next few weeks, we see either a plateauing … of cases or even [the curve] rebounds and slightly goes up.”
Right now, COVID-19 cases in the United Stastes have dropped to their lowest levels since July 2021, according to the latest Centers for Disease Control and Prevention data, with fewer than 30,000 daily cases. At the same time, the rate of decline in cases has slowed significantly and is beginning to plateau.
Public health experts are also pointing to wastewater surveillance data that shows an uptick in viral activity across the country. The CDC’s wastewater dashboard indicates that about 35% of sites that monitor wastewater are seeing an increase, with consistent growth in Florida, Rhode Island, and West Virginia.
“The power of wastewater surveillance is that it’s an early warning system,” Amy Kirby, the program lead for the CDC’s National Wastewater Surveillance System, told NPR.
“We are seeing evidence of increases in some communities across the country,” she said. “What looked like noise at the beginning of the week is starting to look like a true signal here at the end of the week.”
The wastewater system doesn’t distinguish between Omicron and subvariants such as BA.2. However, other CDC data has found an increase in BA.2 cases in the United States, making up about a quarter of new COVID-19 cases.
The BA.2 variant has roughly doubled each week for the last month, which means it could become the dominant coronavirus strain in the United States in coming weeks, according to USA Today. Cases appear to be spreading more quickly in the Northeast and West, making up about 39% of cases in New York and New Jersey last week.
BA.2 also accounts for nearly 39% of cases across the Northeast, including Connecticut, Maine, Massachusetts, New Hampshire, Rhode Island and Vermont, USA Today reported. In the West, which includes Arizona, California and Nevada, the subvariant makes up about 28% of new cases. In the upper West, which includes Alaska, Oregon and Washington, about 26% of cases are BA.2.
The good news is that BA.2 “doesn’t seem to evade our vaccines or immunity any more than the prior Omicron [variant]. And it doesn’t seem to lead to any more increased severity of disease,” Rochelle Walensky, MD, the CDC director, told NPR’s Morning Edition on March 18.
The effects of BA.2 will likely depend on the immunity profile in the United States, including how long it’s been since someone was vaccinated, boosted, or recovered from an infection, she said.
Health officials are watching other countries with BA.2 increases, such as Germany, Italy, and the Netherlands. Many European countries have been reporting an uptick but not implementing major restrictions or shutdowns, USA Today reported.
The BA.2 variant likely won’t lead to a major surge in severe disease or strict COVID-19 measures, Dr. Fauci told NPR, but some coronavirus protocols may need to be implemented again if cases grow dramatically.
“We must be ready to pivot and, if necessary, to go back to stricter mitigation with regard to masks,” he said.
A version of this article first appeared on WebMD.com.
COVID surge in Western Europe puts U.S. health experts on alert
, even as states and cities continue to lift restrictions amid low case numbers.
Infectious disease experts are watching BA.2, the Omicron subvariant that appears to be more transmissible than the original strain. BA.2 is fueling outbreaks across Europe and is growing in dominance across the United States.
“It’s picking up steam. It’s across at least 12 countries … from Finland to Greece,” Eric Topol, MD, director of the Scripps Research Translational Institute, told The Washington Post.
He has been following the surge and has posted recent charts of the outbreak on Twitter. Hospitalizations appear to be increasing in some places as well, he noted, despite the higher vaccination rates of many Western European countries.
“There’s no question there’s a significant wave there,” Dr. Topol said.
Germany recorded more than 260,000 new cases on March 15, according to the data tracker from the New York Times, but coronavirus restrictions are still being lifted this week. The U.K. is reporting more than 75,000 daily cases, and the Netherlands is reporting more than 60,000 daily cases, which are considered major numbers, compared to their population sizes. Meanwhile, France, Italy, and Switzerland are also reporting large increases in infections.
During the past 2 years, widespread outbreaks in Europe have been followed by similar surges in the U.S. weeks later. Most experts interviewed by the Post predicted that it’s likely to happen again.
In the United States, the BA.2 subvariant accounted for 23% of new COVID-19 cases for the week ending March 12, according to the latest estimate from the Centers for Disease Control and Prevention, while the original Omicron strain made up about 66% of cases. The BA.2 percentage is up from 13.7% of new cases for the week ending March 5, 7.1% the previous week, and 4.1% the week before that. In parts of the Northeast and New England, BA.2 makes up more than 38% of new cases.
At the same time, the 7 -day average of COVID-19 cases continues to drop in the United States, with about 31,000 daily cases currently, the New York Times data tracker shows. About 25,000 COVID-19 patients are hospitalized across the country, which has fallen 44% in the past 2 weeks, and about 1,200 deaths are being reported daily.
Several variables could affect the course of a future surge, the Post reported. Vaccination rates, coronavirus safety protocols, and access to antiviral medications could dictate how another wave unfolds across the country.
About 82% of the eligible U.S. population has received at least one vaccine dose, and 69% is fully vaccinated, according to the latest CDC data. About half of those who are eligible for booster doses have received one. In Germany, nearly 76% of people are fully vaccinated, the newspaper reported, and in the United Kingdom, about 74% are fully vaccinated.
Health experts are also considering how natural immunity from a previous infection could affect a BA.2 surge. Millions of Americans were infected with the original Omicron strain, BA.1, which could provide protection. That said, researchers aren’t quite sure whether BA.1 infection protects against BA.2.
“It’s like a weather alert. Right now, the skies are sunny and bright, and we hope they stay that way,” Michael Osterholm, PhD, director of the University of Minnesota’s Center for Infectious Disease Research and Policy, told CNN.
“But we could have some bad weather by evening,” he said. “We just don’t know.”
A version of this article first appeared on WebMD.com.
, even as states and cities continue to lift restrictions amid low case numbers.
Infectious disease experts are watching BA.2, the Omicron subvariant that appears to be more transmissible than the original strain. BA.2 is fueling outbreaks across Europe and is growing in dominance across the United States.
“It’s picking up steam. It’s across at least 12 countries … from Finland to Greece,” Eric Topol, MD, director of the Scripps Research Translational Institute, told The Washington Post.
He has been following the surge and has posted recent charts of the outbreak on Twitter. Hospitalizations appear to be increasing in some places as well, he noted, despite the higher vaccination rates of many Western European countries.
“There’s no question there’s a significant wave there,” Dr. Topol said.
Germany recorded more than 260,000 new cases on March 15, according to the data tracker from the New York Times, but coronavirus restrictions are still being lifted this week. The U.K. is reporting more than 75,000 daily cases, and the Netherlands is reporting more than 60,000 daily cases, which are considered major numbers, compared to their population sizes. Meanwhile, France, Italy, and Switzerland are also reporting large increases in infections.
During the past 2 years, widespread outbreaks in Europe have been followed by similar surges in the U.S. weeks later. Most experts interviewed by the Post predicted that it’s likely to happen again.
In the United States, the BA.2 subvariant accounted for 23% of new COVID-19 cases for the week ending March 12, according to the latest estimate from the Centers for Disease Control and Prevention, while the original Omicron strain made up about 66% of cases. The BA.2 percentage is up from 13.7% of new cases for the week ending March 5, 7.1% the previous week, and 4.1% the week before that. In parts of the Northeast and New England, BA.2 makes up more than 38% of new cases.
At the same time, the 7 -day average of COVID-19 cases continues to drop in the United States, with about 31,000 daily cases currently, the New York Times data tracker shows. About 25,000 COVID-19 patients are hospitalized across the country, which has fallen 44% in the past 2 weeks, and about 1,200 deaths are being reported daily.
Several variables could affect the course of a future surge, the Post reported. Vaccination rates, coronavirus safety protocols, and access to antiviral medications could dictate how another wave unfolds across the country.
About 82% of the eligible U.S. population has received at least one vaccine dose, and 69% is fully vaccinated, according to the latest CDC data. About half of those who are eligible for booster doses have received one. In Germany, nearly 76% of people are fully vaccinated, the newspaper reported, and in the United Kingdom, about 74% are fully vaccinated.
Health experts are also considering how natural immunity from a previous infection could affect a BA.2 surge. Millions of Americans were infected with the original Omicron strain, BA.1, which could provide protection. That said, researchers aren’t quite sure whether BA.1 infection protects against BA.2.
“It’s like a weather alert. Right now, the skies are sunny and bright, and we hope they stay that way,” Michael Osterholm, PhD, director of the University of Minnesota’s Center for Infectious Disease Research and Policy, told CNN.
“But we could have some bad weather by evening,” he said. “We just don’t know.”
A version of this article first appeared on WebMD.com.
, even as states and cities continue to lift restrictions amid low case numbers.
Infectious disease experts are watching BA.2, the Omicron subvariant that appears to be more transmissible than the original strain. BA.2 is fueling outbreaks across Europe and is growing in dominance across the United States.
“It’s picking up steam. It’s across at least 12 countries … from Finland to Greece,” Eric Topol, MD, director of the Scripps Research Translational Institute, told The Washington Post.
He has been following the surge and has posted recent charts of the outbreak on Twitter. Hospitalizations appear to be increasing in some places as well, he noted, despite the higher vaccination rates of many Western European countries.
“There’s no question there’s a significant wave there,” Dr. Topol said.
Germany recorded more than 260,000 new cases on March 15, according to the data tracker from the New York Times, but coronavirus restrictions are still being lifted this week. The U.K. is reporting more than 75,000 daily cases, and the Netherlands is reporting more than 60,000 daily cases, which are considered major numbers, compared to their population sizes. Meanwhile, France, Italy, and Switzerland are also reporting large increases in infections.
During the past 2 years, widespread outbreaks in Europe have been followed by similar surges in the U.S. weeks later. Most experts interviewed by the Post predicted that it’s likely to happen again.
In the United States, the BA.2 subvariant accounted for 23% of new COVID-19 cases for the week ending March 12, according to the latest estimate from the Centers for Disease Control and Prevention, while the original Omicron strain made up about 66% of cases. The BA.2 percentage is up from 13.7% of new cases for the week ending March 5, 7.1% the previous week, and 4.1% the week before that. In parts of the Northeast and New England, BA.2 makes up more than 38% of new cases.
At the same time, the 7 -day average of COVID-19 cases continues to drop in the United States, with about 31,000 daily cases currently, the New York Times data tracker shows. About 25,000 COVID-19 patients are hospitalized across the country, which has fallen 44% in the past 2 weeks, and about 1,200 deaths are being reported daily.
Several variables could affect the course of a future surge, the Post reported. Vaccination rates, coronavirus safety protocols, and access to antiviral medications could dictate how another wave unfolds across the country.
About 82% of the eligible U.S. population has received at least one vaccine dose, and 69% is fully vaccinated, according to the latest CDC data. About half of those who are eligible for booster doses have received one. In Germany, nearly 76% of people are fully vaccinated, the newspaper reported, and in the United Kingdom, about 74% are fully vaccinated.
Health experts are also considering how natural immunity from a previous infection could affect a BA.2 surge. Millions of Americans were infected with the original Omicron strain, BA.1, which could provide protection. That said, researchers aren’t quite sure whether BA.1 infection protects against BA.2.
“It’s like a weather alert. Right now, the skies are sunny and bright, and we hope they stay that way,” Michael Osterholm, PhD, director of the University of Minnesota’s Center for Infectious Disease Research and Policy, told CNN.
“But we could have some bad weather by evening,” he said. “We just don’t know.”
A version of this article first appeared on WebMD.com.
Waiting for the under-5 COVID-19 vaccine
In February, citing the need for more data, Pfizer and BioNTech announced that they were delaying the application for their COVID-19 vaccine for children under the age of 5. Earlier evidence suggests that two doses may not provide adequate protection in the 2- to 4-year old age group. With the larger number of infections and illness in the younger age group from the Omicron variant, Pfizer and BioNTech felt they needed more data on the effectiveness of a third dose.
This delay came as a disappointment to parents of children under 5 who have been eager to have them receive the vaccination. However, Peter Marks, MD, director of the Center for Biologics Evaluation and Research at the Food and Drug Administration, told parents that this delay should be reassuring – that the companies were doing important due diligence before releasing a product that is both safe and effective. The American Academy of Pediatrics wisely released a similar statement of reassurance and support.
It is difficult to know how many parents will eventually immunize their young children once the vaccine is approved. Any survey done more than a few weeks ago must be viewed cautiously as “the COVID numbers” around the country continue to improve and parental attitudes are likely to change.
There will always remain subgroups of parents on either extreme of the bell-shaped curve. Some will reject the under-5 vaccine simply because it is a vaccine. Some parents are so anxious to vaccinate that they will want to be first in line even if waiting is the more prudent approach. In a recent opinion piece appearing in the New York Times, a statistician writes that he is so eager to have his young children immunized that he is encouraging the FDA to replace its traditional reliance on “statistical significance” with a less rigid and binary method such as one based on Bayesian theory (Aubrey Carlton, “I’m a parent and a statistician. There’s a smarter way to think about the under-5 vaccine.” The New York Times. 2022 Mar 1.). However, what this statistician misses in his haste to vaccinate his own children is that we are dealing with an entire population with varying levels of scientific sophistication and appetite for risk. While “statistical significance” may no longer be cutting edge to some statisticians, most of the rest of the country finds the term reassuring.
It will be interesting to see what happens if and when the vaccine is approved. Will the American Academy of Pediatrics come out with a strong recommendation? I hope they are careful and provide a sufficient number of caveats, otherwise we in the trenches will again be left to provide more nuanced advice to families who are both anxious and hesitant.
Despite the recent surge in cases among young children, apparently as a result of the Omicron variant, the disease continues to cause less and milder disease among young children than it does in adults. And the degree to which illness in the pediatric population contributes to the health of the general population appears to still be a matter of debate. This may be yet another instance of when the crafty COVID-19 has moved with a pace that will make an under–age-5 vaccine of relatively little value.
First, we must be careful to assure ourselves that any side effects the vaccine might generate are well within an even more restricted acceptable range. Second, we must be careful not to squander our persuasive currency by promoting a vaccine that in retrospect may turn out to be of relatively little value.
Although there is ample evidence that education often fails to convince the committed anti-vaxxers, pediatricians continue to be held in high regard by most parents, many of whom are understandably confused by the tsunami of health information of mixed quality generated by the pandemic. We must be cautious not to cast ourselves as a group whose knee-jerk reaction is to recommend every vaccine with equal vigor. All vaccines are not created equal. We must be patient and prepared to adjust the level of our enthusiasm. We must continue to tailor our advice based on the hard data. Otherwise, parents will stop asking for our advice because they will believe that they already know what we’re going to say.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at [email protected].
In February, citing the need for more data, Pfizer and BioNTech announced that they were delaying the application for their COVID-19 vaccine for children under the age of 5. Earlier evidence suggests that two doses may not provide adequate protection in the 2- to 4-year old age group. With the larger number of infections and illness in the younger age group from the Omicron variant, Pfizer and BioNTech felt they needed more data on the effectiveness of a third dose.
This delay came as a disappointment to parents of children under 5 who have been eager to have them receive the vaccination. However, Peter Marks, MD, director of the Center for Biologics Evaluation and Research at the Food and Drug Administration, told parents that this delay should be reassuring – that the companies were doing important due diligence before releasing a product that is both safe and effective. The American Academy of Pediatrics wisely released a similar statement of reassurance and support.
It is difficult to know how many parents will eventually immunize their young children once the vaccine is approved. Any survey done more than a few weeks ago must be viewed cautiously as “the COVID numbers” around the country continue to improve and parental attitudes are likely to change.
There will always remain subgroups of parents on either extreme of the bell-shaped curve. Some will reject the under-5 vaccine simply because it is a vaccine. Some parents are so anxious to vaccinate that they will want to be first in line even if waiting is the more prudent approach. In a recent opinion piece appearing in the New York Times, a statistician writes that he is so eager to have his young children immunized that he is encouraging the FDA to replace its traditional reliance on “statistical significance” with a less rigid and binary method such as one based on Bayesian theory (Aubrey Carlton, “I’m a parent and a statistician. There’s a smarter way to think about the under-5 vaccine.” The New York Times. 2022 Mar 1.). However, what this statistician misses in his haste to vaccinate his own children is that we are dealing with an entire population with varying levels of scientific sophistication and appetite for risk. While “statistical significance” may no longer be cutting edge to some statisticians, most of the rest of the country finds the term reassuring.
It will be interesting to see what happens if and when the vaccine is approved. Will the American Academy of Pediatrics come out with a strong recommendation? I hope they are careful and provide a sufficient number of caveats, otherwise we in the trenches will again be left to provide more nuanced advice to families who are both anxious and hesitant.
Despite the recent surge in cases among young children, apparently as a result of the Omicron variant, the disease continues to cause less and milder disease among young children than it does in adults. And the degree to which illness in the pediatric population contributes to the health of the general population appears to still be a matter of debate. This may be yet another instance of when the crafty COVID-19 has moved with a pace that will make an under–age-5 vaccine of relatively little value.
First, we must be careful to assure ourselves that any side effects the vaccine might generate are well within an even more restricted acceptable range. Second, we must be careful not to squander our persuasive currency by promoting a vaccine that in retrospect may turn out to be of relatively little value.
Although there is ample evidence that education often fails to convince the committed anti-vaxxers, pediatricians continue to be held in high regard by most parents, many of whom are understandably confused by the tsunami of health information of mixed quality generated by the pandemic. We must be cautious not to cast ourselves as a group whose knee-jerk reaction is to recommend every vaccine with equal vigor. All vaccines are not created equal. We must be patient and prepared to adjust the level of our enthusiasm. We must continue to tailor our advice based on the hard data. Otherwise, parents will stop asking for our advice because they will believe that they already know what we’re going to say.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at [email protected].
In February, citing the need for more data, Pfizer and BioNTech announced that they were delaying the application for their COVID-19 vaccine for children under the age of 5. Earlier evidence suggests that two doses may not provide adequate protection in the 2- to 4-year old age group. With the larger number of infections and illness in the younger age group from the Omicron variant, Pfizer and BioNTech felt they needed more data on the effectiveness of a third dose.
This delay came as a disappointment to parents of children under 5 who have been eager to have them receive the vaccination. However, Peter Marks, MD, director of the Center for Biologics Evaluation and Research at the Food and Drug Administration, told parents that this delay should be reassuring – that the companies were doing important due diligence before releasing a product that is both safe and effective. The American Academy of Pediatrics wisely released a similar statement of reassurance and support.
It is difficult to know how many parents will eventually immunize their young children once the vaccine is approved. Any survey done more than a few weeks ago must be viewed cautiously as “the COVID numbers” around the country continue to improve and parental attitudes are likely to change.
There will always remain subgroups of parents on either extreme of the bell-shaped curve. Some will reject the under-5 vaccine simply because it is a vaccine. Some parents are so anxious to vaccinate that they will want to be first in line even if waiting is the more prudent approach. In a recent opinion piece appearing in the New York Times, a statistician writes that he is so eager to have his young children immunized that he is encouraging the FDA to replace its traditional reliance on “statistical significance” with a less rigid and binary method such as one based on Bayesian theory (Aubrey Carlton, “I’m a parent and a statistician. There’s a smarter way to think about the under-5 vaccine.” The New York Times. 2022 Mar 1.). However, what this statistician misses in his haste to vaccinate his own children is that we are dealing with an entire population with varying levels of scientific sophistication and appetite for risk. While “statistical significance” may no longer be cutting edge to some statisticians, most of the rest of the country finds the term reassuring.
It will be interesting to see what happens if and when the vaccine is approved. Will the American Academy of Pediatrics come out with a strong recommendation? I hope they are careful and provide a sufficient number of caveats, otherwise we in the trenches will again be left to provide more nuanced advice to families who are both anxious and hesitant.
Despite the recent surge in cases among young children, apparently as a result of the Omicron variant, the disease continues to cause less and milder disease among young children than it does in adults. And the degree to which illness in the pediatric population contributes to the health of the general population appears to still be a matter of debate. This may be yet another instance of when the crafty COVID-19 has moved with a pace that will make an under–age-5 vaccine of relatively little value.
First, we must be careful to assure ourselves that any side effects the vaccine might generate are well within an even more restricted acceptable range. Second, we must be careful not to squander our persuasive currency by promoting a vaccine that in retrospect may turn out to be of relatively little value.
Although there is ample evidence that education often fails to convince the committed anti-vaxxers, pediatricians continue to be held in high regard by most parents, many of whom are understandably confused by the tsunami of health information of mixed quality generated by the pandemic. We must be cautious not to cast ourselves as a group whose knee-jerk reaction is to recommend every vaccine with equal vigor. All vaccines are not created equal. We must be patient and prepared to adjust the level of our enthusiasm. We must continue to tailor our advice based on the hard data. Otherwise, parents will stop asking for our advice because they will believe that they already know what we’re going to say.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at [email protected].
New ACC guidance on cardiovascular consequences of COVID-19
The American College of Cardiology has issued an expert consensus clinical guidance document for the evaluation and management of adults with key cardiovascular consequences of COVID-19.
The document makes recommendations on how to evaluate and manage COVID-associated myocarditis and long COVID and gives advice on resumption of exercise following COVID-19 infection.
The clinical guidance was published online March 16 in the Journal of the American College of Cardiology.
“The best means to diagnose and treat myocarditis and long COVID following SARS-CoV-2 infection continues to evolve,” said Ty Gluckman, MD, MHA, cochair of the expert consensus decision pathway. “This document attempts to provide key recommendations for how to evaluate and manage adults with these conditions, including guidance for safe return to play for both competitive and noncompetitive athletes.”
The authors of the guidance note that COVID-19 can be associated with various abnormalities in cardiac testing and a wide range of cardiovascular complications. For some patients, cardiac symptoms such as chest pain, shortness of breath, fatigue, and palpitations persist, lasting months after the initial illness, and evidence of myocardial injury has also been observed in both symptomatic and asymptomatic individuals, as well as after receipt of the COVID-19 mRNA vaccine.
“For clinicians treating these individuals, a growing number of questions exist related to evaluation and management of these conditions, as well as safe resumption of physical activity,” they say. This report is intended to provide practical guidance on these issues.
Myocarditis
The report states that myocarditis has been recognized as a rare but serious complication of SARS-CoV-2 infection as well as COVID-19 mRNA vaccination.
It defines myocarditis as: 1.cardiac symptoms such as chest pain, dyspnea, palpitations, or syncope; 2. elevated cardiac troponin; and 3. abnormal electrocardiographic, echocardiographic, cardiac MRI, and/or histopathologic findings on biopsy.
The document makes the following recommendations in regard to COVID-related myocarditis:
When there is increased suspicion for cardiac involvement with COVID-19, initial testing should consist of an ECG, measurement of cardiac troponin, and an echocardiogram. Cardiology consultation is recommended for those with a rising cardiac troponin and/or echocardiographic abnormalities. Cardiac MRI is recommended in hemodynamically stable patients with suspected myocarditis.
Hospitalization is recommended for patients with definite myocarditis, ideally at an advanced heart failure center. Patients with fulminant myocarditis should be managed at centers with an expertise in advanced heart failure, mechanical circulatory support, and other advanced therapies.
Patients with myocarditis and COVID-19 pneumonia (with an ongoing need for supplemental oxygen) should be treated with corticosteroids. For patients with suspected pericardial involvement, treatment with NSAIDs, colchicine, and/or prednisone is reasonable. Intravenous corticosteroids may be considered in those with suspected or confirmed COVID-19 myocarditis with hemodynamic compromise or MIS-A (multisystem inflammatory syndrome in adults). Empiric use of corticosteroids may also be considered in those with biopsy evidence of severe myocardial infiltrates or fulminant myocarditis, balanced against infection risk.
As appropriate, guideline-directed medical therapy for heart failure should be initiated and continued after discharge.
The document notes that myocarditis following COVID-19 mRNA vaccination is rare, with highest rates seen in young males after the second vaccine dose. As of May 22, 2021, the U.S. Vaccine Adverse Event Reporting System noted rates of 40.6 cases per million after the second vaccine dose among male individuals aged 12-29 years and 2.4 cases per million among male individuals aged 30 and older. Corresponding rates in female individuals were 4.2 and 1 cases per million, respectively.
But the report says that COVID-19 vaccination is associated with “a very favorable benefit-to-risk ratio” for all age and sex groups evaluated thus far.
In general, vaccine-associated myocarditis should be diagnosed, categorized, and treated in a manner analogous to myocarditis following SARS-CoV-2 infection, the guidance advises.
Long COVID
The document refers to long COVID as postacute sequelae of SARS-CoV-2 infection (PASC), and reports that this condition is experienced by up to 10%-30% of infected individuals. It is defined by a constellation of new, returning, or persistent health problems experienced by individuals 4 or more weeks after COVID-19 infection.
Although individuals with this condition may experience wide-ranging symptoms, the symptoms that draw increased attention to the cardiovascular system include tachycardia, exercise intolerance, chest pain, and shortness of breath.
Nicole Bhave, MD, cochair of the expert consensus decision pathway, says: “There appears to be a ‘downward spiral’ for long-COVID patients. Fatigue and decreased exercise capacity lead to diminished activity and bed rest, in turn leading to worsening symptoms and decreased quality of life.” She adds that “the writing committee recommends a basic cardiopulmonary evaluation performed up front to determine if further specialty care and formalized medical therapy is needed for these patients.”
The authors propose two terms to better understand potential etiologies for those with cardiovascular symptoms:
PASC-CVD, or PASC-cardiovascular disease, refers to a broad group of cardiovascular conditions (including myocarditis) that manifest at least 4 weeks after COVID-19 infection.
PASC-CVS, or PASC-cardiovascular syndrome, includes a wide range of cardiovascular symptoms without objective evidence of cardiovascular disease following standard diagnostic testing.
The document makes the following recommendations for the management of PASC-CVD and PASC-CVS.
For patients with cardiovascular symptoms and suspected PASC, the authors suggest that a reasonable initial testing approach includes basic laboratory testing, including cardiac troponin, an ECG, an echocardiogram, an ambulatory rhythm monitor, chest imaging, and/or pulmonary function tests.
Cardiology consultation is recommended for patients with PASC who have abnormal cardiac test results, known cardiovascular disease with new or worsening symptoms, documented cardiac complications during SARS-CoV-2 infection, and/or persistent cardiopulmonary symptoms that are not otherwise explained.
Recumbent or semirecumbent exercise (for example, rowing, swimming, or cycling) is recommended initially for PASC-CVS patients with tachycardia, exercise/orthostatic intolerance, and/or deconditioning, with transition to upright exercise as orthostatic intolerance improves. Exercise duration should also be short (5-10 minutes/day) initially, with gradual increases as functional capacity improves.
Salt and fluid loading represent nonpharmacologic interventions that may provide symptomatic relief for patients with tachycardia, palpitations, and/or orthostatic hypotension.
Beta-blockers, nondihydropyridine calcium-channel blockers, ivabradine, fludrocortisone, and midodrine may be used empirically as well.
Return to play for athletes
The authors note that concerns about possible cardiac injury after COVID-19 fueled early apprehension regarding the safety of competitive sports for athletes recovering from the infection.
But they say that subsequent data from large registries have demonstrated an overall low prevalence of clinical myocarditis, without a rise in the rate of adverse cardiac events. Based on this, updated guidance is provided with a practical, evidence-based framework to guide resumption of athletics and intense exercise training.
They make the following recommendations:
- For athletes recovering from COVID-19 with ongoing cardiopulmonary symptoms (chest pain, shortness of breath, palpitations, lightheadedness) or those requiring hospitalization with increased suspicion for cardiac involvement, further evaluation with triad testing – an ECG, measurement of cardiac troponin, and an echocardiogram – should be performed.
- For those with abnormal test results, further evaluation with cardiac MRI should be considered. Individuals diagnosed with clinical myocarditis should abstain from exercise for 3-6 months.
- Cardiac testing is not recommended for asymptomatic individuals following COVID-19 infection. Individuals should abstain from training for 3 days to ensure that symptoms do not develop.
- For those with mild or moderate noncardiopulmonary symptoms (fever, lethargy, muscle aches), training may resume after symptom resolution.
- For those with remote infection (≥3 months) without ongoing cardiopulmonary symptoms, a gradual increase in exercise is recommended without the need for cardiac testing.
Based on the low prevalence of myocarditis observed in competitive athletes with COVID-19, the authors note that these recommendations can be reasonably applied to high-school athletes (aged 14 and older) along with adult recreational exercise enthusiasts.
Future study is needed, however, to better understand how long cardiac abnormalities persist following COVID-19 infection and the role of exercise training in long COVID.
The authors conclude that the current guidance is intended to help clinicians understand not only when testing may be warranted, but also when it is not.
“Given that it reflects the current state of knowledge through early 2022, it is anticipated that recommendations will change over time as our understanding evolves,” they say.
The 2022 ACC Expert Consensus Decision Pathway on Cardiovascular Sequelae of COVID-19: Myocarditis, Post-Acute Sequelae of SARS-CoV-2 Infection (PASC), and Return to Play will be discussed in a session at the American College of Cardiology’s annual scientific session meeting in Washington in April.
A version of this article first appeared on Medscape.com.
The American College of Cardiology has issued an expert consensus clinical guidance document for the evaluation and management of adults with key cardiovascular consequences of COVID-19.
The document makes recommendations on how to evaluate and manage COVID-associated myocarditis and long COVID and gives advice on resumption of exercise following COVID-19 infection.
The clinical guidance was published online March 16 in the Journal of the American College of Cardiology.
“The best means to diagnose and treat myocarditis and long COVID following SARS-CoV-2 infection continues to evolve,” said Ty Gluckman, MD, MHA, cochair of the expert consensus decision pathway. “This document attempts to provide key recommendations for how to evaluate and manage adults with these conditions, including guidance for safe return to play for both competitive and noncompetitive athletes.”
The authors of the guidance note that COVID-19 can be associated with various abnormalities in cardiac testing and a wide range of cardiovascular complications. For some patients, cardiac symptoms such as chest pain, shortness of breath, fatigue, and palpitations persist, lasting months after the initial illness, and evidence of myocardial injury has also been observed in both symptomatic and asymptomatic individuals, as well as after receipt of the COVID-19 mRNA vaccine.
“For clinicians treating these individuals, a growing number of questions exist related to evaluation and management of these conditions, as well as safe resumption of physical activity,” they say. This report is intended to provide practical guidance on these issues.
Myocarditis
The report states that myocarditis has been recognized as a rare but serious complication of SARS-CoV-2 infection as well as COVID-19 mRNA vaccination.
It defines myocarditis as: 1.cardiac symptoms such as chest pain, dyspnea, palpitations, or syncope; 2. elevated cardiac troponin; and 3. abnormal electrocardiographic, echocardiographic, cardiac MRI, and/or histopathologic findings on biopsy.
The document makes the following recommendations in regard to COVID-related myocarditis:
When there is increased suspicion for cardiac involvement with COVID-19, initial testing should consist of an ECG, measurement of cardiac troponin, and an echocardiogram. Cardiology consultation is recommended for those with a rising cardiac troponin and/or echocardiographic abnormalities. Cardiac MRI is recommended in hemodynamically stable patients with suspected myocarditis.
Hospitalization is recommended for patients with definite myocarditis, ideally at an advanced heart failure center. Patients with fulminant myocarditis should be managed at centers with an expertise in advanced heart failure, mechanical circulatory support, and other advanced therapies.
Patients with myocarditis and COVID-19 pneumonia (with an ongoing need for supplemental oxygen) should be treated with corticosteroids. For patients with suspected pericardial involvement, treatment with NSAIDs, colchicine, and/or prednisone is reasonable. Intravenous corticosteroids may be considered in those with suspected or confirmed COVID-19 myocarditis with hemodynamic compromise or MIS-A (multisystem inflammatory syndrome in adults). Empiric use of corticosteroids may also be considered in those with biopsy evidence of severe myocardial infiltrates or fulminant myocarditis, balanced against infection risk.
As appropriate, guideline-directed medical therapy for heart failure should be initiated and continued after discharge.
The document notes that myocarditis following COVID-19 mRNA vaccination is rare, with highest rates seen in young males after the second vaccine dose. As of May 22, 2021, the U.S. Vaccine Adverse Event Reporting System noted rates of 40.6 cases per million after the second vaccine dose among male individuals aged 12-29 years and 2.4 cases per million among male individuals aged 30 and older. Corresponding rates in female individuals were 4.2 and 1 cases per million, respectively.
But the report says that COVID-19 vaccination is associated with “a very favorable benefit-to-risk ratio” for all age and sex groups evaluated thus far.
In general, vaccine-associated myocarditis should be diagnosed, categorized, and treated in a manner analogous to myocarditis following SARS-CoV-2 infection, the guidance advises.
Long COVID
The document refers to long COVID as postacute sequelae of SARS-CoV-2 infection (PASC), and reports that this condition is experienced by up to 10%-30% of infected individuals. It is defined by a constellation of new, returning, or persistent health problems experienced by individuals 4 or more weeks after COVID-19 infection.
Although individuals with this condition may experience wide-ranging symptoms, the symptoms that draw increased attention to the cardiovascular system include tachycardia, exercise intolerance, chest pain, and shortness of breath.
Nicole Bhave, MD, cochair of the expert consensus decision pathway, says: “There appears to be a ‘downward spiral’ for long-COVID patients. Fatigue and decreased exercise capacity lead to diminished activity and bed rest, in turn leading to worsening symptoms and decreased quality of life.” She adds that “the writing committee recommends a basic cardiopulmonary evaluation performed up front to determine if further specialty care and formalized medical therapy is needed for these patients.”
The authors propose two terms to better understand potential etiologies for those with cardiovascular symptoms:
PASC-CVD, or PASC-cardiovascular disease, refers to a broad group of cardiovascular conditions (including myocarditis) that manifest at least 4 weeks after COVID-19 infection.
PASC-CVS, or PASC-cardiovascular syndrome, includes a wide range of cardiovascular symptoms without objective evidence of cardiovascular disease following standard diagnostic testing.
The document makes the following recommendations for the management of PASC-CVD and PASC-CVS.
For patients with cardiovascular symptoms and suspected PASC, the authors suggest that a reasonable initial testing approach includes basic laboratory testing, including cardiac troponin, an ECG, an echocardiogram, an ambulatory rhythm monitor, chest imaging, and/or pulmonary function tests.
Cardiology consultation is recommended for patients with PASC who have abnormal cardiac test results, known cardiovascular disease with new or worsening symptoms, documented cardiac complications during SARS-CoV-2 infection, and/or persistent cardiopulmonary symptoms that are not otherwise explained.
Recumbent or semirecumbent exercise (for example, rowing, swimming, or cycling) is recommended initially for PASC-CVS patients with tachycardia, exercise/orthostatic intolerance, and/or deconditioning, with transition to upright exercise as orthostatic intolerance improves. Exercise duration should also be short (5-10 minutes/day) initially, with gradual increases as functional capacity improves.
Salt and fluid loading represent nonpharmacologic interventions that may provide symptomatic relief for patients with tachycardia, palpitations, and/or orthostatic hypotension.
Beta-blockers, nondihydropyridine calcium-channel blockers, ivabradine, fludrocortisone, and midodrine may be used empirically as well.
Return to play for athletes
The authors note that concerns about possible cardiac injury after COVID-19 fueled early apprehension regarding the safety of competitive sports for athletes recovering from the infection.
But they say that subsequent data from large registries have demonstrated an overall low prevalence of clinical myocarditis, without a rise in the rate of adverse cardiac events. Based on this, updated guidance is provided with a practical, evidence-based framework to guide resumption of athletics and intense exercise training.
They make the following recommendations:
- For athletes recovering from COVID-19 with ongoing cardiopulmonary symptoms (chest pain, shortness of breath, palpitations, lightheadedness) or those requiring hospitalization with increased suspicion for cardiac involvement, further evaluation with triad testing – an ECG, measurement of cardiac troponin, and an echocardiogram – should be performed.
- For those with abnormal test results, further evaluation with cardiac MRI should be considered. Individuals diagnosed with clinical myocarditis should abstain from exercise for 3-6 months.
- Cardiac testing is not recommended for asymptomatic individuals following COVID-19 infection. Individuals should abstain from training for 3 days to ensure that symptoms do not develop.
- For those with mild or moderate noncardiopulmonary symptoms (fever, lethargy, muscle aches), training may resume after symptom resolution.
- For those with remote infection (≥3 months) without ongoing cardiopulmonary symptoms, a gradual increase in exercise is recommended without the need for cardiac testing.
Based on the low prevalence of myocarditis observed in competitive athletes with COVID-19, the authors note that these recommendations can be reasonably applied to high-school athletes (aged 14 and older) along with adult recreational exercise enthusiasts.
Future study is needed, however, to better understand how long cardiac abnormalities persist following COVID-19 infection and the role of exercise training in long COVID.
The authors conclude that the current guidance is intended to help clinicians understand not only when testing may be warranted, but also when it is not.
“Given that it reflects the current state of knowledge through early 2022, it is anticipated that recommendations will change over time as our understanding evolves,” they say.
The 2022 ACC Expert Consensus Decision Pathway on Cardiovascular Sequelae of COVID-19: Myocarditis, Post-Acute Sequelae of SARS-CoV-2 Infection (PASC), and Return to Play will be discussed in a session at the American College of Cardiology’s annual scientific session meeting in Washington in April.
A version of this article first appeared on Medscape.com.
The American College of Cardiology has issued an expert consensus clinical guidance document for the evaluation and management of adults with key cardiovascular consequences of COVID-19.
The document makes recommendations on how to evaluate and manage COVID-associated myocarditis and long COVID and gives advice on resumption of exercise following COVID-19 infection.
The clinical guidance was published online March 16 in the Journal of the American College of Cardiology.
“The best means to diagnose and treat myocarditis and long COVID following SARS-CoV-2 infection continues to evolve,” said Ty Gluckman, MD, MHA, cochair of the expert consensus decision pathway. “This document attempts to provide key recommendations for how to evaluate and manage adults with these conditions, including guidance for safe return to play for both competitive and noncompetitive athletes.”
The authors of the guidance note that COVID-19 can be associated with various abnormalities in cardiac testing and a wide range of cardiovascular complications. For some patients, cardiac symptoms such as chest pain, shortness of breath, fatigue, and palpitations persist, lasting months after the initial illness, and evidence of myocardial injury has also been observed in both symptomatic and asymptomatic individuals, as well as after receipt of the COVID-19 mRNA vaccine.
“For clinicians treating these individuals, a growing number of questions exist related to evaluation and management of these conditions, as well as safe resumption of physical activity,” they say. This report is intended to provide practical guidance on these issues.
Myocarditis
The report states that myocarditis has been recognized as a rare but serious complication of SARS-CoV-2 infection as well as COVID-19 mRNA vaccination.
It defines myocarditis as: 1.cardiac symptoms such as chest pain, dyspnea, palpitations, or syncope; 2. elevated cardiac troponin; and 3. abnormal electrocardiographic, echocardiographic, cardiac MRI, and/or histopathologic findings on biopsy.
The document makes the following recommendations in regard to COVID-related myocarditis:
When there is increased suspicion for cardiac involvement with COVID-19, initial testing should consist of an ECG, measurement of cardiac troponin, and an echocardiogram. Cardiology consultation is recommended for those with a rising cardiac troponin and/or echocardiographic abnormalities. Cardiac MRI is recommended in hemodynamically stable patients with suspected myocarditis.
Hospitalization is recommended for patients with definite myocarditis, ideally at an advanced heart failure center. Patients with fulminant myocarditis should be managed at centers with an expertise in advanced heart failure, mechanical circulatory support, and other advanced therapies.
Patients with myocarditis and COVID-19 pneumonia (with an ongoing need for supplemental oxygen) should be treated with corticosteroids. For patients with suspected pericardial involvement, treatment with NSAIDs, colchicine, and/or prednisone is reasonable. Intravenous corticosteroids may be considered in those with suspected or confirmed COVID-19 myocarditis with hemodynamic compromise or MIS-A (multisystem inflammatory syndrome in adults). Empiric use of corticosteroids may also be considered in those with biopsy evidence of severe myocardial infiltrates or fulminant myocarditis, balanced against infection risk.
As appropriate, guideline-directed medical therapy for heart failure should be initiated and continued after discharge.
The document notes that myocarditis following COVID-19 mRNA vaccination is rare, with highest rates seen in young males after the second vaccine dose. As of May 22, 2021, the U.S. Vaccine Adverse Event Reporting System noted rates of 40.6 cases per million after the second vaccine dose among male individuals aged 12-29 years and 2.4 cases per million among male individuals aged 30 and older. Corresponding rates in female individuals were 4.2 and 1 cases per million, respectively.
But the report says that COVID-19 vaccination is associated with “a very favorable benefit-to-risk ratio” for all age and sex groups evaluated thus far.
In general, vaccine-associated myocarditis should be diagnosed, categorized, and treated in a manner analogous to myocarditis following SARS-CoV-2 infection, the guidance advises.
Long COVID
The document refers to long COVID as postacute sequelae of SARS-CoV-2 infection (PASC), and reports that this condition is experienced by up to 10%-30% of infected individuals. It is defined by a constellation of new, returning, or persistent health problems experienced by individuals 4 or more weeks after COVID-19 infection.
Although individuals with this condition may experience wide-ranging symptoms, the symptoms that draw increased attention to the cardiovascular system include tachycardia, exercise intolerance, chest pain, and shortness of breath.
Nicole Bhave, MD, cochair of the expert consensus decision pathway, says: “There appears to be a ‘downward spiral’ for long-COVID patients. Fatigue and decreased exercise capacity lead to diminished activity and bed rest, in turn leading to worsening symptoms and decreased quality of life.” She adds that “the writing committee recommends a basic cardiopulmonary evaluation performed up front to determine if further specialty care and formalized medical therapy is needed for these patients.”
The authors propose two terms to better understand potential etiologies for those with cardiovascular symptoms:
PASC-CVD, or PASC-cardiovascular disease, refers to a broad group of cardiovascular conditions (including myocarditis) that manifest at least 4 weeks after COVID-19 infection.
PASC-CVS, or PASC-cardiovascular syndrome, includes a wide range of cardiovascular symptoms without objective evidence of cardiovascular disease following standard diagnostic testing.
The document makes the following recommendations for the management of PASC-CVD and PASC-CVS.
For patients with cardiovascular symptoms and suspected PASC, the authors suggest that a reasonable initial testing approach includes basic laboratory testing, including cardiac troponin, an ECG, an echocardiogram, an ambulatory rhythm monitor, chest imaging, and/or pulmonary function tests.
Cardiology consultation is recommended for patients with PASC who have abnormal cardiac test results, known cardiovascular disease with new or worsening symptoms, documented cardiac complications during SARS-CoV-2 infection, and/or persistent cardiopulmonary symptoms that are not otherwise explained.
Recumbent or semirecumbent exercise (for example, rowing, swimming, or cycling) is recommended initially for PASC-CVS patients with tachycardia, exercise/orthostatic intolerance, and/or deconditioning, with transition to upright exercise as orthostatic intolerance improves. Exercise duration should also be short (5-10 minutes/day) initially, with gradual increases as functional capacity improves.
Salt and fluid loading represent nonpharmacologic interventions that may provide symptomatic relief for patients with tachycardia, palpitations, and/or orthostatic hypotension.
Beta-blockers, nondihydropyridine calcium-channel blockers, ivabradine, fludrocortisone, and midodrine may be used empirically as well.
Return to play for athletes
The authors note that concerns about possible cardiac injury after COVID-19 fueled early apprehension regarding the safety of competitive sports for athletes recovering from the infection.
But they say that subsequent data from large registries have demonstrated an overall low prevalence of clinical myocarditis, without a rise in the rate of adverse cardiac events. Based on this, updated guidance is provided with a practical, evidence-based framework to guide resumption of athletics and intense exercise training.
They make the following recommendations:
- For athletes recovering from COVID-19 with ongoing cardiopulmonary symptoms (chest pain, shortness of breath, palpitations, lightheadedness) or those requiring hospitalization with increased suspicion for cardiac involvement, further evaluation with triad testing – an ECG, measurement of cardiac troponin, and an echocardiogram – should be performed.
- For those with abnormal test results, further evaluation with cardiac MRI should be considered. Individuals diagnosed with clinical myocarditis should abstain from exercise for 3-6 months.
- Cardiac testing is not recommended for asymptomatic individuals following COVID-19 infection. Individuals should abstain from training for 3 days to ensure that symptoms do not develop.
- For those with mild or moderate noncardiopulmonary symptoms (fever, lethargy, muscle aches), training may resume after symptom resolution.
- For those with remote infection (≥3 months) without ongoing cardiopulmonary symptoms, a gradual increase in exercise is recommended without the need for cardiac testing.
Based on the low prevalence of myocarditis observed in competitive athletes with COVID-19, the authors note that these recommendations can be reasonably applied to high-school athletes (aged 14 and older) along with adult recreational exercise enthusiasts.
Future study is needed, however, to better understand how long cardiac abnormalities persist following COVID-19 infection and the role of exercise training in long COVID.
The authors conclude that the current guidance is intended to help clinicians understand not only when testing may be warranted, but also when it is not.
“Given that it reflects the current state of knowledge through early 2022, it is anticipated that recommendations will change over time as our understanding evolves,” they say.
The 2022 ACC Expert Consensus Decision Pathway on Cardiovascular Sequelae of COVID-19: Myocarditis, Post-Acute Sequelae of SARS-CoV-2 Infection (PASC), and Return to Play will be discussed in a session at the American College of Cardiology’s annual scientific session meeting in Washington in April.
A version of this article first appeared on Medscape.com.
Children and COVID: Decline in new cases reaches 7th week
New cases of COVID-19 in U.S. children have fallen to their lowest level since the beginning of the Delta surge in July of 2021, according to the American Academy of Pediatrics and the Children’s Hospital Association.
. Over those 7 weeks, new cases dropped over 96% from the 1.15 million reported for Jan. 14-20, based on data collected by the AAP and CHA from state and territorial health departments.
The last time that the weekly count was below 42,000 was July 16-22, 2021, when almost 39,000 cases were reported in the midst of the Delta upsurge. That was shortly after cases had reached their lowest point, 8,447, since the early stages of the pandemic in 2020, the AAP/CHA data show.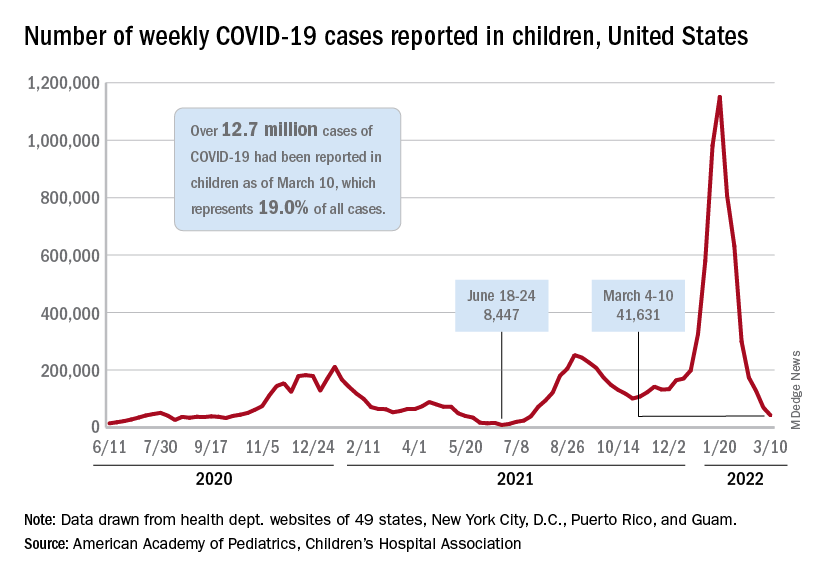
The cumulative number of pediatric cases is now up to 12.7 million, while the overall proportion of cases occurring in children held steady at 19.0% for the 4th week in a row, the AAP and CHA said in their weekly COVID-19 report. The Centers for Disease Control and Prevention, using an age range of 0-18 versus the states’ variety of ages, puts total cases at 11.7 million and deaths at 1,656 as of March 14.
Data from the CDC’s COVID-19–Associated Hospitalization Surveillance Network show that hospitalizations with laboratory-confirmed infection were down by 50% in children aged 0-4 years, by 63% among 5- to 11-year-olds, and by 58% in those aged 12-17 years for the week of Feb. 27 to March 5, compared with the week before.
The pace of vaccination continues to follow a similar trend, as the declines seen through February have continued into March. Cumulatively, 33.7% of children aged 5-11 have received at least one dose, and 26.8% are fully vaccinated, with corresponding numbers of 68.0% and 58.0% for children aged 12-17, the CDC reported on its COVID Data Tracker.
State-level data show that children aged 5-11 in Vermont, with a rate of 65%, are the most likely to have received at least one dose of COVID vaccine, while just 15% of 5- to 11-year-olds in Alabama, Louisiana, and Mississippi have gotten their first dose. Among children aged 12-17, that rate ranges from 40% in Wyoming to 94% in Hawaii, Massachusetts, and Rhode Island, the AAP said in a separate report based on CDC data.
In a recent report involving 1,364 children aged 5-15 years, two doses of the COVID-19 vaccine reduced the risk of infection from the Omicron variant by 31% in children aged 5-11 years and by 59% among children aged 12-15 years, said Ashley L. Fowlkes, ScD, of the CDC’s COVID-19 Emergency Response Team, and associates (MMWR 2022 Mar 11;71).
New cases of COVID-19 in U.S. children have fallen to their lowest level since the beginning of the Delta surge in July of 2021, according to the American Academy of Pediatrics and the Children’s Hospital Association.
. Over those 7 weeks, new cases dropped over 96% from the 1.15 million reported for Jan. 14-20, based on data collected by the AAP and CHA from state and territorial health departments.
The last time that the weekly count was below 42,000 was July 16-22, 2021, when almost 39,000 cases were reported in the midst of the Delta upsurge. That was shortly after cases had reached their lowest point, 8,447, since the early stages of the pandemic in 2020, the AAP/CHA data show.
The cumulative number of pediatric cases is now up to 12.7 million, while the overall proportion of cases occurring in children held steady at 19.0% for the 4th week in a row, the AAP and CHA said in their weekly COVID-19 report. The Centers for Disease Control and Prevention, using an age range of 0-18 versus the states’ variety of ages, puts total cases at 11.7 million and deaths at 1,656 as of March 14.
Data from the CDC’s COVID-19–Associated Hospitalization Surveillance Network show that hospitalizations with laboratory-confirmed infection were down by 50% in children aged 0-4 years, by 63% among 5- to 11-year-olds, and by 58% in those aged 12-17 years for the week of Feb. 27 to March 5, compared with the week before.
The pace of vaccination continues to follow a similar trend, as the declines seen through February have continued into March. Cumulatively, 33.7% of children aged 5-11 have received at least one dose, and 26.8% are fully vaccinated, with corresponding numbers of 68.0% and 58.0% for children aged 12-17, the CDC reported on its COVID Data Tracker.
State-level data show that children aged 5-11 in Vermont, with a rate of 65%, are the most likely to have received at least one dose of COVID vaccine, while just 15% of 5- to 11-year-olds in Alabama, Louisiana, and Mississippi have gotten their first dose. Among children aged 12-17, that rate ranges from 40% in Wyoming to 94% in Hawaii, Massachusetts, and Rhode Island, the AAP said in a separate report based on CDC data.
In a recent report involving 1,364 children aged 5-15 years, two doses of the COVID-19 vaccine reduced the risk of infection from the Omicron variant by 31% in children aged 5-11 years and by 59% among children aged 12-15 years, said Ashley L. Fowlkes, ScD, of the CDC’s COVID-19 Emergency Response Team, and associates (MMWR 2022 Mar 11;71).
New cases of COVID-19 in U.S. children have fallen to their lowest level since the beginning of the Delta surge in July of 2021, according to the American Academy of Pediatrics and the Children’s Hospital Association.
. Over those 7 weeks, new cases dropped over 96% from the 1.15 million reported for Jan. 14-20, based on data collected by the AAP and CHA from state and territorial health departments.
The last time that the weekly count was below 42,000 was July 16-22, 2021, when almost 39,000 cases were reported in the midst of the Delta upsurge. That was shortly after cases had reached their lowest point, 8,447, since the early stages of the pandemic in 2020, the AAP/CHA data show.
The cumulative number of pediatric cases is now up to 12.7 million, while the overall proportion of cases occurring in children held steady at 19.0% for the 4th week in a row, the AAP and CHA said in their weekly COVID-19 report. The Centers for Disease Control and Prevention, using an age range of 0-18 versus the states’ variety of ages, puts total cases at 11.7 million and deaths at 1,656 as of March 14.
Data from the CDC’s COVID-19–Associated Hospitalization Surveillance Network show that hospitalizations with laboratory-confirmed infection were down by 50% in children aged 0-4 years, by 63% among 5- to 11-year-olds, and by 58% in those aged 12-17 years for the week of Feb. 27 to March 5, compared with the week before.
The pace of vaccination continues to follow a similar trend, as the declines seen through February have continued into March. Cumulatively, 33.7% of children aged 5-11 have received at least one dose, and 26.8% are fully vaccinated, with corresponding numbers of 68.0% and 58.0% for children aged 12-17, the CDC reported on its COVID Data Tracker.
State-level data show that children aged 5-11 in Vermont, with a rate of 65%, are the most likely to have received at least one dose of COVID vaccine, while just 15% of 5- to 11-year-olds in Alabama, Louisiana, and Mississippi have gotten their first dose. Among children aged 12-17, that rate ranges from 40% in Wyoming to 94% in Hawaii, Massachusetts, and Rhode Island, the AAP said in a separate report based on CDC data.
In a recent report involving 1,364 children aged 5-15 years, two doses of the COVID-19 vaccine reduced the risk of infection from the Omicron variant by 31% in children aged 5-11 years and by 59% among children aged 12-15 years, said Ashley L. Fowlkes, ScD, of the CDC’s COVID-19 Emergency Response Team, and associates (MMWR 2022 Mar 11;71).
‘Overwhelming’ need to study COVID vaccine–associated tinnitus
It’s now known that tinnitus may be an unexpected side effect of SARS-CoV-2 vaccination, and there is an urgent need to understand the precise mechanisms and best treatment for vaccine-associated tinnitus, researchers say.
As of mid-September 2021, 12,247 cases of tinnitus, or ringing in the ears, following COVID-19 vaccination had been reported to the Vaccine Adverse Event Reporting System of the U.S. Centers for Disease Control and Prevention.
“Despite several cases of tinnitus being reported following SARS-CoV-2 vaccination, the precise pathophysiology is still not clear,” write Syed Hassan Ahmed, 3rd-year MBBS student, Dow University of Health Sciences, Karachi, Pakistan, and coauthors.
The researchers review what is known and unknown about SARS-CoV-2 vaccine-associated tinnitus in an article published online Feb. 11 in Annals of Medicine and Surgery.
Molecular mimicry?
The researchers say cross-reactivity between anti-spike SARS-CoV-2 antibodies and otologic antigens is one possibility, based on the mechanisms behind other COVID-19 vaccine–induced disorders and the phenomenon of molecular mimicry.
“The heptapeptide resemblance between coronavirus spike glycoprotein and numerous human proteins further supports molecular mimicry as a potential mechanism behind such vaccine-induced disorders,” they write.
Anti-spike antibodies may react with antigens anywhere along the auditory pathway and fuel an inflammatory reaction, they point out.
“Therefore, understanding the phenomenon of cross-reactivity and molecular mimicry may be helpful in postulating potential treatment behind not only tinnitus but also the rare events of vaccination associated hearing loss and other otologic manifestations,” the authors say.
Genetic predispositions and associated conditions may also play a significant role in determining whether an individual develops vaccine-induced tinnitus.
Stress and anxiety following COVID vaccination may also play a role, inasmuch as anxiety-related adverse events following vaccination have been reported. Vaccine-related anxiety as a potential cause of tinnitus developing after vaccination needs to be explored, they write.
Jury out on best management
How best to manage COVID vaccine-associated tinnitus also remains unclear, but it starts with a well-established diagnosis, the authors say.
A well-focused and detailed history and examination are essential, with particular emphasis placed on preexisting health conditions, specifically, autoimmune diseases, such as Hashimoto thyroiditis; otologic conditions, such as sensorineural hearing loss; glaucoma; and psychological well-being. According to the review, patients often present with a history of one or more of these disorders.
“However, any such association has not yet been established and requires further investigation to be concluded as potential risk factors for vaccine-induced tinnitus,” they caution.
Routine cranial nerve examination, otoscopy, Weber test, and Rinne test, which are used for tinnitus diagnosis in general, may be helpful for confirmation of vaccine-associated tinnitus.
Owing to the significant association between tinnitus and hearing impairment, audiology should also performed, the authors say.
Although treatments for non–vaccine-induced tinnitus vary significantly, corticosteroids are the top treatment choice for SARS-CoV-2 vaccine-induced tinnitus reported in the literature.
Trials of other drug and nondrug interventions that may uniquely help with vaccine-associated tinnitus are urgently needed, the authors say.
Summing up, the reviewers say, “Although the incidence of COVID-19 vaccine-associated tinnitus is rare, there is an overwhelming need to discern the precise pathophysiology and clinical management as a better understanding of adverse events may help in encountering vaccine hesitancy and hence fostering the COVID-19 global vaccination program.
“Despite the incidence of adverse events, the benefits of the SARS-CoV-2 vaccine in reducing hospitalization and deaths continue to outweigh the rare ramifications,” they conclude.
The research had no specific funding. The authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
It’s now known that tinnitus may be an unexpected side effect of SARS-CoV-2 vaccination, and there is an urgent need to understand the precise mechanisms and best treatment for vaccine-associated tinnitus, researchers say.
As of mid-September 2021, 12,247 cases of tinnitus, or ringing in the ears, following COVID-19 vaccination had been reported to the Vaccine Adverse Event Reporting System of the U.S. Centers for Disease Control and Prevention.
“Despite several cases of tinnitus being reported following SARS-CoV-2 vaccination, the precise pathophysiology is still not clear,” write Syed Hassan Ahmed, 3rd-year MBBS student, Dow University of Health Sciences, Karachi, Pakistan, and coauthors.
The researchers review what is known and unknown about SARS-CoV-2 vaccine-associated tinnitus in an article published online Feb. 11 in Annals of Medicine and Surgery.
Molecular mimicry?
The researchers say cross-reactivity between anti-spike SARS-CoV-2 antibodies and otologic antigens is one possibility, based on the mechanisms behind other COVID-19 vaccine–induced disorders and the phenomenon of molecular mimicry.
“The heptapeptide resemblance between coronavirus spike glycoprotein and numerous human proteins further supports molecular mimicry as a potential mechanism behind such vaccine-induced disorders,” they write.
Anti-spike antibodies may react with antigens anywhere along the auditory pathway and fuel an inflammatory reaction, they point out.
“Therefore, understanding the phenomenon of cross-reactivity and molecular mimicry may be helpful in postulating potential treatment behind not only tinnitus but also the rare events of vaccination associated hearing loss and other otologic manifestations,” the authors say.
Genetic predispositions and associated conditions may also play a significant role in determining whether an individual develops vaccine-induced tinnitus.
Stress and anxiety following COVID vaccination may also play a role, inasmuch as anxiety-related adverse events following vaccination have been reported. Vaccine-related anxiety as a potential cause of tinnitus developing after vaccination needs to be explored, they write.
Jury out on best management
How best to manage COVID vaccine-associated tinnitus also remains unclear, but it starts with a well-established diagnosis, the authors say.
A well-focused and detailed history and examination are essential, with particular emphasis placed on preexisting health conditions, specifically, autoimmune diseases, such as Hashimoto thyroiditis; otologic conditions, such as sensorineural hearing loss; glaucoma; and psychological well-being. According to the review, patients often present with a history of one or more of these disorders.
“However, any such association has not yet been established and requires further investigation to be concluded as potential risk factors for vaccine-induced tinnitus,” they caution.
Routine cranial nerve examination, otoscopy, Weber test, and Rinne test, which are used for tinnitus diagnosis in general, may be helpful for confirmation of vaccine-associated tinnitus.
Owing to the significant association between tinnitus and hearing impairment, audiology should also performed, the authors say.
Although treatments for non–vaccine-induced tinnitus vary significantly, corticosteroids are the top treatment choice for SARS-CoV-2 vaccine-induced tinnitus reported in the literature.
Trials of other drug and nondrug interventions that may uniquely help with vaccine-associated tinnitus are urgently needed, the authors say.
Summing up, the reviewers say, “Although the incidence of COVID-19 vaccine-associated tinnitus is rare, there is an overwhelming need to discern the precise pathophysiology and clinical management as a better understanding of adverse events may help in encountering vaccine hesitancy and hence fostering the COVID-19 global vaccination program.
“Despite the incidence of adverse events, the benefits of the SARS-CoV-2 vaccine in reducing hospitalization and deaths continue to outweigh the rare ramifications,” they conclude.
The research had no specific funding. The authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
It’s now known that tinnitus may be an unexpected side effect of SARS-CoV-2 vaccination, and there is an urgent need to understand the precise mechanisms and best treatment for vaccine-associated tinnitus, researchers say.
As of mid-September 2021, 12,247 cases of tinnitus, or ringing in the ears, following COVID-19 vaccination had been reported to the Vaccine Adverse Event Reporting System of the U.S. Centers for Disease Control and Prevention.
“Despite several cases of tinnitus being reported following SARS-CoV-2 vaccination, the precise pathophysiology is still not clear,” write Syed Hassan Ahmed, 3rd-year MBBS student, Dow University of Health Sciences, Karachi, Pakistan, and coauthors.
The researchers review what is known and unknown about SARS-CoV-2 vaccine-associated tinnitus in an article published online Feb. 11 in Annals of Medicine and Surgery.
Molecular mimicry?
The researchers say cross-reactivity between anti-spike SARS-CoV-2 antibodies and otologic antigens is one possibility, based on the mechanisms behind other COVID-19 vaccine–induced disorders and the phenomenon of molecular mimicry.
“The heptapeptide resemblance between coronavirus spike glycoprotein and numerous human proteins further supports molecular mimicry as a potential mechanism behind such vaccine-induced disorders,” they write.
Anti-spike antibodies may react with antigens anywhere along the auditory pathway and fuel an inflammatory reaction, they point out.
“Therefore, understanding the phenomenon of cross-reactivity and molecular mimicry may be helpful in postulating potential treatment behind not only tinnitus but also the rare events of vaccination associated hearing loss and other otologic manifestations,” the authors say.
Genetic predispositions and associated conditions may also play a significant role in determining whether an individual develops vaccine-induced tinnitus.
Stress and anxiety following COVID vaccination may also play a role, inasmuch as anxiety-related adverse events following vaccination have been reported. Vaccine-related anxiety as a potential cause of tinnitus developing after vaccination needs to be explored, they write.
Jury out on best management
How best to manage COVID vaccine-associated tinnitus also remains unclear, but it starts with a well-established diagnosis, the authors say.
A well-focused and detailed history and examination are essential, with particular emphasis placed on preexisting health conditions, specifically, autoimmune diseases, such as Hashimoto thyroiditis; otologic conditions, such as sensorineural hearing loss; glaucoma; and psychological well-being. According to the review, patients often present with a history of one or more of these disorders.
“However, any such association has not yet been established and requires further investigation to be concluded as potential risk factors for vaccine-induced tinnitus,” they caution.
Routine cranial nerve examination, otoscopy, Weber test, and Rinne test, which are used for tinnitus diagnosis in general, may be helpful for confirmation of vaccine-associated tinnitus.
Owing to the significant association between tinnitus and hearing impairment, audiology should also performed, the authors say.
Although treatments for non–vaccine-induced tinnitus vary significantly, corticosteroids are the top treatment choice for SARS-CoV-2 vaccine-induced tinnitus reported in the literature.
Trials of other drug and nondrug interventions that may uniquely help with vaccine-associated tinnitus are urgently needed, the authors say.
Summing up, the reviewers say, “Although the incidence of COVID-19 vaccine-associated tinnitus is rare, there is an overwhelming need to discern the precise pathophysiology and clinical management as a better understanding of adverse events may help in encountering vaccine hesitancy and hence fostering the COVID-19 global vaccination program.
“Despite the incidence of adverse events, the benefits of the SARS-CoV-2 vaccine in reducing hospitalization and deaths continue to outweigh the rare ramifications,” they conclude.
The research had no specific funding. The authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM ANNALS OF MEDICINE AND SURGERY