User login
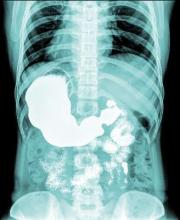
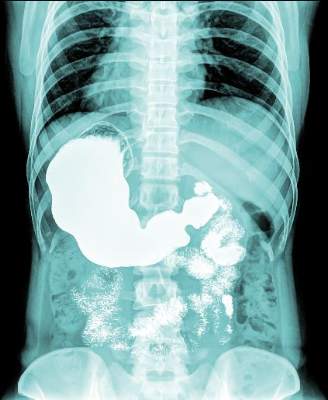
VIDEO: Duodenal bulb sampling barely increased celiac yield in low-probability cohort
Separate sampling of the duodenal bulb increased detection of celiac disease by only 0.1% when endoscopy patients had a low pretest probability of celiac disease, according to research published in the November issue of Clinical Gastroenterology and Hepatology.
Duodenal bulb histology did reveal other abnormal findings, such as chronic peptic duodenitis, gastric heterotopia, and Brunner gland hyperplasia, wrote Samantha Stoven, MD, of Mayo Clinic, Rochester, Minn., and her associates. These findings did not seem to impede the identification of celiac disease, but their clinical implications were unclear, the researchers noted.
Most studies of the diagnostic yield of duodenal bulb specimens have been performed in patients with known celiac disease or positive serology. In past studies of these high-probability cohorts, duodenal bulb sampling increased the diagnostic yield of celiac disease anywhere from 1.8% to 18%, but whether and how that finding translates to low-probability cohorts is unclear, the researchers said. Therefore, they retrospectively analyzed data from 679 endoscopy patients who had both duodenal bulb and small bowel biopsies collected at three Mayo Clinic sites in 2011. These sites are “open access,” meaning that patients can be referred for endoscopy without the approval of a gastroenterologist.
The average age of the patients was 50 years, and 63% were female. They were most commonly referred for duodenal biopsy because of chronic dyspepsia (46% of patients), diarrhea (35%), or nausea (17%). Patients with either known celiac disease or positive serology were excluded from the study (Clin Gastroenterol Hepatol. 2016 Mar 7. doi: 10.1016/j.cgh.2016.02.026). A total of 265 patients (39%) had abnormal duodenal histology, which was most often diagnosed as chronic peptic duodenitis, the researchers said. Histologic abnormalities usually involved the duodenal bulb (36% of cases), not the distal duodenum (15%; P less than .0001). However, among the 16 patients (2%) found to have celiac disease, just one patient had disease only in the duodenal bulb. Thus, duodenal bulb sampling increased the diagnostic yield of celiac disease by only 0.1% when considering the overall cohort. The patient with celiac disease limited to the bulb was a 46-year-old female presenting with diarrhea and anemia who had normal serologies but a permissive human leukocyte antigen test. Her duodenal bulb had villous atrophy and more than 25 intraepithelial lymphocytes per 100 epithelial cells, while her distal duodenum was normal.
Among the 85% of patients who had normal distal duodenums, 28% had abnormal bulb histology, most often chronic peptic duodenitis, active chronic peptic duodenitis, or gastric heterotopia, the researchers said. Among the 59% of patients whose celiac serology before endoscopy was truly unknown, only two (0.5%) had histologic changes consistent with celiac disease, which in both cases were located in the distal duodenum.
SOURCE: American Gastroenterological Association
“Individual sampling of the duodenal bulb in patients with either negative or unknown celiac serologic status can be considered in practices where expert gastrointestinal pathologists are present and there is agreement that both samples can be submitted in the same bottle, or there is not a separate charge for the additional container. Further studies may be needed to assess the diagnostic yield of separate bulb biopsies for celiac detection in all comers.”
An American College of Gastroenterology Junior Faculty Development Award helped support the work. Senior author Joseph A. Murray, MD, disclosed ties to Alba Therapeutics, Alvine Pharmaceuticals, AMAG Pharmaceuticals, and several other corporate entities. The remaining authors had no disclosures.
Histologic diagnosis of celiac disease has traditionally relied upon endoscopic biopsies from the second and third portions of the duodenum. However, several recent studies indicate that duodenal bulb biopsies may show changes of celiac disease, despite normal histology in the more distal duodenum.
In their study, Dr. Stoven and her colleagues evaluated the diagnostic utility of endoscopic duodenal bulb biopsy in patients with a low probability for celiac disease. A new diagnosis of celiac disease was made in 16 of their 679 patients (2.4%). Only one patient showed villous atrophy of the duodenal bulb with normal histology of the more distal duodenum. Although a diagnosis of celiac disease was made, the case was atypical not only because distal duodenal biopsies were normal but also because multiple celiac serology tests were negative, raising the possibility of nonceliac villous atrophy. Thus, the added diagnostic yield of duodenal bulb biopsies in this low-risk population was extremely low (0.15% at most).
Ciaran P. Kelly, MD, AGAF, professor of medicine, Harvard Medical School, director Celiac Center, Beth Israel Deaconess Medical Center, Boston, has acted as a scientific adviser to companies including Celimmune, Cour Pharmaceuticals, ImmunogenX, and Takeda; he also acts as principal investigator on a research grant on celiac disease supported by Aptalis.
Histologic diagnosis of celiac disease has traditionally relied upon endoscopic biopsies from the second and third portions of the duodenum. However, several recent studies indicate that duodenal bulb biopsies may show changes of celiac disease, despite normal histology in the more distal duodenum.
In their study, Dr. Stoven and her colleagues evaluated the diagnostic utility of endoscopic duodenal bulb biopsy in patients with a low probability for celiac disease. A new diagnosis of celiac disease was made in 16 of their 679 patients (2.4%). Only one patient showed villous atrophy of the duodenal bulb with normal histology of the more distal duodenum. Although a diagnosis of celiac disease was made, the case was atypical not only because distal duodenal biopsies were normal but also because multiple celiac serology tests were negative, raising the possibility of nonceliac villous atrophy. Thus, the added diagnostic yield of duodenal bulb biopsies in this low-risk population was extremely low (0.15% at most).
Ciaran P. Kelly, MD, AGAF, professor of medicine, Harvard Medical School, director Celiac Center, Beth Israel Deaconess Medical Center, Boston, has acted as a scientific adviser to companies including Celimmune, Cour Pharmaceuticals, ImmunogenX, and Takeda; he also acts as principal investigator on a research grant on celiac disease supported by Aptalis.
Histologic diagnosis of celiac disease has traditionally relied upon endoscopic biopsies from the second and third portions of the duodenum. However, several recent studies indicate that duodenal bulb biopsies may show changes of celiac disease, despite normal histology in the more distal duodenum.
In their study, Dr. Stoven and her colleagues evaluated the diagnostic utility of endoscopic duodenal bulb biopsy in patients with a low probability for celiac disease. A new diagnosis of celiac disease was made in 16 of their 679 patients (2.4%). Only one patient showed villous atrophy of the duodenal bulb with normal histology of the more distal duodenum. Although a diagnosis of celiac disease was made, the case was atypical not only because distal duodenal biopsies were normal but also because multiple celiac serology tests were negative, raising the possibility of nonceliac villous atrophy. Thus, the added diagnostic yield of duodenal bulb biopsies in this low-risk population was extremely low (0.15% at most).
Ciaran P. Kelly, MD, AGAF, professor of medicine, Harvard Medical School, director Celiac Center, Beth Israel Deaconess Medical Center, Boston, has acted as a scientific adviser to companies including Celimmune, Cour Pharmaceuticals, ImmunogenX, and Takeda; he also acts as principal investigator on a research grant on celiac disease supported by Aptalis.
Separate sampling of the duodenal bulb increased detection of celiac disease by only 0.1% when endoscopy patients had a low pretest probability of celiac disease, according to research published in the November issue of Clinical Gastroenterology and Hepatology.
Duodenal bulb histology did reveal other abnormal findings, such as chronic peptic duodenitis, gastric heterotopia, and Brunner gland hyperplasia, wrote Samantha Stoven, MD, of Mayo Clinic, Rochester, Minn., and her associates. These findings did not seem to impede the identification of celiac disease, but their clinical implications were unclear, the researchers noted.
Most studies of the diagnostic yield of duodenal bulb specimens have been performed in patients with known celiac disease or positive serology. In past studies of these high-probability cohorts, duodenal bulb sampling increased the diagnostic yield of celiac disease anywhere from 1.8% to 18%, but whether and how that finding translates to low-probability cohorts is unclear, the researchers said. Therefore, they retrospectively analyzed data from 679 endoscopy patients who had both duodenal bulb and small bowel biopsies collected at three Mayo Clinic sites in 2011. These sites are “open access,” meaning that patients can be referred for endoscopy without the approval of a gastroenterologist.
The average age of the patients was 50 years, and 63% were female. They were most commonly referred for duodenal biopsy because of chronic dyspepsia (46% of patients), diarrhea (35%), or nausea (17%). Patients with either known celiac disease or positive serology were excluded from the study (Clin Gastroenterol Hepatol. 2016 Mar 7. doi: 10.1016/j.cgh.2016.02.026). A total of 265 patients (39%) had abnormal duodenal histology, which was most often diagnosed as chronic peptic duodenitis, the researchers said. Histologic abnormalities usually involved the duodenal bulb (36% of cases), not the distal duodenum (15%; P less than .0001). However, among the 16 patients (2%) found to have celiac disease, just one patient had disease only in the duodenal bulb. Thus, duodenal bulb sampling increased the diagnostic yield of celiac disease by only 0.1% when considering the overall cohort. The patient with celiac disease limited to the bulb was a 46-year-old female presenting with diarrhea and anemia who had normal serologies but a permissive human leukocyte antigen test. Her duodenal bulb had villous atrophy and more than 25 intraepithelial lymphocytes per 100 epithelial cells, while her distal duodenum was normal.
Among the 85% of patients who had normal distal duodenums, 28% had abnormal bulb histology, most often chronic peptic duodenitis, active chronic peptic duodenitis, or gastric heterotopia, the researchers said. Among the 59% of patients whose celiac serology before endoscopy was truly unknown, only two (0.5%) had histologic changes consistent with celiac disease, which in both cases were located in the distal duodenum.
SOURCE: American Gastroenterological Association
“Individual sampling of the duodenal bulb in patients with either negative or unknown celiac serologic status can be considered in practices where expert gastrointestinal pathologists are present and there is agreement that both samples can be submitted in the same bottle, or there is not a separate charge for the additional container. Further studies may be needed to assess the diagnostic yield of separate bulb biopsies for celiac detection in all comers.”
An American College of Gastroenterology Junior Faculty Development Award helped support the work. Senior author Joseph A. Murray, MD, disclosed ties to Alba Therapeutics, Alvine Pharmaceuticals, AMAG Pharmaceuticals, and several other corporate entities. The remaining authors had no disclosures.
Separate sampling of the duodenal bulb increased detection of celiac disease by only 0.1% when endoscopy patients had a low pretest probability of celiac disease, according to research published in the November issue of Clinical Gastroenterology and Hepatology.
Duodenal bulb histology did reveal other abnormal findings, such as chronic peptic duodenitis, gastric heterotopia, and Brunner gland hyperplasia, wrote Samantha Stoven, MD, of Mayo Clinic, Rochester, Minn., and her associates. These findings did not seem to impede the identification of celiac disease, but their clinical implications were unclear, the researchers noted.
Most studies of the diagnostic yield of duodenal bulb specimens have been performed in patients with known celiac disease or positive serology. In past studies of these high-probability cohorts, duodenal bulb sampling increased the diagnostic yield of celiac disease anywhere from 1.8% to 18%, but whether and how that finding translates to low-probability cohorts is unclear, the researchers said. Therefore, they retrospectively analyzed data from 679 endoscopy patients who had both duodenal bulb and small bowel biopsies collected at three Mayo Clinic sites in 2011. These sites are “open access,” meaning that patients can be referred for endoscopy without the approval of a gastroenterologist.
The average age of the patients was 50 years, and 63% were female. They were most commonly referred for duodenal biopsy because of chronic dyspepsia (46% of patients), diarrhea (35%), or nausea (17%). Patients with either known celiac disease or positive serology were excluded from the study (Clin Gastroenterol Hepatol. 2016 Mar 7. doi: 10.1016/j.cgh.2016.02.026). A total of 265 patients (39%) had abnormal duodenal histology, which was most often diagnosed as chronic peptic duodenitis, the researchers said. Histologic abnormalities usually involved the duodenal bulb (36% of cases), not the distal duodenum (15%; P less than .0001). However, among the 16 patients (2%) found to have celiac disease, just one patient had disease only in the duodenal bulb. Thus, duodenal bulb sampling increased the diagnostic yield of celiac disease by only 0.1% when considering the overall cohort. The patient with celiac disease limited to the bulb was a 46-year-old female presenting with diarrhea and anemia who had normal serologies but a permissive human leukocyte antigen test. Her duodenal bulb had villous atrophy and more than 25 intraepithelial lymphocytes per 100 epithelial cells, while her distal duodenum was normal.
Among the 85% of patients who had normal distal duodenums, 28% had abnormal bulb histology, most often chronic peptic duodenitis, active chronic peptic duodenitis, or gastric heterotopia, the researchers said. Among the 59% of patients whose celiac serology before endoscopy was truly unknown, only two (0.5%) had histologic changes consistent with celiac disease, which in both cases were located in the distal duodenum.
SOURCE: American Gastroenterological Association
“Individual sampling of the duodenal bulb in patients with either negative or unknown celiac serologic status can be considered in practices where expert gastrointestinal pathologists are present and there is agreement that both samples can be submitted in the same bottle, or there is not a separate charge for the additional container. Further studies may be needed to assess the diagnostic yield of separate bulb biopsies for celiac detection in all comers.”
An American College of Gastroenterology Junior Faculty Development Award helped support the work. Senior author Joseph A. Murray, MD, disclosed ties to Alba Therapeutics, Alvine Pharmaceuticals, AMAG Pharmaceuticals, and several other corporate entities. The remaining authors had no disclosures.
FROM CLINICAL GASTROENTEROLOGY AND HEPATOLOGY
Key clinical point: Separate sampling of the duodenal bulb increased detection of celiac disease by only 0.1% when endoscopy patients had a low pretest probability of celiac disease.
Major finding: One (0.1%) patient had celiac disease limited to the duodenal bulb.
Data source: A multicenter retrospective study of 679 patients without celiac disease or positive serology from whom duodenal bulb and small bowel biopsies were collected during endoscopy.
Disclosures: An American College of Gastroenterology Junior Faculty Development Award helped support the work. Senior author Joseph A. Murray, MD, disclosed ties to Alba Therapeutics, Alvine Pharmaceuticals, AMAG Pharmaceuticals, and several other corporate entities. The remaining authors had no disclosures.
Ombitasvir, paritaprevir, ritonavir, and dasabuvir in CKD patients with HCV
Twelve weeks of ombitasvir, paritaprevir, ritonavir, and dasabuvir (Viekira Pak) achieved sustained viral response in 90% of patients with noncirrhotic chronic hepatitis C virus (HCV) genotype 1 infection and comorbid stage 4 or 5 chronic kidney disease, according to a small, single-arm, industry-sponsored trial reported in the November issue of Gastroenterology.
Adverse effects were usually mild or moderate, and serious adverse effects were considered unrelated to treatment, Paul Pockros, MD, at Scripps Clinic and Scripps Translational Science Institute in La Jolla, Calif., and his associates reported in Gastroenterology. No patients stopped direct-acting antivirals because of adverse effects, although nearly half had to interrupt or discontinue ribavirin because of worsening anemia. “The results of this study are important for hepatologists, gastroenterologists, and infectious disease specialists who are accustomed to treating HCV-infected patients with direct-acting antiviral therapy but who may not yet have seen sufficient data to initiate [it] in patients with end-stage renal disease,” the researchers said. “Nephrologists, who may not be accustomed to treating HCV, should also be aware that treatment options may now be available that can help prevent end-stage sequelae of HCV.”
All patients completed treatment, and 18 (90%) achieved sustained viral response (95% confidence interval, 70%-97%). The most common adverse events were anemia (45% of patients), fatigue (35%), diarrhea (25%), and nausea (25%). Among the two patients who did not achieve sustained viral response, one relapsed and the other died. The relapse occurred in a 49-year-old black man on hemodialysis who took about 91% of his medication doses, compared with about 97% for the rest of the cohort, the investigators said. This patient also had to interrupt ribavirin after his hemoglobin level dropped below 10 g/dL. The death occurred in a 60-year-old male hemodialysis patient who had hypertensive nephropathy and developed hypertensive urgency and cardiomyopathy soon after finishing treatment. His death, although considered unrelated to HCV treatment, underscores the need for close monitoring and collaboration between physicians treating HCV and nephrologists, the researchers said.
Most patients in this study were in stage 5 chronic kidney disease. However, the median baseline hemoglobin level was relatively high at 12 g/dL, implying that these patients would tolerate ribavirin better than would those with more pronounced anemia, the researchers noted. Nonetheless, 9 of 13 patients had to interrupt discontinue ribavirin because of worsening anemia. “Therefore, this study does not provide guidance for chronic kidney disease patients with much lower baseline hemoglobin levels, who might not tolerate even a small decrease,” the investigators cautioned.
AbbVie makes Viekira Pak and sponsored the study. Dr. Pockros disclosed ties to AbbVie, Bristol-Myers Squibb, Gilead, Janssen, and Merck.
Twelve weeks of ombitasvir, paritaprevir, ritonavir, and dasabuvir (Viekira Pak) achieved sustained viral response in 90% of patients with noncirrhotic chronic hepatitis C virus (HCV) genotype 1 infection and comorbid stage 4 or 5 chronic kidney disease, according to a small, single-arm, industry-sponsored trial reported in the November issue of Gastroenterology.
Adverse effects were usually mild or moderate, and serious adverse effects were considered unrelated to treatment, Paul Pockros, MD, at Scripps Clinic and Scripps Translational Science Institute in La Jolla, Calif., and his associates reported in Gastroenterology. No patients stopped direct-acting antivirals because of adverse effects, although nearly half had to interrupt or discontinue ribavirin because of worsening anemia. “The results of this study are important for hepatologists, gastroenterologists, and infectious disease specialists who are accustomed to treating HCV-infected patients with direct-acting antiviral therapy but who may not yet have seen sufficient data to initiate [it] in patients with end-stage renal disease,” the researchers said. “Nephrologists, who may not be accustomed to treating HCV, should also be aware that treatment options may now be available that can help prevent end-stage sequelae of HCV.”
All patients completed treatment, and 18 (90%) achieved sustained viral response (95% confidence interval, 70%-97%). The most common adverse events were anemia (45% of patients), fatigue (35%), diarrhea (25%), and nausea (25%). Among the two patients who did not achieve sustained viral response, one relapsed and the other died. The relapse occurred in a 49-year-old black man on hemodialysis who took about 91% of his medication doses, compared with about 97% for the rest of the cohort, the investigators said. This patient also had to interrupt ribavirin after his hemoglobin level dropped below 10 g/dL. The death occurred in a 60-year-old male hemodialysis patient who had hypertensive nephropathy and developed hypertensive urgency and cardiomyopathy soon after finishing treatment. His death, although considered unrelated to HCV treatment, underscores the need for close monitoring and collaboration between physicians treating HCV and nephrologists, the researchers said.
Most patients in this study were in stage 5 chronic kidney disease. However, the median baseline hemoglobin level was relatively high at 12 g/dL, implying that these patients would tolerate ribavirin better than would those with more pronounced anemia, the researchers noted. Nonetheless, 9 of 13 patients had to interrupt discontinue ribavirin because of worsening anemia. “Therefore, this study does not provide guidance for chronic kidney disease patients with much lower baseline hemoglobin levels, who might not tolerate even a small decrease,” the investigators cautioned.
AbbVie makes Viekira Pak and sponsored the study. Dr. Pockros disclosed ties to AbbVie, Bristol-Myers Squibb, Gilead, Janssen, and Merck.
Twelve weeks of ombitasvir, paritaprevir, ritonavir, and dasabuvir (Viekira Pak) achieved sustained viral response in 90% of patients with noncirrhotic chronic hepatitis C virus (HCV) genotype 1 infection and comorbid stage 4 or 5 chronic kidney disease, according to a small, single-arm, industry-sponsored trial reported in the November issue of Gastroenterology.
Adverse effects were usually mild or moderate, and serious adverse effects were considered unrelated to treatment, Paul Pockros, MD, at Scripps Clinic and Scripps Translational Science Institute in La Jolla, Calif., and his associates reported in Gastroenterology. No patients stopped direct-acting antivirals because of adverse effects, although nearly half had to interrupt or discontinue ribavirin because of worsening anemia. “The results of this study are important for hepatologists, gastroenterologists, and infectious disease specialists who are accustomed to treating HCV-infected patients with direct-acting antiviral therapy but who may not yet have seen sufficient data to initiate [it] in patients with end-stage renal disease,” the researchers said. “Nephrologists, who may not be accustomed to treating HCV, should also be aware that treatment options may now be available that can help prevent end-stage sequelae of HCV.”
All patients completed treatment, and 18 (90%) achieved sustained viral response (95% confidence interval, 70%-97%). The most common adverse events were anemia (45% of patients), fatigue (35%), diarrhea (25%), and nausea (25%). Among the two patients who did not achieve sustained viral response, one relapsed and the other died. The relapse occurred in a 49-year-old black man on hemodialysis who took about 91% of his medication doses, compared with about 97% for the rest of the cohort, the investigators said. This patient also had to interrupt ribavirin after his hemoglobin level dropped below 10 g/dL. The death occurred in a 60-year-old male hemodialysis patient who had hypertensive nephropathy and developed hypertensive urgency and cardiomyopathy soon after finishing treatment. His death, although considered unrelated to HCV treatment, underscores the need for close monitoring and collaboration between physicians treating HCV and nephrologists, the researchers said.
Most patients in this study were in stage 5 chronic kidney disease. However, the median baseline hemoglobin level was relatively high at 12 g/dL, implying that these patients would tolerate ribavirin better than would those with more pronounced anemia, the researchers noted. Nonetheless, 9 of 13 patients had to interrupt discontinue ribavirin because of worsening anemia. “Therefore, this study does not provide guidance for chronic kidney disease patients with much lower baseline hemoglobin levels, who might not tolerate even a small decrease,” the investigators cautioned.
AbbVie makes Viekira Pak and sponsored the study. Dr. Pockros disclosed ties to AbbVie, Bristol-Myers Squibb, Gilead, Janssen, and Merck.
FROM GASTROENTEROLOGY
Key clinical point: Twelve weeks of ombitasvir, paritaprevir, ritonavir, and dasabuvir with or without ribavirin was relatively well tolerated and cured most genotype 1 chronic hepatitis C virus infections in patients with severe or end-stage renal disease.
Major finding: All patients completed treatment and 18 (90%) achieved sustained viral response. The most common adverse effect was anemia (45% of patients).
Data source: A single-arm, multicenter study of 20 treatment-naive, noncirrhotic adults with HCV genotype 1 infection and stage 4 or 5 chronic kidney disease.
Disclosures: AbbVie makes Viekira Pak and sponsored the study. Dr. Pockros disclosed ties to AbbVie, Bristol-Myers Squibb, Gilead, Janssen, and Merck.
Analysis yields ‘strong evidence’ for benefit of physical activity in NAFLD
Regular physical exercise significantly improved measures of nonalcoholic fatty liver disease independently of dietary changes, according to a meta-analysis of randomized controlled* trials published in the October issue of Clinical Gastroenterology and Hepatology.
“On the basis of the current findings, physical activity should be recommended not only in combination with dietary changes but also independently as an effective approach to manage NAFLD,” wrote Lorenzo Orci, MD, and his associates at the University of Geneva. “We propose that the level of evidence surrounding the specific role of physical activity in the management of NAFLD is now sufficient to be awarded a grade of Ia.”
Nonalcoholic fatty liver disease, “the hepatic manifestation of metabolic syndrome,” affects at least one in four U.S. adults and 15%-35% of individuals in Europe, the Middle East, China, and Japan, the researchers noted. Dietary changes are the cornerstone of NAFLD management, and there is less evidence for how physical exercise affects liver fat content. Therefore, the researchers searched MEDLINE, Embase, and the Cochrane databases from inception through October 2015 to find randomized trials of the impact of physical activity on markers of liver steatosis and liver inflammation in patients diagnosed with NAFLD, obesity, type 2 diabetes, or metabolic syndrome. This approach yielded 28 trials with data from more than 1,600 patients. Only two trials were multicenter, 13 required participants to have an NAFLD diagnosis, four focused on type 2 diabetes, and most of the rest included sedentary obese patients without requiring a diagnosis of NAFLD, the researchers said (Clin Gastroenterol Hepatol. 2016 May 4. doi: 10.1016/j.cgh.2016.04.036).
After researchers accounted for dietary changes, physical activity led to a significant drop in intrahepatic lipid content with a standardized mean difference of –0.69 compared with controls (95% confidence interval, –0.90 to –0.48; P less than .0001). “Because effect sizes such as standard mean difference [SMD] are difficult to interpret, the translation of such a statistical measure into a clinically relevant notion has been the focus of research for more than a decade,” the investigators added. “A commonly used interpretation was proposed by Cohen, who suggested that SMDs of 0.2, 0.5, and 0.8 correspond to small, moderate, and large effect sizes, respectively. By using this rule of thumb, our results indicate that physical activity exerts a moderate-to-large impact on the reduction of intrahepatic lipid content.”
Exercise reduced liver fat content even more in pediatric patients (SMD, –0.75; 95% CI, –0.1 to –0.5; P less than .0001) and in patients who had been specifically diagnosed with NAFLD (SMD, –0.86; 95% CI, –1.26 to –0.46; P less than .0001). Patients with the highest baseline body mass index also seemed to benefit more than patients with lower baseline BMI (P = .04). Indeed, exercise reduced BMI itself by a weighted mean difference of 0.8 (95% CI, –1.22 to 0.38; P less than .001), the researchers noted. Exercise intensity did not seem to affect the likelihood of benefit. There was a trend toward a greater effect of aerobic over resistance training (P = .06), and few studies examined the effects of combining both types of exercise.
The multivariable analysis also linked physical activity to an average 3.30 IU/L drop in alanine aminotransferase levels (95% CI, –5.57 to –1.04) and to a 4.9 IU/L decrease in aspartate aminotransferase levels (95% CI, –8.68 to –1.02). The investigators were unable to assess the long-term effects of physical exercise, nor its effects on hepatic fibrosis or inflammation, they noted. Nonetheless, the moderate to large effect size “provides strong evidence for the recommendation of physical activity as an effective intervention in the treatment of NAFLD,” they concluded. “Physical activity is also associated with an improvement in blood levels of aminotransferases and is particularly beneficial in patients presenting with severe obesity at baseline.”
The work was funded by the Ligue Genevoise contre le Cancer and the Dr Henri Dubois-Ferrière/Dinu Lipatti Foundation and by the Swiss National Science Foundation. The investigators had no disclosures.
*Content was updated on 10/25/2016
There has been tremendous interest in developing pharmacologic treatments for nonalcoholic steatohepatitis, especially in the Western world. There has not been significant enthusiasm for investigating exercise-based lifestyle modification as a primary treatment for NASH. Although the meta-analysis by Orci et al. included 28 studies, there are only 2 studies (combined, fewer than 100 patients) that examined the effect of exercise on liver histology in NASH and they both suggest that lifestyle modification consisting of exercise in addition to dietary modification improves liver histology in NASH. A seminal study was published by Vilar-Gomez et al. (Gastroenterology. 2015;149:367-78) that showed that a lifestyle modification consisting of reduction in caloric intake by 750 kcal/d along with low-intensity exercise (200 minutes of walking each week) led to significant improvement in liver histology, especially in those who lost at least 5% of their body weight.
Naga Chalasani, MD, AGAF, FACG, FAASLD, is the David W. Crabb Professor and director of the division of gastroenterology and hepatology, Indiana University, Purdue. He had no relevant conflicts.
There has been tremendous interest in developing pharmacologic treatments for nonalcoholic steatohepatitis, especially in the Western world. There has not been significant enthusiasm for investigating exercise-based lifestyle modification as a primary treatment for NASH. Although the meta-analysis by Orci et al. included 28 studies, there are only 2 studies (combined, fewer than 100 patients) that examined the effect of exercise on liver histology in NASH and they both suggest that lifestyle modification consisting of exercise in addition to dietary modification improves liver histology in NASH. A seminal study was published by Vilar-Gomez et al. (Gastroenterology. 2015;149:367-78) that showed that a lifestyle modification consisting of reduction in caloric intake by 750 kcal/d along with low-intensity exercise (200 minutes of walking each week) led to significant improvement in liver histology, especially in those who lost at least 5% of their body weight.
Naga Chalasani, MD, AGAF, FACG, FAASLD, is the David W. Crabb Professor and director of the division of gastroenterology and hepatology, Indiana University, Purdue. He had no relevant conflicts.
There has been tremendous interest in developing pharmacologic treatments for nonalcoholic steatohepatitis, especially in the Western world. There has not been significant enthusiasm for investigating exercise-based lifestyle modification as a primary treatment for NASH. Although the meta-analysis by Orci et al. included 28 studies, there are only 2 studies (combined, fewer than 100 patients) that examined the effect of exercise on liver histology in NASH and they both suggest that lifestyle modification consisting of exercise in addition to dietary modification improves liver histology in NASH. A seminal study was published by Vilar-Gomez et al. (Gastroenterology. 2015;149:367-78) that showed that a lifestyle modification consisting of reduction in caloric intake by 750 kcal/d along with low-intensity exercise (200 minutes of walking each week) led to significant improvement in liver histology, especially in those who lost at least 5% of their body weight.
Naga Chalasani, MD, AGAF, FACG, FAASLD, is the David W. Crabb Professor and director of the division of gastroenterology and hepatology, Indiana University, Purdue. He had no relevant conflicts.
Regular physical exercise significantly improved measures of nonalcoholic fatty liver disease independently of dietary changes, according to a meta-analysis of randomized controlled* trials published in the October issue of Clinical Gastroenterology and Hepatology.
“On the basis of the current findings, physical activity should be recommended not only in combination with dietary changes but also independently as an effective approach to manage NAFLD,” wrote Lorenzo Orci, MD, and his associates at the University of Geneva. “We propose that the level of evidence surrounding the specific role of physical activity in the management of NAFLD is now sufficient to be awarded a grade of Ia.”
Nonalcoholic fatty liver disease, “the hepatic manifestation of metabolic syndrome,” affects at least one in four U.S. adults and 15%-35% of individuals in Europe, the Middle East, China, and Japan, the researchers noted. Dietary changes are the cornerstone of NAFLD management, and there is less evidence for how physical exercise affects liver fat content. Therefore, the researchers searched MEDLINE, Embase, and the Cochrane databases from inception through October 2015 to find randomized trials of the impact of physical activity on markers of liver steatosis and liver inflammation in patients diagnosed with NAFLD, obesity, type 2 diabetes, or metabolic syndrome. This approach yielded 28 trials with data from more than 1,600 patients. Only two trials were multicenter, 13 required participants to have an NAFLD diagnosis, four focused on type 2 diabetes, and most of the rest included sedentary obese patients without requiring a diagnosis of NAFLD, the researchers said (Clin Gastroenterol Hepatol. 2016 May 4. doi: 10.1016/j.cgh.2016.04.036).
After researchers accounted for dietary changes, physical activity led to a significant drop in intrahepatic lipid content with a standardized mean difference of –0.69 compared with controls (95% confidence interval, –0.90 to –0.48; P less than .0001). “Because effect sizes such as standard mean difference [SMD] are difficult to interpret, the translation of such a statistical measure into a clinically relevant notion has been the focus of research for more than a decade,” the investigators added. “A commonly used interpretation was proposed by Cohen, who suggested that SMDs of 0.2, 0.5, and 0.8 correspond to small, moderate, and large effect sizes, respectively. By using this rule of thumb, our results indicate that physical activity exerts a moderate-to-large impact on the reduction of intrahepatic lipid content.”
Exercise reduced liver fat content even more in pediatric patients (SMD, –0.75; 95% CI, –0.1 to –0.5; P less than .0001) and in patients who had been specifically diagnosed with NAFLD (SMD, –0.86; 95% CI, –1.26 to –0.46; P less than .0001). Patients with the highest baseline body mass index also seemed to benefit more than patients with lower baseline BMI (P = .04). Indeed, exercise reduced BMI itself by a weighted mean difference of 0.8 (95% CI, –1.22 to 0.38; P less than .001), the researchers noted. Exercise intensity did not seem to affect the likelihood of benefit. There was a trend toward a greater effect of aerobic over resistance training (P = .06), and few studies examined the effects of combining both types of exercise.
The multivariable analysis also linked physical activity to an average 3.30 IU/L drop in alanine aminotransferase levels (95% CI, –5.57 to –1.04) and to a 4.9 IU/L decrease in aspartate aminotransferase levels (95% CI, –8.68 to –1.02). The investigators were unable to assess the long-term effects of physical exercise, nor its effects on hepatic fibrosis or inflammation, they noted. Nonetheless, the moderate to large effect size “provides strong evidence for the recommendation of physical activity as an effective intervention in the treatment of NAFLD,” they concluded. “Physical activity is also associated with an improvement in blood levels of aminotransferases and is particularly beneficial in patients presenting with severe obesity at baseline.”
The work was funded by the Ligue Genevoise contre le Cancer and the Dr Henri Dubois-Ferrière/Dinu Lipatti Foundation and by the Swiss National Science Foundation. The investigators had no disclosures.
*Content was updated on 10/25/2016
Regular physical exercise significantly improved measures of nonalcoholic fatty liver disease independently of dietary changes, according to a meta-analysis of randomized controlled* trials published in the October issue of Clinical Gastroenterology and Hepatology.
“On the basis of the current findings, physical activity should be recommended not only in combination with dietary changes but also independently as an effective approach to manage NAFLD,” wrote Lorenzo Orci, MD, and his associates at the University of Geneva. “We propose that the level of evidence surrounding the specific role of physical activity in the management of NAFLD is now sufficient to be awarded a grade of Ia.”
Nonalcoholic fatty liver disease, “the hepatic manifestation of metabolic syndrome,” affects at least one in four U.S. adults and 15%-35% of individuals in Europe, the Middle East, China, and Japan, the researchers noted. Dietary changes are the cornerstone of NAFLD management, and there is less evidence for how physical exercise affects liver fat content. Therefore, the researchers searched MEDLINE, Embase, and the Cochrane databases from inception through October 2015 to find randomized trials of the impact of physical activity on markers of liver steatosis and liver inflammation in patients diagnosed with NAFLD, obesity, type 2 diabetes, or metabolic syndrome. This approach yielded 28 trials with data from more than 1,600 patients. Only two trials were multicenter, 13 required participants to have an NAFLD diagnosis, four focused on type 2 diabetes, and most of the rest included sedentary obese patients without requiring a diagnosis of NAFLD, the researchers said (Clin Gastroenterol Hepatol. 2016 May 4. doi: 10.1016/j.cgh.2016.04.036).
After researchers accounted for dietary changes, physical activity led to a significant drop in intrahepatic lipid content with a standardized mean difference of –0.69 compared with controls (95% confidence interval, –0.90 to –0.48; P less than .0001). “Because effect sizes such as standard mean difference [SMD] are difficult to interpret, the translation of such a statistical measure into a clinically relevant notion has been the focus of research for more than a decade,” the investigators added. “A commonly used interpretation was proposed by Cohen, who suggested that SMDs of 0.2, 0.5, and 0.8 correspond to small, moderate, and large effect sizes, respectively. By using this rule of thumb, our results indicate that physical activity exerts a moderate-to-large impact on the reduction of intrahepatic lipid content.”
Exercise reduced liver fat content even more in pediatric patients (SMD, –0.75; 95% CI, –0.1 to –0.5; P less than .0001) and in patients who had been specifically diagnosed with NAFLD (SMD, –0.86; 95% CI, –1.26 to –0.46; P less than .0001). Patients with the highest baseline body mass index also seemed to benefit more than patients with lower baseline BMI (P = .04). Indeed, exercise reduced BMI itself by a weighted mean difference of 0.8 (95% CI, –1.22 to 0.38; P less than .001), the researchers noted. Exercise intensity did not seem to affect the likelihood of benefit. There was a trend toward a greater effect of aerobic over resistance training (P = .06), and few studies examined the effects of combining both types of exercise.
The multivariable analysis also linked physical activity to an average 3.30 IU/L drop in alanine aminotransferase levels (95% CI, –5.57 to –1.04) and to a 4.9 IU/L decrease in aspartate aminotransferase levels (95% CI, –8.68 to –1.02). The investigators were unable to assess the long-term effects of physical exercise, nor its effects on hepatic fibrosis or inflammation, they noted. Nonetheless, the moderate to large effect size “provides strong evidence for the recommendation of physical activity as an effective intervention in the treatment of NAFLD,” they concluded. “Physical activity is also associated with an improvement in blood levels of aminotransferases and is particularly beneficial in patients presenting with severe obesity at baseline.”
The work was funded by the Ligue Genevoise contre le Cancer and the Dr Henri Dubois-Ferrière/Dinu Lipatti Foundation and by the Swiss National Science Foundation. The investigators had no disclosures.
*Content was updated on 10/25/2016
Key clinical point: Physical activity benefits measures of nonalcoholic fatty liver disease independently of diet.
Major finding: After researchers accounted for dietary changes, physical activity led to a significant drop in intrahepatic lipid content with a standardized mean difference of –0.69 compared with controls (95% confidence interval, –0.90 to –0.48; P less than .0001).
Data source: A systematic review and meta-analysis of 28 randomized controlled trials comprising more than 16,000 patients.
Disclosures: The work was funded by the Ligue Genevoise contre le Cancer and the Dr Henri Dubois-Ferrière/Dinu Lipatti Foundation and by the Swiss National Science Foundation. The researchers had no disclosures.
VIDEO: Transcriptomics link gastric cancer to RNA misediting
Changes in the sequence of RNA – also known as RNA editing – might contribute to the pathogenesis and prognosis of gastric cancer, investigators reported in the October issue of Gastroenterology.
By performing high-throughput transcriptome sequencing (or RNA-Seq) of gastric tumor tissue and cell lines, they identified “a clear RNA misediting phenotype” characterized by an imbalance in enzymes called adenosine deaminases that act on RNA, or ADAR, Tim Hon Man Chan, MD, of the National University of Singapore and his associates said. Normal gastric tissue did not show these changes, and they were associated with tumor progression and a poor prognosis, the investigators added.
Gastric cancer is the third most lethal malignancy worldwide. RNA editing in humans usually involves the conversion of adenosine-to-inosine (A-to-I), which is catalyzed by the ADAR1 and ADAR2 enzymes and has been linked to liver and esophageal cancer. To explore the role of A-to-I RNA editing in gastric cancer, the researchers performed next-generation sequencing transcriptomics of 14 tissue specimens from primary gastric tumors and 14 matched samples from noncancerous gastric tissue. They also sequenced gastric cancer cell lines and analyzed single nucleotide polymorphism array and RNA-Seq data from the gastric cancer datasets of The Cancer Genome Atlas (Gastroenterology. 2016 Jun 30. doi: 10.1053/j.gastro.2016.06.043).
Compared with normal tissue specimens, almost all the samples of gastric cancers showed clear evidence of ADAR dysregulation – specifically, the gain of the ADAR1 gene and the loss of the ADAR2 gene, the researchers said. Furthermore, both the in vivo and in vitro studies indicated that RNA editing enabled ADAR1 to function as an oncogene in gastric cancer, while ADAR2 functioned as a tumor suppressor gene. In The Cancer Genome Atlas, about 36% of gastric cancer patients had gained ADAR1, while 44% of patients had lost ADAR2. Not only did these changes lead to a significant rise in ADAR1 mRNA expression and a significant decrease in ADAR2 mRNA expression, but patients with the most developed gastric tumors had the highest levels of ADAR1/ADAR2 dysregulation and the worst clinical prognosis, the investigators said.
SOURCE: American Gastroenterological Association
Additional tests of a model target gene called PODXL (podocalyxin-like) showed that ADAR2 regulates the editing of codon 241 such that histidine is replaced with arginine, which then neutralizes the normal tumorigenic ability of unedited or wild-type PODXL, the researchers reported. “Our data suggest that dysregulation of RNA editing is pervasive in gastric cancer, approaching levels reported for other types of epigenetic dysregulation including DNA methylation and histone modification,” they added. Taken together, the findings “highlight RNA editing as an important pathogenic mechanism in gastric carcinogenesis, and ADAR enzymes as potential gastric cancer therapeutic targets.”
These findings are especially important because the identification of DNA mutations in gastric cancer has not led to viable therapies except for traztuzumab in ERBB2-positive gastric cancer and ramucirumab in advanced gastric cancer, the investigators said. “Our study demonstrates that differentially expressed ADARs in gastric tumors with consequent A-to-I RNA editing dysregulation may serve as a second layer of ‘somatic mutations’ and a novel contributor to gastric cancer,” they concluded.
The work was funded by the National Research Foundation Singapore, the Singapore Ministry of Education, and several other noncorporate entities. The researchers had no disclosures.
Changes in the sequence of RNA – also known as RNA editing – might contribute to the pathogenesis and prognosis of gastric cancer, investigators reported in the October issue of Gastroenterology.
By performing high-throughput transcriptome sequencing (or RNA-Seq) of gastric tumor tissue and cell lines, they identified “a clear RNA misediting phenotype” characterized by an imbalance in enzymes called adenosine deaminases that act on RNA, or ADAR, Tim Hon Man Chan, MD, of the National University of Singapore and his associates said. Normal gastric tissue did not show these changes, and they were associated with tumor progression and a poor prognosis, the investigators added.
Gastric cancer is the third most lethal malignancy worldwide. RNA editing in humans usually involves the conversion of adenosine-to-inosine (A-to-I), which is catalyzed by the ADAR1 and ADAR2 enzymes and has been linked to liver and esophageal cancer. To explore the role of A-to-I RNA editing in gastric cancer, the researchers performed next-generation sequencing transcriptomics of 14 tissue specimens from primary gastric tumors and 14 matched samples from noncancerous gastric tissue. They also sequenced gastric cancer cell lines and analyzed single nucleotide polymorphism array and RNA-Seq data from the gastric cancer datasets of The Cancer Genome Atlas (Gastroenterology. 2016 Jun 30. doi: 10.1053/j.gastro.2016.06.043).
Compared with normal tissue specimens, almost all the samples of gastric cancers showed clear evidence of ADAR dysregulation – specifically, the gain of the ADAR1 gene and the loss of the ADAR2 gene, the researchers said. Furthermore, both the in vivo and in vitro studies indicated that RNA editing enabled ADAR1 to function as an oncogene in gastric cancer, while ADAR2 functioned as a tumor suppressor gene. In The Cancer Genome Atlas, about 36% of gastric cancer patients had gained ADAR1, while 44% of patients had lost ADAR2. Not only did these changes lead to a significant rise in ADAR1 mRNA expression and a significant decrease in ADAR2 mRNA expression, but patients with the most developed gastric tumors had the highest levels of ADAR1/ADAR2 dysregulation and the worst clinical prognosis, the investigators said.
SOURCE: American Gastroenterological Association
Additional tests of a model target gene called PODXL (podocalyxin-like) showed that ADAR2 regulates the editing of codon 241 such that histidine is replaced with arginine, which then neutralizes the normal tumorigenic ability of unedited or wild-type PODXL, the researchers reported. “Our data suggest that dysregulation of RNA editing is pervasive in gastric cancer, approaching levels reported for other types of epigenetic dysregulation including DNA methylation and histone modification,” they added. Taken together, the findings “highlight RNA editing as an important pathogenic mechanism in gastric carcinogenesis, and ADAR enzymes as potential gastric cancer therapeutic targets.”
These findings are especially important because the identification of DNA mutations in gastric cancer has not led to viable therapies except for traztuzumab in ERBB2-positive gastric cancer and ramucirumab in advanced gastric cancer, the investigators said. “Our study demonstrates that differentially expressed ADARs in gastric tumors with consequent A-to-I RNA editing dysregulation may serve as a second layer of ‘somatic mutations’ and a novel contributor to gastric cancer,” they concluded.
The work was funded by the National Research Foundation Singapore, the Singapore Ministry of Education, and several other noncorporate entities. The researchers had no disclosures.
Changes in the sequence of RNA – also known as RNA editing – might contribute to the pathogenesis and prognosis of gastric cancer, investigators reported in the October issue of Gastroenterology.
By performing high-throughput transcriptome sequencing (or RNA-Seq) of gastric tumor tissue and cell lines, they identified “a clear RNA misediting phenotype” characterized by an imbalance in enzymes called adenosine deaminases that act on RNA, or ADAR, Tim Hon Man Chan, MD, of the National University of Singapore and his associates said. Normal gastric tissue did not show these changes, and they were associated with tumor progression and a poor prognosis, the investigators added.
Gastric cancer is the third most lethal malignancy worldwide. RNA editing in humans usually involves the conversion of adenosine-to-inosine (A-to-I), which is catalyzed by the ADAR1 and ADAR2 enzymes and has been linked to liver and esophageal cancer. To explore the role of A-to-I RNA editing in gastric cancer, the researchers performed next-generation sequencing transcriptomics of 14 tissue specimens from primary gastric tumors and 14 matched samples from noncancerous gastric tissue. They also sequenced gastric cancer cell lines and analyzed single nucleotide polymorphism array and RNA-Seq data from the gastric cancer datasets of The Cancer Genome Atlas (Gastroenterology. 2016 Jun 30. doi: 10.1053/j.gastro.2016.06.043).
Compared with normal tissue specimens, almost all the samples of gastric cancers showed clear evidence of ADAR dysregulation – specifically, the gain of the ADAR1 gene and the loss of the ADAR2 gene, the researchers said. Furthermore, both the in vivo and in vitro studies indicated that RNA editing enabled ADAR1 to function as an oncogene in gastric cancer, while ADAR2 functioned as a tumor suppressor gene. In The Cancer Genome Atlas, about 36% of gastric cancer patients had gained ADAR1, while 44% of patients had lost ADAR2. Not only did these changes lead to a significant rise in ADAR1 mRNA expression and a significant decrease in ADAR2 mRNA expression, but patients with the most developed gastric tumors had the highest levels of ADAR1/ADAR2 dysregulation and the worst clinical prognosis, the investigators said.
SOURCE: American Gastroenterological Association
Additional tests of a model target gene called PODXL (podocalyxin-like) showed that ADAR2 regulates the editing of codon 241 such that histidine is replaced with arginine, which then neutralizes the normal tumorigenic ability of unedited or wild-type PODXL, the researchers reported. “Our data suggest that dysregulation of RNA editing is pervasive in gastric cancer, approaching levels reported for other types of epigenetic dysregulation including DNA methylation and histone modification,” they added. Taken together, the findings “highlight RNA editing as an important pathogenic mechanism in gastric carcinogenesis, and ADAR enzymes as potential gastric cancer therapeutic targets.”
These findings are especially important because the identification of DNA mutations in gastric cancer has not led to viable therapies except for traztuzumab in ERBB2-positive gastric cancer and ramucirumab in advanced gastric cancer, the investigators said. “Our study demonstrates that differentially expressed ADARs in gastric tumors with consequent A-to-I RNA editing dysregulation may serve as a second layer of ‘somatic mutations’ and a novel contributor to gastric cancer,” they concluded.
The work was funded by the National Research Foundation Singapore, the Singapore Ministry of Education, and several other noncorporate entities. The researchers had no disclosures.
FROM GASTROENTEROLOGY
Key clinical point: Sequence alterations at the RNA level may contribute to the pathology and prognosis of gastric cancer.
Major finding: Compared with normal gastric tissue, almost all gastric cancer specimens exhibited an RNA misediting phenotype characterized by the respective gain and loss of the ADAR1 and ADAR2 genes.
Data source: High-throughput transcriptome sequencing (RNA-Seq) of gastric cancer and normal tissue, sequencing of gastric cancer cell lines, and analyses of The Cancer Genome Atlas.
Disclosures: The work was funded by the National Research Foundation Singapore, the Singapore Ministry of Education, and several other noncorporate entities. The investigators had no disclosures.
CSF2RB mutation, common in Ashkenazim, linked to Crohn’s
A frameshift mutation in the CSF2RB gene that is common in Ashkenazi Jews has been linked to Crohn’s disease, according to a report in Gastroenterology.
The Ashkenazi Jewish population has a four- to sevenfold higher prevalence of Crohn’s disease than does the general population. To identify possible genetic mutations associated with the disease, researchers performed exome sequencing of samples from 1,477 Ashkenazi Jewish patients with Crohn’s disease and 2,614 Ashkenazi Jewish control subjects who didn’t have the disorder. All the study participants were enrolled at medical centers throughout North America, Europe, and Israel, noted Ling-Shiang Chuang, PhD, a postdoctoral fellow in genetics and genomic sciences, Mount Sinai Medical Center, New York, and his associates.
They genotyped 224 frameshift mutations and identified one in the colony-stimulating factor 2–receptor beta common subunit (CSF2RB) gene that was significantly associated with Crohn’s disease. They validated this association in a replication cohort of 1,515 Ashkenazi Jewish patients with Crohn’s disease and 7,052 health Ashkenazi control subjects, observing nearly identical allele frequencies and odds ratios as in the discovery cohort.
Since the CSF2RB gene encodes for the receptor for GM-CSF (granulocyte-macrophage colony-stimulating factor) cytokines, the mutation would be expected to reduce GM-CSF signaling in monocytes obtained from carriers. The investigators found this was true when they examined monocytes taken from intestinal-tissue samples and peripheral-blood samples from affected patients.
“The present findings of a loss-of-function frameshift variant in the CSF2RB gene argue for a primary pathogenic role of decreased GM-CSF signaling in driving a subset of Crohn’s disease cases. As many as 30% of cases have increased levels of anti-GM-CSF antibodies, which can neutralize GM-CSF activity, indicating that impaired GM-CSF signaling plays a major role in Crohn’s disease,” Dr. Chuang and his associates wrote (Gastroenterol. 2016;151:710-23.e2. doi: 10.1053/j.gastro.2016.06.045).
Future research may identify subgroups of Crohn’s patients in which treatments that target GM-CSF signaling may be effective, and it may also point the way to novel therapies for the broader population of patients with Crohn’s disease, they added.
This study was supported by the National Institutes of Health, the Inflammatory Bowel Disease Genetics Consortium, the Genetic Research Center at Mount Sinai Medical Center, New York’s Crohn’s Foundation, and several others. Dr. Chuang and his associates reported having no relevant financial disclosures.
Genome-wide association studies (GWASs) have identified over 200 DNA mutations (mainly single nucleotide polymorphisms, SNPs) contributing to inflammatory bowel disease. However, their causality in IBD remains unknown. In the post-GWAS era, functional characterization and mechanistic elucidation of these SNPs is a major challenge. SNPs impacting the protein-coding genes can drastically alter protein function and play an important role in molecular pathogenesis. However, most SNPs are in noncoding genes and their impact on gene regulation and disease outcome remains largely unknown. This study reveals a frameshift mutation in the CSF2RB gene associated with Crohn’s disease in an Ashkenazi Jewish population. A frameshift mutation results in the deletion or insertion in a DNA sequence that shifts the way the sequence is transcribed into RNA. The most well described frameshift mutation in Crohn’s disease with clinical relevance is in the NOD2 gene, which results in impaired immune response to microbial stimuli. Because GWASs show association not causality, the biological consequence of the CSF2RB frameshift mutation in intestinal macrophages leads to a decrease in its response to granulocyte-monocyte stimulating factor (GM-CSF) providing a potential mechanistic role for the mutation in Crohn’s disease pathogenesis in a disease-relevant cell and site. This study will pave the way for future important studies that reveal the impact of SNPs on Crohn’s disease development, prognosis, and eventually response to therapy.
Shehzad Z. Sheikh MD, PhD, assistant professor of medicine, department of medicine, division of gastroenterology and hepatology, University of North Carolina, Chapel Hill.
Genome-wide association studies (GWASs) have identified over 200 DNA mutations (mainly single nucleotide polymorphisms, SNPs) contributing to inflammatory bowel disease. However, their causality in IBD remains unknown. In the post-GWAS era, functional characterization and mechanistic elucidation of these SNPs is a major challenge. SNPs impacting the protein-coding genes can drastically alter protein function and play an important role in molecular pathogenesis. However, most SNPs are in noncoding genes and their impact on gene regulation and disease outcome remains largely unknown. This study reveals a frameshift mutation in the CSF2RB gene associated with Crohn’s disease in an Ashkenazi Jewish population. A frameshift mutation results in the deletion or insertion in a DNA sequence that shifts the way the sequence is transcribed into RNA. The most well described frameshift mutation in Crohn’s disease with clinical relevance is in the NOD2 gene, which results in impaired immune response to microbial stimuli. Because GWASs show association not causality, the biological consequence of the CSF2RB frameshift mutation in intestinal macrophages leads to a decrease in its response to granulocyte-monocyte stimulating factor (GM-CSF) providing a potential mechanistic role for the mutation in Crohn’s disease pathogenesis in a disease-relevant cell and site. This study will pave the way for future important studies that reveal the impact of SNPs on Crohn’s disease development, prognosis, and eventually response to therapy.
Shehzad Z. Sheikh MD, PhD, assistant professor of medicine, department of medicine, division of gastroenterology and hepatology, University of North Carolina, Chapel Hill.
Genome-wide association studies (GWASs) have identified over 200 DNA mutations (mainly single nucleotide polymorphisms, SNPs) contributing to inflammatory bowel disease. However, their causality in IBD remains unknown. In the post-GWAS era, functional characterization and mechanistic elucidation of these SNPs is a major challenge. SNPs impacting the protein-coding genes can drastically alter protein function and play an important role in molecular pathogenesis. However, most SNPs are in noncoding genes and their impact on gene regulation and disease outcome remains largely unknown. This study reveals a frameshift mutation in the CSF2RB gene associated with Crohn’s disease in an Ashkenazi Jewish population. A frameshift mutation results in the deletion or insertion in a DNA sequence that shifts the way the sequence is transcribed into RNA. The most well described frameshift mutation in Crohn’s disease with clinical relevance is in the NOD2 gene, which results in impaired immune response to microbial stimuli. Because GWASs show association not causality, the biological consequence of the CSF2RB frameshift mutation in intestinal macrophages leads to a decrease in its response to granulocyte-monocyte stimulating factor (GM-CSF) providing a potential mechanistic role for the mutation in Crohn’s disease pathogenesis in a disease-relevant cell and site. This study will pave the way for future important studies that reveal the impact of SNPs on Crohn’s disease development, prognosis, and eventually response to therapy.
Shehzad Z. Sheikh MD, PhD, assistant professor of medicine, department of medicine, division of gastroenterology and hepatology, University of North Carolina, Chapel Hill.
A frameshift mutation in the CSF2RB gene that is common in Ashkenazi Jews has been linked to Crohn’s disease, according to a report in Gastroenterology.
The Ashkenazi Jewish population has a four- to sevenfold higher prevalence of Crohn’s disease than does the general population. To identify possible genetic mutations associated with the disease, researchers performed exome sequencing of samples from 1,477 Ashkenazi Jewish patients with Crohn’s disease and 2,614 Ashkenazi Jewish control subjects who didn’t have the disorder. All the study participants were enrolled at medical centers throughout North America, Europe, and Israel, noted Ling-Shiang Chuang, PhD, a postdoctoral fellow in genetics and genomic sciences, Mount Sinai Medical Center, New York, and his associates.
They genotyped 224 frameshift mutations and identified one in the colony-stimulating factor 2–receptor beta common subunit (CSF2RB) gene that was significantly associated with Crohn’s disease. They validated this association in a replication cohort of 1,515 Ashkenazi Jewish patients with Crohn’s disease and 7,052 health Ashkenazi control subjects, observing nearly identical allele frequencies and odds ratios as in the discovery cohort.
Since the CSF2RB gene encodes for the receptor for GM-CSF (granulocyte-macrophage colony-stimulating factor) cytokines, the mutation would be expected to reduce GM-CSF signaling in monocytes obtained from carriers. The investigators found this was true when they examined monocytes taken from intestinal-tissue samples and peripheral-blood samples from affected patients.
“The present findings of a loss-of-function frameshift variant in the CSF2RB gene argue for a primary pathogenic role of decreased GM-CSF signaling in driving a subset of Crohn’s disease cases. As many as 30% of cases have increased levels of anti-GM-CSF antibodies, which can neutralize GM-CSF activity, indicating that impaired GM-CSF signaling plays a major role in Crohn’s disease,” Dr. Chuang and his associates wrote (Gastroenterol. 2016;151:710-23.e2. doi: 10.1053/j.gastro.2016.06.045).
Future research may identify subgroups of Crohn’s patients in which treatments that target GM-CSF signaling may be effective, and it may also point the way to novel therapies for the broader population of patients with Crohn’s disease, they added.
This study was supported by the National Institutes of Health, the Inflammatory Bowel Disease Genetics Consortium, the Genetic Research Center at Mount Sinai Medical Center, New York’s Crohn’s Foundation, and several others. Dr. Chuang and his associates reported having no relevant financial disclosures.
A frameshift mutation in the CSF2RB gene that is common in Ashkenazi Jews has been linked to Crohn’s disease, according to a report in Gastroenterology.
The Ashkenazi Jewish population has a four- to sevenfold higher prevalence of Crohn’s disease than does the general population. To identify possible genetic mutations associated with the disease, researchers performed exome sequencing of samples from 1,477 Ashkenazi Jewish patients with Crohn’s disease and 2,614 Ashkenazi Jewish control subjects who didn’t have the disorder. All the study participants were enrolled at medical centers throughout North America, Europe, and Israel, noted Ling-Shiang Chuang, PhD, a postdoctoral fellow in genetics and genomic sciences, Mount Sinai Medical Center, New York, and his associates.
They genotyped 224 frameshift mutations and identified one in the colony-stimulating factor 2–receptor beta common subunit (CSF2RB) gene that was significantly associated with Crohn’s disease. They validated this association in a replication cohort of 1,515 Ashkenazi Jewish patients with Crohn’s disease and 7,052 health Ashkenazi control subjects, observing nearly identical allele frequencies and odds ratios as in the discovery cohort.
Since the CSF2RB gene encodes for the receptor for GM-CSF (granulocyte-macrophage colony-stimulating factor) cytokines, the mutation would be expected to reduce GM-CSF signaling in monocytes obtained from carriers. The investigators found this was true when they examined monocytes taken from intestinal-tissue samples and peripheral-blood samples from affected patients.
“The present findings of a loss-of-function frameshift variant in the CSF2RB gene argue for a primary pathogenic role of decreased GM-CSF signaling in driving a subset of Crohn’s disease cases. As many as 30% of cases have increased levels of anti-GM-CSF antibodies, which can neutralize GM-CSF activity, indicating that impaired GM-CSF signaling plays a major role in Crohn’s disease,” Dr. Chuang and his associates wrote (Gastroenterol. 2016;151:710-23.e2. doi: 10.1053/j.gastro.2016.06.045).
Future research may identify subgroups of Crohn’s patients in which treatments that target GM-CSF signaling may be effective, and it may also point the way to novel therapies for the broader population of patients with Crohn’s disease, they added.
This study was supported by the National Institutes of Health, the Inflammatory Bowel Disease Genetics Consortium, the Genetic Research Center at Mount Sinai Medical Center, New York’s Crohn’s Foundation, and several others. Dr. Chuang and his associates reported having no relevant financial disclosures.
FROM GASTROENTEROLOGY
Key clinical point: A frameshift mutation in the CSF2RB gene that is common in Ashkenazi Jews has been linked to Crohn’s disease.
Major finding: Genotyping of 224 frameshift mutations identified one in the colony-stimulating factor 2–receptor common subunit (CSF2RB) gene that was significantly associated with Crohn’s disease.
Data source: Exome sequencing and genotype analyses of samples from 1,477 Ashkenazi Jewish people with Crohn’s disease and 2,614 without it.
Disclosures: This study was supported by the National Institutes of Health, the Inflammatory Bowel Disease Genetics Consortium, the Genetic Research Center at Mount Sinai Medical Center, New York’s Crohn’s Foundation, and several others. Dr. Chuang and his associates reported having no relevant financial disclosures.
Real-world study: 8 weeks of two-drug combo highly effective against HCV
Eight weeks of a two-drug combination led to sustained viral response (SVR) in 94% of noncirrhotic, treatment-naive, genotype 1 hepatitis C virus–infected patients, based on a retrospective study of Veterans Affairs health system data presented in the September issue of Gastroenterology.
But VA clinicians prescribed the 8-week regimen to less than half of eligible patients, said George Ioannou, MD, of the Veterans Affairs Puget Sound Health Care System in Seattle. Increasing its use, when appropriate, “could save on costs,” although the currently available interferon-free regimens “leave substantial room for improvement in SVRs among persons with cirrhosis and genotype 2 or 3 infections,” he and his associates wrote.
The two-drug regimen contained sofosbuvir and ledipasvir. Clinical trials of sofosbuvir, ledipasvir/sofosbuvir, and paritaprevir/ritonavir/ombitasvir and dasabuvir (PrOD) have reported SVR rates well above 90%, “with the exception of certain subgroups, such as patients with Child’s B or C cirrhosis and those infected with genotype 3 HCV,” the researchers noted. However, older interferon-based regimens did not perform as well in the real world as in trials, and “it is unclear if this is the case for current interferon-free regimens, or whether the relative ease of administration of these regimens has narrowed the SVR gap between clinical trials and clinical practice.” Questions also persist about how effective the interferon-free regimens are in various HCV genotypes and subgroups, they added (Gastroenterology 2016 Jul 18. doi: 10.1053/j.gastro.2016.05.049). To help answer these questions, they analyzed data from more than 17,000 HCV patients in the VA health care system who received sofosbuvir, ledipasvir/sofosbuvir, or paritaprevir/ritonavir/ombitasvir and dasabuvir between January 2014 and June 2015. The cohort included about 14,000 patients with genotype 1 infections, about 2,100 patients with genotype 2 infections, about 1,200 patients with genotype 3 infections, and 135 patients with genotype 4 infections. Patients averaged 62 years of age, about half were non-Hispanic white, 29% were non-Hispanic black, and nearly a third had been diagnosed with cirrhosis, including 10% with decompensated cirrhosis.
The VA guidelines recommended 8 weeks instead of 12 weeks of ledipasvir/sofosbuvir for treatment-naive, noncirrhotic, genotype 1 patients with a viral load under 6 million IU/mL, although that recommendation was not FDA approved and was based only on a post hoc analysis of the ION-3 trial, the investigators noted. These concerns seemed to affect practice – of 4,066 eligible patients, only 1,975 (49%) received the 8-week regimen. Notably, however, their rate of SVR 12 weeks after treatment (SVR12) was 95.1% (95% confidence interval, 94% to 96%) – nearly identical to that of patients with the same characteristics who received 12 weeks of treatment (95.8%; 94.7% to 96.8%; P = .6).
Rates of SVR12 did not significantly differ between ledipasvir/sofosbuvir and PrOD regimens, including in multivariable and propensity score–adjusted analyses, the researchers reported. Rates of SVR12 also exceeded 90% in subgroups of treatment-experienced and cirrhotic genotype 1 patients, they added. However, rates of SVR12 were lower for nongenotype 1 infections, as has been observed in trials. Specifically, rates of SVR12 were 90% for genotype 4 patients, 86% for genotype 2 patients who received sofosbuvir and ribavirin, and 75% for genotype 3 patients (including 78% for patients given ledipasvir/sofosbuvir plus ribavirin, 87% for patients given sofosbuvir and pegylated interferon plus ribavirin, and 71% for patients given sofosbuvir monotherapy). For cirrhotic patients, rates of SVR12 were 91% for genotype 1, 77% for genotype 2, 66% for genotype 3, and 84% for genotype 4.
The findings confirm that the new interferon-free regimens “can achieve remarkably high SVR rates in real-world clinical practice, especially in genotype 1–infected patients,” the researchers wrote. The cost of these regimens is “the main obstacle to curing HCV” in as many patients as possible, but is expected to “decline dramatically” as the FDA approves new regimens, they noted. “In fact, costs decreased dramatically within the VA after the completion of our study and after the FDA approved elbasvir/grazoprevir in January 2016.”
The study was funded by the Department of Veterans Affairs. The investigators had no disclosures.
Eight weeks of a two-drug combination led to sustained viral response (SVR) in 94% of noncirrhotic, treatment-naive, genotype 1 hepatitis C virus–infected patients, based on a retrospective study of Veterans Affairs health system data presented in the September issue of Gastroenterology.
But VA clinicians prescribed the 8-week regimen to less than half of eligible patients, said George Ioannou, MD, of the Veterans Affairs Puget Sound Health Care System in Seattle. Increasing its use, when appropriate, “could save on costs,” although the currently available interferon-free regimens “leave substantial room for improvement in SVRs among persons with cirrhosis and genotype 2 or 3 infections,” he and his associates wrote.
The two-drug regimen contained sofosbuvir and ledipasvir. Clinical trials of sofosbuvir, ledipasvir/sofosbuvir, and paritaprevir/ritonavir/ombitasvir and dasabuvir (PrOD) have reported SVR rates well above 90%, “with the exception of certain subgroups, such as patients with Child’s B or C cirrhosis and those infected with genotype 3 HCV,” the researchers noted. However, older interferon-based regimens did not perform as well in the real world as in trials, and “it is unclear if this is the case for current interferon-free regimens, or whether the relative ease of administration of these regimens has narrowed the SVR gap between clinical trials and clinical practice.” Questions also persist about how effective the interferon-free regimens are in various HCV genotypes and subgroups, they added (Gastroenterology 2016 Jul 18. doi: 10.1053/j.gastro.2016.05.049). To help answer these questions, they analyzed data from more than 17,000 HCV patients in the VA health care system who received sofosbuvir, ledipasvir/sofosbuvir, or paritaprevir/ritonavir/ombitasvir and dasabuvir between January 2014 and June 2015. The cohort included about 14,000 patients with genotype 1 infections, about 2,100 patients with genotype 2 infections, about 1,200 patients with genotype 3 infections, and 135 patients with genotype 4 infections. Patients averaged 62 years of age, about half were non-Hispanic white, 29% were non-Hispanic black, and nearly a third had been diagnosed with cirrhosis, including 10% with decompensated cirrhosis.
The VA guidelines recommended 8 weeks instead of 12 weeks of ledipasvir/sofosbuvir for treatment-naive, noncirrhotic, genotype 1 patients with a viral load under 6 million IU/mL, although that recommendation was not FDA approved and was based only on a post hoc analysis of the ION-3 trial, the investigators noted. These concerns seemed to affect practice – of 4,066 eligible patients, only 1,975 (49%) received the 8-week regimen. Notably, however, their rate of SVR 12 weeks after treatment (SVR12) was 95.1% (95% confidence interval, 94% to 96%) – nearly identical to that of patients with the same characteristics who received 12 weeks of treatment (95.8%; 94.7% to 96.8%; P = .6).
Rates of SVR12 did not significantly differ between ledipasvir/sofosbuvir and PrOD regimens, including in multivariable and propensity score–adjusted analyses, the researchers reported. Rates of SVR12 also exceeded 90% in subgroups of treatment-experienced and cirrhotic genotype 1 patients, they added. However, rates of SVR12 were lower for nongenotype 1 infections, as has been observed in trials. Specifically, rates of SVR12 were 90% for genotype 4 patients, 86% for genotype 2 patients who received sofosbuvir and ribavirin, and 75% for genotype 3 patients (including 78% for patients given ledipasvir/sofosbuvir plus ribavirin, 87% for patients given sofosbuvir and pegylated interferon plus ribavirin, and 71% for patients given sofosbuvir monotherapy). For cirrhotic patients, rates of SVR12 were 91% for genotype 1, 77% for genotype 2, 66% for genotype 3, and 84% for genotype 4.
The findings confirm that the new interferon-free regimens “can achieve remarkably high SVR rates in real-world clinical practice, especially in genotype 1–infected patients,” the researchers wrote. The cost of these regimens is “the main obstacle to curing HCV” in as many patients as possible, but is expected to “decline dramatically” as the FDA approves new regimens, they noted. “In fact, costs decreased dramatically within the VA after the completion of our study and after the FDA approved elbasvir/grazoprevir in January 2016.”
The study was funded by the Department of Veterans Affairs. The investigators had no disclosures.
Eight weeks of a two-drug combination led to sustained viral response (SVR) in 94% of noncirrhotic, treatment-naive, genotype 1 hepatitis C virus–infected patients, based on a retrospective study of Veterans Affairs health system data presented in the September issue of Gastroenterology.
But VA clinicians prescribed the 8-week regimen to less than half of eligible patients, said George Ioannou, MD, of the Veterans Affairs Puget Sound Health Care System in Seattle. Increasing its use, when appropriate, “could save on costs,” although the currently available interferon-free regimens “leave substantial room for improvement in SVRs among persons with cirrhosis and genotype 2 or 3 infections,” he and his associates wrote.
The two-drug regimen contained sofosbuvir and ledipasvir. Clinical trials of sofosbuvir, ledipasvir/sofosbuvir, and paritaprevir/ritonavir/ombitasvir and dasabuvir (PrOD) have reported SVR rates well above 90%, “with the exception of certain subgroups, such as patients with Child’s B or C cirrhosis and those infected with genotype 3 HCV,” the researchers noted. However, older interferon-based regimens did not perform as well in the real world as in trials, and “it is unclear if this is the case for current interferon-free regimens, or whether the relative ease of administration of these regimens has narrowed the SVR gap between clinical trials and clinical practice.” Questions also persist about how effective the interferon-free regimens are in various HCV genotypes and subgroups, they added (Gastroenterology 2016 Jul 18. doi: 10.1053/j.gastro.2016.05.049). To help answer these questions, they analyzed data from more than 17,000 HCV patients in the VA health care system who received sofosbuvir, ledipasvir/sofosbuvir, or paritaprevir/ritonavir/ombitasvir and dasabuvir between January 2014 and June 2015. The cohort included about 14,000 patients with genotype 1 infections, about 2,100 patients with genotype 2 infections, about 1,200 patients with genotype 3 infections, and 135 patients with genotype 4 infections. Patients averaged 62 years of age, about half were non-Hispanic white, 29% were non-Hispanic black, and nearly a third had been diagnosed with cirrhosis, including 10% with decompensated cirrhosis.
The VA guidelines recommended 8 weeks instead of 12 weeks of ledipasvir/sofosbuvir for treatment-naive, noncirrhotic, genotype 1 patients with a viral load under 6 million IU/mL, although that recommendation was not FDA approved and was based only on a post hoc analysis of the ION-3 trial, the investigators noted. These concerns seemed to affect practice – of 4,066 eligible patients, only 1,975 (49%) received the 8-week regimen. Notably, however, their rate of SVR 12 weeks after treatment (SVR12) was 95.1% (95% confidence interval, 94% to 96%) – nearly identical to that of patients with the same characteristics who received 12 weeks of treatment (95.8%; 94.7% to 96.8%; P = .6).
Rates of SVR12 did not significantly differ between ledipasvir/sofosbuvir and PrOD regimens, including in multivariable and propensity score–adjusted analyses, the researchers reported. Rates of SVR12 also exceeded 90% in subgroups of treatment-experienced and cirrhotic genotype 1 patients, they added. However, rates of SVR12 were lower for nongenotype 1 infections, as has been observed in trials. Specifically, rates of SVR12 were 90% for genotype 4 patients, 86% for genotype 2 patients who received sofosbuvir and ribavirin, and 75% for genotype 3 patients (including 78% for patients given ledipasvir/sofosbuvir plus ribavirin, 87% for patients given sofosbuvir and pegylated interferon plus ribavirin, and 71% for patients given sofosbuvir monotherapy). For cirrhotic patients, rates of SVR12 were 91% for genotype 1, 77% for genotype 2, 66% for genotype 3, and 84% for genotype 4.
The findings confirm that the new interferon-free regimens “can achieve remarkably high SVR rates in real-world clinical practice, especially in genotype 1–infected patients,” the researchers wrote. The cost of these regimens is “the main obstacle to curing HCV” in as many patients as possible, but is expected to “decline dramatically” as the FDA approves new regimens, they noted. “In fact, costs decreased dramatically within the VA after the completion of our study and after the FDA approved elbasvir/grazoprevir in January 2016.”
The study was funded by the Department of Veterans Affairs. The investigators had no disclosures.
FROM GASTROENTEROLOGY
Key clinical point: Eight-week and 12-week regimens of ledipasvir/sofosbuvir achieved similarly high rates of sustained viral response (SVR) among noncirrhotic, treatment-naive, genotype 1 hepatitis C virus–infected patients with viral loads under 6 million IU/mL.
Major finding: Twelve weeks after treatment, rates of SVR were 95.1% for the 8-week regimen and 95.8% for the 12-week regimen (P = .6).
Data source: A retrospective analysis of data from 17,487 patients with HCV infection.
Disclosures: The study was funded by the Department of Veterans Affairs. The investigators had no disclosures.
AGA Clinical Practice Update: Experts carve pathway for celiac trials
Celiac disease’s only treatment is far from ideal. Not only is the gluten-free diet hard to follow, costly, and socially isolating, but even adherent patients can suffer persistent and disabling symptoms, Daniel A. Leffler, MD, MS, of Beth Israel Deaconess Medical Center in Boston, and his associates noted. This “high unmet medical need” inspired their discussion of clinical trials in celiac disease at the third Gastroenterology Regulatory Endpoints and Advancement of Therapeutics (GREAT 3). A summary appears as a Clinical Practice Update in Gastroenterology (2016 Jul 22. doi: 10.1053/j.gastro.2016.07.025).
Celiac is the “outlier among intestinal diseases,” the experts noted. Few randomized trials have assessed nondietary celiac disease therapies, drug developers lack a precedent approval to clarify a “guiding regulatory pathway.” The first step toward bridging these gaps is to better define target populations for clinical trials, meeting attendees agreed. Such groups might include patients with diet-refractory symptoms; newly diagnosed patients who need pharmacologic support for symptom resolution and duodenal healing; patients with asymptomatic mucosal damage, if such damage is shown to cause malignancy; and patients with neurobehavioral disorders that impede gluten avoidance. Medications that enable patients to safely consume gluten could benefit even more, including those who already face “arduous” dietary controls from comorbidities such as type 1 diabetes, the experts added.
Defining “clinical benefit” in pivotal trials of celiac disease also poses a challenge. “While every patient might desire something slightly different, the overarching theme of clinical benefit from the patient’s perspective appears to be quality of life – free of symptoms and inflammation without worry about [gluten] contamination,” attendees emphasized. Indeed, one recent survey found that patients prioritized protection against cross-contamination over being able to consume gluten at will. But initial trials should focus on gastrointestinal symptoms, which are common, affect patients of all ages, and “can be measured in a reasonable time frame,” they concluded.
Unfortunately, defining and measuring atypical symptoms can be difficult, particularly in children and adolescents. In an unpublished study at the Nationwide Children’s Hospital, gastrointestinal symptoms affected 77% of celiac patients, while only about 5% experienced atypical or nongastrointestinal symptoms, the experts noted. Such low percentages could make it difficult to adequately power studies of these nongastrointestinal outcomes. Furthermore, measuring sequelae such as osteoporosis or anemia “would require longer studies, as these conditions do not resolve quickly.”
Phase II and III trials cannot secure marketing approvals without clearly defining and measuring “clinical benefit,” Food and Drug Administration representatives at the meeting noted. Accordingly, diarrhea and abdominal pain will be key patient-reported outcome measures, and pivotal trials of young children with celiac disease will require observer-reported outcomes, attendees agreed. Although both celiac disease and the gluten-free diet profoundly undercut health-related quality of life, this measure is too broad and easily confounded to be a good endpoint in pivotal trials of celiac therapies. Likewise, histology is valuable for relating symptoms to active disease, but mucosal healing is too variable and unpredictable to serve as a primary endpoint, experts noted.
In contrast, serologic tests could serve as enrollment criteria, stratification measures, and endpoints if these tests received the appropriate FDA approvals, experts asserted. The endomysial antibody, tissue transglutaminase antibody, and deamidated gliadin peptide tests are most often used in practice, but have only been approved for diagnosing celiac disease – not as a replacement for biopsy or as a measure of disease progression or therapeutic response, FDA representatives noted. Although drug developers need tools to measure therapeutic efficacy in celiac disease, FDA recommended soliciting advice from its Center for Drug Evaluation and Research before testing these tools or kicking off monitoring studies.
The meeting was supported by the Celiac Disease Foundation and Beyond Celiac, and was sponsored by the FDA Center for Drug Evaluation and Research; the American Gastroenterological Association; the American College of Gastroenterology; the American Society for Pediatric Gastroenterology, Hepatology, and Nutrition; and the North American Society for the Study of Celiac Disease. Dr. Leffler disclosed ties to Alba Therapeutics, Alvine Pharmaceuticals, INOVA Diagnostics, Coronado Biosciences, Pfizer, and GI Supply. One coauthor and senior author, Dr. Sheila Crowe, disclosed ties to Alvine Pharmaceuticals, Ferring, and Celimmune.
Celiac disease’s only treatment is far from ideal. Not only is the gluten-free diet hard to follow, costly, and socially isolating, but even adherent patients can suffer persistent and disabling symptoms, Daniel A. Leffler, MD, MS, of Beth Israel Deaconess Medical Center in Boston, and his associates noted. This “high unmet medical need” inspired their discussion of clinical trials in celiac disease at the third Gastroenterology Regulatory Endpoints and Advancement of Therapeutics (GREAT 3). A summary appears as a Clinical Practice Update in Gastroenterology (2016 Jul 22. doi: 10.1053/j.gastro.2016.07.025).
Celiac is the “outlier among intestinal diseases,” the experts noted. Few randomized trials have assessed nondietary celiac disease therapies, drug developers lack a precedent approval to clarify a “guiding regulatory pathway.” The first step toward bridging these gaps is to better define target populations for clinical trials, meeting attendees agreed. Such groups might include patients with diet-refractory symptoms; newly diagnosed patients who need pharmacologic support for symptom resolution and duodenal healing; patients with asymptomatic mucosal damage, if such damage is shown to cause malignancy; and patients with neurobehavioral disorders that impede gluten avoidance. Medications that enable patients to safely consume gluten could benefit even more, including those who already face “arduous” dietary controls from comorbidities such as type 1 diabetes, the experts added.
Defining “clinical benefit” in pivotal trials of celiac disease also poses a challenge. “While every patient might desire something slightly different, the overarching theme of clinical benefit from the patient’s perspective appears to be quality of life – free of symptoms and inflammation without worry about [gluten] contamination,” attendees emphasized. Indeed, one recent survey found that patients prioritized protection against cross-contamination over being able to consume gluten at will. But initial trials should focus on gastrointestinal symptoms, which are common, affect patients of all ages, and “can be measured in a reasonable time frame,” they concluded.
Unfortunately, defining and measuring atypical symptoms can be difficult, particularly in children and adolescents. In an unpublished study at the Nationwide Children’s Hospital, gastrointestinal symptoms affected 77% of celiac patients, while only about 5% experienced atypical or nongastrointestinal symptoms, the experts noted. Such low percentages could make it difficult to adequately power studies of these nongastrointestinal outcomes. Furthermore, measuring sequelae such as osteoporosis or anemia “would require longer studies, as these conditions do not resolve quickly.”
Phase II and III trials cannot secure marketing approvals without clearly defining and measuring “clinical benefit,” Food and Drug Administration representatives at the meeting noted. Accordingly, diarrhea and abdominal pain will be key patient-reported outcome measures, and pivotal trials of young children with celiac disease will require observer-reported outcomes, attendees agreed. Although both celiac disease and the gluten-free diet profoundly undercut health-related quality of life, this measure is too broad and easily confounded to be a good endpoint in pivotal trials of celiac therapies. Likewise, histology is valuable for relating symptoms to active disease, but mucosal healing is too variable and unpredictable to serve as a primary endpoint, experts noted.
In contrast, serologic tests could serve as enrollment criteria, stratification measures, and endpoints if these tests received the appropriate FDA approvals, experts asserted. The endomysial antibody, tissue transglutaminase antibody, and deamidated gliadin peptide tests are most often used in practice, but have only been approved for diagnosing celiac disease – not as a replacement for biopsy or as a measure of disease progression or therapeutic response, FDA representatives noted. Although drug developers need tools to measure therapeutic efficacy in celiac disease, FDA recommended soliciting advice from its Center for Drug Evaluation and Research before testing these tools or kicking off monitoring studies.
The meeting was supported by the Celiac Disease Foundation and Beyond Celiac, and was sponsored by the FDA Center for Drug Evaluation and Research; the American Gastroenterological Association; the American College of Gastroenterology; the American Society for Pediatric Gastroenterology, Hepatology, and Nutrition; and the North American Society for the Study of Celiac Disease. Dr. Leffler disclosed ties to Alba Therapeutics, Alvine Pharmaceuticals, INOVA Diagnostics, Coronado Biosciences, Pfizer, and GI Supply. One coauthor and senior author, Dr. Sheila Crowe, disclosed ties to Alvine Pharmaceuticals, Ferring, and Celimmune.
Celiac disease’s only treatment is far from ideal. Not only is the gluten-free diet hard to follow, costly, and socially isolating, but even adherent patients can suffer persistent and disabling symptoms, Daniel A. Leffler, MD, MS, of Beth Israel Deaconess Medical Center in Boston, and his associates noted. This “high unmet medical need” inspired their discussion of clinical trials in celiac disease at the third Gastroenterology Regulatory Endpoints and Advancement of Therapeutics (GREAT 3). A summary appears as a Clinical Practice Update in Gastroenterology (2016 Jul 22. doi: 10.1053/j.gastro.2016.07.025).
Celiac is the “outlier among intestinal diseases,” the experts noted. Few randomized trials have assessed nondietary celiac disease therapies, drug developers lack a precedent approval to clarify a “guiding regulatory pathway.” The first step toward bridging these gaps is to better define target populations for clinical trials, meeting attendees agreed. Such groups might include patients with diet-refractory symptoms; newly diagnosed patients who need pharmacologic support for symptom resolution and duodenal healing; patients with asymptomatic mucosal damage, if such damage is shown to cause malignancy; and patients with neurobehavioral disorders that impede gluten avoidance. Medications that enable patients to safely consume gluten could benefit even more, including those who already face “arduous” dietary controls from comorbidities such as type 1 diabetes, the experts added.
Defining “clinical benefit” in pivotal trials of celiac disease also poses a challenge. “While every patient might desire something slightly different, the overarching theme of clinical benefit from the patient’s perspective appears to be quality of life – free of symptoms and inflammation without worry about [gluten] contamination,” attendees emphasized. Indeed, one recent survey found that patients prioritized protection against cross-contamination over being able to consume gluten at will. But initial trials should focus on gastrointestinal symptoms, which are common, affect patients of all ages, and “can be measured in a reasonable time frame,” they concluded.
Unfortunately, defining and measuring atypical symptoms can be difficult, particularly in children and adolescents. In an unpublished study at the Nationwide Children’s Hospital, gastrointestinal symptoms affected 77% of celiac patients, while only about 5% experienced atypical or nongastrointestinal symptoms, the experts noted. Such low percentages could make it difficult to adequately power studies of these nongastrointestinal outcomes. Furthermore, measuring sequelae such as osteoporosis or anemia “would require longer studies, as these conditions do not resolve quickly.”
Phase II and III trials cannot secure marketing approvals without clearly defining and measuring “clinical benefit,” Food and Drug Administration representatives at the meeting noted. Accordingly, diarrhea and abdominal pain will be key patient-reported outcome measures, and pivotal trials of young children with celiac disease will require observer-reported outcomes, attendees agreed. Although both celiac disease and the gluten-free diet profoundly undercut health-related quality of life, this measure is too broad and easily confounded to be a good endpoint in pivotal trials of celiac therapies. Likewise, histology is valuable for relating symptoms to active disease, but mucosal healing is too variable and unpredictable to serve as a primary endpoint, experts noted.
In contrast, serologic tests could serve as enrollment criteria, stratification measures, and endpoints if these tests received the appropriate FDA approvals, experts asserted. The endomysial antibody, tissue transglutaminase antibody, and deamidated gliadin peptide tests are most often used in practice, but have only been approved for diagnosing celiac disease – not as a replacement for biopsy or as a measure of disease progression or therapeutic response, FDA representatives noted. Although drug developers need tools to measure therapeutic efficacy in celiac disease, FDA recommended soliciting advice from its Center for Drug Evaluation and Research before testing these tools or kicking off monitoring studies.
The meeting was supported by the Celiac Disease Foundation and Beyond Celiac, and was sponsored by the FDA Center for Drug Evaluation and Research; the American Gastroenterological Association; the American College of Gastroenterology; the American Society for Pediatric Gastroenterology, Hepatology, and Nutrition; and the North American Society for the Study of Celiac Disease. Dr. Leffler disclosed ties to Alba Therapeutics, Alvine Pharmaceuticals, INOVA Diagnostics, Coronado Biosciences, Pfizer, and GI Supply. One coauthor and senior author, Dr. Sheila Crowe, disclosed ties to Alvine Pharmaceuticals, Ferring, and Celimmune.
FROM GASTROENTEROLOGY
Celiac disease most common among Indians from Punjab study found
Americans from the Punjab region of India had the highest prevalence of celiac disease in a national cross-sectional study of duodenal mucosal biopsies according to a study reported in the August issue of Clinical Gastroenterology and Hepatology.
In contrast, persons of South Asian, East Asian, and Hispanic descent are significantly less likely to receive a biopsy-based diagnosis of celiac disease than were other Americans, Anna Krigel, MD, at Columbia University, New York, reported with her associates. The prevalence of celiac disease among Jewish and Middle Eastern individuals resembled that of other Americans and did not differ by sex, the researchers added. “These findings may have clinical relevance to gastroenterologists across the United States and may aid in their diagnostic practices.”
Dr. Krigel and her associates analyzed a national laboratory pathology registry of 454,885 patients who underwent esophagogastroduodenoscopy with duodenal biopsy between January 2008 and April 2015. Mucosal biopsy specimens were analyzed at three laboratories by histopathologists who had completed fellowships in gastrointestinal pathology. Patients were categorized based on their first and last names as North Indian, South Indian, East Asian, Hispanic, Middle Eastern, Jewish, or as “other Americans,” using a published algorithm. The researchers further validated this algorithm by adjusting it against a list of individuals of known ethnicities until its specificity reached 95%. Two-thirds of patients in the cohort were female, and the median age was 53 years (Clin Gastroenterol Hepatol. 2016 May 31. doi: 10.1016/j.cgh.2016.04.032).
Overall, 7,928 patients (1.7%) had duodenal villous atrophy indicative of disease, including 2% of North Indians, 1.8% of Jewish individuals, 1.8% of other Americans, 1.5% of Middle Easterners, 1.1% of Hispanics, and 0.15% of East Asians, said the investigators. Thus, Jewish persons and Middle Eastern persons had a prevalence of celiac disease similar to that of other Americans. Prevalence also was similar among Ashkenazi and Sephardic Jews. In contrast, none of the 177 South Indians in the study were diagnosed with celiac disease, and both East Asians and Hispanics were significantly less likely to be receive a diagnosis than other Americans, with odds ratios of 0.08 (95% confidence interval, 0.04-0.17) and 0.58 (0.52-0.64), respectively, and P values less than .0001.
Importantly, celiac disease was significantly more common among patients from the Punjab region of India (3.1%) than among other North Indians (1.5%; P = .02). Past studies of celiac disease in India reported similar prevalences of compatible human leukocyte antigen (HLA) haplotypes as in Western countries, without notable regional trends within India, the researchers noted. Substantial regional variations in wheat consumption in India are more likely to explain their findings and patterns of case reports in past studies, they added.
Because the registry lacked serology data, patients diagnosed with celiac disease could have actually had tropical sprue or sprue-like enteropathy due to olmesartan, the researchers acknowledged. “In particular, multiple studies have shown that tropical sprue is still the most common cause of malabsorption syndrome in India, whereas celiac disease is emerging as a more important cause of malabsorption than previously thought,” they said. “However, such cases of tropical sprue and sprue-like enteropathy due to olmesartan are far less common than celiac disease in the United States.” The study also did not account for patients whose celiac disease only was diagnosed by serology and clinical symptoms, and the algorithm probably assigned some individuals who were Hispanic to other ethnicities, because only 7% of the cohort was classified as Hispanic, compared with about 16% of Americans in the 2010 U.S. Census, they noted. But ethnic misclassification was unlikely to have differed by celiac disease status, they said.
The National Institutes of Health partially supported the work. The authors had no disclosures.
Americans from the Punjab region of India had the highest prevalence of celiac disease in a national cross-sectional study of duodenal mucosal biopsies according to a study reported in the August issue of Clinical Gastroenterology and Hepatology.
In contrast, persons of South Asian, East Asian, and Hispanic descent are significantly less likely to receive a biopsy-based diagnosis of celiac disease than were other Americans, Anna Krigel, MD, at Columbia University, New York, reported with her associates. The prevalence of celiac disease among Jewish and Middle Eastern individuals resembled that of other Americans and did not differ by sex, the researchers added. “These findings may have clinical relevance to gastroenterologists across the United States and may aid in their diagnostic practices.”
Dr. Krigel and her associates analyzed a national laboratory pathology registry of 454,885 patients who underwent esophagogastroduodenoscopy with duodenal biopsy between January 2008 and April 2015. Mucosal biopsy specimens were analyzed at three laboratories by histopathologists who had completed fellowships in gastrointestinal pathology. Patients were categorized based on their first and last names as North Indian, South Indian, East Asian, Hispanic, Middle Eastern, Jewish, or as “other Americans,” using a published algorithm. The researchers further validated this algorithm by adjusting it against a list of individuals of known ethnicities until its specificity reached 95%. Two-thirds of patients in the cohort were female, and the median age was 53 years (Clin Gastroenterol Hepatol. 2016 May 31. doi: 10.1016/j.cgh.2016.04.032).
Overall, 7,928 patients (1.7%) had duodenal villous atrophy indicative of disease, including 2% of North Indians, 1.8% of Jewish individuals, 1.8% of other Americans, 1.5% of Middle Easterners, 1.1% of Hispanics, and 0.15% of East Asians, said the investigators. Thus, Jewish persons and Middle Eastern persons had a prevalence of celiac disease similar to that of other Americans. Prevalence also was similar among Ashkenazi and Sephardic Jews. In contrast, none of the 177 South Indians in the study were diagnosed with celiac disease, and both East Asians and Hispanics were significantly less likely to be receive a diagnosis than other Americans, with odds ratios of 0.08 (95% confidence interval, 0.04-0.17) and 0.58 (0.52-0.64), respectively, and P values less than .0001.
Importantly, celiac disease was significantly more common among patients from the Punjab region of India (3.1%) than among other North Indians (1.5%; P = .02). Past studies of celiac disease in India reported similar prevalences of compatible human leukocyte antigen (HLA) haplotypes as in Western countries, without notable regional trends within India, the researchers noted. Substantial regional variations in wheat consumption in India are more likely to explain their findings and patterns of case reports in past studies, they added.
Because the registry lacked serology data, patients diagnosed with celiac disease could have actually had tropical sprue or sprue-like enteropathy due to olmesartan, the researchers acknowledged. “In particular, multiple studies have shown that tropical sprue is still the most common cause of malabsorption syndrome in India, whereas celiac disease is emerging as a more important cause of malabsorption than previously thought,” they said. “However, such cases of tropical sprue and sprue-like enteropathy due to olmesartan are far less common than celiac disease in the United States.” The study also did not account for patients whose celiac disease only was diagnosed by serology and clinical symptoms, and the algorithm probably assigned some individuals who were Hispanic to other ethnicities, because only 7% of the cohort was classified as Hispanic, compared with about 16% of Americans in the 2010 U.S. Census, they noted. But ethnic misclassification was unlikely to have differed by celiac disease status, they said.
The National Institutes of Health partially supported the work. The authors had no disclosures.
Americans from the Punjab region of India had the highest prevalence of celiac disease in a national cross-sectional study of duodenal mucosal biopsies according to a study reported in the August issue of Clinical Gastroenterology and Hepatology.
In contrast, persons of South Asian, East Asian, and Hispanic descent are significantly less likely to receive a biopsy-based diagnosis of celiac disease than were other Americans, Anna Krigel, MD, at Columbia University, New York, reported with her associates. The prevalence of celiac disease among Jewish and Middle Eastern individuals resembled that of other Americans and did not differ by sex, the researchers added. “These findings may have clinical relevance to gastroenterologists across the United States and may aid in their diagnostic practices.”
Dr. Krigel and her associates analyzed a national laboratory pathology registry of 454,885 patients who underwent esophagogastroduodenoscopy with duodenal biopsy between January 2008 and April 2015. Mucosal biopsy specimens were analyzed at three laboratories by histopathologists who had completed fellowships in gastrointestinal pathology. Patients were categorized based on their first and last names as North Indian, South Indian, East Asian, Hispanic, Middle Eastern, Jewish, or as “other Americans,” using a published algorithm. The researchers further validated this algorithm by adjusting it against a list of individuals of known ethnicities until its specificity reached 95%. Two-thirds of patients in the cohort were female, and the median age was 53 years (Clin Gastroenterol Hepatol. 2016 May 31. doi: 10.1016/j.cgh.2016.04.032).
Overall, 7,928 patients (1.7%) had duodenal villous atrophy indicative of disease, including 2% of North Indians, 1.8% of Jewish individuals, 1.8% of other Americans, 1.5% of Middle Easterners, 1.1% of Hispanics, and 0.15% of East Asians, said the investigators. Thus, Jewish persons and Middle Eastern persons had a prevalence of celiac disease similar to that of other Americans. Prevalence also was similar among Ashkenazi and Sephardic Jews. In contrast, none of the 177 South Indians in the study were diagnosed with celiac disease, and both East Asians and Hispanics were significantly less likely to be receive a diagnosis than other Americans, with odds ratios of 0.08 (95% confidence interval, 0.04-0.17) and 0.58 (0.52-0.64), respectively, and P values less than .0001.
Importantly, celiac disease was significantly more common among patients from the Punjab region of India (3.1%) than among other North Indians (1.5%; P = .02). Past studies of celiac disease in India reported similar prevalences of compatible human leukocyte antigen (HLA) haplotypes as in Western countries, without notable regional trends within India, the researchers noted. Substantial regional variations in wheat consumption in India are more likely to explain their findings and patterns of case reports in past studies, they added.
Because the registry lacked serology data, patients diagnosed with celiac disease could have actually had tropical sprue or sprue-like enteropathy due to olmesartan, the researchers acknowledged. “In particular, multiple studies have shown that tropical sprue is still the most common cause of malabsorption syndrome in India, whereas celiac disease is emerging as a more important cause of malabsorption than previously thought,” they said. “However, such cases of tropical sprue and sprue-like enteropathy due to olmesartan are far less common than celiac disease in the United States.” The study also did not account for patients whose celiac disease only was diagnosed by serology and clinical symptoms, and the algorithm probably assigned some individuals who were Hispanic to other ethnicities, because only 7% of the cohort was classified as Hispanic, compared with about 16% of Americans in the 2010 U.S. Census, they noted. But ethnic misclassification was unlikely to have differed by celiac disease status, they said.
The National Institutes of Health partially supported the work. The authors had no disclosures.
FROM CLINICAL GASTROENTEROLOGY AND HEPATOLOGY
Key clinical point: Duodenal villous atrophy consistent with celiac disease was most common among Americans from, or descended from, the Punjab region of India.
Major finding: The prevalence of celiac disease was highest among Punjabis (3.1%), and lowest among South Indians (0%).
Data source: A cross-sectional study of duodenal biopsies and associated demographic data for 454,885 patients in the United States.
Disclosures: The National Institutes of Health partially supported the work. The authors had no disclosures.
Rectal indomethacin cut odds of post-ERCP pancreatitis in real-world study
A single, 100-mg rectal dose of indomethacin cut the odds of moderate to severe pancreatitis after endoscopic retrograde cholangiopancreatography (ERCP) by 85% in a single-center retrospective study of more than 4,000 patients reported in the August issue of Gastroenterology.
The effect extended to low-risk patients and those with malignant biliary obstruction, who make up the majority of ERCP patients in community practice, said Nikhil R. Thiruvengadam, MD, and his associates at the University of Pennsylvania. “Usage of rectal indomethacin in current clinical practice is low, as most endoscopists outside of referral centers perform ERCP for indications that are considered low-risk for PEP [post-ERCP pancreatitis], and until now, there were no data to support a benefit of rectal NSAIDs in this population,” they wrote in Gastroenterology. Their “real-world analysis” clearly shows the benefits of rectal indomethacin in low-risk patients and supports its increased use after ERCP, they added.
Pancreatitis, the most common complication of ERCP, affected 2%-9% of patients in prior studies and costs about $200 million in the United States annually, the investigators noted. Pancreatic duct stents help prevent post-ERCP pancreatitis, but require experience to place and have their own complications that limit their use in low-risk patients. Past studies of rectal indomethacin after ERCP reported mixed results and mainly focused on high-risk patients, leaving questions about whether to routinely use this NSAID after ERCP, said the researchers (Gastroenterology. 2016 May 20. doi: 10.1053/j.gastro.2016.04.048). Their study included 4,017 patients who underwent ERCP at the University of Pennsylvania between 2009 and 2015. From 2012 onward, nearly all patients received 100 mg rectal indomethacin immediately after the duodenoscope was withdrawn. This indomethacin group included 2,007 patients, while 2,010 patients in the study did not receive rectal indomethacin. In all, 95 (4.73%) untreated patients developed post-ERCP pancreatitis, compared with only 40 (1.99%) patients who received indomethacin, for a 65% reduction in the odds of post-ERCP pancreatitis (odds ratio, 0.35; 95% confidence interval, 0.24-0.51; P less than .001). Rectal indomethacin also led to an 83% drop in the odds of moderate to severe post-ERCP pancreatitis (OR, 0.17; 95% CI, 0.09-0.32; P less than .001) and showed very similar protective effects for patients with malignant obstruction (OR, 0.35; 95% CI, 0.17-0.75; P less than .001] and 0.20; 95% CI, 0.07-0.63; P less than 0.001, respectively).
Rectal indomethacin was particularly beneficial for patients with malignant obstruction and pancreatic adenocarcinoma, the investigators noted. Such patients had post-ERCP rates of 2.31% when they received rectal indomethacin and 7.53% otherwise (P less than .001). They also had a nearly sevenfold lower rate of moderate to severe post-ERCP pancreatitis when they received rectal indomethacin (P = .001).
Treatment did not affect the chances of perforation and did not cause anaphylaxis, but was tied to a slightly higher rate of postprocedural gastrointestinal bleeding among sphincterotomy patients (0.65% with treatment versus 0.45% without; P = .52). However, sphincterotomy patients were much less likely to develop pancreatitis when they received rectal indomethacin than when they did not (0% and 9.58% of patients, respectively; P = .003).
“The majority of ERCPs were performed by experienced endoscopists at a tertiary care center, which may have limited the effects of variable procedural skills on the risk of PEP,” the researchers said. “Therefore, generalizability of our findings to other populations may be limited. However, it should be noted that the overall PEP rate in both the unexposed and indomethacin groups was fairly low and similar to large community-based estimates, suggesting that our overall patient population was of similar overall risk.” The study was not powered to assess the combined effects of rectal indomethacin and pancreatic duct stents, they noted.
The investigators reported no funding sources and had no disclosures.
A single, 100-mg rectal dose of indomethacin cut the odds of moderate to severe pancreatitis after endoscopic retrograde cholangiopancreatography (ERCP) by 85% in a single-center retrospective study of more than 4,000 patients reported in the August issue of Gastroenterology.
The effect extended to low-risk patients and those with malignant biliary obstruction, who make up the majority of ERCP patients in community practice, said Nikhil R. Thiruvengadam, MD, and his associates at the University of Pennsylvania. “Usage of rectal indomethacin in current clinical practice is low, as most endoscopists outside of referral centers perform ERCP for indications that are considered low-risk for PEP [post-ERCP pancreatitis], and until now, there were no data to support a benefit of rectal NSAIDs in this population,” they wrote in Gastroenterology. Their “real-world analysis” clearly shows the benefits of rectal indomethacin in low-risk patients and supports its increased use after ERCP, they added.
Pancreatitis, the most common complication of ERCP, affected 2%-9% of patients in prior studies and costs about $200 million in the United States annually, the investigators noted. Pancreatic duct stents help prevent post-ERCP pancreatitis, but require experience to place and have their own complications that limit their use in low-risk patients. Past studies of rectal indomethacin after ERCP reported mixed results and mainly focused on high-risk patients, leaving questions about whether to routinely use this NSAID after ERCP, said the researchers (Gastroenterology. 2016 May 20. doi: 10.1053/j.gastro.2016.04.048). Their study included 4,017 patients who underwent ERCP at the University of Pennsylvania between 2009 and 2015. From 2012 onward, nearly all patients received 100 mg rectal indomethacin immediately after the duodenoscope was withdrawn. This indomethacin group included 2,007 patients, while 2,010 patients in the study did not receive rectal indomethacin. In all, 95 (4.73%) untreated patients developed post-ERCP pancreatitis, compared with only 40 (1.99%) patients who received indomethacin, for a 65% reduction in the odds of post-ERCP pancreatitis (odds ratio, 0.35; 95% confidence interval, 0.24-0.51; P less than .001). Rectal indomethacin also led to an 83% drop in the odds of moderate to severe post-ERCP pancreatitis (OR, 0.17; 95% CI, 0.09-0.32; P less than .001) and showed very similar protective effects for patients with malignant obstruction (OR, 0.35; 95% CI, 0.17-0.75; P less than .001] and 0.20; 95% CI, 0.07-0.63; P less than 0.001, respectively).
Rectal indomethacin was particularly beneficial for patients with malignant obstruction and pancreatic adenocarcinoma, the investigators noted. Such patients had post-ERCP rates of 2.31% when they received rectal indomethacin and 7.53% otherwise (P less than .001). They also had a nearly sevenfold lower rate of moderate to severe post-ERCP pancreatitis when they received rectal indomethacin (P = .001).
Treatment did not affect the chances of perforation and did not cause anaphylaxis, but was tied to a slightly higher rate of postprocedural gastrointestinal bleeding among sphincterotomy patients (0.65% with treatment versus 0.45% without; P = .52). However, sphincterotomy patients were much less likely to develop pancreatitis when they received rectal indomethacin than when they did not (0% and 9.58% of patients, respectively; P = .003).
“The majority of ERCPs were performed by experienced endoscopists at a tertiary care center, which may have limited the effects of variable procedural skills on the risk of PEP,” the researchers said. “Therefore, generalizability of our findings to other populations may be limited. However, it should be noted that the overall PEP rate in both the unexposed and indomethacin groups was fairly low and similar to large community-based estimates, suggesting that our overall patient population was of similar overall risk.” The study was not powered to assess the combined effects of rectal indomethacin and pancreatic duct stents, they noted.
The investigators reported no funding sources and had no disclosures.
A single, 100-mg rectal dose of indomethacin cut the odds of moderate to severe pancreatitis after endoscopic retrograde cholangiopancreatography (ERCP) by 85% in a single-center retrospective study of more than 4,000 patients reported in the August issue of Gastroenterology.
The effect extended to low-risk patients and those with malignant biliary obstruction, who make up the majority of ERCP patients in community practice, said Nikhil R. Thiruvengadam, MD, and his associates at the University of Pennsylvania. “Usage of rectal indomethacin in current clinical practice is low, as most endoscopists outside of referral centers perform ERCP for indications that are considered low-risk for PEP [post-ERCP pancreatitis], and until now, there were no data to support a benefit of rectal NSAIDs in this population,” they wrote in Gastroenterology. Their “real-world analysis” clearly shows the benefits of rectal indomethacin in low-risk patients and supports its increased use after ERCP, they added.
Pancreatitis, the most common complication of ERCP, affected 2%-9% of patients in prior studies and costs about $200 million in the United States annually, the investigators noted. Pancreatic duct stents help prevent post-ERCP pancreatitis, but require experience to place and have their own complications that limit their use in low-risk patients. Past studies of rectal indomethacin after ERCP reported mixed results and mainly focused on high-risk patients, leaving questions about whether to routinely use this NSAID after ERCP, said the researchers (Gastroenterology. 2016 May 20. doi: 10.1053/j.gastro.2016.04.048). Their study included 4,017 patients who underwent ERCP at the University of Pennsylvania between 2009 and 2015. From 2012 onward, nearly all patients received 100 mg rectal indomethacin immediately after the duodenoscope was withdrawn. This indomethacin group included 2,007 patients, while 2,010 patients in the study did not receive rectal indomethacin. In all, 95 (4.73%) untreated patients developed post-ERCP pancreatitis, compared with only 40 (1.99%) patients who received indomethacin, for a 65% reduction in the odds of post-ERCP pancreatitis (odds ratio, 0.35; 95% confidence interval, 0.24-0.51; P less than .001). Rectal indomethacin also led to an 83% drop in the odds of moderate to severe post-ERCP pancreatitis (OR, 0.17; 95% CI, 0.09-0.32; P less than .001) and showed very similar protective effects for patients with malignant obstruction (OR, 0.35; 95% CI, 0.17-0.75; P less than .001] and 0.20; 95% CI, 0.07-0.63; P less than 0.001, respectively).
Rectal indomethacin was particularly beneficial for patients with malignant obstruction and pancreatic adenocarcinoma, the investigators noted. Such patients had post-ERCP rates of 2.31% when they received rectal indomethacin and 7.53% otherwise (P less than .001). They also had a nearly sevenfold lower rate of moderate to severe post-ERCP pancreatitis when they received rectal indomethacin (P = .001).
Treatment did not affect the chances of perforation and did not cause anaphylaxis, but was tied to a slightly higher rate of postprocedural gastrointestinal bleeding among sphincterotomy patients (0.65% with treatment versus 0.45% without; P = .52). However, sphincterotomy patients were much less likely to develop pancreatitis when they received rectal indomethacin than when they did not (0% and 9.58% of patients, respectively; P = .003).
“The majority of ERCPs were performed by experienced endoscopists at a tertiary care center, which may have limited the effects of variable procedural skills on the risk of PEP,” the researchers said. “Therefore, generalizability of our findings to other populations may be limited. However, it should be noted that the overall PEP rate in both the unexposed and indomethacin groups was fairly low and similar to large community-based estimates, suggesting that our overall patient population was of similar overall risk.” The study was not powered to assess the combined effects of rectal indomethacin and pancreatic duct stents, they noted.
The investigators reported no funding sources and had no disclosures.
FROM GASTROENTEROLOGY
Key clinical point: A single 100-mg rectal dose of indomethacin given immediately after endoscopic retrograde cholangiopancreatography significantly reduced the odds of postprocedural pancreatitis, including in low-risk patients and those with malignant obstruction.
Major finding: The odds of pancreatitis were 65% lower when patients received rectal indomethacin than otherwise.
Data source: A single-center retrospective cohort study of 4,017 patients undergoing ERCP.
Disclosures: The investigators reported no funding sources and had no disclosures.
Introduction to Clinical Practice Update Committee articles
In this issue of Gastroenterology and in an upcoming issue of Clinical Gastroenterology and Hepatology, an important behind-the-scenes change is occurring. Specifically, both journals will continue to publish important and timely reviews and commentaries, but the formulation and design of some of these articles will now be driven by a new committee. The newly formed American Gastroenterological Association (AGA) Institute Clinical Practice Updates Committee (CPUC) is charged with providing authoritative and balanced articles for the two journals that have the greatest impact on the clinical practice of gastroenterology and hepatology. Potential subjects for these articles will derive from several sources in coordination with the AGA Institute Clinical Guidelines Committee.
First, important topics that are proposed initially for an AGA guideline but do not meet rigid criteria for guideline grading will be sent to the CPUC for review and consideration. Second, ideas for topics will also be generated by the CPUC members and journal editors, as well as by other AGA committees and AGA members themselves. Third, summaries of important symposia such as the yearly Freston Conference will be included. Topics can be presented in the form of a comprehensive, narrative review or, for particularly timely issues in gastroenterology, hepatology, and health care in general, as a rapid commentary.
We believe that the vetting process of these articles is important. All prospective topics and selected authors will be approved by the CPUC with guidance from the AGA Institute Governing Board. At least 2 authors who are recognized authorities will be invited to write an article and will be chosen from different institutions. It is anticipated that the authors will often have differing opinions, to help ensure balance and achieve consensus. A member of the CPUC with expertise in the field will guide the writing to ensure the highest quality and greatest balance in the final product. Once an article is written, it will be submitted for evaluation by the Editors of Gastroenterology and Clinical Gastroenterology and Hepatology, who will decide whether to accept the paper or send it for further review before acceptance.
With seasoned and motivated authors and the guidance of the CPUC, we expect outstanding reviews that will be published in a timely manner and provide AGA members and readers of the journals the most up-to-date and scholarly information across the spectrum of gastroenterology, hepatology, and nutrition. We are excited about this new process as a means to enhance our already outstanding journals, which are dedicated to publishing the best research and practice information in our field. Because this is a new process, we welcome feedback and suggestions for improvement and look forward to providing content that helps your research and clinical practice.
Dr. Katzka is at Mayo Clinic, Rochester, Minn.; Dr. Friedman is at Newton-Wellesley Hospital, Newton, Mass.; Dr. Inadomi is at University of Washington School of Medicine, Seattle; Dr. Kalloo is at Johns Hopkins Hospital, Baltimore; Dr. Kim is at South Denver Gastroenterology, P.C., Littleton, Colo.; Dr. Koch is at Virginia Mason Medical Center, Seattle; Dr. Lieberman is at Oregon Health and Science University, Portland; Dr. Lichtenstein is at the Hospital of the University of Pennsylvania, Merion Station, Penn.: Dr. Lim is at Yale University School of Medicine, New Haven, Conn.; Dr. Pandolfino is at Northwestern University, Chicago; Dr. Shin is at Indiana University School of Medicine, Indianapolis; and Dr. Siedler is Committee Liaison, American Gastroenterological Association.
In this issue of Gastroenterology and in an upcoming issue of Clinical Gastroenterology and Hepatology, an important behind-the-scenes change is occurring. Specifically, both journals will continue to publish important and timely reviews and commentaries, but the formulation and design of some of these articles will now be driven by a new committee. The newly formed American Gastroenterological Association (AGA) Institute Clinical Practice Updates Committee (CPUC) is charged with providing authoritative and balanced articles for the two journals that have the greatest impact on the clinical practice of gastroenterology and hepatology. Potential subjects for these articles will derive from several sources in coordination with the AGA Institute Clinical Guidelines Committee.
First, important topics that are proposed initially for an AGA guideline but do not meet rigid criteria for guideline grading will be sent to the CPUC for review and consideration. Second, ideas for topics will also be generated by the CPUC members and journal editors, as well as by other AGA committees and AGA members themselves. Third, summaries of important symposia such as the yearly Freston Conference will be included. Topics can be presented in the form of a comprehensive, narrative review or, for particularly timely issues in gastroenterology, hepatology, and health care in general, as a rapid commentary.
We believe that the vetting process of these articles is important. All prospective topics and selected authors will be approved by the CPUC with guidance from the AGA Institute Governing Board. At least 2 authors who are recognized authorities will be invited to write an article and will be chosen from different institutions. It is anticipated that the authors will often have differing opinions, to help ensure balance and achieve consensus. A member of the CPUC with expertise in the field will guide the writing to ensure the highest quality and greatest balance in the final product. Once an article is written, it will be submitted for evaluation by the Editors of Gastroenterology and Clinical Gastroenterology and Hepatology, who will decide whether to accept the paper or send it for further review before acceptance.
With seasoned and motivated authors and the guidance of the CPUC, we expect outstanding reviews that will be published in a timely manner and provide AGA members and readers of the journals the most up-to-date and scholarly information across the spectrum of gastroenterology, hepatology, and nutrition. We are excited about this new process as a means to enhance our already outstanding journals, which are dedicated to publishing the best research and practice information in our field. Because this is a new process, we welcome feedback and suggestions for improvement and look forward to providing content that helps your research and clinical practice.
Dr. Katzka is at Mayo Clinic, Rochester, Minn.; Dr. Friedman is at Newton-Wellesley Hospital, Newton, Mass.; Dr. Inadomi is at University of Washington School of Medicine, Seattle; Dr. Kalloo is at Johns Hopkins Hospital, Baltimore; Dr. Kim is at South Denver Gastroenterology, P.C., Littleton, Colo.; Dr. Koch is at Virginia Mason Medical Center, Seattle; Dr. Lieberman is at Oregon Health and Science University, Portland; Dr. Lichtenstein is at the Hospital of the University of Pennsylvania, Merion Station, Penn.: Dr. Lim is at Yale University School of Medicine, New Haven, Conn.; Dr. Pandolfino is at Northwestern University, Chicago; Dr. Shin is at Indiana University School of Medicine, Indianapolis; and Dr. Siedler is Committee Liaison, American Gastroenterological Association.
In this issue of Gastroenterology and in an upcoming issue of Clinical Gastroenterology and Hepatology, an important behind-the-scenes change is occurring. Specifically, both journals will continue to publish important and timely reviews and commentaries, but the formulation and design of some of these articles will now be driven by a new committee. The newly formed American Gastroenterological Association (AGA) Institute Clinical Practice Updates Committee (CPUC) is charged with providing authoritative and balanced articles for the two journals that have the greatest impact on the clinical practice of gastroenterology and hepatology. Potential subjects for these articles will derive from several sources in coordination with the AGA Institute Clinical Guidelines Committee.
First, important topics that are proposed initially for an AGA guideline but do not meet rigid criteria for guideline grading will be sent to the CPUC for review and consideration. Second, ideas for topics will also be generated by the CPUC members and journal editors, as well as by other AGA committees and AGA members themselves. Third, summaries of important symposia such as the yearly Freston Conference will be included. Topics can be presented in the form of a comprehensive, narrative review or, for particularly timely issues in gastroenterology, hepatology, and health care in general, as a rapid commentary.
We believe that the vetting process of these articles is important. All prospective topics and selected authors will be approved by the CPUC with guidance from the AGA Institute Governing Board. At least 2 authors who are recognized authorities will be invited to write an article and will be chosen from different institutions. It is anticipated that the authors will often have differing opinions, to help ensure balance and achieve consensus. A member of the CPUC with expertise in the field will guide the writing to ensure the highest quality and greatest balance in the final product. Once an article is written, it will be submitted for evaluation by the Editors of Gastroenterology and Clinical Gastroenterology and Hepatology, who will decide whether to accept the paper or send it for further review before acceptance.
With seasoned and motivated authors and the guidance of the CPUC, we expect outstanding reviews that will be published in a timely manner and provide AGA members and readers of the journals the most up-to-date and scholarly information across the spectrum of gastroenterology, hepatology, and nutrition. We are excited about this new process as a means to enhance our already outstanding journals, which are dedicated to publishing the best research and practice information in our field. Because this is a new process, we welcome feedback and suggestions for improvement and look forward to providing content that helps your research and clinical practice.
Dr. Katzka is at Mayo Clinic, Rochester, Minn.; Dr. Friedman is at Newton-Wellesley Hospital, Newton, Mass.; Dr. Inadomi is at University of Washington School of Medicine, Seattle; Dr. Kalloo is at Johns Hopkins Hospital, Baltimore; Dr. Kim is at South Denver Gastroenterology, P.C., Littleton, Colo.; Dr. Koch is at Virginia Mason Medical Center, Seattle; Dr. Lieberman is at Oregon Health and Science University, Portland; Dr. Lichtenstein is at the Hospital of the University of Pennsylvania, Merion Station, Penn.: Dr. Lim is at Yale University School of Medicine, New Haven, Conn.; Dr. Pandolfino is at Northwestern University, Chicago; Dr. Shin is at Indiana University School of Medicine, Indianapolis; and Dr. Siedler is Committee Liaison, American Gastroenterological Association.