User login
Flu season showing its staying power
Like an unwelcome guest, the 2018-2019 flu season seems to be settling in for a lengthy stay as three more states have reached the highest level of influenza-like illness (ILI) activity, according to the Centers for Disease Control and Prevention.
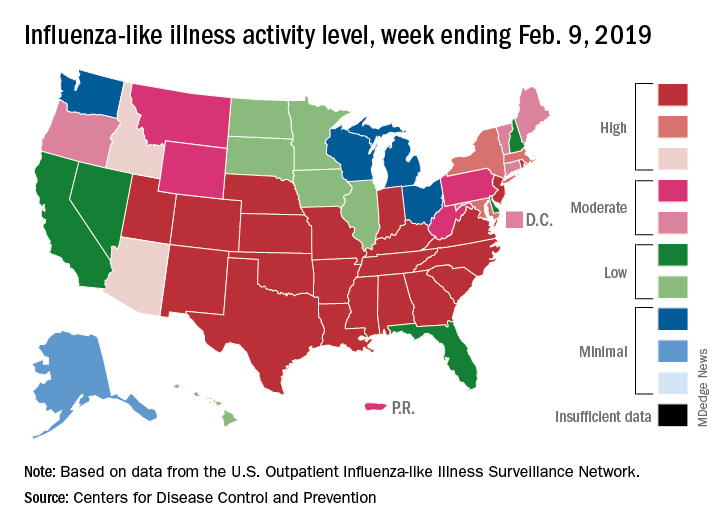
There are now 21 states at level 10 on the CDC’s 1-10 scale, with the South showing up almost solidly red on the flu activity map for the week ending Feb. 9. Another five states are at levels 8 and 9, bringing the total in the high range to 26 for the week, compared with 24 the previous week, the CDC’s influenza division reported Feb. 15.
National activity, reflected in the proportion of outpatient visits involving ILI, took a step up from 4.3% the week before to 4.8% for the week ending Feb. 9. The national baseline rate is 2.2% for ILI, which the CDC defines “as fever (temperature of 100°F [37.8°C] or greater) and cough and/or sore throat.”
Two flu-related pediatric deaths occurred during the week ending Feb. 9, and another four were reported from earlier weeks, which brings the total for the 2018-2019 season to 34, the CDC said. At the same point in last year’s flu season, there had been 84 flu-related deaths in children.
In a separate report, the CDC said that, based on data collected from Nov. 23, 2018 to Feb. 2, 2019, “the influenza vaccine has been 47% effective in preventing medically attended acute respiratory virus infection across all age groups and specifically was 46% effective in preventing medical visits associated with influenza A(H1N1)pdm09.” The effectiveness of the vaccine was 61% for children aged 6 months to 17 years, the CDC said (MMWR. 2019 Feb 15;68[6];135-9).
Flu vaccination during the 2017-2018 season prevented 7.1 million illnesses, 3.7 million medical visits, 109,000 hospitalizations, and 8,000 flu-related deaths, the CDC said, adding that “vaccination has been found to reduce deaths, intensive care unit admissions and length of stay, and overall duration of hospitalization among hospitalized influenza patients.”
Forecasts for the rest of the 2018-2019 season “predict that elevated influenza activity in parts of the United States will continue for several more weeks,” the CDC said.
Like an unwelcome guest, the 2018-2019 flu season seems to be settling in for a lengthy stay as three more states have reached the highest level of influenza-like illness (ILI) activity, according to the Centers for Disease Control and Prevention.

There are now 21 states at level 10 on the CDC’s 1-10 scale, with the South showing up almost solidly red on the flu activity map for the week ending Feb. 9. Another five states are at levels 8 and 9, bringing the total in the high range to 26 for the week, compared with 24 the previous week, the CDC’s influenza division reported Feb. 15.
National activity, reflected in the proportion of outpatient visits involving ILI, took a step up from 4.3% the week before to 4.8% for the week ending Feb. 9. The national baseline rate is 2.2% for ILI, which the CDC defines “as fever (temperature of 100°F [37.8°C] or greater) and cough and/or sore throat.”
Two flu-related pediatric deaths occurred during the week ending Feb. 9, and another four were reported from earlier weeks, which brings the total for the 2018-2019 season to 34, the CDC said. At the same point in last year’s flu season, there had been 84 flu-related deaths in children.
In a separate report, the CDC said that, based on data collected from Nov. 23, 2018 to Feb. 2, 2019, “the influenza vaccine has been 47% effective in preventing medically attended acute respiratory virus infection across all age groups and specifically was 46% effective in preventing medical visits associated with influenza A(H1N1)pdm09.” The effectiveness of the vaccine was 61% for children aged 6 months to 17 years, the CDC said (MMWR. 2019 Feb 15;68[6];135-9).
Flu vaccination during the 2017-2018 season prevented 7.1 million illnesses, 3.7 million medical visits, 109,000 hospitalizations, and 8,000 flu-related deaths, the CDC said, adding that “vaccination has been found to reduce deaths, intensive care unit admissions and length of stay, and overall duration of hospitalization among hospitalized influenza patients.”
Forecasts for the rest of the 2018-2019 season “predict that elevated influenza activity in parts of the United States will continue for several more weeks,” the CDC said.
Like an unwelcome guest, the 2018-2019 flu season seems to be settling in for a lengthy stay as three more states have reached the highest level of influenza-like illness (ILI) activity, according to the Centers for Disease Control and Prevention.

There are now 21 states at level 10 on the CDC’s 1-10 scale, with the South showing up almost solidly red on the flu activity map for the week ending Feb. 9. Another five states are at levels 8 and 9, bringing the total in the high range to 26 for the week, compared with 24 the previous week, the CDC’s influenza division reported Feb. 15.
National activity, reflected in the proportion of outpatient visits involving ILI, took a step up from 4.3% the week before to 4.8% for the week ending Feb. 9. The national baseline rate is 2.2% for ILI, which the CDC defines “as fever (temperature of 100°F [37.8°C] or greater) and cough and/or sore throat.”
Two flu-related pediatric deaths occurred during the week ending Feb. 9, and another four were reported from earlier weeks, which brings the total for the 2018-2019 season to 34, the CDC said. At the same point in last year’s flu season, there had been 84 flu-related deaths in children.
In a separate report, the CDC said that, based on data collected from Nov. 23, 2018 to Feb. 2, 2019, “the influenza vaccine has been 47% effective in preventing medically attended acute respiratory virus infection across all age groups and specifically was 46% effective in preventing medical visits associated with influenza A(H1N1)pdm09.” The effectiveness of the vaccine was 61% for children aged 6 months to 17 years, the CDC said (MMWR. 2019 Feb 15;68[6];135-9).
Flu vaccination during the 2017-2018 season prevented 7.1 million illnesses, 3.7 million medical visits, 109,000 hospitalizations, and 8,000 flu-related deaths, the CDC said, adding that “vaccination has been found to reduce deaths, intensive care unit admissions and length of stay, and overall duration of hospitalization among hospitalized influenza patients.”
Forecasts for the rest of the 2018-2019 season “predict that elevated influenza activity in parts of the United States will continue for several more weeks,” the CDC said.
The amazing work we get to do
Serving people, connecting, and improving care
Stories are told of the first meeting, 20 years ago, where a hat was passed to collect donations to develop a fledgling organization of inpatient physicians. Today, 90% of hospitals with 200+ beds operate with a hospitalist model. Today, we are the Society of Hospital Medicine.
In the early 1900s, health care in many ways was simple. It was a doctor with a shingle hung. It was house calls. Remedies were limited. In the 1940s, companies developed insurance benefits to lure workers during World War II; this third party, the payer, added complexity. Meanwhile, treatment options began to diversify. Then, in the 1960s, Medicare was passed, and the government came into the mix, further increasing this complexity. Diagnostic and treatment options continued to diversify, seemingly exponentially. Some would say it took 30 years for our country to recognize that it had created the most advanced and expensive, as well as one of the least quality-controlled, health systems in the world. Thus, as hospital medicine was conceived in the 1990s, our national health system was awakening to the need – the creative niche – that hospitalists would fill.
When I began my career, I was unaware that I was a hospitalist. The title didn’t exist. Yes, I was working solely in the hospital. I was developing programs to improve care delivery. I was rounding, teaching, collaborating, connecting – everything that we now call hospital medicine. That first job has evolved into my career, one that I find both honorable and enjoyable.
As health care changes with the passing years, being a hospitalist continues to be about serving people, connecting, and improving care. Being a hospitalist is being innovative, willing, and even daring. Dare to try, dare to fail, dare to redesign and try again. Hospital medicine is thinking outside of the box while knowing how to color between the lines. In the coming decades, health care will continue to evolve, and hospital medicine will too. We now encompass surgical comanagement and perioperative care, palliative care, postacute care, and transitions of care services. In corners, hospital medicine is already experimenting with telecare, virtual health, and hospital at home. Our hospital medicine workforce is innovative, diverse, tech savvy and poised for leading.
We are ready and willing to face the pressures affecting health care in the United States today: the recognition of an overwhelming expense to society, the relative underperformance with regard to quality, the increasing complexity of illness and treatment options, the worsening health of the average American citizen, the aging population, the role of medical error in patient harm, the increasing engagement of people in their own care, and the desire to make care better. What our country is facing is actually a phenomenal opportunity, no matter what side of the political aisle you live on. Being in hospital medicine today is being at the center front of this evolutionary stage.
Since joining the SHM board of directors, I continue to find examples of the stellar work of our staff and our members across the country. Having the privilege as a board member to join several Chapter meetings, I have witnessed firsthand the camaraderie, the compassion, the team that makes our Society work. From Houston to San Francisco and from St. Louis to Seattle, I have been honored to work with SHM members that create and nurture local and regional networks with the support of SHM’s Chapters program – a program that now houses more than 50 chapters and has launched regional districts to further support networking and growth. SHM’s chapter venues allow our larger hospital medicine team – yes, the national one – to connect and collaborate.
Take the Pacific Northwest Chapter. In its early years hospitalists from various and competing health systems would convene at a restaurant and just talk. They spoke of how to staff, how to pay, and how to negotiate with hospital leadership. As I have joined chapter meetings in recent years, meetings continue to be the place to share ideas – how to develop new programs; what is the most recent approach to glycemic control, sepsis care, or antibiotic stewardship; how best to approach diagnosis without “anchoring”; and even how to care for each other in the time of loss of a colleague to suicide. It is here in our community that we share experiences, knowledge, new ideas – and this sharing makes us all stronger.
Our hospital medicine community also comes together through areas of shared interest. There are 18 Special Interest Groups (SIGs), focused on specific topic areas. I have been privileged as a board member to work with our Perioperative/Comanagement SIG as it launched in 2017 and has grown rapidly. Currently, the community hosts a “case of the month” hospital medicine discussion as well as a regular journal club webinar that allows participants to review recent literature and interact directly with the authors. As this SIG has grown, shared resources and ideas have allowed for diffusion of knowledge, providing our nation with infrastructure for improving perioperative care. It is networks like this that support our national hospital medicine team to build strength through sharing.
It is our society, our people, that have taken us from the passing of a hat to developing our national community and network. This March, we get to celebrate our field in a new way – Thursday, March 7, 2019, marks the inaugural National Hospitalist Day. Then, March 24-27, our annual conference, Hospital Medicine 2019, will bring thousands of our national team to National Harbor, Maryland. Join your colleagues. Find your niche and your community. Be a part of the change you want to see. While you are there, come introduce yourself to me and let me thank you for the amazing work you are doing.
We are all a part of this movement transforming patient care both on a local and national level. As we move to the future, our innovative, diverse, and connected network of hospital medicine will continue to create and guide health care advances in our country.
Dr. Thompson is professor and chief of the section of hospital medicine at University of Nebraska Medical Center, and medical director of clinical care transitions at Nebraska Medicine, Omaha.
Serving people, connecting, and improving care
Serving people, connecting, and improving care
Stories are told of the first meeting, 20 years ago, where a hat was passed to collect donations to develop a fledgling organization of inpatient physicians. Today, 90% of hospitals with 200+ beds operate with a hospitalist model. Today, we are the Society of Hospital Medicine.
In the early 1900s, health care in many ways was simple. It was a doctor with a shingle hung. It was house calls. Remedies were limited. In the 1940s, companies developed insurance benefits to lure workers during World War II; this third party, the payer, added complexity. Meanwhile, treatment options began to diversify. Then, in the 1960s, Medicare was passed, and the government came into the mix, further increasing this complexity. Diagnostic and treatment options continued to diversify, seemingly exponentially. Some would say it took 30 years for our country to recognize that it had created the most advanced and expensive, as well as one of the least quality-controlled, health systems in the world. Thus, as hospital medicine was conceived in the 1990s, our national health system was awakening to the need – the creative niche – that hospitalists would fill.
When I began my career, I was unaware that I was a hospitalist. The title didn’t exist. Yes, I was working solely in the hospital. I was developing programs to improve care delivery. I was rounding, teaching, collaborating, connecting – everything that we now call hospital medicine. That first job has evolved into my career, one that I find both honorable and enjoyable.
As health care changes with the passing years, being a hospitalist continues to be about serving people, connecting, and improving care. Being a hospitalist is being innovative, willing, and even daring. Dare to try, dare to fail, dare to redesign and try again. Hospital medicine is thinking outside of the box while knowing how to color between the lines. In the coming decades, health care will continue to evolve, and hospital medicine will too. We now encompass surgical comanagement and perioperative care, palliative care, postacute care, and transitions of care services. In corners, hospital medicine is already experimenting with telecare, virtual health, and hospital at home. Our hospital medicine workforce is innovative, diverse, tech savvy and poised for leading.
We are ready and willing to face the pressures affecting health care in the United States today: the recognition of an overwhelming expense to society, the relative underperformance with regard to quality, the increasing complexity of illness and treatment options, the worsening health of the average American citizen, the aging population, the role of medical error in patient harm, the increasing engagement of people in their own care, and the desire to make care better. What our country is facing is actually a phenomenal opportunity, no matter what side of the political aisle you live on. Being in hospital medicine today is being at the center front of this evolutionary stage.
Since joining the SHM board of directors, I continue to find examples of the stellar work of our staff and our members across the country. Having the privilege as a board member to join several Chapter meetings, I have witnessed firsthand the camaraderie, the compassion, the team that makes our Society work. From Houston to San Francisco and from St. Louis to Seattle, I have been honored to work with SHM members that create and nurture local and regional networks with the support of SHM’s Chapters program – a program that now houses more than 50 chapters and has launched regional districts to further support networking and growth. SHM’s chapter venues allow our larger hospital medicine team – yes, the national one – to connect and collaborate.
Take the Pacific Northwest Chapter. In its early years hospitalists from various and competing health systems would convene at a restaurant and just talk. They spoke of how to staff, how to pay, and how to negotiate with hospital leadership. As I have joined chapter meetings in recent years, meetings continue to be the place to share ideas – how to develop new programs; what is the most recent approach to glycemic control, sepsis care, or antibiotic stewardship; how best to approach diagnosis without “anchoring”; and even how to care for each other in the time of loss of a colleague to suicide. It is here in our community that we share experiences, knowledge, new ideas – and this sharing makes us all stronger.
Our hospital medicine community also comes together through areas of shared interest. There are 18 Special Interest Groups (SIGs), focused on specific topic areas. I have been privileged as a board member to work with our Perioperative/Comanagement SIG as it launched in 2017 and has grown rapidly. Currently, the community hosts a “case of the month” hospital medicine discussion as well as a regular journal club webinar that allows participants to review recent literature and interact directly with the authors. As this SIG has grown, shared resources and ideas have allowed for diffusion of knowledge, providing our nation with infrastructure for improving perioperative care. It is networks like this that support our national hospital medicine team to build strength through sharing.
It is our society, our people, that have taken us from the passing of a hat to developing our national community and network. This March, we get to celebrate our field in a new way – Thursday, March 7, 2019, marks the inaugural National Hospitalist Day. Then, March 24-27, our annual conference, Hospital Medicine 2019, will bring thousands of our national team to National Harbor, Maryland. Join your colleagues. Find your niche and your community. Be a part of the change you want to see. While you are there, come introduce yourself to me and let me thank you for the amazing work you are doing.
We are all a part of this movement transforming patient care both on a local and national level. As we move to the future, our innovative, diverse, and connected network of hospital medicine will continue to create and guide health care advances in our country.
Dr. Thompson is professor and chief of the section of hospital medicine at University of Nebraska Medical Center, and medical director of clinical care transitions at Nebraska Medicine, Omaha.
Stories are told of the first meeting, 20 years ago, where a hat was passed to collect donations to develop a fledgling organization of inpatient physicians. Today, 90% of hospitals with 200+ beds operate with a hospitalist model. Today, we are the Society of Hospital Medicine.
In the early 1900s, health care in many ways was simple. It was a doctor with a shingle hung. It was house calls. Remedies were limited. In the 1940s, companies developed insurance benefits to lure workers during World War II; this third party, the payer, added complexity. Meanwhile, treatment options began to diversify. Then, in the 1960s, Medicare was passed, and the government came into the mix, further increasing this complexity. Diagnostic and treatment options continued to diversify, seemingly exponentially. Some would say it took 30 years for our country to recognize that it had created the most advanced and expensive, as well as one of the least quality-controlled, health systems in the world. Thus, as hospital medicine was conceived in the 1990s, our national health system was awakening to the need – the creative niche – that hospitalists would fill.
When I began my career, I was unaware that I was a hospitalist. The title didn’t exist. Yes, I was working solely in the hospital. I was developing programs to improve care delivery. I was rounding, teaching, collaborating, connecting – everything that we now call hospital medicine. That first job has evolved into my career, one that I find both honorable and enjoyable.
As health care changes with the passing years, being a hospitalist continues to be about serving people, connecting, and improving care. Being a hospitalist is being innovative, willing, and even daring. Dare to try, dare to fail, dare to redesign and try again. Hospital medicine is thinking outside of the box while knowing how to color between the lines. In the coming decades, health care will continue to evolve, and hospital medicine will too. We now encompass surgical comanagement and perioperative care, palliative care, postacute care, and transitions of care services. In corners, hospital medicine is already experimenting with telecare, virtual health, and hospital at home. Our hospital medicine workforce is innovative, diverse, tech savvy and poised for leading.
We are ready and willing to face the pressures affecting health care in the United States today: the recognition of an overwhelming expense to society, the relative underperformance with regard to quality, the increasing complexity of illness and treatment options, the worsening health of the average American citizen, the aging population, the role of medical error in patient harm, the increasing engagement of people in their own care, and the desire to make care better. What our country is facing is actually a phenomenal opportunity, no matter what side of the political aisle you live on. Being in hospital medicine today is being at the center front of this evolutionary stage.
Since joining the SHM board of directors, I continue to find examples of the stellar work of our staff and our members across the country. Having the privilege as a board member to join several Chapter meetings, I have witnessed firsthand the camaraderie, the compassion, the team that makes our Society work. From Houston to San Francisco and from St. Louis to Seattle, I have been honored to work with SHM members that create and nurture local and regional networks with the support of SHM’s Chapters program – a program that now houses more than 50 chapters and has launched regional districts to further support networking and growth. SHM’s chapter venues allow our larger hospital medicine team – yes, the national one – to connect and collaborate.
Take the Pacific Northwest Chapter. In its early years hospitalists from various and competing health systems would convene at a restaurant and just talk. They spoke of how to staff, how to pay, and how to negotiate with hospital leadership. As I have joined chapter meetings in recent years, meetings continue to be the place to share ideas – how to develop new programs; what is the most recent approach to glycemic control, sepsis care, or antibiotic stewardship; how best to approach diagnosis without “anchoring”; and even how to care for each other in the time of loss of a colleague to suicide. It is here in our community that we share experiences, knowledge, new ideas – and this sharing makes us all stronger.
Our hospital medicine community also comes together through areas of shared interest. There are 18 Special Interest Groups (SIGs), focused on specific topic areas. I have been privileged as a board member to work with our Perioperative/Comanagement SIG as it launched in 2017 and has grown rapidly. Currently, the community hosts a “case of the month” hospital medicine discussion as well as a regular journal club webinar that allows participants to review recent literature and interact directly with the authors. As this SIG has grown, shared resources and ideas have allowed for diffusion of knowledge, providing our nation with infrastructure for improving perioperative care. It is networks like this that support our national hospital medicine team to build strength through sharing.
It is our society, our people, that have taken us from the passing of a hat to developing our national community and network. This March, we get to celebrate our field in a new way – Thursday, March 7, 2019, marks the inaugural National Hospitalist Day. Then, March 24-27, our annual conference, Hospital Medicine 2019, will bring thousands of our national team to National Harbor, Maryland. Join your colleagues. Find your niche and your community. Be a part of the change you want to see. While you are there, come introduce yourself to me and let me thank you for the amazing work you are doing.
We are all a part of this movement transforming patient care both on a local and national level. As we move to the future, our innovative, diverse, and connected network of hospital medicine will continue to create and guide health care advances in our country.
Dr. Thompson is professor and chief of the section of hospital medicine at University of Nebraska Medical Center, and medical director of clinical care transitions at Nebraska Medicine, Omaha.
Deferoxamine does not improve 90-day outcomes after ICH
HONOLULU – (ICH), according to trial results described at the International Stroke Conference sponsored by the American Heart Association. However, the drug is safe and well tolerated and data suggest that it may improve outcomes at 180 days.
Animal studies indicate that iron, which is released from hemolyzed red blood cells, accumulates in the brain after ICH and is associated with secondary neuronal injury and death. Researchers have found that deferoxamine, an iron chelator, provides neuroprotection and improves recovery after experimental ICH. The drug also has anti-inflammatory, antiapoptotic, and BP-lowering effects. Deferoxamine has been approved since the 1960s.
Magdy H. Selim, MD, PhD, a neurologist at Beth Israel Deaconess Medical Center in Boston, and colleagues hypothesized that treatment with deferoxamine could improve outcomes in patients with ICH. The researchers conducted a phase 2 clinical trial to evaluate whether deferoxamine should be studied in a phase 3 efficacy trial. In their multicenter, double-blind study, Dr. Selim and his colleagues randomized patients with spontaneous supratentorial ICH in equal groups to 32 mg/kg per day of deferoxamine or saline placebo. Treatments were administered as intravenous infusions for 3 consecutive days, and therapy was initiated within 24 hours after ICH onset. The follow-up period was 6 months.
Eligible participants had an National Institutes of Health Stroke Scale score of 6 or higher, a Glasgow Coma Scale score greater than 6, and had been functionally independent before the hemorrhage. The researchers excluded patients with a secondary cause for ICH or coagulopathy.
The primary endpoint in the futility analysis was the proportion of participants with a good clinical outcome – defined as a modified Rankin Scale (mRS) score of 0-2 – at 90 days and 180 days. The secondary endpoint was good outcome, defined as an mRS score of 0-3, at 90 days. Safety endpoints included all deferoxamine-related adverse events until day 7 or discharge (whichever was earlier) and serious adverse events through day 90.
Dr. Selim and his colleagues enrolled 294 participants in their trial, 3 of whom did not receive treatment. Of these included participants, 147 (50.5%) were randomized to placebo and 144 (49.5%) were randomized to deferoxamine. Participants’ mean age was 60.3 years, and 38.5% of the population was female.
Overall, the two study arms did not differ significantly according to demographic and clinical characteristics, however, there were more nonwhite patients in the deferoxamine arm than in the placebo arm, however. In addition, thalamic hemorrhage and intraventricular hemorrhage were more common in the placebo-treated group and hemorrhages in the putamen and basal ganglia were more common in the deferoxamine-treated group.
The rates of adverse events were comparable between the two study arms. Dr. Selim and his colleagues found no unexpected safety issues. Mortality was low, and the 90-day and 180-day mortality rates were comparable between the two treatment arms.
Approximately 34% of deferoxamine-treated patients and 33% of placebo-treated patients had an mRS score of 0-2 at 90 days. The adjusted absolute risk difference between arms was 0.6%; this result did not surpass the predefined futility threshold. The risk difference between groups for mRS score of 0-2 at 180 days was 8.6% in favor of deferoxamine, which did surpass the futility threshold.
The risk difference for meeting the secondary endpoint was 6.2% in favor of deferoxamine; this result did not surpass the futility threshold. Patients in both treatment groups improved between day 90 and day 180. The likelihood of good outcome was approximately 10% higher in the deferoxamine group at day 90 and 26% higher in the deferoxamine group at day 180.
“It is futile to conduct a phase 3 trial with the anticipation that treatment with deferoxamine would improve outcome, defined as mRS score of 0-2 at 90 days,” said Dr. Selim. “These data, together with the data from MISTIE and CLEAR, suggest that ICH trials need to have a longer follow-up period to capture the full extent of recovery after ICH. Several of our secondary analyses tended to favor deferoxamine over the placebo arm and leave open the possibility that deferoxamine might lead to improved outcome at 180 days.”
The researchers received support from the NIH and the National Institute of Neurological Disorders and Stroke.
SOURCE: Selim MH et al. ISC 2019, Abstract LB22.
HONOLULU – (ICH), according to trial results described at the International Stroke Conference sponsored by the American Heart Association. However, the drug is safe and well tolerated and data suggest that it may improve outcomes at 180 days.
Animal studies indicate that iron, which is released from hemolyzed red blood cells, accumulates in the brain after ICH and is associated with secondary neuronal injury and death. Researchers have found that deferoxamine, an iron chelator, provides neuroprotection and improves recovery after experimental ICH. The drug also has anti-inflammatory, antiapoptotic, and BP-lowering effects. Deferoxamine has been approved since the 1960s.
Magdy H. Selim, MD, PhD, a neurologist at Beth Israel Deaconess Medical Center in Boston, and colleagues hypothesized that treatment with deferoxamine could improve outcomes in patients with ICH. The researchers conducted a phase 2 clinical trial to evaluate whether deferoxamine should be studied in a phase 3 efficacy trial. In their multicenter, double-blind study, Dr. Selim and his colleagues randomized patients with spontaneous supratentorial ICH in equal groups to 32 mg/kg per day of deferoxamine or saline placebo. Treatments were administered as intravenous infusions for 3 consecutive days, and therapy was initiated within 24 hours after ICH onset. The follow-up period was 6 months.
Eligible participants had an National Institutes of Health Stroke Scale score of 6 or higher, a Glasgow Coma Scale score greater than 6, and had been functionally independent before the hemorrhage. The researchers excluded patients with a secondary cause for ICH or coagulopathy.
The primary endpoint in the futility analysis was the proportion of participants with a good clinical outcome – defined as a modified Rankin Scale (mRS) score of 0-2 – at 90 days and 180 days. The secondary endpoint was good outcome, defined as an mRS score of 0-3, at 90 days. Safety endpoints included all deferoxamine-related adverse events until day 7 or discharge (whichever was earlier) and serious adverse events through day 90.
Dr. Selim and his colleagues enrolled 294 participants in their trial, 3 of whom did not receive treatment. Of these included participants, 147 (50.5%) were randomized to placebo and 144 (49.5%) were randomized to deferoxamine. Participants’ mean age was 60.3 years, and 38.5% of the population was female.
Overall, the two study arms did not differ significantly according to demographic and clinical characteristics, however, there were more nonwhite patients in the deferoxamine arm than in the placebo arm, however. In addition, thalamic hemorrhage and intraventricular hemorrhage were more common in the placebo-treated group and hemorrhages in the putamen and basal ganglia were more common in the deferoxamine-treated group.
The rates of adverse events were comparable between the two study arms. Dr. Selim and his colleagues found no unexpected safety issues. Mortality was low, and the 90-day and 180-day mortality rates were comparable between the two treatment arms.
Approximately 34% of deferoxamine-treated patients and 33% of placebo-treated patients had an mRS score of 0-2 at 90 days. The adjusted absolute risk difference between arms was 0.6%; this result did not surpass the predefined futility threshold. The risk difference between groups for mRS score of 0-2 at 180 days was 8.6% in favor of deferoxamine, which did surpass the futility threshold.
The risk difference for meeting the secondary endpoint was 6.2% in favor of deferoxamine; this result did not surpass the futility threshold. Patients in both treatment groups improved between day 90 and day 180. The likelihood of good outcome was approximately 10% higher in the deferoxamine group at day 90 and 26% higher in the deferoxamine group at day 180.
“It is futile to conduct a phase 3 trial with the anticipation that treatment with deferoxamine would improve outcome, defined as mRS score of 0-2 at 90 days,” said Dr. Selim. “These data, together with the data from MISTIE and CLEAR, suggest that ICH trials need to have a longer follow-up period to capture the full extent of recovery after ICH. Several of our secondary analyses tended to favor deferoxamine over the placebo arm and leave open the possibility that deferoxamine might lead to improved outcome at 180 days.”
The researchers received support from the NIH and the National Institute of Neurological Disorders and Stroke.
SOURCE: Selim MH et al. ISC 2019, Abstract LB22.
HONOLULU – (ICH), according to trial results described at the International Stroke Conference sponsored by the American Heart Association. However, the drug is safe and well tolerated and data suggest that it may improve outcomes at 180 days.
Animal studies indicate that iron, which is released from hemolyzed red blood cells, accumulates in the brain after ICH and is associated with secondary neuronal injury and death. Researchers have found that deferoxamine, an iron chelator, provides neuroprotection and improves recovery after experimental ICH. The drug also has anti-inflammatory, antiapoptotic, and BP-lowering effects. Deferoxamine has been approved since the 1960s.
Magdy H. Selim, MD, PhD, a neurologist at Beth Israel Deaconess Medical Center in Boston, and colleagues hypothesized that treatment with deferoxamine could improve outcomes in patients with ICH. The researchers conducted a phase 2 clinical trial to evaluate whether deferoxamine should be studied in a phase 3 efficacy trial. In their multicenter, double-blind study, Dr. Selim and his colleagues randomized patients with spontaneous supratentorial ICH in equal groups to 32 mg/kg per day of deferoxamine or saline placebo. Treatments were administered as intravenous infusions for 3 consecutive days, and therapy was initiated within 24 hours after ICH onset. The follow-up period was 6 months.
Eligible participants had an National Institutes of Health Stroke Scale score of 6 or higher, a Glasgow Coma Scale score greater than 6, and had been functionally independent before the hemorrhage. The researchers excluded patients with a secondary cause for ICH or coagulopathy.
The primary endpoint in the futility analysis was the proportion of participants with a good clinical outcome – defined as a modified Rankin Scale (mRS) score of 0-2 – at 90 days and 180 days. The secondary endpoint was good outcome, defined as an mRS score of 0-3, at 90 days. Safety endpoints included all deferoxamine-related adverse events until day 7 or discharge (whichever was earlier) and serious adverse events through day 90.
Dr. Selim and his colleagues enrolled 294 participants in their trial, 3 of whom did not receive treatment. Of these included participants, 147 (50.5%) were randomized to placebo and 144 (49.5%) were randomized to deferoxamine. Participants’ mean age was 60.3 years, and 38.5% of the population was female.
Overall, the two study arms did not differ significantly according to demographic and clinical characteristics, however, there were more nonwhite patients in the deferoxamine arm than in the placebo arm, however. In addition, thalamic hemorrhage and intraventricular hemorrhage were more common in the placebo-treated group and hemorrhages in the putamen and basal ganglia were more common in the deferoxamine-treated group.
The rates of adverse events were comparable between the two study arms. Dr. Selim and his colleagues found no unexpected safety issues. Mortality was low, and the 90-day and 180-day mortality rates were comparable between the two treatment arms.
Approximately 34% of deferoxamine-treated patients and 33% of placebo-treated patients had an mRS score of 0-2 at 90 days. The adjusted absolute risk difference between arms was 0.6%; this result did not surpass the predefined futility threshold. The risk difference between groups for mRS score of 0-2 at 180 days was 8.6% in favor of deferoxamine, which did surpass the futility threshold.
The risk difference for meeting the secondary endpoint was 6.2% in favor of deferoxamine; this result did not surpass the futility threshold. Patients in both treatment groups improved between day 90 and day 180. The likelihood of good outcome was approximately 10% higher in the deferoxamine group at day 90 and 26% higher in the deferoxamine group at day 180.
“It is futile to conduct a phase 3 trial with the anticipation that treatment with deferoxamine would improve outcome, defined as mRS score of 0-2 at 90 days,” said Dr. Selim. “These data, together with the data from MISTIE and CLEAR, suggest that ICH trials need to have a longer follow-up period to capture the full extent of recovery after ICH. Several of our secondary analyses tended to favor deferoxamine over the placebo arm and leave open the possibility that deferoxamine might lead to improved outcome at 180 days.”
The researchers received support from the NIH and the National Institute of Neurological Disorders and Stroke.
SOURCE: Selim MH et al. ISC 2019, Abstract LB22.
REPORTING FROM ISC 2019
Key clinical point: Deferoxamine does not improve disability at 90 days after intracranial hemorrhage.
Major finding: Approximately one-third of patients in both treatment groups had a good outcome.
Study details: A multicenter, randomized, double-blind study of 294 participants with intracranial hemorrhage.
Disclosures: The National Institutes of Health and National Institute of Neurological Disorders and Stroke supported this study.
Source: Selim MH et al. ISC 2019, Abstract LB22.
An unplanned career
A focus on health system transformation
I have to admit that I am not sure I am a legacy in hospital medicine, and the term legacy throws me off a bit. I came to medical school after working at McKinsey & Co. consulting, and I chose pediatrics because of my love of working with children and families, as well as a vague notion that I wanted to work on “system” issues, and therefore, more generalist-type training seemed applicable.
I met Chris Landrigan, MD, MPH, and Vinny Chiang, MD, and learned what a hospitalist was, as an intern in 2002. We had a research elective and I was able to publish a couple of papers in Pediatrics on pediatric hospital medicine with Chris and Raj Srivastava, MD, MPH. In 2004, I went to my first Society of Hospital Medicine meeting and met Larry Wellikson, MD, MHM, and others. From there, I went to the Robert Wood Johnson Clinical Scholars Program, with Ron Keren, MD, MPH, and others, and along with faculty from the Cincinnati Children’s in hospital medicine.
In 2007, I applied for a White House Fellowship and told my wife that I didn’t think there was a chance that I would get it, so we should keep building our new home in Cincinnati. We were both surprised when I was selected. I served Michael Leavitt, the then-Secretary of the Department of Health & Human Services, as his White House fellow during the Bush administration, and then served as his chief medical officer. Exposure to health policy and leadership at that level was career shaping. Cincinnati Children’s was searching for a leader for the conversion of pediatric hospital medicine into a full division in 2009. So I returned to Cincinnati to take on leading pediatric hospital medicine, and a role leading quality measurement and improvement efforts for the entire health system. I loved the work and thought I would remain in that role, and our family would be in Cincinnati for a long time. Best laid plans …
In early 2011, Don Berwick, MD, who was then the administrator of the Centers for Medicare & Medicaid Services called and asked whether I “would come talk with him in D.C.” That talk quickly became a series of interviews, and he offered me the opportunity to be chief medical officer of CMS. He said “this platform is like no other to drive change.” He was right. I have been fortunate to have a few step-change opportunities in my life, and that was one.
On my first day at CMS, I looked around the table of senior executives reporting to me and realized they had more than 200 years of CMS experience. I was a bit scared. Together, we led the implementation of Hospital Value-Based Purchasing, the Compare websites, and numerous quality measurement and improvement programs. Partnership for Patients works on patient safety and was associated with preventing more than 3 million infections and adverse events, over 125,000 lives saved, and more than $26 billion in savings.
In early 2013, I was asked to lead the CMS Innovation Center (CMMI). The goal was to launch new payment and service delivery models to improve quality and lower costs. We launched Accountable Care Organizations, Bundled Payment programs, primary care medical homes, state-based innovation, and so much more. Medicare went from zero dollars in alternative payment models, where providers are accountable for quality and total cost of care, to more than 30% of Medicare payments, representing over $200 billion through agreements with more than 200,000 providers in these alternative payment models. It was the biggest shift in U.S. history in how CMS paid for care. Later, I became principal deputy administrator and acting administrator of CMS, leading an agency that spends over $1 trillion per year, or more than $2.5 billion per day and insures over 130 million Americans. We also improved from being bottom quintile in employee engagement and satisfaction across the federal government to No. 2.
I had assumed that, after working at CMS, I would return to a hospital/health system leadership role. But then, a recruiter called about the CEO role at Blue Cross Blue Shield of North Carolina. It is one of the largest not-for-profit health plans in the country and insures most of the people in North Carolina, many for most of their lives. I met a 75-year-old woman the other day that we have insured every day of her life. I am almost a year into the role and it is a mission-driven organization that drives positive change. I love it so far.
We are going to partner with providers, so that more than half of our payments will be in advanced alternative payment models. No payer in the United States has done that yet. This allows us to innovate and decrease friction in the system (e.g., turn off prior authorization) and be jointly accountable with providers for quality and total cost of care. We insure people through the ACA [Affordable Care Act], commercial, and Medicare markets, and are competing to serve Medicaid as well. We have invested more than $50 million to address social determinants of health across the state. We are making major investments in primary care, and mental and behavioral health. Our goal is to be a Model Blue – or a Model of Health Transformation for our state and nation – and achieve better health outcomes, lower costs, and best-in-class experience for all people we serve. I have learned that no physician leads a health plan of this size, and apparently, no practicing physician has ever led a health plan of this size.
What are some lessons learned over my career? I have had five criteria for all my career decisions: 1) family; 2) impact – better care and outcomes, lower costs, and exceptional experience for populations of patients; 3) people – mentors and colleagues; 4) learning; and 5) joy in work. If someone gives you a chance to lead people in your career as a physician, jump at the chance. We do a relatively poor job of providing this type of opportunity to those early in their careers in medicine, and learning how to manage people and money allows you to progress as a leader and manager.
Don’t listen to the people who say “you must do X before Y” or “you must take this path.” They are usually wrong. Take chances. I applied for many roles for which I was a long shot, and I didn’t always succeed. That’s life and learning. Hospital medicine is a great career. I worked in the hospital on a recent weekend and was able to help families through everything from palliative care decisions and new diagnoses, to recovering from illness. It is an honor to serve and help families in their time of need. Hospitalists have been – and should continue to be – primary drivers of the shift in our health system to value-based care.
As I look back on my career (and I hope I am only halfway done), I could not have predicted more than 90% of it. I was blessed with many opportunities, mentors, and teachers along the way. I try to pass this on by mentoring and teaching others. How did my career happen? I am not sure, but it has been a fun ride! And hopefully I have helped improve the health system some, along the way.
Dr. Conway is president and CEO of Blue Cross and Blue Shield of North Carolina. He is a hospitalist and former deputy administrator for innovation and quality at the Centers for Medicare and Medicaid Services.
A focus on health system transformation
A focus on health system transformation
I have to admit that I am not sure I am a legacy in hospital medicine, and the term legacy throws me off a bit. I came to medical school after working at McKinsey & Co. consulting, and I chose pediatrics because of my love of working with children and families, as well as a vague notion that I wanted to work on “system” issues, and therefore, more generalist-type training seemed applicable.
I met Chris Landrigan, MD, MPH, and Vinny Chiang, MD, and learned what a hospitalist was, as an intern in 2002. We had a research elective and I was able to publish a couple of papers in Pediatrics on pediatric hospital medicine with Chris and Raj Srivastava, MD, MPH. In 2004, I went to my first Society of Hospital Medicine meeting and met Larry Wellikson, MD, MHM, and others. From there, I went to the Robert Wood Johnson Clinical Scholars Program, with Ron Keren, MD, MPH, and others, and along with faculty from the Cincinnati Children’s in hospital medicine.
In 2007, I applied for a White House Fellowship and told my wife that I didn’t think there was a chance that I would get it, so we should keep building our new home in Cincinnati. We were both surprised when I was selected. I served Michael Leavitt, the then-Secretary of the Department of Health & Human Services, as his White House fellow during the Bush administration, and then served as his chief medical officer. Exposure to health policy and leadership at that level was career shaping. Cincinnati Children’s was searching for a leader for the conversion of pediatric hospital medicine into a full division in 2009. So I returned to Cincinnati to take on leading pediatric hospital medicine, and a role leading quality measurement and improvement efforts for the entire health system. I loved the work and thought I would remain in that role, and our family would be in Cincinnati for a long time. Best laid plans …
In early 2011, Don Berwick, MD, who was then the administrator of the Centers for Medicare & Medicaid Services called and asked whether I “would come talk with him in D.C.” That talk quickly became a series of interviews, and he offered me the opportunity to be chief medical officer of CMS. He said “this platform is like no other to drive change.” He was right. I have been fortunate to have a few step-change opportunities in my life, and that was one.
On my first day at CMS, I looked around the table of senior executives reporting to me and realized they had more than 200 years of CMS experience. I was a bit scared. Together, we led the implementation of Hospital Value-Based Purchasing, the Compare websites, and numerous quality measurement and improvement programs. Partnership for Patients works on patient safety and was associated with preventing more than 3 million infections and adverse events, over 125,000 lives saved, and more than $26 billion in savings.
In early 2013, I was asked to lead the CMS Innovation Center (CMMI). The goal was to launch new payment and service delivery models to improve quality and lower costs. We launched Accountable Care Organizations, Bundled Payment programs, primary care medical homes, state-based innovation, and so much more. Medicare went from zero dollars in alternative payment models, where providers are accountable for quality and total cost of care, to more than 30% of Medicare payments, representing over $200 billion through agreements with more than 200,000 providers in these alternative payment models. It was the biggest shift in U.S. history in how CMS paid for care. Later, I became principal deputy administrator and acting administrator of CMS, leading an agency that spends over $1 trillion per year, or more than $2.5 billion per day and insures over 130 million Americans. We also improved from being bottom quintile in employee engagement and satisfaction across the federal government to No. 2.
I had assumed that, after working at CMS, I would return to a hospital/health system leadership role. But then, a recruiter called about the CEO role at Blue Cross Blue Shield of North Carolina. It is one of the largest not-for-profit health plans in the country and insures most of the people in North Carolina, many for most of their lives. I met a 75-year-old woman the other day that we have insured every day of her life. I am almost a year into the role and it is a mission-driven organization that drives positive change. I love it so far.
We are going to partner with providers, so that more than half of our payments will be in advanced alternative payment models. No payer in the United States has done that yet. This allows us to innovate and decrease friction in the system (e.g., turn off prior authorization) and be jointly accountable with providers for quality and total cost of care. We insure people through the ACA [Affordable Care Act], commercial, and Medicare markets, and are competing to serve Medicaid as well. We have invested more than $50 million to address social determinants of health across the state. We are making major investments in primary care, and mental and behavioral health. Our goal is to be a Model Blue – or a Model of Health Transformation for our state and nation – and achieve better health outcomes, lower costs, and best-in-class experience for all people we serve. I have learned that no physician leads a health plan of this size, and apparently, no practicing physician has ever led a health plan of this size.
What are some lessons learned over my career? I have had five criteria for all my career decisions: 1) family; 2) impact – better care and outcomes, lower costs, and exceptional experience for populations of patients; 3) people – mentors and colleagues; 4) learning; and 5) joy in work. If someone gives you a chance to lead people in your career as a physician, jump at the chance. We do a relatively poor job of providing this type of opportunity to those early in their careers in medicine, and learning how to manage people and money allows you to progress as a leader and manager.
Don’t listen to the people who say “you must do X before Y” or “you must take this path.” They are usually wrong. Take chances. I applied for many roles for which I was a long shot, and I didn’t always succeed. That’s life and learning. Hospital medicine is a great career. I worked in the hospital on a recent weekend and was able to help families through everything from palliative care decisions and new diagnoses, to recovering from illness. It is an honor to serve and help families in their time of need. Hospitalists have been – and should continue to be – primary drivers of the shift in our health system to value-based care.
As I look back on my career (and I hope I am only halfway done), I could not have predicted more than 90% of it. I was blessed with many opportunities, mentors, and teachers along the way. I try to pass this on by mentoring and teaching others. How did my career happen? I am not sure, but it has been a fun ride! And hopefully I have helped improve the health system some, along the way.
Dr. Conway is president and CEO of Blue Cross and Blue Shield of North Carolina. He is a hospitalist and former deputy administrator for innovation and quality at the Centers for Medicare and Medicaid Services.
I have to admit that I am not sure I am a legacy in hospital medicine, and the term legacy throws me off a bit. I came to medical school after working at McKinsey & Co. consulting, and I chose pediatrics because of my love of working with children and families, as well as a vague notion that I wanted to work on “system” issues, and therefore, more generalist-type training seemed applicable.
I met Chris Landrigan, MD, MPH, and Vinny Chiang, MD, and learned what a hospitalist was, as an intern in 2002. We had a research elective and I was able to publish a couple of papers in Pediatrics on pediatric hospital medicine with Chris and Raj Srivastava, MD, MPH. In 2004, I went to my first Society of Hospital Medicine meeting and met Larry Wellikson, MD, MHM, and others. From there, I went to the Robert Wood Johnson Clinical Scholars Program, with Ron Keren, MD, MPH, and others, and along with faculty from the Cincinnati Children’s in hospital medicine.
In 2007, I applied for a White House Fellowship and told my wife that I didn’t think there was a chance that I would get it, so we should keep building our new home in Cincinnati. We were both surprised when I was selected. I served Michael Leavitt, the then-Secretary of the Department of Health & Human Services, as his White House fellow during the Bush administration, and then served as his chief medical officer. Exposure to health policy and leadership at that level was career shaping. Cincinnati Children’s was searching for a leader for the conversion of pediatric hospital medicine into a full division in 2009. So I returned to Cincinnati to take on leading pediatric hospital medicine, and a role leading quality measurement and improvement efforts for the entire health system. I loved the work and thought I would remain in that role, and our family would be in Cincinnati for a long time. Best laid plans …
In early 2011, Don Berwick, MD, who was then the administrator of the Centers for Medicare & Medicaid Services called and asked whether I “would come talk with him in D.C.” That talk quickly became a series of interviews, and he offered me the opportunity to be chief medical officer of CMS. He said “this platform is like no other to drive change.” He was right. I have been fortunate to have a few step-change opportunities in my life, and that was one.
On my first day at CMS, I looked around the table of senior executives reporting to me and realized they had more than 200 years of CMS experience. I was a bit scared. Together, we led the implementation of Hospital Value-Based Purchasing, the Compare websites, and numerous quality measurement and improvement programs. Partnership for Patients works on patient safety and was associated with preventing more than 3 million infections and adverse events, over 125,000 lives saved, and more than $26 billion in savings.
In early 2013, I was asked to lead the CMS Innovation Center (CMMI). The goal was to launch new payment and service delivery models to improve quality and lower costs. We launched Accountable Care Organizations, Bundled Payment programs, primary care medical homes, state-based innovation, and so much more. Medicare went from zero dollars in alternative payment models, where providers are accountable for quality and total cost of care, to more than 30% of Medicare payments, representing over $200 billion through agreements with more than 200,000 providers in these alternative payment models. It was the biggest shift in U.S. history in how CMS paid for care. Later, I became principal deputy administrator and acting administrator of CMS, leading an agency that spends over $1 trillion per year, or more than $2.5 billion per day and insures over 130 million Americans. We also improved from being bottom quintile in employee engagement and satisfaction across the federal government to No. 2.
I had assumed that, after working at CMS, I would return to a hospital/health system leadership role. But then, a recruiter called about the CEO role at Blue Cross Blue Shield of North Carolina. It is one of the largest not-for-profit health plans in the country and insures most of the people in North Carolina, many for most of their lives. I met a 75-year-old woman the other day that we have insured every day of her life. I am almost a year into the role and it is a mission-driven organization that drives positive change. I love it so far.
We are going to partner with providers, so that more than half of our payments will be in advanced alternative payment models. No payer in the United States has done that yet. This allows us to innovate and decrease friction in the system (e.g., turn off prior authorization) and be jointly accountable with providers for quality and total cost of care. We insure people through the ACA [Affordable Care Act], commercial, and Medicare markets, and are competing to serve Medicaid as well. We have invested more than $50 million to address social determinants of health across the state. We are making major investments in primary care, and mental and behavioral health. Our goal is to be a Model Blue – or a Model of Health Transformation for our state and nation – and achieve better health outcomes, lower costs, and best-in-class experience for all people we serve. I have learned that no physician leads a health plan of this size, and apparently, no practicing physician has ever led a health plan of this size.
What are some lessons learned over my career? I have had five criteria for all my career decisions: 1) family; 2) impact – better care and outcomes, lower costs, and exceptional experience for populations of patients; 3) people – mentors and colleagues; 4) learning; and 5) joy in work. If someone gives you a chance to lead people in your career as a physician, jump at the chance. We do a relatively poor job of providing this type of opportunity to those early in their careers in medicine, and learning how to manage people and money allows you to progress as a leader and manager.
Don’t listen to the people who say “you must do X before Y” or “you must take this path.” They are usually wrong. Take chances. I applied for many roles for which I was a long shot, and I didn’t always succeed. That’s life and learning. Hospital medicine is a great career. I worked in the hospital on a recent weekend and was able to help families through everything from palliative care decisions and new diagnoses, to recovering from illness. It is an honor to serve and help families in their time of need. Hospitalists have been – and should continue to be – primary drivers of the shift in our health system to value-based care.
As I look back on my career (and I hope I am only halfway done), I could not have predicted more than 90% of it. I was blessed with many opportunities, mentors, and teachers along the way. I try to pass this on by mentoring and teaching others. How did my career happen? I am not sure, but it has been a fun ride! And hopefully I have helped improve the health system some, along the way.
Dr. Conway is president and CEO of Blue Cross and Blue Shield of North Carolina. He is a hospitalist and former deputy administrator for innovation and quality at the Centers for Medicare and Medicaid Services.
ONC aims to help docs, patients with information sharing in proposed rule
The Office of the National Coordinator of Health Information Technology is looking to adopt standardized application programming interfaces (APIs) in an effort to boost interoperability of health data.
The Department of Health & Human Services office posted a proposed rule Feb. 11, 2019, that would, according to an agency press release, “help allow individuals to securely and easily access structured and unstructured EHI [electronic health information] formats using smartphones and other mobile devices.”
“We think our rule is going to help reduce burden and improve care,” Michael Lipinski, director of the Regulatory Affairs Division in the ONC Office of Policy, said in an interview. “It is going to do that through technology. With the APIs, you should be able to get to your information easier and have it readily available. Whether that is from another health care provider or using other health care products through the API to improve care, you will have that ability between the certified API and the information blocking policies to use third party developers and their products.”
The proposed rule also included a requirement that EHRs certified by ONC be able to easily export information contained within the EHR and make the format used to extract and export the data contained within the EHR publicly available.
“Another third party developer can build to that and offer competing services to pull that information out,” Mr. Lipinski said. “That would obviously help if you were choosing to switch [EHRs] if you didn’t like the features you were getting from your EHR. ... That functionality should help if you want to do that.”
The standardizing of APIs to help the delivery of data will go hand in hand with information blocking aspects of the proposed rule, which defines the few exceptions where an activity would not be considered information blocking, such as when engaging in practices will prevent patient harm; engaging in consistent, nondiscriminatory practices to protect patient privacy; and implementing practices to promote the security of health information.
Mr. Lipinski said these changes will help prevent providers from hiding behind HIPAA rules as the excuse to not share patient information, which will help with care coordination. “From a provider’s perspective, this should help them get more access to information, more access in a structured way and then easily get and share that information.”
Ultimately, Mr. Lipinski said, the goal is “to increase competition and lower cost while still improving the quality of care for patients.”
The Office of the National Coordinator of Health Information Technology is looking to adopt standardized application programming interfaces (APIs) in an effort to boost interoperability of health data.
The Department of Health & Human Services office posted a proposed rule Feb. 11, 2019, that would, according to an agency press release, “help allow individuals to securely and easily access structured and unstructured EHI [electronic health information] formats using smartphones and other mobile devices.”
“We think our rule is going to help reduce burden and improve care,” Michael Lipinski, director of the Regulatory Affairs Division in the ONC Office of Policy, said in an interview. “It is going to do that through technology. With the APIs, you should be able to get to your information easier and have it readily available. Whether that is from another health care provider or using other health care products through the API to improve care, you will have that ability between the certified API and the information blocking policies to use third party developers and their products.”
The proposed rule also included a requirement that EHRs certified by ONC be able to easily export information contained within the EHR and make the format used to extract and export the data contained within the EHR publicly available.
“Another third party developer can build to that and offer competing services to pull that information out,” Mr. Lipinski said. “That would obviously help if you were choosing to switch [EHRs] if you didn’t like the features you were getting from your EHR. ... That functionality should help if you want to do that.”
The standardizing of APIs to help the delivery of data will go hand in hand with information blocking aspects of the proposed rule, which defines the few exceptions where an activity would not be considered information blocking, such as when engaging in practices will prevent patient harm; engaging in consistent, nondiscriminatory practices to protect patient privacy; and implementing practices to promote the security of health information.
Mr. Lipinski said these changes will help prevent providers from hiding behind HIPAA rules as the excuse to not share patient information, which will help with care coordination. “From a provider’s perspective, this should help them get more access to information, more access in a structured way and then easily get and share that information.”
Ultimately, Mr. Lipinski said, the goal is “to increase competition and lower cost while still improving the quality of care for patients.”
The Office of the National Coordinator of Health Information Technology is looking to adopt standardized application programming interfaces (APIs) in an effort to boost interoperability of health data.
The Department of Health & Human Services office posted a proposed rule Feb. 11, 2019, that would, according to an agency press release, “help allow individuals to securely and easily access structured and unstructured EHI [electronic health information] formats using smartphones and other mobile devices.”
“We think our rule is going to help reduce burden and improve care,” Michael Lipinski, director of the Regulatory Affairs Division in the ONC Office of Policy, said in an interview. “It is going to do that through technology. With the APIs, you should be able to get to your information easier and have it readily available. Whether that is from another health care provider or using other health care products through the API to improve care, you will have that ability between the certified API and the information blocking policies to use third party developers and their products.”
The proposed rule also included a requirement that EHRs certified by ONC be able to easily export information contained within the EHR and make the format used to extract and export the data contained within the EHR publicly available.
“Another third party developer can build to that and offer competing services to pull that information out,” Mr. Lipinski said. “That would obviously help if you were choosing to switch [EHRs] if you didn’t like the features you were getting from your EHR. ... That functionality should help if you want to do that.”
The standardizing of APIs to help the delivery of data will go hand in hand with information blocking aspects of the proposed rule, which defines the few exceptions where an activity would not be considered information blocking, such as when engaging in practices will prevent patient harm; engaging in consistent, nondiscriminatory practices to protect patient privacy; and implementing practices to promote the security of health information.
Mr. Lipinski said these changes will help prevent providers from hiding behind HIPAA rules as the excuse to not share patient information, which will help with care coordination. “From a provider’s perspective, this should help them get more access to information, more access in a structured way and then easily get and share that information.”
Ultimately, Mr. Lipinski said, the goal is “to increase competition and lower cost while still improving the quality of care for patients.”
Fund projects, not people to address gender bias in research funding
LONDON – Female investigators are less likely to secure research funding than male investigators, not because their proposed project is of lesser scientific merit, but simply because they are women, according to research published in The Lancet.
Women had a 30% lower chance of success in getting funding for a project than did their male counterparts when the caliber of the principal investigator was considered as an explicit part of the grant application process, with an 8.8% probability of getting funded versus 12.7%, respectively. If the application was considered solely on a project basis, however, the gender bias was less (12.1% vs. 12.9%).
The overall success of grant applications was 15.8% in the analysis, which considered almost 24,000 grant applications from more than 7,000 principal investigators submitted to the Canadian Institutes of Health Research (CIHR) between 2011 and 2016.
“I see our study as basically one good thwack in a long game of whack-a-mole,” lead study author Holly O. Witteman, PhD, said during an event to launch a special edition of The Lancet focusing on advancing women in science, medicine, and global health.
Dr. Witteman’s research is one of three original articles included in the thematic issue that brings together female authors and commentators to look at gender equity and what needs to be done to address imbalances. The issue is the result of a call for papers that led to more than 300 submissions from more than 40 countries and, according to an editorial from The Lancet, highlights that gender equity in medicine “is not only a matter of justice and rights, it is crucial for producing the best research and providing the best care to patients.”
That there are discrepancies in research funding awarded to female and male investigators has been known for years, Dr. Witteman, associate professor of family and emergency medicine at Laval University, Quebec City, said at the London press conference. To learn how and why, a “quasiexperimental” approach was used to find out what factors might be influencing the gender gap.
“Women are scored lower for competence compared to men with the same publication record,” she said. It’s not that they publish less or do easier research, or that the quality is lower, they are just viewed less favorably overall throughout their careers. Even when you control for confounding factors, “they still don’t advance as quickly,” she said.
“It had been documented for a while that, overall, women tend to get less grant funding and there hasn’t been any evidence to show either way if maybe women’s grant applications weren’t as good,” Dr. Witteman explained.
In 2014, the CIHR changed the way it funded research projects, creating a “natural experiment.” Two new grant application programs were put in place which largely differed by whether or not an explicit review of the principal investigator and their ability to conduct the research was included.
Adjusting for age and type of research, Dr. Witteman and her coauthors found that there was little difference in the success of women in securing research funding when their grant applications were judged solely on a scientific basis; however, when the focus was placed on the principal investigator, women were disadvantaged.
Dr. Witteman said that “this provides robust evidence in support of the idea that women write equally good grant applications but aren’t evaluated as being equally good scientists.”
So how to redress the balance? Dr. Witteman suggested that one way was for funders to collect robust evidence on the success of grant applications and be transparent who is getting funded and how much funding is being awarded. Institutions should invest in and support young investigators, distributing power and flattening traditionally male-led hierarchies. Salaries should be aligned and research support evened out, she said.
Investigators themselves also have a role to play to do the best possible work and try to change the system. “Advocate for others,” she said. That included advocating for others in groups that you may not be part of – which can be easier in some respects than advocating for a group that you are in.
“Funders should evaluate projects, not people,” Jennifer L. Raymond, PhD, and Miriam B. Goodman, PhD, both professors at Stanford (Calif.) University wrote in a comment in The Lancet special issue. They suggested that people-based funding had been gaining popularity but that funders would be better off funding by project to achieve scientific and clinical goals. “Assess the investigator only after double-blind review of the proposed research is complete,” they suggested. “Reduce the assessment of the investigator to a binary judgment of whether or not the investigator has the expertise and resources needed do the proposed research.”
During a panel discussion at The Lancet event, Cassidy R. Sugimoto, PhD, associate professor of informatics at Indiana University in Bloomington and a program director for the Science and Innovation Policy Program at the National Science Foundation (NSF) observed that data on gender equality in research funding were already being collected and will be used to determine how best to adjust funding policies.
“Looking from the 1980s to the present, women make up shy of 20% of the funds given by the National Science Foundation,” Dr. Sugimoto said. “That’s improved over time, and it’s at 28% currently, which is less than their authorship.”
Tammy Clifford, PhD, vice president of research programs at the CIHR observed that data collection was “a critically important step, but of course that’s not the only step,” she said. “We need to look at and analyze the data regularly, and then when you see things that are not on track, you make changes.”
One of the changes the CIHR has made is to train people who are reviewing grant applications on factors that may unconsciously affect their decisions. “There are things to be done, and I don’t think we are quite there yet, but we are committed to continually looking at those data, to making the changes that are required.”
Representing the Wellcome Trust, Ed Whiting, director of policy and chief of staff, said that the funding of projects led by female investigators was moving in the right direction. He noted that there was still a lower rate of applications from women for senior award levels, but that the panels that decide upon the funding were moving toward equal gender representation. The aim was to get to a 50/50 female to male ratio on the panels by 2020, he said; it is was at 46%-52% in 2018.
Dr. Witteman and all other commentators had no financial disclosures.
SOURCE: Witteman HO et al. Lancet. 2019. doi: 10.1016/S0140-6736(18)32611-4
LONDON – Female investigators are less likely to secure research funding than male investigators, not because their proposed project is of lesser scientific merit, but simply because they are women, according to research published in The Lancet.
Women had a 30% lower chance of success in getting funding for a project than did their male counterparts when the caliber of the principal investigator was considered as an explicit part of the grant application process, with an 8.8% probability of getting funded versus 12.7%, respectively. If the application was considered solely on a project basis, however, the gender bias was less (12.1% vs. 12.9%).
The overall success of grant applications was 15.8% in the analysis, which considered almost 24,000 grant applications from more than 7,000 principal investigators submitted to the Canadian Institutes of Health Research (CIHR) between 2011 and 2016.
“I see our study as basically one good thwack in a long game of whack-a-mole,” lead study author Holly O. Witteman, PhD, said during an event to launch a special edition of The Lancet focusing on advancing women in science, medicine, and global health.
Dr. Witteman’s research is one of three original articles included in the thematic issue that brings together female authors and commentators to look at gender equity and what needs to be done to address imbalances. The issue is the result of a call for papers that led to more than 300 submissions from more than 40 countries and, according to an editorial from The Lancet, highlights that gender equity in medicine “is not only a matter of justice and rights, it is crucial for producing the best research and providing the best care to patients.”
That there are discrepancies in research funding awarded to female and male investigators has been known for years, Dr. Witteman, associate professor of family and emergency medicine at Laval University, Quebec City, said at the London press conference. To learn how and why, a “quasiexperimental” approach was used to find out what factors might be influencing the gender gap.
“Women are scored lower for competence compared to men with the same publication record,” she said. It’s not that they publish less or do easier research, or that the quality is lower, they are just viewed less favorably overall throughout their careers. Even when you control for confounding factors, “they still don’t advance as quickly,” she said.
“It had been documented for a while that, overall, women tend to get less grant funding and there hasn’t been any evidence to show either way if maybe women’s grant applications weren’t as good,” Dr. Witteman explained.
In 2014, the CIHR changed the way it funded research projects, creating a “natural experiment.” Two new grant application programs were put in place which largely differed by whether or not an explicit review of the principal investigator and their ability to conduct the research was included.
Adjusting for age and type of research, Dr. Witteman and her coauthors found that there was little difference in the success of women in securing research funding when their grant applications were judged solely on a scientific basis; however, when the focus was placed on the principal investigator, women were disadvantaged.
Dr. Witteman said that “this provides robust evidence in support of the idea that women write equally good grant applications but aren’t evaluated as being equally good scientists.”
So how to redress the balance? Dr. Witteman suggested that one way was for funders to collect robust evidence on the success of grant applications and be transparent who is getting funded and how much funding is being awarded. Institutions should invest in and support young investigators, distributing power and flattening traditionally male-led hierarchies. Salaries should be aligned and research support evened out, she said.
Investigators themselves also have a role to play to do the best possible work and try to change the system. “Advocate for others,” she said. That included advocating for others in groups that you may not be part of – which can be easier in some respects than advocating for a group that you are in.
“Funders should evaluate projects, not people,” Jennifer L. Raymond, PhD, and Miriam B. Goodman, PhD, both professors at Stanford (Calif.) University wrote in a comment in The Lancet special issue. They suggested that people-based funding had been gaining popularity but that funders would be better off funding by project to achieve scientific and clinical goals. “Assess the investigator only after double-blind review of the proposed research is complete,” they suggested. “Reduce the assessment of the investigator to a binary judgment of whether or not the investigator has the expertise and resources needed do the proposed research.”
During a panel discussion at The Lancet event, Cassidy R. Sugimoto, PhD, associate professor of informatics at Indiana University in Bloomington and a program director for the Science and Innovation Policy Program at the National Science Foundation (NSF) observed that data on gender equality in research funding were already being collected and will be used to determine how best to adjust funding policies.
“Looking from the 1980s to the present, women make up shy of 20% of the funds given by the National Science Foundation,” Dr. Sugimoto said. “That’s improved over time, and it’s at 28% currently, which is less than their authorship.”
Tammy Clifford, PhD, vice president of research programs at the CIHR observed that data collection was “a critically important step, but of course that’s not the only step,” she said. “We need to look at and analyze the data regularly, and then when you see things that are not on track, you make changes.”
One of the changes the CIHR has made is to train people who are reviewing grant applications on factors that may unconsciously affect their decisions. “There are things to be done, and I don’t think we are quite there yet, but we are committed to continually looking at those data, to making the changes that are required.”
Representing the Wellcome Trust, Ed Whiting, director of policy and chief of staff, said that the funding of projects led by female investigators was moving in the right direction. He noted that there was still a lower rate of applications from women for senior award levels, but that the panels that decide upon the funding were moving toward equal gender representation. The aim was to get to a 50/50 female to male ratio on the panels by 2020, he said; it is was at 46%-52% in 2018.
Dr. Witteman and all other commentators had no financial disclosures.
SOURCE: Witteman HO et al. Lancet. 2019. doi: 10.1016/S0140-6736(18)32611-4
LONDON – Female investigators are less likely to secure research funding than male investigators, not because their proposed project is of lesser scientific merit, but simply because they are women, according to research published in The Lancet.
Women had a 30% lower chance of success in getting funding for a project than did their male counterparts when the caliber of the principal investigator was considered as an explicit part of the grant application process, with an 8.8% probability of getting funded versus 12.7%, respectively. If the application was considered solely on a project basis, however, the gender bias was less (12.1% vs. 12.9%).
The overall success of grant applications was 15.8% in the analysis, which considered almost 24,000 grant applications from more than 7,000 principal investigators submitted to the Canadian Institutes of Health Research (CIHR) between 2011 and 2016.
“I see our study as basically one good thwack in a long game of whack-a-mole,” lead study author Holly O. Witteman, PhD, said during an event to launch a special edition of The Lancet focusing on advancing women in science, medicine, and global health.
Dr. Witteman’s research is one of three original articles included in the thematic issue that brings together female authors and commentators to look at gender equity and what needs to be done to address imbalances. The issue is the result of a call for papers that led to more than 300 submissions from more than 40 countries and, according to an editorial from The Lancet, highlights that gender equity in medicine “is not only a matter of justice and rights, it is crucial for producing the best research and providing the best care to patients.”
That there are discrepancies in research funding awarded to female and male investigators has been known for years, Dr. Witteman, associate professor of family and emergency medicine at Laval University, Quebec City, said at the London press conference. To learn how and why, a “quasiexperimental” approach was used to find out what factors might be influencing the gender gap.
“Women are scored lower for competence compared to men with the same publication record,” she said. It’s not that they publish less or do easier research, or that the quality is lower, they are just viewed less favorably overall throughout their careers. Even when you control for confounding factors, “they still don’t advance as quickly,” she said.
“It had been documented for a while that, overall, women tend to get less grant funding and there hasn’t been any evidence to show either way if maybe women’s grant applications weren’t as good,” Dr. Witteman explained.
In 2014, the CIHR changed the way it funded research projects, creating a “natural experiment.” Two new grant application programs were put in place which largely differed by whether or not an explicit review of the principal investigator and their ability to conduct the research was included.
Adjusting for age and type of research, Dr. Witteman and her coauthors found that there was little difference in the success of women in securing research funding when their grant applications were judged solely on a scientific basis; however, when the focus was placed on the principal investigator, women were disadvantaged.
Dr. Witteman said that “this provides robust evidence in support of the idea that women write equally good grant applications but aren’t evaluated as being equally good scientists.”
So how to redress the balance? Dr. Witteman suggested that one way was for funders to collect robust evidence on the success of grant applications and be transparent who is getting funded and how much funding is being awarded. Institutions should invest in and support young investigators, distributing power and flattening traditionally male-led hierarchies. Salaries should be aligned and research support evened out, she said.
Investigators themselves also have a role to play to do the best possible work and try to change the system. “Advocate for others,” she said. That included advocating for others in groups that you may not be part of – which can be easier in some respects than advocating for a group that you are in.
“Funders should evaluate projects, not people,” Jennifer L. Raymond, PhD, and Miriam B. Goodman, PhD, both professors at Stanford (Calif.) University wrote in a comment in The Lancet special issue. They suggested that people-based funding had been gaining popularity but that funders would be better off funding by project to achieve scientific and clinical goals. “Assess the investigator only after double-blind review of the proposed research is complete,” they suggested. “Reduce the assessment of the investigator to a binary judgment of whether or not the investigator has the expertise and resources needed do the proposed research.”
During a panel discussion at The Lancet event, Cassidy R. Sugimoto, PhD, associate professor of informatics at Indiana University in Bloomington and a program director for the Science and Innovation Policy Program at the National Science Foundation (NSF) observed that data on gender equality in research funding were already being collected and will be used to determine how best to adjust funding policies.
“Looking from the 1980s to the present, women make up shy of 20% of the funds given by the National Science Foundation,” Dr. Sugimoto said. “That’s improved over time, and it’s at 28% currently, which is less than their authorship.”
Tammy Clifford, PhD, vice president of research programs at the CIHR observed that data collection was “a critically important step, but of course that’s not the only step,” she said. “We need to look at and analyze the data regularly, and then when you see things that are not on track, you make changes.”
One of the changes the CIHR has made is to train people who are reviewing grant applications on factors that may unconsciously affect their decisions. “There are things to be done, and I don’t think we are quite there yet, but we are committed to continually looking at those data, to making the changes that are required.”
Representing the Wellcome Trust, Ed Whiting, director of policy and chief of staff, said that the funding of projects led by female investigators was moving in the right direction. He noted that there was still a lower rate of applications from women for senior award levels, but that the panels that decide upon the funding were moving toward equal gender representation. The aim was to get to a 50/50 female to male ratio on the panels by 2020, he said; it is was at 46%-52% in 2018.
Dr. Witteman and all other commentators had no financial disclosures.
SOURCE: Witteman HO et al. Lancet. 2019. doi: 10.1016/S0140-6736(18)32611-4
FROM A LAUNCH EVENT HELD BY THE LANCET
Key clinical point: Funding bodies should focus on the science of a research project not on who is conducting the research.
Major finding: Between 2011 and 2016, 8.8% of projects proposed by female researchers and 12.7% of those proposed by male researchers were funded.
Study details: Analysis of nearly 24,000 grant applications from more than 7,000 principal investigators submitted to the Canadian Institutes of Health Research during 2011-2016.
Disclosures: The research was unfunded. Dr. Witteman and all other commentators had no financial disclosures.
Source: Witteman HO et al. Lancet. 2019. doi: 10.1016/S0140-6736(18)32611-4.
In California, opioids most often prescribed in low-income, mostly white areas
There is a higher prevalence of opioid prescribing and opioid-related overdose deaths concentrated in regions with mostly low-income, white residents, compared with regions with high income and the lowest proportion of white residents, according to a new analysis of data on people living in California.
The findings of this study provide further evidence that the opioid epidemic affects a large proportion of low-income white communities (JAMA Intern Med. 2019 Feb 11. doi: 10.1001/jamainternmed.2018.6721).
“Whereas most epidemics predominate within social minority groups and previous US drug epidemics have typically been concentrated in nonwhite communities, Joseph Friedman, MPH, from the University of California, Los Angeles, and his colleagues wrote in their study. “Our analysis suggests that, at least in California, an important determinant of this phenomenon may be that white individuals have a higher level of exposure than nonwhite individuals to opioid prescriptions on a per capita basis through the health care system.”
Mr. Friedman and his colleagues analyzed 29.7 million prescription drug records from California’s Controlled Substance Utilization Review and Evaluation System in and examined the prevalence of opioids, benzodiazepines, and stimulants by race, ethnicity, and income level in 1,760 zip codes during 2011-2015. The researchers estimated the prevalence of opioid prescriptions in each zip code by calculating the number of people per zip code receiving an opioid prescription divided by the population of the zip code during each year.
Overall, 23.6% of California residents received at least one opioid prescription each year of the study. The researchers found 44.2% of individuals in zip codes with the lowest income but highest proportion of white residents and 16.1% of individuals in areas with the highest income and lowest proportion of white residents had received a minimum of one opioid prescription each year. The prevalence of stimulant prescriptions was 3.8% in zip codes with high income, and a high proportion of white population, compared with a prevalence of 0.6% in areas with low income and a low proportion of white residents. The researchers noted there was no association between income and benzodiazepine prescription, but the prevalence of benzodiazepine prescriptions was 15.7% in zip codes with the highest proportion of white residents, compared with 7.0% in zip codes with a low proportion of white residents.
During the same time period, there were 9,534 opioid overdose deaths in California from causes such as fentanyl, synthetic opioids, and prescription opioids. “Overdose deaths were highly concentrated in lower-income and mostly white areas,” Mr. Friedman and his colleagues wrote. “We observed an approximate 10-fold difference in overdose rates across the race/ethnicity–income gradient in California.”
Although the number of opioids prescribed each year has decreased since 2012, in a research letter published in the same issue noted that the rate of prescribing is still higher than it was in 1999 (JAMA Intern Med. 2019 Feb 11. doi: 10.1001/jamainternmed.2018.6989). The authors also pointed out increases in the duration of opioid prescriptions and wide regional variations in opioid prescribing rates.
In their study, Gery P. Guy Jr., PhD, and his colleagues used data from the IQVIA Xponent database from approximately 50,400 retail pharmacies and discovered the average morphine milligram equivalent (MME) per capita had decreased from 641.4 MME per capita in 2015 to 512.6 MME per capita in 2017 (20.1%). The number of opioid prescriptions also decreased from 6.7 per 100 persons in 2015 to 5.0 per 100 persons in 2017 (25.3%). However, during 2015-2017, the average duration of opioid prescriptions increased from 17.7 days to 18.3 days (3.4%), while the median duration increased during the same time from 15.0 days to 20.0 days (33.3%).
While 74.7% of counties reduced the number of opioids prescribed during 2015-2017 and there also were reductions in the rate of high-dose prescribing (76.6%) and overall prescribing rates (74.7%), Dr. Guy of the Centers for Disease Control and Prevention and his colleagues found “substantial variation” in 2017 prescription rates at the county level, with opioids prescribed at 1,061.0 MME per capita at the highest quartile, compared with 182.8 MME per capita at the lowest quartile.
“Recent reductions could be related to policies and strategies aimed at reducing inappropriate prescribing, increased awareness of the risks associated with opioids, and release of the CDC Guideline for Prescribing Opioids for Chronic Pain–United States, 2016,” Dr. Guy and his colleagues noted.
In an additional article published in the same JAMA Internal Medicine issue, Bennett Allen, a research associate at the New York City Department of Health and Mental Hygiene and his colleagues examined the rate of opioid overdose deaths for non-Hispanic white, non-Hispanic black, Hispanic, and undefined other races in New York (JAMA Intern Med. 2019 Feb 11. doi: 10.1001/jamainternmed.2018.7700). They identified 1,487 deaths in 2017, which included 556 white (37.0%), 421 black (28.0%), 455 Hispanic (31.0%), and 55 undefined (4.0%) opioid overdose deaths. There was a higher rate of fentanyl and/or heroin overdose deaths from younger (aged 15-34 years) white New Yorkers (22.2/100,000 persons; 95% confidence interval, 19.0-25.5), compared with younger black New Yorkers (5.8/100,000; 95% CI, 4.0-8.2) and Hispanic (9.7/100,000; 95% CI, 7.6-12.1).
Among older residents (aged 55-84 years), Mr. Allen and his colleagues found higher rates of fentanyl and/or heroin overdose for black New Yorkers (25.4/100,000 persons; 95% CI, 20.9-30.0), compared with older white New Yorkers (9.4/100,000 persons; 95% CI, 7.3-11.8), as well as significantly higher rates of cocaine overdose (25.4/100,000 persons; 95% CI, 20.9-30.0), compared with white (5.1/100,000 persons; 95% CI, 3.6-7.0) and Hispanic residents (11.8/100,000 persons; 95% CI, 8.9-15.4).
“The distinct age distribution and drug involvement of overdose deaths among New York City blacks, Latinos, and whites, along with complementary evidence about drug use trajectories, highlight the need for heterogeneous approaches to treatment and the equitable allocation of treatment and health care resources to reach diverse populations at risk of overdose,” Mr. Allen and his colleagues wrote.
Dr. Schriger reported support from Korein Foundation for his time working on the study by Friedman et al. The other authors reported no conflicts of interest.
The results published by Friedman et al. are a reminder that we can use regional prescribing trends to identify communities most susceptible to the opioid epidemic and give them the resources they need to combat opioid addiction, Vice Adm. Jerome M. Adams, MD, MPH, and Adm. Brett P. Giroir, MD, wrote in a related editorial.
“Discussion of overdose risks and coprescribing of naloxone must become routine if we are to make opioid prescribing safer,” the authors wrote.
Physicians also can help respond to the opioid epidemic outside of prescribing by promoting evidence-based nonopioid and nonpharmaceutical pain treatments, screening their patients for OUD and OUD risks, and acknowledging “that the problem cannot be solved by medical interventions alone.” Individual, environmental, and societal factors also contribute to the opioid epidemic, and physicians are uniquely suited to spearhead efforts aimed at addressing comprehensive opioid misuse.
“Physicians stand out as natural leaders to help solve the crises because of the depth of their knowledge, immediacy of their contact with patients, and relatively high level of respect their profession enjoys,” Dr. Adams and Dr. Giroir wrote. “We thereby call on our nation’s doctors to embrace their roles in the clinic and beyond to help educate communities, bring together stakeholders, and be part of the cultural change to support people living free from addiction.”
Dr. Adams is the 20th surgeon general of the United States at the U.S. Public Health Service and HHS; Dr. Giroir is the 16th U.S. assistant secretary for health at the U.S. Public Health Service and HHS. They reported no relevant conflicts of interest. Their invited commentary accompanied the three related articles in the publication (JAMA Intern Med. 2019 Feb 11. doi: 10.1001/jamainternmed.2018.7934 ).
The results published by Friedman et al. are a reminder that we can use regional prescribing trends to identify communities most susceptible to the opioid epidemic and give them the resources they need to combat opioid addiction, Vice Adm. Jerome M. Adams, MD, MPH, and Adm. Brett P. Giroir, MD, wrote in a related editorial.
“Discussion of overdose risks and coprescribing of naloxone must become routine if we are to make opioid prescribing safer,” the authors wrote.
Physicians also can help respond to the opioid epidemic outside of prescribing by promoting evidence-based nonopioid and nonpharmaceutical pain treatments, screening their patients for OUD and OUD risks, and acknowledging “that the problem cannot be solved by medical interventions alone.” Individual, environmental, and societal factors also contribute to the opioid epidemic, and physicians are uniquely suited to spearhead efforts aimed at addressing comprehensive opioid misuse.
“Physicians stand out as natural leaders to help solve the crises because of the depth of their knowledge, immediacy of their contact with patients, and relatively high level of respect their profession enjoys,” Dr. Adams and Dr. Giroir wrote. “We thereby call on our nation’s doctors to embrace their roles in the clinic and beyond to help educate communities, bring together stakeholders, and be part of the cultural change to support people living free from addiction.”
Dr. Adams is the 20th surgeon general of the United States at the U.S. Public Health Service and HHS; Dr. Giroir is the 16th U.S. assistant secretary for health at the U.S. Public Health Service and HHS. They reported no relevant conflicts of interest. Their invited commentary accompanied the three related articles in the publication (JAMA Intern Med. 2019 Feb 11. doi: 10.1001/jamainternmed.2018.7934 ).
The results published by Friedman et al. are a reminder that we can use regional prescribing trends to identify communities most susceptible to the opioid epidemic and give them the resources they need to combat opioid addiction, Vice Adm. Jerome M. Adams, MD, MPH, and Adm. Brett P. Giroir, MD, wrote in a related editorial.
“Discussion of overdose risks and coprescribing of naloxone must become routine if we are to make opioid prescribing safer,” the authors wrote.
Physicians also can help respond to the opioid epidemic outside of prescribing by promoting evidence-based nonopioid and nonpharmaceutical pain treatments, screening their patients for OUD and OUD risks, and acknowledging “that the problem cannot be solved by medical interventions alone.” Individual, environmental, and societal factors also contribute to the opioid epidemic, and physicians are uniquely suited to spearhead efforts aimed at addressing comprehensive opioid misuse.
“Physicians stand out as natural leaders to help solve the crises because of the depth of their knowledge, immediacy of their contact with patients, and relatively high level of respect their profession enjoys,” Dr. Adams and Dr. Giroir wrote. “We thereby call on our nation’s doctors to embrace their roles in the clinic and beyond to help educate communities, bring together stakeholders, and be part of the cultural change to support people living free from addiction.”
Dr. Adams is the 20th surgeon general of the United States at the U.S. Public Health Service and HHS; Dr. Giroir is the 16th U.S. assistant secretary for health at the U.S. Public Health Service and HHS. They reported no relevant conflicts of interest. Their invited commentary accompanied the three related articles in the publication (JAMA Intern Med. 2019 Feb 11. doi: 10.1001/jamainternmed.2018.7934 ).
There is a higher prevalence of opioid prescribing and opioid-related overdose deaths concentrated in regions with mostly low-income, white residents, compared with regions with high income and the lowest proportion of white residents, according to a new analysis of data on people living in California.
The findings of this study provide further evidence that the opioid epidemic affects a large proportion of low-income white communities (JAMA Intern Med. 2019 Feb 11. doi: 10.1001/jamainternmed.2018.6721).
“Whereas most epidemics predominate within social minority groups and previous US drug epidemics have typically been concentrated in nonwhite communities, Joseph Friedman, MPH, from the University of California, Los Angeles, and his colleagues wrote in their study. “Our analysis suggests that, at least in California, an important determinant of this phenomenon may be that white individuals have a higher level of exposure than nonwhite individuals to opioid prescriptions on a per capita basis through the health care system.”
Mr. Friedman and his colleagues analyzed 29.7 million prescription drug records from California’s Controlled Substance Utilization Review and Evaluation System in and examined the prevalence of opioids, benzodiazepines, and stimulants by race, ethnicity, and income level in 1,760 zip codes during 2011-2015. The researchers estimated the prevalence of opioid prescriptions in each zip code by calculating the number of people per zip code receiving an opioid prescription divided by the population of the zip code during each year.
Overall, 23.6% of California residents received at least one opioid prescription each year of the study. The researchers found 44.2% of individuals in zip codes with the lowest income but highest proportion of white residents and 16.1% of individuals in areas with the highest income and lowest proportion of white residents had received a minimum of one opioid prescription each year. The prevalence of stimulant prescriptions was 3.8% in zip codes with high income, and a high proportion of white population, compared with a prevalence of 0.6% in areas with low income and a low proportion of white residents. The researchers noted there was no association between income and benzodiazepine prescription, but the prevalence of benzodiazepine prescriptions was 15.7% in zip codes with the highest proportion of white residents, compared with 7.0% in zip codes with a low proportion of white residents.
During the same time period, there were 9,534 opioid overdose deaths in California from causes such as fentanyl, synthetic opioids, and prescription opioids. “Overdose deaths were highly concentrated in lower-income and mostly white areas,” Mr. Friedman and his colleagues wrote. “We observed an approximate 10-fold difference in overdose rates across the race/ethnicity–income gradient in California.”
Although the number of opioids prescribed each year has decreased since 2012, in a research letter published in the same issue noted that the rate of prescribing is still higher than it was in 1999 (JAMA Intern Med. 2019 Feb 11. doi: 10.1001/jamainternmed.2018.6989). The authors also pointed out increases in the duration of opioid prescriptions and wide regional variations in opioid prescribing rates.
In their study, Gery P. Guy Jr., PhD, and his colleagues used data from the IQVIA Xponent database from approximately 50,400 retail pharmacies and discovered the average morphine milligram equivalent (MME) per capita had decreased from 641.4 MME per capita in 2015 to 512.6 MME per capita in 2017 (20.1%). The number of opioid prescriptions also decreased from 6.7 per 100 persons in 2015 to 5.0 per 100 persons in 2017 (25.3%). However, during 2015-2017, the average duration of opioid prescriptions increased from 17.7 days to 18.3 days (3.4%), while the median duration increased during the same time from 15.0 days to 20.0 days (33.3%).
While 74.7% of counties reduced the number of opioids prescribed during 2015-2017 and there also were reductions in the rate of high-dose prescribing (76.6%) and overall prescribing rates (74.7%), Dr. Guy of the Centers for Disease Control and Prevention and his colleagues found “substantial variation” in 2017 prescription rates at the county level, with opioids prescribed at 1,061.0 MME per capita at the highest quartile, compared with 182.8 MME per capita at the lowest quartile.
“Recent reductions could be related to policies and strategies aimed at reducing inappropriate prescribing, increased awareness of the risks associated with opioids, and release of the CDC Guideline for Prescribing Opioids for Chronic Pain–United States, 2016,” Dr. Guy and his colleagues noted.
In an additional article published in the same JAMA Internal Medicine issue, Bennett Allen, a research associate at the New York City Department of Health and Mental Hygiene and his colleagues examined the rate of opioid overdose deaths for non-Hispanic white, non-Hispanic black, Hispanic, and undefined other races in New York (JAMA Intern Med. 2019 Feb 11. doi: 10.1001/jamainternmed.2018.7700). They identified 1,487 deaths in 2017, which included 556 white (37.0%), 421 black (28.0%), 455 Hispanic (31.0%), and 55 undefined (4.0%) opioid overdose deaths. There was a higher rate of fentanyl and/or heroin overdose deaths from younger (aged 15-34 years) white New Yorkers (22.2/100,000 persons; 95% confidence interval, 19.0-25.5), compared with younger black New Yorkers (5.8/100,000; 95% CI, 4.0-8.2) and Hispanic (9.7/100,000; 95% CI, 7.6-12.1).
Among older residents (aged 55-84 years), Mr. Allen and his colleagues found higher rates of fentanyl and/or heroin overdose for black New Yorkers (25.4/100,000 persons; 95% CI, 20.9-30.0), compared with older white New Yorkers (9.4/100,000 persons; 95% CI, 7.3-11.8), as well as significantly higher rates of cocaine overdose (25.4/100,000 persons; 95% CI, 20.9-30.0), compared with white (5.1/100,000 persons; 95% CI, 3.6-7.0) and Hispanic residents (11.8/100,000 persons; 95% CI, 8.9-15.4).
“The distinct age distribution and drug involvement of overdose deaths among New York City blacks, Latinos, and whites, along with complementary evidence about drug use trajectories, highlight the need for heterogeneous approaches to treatment and the equitable allocation of treatment and health care resources to reach diverse populations at risk of overdose,” Mr. Allen and his colleagues wrote.
Dr. Schriger reported support from Korein Foundation for his time working on the study by Friedman et al. The other authors reported no conflicts of interest.
There is a higher prevalence of opioid prescribing and opioid-related overdose deaths concentrated in regions with mostly low-income, white residents, compared with regions with high income and the lowest proportion of white residents, according to a new analysis of data on people living in California.
The findings of this study provide further evidence that the opioid epidemic affects a large proportion of low-income white communities (JAMA Intern Med. 2019 Feb 11. doi: 10.1001/jamainternmed.2018.6721).
“Whereas most epidemics predominate within social minority groups and previous US drug epidemics have typically been concentrated in nonwhite communities, Joseph Friedman, MPH, from the University of California, Los Angeles, and his colleagues wrote in their study. “Our analysis suggests that, at least in California, an important determinant of this phenomenon may be that white individuals have a higher level of exposure than nonwhite individuals to opioid prescriptions on a per capita basis through the health care system.”
Mr. Friedman and his colleagues analyzed 29.7 million prescription drug records from California’s Controlled Substance Utilization Review and Evaluation System in and examined the prevalence of opioids, benzodiazepines, and stimulants by race, ethnicity, and income level in 1,760 zip codes during 2011-2015. The researchers estimated the prevalence of opioid prescriptions in each zip code by calculating the number of people per zip code receiving an opioid prescription divided by the population of the zip code during each year.
Overall, 23.6% of California residents received at least one opioid prescription each year of the study. The researchers found 44.2% of individuals in zip codes with the lowest income but highest proportion of white residents and 16.1% of individuals in areas with the highest income and lowest proportion of white residents had received a minimum of one opioid prescription each year. The prevalence of stimulant prescriptions was 3.8% in zip codes with high income, and a high proportion of white population, compared with a prevalence of 0.6% in areas with low income and a low proportion of white residents. The researchers noted there was no association between income and benzodiazepine prescription, but the prevalence of benzodiazepine prescriptions was 15.7% in zip codes with the highest proportion of white residents, compared with 7.0% in zip codes with a low proportion of white residents.
During the same time period, there were 9,534 opioid overdose deaths in California from causes such as fentanyl, synthetic opioids, and prescription opioids. “Overdose deaths were highly concentrated in lower-income and mostly white areas,” Mr. Friedman and his colleagues wrote. “We observed an approximate 10-fold difference in overdose rates across the race/ethnicity–income gradient in California.”
Although the number of opioids prescribed each year has decreased since 2012, in a research letter published in the same issue noted that the rate of prescribing is still higher than it was in 1999 (JAMA Intern Med. 2019 Feb 11. doi: 10.1001/jamainternmed.2018.6989). The authors also pointed out increases in the duration of opioid prescriptions and wide regional variations in opioid prescribing rates.
In their study, Gery P. Guy Jr., PhD, and his colleagues used data from the IQVIA Xponent database from approximately 50,400 retail pharmacies and discovered the average morphine milligram equivalent (MME) per capita had decreased from 641.4 MME per capita in 2015 to 512.6 MME per capita in 2017 (20.1%). The number of opioid prescriptions also decreased from 6.7 per 100 persons in 2015 to 5.0 per 100 persons in 2017 (25.3%). However, during 2015-2017, the average duration of opioid prescriptions increased from 17.7 days to 18.3 days (3.4%), while the median duration increased during the same time from 15.0 days to 20.0 days (33.3%).
While 74.7% of counties reduced the number of opioids prescribed during 2015-2017 and there also were reductions in the rate of high-dose prescribing (76.6%) and overall prescribing rates (74.7%), Dr. Guy of the Centers for Disease Control and Prevention and his colleagues found “substantial variation” in 2017 prescription rates at the county level, with opioids prescribed at 1,061.0 MME per capita at the highest quartile, compared with 182.8 MME per capita at the lowest quartile.
“Recent reductions could be related to policies and strategies aimed at reducing inappropriate prescribing, increased awareness of the risks associated with opioids, and release of the CDC Guideline for Prescribing Opioids for Chronic Pain–United States, 2016,” Dr. Guy and his colleagues noted.
In an additional article published in the same JAMA Internal Medicine issue, Bennett Allen, a research associate at the New York City Department of Health and Mental Hygiene and his colleagues examined the rate of opioid overdose deaths for non-Hispanic white, non-Hispanic black, Hispanic, and undefined other races in New York (JAMA Intern Med. 2019 Feb 11. doi: 10.1001/jamainternmed.2018.7700). They identified 1,487 deaths in 2017, which included 556 white (37.0%), 421 black (28.0%), 455 Hispanic (31.0%), and 55 undefined (4.0%) opioid overdose deaths. There was a higher rate of fentanyl and/or heroin overdose deaths from younger (aged 15-34 years) white New Yorkers (22.2/100,000 persons; 95% confidence interval, 19.0-25.5), compared with younger black New Yorkers (5.8/100,000; 95% CI, 4.0-8.2) and Hispanic (9.7/100,000; 95% CI, 7.6-12.1).
Among older residents (aged 55-84 years), Mr. Allen and his colleagues found higher rates of fentanyl and/or heroin overdose for black New Yorkers (25.4/100,000 persons; 95% CI, 20.9-30.0), compared with older white New Yorkers (9.4/100,000 persons; 95% CI, 7.3-11.8), as well as significantly higher rates of cocaine overdose (25.4/100,000 persons; 95% CI, 20.9-30.0), compared with white (5.1/100,000 persons; 95% CI, 3.6-7.0) and Hispanic residents (11.8/100,000 persons; 95% CI, 8.9-15.4).
“The distinct age distribution and drug involvement of overdose deaths among New York City blacks, Latinos, and whites, along with complementary evidence about drug use trajectories, highlight the need for heterogeneous approaches to treatment and the equitable allocation of treatment and health care resources to reach diverse populations at risk of overdose,” Mr. Allen and his colleagues wrote.
Dr. Schriger reported support from Korein Foundation for his time working on the study by Friedman et al. The other authors reported no conflicts of interest.
FROM JAMA INTERNAL MEDICINE
Key clinical point: The most common users of opioids according to prescription drug records are residents of mostly low-income, white neighborhoods.
Major finding: Compared with 23.6% of all Californians, 44.2% of individuals in zip codes containing mostly low-income, white residents had at least one opioid prescription each year, compared with 16.1% of individuals in high-income zip codes with the lowest population of white residents.
Study details: An analysis of 29.7 million opioid prescription drug records by race and income in California during 2011-2015.
Disclosures: Dr. Schriger reported support from the Korein Foundation for his time working on the study by Friedman et al. The other authors from Friedman et al. reported no conflicts of interest.
Improving interview skills for hospitalists
Standardized prep courses are helpful
Are residents generally prepared for fellowship interviews? Applications to the Fellowship Match through the National Residency Matching Program (NRMP) Specialties Matching Service (SMS) are at an all-time high, but there is limited data regarding the preparedness of residents who go through the fellowship interview process, said Kelvin Wong, MD, a coauthor of research describing a new approach to fellowship interview preparation, which may be generalizable to hospitalists in applying for other positions.
“Applicants receive little to no feedback after their interviews and are thus likely to repeat the same mistakes throughout the process. Verbal feedback from our own fellowship directors indicated that residents as a whole are unprepared to interview,” according to an abstract written by Dr. Wong and his colleagues.
Dr. Wong wanted to investigate the effects of a standardized fellowship interview preparation course on resident preparedness. He and his coauthors developed a formal preparation course for the applicants in the summer of 2017.
Precourse surveys showed that only 17.65% of residents felt prepared to go on interviews; postcourse surveys showed a rise in this number to 82.35%. Immediately after their mock interview, only 27.78% of residents rated their overall interview skills as “very good” or “excellent,” whereas 87.5% of interviewers and 70.59% of observers rated their skills to be “very good” or “excellent.”
A final survey will be given to the applicants and the fellowship program directors once the applicants have completed all of their actual interviews.
“This demonstrates the potential positive impact that mock interviews and standardized interview preparation courses can have, which may be generalizable to hospitalists applying for other positions,” Dr. Wong said. “Specifically for teaching hospitalists in teaching hospitals, the institution of such fellowship interview preparation courses may improve resident preparedness for the fellowship application process.”
Reference
1. Wong K et al. A novel approach to improve fellowship interview skills [abstract]. https://www.shmabstracts.com/abstract/a-novel-approach-to-improve-fellowship-interview-skills/.
Standardized prep courses are helpful
Standardized prep courses are helpful
Are residents generally prepared for fellowship interviews? Applications to the Fellowship Match through the National Residency Matching Program (NRMP) Specialties Matching Service (SMS) are at an all-time high, but there is limited data regarding the preparedness of residents who go through the fellowship interview process, said Kelvin Wong, MD, a coauthor of research describing a new approach to fellowship interview preparation, which may be generalizable to hospitalists in applying for other positions.
“Applicants receive little to no feedback after their interviews and are thus likely to repeat the same mistakes throughout the process. Verbal feedback from our own fellowship directors indicated that residents as a whole are unprepared to interview,” according to an abstract written by Dr. Wong and his colleagues.
Dr. Wong wanted to investigate the effects of a standardized fellowship interview preparation course on resident preparedness. He and his coauthors developed a formal preparation course for the applicants in the summer of 2017.
Precourse surveys showed that only 17.65% of residents felt prepared to go on interviews; postcourse surveys showed a rise in this number to 82.35%. Immediately after their mock interview, only 27.78% of residents rated their overall interview skills as “very good” or “excellent,” whereas 87.5% of interviewers and 70.59% of observers rated their skills to be “very good” or “excellent.”
A final survey will be given to the applicants and the fellowship program directors once the applicants have completed all of their actual interviews.
“This demonstrates the potential positive impact that mock interviews and standardized interview preparation courses can have, which may be generalizable to hospitalists applying for other positions,” Dr. Wong said. “Specifically for teaching hospitalists in teaching hospitals, the institution of such fellowship interview preparation courses may improve resident preparedness for the fellowship application process.”
Reference
1. Wong K et al. A novel approach to improve fellowship interview skills [abstract]. https://www.shmabstracts.com/abstract/a-novel-approach-to-improve-fellowship-interview-skills/.
Are residents generally prepared for fellowship interviews? Applications to the Fellowship Match through the National Residency Matching Program (NRMP) Specialties Matching Service (SMS) are at an all-time high, but there is limited data regarding the preparedness of residents who go through the fellowship interview process, said Kelvin Wong, MD, a coauthor of research describing a new approach to fellowship interview preparation, which may be generalizable to hospitalists in applying for other positions.
“Applicants receive little to no feedback after their interviews and are thus likely to repeat the same mistakes throughout the process. Verbal feedback from our own fellowship directors indicated that residents as a whole are unprepared to interview,” according to an abstract written by Dr. Wong and his colleagues.
Dr. Wong wanted to investigate the effects of a standardized fellowship interview preparation course on resident preparedness. He and his coauthors developed a formal preparation course for the applicants in the summer of 2017.
Precourse surveys showed that only 17.65% of residents felt prepared to go on interviews; postcourse surveys showed a rise in this number to 82.35%. Immediately after their mock interview, only 27.78% of residents rated their overall interview skills as “very good” or “excellent,” whereas 87.5% of interviewers and 70.59% of observers rated their skills to be “very good” or “excellent.”
A final survey will be given to the applicants and the fellowship program directors once the applicants have completed all of their actual interviews.
“This demonstrates the potential positive impact that mock interviews and standardized interview preparation courses can have, which may be generalizable to hospitalists applying for other positions,” Dr. Wong said. “Specifically for teaching hospitalists in teaching hospitals, the institution of such fellowship interview preparation courses may improve resident preparedness for the fellowship application process.”
Reference
1. Wong K et al. A novel approach to improve fellowship interview skills [abstract]. https://www.shmabstracts.com/abstract/a-novel-approach-to-improve-fellowship-interview-skills/.
Quick Byte: Needle-free injections
A start-up operating out of MIT in Cambridge, Mass., called Portal Instruments has developed a needleless injection system.
The device, called PRIME, delivers medication into the bloodstream in a high-pressure stream that travels at Mach 0.7 – the speed of a jet. The makers signed a commercial deal in December 2017, and the device is expected to be available soon.
Reference
1. Kerrigan S. The 16 Most Remarkable Healthcare Innovations, Events, and Discoveries of 2018 For World Health Day. https://interestingengineering.com/the-16-most-remarkable-healthcare-innovations-events-and-discoveries-of-2018-for-world-health-day. April 7, 2018. Accessed June 4, 2018.
A start-up operating out of MIT in Cambridge, Mass., called Portal Instruments has developed a needleless injection system.
The device, called PRIME, delivers medication into the bloodstream in a high-pressure stream that travels at Mach 0.7 – the speed of a jet. The makers signed a commercial deal in December 2017, and the device is expected to be available soon.
Reference
1. Kerrigan S. The 16 Most Remarkable Healthcare Innovations, Events, and Discoveries of 2018 For World Health Day. https://interestingengineering.com/the-16-most-remarkable-healthcare-innovations-events-and-discoveries-of-2018-for-world-health-day. April 7, 2018. Accessed June 4, 2018.
A start-up operating out of MIT in Cambridge, Mass., called Portal Instruments has developed a needleless injection system.
The device, called PRIME, delivers medication into the bloodstream in a high-pressure stream that travels at Mach 0.7 – the speed of a jet. The makers signed a commercial deal in December 2017, and the device is expected to be available soon.
Reference
1. Kerrigan S. The 16 Most Remarkable Healthcare Innovations, Events, and Discoveries of 2018 For World Health Day. https://interestingengineering.com/the-16-most-remarkable-healthcare-innovations-events-and-discoveries-of-2018-for-world-health-day. April 7, 2018. Accessed June 4, 2018.
United States now over 100 measles cases for the year
according to the Centers for Disease Control and Prevention.
Just over half of the cases in 2019 have occurred in Clark County, Wash., which has reported 53 cases. That outbreak led Gov. Jay Inslee to declare a public health emergency for the entire state on Jan. 25.
The cases in Washington represent one of the five outbreaks – the CDC defines an outbreak as three or more cases – that have occurred so far this year, with three reported in New York State (Rockland County, Monroe County, and New York City) and one in Texas, which has been spread out over five counties, the CDC reported Feb. 11.
“These outbreaks are linked to travelers who brought measles back from other countries such as Israel and Ukraine, where large measles outbreaks are occurring,” the CDC noted. The other states with confirmed cases are California, Colorado, Connecticut, Georgia, Illinois, New Jersey, and Oregon.
In a video released Feb. 1, Surgeon General Jerome Adams stressed the importance of getting vaccinated and noted that an infected person can transmit the measles virus up to 4 days before he or she develops symptoms.
according to the Centers for Disease Control and Prevention.

Just over half of the cases in 2019 have occurred in Clark County, Wash., which has reported 53 cases. That outbreak led Gov. Jay Inslee to declare a public health emergency for the entire state on Jan. 25.
The cases in Washington represent one of the five outbreaks – the CDC defines an outbreak as three or more cases – that have occurred so far this year, with three reported in New York State (Rockland County, Monroe County, and New York City) and one in Texas, which has been spread out over five counties, the CDC reported Feb. 11.
“These outbreaks are linked to travelers who brought measles back from other countries such as Israel and Ukraine, where large measles outbreaks are occurring,” the CDC noted. The other states with confirmed cases are California, Colorado, Connecticut, Georgia, Illinois, New Jersey, and Oregon.
In a video released Feb. 1, Surgeon General Jerome Adams stressed the importance of getting vaccinated and noted that an infected person can transmit the measles virus up to 4 days before he or she develops symptoms.
according to the Centers for Disease Control and Prevention.

Just over half of the cases in 2019 have occurred in Clark County, Wash., which has reported 53 cases. That outbreak led Gov. Jay Inslee to declare a public health emergency for the entire state on Jan. 25.
The cases in Washington represent one of the five outbreaks – the CDC defines an outbreak as three or more cases – that have occurred so far this year, with three reported in New York State (Rockland County, Monroe County, and New York City) and one in Texas, which has been spread out over five counties, the CDC reported Feb. 11.
“These outbreaks are linked to travelers who brought measles back from other countries such as Israel and Ukraine, where large measles outbreaks are occurring,” the CDC noted. The other states with confirmed cases are California, Colorado, Connecticut, Georgia, Illinois, New Jersey, and Oregon.
In a video released Feb. 1, Surgeon General Jerome Adams stressed the importance of getting vaccinated and noted that an infected person can transmit the measles virus up to 4 days before he or she develops symptoms.