User login
Phone app diagnoses STEMI nearly as well as ECG
CHICAGO – A novel smartphone app performed nearly as well as a standard 12-lead ECG for diagnosis of ST-segment elevation MI (STEMI) in patients presenting with chest pain in ST LEUIS, an international, multicenter study.
“This study demonstrates that a 12-lead-equivalent ECG obtained using a smartphone coupled with a software application and inexpensive two-wire attachment can identify STEMI versus non-STEMI with an excellent correlation to a traditional 12-lead ECG. This technology holds substantial promise to improve outcomes in STEMI by enabling more rapid diagnosis and treatment anywhere in the world for inexpensive cost,” J. Brent Muhlestein, MD, said while presenting the ST LEUIS results at the American Heart Association scientific sessions.
This technology could provide a long-sought breakthrough in overcoming patient denial and motivating hard-headed individuals with a life-threatening MI to get to the hospital more quickly after symptom onset, instead of initially shrugging off the matter as indigestion or another nuisance. If individuals can use their handy cell phone or smartwatch to quickly obtain an ECG that shows they’re having a STEMI, they’re going to seek medical attention much sooner, with resultant greater salvage of heart muscle, noted Dr. Muhlestein of Intermountain Healthcare in Salt Lake City.
ST LEUIS tested whether a smartphone ECG app developed by AliveCor can accurately diagnose STEMI in patients with chest pain. The study, which took place at Intermountain Medical Center and a handful of other sites associated with the Duke University Cooperative Cardiovascular Society, included 204 patients who presented to EDs with chest pain. They simultaneously received both a standard 12-lead ECG and an ECG obtained using the AliveCor smartphone app. The matched ECG pairs were evaluated separately, both quantitatively and qualitatively, by a blinded panel of experienced cardiologists and classified as STEMI, left bundle branch block, non-STEMI, or uninterpretable. The study population included 92 patients with chest pain and activation of a STEMI protocol and 112 who came through the ED chest pain protocol.
Side-by-side ECG comparisons weren’t attempted in 14 pairs deemed not interpretable. In 13 cases this was because of technical problems with the smartphone ECG, and in the 14th because of ventricular pacing in the standard 12-lead ECG.
STEMI was diagnosed in 22.5% of the study population by 12-lead ECG and in 29.4% by smartphone app. The discrepancy was explained by small voltage differences in the ST-segment elevation which met criteria for STEMI by smartphone but not standard 12-lead ECG in 15 cases.
“It appears that the ST elevation was a little bit more obvious in the smartphone ECG,” Dr. Muhlestein observed.
Left bundle branch block was identified in 5.4% of patients by both methods.
The key performance numbers: The smartphone ECG had a sensitivity of 89%, specificity of 84%, positive predictive value of 70%, and negative predictive value of 95% for diagnosis of STEMI or left bundle branch block. The positive predictive value was diminished by the increased likelihood that the smartphone would call STEMI in discordant cases.
Dr. Muhlestein said that, despite the AliveCor device’s very good correlation with the standard 12-lead ECG, the system needs further tweaking.
“We definitely think this is not ready for prime time. Further refinements of the software and hardware may improve on our study results and broaden potential applications through increased ease of use and reliability. I’m sure smart engineers can make a much more simple, really user-friendly device now that we know it’s actually feasible. I envision a time when you turn it on and it speaks loud and tells you what to do and how to do it – like an AED [automated external defibrillator] – then uploads the ECG to the cloud, interprets it, and tells you whether you should go to the emergency department or not,” Dr. Muhlestein said.
This is a device that’s going to be a boon not only in the United States but also in developing countries, where even people living without electricity or running water often have cell phones, the cardiologist noted.
Dr. Muhlestein reported having no financial conflicts of interest regarding the study, which was sponsored by the participating medical institutions.
CHICAGO – A novel smartphone app performed nearly as well as a standard 12-lead ECG for diagnosis of ST-segment elevation MI (STEMI) in patients presenting with chest pain in ST LEUIS, an international, multicenter study.
“This study demonstrates that a 12-lead-equivalent ECG obtained using a smartphone coupled with a software application and inexpensive two-wire attachment can identify STEMI versus non-STEMI with an excellent correlation to a traditional 12-lead ECG. This technology holds substantial promise to improve outcomes in STEMI by enabling more rapid diagnosis and treatment anywhere in the world for inexpensive cost,” J. Brent Muhlestein, MD, said while presenting the ST LEUIS results at the American Heart Association scientific sessions.
This technology could provide a long-sought breakthrough in overcoming patient denial and motivating hard-headed individuals with a life-threatening MI to get to the hospital more quickly after symptom onset, instead of initially shrugging off the matter as indigestion or another nuisance. If individuals can use their handy cell phone or smartwatch to quickly obtain an ECG that shows they’re having a STEMI, they’re going to seek medical attention much sooner, with resultant greater salvage of heart muscle, noted Dr. Muhlestein of Intermountain Healthcare in Salt Lake City.
ST LEUIS tested whether a smartphone ECG app developed by AliveCor can accurately diagnose STEMI in patients with chest pain. The study, which took place at Intermountain Medical Center and a handful of other sites associated with the Duke University Cooperative Cardiovascular Society, included 204 patients who presented to EDs with chest pain. They simultaneously received both a standard 12-lead ECG and an ECG obtained using the AliveCor smartphone app. The matched ECG pairs were evaluated separately, both quantitatively and qualitatively, by a blinded panel of experienced cardiologists and classified as STEMI, left bundle branch block, non-STEMI, or uninterpretable. The study population included 92 patients with chest pain and activation of a STEMI protocol and 112 who came through the ED chest pain protocol.
Side-by-side ECG comparisons weren’t attempted in 14 pairs deemed not interpretable. In 13 cases this was because of technical problems with the smartphone ECG, and in the 14th because of ventricular pacing in the standard 12-lead ECG.
STEMI was diagnosed in 22.5% of the study population by 12-lead ECG and in 29.4% by smartphone app. The discrepancy was explained by small voltage differences in the ST-segment elevation which met criteria for STEMI by smartphone but not standard 12-lead ECG in 15 cases.
“It appears that the ST elevation was a little bit more obvious in the smartphone ECG,” Dr. Muhlestein observed.
Left bundle branch block was identified in 5.4% of patients by both methods.
The key performance numbers: The smartphone ECG had a sensitivity of 89%, specificity of 84%, positive predictive value of 70%, and negative predictive value of 95% for diagnosis of STEMI or left bundle branch block. The positive predictive value was diminished by the increased likelihood that the smartphone would call STEMI in discordant cases.
Dr. Muhlestein said that, despite the AliveCor device’s very good correlation with the standard 12-lead ECG, the system needs further tweaking.
“We definitely think this is not ready for prime time. Further refinements of the software and hardware may improve on our study results and broaden potential applications through increased ease of use and reliability. I’m sure smart engineers can make a much more simple, really user-friendly device now that we know it’s actually feasible. I envision a time when you turn it on and it speaks loud and tells you what to do and how to do it – like an AED [automated external defibrillator] – then uploads the ECG to the cloud, interprets it, and tells you whether you should go to the emergency department or not,” Dr. Muhlestein said.
This is a device that’s going to be a boon not only in the United States but also in developing countries, where even people living without electricity or running water often have cell phones, the cardiologist noted.
Dr. Muhlestein reported having no financial conflicts of interest regarding the study, which was sponsored by the participating medical institutions.
CHICAGO – A novel smartphone app performed nearly as well as a standard 12-lead ECG for diagnosis of ST-segment elevation MI (STEMI) in patients presenting with chest pain in ST LEUIS, an international, multicenter study.
“This study demonstrates that a 12-lead-equivalent ECG obtained using a smartphone coupled with a software application and inexpensive two-wire attachment can identify STEMI versus non-STEMI with an excellent correlation to a traditional 12-lead ECG. This technology holds substantial promise to improve outcomes in STEMI by enabling more rapid diagnosis and treatment anywhere in the world for inexpensive cost,” J. Brent Muhlestein, MD, said while presenting the ST LEUIS results at the American Heart Association scientific sessions.
This technology could provide a long-sought breakthrough in overcoming patient denial and motivating hard-headed individuals with a life-threatening MI to get to the hospital more quickly after symptom onset, instead of initially shrugging off the matter as indigestion or another nuisance. If individuals can use their handy cell phone or smartwatch to quickly obtain an ECG that shows they’re having a STEMI, they’re going to seek medical attention much sooner, with resultant greater salvage of heart muscle, noted Dr. Muhlestein of Intermountain Healthcare in Salt Lake City.
ST LEUIS tested whether a smartphone ECG app developed by AliveCor can accurately diagnose STEMI in patients with chest pain. The study, which took place at Intermountain Medical Center and a handful of other sites associated with the Duke University Cooperative Cardiovascular Society, included 204 patients who presented to EDs with chest pain. They simultaneously received both a standard 12-lead ECG and an ECG obtained using the AliveCor smartphone app. The matched ECG pairs were evaluated separately, both quantitatively and qualitatively, by a blinded panel of experienced cardiologists and classified as STEMI, left bundle branch block, non-STEMI, or uninterpretable. The study population included 92 patients with chest pain and activation of a STEMI protocol and 112 who came through the ED chest pain protocol.
Side-by-side ECG comparisons weren’t attempted in 14 pairs deemed not interpretable. In 13 cases this was because of technical problems with the smartphone ECG, and in the 14th because of ventricular pacing in the standard 12-lead ECG.
STEMI was diagnosed in 22.5% of the study population by 12-lead ECG and in 29.4% by smartphone app. The discrepancy was explained by small voltage differences in the ST-segment elevation which met criteria for STEMI by smartphone but not standard 12-lead ECG in 15 cases.
“It appears that the ST elevation was a little bit more obvious in the smartphone ECG,” Dr. Muhlestein observed.
Left bundle branch block was identified in 5.4% of patients by both methods.
The key performance numbers: The smartphone ECG had a sensitivity of 89%, specificity of 84%, positive predictive value of 70%, and negative predictive value of 95% for diagnosis of STEMI or left bundle branch block. The positive predictive value was diminished by the increased likelihood that the smartphone would call STEMI in discordant cases.
Dr. Muhlestein said that, despite the AliveCor device’s very good correlation with the standard 12-lead ECG, the system needs further tweaking.
“We definitely think this is not ready for prime time. Further refinements of the software and hardware may improve on our study results and broaden potential applications through increased ease of use and reliability. I’m sure smart engineers can make a much more simple, really user-friendly device now that we know it’s actually feasible. I envision a time when you turn it on and it speaks loud and tells you what to do and how to do it – like an AED [automated external defibrillator] – then uploads the ECG to the cloud, interprets it, and tells you whether you should go to the emergency department or not,” Dr. Muhlestein said.
This is a device that’s going to be a boon not only in the United States but also in developing countries, where even people living without electricity or running water often have cell phones, the cardiologist noted.
Dr. Muhlestein reported having no financial conflicts of interest regarding the study, which was sponsored by the participating medical institutions.
REPORTING FROM THE AHA SCIENTIFIC SESSIONS
Key clinical point:
Major finding: The app, designed to diagnose ST-segment elevation MI, had a sensitivity of 89%, specificity of 84%, and negative predictive value of 95% for this purpose.
Study details: This multicenter, international study featured blinded expert side-by-side comparisons of standard 12-lead ECGs and ECGs obtained via a smartphone app in 204 patients who presented with chest pain.
Disclosures: The presenter reported having no financial conflicts of interest regarding the study, which was fully sponsored by the participating medical institutions.
Bring Schwartz Rounds to your hospital
A more emotional approach to rounds
If you are not doing Schwartz Rounds, get them started. ASAP.
I recently completed a 4-year tenure as physician moderator for our hospital’s Schwartz Rounds. An amazing team at my hospital helped pull the bimonthly sessions together. These compassionate care rounds are a national initiative to help foster empathy and compassion in the health care setting.
We gather a panel of two to three people involved in our patient presentation who share and move quickly through the clinical details, and head on toward the thornier ethical issues, emotional triggers, and responses. The best sessions are when the audience’s voice is heard for the bulk of the time.
The emotional cadence flows from boiling in frustration, drowning in tears, followed by comfort, and ending in thoughts for the next session. It is a more powerful arc than an episode of the television program “This is Us.” Largely, because this was us. This was real life. Real-time catharsis in the hospital.
In the daily grind, we often skip the step of processing our frustration, sadness, and anger, moving right on to the next patient and walking into the next room with that stoic layer of equanimity. I walk the hallways and find I grab my phone to catch up on emails, walking to the wrong floor because I’m not paying attention. Always something to do, someone to talk to, a family to call, pagers going off, phone calls. When do we sit and reflect?
These Schwartz Rounds are those moments of reflection – a slowdown in the day to think more deeply about the case. We talk about everything and anything. We have discussions with opposing views:
“Everything should have been done!”
“How did you not stop care?!”
“I agree with the doctors.”
“I can see the patient’s view more clearly now.”
Our first Schwartz Rounds tended to be end-of-life stories, particularly regarding the family mantra of “Do everything.” The health care team watches the suffering of a patient, a family, in a seemingly futile situation. Conversations around the end of life, choices, and quality of life are cut short daily by family members who simply recite, “Do everything.”
After several of these sessions, a case swings us in the other direction. The elderly gentleman with treatable cancer, who could easily survive another 20 years, declines treatment. “I’m fine, doc; I’ve lived long enough.” His wife at his bedside, shaking her head, tells us, “I don’t know why he wants to give up. He’s been as stubborn as a mule since the day I met him.” I spend 30 minutes convincing him to stay. The nurse does the same. Now we have a patient with a “Do nothing.” The patient’s decisions conflict with the family and the health care team.
Every day in the hospital provides a new ethical dilemma, a frustrating case, a challenging patient. Fodder for rounds.
Read the full post at hospitalleader.org.
Dr. Messler is a hospitalist at Morton Plant Hospitalist group in Clearwater, Fla. He previously chaired SHM’s Quality and Patient Safety Committee and has been active in several SHM mentoring programs, most recently with Project BOOST and Glycemic Control.
Also on The Hospital Leader
- Incubating Success: How We Used Structured Feedback to Reduce A Dangerous Practice by Rich Bottner, PA-C & Victoria Valencia, MPH
- New SoHM Report Provides Unique Window into Hospital Medicine Practice Trends by Leslie Flores, MHA, SFHM
- IGNITE Change: Improving Care via Interprofessional Clinical Learning Environments by Vineet Arora, MD, MAPP, MHM
A more emotional approach to rounds
A more emotional approach to rounds
If you are not doing Schwartz Rounds, get them started. ASAP.
I recently completed a 4-year tenure as physician moderator for our hospital’s Schwartz Rounds. An amazing team at my hospital helped pull the bimonthly sessions together. These compassionate care rounds are a national initiative to help foster empathy and compassion in the health care setting.
We gather a panel of two to three people involved in our patient presentation who share and move quickly through the clinical details, and head on toward the thornier ethical issues, emotional triggers, and responses. The best sessions are when the audience’s voice is heard for the bulk of the time.
The emotional cadence flows from boiling in frustration, drowning in tears, followed by comfort, and ending in thoughts for the next session. It is a more powerful arc than an episode of the television program “This is Us.” Largely, because this was us. This was real life. Real-time catharsis in the hospital.
In the daily grind, we often skip the step of processing our frustration, sadness, and anger, moving right on to the next patient and walking into the next room with that stoic layer of equanimity. I walk the hallways and find I grab my phone to catch up on emails, walking to the wrong floor because I’m not paying attention. Always something to do, someone to talk to, a family to call, pagers going off, phone calls. When do we sit and reflect?
These Schwartz Rounds are those moments of reflection – a slowdown in the day to think more deeply about the case. We talk about everything and anything. We have discussions with opposing views:
“Everything should have been done!”
“How did you not stop care?!”
“I agree with the doctors.”
“I can see the patient’s view more clearly now.”
Our first Schwartz Rounds tended to be end-of-life stories, particularly regarding the family mantra of “Do everything.” The health care team watches the suffering of a patient, a family, in a seemingly futile situation. Conversations around the end of life, choices, and quality of life are cut short daily by family members who simply recite, “Do everything.”
After several of these sessions, a case swings us in the other direction. The elderly gentleman with treatable cancer, who could easily survive another 20 years, declines treatment. “I’m fine, doc; I’ve lived long enough.” His wife at his bedside, shaking her head, tells us, “I don’t know why he wants to give up. He’s been as stubborn as a mule since the day I met him.” I spend 30 minutes convincing him to stay. The nurse does the same. Now we have a patient with a “Do nothing.” The patient’s decisions conflict with the family and the health care team.
Every day in the hospital provides a new ethical dilemma, a frustrating case, a challenging patient. Fodder for rounds.
Read the full post at hospitalleader.org.
Dr. Messler is a hospitalist at Morton Plant Hospitalist group in Clearwater, Fla. He previously chaired SHM’s Quality and Patient Safety Committee and has been active in several SHM mentoring programs, most recently with Project BOOST and Glycemic Control.
Also on The Hospital Leader
- Incubating Success: How We Used Structured Feedback to Reduce A Dangerous Practice by Rich Bottner, PA-C & Victoria Valencia, MPH
- New SoHM Report Provides Unique Window into Hospital Medicine Practice Trends by Leslie Flores, MHA, SFHM
- IGNITE Change: Improving Care via Interprofessional Clinical Learning Environments by Vineet Arora, MD, MAPP, MHM
If you are not doing Schwartz Rounds, get them started. ASAP.
I recently completed a 4-year tenure as physician moderator for our hospital’s Schwartz Rounds. An amazing team at my hospital helped pull the bimonthly sessions together. These compassionate care rounds are a national initiative to help foster empathy and compassion in the health care setting.
We gather a panel of two to three people involved in our patient presentation who share and move quickly through the clinical details, and head on toward the thornier ethical issues, emotional triggers, and responses. The best sessions are when the audience’s voice is heard for the bulk of the time.
The emotional cadence flows from boiling in frustration, drowning in tears, followed by comfort, and ending in thoughts for the next session. It is a more powerful arc than an episode of the television program “This is Us.” Largely, because this was us. This was real life. Real-time catharsis in the hospital.
In the daily grind, we often skip the step of processing our frustration, sadness, and anger, moving right on to the next patient and walking into the next room with that stoic layer of equanimity. I walk the hallways and find I grab my phone to catch up on emails, walking to the wrong floor because I’m not paying attention. Always something to do, someone to talk to, a family to call, pagers going off, phone calls. When do we sit and reflect?
These Schwartz Rounds are those moments of reflection – a slowdown in the day to think more deeply about the case. We talk about everything and anything. We have discussions with opposing views:
“Everything should have been done!”
“How did you not stop care?!”
“I agree with the doctors.”
“I can see the patient’s view more clearly now.”
Our first Schwartz Rounds tended to be end-of-life stories, particularly regarding the family mantra of “Do everything.” The health care team watches the suffering of a patient, a family, in a seemingly futile situation. Conversations around the end of life, choices, and quality of life are cut short daily by family members who simply recite, “Do everything.”
After several of these sessions, a case swings us in the other direction. The elderly gentleman with treatable cancer, who could easily survive another 20 years, declines treatment. “I’m fine, doc; I’ve lived long enough.” His wife at his bedside, shaking her head, tells us, “I don’t know why he wants to give up. He’s been as stubborn as a mule since the day I met him.” I spend 30 minutes convincing him to stay. The nurse does the same. Now we have a patient with a “Do nothing.” The patient’s decisions conflict with the family and the health care team.
Every day in the hospital provides a new ethical dilemma, a frustrating case, a challenging patient. Fodder for rounds.
Read the full post at hospitalleader.org.
Dr. Messler is a hospitalist at Morton Plant Hospitalist group in Clearwater, Fla. He previously chaired SHM’s Quality and Patient Safety Committee and has been active in several SHM mentoring programs, most recently with Project BOOST and Glycemic Control.
Also on The Hospital Leader
- Incubating Success: How We Used Structured Feedback to Reduce A Dangerous Practice by Rich Bottner, PA-C & Victoria Valencia, MPH
- New SoHM Report Provides Unique Window into Hospital Medicine Practice Trends by Leslie Flores, MHA, SFHM
- IGNITE Change: Improving Care via Interprofessional Clinical Learning Environments by Vineet Arora, MD, MAPP, MHM
Acute stroke thrombolysis worked safely despite GI bleed or malignancy
CHICAGO – A recent history of GI bleeding or malignancy may not be a valid contraindication to thrombolytic therapy in patients with an acute ischemic stroke, based on a review of outcomes from more than 40,000 U.S. stroke patients.
The analysis showed that, among 40,396 U.S. patients who had an acute ischemic stroke during 2009-2015 and received timely treatment with alteplase, “we did not find statistically significant increased rates of in-hospital mortality or bleeding” in the small number of patients who received alteplase (Activase) despite a recent GI bleed or diagnosed GI malignancy, Taku Inohara, MD, said at the American Heart Association scientific sessions. The 2018 Guidelines for the Early Management of Patients With Acute Ischemic Stroke deemed thrombolytic therapy with alteplase in these types of patients contraindicated, based on consensus expert opinion (Stroke. 2018 March;49[3]:e66-e110).
“Further study is needed to evaluate the safety of recombinant tissue–type plasminogen activator [alteplase] in this specific population,” suggested Dr. Inohara, a cardiologist and research fellow at Duke University, Durham, N.C.
His analysis used data collected by the Get With the Guidelines–Stroke program, a voluntary quality promotion and improvement program that during 2009-2015 included records for more than 633,000 U.S. stroke patients that could be linked with records kept by the Centers for Medicare & Medicaid Services. From this database, 40,396 patients (6%) treated with alteplase within 4.5 hours of stroke onset were identified. The alteplase-treated patients included 93 with a diagnosis code during the prior year for a GI malignancy and 43 with a diagnostic code within the prior 21 days for a GI bleed.
Dr. Inohara and his associates determined patients’ mortality during their stroke hospitalization, as well as several measures of functional recovery at hospital discharge and thrombolysis-related complications. For each of these endpoints, the rate among patients with a GI malignancy, a GI bleed, or the rate among a combined group of both patients showed no statistically significant differences, compared with the more than 40,000 other patients without a GI complication after adjustment for several demographic and clinical between-group differences. However, Dr. Inohara cautioned that residual or unmeasured confounding may exist that distorts these findings. The rate of in-hospital mortality, the prespecified primary endpoint for the analysis, was 10% among patients with either type of GI complication and 9% in those without. The rate of serious thrombolysis-related complications was 7% in the patients with GI disease and 9% in those without.
In a separate analysis of the complete database of more than 633,000 patients, Dr. Inohara and his associates found 148 patients who had either a GI bleed or malignancy and otherwise qualified for thrombolytic therapy but did not receive this treatment. This meant that overall, in this large U.S. experience, 136 of 284 (48%) acute ischemic stroke patients who qualified for thrombolysis but had a GI complication nonetheless received thrombolysis. Further analysis showed that the patients not treated with thrombolysis had at admission an average National Institutes of Health Stroke Scale score of 11, compared with an average score of 14 among patients who received thrombolysis.
This apparent selection for thrombolytic treatment of patients with more severe strokes “may have overestimated risk in the patients with GI disease,” Dr. Inohara said.
Dr. Inohara reported receiving research funding from Boston Scientific.
SOURCE: Inohara T et al. Circulation. 2018 Nov 6;138[suppl 1], Abstract 12291.
CHICAGO – A recent history of GI bleeding or malignancy may not be a valid contraindication to thrombolytic therapy in patients with an acute ischemic stroke, based on a review of outcomes from more than 40,000 U.S. stroke patients.
The analysis showed that, among 40,396 U.S. patients who had an acute ischemic stroke during 2009-2015 and received timely treatment with alteplase, “we did not find statistically significant increased rates of in-hospital mortality or bleeding” in the small number of patients who received alteplase (Activase) despite a recent GI bleed or diagnosed GI malignancy, Taku Inohara, MD, said at the American Heart Association scientific sessions. The 2018 Guidelines for the Early Management of Patients With Acute Ischemic Stroke deemed thrombolytic therapy with alteplase in these types of patients contraindicated, based on consensus expert opinion (Stroke. 2018 March;49[3]:e66-e110).
“Further study is needed to evaluate the safety of recombinant tissue–type plasminogen activator [alteplase] in this specific population,” suggested Dr. Inohara, a cardiologist and research fellow at Duke University, Durham, N.C.
His analysis used data collected by the Get With the Guidelines–Stroke program, a voluntary quality promotion and improvement program that during 2009-2015 included records for more than 633,000 U.S. stroke patients that could be linked with records kept by the Centers for Medicare & Medicaid Services. From this database, 40,396 patients (6%) treated with alteplase within 4.5 hours of stroke onset were identified. The alteplase-treated patients included 93 with a diagnosis code during the prior year for a GI malignancy and 43 with a diagnostic code within the prior 21 days for a GI bleed.
Dr. Inohara and his associates determined patients’ mortality during their stroke hospitalization, as well as several measures of functional recovery at hospital discharge and thrombolysis-related complications. For each of these endpoints, the rate among patients with a GI malignancy, a GI bleed, or the rate among a combined group of both patients showed no statistically significant differences, compared with the more than 40,000 other patients without a GI complication after adjustment for several demographic and clinical between-group differences. However, Dr. Inohara cautioned that residual or unmeasured confounding may exist that distorts these findings. The rate of in-hospital mortality, the prespecified primary endpoint for the analysis, was 10% among patients with either type of GI complication and 9% in those without. The rate of serious thrombolysis-related complications was 7% in the patients with GI disease and 9% in those without.
In a separate analysis of the complete database of more than 633,000 patients, Dr. Inohara and his associates found 148 patients who had either a GI bleed or malignancy and otherwise qualified for thrombolytic therapy but did not receive this treatment. This meant that overall, in this large U.S. experience, 136 of 284 (48%) acute ischemic stroke patients who qualified for thrombolysis but had a GI complication nonetheless received thrombolysis. Further analysis showed that the patients not treated with thrombolysis had at admission an average National Institutes of Health Stroke Scale score of 11, compared with an average score of 14 among patients who received thrombolysis.
This apparent selection for thrombolytic treatment of patients with more severe strokes “may have overestimated risk in the patients with GI disease,” Dr. Inohara said.
Dr. Inohara reported receiving research funding from Boston Scientific.
SOURCE: Inohara T et al. Circulation. 2018 Nov 6;138[suppl 1], Abstract 12291.
CHICAGO – A recent history of GI bleeding or malignancy may not be a valid contraindication to thrombolytic therapy in patients with an acute ischemic stroke, based on a review of outcomes from more than 40,000 U.S. stroke patients.
The analysis showed that, among 40,396 U.S. patients who had an acute ischemic stroke during 2009-2015 and received timely treatment with alteplase, “we did not find statistically significant increased rates of in-hospital mortality or bleeding” in the small number of patients who received alteplase (Activase) despite a recent GI bleed or diagnosed GI malignancy, Taku Inohara, MD, said at the American Heart Association scientific sessions. The 2018 Guidelines for the Early Management of Patients With Acute Ischemic Stroke deemed thrombolytic therapy with alteplase in these types of patients contraindicated, based on consensus expert opinion (Stroke. 2018 March;49[3]:e66-e110).
“Further study is needed to evaluate the safety of recombinant tissue–type plasminogen activator [alteplase] in this specific population,” suggested Dr. Inohara, a cardiologist and research fellow at Duke University, Durham, N.C.
His analysis used data collected by the Get With the Guidelines–Stroke program, a voluntary quality promotion and improvement program that during 2009-2015 included records for more than 633,000 U.S. stroke patients that could be linked with records kept by the Centers for Medicare & Medicaid Services. From this database, 40,396 patients (6%) treated with alteplase within 4.5 hours of stroke onset were identified. The alteplase-treated patients included 93 with a diagnosis code during the prior year for a GI malignancy and 43 with a diagnostic code within the prior 21 days for a GI bleed.
Dr. Inohara and his associates determined patients’ mortality during their stroke hospitalization, as well as several measures of functional recovery at hospital discharge and thrombolysis-related complications. For each of these endpoints, the rate among patients with a GI malignancy, a GI bleed, or the rate among a combined group of both patients showed no statistically significant differences, compared with the more than 40,000 other patients without a GI complication after adjustment for several demographic and clinical between-group differences. However, Dr. Inohara cautioned that residual or unmeasured confounding may exist that distorts these findings. The rate of in-hospital mortality, the prespecified primary endpoint for the analysis, was 10% among patients with either type of GI complication and 9% in those without. The rate of serious thrombolysis-related complications was 7% in the patients with GI disease and 9% in those without.
In a separate analysis of the complete database of more than 633,000 patients, Dr. Inohara and his associates found 148 patients who had either a GI bleed or malignancy and otherwise qualified for thrombolytic therapy but did not receive this treatment. This meant that overall, in this large U.S. experience, 136 of 284 (48%) acute ischemic stroke patients who qualified for thrombolysis but had a GI complication nonetheless received thrombolysis. Further analysis showed that the patients not treated with thrombolysis had at admission an average National Institutes of Health Stroke Scale score of 11, compared with an average score of 14 among patients who received thrombolysis.
This apparent selection for thrombolytic treatment of patients with more severe strokes “may have overestimated risk in the patients with GI disease,” Dr. Inohara said.
Dr. Inohara reported receiving research funding from Boston Scientific.
SOURCE: Inohara T et al. Circulation. 2018 Nov 6;138[suppl 1], Abstract 12291.
REPORTING FROM THE AHA SCIENTIFIC SESSIONS
Key clinical point:
Major finding: In-hospital mortality after thrombolysis was 10% in those with a GI bleed or malignancy and 9% in those without.
Study details: A review of Medicare records for 40,396 acute ischemic stroke patients treated with thrombolysis during 2009-2015.
Disclosures: Dr. Inohara reported receiving research funding from Boston Scientific.
Source: Inohara T et al. Circulation. 2018 Nov 6;138[suppl 1], Abstract A12291.
ICU-acquired pneumonia mortality risk may be underestimated
In a large prospectively collected database, the risk of death at 30 days in ICU patients was far greater in those with hospital-acquired pneumonia (HAP) than in those with ventilator-associated pneumonia (VAP) even after adjustment for prognostic factors, according to a large study that compared mortality risk for these complications.
The data for this newly published study were drawn from an evaluation of 14,212 patients treated at 23 ICUs participating in a collaborative French network OUTCOMEREA and published Critical Care Medicine.
HAP in ICU patients “was associated with an 82% increase in the risk of death at day 30,” reported a team of investigators led by Wafa Ibn Saied, MD, of the Université Paris Diderot. Although VAP and HAP were independent risk factors (P both less than .0001) for death at 30 days, VAP increased risk by 38%, less than half of HAP, which increased risk by 82%.
From an observational but prospective database initiated in 1997, this study evaluated 7,735 ICU patients at risk for VAP and 9,747 at risk for HAP. Of those at risk, defined by several factors including an ICU stay of more than 48 hours, HAP developed in 8% and VAP developed in 1%.
The 30-day mortality rates at 30 days after pneumonia were 23.9% for HAP and 28.4% for VAP. The greater risk of death by HR was identified after an analysis that adjusted for mortality risk factors, the adequacy of initial treatment, and other factors, such as prior history of pneumonia.
In HAP patients, the rate of mortality at 30 days was 32% in the 75 who were reintubated but only 16% in the 101 who were not. Adequate empirical therapy within the first 24 hours for HAP was not associated with a reduction in the risk of death.
As in the HAP patients, mortality was not significantly higher in VAP patients who received inadequate empirical therapy, compared with those who did, according to the authors.
Previous studies have suggested that both HAP and VAP increase risk of death in ICU patients, but the authors of this study believe that the relative risk of HAP “is underappreciated.” They asserted, based on these most recent data as well as on previously published analyses, that nonventilated HAP results in “significant increases in cost, length of stay, and mortality.”
The researchers had no disclosures.
SOURCE: Saied WI et al. Crit Care Med. 2018 Nov 7. doi: 10.1097/CCM.0000000000003553.
In a large prospectively collected database, the risk of death at 30 days in ICU patients was far greater in those with hospital-acquired pneumonia (HAP) than in those with ventilator-associated pneumonia (VAP) even after adjustment for prognostic factors, according to a large study that compared mortality risk for these complications.
The data for this newly published study were drawn from an evaluation of 14,212 patients treated at 23 ICUs participating in a collaborative French network OUTCOMEREA and published Critical Care Medicine.
HAP in ICU patients “was associated with an 82% increase in the risk of death at day 30,” reported a team of investigators led by Wafa Ibn Saied, MD, of the Université Paris Diderot. Although VAP and HAP were independent risk factors (P both less than .0001) for death at 30 days, VAP increased risk by 38%, less than half of HAP, which increased risk by 82%.
From an observational but prospective database initiated in 1997, this study evaluated 7,735 ICU patients at risk for VAP and 9,747 at risk for HAP. Of those at risk, defined by several factors including an ICU stay of more than 48 hours, HAP developed in 8% and VAP developed in 1%.
The 30-day mortality rates at 30 days after pneumonia were 23.9% for HAP and 28.4% for VAP. The greater risk of death by HR was identified after an analysis that adjusted for mortality risk factors, the adequacy of initial treatment, and other factors, such as prior history of pneumonia.
In HAP patients, the rate of mortality at 30 days was 32% in the 75 who were reintubated but only 16% in the 101 who were not. Adequate empirical therapy within the first 24 hours for HAP was not associated with a reduction in the risk of death.
As in the HAP patients, mortality was not significantly higher in VAP patients who received inadequate empirical therapy, compared with those who did, according to the authors.
Previous studies have suggested that both HAP and VAP increase risk of death in ICU patients, but the authors of this study believe that the relative risk of HAP “is underappreciated.” They asserted, based on these most recent data as well as on previously published analyses, that nonventilated HAP results in “significant increases in cost, length of stay, and mortality.”
The researchers had no disclosures.
SOURCE: Saied WI et al. Crit Care Med. 2018 Nov 7. doi: 10.1097/CCM.0000000000003553.
In a large prospectively collected database, the risk of death at 30 days in ICU patients was far greater in those with hospital-acquired pneumonia (HAP) than in those with ventilator-associated pneumonia (VAP) even after adjustment for prognostic factors, according to a large study that compared mortality risk for these complications.
The data for this newly published study were drawn from an evaluation of 14,212 patients treated at 23 ICUs participating in a collaborative French network OUTCOMEREA and published Critical Care Medicine.
HAP in ICU patients “was associated with an 82% increase in the risk of death at day 30,” reported a team of investigators led by Wafa Ibn Saied, MD, of the Université Paris Diderot. Although VAP and HAP were independent risk factors (P both less than .0001) for death at 30 days, VAP increased risk by 38%, less than half of HAP, which increased risk by 82%.
From an observational but prospective database initiated in 1997, this study evaluated 7,735 ICU patients at risk for VAP and 9,747 at risk for HAP. Of those at risk, defined by several factors including an ICU stay of more than 48 hours, HAP developed in 8% and VAP developed in 1%.
The 30-day mortality rates at 30 days after pneumonia were 23.9% for HAP and 28.4% for VAP. The greater risk of death by HR was identified after an analysis that adjusted for mortality risk factors, the adequacy of initial treatment, and other factors, such as prior history of pneumonia.
In HAP patients, the rate of mortality at 30 days was 32% in the 75 who were reintubated but only 16% in the 101 who were not. Adequate empirical therapy within the first 24 hours for HAP was not associated with a reduction in the risk of death.
As in the HAP patients, mortality was not significantly higher in VAP patients who received inadequate empirical therapy, compared with those who did, according to the authors.
Previous studies have suggested that both HAP and VAP increase risk of death in ICU patients, but the authors of this study believe that the relative risk of HAP “is underappreciated.” They asserted, based on these most recent data as well as on previously published analyses, that nonventilated HAP results in “significant increases in cost, length of stay, and mortality.”
The researchers had no disclosures.
SOURCE: Saied WI et al. Crit Care Med. 2018 Nov 7. doi: 10.1097/CCM.0000000000003553.
FROM CRITICAL CARE MEDICINE
Key clinical point: Hospital-acquired pneumonia poses a greater risk of death in the ICU than ventilator-associated pneumonia.
Major finding: After prognostic adjustment, the mortality hazard ratios were 1.82 and 1.38 for HAP and VAP, respectively.
Study details: Observational cohort study.
Disclosures: The researchers had no disclosures.
Source: Saied WI et al. Crit Care Med. 2018 Nov 7; doi: 10.1097/CCM.0000000000003553.
Unit-based assignments: Pros and cons
Geographic cohorting shows ‘varying success’
A relatively recent practice catching on in many different hospitalist groups is geographic cohorting, or unit-based assignments. Traditionally, most hospitalists have had patients assigned on multiple different units. Unit-based assignments have been touted as a way of improving interdisciplinary communication and provider and patient satisfaction.1
How frequently are hospital medicine groups using unit-based assignments? SHM sought to quantify this trend in the recently published 2018 State of Hospital Medicine Report. Overall, among hospital medicine groups serving adults only, a little over one-third (36.4%) of groups reported utilizing unit-based assignments. However, there was significant variation, particularly dependent on group size. Geographic cohorting was used only in 7.6% of groups with 4 or fewer full-time equivalents, and in 68.8% of groups with 30 or more FTE. These data seem logical, as the potential gains from cohorting likely increase with group/hospital size, where physicians would otherwise round on an increasingly large number of units.
As has been shared in the hospital medicine literature, groups have experienced variable success with geographic cohorting. Improvements have been achieved in interprofessional collaboration, efficiency, nursing satisfaction,2 and, in some instances, length of stay. Unit-based assignments have allowed some groups to pilot other interventions, such as interdisciplinary rounds.
But geographic cohorting comes with its implementation challenges, too. For example, in many hospitals, some units have differing telemetry or nursing capabilities. And, in other institutions, there are units providing specialized care, such as care for neurology or oncology patients. The workload for hospitalists caring for particular types of patients may vary, and with specialty units, it may be more difficult to keep a similar census assigned to each hospitalist.
While some groups have noted increased professional satisfaction, others have noted decreases in satisfaction. One reason is that, while the frequency of paging may decrease, this is replaced by an increase in face-to-face interruptions. Also, unit-based assignments in some groups have resulted in hospitalists perceiving they are working in silos because of a decrease in interactions and camaraderie among providers in the same hospital medicine group.
At my home institution, University of California, San Diego, geographic cohorting has largely been a successful and positively perceived change. Our efforts have been particularly successful at one of our two campuses where most units have telemetry capabilities and where we have a dedicated daytime admitter (there are data on this in the Report as well, and a dedicated daytime admitter is the topic of a future Survey Insights column). Unit-based assignments have allowed the implementation of what we’ve termed focused interdisciplinary rounds.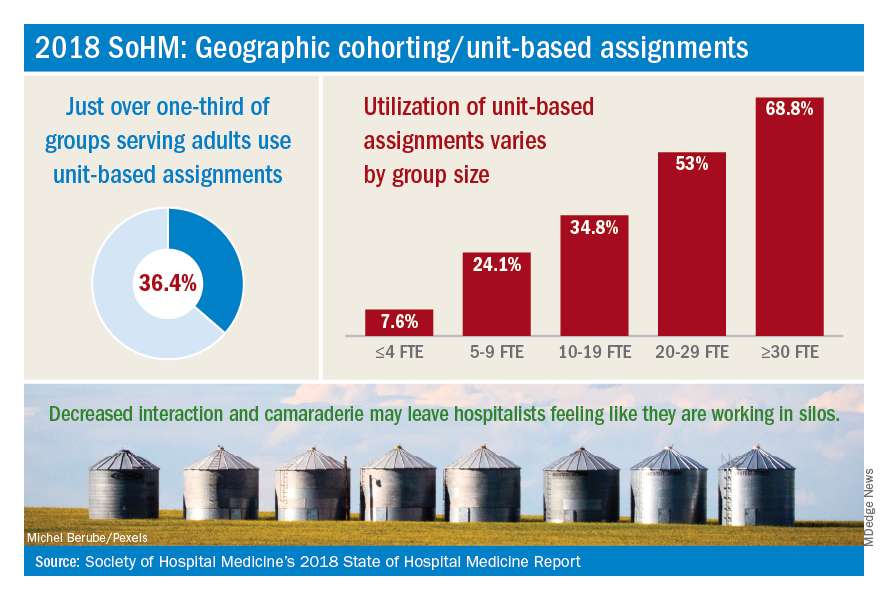
Our unit-based assignments are not perfect – we re-cohort each week when new hospitalists come on service, and some hospitalists are assigned a small number of patients off their home unit. Our internal data have shown a significant increase in patient satisfaction scores, but we have not realized a decrease in length of stay. Despite an overall positive perception, hospitalists have sometimes noted an imbalanced workload – we have a particularly challenging oncology/palliative unit and a daytime admitter that is at times very busy. Our system also requires the use of physician time to assign patients each morning and each week.
In contrast, while we’ve aimed to achieve the same success with unit-based assignments at our other campus, we’ve faced more challenges there. Our other facility is older, and fewer units have telemetry capabilities. A more traditional teaching structure also means that teams take turns with on-call admitting days, as opposed to a daytime admitter structure, and there may not be beds available in the unit assigned to the admitting team of the day.
Overall, geographic cohorting is likely to be considered or implemented in many hospital medicine groups, and efforts have met with varying success. There are certainly pros and cons to every model, and if your group is looking at redesigning services to include unit-based assignments, it’s worth examining the intended outcomes. While unit-based assignments are not for every group, there’s no doubt that this trend has been driven by our specialty’s commitment to outcome-driven process improvement.
Addendum added Feb. 15, 2019: The impact of UC San Diego's efforts discussed in this article are the author's own opinions through limited participation in focused interdisciplinary rounds, and have not been validated with formal data analysis. More study is in progress on the impact of focused interdiscplinary rounds on communication, utilization, and quality metrics. Sarah Horman, MD ([email protected]), Daniel Bouland, MD ([email protected]), and William Frederick, MD ([email protected]), have led efforts at UC San Diego to develop and implement focused interdisciplinary rounds, and may be contacted for further information.
Dr. Huang is physician advisor for care management and associate clinical professor in the division of hospital medicine at the University of California, San Diego. He is a member of SHM’s practice analysis subcommittee.
References
1. O’Leary KJ et al. Interdisciplinary teamwork in hospitals: A review and practical recommendations for improvement. J Hosp Med. 2012 Jan;7(1):48-54.
2. Kara A et al. Hospital-based clinicians’ perceptions of geographic cohorting: Identifying opportunities for improvement. Am J Med Qual. 2018 May/Jun;33(3):303-12.
Geographic cohorting shows ‘varying success’
Geographic cohorting shows ‘varying success’
A relatively recent practice catching on in many different hospitalist groups is geographic cohorting, or unit-based assignments. Traditionally, most hospitalists have had patients assigned on multiple different units. Unit-based assignments have been touted as a way of improving interdisciplinary communication and provider and patient satisfaction.1
How frequently are hospital medicine groups using unit-based assignments? SHM sought to quantify this trend in the recently published 2018 State of Hospital Medicine Report. Overall, among hospital medicine groups serving adults only, a little over one-third (36.4%) of groups reported utilizing unit-based assignments. However, there was significant variation, particularly dependent on group size. Geographic cohorting was used only in 7.6% of groups with 4 or fewer full-time equivalents, and in 68.8% of groups with 30 or more FTE. These data seem logical, as the potential gains from cohorting likely increase with group/hospital size, where physicians would otherwise round on an increasingly large number of units.
As has been shared in the hospital medicine literature, groups have experienced variable success with geographic cohorting. Improvements have been achieved in interprofessional collaboration, efficiency, nursing satisfaction,2 and, in some instances, length of stay. Unit-based assignments have allowed some groups to pilot other interventions, such as interdisciplinary rounds.
But geographic cohorting comes with its implementation challenges, too. For example, in many hospitals, some units have differing telemetry or nursing capabilities. And, in other institutions, there are units providing specialized care, such as care for neurology or oncology patients. The workload for hospitalists caring for particular types of patients may vary, and with specialty units, it may be more difficult to keep a similar census assigned to each hospitalist.
While some groups have noted increased professional satisfaction, others have noted decreases in satisfaction. One reason is that, while the frequency of paging may decrease, this is replaced by an increase in face-to-face interruptions. Also, unit-based assignments in some groups have resulted in hospitalists perceiving they are working in silos because of a decrease in interactions and camaraderie among providers in the same hospital medicine group.
At my home institution, University of California, San Diego, geographic cohorting has largely been a successful and positively perceived change. Our efforts have been particularly successful at one of our two campuses where most units have telemetry capabilities and where we have a dedicated daytime admitter (there are data on this in the Report as well, and a dedicated daytime admitter is the topic of a future Survey Insights column). Unit-based assignments have allowed the implementation of what we’ve termed focused interdisciplinary rounds.
Our unit-based assignments are not perfect – we re-cohort each week when new hospitalists come on service, and some hospitalists are assigned a small number of patients off their home unit. Our internal data have shown a significant increase in patient satisfaction scores, but we have not realized a decrease in length of stay. Despite an overall positive perception, hospitalists have sometimes noted an imbalanced workload – we have a particularly challenging oncology/palliative unit and a daytime admitter that is at times very busy. Our system also requires the use of physician time to assign patients each morning and each week.
In contrast, while we’ve aimed to achieve the same success with unit-based assignments at our other campus, we’ve faced more challenges there. Our other facility is older, and fewer units have telemetry capabilities. A more traditional teaching structure also means that teams take turns with on-call admitting days, as opposed to a daytime admitter structure, and there may not be beds available in the unit assigned to the admitting team of the day.
Overall, geographic cohorting is likely to be considered or implemented in many hospital medicine groups, and efforts have met with varying success. There are certainly pros and cons to every model, and if your group is looking at redesigning services to include unit-based assignments, it’s worth examining the intended outcomes. While unit-based assignments are not for every group, there’s no doubt that this trend has been driven by our specialty’s commitment to outcome-driven process improvement.
Addendum added Feb. 15, 2019: The impact of UC San Diego's efforts discussed in this article are the author's own opinions through limited participation in focused interdisciplinary rounds, and have not been validated with formal data analysis. More study is in progress on the impact of focused interdiscplinary rounds on communication, utilization, and quality metrics. Sarah Horman, MD ([email protected]), Daniel Bouland, MD ([email protected]), and William Frederick, MD ([email protected]), have led efforts at UC San Diego to develop and implement focused interdisciplinary rounds, and may be contacted for further information.
Dr. Huang is physician advisor for care management and associate clinical professor in the division of hospital medicine at the University of California, San Diego. He is a member of SHM’s practice analysis subcommittee.
References
1. O’Leary KJ et al. Interdisciplinary teamwork in hospitals: A review and practical recommendations for improvement. J Hosp Med. 2012 Jan;7(1):48-54.
2. Kara A et al. Hospital-based clinicians’ perceptions of geographic cohorting: Identifying opportunities for improvement. Am J Med Qual. 2018 May/Jun;33(3):303-12.
A relatively recent practice catching on in many different hospitalist groups is geographic cohorting, or unit-based assignments. Traditionally, most hospitalists have had patients assigned on multiple different units. Unit-based assignments have been touted as a way of improving interdisciplinary communication and provider and patient satisfaction.1
How frequently are hospital medicine groups using unit-based assignments? SHM sought to quantify this trend in the recently published 2018 State of Hospital Medicine Report. Overall, among hospital medicine groups serving adults only, a little over one-third (36.4%) of groups reported utilizing unit-based assignments. However, there was significant variation, particularly dependent on group size. Geographic cohorting was used only in 7.6% of groups with 4 or fewer full-time equivalents, and in 68.8% of groups with 30 or more FTE. These data seem logical, as the potential gains from cohorting likely increase with group/hospital size, where physicians would otherwise round on an increasingly large number of units.
As has been shared in the hospital medicine literature, groups have experienced variable success with geographic cohorting. Improvements have been achieved in interprofessional collaboration, efficiency, nursing satisfaction,2 and, in some instances, length of stay. Unit-based assignments have allowed some groups to pilot other interventions, such as interdisciplinary rounds.
But geographic cohorting comes with its implementation challenges, too. For example, in many hospitals, some units have differing telemetry or nursing capabilities. And, in other institutions, there are units providing specialized care, such as care for neurology or oncology patients. The workload for hospitalists caring for particular types of patients may vary, and with specialty units, it may be more difficult to keep a similar census assigned to each hospitalist.
While some groups have noted increased professional satisfaction, others have noted decreases in satisfaction. One reason is that, while the frequency of paging may decrease, this is replaced by an increase in face-to-face interruptions. Also, unit-based assignments in some groups have resulted in hospitalists perceiving they are working in silos because of a decrease in interactions and camaraderie among providers in the same hospital medicine group.
At my home institution, University of California, San Diego, geographic cohorting has largely been a successful and positively perceived change. Our efforts have been particularly successful at one of our two campuses where most units have telemetry capabilities and where we have a dedicated daytime admitter (there are data on this in the Report as well, and a dedicated daytime admitter is the topic of a future Survey Insights column). Unit-based assignments have allowed the implementation of what we’ve termed focused interdisciplinary rounds.
Our unit-based assignments are not perfect – we re-cohort each week when new hospitalists come on service, and some hospitalists are assigned a small number of patients off their home unit. Our internal data have shown a significant increase in patient satisfaction scores, but we have not realized a decrease in length of stay. Despite an overall positive perception, hospitalists have sometimes noted an imbalanced workload – we have a particularly challenging oncology/palliative unit and a daytime admitter that is at times very busy. Our system also requires the use of physician time to assign patients each morning and each week.
In contrast, while we’ve aimed to achieve the same success with unit-based assignments at our other campus, we’ve faced more challenges there. Our other facility is older, and fewer units have telemetry capabilities. A more traditional teaching structure also means that teams take turns with on-call admitting days, as opposed to a daytime admitter structure, and there may not be beds available in the unit assigned to the admitting team of the day.
Overall, geographic cohorting is likely to be considered or implemented in many hospital medicine groups, and efforts have met with varying success. There are certainly pros and cons to every model, and if your group is looking at redesigning services to include unit-based assignments, it’s worth examining the intended outcomes. While unit-based assignments are not for every group, there’s no doubt that this trend has been driven by our specialty’s commitment to outcome-driven process improvement.
Addendum added Feb. 15, 2019: The impact of UC San Diego's efforts discussed in this article are the author's own opinions through limited participation in focused interdisciplinary rounds, and have not been validated with formal data analysis. More study is in progress on the impact of focused interdiscplinary rounds on communication, utilization, and quality metrics. Sarah Horman, MD ([email protected]), Daniel Bouland, MD ([email protected]), and William Frederick, MD ([email protected]), have led efforts at UC San Diego to develop and implement focused interdisciplinary rounds, and may be contacted for further information.
Dr. Huang is physician advisor for care management and associate clinical professor in the division of hospital medicine at the University of California, San Diego. He is a member of SHM’s practice analysis subcommittee.
References
1. O’Leary KJ et al. Interdisciplinary teamwork in hospitals: A review and practical recommendations for improvement. J Hosp Med. 2012 Jan;7(1):48-54.
2. Kara A et al. Hospital-based clinicians’ perceptions of geographic cohorting: Identifying opportunities for improvement. Am J Med Qual. 2018 May/Jun;33(3):303-12.
No difference between PPI prophylaxis, placebo for GI bleeding
There was no significant difference in mortality between critically ill patients who received pantoprazole prophylaxis for gastrointestinal bleeding, and those who received placebo, new findings suggest.
In a multicenter, randomized trial of 3,298 adult patients at risk for gastrointestinal bleeding, 510 patients (31.1%) in the pantoprazole group and 499 (30.4%) in the placebo group had died at 90 days (relative risk, 1.02; 95% confidence interval, 0.91-1.13; P = .76). The results were published in the New England Journal of Medicine.
Patients were aged 18 years or older; had been admitted to the ICU for an acute condition in one of six international centers; and had at least one risk factor for gastrointestinal bleeding including shock, use of anticoagulant agents, renal replacement therapy, mechanical ventilation (expected to last more than 24 hours), any history of liver disease, or any history of or ongoing coagulopathy. A total of 1,645 patients were randomly assigned to receive 40 mg of intravenous pantoprazole once daily and 1,653 received placebo, reported Mette Krag, MD, of the department of intensive care at Rigshospitalet in Copenhagen, and her coauthors.
The primary outcome was 90-day mortality. Secondary outcomes were clinically important events in the ICU, clinically important gastrointestinal bleeding in the ICU, infectious adverse events in the ICU, and days alive without the use of life support within the 90-day period.
One or more clinically important events occurred in 21.9% of patients in the pantoprazole group and in 22.6% in the placebo group (RR, 0.96; 95% CI, 0.83-1.11). In the pantoprazole group, 2.5% of patients had clinically important gastrointestinal bleeding, compared with 4.2% in the placebo group, Dr. Krag and her coauthors wrote.
The findings are similar to other recently published results, which showed “no significant differences ... in the rates of death or infectious complications between patients receiving placebo or no prophylaxis and those receiving proton pump inhibitors,” the authors wrote.
Dr. Krag reported financial support from Innovation Fund Denmark, Ehrenreich’s Foundation, and several other organizations.
SOURCE: Krag M et al. N Engl J Med. 2018 Dec 6. doi: 10.1056/NEJMoa1714919.
This article was updated 12/6/18.
“The take-home message from this trial is that, given the low incidence of clinically important upper gastrointestinal bleeding in the ICU, prophylaxis with a PPI [proton pump inhibitor], if initiated, should be reserved for seriously ill patients who are at high risk for this complication,” wrote Alan Barkun, MD, CM, of McGill University, Montreal, and Marc Bardou, MD, PhD, of the Centre Hospitalier Universitaire Dijon–Bourgogne (France), in an editorial published with the study.
Though 90-day mortality was similar between groups in this trial, “the between-group difference in the rate of important upper gastrointestinal bleeding may still support the recommendation of using a prophylactic PPI” given the absence of a difference in the rate of adverse events between the two groups, they added.
Dr. Barkun reported no disclosures; Dr. Bardou reported support from the French Medicines Agency.
“The take-home message from this trial is that, given the low incidence of clinically important upper gastrointestinal bleeding in the ICU, prophylaxis with a PPI [proton pump inhibitor], if initiated, should be reserved for seriously ill patients who are at high risk for this complication,” wrote Alan Barkun, MD, CM, of McGill University, Montreal, and Marc Bardou, MD, PhD, of the Centre Hospitalier Universitaire Dijon–Bourgogne (France), in an editorial published with the study.
Though 90-day mortality was similar between groups in this trial, “the between-group difference in the rate of important upper gastrointestinal bleeding may still support the recommendation of using a prophylactic PPI” given the absence of a difference in the rate of adverse events between the two groups, they added.
Dr. Barkun reported no disclosures; Dr. Bardou reported support from the French Medicines Agency.
“The take-home message from this trial is that, given the low incidence of clinically important upper gastrointestinal bleeding in the ICU, prophylaxis with a PPI [proton pump inhibitor], if initiated, should be reserved for seriously ill patients who are at high risk for this complication,” wrote Alan Barkun, MD, CM, of McGill University, Montreal, and Marc Bardou, MD, PhD, of the Centre Hospitalier Universitaire Dijon–Bourgogne (France), in an editorial published with the study.
Though 90-day mortality was similar between groups in this trial, “the between-group difference in the rate of important upper gastrointestinal bleeding may still support the recommendation of using a prophylactic PPI” given the absence of a difference in the rate of adverse events between the two groups, they added.
Dr. Barkun reported no disclosures; Dr. Bardou reported support from the French Medicines Agency.
There was no significant difference in mortality between critically ill patients who received pantoprazole prophylaxis for gastrointestinal bleeding, and those who received placebo, new findings suggest.
In a multicenter, randomized trial of 3,298 adult patients at risk for gastrointestinal bleeding, 510 patients (31.1%) in the pantoprazole group and 499 (30.4%) in the placebo group had died at 90 days (relative risk, 1.02; 95% confidence interval, 0.91-1.13; P = .76). The results were published in the New England Journal of Medicine.
Patients were aged 18 years or older; had been admitted to the ICU for an acute condition in one of six international centers; and had at least one risk factor for gastrointestinal bleeding including shock, use of anticoagulant agents, renal replacement therapy, mechanical ventilation (expected to last more than 24 hours), any history of liver disease, or any history of or ongoing coagulopathy. A total of 1,645 patients were randomly assigned to receive 40 mg of intravenous pantoprazole once daily and 1,653 received placebo, reported Mette Krag, MD, of the department of intensive care at Rigshospitalet in Copenhagen, and her coauthors.
The primary outcome was 90-day mortality. Secondary outcomes were clinically important events in the ICU, clinically important gastrointestinal bleeding in the ICU, infectious adverse events in the ICU, and days alive without the use of life support within the 90-day period.
One or more clinically important events occurred in 21.9% of patients in the pantoprazole group and in 22.6% in the placebo group (RR, 0.96; 95% CI, 0.83-1.11). In the pantoprazole group, 2.5% of patients had clinically important gastrointestinal bleeding, compared with 4.2% in the placebo group, Dr. Krag and her coauthors wrote.
The findings are similar to other recently published results, which showed “no significant differences ... in the rates of death or infectious complications between patients receiving placebo or no prophylaxis and those receiving proton pump inhibitors,” the authors wrote.
Dr. Krag reported financial support from Innovation Fund Denmark, Ehrenreich’s Foundation, and several other organizations.
SOURCE: Krag M et al. N Engl J Med. 2018 Dec 6. doi: 10.1056/NEJMoa1714919.
This article was updated 12/6/18.
There was no significant difference in mortality between critically ill patients who received pantoprazole prophylaxis for gastrointestinal bleeding, and those who received placebo, new findings suggest.
In a multicenter, randomized trial of 3,298 adult patients at risk for gastrointestinal bleeding, 510 patients (31.1%) in the pantoprazole group and 499 (30.4%) in the placebo group had died at 90 days (relative risk, 1.02; 95% confidence interval, 0.91-1.13; P = .76). The results were published in the New England Journal of Medicine.
Patients were aged 18 years or older; had been admitted to the ICU for an acute condition in one of six international centers; and had at least one risk factor for gastrointestinal bleeding including shock, use of anticoagulant agents, renal replacement therapy, mechanical ventilation (expected to last more than 24 hours), any history of liver disease, or any history of or ongoing coagulopathy. A total of 1,645 patients were randomly assigned to receive 40 mg of intravenous pantoprazole once daily and 1,653 received placebo, reported Mette Krag, MD, of the department of intensive care at Rigshospitalet in Copenhagen, and her coauthors.
The primary outcome was 90-day mortality. Secondary outcomes were clinically important events in the ICU, clinically important gastrointestinal bleeding in the ICU, infectious adverse events in the ICU, and days alive without the use of life support within the 90-day period.
One or more clinically important events occurred in 21.9% of patients in the pantoprazole group and in 22.6% in the placebo group (RR, 0.96; 95% CI, 0.83-1.11). In the pantoprazole group, 2.5% of patients had clinically important gastrointestinal bleeding, compared with 4.2% in the placebo group, Dr. Krag and her coauthors wrote.
The findings are similar to other recently published results, which showed “no significant differences ... in the rates of death or infectious complications between patients receiving placebo or no prophylaxis and those receiving proton pump inhibitors,” the authors wrote.
Dr. Krag reported financial support from Innovation Fund Denmark, Ehrenreich’s Foundation, and several other organizations.
SOURCE: Krag M et al. N Engl J Med. 2018 Dec 6. doi: 10.1056/NEJMoa1714919.
This article was updated 12/6/18.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Key clinical point: There was no significant difference in mortality between patients who received pantoprazole prophylaxis for gastrointestinal bleeding, and those who received placebo.
Major finding: Just over 31% of patients in the pantoprazole group and 30.4% in the placebo group had died at 90 days (relative risk, 1.02; 95% confidence interval, 0.91-1.13; P = .76).
Study details: A multicenter, randomized trial of 3,298 adult ICU patients at risk for gastrointestinal bleeding.
Disclosures: Dr. Krag reported financial support from Innovation Fund Denmark, Ehrenreich’s Foundation, and several other organizations.
Source: Krag M et al. N Engl J Med. 2018 Dec 6. doi: 10.1056/NEJMoa1714919.
ONC releases draft strategy on reducing EHR burden
A new federal proposal aims to move you away from the keyboard and back face-to-face with your patients.
The draft strategy from the Office of the National Coordinator for Health IT has three aims: to reduce the time and effort to record information in EHRs; to reduce the time and effort required to meet regulatory requirements; and to improve the functionality and ease of use of EHRs.
“This draft strategy includes recommendations that will allow physicians and other clinicians to provide effective care to their patients with a renewed sense of satisfaction for them and their patients,” Andrew Gettinger, MD, chief clinical officer at ONC, and Kate Goodrich, MD, chief medical officer at the Centers for Medicare & Medicaid Services, wrote in a recent blog post. “We are taking one more step toward improving the interoperability and usability of health information by establishing a goal, strategy, and recommendations to reduce regulatory and administrative burdens relating to the use of EHRs.”
To ease documentation burdens, the proposal seeks to “mitigate the EHR-related burden associated with a variety of administrative processes,” the draft strategy notes. “We are considering how reforming certain administrative requirements or optimizing out-of-date requirements for health IT–enabled health care provider work flows can reduce the burden of clinical documentation.”
Specifically, ONC proposes to reduce the overall regulatory burden, leverage data present in the electronic record to reduce the redocumentation, waive certain documentation requirements for participants in advanced alternative payment models (APMs), and promote standardized documentation for ordering and prior authorization.
To improve health IT usability, the draft strategy aims to “address how improvements in the design and use of health IT systems” can reduce burden and calls on clinicians, software developers, and other vendors to collaborate.
To do so, ONC recommends better alignment between EHR design and clinical work flow and making improvements to clinical decision support, as well as improving the presentation of clinical data within EHRs and clinical documentation functionality.
ONC also recommends standardizing basic clinical operations across all EHRs, designing EHR interfaces that are standard to health care delivery, and better integration of the EHR with the exam room.
The draft strategy also includes recommendations to help doctors better understand the financial requirements for successful implementation and optimize the log-in procedures to help reduce burden.
EHR reporting strategies “are designed to address many of the programmatic, technical, and operational challenges raised by stakeholders to reduce EHR-related burden associated with program reporting.”
ONC wants to simplify scoring for the “promoting interoperability” performance category in the Merit-based Incentive Payment System (MIPS) track of the Quality Payment Program and improving other measures of health IT usage; applying additional data standards to make data access, extraction, and integration across multiple systems easier and less costly; and exploring alternate, less burdensome approaches to electronic quality measurement through pilot programs and reporting program incentives.
Finally, public health reporting strategies “look at a set of topics linked to federal, state, local, territorial, and tribal government policies and public health programs, with a specific focus on EPCS [electronic prescribing for controlled substances] and PDMPs [prescription drug monitoring programs]. Where EHR-related burden remains a key barrier to progress in these areas, there are several recommendations for how stakeholders can advance these burden reduction goals related to public health.”
In this area, ONC is recommending increasing adoption of e-prescribing of controlled substances with access to medication history to better inform prescribing of controlled substances, harmonizing reporting requirements across federally funded programs to streamline reporting requirements, and providing better guidance about HIPPA and federal confidentially requirements governing substance abuse disorder to better facilitate the electronic exchange of health information for patient care.
Comments on the report may be submitted electronically through Jan. 28, 2019.
A new federal proposal aims to move you away from the keyboard and back face-to-face with your patients.
The draft strategy from the Office of the National Coordinator for Health IT has three aims: to reduce the time and effort to record information in EHRs; to reduce the time and effort required to meet regulatory requirements; and to improve the functionality and ease of use of EHRs.
“This draft strategy includes recommendations that will allow physicians and other clinicians to provide effective care to their patients with a renewed sense of satisfaction for them and their patients,” Andrew Gettinger, MD, chief clinical officer at ONC, and Kate Goodrich, MD, chief medical officer at the Centers for Medicare & Medicaid Services, wrote in a recent blog post. “We are taking one more step toward improving the interoperability and usability of health information by establishing a goal, strategy, and recommendations to reduce regulatory and administrative burdens relating to the use of EHRs.”
To ease documentation burdens, the proposal seeks to “mitigate the EHR-related burden associated with a variety of administrative processes,” the draft strategy notes. “We are considering how reforming certain administrative requirements or optimizing out-of-date requirements for health IT–enabled health care provider work flows can reduce the burden of clinical documentation.”
Specifically, ONC proposes to reduce the overall regulatory burden, leverage data present in the electronic record to reduce the redocumentation, waive certain documentation requirements for participants in advanced alternative payment models (APMs), and promote standardized documentation for ordering and prior authorization.
To improve health IT usability, the draft strategy aims to “address how improvements in the design and use of health IT systems” can reduce burden and calls on clinicians, software developers, and other vendors to collaborate.
To do so, ONC recommends better alignment between EHR design and clinical work flow and making improvements to clinical decision support, as well as improving the presentation of clinical data within EHRs and clinical documentation functionality.
ONC also recommends standardizing basic clinical operations across all EHRs, designing EHR interfaces that are standard to health care delivery, and better integration of the EHR with the exam room.
The draft strategy also includes recommendations to help doctors better understand the financial requirements for successful implementation and optimize the log-in procedures to help reduce burden.
EHR reporting strategies “are designed to address many of the programmatic, technical, and operational challenges raised by stakeholders to reduce EHR-related burden associated with program reporting.”
ONC wants to simplify scoring for the “promoting interoperability” performance category in the Merit-based Incentive Payment System (MIPS) track of the Quality Payment Program and improving other measures of health IT usage; applying additional data standards to make data access, extraction, and integration across multiple systems easier and less costly; and exploring alternate, less burdensome approaches to electronic quality measurement through pilot programs and reporting program incentives.
Finally, public health reporting strategies “look at a set of topics linked to federal, state, local, territorial, and tribal government policies and public health programs, with a specific focus on EPCS [electronic prescribing for controlled substances] and PDMPs [prescription drug monitoring programs]. Where EHR-related burden remains a key barrier to progress in these areas, there are several recommendations for how stakeholders can advance these burden reduction goals related to public health.”
In this area, ONC is recommending increasing adoption of e-prescribing of controlled substances with access to medication history to better inform prescribing of controlled substances, harmonizing reporting requirements across federally funded programs to streamline reporting requirements, and providing better guidance about HIPPA and federal confidentially requirements governing substance abuse disorder to better facilitate the electronic exchange of health information for patient care.
Comments on the report may be submitted electronically through Jan. 28, 2019.
A new federal proposal aims to move you away from the keyboard and back face-to-face with your patients.
The draft strategy from the Office of the National Coordinator for Health IT has three aims: to reduce the time and effort to record information in EHRs; to reduce the time and effort required to meet regulatory requirements; and to improve the functionality and ease of use of EHRs.
“This draft strategy includes recommendations that will allow physicians and other clinicians to provide effective care to their patients with a renewed sense of satisfaction for them and their patients,” Andrew Gettinger, MD, chief clinical officer at ONC, and Kate Goodrich, MD, chief medical officer at the Centers for Medicare & Medicaid Services, wrote in a recent blog post. “We are taking one more step toward improving the interoperability and usability of health information by establishing a goal, strategy, and recommendations to reduce regulatory and administrative burdens relating to the use of EHRs.”
To ease documentation burdens, the proposal seeks to “mitigate the EHR-related burden associated with a variety of administrative processes,” the draft strategy notes. “We are considering how reforming certain administrative requirements or optimizing out-of-date requirements for health IT–enabled health care provider work flows can reduce the burden of clinical documentation.”
Specifically, ONC proposes to reduce the overall regulatory burden, leverage data present in the electronic record to reduce the redocumentation, waive certain documentation requirements for participants in advanced alternative payment models (APMs), and promote standardized documentation for ordering and prior authorization.
To improve health IT usability, the draft strategy aims to “address how improvements in the design and use of health IT systems” can reduce burden and calls on clinicians, software developers, and other vendors to collaborate.
To do so, ONC recommends better alignment between EHR design and clinical work flow and making improvements to clinical decision support, as well as improving the presentation of clinical data within EHRs and clinical documentation functionality.
ONC also recommends standardizing basic clinical operations across all EHRs, designing EHR interfaces that are standard to health care delivery, and better integration of the EHR with the exam room.
The draft strategy also includes recommendations to help doctors better understand the financial requirements for successful implementation and optimize the log-in procedures to help reduce burden.
EHR reporting strategies “are designed to address many of the programmatic, technical, and operational challenges raised by stakeholders to reduce EHR-related burden associated with program reporting.”
ONC wants to simplify scoring for the “promoting interoperability” performance category in the Merit-based Incentive Payment System (MIPS) track of the Quality Payment Program and improving other measures of health IT usage; applying additional data standards to make data access, extraction, and integration across multiple systems easier and less costly; and exploring alternate, less burdensome approaches to electronic quality measurement through pilot programs and reporting program incentives.
Finally, public health reporting strategies “look at a set of topics linked to federal, state, local, territorial, and tribal government policies and public health programs, with a specific focus on EPCS [electronic prescribing for controlled substances] and PDMPs [prescription drug monitoring programs]. Where EHR-related burden remains a key barrier to progress in these areas, there are several recommendations for how stakeholders can advance these burden reduction goals related to public health.”
In this area, ONC is recommending increasing adoption of e-prescribing of controlled substances with access to medication history to better inform prescribing of controlled substances, harmonizing reporting requirements across federally funded programs to streamline reporting requirements, and providing better guidance about HIPPA and federal confidentially requirements governing substance abuse disorder to better facilitate the electronic exchange of health information for patient care.
Comments on the report may be submitted electronically through Jan. 28, 2019.
Giving hospitalists a larger clinical footprint
Something you did not know about warm handoffs
I am going to teach you something you do not know. I am almost sure of it.
Warm handoffs – a term you often hear within the confines of hospital walls when transferring a patient service to service or ward to ward. You do it in-house, but it’s unlikely you make the same connection when you discharge the same patient or transfer them to an outside entity.
But you have to be asleep under a rock not to have heard or read the changes afoot in the skilled nursing facility (SNF) realm, including the rise of the “SNFist”. Too much variation in use and spending; plus, we are learning patients do not need 25 days cooped up in a rehabilitation facility when 15 might do with a segue into home health for another 10 or 14. Patients like being home, and it costs a lot less.
Unfortunately, we do not do SNF handoffs in the same manner as ICUs. Our bad, and inpatient providers better adapt.
As hospitals decant and quality measures get an intimate look in the rehab space, SNFs will notice sicker patients, and the staff there will be more mindful of the sign outs and the data they receive. Know a Centers for Medicare & Medicaid Services value-based program started on October 1 (just like hospitals – penalties and all) and SNFists, whoever they might be – NP/PA/DO/MD – will also require of hospitals a step-up in information transfer, both in quality and timeliness.
Read the full post at hospitalleader.org.
Also on The Hospital Leader
- “Immigration & the Future of Healthcare: Looking to a Greater Good,” by Jordan Messler, MD, SFHM
- “We Must Become Comfortable Talking about Physician Suicide,” by Tracy Cardin, ACNP-BC, SFHM
- “A New Light in the Darkness: Using Hospital-Based Medication-Assisted Treatment to Tackle the Opioid Crisis,” by Richard Bottner, PA-C
Something you did not know about warm handoffs
Something you did not know about warm handoffs
I am going to teach you something you do not know. I am almost sure of it.
Warm handoffs – a term you often hear within the confines of hospital walls when transferring a patient service to service or ward to ward. You do it in-house, but it’s unlikely you make the same connection when you discharge the same patient or transfer them to an outside entity.
But you have to be asleep under a rock not to have heard or read the changes afoot in the skilled nursing facility (SNF) realm, including the rise of the “SNFist”. Too much variation in use and spending; plus, we are learning patients do not need 25 days cooped up in a rehabilitation facility when 15 might do with a segue into home health for another 10 or 14. Patients like being home, and it costs a lot less.
Unfortunately, we do not do SNF handoffs in the same manner as ICUs. Our bad, and inpatient providers better adapt.
As hospitals decant and quality measures get an intimate look in the rehab space, SNFs will notice sicker patients, and the staff there will be more mindful of the sign outs and the data they receive. Know a Centers for Medicare & Medicaid Services value-based program started on October 1 (just like hospitals – penalties and all) and SNFists, whoever they might be – NP/PA/DO/MD – will also require of hospitals a step-up in information transfer, both in quality and timeliness.
Read the full post at hospitalleader.org.
Also on The Hospital Leader
- “Immigration & the Future of Healthcare: Looking to a Greater Good,” by Jordan Messler, MD, SFHM
- “We Must Become Comfortable Talking about Physician Suicide,” by Tracy Cardin, ACNP-BC, SFHM
- “A New Light in the Darkness: Using Hospital-Based Medication-Assisted Treatment to Tackle the Opioid Crisis,” by Richard Bottner, PA-C
I am going to teach you something you do not know. I am almost sure of it.
Warm handoffs – a term you often hear within the confines of hospital walls when transferring a patient service to service or ward to ward. You do it in-house, but it’s unlikely you make the same connection when you discharge the same patient or transfer them to an outside entity.
But you have to be asleep under a rock not to have heard or read the changes afoot in the skilled nursing facility (SNF) realm, including the rise of the “SNFist”. Too much variation in use and spending; plus, we are learning patients do not need 25 days cooped up in a rehabilitation facility when 15 might do with a segue into home health for another 10 or 14. Patients like being home, and it costs a lot less.
Unfortunately, we do not do SNF handoffs in the same manner as ICUs. Our bad, and inpatient providers better adapt.
As hospitals decant and quality measures get an intimate look in the rehab space, SNFs will notice sicker patients, and the staff there will be more mindful of the sign outs and the data they receive. Know a Centers for Medicare & Medicaid Services value-based program started on October 1 (just like hospitals – penalties and all) and SNFists, whoever they might be – NP/PA/DO/MD – will also require of hospitals a step-up in information transfer, both in quality and timeliness.
Read the full post at hospitalleader.org.
Also on The Hospital Leader
- “Immigration & the Future of Healthcare: Looking to a Greater Good,” by Jordan Messler, MD, SFHM
- “We Must Become Comfortable Talking about Physician Suicide,” by Tracy Cardin, ACNP-BC, SFHM
- “A New Light in the Darkness: Using Hospital-Based Medication-Assisted Treatment to Tackle the Opioid Crisis,” by Richard Bottner, PA-C
DOAC pause yields favorable outcomes for AF patients
San Diego – In patients with atrial fibrillation who had direct oral anticoagulant (DOAC) interruption for an elective surgery, a simple and standardized management strategy yielded low rates of bleeding and thromboembolism, according to results of a prospective study of more than 3,000 patients.
Rates of major bleeding were less than 2% and rates of arterial thromboembolism were less than 1% in patients managed in accordance with the strategy, which foregoes heparin bridging and preoperative coagulation testing, according to investigator James D. Douketis, MD, of St. Joseph’s Healthcare and McMaster University, Hamilton, Ont.
“This is the first study to demonstrate the safety of a standardized perioperative management approach in a patients with atrial fibrillation who are taking a DOAC, and we hope will establish a standard and will have an effect on our clinical practice guidelines,” Dr. Douketis said during a press briefing at the annual meeting of the American Society of Hematology.
This trial offers the “most definitive evidence to date” that atrial fibrillation patients can – in an organized fashion based on bleeding risk – safely stop taking DOACs, said Mark Crowther, MD, chair and professor of medicine at McMaster University.
“This study will almost instantaneously establish a treatment practice and a treatment standard for the vast number of patients in North America and around the world who take these drugs,” added Dr. Crowther, who moderated the press briefing.
The PAUSE study included three parallel cohorts of atrial fibrillation patients taking DOACs (apixaban, dabigatran, or rivaroxaban) who required anticoagulant interruption for an elective surgery or procedure.
The DOAC interruptions were done using standardized protocols based on the pharmacokinetic properties of each DOAC, procedure-associated bleeding risk, and creatinine clearance, the investigators reported.
The interruptions occurred 1 day before and after low bleeding risk surgeries, and 2 days before and after high bleeding risk surgeries, while longer interruptions were used in patients receiving dabigatran who had a creatinine clearance below 50 mL/min.
A total of 3,007 patients at 23 sites in Canada, the United States, and Europe were managed by this approach in the PAUSE study – 1,257 patients receiving apixaban, 668 receiving dabigatran, and 1,082 receiving rivaroxaban – and were evaluated weekly for 30 days post-procedure.
PAUSE is the largest study to date that addresses how to manage the common problem of perioperative DOAC management. It is likely to have a practice-changing impact and will inform future practice guidelines in perioperative care.
The 30-day postoperative rate of major bleeding was low, according to investigators, at 1.35% (95% confidence interval, 0-2.00%) for apixaban, 0.90% (95% CI, 0-1.73%) for dabigatran, and 1.85% (95% CI, 0-2.65%) for rivaroxaban, Dr. Douketis reported.
Likewise, the rate of arterial thromboembolism was low at 0.16% (95% CI, 0-0.48%) for apixaban, 0.6% (95% CI, 0-1.33%) for dabigatran, and 0.37% (95% CI, 0-0.82%) for rivaroxaban, he said.
Most patients (greater than 90%) had minimal to no residual DOAC levels at the time of surgery, the investigator added.
The study was funded by the Canadian Institutes of Health Research and the H&S Foundation of Canada. Dr. Douketis reported disclosures related to Janssen, which makes rivaroxaban; Boehringer-Ingelheim, which makes dabigatran; and other companies. Dr. Crowther reported financial relationships with Bristol-Myers Squibb and other companies.
SOURCE: Douketis J et al. ASH 2018, Abstract LBA-5.
San Diego – In patients with atrial fibrillation who had direct oral anticoagulant (DOAC) interruption for an elective surgery, a simple and standardized management strategy yielded low rates of bleeding and thromboembolism, according to results of a prospective study of more than 3,000 patients.
Rates of major bleeding were less than 2% and rates of arterial thromboembolism were less than 1% in patients managed in accordance with the strategy, which foregoes heparin bridging and preoperative coagulation testing, according to investigator James D. Douketis, MD, of St. Joseph’s Healthcare and McMaster University, Hamilton, Ont.
“This is the first study to demonstrate the safety of a standardized perioperative management approach in a patients with atrial fibrillation who are taking a DOAC, and we hope will establish a standard and will have an effect on our clinical practice guidelines,” Dr. Douketis said during a press briefing at the annual meeting of the American Society of Hematology.
This trial offers the “most definitive evidence to date” that atrial fibrillation patients can – in an organized fashion based on bleeding risk – safely stop taking DOACs, said Mark Crowther, MD, chair and professor of medicine at McMaster University.
“This study will almost instantaneously establish a treatment practice and a treatment standard for the vast number of patients in North America and around the world who take these drugs,” added Dr. Crowther, who moderated the press briefing.
The PAUSE study included three parallel cohorts of atrial fibrillation patients taking DOACs (apixaban, dabigatran, or rivaroxaban) who required anticoagulant interruption for an elective surgery or procedure.
The DOAC interruptions were done using standardized protocols based on the pharmacokinetic properties of each DOAC, procedure-associated bleeding risk, and creatinine clearance, the investigators reported.
The interruptions occurred 1 day before and after low bleeding risk surgeries, and 2 days before and after high bleeding risk surgeries, while longer interruptions were used in patients receiving dabigatran who had a creatinine clearance below 50 mL/min.
A total of 3,007 patients at 23 sites in Canada, the United States, and Europe were managed by this approach in the PAUSE study – 1,257 patients receiving apixaban, 668 receiving dabigatran, and 1,082 receiving rivaroxaban – and were evaluated weekly for 30 days post-procedure.
PAUSE is the largest study to date that addresses how to manage the common problem of perioperative DOAC management. It is likely to have a practice-changing impact and will inform future practice guidelines in perioperative care.
The 30-day postoperative rate of major bleeding was low, according to investigators, at 1.35% (95% confidence interval, 0-2.00%) for apixaban, 0.90% (95% CI, 0-1.73%) for dabigatran, and 1.85% (95% CI, 0-2.65%) for rivaroxaban, Dr. Douketis reported.
Likewise, the rate of arterial thromboembolism was low at 0.16% (95% CI, 0-0.48%) for apixaban, 0.6% (95% CI, 0-1.33%) for dabigatran, and 0.37% (95% CI, 0-0.82%) for rivaroxaban, he said.
Most patients (greater than 90%) had minimal to no residual DOAC levels at the time of surgery, the investigator added.
The study was funded by the Canadian Institutes of Health Research and the H&S Foundation of Canada. Dr. Douketis reported disclosures related to Janssen, which makes rivaroxaban; Boehringer-Ingelheim, which makes dabigatran; and other companies. Dr. Crowther reported financial relationships with Bristol-Myers Squibb and other companies.
SOURCE: Douketis J et al. ASH 2018, Abstract LBA-5.
San Diego – In patients with atrial fibrillation who had direct oral anticoagulant (DOAC) interruption for an elective surgery, a simple and standardized management strategy yielded low rates of bleeding and thromboembolism, according to results of a prospective study of more than 3,000 patients.
Rates of major bleeding were less than 2% and rates of arterial thromboembolism were less than 1% in patients managed in accordance with the strategy, which foregoes heparin bridging and preoperative coagulation testing, according to investigator James D. Douketis, MD, of St. Joseph’s Healthcare and McMaster University, Hamilton, Ont.
“This is the first study to demonstrate the safety of a standardized perioperative management approach in a patients with atrial fibrillation who are taking a DOAC, and we hope will establish a standard and will have an effect on our clinical practice guidelines,” Dr. Douketis said during a press briefing at the annual meeting of the American Society of Hematology.
This trial offers the “most definitive evidence to date” that atrial fibrillation patients can – in an organized fashion based on bleeding risk – safely stop taking DOACs, said Mark Crowther, MD, chair and professor of medicine at McMaster University.
“This study will almost instantaneously establish a treatment practice and a treatment standard for the vast number of patients in North America and around the world who take these drugs,” added Dr. Crowther, who moderated the press briefing.
The PAUSE study included three parallel cohorts of atrial fibrillation patients taking DOACs (apixaban, dabigatran, or rivaroxaban) who required anticoagulant interruption for an elective surgery or procedure.
The DOAC interruptions were done using standardized protocols based on the pharmacokinetic properties of each DOAC, procedure-associated bleeding risk, and creatinine clearance, the investigators reported.
The interruptions occurred 1 day before and after low bleeding risk surgeries, and 2 days before and after high bleeding risk surgeries, while longer interruptions were used in patients receiving dabigatran who had a creatinine clearance below 50 mL/min.
A total of 3,007 patients at 23 sites in Canada, the United States, and Europe were managed by this approach in the PAUSE study – 1,257 patients receiving apixaban, 668 receiving dabigatran, and 1,082 receiving rivaroxaban – and were evaluated weekly for 30 days post-procedure.
PAUSE is the largest study to date that addresses how to manage the common problem of perioperative DOAC management. It is likely to have a practice-changing impact and will inform future practice guidelines in perioperative care.
The 30-day postoperative rate of major bleeding was low, according to investigators, at 1.35% (95% confidence interval, 0-2.00%) for apixaban, 0.90% (95% CI, 0-1.73%) for dabigatran, and 1.85% (95% CI, 0-2.65%) for rivaroxaban, Dr. Douketis reported.
Likewise, the rate of arterial thromboembolism was low at 0.16% (95% CI, 0-0.48%) for apixaban, 0.6% (95% CI, 0-1.33%) for dabigatran, and 0.37% (95% CI, 0-0.82%) for rivaroxaban, he said.
Most patients (greater than 90%) had minimal to no residual DOAC levels at the time of surgery, the investigator added.
The study was funded by the Canadian Institutes of Health Research and the H&S Foundation of Canada. Dr. Douketis reported disclosures related to Janssen, which makes rivaroxaban; Boehringer-Ingelheim, which makes dabigatran; and other companies. Dr. Crowther reported financial relationships with Bristol-Myers Squibb and other companies.
SOURCE: Douketis J et al. ASH 2018, Abstract LBA-5.
REPORTING FROM ASH 2018
Key clinical point:
Major finding: The 30-day postoperative rate of major bleeding was 1.35% (95% CI, 0-2.00%) for apixaban, 0.90% (95% CI, 0-1.73%) for dabigatran, and 1.85% (95% CI, 0-2.65%) for rivaroxaban.
Study details: A prospective study of more than 3,000 subjects with atrial fibrillation who underwent DOAC interruption due to an elective surgery or procedure.
Disclosures: The study was funded by the Canadian Institutes of Health Research and H&S Foundation of Canada. Dr. Douketis reported disclosures related to Janssen, which makes rivaroxaban; Boehringer-Ingelheim, which makes dabigatran; and other companies.
Source: Douketis J et al. ASH 2018, Abstract LBA-5.
EARLY: Angiography within 2 hours of acute non-ST event cut recurrent ischemic events
CHICAGO – Coronary angiography within 2 hours of a diagnosis of non–ST-segment elevation acute coronary syndrome (NSTE-ACS) significantly reduced the risk of recurrent ischemic events as compared to angiography delayed for 12 hours or more, based on the results of the EARLY trial presented at the American Heart Association scientific sessions.
EARLY examined the impacts of not pretreating with a P2Y12-ADP antagonist and of delay before coronary angiography; all study participants received the loading dose of a P2Y12-ADP antagonist at the time of intervention. The early group received angiography within 2 hours, and a delayed group received angiography 12 or more hours after NSTE-ACS.
“Regarding the primary endpoint at 30 days, which is a composite of cardiovascular death and recurrent ischemic event, there is a fivefold lower rate of MACE [major adverse cardiovascular events] in the very-early [group as] compared to the control group,” said Laurent Bonello, MD, PhD, of University Hospital North in Marseilles, France.
The MACE rate was 4.4% in the early group and 21.3% in the delayed group. However, the reduction in MACE was largely because of a reduction in recurrent ischemic events; death rates were similar in the two groups.
The EARLY trial randomized 740 patients at 16 hospitals in France with NSTE-ACS to one of two timing strategies for intervention: within 2 hours of diagnosis, the early-intervention group, and between 12 and 72 hours after diagnosis, the delayed group. Intermediate- and high-risk patients did not receive pretreatment with a P2Y12-ADP antagonist such as clopidogrel before angiography; they received the loading dose at the time of the intervention.
On average, angiography was done within 1 hour in the early group and at 18 hours in the delayed group. Percutaneous coronary intervention (PCI) was performed on 75% of the study population; 3% underwent coronary artery bypass grafting; and 20% received medical therapy.
Dr. Bonello said the purpose of the trial was to settle some uncertainties over the management of NSTE-ACS patients regarding the benefit of pretreatment with P2Y12-ADP antagonists – namely, to evaluate the impact of the lack of pretreatment on the optimal timing of the intervention. “There are no randomized clinical trials available on this specific group of non-ST elevation acute coronary syndrome patients not pretreated for the timing of the invasive strategy,” he said.
Both groups had similar baseline characteristics, such as history of MI, PCI, and aspirin and P2Y12-ADP use, although the delayed group had a higher rate of diabetes (35% vs. 28.3%).
Regarding secondary endpoints, rates of recurrent ischemic events were 2.9% for the early group and 19.8% for the delayed group during hospitalization, and 4.1% vs. 20.7% at 30 days.
Dr. Bonello noted that rates of cardiovascular death were similar for both groups: 0.3% and 0.8% in-hospital deaths, and 0.6% and 1.1% deaths at 30 days.
The disparity in MACE between all subgroups paralleled that of the overall results with two exceptions, Dr. Bonello said: The positive effect of early intervention was less pronounced in women, and there were no differences in MACE rates among those who had interventions other than PCI.
In his discussion of the trial, Gilles Montalescot, MD, PhD, of the Institute of Cardiology at Pitié-Salpêtrière Hospital in Paris, said the EARLY trial with no P2Y12-ADP pretreatment confirms findings of studies before the ACCOAST trial (N Engl J Med. 2013;369:999-1010), that early angiography has no benefit on survival, recurrent MI, revascularization, or bleeding. While the ACCOAST trial, of which Dr. Montalescot was a principal investigator, found no benefit of pretreatment with prasugrel in patients with NTSE-ASC, the EARLY trial extends those findings to other P2Y12-ADP antagonists. “With the immediate angiography strategy, there is a trivial benefit on recurrent ischemia and length of stay, like in the previous studies, thus not related to pretreatment,” he said.
Dr. Montalescot cautioned against embracing this early-intervention strategy with no P2Y12-ADP pretreatment in all situations.
“If you have a conservative strategy for managing the NTSE-ASC patient or if you are in a center far away from a cath lab and your patients have to wait days for a test, yes, you should consider administration of the P2Y12-ADP antagonist,” Dr. Montalescot said.
Dr. Montalescot disclosed receiving grants or honoraria from ADIR, Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, Bristol-Myers Squibb, Beth Israel Deaconess Medical, and Action Coeur Academic Research Organization.
Dr. Bonello reported financial relationships with AstraZeneca, Boston Scientific, Abbott, and Biotronik. The EARLY trial received funding from the French Ministry of Health.
SOURCE: Bonello B et al. AHA scientific sessions, Session LBS.04 19343.
CHICAGO – Coronary angiography within 2 hours of a diagnosis of non–ST-segment elevation acute coronary syndrome (NSTE-ACS) significantly reduced the risk of recurrent ischemic events as compared to angiography delayed for 12 hours or more, based on the results of the EARLY trial presented at the American Heart Association scientific sessions.
EARLY examined the impacts of not pretreating with a P2Y12-ADP antagonist and of delay before coronary angiography; all study participants received the loading dose of a P2Y12-ADP antagonist at the time of intervention. The early group received angiography within 2 hours, and a delayed group received angiography 12 or more hours after NSTE-ACS.
“Regarding the primary endpoint at 30 days, which is a composite of cardiovascular death and recurrent ischemic event, there is a fivefold lower rate of MACE [major adverse cardiovascular events] in the very-early [group as] compared to the control group,” said Laurent Bonello, MD, PhD, of University Hospital North in Marseilles, France.
The MACE rate was 4.4% in the early group and 21.3% in the delayed group. However, the reduction in MACE was largely because of a reduction in recurrent ischemic events; death rates were similar in the two groups.
The EARLY trial randomized 740 patients at 16 hospitals in France with NSTE-ACS to one of two timing strategies for intervention: within 2 hours of diagnosis, the early-intervention group, and between 12 and 72 hours after diagnosis, the delayed group. Intermediate- and high-risk patients did not receive pretreatment with a P2Y12-ADP antagonist such as clopidogrel before angiography; they received the loading dose at the time of the intervention.
On average, angiography was done within 1 hour in the early group and at 18 hours in the delayed group. Percutaneous coronary intervention (PCI) was performed on 75% of the study population; 3% underwent coronary artery bypass grafting; and 20% received medical therapy.
Dr. Bonello said the purpose of the trial was to settle some uncertainties over the management of NSTE-ACS patients regarding the benefit of pretreatment with P2Y12-ADP antagonists – namely, to evaluate the impact of the lack of pretreatment on the optimal timing of the intervention. “There are no randomized clinical trials available on this specific group of non-ST elevation acute coronary syndrome patients not pretreated for the timing of the invasive strategy,” he said.
Both groups had similar baseline characteristics, such as history of MI, PCI, and aspirin and P2Y12-ADP use, although the delayed group had a higher rate of diabetes (35% vs. 28.3%).
Regarding secondary endpoints, rates of recurrent ischemic events were 2.9% for the early group and 19.8% for the delayed group during hospitalization, and 4.1% vs. 20.7% at 30 days.
Dr. Bonello noted that rates of cardiovascular death were similar for both groups: 0.3% and 0.8% in-hospital deaths, and 0.6% and 1.1% deaths at 30 days.
The disparity in MACE between all subgroups paralleled that of the overall results with two exceptions, Dr. Bonello said: The positive effect of early intervention was less pronounced in women, and there were no differences in MACE rates among those who had interventions other than PCI.
In his discussion of the trial, Gilles Montalescot, MD, PhD, of the Institute of Cardiology at Pitié-Salpêtrière Hospital in Paris, said the EARLY trial with no P2Y12-ADP pretreatment confirms findings of studies before the ACCOAST trial (N Engl J Med. 2013;369:999-1010), that early angiography has no benefit on survival, recurrent MI, revascularization, or bleeding. While the ACCOAST trial, of which Dr. Montalescot was a principal investigator, found no benefit of pretreatment with prasugrel in patients with NTSE-ASC, the EARLY trial extends those findings to other P2Y12-ADP antagonists. “With the immediate angiography strategy, there is a trivial benefit on recurrent ischemia and length of stay, like in the previous studies, thus not related to pretreatment,” he said.
Dr. Montalescot cautioned against embracing this early-intervention strategy with no P2Y12-ADP pretreatment in all situations.
“If you have a conservative strategy for managing the NTSE-ASC patient or if you are in a center far away from a cath lab and your patients have to wait days for a test, yes, you should consider administration of the P2Y12-ADP antagonist,” Dr. Montalescot said.
Dr. Montalescot disclosed receiving grants or honoraria from ADIR, Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, Bristol-Myers Squibb, Beth Israel Deaconess Medical, and Action Coeur Academic Research Organization.
Dr. Bonello reported financial relationships with AstraZeneca, Boston Scientific, Abbott, and Biotronik. The EARLY trial received funding from the French Ministry of Health.
SOURCE: Bonello B et al. AHA scientific sessions, Session LBS.04 19343.
CHICAGO – Coronary angiography within 2 hours of a diagnosis of non–ST-segment elevation acute coronary syndrome (NSTE-ACS) significantly reduced the risk of recurrent ischemic events as compared to angiography delayed for 12 hours or more, based on the results of the EARLY trial presented at the American Heart Association scientific sessions.
EARLY examined the impacts of not pretreating with a P2Y12-ADP antagonist and of delay before coronary angiography; all study participants received the loading dose of a P2Y12-ADP antagonist at the time of intervention. The early group received angiography within 2 hours, and a delayed group received angiography 12 or more hours after NSTE-ACS.
“Regarding the primary endpoint at 30 days, which is a composite of cardiovascular death and recurrent ischemic event, there is a fivefold lower rate of MACE [major adverse cardiovascular events] in the very-early [group as] compared to the control group,” said Laurent Bonello, MD, PhD, of University Hospital North in Marseilles, France.
The MACE rate was 4.4% in the early group and 21.3% in the delayed group. However, the reduction in MACE was largely because of a reduction in recurrent ischemic events; death rates were similar in the two groups.
The EARLY trial randomized 740 patients at 16 hospitals in France with NSTE-ACS to one of two timing strategies for intervention: within 2 hours of diagnosis, the early-intervention group, and between 12 and 72 hours after diagnosis, the delayed group. Intermediate- and high-risk patients did not receive pretreatment with a P2Y12-ADP antagonist such as clopidogrel before angiography; they received the loading dose at the time of the intervention.
On average, angiography was done within 1 hour in the early group and at 18 hours in the delayed group. Percutaneous coronary intervention (PCI) was performed on 75% of the study population; 3% underwent coronary artery bypass grafting; and 20% received medical therapy.
Dr. Bonello said the purpose of the trial was to settle some uncertainties over the management of NSTE-ACS patients regarding the benefit of pretreatment with P2Y12-ADP antagonists – namely, to evaluate the impact of the lack of pretreatment on the optimal timing of the intervention. “There are no randomized clinical trials available on this specific group of non-ST elevation acute coronary syndrome patients not pretreated for the timing of the invasive strategy,” he said.
Both groups had similar baseline characteristics, such as history of MI, PCI, and aspirin and P2Y12-ADP use, although the delayed group had a higher rate of diabetes (35% vs. 28.3%).
Regarding secondary endpoints, rates of recurrent ischemic events were 2.9% for the early group and 19.8% for the delayed group during hospitalization, and 4.1% vs. 20.7% at 30 days.
Dr. Bonello noted that rates of cardiovascular death were similar for both groups: 0.3% and 0.8% in-hospital deaths, and 0.6% and 1.1% deaths at 30 days.
The disparity in MACE between all subgroups paralleled that of the overall results with two exceptions, Dr. Bonello said: The positive effect of early intervention was less pronounced in women, and there were no differences in MACE rates among those who had interventions other than PCI.
In his discussion of the trial, Gilles Montalescot, MD, PhD, of the Institute of Cardiology at Pitié-Salpêtrière Hospital in Paris, said the EARLY trial with no P2Y12-ADP pretreatment confirms findings of studies before the ACCOAST trial (N Engl J Med. 2013;369:999-1010), that early angiography has no benefit on survival, recurrent MI, revascularization, or bleeding. While the ACCOAST trial, of which Dr. Montalescot was a principal investigator, found no benefit of pretreatment with prasugrel in patients with NTSE-ASC, the EARLY trial extends those findings to other P2Y12-ADP antagonists. “With the immediate angiography strategy, there is a trivial benefit on recurrent ischemia and length of stay, like in the previous studies, thus not related to pretreatment,” he said.
Dr. Montalescot cautioned against embracing this early-intervention strategy with no P2Y12-ADP pretreatment in all situations.
“If you have a conservative strategy for managing the NTSE-ASC patient or if you are in a center far away from a cath lab and your patients have to wait days for a test, yes, you should consider administration of the P2Y12-ADP antagonist,” Dr. Montalescot said.
Dr. Montalescot disclosed receiving grants or honoraria from ADIR, Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, Bristol-Myers Squibb, Beth Israel Deaconess Medical, and Action Coeur Academic Research Organization.
Dr. Bonello reported financial relationships with AstraZeneca, Boston Scientific, Abbott, and Biotronik. The EARLY trial received funding from the French Ministry of Health.
SOURCE: Bonello B et al. AHA scientific sessions, Session LBS.04 19343.
REPORTING FROM THE AHA SCIENTIFIC SESSIONS
Key clinical point: Coronary angiography within 2 hours of non–ST-segment elevation acute coronary syndrome yielded improved outcomes.
Major finding: Rates of major cardiovascular events were 4.4% with early intervention and 21.3% with delayed intervention.
Study details: Prospective, multicenter, randomized clinical trial of 709 patients.
Disclosures: Dr. Bonello reported financial relationships with AstraZeneca, Boston Scientific, Abbott, and Biotronik. The trial received funding from the French Ministry of Health.
Source: Bonello B et al. AHA scientific sessions, Session LBS.04 19343.
















